false000144702812/312022Q300014470282022-01-012022-09-3000014470282022-11-08xbrli:shares00014470282022-09-30iso4217:USD00014470282021-12-310001447028abus:CollaborationAndContractsMember2022-07-012022-09-300001447028abus:CollaborationAndContractsMember2021-07-012021-09-300001447028abus:CollaborationAndContractsMember2022-01-012022-09-300001447028abus:CollaborationAndContractsMember2021-01-012021-09-300001447028abus:NonCashRoyaltyMember2022-07-012022-09-300001447028abus:NonCashRoyaltyMember2021-07-012021-09-300001447028abus:NonCashRoyaltyMember2022-01-012022-09-300001447028abus:NonCashRoyaltyMember2021-01-012021-09-3000014470282022-07-012022-09-3000014470282021-07-012021-09-3000014470282021-01-012021-09-30iso4217:USDxbrli:shares0001447028us-gaap:CommonStockMember2021-12-310001447028us-gaap:AdditionalPaidInCapitalMember2021-12-310001447028us-gaap:RetainedEarningsMember2021-12-310001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310001447028us-gaap:AdditionalPaidInCapitalMember2022-01-012022-03-3100014470282022-01-012022-03-310001447028abus:OpenMarketSaleAgreementMemberus-gaap:CommonStockMember2022-01-012022-03-310001447028abus:OpenMarketSaleAgreementMember2022-01-012022-03-310001447028us-gaap:CommonStockMember2022-01-012022-03-310001447028abus:SharePurchaseAgreementMemberus-gaap:CommonStockMember2022-01-012022-03-310001447028abus:SharePurchaseAgreementMember2022-01-012022-03-310001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-03-310001447028us-gaap:RetainedEarningsMember2022-01-012022-03-310001447028us-gaap:CommonStockMember2022-03-310001447028us-gaap:AdditionalPaidInCapitalMember2022-03-310001447028us-gaap:RetainedEarningsMember2022-03-310001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-03-3100014470282022-03-310001447028us-gaap:AdditionalPaidInCapitalMember2022-04-012022-06-3000014470282022-04-012022-06-300001447028us-gaap:CommonStockMember2022-04-012022-06-300001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-04-012022-06-300001447028us-gaap:RetainedEarningsMember2022-04-012022-06-300001447028us-gaap:CommonStockMember2022-06-300001447028us-gaap:AdditionalPaidInCapitalMember2022-06-300001447028us-gaap:RetainedEarningsMember2022-06-300001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-06-3000014470282022-06-300001447028us-gaap:AdditionalPaidInCapitalMember2022-07-012022-09-300001447028us-gaap:CommonStockMember2022-07-012022-09-300001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-07-012022-09-300001447028us-gaap:RetainedEarningsMember2022-07-012022-09-300001447028us-gaap:CommonStockMember2022-09-300001447028us-gaap:AdditionalPaidInCapitalMember2022-09-300001447028us-gaap:RetainedEarningsMember2022-09-300001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-09-300001447028us-gaap:PreferredStockMember2020-12-310001447028us-gaap:CommonStockMember2020-12-310001447028us-gaap:AdditionalPaidInCapitalMember2020-12-310001447028us-gaap:RetainedEarningsMember2020-12-310001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-3100014470282020-12-310001447028us-gaap:PreferredStockMember2021-01-012021-03-310001447028us-gaap:RetainedEarningsMember2021-01-012021-03-3100014470282021-01-012021-03-310001447028us-gaap:AdditionalPaidInCapitalMember2021-01-012021-03-310001447028us-gaap:CommonStockMember2021-01-012021-03-310001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-03-310001447028us-gaap:PreferredStockMember2021-03-310001447028us-gaap:CommonStockMember2021-03-310001447028us-gaap:AdditionalPaidInCapitalMember2021-03-310001447028us-gaap:RetainedEarningsMember2021-03-310001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-03-3100014470282021-03-310001447028us-gaap:PreferredStockMember2021-04-012021-06-300001447028us-gaap:RetainedEarningsMember2021-04-012021-06-300001447028us-gaap:AdditionalPaidInCapitalMember2021-04-012021-06-3000014470282021-04-012021-06-300001447028us-gaap:CommonStockMember2021-04-012021-06-300001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-04-012021-06-300001447028us-gaap:PreferredStockMember2021-06-300001447028us-gaap:CommonStockMember2021-06-300001447028us-gaap:AdditionalPaidInCapitalMember2021-06-300001447028us-gaap:RetainedEarningsMember2021-06-300001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-06-3000014470282021-06-300001447028us-gaap:PreferredStockMember2021-07-012021-09-300001447028us-gaap:RetainedEarningsMember2021-07-012021-09-300001447028us-gaap:AdditionalPaidInCapitalMember2021-07-012021-09-300001447028us-gaap:CommonStockMember2021-07-012021-09-300001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-07-012021-09-300001447028us-gaap:PreferredStockMember2021-09-300001447028us-gaap:CommonStockMember2021-09-300001447028us-gaap:AdditionalPaidInCapitalMember2021-09-300001447028us-gaap:RetainedEarningsMember2021-09-300001447028us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-09-3000014470282021-09-300001447028abus:SharePurchaseAgreementMember2022-01-012022-09-300001447028abus:SharePurchaseAgreementMember2021-01-012021-09-300001447028abus:OpenMarketSaleAgreementMember2022-01-012022-09-300001447028abus:OpenMarketSaleAgreementMember2021-01-012021-09-30abus:subsidiary0001447028us-gaap:EmployeeStockOptionMember2022-01-012022-09-3000014470282021-10-180001447028us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2022-09-300001447028us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2022-09-300001447028us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2022-09-300001447028us-gaap:FairValueMeasurementsRecurringMember2022-09-300001447028us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2021-12-310001447028us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-12-310001447028us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2021-12-310001447028us-gaap:FairValueMeasurementsRecurringMember2021-12-310001447028us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-09-300001447028us-gaap:FairValueMeasurementsRecurringMember2021-09-300001447028abus:EmployeeStockOptionLiabilityClassifiedMember2021-12-310001447028abus:EmployeeStockOptionLiabilityClassifiedMember2022-01-012022-09-300001447028abus:EmployeeStockOptionLiabilityClassifiedMember2022-09-300001447028abus:EmployeeStockOptionLiabilityClassifiedMember2020-12-310001447028abus:EmployeeStockOptionLiabilityClassifiedMember2021-01-012021-09-300001447028abus:EmployeeStockOptionLiabilityClassifiedMember2021-09-300001447028us-gaap:CashEquivalentsMemberabus:USGovernmentMoneyMarkeTFundMember2022-09-300001447028us-gaap:CashEquivalentsMember2022-09-300001447028abus:USGovernmentAgencyBondsMember2022-09-300001447028abus:USCorporateBondsMember2022-09-300001447028us-gaap:ShortTermInvestmentsMemberabus:USCorporateBondsMember2022-09-300001447028us-gaap:USTreasuryBillSecuritiesMember2022-09-300001447028us-gaap:ShortTermInvestmentsMemberus-gaap:USTreasuryBillSecuritiesMember2022-09-300001447028abus:USGovernmentBondsMember2022-09-300001447028abus:USGovernmentBondsMemberus-gaap:ShortTermInvestmentsMember2022-09-300001447028us-gaap:ShortTermInvestmentsMember2022-09-300001447028us-gaap:ShortTermInvestmentsMember2022-09-300001447028abus:USGovernmentAgencyBondsMemberabus:LongTermInvestmentsMember2022-09-300001447028abus:USCorporateBondsMemberabus:LongTermInvestmentsMember2022-09-300001447028abus:USGovernmentBondsMemberabus:LongTermInvestmentsMember2022-09-300001447028abus:LongTermInvestmentsMember2022-09-300001447028us-gaap:CashEquivalentsMemberabus:USGovernmentMoneyMarkeTFundMember2021-12-310001447028us-gaap:CashEquivalentsMember2021-12-310001447028abus:USGovernmentAgencyBondsMember2021-09-300001447028us-gaap:USTreasuryBillSecuritiesMember2021-09-300001447028us-gaap:ShortTermInvestmentsMemberus-gaap:USTreasuryBillSecuritiesMember2021-09-300001447028us-gaap:ShortTermInvestmentsMember2021-09-300001447028us-gaap:ShortTermInvestmentsMember2021-09-300001447028abus:USGovernmentAgencyBondsMember2021-12-310001447028abus:USGovernmentAgencyBondsMemberabus:LongTermInvestmentsMember2021-12-310001447028abus:USGovernmentBondsMember2021-12-310001447028abus:USGovernmentBondsMemberabus:LongTermInvestmentsMember2021-12-310001447028abus:LongTermInvestmentsMember2021-12-310001447028abus:GenevantSciencesCorporationMember2022-09-30xbrli:pure0001447028abus:GenevantSciencesCorporationMember2022-01-012022-09-300001447028abus:OMERSMembersrt:MinimumMember2019-07-022019-07-020001447028abus:OMERSMembersrt:MaximumMember2019-07-022019-07-020001447028abus:ONPATTROGlobalNetSalesMember2019-07-022019-07-020001447028abus:OMERSMember2019-01-012019-01-010001447028abus:OMERSMember2019-07-022019-07-020001447028abus:OMERSMember2019-07-020001447028abus:OMERSMember2022-01-012022-09-300001447028abus:OMERSMember2019-07-022022-06-300001447028abus:OMERSMember2021-01-012021-09-300001447028srt:MinimumMember2022-01-012022-09-300001447028srt:MaximumMember2022-01-012022-09-300001447028abus:ONPATTROGlobalNetSalesMember2022-01-012022-09-300001447028abus:ArbitrationWithTheUniversityOfBritishColumbiaMember2020-12-182020-12-180001447028abus:ArbitrationWithTheUniversityOfBritishColumbiaMember2022-01-012022-09-300001447028abus:ArbutusInc.Memberabus:EnantigensSellingShareholdersMember2014-10-310001447028us-gaap:FairValueMeasurementsRecurringMemberabus:EnantigensSellingShareholdersMember2014-10-310001447028us-gaap:FairValueMeasurementsRecurringMemberabus:EnantigensSellingShareholdersMember2022-09-300001447028abus:QiluPharmaceuticalCoLTDMemberabus:OneTimeUpfrontCashPaymentMember2022-01-052022-01-050001447028abus:QiluPharmaceuticalCoLTDMember2021-12-130001447028abus:QiluPharmaceuticalCoLTDMember2022-01-012022-09-300001447028abus:QiluPharmaceuticalCoLTDMemberus-gaap:CommonStockMember2021-12-130001447028abus:QiluPharmaceuticalCoLTDMemberus-gaap:CommonStockMember2022-01-062022-01-060001447028us-gaap:CommonStockMemberabus:QiluPharmaceuticalCoLTDMember2021-12-130001447028abus:QiluPharmaceuticalCoLTDMember2022-01-012022-09-300001447028abus:QiluPharmaceuticalCoLTDMember2022-09-300001447028abus:QiluPharmaceuticalCoLTDMember2022-09-300001447028abus:QiluPharmaceuticalCoLTDMemberus-gaap:LicenseMember2022-07-012022-09-300001447028abus:QiluPharmaceuticalCoLTDMemberus-gaap:LicenseMember2022-01-012022-09-300001447028abus:QiluPharmaceuticalCoLTDMember2022-07-012022-09-300001447028abus:VaccitechMember2022-07-012022-09-300001447028abus:VaccitechMember2022-01-012022-09-300001447028abus:VaccitechMember2021-01-012021-09-300001447028abus:VaccitechMember2021-07-012021-09-300001447028abus:AssemblyBiosciencesIncMember2022-07-012022-09-300001447028abus:AssemblyBiosciencesIncMember2022-01-012022-09-300001447028abus:AssemblyBiosciencesIncMember2021-07-012021-09-300001447028abus:AssemblyBiosciencesIncMember2021-01-012021-09-300001447028abus:XChemIncAndProterosMember2022-07-012022-09-300001447028abus:XChemIncAndProterosMember2022-01-012022-09-300001447028abus:XChemIncAndProterosMember2021-07-012021-09-300001447028abus:XChemIncAndProterosMember2021-01-012021-09-30abus:product0001447028abus:OMERSMembersrt:MinimumMember2013-01-012013-12-310001447028abus:OMERSMembersrt:MaximumMember2013-01-012013-12-310001447028abus:ONPATTROGlobalNetSalesMember2013-01-012013-12-310001447028abus:OMERSMember2019-01-010001447028us-gaap:LicenseMemberabus:AcuitasTherapeuticsInc.Member2022-07-012022-09-300001447028us-gaap:LicenseMemberabus:AcuitasTherapeuticsInc.Member2021-07-012021-09-300001447028us-gaap:LicenseMemberabus:AcuitasTherapeuticsInc.Member2022-01-012022-09-300001447028us-gaap:LicenseMemberabus:AcuitasTherapeuticsInc.Member2021-01-012021-09-300001447028abus:QiluPharmaceuticalCoLTDMemberus-gaap:LicenseMember2021-07-012021-09-300001447028abus:QiluPharmaceuticalCoLTDMemberus-gaap:LicenseMember2021-01-012021-09-300001447028us-gaap:LicenseMemberabus:OtherMilestoneandRoyaltyPaymentsMember2022-07-012022-09-300001447028us-gaap:LicenseMemberabus:OtherMilestoneandRoyaltyPaymentsMember2021-07-012021-09-300001447028us-gaap:LicenseMemberabus:OtherMilestoneandRoyaltyPaymentsMember2022-01-012022-09-300001447028us-gaap:LicenseMemberabus:OtherMilestoneandRoyaltyPaymentsMember2021-01-012021-09-300001447028abus:NonCashRoyaltyMemberabus:AlnylamPharmaceuticalsIncMember2022-07-012022-09-300001447028abus:NonCashRoyaltyMemberabus:AlnylamPharmaceuticalsIncMember2021-07-012021-09-300001447028abus:NonCashRoyaltyMemberabus:AlnylamPharmaceuticalsIncMember2022-01-012022-09-300001447028abus:NonCashRoyaltyMemberabus:AlnylamPharmaceuticalsIncMember2021-01-012021-09-300001447028abus:Jan2020RegistrationStmtMemberus-gaap:CommonStockMember2020-01-100001447028abus:Jan2020ProspectusSupplementMemberabus:JefferiesLLCMemberus-gaap:CommonStockMemberabus:Jan2020RegistrationStmtMember2020-01-100001447028abus:JefferiesLLCMemberus-gaap:CommonStockMemberabus:Jan2020RegistrationStmtMemberabus:Aug2020ProspectusSupplementMember2020-08-070001447028us-gaap:CommonStockMemberabus:Oct2020RegistrationStmtMember2020-10-220001447028abus:JefferiesLLCMemberus-gaap:CommonStockMemberabus:Mar2021ProspectusSupplementAgreementMemberabus:Oct2020RegistrationStmtMember2021-03-040001447028abus:JefferiesLLCMemberabus:October2021ProspectusSupplementMemberus-gaap:CommonStockMemberabus:Oct2020RegistrationStmtMember2021-10-080001447028abus:Nov2021RegistrationStmtMemberus-gaap:CommonStockMember2021-11-040001447028abus:JefferiesLLCMemberus-gaap:CommonStockMemberabus:Jan2020Oct2020AndNov2021RegistrationStmtsMemberabus:March2022ProspectusSupplementMember2022-03-030001447028abus:JefferiesLLCMemberus-gaap:CommonStockMember2022-01-012022-09-300001447028abus:JefferiesLLCMemberus-gaap:CommonStockMemberabus:SaleAgreementMember2022-01-012022-09-300001447028abus:JefferiesLLCMemberus-gaap:CommonStockMember2021-07-012021-09-300001447028abus:JefferiesLLCMemberus-gaap:CommonStockMemberabus:SaleAgreementMember2021-07-012021-09-300001447028abus:JefferiesLLCMemberus-gaap:CommonStockMemberabus:March2022ProspectusSupplementMember2022-09-300001447028abus:JefferiesLLCMemberabus:October2021ProspectusSupplementMemberus-gaap:CommonStockMember2022-09-300001447028abus:ArbutusPlansMember2022-07-012022-09-300001447028abus:ArbutusPlansMember2021-07-012021-09-300001447028abus:ArbutusPlansMember2022-01-012022-09-300001447028abus:ArbutusPlansMember2021-01-012021-09-300001447028us-gaap:ResearchAndDevelopmentExpenseMember2022-07-012022-09-300001447028us-gaap:ResearchAndDevelopmentExpenseMember2021-07-012021-09-300001447028us-gaap:ResearchAndDevelopmentExpenseMember2022-01-012022-09-300001447028us-gaap:ResearchAndDevelopmentExpenseMember2021-01-012021-09-300001447028us-gaap:GeneralAndAdministrativeExpenseMember2022-07-012022-09-300001447028us-gaap:GeneralAndAdministrativeExpenseMember2021-07-012021-09-300001447028us-gaap:GeneralAndAdministrativeExpenseMember2022-01-012022-09-300001447028us-gaap:GeneralAndAdministrativeExpenseMember2021-01-012021-09-300001447028abus:RoivantSciencesLtdMemberus-gaap:ConvertiblePreferredStockMember2017-10-022017-10-020001447028abus:RoivantSciencesLtdMemberus-gaap:ConvertiblePreferredStockMember2017-10-020001447028abus:RoivantSciencesLtdMember2022-09-300001447028abus:RoivantSciencesLtdMemberus-gaap:ConvertiblePreferredStockMember2022-01-012022-09-300001447028abus:ResearchAndDevelopmentServicesMemberabus:GenevantSciencesCorporationMember2021-01-012021-09-300001447028abus:ResearchAndDevelopmentServicesMemberabus:GenevantSciencesCorporationMember2021-07-012021-09-300001447028abus:ResearchAndDevelopmentServicesMemberabus:GenevantSciencesCorporationMember2022-07-012022-09-300001447028abus:ResearchAndDevelopmentServicesMemberabus:GenevantSciencesCorporationMember2022-01-012022-09-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended September 30, 2022
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Transition Period from to
Commission File Number: 001-34949
ARBUTUS BIOPHARMA CORPORATION
(Exact Name of Registrant as Specified in Its Charter)
| | | | | | | | |
| British Columbia, Canada | | 98-0597776 |
| (State or Other Jurisdiction of | | (I.R.S. Employer |
| Incorporation or Organization) | | Identification No.) |
701 Veterans Circle, Warminster, PA 18974
(Address of Principal Executive Offices and Zip Code)
267-469-0914
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Shares, without par value | ABUS | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | Accelerated filer | Non-accelerated filer | Smaller reporting company | Emerging growth company |
| ☐ | ☐ | ☒ | ☒ | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
As of November 8, 2022, the registrant had 156,467,085 common shares, without par value, outstanding.
ARBUTUS BIOPHARMA CORPORATION
PART I. FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS (UNAUDITED)
ARBUTUS BIOPHARMA CORPORATION
Condensed Consolidated Balance Sheets
(Unaudited)
(In thousands of U.S. Dollars, except share and per share amounts)
| | | | | | | | | | | |
| September 30, 2022 | | December 31, 2021 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 24,004 | | | $ | 109,282 | |
| Investments in marketable securities, current | 110,714 | | | 46,035 | |
| Accounts receivable | 1,835 | | | 899 | |
| Prepaid expenses and other current assets | 4,583 | | | 4,445 | |
| Total current assets | 141,136 | | | 160,661 | |
Property and equipment, net of accumulated depreciation of $10,475 (December 31, 2021: $9,374) | 5,241 | | | 5,983 | |
| Investments in marketable securities, non-current | 55,436 | | | 35,688 | |
| Right of use asset | 1,821 | | | 2,092 | |
| Other non-current assets | 167 | | | 61 | |
| Total assets | $ | 203,801 | | | $ | 204,485 | |
| Liabilities and stockholders’ equity | | | |
| Current liabilities: | | | |
| Accounts payable and accrued liabilities | $ | 12,268 | | | $ | 10,838 | |
| Deferred license revenue, current | 14,878 | | | — | |
| Lease liability, current | 360 | | | 383 | |
| Total current liabilities | 27,506 | | | 11,221 | |
| Liability related to sale of future royalties | 12,316 | | | 16,296 | |
| Deferred license revenue, non-current | 10,585 | | | — | |
| Contingent consideration | 5,922 | | | 5,298 | |
| Lease liability, non-current | 1,955 | | | 2,231 | |
| Total liabilities | 58,284 | | | 35,046 | |
| Stockholders’ equity | | | |
| Common shares | | | |
| Authorized: unlimited number without par value | | | |
Issued and outstanding: 152,711,702 (December 31, 2021: 144,987,736) | 1,307,654 | | | 1,286,636 | |
| Additional paid-in capital | 70,738 | | | 65,485 | |
| Deficit | (1,181,871) | | | (1,134,347) | |
| Accumulated other comprehensive loss | (51,004) | | | (48,335) | |
Total stockholders’ equity | 145,517 | | | 169,439 | |
| Total liabilities and stockholders’ equity | $ | 203,801 | | | $ | 204,485 | |
See accompanying notes to the condensed consolidated financial statements.
ARBUTUS BIOPHARMA CORPORATION
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
(In thousands of U.S. Dollars, except share and per share amounts)
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, | | Nine Months Ended September 30, |
| 2022 | | 2021 | | 2022 | | 2021 |
| Revenue | | | | | | | |
| Collaborations and licenses | $ | 3,607 | | | $ | 1,480 | | | $ | 27,381 | | | $ | 3,819 | |
| Non-cash royalty revenue | 2,345 | | | 1,860 | | | 5,393 | | | 3,963 | |
| Total Revenue | 5,952 | | | 3,340 | | | 32,774 | | | 7,782 | |
| Operating expenses | | | | | | | |
| Research and development | 20,055 | | | 16,709 | | | 61,459 | | | 46,290 | |
| General and administrative | 3,493 | | | 4,183 | | | 13,585 | | | 12,539 | |
| Change in fair value of contingent consideration | 215 | | | 856 | | | 624 | | | 1,679 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Total operating expenses | 23,763 | | | 21,748 | | | 75,668 | | | 60,508 | |
| Loss from operations | (17,811) | | | (18,408) | | | (42,894) | | | (52,726) | |
| Other income (loss) | | | | | | | |
| Interest income | 694 | | | 27 | | | 1,249 | | | 97 | |
| Interest expense | (429) | | | (762) | | | (1,417) | | | (2,297) | |
| Foreign exchange loss | (21) | | | (15) | | | (18) | | | — | |
| | | | | | | |
| Total other income (loss) | 244 | | | (750) | | | (186) | | | (2,200) | |
| Loss before income taxes | (17,567) | | | (19,158) | | | (43,080) | | | (54,926) | |
| Income tax expense | — | | | — | | | (4,444) | | | — | |
| Net loss | $ | (17,567) | | | $ | (19,158) | | | $ | (47,524) | | | $ | (54,926) | |
| Items applicable to preferred shares: | | | | | | | |
| Dividend accretion of convertible preferred shares | — | | | (5,087) | | | — | | | (11,565) | |
| Net loss attributable to common shares | $ | (17,567) | | | $ | (24,245) | | | $ | (47,524) | | | $ | (66,491) | |
| | | | | | | |
| Loss per share | | | | | | | |
| Basic and diluted | $ | (0.12) | | | $ | (0.24) | | | $ | (0.32) | | | $ | (0.68) | |
| Weighted average number of common shares | | | | | | | |
| Basic and diluted | 150,995,191 | | | 101,286,351 | | | 149,385,999 | | | 97,174,253 | |
| | | | | | | |
| Comprehensive loss | | | | | | | |
| Unrealized loss on available-for-sale securities | $ | (907) | | | $ | (31) | | | $ | (2,669) | | | $ | (16) | |
| Comprehensive loss | $ | (18,474) | | | $ | (19,189) | | | $ | (50,193) | | | $ | (54,942) | |
See accompanying notes to the condensed consolidated financial statements.
ARBUTUS BIOPHARMA CORPORATION
Condensed Consolidated Statement of Stockholders’ Equity
(Unaudited)
(In thousands of U.S. Dollars, except share and per share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Shares | | | | | | | | |
| | Number of Shares | | Share Capital | | Additional Paid-In Capital | | Deficit | | Accumulated Other Comprehensive Loss | | Total Stockholders' Equity |
| Balance December 31, 2021 | 144,987,736 | | | $ | 1,286,636 | | | $ | 65,485 | | | $ | (1,134,347) | | | $ | (48,335) | | | $ | 169,439 | |
| Stock-based compensation | — | | | — | | | 1,736 | | | — | | | — | | | 1,736 | |
| Certain fair value adjustments to liability stock option awards | — | | | — | | | 21 | | | — | | | — | | | 21 | |
| Issuance of common shares pursuant to the Open Market Sale Agreement | 69,048 | | | 268 | | | — | | | — | | | — | | | 268 | |
| Issuance of common shares pursuant to exercise of options | 5,000 | | | 18 | | | (10) | | | — | | | — | | | 8 | |
| Issuance of common shares pursuant to ESPP | 86,501 | | | 317 | | | (81) | | | — | | | — | | | 236 | |
| Issuance of common shares pursuant to Share Purchase Agreement | 3,579,952 | | | 10,973 | | | — | | | — | | | — | | | 10,973 | |
| Unrealized loss on available-for-sale securities | — | | | — | | | — | | | — | | | (1,071) | | | (1,071) | |
| Net loss | — | | | — | | | — | | | (15,765) | | | — | | | (15,765) | |
| Balance March 31, 2022 | 148,728,237 | | | $ | 1,298,212 | | | $ | 67,151 | | | $ | (1,150,112) | | | $ | (49,406) | | | $ | 165,845 | |
| Stock-based compensation | — | | | — | | | 2,064 | | | — | | | — | | | 2,064 | |
| Certain fair value adjustments to liability stock option awards | — | | | — | | | 3 | | | — | | | — | | | 3 | |
| | | | | | | | | | | |
| Issuance of common shares pursuant to exercise of options | 66,025 | | | 197 | | | (84) | | | — | | | — | | | 113 | |
| | | | | | | | | | | |
| Unrealized loss on available-for-sale securities | — | | | — | | | — | | | — | | | (691) | | | (691) | |
| Net loss | — | | | — | | | — | | | (14,192) | | | — | | | (14,192) | |
| Balance June 30, 2022 | 148,794,262 | | | $ | 1,298,409 | | | $ | 69,134 | | | $ | (1,164,304) | | | $ | (50,097) | | | $ | 153,142 | |
| Stock-based compensation | — | | | — | | | 1,715 | | | — | | | — | | | 1,715 | |
| Certain fair value adjustments to liability stock option awards | — | | | — | | | 2 | | | — | | | — | | | 2 | |
| Issuance of common shares pursuant to the Open Market Sale Agreement | 3,832,717 | | | 8,973 | | | — | | | — | | | — | | | 8,973 | |
| | | | | | | | | | | |
| Issuance of common shares pursuant to ESPP | 84,723 | | | 272 | | | (113) | | | — | | | — | | | 159 | |
| Unrealized loss on available-for-sale securities | — | | | — | | | — | | | — | | | (907) | | | (907) | |
| Net loss | — | | | — | | | — | | | (17,567) | | | — | | | (17,567) | |
| Balance September 30, 2022 | 152,711,702 | | | $ | 1,307,654 | | | $ | 70,738 | | | $ | (1,181,871) | | | $ | (51,004) | | | $ | 145,517 | |
See accompanying notes to the condensed consolidated financial statements.
ARBUTUS BIOPHARMA CORPORATION
Condensed Consolidated Statement of Stockholders’ Equity
(Unaudited)
(In thousands of U.S. Dollars, except share and per share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Convertible Preferred Shares | | Common Shares | | | | | | | | |
| | Number of Shares | | Share Capital | | Number of Shares | | Share Capital | | Additional Paid-In Capital | | Deficit | | Accumulated Other Comprehensive Loss | | Total Stockholders' Equity |
| Balance December 31, 2020 | 1,164,000 | | | $ | 149,408 | | | 89,678,722 | | | $ | 985,939 | | | $ | 60,751 | | | $ | (1,045,961) | | | $ | (48,171) | | | $ | 101,966 | |
| Accretion of accumulated dividends on Preferred Shares | — | | | 3,212 | | | — | | | — | | | — | | | (3,212) | | | — | | | — | |
| Stock-based compensation | — | | | — | | | — | | | — | | | 1,647 | | | — | | | — | | | 1,647 | |
| Certain fair value adjustments to liability stock option awards | — | | | — | | | — | | | — | | | 40 | | | — | | | — | | | 40 | |
| Issuance of common shares pursuant to the Open Market Sale Agreement | — | | | — | | | 6,395,780 | | | 26,419 | | | — | | | — | | | — | | | 26,419 | |
| Issuance of common shares pursuant to exercise of options | — | | | — | | | 65,952 | | | 335 | | | (127) | | | — | | | — | | | 208 | |
| Issuance of common shares pursuant to ESPP | — | | | — | | | 104,917 | | | 425 | | | (178) | | | — | | | — | | | 247 | |
| Unrealized gain on available-for-sale securities | — | | | — | | | — | | | — | | | — | | | — | | | 3 | | | 3 | |
| Net loss | — | | | — | | | — | | | — | | | — | | | (16,381) | | | — | | | (16,381) | |
| Balance March 31, 2021 | 1,164,000 | | | $ | 152,620 | | | 96,245,371 | | | $ | 1,013,118 | | | $ | 62,133 | | | $ | (1,065,554) | | | $ | (48,168) | | | $ | 114,149 | |
| Accretion of accumulated dividends on Preferred Shares | — | | | 3,266 | | | — | | | — | | | | | (3,266) | | | — | | | — | |
| Stock-based compensation | — | | | — | | | — | | | — | | | 1,758 | | | — | | | — | | | 1,758 | |
| Certain fair value adjustments to liability stock option awards | — | | | — | | | — | | | — | | | 51 | | | — | | | — | | | 51 | |
| Issuance of common shares pursuant to the Open Market Sale Agreement | — | | | — | | | 1,450,145 | | | 4,274 | | | — | | | — | | | — | | | 4,274 | |
| Issuance of common shares pursuant to exercise of options | — | | | — | | | 4,500 | | | 24 | | | (9) | | | — | | | — | | | 15 | |
| | | | | | | | | | | | | | | |
| Unrealized gain on available-for-sale securities | — | | | — | | | — | | | — | | | — | | | — | | | (31) | | | (31) | |
| Net loss | — | | | — | | | — | | | — | | | — | | | (19,387) | | | — | | | (19,387) | |
| Balance June 30, 2021 | 1,164,000 | | | $ | 155,886 | | | 97,700,016 | | | $ | 1,017,416 | | | $ | 63,933 | | | $ | (1,088,207) | | | $ | (48,199) | | | $ | 100,829 | |
| Accretion of accumulated dividends on Preferred Shares | — | | | 5,087 | | | — | | | — | | | — | | | (5,087) | | | — | | | — | |
| Stock-based compensation | — | | | — | | | — | | | — | | | 1,549 | | | — | | | — | | | 1,549 | |
| Certain fair value adjustments to liability stock option awards | — | | | — | | | — | | | — | | | (44) | | | — | | | — | | | (44) | |
| Issuance of common shares pursuant to the Open Market Sale Agreement | — | | | — | | | 11,869 | | | 44,736 | | | — | | | — | | | — | | | 44,736 | |
| Issuance of common shares pursuant to exercise of options | — | | | — | | | 604 | | | 3,166 | | | (1,164) | | | — | | | — | | | 2,002 | |
| Issuance of common shares pursuant to ESPP | — | | | — | | | 91 | | | 392 | | | (178) | | | — | | | — | | | 214 | |
| Unrealized gain on available-for-sale securities | — | | | — | | | — | | | — | | | — | | | — | | | 12 | | | 12 | |
| Net loss | — | | | — | | | — | | | — | | | — | | | (19,158) | | | — | | | (19,158) | |
| Balance September 30, 2021 | 1,164,000 | | | $ | 160,973 | | | 110,264,915 | | | $ | 1,065,710 | | | $ | 64,096 | | | $ | (1,112,452) | | | $ | (48,187) | | | $ | 130,140 | |
See accompanying notes to the condensed consolidated financial statements.
ARBUTUS BIOPHARMA CORPORATION
Condensed Consolidated Statements of Cash Flow
(Unaudited)
(In thousands of U.S. Dollars)
| | | | | | | | | | | |
| | Nine Months Ended September 30, |
| | 2022 | | 2021 |
| OPERATING ACTIVITIES | | | |
| Net loss | $ | (47,524) | | | $ | (54,926) | |
| Non-cash items: | | | |
| Depreciation | 1,120 | | | 1,326 | |
| Gain on sale of property and equipment | (20) | | | — | |
| Stock-based compensation expense | 5,515 | | | 4,993 | |
| Change in fair value of contingent consideration | 624 | | | 1,679 | |
| Non-cash royalty revenue | (5,393) | | | (3,963) | |
| Non-cash interest expense | 1,413 | | | 2,292 | |
| Net accretion and amortization of investments in marketable securities | 170 | | | 753 | |
| Net change in operating items: | | | |
| Accounts receivable | (936) | | | (355) | |
| Prepaid expenses and other assets | 27 | | | (67) | |
| Accounts payable and accrued liabilities | 1,456 | | | 585 | |
| Deferred license revenue | 25,463 | | | — | |
| Other liabilities | (281) | | | (243) | |
| Net cash used in operating activities | (18,366) | | | (47,926) | |
| INVESTING ACTIVITIES | | | |
| Purchase of investments | (117,266) | | | (54,156) | |
| Disposition of investments | 30,000 | | | 50,350 | |
| Proceeds from sale of property and equipment | 20 | | | — | |
| Acquisition of property and equipment | (378) | | | (751) | |
| Net cash used in investing activities | (87,624) | | | (4,557) | |
| FINANCING ACTIVITIES | | | |
| Issuance of common shares pursuant to Share Purchase Agreement | 10,973 | | | — | |
| Issuance of common shares pursuant to the Open Market Sale agreement | 9,241 | | | 75,429 | |
| Issuance of common shares pursuant to exercise of options | 121 | | | 2,225 | |
| Issuance of common shares pursuant to ESPP | 395 | | | 461 | |
| Net cash provided by financing activities | 20,730 | | | 78,115 | |
| Effect of foreign exchange rate changes on cash and cash equivalents | (18) | | | — | |
| (Decrease)/increase in cash and cash equivalents | (85,278) | | | 25,632 | |
| Cash and cash equivalents, beginning of period | 109,282 | | | 52,251 | |
| Cash and cash equivalents, end of period | $ | 24,004 | | | $ | 77,883 | |
| | | |
| Supplemental cash flow information | | | |
| Preferred shares dividends accrued | $ | — | | | $ | (11,565) | |
See accompanying notes to the condensed consolidated financial statements.
ARBUTUS BIOPHARMA CORPORATION
Notes to Condensed Consolidated Financial Statements
(Tabular amounts in thousands of U.S. Dollars, except share and per share amounts)
1. Nature of business and future operations
Description of the Business
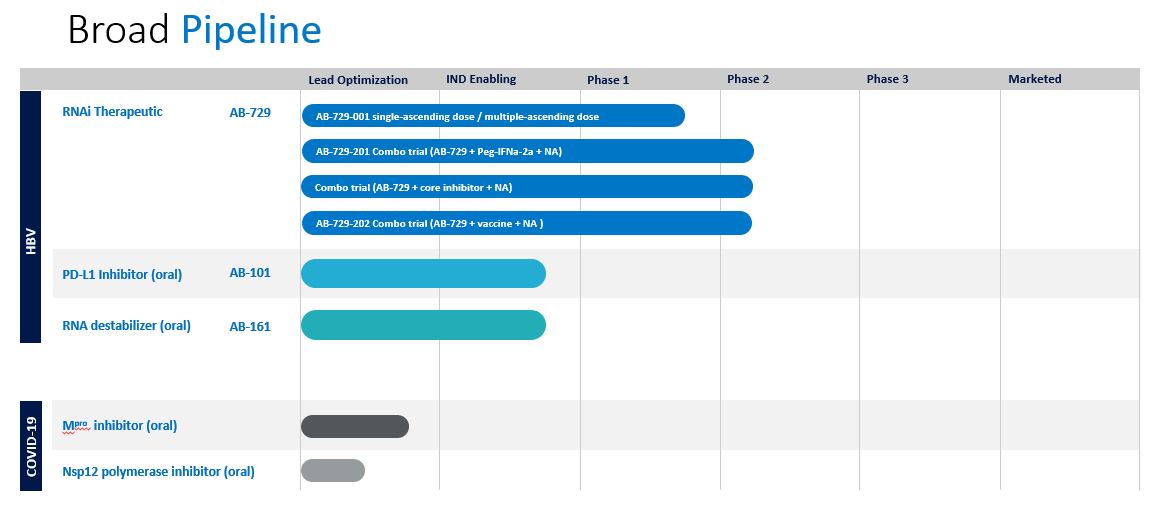
Arbutus Biopharma Corporation (“Arbutus” or the “Company”) is a clinical-stage biopharmaceutical company leveraging its extensive virology expertise to develop novel therapeutics that target specific viral diseases. The Company’s current focus areas include Hepatitis B virus (“HBV”), SARS-CoV-2, and coronaviruses. In HBV, the Company is developing an RNA interference (“RNAi”) therapeutic, an oral PD-L1 inhibitor, and an oral RNA destabilizer to potentially identify a combination regimen with the aim of providing a functional cure for patients with chronic HBV infection (“cHBV”) by suppressing viral replication, reducing surface antigen and reawakening the immune system. The Company believes its lead compound, AB-729, is the only RNAi therapeutic with evidence of immune re-awakening, and is currently being evaluated in multiple phase 2 clinical trials. The Company has an ongoing drug discovery and development program directed to identifying novel, orally active agents for treating coronaviruses, including SARS-CoV-2. The Company is also exploring oncology applications for its internal PD-L1 portfolio.
Liquidity
At September 30, 2022, the Company had an aggregate of $190.2 million in cash, cash equivalents and investments in marketable securities. The Company had no outstanding debt as of September 30, 2022. The Company believes it has sufficient cash resources to fund its operations for at least the next 12 months.
The success of the Company is dependent on obtaining the necessary regulatory approvals to bring its products to market and achieve profitable operations. The Company’s research and development activities and the commercialization of its products are dependent on its ability to successfully complete these activities and to obtain adequate financing through a combination of financing activities and operations. It is not possible to predict either the outcome of the Company’s existing or future research and development programs or the Company’s ability to continue to fund these programs in the future.
COVID-19 Impact
The COVID-19 pandemic has resulted in and will likely continue to result in significant disruptions to businesses. Measures implemented around the world in attempts to slow the spread of COVID-19 have had, and will likely continue to have, a major impact on clinical development, at least in the near-term, including shortages and delays in the supply chain and prohibitions in certain countries on enrolling patients in new clinical trials. While the Company has been able to progress with its clinical and pre-clinical activities to date, it is not possible to predict if the COVID-19 pandemic will materially impact the Company’s plans and timelines in the future.
2. Significant accounting policies
Basis of presentation and principles of consolidation
These unaudited condensed consolidated financial statements have been prepared in accordance with United States generally accepted accounting principles (“GAAP”) for interim financial statements and accordingly, do not include all disclosures required for annual financial statements. These statements should be read in conjunction with the Company’s audited consolidated financial statements and notes thereto for the year ended December 31, 2021 included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2021. These unaudited condensed consolidated financial statements include the accounts of Arbutus Biopharma Corporation and its one wholly-owned subsidiary, Arbutus Biopharma, Inc., and reflect, in the opinion of management, all adjustments and reclassifications necessary to fairly present the Company’s financial position as of September 30, 2022 and December 31, 2021, the Company’s results of operations for the three and nine months ended September 30, 2022 and 2021, and the Company’s cash flows for the nine months ended September 30, 2022 and 2021. Such adjustments are of a normal recurring nature. The results of operations for the three and nine months ended September 30, 2022 are not necessarily indicative of the results for the full year. These unaudited condensed consolidated financial statements follow the same significant accounting policies as those described in the notes to the audited consolidated financial statements of the Company for the year ended December 31, 2021, except as described below under Recent Accounting Pronouncements.
All intercompany balances and transactions have been eliminated. Certain prior year amounts have been reclassified to conform to the current year presentation.
Net loss attributable to common shareholders per share
Net loss attributable to common shareholders per share is calculated based on the weighted average number of common shares outstanding. Diluted net loss attributable to common shareholders per share does not differ from basic net loss attributable to common shareholders per share for the three and nine months ended September 30, 2022 and 2021, since the effect of including potential common shares would be anti-dilutive. For the nine months ended September 30, 2022, potential common shares of 15.9 million pertaining to outstanding stock options were excluded from the calculation of net loss attributable to common shareholders per share. A total of approximately 34.3 million outstanding stock options and if-converted Series A participating convertible preferred shares (“Preferred Shares”) were excluded from the calculation for the nine months ended September 30, 2021.
On October 18, 2021, the Company’s outstanding Preferred Shares were converted into 22,833,922 common shares. Prior to that date, the Company followed the two-class method when computing net loss attributable to common shareholders per share as the Preferred Shares met the definition of participating securities. The Preferred Shares entitled the holders to participate in dividends but did not require the holders to participate in losses of the Company. Accordingly, net losses attributable to holders of the Company’s common shares were not allocated to holders of the Preferred Shares.
Revenue from collaborations and licenses
The Company generates revenue through certain collaboration agreements and license agreements. Such agreements may require the Company to deliver various rights and/or services, including intellectual property rights or licenses and research and development services. Under such agreements, the Company is generally eligible to receive non-refundable upfront payments, funding for research and development services, milestone payments and royalties.
The Company’s collaboration agreements fall under the scope of Accounting Standards Codification (“ASC”) Topic 808, Collaborative Arrangements (“ASC 808”) when both parties are active participants in the arrangement and are exposed to significant risks and rewards. For certain arrangements under the scope of ASC 808, the Company analogizes to ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”) for some aspects, including for the delivery of a good or service (i.e., a unit of account).
ASC 606 requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers under a five-step model: (i) identify contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when or as a performance obligation is satisfied.
In contracts where the Company has more than one performance obligation to provide its customer with goods or services, each performance obligation is evaluated to determine whether it is distinct based on whether (i) the customer can benefit from the
good or service either on its own or together with other resources that are readily available and (ii) the good or service is separately identifiable from other promises in the contract. The consideration under the contract is then allocated between the distinct performance obligations based on their respective relative stand-alone selling prices. The estimated stand-alone selling price of each deliverable reflects the Company’s best estimate of what the selling price would be if the deliverable was regularly sold on a stand-alone basis and is determined by reference to market rates for the good or service when sold to others or by using an adjusted market assessment approach if the selling price on a stand-alone basis is not available.
The consideration allocated to each distinct performance obligation is recognized as revenue when control is transferred to the customer for the related goods or services. Consideration associated with at-risk substantive performance milestones, including sales-based milestones, is recognized as revenue when it is probable that a significant reversal of the cumulative revenue recognized will not occur. Sales-based royalties received in connection with licenses of intellectual property are subject to a specific exception in the revenue standards, whereby the consideration is not included in the transaction price and recognized in revenue until the customer’s subsequent sales or usages occur.
Deferred Revenue
When consideration is received or is unconditionally due from a customer, collaborator or licensee prior to the Company completing its performance obligation to the customer, collaborator or licensee under the terms of a contract, deferred revenue is recorded. Deferred revenue expected to be recognized as revenue within the 12 months following the balance sheet date is classified as a current liability. Deferred revenue not expected to be recognized as revenue within the 12 months following the balance sheet date is classified as a long-term liability. In accordance with ASC Topic 210-20, Balance Sheet - Offsetting the Company’s deferred revenue is offset by a contract asset as further discussed in Note 9.
Segment information
The Company operates as a single segment.
Recent accounting pronouncements
The Company has reviewed all recently issued standards and has determined that such standards will not have a material impact on the Company’s financial statements or do not otherwise apply to the Company’s operations.
3. Fair value measurements
The Company measures certain financial instruments and other items at fair value.
To determine the fair value, the Company uses the fair value hierarchy for inputs used in measuring fair value that maximize the use of observable inputs and minimize the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use to value an asset or liability and are developed based on market data obtained from independent sources. Unobservable inputs are inputs based on assumptions about the factors market participants would use to value an asset or liability. The three levels of inputs that may be used to measure fair value are as follows:
•Level 1 inputs are quoted market prices for identical instruments available in active markets.
•Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability either directly or indirectly. If the asset or liability has a contractual term, the input must be observable for substantially the full term. An example includes quoted market prices for similar assets or liabilities in active markets.
•Level 3 inputs are unobservable inputs for the asset or liability and will reflect management’s assumptions about market assumptions that would be used to price the asset or liability.
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Changes in the observability of valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy.
The carrying values of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities approximate their fair values due to the immediate or short-term maturity of these financial instruments.
To determine the fair value of the contingent consideration (note 8), the Company uses a probability weighted assessment of the likelihood the milestones would be met and the estimated timing of such payments, and then the potential contingent payments were discounted to their present value using a probability adjusted discount rate that reflects the early stage nature of the development program, the time to complete the program development, and overall biotech indices. The Company determined the fair value of the contingent consideration was $5.9 million as of September 30, 2022 and the increase of $0.6 million from December 31, 2021 has been recorded as a component of total operating expenses in the statement of operations and comprehensive loss for the nine months ended September 30, 2022. The assumptions used in the discounted cash flow model are level 3 inputs as defined above. The Company assessed the sensitivity of the fair value measurement to changes in these unobservable inputs, and determined that changes within a reasonable range would not result in a materially different assessment of fair value.
The following tables present information about the Company’s assets and liabilities that are measured at fair value on a recurring basis, and indicates the fair value hierarchy of the valuation techniques used to determine such fair value:
| | | | | | | | | | | | | | | | | | | | | | | |
| Level 1 | | Level 2 | | Level 3 | | Total |
| As of September 30, 2022 | (in thousands) |
| Assets | | | | | | | |
| Cash and cash equivalents | $ | 24,004 | | | $ | — | | | $ | — | | | $ | 24,004 | |
| Investments in marketable securities, current | — | | | 110,714 | | | — | | | 110,714 | |
| Investments in marketable securities, non-current | — | | | 55,436 | | | — | | | 55,436 | |
| Total | $ | 24,004 | | | $ | 166,150 | | | $ | — | | | $ | 190,154 | |
| Liabilities | | | | | | | |
| Liability-classified stock options | $ | — | | | $ | — | | | $ | 1 | | | $ | 1 | |
| Contingent consideration | — | | | — | | | 5,922 | | | 5,922 | |
| Total | $ | — | | | $ | — | | | $ | 5,923 | | | $ | 5,923 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Level 1 | | Level 2 | | Level 3 | | Total |
| As of December 31, 2021 | (in thousands) |
| Assets | | | | | | | |
| Cash and cash equivalents | $ | 109,282 | | | $ | — | | | $ | — | | | $ | 109,282 | |
| Investments in marketable securities, current | — | | | 46,035 | | | — | | | 46,035 | |
| Investments in marketable securities, non-current | — | | | 35,688 | | — | | | 35,688 | |
| Total | $ | 109,282 | | | $ | 81,723 | | | $ | — | | | $ | 191,005 | |
| Liabilities | | | | | | | |
| Liability-classified stock options | $ | — | | | $ | — | | | $ | 26 | | | $ | 26 | |
| Contingent consideration | — | | | — | | | 5,298 | | | 5,298 | |
| Total | $ | — | | | $ | — | | | $ | 5,324 | | | $ | 5,324 | |
The following table presents the changes in fair value of the Company’s liability-classified stock options:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Liability at beginning of the period | | Fair value of liability-classified options exercised in the period | | Decrease in fair value of liability | | Liability at end of the period |
| (in thousands) |
| Nine Months Ended September 30, 2022 | $ | 26 | | | $ | — | | | $ | (25) | | | $ | 1 | |
| Nine Months Ended September 30, 2021 | $ | 250 | | | $ | (96) | | | $ | (117) | | | $ | 37 | |
The following table presents the changes in fair value of the Company’s contingent consideration:
| | | | | | | | | | | | | | | | | |
| | Liability at beginning of the period | | Increase in fair value of liability | | Liability at end of the period |
| (in thousands) |
| Nine Months Ended September 30, 2022 | $ | 5,298 | | | $ | 624 | | | $ | 5,922 | |
| Nine Months Ended September 30, 2021 | $ | 3,426 | | | $ | 1,679 | | | $ | 5,105 | |
4. Investments in marketable securities
Investments in marketable securities consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| Amortized Cost | | Gross Unrealized Gain(1) | | Gross Unrealized Loss(1) | | Fair Value |
| As of September 30, 2022 | (in thousands) |
| Cash equivalents | | | | | | | |
| US government money market fund | $ | 7,815 | | | $ | — | | | $ | — | | | $ | 7,815 | |
| | | | | | | |
| Total | $ | 7,815 | | | $ | — | | | $ | — | | | $ | 7,815 | |
| Investments in marketable short-term securities | | | | | | | |
| US government agency bonds | $ | 23,103 | | | $ | — | | | $ | (306) | | | $ | 22,797 | |
| US corporate bonds | 11,014 | | | — | | | (120) | | | 10,894 | |
| US treasury bills | 8,944 | | | — | | | (50) | | | 8,894 | |
| US government bonds | 69,135 | | | — | | | (1,006) | | | 68,129 | |
| Total | $ | 112,196 | | | $ | — | | | $ | (1,482) | | | $ | 110,714 | |
| Investments in marketable long-term securities | | | | | | | |
| US government agency bonds | $ | 13,380 | | | $ | — | | | $ | (346) | | | $ | 13,034 | |
| US corporate bonds | 28,478 | | | — | | | (493) | | | 27,985 | |
| | | | | | | |
| US government bonds | 14,915 | | | — | | | (498) | | | 14,417 | |
| Total | $ | 56,773 | | | $ | — | | | $ | (1,337) | | | $ | 55,436 | |
(1) Gross unrealized gain (loss) is pre-tax and is reported in accumulated other comprehensive loss.
| | | | | | | | | | | | | | | | | | | | | | | |
| Amortized Cost | | Gross Unrealized Gain(1) | | Gross Unrealized Loss(1) | | Fair Value |
| As of December 31, 2021 | (in thousands) |
| Cash equivalents | | | | | | | |
| US government money market fund | $ | 62,836 | | | $ | — | | | $ | — | | | $ | 62,836 | |
| | | | | | | |
| | | | | | | |
| Total | $ | 62,836 | | | $ | — | | | $ | — | | | $ | 62,836 | |
| Investments in marketable short-term securities | | | | | | | |
| US government agency bonds | $ | 8,131 | | | $ | — | | | $ | (11) | | | $ | 8,120 | |
| US treasury bills | 37,968 | | | — | | | (53) | | | 37,915 | |
| Total | $ | 46,099 | | | $ | — | | | $ | (64) | | | $ | 46,035 | |
| Investments in marketable long-term securities | | | | | | | |
| US government agency bonds | $ | 13,068 | | | $ | — | | | $ | (29) | | | $ | 13,039 | |
| US treasury bills | 22,707 | | | — | | | (58) | | | 22,649 | |
| Total | $ | 35,775 | | | $ | — | | | $ | (87) | | | $ | 35,688 | |
(1) Gross unrealized gain (loss) is pre-tax and is reported in accumulated other comprehensive loss.
The contractual term to maturity of the $110.7 million of short-term marketable securities held by the Company as of September 30, 2022 is less than one year. As of September 30, 2022, the Company held $55.4 million of long-term marketable securities with contractual maturities of more than one year, but less than five years. As of December 31, 2021, the Company’s $46.0 million of short-term marketable securities had contractual maturities of less than one year, while the Company’s $35.7 million of long-term marketable securities had maturities of more than one year, but less than five years.
There were realized gains of less than $0.1 million during each of the three and nine months ended September 30, 2022. There were no realized gains or losses for the three and nine months ended September 30, 2021.
5. Investment in Genevant
In April 2018, the Company entered into an agreement with Roivant Sciences Ltd. (“Roivant”), its largest shareholder, to launch Genevant Sciences Ltd. (“Genevant”), a company focused on the discovery, development, and commercialization of a broad range of RNA-based therapeutics enabled by the Company’s lipid nanoparticle (“LNP”) and ligand conjugate delivery technologies. The Company licensed exclusive rights to its LNP and ligand conjugate delivery platforms to Genevant for RNA-based applications outside of HBV, except to the extent certain rights had already been licensed to other third parties (the “Genevant License”). The Company retained all rights to its LNP and conjugate delivery platforms for HBV.
Under the Genevant License, as amended, if a third party sublicensee of intellectual property licensed by Genevant from the Company commercializes a sublicensed product, the Company becomes entitled to receive a specified percentage of certain revenue that may be received by Genevant for such sublicense, including royalties, commercial milestones and other sales-related revenue, or, if less, tiered low single-digit royalties on net sales of the sublicensed product. The specified percentage is 20% in the case of a mere sublicense (i.e., naked sublicense) by Genevant without additional contribution and 14% in the case of a bona fide collaboration with Genevant.
Additionally, if Genevant receives proceeds from an action for infringement by any third parties of the Company’s intellectual property licensed to Genevant, the Company would be entitled to receive, after deduction of litigation costs, 20% of the proceeds received by Genevant or, if less, tiered low single-digit royalties on net sales of the infringing product (inclusive of the proceeds from litigation or settlement, which would be treated as net sales).
The Company accounts for its interest in Genevant as equity securities without readily determinable fair values. Accordingly, an estimate of the fair value of the securities is based on the original cost less previously recognized equity method losses, less impairments, plus or minus changes resulting from observable price changes in orderly transactions for identical or a similar Genevant securities. As of September 30, 2022, the carrying value of the Company’s investment in Genevant was zero and the Company owned approximately 16% of the common equity of Genevant.
6. Accounts payable and accrued liabilities
Accounts payable and accrued liabilities are comprised of the following:
| | | | | | | | | | | |
| | September 30, 2022 | | December 31, 2021 |
| (in thousands) |
| Trade accounts payable | $ | 872 | | | $ | 3,174 | |
| Research and development accruals | 8,251 | | | 2,371 | |
| Professional fee accruals | 315 | | | 983 | |
| Payroll accruals | 2,824 | | | 4,279 | |
| Other accrued liabilities | 6 | | | 31 | |
| Total accounts payable and accrued liabilities | $ | 12,268 | | | $ | 10,838 | |
7. Sale of future royalties
On July 2, 2019, the Company entered into a Purchase and Sale Agreement (the “Agreement”) with the Ontario Municipal Employees Retirement System (“OMERS”), pursuant to which the Company sold to OMERS part of its royalty interest on future global net sales of ONPATTRO® (Patisiran) (“ONPATTRO”), an RNA interference therapeutic currently being sold by Alnylam Pharmaceuticals, Inc. (“Alnylam”).
ONPATTRO utilizes the Company’s LNP technology, which was licensed to Alnylam pursuant to the Cross-License Agreement, dated November 12, 2012, by and between the Company and Alnylam (the “LNP License Agreement”). Under the terms of the LNP License Agreement, the Company is entitled to tiered royalty payments on global net sales of ONPATTRO ranging from 1.00% to 2.33% after offsets, with the highest tier applicable to annual net sales above $500 million. This royalty interest was sold to OMERS, effective as of January 1, 2019, for $20 million in gross proceeds before advisory fees. OMERS will retain this entitlement until it has received $30 million in royalties, at which point 100% of such royalty interest on future global net sales of ONPATTRO will revert to the Company. OMERS has assumed the risk of collecting up to $30 million of future royalty payments from Alnylam and the Company is not obligated to reimburse OMERS if they fail to collect any such future royalties.
The $30 million in royalties to be paid to OMERS is accounted for as a liability, with the difference between the liability and the gross proceeds received accounted for as a discount. The discount, as well as $1.5 million of transaction costs, will be amortized as interest expense based on the projected balance of the liability as of the beginning of each period. As of September 30, 2022, the Company estimated an effective annual interest rate of approximately 12%. Over the course of the Agreement, the actual interest rate will be affected by the amount and timing of royalty revenue recognized and changes in the timing of forecasted royalty revenue. On a quarterly basis, the Company will reassess the expected timing of the royalty revenue, recalculate the amortization and effective interest rate and adjust the accounting prospectively as needed.
The Company recognizes non-cash royalty revenue related to the sales of ONPATTRO during the term of the Agreement. As royalties are remitted to OMERS from Alnylam, the balance of the recognized liability is effectively repaid over the life of the Agreement. From the inception of the royalty sale through September 30, 2022, the Company has recorded an aggregate of $16.5 million of non-cash royalty revenue for royalties earned by OMERS. There are a number of factors that could materially affect the amount and timing of royalty payments from Alnylam, none of which are within the Company’s control.
During the nine months ended September 30, 2022, the Company recognized non-cash royalty revenue of $5.4 million and non-cash interest expense of $1.4 million. During the nine months ended September 30, 2021, the Company recognized non-cash royalty revenue of $4.0 million and related non-cash interest expense of $2.3 million.
The table below shows the activity related to the net liability for the nine months ended September 30, 2022 and 2021:
| | | | | | | | | | | |
| Nine Months Ended September 30, |
| 2022 | | 2021 |
| (in thousands) |
| Net liability related to sale of future royalties - beginning balance | $ | 16,296 | | | $ | 19,554 | |
| Non-cash royalty revenue | (5,393) | | | (3,963) | |
| Non-cash interest expense | 1,413 | | | 2,292 | |
| Net liability related to sale of future royalties - ending balance | $ | 12,316 | | | $ | 17,883 | |
In addition to the royalty from the LNP License Agreement, the Company is also receiving a second royalty interest ranging from 0.75% to 1.125% on global net sales of ONPATTRO, with 0.75% applying to sales greater than $500 million, originating from a settlement agreement and subsequent license agreement with Acuitas Therapeutics, Inc. (“Acuitas”). The royalty from Acuitas has been retained by the Company and was not part of the royalty sale to OMERS.
8. Contingencies and commitments
Arbitration with the University of British Columbia
Certain early work on lipid nanoparticle delivery systems and related inventions was undertaken at the University of British Columbia (“UBC”), as well as by the Company that was subsequently assigned to UBC. These inventions are licensed to the Company by UBC under a license agreement, initially entered into in 1998 and as amended in 2001, 2006 and 2007. The Company has granted sublicenses under the UBC license to certain third parties, including Alnylam.
On December 18, 2020, UBC delivered to the Company a notice of arbitration alleging that under the cross license between UBC and Arbutus, it is due royalties of $2.0 million plus interest arising from the Company’s sale to OMERS of part of its royalty interest on future global net sales of ONPATTRO, currently being sold by Alnylam. Oral hearings for this matter were held in April 2022 and, on July 11, 2022, the arbitrator issued his decision fully dismissing UBC’s claim for royalties. As a result, no payments are owed to UBC. In September 2022, the arbitrator awarded the Company $0.5 million for reimbursement of costs and attorneys’ fees, which the Company received from UBC in October 2022. This matter is now fully resolved.
Stock Purchase Agreement with Enantigen
In October 2014, Arbutus Inc., the Company’s wholly-owned subsidiary, acquired all of the outstanding shares of Enantigen Therapeutics, Inc. (“Enantigen”) pursuant to a stock purchase agreement. The amount paid to Enantigen’s selling shareholders could be up to an additional $102.5 million in sales performance milestones in connection with the sale of the first commercialized product by the Company for the treatment of HBV, regardless of whether such product is based upon assets acquired under this agreement, and a low single-digit royalty on net sales of such first commercialized HBV product, up to a maximum royalty payment of $1.0 million that, if paid, would be offset against the Company’s milestone payment obligations. Certain other development milestones related to the acquisition were tied to programs which are no longer under development by the Company, and therefore the contingency related to those development milestones is zero.
The contingent consideration is a financial liability and is measured at its fair value at each reporting period, with any changes in fair value from the previous reporting period recorded in the statements of operations and comprehensive loss (see note 3).
The fair value of the contingent consideration was $5.9 million as of September 30, 2022.
9. Collaborations, contracts and licensing agreements
Collaborations
Qilu Pharmaceutical Co., Ltd.
In December 2021, the Company entered into a technology transfer and licensing agreement (the “License Agreement”) with Qilu Pharmaceutical Co., Ltd. (“Qilu”), pursuant to which the Company granted Qilu a sublicensable, royalty-bearing license, under certain intellectual property owned by the Company, which is non-exclusive as to development and manufacturing and exclusive with respect to commercialization of AB-729, including pharmaceutical products that include AB-729, for the treatment or prevention of hepatitis B in China, Hong Kong, Macau and Taiwan (the “Territory”).
In partial consideration for the rights granted by the Company, Qilu paid the Company a one-time upfront cash payment of $40.0 million, net of withholding taxes, on January 5, 2022, and agreed to pay the Company milestone payments totaling up to $245.0 million, net of withholding taxes, upon the achievement of certain technology transfer, development, regulatory and commercialization milestones. Qilu paid $4.4 million of withholding taxes to the Chinese taxing authority on the Company’s behalf, related to the upfront cash payment. In addition, Qilu agreed to pay the Company double digit royalties into the low twenties percent based upon annual net sales of AB-729 in the Territory. The royalties are payable on a product-by-product and region-by-region basis, subject to certain limitations.
Qilu is responsible for all costs related to developing, obtaining regulatory approval for, and commercializing AB-729 for the treatment or prevention of hepatitis B in the Territory. Qilu is required to use commercially reasonable efforts to develop, seek regulatory approval for, and commercialize at least one AB-729 product candidate in the Territory. A joint development committee has been established between the Company and Qilu to coordinate and review the development, manufacturing and commercialization plans. Both parties also have entered into a supply agreement and related quality agreement pursuant to which the Company will manufacture or have manufactured and supply Qilu with all quantities of AB-729 necessary for Qilu to develop and commercialize in the Territory until the Company has completed manufacturing technology transfer to Qilu and approval of a product manufactured by Qilu, or its designated contract manufacturing organization, by the National Medical Products Administration in China for AB-729.
Concurrent with the execution of the License Agreement, the Company entered into a Share Purchase Agreement (the “Share Purchase Agreement”) with Anchor Life Limited, a company established pursuant to the applicable laws and regulations of Hong Kong and an affiliate of Qilu (the “Investor”), pursuant to which the Investor purchased 3,579,952 of the Company’s common shares, without par value (the “Common Shares”), at a purchase price of USD $4.19 per share, which was a 15% premium on the thirty-day average closing price of the Common Shares as of the close of trading on December 10, 2021 (the “Share Transaction”). The Company received $15.0 million of gross proceeds from the Share Transaction on January 6, 2022. The Common Shares sold to the Investor in the Share Transaction represented approximately 2.5% of the Common Shares outstanding immediately prior to the execution of the Share Purchase Agreement.
The License Agreement falls under the scope of ASC 808 as both parties are active participants in the arrangement and are exposed to significant risks and rewards. While this arrangement is in the scope of ASC 808, the Company analogizes to ASC 606 for some aspects of this arrangement, including for the delivery of a good or service (i.e., a unit of account). In accordance
with the guidance, the Company identified the following commitments under the arrangement: (i) rights to develop, use, sell, have sold, offer for sale and import any product comprised of Licensed Product (the “Qilu License”) and (ii) drug supply obligations and manufacturing technology transfer (the “Manufacturing Obligations”). The Company determined that these two commitments are not distinct performance obligations for purposes of recognizing revenue as the manufacturing process is highly specialized and Qilu would not be able to benefit from the Qilu License without the Company’s involvement in the manufacturing activities until the transfer of the manufacturing know-how is complete. As such, the Company will combine these commitments into one performance obligation to which the transaction price will be allocated to and will recognize this transaction price associated with the bundled performance obligation over time using an inputs method based on labor hours expended by the Company on its Manufacturing Obligations.
The Company determined the initial transaction price of the combined performance obligation to be $49.3 million, which includes the $40.0 million upfront fee, $4.4 million of withholding taxes paid by Qilu on behalf of the Company, the premium paid for the Share Transaction of $4.1 million, and $0.8 million associated with certain manufacturing costs expected to be reimbursed by Qilu. The Company determined the Milestone Payments to be variable consideration subject to constraint at inception. At the end of each subsequent reporting period, the Company will reevaluate the probability of achievement of the future development, regulatory, and sales milestones subject to constraint and, if necessary, will adjust its estimate of the overall transaction price. Any such adjustments will be recorded on a cumulative catch-up basis, which would affect revenues and earnings in the period of adjustment. The following table outlines the transaction price and the changes to the related asset and liability balances during the nine months ended September 30, 2022:
| | | | | | | | | | | | | | | | | |
| Nine Months Ended September 30, 2022 |
| Transaction Price | | Cumulative Collaboration Revenue Recognized | | Deferred License Revenue |
| (in thousands) |
| Combined performance obligation | $ | 49,270 | | | $ | 23,007 | | | $ | 26,263 | |
| Less contract asset | | | | | (800) | |
| Total deferred license revenue | | | | | 25,463 | |
| Less current portion of deferred license revenue | | | | | 14,878 | |
| Non-current deferred license revenue | | | | | $ | 10,585 | |
The Company recognized $2.4 million and $23.0 million of revenue based on labor hours expended by the Company on its Manufacturing Obligations during the three and nine months ended September 30, 2022.
As of September 30, 2022, the balance of the deferred license revenue was $26.3 million, which, in accordance with ASC 210-20, was partially offset by the contract asset associated with the manufacturing cost reimbursement of $0.8 million, resulting in a net deferred license revenue liability of $25.5 million. The $4.4 million of withholding taxes paid by Qilu on behalf of the Company was recorded as income tax expense during the nine months ended September 30, 2022.
The Company incurred $0.6 million of incremental costs in obtaining the Qilu License, which the Company capitalized in other current assets and other assets and amortizes as a component of general and administrative expense commensurate with the recognition of the combined performance obligation. The Company recognized less than $0.1 million and $0.3 million of related amortization expense for the three and nine months ended September 30, 2022, respectively.
The Company reevaluates the transaction price and the total estimated labor hours expected to be incurred to satisfy the performance obligations and adjusts the deferred revenue at the end of each reporting period. Such changes will result in a change to the amount of collaboration revenue recognized and deferred revenue.
Vaccitech plc
In July 2021, the Company entered into a clinical collaboration agreement with Vaccitech plc (“Vaccitech”) to evaluate AB-729 followed by Vaccitech’s VTP-300, a proprietary T cell stimulating therapeutic vaccine, in nucleos(t)ide reverse transcriptase inhibitor (“NrtI”)-suppressed patients with cHBV.
The Company is responsible for managing this Phase 2a proof-of-concept clinical trial, subject to oversight by a joint
development committee comprised of representatives from the Company and Vaccitech. The Company and Vaccitech retain full rights to their respective product candidates and will split all costs associated with the clinical trial. The Company incurred $0.3 million and $0.7 million of expenses, net of reimbursements from Vaccitech, related to the collaboration during the three and nine months ended September 30, 2022, respectively and reflected those costs in research and development in the statement of operations and comprehensive loss. There were no such costs during the three and nine months ended September 30, 2021.
Assembly Biosciences, Inc.
In August 2020, the Company entered into a clinical collaboration agreement with Assembly Biosciences, Inc. (“Assembly”) to evaluate AB-729 in combination with Assembly’s first-generation HBV core inhibitor (capsid inhibitor) candidate vebicorvir (“VBR”) and standard-of-care NA therapy for the treatment of patients with HBV infection. Assembly has completed enrollment in the clinical trial. In July 2022, Assembly announced its plan to discontinue development of VBR. Despite this, in consultation with Assembly, the Company plans to continue dosing patients in the Phase 2a proof-of-concept clinical trial in order to fully and accurately assess the results. The Company and Assembly are sharing in the costs of the collaboration. The Company incurred $0.6 million and $2.1 million of expenses related to the collaboration during the three and nine months ended September 30, 2022, respectively, and $0.9 million and $2.1 million during the three and nine months ended September 30, 2021, respectively. Those costs are reflected in research and development in the statement of operations and comprehensive loss. Except to the extent necessary to carry out Assembly’s responsibilities with respect to the collaboration trial, the Company has not provided any license grant to Assembly for use of its AB-729 compound.
X-Chem, Inc. and Proteros biostructures GmbH
In March 2021, the Company entered into a discovery research and license agreement with X-Chem, Inc. (“X-Chem”) and Proteros biostructures GmbH (“Proteros”) to focus on the discovery of novel inhibitors targeting the SARS-CoV-2 nsp5 main protease (Mpro). This collaboration brings together the Company’s expertise in the discovery and development of antiviral agents with X-Chem’s industry leading DNA-encoded library (DEL) technology and Proteros’ protein sciences, biophysics and structural biology capabilities and provides important synergies to potentially identify safe and effective therapies against coronaviruses including SARS-CoV-2. The collaboration allows for the rapid screening of one of the largest small molecule libraries against Mpro (an essential protein required for the virus to replicate itself) and the use of state-of-the-art structure guided methods to rapidly optimize Mpro inhibitors, which the Company could potentially progress to clinical candidates. The agreement provides for payments by the Company to X-Chem and Proteros upon satisfaction of certain development, regulatory and commercial milestones, as well as royalties on sales. The agreement with X-Chem and Proteros was amended effective March 31, 2022 primarily to extend the term of the collaboration and update the funding and fee structure. Through this collaboration, the Company has identified and obtained a worldwide exclusive license to several molecules that inhibit Mpro, a validated target for the treatment of COVID-19 and potential future coronavirus outbreaks. The Company incurred $0.6 million and $0.9 million of expenses related to the collaboration during the three and nine months ended September 30, 2022, respectively, and $0.3 million and $1.5 million of expenses related to the collaboration during the three and nine months ended September 30, 2021, respectively. Those costs are reflected in research and development in the statements of operations and comprehensive loss.
Royalty Entitlements
Alnylam Pharmaceuticals, Inc. and Acuitas Therapeutics, Inc.
The Company has two royalty entitlements to Alnylam Pharmaceuticals, Inc.’s (“Alnylam”) global net sales of ONPATTRO.
In 2012, the Company entered into a license agreement with Alnylam that entitles Alnylam to develop and commercialize products with the Company’s LNP technology. Alnylam launched ONPATTRO, the first approved application of the Company’s LNP technology, in 2018. Under the terms of this license agreement, the Company is entitled to tiered royalty payments on global net sales of ONPATTRO ranging from 1.00% - 2.33% after offsets, with the highest tier applicable to annual net sales above $500 million. This royalty interest was sold to OMERS, effective as of January 1, 2019, for $20 million in gross proceeds before advisory fees. OMERS will retain this entitlement until it has received $30 million in royalties, at which point 100% of this royalty entitlement on future global net sales of ONPATTRO will revert back to the Company. OMERS has assumed the risk of collecting up to $30 million of future royalty payments from Alnylam and the Company is not obligated to reimburse OMERS if they fail to collect any such future royalties. If this royalty entitlement reverts to the Company, it has the potential to provide an active royalty stream or to be otherwise monetized again in full or in part. From the inception of the royalty sale through September 30, 2022, an aggregate of $16.5 million of royalties have been earned by OMERS.
The Company also is receiving a second royalty interest of 0.75% to 1.125% on global net sales of ONPATTRO, with 0.75% applying to sales greater than $500 million, originating from a settlement agreement and subsequent license agreement with Acuitas Therapeutics, Inc. (“Acuitas”). This royalty entitlement from Acuitas has been retained by the Company and was not part of the royalty entitlement sale to OMERS.
Revenues are summarized in the following table:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, | | Nine Months Ended September 30, |
| 2022 | | 2021 | | 2022 | | 2021 |
| (in thousands) | | (in thousands) |
| Revenue from collaborations and licenses |
| Acuitas Therapeutics, Inc. | $ | 1,256 | | | $ | 1,425 | | | $ | 4,339 | | | $ | 3,683 | |
| Qilu Pharmaceutical Co., Ltd. | 2,352 | | | — | | | 23,007 | | | — | |
| Other milestone and royalty payments | — | | | 55 | | | 35 | | | 136 | |
| Non-cash royalty revenue | | | | | | | |
| Alnylam Pharmaceuticals, Inc. | 2,344 | | | 1,860 | | | 5,393 | | | 3,963 | |
| Total revenue | $ | 5,952 | | | $ | 3,340 | | | $ | 32,774 | | | $ | 7,782 | |
10. Shareholders’ equity
Authorized share capital
The Company’s authorized share capital consists of an unlimited number of common shares and preferred shares, without par value, and 1,164,000 Series A participating convertible preferred shares, without par value.
Open Market Sale Agreement
The Company has an Open Market Sale Agreement with Jefferies LLC dated December 20, 2018, as amended by Amendment No. 1, dated December 20, 2019, Amendment No. 2, dated August 7, 2020 and Amendment No. 3, dated March 4, 2021 (as amended, the “Sale Agreement”), under which it may issue and sell common shares, from time to time.
On December 23, 2019, the Company filed a shelf registration statement on Form S-3 with the Securities and Exchange Commission (the “SEC”) (File No. 333-235674) and accompanying base prospectus, which was declared effective by the SEC on January 10, 2020 (the “January 2020 Registration Statement”), for the offer and sale of up to $150.0 million of the Company’s securities. The January 2020 Registration Statement also contained a prospectus supplement in connection with the offering of up to $50.0 million of the Company’s common shares pursuant to the Sale Agreement. This prospectus supplement was fully utilized during 2020. On August 7, 2020, the Company filed a prospectus supplement with the SEC (the “August 2020 Prospectus Supplement”) in connection with the offering of up to an additional $75.0 million of its common shares pursuant to the Sale Agreement under the January 2020 Registration Statement. The August 2020 Prospectus Supplement was fully utilized during 2020.
On August 28, 2020, the Company filed a shelf registration statement on Form S-3 with the SEC (File No. 333-248467) and accompanying base prospectus, which was declared effective by the SEC on October 22, 2020 (the “October 2020 Registration Statement”), for the offer and sale of up to $200.0 million of the Company’s securities. On March 4, 2021, the Company filed a prospectus supplement with the SEC (the “March 2021 Prospectus Supplement”) in connection with the offering of up to an additional $75.0 million of its common shares pursuant to the Sale Agreement under the October 2020 Registration Statement. The March 2021 Prospectus Supplement was fully utilized during 2021. On October 8, 2021, the Company filed a prospectus supplement with the SEC (the “October 2021 Prospectus Supplement”) in connection with the offering of up to an additional $75.0 million of its common shares pursuant to the Sale Agreement under the October 2020 Registration Statement.
On November 4, 2021, the Company filed a shelf registration statement on Form S-3 with the SEC (File No. 333-260782) and accompanying base prospectus, declared effective by the SEC on November 18, 2021 (the “November 2021 Registration Statement”), for the offer and sale of up to $250.0 million of the Company’s securities.
On March 3, 2022, the Company filed a prospectus supplement with the SEC (the “March 2022 Prospectus Supplement”) in connection with the offering of up to an additional $100.0 million of its common shares pursuant to the Sale Agreement under:
(i) the January 2020 Registration Statement; (ii) the October 2020 Registration Statement; and (iii) the November 2021 Registration Statement.
During the nine months ended September 30, 2022, the Company issued 3,901,765 common shares pursuant to the Sale Agreement, resulting in net proceeds of approximately $9.2 million. For the nine months ended September 30, 2021, the Company issued 19,715,142 common shares pursuant to the Sale Agreement, resulting in net proceeds of approximately $75.4 million. As of September 30, 2022, there was approximately $142.6 million remaining available in aggregate under the October 2021 Prospectus Supplement and the March 2022 Prospectus Supplement.
Stock-based compensation
The table below summarizes information about the Company’s stock-based compensation for the three and nine months ended September 30, 2022 and 2021 and the expense recognized in the condensed consolidated statements of operations:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, | | Nine Months Ended September 30, |
| 2022 | | 2021 | | 2022 | | 2021 |
| (in thousands, except share and per share data) |
| Options granted during period | 62,700 | | | 242,400 | | | 4,797,275 | | | 3,369,750 | |
| Weighted average exercise price | $ | 2.37 | | | $ | 3.70 | | | $ | 2.77 | | | $ | 4.19 | |
| Stock compensation expense | | | | | | | |
| Research and development | $ | 736 | | | $ | 729 | | | $ | 2,191 | | | $ | 2,147 | |
| General and administrative | 978 | | | 886 | | | 3,324 | | | 2,846 | |
| Total stock compensation expense | $ | 1,714 | | | $ | 1,615 | | | $ | 5,515 | | | $ | 4,993 | |
Series A Preferred Shares
In October 2017, the Company entered into a subscription agreement with Roivant for the sale of Preferred Shares to Roivant for gross proceeds of $116.4 million. The Preferred Shares were non-voting and were convertible into common shares at a conversion price of $7.13 per share (which represented a 15% premium to the closing price of $6.20 per share). The purchase price for the Preferred Shares plus an amount equal to 8.75% per annum, compounded annually, was subject to mandatory conversion into common shares on October 18, 2021, at which time the Preferred Shares were converted into 22,833,922 common shares and both the lockup and standstill periods that Roivant had previously agreed to expired. As of September 30, 2022, Roivant owned approximately 25% of the Company’s outstanding common shares.
The Company recorded the Preferred Shares wholly as equity with no bifurcation of the conversion feature from the host contract, given that the Preferred Shares could not be cash settled and the redemption features were within the Company’s control, which included a fixed conversion ratio with predetermined timing and proceeds. The Company accrued for the 8.75% per annum compounding coupon at each reporting period end date as an increase to preferred share capital, and an increase to deficit (see statement of stockholders’ equity).
11. Related party transactions
During the three and nine months ended September 30, 2022 and 2021, Genevant purchased certain administrative services from the Company. Income from these services was less than $0.1 million in both periods and is netted against research and development expenses in the condensed consolidated statements of operations.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis by our management of our financial position and results of operations in conjunction with our audited consolidated financial statements and related notes thereto included as part of our Annual Report on Form 10-K for the year ended December 31, 2021 and our unaudited condensed consolidated financial statements for the three and nine months ended September 30, 2022. Our consolidated financial statements have been prepared in accordance with United States generally accepted accounting principles and are presented in U.S. dollars.
REFERENCES TO ARBUTUS BIOPHARMA CORPORATION
Throughout this Quarterly Report on Form 10-Q (“Form 10-Q”), the “Company,” “Arbutus,” “we,” “us,” and “our,” except where the context requires otherwise, refer to Arbutus Biopharma Corporation and its consolidated subsidiaries, and “our board of directors” refers to the board of directors of Arbutus Biopharma Corporation.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Form 10-Q contains “forward-looking statements” or “forward-looking information” within the meaning of applicable United States and Canadian securities laws (we collectively refer to these items as “forward-looking statements”). Forward-looking statements are generally identifiable by use of the words “believes,” “may,” “plans,” “will,” “anticipates,” “intends,” “budgets,” “could,” “estimates,” “expects,” “forecasts,” “projects” and similar expressions that are not based on historical fact or that are predictions of or indicate future events and trends, and the negative of such expressions. Forward-looking statements in this Form 10-Q, including the documents incorporated by reference, include statements about, among other things:
•our strategy, future operations, pre-clinical research, pre-clinical studies, clinical trials, prospects and the plans of management;
•the potential for our product candidates to achieve their desired or anticipated outcomes;
•the expected cost, timing and results of our clinical development plans and clinical trials, including our clinical collaborations with third parties;
•the potential impact of the COVID-19 pandemic on our business and clinical trials;
•the discovery, development and commercialization of a curative combination regimen for chronic hepatitis B infection, a disease of the liver caused by the hepatitis B virus (“HBV”);
•the potential of our product candidates to improve upon the standard of care and contribute to a functional curative combination treatment regimen;
•obtaining necessary regulatory approvals;
•obtaining adequate financing through a combination of financing activities and operations;
•the potential for us to discover and/or develop new molecular entities for treating coronaviruses, including COVID-19;
•the expected returns and benefits from strategic alliances, licensing agreements, and research collaborations with third parties, and the timing thereof;
•our expectations regarding our technology licensed to third parties, and the timing thereof;
•our anticipated revenue and expense fluctuation and guidance;
•our expectations regarding the timing of announcing data from our ongoing clinical trials;
•our expectations regarding current patent disputes and litigation;
•our expectation of a net cash burn between $90 million and $95 million in 2022;
•our belief that we have sufficient cash resources to fund our operations into the second quarter of 2024; and
•the possibility that our clinical development plans could be further delayed or suspended as a result of the military action by Russia in Ukraine.
as well as other statements relating to our future operations, financial performance or financial condition, prospects or other future events. Forward-looking statements appear primarily in the sections of this Form 10-Q entitled “Part I, Item 1- Financial Statements (Unaudited),” and “Part I, Item 2-Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Forward-looking statements are based upon current expectations and assumptions and are subject to a number of known and unknown risks, uncertainties and other factors that could cause actual results to differ materially and adversely from those expressed or implied by such statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in this Form 10-Q and our Annual Report on Form 10-K for the year ended December 31, 2021 (the “Form 10-K”), and in particular the risks and uncertainties discussed under “Item 1A-Risk Factors” of this Form 10-Q and the Form 10-K. As a result, you should not place undue reliance on forward-looking statements.
Additionally, the forward-looking statements contained in this Form 10-Q represent our views only as of the date of this Form 10-Q (or any earlier date indicated in such statement). While we may update certain forward-looking statements from time to time, we specifically disclaim any obligation to do so, even if new information becomes available in the future. However, you are advised to consult any further disclosures we make on related subjects in the periodic and current reports that we file with the Securities and Exchange Commission.
The foregoing cautionary statements are intended to qualify all forward-looking statements wherever they may appear in this Form 10-Q. For all forward-looking statements, we claim protection of the safe harbor for the forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
This Form 10-Q also contains estimates, projections and other information concerning our industry, our business, and the markets for certain diseases, including data regarding the estimated size of those markets, and the incidence and prevalence of certain medical conditions. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources.
OVERVIEW
Arbutus Biopharma Corporation (“Arbutus”, the “Company”, “we”, “us”, and “our”) is a clinical-stage biopharmaceutical company leveraging its extensive virology expertise to develop novel therapeutics that target specific viral diseases. Our current focus areas include Hepatitis B virus (“HBV”), SARS-CoV-2 and other coronaviruses. In HBV, we are developing an RNA interference (“RNAi”) therapeutic, an oral PD-L1 inhibitor, and an oral RNA destabilizer to potentially identify a combination regimen with the aim of providing a functional cure for patients with chronic HBV infection (“cHBV”) by suppressing viral replication, reducing surface antigen and reawakening the immune system. We believe our lead compound, AB-729, is the only RNAi therapeutic with evidence of immune re-awakening and is currently being evaluated in multiple phase 2 clinical trials. We have an ongoing drug discovery and development program directed to identifying novel, orally active agents for treating coronaviruses, including SARS-CoV-2. We are also exploring oncology applications for our internal PD-L1 portfolio.
Strategy
The core elements of our strategy include:
•Developing a broad portfolio of compounds that target cHBV. Our HBV product pipeline includes a subcutaneously-delivered RNAi therapeutic, an oral HBV RNA destabilizer compound and an oral PD-L1 inhibitor. We believe that by combining these compounds to suppress HBV DNA replication and hepatitis B surface antigen (“HBsAg”) expression as well as reawaken patients’ HBV-specific immune response, we can address the most important elements to achieving a functional cure. We define a functional cure as unquantifiable plasma HBV DNA and HBsAg levels more than six months after treatment with or without quantifiable anti-HBsAg antibodies.
AB-729, our proprietary subcutaneously-delivered RNAi therapeutic product candidate that suppresses HBsAg expression, which is thought to be a key prerequisite to enable reawakening of a patient’s immune system to respond to HBV, is currently in one ongoing Phase 1a/1b clinical trial (“AB-729-001”) and three Phase 2a proof-of-concept clinical trials in combination with other agents with potentially complementary mechanisms of action. Preliminary data from AB-729-001 has shown that treatment with AB-729 resulted in meaningful declines in HBsAg while being well tolerated with no serious adverse events (SAEs) noted after both single and repeat dosing. Preliminary data also suggests that long-term suppression of HBsAg with AB-729 results in increased HBV-specific immune response. At the American Association for the Study of Liver Diseases (“AASLD”) Liver Meeting held in November 2022 (the “2022 AASLD Liver Meeting”), we presented additional off-treatment data from Part 3 of the AB-729-001 Phase 1a/1b clinical trial, which included nine patients who had completed 12 to 44 weeks of follow-up after discontinuing their nucleos(t)ide analogue (“NA”) therapy. Protocol-defined criteria to restart NA therapy was not met by any patient and there was no evidence of clinical or biochemical relapse. HBsAg levels remained at 1.05 to 2.35 log10 below pre-trial levels in all nine patients, which further supports AB-729’s potential for immunological control. One patient restarted NA therapy at the investigator’s request after the week 20 visit; no alanine transaminase (“ALT”) elevation or safety signals were observed. Eight patients remain off NA therapy and are continuing to be followed for an additional two years to monitor for sustained viral response and potential functional cure. The new clinical data for AB-729 continues to support its development as a potential cornerstone agent for the treatment of cHBV infection.
AB-101, our oral PD-L1 inhibitor that has the potential to reawaken patients’ HBV-specific immune response by inhibiting PD-L1, is advancing through investigational new drug (“IND”)-enabling studies that are anticipated to be completed in the fourth quarter of 2022. Preclinical data in an HBV mouse model was presented at the 2022 AASLD Liver Meeting showing that combination treatment with AB-101 and an HBV-targeting GalNAc-siRNA agent resulted in activation and increased frequency of HBV-specific T-cells and greater anti-HBsAg antibody production. This favorable preclinical profile supports further development of AB-101 as a therapeutic modality for cHBV treatment. We are also exploring potential oncology applications for our internal PD-L1 portfolio.
AB-161 is our next-generation oral HBV specific RNA destabilizer. We have conducted extensive non-clinical safety evaluations with AB-161 that gives us confidence in this molecule’s ability to circumvent the peripheral neuropathy findings seen in non-clinical safety studies with our first-generation oral RNA destabilizer, AB-452. We recently presented preclinical data at the Discovery on Target Conference showing that AB-161 reduced HBV RNA and HBsAg in multiple preclinical models, with favorable liver centricity and lack of observed peripheral neuropathy. We are conducting the remaining IND-enabling studies, which are anticipated to be completed in the fourth quarter of 2022.
• Combining therapeutic product candidates with complementary mechanisms of action to find a functional cure for people with cHBV. We believe that our proprietary product candidates AB-729, AB-101 and AB-161, along with existing approved therapies, may provide our first proprietary combination therapy for patients with cHBV. In-line
with our strategy to position AB-729 as a potential cornerstone therapeutic in future HBV combination regimens, and to help guide future development of combination therapies of AB-729 with other compounds from our proprietary HBV portfolio, we are evaluating AB-729 in combination with other agents with potentially complementary mechanisms of action, including the following:
•Enrollment is ongoing in a Phase 2a proof-of-concept clinical trial (“AB-729-201”) to evaluate AB-729 in combination with ongoing standard-of-care NA therapy and short courses of Peg-IFNα-2a in patients with cHBV, with preliminary data anticipated in the fourth quarter of 2022.
•Through our collaboration with Assembly BioSciences, Inc. (“Assembly”), patients with cHBV were enrolled in a Phase 2a proof-of-concept clinical trial evaluating a triple combination of AB-729, Assembly’s first-generation HBV core inhibitor (capsid inhibitor) product candidate, vebicorvir (“VBR”), and NA therapy. In July 2022, Assembly announced its plans to discontinue development of VBR. Despite this, in consultation with Assembly, we continued dosing patients in the Phase 2a proof-of-concept clinical trial in order to fully and accurately assess the results. Preliminary data from sixty-five patients in this clinical trial was presented as a poster presentation at the 2022 AASLD Liver Meeting and showed that adding VBR to AB-729 and NA therapy did not result in greater on-treatment improvements in markers of active HBV infection as compared to AB-729 and NA therapy alone. The addition of VBR did not negatively impact the reduction of HBsAg in the triple combination arm. All regimens were safe and well-tolerated in this trial. Patients are continuing to be followed.
•Through our collaboration with Vaccitech plc (“Vaccitech”), we are enrolling patients in a Phase 2a clinical trial (“AB-729-202”) to evaluate a triple combination of AB-729 with Vaccitech’s VTP-300, a proprietary T cell stimulating therapeutic vaccine, and NA therapy for the treatment of patients with cHBV.
• Advancing small molecule antiviral product candidates to treat COVID-19 and future coronavirus outbreaks. This program is focused on the discovery and development of new molecular entities for treating coronaviruses (including COVID-19) that address specific viral targets including the nsp12 viral polymerase and the nsp5 viral protease. We see an opportunity to pursue a potential combination therapy to achieve better patient treatment outcomes and use in prophylactic settings. Through our collaboration with X-Chem, Inc. (“X-Chem”) and Proteros biostructures GmbH (“Proteros”), we have identified and obtained a worldwide exclusive license to several molecules that inhibit the SARS-CoV-2 nsp5 main protease (“Mpro”), a validated target for the treatment of COVID-19 and potential future coronavirus outbreaks. We expect to nominate a candidate that inhibits Mpro in the fourth quarter of 2022 and advance that compound into IND-enabling studies. We are also continuing lead optimization activities for an nsp12 viral polymerase candidate.
Our Product Candidates
Our product pipeline includes multiple product candidates that target various steps in the HBV viral lifecycle and pan-coronavirus compounds that target essential enzymes for replication, the viral protease (Mpro) and polymerase (nsp12).
Our product pipeline consists of the following programs:
We continue to explore expansion opportunities for our pipeline through internal discovery and development activities and through potential strategic alliances.
GalNAc RNAi (AB-729)
RNAi therapeutics represent a recent significant advancement in drug development. RNAi therapeutics utilize a natural pathway within cells to silence genes by eliminating the disease-causing proteins that they code for. We are developing RNAi therapeutics that are designed to reduce HBsAg expression and other HBV antigens in people with cHBV. Reducing HBsAg is widely believed to be a key prerequisite to enable a patient’s immune system to reawaken and respond against the virus.
AB-729 is a subcutaneously-delivered RNAi therapeutic targeted to hepatocytes using our proprietary covalently conjugated GalNAc delivery technology. AB-729 reduces all HBV antigens and inhibits viral replication. AB-729-001 is our three-part clinical trial designed to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of single- and multi-dose AB-729 in healthy subjects and in cHBV patients and to determine the most appropriate doses and dosing intervals to take forward into Phase 2 clinical development.
Part 1 of the trial dosed healthy subjects, and upon completion, supported advancing doses ranging from 60 mg to 180 mg into Part 2. Part 2 of the trial dosed patients with cHBV with single doses (60, 90 and 180 mg) of AB-729, and upon completion, showed that single doses of AB-729 result in comparable mean HBsAg declines at week 12 followed by a sustained plateau phase. Part 3 of the trial dosed HBV DNA negative and positive patients with multiple doses of AB-729 every 4, 8 or twelve weeks. Dosing of patients in Part 3 has been completed and we are continuing to follow these patients for one year. A total of 41 patients were dosed with AB-729 during Parts 2 and 3 of this Phase 1a/1b clinical trial.
At the 2022 AASLD Liver Meeting, we presented additional off-treatment data from Part 3 of the AB-729-001 Phase 1a/1b clinical trial, which included nine patients who had had previously completed 48 weeks of treatment with AB-729, and 24 weeks later met protocol-defined criteria to also stop NA therapy. These nine patients had completed 12 to 44 weeks of follow-up after discontinuing their NA therapy and none had met the protocol-defined criteria to restart NA therapy and there was no evidence of clinical or biochemical relapse. HBsAg levels remained at 1.05 log10 to 2.35 log10 below pre-trial levels in all nine patients. Three patients experienced transient HBV DNA elevations that spontaneously resolved without intervention, which further supports AB-729’s potential for immunological control. One patient restarted NA therapy at the investigator’s request after the week 20 visit; no ALT elevation or safety signals were observed. Eight patients remain off NA therapy and are continuing to be followed for an additional two years to monitor for sustained viral response and potential functional cure. There were no adverse events (“AEs”) reported and no ALT elevations observed. The new clinical data for AB-729 continues to support its development as a potential cornerstone agent for the treatment of cHBV infection.
Data presented earlier this year at the 2022 European Association for the Study of the Liver (EASL) International Liver Congress™ (ILC) showed that repeat dosing of 60 mg and 90 mg of AB-729 resulted in robust mean declines in HBsAg in
HBeAg positive/negative and HBV DNA positive/negative patients that were sustained up to 48 weeks, with no statistically significant differences observed to date between the 60 mg and 90 mg dose and/or dosing intervals.
The reported data for patients from Cohorts E, F, G, I and J showed:
•76% (26 of 34) patients had HBsAg <100 IU/mL at some point during the trial and 50% (16 out of 32) of patients maintained HBsAg levels below 100 IU/mL 24 weeks after their last AB-729 dose;
•Most patients had a robust decline in HBsAg that was maintained well after cessation of AB-729 treatment, mean log change from baseline to 24 weeks post last dose was approximately -1.5 log10 across cohorts;
•Repeat dosing of AB-729 continues to be generally safe and well-tolerated with only transient Grade 1 or 2 ALT elevations; and
•AB-729 continues to result in HBV-specific T-cell immune restoration and decrease of exhausted T-cells.
The reported data for patients from Cohort K, which included HBeAg positive patients only, showed:
•All patients had HBsAg levels <100 IU/mL during AB-729 treatment or follow-up with two patients reaching HBsAg below levels of quantification on multiple visits;
•All seven patients had residual detectable HBeAg and therefore did not meet the protocol-defined discontinuation criteria for their NA therapy;
•One patient reached HBeAg less than lower levels of quantification intermittently; and
•No safety events were noted during the follow-up period.
The efficacy and safety data for AB-729, derived from up to one year of dosing, supported our view that 60 mg every 8 weeks was an appropriate dose to move forward in our Phase 2a clinical trials. To advance our efforts to position AB-729 as a potential cornerstone therapeutic in future HBV combination regimens, we are evaluating AB-729 in several Phase 2a proof-of-concept combination clinical trials with other agents with potentially complementary mechanisms of action via clinical collaborations with other companies as described below.
Phase 2a proof-of-concept clinical trial to evaluate AB-729 in combination with Peg-IFNα-2a (AB-729-201)
We are evaluating AB-729-201 in a randomized, open label, multicenter Phase 2a proof-of-concept clinical trial investigating the safety and antiviral activity of AB-729 in combination with ongoing NA therapy and short courses of Peg-IFNα-2a in up to 40 stably NA-suppressed, HBeAg negative, non-cirrhotic patients with cHBV. After 24-weeks of dosing with AB-729 (60 mg every 8 weeks), patients will be randomized into one of four groups to receive either AB-729 plus NA therapy plus Peg-IFNα-2a or NA therapy plus Peg-IFNα-2a for either 24 or 12 weeks. After completion of the assigned Peg-IFNα-2a treatment period, all patients will remain on NA therapy for the initial 24-week follow-up period, and will then discontinue NA treatment, provided they meet certain stopping criteria. If patients stop NA therapy, they will enter an intensive follow-up period for 48 weeks. Enrollment is ongoing in this clinical trial and we anticipate preliminary data in the fourth quarter of 2022.
Collaboration with Assembly
Through a clinical collaboration agreement with Assembly that we entered into in August 2020, Assembly is evaluating AB-729 in combination with its first-generation HBV core inhibitor (capsid inhibitor) candidate VBR and standard-of-care NA therapy for the treatment of HBeAg negative patients with cHBV. The randomized, multi-center, open-label Phase 2a proof-of-concept clinical trial was designed to evaluate the safety, pharmacokinetics, and antiviral activity of the triple combination of AB-729, VBR, and an NA (n=32) compared to the double combinations of VBR with an NA (n=16) and AB-729 with an NA (n=17). Patients are dosed for 48 weeks with AB-729 (60 mg subcutaneously every 8 weeks) and VBR (300 mg orally once daily), with a 48-week follow-up period. Both parties share in the costs of the collaboration. Assembly has completed enrollment in the clinical trial. In July 2022, Assembly announced its plans to discontinue development of VBR. Despite this, in consultation with Assembly, we continued this Phase 2a proof-of-concept clinical trial in order to fully and accurately assess the results. Preliminary data from this clinical trial were presented as a poster presentation at the 2022 AASLD Liver Meeting. The preliminary data indicate that adding VBR to AB-729 and NA therapy does not result in greater on-treatment improvements in markers of active HBV infection as compared to AB-729 and NA therapy alone. The addition of VBR did not negatively impact the reduction of HBsAg in the triple combination arm. All regimens were safe and well-tolerated in this trial. Patients are continuing to be followed. Except to the extent necessary to carry out Assembly’s responsibilities with respect to the collaboration trial, we have not provided any license grant to Assembly for use of AB-729.
Collaboration with Vaccitech (AB-729-202)
Through a clinical collaboration agreement with Vaccitech that we entered into in July 2021, we are enrolling patients in AB-729-202, a Phase 2a proof-of-concept clinical trial evaluating the safety, antiviral activity and immunogenicity of Vaccitech’s VTP-300, a proprietary T cell stimulating therapeutic vaccine, administered after AB-729 in NA-suppressed patients with cHBV. The trial is designed to enroll 40 NA-suppressed, HBeAg negative or positive, non-cirrhotic cHBV patients. All patients will receive AB-729 (60mg every 8 weeks) plus NA therapy for 24 weeks. At week 24, treatment with AB-729 will stop. Patients will continue only their NA therapy and will be randomized to receive VTP-300 or placebo for an additional 24 weeks. At week 48, all patients will be evaluated for eligibility to either discontinue or remain on NA therapy. This clinical trial is being managed by us, subject to oversight by a joint development committee comprised of representatives from both companies. We and Vaccitech retain full rights to our respective product candidates and will split all costs associated with the clinical trial. Pursuant to the agreement, the parties intend to undertake a larger Phase 2b clinical trial depending on the results of the initial Phase 2a clinical trial.
Collaboration with Antios
We have terminated our clinical collaboration agreement with Antios Therapeutics, Inc. (“Antios”) that we entered into in June 2021. Antios completed enrollment in a single cohort of its ongoing Antios Phase 2a ANTT201 clinical trial evaluating its proprietary Active Site Polymerase Inhibitor Nucleotide (ASPIN), ATI-2173, in combination with AB-729 and Viread (tenofovir disoproxil fumarate), a nucleos(t)ide reverse transcriptase inhibitor which is currently approved by the FDA, for the treatment of patients with cHBV. Antios was responsible for conducting this clinical trial and for the costs of adding this single cohort to its existing clinical trial. We were responsible for the manufacture and supply of AB-729. Except to the extent necessary to carry out Antios’ responsibilities with respect to the collaboration trial, we did not provide any license grant to Antios for use of AB-729.
A majority of patients in this cohort were enrolled in Ukraine, which is currently in a state of war, and as a result these patients were lost to follow-up before completing the clinical trial. Antios recently terminated this clinical trial.
Oral Capsid Inhibitor (AB-836)
HBV core protein assembles into a capsid structure, which is required for viral replication. The current commercially available therapies (NAs or Peg-IFN) significantly reduce HBV DNA levels in the serum, but HBV replication continues in the liver, thereby enabling HBV infection to persist. More effective therapies for patients require new agents which will further block viral replication. Oral capsid inhibitors (also known as core protein inhibitors), in combination with NAs, could further reduce HBV replication. By inhibiting assembly of functional viral capsids, the ability of HBV to replicate is impaired. Capsid inhibitor molecules also inhibit the uncoating step of the viral life cycle and thus reduce the formation of cccDNA, the viral reservoir which resides in the cell nucleus, and which is believed to play a role in viral persistence.
We enrolled patients in a double-blind, randomized, placebo-controlled Phase 1a/1b clinical trial (“AB-836-001”) designed to evaluate the safety, tolerability, pharmacokinetics and antiviral activity of single and multiple doses of AB-836 in healthy subjects and patients with cHBV. In June 2022, we presented a poster at the 2022 EASL ILC highlighting the most recent data from AB-836-001 showing that the 100mg and 200mg doses of AB-836 provided potent inhibition of HBV replication with mean declines in HBV DNA at Day 28 of 3.04 and 3.55 log10 IU/mL, respectively. From a safety standpoint, there were no deaths or SAEs observed. Two HBeAg positive patients in the 100mg dose cohort had transient Grade 3 ALT elevations that resolved with continued dosing and were not considered treatment emergent adverse events (TEAEs). Two patients in the 200mg cohort had Grade 3 and Grade 4 ALT elevations on the last day of dosing (Day 28) that returned to baseline during follow up, which were reported as TEAEs. The Grade 3 and Grade 4 ALT elevations seen in the 200 mg cohort were accompanied by serum IP-10 increases, an exploratory and hence not a definitive cytokine biomarker, which we had previously observed to be associated with potential liver toxicity in the capsid inhibitor space. All patients with ALT elevations were asymptomatic and none had changes in bilirubin or met drug-induced liver injury (DILI) criteria. There were no other clinically significant lab abnormalities, ECG or vital sign changes observed.
Based on these ALT findings, an additional arm was added to the AB-836-001 clinical trial to evaluate the safety of dosing AB-836 for a longer period of time to help determine if the previously seen ALT elevations were beneficial or were instead the result of liver toxicity. In this healthy volunteer arm, two subjects dosed with AB-836 experienced low grade ALT elevations after more than 20 days of dosing, causing us to stop dosing. Based on these additional safety findings, we decided to discontinue clinical development of AB-836.
Oral PD-L1 Inhibitor (AB-101)
PD-L1 inhibitors complement our pipeline of agents and could potentially be an important part of a combination therapy for the treatment of HBV by reawakening the immune system. Highly functional HBV-specific T cells within our immune system are believed to be required for long-term HBV viral resolution. However, HBV-specific T cells become functionally defective, and greatly reduced in their frequency during cHBV. One approach to boost HBV-specific T cells is to prevent PD-L1 proteins from binding to PD-1 and thus inhibiting the HBV-specific immune function of T cells.
AB-101 is our oral PD-L1 inhibitor, which we believe has the potential to reawaken patients’ HBV-specific immune response. In June 2022, we presented a poster at the 2022 EASL ILC highlighting data from a study that was designed to assess the preclinical activity of AB-101 and the compound’s ability to reinvigorate patient HBV-specific T-cells. Studies were conducted using a transgenic MC38 tumor mouse model and peripheral blood mononuclear cells (PBMCs) from cHBV patients. The data presented showed that once daily oral administration of AB-101 resulted in profound tumor reduction that was associated with T-cell activation. In addition, AB-101 activates and reinvigorates HBV-specific T-cells in vitro.
Additionally, preclinical data in an HBV mouse model was presented at the 2022 AASLD Liver Meeting showing that monotherapy with AB-101 reduced PD-L1 in liver immune cells, confirming liver target engagement of the compound. Combination treatment with AB-101 and an HBV-targeting GalNAc-siRNA agent resulted in activation and increased frequency of HBV-specific T-cells and greater anti-HBsAg antibody production. This favorable preclinical profile supports further development of AB-101 as a therapeutic modality for cHBV treatment. We anticipate completing IND-enabling studies for AB-101 in the fourth quarter of 2022.
We are also exploring potential oncology applications for our internal PD-L1 portfolio. Preclinical data was selected for publication at the American Society of Clinical Oncology (ASCO) Annual Meeting in June 2022 showing that our oral small-molecule PD-L1 inhibitors in development, which possess a novel mechanism of action, have the ability to mediate T-cell activation in primary human immune cells. The anti-tumor efficacy seen in vivo was comparable to anti-PD-L1 antibodies. The data is published in the Journal of Clinical Oncology.
Oral HBV RNA Destabilizer (AB-161)
HBV RNA destabilizers are small molecule orally available agents that cause the destabilization and ultimate degradation of HBV RNAs. These agents result in the reduction of HBsAg and other viral proteins in both whole cell systems and animal models. They have the potential to selectively impact HBV versus other RNA or DNA viruses and demonstrate pangenotypic characteristics. HBV RNA destabilizers have demonstrated additive effects in combination with other anti-HBV mechanisms of action. HBV RNA destabilizers have the potential to complement or replace subcutaneously delivered RNAi agents, such as AB-729, with an oral therapy in combination with a capsid inhibitor and an approved NA.
AB-161 is our next-generation oral HBV specific RNA destabilizer. We have conducted extensive non-clinical safety evaluations with AB-161 that provide confidence in this molecule’s ability to circumvent the peripheral neuropathy findings seen in non-clinical safety studies with our first-generation oral RNA destabilizer, AB-452. We recently presented preclinical data at the Discovery on Target Conference showing that AB-161 reduced HBV RNA and HBsAg in multiple preclinical models, with favorable liver centricity and lack of observed peripheral neuropathy. We are conducting the remaining IND-enabling studies, which are anticipated to be completed in the fourth quarter of 2022.
COVID-19 Research Efforts
While our core mission is to find a cure for HBV, the magnitude of the coronavirus pandemic is undeniable. Given our science team’s proven expertise in the discovery of new antiviral therapies, in 2020 we initiated a drug discovery effort for treating coronaviruses, including COVID-19. To that end, we have assembled an internal team of expert scientists under the direction of our Chief Scientific Officer, Dr. Michael Sofia, to identify novel small molecule therapies to treat COVID-19 and future coronavirus outbreaks. Dr. Sofia, who was awarded the Lasker-DeBakey Award for his discovery of sofosbuvir, brings extensive antiviral drug discovery experience to this new program. Our COVID-19 research program is focused on the discovery and development of new molecular entities that address specific viral targets including the nsp12 viral polymerase and the nsp5 viral protease. These targets are essential viral proteins which our science team has experience in targeting. We see an opportunity to pursue a potential combination therapy to achieve better patient treatment outcomes and use in prophylactic settings.
Collaboration with X-Chem, Inc. and Proteros biostructures GmbH
In March 2021, we entered into a discovery research and license agreement, as amended, with X-Chem and Proteros to focus on the discovery of novel inhibitors targeting the SARS-CoV-2 nsp5 main protease (Mpro). The agreement is designed to accelerate the development of pan-coronavirus agents to treat COVID-19 and potential future coronavirus outbreaks. This collaboration brought together our expertise in the discovery and development of antiviral agents with X-Chem’s industry leading DNA-encoded library (DEL) technology and Proteros’ protein sciences, biophysics and structural biology capabilities and provides important synergies to potentially identify safe and effective therapies against coronaviruses, including SARS-CoV-2. The collaboration allows for the rapid screening of one of the largest small molecule libraries against Mpro (an essential protein required for the virus to replicate itself) and the use of state-of-the-art structure guided methods to rapidly optimize Mpro inhibitors, which we could potentially progress to clinical candidates. The agreement provides for payments by us to X-Chem and Proteros upon satisfaction of certain development, regulatory and commercial milestones, as well as royalties on sales. Through this collaboration, we have identified and obtained a worldwide exclusive license to several molecules that inhibit Mpro, a validated target for the treatment of COVID-19 and potential future coronavirus outbreaks. We expect to nominate an Mpro product candidate in the fourth quarter of 2022 and advance into IND-enabling studies. We are also continuing lead optimization activities for an Nsp12 viral polymerase candidate.
COVID-19 Impact
The COVID-19 pandemic has resulted in and will likely continue to result in significant disruptions to businesses. Measures implemented around the world in attempts to slow the spread of COVID-19 have had, and will likely continue to have, a major impact on clinical development, at least in the near-term, including shortages and delays in the supply chain and prohibitions in certain countries on enrolling patients in new clinical trials. While we have been able to progress with our clinical and pre-clinical activities to date, it is not possible to predict if the COVID-19 pandemic will materially impact our plans and timelines in the future.
Other Collaborations and Royalty Entitlements
Qilu Pharmaceutical Co., Ltd.
In December 2021, we entered into a technology transfer and license agreement (the “License Agreement”) with Qilu Pharmaceutical Co., Ltd. (“Qilu”), pursuant to which we granted Qilu a sublicensable, royalty-bearing license, under certain intellectual property owned by us, which is non-exclusive as to development and manufacturing and exclusive with respect to commercialization of AB-729, including pharmaceutical products that include AB-729, for the treatment or prevention of hepatitis B in China, Hong Kong, Macau and Taiwan (the “Territory”).
In partial consideration for the rights granted by us, Qilu paid us a one-time upfront cash payment of $40 million on January 5, 2022 and agreed to pay us milestone payments totaling up to $245 million, net of withholding taxes, upon the achievement of certain technology transfer, development, regulatory and commercialization milestones. Qilu also agreed to pay us double digit royalties into the low twenties percent based upon annual net sales of AB-729 in the Territory. The royalties are payable on a product-by-product and region-by-region basis, subject to certain limitations.
Qilu is responsible for all costs related to developing, obtaining regulatory approval for, and commercializing AB-729 for the treatment or prevention of hepatitis B in the Territory. Qilu is required to use commercially reasonable efforts to develop, seek regulatory approval for, and commercialize at least one AB-729 product candidate in the Territory. A joint development committee has been established between us and Qilu to coordinate and review the development, manufacturing and commercialization plans. Both parties also have entered into a supply agreement and related quality agreement pursuant to which we will manufacture or have manufactured and supply Qilu with all quantities of AB-729 necessary for Qilu to develop and commercialize in the Territory until we have completed manufacturing technology transfer to Qilu and approval of a product manufactured by Qilu, or its designated contract manufacturing organization, by National Medical Products Administration in China for AB-729.
Concurrent with the execution of the License Agreement, we entered into a Share Purchase Agreement (the “Share Purchase Agreement”) with Anchor Life Limited, a company established pursuant to the applicable laws and regulations of Hong Kong and an affiliate of Qilu (the “Investor”), pursuant to which the Investor purchased 3,579,952 of our common shares, without par value (the “Common Shares”), at a purchase price of USD $4.19 per share, which was a 15% premium on the thirty-day average closing price of the Common Shares as of the close of trading on December 10, 2021 (the “Share Transaction”). We received $15.0 million of gross proceeds from the Share Transaction on January 6, 2022. The Common Shares sold to the Investor in the Share Transaction represented approximately 2.5% of the Common Shares outstanding immediately prior to the execution of the Share Purchase Agreement.
Alnylam Pharmaceuticals, Inc. and Acuitas Therapeutics, Inc.
We have two royalty entitlements to Alnylam’s global net sales of ONPATTRO.
In 2012, we entered into a license agreement with Alnylam that entitles Alnylam to develop and commercialize products with our lipid nanoparticle (“LNP”) delivery technology. Alnylam’s ONPATTRO, which represents the first approved application of our LNP technology, was approved by the United States FDA and the European Medicines Agency (“EMA”) during the third quarter of 2018 and was launched by Alnylam immediately upon approval in the United States. Under the terms of this license agreement, we are entitled to tiered royalty payments on global net sales of ONPATTRO ranging from 1.00% - 2.33% after offsets, with the highest tier applicable to annual net sales above $500 million. This royalty interest was sold to the Ontario Municipal Employees Retirement System (“OMERS”), effective as of January 1, 2019, for $20 million in gross proceeds before advisory fees. OMERS will retain this entitlement until it has received $30 million in royalties, at which point 100% of this royalty entitlement on future global net sales of ONPATTRO will revert to us. OMERS has assumed the risk of collecting up to $30 million of future royalty payments from Alnylam and we are not obligated to reimburse OMERS if they fail to collect any such future royalties. If this royalty entitlement reverts to us, it has the potential to provide an active royalty stream or to be otherwise monetized again in full or in part. From the inception of the royalty sale through September 30, 2022, an aggregate of $16.5 million of royalties have been collected by OMERS.
We also have rights to a second royalty interest ranging from 0.75% to 1.125% on global net sales of ONPATTRO, with 0.75% applying to sales greater than $500 million, originating from a settlement agreement and subsequent license agreement with Acuitas Therapeutics, Inc. (“Acuitas”). This royalty entitlement from Acuitas has been retained by us and was not part of the royalty entitlement sale to OMERS.
Genevant Sciences, Ltd.
In April 2018, we entered into an agreement with Roivant Sciences Ltd. (“Roivant”), our largest shareholder, to launch Genevant Sciences Ltd. (“Genevant”), a company focused on a broad range of RNA-based therapeutics enabled by our LNP and ligand conjugate delivery technologies. We licensed rights to our LNP and ligand conjugate delivery platforms to Genevant for RNA-based applications outside of HBV, except to the extent certain rights had already been licensed to other third parties (the “Genevant License”). We retained all rights to our LNP and conjugate delivery platforms for HBV.
Under the Genevant License, as amended, if a third party sublicensee of intellectual property licensed by Genevant from us commercializes a sublicensed product, we become entitled to receive a specified percentage of certain revenue that may be received by Genevant for such sublicense, including royalties, commercial milestones and other sales-related revenue, or, if less, tiered low single-digit royalties on net sales of the sublicensed product. The specified percentage is 20% in the case of a mere sublicense (i.e., naked sublicense) by Genevant without additional contribution and 14% in the case of a bona fide collaboration with Genevant.
Additionally, if Genevant receives proceeds from an action for infringement by any third parties of our intellectual property licensed to Genevant, we would be entitled to receive, after deduction of litigation costs, 20% of the proceeds received by Genevant or, if less, tiered low single-digit royalties on net sales of the infringing product (inclusive of the proceeds from litigation or settlement, which would be treated as net sales).
In July 2020, Roivant recapitalized Genevant through an equity investment and conversion of previously issued convertible debt securities held by Roivant. We participated in the recapitalization of Genevant with an equity investment of $2.5 million. In connection with the recapitalization, the three parties entered into an Amended and Restated Shareholders Agreement that provides Roivant with substantial control of Genevant. We have a non-voting observer seat on Genevant’s Board of Directors. As of September 30, 2022, we owned approximately 16% of the common equity of Genevant and the carrying value of our investment in Genevant was zero. Our entitlement to receive future royalties or sublicensing revenue from Genevant was not impacted by the recapitalization.
Moderna Inter Partes Review Petitions
On February 21, 2018 and March 5, 2018, Moderna Therapeutics, Inc. (“Moderna”) filed petitions requesting the United States Patent and Trademark Office to institute an Inter Partes Review of Arbutus United States Patents 9,404,127 (the “’127 Patent”) and 9,364,435 (the “’435 Patent”). In its petitions, Moderna sought to invalidate all claims of each patent based on Moderna’s allegation that the claims are anticipated and/or obvious. We filed a response to Moderna’s petitions on June 14, 2018. On
September 12, 2018, the Patent Trial and Appeal Board (the “PTAB”) rendered its decision to institute Inter Partes Review of both the ‘127 Patent and the ‘435 Patent.
The status of these patents, which collectively represent only a fraction of our extensive LNP patent portfolio, is as follows:
‘127 Patent
With respect to the ‘127 Patent, the PTAB held all claims as invalid on September 10, 2019, by reason of anticipatory prior art. However this decision was vacated and sent back (remanded) to the PTAB for a rehearing, pending the Supreme Court’s decision whether to grant certiorari in a different case, United States v. Athrex, Inc. (“US v. Athrex”), the holding of which could impact the findings in the ‘127 Patent matter. The Supreme Court granted certiorari in US v. Athrex on October 13, 2020 (i.e. agreed to review the decision appealed from a lower court). Until the Supreme Court rendered its opinion in US v. Athrex, the ‘127 Patent hearing remained in abeyance, with no decision reached as to the validity of its claims. The Supreme Court decided on the US v. Athrex case on June 21, 2021, following which the Federal Circuit reinstated the appeal sua sponte, requiring the parties to brief how the case should proceed in light of the Supreme Court’s opinion or for the Appellant to waive the challenge. We elected to waive the challenge and proceed with the appeal at the Federal Circuit. The opening brief was filed on October 25, 2021. Moderna’s responsive brief was filed on February 24, 2022 and our reply brief was filed on April 26, 2022. A oral hearing for this matter was held on November 4, 2022.
‘435 Patent
With respect to the ‘435 Patent, the PTAB rendered its decision on September 11, 2019, holding certain claims invalid and upholding other claims as valid. On November 13, 2019, we and Moderna both appealed the decision. Moderna filed its opening brief on May 4, 2020 and we provided our opening and responsive brief on July 27, 2020. Moderna subsequently filed its reply and responsive brief on October 5, 2020, and we filed our reply brief on November 9, 2020. An oral hearing on the ‘435 Patent was held on October 7, 2021. On December 1, 2021, the Federal Circuit issued its opinion, leaving intact the PTAB’s holding regarding the validity of certain claims in the ‘435 Patent and the invalidity of other claims in the ‘435 Patent. The decision in the ‘435 appeal was rendered final by mandate on January 25, 2022.
‘069 Patent
On January 9, 2019, Moderna filed an additional petition requesting Inter Partes Review of Arbutus United States Patent 8,058,069 (the “’069 Patent”). The PTAB instituted Inter Partes Review of the ‘069 Patent and, on July 23, 2020, issued a decision upholding all claims as valid. On September 23, 2020, Moderna appealed the ‘069 Inter Partes Review decision to the Federal Circuit Court of Appeals. Moderna filed its opening brief in that appeal on February 23, 2021, we filed our responsive brief on May 11, 2021, and Moderna filed its reply brief on July 1, 2021. An oral hearing on the ‘069 Patent was held on October 7, 2021, in a joint hearing with the hearing regarding the ‘435 patent, before the U.S. Court of Appeals for the Federal Circuit. On December 1, 2021, the Federal Circuit also issued its ruling with respect to the ‘069 Patent, affirming the PTAB’s finding that all claims were valid. The Federal Circuit’s decision in the ‘069 appeal was rendered final by mandate on January 10, 2022.
Moderna and Merck European Oppositions
On April 5, 2018, Moderna and Merck, Sharp & Dohme Corporation (“Merck”) filed Notices of Opposition to Arbutus’ European patent EP 2279254 (“the ’254 Patent”) with the European Patent Office (“EPO”), requesting that the ‘254 Patent be revoked in its entirety for all contracting states. We filed a response to Moderna and Merck’s oppositions on September 3, 2018. A hearing was conducted before the Opposition Division of the EPO on October 10, 2019. At the conclusion of the hearing, the EPO upheld an auxiliary request adopting the amendment, as put forth by us, of certain claims of the ‘254 Patent. In February 2020 Moderna and Merck filed Notices of Appeal challenging the EPO’s grant of the auxiliary request. Merck filed its notice of appeal on February 24, 2020 and Moderna on February 27, 2020. Both Merck and Moderna perfected their appeals by filing Grounds of Appeal on April 30, 2020. We filed our response to the appeals on September 18, 2020. On March 22, 2022, Moderna filed further written submissions. The date for the oral proceedings has not been set.
While we are the patent holder, the ‘127 Patent, the ‘435 Patent, the ‘069 Patent and the ‘254 Patent have been licensed to Genevant and are included in the rights licensed by us to Genevant under the Genevant License.
Patent Infringement Litigation vs. Moderna
On February 28, 2022, we and Genevant filed a lawsuit in the U.S. District Court for the District of Delaware against Moderna, Inc. and a Moderna affiliate seeking damages for infringement of U.S. Patent Nos. 8,058,069, 8,492,359, 8,822,668, 9,364,435, 9,504,651, and 11,141,378 in the manufacture and sale of MRNA-1273, Moderna’s vaccine for COVID-19. The patents relate to nucleic acid-lipid particles and lipid vesicles, as well as compositions and methods for their use. The lawsuit does not seek an injunction or otherwise seek to impede the sale, manufacture or distribution of MRNA-1273. However, we seek fair compensation for Moderna’s use of our patented technology that was developed with great effort and at great expense, without which Moderna’s COVID-19 vaccine would not have been successful. On May 6, 2022, Moderna filed a partial motion to dismiss the claims “relating to Moderna’s sale and provision of COVID-19 vaccine doses to the U.S. Government.” On November 2, 2022, the Court issued an Order denying Moderna’s motion.
Acuitas Declaratory Judgment Lawsuit
On March 18, 2022, Acuitas Therapeutics Inc. (“Acuitas”) filed a lawsuit against us and Genevant in the Southern District of New York, asking the court to enter declaratory judgment that Arbutus patent Nos. 8,058,069, 8,492,359, 8,822,668, 9,006,417, 9,364,435, 9,404,127, 9,504,651, 9,518,272, and 11,141,378 do not infringe Pfizer and BioNTech’s COVID-19 vaccine, COMIRNATY, which uses an mRNA lipid provided, under license, by Acuitas. Acuitas also seeks a declaration that each of the listed patents is invalid. On June 24, 2022, we and Genevant sought a pre-motion conference concerning our anticipated motion to dismiss all of Acuitas’ claims due to lack of subject matter jurisdiction. The request for a pre-motion conference was granted, but the case was subsequently re-assigned to a new judge who entered an order directing: (i) Acuitas to inform the court whether it intended to file an amended complaint; (ii) that Acuitas must file any amended complaint by a certain date; and (iii) that if Acuitas did not file an amended complaint, we and Genevant must file our motion to dismiss by a certain date. Acuitas filed its amended complaint on September 6, 2022. On October 4, 2022, we and Genevant filed our motion to dismiss the Acuitas action for lack of subject matter jurisdiction based on the lack of a case or controversy. Acuitas’ filed its opposition to the motion to dismiss on November 1, 2022, and we and Genevant intend to file our reply brief on November 16, 2022. No case schedule is yet in place.
CRITICAL ACCOUNTING POLICIES AND SIGNIFICANT JUDGEMENTS AND ESTIMATES
This management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with United States generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, and expenses. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe there have been no significant changes in our critical accounting policies and estimates as discussed in “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our Annual Report on Form 10-K for the year ended December 31, 2021.
RECENT ACCOUNTING PRONOUNCEMENTS
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board or other standard setting bodies that are adopted by us as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption.
Please refer to note 2 to our condensed consolidated financial statements included in Part I, Item 1, “Financial Statements (Unaudited)” of this Quarterly Report on Form 10-Q for a description of recent accounting pronouncements applicable to our business.
RESULTS OF OPERATIONS
The following summarizes the results of our operations for the periods shown:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended September 30, | | Nine Months Ended September 30, |
| | 2022 | | 2021 | | 2022 | | 2021 |
| (in thousands) |
| Total revenue | $ | 5,952 | | | $ | 3,340 | | | $ | 32,774 | | | $ | 7,782 | |
| Operating expenses | 23,763 | | | 21,748 | | | 75,668 | | | 60,508 | |
| Loss from operations | (17,811) | | | (18,408) | | | (42,894) | | | (52,726) | |
| Other loss | 244 | | | (750) | | | (186) | | | (2,200) | |
| Loss before income taxes | (17,567) | | | (19,158) | | | (43,080) | | | (54,926) | |
| Income tax expense | — | | | — | | | (4,444) | | | — | |
| Net loss | (17,567) | | | (19,158) | | | (47,524) | | | (54,926) | |
| Dividend accretion of convertible preferred shares | — | | | (5,087) | | | — | | | (11,565) | |
| Net loss attributable to common shares | $ | (17,567) | | | $ | (24,245) | | | $ | (47,524) | | | $ | (66,491) | |
Revenue
Revenues are summarized in the following table:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, |
| 2022 | | % of Total | | 2021 | | % of Total |
| (in thousands, except percentages) |
| Revenue from collaborations and licenses | | | | | | | |
| Acuitas Therapeutics, Inc. | $ | 1,256 | | | 21 | % | | $ | 1,425 | | | 43 | % |
| Qilu Pharmaceutical Co., Ltd. | 2,352 | | | 40 | % | | — | | | — | % |
| Other milestone and royalty payments | — | | | — | % | | 55 | | | 2 | % |
| Non-cash royalty revenue | | | | | | | |
| Alnylam Pharmaceuticals, Inc. | 2,344 | | | 39 | % | | 1,860 | | | 56 | % |
| Total revenue | $ | 5,952 | | | 100 | % | | $ | 3,340 | | | 100 | % |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| Nine Months Ended September 30, |
| 2022 | | % of Total | | 2021 | | % of Total |
| (in thousands, except percentages) |
| Revenue from collaborations and licenses | | | | | | | |
| Acuitas Therapeutics, Inc. | $ | 4,339 | | | 13 | % | | $ | 3,683 | | | 47 | % |
| Qilu Pharmaceutical Co., Ltd. | 23,007 | | | 70 | % | | — | | | — | % |
| Other milestone and royalty payments | 35 | | | — | % | | 136 | | | 2 | % |
| Non-cash royalty revenue | | | | | | | |
| Alnylam Pharmaceuticals, Inc. | 5,393 | | | 16 | % | | 3,963 | | | 51 | % |
| Total revenue | $ | 32,774 | | | 100 | % | | $ | 7,782 | | | 100 | % |
Total revenue increased $2.6 million and $25.0 million for the three and nine months ended September 30, 2022, respectively, compared to the same periods in 2021, primarily due to license revenue recognized related to our progress towards the satisfaction of our performance obligations with respect to the technology transfer and licensing agreement with Qilu, which closed in January 2022, as well as an increase in license royalty revenue from Alnylam and Acuitas due to the growth of Alnylam’s sales of ONPATTRO.
Operating expenses
Operating expenses are summarized in the following table:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, |
| 2022 | | % of Total | | 2021 | | % of Total |
| (in thousands, except percentages) |
| Research and development | $ | 20,055 | | | 84 | % | | $ | 16,709 | | | 77 | % |
| General and administrative | 3,493 | | | 15 | % | | 4,183 | | | 19 | % |
| Change in fair value of contingent consideration | 215 | | | 1 | % | | 856 | | | 4 | % |
| Total operating expenses | $ | 23,763 | | | 100 | % | | $ | 21,748 | | | 100 | % |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| Nine Months Ended September 30, |
| 2022 | | % of Total | | 2021 | | % of Total |
| (in thousands, except percentages) |
| Research and development | $ | 61,459 | | | 81 | % | | $ | 46,290 | | | 77 | % |
| General and administrative | 13,585 | | | 18 | % | | 12,539 | | | 21 | % |
| Change in fair value of contingent consideration | 624 | | | 1 | % | | 1,679 | | | 3 | % |
| Total operating expenses | $ | 75,668 | | | 100 | % | | $ | 60,508 | | | 100 | % |
Research and development
Research and development expenses consist primarily of personnel expenses, fees paid to clinical research organizations and contract manufacturers, consumables and materials, consulting, and other third party expenses to support our clinical and pre-clinical activities, as well as a portion of stock-based compensation and general overhead costs.
Research and development expenses increased $3.3 million and $15.2 million for the three and nine months ended September 30, 2022, respectively, compared to the same periods in 2021. The increase was due primarily to an increase in expenses for our ongoing AB-729 Phase 2a clinical trials, including our collaborations with Assembly and Vaccitech, and an increase in expenses for our early-stage development programs, including AB-101 and AB-161.
A significant portion of our research and development expenses are not tracked by project as they benefit multiple projects or our technology platform and because our most-advanced programs are not yet in late-stage clinical development.
General and administrative
General and administrative expenses decreased $0.7 million for the three months ended September 30, 2022 as compared to the same period in 2021 due primarily to an award of $0.5 million during the third quarter of 2022 from the arbitrator in the UBC matter for reimbursement of costs and attorneys’ fees. General and administrative expenses increased $1.0 million for the nine months ended September 30, 2022 as compared to the same period in 2021, due primarily to increases in employee compensation costs and non-cash stock-based compensation expense.
Change in fair value of contingent consideration
Contingent consideration is a liability related to our acquisition of Enantigen Therapeutics, Inc. in October 2014. In general, as time passes and assuming no changes to the assumptions related to the contingency, the fair value of the contingent consideration increases as the progress of our programs get closer to triggering contingent payments based on certain sales milestones of our first commercial product for cHBV. As AB-729 continues to progress through Phase 2a proof-of-concept clinical trials, we adjust our assumption regarding probability of success commensurate with the progression of the program, which will increase the fair value of the liability.
Other income (loss)
The components of our other income (loss) are summarized in the following table:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended September 30, | | Nine Months Ended September 30, |
| | 2022 | | 2021 | | 2022 | | 2021 |
| (in thousands) |
| Interest income | $ | 694 | | | $ | 27 | | | $ | 1,249 | | | $ | 97 | |
| Interest expense | (429) | | | (762) | | | (1,417) | | | (2,297) | |
| Foreign exchange loss | (21) | | | (15) | | | (18) | | | — | |
| | | | | | | |
| Total other loss | $ | 244 | | | $ | (750) | | | $ | (186) | | | $ | (2,200) | |
Interest income
The increase in interest income for the three and nine months ended September 30, 2022 compared to the same periods in 2021 was due primarily to higher interest earned on higher average cash and investment balances.
Interest expense
Interest expense for both the three and nine months ended September 30, 2022 and 2021 consisted primarily of non-cash amortization of discount and issuance costs related to the sale of a portion of our ONPATTRO royalty interest to OMERS in July 2019. The decreases are related to the declining balance of the unamortized discount and issuance costs.
Foreign exchange gains
In connection with our site consolidation to Warminster, PA, our Canadian dollar-denominated expenses and cash balances have decreased significantly now that a majority of our business transactions are based in the United States. We continue to incur expenses and hold some cash balances in Canadian dollars, and as such, we will remain subject to risks associated with foreign currency fluctuations.
Income Tax Expense
During the nine months ended September 30, 2022, we recognized income tax expense of $4.4 million for withholding taxes paid to the Chinese taxing authority by Qilu on our behalf in connection with the upfront license fee Qilu paid us.
LIQUIDITY AND CAPITAL RESOURCES
The following table summarizes our cash flow activities for the periods indicated:
| | | | | | | | | | | |
| | Nine Months Ended September 30, |
| | 2022 | | 2021 |
| (in thousands) |
| Net loss | $ | (47,524) | | | $ | (54,926) | |
| Non-cash items | 3,429 | | | 7,080 | |
| Change in deferred license revenue | 25,463 | | | — | |
| Net change in operating items | 266 | | | (80) | |
| Net cash used in operating activities | (18,366) | | | (47,926) | |
| Net cash used in investing activities | (87,624) | | | (4,557) | |
| Issuance of common shares pursuant to Share Purchase Agreement | 10,973 | | | — | |
| Cash provided by other financing activities | 9,757 | | | 78,115 | |
| Net cash provided by financing activities | 20,730 | | | 78,115 | |
| Effect of foreign exchange rate changes on cash and cash equivalents | (18) | | | — | |
| (Decrease)/increase in cash and cash equivalents | (85,278) | | | 25,632 | |
| Cash and cash equivalents, beginning of period | 109,282 | | | 52,251 | |
| Cash and cash equivalents, end of period | $ | 24,004 | | | $ | 77,883 | |
Since our incorporation, we have financed our operations through sales of equity, debt, revenues from research and development collaborations and licenses with corporate partners, royalty monetization, interest income on funds available for investment, and government contracts, grants and tax credits.
For the nine months ended September 30, 2022, $18.4 million of cash was used in operating activities compared to $47.9 million used in operating activities for the nine months ended September 30, 2021, a decrease of $29.6 million. The decrease was due primarily to a January 2022 upfront cash payment of $40.0 million from Qilu and a $4.0 million premium paid by Qilu as part of their $15.0 million equity investment. These cash inflows were offset by $62.4 million of cash used in operations.
For the nine months ended September 30, 2022, net cash used in investing activities was $87.6 million, consisting primarily of additional investments in marketable securities of $117.3 million, partially offset by maturities of investments in marketable securities of $30.0 million. For the nine months ended September 30, 2021, net cash used in investing activities was $4.6 million, which consisted primarily of maturities of investments in marketable securities of $50.4 million, partially offset by additional investments in marketable securities of $54.2 million.
For the nine months ended September 30, 2022, net cash provided by financing activities was $20.7 million, which included $11.0 million for the fair value of the shares purchased by Qilu as part of their $15.0 million equity investment, of which the remaining $4.0 million was a premium paid by Qilu on the equity investment and was allocated to deferred revenue. Also contributing to net cash provided by financing activities was $9.2 million in proceeds from sales of common shares under the Sale Agreement. For the nine months ended September 30, 2021, net cash provided by financing activities was $78.1 million, which was primarily driven by $75.4 million in proceeds from sales of common shares under the Sale Agreement.
Sources of Liquidity
As of September 30, 2022, we had cash, cash equivalents and investments in marketable securities of $190.2 million. We had no outstanding debt as of September 30, 2022.
Open Market Sale Agreement
We have an Open Market Sale AgreementSM with Jefferies LLC dated December 20, 2018, as amended by Amendment No. 1, dated December 20, 2019, Amendment No. 2, dated August 7, 2020 and Amendment No. 3, dated March 4, 2021 (as amended, the “Sale Agreement”), under which we may offer and sell common shares, from time to time.
On December 23, 2019, we filed a shelf registration statement on Form S-3 with the Securities and Exchange Commission (the “SEC”) (File No. 333-235674) and accompanying base prospectus, declared effective by the SEC on January 10, 2020 (the “January 2020 Registration Statement”), for the offer and sale of up to $150.0 million of our securities. The January 2020 Registration Statement also contained a prospectus supplement in connection with the offering of up to $50.0 million of our common shares pursuant to the Sale Agreement. This prospectus supplement was fully utilized during 2020. On August 7, 2020, we filed a prospectus supplement with the SEC (the “August 2020 Prospectus Supplement”) in connection with the offering of up to an additional $75.0 million of our common shares pursuant to the Sale Agreement under the January 2020 Registration Statement. The August 2020 Prospectus Supplement was fully utilized during 2020.
On August 28, 2020, we filed a shelf registration statement on Form S-3 with the SEC (File No. 333-248467) and accompanying base prospectus, which was declared effective by the SEC on October 22, 2020 (the “October 2020 Registration Statement”), for the offer and sale of up to $200.0 million of our securities. On March 4, 2021, we filed a prospectus supplement with the SEC (the “March 2021 Prospectus Supplement”) in connection with the offering of up to an additional $75.0 million of our common shares pursuant to the Sale Agreement under the October 2020 Registration Statement. We fully utilized the March 2021 Prospectus Supplement during 2021. On October 8, 2021, we filed a prospectus supplement with the SEC (the “October 2021 Prospectus Supplement”) for the offer and sale of up to an additional $75.0 million of our common shares pursuant to the Sale Agreement under the October 2020 Registration Statement.
On November 4, 2021, we filed a shelf registration statement on Form S-3 with the SEC (File No. 333-260782) and accompanying base prospectus, declared effective by the SEC on November 18, 2021 (the “November 2021 Registration Statement”), for the offer and sale of up to $250.0 million of our securities.
On March 3, 2022, we filed a prospectus supplement with the SEC (the “March 2022 Prospectus Supplement”) in connection with the offering of up to an additional $100.0 million of our common shares pursuant to the Sale Agreement under: (i) the January 2020 Registration Statement; (ii) the October 2020 Registration Statement; and (iii) the November 2021 Registration Statement.
During the nine months ended September 30, 2022, we issued 3,901,765 common shares pursuant to the Sale Agreement, as amended, resulting in net proceeds of approximately $9.2 million. For the nine months ended September 30, 2021, we issued 19,715,142 common shares pursuant to the Sale Agreement, resulting in net proceeds of approximately $75.4 million. As of September 30, 2022, there was approximately $142.6 million available in aggregate under the October 2021 Prospectus Supplement and the March 2022 Prospectus Supplement.
Royalty Entitlements
Additionally, we have a royalty entitlement on ONPATTRO, a drug developed by Alnylam that incorporates our LNP technology and was approved by the FDA and the EMA during the third quarter of 2018 and was launched by Alnylam immediately upon approval in the United States. In July 2019, we sold a portion of this royalty interest to OMERS, effective as of January 1, 2019, for $20 million in gross proceeds before advisory fees. OMERS will retain this entitlement until it has received $30 million in royalties, at which point 100% of such royalty interest on future global net sales of ONPATTRO will revert to us. OMERS has assumed the risk of collecting up to $30 million of future royalty payments from Alnylam and we are not obligated to reimburse OMERS if they fail to collect any such future royalties. If this royalty entitlement reverts to us, it has the potential to provide an active royalty stream or to be otherwise monetized again in full or in part. In addition to the royalty from the Alnylam LNP license agreement, we are also receiving a second, lower royalty interest on global net sales of ONPATTRO originating from a settlement agreement and subsequent license agreement with Acuitas. The royalty from Acuitas has been retained by us and was not part of the royalty sale to OMERS.
In December 2021, we entered into a technology transfer and licensing agreement with Qilu pursuant to which we granted Qilu a sublicensable, royalty-bearing license, under certain intellectual property owned by us, which is non-exclusive as to development and manufacturing and exclusive with respect to commercialization of AB-729, including pharmaceutical products that include AB-729, for the treatment or prevention of hepatitis B in the Territory. In partial consideration for the rights granted by us, Qilu paid us a one-time upfront cash payment of $40 million and made an equity investment of $15 million, both received in January 2022, and agreed to pay us milestone payments totaling up to $245 million, net of withholding taxes, upon the achievement of certain technology transfer, development, regulatory and commercialization milestones. Qilu also agreed to pay us double digit royalties into the low twenties percent based upon annual net sales of AB-729 in the Territory. The royalties are payable on a product-by-product and region-by-region basis, subject to certain limitations.
Cash requirements
We believe that our $190.2 million of cash, cash equivalents and investments in marketable securities as of September 30, 2022 will be sufficient to fund our operations into the second quarter of 2024 based on our expectation of a net cash burn between $90 million and $95 million in 2022. In the future, substantial additional funds will be required to continue with the active development of our pipeline products and technologies.
In particular, our funding needs may vary depending on a number of factors including:
•the effects of the COVID-19 pandemic on our business, the medical community and the global economy;
•revenue earned from our legacy collaborative partnerships and licensing agreements, including potential royalty payments from Alnylam’s ONPATTRO;
•revenue earned from ongoing collaborative partnerships, including milestone and royalty payments;
•the potential requirement to make milestone payments related to our legacy agreements;
•the extent to which we continue the development of our product candidates, add new product candidates to our pipeline, or form collaborative relationships or licensing arrangements to advance our product candidates;
•delays in the development of our product candidates due to pre-clinical and clinical findings;
•our decisions to in-license or acquire additional products, product candidates or technology for development;
•our ability to attract and retain development or commercialization partners, and their effectiveness in carrying out the development and ultimate commercialization of one or more of our product candidates;
•whether batches of product candidates that we manufacture fail to meet specifications resulting in clinical trial delays and investigational and remanufacturing costs;
•the decisions, and the timing of decisions, made by health regulatory agencies regarding our technology and product candidates;
•competing products, product candidates and technological and market developments; and
•costs associated with prosecuting and enforcing our patent claims and other intellectual property rights, including litigation and arbitration arising in the course of our business activities.
We intend to seek funding to maintain and advance our business from a variety of sources including public or private equity or debt financing, potential monetization transactions, collaborative or licensing arrangements with pharmaceutical companies and government grants and contracts. There can be no assurance that funding will be available at all or on acceptable terms to permit further development of our research and development programs. Further, the continued spread of COVID-19 has also led to severe disruption and volatility in the global capital markets, which could increase our cost of capital and adversely affect our ability to access the capital markets in the future.
If adequate funding is not available, we may be required to delay, reduce or eliminate one or more of our research or development programs or reduce expenses associated with our non-core activities. We may need to obtain funds through arrangements with collaborators or others that may require us to relinquish most or all of our rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise seek if we were better funded. Insufficient financing may also mean failing to prosecute our patents or relinquishing rights to some of our technologies that we would otherwise develop or commercialize.
OFF-BALANCE SHEET ARRANGEMENTS
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The information under this item is not required to be provided by smaller reporting companies.
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures as of September 30, 2022. The term “disclosure controls and procedures”, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure, particularly during the period in which this Quarterly Report on Form 10-Q was being prepared. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their desired objectives, and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of September 30, 2022, our principal executive officer and principal financial officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting (as defined in Rules 13a–15(f) and 15d–15(f) under the Exchange Act) during the three months ended September 30, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
Patent Infringement Litigation vs. Moderna
On February 28, 2022, we and Genevant filed a lawsuit in the U.S. District Court for the District of Delaware against Moderna, Inc. and a Moderna affiliate (collectively, “Moderna”) seeking damages for infringement of U.S. Patent Nos. 8,058,069, 8,492,359, 8,822,668, 9,364,435, 9,504,651, and 11,141,378 in the manufacture and sale of MRNA-1273, Moderna’s vaccine for COVID-19. The patents relate to nucleic acid-lipid particles and lipid vesicles, as well as compositions and methods for their use. The lawsuit does not seek an injunction or otherwise seek to impede the sale, manufacture or distribution of MRNA-1273. However, the Company seeks fair compensation for Moderna’s use of its patented technology that was developed with great effort and at great expense, without which Moderna’s COVID-19 vaccine would not have been successful. On May 6, 2022, Moderna filed a partial motion to dismiss the claims “relating to Moderna’s sale and provision of COVID-19 vaccine doses to the U.S. Government.” On November 2, 2022, the Court issued an Order denying Moderna’s motion.
Acuitas Declaratory Judgment Lawsuit
On March 18, 2022, Acuitas Therapeutics Inc. (“Acuitas”) filed a lawsuit against us and Genevant in the U.S. District Court for the Southern District of New York, asking the court to enter declaratory judgment that Arbutus patent Nos. 8,058,069, 8,492,359, 8,822,668, 9,006,417, 9,364,435, 9,404,127, 9,504,651, 9,518,272, and 11,141,378 do not infringe Pfizer and BioNTech’s COVID-19 vaccine, COMIRNATY, which uses an mRNA lipid provided, under license, by Acuitas. Acuitas also seeks a declaration that each of the listed patents is invalid. On June 24, 2022, we and Genevant sought a pre-motion conference concerning our anticipated motion to dismiss all of Acuitas’ claims due to lack of subject matter jurisdiction. The request for a pre-motion conference was granted, but the case was subsequently re-assigned to a new judge who entered an order directing: (i) Acuitas to inform the court whether it intended to file an amended complaint; (ii) that Acuitas must file any amended complaint by a certain date; and (iii) that if Acuitas did not file an amended complaint, we and Genevant must file our motion to dismiss by a certain date. Acuitas filed its amended complaint on September 6, 2022. On October 4, 2022, we and Genevent filed our motion to dismiss the Acuitas action for lack of subject matter jurisdiction based on the lack of a case or controversy. Acuitas filed its opposition to the motion to dismiss on November 1, 2022, and we and Genevant intend to file our reply brief on November 16, 2022. No case schedule is yet in place.
University of British Columbia
Certain early work on lipid nanoparticle delivery systems and related inventions was undertaken at the University of British Columbia (“UBC”), as well as by us that was subsequently assigned to UBC. These inventions are licensed to us by UBC under a license agreement, initially entered into in 1998 and as amended in 2001, 2006 and 2007. We granted sublicenses under the UBC license to certain third parties, including Alnylam. In November 2014, UBC filed a demand for arbitration against us which alleged entitlement to unpaid royalties. In August 2019, the arbitrator issued his decision for the second phase of the arbitration, awarding UBC $5.9 million, which included interest of approximately $2.6 million. We paid the $5.9 million award to UBC in September 2019 and paid an additional $0.2 million award for costs and attorneys’ fees in March 2021, and this matter is now fully resolved.
On December 18, 2020, UBC delivered to us a notice of arbitration alleging that under its cross license with us, it is due royalties of $2.0 million plus interest arising from our sale to OMERS of part of our royalty interest on future global net sales of ONPATTRO, currently being sold by Alnylam. Oral hearings for this matter were held in April 2022 and, on July 11, 2022, the arbitrator issued his decision fully dismissing UBC’s claim for royalties. As a result, no payments are owed to UBC. In September 2022, the arbitrator awarded the Company $0.5 million for reimbursement of costs and attorneys’ fees, which the Company received from UBC in October 2022. This matter is now fully resolved.
ITEM 1A. RISK FACTORS
Several of our and our collaboration partners’ current and planned clinical trials have been impacted and could be further delayed or suspended as a result of the military action by Russia in Ukraine.
In February 2022, Russia commenced a military invasion of Ukraine. A portion of our clinical trial evaluating AB-836 and a cohort of Antios Therapeutics, Inc.’s (“Antios”) clinical trial evaluating a triple combination including AB-729 were being conducted in Ukraine. We had also planned to conduct a portion of the following clinical trials in Ukraine: (i) our Phase 2a clinical trial evaluating AB-729 in combination with ongoing NA therapy and short courses of PEG-IFNα-2a in cHBV patients
and (ii) our planned Phase 2a clinical trial to evaluate a triple combination of AB-729 with Vaccitech’s VTP-300 and a NA. We intend to utilize alternative clinical trial sites for our ongoing and planned clinical trials impacted by the military action in Ukraine.
Russia’s invasion and the ensuing response by Ukraine has disrupted our and our collaboration partners’ current clinical trials in such jurisdictions and could increase our costs and disrupt future planned clinical development activities. For example, enrollment was completed in a cohort of patients in Antios’ ongoing Phase 2a proof-of-concept clinical trial evaluating a triple combination of AB-729, Antios’ proprietary Active Site Polymerase Inhibitor Nucleotide (ASPIN), ATI-2173, and Viread (tenofovir disoproxil fumarate), a nucleos(t)ide reverse transcriptase inhibitor. However, the majority of patients in this cohort were enrolled in Ukraine and, as a result, these patients have been lost to follow-up before completing the clinical trial. Antios recently terminated this clinical trial and we have terminated our clinical collaboration agreement with Antios.
Although the length and impact of Russia’s military action is highly unpredictable, actions by Russia, or potentially other countries, against Ukraine and surrounding areas may adversely affect our ability to adequately conduct or complete certain clinical trials and maintain compliance with relevant protocols due to, among other reasons, the prioritization of hospital resources away from clinical trials, reallocation or evacuation of site staff and subjects, or as a result of government-imposed curfews, warfare, violence, or other governmental action or events that restrict movement. These developments may also result in our inability to access sites for monitoring or to obtain data from affected sites or patients going forward. We could also experience disruptions in our supply chain or limits to our ability to provide sufficient investigational materials in Ukraine and surrounding regions. Alternative sites to fully and timely compensate for our clinical trial activities in Ukraine may not be available and we may need to find other countries to conduct these clinical trials. If these clinical trials are further interrupted, our clinical development plans for these product candidates could be significantly delayed, which would increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues.
There have been no other material changes in our risk factors from those disclosed in our Annual Report on Form 10-K for the fiscal year-ended December 31, 2021.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
None.
ITEM 6. EXHIBITS
EXHIBIT INDEX
| | | | | | | | |
| Number | | Description |
| 3.1 | | |
| | |
| 3.2 | | |
| | |
| 10.1 | |
|
| | |
| 31.1* | | |
| | |
| 31.2* | | |
| | |
| 32.1** | | |
| | |
| 32.2** | | |
| | |
| 101 | | The following materials from Arbutus Biopharma Corporation’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2022, formatted in inline XBRL (eXtensible Business Reporting Language): (i) Condensed Consolidated Balance Sheets; (ii) Condensed Consolidated Statements of Operations; (iii) Condensed Consolidated Statements of Comprehensive Loss; (iv) Condensed Consolidated Statements of Stockholders’ Equity; (v) Condensed Consolidated Statements of Cash Flows; and (vi) Notes to Condensed Consolidated Financial Statements |
| | |
| 104 | | Cover page interactive data file (embedded within the inline XBRL document and included in Exhibit 101) |
* Filed herewith.
** Furnished herewith.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on November 9, 2022.
| | | | | | | | |
| ARBUTUS BIOPHARMA CORPORATION |
| | |
| | By: | /s/ William H Collier |
| | | William H Collier |
| | | President and Chief Executive Officer |
| | (Principal Executive Officer) |
| | |
| | |
| By: | /s/ David C. Hastings |
| | David C. Hastings |
| | Chief Financial Officer |
| | (Principal Financial Officer and Principal Accounting Officer) |