UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF THE
SECURITIES EXCHANGE ACT OF 1934
For the month July 2017
(Commission File No. 001-35193)
Grifols, S.A.
(Translation of registrant’s name into English)
Avinguda de la Generalitat, 152-158
Parc de Negocis Can Sant Joan
Sant Cugat del Valles 08174
Barcelona, Spain
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F x Form 40-F o
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (1):
Yes o No x
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (7):
Yes o No x
Indicate by check mark whether the registrant by furnishing the information contained in this Form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes o No x
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82- . .
Grifols, S.A.
|
Item |
|
|
Sequential Page Number |
|
|
|
|
|
|
|
1 | ||
|
|
14 | ||
|
|
29 |
|
|
Free translation from the original in Spanish |
Grifols increases its revenues by 12.3% to
EUR 2,192 million driven by growth
of its main divisions
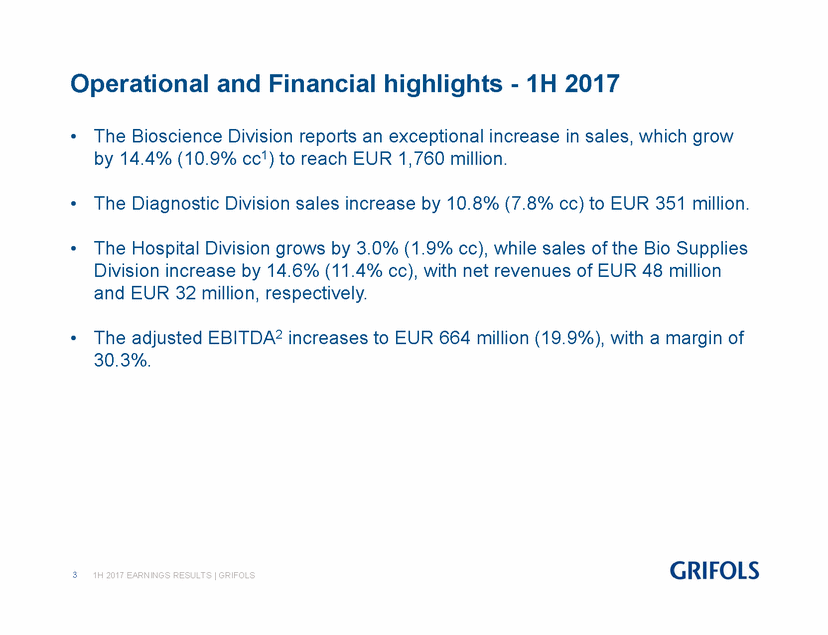
· The Bioscience Division reports an exceptional increase in sales, which grow by 14.4% (10.9% cc(1)) to reach EUR 1,760 million.
· The Diagnostic Division sales increase by 10.8% (7.8% cc) to EUR 351 million.
· The Hospital Division grows by 3.0% (1.9% cc), while sales of the Bio Supplies Division increase by 14.6% (11.4% cc), with net revenues of EUR 48 million and EUR 32 million, respectively.
· The adjusted EBITDA(2) increases to EUR 664 million (19.9%), with a margin of 30.3%.
· The company increases its net investments in R&D+i by 19.0% to EUR 129 million. Grifols manages its innovation strategy through both internal and investee projects, such as the recent acquisition of a 44% stake in the U.S. firm GigaGen for USD 35 million.
· The group expands its portfolio of plasma products with a biological sealant for biosurgical use; promotes the Diagnostic Division hemostasis line with a new global distribution contract; and boosts the presence of its Hospital Division in the U.S. after obtaining FDA approval to commercialize its saline solution in this market.
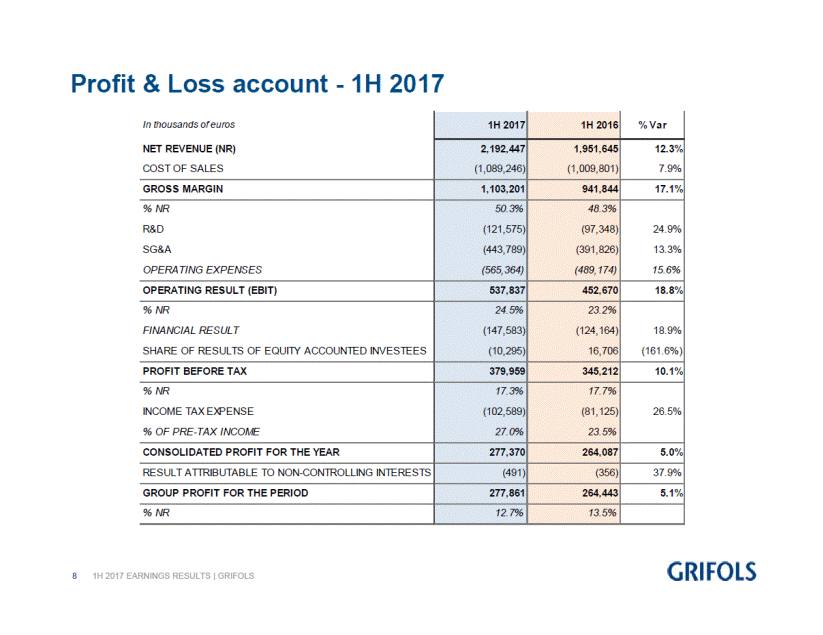
Barcelona, July 28, 2017. - Grifols (MCE: GRF, MCE: GRF.P and NASDAQ: GRFS) increased its net revenue by 12.3% (9.0% cc) in the first half of 2017 to reach EUR 2,192.4 million. The company reported significant revenue increases in all of its divisions and geographical areas where it operates.
Grifols is one of the world top three producers of plasma-derived therapies. The Bioscience Division sales reached EUR 1,759.9 million in the first half, which represents a 14.4% (10.9% cc) increase with respect to the same period in 2016.
Global demand for plasma proteins, which has remained strong as projected in previous periods, coupled with price increases in some markets, have exceptionally boosted revenue of Grifols main plasma-derived products.
Diagnostic Division sales increased by 10.8% (7.8% cc) to EUR 351.1 million, compared to EUR 316.8 million reported in the same period in 2016. The worldwide leader in transfusion medicine, Grifols continued to notably boost sales revenues for virological screening of blood donations. Higher sales of both its NAT technology systems (Procleix® NAT Solutions) in core markets such
(1) Cc: Constant currency.
(2) Excludes non-recurring costs and associated with recent acquisitions.
as the U.S., China and Japan, and its antigens used to manufacture diagnostic immunoassays led to the increase.
The Hospital Division reached sales of EUR 47.9 million, representing a 3.0% (1.9% cc) increase, driven largely by favorable activity growth of equipment and systems for hospital logistics (Pharmatech) in global markets.
As of January 2017, the company also has a new unit - the Bio Supplies Division - that primarily oversees sales of biological products for non-therapeutic use. The division reported revenues of EUR 32.1 million for the first half of 2017 and growth of 14.6% (11.4% cc), following the agreement reached with Access Biologicals to commercialize Grifols biological products for non-therapeutic use, as well as income derived from its contract with Kedrion.
The adjusted EBITDA(2) for the first half of 2017 increased by 19.9% to EUR 663.9 million, which represents a 30.3% margin. Taking into account the non-recurring costs associated with the acquisition of Hologic NAT donor-screening unit, Grifols EBITDA from January to June 2017 is EUR 644.4 million, which represents a 29.4% margin.
In line with company forecasts, Grifols acquisition of Hologic share of the NAT donor-screening unit at the beginning of 2017 has had a positive impact on the group margins. The EBITDA margin continues to reflect the effect of increased plasma costs related mainly to the expansion of plasma donation centers.
Grifols is the global leader in plasma donation centers, with a network of over 180 centers throughout the U.S. During the first half of the year, the company continued to invest in opening new centers, as well as allocating resources to expand, renovate and relocate existing ones. Grifols aims to have 190 centers by the end of the fiscal year and 230 centers by 2019.
The company also intensified its total net investments in R&D+i, which increased by 19.0% compared to the same period last year, to EUR 129.3 million. This figure includes both internal and investee projects, which are managed through Grifols Innovation and New Technologies Limited (GIANT). Grifols continues to make significant progress in R&D+i through its investee companies, the most recent of which is GigaGen. The company acquired a 44% stake of this U.S. firm for USD 35 million after the close of the period. Headquartered in San Francisco (California, U.S.), GigaGen specializes in the development of biotherapeutic therapies.
Grifols financial result was EUR 147.6 million, compared to EUR 124.2 million reported for the same period last year. The refinancing process has enabled the company to optimize its financial expenses resulting from the higher levels of debt assumed to acquire Hologic share of the NAT donor-screening unit. In the second quarter, exchange-rate differences had a negative impact of EUR 14.0 million.
Grifols effective tax rate was 27.0% in line with the figure stated in the first quarter of 2017. It exceeds the rate reported in the same period last year, due mainly to the higher profits generated by the Bioscience and Diagnostic Divisions in the U.S. market.
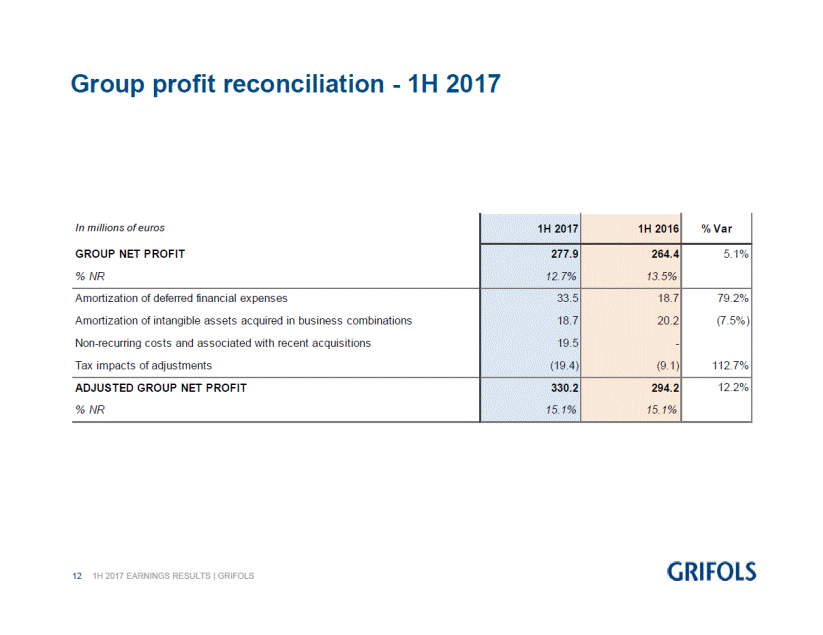
The adjusted net profit(3) reached EUR 330.2 million, increasing 12.2% in relation to the EUR 294.2 million for the same period last year. The reported net profit rose by 5.1% to EUR 277.9 million, which represents 12.7% of the company net revenue.
At the close of the first half of 2017, Grifols net financial debt was EUR 5,440.5 million, including EUR 750.2 million in cash, after taking into account the acquisition of a 49% stake in Access Biologicals for USD 51 million, the acquisition of six plasma centers from Kedplasma for USD 47 million, and deducting EUR 95.3 million corresponding to the final dividend for the 2016 fiscal year, which was approved in the Ordinary General Shareholders Meeting.
This final dividend, distributed in June 2017 (Euros 0.1356 gross dividend per share) and the one paid in December 2016 (Euros 0.18 gross dividend per share), amount to a total of EUR 218.2 million(4) paid in dividends for the 2016 fiscal year and a sustained payout of 40% of consolidated net profit. Grifols dividends have had an annual compound growth of 16% over the last four years, evidence of the company commitment to its shareholders.
Grifols net debt ratio is 4.10x EBITDA, lower than the 4.45x recorded in March 2017. Continued efforts to reduce its levels of financial leverage remain a priority for the company.
As of June 30, 2017, Grifols had EUR 420 million in undrawn credit lines and its liquidity position was approximately EUR 1,200 million.
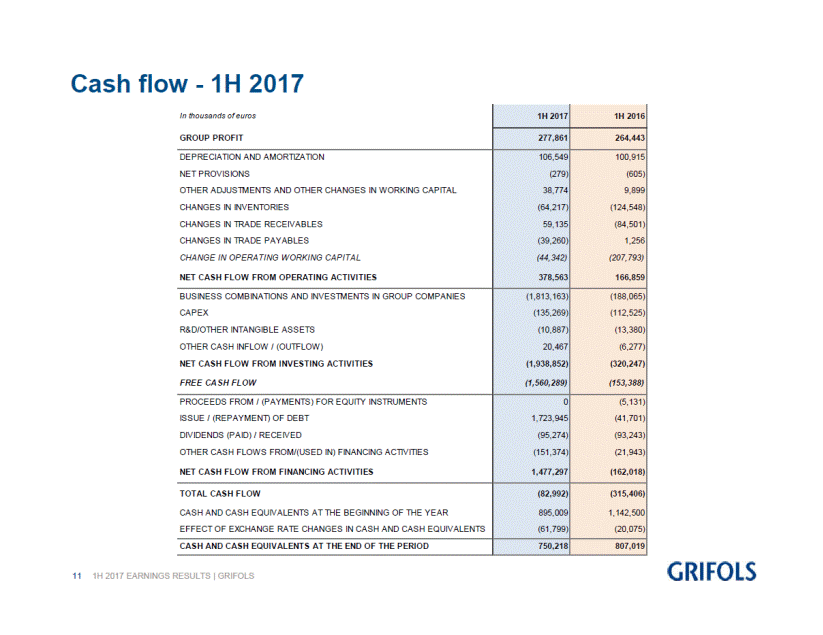
Grifols cash generation remains high, providing the necessary solvency for the company to advance its expansion and investment plans, and continue its deleveraging process. The operating cash flow in the first half of 2017 was EUR 378.6 million, compared to EUR 166.9 million reported for the same period last year. Operating cash-flow generation remains high, taking into account the higher inventory levels related to higher volume of sales.
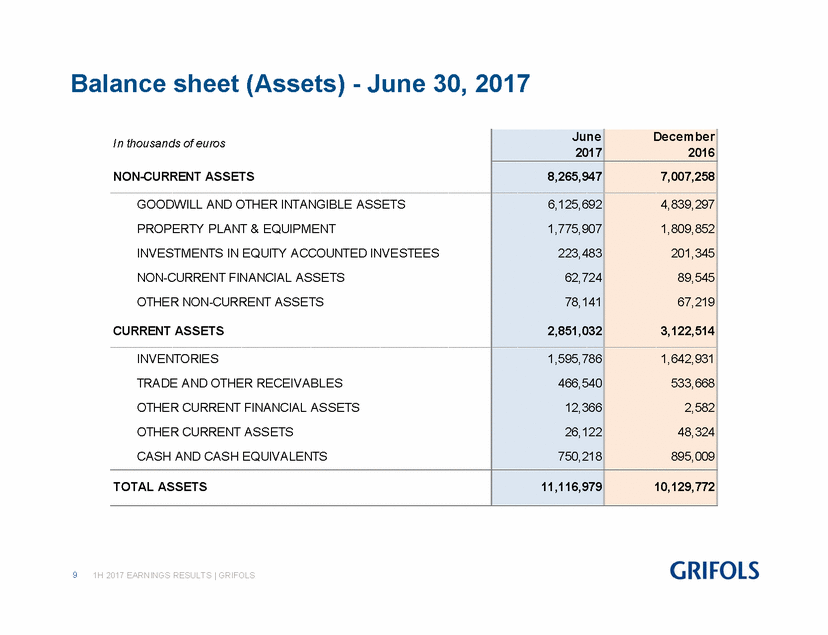
As of June 2017, total consolidated assets rose to EUR 11,117.0 million, compared to EUR 10,129.8 million in December 2016. The increase stems primarily from the new assets acquired from the purchase of Hologic share of the NAT donor-screening unit, although it was partially offset by the effect of the Euro appreciation against the U.S. dollar.
(3) Excludes non-recurring costs and associated with recent acquisitions, amortization of deferred expenses associated to the refinancing and amortization of intangible assets related to acquisitions.
(4) Includes the preferred dividend of Euros 0.01 gross per share associated with each Class B share.
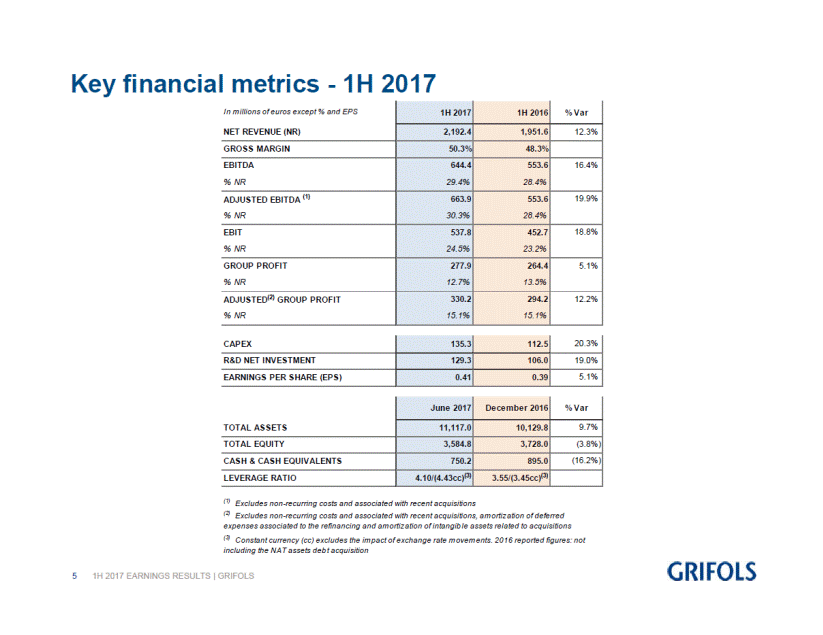
Key financial metrics for the first half of 2017:
|
In millions of euros except % and EPS |
|
1H 2017 |
|
1H 2016 |
|
% Var |
|
|
NET REVENUE (NR) |
|
2,192.4 |
|
1,951.6 |
|
12.3 |
% |
|
GROSS MARGIN |
|
50.3 |
% |
48.3 |
% |
|
|
|
EBITDA |
|
644.4 |
|
553.6 |
|
16.4 |
% |
|
% NR |
|
29.4 |
% |
28.4 |
% |
|
|
|
ADJUSTED EBITDA (1) |
|
663.9 |
|
553.6 |
|
19.9 |
% |
|
% NR |
|
30.3 |
% |
28.4 |
% |
|
|
|
EBIT |
|
537.8 |
|
452.7 |
|
18.8 |
% |
|
% NR |
|
24.5 |
% |
23.2 |
% |
|
|
|
GROUP PROFIT |
|
277.9 |
|
264.4 |
|
5.1 |
% |
|
% NR |
|
12.7 |
% |
13.5 |
% |
|
|
|
ADJUSTED(2) GROUP PROFIT |
|
330.2 |
|
294.2 |
|
12.2 |
% |
|
% NR |
|
15.1 |
% |
15.1 |
% |
|
|
|
CAPEX |
|
135.3 |
|
112.5 |
|
20.3 |
% |
|
R&D NET INVESTMENT |
|
129.3 |
|
106.0 |
|
19.0 |
% |
|
EARNINGS PER SHARE (EPS) |
|
0.41 |
|
0.39 |
|
5.1 |
% |
|
|
|
June 2017 |
|
December 2016 |
|
% Var |
|
|
TOTAL ASSETS |
|
11,117.0 |
|
10,129.8 |
|
9.7 |
% |
|
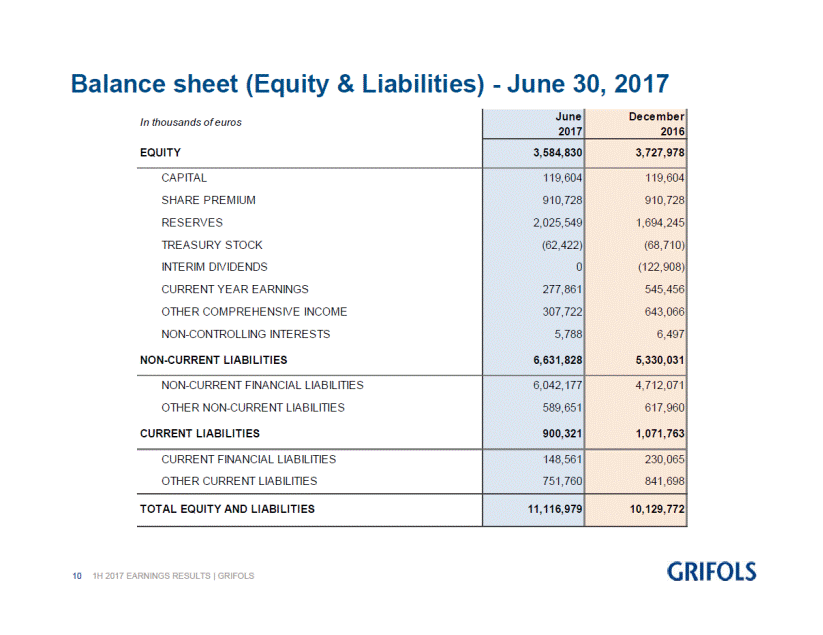
TOTAL EQUITY |
|
3,584.8 |
|
3,728.0 |
|
(3.8 |
)% |
|
CASH & CASH EQUIVALENTS |
|
750.2 |
|
895.0 |
|
(16.2 |
)% |
|
LEVERAGE RATIO |
|
4.10/(4.43cc)(3) |
|
3.55/(3.45cc)(3) |
|
|
|
(1) Excludes non-recurring costs and associated with recent acquisitions
(2) Excludes non-recurring costs and associated with recent acquisitions, amortization of deferred expenses associated to the refinancing and amortization of intangible assets related to acquisitions
(3) Constant currency (cc) excludes the impact of exchange rate movements. 2016 reported figures: not including the NAT assets debt acquisition
REVENUE PERFORMANCE
· Bioscience Division: 80.3% of total revenues
The Bioscience Division is the company main driver of growth. In the second quarter of 2017, demand for the main plasma proteins remained strong, as anticipated in previous periods. During the first half of the year, greater market demand led to a 14.4% (10.9% cc) growth in revenues to EUR 1,759.9 million. Increased sales volumes of the main plasma proteins and a positive price impact in some markets have propelled this growth.
Immunoglobulin sales were one of the growth drivers in the first half. Demand for this plasma protein continues to be robust, supported by growth in the United States, Canada and certain core markets in the European Union (EU). Immunoglobulin use in the neurology field continued its upward trend, particularly in markets with higher per capita consumption like the U.S. and Canada. Grifols also remains steadfast in its efforts to promote the use of this protein in the treatment of primary immunodeficiencies. In this regard, demand has risen significantly in specific markets in Latin America and the Asia Pacific that are currently expanding their healthcare coverage.
Grifols is the leader in the manufacture and sales of alpha-1 antitrypsin. The on-going improvement in the diagnosis of alpha-1 antitrypsin deficiency (AATD) in the U.S. and Europe, and more incipiently in Latin America, continue to be the main driver of growth, as the number of undiagnosed patients is high. Grifols also promotes disease-management programs for patients with this genetic disorder, whose symptoms are similar to those of chronic obstructive pulmonary disease (COPD). In this regard, the company continues its efforts to develop new formulations to have differentiated products that widen the treatment options for its patients. Grifols has developed a liquid formulation of its alpha 1-antitrypsin that, once approved, will expand the portfolio of products.
Meanwhile, albumin sales continue to grow, particularly in China where demand remains high, and in certain countries in Europe and Asia Pacific.
Sales of factor VIII continue to grow, most notably in the U.S. for the treatment of hemophilia A including patients with inhibitors. The company also heightened its role in the treatment of previously untreated patients with severe hemophilia A, a shift mainly driven by the results of the SIPPET(5) study (Survey of Inhibitors in Plasma Products Exposed Toddlers) released last year.
The group remains committed to offering other specialty proteins that enhance its differentiated portfolio of products, optimizing raw material costs and production capacities, and delivering added value for patients. Of note is the upturn in sales of hyperimmune immunoglobulins used to treat rabies and tetanus. In addition, Grifols signed an exclusive 10-year contract with the U.S. firm Ethicon for the manufacture and supply of plasma-derived therapies used for biosurgery (biologic fibrin sealant). This agreement is extendible for 5-year periods and subject to product authorization by regulatory bodies.
(5) The SIPPET study demonstrated that treatment with recombinant factor VIII (rFVIII) is associated with an 87% greater incidence of inhibitors than when using plasma-derived factor VIII with von Willebrand factor (pdFVIII/VWF) in previously untreated patients with severe hemophilia A.
· Diagnostic Division: 16.0% of total revenues
The Diagnostic Divisions continues its positive revenue trend, achieving EUR 351.1 million in sales and growth of 10.8% (7.8% cc) compared to the same period last year.
There was a significant uptick in sales of NAT technology systems (Procleix® NAT Solutions), used for the virological screening of blood and plasma donations. This boost in revenues was triggered mainly by greater market penetration in the Asia Pacific and the U.S. rollout of a Zika virus screening test, which obtained FDA authorization for Investigational New Drug (IND) use in 2016. Since May 2017, this test is also available in blood banks that accept the CE Mark, as the product complies with the legislation for commercialization in the European Economic Area.
As the market leader in the NAT segment, Grifols is equipped to meet the demand in new markets that opt to include this analysis in their blood and plasma donations processes as their healthcare systems develop. In addition, the division continues to develop new tests for emerging viruses. In the second quarter of the year, the FDA granted IND status for a new test for babesiosis to be applied in U.S. blood banks. The test detects the presence of the four species of the babesiosis parasite that can be transmitted to humans.
Sales of antigens used in the production of diagnostic immunoassays, marketed within the framework of its joint-business agreement with Ortho Clinical Diagnostics, also contributed to the division upturn in revenues. In addition, the company signed a five-year extension of its agreement with OraSure Technologies, a leader in infectious disease diagnostic tests. In this way, Grifols strengthens its position as a global, flexible provider of antigens with scalable capabilities. The group continues the approval process of its new plant in Emeryville, California, which will optimize and boost its productive capacity.
Grifols continues to expand the presence of its blood typing line in the U.S. market. In the second quarter of the year, the company launched Erytra Eflexis®, a fully automatic, mid-sized analyzer that performs pre-transfusion compatibility tests using DG Gel® technology. The system optimizes workflow efficiency and improves daily workloads by allowing laboratories to adapt the system to their specific needs.
In addition to its progress in transfusional medicine, Grifols reinforced its position in specialty diagnostics.
In order to increase its presence in the hemostasis field and support its global expansion strategy, the company signed an exclusive distribution agreement with Beckman Coulter, a leading provider of diagnostics solutions. The long-term agreement includes the global distribution of Grifols hemostasis instruments, reagents and consumables. The commercialization of these systems under this agreement is expected in Europe in early 2018.
In terms of expanding its specialty diagnostics product portfolio, during the second quarter Grifols added a new diagnostic test based on the human genomic DNA ID RhD XT, which enables the molecular detection of the most relevant variants of the RhD gene that determine Factor D, of particular importance in pregnant women. Furthermore, in May 2017, the company CLIA-certified laboratory in San Marcos, (Texas, U.S.) launched a series of test under the TDMonitor brand. The new tests offered are used to monitor biological therapies.
· Hospital Division: 2.2% of total revenues
The Hospital Division increased sales by 3.0% (1.9% cc) to EUR 47.9 million, driven primarily by positive growth of its Pharmatech line (including hospital logistics) in certain Latin American markets and in the U.S. It also expanded its third-party manufacturing services.
The U.S. is a critical market for the division growth strategy. In the first half of the year, Grifols saline solution produced in its Murcia, Spain facility, received FDA approval allowing the company to market its IV solution in the U.S. The approval also guarantees the group self-sufficiency since its U.S. network of plasma-donation centers will use the product to restore donors’ circulatory volume.
As Grifols continues to drive the internationalization of its Hospital Division, this approval opens up new opportunities for future authorizations to sell other products manufactured in its Barcelona and Murcia plants. It also confirms the company strategy to promote complementarity of its products and services among business divisions.
· Bio Supplies Division: 1.5% of total revenues
From January 2017, revenues previously recorded in Raw Materials are now part of the new Bio Supplies Division. The division also encompasses sales of biological products for non-therapeutic use and other biologicals, and income derived from its manufacturing agreement with Kedrion.
Division sales reached EUR 32.1 million in the first half of 2017, compared to EUR 28.0 million reported the previous year.
In order to strengthen this business line, Grifols acquired a 49% stake in Access Biologicals in January of 2017. As part of this agreement, Grifols also signed a supply contract with Access Biologicals to sell its biological products for non-therapeutic use.
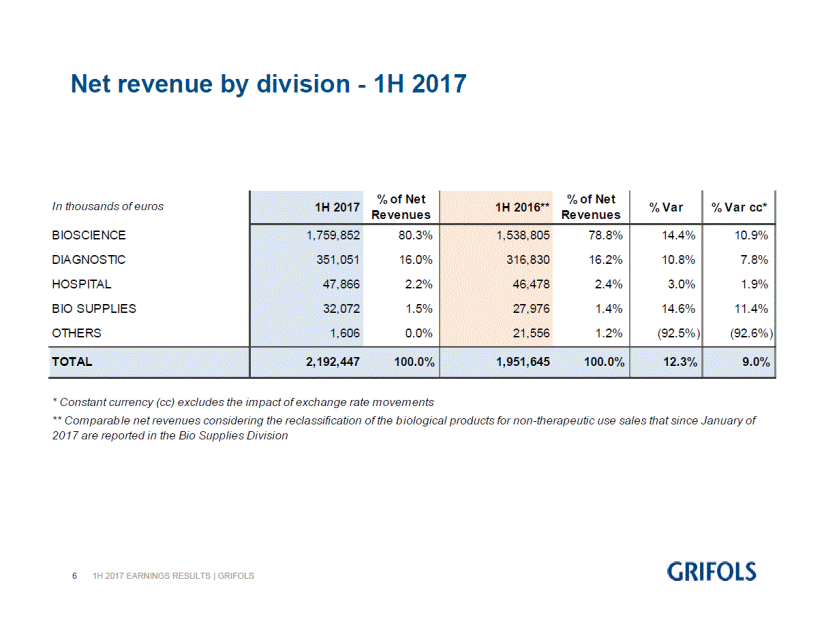
First half 2017 net revenue by division:
|
In thousands of euros |
|
1H 2017 |
|
% of Net |
|
1H 2016** |
|
% of Net |
|
% Var |
|
% Var cc* |
|
|
BIOSCIENCE |
|
1,759,852 |
|
80.3 |
% |
1,538,805 |
|
78.8 |
% |
14.4 |
% |
10.9 |
% |
|
DIAGNOSTIC |
|
351,051 |
|
16.0 |
% |
316,830 |
|
16.2 |
% |
10.8 |
% |
7.8 |
% |
|
HOSPITAL |
|
47,866 |
|
2.2 |
% |
46,478 |
|
2.4 |
% |
3.0 |
% |
1.9 |
% |
|
BIO SUPPLIES |
|
32,072 |
|
1.5 |
% |
27,976 |
|
1.4 |
% |
14.6 |
% |
11.4 |
% |
|
OTHERS |
|
1,606 |
|
0.0 |
% |
21,556 |
|
1.2 |
% |
(92.5 |
)% |
(92.6 |
)% |
|
TOTAL |
|
2,192,447 |
|
100.0 |
% |
1,951,645 |
|
100.0 |
% |
12.3 |
% |
9.0 |
% |
* Constant currency (cc) excludes the impact of exchange rate movements
** Comparable net revenues considering the reclassification of the biological products for non-therapeutic use sales that since January of 2017 are reported in the Bio Supplies Division
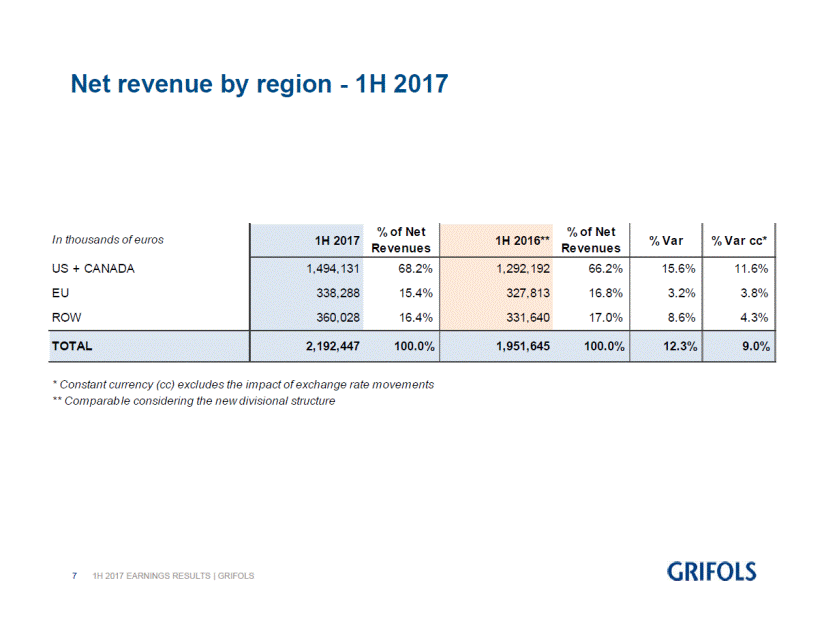
First half 2017 net revenue by region:
|
|
|
|
|
% of Net |
|
|
|
% of Net |
|
|
|
|
|
|
In thousands of euros |
|
1H 2017 |
|
Revenues |
|
1H 2016** |
|
Revenues |
|
% Var |
|
% Var cc* |
|
|
US + CANADA |
|
1,494,131 |
|
68.2 |
% |
1,292,192 |
|
66.2 |
% |
15.6 |
% |
11.6 |
% |
|
EU |
|
338,288 |
|
15.4 |
% |
327,813 |
|
16.8 |
% |
3.2 |
% |
3.8 |
% |
|
ROW |
|
360,028 |
|
16.4 |
% |
331,640 |
|
17.0 |
% |
8.6 |
% |
4.3 |
% |
|
TOTAL |
|
2,192,447 |
|
100.0 |
% |
1,951,645 |
|
100.0 |
% |
12.3 |
% |
9.0 |
% |
* Constant currency (cc) excludes the impact of exchange rate movements
** Comparable considering the new divisional structure
SECOND QUARTER 2017
· Sales increase in the main divisions and geographic regions
Grifols reported revenues of EUR 1,130.8 million in the second quarter of 2017, compared to EUR 992.7 million for the same period in 2016, which represents growth of 13.9% (10.3% cc). Sales performance has been particularly positive in all four of its main divisions, as well as in the regions where it operates.
The Bioscience Division acted as the main growth engine, increasing its revenue by 13.7% (10.0% cc) to reach EUR 906.2 million. The increase in immunoglobulin sales in the U.S. and Canada, alpha-1 antitrypsin sales growth in North America and Europe and albumin sales in China, were especially remarkable. The company revenues from its differentiated specialty products, such as its hyperimmune immunoglobulins have also seen a marked increase.
The second quarter also includes the income derived from the agreement reached with the Spanish Ministry of Health to meet the country supply needs for tetanus and diphtheria (TD) vaccinations. These sales were made possible through a sales agreement between Grifols and MassBiologics of the University of Massachusetts Medical School (U.S).
The Diagnostic Division revenues rose sharply by 15.8% (12.5% cc) in the second quarter to EUR 180.4 million. The division continues to build on the growth reported in the first quarter of the year, fueled primarily by an upsurge in sales of its NAT technology systems - especially for the Zika screening test - as well as an increase in sales of antigens used in the manufacture of immunoassays.
The Hospital Division grew by 5.3% (4.1% cc) to EUR 24.9 million, while Bio Supplies grew by 70.9% (65.7% cc), with sales of EUR 17.7 million. Since January 2017, this division includes sales of biological products for non-therapeutic use, as well as income generated from the Kedrion agreement.
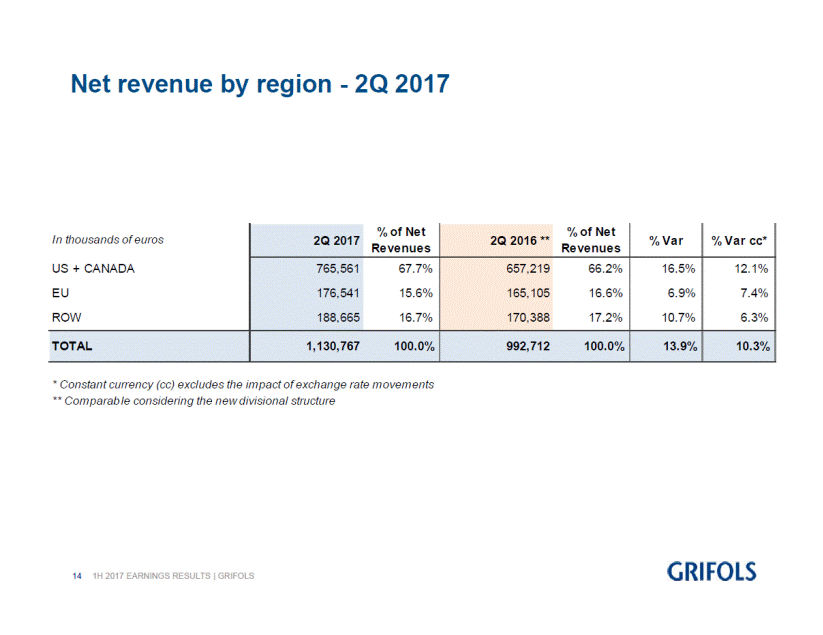
In comparison to the previous year, the company has significantly increased sales in all regions. Revenues in the U.S. and Canada reached EUR 765.6 million, denoting a 16.5% (12.1% cc). The company also reported higher revenues in the rest of the world (ROW), with a 10.7% (6.3% cc) growth to reach close to EUR 189 million, and a recovery in the European Union, with sales of EUR 176.5 million and growth of 6.9% (7.4% cc).
Second quarter 2017 net revenues by division:
|
|
|
|
|
% of Net |
|
|
|
% of Net |
|
|
|
|
|
|
In thousands of euros |
|
2Q 2017 |
|
Revenues |
|
2Q 2016 ** |
|
Revenues |
|
% Var |
|
% Var cc* |
|
|
BIOSCIENCE |
|
906,213 |
|
80.1 |
% |
796,946 |
|
80.3 |
% |
13.7 |
% |
10.0 |
% |
|
DIAGNOSTIC |
|
180,408 |
|
16.0 |
% |
155,790 |
|
15.7 |
% |
15.8 |
% |
12.5 |
% |
|
HOSPITAL |
|
24,902 |
|
2.2 |
% |
23,640 |
|
2.4 |
% |
5.3 |
% |
4.1 |
% |
|
BIO SUPPLIES |
|
17,671 |
|
1.6 |
% |
10,342 |
|
1.0 |
% |
70.9 |
% |
65.7 |
% |
|
OTHERS |
|
1,573 |
|
0.1 |
% |
5,994 |
|
0.6 |
% |
(73.8 |
)% |
(75.1 |
)% |
|
TOTAL |
|
1,130,767 |
|
100.0 |
% |
992,712 |
|
100.0 |
% |
13.9 |
% |
10.3 |
% |
* Constant currency (cc) excludes the impact of exchange rate movements
** Comparable net revenues considering the reclassification of the biological products for non-therapeutic use sales that since January of 2017 are reported in the Bio Supplies Division
Second quarter 2017 net revenues by region:
|
|
|
|
|
% of Net |
|
|
|
% of Net |
|
|
|
|
|
|
In thousands of euros |
|
2Q 2017 |
|
Revenues |
|
2Q 2016 ** |
|
Revenues |
|
% Var |
|
% Var cc* |
|
|
US + CANADA |
|
765,561 |
|
67.7 |
% |
657,219 |
|
66.2 |
% |
16.5 |
% |
12.1 |
% |
|
EU |
|
176,541 |
|
15.6 |
% |
165,105 |
|
16.6 |
% |
6.9 |
% |
7.4 |
% |
|
ROW |
|
188,665 |
|
16.7 |
% |
170,388 |
|
17.2 |
% |
10.7 |
% |
6.3 |
% |
|
TOTAL |
|
1,130,767 |
|
100.0 |
% |
992,712 |
|
100.0 |
% |
13.9 |
% |
10.3 |
% |
* Constant currency (cc) excludes the impact of exchange rate movements
** Comparable considering the new divisional structure
INVESTMENT ACTIVITIES: R&D+INNOVATION, ACQUISITIONS AND CAPEX
· Grifols allocates nearly EUR 130 million toward R&D+i in the first half
The company allocated EUR 129.3 million for R&D+i activities in the first half of 2017, taking into account net internal investments and external investments carried out through its investee companies. This figure represents a 19.0% increase with respect to the same period in 2016 and 5.9% of revenues.
During the second quarter of 2017, Grifols investee company, Araclon Biotech initiated the phase II clinical trial for an Alzheimer vaccine after receiving authorization from the Spanish Agency of Medicinal Products and Medical Devices. Phase II aims to establish guidelines for product dosage, as well as corroborate the data collected in phase I concerning product safety and tolerability.
· New research lines through investee companies: acquisition of a 44% stake in GigaGen for USD 35 million
Headquartered in San Francisco, GigaGen is a biopharmaceutical firm specialized in the development of pre-clinical biotherapeutic therapies that use human B-cells(6) to capture the genetic diversity of antibodies and transform them into therapies to treat severe diseases. Specifically, GigaGen discovers and develops innovative recombinant monoclonal and polyclonal antibody therapies.
In addition to the financial transaction, Grifols and GigaGen have entered into a research and collaboration agreement whereby, in exchange of a collaboration fee of USD 15 million in the aggregate, GigaGen will commit to carry out research activities to develop a recombinant polyclonal immunoglobulin drug product derived from human B cells for the treatment of any human diseases.
With this transaction, Grifols further strengthens its portfolio of R&D+i projects, which includes holdings in research projects and companies that complement its activity and hold the potential of generating added value for the group. The acquisition has been carried out through Grifols Innovation and New Technologies (GIANT), responsible for overseeing the company external R&D+i investments. The transaction was completed after June 30, 2017.
· Capital Investments (CAPEX)
In the first six months of the year, Grifols invested EUR 135.3 million to enhance and expand the production facilities of its four divisions. These on-going investments progress as outlined in the 2016-2020 Capital Investment Plan, endowed with EUR 1,200 million to guarantee the company long-term sustainable growth.
Of strategic importance are Grifols investments to increase the supply of plasma, including the opening of new donation centers in the U.S., as well as the expansion, renovation and relocation of existing centers. Grifols is currently the market leader, with a network of more than 180 plasma collection centers in the U.S. that it aims to expand to 230 centers by 2019.
In accordance to plans, the company continues to make progress on the construction of new facilities for plasma fractionation, and the purification and sterile filling of plasma proteins.
CORPORATE RESPONSIBILITY
· Human Resources: more employees and more development
Grifols currently has 16,808 employees, representing a 13.0% growth in the first half of the year with respect to the close of the 2016 fiscal year. The employee base grew in all geographic regions, especially in North America, where headcount increased by 16.6%. Grifols continues to generate employment in Spain, where the workforce grew by 3.2% to 3,539 employees. Meanwhile, headcount in the rest of the world (ROW) grew by 7.8%.
(6) Type of white blood cell that makes antibodies. B cells are part of the immune system and develop from stem cells in the bone marrow. They are also known as B-lymphocytes.
The average seniority of Grifols personnel is 6.1 years and the average age is 38.3, although 57% is aged 40 or younger. The gender breakdown (44% men and 56% women) reflects a balanced workforce.
Health and safety and training and development are the cornerstones of the company human resource strategy. In terms of health, the company is promoting awareness of healthy lifestyle habits as a preventative measure.
In terms of training and development, of particular note are the new-employee integration programs; performance-review programs; and a five-year leadership development program aimed at 1,500 mid-level managers and directors throughout the world. In addition, the company has expanded other programs, including technical development and training on concrete issues such as good manufacturing practices (GMP), compliance and the corporate equality plan, among others.
· 2017-2019 Environmental Program
Grifols initiated the implementation of its 2017-2019 Environmental Plan, whose main objectives are reducing the electrical consumption in its production facilities by 8.3 million kWh per year; decreasing its natural-gas consumption by 20.6 million kWh per year; and cutting its annual water consumption by 265,000 cubic meters. The plan also aims to increase the recovery of waste by 270 tons per year.
External audits according to the ISO 14001 standard were carried out in the first half of the year in the company facilities in Spain and in Clayton, (North Carolina, U.S.) with satisfactory results. The company plans to extend this certification to its Diagnostic Division facilities in Emeryville and the Bioscience Division plant in Los Angeles (California, U.S.).
In June, Grifols submitted the questionnaire to participate in the 2016 Carbon Disclosure Project (CDP), a program that evaluates the organization strategy and progress on the issue of climate change. Included in the information provided is the calculation of Grifols carbon footprint, estimated at 292,437 tons of CO2 equivalent emissions. This figure is similar to the increase in production at its various facilities.
· Transparency: Grifols voluntarily discloses transfers of value to health professionals and healthcare organizations in Europe made in 2016
Grifols voluntarily adopted the Code of Conduct on Industry Interactions with Healthcare Professionals and Healthcare Organizations of the European Federation of Pharmaceutical Industries and Associations (EFPIA) in 2015. In 2016, the company disclosed, for the second consecutive year, all of its payments and other transfers of value to health professionals and health sector organizations in 33 European countries, including Spain.
The EFPIA Code applies specifically to medicines; nonetheless, Grifols opted to expand its scope of application to include transfers unrelated to medicines, as well as to the company three divisions: Bioscience, Diagnostic and Hospital. Grifols applies this transparency policy in the United States as required by the regulatory body (Centers for Medicaid and Medicare Services, or CMS) and, in addition to Europe, also plans to implement it in countries such as Australia and Japan.
· Annual Capital Markets Day
Grifols held its annual meeting for analysts and investors in Emeryville at the beginning of June. The company senior executives presented status overviews of the main divisions, investment plans and key research projects, followed by an in-depth analysis of Grifols financials. The meeting included a tour of the Diagnostics Division new production facilities in Emeryville.
· Our commitment to patients: Grifols to support Alpha-1 Foundation John W. Walsh Research Fund
Grifols awarded a USD 1 million grant to support the John. W. Walsh Research Fund, dedicated to promoting research that will improve the health of patients with Alpha-1 antitrypsin deficiency. The program supports basic science and clinical research, improved understanding of the pathogenesis of the clinical manifestations of AATD, the development and testing of treatments for the disease, bioethics and social research, and the promotion of education of members of the medical community regarding AATD. This announcement is a continuation of Grifols longstanding commitment to improving the lives of patients worldwide.
· Our commitments to patients: 140 million international units (IU) of blood clotting factors donated to the World Federation of Hemophilia (WFH) Humanitarian Aid Program
This donation represents Grifols most significant contribution to the WFH Humanitarian Aid Program to date. According to the WFH, the donation ensures around 10,300 doses until 2021 to treat approximately 6,000 patients in emerging countries where access to adequate healthcare is limited or non-existent.
For over a decade, Grifols has been a proud supporter of the WFH and its efforts to improve access to treatment of bleeding disorders around the world. The renewed partnership builds upon the company three-year commitment from 2014 and brings the total humanitarian aid commitment to more than 200 million IU of Factor VIII over eight years.
The financial information corresponding to the first half of the 2017 fiscal year is included in an annex as part of the company interim financial information. All of the documents are available for download on the Grifols corporate website (www.grifols.com).
About Grifols
Grifols is a global healthcare company founded in 1940. Grifols has over 75 years improving people’s health and wellbeing through the development of life-saving plasma medicines, diagnostics systems, and hospital pharmacy products.
The company is present in more than 100 countries worldwide and is headquartered in Barcelona, Spain. Grifols is a leader in plasma collection with a network of close to 180 plasma donor centers in the U.S., and a leading producer of plasma-derived biological medicines. The company also provides a comprehensive range of transfusion medicine, hemostasis, and immunoassay solutions for clinical laboratories, blood banks and transfusion centers, and is a recognized leader in transfusion medicine.
In 2016, sales exceeded 4,000 million euros with a headcount close to 15,000 employees. Grifols demonstrates its commitment to advancing healthcare by allocating a significant portion of its annual income to R&D.
The company class A shares are listed on the Spanish Stock Exchange, where they are part of the Ibex-35 (MCE: GRF). Its non-voting class B shares are listed on the Mercado Continuo (MCE: GRF.P) and on the U.S. NASDAQ via ADRs (NASDAQ: GRFS). For more information visit www.grifols.com
LEGAL DISCLAIMER
The facts and figures contained in this report that do not refer to historical data are “future projections and assumptions”. Words and expressions such as “believe”, “hope”, “anticipate”, “predict”, “expect”, “intend”, “should”, “will seek to achieve”, “it is estimated”, “future” and similar expressions, in so far as they relate to the Grifols group, are used to identify future projections and assumptions. These expressions reflect the assumptions, hypotheses, expectations and predictions of the management team at the time of writing this report, and these are subject to a number of factors that mean that the actual results may be materially different. The future results of the Grifols group could be affected by events relating to its own activities, such as a shortage of supplies of raw materials for the manufacture of its products, the appearance of competitor products on the market, or changes to the regulatory framework of the markets in which it operates, among others. At the date of compiling this report, the Grifols group has adopted the necessary measures to mitigate the potential impact of these events. Grifols, S.A. does not accept any obligation to publicly report, revise or update future projections or assumptions to adapt them to events or circumstances subsequent to the date of writing this report, except where expressly required by the applicable legislation. This document does not constitute an offer or invitation to buy or subscribe shares in accordance with the provisions of the following Spanish legislation: Royal Legislative Decree 4/2015, of 23 October, approving recast text of Securities Market Law; Royal Decree Law 5/2005, of 11 March and/or Royal Decree 1310/2005, of 4 November, and any regulations developing this legislation. In addition, this document does not constitute an offer of purchase, sale or exchange, or a request for an offer of purchase, sale or exchange of securities, or a request for any vote or approval in any other jurisdiction. The information included in this document has not been verified nor reviewed by the external auditors of the Grifols group.
The facts and figures contained in this report that do not refer to historical data are “future projections and assumptions”. Words and expressions such as “believe”, “hope”, “anticipate”, “predict”, “expect”, “intend”, “should”, “will seek to achieve”, “it is estimated”, “future” and similar expressions, in so far as they relate to the Grifols group, are used to identify future projections and assumptions. These expressions reflect the assumptions, hypotheses, expectations and predictions of the management team at the time of writing this report, and these are subject to a number of factors that mean that the actual results may be materially different. The future results of the Grifols group could be affected by events relating to its own activities, such as a shortage of supplies of raw materials for the manufacture of its products, the appearance of competitor products on the market, or changes to the regulatory framework of the markets in which it operates, among others. At the date of compiling this report, the Grifols group has adopted the necessary measures to mitigate the potential impact of these events. Grifols, S.A. does not accept any obligation to publicly report, revise or update future projections or assumptions to adapt them to events or circumstances subsequent to the date of writing this report, except where expressly required by the applicable legislation. This document does not constitute an offer or invitation to buy or subscribe shares in accordance with the provisions of the following Spanish legislation: Royal Legislative Decree 4/2015, of 23 October, approving recast text of Securities Market Law; Royal Decree Law 5/2005, of 11 March and/or Royal Decree 1310/2005, of 4 November, and any regulations developing this legislation. In addition, this document does not constitute an offer of purchase, sale or exchange, or a request for an offer of purchase, sale or exchange of securities, or a request for any vote or approval in any other jurisdiction. The information included in this document has not been verified nor reviewed by the external auditors of the Grifols group. 21H 2017 EARNINGS RESULTS | GRIFOLS

•The Bioscience Division reports an exceptional increase in sales, which grow by 14.4% (10.9% cc1) to reach EUR 1,760 million. •The Diagnostic Division sales increase by 10.8% (7.8% cc) to EUR 351 million. •The Hospital Division grows by 3.0% (1.9% cc), while sales of the Bio Supplies Division increase by 14.6% (11.4% cc), with net revenues of EUR 48 million and EUR 32 million, respectively. •The adjusted EBITDA2 increases to EUR 664 million (19.9%), with a margin of 30.3%. 31H 2017 EARNINGS RESULTS | GRIFOLS
•The company increases its net investments in R&D+i by 19.0% to EUR 129 million. Grifols manages its innovation strategy through both internal and investee projects, such as the recent acquisition of a 44% stake in the U.S. firm GigaGen for USD 35 million. •The group expands its portfolio of plasma products with a biological sealant for biosurgical use; promotes the Diagnostic Division hemostasis line with a new global distribution contract; and boosts the presence of its Hospital Division in the U.S. after obtaining FDA approval to commercialize its saline solution in this market. 1.cc: at constant currency rates. 2.Excludes non-recurring costs and associated with recent acquisitions. 41H 2017 EARNINGS RESULTS | GRIFOLS

(1) Excludes non-recurring costs and associated with recent acquisitions (2) Excludes non-recurring costs and associated with recent acquisitions, am ortiz ation of deferred expenses associated to the refinancing and am ortiz ation of intangib le assets related to acquisitions (3) Constant currency (cc) excludes the im pact of exchange rate m ovem ents. 2016 reported figures: not including the NAT assets deb t acquisition 51H 2017 EARNINGS RESULTS | GRIFOLS
% Var 14.4% % Var cc* 10.9% 10.8% 7.8% 3.0% 1.9% 14.6% 11.4% (92.5%) (92.6%) ** Com parab le net revenues considering the reclassification of the b iological products for non-therapeutic use sales that since January of 2017 are reported in the Bio Supplies Division 61H 2017 EARNINGS RESULTS | GRIFOLS
% Var% Var cc* 15.6%11.6% 3.2%3.8% 8.6%4.3% In thousands of euros US + CANADA EU ROW 1H 2017 1,494,131 338,288 360,028 % of Net Revenues 68.2% 15.4% 16.4% 1H 2016** 1,292,192 327,813 331,640 % of Net Revenues 66.2% 16.8% 17.0% TOTAL 2,192,447 100.0% 1,951,645 100.0% 12.3% 9.0% ** Com parab le considering the new divisional structure 71H 2017 EARNINGS RESULTS | GRIFOLS
81H 2017 EARNINGS RESULTS | GRIFOLS
91H 2017 EARNINGS RESULTS | GRIFOLS
101H 2017 EARNINGS RESULTS | GRIFOLS
111H 2017 EARNINGS RESULTS | GRIFOLS
121H 2017 EARNINGS RESULTS | GRIFOLS
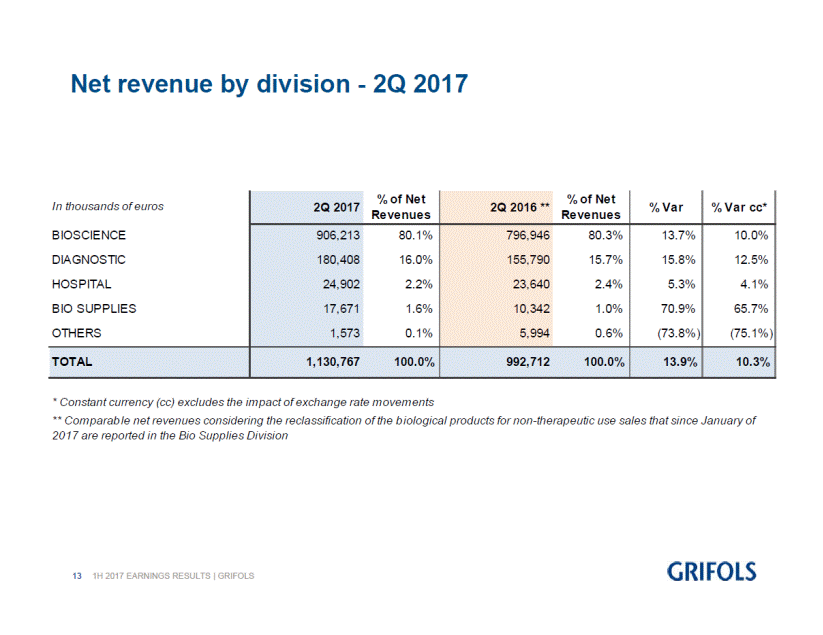
% Var 13.7% % Var cc* 10.0% 15.8% 12.5% 5.3% 4.1% 70.9% 65.7% (73.8%) (75.1%) ** Com parab le net revenues considering the reclassification of the b iological products for non-therapeutic use sales that since January of 2017 are reported in the Bio Supplies Division 131H 2017 EARNINGS RESULTS | GRIFOLS
% Var% Var cc* 16.5%12.1% 6.9%7.4% 10.7%6.3% In thousands of euros US + CANADA EU ROW 2Q 2017 765,561 176,541 188,665 % of Net Revenues 67.7% 15.6% 16.7% 2Q 2016 ** 657,219 165,105 170,388 % of Net Revenues 66.2% 16.6% 17.2% TOTAL 1,130,767 100.0% 992,712 100.0% 13.9% 10.3% ** Com parab le considering the new divisional structure 141H 2017 EARNINGS RESULTS | GRIFOLS
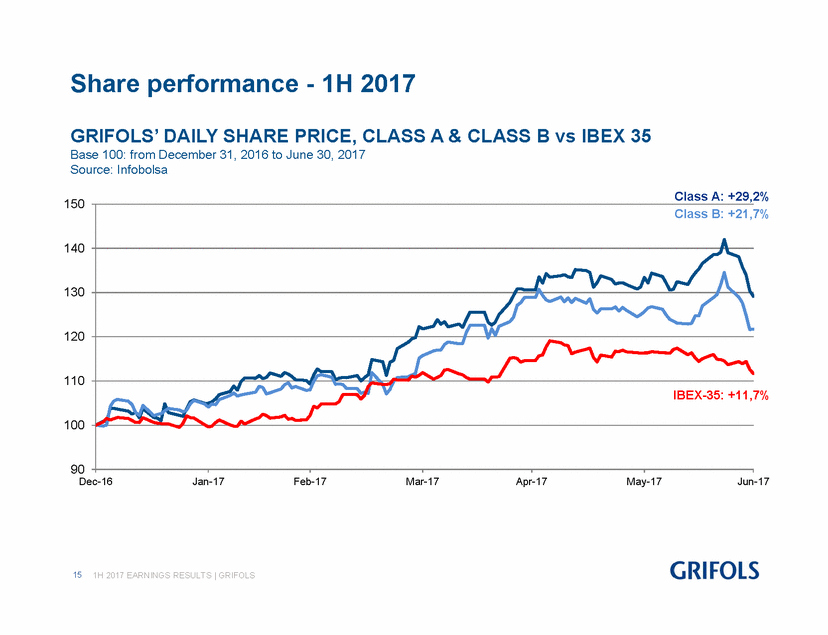
GRIFOLS’ DAILY SHARE PRICE, CLASS A & CLASS B vs IBEX 35 Base 100: from December 31, 2016 to June 30, 2017 Source: Infobolsa 150 Class A: +29,2% Class B: +21,7% 140 130 120 110 IBEX-35: +11,7% 100 90 Dec-16Jan-17Feb-17Mar-17Apr-17May-17Jun-17 151H 2017 EARNINGS RESULTS | GRIFOLS

Condensed Consolidated Interim Financial
30 June 2017
Interim Consolidated Directors’ Report
30 June 2017
(With Limited Review Report thereon)
(Free translation from the original in Spanish. In the event of
discrepancy, the Spanish-language version prevails.)

KPMG Auditores, S.L.
Torre Realia
Plaça d’Europa, 41-43
08908 L’Hospitalet de Llobregat
(Barcelona)
(Translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails.)
Limited Review on the Condensed Consolidated Interim Financial Statements
To the shareholders of
Grifols, S.A. commissioned by the Directors
Report on the Condensed Consolidated Interim Financial Statements
Introduction
We have carried out a limited review of the accompanying condensed consolidated interim financial statements (the “interim financial statements”) of Grifols, S.A. (the “Company”) and subsidiaries (the “Group”), which comprise the balance sheet at 30 June 2017, the income statement, statement of comprehensive income, statement of changes in equity, statement of cash flows and the explanatory notes for the 6-month period then ended (all condensed and consolidated). Pursuant to article 12 of Royal Decree 1362/2007 the Directors of the Company are responsible for the preparation of these interim financial statements in accordance with International Accounting Standard (IAS) 34 Interim Financial Reporting as adopted by the European Union. Our responsibility is to express a conclusion on these interim financial statements based on our limited review.
Scope of Review
We conducted our limited review in accordance with the International Standard on Review Engagements 2410, “Review of Interim Financial Information Performed by the Independent Auditor of the Entity”. A limited review of interim financial statements consists of making inquiries, primarily of persons responsible for financial and accounting matters, and applying analytical and other review procedures. A limited review is substantially less in scope than an audit conducted in accordance with prevailing legislation regulating the audit of accounts in Spain and, consequently, does not enable us to obtain assurance that we would become aware of all significant matters that might be identified in an audit. Accordingly, we do not express an audit opinion on the accompanying interim financial statements.
Conclusion
Based on our limited review, which can under no circumstances be considered an audit, nothing has come to our attention that causes us to believe that the accompanying interim financial statements for the 6-month period ended 30 June 2017 have not been prepared, in all material respects, in accordance with International Accounting Standard (IAS) 34 Interim Financial Reporting, as adopted by the European Union, for the preparation of condensed interim financial statements, pursuant to article 12 of Royal Decree 1362/2007.
Emphasis of Matter
We draw your attention to note 2 to the accompanying interim financial statements, which states that these interim financial statements do not include all the information required in complete consolidated financial statements prepared in accordance with International Financial Reporting Standards as adopted by the European Union. The accompanying interim financial statements should therefore be read in conjunction with the Group’s consolidated annual accounts for the year ended 31 December 2016. This matter does not modify our conclusion.
|
KPMG Auditores S.L., a limited liability Spanish company and a member firm of the KPMG network of independent member firms affiliated with KPMG International Cooperative (“KPMG International”), a Swiss entity. |
|
Reg. Mer Madrid, T. 11.961, F.90, Sec. 8, H. M -188.007, Inscrip. 9 N.I.F. B-78510153 |
|
Report on Other Legal and Regulatory Requirements
The accompanying consolidated interim directors’ report for the 6-month period ended 30 June 2017 contains such explanations as the Directors of the Company consider relevant with respect to the significant events that have taken place in this period and their effect on the consolidated interim financial statements, as well as the disclosures required by article 15 of Royal Decree 1362/2007. The consolidated interim directors’ report is not an integral part of the consolidated interim financial statements. We have verified that the accounting information contained therein is consistent with that disclosed in the interim financial statements for the 6-month period ended 30 June 2017. Our work is limited to the verification of the consolidated interim directors’ report within the scope described in this paragraph and does not include a review of information other than that obtained from the accounting records of Grifols, S.A. and subsidiaries.
Paragraph on Other Matters
This report has been prepared at the request of the Company’s Directors in relation to the publication of the six-monthly financial report required by article 119 of the Revised Securities Market Law, enacted by Royal Decree 1362/2007 of 19 October 2007.
KPMG Auditores, S.L.
(Signed on original in Spanish)
Olga Sánchez López
27 July 2017
GRIFOLS, S.A. and Subsidiaries
Notes to Condensed Consolidated Interim Financial Statements for the
six-month period ended 30 June 2017
CONTENTS
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language
version prevails)
· Condensed Consolidated Interim Financial Statements
· Balance Sheet
· Statement of Profit or Loss
· Statement of Comprehensive Income
· Statement of Cash Flows
· Statement of Changes in Equity
· Notes to Condensed Consolidated Interim Financial Statements
(1) General Information
(2) Basis of Presentation and Accounting Principles Applied
(3) Changes in the composition of the Group
(4) Financial Risk Management Policy
(5) Segment Reporting
(6) Goodwill
(7) Other Intangible Assets and Property, Plant and Equipment
(8) Non-Current Financial Assets
(9) Trade and Other Receivables
(10) Equity
(11) Financial Liabilities
(12) Expenses by Nature
(13) Finance Result
(14) Taxation
(15) Discontinued Operations
(16) Contingencies
(17) Related Parties
(18) Subsequent Events
GRIFOLS, S.A. AND SUBSIDIARIES
Condensed Consolidated Balance Sheets
as of 30 June 2017 and 31 December 2016
(Expressed in thousands of Euros)
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
Assets |
|
30/06/2017 |
|
31/12/2016 |
|
|
|
|
(unaudited) |
|
|
|
|
|
|
|
|
|
|
|
Non-current assets |
|
|
|
|
|
|
Goodwill (note 6) |
|
5,023,903 |
|
3,643,995 |
|
|
Other intangible assets (note 7) |
|
1,101,789 |
|
1,195,302 |
|
|
Property, plant and equipment (note 7) |
|
1,775,907 |
|
1,809,852 |
|
|
Investments in equity accounted investees (note 3) |
|
223,483 |
|
201,345 |
|
|
Non-current financial assets (note 8) |
|
|
|
|
|
|
Non-current financial assets measured at fair value |
|
42,211 |
|
43,663 |
|
|
Non-current financial assets at amortized cost |
|
20,513 |
|
45,882 |
|
|
Deferred tax assets |
|
78,141 |
|
67,219 |
|
|
Total non-current assets |
|
8,265,947 |
|
7,007,258 |
|
|
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
Inventories |
|
1,595,786 |
|
1,642,931 |
|
|
Trade and other receivables |
|
|
|
|
|
|
Trade receivables (note 9) |
|
330,309 |
|
413,656 |
|
|
Other receivables (note 9) |
|
60,603 |
|
42,299 |
|
|
Current tax assets |
|
75,628 |
|
77,713 |
|
|
Trade and other receivables |
|
466,540 |
|
533,668 |
|
|
Other current financial assets (note 8) |
|
12,366 |
|
2,582 |
|
|
Other current assets |
|
26,122 |
|
48,324 |
|
|
Cash and cash equivalents |
|
750,218 |
|
895,009 |
|
|
Total current assets |
|
2,851,032 |
|
3,122,514 |
|
|
|
|
|
|
|
|
|
Total assets |
|
11,116,979 |
|
10,129,772 |
|
GRIFOLS, S.A. AND SUBSIDIARIES
Condensed Consolidated Balance Sheets
as of 30 June 2017 and 31 December 2016
(Expressed in thousands of Euros)
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
Equity and liabilities |
|
30/06/2017 |
|
31/12/2016 |
|
|
|
|
(unaudited) |
|
|
|
|
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
Share capital (note 10) |
|
119,604 |
|
119,604 |
|
|
Share premium (note 10) |
|
910,728 |
|
910,728 |
|
|
Reserves (note 10) |
|
2,025,549 |
|
1,694,245 |
|
|
Treasury stock (note 10) |
|
(62,422 |
) |
(68,710 |
) |
|
Interim dividend |
|
0 |
|
(122,908 |
) |
|
Profit attributable to the Parent |
|
277,861 |
|
545,456 |
|
|
Total |
|
3,271,320 |
|
3,078,415 |
|
|
Available for sale financial assets |
|
(5,196 |
) |
(5,219 |
) |
|
Other comprehensive Income |
|
(642 |
) |
(642 |
) |
|
Translation differences |
|
313,560 |
|
648,927 |
|
|
Other comprehensive income |
|
307,722 |
|
643,066 |
|
|
Equity attributable to the Parent |
|
3,579,042 |
|
3,721,481 |
|
|
Non-controlling interests |
|
5,788 |
|
6,497 |
|
|
|
|
|
|
|
|
|
Total equity |
|
3,584,830 |
|
3,727,978 |
|
|
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
Non-current liabilities |
|
|
|
|
|
|
Grants |
|
11,992 |
|
12,196 |
|
|
Provisions |
|
5,511 |
|
5,118 |
|
|
Non-current financial liabilities (note 11) |
|
6,042,177 |
|
4,712,071 |
|
|
Deferred tax liabilities |
|
572,148 |
|
600,646 |
|
|
Total non-current liabilities |
|
6,631,828 |
|
5,330,031 |
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
Provisions |
|
77,564 |
|
89,588 |
|
|
Current financial liabilities (note 11) |
|
148,561 |
|
230,065 |
|
|
Trade and other payables |
|
|
|
|
|
|
Suppliers |
|
394,821 |
|
461,073 |
|
|
Other payables |
|
129,705 |
|
142,894 |
|
|
Current income tax liabilities |
|
20,936 |
|
7,957 |
|
|
Total trade and other payables |
|
545,462 |
|
611,924 |
|
|
Other current liabilities |
|
128,734 |
|
140,186 |
|
|
|
|
|
|
|
|
|
Total current liabilities |
|
900,321 |
|
1,071,763 |
|
|
|
|
|
|
|
|
|
Total liabilities |
|
7,532,149 |
|
6,401,794 |
|
|
|
|
|
|
|
|
|
Total equity and liabilities |
|
11,116,979 |
|
10,129,772 |
|
GRIFOLS, S.A. AND SUBSIDIARIES
Condensed Consolidated Statements of Profit or Loss
for each of the three-and six-month periods ended 30 June 2017 and 2016
(Expressed in thousands of Euros)
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
|
|
Six-Months Ended |
|
Three-Months Ended |
| ||||
|
|
|
30/06/2017 |
|
30/06/2016 |
|
30/06/2017 |
|
30/06/2016 |
|
|
|
|
(unaudited) |
|
(unaudited) |
|
(unaudited) |
|
(unaudited) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Continuing Operations |
|
|
|
|
|
|
|
|
|
|
Net revenue (note 5) |
|
2,192,447 |
|
1,951,645 |
|
1,130,767 |
|
992,712 |
|
|
Cost of sales |
|
(1,089,246 |
) |
(1,009,801 |
) |
(569,463 |
) |
(525,047 |
) |
|
Gross Margin |
|
1,103,201 |
|
941,844 |
|
561,304 |
|
467,665 |
|
|
Research and Development |
|
(121,575 |
) |
(97,348 |
) |
(62,404 |
) |
(49,683 |
) |
|
Sales, General and Administration expenses |
|
(443,789 |
) |
(391,826 |
) |
(213,775 |
) |
(196,765 |
) |
|
Operating Expenses |
|
(565,364 |
) |
(489,174 |
) |
(276,179 |
) |
(246,448 |
) |
|
Operating Results |
|
537,837 |
|
452,670 |
|
285,125 |
|
221,217 |
|
|
Finance income |
|
4,164 |
|
3,924 |
|
2,152 |
|
2,010 |
|
|
Finance costs |
|
(135,487 |
) |
(124,071 |
) |
(69,493 |
) |
(60,841 |
) |
|
Change in fair value of financial instruments |
|
0 |
|
(7,426 |
) |
0 |
|
(2,870 |
) |
|
Impairment of financial instruments |
|
(5,500 |
) |
0 |
|
0 |
|
0 |
|
|
Exchange differences |
|
(10,760 |
) |
3,409 |
|
(14,017 |
) |
6,103 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance Result (note 13) |
|
(147,583 |
) |
(124,164 |
) |
(81,358 |
) |
(55,598 |
) |
|
Share of income/(losses) of equity accounted investees |
|
|
|
|
|
|
|
|
|
|
|
|
(10,295 |
) |
16,706 |
|
(7,007 |
) |
15,355 |
|
|
Profit before income tax from continuing operations |
|
|
|
|
|
|
|
|
|
|
|
|
379,959 |
|
345,212 |
|
196,760 |
|
180,974 |
|
|
Income tax expense (note 14) |
|
(102,589 |
) |
(81,125 |
) |
(53,125 |
) |
(41,708 |
) |
|
Profit after income tax from continuing operations |
|
277,370 |
|
264,087 |
|
143,635 |
|
139,266 |
|
|
Consolidated profit for the period |
|
277,370 |
|
264,087 |
|
143,635 |
|
139,266 |
|
|
Profit attributable to the Parent |
|
277,861 |
|
264,443 |
|
143,868 |
|
139,198 |
|
|
(Profit) attributable to non-controlling interest |
|
(491 |
) |
(356 |
) |
(233 |
) |
68 |
|
|
Basic earnings per share (Euros) |
|
0.41 |
|
0.39 |
|
0.21 |
|
0.20 |
|
|
Diluted earnings per share (Euros) |
|
0.41 |
|
0.39 |
|
0.21 |
|
0.20 |
|
GRIFOLS, S.A. AND SUBSIDIARIES
Condensed Consolidated Statements of Comprehensive Income
for each of the three-and six-month periods ended 30 June 2017 and 2016
(Expressed in thousands of Euros)
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
|
|
Six-Months’ Ended |
|
Three-Months’ Ended |
| ||||
|
|
|
30/06/2017 |
|
30/06/2016 |
|
30/06/2017 |
|
30/06/2016 |
|
|
|
|
(unaudited) |
|
(unaudited) |
|
(unaudited) |
|
(unaudited) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the period |
|
277,370 |
|
264,087 |
|
143,635 |
|
139,266 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Items for reclassification to profit or loss |
|
|
|
|
|
|
|
|
|
|
Translation differences |
|
(319,057 |
) |
(57,093 |
) |
(276,585 |
) |
76,764 |
|
|
Translation differences / Cash Flow Hedge |
|
— |
|
(6,809 |
) |
— |
|
(6,809 |
) |
|
Equity accounted investees / Translation differences |
|
(16,425 |
) |
1,965 |
|
(13,326 |
) |
3,926 |
|
|
Cash flow hedges - effective part of changes in fair value |
|
— |
|
14,682 |
|
— |
|
5,912 |
|
|
Cash flow hedges - others |
|
— |
|
(181 |
) |
— |
|
(181 |
) |
|
Cash flow hedges - amounts taken to profit or loss |
|
— |
|
(7,426 |
) |
— |
|
(2,870 |
) |
|
Other |
|
— |
|
(4,532 |
) |
— |
|
— |
|
|
Tax effect |
|
— |
|
(2,462 |
) |
— |
|
(1,707 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income for the period, after tax |
|
(335,482 |
) |
(61,856 |
) |
(289,911 |
) |
75,035 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the period |
|
(58,112 |
) |
202,231 |
|
(146,276 |
) |
214,301 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income attributable to the Parent |
|
(57,506 |
) |
202,682 |
|
(145,806 |
) |
214,403 |
|
|
Total comprehensive (income)/ loss attributable to non-controlling interests |
|
(606 |
) |
(451 |
) |
(470 |
) |
(102 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the period |
|
(58,112 |
) |
202,231 |
|
(146,276 |
) |
214,301 |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the unaudited condensed consolidated interim financial statements.
GRIFOLS, S.A. AND SUBSIDIARIES
Condensed Consolidated Statements of Cash Flows
for each of the six-month periods ended 30 June 2017 and 2016
(Expressed in thousands of Euros)
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
|
|
30/06/2017 |
|
30/06/2016 |
|
|
|
|
(unaudited) |
| ||
|
|
|
|
|
|
|
|
Cash flows from operating activities |
|
|
|
|
|
|
Profit before tax |
|
379,959 |
|
345,212 |
|
|
Adjustments for: |
|
249,022 |
|
203,443 |
|
|
Amortisation and depreciation |
|
106,549 |
|
100,915 |
|
|
Other adjustments: |
|
142,473 |
|
102,528 |
|
|
(Profit)/Losses on equity accounted investments |
|
10,295 |
|
(16,706 |
) |
|
Impairment of Assets and net provision changes |
|
(279 |
) |
(605 |
) |
|
Loss on disposal of fixed assets |
|
249 |
|
818 |
|
|
Government grants taken to income |
|
(707 |
) |
(795 |
) |
|
Finance cost |
|
130,897 |
|
123,688 |
|
|
Other adjustments |
|
2,018 |
|
(3,872 |
) |
|
Changes operating assets and liabilities |
|
(69,264 |
) |
(231,547 |
) |
|
Change in inventories |
|
(64,217 |
) |
(124,548 |
) |
|
Change in trade and other receivables |
|
39,078 |
|
(87,584 |
) |
|
Change in current financial assets and other current assets |
|
5,205 |
|
4,636 |
|
|
Change in current trade and other payables |
|
(49,330 |
) |
(24,051 |
) |
|
Other cash flows used in operating activities |
|
(181,154 |
) |
(150,249 |
) |
|
Interest paid |
|
(106,706 |
) |
(90,300 |
) |
|
Interest recovered |
|
2,993 |
|
4,006 |
|
|
Income tax paid |
|
(77,075 |
) |
(63,955 |
) |
|
Other paid |
|
(366 |
) |
0 |
|
|
|
|
|
|
|
|
|
Net cash from operating activities |
|
378,563 |
|
166,859 |
|
|
|
|
|
|
|
|
|
Cash flows from investing activities |
|
|
|
|
|
|
Payments for investments |
|
(1,959,854 |
) |
(322,001 |
) |
|
Group companies and business units |
|
(1,813,163 |
) |
(188,065 |
) |
|
Property, plant and equipment and intangible assets |
|
(146,155 |
) |
(125,905 |
) |
|
Property, plant and equipment |
|
(125,562 |
) |
(105,552 |
) |
|
Intangible assets |
|
(20,593 |
) |
(20,353 |
) |
|
Other financial assets |
|
(536 |
) |
(8,031 |
) |
|
Proceeds from the sale of financial investments |
|
20,451 |
|
0 |
|
|
Proceeds from the sale of property, plant and equipment |
|
551 |
|
1,754 |
|
|
|
|
|
|
|
|
|
Net cash used in investing activities |
|
(1,938,852 |
) |
(320,247 |
) |
|
|
|
|
|
|
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments |
|
0 |
|
(5,131 |
) |
|
Acquisition of treasury stock |
|
0 |
|
(5,131 |
) |
|
Proceeds from and payments for financial liability intruments |
|
1,723,945 |
|
(41,701 |
) |
|
Dividends and interest on other equity instruments paid and received |
|
(95,274 |
) |
(93,243 |
) |
|
Dividends paid |
|
(95,274 |
) |
(93,243 |
) |
|
Other cash flows from financing activities |
|
(151,374 |
) |
(21,943 |
) |
|
Costs of financial instruments issued |
|
(142,288 |
) |
— |
|
|
Other payments from financing activities |
|
(9,086 |
) |
(21,943 |
) |
|
Net cash used in financing activities |
|
1,477,297 |
|
(162,018 |
) |
|
Effect of exchange rate fluctuations on cash and cash equivalents |
|
(61,799 |
) |
(20,075 |
) |
|
Net decrease in cash and cash equivalents |
|
(144,791 |
) |
(335,481 |
) |
|
Cash and cash equivalents at beginning of the period |
|
895,009 |
|
1,142,500 |
|
|
Cash and cash equivalents at end of period |
|
750,218 |
|
807,019 |
|
The accompanying notes form an integral part of the unaudited condensed consolidated interim financial statements.
GRIFOLS, S.A. AND SUBSIDIARIES
Condensed Consolidated Statements of Changes in Equity
for each of the six-month periods ended 30 June 2017 and 2016
(Expressed in thousands of Euros)
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
|
|
Attributable to equity holders of the Parent |
|
|
|
|
|
|
| ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income |
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable |
|
|
|
|
|
|
|
|
|
|
|
|
|
attributable |
|
|
|
|
|
|
|
|
Share |
|
Share |
|
|
|
to |
|
Interim |
|
Treasury |
|
Translation |
|
Available for sale |
|
Other comprehensive |
|
Cash flow |
|
to |
|
Non- controlling |
|
|
|
|
|
|
capital |
|
premium |
|
Reserves |
|
Parent |
|
dividend |
|
Stock |
|
differences |
|
financial assets |
|
income |
|
hedges |
|
Parent |
|
interests |
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at 31 December 2015 |
|
119,604 |
|
910,728 |
|
1,371,061 |
|
532,145 |
|
(119,615 |
) |
(58,575 |
) |
534,491 |
|
— |
|
3,035 |
|
3,329 |
|
3,296,203 |
|
5,187 |
|
3,301,390 |
|
|
Translation differences |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(55,033 |
) |
— |
|
— |
|
— |
|
(55,033 |
) |
(95 |
) |
(55,128 |
) |
|
Cash flow hedges |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(3,329 |
) |
(3,329 |
) |
— |
|
(3,329 |
) |
|
Other Comprehensive income |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(3,399 |
) |
— |
|
(3,399 |
) |
— |
|
(3,399 |
) |
|
Other comprehensive income for the period |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
|
(55,033 |
) |
0 |
|
(3,399 |
) |
(3,329 |
) |
(61,761 |
) |
(95 |
) |
(61,856 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit/(loss) for the period |
|
— |
|
— |
|
— |
|
264,443 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
264,443 |
|
(356 |
) |
264,087 |
|
|
Total comprehensive income for the period |
|
0 |
|
0 |
|
0 |
|
264,443 |
|
0 |
|
0 |
|
(55,033 |
) |
0 |
|
(3,399 |
) |
(3,329 |
) |
202,682 |
|
(451 |
) |
202,231 |
|
|
Net change in treasury stock |
|
— |
|
— |
|
(232 |
) |
— |
|
— |
|
(3,450 |
) |
— |
|
— |
|
— |
|
— |
|
(3,682 |
) |
— |
|
(3,682 |
) |
|
Acquisition of non-controlling interests |
|
— |
|
— |
|
(570 |
) |
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(570 |
) |
(588 |
) |
(1,158 |
) |
|
Other changes |
|
— |
|
— |
|
6,813 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
6,813 |
|
— |
|
6,813 |
|
|
Distribution of 2015 profit |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves |
|
— |
|
— |
|
412,530 |
|
(412,530 |
) |
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
0 |
|
— |
|
0 |
|
|
Dividends |
|
— |
|
— |
|
(93,243 |
) |
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(93,243 |
) |
— |
|
(93,243 |
) |
|
Interim dividend |
|
— |
|
— |
|
— |
|
(119,615 |
) |
119,615 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
0 |
|
— |
|
0 |
|
|
Operations with equity holders or owners |
|
0 |
|
0 |
|
325,298 |
|
(532,145 |
) |
119,615 |
|
(3,450 |
) |
0 |
|
0 |
|
0 |
|
0 |
|
(90,682 |
) |
(588 |
) |
(91,270 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at 30 June 2016 (unaudited) |
|
119,604 |
|
910,728 |
|
1,696,359 |
|
264,443 |
|
0 |
|
(62,025 |
) |
479,458 |
|
0 |
|
(364 |
) |
0 |
|
3,408,203 |
|
4,148 |
|
3,412,351 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at 31 December 2016 |
|
119,604 |
|
910,728 |
|
1,694,245 |
|
545,456 |
|
(122,908 |
) |
(68,710 |
) |
648,927 |
|
(5,219 |
) |
(642 |
) |
— |
|
3,721,481 |
|
6,497 |
|
3,727,978 |
|
|
Translation differences |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(335,367 |
) |
— |
|
— |
|
— |
|
(335,367 |
) |
(115 |
) |
(335,482 |
) |
|
Other comprehensive income for the period |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
|
(335,367 |
) |
0 |
|
0 |
|
0 |
|
(335,367 |
) |
(115 |
) |
(335,482 |
) |
|
Profit/(loss) for the period |
|
— |
|
— |
|
— |
|
277,861 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
277,861 |
|
(491 |
) |
277,370 |
|
|
Total comprehensive income for the period |
|
0 |
|
0 |
|
0 |
|
277,861 |
|
0 |
|
0 |
|
(335,367 |
) |
0 |
|
0 |
|
0 |
|
(57,506 |
) |
(606 |
) |
(58,112 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net change in treasury stock |
|
— |
|
— |
|
— |
|
— |
|
— |
|
6,288 |
|
— |
|
— |
|
— |
|
— |
|
6,288 |
|
— |
|
6,288 |
|
|
Acquisition of non-controlling interests |
|
— |
|
— |
|
27 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
27 |
|
(27 |
) |
0 |
|
|
Other changes |
|
— |
|
— |
|
4,003 |
|
— |
|
— |
|
— |
|
— |
|
23 |
|
— |
|
— |
|
4,026 |
|
(76 |
) |
3,950 |
|
|
Distribution of 2016 profit |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves |
|
— |
|
— |
|
422,548 |
|
(422,548 |
) |
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
0 |
|
— |
|
0 |
|
|
Dividends |
|
— |
|
— |
|
(95,274 |
) |
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(95,274 |
) |
— |
|
(95,274 |
) |
|
Interim dividend |
|
— |
|
— |
|
— |
|
(122,908 |
) |
122,908 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
0 |
|
— |
|
0 |
|
|
Operations with equity holders or owners |
|
0 |
|
0 |
|
331,304 |
|
(545,456 |
) |
122,908 |
|
6,288 |
|
0 |
|
23 |
|
0 |
|
0 |
|
(84,933 |
) |
(103 |
) |
(85,036 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at 30 June 2017 (unaudited) |
|
119,604 |
|
910,728 |
|
2,025,549 |
|
277,861 |
|
0 |
|
(62,422 |
) |
313,560 |
|
(5,196 |
) |
(642 |
) |
0 |
|
3,579,042 |
|
5,788 |
|
3,584,830 |
|
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
(1) General Information
Grifols, S.A. (hereinafter the Company) was incorporated with limited liability under Spanish law on 22 June 1987. It’s registered and tax offices are in Barcelona. The Company’s statutory activity consists of providing corporate and business administrative, management and control services, as well as investing in assets and property. Its principal activity involves rendering administrative, management and control services to its subsidiaries.
All the Company’s shares are listed in the Barcelona, Madrid, Valencia, and Bilbao securities markets and on the Spanish Automated Quotation System (SIBE/Continuous Market). On 2 June 2011, Class B non-voting shares were listed on the NASDAQ (USA) and on the Spanish Automated Quotation System (SIBE/Continuous Market).
Grifols, S.A. is the parent company of the Group (hereinafter the Group or Grifols) which acts on an integrated basis under a common management and whose main activity is the procurement, manufacture, preparation, and sale of therapeutic products.
The main factory locations of the Group’s Spanish companies are in Parets del Vallés (Barcelona) and Torres de Cotilla (Murcia), while the US companies are located in Los Angeles, (California, USA), Clayton (North Carolina, USA), Emeryville (California, USA) and San Diego (California, USA).
(2) Basis of Presentation and Accounting Principles Applied
These condensed consolidated interim financial statements for the six-month period ended 30 June 2017 have been prepared under International Financial Reporting Standards as adopted by the European Union (IFRS-EU) and specifically, with that provided by the guidelines of International Accounting Standard (hereinafter IAS) 34 on Interim Financial Reporting. They do not include all of the information required for full annual financial statements, and should be read in conjunction with the consolidated financial statements of the Group for the year ended 31 December 2016.
The Board of Directors of Grifols, S.A. authorised these condensed consolidated interim financial statements for issue at their meeting held on 26 July 2017.
Amounts contained in these condensed consolidated interim financial statements are expressed in thousands of Euros.
The condensed consolidated interim financial statements of Grifols for the six-month period ended 30 June 2017 have been prepared based on the accounting records maintained by Grifols and subsidiaries. We also have included for information purposes the three-month period ended 30 June 2017.
Accounting principles and basis of consolidation applied
Except as noted below, the accounting principles and basis of consolidation applied in the preparation of these condensed consolidated interim financial statements are the same as those applied by the Group in its consolidated financial statements as at and for the year ended 31 December 2016.
At the date of presentation of these condensed consolidated interim financial statements, the following IFRS standards, amendments and IFRIC interpretations have been issued by the European Union but their application is not mandatory until future periods as described below:
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
|
|
|
|
Mandatory application for |
|
Mandatory application for |
|
|
|
|
|
annual periods beginning on |
|
annual periods beginning |
|
|
|
|
|
or after: |
|
on or after: |
|
Standards |
|
EU effective date |
|
IASB effective date | ||
|
|
|
|
|
|
|
|
|
IAS 12 |
|
Recognition of Deferred Tax Assets for Unrealized Losses (issued on 19 January 2016) |
|
pending |
|
1-Jan-17 |
|
|
|
|
|
|
|
|
|
IAS 7 |
|
Disclosure Initiative (issued on 29 January 2016) |
|
pending |
|
1-Jan-17 |
|
|
|
|
|
|
|
|
|
Various |
|
Annual improvements to IFRSs 2014 - 2016 cycle (issued on 8 December 2016) - IFRS 12 |
|
pending |
|
1-Jan-17 |
|
|
|
|
|
|
|
|
|
IFRS 15 |
|
Revenue from contracts with Customers (issued on 28 May 2014) |
|
1 January 2018 |
|
1 January 2018 |
|
|
|
|
|
|
|
|
|
IFRS 15 |
|
Clarification to IFRS15 Revenue from Contracts with Customers (issued on 12 April 2016) |
|
pending |
|
1 January 2018 |
|
|
|
|
|
|
|
|
|
IFRS 9 |
|
Financial instruments (issued on 24 July 2014) |
|
1 January 2018 |
|
1 January 2018 |
|
|
|
|
|
|
|
|
|
IFRS 2 |
|
Classification and Measurement of Share-based Payment Transactions (issued on 20 June 2016) |
|
pending |
|
1 January 2018 |
|
|
|
|
|
|
|
|
|
IFRS 4 IFRS 9 |
|
Applying IFRS 9 Financial Instruments with IFRS 4 Insurance Contracts (issued on 12 September 2016) |
|
pending |
|
1 January 2018 |
|
|
|
|
|
|
|
|
|
IFRIC 22 |
|
IFRIC 22 Interpretation: Foreign currency translations and Advance Consideration |
|
pending |
|
1 January 2018 |
|
|
|
|
|
|
|
|
|
IAS 40 |
|
Amendments to IAS 40: Transfers of Investment Property |
|
pending |
|
1 January 2018 |
|
|
|
|
|
|
|
|
|
Various |
|
Annual improvements to IFRSs 2014 - 2016 cycle (issued on 8 December 2016) |
|
pending |
|
1 January 2018 |
|
|
|
|
|
|
|
|
|
IFRS 16 |
|
Leases (Issued on 13 January 2016) |
|
pending |
|
1 January 2019 |
|
|
|
|
|
|
|
|
|
IFRIC 23 |
|
Uncertainty over Income Tax Treatments (issued on 7 June 2017) |
|
pending |
|
1 January 2019 |
|
|
|
|
|
|
|
|
|
IFRS 17 |
|
Insurance Contracts (issued on 18 May 2017) |
|
pending |
|
1 January 2021 |
The Group has not applied any of the standards or interpretations issued prior to their effective date.
At the date of issue of these condensed consolidated interim financial statements, the Group is analyzing the impact of the application of the above standards or interpretations published by the European Union (EU).
Modifications on IAS 12, IAS 7 and IFRS 12 standards include minor changes that clarify requirements, add new information or do not apply to the Group. Consequently, the Group confirms that even though the existent divergence between EU and IASB effective date, we fulfil both IASB-IFRS and EU-IFRS standards at 30 June 2017.
Based on the preliminary analysis of the impact of the application of IFRS 15 in 2018, the Group does not expect significant impacts to occur in the consolidated interim financial statements. The Group is currently in the process of evaluating the possible impacts of the application of IFRS9 in the light of the latest IASB pronouncements.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
Responsibility regarding information, estimates, and relevant judgments in the application of accounting policies
The information contained in these condensed consolidated interim financial statements for the six-month period ended 30 June 2017 is the responsibility of the Directors of the Company. The preparation of the condensed consolidated interim financial statements requires management to make judgements, estimates and assumptions that affect the application of Group accounting policies. The following notes include a summary of the relevant accounting estimates and judgements used to apply accounting policies which have the most significant effect on the accounts recognised in these condensed consolidated interim financial statements.
· Assumptions used to test non-current assets and goodwill for impairment. Relevant cash generating units are tested annually for impairment. These are based on risk-adjusted future cash flows discounted using appropriate interest rates. Assumptions relating to risk-adjusted future cash flows and discount rates are based on business forecasts and are therefore inherently subjective. Future events could cause a change in business forecasts, with a consequent adverse effect on the future results of the Group. To the extent considered a reasonably possible change in key assumptions could result in an impairment of goodwill, a sensitivity analysis has been disclosed in note 7 of the consolidated financial statements as at and for the year ended 31 December 2016 to show the effect of changes to these assumptions and the effect of the cash generating unit (CGU) on the recoverable amount.
· Useful lives of property, plant and equipment and intangible assets. The estimated useful lives of each category of property, plant and equipment and intangible assets are set out in notes 4(g) and 4(h) of the consolidated financial statements as at and for the year ended 31 December 2016. Although estimates are calculated by the Company’s management based on the best information available at the reporting date, future events may require changes to these estimates in subsequent years. Given the large number of individual items of property, plant and equipment it is not considered likely that a reasonably possible change in the assumptions would lead to a material adverse effect. Potential changes to the useful lives of intangible assets are mainly related to the currently marketed products and the useful lives will depend on the life cycle of the same. No significant changes to useful lives are expected. Adjustments made in subsequent years are recognised prospectively.
· Evaluation of the nature of leases (operating or finance). The Group analyses the conditions of the lease contracts at their inception in order to conclude if the risks and rewards have been transferred. If the lease contract is renewed or amended the Group conducts a new evaluation.
· Assumptions used to determine the fair value of assets, liabilities and contingent liabilities related to business combinations.
· Evaluation of the capitalisation of development costs. The key assumption is related to the estimation of sufficient future economic benefits of the projects.
· Evaluation of provisions and contingencies. Key assumptions relate to the evaluation of the likelihood of an outflow of resources due to a past event, as well as to the evaluation of the best estimate of the likely outcome. These estimates take into account the specific circumstances of each dispute and relevant external advice and therefore are inherently subjective and could change substantially over time as new facts arise and each dispute progresses. Details of the status of various uncertainties involved in significant unresolved disputes are set out in note 16.
· Evaluation of the recoverability of tax credits, including tax loss carryforwards and rights for deductions. Deferred tax assets are recognized to the extent that future taxable profits will be available against which the temporary differences can be utilized, based on management’s assumptions relating to the amount and timing of future taxable profits.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version
prevails)
No changes have been made to prior year judgements relating to existing uncertainties.
The Group is also exposed to interest rate and currency risks.
Grifols’ management does not consider that there are any assumptions or causes for uncertainty in the estimates which could imply a significant risk of material adjustments arising in the next financial year.
The estimates and relevant judgments used in the preparation of these condensed consolidated interim financial statements do not significantly differ from those applied in the preparation of the consolidated financial statements as at and for the year ended 31 December 2016.
Seasonality of transactions during this period
Given the nature of the activities conducted by the Group, there are no factors that determine any significant seasonality in the Group’s operations that could affect the interpretation of these condensed consolidated interim financial statements for the six-month period ended 30 June 2017 in comparison with the financial statements for a full fiscal year.
Relative importance
When determining the information to be disclosed in these Notes, in accordance with IAS 34, the relative importance in relation to these condensed consolidated interim financial statements has been taken into account.
(3) Changes in the composition of the Group
For the preparation of its condensed consolidated interim financial statements, the Group has included its investments in all subsidiaries, associates and joint ventures. Appendix I of the consolidated financial statements as at 31 December 2016 lists the subsidiaries, associates and joint ventures in which Grifols, S.A. holds a direct or indirect stake and that were included in the scope of consolidation at that date.
The main changes in the scope of consolidation during the interim period ended 30 June 2017 are detailed below:
· Access Biologicals Acquisition
On 12 January 2017, the group has announced the acquisition of 49% of the voting rights in Access Biologicals LLC, a company based in San Diego, California, USA, for the amount of US Dollar 51 million. Grifols has entered into an option agreement to purchase the remaining 51% voting rights in five years, in 2022. Grifols has also signed a supply agreement to sell to Access Biologicals biological products not meant for therapeutic use.
The principal business activity of Access Biologicals is the collection and manufacturing of an extensive portfolio of biologicals products. Combined with closed-loop material sourcing, it provides critical support for various markets such as in-vitro diagnostic manufacturing, biopharmaceutical, cell culture and diagnostic research & development.
Details of the aggregate business combination cost, the provisional fair value of the net assets acquired and provisional goodwill at the acquisition date are provided below. The values shown in the table below should be considered provisional.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version
prevails)
|
|
|
Thousand |
|
Thousand of |
|
|
|
|
of Euros |
|
US Dollars |
|
|
Cost of the business combination |
|
|
|
|
|
|
|
|
|
|
|
|
|
Payment in cash |
|
48,383 |
|
51,010 |
|
|
|
|
|
|
|
|
|
Total business combination cost |
|
48,383 |
|
51,010 |
|
|
|
|
|
|
|
|
|
Non-current assets |
|
1,147 |
|
1,209 |
|
|
Current assets |
|
9,697 |
|
10,224 |
|
|
Current liabilities |
|
(1,866 |
) |
(1,967 |
) |
|
Total net assets (100%) |
|
8,978 |
|
9,466 |
|
|
|
|
|
|
|
|
|
Group’s share of net assets (49%) |
|
4,399 |
|
4,638 |
|
|
|
|
|
|
|
|
|
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
43,984 |
|
46,372 |
|
· Hologic acquisition
On 14 December 2016 Grifols entered into and asset purchase agreement to acquire Hologic’s business of NAT (Nucleic Acid Testing) donor screening unit for US Dollar 1,865 million. The transaction was closed on 31 January 2017. The agreement encompasses the acquisition of the Hologic unit engaged in research, development and manufacture of assays and instruments based on NAT technology for transfusion and transplantation screening. In addition, it was agreed the cancelation of the existing joint-collaboration agreement for the commercialization of NAT donor screening products by Grifols. NAT technology makes possible to detect the presence of infectious agents in blood and plasma donations, contributing to greater transfusion safety.
The assets acquired comprise a plant in San Diego, CA (United States) as well as development rights, licenses to patents, know-how and access to product manufacturers.
The acquisition is structured through Grifols Diagnostic Solutions, Inc., a U.S. incorporated and wholly-owned subsidiary of Grifols, S.A.
Grifols consolidates itself as one of the only vertically integrated providers capable of offering comprehensive solutions to blood and plasma donation centers.
This acquisition strengthens cash flows and positively impacts the group’s margins. The revenues of the Diagnostic Division will not change as a result of the acquisition due to the existing joint-business between Grifols and Hologic in place since 2014, under which Grifols already owns customer facing activities and records all revenues.
It is expected that this acquisition will strengthen the position of the Grifols Diagnostic Division in transfusion medicine and will increase significantly the profitability of Grifols Diagnostic Division having a direct impact on the group’s EBITDA margin. By streamlining and integrating the NAT business, operational efficiency will be in terms of production, R&D, overheads and administrative expenses.
At the date of issue of these condensed consolidated interim financial statements, the Group is working to determine the definitive fair value of intangible assets, liabilities and contingent liabilities acquired in the business combination.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version
prevails)
Details of the aggregate business combination cost, the provisional fair value of the net assets acquired and provisional goodwill at the acquisition date are provided below. The values shown in the table below should be considered provisional.
|
|
|
Thousands of Euros |
|
Thousands of |
|
|
|
|
|
|
|
|
|
Cost of the business combination |
|
|
|
|
|
|
|
|
|
|
|
|
|
Payment in cash |
|
1,734,077 |
|
1,865,000 |
|
|
|
|
|
|
|
|
|
Total business combination cost |
|
1,734,077 |
|
1,865,000 |
|
|
|
|
|
|
|
|
|
Fair value of net assets acquired |
|
22,091 |
|
23,759 |
|
|
|
|
|
|
|
|
|
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
1,711,986 |
|
1,841,241 |
|
Provisional Goodwill shown in the table above includes workforce of the acquired business and also the separable intangible assets which will be recorded retroactively at 31 January 2017 once the purchase price allocation exercise is finalized. The fair value of net assets acquired includes mainly property, plant and equipment amounting to Euros 24,490 thousand .
The expenses incurred in this transaction in 2017 amount to approximately Euros 13 million (Euros 5.1 million in 2016).
Goodwill is allocated to the Diagnostic segment.
· Kedplasma acquisition
On 27 December 2016 Grifols entered into an agreement to acquire six new Plasma Donor Centers to the company Kedplasma, LLC, with a purchase price of US Dollar 47 million. Delivery of these centers has been made in February 2017.
Aggregate details of the combination cost, fair value of the net assets acquired and goodwill at the acquisition date (or surplus net assets acquired over the combination cost) are as follows:
|
|
|
Thousands of Euros |
|
Thousands of US Dollars |
|
|
|
|
|
|
|
|
|
Cost of the business combination |
|
|
|
|
|
|
|
|
|
|
|
|
|
Payment in cash |
|
44,238 |
|
47,083 |
|
|
|
|
|
|
|
|
|
Total business combination cost |
|
44,238 |
|
47,083 |
|
|
|
|
|
|
|
|
|
Fair value of net assets acquired |
|
4,137 |
|
4,403 |
|
|
|
|
|
|
|
|
|
Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) |
|
40,101 |
|
42,680 |
|
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version
prevails)
The fair value of net assets acquired includes property, plant and equipment amounting to Euros 3,703 thousand.
Goodwill is allocated to the Bioscience segment and includes plasma donor center base, FDA licenses and workforce retained.
At 31 December 2016, the group advanced the sum of US Dollar 15 million related to this acquisition.
(4) Financial Risk Management Policy
At 30 June 2017 the Group’s financial risk management objectives and policies are consistent with those disclosed in the consolidated financial statements for the year ended 31 December 2016.
(5) Segment Reporting
The distribution by business segments of the Group’s net revenues and consolidated income for the three- and six-month periods ended 30 June 2017 and 30 June 2016 is as follows:
|
|
|
Net revenues (Thousands of Euros) |
| ||||||
|
|
|
Six-Months Ended 30 |
|
Six-Months Ended |
|
Three-Months |
|
Three-Months |
|
|
Segments |
|
June 2017 |
|
30 June 2016 |
|
Ended 30 June 2017 |
|
Ended 30 June 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Bioscience |
|
1,759,852 |
|
1,538,805 |
|
906,213 |
|
796,946 |
|
|
Hospital |
|
47,866 |
|
46,478 |
|
24,902 |
|
23,640 |
|
|
Diagnostic |
|
351,051 |
|
316,830 |
|
180,408 |
|
155,790 |
|
|
Bio supplies |
|
32,072 |
|
27,976 |
|
17,671 |
|
10,342 |
|
|
Other |
|
1,606 |
|
21,556 |
|
1,573 |
|
5,994 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Revenues |
|
2,192,447 |
|
1,951,645 |
|
1,130,767 |
|
992,712 |
|
|
|
|
Profit/(loss) (Thousands of Euros) |
| ||||||
|
|
|
Six-Months |
|
Six-Months |
|
Three-Months |
|
Three-Months |
|
|
|
|
Ended 30 June |
|
Ended 30 June |
|
Ended 30 June |
|
Ended 30 June |
|
|
Segments |
|
2017 |
|
2016 |
|
2017 |
|
2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Bioscience |
|
509,650 |
|
454,225 |
|
257,562 |
|
235,615 |
|
|
Hospital |
|
(12,211 |
) |
(7,017 |
) |
(6,770 |
) |
(4,374 |
) |
|
Diagnostic |
|
123,023 |
|
39,902 |
|
65,430 |
|
13,554 |
|
|
Bio supplies |
|
18,352 |
|
14,853 |
|
10,379 |
|
4,751 |
|
|
Other |
|
(11,966 |
) |
37,001 |
|
(4,903 |
) |
21,900 |
|
|
Total income of reported segments |
|
626,848 |
|
538,964 |
|
321,698 |
|
271,446 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unallocated expenses plus net financial result |
|
(246,889 |
) |
(193,752 |
) |
(124,938 |
) |
(90,472 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax from continuing operations |
|
379,959 |
|
345,212 |
|
196,760 |
|
180,974 |
|
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version
prevails)
Since January 2017, the company is including all transactions related to biological products for non-therapeutic use in the new Bio Supplies Division. Consequently, the comparative figures for 2016 have been restated accordingly, resulting in a reclassification from Bioscience Division to Bio Supplies Division.
(6) Goodwill
Details and movement in goodwill during the six month period ended 30 June 2017 is as follows:
|
|
|
Thousands of Euros |
| ||||||||
|
|
|
|
|
Balance at |
|
Business |
|
Translation |
|
Balance at |
|
|
|
|
Segment |
|
31/12/2016 |
|
Combination |
|
differences |
|
30/06/2017 |
|
|
Net value |
|
|
|
|
|
|
|
|
|
|
|
|
Grifols UK.Ltd. (UK) |
|
Bioscience |
|
8,025 |
|
— |
|
(211 |
) |
7,814 |
|
|
Grifols Italia.S.p.A. (Italy ) |
|
Bioscience |
|
6,118 |
|
— |
|
— |
|
6,118 |
|
|
Biomat USA, Inc. (USA) |
|
Bioscience |
|
193,039 |
|
40,101 |
|
(17,435 |
) |
215,705 |
|
|
Grifols Australia Pty Ltd. |
|
|
|
|
|
|
|
|
|
|
|
|
(Australia) / Medion Diagnostics AG |
|
Diagnostic |
|
10,134 |
|
— |
|
(175 |
) |
9,959 |
|
|
(Switzerland) |
|
|
|
|
|
|
|
|
|
|
|
|
Grifols Therapeutics, Inc. (USA) |
|
Bioscience |
|
2,108,139 |
|
— |
|
(160,900 |
) |
1,947,239 |
|
|
Araclon Biotech, S.L. (Spain) |
|
Diagnostic |
|
6,000 |
|
— |
|
— |
|
6,000 |
|
|
Progenika Biopharma, S.A. (Spain) |
|
Diagnostic |
|
40,516 |
|
— |
|
— |
|
40,516 |
|
|
Grifols Diagnostic (Novartis & Hologic) |
|
Diagnostic |
|
1,272,024 |
|
1,711,986 |
|
(193,458 |
) |
2,790,552 |
|
|
(USA, Spain and Hong Kong) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,643,995 |
|
1,752,087 |
|
(372,179 |
) |
5,023,903 |
|
|
|
|
|
|
|
|
(note 3) |
|
|
|
|
|
Impairment testing:
As a result of the acquisition of Talecris in 2011, and for impairment testing purposes, the Group combines the CGUs allocated to the Bioscience segment, grouping them together at segment level, because substantial synergies were expected to arise on the acquisition of Talecris, and due to the vertical integration of the business and the lack of an independent organized market for the products. Because the synergies benefit the Bioscience segment globally they cannot be allocated to individual CGUs. The Bioscience segment represents the lowest level to which goodwill is allocated and is subject to control by Group management for internal control purposes.
Due to the acquisition of Novartis’ Diagnostic business unit in 2014, the Group decided to group Araclon, Progenika, Australia and Hologic into a single CGU for the Diagnostic business since the acquisition is supporting not only the vertically integration business but also cross-selling opportunities. In addition, for management purposes, the Group’s management is focused on the business more than geographical areas or individual companies.
At 30 June 2017, the Group has not identified any triggering event that would make it necessary to perform the impairment test of the respective CGU’s for this interim period.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version
prevails)
(7) Other Intangible Assets and Property, Plant, and Equipment
Movement of Other Intangible Assets and Property, Plant and Equipment during the six-month period ended 30 June 2017 is as follows:
|
|
|
Thousands of Euros |
| ||||
|
|
|
Other intangible |
|
Property, plant |
|
|
|
|
|
|
assets |
|
and equipment |
|
Total |
|
|
Total Cost at 31/12/2016 |
|
1,682,673 |
|
2,618,696 |
|
4,301,369 |
|
|
Total depreciation and amortization at 31/12/2016 |
|
(487,371 |
) |
(805,644 |
) |
(1,293,015 |
) |
|
Impairment at 31/12/2016 |
|
— |
|
(3,200 |
) |
(3,200 |
) |
|
Balance at 31/12/2016 |
|
1,195,302 |
|
1,809,852 |
|
3,005,154 |
|
|
|
|
|
|
|
|
|
|
|
Cost |
|
|
|
|
|
|
|
|
Additions |
|
20,593 |
|
130,993 |
|
151,586 |
|
|
Business combination |
|
7 |
|
28,188 |
|
28,195 |
|
|
Disposals |
|
(95 |
) |
(5,655 |
) |
(5,750 |
) |
|
Transfers |
|
370 |
|
(404 |
) |
(34 |
) |
|
Translation differences |
|
(111,594 |
) |
(159,510 |
) |
(271,104 |
) |
|
Total Cost at 30/06/2017 |
|
1,591,954 |
|
2,612,308 |
|
4,204,262 |
|
|
|
|
|
|
|
|
|
|
|
Depreciation & amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additions |
|
(30,499 |
) |
(76,050 |
) |
(106,549 |
) |
|
Disposals |
|
94 |
|
4,856 |
|
4,950 |
|
|
Transfers |
|
— |
|
35 |
|
35 |
|
|
Translation differences |
|
27,611 |
|
43,411 |
|
71,022 |
|
|
Total depreciation and amortization at 30/06/2017 |
|
(490,165 |
) |
(833,392 |
) |
(1,323,557 |
) |
|
|
|
|
|
|
|
|
|
|
Impairment |
|
|
|
|
|
|
|
|
Additions |
|
— |
|
86 |
|
86 |
|
|
Translation differences |
|
— |
|
105 |
|
105 |
|
|
Impairment at 30/06/2017 |
|
— |
|
(3,009 |
) |
(3,009 |
) |
|
Balance at 30/06/2017 |
|
1,101,789 |
|
1,775,907 |
|
2,877,696 |
|
At 30 June 2017 there are no indications that these assets have been impaired beyond recognized impairment.
Intangible assets acquired from Talecris mainly include currently marketed products. Identifiable intangible assets correspond to Gamunex and have been recognised at fair value at the acquisition date of Talecris and classified as currently marketed products. Intangible assets recognised comprise the rights on the Gamunex product, its commercialisation and distribution license, trademark, as well as relations with hospitals. Each of these components are closely linked and fully complementary, are subject to similar risks and have a similar regulatory approval process.
Intangible assets acquired from Progenika mainly include currently marketed products. Identifiable intangible assets correspond to blood, immunology and cardiovascular genotyping. These assets have been recognised at fair value at the acquisition date of Progenika and classified as currently marketed products.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version
prevails)
The cost and accumulated amortisation of currently marketed products acquired from Talecris and Progenika at 30 June 2017 is as follows:
|
|
|
Thousands of Euros |
| ||||||
|
|
|
Balance at |
|
|
|
Translation |
|
Balance at |
|
|
|
|
31/12/2016 |
|
Additions |
|
differences |
|
30/06/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of currently marketed products - Gamunex |
|
1,138,412 |
|
— |
|
(86,887 |
) |
1,051,525 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of currently marketed products - Progenika |
|
23,792 |
|
— |
|
— |
|
23,792 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated amortisation of currently marketed products - Gamunex |
|
(211,871 |
) |
(18,677 |
) |
17,322 |
|
(213,226 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated amortisation of currently marketed products - Progenika |
|
(9,117 |
) |
(1,190 |
) |
— |
|
(10,307 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Net carrying amount of currently marketed products |
|
941,216 |
|
(19,867 |
) |
(69,565 |
) |
851,784 |
|
The estimated useful life of the currently marketed products acquired from Talecris is considered limited, has been estimated at 30 years on the basis of the expected life cycle of the product (Gamunex) and is amortised on a straight-line basis.
At 30 June 2017 the residual useful life of currently marketed products from Talecris is 23 years and 11 months (24 years and 11 months at 30 June 2016).
The estimated useful life of the currently marketed products acquired from Progenika is considered limited, has been estimated at 10 years on the basis of the expected life cycle of the product and is amortised on a straight-line basis.
At 30 June 2017 the residual useful life of currently marketed products from Progenika is 5 years and 8 months (6 years and 8 months at 30 June 2016).
(8) Financial Assets
|
|
|
Thousands of Euros |
| ||
|
|
|
30/06/2017 |
|
31/12/2016 |
|
|
|
|
|
|
|
|
|
Non-current derivatives (b) |
|
12,622 |
|
13,665 |
|
|
Non-current investment in quoted shares |
|
29,589 |
|
29,998 |
|
|
Total Non-current financial assets measured at fair value |
|
42,211 |
|
43,663 |
|
|
|
|
|
|
|
|
|
Convertible Bond (a) |
|
14,471 |
|
40,201 |
|
|
Non-current guarantee deposits |
|
4,706 |
|
4,603 |
|
|
Other non-current financial assets |
|
1,336 |
|
1,078 |
|
|
Total Non-current financial assets at amortized cost |
|
20,513 |
|
45,882 |
|
Details of other current financial assets on the consolidated balance sheet at 30 June 2017 and 31 December 2016 are as follows:
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version
prevails)
|
|
|
Thousands of Euros |
| ||
|
|
|
30/06/2017 |
|
31/12/2016 |
|
|
Deposits and guarantees |
|
868 |
|
957 |
|
|
Current loans to third parties |
|
60 |
|
832 |
|
|
Current loans to associates |
|
11,438 |
|
793 |
|
|
|
|
|
|
|
|
|
Total other current financial assets |
|
12,366 |
|
2,582 |
|
(a) Convertible Bond
On April 22, 2016, our subsidiary, Grifols Worldwide Operations Limited, subscribed US Dollars 19,950 thousand (Euros 17,997 thousand) aggregate principal amounts of 9% convertible bonds due 2021 issued by Aradigm. The Group indirectly owns 35.13% of the common stock of Aradigm. Interest on the convertible bonds is payable on May 1 and November 1 of each year.
During the six-month period ended 30 June 2017, Aradigm has paid us an amount of Euros 837 thousand of interest on the convertible bonds.
During the periods or upon the events described in the indenture governing the convertible bonds, the convertible bonds are convertible into common stock of Aradigm. At the date of this condensed consolidated interim financial statements, the conversion rate is 191.94 shares of Aradigm common stock per US Dollar 1,000 principal amount of convertible bonds.
The conversion feature to convert the liablility in to equity of the issuer at a price that can be adjusted results in an embedded derivative measured at fair value. All changes in fair value are recognized in the profit and loss account.
Aradigm intends to use the net proceeds from the offering to fund the current clinical development and regulatory submission for licensure of Pulmaquin and for general corporate purposes.
On February 2, 2017 Grifols Worldwide Operations Limited sold to Nomura International PLC the convertible bonds issued by TiGenix that the Group subscribed on March 6, 2015. The settlement amount was Euros 20.5 million resulting in a loss of Euros 5.5 million.
(b) ( b) Non-current derivatives
Non-current derivatives includes an amount of Euros 8,763 thousand in respect of the call right for the Interstate Blood Bank, Inc. shares, Bio-Blood Components, Inc. shares and Plasma Biological Services, LLC. units that are not owned by the Group. The call right can be exercised by the Group by delivering written notice of its intention at any time on or after February 1, 2019 and on or before April 30, 2019
(9) Trade and Other Receivables
At 30 June 2017, certain companies of the group had signed sales agreements for credit rights without recourse with certain financial institutions.
The total sum of credit rights sold without recourse, for which ownership was transferred to financial entities pursuant to the aforementioned agreements, amounts to Euros 446,820 thousand for the six-month period ended at 30 June 2017 (Euros 434,507 thousand for the six-month period ended 30 June 2016 and Euros 870,324 thousand for the year ended 31 December 2016).
The deferred collection equivalent to the amount pending to be received from a financial entity is presented in the balance sheet under “Other receivables” for an amount of Euros 1,961 thousand as at 30 June 2017 (Euros
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
2,560 thousand as at 31 December 2016) which does not differ significantly from their fair value and is also the amount of the maximum exposure to loss.
The finance cost of credit rights sold amounts to Euros 1,908 thousand for the six-month period ended 30 June 2017 (Euros 2,765 thousand for the six-month period ended 30 June 2016) (see note 13).
The recoverability of receivables from public entities in countries facing liquidity problems, specifically in Italy, Greece, Portugal and Spain, has not significantly changed compared to 31 December 2016.
(10) Equity
Details of consolidated equity and changes are shown in the condensed consolidated statement of changes in equity, which forms part of the condensed consolidated interim financial statements.
(a) Share Capital and Share Premium
On 4 January 2016 the Company’s new shares resulting from the share split ruling on 3 December 2015 by the Company’s board of directors (relevant event nº 231793) started to be traded in accordance with the delegation of authorities by the shareholders at the general shareholders’ meeting held on 29 May 2015. This share split entails that the nominal value of the new Class A shares will be Euro 0.25 per share (previously Euro 0.50 per share), whilst the nominal value of the new Class B shares will be Euro 0.05 per share (previously Euro 0.10 per share).
At 30 June 2017 the Company’s share capital was represented by 426,129,798 Class A shares and 261,425,110 Class B shares.
(b) Reserves
The availability of the reserves for distribution is subject to legislation applicable to each of the Group companies. At 30 June 2017, Euros 44,920 thousand equivalent to the carrying amount of development costs pending amortisation of certain Spanish companies (Euros 50,680 thousand at 31 December 2016) are, in accordance with applicable legislation, restricted reserves which cannot be distributed until these development costs have been amortised.
Companies in Spain are obliged to transfer 10% of each year’s profits to a legal reserve until this reserve reaches an amount equal to 20% of share capital. This reserve is not distributable to shareholders and may only be used to offset losses if no other reserves are available. Under certain conditions it may be used to increase share capital provided that the balance left on the reserve is at least equal to 10% of the nominal value of the total share capital after the increase.
At 30 June 2017 and 31 December 2016 the legal reserve of the Company amounts to Euros 23,921 thousand.
(c) Treasury Stock
At 30 June 2017 and 30 June 2016 the company does not have Class A treasury stock.
Movement in Class B treasury stock during the six-month period ended 30 June 2017 is as follows:
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
|
|
No. of Class B shares |
|
Thousand Euros |
|
|
|
|
|
|
|
|
|
Balance at 1 January 2017 |
|
4,730,735 |
|
68,710 |
|
|
Disposals Class B shares |
|
(432,929 |
) |
(6,288 |
) |
|
|
|
|
|
|
|
|
Balance at 30 June 2017 |
|
4,297,806 |
|
62,422 |
|
In March 2017 the company delivered 432,929 treasury stocks (Class B shares) to eligible employees as a compensation of the Restricted Share Unit Retention Plan (see note 16 (b)).
Movement in Class B treasury stock during the six-month period ended 30 June 2016 is as follows:
|
|
|
No. of Class B shares |
|
Thousand Euros |
|
|
|
|
|
|
|
|
|
Balance at 1 January 2016 |
|
4,038,570 |
|
58,575 |
|
|
Acquisitions Class B shares |
|
1,132,322 |
|
16,166 |
|
|
Non Cash Disposal Class B shares |
|
(876,777 |
) |
(12,716 |
) |
|
|
|
|
|
|
|
|
Balance at 30 June 2016 |
|
4,294,115 |
|
62,025 |
|
In March 2016 the company delivered 876,777 treasury stocks (Class B Shares) to the Progenika’s non-controlling interests in exchange of the 16.465% acquired to them.
Acquisitions Class B shares include the purchase of the Class B shares from the vendor shareholders of Progenika for which Grifols exercised the cash option for an amount of Euros 11,035 thousand. This amount has been considered as cash used in investing activities in the statement of cash flows.
(d) Distribution of profits
The profits of Grifols, S.A. and subsidiaries will be allocated as agreed by respective shareholders at their general meetings and the proposed allocation of the profit for the year ended 31 December 2016 is presented in the consolidated statements of changes in equity.
The dividends paid during the six months period ended 30 June 2017 is as follows:
|
|
|
Six-Months’ Ended 30 June 2017 |
| ||||
|
|
|
% over |
|
Euros |
|
Amount in |
|
|
|
|
par value |
|
per shares |
|
thousand of Euros |
|
|
Ordinary Shares |
|
54 |
% |
0.14 |
|
57,790 |
|
|
Non-voting shares |
|
271 |
% |
0.14 |
|
34,870 |
|
|
Non-voting shares (Preferred Dividend) |
|
20 |
% |
0.01 |
|
2,614 |
|
|
|
|
|
|
|
|
|
|
|
Total Dividends Paid |
|
|
|
|
|
95,274 |
|
The dividends paid during the six-month period ended 30 June 2016 were as follows:
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
|
|
Six-Months’ Ended 30 June 2016 |
| ||||
|
|
|
% over |
|
Euros |
|
Amount in |
|
|
|
|
par value |
|
per shares |
|
thousand of Euros |
|
|
|
|
|
|
|
|
|
|
|
Ordinary Shares |
|
53 |
% |
0.13 |
|
56,493 |
|
|
Non-voting shares |
|
265 |
% |
0.13 |
|
34,136 |
|
|
Non-voting shares (Preferred Dividend) |
|
20 |
% |
0.01 |
|
2,614 |
|
|
|
|
|
|
|
|
|
|
|
Total Dividends Paid |
|
|
|
|
|
93,243 |
|
(e) Restricted Share Unit Compensation
The Group has set up a Restricted Share Unit Retention Plan (hereinafter RSU) for certain employees (see note 16 (b) ). This commitment is settled using equity instruments and the cumulative accrual amounts to Euros 11,901 thousand in June 2017 (Euros 9,132 thousand in June 2016).
(11) Financial Liabilities
The detail of financial liabilities at 30 June 2017 and 31 December 2016 is as follows:
|
|
|
Thousands of Euros |
| ||
|
|
|
30/06/2017 |
|
31/12/2016 |
|
|
Financial liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current obligations (a) |
|
844,206 |
|
831,417 |
|
|
Senior secured debt (b) |
|
5,056,686 |
|
3,728,695 |
|
|
Other loans |
|
112,140 |
|
114,898 |
|
|
Finance lease liabilities |
|
6,627 |
|
6,086 |
|
|
Other non-current financial liabilities |
|
22,518 |
|
30,975 |
|
|
|
|
|
|
|
|
|
Total non-current financial liabilities |
|
6,042,177 |
|
4,712,071 |
|
|
|
|
|
|
|
|
|
Current obligations (a) |
|
94,549 |
|
95,524 |
|
|
Senior secured debt (b) |
|
20,083 |
|
81,273 |
|
|
Other loans |
|
10,791 |
|
23,288 |
|
|
Finance lease liabilities |
|
3,910 |
|
3,859 |
|
|
Other current financial liabilities |
|
19,228 |
|
26,121 |
|
|
|
|
|
|
|
|
|
Total current financial liabilities |
|
148,561 |
|
230,065 |
|
On 06 February 2017 the Group concluded the refinancing process of its senior debt. The total debt refinanced amounts to US Dollars 6,300 million (Euros 5,800 million), including the US Dollars 1,816 million loan obtained for the acquisition of Hologic’s transfusional diagnostics unit. Following the refinancing process, Grifols’ debt structure consists of a US Dollars 6,000 million long-term loan with institutional investors and banks segmented in two tranches (Term Loan A and Term Loan B), and a US Dollars 300 million undrawn revolving credit facility.
On 18 April 2017 the Group concluded the refinancing process of the Senior Unsecured Notes. The total bond issuance amounted to Euros 1,000 million.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
On 28 October 2015 the Group received an additional loan from the European Investment Bank up to Euros 100 million at a fixed interest rate for a tenor of ten years with a grace period of two years. The loan will be used to support some investments in R&D which are mainly focused on searching new applications for plasmatic proteins.
(a) Senior Unsecured Notes
On 18 April 2017, Grifols, S.A. issued Euros 1,000 million Senior Unsecured Notes (the “Notes”) that will mature in 2025 and will bear an annual coupon of 3.20%. These notes have been exchanged with the 97.1% of the Senior Unsecured Notes issued in 2014 by Grifols Worldwide Operations Limited, a 100% subsidiary of Grifols, S.A., amounting to US Dollars 1,000 million, with a maturity in 2022 and at interest rate of 5.25% that was owned by a financial institution. The remaining 2.9% of the existing notes was redeemed before the exchange by an amount of Euros 27,098 thousand. The corresponding deferred costs of the notes has been removed to profit and loss. On 2 May 2017 the Notes have been admitted to listing in the Irish Stock Exchange.
The present value of discounted cash flows of the new Notes under the new agreement, including costs for fees paid and discounted using the original effective interest rate differs by less than 10% of the present value discounted cash flows remaining in the original debt, whereby the new agreement is not substantially different to the original agreement.
The costs of the refinancing Senior Unsecured Notes have amounted to Euros 57.5 million, including the redemptions costs. These costs were included as transaction costs together with the costs deriving from the debt issue and will be taken to profit or loss in accordance with the new effective interest rate. Based on the analysis of the quantitative and qualitative factors, the Group has concluded that the renegotiation of conditions of the Senior Unsecured Notes does not trigger a derecognition of the liability. Unamortized financing costs from the Senior Unsecured Notes amount to Euros 156 million at 30 June 2017 (Euros 117 million at 31 December 2016).
The total principal plus interest of the Senior Unsecured Notes to be paid is detailed as follows:
|
|
|
Senior Unsecured Notes |
| ||
|
|
|
Principal+Interests in |
| ||
|
|
|
Thousand of Euros |
| ||
|
Maturity |
|
|
|
|
|
|
|
|
|
|
|
|
|
2017 |
|
|
|
16,356 |
|
|
2018 |
|
|
|
32,000 |
|
|
2019 |
|
|
|
32,000 |
|
|
2020 |
|
|
|
32,000 |
|
|
2021 |
|
|
|
32,000 |
|
|
2022 |
|
|
|
32,000 |
|
|
2023 |
|
|
|
32,000 |
|
|
2024 |
|
|
|
32,000 |
|
|
2025 |
|
|
|
1,016,000 |
|
|
Total |
|
|
|
1,256,356 |
|
(b) Senior Secured Debt
On 06 February 2017 the Group refinanced its Senior Secured Debt with the existing lenders and obtained the additional debt for the acquisition of Hologic by an amount of US Dollars 1,816 million. The new senior debt consists of a Term Loan A (“TLA”), which amounts to US Dollars 2,350 million and Euros 607 million with a 1.75% margin over Libor and Euribor respectively, maturity in 2023 and quasi-bullet amortization structure, and a Term Loan B (“TLB”) that amounts to US Dollars 3,000 million with a
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
2.25% margin over Libor, maturity in 2025 and quasi-bullet amortization. The borrowers of the total debt are Grifols Worldwide Operations Limited and Grifols, S.A. for the Term Loan A and Grifols Worldwide Operations USA, Inc. for the Term Loan B.
The present value of cash flows under the refinanced agreement, including any fees paid and discounted using the original effective interest rate differs by less than 10% of the present value of cash flows remaining in the original debt, whereby it is considered that the debt instrument has not been substantially modified
The costs of refinancing the senior debt have amounted to Euros 84.8 million. Based on the analysis of the quantitative and qualitative factors, the Group has concluded that the renegotiation of conditions of the senior debt does not trigger a derecognition of the liability. Unamortized financing costs from the senior secured debt amount to Euros 212 million at 30 June 2017 and Euros 154 million at 31 December 2016.
The terms and conditions of the senior secured debt are as follows:
· Tranche A: six year loan divided into two tranches: US Tranche A and Tranche A in Euros.
· US Tranche A:
· Original Principal Amount of US Dollars 2,350 million.
· Applicable margin of 175 basis points (bp) linked to US Libor.
· Quasi-bullet amortization structure.
· Maturity in 2023
· Tranche A in Euros:
· Original Principal Amount of Euros 607 million.
· Applicable margin of 175 basis points (bp) linked to Euribor.
· Quasi-bullet amortization structure.
· Maturity in 2023
The detail of the Tranche A by maturity as at 30 June 2017 is as follows:
|
|
|
US Tranche A |
|
Tranche A in Euros |
| ||||||
|
|
|
|
|
Principal in thousands |
|
Principal in thousands |
|
|
|
Principal in |
|
|
|
|
Currency |
|
of US Dollars |
|
of Euros |
|
Currency |
|
thousands of Euros |
|
|
Maturity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2019 |
|
US Dollars |
|
117,500 |
|
102,962 |
|
Euros |
|
30,350 |
|
|
2020 |
|
US Dollars |
|
235,000 |
|
205,924 |
|
Euros |
|
60,700 |
|
|
2021 |
|
US Dollars |
|
235,000 |
|
205,924 |
|
Euros |
|
60,700 |
|
|
2022 |
|
US Dollars |
|
1,762,500 |
|
1,544,427 |
|
Euros |
|
455,250 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
US Dollars |
|
2,350,000 |
|
2,059,237 |
|
Euros |
|
607,000 |
|
· Tranche B: Senior Debt Loan repayable in eight years.
· US Tranche B :
· Original Principal Amount of US Dollars 3,000 million.
· Applicable margin of 225 basis points (bp) linked to US Libor.
· Quasi-bullet amortization structure.
· Maturity in 2025
The detail of the Tranche B by maturity as at 30 June 2017 is as follows:
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
|
|
US Tranche B |
| ||||
|
|
|
|
|
Principal in thousands of US |
|
Principal in thousands of |
|
|
|
|
Currency |
|
Dollars |
|
Euros |
|
|
Maturity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2017 |
|
US Dollars |
|
15,000 |
|
13,144 |
|
|
2018 |
|
US Dollars |
|
30,000 |
|
26,288 |
|
|
2019 |
|
US Dollars |
|
30,000 |
|
26,288 |
|
|
2020 |
|
US Dollars |
|
30,000 |
|
26,288 |
|
|
2021 |
|
US Dollars |
|
30,000 |
|
26,288 |
|
|
2022 |
|
US Dollars |
|
30,000 |
|
26,288 |
|
|
2023 |
|
US Dollars |
|
30,000 |
|
26,288 |
|
|
2024 |
|
US Dollars |
|
30,000 |
|
26,288 |
|
|
2025 |
|
US Dollars |
|
2,767,500 |
|
2,425,080 |
|
|
|
|
|
|
|
|
|
|
|
Total |
|
US Dollars |
|
2,992,500 |
|
2,622,240 |
|
· US Dollar 300 Million committed credit revolving facility: Amount maturing on 2023 and applicable margin of 175 basis points (bp) linked to US Libor. At 30 June 2017 no amount has been drawn down on this facility.
The total principal plus interest of the Tranche A & B Senior Loan is detailed as follows:
|
|
|
Thousands of Euros |
| ||
|
|
|
Tranche A Senior Loan |
|
Tranche B Senior Loan |
|
|
Maturity |
|
|
|
|
|
|
2017 |
|
63,348 |
|
80,426 |
|
|
2018 |
|
72,071 |
|
116,839 |
|
|
2019 |
|
206,337 |
|
115,923 |
|
|
2020 |
|
343,333 |
|
115,251 |
|
|
2021 |
|
348,182 |
|
114,091 |
|
|
2022 |
|
2,098,683 |
|
113,176 |
|
|
2023 |
|
— |
|
112,260 |
|
|
2024 |
|
— |
|
111,578 |
|
|
2025 |
|
— |
|
2,432,256 |
|
|
|
|
|
|
|
|
|
Total |
|
3,131,954 |
|
3,311,800 |
|
The issue of senior unsecured notes and senior secured debt is subject to compliance with the leverage ratio covenant. At 30 June 2017 the Group complies with this covenant.
Both the Senior Term Loans and the Revolving Loans are guaranteed by Grifols, S.A. and certain significant subsidiaries of Grifols, S.A. that together with Grifols, S.A. represent, in the aggregate, at least 80% of the consolidated assets and consolidated EBITDA of Grifols, S.A. and its subsidiaries.
The Notes have been issued by Grifols, S.A. and are guaranteed on a senior unsecured basis by subsidiaries of Grifols, S.A. that are guarantors and co-borrowers under the New Credit Facilities. Guarantors are Grifols Worldwide Operations Limited, Biomat USA, Inc., Grifols Biologicals Inc., Grifols Shared Services North America, Inc., Grifols Diagnostic Solutions Inc., Grifols Therapeutics, Inc., Instituto Grifols, S.A. Grifols Worldwide Operations USA, Inc. and Grifols USA, Llc.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
(12) Expenses by Nature
Details of wages and other employee benefits expenses by function are as follows:
|
|
|
Thousands of Euros |
| ||||||
|
|
|
Six-Months |
|
Six-Months |
|
Three-Months |
|
Three-Months |
|
|
|
|
Ended 30 June |
|
Ended 30 June |
|
Ended 30 June |
|
Ended 30 June |
|
|
|
|
2017 |
|
2016 |
|
2017 |
|
2016 |
|
|
Cost of sales |
|
368,263 |
|
316,710 |
|
186,343 |
|
154,283 |
|
|
Research and development |
|
45,050 |
|
39,954 |
|
22,954 |
|
19,560 |
|
|
Selling, general & administrative |
|
165,879 |
|
151,180 |
|
83,348 |
|
77,684 |
|
|
expenses |
|
|
|
|
|
|
|
|
|
|
|
|
579,192 |
|
507,844 |
|
292,645 |
|
251,527 |
|
Details of amortisation and depreciation expenses by function are as follows:
|
|
|
Thousands of Euros |
| ||||||
|
|
|
Six-Months |
|
Six-Months |
|
Three-Months |
|
Three-Months |
|
|
|
|
Ended 30 June |
|
Ended 30 June |
|
Ended 30 June |
|
Ended 30 June |
|
|
|
|
2017 |
|
2016 |
|
2017 |
|
2016 |
|
|
Cost of sales |
|
68,142 |
|
63,753 |
|
34,087 |
|
31,443 |
|
|
Research and development |
|
7,062 |
|
6,619 |
|
3,600 |
|
3,219 |
|
|
Selling, general & administrative |
|
31,345 |
|
30,543 |
|
15,548 |
|
15,185 |
|
|
expenses |
|
|
|
|
|
|
|
|
|
|
|
|
106,549 |
|
100,915 |
|
53,235 |
|
49,847 |
|
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
(13) Finance Result
Details are as follows:
|
|
|
Thousands of Euros |
| ||||||
|
|
|
Six-Months |
|
Six-Months |
|
Three-Months |
|
Three-Months |
|
|
|
|
Ended 30 June |
|
Ended 30 June |
|
Ended 30 June |
|
Ended 30 June |
|
|
|
|
2017 |
|
2016 |
|
2017 |
|
2016 |
|
|
Finance income |
|
4,164 |
|
3,924 |
|
2,152 |
|
2,010 |
|
|
Finance cost from Senior Unsecured |
|
(38,221 |
) |
(36,701 |
) |
(19,081 |
) |
(18,130 |
) |
|
Notes |
|
|
|
|
|
|
|
|
|
|
Finance cost from Senior debt |
|
(96,205 |
) |
(84,196 |
) |
(50,008 |
) |
(41,465 |
) |
|
Finance cost from sale of receivables (note 9) |
|
(1,908 |
) |
(2,765 |
) |
(943 |
) |
(1,086 |
) |
|
Capitalised interest |
|
5,429 |
|
4,936 |
|
2,676 |
|
2,489 |
|
|
Other finance costs |
|
(4,582 |
) |
(5,345 |
) |
(2,137 |
) |
(2,649 |
) |
|
Finance costs |
|
(135,487 |
) |
(124,071 |
) |
(69,493 |
) |
(60,841 |
) |
|
Change in fair value of financial derivatives |
|
— |
|
(7,426 |
) |
— |
|
(2,870 |
) |
|
Impairment financial instruments (note 8) |
|
(5,500 |
) |
— |
|
— |
|
— |
|
|
Exchange differences |
|
(10,760 |
) |
3,409 |
|
(14,017 |
) |
6,103 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance result |
|
(147,583 |
) |
(124,164 |
) |
(81,358 |
) |
(55,598 |
) |
(14) Taxation
Income tax expense is recognised based on management’s best estimate of the weighted average annual income tax rate expected for the full financial year applied to the pre-tax income of the interim period. The Group’s consolidated effective tax rate has increased from 23,5% for the six-month period ended 30 June 2016 to 27 % for the six-month period ended 30 June 2017 mainly due to a change of country mix of profits.
The following events have arisen regarding income tax audits during the six-month period ended 30 June 2017:
· Grifols Share Services North America, Inc: Income Tax Audit for the tax year ending, 2015 was initiated from May, 2017.
The Group does not expect any significant impact affecting the financial statements to arise from these tax audits.
(15) Discontinued operations
The Group does not consider any operations as discontinued for the six-month period ended 30 June 2017 and 2016.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
(16) Contingencies and Commitments
(a) Contingencies
There have been no significant changes to the Group’s contingencies during the six month period ended 30 June 2017.
(b) Commitments
· Restricted Share Unit Retention Plan
For the annual bonus, the Group established a Restricted Share Unit Retention Plan (RSU Plan), for eligible employees. By these plans, the employee could elect to receive up to 50% of its yearly bonus in non-voting Class B ordinary shares (Grifols Class B Shares) or Grifols American Depositary Shares (Grifols ADS), and the Group will match with an additional 50% of the employee election of RSUs (additional RSUs).
Grifols Class B Shares and Grifols ADS are valued at grant date.
These RSUs will have a vesting period of 2 years and 1 day and, subsequently, the RSU’s will be exchanged for Grifols Class B Shares or Grifols ADS (American Depositary Share representing 1 Class B Share).
If an eligible employee leaves the Company or is terminated before the vesting period, he will not be entitled to the additional RSU.
At 30 June 2017, the Group has settled the RSU plan of 2014 for an amount of Euros 7,303 thousand.
This commitment is treated as equity-settled and the accumulated amount recognized as at 30 June 2017 as share based payments costs of employees is Euros 11,901 thousand (Euros 10,594 thousand at December 2016).
(17) Related Parties
Transactions with related parties have been performed as part of the Group’s ordinary course of business and have been performed at arm’s length.
Group transactions with related parties during the six-months ended 30 June 2017 were as follows:
|
|
|
Thousands of Euros |
| ||||||
|
|
|
|
|
Key management |
|
Other related |
|
Board of directors |
|
|
|
|
Associates |
|
personnel |
|
parties |
|
of the company |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales |
|
1,646 |
|
— |
|
— |
|
— |
|
|
Purchases of inventory |
|
(30,203 |
) |
— |
|
— |
|
— |
|
|
Other service expenses |
|
(5,838 |
) |
— |
|
(3,595 |
) |
(457 |
) |
|
Operating leases expenses |
|
— |
|
— |
|
(2,855 |
) |
— |
|
|
Remuneration |
|
— |
|
(6,741 |
) |
— |
|
(1,938 |
) |
|
Financial costs / income |
|
853 |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(33,542 |
) |
(6,741 |
) |
(6,450 |
) |
(2,395 |
) |
Group transactions with related parties during the six-months ended 30 June 2016 were as follows:
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
|
|
|
Thousands of Euros |
| ||||||
|
|
|
|
|
Key management |
|
Other related |
|
Board of directors |
|
|
|
|
Associates |
|
personnel |
|
parties |
|
of the company |
|
|
Purchases of inventory |
|
(7,011 |
) |
— |
|
— |
|
— |
|
|
Other service expenses |
|
(3,067 |
) |
— |
|
(2,600 |
) |
(454 |
) |
|
Operating leases expenses |
|
— |
|
— |
|
(2,496 |
) |
— |
|
|
Remuneration |
|
— |
|
(4,288 |
) |
— |
|
(1,577 |
) |
|
Financial income |
|
1,294 |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(8,784 |
) |
(4,288 |
) |
(5,096 |
) |
(2,031 |
) |
Group transactions with related parties during the three-months period ended 30 June 2017 were as follows:
|
|
|
Thousands of Euros |
| ||||||
|
|
|
|
|
Key management |
|
Other related |
|
Board of directors |
|
|
|
|
Associates |
|
personnel |
|
parties |
|
of the company |
|
|
Net sales |
|
1,099 |
|
— |
|
— |
|
— |
|
|
Purchases of inventory |
|
(13,298 |
) |
— |
|
— |
|
— |
|
|
Other service expenses |
|
(2,752 |
) |
— |
|
(1,753 |
) |
(231 |
) |
|
Operating leases expenses |
|
— |
|
— |
|
(1,594 |
) |
— |
|
|
Remuneration |
|
— |
|
(3,423 |
) |
— |
|
(1,091 |
) |
|
Financial costs / income |
|
454 |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(14,497 |
) |
(3,423 |
) |
(3,347 |
) |
(1,322 |
) |
Group transactions with related parties during the three-months period ended 30 June 2016 were as follows:
|
|
|
Thousands of Euros |
| ||||||
|
|
|
|
|
Key management |
|
Other related |
|
Board of directors |
|
|
|
|
Associates |
|
personnel |
|
parties |
|
of the company |
|
|
Purchases of inventory |
|
(7,011 |
) |
— |
|
— |
|
— |
|
|
Other service expenses |
|
(1,737 |
) |
— |
|
(1,300 |
) |
(226 |
) |
|
Operating leases expenses |
|
— |
|
— |
|
(1,248 |
) |
— |
|
|
Remuneration |
|
— |
|
(1,826 |
) |
— |
|
(789 |
) |
|
Financial costs / income |
|
729 |
|
— |
|
— |
|
— |
|
|
|
|
(8,019 |
) |
(1,826 |
) |
(2,548 |
) |
(1,015 |
) |
The Group has not extended any advances or loans to the members of the board of directors or key management personnel nor has it assumed any guarantee commitments on their behalf. It has also not assumed any pension or life insurance obligations on behalf of former or current members of the board of directors or key management personnel. In addition, as disclosed in note 29(c) of the consolidated financial statements as at and for the year ended 31 December 2016, certain Company directors and key management personnel are entitled to termination benefits.
GRIFOLS, S.A. AND SUBSIDIARIES
Notes to Condensed Consolidated Interim Financial Statements
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails)
(18) Subsequent Events
a) GigaGen acquisition
On 5 July 2017 Grifols, through its 100% subsidiary Grifols Innovation and New Technologies Limited (“GIANT”), has acquired a 43.96% shareholding in GigaGen, Inc., a company based in San Francisco (USA) for the amount of US Dollars 35 million.
GIANT and GigaGen have also entered into a Research and Collaboration Agreement whereby in exchange of a collaboration fee of US Dollars 15 million in the aggregate, GigaGen will commit to carry out research activities to develop recombinant polyclonal immunoglobulin therapies derived from human B cells for the treatment of human diseases.
b) Kiro Grifols additional acquisition
On 25 July 2017, Grifols has acquired an additional 40% equity stake in Kiro Grifols, S.L. for a purchase price of EUR 12.8 million. In September 2014, Grifols subscribed to a capital increase by virtue of which Grifols acquired 50% of Kiro Grifols´ economic and voting rights. With this new acquisition, Grifols has reached a 90% stake in the equity of Kiro Grifols. The remaining 10% will continue to be held by Socios Fundadores Kiro, S.L. a company wholly owned by cooperatives of the Mondragon Corporation.
GRIFOLS, S.A. AND SUBSIDIARIES
Consolidated Management Report
for the six-month period ended 30 June 2017
(Free translation from the original in Spanish. In the event of discrepancy, the Spanish language version will prevail)
You are encouraged to read the following discussion and analysis of Grifols’ financial condition and results of operations together with their six months period ended June 30 2017 condensed consolidated interim financial statements and related footnotes. This discussion and analysis may contain forward-looking statements that involve risks and uncertainties. See the section “Cautionary Statement Regarding Forward-Looking Statements” included in this document.
RESULTS OF OPERATIONS
Six months ended June 30, 2017 compared to six months ended June 30, 2016
Key financial figures — First Half 2017
Grifols increased its net revenue by 12.3% (9.0% cc(1)) in the first half of 2017 to reach EUR 2,192.4 million. The company reported significant revenue increases in all of its divisions and geographical areas where it operates.
Grifols is one of the world top three producers of plasma-derived therapies. The Bioscience Division sales reached EUR 1,759.9 million in the first half, which represents a 14.4% (10.9% cc) increase with respect to the same period in 2016.
Global demand for plasma proteins, which has remained strong as projected in previous periods, coupled with price increases in some markets, have exceptionally boosted revenue of Grifols main plasma-derived products.
Diagnostic Division sales increased by 10.8% (7.8% cc) to EUR 351.1 million, compared to EUR 316.8 million reported in the same period in 2016. The worldwide leader in transfusion medicine, Grifols continued to notably boost sales revenues for virological screening of blood donations. Higher sales of both its NAT technology systems (Procleix® NAT Solutions) in core markets such as the U.S., China and Japan, and its antigens used to manufacture diagnostic immunoassays led to the increase.
The Hospital Division reached sales of EUR 47.9 million, representing a 3.0% (1.9% cc) increase, driven largely by favorable activity growth of equipment and systems for hospital logistics (Pharmatech) in global markets.
As of January 2017, the company also has a new unit - the Bio Supplies Division - that primarily oversees sales of biological products for non-therapeutic use. The division reported revenues of EUR 32.1 million for the first half of 2017 and growth of 14.6% (11.4% cc), following the agreement reached with Access Biologicals to commercialize Grifols biological products for non-therapeutic use, as well as income derived from its contract with Kedrion.
The adjusted EBITDA(2) for the first half of 2017 increased by 19.9% to EUR 663.9 million, which represents a 30.3% margin. Taking into account the non-recurring costs associated with the acquisition of Hologic NAT donor-screening unit, Grifols EBITDA from January to June 2017 is EUR 644.4 million, which represents a 29.4% margin.
In line with company forecasts, Grifols acquisition of Hologic share of the NAT donor-screening unit at the beginning of 2017 has had a positive impact on the group margins. The EBITDA margin continues to reflect the effect of increased plasma costs related mainly to the expansion of plasma donation centers.
(1) CC: Constant Currency.
(2) Excludes non-recurring costs and associated with recent acquisitions.
Grifols is the global leader in plasma donation centers, with a network of over 180 centers throughout the U.S. During the first half of the year, the company continued to invest in opening new centers, as well as allocating resources to expand, renovate and relocate existing ones. Grifols aims to have 190 centers by the end of the fiscal year and 230 centers by 2019.
The company also intensified its total net investments in R&D+i, which increased by 19.0% compared to the same period last year, to EUR 129.3 million. This figure includes both internal and investee projects, which are managed through Grifols Innovation and New Technologies Limited (GIANT). Grifols continues to make significant progress in R&D+i through its investee companies, the most recent of which is GigaGen. The company acquired a 44% stake of this U.S. firm for USD 35 million after the close of the period. Headquartered in San Francisco (California, U.S.), GigaGen specializes in the development of biotherapeutic therapies.
Grifols financial result was EUR 147.6 million, compared to EUR 124.2 million reported for the same period last year. The refinancing process has enabled the company to optimize its financial expenses resulting from the higher levels of debt assumed to acquire Hologic share of the NAT donor-screening unit. In the second quarter, exchange-rate differences had a negative impact of EUR 14.0 million.
Grifols effective tax rate was 27.0% in line with the figure stated in the first quarter of 2017. It exceeds the rate reported in the same period last year, due mainly to the higher profits generated by the Bioscience and Diagnostic Divisions in the U.S. market.
The adjusted net profit(3) reached EUR 330.2 million, increasing 12.2% in relation to the EUR 294.2 million for the same period last year. The reported net profit rose by 5.1% to EUR 277.9 million, which represents 12.7% of the company net revenue.
At the close of the first half of 2017, Grifols net financial debt was EUR 5,440.5 million, including EUR 750.2 million in cash, after taking into account the acquisition of a 49% stake in Access Biologicals for USD 51 million, the acquisition of six plasma centers from Kedplasma for USD 47 million, and deducting EUR 95.3 million corresponding to the final dividend for the 2016 fiscal year, which was approved in the Ordinary General Shareholders Meeting.
This final dividend, distributed in June 2017 (Euros 0.1356 gross dividend per share) and the one paid in December 2016 (Euros 0.18 gross dividend per share), amount to a total of EUR 218.2(4) million paid in dividends for the 2016 fiscal year and a sustained payout of 40% of consolidated net profit. Grifols dividends have had an annual compound growth of 16% over the last four years, evidence of the company commitment to its shareholders.
Grifols net debt ratio is 4.10x EBITDA, lower than the 4.45x recorded in March 2017. Continued efforts to reduce its levels of financial leverage remain a priority for the company.
As of June 30, 2017, Grifols had EUR 420 million in undrawn credit lines and its liquidity position was approximately EUR 1,200 million.
Grifols cash generation remains high, providing the necessary solvency for the company to advance its expansion and investment plans, and continue its deleveraging process. The operating cash flow in the first half of 2017 was EUR 378.6 million, compared to EUR 166.9 million reported for the same period last year. Operating cash-flow generation remains high, taking into account the higher inventory levels related to higher volume of sales.
(3) Excludes non-recurring costs and associated with recent acquisitions, amortization of deferred expenses associated to the refinancing and amortization of intangible assets related to acquisitions.
(4) Includes the preferred dividend of Euros 0.01 gross per share associated with each Class B share.
As of June 2017, total consolidated assets rose to EUR 11,117.0 million, compared to EUR 10,129.8 million in December 2016. The increase stems primarily from the new assets acquired from the purchase of Hologic share of the NAT donor-screening unit, although it was partially offset by the effect of the Euro appreciation against the U.S. dollar.
Key financial metrics for the first half of 2017:
|
In millions of euros except % and EPS |
|
1H 2017 |
|
1H 2016 |
|
% Var |
|
|
NET REVENUE (NR) |
|
2,192.4 |
|
1,951.6 |
|
12.3 |
% |
|
GROSS MARGIN |
|
50.3 |
% |
48.3 |
% |
|
|
|
EBITDA |
|
644.4 |
|
553.6 |
|
16.4 |
% |
|
% NR |
|
29.4 |
% |
28.4 |
% |
|
|
|
ADJUSTED EBITDA (1) |
|
663.9 |
|
553.6 |
|
19.9 |
% |
|
% NR |
|
30.3 |
% |
28.4 |
% |
|
|
|
EBIT |
|
537.8 |
|
452.7 |
|
18.8 |
% |
|
% NR |
|
24.5 |
% |
23.2 |
% |
|
|
|
GROUP PROFIT |
|
277.9 |
|
264.4 |
|
5.1 |
% |
|
% NR |
|
12.7 |
% |
13.5 |
% |
|
|
|
ADJUSTED(2) GROUP PROFIT |
|
330.2 |
|
294.2 |
|
12.2 |
% |
|
% NR |
|
15.1 |
% |
15.1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
CAPEX |
|
135.3 |
|
112.5 |
|
20.3 |
% |
|
R&D NET INVESTMENT |
|
129.3 |
|
106.0 |
|
19.0 |
% |
|
EARNINGS PER SHARE (EPS) |
|
0.41 |
|
0. 39 |
|
5.1 |
% |
|
|
|
June 2017 |
|
December 2016 |
|
% Var |
|
|
TOTAL ASSETS |
|
11,117.0 |
|
10,129.8 |
|
9.7 |
% |
|
TOTAL EQUITY |
|
3,584.8 |
|
3,728.0 |
|
(3.8 |
)% |
|
CASH & CASH EQUIVALENTS |
|
750.2 |
|
895.0 |
|
(16.2 |
)% |
|
LEVERAGE RATIO |
|
4.10/(4.43cc)(3) |
|
3.55/(3.45cc)(3) |
|
|
|
(1) Excludes non-recurring costs and associated with recent acquisitions
(2) Excludes non-recurring costs and associated with recent acquisitions, amortization of deferred expenses associated to the refinancing and amortization of intangible assets related to acquisitions
(3) Constant currency (cc) excludes the impact of exchange rate movements. 2016 reported figures: not including the NAT assets debt acquisition
Group Net Profit Reconciliation for the first half of 2017:
|
In millions of euros |
|
1H 2017 |
|
1H 2016 |
|
% Var |
|
|
GROUP NET PROFIT |
|
277.9 |
|
264.4 |
|
5.1 |
% |
|
% NR |
|
12.7 |
% |
13.5 |
% |
|
|
|
Amortization of deferred financial expenses |
|
33.5 |
|
18.7 |
|
79.2 |
% |
|
Amortization of intangible assets acquired in business combinations |
|
18.7 |
|
20.2 |
|
(7.5 |
)% |
|
Non-recurring costs and associated with recent acquisitions |
|
19.5 |
|
— |
|
|
|
|
Tax impacts of adjustments |
|
(19.4 |
) |
(9.1 |
) |
112.7 |
% |
|
ADJUSTED GROUP NET PROFIT |
|
330.2 |
|
294.2 |
|
12.2 |
% |
|
% NR |
|
15.1 |
% |
15.1 |
% |
|
|
REVENUE PERFORMANCE
· Bioscience Division: 80.3% of total revenues
The Bioscience Division is the company main driver of growth. In the second quarter of 2017, demand for the main plasma proteins remained strong, as anticipated in previous periods. During the first half of the year, greater market demand led to a 14.4% (10.9% cc) growth in revenues to EUR 1,759.9 million. Increased sales volumes of the main plasma proteins and a positive price impact in some markets have propelled this growth.
Immunoglobulin sales were one of the growth drivers in the first half. Demand for this plasma protein continues to be robust, supported by growth in the United States, Canada and certain core markets in the European Union (EU). Immunoglobulin use in the neurology field continued its upward trend, particularly in markets with higher per capita consumption like the U.S. and Canada. Grifols also remains steadfast in its efforts to promote the use of this protein in the treatment of primary immunodeficiencies. In this regard, demand has risen significantly in specific markets in Latin America and the Asia Pacific that are currently expanding their healthcare coverage.
Grifols is the leader in the manufacture and sales of alpha-1 antitrypsin. The on-going improvement in the diagnosis of alpha-1 antitrypsin deficiency (AATD) in the U.S. and Europe, and more incipiently in Latin America, continue to be the main driver of growth, as the number of undiagnosed patients is high. Grifols also promotes disease-management programs for patients with this genetic disorder, whose symptoms are similar to those of chronic obstructive pulmonary disease (COPD). In this regard, the company continues its efforts to develop new formulations to have differentiated products that widen the treatment options for its patients. Grifols has developed a liquid formulation of its alpha 1-antitrypsin that, once approved, will expand the portfolio of products.
Meanwhile, albumin sales continue to grow, particularly in China where demand remains high and in certain countries in Europe and Asia Pacific.
Sales of factor VIII continue to grow, most notably in the U.S. for the treatment of hemophilia A including patients with inhibitors. The company also heightened its role in the treatment of previously untreated patients with severe hemophilia A, a shift mainly driven by the results of the SIPPET(5) study (Survey of Inhibitors in Plasma Products Exposed Toddlers) released last year.
(5) The SIPPET study demonstrated that treatment with recombinant factor VIII (rFVIII) is associated with an 87% greater incidence of inhibitors than when using plasma-derived factor VIII with von Willebrand factor (pdFVIII/VWF) in previously untreated patients with severe hemophilia A.
The group remains committed to offering other specialty proteins that enhance its differentiated portfolio of products, optimizing raw material costs and production capacities, and delivering added value for patients. Of note is the upturn in sales of hyperimmune immunoglobulins used to treat rabies and tetanus. In addition, Grifols signed an exclusive 10-year contract with the U.S. firm Ethicon for the manufacture and supply of plasma-derived therapies used for biosurgery (biologic fibrin sealant). This agreement is extendible for 5-year periods and subject to product authorization by regulatory bodies.
· Diagnostic Division: 16.0% of total revenues
The Diagnostic Divisions continues its positive revenue trend, achieving EUR 351.1 million in sales and growth of 10.8% (7.8% cc) compared to the same period last year.
There was a significant uptick in sales of NAT technology systems (Procleix® NAT Solutions), used for the virological screening of blood and plasma donations. This boost in revenues was triggered mainly by greater market penetration in the Asia Pacific and the U.S. rollout of a Zika virus screening test, which obtained FDA authorization for Investigational New Drug (IND) use in 2016. Since May 2017, this test is also available in blood banks that accept the CE Mark, as the product complies with the legislation for commercialization in the European Economic Area.
As the market leader in the NAT segment, Grifols is equipped to meet the demand in new markets that opt to include this analysis in their blood and plasma donations processes as their healthcare systems develop. In addition, the division continues to develop new tests for emerging viruses. In the second quarter of the year, the FDA granted IND status for a new test for babesiosis to be applied in U.S. blood banks. The test detects the presence of the four species of the babesiosis parasite that can be transmitted to humans.
Sales of antigens used in the production of diagnostic immunoassays, marketed within the framework of its joint-business agreement with Ortho Clinical Diagnostics, also contributed to the division upturn in revenues. In addition, the company signed a five-year extension of its agreement with OraSure Technologies, a leader in infectious disease diagnostic tests. In this way, Grifols strengthens its position as a global, flexible provider of antigens with scalable capabilities. The group continues the approval process of its new plant in Emeryville, California, which will optimize and boost its productive capacity.
Grifols continues to expand the presence of its blood typing line in the U.S. market. In the second quarter of the year, the company launched Erytra Eflexis®, a fully automatic, mid-sized analyzer that performs pre-transfusion compatibility tests using DG Gel® technology. The system optimizes workflow efficiency and improves daily workloads by allowing laboratories to adapt the system to their specific needs.
In addition to its progress in transfusional medicine, Grifols reinforced its position in specialty diagnostics.
In order to increase its presence in the hemostasis field and support its global expansion strategy, the company signed an exclusive distribution agreement with Beckman Coulter, a leading provider of diagnostics solutions. The long-term agreement includes the global distribution of Grifols hemostasis instruments, reagents and consumables. The commercialization of these systems under this agreement is expected in Europe in early 2018.
In terms of expanding its specialty diagnostics product portfolio, during the second quarter Grifols added a new diagnostic test based on the human genomic DNA ID RhD XT, which enables the molecular detection of the most relevant variants of the RhD gene that determine Factor D, of particular importance in pregnant women. Furthermore, in May 2017, the company CLIA-certified laboratory in San Marcos, (Texas, U.S.) launched a series of test under the TDMonitor brand. The new tests offered are used to monitor biological therapies.
· Hospital Division: 2.2% of total revenues
The Hospital Division increased sales by 3.0% (1.9% cc) to EUR 47.9 million, driven primarily by positive growth of its Pharmatech line (including hospital logistics) in certain Latin American markets and in the U.S. It also expanded its third-party manufacturing services.
The U.S. is a critical market for the division growth strategy. In the first half of the year, Grifols saline solution produced in its Murcia, Spain facility, received FDA approval allowing the company to market its IV solution in the U.S. The approval also guarantees the group self-sufficiency since its U.S. network of plasma-donation centers will use the product to restore donors’ circulatory volume.
As Grifols continues to drive the internationalization of its Hospital Division, this approval opens up new opportunities for future authorizations to sell other products manufactured in its Barcelona and Murcia plants. It also confirms the company strategy to promote complementarity of its products and services among business divisions.
· Bio Supplies Division: 1.5% of total revenues
From January 2017, revenues previously recorded in Raw Materials are now part of the new Bio Supplies Division. The division also encompasses sales of biological products for non-therapeutic use and other biologicals, and income derived from its manufacturing agreement with Kedrion.
Division sales reached EUR 32.1 million in the first half of 2017, compared to EUR 28.0 million reported the previous year.
In order to strengthen this business line, Grifols acquired a 49% stake in Access Biologicals in January of 2017. As part of this agreement, Grifols also signed a supply contract with Access Biologicals to sell its biological products for non-therapeutic use.
First half 2017 net revenue by division:
|
|
|
|
|
% of Net |
|
|
|
% of Net |
|
|
|
|
|
|
In thousands of euros |
|
1H 2017 |
|
Revenues |
|
1H 2016** |
|
Revenues |
|
% Var |
|
% Var cc* |
|
|
BIOSCIENCE |
|
1,759,852 |
|
80.3 |
% |
1,538,805 |
|
78.8 |
% |
14.4 |
% |
10.9 |
% |
|
DIAGNOSTIC |
|
351,051 |
|
16.0 |
% |
316,830 |
|
16.2 |
% |
10.8 |
% |
7.8 |
% |
|
HOSPITAL |
|
47,866 |
|
2.2 |
% |
46,478 |
|
2.4 |
% |
3.0 |
% |
1.9 |
% |
|
BIO SUPPLIES |
|
32,072 |
|
1.5 |
% |
27,976 |
|
1.4 |
% |
14.6 |
% |
11.4 |
% |
|
OTHERS |
|
1,606 |
|
0.0 |
% |
21,556 |
|
1.2 |
% |
(92.5 |
)% |
(92.6 |
)% |
|
TOTAL |
|
2,192,447 |
|
100.0 |
% |
1,951,645 |
|
100.0 |
% |
12.3 |
% |
9.0 |
% |
* Constant currency (cc) excludes the impact of exchange rate movements
** Comparable net revenues considering the reclassification of the biological products for non-therapeutic use sales that since January of 2017 are reported in the Bio Supplies Division
First half 2017 net revenue by region:
|
|
|
|
|
% of Net |
|
|
|
% of Net |
|
|
|
|
|
|
In thousands of euros |
|
1H 2017 |
|
Revenues |
|
1H 2016** |
|
Revenues |
|
% Var |
|
% Var cc* |
|
|
US + CANADA |
|
1,494,131 |
|
68.2 |
% |
1,292,192 |
|
66.2 |
% |
15.6 |
% |
11.6 |
% |
|
EU |
|
338,288 |
|
15.4 |
% |
327,813 |
|
16.8 |
% |
3.2 |
% |
3.8 |
% |
|
ROW |
|
360,028 |
|
16.4 |
% |
331,640 |
|
17.0 |
% |
8.6 |
% |
4.3 |
% |
|
TOTAL |
|
2,192,447 |
|
100.0 |
% |
1,951,645 |
|
100.0 |
% |
12.3 |
% |
9.0 |
% |
* Constant currency (cc) excludes the impact of exchange rate movements
** Comparable considering the new divisional structure
SECOND QUARTER 2017
· Sales increase in the main divisions and geographic regions
Grifols reported revenues of EUR 1,130.8 million in the second quarter of 2017, compared to EUR 992.7 million for the same period in 2016, which represents growth of 13.9% (10.3% cc). Sales performance has been particularly positive in all four of its main divisions, as well as in the regions where it operates.
The Bioscience Division acted as the main growth engine, increasing its revenue by 13.7% (10.0% cc) to reach EUR 906.2 million. The increase in immunoglobulin sales in the U.S. and Canada, alpha-1 antitrypsin sales growth in North America and Europe and albumin sales in China, were especially remarkable. The company revenues from its differentiated specialty products, such as its hyperimmune immunoglobulins have also seen a marked increase.
The second quarter also includes the income derived from the agreement reached with the Spanish Ministry of Health to meet the country supply needs for tetanus and diphtheria (TD) vaccinations. These sales were made possible through a sales agreement between Grifols and MassBiologics of the University of Massachusetts Medical School (U.S).
The Diagnostic Division revenues rose sharply by 15.8% (12.5% cc) in the second quarter to EUR 180.4 million. The division continues to build on the growth reported in the first quarter of the year, fueled primarily by an upsurge in sales of its NAT technology systems - especially for the Zika screening test - as well as an increase in sales of antigens used in the manufacture of immunoassays.
The Hospital Division grew by 5.3% (4.1% cc) to EUR 24.9 million, while Bio Supplies grew by 70.9% (65.7% cc), with sales of EUR 17.7 million. Since January 2017, this division includes sales of biological products for non-therapeutic use, as well as income generated from the Kedrion agreement.
In comparison to the previous year, the company has significantly increased sales in all regions. Revenues in the U.S. and Canada reached EUR 765.6 million, denoting a 16.5% (12.1% cc). The company also reported higher revenues in the rest of the world (ROW), with a 10.7% (6.3% cc) growth to reach close to EUR 189 million, and a recovery in the European Union, with sales of EUR 176.5 million and growth of 6.9% (7.4% cc).
Second quarter 2017 net revenues by division:
|
|
|
|
|
% of Net |
|
|
|
% of Net |
|
|
|
|
|
|
In thousands of euros |
|
2Q 2017 |
|
Revenues |
|
2Q 2016 ** |
|
Revenues |
|
% Var |
|
% Var cc* |
|
|
BIOSCIENCE |
|
906,213 |
|
80.1 |
% |
796,946 |
|
80.3 |
% |
13.7 |
% |
10.0 |
% |
|
DIAGNOSTIC |
|
180,408 |
|
16.0 |
% |
155,790 |
|
15.7 |
% |
15.8 |
% |
12.5 |
% |
|
HOSPITAL |
|
24,902 |
|
2.2 |
% |
23,640 |
|
2.4 |
% |
5.3 |
% |
4.1 |
% |
|
BIO SUPPLIES |
|
17,671 |
|
1.6 |
% |
10,342 |
|
1.0 |
% |
70.9 |
% |
65.7 |
% |
|
OTHERS |
|
1,573 |
|
0.1 |
% |
5,994 |
|
0.6 |
% |
(73.8 |
)% |
(75.1 |
)% |
|
TOTAL |
|
1,130,767 |
|
100.0 |
% |
992,712 |
|
100.0 |
% |
13.9 |
% |
10.3 |
% |
* Constant currency (cc) excludes the impact of exchange rate movements
** Comparable net revenues considering the reclassification of the biological products for non-therapeutic use sales that since January of 2017 are reported in the Bio Supplies Division
Second quarter 2017 net revenues by region:
|
|
|
|
|
% of Net |
|
|
|
% of Net |
|
|
|
|
|
|
In thousands of euros |
|
2Q 2017 |
|
Revenues |
|
2Q 2016 ** |
|
Revenues |
|
% Var |
|
% Var cc* |
|
|
US + CANADA |
|
765,561 |
|
67.7 |
% |
657,219 |
|
66.2 |
% |
16.5 |
% |
12.1 |
% |
|
EU |
|
176,541 |
|
15.6 |
% |
165,105 |
|
16.6 |
% |
6.9 |
% |
7.4 |
% |
|
ROW |
|
188,665 |
|
16.7 |
% |
170,388 |
|
17.2 |
% |
10.7 |
% |
6.3 |
% |
|
TOTAL |
|
1,130,767 |
|
100.0 |
% |
992,712 |
|
100.0 |
% |
13.9 |
% |
10.3 |
% |
* Constant currency (cc) excludes the impact of exchange rate movements
** Comparable considering the new divisional structure
INVESTMENT ACTIVITIES: R&D+INNOVATION, ACQUISITIONS AND CAPEX
· Grifols allocates nearly EUR 130 million toward R&D+i in the first half
The company allocated EUR 129.3 million for R&D+i activities in the first half of 2017, taking into account net internal investments and external investments carried out through its investee companies. This figure represents a 19.0% increase with respect to the same period in 2016 and 5.9% of revenues.
During the second quarter of 2017, Grifols investee company, Araclon Biotech initiated the phase II clinical trial for an Alzheimer vaccine after receiving authorization from the Spanish Agency of Medicinal Products and Medical Devices. Phase II aims to establish guidelines for product dosage, as well as corroborate the data collected in phase I concerning product safety and tolerability.
· New research lines through investee companies: acquisition of a 44% stake in GigaGen for USD 35 million
Headquartered in San Francisco, GigaGen is a biopharmaceutical firm specialized in the development of pre-clinical biotherapeutic therapies that use human B-cells(6) to capture the genetic diversity of antibodies and transform them into therapies to treat severe diseases. Specifically, GigaGen discovers and develops innovative recombinant monoclonal and polyclonal antibody therapies.
In addition to the financial transaction, Grifols and GigaGen have entered into a research and collaboration agreement whereby, in exchange of a collaboration fee of USD 15 million in the aggregate, GigaGen will commit to carry out research activities to develop a recombinant polyclonal immunoglobulin drug product derived from human B cells for the treatment of any human diseases.
With this transaction, Grifols further strengthens its portfolio of R&D+i projects, which includes holdings in research projects and companies that complement its activity and hold the potential of generating added value for the group. The acquisition has been carried out through Grifols Innovation and New Technologies (GIANT), responsible for overseeing the company external R&D+i investments. The transaction was completed after June 30, 2017.
· Capital Investments (CAPEX)
In the first six months of the year, Grifols invested EUR 135.3 million to enhance and expand the production facilities of its four divisions. These on-going investments progress as outlined in the 2016-2020 Capital Investment Plan, endowed with EUR 1,200 million to guarantee the company long-term sustainable growth.
Of strategic importance are Grifols investments to increase the supply of plasma, including the opening of new donation centers in the U.S., as well as the expansion, renovation and relocation of existing centers. Grifols is currently the market leader, with a network of more than 180 plasma collection centers in the U.S. that it aims to expand to 230 centers by 2019.
In accordance to plans, the company continues to make progress on the construction of new facilities for plasma fractionation, and the purification and sterile filling of plasma proteins.
CORPORATE RESPONSIBILITY
· Human Resources: more employees and more development
Grifols currently has 16,808 employees, representing a 13.0% growth in the first half of the year with respect to the close of the 2016 fiscal year. The employee base grew in all geographic regions, especially in North America, where headcount increased by 16.6%. Grifols continues to generate employment in Spain, where the workforce grew by 3.2% to 3,539 employees. Meanwhile, headcount in the rest of the world (ROW) grew by 7.8%.
The average seniority of Grifols personnel is 6.1 years and the average age is 38.3, although 57% is aged 40 or younger. The gender breakdown (44% men and 56% women) reflects a balanced workforce.
Health and safety and training and development are the cornerstones of the company human resource strategy. In terms of health, the company is promoting awareness of healthy lifestyle habits as a preventative measure.
(6) Type of white blood cell that makes antibodies. B cells are part of the immune system and develop from stem cells in the bone marrow. They are also known as B-lymphocytes.
In terms of training and development, of particular note are the new-employee integration programs; performance-review programs; and a five-year leadership development program aimed at 1,500 mid-level managers and directors throughout the world. In addition, the company has expanded other programs, including technical development and training on concrete issues such as good manufacturing practices (GMP), compliance and the corporate equality plan, among others.
· 2017-2019 Environmental Program
Grifols initiated the implementation of its 2017-2019 Environmental Plan, whose main objectives are reducing the electrical consumption in its production facilities by 8.3 million kWh per year; decreasing its natural-gas consumption by 20.6 million kWh per year; and cutting its annual water consumption by 265,000 cubic meters. The plan also aims to increase the recovery of waste by 270 tons per year.
External audits according to the ISO 14001 standard were carried out in the first half of the year in the company facilities in Spain and in Clayton, (North Carolina, U.S.) with satisfactory results. The company plans to extend this certification to its Diagnostic Division facilities in Emeryville and the Bioscience Division plant in Los Angeles (California, U.S.).
In June, Grifols submitted the questionnaire to participate in the 2016 Carbon Disclosure Project (CDP), a program that evaluates the organization strategy and progress on the issue of climate change. Included in the information provided is the calculation of Grifols carbon footprint, estimated at 292,437 tons of CO2 equivalent emissions. This figure is similar to the increase in production at its various facilities.
· Transparency: Grifols voluntarily discloses transfers of value to health professionals and healthcare organizations in Europe made in 2016
Grifols voluntarily adopted the Code of Conduct on Industry Interactions with Healthcare Professionals and Healthcare Organizations of the European Federation of Pharmaceutical Industries and Associations (EFPIA) in 2015. In 2016, the company disclosed, for the second consecutive year, all of its payments and other transfers of value to health professionals and health sector organizations in 33 European countries, including Spain.
The EFPIA Code applies specifically to medicines; nonetheless, Grifols opted to expand its scope of application to include transfers unrelated to medicines, as well as to the company three divisions: Bioscience, Diagnostic and Hospital. Grifols applies this transparency policy in the United States as required by the regulatory body (Centers for Medicaid and Medicare Services, or CMS) and, in addition to Europe, also plans to implement it in countries such as Australia and Japan.
· Annual Capital Markets Day
Grifols held its annual meeting for analysts and investors in Emeryville at the beginning of June. The company senior executives presented status overviews of the main divisions, investment plans and key research projects, followed by an in-depth analysis of Grifols financials. The meeting included a tour of the Diagnostics Division new production facilities in Emeryville.
· Our commitment to patients: Grifols to support Alpha-1 Foundation John W. Walsh Research Fund
Grifols awarded a USD 1 million grant to support the John. W. Walsh Research Fund, dedicated to promoting research that will improve the health of patients with Alpha-1 antitrypsin deficiency. The program supports basic science and clinical research, improved understanding of the pathogenesis of the clinical manifestations of AATD, the development and testing of treatments for the disease, bioethics and social research, and the promotion of education of members of the medical community regarding AATD. This announcement is a continuation of Grifols longstanding commitment to improving the lives of patients worldwide.
Our commitments to patients: 140 million international units (IU) of blood clotting factors donated to the World Federation of Hemophilia (WFH) Humanitarian Aid Program
This donation represents Grifols most significant contribution to the WFH Humanitarian Aid Program to date. According to the WFH, the donation ensures around 10,300 doses until 2021 to treat approximately 6,000 patients in emerging countries where access to adequate healthcare is limited or non-existent.
For over a decade, Grifols has been a proud supporter of the WFH and its efforts to improve access to treatment of bleeding disorders around the world. The renewed partnership builds upon the company three-year commitment from 2014 and brings the total humanitarian aid commitment to more than 200 million IU of Factor VIII over eight years.
Risks
At 30 June 2017 the Group’s financial risk management objectives and policies are consistent with those disclosed in the consolidated financial statements for the year ended 31 December 2016.
“Cautionary Statement Regarding Forward-Looking Statements”
The facts and figures contained in this report that do not refer to historical data are “future projections and assumptions”. Words and expressions such as “believe”, “hope”, “anticipate”, “predict”, “expect”, “intend”, “should”, “will seek to achieve”, “it is estimated”, “future” and similar expressions, in so far as they relate to the Grifols group, are used to identify future projections and assumptions. These expressions reflect the assumptions, hypotheses, expectations and predictions of the management team at the time of writing this report, and these are subject to a number of factors that mean that the actual results may be materially different. The future results of the Grifols group could be affected by events relating to its own activities, such as a shortage of supplies of raw materials for the manufacture of its products, the appearance of competitor products on the market, or changes to the regulatory framework of the markets in which it operates, among others. At the date of compiling this report, the Grifols group has adopted the necessary measures to mitigate the potential impact of these events. Grifols, S.A. does not accept any obligation to publicly report, revise or update future projections or assumptions to adapt them to events or circumstances subsequent to the date of writing this report, except where expressly required by the applicable legislation. This document does not constitute an offer or invitation to buy or subscribe shares in accordance with the provisions of the following Spanish legislation: Royal Legislative Decree 4/2015, of 23 October, approving recast text of Securities Market Law; Royal Decree Law 5/2005, of 11 March and/or Royal Decree 1310/2005, of 4 November, and any regulations developing this legislation. In addition, this document does not constitute an offer of purchase, sale or exchange, or a request for an offer of purchase, sale or exchange of securities, or a request for any vote or approval in any other jurisdiction.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
|
|
Grifols, S.A. | ||
|
|
| ||
|
|
By: |
/s/ David I. Bell | |
|
|
|
Name: |
David I. Bell |
|
|
|
Title: |
Authorized Signatory |
|
|
| ||
|
Date: July 28, 2017 |
| ||
