Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
(Mark One)
| ☐ | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
OR
| ☐ | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report
Commission file number 001-37384
GALAPAGOS NV
(Exact name of Registrant as specified in its charter and translation of Registrant’s name into English)
Belgium
(Jurisdiction of incorporation or organization)
Generaal De Wittelaan L11 A3
2800 Mechelen, Belgium
(Address of principal executive offices)
Onno van de Stolpe
Chief Executive Officer
Galapagos NV
Generaal De Wittelaan L11 A3
2800 Mechelen, Belgium
Tel: +32 15 342 900 Fax: +32 15 342 901
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
| Title of each class |
Name of each exchange on which registered | |
| American Depositary Shares, each representing one ordinary share, no par value per share |
The Nasdaq Stock Market LLC | |
| Ordinary shares, no par value per share* | The Nasdaq Stock Market LLC* |
* Not for trading, but only in connection with the registration of the American Depositary Shares.
Securities registered or to be registered pursuant to Section 12(g) of the Act. None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act. None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
Ordinary shares, no par value per share: 46,256,078 as of December 31, 2016
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☒ Yes ☐ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files. ☐ Yes ☒ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP ☐ | International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ |
Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. ☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
Table of Contents
| Page | ||||||||
| 1 | ||||||||
| PART I |
3 | |||||||
| Item 1 | 3 | |||||||
| Item 2 | 3 | |||||||
| Item 3 | 3 | |||||||
| A. | 3 | |||||||
| B. | 5 | |||||||
| C. | 5 | |||||||
| D. | 5 | |||||||
| Item 4 | 47 | |||||||
| A. | 47 | |||||||
| B. | 48 | |||||||
| C. | 96 | |||||||
| D. | 96 | |||||||
| Item 4B | 97 | |||||||
| Item 5 | 97 | |||||||
| A. | 107 | |||||||
| B. | 120 | |||||||
| C. | 124 | |||||||
| D. | 124 | |||||||
| E. | 125 | |||||||
| F. | 125 | |||||||
| G. | 126 | |||||||
| Item 6 | 126 | |||||||
| A. | 126 | |||||||
| B. | 130 | |||||||
| C. | 139 | |||||||
| D. | 143 | |||||||
| E. | 143 | |||||||
| Item 7 | 143 | |||||||
| A. | 143 | |||||||
| B. | 145 | |||||||
| C. | 149 | |||||||
| Item 8 | 149 | |||||||
| A. | 149 | |||||||
| B. | 149 | |||||||
| Item 9 | 150 | |||||||
| A. | 150 | |||||||
| B. | 151 | |||||||
| C. | 151 | |||||||
Table of Contents
| Page | ||||||||
| D. | 151 | |||||||
| E. | 151 | |||||||
| F. | 152 | |||||||
| Item 10 | 152 | |||||||
| A. | 152 | |||||||
| B. | 152 | |||||||
| C. | 152 | |||||||
| D. | 152 | |||||||
| E. | 152 | |||||||
| F. | 162 | |||||||
| G. | 162 | |||||||
| H. | 162 | |||||||
| I. | 162 | |||||||
| Item 11 | 162 | |||||||
| Item 12 | 164 | |||||||
| A. | 164 | |||||||
| B. | 164 | |||||||
| C. | 165 | |||||||
| D. | 165 | |||||||
| PART II |
167 | |||||||
| Item 13 | 167 | |||||||
| Item 14 | Material Modifications to the Rights of Security Holders and Use of Proceeds. |
167 | ||||||
| Item 15 | 167 | |||||||
| Item 16 | 168 | |||||||
| Item 16A | 168 | |||||||
| Item 16B | 168 | |||||||
| Item 16C | 169 | |||||||
| Item 16D | 170 | |||||||
| Item 16E | Purchases of Equity Securities by the Issuer and Affiliated Purchasers. |
170 | ||||||
| Item 16F | 170 | |||||||
| Item 16G | 170 | |||||||
| Item 16H | 171 | |||||||
| PART III |
172 | |||||||
| Item 17 | 172 | |||||||
| Item 18 | 172 | |||||||
| Item 19 | 172 | |||||||
ii
Table of Contents
Unless otherwise indicated, “GLPG,” “the company,” “our company,” “we,” “us,” and “our” refer to Galapagos NV and its consolidated subsidiaries.
We own various trademark registrations and applications, and unregistered trademarks, including GALAPAGOS, FIDELTA, and our corporate logo. All other trade names, trademarks and service marks referred to in this annual report on Form 20-F, or this annual report, are the property of their respective owners. Trade names, trademarks and service marks of other companies appearing in this annual report are the property of their respective holders. Solely for convenience, the trademarks and trade names in this annual report may be referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend to use or display other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Our audited consolidated financial statements have been prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB. Our consolidated financial statements are presented in euros. All references in this annual report to “$,” “US$,” “U.S.$,” “U.S. dollars,” “dollars,” and “USD” mean U.S. dollars and all references to “€” and “euros” mean euros, unless otherwise noted. Throughout this annual report, references to “ADSs” mean American Depositary Shares or ordinary shares represented by American Depositary Shares, as the case may be.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, that are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than present and historical facts and conditions contained in this annual report, including statements regarding our future results of operations and financial positions, business strategy, plans and our objectives for future operations, are forward-looking statements. When used in this annual report, the words “anticipate,” “believe,” “can,” “could,” “estimate,” “expect,” “intend,” “is designed to,” “may,” “might,” “plan,” “potential,” “predict,” “objective,” “should,” or the negative of these and similar expressions identify forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| • | the initiation, timing, progress and results of our pre-clinical studies and clinical trials, and our research and development programs; |
| • | our ability to advance product candidates into, and successfully complete, clinical trials; |
| • | our reliance on the success of our product candidate filgotinib and certain other product candidates; |
| • | the timing or likelihood of regulatory filings and approvals; |
| • | our ability to develop sales and marketing capabilities; |
| • | the commercialization of our product candidates, if approved; |
| • | the pricing and reimbursement of our product candidates, if approved; |
| • | the implementation of our business model, strategic plans for our business, product candidates and technology; |
| • | the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology; |
| • | our ability to operate our business without infringing the intellectual property rights and proprietary technology of third parties; |
Table of Contents
| • | cost associated with defending intellectual property infringement, product liability, and other claims; |
| • | regulatory development in the United States, Europe, and other jurisdictions; |
| • | estimates of our expenses, future revenues, capital requirements and our needs for additional financing; |
| • | the potential benefits of strategic collaboration agreements and our ability to enter into strategic arrangements; |
| • | our ability to maintain and establish collaborations or obtain additional grant funding; |
| • | the rate and degree of market acceptance of our product candidates if approved by regulatory authorities; |
| • | our financial performance; |
| • | developments relating to our competitors and our industry, including competing therapies; |
| • | our ability to effectively manage and anticipated growth; |
| • | our ability to attract and retain qualified employees and key personnel; |
| • | statements regarding future revenue, hiring plans, expenses, capital expenditures, capital requirements and share performance; and |
| • | other risks and uncertainties, including those listed in the section of this annual report titled “Item 3.D.—Risk Factors.” |
You should refer to the section of this annual report titled “Item 3.D.—Risk Factors” for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this annual report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
You should read this annual report and the documents that we reference in this annual report and have filed as exhibits to this annual report completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
This annual report contains market data and industry forecasts that were obtained from industry publications. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified any third-party information. While we believe the market position, market opportunity and market size information included in this annual report is generally reliable, such information is inherently imprecise.
Please see the Glossary of Terms on page 87 for definitions of scientific and other terms used in this annual report.
2
Table of Contents
PART I
| Item 1 | Identity of Directors, Senior Management and Employee. |
Not applicable.
| Item 2 | Offer Statistics and Expected Timetable. |
Not applicable.
| Item 3 | Key Information. |
| A. | Selected Financial Data |
Our consolidated audited financial statements have been prepared in accordance with IFRS, as issued by the IASB. We derived the selected statements of consolidated operations data, selected statements of consolidated financial position and selected statements of consolidated cash flows, each as of December 31, 2012, 2013, 2014, 2015 and 2016 from our consolidated audited financial statements. This data should be read together with, and is qualified in its entirety by reference to, “Item 5—Operating and Financial Review and Prospects” as well as our financial statements and notes thereto appearing elsewhere in this annual report. Our historical results are not necessarily indicative of the results to be expected in the future.
| Consolidated statement of operations: | Year ended December 31, | |||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (Euro, in thousands, except share and per share data) | ||||||||||||||||||||
| Revenues |
€ | 129,519 | € | 39,563 | € | 69,368 | € | 76,625 | € | 74,504 | ||||||||||
| Other income |
22,093 | 21,017 | 20,653 | 19,947 | 17,722 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenues and other income |
151,612 | 60,579 | 90,021 | 96,572 | 92,226 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Service cost of sales |
(5,584 | ) | ||||||||||||||||||
| Research and development expenditure |
(139,573 | ) | (129,714 | ) | (111,110 | ) | (99,380 | ) | (80,259 | ) | ||||||||||
| General and administrative expenses |
(21,744 | ) | (19,127 | ) | (13,875 | ) | (12,353 | ) | (12,118 | ) | ||||||||||
| Sales and marketing expenses |
(1,785 | ) | (1,182 | ) | (992 | ) | (1,464 | ) | (1,285 | ) | ||||||||||
| Restructuring and integration costs |
— | — | (669 | ) | (290 | ) | (2,506 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
(163,103 | ) | (150,023 | ) | (126,646 | ) | (113,487 | ) | (101,751 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(11,491 | ) | (89,444 | ) | (36,624 | ) | (16,915 | ) | (9,526 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Fair value re-measurement of share subscription agreement |
57,479 | (30,632 | ) | — | — | — | ||||||||||||||
| Other financial income |
9,950 | 1,987 | 2,291 | 2,182 | 3,103 | |||||||||||||||
| Other financial expenses |
(1,692 | ) | (1,539 | ) | (867 | ) | (1,402 | ) | (1,176 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income / loss (-) before tax |
54,246 | (119,627 | ) | (35,201 | ) | (16,135 | ) | (7,599 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income taxes |
(235 | ) | 1,218 | (2,103 | ) | (676 | ) | 164 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income / loss (-) from continuing operations |
54,012 | (118,410 | ) | (37,303 | ) | (16,811 | ) | (7,435 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income from discontinued operations |
— | — | 70,514 | 8,732 | 1,714 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income / loss (-) |
€ | 54,012 | € | (118,410 | ) | € | 33,211 | € | (8,079 | ) | € | (5,721 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
3
Table of Contents
| Consolidated statement of operations: | Year ended December 31, | |||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (Euro, in thousands, except share and per share data) | ||||||||||||||||||||
| Net income / loss (-) attributable to: |
||||||||||||||||||||
| Owners of the parent |
54,012 | (118,410 | ) | 33,211 | (8,079 | ) | (5,721 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic income / loss (-) per share |
€ | 1.18 | € | (3.32 | ) | € | 1.10 | € | (0.28 | ) | € | (0.22 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted income / loss (-) per share |
€ | 1.14 | € | (3.32 | ) | € | 1.10 | € | (0.28 | ) | € | (0.22 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic income/ loss (-) per share from continuing operations |
€ | 1.18 | € | (3.32 | ) | € | (1.24 | ) | € | (0.58 | ) | € | (0.28 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted income/ loss (-) per share from continuing operations |
€ | 1.14 | € | (3.32 | ) | € | (1.24 | ) | € | (0.58 | ) | € | (0.28 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of shares - Basic (in ‘000 shares) |
45,696 | 35,700 | 30,108 | 28,787 | 26,545 | |||||||||||||||
| Weighted average number of shares - Diluted (in ‘000 shares) |
47,308 | 35,700 | 30,108 | 28,787 | 26,545 | |||||||||||||||
| Condensed consolidated statement of financial position: | ||||||||||||||||||||
| December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (Euro, in thousands) | ||||||||||||||||||||
| Cash and cash equivalents |
€ | 973,241 | € | 340,314 | € | 187,712 | € | 138,175 | € | 94,369 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
1,083,338 | 442,514 | 270,467 | 287,374 | 94,369 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Share capital |
223,928 | 185,399 | 157,274 | 154,542 | 139,347 | |||||||||||||||
| Share premium account |
649,135 | 357,402 | 114,182 | 112,484 | 72,876 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total equity / net assets |
758,701 | 364,999 | 206,135 | 167,137 | 118,447 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total non-current liabilities |
220,846 | 5,103 | 3,976 | 7,678 | 7,867 | |||||||||||||||
| Total current liabilities |
103,791 | 72,412 | 60,356 | 112,559 | 109,014 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
324,637 | 77,515 | 64,332 | 120,237 | 116,881 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities and equity |
€ | 1,083,338 | € | 442,514 | € | 270,467 | € | 287,374 | € | 235,328 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Condensed consolidated statement of cash flows: | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (Euro, in thousands) | ||||||||||||||||||||
| Cash and cash equivalents at beginning of the period |
€ | 340,314 | € | 187,712 | € | 138,175 | € | 94,369 | € | 32,277 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash flows generated / used (-) in operating activities |
239,403 | (114,590 | ) | (75,555 | ) | 1,846 | 65,873 | |||||||||||||
| Net cash flows generated / used (-) in investing activities |
(7,287 | ) | (4,297 | ) | 120,606 | (11,988 | ) | (6,437 | ) | |||||||||||
| Net cash flows generated in financing activities |
395,996 | 271,370 | 4,214 | 54,495 | 2,265 | |||||||||||||||
| Effect of exchange rate differences on cash and cash equivalents |
4,816 | 118 | 271 | (548 | ) | 391 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of the period |
€ | 973,241 | € | 340,314 | € | 187,712 | € | 138,175 | € | 94,369 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
4
Table of Contents
Exchange Rate Information
The following table sets forth, for each period indicated, the low and high exchange rates of U.S. dollars per euro, the exchange rate at the end of such period and the average of such exchange rates on the last day of each month during such period, based on the noon buying rate of the Federal Reserve Bank of New York for the euro. As used in this document, the term “noon buying rate” refers to the rate of exchange for the euro, expressed in U.S. dollars per euro, as certified by the Federal Reserve Bank of New York for customs purposes. The exchange rates set forth below demonstrate trends in exchange rates, but the actual exchange rates used throughout this annual report may vary.
| Year ended December 31, | ||||||||||||||||||||
| 2012 | 2013 | 2014 | 2015 | 2016 | ||||||||||||||||
| High |
1.3463 | 1.3816 | 1.3927 | 1.2015 | 1.1516 | |||||||||||||||
| Low |
1.2062 | 1.2774 | 1.2101 | 1.0524 | 1.0375 | |||||||||||||||
| Rate at end of period |
1.3186 | 1.3779 | 1.2101 | 1.0859 | 1.0552 | |||||||||||||||
| Average rate per period |
1.2859 | 1.3281 | 1.3297 | 1.1096 | 1.1070 | |||||||||||||||
The following table sets forth, for each of the last six months, the low and high exchange rates for euros expressed in U.S. dollars and the exchange rate at the end of the month based on the noon buying rate as described above.
| September 2016 |
October 2016 |
November 2016 |
December 2016 |
January 2017 |
February 2017 |
|||||||||||||||||||
| High |
1.1271 | 1.1212 | 1.1121 | 1.0758 | 1.0794 | 1.0802 | ||||||||||||||||||
| Low |
1.1158 | 1.0866 | 1.0560 | 1.0375 | 1.0416 | 1.0551 | ||||||||||||||||||
| Rate at end of period |
1.1238 | 1.0962 | 1.0578 | 1.0552 | 1.0794 | 1.0580 | ||||||||||||||||||
On December 31, 2016, the noon buying rate of the Federal Reserve Bank of New York for the euro was €1.00 = US$1.0552. Unless otherwise indicated, currency translations in this annual report reflect the December 31, 2016 exchange rate.
On March 17, 2017, the noon buying rate of the Federal Reserve Bank of New York for the euro was €1.00 = $1.0742.
| B. | Capitalization and Indebtedness |
Not applicable.
| C. | Reasons for the Offer and Use of Proceeds |
Not applicable.
| D. | Risk Factors |
Our business faces significant risks. You should carefully consider all of the information set forth in this annual report and in our other filings with the U.S. Securities and Exchange Commission, or the SEC, including the following risk factors which we face and which are faced by our industry. Our business, financial condition or results of operations could be materially adversely affected by any of these risks. This report also contains forward-looking statements that involve risks and uncertainties. Our results could materially differ from those anticipated in these forward-looking statements, as a result of certain factors including the risks described below and elsewhere in this annual report and our other SEC filings. See “Special Note Regarding Forward-Looking Statements” above.
5
Table of Contents
Risks Related to Product Development, Regulatory Approval and Commercialization
We are heavily dependent on the success of our product candidate filgotinib. We are also dependent on the success of our other product candidates, such as our CF candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737), GLPG1690, GLPG1972 and MOR106. We cannot give any assurance that any product candidate will successfully complete clinical trials or receive regulatory approval, which is necessary before it can be commercialized.
Filgotinib is currently undergoing Phase 3 studies in rheumatoid arthritis, or RA, and in Crohn’s disease, or CD, and a Phase 2b/3 trial in ulcerative colitis, or UC, by our collaboration partner Gilead. Our business and future success is substantially dependent on our ability to develop, obtain regulatory approval for, and then successfully commercialize our product candidate filgotinib, either alone or in a partnership. Our business and future success also depend on our ability to develop successfully, obtain regulatory approval for, and then successfully commercialize our other product candidates, such as our cystic fibrosis, or CF, candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737), GLPG1690, GLPG1972 and MOR106. We completed Phase 2 trials in certain mutations of CF with potentiator GLPG1837 in 2016; we completed a Phase 1 trial and submitted an application for a Phase 2 safety and pharmacokinetics study in 2016 for GLPG2222, a CF corrector candidate; we initiated a Phase 1 trial in May 2016 for GLPG2451, a CF corrector candidate; we initiated a Phase 1 trial in November 2016 with GLPG2737, a CF corrector candidate; we initiated a Phase 2a trial for idiopathic pulmonary fibrosis, or IPF, with GLPG1690 in April 2016; we plan to initiate a Phase 1b trial with GLPG1972 in osteoarthritis, or OA, patients in 2017; and we initiated a Phase 1b trial in April 2016 with MOR106, a human monoclonal antibody, in patients with atopic dermatitis, or AtD. Our product candidates will require additional clinical development, management of clinical and manufacturing activities, regulatory approval in multiple jurisdictions (if regulatory approval can be obtained at all), securing sources of commercial manufacturing supply, building of, or partnering with, a commercial organization, substantial investment and significant marketing efforts before any revenues can be generated from product sales. We are not permitted to market or promote any of our product candidates before we receive regulatory approval from the FDA, the EMA, or any other comparable regulatory authority, and we may never receive such regulatory approval for any of our product candidates. We cannot assure you that our clinical trials for filgotinib, our CF candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737), GLPG1690, GLPG1972 or MOR106 will be completed in a timely manner, or at all, or that we will be able to obtain approval from the U.S. Food and Drug Administration, or FDA, the European Medicines Agency, or EMA, or any other comparable regulatory authority for any of these product candidates. We cannot be certain that we will advance any other product candidates into clinical trials. If any of filgotinib, our CF candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737), GLPG1690, GLPG1972 or MOR106 or any future product candidate is not approved and commercialized, we will not be able to generate any product revenues for that product candidate. Moreover, any delay or setback in the development of any product candidate could adversely affect our business and cause the price of the ADSs or our ordinary shares to fall.
Due to our limited resources and access to capital, we must and have in the past decided to prioritize development of certain product candidates; these decisions may prove to have been wrong and may adversely affect our revenues.
Because we have limited resources and access to capital to fund our operations, we must decide which product candidates to pursue and the amount of resources to allocate to each. As such, we are currently primarily focused on the development of filgotinib, our CF candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737), GLPG1690, GLPG1972, and MOR106. Our decisions concerning the allocation of research, collaboration, management and financial resources toward particular compounds, product candidates or therapeutic areas may not lead to the development of viable commercial products and may divert resources away from better opportunities. Similarly, our potential decisions to delay, terminate or collaborate with third parties in respect of certain product development programs may also prove not to be optimal and could cause us to miss valuable opportunities. If we make incorrect determinations regarding the market potential of our product candidates or misread trends in the pharmaceutical industry, our business, financial condition and results of operations could be materially adversely affected.
6
Table of Contents
The regulatory approval processes of the FDA, the EMA and other comparable regulatory authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA, the EMA and other comparable regulatory authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate and it is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval.
Our product candidates could fail to receive regulatory approval for many reasons, including the following:
| • | the FDA, the EMA or other comparable regulatory authorities may disagree with the design or implementation of our clinical trials; |
| • | we may be unable to demonstrate to the satisfaction of the FDA, the EMA or other comparable regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| • | the results of clinical trials may not meet the level of statistical significance required by the FDA, the EMA or other comparable regulatory authorities for approval; |
| • | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| • | filgotinib and our other product candidates (except for our CF program) are developed to act against targets discovered by us, and because our product candidates are novel mode of action products, they carry an additional risk regarding desired level of efficacy and safety profile; |
| • | the FDA, the EMA or other comparable regulatory authorities may disagree with our interpretation of data from pre-clinical studies or clinical trials; |
| • | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of a new drug application, or NDA, supplemental NDA, biologics license application, or BLA, or other submission or to obtain regulatory approval in the United States, Europe or elsewhere; |
| • | the FDA, the EMA or other comparable regulatory authorities may find deficiencies with or fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies or such processes or facilities may not pass a pre-approval inspection; and |
| • | the approval policies or regulations of the FDA, the EMA or other comparable regulatory authorities may change or differ from one another significantly in a manner rendering our clinical data insufficient for approval. |
This lengthy approval process as well as the unpredictability of future clinical trial results may result in our or our collaboration partners’ failure to obtain regulatory approval to market filgotinib, GLPG1837, GLPG2222, GLPG1690, GLPG1972 and/or other product candidates, which would harm our business, results of operations and prospects significantly. In addition, even if we were to obtain approval, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. In certain jurisdictions, regulatory authorities may not approve the price we intend to charge for our products. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
We have not previously submitted an NDA, a BLA, a marketing authorization application, or any similar drug approval filing to the FDA, the EMA or any comparable regulatory authority for any product candidate, and we cannot be certain that any of our product candidates will be successful in clinical trials or receive regulatory
7
Table of Contents
approval. Further, our product candidates may not receive regulatory approval even if they are successful in clinical trials. If we do not receive regulatory approvals for our product candidates, we may not be able to continue our operations. Even if we successfully obtain regulatory approvals to market one or more of our product candidates, our revenues will be dependent, to a significant extent, upon the size of the markets in the territories for which we gain regulatory approval and have commercial rights or share in revenues from the exercise of such rights. If the markets for patient subsets that we are targeting (such as RA, CD, UC, or CF) are not as significant as we estimate, we may not generate significant revenues from sales of such products, if approved.
In connection with our global clinical trials, local regulatory authorities may have differing perspectives on clinical protocols and safety parameters, which impacts the manner in which we conduct these global clinical trials and could negatively impact our chances for obtaining regulatory approvals or marketing authorization in these jurisdictions, or for obtaining the requested dosage for our product candidates, if regulatory approvals or marketing authorizations are obtained.
In connection with our global clinical trials, we are obliged to comply with the requirements of local regulatory authorities in each jurisdiction where we execute and locate a clinical trial. Local regulatory authorities can request specific changes to the clinical protocol or specific safety measures that differ from the positions taken in other jurisdictions. For example, in our DARWIN Phase 2 clinical trials for filgotinib in subjects with RA, we agreed with the FDA to exclude the 200 mg filgotinib daily dose for male subjects enrolled in the United States pending further data to demonstrate a wider exposure margin in patients versus the safe exposure in animal studies, while there is no such restriction by health authorities outside the United States. We cannot assure you that this view will not be adopted by other regulatory authorities in later stage trials or at the marketing authorization stage, if filgotinib successfully completes the registrational trials. Even if filgotinib does receive regulatory approval or marketing authorization, the FDA or other regulatory authorities may impose dosing restrictions that differ from the approved dosing regimen in other jurisdictions, and these differences could have a material adverse effect on our ability to commercialize our products in these jurisdictions.
Even if we receive regulatory approval for any of our product candidates, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates, if approved, could be subject to labeling and other restrictions and market withdrawal and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our products.
Any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate, and we may be required to include labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings.
If the FDA, EMA or any other comparable regulatory authority approves any of our product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration requirements and continued compliance with current good manufacturing practices, or cGMPs, and good clinical practices, or GCPs, for any clinical trials that we conduct post-approval. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| • | fines, untitled or warning letters or holds on clinical trials; |
8
Table of Contents
| • | refusal by the FDA, the EMA or any other comparable regulatory authority to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product approvals or licenses; |
| • | product seizure or detention, or refusal to permit the import or export of products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
The policies of the FDA, the EMA and other comparable regulatory authorities may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
Filgotinib, if approved, may be subject to box warnings, labeling restrictions or dose limitations in certain jurisdictions, which could have a material adverse impact on our ability to market filgotinib in these jurisdictions.
Based on pre-clinical findings, we expect that filgotinib, if approved, may have a labeling statement warning female patients of child-bearing age to take precautionary measures of birth control to protect against pregnancy, similar to warnings included with other frequently used medications in RA, such as methotrexate, or MTX.
In addition, there may be dose limitations imposed for male patients who are prescribed filgotinib, if approved. In connection with the DARWIN clinical program, we agreed with the FDA to exclude the 200 mg filgotinib daily dose for male subjects in the United States; males received a maximum daily dose of 100 mg in the U.S. sites in these trials. This limitation was not imposed by any other regulatory agency in any other jurisdiction in which the Phase 2 DARWIN clinical program is being conducted. We agreed to this limitation because in both rat and dog toxicology studies, filgotinib induced adverse effects on the male reproductive system and the FDA determined there was not a sufficient safety margin between the filgotinib exposure at the no-observed-adverse-effect-level observed in these studies and the anticipated human exposure at the 200 mg daily filgotinib dose. Accordingly, in connection with the DARWIN 3 open-label, long-term extension clinical trial, in the United States, male subjects are dosed at 100-mg-daily-dose only. Male participants in this study and their partners are required to use highly effective contraceptive measures for the duration of the study and during a washout period thereafter. As an additional safety measure, we monitor clinical laboratory changes in hormone levels for subjects in the DARWIN 3 clinical trial.
Recently generated nonclinical data showed filgotinib did not induce any macroscopic or microscopic findings in the male reproductive system in animals with higher filgotinib exposure versus previous studies.
The Phase 3 FINCH program, led by our collaboration partner Gilead, is evaluating 100 mg and 200 mg filgotinib in both males and females in major RA patient populations worldwide. Men and women in both the Phase 2b/3 SELECTION and Phase 3 DIVERSITY trials in UC and CD, respectively, will be randomized to receive placebo, 100 mg or 200 mg filgotinib. In these SELECTION and DIVERSITY trials in the United States, males may receive 200 mg only if they failed conventional therapy, anti-TNF and vedolizumab. The filgotinib Phase 3 programs will also contain a dedicated male patient testicular safety study.
Even if filgotinib does receive regulatory approval or marketing authorization, the FDA or other regulatory authorities may impose dosing restrictions that differ from the approved dosing regimen in other jurisdictions.
Box warnings, labeling restrictions, dose limitations and similar restrictions on use could have a material adverse effect on our ability to commercialize filgotinib in those jurisdictions where such restrictions apply.
9
Table of Contents
Clinical development is a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials as well as data from any interim analysis of ongoing clinical trials may not be predictive of future trial results. Clinical failure can occur at any stage of clinical development. We have never completed a Phase 3 trial or submitted an NDA or BLA.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. Although product candidates may demonstrate promising results in early clinical (human) trials and pre-clinical (animal) studies, they may not prove to be effective in subsequent clinical trials. For example, testing on animals may occur under different conditions than testing in humans and therefore the results of animal studies may not accurately predict human experience. Likewise, early clinical studies may not be predictive of eventual safety or effectiveness results in larger-scale pivotal clinical trials. The results of pre-clinical studies and previous clinical trials as well as data from any interim analysis of ongoing clinical trials of our product candidates, as well as studies and trials of other products with similar mechanisms of action to our product candidates, may not be predictive of the results of ongoing or future clinical trials. For example, the positive results generated to date in pre-clinical studies and Phase 1, Phase 2a and Phase 2b clinical trials for filgotinib in RA or Phase 2 clinical trials for CD do not ensure that later clinical trials will continue to demonstrate similar results or observations, including the Phase 3 studies in RA, UC, and CD currently ongoing. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through pre-clinical studies and earlier clinical trials. In addition to the safety and efficacy traits of any product candidate, clinical trial failures may result from a multitude of factors including flaws in trial design, dose selection, placebo effect and patient enrollment criteria. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials, and it is possible that we will as well. Based upon negative or inconclusive results, we or our collaboration partners may decide, or regulators may require us, to conduct additional clinical trials or pre-clinical studies. In addition, data obtained from trials and studies are susceptible to varying interpretations, and regulators may not interpret our data as favorably as we do, which may delay, limit or prevent regulatory approval.
We may experience delays in our ongoing clinical trials and we do not know whether planned clinical trials will begin on time, need to be redesigned, enroll patients on time or be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including delays related to:
| • | obtaining regulatory approval to commence a trial; |
| • | reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| • | obtaining Institutional Review Board, or IRB, or ethics committee approval at each site; |
| • | obtaining regulatory concurrence on the design and parameters for the trial; |
| • | obtaining approval for the designs of our clinical development programs for each country targeted for trial enrollment; |
| • | recruiting suitable patients to participate in a trial, which may be impacted by the number of competing trials that are enrolling patients; |
| • | having patients complete a trial or return for post-treatment follow-up; |
| • | clinical sites deviating from trial protocol or dropping out of a trial; |
| • | adding new clinical trial sites; |
| • | manufacturing sufficient quantities of product candidate or obtaining sufficient quantities of comparator drug for use in clinical trials; or |
| • | the availability of adequate financing and other resources. |
10
Table of Contents
We could encounter delays if a clinical trial is suspended or terminated by us, by the IRBs or ethics committees of the institutions in which such trials are being conducted, or by the FDA, the EMA or other comparable regulatory authorities, or recommended for suspension or termination by the Data Monitoring Committee, or the DMC, for such trial. A suspension or termination may be imposed due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA, the EMA or other comparable regulatory authorities resulting in the imposition of a clinical hold, safety issues or adverse side effects, including those seen in the class to which our product candidates belong, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions, manufacturing issues or lack of adequate funding to continue the clinical trial. For example, it is possible that safety issues or adverse side effects could be observed in trials for filgotinib in RA, CD and UC and other potential indications; for our CF candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737) in CF; for GLPG1690 in IPF; for GLPG1972 in OA; or for MOR106 in AtD, which could result in a delay, suspension or termination of the ongoing trials of filgotinib (in one or more indications), our CF candidates, GLPG1690, GLPG1972 or MOR106. If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause or lead to a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
If filgotinib, our CF candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737), GLPG1690, GLPG1972, MOR106 or any other product candidate is found to be unsafe or lack efficacy, we will not be able to obtain regulatory approval for it and our business would be materially harmed. For example, if the results of ongoing or future trials for filgotinib do not achieve the primary efficacy endpoints or demonstrate unexpected safety findings, the prospects for approval of filgotinib, as well as the price of the ADSs or our ordinary shares and our ability to create shareholder value could be materially and adversely affected.
In some instances, there can be significant variability in safety and/or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in composition of the patient populations, adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. We do not know whether any Phase 2, Phase 3 or other clinical trials we or any of our collaboration partners may conduct will demonstrate consistent or adequate efficacy and safety to obtain regulatory approval to market our product candidates. If we are unable to bring any of our current or future product candidates to market, our ability to create long-term shareholder value will be limited.
We initiated our first clinical study in 2009, and for eight of our compounds, Phase 2 studies have been initiated. Phase 3 studies in RA and CD and a Phase 2b/3 trial in UC have been initiated by our collaboration partner Gilead for filgotinib.
The rates at which we complete our scientific studies and clinical trials depend on many factors, including, but not limited to, patient enrollment.
Patient enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating. With respect to clinical development of our CF candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737), the availability of, for example, Kalydeco® (ivacaftor), which is a drug developed by Vertex to be used to treat patients with a certain mutation of CF, may cause patients to be less willing to participate in our clinical trial in regions in which
11
Table of Contents
therapy has been approved. Since CF is a competitive market in certain regions such as the United States and the European Union with a number of product candidates in development, patients may have other choices with respect to potential clinical trial participation and we may have difficulty in reaching our enrollment targets. In addition, the relatively limited number of patients worldwide (estimated to be 80,000) may make enrollment more challenging. Any of these occurrences may harm our clinical trials and by extension, our business, financial condition and prospects.
We may not be successful in our efforts to use and expand our novel, proprietary target discovery platform to build a pipeline of product candidates.
A key element of our strategy is to use and expand our novel, proprietary target discovery platform to build a pipeline of product candidates and progress these product candidates through clinical development for the treatment of a variety of diseases. Although our research and development efforts to date have resulted in a pipeline of product candidates directed at various diseases, we may not be able to develop product candidates that are safe and effective. Even if we are successful in continuing to build our pipeline, the potential product candidates that we identify may not be suitable for clinical development, including as a result of being shown to have harmful side effects or other characteristics that indicate that they are unlikely to be products that will receive marketing approval and achieve market acceptance. If we do not continue to successfully develop and begin to commercialize product candidates, we will face difficulty in obtaining product revenues in future periods, which could result in significant harm to our financial position and adversely affect the price of the ADSs or our ordinary shares.
Our commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among physicians, healthcare payors, patients and the medical community.
Even if we obtain regulatory approval for one or more of our product candidates, the product may not gain market acceptance among physicians, healthcare payors, patients and the medical community, which is critical to commercial success. Market acceptance of any product candidate for which we receive approval depends on a number of factors, including:
| • | the efficacy and safety as demonstrated in clinical trials; |
| • | the timing of market introduction of the product candidate as well as competitive products; |
| • | the clinical indications for which the product candidate is approved; |
| • | acceptance by physicians, the medical community and patients of the product candidate as a safe and effective treatment; |
| • | the convenience of prescribing and initiating patients on the product candidate; |
| • | the potential and perceived advantages of such product candidate over alternative treatments; |
| • | the cost of treatment in relation to alternative treatments, including any similar generic treatments; |
| • | the availability of coverage and adequate reimbursement and pricing by third-party payors and government authorities; |
| • | relative convenience and ease of administration; |
| • | the prevalence and severity of adverse side effects; and |
| • | the effectiveness of sales and marketing efforts. |
If our product candidates are approved but fail to achieve an adequate level of acceptance by physicians, healthcare payors, patients and the medical community, we will not be able to generate significant revenues, and we may not become or remain profitable.
12
Table of Contents
We currently have no marketing and sales organization. To the extent any of our product candidates for which we maintain commercial rights is approved for marketing, if we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates, we may not be able to effectively market and sell any product candidates, or generate product revenues.
We currently do not have a marketing or sales organization for the marketing, sales and distribution of pharmaceutical products. In order to independently commercialize any product candidates that receive marketing approval and for which we maintain commercial rights, we would have to build marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. In the event of successful development of filgotinib, GLPG1837, GLPG2451, GLPG2222, GLPG2737, GLPG1690, GLPG1972, MOR106 or any other product candidates for which we maintain commercial rights, we may elect to build a targeted specialty sales force which will be expensive and time consuming. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of these products. With respect to our product candidates, we may choose to partner with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. In the instance of filgotinib, under our collaboration agreement with Gilead, if we exercise our co-promotion option with respect to licensed products, we would assume a portion of the co-promotion effort in the United Kingdom, Germany, France, Italy, Spain, the Netherlands, Belgium, and/or Luxembourg and share equally in the net profit and net losses in these territories instead of receiving royalties in those territories during the period of co-promotion. In the instance of our CF portfolio of drugs aimed at a triple combination therapy, under our collaboration agreement with AbbVie, if we exercise our co-promotion option with respect to a licensed product, we would assume a portion of the co-promotion effort in the Netherlands, Belgium and Luxembourg and share in the net profit and net losses in these territories instead of receiving royalties in those territories during the period of co-promotion. If we are unable to enter into collaborations with third parties for the commercialization of approved products, if any, on acceptable terms or at all, or if any such partner does not devote sufficient resources to the commercialization of our product or otherwise fails in commercialization efforts, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval. If we are not successful in commercializing our product candidates, either on our own or through collaborations with one or more third parties, our future revenue will be materially and adversely impacted.
Coverage and reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance.
There is significant uncertainty related to the third-party coverage and reimbursement of newly approved drugs. To the extent that we retain commercial rights following clinical development, we would seek approval to market our product candidates in the United States, the European Union and other selected jurisdictions. Market acceptance and sales of our product candidates, if approved, in both domestic and international markets will depend significantly on the availability of adequate coverage and reimbursement from third-party payors for any of our product candidates and may be affected by existing and future healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs they will cover and establish payment levels. We cannot be certain that coverage and adequate reimbursement will be available for any of our product candidates, if approved. Also, we cannot be certain that reimbursement policies will not reduce the demand for, or the price paid for, any of our product candidates, if approved. If reimbursement is not available or is available on a limited basis for any of our product candidates, if approved, we may not be able to successfully commercialize any such product candidate. Reimbursement by a third-party payor may depend upon a number of factors, including, without limitation, the third-party payor’s determination that use of a product is:
| • | a covered benefit under its health plan; |
| • | safe, effective and medically necessary; |
| • | appropriate for the specific patient; |
13
Table of Contents
| • | cost-effective; and |
| • | neither experimental nor investigational. |
Obtaining coverage and reimbursement approval for a product from a government or other third-party payor is a time consuming and costly process that could require us to provide supporting scientific, clinical and cost- effectiveness data for the use of our products to the payor. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement or to have pricing set at a satisfactory level. If reimbursement of our future products, if any, is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels such as may result where alternative or generic treatments are available, we may be unable to achieve or sustain profitability.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or MMA, changed the way Medicare covers and pays for pharmaceutical products. The legislation established Medicare Part D, which expanded Medicare coverage for outpatient prescription drug purchases by the elderly but provided authority for limiting the number of drugs that will be covered in any therapeutic class. The MMA also introduced a new reimbursement methodology based on average sales prices for physician-administered drugs. Any negotiated prices for any of our product candidates, if approved, covered by a Part D prescription drug plan will likely be lower than the prices we might otherwise obtain outside of the Medicare Part D prescription drug plan. Moreover, while Medicare Part D applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment under Medicare Part D may result in a similar reduction in payments from non-governmental payors.
In certain countries, particularly in the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct additional clinical trials that compare the cost-effectiveness of our product candidates to other available therapies. If reimbursement of any of our product candidates, if approved, is unavailable or limited in scope or amount in a particular country, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability of our products in such country.
The delivery of healthcare in the European Union, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of healthcare and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize any products for which we obtain marketing approval.
Legislative and regulatory activity may exert downward pressure on potential pricing and reimbursement for any of our product candidates, if approved, that could materially affect the opportunity to commercialize.
The United States and several other jurisdictions are considering, or have already enacted, a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell any of our product candidates profitably, if approved. Among policy-makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access to healthcare. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. There have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future.
14
Table of Contents
The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare may adversely affect:
| • | the demand for any of our product candidates, if approved; |
| • | the ability to set a price that we believe is fair for any of our product candidates, if approved; |
| • | our ability to generate revenues and achieve or maintain profitability; |
| • | the level of taxes that we are required to pay; and |
| • | the availability of capital. |
In 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or, collectively, the ACA, became law in the United States. The goal of the ACA is to reduce the cost of healthcare and substantially change the way healthcare is financed by both governmental and private insurers. The ACA may result in downward pressure on pharmaceutical reimbursement, which could negatively affect market acceptance of any of our product candidates, if they are approved. Provisions of the ACA relevant to the pharmaceutical industry include the following:
| • | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic products, apportioned among these entities according to their market share in certain government healthcare programs; |
| • | an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively; |
| • | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts on negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; |
| • | extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
| • | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
| • | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
| • | requirements under the federal Open Payments program and its implementing regulations for the disclosure by certain drug, biologic product, device and medical supply manufacturers of payments made to physicians and teaching hospitals and of ownership or investment interests held by physicians and their immediate family members in these manufacturers; |
| • | expansion of healthcare fraud and abuse laws, including the federal False Claims Act and the federal Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance; |
| • | a licensure framework for follow-on biologic products; and |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research, along with funding for such research. |
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013. In January 2013, then President Obama signed into law the American Taxpayer Relief
15
Table of Contents
Act of 2012, which, among other things, reduced Medicare payments to several types of providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These laws may result in additional reductions in Medicare and other healthcare funding.
As a result of the 2016 election in the United States, there is great political uncertainty concerning the fate of the ACA and other healthcare laws. The United States Congress is expected to draft legislation to repeal parts of the ACA, but it is uncertain when such legislation would be passed and whether Congress would replace the law and what any replacement law would encompass. We cannot predict any initiatives that may be adopted in the future.
We face significant competition for our drug discovery and development efforts, and if we do not compete effectively, our commercial opportunities will be reduced or eliminated.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. Our drug discovery and development efforts may target diseases and conditions that are already subject to existing therapies or that are being developed by our competitors, many of which have substantially greater resources, larger research and development staffs and facilities, more experience in completing pre-clinical testing and clinical trials, and formulation, marketing and manufacturing capabilities than we do. As a result of these resources, our competitors may develop drug products that render our products obsolete or noncompetitive by developing more effective drugs or by developing their products more efficiently. Our ability to develop competitive products would be limited if our competitors succeeded in obtaining regulatory approvals for product candidates more rapidly than we were able to or in obtaining patent protection or other intellectual property rights that limited our drug development efforts. Any drug products resulting from our research and development efforts, or from our joint efforts with collaboration partners or licensees, might not be able to compete successfully with our competitors’ existing and future products, or obtain regulatory approval in the United States, European Union or elsewhere. Further, we may be subject to additional competition from alternative forms of treatment, including generic or over-the-counter drugs.
In the field of RA, therapeutic approaches have traditionally relied on disease-modifying anti-rheumatic drugs, or DMARDS, such as MTX and sulphasalazine as first-line therapy. These oral drugs work primarily to suppress the immune system and, while effective in this regard, the suppression of the immune system leads to an increased risk of infections and other side effects. Accordingly, in addition to DMARDS, monoclonal antibodies targeting tumor necrosis factor, or TNF, like AbbVie’s Humira, or IL-6 like Roche’s Actemra, have been developed. These biologics, which must be delivered via injection, are currently the standard of care as first- and second-line therapies for RA patients who have an inadequate response to DMARDS. In November 2012, Xeljanz (tofacitinib citrate), marketed by Pfizer, was approved by the FDA as an oral treatment for the treatment of adult patients with RA who have had an inadequate response to, or who are intolerant of, MTX. Xeljanz is the first Janus kinase, or JAK, inhibitor for RA approved for commercial sale in the United States. We are aware of other JAK inhibitors in development for patients with RA, including a once-daily JAK1/2 inhibitor called baricitinib which is being developed by Lilly, is approved by the EMA for RA and expected to be approved by the FDA for RA as early as March 2017, a JAK3/2/1 inhibitor called ASP015k which is being developed in Japan by Astellas, and a JAK inhibitor called ABT-494 which is being developed in Phase 3 in RA by AbbVie. Filgotinib, a selective JAK1 inhibitor, was developed in collaboration with AbbVie until AbbVie terminated the collaboration agreement on September 25, 2015. On December 16, 2015, we entered into a collaboration agreement with Gilead, under which Gilead initiated a Phase 3 trial for filgotinib in August 2016. We expect that filgotinib, which we are developing to treat patients with moderate to severe RA who have an inadequate response to MTX, will compete with all of these therapies. If generic or biosimilar versions of these therapies are approved we would expect to also compete against these versions of the therapies.
In the field of inflammatory bowel disease, or IBD, first line therapies are oral (or local) treatments with several low-cost generic compounds like mesalazine, more effective in UC and azathioprine, more effective in CD. Steroids like budesonide are used in both UC and CD. Companies like Santarus have developed controlled-
16
Table of Contents
release oral formulation with the aim to have local intestinal delivery of budesonide thereby limiting systemic side effects. For more advanced therapy, monoclonal antibodies with various targets such as TNF and more recently, integrins by vedoluzimab (Entyvio), marketed by Takeda, are approved. We are also aware of other biologics in clinical development for these indications, such as: ustekinumab, developed by Johnson & Johnson, which is in Phase 3 clinical trials and RPC1063, which is being developed by Celgene and has shown efficacy in a Phase 2 trial in UC. There are also several novel oral treatments being explored in Phase 2 and Phase 3, including Pfizer’s Xeljanz, which has been filed for approval in UC. The large number of treatments for UC, and somewhat less for CD, presents a substantial level of competition for any new treatment entering the IBD market. Gilead, under our collaboration agreement, initiated a Phase 3 trial for filgotinib for CD in December 2016 and a Phase 2b/3 trial for filgotinib for UC in December 2016. We expect that filgotinib, which we are developing to treat patients with moderately to severely active CD and UC, will compete with all of these therapies. If generic or biosimilar versions of these therapies are approved we would expect to also compete against these versions of the therapies.
In the field of CF, all but two of the approved therapies to treat CF patients have been designed to treat the symptoms of the disease rather than its cause. Kalydeco, marketed by Vertex, is currently the only approved therapy to address the cause of Class III mutation CF. Kalydeco is a CF transmembrane conductance regulator, or CFTR, potentiator to treat CF in patients with a Class III (G551D) mutation of the CFTR gene. Vertex also markets Orkambi, which is Kalydeco and lumacaftor, a corrector molecule for patients with a Class II (F508del) mutation of the CFTR gene, a broader patient population. Vertex obtained FDA approval in July 2015 for Orkambi in the United States and obtained European Commission Marketing Authorization for Orkambi in Europe in November 2015. We are also aware of other companies, including Novartis, Nivalis, Pfizer, Proteostasis and ProQR, and not-for-profit organizations like Flatley Discovery Lab, which are actively developing product candidates for the treatment of CF. These typically target the CFTR protein as potentiators, correctors, or other modulators of its activity. On September 23, 2013, we entered into a global collaboration agreement with AbbVie, focused on the discovery, worldwide development and commercialization of potentiator and corrector molecules for the treatment of CF. On April 28, 2016, we expanded this collaboration to provide for the potential development and commercialization of triple combination products consisting of a potentiator molecule, a corrector 1 molecule and a corrector 2 molecule to treat specified populations of patients with CF. We expect that our CF portfolio of drugs aimed at a triple combination therapy will compete with all these therapies. If generic or biosimilar versions of these therapies are approved we would expect to also compete against these versions of the therapies.
In the field of IPF there are two approved disease modifying drugs: pirfenidone (Esbriet), marketed by Roche, and nintedanib (Ofev), marketed by Boehringer Ingelheim. These drugs prolong life for IPF patients by months, leaving an unmet medical need for those developing disease-modifying drugs in this field.
In the field of OA, there are currently no disease-modifying drugs approved. Current treatment involves weight loss, physical therapy, and pain management.
In the field of AtD, generic drugs are approved standard of care, including immunomodulators cyclosporine and mycophenolate mofetil and topical treatments. There are disease-modifying biologics in currently in development.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, the EMA or other comparable regulatory authorities. Results of our trials could reveal a high and unacceptable severity and prevalence of certain side effects. In such an event, our trials could be suspended or terminated and the FDA, the EMA or comparable regulatory authorities could order us to cease
17
Table of Contents
further development of or deny approval of our product candidates for any or all targeted indications. The drug- related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition and prospects significantly.
If one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including:
| • | regulatory authorities may withdraw approvals of such product; |
| • | regulatory authorities may require additional warnings on the label; |
| • | we may be required to create a medication guide outlining the risks of such side effects for distribution to patients; |
| • | we could be sued and held liable for harm caused to patients; and |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results of operations and prospects.
Combination therapies involve unique adverse events that could be exacerbated compared to adverse events from monotherapies or could lead to unfavorable drug-drug interactions.
Combination therapies, such as using our wholly-owned product candidates as well as third-party agents, involve unique adverse events that could be exacerbated compared to adverse events from monotherapies or could lead to unfavorable drug-drug interactions. These types of adverse events could be caused by our product candidates or drug interactions and could also cause us, our collaboration partners or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, EMA or other comparable regulatory authority. For example, we or our collaboration partners may voluntarily suspend or terminate clinical trials if at any time one of our product candidates, or a combination therapy including any of them presents an unacceptable safety risk to the clinical trial patients. This, in turn, could prevent us or our collaboration partners from commercializing our product candidates. Results of our trials could reveal a high and unacceptable severity or prevalence of adverse events.
Risks Related to Our Financial Position and Need for Additional Capital
We are a clinical-stage company with no approved products and no historical product revenues, which makes it difficult to assess our future prospects and financial results.
We are a clinical-stage biotechnology company and we have not yet generated any product income. Pharmaceutical product development is a highly speculative undertaking and involves a substantial degree of uncertainty. Our operations to date have been limited to developing our technology and undertaking pre-clinical studies and clinical trials of our product candidates, including filgotinib, our CF candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737), GLPG1690, GLPG1972 and MOR106. We may not have the ability to overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the pharmaceutical area. Consequently, the ability to predict our future operating results or business prospects is more limited than if we had a longer operating history or approved products on the market.
We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. We have never generated any revenue from product sales and may never be profitable.
We have incurred significant operating losses since our inception in 1999. We have incurred net profits of €33.2 million for the year ended December 31, 2014, net losses of €118.4 million for the year ended
18
Table of Contents
December 31, 2015 and net profits of €54.0 million for the year ended December 31, 2016. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our shareholders’ equity and working capital. In April 2014, we sold our service division for net proceeds of €130.8 million, which explains the net profits recorded in 2014. In January 2016, Gilead made an equity investment in Galapagos through a subscription of new ordinary shares, which resulted in a positive non-cash fair value gain of €57.5 million in the financial result of 2016, contributing significantly to net profits recorded in 2016. We expect to continue incurring significant research, development and other expenses related to our ongoing operations, and to continue incurring operating losses for the foreseeable future. We also expect these losses to increase, due to higher costs of later stage development, as we continue our development of, and to seek regulatory approvals for, our product candidates.
We do not anticipate generating revenues from sales of products for the foreseeable future, if ever. If any of our product candidates fail in clinical trials or do not gain regulatory approval, or if any of our product candidates, if approved, fail to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
If one or more of our product candidates is approved for commercial sale and we retain commercial rights, we anticipate incurring significant costs associated with commercializing any such approved product candidate. Therefore, even if we are able to generate revenues from the sale of any approved product, we may never become profitable. Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to predict the timing or amount of expenses and when we will be able to achieve or maintain profitability, if ever.
We will require substantial additional funding, which may not be available to us on acceptable terms, or at all.
Our operations have consumed substantial amounts of cash since inception. We are currently conducting clinical trials for filgotinib, our CF candidates (including GLPG1837, GLPG2451, GLPG2222, and GLPG2737), GLPG1690, GLPG1972 and MOR106. Developing pharmaceutical product candidates, including conducting clinical trials, is expensive. We will require substantial additional future capital in order to complete clinical development and, if we are successful, to commercialize any of our current product candidates. If the FDA, or any other comparable regulatory agency, such as the EMA, requires that we perform studies or trials in addition to those that we currently anticipate with respect to the development of our product candidates, or repeat studies or trials, our expenses would further increase beyond what we currently expect, and any delay resulting from such further or repeat studies or trials could also result in the need for additional financing.
Our existing cash and cash equivalents will not be sufficient for us to complete advanced clinical development of any of our product candidates or, if applicable, to commercialize any product candidate that is approved. Accordingly, we will continue to require substantial additional capital to continue our clinical development activities and potentially engage in commercialization activities. Because successful development of our product candidates is uncertain, we are unable to estimate the actual funds we will require to complete research and development and commercialize our product candidates. The amount and timing of our future funding requirements will depend on many factors, including but not limited to:
| • | the progress, costs, results of and timing of our ongoing and planned clinical trials; |
| • | our ability to reach milestones under our existing collaboration arrangements and enter into additional collaborative agreements for the development and commercialization of our product candidates; |
| • | the willingness of the FDA, EMA and other comparable regulatory authorities to accept our clinical trials and pre-clinical studies and other work as the basis for review and approval of product candidates; |
| • | the outcome, costs and timing of seeking and obtaining regulatory approvals from the FDA, EMA and other comparable regulatory authorities; |
19
Table of Contents
| • | whether our collaboration partners continue to collaborate with us on the development and commercialization of our product candidates; |
| • | the number of product candidates and indications that we pursue, whether developed from our novel, proprietary target discovery platform, otherwise developed internally or in-licensed; |
| • | the timing and costs associated with manufacturing our product candidates for clinical trials and other studies and, if approved, for commercial sale; |
| • | our need to expand our development activities and, potentially, our research activities; |
| • | the timing and costs associated with establishing sales and marketing capabilities; |
| • | market acceptance of any approved product candidates; |
| • | the costs of acquiring, licensing or investing in additional businesses, products, product candidates and technologies; |
| • | the cost to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| • | the extent to which we may be required to pay milestone or other payments under our in-license agreements and the timing of such payments; |
| • | our need and ability to hire additional management, development and scientific personnel; and |
| • | our need to implement additional internal systems and infrastructure, including financial and reporting systems. |
Some of these factors are outside of our control. Based upon our current expected level of operating expenditures and our existing cash and cash equivalents, we believe that we will be able to fund our operating expenses and capital expenditure requirements at least for the next two to three years. This period could be shortened if there are any significant increases beyond our expectations in spending on development programs or more rapid progress of development programs than anticipated. Accordingly, we expect that we will need to raise substantial additional funds in the future. Additional funding may not be available to us on acceptable terms, or at all. If we are unable to obtain funding from equity offerings or debt financings, including on a timely basis, we may be required to:
| • | seek collaboration partners for one or more of our product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; |
| • | relinquish or license on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves; or |
| • | significantly curtail one or more of our research or development programs or cease operations altogether. |
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our product candidates or technologies.
We may seek additional funding through a combination of equity offerings, debt financings, collaborations and/or licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a holder of the ADSs or our ordinary shares. The incurrence of additional indebtedness and/or the issuance of certain equity securities could result in increased fixed payment obligations and could also result in certain additional restrictive covenants, such as limitations on our ability to
20
Table of Contents
incur additional debt and/or issue additional equity, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. In addition, issuance of additional equity securities, or the possibility of such issuance, may cause the market price of the ADSs or our ordinary shares to decline. In the event that we enter into collaborations and/or licensing arrangements in order to raise capital, we may be required to accept unfavorable terms, including relinquishing or licensing to a third party on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves or potentially reserve for future potential arrangements when we might be able to achieve more favorable terms.
Risks Related to Our Reliance on Third Parties
We may not be successful in maintaining development and commercialization collaborations, and any collaboration partner may not devote sufficient resources to the development or commercialization of our product candidates or may otherwise fail in development or commercialization efforts, which could adversely affect our ability to develop certain of our product candidates and our financial condition and operating results.
The collaboration arrangements that we have established, and any collaboration arrangements that we may enter into in the future may not ultimately be successful, which could have a negative impact on our business, results of operations, financial condition and growth prospects. If we partner with a third party for development and commercialization of a product candidate, we can expect to relinquish some or all of the control over the future success of that product candidate to the third party. It is possible that a collaboration partner may not devote sufficient resources to the development or commercialization of our product candidate or may otherwise fail in development or commercialization efforts, in which event the development and commercialization of such product candidate could be delayed or terminated and our business could be substantially harmed. In particular, we are heavily dependent on Gilead for its further development of our product candidate filgotinib and on AbbVie for its further development of our triple combination product candidate for the treatment of CF. Gilead and AbbVie may not devote sufficient resources or give sufficient priority to the filgotinib program or CF collaboration, respectively. Our collaborators may not elect to advance the product candidates on which we collaborate. Gilead may not be successful in the further development and commercialization of filgotinib, even when they do devote resources and prioritize their efforts for filgotinib. AbbVie may not be successful in the further development and commercialization of our potential triple combination product for the treatment of CF.
In addition, the terms of any collaboration or other arrangement that we establish may not be favorable to us or may not be perceived as favorable, which may negatively impact the trading price of the ADSs or our ordinary shares. In some cases, we may be responsible for continuing development of a product candidate or research program under a collaboration and the payment we receive from our collaboration partner may be insufficient to cover the cost of this development. Moreover, collaborations and sales and marketing arrangements are complex and time consuming to negotiate, document and implement and they may require substantial resources to maintain.
We are subject to a number of additional risks associated with our dependence on collaborations with third parties, the occurrence of which could cause our collaboration arrangements to fail. Conflicts may arise between us and collaboration partners, such as conflicts concerning the interpretation of clinical data, the achievement of milestones, the interpretation of financial provisions or the ownership of intellectual property developed during the collaboration. If any such conflicts arise, a collaboration partner could act in its own self-interest, which may be adverse to our best interests. Any such disagreement between us and a collaboration partner could result in one or more of the following, each of which could delay or prevent the development or commercialization of our product candidates, and in turn prevent us from generating sufficient revenues to achieve or maintain profitability:
| • | reductions in the payment of royalties or other payments we believe are due pursuant to the applicable collaboration arrangement; |
21
Table of Contents
| • | actions taken by a collaboration partner inside or outside our collaboration which could negatively impact our rights or benefits under our collaboration including termination of the collaboration for convenience by the partner; or |
| • | unwillingness on the part of a collaboration partner to keep us informed regarding the progress of its development and commercialization activities or to permit public disclosure of the results of those activities. |
If our collaborations on research and development candidates do not result in the successful development and commercialization of products or if one of our collaboration partners terminates its agreement with us, we may not receive any future research funding or milestone or royalty payments under the collaboration. If we do not receive the funding we expect under these agreements, our development of our product candidates could be delayed and we may need additional resources to develop product candidates.
We may not be successful in establishing development and commercialization collaborations, which could adversely affect, and potentially prohibit, our ability to develop our product candidates.
Developing pharmaceutical products, conducting clinical trials, obtaining regulatory approval, establishing manufacturing capabilities and marketing approved products are expensive. Accordingly, we have sought and may in the future seek to enter into collaborations with companies that have more resources and experience. If we are unable to obtain a collaboration partner for our product candidates, we may be unable to advance the development of our product candidates through late-stage clinical development and seek approval in any market. In situations where we enter into a development and commercial collaboration arrangement for a product candidate, we may also seek to establish additional collaborations for development and commercialization in territories outside of those addressed by the first collaboration arrangement for such product candidate. If any of our product candidates receives marketing approval, we may enter into sales and marketing arrangements with third parties with respect to otherwise unlicensed or unaddressed territories. There are a limited number of potential collaboration partners, and we expect to face competition in seeking appropriate collaboration partners. If we are unable to enter into any development and commercial collaborations and/ or sales and marketing arrangements on acceptable terms, or at all, we may be unable to successfully develop and seek regulatory approval for our product candidates and/or effectively market and sell approved products, if any.
We rely on third parties to conduct our pre-clinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon CROs to monitor and manage data for our pre-clinical and clinical programs. We rely on these parties for execution of our pre-clinical studies and clinical trials, and we control only certain aspects of their activities. We and our CROs also rely upon clinical sites and investigators for the performance of our clinical trials in accordance with the applicable protocols and applicable legal and regulatory requirements and scientific standards. Nevertheless, we are responsible for ensuring that each of our studies and trials is conducted in accordance with the applicable protocol and applicable legal and regulatory requirements and scientific standards, and our reliance on CROs as well as clinical sites and investigators does not relieve us of our regulatory responsibilities. We, our CROs, as well as the clinical sites and investigators are required to comply with current GCPs, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area, or EEA, and comparable regulatory authorities for all of our products in clinical development. Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, investigators and clinical sites. If we, any of our CROs or any of the clinical sites or investigators fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, EMA or comparable regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon
22
Table of Contents
inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations. We also cannot assure you that our CROs, as well as the clinical sites and investigators, will perform our clinical trials in accordance with the applicable protocols as well as applicable legal and regulatory requirements and scientific standards, or report the results obtained in a timely and accurate manner. In addition to GCPs, our clinical trials must be conducted with product produced under cGMP regulations. While we have agreements governing activities of our CROs, we have limited influence over the actual performance of our CROs as well as the performance of clinical sites and investigators. In addition, significant portions of the clinical trials for our product candidates are and will continue to be conducted outside of Belgium, which will make it more difficult for us to monitor CROs as well as clinical sites and investigators and perform visits of our clinical sites, and will force us to rely heavily on CROs to ensure the proper and timely conduct of our clinical trials in accordance with the applicable protocols and compliance with applicable regulations, including GCPs. Failure to comply with applicable protocols and regulations in the conduct of the clinical trials for our product candidates may require us to repeat clinical trials, which would delay the regulatory approval process.
Some of our CROs have an ability to terminate their respective agreements with us if it can be reasonably demonstrated that the safety of the subjects participating in our clinical trials warrants such termination, if we make a general assignment for the benefit of our creditors or if we are liquidated.
If any of our relationships with these CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms. In addition, our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our pre-clinical and clinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure (including by clinical sites or investigators) to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase substantially and our ability to generate revenues could be delayed significantly.
Switching or adding additional CROs involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays may occur, which can materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
We rely completely on third parties to manufacture our pre-clinical and clinical drug supplies and we intend to rely on third parties to produce commercial supplies of any approved product candidate.
If, for any reason, we were to experience an unexpected loss of supply of our product candidates or placebo or comparator drug used in certain of our clinical trials, whether as a result of manufacturing, supply or storage issues or otherwise, we could experience delays, disruptions, suspensions or terminations of, or be required to restart or repeat, any pending or ongoing clinical trials. We do not currently have, nor do we plan to acquire, the infrastructure or capability internally to manufacture our pre-clinical and clinical drug supplies and we lack the resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. The facilities used by our contract manufacturers or other third-party manufacturers to manufacture our product candidates are subject to the FDA’s, EMA’s and other comparable regulatory authorities’ pre-approval inspections that will be conducted after we submit our NDA or BLA to the FDA or the required approval applications to any other relevant regulatory authority. We do not control the implementation of the manufacturing process of, and are completely dependent on, our contract manufacturers or other third-party
23
Table of Contents
manufacturers for compliance with cGMP regulatory requirements for manufacture of both active drug substances and finished drug products. If our contract manufacturers or other third-party manufacturers cannot successfully manufacture material that conforms to applicable specifications and the strict regulatory requirements of the FDA, EMA or others, we will not be able to secure and/or maintain regulatory approvals for our products manufactured at these facilities. In addition, we have no control over the ability of our contract manufacturers or other third-party manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA, EMA or other comparable regulatory authority finds deficiencies at these facilities for the manufacture of our product candidates or if it withdraws any approval because of deficiencies at these facilities in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved.
We rely on our manufacturers to purchase from third-party suppliers the materials necessary to produce our product candidates for our clinical trials. There are a limited number of suppliers for raw materials that we use to manufacture our drugs and there may be a need to assess alternate suppliers to prevent a possible disruption of the manufacture of the materials necessary to produce our product candidates for our clinical trials, and if approved, for commercial sale. We do not have any control over the process or timing of the acquisition of these raw materials by our manufacturers. Moreover, we currently do not have any agreements for the commercial production of these raw materials. Although we generally do not begin a clinical trial unless we believe we have access to a sufficient supply of a product candidate to complete the clinical trial, any significant delay in the supply of a product candidate, or the raw material components thereof, for an ongoing clinical trial due to the need to replace a contract manufacturer or other third-party manufacturer could considerably delay completion of our clinical trials, product testing and potential regulatory approval of our product candidates. If our manufacturers or we are unable to purchase these raw materials after regulatory approval has been obtained for our product candidates, the commercial launch of our product candidates would be delayed or there would be a shortage in supply, which would impair our ability to generate revenues from the sale of our product candidates. Additionally, if we receive regulatory approval for our product candidates, we may experience unforeseen difficulties or challenges in the manufacture of our product candidates on a commercial scale compared to the manufacture for clinical purposes.
We expect to continue to depend on contract manufacturers or other third-party manufacturers for the foreseeable future. We currently obtain our supplies of finished drug product through individual purchase orders. We have not entered into long-term agreements with our current contract manufacturers or with any alternate fill/ finish suppliers. Although we intend to do so prior to any commercial launch in order to ensure that we maintain adequate supplies of finished drug product, we may be unable to enter into such an agreement or do so on commercially reasonable terms, which could have a material adverse impact upon our business.
We rely on clinical data and results obtained by third parties that could ultimately prove to be inaccurate or unreliable.
As part of our strategy to mitigate development risk, we seek to develop product candidates with validated mechanisms of action and we utilize biomarkers to assess potential clinical efficacy early in the development process. This strategy necessarily relies upon clinical data and other results obtained by third parties that may ultimately prove to be inaccurate or unreliable. Further, such clinical data and results may, at times, be based on products or product candidates that are significantly different from our product candidates. If the third-party data and results we rely upon prove to be inaccurate, unreliable or not applicable to our product candidates, we could make inaccurate assumptions and conclusions about our product candidates and our research and development efforts could be materially adversely affected.
Risks Related to Our Intellectual Property
Our ability to compete may decline if we do not adequately protect our proprietary rights.
Our commercial success depends on obtaining and maintaining proprietary rights to our product candidates for the treatment of RA, CD, UC, CF and other diseases, as well as successfully defending these rights against
24
Table of Contents
third-party challenges. We will only be able to protect our product candidates, and their uses from unauthorized use by third parties to the extent that valid and enforceable patents, or effectively protected trade secrets, cover them. Our ability to obtain patent protection for our product candidates is uncertain due to a number of factors, including:
| • | we may not have been the first to make the inventions covered by pending patent applications or issued patents; |
| • | we may not have been the first to file patent applications for our product candidates or the compositions we developed or for their uses; |
| • | others may independently develop identical, similar or alternative products or compositions and uses thereof; |
| • | our disclosures in patent applications may not be sufficient to meet the statutory requirements for patentability; |
| • | any or all of our pending patent applications may not result in issued patents; |
| • | we may not seek or obtain patent protection in countries that may eventually provide us a significant business opportunity; |
| • | any patents issued to us may not provide a basis for commercially viable products, may not provide any competitive advantages, or may be successfully challenged by third parties; |
| • | our compositions and methods may not be patentable; |
| • | others may design around our patent claims to produce competitive products which fall outside of the scope of our patents; or |
| • | others may identify prior art or other bases which could invalidate our patents. |
Even if we have or obtain patents covering our product candidates or compositions, we may still be barred from making, using and selling our product candidates or technologies because of the patent rights of others. Others may have filed, and in the future may file, patent applications covering compositions or products that are similar or identical to ours. If a patent owned by a third party covers one of our product candidates or its use, this could materially affect our ability to develop the product candidate or sell the resulting product if approved. Because patent applications are not published until 18 months from their priority date, there may be currently pending applications unknown to us that may later result in issued patents that our product candidates or compositions may infringe. Additionally, because the scope of claims in pending patent applications can change, there may be pending applications whose claims do not currently cover any of our product candidates but may be altered such that one or more of our product candidates are covered when the resulting patent issues. These patent applications may have priority over patent applications filed by us.
Moreover, even if we are able to obtain patent protection, such patent protection may be of insufficient scope to achieve our business objectives. For example, others may be able to develop a product that is similar to, or better than, ours in a way that is not covered by the claims of our patents.
Obtaining and maintaining a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity fees, various other governmental fees on patents and/or applications due in several stages over the lifetime of patents and/or applications, as well as the cost associated with complying with numerous procedural provisions during the patent application process. We may or may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we choose to forgo patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer. Moreover, in some circumstances, we do not
25
Table of Contents
have the right to control the preparation, filing or prosecution of patent applications, or to maintain the patents, covering technology subject to our collaboration or license agreements with third parties. For example, under our collaboration agreement with AbbVie for CF, AbbVie has the right to control prosecution and maintenance of any patent rights covering inventions that are jointly discovered or developed by us and AbbVie and patent rights that we control which relate to the compounds or products subject to the collaboration. In addition, in some circumstances, our counterparty has the right to enforce the patent rights subject to the applicable agreement without our involvement or consent or to otherwise control the enforcement of such patent rights. For example, under our collaboration agreement with AbbVie for CF, AbbVie controls the enforcement of the patent rights subject to the agreement, although we may elect to participate in such enforcement proceedings and under our collaboration agreement with Gilead, Gilead controls any litigation on our patents for filgotinib. Therefore, these patents and patent applications may not be prosecuted or enforced in a manner consistent with the best interests of our business.
Legal actions to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or other actions against those that have infringed on our patents, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our results of operations.
Pharmaceutical patents and patent applications involve highly complex legal and factual questions, which, if determined adversely to us, could negatively impact our patent position.
The patent positions of biotechnology and pharmaceutical companies can be highly uncertain and involve complex legal and factual questions. The interpretation and breadth of claims allowed in some patents covering pharmaceutical compositions may be uncertain and difficult to determine, and are often affected materially by the facts and circumstances that pertain to the patented compositions and the related patent claims. The standards of the United States Patent and Trademark Office, or USPTO, the European Patent Office, and other foreign counterparts are sometimes uncertain and could change in the future. Consequently, the issuance and scope of patents cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated or circumvented. Certain U.S. patents and patent applications may also be subject to interference proceedings, and U.S. patents may be subject to reexamination proceedings, post-grant review and/or inter partes review in the USPTO. European patents and other foreign patents may be subject also to opposition or comparable proceedings in the corresponding foreign patent office, which could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, such interference, reexamination, post-grant review, inter partes review and opposition proceedings may be costly. Accordingly, rights under any issued patents may not provide us with sufficient protection against competitive products or processes.
In addition, changes in or different interpretations of patent laws in the United States, Europe, and other jurisdictions may permit others to use our discoveries or to develop and commercialize our technology and products without providing any compensation to us, or may limit the number of patents or claims we can obtain. The laws of some countries do not protect intellectual property rights to the same extent as U.S. and European laws and those countries may lack adequate rules and procedures for defending our intellectual property rights.
If we fail to obtain and maintain patent protection and trade secret protection of our product candidates, we could lose our competitive advantage and competition we face would increase, reducing any potential revenues and adversely affecting our ability to attain or maintain profitability.
Developments in patent law could have a negative impact on our business.
From time to time, courts and other governmental authorities in the United States, Europe and other jurisdictions may change the standards of patentability and any such changes could have a negative impact on our business.
26
Table of Contents
For example, the Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged, and changes to the way patent applications are disputed during the examination process. These changes may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. Substantive changes to patent law associated with the America Invents Act may affect our ability to obtain patents, and if obtained, to enforce or defend them. Accordingly, it is not clear what impact, if any, the America Invents Act will have on the cost of prosecuting our patent applications, our ability to obtain patents based on our discoveries and our ability to enforce or defend any patents that may issue from our patent applications, all of which could have a material adverse effect on our business.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to patent protection, because we operate in the highly technical field of development of therapies, we rely in part on trade secret protection in order to protect our proprietary technology and processes. However, trade secrets are difficult to protect. It is our policy to enter into confidentiality and intellectual property assignment agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. These agreements also generally provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. Adequate remedies may not exist in the event of unauthorized use or disclosure of our confidential information. The disclosure of our trade secrets would impair our competitive position and may materially harm our business, financial condition and results of operations.
In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or a third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and recourse we take against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets, with protection varying across Europe and in other countries. Trade secrets may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed.
We will not seek to protect our intellectual property rights in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection.
Filing, prosecuting and defending patents on our product candidates in all countries and jurisdictions throughout the world would be prohibitively expensive, and our intellectual property rights in some countries could be less extensive than those in the United States and Europe, assuming that rights are obtained in the United States and Europe. Furthermore, even if patents are granted based on our European patent applications, we may not choose to perfect or maintain our rights in all available European countries. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as laws in the United States and Europe. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries, or from selling or importing products made using our inventions. The statutory deadlines for pursuing patent protection in individual foreign jurisdictions are based on the priority dates of each of our patent applications.
27
Table of Contents
Competitors may use our technologies in jurisdictions where we do not pursue and obtain patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States and Europe. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing. Even if we pursue and obtain issued patents in particular jurisdictions, our patent claims or other intellectual property rights may not be effective or sufficient to prevent third parties from so competing.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States and Europe. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to pharmaceuticals or biotechnologies. This could make it difficult for us to stop the infringement of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. In addition, changes in the law and legal decisions by courts in the United States, Europe and other jurisdictions may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
We may be subject to claims by third parties asserting ownership or commercial rights to inventions we develop or obligations to make compensatory payments to employees.
Third parties may in the future make claims challenging the inventorship or ownership of our intellectual property. We have written agreements with collaboration partners that provide for the ownership of intellectual property arising from our collaborations. These agreements provide that we must negotiate certain commercial rights with collaboration partners with respect to joint inventions or inventions made by our collaboration partners that arise from the results of the collaboration. In some instances, there may not be adequate written provisions to address clearly the resolution of intellectual property rights that may arise from a collaboration. If we cannot successfully negotiate sufficient ownership and commercial rights to the inventions that result from our use of a third-party collaboration partner’s materials where required, or if disputes otherwise arise with respect to the intellectual property developed with the use of a collaboration partner’s samples, we may be limited in our ability to capitalize on the market potential of these inventions. In addition, we may face claims by third parties that our agreements with employees, contractors, or consultants obligating them to assign intellectual property to us are ineffective, or in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes regarding intellectual property we have developed or will develop and interfere with our ability to capture the commercial value of such inventions. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property, or may lose our exclusive rights in that intellectual property. Either outcome could have an adverse impact on our business.
28
Table of Contents
While it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing or obtaining such an agreement with each party who, in fact, develops intellectual property that we regard as our own. In addition, such agreements may be breached or may not be self-executing, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We employ individuals who were previously employed at universities, pharmaceutical companies or biopharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
A dispute concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time consuming and costly, and an unfavorable outcome could harm our business.
Our success will depend in part on our ability to operate without infringing the intellectual property and proprietary rights of third parties. We cannot assure you that our business, products and methods do not or will not infringe the patents or other intellectual property rights of third parties.
There is significant litigation in the pharmaceutical industry regarding patent and other intellectual property rights. While we are not currently subject to any pending intellectual property litigation, and are not aware of any such threatened litigation, we may be exposed to future litigation by third parties based on claims that our product candidates, technologies or activities infringe the intellectual property rights of others. If our development activities are found to infringe any such patents, we may have to pay significant damages or seek licenses to such patents. A patentee could prevent us from using the patented drugs or compositions. We may need to resort to litigation to enforce a patent issued to us, to protect our trade secrets, or to determine the scope and validity of third-party proprietary rights. From time to time, we may hire scientific personnel or consultants formerly employed by other companies involved in one or more areas similar to the activities conducted by us. Either we or these individuals may be subject to allegations of trade secret misappropriation or other similar claims as a result of prior affiliations. Even if we are successful in these proceedings, we may incur substantial costs and divert management time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court, or redesign our products. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a material adverse impact on our cash position and the price of the ADSs or our ordinary shares. Any legal action against us or our collaboration partners could lead to:
| • | payment of substantial damages for past use of the asserted intellectual property and potentially treble damages, if we are found to have willfully infringed a party’s patent rights; |
| • | injunctive or other equitable relief that may effectively block our ability to further develop, commercialize, and sell our product candidates; or |
29
Table of Contents
| • | us or our collaboration partners having to enter into license arrangements that may not be available on commercially acceptable terms, if at all, all of which could have a material adverse impact on our cash position and business and financial condition. As a result, we could be prevented from commercializing current or future product candidates. |
Any of these risks coming to fruition could have a material adverse effect on our business, results of operations, financial condition and prospects.
Issued patents covering our product candidates could be found to be invalid or unenforceable if challenged in court.
If we or one of our licensing partners initiated legal proceedings against a third party to enforce a patent covering our product candidate, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include alleged failures to meet any of several statutory requirements in most jurisdictions, including lack of novelty, obviousness or non-enablement. In the United States, grounds for unenforceability assertions include allegations that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review and equivalent proceedings in foreign jurisdictions, e.g., opposition proceedings. Such proceedings could result in revocation or amendment of our patents in such a way that they no longer cover our product candidates or competitive products. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to validity, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection would have a material adverse impact on our business.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition by potential partners or customers in our markets of interest. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected.
Risks Related to Our Organization, Structure and Operation
Our future success depends on our ability to retain the members of our executive committee and to attract, retain and motivate qualified personnel. If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our industry has experienced a high rate of turnover of management personnel in recent years. Our ability to compete in the highly competitive biotechnology and pharmaceutical industries depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. We are highly dependent on our management, scientific and medical personnel, especially our executive committee comprised of: Onno van de Stolpe, our chief executive officer; Bart Filius, our chief financial officer; Piet Wigerinck, our chief scientific officer; and Andre Hoekema, our senior vice president of corporate development, whose services are critical to the successful implementation of our product candidate acquisition, development and regulatory strategies. We are not aware of any present intention of any of these individuals to leave our company. In order to induce valuable employees to continue their employment with us, we have provided warrants that vest over time. The
30
Table of Contents
value to employees of warrants that vest over time is significantly affected by movements in our share price that are beyond our control, and may at any time be insufficient to counteract more lucrative offers from other companies.
Despite our efforts to retain valuable employees, members of our management, scientific and development teams may terminate their employment with us at any time, with or without notice. The loss of the services of any of the members of our executive committee or other key employees and our inability to find suitable replacements could harm our business, financial condition and prospects. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level, and senior managers as well as junior, mid-level, and senior scientific and medical personnel.
We may not be able to attract or retain qualified management and scientific personnel in the future due to the intense competition for a limited number of qualified personnel among biopharmaceutical, biotechnology, pharmaceutical and other businesses. Many of the other pharmaceutical companies that we compete against for qualified personnel have greater financial and other resources, different risk profiles and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high quality candidates than what we have to offer. If we are unable to continue to attract and retain high quality personnel, the rate and success at which we can develop and commercialize product candidates will be limited.
If we fail to manage our growth effectively, our ability to develop and commercialize products could suffer.
We expect that if our drug discovery efforts continue to generate product candidates, our clinical product candidates continue to progress in development, and we continue to build our development, medical and commercial organizations, we will require significant additional investment in personnel, management and resources. Our ability to achieve our research, development and commercialization objectives depends on our ability to respond effectively to these demands and expand our internal organization, systems, controls and facilities to accommodate additional anticipated growth. If we are unable to manage our growth effectively, our business could be harmed and our ability to execute our business strategy could suffer.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of any of our product candidates, if approved.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. Physicians and patients may not comply with any warnings that identify known potential adverse effects and patients who should not use our products. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to stop development or, if approved, limit commercialization of our product candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| • | delay or termination of clinical trials; |
| • | injury to our reputation; |
| • | withdrawal of clinical trial participants; |
| • | initiation of investigations by regulators; |
| • | costs to defend the related litigation; |
31
Table of Contents
| • | a diversion of management’s time and our resources; |
| • | substantial monetary awards to trial participants or patients; |
| • | decreased demand for our product candidates; |
| • | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| • | loss of revenues from product sales; and |
| • | the inability to commercialize any our product candidates, if approved. |
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the development or commercialization of our product candidates. We currently carry clinical trial liability insurance at levels which we believe are appropriate for our clinical trials. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Risks from the improper conduct of employees, agents, contractors, or collaboration partners could adversely affect our reputation and our business, prospects, operating results, and financial condition.
We cannot ensure that our compliance controls, policies, and procedures will in every instance protect us from acts committed by our employees, agents, contractors, or collaboration partners that would violate the laws or regulations of the jurisdictions in which we operate, including, without limitation, healthcare, employment, foreign corrupt practices, environmental, competition, and patient privacy and other privacy laws and regulations. Such improper actions could subject us to civil or criminal investigations, and monetary and injunctive penalties, and could adversely impact our ability to conduct business, operating results, and reputation.
In particular, our business activities may be subject to the Foreign Corrupt Practices Act, or FCPA, and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate, including the U.K. Bribery Act. The FCPA generally prohibits offering, promising, giving, or authorizing others to give anything of value, either directly or indirectly, to a non-U.S. government official in order to influence official action, or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. Additionally, in many other countries, the health care providers who prescribe pharmaceuticals are employed by their government, and the purchasers of pharmaceuticals are government entities; therefore, our dealings with these prescribers and purchasers are subject to regulation under the FCPA. Recently the SEC and U.S. Department of Justice have increased their FCPA enforcement activities with respect to pharmaceutical companies. There is no certainty that all of our employees, agents, contractors, or collaboration partners, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers, or our employees, the closing down of our facilities, requirements to obtain export licenses, cessation of business activities in sanctioned countries, implementation of compliance programs, and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries and could materially damage our reputation, our brand, our international expansion efforts, our ability to attract and retain employees, and our business, prospects, operating results, and financial condition.
32
Table of Contents
We could be subject to liabilities under environmental, health and safety laws or regulations, or fines, penalties or other sanctions, if we fail to comply with such laws or regulations or otherwise incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws, regulations, and permitting requirements, including those governing laboratory procedures, decontamination activities and the handling, transportation, use, remediation, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals, radioactive isotopes and biological materials and produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials or wastes either at our sites or at third-party disposal sites. In the event of such contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties. Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws, regulations or permitting requirements. These current or future laws, regulations and permitting requirements may impair our research, development or production efforts. Failure to comply with these laws, regulations and permitting requirements also may result in substantial fines, penalties or other sanctions.
Any future relationships with customers and third-party payors may be subject, directly or indirectly, to applicable anti-kickback laws, fraud and abuse laws, false claims laws, health information privacy and security laws and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens and diminished profits and future earnings.
If we obtain FDA, EMA or any other comparable regulatory authority approval for any of our product candidates and begin commercializing those products in the United States, European Union or other jurisdiction, our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any products for which we obtain marketing approval. In addition, we may be subject to health information privacy and security regulation of the European Union, the United States and other jurisdictions in which we conduct our business. For example, the laws that may affect our ability to operate include:
| • | the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, either the referral of an individual, or the purchase or recommendation of an item or service for which payment may be made under a federal healthcare program, such as the Medicare and Medicaid programs; |
| • | U.S. federal civil and criminal false claims laws and civil monetary penalty laws, including the federal False Claims Act, which impose criminal and civil penalties against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the U.S. federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which imposes certain obligations, |
33
Table of Contents
| including mandatory contractual terms, on covered healthcare providers, health plans, and healthcare clearinghouses, as well as their business associates, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; and |
| • | analogous state and laws and regulations in other jurisdictions, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and state and laws in other jurisdiction governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, fines, imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations or other sanctions. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws and regulations, that person or entity may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government funded healthcare programs.
We must maintain effective internal control over financial reporting, and if we are unable to do so, the accuracy and timeliness of our financial reporting may be adversely affected, which could have a material adverse effect on our business, investor confidence and market price.
We must maintain effective internal control over financial reporting in order to accurately and timely report our results of operations and financial condition. We often use estimates and assumptions concerning the future, especially when performing impairment tests on (in)tangible assets. We perform these tests whenever there is an impairment indicator. In addition, because we are a U.S. public company, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, requires, among other things, that we assess the effectiveness of our disclosure controls and procedures annually and the effectiveness of our internal control over financial reporting at the end of each fiscal year.
We are no longer an “emerging growth company” and we will no longer be able to avail ourselves of exemptions from various reporting requirements applicable to other public companies but not to “emerging growth companies.” For example, the Sarbanes-Oxley Act requires, among other things, that we assess the effectiveness of our internal control over financial reporting annually and the effectiveness of our disclosure controls and procedures quarterly. Section 404 of the Sarbanes-Oxley Act (“Section 404”) requires us to perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on, and our independent registered public accounting firm potentially to attest to, the effectiveness of our internal control over financial reporting. We previously availed ourselves of the exemption from the requirement that our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting under Section 404. However, we are no longer able to avail ourselves of this exemption. Our independent registered public accounting firm is now required to undertake an assessment of our internal control over financial reporting, and as a result the cost of our compliance with Section 404 will correspondingly increase. The rules governing the standards that must be met for our management to assess our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act are complex and require significant documentation, testing and possible remediation. These stringent standards require that our audit committee be advised and regularly updated on management’s review of internal control over financial reporting. Our compliance with applicable provisions of Section 404 will require that we incur substantial accounting expense
34
Table of Contents
and expend significant management time on compliance-related issues as we implement additional corporate governance practices and comply with reporting requirements. Moreover, if we are not able to comply with the requirements of Section 404 applicable to us in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of the ADSs or our ordinary shares could decline and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources. Furthermore, investor perceptions of our company may suffer if deficiencies are found, and this could cause a decline in the market price of the ADSs or our ordinary shares. Irrespective of compliance with Section 404, any failure of our internal control over financial reporting could have a material adverse effect on our stated operating results and harm our reputation. If we are unable to implement these requirements effectively or efficiently, it could harm our operations, financial reporting, or financial results and could result in an adverse opinion on our internal control over financial reporting from our independent registered public accounting firm.
Our information technology systems could face serious disruptions that could adversely affect our business.
Our information technology and other internal infrastructure systems, including corporate firewalls, servers, leased lines and connection to the Internet, face the risk of systemic failure that could disrupt our operations. A significant disruption in the availability of our information technology and other internal infrastructure systems could cause interruptions in our collaborations with our partners and delays in our research and development work. The loss of product development or clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our development programs and the development of our product candidates could be delayed.
Business interruptions could delay us in the process of developing our product candidates.
Loss of our laboratory facilities through fire or other causes could have an adverse effect on our ability to continue to conduct our business. We currently have insurance coverage to compensate us for such business interruptions; however, such coverage may prove insufficient to fully compensate us for the damage to our business resulting from any significant property or casualty loss to our facilities.
We may undertake strategic acquisitions in the future and any difficulties from integrating such acquisitions could adversely affect our share price, operating results and results of operations.
We may acquire companies, businesses and products that complement or augment our existing business. We may not be able to integrate any acquired business successfully or operate any acquired business profitably. Integrating any newly acquired business could be expensive and time-consuming. Integration efforts often take a significant amount of time, place a significant strain on managerial, operational and financial resources, result in loss of key personnel and could prove to be more difficult or expensive than we predict. The diversion of our management’s attention and any delay or difficulties encountered in connection with any future acquisitions we may consummate could result in the disruption of our on-going business or inconsistencies in standards and controls that could negatively affect our ability to maintain third-party relationships. Moreover, we may need to raise additional funds through public or private debt or equity financing, or issue additional shares, to acquire any businesses or products, which may result in dilution for shareholders or the incurrence of indebtedness.
As part of our efforts to acquire companies, business or product candidates or to enter into other significant transactions, we conduct business, legal and financial due diligence with the goal of identifying and evaluating material risks involved in the transaction. Despite our efforts, we ultimately may be unsuccessful in ascertaining or evaluating all such risks and, as a result, might not realize the intended advantages of the transaction. If we fail to realize the expected benefits from acquisitions we may consummate in the future or have consummated in the
35
Table of Contents
past, whether as a result of unidentified risks or liabilities, integration difficulties, regulatory setbacks, litigation with current or former employees and other events, our business, results of operations and financial condition could be adversely affected. If we acquire product candidates, we will also need to make certain assumptions about, among other things, development costs, the likelihood of receiving regulatory approval and the market for such product candidates. Our assumptions may prove to be incorrect, which could cause us to fail to realize the anticipated benefits of these transactions.
In addition, we will likely experience significant charges to earnings in connection with our efforts, if any, to consummate acquisitions. For transactions that are ultimately not consummated, these charges may include fees and expenses for investment bankers, attorneys, accountants and other advisors in connection with our efforts. Even if our efforts are successful, we may incur, as part of a transaction, substantial charges for closure costs associated with elimination of duplicate operations and facilities and acquired in-process research and development charges. In either case, the incurrence of these charges could adversely affect our results of operations for particular periods.
Our collaboration arrangements with our strategic partners may make us an attractive target for potential acquisitions under certain circumstances.
Under certain circumstances, due to the structure of our collaboration arrangements with our strategic partners, our strategic partners may prefer to acquire us rather than paying the milestone payments or royalties under the collaboration arrangements, which may bring additional uncertainties to our business development and prospects.
Our international operations subject us to various risks, and our failure to manage these risks could adversely affect our results of operations.
We face significant operational risks as a result of doing business internationally, such as:
| • | fluctuations in foreign currency exchange rates; |
| • | potentially adverse and/or unexpected tax consequences, including penalties due to the failure of tax planning or due to the challenge by tax authorities on the basis of transfer pricing and liabilities imposed from inconsistent enforcement; |
| • | potential changes to the accounting standards, which may influence our financial situation and results; |
| • | becoming subject to the different, complex and changing laws, regulations and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties and regulations; |
| • | reduced protection of, or significant difficulties in enforcing, intellectual property rights in certain countries; |
| • | difficulties in attracting and retaining qualified personnel; |
| • | restrictions imposed by local labor practices and laws on our business and operations, including unilateral cancellation or modification of contracts; |
| • | rapid changes in global government, economic and political policies and conditions, political or civil unrest or instability, terrorism or epidemics and other similar outbreaks or events, and potential failure in confidence of our suppliers or customers due to such changes or events; and |
| • | tariffs, trade protection measures, import or export licensing requirements, trade embargoes and other trade barriers. |
Recent developments relating to the United Kingdom’s referendum vote in favor of withdrawal from the European Union could adversely affect us.
The United Kingdom held a referendum on June 23, 2016 in which a majority voted for the United Kingdom’s withdrawal from the European Union (referred to as “Brexit”). As a result of this vote, negotiations
36
Table of Contents
are expected to commence to determine the terms of the United Kingdom’s withdrawal from the European Union as well as its relationship with the European Union going forward, including the terms of trade between the United Kingdom and the European Union. The effects of Brexit have been and are expected to continue to be far-reaching. Brexit and the perceptions as to its impact may adversely affect business activity and economic conditions in Europe and globally and could continue to contribute to instability in global financial and foreign exchange markets. Brexit could also have the effect of disrupting the free movement of goods, services and people between the United Kingdom and the European Union; however, the full effects of Brexit are uncertain and will depend on any agreements the United Kingdom may make to retain access to European Union markets.
In addition, we expect that Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the United Kingdom determines which European Union laws to replicate or replace. If the United Kingdom were to significantly alter its regulations affecting the pharmaceutical industry, we could face significant new costs. It may also be time-consuming and expensive for us to alter our internal operations in order to comply with new regulations. Altered regulations could also add time and expense to the process by which our product candidates receive regulatory approval in the United Kingdom and European Union. Similarly, it is unclear at this time what impact Brexit will have on our intellectual property rights and the process for obtaining, maintaining and defending such rights. It is possible that certain intellectual property rights, such as trademarks, granted by the European Union will cease being enforceable in the United Kingdom absent special arrangements to the contrary, and we are required to refile our trademarks and other intellectual property applications domestically in the United Kingdom.
Lastly, as a result of Brexit, other European countries may seek to conduct referenda with respect to their continuing membership in the European Union. Given these possibilities and others we may not anticipate, as well as the lack of comparable precedent, the full extent to which our business, results of operations and financial condition could be adversely affected by Brexit is uncertain.
If we are unable to use tax loss carryforwards to reduce future taxable income or benefit from favorable tax legislation, our business, results of operations and financial condition may be adversely affected.
At December 31, 2016, we had cumulative carry forward tax losses of €230.9 million in Belgium, €61.5 million in France (when taking into account pending tax litigation effect), and €18.7 million related to the other entities of our company. These are available to carry forward and offset against future taxable income for an indefinite period in Belgium and France, but approximately €17.8 million of these tax loss carryforwards in Switzerland, Croatia, the United States and the Netherlands will expire between 2018 and 2030. If we are unable to use tax loss carryforwards to reduce future taxable income, our business, results of operations and financial condition may be adversely affected. As a company active in research and development in Belgium and France, we have benefited from certain research and development incentives including, for example, the Belgian research and development tax credit and the French research tax credit (crédit d’impôt recherche). These tax credits can be offset against Belgian and French corporate income tax due, respectively. The excess portion may be refunded as from the end of a five-year fiscal period for the Belgian research and development incentive, and at the end of a three-year fiscal period for the French research and development incentive. The research and development incentives are both calculated based on the amount of eligible research and development expenditure. The Belgian tax credit represented €4.3 million for the year ended December 31, 2014, €5.4 million for the year ended December 31, 2015 and €5.7 million for the year ended December 31, 2016. The French tax credit amounted to €7.8 million for the year ended December 31, 2014, €8.7 million for the year ended December 31, 2015 and €9.5 million for the year ended December 31, 2016. The Belgian and/or French tax authorities may audit each research and development program in respect of which a tax credit has been claimed and assess whether it qualifies for the tax credit regime. The tax authorities may challenge our eligibility for, or our calculation of, certain tax reductions and/or deductions in respect of our research and development activities and, should the Belgian and/or French tax authorities be successful, we may be liable for additional corporate income tax, and penalties and interest related thereto, which could have a significant impact on our results of operations and future cash flows. Furthermore, if the Belgian and/or the French government decide to eliminate, or reduce
37
Table of Contents
the scope or the rate of, the research and development incentive benefit, either of which it could decide to do at any time, our results of operations could be adversely affected.
As a company active in research and development in Belgium, we also expect to benefit in the future from the “patent income deduction” or the replacing “innovation deduction” in Belgium. The patent income deduction regime allows, in the case of taxable income, gross profits attributable to revenue from patented products to be taxed at a lower rate than other revenues, i.e., 6.8%. The innovation deduction regime allows net profits attributable to revenue from among others patented products (or products for which the patent application is pending) to be taxed at a lower rate than other revenues, i.e., 5.1%. When taken in combination with tax losses carried forward and research and development incentives mentioned above, we expect that this will result in a long-term low rate of corporation tax for us. The innovation deduction applies as of July 1, 2016, although subject to various conditions, the patent income deduction can continue to apply until June 30, 2021 at the latest.
Our inability to qualify for such advantageous tax legislation, as well as the aforementioned future alteration of the Belgian patent income deduction regime, as well as any other unexpected adverse changes of Belgian tax legislation, may adversely affect our business, results of operations and financial condition.
We may be forced to repay the technological innovation grants if we fail to comply with our contractual obligations under the applicable grant agreements.
We have received several technological innovation grants to date, totaling €26.8 million as of December 31, 2016, to support various research programs from an agency of the Flemish government to support technological innovation in Flanders. These grants carry clauses which require us to maintain a presence in the Flemish region for a number of years and invest according to pre-agreed budgets. If we fail to comply with our contractual obligations under the applicable technological innovation grant agreements, we could be forced to repay all or part of the grants received. Such repayment could adversely affect our ability to finance our research and development projects. In addition, we cannot ensure that we will then have the additional financial resources needed, the time or the ability to replace these financial resources with others.
We may be exposed to significant foreign exchange risk.
We incur portions of our expenses, and may in the future derive revenues, in currencies other than the euro, in particular, the U.S. dollar. As a result, we are exposed to foreign currency exchange risk as our results of operations and cash flows are subject to fluctuations in foreign currency exchange rates. We currently do not engage in hedging transactions to protect against uncertainty in future exchange rates between particular foreign currencies and the euro. Therefore, for example, an increase in the value of the euro against the U.S. dollar could be expected to have a negative impact on our revenue and earnings growth as U.S. dollar revenue and earnings, if any, would be translated into euros at a reduced value. We cannot predict the impact of foreign currency fluctuations, and foreign currency fluctuations in the future may adversely affect our financial condition, results of operations and cash flows.
The instability of the euro or the inability of countries to refinance their debts could have a material adverse effect on our revenue, profitability and financial position.
As a result of the credit crisis in Europe, in particular in Greece, Italy, Ireland, Portugal and Spain, the European Commission created the European Financial Stability Facility, or the EFSF, and the European Financial Stability Mechanism, or the EFSM, to provide funding to Eurozone countries in financial difficulties that seek such support. In March 2011, the European Council agreed on the need for Eurozone countries to establish a permanent stability mechanism, the European Stability Mechanism, which was established on September 27, 2012 to assume the role of the EFSF and the EFSM in providing external financial assistance to Eurozone countries. Despite these measures, concerns persist regarding the debt burden of certain Eurozone countries and their ability to meet future financial obligations and the overall stability of the euro. An extended
38
Table of Contents
period of adverse development in the outlook for European countries could reduce the expenditures on drugs through reduced volumes and lower prices, which could have negative impact on the development and commercialization of our product candidates. In addition, the European credit crisis could affect the availability and cost of debt, if and when needed by us to finance our operations and research and development. These potential developments, or market perceptions concerning these and related issues, could affect our financial position, results of operations and cash flow.
The requirements of being a U.S. public company may strain our resources and divert management’s attention.
We are required to comply with various corporate governance and financial reporting requirements under the Sarbanes-Oxley Act of 2002, the Exchange Act, and the rules and regulations adopted by the SEC and the U.S. Public Corporation Accounting Oversight Board, or PCAOB. Further, compliance with various regulatory reporting requires significant commitments of time from our management and our directors, which reduces the time available for the performance of their other responsibilities. Our failure to track and comply with the various rules may materially adversely affect our reputation, ability to obtain the necessary certifications to financial statements, lead to additional regulatory enforcement actions, and could adversely affect the value of the ADSs or our ordinary shares.
If a claim is introduced by Charles River with regard to our former service division, our results of operations and financial condition may be adversely affected.
On March 13, 2014, we announced the signing of a definitive agreement to sell the service division operations to Charles River Laboratories International, Inc., or CRL, for a total consideration of up to €134 million. CRL agreed to pay us an immediate cash consideration of €129 million. The potential earn-out of €5 million due upon achievement of a revenue target 12 months after transaction closing has not been obtained. Approximately 5% of the total consideration, including price adjustments, was being held on an escrow account. Following common practice, we have given customary representations and warranties with customary caps and limitations which are capped and limited in time (since April 1, 2016, CRL can only introduce a claim covered by the Tax Deed (during a period of 5 years), other claims related to the sale cannot be submitted anymore). If Charles River makes a claim with respect to the sale of the service division, we could incur significant costs and expenses associated with the claim. To date, four claims have been introduced by CRL, which have all been settled for a total amount of €1.3 million. On January 17, 2017 an amount of €4.1 million was released from the escrow account. The release of the remaining balance of the escrow account will be possible after final agreement between the parties on the amounts at stake.
The audit report included in this annual report is prepared by an auditor who is not inspected by the PCAOB, and, as such, you are deprived of the benefits of such inspection.
Auditors of companies that are registered with the SEC and traded publicly in the United States, including our auditors, must be registered with the PCAOB and are required by the laws of the United States to undergo regular inspections by the PCAOB to assess their compliance with the laws of the United States and professional standards. Although our auditors are registered with the PCAOB, because our auditors are located in Belgium, a jurisdiction where the PCAOB is currently unable to conduct inspections without the approval of the Belgian authorities, our auditors are not currently inspected by the PCAOB. This lack of PCAOB inspections in Belgium currently prevents the PCAOB from regularly evaluating audits and quality control procedures of any auditors operating in Belgium, including our auditors. The inability of the PCAOB to conduct inspections of auditors in Belgium makes it more difficult to evaluate the effectiveness of our auditors’ audit procedures or quality control procedures as compared to auditors outside of Belgium that are subject to PCAOB inspections. As a result, investors may be deprived of the benefits of PCAOB inspections.
39
Table of Contents
Risks Related to Ownership of Our Ordinary Shares and ADSs
The market price of the ADSs could be subject to wide fluctuations.
The market price of the ADSs could be subject to wide fluctuations in response to many risk factors listed in this section, and others beyond our control, including:
| • | actual or anticipated fluctuations in our financial condition and operating results; |
| • | actual or anticipated changes in our growth rate relative to our competitors; |
| • | competition from existing products or new products that may emerge; |
| • | announcements by us, our partners or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations, or capital commitments; |
| • | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; |
| • | issuance of new or updated research or reports by securities analysts; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| • | additions or departures of key management or scientific personnel; |
| • | disputes or other developments related to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies; |
| • | changes to coverage policies or reimbursement levels by commercial third-party payors and government payors and any announcements relating to coverage policies or reimbursement levels; |
| • | announcement or expectation of additional debt or equity financing efforts; |
| • | sales of the ADSs by us, our insiders or our other shareholders; and |
| • | general economic and market conditions. |
These and other market and industry factors may cause the market price and demand for the ADSs to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their ADSs and may otherwise negatively affect the liquidity of our capital shares. In addition, the stock market in general, and biotechnology and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies.
Share ownership is concentrated in the hands of our principal shareholders and management, which may have the effect of delaying or preventing a change of control of our company.
Our executive officers, directors, current 5% or greater shareholders and their affiliated entities, including Gilead, together beneficially own approximately 33% of our ordinary shares, including shares in the form of ADSs. This concentration of ownership might have the effect of delaying or preventing a change of control of our company that other shareholders may view as beneficial.
Fluctuations in the exchange rate between the U.S. dollar and the euro may increase the risk of holding the ADSs.
Our shares currently trade on Euronext Brussels and Euronext Amsterdam in euros, while the ADSs trade on NASDAQ in U.S. dollars. Fluctuations in the exchange rate between the U.S. dollar and the euro may result in temporary differences between the value of the ADSs and the value of our ordinary shares, which may result in heavy trading by investors seeking to exploit such differences.
40
Table of Contents
In addition, as a result of fluctuations in the exchange rate between the U.S. dollar and the euro, the U.S. dollar equivalent of the proceeds that a holder of the ADSs would receive upon the sale in Belgium of any shares withdrawn from the depositary and the U.S. dollar equivalent of any cash dividends paid in euros on our shares represented by the ADSs could also decline.
If securities or industry analysts do not publish research or publish inaccurate research or unfavorable research about our business, the price of the ordinary shares and ADSs and trading volume could decline.
The trading market for the ordinary shares and ADSs depends in part on the research and reports that securities or industry analysts publish about us or our business. If no or few securities or industry analysts cover our company, the trading price for the ordinary shares and ADSs would be negatively impacted. If one or more of the analysts who covers us downgrades the ordinary shares and ADSs or publishes incorrect or unfavorable research about our business, the price of the ordinary shares and ADSs would likely decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, or downgrades the ordinary shares and ADSs, demand for the ordinary shares and ADSs could decrease, which could cause the price of the ordinary shares and ADSs or trading volume to decline.
We have no present intention to pay dividends on our ordinary shares in the foreseeable future and, consequently, your only opportunity to achieve a return on your investment during that time is if the price of the ADSs appreciates.
We have no present intention to pay dividends in the foreseeable future. Any recommendation by our board of directors to pay dividends will depend on many factors, including our financial condition (including losses carried-forward), results of operations, legal requirements and other factors. Furthermore, pursuant to Belgian law, the calculation of amounts available for distribution to shareholders, as dividends or otherwise, must be determined on the basis of our non-consolidated statutory accounts prepared in accordance with Belgian accounting rules. In addition, in accordance with Belgian law and our articles of association, we must allocate each year an amount of at least 5% of our annual net profit under our non-consolidated statutory accounts to a legal reserve until the reserve equals 10% of our share capital. Therefore, we are unlikely to pay dividends or other distributions in the foreseeable future. If the price of the ADSs declines before we pay dividends, you will incur a loss on your investment, without the likelihood that this loss will be offset in part or at all by potential future cash dividends.
Our shareholders residing in countries other than Belgium may be subject to double withholding taxation with respect to dividends or other distributions made by us.
Any dividends or other distributions we make to shareholders will, in principle, be subject to withholding tax in Belgium at a rate of 30%, except for shareholders which qualify for an exemption of withholding tax such as, among others, qualifying pension funds or a company qualifying as a parent company in the sense of the Council Directive (90/435/EEC) of July 23, 1990, or the Parent-Subsidiary Directive, or that qualify for a lower withholding tax rate or an exemption by virtue of a tax treaty. Various conditions may apply and shareholders residing in countries other than Belgium are advised to consult their advisers regarding the tax consequences of dividends or other distributions made by us. Our shareholders residing in countries other than Belgium may not be able to credit the amount of such withholding tax to any tax due on such dividends or other distributions in any other country than Belgium. As a result, such shareholders may be subject to double taxation in respect of such dividends or other distributions. Belgium and the United States have concluded a double tax treaty concerning the avoidance of double taxation, or the U.S.-Belgium Tax Treaty. The U.S.-Belgium Tax Treaty reduces the applicability of Belgian withholding tax to 15%, 5% or 0% for U.S. taxpayers, provided that the U.S. taxpayer meets the limitation of benefits conditions imposed by the U.S.-Belgium Tax Treaty. The Belgian withholding tax is generally reduced to 15% under the U.S.-Belgium Tax Treaty. The 5% withholding tax applies in case where the U.S. shareholder is a company which holds at least 10% of the shares in the company. A 0% Belgian withholding tax applies when the shareholder is a company which has held at least 10% of the shares in
41
Table of Contents
the company for at least 12 months, or is, subject to certain conditions, a U.S. pension fund. The U.S. shareholders are encouraged to consult their own tax advisers to determine whether they can invoke the benefits and meet the limitation of benefits conditions as imposed by the U.S.-Belgium Tax Treaty.
Future sales of ordinary shares or ADSs by existing shareholders could depress the market price of the ordinary shares and ADSs.
If our existing shareholders sell, or indicate an intent to sell, substantial amounts of ordinary shares or ADSs in the public market, the trading price of the ADSs could decline significantly. As of March 15, 2017, 39,495,377 shares were eligible for sale in the public market, 563,485 of which shares are held by directors, executive officers and other affiliates and are subject to volume limitations under Rule 144 under the Securities Act . In addition, the ordinary shares subject to outstanding warrants will become eligible for sale in the public market in the future, subject to certain legal and contractual limitations. We have filed registration statements on Form S-8 with the SEC covering ordinary shares available for future issuance under our warrant plans. Sales of a large number of the shares issued under these plans in the public market could have an adverse effect on the market price of the ADSs.
We are a Belgian public limited liability company, and shareholders of our company may have different and in some cases more limited shareholder rights than shareholders of a U.S. listed corporation.
We are a public limited liability company incorporated under the laws of Belgium. Our corporate affairs are governed by Belgian corporate law. The rights provided to our shareholders under Belgian corporate law and our articles of association differ in certain respects from the rights that you would typically enjoy as a shareholder of a U.S. corporation under applicable U.S. federal and state laws.
Under Belgian corporate law, other than certain limited information that we must make public and except in certain limited circumstances, our shareholders may not ask for an inspection of our corporate records, while under Delaware corporate law any shareholder, irrespective of the size of its shareholdings, may do so. Shareholders of a Belgian corporation are also unable to initiate a derivative action, a remedy typically available to shareholders of U.S. companies, in order to enforce a right of our company, in case we fail to enforce such right ourselves, other than in certain cases of director liability under limited circumstances. In addition, a majority of our shareholders present or represented at our meeting of shareholders may release a director from any claim of liability we may have, including if he or she has acted in bad faith or has breached his or her duty of loyalty, provided, in some cases, that the relevant acts were specifically mentioned in the convening notice to the meeting of shareholders deliberating on the discharge. In contrast, most U.S. federal and state laws prohibit a company or its shareholders from releasing a director from liability altogether if he or she has acted in bad faith or has breached his or her duty of loyalty to the company. Finally, Belgian corporate law does not provide any form of appraisal rights in the case of a business combination. Please see the section of this annual report titled “Item 10.B.—Memorandum and Articles of Association.”
As a result of these differences between Belgian corporate law and our articles of association, on the one hand, and the U.S. federal and state laws, on the other hand, in certain instances, you could receive less protection as an ADS holder of our company than you would as a shareholder of a listed U.S. company.
Takeover provisions in Belgian law may make a takeover difficult.
Public takeover bids on our shares and other voting securities, such as warrants or convertible bonds, if any, are subject to the Belgian Act of April 1, 2007 and to the supervision by the Belgian Financial Services and Markets Authority, or FSMA. Public takeover bids must be made for all of our voting securities, as well as for all other securities that entitle the holders thereof to the subscription to, the acquisition of or the conversion into voting securities. Prior to making a bid, a bidder must issue and disseminate a prospectus, which must be approved by the Belgian FSMA. The bidder must also obtain approval of the relevant competition authorities, where such approval is legally required for the acquisition of our company.
42
Table of Contents
The Belgian Act of April 1, 2007 provides that a mandatory bid will be triggered if a person, as a result of its own acquisition or the acquisition by persons acting in concert with it or by persons acting on their account, directly or indirectly holds more than 30% of the voting securities in a company that has its registered office in Belgium and of which at least part of the voting securities are traded on a regulated market or on a multilateral trading facility designated by the Royal Decree of April 27, 2007 on public takeover bids. The mere fact of exceeding the relevant threshold through the acquisition of one or more shares will give rise to a mandatory bid, irrespective of whether or not the price paid in the relevant transaction exceeds the current market price.
There are several provisions of Belgian company law and certain other provisions of Belgian law, such as the obligation to disclose important shareholdings and merger control, that may apply to us and which may make an unfriendly tender offer, merger, change in management or other change in control, more difficult. These provisions could discourage potential takeover attempts that third parties may consider and thus deprive the shareholders of the opportunity to sell their shares at a premium (which is typically offered in the framework of a takeover bid).
Holders of the ADSs are not treated as shareholders of our company, do not have the same voting rights as the holders of our ordinary shares and may not receive voting materials in time to be able to exercise your right to vote.
Holders of the ADSs are not treated as shareholders of our company, unless they withdraw our ordinary shares underlying the ADSs. The depositary, or its nominee, is the holder of the ordinary shares underlying the ADSs. Holders of ADSs therefore do not have any rights as shareholders of our company, other than the rights that they have pursuant to the deposit agreement.
Holders of ADSs may exercise voting rights with respect to the ordinary shares represented by the ADSs only in accordance with the provisions of the deposit agreement. The deposit agreement provides that, upon receipt of notice of any meeting of holders of our ordinary shares, the depositary will fix a record date for the determination of ADS holders who shall be entitled to give instructions for the exercise of voting rights. Upon timely receipt of notice from us, if we so request, the depositary shall distribute to the holders as of the record date (1) the notice of the meeting or solicitation of consent or proxy sent by us and (2) a statement as to the manner in which instructions may be given by the holders.
Holders of ADSs may instruct the depositary of their ADSs to vote the ordinary shares underlying their ADSs. Otherwise, ADS holders will not be able to exercise their right to vote, unless they withdraw the ordinary shares underlying the ADSs they hold. However, ADS holders may not know about the meeting far enough in advance to withdraw those ordinary shares. We cannot guarantee ADS holders that they will receive the voting materials in time to ensure that they can instruct the depositary to vote their ordinary shares or to withdraw their ordinary shares so that they can vote them themselves. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that ADS holders may not be able to exercise their right to vote, and there may be nothing they can do if the ordinary shares underlying their ADSs are not voted as they requested.
We may not be able to complete equity offerings without cancellation or limitation of the preferential subscription rights of our existing shareholders, which may as a practical matter preclude us from timely completion of offerings.
In accordance with the Belgian Companies Code, our articles of association provide for preferential subscription rights to be granted to our existing shareholders to subscribe on a pro rata basis for any issue for cash of new shares, convertible bonds or warrants that are exercisable for cash, unless such rights are cancelled or limited either by resolution of our shareholders’ meeting or by our board of directors in the framework of the authorized capital, as described below. On April 26, 2016, our shareholders authorized our board to increase our share capital (possibly with cancellation or limitation of the preferential subscription rights of our existing
43
Table of Contents
shareholders at the discretion of our board), subject to certain limitations, for a period of five years. We refer to this authority for our board to increase our share capital as our authorized capital. As of the date of this annual report, our board of directors may decide to issue up to 8,557,345 ordinary shares pursuant to this authorization, without taking into account however subsequent issuances under our warrant programs or otherwise. Please see the section of this annual report titled “Item 10.B.—Memorandum and Articles of Association.” Absent renewal by our shareholders of this authorization of the board or absent cancellation or limitation by our shareholders of the preferential subscription rights of our existing shareholders, the requirement to offer our existing shareholders the preferential right to subscribe, pro rata, for new shares being offered may as a practical matter preclude us from timely raising capital on commercially acceptable terms or at all.
Shareholders may not be able to participate in equity offerings we may conduct from time to time.
If we conduct equity offerings in the future, certain shareholders, including those in the United States, may, even in the case where preferential subscription rights have not been cancelled or limited, not be entitled to exercise such rights, unless the offering is registered or the shares are qualified for sale under the relevant regulatory framework. As a result, there is the risk that investors may suffer dilution of their shareholdings should they not be permitted to participate in preference right equity or other offerings that we may conduct in the future.
Holders of ADSs may be subject to limitations on the transfer of their ADSs and the withdrawal of the underlying ordinary shares.
ADSs are transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when it deems expedient in connection with the performance of its duties. The depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary think it is advisable to do so because of any requirement of law, government or governmental body, or under any provision of the deposit agreement, or for any other reason, subject to the right of ADS holders to cancel their ADSs and withdraw the underlying ordinary shares. Temporary delays in the cancellation of your ADSs and withdrawal of the underlying ordinary shares may arise because the depositary has closed its transfer books or we have closed our transfer books, the transfer of ordinary shares is blocked to permit voting at a shareholders’ meeting or we are paying a dividend on our ordinary shares. In addition, ADS holders may not be able to cancel their ADSs and withdraw the underlying ordinary shares when they owe money for fees, taxes and similar charges and when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities.
As a foreign private issuer, we are exempt from a number of rules under the U.S. securities laws and are permitted to file less information with the SEC than a U.S. company. This may limit the information available to holders of ADSs or our ordinary shares.
We are a “foreign private issuer,” as defined in the SEC’s rules and regulations and, consequently, we are not subject to all of the disclosure requirements applicable to public companies organized within the United States. For example, we are exempt from certain rules under the Exchange Act, that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act, including the U.S. proxy rules under Section 14 of the Exchange Act. In addition, our officers and directors are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. Moreover, while we currently make annual and semi-annual filings with respect to our listing on Euronext Brussels and Euronext Amsterdam and voluntarily report our results of operations on a quarterly basis, we will not be required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. domestic issuers and will not be required to file quarterly reports on Form 10-Q or current reports on Form 8-K under the Exchange Act. Accordingly, there is and will continue to be less publicly available information concerning our company than there would be if we were not a foreign private issuer.
44
Table of Contents
As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from NASDAQ corporate governance listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards.
As a foreign private issuer listed on the NASDAQ Global Select Market, we are subject to corporate governance listing standards. However, rules permit a foreign private issuer like us to follow the corporate governance practices of its home country. Certain corporate governance practices in Belgium, which is our home country, may differ significantly from corporate governance listing standards. For example, neither the corporate laws of Belgium nor our articles of association require a majority of our directors to be independent and we could include non-independent directors as members of our nomination and remuneration committee, and our independent directors would not necessarily hold regularly scheduled meetings at which only independent directors are present. Currently, we intend to follow home country practice to the maximum extent possible. Therefore, our shareholders may be afforded less protection than they otherwise would have under corporate governance listing standards applicable to U.S. domestic issuers. See the sections of this annual report titled “Item 6—Directors, Senior Management and Employees” and “Item 16G—Corporate Governance.”
We may lose our foreign private issuer status in the future, which could result in significant additional cost and expense.
While we currently qualify as a foreign private issuer, the determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter and, accordingly, the next determination will be made with respect to us on June 30, 2017.
In the future, we would lose our foreign private issuer status if we to fail to meet the requirements necessary to maintain our foreign private issuer status as of the relevant determination date. For example, if more than 50% of our securities are held by U.S. residents and more than 50% of the members of our executive committee or members of our board of directors are residents or citizens of the United States, we could lose our foreign private issuer status.
The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly more than costs we incur as a foreign private issuer. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive in certain respects than the forms available to a foreign private issuer. We would be required under current SEC rules to prepare our financial statements in accordance with U.S. GAAP, rather than IFRS, in U.S. dollars rather than euros and modify certain of our policies to comply with corporate governance practices associated with U.S. domestic issuers. Such conversion of our financial statements to U.S. GAAP will involve significant time and cost. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers such as the ones described above and exemptions from procedural requirements related to the solicitation of proxies.
It may be difficult for investors outside Belgium to serve process on, or enforce foreign judgments against, us or our directors and senior management.
We are a Belgian public limited liability company. Less than a majority of the members of our board of directors and members of our executive committee are residents of the United States. All or a substantial portion of the assets of such non-resident persons and most of our assets are located outside the United States. As a result, it may not be possible for investors to effect service of process upon such persons or on us or to enforce against them or us a judgment obtained in U.S. courts. Original actions or actions for the enforcement of judgments of U.S. courts relating to the civil liability provisions of the federal or state securities laws of the United States are not directly enforceable in Belgium. The United States and Belgium do not currently have a multilateral or bilateral treaty providing for reciprocal recognition and enforcement of judgments, other than
45
Table of Contents
arbitral awards, in civil and commercial matters. In order for a final judgment for the payment of money rendered by U.S. courts based on civil liability to produce any effect on Belgian soil, it is accordingly required that this judgment be recognized or be declared enforceable by a Belgian court in accordance with Articles 22 to 25 of the 2004 Belgian Code of Private International Law. Recognition or enforcement does not imply a review of the merits of the case and is irrespective of any reciprocity requirement. A U.S. judgment will, however, not be recognized or declared enforceable in Belgium if it infringes upon one or more of the grounds for refusal that are exhaustively listed in Article 25 of the Belgian Code of Private International Law. Actions for the enforcement of judgments of U.S. courts might be successful only if the Belgian court confirms the substantive correctness of the judgment of the U.S. court and is satisfied that:
| • | the effect of the enforcement judgment is not manifestly incompatible with Belgian public policy; |
| • | the judgment did not violate the rights of the defendant; |
| • | the judgment was not rendered in a matter where the parties transferred rights subject to transfer restrictions with the sole purpose of avoiding the application of the law applicable according to Belgian international private law; |
| • | the judgment is not subject to further recourse under U.S. law; |
| • | the judgment is not compatible with a judgment rendered in Belgium or with a subsequent judgment rendered abroad that might be enforced in Belgium; |
| • | a claim was not filed outside Belgium after the same claim was filed in Belgium, while the claim filed in Belgium is still pending; |
| • | the Belgian courts did not have exclusive jurisdiction to rule on the matter; |
| • | the U.S. court did not accept its jurisdiction solely on the basis of either the nationality of the plaintiff or the location of the disputed goods; and |
| • | the judgment submitted to the Belgian court is authentic. |
In addition to recognition or enforcement, a judgment by a federal or state court in the United States against us may also serve as evidence in a similar action in a Belgian court if it meets the conditions required for the authenticity of judgments according to the law of the state where it was rendered. The findings of a federal or state court in the United States will not, however, be taken into account to the extent they appear incompatible with Belgian public policy.
We may be at an increased risk of securities class action litigation.
Historically, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and biopharmaceutical companies have experienced significant share price volatility in recent years. If we were to be sued, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
We believe that we should not be a passive foreign investment company, or PFIC, for U.S. federal income tax purposes for the 2016 taxable year and we do not anticipate being a PFIC for the current taxable year, but this conclusion is a factual determination that is made annually and thus may be subject to change. If we were a PFIC, this could result in adverse U.S. tax consequences to certain U.S. holders.
Generally, if, for any taxable year, at least 75% of our gross income is passive income, or at least 50% of the value of our assets is attributable to assets that produce passive income or are held for the production of passive income, including cash, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. For purposes of these tests, passive income includes dividends, interest, and gains
46
Table of Contents
from the sale or exchange of investment property and rents and royalties other than rents and royalties which are received from unrelated parties in connection with the active conduct of a trade or business. Our status as a PFIC depends on the composition of our income and the composition and value of our assets (for which purpose the total value of our assets may be determined in part by reference to the market value of the ADSs and our ordinary shares, which are subject to change) from time to time. If we are a PFIC for any taxable year, U.S. holders of the ADSs may suffer adverse tax consequences, including having gains realized on the sale of the ADSs treated as ordinary income, rather than capital gain, losing the preferential rate applicable to dividends received on the ADSs by individuals who are U.S. holders, and having interest charges apply to distributions by us and the proceeds of sales of the ADSs. See “Item 10.E.—Taxation—Certain Material U.S. Federal Income Tax Considerations to U.S. Holders—Passive Foreign Investment Company Considerations.”
Based upon the expected value of our assets, including any goodwill, and the expected composition of our income and assets, we believe that we should not be a PFIC for the 2016 taxable year and we do not anticipate that we will be a PFIC with respect to the current taxable year. However, our status as a PFIC is a fact-intensive determination made on an annual basis, and we cannot provide any assurances regarding our PFIC status for the current, prior or future taxable years. We do not currently intend to provide the information necessary for U.S. holders to make a “qualified electing fund,” or QEF, election if we are treated as a PFIC for any taxable year, and prospective investors should assume that a QEF election will not be available.
We believe that we were not a controlled foreign corporation, or CFC, for U.S. federal income tax purposes for the 2016 taxable year. If we were to qualify as a CFC, this could result in adverse U.S. federal income tax consequences to certain U.S. holders.
Each “Ten Percent Shareholder” (as defined below) in a non-U.S. corporation that is classified as a “controlled foreign corporation,” or a CFC, for U.S. federal income tax purposes generally is required to include in income for U.S. federal tax purposes such Ten Percent Shareholder’s pro rata share of the CFC’s “Subpart F income” and investment of earnings in U.S. property, even if the CFC has made no distributions to its shareholders. Subpart F income generally includes dividends, interest, rents and royalties, gains from the sale of securities and income from certain transactions with related parties. In addition, a Ten Percent Shareholder that realizes gain from the sale or exchange of shares in a CFC may be required to classify a portion of such gain as dividend income rather than capital gain. A non-U.S. corporation generally will be classified as a CFC for United States federal income tax purposes if Ten Percent Shareholders own, directly or indirectly, more than 50% of either the total combined voting power of all classes of stock of such corporation entitled to vote or of the total value of the stock of such corporation. A “Ten Percent Shareholder” is a United States person (as defined by the U.S. Internal Revenue Code of 1986, as amended (the “Code”)) who owns or is considered to own 10% or more of the total combined voting power of all classes of stock entitled to vote of such corporation. The determination of CFC status is complex and includes attribution rules, the application of which is not entirely certain.
We do not believe that we were a CFC for the taxable year ended December 31, 2016. However, we cannot provide any assurances regarding our status as a CFC for the 2016 taxable year or any future taxable years.
| Item 4 | Information on the Company. |
| A. | History and Development of the Company |
Our legal and commercial name is Galapagos NV. We are a limited liability company incorporated in the form of a naamloze vennootschap / société anonyme under Belgian law. We were incorporated in Belgium on June 30, 1999 for an unlimited duration. We are registered with the Register of Legal Entities (Antwerp, division Mechelen) under the enterprise number 0466.460.429. Our principal executive and registered offices are located at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium and our telephone number is +32 15 34 29 00. Our agent for service of process in the United States is CT Corporation System.
47
Table of Contents
Our fiscal year ends December 31. We also maintain a corporate website at www.glpg.com. Information contained on, or that can be accessed through, our website does not constitute a part of this annual report. We have included our website address in this annual report solely as an inactive textual reference.
Our actual capital expenditures for the years ended December 31, 2014, 2015 and 2016 amounted to €2.8 million, €6.7 million, and €4.8 million respectively. These capital expenditures primarily consisted of laboratory equipment and leasehold improvements. We expect our capital expenditures to increase in absolute terms in the near term as we continue to advance our research and development programs and grow our operations. We anticipate our capital expenditure in 2017 to be financed from the cash flows from operating activities and cash reserves . For more information on our capital expenditures, see the section of this annual report titled “Item 6.B.—Liquidity and Capital Resources—Capital Expenditures.”
| B. | Business Overview |
We are a clinical-stage biotechnology company specialized in the discovery and development of medicines with novel modes of action, addressing disease areas of high unmet medical need. Our pipeline comprises programs ranging from discovery to Phase 3 clinical trials in inflammation, cystic fibrosis, fibrosis, osteoarthritis and other indications. Our highly flexible platform is applicable across many therapeutic areas. Our clinical stage programs include: filgotinib, which is currently in Phase 3 trials in rheumatoid arthritis (RA), Crohn’s disease (CD), and ulcerative colitis (UC); our cystic fibrosis (CF) portfolio of drugs aimed at a triple combination therapy for 90% of CF patients, for which we plan to initiate patient clinical trials by mid-2017; GLPG1690, our fully proprietary autotaxin inhibitor, which is currently in a Phase 2a trial for idiopathic pulmonary fibrosis (IPF); GLPG1972 for osteoarthritis (OA), which is expected to be dosed in a Phase 1b trial in U.S. patients in 2017; and MOR106, which is currently being dosed in atopic dermatitis (AtD) patients in a Phase 1b trial. Except for our CF program, these programs are derived from our proprietary target discovery platform.
We have collaborations with Gilead for filgotinib, with AbbVie for CF, with Servier for GLPG1972, and with MorphoSys for MOR106. For more information on our collaborations, see “—Collaborations.” The following table summarizes key information on our lead development programs as of the date of this annual report:
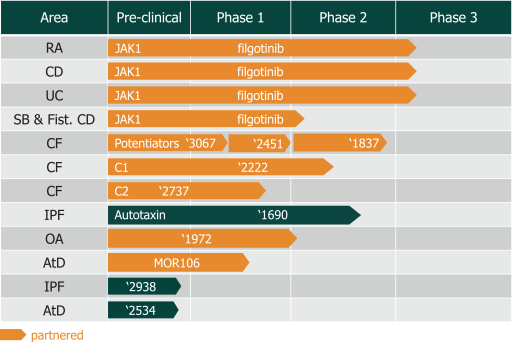
48
Table of Contents
Lead Programs
Filgotinib: Selective JAK1 Inhibitor with a Potential Best-in-class Product Profile
Based on results from our Phase 2 trials, we believe that filgotinib is a promising candidate for the treatment of RA, CD and potentially other inflammatory diseases. We are party to an exclusive collaboration agreement with Gilead to develop and commercialize filgotinib in multiple diseases. Under the terms of the collaboration, Gilead is primarily responsible for development and seeking regulatory approval of the licensed product. We are required to use commercially reasonable efforts as requested by Gilead to assist Gilead with certain development activities. Gilead initiated Phase 3 clinical programs in RA and CD and a Phase 2b/3 program in UC in 2016.
Our Filgotinib Program in RA
RA is a chronic autoimmune disease that affects 2.9 million patients (of which approximately 1.5 million are being treated with biologics) in the United States and Europe. RA is characterized by inflammation and degeneration of the joints. Patients suffer from pain, stiffness, and restricted mobility due to a persistent inflammation of multiple joints, ultimately resulting in irreversible damage of the joint cartilage and bone. According to GlobalData, sales of RA therapeutics across the 10 main healthcare markets was $19.5 billion in 2015, with the current market being dominated by injectable, biological therapies. Biologics, mostly TNF therapies, need to be injected and often lose their effect over time, so there continues to be a considerable unmet need with regard to efficacy, safety, and convenience of use with existing treatments.
New oral therapies that target the Janus kinase, or JAK, signaling pathway are emerging to treat inflammatory diseases; JAK inhibitors, however, are associated with a range of side effects, including aberrations in low-density lipoprotein, or LDL, cholesterol and red blood and NK cell counts. In a human whole blood assay we demonstrated that filgotinib, with a 30-fold selectivity for JAK1 over JAK2, was more selective for JAK1 than any other compound known to us to be either approved for sale or in clinical development. We believe the high selectivity of filgotinib for JAK1 may allow for a positive efficacy profile, with an improved safety profile for filgotinib due to the improved selectivity over JAK2 and JAK3.
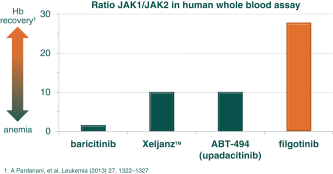
Our Clinical Program for Filgotinib for RA
Clinical trials to date have shown that filgotinib is well-tolerated, with atherogenic index improvement and absence of anemia. We believe its once-a-day oral dosage and its low risk for drug-drug interactions make it convenient for patient use.
We reported final 24-week data from DARWIN 1 and DARWIN 2 Phase 2b dose-range finding clinical trials in 2015. Both trials were double-blind, placebo-controlled for 24 weeks of treatment in patients with moderate to severe rheumatoid arthritis who showed an inadequate response to methotrexate. DARWIN 1 (594 patients) evaluated filgotinib as an addition to methotrexate, as once- and twice-daily administration (qd and bid dosing, respectively) at three daily dose levels. DARWIN 2 (283 patients) evaluated filgotinib as once-daily
49
Table of Contents
monotherapy administration (qd dosing) at three dose levels. Both trials achieved the primary endpoints (ACR20). Below are the ACR50 and ACR70 scores at 12 and 24 weeks for 100 and 200 mg qd in both DARWIN 1 and DARWIN 2:
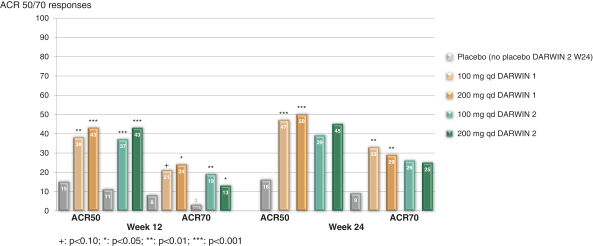
Overall, there was no statistically relevant difference between the once-daily and twice-daily dosing regimens in DARWIN 1. Both trials showed a rapid onset of activity, as of week one for ACR and DAS28(CRP) responses. In DARWIN 1 (200 mg bid) and in DARWIN 2 (100 mg qd) up to 50% of the patients reached low disease activity or remission. The 100 mg and 200 mg qd doses achieved similar levels of activity overall.
Safety data in both trials was similarly promising. In dose groups including placebo in both studies, 3.9% of patients stopped treatment during the trial for safety reasons. In DARWIN 1 patients reporting serious (2.5% overall) and non-serious treatment-emergent adverse events were evenly spread over the dose groups including placebo. Serious infections were reported in six patients, including one death on active treatment in the second half of the trial and for which the Data Safety Monitoring Board did not see a reason to pause or change the trial. No opportunistic infections were reported. Herpes zoster infection occurred in five patients, equally spread over placebo and filgotinib groups. In DARWIN 2 a higher discontinuation rate for safety was observed for placebo (5.6%) during the first 12 weeks of the trial compared to filgotinib treated patients (2.5%) up to week 24. Similar incidence of serious and non-serious treatment-emergent adverse events was reported, evenly spread over the dose groups including placebo. A higher rate of infections was observed in filgotinib (19% over 24 weeks) compared to placebo (10% up to week 12), with serious infections remaining limited (1.4% of filgotinib patients). No malignancies, tuberculosis, major adverse cardiac events, opportunistic infections, or deaths were reported in DARWIN 2.
On the basis of pre-clinical findings, males in the United States were restricted by the FDA to the 100 mg dose for DARWIN 1 and 2. Male reproductive hormones consequently were monitored in male patients taking 200 mg in DARWIN 1 and 2 outside the United States. No clinically significant changes or discontinuations were observed for male reproductive hormones in either trial.
Consistent with its selective JAK1 inhibition, filgotinib treatment led to an improvement in hemoglobin (DARWIN 1 up to 0.5 g/dL, or a 4% increase from baseline, DARWIN 2 up to 0.4 g/dL, or 3.6% increase from baseline). In DARWIN 1, all lipid fractions including HDL and LDL increased, with the largest percentage increase in HDL, while in DARWIN 2 similar increases in LDL and HDL were maintained. Neutrophil levels remained stable after initial decline to mid-normal range at week four. Neither lymphocytes nor liver enzymes were impacted by treatment with filgotinib in either study.
50
Table of Contents
Filgotinib’s improvement in hemoglobin shown in DARWIN 1 and 2 potentially differentiates it when compared to impact on hemoglobin shown by other JAK inhibitors in RA trials:
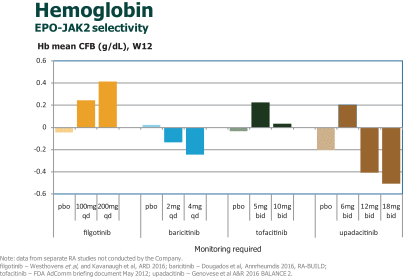
Filgotinib’s lack of impact on natural killer (NK) cells shown in DARWIN 1 and 2 potentially differentiates it when compared to the impact on NK cells shown by other JAK inhibitors in RA trials:
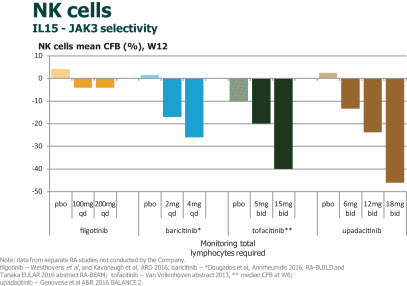
Of the patients who have completed DARWIN 1 and DARWIN 2 and were eligible to continue, approximately 98% elected to participate in the DARWIN 3 follow-up trial. DARWIN 3 is a multi-center, open-label, long-term follow-up safety and efficacy trial of subjects who have completed either DARWIN 1 or DARWIN 2. All subjects have started the trial at the same dose level, either at 200 mg once per day or at 100 mg twice per day (except for males in the U.S. sites of these trials who receive a maximum daily dose of 100 mg), depending on the regimen administered during the preceding trial, with DARWIN 1 subjects continuing to use filgotinib in combination with MTX.
51
Table of Contents
FINCH Phase 3 Program with Filgotinib in RA
In August 2016, our collaboration partner Gilead initiated the FINCH global Phase 3 program investigating the efficacy and safety of 100 mg and 200 mg filgotinib once daily, in RA patient populations, ranging from early stage to biologic-experienced patients:
FINCH 1 is a 52-week, randomized, placebo- and adalimumab-controlled trial in combination with methotrexate (MTX) in an expected 1,650 patients who have had inadequate response to MTX. The primary endpoint is ACR20 at week 12. American College of Rheumatology 20% (ACR20) response rate signifies a 20% or greater improvement in the number of swollen and tender joints as well as a 20% or greater improvement in three out of five other disease-activity measures. ACR50 and ACR70 reflect the same, respectively for 50% and 70% response rates. The study will also include radiographic assessment at weeks 24 and 52.
FINCH 2 is a 24-week, randomized, placebo-controlled trial in an expected 423 patients who are on conventional disease-modifying anti-rheumatic drugs (cDMARD), and have had an inadequate response to biological treatment. The primary endpoint is ACR20 at week 12.
FINCH 3 is a 52-week, randomized trial in an expected 1,200 MTX-naïve patients to study filgotinib in combination with MTX, as well as monotherapy. The primary endpoint is ACR20 at week 24. Radiographic progression will also be assessed.
Gilead will perform a single dedicated male patient safety study concurrent to all Phase 3 programs. This study is expected to include RA, CD, and UC patients.
Our Filgotinib Program in Inflammatory Bowel Disease (IBD)
IBD includes CD and UC. We observed high activity and a favorable safety profile in a Phase 2 trial with filgotinib in CD. The profile we saw with filgotinib in this CD patient trial leads us to believe the product candidate may show activity and tolerability in UC patient studies as well. IBD affects approximately 2 million patients (of which approximately 0.5 million are being treated with biologics) in the United States and Europe, and the market for IBD therapies is approximately $9 billion today, according to GlobalData. Current treatments are dominated by anti-TNF agents, with new biologic products gaining some ground in second line treatment.
CD is an IBD of unknown cause, resulting in chronic inflammation of the gastrointestinal (GI) tract with a relapsing and remitting course. Today, only 10% of CD patients achieve prolonged clinical remission. There are currently no highly effective oral therapies approved for CD and, similar to RA, treatment is dominated by injectable, biologic treatments including anti-TNF therapies. Anti-TNF agents have improved the management of CD; however, not all patients respond to these drugs, and secondary loss of response is reported in up to 50% of patients per year in placebo-controlled trials. There continues to be a considerable unmet need with these existing treatments. Dysregulation of the JAK signaling pathway has also been associated with CD, and we believe that filgotinib, with its high selectivity for JAK1, is a highly attractive candidate for the treatment of CD. By inhibiting JAK1 but not JAK2, unwanted effects such as anemia may be prevented. This absence of anemia is of particular importance to IBD patients, who frequently experience fecal blood loss.
Increased activity and focus from pharmaceutical companies has led to more clinical trials of oral therapies in CD. For example, AbbVie is conducting a Phase 2 trial with upadacitinib (pan-JAK inhibitor) which should read out in 2017. Celgene announced Phase 1b results with GED-0301 (SMAD-7), which showed early activity but limited endoscopic improvement. Celgene is also investigating ozanimod (S1P 1 and 5 receptor modulator) in Phase 2 in CD, with topline results expected in 2017. After two Phase 2 trials, Pfizer announced Xeljanz (pan-JAK inhibitor) will not be developed further in CD.
Our Clinical Program with Filgotinib in CD
Our FITZROY Phase 2 trial (174 patients) evaluated filgotinib once-daily versus placebo in patients with moderate to severely active CD and mucosal ulceration. Patients recruited were either anti-TNF naïve or
52
Table of Contents
anti-TNF failures. FITZROY was the first trial in CD to require endoscopic confirmation of lesions at entry, and also to include a placebo control on endoscopy. The trial comprised two parts, each of 10 weeks duration: the first part investigated the safety and efficacy of filgotinib 200 mg once daily versus placebo, while the second part of the study investigated continued treatment through 20 weeks in an observational exploratory design. The FITZROY trial achieved the primary endpoint of clinical remission at 10 weeks: the percentage of patients achieving a Crohn’s Disease Activity Index (CDAI) score lower than 150 was statistically significantly higher in patients treated with filgotinib (47%) versus patients receiving placebo (23%). The share of patients achieving 100-points clinical response (60%) also was significant versus those receiving placebo (41%). Improvement in quality of life, histopathology, endoscopy assessment and biomarkers of inflammatory activity were also observed at week 10. Overall mean change in histopathology scores at week 10 for patients treated with filgotinib (-3.5) versus placebo (-0.6) was significantly different, confirming the clinical responses in the tissues of patients. More patients on filgotinib showed >50% improvement in SES-CD (endoscopy) scores versus placebo patients at week 10:
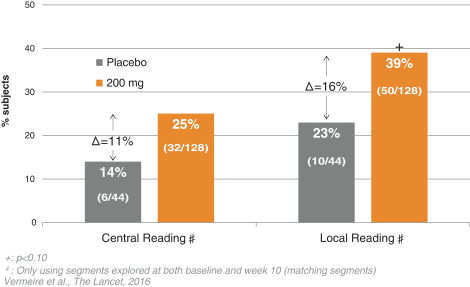
Clinical responses were maintained from week 10 to week 20. Non-responders in the placebo arm from the first ten weeks received filgotinib 100 mg in the second ten weeks and showed improvement in clinical remission during the second part of the trial.
Overall, in the FITZROY study at 20 weeks of treatment, filgotinib demonstrated a favorable safety profile consistent with the DARWIN studies in RA. An increase in hemoglobin was also observed in FITZROY, without difference between filgotinib and placebo. No clinically significant changes from baseline in neutrophils or liver function tests were observed.
Gilead initiated a Phase 3 trial (DIVERSITY) with filgotinib in CD in November 2016. The DIVERSITY Phase 3 trial investigates efficacy and safety of 100 mg and 200 mg filgotinib once-daily compared to placebo in patients with moderately to severely active disease including those with prior antibody therapy failure. Gilead will recruit approximately 1,300 patients from the United States, Europe, Latin America, Canada, and Asia/Pacific regions. Men and women in the DIVERSITY trial will be randomized to receive placebo, 100 mg or 200 mg filgotinib. In the United States, males may receive 200 mg if they failed at least one anti-TNF and vedolizumab, a monoclonal anti-integrin antibody marketed by Takeda.
In March 2017, Gilead initiated a Phase 2 study in small bowel CD and a Phase 2 study in fistulizing CD.
53
Table of Contents
Our Clinical Program with Filgotinib in UC
UC is an inflammatory bowel disease resulting in ulcerations and inflammation of the colon and rectum. Unlike CD, UC involves damaging inflammation of only the colon and rectum. Although the introduction of anti-TNF biologics has improved the treatment of some patients, only 33% of patients will achieve long-term remission, and many patients lose their response to treatment over time. The medical need for improved efficacy is high and could likely be achieved by a new mechanism of action.
Increased activity and focus from potential competitors has led to more clinical trials of oral therapies in UC. Pfizer showed activity with Xeljanz (pan-JAK inhibitor) in Phase 3 UC trials and has filed for approval. AbbVie is conducting a Phase 2 trial with ABT-494 (pan-JAK inhibitor) which should read out in 2017. Celgene is investigating ozanimod (S1P 1 and 5 receptor modulator) in Phase 3 and GED-0301 (SMAD-7) in Phase 2 in UC.
Gilead initiated the SELECTION Phase 2b/3 trial in UC with filgotinib in December 2016. SELECTION investigates efficacy and safety of 100 mg and 200 mg filgotinib once-daily compared to placebo in patients with moderately to severely active disease including those with prior antibody therapy failure. Gilead will recruit approximately 1,300 patients from the United States, Europe, Latin America, Canada, and Asia/Pacific regions. The SELECTION Phase 2b/3 trial in UC will include a futility analysis, serving as the Phase 2b part of this integrated Phase 2b/3 study. Men and women in SELECTION will be randomized to receive placebo, 100 mg or 200 mg filgotinib. In the United States, males may receive 200 mg if they failed at least one anti-TNF and vedolizumab.
Our CF Program
CF is a rare, life-threatening, genetic disease affecting the lungs and the digestive system, impacting approximately 80,000 patients worldwide with approximately 30,000 patients in the United States. CF patients carry a defective cystic fibrosis transmembrane conductance regulator, or CFTR, gene and are classified based on their specific mutation of the CFTR gene. The Class II mutation is present in approximately 90% of CF patients, with Orkambi being the only approved therapy for the underlying cause of CF in this mutation. Kalydeco is a disease-modifying treatment for Class II mutations, representing 4% of total CF patients. The market for CF therapies is robust and growing. According to Vertex Pharmaceuticals, approximately 9,000 patients were treated with Vertex therapies in 2016, and this they expect to grow to approximately 75,000 patients by 2024. Combined sales of Kalydeco and Orkambi were approximately $1.7 billion in 2016.
Despite the approval of Kalydeco and Orkambi, there is need for better therapies to improve pulmonary function for a large majority of the patient population. Though many pediatric patients have normal lung function at the time of diagnosis, physicians generally believe that earlier treatments can have downstream benefits for the patient by slowing the deterioration in lung function.
Two types of disease-modifying CFTR modulators have the current focus of CF drug developers. Potentiator molecules aim to restore the flow of ions through an activated CFTR by influencing the channel’s opening. Corrector molecules aim to overcome defective protein processing by restoring proper folding of CFTR and allowing for increased cell surface expression. In order to improve CFTR function meaningfully for the largest patient group with Class II and other mutations, we believe a combination of medicines will be required, comprising a potentiator and two novel corrector (which we refer to as C1 and C2) molecules.
54
Table of Contents
In pre-clinical cellular assay studies, we consistently observed that combinations of potentiator, C1, and C2 correctors restore close to healthy CFTR function in lung epithelial cells and organoids cultured from Class II and Class III/IV patients, both homozygous and heterozygous for F508del, respectively:
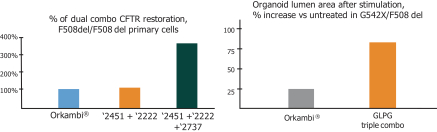
These results are suggestive of a compelling therapeutic option for these patients. We believe that our CF combination therapy may address the unmet need in both homozygous and heterozygous Class II patients, based on these in vitro results.
We aim to evaluate a once-daily, oral, triple combination CF therapy in patients starting in mid-2017, with additional trials with novel CF compounds initiating throughout 2017. We have developed a portfolio of lead and follow-on compounds from which to select the best potentiator and corrector molecules for our triple combination therapy:
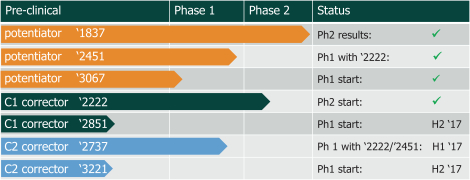
Our Clinical Program for CF
Our most advanced product candidate, potentiator GLPG1837, was the first potentiator since Kalydeco to show comparable results in G551D patients in the SAPHIRA 1 Phase 2a trial in late 2016.
SAPHIRA 1 was an open-label study of three doses of GLPG1837 in 26 patients with the G551D mutation. Of these patients, 25 patients were on stable Kalydeco treatment at screening and agreed to a one week washout prior to the start of dosing GLPG1837. One patient was naïve to Kalydeco. All subjects received GLPG1837 125 mg bid (twice-daily) for 7 days, immediately followed by 250 mg bid for 7 days and subsequently by 500 mg bid for 14 days.
55
Table of Contents
A statistically significant dose dependent decrease in sweat chloride concentration was observed. At the 500 mg bid dose, sweat chloride decreased from a mean value of 98 mmol/L at baseline to 66 mmol/L (p <0.0001). For those patients exceeding the predicted target concentration, sweat chloride changed from a mean value of 94 mmol/L at baseline to 52 mmol/L. Below is an illustration of the changes in sweat chloride at increasing pre-dose exposures of GLPG1837 in blood plasma from SAPHIRA 1 in G551D patients who had washed out of Kalydeco prior to being treated with GLPG1837:
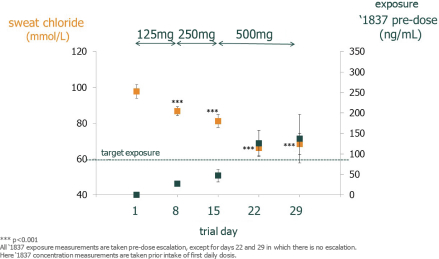
Twenty-five patients were on stable treatment with Kalydeco prior to this study. For these patients, mean percent predicted FEV1 (ppFEV1) levels were 74% at screening (prior to Kalydeco washout). The one week wash-out resulted in a 5.4% mean decrease in absolute ppFEV1. At the end of treatment with GLPG1837, the ppFEV1 levels returned to the Kalydeco pre-washout levels. The figure below illustrates this restoration of ppFEV1 to screening levels, after washout and treatment with GLPG1837 in SAPHIRA 1:
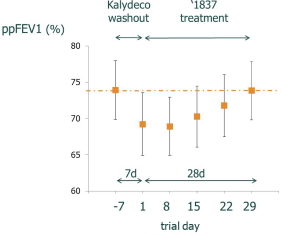
Overall GLPG1837 was well tolerated in SAPHIRA 1, with observed treatment emergent adverse events being predominantly mild or moderate, and typical for a CF patient population. One patient dropped out of the study due to an increase in non-cardiac creatine phosphokinase.
We believe that SAPHIRA 1 represents a clinical validation of our in vitro systems, reinforcing our confidence in our approach to get to a triple combination therapy.
56
Table of Contents
We initiated a Phase 1 trial for a second potentiator candidate GLPG2451 in May 2016. Follow-on potentiator GLPG3067 entered Phase 1 in March 2017. GLPG1837 has a twice-daily dosing profile, while GLPG2451 has potential for once-daily dosing.
We reported that GLPG2222, the first early binding (C1) corrector, showed favorable safety and tolerability in Phase 1 trials in healthy volunteers in June 2016. GLPG2222 was tested in single ascending doses up to 800 mg, and in multiple ascending doses up to 600 mg qd for 14 days in a double-blind, randomized, placebo-controlled study. The product candidate was shown to be well-tolerated and no emerging safety signals observed in the dose range studied. Absorption of GLPG2222 was rapid and favorable. Pharmacokinetics of GLPG2222 support once-daily dosing regimens to be explored in further development. We believe GLPG2222 is well positioned for selection for the first triple combination therapy in 2017 and will be further explored in the clinic throughout the year to improve our understanding of dosing for the triple combination, including a Phase 2 trial on top of Kalydeco in Class III mutation patients initiated in January 2017. We dosed the first healthy volunteer with a combination of GLPG2451 and GLPG2222 in February 2017. Backup C1 corrector GLPG2851 will enter Phase 1 trials in 2017.
We initiated a Phase 1 trial for our first late binding (C2) corrector GLPG2737, the final component needed for a triple combination therapy, in November 2016. Topline results from this trial are expected in Q2 2017. We are developing follow-on C2 correctors which are expected to enter Phase 1 in 2017.
Our IPF Programs
IPF is a chronic, relentlessly progressive fibrotic disorder of the lungs that typically affects adults over the age of 40. According to GlobalData, IPF affects approximately 109,000 patients in the United States and Europe and, as such, we have received orphan designation for our product candidate GLPG1690 in this indication from the European Commission and we intend to seek orphan designation in the United States for our product candidates in IPF. The clinical prognosis of patients with IPF is poor, as the median survival at diagnosis is two to four years. Currently, no medical therapies have been found to cure IPF. The medical treatment strategy aims to slow disease progression and improve quality of life. Lung transplantation may be an option for appropriate patients with progressive disease and minimal comorbidities.
Regulatory agencies have approved Esbriet and Ofev for the treatment of mild to moderate IPF. Both Esbriet and Ofev have been shown to slow the rate of functional decline in IPF and are gaining ground as the standard of care worldwide. Combined sales of both drugs reached $1.1 billion in 2016, with 74% of global revenues being in the United States. These regulatory approvals represent a major breakthrough for IPF patients; yet neither drug improves lung function, and the disease in most patients on these therapies continues to progress. Moreover, the adverse effects associated with these therapies are considerable (e.g., diarrhea, liver function test abnormalities with Ofev, nausea and rash with Esbriet). Therefore, there is still a large unmet medical need as IPF remains a major cause of morbidity and mortality. GlobalData estimates global sales of approved IPF drugs will grow to nearly $2.4 billion in 2022.
GLPG1690 is a potent and selective inhibitor of autotaxin (ATX) and is fully proprietary to us. We identified ATX as a potential target for IPF, after finding the target using an inflammation assay in our target discovery platform. Pharmacology and translational studies published by other parties since then suggest that ATX may also play a role in metabolic disease, arthritic pain, oncology, and lung disease. We evaluated GLPG1690 in a pre-clinical lung fibrosis model (bleomycin-treated mice) and observed effects on reducing the fibrotic score, numerically favoring GLPG1690 over Esbriet.
GLPG1690 completed a Phase 1 first-in-human trial in February 2015. In this trial, GLPG1690 was shown to be well-tolerated in up to 1000 mg daily dose and demonstrated a favorable pharmacokinetic profile.
57
Table of Contents
Moreover, in this trial GLPG1690 demonstrated the ability to reduce plasma lysophosphatidic acid (LPA) levels on a sustained basis, implying ATX engagement.
We are currently conducting a Phase 2a trial (called FLORA) in IPF patients. FLORA is fully recruited and we expect topline results in the second half of 2017. This randomized, placebo-controlled double-blind trial includes up to 24 patients with IPF from 17 centers in Europe and evaluates treatment with 600mg of GLPG1690 for 12 weeks. Primary objectives are to assess safety, tolerability, and pharmacokinetics and pharmacodynamics of GLPG1690 in IPF patients. Target engagement will be measured by LPA in plasma and bronchoalveolar lavage fluid, both at baseline and through 12 weeks of treatment. Secondary objectives include the evaluation of lung function, changes in disease biomarkers and quality of life.
In June 2016, we nominated a second product candidate for IPF, GLPG2938, with an undisclosed novel mechanism of action. This candidate is expected to enter Phase 1 trials in 2017.
Our OA Program
Sometimes called degenerative joint disease or degenerative arthritis, OA is the most common chronic condition of the joints. OA can affect any joint, but it occurs most often in the small joints of the fingers, knees, hips, lower back and neck, and the bases of the thumb and big toe. According to GlobalData, OA will be the fourth leading cause of disability by the year 2020. There are limited data on the total prevalence of OA. GlobalData estimates that diagnosed cases will grow from approximately 117 million cases in 2016 to approximately 131 million cases by 2024, with cases affecting hand, knee, and hip in that order of prevalence.
In normal joints, a firm, rubbery material called cartilage covers the end of each bone. Cartilage provides a smooth, gliding surface for joint motion and acts as a cushion between the bones. In OA, the cartilage breaks down, causing pain, swelling and problems moving the joint. As OA worsens over time, bones may break down and develop growths called spurs. Bits of bone or cartilage may chip off and float around in the joint. In the body, an inflammatory process occurs and cytokines (proteins) and enzymes develop that further damage the cartilage. In the final stages of OA, the cartilage wears away and bone rubs against bone leading to joint damage and more pain.
Although OA occurs in people of all ages, it is most common in people older than 65. Common risk factors include obesity, previous joint injury, over-use of the joint, and weak thigh muscles. One in two adults will develop symptoms of knee OA during their lives. One in four adults will develop symptoms of hip OA by age 85. Current treatments for OA include weight loss, physical therapy, pain and anti-inflammatory medicines, and surgery, all of which address only the symptoms of the disease. There are currently no disease-modifying therapies available for OA, with drug sales for OA patients amounting to approximately $4 billion in generic painkillers in 2016.
GLPG1972 has a novel mode of action with potential application in OA and was discovered by us under our collaboration agreement with Servier, a French pharmaceutical company.
In June 2016, we announced that GLPG1972, a first-in-class product candidate aimed at treating OA, was shown to be safe and well tolerated in healthy human volunteers in a Phase 1 first-in-human trial. In this trial, dosing with GLPG1972 reduced a cartilage breakdown biomarker by up to 60% in these volunteers within two weeks. In 2017, we intend to conduct a Phase 1b patient clinical trial of GLPG1972 in the United States, where we retained full commercial rights. Additional data resulting from the ongoing nonclinical program expected in the second quarter of 2017 is expected to enable our collaboration partner Servier to decide on the exercise of the option to license the compound for further development into OA patient trials outside the United States.
58
Table of Contents
Our AtD Programs
AtD, also known as atopic eczema, is a chronic pruritic (itching) inflammatory skin disease that most frequently starts in early childhood, often persists into adulthood, but may also have an adult onset. According to GlobalData, sales of AtD therapies in the seven major healthcare markets may reach $4 billion in 2016, with 35 million patients diagnosed with the disease and 10 million patients being treated in those markets. The main features of AtD are the impairment of the skin barrier and dysfunction of the immune system accompanied with dry skin and severe pruritus that is associated with cutaneous hyperactivity to various environmental stimuli. The pruritus (itching) may lead to sleep loss, anxiety, depression and impaired social life and is therefore considered as highest therapeutic need in AtD. Generic drugs are the approved standard of care, including immunomodulators cyclosporine and mycophenolate mofetil and topical treatments. There are disease-modifying biologics in currently in development.
MOR106 is a human monoclonal antibody designed to selectively target IL-17C in clinical development worldwide. IL-17C is a target discovered by us and has been shown to be distinct from other members of the IL-17 cytokine family, playing an important and pro-inflammatory role in certain skin disorders. MOR106 potently inhibits the binding of IL-17C to its receptor and thus inhibits its biological activity.
MOR106 arises from a strategic discovery and co-development alliance between us and MorphoSys, in which both companies contribute their core technologies and expertise.
We are currently evaluating MOR106 in a randomized, double-blind, placebo-controlled Phase 1 trial, with the aim to evaluate safety and tolerability. As secondary endpoints, the trial will assess pharmacokinetics and potential immunogenicity of MOR106.
The first part of the trial was conducted in a single center in 56 healthy volunteers, evaluating single ascending doses (SAD) as intravenous infusion compared to placebo. MOR106 showed favorable safety and PK results when administered to healthy volunteers in the ongoing trial. Subsequently investigation was started of multiple ascending doses (MAD) compared to placebo in approximately 24 patients with moderate to severe AtD in several European centers. Topline results of the complete trial, including the MAD part in patients and further results from the SAD part in healthy volunteers, are expected in the second half of 2017.
In June 2016, we nominated a second product candidate for AtD, GLPG2534, with a novel mechanism of action, which is different from the target of MOR106. This small molecule candidate is fully proprietary to us and is expected to enter Phase 1 trials in 2017.
For a breakdown of our total revenues by activity and geographic market, please see “Note 3—Segment information—Geographical information” in our consolidated financial statements appended to this annual report.
Our Strategy
We seek to develop a robust portfolio of breakthrough therapies. Our ambition is to become a fully integrated biopharmaceutical company focused on the development and commercialization of novel medicines. Our strategy is to leverage our unique and proprietary target discovery platform, which facilitates discovery and development of therapies with novel modes of action which address the root causes of the disease.
Key elements of our strategy include:
| • | Rapidly advance the development and commercialization of filgotinib with our collaboration partner Gilead in RA, CD, UC, and potentially other inflammatory diseases |
Based on the results from our Phase 2 clinical trials, we believe that filgotinib is a promising candidate for the treatment of RA, CD, UC and potentially other inflammatory diseases. Our collaboration partner Gilead
59
Table of Contents
initiated Phase 3 clinical programs in RA, CD and UC in 2016. We retained an option to co-promote filgotinib with Gilead in the UK, Germany, France, Italy, Spain, the Netherlands, Belgium, and Luxembourg; exercising this option would enable us to build a commercial organization and further progress our ambition to become a fully integrated biopharmaceutical company.
| • | Collaborate with our collaboration partner AbbVie to develop a CF franchise of triple combination oral therapies |
In order to address the unmet need in CF patients with Class II and other mutations in the CFTR gene, we aim to develop a triple combination therapy comprising a potentiator and two corrector molecules. Our most advanced product candidate for this triple combination therapy, potentiator GLPG1837, showed promising activity, with observed treatment emergent adverse events being predominantly mild or moderate, in CF patients with the Class III mutation of the CFTR gene in a Phase 2 trial late in 2016. This trial also provided data necessary to validate our in vitro assays and dosing modelling for developing a successful triple combination therapy. We initiated a Phase 1 trial for a second potentiator candidate GLPG2451 in May 2016. We reported positive topline results from a Phase 1 trial for our C1 corrector candidate, GLPG2222, in June 2016 and initiated a Phase 2 trial on top of Kalydeco in Class III mutation patients in January 2017. We initiated a Phase 1 trial for our C2 corrector GLPG2737 in November 2016. We dosed the first healthy volunteer with a combination of GLPG2451 and GLPG2222 in February 2017. We aim to evaluate a once-daily, oral, triple combination therapy in CF patients starting in mid-2017, with additional trials with novel CF compounds initiating throughout 2017. We initiated a Phase 1 study with potentiator GLPG3067 in March 2017. We have an exclusive collaboration agreement with AbbVie to jointly discover, develop, and commercialize these novel CF modulators.
| • | Advance GLPG1690 in patient clinical trials in IPF |
We have completed the enrollment of IPF patients in a Phase 2a trial evaluating target and disease biomarker changes during three months’ treatment with autotaxin inhibitor GLPG1690 or placebo, and we intend to disclose topline results of this trial in the second half of 2017. We have worldwide development and commercialization rights for GLPG1690.
| • | Advance GLPG1972 in OA patient clinical trials in the United States |
In June 2016, we announced that a Phase 1 first-in-human trial of GLPG1972, a novel mechanism of action product candidate for the treatment of OA, showed the product candidate reduced a cartilage breakdown biomarker in healthy volunteers up to 60% within two weeks. We retain all development and commercialization rights to this compound in the United States, and we intend to initiate clinical trials of GLPG1972 in the United States in 2017. Additional data resulting from an ongoing nonclinical program expected in the second quarter of 2017 will enable our collaboration partner Servier to make a decision regarding exercising its option to license the compound for further development in OA patient trials outside the United States.
| • | Advance MOR106 in AtD patient clinical trials with our collaboration partner MorphoSys |
In April 2016, we initiated a Phase 1 first-in-human trial and in October 2016, we announced first dosing of an AtD patient with MOR106, a novel human monoclonal antibody medicine in development with MorphoSys, in a Phase 1b trial. MOR106 targets IL17-C, a novel antibody target discovered by us. MorphoSys and we share costs and potential benefits equally in this collaboration. Topline data from the Phase 1b trial with MOR106 are expected in the second half of 2017.
| • | Maximize and capture the value of our target discovery platform by becoming a fully integrated biotechnology company |
Our platform has yielded many new mode-of-action investigational therapies across multiple therapeutic areas. Our most mature pre-clinical programs are GLPG2534 for AtD and GLPG2938 for IPF, both of which we plan to take into Phase 1 trials in 2017. Additionally, we are exploring the potential of development programs
60
Table of Contents
and pre-clinical product candidates in ankylosing spondylitis, psoriatic arthritis, lupus, nonalcoholic steatohepatitis, type 2 diabetes, and hepatitis B. We aim to initiate a Phase 3 trial every other year, while conducting three proof-of-concept trials, delivering eight new validated targets and three pre-clinical product candidates every year. We aim to select promising programs for internal development and commercialization to capture greater value for shareholders and establish ourselves as a fully integrated biopharmaceutical company.
Our Proprietary Target Discovery Platform
Our target discovery platform provides a significant and substantial competitive advantage in our portfolio of novel mode of action product candidates as it:
| • | closely mimics the in vivo situation through the use of primary human cell with relevant trigger and readout for a specific disease phenotype |
| • | identifies the optimal point to intervene in a disease pathway by knocking down of a given protein in these assays |
| • | enables us to rapidly analyze all of the drugable genome and select pharmaceutically tractable protein targets directly by their ability to regulate key disease biology |
Our product candidate filgotinib acts on a target whose role in the specific disease was discovered by us using our discovery platform and we believe is a proof of success of this approach. Filgotinib acts on JAK1 and we believe has potential for a best-in-class profile in Phase 3 clinical trials in RA, CD, and UC.
The human genome is made up of tens of thousands of genes which code for the proteins that make up the human body. Nearly all chronic diseases and disorders are caused by a disruption in the normal function of certain proteins. The main goal of pharmaceutical companies is to design drugs that alter the activity of these proteins so that normal function returns and the cause of the disease is minimized or eliminated. One of the main obstacles in discovering new drugs is to understand exactly which of the body’s thousands of proteins play a key role in a particular disease. Once these proteins are discovered, they become targets for drug design. Finding these targets is one of the critical steps in the drug discovery process. Our approach to target discovery is unique as our discovery platform focuses on target identification using primary human cells, which we believe provides a good system to study the effect that a protein might have on the disease in the human body.
In order to study proteins in human cells, we take advantage of the distinctive properties of adenoviruses. Adenovirus is the virus that causes the common cold and has the capability to infect almost every type of human cell. The adenoviruses we work with have been engineered to act as a shuttle vehicle, allowing the delivery of specific pieces of DNA into human cells. Additionally, these viruses have been made replication incompetent, meaning they do not replicate in the human cell they infect, and so do not interfere with the processes in the cell. We engineered the viruses to carry small pieces of DNA, specific for individual human genes. When the virus enters the cell, this DNA piece leads to the production of a short sequence of RNA that is processed in the cell to become “short interfering RNA,” or siRNA, which specifically interferes with the mRNA of the protein it was designed for. By using these viruses, we can cause the cells to block, or “knock-down,” the production of a certain protein, mimicking what a small molecule drug does in the human body. We built a collection with these adenoviruses, now in excess of 20,000 viruses, that addresses over 6,000 drugable genes.
Our drug discovery research is based on the targets discovered using this technology. Once a target is validated, it is tested against large collections of chemical small molecules to identify chemical structures that interact with the target and block or activate protein production. These chemical structures are then optimized to obtain “drug-like” characteristics followed by testing of the product candidate in the clinic.
We believe that this discovery approach may increase the chances of success in bringing new mode of action drugs to the market. Since 2009, we have generated 32 pre-clinical candidates of which 24 have novel modes of action. Of these, 15 have entered the clinic, 11 with novel modes of action.
61
Table of Contents
In addition to our pipeline of molecules in the clinic, we have multiple discovery programs which are advancing toward clinical development. Further to targets and molecules in RA, IBD, and CF, we are exploring new modes of action in OA, metabolic diseases, fibrosis, Hepatitis B virus, and immune inflammation.
Intellectual Property
The proprietary nature of, and protection for, our product candidates, their methods of use, and our platform technologies are an important part of our strategy to develop and commercialize novel medicines. We have obtained patents relating to certain of our product candidates, and are pursuing additional patent protection for them and for our other product candidates and technologies. We also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. Additionally, we have registered and unregistered trademarks, including amongst others our company name.
Our success will depend significantly on our ability to obtain and maintain patent and other proprietary protection for commercially important products, technologies, inventions and know-how related to our business and our ability to defend and enforce our patents, preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and proprietary rights of third parties. We also rely on know-how, continuing technological innovation and in-licensing opportunities to develop, strengthen and maintain the proprietary position of our development programs.
As of March 24, 2017, patent rights held by Galapagos NV relating to our product candidates include the following:
Filgotinib Product Candidate: We have four U.S. patents relating to filgotinib, one pending U.S. patent application, one patent granted via the EPO and counterpart patent applications that are pending in Australia, Canada, Europe and other foreign countries. The four issued U.S. patents, and any additional patents that may be granted based on our pending U.S. and foreign patent applications, are currently expected to expire in 2030, not including any potential extensions for the marketed product that may be available via supplementary protection certificates or patent term extensions. In addition, we have one granted U.S. patent and two pending U.S. applications, with counterpart applications pending in other foreign countries, which are directed to certain physical forms, including polymorphic forms and compositions, of our filgotinib product candidate, and patents, if granted, based on these patent applications are estimated to expire in 2035, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions. We also have a pending application under the Patent Cooperation Treaty, or PCT, related to the use of our filgotinib product candidate in cardiovascular disorders, and a pending PCT application related to the specific use of our filgotinib product candidate at particular doses in inflammatory conditions. Any patents, if granted, based on these patent applications are estimated to expire in 2036. We additionally have rights in six pending U.K. applications which relate to methods of treatment using filgotinib in additional indications. Any patents, if granted, based on these patent applications are estimated to expire in 2037. We also have two pending U.K. applications related to the use of combinations of filgotinib with other Galapagos proprietary compounds. Any patents, if granted, based on these patent applications are estimated to expire in 2038. We have additional patents and pending patent applications directed to the use of compounds related to our filgotinib product candidate and these patents, and patents that may be issued based on these pending patent applications, are currently expected to expire from 2029 to 2033, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG1837 Product Candidate: We have two issued U.S. patents relating to GLPG1837, one pending U.S. patent application and counterpart foreign patent applications that are pending in Australia, Canada, Europe and other foreign countries. Patents, if any, that issue based on these pending patent applications are estimated to expire in 2034, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG2222 Product Candidate: We have rights in one pending U.S. patent application, a pending patent application under the PCT, as well as patent applications pending in Taiwan and other foreign countries relating
62
Table of Contents
to GLPG2222. Patents, if any, that issue based on these pending patent applications are estimated to expire in 2035, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG2451 Product Candidate: We have rights in one pending U.S. patent application, a pending patent application under the PCT, as well as patent applications pending in Taiwan and other foreign countries relating to GLPG2451. Patents, if any, that issue based on these pending patent applications are estimated to expire in 2036, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG2737 Product Candidate: We have rights in a pending U.S. patent application, a pending patent application under the PCT, as well as patent applications pending in Taiwan and other foreign countries relating to GLPG2737. Patents, if any, that issue, based on this pending patent application are estimated to expire in 2036, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG3067 Product Candidate: We have rights in a pending U.S. patent application relating to GLPG3067. Patents, if any, that issue, based on this pending patent application are estimated to expire in 2037, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG2851 Product Candidate: We have rights in a pending U.S. patent application, a pending patent application under the PCT, as well as patent applications pending in Taiwan and other foreign countries relating to GLPG2851. Patents, if any, that issue, based on this pending patent application are estimated to expire in 2036, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG3221 Product Candidate: We have rights in a pending U.S. patent application relating to GLPG3221. Patents, if any, that issue, based on this pending patent application are estimated to expire in 2037, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG1690 Product Candidate: We have two issued U.S. patents relating to GLPG1690, one pending U.S. patent application and counterpart foreign patent applications that are pending in Australia, Canada, Europe and other foreign countries. Patents, if any, that issue based on these pending patent applications are estimated to expire in 2034, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG1972 Product Candidate: We have rights, jointly with our alliance partner Servier, in a pending patent application under the PCT, as well as patent applications pending in Taiwan and other foreign countries relating to GLPG1972. Patents, if any, that issue based on these pending patent applications are estimated to expire in 2035, not including any potential extensions that may be available for the marketed product via supplementary protection certificates or patent term extensions.
MOR106 Product Candidate: We have rights in a pending patent application under the PCT, as well as patent applications pending in Taiwan and other foreign countries relating to MOR106. Patents, if any, that issue based on this pending patent application are estimated to expire in 2037, not including any potential extension that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG2534 Product Candidate: We have a pending patent application under the PCT, as well as patent applications pending in Taiwan and other foreign countries relating to GLPG2534. Patents, if any, that issue
63
Table of Contents
based on this pending patent application are estimated to expire in 2036, not including any potential extension that may be available for the marketed product via supplementary protection certificates or patent term extensions.
GLPG2938 Product Candidate: We have a pending patent application under the PCT, as well as patent applications pending in Taiwan and other foreign countries relating to GLPG2938. Patents, if any, that issue based on this pending patent application are estimated to expire in 2037, not including any potential extension that may be available for the marketed product via supplementary protection certificates or patent term extensions.
We also own or have rights in patents relating to our target discovery platform.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the date of filing the application. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the United States Patent and Trademark Office, or USPTO, in granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier-filed co-owned patent. In addition, in certain instances, a patent term can be extended to recapture a portion of the term effectively lost as a result of the FDA regulatory review period. However, the restoration period cannot be longer than five years and the total patent term including the restoration period must not exceed 14 years following FDA approval. In certain foreign jurisdictions similar extensions as compensation for regulatory delays are also available. The actual protection afforded by a patent varies on a claim by claim and country to country basis for each applicable product and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
Furthermore, the patent positions of biotechnology and pharmaceutical products and processes like those we intend to develop and commercialize are generally uncertain and involve complex legal and factual questions. No consistent policy regarding the breadth of claims allowed in such patents has emerged to date in the United States. The patent situation outside the United States is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States and other countries can diminish our ability to protect our inventions, and enforce our intellectual property rights and more generally, could affect the value of intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents.
The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights. Our ability to maintain and solidify our proprietary position for our product candidates and technology will depend on our success in obtaining effective claims and enforcing those claims once granted. We do not know whether any of the patent applications that we may file or license from third parties will result in the issuance of any patents. The issued patents that we own or may receive in the future, may be challenged, invalidated or circumvented, and the rights granted under any issued patents may not provide us with proprietary protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may be able to independently develop and commercialize similar drugs or duplicate our technology, business model or strategy without infringing our patents. Because of the extensive time required for clinical development and regulatory review of a drug we may develop, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of any such patent.
We may rely, in some circumstances, on trade secrets and unpatented know-how to protect our technology. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our consultants, scientific advisors and contractors and invention assignment agreements with our employees. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security
64
Table of Contents
measures may be breached and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants, contractors or collaboration partners use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Our commercial success will also depend in part on not infringing the proprietary rights of third parties. It is uncertain whether the issuance of any third-party patent would require us to alter our development or commercial strategies, or our product candidates or processes, obtain licenses or cease certain activities. Our breach of any license agreements or failure to obtain a license to proprietary rights that we may require to develop or commercialize our product candidates may have a material adverse impact on us. If third parties have prepared and filed patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference proceedings in the USPTO, to determine priority of invention if the patent applications were filed before March 16, 2013, or in derivation proceedings to determine inventorship for patent applications filed after such date.
In addition, substantial scientific and commercial research has been conducted for many years in the areas in which we have focused our development efforts, which has resulted in third parties having a number of issued patents and pending patent applications relating to such areas. Patent applications in the United States and elsewhere are generally published only after 18 months from the priority date. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made. Therefore, patent applications relating to drugs similar to our current product candidates and any future drugs, discoveries or technologies we might develop may have already been filed by others without our knowledge. For more information on these and other risks related to intellectual property, see “Item 3.D.—Risk Factors—Risks Related to Our Intellectual Property.”
Collaborations
We have entered into multiple collaboration agreements with pharmaceutical partners, which have generated approximately $896 million in cash at December 31, 2016 to fund discovery and development. We expect to continue to collaborate selectively with pharmaceutical and biotechnology companies to leverage our discovery platform and accelerate product candidate development. Our current alliances include the following alliances with AbbVie and Gilead:
Amended and Restated Collaboration with AbbVie for CFTR Modulators (CF)
On September 23, 2013, we entered into a global collaboration agreement with AbbVie focused on the discovery and worldwide development and commercialization of potentiator and corrector molecules for the treatment of CF. In connection with our entry into the collaboration agreement we received a one-time, non-refundable, non-creditable upfront payment in the amount of $45 million. On April 28, 2016, we and AbbVie entered into the amended and restated agreement which expanded the parties’ CF collaboration by amending and restating the collaboration agreement to, among other things, increase the remaining total milestones under the amended and restated agreement up to approximately $600 million from $350 million. As amended, the collaboration will provide for the potential development and commercialization of triple combination products consisting of a potentiator molecule, a corrector 1 molecule and a corrector 2 molecule to treat specified populations of patients with CF. As of the date of this annual report, we have achieved $57.5 million as milestones under this agreement (of which $50 million has been paid to date), in addition to the $45 million upfront payment.
The collaboration is managed by a set of joint committees comprised of equal numbers of representatives from each party. The joint steering committee oversees and coordinates the overall conduct of the collaboration. The joint research committee, or JRC, oversees and coordinates the discovery phase of the collaboration. The joint development committee, or JDC, oversees and coordinates the development phase of the collaboration. The
65
Table of Contents
joint commercialization committee will oversee and develop the strategies for commercialization of co-promoted licensed products in the Netherlands, Belgium and Luxembourg if we elect to exercise our co-promotion option, as described below.
Under the terms of the collaboration, both parties are required to use commercially reasonable efforts to identify and deliver a specified number of potentiator molecules which may be used in combination with a corrector molecule as a dual combination product, a specified number of corrector 1 molecules to be used in combination with a potentiator molecule and a corrector 2 molecule as a triple combination product and a specified number of corrector 2 molecules which may be used in combination with a potentiator molecule and a corrector 1 molecule as a triple combination product. The parties are also required to use commercially reasonable efforts to identify and deliver a specified number of backup molecules for each of the molecules described above. Each of the above molecules is to be measured against agreed-to success criteria.
If (i) the JRC determines that a potentiator molecule, a corrector 1 molecule and/or a corrector 2 molecule have met certain specified criteria by a specified date, or AbbVie otherwise decides to continue development of such molecule(s), and (ii) an investigational new drug application has been accepted for such molecules(s), then we and AbbVie will develop and approve (through the JDC) a plan in connection with development of such molecule and, when appropriate, combination product(s) including such molecule, with the goal of achieving agreed-to proof of concept criteria. We are generally responsible for the costs of such development activities at our expense up to an agreed cost cap, and then each party will be responsible for the excess costs associated with its respective agreed upon development activities.
If the applicable proof of concept criteria are met or AbbVie otherwise decides to continue development, we and AbbVie will develop and approve (through the JDC) a plan in connection with Phase 3 clinical trials for the molecule or molecules, in which we are responsible for a specified percentage of the costs.
Subject to certain exceptions, following approval, AbbVie will have the sole right to commercialize licensed products worldwide, except in China and South Korea, in which we will have the sole right to commercialize licensed products, and further subject to our co-promotion option in the Netherlands, Belgium and Luxembourg. We will be solely responsible for obtaining regulatory and other approvals required for commercialization of licensed products in China and South Korea.
Under the amended and restated agreement, we are still eligible to receive up to $567.5 million in total additional payments for developmental, regulatory and sales-based milestones. In March 2017, we achieved a $7.5 million milestone, for which the payment is not yet received to date. In addition, we will be eligible to receive tiered royalties ranging from the mid-teens to 20% on net sales of licensed products payable on a product-by-product basis. The royalties payable to us under the amended and restated agreement may be reduced under certain circumstances, including if generic competition on an active ingredient of a licensed product in a particular territory results in market share losses of a certain amount. Our right to receive royalties under the amended and restated agreement expires, on a product-by-product and country-by-country basis, on the later of (1) the last day that at least one valid patent claim subject to the amended and restated agreement and covering the licensed product exists, (2) the expiry of a mutually agreed upon time period after the first commercial sale of the licensed product in the applicable country, or (3) the expiration of regulatory exclusivity for the licensed product in the applicable country. In the event we exercise our co-promotion option with respect to a licensed product, we would assume a portion of the co-promotion effort in The Netherlands, Belgium and Luxembourg and share in the net profit and net losses in these territories instead of receiving royalties in those territories during the period of co-promotion.
Under the amended and restated agreement, subject to certain exceptions, neither party may directly or indirectly (including by means of licensing, acquisition or otherwise), on its own or through a third party, research, develop, commercialize or manufacture any molecule, compound or product that has as one of its primary mechanisms of action modulation of the activity of the CF transmembrane conductance regulator.
66
Table of Contents
The amended and restated agreement will expire upon the expiration of the longest royalty term applicable to licensed products under the agreement as described above. Either party may terminate the amended and restated agreement on a country-by-country basis in their respective jurisdictions if they are unable to secure or maintain regulatory approval for the licensed product. After certain discovery activities, but before the first commercial sale of any licensed product by AbbVie, AbbVie may terminate the amended and restated agreement for convenience in its entirety or on a country-by-country basis upon prior written notice to us. Either we or AbbVie may terminate the agreement for the other party’s uncured material breach; however, if such breach relates solely to a breach with respect to our diligence obligations in China or South Korea or AbbVie’s commercialization diligence obligations in the United States, France, Italy, Spain, the United Kingdom or Germany, we or AbbVie may only terminate the amended and restated agreement with respect to such country. Either party may terminate the amended and restated agreement in the event of specified insolvency events involving the other party.
If the amended and restated agreement terminates due to our material breach or as a result of a change of control, all rights and licenses granted to AbbVie will become exclusive or non-exclusive at AbbVie’s sole option, irrevocable, unrestricted and perpetual, and AbbVie will provide consideration for such rights and licenses in an amount to be mutually agreed between us and AbbVie. If the amended and restated agreement terminates in its entirety for any other reason, all rights and licenses granted by either party will terminate, and we will have an exclusive option to obtain an exclusive or non-exclusive license from AbbVie under certain intellectual property rights to exploit the licensed product that is the subject of development or commercialization at the time of termination. If we exercise such option, we and AbbVie will then negotiate a transition agreement which will, in most termination cases, include reasonable financial consideration to AbbVie.
If the amended and restated agreement is terminated in a specific territory because of AbbVie’s material, uncured breach in such territory, or due to an inability by AbbVie to obtain regulatory approval, all rights and licenses granted by us will be deemed amended not to include such territory, and we will have specified rights for, and AbbVie will take specified actions to assist us in continuing the development, manufacture and commercialization of the licensed product in such territory. If the amended and restated agreement is terminated in a specific territory because of our material, uncured breach in such territory, or because of our inability to obtain regulatory approval, all rights and licenses granted to AbbVie with respect to that country will become exclusive or non-exclusive at AbbVie’s sole option, irrevocable, unrestricted and perpetual, and AbbVie will provide consideration for such rights and licenses in an amount to be mutually agreed between us and AbbVie. In addition, AbbVie will have specified rights for, and we will take specified actions to assist AbbVie in, continuing the development, manufacture and commercialization of the licensed product in such territory.
Either party may, without the consent of the other party, assign the amended and restated agreement to an affiliate or successor. Any other assignment requires written consent of the other party. However, with respect to an assignment to an affiliate, the assigning party will remain responsible. If we undergo a change in control prior to the first commercial sale of a product, AbbVie has the right to terminate the amended and restated agreement. At any time, if we undergo a change in control, AbbVie may disband all joint committees and undertake exclusive control of their activities, terminate our right to co-promote and/or terminate our rights and licenses in connection with development and sale of any product in China and South Korea.
Exclusive Collaboration Agreement with Gilead for Filgotinib
In September 2015, our exclusive collaboration with AbbVie for JAK1 inhibitors was terminated, following which we regained all unencumbered rights to filgotinib.
In December 2015, we entered into a global collaboration agreement with Gilead to develop and commercialize filgotinib for the treatment of inflammatory indications. On January 13, 2016, the parties announced that the U.S. Federal Trade Commission provided early termination of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and we closed this transaction on January 19, 2016.
67
Table of Contents
In connection with our entry into the collaboration agreement, we received an upfront payment of $725 million consisting of a one-time, non-refundable, non-creditable license fee in the amount of $300 million and a $425 million equity investment. All payments by Gilead to us are made in U.S. dollars. As of the date of this annual report, we have received an additional $60 million as payments under this agreement.
In addition, we will be eligible to receive development and regulatory milestone-based payments of up to $695 million and sales-based milestone payments of up to $600 million. We will be eligible to receive tiered royalty percentages starting at 20% on global net sales of licensed products. The royalties payable to us under the collaboration agreement may be reduced under certain circumstances. Our right to receive royalties under the collaboration agreement continues, on a country-by-country basis, until the later to occur of certain specified events. In the event we exercise our co-promotion option with respect to licensed products in one or more of the territories eligible for co-promotion, we would assume a portion of the co-promotion effort in the United Kingdom, Germany, France, Italy, Spain, the Netherlands, Belgium, and/or Luxembourg and share equally in the net profit and net losses in these territories instead of receiving royalties in those territories during the period of co-promotion.
The collaboration is managed by a set of joint committees comprised of equal numbers of representatives from each of us and Gilead. The joint steering committee monitors and provides strategic oversight of the activities under the collaboration and facilitates communications between the parties. The joint development committee oversees and coordinates the development of the licensed products. The joint commercialization committee will oversee commercialization of licensed products and co-promoted licensed products in co-promotion territories if we elect to exercise our co-promotion option, as described below.
Under the terms of the collaboration, Gilead is primarily responsible for development and for seeking regulatory approval of the licensed product. We are required to use commercially reasonable efforts as requested by Gilead to assist Gilead with certain development activities.
The collaboration agreement will expire (a) on a country-by-country basis at the end of the royalty term in such country and (b) at such time as a generic product is first sold in a co-promotion country in the event we exercise our co-promotion option with respect to licensed products in The United Kingdom, Germany, France, Italy, Spain, The Netherlands, Belgium, and/or Luxembourg. Upon expiration of the collaboration agreement, the licenses will become fully-paid, perpetual and irrevocable. Either we or Gilead may terminate the collaboration agreement for the other party’s uncured material breach. Either we or Gilead may terminate the collaboration agreement in the event of specified insolvency events involving the other party. Gilead may also terminate the collaboration agreement in its entirety for convenience following a certain period upon prior written notice.
If the collaboration agreement terminates in its entirety for any reason, all rights and licenses granted by either party will terminate, and we will obtain an exclusive, perpetual, irrevocable, royalty-bearing license from Gilead under certain intellectual property rights to exploit the licensed product that is the subject of development or commercialization at the time of termination. If the collaboration agreement is terminated in a specific territory, all rights and licenses granted by us will be deemed to be amended not to include such territory, and we will have a corresponding license with respect to such terminated country. The collaboration agreement also contains other termination rights specified therein.
Either party may, without the consent of the other party, assign the collaboration agreement to an affiliate or successor. Any other assignment requires written consent of the other party. However, with respect to an assignment to an affiliate, the assigning party will remain bound by the terms of the collaboration agreement. If we undergo a change in control, Gilead has the right to terminate the co-promotion option or, if the option has already been exercised, our right to co-promote, and disband all joint committees and undertake exclusive control of their activities; provided, that Gilead has no right to exercise such rights if we undergo a change in control with a drug company that has a market capitalization less than a certain percentage of our market capitalization.
Seasonality
Our business is currently not materially affected by seasonality.
68
Table of Contents
Manufacturing and Supply
We currently do not own or operate manufacturing facilities for the production of product candidates for pre-clinical, clinical or commercial use. We currently outsource to a limited number of external service providers the production of all drug substances and drug products, and we expect to continue to do so to meet the pre-clinical and clinical requirements of our product candidates. We do not have long-term agreements with these third parties. We have framework agreements with most of our external service providers, under which they generally provide services to us on a short-term, project-by-project basis.
Currently, our drug raw materials which support our clinical studies are manufactured by multiple suppliers. We have agreements for the supply of such drug materials with manufacturers or suppliers that we believe have sufficient capacity to meet our demands. In addition, we believe that adequate alternative sources for such supplies exist. However, there is a risk that, if supplies are interrupted, it would materially harm our business. We typically order raw materials and services on a purchase order basis and do not enter into long-term dedicated capacity or minimum supply arrangements. To date, the prices of our principal raw materials have not been volatile.
Manufacturing is subject to extensive regulations that impose various procedural and documentation requirements, which govern record keeping, manufacturing processes and controls, personnel, quality control and quality assurance, among others. The contract manufacturing organizations we use to manufacture our product candidates operate under current good manufacturing practice, or cGMP, conditions. cGMPs are regulatory requirements for the production of pharmaceuticals that will be used in humans. For most of our manufacturing processes a back-up GMP manufacturer is in place or can easily be identified.
Competition
Our industry is highly competitive and subject to rapid and significant change. While we believe that our development and commercialization experience, scientific knowledge and industry relationships provide us with competitive advantages, we face competition from pharmaceutical, medical device and biotechnology companies, including specialty pharmaceutical companies, and generic drug companies, academic institutions, government agencies and research institutions.
In the field of RA, therapeutic approaches have traditionally relied on DMARDS such as MTX and sulphasalazine as first-line therapy. These oral drugs work primarily to suppress the immune system and, while effective in this regard, the suppression of the immune system leads to an increased risk of infections and other side effects. Accordingly, in addition to DMARDS, monoclonal antibodies targeting TNF, like AbbVie’s Humira, or IL-6 like Roche’s Actemra, have been developed. These biologics, which must be delivered via injection, are currently the standard of care as first- and second-line therapies for RA patients who have an inadequate response to DMARDS. In November 2012, Xeljanz, marketed by Pfizer, was approved by the FDA as an oral treatment of adult patients with RA who have had an inadequate response to, or who are intolerant of, MTX. Xeljanz is the first JAK inhibitor for RA approved for commercial sale in the United States. We are aware of other JAK inhibitors in development for patients with RA, including a once- daily JAK1/2 inhibitor called baricitinib being developed by Lilly which is approved by the EMA for RA and is expected to be approved by the FDA as early as March 2017, a JAK3/2/1 inhibitor called ASP015k which is being developed in Japan by Astellas, and a JAK inhibitor called ABT-494 which is being developed by AbbVie. Filgotinib, which is a selective JAK1 inhibitor currently in three Phase 3 studies, is being developed in collaboration with Gilead.
We expect that filgotinib, for which we have completed a Phase 2 program in patients with moderate to severe RA who have an inadequate response to MTX, will compete with all of these therapies when marketed. If generic or biosimilar versions of these therapies are approved we would also expect to compete against these versions of the therapies.
In the field of IBD, first line therapies are oral (or local) treatments with several low-cost generic compounds such as mesalazine, more effective in UC, and azathioprine, more effective in CD. Steroids such as
69
Table of Contents
budesonide are used in both UC and CD. Companies such as Santarus have developed controlled-release oral formulation with the aim to have local intestinal delivery of budesonide thereby limiting systemic side effects. For more advanced therapy, monoclonal antibodies with various targets such as TNF and more recently, integrins by vedoluzimab (Entyvio) are approved. We are also aware of other biologics in clinical development for these indications, such as: ustekinumab, developed by Johnson & Johnson, which is in Phase 3 clinical trials. Celgene has two new oral therapies in development: ozanimod, currently in Phase 3 in UC and Phase 2 in CD, and mongersen in a Phase 3 study in CD and Phase 2 study in UC. Pfizer’s Xeljanz showed activity in Phase 3 and has been filed for approval in UC. The large number of treatments for UC, and somewhat less for CD, presents a substantial level of competition for any new treatment entering the IBD market.
In the field of CF, the approved therapies to treat CF patients have mostly been designed to treat the symptoms of the disease rather than its cause. Kalydeco and more recently Orkambi, both from Vertex, are currently the only two FDA-approved therapies to address the cause of Class III and Class II mutation CF, respectively. Kalydeco, also approved in Europe, is a CFTR potentiator to treat CF in patients with a Class III (G551D) mutation of the CFTR gene. Vertex also developed lumacaftor, a corrector molecule to address a broader patient population, including patients with a Class II (F508del) mutation of the CFTR gene. Vertex obtained approval in July 2015 in the United States for Orkambi, a combination product (Kalydeco + lumacaftor) and obtained approval in November 2015 in Europe. We are also aware of other companies, including Novartis, Nivalis, Pfizer, Proteostasis, and ProQR, and not-for-profit organizations like Flatley Discovery Lab, which are actively developing product candidates for the treatment of CF. These typically target the CFTR protein as potentiators, correctors or other modulators of its activity.
In the field of IPF, there are two approved disease modifying drugs, pirfenidone, marketed by Roche, and nintenanib, marketed by Boehringer Ingelheim. These drugs are not well tolerated by patients and prolong life for IPF patients by a matter of months, leaving an unmet medical need for those developing disease-modifying drugs in this field.
In the field of OA, there are currently no disease-modifying drugs approved. Current treatment involves weight loss, physical therapy, prednisolone, non-steroidal anti-inflammatory drugs, and pain management.
In the field of AtD, immunomodulators such as cyclosporine and mycophenolate mofetil and topical calcineurin inhibitors tacrolimus, marketed by Astellas, and pimecrolimus, marketed by Meda, currently dominate treatment share, according to a GlobalData 2015 report. Key opinion leaders interviewed by GlobalData for that same report indicated that a high unmet need remains for a better treatment armamentarium for severe, recalcitrant patients. This patient segment remains underserved as physicians have few to no pharmacological options following treatment failure with or intolerability to cyclosporine, and as a result physicians often resort to prescribing off-label therapies. Further compounding this issue is the fact that although cyclosporine is indicated for the short-term treatment of AtD in some European countries, leading dermatologists believe that no true systemic drug for the disease exists, and this remains the most pressing unmet need for developers to address.
Many of our competitors have significantly greater financial, technical and human resources than we have. Mergers and acquisitions in the pharmaceutical, medical device and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Our commercial opportunity could be reduced or eliminated if our competitors develop or market products or other novel therapies that are more effective, safer or less costly than our current or future product candidates, or obtain regulatory approval for their products more rapidly than we may obtain approval for our product candidates. Our success will be based in part on our ability to identify, develop and manage a portfolio of product candidates that are safer and more effective than competing products.
70
Table of Contents
Government Regulation
Government Regulation and Product Approval
Government authorities in the United States at the federal, state and local level, and in other countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing, export and import of products such as those we are developing.
U.S. Regulation
U.S. Drug Development Process
The process of obtaining regulatory approvals and compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process, or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, untitled or warning letters, voluntary product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| • | completion of pre-clinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices regulations; |
| • | submission to the FDA of an investigational new drug application, or IND, which must become effective before human clinical trials may begin; |
| • | performance of adequate and well-controlled human clinical trials according to good clinical practices, or GCP, to establish the safety and efficacy of the proposed drug for its intended use; |
| • | preparation and submission to the FDA of a new drug application, or NDA; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with cGMP; |
| • | potential FDA audit of the clinical trial sites that generated the data in support of the NDA; and |
| • | FDA review and approval of the NDA. |
The testing and approval process requires substantial time, effort and financial resources and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all.
Once a pharmaceutical product candidate is identified for development, it enters the pre-clinical testing stage. Pre-clinical tests include laboratory evaluations of product chemistry, toxicity, formulation and stability, as well as animal studies. An IND sponsor must submit the results of the pre-clinical tests, together with manufacturing information, analytical data and any available clinical data or literature, to the FDA as part of the IND. The sponsor must also include a protocol detailing, among other things, the objectives of the initial clinical trial, dosing procedures, subject selection and exclusion criteria, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated if the initial clinical trial lends itself to an efficacy evaluation. Some pre-clinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions related to a proposed clinical trial and places the trial on a clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during clinical trials due to safety concerns or non-compliance, and may be imposed on all drug products within a certain class of drugs. The FDA also can impose partial clinical holds, for example, prohibiting the initiation of clinical trials of a certain duration or for a certain dose.
71
Table of Contents
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with GCP regulations. These regulations include the requirement that all research subjects provide informed consent in writing before their participation in any clinical trial. Further, an institutional review board, or IRB, must review and approve the plan for any clinical trial before it commences at any institution, and the IRB must conduct continuing review and reapprove the study at least annually. An IRB considers, among other things, whether the risks to individuals participating in the clinical trial are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the information regarding the clinical trial and the consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. Each new clinical protocol and any amendments to the protocol must be submitted for FDA review, and to the IRBs for approval.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| • | Phase 1. The product is initially introduced into a small number of healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain early evidence on effectiveness. In the case of some products for severe or life- threatening diseases, especially when the product is suspected or known to be unavoidably toxic, the initial human testing may be conducted in patients. |
| • | Phase 2. Involves clinical trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage and schedule. |
| • | Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit relationship of the product and provide an adequate basis for physician labeling. |
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA. Written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected suspected adverse events, findings from other studies or animal or in vitro testing that suggest a significant risk to humans, and any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. Phase 1, Phase 2 and Phase 3 testing may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the product and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
A drug being studied in clinical trials may be made available to individual patients in certain circumstances. Pursuant to the 21st Century Cures Act, or Cures Act, which was signed into law in December 2016, the manufacturer of an investigational drug for a serious disease or condition is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for individual patient access to such investigational drug. This requirement applies on the later of 60 calendar days after the date of enactment of the Cures Act or the first initiation of a Phase 2 or Phase 3 trial of the investigational drug.
72
Table of Contents
U.S. Review and Approval Processes
The results of product development, pre-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the drug, proposed labeling and other relevant information, are submitted to the FDA as part of an NDA for a new drug, requesting approval to market the product. The submission of an NDA is subject to the payment of a substantial user fee; although a waiver of such fee may be obtained under certain limited circumstances. For example, the agency will waive the application fee for the first human drug application that a small business or its affiliate submits for review. The sponsor of an approved NDA is also subject to annual product and establishment user fees.
The FDA reviews all NDAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be re-submitted with the additional information. The re-submitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure the product’s identity, strength, quality and purity. Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is or will be manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. The FDA may refer the NDA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. An advisory committee is a panel of experts, including clinicians and other scientific experts, who provide advice and recommendations when requested by the FDA. The FDA is not bound by the recommendation of an advisory committee, but it considers such recommendations when making decisions.
The approval process is lengthy and difficult and the FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical data or other data and information. Even if such data and information are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data. The FDA will issue a complete response letter if the agency decides not to approve the NDA in its present form. The complete response letter usually describes all of the specific deficiencies that the FDA identified in the NDA that must be satisfactorily addressed before it can be approved. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical trials. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application or request an opportunity for a hearing.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may require post-approval studies, including Phase 4 clinical trials, to further assess a drug’s safety and effectiveness after NDA approval and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized. As part of the NDA, the FDA also may require the submission of a risk evaluation and mitigation strategy, or REMS, to ensure that the benefits of the drug outweigh the risks of the drug. The REMS plan could include medication guides, physician communication plans, and elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools. An assessment of the REMS must be conducted at set intervals. Following product approval, a REMS also may be required by the FDA if new safety information is discovered and the FDA determines that a REMS is necessary to ensure that the benefits of the drug outweigh the risks of the drug.
73
Table of Contents
Expedited Programs
Fast Track Designation
The FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new drugs that meet certain criteria. Specifically, new drugs are eligible for Fast Track designation if they are intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new drug may request the FDA to designate the drug as a Fast Track product concurrently with, or at any time after, submission of an IND, and the FDA must determine if the product candidate qualifies for fast track designation within 60 days of receipt of the sponsor’s request.
In addition to other benefits, such as the ability to engage in more frequent interactions with the FDA, the FDA may initiate review of sections of a Fast Track drug’s NDA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submission of each portion of the NDA and the applicant pays applicable user fees. However, the FDA’s time period goal for reviewing an application does not begin until the last section of the NDA is submitted. Additionally, the Fast Track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Accelerated Approval
Under FDA’s accelerated approval regulations, the FDA may approve a drug product for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. In clinical trials, a surrogate endpoint is a marker, such as a measurement of laboratory or clinical signs of a disease or condition that is thought to predict clinical benefit, but is not itself a measure of clinical benefit. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of post-approval clinical trials sometimes referred to as Phase 4 trials to confirm the effect on the clinical endpoint. Failure to conduct required post- approval studies, or to confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the drug product from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
Breakthrough Designation
The Food and Drug Administration Safety and Innovation Act, or FDASIA, amended the FDCA to require the FDA to expedite the development and review of a breakthrough therapy. A drug product can be designated as a breakthrough therapy if it is intended to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that it may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. A sponsor may request that a drug product be designated as a breakthrough therapy concurrently with, or at any time after, the submission of an IND, and the FDA must determine if the product candidate qualifies for breakthrough therapy designation within 60 days of receipt of the sponsor’s request. If so designated, the FDA shall act to expedite the development and review of the product’s marketing application, including by meeting with the sponsor throughout the product’s development, providing timely advice to the sponsor to ensure that the development program to gather pre-clinical and clinical data is as efficient as practicable, involving senior managers and experienced review staff in a cross-disciplinary review, assigning a cross-disciplinary project lead for the FDA review team to facilitate an efficient review of the development program and to serve as a scientific liaison between the review team and the sponsor, and taking steps to ensure that the design of the clinical trials is as efficient as practicable.
74
Table of Contents
Priority Review
Priority review is granted where there is evidence that the proposed product would be a significant improvement in the safety or effectiveness of the treatment, diagnosis, or prevention of a serious condition. If a product that contains a new molecular entity is granted priority review, the FDA aims to review the application six months after it accepts the application for filing. If criteria are not met for priority review, the application is subject to the standard FDA review period of ten months after FDA accepts the application for filing. Priority review designation does not change the scientific/ medical standard for approval or the quality of evidence necessary to support approval.
Post-Approval Requirements
Any products for which we receive FDA approval are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements. The FDA strictly regulates labeling, advertising, promotion and other types of information on products that are placed on the market. Products may be promoted only for the approved indications and in accordance with the provisions of the approved label. Further, manufacturers must continue to comply with cGMP requirements, which are extensive and require considerable time, resources and ongoing investment to ensure compliance. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented and other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
Manufacturers and other entities involved in the manufacturing and distribution of approved products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. The cGMP requirements apply to all stages of the manufacturing process, including the production, processing, sterilization, packaging, labeling, storage and shipment of the product. Manufacturers must establish validated systems to ensure that products meet specifications and regulatory standards, and test each product batch or lot prior to its release. We rely, and expect to continue to rely, on third parties for the production of clinical quantities of our product candidates. Future FDA and state inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution or may require substantial resources to correct.
The FDA may withdraw a product approval if compliance with regulatory requirements is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Further, the failure to maintain compliance with regulatory requirements may result in administrative or judicial actions, such as fines, untitled or warning letters, holds on clinical trials, voluntary product recalls, product seizures, product detention or refusal to permit the import or export of products, refusal to approve pending applications or supplements, restrictions on marketing or manufacturing, injunctions or civil or criminal penalties.
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations, guidances, and policies are often revised or reinterpreted by the agency in ways that may significantly affect our business and our product candidates. It is impossible to predict whether further legislative or FDA regulation or policy changes will be enacted or implemented and what the impact of such changes, if any, may be.
75
Table of Contents
Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of the use of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA plus the time between the submission date of an NDA and the approval of that application, less any time the applicant did not act with due diligence. Only one patent applicable to an approved drug is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restorations of patent term for some of our currently owned or licensed patents to add patent life beyond their current expiration dates, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant NDA; however, there can be no assurance that any such extension will be granted to us.
Market exclusivity provisions under the FDCA can also delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the pre-clinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Pediatric exclusivity is another type of exclusivity in the United States. Pediatric exclusivity, if granted, provides an additional six months to an existing exclusivity or statutory delay in approval resulting from a patent certification. This six-month exclusivity, which runs from the end of other exclusivity protection or patent delay, may be granted based on the voluntary completion of a pediatric clinical trial in accordance with an FDA-issued “Written Request” for such a clinical trial.
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition—generally a disease or condition that affects fewer than 200,000 individuals in the United States or that affects more than 200,000 individuals in the United States and for which there is no reasonable expectation that costs of research and development of the drug for the indication can be recovered by sales of the drug in the United States. Orphan drug designation must be requested before submitting an NDA.
After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the
76
Table of Contents
duration of, the regulatory review and approval process. The first NDA applicant to receive FDA approval for a particular active ingredient to treat a particular disease or condition with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the United States for that product, for that indication. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee.
During the exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease or condition, except in limited circumstances, such as if the second applicant demonstrates the clinical superiority of its product to the product with orphan drug exclusivity through a demonstration of superior safety, superior efficacy, or a major contribution to patient care. “Same drug” means, a drug that contains the same identity of the active moiety if it is a drug composed of small molecules, or of the principal molecular structural features if it is composed of macromolecules and is intended for the same use as a previously approved drug, except that if the subsequent drug can be shown to be clinically superior to the first drug, it will not be considered to be the same drug. Drug exclusivity does not prevent FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition.
Pediatric Information
Under the Pediatric Research Equity Act of 2003, or PREA, NDAs or supplements to NDAs must contain data adequate to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. The FDASIA amended the FDCA to require that a sponsor who is planning to submit a marketing application for a drug product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan, or PSP, within sixty days of an end-of-phase 2 meeting or as may be agreed between the sponsor and the FDA. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of data or full or partial waivers. The FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from pre-clinical studies, early phase clinical trials, and/or other clinical development programs. At this time, the requirements of PREA do not apply to an application to market a drug for an orphan-designated indication.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA-regulated products, including drugs, are required to register and disclose certain clinical trial information, which is publicly available at www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed until the new product or new indication being studied has been approved. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we may obtain regulatory approval. In the United States, sales of any products for which we may receive regulatory approval for commercial sale will depend in part on the availability of coverage and reimbursement from third-party payors. Third-party payors include government authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a
77
Table of Contents
drug product may be separate from the process for setting the reimbursement rate that the payor will pay for the drug product. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular indication. Moreover, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Third-party payors are increasingly challenging the price and examining the medical necessity and cost- effectiveness of medical products and services, in addition to their safety and efficacy. In order to obtain coverage and reimbursement for any product that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of any products, in addition to the costs required to obtain regulatory approvals. Our product candidates may not be considered medically necessary or cost-effective. If third-party payors do not consider a product to be cost-effective compared to other available therapies, they may not cover the product after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell its products at a profit.
The U.S. government and state legislatures have shown significant interest in implementing cost containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. For example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, collectively, the Affordable Care Act, contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs reimbursed by Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal health care programs. Adoption of government controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, an increasing emphasis on cost containment measures in the United States has increased and we expect will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other Healthcare Laws and Compliance Requirements
If we obtain regulatory approval of our products, we may be subject to various federal and state laws targeting fraud and abuse in the healthcare industry. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The U.S. laws that may affect our ability to operate include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order, or recommendation of, an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, or making a false statement or record material to payment of a false claim or avoiding, decreasing, or concealing an obligation to pay money to the federal government; |
78
Table of Contents
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
| • | the federal transparency laws, including the provision of the Affordable Care Act commonly referred to as the federal Physician Payment Sunshine Act, that requires applicable manufacturers of covered drugs to disclose payments and other transfers of value provided to physicians and teaching hospitals and physician ownership and investment interests; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
The Affordable Care Act broadened the reach of the fraud and abuse laws by, among other things, amending the intent requirement of the federal Anti-Kickback Statute and the applicable criminal healthcare fraud statutes contained within 42 U.S.C. § 1320a-7b. Pursuant to the statutory amendment, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act or the civil monetary penalties statute.
The U.S. federal False Claims Act prohibits anyone from, among other things, knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services that are false or fraudulent. Although we would not submit claims directly to payors, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. In addition, our future activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state, and third-party reimbursement for our products, and the sale and marketing of our products, are subject to scrutiny under this law. For example, pharmaceutical companies have been prosecuted under the federal False Claims Act in connection with their off-label promotion of drugs. Penalties for a False Claims Act violation include three times the actual damages sustained by the government, plus mandatory civil penalties of between $10,781 and $21,563 for each separate false claim, the potential for exclusion from participation in federal healthcare programs, and, although the federal False Claims Act is a civil statute, conduct that results in a False Claims Act violation may also implicate various federal criminal statutes. In addition, private individuals have the ability to bring actions under the federal False Claims Act and certain states have enacted laws modeled after the federal False Claims Act.
Patient Protection and Affordable Care Act
In March 2010, the Affordable Care Act was enacted, which includes measures that have or will significantly change the way health care is financed by both U.S. governmental and private insurers. Among the provisions of the Affordable Care Act of greatest importance to the pharmaceutical industry are the following:
| • | The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. The Affordable Care Act made several changes to the Medicaid Drug |
79
Table of Contents
| Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs from 15.1% of average manufacturer price, or AMP, to 23.1% of average manufacturer price, or AMP, and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral dosage forms of branded products, as well as potentially impacting their rebate liability by modifying the statutory definition of AMP. The Affordable Care Act also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization and by expanding the population potentially eligible for Medicaid drug benefits. In addition, the Affordable Care Act provides for the public availability of retail survey prices and certain weighted average AMPs under the Medicaid program. |
| • | In order for a pharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer. The Affordable Care Act expanded the types of entities eligible to receive discounted 340B pricing, although, under the current state of the law, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs when used for the orphan indication. In addition, as 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase. |
| • | The Affordable Care Act imposed a requirement on manufacturers of branded drugs to provide a 50% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap. |
| • | The Affordable Care Act imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications. |
| • | The Affordable Care Act requires pharmaceutical manufacturers to track certain financial arrangements with physicians and teaching hospitals, including any “transfer of value” made or distributed to such entities, as well as any ownership and investment interests held by physicians and their immediate family members. Manufacturers annually report this information to CMS and the information is publicly available in a searchable format on a CMS website. |
| • | A new Patient-Centered Outcomes Research Institute was established pursuant to the Affordable Care Act to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. The research conducted by the Patient- Centered Outcomes Research Institute may affect the market for certain pharmaceutical products. |
| • | The Affordable Care Act created the Independent Payment Advisory Board which has authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription drugs. Under certain circumstances, these recommendations will become law unless Congress enacts legislation that will achieve the same or greater Medicare cost savings. |
| • | The Affordable Care Act established the Center for Medicare and Medicaid Innovation within CMS potentially including prescription drug spending. Funding has been allocated to support the mission of the Center for Medicare and Medicaid Innovation from 2011 to 2019. |
Some of the provisions of the Affordable Care Act have yet to be fully implemented, while certain provisions have been subject to judicial and Congressional challenges. In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, that while not a law, is widely viewed as the first step toward the passage
80
Table of Contents
of legislation that would repeal certain aspects of the Affordable Care Act. Further, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the Affordable Care Act to waive, defer, grant exemptions from, or delay the implementation of any provision of the Affordable Care Act that would impose a fiscal burden on states or a cost, fee, tax, penalty or regulatory burden on individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Congress also could consider subsequent legislation to replace elements of the Affordable Care Act that are repealed. Thus, the full impact of the Affordable Care Act, any law replacing elements of it, or the political uncertainty surrounding its repeal or replacement on our business remains unclear.
European Union Regulation
European Union Drug Review and Approval
In the EEA (which is comprised of the 28 Member States of the European Union plus Norway, Iceland and Liechtenstein), medicinal products can only be commercialized after obtaining a Marketing Authorization, or MA. There are two types of marketing authorizations: the Community MA, which is issued by the European Commission through the Centralized Procedure based on the opinion of the Committee for Medicinal Products for Human Use, or CHMP, a body of the European Medicines Agency, or EMA, and which is valid throughout the entire territory of the EEA; and the National MA, which is issued by the competent authorities of the Member States of the EEA and only authorizes marketing in that Member State’s national territory and not the EEA as a whole.
The Centralized Procedure is compulsory for human medicines for the treatment of human immunodeficiency virus (HIV) or acquired immune deficiency syndrome (AIDS), cancer, diabetes, neurodegenerative diseases, auto-immune and other immune dysfunctions, and viral diseases; for veterinary medicines for use as growth or yield enhancers; for medicines derived from biotechnology processes, such as genetic engineering; for advanced-therapy medicines, such as gene-therapy, somatic cell-therapy or tissue- engineered medicines; and for officially designated “orphan medicines” (medicines used for rare human diseases). The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or for products which are in the interest of public health in the European Union. The National MA is for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketing in a Member State of the EEA, this National MA can be recognized in another Member State through the Mutual Recognition Procedure. If the product has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. Under the Decentralized Procedure an identical dossier is submitted to the competent authorities of each of the Member States in which the MA is sought, one of which is selected by the applicant as the Reference Member State, or RMS. If the RMS proposes to authorize the product, and the other Member States do not raise objections, the product is granted a national MA in all the Member States where the authorization was sought. Before granting the MA, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
Regulation in the European Union
Product development, the regulatory approval process, and safety monitoring of medicinal products and their manufacturers in the European Union proceed in much the same manner as they do in the United States. Therefore, many of the issues discussed above apply similarly in the context of the European Union. In addition, drugs are subject to the extensive price and reimbursement regulations of the various European Union Member States.
81
Table of Contents
Clinical Trials
As is the case in the United States, the various phases of pre-clinical and clinical research in the European Union are subject to significant regulatory controls. The Clinical Trials Directive 2001/20/EC, as amended, (and which will be replaced by Regulation (EU) No 536/2014, currently expected to be in October 2018) provides a system for the approval of clinical trials in the European Union via implementation through national legislation of the Member States. Under this system, approval must be obtained from the competent national authorities of the European Union Member States in which the clinical trial is to be conducted. Furthermore, a clinical trial may only be started after a competent ethics committee has issued a favorable opinion on the clinical trial application, which must be supported by an investigational medicinal product dossier with supporting information prescribed by the Clinical Trials Directive and corresponding national laws of the Member States and further detailed in applicable guidance documents. A clinical trial may only be undertaken if provision has been made for insurance or indemnity to cover the liability of the investigator or sponsor. In certain countries, the sponsor of a clinical trial has a strict (faultless) liability for any (direct or indirect) damage suffered by trial subjects. The sponsor of a clinical trial, or its legal representative, must be based in the European Economic Area. European regulators and ethics committees also require the submission of adverse event reports during a study and a copy of the final study report.
Marketing Approval
Marketing approvals under the European Union regulatory system may be obtained through a centralized or decentralized procedure. The centralized procedure results in the grant of a single marketing authorization that is valid for all (currently 28) European Union Member States and three European Free Trade Association members (Norway, Iceland, Liechtenstein).
Pursuant to Regulation (EC) No. 726/2004, as amended, the centralized procedure is mandatory for drugs developed by means of specified biotechnological processes, advanced therapy medicinal products, drugs for human use containing a new active substance for which the therapeutic indication is the treatment of specified diseases, including but not limited to acquired immune deficiency syndrome, neurodegenerative disorders, auto- immune diseases and other immune dysfunctions, as well as drugs designated as orphan drugs. The CHMP also has the discretion to permit other products to use the centralized procedure if it considers them sufficiently innovative or they contain a new active substance or they may be of benefit to public health at the Community level.
In the marketing authorization application, or MAA, the applicant has to properly and sufficiently demonstrate the quality, safety and efficacy of the drug. Under the centralized approval procedure, the CHMP, possibly in conjunction with other committees, is responsible for drawing up the opinion of the EMA on any matter concerning the admissibility of the files submitted in accordance with the centralized procedure, such as an opinion on the granting, variation, suspension or revocation of a marketing authorization, and pharmacovigilance.
The CHMP and other committees are also responsible for providing guidelines and have published numerous guidelines that may apply to our product candidates. These guidelines provide additional guidance on the factors that the EMA will consider in relation to the development and evaluation of drug products and may include, among other things, the pre-clinical studies required in specific cases; and the manufacturing and control information that should be submitted in a MAA; and post-approval measures required to monitor patients and evaluate the long-term efficacy and potential adverse reactions. Although these guidelines are not legally binding, we believe that our compliance with them is likely necessary to gain approval for any of our product candidates.
Following Article 6(3), first subparagraph, of Regulation (EC) No. 726/2004, the maximum timeframe for the evaluation of an MAA by the CHMP under the centralized procedure is 210 days after receipt of a valid application. This period will be suspended until such time as the supplementary information requested by the CHMP, has been provided by the applicant. Likewise, this time-limit will be suspended for the time allowed for
82
Table of Contents
the applicant to prepare oral or written explanations. When an application is submitted for a marketing authorization in respect of a drug which is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation, the applicant may request an accelerated assessment procedure. If the CHMP accepts such request, the time-limit of 210 days will be reduced to 150 days, according to Article 14(9) of Regulation (EC) No. 726/2004, but it is possible that the CHMP can revert to the standard time-limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment.
If the CHMP concludes that the quality, safety and efficacy of the product are sufficiently proven, it adopts a positive opinion. This is sent to the European Commission which drafts a decision. After consulting with the Member States, the European Commission adopts a decision and grants a marketing authorization, which is valid for the whole of the European Economic Area, or EEA. The marketing authorization may be subject to certain conditions, which may include, without limitation, the performance of post-authorization safety and/or efficacy studies. Pursuant to Regulation (EC) No. 726/2004, a new marketing authorization is valid for five years and may be renewed for an unlimited period on the basis of a re-evaluation of the risk-benefit balance after submission of a consolidated version of the initial marketing authorization application in addition to the pharmacovigilance data reported and all variations introduced since granting of the marketing authorization. The marketing authorization shall cease to be valid if any marketing authorization granted is not followed by the actual launch of the product on the market within three years or, if the product is no longer available on the market for three consecutive years.
European Union legislation also provides for a system of regulatory data and market exclusivity. According to Article 14(11) of Regulation (EC) No. 726/2004, as amended, and Article 10(1) of Directive 2001/83/EC, as amended, upon receiving marketing authorization, new chemical entities approved on the basis of a complete independent data package benefit from eight years of data exclusivity and an additional two years of market exclusivity. Data exclusivity prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic (abbreviated) application. During the additional two-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic medicinal product can be marketed until the expiration of the market exclusivity. The overall ten-year period will be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder, or MAH, obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity and the innovator is able to gain the period of data exclusivity, another company nevertheless could also market another version of the drug if such company obtained marketing authorization based on an MAA with a complete independent data package of pharmaceutical test, pre-clinical tests and clinical trials. However, products designated as orphan medicinal products enjoy, upon receiving marketing authorization, a period of 10 years of orphan market exclusivity limited to the therapeutic indication for which orphan designation has been obtained—see also “—Orphan Drug Regulation.” Depending upon the timing and duration of the EU marketing authorization process, products may be eligible for up to five years’ supplementary protection certification, or SPC, pursuant to Regulation (EC) No. 469/2009. Such SPCs extend the rights under the basic patent for the drug.
Additional rules apply to medicinal products for pediatric use under Regulation (EC) No. 1901/2006. Potential incentives include a six-month extension of any supplementary protection certificate granted pursuant to Regulation (EC) No. 469/2009, however not in cases in which the relevant product is designated as orphan medicinal products pursuant to Regulation (EC) No. 141/2000, as amended. Instead, medicinal products designated as orphan medicinal product may enjoy an extension of the ten-year market exclusivity period granted under Regulation (EC) No. 141/2000 to twelve years subject to the conditions applicable to orphan drugs.
83
Table of Contents
Orphan Drug Regulation
In the European Union, Regulation (EC) No. 141/2000, as amended, states that a drug will be designated as an orphan drug if its sponsor can establish:
| • | that it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in ten thousand persons in the Community when the application is made, or that it is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the European Union and that without incentives it is unlikely that the marketing of the drug in the European Union would generate sufficient return to justify the necessary investment; and |
| • | that there exists no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the European Union or, if such method exists, that the drug will be of significant benefit to those affected by that condition. |
Regulation (EC) No. 847/2000 sets out further provisions for implementation of the criteria for designation of a drug as an orphan drug. An application for the designation of a drug as an orphan drug must be submitted at any stage of development of the drug before filing of a marketing authorization application.
If a European Union-wide community marketing authorization in respect of an orphan drug is granted or if all the European Union Member States have granted marketing authorizations in accordance with the procedures for mutual recognition, the European Union and the Member States will not, for a period of 10 years, accept another application for a marketing authorization, or grant a marketing authorization or accept an application to extend an existing marketing authorization, for the same therapeutic indication, in respect of a similar drug. This period may however be reduced to six years if, at the end of the fifth year, it is established, with respect to the drug concerned, that the criteria for orphan drug designation are no longer met, in other words, when it is shown on the basis of available evidence that the product is sufficiently profitable not to justify maintenance of market exclusivity (cfr. Article 8(s) of Regulation (EC) No. 141/200). Notwithstanding the foregoing, Regulation (EC) No. 141/2000 states that a marketing authorization may be granted, for the same therapeutic indication, to a similar drug if:
| • | the holder of the marketing authorization for the original orphan drug has given its consent to the second applicant; |
| • | the holder of the marketing authorization for the original orphan drug is unable to supply sufficient quantities of the drug; or |
| • | the second applicant can establish in the application that the second drug, although similar to the orphan drug already authorized, is safer, more effective or otherwise clinically superior. |
Other incentives available to orphan drugs in the European Union include financial incentives such as a reduction of fees or fee waivers and protocol assistance. Orphan drug designation does not shorten the duration of the regulatory review and approval process.
Pediatric Investigation Plan
An application for marketing authorization of a medicinal product for human use which is not yet authorized in the European Union shall be considered valid only if it includes a Pediatric Investigational Plan, or PIP, according to Regulation (EC) No. 1901/2006. The PIP or the application for waiver shall be submitted with a request for agreement, except in duly justified cases, early during the product development phase and not later than upon completion of the human pharmacokinetic studies in healthy subjects. The end of Phase 1 pharmacokinetic studies can coincide with the initial tolerability studies, or the initiation of the adult Phase 2 studies (proof-of-concept studies); in any case, submission of the PIP cannot be after initiation of pivotal trials or confirmatory (Phase 3) trials.
84
Table of Contents
The Pediatric Committee, a scientific committee established at Community level, shall assess the content of any PIP, waivers and deferrals for a medicinal product submitted to it in accordance with the regulation on medicinal products for pediatric use and formulate an opinion thereon.
Manufacturing and Manufacturers’ License
Pursuant to Directive 2003/94/EC, as transposed into the national laws of the Member States, the manufacturing of investigational medicinal products and approved drugs is subject to a separate manufacturer’s license and must be conducted in strict compliance with cGMP requirements, which mandate the methods, facilities, and controls used in manufacturing, processing, and packing of drugs to assure their safety and identity. Manufacturers must have at least one qualified person permanently and continuously at their disposal. The qualified person is ultimately responsible for certifying that each batch of finished product released onto the market has been manufactured in accordance with cGMP and the specifications set out in the marketing authorization or investigational medicinal product dossier. cGMP requirements are enforced through mandatory registration of facilities and inspections of those facilities. Failure to comply with these requirements could interrupt supply and result in delays, unanticipated costs and lost revenues, and subject the applicant to potential legal or regulatory action, including but not limited to warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil and criminal penalties.
Wholesale Distribution and License
Pursuant to Directive 2001/83/EC, the wholesale distribution of medicinal products is subject to the possession of an authorization to engage in activity as a wholesaler in medicinal products. Possession of a manufacturing authorization includes authorization to distribute by wholesale the medicinal products covered by that authorization. The distribution of medicinal products must comply with the principles and guidelines of good distribution practices, or GDP.
Advertising
In the European Union, the promotion of prescription medicines is subject to intense regulation and control, including EU and national legislation as well as self-regulatory codes (industry codes). Advertising legislation inter alia includes a prohibition on direct-to-consumer advertising. All prescription medicines advertising must be consistent with the product’s approved summary of products characteristics, and must be factual, accurate, balanced and not misleading. Advertising of prescription medicines pre-approval or off-label is not allowed.
Some jurisdictions require that all promotional materials for prescription medicines be subjected to either prior internal review and approval or regulatory review and approval.
Other Regulatory Requirements
A marketing authorization holder, or MAH, for a medicinal product is legally obliged to fulfill a number of obligations by virtue of its status as an MAH. The MAH can delegate the performance of related tasks to third parties, such as distributors or marketing partners, provided that this delegation is appropriately documented and the MAH maintains legal responsibility and liability.
The obligations of an MAH include:
Manufacturing and batch release. MAHs should guarantee that all manufacturing operations comply with relevant laws and regulations, applicable good manufacturing practices, with the product specifications and manufacturing conditions set out in the marketing authorization and that each batch of product is subject to appropriate release formalities.
85
Table of Contents
Availability and continuous supply. Pursuant to Directive 2001/83/EC, as transposed into the national laws of the Member States, the MAH for a medicinal product and the distributors of the said medicinal product actually placed on the market in a Member State shall, within the limits of their responsibilities, ensure appropriate and continued supplies of that medicinal product to pharmacies and persons authorized to supply medicinal products so that the needs of patients in the Member State in question are covered.
Pharmacovigilance. MAHs are obliged to establish and maintain a pharmacovigilance system, including a qualified person responsible for oversight, submit safety reports to the regulators and comply with the good pharmacovigilance practice guidelines adopted by the EMA.
Advertising and promotion. MAHs remain responsible for all advertising and promotion of its products, including promotional activities by other companies or individuals on their behalf and in some cases must conduct internal or regulatory pre-approval of promotional materials. Regulation in this area also covers interactions with healthcare practitioners and/or patient groups, and in some jurisdictions legal or self-regulatory obligations to disclose such interactions exist.
Medical affairs/scientific service. MAHs are required to disseminate scientific and medical information on its medicinal products to healthcare professionals, regulators and patients. Legal representation and distributor issues. MAHs are responsible for regulatory actions or inactions of their distributors and agents.
Preparation, filing and maintenance of the application and subsequent marketing authorization. MAHs must maintain appropriate records, comply with the marketing authorization’s terms and conditions, fulfill reporting obligations to regulators, submit renewal applications and pay all appropriate fees to the authorities. We may hold any future marketing authorizations granted for our product candidates in our own name, or appoint an affiliate or a collaboration partner to hold marketing authorizations on our behalf. Any failure by an MAH to comply with these obligations may result in regulatory action against an MAH and ultimately threaten our ability to commercialize our products.
Price and Reimbursement
In the European Union, the pricing and reimbursement mechanisms by private and public health insurers vary largely by country and even within countries. The public systems reimbursement for standard drugs is determined by guidelines established by the legislator or responsible national authority. The approach taken varies by Member State. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other Member States allow companies to fix their own prices for medicines, but monitor and control company profits and may limit or restrict reimbursement. The downward pressure on healthcare costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products and some of EU countries require the completion of studies that compare the cost-effectiveness of a particular product candidate to currently available therapies in order to obtain reimbursement or pricing approval. Special pricing and reimbursement rules may apply to orphan drugs. Inclusion of orphan drugs in reimbursement systems tend to focus on the medical usefulness, need, quality and economic benefits to patients and the healthcare system as for any drug. Acceptance of any medicinal product for reimbursement may come with cost, use and often volume restrictions, which again can vary by country. In addition, results based rules of reimbursement may apply.
Legal Proceedings
From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceedings that, if determined adversely to us, would individually or taken together have a material adverse effect on our business, results of operations, financial condition or cash flows. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
86
Table of Contents
Glossary of Terms
Glossary of terms, to be read only in conjunction with this annual report.
| 100 points clinical response |
Percentage of patients achieving a 100-point decrease in CDAI score during a clinical trial in Crohn’s disease patients |
| ACR |
American College of Rheumatology |
| ACR20 (ACR 20/50/70) |
American College of Rheumatology 20% response rate signifies a 20% or greater improvement in the number of swollen and tender joints as well as a 20% or greater improvement in three out of five other disease-activity measures. ACR50 and ACR70 reflect the same, for 50% and 70% response rates, respectively |
| ADR |
American Depositary Receipt; Galapagos has a Level 3 ADR listed on NASDAQ with ticker symbol GLPG and CUSIP number 36315X101. One ADR is equivalent to one ordinary share in Galapagos NV |
| AFM |
Dutch Authority for the Financial Markets |
| Anemia |
Condition in which the patient has an inadequate number of red blood cells to carry oxygen to the body’s tissues |
| Atherogenic index |
Total cholesterol over HDL ratio. Improvement of the atherogenic index may be a forecast of cardiovascular health |
| Atopic dermatitis |
Also known as atopic eczema, atopic dermatitis is a common pruritis inflammatory condition affecting the skin, which most frequently starts in childhood |
| Attrition rate |
The historical success rate for drug discovery and development, based on publicly known development paths. Statistically seen, investment in at least 12 target-based programs is required to ensure that at least one of these will reach a Phase 3 study. Most new drug R&D programs are discontinued before reaching Phase 3 because they are not successful enough to be approved |
| Autotaxin (ATX) |
An enzyme important for generating the signaling molecule lypophosphatidic acid (LPA). GLPG1690 targets autotaxin for IPF |
| BID dosing |
Twice daily dosing (bis in die) |
| Bioavailability |
Assessment of the amount of product candidate that reaches a body’s systemic circulation after (oral) administration |
| Biomarker |
Substance used as an indicator of a biological process, particularly to determine whether a product candidate has a biological effect |
| Black & Scholes model |
A mathematical description of financial markets and derivative investment instruments that is widely used in the pricing of European options and warrants |
87
Table of Contents
| Bleomycin model |
A pre-clinical model involving use of bleomycin (a cancer medication) to induce IPF symptoms |
| CDAI |
Crohn’s Disease Activity Index, evaluating patients on 8 different factors, each of which has a pre-defined weight as a way to quantify the impact of Crohn’s disease |
| CFTR |
Cystic Fibrosis Transmembrane conductance Regulator protein. CFTR is an ion channel that transports chloride and thiocyanate ions across epithelial cell membranes. Mutations in the CFTR gene, that codes for the CFTR protein, cause cystic fibrosis |
| CIR |
Crédit d’Impôt Recherche, or research credit. Under the CIR, the French government refunds up to 30% of the annual investment in French R&D operations, over a period of three years. Galapagos benefits from the CIR through its operations in Romainville, just outside Paris |
| Class II mutation |
A genetic mutation in cystic fibrosis resulting in errors in CFTR folding, transport of functional CFTR to the cell membrane, and CFTR channel opening, whereby chloride ion flow at the cell surface in the membrane of affected organs is impacted negatively. More than 90% of cystic fibrosis patients are carriers of the Class II mutation. It is believed that a potentiator and multiple correctors will be needed to address the CFTR malfunction of Class II mutation patients. Orkambi is the only approved disease-modifying therapy for Class II mutation patients today |
| Class III mutation |
A genetic mutation in cystic fibrosis resulting in errors in CFTR channel opening, whereby chloride ion flow at the cell surface in the membrane of affected organs is impacted negatively. Approximately 4% of cystic fibrosis patients are carriers of the Class III mutation. It is believed that a potentiator is needed to address the malfunction of Class III mutation patients. Kalydeco is the only approved disease-modifying therapy for Class III mutation patients today |
| Clinical Proof of Concept (PoC) |
Point in the drug development process where the product candidate shows efficacy in a therapeutic setting |
| Compound |
A chemical substance, often a small molecule with drug-like properties |
| Contract research organization |
Organization which provides drug discovery and development services |
| Corrector drug |
Drug that restores the correct protein formation in cystic fibrosis patients. In most CF patients, a potentiator and corrector drug are needed in combination to restore the channel function of the CFTR. Galapagos and AbbVie are planning to combine a potentiator with two correctors to be investigated in CF patients with the most prevalent mutation of CFTR |
88
Table of Contents
| Crohn’s disease (CD) |
An inflammatory bowel disease involving inflammation of the small and large intestines, leading to pain, bleeding, and ultimately in some cases surgical removal of parts of the bowel |
| CRP |
C-reactive protein is a protein found in the blood, the levels of which rise in response to inflammation |
| Cystic fibrosis (CF) |
A life-threatening genetic disease that affects approximately 80,000 people worldwide. Although the disease affects the entire body, difficulty breathing is the most serious symptom as a result of clogging of the airways due to mucus build-up and frequent lung infections |
| Cytokine |
A category of small proteins which play important roles in signaling in processes in the body |
| DARWIN |
Phase 2 program for filgotinib in rheumatoid arthritis; completed and reported in 2015 (except for the currently still ongoing DARWIN 3 study). DARWIN 1 explored three doses, in BID and QD administration, for up to 24 weeks in RA patients with insufficient response to methotrexate (MTX) and who remained on their stable background treatment with MTX. DARWIN 2 explored three QD doses for up to 24 weeks in RA patients with insufficient response to methotrexate (MTX) and who washed out of their treatment with MTX. DARWIN 1 and 2 were double-blind, placebo-controlled trials which recruited approximately 900 patients globally. DARWIN 3 is a long term extension trial currently ongoing; all patients are on 200 mg filgotinib, except for U.S. males who are on 100 mg. |
| DAS28(CRP) |
DAS28 is an RA Disease Activity Score based on a calculation that uses tender and swollen joint counts of 28 defined joints, the physician’s global health assessment and a serum marker for inflammation, such as C-reactive protein. DAS28(CRP) includes c-reactive protein the score calculation: scores range from 2.0 to 10.0, with scores below 2.6 being considered remission. |
| Development |
All activities required to bring a new drug to the market. This includes pre-clinical and clinical development research, chemical and pharmaceutical development and regulatory filings of product candidates |
| Discovery |
Process by which new medicines are discovered and/or designed. At Galapagos, this is the department that oversees target and drug discovery research through to nomination of pre-clinical candidates |
| Disease-modifying |
Addresses the cause of disease and modifying the disease progression, not just the symptoms of the disease |
| DIVERSITY |
Phase 3 program evaluating filgotinib in Crohn’s disease |
| Dose-range finding study |
Phase 2 clinical study exploring the balance between efficacy and safety among various doses of treatment in patients. Results are used to determine doses for later studies |
89
Table of Contents
| Double-blind |
Term to characterize a clinical trial in which neither the physician nor the patient knows if the patient is taking placebo or the treatment being evaluated |
| Drug development |
See: Development |
| Drug discovery |
See: Discovery |
| Efficacy |
Effectiveness for intended use |
| EMA |
European Medicines Agency, in charge of European market authorization of new medications |
| Endoscopy |
A non-surgical procedure involving use of an endoscope to examine a person’s digestive tract |
| Esbriet |
An approved drug (pirfenidone) for IPF, marketed by Roche |
| FDA |
The U.S. Food and Drug Administration is an agency responsible for protecting and promoting public health and in charge of American market approval of new medications |
| Fee-for-service |
Payment system where the service provider is paid a specific amount for each procedure or service performed |
| Fibrotic score |
The Ashcroft fibrotic score involves measuring pulmonary fibrosis through examination of histopathology tissue |
| FIH |
First-in-human clinical trial, usually conducted in healthy volunteers with the aim to assess the safety, tolerability and pharmacokinetics of the product candidate |
| Filgotinib |
Formerly known as GLPG0634. Small molecule selective JAK1 inhibitor which showed activity and favorable safety in rheumatoid arthritis and Crohn’s disease patients in Phase 2 trials. Filgotinib is partnered with Gilead. Galapagos and Gilead are running Phase 3 trials with filgotinib in RA, Crohn’s, and ulcerative colitis and expect to initiate Phase 2 trials with filgotinib in new indications in the course of 2017. Filgotinib is an investigational drug and its efficacy and safety have not been established. |
| FINCH |
Phase 3 program evaluating filgotinib in rheumatoid arthritis |
| Fistulizing CD |
Fistulae are inflammatory tracts that most often occur between the distal colon and the perianal region. Fistulae are one of the most severe sequelae of luminal CD and the lifetime risk of occurrence is close to 50% of those with active CD. |
| FITZROY |
A double-blind, placebo controlled Phase 2 trial with filgotinib in 177 Crohn’s disease patients for up to 20 weeks; full results were published in The Lancet in 2016 |
90
Table of Contents
| FLORA |
A double-blind, placebo-controlled exploratory Phase 2a trial with GLPG1690 in up to 24 idiopathic pulmonary fibrosis patients; topline results are expected in the second half of 2017 |
| FSMA |
The Belgian market authority: Financial Services and Markets Authority, or Autoriteit voor Financiële Diensten en Markten |
| FTE |
Full-time equivalent; a way to measure a worker’s involvement in a project. For example, an FTE of 1.0 means that the equivalent work of one full-time worker was used on the project |
| GLPG0634 |
Molecule number currently known as filgotinib |
| GLPG1690 |
A novel drug targeting autotaxin, with potential application in idiopathic pulmonary fibrosis. Fully proprietary to Galapagos. Testing in Phase 2 proof-of-concept FLORA study in IPF underway, with topline results expected in the second half of 2017 |
| GLPG1837 |
A potentiator product candidate which showed activity and favorable safety in the Phase 2 SAPHIRA 1 and 2 trials in Class III cystic fibrosis mutation patients |
| GLPG1972 |
A novel mode-of-action product candidate that is part of the osteoarthritis alliance with Servier. GLPG1972 was in a Phase 1 trial with healthy volunteers. Galapagos expects to initiate a Phase 1b trial with GLPG1972 in osteoarthritis patients in the U.S. in 2017 |
| GLPG2222 |
A C1 (early) corrector drug candidate which showed favorable safety in Phase 1 and is currently being tested in the ALBATROSS Phase 2 study in combination with Kalydeco in Class III mutation patients. In February 2017 Galapagos announced first dosing of GLPG2222 with GLPG2451 in healthy volunteers |
| GLPG2451 |
A potentiator drug candidate currently undergoing a Phase 1 safety trial. In February 2017 Galapagos announced first dosing of GLPG2222 with GLPG2451 in healthy volunteers |
| GLPG2534 |
A pre-clinical candidate with novel mode of action with potential application in atopic dermatitis. GLPG2534 is expected to enter Phase 1 trials in 2017 |
| GLPG2737 |
A C2 (late) corrector drug candidate currently in a Phase 1 safety trial |
| GLPG2851 |
A C1 (early) corrector drug candidate currently at the pre-clinical stage. GLPG2851 is expected to enter Phase 1 trials in 2017 |
| GLPG2938 |
A pre-clinical candidate with novel mode of action with potential application in IPF. GLPG 2938 is expected to enter Phase 1 trials in 2017 |
91
Table of Contents
| GLPG3067 |
A potentiator drug candidate. GLPG3067 started a Phase 1 trial in March 2017 |
| GLPG3221 |
A C2 (late) corrector drug candidate currently at the pre-clinical stage. GLPG3221 is expected to enter Phase 1 trials in 2017 |
| HDL |
High-density lipoprotein. HDL scavenges and reduces low-density lipoprotein (LDL) which contributes to heart disease at high levels. High levels of HDL reduce the risk for heart disease, while low levels of HDL increase the risk of heart disease |
| Hemoglobin |
A protein inside red blood cells that carries oxygen from the lungs to tissues and organs in the body and carries carbon dioxide back to the lungs |
| Heterozygous |
Genetic term meaning a cell containing different alleles for a gene |
| Histopathology |
Microscopic examination of tissues for manifestations of a disease |
| Homozygous |
Genetic term meaning identical alleles of the gene are present on both homologous chromosomes |
| IBD |
Inflammatory Bowel Disease. This is a general term for an autoimmune disease affecting the bowel, including Crohn’s disease and ulcerative colitis. Crohn’s disease affects the small and large intestine, while ulcerative colitis affects the large intestine. Both diseases involve inflammation of the intestinal wall, leading to pain, bleeding, and ultimately, in some cases, surgical removal of part of the bowel |
| IL-17C |
IL-17C has been shown to be distinct from other members of the IL-17 family of cytokines. IL-17C has been shown to be an important mediator in inflammatory skin diseases, and is the target of MOR106 |
| IPF |
Idiopathic pulmonary fibrosis. A chronic and ultimately fatal disease characterized by a progressive decline in lung function. Pulmonary fibrosis involves scarring of lung tissue and is the cause of shortness of breath. Fibrosis is usually associated with a poor prognosis. The term “idiopathic” is used because the cause of pulmonary fibrosis is still unknown |
| Inflammatory diseases |
A large, unrelated group of disorders associated with abnormalities in inflammation |
| In-/out-licensing |
Receiving/granting permission from/to another company or institution to use a brand name, patent, or other proprietary right, in exchange for a fee and/or royalty |
| Intellectual property |
Creations of the mind that have commercial value and are protected, including by patents, trademarks or copyrights |
| Intersegment |
Occurring between the different operations of a company |
92
Table of Contents
| Investigational New Drug (IND) application |
United States Federal law requires a pharmaceutical company to obtain an exemption to ship an experimental drug across state lines, usually to clinical investigators, before a marketing application for the drug has been approved. The IND is the means by which the sponsor obtains this exemption, allowing them to perform clinical studies |
| In vitro |
Studies performed with cells outside their natural context, for example in a laboratory |
| JAK |
Janus kinases (JAK) are critical components of signaling mechanisms utilized by a number of cytokines and growth factors, including those that are elevated in rheumatoid arthritis. Filgotinib is a selective JAK1 inhibitor |
| Kalydeco |
A potentiator drug marketed by Vertex Pharmaceuticals |
| LDL |
Low-density lipoprotein. LDL contributes to heart disease at high levels |
| Liver enzymes |
Inflamed or injured liver cells secrete higher than normal amounts of certain chemicals, including liver enzymes, into the bloodstream |
| Lymphocyte |
Type of white blood cell that is part of the immune system. |
| LPA |
Lysophosphatidic acid, or LPA, is a signaling molecule involved in fibrosis |
| Milestone |
Major achievement in a project or program; in our alliances, this is usually associated with a payment |
| MTX |
Methotrexate; a first-line therapy for inflammatory diseases |
| Molecule collections |
Chemical libraries, usually consisting of drug-like small molecules that are designed to interact with specific target classes. These collections can be screened against a target to generate initial “hits” in a drug discovery program |
| MOR106 |
A novel mode-of-action antibody product candidate currently being evaluated in atopic dermatitis patients in a Phase 1b trial. MOR106 acts on IL-17C, a novel antibody target discovered by Galapagos. MOR106 is part of the alliance with MorphoSys |
| NDA |
New Drug Application |
| Neutrophil |
Type of immune system cell which is one of the first cell types to travel to the site of an infection in the body. Neutrophils are another type of white blood cell which fight infection by ingesting and killing microorganisms |
| NK cells |
Natural killer cells, type of white blood cell with granules of enzymes which can attack tumors or viruses |
93
Table of Contents
| Ofev |
An approved drug (nintedanib) for IPF, marketed by Boehringer Ingelheim |
| Oral dosing |
Administration of medicine by the mouth, either as a solution or solid (capsule, pill) form |
| Organoids |
Miniature organ produced from cells from a donor; organoids have all the phenotypic characteristics of the patient donor, making them useful tools for in vitro drug research |
| Orkambi |
A combination potentiator-corrector therapy marketed by Vertex Pharmaceuticals |
| Osteoarthritis |
The most common form of arthritis, usually occurring after middle age, marked by chronic breakdown of cartilage in the joints leading to pain, stiffness, and swelling |
| Outsourcing |
Contracting work to a third party |
| Pharmacokinetics (PK) |
Study of what a body does to a drug; the fate of a substance delivered to a body. This includes absorption, distribution to the tissues, metabolism and excretion. These processes determine the blood concentration of the drug and its metabolite(s) as a function of time from dosing |
| Phase 1 |
First stage of clinical testing of an investigational drug designed to assess the safety and tolerability, pharmacokinetics of a drug, usually performed in a small number of healthy human volunteers |
| Phase 2 |
Second stage of clinical testing, usually performed in no more than several hundred patients, in order to determine efficacy, tolerability and the dose to use |
| Phase 3 |
Large clinical trials, usually conducted in several hundred to several thousand patients to gain a definitive understanding of the efficacy and tolerability of the candidate treatment; serves as the principal basis for regulatory approval |
| Placebo-controlled |
A substance having no pharmacological effect but administered as a control in testing a biologically active preparation |
| Potentiator drug |
Drug that restores the CFTR ion channel opening in cystic fibrosis patients. In most CF patients, a potentiator and corrector drug are needed in combination to restore the genetic defect causing CF. Galapagos and AbbVie are planning to combine a potentiator with two correctors to investigate in CF patients with the most prevalent mutation of CFTR |
| Pre-clinical |
Stage of drug research development, undertaken prior to the administration of the drug to humans. Consists of in vitro and in vivo screening, pharmacokinetics, toxicology, and chemical upscaling |
94
Table of Contents
| Pre-clinical candidate (PCC) |
A new molecule and potential drug that meets chemical and biological criteria to begin the development process |
| Product candidate |
Substance that has satisfied the requirements of early pre-clinical testing and has been selected for development, starting with formal pre-clinical safety evaluation followed by clinical testing for the treatment of a certain disorder in humans |
| Proof of Concept study |
Phase 2 patient study in which activity as well as safety in patients is evaluated, usually for a new mechanism of action |
| Pruritis |
Extreme itching, as observed in atopic dermatitis patients |
| QD dosing |
Once daily dosing (qd from the Latin quaque die) |
| Rheumatoid arthritis (RA) |
A chronic, systemic inflammatory disease that causes joint inflammation, and usually leads to cartilage destruction, bone erosion and disability |
| R&D operations |
Research and development operations; unit responsible for discovery and developing new product candidates for internal pipeline or as part of risk/reward sharing alliances with partners |
| SAPHIRA |
A Phase 2 trial of potentiator GLPG1837 in cystic fibrosis patients carrying a Class III mutation. Results were reported in 2016, showing activity and favorable safety in two Class III mutations. |
| Screening |
Method usually applied at the beginning of a drug discovery campaign, where a target is tested in a biochemical assay against a series of small molecules or antibodies to obtain an initial set of “hits” that show activity against the target. These hits are then further tested or optimized |
| SELECTION |
Phase 2/3 program evaluating filgotinib in ulcerative colitis patients. Galapagos expects an interim readout for the Phase 2 portion of the program in late 2017 |
| Service operations |
Business unit primarily focused on delivering products and conducting fee-for-service work for clients. Our service operations included the BioFocus and Argenta business units, which were both sold in April 2014 to Charles River Laboratories |
| SES-CD scores |
Simple endoscopic score for Crohn’s disease, involving review of 5 pre-defined bowel segments, assigning values from 0 (unaffected) to 3 (highly affected) |
| Small bowel CD (SBCD) |
CD causes chronic inflammation and erosion of the intestines. It can affect different regions of gastrointestinal tract including the stomach and small and large intestines. While isolated SBCD is an uncommon presentation of CD, involvement of some portion of the small bowel, particularly the ileum, is common |
95
Table of Contents
| Target |
Protein that has been shown to be involved in a disease process and forms the basis of therapeutic intervention or drug discovery |
| Target discovery |
Identification and validation of proteins that have been shown to play a role in a disease process |
| Technology access fee |
License payment made in return for access to specific technology (e.g. compound or virus collections) |
| (anti-)TNF |
Tumor necrosis factor. An anti-TNF drug acts by modulation of TNF |
| Ulcerative colitis (UC) |
UC is an inflammatory bowel disease (IBD) causing chronic inflammation of the lining of the colon and rectum (unlike CD with inflammation throughout the gastrointestinal tract) |
| C. | Organizational Structure. |
As of December 31, 2016, we had eight subsidiaries. The following table sets out for each of our principal subsidiaries, the country of incorporation, and percentage ownership and voting interest held by us (directly or indirectly through subsidiaries).
| Company | Country of incorporation | Percentage ownership and voting interest | ||
| Galapagos B.V. | The Netherlands | 100.0% | ||
| BioFocus DPI AG | Switzerland | 100.0% | ||
| Inpharmatica Ltd | United Kingdom | 100.0% | ||
| Galapagos SASU | France | 100.0% | ||
| Fidelta d.o.o. | Croatia | 100.0% | ||
| Discovery Partners International GmbH | Germany | 100.0% | ||
| BioFocus, Inc. | United States | 100.0% | ||
| Xenometrix, Inc. | United States | 100.0% |
| D. | Property, Plants and Equipment. |
We have our principal executive, operational offices and laboratory space located in Mechelen, Belgium. We believe our current facility is sufficient to meet our current needs, but we intend to expand our facilities in Belgium by 2019 at the earliest in order to meet our future needs. We had a total of four facilities worldwide owned or leased as of December 31, 2016, as set forth in the following table:
| Facility location | Use | Approx. size (m2) | Lease expiry | |||
| Mechelen, Belgium (leased) |
Headquarters, R&D, Operations | 6,800 (1) | May 31, 2024 | |||
|
Romainville, France (leased) |
R&D | 6,000 | February 28, 2027 | |||
| Zagreb, Croatia (leased) |
Research Services | 6,000 | May 4, 2018(2) | |||
| Leiden, the Netherlands (leased) |
R&D | 3,000 | September 1, 2025 |
| (1) | 6,800 m2 per December 31, 2016, which was increased to 7,200 m2 on January 1, 2017. |
| (2) | With the exception of approximately 545 m² of laboratory and office space, for which the lease expires on January 1, 2021 |
96
Table of Contents
Environmental Issues
For more information on environmental issues that may affect our utilization of our facilities, please see the section of this annual report titled “Item 3.D.—Risk Factors—Risks Related to Our Organization, Structure and Operation—We could be subject to liabilities under environmental, health and safety laws or regulations, or fines, penalties or other sanctions, if we fail to comply with such laws or regulations or otherwise incur costs that could have a material adverse effect on the success of our business.”
| Item 4B | Unresolved Staff Comments. |
Not applicable.
| Item 5 | Operating and Financial Review and Prospects. |
Overview
We are a clinical-stage biotechnology company specialized in the discovery and development of medicines with novel modes of action, addressing disease areas of high unmet medical need. Our pipeline comprises programs ranging from discovery to Phase 3 clinical trials in inflammation, cystic fibrosis, fibrosis, osteoarthritis and other indications. Our highly flexible platform is applicable across many therapeutic areas. Our clinical stage programs include: filgotinib, which is currently in Phase 3 trials in rheumatoid arthritis (RA), Crohn’s disease (CD), and ulcerative colitis (UC); our cystic fibrosis (CF) portfolio of drugs aimed at a triple combination therapy for 90% of CF patients, for which we plan to initiate patient clinical trials by mid-2017; GLPG1690, our fully proprietary autotaxin inhibitor, which is currently in a Phase 2a trial for idiopathic pulmonary fibrosis (IPF); GLPG1972 for osteoarthritis (OA), which is expected to be dosed in a Phase 1b trial in U.S. patients in 2017; and MOR106, which is currently being dosed in atopic dermatitis (AtD) patients in a Phase 1b trial. Except for our CF program, these programs are derived from our proprietary target discovery platform. We have collaborations with Gilead for filgotinib, with AbbVie for CF, with Servier for GLPG1972, and with MorphoSys for MOR106.
We devote substantially all of our resources to our drug discovery efforts from target discovery through to clinical development. To date, we do not have any products approved for sale and have not generated any revenue from product sales. We sold our service division to Charles River Laboratories International, Inc., or Charles River, on April 1, 2014. As a result, the service division has been reported under discontinued operations, although certain entities of the service division were not sold and are therefore still reported under continuing operations.
To date, we funded our operations through public and private placements of equity securities, upfront and milestone payments received from pharmaceutical partners under our collaboration and alliance agreements, payments under our fee-for-service contracts, funding from governmental bodies, interest income as well as the net proceeds from the sale of our service division. From January 1, 2014 until December 31, 2016, we raised net proceeds of €651.2 million from a global offering of ordinary shares in May 2015 and from an equity investment by Gilead in January 2016, and we also received €395.6 million in payments through our collaboration and alliance agreements. These are non-recurring items which have a significant impact upon the profitability or cash flow of our business in each year in which they are received and earned. Fee-for-service payments and payments from governmental bodies contributed €18.3 million and €41.4 million, respectively. Over the same period, we also received €3.1 million in interest payments. In April 2014, the sale of our service division generated net proceeds of €130.8 million. As of December 31, 2016, we had cash and cash equivalents of €973.2 million.
Due to the sale of the service division, we realized a net income of €33.2 million for the year ended December 31, 2014. For the year ended December 31, 2015, we incurred a net loss of €118.4 million. Due to a
97
Table of Contents
non-cash adjustment on a short term financial asset, as described below, with regard to the share subscription agreement with Gilead on January 19, 2016, we realized a net income of €54.0 million for the year ended December 31, 2016. Excluding the impact of possible upfront and in-licensing payments we may receive from our collaborations, we forecast to continue incurring losses as we continue to invest in our clinical and pre-clinical development programs and our discovery platform.
In 2015, we recognized a short term financial asset worth €39 million and an offsetting deferred income of €39 million upon signing of the share subscription agreement with Gilead, as required under IAS 39. This financial asset initially reflected the share premium that Gilead committed to pay above the closing stock price of our ordinary shares on the day of signing of the subscription agreement. Under IAS 39, the fair value of the financial asset was re-measured at year end and again upon entering into force of the subscription agreement on January 19, 2016, when the financial asset expired. Variations in fair value of the financial asset were recorded in the income statement. The decrease in the fair value of the financial asset resulting from the increase in our share price between signing of the subscription agreement and December 31, 2015, resulted in a negative, non-cash fair value charge of €30.6 million in the 2015 financial results. The subsequent increase in the fair value of the financial asset resulting from the decrease in our share price between January 1, 2016 and January 19, 2016 resulted in a positive non-cash gain of €57.5 million in the financial result of 2016. The €65.9 million current financial asset from the share subscription agreement reflected the premium that Gilead paid compared to the closing price of our shares on the day of the capital increase. This financial asset expired on January 19, 2016, the effective date of the share subscription agreement and was derecognized through the share premium account.
Collaboration and Alliance Agreements
Our main collaborations and alliance agreements are summarized below. All U.S. dollar payment amounts which have been received in cash regarding our AbbVie collaboration in this Item 5 are converted into euros as per historical exchange rates (i.e., the spot rate at the moment of the transaction).
Amended AbbVie Collaboration Agreement for CF
In September 2013, we entered into a global collaboration agreement with AbbVie focused on the discovery and worldwide development and commercialization of potentiator and corrector molecules for the treatment of CF. On April 28, 2016, we and AbbVie entered into the amended and restated agreement, which expanded the parties’ CF collaboration by amending and restating the collaboration agreement to, among other things, increase the remaining total milestones under the amended and restated agreement up to approximately $600 million from $350 million. As amended, the collaboration will provide for the potential development and commercialization of triple combination products consisting of a potentiator molecule, a corrector 1 molecule and a corrector 2 molecule to treat specified populations of patients with CF. A detailed summary of this collaboration agreement is set forth in the section of this annual report titled “Item 4.B.—Business Overview—Collaborations—Amended and Restated Collaboration with AbbVie for CFTR Modulators (CF).”
Upon execution of the collaboration agreement, we received a one-time non-refundable, non-creditable upfront payment of $45.0 million (€34.0 million), which has been fully recognized as of June 2015. In December 2014, we initiated a Phase 1 trial for GLPG1837 for which we received a milestone payment of $10.0 million (€8.0 million). In November 2015, we initiated a Phase 1 trial for GLPG2222, for which we received a $10.0 million (€8.8 million) payment from AbbVie in January 2016. In April 2016, we initiated a Phase 1 trial for GLPG2451, for which we received a $10.0 million (€8.8 million) payment. In November 2016, we initiated a Phase 1 trial for GLPG2737, for which we received a $10.0 million (€9.1 million) payment. In January 2017, we received IND acceptance in U.S. from FDA for GLPG2222, for which we received a $10.0 million (€9.5 million) payment. In March 2017, we initiated a Phase 1 trial for GLPG3067, for which we will receive a $7.5 million (€7.1 million) payment. All payments by AbbVie to us are made in U.S. dollars.
Under the agreement, we are still eligible to receive up to $567.5 million in total additional developmental, regulatory, and sales-based milestones. In March 2017, we achieved a $7.5 million milestone, for which the
98
Table of Contents
payment is not yet received to date. In addition, we will be eligible to receive tiered royalty percentages ranging from the mid-teens to 20% on net sales of licensed products payable on a product-by-product basis. In the event we exercise our co-promotion option with respect to a licensed product, we would assume a portion of the co-promotion effort in the Netherlands, Belgium, and Luxembourg and share in the net profit and net losses in these territories instead of receiving royalties in those territories during the period of co-promotion.
Gilead Collaboration Agreement for Filgotinib
In December 2015, we entered into a global collaboration agreement with Gilead to develop and commercialize filgotinib for the treatment of inflammatory indications. A detailed summary of this collaboration agreement is set forth in the section of this annual report titled “Item 4.B.—Business Overview—Collaborations—Exclusive Collaboration Agreement with Gilead for Filgotinib.”
In connection with our entry into the collaboration agreement, we received in January 2016 an upfront payment of $725 million consisting of a one-time, non-refundable, non-creditable license fee in the amount of $300 million and a $425 million equity investment. In November 2016, Gilead initiated a Phase 3 trial in CD, for which we received a $50.0 million (€45.7 million) payment. In December 2016, Gilead initiated a Phase 2 trial in UC for which we received a $10.0 million (€9.4 million) payment. All payments by Gilead to us are made in U.S. dollars. In addition, we will be eligible to receive development and regulatory milestone-based payments of up to $695 million and sales-based milestone payments of up to $600 million. We will be eligible to receive tiered royalty percentages starting at 20% on global net sales of licensed products. The royalties payable to us under the collaboration agreement may be reduced under certain circumstances. Our right to receive royalties under the collaboration agreement continues, on a country-by-country basis, until the later to occur of certain specified events. In the event we exercise our co-promotion option with respect to licensed products in one or more of the territories eligible for co-promotion, we would assume a portion of the co-promotion effort in the United Kingdom, Germany, France, Italy, Spain, the Netherlands, Belgium, and/or Luxembourg and share equally in the net profit and net losses in these territories instead of receiving royalties in those territories during the period of co-promotion.
Financial Operations Overview
Revenue
Our revenues in our continuing operations to date have consisted principally of milestones, reimbursement income, license fees, and upfront payments received in connection with our collaboration and alliance agreements. Additionally, we have generated revenue from our fee-for-service activities and various R&D incentives and grants.
Collaboration and alliance agreements with our commercial partners for R&D activities generally include non-refundable, upfront fees; milestone payments, the receipt of which is dependent upon the achievement of certain clinical, regulatory or commercial milestones; license fees; and royalties on sales.
Our revenue recognition policies are as follows:
Upfront Payments
Non-refundable, upfront payments received in connection with R&D collaboration agreements are deferred and recognized over the relevant period of our involvement. At inception, management estimates the period of our involvement as well as the cost involved in the project. Upfront payments are recognized over the estimated period of involvement, either on a straight line basis or based on the cost incurred under the project if such cost can be reliably estimated. Periodically, we reassess the estimated time and cost to complete the project phase and adjust the time period over which the revenue is deferred accordingly.
99
Table of Contents
Milestone Payments
Research milestone payments are recognized as revenues when milestones are achieved. In addition, the payments have to be acquired irrevocably and the milestone payment amount needs to be substantive and commensurate with the magnitude of the related achievement. Milestone payments that are not substantive, not commensurate, or that are not irrevocable are recorded as deferred revenue. Revenue from these activities can vary significantly from period to period due to the timing of milestones.
Reimbursement Income
Cost reimbursements resulting from license and collaboration agreements with our commercial partners are recognized as reimbursement income in revenue as the related costs are incurred and upon agreement by the parties involved. The corresponding expenses are included in research and development expenditure.
Cost reimbursements from collaboration in which we share equally in the risks and benefits associated with development of a specific drug with a collaboration partner are recognized as decrease of the related incurred research and development expenditure.
License Fees
Revenues from term licenses are spread over the period to which the licenses relate, reflecting the obligation over the term, to update content and provide ongoing maintenance. Revenues from perpetual licenses are recognized immediately upon sale to the extent that there are no further obligations.
Royalties
Royalty revenues are recognized when we can reliably estimate such amounts and collectability is reasonably assured. As such, we generally recognize royalty revenues in the period in which our licensees are reporting the royalties to us through royalty reports, that is, royalty revenues are generally recognized in arrears, i.e., after the period in which sales by our licensees occurred. Under this accounting policy, the royalty revenues we report are not based upon our estimates and such royalty revenues are typically reported in the same period in which we receive payment from our licensees.
Grants and R&D Incentives
We benefit from various grants and R&D incentives from certain governmental agencies. These grants and R&D incentives generally aim to partly reimburse approved expenditures incurred in our R&D efforts and are credited to the income statement, under other income, when the relevant expenditure has been incurred and there is reasonable assurance that the grant or R&D incentive is receivable. The main grants and R&D incentives are as follows:
| • | Companies in Belgium are eligible to receive R&D incentives linked to R&D investments (cash rebates equaling 33.99% of 13.5% of the investment value in 2016, 33.99% of 13.5% of the investment value in 2015, or 33.99% of 13.5% of the investment value in 2014). This R&D tax credit results in a cash inflow to us from the tax authorities five years after the investment was made and capitalized in our standalone financial statements under Belgian GAAP for the portion that has not been used to offset the payment of corporate tax or is paid to us for the portion that remains unused. We also received several grants from an agency of the Flemish government to support various research programs focused on technological innovation in Flanders. These grants carry clauses which require us to maintain a presence in the Flemish region for a number of years and invest according to pre-agreed budgets. Finally, we also benefit from certain rebates on payroll withholding taxes for scientific personnel. |
| • | In France, we benefit from R&D incentives from the French Government for R&D activities whereby 30% of qualifying R&D expenses can be recuperated. This research tax credit (crédit d’impôt |
100
Table of Contents
| recherche), results in a cash inflow to us from the tax authorities after three years, i.e., it is used to offset the payment of corporate tax or is paid to us for the portion that remains unused. Qualifying expenditures largely comprise employment costs for research staff, consumables, and certain overhead costs as well as capped outsourcing costs incurred as part of R&D projects. |
R&D Expenditure
Expenses on R&D activities are recognized as an expense in the period in which the expense is incurred.
An internally-generated intangible asset arising from our R&D activities would be recognized only when an asset is created that can be identified, it is probable that the asset created will generate future economic benefits, and the development cost of the asset can be measured reliably.
Our Funded R&D Expenditure
Our funded R&D expenditure consists of costs associated with our R&D activities such as:
| • | personnel costs associated with employing our team of R&D staff, including salaries, social security costs, and share-based compensation expenses; |
| • | disposables and lab consumables used in the conduct of our in-house research programs; |
| • | payments for research work conducted by sub-contractors and sponsorship of work by our network of academic collaborative research scientists; |
| • | subcontracting costs paid to contracted research organizations, or CROs, for our pre-clinical studies or clinical trials, as well as costs associated with safety studies; |
| • | premises costs associated with our laboratory and office space to accommodate our teams; |
| • | depreciation of fixed assets used to develop our product candidates; and |
| • | other operating expenses, namely software and licenses, maintenance costs for equipment, travel costs, and office expenses. |
We expect to increase our investment in our funded R&D in the future as we seek to advance our most promising pipeline product candidates through further clinical development.
Alliance R&D Expenditure
R&D expenditure under alliance represent costs incurred by us in conducting R&D plans under our collaborations and alliance agreements. Our expenses primarily relate to the following key programs:
| • | Development costs for the development of filgotinib in RA and IBD (currently in collaboration with Gilead, previously with AbbVie) : these costs relate to the Phase 2 and Phase 3 trials and mainly consist of costs recharged by our collaboration partner as we are co-funding 20% of the global development activities, as well as costs paid to CROs in conjunction with clinical trials, costs for production of the compound for clinical testing, and, to a smaller extent, personnel costs and consultancy costs. |
| • | Costs for the CF collaboration with AbbVie: these costs are primarily composed of (1) personnel costs, (2) internal laboratory costs, and (3) costs incurred in carrying out our pre-clinical toxicology, pharmacology, and both in vitro and in vivo pre-clinical models in the fields of CF. |
| • | Other R&D programs: these expenses primarily consist of personnel costs, costs for production of the pre-clinical compounds, and costs paid to CROs in conjunction with pre-clinical studies and clinical trials. |
101
Table of Contents
Our R&D expenses under alliance are expected to increase as we advance our filgotinib program, our CF program and any other alliance product candidate into clinical trials.
Since 2014, we cumulatively have spent approximately €380.4 million on R&D activities which can be split as follows between the key programs:
| Year ended December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||
| (Euro, in thousands) | cumulative | |||||||||||||||||||
| Filgotinib program (partnered) |
€ | (22,376 | ) | (35,404 | ) | € | (33,843 | ) | € | (91,622 | ) | 24 | % | |||||||
| CF program (partnered) |
(31,203 | ) | (25,634 | ) | (14,894 | ) | (71,731 | ) | 19 | % | ||||||||||
| IPF program on GLPG1690 (proprietary) |
(7,129 | ) | (4,612 | ) | (4,592 | ) | (16,333 | ) | 4 | % | ||||||||||
| OA program on GLPG1972 (partnered) |
(6,538 | ) | (5,832 | ) | (801 | ) | (13,171 | ) | 3 | % | ||||||||||
| AtD program on MOR106 (partnered) |
(3,491 | ) | (4,651 | ) | (894 | ) | (9,036 | ) | 2 | % | ||||||||||
| Other |
(68,836 | ) | (53,582 | ) | (56,086 | ) | (178,504 | ) | 47 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total R&D expenditure |
€ | (139,573 | ) | € | (129,714 | ) | € | (111,110 | ) | € | (380,397 | ) | 100 | % | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
As illustrated above the R&D expenditures have shown a growth trend over the three years from €111.1 million for the year ended December 31, 2014 to €139.6 million for the year ended December 31, 2016. The increase is driven by the maturing pipeline of our R&D projects. As progressively product candidate compounds have been entering the clinic, costs for development of these molecules increased as well, specifically with regard to third-party CRO costs for conducting these clinical trials. Our program filgotinib accounts for 24% of the cumulative spend over the last three years with a total cost of €91.6 million. Costs reported under other programs relate to investments in own funded discovery and development projects, and in our discovery platform, as well as costs related to other collaborations and alliance contracts.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and benefits related to our executive, finance, business development, legal, intellectual property, and information technology support functions. Professional fees reported under general and administrative expenses mainly include legal fees, accounting fees, audit fees, and fees for taxation advisory. Other general and administrative operating expenses primarily encompass software and license costs, equipment maintenance and leasing costs, consultancy costs, insurance costs, office expenses, and travel costs.
We expect our general and administrative expenses to increase as we continue to support our growth and as we operate as a U.S.-listed company. Such costs include increases in our finance and legal personnel, additional external legal and audit fees, and expenses and costs associated with compliance with the regulations governing public companies. We also expect to incur increased costs for directors’ and officers’ liability insurance and an enhanced investor relations function.
Sales and Marketing Expenses
Sales and marketing expenses include costs associated with managing our commercial activities and the costs of compliance with the day-to-day requirements of being a listed public company in Belgium and the United States, such as:
| • | Headquarters costs related to investor relations and corporate communications in Belgium and the Netherlands. |
| • | Sales and marketing department in Croatia as from 2013. |
102
Table of Contents
Interest Expense and Interest Income
Interest expense consists primarily of interest expense incurred on finance leases.
Interest income consists primarily of interest earned by investing our cash reserves in short-term, interest-bearing deposit accounts.
Taxation
We have a history of losses. Excluding the impact of possible upfront or milestone payments we may receive from our collaborations, we forecast to continue incurring losses as we continue to invest in our clinical and pre-clinical development programs and our discovery platform. Consequently, we do not have any deferred tax asset on the balance sheet as at December 31, 2016, except for two subsidiaries operating on a cost plus basis for the company a deferred tax asset was set up for an amount of €2.0 million as of December 31, 2016.
As a company active in research and development in Belgium, we also expect to benefit in the future from the “patent income deduction” or the replacing “innovation deduction” in Belgium. The patent income deduction regime allows, in the case of taxable income, gross profits attributable to revenue from patented products to be taxed at a lower rate than other revenues, i.e., 6.8%. The innovative deduction regime allows net profits attributable to revenue from among others patented products (or products for which the patent application is pending) to be taxed at a lower rate than other revenues, i.e., 5.1%. When taken in combination with tax losses carried forward and research and development incentives mentioned above, we expect that this will result in a long-term low rate of corporation tax for us. The innovation deduction applies as of July 1, 2016, although subject to various conditions, the patent income deduction can continue to apply until June 30, 2021 at the latest.
Operating Segments
Following the sale of the service division on April 1, 2014, the continuing operations related primarily to R&D activities. Consequently, in 2014, we only had one reportable segment.
In 2015, the IFRS8 threshold of 10% of the combined revenues, external and inter-segment, of all segments was met by the external and internal revenues reported by our fee-for-service business located in Croatia. Consequently, there are two reportable segments in 2015 and 2016, R&D and fee-for-service business.
Financial information related to our two reportable segments and geographic information is contained in “Note 3—Segment information” in our consolidated financial statements appended to this annual report.
Risks
For further information regarding governmental economic, fiscal, monetary or political policies or factors that have materially affected, or could materially affect, directly or indirectly, our operations, please see the section of this annual report titled “Item 3.D.—Risk Factors.”
Critical Accounting Policies and Estimates
In the application of our accounting policies, we are required to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates.
Our estimates and assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revisions and future periods if the revision affects both current and future periods.
103
Table of Contents
Drafting financial statements in accordance with IFRS requires management to make judgments and estimates and to use assumptions that influence the reported amounts of assets and liabilities, the notes on contingent assets and liabilities on the date of the financial statements and the reported amounts of income and expenses during the reporting period. Actual results may differ from these estimates.
The following are our critical judgments and estimates that we have made in the process of applying our accounting policies and that have the most significant effect on the amounts recognized in our consolidated financial statements presented elsewhere in this annual report.
Critical Judgments in Applying Accounting Policies
Share Subscription Agreement with Gilead: Classification as Derivative Financial Asset or Equity Instrument
As described in the section of this annual report titled “Item 5.A.—Operating Results—Comparison of Years Ended December 31, 2016 and 2015—Fair Value Re-measurement of Share Subscription Agreement,” as well as “Item 5.A.—Operating Results—Comparison of Years Ended December 31, 2015 and 2014—Fair Value Re-measurement of Share Subscription Agreement,” Gilead committed itself on December 16, 2015 to make a $425 million equity investment in our company by subscribing to new shares at a fixed price of €58 per share, including issuance premium upon completion of the license and collaboration agreement with us that took place on January 19, 2016.
Significant judgment had to be applied in assessing whether this forward subscription commitment of Gilead over the shares of our company shall be classified as an equity instrument or as a derivative financial asset of our company. IAS 32 requires that for a derivative to meet the definition of equity it must be settled only by the issuer exchanging a “fixed amount of cash or another financial asset for a fixed number of its own equity instruments.” Because the above-mentioned commitment by Gilead was made in U.S. dollars, the actual number of shares finally issued by us varied with the fluctuation in the $/€ exchange rate until the settlement date on January 19, 2016.
Despite the fact that this foreign currency exchange exposure was limited, management judged that this variability prevents the instrument from being classified as equity under IAS 32 and was therefore treated as a derivative at fair value through profit and loss.
Revenue Recognition
Evaluating the criteria for revenue recognition with respect to our R&D and collaboration agreements requires management’s judgment to ensure that all criteria have been fulfilled prior to recognizing any amount of revenue. In particular, such judgments are made with respect to determination of the nature of transactions, whether simultaneous transactions shall be considered as one or more revenue-generating transactions, allocation of the contractual price (upfront and milestone payments in connection with a collaboration agreement) to several elements included in an agreement, and the determination of whether the significant risks and rewards have been transferred to the buyer. Collaboration agreements are reviewed carefully to understand the nature of risks and rewards of the arrangement. All of our revenue-generating transactions have been subject to such evaluation by management.
104
Table of Contents
Critical Accounting Estimates
Fair Value Re-measurement of Our Share Subscription Agreement with Gilead (Derivative Financial Asset Instrument)
| Current financial asset from share subscription agreement: |
| |||
| (Euro, in thousands) | ||||
| Fair value at inception |
39,003 | |||
| Movement of 2015 ( recognized in the income statement) |
(30,632 | ) | ||
|
|
|
|||
| Fair value per December 31, 2015 |
8,371 | |||
|
|
|
|||
| Movement of period January–19, 2016 (recognized in the income statement) |
57,479 | |||
|
|
|
|||
| Fair value per January 19, 2016 |
65,850 | |||
|
|
|
|||
| Derecognition of the financial asset through the share premium account |
(65,850 | ) | ||
|
|
|
|||
| Fair value per December 31, 2016 |
— | |||
|
|
|
|||
The fair value measurement of this derivative financial asset was categorized as a level 3 in the fair value hierarchy of the IFRS 13 Fair Value Measurement.
Its measurement was based on computing the difference between the strike price (€58 per share) and our anticipated forward price, discounted to the valuation date. The notional was converted from U.S. dollars to euros by the currency exchange forward rate, and the number of shares was computed by dividing the euro notional by the strike price.
Input data were taken from Bloomberg as of December 16, 2015 and December 31, 2015, including:
| • | Euro overnight index swap discount rates (curve 133); |
| • | Implied forward rate of the GLPG shares at January 31, 2016; and |
| • | Implied currency exchange forward rate at January 31, 2016. |
This computation was based on the following unobservable assumptions:
| (1) | Between the date that the deal was signed (December 16, 2015) until the date that the deal was complete, the two counterparties could not back off from the deal, and it was 100% certain that the regulator would give the green light. |
| (2) | At the two valuation dates, it was assumed that the date when the deal will be complete would be January 31, 2016. This was the forward date from where all the market data was taken from. |
| (3) | The effect of the correlation between our share price and the $/€ currency exchange rate was negligible. This was reasonable given the very short maturity of the deal. |
Relationship of unobservable inputs to the fair value measurement:
| • | If one would have assumed that the closing date of the deal was January 19, 2016 (the actual closing date), the fair value of the derivative financial asset at December 31, 2015 would have been €8,367 thousand. |
On January 19, 2016, the value of the financial asset at maturity amounted to €65.9 million, reflecting the share premium that Gilead paid above our closing share price on the day of the capital increase. This financial asset expired on the effective date of the share subscription agreement and was derecognized through the share premium account.
105
Table of Contents
Share-based Payments Plans
We determine the costs of the share-based payments plans (i.e., our warrant plans) on the basis of the fair value of the equity instrument at grant date. Determining the fair value assumes choosing the most suitable valuation model for these equity instruments, by which the characteristics of the grant have a decisive influence. This assumes also the input into the valuation model of some relevant judgments, like the estimated useful life of the warrant and the volatility.
Pension Obligations
The cost of a defined pension arrangement is determined based on actuarial valuations. An actuarial valuation assumes the estimation of discount rates, estimated returns on assets, future salary increases, mortality figures and future pension increases. Because of the long-term nature of these pension plans, the valuation of these is subject to important uncertainties.
Corporate Income Taxes
Significant judgment is required in determining the use of tax loss carry forwards. We recognize deferred tax assets arising from unused tax losses or tax credits only to the extent that we have sufficient taxable temporary differences or there is convincing evidence that sufficient taxable profit will be available against which the unused tax losses or unused tax credits can be utilized by us. Management’s judgment is that such convincing evidence is currently not sufficiently available and a deferred tax asset is therefore not yet recognized, except for two subsidiaries operating on a cost plus basis for the company a deferred tax asset was set up for an amount of €2.0 million as of December 31, 2016.
As of December 31, 2016, we had a total of approximately €311 million of statutory tax losses carried forward which may be partially offset by future statutory taxable profits for an indefinite period, except for an amount of approximately €18 million in Switzerland, Croatia, the United States and the Netherlands with expiry dates between 2018 and 2030. As of December 31, 2016, the available tax losses carried forward in Belgium amounted to €231 million.
Long-term Management Bonus Provision
Our executive committee members, together with other senior managers, are eligible to receive bonuses under the Senior Management Bonus Scheme established in 2006. Pursuant to the rules of the Senior Management Bonus Scheme, 50% of the bonus is paid immediately around year-end and the payment of the remaining 50% is deferred for three years. The deferred 50% component is dependent on our share price change relative to the Next Biotech Index (which tracks our peers). Our share price and the Next Biotech Index at the start and end of the three-year period is calculated by the average price over the preceding and last month of the three-year period, respectively.
| • | If our share price change is better than or equal to the change in the Next Biotech Index, the deferred bonus will be adjusted by the share price increase/decrease and paid out. |
| • | If our share price change is up to 10% worse than the change in the Next Biotech Index, 50% of the deferred bonus will be adjusted by the share price increase/decrease and paid out, and the remainder will be forfeited. |
| • | If our share price change is more than 10% worse than the change in the Next Biotech Index, the deferred bonus will be forfeited. |
Since the bonus is calculated by reference to our share price, it is accounted for as a cash-settled share-based payment under IFRS 2. The liability incurred is measured at the fair value of the liability. Until the liability is settled, the fair value of the liability is re-measured at the end of each reporting period and at the date of settlement, with any changes in fair value recognized in profit or loss for the period. Management judgment is required in determining the fair value.
106
Table of Contents
| A. | Operating Results |
Comparison of Years Ended December 31, 2016 and 2015
The following table summarizes the results of our operations for the years ended December 31, 2016 and 2015, together with the changes to those items.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | % Change | ||||||||||
| (Euro, in thousands, except share and per share data) |
||||||||||||
| Revenues |
€ | 129,519 | € | 39,563 | 227 | % | ||||||
| Other income |
22,093 | 21,017 | 5 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues and other income |
151,612 | 60,579 | 150 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Research and development expenditure |
(139,573 | ) | (129,714 | ) | 8 | % | ||||||
| General and administrative expenses |
(21,744 | ) | (19,127 | ) | 14 | % | ||||||
| Sales and marketing expenses |
(1,785 | ) | (1,182 | ) | 51 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
(163,103 | ) | (150,023 | ) | 9 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(11,491 | ) | (89,444 | ) | (87 | %) | ||||||
|
|
|
|
|
|
|
|||||||
| Fair value re-measurement of share subscription agreement |
57,479 | (30,632 | ) | (288 | %) | |||||||
| Other financial income |
9,950 | 1,987 | 401 | % | ||||||||
| Other financial expenses |
(1,692 | ) | (1,539 | ) | 10 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Loss before tax |
54,246 | (119,627 | ) | (145 | %) | |||||||
|
|
|
|
|
|
|
|||||||
| Income taxes |
(235 | ) | 1,218 | (119 | %) | |||||||
|
|
|
|
|
|
|
|||||||
| Net income / loss (-) from continuing operations |
54,012 | (118,410 | ) | (146 | %) | |||||||
|
|
|
|
|
|
|
|||||||
| Net income from discontinued operations |
— | — | ||||||||||
|
|
|
|
|
|||||||||
| Net income / loss (-) |
€ | 54,012 | € | (118,410 | ) | |||||||
|
|
|
|
|
|||||||||
| Net income / loss (-) attributable to: |
||||||||||||
| Owners of the parent |
54,012 | (118,410 | ) | |||||||||
|
|
|
|
|
|||||||||
| Basic income / loss (-) per share |
€ | 1.18 | € | (3.32 | ) | |||||||
|
|
|
|
|
|||||||||
| Diluted income / loss (-) per share |
€ | 1.14 | € | (3.32 | ) | |||||||
|
|
|
|
|
|||||||||
| Basic income/ loss (-) per share from continuing operations |
€ | 1.18 | € | (3.32 | ) | |||||||
|
|
|
|
|
|||||||||
| Diluted income/ loss (-) per share from continuing operations |
€ | 1.14 | € | (3.32 | ) | |||||||
|
|
|
|
|
|||||||||
| Weighted average number of shares - Basic (in ‘000 shares) |
45,696 | 35,700 | ||||||||||
| Weighted average number of shares - Diluted (in ‘000 shares) |
47,308 | 35,700 | ||||||||||
Revenues
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Recognition of non-refundable upfront payments |
€ | 30,257 | € | 26,419 | 15 | % | ||||||
| Milestone payments |
81,784 | 3,835 | 2033 | % | ||||||||
| Reimbursement income |
9,699 | 3,807 | 155 | % | ||||||||
| Other revenues |
7,777 | 5,501 | 41 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
€ | 129,519 | € | 39,563 | 227 | % | ||||||
|
|
|
|
|
|
|
|||||||
107
Table of Contents
Total revenues increased by €90.0 million, or 227%, to €129.5 million for the year ended December 31, 2016, from €39.6 million for the year ended December 31, 2015. This increase was mainly driven by a substantial increase in milestone payments, as explained below.
Revenue recognized in 2015 from upfront non-refundable payments related to the CF collaboration agreement with AbbVie signed in September 2013 and the contract signed with AbbVie in February 2012 for our filgotinib program (including the extension signed in March 2013). Those upfront payments were fully recognized into revenues by the end of August 2015.
In September 2015 AbbVie decided not to opt in, which ended the collaboration agreement regarding our filgotinib program and consequently the period of our involvement. There are no outstanding commitments for us regarding this terminated collaboration for our filgotinib program.
On December 16, 2015, we entered into a global collaboration with Gilead Sciences, Inc. for the development and commercialization of the JAK1-selective inhibitor filgotinib for inflammatory indications. On January 19, 2016, we completed the closing of the global collaboration agreement with Gilead, in the framework of which Gilead made a $425 million (or €392 million) equity investment in Galapagos NV by subscribing to new shares at a price of €58 per share, including issuance premium. This resulted in Gilead owning 6,760,701 ordinary shares of Galapagos NV, representing 14.75% percent of the then-outstanding share capital of Galapagos. We also received a license fee of $300 million. In addition, we are eligible for payments of up to $755 million in development and regulatory milestones and $600 million in sales milestones, with tiered royalties starting at 20% and a profit split in co-promotion territories. Finally, we agreed on a 20-80 cost split for development costs of the licensed product, i.e. we will support 20% of all development costs. As we do not expect to have a statutory taxable base in the foreseeable future, we did not recognize any additional deferred tax asset following the signing of this new collaboration.
The global collaboration with Gilead foresees continuous involvement from us, since we will perform certain R&D activities in the development phase of the filgotinib program; therefore, management assessed that the upfront payment of $300 million (or €275.6 million) received in January 2016 from Gilead should be spread as a function of the costs incurred for this program, applying the percentage of completion method. In the year ended December 31, 2016, €25.6 million revenues were recognized regarding this upfront payment.
In connection with the agreement with Gilead, we recognized a deferred income and an offsetting short-term financial asset (derivative) of €39 million upon signing of the share subscription agreement with Gilead, as required under IAS 39. The deferred income will be recognized in function of the costs incurred for this program, applying the percentage of completion method, along with the upfront payment. In the year ended December 31, 2016, €3.6 million revenues were recognized in the income statement.
In 2016, Galapagos signed a license agreement with ThromboGenics for an integrin antagonist (formerly GLPG0187), for which an upfront payment of €1 million was invoiced and fully recognized, as Galapagos has no further involvement or obligation in the contract.
108
Table of Contents
The following table summarizes the upfront payments recognition for years ended December 31, 2016 and 2015.
| Agreement |
Upfront received |
Upfront received |
Date of receipt |
Revenue recognized, year ended December 31, 2016 |
Revenue recognized, year ended December 31, 2015 |
Outstanding balance in deferred income as at December 31, 2016 |
||||||||||||||||||
| (USD, in thousands) |
(Euro, in thousands) |
(Euro, in thousands) | ||||||||||||||||||||||
| AbbVie collaboration agreement for CF
|
$45,000 | €34,001 | September 2013 | €11,401 | ||||||||||||||||||||
| AbbVie collaboration agreement for RA and CD (filgotinib)
|
$150,000 | €111,582 | February 2012 | €12,045 | ||||||||||||||||||||
| First amendment to AbbVie collaboration agreement for RA and CD (filgotinib)
|
$20,000 | €15,619 | March 2013 | €2,973 | ||||||||||||||||||||
| Gilead collaboration agreement for filgotinib
|
$300,000 | €275,558 | January 2016 | €25,621 | €249,937 | |||||||||||||||||||
| Gilead collaboration agreement for filgotinib
|
N.A. | €39,003 (*) | January 2016 | €3,626 | €35,376 | |||||||||||||||||||
| ThromboGenics license agreement for integrin antagonists
|
N.A. | €1,000 | April 2016 | €1,000 | ||||||||||||||||||||
| Sirion Biotech license agreement for RNA interference (RNAi) technologies
|
N.A. | €10 | June 2016 | €10 | ||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Total recognition of non-refundable upfront payments |
|
€30,257 | €26,419 | €285,314 | ||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| (*) | deferred income of €39 million booked upon signing of the share subscription agreement with Gilead as required under IAS 39 |
Milestone revenues increased substantially by €77.9 million, to €81.8 million for the year ended December 31, 2016 compared to €3.8 million for the year ended December 31, 2015. Milestones in 2016 related to the filgotinib program with Gilead in CD and UC, and the CF program in with AbbVie. Milestones in 2015 related to our OA program with Servier.
Reimbursement income increased by €5.9 million or 155%, to €9.7 million for the year ended December 31, 2016 compared to €3.8 million for the year ended December 31, 2015, due to higher reimbursements in relation with the CF program with AbbVie and the filgotinib program with Gilead (which was partnered with AbbVie in 2015). The reimbursement of certain research and development costs related to the development work under our collaboration agreements amounted to €5.9 million for our CF program with AbbVie and €3.5 million for our filgotinib program with Gilead for the year ended December 31, 2016. For the year ended December 31, 2015, €2.2 million and €1.2 million of costs were reimbursed in relation with the CF and filgotinib collaboration agreements with AbbVie, respectively,
Other revenues increased by €2.3 million, or 41%, to €7.8 million for the year ended December 31, 2016 compared to €5.5 million for the year ended December 31, 2015, principally due to higher revenues from fee-for-service activities.
109
Table of Contents
Other Income
The following table summarizes our other income for the years ended December 31, 2016 and 2015, together with the changes to those items.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Grant income |
€ | 2,329 | € | 3,095 | (25 | %) | ||||||
| Other income |
19,764 | 17,922 | 10 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other income |
€ | 22,093 | € | 21,017 | 5 | % | ||||||
|
|
|
|
|
|
|
|||||||
Total other income was composed of grant income and other income and increased by €1.1 million, or 5%, from €21.0 million for the year ended December 31, 2015 to €22.1 million for the year ended December 31, 2016.
Grant income decreased by €0.8 million, or 25%, from €3.1 million for the year ended December 31, 2015 to €2.3 million for the year ended December 31, 2016. The majority of this grant income is related to grants from a Flemish agency, representing approximately 88% of all reported grant income in 2016 (2015: 94%). In many cases these carry clauses which require us to maintain a presence in the same region for a number of years and invest according to pre-agreed budgets.
The decrease in grant income was more than offset by an increase in other income of €1.8 million, or 10%, from €17.9 million for the year ended December 31, 2015 to €19.8 million for the year ended December 31, 2016. Other income was primarily composed of:
| • | Income from an innovation incentive system of the French government, which represented €9.5 million of other income for the year ended December 31, 2016 compared to €8.7 million for the year ended December 31, 2015 |
| • | Income from Belgian R&D incentives with regard to incurred R&D expenses, which represented €5.8 million of other income for the year ended December 31, 2016 compared to €5.3 million for the year ended December 31, 2015 |
| • | Tax rebates on payroll withholding taxes of R&D personnel in Belgium and the Netherlands, representing €3.8 million of other income for the year ended December 31, 2016 compared to €3.0 million for the year ended December 31, 2015 |
R&D Expenditure
The following table summarizes our R&D expenditure for the years ended December 31, 2016 and 2015, together with the changes to those items.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Personnel costs |
€ | (42,315 | ) | € | (35,875 | ) | 18 | % | ||||
| Subcontracting |
(65,649 | ) | (65,883 | ) | (0 | %) | ||||||
| Disposables and lab fees and premises costs |
(20,414 | ) | (18,696 | ) | 9 | % | ||||||
| Other operating expenses |
(11,196 | ) | (9,260 | ) | 21 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total R&D expenditure |
€ | (139,573 | ) | € | (129,714 | ) | 8 | % | ||||
|
|
|
|
|
|
|
|||||||
110
Table of Contents
R&D expenditure increased by €9.9 million, or 8%, to €139.6 million for the year ended December 31, 2016, from €129.7 million for the year ended December 31, 2015. This increase was principally due to:
| • | Increased R&D personnel costs of €6.4 million, or 18%, from €35.9 million for the year ended December 31, 2015 to €42.3 million for the year ended December 31, 2016, which was explained by an enlarged workforce, higher warrant costs and a higher liability for short term and long term management bonus, mainly as a result of the increase of our share price change relative to the Next Biotech Index on Euronext. |
| • | Subcontracting costs were relatively stable and decreased slightly by €0.2 million, or 0.4%, from €65.9 million for the year ended December 31, 2015 to €65.6 million for the year ended December 31, 2016. |
| • | Intensified use of lab consumables was the main driver of the increase in disposables, lab fees and premises costs of €1.7 million, or 9%, from €18.7 million for the year ended December 31, 2015 to €20.4 million for the year ended December 31, 2016. |
| • | Other operating expenses increased by €1.9 million, or 21%, from €9.3 million for the year ended December 31, 2015 to €11.2 million for the year ended December 31, 2016, primarily due to an increase in depreciation of €1.0 million. |
The table below summarizes our R&D expenditure for the years ended December 31, 2016 and 2015, broken down by R&D expenses under alliance and own funded R&D expenses. All filgotinib costs (both costs incurred in the period under alliance (with AbbVie) and costs incurred after AbbVie’s opt-out decision in September 2015) are presented as “R&D under alliance” or as “partnered” in the tables in this section for the year ended December 31, 2015, as a new alliance was signed in December 2015 with Gilead for this program.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| R&D under alliance |
€ | (71,980 | ) | € | (80,832 | ) | (11 | %) | ||||
| Galapagos funded R&D |
(67,593 | ) | (48,882 | ) | 38 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total R&D expenditure |
€ | (139,573 | ) | € | (129,714 | ) | 8 | % | ||||
|
|
|
|
|
|
|
|||||||
We track all R&D expenditures against detailed budgets and allocated them by individual project. The table below summarizes our R&D expenditure for the years ended December 31, 2016 and 2015, broken down by program.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Filgotinib program (partnered) |
€ | (22,376 | ) | € | (35,404 | ) | (37 | %) | ||||
| CF program (partnered) |
(31,203 | ) | (25,634 | ) | 22 | % | ||||||
| IPF program on GLPG1690 (proprietary) |
(7,129 | ) | (4,612 | ) | 55 | % | ||||||
| OA program on GLPG1972 (partnered) |
(6,538 | ) | (5,832 | ) | 12 | % | ||||||
| AtD program on MOR106 (partnered) |
(3,491 | ) | (4,651 | ) | (25 | %) | ||||||
| Other |
(68,836 | ) | (53,582 | ) | 28 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total R&D expenditure |
€ | (139,573 | ) | € | (129,714 | ) | 8 | % | ||||
|
|
|
|
|
|
|
|||||||
R&D expenditure under alliance decreased by €8.9 million, or 11%, from €80.8 million for the year ended December 31, 2015 to €72.0 million for the year ended December 31, 2016, mainly due to our RA and IBD program on filgotinib (partnered with AbbVie in 2015 and partnered with Gilead in 2016), which has been
111
Table of Contents
partially offset by increased R&D spending on our CF program in collaboration with AbbVie. We increased our investments in our own funded portfolio by €18.7 million, or 38%, from €48.9 million for the year ended December 31, 2015 to €67.6 million for the year ended December 31, 2016, primarily because of intensified research investments in our proprietary programs on inflammation, HBV and fibrosis, as well as increased spending on our proprietary IPF program GLPG1690.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the years ended December 31, 2016 and 2015, together with the changes to those items.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Personnel costs and directors fees |
€ | (15,160 | ) | € | (12,739 | ) | 19 | % | ||||
| Other operating expenses |
(6,584 | ) | (6,388 | ) | 3 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total general and administrative expenses |
€ | (21,744 | ) | € | (19,127 | ) | 14 | % | ||||
|
|
|
|
|
|
|
|||||||
General and administrative expenses amounted to €19.1 million for the year ended December 31, 2015 and increased by €2.6 million, or 14%, to €21.7 million for the year ended December 31, 2016. This increase was principally due to directors fees, which increased by €2.7 million, or 116%, from €2.4 million for the year ended December 31, 2015 to €5.1 million for the year ended December 31, 2016, resulting from various effects, such as increased costs of share-based payments plans (our warrant plans) and increased liability for short and long term management bonus, mainly as a result of the increase of our share price change relative to the Next Biotech Index on Euronext.
Sales and Marketing Expenses
The following table summarizes our sales and marketing expenses for the years ended December 31, 2016 and 2015, together with the changes to those items.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Personnel costs |
€ | (1,167 | ) | € | (785 | ) | 49 | % | ||||
| Other operating expenses |
(618 | ) | (397 | ) | 56 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total sales and marketing expenses |
€ | (1,785 | ) | € | (1,182 | ) | 51 | % | ||||
|
|
|
|
|
|
|
|||||||
Sales and marketing expenses increased by €0.6 million, or 51%, from €1.2 million for the year ended December 31, 2015 to €1.8 million for the year ended December 31, 2016.
Fair Value Re-measurement of Share Subscription Agreement
On December 16, 2015, Gilead Sciences, Inc. and Galapagos NV entered into a global collaboration for the development and commercialization of filgotinib, in the framework of which Gilead committed to an upfront payment of $725 million consisting of a license fee of $300 million and a $425 million equity investment in Galapagos NV by subscribing to new shares at a price of €58 per share, including issuance premium. This agreement was effectively completed and entered into force on January 19, 2016 and the full payment was received.
In connection with the agreement, we recognized a deferred income and an offsetting short term financial asset (derivative) of €39 million upon signing of the share subscription agreement with Gilead as required under IAS 39.
112
Table of Contents
This financial asset initially reflected the share premium that Gilead committed to pay above our closing share price on the day of entering into the subscription agreement. Under IAS 39 the fair value of the financial asset is re-measured at year-end and again upon entering into force of the share subscription agreement on January 19, 2016, when the financial asset expired. Variations in fair value of the financial asset are recorded in the income statement.
The decrease in the fair value of the financial asset resulting from the increase in the Galapagos share price between signing of the subscription agreement and December 31, 2015 resulted in a negative, non-cash fair value charge of €30.6 million in the financial results. The subsequent increase in the fair value of the financial asset resulting from the decrease in our share price between January 1, 2016 and January 19, 2016 resulted in a positive non-cash gain of €57.5 million in the financial result of 2016.
On January 19, 2016, the value of the financial asset at maturity amounted to €65.9 million, reflecting the share premium that Gilead paid above our closing share price on the day of the capital increase. This amount was composed of (1) the initial measurement on the day of entering into the share subscription agreement for an amount of €39 million which was reported in deferred income and (2) the subsequent re-measurements of the financial asset, reported as financial result under IAS 39: €30.6 million fair value loss reported in the year 2015 and €57.5 million fair value gain reported in the first quarter of 2016, together a net fair value gain of €26.8 million. This financial asset expired on the effective date of the share subscription agreement and was derecognized through the share premium account.
Other Financial Income and Expense
The following table summarizes other financial income and expense for the years ended December 31, 2016 and 2015.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Other financial income: |
||||||||||||
| Interest on bank deposit |
€ | 1,614 | € | 1,246 | 29 | % | ||||||
| Effect of discounting long term R&D incentives receivables |
99 | 99 | — | |||||||||
| Currency exchange gain |
8,150 | 636 | 1182 | % | ||||||||
| Other finance income |
87 | 7 | 1142 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other financial income |
9,950 | 1,987 | 401 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Other financial expenses: |
||||||||||||
| Interest expenses |
(47 | ) | (46 | ) | 2 | % | ||||||
| Currency exchange loss |
(1,453 | ) | (1,310 | ) | 11 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Other finance charges |
(191 | ) | (182 | ) | 5 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total other financial expense |
(1,692 | ) | (1,539 | ) | 10 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total other net financial income |
€ | 8,257 | € | 448 | 1742 | % | ||||||
|
|
|
|
|
|
|
|||||||
Other financial income increased significantly by €8.0 million, or 401%, from €2.0 million for the year ended December 31, 2015 to €10.0 million for the year ended December 31, 2016. The increase relates to an exchange gain of €4.8 million on deposits held in U.S. dollar and exchange gains of €2.0 million realized on milestone payments from AbbVie and Gilead in U.S. dollar. For more information on currency exchange fluctuations on our business, please see the section of this annual report titled “Item 11—Quantitative and Qualitative Disclosures About Market Risk—Foreign Exchange Risk.”
Other financial expenses increased by €0.2 million, or 10% from €1.5 million for the year ended December 31, 2015 to €1.7 million for the year ended December 31, 2016. Net exchange profit amounts to €6.7 million for the year ended December 31, 2016, compared to a loss of €0.7 million for the year ended December 31, 2015. Interest expenses are related to interests paid on financial lease.
113
Table of Contents
Tax
The following table summarizes our tax result for the years ended December 31, 2016 and 2015.
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| (Euro, in thousands) | ||||||||
| Current tax |
€ | (466 | ) | € | (215 | ) | ||
| Deferred tax |
231 | 1,433 | ||||||
|
|
|
|
|
|||||
| Total taxes |
€ | (235 | ) | € | 1,218 | |||
|
|
|
|
|
|||||
Current tax representing €0.5 million for the year ended December 31, 2016 and €0.2 million for the year ended December 31, 2015 was related to taxes for subsidiaries operating on cost plus basis.
Deferred tax income of €0.2 million for the year ended December 31, 2016 and of €1.4 million for the year ended December 31, 2015 related to subsidiaries working on a cost plus basis.
Comparison of Years Ended December 31, 2015 and 2014
The following table summarizes the results of our operations for the years ended December 31, 2015 and 2014, together with the changes to those items
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | % Change | ||||||||||
| (Euro, in thousands, except share and per share data) |
||||||||||||
| Revenues |
€ | 39,563 | € | 69,368 | (43 | %) | ||||||
| Other income |
21,017 | 20,653 | 2 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues and other income |
60,579 | 90,021 | (33 | %) | ||||||||
|
|
|
|
|
|
|
|||||||
| Research and development expenditure |
(129,714 | ) | (111,110 | ) | 17 | % | ||||||
| General and administrative expenses |
(19,127 | ) | (13,875 | ) | 38 | % | ||||||
| Sales and marketing expenses |
(1,182 | ) | (992 | ) | 19 | % | ||||||
| Restructuring and integration costs |
— | (669 | ) | (100 | %) | |||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
(150,023 | ) | (126,646 | ) | 18 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(89,444 | ) | (36,624 | ) | 144 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Fair value re-measurement of share subscription agreement |
(30,632 | ) | ||||||||||
| Other financial income |
1,987 | 2,291 | (13 | %) | ||||||||
| Other financial expenses |
(1,539 | ) | (867 | ) | 78 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Loss before tax |
(119,627 | ) | (35,201 | ) | 240 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Income taxes |
1,218 | (2,103 | ) | (158 | %) | |||||||
|
|
|
|
|
|
|
|||||||
| Net loss from continuing operations |
(118,410 | ) | (37,303 | ) | 217 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Net income from discontinued operations |
— | 70,514 | ||||||||||
|
|
|
|
|
|||||||||
| Net income / loss (-) |
€ | (118,410 | ) | € | 33,211 | |||||||
|
|
|
|
|
|||||||||
| Net income / loss (-) attributable to: |
||||||||||||
| Owners of the parent |
(118,410 | ) | 33,211 | |||||||||
|
|
|
|
|
|||||||||
| Basic and diluted income / loss (-) per share |
€ | (3.32 | ) | € | 1.10 | |||||||
|
|
|
|
|
|||||||||
| Basic and diluted loss per share from continuing operations |
€ | (3.32 | ) | € | (1.24 | ) | ||||||
| Weighted average number of shares (in ‘000 shares) |
35,700 | 30,108 | ||||||||||
114
Table of Contents
Revenues
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Recognition of non-refundable upfront payments |
€ | 26,419 | € | 45,838 | (42 | %) | ||||||
| Milestone payments |
3,835 | 19,039 | (80 | %) | ||||||||
| Reimbursement income |
3,807 | 729 | 422 | % | ||||||||
| Other revenues |
5,501 | 3,762 | 46 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
€ | 39,563 | € | 69,368 | (43 | %) | ||||||
|
|
|
|
|
|
|
|||||||
Total revenues decreased by €29.8 million, or 43%, to €39.6 million for the year ended December 31, 2015, from €69.4 million for the year ended December 31, 2014. This decrease was mainly driven by lower recognition of non-refundable upfront payments and reduced milestone payments, as explained below.
Revenue from non-refundable upfront payments related to the deferred recognition of upfront payments received under the agreements with AbbVie, amounting to €111.6 million in 2012 and €49.6 million in 2013, which were amortized over a period ranging from 21 to 42 months, based on the estimated period of our involvement.
At inception and as of December 31, 2012, the period of involvement was estimated at 30 months starting in March 2012. As from April 2013 and as of December 31, 2013, we changed the estimate of our period of involvement to 34 months due to delays that occurred in clinical trials and changed our recognition of the remaining unrecognized upfront payments accordingly. As of June 30, 2014 and December 31, 2014, we changed the estimate of our period of involvement from 34 months to 39 months and 40 months, respectively, due to additional delays and changed our recognition of the remaining unrecognized upfront payments accordingly. As of June 30, 2015, we changed the estimate of our period of involvement from 40 months to 42 months, due to additional delays and changed our recognition of the remaining unrecognized upfront payments accordingly.
Milestone revenues and costs reimbursements decreased by €12.1 million, or 61%, to €7.6 million for the year ended December 31, 2015 compared to €19.8 million for the year ended December 31, 2014. This decrease was primarily related to fewer milestones achieved in 2015 compared to 2014 as a result of the increasing proprietary nature of our pipeline programs. For the year ended December 31, 2015, €2.2 million and €1.2 million of costs were reimbursed in relation with the CF and filgotinib collaboration agreements with AbbVie, respectively, and €3.8 million of milestones related to partnered programs with Servier were recognized. For the year ended December 31, 2014, €8.3 million of milestones were recognized in relation with the CF collaboration agreement with AbbVie and €11.5 million of milestones primarily related to partnered programs with Janssen Pharmaceutica, Servier and GSK.
Other revenues increased by €1.7 million, or 46%, to €5.5 million for the year ended December 31, 2015 compared to €3.8 million for the year ended December 31, 2014, principally due to higher revenues from fee-for-service activities.
Other Income
The following table summarizes our other income for the years ended December 31, 2015 and 2014, together with the changes to those items.
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Grant income |
€ | 3,095 | € | 5,646 | (45 | %) | ||||||
| Other income |
17,922 | 15,008 | 19 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other income |
€ | 21,017 | € | 20,653 | 2 | % | ||||||
|
|
|
|
|
|
|
|||||||
115
Table of Contents
Total other income was composed of grant income and other income and increased by €0.4 million, or 2%, from €20.7 million for the year ended December 31, 2014 to €21.0 million for the year ended December 31, 2015.
Grant income decreased by €2.6 million, or 45%, from €5.6 million for the year ended December 31, 2014 to €3.1 million for the year ended December 31, 2015. The majority of this grant income was related to grants from a Flemish agency, representing approximately 94% of all reported grant income in both years. In many cases these carry clauses require us to maintain a presence in the same region for a number of years and invest according to pre-agreed budgets.
The decrease in grant income was compensated by an increase in other income of €2.9 million, or 19%, from €15.0 million for the year ended December 31, 2014 to €17.9 million for the year ended December 31, 2015. Other income was primarily composed of:
| • | Income from an innovation incentive system of the French government, which represented €8.7 million of other income for the year ended December 31, 2015 compared to €7.8 million for the year ended December 31, 2014 |
| • | Income from Belgian R&D incentives with regard to incurred R&D expenses, which represented €5.3 million of other income for the year ended December 31, 2015 compared to €4.3 million for the year ended December 31, 2014 |
| • | Tax rebates on payroll withholding taxes of R&D personnel in Belgium and the Netherlands, representing €3.0 million of other income for the year ended December 31, 2015 compared to €2.4 million for the year ended December 31, 2014 |
R&D Expenditure
The following table summarizes our R&D expenditure for the years ended December 31, 2015 and 2014, together with the changes to those items.
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Personnel costs |
€ | (35,875 | ) | € | (31,038 | ) | 16 | % | ||||
| Subcontracting |
(65,883 | ) | (54,293 | ) | 21 | % | ||||||
| Disposables and lab fees and premises costs |
(18,696 | ) | (16,830 | ) | 11 | % | ||||||
| Other operating expenses |
(9,260 | ) | (8,949 | ) | (3 | %) | ||||||
|
|
|
|
|
|
|
|||||||
| Total R&D expenditure |
€ | (129,714 | ) | € | (111,110 | ) | 17 | % | ||||
|
|
|
|
|
|
|
|||||||
R&D expenditure increased by €18.6 million, or 17%, to €129.7 million for the year ended December 31 2015, from €111.1 million for the year ended December 31, 2014. This increase was principally due to:
| • | Increased R&D personnel costs of €4.8 million, or 16%, from €31.0 million for the year ended December 31, 2014 to €35.9 million for the year ended December 31, 2015, which was explained by an enlarged workforce, higher warrant costs and a higher liability for short-term and long-term management bonus, mainly as a result of the increase of our share price change relative to the Next Biotech Index on Euronext. Increased subcontracting costs of €11.6 million, or 21%, from €54.3 million for the year ended December 31, 2014 to €65.9 million for the year ended December 31, 2015. This cost increase was mainly driven by increased subcontracting costs of €8.4 million for the CF collaboration with AbbVie and to a lesser extent by the increase of €4.2 million in subcontracting costs for our other partnered and internal programs. |
116
Table of Contents
| • | Intensified use of lab consumables was the main driver of the increase in disposables, lab fees and premises costs of €1.9 million, or 11%, from €16.8 million for the year ended December 31, 2014 to €18.7 million for the year ended December 31, 2015. |
| • | Other operating expenses slightly increased by €0.3 million, or 3%, from €8.9 million for the year ended December 31, 2014 to €9.3 million for the year ended December 31, 2015. |
The table below summarizes our R&D expenditure for the years ended December 31, 2015 and 2014, broken down by R&D expenses under alliance and own funded R&D expenses. All filgotinib costs (both costs incurred in the period under alliance (with AbbVie) and costs incurred after AbbVie’s opt-out decision in September 2015) are presented as “R&D under alliance” or as “partnered” in the tables in this section for the year ended December 31, 2015, as a new alliance was signed in December 2015 with Gilead for this program.
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| R&D under alliance |
€ | (80,832 | ) | € | (76,297 | ) | 6 | % | ||||
| Galapagos funded R&D |
(48,882 | ) | (34,813 | ) | 40 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total R&D expenditure |
€ | (129,714 | ) | € | (111,110 | ) | 17 | % | ||||
|
|
|
|
|
|
|
|||||||
We track all R&D expenditures against detailed budgets and allocated them by individual project. The table below summarizes our R&D expenditure for the years ended December 31, 2015 and 2014, broken down by program.
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| RA program on filgotinib with AbbVie |
€ | (30,998 | ) | € | (30,437 | ) | 2 | % | ||||
| IBD program on filgotinib with AbbVie |
(4,406 | ) | (3,406 | ) | 29 | % | ||||||
| IBD program on GLPG1205 |
(5,769 | ) | (6,020 | ) | (4 | %) | ||||||
| CF program with AbbVie |
(25,634 | ) | (14,894 | ) | 72 | % | ||||||
| Pulmonary program on GLPG1690 |
(4,612 | ) | (4,592 | ) | 0 | % | ||||||
| Other |
(58,295 | ) | (51,762 | ) | (13 | %) | ||||||
|
|
|
|
|
|
|
|||||||
| Total R&D expenditure |
€ | (129,714 | ) | € | (111,110 | ) | 17 | % | ||||
|
|
|
|
|
|
|
|||||||
R&D expenditure under alliance increased by €4.5 million, or 6%, from €76.3 million for year ended December 31, 2014 to €80.8 million for the year ended December 31, 2015, mainly due to our CF program in collaboration with AbbVie. We also increased our investments in our own funded portfolio by €14.1 million, or 40%, from €34.8 million for the year ended December 31, 2014 to €48.9 million for the year ended December 31, 2015, primarily because GLPG1205 and GLPG1690 programs became own funded.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the years ended December 31, 2015 and 2014, together with the changes to those items.
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Personnel costs and directors fees |
€ | (12,739 | ) | € | (8,087 | ) | 58 | % | ||||
| Other operating expenses |
(6,388 | ) | (5,788 | ) | 10 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total general and administrative expenses |
€ | (19,127 | ) | € | (13,875 | ) | 38 | % | ||||
|
|
|
|
|
|
|
|||||||
117
Table of Contents
General and administrative expenses amounted to €13.9 million for the year ended December 31, 2014 and increased by €5.2 million, or 38%, to €19.1 million for the year ended December 31, 2015. This increase was principally due to personnel costs and directors fees, which increased by €4.6 million, or 58%, from €8.1 million for the year ended December 31, 2014 to €12.7 million for the year ended December 31, 2015, resulting from various effects, such as increased costs of share-based payments plans (warrant plans) and increased liability for short- and long-term management bonus, mainly as a result of the increase of our share price change relative to the Next Biotech Index on Euronext. In addition, other operating expenses increased by €0.6 million, or 10%, from €5.8 million for the year ended December 31, 2014 to €6.4 million for the year ended December 31, 2015, mainly due to higher professional fees.
Sales and Marketing Expenses
The following table summarizes our sales and marketing expenses for the years ended December 31, 2015 and 2014, together with the changes to those items.
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Personnel costs |
€ | (785 | ) | € | (579 | ) | 36 | % | ||||
| Other operating expenses |
(397 | ) | (412 | ) | (4 | %) | ||||||
|
|
|
|
|
|
|
|||||||
| Total sales and marketing expenses |
€ | (1,182 | ) | € | (992 | ) | 19 | % | ||||
|
|
|
|
|
|
|
|||||||
Sales and marketing expenses increased by €0.2 million, or 19%, from €1.0 million for the year ended December 31, 2014 to €1.2 million for the year ended December 31, 2015.
Restructuring and Integration Costs
The restructuring and integration costs amounted to €0.7 million for the year ended December 31, 2014 and were entirely related to workforce reductions within certain of the R&D operations.
Fair Value Re-measurement of Share Subscription Agreement
On December 16, 2015, we entered into a global collaboration for the development and commercialization of filgotinib with Gilead, in the framework of which Gilead committed to an upfront payment of $725 million consisting of a license fee of $300 million and a $425 million equity investment in our company by subscribing to new shares at a price of €58 per share, including issuance premium. This agreement was effectively completed and entered into force on January 19, 2016 and the full payment was received.
In connection with the agreement, we recognized a deferred income and an offsetting short-term financial asset (derivative) of €39 million upon signing of the share subscription agreement with Gilead as required under IAS 39. This financial asset initially reflects the share premium that Gilead committed to pay above our closing share price on the day of entering into the subscription agreement. Under IAS 39 the fair value of the financial asset is re-measured at year-end and again upon entering into force of the share subscription agreement on January 19, 2016, when the financial asset expired. Variations in fair value of the financial asset are recorded in the income statement included in our consolidated financial statements appended to this annual report.
The decrease in the fair value of the financial asset resulting from the increase in our share price between signing of the subscription agreement and December 31, 2015 resulted in a negative, non-cash adjustment fair value charge of €30.6 million in the financial results. The subsequent increase in the fair value of the financial asset resulting from the decrease in our share price between January 1, 2016 and January 19, 2016 will result in a positive non-cash gain of €57.5 million in the financial result of the first quarter of 2016.
118
Table of Contents
Other Financial Income and Expense
The following table summarizes other financial income and expense for the years ended December 31, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | % Change | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Other financial income: |
||||||||||||
| Interest on bank deposit |
€ | 1,246 | € | 1,155 | 8 | % | ||||||
| Effect of discounting long term R&D incentives receivables |
99 | 920 | (89 | %) | ||||||||
| Currency exchange gain |
636 | 198 | 221 | % | ||||||||
| Other finance income |
7 | 17 | (59 | %) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other financial income |
1,987 | 2,291 | (13 | %) | ||||||||
|
|
|
|
|
|
|
|||||||
| Other financial expenses: |
||||||||||||
| Interest expenses |
(46 | ) | (110 | ) | (58 | %) | ||||||
| Currency exchange loss |
(1,310 | ) | (652 | ) | 101 | % | ||||||
| Other finance charges |
(182 | ) | (105 | ) | 73 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total other financial expense |
(1,539 | ) | (867 | ) | 77 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total other net financial income |
€ | 448 | € | 1,424 | (69 | %) | ||||||
|
|
|
|
|
|
|
|||||||
Other financial income decreased slightly by €0.3 million, or 13%, from €2.3 million for the year ended December 31, 2014 to €2.0 million for the year ended December 31, 2015. The €0.8 million decrease in the effect of discounting long-term R&D incentives receivables was partly compensated by a €0.4 million increase in currency exchange gains. For more information on currency exchange fluctuations on our business, please see the section of this annual report titled “Item 11—Quantitative and Qualitative Disclosures About Market Risk—Foreign Exchange Risk.”
Other financial expense increased by €0.6 million, or 77%, from €0.9 million for the year ended December 31, 2014 to €1.5 million for the year ended December 31, 2015. Net exchange loss amounts to €0.7 million for the year ended December 31, 2015, as compared to €0.5 million for the year ended December 31, 2014. Interest expenses are related to interests paid on financial lease.
Tax
The following table summarizes our tax result for the years ended December 31, 2015 and 2014.
| Year ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| (Euro, in thousands) | ||||||||
| Current tax |
€ | (215 | ) | € | (2,396 | ) | ||
| Deferred tax |
1,433 | 293 | ||||||
|
|
|
|
|
|||||
| Total taxes |
€ | 1,218 | € | (2,103 | ) | |||
|
|
|
|
|
|||||
Current tax representing €0.2 million for the year ended December 31, 2015 was related to taxes for subsidiaries operating on cost plus basis.
Current tax recorded in 2014 for an amount of €2.4 million related to a tax provision for subsidiaries operating under cost plus transfer pricing arrangements, triggered by a tax audit.
119
Table of Contents
Deferred tax income of €1.4 million for the year ended December 31, 2015 and €0.3 million for the year ended December 31, 2014 both related to subsidiaries working on a cost plus basis.
Results from Discontinued Operations
The following table summarizes the results from discontinued operations for the years ended December 31, 2015 and 2014.
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| (Euro, in thousands, except share and per share data) |
||||||||
| Service revenues |
— | € | 17,502 | |||||
| Other income |
— | 669 | ||||||
|
|
|
|
|
|||||
| Total revenues and other income |
— | 18,171 | ||||||
|
|
|
|
|
|||||
| Services cost of sales |
— | (11,283 | ) | |||||
| General and administrative expenses |
— | (3,772 | ) | |||||
| Sales and marketing expenses |
— | (255 | ) | |||||
| Restructuring and integration costs |
— | (38 | ) | |||||
| Loss on divestment |
— | — | ||||||
| Gain on sale of service division |
— | 67,508 | ||||||
|
|
|
|
|
|||||
| Operating income |
— | 70,331 | ||||||
|
|
|
|
|
|||||
| Finance income / expense (-) |
— | 417 | ||||||
|
|
|
|
|
|||||
| Income before tax |
— | 70,748 | ||||||
|
|
|
|
|
|||||
| Income taxes |
— | (234 | ) | |||||
|
|
|
|
|
|||||
| Net income from discontinued operations |
— | € | 70,514 | |||||
|
|
|
|
|
|||||
| Basic and diluted income per share from discontinued operations |
— | € | 2.34 | |||||
|
|
|
|
|
|||||
| Weighted average number of shares (in ‘000 shares) |
— | 30,108 | ||||||
The service division was sold on April 1, 2014. The above table illustrates the results of the discontinued operations included in the consolidated results of operations for the years ended December 31, 2015 and 2014. For the year ended December 31, 2014, results only relate to the period from January 1, 2014 through the disposal on April 1, 2014.
Net income amounting to €70.5 million in 2014 was mainly driven by the €67.5 million gain on disposal of the service division.
| B. | Liquidity and Capital Resources |
To date, we have incurred significant operating losses. We have funded our operations through public and private placements of equity securities, upfront and milestone payments received from pharmaceutical partners under our collaboration and alliance agreements, payments under our fee-for-service contracts, funding from governmental bodies, interest income as well as the net proceeds from the sale of our service division. Our cash flows may fluctuate and are difficult to forecast and will depend on many factors. As at December 31, 2016, our cash and cash equivalents amounted to €973.2 million. For more information on our policies regarding financial instruments, please see “Note 2—Significant accounting policies—Financial instruments” included in our consolidated financial statements appended to this annual report.
120
Table of Contents
Cash Flows
Comparison for the Years Ended December 31, 2016 and 2015
The following table summarizes the results of our consolidated audited statement of cash flows for the years ended December 31, 2016 and 2015.
| 2016 | 2015 | Variance | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Cash and cash equivalents at beginning of the period |
€ | 340,314 | € | 187,712 | € | 152,601 | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash flows generated / used (-) in operating activities |
239,403 | (114,590 | ) | 353,993 | ||||||||
| Net cash flows used in investing activities |
(7,287 | ) | (4,297 | ) | (2,990 | ) | ||||||
| Net cash flows generated in financing activities |
395,996 | 271,370 | 124,626 | |||||||||
| Effect of exchange rate differences on cash and cash equivalents |
4,816 | 118 | 4,697 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of the period |
€ | 973,241 | € | 340,314 | € | 632,927 | ||||||
|
|
|
|
|
|
|
|||||||
Cash and cash equivalents at December 31, 2016 amounted to €973.2 million.
Net cash outflow from operating activities decreased by €354.0 million to a €239.4 million inflow for the year ended December 31, 2016 compared to a €114.6 million outflow for the year ended December 31, 2015. This net cash inflow from operations recorded in 2016 was primarily due to the license fee of $300 million (€275.6 million) received from Gilead in relation with our collaboration agreement on filgotinib. In addition, milestone payments increased substantially in 2016 compared to 2015, which contributed significantly to the net cash inflow from operations in 2016.
The net cash outflow from investing activities increased by €3.0 million to €7.3 million net cash outflow for the year ended December 31, 2016 compared to €4.3 million net cash outflow for the year ended December 31, 2015, which was principally related to an acquisition of available-for-sale financial assets, as well as a lower decrease in restricted cash compared to previous year. Restricted cash amounted to €7.9 million for the year ended December 31, 2015, and decreased to €7.7 million for the year ended December 31, 2016. This decrease is related to a payment of a claim to Charles River by decrease of the escrow account for €0.3 million, which has been slightly offset by an increase in non-current restricted cash of €0.1 million related to an increase in the bank guarantee with regard to the rental of additional office space for the Belgian premises.
The net cash inflow from financing activities increased by €124.6 million, from €271.4 million net cash inflow for the year ended December 31, 2015 to €396.0 million net cash inflow for the year ended December 31, 2016. The net cash inflow in 2016 can mainly be attributed to the subscription on Galapagos shares by Gilead on January 19, 2016 for which the cash proceeds from capital and share premium increases amounted to €391.9 million, net of issue costs. The net cash inflow in 2015 can primarily be attributed to €259.4 million of net new funds from the global offering and concurrent listing on the NASDAQ Global Select Market on May 19, 2015. In addition, proceeds received on exercise of warrants contributed to cash generated by financing activities in 2016 for €4.3 million and to a greater extent for €12.0 million in 2015.
121
Table of Contents
Comparison of Years Ended December 31, 2015 and 2014
The following table summarizes the results of our consolidated statement of cash flows for the years ended December 31, 2015 and 2014.
| 2015 | 2014 | Variance | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Cash and cash equivalents at beginning of the period |
€ | 187,712 | € | 138,175 | € | 49,538 | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash flows used in operating activities |
(114,590 | ) | (75,555 | ) | (39,035 | ) | ||||||
| Net cash flows generated / used (-) in investing activities |
(4,297 | ) | 120,606 | (124,904 | ) | |||||||
| Net cash flows generated in financing activities |
271,370 | 4,214 | 267,156 | |||||||||
| Effect of exchange rate differences on cash and cash equivalents |
118 | 271 | (153 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of the period |
€ | 340,314 | € | 187,712 | € | 152,601 | ||||||
|
|
|
|
|
|
|
|||||||
Cash and cash equivalents at December 31, 2015 amounted to €340.3 million.
Net cash outflow from operating activities increased by €39.0 million to a €114.6 million outflow for the year ended December 31, 2015 compared to a €75.6 million outflow for the year ended December 31, 2014. The higher cash burn from operations recorded in 2015 was primarily explained by increased R&D investments, €15.9 million less cash received from milestones and costs reimbursement, of which mainly €5.9 million in alliance related receivables for which revenues were recorded in 2013 and for which payment came in the first half of 2014.
The net cash inflow from investing activities decreased by €124.9 million to €4.3 million net cash outflow for the year ended December 31, 2015 compared to €120.6 million net cash inflow for the year ended December 31, 2014, which reflected €130.8 million of net cash and cash equivalents proceeds from the sale of the service operations to Charles River on April 1, 2014 (€129 million headline consideration adjusted with agreed price adjustments and costs of the sale for a total amount of €1.9 million), decreased by €7.4 million held as escrow account and presented as restricted cash in our statement of financial position. Restricted cash amounted to €10.7 million for the year ended December 31, 2014, and decreased to €7.9 million for the year ended December 31, 2015. This decrease is related to (i) the release of the €3 million bank guarantee issued in 2013 for the rental of the new premises in France which expired on June 30, 2015 following the move to the new offices, (ii) the payment of a claim to Charles River by decrease of the escrow account, and (iii) a €0.7 million bank guarantee issued in September 2015 for the rental of new premises in the Netherlands (to replace the current premises) which will expire on October 1, 2025.
The net cash inflow from financing activities have increased by €267.2 million, from €4.2 million net cash inflow for the year ended December 31, 2014, to €271.4 million net cash inflow for the year ended December 31, 2015. The substantial net cash inflow in 2015 can primarily be attributed to €259.4 million of net new funds from the recent global offering and concurrent listing on the NASDAQ Global Select Market on May 19, 2015. In addition, proceeds received on exercise of warrants contributed to cash generated by financing activities in 2015 for €12.0 million and to a lesser extent for €4.4 million in 2014.
The consolidated cash flow table above included both continuing and discontinued operations. The table below summarizes our statement of cash flows from discontinued operations included in the table above for the years ended December 31, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2015 | 2014 | Variance | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Net cash flows used in operating activities |
€ | — | € | (1,722 | ) | € | 1,722 | |||||
| Net cash flows generated in investing activities |
— | 122,580 | (122,580 | ) | ||||||||
| Net cash flows generated/ used (-) in financing activities |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash generated |
€ | — | € | 120,858 | € | (120,858 | ) | |||||
|
|
|
|
|
|
|
|||||||
122
Table of Contents
Cash and Funding Sources
The table below summarizes our sources of financing, excluding warrant exercises, for the years ended December 31, 2016, 2015 and 2014.
| Private placement | ||||
| (Euro, in thousands) | ||||
| 2014 |
€ | — | ||
| 2015 |
259,343 | |||
| 2016 |
391,852 | |||
|
|
|
|||
| Total sources of financing |
€ | 651,195 | ||
|
|
|
|||
On May 19, 2015, we completed a global offering of 7,532,499 ordinary shares, a concurrent public offering in the United States and private placement in Europe, in which framework we offered 5,746,000 ordinary shares through a public offering in the United States in the form of ADSs, at a price of $42.05 per ADS, before underwriting discounts and 1,786,499 ordinary shares through a European private placement at a price of €37.00 per share, before underwriting discounts. The ADSs were evidenced by American Depositary Receipts, and each ADS represents the right to receive one ordinary share. The ADSs are listed on the NASDAQ Global Select Market under the symbol “GLPG”. We received €278.7 million of gross proceeds from the global offering, decreased by €19.4 million of underwriter discounts and commission, and offering expenses, of which €19.3 million has been paid at December 31, 2015 and €0.1 million (remainder) has been settled at December 31, 2016. Total net cash proceeds from the global offering amounted to €259.3 million. On January 19, 2016, Gilead made a $425 million equity investment in Galapagos NV by subscribing to 6,760,701 new ordinary shares at a price of €58 per share, including issuance premium. Galapagos received €392.1 million of gross proceeds, decreased by €0.26 million of expenses, of which all has been paid at December 31, 2016. The total net cash proceeds from the share subscription by Gilead amounts to €391.9 million. The €65.9 million current financial asset from the share subscription agreement reflecting the premium that Gilead paid compared to the closing price of our shares on January 19, 2016 was derecognized through the share premium account.
As of December 31, 2016, we had no long-term debt, other than finance leases and advances from Oseo, a French public organization for innovation support, for €0.1 million.
Our ongoing financial commitments are listed in the section of this annual report titled “Item 5.F.—Tabular Disclosure of Contractual Obligations” and mainly consist of operating lease obligations and purchase commitments.
Payment of Dividends by Subsidiaries
The amount of dividends payable by our subsidiaries to us is subject to, among other restrictions, general limitations imposed by the corporate laws, capital transfer restrictions and exchange control restrictions of the respective jurisdictions where those subsidiaries are organized and operate.
Of our cash and cash equivalents held outside of Belgium as of December 31, 2016 and 2015, the amount of cash that would have been subject to withholding taxes if transferred to us by way of dividends and the amount of cash that could not have been transferred by law was in each case immaterial.
Funding Requirements
Based on conservative assumptions, we believe that our existing cash and cash equivalents will enable us to fund our operating expenses and capital expenditure requirements at least through the next two to three years. We have based this estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect.
123
Table of Contents
Our present and future funding requirements will depend on many factors, including, among other things:
| • | the terms and timing of milestones, in-licensing payments and expense reimbursement payments, if any, from our collaboration and alliance agreements; |
| • | the progress, timing, scope and costs of pre-clinical testing and clinical trials for any current or future compounds; |
| • | the number and characteristics of potential new compounds we identify and decide to develop; |
| • | our need to expand our development activities and, potentially, our research activities; |
| • | the costs involved in filing patent applications and maintaining and enforcing patents; |
| • | the cost, timing and outcomes of regulatory approvals; |
| • | selling and marketing activities undertaken in connection with the anticipated commercialization of any of our current or future compounds; and |
| • | the amount of revenues, if any, we may derive either directly or in the form of royalty payments from future sales of our products. |
We may raise additional capital through the sale of equity or convertible debt securities. In such an event, your ownership interest may be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a holder of the ADSs or our ordinary shares.
For more information as to the risks associated with our future funding needs, see the section of this annual report titled “Item 3.D.—Risk Factors—Risks Related to Our Financial Position and Need for Additional Capital.”
Capital Expenditures
Our commitments for capital expenditures as of December 31, 2016 amount to €0.3 million.
Our capital expenditures amounted to €4.8 million, €6.7 million and €2.8 million for the years ended December 31, 2016, 2015 and 2014 respectively.
In 2016, our capital expenditures were primarily related to laboratory equipment for €3.3 million, €0.6 million for other tangible fixed assets and €0.3 million of intangible assets primarily related to software development.
In 2015, our capital expenditures were primarily related to laboratory equipment for €2.2 million, leasehold improvements mainly for our new building in Leiden (The Netherlands) for €2.2 million, €1.7 million for other tangible fixed assets and €0.6 million of intangible assets primarily related to software development.
In 2014, we invested €1.2 million in laboratory equipment, €0.9 million in other tangible assets and €0.7 million in intangible assets primarily related to software development.
| C. | Research and Development |
For a discussion of our R&D activities, see “Item 4.B.—Business Overview” and “Item 5.A.—Operating Results.”
| D. | Trend Information |
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the period from January 1, 2016 to December 31, 2016 that are reasonably likely to have a material adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial conditions. For a discussion of trends, see “Item 4.B.—Business Overview,” “Item 5.A.—Operating Results,” and “Item 5.B.—Liquidity and Capital Resources.”
124
Table of Contents
| E. | Off-Balance Sheet Arrangements |
During the periods presented, we did not and do not currently have any off-balance sheet arrangements as defined under SEC rules, such as relationships with unconsolidated entities or financial partnerships, which are often referred to as structured finance or special purpose entities, established for the purpose of facilitating financing transactions that are not required to be reflected on our balance sheets.
Contingent Liabilities and Assets
On March 13, 2014, we announced the signing of a definitive agreement to sell the service division operations to Charles River Laboratories International, Inc., or CRL, for a total consideration of up to €134 million. CRL agreed to pay us an immediate cash consideration of €129 million. The potential earn-out of €5 million due upon achievement of a revenue target 12 months after transaction closing has not been obtained. Approximately 5% of the total consideration, including price adjustments, was being held on an escrow account. To date, four claims have been introduced by CRL, which have all been settled for a total amount of €1.3 million. On January 17, 2017 an amount of €4.1 million was released from the escrow account. The release of the remaining balance of the escrow account will be possible after final agreement between the parties on the amounts at stake.
Following the divestment, we remained guarantor until early February 2017 in respect of the lease obligations for certain U.K. premises amounting to £3 million future rent payments. CRL shall fully indemnify us against all liabilities arising in connection with the lease obligations. We evaluated the risk to be remote. Finally, following common practice, we have given customary representations and warranties which are capped and limited in time (since April 1, 2016, CRL can only introduce a claim covered by the Tax Deed (during a period of 5 years), other claims related to the sale cannot be submitted anymore).
In the course of 2008, a former director of one of the subsidiaries sued for wrongful termination and seeks damages of €1.5 million. We believe that the amount of damages claimed is unrealistically high. In 2014, the court requested an external advisor to evaluate the exact amount of damages. On January 29, 2016, the court made a 1st degree judgment, dismissing all claims in full. In appeal, the 2nd degree court instructed the 1st degree court to conduct a new trial, which is currently pending. So far, no hearings have been scheduled and no decisions have been made. Considering the defense elements provided, as well as the fact that so far the court has made no decision indicating that the claim would be sustained, our board and management evaluated the risk to be remote to possible, but not likely. Accordingly, it was decided not to record any provision in 2016 as the exposure was considered to be limited.
| F. | Tabular Disclosure of Contractual Obligations |
We entered into lease agreements for office and laboratories which qualify as operating leases. We also have certain purchase commitments with contract research organization subcontractors principally. Future events could cause actual payments to differ from these estimates. On December 31, 2016, we had outstanding obligations for future minimum rent payments and purchase commitments, which become due as follows:
| Total | Less than 1 year |
1–3 years |
3–5 years |
More than 5 years |
||||||||||||||||
| (thousands of €) | ||||||||||||||||||||
| Operating lease obligations |
€ | 27,263 | € | 4,114 | € | 6,494 | € | 5,504 | € | 11,151 | ||||||||||
| Purchase commitments |
27,579 | 27,084 | 495 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations & commitments |
€ | 54,842 | € | 31,198 | € | 6,989 | € | 5,504 | € | 11,151 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The table above does not include pension liabilities, non-current deferred income and other non-current liabilities.
We provide retirement benefit plans for all of our qualifying employees. We classify these benefits on the basis of the type of benefit provided and in particular as defined contribution plans, defined benefit obligations
125
Table of Contents
and other provisions for employees. At December 31, 2016 the liability for such obligations amounted to €3.5 million (€2.7 million at December 31, 2015). See note 29 to the consolidated financial statements.
Non-current deferred income amounted to €214.8 million at December 31, 2016 (nil at December 31, 2015) and related to the recognition of a deferred income upon signing of the share subscription agreement with Gilead, as well as an upfront payment from Gilead for an amount of $300 million that we received in January 2016. See note 24 to the consolidated financial statements.
Other non-current liabilities amounted to €2.5 million at December 31, 2016 (€2.3 million at December 31, 2015) and primarily related to deferred management bonuses. The executive committee members, together with other senior managers, are eligible to receive bonuses under the Senior Management Bonus Scheme. Pursuant to the rules of the Senior Management Bonus Scheme, 50% of the bonus is paid immediately around year-end and the payment of the remaining 50% is deferred for three years. The deferred 50% component is dependent on the Galapagos share price change relative to the Next Biotech Index (which tracks Euronext-listed biotech companies). See notes 2 and 24 to the consolidated financial statements.
| G. | Safe Harbor. |
This annual report contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act and as defined in the Private Securities Litigation Reform Act of 1995. See “Special Note Regarding Forward-Looking Statements” at the beginning of this annual report.
| Item 6 | Directors, Senior Management and Employees. |
| A. | Directors and Senior Management. |
Our Board of Directors
We currently have eight directors, less than a majority of whom are citizens or residents of the United States.
Under our articles of association, our board of directors must be composed of between five and nine members, of which at least three are independent directors as defined by the Belgian Companies Code. Half of the members of our board of directors must be non-executive directors. Within these limits, the number of directors is determined by our shareholders. Directors are elected, re-elected and may be removed at a shareholders’ general meeting with a simple majority vote of our shareholders. Pursuant to our articles of association, our directors serve four-year terms.
The following table sets forth certain information with respect to the current members of our board of directors, including their ages, as of December 31, 2016:
| Name |
Age | Date service began in current term |
Date of expiration of current term (1) |
Position(s) | ||||||||||
| Onno van de Stolpe |
57 | 2013 | 2017 | Director and Chief Executive Officer | ||||||||||
| Rajesh Parekh, MA, DPhil(2) |
56 | 2013 | 2017 | Chairman of the board of directors | ||||||||||
| Harrold van Barlingen, Ph.D.(3) |
51 | 2014 | 2018 | Director | ||||||||||
| Werner Cautreels, Ph.D.(2)(3) |
64 | 2014 | 2018 | Director | ||||||||||
| Howard Rowe, JD(3) |
47 | 2014 | 2018 | Director | ||||||||||
| Katrine Bosley(2) |
48 | 2013 | 2017 | Director | ||||||||||
| Christine Mummery, Ph.D. |
63 | 2015 | 2019 | Director | ||||||||||
| Mary Kerr, Ph.D. |
56 | 2016 | 2020 | Director | ||||||||||
| (1) | The term of the mandates of the directors will expire immediately after the annual shareholders’ meeting held in the year set forth next to the director’s name. |
| (2) | Member of the nomination and remuneration committee. |
| (3) | Member of the audit committee. |
126
Table of Contents
The address for our directors is Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium.
Our board of directors has determined that seven out of eight of the members of the board are independent under the NASDAQ Stock Market listing requirements and that five out of eight of the members of the board of directors are independent under Belgian law.
The following is the biographical information of the members of our board of directors:
Onno van de Stolpe founded our company in 1999 and has served as our Chief Executive Officer and a member of our board of directors from 1999 to the present. From 1998 to 1999, he was the Managing Director of Genomics at IntroGene B.V. (later Crucell N.V., which was acquired by Johnson & Johnson Services, Inc. in 2011). Prior to joining IntroGene in 1998, he was Managing Director of Molecular Probes Europe B.V. He established this European headquarters after joining Molecular Probes, Inc. in the United States. Previously, he worked for The Netherlands Foreign Investment Agency in California, where he was responsible for recruiting biotechnology and medical device companies to locate in the Netherlands. Mr. Van de Stolpe started his career as Manager of Business Development at MOGEN International N.V. in Leiden. He received an MSc degree from Wageningen University. Mr. Van de Stolpe currently also serves as a member of the supervisory board of the Stichting Institute for Human Organ and Disease Model Technologies and has previously served as a member of the board of directors of DCPrime B.V.
Rajesh Parekh, MA, DPhil has served as the Chairman of our board of directors since 2004. Dr. Parekh is a General Partner at Advent Life Sciences LLP, which he joined in 2005. During an academic career at Oxford University, he co-founded Oxford GlycoSciences PLC, where he served as Chief Scientific Officer and Chief Executive Officer from 1988 until its sale to Celltech Group PLC (now UCB SA) in 2003. He has founded or served on the boards of several life sciences companies in the United States and Europe including Celldex Therapeutics, Inc.; Avila Therapeutics, Inc.; EUSA Pharma (Europe) Limited; Thiakis Limited; Biocartis NV; and Amsterdam Molecular Therapeutics (AMT) Holding N.V. (now uniQure). Dr. Parekh currently serves as a member of the board of directors of Advent Venture Partners; Advent Life Sciences LLP; Aleta Inc.; Arrakis, Inc.; Aura Inc.; Artax Inc.; Capella BioSciences Ltd.; Cellnovo Limited; Itara Ltd.; Levicept Limited; PE Limited; and Project Paradise Limited. He is also a member of the Supervisory Board of the Novartis Venture Fund. He received his MA in Biochemistry and DPhil in Molecular Medicine from the University of Oxford, where he has also been a Senior Research Fellow and Professor.
Harrold van Barlingen, Ph.D. has served as a member of our board of directors since 2005. Dr. Van Barlingen is the managing director and founder of Thuja Capital B.V., Thuja Capital Holding B.V. and Thuja Capital Management B.V. Prior to founding Thuja Capital, he headed the life sciences effort of AlpInvest Partners B.V. from 2001 to 2006, managing a portfolio of over 30 companies. Previously, he was at the Boston Consulting Group, or BCG, where he worked as a consultant in management and strategy from 1999 to 2002. Prior to BCG, Dr. Van Barlingen headed the continental activities of The Lewin Group (a Quintiles subsidiary), an internationally active firm specialized in the field of health economics. He holds an MSc in Medical Biology and a PhD in Medicine, both from Utrecht University. From 1991 to 1992 he was a visiting scientist at the University of Chicago. He is the author of a wide variety of peer-reviewed scientific and pharmaco-economics papers. He currently serves on the supervisory boards of Encare Biotech B.V., TheraSolve NV, Indigo Diabetes NV (chairman) (chairman), and Hemics B.V. (chairman). In addition, during the last five years he also served on the boards of Okapi Sciences NV and arGEN-X N.V.
Werner Cautreels, Ph.D. has served as a member of our board of directors since 2009. Dr. Cautreels is the President, Chief Executive Officer and member of the board of Selecta Biosciences, Inc. Previously, Dr. Cautreels joined Solvay Pharmaceuticals SA in 1998 where he was Global Head of R&D and later Global Chief Executive Officer from 2005 onwards, until it was acquired by Abbott Laboratories Inc. in February 2010. Prior to joining Solvay he was employed by Sanofi S.A., Sterling Winthrop, Inc. and Nycomed Amersham PLC in a variety of R&D management positions in Europe and in the United States from 1979 to 1998. Dr. Cautreels was a director of Innogenetics NV and ArQule, Inc. from 1999 until 2006 and of Seres Therapeutics Inc. from
127
Table of Contents
2012 until 2016. He was the President of the Belgian-Luxemburg Chamber of Commerce for Russia and Belarus until June 2010. He graduated from the University of Antwerp, with a Doctorate in Chemistry, specializing in mass spectrometry. He received his management and financial education from the Harvard Business School.
Howard Rowe, JD has served as a member of our board of directors since 2010. Mr. Rowe is Managing Director at Hayfin Capital Management LLP. Prior to joining Hayfin Capital Management, Mr. Rowe was a Managing Director with The Goldman Sachs Group, Inc. where he had multiple healthcare responsibilities over his 12 years at the firm. His most recent roles at Goldman Sachs were as part of the European Special Situations and Principal Strategies teams where he established and led the private healthcare investing effort. During that time he served on the boards of EUSA Pharma (Europe) Limited, Healthcare Brands International Limited, SmallBone Innovations, Inc., MedAvante, Inc. and Ikonisys, Inc. Prior to his investing activities, Mr. Rowe was a senior member of the European Healthcare Investment Banking team, where he advised numerous corporate clients on M&A and corporate finance activities. Before joining Goldman Sachs, he was a corporate lawyer with the law firm Sullivan & Cromwell LLP. Mr. Rowe received his Bachelor of Science in Psychobiology from the University of Southern California and his JD from Harvard Law School.
Katrine Bosley has served as a member of our board of directors since 2013. Ms. Bosley has served as the President, Chief Executive Officer and member of the board of directors of Editas Medicine, Inc. since June 2014. Prior to joining Editas, Ms. Bosley was the Entrepreneur-in-Residence at The Broad Institute from 2013 to 2014. From 2009 to 2012, Ms. Bosley was President, Chief Executive Officer and member of the board of directors of Avila Therapeutics, Inc., which was acquired by Celgene Corporation in 2012. Ms. Bosley served as President, Celgene Avilomics Research at Celgene in 2012. Prior to her time at Avila Therapeutics, Ms. Bosley was Vice President, Strategic Operations at Adnexus, a Bristol-Myers Squibb R&D Company, and was Vice President, Business Development at Adnexus Therapeutics, Inc. before that. Ms. Bosley joined Adnexus Therapeutics from Biogen Idec, Inc. where she had roles in business development, commercial operations and portfolio strategy in the United States and Europe. Earlier, she was part of the healthcare team at the venture firm Highland Capital Partners, Inc. Ms. Bosley graduated from Cornell University with a B.A. in Biology. Ms. Bosley currently serves as chairman of the board of Genocea Biosciences, Inc. and as a director of Scholar Rock, LLC. She also serves on the board of directors of the Biotechnology Innovation Organization and is a review committee member of the Wellcome Trust.
Christine Mummery, Ph.D. has served as a member of our board of directors since September 30, 2015. Dr. Mummery has served as a Professor of Developmental Biology and Chair of the Department of Anatomy and Embryology at the Leiden University Medical Centre (LUMC) since 2008 and a Professor of Vascular Modelling at the Technical University of Twente in the Netherlands since September 2015. In 2007, she was a Radcliffe fellow at the Harvard Stem Cell Institute and Massachusetts General Hospital when human-induced pluripotent stem cells were being developed, and she was the first to derive these from patients in the Netherlands. In 2002, she became a Professor at the Utrecht University Medical Centre in the Netherlands. She was a postdoctoral fellow from 1981 to 1984 at the Hubrecht Institute in Utrecht, where she later also served as a staff scientist and group leader until 2008. Dr. Mummery obtained her B.S. in Physics, Electronics, and Mathematics at the University of Nottingham and her Ph.D. in BioPhysics at London University in the United Kingdom. Her primary research focus is currently the development and use of stem cells in cardiovascular development and disease. She served on the Ethical Councils of the Dutch Ministry of Health, is member of the Royal Netherlands Academy of Arts and Sciences (KNAW), editor-in-chief of the Cell Press journal Stem Cell Reports, former board member of the International Society for Stem Cell Research and past-president of the International Society of Differentiation. She was co-founder of Pluriomics B.V. In addition, she is on the board of ZonMw (Dutch Medical Research Council) and chairs the executive board of the Institute for human Organ and Disease Model Technologies (hDMT), a non-profit R&D institute of which we are a founding partner. She is a review committee member of the European Research Council, the Wellcome Trust ( ad hoc ) and the Heineken Jury Prize (KNAW).
Mary Kerr, Ph.D. has served as a member of our board of directors since July 26, 2016. Dr. Kerr, a UK national, is Chief Executive Officer and director at NeRRe Therapeutics. Prior to her appointment at NeRRe,
128
Table of Contents
Dr. Kerr held a range of senior leadership roles at GSK over more than 20 years, most recently as Senior Vice President and Global Franchise leader for the Immuno-inflammation and Infectious Diseases franchise. Dr. Kerr was a founding member and on the Corporate Executive team of ViiV Healthcare where she led a turnaround in the performance of the HIV business in Europe. She has spent the majority of her career on the R&D commercial interface in global strategy and regional operational roles, predominantly in the specialty and orphan space. Dr. Kerr gained a Ph.D. in Pharmacology at the University of Bradford, did post-doctoral research at the Michigan Cancer Foundation in Detroit and has an MBA from the University of Kingston.
Executive Committee
Our board of directors has established an executive committee in accordance with article 524bis of the Belgian Companies Code. The following table sets forth certain information with respect to the members of our executive committee as of December 31, 2016:
| Name |
Age | Position(s) | ||||
| Onno van de Stolpe |
57 | Chief Executive Officer | ||||
| Piet Wigerinck, Ph.D. |
52 | Chief Scientific Officer | ||||
| Bart Filius, MBA |
46 | Chief Financial Officer | ||||
| Andre Hoekema, Ph.D. |
59 | Senior Vice President Corporate Development | ||||
The address for the members of our executive committee is Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium.
There is no potential conflict of interest between the private interests or other duties of the members of the executive committee listed above and their duties to us.
Below are the biographies of those members of our executive committee who do not also serve on our board of directors:
Piet Wigerinck, Ph.D. joined our company in April 2008 from Tibotec-Virco Comm. VA (a subsidiary of Johnson & Johnson Services, Inc.) where he was the Vice President, Drug Discovery, Early Development and CM&C, and a member of the Management Board. He started his professional career as a medicinal chemist at Janssen Research Foundation in 1992. He then joined Tibotec Group NV in 1998, where, under his leadership, TMC114 (Prezista™) and TMC435 (Olysio™) were selected and moved forward into clinical trials. Dr. Wigerinck played a key role in Tibotec’s expansion into novel diseases such as Hepatitis C and advanced several compounds into Phase 1 and Phase 2 clinical trials. He brings over 25 years of R&D experience from both large pharmaceutical companies and biotechnology companies to our company. Dr. Wigerinck holds a Ph.D. from the K.U. Leuven and is inventor on more than 25 patent applications.
Bart Filius, MBA has served as our Chief Financial Officer since December 2014. Prior to that, Mr. Filius worked over 13 years at Sanofi S.A., where he was the Chief Financial Officer of Sanofi Europe during the last three years. Earlier at Sanofi, Mr. Filius was the Country Manager and Chief Financial Officer of Sanofi in the Netherlands. Before that, he was Vice President for Mergers & Acquisitions, during which time Mr. Filius led and completed the divestiture of various franchises. Prior to joining Sanofi, Bart was a strategy consultant at Arthur D. Little. Mr. Filius has an MBA degree from INSEAD and a bachelor’s degree in business from Nyenrode Business University.
Andre Hoekema, Ph.D. is responsible for M&A, licensing and Intellectual Property at Galapagos. He had the lead in rolling out our pharmaceutical alliance strategy since its start in 2006, and is the architect of our collaboration with AbbVie in CF. Dr. Hoekema joined Galapagos in March 2005 from Invitrogen Corporation, where he was Managing Director of Corporate Development Europe, overseeing licensing and M&A for Invitrogen Europe. He brings 30 years of biotech experience from positions at Molecular Probes Europe B.V. (Managing Director of the European office), Crucell N.V. (Director of Business Development and Intellectual
129
Table of Contents
Property), Koninklijke DSM N.V., MOGEN International N.V. (Research and Project Management), and Genentech, Inc. (postdoctoral researcher). Dr. Hoekema studied Chemistry and holds a Ph.D. from Leiden University. During his Ph.D. work, he invented the binary vector system for the genetic modification of plants, which he published in Nature in 1983; this has since then become the global standard in the field of agricultural biotech. He is the author of more than 30 peer-reviewed scientific papers, and an inventor of over 20 series of patent applications, resulting in 15 patents issued in the United States. Dr. Hoekema currently also serves as a member of the supervisory board of Mimetas B.V. and has previously served as a member of the supervisory board of VitalNext B.V.
On March 1, 2017, Dr. Walid Abi-Saab joined our company as Chief Medical Officer and member of the executive committee.
The executive committee exercises the powers delegated to it by the board of directors, such powers not being related to the general strategy of the company or to other actions which are reserved for the board of directors according to legal requirements, articles of association or the corporate governance charter of the company.
The tasks of the executive committee include the following matters: the research, identification and development of strategic possibilities and proposals which may contribute to our company’s development in general, the drafting and development of policy guidelines to be approved by our board of directors, our company’s management through, among other things, the implementation of policy guidelines, the supervision of the performance of the business in comparison with the strategic goals, plans and budgets, and the support of the chief executive officer with the day-to-day management of our company.
Notwithstanding the above, and according to its “evocation right,” our board of directors retains the right to deliberate and decide on matters which have in principle been delegated to our executive committee, but for which our board of directors is of the opinion that they require deliberation at the board of directors’ level.
Family Relationships
There are no family relationships among any of the members of our executive committee or directors.
| B. | Compensation. |
The aggregate compensation paid and benefits in kind granted by us to our current members of the executive committee and directors, excluding share-based compensation, for the year ended December 31, 2016, was €3,455,648.97. For the year ended December 31, 2016, the total amounts set aside or accrued to provide pension, retirement or similar benefits to our executive committee amounted to €227,554.91.
For a discussion of our employment arrangements with the members of our executive committee and consulting arrangement with our directors, see the section of this annual report titled “Item 7.B.—Related Party Transactions—Agreements with Our Directors and Members of Executive Committee.” For more information regarding warrant grants, see “—Warrant Plans” below.
Compensation of Our Board of Directors
The remuneration of our directors (other than our chief executive officer) and the grant of warrants to our directors is submitted by our board of directors for approval to the shareholders’ meeting and is only implemented after such approval. The procedure for establishing the remuneration policy and setting remuneration for members of our board of directors is determined by our board of directors on the basis of proposals from the nomination and remuneration committee, taking into account relevant benchmarks from the biotechnology industry.
130
Table of Contents
The annual shareholders’ meeting of April 26, 2016 determined, upon recommendation of the nomination and remuneration committee, that the compensation (excluding expenses) of the non-executive directors for the exercise of their mandate during the financial year ending December 31, 2016 is as follows: (i) Chairman of the Board (i.e. Raj Parekh): €80,000; (ii) other non-executive board members (i.e., Werner Cautreels, Harrold van Barlingen, Howard Rowe, Katrine Bosley and Christine Mummery): €40,000 each; (iii) annual additional compensation for membership of a board committee (audit committee: Harrold van Barlingen and Howard Rowe; nomination and remuneration committee: Werner Cautreels and Katrine Bosley): €5,000; (iv) annual additional compensation for the chairmanship of a board committee (audit committee: Werner Cautreels; nomination and remuneration committee: Rajesh Parekh): €10,000. The same annual shareholders’ meeting granted a power of attorney to our board of directors to determine the total remuneration package of our managing director (CEO) for his management function in Galapagos. The special shareholders’ meeting of July 26, 2016, which resolved upon the appointment of Mary Kerr as a non-executive director of Galapagos NV determined that the remuneration principles for the financial year ending on December 31, 2016 approved by the annual shareholders’ meeting of April 26, 2016 shall apply to determine the compensation (excluding expenses) of Ms. Kerr, pro rata temporis, for the period starting on the date of her appointment and ending on December 31, 2016. Directors representing a shareholder on the board of directors would only receive reimbursement of the expenses incurred for participating in the board of directors (there were no such directors in 2016, nor are there currently).
The remuneration of the non-executive directors does not contain a variable part; hence no performance criteria apply to the remuneration of the non-executive directors.
The following table sets forth the fees (excluding expenses) received by our non-executive directors for the performance of their mandate as a board member, during the year ended December 31, 2016:
| Name |
Fees earned (€) |
|||
| Rajesh Parekh(1) |
69,736.53 | |||
| Harrold van Barlingen |
45,000 | |||
| Werner Cautreels |
55,000 | |||
| Howard Rowe |
45,000 | |||
| Christine Mummery |
40,000 | |||
| Katrine Bosley |
45,000 | |||
| Mary Kerr(2) |
17,282.31 | |||
| Total |
317,018.84 | |||
| (1) | During the first four months of 2016, Dr. Parekh did not receive remuneration for his director’s mandate, but was compensated through a consultancy agreement with Parekh Enterprises only (consultancy fee of €20,263 in 2016). |
| (2) | Mary Kerr joined our board of directors effective July 26, 2016. |
In addition to the benefits set forth above, our non-executive directors also received benefits consisting of tax advisory services in 2016 for an amount of €14,495.
Our executive director, Onno van de Stolpe, does not receive any specific or additional remuneration for his service on our board of directors, as this is included in his total remuneration package in his capacity as member of our executive committee. For more information regarding Mr. Van de Stolpe’s compensation, see “—Compensation of Members of the Executive Committee” below.
131
Table of Contents
The table below provides an overview as of December 31, 2016 of the warrants held by the non-executive directors.
| Warrant award | ||||||||||||
| Name |
Number of ordinary shares underlying warrants |
Warrant exercise price (€) |
Warrant expiration date |
|||||||||
| Rajesh Parekh |
5,400 | 19.38 | 5/15/2021 | |||||||||
| 5,400 | 14.54 | 7/24/2022 | ||||||||||
| 5,400 | 28.75 | 4/29/2023 | ||||||||||
| 15,000 | 49.00 | 12/21/2023 | ||||||||||
| 15,000 | 46.10 | 5/31/2024 | ||||||||||
|
|
|
|||||||||||
| Total |
46,200 | |||||||||||
|
|
|
|||||||||||
| Harrold van Barlingen |
2,520 | 14.19 | 9/2/2020 | |||||||||
| 2,520 | 19.38 | 5/15/2021 | ||||||||||
| 2,520 | 14.54 | 7/24/2022 | ||||||||||
| 2,520 | 28.75 | 4/29/2023 | ||||||||||
| 7,500 | 49.00 | 12/21/2023 | ||||||||||
| 7,500 | 46.10 | 5/31/2024 | ||||||||||
|
|
|
|||||||||||
| Total |
25,080 | |||||||||||
|
|
|
|||||||||||
| Werner Cautreels |
3,780 | 19.38 | 5/15/2021 | |||||||||
| 3,780 | 14.54 | 7/24/2022 | ||||||||||
| 3,780 | 28.75 | 4/29/2023 | ||||||||||
| 7,500 | 49.00 | 12/21/2023 | ||||||||||
| 7,500 | 46.10 | 5/31/2024 | ||||||||||
|
|
|
|||||||||||
| Total |
26,340 | |||||||||||
|
|
|
|||||||||||
| Howard Rowe |
2,520 | 14.19 | 9/2/2020 | |||||||||
| 2,520 | 19.38 | 5/15/2021 | ||||||||||
| 2,520 | 14.54 | 7/24/2022 | ||||||||||
| 2,520 | 28.75 | 4/29/2023 | ||||||||||
| 7,500 | 49.00 | 12/21/2023 | ||||||||||
| 7,500 | 46.10 | 5/31/2024 | ||||||||||
|
|
|
|||||||||||
| Total |
25,080 | |||||||||||
|
|
|
|||||||||||
| Katrine Bosley |
7,500 | 19.38 | 5/15/2021 | |||||||||
| 2,520 | 14.54 | 7/24/2022 | ||||||||||
| 2,520 | 28.75 | 4/29/2023 | ||||||||||
| 7,500 | 49.00 | 12/21/2023 | ||||||||||
| 7,500 | 46.10 | 5/31/2024 | ||||||||||
|
|
|
|||||||||||
| Total |
27,540 | |||||||||||
|
|
|
|||||||||||
| Christine Mummery |
7,500 | 49.00 | 12/21/2023 | |||||||||
| 7,500 | 46.10 | 5/31/2024 | ||||||||||
|
|
|
|||||||||||
| Total |
15,000 | |||||||||||
|
|
|
|||||||||||
Mary Kerr did not hold any warrants as of December 31, 2016.
No loans, quasi-loans or other guarantees were given to the non-executive directors during the year ended December 31, 2016.
132
Table of Contents
Compensation of Members of the Executive Committee
The compensation of the members of our executive committee is determined by our board of directors based on the recommendations by our nomination and remuneration committee.
The remuneration of the members of our executive committee consists of different components:
| • | Fixed remuneration: a basic fixed fee designed to fit responsibilities, relevant experience and competences, in line with market rates for equivalent positions. The amount of fixed remuneration is evaluated and determined by the board of directors every year, upon recommendation of the nomination and remuneration committee. |
| • | Variable remuneration (short-term and long-term): members of the executive committee may be entitled to a bonus, depending on the level of achievement of the criteria from the Senior Management Bonus Scheme (i.e., corporate objective for that year). The maximum bonus of the chief executive officer is set at 100% of his yearly fixed salary. The actual bonus of the chief executive officer is determined by our board of directors, upon recommendation of the nomination and remuneration committee, and is based on the achievement of corporate and individual objectives. The maximum aggregate bonus pot for the other members of the executive committee is set at 75% of their combined salaries. The actual bonuses of these executive officers are determined by our board of directors, upon recommendation of the nomination and remuneration committee, and are based on the achievement of corporate and individual objectives. In addition, exceptional special bonuses, outside the scope of the regular bonus schemes, can be considered by the board of directors, upon recommendation of the nomination and remuneration committee, in the event of and for exceptional achievements. For each year, 50% of this variable remuneration is paid in early January of the following year, and the other 50% is deferred for three years and is adjusted in light of the change of the company’s share price relative to the Euronext Next Biotech Index. |
| • | Incentive plan: warrants have been granted and may be granted in the future, to the members of the executive committee. For a description of the main characteristics of our warrant plans, see “—Warrant Plans” below. |
| • | Other: our pension, company car, tax advisory services and payments for invalidity and healthcare cover and other fringe benefits of non-material value. |
No loans, quasi-loans or other guarantees were given to members of our executive committee during the year ended December 31, 2016.
The following table sets forth information regarding compensation earned by Onno van de Stolpe, our chief executive officer, during the year ended December 31, 2016.
| Compensation (€) |
||||
| Fixed remuneration (gross) |
453,407.56 | |||
| Variable remuneration (short-term)(1) |
236,133.50 | |||
| Variable remuneration (long-term)(2) |
652,974.18 | |||
| Pension/life |
62,843.00 | |||
| Other benefits |
39,384.25 | |||
| Total |
1,444,742.49 | |||
| (1) | 50% of the performance bonus for the year 2016, paid in January 2017. The remaining 50% is deferred for three years and is adjusted in light of the change of our company’s share price relative to the Euronext Next Biotech Index. |
| (2) | The value of the 50% deferred part of the bonus awarded over 2013 was established at the end of 2016 and resulted in a payment in early January 2017 of an amount of €652,974.18 (a multiple of 4.01 of the deferred |
133
Table of Contents
| bonus, as a result of the share price performance over the period 2013–2016 as per the provisions of the Senior Management Bonus Scheme). |
In addition, Mr. Van de Stolpe was granted (and accepted) 100,000 warrants under Warrant Plan 2016. The exercise price of these warrants is €46.10. These warrants are exercisable as from January 1, 2020.
The following table sets forth information concerning the aggregate compensation earned during the year ended December 31, 2016 by the other current members of our executive committee.
| Compensation (€) |
||||
| Fixed remuneration (gross) |
838,435.00 | |||
| Variable remuneration (short-term)(1) |
336,913.00 | |||
| Variable remuneration (long-term)(2) |
521,192.63 | |||
| Pension/life |
164,711.91 | |||
| Other benefits |
45,695.00 | |||
| Total |
1,906,947.54 | |||
| (1) | 50% of the performance bonus for the year 2016, paid in January 2017. The remaining 50% is deferred for three years and is adjusted in light of the change of our company’s share price relative to the Euronext Next Biotech Index. |
| (2) | The value of the 50% deferred part of the bonus awarded over 2013 was established at the end of 2016 and resulted in a payment in early January 2017 of an amount of €521,192.63 (a multiple of 4.01 of the deferred bonus, as a result of the share price performance over the period 2013–2016 as per the provisions of the Senior Management Bonus Scheme). |
In addition, the other members of the executive committee were granted (and accepted) an aggregate amount of 175,000 warrants under Warrant Plan 2016, with an exercise price of €46.10.
134
Table of Contents
The table below provides an overview as of December 31, 2016 of the warrants held by the members of our executive committee.
| Warrant awards | ||||||||||||
| Name |
Number of ordinary shares underlying warrants |
Warrant exercise price (€) |
Warrant expiration date |
|||||||||
| Onno van de Stolpe |
90,000 | 6.91 | 7/3/2018 | |||||||||
| 16,874 | 8.65 | 6/27/2020 | ||||||||||
| 100,000 | 14.19 | 9/2/2020 | ||||||||||
| 100,000 | 19.38 | 5/15/2021 | ||||||||||
| 100,000 | 14.54 | 7/24/2022 | ||||||||||
| 100,000 | 28.75 | 4/29/2023 | ||||||||||
| 100,000 | 49.00 | 12/21/2023 | ||||||||||
| 100,000 | 46.10 | 5/31/2024 | ||||||||||
|
|
|
|||||||||||
| Total |
706,874 | |||||||||||
|
|
|
|||||||||||
| Other officers |
25,000 | 6.76 | 2/1/2017 | |||||||||
| 12,500 | 8.60 | 12/14/2018 | ||||||||||
| 30,000 | 8.65 | 6/27/2020 | ||||||||||
| 77,500 | 5.60 | 6/25/2021 | ||||||||||
| 35,000 | 11.55 | 4/26/2018 | ||||||||||
| 50,000 | 9.95 | 5/22/2019 | ||||||||||
| 70,000 | 14.19 | 9/2/2020 | ||||||||||
| 50,000 | 19.38 | 5/15/2021 | ||||||||||
| 80,000 | 14.54 | 7/24/2022 | ||||||||||
| 150,000 | 11.93 | 10/13/2022 | ||||||||||
| 75,000 | 28.75 | 4/29/2023 | ||||||||||
| 140,000 | 49.00 | 12/21/2023 | ||||||||||
| 175,000 | 46.10 | 5/31/2024 | ||||||||||
|
|
|
|||||||||||
| Total |
970,000 | |||||||||||
|
|
|
|||||||||||
Limitations on Liability and Indemnification Matters
Under Belgian law, the directors of a company may be liable for damages to the company in case of improper performance of their duties. Our directors may be liable to our company and to third parties for infringement of our articles of association or Belgian company law. Under certain circumstances, directors may be criminally liable.
We maintain liability insurance for our directors and officers, including insurance against liability under the Securities Act.
Certain of our non-executive directors may, through their relationships with their employers or partnerships, be insured and/or indemnified against certain liabilities in their capacity as members of our board of directors.
In the underwriting agreement we entered into in connection with our May 2015 global offering, the underwriters agreed to indemnify, under certain conditions, us, the members of our board of directors and persons who control our company within the meaning of the Securities Act against certain liabilities, but only to the extent that such liabilities are caused by information relating to the underwriters furnished to us in writing expressly for use in our registration statement and certain other disclosure documents.
135
Table of Contents
Warrant Plans
We have established a number of warrant plans, under which we have granted warrants free of charge to the recipients, i.e., employees, directors and independent consultants of our company. For warrant plans issued prior to 2011, the warrants offered to the employees and independent consultants vest according to the following schedule: 10% of the warrants vest on the date of the grant; an additional 10% vest at the first anniversary of the grant; an additional 20% vest at the second anniversary of the grant; an additional 20% vest at the third anniversary of the grant; and an additional 40% vest at the end of the third calendar year following the grant. The warrants granted under warrant plans created from 2011 up to (and including) Warrant Plan 2016 and Warrant Plan 2016 RMV, with the exception of Warrant Plan 2015 (B) and Warrant Plan 2015 RMV, vest at the end of the third calendar year following the year of the grant, with no intermediate vesting. The warrants granted under Warrant Plan 2015 (B) and Warrant Plan 2015 RMV vest on the third anniversary of the notary deed enacting the acceptance of the warrants. The warrants offered to directors vest over a period of 36 months at a rate of 1/36th per month. Warrants cannot be exercised before the end of the third calendar year following the year of the grant, except for warrants granted under Warrant Plan 2015 (B) and Warrant Plan 2015 RMV, which become exercisable on the third anniversary of the notary deed enacting the acceptance of the warrants. Pursuant to a resolution adopted at the extraordinary general shareholders’ meeting held on May 23, 2011, a provision has been incorporated in the warrant plans, which provides that in the event of a change of control of our company, all outstanding warrants vest immediately and will be immediately exercisable.
After the reverse 4:1 share split approved by the shareholders’ meeting held on March 29, 2005, four warrants under Warrant Plan 2002 Belgium entitle the warrant holder to subscribe for one ordinary share. For the warrant plans created from 2005 onwards, one warrant entitles the warrant holder to subscribe for one ordinary share. In the summaries and tables below, the numbers of warrants issued under Warrant Plan 2002 Belgium are divided by four to avoid a mixture of rights.
Generally, unless our board of directors at the time of the grant of the warrant determines a higher exercise price, the exercise price of a warrant will at least be equal to:
| • | the last closing price of our ordinary shares on Euronext Amsterdam prior to the date on which the warrant is offered; or |
| • | the average closing price of our ordinary shares on Euronext Amsterdam over the thirty-day period preceding the date on which the warrant is offered. |
For beneficiaries of the warrant plan that are not employees of our company, the exercise price cannot be lower than the average closing price of our ordinary shares on Euronext Amsterdam over the thirty-day period preceding the date of the offer of the warrants.
However, for the warrants offered under Warrant Plan 2002 Belgium, since the ordinary shares of our company were not yet traded or listed on a stock exchange at the time of the relevant offers, the exercise price was to be determined by our board of directors at the time of the offer and had to be at least equal to the market value of the former Class D shares, as determined by the board of directors and as certified by the auditor of our company. In addition, the exercise price could not be lower than (1) the book value of the existing shares as appearing from the last approved annual accounts of the company at the date of the offer and (2) €1.
Since 2002 until December 31, 2016, an aggregate of 8,514,467 warrants were granted. Of these 8,514,467 warrants:
| • | 147,112 warrants lapsed because they were not timely exercised by their beneficiaries; |
| • | 1,188,433 warrants lapsed due to their beneficiaries no longer being employed by the company or because another condition for vesting was not met; and |
| • | 3,712,515 warrants have been exercised. |
136
Table of Contents
As a result, as of December 31, 2016, there were 3,466,407 warrants outstanding which represent approximately 7.5% of the total number of all our issued and outstanding voting financial instruments.
The table below sets forth the details of all warrants granted under the warrant plans in force as per December 31, 2016, including the plan under which the warrants were granted, the offer date, exercise price, expiry date, number of warrants exercised, number of warrants voided and number of warrants outstanding. Aside from the warrants set forth in the below table, there are currently no other stock options, options to purchase securities, convertible securities or other rights to subscribe for or purchase outstanding securities.
| Warrant plan |
Offer date | Exercise price (€) |
Number of warrants granted |
Number of warrants exercised |
Number of warrants voided |
Number of warrants still outstanding |
Exercisable from |
Expiry date | ||||||||||||||||||||||||
| 2002 Belgium |
3/6/2002 | 4.00 | 553,705 | 423,698 | 130,007 | — | 1/1/2006 | 3/6/2010 | ||||||||||||||||||||||||
| 9/2/2002 | 4.00 | 27,125 | 14,150 | 12,975 | — | 1/1/2006 | 9/2/2010 | |||||||||||||||||||||||||
| 3/6/2003 | 4.00 | 5,250 | 1,287 | 3,963 | — | 1/1/2007 | 3/31/2007 | |||||||||||||||||||||||||
| 4/1/2003 | 4.00 | 7,500 | 7,500 | — | — | 1/1/2007 | 4/1/2011 | |||||||||||||||||||||||||
| 6/15/2004 | 4.00 | 2,000 | 2,000 | — | — | 1/1/2008 | 6/15/2012 | |||||||||||||||||||||||||
| 7/9/2004 | 4.00 | 31,250 | 31,250 | — | — | 1/1/2008 | 2/1/2017 | |||||||||||||||||||||||||
| 7/22/2004 | 4.00 | 7,500 | — | 7,500 | — | 1/1/2008 | 3/31/2008 | |||||||||||||||||||||||||
| 1/31/2005 | 6.76 | 159,375 | 90,000 | 44,375 | 25,000 | 1/1/2009 | 2/1/2017 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
793,705 | 569,885 | 198,820 | 25,000 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2005 |
7/4/2005 | 6.91 | 145,000 | 55,000 | — | 90,000 | 1/1/2009 | 7/3/2018 | ||||||||||||||||||||||||
| 11/23/2005 | 8.35 | 125,000 | 75,000 | 50,000 | — | 1/1/2009 | 11/22/2018 | |||||||||||||||||||||||||
| 12/15/2005 | 8.60 | 12,500 | — | — | 12,500 | 1/1/2009 | 12/14/2018 | |||||||||||||||||||||||||
| 2/13/2006 | 8.61 | 40,000 | 8,000 | 32,000 | — | 1/1/2010 | 3/31/2010 | |||||||||||||||||||||||||
| 2/13/2006 | 8.73 | 53,500 | 50,972 | 2,528 | — | 1/1/2010 | 3/31/2010 | |||||||||||||||||||||||||
| 11/22/2006 | 8.65 | 82,600 | 61,285 | 21,315 | — | 1/1/2010 | 11/21/2019 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
458,600 | 250,257 | 105,843 | 102,500 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2006 BNL |
2/13/2006 | 8.61 | 112,953 | 100,662 | 12,291 | — | 1/1/2010 | 2/12/2019 | ||||||||||||||||||||||||
| 11/22/2006 | 8.65 | 87,090 | 16,450 | 70,640 | — | 1/1/2010 | 11/21/2019 | |||||||||||||||||||||||||
| 2/14/2007 | 9.57 | 102,900 | 9,170 | 93,730 | — | 1/1/2011 | 08/31/2011 | |||||||||||||||||||||||||
| 5/4/2007 | 9.22 | 17,500 | 10,000 | — | 7,500 | 1/1/2011 | 5/3/2020 | |||||||||||||||||||||||||
| 6/28/2007 | 8.65 | 735 | — | — | 735 | 1/1/2011 | 6/27/2020 | |||||||||||||||||||||||||
| 12/21/2007 | 7.12 | 25,110 | 12,121 | 11,939 | 1,050 | 1/1/2011 | 12/20/2020 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
346,288 | 148,403 | 188,600 | 9,285 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2006 UK |
6/1/2006 | 8.70 | 302,191 | 230,963 | 71,228 | — | 1/1/2010 | 9/30/2014 | ||||||||||||||||||||||||
| 11/22/2006 | 8.65 | 13,965 | 11,907 | 2,058 | — | 1/1/2010 | 11/21/2014 | |||||||||||||||||||||||||
| 12/19/2006 | 9.18 | 77,700 | 31,885 | 45,815 | — | 1/1/2010 | 12/18/2014 | |||||||||||||||||||||||||
| 6/28/2007 | 8.43 | 30,585 | 20,085 | 10,500 | — | 1/1/2011 | 6/27/2015 | |||||||||||||||||||||||||
| 12/21/2007 | 7.25 | 945 | 945 | — | — | 1/1/2011 | 12/20/2015 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
425,386 | 295,785 | 129,601 | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2007 |
6/28/2007 | 8.65 | 108,126 | 108,126 | — | — | 1/1/2011 | 6/27/2015 | ||||||||||||||||||||||||
| 6/28/2007 | 8.65 | 256,314 | 154,232 | 53,173 | 48,909 | 1/1/2011 | 6/27/2020 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
364,440 | 262,358 | 53,173 | 48,909 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2007 RMV |
10/25/2007 | 8.65 | 108,850 | 66,300 | 4,900 | 37,650 | 1/1/2011 | 10/24/2020 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
108,850 | 66,300 | 4,900 | 37,650 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2008 |
6/26/2008 | 5.60 | 201,445 | 114,519 | 7,326 | 79,600 | 1/1/2012 | 6/25/2021 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
201,445 | 114,519 | 7,326 | 79,600 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
137
Table of Contents
| Warrant plan |
Offer date | Exercise price (€) |
Number of warrants granted |
Number of warrants exercised |
Number of warrants voided |
Number of warrants still outstanding |
Exercisable from |
Expiry date | ||||||||||||||||||||||||
| 2008 (B) |
6/26/2008 | 5.60 | 57,500 | 50,000 | 7,500 | — | 1/1/2012 | 6/25/2013 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
57,500 | 50,000 | 7,500 | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2009 |
4/1/2009 | 5.87 | 555,000 | 482,500 | 65,000 | 7,500 | 1/1/2013 | 3/31/2017 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
555,000 | 482,500 | 65,000 | 7,500 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2009 (B) |
6/2/2009 | 7.09 | 135,100 | 131,670 | 3,430 | — | 1/1/2013 | 6/1/2014 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
135,100 | 131,670 | 3,430 | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2010 |
4/27/2010 | 11.55 | 466,500 | 363,750 | 49,750 | 53,000 | 1/1/2014 | 4/26/2018 | ||||||||||||||||||||||||
| 4/27/2010 | 11.55 | 40,000 | 40,000 | — | — | 4/27/2014 | 4/26/2018 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
506,500 | 403,750 | 49,750 | 53,000 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2010 (B) |
4/27/2010 | 11.55 | 195,040 | 190,108 | 4,932 | — | 1/1/2014 | 4/26/2015 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
195,040 | 190,108 | 4,932 | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2010 (C) |
12/23/2010 | 11.74 | 75,000 | 75,000 | — | — | 1/1/2014 | 12/22/2018 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
75,000 | 75,000 | — | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2011 |
5/23/2011 | 9.95 | 561,500 | 375,000 | 129,000 | 57,500 | 1/1/2015 | 5/22/2019 | ||||||||||||||||||||||||
| 5/23/2011 | 9.95 | 57,500 | 48,400 | 7,500 | 1,600 | 5/23/2015 | 5/22/2019 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
619,000 | 423,400 | 136,500 | 59,100 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2011 (B) |
5/23/2011 | 9.95 | 129,220 | 127,750 | 1,470 | — | 1/1/2015 | 5/22/2016 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
129,220 | 127,750 | 1,470 | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2012 |
9/3/2012 | 14.19 | 448,640 | 110,830 | 103,150 | 234,660 | 1/1/2016 | 9/2/2020 | ||||||||||||||||||||||||
| 9/3/2012 | 14.19 | 32,500 | 10,000 | 10,000 | 12,500 | 9/3/2016 | 9/2/2020 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
481,140 | 120,830 | 113,150 | 247,160 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2013 |
5/16/2013 | 19.38 | 602,790 | — | 170,550 | 432,240 | 1/1/2017 | 5/15/2021 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
602,790 | — | 170,550 | 432,240 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2013 (B) |
9/18/2013 | 15.18 | 75,000 | — | 45,000 | 30,000 | 1/1/2017 | 6/30/2017 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
75,000 | — | 45,000 | 30,000 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2014 |
7/25/2014 | 14.54 | 571,660 | — | 35,000 | 536,660 | 1/1/2018 | 7/24/2022 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
571,660 | — | 35,000 | 536,660 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2014 (B) |
10/14/2014 | 11.93 | 150,000 | — | — | 150,000 | 1/1/2018 | 10/13/2022 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
150,000 | — | — | 150,000 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2015 |
4/30/2015 | 28.75 | 532,053 | — | 15,000 | 517,053 | 1/1/2019 | 4/29/2023 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
532,053 | — | 15,000 | 517,053 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2015 (B) |
12/22/2015 | 49.00 | 399,000 | — | — | 399,000 | 03/02/2019 | 12/21/2023 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
399,000 | — | — | 399,000 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
138
Table of Contents
| Warrant plan |
Offer date | Exercise price (€) |
Number of warrants granted |
Number of warrants exercised |
Number of warrants voided |
Number of warrants still outstanding |
Exercisable from |
Expiry date | ||||||||||||||||||||||||
| 2015 RMV |
12/22/2015 | 49.00 | 97,500 | — | — | 97,500 | 03/02/2019 | 12/21/2023 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
97,500 | — | — | 97,500 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2016 |
06/01/2016 | 46.1 | 514,250 | — | — | 514,250 | 01/01/2020 | 05/31/2024 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
514,250 | — | — | 514,250 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| 2016 RMV |
06/01/2016 | 46.1 | 120,000 | — | — | 120,000 | 01/01/2020 | 05/31/2024 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total |
120,000 | — | — | 120,000 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Grand Total |
8,514,467 | 3,712,515 | 1,335,545 | 3,466,407 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| C. | Board Practices. |
Our board of directors can set up specialized committees to analyze specific issues and advise the board of directors on those issues. Except for our executive committee, the committees are advisory bodies only and the decision-making remains within the collegial responsibility of the board of directors. The board of directors determines the terms of reference of each committee with respect to the organization, procedures, policies and activities of the committee.
Our board of directors has set up and appointed an executive committee, an audit committee and a nomination and remuneration committee. The composition and function of all of our committees will comply with all applicable requirements of the Belgian Companies Code, the Exchange Act, the exchanges on which the ordinary shares and ADSs are listed and SEC rules and regulations.
Except the arrangements described in the section of this annual report titled “Item 7.B.—Related-Party Transactions—Agreements with Our Directors and Members of Executive Committee,” there are no arrangements or understanding between us and any of the members of our executive committee or directors providing for benefits upon termination of their employment, other than as required by applicable law. For information regarding the expiration of our directors’ current terms of office and the period each director has served in that office, see “Item 6.A.—Directors and Senior Management—Our Board of Directors.”
Director Independence
As a foreign private issuer, under the listing requirements and rules of NASDAQ, we are not required to have independent directors on our board of directors, except that our audit committee is required to consist fully of independent directors, subject to certain phase-in schedules. However, our board of directors has determined that, under current listing requirements and rules of NASDAQ and taking into account any applicable committee independence standards, Rajesh Parekh, Harrold van Barlingen, Werner Cautreels, Howard Rowe, Katrine Bosley, Christine Mummery and Mary Kerr are “independent directors.” In making such determination, our board of directors considered the relationships that each non-executive director has with us and all other facts and circumstances our board of directors deemed relevant in determining the director’s independence, including the number of ordinary shares beneficially owned by the director and his or her affiliated entities (if any).
The independence criteria under the applicable NASDAQ Stock Market Listing Rules differ from the independence criteria set forth in Article 526ter of the Belgian Companies Code. Under Article 526ter of the Belgian Companies Code, Werner Cautreels, Howard Rowe, Katrine Bosley, Christine Mummery and Mary Kerr are “independent directors.”
139
Table of Contents
Role of the Board in Risk Oversight
Our board of directors is responsible for the oversight of our risk management activities and has delegated to the audit committee the responsibility to assist our board in this task. While our board oversees our risk management, our management is responsible for day-to-day risk management processes. Our board of directors expects our management to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively implement risk management strategies adopted by the board of directors. We believe this division of responsibilities is the most effective approach for addressing the risks we face.
Corporate Governance Practices
Along with our articles of association, we adopted a corporate governance charter in accordance with the recommendations set out in the Belgian Corporate Governance Code issued on March 12, 2009 by the Belgian Corporate Governance Committee. The Belgian Corporate Governance Code is based on a “comply or explain” system: Belgian listed companies are expected to follow the Belgian Corporate Governance Code, but can deviate from specific provisions and guidelines (though not the principles) provided they disclose the justification for such deviations.
Our board of directors complies with the Belgian Corporate Governance Code, but believes that certain deviations from its provisions are justified in view of our particular situation. These deviations include the grant of warrants to non-executive directors. In this way, we have additional possibilities to attract competent non- executive directors and to offer them an attractive additional remuneration without the consequence that this additional remuneration weighs on our financial results. Furthermore, the grant of warrants is a commonly used method in the sector in which we operate. Without this possibility, we would be subject to a considerable disadvantage compared to competitors who do offer warrants to their non-executive directors. Our board of directors is of the opinion that the grant of warrants has no negative impact on the functioning of the non-executive directors.
Our board of directors reviews its corporate governance charter from time to time and makes such changes as it deems necessary and appropriate. Additionally, our board of directors adopted written terms of reference for each of the executive committee, the audit committee and the nomination and remuneration committee, which are part of the corporate governance charter.
Board Committees
The board of directors has established an audit committee and a nomination and remuneration committee, which operate pursuant to the written terms of reference for each of the audit committee and the nomination and remuneration committee that are part of the corporate governance charter adopted by our board of directors. The composition and functioning of all of our committees will comply with all applicable requirements of the Belgian Companies Code and the Belgian Corporate Governance Code, the Exchange Act, the exchange on which the ADSs are listed, and SEC rules and regulations, taking into account the differences set out below and the company’s status as a foreign private issuer.
The Listing Rules of the NASDAQ Stock Market include certain accommodations in the corporate governance requirements that allow foreign private issuers, to follow “home country” corporate governance practices in lieu of the otherwise applicable corporate governance standards of the NASDAQ Stock Market. The application of such exceptions requires that we disclose each of the NASDAQ Stock Market Listing Rules that we do not follow and describe the Belgian corporate governance practices we do follow in lieu of the relevant NASDAQ Stock Market corporate governance standard.
140
Table of Contents
We follow Belgian corporate governance practices in lieu of the corporate governance requirements of the NASDAQ Stock Market in respect of the following rules applicable to board committees:
| • | Compensation Committee. NASDAQ Stock Market Listing Rule 5605(d)(2) requires that compensation of officers must be determined by, or recommended to, the board of directors for determination, either by a majority of the independent directors, or a compensation committee comprised solely of independent directors. NASDAQ Stock Market Listing Rule 5605(e) requires that director nominees be selected, or recommended for selection, either by a majority of the independent directors or a nominations committee comprised solely of independent directors. Under Belgian law, we are not subject to such composition requirements. Pursuant to Article 526quater of the Belgian Companies Code and the principles and guidelines of the Belgian Corporate Governance Code, we are required to set up a remuneration committee within our board of directors. In addition, the Belgian Corporate Governance Code provides that the board of directors should set up a nomination committee, which can be combined with the remuneration committee. Our board of directors has set up and appointed a nomination and remuneration committee. |
| • | Charters. NASDAQ Stock Market Listing Rules 5605(c)(1), (d)(1) and (e)(2) require that each committee of the board of directors must have a formal written charter. Pursuant to the Belgian Corporate Governance Code, our board of directors has drawn up a corporate governance charter including, amongst others, the internal rules of our committees. |
Audit Committee
Our audit committee consists of three members: Werner Cautreels (Chairman), Harrold van Barlingen and Howard Rowe.
Our board of directors has determined that all members of our audit committee are independent under Rule 10A-3 of the Exchange Act and the applicable rules of the NASDAQ Stock Market and that Werner Cautreels qualifies as an “audit committee financial expert” as defined under the Exchange Act.
Our audit committee assists our board of directors in overseeing the accuracy and integrity of our accounting and financial reporting processes and audits of our consolidated financial statements, the implementation and effectiveness of an internal control system and our compliance with legal and regulatory requirements, the independent auditors’ qualifications and independence and the performance of the independent auditors.
Our audit committee’s duties and responsibilities to carry out its purposes include, among others:
| • | ensuring the integrity of our financial reporting, including review of period information before it is made public; |
| • | evaluating our system of internal controls set up by our executive committee, including evaluation and approval of the explanatory notes on internal controls in our annual reports; |
| • | reviewing the functions of our internal risk management system and the efficacy of these systems; |
| • | assessing the necessity for setting up an internal audit function; and |
| • | supervising our relationship with our external auditors during the external audit process, including evaluation of our auditors’ independence. |
The committee reports regularly to our board of directors on the exercise of its functions. It informs our board of directors about all areas in which action or improvement is necessary in its opinion and produces recommendations concerning the necessary steps that need to be taken. The audit review and the reporting on that review cover us and our subsidiaries as a whole. The members of the audit committee are entitled to receive
141
Table of Contents
all information which they need for the performance of their function, from our board of directors, executive committee and employees. Every member of the audit committee shall exercise this right in consultation with the chairman of the audit committee.
Nomination and Remuneration Committee
Our nomination and remuneration committee consists of three members: Rajesh Parekh (Chairman), Katrine Bosley and Werner Cautreels.
Our board of directors has determined that all members of our nomination and remuneration committee are independent under the applicable rules of the NASDAQ Stock Market.
Concerning our company’s nomination policy, this committee’s duties and responsibilities to carry out its purposes include, among others:
| • | making and evaluating proposals to our board of directors with regard to the election and re-election of non-executive directors; |
| • | advising on the size and composition of the board of directors periodically; |
| • | making selection criteria and nomination procedures for members of the executive committee; and |
| • | advising on proposals relating to the appointment or dismissal of the members of the executive committee. |
Concerning our company’s remuneration policy, this committee’s duties and responsibilities to carry out its purposes include, among others:
| • | making and evaluating proposals to our board of directors with regard to the remuneration policy for non-executive directors and the proposals which have to be submitted to the shareholders; |
| • | making and evaluating proposals to our board of directors relating to the remuneration policy for members of our executive committee; |
| • | making proposals relating to individual remuneration, including bonuses; and |
| • | discussing and evaluating the operations and performance of the executive committee at least once a year. |
142
Table of Contents
| D. | Employees. |
As of December 31, 2016, we had 508 employees. Our employees in France and Croatia are represented by a labor union and/or covered by a collective bargaining agreement. We have never experienced any employment related work stoppages, and we consider our relations with our employees to be good. We have also engaged and may continue to engage independent contractors to assist us with our clinical project activities. At each date shown, we had the following employees (excluding certain employees of our service division that was sold in April 2014), broken out by department and geography:
| December 31, | ||||||||||||
| 2014 | 2015 | 2016 | ||||||||||
| Function: |
||||||||||||
| Executive officers |
4 | 4 | 4 | |||||||||
| Research |
213 | 205 | 216 | |||||||||
| Development |
38 | 53 | 88 | |||||||||
| Research services |
102 | 102 | 116 | |||||||||
| Corporate and support |
60 | 71 | 84 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
417 | 435 | 508 | |||||||||
| Geography: |
||||||||||||
| Leiden, the Netherlands |
31 | 34 | 45 | |||||||||
| Mechelen, Belgium |
138 | 151 | 189 | |||||||||
| Romainville, France |
128 | 129 | 140 | |||||||||
| Zagreb, Croatia |
120 | 121 | 134 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
417 | 435 | 508 | |||||||||
| E. | Share Ownership. |
For information regarding the share ownership of our directors and members of our executive committee, see “Item 6.B.—Compensation” and “Item 7.A.—Major Shareholders.”
| Item 7 | Major Shareholders and Related Party Transactions. |
| A. | Major Shareholders. |
The following table sets forth information with respect to the beneficial ownership of our ordinary shares as of March 15, 2017 for:
| • | each person who is known by us to own beneficially more than 5% of our outstanding ordinary shares; |
| • | each member of our board of directors; |
| • | our executive committee, excluding our chief executive officer, as a group; and |
| • | all members of our board of directors and executive committee as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include ordinary shares that can be acquired within 60 days of March 15, 2017. The percentage ownership information shown in the table is based upon 46,256,078 ordinary shares outstanding as of March 15, 2017.
Except as otherwise indicated, all of the shares reflected in the table are ordinary shares or ADSs, and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose.
143
Table of Contents
In computing the number of ordinary shares beneficially owned by a person and the percentage ownership of that person, we deemed outstanding ordinary shares subject to warrants held by that person that are immediately exercisable or exercisable within 60 days of March 15, 2017. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person. Beneficial ownership representing less than 1% is denoted with an asterisk (*). The information in the table below is based on information known to us or ascertained by us from public filings made by the shareholders. Except as otherwise indicated in the table below, addresses of the directors, members of our executive committee and named beneficial owners are in care of Galapagos NV, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium.
| Shares beneficially owned | ||||||||
| Name of beneficial owner |
Number | Percentage | ||||||
| 5% shareholders: |
||||||||
| Gilead Sciences, Inc. |
6,760,701 | (1)(2) | 14.62 | % | ||||
| FMR LLC |
4,616,982 | (1)(3) | 9.98 | % | ||||
| Van Herk Investments B.V. |
3,943,150 | (1)(4) | 8.52 | % | ||||
| Directors and members of executive committee: |
||||||||
| Rajesh Parekh, MA, DPhil |
36,650 | (5) | * | |||||
| Onno van de Stolpe |
785,163 | (6) | 1.69 | % | ||||
| Werner Cautreels, Ph.D. |
6,300 | (7) | * | |||||
| Harrold van Barlingen, Ph.D. |
20,660 | (8) | * | |||||
| Howard Rowe, JD |
5,040 | (9) | * | |||||
| Katrine Bosley |
7,500 | (10) | — | |||||
| Christine Mummery, Ph.D. |
454 | (11) | * | |||||
| Mary Kerr, Ph.D. |
— | — | ||||||
| Executive committee excluding Onno van de Stolpe |
385,352 | (12) | * | |||||
| All members of our board of directors and executive committee as a group (11 persons) |
1,247,119 | (13) | 2.66 | % | ||||
| (1) | At the time of the most recent transparency notification or filing of a statement of beneficial ownership with the SEC. |
| (2) | Consists of 6,760,701 shares held by Gilead Biopharmaceutics Ireland Unlimited Company, which is a direct subsidiary of Gilead Sciences, Inc., which has the sole voting and investment power with respect to these shares. The address of Gilead Sciences, Inc. is 1209 Orange Street, Wilmington, DE, 19801, United States of America. |
| (3) | Consists of 4,616,982 shares held by FMR LLC, based on the transparency notification received on October 7, 2016. The address of FMR LLC is 1209 Orange Street, Wilmington, DE, 19801, United States of America. |
| (4) | Consists of 3,943,150 shares held by Van Herk Investments B.V. Van Herk Private Equity Investments B.V. holds all shares in Van Herk Investments B.V. Adrianus van Herk holds all shares in Van Herk Private Equity Investments B.V. and has sole voting and investment power with respect to these shares. The address of Van Herk Investments B.V. is Lichtenauerlaan 30, 3062 ME Rotterdam, the Netherlands. |
| (5) | Consists of (i) 31,250 shares and (ii) 5,400 shares issuable upon the exercise of warrants that are immediately exercisable or exercisable within 60 days of March 15, 2017. |
| (6) | Consists of (i) 478,289 shares and (ii) 306,874 shares issuable upon the exercise of warrants that are immediately exercisable or exercisable within 60 days of March 15, 2017. |
| (7) | Consists of (i) 2,520 shares and (ii) 3,780 shares issuable upon the exercise of warrants that are immediately exercisable or exercisable within 60 days of March 15, 2017. |
| (8) | Consists of (i) 15,620 shares and (ii) 5,040 shares issuable upon the exercise of warrants that are immediately exercisable or exercisable within 60 days of March 15, 2017. |
| (9) | Consists of 5,040 shares issuable upon the exercise of warrants that are immediately exercisable or exercisable within 60 days of March 15, 2017. |
| (10) | Consists of 7,500 shares issuable upon the exercise of warrants that are immediately exercisable or exercisable within 60 days of March 15, 2017. |
| (11) | Consists of 454 shares. |
144
Table of Contents
| (12) | Consists of (i) 35,352 shares and (ii) 350,000 shares issuable upon the exercise of warrants that are immediately exercisable or exercisable within 60 days of March 15, 2017. |
| (13) | Includes 683,634 shares issuable upon the exercise of warrants that are immediately exercisable or exercisable within 60 days of March 15, 2017. |
Each of our shareholders is entitled to one vote per ordinary share. None of the holders of our shares will have different voting rights from other holders of shares after the closing of this offering. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
As of March 15, 2017, assuming that all of our ordinary shares represented by ADSs are held by residents of the United States, we estimate that approximately 36% of our outstanding ordinary shares were held in the United States by approximately 87 institutional holders of record, excluding Gilead Sciences, Inc., or Gilead. At such date, there were outstanding 9,686,518 ADSs, each representing one ordinary share, and in the aggregate representing 21% of our outstanding ordinary shares. The actual number of holders is greater than these numbers of record holders, and includes beneficial owners whose ADSs are held in street name by brokers and other nominees. This number of holders of record also does not include holders whose shares may be held in trust by other entities.
On January 28, 2016, we received a transparency notification from Wellington Management Group LLP, confirming that, as a result of the capital increase through which Gilead acquired 6,760,701 of our ordinary shares, its shareholding had passively decreased below the lowest 5% notification threshold of Galapagos NV’s voting rights. On March 1, 2016, we received a transparency notification from Johnson & Johnson, indicating that affiliates under its control sold 2,350,061 shares, as a result of which its shareholding decreased below the lowest 5% notification threshold of Galapagos NV’s voting rights. On July 20, 2016, we received a transparency notice from Federated Equity Management Company of Pennsylvania indicating that as a result of a sale of shares, its shareholding had decreased below the 5% notification threshold of Galapagos NV’s voting rights. On October 7, 2016, we received a transparency notice from FMR LLC indicating that, as a result of an acquisition of voting securities, affiliates under its control reached the 10% threshold of Galapagos NV’s voting rights. For information regarding the acquisition of 6,760,701 of our ordinary shares by Gilead in connection with its affiliate’s entry into an exclusive license and collaboration agreement relating to filgotinib, please see “Item 7.B.—Related Party Transactions” below.
| B. | Related Party Transactions. |
Since January 1, 2016, we have engaged in the following transactions with our directors, members of our executive committee and holders of more than 10% of our outstanding voting securities and their affiliates.
Transaction with Major Shareholder
On December 16, 2015, we signed an exclusive license and collaboration agreement to develop and commercialize filgotinib in multiple indications with Gilead Biopharmaceutics Ireland Unlimited Company. Under the terms of the collaboration, Gilead is primarily responsible for development and for seeking regulatory approval of the licensed product. We are required to use commercially reasonable efforts as requested by Gilead to assist Gilead with certain development activities. In addition, we agreed on a 20-80 cost split for development costs of the licensed product, i.e. we will bear 20% of all development costs. In the framework of the closing of the transaction on January 19, 2016, Gilead Biopharmaceutics Ireland Unlimited Company paid a license fee of $300 million (or €275.6 million) and made a $425 million (or €392 million) equity investment in our share capital by subscribing to new ordinary shares at an issue price of €58.00 per share, including issuance premium. This resulted in Gilead owning 6,760,701 ordinary shares, representing 14.75% of our outstanding share capital as of the date of the capital increase. Moreover, under the subscription agreement relating hereto, the parties agreed to a lock-up and standstill arrangement. In the year ended December 31, 2016, we received $60.0 million (or €55.1 million) in milestone payments from Gilead under this exclusive license and collaboration agreement.
145
Table of Contents
We incurred €22.4 million in development costs for the development of filgotinib in collaboration with Gilead: these costs relate to the Phase 2b and Phase 3 trials and mainly consist of costs recharged by Gilead as we are co-funding 20% of the global development activities, as well as costs paid to CROs in conjunction with clinical trials, costs for production of the compound for clinical testing, and, to a smaller extent, personnel costs and consultancy costs. The reimbursement of research and development costs under the 20-80 cost split mechanism by Gilead to us amounted to €3.5 million for the year ended December 31, 2016. For further information on our exclusive license and collaboration agreement with Gilead, see the section of this annual report titled “Item 4.B.—Business Overview—Collaborations—Exclusive Collaboration Agreement with Gilead for Filgotinib.”
Transactions with Related Companies
From time to time, in the ordinary course of our business we may contract for services from companies in which certain of the members of our executive committee or directors may serve as director or advisor. The cost of these services is negotiated on an arm’s length basis and none of these arrangements is material to us.
Agreements with Our Directors and Members of Executive Committee
Employment and Management Arrangements
Onno van de Stolpe
On March 1, 2002, we entered into a management agreement with Onno van de Stolpe for the position of Managing Director and Chief Executive Officer for an indefinite period. Effective March 1, 2011, Mr. Van de Stolpe’s management agreement with Galapagos NV was reduced from a full-time basis to a part-time basis, for approximately 40% of his time, at which time he entered into (1) an employment agreement with Galapagos B.V. on a part-time basis, for approximately 35% of his time, and (2) a management agreement with Galapagos SASU for approximately 25% of his time. Mr. Van de Stolpe currently receives (1) a base remuneration from Galapagos NV of €188,907 (including €18,859.44 in the form of pension contributions), (2) a base salary from Galapagos B.V. of €165,294 (including an 8% holiday bonus) and (3) a base salary from Galapagos SASU of €118,067.
Bart Filius
On September 15, 2014, Galapagos B.V. entered into an employment agreement, subject to Dutch law, with Bart Filius for the position of Chief Financial Officer, starting December 1, 2014 for an indefinite period. Effective December 1, 2014, Mr. Filius’ employment agreement with Galapagos B.V. was reduced from a full-time basis to a part-time basis, for approximately 60% of his time, and he entered into a management agreement with Galapagos NV for approximately 40% of his time.
Andre Hoekema
On January 31, 2005, Galapagos B.V. entered into an employment agreement, subject to Dutch law, with Andre Hoekema for the position of Senior Vice President Corporate Development and member of the executive committee, for an indefinite period.
Piet Wigerinck
On February 28, 2008, we entered into a management agreement with Piet Wigerinck for the position of Senior Vice President Drug Development and member of the executive committee, for an indefinite period. Mr. Wigerinck was appointed Chief Scientific Officer effective March 1, 2012. The management agreement stipulates that Mr. Wigerinck shall perform his duties thereunder on an independent basis.
146
Table of Contents
Walid Abi-Saab
On October 26, 2016, Galapagos NV entered into a management agreement, subject to Belgian law, with Walid Abi-Saab for the position of Chief Medical Officer, starting March 1, 2017, for an indefinite period. Effective March 1, 2017, Mr. Abi-Saab’s management agreement with Galapagos NV was reduced from a full-time basis to a part-time basis, for approximately 95% of his time, and he entered into an employment agreement with Galapagos B.V. for approximately 5% of his time.
Severance Payments Upon Change of Control
The abovementioned agreements with the members of our executive committee do not provide for severance compensation. They do not contain notice periods that exceed six months. However, we entered into undertakings with the members of our executive committee providing that, in case their contract with us is terminated as a result of a change of control of our company, they would be entitled to a severance compensation of 12 months’ base salary for our chief executive officer and nine months’ base salary for the other executive committee members.
Consulting Arrangements
Parekh Enterprises Ltd
On August 1, 2005, we entered into a management agreement with Parekh Enterprises Ltd, duly represented by Rajesh Parekh, for the provision of consultancy services to the company consisting of the strategic positioning of our company, the evaluation of corporate transactions, the managing of relations with existing and potential investors and with stock markets and other matters of strategic importance for the company. This management agreement was terminated effective April 30, 2016. Parekh Enterprises Ltd received a fee of €20,263 for the provision of consultancy services during the first four months of 2016.
Director and Executive Committee Compensation
See the sections of this annual report in “Item 6.B.—Compensation” titled “—Compensation of Our Board of Directors” and “—Compensation of Members of the Executive Committee” for information regarding compensation of directors and members of our executive committee.
Equity Awards
Since January 1, 2016, we have granted warrants to certain of our directors and members of our executive committee.
See the section of this annual report titled “Item 7.A.—Major Shareholders” for information regarding equity awards to members of our executive committee.
Bonus Plans
See the section of this annual report titled “Item 6.B.—Compensation—Compensation of Members of the Executive Committee” for information regarding bonus plans for members of our executive committee.
Related-party Transactions Policy
Article 524 of the Belgian Companies Code provides for a special procedure that applies to intra-group or related party transactions with affiliates. The procedure applies to decisions or transactions between us and our affiliates that are not one of our subsidiaries. Prior to any such decision or transaction, our board of directors must appoint a special committee consisting of three independent directors, assisted by one or more independent
147
Table of Contents
experts. This committee must assess the business advantages and disadvantages of the decision or transaction, quantify its financial consequences and determine whether the decision or transaction causes a disadvantage to us that is manifestly illegitimate in view of our policy. If the committee determines that the decision or transaction is not illegitimate but will prejudice us, it must analyze the advantages and disadvantages of such decision or transaction and set out such considerations as part of its advice. Our board of directors must then make a decision, taking into account the opinion of the committee. Any deviation from the committee’s advice must be justified. Directors who have a conflict of interest are not entitled to participate in the deliberation and vote. The committee’s advice and the decision of the board of directors must be notified to our auditor, who must render a separate opinion. The conclusion of the committee, an excerpt from the minutes of the board of directors and the opinion by the auditor must be included in our annual report. This procedure does not apply to decisions or transactions in the ordinary course of business under customary market conditions and security documents, or to transactions or decisions with a value of less than 1% of our net assets as shown in our consolidated annual accounts.
In addition to this, our corporate governance charter provides for guidelines for transactions between our company and our directors or members of the executive committee. According to such guidelines:
| • | it is expected from all directors and members of the executive committee that they avoid all acts, standpoints or interests which are conflicting with, or which give the impression that they are conflicting with, the interests of our company; |
| • | all transactions between our company and our directors, members of the executive committee or representatives need the approval of our board of directors. Such transactions could only be allowed at arm’s length (normal market conditions); |
| • | our directors and members of the executive committee are, by way of example, not allowed, directly or indirectly, to enter into agreements with our company which relate to supply of materials or delivery of services (other than in the framework of their mandate for our company), except with the explicit approval of our board of directors; |
| • | in the event our directors, members of the executive committee or their permanent representatives are confronted with a potential conflict of interest with regard to a decision or a transaction of our company, they shall immediately inform the chairman of the board of directors thereof. Conflict of interest means a conflict of proprietary interest, but also functional conflict of interest or conflicts of a family nature (up to second degree); |
| • | in the event Article 523 of the Belgian Companies Code applies, our director or the member of the executive committee shall not participate in the deliberation on the subject matter; and |
| • | in the event Article 523 of the Belgian Companies Code does not apply, the existence of the conflict of interest shall be written down in the minutes (but shall not be published) and the director or the member of the executive committee shall not vote. |
We have adopted a related-party transaction policy that sets forth our procedures for the identification, review, consideration and approval or ratification of related-party transactions. For purposes of our policy only, a related-party transaction is a transaction in which we are a participant and a related party has a direct or indirect material interest. For purposes of this policy, a related party is any executive officer, director (or nominee for director) or beneficial owner of more than 5% of any class of our voting securities, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, if a transaction has been identified as a related-party transaction, our audit committee will review and consider information regarding the related-party transaction. In reviewing any related-party transaction, the committee will take into account, among other factors it deems appropriate, (i) whether the transaction is on terms no less favorable to us than terms generally available in a transaction with an unaffiliated third party under the same or similar circumstances; and (ii) the extent of the related party’s interest in the
148
Table of Contents
related-party transaction. Additionally, we will provide the audit committee with all material information regarding the related-party transaction, the interest of the related party, and any potential disclosure obligations in connection therewith. In addition, under our Code of Business Conduct and Ethics, our employees and directors have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest.
| C. | Interests of Experts and Counsel. |
Not applicable.
| Item 8 | Financial Information |
| A. | Consolidated Statements and Other Financial Information. |
Consolidated Financial Statements
Our consolidated financial statements are appended at the end of this annual report, starting at page F-1, and incorporated herein by reference.
Legal Proceedings
From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceedings that, if determined adversely to us, would individually or taken together have a material adverse effect on our business, results of operations, financial condition or cash flows. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Dividend Distribution Policy
We have never declared or paid any cash dividends on our ordinary shares. We do not anticipate paying cash dividends on our equity securities in the foreseeable future and intend for the foreseeable future to retain all available funds and any future earnings for use in the operation and expansion of our business. In general, distributions of dividends proposed by our board of directors require the approval of our shareholders at a shareholders’ meeting with a simple majority vote, although our board of directors may declare interim dividends without shareholder approval, subject to the terms and conditions of the Belgian Companies Code.
Pursuant to Belgian law, the calculation of amounts available for distribution to shareholders, as dividends or otherwise, must be determined on the basis of our non-consolidated statutory financial accounts. In addition, under the Belgian Companies Code, we may declare or pay dividends only if, following the declaration and issuance of the dividends, the amount of our net assets on the date of the closing of the last financial year according to our statutory annual accounts (i.e., the amount of the assets as shown in the balance sheet, decreased with provisions and liabilities, all as prepared in accordance with Belgian accounting rules), decreased with the non-amortized costs of incorporation and expansion and the non-amortized costs for research and development, does not fall below the amount of the paid-up capital (or, if higher, the called capital), increased with the amount of non-distributable reserves. Finally, prior to distributing dividends, we must allocate at least 5% of our annual net profits (under our non-consolidated statutory accounts prepared in accordance with Belgian accounting rules) to a legal reserve, until the reserve amounts to 10% of our share capital.
| B. | Significant Changes. |
On January 20, 2017, our board of directors conditionally issued up to 150,000 warrants (subject to acceptance by the beneficiary) within the framework of the authorized capital for the benefit of our chief medical officer under a new warrant plan: Warrant Plan 2016 (B). The warrants to be issued under Warrant Plan 2016 (B) have a term of eight years and an exercise price of €62.50.
149
Table of Contents
On February 1, 2017, we announced the opening of an Investigational New Drug file with the U.S. Food and Drug Administration for GLPG2222, which triggered a $10 million milestone payment from our collaboration partner AbbVie.
On March 22, 2017, we announced the initiation of a Phase 1 trial with GLPG3067, triggering a $7.5 million milestone payment from AbbVie.
| Item 9 | The Offer and Listing. |
| A. | Offer and Listing Details. |
The ADSs have been listed on the NASDAQ Global Select Market, or NASDAQ, under the symbol “GLPG” since May 14, 2015. Prior to that date, there was no public trading market for the ADSs. Our ordinary shares have been trading on Euronext Amsterdam and Euronext Brussels under the symbol “GLPG” since May 6, 2005. Prior to that date, there was no public trading market for the ADSs or our ordinary shares. Our global offering in May 2015 was priced at $42.05 per ADS and €37.00 per ordinary share based on an exchange rate of $1.1365 per euro. The following tables set forth for the periods indicated the reported high and low sale prices per ADS on NASDAQ in U.S. dollars and per ordinary share on Euronext Amsterdam in euros.
NASDAQ
| Per ADS | ||||||||
| Period |
High | Low | ||||||
| Annual: |
||||||||
| 2015 (beginning May 14) |
$ | 65.54 | $ | 38.28 | ||||
| 2016 |
$ | 73.37 | $ | 37.03 | ||||
| Quarterly: |
||||||||
| Second Quarter 2015 (beginning May 14) |
$ | 58.79 | $ | 44.36 | ||||
| Third Quarter 2015 |
$ | 65.54 | $ | 38.28 | ||||
| Fourth Quarter 2015 |
$ | 63.50 | $ | 39.03 | ||||
| First Quarter 2016 |
$ | 61.69 | $ | 37.03 | ||||
| Second Quarter 2016 |
$ | 61.02 | $ | 41.21 | ||||
| Third Quarter 2016 |
$ | 73.37 | $ | 51.91 | ||||
| Fourth Quarter 2016 |
$ | 67.56 | $ | 57.16 | ||||
| First Quarter 2017 (through March 17) |
$ | 82.14 | $ | 63.69 | ||||
| Month Ended: |
||||||||
| September 2016 |
$ | 73.37 | $ | 53.78 | ||||
| October 2016 |
$ | 67.56 | $ | 59.93 | ||||
| November 2016 |
$ | 62.65 | $ | 57.16 | ||||
| December 2016 |
$ | 64.66 | $ | 57.86 | ||||
| January 2017 |
$ | 70.10 | $ | 63.69 | ||||
| February 2017 |
$ | 71.01 | $ | 65.78 | ||||
| March 2017 (through March 17) |
$ | 82.14 | $ | 70.51 | ||||
150
Table of Contents
Euronext Amsterdam
| Per Ordinary Share | ||||||||
| Period |
High | Low | ||||||
| Annual: |
||||||||
| 2012 |
€ | 17.95 | € | 9.75 | ||||
| 2013 |
€ | 20.70 | € | 13.40 | ||||
| 2014 |
€ | 18.42 | € | 10.00 | ||||
| 2015 |
€ | 60.55 | € | 14.81 | ||||
| 2016 |
€ | 66.19 | € | 32.50 | ||||
| Quarterly: |
||||||||
| First Quarter 2015 |
€ | 24.68 | € | 14.81 | ||||
| Second Quarter 2015 |
€ | 55.40 | € | 22.00 | ||||
| Third Quarter 2015 |
€ | 59.00 | € | 31.15 | ||||
| Fourth Quarter 2015 |
€ | 60.55 | € | 34.54 | ||||
| First Quarter 2016 |
€ | 57.68 | € | 32.50 | ||||
| Second Quarter 2016 |
€ | 53.70 | € | 36.20 | ||||
| Third Quarter 2016 |
€ | 66.19 | € | 46.07 | ||||
| Fourth Quarter 2016 |
€ | 62.16 | € | 51.15 | ||||
| First Quarter 2017 (through March 17) |
€ | 76.15 | € | 59.13 | ||||
| Month Ended: |
||||||||
| September 2016 |
€ | 66.19 | € | 47.52 | ||||
| October 2016 |
€ | 62.16 | € | 54.84 | ||||
| November 2016 |
€ | 58.21 | € | 51.15 | ||||
| December 2016 |
€ | 61.94 | € | 53.84 | ||||
| January 2017 |
€ | 66.95 | € | 59.13 | ||||
| February 2017 |
€ | 67.90 | € | 60.41 | ||||
| March 2017 (through March 17) |
€ | 76.15 | € | 65.00 | ||||
On March 17, 2017, the last reported sale price of the ADSs on NASDAQ was $81.86 per ADS, and the last reported sale price of the ordinary shares on Euronext Amsterdam was €76.15 per share.
| B. | Plan of Distribution. |
Not applicable.
| C. | Markets. |
The ADSs have been listed on NASDAQ under the symbol “GLPG” since May 14, 2015, and our ordinary shares have been listed on Euronext Amsterdam and Euronext Brussels under the symbol “GLPG” since May 6, 2005.
| D. | Selling Shareholders. |
Not applicable.
| E. | Dilution. |
Not applicable.
151
Table of Contents
| F. | Expenses of the Issue. |
Not applicable.
| Item 10 | Additional Information. |
| A. | Share Capital. |
Not applicable.
| B. | Memorandum and Articles of Association. |
The information set forth in our prospectus dated May 13, 2015, filed with the SEC pursuant to Rule 424(b), under the headings “Description of Share Capital—Articles of Association and Other Share Information,” “Description of Share Capital—Board of Directors,” “Description of Share Capital—Description of the Rights and Benefits Attached to Our Shares,” “Description of Share Capital—Belgian Legislation” and “Description of Share Capital—Limitations on the Right to Own Securities” is incorporated herein by reference.
| C. | Material Contracts. |
We entered into an underwriting agreement among Morgan Stanley & Co. LLC and Credit Suisse Securities (USA) LLC, as representatives of the underwriters, on May 13, 2015, with respect to the ADSs and ordinary shares sold in our global offering. We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect of such liabilities. For additional information on our material contracts, please see the sections of this annual report titled “Item 4—Information on the Company” and “Item 7—Major Shareholders and Related Party Transactions.”
| D. | Exchange Controls. |
There are no Belgian exchange control regulations that impose limitations on our ability to make, or the amount of, cash payments to residents of the United States.
We are in principle under an obligation to report to the National Bank of Belgium certain cross-border payments, transfers of funds, investments and other transactions in accordance with applicable balance-of-payments statistical reporting obligations. Where a cross-border transaction is carried out by a Belgian credit institution on our behalf, the credit institution will in certain circumstances be responsible for the reporting obligations.
| E. | Taxation. |
Certain Material U.S. Federal Income Tax Considerations to U.S. Holders
The following is a summary of certain material U.S. federal income tax considerations relating to the ownership and disposition of ADSs by a U.S. holder (as defined below). This summary addresses only the U.S. federal income tax considerations for U.S. holders that hold such ADSs as capital assets for U.S. federal income tax purposes. This summary does not address all U.S. federal income tax matters that may be relevant to a particular U.S. holder. This summary does not address tax considerations applicable to a holder of ADSs that may be subject to special tax rules including, without limitation, the following:
| • | banks, financial institutions or insurance companies; |
| • | brokers, dealers or traders in securities, currencies, commodities, or notional principal contracts; |
| • | tax-exempt entities or organizations, including an “individual retirement account” or “Roth IRA” as defined in Section 408 or 408A of the Code (as defined below), respectively; |
152
Table of Contents
| • | real estate investment trusts, regulated investment companies or grantor trusts; |
| • | persons that hold the ADSs as part of a “hedging,” “integrated” or “conversion” transaction or as a position in a “straddle” for U.S. federal income tax purposes; |
| • | partnerships (including entities classified as partnerships for U.S. federal income tax purposes) or other pass-through entities, or persons that will hold the ADSs through such an entity; |
| • | certain former citizens or long-term residents of the United States; |
| • | holders that own directly, indirectly, or through attribution 10% or more of the voting power or value of the ADSs and shares; and |
| • | holders that have a “functional currency” for U.S. federal income tax purposes other than the U.S. dollar. |
Further, this summary does not address the U.S. federal estate, gift, or alternative minimum tax considerations, or any U.S. state, local, or non-U.S. tax considerations of the ownership and disposition of the ADSs.
This description is based on the U.S. Internal Revenue Code of 1986, as amended, or the Code; existing, proposed and temporary U.S. Treasury Regulations promulgated thereunder, administrative and judicial interpretations thereof; and the income tax treaty between Belgium and the United States in each case as in effect and available on the date hereof. All the foregoing is subject to change, which change could apply retroactively, and to differing interpretations, all of which could affect the tax considerations described below. There can be no assurances that the U.S. Internal Revenue Service, or the IRS, will not take a contrary or different position concerning the tax consequences of the ownership and disposition of the ADSs or that such a position would not be sustained. Holders should consult their own tax advisers concerning the U.S. federal, state, local and non-U.S. tax consequences of owning, and disposing of the ADSs in their particular circumstances.
For the purposes of this summary, a “U.S. holder” is a beneficial owner of ADSs that is (or is treated as), for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation, or other entity that is treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof, or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust, if a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of the substantial decisions of such trust or has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a United States person. |
If a partnership (or any other entity treated as a partnership for U.S. federal income tax purposes) holds ADSs, the U.S. federal income tax consequences relating to an investment in the ADSs will depend in part upon the status of the partner and the activities of the partnership. Such a partner or partnership should consult its tax advisor regarding the U.S. federal income tax considerations of owning and disposing of the ADSs in its particular circumstances.
In general, a U.S. holder who owns ADSs will be treated as the beneficial owner of the underlying shares represented by those ADSs for U.S. federal income tax purposes. Accordingly, no gain or loss will generally be recognized if a U.S. holder exchanges ADSs for the underlying shares represented by those ADSs.
The U.S. Treasury has expressed concern that parties to whom ADSs are released before shares are delivered to the depositary (“pre-release”), or intermediaries in the chain of ownership between holders and the issuer of the
153
Table of Contents
security underlying the ADSs, may be taking actions that are inconsistent with the claiming of foreign tax credits by holders of ADSs. These actions would also be inconsistent with the claiming of the reduced rate of tax, described below, applicable to dividends received by certain non-corporate holders. Accordingly, the creditability of Belgian taxes, and the availability of the reduced tax rate for dividends received by certain non-corporate U.S. holders, each described below, could be affected by actions taken by such parties or intermediaries.
As indicated below, this discussion is subject to U.S. federal income tax rules applicable to a “passive foreign investment company,” or a PFIC.
Persons considering an investment in the ADSs should consult their own tax advisors as to the particular tax consequences applicable to them relating to the ownership and disposition of the ADSs, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
Distributions. Although we do not currently plan to pay dividends, and subject to the discussion under “—Passive Foreign Investment Company Considerations” below, the gross amount of any distribution (before reduction for any amounts withheld in respect of Belgian withholding tax) actually or constructively received by a U.S. holder with respect to ADSs will be taxable to the U.S. holder as a dividend to the extent of the U.S. holder’s pro rata share of our current and accumulated earnings and profits as determined under U.S. federal income tax principles. Distributions in excess of earnings and profits will be non-taxable to the U.S. holder to the extent of, and will be applied against and reduce, the U.S. holder’s adjusted tax basis in the ADSs. Distributions in excess of earnings and profits and such adjusted tax basis will generally be taxable to the U.S. holder as either long-term or short-term capital gain depending upon whether the U.S. holder has held the ADSs for more than one year as of the time such distribution is received. However, since we do not calculate our earnings and profits under U.S. federal income tax principles, it is expected that any distribution will be reported as a dividend, even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above. Non-corporate U.S. holders may qualify for the preferential rates of taxation with respect to dividends on ADSs applicable to long-term capital gains (i.e., gains from the sale of capital assets held for more than one year) applicable to qualified dividend income (as discussed below) if we are a “qualified foreign corporation” and certain other requirements (discussed below) are met. A non-U.S. corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (a) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information provision, or (b) with respect to any dividend it pays on ADSs which are readily tradable on an established securities market in the United States. The ADSs are listed on the NASDAQ Global Select Market, or NASDAQ, which is an established securities market in the United States, and we expect the ADSs to be readily tradable on NASDAQ. However, there can be no assurance that the ADSs will be considered readily tradable on an established securities market in the United States in later years. The company, which is incorporated under the laws of Belgium, believes that it qualifies as a resident of Belgium for purposes of, and is eligible for the benefits of, The Convention between the Government of the United States of America and the Government of the Kingdom of Belgium for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income, signed on November 27, 2006, or the U.S.-Belgium Tax Treaty, although there can be no assurance in this regard. Further, the IRS has determined that the U.S.-Belgium Tax Treaty is satisfactory for purposes of the qualified dividend rules and that it includes an exchange-of-information program. Therefore, subject to the discussion under “—Passive Foreign Investment Company Considerations” below, such dividends will generally be “qualified dividend income” in the hands of individual U.S. holders, provided that a holding period requirement (more than 60 days of ownership, without protection from the risk of loss, during the 121-day period beginning 60 days before the ex-dividend date) and certain other requirements are met. The dividends will not be eligible for the dividends-received deduction generally allowed to corporate U.S. holders.
A U.S. holder generally may claim the amount of any Belgian withholding tax as either a deduction from gross income or a credit against U.S. federal income tax liability. However, the foreign tax credit is subject to
154
Table of Contents
numerous complex limitations that must be determined and applied on an individual basis. Generally, the credit cannot exceed the proportionate share of a U.S. holder’s U.S. federal income tax liability that such U.S. holder’s taxable income bears to such U.S. holder’s worldwide taxable income. In applying this limitation, a U.S. holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source.” In addition, this limitation is calculated separately with respect to specific categories of income. The amount of a distribution with respect to the ADSs that is treated as a “dividend” may be lower for U.S. federal income tax purposes than it is for Belgian income tax purposes, potentially resulting in a reduced foreign tax credit for the U.S. holder. Each U.S. holder should consult its own tax advisors regarding the foreign tax credit rules.
In general, the amount of a distribution paid to a U.S. holder in a foreign currency will be the dollar value of the foreign currency calculated by reference to the spot exchange rate on the day the U.S. holder receives the distribution, regardless of whether the foreign currency is converted into U.S. dollars at that time. Any foreign currency gain or loss a U.S. holder realizes on a subsequent conversion of foreign currency into U.S. dollars will be U.S. source ordinary income or loss. If dividends received in a foreign currency are converted into U.S. dollars on the day they are received, a U.S. holder should not be required to recognize foreign currency gain or loss in respect of the dividend.
Sale, Exchange or Other Taxable Disposition of the ADSs. A U.S. holder will generally recognize gain or loss for U.S. federal income tax purposes upon the sale, exchange or other taxable disposition of ADSs in an amount equal to the difference between the U.S. dollar value of the amount realized from such sale or exchange and the U.S. holder’s tax basis for those ADSs. Subject to the discussion under “—Passive Foreign Investment Company Considerations” below, this gain or loss will generally be a capital gain or loss. The adjusted tax basis in the ADSs generally will be equal to the cost of such ADSs. Capital gain from the sale, exchange or other taxable disposition of ADSs of a non-corporate U.S. holder is generally eligible for a preferential rate of taxation applicable to capital gains, if the non-corporate U.S. holder’s holding period determined at the time of such sale, exchange or other taxable disposition for such ADSs exceeds one year (i.e., such gain is long-term taxable gain). The deductibility of capital losses for U.S. federal income tax purposes is subject to limitations under the Code. Any such gain or loss that a U.S. holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes.
Medicare Tax. Certain U.S. holders that are individuals, estates or trusts are subject to a 3.8% tax on all or a portion of their “net investment income,” which may include all or a portion of their dividend income and net gains from the disposition of ADSs. Each U.S. holder that is an individual, estate or trust is urged to consult its tax advisors regarding the applicability of the Medicare tax to its income and gains in respect of its investment in the ADSs.
Passive Foreign Investment Company Considerations. If we are classified as a PFIC in any taxable year, a U.S. holder would be subject to special rules generally intended to reduce or eliminate any benefits from the deferral of U.S. federal income tax that a U.S. holder could derive from investing in a non-U.S. company that does not distribute all of its earnings on a current basis.
A corporation organized outside the United States generally will be classified as a PFIC for U.S. federal income tax purposes in any taxable year in which, after applying certain look-through rules with respect to the income and assets of its subsidiaries, either: (i) at least 75% of its gross income is “passive income” or (ii) at least 50% of the average quarterly value of its total gross assets (which, assuming we are not a controlled foreign corporation for the year being tested, would be measured by the fair market value of our assets, and for which purpose the total value of our assets may be determined in part by the market value of the ADSs and our ordinary shares, which are subject to change) is attributable to assets that produce “passive income” or are held for the production of “passive income.”
Passive income for this purpose generally includes dividends, interest, royalties, rents, gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which
155
Table of Contents
produce passive income, and includes amounts derived by reason of the temporary investment of funds raised in offerings of the ADSs. If a non-U.S. corporation owns directly or indirectly at least 25% by value of the stock of another corporation, the non-U.S. corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly its proportionate share of the other corporation’s income. If we are classified as a PFIC in any year with respect to which a U.S. holder owns the ADSs, we will continue to be treated as a PFIC with respect to such U.S. holder in all succeeding years during which the U.S. holder owns the ADSs, regardless of whether we continue to meet the tests described above.
Whether we are a PFIC for any taxable year will depend on the composition of our income and the projected composition and estimated fair market values of our assets in each year, and because this is a factual determination made annually after the end of each taxable year, there can be no assurance that we will not be considered a PFIC in any taxable year. The market value of our assets may be determined in large part by reference to the market price of the ADSs and our ordinary shares, which is likely to fluctuate after the offering. Based on the foregoing, with respect to the 2016 taxable year and foreseeable future tax years, we do not anticipate that we will be a PFIC based upon the expected value of our assets, including any goodwill, and the expected composition of our income and assets, however, as previously mentioned, we cannot provide any assurances regarding our PFIC status for the current, prior or future taxable years.
If we are a PFIC, and you are a U.S. holder, then unless you make one of the elections described below, a special tax regime will apply to both (a) any “excess distribution” by us to you (generally, your ratable portion of distributions in any year which are greater than 125% of the average annual distribution received by you in the shorter of the three preceding years or your holding period for the ADSs) and (b) any gain realized on the sale or other disposition of the ADSs. Under this regime, any excess distribution and realized gain will be treated as ordinary income and will be subject to tax as if (a) the excess distribution or gain had been realized ratably over your holding period, (b) the amount deemed realized in each year had been subject to tax in each year of that holding period at the highest marginal rate for such year (other than income allocated to the current period or any taxable period before we became a PFIC, which would be subject to tax at the U.S. holder’s regular ordinary income rate for the current year and would not be subject to the interest charge discussed below), and (c) the interest charge generally applicable to underpayments of tax had been imposed on the taxes deemed to have been payable in those years. In addition, dividend distributions made to you will not qualify for the lower rates of taxation applicable to long-term capital gains discussed above under “—Distributions.”
Certain elections exist that may alleviate some of the adverse consequences of PFIC status and would result in an alternative treatment (such as mark-to-market treatment) of the ADSs. If a U.S. holder makes the mark-to- market election, the U.S. holder generally will recognize as ordinary income any excess of the fair market value of the ADSs at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ADSs over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. holder makes the election, the U.S. holder’s tax basis in the ADSs will be adjusted to reflect these income or loss amounts. Any gain recognized on the sale or other disposition of ADSs in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). The mark-to- market election is available only if we are a PFIC and the ADSs are “regularly traded” on a “qualified exchange.” The ADSs will be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of the ADSs are traded on a qualified exchange on at least 15 days during each calendar quarter (subject to the rule that trades that have as one of their principal purposes the meeting of the trading requirement as disregarded). NASDAQ is a qualified exchange for this purpose and, consequently, if the ADSs are regularly traded, the mark- to-market election will be available to a U.S. holder.
If we are a PFIC for any year during which a U.S. holder holds the ADSs, we must generally continue to be treated as a PFIC by that U.S. holder for all succeeding years during which the U.S. holder holds the ADSs, unless we cease to meet the requirements for PFIC status and the U.S. holder makes a “deemed sale” election
156
Table of Contents
with respect to the ADSs. If such election is made, the U.S. holder will be deemed to have sold the ADSs it holds at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain from such deemed sale would be subject to the consequences described above. After the deemed sale election, the U.S. holder’s ADSs with respect to which the deemed sale election was made will not be treated as shares in a PFIC unless we subsequently become a PFIC.
The tax consequences that would apply if we were a PFIC would also be different from those described above if a U.S. holder were able to make a valid “qualified electing fund,” or QEF, election. However, we do not currently intend to provide the information necessary for U.S. holders to make a QEF election if we were treated as a PFIC for any taxable year and prospective investors should assume that a QEF election will not be available. U.S. holders should consult their tax advisors to determine whether any of these above elections would be available and if so, what the consequences of the alternative treatments would be in their particular circumstances.
If we are determined to be a PFIC, the general tax treatment for U.S. holders described in this section would apply to indirect distributions and gains deemed to be realized by U.S. holders in respect of any of our subsidiaries that also may be determined to be PFICs.
If a U.S. holder owns ADSs during any taxable year in which we are a PFIC, the U.S. holder generally will be required to file an IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) with respect to the company, generally with the U.S. holder’s federal income tax return for that year. If our company were a PFIC for a given taxable year, then you should consult your tax advisor concerning your annual filing requirements.
The U.S. federal income tax rules relating to PFICs are complex. Prospective U.S. investors are urged to consult their own tax advisers with respect to the ownership and disposition of the ADSs, the consequences to them of an investment in a PFIC, any elections available with respect to the ADSs and the IRS information reporting obligations with respect to the ownership and disposition of the ADSs.
Backup Withholding and Information Reporting. U.S. holders generally will be subject to information reporting requirements with respect to dividends on ADSs and on the proceeds from the sale, exchange or disposition of ADSs that are paid within the United States or through U.S.-related financial intermediaries, unless the U.S. holder is an “exempt recipient.” In addition, U.S. holders may be subject to backup withholding on such payments, unless the U.S. holder provides a taxpayer identification number and a duly executed IRS Form W-9 or otherwise establishes an exemption. Backup withholding is not an additional tax, and the amount of any backup withholding will be allowed as a credit against a U.S. holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Asset Reporting. Certain U.S. holders who are individuals are required to report information relating to an interest in the ADSs, subject to certain exceptions (including an exception for shares held in accounts maintained by U.S. financial institutions) by filing IRS Form 8938 (Statement of Specified Foreign Financial Assets) with their federal income tax return. U.S. holders are urged to consult their tax advisors regarding their information reporting obligations, if any, with respect to their ownership and disposition of the ADSs.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR. EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN ADSS IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
157
Table of Contents
Belgian Tax Consequences
The following paragraphs are a summary of material Belgian tax consequences of the ownership of ADSs by an investor. The summary is based on laws, treaties and regulatory interpretations in effect in Belgium on the date of this annual report, all of which are subject to change, including changes that could have retroactive effect.
The summary only discusses Belgian tax aspects which are relevant to U.S. holders of ADSs, or Holders. This summary does not address Belgian tax aspects which are relevant to persons who are fiscally resident in Belgium or who avail of a permanent establishment or a fixed base in Belgium to which the ADSs are effectively connected.
This summary does not purport to be a description of all of the tax consequences of the ownership of ADSs, and does not take into account the specific circumstances of any particular investor, some of which may be subject to special rules, or the tax laws of any country other than Belgium. This summary does not describe the tax treatment of investors that are subject to special rules, such as banks, insurance companies, collective investment undertakings, dealers in securities or currencies, persons that hold, or will hold, ADSs in a position in a straddle, share-repurchase transaction, conversion transactions, synthetic security or other integrated financial transactions. Investors should consult their own advisers regarding the tax consequences of an investment in ADSs in the light of their particular circumstances, including the effect of any state, local or other national laws.
In addition to the assumptions mentioned above, it is also assumed in this discussion that for purposes of the domestic Belgian tax legislation, the owners of ADSs will be treated as the owners of the ordinary shares represented by such ADSs. However, the assumption has not been confirmed by or verified with the Belgian Tax Authorities.
Dividend Withholding Tax
As a general rule, a withholding tax of 30% is levied on the gross amount of dividends paid on the ordinary shares represented by the ADSs, subject to such relief as may be available under applicable domestic or tax treaty provisions.
Dividends subject to the dividend withholding tax include all benefits attributed to the ordinary shares represented by the ADSs, irrespective of their form, as well as reimbursements of statutory share capital by us, except reimbursements of fiscal capital made in accordance with the Belgian Companies Code. In principle, fiscal capital includes paid-up statutory share capital, and subject to certain conditions, the paid-up issue premiums and the amounts subscribed to at the time of the issue of profit sharing certificates.
In case of a redemption by us of our own shares represented by ADSs, the redemption distribution (after deduction of the portion of fiscal capital represented by the redeemed shares) will be treated as a dividend which in principle is subject to the withholding tax of 30%, subject to such relief as may be available under applicable domestic or tax treaty provisions. In case of a liquidation of our company, any amounts distributed in excess of the fiscal capital will also be treated as a dividend, and will in principle be subject to a 30% withholding tax, subject to such relief as may be available under applicable domestic or tax treaty provisions.
For non-residents the dividend withholding tax, if any, will be the only tax on dividends in Belgium, unless the non-resident avails of a fixed base in Belgium or a Belgian permanent establishment to which the ADSs are effectively connected.
Relief of Belgian Dividend Withholding Tax
Under the U.S.-Belgium Tax Treaty, under which we are entitled to benefits accorded to residents of Belgium, there is a reduced Belgian withholding tax rate of 15% on dividends paid by us to a U.S. resident which beneficially owns the dividends and is entitled to claim the benefits of the U.S.-Belgium Tax Treaty under the limitation of benefits article included in the U.S.-Belgium Tax Treaty, or Qualifying Holders.
158
Table of Contents
If such Qualifying Holder is a company that owns directly at least 10% of our voting stock, the Belgian withholding tax rate is further reduced to 5%. No withholding tax is however applicable if the Qualifying Holder, is either of the following:
| • | a company that is a resident of the United States that has owned directly ADSs representing at least 10% of our capital for a twelve-month period ending on the date the dividend is declared, or |
| • | a pension fund that is a resident of the United States, provided that such dividends are not derived from the carrying on of a business by the pension fund or through an associated enterprise. |
Under the normal procedure, we or our paying agent must withhold the full Belgian withholding tax, without taking into account the reduced U.S.-Belgium Tax Treaty rate. Qualifying Holders may then make a claim for reimbursement for amounts withheld in excess of the rate defined by the U.S.-Belgium Tax Treaty. The reimbursement form (Form 276 Div-Aut.) can be obtained as follows:
| • | by letter from the Bureau Central de Taxation Bruxelles-Etranger, Boulevard du Jardin Botanique 50 boîte 3429, 1000 Brussels, Belgium; |
| • | by fax at +32 (0) 257/968 42; |
| • | via e-mail at ctk.db.brussel.buitenland@minfin.fed.be; or at |
| • | http://financien.belgium.be/nl/ondernemingen/vennootschapsbelasting/voorheffingen/roerende_voorheffing/formulieren. |
The reimbursement form is to be sent to the Bureau Central de Taxation Bruxelles-Etranger, Boulevard du Jardin Botanique 50 boîte 3429, 1000 Brussels, Belgium as soon as possible and in each case within a term of five years starting from the first of January of the year the withholding tax was withheld.
Qualifying Holders may also, subject to certain conditions, obtain the reduced U.S.-Belgium Tax Treaty rate at source. Qualifying Holders should deliver a duly completed Form 276 Div-Aut. no later than ten days after the date on which the dividend has been paid or attributed (whichever comes first).
Additionally, pursuant to Belgian domestic tax law, dividends distributed to corporate Holders that qualify as a parent company will be exempt from Belgian withholding tax provided that the ADSs held by the Holder, upon payment or attribution of the dividends, amount to at least 10% of our share capital and are held or will be held during an uninterrupted period of at least one year, and provided the anti-abuse provision does not apply. A Holder qualifies as a parent company if it has a legal form similar to the ones listed in the annex to the EU Parent-Subsidiary Directive of July 23, 1990 (90/435/EC), if it is considered to be a tax resident according to the laws of the United States of America and the U.S.-Belgium Tax Treaty, and if it is subject to a tax similar to the Belgian corporate income tax without benefiting from a tax regime that derogates from the ordinary tax regime.
In order to benefit from this exemption, the Holder must provide us or its paying agent with a certificate confirming its qualifying status and the fact that it satisfies the required conditions. If the Holder holds the ADSs for less than one year, at the time the dividends are paid on or attributed to the shares represented by the ADSs, we must deduct the withholding tax but we do not need to transfer it to the Belgian Treasury provided that the Holder certifies its qualifying status, the date from which the Holder has held the ADSs, and the Holder’s commitment to hold the shares for an uninterrupted period of at least one year. The Holder must also inform us or its paying agent when the one-year period has expired or if its shareholding drops below 10% of our share capital before the end of the one-year holding period. Upon satisfying the one-year shareholding requirement, the deducted dividend withholding tax will be paid to the Holder.
Dividends paid or attributable to a corporate Holder will under certain conditions be subject to a reduced 1.6995% withholding tax (5% of 33.99%), provided that the Holder has a legal form similar to the ones listed in Annex I, Part A to Council Directive 2011/96/EU of November 30, 2011 on the common system of taxation
159
Table of Contents
applicable in the case of parent companies and subsidiaries of different Member States, as amended by the Council Directive of July 8, 2014 (2014/86/EU) and holds a share participation in our share capital, upon payment or attribution of the dividends, of less than 10% but with an acquisition value of at least EUR 2,500,000 and has held this share participation in full legal ownership during an uninterrupted period of at least one year.
The reduced 1.6995% withholding tax is only applied to the extent that the Belgian withholding tax cannot be credited nor reimbursed at the level of the qualifying, dividend receiving, Holder. The Holder must provide us or its paying agent with a certificate confirming its qualifying status and the fact that it meets the required conditions.
Withholding tax is also not applicable, pursuant to Belgian domestic tax law, on dividends paid to a U.S. pension fund which satisfies the following conditions:
| (i) | to be a legal entity with fiscal residence in the United States and without a permanent establishment or fixed base in Belgium, |
| (ii) | whose corporate purpose consists solely in managing and investing funds collected in order to pay legal or complementary pensions, |
| (iii) | whose activity is limited to the investment of funds collected in the exercise of its statutory mission, without any profit making aim and without operating a business in Belgium, |
| (iv) | which is exempt from income tax in the United States, and |
| (v) | provided that it (save in certain particular cases as described in Belgian law) is not contractually obligated to redistribute the dividends to any ultimate beneficiary of such dividends for whom it would manage the shares or ADSs, nor obligated to pay a manufactured dividend with respect to the shares or ADSs under a securities borrowing transaction. The exemption will only apply if the U.S. pension fund provides an affidavit confirming that it is the full legal owner or usufruct holder of the shares or ADSs and that the above conditions are satisfied. The organization must then forward that affidavit to us or our paying agent. |
Prospective Holders are encouraged to consult their own tax advisers to determine whether they qualify for an exemption or a reduction of the withholding tax rate upon payment of dividends and, if so, the procedural requirements for obtaining such an exemption or a reduction upon the payment of dividends or making claims for reimbursement.
Capital Gains and Losses
Pursuant to the U.S.-Belgium Tax Treaty, capital gains and/or losses realized by a Qualifying Holder from the sale, exchange or other disposition of ADSs are exempt from tax in Belgium.
Capital gains realized on ADSs by a corporate Holder who is not a Qualifying Holder are generally not subject to taxation in Belgium unless such Holder is acting through a Belgian permanent establishment or a fixed place in Belgium to which the ADSs are effectively connected (in which case a 33.99%, 25.75%, 0.412% or 0% tax on the capital gain may apply, depending on the particular circumstances). Capital losses are generally not tax deductible.
Private individual Holders who are not Qualifying Holders and who are holding ADSs as a private investment will, as a rule, not be subject to tax in Belgium on any capital gains arising out of a disposal of ADSs. Losses will, as a rule, not be tax deductible.
Capital gains realized by a Holder upon the redemption of ADSs or upon our liquidation will generally be taxable as a dividend. See “—Dividend Withholding Tax” above.
160
Table of Contents
Estate and Gift Tax
There is no Belgium estate tax on the transfer of ADSs on the death of a Belgian non-resident. Donations of ADSs made in Belgium may or may not be subject to gift tax depending on the modalities under which the donation is carried out.
Belgian Tax on Stock Exchange Transactions
A stock market tax is normally levied on the purchase and the sale and on any other acquisition and transfer for consideration in Belgium of ADSs through a professional intermediary established in Belgium on the secondary market, so-called “secondary market transactions.” The tax is due from the transferor and the transferee separately. The applicable rate amounts to 0.27% with a cap of €1,600 per transaction and per party. Such tax is also due for transactions for which the order is directly or indirectly given by an individual with habitual abode in Belgium, or by a legal entity on account of its Belgian seat or establishment, to an intermediary established outside Belgium. In such case, this individual or legal entity should declare and pay the tax on stock exchange transactions due, unless if he can prove that it was already paid.
Belgian non-residents who purchase or otherwise acquire or transfer, for consideration, ADSs in Belgium for their own account through a professional intermediary may be exempt from the stock market tax if they deliver a sworn affidavit to the intermediary in Belgium confirming their non-resident status, except in case they would be considered to have their habitual abode or their seat or establishment in Belgium.
In addition to the above, no stock market tax is payable by: (i) professional intermediaries described in Article 2, 9 and 10 of the Law of August 2, 2002 acting for their own account, (ii) insurance companies described in Article 2, §1 of the Law of July 9, 1975 acting for their own account, (iii) professional retirement institutions referred to in Article 2, §1 of the Law of October 27, 2006 relating to the control of professional retirement institutions acting for their own account, (iv) collective investment institutions acting for their own account, (v) the aforementioned non-residents acting for their own account (upon delivery of a certificate of non-residency in Belgium), or (vi) regulated real estate companies acting for their own account.
No stock exchange tax will thus be due by Holders on the subscription, purchase or sale of ADSs, if the Holders are acting for their own account, except in case they would be considered to have their habitual abode or their seat or establishment in Belgium. In order to benefit from this exemption, the Holders must file with the professional intermediary in Belgium a sworn affidavit evidencing that they are non-residents for Belgian tax purposes.
Proposed Financial Transactions Tax
On February 14, 2013 the EU Commission adopted a Draft Directive on a common Financial Transaction Tax (the “FTT”). Earlier negotiations for a common transaction tax among all 28 EU Member States had failed. The current negotiations between Austria, Belgium, France, Germany, Greece, Italy, Portugal, Slovakia, Slovenia and Spain (the Participating Member States) are seeking a compromise under “enhanced cooperation” rules, which require consensus from at least nine nations. Earlier Estonia dropped out of the negotiations by declaring it would not introduce the FTT.
The Draft Directive currently stipulates that once the FTT enters into force, the Participating Member States shall not maintain or introduce taxes on financial transactions other than the FTT (or VAT as provided in the Council Directive 2006/112/EC of November 28, 2006 on the common system of value added tax). For Belgium, the tax on stock exchange transactions should thus be abolished once the FTT enters into force.
However, the Draft Directive on the FTT remains subject to negotiations between the Participating Member States. It may therefore be altered prior to any implementation, of which the eventual timing and outcome remains unclear. Additional EU Member States may decide to participate or drop out of the negotiations. If the number of Participating Member States would fall below nine, it would put an end to the legislative project.
161
Table of Contents
In June 2016, the Participating Member States declared that they would continue their efforts in the second half of the year but since then the negotiating parties have not been successful in reaching an agreement.
Prospective investors should consult their own professional advisors in relation to the FTT.
| F. | Dividends and Paying Agents. |
Not applicable.
| G. | Statement by Experts. |
Not applicable.
| H. | Documents on Display. |
We are subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers and under those requirements will file reports with the SEC. Those reports may be inspected without charge at the locations described below. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. Nevertheless, we will file with the SEC an annual report containing financial statements that have been examined and reported on, with and opinion expressed by an independent registered public accounting firm.
We maintain a corporate website at www.glpg.com. We intend to post a link to our annual report on Form 20-F as filed with the SEC on our website promptly following it being filed with the SEC. Information contained on, or that can be accessed through, our website does not constitute a part of this annual report. We have included our website address in this annual report solely as an inactive textual reference.
You may also review a copy of this annual report, including exhibits and any schedule filed herewith, and obtain copies of such materials at prescribed rates, at the SEC’s Public Reference Room in Room 1580, 100 F Street, NE, Washington, D.C. 20549-0102. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains a website ( www.sec.gov ) that contains reports, proxy and information statements and other information regarding registrants, such as Galapagos NV, that file electronically with the SEC.
With respect to references made in this annual report to any contract or other document of Galapagos NV, such references are not necessarily complete and you should refer to the exhibits attached or incorporated by reference to this annual report for copies of the actual contract or document.
| I. | Subsidiary Information. |
Not applicable.
| Item 11 | Quantitative and Qualitative Disclosures About Market Risk. |
Our financial risks are managed centrally. Our finance department coordinates the access to national and international financial markets and considers and manages continuously the financial risks concerning our activities. These relate to the financial markets risk, credit risk, liquidity risk and currency risk. There are no other important risks, such as interest rate risk, because we have nearly no financial debt and have a strong cash position. We do not buy or trade financial instruments for speculative purposes. For additional information on general risk factors, please see the section of this annual report titled “Item 3.D.—Risk Factors.”
162
Table of Contents
Liquidity Risk
Our consolidated balance sheet shows an amount of €112.3 million as incurred losses at the end of 2016. Management forecasts our liquidity requirements to ensure that there is sufficient cash to meet operational needs. We have no credit lines. Such forecasting is based on realistic assumptions with regards to milestone and upfront payments to be received, taking into account our past track record, including the assumption that not all new projects that are being planned will be realized.
Credit Risk
The term “credit risk” refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss.
Our trade receivables consist of a limited amount of creditworthy customers, many of which are large pharmaceutical companies, spread over different geographical areas. To limit the risk of financial losses, a policy of only dealing with creditworthy counterparties has been developed.
We grant credit to our clients in the framework of our normal business activities. Usually, we require no pledge or other collateral to cover the amounts due. Management continuously evaluates the client portfolio for creditworthiness. All receivables are considered collectable, except for these for which a provision for doubtful debtors has been established. The aging balance of receivables that are due, but that are still considered collectable is set forth in the table below:
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| 60–90 days |
€ | 170 | € | 86 | € | 17 | ||||||
| 90–120 days |
— | — | — | |||||||||
| More than 120 days |
€ | 54 | € | 17 | € | 45 | ||||||
Our cash and cash equivalents are invested primarily in savings and deposit accounts. Saving and deposit accounts generate a small amount of interest income. For banks and financial institutions, only independently rated parties with a minimum rating of ‘A’ are accepted at the beginning of the term.
Interest Rate Risk
The only variable interest-bearing financial asset is cash and cash equivalents.
Changes in interest rates may cause variations in interest income and expenses resulting from short term interest-bearing assets. Management does not expect the short term interest rates to decrease significantly in the immediate foreseeable future, which limits the interest exposure on our cash and cash equivalents.
Effect of Interest Rate Fluctuation
A 100 basis point increase in interest rates at balance sheet date would have increased profit and loss by approximately €10 million (2015: €3 million); a 100 basis point decrease in interest rates would have decreased profit and loss by approximately €10 million (2015: €3 million).
Foreign Exchange Risk
We are exposed to foreign exchange risk arising from various currency exposures. Our functional currency is euro, but we receive payments from our main business partners AbbVie and Gilead in U.S. dollars and acquire some consumables and materials in U.S. dollars, Swiss Francs, GB Pounds and Croatian Kuna.
163
Table of Contents
To limit this risk, we attempt to align incoming and outgoing cash flows in currencies other than the euro. In addition, contracts closed by our different entities are mainly in the functional currencies of that entity, except for the alliance agreements signed with AbbVie and Gilead for which payments are denominated in U.S. dollars.
In order to further reduce this risk, a netting system was implemented in the course of 2012, which restrains intra-group payments between entities with a different functional currency.
The exchange rate risk in case of a 10% change in the exchange rate amounts to:
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Net book value: |
||||||||||||
| Increase in Euros - U.S. Dollars |
€ | (16,863 | ) | € | 506 | € | 589 | |||||
| Increase in Euros - GB Pounds |
130 | 164 | 138 | |||||||||
| Increase in Euros - CH Francs |
165 | 169 | 181 | |||||||||
| Increase in Euros - HR Kunas |
(95 | ) | (50 | ) | 215 | |||||||
| Increase in U.S. Dollars - GB Pounds |
€ | (913 | ) | € | (907 | ) | € | (807 | ) | |||
The exchange rate risk on the U.S. dollar is primarily related to our cash and cash equivalents held in U.S dollars.
Capital Risk Factors
We manage our capital to safeguard that we will be able to continue as a going concern. At the same time, we want to ensure the return to our shareholders through the results from our research and development activities.
Our capital structure consists of cash-at-bank and in-hand and cash equivalents, financial debt (which as of December 31, 2016, consists of finance leases and advances from Oseo, a French public organization for innovation support, for €0.1 million), and equity attributed to the holders of our equity instruments, such as capital, reserves and results carried forward, as mentioned in the consolidated statement of changes in equity.
We manage our capital structure and make the necessary adjustments in the light of changes of economic circumstances, the risk characteristics of underlying assets and the projected cash needs of the current research and development activities.
The adequacy of the capital structure will depend on many factors, including scientific progress in the research and development programs, the magnitude of those programs, the commitments to existing and new clinical contract research organizations, the ability to establish new alliance or collaboration agreements, the capital expenditures, market developments and any future acquisition.
Neither we nor any of our subsidiaries are subject to any externally imposed capital requirements, other than those imposed by generally applicable company law requirements.
| Item 12 | Description of Securities Other than Equity Securities. |
| A. | Debt Securities. |
Not applicable.
| B. | Warrants and Rights. |
Not applicable.
164
Table of Contents
| C. | Other Securities. |
Not applicable.
| D. | American Depositary Shares. |
Citibank, N.A., as depositary, registers and delivers ADSs. Each ADS represents one ordinary share (or a right to receive one ordinary share) deposited with Citibank International Limited, located at EGSP 186, 1 North Wall Quay, Dublin 1 Ireland or any successor, as custodian for the depositary. Each ADS will also represent any other securities, cash or other property which may be held by the depositary in respect of the depositary facility. The depositary’s corporate trust office at which the ADSs are administered is located at 388 Greenwich Street, New York, New York 10013.
A deposit agreement among us, the depositary and the ADS holders sets out the ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs. A copy of the deposit agreement is incorporated by reference as an exhibit to this annual report.
Fees and Charges
Pursuant to the terms of the deposit agreement, the holders of ADSs will be required to pay the following fees:
| Service | Fees | |
| • Issuance of ADSs |
Up to U.S. $0.05 per ADS issued | |
| • Cancellation of ADSs |
Up to U.S. $0.05 per ADS canceled | |
| • Distribution of cash dividends or other cash distributions |
Up to U.S. $0.05 per ADS held | |
| • Distribution of ADSs pursuant to stock dividends, free stock distributions or exercise of rights. |
Up to U.S. $0.05 per ADS held | |
| • Distribution of securities other than ADSs or rights to purchase additional ADSs |
Up to U.S. $0.05 per ADS held | |
| • ADS Services |
Up to U.S. $0.05 per ADS held on the applicable record date(s) established by the depositary | |
The holders of ADSs will also be responsible to pay certain fees and expenses incurred by the depositary and certain taxes and governmental charges such as:
| • | fees for the transfer and registration of ordinary shares charged by the registrar and transfer agent for the ordinary shares in France (i.e., upon deposit and withdrawal of ordinary shares); |
| • | expenses incurred for converting foreign currency into U.S. dollars; |
| • | expenses for cable, telex and fax transmissions and for delivery of securities; |
| • | taxes and duties upon the transfer of securities (i.e., when ordinary shares are deposited or withdrawn from deposit); and |
| • | fees and expenses incurred in connection with compliance with exchange control regulations and other regulatory requirements applicable to ordinary shares, ADSs or American Depositary Receipts, or ADRs, or in connection with the delivery or servicing of ordinary shares on deposit. |
Depositary fees payable upon the issuance and cancellation of ADSs are typically paid to the depositary by the brokers (on behalf of their clients) receiving the newly issued ADSs from the depositary and by the brokers
165
Table of Contents
(on behalf of their clients) delivering the ADSs to the depositary for cancellation. The brokers in turn charge these fees to their clients. Depositary fees payable in connection with distributions of cash or securities to ADS holders and the depositary services fee are charged by the depositary to the holders of record of ADSs as of the applicable ADS record date.
The depositary fees payable for cash distributions are generally deducted from the cash being distributed. In the case of distributions other than cash (i.e., stock dividend, rights), the depositary charges the applicable fee to the ADS record date holders concurrent with the distribution. In the case of ADSs registered in the name of the investor (whether certificated or uncertificated in direct registration), the depositary sends invoices to the applicable record date ADS holders. In the case of ADSs held in brokerage and custodian accounts (via DTC), the depositary generally collects its fees through the systems provided by DTC (whose nominee is the registered holder of the ADSs held in DTC) from the brokers and custodians holding ADSs in their DTC accounts. The brokers and custodians who hold their clients’ ADSs in DTC accounts in turn charge their clients’ accounts the amount of the fees paid to the depositary.
In the event of refusal to pay the depositary fees, the depositary may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary fees from any distribution to be made to the ADS holder.
Note that the fees and charges the holders of ADSs may be required to pay may vary over time and may be changed by us and by the depositary. The holders of ADSs will receive prior notice of such changes.
The depositary may reimburse us for certain expenses incurred by us in respect of the ADR program established pursuant to the deposit agreement, by making available a portion of the depositary fees charged in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary may agree from time to time.
166
Table of Contents
PART II
| Item 13 | Defaults, Dividend Arrearages and Delinquencies. |
Not applicable.
| Item 14 | Material Modifications to the Rights of Security Holders and Use of Proceeds. |
Global Offering
In May 2015, we sold 5,746,000 ADSs, each representing one ordinary share, no nominal value, and 1,786,499 ordinary shares, in our global offering at a price of $42.05 per ADS and €37.00 per share, including the exercise in full by the underwriters of their option to purchase additional ADSs and ordinary shares, for aggregate gross proceeds to us of approximately €278.7 million. The net offering proceeds to us, after deducting underwriting discounts and commissions and offering expenses, were approximately €259.3 million. The offering commenced on May 6, 2015 and did not terminate before all of the securities registered in the registration statement were sold. The effective date of the registration statement, File No. 333-203435, for our global offering was May 13, 2015. Morgan Stanley & Co. LLC, Credit Suisse Securities (USA) LLC and Cowen and Company, LLC acted as joint book-running managers, and Nomura Securities International, Inc. and Bryan, Garnier & Co. acted as co-managers, of the global offering.
A portion of the net proceeds from our global offering was used to advance our CF program and our other discovery and development programs, as well as for general corporate and working capital purposes. The balance is held in cash and cash equivalents and is intended to be used to advance the discovery and development of our programs, mainly our CF and IPF programs, and for working capital and general corporate purposes. We currently expect that our cash needs for developing our filgotinib program with Gilead will be entirely financed by the equity investment and upfront payment which were received in January 2016.
None of the net proceeds of our global offering were paid directly or indirectly to any director, officer, general partner of ours or to their associates, persons owning 10% or more of any class of our equity securities, or to any of our affiliates.
| Item 15 | Controls and Procedures. |
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Exchange Act Rule 13a-15(b) as of December 31, 2016. While there are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures, our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives. Based upon our evaluation, as of December 31, 2016, the Chief Executive Officer and Chief Financial Officer have concluded that the disclosure controls and procedures, in accordance with Exchange Act Rule 13a-15(e), (i) are effective at that level of reasonable assurance in ensuring that information required to be disclosed in the reports that are filed or submitted under the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in the Commission’s rules and forms, and (ii) are effective at that level of reasonable assurance in ensuring that information to be disclosed in the reports that are filed or submitted under the Exchange Act is accumulated and communicated to the management of our company, including the Chief Executive Officer and the Chief Financial Officer, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is a process designed, under the supervision of the Chief
167
Table of Contents
Executive Officer and Chief Financial Officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external reporting purposes in accordance with generally accepted accounting principles.
Our internal control over financial reporting includes policies and procedures that pertain to the maintenance of records that, in reasonable detail, accurately and fairly, reflect transactions and dispositions of assets, provide reasonable assurance that transactions are recorded in the manner necessary to permit the preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are only carried out in accordance with the authorization of our management and directors, and provide reasonable assurance regarding the prevention or timely detection of any unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Moreover, projections of any evaluation of the effectiveness of internal control to future periods are subject to a risk that controls may become inadequate because of changes in conditions and that the degree of compliance with the policies or procedures may deteriorate.
Our management has assessed the effectiveness of internal control over financial reporting based on the Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013. Based on this assessment, our management has concluded that our internal control over financial reporting as of December 31, 2016 was effective.
The effectiveness of internal control over financial reporting as of December 31, 2016 has been audited by Deloitte Bedrijfsrevisoren BV o.v.v.e. CVBA, our independent registered public accounting firm. Their audit report, including their opinion on management’s assessment of internal control over financial reporting, is included in our audited consolidated financial statements included in this annual report.
Changes in Internal Control over Financial Reporting
During the period covered by this annual report, we have not made any change to our internal control over financial reporting that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| Item 15T. | Controls and Procedures. |
Not applicable.
| Item 16 | Reserved. |
Not applicable.
| Item 16A | Audit Committee Financial Expert. |
Our board of directors has determined that Werner Cautreels is an audit committee financial expert as defined by SEC rules and has the requisite financial sophistication under the applicable rules and regulations of the NASDAQ Stock Market. Dr. Cautreels is independent as such term is defined in Rule 10A-3 under the Exchange Act and under the listing standards of the NASDAQ Stock Market.
| Item 16B | Code of Business Conduct and Ethics. |
We have adopted a Code of Business Conduct and Ethics, or the Code of Conduct, that is applicable to all of our employees, members of our executive committee and directors. The Code of Conduct is available on our website at www.glpg.com . Our board of directors is responsible for administering the Code of Conduct and will
168
Table of Contents
be required to approve any waivers of the Code of Conduct for directors or members of our executive committee. Any waivers of the Code of Conduct for other employees may also be made by the compliance officer. We expect that any amendments to the Code of Conduct, or any waivers of its requirements, will be disclosed on our website.
| Item 16C | Principal Accountant Fees and Services. |
Deloitte Bedrijfsrevisoren BV o.v.v.e. CVBA has served as our independent registered public accounting firm for 2015 and 2016. Our accountants billed the following fees to us for professional services in each of those fiscal years:
| Year ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| (Euro, in thousands) | ||||||||
| Audit fees |
€ | 515.0 | € | 280.0 | ||||
| Audit-related fees |
186.0 | 538.4 | ||||||
| Tax fees |
— | 7.9 | ||||||
| All other fees |
— | — | ||||||
|
|
|
|
|
|||||
| Total |
€ | 701.0 | € | 826.3 | ||||
|
|
|
|
|
|||||
“Audit Fees” are the aggregate fees billed for the audit of our annual financial statements. This category also includes services that generally the independent accountant provides, such as consents and assistance with and review of documents filed with the SEC.
“Audit-Related Fees” are the aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit and are not reported under Audit Fees. In 2015, “Audit Related Fees” also include fees billed for assurance and audit-related services regarding our global offering.
“Tax Fees” are the aggregate fees billed for professional services rendered by the principal accountant for tax compliance, tax advice and tax planning related services.
“All Other Fees” are any additional amounts billed for products and services provided by the principal accountant. No other fees were paid to Deloitte Bedrijfsrevisoren BV o.v.v.e. CVBA for the fiscal years ended December 31, 2016 and 2015 .
Audit and Non-Audit Services Pre-Approval Policy
The audit committee has responsibility for, among other things, appointing, setting compensation of and overseeing the work of our independent registered public accounting firm, or external auditor. In recognition of these responsibilities, the audit committee has adopted a policy governing the pre-approval of all audit and permitted non-audit services performed by our external auditor to ensure that the provision of such services does not impair the external auditor’s independence from us and our management. Unless a type of service to be provided by our external auditor has received general pre-approval from the audit committee, it requires specific pre-approval by the audit committee. The payment for any proposed services in excess of pre-approved cost levels requires specific pre-approval by the audit committee.
Pursuant to its pre-approval policy, the audit committee may delegate its authority to pre-approve services to the chairperson of the Audit Committee. The decisions of the chairperson to grant pre-approvals must be presented to the full audit committee at its next scheduled meeting. The audit committee may not delegate its responsibilities to pre-approve services to the management.
The audit committee has considered the non-audit services provided by Deloitte Bedrijfsrevisoren BV o.v.v.e. CVBA as described above and believes that they are compatible with maintaining Deloitte Bedrijfsrevisoren BV o.v.v.e. CVBA’s independence as our external auditor.
169
Table of Contents
| Item 16D | Exemptions from the Listing Standards for Audit Committees. |
Not applicable.
| Item 16E | Purchases of Equity Securities by the Issuer and Affiliated Purchasers. |
Not applicable.
| Item 16F | Change in Registrant’s Certifying Accountant. |
Not applicable.
| Item 16G | Corporate Governance. |
As a Belgian naamloze vennootschap / société anonyme, we are subject to various corporate governance requirements under Belgian law. In addition, as a foreign private issuer listed on the NASDAQ Global Select Market, we will be subject to NASDAQ corporate governance listing standards. However, the NASDAQ Global Select Market’s listing standards provide that foreign private issuers are permitted to follow home country corporate governance practices in lieu of the NASDAQ rules, with certain exceptions. We intend to rely on the certain exemptions for foreign private issuers and follow Belgian corporate governance practices in lieu of the NASDAQ corporate governance rules.
Differences Between Our Corporate Governance Practices and the Listing Rules of the NASDAQ Stock Market
The following are the significant ways in which our corporate governance practices differ from those required for U.S. companies listed on NASDAQ:
| • | Quorum At Shareholder Meetings. NASDAQ Stock Market Listing Rule 5620(c) requires that for any shareholders’ meeting, the quorum must be no less than 33 1/3 % of the outstanding ordinary shares. There is no quorum requirement under Belgian law for our shareholders’ meetings, except as provided for by law in relation to decisions regarding certain matters. |
| • | Committee. NASDAQ Stock Market Listing Rule 5605(d)(2) requires that compensation of officers must be determined by, or recommended to, the board of directors for determination, either by a majority of the independent directors, or a compensation committee comprised solely of independent directors. NASDAQ Stock Market Listing Rule 5605(e) requires that director nominees be selected, or recommended for selection, either by a majority of the independent directors or a nominations committee comprised solely of independent directors. Under Belgian law, we are not subject to such composition requirements. Pursuant to Article 526quater of the Belgian Companies Code and the principles and guidelines of the Belgian Corporate Governance Code, we are required to set up a remuneration committee within our board of directors. In addition, the Belgian Corporate Governance Code provides that the board of directors should set up a nomination committee, which can be combined with the remuneration committee. Our board of directors has set up and appointed a nomination and remuneration committee. |
| • | Executive Session. NASDAQ Stock Market Listing Rule 5605(b)(2) requires that independent directors must have regularly scheduled meetings at which only independent directors are present. We do not intend to require our independent directors to meet separately from the full board of directors on a regular basis or at all, although the board of directors is supportive of its independent members voluntarily arranging to meet separately from the other members of our board of directors when and if they wish to do so. |
| • | Charters. NASDAQ Stock Market Listing Rules 5605(c)(1), (d)(1) and (e)(2) require that each committee of the board of directors must have a formal written charter. Pursuant to the Belgian Corporate Governance Code, our board of directors has drawn up a corporate governance charter including, amongst others, the internal rules of our committees. |
170
Table of Contents
| • | Shareholder Approval for Certain Issuances of Securities. NASDAQ Stock Market Listing Rule 5635 requires that a company obtains shareholder approval prior to making certain issuances of securities. Pursuant to the Belgian Companies Code and subject to the conditions set forth therein and in our articles of association, our board of directors is allowed to issue shares through the use of authorized capital limited to the maximum amount of our share capital. The authorized capital may however not be used for (i) capital increases by contribution in kind exclusively reserved for one of our shareholders holding shares to which more than 10% of the voting rights are attached, (ii) the issuance of shares at a price lower than the accounting par value (fractiewaarde/pair comptable) of the then outstanding shares of the same class, or (iii) the issuance of warrants intended mainly for one or more specified persons other than our or our subsidiaries’ employees. Restrictions on the use of the authorized capital also exist in case a public take-over bid on us has been announced. |
| Item 16H | Mine Safety Disclosure. |
Not applicable.
171
Table of Contents
PART III
| Item 17 | Financial Statements. |
See pages F-1 through F-71 of this annual report.
| Item 18 | Financial Statements. |
Not applicable.
| Item 19 | Exhibits. |
The Exhibits listed in the Exhibit Index at the end of this annual report are filed as Exhibits to this annual report.
172
Table of Contents
Index to Financial Statements
FINANCIAL SECTION
Audited consolidated Financial Statements as of and for the years ended December 31, 2016, 2015 and 2014
| F-2 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 | ||||
| F-9 | ||||
F-1
Table of Contents
REPORTS OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
21 March 2017
To the board of directors and shareholders of Galapagos NV and subsidiaries
We have audited the accompanying consolidated statements of financial position of Galapagos NV and its subsidiaries (the “Company”) as of 31 December 2016, 2015 and 2014, and the related consolidated statements of operations, comprehensive income, changes in equity, and cash flows for each of the three years in the period ended 31 December 2016, 2015 and 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such financial statements present fairly, in all material respects, the financial position of Galapagos NV and its subsidiaries as of 31 December 2016, 2015 and 2014, and the results of their operations, their comprehensive income, their changes in equity and their cash flows for each of the three years in the period ended 31 December 2016, 2015 and 2014, in conformity with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of 31 December 2016, based on the criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated 21 March 2017 expressed an unqualified opinion on the Company’s internal control over financial reporting.
Zaventem, 21 March 2017
The statutory auditor
| /s/ Gert Vanhees |
| DELOITTE Bedrijfsrevisoren / Reviseurs d’Entreprises |
| BV o.v.v.e. CVBA / SC s.f.d. SCRL |
| Represented by Gert Vanhees |
F-2
Table of Contents
21 March 2017
To the board of directors and shareholders of Galapagos NV and subsidiaries
We have audited the internal control over financial reporting of Galapagos NV and its subsidiaries (the “Company”) as of 31 December 2016, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying consolidated statements of financial position. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of 31 December 2016, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated statements of financial position as of and for the year ended 31 December 2016 of the Company and our report dated 21 March 2017, expressed an unqualified opinion on those financial statements.
Zaventem, 21 March 2017
The statutory auditor
| /s/ Gert Vanhees |
| DELOITTE Bedrijfsrevisoren / Reviseurs d’Entreprises |
| BV o.v.v.e. CVBA / SC s.f.d. SCRL |
| Represented by Gert Vanhees |
F-3
Table of Contents
Consolidated Statement of Financial Position
| (Euro, in thousands) | ||||||||||||||||
| December 31, | ||||||||||||||||
| 2016 | 2015 | 2014 | Notes | |||||||||||||
| Assets |
||||||||||||||||
| Intangible assets |
€ | 1,023 | € | 1,550 | € | 2,015 | 12 | |||||||||
| Property, plant and equipment |
14,961 | 13,782 | 10,091 | 13 | ||||||||||||
| Deferred tax assets |
1,957 | 1,726 | 293 | 22 | ||||||||||||
| Non-current R&D incentives receivables |
54,188 | 49,384 | 43,944 | 15 | ||||||||||||
| Non-current restricted cash |
1,098 | 1,046 | 306 | 16 | ||||||||||||
| Other non-current assets |
2,880 | 557 | 215 | 14 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Non-currents assets |
76,107 | 68,044 | 56,864 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Inventories |
300 | 325 | 281 | |||||||||||||
| Trade and other receivables |
9,728 | 3,931 | 3,211 | 17 | ||||||||||||
| Current R&D incentives receivables |
10,154 | 9,161 | 7,351 | 15 | ||||||||||||
| Cash and cash equivalents |
973,241 | 340,314 | 187,712 | 18 | ||||||||||||
| Current restricted cash |
6,570 | 6,857 | 10,422 | 16 | ||||||||||||
| Current financial asset from share subscription agreement |
— | 8,371 | — | 7 | ||||||||||||
| Other current assets |
7,239 | 5,512 | 4,625 | 17 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Current assets |
1,007,232 | 374,470 | 213,603 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total assets |
€ | 1,083,338 | € | 442,514 | € | 270,467 | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Equity and liabilities |
||||||||||||||||
| Share capital |
€ | 223,928 | € | 185,399 | € | 157,274 | 19 | |||||||||
| Share premium account |
649,135 | 357,402 | 114,182 | 19 | ||||||||||||
| Other reserves |
(1,000 | ) | (18 | ) | (220 | ) | 20 | |||||||||
| Translation differences |
(1,090 | ) | (467 | ) | (1,157 | ) | 21 | |||||||||
| Accumulated losses |
(112,272 | ) | (177,317 | ) | (63,944 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total equity |
758,701 | 364,999 | 206,135 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Pension liabilities |
3,520 | 2,693 | 2,865 | 29 | ||||||||||||
| Provisions |
63 | 55 | 72 | 25 | ||||||||||||
| Finance lease liabilities |
9 | 63 | 115 | 23 | ||||||||||||
| Other non-current liabilities |
2,469 | 2,291 | 923 | 24 | ||||||||||||
| Non-current deferred income |
214,785 | — | — | 24 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Non-current liabilities |
220,846 | 5,103 | 3,976 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Provisions |
— | — | 105 | 25 | ||||||||||||
| Finance lease liabilities |
54 | 52 | 52 | 23 | ||||||||||||
| Trade and other payables |
31,269 | 29,482 | 30,007 | 24 | ||||||||||||
| Current tax payable |
1,022 | 2,583 | 2,582 | 9 | ||||||||||||
| Accrued charges |
619 | 490 | 585 | 24 | ||||||||||||
| Deferred income |
70,827 | 39,806 | 27,026 | 24 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Current liabilities |
103,791 | 72,412 | 60,356 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
324,637 | 77,515 | 64,332 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total equity and liabilities |
€ | 1,083,338 | € | 442,514 | € | 270,467 | ||||||||||
|
|
|
|
|
|
|
|||||||||||
The accompanying notes form an integral part of these financial statements.
F-4
Table of Contents
Consolidated Statement of Operations
| (Euro, in thousands, except share and per share data) | ||||||||||||||||
| Year Ended December 31, | ||||||||||||||||
| 2016 | 2015 | 2014 | Notes | |||||||||||||
| Revenues |
€ | 129,519 | € | 39,563 | € | 69,368 | 4 | |||||||||
| Other income |
22,093 | 21,017 | 20,653 | 4 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total revenues and other income |
151,612 | 60,579 | 90,021 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Service cost of sales |
||||||||||||||||
| Research and development expenditure |
(139,573 | ) | (129,714 | ) | (111,110 | ) | 5 | |||||||||
| General and administrative expenses |
(21,744 | ) | (19,127 | ) | (13,875 | ) | 5 | |||||||||
| Sales and marketing expenses |
(1,785 | ) | (1,182 | ) | (992 | ) | 5 | |||||||||
| Restructuring and integration costs |
— | — | (669 | ) | 5 | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
(163,103 | ) | (150,023 | ) | (126,646 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(11,491 | ) | (89,444 | ) | (36,624 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Fair value re-measurement of share subscription agreement |
57,479 | (30,632 | ) | — | 7 | |||||||||||
| Other financial income |
9,950 | 1,987 | 2,291 | 8 | ||||||||||||
| Other financial expenses |
(1,692 | ) | (1,539 | ) | (867 | ) | 8 | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Income / loss (-) before tax |
54,246 | (119,627 | ) | (35,201 | ) | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Income taxes |
(235 | ) | 1,218 | (2,103 | ) | 9 | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net income / loss (-) from continuing operations |
54,012 | (118,410 | ) | (37,303 | ) | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net income from discontinued operations |
— | — | 70,514 | 10 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net income / loss (-) |
€ | 54,012 | € | (118,410 | ) | € | 33,211 | 11 | ||||||||
|
|
|
|
|
|
|
|||||||||||
| Net income / loss (-) attributable to: |
||||||||||||||||
| Owners of the parent |
54,012 | (118,410 | ) | 33,211 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Basic income / loss (-) per share |
€ | 1.18 | € | (3.32 | ) | € | 1.10 | 11 | ||||||||
|
|
|
|
|
|
|
|||||||||||
| Diluted income / loss (-) per share |
€ | 1.14 | € | (3.32 | ) | € | 1.10 | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Basic income/ loss (-) per share from continuing operations |
€ | 1.18 | € | (3.32 | ) | € | (1.24 | ) | ||||||||
|
|
|
|
|
|
|
|||||||||||
| Diluted income/ loss (-) per share from continuing operations |
€ | 1.14 | € | (3.32 | ) | € | (1.24 | ) | ||||||||
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of shares—Basic (in ‘000 shares) |
45,696 | 35,700 | 30,108 | 11 | ||||||||||||
| Weighted average number of shares—Diluted (in ‘000 shares) |
47,308 | 35,700 | 30,108 | |||||||||||||
The accompanying notes form an integral part of these financial statements.
F-5
Table of Contents
Consolidated Statement of Comprehensive Income
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Net income / loss (-) |
€ | 54,012 | € | (118,410 | ) | € | 33,211 | |||||
|
|
|
|
|
|
|
|||||||
| Items that will not be reclassified subsequently to profit or loss: |
||||||||||||
| Re-measurement of defined benefit obligation |
(583 | ) | 202 | (267 | ) | |||||||
| Items that may be reclassified subsequently to profit or loss: |
||||||||||||
| Fair value adjustment of financial assets available for sale |
(399 | ) | — | — | ||||||||
| Translation differences, arisen from translating foreign activities |
(623 | ) | 690 | 460 | ||||||||
| Translation differences, arisen from the sale of service division |
— | — | (1,787 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Other comprehensive income, net of income tax |
(1,605 | ) | 892 | (1,594 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total comprehensive income attributable to: |
||||||||||||
| Owners of the parent |
€ | 52,406 | € | (117,517 | ) | € | 31,617 | |||||
The accompanying notes form an integral part of these financial statements.
F-6
Table of Contents
Consolidated Statement of Changes in Equity
| Share capital |
Share premium account |
Translation differences |
Other reserves |
Accumul. losses |
Total | |||||||||||||||||||
| On January 1, 2014 |
€ | 154,542 | € | 112,484 | € | 170 | € | 47 | € | (100,107 | ) | € | 167,137 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income |
— | — | — | — | 33,211 | 33,211 | ||||||||||||||||||
| Other comprehensive income |
— | — | (1,327 | ) | (267 | ) | — | (1,594 | ) | |||||||||||||||
| Total comprehensive income |
— | — | (1,327 | ) | (267 | ) | 33,211 | 31,617 | ||||||||||||||||
| Share-based compensation |
— | — | — | — | 2,952 | 2,952 | ||||||||||||||||||
| Exercise of warrants |
2,732 | 1,698 | — | — | — | 4,430 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| On December 31, 2014 |
€ | 157,274 | € | 114,182 | € | (1,157 | ) | € | (220 | ) | € | (63,944 | ) | € | 206,135 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income |
— | — | — | — | (118,410 | ) | (118,410 | ) | ||||||||||||||||
| Other comprehensive income |
— | — | 690 | 202 | — | 892 | ||||||||||||||||||
| Total comprehensive income |
— | — | 690 | 202 | (118,410 | ) | (117,517 | ) | ||||||||||||||||
| Share-based compensation |
— | — | — | — | 5,036 | 5,036 | ||||||||||||||||||
| Issue of new shares |
40,751 | 237,952 | — | — | — | 278,703 | ||||||||||||||||||
| Share issue costs |
(19,360 | ) | — | — | — | — | (19,360 | ) | ||||||||||||||||
| Exercise of warrants |
6,734 | 5,269 | — | — | — | 12,002 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| On December 31, 2015 |
€ | 185,399 | € | 357,402 | € | (467 | ) | € | (18 | ) | € | (177,317 | ) | € | 364,999 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income |
— | — | — | — | 54,012 | 54,012 | ||||||||||||||||||
| Other comprehensive income |
— | — | (623 | ) | (982 | ) | — | (1,605 | ) | |||||||||||||||
| Total comprehensive income |
— | — | (623 | ) | (982 | ) | 54,012 | 52,406 | ||||||||||||||||
| Share-based compensation |
— | — | — | — | 11,034 | 11,034 | ||||||||||||||||||
| Issue of new shares |
36,575 | 289,696 | — | — | — | 326,271 | ||||||||||||||||||
| Share issue costs |
(269 | ) | — | — | — | — | (269 | ) | ||||||||||||||||
| Exercise of warrants |
2,223 | 2,037 | — | — | — | 4,261 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| On December 31, 2016 |
€ | 223,928 | € | 649,135 | € | (1,090 | ) | € | (1,000 | ) | € | (112,272 | ) | € | 758,701 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes form an integral part of these financial statements.
F-7
Table of Contents
Consolidated Statement of Cash Flows
| (Euro, in thousands) | ||||||||||||||||
| 2016 | 2015 | 2014 | Notes | |||||||||||||
| Cash and cash equivalents at beginning of year |
€ | 340,314 | € | 187,712 | € | 138,175 | 18 | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Net income / loss (-) |
54,012 | (118,410 | ) | 33,211 | ||||||||||||
| Adjustments for: |
||||||||||||||||
| Tax income (-) /expenses |
235 | (1,218 | ) | 2,337 | 9 | |||||||||||
| Other net financial income |
(8,258 | ) | (448 | ) | (1,841 | ) | 8 | |||||||||
| Fair value re-measurement of share subscription agreement |
(57,479 | ) | 30,632 | — | 7 | |||||||||||
| Depreciation of property, plant and equipment |
3,322 | 2,372 | 3,582 | 13 | ||||||||||||
| Amortization of intangible fixed assets |
860 | 1,030 | 1,067 | 12 | ||||||||||||
| Net realized gain / loss (-) on foreign exchange transactions |
1,229 | (398 | ) | (261 | ) | |||||||||||
| Share-based compensation |
11,034 | 5,036 | 2,952 | 30 | ||||||||||||
| Increase / decrease (-) in provisions |
7 | (125 | ) | 27 | 25 | |||||||||||
| Increase in pension liabilities |
244 | 30 | 409 | 29 | ||||||||||||
| Gain on disposal of fixed assets |
(14 | ) | (62 | ) | — | |||||||||||
| Gain on sale of service division |
— | — | (67,508 | ) | 33 | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating cash flows before movements in working capital |
5,192 | (81,560 | ) | (26,025 | ) | |||||||||||
| Increase (-) / decrease in inventories |
25 | (44 | ) | (32 | ) | |||||||||||
| Increase in receivables |
(12,978 | ) | (7,220 | ) | (10,110 | ) | 17 | |||||||||
| Increase / decrease (-) in payables |
2,102 | (39,508 | ) | 11,642 | 24 | |||||||||||
| Increase / decrease (-) in deferred income |
245,806 | 12,780 | (51,953 | ) | 24 | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Cash generated / used (-) from operations |
240,148 | (115,553 | ) | (76,479 | ) | |||||||||||
| Interest paid |
(47 | ) | (49 | ) | (113 | ) | ||||||||||
| Interest received |
1,066 | 1,106 | 951 | |||||||||||||
| Income taxes paid (-) / received |
(1,763 | ) | (94 | ) | 86 | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net cash flows generated/used (-) in operating activities |
239,403 | (114,590 | ) | (75,555 | ) | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Purchase of property, plant and equipment |
(4,458 | ) | (6,100 | ) | (2,061 | ) | 13 | |||||||||
| Purchase of and expenditure in intangible fixed assets |
(332 | ) | (565 | ) | (743 | ) | 12 | |||||||||
| Proceeds from disposal of intangible assets |
18 | 110 | — | 12 | ||||||||||||
| Proceeds from disposal of property, plant and equipment |
— | — | 45 | 13 | ||||||||||||
| Disposals of subsidiaries, net of cash disposed |
— | — | 130,787 | 33 | ||||||||||||
| Increase (-) / decrease in restricted cash |
235 | 2,258 | (7,422 | ) | 16 | |||||||||||
| Acquisition of available-for-sale financial assets |
(2,750 | ) | — | — | 14 | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net cash flows generated/used (-) in investing activities |
(7,287 | ) | (4,297 | ) | 120,606 | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Repayment of obligations under finance leases and other debts |
(49 | ) | (43 | ) | (216 | ) | 23 | |||||||||
| Proceeds from capital and share premium increases, net of issue costs |
391,784 | 259,410 | — | 19 | ||||||||||||
| Proceeds from capital and share premium increases from exercise of warrants |
4,261 | 12,003 | 4,430 | 19 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net cash flows generated in financing activities |
395,996 | 271,370 | 4,214 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Effect of exchange rate differences on cash and cash equivalents |
4,816 | 118 | 271 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Increase in cash and cash equivalents |
632,927 | 152,601 | 49,537 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of year |
€ | 973,241 | € | 340,314 | € | 187,712 | 18 | |||||||||
|
|
|
|
|
|
|
|||||||||||
The accompanying notes form an integral part of these financial statements.
F-8
Table of Contents
Notes to Consolidated Financial Statements
1. General information
Galapagos NV is a limited liability company incorporated in Belgium and has its registered office at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium. In the notes to the consolidated financial statements, references to “we,” “us,” “the group” or “Galapagos” include Galapagos NV together with its subsidiaries.
R&D
The R&D operations are specialized in the discovery and development of small molecules. Our ambition is to become a leading global biotechnology company focused on the development and commercialization of novel medicines. Our strategy is to leverage our unique and proprietary target discovery platform, which facilitates our discovery and development of therapies with novel modes of action.
The components of the operating result for continuing operations presented in the financial statements include the following companies: Galapagos NV (Mechelen, Belgium); Galapagos SASU (Romainville, France); Galapagos B.V. (Leiden, the Netherlands); Fidelta d.o.o. (Zagreb, Croatia); BioFocus, Inc. and its subsidiaries, BioFocus DPI LLC, and Xenometrix, Inc.; BioFocus DPI AG (Basel, Switzerland) and its subsidiary Discovery Partners International GmbH (Heidelberg, Germany); and Inpharmatica Ltd. (Saffron Walden, UK).
Our continuing operations have around 508 employees working in the operating facilities in Mechelen (the Belgian headquarters), the Netherlands, France, and Croatia.
SERVICES
We sold our service division to Charles River Laboratories International, Inc. on April 1, 2014.
The legal entities that were sold as part of this transaction were BioFocus DPI (Holdings) Ltd., BioFocus DPI Ltd., Argenta Discovery 2009 Ltd. and Cangenix Ltd. Galapagos B.V. was not sold; its service division operations were carved out by means of an asset deal.
As a result of this sale, the service division is reported as discontinued operations.
2. Significant accounting policies
Our principal accounting policies are summarized below.
BASIS OF PREPARATION AND GOING CONCERN ASSUMPTION
The consolidated financial statements are prepared in accordance with the International Financing Reporting Standards (IFRS), issued by the International Accounting Standard Board (IASB) and the interpretations issued by the IASB’s International Financial Reporting Interpretation Committee. The consolidated financial statements provide a general overview of our activities and the results achieved. They give a true and fair view of our financial position, our financial performance and cash flows, on a going concern basis.
NEW STANDARDS AND INTERPRETATIONS APPLICABLE FOR THE ANNUAL PERIOD BEGINNING ON JANUARY 1, 2016
| • | Amendments to IAS 1 Presentation of Financial Statements—Disclosure Initiative (applicable for annual periods beginning on or after 1 January 2016) |
| • | Amendments to IAS 16 and IAS 38 Property, Plant and Equipment and Intangible Assets—Clarification of Acceptable Methods of Depreciation and Amortization (applicable for annual periods beginning on or after 1 January 2016) |
| • | Amendments to IAS 19 Employee Benefits—Employee Contributions (applicable for annual periods beginning on or after 1 February 2015) |
F-9
Table of Contents
STANDARDS AND INTERPRETATIONS PUBLISHED, BUT NOT YET APPLICABLE FOR THE ANNUAL PERIOD BEGINNING ON JANUARY 1, 2016
| • | IFRS 9 Financial Instruments and subsequent amendments (applicable for annual periods beginning on or after 1 January 2018) |
| • | IFRS 15 Revenue from Contracts with Customers (applicable for annual periods beginning on or after 1 January 2018) |
| • | IFRS 16 Leases (applicable for annual periods beginning on or after 1 January 2019, but not yet endorsed in the EU) |
| • | Improvements to IFRS (2014-2016) (applicable for annual periods beginning on or after 1 January 2017 or 2018, but not yet endorsed in the EU) |
| • | Amendments to IFRS 2 Classification and Measurement of Share-based Payment Transactions (applicable for annual periods beginning on or after 1 January 2018, but not yet endorsed in the EU) |
| • | Amendments to IFRS 4 Insurance Contracts—Applying IFRS 9 Financial Instruments with IFRS 4 (applicable for annual periods beginning on or after 1 January 2018, but not yet endorsed in the EU) |
| • | Amendments to IAS 7 Statement of Cash Flows—Disclosure Initiative (applicable for annual periods beginning on or after 1 January 2017, but not yet endorsed in the EU) |
| • | Amendments to IAS 12 Income Taxes—Recognition of Deferred Tax Assets for Unrealized Losses (applicable for annual periods beginning on or after 1 January 2017, but not yet endorsed in the EU) |
| • | IFRIC 22 Foreign Currency Transactions and Advance Consideration (applicable for annual periods beginning on or after 1 January 2018, but not yet endorsed in the EU) |
The new standards applicable did not have any impact on our financials.
Assessment of the impact of IFRS 15 Revenue from Contracts with Customers (applicable for annual periods beginning on or after January 1, 2018) on the revenue recognition of our current material license and collaboration agreements.
The IASB has issued IFRS 15 Revenue from Contracts with Customers, with an effective date of January 1, 2018. It was endorsed by the EU in third quarter of 2016.
The IASB issued clarifications to IFRS 15 Amendments to IFRS 15 - Clarifications to IFRS 15 Revenue from Contracts with Customers, with an effective date of January 1, 2018, currently awaiting EU endorsement. The clarifications address how to identify the performance obligations in a contract, how to determine whether a party involved in a transaction is a principal or an agent, how to determine whether a license provides the customer with a right to access or a right to use the entity’s intellectual property, and added practical expedients to the transition requirements of IFRS 15.
Entities will apply a five step model to determine when, how and at what amount revenue is to be recognized depending on whether certain criteria are met.
The company is currently in process of reviewing all its research and development, license, and collaboration agreements to ascertain how IFRS 15 will impact the identification of performance obligations and the allocation of consideration to them. We performed preliminary qualitative assessments of the consequences of IFRS 15, which are however subject to change arising from a more detailed ongoing analysis.
| 1. | Identify the contracts |
The substance of our current arrangements is that Galapagos is licensing its IP or selling its compounds to collaborative partner entities and providing research and development (“R&D”) services. Such activities result in a good or service that is an output of Galapagos’ ordinary activities.
F-10
Table of Contents
We generate revenue through a number of these arrangements which include license fees, milestone payments, reimbursement income and future sales based milestones and sales based royalties.
Certain revenues from our current material licensing and collaboration agreements could be in the scope of IFRS 15.
| 2. | Identify performance obligations |
We assessed that there could be one single combined performance obligation for certain arrangements in our material ongoing license and collaboration arrangements under the new standards of IFRS 15; the transfer of a license combined with performance of R&D services. This is because we could consider that the license has no stand-alone value without Galapagos being further involved in the R&D collaboration and that there is interdependence between the license and the R&D services to be provided. For certain arrangements, we could consider that there is a transformational relationship between the license and the R&D services to be delivered. We could estimate that the Galapagos’ activities during the R&D collaboration are going to significantly add to Intellectual Property (IP) and thereby the value of the programs.
| 3. | Determine the transaction price |
We analyzed the transaction prices of our material ongoing license and collaboration agreements currently composed of upfront license fees, R&D milestones and cost reimbursements for R&D services being delivered. Sales based milestones and sales based royalties are part of certain of our arrangements but are not yet included in our revenues as our most advanced license and collaboration arrangement is entering into a late development phase. Transaction price must be re-assessed at each reporting periods under IFRS 15.
| 4. | Allocate the transaction price |
An entity shall allocate the transaction price to each performance obligation identified in the contract on a relative stand-alone selling price. The transaction price of certain of our arrangements could be allocated to a single combined performance obligation when the transfer of a license is considered to be combined with performance of R&D services. R&D milestone payment is variable consideration that could be entirely allocated to a specific performance obligation or to a distinct good or service that forms part of a single performance obligation if certain criteria under IFRS 15 are met.
| 5. | Recognize revenue |
Revenue from certain arrangements could be recognized as Galapagos satisfies a combined performance obligation.
Galapagos could recognize revenues allocated to a combined performance obligation over the estimated service period based on a pattern that reflects the transfer of the services. The revenues recognized would reflect the level of service each period. In this case, Galapagos would use an output model that considers estimates of the percentage of total R&D service costs that are completed each period compared to the total estimated services costs (% of completion method).
Milestone payments could be recognized in revenues entirely when achieved as we achieved a specific performance obligation or to a distinct good or service that forms part of a single performance obligation if certain criteria under IFRS 15 are met.
Costs reimbursements could be recognized in revenues when costs are incurred and agreed by the parties as Galapagos is acting as a principal in the scope of its stake of the R&D activities of its ongoing license and collaboration agreements.
F-11
Table of Contents
Assessment of the impact of IFRS 15
As the company’s assessment of all contracts, potential performance obligations, and potential allocation of the revenue is still ongoing, the company is not able at this stage to provide a final estimate of the impact of IFRS 15 on its consolidated financial statements. The company plans to adopt IFRS 15 on the effective date.
Assessment of the impact of the implementation of IFRS 9 Financial Instruments and subsequent amendments (applicable for annual periods beginning on or after January 1, 2018) on our consolidated financial statements.
The IASB has issued IFRS 9 Financial Instruments, with an effective date of January 1, 2018, endorsed by the EU in the fourth quarter of 2016. IFRS 9 addresses the classification, measurement and de-recognition of financial assets and financial liabilities and introduces new rules for hedge accounting. The new standard also introduces expanded disclosure requirements and changes in presentation.
We performed a preliminary assessment evaluating the guidance to determine the potential impact on the consolidated financial statements.
Financial assets and financial liabilities are recognized on our balance sheet when we become a party to the contractual provisions of the instrument. Hedging and derivatives have never been used: we do not actively use currency derivatives to hedge planned future cash flows, nor do we make use of forward foreign exchange contracts. However, at year-end 2015 and until 19 January 2016, an embedded derivative existed under the terms of the Gilead contract (see note 7).
As of December 31, 2016, some equity instruments held by the group were classified as “available-for-sale”. The group applies IAS 39 for its equity instruments.
We performed a preliminary assessment of the impact of the implementation of the new standards of IFRS 9 on our current accounting policies under IAS 39, and primarily on the current accounting treatment applied for our available-for-sale financial assets.
All equity investments in scope of IFRS 9 are to be measured at fair value in the statement of financial position, with value changes recognized in profit or loss, except for those equity investments for which the entity has elected to present value changes in ‘other comprehensive income’. There is no ‘cost exception’ for unquoted equities.
‘Other comprehensive income’ option under IFRS 9
If an equity investment is not held for trading, an entity can make an irrevocable election at initial recognition to measure it at fair value through other comprehensive income with only dividend income recognized in profit or loss.
Assessment of the impact of IFRS 9
Galapagos’ preliminary assessment is that the coming new standards IFRS 9 Financial Instruments and subsequent amendments (applicable for annual periods beginning on or after January 1, 2018) should not have a material impact on our consolidated financial statements. The company plans to adopt IFRS 9 on the effective date.
IFRS 16 Leases
The IASB has issued IFRS 16 Leases (applicable for annual periods beginning on or after January 1, 2019) currently awaiting EU endorsement. The standard requires that all leases be recognized in the balance sheet with
F-12
Table of Contents
a corresponding lease liability, except for short term assets and minor assets. IFRS 16 required leased assets to be amortized over the lease term, and payments will be allocated between instalments on the lease obligation and interest expense, classified as financial items. In addition, the nature of the expenses related to those leases will change as IFRS 16 replaces the straight-line operating lease expense with a depreciation charge for right of the use assets and interest expense on lease liabilities.
We know that this new coming standard will have an impact on our consolidated financial statements in 2019 and we are currently evaluating the guidance to determine this impact. We plan to adopt IFRS 16 on the effective date.
CONSOLIDATED REPORTING
The consolidated financial statements comprise the financial statements of Galapagos NV and entities controlled by Galapagos NV. Control is achieved where Galapagos NV has the power to govern the financial and operating policies of another entity so as to obtain benefits from its activities. The results of subsidiaries are included in the income statement and statement of comprehensive income from the effective date of acquisition up to the date when control ceases to exist. Where necessary, adjustments are made to the financial statements of subsidiaries to ensure consistency with our accounting policies. All intra-group transactions, balances, income and expenses are eliminated when preparing the consolidated financial statements.
BUSINESS COMBINATIONS
The acquisition of subsidiaries is accounted for using the acquisition method. The cost of the acquisition is measured as the aggregate of the fair values, at the date of exchange, of assets given, liabilities incurred or assumed, and equity instruments issued by us in exchange for control of the acquired entity.
The acquired entity’s identifiable assets, liabilities and contingent liabilities that meet the conditions for recognition under IFRS 3 are recognized at their fair value at the acquisition date.
Goodwill arising on business combinations is recognized as an asset and initially measured as excess of the cost of acquisition over our interest in the fair value of the identifiable assets, liabilities and contingent liabilities of the acquired subsidiary less the value of the non-controlling interests at date of the acquisition. Goodwill is not amortized but tested for impairment on an annual basis and whenever there is an indication that the cash generating unit to which goodwill has been allocated may be impaired. Goodwill is stated at cost less accumulated impairment losses. An impairment loss recognized for goodwill is not reversed in a subsequent period.
In cases in which the acquirer’s interest in the net fair value of the acquired entity’s identifiable assets, liabilities and contingent liabilities less the value of the non-controlling interests exceeds cost, all fair values and cost calculations are reassessed. In the event that an excess still exists, it is immediately recognized in the profit or loss statement.
INTANGIBLE ASSETS
Expenditure on research activities is recognized as an expense in the period in which it is incurred.
An internally generated intangible asset arising from our development activities is recognized only if all of the following conditions are met:
| • | Technically feasible to complete the intangible asset so that it will be available for use or sale |
| • | We have the intention to complete the intangible assets and use or sell it |
| • | We have the ability to use or sell the intangible assets |
F-13
Table of Contents
| • | The intangible asset will generate probable future economic benefits, or indicate the existence of a market |
| • | Adequate technical, financial and other resources to complete the development are available |
| • | We are able to measure reliably the expenditure attributable to the intangible asset during its development. |
The amount capitalized as internally generated intangible assets is the sum of the development costs incurred as of the date that the asset meets the conditions described above.
Internally generated intangible assets are amortized on a straight-line basis over their estimated useful lives. If the recognition criteria for accounting as an intangible asset are not met, development costs are recognized as an expense in the period in which they are incurred.
Intellectual property, which comprises patents, licenses and rights, is measured internally at purchase cost and is amortized on a straight-line basis over the estimated useful life on the following bases:
| • | Customer relationships: 1–10 years |
| • | In process technology: 3–5 years |
| • | Software & databases: 3–5 years |
| • | Brands, licenses, patents & know how: 5–15 years |
In the event an asset has an indefinite life, this fact is disclosed along with the reasons for being deemed to have an indefinite life.
PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment are recognized at cost less accumulated depreciation and any impairment loss. Depreciation is recognized so as to write off the cost or valuation of assets over their useful lives, using the straight-line method, on the following bases:
| • | Installation & machinery: 4–15 years |
| • | Furniture, fixtures & vehicles: 4–10 years |
Any gain or loss incurred at the disposal of an asset is determined as the difference between the sale proceeds and the carrying amount of the asset, and is recognized in profit or loss.
LEASEHOLD IMPROVEMENTS
Leasehold improvements are depreciated over the term of the lease, unless a shorter useful life is expected.
ASSETS HELD UNDER FINANCE LEASE
Assets held under finance leases are depreciated over their useful lives on the same bases as owned assets or, where shorter, over the term of the related lease agreement.
INVENTORIES
Inventories are valued at the lower of cost and net realizable value. The net realizable value represents the estimated sales price less all estimated costs for completion and costs for marketing, sales and logistics.
Cost of raw materials comprises mainly purchase costs. Raw materials are not ordinarily interchangeable, and they are as such accounted for using the specific identification of their individual cost.
F-14
Table of Contents
FINANCIAL INSTRUMENTS
Financial assets and financial liabilities are recognized on our balance sheet when we become a party to the contractual provisions of the instrument. Hedging and derivatives have never been used: we do not actively use currency derivatives to hedge planned future cash flows, nor do we make use of forward foreign exchange contracts. However, at year-end 2015 and until January 19, 2016 an embedded derivative existed under the terms of the Gilead contract (see note 7).
Available-for-sale financial assets
The group applies IAS 39 for its equity instruments. At the time of purchase, management determines the financial instrument’s classification and reviews this classification at each reporting date. The classification depends on the purpose of acquiring the financial instrument. As of December 31, 2016, some financial instruments held by the group were classified as “available-for-sale”. These financial instruments are recognized or derecognized as of the date of settlement. Following their initial recognition, available-for-sale financial assets are measured at fair value, and any resulting gain or loss is reported directly in the revaluation reserve within equity until the financial instruments are sold, redeemed, otherwise disposed of or considered impaired, at which time the accumulated gain or loss is reported in profit and loss. Initial recognition at fair value is defined as the fair value of the consideration provided net of transaction costs. However, when investments in equity instruments do not have a quoted market price in an active market and the fair value cannot be reliably measured; those equity instruments are measured at cost.
RESEARCH AND DEVELOPMENT INCENTIVES RECEIVABLES
The R&D incentives receivables relate to refunds resulting from R&D incentives on research and development expenses in France and Belgium. Non-current research and development incentives receivables are discounted over the period until maturity date according to the appropriate discount rates.
TRADE RECEIVABLES
Trade receivables do not carry any interest and are stated at their nominal value reduced by appropriate allowances for irrecoverable amounts.
CASH AND CASH EQUIVALENTS
Cash and cash equivalents are measured at nominal value. For the purposes of the cash flow statements, cash and cash equivalents comprise cash on hand, deposits held on call with banks, other short-term deposits and highly liquid investments. Cash and cash equivalents exclude restricted cash which is presented separately in the statement of financial position.
TRADE PAYABLES
Trade payables bear no interest and are measured at their nominal value.
TAXATION
Income tax in the profit or loss accounts represents the sum of the current tax and deferred tax.
Current tax is the expected tax payable on the taxable profit of the year. The taxable profit of the year differs from the profit as reported in the financial statements as it excludes items of income or expense that are taxable or deductible in other years and it further excludes items that are never taxable or deductible. Our liability for current tax is calculated using tax rates that have been enacted or substantively enacted by the balance sheet date.
F-15
Table of Contents
Deferred income tax is provided in full, using the liability-method, on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the financial statements. However, the deferred income tax is not accounted for if it arises from the initial recognition of an asset or liability in a transaction other than a business combination that at the time of the transaction affects neither accounting nor taxable profit nor loss.
Deferred income tax is determined using tax rates (and laws) that have been enacted or substantially enacted by the balance sheet date and are expected to apply when the related deferred income tax asset is realized or the deferred income tax liability is settled. Deferred tax assets are recognized to the extent that it is probable that future taxable profit will be available against which the temporary differences can be utilized. As such, a deferred tax asset for the carry forward of unused tax losses will be recognized to the extent that is probable that future taxable profits will be available.
FOREIGN CURRENCIES
| • | Functional and presentation currency |
Items included in the financial statements of each of our entities are valued using the currency of the primary economic environment in which the entity operates. The consolidated financial statements are presented in Euros, which is our functional and presentation currency.
| • | Transactions and balances in foreign currency |
Foreign currency transactions are translated into the functional currency using the exchange rates prevailing at the dates of transaction. We are using monthly transaction rates based on the closing exchange rates of the foreign currencies on the last business day of the month preceding the date of the transaction. Foreign currency gains and losses resulting from the settlement of such transactions and from the translation at closing rates of monetary assets and liabilities denominated in foreign currencies are recognized in the income statement.
Non-monetary assets and liabilities measured at historical cost that are denominated in foreign currencies are translated using the exchange rate at the date of the transaction.
| • | Financial statements of foreign group companies |
The results and financial position of all our entities that have a functional currency different from Euro are translated as follows:
| • | Assets and liabilities for each balance sheet presented are translated at the closing rate at the date of that balance sheet; |
| • | Income and expenses for each income statement are translated at average exchange rates; |
| • | All resulting cumulative exchange differences are recognized as a separate component of equity; |
| • | Such cumulative exchange differences are recognized in profit or loss in the period in which the foreign operation is disposed of. |
RECOGNITION OF EXPENSES LINKED TO CLINICAL TRIAL MILESTONES
We recognize expenses specifically linked to clinical trial milestones with regard to patient recruitment and patient treatment (i.e. completion), incurred in carrying out clinical trials, in line with actual patient recruitment or treatment at each period end, in reference to the milestone targets for patient recruitment or treatment.
This involves the calculation of clinical trial accruals at each period end, for which an estimation of the expected full clinical trial milestone cost is required, as well as the current stage of patient recruitment or treatment.
F-16
Table of Contents
Clinical trials usually take place over extended time periods and typically involve a set-up phase, a recruitment phase and a completion phase which ends upon the receipt of a final report containing full statistical analysis of trial results. Accruals for patient recruitment and patient completion are prepared separately for each clinical trial in progress and take into consideration the stage of completion of each trial including the number of patients that have entered the trial and the number of patients that have been treated in the trial. In all cases, the full cost of each trial is expensed by the time the final report is received.
REVENUE RECOGNITION
Revenues to date have consisted principally of milestones, license fees and upfront payments received in connection with collaboration and alliance agreements. We also generate revenue from our fee-for-service activities, and various research and development incentives and grants.
Collaboration and alliance agreements with our commercial partners for research and development activities generally include non-refundable upfront fees; costs reimbursements; milestone payments, the receipt of which is dependent upon the achievement of certain clinical, regulatory or commercial milestones; license fees and royalties on sales.
The revenue recognition policies can be summarized as follows:
Upfront payments
Non-refundable, upfront payments received in connection with research and development collaboration agreements are deferred and recognized over the relevant, required periods of our involvement. The payments and our involvement relate to a contractually defined phase of the project. At inception management estimates the period of our involvement as well as the cost involved in the project. Upfront payments are recognized over the estimated period of involvement, either on a straight line basis or based on the cost incurred under the project if such cost can be reliably estimated. Periodically we reassess the estimated time and cost to complete the project phase and adjust the time period over which the revenue is deferred accordingly.
Milestone payments
Research milestone payments are recognized as revenues when achieved. In addition, the payments have to be acquired irrevocably and the milestone payment amount needs to be substantive and commensurate with the magnitude of the related achievement. Milestone payments that are not substantive, not commensurate or that are not irrevocable are recorded as deferred revenue. Revenue from these activities can vary significantly from period to period due to the timing of milestones.
Reimbursement income
Cost reimbursements resulting from license and collaboration agreements with our commercial partners are recognized as reimbursement income in revenue as the related costs are incurred and upon agreement by the parties involved. The corresponding expenses are included in research and development expenditure.
Cost reimbursements from collaboration in which we share equally in the risks and benefits associated with development of a specific drug with a collaboration partner are recognized as decrease of the related incurred research and development expenditure.
Licenses
Revenues from term licenses are spread over the period to which the licenses relate, reflecting the obligation over the term, to update content and provide ongoing maintenance. Revenues from perpetual licenses are recognized immediately upon sale to the extent that there are no further obligations.
F-17
Table of Contents
Royalties
Royalty revenues are recognized when we can reliably estimate such amounts and collectability is reasonably assured. As such, we generally recognize royalty revenues in the period in which the licensees are reporting the royalties to us through royalty reports, that is, royalty revenues are generally recognized in arrears, i.e. after the period in which sales by the licensees occurred. Under this accounting policy, the royalty revenues we report are not based upon our estimates and such royalty revenues are typically reported in the same period in which we receive payment from our licensees.
Grants and R&D incentives
As we carry out extensive research and development activities, we benefit from various grants and R&D incentives from certain governmental agencies. These grants and R&D incentives generally aim to partly reimburse approved expenditures incurred in our research and development efforts and are credited to the income statement, under other income, when the relevant expenditure has been incurred and there is reasonable assurance that the grants or R&D incentives are receivable.
INTERESTS IN JOINT OPERATIONS
A joint operation is a joint arrangement whereby the parties that have joint control of the arrangement have rights to the assets and obligations for the liabilities, relating to the arrangement. Joint control is the contractually agreed sharing of control of an arrangement, which exists only when decisions about the relevant activities require unanimous consent of the parties sharing control.
When we undertake our activities under joint operations, we as a joint operator recognize in relation to our interest in a joint operation:
| • | Our assets, including our share of any assets held jointly |
| • | Our liabilities, including our share of any liabilities incurred jointly |
| • | Our revenue from the sale of our share of the output arising from the joint operation |
| • | Our share of the revenue from the sale of the output by the joint operation |
| • | Our expenses, including our share of any expenses incurred jointly |
We account for the assets, liabilities, revenues and expenses relating to our interest in a joint operation in accordance with IFRSs applicable to the particular assets, liabilities, revenues and expenses.
When we transact with a joint operation in which we are a joint operator (such as sale or contribution of assets), we are considered to be concluding the transaction with the other parties to the joint operation, and gains and losses resulting from the transactions are recognized in our consolidated financial statements only to the extent of other parties’ interests in the joint operation.
When we transact with a joint operation in which we are a joint operator (such as purchase of assets), we do not recognize our share of the gains and losses until we resell those assets to a third party.
EQUITY INSTRUMENTS
Equity instruments issued by us are measured by the fair value of the proceeds received, net of direct issue costs.
EMPLOYEE BENEFITS
a/ Defined contribution plans
Contributions to defined contribution pension plans are recognized as an expense in the income statement as incurred.
F-18
Table of Contents
b/ Defined benefit plans
For defined retirement benefit plans, the cost of providing benefits is determined using the projected unit credit method, with actuarial valuations being carried out at the end of each annual reporting period. Re-measurement, comprising actuarial gains and losses, the effect of the changes to the asset ceiling (if applicable) and the return on plan assets (excluding interest), is reflected immediately in the statement of financial position with a charge or credit recognized in other comprehensive income in the period in which they occur. Re-measurement recognized in other comprehensive income is reflected immediately in retained earnings and will not be reclassified to profit or loss. Past service cost is recognized in profit or loss in the period of a plan amendment. Net interest is calculated by applying the discount rate at the beginning of the period to the net defined benefit liability or asset. Defined benefit costs are categorized as follows:
| • | Service cost (including current service cost, past service cost, as well as gains and losses on curtailments and settlements) |
| • | Net interest expenses or income |
| • | Re-measurement |
The retirement benefit obligation recognized in the consolidated statement of financial position represents the actual deficit or surplus in the defined benefit plans. Any surplus resulting from this calculation is limited to the present value of any economic benefits available in the form of refunds from the plans or a reduction in future contributions to the plans. A liability for a termination benefit is recognized at the earlier of when we can no longer withdraw the offer of the termination benefit and when we recognize any related restructuring costs.
c/ Staff bonus plan
We recognize an expense in the income statement for staff bonus plans.
d/ Management bonus plan
The executive committee members, together with other senior managers, are eligible to receive bonuses under the Senior Management Bonus Scheme established in 2006. Pursuant to the rules of the Senior Management Bonus Scheme, 50% of the bonus is paid immediately around year-end and the payment of the remaining 50% is deferred for three years. The deferred 50% component is dependent on the Galapagos share price change relative to the Next Biotech Index (which tracks Euronext-listed biotech companies). The Galapagos share price and the Next Biotech Index at the start and end of the 3-year period is calculated by the average price over the preceding and last month of the 3-year period, respectively.
| • | If the Galapagos share price change is better than or equal to the change in the Next Biotech Index, the deferred bonus will be adjusted by the share price increase/decrease and paid out |
| • | If the Galapagos share price change is up to 10% worse than the change in the Next Biotech Index, 50% of the deferred bonus will be adjusted by the share price increase/decrease and paid out, and the remainder will be forfeited |
| • | If the Galapagos share price change is more than 10% worse than the change in the Next Biotech Index the deferred bonus will be forfeited |
The possible payment of the deferred component of the Senior Management Bonus Schemes within three years is recognized at the moment that the bonus amount is determined, based on the fair value of the liability at each reporting period. The fair value of the liability is measured by use of the Monte Carlo valuation model taking into consideration (a) the average reference price of the Galapagos share and Next Biotech Index, (b) the average price of the reporting period of the Galapagos share and the Next Biotech Index, (c) the simulation of the evolution of the Galapagos share price and the Next Biotech Index based on their volatility and correlation until
F-19
Table of Contents
maturity of the bonus, (d) the applicable discount rates at the end of the reporting period and (e) the probability of the number of beneficiaries assumed to stay with us until maturity of the bonus. The changes in fair value are recognized in profit or loss for the period.
SHARE-BASED PAYMENTS
We grant equity-settled incentives to certain employees, directors and consultants in the form of warrants. Equity-settled warrants are measured at fair value at the date of acceptance. The fair value determined at the acceptance date of the warrants is expensed over the vesting period, based on our estimate of warrants that are expected to be exercised. Fair value is measured by use of the Black & Scholes model. The expected life used in the model has been adjusted, based on management’s best estimate, for the effects of non-transferability, exercise restrictions, and behavioral considerations.
PROVISIONS
Provisions are recognized on the balance sheet when we have a present obligation as a result of a past event; when it is probable that an outflow of resources embodying economic benefits will be required to settle the obligations and a reliable estimate can be made of the amount of the obligations. The amount recognized as a provision is the best estimate of the expenditure required to settle the present obligation at the balance sheet date. If the effect is material, provisions are determined by discounting the expected future cash flows at a pre-tax rate that reflects current market assessments of the time value of the money and, when appropriate, the risk specified to the liability.
FINANCE AND OPERATING LEASES
Leases are classified as finance leases whenever the terms of the lease substantially transfer all the risks and rewards of ownership to the lessee. All other leases are classified as operating leases.
Assets held under finance leases are recognized as our assets at their fair value or, if lower, at the present value of the minimum lease payments, each determined at the inception of the lease. The corresponding liability to the lessor is included in the balance sheet as a finance lease obligation. The payments are divided proportionally between the financial costs and a diminution of the outstanding balance of the obligation, so that the periodic interest rate on the outstanding balance of the obligation would be constant. Interest is recognized in the income statement, unless it is directly attributable to the corresponding asset, in which case they are capitalized.
Rents paid on operating leases are charged to income on a straight-line basis over the term of the relevant lease. Benefits received and receivable as an incentive to enter into an operating lease are also spread on a straight-line basis over the lease term.
IMPAIRMENT OF TANGIBLE AND INTANGIBLE ASSETS
At each balance sheet date, we review the carrying amount of our tangible and intangible assets to determine whether there is any indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss (if any). Where the asset does not generate cash flows that are independent from other assets, we estimate the recoverable amount of the cash-generating unit to which the asset belongs.
An intangible asset with an indefinite useful life is tested for impairment annually, and whenever there is an indication that the asset might be impaired. The recoverable amount is the higher of fair value less costs to sell and value in use.
If the recoverable amount of an asset or cash generating unit is estimated to be less than the carrying amount, the carrying amount of the asset is reduced to its recoverable amount. An impairment loss is recognized as an expense immediately.
F-20
Table of Contents
When an impairment loss subsequently reverses, the carrying amount of the asset is increased to the revised estimate of its recoverable amount, but so that the increased carrying amount does not exceed the carrying amount that would have been determined, had no impairment loss been recognized for the asset in prior years. A reversal of an impairment loss resulting from a sale of a subsidiary is recognized as income. In other cases impairment losses of goodwill are never reversed.
NET INCOME / LOSS PER SHARE
Basic net income/loss per share is computed based on the weighted average number of shares outstanding during the period. Diluted net income per share is computed based on the weighted average number of shares outstanding including the dilutive effect of warrants, if any.
DISCONTINUED OPERATIONS
A discontinued operation is a component of us that either has been disposed of or is classified as held for sale and (a) represents a separate major line of business or geographical area of operations, (b) is part of a single coordinated plan to dispose of a separate major line of business or geographical area of operations, or (c) is a subsidiary acquired exclusively with a view to resale.
SEGMENT REPORTING
Segment results include revenue and expenses directly attributable to a segment and the relevant portion of revenue and expenses that can be allocated on a reasonable basis to a segment. Segment assets and liabilities comprise those operating assets and liabilities that are directly attributable to the segment or can be allocated to the segment on a reasonable basis; and do not include income tax items. We have only two segments.
3. Segment information
In 2015, the IFRS 8 threshold of 10% of the combined revenues, external and inter-segment, of all segments was met by the external and internal revenues reported by our fee-for-service business located in Croatia. Consequently, there were two reportable segments in 2015 and 2016, R&D and fee-for-service business.
In 2014, following the sale of the service division on April 1, 2014, the continuing operations related primarily to R&D activities. Consequently, there was one reportable segment as at December 31, 2014.
However, our fee-for-service business was presented separately for the year 2014 for comparison with the years 2015 and 2016.
Segment information for year 2016
(Euro, in thousands)
| R&D | Fee-for-services | Inter-segment elimination |
Group | |||||||||||||
| External revenue |
121,616 | 7,903 | — | 129,519 | ||||||||||||
| Internal revenue |
— | 4,379 | (4,379 | ) | — | |||||||||||
| Other income |
21,922 | 171 | — | 22,093 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Revenues & other income |
143,538 | 12,453 | (4,379 | ) | 151,612 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Segment result |
1,138 | (1,787 | ) | (649 | ) | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Unallocated expenses(1) |
(10,841 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Operating loss |
(11,491 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Financial income(2) |
65,737 | |||||||||||||||
|
|
|
|||||||||||||||
| Result before tax |
54,246 | |||||||||||||||
|
|
|
|||||||||||||||
| Incomes taxes(2) |
(235 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Net profit |
54,012 | |||||||||||||||
|
|
|
|||||||||||||||
F-21
Table of Contents
| (1) | The unallocated expenses of €10,841 thousand were composed of (a) €11,034 thousand of warrant costs; and (b) €193 thousand of reduced cost from the IAS19R reclassification of actuarial losses on long term defined post-employment benefit obligations, from profit or loss accounts to other comprehensive income. The above listed items are not presented to management in our management reporting as segment results, and are, therefore, presented on the line “unallocated expenses” in our segment reporting. |
| (2) | Cash and taxes are handled at group level and are therefore presented under unallocated (expenses) / income. |
Segment information for year 2015
(Euro, in thousands)
| R&D | Fee-for-services | Inter-segment elimination |
Group | |||||||||||||
| External revenue |
34,129 | 5,434 | — | 39,563 | ||||||||||||
| Internal revenue |
— | 5,459 | (5,459 | ) | ||||||||||||
| Other income |
20,778 | 238 | — | 21,017 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Revenues & other income |
54,907 | 11,131 | (5,459 | ) | 60,579 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Segment result |
(82,024 | ) | (2,690 | ) | (84,713 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Unallocated expenses(1) |
(4,731 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Operating loss |
(89,444 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Financial (expenses)/income(2) |
(30,184 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Result before tax |
(119,627 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Incomes taxes(2) |
1,218 | |||||||||||||||
|
|
|
|||||||||||||||
| Net loss |
(118,410 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| (1) | The unallocated expenses of €4,731 thousand were composed of (a) €5,036 thousand of warrant costs; (b) €507 thousand of decrease in depreciation cost triggered by an IFRS adjustment on the depreciation charges reported by Fidelta (Croatia) reflecting the expected useful lifetime following the purchase accounting of the acquisition of the Zagreb Research operations of GSK in 2010; and (c) €202 thousand of cost from the IAS19R reclassification of actuarial gains on long term defined post-employment benefit obligations, from profit or loss accounts to other comprehensive income. The above listed items are not presented to management in our management reporting as segment results, and are, therefore, presented on the line “unallocated expenses” in our segment reporting. |
| (2) | Cash and taxes are handled at group level and are therefore presented under unallocated (expenses) / income. |
F-22
Table of Contents
Segment information for the year 2014
(Euro, in thousands)
| R&D | Fee-for-services | Inter-segment elimination |
Group | |||||||||||||
| External revenue |
65,642 | 3,726 | — | 69,368 | ||||||||||||
| Internal revenue |
— | 4,083 | (4,083 | ) | — | |||||||||||
| Other income |
20,437 | 217 | — | 20,653 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Revenues & other income |
86,079 | 8,025 | (4,083 | ) | 90,021 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Segment result |
(30,369 | ) | (4,704 | ) | (35,073 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Unallocated expenses(1) |
(1,551 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Operating loss |
(36,624 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Financial (expenses)/income(2) |
1,424 | |||||||||||||||
|
|
|
|||||||||||||||
| Result before tax |
(35,201 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Incomes taxes(2) |
(2,103 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Net loss from continuing operations |
(37,303 | ) | ||||||||||||||
|
|
|
|||||||||||||||
| Net income from discontinued operations |
70,514 | |||||||||||||||
|
|
|
|||||||||||||||
| Net income |
33,211 | |||||||||||||||
|
|
|
|||||||||||||||
| (1) | Unallocated expenses consisted mainly of expenses for warrant plans under IFRS 2. |
| (2) | Cash and taxes are handled at group level and are therefore presented under unallocated (expenses)/income. |
Segment assets and liabilities are not information being provided to the chief operating decision maker on a recurring basis. This information is therefore not disclosed in our segment information.
GEOGRAPHICAL INFORMATION
In 2014, 2015 and 2016, our operations were located in Belgium, Croatia, France and the Netherlands.
In 2016 our top 10 customers represented 98% of the revenues. In 2015 our top 10 customers represents 97% of the revenues. In 2014 the continuing operations top 10 customers represents 98% of the revenues. Our client base in 2016, 2015 and 2014 included six of the top 20 pharmaceutical companies in the world.
Following table summarizes the revenues by destination of customer:
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| United States |
€ | 88,628 | € | 17,077 | € | 31,100 | ||||||
| Europe |
40,884 | 22,446 | 38,169 | |||||||||
| Asia Pacific |
6 | 40 | 100 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
€ | 129,519 | € | 39,563 | € | 69,368 | ||||||
|
|
|
|
|
|
|
|||||||
F-23
Table of Contents
Following table summarizes the revenues by major customers:
| Year ended December 31, | ||||||||||||||||||||||||
| Spilt up of revenues by major customers | 2016 | 2015 | 2014 | |||||||||||||||||||||
| (Euro, in thousands) |
% | (Euro, in thousands) |
% | (Euro, in thousands) |
% | |||||||||||||||||||
| Gilead |
87,813 | 68 | % | — | 0 | % | — | 0 | % | |||||||||||||||
| United States |
87,813 | 68 | % | — | 0 | % | — | 0 | % | |||||||||||||||
| AbbVie |
32,596 | 25 | % | 29,870 | 75 | % | 54,092 | 78 | % | |||||||||||||||
| Europe |
32,596 | 25 | % | 13,640 | 34 | % | 24,054 | 35 | % | |||||||||||||||
| United States |
— | 0 | % | 16,229 | 41 | % | 30,038 | 43 | % | |||||||||||||||
| Janssen Pharmaceutica |
243 | 0 | % | 566 | 1 | % | 8,662 | 12 | % | |||||||||||||||
| Europe |
156 | 0 | % | 112 | 0 | % | 8,662 | 12 | % | |||||||||||||||
| United States |
87 | 0 | % | 454 | 1 | % | — | 0 | % | |||||||||||||||
| Les Laboratoires Servier |
265 | 0 | % | 3,835 | 10 | % | 2,095 | 3 | % | |||||||||||||||
| Europe |
265 | 0 | % | 3,835 | 10 | % | 2,095 | 3 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total revenues |
120,917 | 93 | % | 34,271 | 87 | % | 64,849 | 93 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Following table summarizes the revenues of the continuing operations by destination:
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Galapagos NV (Belgium) |
€ | 121,703 | € | 34,082 | € | 65,448 | ||||||
| Galapagos SASU (France) |
84 | 25 | 108 | |||||||||
| Fidelta d.o.o. (Croatia) |
7,732 | 5,440 | 3,726 | |||||||||
| Xenometrix, Inc. (United States) |
— | 16 | 86 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
€ | 129,519 | € | 39,563 | € | 69,368 | ||||||
|
|
|
|
|
|
|
|||||||
In 2016, we held €76 million of non-current assets (€68 million in 2015; €57 million in 2014) distributed as follows:
| • | Belgium: €37 million (€30 million in 2015; €25 million in 2014) |
| • | France: €31 million (€29 million in 2015; €26 million in 2014) |
| • | Croatia: €4 million (€5 million in 2015; €4 million in 2014) |
| • | The Netherlands: €4 million (€4 million in 2015; €1 million in 2014) |
The increase in non-current assets 2016 vs 2015 was mainly explained by the increase in non-current R&D incentives receivables (see note 15).
4. Total revenues and other income
REVENUES
The following table summarizes the revenues for the years ended December 31, 2016, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Recognition of non-refundable upfront payments |
€ | 30,257 | € | 26,419 | € | 45,838 | ||||||
| Milestone payments |
81,784 | 3,835 | 19,039 | |||||||||
| Reimbursement income |
9,699 | 3,807 | 729 | |||||||||
| Other revenues |
7,777 | 5,501 | 3,762 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
€ | 129,519 | € | 39,563 | € | 69,368 | ||||||
|
|
|
|
|
|
|
|||||||
F-24
Table of Contents
Total revenues decreased by €29.8 million, or 43%, to €39.6 million for the year ended December 31, 2015, from €69.4 million for the year ended December 31, 2014. This decrease was mainly driven by lower recognition of non-refundable upfront payments and reduced milestone payments, as explained below.
| • | Revenue from non-refundable upfront payments related to the deferred recognition of upfront payments received under the agreements with AbbVie, amounting to €111.6 million in 2012 and €49.6 million in 2013, which were amortized over a period ranging from 21 to 42 months, based on the estimated period of the involvement. |
| • | Milestone revenues and costs reimbursements decreased by €12.1 million, or 61%, to €7.6 million for the year ended December 31, 2015 compared to €19.8 million for the year ended December 31, 2014. This decrease was primarily related to fewer milestones achieved in 2015 compared to 2014 as a result of the increasing proprietary nature of the pipeline programs. For the year ended December 31, 2015 €2.2 million and €1.2 million of costs were reimbursed in relation with respectively the CF and Filgotinib collaboration agreements with AbbVie, and €3.8 million of milestones related to partnered programs with Servier were recognized. |
| • | Other revenues increased by €1.7 million, or 46%, to €5.5 million for the year ended December 31, 2015 compared to €3.8 million for the year ended December 31, 2014, principally due to higher revenues from fee-for-service activities. |
Total revenues increased by €90.0 million, or 227%, to €129.5 million for the year ended December 31, 2016, from €39.6 million for the year ended December 31, 2015. This increase was mainly driven by a substantial increase in milestone payments, as explained below.
Revenue recognized in 2015 from upfront non-refundable payments related to the CF collaboration agreement with AbbVie signed in September 2013 and the contract signed with AbbVie in February 2012 for our filgotinib program (including the extension signed in March 2013). Those upfront payments were fully recognized into revenues by the end of August 2015.
In September 2015 AbbVie decided not to opt in, which ended the collaboration agreement regarding our filgotinib program and consequently the period of our involvement. There are no outstanding commitments for us regarding this terminated collaboration for our filgotinib program.
On December 16, 2015, we entered into a global collaboration with Gilead Sciences, Inc. for the development and commercialization of the JAK1-selective inhibitor filgotinib for inflammatory indications. On January 19, 2016, we completed the closing of the global collaboration agreement with Gilead, in the framework of which Gilead made a $425 million (or €392 million) equity investment in Galapagos NV by subscribing to new shares at a price of €58 per share, including issuance premium. This resulted in Gilead owning 6,760,701 ordinary shares of Galapagos NV, representing 14.75% percent of the then-outstanding share capital of Galapagos. We also received a license fee of $300 million. In addition, we are eligible for payments of up to $695 million in additional development and regulatory milestones and $600 million in sales milestones, with tiered royalties starting at 20% and a profit split in co-promotion territories. In 2016, $60 million of development milestones was already achieved and paid by Gilead. Furthermore, development costs of the licensed product will be split 20-80. As such Galapagos will support 20% of all development costs. As we do not expect to have a statutory taxable base in the foreseeable future, we did not recognize any additional deferred tax asset following the signing of this new collaboration.
The global collaboration with Gilead foresees continuous involvement from us, since we will perform certain R&D activities in the development phase of the filgotinib program; therefore, management assessed that the upfront payment of $300 million (or €275.6 million) received in January 2016 from Gilead should be spread as a function of the costs incurred for this program, applying the percentage of completion method. In the year ended December 31, 2016, €25.6 million revenues were recognized regarding this upfront payment.
In connection with the agreement with Gilead, we recognized a deferred income and an offsetting short-term financial asset (derivative) of €39 million upon signing of the share subscription agreement with Gilead, as
F-25
Table of Contents
required under IAS 39. We refer to note 7 for further details. The deferred income will be recognized in function of the costs incurred for this program, applying the percentage of completion method, along with the upfront payment. In the year ended December 31, 2016, €3.6 million revenues were recognized in the income statement.
In 2016, Galapagos signed a license agreement with ThromboGenics for an integrin antagonist (formerly GLPG0187), for which an upfront payment of €1 million was invoiced and fully recognized, as Galapagos has no further involvement or obligation in the contract.
The following table summarizes the upfront payments recognition for years ended December 31, 2016 and 2015.
| Agreement |
Upfront received |
Upfront received |
Date of receipt |
Revenue recognized, year ended December 31, 2016 |
Revenue recognized, year ended December 31, 2015 |
Outstanding balance in deferred income as at December 31, 2016 |
||||||||||||||||||
| (USD, in thousands) |
(Euro, in thousands) |
(Euro, in thousands) | ||||||||||||||||||||||
| AbbVie collaboration agreement for CF |
$ | 45,000 | € | 34,001 | September 2013 | — | € | 11,401 | — | |||||||||||||||
| AbbVie collaboration agreement for RA and CD (filgotinib) |
$ | 150,000 | € | 111,582 | February 2012 | — | € | 12,045 | — | |||||||||||||||
| First amendment to AbbVie collaboration agreement for RA and CD (filgotinib) |
$ | 20,000 | € | 15,619 | March 2013 | — | € | 2,973 | — | |||||||||||||||
| Gilead collaboration agreement for filgotinib |
$ | 300,000 | € | 275,558 | January 2016 | € | 25,621 | — | € | 249,937 | ||||||||||||||
| Gilead collaboration agreement for filgotinib |
N.A. | € | 39,003 | (*) | January 2016 | € | 3,626 | — | € | 35,376 | ||||||||||||||
| ThromboGenics license agreement for integrin antagonists |
N.A. | € | 1,000 | April 2016 | € | 1,000 | — | — | ||||||||||||||||
| Sirion Biotech license agreement for RNA interference (RNAi) technologies |
N.A. | € | 10 | June 2016 | € | 10 | — | — | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Total recognition of non-refundable |
€ | 30,257 | € | 26,419 | € | 285,314 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| (*) | deferred income of €39 million booked upon signing of the share subscription agreement with Gilead as required under IAS 39 |
Milestone revenues increased substantially by €77.9 million to €81.8 million for the year ended December 31, 2016 compared to €3.8 million for the year ended December 31, 2015. Milestones in 2016 related to the filgotinib program with Gilead in CD and UC, and the CF program with AbbVie.
Reimbursement income increased by €5.9 million or 155%, to €9.7 million for the year ended December 31, 2016 compared to €3.8 million for the year ended December 31, 2015, due to higher reimbursements in relation with the CF program with AbbVie and the filgotinib program with Gilead (which was partnered with AbbVie in 2015). The reimbursement of certain research and development costs related to the development work under the Galapagos’ collaboration agreements amounted to €5.9 million for our CF program with AbbVie and €3.5 million for our filgotinib program with Gilead for the year ended December 31, 2016.
Other revenues increased by €2.3 million, or 41%, to €7.8 million for the year ended December 31, 2016 compared to €5.5 million for the year ended December 31, 2015, principally due to higher revenues from fee-for-service activities.
F-26
Table of Contents
OTHER INCOME
The following table summarizes other income for the years ended December 31, 2016, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Grant income |
€ | 2,329 | € | 3,095 | € | 5,646 | ||||||
| Other income |
19,764 | 17,922 | 15,008 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other income |
€ | 22,093 | € | 21,017 | € | 20,653 | ||||||
|
|
|
|
|
|
|
|||||||
Total other income was composed of grant income and other income and increased by €0.4 million, or 2%, from €20.7 million for the year ended December 31, 2014 to €21.0 million for the year ended December 31, 2015. Grant income decreased by €2.6 million, or 45%, from €5.6 million for the year ended December 31, 2014 to €3.1 million for the year ended December 31, 2015. The majority of this grant income was related to grants from a Flemish agency, representing approximately 94% of all reported grant income in both years. In many cases these carry clauses which require us to maintain a presence in the same region for a number of years and invest according to pre-agreed budgets.
The decrease in grant income was compensated by an increase in other income of €2.9 million, or 19%, from €15.0 million for the year ended December 31, 2014 to €17.9 million for the year ended December 31, 2015. Other income was primarily composed of:
| • | Income from an innovation incentive system of the French government, which represented €8.7 million of other income for the year ended December 31, 2015 compared to €7.8 million for the year ended December 31, 2014 |
| • | Income from Belgian R&D incentives with regard to incurred R&D expenses, which represented €5.3 million of other income for the year ended December 31, 2015 compared to €4.3 million for the year ended December 31, 2014 |
| • | Tax rebates on payroll withholding taxes of R&D personnel in Belgium and the Netherlands, representing €3.0 million of other income for the year ended December 31, 2015 compared to €2.4 million for the year ended December 31, 2014. |
Total other income was composed of grant income and other income and increased by €1.1 million, or 5%, from €21.0 million for the year ended December 31, 2015 to €22.1 million for the year ended December 31, 2016.
Grant income decreased by €0.8 million, or 25%, from €3.1 million for the year ended December 31, 2015 to €2.3 million for the year ended December 31, 2016. The majority of this grant income is related to grants from a Flemish agency, representing approximately 88% of all reported grant income in 2016 (2015: 94%). In many cases these carry clauses which require us to maintain a presence in the same region for a number of years and invest according to pre-agreed budgets.
The decrease in grant income was more than offset by an increase in other income of €1.8 million, or 10%, from €17.9 million for the year ended December 31, 2015 to €19.8 million for the year ended December 31, 2016. Other income was primarily composed of:
| • | Income from an innovation incentive system of the French government, which represented €9.5 million of other income for the year ended December 31, 2016 compared to €8.7 million for the year ended December 31, 2015 |
| • | Income from Belgian R&D incentives with regard to incurred R&D expenses, which represented €5.8 million of other income for the year ended December 31, 2016 compared to €5.3 million for the year ended December 31, 2015 |
F-27
Table of Contents
| • | Tax rebates on payroll withholding taxes of R&D personnel in Belgium and the Netherlands, representing €3.8 million of other income for the year ended December 31, 2016 compared to €3.0 million for the year ended December 31, 2015 |
5. Operating costs
Operating result has been calculated after charging (-) / crediting:
RESEARCH AND DEVELOPMENT EXPENDITURE
The following table summarizes research and development expenditure for the years ended December 31, 2016, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Personnel costs |
€ | (42,315 | ) | € | (35,875 | ) | € | (31,038 | ) | |||
| Subcontracting |
(65,649 | ) | (65,883 | ) | (54,293 | ) | ||||||
| Disposables and lab fees and premises costs |
(20,414 | ) | (18,696 | ) | (16,830 | ) | ||||||
| Other operating expenses |
(11,196 | ) | (9,260 | ) | (8,949 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total R&D expenditure |
€ | (139,573 | ) | € | (129,714 | ) | € | (111,110 | ) | |||
|
|
|
|
|
|
|
|||||||
R&D expenditure increased by €18.6 million, or 17%, to €129.7 million for the year ended December 31, 2015, from €111.1 million for the year ended December 31, 2014. This increase was principally due to:
| • | Increased R&D personnel costs of €4.8 million, or 16%, from €31.0 million for the year ended December 31, 2014 to €35.9 million for the year ended December 31, 2015, which was explained by an enlarged workforce, higher warrant costs and a higher payable for short-term and long-term management bonus, mainly as a result of the evolution of our share price change relative to the Next Biotech Index on Euronext. |
| • | Increased subcontracting costs of €11.6 million, or 21%, from €54.3 million for the year ended December 31, 2014 to €65.9 million for the year ended December 31, 2015. This cost increase was mainly driven by increased subcontracting costs of €8.4 million for the CF collaboration with AbbVie and to a lesser extent by the increase of €4.2 million in subcontracting costs for our other partnered and internal programs. |
| • | Intensified use of lab consumables was the main driver of the increase in disposables, lab fees and premises costs of €1.9 million, or 11%, from €16.8 million for the year ended December 31, 2014 to €18.7 million for the year ended December 31, 2015 |
| • | Other operating expenses slightly increased by €0.3 million, or 3%, from €8.9 million for the year ended December 31, 2014 to €9.3 million for the year ended December 31, 2015. |
R&D expenditure increased by €9.9 million, or 8%, to €139.6 million for the year ended December 31, 2016, from €129.7 million for the year ended December 31, 2015. This increase was principally due to:
| • | Increased R&D personnel costs of €6.4 million, or 18%, from €35.9 million for the year ended December 31, 2015 to €42.3 million for the year ended December 31, 2016, which was explained by an enlarged workforce, higher warrant costs and a higher payable for short term and long term management bonus, mainly as a result of the increase of our share price change relative to the Next Biotech Index on Euronext |
| • | Intensified use of lab consumables was the main driver of the increase in disposables, lab fees and premises costs of €1.7 million, or 9%, from €18.7 million for the year ended December 31, 2015 to €20.4 million for the year ended December 31, 2016 |
F-28
Table of Contents
| • | Other operating expenses increased by €1.9 million, or 21%, from €9.3 million for the year ended December 31, 2015 to €11.2 million for the year ended December 31, 2016, primarily due to an increase in depreciation of €1.0 million. |
Subcontracting costs were relatively stable and decreased slightly by €0.2 million, or 0.4%, from €65.9 million for the year ended December 31, 2015 to €65.6 million for the year ended December 31, 2016.
The table below summarizes our research and development expenditure for the years ended December 31, 2016, 2015 and 2014, broken down by research and development expenses under alliance and own funded research and development expenses. All filgotinib costs (both costs incurred in the period under alliance (with AbbVie) and costs incurred after AbbVie’s opt-out decision in September 2015) are presented as “R&D under alliance” or as “partnered” in the tables in this section for the year ended December 31, 2015, as a new alliance was signed in December 2015 with Gilead for this program.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| R&D under alliance |
€ | (71,980 | ) | € | (80,832 | ) | € | (76,297 | ) | |||
| Galapagos funded R&D |
(67,593 | ) | (48,882 | ) | (34,813 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total R&D expenditure |
€ | (139,573 | ) | € | (129,714 | ) | € | (111,110 | ) | |||
|
|
|
|
|
|
|
|||||||
All research and development expenditures are tracked against detailed budgets and allocated by individual project. The table below summarizes our research and development expenditure for the years ended December 31, 2016, 2015 and 2014, broken down by program.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Filgotinib program (partnered) |
€ | (22,376 | ) | (35,404 | ) | € | (33,843 | ) | ||||
| CF program (partnered) |
(31,203 | ) | (25,634 | ) | (14,894 | ) | ||||||
| IPF program on GLPG1690 (proprietary) |
(7,129 | ) | (4,612 | ) | (4,592 | ) | ||||||
| OA program on GLPG1972 (partnered) |
(6,538 | ) | (5,832 | ) | (801 | ) | ||||||
| AtD program on MOR106 (partnered) |
(3,491 | ) | (4,651 | ) | (894 | ) | ||||||
| Other |
(68,836 | ) | (53,582 | ) | (56,086 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total R&D expenditure |
€ | (139,573 | ) | € | (129,714 | ) | € | (111,110 | ) | |||
|
|
|
|
|
|
|
|||||||
Research and development expenditure under alliance increased by €4.5 million, or 6%, to €80.8 million for the year December 31, 2015, mainly due to our CF program in collaboration with AbbVie. We also increased our investments in our own funded portfolio by €14.1 million, or 40%, from €34.8 million for the year ended December 31, 2014 to €48.9 million for the year ended December 31, 2015, primarily because GLPG1205 and GLPG1690 programs became own funded.
R&D expenditure under alliance decreased by €8.9 million, or 11%, from €80.8 million for the year ended December 31, 2015 to €72.0 million for the year ended December 31, 2016, mainly due to decreased R&D spending in our RA and IBD program on filgotinib (partnered with AbbVie in 2015 and partnered with Gilead in 2016), which has been partially offset by increased R&D spending on our CF program in collaboration with AbbVie. We increased our investments in our own funded portfolio by €18.7 million, or 38%, from €48.9 million for the year ended December 31, 2015 to €67.6 million for the year ended December 31, 2016, primarily because of intensified research investments in our proprietary programs on inflammation, HBV and fibrosis, as well as increased spending on our proprietary IPF program GLPG1690.
F-29
Table of Contents
GENERAL AND ADMINISTRATIVE EXPENSES
The following table summarizes the general and administrative expenses for the years ended December 31, 2016, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Personnel costs and directors fees |
€ | (15,160 | ) | € | (12,739 | ) | € | (8,087 | ) | |||
| Other operating expenses |
(6,584 | ) | (6,388 | ) | (5,788 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total general and administrative expenses |
€ | (21,744 | ) | € | (19,127 | ) | € | (13,875 | ) | |||
|
|
|
|
|
|
|
|||||||
General and administrative expenses increased by €5.2 million, or 38%, to €19.1 million for the year ended December 31, 2015. This increase was principally due to personnel costs and directors fees, which increased by €4.6 million, or 58%, from €8.1 million for the year ended December 31, 2014 to €12.7 million for the year ended December 31, 2015, resulting from various effects, such as increased costs of share-based payments plans (warrant plans) and increased payables for short- and long-term management bonus, mainly as a result of the evolution of our share price change relative to the Next Biotech Index on Euronext. In addition, other operating expenses increased by €0.6 million, or 10%, from €5.8 million for the year ended December 31, 2014 to €6.4 million for the year ended December 31, 2015, mainly due to higher professional fees.
General and administrative expenses amounted to €19.1 million for the year ended December 31, 2015 and increased by €2.6 million, or 14%, to €21.7 million for the year ended December 31, 2016. This increase was principally due to directors fees, which increased by €2.7 million, or 116%, from €2.4 million for the year ended December 31, 2015 to €5.1 million for the year ended December 31, 2016, resulting from various effects, such as increased costs of share-based payments plans (our warrant plans) and increased payables for short and long term management bonus, mainly as a result of the increase of our share price change relative to the Next Biotech Index on Euronext.
SALES AND MARKETING EXPENSES
The following table summarizes the sales and marketing expenses for the years ended December 31, 2016, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Personnel costs |
€ | (1,167 | ) | € | (785 | ) | € | (579 | ) | |||
| Other operating expenses |
(618 | ) | (397 | ) | € | (412 | ) | |||||
|
|
|
|
|
|
|
|||||||
| Total sales and marketing expenses |
€ | (1,785 | ) | € | (1,182 | ) | € | (992 | ) | |||
|
|
|
|
|
|
|
|||||||
Sales and marketing expenses increased by €0.2 million, or 19%, from €1.0 million for the year ended December 31, 2014, to €1.2 million for the year ended December 31, 2015.
Sales and marketing expenses increased by €0.6 million, or 51%, from €1.2 million for the year ended December 31, 2015 to €1.8 million for the year ended December 31, 2016.
F-30
Table of Contents
RESTRUCTURING COSTS
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Restructuring costs |
€ | — | € | — | € | (669 | ) | |||||
|
|
|
|
|
|
|
|||||||
| Total restructuring and integration costs |
€ | — | € | — | € | (669 | ) | |||||
|
|
|
|
|
|
|
|||||||
The restructuring and integration costs amounted to €0.7 million for the year ended December 31, 2014 and were entirely related to workforce reductions within certain of the R&D operations.
6. Staff costs
The following table illustrates the personnel costs of the continuing operations for the years 2016, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Wages and salaries |
€ | (34,857 | ) | € | (33,676 | ) | € | (26,891 | ) | |||
| Social security costs |
(7,328 | ) | (7,328 | ) | (7,468 | ) | ||||||
| Pension costs |
(1,728 | ) | (1,456 | ) | (1,454 | ) | ||||||
| Other personnel costs |
(9,617 | ) | (4,574 | ) | (2,635 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total personnel costs |
€ | (53,530 | ) | € | (47,034 | ) | € | (38,447 | ) | |||
|
|
|
|
|
|
|
|||||||
The other personnel costs mainly related to costs for warrants granted of €6.6 million (2015: €2.9 million). For the costs of warrants granted, see note 30.
7. Fair value re-measurement of share subscription agreement
On December 16, 2015, Gilead Sciences, Inc. and Galapagos NV entered into a global collaboration for the development and commercialization of filgotinib, in the framework of which Gilead committed to an upfront payment of $725 million consisting of a license fee of $300 million and a $425 million equity investment in Galapagos NV by subscribing to new shares at a price of €58 per share, including issuance premium. This agreement was effectively completed and entered into force January 19, 2016 and full payment was received.
In connection with the agreement, we recognized a deferred income and an offsetting short-term financial asset (derivative) of €39 million upon signing of the share subscription agreement with Gilead as required under IAS 39. This financial asset initially reflects the share premium that Gilead committed to pay above the closing stock price of Galapagos on the day of entering into the subscription agreement. Under IAS 39 the fair value of the financial asset is re-measured at year-end and again upon entering into force of the subscription agreement on January 19, 2016, when the financial asset expired. Variations in fair value of the financial asset are recorded in the income statement.
The decrease in the fair value of the financial asset resulting from the increase in the Galapagos share price between signing of the subscription agreement and December 31, 2015 resulted in a negative, non-cash fair value charge of €30.6 million in the financial results. The subsequent increase in the fair value of the financial asset resulting from the decrease in our share price between January 1st, 2016 and January 19, 2016 resulted in a positive non-cash gain of €57.5 million in the financial result of 2016.
On January 19, 2016, the value of the financial asset at maturity amounted to €65.9 million, reflecting the share premium that Gilead paid above our closing share price on the day of the capital increase. This amount was
F-31
Table of Contents
composed of (1) the initial measurement on the day of entering into the share subscription agreement for an amount of €39 million which was reported in deferred income and (2) the subsequent re-measurements of the financial asset, reported as financial result under IAS 39: €30.6 million fair value loss reported in the year 2015 and €57.5 million fair value gain reported in the year 2016, together a net fair value gain of €26.8 million. This financial asset expired on the effective date of the share subscription agreement and was derecognized through the share premium account.
8. Other financial income / expenses
The following table summarizes other financial income and expense for the years ended December 31, 2016, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Other financial income: |
||||||||||||
| Interest on bank deposit |
€ | 1,614 | € | 1,246 | € | 1,155 | ||||||
| Effect of discounting long term R&D incentives receivables |
99 | 99 | 920 | |||||||||
| Currency exchange gain |
8,150 | 636 | 198 | |||||||||
| Other finance income |
87 | 7 | 17 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other financial income |
9,950 | 1,987 | 2,291 | |||||||||
|
|
|
|
|
|
|
|||||||
| Other financial expenses: |
||||||||||||
| Interest expenses |
(47 | ) | (46 | ) | (110 | ) | ||||||
| Currency exchange loss |
(1,453 | ) | (1,310 | ) | (652 | ) | ||||||
| Other finance charges |
(191 | ) | (182 | ) | (105 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total other financial expense |
(1,692 | ) | (1,539 | ) | (867 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total other net financial income |
€ | 8,257 | € | 448 | € | 1,424 | ||||||
|
|
|
|
|
|
|
|||||||
Other finance income decreased slightly by €0.3 million, or 13%, to €2.0 million for the year ended December 31, 2015. The decrease in the effect of discounting long-term R&D incentives receivables (-€0.8 million) was partly compensated by an increase in currency exchange gains (+ €0.4 million).
Other financial income increased significantly by €8.0 million, or 401%, from €2.0 million for the year ended December 31, 2015 to €10.0 million for the year ended December 31, 2016. The increase primarily related to an exchange gain of €4.8 million on deposits held in U.S. dollar and exchange gains of €2.0 million realized on milestone payments from AbbVie and Gilead in U.S. dollar.
Other finance expense increased by €0.6 million, or 77% to €1.5 million for the year ended December 31, 2015. Net exchange loss amounted to €0.7 million for the year ended December 31, 2015, as compared to €0.5 million for the year ended December 31, 2014.
Other financial expenses increased by €0.2 million, or 10% from €1.5 million for the year ended December 31, 2015 to €1.7 million for the year ended December 31, 2016. Net exchange profit amounts to €6.7 million for the year ended December 31, 2016, compared to a net exchange loss of €0.7 million for the year ended December 31, 2015.
Interest expenses were related to interests paid on financial lease.
F-32
Table of Contents
9. Taxes
INCOME TAXES RELATING TO CONTINUING OPERATIONS
The following table summarizes the income tax recognized in profit or loss for the years ended December 31, 2016, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| Taxes recognized in profit or loss | 2016 | 2015 | 2014 | |||||||||
| (Euro, in thousands) | ||||||||||||
| Continuing operations: |
||||||||||||
| Current tax |
€ | (466 | ) | € | (215 | ) | € | (2,396 | ) | |||
| Deferred tax |
231 | 1,433 | 293 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total continuing operations |
(235 | ) | 1,218 | (2,103 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Discontinued operations: |
||||||||||||
| Current tax |
— | — | € | (437 | ) | |||||||
| Deferred tax |
— | — | 203 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total discontinued operations |
— | — | (234 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total taxes |
€ | (235 | ) | € | 1,218 | € | (2,337 | ) | ||||
|
|
|
|
|
|
|
|||||||
Current tax amounted to €0.5 million for the year ended December 31, 2016 and €0.2 million for the year ended December 31, 2015 and was related to taxes for subsidiaries operating on cost plus basis. Current tax recorded in 2014 for an amount of €2.4 million related to a tax provision for subsidiaries operating under cost plus transfer pricing arrangements, triggered by a tax audit.
Deferred tax income of €0.2 million for the year ended December 31, 2016, €1.4 million for the year ended December 31, 2015 and €0.3 million for the year ended December 31, 2014 related to subsidiaries working on a cost plus basis.
TAX LIABILITIES
The below table illustrates the tax liabilities related captions in the balance sheet on December 31, 2016, 2015 and 2014.
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Current tax payable |
€ | 1,022 | € | 2,583 | € | 2,582 | ||||||
|
|
|
|
|
|
|
|||||||
| Total tax liabilities |
€ | 1,022 | € | 2,583 | € | 2,582 | ||||||
|
|
|
|
|
|
|
|||||||
On December 31, 2015 and 2014, tax liabilities included €2.6 million primarily related to the recognition of tax liabilities for two subsidiaries operating on a cost plus basis. This amount was partly due to a tax audit on the years 2008 to 2011 and underlying proposed tax adjustment amounting to €1.9 million in cash and decrease of our tax losses carried forward for €19.5 million. A liability was recognized in 2014 considering this claim and the potential risk, partly under current tax liability for €1.3 million and partly as a decrease of the R&D incentives receivables for €0.6 million. The tax adjustment was settled in cash in the fourth quarter of 2016. However, discussions are still ongoing with regard to this claim.
F-33
Table of Contents
In addition, taxes on gain on the sale of the service division in 2014 were included in the tax liabilities on December 31, 2015 for €0.4 million and were paid in 2016.
On December 31, 2016, €1.0 million of tax liabilities were primarily related to two of our subsidiaries operating on a cost plus basis.
Corporation tax was calculated at 34% (2015 and 2014: 34%)—which is the tax rate applied in Belgium—on the estimated assessable profit for the year. The applied tax rate for other territorial jurisdictions was the tax rate that is applicable in these respective territorial jurisdictions on the estimated taxable result of the accounting year.
| Year ended December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||
| (Euro, in thousands) | ||||||||||||||||||||
| The tax of the year can be reconciled to the accounting result as follows: |
||||||||||||||||||||
| Income / loss (-) before tax from continuing operations |
€ | 54,246 | € | (119,627 | ) | € | (35,201 | ) | ||||||||||||
| Income before tax from discontinued operations |
— | % | % | 70,748 | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Income/ loss (-) before tax |
54,246 | 34 | (119,627 | ) | 34 | 35,548 | ||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Income tax debit / credit (-), calculated using the Belgian statutory tax rate on the accounting income / loss (-) before tax (theoretical) |
18,438 | (40,661 | ) | 12,083 | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Tax expenses / income (-) in income statement (effective) from continuing operations |
235 | (1,218 | ) | 2,103 | ||||||||||||||||
| Tax expenses in income statement (effective) from discontinued operations |
— | — | 234 | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Tax expenses / income (-) in income statement (effective) |
235 | (1,218 | ) | 2,337 | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Difference in tax expense / income to explain |
€ | (18,203 | ) | € | 39,444 | € | (9,746 | ) | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Effect of tax rates in other jurisdictions |
€ | 163 | € | 328 | € | 6 | ||||||||||||||
| Effect of non taxable revenues |
(27,399 | ) | (5,934 | ) | (41,249 | ) | ||||||||||||||
| Effect of consolidation entry without tax impact |
2 | 57 | 12,786 | |||||||||||||||||
| Effect of non tax deductible expenses |
4,387 | 12,378 | 1,459 | |||||||||||||||||
| Effect of recognition of previously non recognized deferred tax assets |
(421 | ) | (1,307 | ) | (293 | ) | ||||||||||||||
| Effect of change in tax rates |
— | — | (165 | ) | ||||||||||||||||
| Effect of tax losses (utilized) reversed |
(655 | ) | (597 | ) | (1,549 | ) | ||||||||||||||
| Effect from under or over provisions in prior periods |
— | 58 | 2,144 | |||||||||||||||||
| Effect of non recognition of deferred tax assets |
5,720 | 34,783 | 17,688 | |||||||||||||||||
| Effect of R&D tax credit claims |
— | (322 | ) | (572 | ) | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total explanations |
€ | (18,203 | ) | € | 39,444 | € | (9,746 | ) | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
The main difference between the theoretical tax and the effective tax for the years 2016 and 2015 was primarily explained by the unrecognized deferred tax assets on tax losses carried forward for which we conservatively assess that it is not likely that these will be realized in the foreseeable future
The main difference between the theoretical tax and the effective tax for the year 2014 was primarily explained by low capital gain tax (less than 1%) under Belgian tax law, on the gain on sale of the service division (see line non-taxable revenues and effect of consolidation entries), and by the unrecognized deferred tax assets on tax losses carried forward.
Non-taxable revenues for the years ended December 31, 2014, 2015 and 2016 related to non-taxable subsidies and tax credits. Non-taxable revenues in 2016 also included the financial profit related to the fair value re-measurement of the share subscription agreement.
F-34
Table of Contents
10. Discontinued operations
The following table summarizes the results from discontinued operations for the years ended December 31, 2016, 2015 and 2014.
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands, except share and per share data) |
||||||||||||
| Service revenues |
€ | 17,502 | ||||||||||
| Other income |
669 | |||||||||||
|
|
|
|||||||||||
| Total revenues and other income |
18,171 | |||||||||||
|
|
|
|||||||||||
| Services cost of sales |
(11,283 | ) | ||||||||||
| General and administrative expenses |
(3,772 | ) | ||||||||||
| Sales and marketing expenses |
(255 | ) | ||||||||||
| Restructuring and integration costs |
(38 | ) | ||||||||||
| Loss on divestment |
— | |||||||||||
| Gain on sale of service division |
67,508 | |||||||||||
|
|
|
|||||||||||
| Operating income |
70,331 | |||||||||||
|
|
|
|||||||||||
| Finance income / expense (-) |
417 | |||||||||||
|
|
|
|||||||||||
| Income before tax |
70,748 | |||||||||||
|
|
|
|||||||||||
| Income taxes |
(234 | ) | ||||||||||
|
|
|
|||||||||||
| Net income from discontinued operations |
€ | 70,514 | ||||||||||
|
|
|
|||||||||||
| Basic and diluted income per share from discontinued operations |
€ | 2.34 | ||||||||||
|
|
|
|||||||||||
| Weighted average number of shares (in ‘000 shares) |
30,108 | |||||||||||
The service division was sold on April 1, 2014. The above table illustrates the results of the discontinued operations included in the consolidated results of operations for the years ended December 31, 2016, 2015 and 2014. For the year ended December 31, 2014, results only relate to the period from January 1, 2014 through the disposal on April 1, 2014.
Net income amounting to €70.5 million in 2014 was mainly driven by the €67.5 million gain on disposal of the service division.
Cash flows from discontinued operations can be summarized as follows:
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Net cash flows used in operating activities |
— | — | € | (1,722 | ) | |||||||
| Net cash flows generated in investing activities |
— | — | 122,580 | |||||||||
| Net cash flows generated/ used (-) in financing activities |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash generated |
€ | — | € | — | € | 120,858 | ||||||
|
|
|
|
|
|
|
|||||||
F-35
Table of Contents
11. Result per share
Basic result per share is calculated by dividing the net result attributable to shareholders by the weighted average number of ordinary shares issued during the year.
Diluted result per share is calculated based on the weighted average number of shares (diluted) also considering outstanding warrants, for which our average share price of the year was higher than the exercise price.
Income / loss per share
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Income/ loss per share: |
||||||||||||
| Result for the purpose of basic income / loss (-) per share (thousands €) |
€ | 54,012 | € | (118,410 | ) | € | 33,211 | |||||
| Number of shares (thousands) |
||||||||||||
| Weighted average number of shares for the purpose of basic income / loss per share |
45,696 | 35,700 | 30,108 | |||||||||
|
|
|
|
|
|
|
|||||||
| Basic income / loss (-) per share (Euros) |
€ | 1.18 | € | (3.32 | ) | € | 1.10 | |||||
|
|
|
|
|
|
|
|||||||
| Result for the purpose of diluted income/ loss (-) per share |
€ | 54,012 | € | (118,410 | ) | € | 33,211 | |||||
| Number of shares (thousands) |
||||||||||||
| Weighted average number of shares for the purpose of diluted income / loss per share |
45,696 | 35,700 | 30,108 | |||||||||
| Number of dilutive potential ordinary shares |
1,612 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted income / loss (-) per share (Euros) |
€ | 1.14 | € | (3.32 | ) | € | 1.10 | |||||
|
|
|
|
|
|
|
|||||||
As our continuing operations report a net loss in 2014 and 2015, the outstanding warrants (specified in note 30) have an anti-dilutive effect rather than a dilutive effect. Consequently, basic and diluted loss per share are the same for 2014 and 2015.
Basic income per share of €1.18 and diluted income per share of €1.14 in 2016 are based on a net income for 2016 which was strongly influenced by the non-cash gain from the fair value re-measurement of the share subscription agreement with Gilead amounting to €57.5 million.
F-36
Table of Contents
12. Intangible assets
| Customer relationships |
In process technology |
Software & databases |
Brands, licenses, patents & know-how |
Total | ||||||||||||||||
| (Euro, in thousands) | ||||||||||||||||||||
| Acquisition value: |
||||||||||||||||||||
| On January 1, 2014 |
€ | 2,055 | € | 5,561 | € | 7,681 | € | 17,698 | € | 32,993 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Additions |
— | — | 728 | 15 | 743 | |||||||||||||||
| Sales and disposals |
— | — | (503 | ) | — | (503 | ) | |||||||||||||
| Sale of the service division |
(2,055 | ) | — | — | (16,227 | ) | (18,282 | ) | ||||||||||||
| Translation differences |
— | — | 183 | 26 | 209 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2014 |
— | 5,561 | 8,088 | 1,512 | 15,161 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Additions |
— | — | 565 | — | 565 | |||||||||||||||
| Sales and disposals |
— | — | (1,512 | ) | — | (1,512 | ) | |||||||||||||
| Reclassifications |
— | — | — | — | — | |||||||||||||||
| Translation differences |
— | — | 177 | — | 177 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2015 |
— | 5,561 | 7,318 | 1,512 | 14,392 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Additions |
— | — | 317 | 15 | 332 | |||||||||||||||
| Sales and disposals |
— | — | (508 | ) | (4 | ) | (512 | ) | ||||||||||||
| Reclassifications |
— | — | — | — | — | |||||||||||||||
| Translation differences |
— | — | 58 | 0 | 58 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2016 |
— | 5,561 | 7,185 | 1,523 | 14,269 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Amortization and impairment: |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On January 1, 2014 |
912 | 5,561 | 6,321 | 12,366 | 25,161 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Amortization |
25 | — | 748 | 294 | 1,067 | |||||||||||||||
| Sales and disposals |
— | — | (500 | ) | — | (500 | ) | |||||||||||||
| Sale of the service division |
(937 | ) | — | — | (11,853 | ) | (12,790 | ) | ||||||||||||
| Reclassifications |
— | — | (666 | ) | 666 | — | ||||||||||||||
| Translation differences |
— | — | 184 | 24 | 208 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2014 |
— | 5,561 | 6,087 | 1,497 | 13,147 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Amortization |
— | — | 1,026 | 4 | 1,030 | |||||||||||||||
| Sales and disposals |
— | — | (1,512 | ) | — | (1,512 | ) | |||||||||||||
| Reclassifications |
— | — | — | — | — | |||||||||||||||
| Translation differences |
— | — | 177 | — | 177 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2015 |
— | 5,561 | 5,777 | 1,501 | 12,841 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Amortization |
— | — | 856 | 4 | 860 | |||||||||||||||
| Sales and disposals |
— | — | (509 | ) | (5 | ) | (514 | ) | ||||||||||||
| Reclassifications |
— | — | — | — | — | |||||||||||||||
| Translation differences |
— | — | 57 | — | 57 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2016 |
— | 5,561 | 6,182 | 1,501 | 13,246 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Carrying amount: |
||||||||||||||||||||
| On December 31, 2014 |
— | — | 2,000 | 15 | 2,015 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2015 |
— | — | 1,540 | 11 | 1,550 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2016 |
€ | — | € | — | € | 1,003 | € | 22 | € | 1,023 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The intangible assets decreased by €0.5 million from €2 million at December 31, 2014, to €1.5 million at December 31, 2015. The amortization of €1.0 million was partly compensated by new additions for €0.5 million.
F-37
Table of Contents
The intangible assets decreased by €0.5 million from €1.5 million as at December 31, 2015, to €1.0 million as at December 31, 2016. The amortization of €0.9 million was partly compensated by new additions for €0.3 million.
13. Property, plant and equipment
| Land & building improvements |
Installation & machinery |
Furniture, fixtures & vehicles |
Other tangible assets |
Total | ||||||||||||||||
| (Euro, in thousands) | ||||||||||||||||||||
| Acquisition value: |
||||||||||||||||||||
| On January 1, 2014 |
€ | 13,898 | € | 52,251 | € | 4,455 | € | 3,565 | € | 74,169 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Additions |
117 | 1,155 | 104 | 685 | 2,061 | |||||||||||||||
| Sales and disposals |
(1,733 | ) | (4,549 | ) | (73 | ) | — | (6,355 | ) | |||||||||||
| Sale of the service division |
(4,022 | ) | (23,677 | ) | (1,919 | ) | (370 | ) | (29,988 | ) | ||||||||||
| Reclassifications |
— | 3,543 | 16 | (3,559 | ) | — | ||||||||||||||
| Translation differences |
26 | 97 | 11 | — | 134 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2014 |
8,286 | 28,820 | 2,594 | 321 | 40,021 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Additions |
2,158 | 2,250 | 285 | 1,407 | 6,100 | |||||||||||||||
| Sales and disposals |
(6,395 | ) | (5,041 | ) | (188 | ) | (11 | ) | (11,635 | ) | ||||||||||
| Reclassifications |
— | 540 | 3 | (543 | ) | — | ||||||||||||||
| Translation differences |
— | 19 | 1 | (1 | ) | 20 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2015 |
4,049 | 26,588 | 2,695 | 1,174 | 34,506 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Additions |
296 | 3,325 | 210 | 627 | 4,458 | |||||||||||||||
| Sales and disposals |
— | (1,315 | ) | (105 | ) | (0 | ) | (1,420 | ) | |||||||||||
| Reclassifications |
67 | 1,064 | 167 | (1,299 | ) | (1 | ) | |||||||||||||
| Translation differences |
— | 70 | 6 | 4 | 81 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2016 |
4,412 | 29,733 | 2,973 | 505 | 37,624 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Depreciations and impairment: |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On January 1, 2014 |
12,715 | 36,720 | 3,086 | 2,123 | 54,644 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Depreciation |
639 | 2,531 | 243 | 168 | 3,581 | |||||||||||||||
| Sales and disposals |
(1,700 | ) | (4,011 | ) | (42 | ) | — | (5,753 | ) | |||||||||||
| Sale of the service division |
(3,694 | ) | (17,404 | ) | (1,247 | ) | (299 | ) | (22,644 | ) | ||||||||||
| Reclassifications |
— | 1,884 | — | (1,884 | ) | — | ||||||||||||||
| Translation differences |
24 | 70 | 6 | 2 | 102 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2014 |
7,984 | 19,790 | 2,046 | 110 | 29,930 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Amortization |
164 | 1,873 | 272 | 63 | 2,372 | |||||||||||||||
| Sales and disposals |
(6,395 | ) | (4,996 | ) | (188 | ) | (7 | ) | (11,587 | ) | ||||||||||
| Reclassifications |
— | 44 | — | (44 | ) | — | ||||||||||||||
| Translation differences |
— | 8 | — | — | 8 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2015 |
1,753 | 16,718 | 2,130 | 122 | 20,724 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Amortization |
272 | 2,752 | 243 | 55 | 3,322 | |||||||||||||||
| Sales and disposals |
— | (1,315 | ) | (100 | ) | — | (1,415 | ) | ||||||||||||
| Reclassifications |
— | 67 | (93 | ) | 26 | — | ||||||||||||||
| Translation differences |
— | 29 | 5 | — | 34 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2016 |
2,025 | 18,252 | 2,184 | 203 | 22,663 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Carrying amount: |
||||||||||||||||||||
| On December 31, 2014 |
302 | 9,031 | 547 | 210 | 10,091 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2015 |
2,296 | 9,870 | 565 | 1,051 | 13,782 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2016 |
€ | 2,387 | € | 11,481 | € | 789 | € | 302 | € | 14,961 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-38
Table of Contents
The property, plant and equipment increased from €10.1 million at December 31, 2014 to €13.8 million at December 31, 2015. This increase was mainly the result of new additions of €6.1 million, partly compensated by a depreciation charge of €2.4 million. The sales and disposals in 2015 related to the move to new premises in France and the Netherlands.
The property, plant and equipment increased from €13.8 million as at December 31, 2015 to €15.0 million as at December 31, 2016. This increase was mainly the result of new additions of €4.5 million, partly compensated by a depreciation charge of €3.3 million. There are no pledged items of property, plant and equipment. There are also no restrictions in use on any items of property, plant and equipment.
14. Other non-current assets
On July 15, 2016, we invested €2.75 million in Pharnext, a French advanced clinical stage biopharmaceutical company developing new therapeutics for severe orphan and common neurological diseases, listed on Euronext. Galapagos has no restrictions on the sale of this equity investment and the asset is not pledged under any Galapagos’ liabilities. This investment is classified as available-for-sale equity investment which qualifies for level 1 fair value measurement based upon the closing price of the PXT securities on Euronext at each reporting date. Fair value changes on available-for-sale financial assets are recognized directly in equity, through the statement of changes in equity.
As of December 31, 2016, other non-current assets mainly consisted of available-for-sale equity investments in Pharnext re-measured at fair value for €2.4 million as follows.
| Fair value of available-for-sale financial assets |
||||
| (thousands of €) | ||||
| Costs at January 1, 2016 |
— | |||
| Additions of the year |
2,750 | |||
|
|
|
|||
| Costs at December 31, 2016 |
2,750 | |||
|
|
|
|||
| Fair value adjustment of the year |
(399 | ) | ||
|
|
|
|||
| Fair value adjustment at December 31, 2016 |
(399 | ) | ||
|
|
|
|||
| Net book value at December 31, 2016 |
2,351 | |||
|
|
|
|||
The unrealized loss of €399 thousand as of December 31, 2016, based on unadjusted quoted market price, was recorded as a separate item within equity (revaluation reserve) in the line “other reserves”.
15. Research and Development incentives receivables
The table below illustrates the R&D incentives receivables related captions in the balance sheet at December 31, 2016, 2015 and 2014:
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Non-current R&D incentives receivables |
€ | 54,188 | € | 49,384 | € | 43,944 | ||||||
| Current R&D incentives receivables |
10,154 | 9,161 | 7,351 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total R&D incentives receivables |
€ | 64,342 | € | 58,545 | € | 51,296 | ||||||
|
|
|
|
|
|
|
|||||||
Total R&D incentives receivables increased by €7.2 million for the year ended December 31, 2015 compared to December 31, 2014. This increase was explained by new R&D incentives reported in 2015 for €14.0 million
F-39
Table of Contents
(€8.7 million related to French R&D incentives and €5.3 million related to Belgian R&D incentives) less the payment received related to French R&D incentives amounting to €6.7 million.
Total R&D incentives receivables increased by €5.8 million for the year ended December 31, 2016, compared to December 31, 2015. This increase is explained by new R&D incentives reported in 2016 for €15.3 million (€9.5 million related to French R&D incentives and €5.8 million related to Belgian R&D incentives) less the payments received related to French R&D incentives amounting to €8.7 million and to Belgian R&D incentives amounting to €0.8 million. The R&D incentives receivables relate to future refunds resulting from R&D incentives on research expenses in France and Belgium. Non-current R&D incentives receivables are discounted over the period until maturity date.
The table below provides detailed information on the maturity of the non-current R&D incentives receivables reported in the balance sheet at December 31, 2016.
Non-current R&D incentives receivables
| December 31, 2016 maturity date | ||||||||||||||||||||||||
| 2018 | 2019 | 2020 | 2021 | 2022–2026 | Total | |||||||||||||||||||
| (Euro, in thousands) | ||||||||||||||||||||||||
| French non-current R&D incentives receivables—nominal value |
€ | 8,214 | € | 8,621 | € | 9,168 | — | — | € | 26,003 | ||||||||||||||
| French non-current R&D incentives receivables—discounted value |
8,214 | 8,621 | 9,168 | — | — | 26,003 | ||||||||||||||||||
| Belgian non-current R&D incentives receivables—nominal value |
2,074 | 2,966 | 3,831 | € | 4,188 | € | 15,235 | 28,294 | ||||||||||||||||
| Belgian non-current R&D incentives receivables—discounted value |
2,074 | 2,966 | 3,831 | 4,188 | 15,126 | 28,185 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total non-current R&D incentives receivables—nominal value |
€ | 10,288 | € | 11,587 | € | 12,999 | € | 4,188 | € | 15,235 | € | 54,297 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total non-current R&D incentives receivables—discounted value |
€ | 10,288 | € | 11,587 | € | 12,999 | € | 4,188 | € | 15,126 | € | 54,188 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
16. Restricted cash
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Non-current restricted cash |
€ | 1,098 | € | 1,046 | € | 306 | ||||||
| Current restricted cash |
6,570 | 6,857 | 10,422 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total restricted cash |
€ | 7,668 | € | 7,903 | € | 10,728 | ||||||
|
|
|
|
|
|
|
|||||||
Restricted cash amounted to €10.7 million on December 31, 2014, and decreased to €7.9 million on December 31, 2015. This decrease was related to (a) the release of the €3 million bank guarantee issued in 2013 for the rental of the new premises in France which expired on June 30, 2015 following the move to the new offices, (b) the payment of a claim to Charles River by decrease of the escrow account, and (c) a €0.7 million bank guarantee issued in September 2015 for the rental of new premises in the Netherlands (to replace the current premises) which will expire on October 1, 2025. Restricted cash on December 31, 2015 was related to €0.3 million and €0.7 million bank guarantees on real estate lease obligations in Belgium and in the Netherlands respectively, and to €6.9 million escrow account containing part of the proceeds from the sale of the service division in 2014.
F-40
Table of Contents
Restricted cash amounted to €7.9 million on December 31, 2015, and decreased to €7.7 million on December 31, 2016. This decrease is related to the payment of a claim to Charles River by decrease of the escrow account for €0.3 million, which has been slightly offset by an increase in non-current restricted cash of €0.1 million related to an increase in the bank guarantee with regard to the rental of additional office space for the Belgian premises. Restricted cash on December 31, 2016 is related to €0.4 million and €0.7 million of bank guarantees on real estate lease obligations in Belgium and in the Netherlands respectively, and to €6.6 million escrow account containing part of the proceeds from the sale of the service division in 2014 for which the release will be possible after final agreement between the parties.
17. Trade and other receivables and other current assets
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Trade receivables |
€ | 6,629 | € | 1,494 | € | 1,340 | ||||||
| Prepayments |
21 | 11 | 9 | |||||||||
| Other receivables |
3,078 | 2,426 | 1,862 | |||||||||
|
|
|
|
|
|
|
|||||||
| Trade and other receivables |
9,728 | 3,931 | 3,211 | |||||||||
|
|
|
|
|
|
|
|||||||
| Accrued income |
3,617 | 2,976 | 3,242 | |||||||||
| Deferred charges |
3,621 | 2,536 | 1,384 | |||||||||
|
|
|
|
|
|
|
|||||||
| Other current assets |
7,239 | 5,512 | 4,625 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total trade and other receivables & other current assets |
€ | 16,966 | € | 9,443 | € | 7,836 | ||||||
|
|
|
|
|
|
|
|||||||
The carrying amount of trade and other receivables approximates their fair value. Other current assets mainly included accrued income from subsidy projects and deferred charges.
On December 31, 2016, we did not have any bad debt allowance.
18. Cash and cash equivalents
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Cash at banks |
€ | 357,630 | € | 240,292 | 137,711 | |||||||
| Term deposits |
515,632 | 100,000 | 50,000 | |||||||||
| Money market funds |
99,977 | — | — | |||||||||
| Cash on hand |
2 | 22 | 1 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cash and cash equivalents |
973,241 | 340,314 | 187,712 | |||||||||
|
|
|
|
|
|
|
|||||||
We reported a cash position of €187.7 million at the end of December 2014 compared to €138.2 million at year-end 2013. The operating activities reported use of €75.6 million of cash in 2014 while the investing activities brought €120.6 million of cash in-flow mainly due the proceeds from the sale of the service division (€130.8 million) and €4.2 million from the financing activities.
We reported a cash position of €340.3 million at the end of December 2015. The operating and investing activities reported use of respectively €114.6 million and €4.3 million of cash in 2015 while the financing activities brought €271.4 million of cash in-flow mainly due to the proceeds of a recent global offering and concurrent listing on NASDAQ (€259.4 million) and due to warrant exercises (€12 million).
F-41
Table of Contents
We reported a cash position of €973.2 million at the end of December 2016 compared to €340.3 million at year-end 2015. Net cash inflow from operating activities amounted to €239.4 million for the year ended December 31, 2016. This net cash inflow from operations recorded in 2016 was primarily due to the license fee of $300 million (€275.6 million) received from Gilead in relation with our collaboration agreement on filgotinib. In addition, milestone payments increased substantially in 2016 (compared to 2015), which contributed significantly to the net cash inflow from operations in 2016. The net cash outflow from investing activities amounted to €7.3 million for the year ended December 31, 2016, which included an acquisition of available-for-sale equity investment (see note 14). The net cash inflow from financing activities amounted to €396.0 million for the year ended December 31, 2016, which can mainly be attributed to the subscription of Galapagos shares by Gilead on January 19, 2016 for which the cash proceeds from capital and share premium increases amounted to €391.9 million, net of issue costs. In addition, proceeds received on exercise of warrants contributed to cash generated by financing activities in 2016 in the amount of €4.3 million.
Cash and cash equivalents comprise cash at banks, short term bank deposits and money market funds that are readily convertible to cash and are subject to an insignificant risk of changes in value. Our cash management strategy monitors and optimizes our liquidity position. Our cash management strategy may allow short term deposits with an original maturity exceeding 3 months while monitoring all liquidity aspects. Cash and cash equivalents comprise €515.6 million of term deposits of which €458.7 million had an original maturity longer than 3 months. All cash and cash equivalents are available upon maximum one month notice period. Cash at banks were mainly composed of savings accounts and current accounts. We maintain our bank deposits in highly rated financial institutions to reduce credit risk. Cash invested in highly liquid money market funds represented €100.0 million and was aimed at meeting short-term cash commitments, while reducing the counterparty risk of investment.
19. Share capital
The share capital of Galapagos NV, as set forth in the articles of association, reconciles to ‘share capital’ on the balance sheet as follows:
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| On January 1 |
€ | 185,399 | € | 157,274 | € | 154,542 | ||||||
|
|
|
|
|
|
|
|||||||
| Share capital increase |
38,798 | 47,485 | 2,732 | |||||||||
| Costs of capital increase |
(269 | ) | (19,360 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Share capital on December 31 |
€ | 223,928 | € | 185,399 | € | 157,274 | ||||||
|
|
|
|
|
|
|
|||||||
| Aggregate share capital |
€ | 250,187 | € | 211,389 | € | 163,904 | ||||||
| Costs of capital increase (accumulated) |
(26,259 | ) | (25,990 | ) | (6,629 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Share capital on December 31 |
€ | 223,928 | € | 185,399 | € | 157,274 | ||||||
|
|
|
|
|
|
|
|||||||
Costs of capital increases are netted against the proceeds of capital increases, in accordance with IAS 32 Financial instruments: disclosure and presentation.
F-42
Table of Contents
HISTORY OF SHARE CAPITAL
The history of the share capital of Galapagos NV between January 1, 2014 and December 31, 2016 is as follows:
| Date |
Share capital increase new shares (in thousands €) |
Share capital increase warrants (in thousands €) |
Number of shares issued (in thousands of shares) |
Aggregate number of shares after transaction (in thousands of shares) |
Aggregate share capital after transaction (in thousands €) |
|||||||||||||||
| January 1, 2014 |
29,794 | € | 161,171 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| April 10, 2014 |
€ | 1,649 | 305 | |||||||||||||||||
| July 4, 2014 |
982 | 182 | ||||||||||||||||||
| September 25, 2014 |
66 | 12 | ||||||||||||||||||
| December 9, 2014 |
35 | 7 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| December 31, 2014 |
30,299 | 163,904 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| March 26, 2015 |
3,092 | 571 | ||||||||||||||||||
| May 19, 2015 |
€ | 40,751 | 7,532 | |||||||||||||||||
| June 19, 2015 |
2,659 | 491 | ||||||||||||||||||
| September 25, 2015 |
640 | 118 | ||||||||||||||||||
| December 4, 2015 |
344 | 64 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| December 31, 2015 |
39,075 | 211,390 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| January 19, 2016 |
36,575 | 6,761 | ||||||||||||||||||
| April 1, 2016 |
668 | 132 | ||||||||||||||||||
| May 19, 2016 |
762 | 141 | ||||||||||||||||||
| September 19, 2016 |
326 | 60 | ||||||||||||||||||
| November 28, 2016 |
467 | 86 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| December 31, 2016 |
46,256 | € | 250,187 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
On January 1, 2014, Galapagos NV’s share capital amounted to €161,171.6 thousand, represented by 29,794,046 shares. All shares were issued, fully paid up and of the same class.
On April 10, 2014, warrants were exercised at various exercise prices (with an average exercise price of €7.81 per warrant) resulting in a share capital increase (including issuance premium) of €2,381.2 thousand and the issuance of 304,791 new ordinary shares. The closing price of the Galapagos share at this date was €16.80.
On July 4, 2014, warrants were exercised at various exercise prices (with an average exercise price of €10.26 per warrant), resulting in a share capital increase (including issuance premium) of €1,862.3 thousand and the issuance of 181,507 new ordinary shares. The closing price of the Galapagos share on July 4, 2014, was €15.13.
On September 25, 2014, warrants were exercised at various exercise prices (with an average exercise price of €10.60 per warrant), resulting in a share capital increase (including issuance premium) of €130.0 thousand and the issuance of 12,260 new ordinary shares. The closing price of the Galapagos share at this date was €12.19.
On December 9, 2014, warrants were exercised at various exercise prices (with an average exercise price of €8.61 per warrant), resulting in a share capital increase (including issuance premium) of €56.2 thousand and the issuance of 6,525 new ordinary shares. The closing price of the Galapagos share on December 9, 2014, was €14.77.
On December 31, 2014, Galapagos NV’s share capital amounted to €163,904.1 thousand, represented by 30,299,129 shares. All shares were issued, fully paid up and of the same class.
F-43
Table of Contents
On March 26, 2015, warrants were exercised at various exercise prices (with an average exercise price of €10.18 per warrant), resulting in a share capital increase (including issuance premium) of €5,819 thousand and the issuance of 571,548 new ordinary shares. The closing price of the Galapagos share at this date was €21.26.
On May 19, 2015, Galapagos completed a global offering of 7,532,499 ordinary shares consisting of a concurrent public offering in the United States and private placement in Europe and countries other than the United States and Canada. Galapagos offered 5,746,000 ordinary shares through a public offering in the United States in the form of American Depositary Shares, or ADSs, at a price of $42.05 per ADS, before underwriting discounts. The ADSs are evidenced by American Depositary Receipts, or ADRs, and each ADS represents the right to receive one ordinary share. The ADSs are listed on the NASDAQ Global Select Market under the symbol “GLPG.” Galapagos offered 1,786,499 ordinary shares through a private placement in Europe and countries other than the United States and Canada at a price of €37.00 per share, before underwriting discounts.
Galapagos received €278.7 million of gross proceeds from the global offering, decreased by €19.4 million of underwriter discounts and commission, and offering expenses, of which €19.3 million has been paid at December 31, 2015 and €0.1 million has been paid in 2016. The total net cash proceeds from the global offering after remaining settlements will amount to €259.3 million.
On June 19, 2015, warrants were exercised at various exercise prices (with an average exercise price of €8.94 per warrant), resulting in a share capital increase (including issuance premium) of €4,395 thousand and the issuance of 491,406 new ordinary shares. The closing price of the Galapagos share on June 19, 2015, was €46.73.
On September 25, 2015, warrants were exercised at various exercise prices (with an average exercise price of €10.13 per warrant), resulting in a share capital increase (including issuance premium) of €1,198 thousand and the issuance of 118,260 new ordinary shares. The closing price of the Galapagos share at this date was €44.75.
On December 4, 2015, warrants were exercised at various exercise prices (with an average exercise price of €9.30 per warrant), resulting in a share capital increase (including issuance premium) of €590.8 thousand and the issuance of 63,500 new ordinary shares. The closing price of the Galapagos share on December 4, 2015, was €44.78.
On December 31, 2015, Galapagos NV’s share capital amounted to €211,388.9 thousand, represented by 39,076,342 shares. All shares were issued, fully paid up and of the same class.
On January 19, 2016, Gilead made a $425 million equity investment in Galapagos NV by subscribing to 6,760,701 new ordinary shares at a price of €58 per share, including issuance premium. Galapagos received €392.1 million of gross proceeds, decreased by €0.26 million of expenses, which were all paid at December 31, 2016. The total net cash proceeds from the share subscription by Gilead amounts to €391.9 million. The closing price of the Galapagos share on January 19, 2016 was €48.26.
On April 1, 2016, warrants were exercised at various exercise prices (with an average exercise price of €10.70 per warrant) resulting in a share capital increase (including issuance premium) of €1,409.3 thousand and the issuance of 131,695 new shares. The closing price of the Galapagos share on this date was €36.64.
On May 19, 2016, warrants were exercised at various exercise prices (with an average exercise price of €10.49 per warrant) resulting in a share capital increase (including issuance premium) of €1,476.4 thousand and the issuance of 140,770 new shares. The closing price of the Galapagos share on this date was €45.41.
On September 19, 2016, warrants were exercised at various exercise prices (with an average exercise price of €10.00 per warrant) resulting in a share capital increase (including issuance premium) of €603.3 thousand and the issuance of 60,320 new shares. The closing price of the Galapagos share on this date was €58.62.
F-44
Table of Contents
On November 28, 2016, warrants were exercised at various exercise prices (with an average exercise price of €8.94 per warrant) resulting in a share capital increase (including issuance premium) of €771.3 thousand and the issuance of 86,250 new shares. The closing price of the Galapagos share on this date was €55.73.
On December 31, 2016, Galapagos NV’s share capital amounted to €250,187 thousand, represented by 46,256,078 shares. All shares were issued, fully paid up and of the same class.
All of the share issuances listed above were for cash consideration.
The below table summarizes the capital increases for the years 2014, 2015 and 2016.
| (thousands of €, except share data) | Number of shares | Share capital |
Share premium |
Share capital and share premium |
||||||||||||
| On January 1, 2014 |
29,794,046 | 154,542 | 112,484 | 267,026 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| April 10, 2014 : exercise of warrants |
304,791 | 1,649 | 732 | 2,381 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| July 4, 2014 : exercise of warrants |
181,507 | 982 | 880 | 1,862 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| September 25, 2014 : exercise of warrants |
12,260 | 66 | 64 | 130 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| December 9, 2014 : exercise of warrants |
6,525 | 35 | 21 | 56 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| On January 1, 2015 |
30,299,129 | 157,274 | 114,182 | 271,456 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| March 26, 2015: exercise of warrants |
571,548 | 3,092 | 2,727 | 5,819 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| May 19, 2015: global offering |
||||||||||||||||
| Ordinary shares (fully paid) |
1,786,499 | 9,665 | 56,436 | 66,100 | ||||||||||||
| ADSs (fully paid) |
5,746,000 | 31,086 | 181,516 | 212,602 | ||||||||||||
| Underwriter discounts and offering expenses (fully paid) |
— | (19,360 | ) | — | (19,293 | ) | ||||||||||
| Total global offering |
7,532,499 | 21,391 | 237,952 | 259,343 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| June 19, 2015: exercise of warrants |
491,406 | 2,659 | 1,737 | 4,395 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| September 25, 2015: exercise of warrants |
118,260 | 640 | 558 | 1,198 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| December 4, 2015: exercise of warrants |
63,500 | 344 | 247 | 591 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| On January 1, 2016 |
39,076,342 | 185,399 | 357,402 | 542,803 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| January 19, 2016 : share subscription from Gilead |
||||||||||||||||
| Ordinary shares (fully paid) |
6,760,701 | 36,575 | 355,546 | 392,121 | ||||||||||||
| Derecognition of financial asset from share subscription agreement |
— | — | (65,850 | ) | (65,850 | ) | ||||||||||
| Capital increase expenses (fully paid) |
— | (269 | ) | — | (269 | ) | ||||||||||
| Total share subscription by Gilead |
6,760,701 | 36,306 | 289,696 | 326,002 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| April 1, 2016 : exercise of warrants |
131,695 | 668 | 741 | 1,409 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| May 19, 2016 : exercise of warrants |
140,770 | 762 | 715 | 1,476 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| September 19, 2016 : exercise of warrants |
60,320 | 326 | 277 | 603 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| November 28, 2016 : exercise of warrants |
86,250 | 467 | 305 | 772 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| On December 31, 2016 |
46,256,078 | 223,928 | 649,135 | 873,063 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Other information
| Ordinary shares | Total | |||||||
| Par value of shares (€) |
5.41 | 5.41 | ||||||
F-45
Table of Contents
The board of directors is authorized for a period of five years starting from the date of the publication in the Annexes to the Belgian State Gazette of the shareholders’ resolution that granted the renewed authorization, being June 3, 2016, to increase the share capital of Galapagos NV within the framework of the authorized capital through contributions in kind or in cash, with limitation or cancellation of the shareholders’ preferential subscription rights. Said authorization can be renewed. The board of directors is currently not authorized to increase the share capital after notification by the FSMA (Financial Services and Markets Authority) of a public takeover bid on Galapagos NV’s shares.
The authorized capital as approved by the extraordinary shareholders’ meeting of April 26, 2016 amounted to €49,726.5 thousand. As of December 31, 2016, €3,431.3 thousand of the authorized capital was used, so that an amount of €46,295.2 thousand still remained available.
20. Other reserves
Actuarial and other gains or losses recognized through other comprehensive income
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| On January 1 |
€ | (18 | ) | € | (220 | ) | € | 47 | ||||
|
|
|
|
|
|
|
|||||||
| Gain or loss (-) on defined benefit obligation recognized through OCI |
(583 | ) | 202 | (267 | ) | |||||||
| Loss on financial asset available-for-sale recognized through OCI |
(399 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Other reserves on December 31 |
€ | (1,000 | ) | € | (18 | ) | € | (220 | ) | |||
|
|
|
|
|
|
|
|||||||
The other reserves amounted to a negative of €18 thousand on December 31, 2015 (2014: €220 thousand; 2013; positive of €47 thousand) and related to the re-measurement of defined benefit obligations recognized through OCI in line with IAS19R.
Other reserves on December 31, 2016 consisted of (1) a negative of €601 thousand, compared to a negative of €18 thousand in 2015 (2014: €220 thousand), which was related to the re-measurement of defined benefit obligations recognized through OCI in line with IAS19R, and (2) a negative of €399 thousand, compared to nil in 2015 and 2014, related to the fair value adjustment on the available-for-sale equity investment in 2016 (see note 14).
DERIVATIVE FINANCIAL INSTRUMENTS: CURRENCY DERIVATIVES
We do not actively use currency derivatives to hedge planned future cash flows. On the balance sheet date, total notional amount of outstanding forward foreign exchange contracts that we have committed were nil (2015: nil, 2014: nil).
On December 31, 2016 the fair value of our currency derivatives was nil (2015: nil, 2014: nil). We do not designate our foreign currency denominated debt as a hedge instrument for the purpose of hedging the translation of our foreign operations.
See note 35 for further information on how we manage financial risks.
21. Translation differences
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| On January 1 |
€ | (467 | ) | € | (1,157 | ) | € | 170 | ||||
|
|
|
|
|
|
|
|||||||
| Translation differences, arisen from translating foreign activities |
(623 | ) | 690 | 460 | ||||||||
| Translation differences, arisen from the sale of the service division |
— | — | (1,787 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Translation differences on December 31 |
€ | (1,090 | ) | € | (467 | ) | € | (1,157 | ) | |||
|
|
|
|
|
|
|
|||||||
F-46
Table of Contents
Translation differences decreased to a negative of €1.2 million at the end of December 2014 mainly due to the sale of the service division which reported positive translation differences of €2.0 million at the end of December 2013.
Translation differences increased from a negative €1.2 million at the end of December 2014 to a negative of €0.5 million at the end of December 2015 mainly due to the increase of the GB pounds and U.S. dollar exchange rates.
Translation differences decreased from a negative €0.5 million at the end of December 2015 to a negative of €1.1 million at the end of December 2016 mainly due to fluctuations of the GB pounds and the U.S. dollar exchange rates.
22. Deferred tax
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Recognized deferred tax assets and liabilities: |
||||||||||||
| Assets |
€ | 1,957 | € | 1,726 | € | 293 | ||||||
| Liabilities |
€ | — | € | — | € | — | ||||||
| Continuing operations |
||||||||||||
| Assets |
1,957 | 1,726 | 293 | |||||||||
| Liabilities |
— | — | — | |||||||||
| Deferred tax assets unrecognized |
€ | 128,377 | € | 145,513 | € | 104,484 | ||||||
|
|
|
|
|
|
|
|||||||
| Deferred taxes in the consolidated statement of operations |
€ | 231 | € | 1,433 | € | 496 | ||||||
|
|
|
|
|
|
|
|||||||
| Continuing operations |
231 | 1,433 | 293 | |||||||||
|
|
|
|
|
|
|
|||||||
| Tax benefit arising from previously unrecognized tax assets used to reduce deferred tax expense (+) |
421 | 1,433 | 293 | |||||||||
| Deferred tax expenses relating to use of previously recognized deferred tax assets |
(190 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Discontinued operations |
203 | |||||||||||
|
|
|
|
|
|
|
|||||||
| Deferred tax expenses net relating to origination and reversal of temporary differences |
— | — | 203 | |||||||||
| Tax benefit arising from previously unrecognized tax assets used to reduce deferred tax expense (+) |
— | — | — | |||||||||
| Deferred tax expenses relating to write down of previously recognized deferred tax assets |
— | — | — | |||||||||
The notional interest deduction for an amount of €2.6 million (2015 and 2014: €2.6 million) and the investment deduction of €1 million (2015 and 2014: €1 million) could give rise to deferred tax assets. The amount of notional interest deduction that has been accumulated in the past can be carried forward for maximum seven years, the notional interest deduction of 2012 and following years will not be carried forward according to a change in the Belgian tax legislation. There is no limit in time for the investment deduction.
The consolidated unused tax losses carried forward at December 31, 2016 amounted to €385 million (2015: €434 million; 2014: €315 million), €17 million were related to unrecognized tax losses with expiry date between 2018 and 2030.
The available statutory tax losses carried forward that can be offset against future statutory taxable profits amounted to €311.1 million on December 31, 2016. These statutory tax losses can be compensated with future statutory profits for an indefinite period except for an amount of €18 million in Switzerland, Croatia, the United States and the Netherlands with expiry date between 2018 and 2030. On December 31, 2016, the available tax losses carried forward in Galapagos NV (Belgium) amounted to €230.9 million.
F-47
Table of Contents
We have a history of losses. Excluding the impact of possible upfront or milestone payments to be received from collaborations, we forecast to continue incurring taxable losses in the foreseeable future as we continue to invest in clinical and pre-clinical development programs and discovery platforms. Consequently, no deferred tax asset was set up as at December 31, 2016, except for two subsidiaries operating on a cost plus basis for which deferred tax assets were recognized for €2.0 million (2015: €1.7 million; 2014: €0.3 million).
23. Finance lease liabilities
| December 31, | December 31, | |||||||||||||||||||||||
| 2016 | 2015 | 2014 | 2016 | 2015 | 2014 | |||||||||||||||||||
| (Euro, in thousands) | ||||||||||||||||||||||||
| Minimum lease payments |
Present value of minimum lease payments |
|||||||||||||||||||||||
| Amounts payable under finance lease: |
||||||||||||||||||||||||
| Within one year |
€ | 56 | € | 56 | € | 58 | € | 54 | € | 52 | € | 52 | ||||||||||||
| In the second to fifth years inclusive |
9 | 65 | 121 | 9 | 63 | 115 | ||||||||||||||||||
| After five years |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| € | 65 | € | 121 | € | 179 | € | 63 | € | 115 | € | 167 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Less future finance charges |
2 | 6 | 12 | |||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Present value of lease obligation |
€ | 63 | € | 115 | € | 167 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Less amount due for settlement within 12 months |
54 | 52 | 52 | |||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Amount due for settlement after 12 months |
€ | 9 | € | 63 | € | 115 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| December 31, | December 31, | |||||||||||||||||||||||
| 2016 | 2015 | 2014 | 2016 | 2015 | 2014 | |||||||||||||||||||
| (Euro, in thousands) | ||||||||||||||||||||||||
| Net book value | Acquisition cost | |||||||||||||||||||||||
| Leased assets: |
||||||||||||||||||||||||
| Installation & machinery |
€ | 58 | € | 109 | € | 161 | € | 251 | € | 251 | € | 295 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total leased assets |
€ | 58 | € | 109 | € | 161 | € | 251 | € | 251 | € | 295 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
We lease certain of our installation and machinery under finance leases. For the year ended December 31, 2016, the average borrowing rate was 4.34% (2015: 4.3%; 2014: 6.27%). The interest rates were fixed at the date of the contracts. All leases are on a fixed repayment basis and no arrangements have been entered into for contingent rental payments.
The fair value of our lease obligations approximates their carrying value.
F-48
Table of Contents
24. Trade and other liabilities
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Trade and other payables |
€ | 31,209 | € | 29,113 | € | 29,344 | ||||||
| Other current liabilities |
60 | 369 | 663 | |||||||||
| Other non-current liabilities |
2,469 | 2,291 | 923 | |||||||||
| Accrued charges |
619 | 490 | 585 | |||||||||
| Deferred income |
285,612 | 39,806 | 27,026 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total trade and other liabilities |
€ | 319,969 | € | 72,068 | € | 58,541 | ||||||
|
|
|
|
|
|
|
|||||||
| Included in current liabilities |
102,715 | 69,777 | 57,618 | |||||||||
| Included in non-current liabilities |
217,254 | 2,291 | 923 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total trade and other liabilities |
€ | 319,969 | € | 72,068 | € | 58,541 | ||||||
|
|
|
|
|
|
|
|||||||
The trade and other liabilities, amounting to €72.1 million as of December 31, 2015, increased by €13.5 million compared to the €58.5 million reported as of December 31, 2014.
| • | The trade and other payables amounting to €29.1 million as of December 31, 2015 remained stable compared to the €29.3 million as of December 31, 2014. Nevertheless, trade payables decreased by €2.7 million compared to the same period last year which fully compensated the increase in other payables by €2.5 million as a result of higher bonus payables. |
| • | Deferred income amounted to €39.8 million at December 31, 2015 and increased by €12.8 million compared to December 31, 2014. On the one hand there was an increase of €39 million due to the recognition of a deferred income upon signing of the share subscription agreement with Gilead (see note 7). On the other hand there was a substantial decrease of €26.4 million, which was mainly explained by revenues from non-refundable upfront payments recognized in the income statement. For the year ended December 31, 2014, €15.0 million revenue was deferred for the filgotinib program for rheumatoid arthritis and Crohn’s disease with AbbVie, and €11.4 million was deferred for the CF program with AbbVie. |
| • | The outstanding deferred income balance at December 31, 2015 included €39.0 million deferred income related to the Gilead share subscription agreement and €0.8 million primarily related to deferred revenues from grants. |
Our trade and other liabilities, amounting to €320.0 million as of December 31, 2016, increased by €247.9 million compared to the €72.1 million reported as of December 31, 2015.
The trade and other payables, amounting to €31.2 million as of December 31, 2016, increased slightly compared to the €29.1 million reported as of December 31, 2015. This increase is mainly due to higher trade payables.
Deferred income (long term and short term) amounted to €285.6 million at December 31, 2016 and increased by €245.8 million compared to €39.8 million as at December 31, 2015. On the one hand we had per December 31, 2015 a deferred income of €39 million due to recognition of a deferred income upon signing of the share subscription agreement with Gilead (see note 7). On the other hand we received in January 2016 an upfront payment from Gilead for an amount of $300 million (or €276 million). The global collaboration with Gilead foresees continuous involvement from us, since we will perform certain R&D activities in the development phase of the filgotinib program; therefore, management assessed that both items of the deferred income should be spread in function of the costs incurred for the filgotinib program, applying the percentage of completion method. For the year ended December 31, 2016, €29.2 million were recognized in revenue, of which €3.6 million were related to the deferred income from the share subscription agreement and €25.6 million were related to the upfront payment.
F-49
Table of Contents
The outstanding deferred income balance at December 31, 2016 included €285.3 million deferred income related to filgotinib (€214.8 million classified as non-current deferred income)—of which €35.4 million deferred income related to the Gilead share subscription agreement, remaining €249.9 million deferred income related to the $300 million upfront payment—and €0.3 million deferred grant income.
25. Provisions
| Post- employment benefits (non-current) |
Other provisions (non-current) |
Restructuring provision (current) |
Other provisions (current) |
Total | ||||||||||||||||
| (Euro, in thousands) | ||||||||||||||||||||
| On January 1, 2014 |
€ | 7 | € | 660 | € | 81 | € | — | € | 747 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Additional provisions |
7 | — | — | 73 | 80 | |||||||||||||||
| Provisions utilized amounts |
— | (3 | ) | (50 | ) | — | (53 | ) | ||||||||||||
| Sale of the service division |
— | (604 | ) | — | — | (604 | ) | |||||||||||||
| Translation differences |
— | 4 | 1 | — | 5 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2014 |
14 | 57 | 32 | 73 | 176 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Additional provisions |
— | — | — | — | — | |||||||||||||||
| Provisions utilized amounts |
(7 | ) | (10 | ) | (35 | ) | (73 | ) | (125 | ) | ||||||||||
| Translation differences |
— | — | 4 | — | 4 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2015 |
8 | 47 | 0 | 0 | 55 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Additional provisions |
— | 10 | — | — | 10 | |||||||||||||||
| Provisions utilized amounts |
(2 | ) | — | — | — | (2 | ) | |||||||||||||
| Translation differences |
0 | 0 | — | — | 1 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| On December 31, 2016 |
€ | 6 | € | 57 | € | — | € | — | € | 63 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The decrease in provisions in 2014 is mainly due to the sale of the service division (€0.6 million).
The decrease in provisions in 2015 is mainly due to the use of the provision for decontamination of the building in France (€0.1 million).
The provisions remained stable at €0.1 million in 2016.
26. Operating lease obligations
We entered into lease agreements for office and laboratories which qualify as operating leases.
Minimum lease payments under operating leases recognized in the income statement for the year
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Continuing operations |
€ | 4,302 | € | 4,020 | € | 3,676 | ||||||
| Discontinued operations |
— | — | 643 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total minimum lease payments under operating leases |
€ | 4,302 | € | 4,020 | € | 4,319 | ||||||
|
|
|
|
|
|
|
|||||||
Regarding outstanding commitments for future minimum lease payments under operating leases, see off-balance sheet arrangements as explained in note 27 below.
F-50
Table of Contents
27. Off-balance sheet arrangements
CONTRACTUAL OBLIGATIONS AND COMMITMENTS
We entered into lease agreements for office and laboratories which qualify as operating leases. We also have certain purchase commitments with CRO subcontractors principally.
On December 31, 2016, we had outstanding obligations for future minimum rent payments and purchase commitments, which become due as follows:
| Total | Less than 1 year |
1–3 years |
3–5 years |
More than 5 years |
||||||||||||||||
| (thousands of €) |
||||||||||||||||||||
| Operating lease obligations |
€ | 27,263 | € | 4,114 | € | 6,494 | € | 5,504 | € | 11,151 | ||||||||||
| Purchase commitments |
27,579 | 27,084 | 495 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations & commitments |
€ | 54,842 | € | 31,198 | € | 6,989 | € | 5,504 | € | 11,151 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
28. Contingent assets and liabilities
On March 13, 2014, we announced the signing of a definitive agreement to sell the service division operations to Charles River Laboratories International, Inc. or CRL for a total consideration of up to €134 million. CRL agreed to pay us an immediate cash consideration of €129 million. The potential earn-out of €5 million due upon achievement of a revenue target 12 months after transaction closing has not been obtained. Approximately 5% of the total consideration, including price adjustments, was being held on an escrow account. To date, four claims have been introduced by CRL, of which all have been settled for a total amount of €1.3 million. On January 17, 2017 an amount of €4.1 million was released from the escrow account. The release of the remaining balance of the escrow account will be possible after final agreement between the parties on the amounts at stake.
Following the divestment, we remained guarantor until early February 2017 in respect of the lease obligations for certain U.K. premises amounting to £3 million future rent payments. CRL shall fully indemnify us against all liabilities arising in connection with the lease obligations. We evaluated the risk to be remote. Finally, following common practice, we have given customary representations and warranties which are capped and limited in time (since April 1, 2016, CRL can only introduce a claim covered by the Tax Deed (during a period of 5 years), other claims related to the sale cannot be submitted anymore).
In the course of 2008, a former director of one of the subsidiaries sued for wrongful termination and seeks damages of €1.5 million. We believe that the amount of damages claimed is unrealistically high. In 2014, the court requested an external advisor to evaluate the exact amount of damages. On January 29, 2016, the court made a 1st degree judgment, dismissing all claims in full. In appeal, the 2nd degree court instructed the 1st degree court to conduct a new trial, which is currently pending. So far, no hearings have been scheduled and no decisions have been made. Considering the defense elements provided, as well as the fact that so far the court has made no decision indicating that the claim would be sustained, our board and management evaluated the risk to be remote to possible, but not likely. Accordingly, it was decided not to record any provision in 2016 as the exposure is considered to be limited.
29. Retirement benefit plans
DEFINED CONTRIBUTION PLANS
We operate defined contribution systems for all of our qualifying employees. The assets of the schemes are held separately from ours in designated pension plans. For defined contribution systems, we pay contributions to publicly or privately administered pension or insurance funds. Once the contribution is paid, we do not have any remaining obligation.
F-51
Table of Contents
DEFINED BENEFIT PLANS IN BELGIUM
Our personnel in Belgium participated in a defined contribution plan (extra-legal pension). The Belgian defined contribution pension plans were by law subject to minimum guaranteed rates of return, 3.25% on employer contributions and 3.75% on employee contributions. These rates, which apply as an average over the entire career, may be modified by Royal Decree. Therefore, those plans were basically accounted for as defined contribution plans.
As a consequence of the law of December 18, 2015, minimum returns were guaranteed by the employer as follows: (a) for the contributions paid as from January 1, 2016, a new variable minimum return based on OLO rates, with a minimum of 1.75% and a maximum of 3.75%. In review of the low rates of the OLO in the last years, the return has been initially set to 1.75%; (b) for the contributions paid until end of December 2015, the previously applied legal returns as mentioned above, continue to apply until the leaving of the employees.
In view of the minimum returns guarantees, the Belgian defined contribution plans classify as defined benefit plans as from end December 2015.
As at December 31, 2015 no net liability was recognized (2014: nil) in the balance sheet as the minimum rates of return to be guaranteed by the employer are closely matched by the rates of return guaranteed by the insurer. As at December 31, 2016 however a net liability of €386.6 thousand was recorded.
The contributions for those plans that were due by the employer for 2016, 2015 and 2014 amounted to respectively €528.0 thousand, €476.3 thousand and €465.6 thousand, of which €42.5 thousand was paid after December 31, 2016 (2015: €35.9 thousand; 2014: €32.9 thousand). No contributions were made by the employees.
The plan assets as at December 31, 2016 consisted of €1,788.7 thousand (2015: 1,063.7 thousand) individual insurance reserves, which benefit from a weighted average guaranteed interest rate of 2.82% (2015 : 3.0%).
DEFINED BENEFIT PLANS IN FRANCE
We use two defined benefit plans for the employees of our French entity. The defined benefit plans are not supported by funds.
The chemical and pharmaceutical industry’s collective bargaining agreements require that the French entity pays a retirement allowance depending on the seniority of the employees at the moment they retire. The benefit obligations for these retirement allowances amounted to €1,808.5 thousand for 2016 (2015: €1,520.9 thousand; 2014: €1,622.3 thousand). The decrease in 2015 compared to 2014 is mainly due to changed actuarial assumptions (increase of discount rate from 1.75% to 2%). The increase in 2016 is mainly due to changed actuarial assumptions (decrease of discount rate from 2% to 1.44%).
Additionally, there are also seniority premiums paid in France. The provisions for these premiums amounted to €1,324.9 thousand in 2016 (2015: €1,172.0 thousand; 2014: €1,242.9 thousand).
Total obligation included in the balance sheet related to the defined benefit plans amounts to €3,133.4 thousand for the year ended December 31, 2016 (2015: €2,692.9 thousand; 2014: €2,865.2 thousand).
Actuarial gains and losses are recognized immediately on the balance sheet, with a charge or credit to other comprehensive income (OCI), in accordance with IAS 19R. They are not recycled subsequently. Actuarial losses of €193.2 thousand have been booked through other comprehensive income (OCI) at the end of 2016 (2015: €201.5 thousand of actuarial gains, 2014: €266.6 thousand of actuarial losses).
Total amounts due by all entities to these pension plans in 2016 were €1.7 million in total (2015: €1.5 million, 2014: €1.5 million).
F-52
Table of Contents
Obligations included in the balance sheet
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Present value of funded defined benefit obligation |
€ | 2,175 | — | — | ||||||||
| Plan assets |
€ | (1,789 | ) | € | (1,064 | ) | — | |||||
| Deficit/ surplus |
387 | (1,064 | ) | — | ||||||||
| Present value of unfunded defined benefit obligation |
3,133 | 2,693 | € | 2,865 | ||||||||
| Reclassification—Belgian contribution plans |
— | 1,064 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Liability included in the balance sheet |
€ | 3,520 | € | 2,693 | € | 2,865 | ||||||
|
|
|
|
|
|
|
|||||||
The present value of the gross obligation developed as follows:
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Opening balance |
€ | 3,757 | € | 2,865 | € | 2,189 | ||||||
| Current service cost |
649 | 194 | 228 | |||||||||
| Actual taxes on contributions paid |
(48 | ) | — | — | ||||||||
| Interest cost |
82 | 50 | 65 | |||||||||
| Benefits paid |
(119 | ) | (44 | ) | (48 | ) | ||||||
| Reclassification—Belgian contribution plans |
— | 1,064 | — | |||||||||
| Actuarial gains (-) or losses due to experience adjustments |
500 | (27 | ) | 82 | ||||||||
| Actuarial losses due to experience adjustments related to new financial assumptions |
432 | (99 | ) | 347 | ||||||||
| Actuarial gains (-) or losses due to experience adjustments related to new demographic assumptions |
56 | (247 | ) | 3 | ||||||||
|
|
|
|
|
|
|
|||||||
| Closing balance |
€ | 5,308 | € | 3,757 | € | 2,865 | ||||||
|
|
|
|
|
|
|
|||||||
The fair value of the plan assets developed as follows:
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Opening balance |
€ | (1,064 | ) | — | — | |||||||
| Interest income on plan assets |
(32 | ) | — | — | ||||||||
| Actual administration costs |
2 | — | — | |||||||||
| Contributions from employer |
(411 | ) | — | — | ||||||||
| Actual taxes on contributions paid |
48 | — | — | |||||||||
| Plan assets gain during the period |
(332 | ) | — | — | ||||||||
| Reclassification—Belgian contribution plans |
— | (1,064 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Closing balance |
€ | (1,788 | ) | € | (1,064 | ) | € | — | ||||
|
|
|
|
|
|
|
|||||||
The expected rate of return on the plan assets is 2%.
The fair value of the plan assets is the fair market value of the plan assets. The fair value of the plan assets was calculated as the reduced lump sums (received from the plan administrators) actualized with the assumptions set (discount rate and mortality tables). The total plan assets are equal to the fair value of the plan assets increased with the financing fund.
F-53
Table of Contents
Amounts recognized in profit or loss for defined benefit plans are as follows:
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Current service cost |
€ | 649 | € | 194 | € | 228 | ||||||
| Interest cost |
82 | 50 | 65 | |||||||||
| Interest income |
(32 | ) | — | — | ||||||||
| Administration expenses |
2 | — | — | |||||||||
| Revaluations of net liability / net asset |
73 | (171 | ) | 165 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total expense |
€ | 773 | € | 73 | € | 457 | ||||||
|
|
|
|
|
|
|
|||||||
Obligation included in the balance sheet reconciles as follows:
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Opening balance |
€ | 2,693 | € | 2,865 | € | 2,189 | ||||||
| Real employer contributions |
(411 | ) | — | — | ||||||||
| Total expense recognized in the income statement |
773 | 73 | 457 | |||||||||
| Re-measurement on the net defined benefit liability |
583 | (202 | ) | 267 | ||||||||
| Benefits paid |
(119 | ) | (44 | ) | (48 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Closing balance |
€ | 3,520 | € | 2,693 | € | 2,865 | ||||||
|
|
|
|
|
|
|
|||||||
The main actuarial assumptions were:
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (%) | ||||||||||||
| Discount rate |
1.60 | % | 2.00 | % | 1.75 | % | ||||||
| Expected salary increase |
2.50 | % | 2.25 | % | 2.25 | % | ||||||
| Inflation rate |
1.75 | % | 1.75 | % | 1.75 | % | ||||||
The discount rate was based on the Merrill Lynch yields for AA rated Eurozone corporate bonds (bonds with maturity dates which correspond with the commitments).
Breakdown of defined benefit obligation by type of plan participants:
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (number of participants) | ||||||||||||
| Active plan participants |
267 | 254 | 125 | |||||||||
Breakdown of defined benefit obligation by type of benefits:
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Retirement and death benefits |
€ | 3,983 | 2,585 | 1,622 | ||||||||
| Other post-employment benefits |
1,325 | 1,172 | 1,243 | |||||||||
F-54
Table of Contents
Major categories of plan assets: fair value plan of assets:
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Equity |
€ | 89 | € | 74 | ||||||||
| Debt |
1,698 | 979 | ||||||||||
| Cash |
11 | |||||||||||
Sensitivity analysis on discount rate: effect on obligation:
| December 31, | ||||||||
| 2016 | ||||||||
| Obligation (Euro, in thousands) |
||||||||
| Discount rate |
1.10 | % | € | 3,792 | ||||
| Discount rate |
1.35 | % | 3,661 | |||||
| Discount rate |
1.60 | % | 3,520 | |||||
| Discount rate |
1.85 | % | 3,419 | |||||
| Discount rate |
2.10 | % | € | 3,312 | ||||
| December 31, | ||||||||
| 2015 | ||||||||
| Obligation (Euro, in thousands) |
||||||||
| Discount rate |
1.50 | % | € | 2,868 | ||||
| Discount rate |
1.75 | % | 2,779 | |||||
| Discount rate |
2.00 | % | 2,693 | |||||
| Discount rate |
2.25 | % | 2,612 | |||||
| Discount rate |
2.50 | % | € | 2,534 | ||||
| December 31, | ||||||||
| 2014 | ||||||||
| Obligation (Euro, in thousands) |
||||||||
| Discount rate |
1.25 | % | € | 3,068 | ||||
| Discount rate |
1.50 | % | 2,964 | |||||
| Discount rate |
1.75 | % | 2,865 | |||||
| Discount rate |
2.00 | % | 2,772 | |||||
| Discount rate |
2.25 | % | € | 2,682 | ||||
30. Warrant plans
Presented below is a summary of warrant plans activities for the reported periods. Various warrant plans were approved for the benefit of our employees, and for directors and independent consultants of Galapagos NV. For warrant plans issued prior to 2011, the warrants offered to the employees and independent consultants vest according to the following schedule: 10% of the warrants vest on the date of the grant; an additional 10% vest at the first anniversary of the grant; an additional 20% vest at the second anniversary of the grant; an additional 20% vest at the third anniversary of the grant; and an additional 40% vest at the end of the third calendar year following the grant. The warrants granted under warrant plans created from 2011 up to (and including) Warrant Plan 2015 vest at the end of the third calendar year following the year of the grant, with no intermediate vesting. The warrants granted under Warrant Plan 2015 (B) and Warrant Plan 2015 RMV vest on the third anniversary of the notary deed enacting the acceptance of the warrants. The warrants granted under Warrant plan 2016 and
F-55
Table of Contents
Warrant Plan 2016 RMV vest at the end of the third calendar year following the year of the grant, with no intermediate vesting.
The warrants offered to directors vest over a period of 36 months at a rate of 1/36th per month. Warrants cannot be exercised before the end of the third calendar year following the year of the grant, except for warrants granted under Warrant Plan 2015 (B) and Warrant Plan 2015 RMV, which become exercisable on the third anniversary of the notary deed enacting the acceptance of the warrants. In the event of a change of control over Galapagos NV, all outstanding warrants vest immediately and will be immediately exercisable.
After the reverse 4:1 share split approved by the extraordinary shareholders’ meeting of March 29, 2005, four warrants under Warrant Plan 2002 Belgium entitle the warrant holder to subscribe for one ordinary share. For the warrant plans created from 2005 onwards, one warrant entitles the warrant holder to subscribe for one ordinary share. In the summaries and tables below, the numbers of warrants issued under Warrant Plan 2002 Belgium are divided by four to avoid confusion in entitlements and rights.
The table below sets forth a summary of warrants outstanding and exercisable at December 31, 2016, per warrant plan:
| Warrantplan |
Allocation date |
Expiry date |
Exercise price (€) |
Outstanding per January 1, 2016 |
Granted during the year |
Exercised during the year |
Forfeited during the year |
Expired during the year |
Outstanding per December 31, 2016 |
Exercisable per December 31, 2016 |
||||||||||||||||||||||||||||||
| 2002 B |
7/9/2004 | 7/8/2017 | 4 | 31,250 | (31,250 | ) | 0 | 0 | ||||||||||||||||||||||||||||||||
| 2002 B |
1/31/2005 | 1/30/2017 | 6.76 | 30,000 | (5,000 | ) | 25,000 | 25,000 | ||||||||||||||||||||||||||||||||
| 2005 |
7/4/2005 | 7/3/2018 | 6.91 | 120,000 | (30,000 | ) | 90,000 | 90,000 | ||||||||||||||||||||||||||||||||
| 2005 |
12/15/2005 | 12/14/2018 | 8.6 | 12,500 | 12,500 | 12,500 | ||||||||||||||||||||||||||||||||||
| 2006 BNL |
5/4/2007 | 5/3/2020 | 9.22 | 7,500 | 7,500 | 7,500 | ||||||||||||||||||||||||||||||||||
| 2006 BNL |
6/28/2007 | 6/27/2020 | 8.65 | 735 | 735 | 735 | ||||||||||||||||||||||||||||||||||
| 2006 BNL |
12/21/2007 | 12/20/2020 | 7.12 | 1,575 | (525 | ) | 1,050 | 1,050 | ||||||||||||||||||||||||||||||||
| 2007 |
6/28/2007 | 6/27/2020 | 8.65 | 48,909 | 48,909 | 48,909 | ||||||||||||||||||||||||||||||||||
| 2007 RMV |
10/25/2007 | 10/24/2020 | 8.65 | 44,125 | (6,475 | ) | 37,650 | 37,650 | ||||||||||||||||||||||||||||||||
| 2008 |
6/26/2008 | 6/25/2021 | 5.6 | 89,915 | (10,315 | ) | 79,600 | 79,600 | ||||||||||||||||||||||||||||||||
| 2009 |
4/1/2009 | 3/31/2017 | 5.87 | 42,500 | (35,000 | ) | 7,500 | 7,500 | ||||||||||||||||||||||||||||||||
| 2010 |
4/27/2010 | 4/26/2018 | 11.55 | 96,300 | (43,300 | ) | 53,000 | 53,000 | ||||||||||||||||||||||||||||||||
| 2011 |
5/23/2011 | 5/22/2019 | 9.95 | 77,500 | (18,400 | ) | 59,100 | 59,100 | ||||||||||||||||||||||||||||||||
| 2011 (B) |
5/23/2011 | 5/22/2016 | 9.95 | 117,940 | (117,940 | ) | 0 | 0 | ||||||||||||||||||||||||||||||||
| 2012 |
9/3/2012 | 9/2/2020 | 14.19 | 370,490 | (120,830 | ) | (2,500 | ) | 247,160 | 247,160 | ||||||||||||||||||||||||||||||
| 2013 |
5/16/2013 | 5/15/2021 | 19.38 | 445,740 | (13,500 | ) | 432,240 | |||||||||||||||||||||||||||||||||
| 2013 (B) |
9/18/2013 | 9/17/2021 | 15.18 | 30,000 | 30,000 | |||||||||||||||||||||||||||||||||||
| 2014 |
7/25/2014 | 7/24/2022 | 14.54 | 556,660 | (20,000 | ) | 536,660 | |||||||||||||||||||||||||||||||||
| 2014 (B) |
10/14/2014 | 10/13/2022 | 11.93 | 150,000 | 150,000 | |||||||||||||||||||||||||||||||||||
| 2015 |
4/30/2015 | 4/29/2023 | 28.75 | 532,053 | (15,000 | ) | 517,053 | |||||||||||||||||||||||||||||||||
| 2015 (B) |
12/22/2015 | 12/21/2023 | 49 | 399,000 | 399,000 | |||||||||||||||||||||||||||||||||||
| 2015 RMV |
12/22/2015 | 12/21/2023 | 49 | 97,500 | 97,500 | |||||||||||||||||||||||||||||||||||
| 2016 |
6/1/2016 | 5/31/2024 | 46.1 | 514,250 | 514,250 | |||||||||||||||||||||||||||||||||||
| 2016 RMV |
6/1/2016 | 5/31/2024 | 46.1 | 120,000 | 120,000 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
| Total |
2,805,692 | 1,130,750 | (419,035 | ) | (48,500 | ) | (2,500 | ) | 3,466,407 | 669,704 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
F-56
Table of Contents
| Warrants | Weighted average exercise price (€) |
|||||||
| Outstanding on January 1, 2014 |
3,627,076 | € | 11.50 | |||||
|
|
|
|
|
|||||
| Exercisable on December 31, 2013 |
1,138,438 | |||||||
| Granted during the period |
721,660 | |||||||
| Forfeited during the year |
(252,800 | ) | ||||||
| Exercised during the period |
(505,083 | ) | ||||||
| Expired during the year |
— | |||||||
|
|
|
|
|
|||||
| Outstanding on December 31, 2014 |
3,590,853 | € | 12.06 | |||||
|
|
|
|
|
|||||
| Exercisable on December 31, 2014 |
1,355,213 | |||||||
| Granted during the period |
532,053 | |||||||
| Forfeited during the year |
(72,500 | ) | ||||||
| Exercised during the period |
(1,244,714 | ) | ||||||
| Expired during the year |
— | |||||||
|
|
|
|
|
|||||
| Outstanding on December 31, 2015 |
2,805,692 | € | 16.22 | |||||
|
|
|
|
|
|||||
| Exercisable on December 31, 2015 |
720,749 | |||||||
| Granted during the period |
1,130,750 | |||||||
| Forfeited during the year |
(48,500 | ) | ||||||
| Exercised during the period |
(419,035 | ) | ||||||
| Expired during the year |
(2,500 | ) | ||||||
|
|
|
|
|
|||||
| Outstanding on December 31, 2016 |
3,466,407 | € | 27.06 | |||||
|
|
|
|
|
|||||
| Exercisable on December 31, 2016 |
669,704 | |||||||
The table below sets forth the inputs into the valuation of the warrants.
| 2016 | 2016 RMV | 2015 (B) | 2015 RMV | 2015 | 2014 | 2014 | ||||||||||||||||||||||
| June 1 | June 1 | December 22 | December 22 | April 30 | Oct 14 | Jul 25 | ||||||||||||||||||||||
| Exercise Price |
€ | 46.10 | € | 46.10 | € | 49.00 | € | 49.00 | € | 28.75 | € | 11.93 | € | 14.54 | ||||||||||||||
| Share price at acceptance date |
€ | 48.71 | € | 47.63 | € | 39.85 | € | 39.78 | € | 46.09 | € | 10.95 | € | 14.38 | ||||||||||||||
| Fair value on the acceptance date |
€ | 21.95 | € | 21.16 | € | 15.41 | € | 15.39 | € | 26.05 | € | 4.35 | € | 6.14 | ||||||||||||||
| Estimated volatility (%) |
40.69 | 40.69 | 41.1 | 41.08 | 39.2 | 38.03 | 38.76 | |||||||||||||||||||||
| Time to expiration (years) |
8 | 8 | 8 | 8 | 8 | 8 | 8 | |||||||||||||||||||||
| Risk free rate (%) |
0 | 0 | 0.24 | 0.28 | 0.39 | 0.58 | 0.58 | |||||||||||||||||||||
| Expected dividends |
None | None | None | None | None | None | None | |||||||||||||||||||||
Warrant Plans
The exercise price of the warrants is determined pursuant to the applicable provisions of the Belgian Companies Code.
The estimated volatility is calculated on the basis of the historical volatility of the share price over the expected life of the warrants, validated by reference to the volatility of a representative biotech index.
The time to expiration of the warrant is calculated as the estimated duration until exercise, taking into account the specific features of the plans.
The warrants were accounted for in accordance with International Financial Reporting Standard 2 on Share Based Payments. IFRS 2 takes effect for all warrants offered after November 7, 2002.
F-57
Table of Contents
Our warrants expense in 2016 amounted to €11,034 thousand (2015: 5,036 thousand; 2014: €2,952 thousand).
The following table provides an overview of the outstanding warrants per category of warrant holders at December 31, 2016, 2015 and 2014.
Category
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (in number of warrants) | ||||||||||||
| Non-executive directors |
165,240 | 115,730 | 199,070 | |||||||||
| Executive team |
1,676,874 | 1,376,874 | 1,520,000 | |||||||||
| Other |
1,624,293 | 1,313,088 | 1,871,783 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total warrants outstanding |
3,466,407 | 2,805,692 | 3,590,853 | |||||||||
|
|
|
|
|
|
|
|||||||
The outstanding warrants at the end of the accounting period have an average exercise price of €27.06 (2015: €16.22; 2014: €12.06) and a weighted average remaining expected life of 1,482 days (2015: 1,469 days; 2014: 1,639 days).
31. Related parties
Relationship and transactions with entities with (joint) control of, or significant influence over, Galapagos
There are no shareholders or other entities who, solely or jointly, control Galapagos or exercise significant influence over Galapagos.
Relationship and transactions with subsidiaries
Please see Note 32 for an overview of the consolidated companies of the group, which are all wholly-owned subsidiaries of Galapagos NV.
Intercompany transactions between Galapagos NV and its subsidiaries, and amongst the subsidiaries, have been eliminated in the consolidation and are not disclosed in this note.
Relationship and transactions with key management personnel
Our key management personnel consists of the members of our executive committee and the members of our board of directors. All amounts mentioned in this section are based on expenses recognized in the financial statements for the relevant financial year.
Remuneration of key management personnel
On December 31, 2016, our executive committee had four members: Mr. Onno van de Stolpe, Mr. Bart Filius, Dr. Piet Wigerinck and Dr. Andre Hoekema. On December 31, 2016, our board of directors consisted of eight members: Mr. Onno van de Stolpe, Dr. Raj Parekh, Dr. Werner Cautreels, Dr. Harrold van Barlingen, Mr. Howard Rowe, Ms. Katrine Bosley, Dr. Christine Mummery and Dr. Mary Kerr.
Only the CEO is a member of both the executive committee and the board of directors. Our CEO does not receive any special remuneration for his board membership, as this is part of his total remuneration package in his capacity as member of the executive committee.
F-58
Table of Contents
Historically, the chairman of the board of directors, Dr. Parekh, did not receive remuneration like the other directors. Between August 1, 2005 and April 30, 2016, Dr. Parekh received an annual consulting fee of £50,000 under a consultancy contract with his management company, Parekh Enterprises Ltd. as compensation for his specific assignment to assist the group in strategic positioning, financing and acquisitions. Since May 1, 2016, Dr. Parekh receives remuneration for his director’s mandate in the same way as the other directors.
The remuneration package of the members of key management personnel comprises:
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands, except for the number of warrants) |
||||||||||||
| Remuneration of key management personnel: |
||||||||||||
| Short-term employee benefits (*) |
||||||||||||
| Executive committee members as a group |
€ | 3,124 | € | 2,937 | € | 1,506 | ||||||
| Raj Parekh (^) |
73 | — | — | |||||||||
| Harrold van Barlingen |
47 | 40 | 20 | |||||||||
| Howard Rowe |
50 | 40 | 20 | |||||||||
| Werner Cautreels |
56 | 45 | 45 | |||||||||
| Katrine Bosley |
45 | 40 | 40 | |||||||||
| Christine Mummery (#) |
43 | 10 | — | |||||||||
| Mary Kerr (##) |
18 | — | — | |||||||||
| Vicki Sato (###) |
— | — | 40 | |||||||||
| Post-employment benefits(°) |
228 | 215 | 184 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total benefits excluding warrants |
€ | 3,683 | € | 3,327 | € | 1,855 | ||||||
|
|
|
|
|
|
|
|||||||
| Number of warrants granted in the year |
||||||||||||
| Executive committee members as a group |
515,000 | 175,000 | 330,000 | |||||||||
| Raj Parekh (^) |
30,000 | 5,400 | 5,400 | |||||||||
| Harrold van Barlingen |
15,000 | 2,520 | 2,520 | |||||||||
| Howard Rowe |
15,000 | 2,520 | 2,520 | |||||||||
| Werner Cautreels |
15,000 | 3,780 | 3,780 | |||||||||
| Katrine Bosley |
15,000 | 2,520 | 2,520 | |||||||||
| Christine Mummery (#) |
15,000 | — | — | |||||||||
| Mary Kerr (##) |
— | — | — | |||||||||
| Vicki Sato (###) |
— | — | 2,520 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total number of warrants granted in the year |
620,000 | 191,740 | 349,260 | |||||||||
|
|
|
|
|
|
|
|||||||
| (*) | Includes for executive committee members: salaries, employer social security contributions, other short-term benefits; includes for board members: board fees, other short-term benefits. |
| (^) | During the first four months of 2016, Dr. Parekh did not receive remuneration for his director’s mandate, but was compensated through a consultancy agreement only (consultancy fee of €20 thousand in 2016). |
| (°) | Only executive committee members are granted post-employment benefits. |
| (#) | Dr. Mummery joined the board on September 30, 2015. |
| (##) | Dr. Kerr joined the board on July 26, 2016. |
| (###) | Dr. Sato resigned from the board on December 31, 2014. |
SHORT-TERM EMPLOYEE BENEFITS AND BOARD FEES
The members of the executive committee provide their services to us on a full-time basis.
F-59
Table of Contents
The four members of the executive committee (including the CEO) who were in function in the course of 2016 were paid an aggregate amount of €1,291.84 in remuneration and received an aggregate amount of €1,747.21 in bonuses (2015: €1,245.5 thousand in remuneration and €1,629.5 thousand in bonuses; 2014, for the six members of the executive committee who were in function in the course of 2014: €1,151.6 thousand in remuneration and €268.6 thousand in bonuses). The aggregate bonus amount for 2016 was composed of two parts: (i) an aggregate bonus of €573.05 thousand, being 50% of the bonus for performance over 2016 (paid in early January 2017), with the other 50% being deferred for 3 years, (ii) an aggregate amount of €1,174.17 thousand as deferred part of the bonus for performance over 2013 (paid in early January 2017). The aggregate bonus amount for 2015 was composed of 3 parts: (i) an aggregate bonus of €488.5 thousand, being 50% of the bonus for performance over 2015 (paid in early January 2016), with the other 50% being deferred for 3 years, (ii) an aggregate amount of €628.5 thousand as deferred part of the bonus for performance over 2012 (paid in early January 2016) and (iii) an aggregate amount of €512.5 thousand, being 50% of the exceptional special bonus awarded for the success of the NASDAQ listing (paid in June 2015), with the other 50% being deferred for 3 years. The aggregate bonus amount for 2014 was composed of 2 parts: (a) an aggregate bonus of €234 thousand, being 50% of the bonus for performance over 2014 (paid in early January 2015), with the other 50% being deferred for 3 years, (b) an aggregate amount of €34.6 thousand as an exceptional special bonus granted to former executive committee member Mr. Smith in connection with his instrumental role in the divestment of the group’s services division. No performance bonus was awarded for the year 2011, as three out of five of the corporate objectives for 2011 were not achieved. Therefore, no deferred part of the bonus for the year 2011 was paid out in 2014.
Other components of the remuneration of the executive committee members included contributions to health insurance schemes, company cars, tax advisory services and certain fringe benefits of non-material value.
Pursuant to the decision of the annual shareholders’ meeting of April 26, 2016, Dr. Parekh received €70 thousand (or, taking into account €20 thousand received in consultancy fees for the first four months of 2016, an aggregate of €90 thousand: €80 thousand as chairman of the board, and €10 thousand as chairman of the nomination and remuneration committee), Dr. Cautreels received €55 thousand (€40 thousand as non-executive director, €10 thousand as chairman of the audit committee and €5 thousand as member of the nomination and remuneration committee), Ms. Bosley, Mr. Rowe and Dr. Van Barlingen each received €45 thousand (€40 thousand as non-executive director and €5 thousand as member of the nomination and remuneration committee or audit committee) and Dr. Mummery received €40 thousand as non-executive director. Dr. Kerr, being appointed as non-executive director as from July 26, 2016, received €17 thousand as remuneration for the performance of her mandate during the remainder of 2016 pursuant to the decision of the special shareholders’ meeting of July 26, 2016. Pursuant to a power of attorney granted by the annual shareholders’ meeting of April 28, 2015, the board determined, after discussion within the nomination and remuneration committee, to allocate the aggregate annual remuneration for directors for 2015 as follows: (a) annual remuneration for each non-executive director (Dr. Cautreels, Dr. Van Barlingen, Mr. Rowe and Ms. Bosley): €40 thousand; and (b) additional remuneration for the chairman of the audit committee (Dr. Cautreels): €5 thousand. Dr. Mummery, being appointed as non-executive director as from September 30, 2015, received €10 thousand as remuneration for the performance of her mandate during the last quarter of 2015. Pursuant to a power of attorney granted by the annual shareholders’ meeting of April 29, 2014, the board determined, after discussion within the nomination and remuneration committee, to allocate the aggregate annual remuneration for directors for 2014 as follows: (a) remuneration for non-executive directors who do not represent a shareholder (Dr. Van Barlingen and Mr. Rowe): €20 thousand; (b) remuneration for non-EU-based directors (who do not represent a shareholder) and/or for directors who actively and on a regular basis provide independent clinical, scientific and/or transactional advice to the board of directors (Dr. Cautreels, Dr. Sato and Ms. Bosley): €40 thousand; (c) additional remuneration for the chairman of the audit committee (Dr. Cautreels): €5 thousand.
The increase in board fees is due to the increased number of directors and the decision of the annual shareholders’ meeting of April 26, 2016 to increase the amount of remuneration paid to the directors, also taking into account their positions as board chairman, committee chairman and committee member. In addition, Dr. Parekh did not receive remuneration for his director’s mandate in 2014, 2015 and the first four months of
F-60
Table of Contents
2016, but was instead compensated only through a consultancy agreement until April 30, 2016. Finally, in 2016, a total amount of €14.5 thousand was paid as other short-term benefit for the non-executive directors (2015: €4.95 thousand). These benefits related to the payment of tax advisory services.
POST-EMPLOYMENT BENEFITS
The post-employment benefits to the members of the executive committee are granted under separate retirement benefit schemes, including pension schemes, post-employment life insurance and additional individual pension contributions.
SEVERANCE PAYMENTS
The employment and management agreements of the members of the executive committee do not provide for severance compensation. They do not contain notice periods that exceed six months. However, Galapagos entered into undertakings with the members of the executive committee providing that, in case their contract with the group is terminated as a result of a change of control of Galapagos NV, they would be entitled to a severance compensation of 12 months’ base salary for the Chief Executive Officer and nine months’ base salary for the other executive committee members.
WARRANTS GRANTED IN 2016
In 2016, 60,000 warrants were granted to independent directors (2015: 8,820; 2014: 11,340) and 45,000 warrants were granted to the other non-executive directors (2015: 7,920; 2014: 7,920). The increase can be explained by the fact that the final acceptance and issuance of the warrants under Warrant Plan 2015 (B) took place in 2016, and are counted as warrants granted in 2016 along with the warrants granted under Warrant Plan 2016. The special shareholders’ meeting of December 22, 2015, upon the proposal of our nomination and remuneration committee, offered additional warrants to our directors under Warrant Plan 2015 (B) in light of an independent benchmarking exercise and recommendation by an external advisor, following the growth of the company and the U.S. listing on Nasdaq in 2015.
OTHER
No loans, quasi-loans or other guarantees were given by Galapagos NV or any of its subsidiaries to members of the board and of the executive committee. We have not entered into transactions with our key management personnel, other than as described above with respect to remuneration arrangements relating to the exercise of their mandates as members of the executive committee and the board of directors.
F-61
Table of Contents
32. Consolidated companies as of December 31, 2016
| Year ended December 31, | ||||||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||
| Name of the subsidiary |
Country |
% voting right Galapagos NV (directly or indirectly through subsidiaries) |
Change in % voting right previous period (2016 vs 2015) |
% voting right Galapagos NV (directly or indirectly through subsidiaries) |
% voting right Galapagos NV (directly or indirectly through subsidiaries) |
|||||||||||||
| Continuing operations: |
||||||||||||||||||
| BioFocus DPI AG |
Switzerland | 100% | 100% | 100% | ||||||||||||||
| BioFocus DPI LLC |
United States | 0% | (100 | %) | 100% | 100% | ||||||||||||
| BioFocus, Inc. |
United States | 100% | 100% | 100% | ||||||||||||||
| Discovery Partners International GmbH |
Germany | 100% | 100% | 100% | ||||||||||||||
| Galapagos B.V. |
The Netherlands | 100% | 100% | 100% | ||||||||||||||
| Galapagos NV |
Belgium | parent company | parent company | parent company | ||||||||||||||
| Fidelta d.o.o. |
Croatia | 100% | 100% | 100% | ||||||||||||||
| Galapagos SASU |
France | 100% | 100% | 100% | ||||||||||||||
| Inpharmatica Ltd. |
United Kingdom | 100% | 100% | 100% | ||||||||||||||
| Xenometrix, Inc. |
United States | 100% | 100% | 100% | ||||||||||||||
| Discontinued operations: * |
||||||||||||||||||
| Argenta Discovery 2009 Ltd. |
United Kingdom | 0% | — | 0% | 0% | |||||||||||||
| BioFocus DPI (Holdings) Ltd. |
United Kingdom | 0% | — | 0% | 0% | |||||||||||||
| BioFocus DPI Ltd. |
United Kingdom | 0% | — | 0% | 0% | |||||||||||||
| Cangenix Ltd. |
United Kingdom | 0% | — | 0% | 0% | |||||||||||||
| * | On April 1, 2014 these entities were sold to Charles River. |
BioFocus DPI LLC was voluntarily cancelled in 2016.
There are no significant restrictions on the ability to access or use assets and settle liabilities.
There are no significant restrictions on the group’s ability to access or use assets, or settle liabilities of one of the group’s subsidiaries.
F-62
Table of Contents
33. Company acquisitions and disposals
COMPANY DISPOSALS: SALE OF SERVICE DIVISION
On April 1, 2014, we sold our service division, comprising all service operations of BioFocus and Argenta in the UK and the Netherlands, to Charles River Laboratories International, Inc. In particular, we disposed of following companies which were previously fully consolidated: BioFocus DPI (Holdings) Ltd. and BioFocus DPI Ltd. (Saffron Walden, UK), Argenta Discovery 2009 Ltd. (Harlow, UK) and its subsidiary Cangenix Ltd. (Canterbury, UK). In addition, also certain assets from Galapagos B.V. (Leiden, the Netherlands) have been acquired by Charles River Laboratories International, Inc.
| April 1, 2014 | ||||
| (Euro, in thousands) | ||||
| Consideration received in cash and cash equivalents |
€ | 137,760 | ||
| Correction on consideration still to settle |
(650 | ) | ||
|
|
|
|||
| Total consideration |
€ | 137,111 | ||
|
|
|
|||
| April 1, 2014 | ||||
| (Euro, in thousands) | ||||
| Cash |
€ | 6,115 | ||
| Trade and other receivables |
18,165 | |||
|
|
|
|||
| Current assets |
24,280 | |||
|
|
|
|||
| Goodwill |
39,246 | |||
| Fixed assets |
13,397 | |||
| Deferred tax assets |
4,588 | |||
|
|
|
|||
| Non-current assets |
57,231 | |||
|
|
|
|||
| Trade payables |
(2,569 | ) | ||
| Other payables |
(5,263 | ) | ||
|
|
|
|||
| Current liabilities |
(7,831 | ) | ||
|
|
|
|||
| Provisions |
(604 | ) | ||
| Deferred tax liabilities |
(1,996 | ) | ||
| Other non-current liabilities |
(549 | ) | ||
|
|
|
|||
| Non-current liabilities |
(3,149 | ) | ||
|
|
|
|||
| Net assets disposed of |
€ | 70,531 | ||
|
|
|
|||
| April 1, 2014 | ||||
| (Euro, in thousands) | ||||
| Total consideration |
€ | 137,111 | ||
| Net assets disposed of |
(70,531 | ) | ||
| Effect from Cumulative Translation Adjustments reclassified from equity |
1,787 | |||
| Costs associated to sale |
(858 | ) | ||
|
|
|
|||
| Gain on disposal |
€ | 67,508 | ||
|
|
|
|||
F-63
Table of Contents
The gain on the sale is included in the income from discontinued operations for the year ended December 31, 2014.
| April 1, 2014 | ||||
| (Euro, in thousands) | ||||
| Consideration received in cash and cash equivalents |
€ | 137,760 | ||
| Less: cash and cash equivalent balances disposed |
(6,115 | ) | ||
|
|
|
|||
| Total consideration received |
131,645 | |||
|
|
|
|||
| Costs associated to sale |
(858 | ) | ||
|
|
|
|||
| Cash in from disposal of subsidiaries, net of cash disposed |
€ | 130,787 | ||
|
|
|
|||
34. Critical accounting estimates and judgments
In the application of the accounting policies, we are required to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates.
Our estimates and assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revisions and future periods if the revision affects both current and future periods.
Drafting financial statements in accordance with IFRS requires management to make judgments and estimates and to use assumptions that influence the reported amounts of assets and liabilities, the notes on contingent assets and liabilities on the date of the financial statements and the reported amounts of income and expenses during the reporting period. Actual results may differ from these estimates.
The following are the critical judgments and estimates that we have made in the process of applying the accounting policies and that have the most significant effect on the amounts recognized in the consolidated financial statements presented elsewhere in this annual report.
Critical judgments in applying accounting policies
Share subscription agreement with Gilead- classification as derivative financial asset or equity instrument
As described in note 7, Gilead Sciences, Inc. (“Gilead”) committed itself on December 16, 2015 to make a $425 million equity investment in Galapagos by subscribing to new shares at a fixed price of €58 per share, including issuance premium upon completion of the license and collaboration agreement with Galapagos that took place on January 19, 2016.
Significant judgment had to be applied in assessing whether this forward subscription commitment of Gilead over the own shares of Galapagos shall be classified as an own equity instrument of Galapagos or as a derivative financial asset. IAS 32 requires that for a derivative to meet the definition of equity it must be settled only by the issuer (Galapagos) exchanging a “fixed amount of cash or another financial asset for a fixed number of its own equity instruments.” Because the above mentioned commitment of Gilead was made in $, the actual number of shares finally issued by Galapagos varied with the fluctuation in the $/€ exchange rate until the settlement date on January 19, 2016.
Despite the fact that this foreign exchange exposure was limited, management judged that this variability prevents the instrument from being classified as equity under IAS 32 and was therefore treated as a derivative at fair value through profit and loss.
F-64
Table of Contents
Revenue recognition
Evaluating the criteria for revenue recognition with respect to our research and development and collaboration agreements requires management’s judgment to ensure that all criteria have been fulfilled prior to recognizing any amount of revenue. In particular, such judgments are made with respect to determination of the nature of transactions, whether simultaneous transactions shall be considered as one or more revenue-generating transactions, allocation of the contractual price (upfront and milestone payments in connection with a collaboration agreement) to several elements included in an agreement, and the determination of whether the significant risks and rewards have been transferred to the buyer. Collaboration agreements are reviewed carefully to understand the nature of risks and rewards of the arrangement. All of our revenue-generating transactions have been subject to such evaluation by management.
Critical accounting estimates
Fair value re-measurement of the Gilead share subscription agreement (derivative financial asset instrument)
| (Euro, in thousands) | ||||
| Fair value at inception |
39,003 | |||
| Movement of 2015 ( recognized in the income statement) |
(30,632 | ) | ||
|
|
|
|||
| Fair value per December 31, 2015 |
8,371 | |||
|
|
|
|||
| Movement of period January 1-19, 2016 ( recognized in the income statement) |
57,479 | |||
|
|
|
|||
| Fair value per January 19, 2016 |
65,850 | |||
|
|
|
|||
| Derecognition of the financial asset through the share premium account |
(65,850 | ) | ||
|
|
|
|||
| Fair value per December 31, 2016 |
— | |||
|
|
|
|||
The fair value measurement of this derivative financial asset was categorized as a level 3 in the fair value hierarchy of IFRS 13 Fair Value Measurement.
Its measurement was based on computing the difference between the strike price (58 EUR / share) and the anticipated Galapagos forward price, discounted to the valuation date. The notional was converted from USD to EUR by the FX forward rate and the number of shares was computed by dividing the EUR notional by the strike.
Input data were taken from Bloomberg as of December 16, 2015 and December 31, 2015, including:
| • | EUR OIS Discount rates (curve 133) |
| • | Implied forward rate of the GLPG share at January 31, 2016 |
| • | Implied FX Forward rate at January 31, 2016. |
This computation was based on the following unobservable assumptions:
| 1) | Between the date that the deal was signed (December 16, 2015) till the date the deal was complete, the two counterparties could not back off from the deal and it was 100% certain that the regulator would give the green light. |
| 2) | At the two valuation dates, it was assumed that the date when the deal will be complete would be January 31, 2016. This was the forward date where all the market data was taken from. |
| 3) | It was assumed that the effect of the correlation between the Galapagos share price and the €/$ FX rate was negligible. This was reasonable given the very short maturity of the deal. |
F-65
Table of Contents
Relationship of unobservable inputs to the fair value measurement:
| • | If one would have assumed that the closing date of the deal was January 19, 2016 (the actual closing date) the fair value of the derivative financial asset at December 31, 2015 would have been €8,367 thousands. |
On January 19, 2016, the value of the financial asset at maturity amounted to €65.9 million, reflecting the share premium that Gilead paid above our closing share price on the day of the capital increase. This financial asset expired on the effective date of the share subscription agreement and was derecognized through the share premium account.
Share-based payments plans
We determine the costs of the share-based payments plans (our warrant plans) on the basis of the fair value of the equity instrument at grant date. Determining the fair value assumes choosing the most suitable valuation model for these equity instruments, by which the characteristics of the grant have a decisive influence. This assumes also the input into the valuation model of some relevant judgments, like the estimated expected life of the warrant and the volatility. The judgments made and the model used are further specified in note 30.
Pension obligations
The cost of a defined pension arrangement is determined based on actuarial valuations. An actuarial valuation assumes the estimation of discount rates, estimated returns on assets, future salary increases, mortality figures and future pension increases. Because of the long-term nature of these pension plans, the valuation of these is subject to important uncertainties. See note 29 for additional details.
Corporate income taxes
Significant judgment is required in determining the use of tax loss carry forwards. Deferred tax assets arising from unused tax losses or tax credits are only recognized to the extent that there are sufficient taxable temporary differences or there is convincing evidence that sufficient taxable profit will be available against which the unused tax losses or unused tax credits can be utilized. Management’s judgment is that such convincing evidence is currently not sufficiently available except for two subsidiaries operating intercompany on a cost plus basis and as such a deferred tax asset is therefore recognized. As of December 31, 2016, we had a total of approximately €311.1 million of statutory tax losses carried forward which can be compensated with future taxable statutory profits for an indefinite period except for an amount of €18 million in Switzerland, Croatia, the United States and the Netherlands with expiry date between 2018 and 2030. As of December 31, 2016, the available tax losses carried forward in Belgium amounted to €230.9 million.
As from July 1, 2016, the existing Belgian patent income deduction (‘PID’) regime has been abolished and replaced by the innovation income deduction (‘IID’) regime (adopted by the Belgian Chamber of Representatives on February 2, 2017 and published in the official Belgian gazette on February 20, 2017).
Taxpayers benefitting from the previous PID regime will be able to still choose for the old PID regime (instead of the new IID regime) for five years (grandfathering until June 30, 2021).
The choice for the PID regime is however irrevocable. An assessment is currently ongoing to determine which regime is the most favorable for Galapagos. Given this ongoing assessment, the company has taken the position to make abstract of the new IID regime when estimating the tax provision in respect of assessment year 2017. In case the newly IID regime would be applied, it is possible that an additional carried-forward tax asset could be recognized (however subject to further analysis).
F-66
Table of Contents
35. Financial risk management
See “Risk Factors” for additional details on general risk factors.
Financial risk factors
Our financial risks are managed centrally. Our finance department coordinates the access to national and international financial markets and considers and manages continuously the financial risks concerning our activities. These relate to the financial markets risk, credit risk, liquidity risk and currency risk. There are no other important risks, such as interest rate risk, because we have nearly no financial debt and have a strong cash position. We do not buy or trade financial instruments for speculative purposes.
Categories of material financial assets and liabilities:
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Financial assets: |
||||||||||||
| Cash and cash equivalents |
€ | 973,241 | € | 340,314 | € | 187,712 | ||||||
| Restricted cash (current and non-current) |
7,668 | 7,903 | 10,728 | |||||||||
| Trade receivables |
6,629 | 1,494 | 1,340 | |||||||||
| R&D incentives receivables (current and non-current) |
64,342 | 58,545 | 51,296 | |||||||||
| Current financial asset from share subscription agreement |
— | 8,371 | — | |||||||||
| Available-for-sale financial assets |
2,351 | — | — | |||||||||
| Other amounts receivable |
3,078 | 2,426 | 1,862 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total financial assets |
€ | 1,057,309 | € | 419,052 | € | 252,937 | ||||||
|
|
|
|
|
|
|
|||||||
| Financial liabilities: |
||||||||||||
| Trade & other payables |
€ | 31,269 | € | 29,482 | € | 30,007 | ||||||
| Other non-current liabilities |
2,469 | 2,291 | 923 | |||||||||
| Leasing debts |
63 | 115 | 167 | |||||||||
| Tax payable |
1,022 | 2,583 | 2,582 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total financial liabilities |
€ | 34,823 | € | 34,471 | € | 33,679 | ||||||
|
|
|
|
|
|
|
|||||||
Share subscription agreement with Gilead
We have been temporarily exposed to financial market and currency risk though our share subscription agreement with Gilead.
On December 16, 2015, Gilead Sciences, Inc. and Galapagos NV entered into a global collaboration for the development and commercialization of filgotinib, in the framework of which Gilead committed to an upfront payment of $725 million consisting of a license fee of $300 million and a $425 million equity investment in Galapagos NV by subscribing to new shares at a price of €58 per share, including issuance premium. This agreement was effectively completed and entered into force January 19, 2016 and full payment was received.
In connection with the agreement, we recognized a deferred income and an offsetting short-term financial asset (derivative) of €39 million upon signing of the share subscription agreement with Gilead as required under IAS 39. This financial asset initially reflected the share premium that Gilead committed to pay above the closing stock price of Galapagos on the day of entering into the subscription agreement. This amount also represented a deferred income that will be recognized in revenues at the same rhythm than the $300 million upfront payment for the license.
F-67
Table of Contents
The fair value of this derivative financial asset was initially measured on December 16, 2015, based on the implied value of the Galapagos share at the end of January 2016, the implied volatility of the €/$ currency exchange rates and applicable discount rates.
Under IAS 39 the fair value of the derivative financial asset is re-measured at year end and again upon execution of the subscription agreement on January 19, 2016, when the financial asset expired. Variations in fair value of the financial asset are recorded in the income statement.
The decrease in the fair value of the financial asset resulting from the increase in the Galapagos share price between signing of the subscription agreement and December 31, 2015 resulted in a non-cash, fair value re-measurement of €30.6 million in the financial results. On December 31, 2015, the fair value of the financial asset was re-measured based on the implied value of the Galapagos share at the end of January 2016, the implied volatility of the €/$ currency exchange rates and applicable discount rates.
On January 19, 2016, the transaction was officially completed materialized by the share subscription of Gilead Biopharmaceutics Ireland Unlimited Company, of 6,760,701 new ordinary shares of Galapagos NV at a price of €58.00 per share including share premium, amounting to $425 million converted to €392,120,658 at a €/$ exchange rate of 1.0839.
The increase in the fair value of the financial asset resulting from the decrease in the Galapagos share price between January 1, 2016 and January 19, 2016 resulted in a positive non-cash gain of €57.5 million in the financial result of 2016.
On January 19, 2016, the value of the financial asset at maturity amounted to €65.9 million, reflecting the share premium that Gilead paid above our closing share price on the day of the capital increase. This amount was composed of (1) the initial measurement on the day of entering into the share subscription agreement for an amount of €39 million which was reported in deferred income and (2) the subsequent re-measurements of the financial asset, reported as financial result under IAS 39: €30.6 million fair value loss reported in the year 2015 and €57.5 million fair value gain reported in the first quarter of 2016, together a net fair value gain of €26.8 million. This financial asset expired on the effective date of the share subscription agreement and was derecognized through the share premium account.
Available-for-sale financial assets
On July 15, 2016, we invested €2.75 million in Pharnext, a French advanced clinical stage biopharmaceutical company developing new therapeutics for severe orphan and common neurological diseases, listed on Euronext. Galapagos has no restrictions on the sale of this equity investment and the asset is not pledged under any Galapagos’ liabilities. This investment is classified as available-for-sale equity investment which qualifies for level 1 fair value measurement based upon the closing price of the PXT securities on Euronext at each reporting date.
The market price of the Pharnext shares might face fluctuations and might be affected by a variety of factors, such as the global economic situation, the business development of competitors, sector mergers and acquisitions; it is difficult to mitigate this risk.
Liquidity risk
Our consolidated balance sheet shows an amount of €112.3 million as incurred losses at the end of 2016. Management forecasts our liquidity requirements to ensure that there is sufficient cash to meet operational needs. We have no credit lines. Such forecasting is based on realistic assumptions with regards to milestone and upfront payments to be received, taking into account our past track record, including the assumption that not all new projects that are being planned will be realized.
F-68
Table of Contents
Credit risk
The term “credit risk” refers to the risk that counterparty will default on its contractual obligations resulting in financial loss.
The trade receivables consist of a limited amount of creditworthy customers, many of which are large pharmaceutical companies, spread over different geographical areas. To limit the risk of financial losses, a policy of only dealing with creditworthy counterparties has been developed.
We grant credit to our clients in the framework of our normal business activities. Usually, we require no pledge or other collateral to cover the amounts due. Management continuously evaluates the client portfolio for creditworthiness. All receivables are considered collectable, except for these for which a provision for doubtful debtors has been established.
Aging balance of receivables that are due, but that are still considered collectable:
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| 60–90 days |
€ | 170 | € | 86 | € | 17 | ||||||
| 90–120 days |
— | — | — | |||||||||
| more than 120 days |
€ | 54 | € | 17 | € | 45 | ||||||
Our cash and cash equivalents are invested primarily in saving and deposit accounts. Saving and deposit accounts generate a small amount of interest income. For banks and financial institutions, only independently rated parties with a minimum rating of ‘A’ are accepted at the beginning of the term.
Interest rate risk
The only variable interest-bearing financial asset is cash and cash equivalents.
Changes in interest rates may cause variations in interest income and expenses resulting from short term interest-bearing assets.
Management does not expect the short term interest rates to decrease significantly in the immediate foreseeable future, which limits the interest exposure on our cash and cash equivalents.
Effect of interest rate fluctuation
A 100 basis point increase in interest rates at balance sheet date would have increased profit and loss by approximately €10 million (2015: €3 million; 2014: €2 million); a 100 basis point decrease in interest rates would have decreased profit and loss by approximately €10 million (2015: €3 million; 2014 : €2 million).
Foreign exchange risk
We are exposed to foreign exchange risk arising from various currency exposures. Our functional currency is euro, but we receive payments from our main collaboration partners AbbVie and Gilead in U.S. dollar and acquire some consumables and materials in U.S. dollars, Swiss Francs, GB Pounds and Croatian Kuna.
To limit this risk, we attempt to align incoming and outgoing cash flows in currencies other than EUR. In addition, contracts closed by our different entities are mainly in the functional currencies of that entity, except for the alliance agreements signed with AbbVie and Gilead for which payments are denominated in U.S. dollars.
F-69
Table of Contents
In order to further reduce this risk, a netting system was implemented in the course of 2012, which restrains intra-group payments between entities with a different functional currency. The exchange rate risk in case of a 10% change in the exchange rate amounts to:
| Year ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Euro, in thousands) | ||||||||||||
| Net book value: |
||||||||||||
| Increase in Euros—U.S. Dollars |
€ | (16,863 | ) | € | 506 | € | 589 | |||||
| Increase in Euros—GB Pounds |
130 | 164 | 138 | |||||||||
| Increase in Euros—CH Francs |
165 | 169 | 181 | |||||||||
| Increase in Euros—HR Kunas |
(95 | ) | (50 | ) | 215 | |||||||
| Increase in U.S. Dollars—GB Pounds |
€ | (913 | ) | € | (907 | ) | € | (807 | ) | |||
The exchange rate risk on the U.S. dollar is primarily related to our cash and cash equivalents held in U.S dollars.
Capital risk factors
We manage our capital to safeguard that we will be able to continue as a going concern. At the same time, we want to ensure the return to our shareholders through the results from our research and development activities.
Our capital structure consists of cash at bank and in hand and cash equivalents, financial debt (which currently we barely have: as of December 31, 2016, we had no financial debt other than finance leases and advances from Oseo, a French public organization for innovation support, for €0.1 million), and equity attributed to the holders of our equity instruments, such as capital, reserves and results carried forward, as mentioned in the consolidated statement of changes in equity.
We manage our capital structure and make the necessary adjustments in the light of changes of economic circumstances, the risk characteristics of underlying assets and the projected cash needs of the current research and development activities.
The adequacy of the capital structure will depend on many factors, including scientific progress in the research and development programs, the magnitude of those programs, the commitments to existing and new clinical CROs, the ability to establish new alliance or collaboration agreements, the capital expenditures, market developments and any future acquisition.
Neither Galapagos NV nor any of its subsidiaries are subject to any externally imposed capital requirements, other than those imposed by generally applicable company law requirements.
36. Auditor’s remuneration
The statutory auditor’s fees for carrying out his mandate at group level amounted to €475.0 thousand in 2016 (2015: €235.0 thousand). The fees for audit-related services executed by the statutory auditor, in particular other assurance engagements primarily related to the performance of the audit or review of the company’s financial statements, amounted to €186.0 thousand in 2016 (2015: €538.4 thousand), of which €6.2 thousand related to legal assignments (2015: €33.0 thousand). Fees for persons related to the statutory auditor for carrying out an auditor’s mandate at group level amounted to €40.0 thousand in 2016 (2015: €45.0 thousand). The audit committee and the board of directors are of the opinion that these non-audit services do not affect the independence of the statutory auditor in the performance of his audit. The abovementioned additional fees were fully approved by the audit committee in accordance with article 133 §6 of the Belgian Companies Code.
F-70
Table of Contents
37. Events after balance sheet date
On January 17, 2017, we announced the appointment of Dr. Walid Abi-Saab as Chief Medical Officer and member of the executive committee, beginning on March 1, 2017.
On January 20, 2017, the board of directors conditionally issued 150,000 warrants within the framework of the authorized capital, for the benefit of Dr. Abi-Saab (“Warrant Plan 2016 (B)”). The issuance of the warrants is subject to acceptance by Dr. Abi-Saab. These warrants have a term of eight years and an exercise price of €62.50.
On February 1, 2017, we announced the dosing of the first patient with CF Class III (F508del and a gating mutation like G551D) with our novel CF corrector GLPG2222 as an add-on to Kalydeco® in a Phase 2a study. We also announced the opening of an Investigational New Drug file with the U.S. Food & Drug Administration for GLPG2222, which triggered a $10 million milestone payment from AbbVie to Galapagos.
On March 10, 2017, we announced the initiation of two additional Phase 2 studies with filgotinib: one in small bowel Crohn’s disease, and one in fistulizing Crohn’s disease.
On March 22, 2017, we announced the initiation of a Phase 1 trial with GLPG3067, triggering a $7.5 million milestone payment from our collaboration partner AbbVie.
F-71
Table of Contents
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| GALAPAGOS NV | ||
| /s/ Onno van de Stolpe | ||
| By: |
Onno van de Stolpe | |
| Title: |
Chief Executive Officer (Principal Executive Officer) | |
Date: March 23, 2017
Table of Contents
| Incorporated by Reference | ||||||||||||||||
| Exhibit |
Description |
Schedule/ Form |
File Number | Exhibit | File Date (mm/dd/yyyy) |
|||||||||||
| 1.1 | Articles of Association (English translation), as amended | Form S-8 | 333-215783 | 4.1 | 01/27/2017 | |||||||||||
| 2.1 | Form of Deposit Agreement | Form F-1/A | 333-203435 | 4.1 | 04/30/2015 | |||||||||||
| 2.2 | Form of American Depositary Receipt (included in Exhibit 2.1) | Form F-1/A | 333-203435 | 4.2 | 04/30/2015 | |||||||||||
| 4.1 | Lease dated June 30, 1999 between the registrant and Innotech N.V., as amended (English translation) | Form F-1 | 333-203435 | 10.1 | 04/15/2015 | |||||||||||
| 4.2† | Warrant Plans (English translation) | Form F-1/A | 333-203435 | 10.3 | 05/11/2015 | |||||||||||
| 4.3** | Collaboration Agreement dated February 28, 2012 between the registrant and Abbot Hospitals Limited, as amended | Form F-1/A | 333-203435 | 10.4 | 05/05/2015 | |||||||||||
| 4.4** | Collaboration Agreement dated September 23, 2013 between the registrant and AbbVie S.à.r.l. | Form F-1/A | 333-203435 | 10.5 | 05/05/2015 | |||||||||||
| 4.5† | Employment and Management Agreements between Onno van de Stolpe and the registrant and its affiliates (English translation) | Form F-1 | 333-203435 | 10.6 | 04/15/2015 | |||||||||||
| 4.6## | Sale & Purchase Agreement dated March 13, 2014 between the registrant and Charles River Laboratories Holding Limited, as amended | Form F-1 | 333-203435 | 10.7 | 04/15/2015 | |||||||||||
| 4.7† | Warrant Plan 2015 (B) (English translation) | Form S-8 | 333-208697 | 99.1 | 12/22/2015 | |||||||||||
| 4.8** | License and Collaboration Agreement dated December 16, 2015 by and between the registrant and Gilead Biopharmaceutics Ireland Unlimited Company | Form 6-K | 001-37384 | 10.1 | 01/19/2016 | |||||||||||
| 4.9** | Amended and Restated Collaboration Agreement dated April 28, 2016 by and between the registrant and AbbVie S.à.r.l. | Form 6-K | 001-37384 | 10.1 | 06/01/2016 | |||||||||||
| 4.10† | Warrant Plan 2016 (English translation) | Form S-8 | 333-211834 | 99.1 | 06/03/2016 | |||||||||||
| 4.11† | Warrant Plan 2016 (B) (English translation) | Form S-8 | 333-215783 | 99.1 | 01/27/2017 | |||||||||||
| 4.12†# | Warrants Plans 2015 RMV and 2016 RMV (English translation) | |||||||||||||||
| 4.13# | Lease Addendum dated April 28, 2016 between the registrant and Intervest Offices & Warehouses NV (English translation) | |||||||||||||||
| 8.1# | List of subsidiaries of the registrant | |||||||||||||||
| 12.1# | Certification by the Principal Executive Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |||||||||||||||
| 12.2# | Certification by the Principal Financial Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |||||||||||||||
Table of Contents
| Incorporated by Reference | ||||||||||||||||
| Exhibit |
Description |
Schedule/ Form |
File Number | Exhibit | File Date (mm/dd/yyyy) |
|||||||||||
| 13.1* | Certification by the Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |||||||||||||||
| 13.2* | Certification by the Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |||||||||||||||
| 15.1# | Consent of Deloitte Bedrijfsrevisoren BV o.v.v.e. CVBA | |||||||||||||||
| # | Filed herewith. |
| * | Furnished herewith. |
| † | Indicates a management contract or any compensatory plan, contract or arrangement. |
| ## | Certain exhibits and schedules to these agreements were omitted from the registration statement pursuant to Item 601(b)(2) of Regulation S-K. The registrant will furnish copies of any of the exhibits and schedules to the U.S. Securities and Exchange Commission upon request. |
| ** | Confidential treatment status has been granted as to certain portions thereto, which portions are omitted and filed separately with the U.S. Securities and Exchange Commission. |