UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
FORM 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2019
OR
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____to_____
Commission File Number: 000-54554
Therapeutic Solutions International, Inc.
(Exact name of registrant as specified in its charter)
Nevada |
| 45-1226465 |
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer Identification No.) |
4093 Oceanside Boulevard, Suite B |
Oceanside, California 92056 |
(Address of principal executive offices, including zip code) |
|
(760) 295-7208 |
(Registrant's telephone number, including area code) |
|
Securities registered pursuant to Section 12(b) of the Act: |
None |
|
Securities registered pursuant to Section 12(g) of the Act: |
|
Title of class |
Common Stock, $0.001 par value per share |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes [ ] No [X]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [ ] No [X]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [ ] No [X]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [X]
1
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
[ ] | Large accelerated filer | [ ] | Accelerated filer |
[X] | Non-accelerated filer | [X] | Smaller reporting company |
[ ] | Emerging growth company |
|
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes [ ] No [X]
The aggregate market value of the voting and non-voting common equity held by non-affiliates was $1,192,453 based on a closing price of $0.015 as of June 30, 2019.
As of May 14, 2020, 1,656,544,305 shares of the registrartant’s common stock, par value of $0.0001 per shares, were outstanding.
2
INDEX
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
|
| PAGE NO |
PART I |
|
|
|
|
|
ITEM 1 | BUSINESS | 6 |
ITEM 1A | RISK FACTORS | 15 |
ITEM 1B | UNRESOLVED STAFF COMMENTS | 19 |
ITEM 2 | PROPERTIES | 19 |
ITEM 3 | LEGAL PROCEEDINGS | 19 |
ITEM 4 | MINE SAFETY DISCLOSURES | 20 |
|
|
|
PART II |
|
|
|
|
|
ITEM 5 | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | 20 |
ITEM 6 | SELECTED FINANCIAL DATA | 21 |
ITEM 7 | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 21 |
ITEM 7A | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 23 |
ITEM 8 | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 23 |
ITEM 9 | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 23 |
ITEM 9A | CONTROLS AND PROCEDURES | 23 |
ITEM 9B | OTHER INFORMATION | 24 |
|
|
|
PART III |
|
|
|
|
|
ITEM 10 | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | 24 |
ITEM 11 | EXECUTIVE COMPENSATION | 28 |
ITEM 12 | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 28 |
ITEM 13 | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 29 |
ITEM 14 | PRINCIPAL ACCOUNTANT FEES AND SERVICES | 29 |
|
|
|
PART IV |
|
|
|
|
|
ITEM 15 | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES | 29 |
|
|
|
SIGNATURES | 30 | |
3
PART I.
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of federal securities laws, which are subject to a number of risks and uncertainties. All statements that are not historical facts are forward-looking statements, including statements about our business strategy, uncertainty regarding our future operating results and our profitability, anticipated sources of funds and all plans, objectives, expectations and intentions and any statements regarding future potential revenue, gross margins and our prospects for 2020 and thereafter. These statements may appear in a number of places and can be identified by the use of forward-looking terminology such as "may," "will," "should," "expect," "plan," "anticipate," "believe," "estimate," "predict," "future," "intend," or "certain" or the negative of these terms or other variations or comparable terminology, or by discussions of strategy.
The following factors are among those that may cause actual results to differ materially from our forward-looking statements:
Limited operating history in our new business model;
Limited experience introducing new products;
Our ability to successfully expand our operations and manage our future growth;
Difficulty in managing our growth and expansion;
Dilutive effects of any raising of additional capital;
The deterioration of global economic conditions and the decline of consumer confidence and spending;
Material weaknesses reported in our internal control over financial reporting;
Our ability to protect intellectual property rights and the value of our products;
The potential for product liability claims against us;
Our dependence on third party manufacturers to manufacture our products;
Our common stock is currently classified as a penny stock;
Our stock price may experience future volatility;
The illiquidity of our common stock; and
Substantial sales of shares of our common stock.
Actual results may vary materially from those in such forward-looking statements as a result of various factors, including those identified in "Item 1A. Risk Factors" and elsewhere in this document. No assurance can be given that the risk factors described in this Annual Report on Form 10-K are all of the factors that could cause actual results to vary materially from the forward-looking statements. Forward-looking statements are not guarantees of future performance. They involve risks, uncertainties, and assumptions. The Company's future results and shareholder values may differ materially from those expressed in these forward-looking statements. Readers are cautioned not to put undue reliance on any forward-looking statements. Forward-looking statements also include statements in which words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “estimate,” “consider,” or similar expressions are used. References in this Annual Report on Form 10-K to the “Company,” “TSOI,” “we,” “our,” and “us” refer to Therapeutic Solutions International, Inc.
4
Corporate History
Therapeutic Solutions International, Inc. (“TSI” or the “Company”) was organized August 6, 2007 under the name Friendly Auto Dealers, Inc., under the laws of the State of Nevada. In the first quarter of 2011, the Company changed its name from Friendly Auto Dealers, Inc. to Therapeutic Solutions International, Inc., and acquired Splint Decisions, Inc., a California corporation.
Currently, the Company is focused on immune modulation for the treatment of several specific diseases. Immune modulation refers to the ability to upregulate (make more active) or downregulate (make less active) one’s immune system.
Activating one’s immune system is now an accepted method to cure certain cancers, reduce recovery time from viral or bacterial infections and to prevent illness. Additionally, inhibiting one’s immune system is vital for reducing inflammation, autoimmune disorders and allergic reactions.
TSI is developing a range of immune-modulatory agents to target certain cancers, improve maternal and fetal health, fight periodontal disease, and for daily health.
Nutraceutical Division – TSI has been producing high quality nutraceuticals. Its current flagship product, NanoStilbene™ PKE, is prepared by low-energy emulsification which allows for better solubility, stability, and the release performance of pterostilbene nanoparticles. The pterostilbene placed in a nanoemulsion droplet is free from air, light, and hard environment; therefore, as a delivery system, nanoemulsion’s can improve the bioavailability of pterostilbene but also protect it from oxidation and hydrolysis, while it possesses an ability of sustained release at the same time.
Emvolio, Inc., – was a wholly-owned subsidiary of TSI, incorporated in the State of Delaware on October 3, 2016, for the purpose of filing an Investigation New Drug application with the FDA for our StemVacs immunotherapy vaccine. In May of 2018, President Donald J. Trump signed into the law, the Right To Try bill. Because of this change, Emvolio has decided to withdraw the IND for a Phase 1 trial in the USA because TSI had previously completed a 10 patient trial in Mexico. TSI has since generated GCP documentation for the previously treated 10 patients into a Phase I trial, which will be presented to the FDA by TSI as part of an Ex-US trial compliant with 21 CFR 312.120 Foreign clinical studies not conducted under an IND. Therefore, we have dissolved Emvolio, Inc. as it is no longer needed.
SandBox Dental Labs, Inc. – is a wholly-owned subsidiary of TSI consisting of a future dental laboratory to manufacture and fill prescriptions from dentists who will use our proprietary Sleep Appliance to treat their patients with mild to moderate obstructive sleep apnea. The Company needs to seek regulatory approval for its device to treat sleep apnea. As of December 31, 2019 and April 1, 2020, formal operations have not commenced.
Management does not expect existing cash as of December 31, 2019 or as of March 31, 2020 to be sufficient to fund the Company’s operations for at least twelve months from the issuance date of these December 31, 2019 financial statements. These financial statements have been prepared on a going concern basis which assumes the Company will continue to realize its assets and discharge its liabilities in the normal course of business. As of December 31, 2019, the Company has incurred losses totaling $8.8 million since inception, has not yet generated material revenue from operations, and will require additional funds to maintain its operations. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern within one year after the consolidated financial statements are issued. The Company’s ability to continue as a going concern is dependent upon its ability to generate future profitable operations and obtain the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they become due. The Company intends to finance operating costs over the next twelve months through its existing financial resources and we may also raise additional capital through equity offerings, debt financings, collaborations and/or licensing arrangements. If adequate funds are not available on acceptable terms, we may be required to delay, reduce the scope of, or curtail, our operations. The accompanying consolidated financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
CURRENT BUSINESS DESCRIPTION
Currently, the Company is focused on immune modulation for the treatment of several specific diseases. Immune modulation refers to the ability to upregulate (make more active) or downregulate (make less active) one’s immune system.
Activating one’s immune system is now an accepted method to treat certain cancers, reduce recovery time from viral or bacterial infections and to prevent illness. Additionally, inhibiting one’s immune system is vital for reducing inflammation, autoimmune disorders and allergic reactions.
5
TSI is developing a range of immune-modulatory agents to target certain cancers, improve maternal and fetal health, fight periodontal disease, and for daily health.
Nutraceutical Division – TSI has been producing high quality nutraceuticals. Its current flagship product, NanoStilbene™ PKE, is prepared by low-energy emulsification which allows for better solubility, stability, and the release performance of pterostilbene nanoparticles. The pterostilbene placed in a nanoemulsion droplet is free from air, light, and hard environment; therefore, as a delivery system, nanoemulsion’s can improve the bioavailability of pterostilbene but also protect it from oxidation and hydrolysis, while it possesses an ability of sustained release at the same time.
Cellular Division – TSI recently obtained exclusive rights to a patented adult stem cell for development of therapeutics in the area of chronic traumatic encephalopathy (CTE) and traumatic brain injury (TBI).
The stem cell licensed, termed “JadiCell” is unique in that it possesses features of mesenchymal stem cells, however, outperforms these cells in terms of a) enhanced growth factor production; b) augmented ability to secrete exosomes; and c) superior angiogenic and neurogenic ability.
Chronic Traumatic Encephalopathy (CTE) is caused by repetitive concussive/sub-concussive hits to the head sustained over a period of years and is often found in football players. The condition is characterized by memory loss, impulsive/erratic behavior, impaired judgment, aggression, depression, and dementia. In many patients with CTE, it is anatomically characterized by brain atrophy, reduced mass of frontal and temporal cortices, and medial temporal lobe. TSOI has previously filed several patents in the area of CTE based on modulating the brain microenvironment to enhance receptivity of regenerative cells such as stem cells.
Nutraceutical Division (TSOI)
ProJuvenol® is a patented, (US No.: 9,682,047) and powerful synergistic blend of complex anti-aging ingredients in capsules.
NanoStilbene is an easily absorbed nanoemulsion of nanoparticle pterostilbene derived from the ‘047 patent.
DermalStilbene is a topical form of pterostilbene delivered via spray application onto skin, derived from the ‘047 patent.
IsoStilbene an injectable formulation of pterostilbene is available by prescription only, derived from the ‘047 patent.
NeuroStilbene is an intranasal form of pterostilbene delivered via spray application inside the nostril, derived from the ‘047 patent.
NanoPlus is a blend of NanoStilbene and Nano Cannabidiol which are an easily absorbed Nanoparticles formulation of Pterostilbene and Cannabidiol.
Nano Cannabidiol is an easily absorbed Nanoparticle formulation of Cannabidiol Isolate in the range of 75-90 nanometers. This product is built on the same nano platform as NanoStilbene and is delivered at a concentration of 200mg per milliliter.
NanoPSA is a blend of NanoStilbene™ and Broccoli Sprout Extract (BSE) providing 74mg of BSE and 125mg of our patented NanoStilbene, a proprietary formulation of nanoparticle pterostilbene.
Nutraceutical Patents:
TSOI filed a patent in July 2015 covering the use of its ProJuvenol® product, as well as various pterostilbene compositions, for use in augmenting efficacy of existing immuno-oncology drugs that are currently on the market. The patent is based on the ability of pterostilbene, one of the major ingredients of ProJuvenol®, to reduce oxidative stress produced by cancer cells, which in turn protects the immune system from cancer mediated immune suppression. That patent, U.S. No.: 9,682,047 was granted on 6-20-2017.
In addition, on April 28, 2016 the Company filed a patent application covering the use of ProJuvenol© and its active ingredient pterostilbene for augmentation of stem cell activity. Diseases such as diabetes, cardiovascular disease, and neurodegenerative diseases are characterized by deficient stem cell activity. The patent covers the stimulation of stem cells that already exist in the patient’s body, as well as stem cells that are administered therapeutically.
Studies have shown that patients who have higher levels of endogenous stem cell activity have reduced cardiovascular disease risk and undergo accelerated neurological recovery after stroke as compared to patients with lower numbers of such stem cells.
6
On October 16, 2017, the Company filed a patent application titled "Synergistic Inhibition of Glioma Using Pterostilbene and Analogues Thereof" which was developed to utilize the ability of the immune system to augment the possibility of increasing overall survival of glioma patients after treatment with conventional therapies. Our data suggests that when pterostilbene is combined with brain cancer therapeutics such as Gefitinib, Sertraline, or Temozolomide, the prognosis is vastly improved.
On August 13, 2018, the Company filed a patent application titled “Enhancement of Ozone Therapy using Pterostilbene” showing pterostilbene potently augments killing of breast cancer, prostate cancer, and ovarian cancer cells by ozone therapy. The data obtained is an extension of ongoing work at the Company seeking to identify means of enhancing the effects of pterostilbene administration for treatment of a variety of cancers, as well as enhancing the efficacy of existing cancer therapies.
On September 17, 2018, the Company filed a patent application titled “Pterostilbene and Compositions Thereof for Prevention and Treatment of Chronic Traumatic Encephalopathy” with new data demonstrating the ability of its NeuroStilbene intranasal formulation of pterostilbene to successfully prevent the development of brain injury in an animal model of Chronic Traumatic Encephalopathy aka CTE.
On September 25, 2018, the Company filed a patent application titled “Pterostilbene and Formulations Thereof for Treatment of Pathological Immune Activation” covering novel clinical data using its NanoStilbene™ formulation to reduce inflammatory cytokine production in cancer patients.
On September 9, 2019, the Company filed a patent application titled “Pterostilbene and Formulations Thereof for Protection of Hematopoiesis from Chemotherapy and Radiation” covering the ability of NanoStilbene™ and its active ingredient, pterostilbene, at accelerating recovery of blood cells after treatment with chemotherapy.
On November 4, 2019, the Company filed a patent application titled "Cellular, Organ, and Whole-Body Rejuvenation Utilizing Cord Blood Plasma and Pterostilbene" suggesting that pterostilbene, the active ingredient in its commercially available NanoStilbene™ product, augments the ability of cord blood plasma to suppress biological properties associated with aging.
On May 15, 2018, TSI announced Institutional Review Board (IRB) clearance to initiate a pilot pharmacokinetic trial of “NanoStilbene.” Then on July 02, 2018 the Company announced receiving pilot clinical data providing proof of concept that NanoStilbene more effectively increases blood levels of the molecule as compared to conventional formulations. The clinical trial involved the administration of NanoStilbene in comparison to powder in capsule form pterostilbene with healthy volunteers, whom underwent a series of blood draws to determine the concentration of the compound.
Cellular, Bioligical, and Pharmaceutical Patents:
On March 29, 2017, the Company filed a patent application titled “Stimulation of Immunity to Tumor Stem Cell Specific Proteins by Peptide Immunization”.
On March 29, 2017, the Company filed a patent application titled “Activated Leukocyte Extract for Repair of Innate Immunity in Cancer Patients”.
On March 29, 2017, the Company filed a patent application titled “Augmentation of Anti-Tumor Immunity by Mifepristone and Analogues Thereof”.
On March 29, 2017, the Company filed a patent application titled “Methods of Re-Activating Dormant Memory Cells with Anticancer Activity”.
On December 5, 2018, the Company filed a patent application titled “Treatment of Chronic Traumatic Encephalopathy via RNA Administration”.
On January 9, 2019, the Company filed a patent application titled “Autologous Neurogenic Cells and Uses Thereof for Professional Athletes at Risk of Chronic Traumatic Encephalopathy”.
On January 9, 2019, the Company filed a patent application titled “Prevention and Reversion of Chronic Traumatic Encephalopathy through Administration of “Educated” Monocytes and Progenitors Thereof”.
7
Nanotechnology
Therapeutic uses of nanotechnology typically involve the delivery of small-molecule drugs, peptides, proteins, and nucleic acids. Nanoparticles have advanced pharmacological effects compared with the therapeutic entities they contain. Active intracellular delivery and improved pharmacokinetics and pharmacodynamics of drug nanoparticles depend on various factors, including their size and surface properties.
Nanoparticle therapeutics is an emerging treatment modality in cancer and other inflammatory disorders. The National Cancer Institute has recognized nanotechnology as an emerging field with the potential to revolutionize modern medicine for detection, treatment, and prevention of cancer.
On May 15, 2018, TSI announced Institutional Review Board (IRB) clearance to initiate a pilot pharmacokinetic trial of “NanoStilbene.” Then on July 2, 2018 the Company announced receiving pilot clinical data providing proof of concept that NanoStilbene more effectively increases blood levels of the molecule as compared to conventional formulations. The clinical trial involved the administration of NanoStilbene in comparison to powder in capsule form pterostilbene with healthy volunteers, whom underwent a series of blood draws to determine the concentration of the compound.
NanoStilbene Administration Results in Superior Pharmacokinetic Profile
Compared to Pterostilbene Administration
Blood was collected in EDTA tubes and plasma collected subsequent to centrifugation at 700g for 10 minutes. Collection time points were prior to administration of test compound, as well as at times 2hr, 4hr, 6hr, 8hr, 10hr, and 12 hrs. Test compounds were 10 ml of NanoStilbene (provided by Therapeutic Solutions International) and 6 capsules of 50 mg pterostilbene (VitaMonk). A wash out period of 3 days was allowed between two test compound administration.
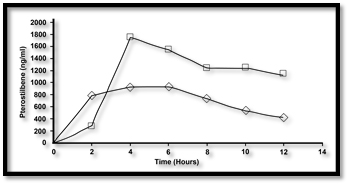
Results
The results were that at peak concentration NanoStilbene (Square) had a 55% increase in serum levels over the traditional powder (Triangle) form of pterostilbene. The data also shows the half-life to be double to that of the powder form.
The data in Granted U.S. Patent No.: 9,682,047 strongly suggest that pterostilbene administration may be an inexpensive and safe method of augmenting efficacy of numerous immunotherapeutic drugs. Although cancer immunotherapy has revolutionized the prognosis of many patients, the majority of patients still possess poor or suboptimal responses to this approach.
8
Clinical Trial of NanoStilbene for Immune Derepression in Advanced Cancer
12 patients with advanced cancer
ECOG >2 300mg NanoStilbene Oral daily (300mg PTER) Assessments pre and 1, 2, and 3 weeks after treatment
Inflammatory Markers Decrease TNF, IL-6, CRP
Immune Markers Increased IFN gamma from stimulated PBMC NK Cytolysis activity |
|
Pterostilbene, being a methyl ether of resveratrol, is known to possess anti-inflammatory and anticancer activity in various model systems. It is known that in advanced cancer, excess inflammatory signaling may be associated with reduction in CD3 zeta chain signaling and inhibited function of natural killer (NK) cells.
Given the importance of NK cells in the activity of various Immunotherapeutics, we sought to determine whether administration of a nanoparticle formulation of pterostilbene may reverse cancer associated suppression of NK activity. An initial study in heathy volunteers was performed to elucidate amount of NanoStilbene needed to be administered to achieve sufficient plasma concentration for induction of anti-inflammatory activity.
Subsequent to this, the selected NanoStilbene dose was administered to twelve patients with advanced solid cancers for 3 weeks. Daily treatment with 300mg of NanoStilbene caused reduction in serum levels of inflammatory markers TNF-alpha, IL-6, and CRP. Assessment of peripheral blood mononuclear cell ability to generate IFN-gamma subsequent to stimulation with anti-CD3 and anti-CD28 was increased.
Additionally, NK cytotoxicity was augmented. These results suggest that NanoStilbene may be a useful adjuvant to immunotherapy of cancer rescuing T cell and NK cell activities.
Augmentation of NK cell function may stimulate efficacy of approved therapies that depend on an active NK compartment such as Herceptin, Rituximab, and Cetuximab.
These results suggest that NanoStilbene may be a useful adjuvant to immunotherapy of cancer rescuing T cell and NK cell activities. Augmentation of NK cell function may stimulate efficacy of approved therapies that depend on an active NK compartment such as Herceptin, Rituximab, and Cetuximab.
*The data provided here is partial and does not contain all materials submitted for publication and is preliminary until peer review is complete. These statements have not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure, or prevent any disease.
Immune-Oncology – Right To Try
In May of 2018, President Donald J. Trump signed into the law, the Right To Try bill. In 2015/2016, TSI began and completed a 10 patient clinical trial of advanced cancer patients in Mexico at the Pan Am Cancer Treatment Center located in Tijuan Mexico using our dendritic cell vaccine code named StemVacs. TSI has since generated GCP documentation for the previously treated 10 patients into a Phase I trial, which will be presented to the FDA by TSI as part of an Ex-US trial compliant with 21 CFR 312.120 Foreign clinical studies not conducted under an IND. This is a required step to conform to the new Right To Try law.
StemVacs is an immunotherapy platform that consists of 5 components. The overarching approach to the StemVacs Immunotherapy Platform is as follows:
9
1.Treat innate immune suppression: Administration of oral apigenin/NanoStilbene (Cancer DeTox Product) to decrease immune suppressive toxic molecules made by tumor and tumor microenvironment.
2.Treat adaptive immune suppression: Administration of MemoryMune to activate dormant memory cells recognizing the tumor. Administration of LymphoBoost to repair deficient IL-12 production.
3.Stimulation of immune response to cancer stem cells (StemVacs).
4.Consolidation and maintenance of immunity: Cycles of StemVacs, supported by innaMune and LymphoBoost
StemVacs: StemVacs is a subcutaneously administered vaccine comprised of immune stimulatory peptides resembling cancer stem cell specific proteins.
Cancer Metabolic DeTox: This is an orally administered agent that is derived from various herbs termed apigenin. The unique property of apigenin is that it inhibits a cancer associated metabolic pathway that degrades the amino acid tryptophan. Specifically, apigenin inhibits the enzyme indolamine 2,3 deoxygenase (IDO), which is responsible for breaking down tryptophan in the vicinity of the tumor and generating by-products such as kynurenine. It is known that immune activation is dependent on tryptophan being present in the tumor environment. The depletion of tryptophan and generation of kynurenine by tumor cells and tumor associated cells is a major cause of immune suppression in cancer. By administering Cancer Metabolic DeTox, the innate arm of the immune system has a chance to regenerate. This positions the patient for better outcome after administration of specific immune stimulating vaccines.
MemoryMune: This is a product derived from a two-step culture process of donor blood cells. The product MemoryMune reawakens dormant immune memory cells. It is known that many cancer patients possess memory T cells that enter the tumor, however, once inside the tumor these cells are inactivated. MemoryMune contains a unique combination of growth factors specific for immune system cells called “cytokines”.
LymphoBoost: LymphoBoost is a proprietary formulation of Misoprostol, a drug approved for another indication, which we have shown to be capable of stimulating lymphocytes, particularly NK cells and T cells, both critical in maintaining anti-tumor immunity.
innaMune: This is a biological product derived from tissue culture of blood cells derived from healthy donors. It is a combination of cytokines that maintain activity of innate immune system cells, as well as having ability to shift M2 macrophages to M1.
Chronic Traumatic Encephalopathy (CTE), and Traumatic Brain Injury (TBI) – Right To Try
On December 10, 2018, Therapeutic Solutions International, Inc., announced the signing of an agreement between TSOI and Jadi Cell LLC for licensing of the Jadi Cell universal donor adult stem cell, as covered in US Patent No.: 9,803,176 B2 for use in Chronic Traumatic Encephalopathy (CTE), and Traumatic Brain Injury (TBI).
The Jadi Cell product, which belongs to the mesenchymal stem cell (MSC) family of cells, is a unique adult stem cell, which produces higher levels of therapeutic factors compared to other stem cells. The cells have demonstrated safety in animal models and pilot human trials. The Jadi Cell product is generated from umbilical cords, which are a source of medical waste and available in large quantities at inexpensive prices.
Chronic Traumatic Encephalopathy (CTE) is caused by repetitive concussive/sub-concussive hits to the head sustained over a period of years and is often found in football players. The condition is characterized by memory loss, impulsive/erratic behavior, impaired judgment, aggression, depression, and dementia. In many patients with CTE, it is anatomically characterized by brain atrophy, reduced mass of frontal and temporal cortices, and medial temporal lobe.
Traumatic brain injury (TBI) is an insult to the brain, not of a degenerative or congenital nature, but caused by external physical force that may produce a diminished or altered state of consciousness, which results in an impairment of cognitive abilities or physical functioning.
CTE represents a significant unmet medical need which we believe is amenable to stem cell intervention. We are eager to accelerate treatments and potential cures for debilitating conditions such as CTE and traumatic brain injury and plan to leverage New regulatory pathways such as the recently approved “Right to Try” Law to deliver these medicines as soon as possible to patients which currently have no other options.
The Jadi Cell product because of its advanced stage of development in contrast to other stem cell types, which require years, if not decades of development before entry into American patients, will allow us we believe to be treating patients within 12 months. Currently means of isolating, producing, scaling up, and delivery of the cells has all been worked out by Jadi Cell and Collaborators.
10
GOVERNMENT REGULATION
The Company’s business is subject to varying degrees of regulation by a number of government authorities in the United States, including the United States Food and Drug Administration (FDA), the Federal Trade Commission (FTC), and the Consumer Product Safety Commission. The Company will be subject to additional agencies and regulations if it enters the manufacturing business. Various agencies of the state and localities in which we operate and in which our products are sold also regulate our business, such as the California Department of Health Services, Food and Drug Branch. The areas of our business that these and other authorities regulate include, among others:
product claims and advertising;
product labels;
product ingredients; and
how we package, distribute, import, export, sell and store our products.
The FDA, in particular, regulates the formulation, manufacturing, packaging, storage, labeling, promotion, distribution and sale of vitamins and other nutritional supplements in the United States, while the FTC regulates marketing and advertising claims. The FDA issued a final rule called “Statements Made for Dietary Supplements Concerning the Effect of the Product on the Structure or Function of the Body,” which includes regulations requiring companies, their suppliers and manufacturers to meet Good Manufacturing Practices in the preparation, packaging, storage and shipment of their products. Management is committed to meeting or exceeding the standards set by the FDA.
The FDA has also issued regulations governing the labeling and marketing of dietary and nutritional supplement products. They include:
the identification of dietary or nutritional supplements and their nutrition and ingredient labeling;
requirements related to the wording used for claims about nutrients, health claims, and statements of nutritional support;
labeling requirements for dietary or nutritional supplements for which “high potency” and “antioxidant” claims are made;
notification procedures for statements on dietary and nutritional supplements; and
pre-market notification procedures for new dietary ingredients in nutritional supplements.
The Dietary Supplement Health and Education Act of 1994 (DSHEA) revised the existing provisions of the Federal Food, Drug and Cosmetic Act concerning the composition and labeling of dietary supplements and defined dietary supplements to include vitamins, minerals, herbs, amino acids and other dietary substances used to supplement diets. DSHEA generally provides a regulatory framework to help ensure safe, quality dietary supplements and the dissemination of accurate information about such products. The FDA is generally prohibited from regulating active ingredients in dietary supplements as drugs unless product claims, such as claims that a product may heal, mitigate, cure or prevent an illness, disease or malady, trigger drug status.
The Company is also subject to a variety of other regulations in the United States, including those relating to taxes, labor and employment, import and export, and intellectual property.
EMPLOYEES
As of December 31, 2019, we had two full-time employees, all non-union. We believe that our relations with our employees are good.
COMPETITION
The bio-technology, bio-pharma, and nutraceutical industries are subject to rapid technological change. Competition from domestic and foreign companies, large pharmaceutical companies and other interested businesses is intense and expected to increase. A number of companies with significantly greater resources are pursuing the development of pharmaceuticals, biologics, and nutraceuticals in our targeted areas.
ITEM 1A RISK FACTORS
As a smaller reporting company we are not required to provide a statement of risk factors. However, we believe this information may be valuable to our shareholders for this filing. We reserve the right to not provide risk factors in our future filings. Our primary risk factors and other considerations include:
This Annual Report on Form 10-K contains forward-looking statements concerning our future programs, expenses, revenue, liquidity and cash needs as well as our plans and strategies. These forward-looking statements are based on current expectations and we assume no obligation to update this information, except as required by applicable laws and regulations. Numerous factors could cause actual results to differ significantly from the results described in these forward-looking statements, including the following risk factors.
11
Internal Control
Our management has concluded that our internal control over financial reporting is not effective. Material weaknesses in our internal control over financial reporting could cause our financial reporting to be unreliable and could lead to misinformation being disseminated to the public.
Our management concluded that as of December 31, 2019, our internal control over financial reporting was not effective, and that material weaknesses existed in the following areas as of December 31, 2019:
(1)we do not employ full time in-house personnel with the technical knowledge to identify and address some of the reporting issues surrounding certain complex or non-routine transactions. With respect to material, complex and non-routine transactions, management has and will continue to seek guidance from third-party experts and/or consultants to gain a thorough understanding of these transactions;
(2)we have inadequate segregation of duties consistent with the control objectives including but not limited to the disbursement process, transaction or account changes, and the performance of account reconciliations and approval; and
(3)we have ineffective controls over the period end financial disclosure and reporting process caused by reliance on third-party experts and/or consultants and insufficient accounting staff.
Based on our current plan, we believe we will need additional capital to support our operations.
Based on our current business plan, we believe that our cash and cash equivalents at December 31, 2019 will not be sufficient to meet our anticipated cash requirements during the twelve-month period subsequent to the issuance of the financial statements included in this Annual Report on Form 10-K. Our current commercialization of products and clinical trial strategy will undergo continual prioritization and in the future we may adjust our commercialization efforts to preserve our existing cash. We need to raise additional capital to fund our operations. We may raise additional capital through equity offerings, debt financings, collaborations and/or licensing arrangements. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available, we may be required to delay, reduce the scope of, or curtail, our operations. To the extent that we raise additional funds by issuing equity securities, our shareholders will experience dilution, and debt financing or other preferred equity instruments, if available, may involve restrictive covenants.
There is substantial doubt about our ability to continue as a going concern.
Our recurring losses from operations raise substantial doubt about our ability to continue as a going concern, and as a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended December 31, 2019 with respect to this uncertainty. This going concern uncertainty, and any future going concern uncertainty, could materially limit our ability to raise additional capital. We have incurred significant losses since our inception, and it is possible we will never achieve future profitability. The Company is currently developing its new NanoStilbene product for commercialization. In addition, we are currently seeking regulatory clearance for our subsidiary SandBox’s product to treat obstructive sleep apnea in the United States. As a result, we continue to incur significant general and administrative expenses related to our operations. As a result, we have incurred substantial net losses to date. Our net losses for the years ended December 31, 2019 and 2018 were $1.7 million and $1.9 million, respectively. As of December 31, 2019, we had an accumulated deficit of $8.808 million. Meaningful revenues will likely not be available until, and unless, the Company can successfully launch our current TSI product line and our SandBox subsidiary’s product is cleared by the FDA and successfully commercialized. The perception that we may not be able to continue as a going concern may cause potential partners or investors to choose not to deal with us due to concerns about our ability to meet our contractual and financial obligations.
We may not be able to effectively manage our potential growth and the execution of our business plan.
Our potential growth and the execution of our business plan together are likely to place significant strain on our managerial, operational and financial resources. To effectively manage our potential growth and execute our business plan, we will need to, among other things:
retain additional personnel across several departments in the Company;
develop strong customer loyalty for new products in a crowded competitive marketplace;
continue to establish and continue to increase awareness of our brands;
12
price our products and services at points which will allow us to maximize sales while at the same time maximizing gross profit margins;
establish, maintain, expand and manage multiple relationships with various vendors, strategic partners, licensees and other third parties, including suppliers of the products we sell on our website and elsewhere, warehousing distributors, shipping companies and others;
rapidly respond to competitive developments, particularly when new high-demand products become available;
build an operations structure to support our business and provide efficient and effective customer service and support;
expand our IT infrastructure to respond to increasing customer traffic to our website, demand for content from site users and to manage growing e-commerce transactions;
establish and maintain effective financial and management controls, reporting systems and procedures;
control our expenses;
provide competitive employee salaries and benefit packages; and,
avoid lawsuits and other adverse claims.
There can be no assurance that we will be able to accomplish any or all of the above goals. If we prove unable to effectively execute our business plan or manage our growth, it is likely to have a material adverse effect on our business, financial condition, including liquidity and profitability, and our results of operations.
If our proposed product sales model does not successfully operate at a profit our growth strategy may be impeded.
To effectively expand and meet our growth objectives our products sales model must be executed upon in a profitable manner. Profitability is dependent upon a variety of factors, some beyond our control, including, but not limited to the amount of traffic we can consistently attract to our brand, to retail sales in “brick and mortar” retailers, to our website, and our ability to stock or otherwise make available products that our customers purchase, our ability to stock or otherwise make available the best new products as they enter the market, our ability to provide consistent and superior customer service, the general economic conditions, particularly in the U.S., that could impact the amount of money customers spend collectively on the products we sell, and/or that could reduce the amount of money our average customer spends, and/or could reduce the number or frequency of repeat orders for products, and/or could result in customers finding products in other venues if they can find those products for a lower price. Other factors that could impact our ability to execute on our business model in a profitable manner include, but are not limited to, competition in our markets, recruiting, training and retaining qualified personnel and management, maintenance of required local, state and federal governmental approvals and permits, costs associated with principal component products and supplies, delivery shortages or interruptions, consumer trends, our ability to finance operations externally, changes in supply or prices of the products we sell and disruptions or business failures among our product suppliers, distributors, warehouses or shippers. Any failure to operate in a profitable manner could hurt our ability to meet our growth objectives by attracting licensees, and our business, financial condition, including liquidity and profitability, and our results of operations would be negatively affected.
We face significant competition for our products.
The markets in which we operate are intensely competitive, continually evolving and, in some cases, subject to rapid change. Our competitors include:
traditional and well established companies with recognized and well patronized brands in the nutritional supplements and health products industry segment;
entrenched nutritional supplements and health products companies with well known customer on-line services and portals and other high-traffic web sites that provide sales access to healthcare and nutritional supplements and related products; and
companies that focus on providing on-line and/or off-line healthcare related content, including some that promote competitor brands.
13
Many of our competitors have greater financial, technical, product development, marketing and other resources than we do. These companies may be better known than we are and have more customers than we do. We cannot provide assurance that we will be able to compete successfully against these companies or any alliances they have formed or may form. If we are unable to compete with one or more of our competitors, our growth strategy may be impeded, which could negatively affect our business, financial condition, including liquidity and profitability, and our results of operations.
Government regulation could adversely affect our business.
Our products and their associated component ingredients are subject to existing and potential government regulation. Our failure, or the failure of our business partners or third party providers, to accurately anticipate the application of laws and regulations affecting our products and the manner in which we deliver them, or any failure to comply, could create liability for us, result in adverse publicity, or negatively affect our business. In addition, new laws and regulations, or new interpretations of existing laws and regulations, may be adopted with respect to consumer protection and other issues, including pricing, products liability, copyrights and patents, distribution and characteristics and quality of products and services. We cannot predict whether these laws or regulations will change or how such changes will affect our business. Any of this government regulation could impact our growth strategy, which could negatively affect our business, financial condition, including liquidity and profitability, and our results of operations.
Third parties may claim that we are infringing their intellectual property, and we could suffer significant litigation or licensing expense or be prevented from providing certain services, and which may otherwise harm our business.
We could be subject to claims that we are misappropriating or infringing intellectual property, trade secrets or other proprietary rights of others. These claims, even if not meritorious, could be expensive to defend and divert management’s attention from our operations. If we become liable to third parties for infringing these rights, we could be required to pay substantial damage awards and to develop non-infringing products, obtain a license or cease selling the products that use or contain the infringing intellectual property. We may be unable to develop non-infringing products or obtain a license on commercially reasonable terms, or at all. Any claims against our company for infringement could impede our growth strategy, which could negatively affect our business, financial condition, including liquidity and profitability, and our results of operations.
We may be subject to claims brought against us as a result of product associated content we provide.
Consumers are reasonably expected to access health-related information regarding our products through our on-line web site. If our content, or content we obtain from third parties, contains inaccuracies, it is possible that consumers or others may sue us for various causes of action. Although our planned web site contains terms and conditions, including disclaimers of liability, that are intended to reduce or eliminate our liability, the law governing the validity and enforceability of on-line agreements with consumers that provide the terms and conditions for use of our public or private portals are unenforceable. A finding by a court that these agreements are invalid and that we are subject to liability could harm our business and require costly changes to our business. We have planned editorial procedures in place to provide quality control of the information that we publish or provide. However, we cannot assure you that our editorial and other quality control procedures will be sufficient to ensure that there are no errors or omissions in particular content. Even if potential claims do not result in liability to us, the fact that we would need to investigate and defend against these claims could be expensive and time consuming and could divert management’s attention away from our operations. In addition, our business is in part based on establishing a reputation amongst consumers that our portals as trustworthy and dependable sources of healthcare information. Allegations of impropriety or inaccuracy, even if unfounded, could therefore harm our reputation and business, which could negatively affect our business, financial condition, including liquidity and profitability, and our results of operations.
Changes in commodity and other operating costs or supply chain and business disruptions could adversely affect our results of operations.
Changes in product costs are a part of our business; any increase in the prices that suppliers charge for their products could adversely affect our operating results. We remain susceptible to increases in prices as a result of factors beyond our control, such as general economic conditions, seasonal fluctuations, weather conditions, demand, safety concerns, product recalls, labor disputes and government regulations. We rely on third-party distribution companies to deliver ingredients to our manufacturers and ultimately our products to customers. Interruption of distribution services due to financial distress or other issues could adversely affect our operations.
14
We face substantial competition in attracting and retaining qualified senior management and key personnel and may be unable to develop and grow our business if we cannot attract and retain such senior management and key personnel.
As an early stage company, our ability to develop and grow our business, to a large extent, depends upon our ability to attract, hire and retain highly qualified and knowledgeable senior management and key personnel who possess the skills and experience necessary to satisfy our business needs. Our ability to attract and retain such senior management and key personnel will depend on numerous factors, including our ability to offer salaries, benefits and professional growth opportunities that are comparable with and competitive to those offered by more established companies operating in our marketplace. We may be required to invest significant time and resources in attracting and retaining additional senior management and key personnel as needed. Moreover, many of the companies with which we will compete for any such individuals have greater financial and other resources, affording them the ability to undertake more extensive and aggressive hiring campaigns, than we can. The normal running of our operations may be interrupted, and our financial condition and results of operations negatively affected, as a result of any inability on our part to attract or retain the services of qualified and experienced senior management and key personnel, or should our prospective key personnel refuse to serve, or, once appointed, leave prior to a suitable replacement being found.
COVID-19
On January 30, 2020, the World Health Organization declared the COVID-19 outbreak a “Public Health Emergency of International Concern” and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread of the COVID-19 include restrictions on travel, quarantines in certain areas, and forced closures for certain types of public places and businesses. COVID-19, and actions taken to mitigate it, have had and are expected to continue to have an adverse impact on the economies and financial markets of many countries, including the geographical area in which the Company operates. While it is unknown how long these conditions will last and what the complete financial effect will be to the Company, COVID-19 has had an adverse effect on our business, including our supply chains and distribution systems. While we are taking diligent steps to mitigate disruptions to our supply chain, we are unable to predict the extent or nature of these impacts, at this time, to our future financial condition and results of operations.
Risks Associated With Our Restricted Securities
Because there is currently a limited public trading market for our common stock, investor may not be able to resell stock.
Our stock is now traded in OTC Markets under the stock symbol TSOI, which results in a very illiquid and limited market for our common stock.
There is currently no liquid trading market for our common stock and we cannot ensure that one will ever develop or be sustained.
The trading market for our common stock is currently not liquid. We cannot predict how liquid the market for our common stock might become. Our common stock is quoted in OTC Markets under the symbol TSOI.
Our common stock may be deemed a “penny stock”, which would make it more difficult for investors to sell their shares.
Our common stock is subject to the “penny stock” rules adopted under the Exchange Act. The penny stock rules apply to companies whose common stock is not listed on the NASDAQ Stock Market or other national securities exchange and trades at less than $4.00 per share, other than companies that have had average revenue of at least $6,000,000 for the last three years or that have tangible net worth of at least $5,000,000 ($2,000,000 if the company has been operating for three or more years). These rules require, among other things, that brokers who trade penny stock to persons other than “established customers” complete certain documentation, make suitability inquiries of investors and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trade penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities and investors may find it more difficult to dispose of our securities.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
If our stockholders have the right to sell substantial amounts of common stock in the public market, e.g. upon the expiration of any statutory holding period under Rule 144, it could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make our ability to raise additional financing through the sale of equity or equity-related securities in the future, at a time and price that we deem reasonable or appropriate, more difficult.
15
The elimination of monetary liability against our directors and officers under the Company’s Articles of Incorporation and Nevada law, and the existence of indemnification rights to our directors, officers and employees, may result in substantial expenditures by the Company.
Article 6 of our Articles of Incorporation exculpates our directors and officers from certain monetary liabilities. Article 7 of our Articles of Incorporation provides that we shall indemnify all directors (and all persons serving at our request as a director or officer of another corporation) to the fullest extent permitted by Nevada law.
Further pursuant to Article 7, the expenses of the indemnified person incurred in defending a civil suit or proceeding must be paid by us as incurred and in advance of the final disposition of the action, suit, or proceeding under receipt of an undertaking by or on behalf of the indemnified person to repay the amount if it is ultimately determined by a court of competent jurisdiction that he or she is not entitled to be indemnified by us.
The foregoing indemnification obligations could result in us incurring substantial expenditures, which we may be unable to recoup. These provisions and resultant costs may also discourage us from bringing a lawsuit against directors and officers for breaches of their fiduciary duties even though such actions, if successful, might otherwise benefit us and our stockholders.
Public company compliance may make it more difficult to attract and retain officers and directors.
The Sarbanes-Oxley Act and related rules implemented by the SEC have required changes in corporate governance practices of public companies. As a public entity, these rules and regulations increase compliance costs and make certain activities more time consuming and costly. As a public entity, these rules and regulations also make it more difficult and expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve as directors or as executive officers.
We do not plan to pay any cash or stock dividends in the foreseeable future.
The payment of dividends upon our capital stock is solely within the discretion of our future board of directors and is dependent upon our financial condition, results of operations, capital requirements, restrictions contained in our future financing instruments and any other factors our board of directors may deem relevant. We have never declared or paid any cash or stock dividends on our capital stock and we currently anticipate that we will retain earnings, if any, to finance the development and expansion of our business and, as such, do not intend on paying any cash or stock dividends in the foreseeable future.
ITEM 1B UNRESOLVED STAFF COMMENTS
As a Smaller Reporting Company, we are not required to furnish information under this Item 1B.
ITEM 2 PROPERTIES.
We do not own any real-estate property or manufacturing equipment. Our business is conducted in approximately 1,300 square feet of rented offices and warehouse space located at 4093 Oceanside Blvd., Suite B, Oceanside, CA 92056.
ITEM 3 LEGAL PROCEEDINGS.
From time to time, claims are made against us in the ordinary course of business, which could result in litigation. Claims and associated litigation are subject to inherent uncertainties and unfavorable outcomes could occur, such as monetary damages, fines, penalties or injunctions prohibiting us from selling one or more products or engaging in other activities. The occurrence of an unfavorable outcome in any specific period could have a material adverse effect on our results of operations for that period or future periods.
However, as of the date of this report, management believes the outcome of currently identified potential claims and lawsuits will not have a material adverse effect on our financial condition or results of operations.
ITEM 4 MINE SAFETY DISCLOSURES.
16
ITEM 5 MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Our stock is now traded in OTC Markets under the stock symbol TSOI, which results in a very illiquid and limited market for our common stock. As of the date of Annual Report on Form 10-K there are approximately 181 stockholders of record of our common stock.
The following table sets forth the quarterly high and low closing sales prices for our common stock from January 1, 2018 through December 31, 2019.
Quarter Ended | High | Low |
December 31, 2019 | $0.0035 | $0.0031 |
September 30, 2019 | $0.0018 | $0.0015 |
June 30, 2019 | $0.0015 | $0.0014 |
March 31, 2019 | $0.0045 | $0.004 |
December 31, 2018 | $0.017 | $0.003 |
September 30, 2018 | $0.032 | $0.008 |
June 30, 2018 | $0.025 | $0.004 |
March 31, 2018 | $0.009 | $0.005 |
Dividends
We did not declare or pay dividends during 2018 to 2019.
Issuances of Unregistered Securities
On January 26, 2018, we issued 2,424,242 shares of common stock for the partial conversion of $8,000 for convertible note dated July 24, 2017.
On February 1, 2018, we issued 6,376,471 shares of common stock for the conversion of the balance of $20,000 for convertible note dated July 24, 2017.
On February 1, 2018, we issued 5,000,000 shares of common stock, valued at $0.009 per share, for consulting services.
On February 1, 2018, we issued 2,500,000 shares of common stock, valued at $0.009 per share, for consulting services.
On February 20, 2018, we issued 15,000,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement to a related party.
On February 20, 2018, we issued 2,500,000 shares of common stock, valued at $0.0062 per share, for consulting services.
On April 14, 2018, we issued 2,500,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement to a related party.
On April 14, 2018, we issued 5,000,000 shares of common stock, valued at $0.0057 per share, for consulting services.
On April 27, 2018, we issued 3,225,806 shares of common stock for the partial conversion of $8,000 for convertible note dated September 7, 2017.
On May 1, 2018, we issued 4,137,931 shares of common stock for the partial conversion of $12,000 for convertible note dated September 7, 2017.
On May 2, 2018, we issued 25,000,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement to a related party.
On May 21, 2018, we issued 2,742,857 shares of common stock for the partial conversion of $6,000 for convertible note dated September 7, 2017.
17
On June 15, 2018, we issued 8,500,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement.
On July 3, 2018, we issued 5,000,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement.
On July 9, 2018, we issued 4,166,667 shares of common stock for the partial conversion of $15,000 for convertible note dated January 3, 2018.
On July 12, 2018, we issued 4,077,778 shares of common stock for the partial conversion of $13,000 for convertible note dated January 3, 2018.
On July 19, 2018, we issued 2,500,000 shares of common stock, valued at $0.015 per share, to a Scientific Advisory Board member for consulting services.
On August 7, 2018, we issued 11,000,000 shares of common stock at $0.011 per share, for consulting services.
On September 5, 2018, we issued 3,260,870 shares of common stock for the partial conversion of $15,000 for convertible note dated February 27, 2018.
On September 10, 2018, we issued 3,262,222 shares of common stock for the partial conversion of $13,000 for convertible note dated February 27, 2018.
On September 19, 2018, we issued 5,000,000 shares of common stock, valued at $0.005 per share, for an investment in the Company’s Private Placement.
On September 19, 2018, we issued 1,500,000 shares of common stock, valued at $0.01 per share, to a Scientific Advisory Board member for consulting services.
On October 25, 2018, we issued 15,000,000 shares of common stock, valued at .0071 each to two officers and one director of the Company under a Restricted Stock Award.
On November 15, 2018, we issued 2,500,000 shares of common stock, valued at $0.008 per share, to a Scientific Advisory Board member for consulting services.
On November 23, 2018, we issued 3,805,899 shares of common stock for the partial conversion of $12,000 for convertible note dated May 1, 2018.
On November 26, 2018, we issued 4,347,826 shares of common stock for the partial conversion of $10,000 for convertible note dated May 1, 2018.
On November 28, 2018, we issued 3,657,143 shares of common stock for the partial conversion of $6,000 for convertible note dated May 1, 2018.
On December 7, 2018, we issued 8,823,529 shares of common stock for the partial conversion of $15,000 for convertible note dated June 5, 2018.
On December 14, 2018, we issued 5,882,353 shares of common stock for the partial conversion of $10,000 for convertible note dated June 5, 2018.
On December 17, 2018, we issued 5,870588 shares of common stock for the partial conversion of $8,000 for convertible note dated June 5, 2018.
On January 3, 2019, we issued 15,000,000 shares of common stock, valued at $0.0071 each to two officers and one director of the Company under a Restricted Stock Award.
On January 3, 2019, we issued 10,000,000 shares of common stock, valued at $0.005 per share, to a Scientific Advisory Board member for consulting services.
18
On January 7, 2019, we issued 7,500,000 shares of common stock for the partial conversion of $12,000 for convertible note dated July 2, 2018.
On January 9, 2019, we issued 6,250,000 shares of common stock for the partial conversion of $10,000 for convertible note dated July 2, 2018.
On January 9, 2019, we issued 4,800,000 shares of common stock for the partial conversion of $7,680 for convertible note dated July 2, 2018.
On February 8, 2019, we issued 8,333,333 shares of common stock for the partial conversion of $10,000 for convertible note dated August 6, 2018.
On February 12, 2019, we issued 8,155,556 shares of common stock for the partial conversion of $14,680 for convertible note dated August 6, 2018.
On April 15, 2019, we issued 6,000,000 shares of common stock for the partial conversion of $12,000 for convertible note dated October 3, 2018.
On April 24, 2019, we issued 11,490,000 shares of common stock for the partial conversion of $22,980 for convertible note dated October 3, 2018.
On May 20, 2019, we issued 6,666,667 shares of common stock for the partial conversion of $12,000 for convertible note dated November 15, 2018.
On May 24, 2019, we issued 12,766,667 shares of common stock for the partial conversion of $22,980 for convertible note dated November 15, 2018.
On June 11, 2019, we issued 21,818,182 shares of common stock for the partial conversion of $24,000 for convertible note dated December 6, 2018.
On June 18, 2019, we issued 95,970,000 shares of common stock, valued at $0.0016 per share, for a license.
On June 18, 2019, we issued 15,000,000 shares of common stock, valued at $0.0016 per share, to a Scientific Advisory Board member for consulting services.
On June 19, 2019, we issued 12,200,000 shares of common stock for the partial conversion of $10,980 for convertible note dated December 6, 2018.
On June 28, 2019, we issued 12,000,000 shares of common stock, valued at $0.0015 per share, for an investment in the Company’s Private Placement.
On July 8, 2019, we issued 24,590,164 shares of common stock for the partial conversion of $15,000 for convertible note dated January 2, 2019.
On July 11, 2019, we issued 32,754,098 shares of common stock for the partial conversion of $19,980 for convertible note dated January 2, 2019.
On July 23, 2019, we issued 56,033,333 shares of common stock, valued at $0.0009 per share, for an investment in the Company’s Private Placement.
On August 5, 2019, we issued 4,000,000 shares of common stock, valued at $0.002 per share, for an investment in the Company’s Private Placement.
On September 12, 2019, we issued 10,000,000 shares of common stock for the partial conversion of $12,000 for convertible note dated March 11, 2019.
On September 18, 2019, we issued 10,909,091 shares of common stock for the partial conversion of $12,000 for convertible note dated March 11, 2019.
19
On September 20, 2019, we issued 5,737,374 shares of common stock for the partial conversion of $5,680 for convertible note dated March 11, 2019.
On October 29, 2019, we issued 12,345,679 shares of common stock for the partial conversion of $10,000 for convertible note dated April 23, 2019.
On October 31, 2019, we issued 18,987,342 shares of common stock for the partial conversion of $15,000 for convertible note dated April 23, 2019.
On November 4, 2019, we issued 14,257,143 shares of common stock for the partial conversion of $9,980 for convertible note dated April 23, 2019.
On December 12, 2019, we issued 30,000,000 shares of common stock, valued at $0.0013 per share, for consulting services.
On December 12, 2019, we issued 100,000,000 shares of common stock, valued at $0.0013 each to one officer and one director of the Company under a Restricted Stock Award.
ITEM 6 SELECTED FINANCIAL DATA.
Not required for a “smaller reporting company”.
ITEM 7 MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following discussion and analysis should be read in conjunction with the consolidated Financial Statements and Notes thereto appearing elsewhere in this Annual Report.
Overview
Currently, the Company is focused on immune modulation for the treatment of several specific diseases. Immune modulation refers to the ability to upregulate (make more active) or downregulate (make less active) one’s immune system.
Activating one’s immune system is now an accepted method to cure certain cancers, reduce recovery time from viral or bacterial infections and to prevent illness. Additionally, inhibiting one’s immune system is vital for reducing inflammation, autoimmune disorders and allergic reactions.
Nutraceutical Division – TSI has been producing high quality nutraceuticals. Its flagship product, ProJuvenol®, is a proprietary mixture containing pterostilbene – one of the most potent antioxidants known. TSI filed a patent application for ProJuvenol® on 07-08-2015 titled: “Augmentation of Oncology Immunotherapies by Pterostilbene Containing Compositions”.
SandBox Dental Labs, Inc. – is a wholly-owned subsidiary of TSI consisting of a future dental laboratory to manufacture and fill prescriptions from dentists who will use our proprietary Sleep Appliance to treat their patients with mild to moderate obstructive sleep apnea. The Company needs to seek regulatory approval for its device to treat sleep apnea. As of December 31, 2019 and April 1, 2020, formal operations have not commenced.
We had a net loss of approximately $1.7 million in 2019 compared to a net loss of approximately $1.9 million in 2018.
Net sales increased $24,011 from $3,484 to $27,495, for the years ended December 31, 2018 and 2019, respectively. This increase was mainly due to an increase in sales of the Company’s nutraceutical line of products.
Cost of goods sold increased $858, from $2,157 to $3,015, for the years ended December 31, 2018 and 2019, respectively. This increase was mainly due to higher net sales of the Company’s new nutraceutical line of products in 2019 vs 2018.
Operating expenses for the years ended December 31, 2019 and 2018 were approximately $1.1 million and $1.2 million, respectively, a decrease of $100,000. This decrease was mainly due a combination of decreased general and administrative expenses, decreased salaries, wages and related costs, an increase in consulting fees, a decrease in legal and accounting fees, and a decrease in research and development.
20
General and administrative expenses decreased approximately $337,000, from $408,000 to $71,000, for the years ended December 31, 2018 and 2019, respectively. This decrease was mainly due to a decrease in bad debt, marketing and insurance during the year.
Salaries, wages and related expenses decreased approximately $110,000, from $415,000 to $305,000, for the years ended December 31, 2018 and 2019, respectively. This decrease was mainly due to a decrease in officers’ salaries.
Consulting fees increased approximately $468,000 from $113,000 to $181,000 for the years ended December 31, 2018 and 2019, respectively, due to an increase in overall consulting services during 2019.
Legal and professional fees decreased approximately $62,000, from $190,000 to $128,000 for the years ended December 31, 2018 and 2019, respectively, due to a decrease in overall accounting, patent and general counsel services.
Research and development costs decreased approximately $47,000 from $75,000 to $28,000, for the years ended December 31, 2018 and 2019, respectively. This decrease was mainly due to research and development expenses related to the Company’s nutraceutical line of products.
Total loss from derivatives liabilities decreased approximately $122,000 from $425,000 to $303,000 for the years ended December 31, 2018 and 2019, respectively. This decrease was due to a derivative liability expense from certain convertible notes in 2019 compared to 2018.
Net interest expense increased approximately $109,000 from $242,000 to $351,000 for the years ended December 31, 2018 and 2019, respectively. This increase was mainly due to increased debt balances.
Liquidity and Capital Resources
We have experienced recurring losses over the past years which have resulted in accumulated deficits of approximately $8.8 million and a working capital deficit of approximately $2 million at December 31, 2019. These conditions raise significant doubt about the Company’s ability to continue as a going concern. The Company’s ability to continue as a going concern is contingent upon its ability to secure additional financing, increase sales of its products and attain profitable operations. It is the intent of management to continue to raise additional capital. However, there can be no assurance that the Company will be able to secure such additional funds or obtain such on terms satisfactory to the Company, if at all.
There is no guarantee we will receive the required financing to complete our business strategies, and it is uncertain whether future financing will be available to us on acceptable terms. If financing is not available on satisfactory terms, we may be unable to continue, develop or expand our operations.
As of December 31, 2019, we had approximately $26,000 in cash and cash equivalents, representing an increase in cash and cash equivalents of approximately $4,000 from December 31, 2018. Sources of cash were predominantly from the sale of equity and debt. We anticipate that our current sources of liquidity, including cash and cash equivalents, together with our current projections of cash flow from operating activities, will provide us with liquidity into the third quarter of 2020.
Cash Flows from Operating Activities
Our cash flows from operating activities are significantly affected by our cash outflows to support the growth of our business in areas such as R&D and G&A expenses. Our operating cash flows are also affected by our working capital needs to support personnel related expenditures, accounts payable and other current assets and liabilities.
During the year ended December 31, 2019, cash used in operating activities was $315,609, which was primarily the result of our net loss incurred of $1,697,322, partially offset by increases in the various accounts payable and accrued liability accounts totaling $283,093 and non-cash expenses including stock-based compensation of $468,000, loss on derivative liabilities of $352,934, and amortization of debt discount of $278,593.
During the year ended December 31, 2018, cash used in operating activities was $469,172, which was primarily the result of our net loss incurred of $1,866,912, and increases in prepaids and other current assets of $112,467 and the right-of-use asset of $47,086. This was partially offset by non-cash expenses including stock-based compensation of $623,250, loss on derivative liabilities of $388,121, and amortization of debt discount of $194,985.
21
Cash Flows from Financing Activities
During the year ended December 31, 2019, net cash provided by financing activities was $314,741, compared to $491,540 during the year ended December 31, 2018. The decrease of approximately $177,000 in net cash provided by financing activities is mainly attributable to a decrease of $173,000 in cash received from the sale of common stock due to the Company’s declining stock price. Other cash flows provided by financing activities during the year ended December 31, 2019 included proceeds from convertible notes payable to related and third parties totaling $275,000 offset by payments on notes payable to related parties of $3,689 and payments on convertible notes payable of $33,000. Other cash flows provided by financing activities during the year ended December 31, 2018 included proceeds from convertible notes payable to third parties totaling $245,000 offset by payments on notes payable to related parties of $2,460.
Off-Balance Sheet Arrangements.
We currently do not have any off-balance sheet arrangements.
ITEM 7A QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Not required for a “smaller reporting company”.
ITEM 8 FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
Our financial statements and the accompanying notes that are filed as part of this Annual Report on Form 10-K are listed and set forth beginning on page F-1 immediately following the signature page of this Form 10-K.
ITEM 9 CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
ITEM 9A CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Securities Exchange Act is recorded, processed , summarized and reported , within the time periods specified in the SEC's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Securities Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
At the end of the period covered by this Annual Report on Form 10-K for the fiscal year ended December 31, 2019, an evaluation was carried out under the supervision of and with the participation of our management, including the Chief Executive Officer (“CEO”), of the effectiveness of the design and operations of our disclosure controls and procedures (as defined in Rule 13a-15(e) and Rule 15d-15(e) under the Exchange Act). Based on that evaluation the CEO has concluded that as of the end of the period covered by this Annual Report, our disclosure controls and procedures were not effective.
Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a- 15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, a company's principal executive and principal financial officers and effected by a company's board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the company;
22
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
All internal control systems, no matter how well designed, have inherent limitations and can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our management, including the Chief Executive Officer, assessed the effectiveness of our internal control over financial reporting as of December 31, 2019. In making our assessment, we used the framework and criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO") (2013) in Internal Control-Integrated Framework. Based on that assessment, our management has identified certain material weaknesses in our internal control over financial reporting.
Our management concluded that as of December 31, 2019 our internal control over financial reporting was not effective, and that material weaknesses existed in the following areas as of December 31, 2019:
(1)we do not employ full time in-house personnel with the technical knowledge to identify and address some of the reporting issues surrounding certain complex or non-routine transactions. With respect to material, complex and non-routine transactions, management has and will continue to seek guidance from third-party experts and/or consultants to gain a thorough understanding of these transactions;
(2)we have inadequate segregation of duties consistent with the control objectives including but not limited to the disbursement process, transaction or account changes, and the performance of account reconciliations and approval ;
(3)we have ineffective controls over the period end financial disclosure and reporting process caused by reliance on third-party experts and/or consultants and insufficient accounting staff.
Changes in Internal Control Over Financial Reporting
No substantial changes in our internal control over financial reporting occurred during 2018 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting, except that we have increased our use of external accounting services, adopted policies to improve timely reviews by management and coordination with accounting consultants, and engaged corporate and securities legal counsel with better capabilities than our previous provider's.
ITEM 9B OTHER INFORMATION.
23
PART III
ITEM 10 DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
The Company’s executive officers and directors and their respective ages as of December 31, 2019 are as follows:
| Age | |
Timothy G. Dixon |
| 61 |
Dr. Thomas E. Ichim |
| 43 |
Executive Officers:
Name of Officer |
| Age |
| Office |
Timothy G. Dixon |
| 61 |
| President & CEO |
Dr. James Veltmeyer |
| 56 |
| Chief Medical Officer |
Dr. Feng Lin |
| 51 |
| Chief Scientific Officer |
The term of office for each director is one year, or until the next annual meeting of the stockholders.
Biographical Information
Timothy G. Dixon
Mr. Dixon currently serves as Chief Executive Officer, President, and Chairman of Therapeutic Solutions International, Inc. Mr. Dixon also serves as President and Chairman of Emvolio, Inc., and President and Chairman of SandBox Dental Labs, Inc. Mr. Dixon previously served as the President of TMD Courses, Inc. from 2006 to 2012 and; as the President of Splint Decisions Inc. from 2010 to 2011. Mr. Dixon has attended hundreds of hours of continuing medical/dental education throughout the years and has produced many educational DVD’s used by dental professionals worldwide on the subject of parafunctional control, migraine prevention, therapeutic Botox injections, migraine pathophysiology, dental sleep medicine, and other therapeutic protocols. Mr. Dixon also has extensive experience in dealing with corporate compliance matters with the U.S. Food and Drug Administration, (FDA) as well as many international regulatory bodies.
Thomas E. Ichim, Ph.D.
Dr. Ichim was appointed to the Board of Directors on January 22, 2016. Dr. Ichim also serves as Chief Executive Officer of Emvolio, Inc. Dr. Ichim is a seasoned biotechnology entrepreneur with a track record of scientific excellence. He has founded/co-founded several companies including Batu Biologics, Inc., Medvax Pharma Corp, ToleroTech, Inc, bioRASI, and OncoMune LLC. To date he has published 125 peer-reviewed articles and is co-editor of the textbooks “RNA Interference: From Bench to Clinical Translation” and “Immuno-Oncology Text Book.”
Dr. Ichim is an ad-hoc editor and sits on several editorial boards. Dr. Ichim is inventor on over 150 patents and patent applications. Dr. Ichim has extensive experience with stem cell therapy and cellular product development through FDA regulatory pathways. Dr. Ichim spent over 7 years as the President and Chief Scientific Officer of Medistem, developing and commercializing a novel stem cell, the Endometrial Regenerative Cell, through drug discovery, optimization, preclinical testing, IND filing, and up through Phase II clinical trials with the FDA. Dr. Ichim has extensive experience in product development, regulatory filings, and business development.
Dr. Ichim has a BSc in Biology from the University of Waterloo, Waterloo, Ontario, Canada, a MSc in Microbiology and Immunology a University of Western Ontario, London, Ontario, Canada and a Ph.D. in Immunology from the University of Sciences Arts and Technology, Olveston Monserrat.
24
James Veltmeyer, M.D., – Chief Medical Officer
Dr. Veltmeyer is a board-certified family physician in La Mesa, California. A graduate of UC San Diego and the Ross University School of Medicine, he completed his residency through the UC San Francisco system where he became Chief of Family Medicine Residency, overseeing 36 doctors.
Dr. Veltmeyer, a member of the San Diego Critical Care Medical Group, has been elected for four years (2012, 2014, 2016, and 2017) by his colleagues in the San Diego County Medical Society as a “Physician of Exceptional Excellence,” the most prestigious honor awarded to a “Top Doctor” in San Diego County. He is among a select group of San Diego physicians who was chosen four of the last fifteen years and he consistently ranks in the top 1% to 2% for patient satisfaction. He is currently the Chief of the Department of Family Medicine at Sharp Grossmont Hospital where he provides senior leadership to over 200 doctors.
Feng Lin, MD, Ph.D., – Chief Scientific Officer
Dr. Lin has a stellar track record of drug development in the area of immunology and immuno-oncology having worked with the public company Inovio Pharmaceuticals, where he developed technologies for gene delivery and therapeutic DNA vaccines against cancer and infectious diseases in both R&D and clinical settings. Subsequently, Dr. Lin served as Director of Chinese Operations for MediStem Inc, which was acquired by Intrexon in May 2014. It was the rapid clinical translation model developed by Dr. Lin at MediStem that resulted in the company’s accelerated FDA clearance to begin clinical trials, which resulted in the sale of the company.
Dr. Lin received his postdoctoral training at the Sanford-Burnham Medical Research Institute and his MD and Ph.D. at the Xiangya Medical School of Central South University, China. He has authored over 20 peer-reviewed scientific publications, including several in top journals such as Science, Cell, and Cancer Cell. He holds several patents.
Information with Respect to Our Board of Directors
The following is a brief description of the structure and certain functions of our Board of Directors. Each of the current directors is serving until his respective successor is duly elected, subject to earlier resignation. We do not have standing audit, compensation or nominating committees of our Board of Directors. However, the full Board of Directors performs all of the functions of a standing audit committee, compensation committee and nominating committee.
Audit Committee Related Function
We do not have a separately designated standing audit committee in place. Our full Board of Directors currently serves in that capacity. This is due to the small number of members of our Board of Directors, the small number of executive officers involved with our company, and the fact that we operate with few employees. Our Board of Directors will continue to evaluate, from time to time, whether a separately designated standing audit committee should be put in place. We do not have an audit committee charter.
The Board of Directors reviews with management and the Company's independent public accountants the Company's financial statements, the accounting principles applied in their preparation, the scope of the audit, any comments made by the independent accountants upon the financial condition of the Company and its accounting controls and procedures and such other matters as the Board of Directors deems appropriate. Because our common stock is traded on the OTC Markets Pink Sheet, we are not subject to the listing requirements of any securities exchange regarding audit committee related matters.
The Board of Directors consisted of two directors: Mr. Dixon and Dr. Ichim. Because we do not have an audit committee at all, we disclose that we do not have any "audit committee financial expert" serving on an audit committee.
Compensation Committee Related Function
We do not currently have a standing compensation committee, and do not have a compensation committee charter. The full Board of Directors currently has the responsibility of reviewing and establishing compensation for executive officers and making policy decisions concerning salaries and incentive compensation for executive officers of the Company.
The Company's executive compensation program is administered by the Board of Directors, which determines the compensation of the Chief/Executive Officer/President and the Chief Financial Officer of the Company. In reviewing the compensation of the individual executive officers, the Board of Directors considers the recommendations of the Chief Executive Officer, other market information and current market conditions, as well as any existing employment agreements with them.
25
Nominating Committee Related Function
We do not currently have a standing nominating committee. We have not adopted procedures by which security holders may recommend nominees to serve on our board of directors.
SCIENTIFIC ADVISORY BOARD
The following are members of the Company’s Scientific Advisory Board as of December 31, 2019:
Santosh Kesari, MD, Ph.D.
Dr. Santosh Kesari is a board-certified neurologist and neuro-oncologist and is currently Chair, Department of Translational Neuro-Oncology and Neurotherapeutics, John Wayne Cancer Institute.
He is also Director of Neuro-Oncology, Providence Saint John’s Health Center and Member, Los Angeles Biomedical Research Institute. Dr. Kesari is ranked among the top 1% of neuro-oncologists and neurologists in the nation, according to Castle Connolly Medical Ltd and an internationally recognized scientist and clinician. He is a winner of an Innovation Award by the San Diego Business Journal. He is on the advisory board of American Brain Tumor Association, San Diego Brain Tumor Foundation, Chris Elliott Fund, Nicolas Conor Institute, Voices Against Brain Cancer, and Philippine Brain Tumor Alliance. He has been the author of over 250 scientific publications, reviews, or books. He is the inventor on several patents and patent applications, and founder and advisor to many cancer and neurosciences focused biotech startups.
Dr. Kesari has had a long-standing interest in cancer stem cells and studies their role in the formation of brain tumors and resistance to treatment. He believes that in order to cure patients with brain tumors we first need to gain a better molecular and biological understanding of the disease. A physician/scientist, Kesari harnesses his experience in surgery, chemotherapy, immunotherapy, radiation therapy and novel devices to help develop Precision Therapeutic Strategies that will advance medicine to a new stage in the battle against brain tumors and eradicate the disease.
Francesco Marincola, MD
Dr. Francesco Marincola is currently Chief Science Officer at Refuge Biotechnologies, Menlo Park, California. Most recently, he served as a distinguished research fellow and strategist for immune oncology discovery at AbbVie, Inc Previously, he was the Inaugural Chief Research Officer of Sidra Medical and Research Centre in Doha, Qatar. He was previously a tenured Senior Investigator at the U.S. National Institutes of Health. He is past-President of the Society for the Immunotherapy of Cancer and Editor-in-Chief of the Journal of Translational Medicine.
Dr. Marincola received his MD summa cum laude from the University of Milan and did his surgery training at Stanford University, California. His scientific work deepened the understanding of the mechanisms leading to rejection of tumors or transplanted organs by the immune system and development of autoimmunity. With over 500 peer-reviewed scientific papers, Dr. Marincola is considered one of the world’s leading experts in cancer immunotherapy.
Pablo Guzman, MD
Dr. Pablo Guzman is a cardiologist in Fort Lauderdale, Florida where he is on staff at Holy Cross Hospital. He received his medical degree from University of Puerto Rico School of Medicine and his Cardiology Fellowship at The Johns Hopkins Hospital where he then spent the first part of his career continuing his basic science and clinical research along with his clinical duties. His CV includes over 25 papers published in peer-reviewed journals and more than 15 abstracts.
He is a Fellow of the American College of Cardiology and practiced for more than 30 years. Dr. Guzman is well experienced in basic and clinical research, having participated in many clinical trials. He is also the acting Chief Medical Officer of Variant Pharmaceuticals, a Specialty Pharma company developing treatments for kidney diseases.
Juergen Winkler, MD
Dr. Juergen Winkler is presently practicing at Quantum Functional Medicine in Carlsbad, CA, which he founded in July of 2012. In 2005 he was the co-founder of Genesis Health Systems (Integrative Cancer and Medical Treatment Center) located in Oceanside, CA. He has been a featured speaker for: the NSCC Women’s Health Seminar, Annual IPT/IPTLD Integrative Cancer Care Conference (Multiple years), Health Freedom Expo 2011 & 2012, the Japanese Society of Oxidative Medicine in Osaka Japan, ACOSPM 2010 & 2011 conferences, NSCC Health and Wellness Series 2013, and various other events. He is the physician author of Chapter 5 in the Defeat Cancer book and has been a featured physician in the Townsend Letter.
26
Nassir Azimi, MD
Dr. Nassir Azimi is a cardiologist in La Mesa, California and attended Dartmouth Medical School and completed his residency at the University of Colorado. He finished his four year fellowship in Cardiovascular and Peripheral Interventions at Yale University in New Haven. Dr. Azimi has been in private practice for over 13 years establishing a thriving clinical practice for cardiac patients as well as treating patients for peripheral vascular disease. He is active in Interventional Cardiology and Peripheral Interventions. Dr. Azimi is the director of La Mesa Cardiac Center’s Nuclear Cardiology Laboratory. He is also an investigator in multiple clinical research studies for various cardiac and peripheral diseases.
He has been recognized as San Diego’s Top Interventional Cardiologists by San Diego Magazine 2013,2014,2016, 2017 and also by Castle Connoly for 2013, 2014, 2015,2016, 2017, and 2018. He is a former chief of biomedical ethics (6 years), former chief of Medicine and former chief of Endovascular Medicine as well as Vice Chief of Cardiology at SGH. He is on the board of directors of the California ACC where he serves as chair of the public relations committee. He is on Editorial Review Board for multiple medical journals. He is a national speaker on various topics in cardiology and internal medicine.
Barry Glassman, DMD
Dr. Barry Glassman, DMD, DAAPM, DAACP, FICCMO, Diplomate ABDSM, FADI, is a Diplomate of the American Academy of Craniofacial Pain and the American Academy of Pain Management, as well as a Fellow of the International College of Craniomandibular Orthopedics and the Academy of Dentistry International, he is also on staff at the Lehigh Valley Hospital where he serves as a resident instructor of Craniofacial Pain and Dysfunction and Dental Sleep Medicine.
Dr. Glassman is a Diplomate of the Academy of Dental Sleep Medicine. He is on the staff at the Sacred Heart Hospital Sleep Disorder Center, as well as serving as the Chief Dental Consultant to three other sleep centers in the Lehigh Valley. A popular and dynamic speaker, Dr. Glassman lectures internationally, as well as throughout the United States. In addition to his extensive schedule which includes guest lecture appearances and in-depth courses on joint dysfunction, chronic pain, headache, sleep disorders, and migraine headache, Dr. Glassman is a frequent speaker at major chronic pain and joint dysfunction professional conferences.
University of Pittsburgh: Bachelor of Science 1969, Pittsburgh, Pennsylvania University of Pittsburgh School of Dental Medicine; D.M.D. 1973, Pittsburgh Pennsylvania Post Graduate Hours in Craniomandibular Dysfunction and Sleep Disorders: Over 2500
Vijay Mahant, Ph.D.
Dr. Vijay Mahant has been involved in Research and Development in the medical industry for close to 30 years. Working in the FDA regulated medical industry, he has headed R&D activities for several bio-medical companies as well as being the founder, CEO & Chairman of MediLite, Inc.
Dr. Mahant has specialized in the areas of assay development, has numerous patents to his credit and has published extensively. Dr. Mahant received his B.S. in Biochemistry from the University of Salford, UK; a M.S. in Medicinal Chemistry and a Ph.D. in Medical Biochemistry from Lougborough University of Technology, UK.
David P. Hajjar, Ph.D.
Dr. David P. Hajjar is currently Professor of Biochemistry, at Weill Cornell Medical College and Professor of Pathology and Laboratory Medicine, Weill Cornell Medical College.
Professor Hajjar was also a Frank H.T. Rhodes Distinguished Professor of Cardiovascular Biology and Genetics, Pathology and Laboratory Medicine, Weill Cornell Medical College from 1998 – 2014. Currently Dr. Hajjar is Dean Emeritus and was Executive Vice Provost at Cornell University.
The principal aim of Dr. Hajjar’s work is to define the mechanisms by which Nitric Oxide (NO) and prostaglandin synthetic pathways interact to alter eicosanoid biosynthesis as well as to investigate the impact of these mediators on atherosclerosis and thrombosis. Over the years, he has defined the roles and mechanisms of these complex signaling interactions in order to gain an understanding of the pathophysiological processes in atherosclerosis using animal models and the consequences of pharmacological interventions.
27
In recent work, he has showed that the enzyme prostaglandin H2 synthase (PGHS, also known as cyclooxygenase) regulates the production of eicosanoids that modulate physiologic processes in the vessel wall, contributing to atherosclerosis and thrombosis. Dr. Hajjar demonstrated that various forms of NOx can have different modulatory effects on the activity of PGHS-1, the predominant isozyme in platelets. These and other studies revealed that the active heme center of PGHS-1 regulates peroxynitrite-induced modification and loss of enzyme reactivity, indicating that heme may play a decisive role in catalyzing these processes in PGHS-1 when exposed to nitrative stress in an inflammatory setting. Collectively, these studies show for the first time that iNOS influences PGHS expression and its activity, which can contribute to modification of an important enzyme involved in inflammation during atherosclerosis. Since iNOS-derived species are required for robust atherosclerosis-associated peroxynitrite production in peripheral organs, these studies have contributed importantly to our understanding of the complex alterations in eicosanoid metabolism that occur during the pathogenesis of heart disease where inflammation occurs.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers, and persons who own more than 10% of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other of our equity securities. Officers, directors and greater than 10% stockholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us during the fiscal year ended December 31, 2019, all Section 16(a) filing requirements applicable to our officers, directors and greater than 10% beneficial owners were complied with.
We have adopted a Code of Ethics for our principal executive and financial officers. Our Code of Ethics was filed as an Exhibit to our Annual Report on Form 10-K for fiscal year 2010. We hereby undertake to provide a copy of this Code of Ethics to any person, without charge, upon request. Requests for a copy of this Code of Ethics may be made in writing addressed to: Therapeutic Solutions International, Inc., 4093 Oceanside Blvd, Suite B, Oceanside, California 92056, Attn: Corporate Secretary.
ITEM 11 EXECUTIVE COMPENSATION.
The following table summarizes the compensation paid, with respect to years ended December 31, 2019 and 2018 for services rendered to us in all capacities, to each person who served as an executive officer of the Company.
Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Nonequity Incentive Plan Compensation ($) | All Other Compensation ($) | Total ($) |
Timothy G. Dixon | 2019 | 145,700 (1) | - | 140,000 | - | - | - | 285,700 |
President, CEO and CFO | 2018 | 174,000 (2) | - | 106,500 | - | - | - | 280,500 |
|
|
|
|
|
|
|
|
|
Dr. James Veltmeyer | 2019 | - | - | 50,000 | - | - | - | 50,000 |
Chief Medical Officer | 2018 | - | - | - | - | - | - | - |
|
|
|
|
|
|
|
|
|
Feng Lin | 2019 | - | - | 26,000 | - | - | - | 26,000 |
Chief Scientific Officer | 2018 | - | - | - | - | - | - | - |
|
|
|
|
|
|
|
|
|
Gerry B. Berg, Former CFO | 2019 | 128,800 (3) | - | 75,000 | - | - | - | 203,800 |
Former Vice President, | 2018 | 174,000 (3) | - | 106,500 | - | - | - | 280,500 |
Former CFO |
|
|
|
|
|
|
|
|
28
(1)$110,000 was accrued and unpaid as of December 31, 2019
(2)$136,000 was accrued and unpaid as of December 31, 2018
(3)$110,000 was accrued and unpaid as of December 31, 2019
(4)$136,000 was accrued and unpaid as of December 31, 2018
Outstanding Equity Awards
None
Employment Agreements
We do not have any employment agreements as of December 31, 2019.
Director Compensation
When our employees serve on our Board of Directors, we do not give them any additional compensation in respect of such Board service. Directors currently serve without compensation.
ITEM 12 SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The following table sets forth, as of December 31, 2019, information regarding the ownership of the Company’s outstanding shares of common stock by (i) each person known to management to own, beneficially or of record, more than 5% of the outstanding shares of our common stock, (ii) each director of the Company, (iii) each executive officer of the Company, and (iv) all directors and executive officers as a group. As of December 31, 2019, a total of 1,614,627,811 shares of our common stock were outstanding.
(1)Under SEC rules (i) a person is deemed to be the beneficial owner of shares if that person has, either alone or with others, the power to vote or dispose of those shares. The persons named in the table have sole voting and dispositive power with respect to all shares shown as beneficially owned by them, subject to community property laws where applicable.
ITEM 13 CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Our Board of Directors currently consists of two directors, one of whom is an officer of the Company. As of December 31, 2019 we disclose that we had no independent directors.
In general, it is our policy to submit all proposed related party transactions (those of the kind and size that may require disclosure under Regulation S-K, Item 404) to the Board of Directors for approval. The Board of Directors only approves those transactions that are on terms comparable to, or more beneficial to us than, those that could be obtained in arm’s length dealings with an unrelated third party. Examples of related party transactions covered by our policy are transactions in which any of the following individuals has or will have a direct or indirect material interest: any of our directors or executive officers, any person who is known to us to be the beneficial owner of more than 5% of our common stock, and any immediate family member of one of our directors or executive officers or person known to us to be the beneficial owner of more than 5% of our common stock.
29
ITEM 14 PRINCIPAL ACCOUNTANT FEES AND SERVICES.
Representatives of our principal accountants, Fruci & Assoicates II, PLLC, will be available for the current year and for the most recently completed fiscal year at the stockholder’s meeting to provide the financial statements and be available for questions. At this time, we are not planning to have a stockholder’s meeting.
Audit Fees
The aggregate fees billed to us by our principal accountants, Fruci & Associates II, PLLC for auditing and accounting services for fiscal year 2019 was $58,000 (inclusive of the review of the quarterly reports on Form 10-Q) and for year 2018 was $37,000.
Audit-Related Fees, Tax Fees and All Other Fees
There were no fees billed to us by our principal accountant for fiscal year 2019 and 2018 for assurance and related services (audit-related fees), tax services or other products and services.
Audit Committee Matters
We do not have an audit committee, the executive officers and BOD perform the role of the audit committee. The executive officers and BOD review and approve the audit The audit fees were 83% and the audit related fees were 17% of the total fees and were approved by the executive officers and BOD.
30
ITEM 15 EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
(a)The following documents have been filed as a part of this Annual Report on Form 10-K.
1.Financial Statements
| Page |
Report of Independent Registered Public Accounting Firm | F-1 |
Consolidated Balance Sheets | F-2 |
Consolidated Statements of Operations | F-3 |
Consolidated Statements of Changes in Stockholders' Deficit | F-4 |
Consolidated Statements of Cash Flows | F-5 |
Consolidated Notes to Financial Statements | F-6 |
2.Financial Statement Schedules.
All schedules are omitted because they are not applicable or not required or because the required information is included in the Financial Statements or the Notes thereto.
3.Exhibits.
The following exhibits are filed as part of, or incorporated by reference into, this Annual Report on Form 10-K:
EXHIBIT NUMBER |
| DESCRIPTION |
3.1 |
| Articles of Incorporation |
3.1.1 |
| Certificate of Merger, filed February 22, 2011 |
| Certificate of Amendment to Articles of Incorporation filed October 15, 2012 (incorporated herein by reference to Form 8-K, filed on October 17, 2012) | |
| Bylaws (incorporated herein by reference to Form SB-2, filed on November 21, 2007) | |
| Bylaws amendments adopted August 22, 2012, August 24, 2012 and September 26, 2012 | |
| Consent of Independent Registered Public Accounting Firm – Squar Milner LLP | |
| Rule 13a-14(a)/Section 302 Certification of Principal Executive Officer | |
| Rule 13a-14(a)/Section 302 Certification of Principal Financial Officer | |
| Certification pursuant to 18 U.S.C. Section 1350/Rule 13a-14(b) |
31
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC. | ||
| ||
By: | /s/ Timothy G. Dixon | |
Timothy G. Dixon | ||
Chief Executive Officer and President | ||
|
| |
May 20, 2020 | ||
|
| |
/s/ Thomas E. Ichim | ||
| Thomas E. Ichim | |
Director | ||
|
| |
May 20, 2020 | ||
32

F-1
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Balance Sheets
|
| December 31, 2019 |
| December 31, 2018 |
ASSETS |
|
|
|
|
Current assets: |
|
|
|
|
Cash and cash equivalents | $ | 26,410 | $ | 22,397 |
Restricted cash |
| 10,187 |
| 10,173 |
Accounts receivable |
| 2,904 |
| - |
Inventory |
| 5,180 |
| - |
Prepaid expenses and other current assets |
| 89,379 |
| 113,521 |
Right-of-use asset |
| 5,619 |
| - |
Total current assets |
| 139,679 |
| 146,091 |
|
|
|
|
|
Other assets |
| 171,322 |
| 60,840 |
|
|
|
|
|
Total assets | $ | 311,001 | $ | 206,931 |
|
|
|
|
|
LIABILITIES AND SHAREHOLDERS' DEFICIT |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
Accounts payable | $ | 324,936 | $ | 320,812 |
Accounts payable-related parties |
| 12,715 |
| 7,981 |
Accrued expenses and other current liabilities |
| 505,072 |
| 717,723 |
Lease liability |
| 5,619 |
| - |
Convertible notes payable, net of discount of $105,525 and $105,556, at December 31, 2019 and 2018, respectively |
| 38,475 |
| 45,784 |
Notes payable-related parties, net |
| 937,528 |
| 458,487 |
Derivative liabilities |
| 521,700 |
| 466,612 |
Total current liabilities |
| 2,346,045 |
| 2,017,399 |
|
|
|
|
|
Commitments and contingencies |
| - |
| - |
|
|
|
|
|
Shareholders' Deficit: |
|
|
|
|
Preferred stock, $ 0.001 par value; 5,000,000 shares authorized |
| - |
| - |
Common stock, $ 0.001 par value; 3,500,000,000 shares authorized; 1,614,627,811 and 1,011,063,182 shares issued and outstanding at December 31, 2019 and 2018, respectively. |
| 1,614,628 |
| 1,011,063 |
Additional paid-in capital |
| 5,183,228 |
| 4,314,047 |
Accumulated deficit |
| (8,832,900) |
| (7,135,578) |
Total shareholders' deficit |
| (2,035,044) |
| (1,810,468) |
|
|
|
|
|
Total liabilities and shareholders' deficit | $ | 311,001 | $ | 206,931 |
|
|
|
|
|
See accompanying notes to consolidated financial statements. | ||||
F-2
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Statements of Operations
|
| For the Year Ended December 31, 2019 |
| For the Year Ended December 31, 2018 |
Net sales | $ | 27,495 | $ | 3,484 |
Cost of goods sold |
| 3,015 |
| 2,157 |
|
|
|
|
|
Gross profit |
| 24,480 |
| 1,327 |
|
|
|
|
|
Operating expenses: |
|
|
|
|
General and administrative |
| 70,537 |
| 408,364 |
Salaries, wages, and related costs |
| 305,133 |
| 415,072 |
Stock compensation |
| 355,000 |
| - |
Consulting fees |
| 181,374 |
| 112,877 |
Legal and professional fees |
| 127,917 |
| 189,853 |
Research and development |
| 27,685 |
| 74,970 |
Total operating expenses |
| 1,067,646 |
| 1,201,136 |
|
|
|
|
|
Loss from operations |
| (1,043,166) |
| (1,199,809) |
|
|
|
|
|
Other income (expense): |
|
|
|
|
Loss on derivatives liabilities |
| (352,934) |
| (388,121) |
Change in fair value of derivative liabilities |
| 49,521 |
| (37,230) |
Interest expense |
| (350,743) |
| (241,752) |
Total other income (expense) |
| (654,156) |
| (667,103) |
|
|
|
|
|
Net loss | $ | (1,697,322) | $ | (1,866,912) |
|
|
|
|
|
Net loss per share - basic and diluted | $ | (0.00) | $ | (0.00) |
|
|
|
|
|
Weighted average shares outstanding - basic and diluted |
| 1,284,150,496 |
| 896,851,647 |
|
|
|
|
|
See accompanying notes to consolidated financial statements. | ||||
See accompanying notes to consolidated financial statements.
F-3
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Statement of Changes in Shareholders' Deficit
For the Years Ended December 31, 2019 and 2018
| Common Stock |
|
|
|
|
|
| ||
| Shares |
| Amount |
| Additional Paid-in Capital |
| Accumulated Deficit |
| Total Shareholders' Deficit |
December 31, 2017 | 806,501,000 | $ | 806,501 | $ | 3,147,811 | $ | (5,268,666) | $ | (1,314,354) |
|
|
|
|
|
|
|
|
|
|
Common stock issued for services | 77,500,000 |
| 77,500 |
| 545,750 |
| - |
| 623,250 |
Common stock issued upon conversion of convertible notes payable | 66,062,182 |
| 66,062 |
| 432,486 |
| - |
| 498,548 |
Common stock sold | 61,000,000 |
| 61,000 |
| 188,000 |
| - |
| 249,000 |
Net loss | - |
| - |
| - |
| (1,866,912) |
| (1,866,912) |
December 31, 2018 | 1,011,063,182 |
| 1,011,063 |
| 4,314,047 |
| (7,135,578) |
| (1,810,468) |
|
|
|
|
|
|
|
|
|
|
Common stock issued for services | 200,000,000 |
| 200,000 |
| 268,000 |
| - |
| 468,000 |
Common stock issued upon conversion of convertible notes payable | 235,561,296 |
| 235,562 |
| 526,702 |
| - |
| 762,264 |
Common stock issued for a license | 95,970,000 |
| 95,970 |
| 57,582 |
| - |
| 153,552 |
Common stock sold | 72,033,333 |
| 72,033 |
| 4,397 |
| - |
| 76,430 |
Beneficial conversion feature on note payable | - |
| - |
| 12,500 |
| - |
| 12,500 |
Net loss | - |
| - |
| - |
| (1,697,322) |
| (1,697,322) |
December 31, 2019 | 1,614,627,811 | $ | 1,614,628 | $ | 5,183,228 | $ | (8,832,900) | $ | (2,035,044) |
|
|
|
|
|
|
|
|
|
|
See accompanying notes to consolidated financial statements.
F-4
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Statements of Cash Flows
|
| For the Year Ended December 31, 2019 |
| For the Year Ended December 31, 2018 |
Cash flows from operating activities |
|
|
|
|
Net loss | $ | (1,697,322) | $ | (1,866,912) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
Stock-based compensation to consultants |
| 113,000 |
| 303,750 |
Stock-based compensation to related parties |
| 355,000 |
| 319,500 |
Loss on derivative liabilities |
| 352,934 |
| 388,121 |
Change in fair value of derivatives liabilities |
| (49,521) |
| 37,230 |
Amortization of debt discount |
| 278,593 |
| 194,985 |
Patent amortization |
| 6,591 |
| - |
Changes in operating assets and liabilities: |
|
|
|
|
Accounts receivable |
| (2,904) |
| - |
Inventory |
| (5,180) |
| 1,515 |
Prepaid expenses and other current assets |
| 60,621 |
| (112,467) |
Right-of-use asset |
| (5,619) |
| (47,086) |
Accounts payable |
| 4,122 |
| (22,998) |
Accounts payable - related parties |
| 4,734 |
| 7,981 |
Accrued expenses and other current liabilities |
| 268,618 |
| 327,209 |
Lease liability |
| 5,619 |
| - |
Net cash used in operating activities |
| (310,714) |
| (469,172) |
|
|
|
|
|
Cash flows from financing activities |
|
|
|
|
Payments on notes payable to related party |
| (3,689) |
| (2,460) |
Proceeds from convertible notes payable to related party |
| 25,000 |
| - |
Payments on convertible notes payable |
| (33,000) |
| - |
Proceeds from convertible notes payable |
| 250,000 |
| 245,000 |
Proceeds from sale of common stock |
| 76,430 |
| 249,000 |
Net cash provided by financing activities |
| 314,741 |
| 491,540 |
|
|
|
|
|
Net increase (decrease) in cash, cash equivalents and restricted cash |
| 4,027 |
| 22,368 |
Cash, cash equivalents and restricted cash at beginning of year |
| 32,570 |
| 10,202 |
Cash, cash equivalents and restricted cash at end of year | $ | 36,597 | $ | 32,570 |
|
|
|
|
|
Supplemental cash flow information: |
|
|
|
|
Cash paid for interest | $ | 21,581 | $ | 4,608 |
Cash paid for income taxes | $ | - | $ | 1,600 |
|
|
|
|
|
Non-cash investing and financing transactions: |
|
|
|
|
Original issuance discount on convertible notes payable | $ | - | $ | 27,000 |
Debt discount recorded in connection with derivative liability | $ | 250,000 | $ | 245,000 |
Common stock issued in conversion of convertible notes payable and interest | $ | 762,264 | $ | 498,548 |
Beneficial conversion feature on convertible note | $ | 12,500 | $ | - |
Common stock issued in payment of license agreement | $ | 153,552 | $ | - |
Formalization of accrued salary into related party note | $ | 430,715 | $ | - |
Accrued interest added to principal | $ | 35,614 | $ | - |
|
|
|
|
|
Reconciliation of cash, cash equivalents and restricted cash to the |
|
|
|
|
consolidated balance sheets: |
|
|
|
|
Cash and cash equivalents | $ | 26,410 | $ | 22,397 |
Restricted cash |
| 10,187 |
| 10,173 |
Total cash, cash equivalents, and restricted cash shown in the |
|
|
|
|
consolidated statements of cash flows: | $ | 36,597 | $ | 32,570 |
See accompanying notes to consolidated financial statements.
F-5
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
Note 1 – Nature of business
Therapeutic Solutions International, Inc. (“TSI” or the “Company”) was organized August 6, 2007 under the name Friendly Auto Dealers, Inc., under the laws of the State of Nevada. In the first quarter of 2011 the Company changed its name from Friendly Auto Dealers, Inc. to Therapeutic Solutions International, Inc., and acquired Splint Decisions, Inc., a California corporation.
Currently, the Company is focused on immune modulation for the treatment of several specific diseases. Immune modulation refers to the ability to upregulate (make more active) or downregulate (make less active) one’s immune system.
Activating one’s immune system is now an accepted method to treat certain cancers, reduce recovery time from viral or bacterial infections and to prevent illness. Additionally, inhibiting one’s immune system is vital for reducing inflammation, autoimmune disorders and allergic reactions.
TSI is developing a range of immune-modulatory agents to target certain cancers, improve maternal and fetal health, fight periodontal disease, and for daily health.
Nutraceutical Division – TSI has been producing high quality nutraceuticals. Its current flagship product, NanoStilbene™ PKE, is prepared by low-energy emulsification which allows for better solubility, stability, and the release performance of pterostilbene nanoparticles. The pterostilbene placed in a nanoemulsion droplet is free from air, light, and hard environment; therefore, as a delivery system, nanoemulsion’s can improve the bioavailability of pterostilbene but also protect it from oxidation and hydrolysis, while it possesses an ability of sustained release at the same time.
Cellular Division – TSI recently obtained exclusive rights to a patented adult stem cell for development of therapeutics in the area of chronic traumatic encephalopathy (CTE) and traumatic brain injury (TBI).
The stem cell licensed, termed “JadiCell” is unique in that it possesses features of mesenchymal stem cells, however, outperforms these cells in terms of a) enhanced growth factor production; b) augmented ability to secrete exosomes; and c) superior angiogenic and neurogenic ability.
Chronic Traumatic Encephalopathy (CTE) is caused by repetitive concussive/sub-concussive hits to the head sustained over a period of years and is often found in football players. The condition is characterized by memory loss, impulsive/erratic behavior, impaired judgment, aggression, depression, and dementia. In many patients with CTE, it is anatomically characterized by brain atrophy, reduced mass of frontal and temporal cortices, and medial temporal lobe. TSOI has previously filed several patents in the area of CTE based on modulating the brain microenvironment to enhance receptivity of regenerative cells such as stem cells.
Management does not expect existing cash as of December 31, 2019 or as of March 31, 2020 to be sufficient to fund the Company’s operations for at least twelve months from the issuance date of these financial statements. These financial statements have been prepared on a going concern basis which assumes the Company will continue to realize its assets and discharge its liabilities in the normal course of business. As of December 31, 2019, the Company has incurred losses totaling $8.8 million since inception, has not yet generated material revenue from operations, and will require additional funds to maintain its operations. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern within one year after the consolidated financial statements are issued. The Company’s ability to continue as a going concern is dependent upon its ability to generate future profitable operations and obtain the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they become due. The Company intends to finance operating costs over the next twelve months through its existing financial resources and we may also raise additional capital through equity offerings, debt financings, collaborations and/or licensing arrangements. If adequate funds are not available on acceptable terms, we may be required to delay, reduce the scope of, or curtail, our operations. The accompanying consolidated financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
F-6
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 2 – Basis of presentation and significant accounting policies
Basis of Presentation
The consolidated financial statements and accompanying notes have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). In the opinion of the Company’s management, the consolidated financial statements include all adjustments, which include only normal recurring adjustments, necessary for the fair presentation of the Company’s financial position for the periods presented.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Therapeutic Solutions International, Inc. and its wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
Revenue Recognition
The Company recognizes revenue in accordance with ASC 606,”Revenue from Contracts with Customers” (“ASC 606”). In accordance with ASC 606, the Company applies the following methodology to recognize revenue:
1)Identify the contract with a customer.
2)Identify the performance obligations in the contract.
3)Determine the transaction price.
4)Allocate the transaction price to the performance obligations in the contract.
5)Recognize revenue when (or as) the entity satisfies a performance obligation.
ASC 606 provides that sales revenue is recognized when control of the promised goods or services is transferred to customers at an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services. The Company generally satisfies performance obligations upon shipment of the product or service to the customer. This is consistent with the time in which the customer obtains control of the product or service.
Wholesale policies:
Delivery. The Goods shall be deemed delivered when Buyer has accepted delivery at the above-referenced location. The shipping method shall be determined by Seller, but Buyer will not be responsible for shipping costs.
Purchase Price & Payments. Seller agrees to sell the Goods to Buyer for Fifty Percent (50%) off Sellers listed retail price (see Exhibit A). Seller will provide an invoice to Buyer at the time of delivery. All invoices must be paid, in full, within thirty (30) days. Any balances not paid within thirty (30) days will be subject to a five percent (5%) late payment penalty. In the event Buyer exceeds the aggregate of $500,000.00 worth of aforementioned products having been purchased, delivered, and paid for, Buyer will be entitled to an additional Five Percent (5%) discount up to the aggregate of $750,000.00. In the event Buyer exceeds the aggregate of $750,000.00 worth of aforementioned products having been purchased, delivered, and paid for, Buyer will be entitled to an additional Five Percent (5%) discount up to the aggregate of $1,500,000.00. All future sales after initial $1,500,000 in aggregate purchases will be sold at 60% off retail.
Inspection of Goods & Rejection. Buyer is entitled to inspect the Goods upon delivery. If the Goods are unacceptable for any reason, Buyer must reject them at the time of delivery up to five (5) business days from the date of delivery. If Buyer has not rejected the Goods within five (5) business days from the date of delivery, Buyer shall have waived any right to reject that specific delivery of Goods. In the event Buyer rejects the Goods, Buyer shall allow Seller a reasonable time to cure the deficiency. A reasonable time period shall be determined by industry standards for the particular Goods, as well as the Seller and Buyer.
Risk of Loss. Risk of loss will be on the Seller until the time when the Buyer accepts delivery. Seller shall maintain any and all necessary insurance in order to insure the Goods against loss at Seller’s own expense
F-7
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 2 – Basis of presentation and significant accounting policies (Continued)
Retail policies of e-commerce:
Returns. We will gladly accept the return of products that are defective due to defects in manufacturing and/or workmanship. Fulfillment mistakes that may be made which result in the shipment of incorrect products to you will also be accepted for return.
Shipping. Shipping Time -- Most orders will ship the next business day, provided the product ordered is in stock. Orders are not processed or shipped on Saturday or Sunday, except by prior arrangement. We cannot guarantee when an order will arrive. Consider any shipping or transit time offered to you by this site or other parties only as an estimate. We encourage you to order in a timely fashion to avoid delays caused by shipping or product availability.
Out of Stock. We will ship your product as it becomes available. Usually, products ship by the next business day. However, there may be times when the product you have ordered is out-of-stock, which will delay fulfilling your order. We will keep you informed of any products that you have ordered that are out-of-stock and unavailable for immediate shipment. You may cancel your order at any time prior to shipping.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with maturity of three months or less at the time of issuance to be cash equivalents.
Inventories
Inventories are stated at lower of cost (using the first-in, first-out method, “FIFO”) or market. Inventories consist of purchased materials and assembly items.
Derivative Liabilities
A derivative is an instrument whose value is “derived” from an underlying instrument or index such as a future, forward, swap, option contract, or other financial instrument with similar characteristics, including certain derivative instruments embedded in other contracts and for hedging activities.
As a matter of policy, the Company does not invest in separable financial derivatives or engage in hedging transactions. However, the Company entered into certain debt financing transactions in fiscal 2019 and 2018, as disclosed in Note 5, containing certain conversion features that have resulted in the instruments being deemed derivatives. We evaluate such derivative instruments to properly classify such instruments within equity or as liabilities in our financial statements. Our policy is to settle instruments indexed to our common shares on a first-in-first-out basis.
The classification of a derivative instrument is reassessed at each reporting date. If the classification changes as a result of events during a reporting period, the instrument is reclassified as of the date of the event that caused the reclassification. There is no limit on the number of times a contract may be reclassified.
Instruments classified as derivative liabilities are remeasured using the Black-Scholes model at each reporting period (or upon reclassification) and the change in fair value is recorded on our consolidated statement of operations. We recorded derivative liabilities of $521,700 and $466,612 at December 31, 2019 and 2018, respectively.
Fair Value of Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, prepaids, convertible notes, and payables. The carrying amount of cash and cash equivalents and payables approximates fair value because of the short-term nature of these items.
F-8
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 2 – Basis of presentation and significant accounting policies (Continued)
Fair value is an exit price, representing the amount that would be received from the sale of an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. Fair value measurements are required to be disclosed by level within the following fair value hierarchy:
Level 1 – Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date.
Level 2 – Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument’s anticipated life.
Level 3 – Inputs lack observable market data to corroborate management’s estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model.
When determining fair value, whenever possible the Company uses observable market data, and relies on unobservable inputs only when observable market data is not available. As of December 31, 2018, the Company has level 3 fair value calculations on derivative liabilities. The table below reflects the results of our Level 3 fair value calculations:
The following is the change in derivative liability for the years ended December 31, 2019 and 2018:
Balance, December 31, 2017 | $ | 107,769 |
|
|
|
Issuance of new derivative liabilities |
| 633,122 |
Conversions to paid-in capital |
| (311,509) |
Change in fair market value of derivative liabilities |
| 37,230 |
|
|
|
Balance, December 31, 2018 |
| 466,612 |
|
|
|
Issuance of new derivative liabilities |
| 602,934 |
Conversions to paid-in capital |
| (498,325) |
Change in fair market value of derivative liabilities |
| (49,521) |
Balance, December 31, 2019 | $ | 521,700 |
Estimates were made relating to valuation allowances, impairment of assets, share-based compensation expense and accruals. Actual results could differ materially from those estimates.
Comprehensive Loss
Comprehensive loss for the periods reported was comprised solely of the Company’s net loss.
Net Loss Per Share
Basic loss per share is computed by dividing net income available to common stockholders by the weighted average number of common shares outstanding during the period of computation. Diluted loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if potential common shares had been issued, if such additional common shares were dilutive. Since we had net losses for all the periods presented, basic and diluted loss per share are the same, and additional potential common shares have been excluded as their effect would be antidilutive.
As of December 31, 2019 and 2018, a total of 181,588,903 and 226,902,346, respectively, potential common shares, consisting of shares underlying outstanding convertible notes payable were excluded as their inclusion would be antidilutive.
F-9
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 2 – Basis of presentation and significant accounting policies (Continued)
Depreciation and Amortization
Depreciation is calculated using the straight line method over the estimated useful lives of the assets. Amortization is computed using the straight line method over the term of the agreement. During the years ended December 31, 2019 and 2018, there was no depreciation or amortization expense as all fixed assets have been fully depreciated.
Intangible Assets
Intangible assets consisted primarily of intellectual properties such as proprietary nutraceutical formulations. Intellectual assets are capitalized in accordance with ASC Topic 350 “Intangibles – Goodwill and Other.” Intangible assets with finite lives are amortized over their respective estimated lives and reviewed for impairment whenever events or other changes in circumstances indicate that the carrying amount may not be recoverable. Amortization expense for the years ended December 31, 2019 and 2018 was $6,591 and $0, respectively.
Long-lived Assets
In accordance with ASC 360, Property, Plant and Equipment, the carrying value of intangible assets and other long-lived assets is reviewed on a regular basis for the existence of facts or circumstances that may suggest impairment. The Company recognizes impairment when the sum of the expected undiscounted future cash flows is less than the carrying amount of the asset. Impairment losses, if any, are measured as the excess of the carrying amount of the asset over its estimated fair value.
Research and Development
Research and Development costs are expensed as incurred. Research and Development expenses were $27,685 and $74,970 for the years ended December 31, 2019 and 2018, respectively.
The Company accounts for income taxes under ASC 740 "Income Taxes," which codified SFAS 109, "Accounting for Income Taxes" and FIN 48 “Accounting for Uncertainty in Income Taxes – an Interpretation of FASB Statement No. 109.” Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under ASC 740, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations.
Stock-Based Compensation
Compensation expense for stock issued to employees is determined as the fair value of consideration or services received or the fair value of the equity instruments issued, whichever is more reliably measured. The Financial Accounting Standards Board (FASB) issued ASU 2018-07 to expand the scope of Topic 718 to include share-based payments issued to nonemployees. The effective date for public companies is for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. For all other entities, the effective date is fiscal years beginning after December 15, 2019. The Company adopted during the year ended December 31, 2018 for which there was no impact on the consolidated financial statements.
Leases
On February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). The new standard requires lessees to recognize most leases on their balance sheets as lease liabilities with corresponding right-of-use assets and eliminates certain real estate-specific provisions. ASU 2016-02 became effective for the Company in the first quarter of 2019 and was adopted on a modified retrospective transition basis for leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements. The Company recorded a Right-of-use asset and a Lease Liability of $5,619 as of December 31, 2019.
F-10
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 2 – Basis of presentation and significant accounting policies (Continued)
Recent Accounting Pronouncements
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820) – Disclosure Framework – Changes to the Disclosure Requirements for Fair Value Measurement. The new guidance improves and clarifies the fair value measurement disclosure requirement of ASC 820. The new disclosure requirements include the changes in unrealized gains or losses included in other comprehensive income for recurring Level 3 fair value measurement held at the end of the reporting period and the explicit requirement to disclose the range and weighted average used to develop significant unobservable inputs for Level 3 fair value measurements. The other provisions of ASU 2018-13 also include eliminated and modified disclosure requirements. The guidance is effective for fiscal years beginning after December 15, 2019, with early adoption permitted, including in an interim period for which financial statements have not been issued or made available for issuance. The Company has evaluated the impact of adoption of this ASU and determined that it will have no significant impact on its consolidated financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. ASU 2019-12 eliminated certain exceptions and changed guidance on other matters. The exceptions relate to the allocation of income taxes in separate company financial statements, tax accounting for equity method investments and accounting for income taxes when the interim period year-to-date loss exceeds the anticipated full year loss. Changes relate to the accounting for franchise taxes that are income-based and non-income-based, determining if a step up in tax basis is part of a business combination or if it is a separate transaction, when enacted tax law changes should be included in the annual effective tax rate computation, and the allocation of taxes in separate company financial statements to a legal entity that is not subject to income tax. The new standard is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted. The Company is currently evaluating the potential impact but does not believe there will be an impact of the adoption of this standard on its results of operations, financial position and cash flows and related disclosures.
Note 3 – Restricted cash
Included in current assets is a $10,000 certificate of deposit with an annual interest rate of 0.6%. This certificate matures on June 17, 2020, and is used as collateral for a Company credit card, pursuant to a security agreement dated June 20, 2011.
Note 4 – Prepaid expense and other current assets
Prepaid expenses and other current assets consist of the following:
|
| December 31, 2019 |
| December 31, 2018 |
Prepaid consulting | $ | 88,261 | $ | 111,655 |
Insurance |
| - |
| 848 |
Prepaid costs |
| 1,118 |
| 1,018 |
Total | $ | 89,379 | $ | 113,521 |
Note 5 – Fixed assets
Fixed assets consist of the following:
|
| December 31, 2019 |
| December 31, 2018 |
Computer hardware | $ | 10,747 | $ | 10,747 |
Office furniture and equipment |
| 3,639 |
| 3,639 |
Shipping and other equipment |
| 1,575 |
| 1,575 |
Total |
| 15,961 |
| 15,961 |
Accumulated depreciation |
| (15,961) |
| (15,961) |
Property and equipment, net | $ | - | $ | - |
Depreciation expense was $0 for December 31, 2019 and 2018.
F-11
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 6 – Other assets
Other assets consist of the following:
|
| December 31, 2019 |
| December 31, 2018 |
Prepaid consulting | $ | 20,238 | $ | 56,717 |
Deposit |
| 4,123 |
| 4,123 |
Licenses, net |
| 146,961 |
| - |
Total | $ | 171,322 | $ | 60,840 |
Prepaid consulting agreements are for one to two years and are expensed monthly over the term of the agreement. The net licenses amount above consists of the following:
|
| December 31, 2019 |
| December 31, 2018 |
|
|
|
|
|
License | $ | 153,552 | $ | - |
Accumulated amortization |
| (6,591) |
| - |
Licenses, net | $ | 146,961 | $ | - |
As of June 1, 2019, we entered into a license agreement, which will be amortized over the life of the Patent. The Patent expires December 31, 2032. The Exclusive Patent License to the Jadi Cell is for use under the designated areas of CTE (Chronic Traumatic Encephalopathy), and TBI (Traumatic Brain Injury). The Jadi Cell is an cGMP grade and Research grade manufactured allogenic mesenchymal stem cells derived from US Patent No.: 9,803,176 B2. Forward looking the Company intends to file an Investigational New Drug Application (IND) for brain injured patients who have been intensively cared for and mechanically ventilated due to covid-19 illness and a second IND for CTE/TBI as well in keeping with the spirit of the licensing agreement to advance the Jadi Cell through to FDA Approval for CTE/TBI.
F-12
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 7 – Convertible notes payable
On January 3, 2018, February 27, 2018, May 1, 2018, June 5, 2018, July 2, 2018, August 6, 2018, October 3, 2018, November 15, 2018, and December 6, 2018, the Company entered into five $28,000 convertible promissory notes and four $33,000 convertible promissory notes with third parties for which the proceeds were used for operations. The Company received net proceeds of $245,000 and a $27,000 original issuance discount was recorded. The convertible promissory notes incur interest at 12% per annum for which $28,000 plus accrued interest were due on October 15, 2018, November 20, 2018, February 15, 2019, April 15, 2019 and May 30, 2019 and $33,000 plus accrued interest were due March 30, 2019, July 30, 2019, August 30, 2019, and September 30, 2019. The convertible promissory notes were convertible to shares of the Company's common stock 180 days after issuance. The conversion price per share was equal to 55% of the average of the three (3) lowest trading price of the Company's common stock during the fifteen (15) trading days immediately preceding the applicable conversion date. The Company had the option to prepay the convertible notes in the first 180 days from closing subject to prepayment penalties ranging from 120 of 145% of principal balance plus interest, depending upon the date of prepayment. The convertible promissory notes included various default provisions for which the default interest rate increases to 22% per annum with the outstanding principal and accrued interest increasing by 150%.
On January 2, 2019, February 7, 2019, March 11, 2019, April 23, 2019, August 28, 2019, October 30, 2019 and December 13, 2019, the Company entered into two $28,000 convertible promissory notes, three $33,000 convertible promissory notes, one $78,000 convertible promissory note and one $38,000 convertible promissory note with a third party for which the proceeds were used for operations. The Company received net proceeds of $250,000 and a $21,000 original issuance discount was recorded. The convertible promissory notes incur interest at 12% per annum for which $28,000 plus accrued interest are due on January 30, 2020 and October 30, 2020 and $33,000 plus accrued interest are/were due October 30, 2019, November 30, 2019 and February 28, 2020 and $78,000 plus accrued interest is due June 30, 2020 and $38,000 plus accrued interest is due June 30, 2020. The convertible promissory notes are convertible to shares of the Company's common stock 180 days after issuance. The conversion price per share is equal to 55% of the average of the three (3) lowest trading prices of the Company's common stock during the fifteen (15) trading days immediately preceding the applicable conversion date. The Company has the option to prepay the convertible notes in the first 180 days from closing subject to prepayment penalties ranging from 120% to 145% of principal balance plus interest, depending upon the date of prepayment. The convertible promissory notes include various default provisions for which the default interest rate increases to 22% per annum with the outstanding principal and accrued interest increasing by 150%. The convertible proissory note dated February 7, 2019 was paid in full on July 26, 2019. The Company was required to reserve at December 31, 2019, a total of 872,670,108 common shares in connection with the promissory notes.
Derivative liabilities
These convertible promissory notes are convertible into a variable number of shares of common stock for which there is not a floor to the number of common stock we might be required to issue. Based on the requirements of ASC 815 Derivatives and Hedging, the conversion feature represented an embedded derivative that is required to be bifurcated and accounted for as a separate derivative liability. The derivative liability is originally recorded at its estimated fair value and is required to be revalued at each conversion event and reporting period. Changes in the derivative liability fair value are reported in operating results each reporting period.
For the nine notes issued during the year ended December 31, 2018, the Company valued the conversion feature on the date of issuance resulting in initial liability of $633,121. Since the fair value of the derivatives were in excess of the proceeds received of $245,000, a full discount to convertible notes payable and a day one loss on derivative liabilities of $388,121 was recorded during the year ended December 31, 2018. Upon issuance, the Company valued the conversion feature using the Black-Scholes option pricing model with the following assumptions: conversion prices ranging from $0.002 to $0.006, the closing stock price of the Company's common stock on the date of valuation ranging from $0.0045 to $0.0220, an expected dividend yield of 0%, expected volatility ranging from 214% to 304%, risk-free interest rates ranging from 1.81% to 2.70%, and an expected term ranging from 0.76 to 0.82 years.
F-13
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 7 – Convertible notes payable (Continued)
During the year ended December 31, 2018, five of the $28,000 convertible notes and one of the $33,000 convertible notes were converted into 63,848,737 shares of common stock. At each conversion date, the Company recalculated the value of the derivative liability associated with the convertible note recording a gain (loss) in connection with the change in fair market value. In addition, the pro-rata portion of the derivative liability as compared to the portion of the convertible note converted was reclassed to additional paid-in capital. During the year ended December 31, 2018, the Company recorded a gain of $165,563 related to the change of fair value of the derivative liability and recorded $311,509 to additional paid-in capital. The derivative liabilities were revalued using the Black-Scholes option pricing model with the following assumptions: conversion prices ranging from $0.0033 to $0.005 the closing stock price of the Company's common stock on the date of valuation ranging from $0.0028 to $0.02720, an expected dividend yield of 0%, expected volatility ranging from 185% to 277%, risk-free interest rates ranging from 1.81% to 2.70%, and expected terms ranging from 0.07 to 0.75 years.
On December 31, 2018, the derivative liabilities on the remaining five convertible notes were revalued at $466,612 resulting in a loss of $202,793 for the year ended December 31, 2018 related to the change in fair value of the derivative liabilities. The derivative liabilities were revalued using the Black-Scholes option pricing model with the following assumptions: exercise price of $0.002, the closing stock price of the Company's common stock on the date of valuation of $0.0055, an expected dividend yield of 0%, expected volatility ranging from 248% to 279%, risk-free interest rate of 2.63%, and an expected term ranging from 0.29 to 0.75 years.
For the seven notes issued during the year ended December 31, 2019, the Company valued the conversion feature on the date of issuance resulting in initial liability of $602,934. Since the fair value of the derivatives were in excess of the proceeds received of $250,000, a full discount to convertible notes payable and a day one loss on derivative liabilities of $352,934 was recorded during the year ended December 31, 2019. Upon issuance, the Company valued the conversion feature using the Black-Scholes option pricing model with the following assumptions: conversion prices ranging from $0.001 to $0.003, the closing stock price of the Company's common stock on the date of valuation ranging from $0.002 to $0.009, an expected dividend yield of 0%, expected volatility ranging from 236% to 262%, risk-free interest rates ranging from 1.55% to 2.60%, and an expected term ranging from 0.81 to 1 years.
During the year ended December 31, 2019, three of the $28,000 convertible notes and five of the $33,000 convertible notes were converted into 235,561,296 shares of common stock. At each conversion date, the Company recalculated the value of the derivative liability associated with the convertible note recording a gain (loss) in connection with the change in fair market value. In addition, the pro-rata portion of the derivative liability as compared to the portion of the convertible note converted was reclassed to additional paid-in capital. During the year ended December 31, 2019, the Company recorded a gain of $310,347 related to the change of fair value of the derivative liability and recorded $498,324 to additional paid-in capital. The derivative liabilities were revalued using the Black-Scholes option pricing model with the following assumptions: conversion prices ranging from $0.0004 to $0.002, the closing stock price of the Company's common stock on the date of valuation ranging from $0.001 to $0.009, an expected dividend yield of 0%, expected volatility ranging from 214% to 263%, risk-free interest rates ranging from 1.56% to 2.59%, and expected terms ranging from 0.26 to 0.38 years.
On December 31, 2019, the derivative liabilities on the remaining three convertible notes were revalued at $521,700 resulting in a loss of $260,826 for the year ended December 31, 2019 related to the change in fair value of the derivative liabilities. The derivative liabilities were revalued using the Black-Scholes option pricing model with the following assumptions: exercise price of $0.001, the closing stock price of the Company's common stock on the date of valuation of $0.003, an expected dividend yield of 0%, expected volatility ranging from 245% to 262%, risk-free interest rate of 1.59%, and an expected term ranging from 0.5 to 0.95 years.
The Company amortizes the discounts over the term of the convertible promissory notes using the straight line method which is similar to the effective interest method. During the years ended December 31, 2019 and 2018, the Company amortized $278,593 and $194,985 to interest expense, respectively. As of December 31, 2019, discounts of $105,525 remained for which will be amortized through December 2020.
F-14
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 8 – Notes payable-related parties
Notes payable-related parties consist of:
|
| December 31, 2019 |
| December 31, 2018 |
|
|
|
|
|
Note payable – Scientific Advisory Board Member, unsecured, including interest at 10% per annum, with a maturity date of December 31, 2019 |
| 18,162 | $ | 17,015 |
|
|
|
|
|
Three notes payable – Chief Executive Officer, unsecured, including interest at 8%, 10% and 10% per annum, respectively, with maturity date of December 31, 2019 |
| 37,671 |
| 138,105 |
|
|
|
|
|
One note payable – Chief Executive Officer, unsecured, no interest, paid from a % of revenues |
| 534,700 |
| - |
|
|
|
|
|
Note payable – Chief Financial Officer, unsecured, including interest at 8% per annum, with a maturity date of December 31, 2019 |
| 105,600 |
| 99,200 |
|
|
|
|
|
Three notes payable – Business Advisory Board Member, unsecured, including interest at 8% and 10% per annum, convertible into common stock at $0.005 and $0.004,respectively, with maturity date of April 20, 2019 |
| 246,334 |
| 204,167 |
|
| 942,467 |
| 458,487 |
Less debt discount |
| (4,939) |
| - |
| $ | 937,528 | $ | 458,487 |
Note 9 – Related party transactions
As of December 31, 2019 and 2018, the Company had accrued officers’ salary of $439,534 and $663,100, respectively. One of the officers settled with the company for a note payable that is unsecured and doesn’t accrue interest and will be paid as 0.5% of revenues. This decreased accrued officers’ salary.
On October 25, 2018, we issued 15,000,000 shares of common stock, valued at $0.0071 each to two officers and one director of the Company under a Restricted Stock Award.
On December 12, 2019, we issued 100,000,000 shares of common stock, valued at $0.0013 each to one officer and one director of the Company under a Restricted Stock Award.
F-15
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
The Company is subject to United States federal and state income taxes at an approximate rate of 30%. The reconciliation of the provision for income taxes at the United States federal statutory rate compared to the Company’s income tax expense as reported is as follows:
|
| December 31, 2019 |
| December 31, 2018 |
Expected income tax at statutory rate | $ | (356,438) | $ | (391,884) |
State tax |
| 168 |
| 168 |
Permanent differences |
| 220,501 |
| 253,768 |
Other |
| 6,556 |
| (11,756) |
Change in valuation allowance |
| 129,213 |
| 149,704 |
Provision for income taxes | $ | - | $ | - |
The significant components of deferred income tax assets and liabilities at December 31, 2019 and 2018 are as follows:
|
| December 31, 2019 |
| December 31, 2018 |
Net operating loss carry-forward | $ | 1,450,896 | $ | 1,321,683 |
Valuation allowance |
| (1,450,896) |
| (1,321,683) |
Net deferred tax asset | $ | - | $ | - |
The Company has net operating losses carried forward of approximately $5.7 million and $5 million as of December 31, 2019 and 2018, respectively, available to offset taxable income in future years which expire beginning in fiscal 2032.
As of and for the years ended December 31, 2019 and 2018, management does not believe the Company has any uncertain tax positions. Accordingly, there are no recognized tax benefits at December 31, 2019 and 2018.
The Company is subject to tax in the United States and files tax returns in the U.S. Federal jurisdiction and California state jurisdiction. The Company is subject to U.S. Federal, state and local income tax examinations by tax authorities starting in 2016. The Company currently is not under examination by any tax authority.
Note 11 – Equity
Our authorized capital stock consists of an aggregate of 3,505,000,000 shares, comprised of 3,500,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, which may be issued in various series from time to time and the rights, preferences, privileges and restrictions of which shall be established by our board of directors. As of December 31, 2019, we have 1,614,627,811 shares of common stock and no preferred shares issued and outstanding.
On January 26, 2018, we issued 2,424,242 shares of common stock for the partial conversion of $8,000 for convertible note dated July 24, 2017.
On February 1, 2018, we issued 6,376,471 shares of common stock for the conversion of the balance of $20,000 for convertible note dated July 24, 2017.
On February 1, 2018, we issued 5,000,000 shares of common stock, valued at $0.009 per share, for consulting services.
On February 1, 2018, we issued 2,500,000 shares of common stock, valued at $0.009 per share, for consulting services.
On February 20, 2018, we issued 15,000,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement to a related party.
F-16
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 11 – Equity (Continued)
On February 20, 2018, we issued 2,500,000 shares of common stock, valued at $0.0062 per share, for consulting services.
On April 14, 2018, we issued 2,500,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement to a related party.
On April 14, 2018, we issued 5,000,000 shares of common stock, valued at $0.0057 per share, for consulting services.
On April 27, 2018, we issued 3,225,806 shares of common stock for the partial conversion of $8,000 for convertible note dated September 7, 2017.
On May 1, 2018, we issued 4,137,931 shares of common stock for the partial conversion of $12,000 for convertible note dated September 7, 2017.
On May 2, 2018, we issued 25,000,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement to a related party.
On May 21, 2018, we issued 2,742,857 shares of common stock for the partial conversion of $6,000 for convertible note dated September 7, 2017.
On June 15, 2018, we issued 8,500,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement.
On July 3, 2018, we issued 5,000,000 shares of common stock, valued at $0.004 per share, for an investment in the Company’s Private Placement.
On July 9, 2018, we issued 4,166,667 shares of common stock for the partial conversion of $15,000 for convertible note dated January 3, 2018.
On July 12, 2018, we issued 4,077,778 shares of common stock for the partial conversion of $13,000 for convertible note dated January 3, 2018.
On July 19, 2018, we issued 2,500,000 shares of common stock, valued at $0.015 per share, to a Scientific Advisory Board member for consulting services.
On August 7, 2018, we issued 11,000,000 shares of common stock at $0.011 per share, for consulting services.
On September 5, 2018, we issued 3,260,870 shares of common stock for the partial conversion of $15,000 for convertible note dated February 27, 2018.
On September 10, 2018, we issued 3,262,222 shares of common stock for the partial conversion of $13,000 for convertible note dated February 27, 2018.
On September 19, 2018, we issued 5,000,000 shares of common stock, valued at $0.005 per share, for an investment in the Company’s Private Placement.
On September 19, 2018, we issued 1,500,000 shares of common stock, valued at $0.01 per share, to a Scientific Advisory Board member for consulting services.
On October 25, 2018, we issued 15,000,000 shares of common stock, valued at .0071 each to two officers and one director of the Company under a Restricted Stock Award.
On November 15, 2018, we issued 2,500,000 shares of common stock, valued at $0.008 per share, to a Scientific Advisory Board member for consulting services.
F-17
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 11 – Equity (Continued)
On November 23, 2018, we issued 3,805,899 shares of common stock for the partial conversion of $12,000 for convertible note dated May 1, 2018.
On November 26, 2018, we issued 4,347,826 shares of common stock for the partial conversion of $10,000 for convertible note dated May 1, 2018.
On November 28, 2018, we issued 3,657,143 shares of common stock for the partial conversion of $6,000 for convertible note dated May 1, 2018.
On December 7, 2018, we issued 8,823,529 shares of common stock for the partial conversion of $15,000 for convertible note dated June 5, 2018.
On December 14, 2018, we issued 5,882,353 shares of common stock for the partial conversion of $10,000 for convertible note dated June 5, 2018.
On December 17, 2018, we issued 5,870588 shares of common stock for the partial conversion of $8,000 for convertible note dated June 5, 2018.
On January 3, 2019, we issued 15,000,000 shares of common stock, valued at $0.0071 each to two officers and one director of the Company under a Restricted Stock Award.
On January 3, 2019, we issued 10,000,000 shares of common stock, valued at $0.005 per share, to a Scientific Advisory Board member for consulting services.
On January 7, 2019, we issued 7,500,000 shares of common stock for the partial conversion of $12,000 for convertible note dated July 2, 2018.
On January 9, 2019, we issued 6,250,000 shares of common stock for the partial conversion of $10,000 for convertible note dated July 2, 2018.
On January 9, 2019, we issued 4,800,000 shares of common stock for the partial conversion of $7,680 for convertible note dated July 2, 2018.
On February 8, 2019, we issued 8,333,333 shares of common stock for the partial conversion of $10,000 for convertible note dated August 6, 2018.
On February 12, 2019, we issued 8,155,556 shares of common stock for the partial conversion of $14,680 for convertible note dated August 6, 2018.
On April 15, 2019, we issued 6,000,000 shares of common stock for the partial conversion of $12,000 for convertible note dated October 3, 2018.
On April 24, 2019, we issued 11,490,000 shares of common stock for the partial conversion of $22,980 for convertible note dated October 3, 2018.
On May 20, 2019, we issued 6,666,667 shares of common stock for the partial conversion of $12,000 for convertible note dated November 15, 2018.
On May 24, 2019, we issued 12,766,667 shares of common stock for the partial conversion of $22,980 for convertible note dated November 15, 2018.
On June 11, 2019, we issued 21,818,182 shares of common stock for the partial conversion of $24,000 for convertible note dated December 6, 2018.
F-18
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 11 – Equity (Continued)
On June 18, 2019, we issued 95,970,000 shares of common stock, valued at $0.0016 per share, for a license.
On June 18, 2019, we issued 15,000,000 shares of common stock, valued at $0.0016 per share, to a Scientific Advisory Board member for consulting services.
On June 19, 2019, we issued 12,200,000 shares of common stock for the partial conversion of $10,980 for convertible note dated December 6, 2018.
On June 28, 2019, we issued 12,000,000 shares of common stock, valued at $0.0015 per share, for an investment in the Company’s Private Placement.
On July 8, 2019, we issued 24,590,164 shares of common stock for the partial conversion of $15,000 for convertible note dated January 2, 2019.
On July 11, 2019, we issued 32,754,098 shares of common stock for the partial conversion of $19,980 for convertible note dated January 2, 2019.
On July 23, 2019, we issued 56,033,333 shares of common stock, valued at $0.0009 per share, for an investment in the Company’s Private Placement.
On August 5, 2019, we issued 4,000,000 shares of common stock, valued at $0.002 per share, for an investment in the Company’s Private Placement.
On September 12, 2019, we issued 10,000,000 shares of common stock for the partial conversion of $12,000 for convertible note dated March 11, 2019.
On September 18, 2019, we issued 10,909,091 shares of common stock for the partial conversion of $12,000 for convertible note dated March 11, 2019.
On September 20, 2019, we issued 5,737,374 shares of common stock for the partial conversion of $5,680 for convertible note dated March 11, 2019.
On October 29, 2019, we issued 12,345,679 shares of common stock for the partial conversion of $10,000 for convertible note dated April 23, 2019.
On October 31, 2019, we issued 18,987,342 shares of common stock for the partial conversion of $15,000 for convertible note dated April 23, 2019.
On November 4, 2019, we issued 14,257,143 shares of common stock for the partial conversion of $9,980 for convertible note dated April 23, 2019.
On December 12, 2019, we issued 30,000,000 shares of common stock, valued at $0.0013 per share, for consulting services.
On December 12, 2019, we issued 100,000,000 shares of common stock, valued at $0.0013 each to one officer and one director of the Company under a Restricted Stock Award.
F-19
THERAPEUTIC SOLUTIONS INTERNATIONAL, INC.
Consolidated Notes to Financial Statements
December 31, 2019
Note 12 – Legal proceedings
From time to time, claims are made against us in the ordinary course of business, which could result in litigation. Claims and associated litigation are subject to inherent uncertainties and unfavorable outcomes could occur, such as monetary damages, fines, penalties or injunctions prohibiting us from selling one or more products or engaging in other activities. The occurrence of an unfavorable outcome in any specific period could have a material adverse effect on our results of operations for that period or future periods.
However, as of the date of this report, management believes the outcome of currently identified potential claims and lawsuits will not have a material adverse effect on our financial condition or results of operations.
Note 13 – Commitments and Contingencies
Effective May 1, 2017, the Company entered into a fourth amendment to a Lease Agreement for property located in Oceanside, CA. The lease consists of approximately 1,700 square feet and the amendment is for a term of 36 months and expires on April 30, 2020.
During the year ended December 31, 2019 and 2018, the Company incurred rent expense of $22,494 and $21,774.
Future minimum lease payments as of December 31, 2019 are as follows:
For the year ending December 31, |
|
|
|
|
|
2020 | $ | 7,492 |
Effective November 8, 2019, the Company entered into a royalty agreement with one of the officers, refer to Note 9.
On February 3, 2020. we issued a annual convertible note in the amount of $33,000 with an annual interest rate of 12%.
On March 1, 2020, the Company entered into a fifth amendment to the lease agreement for property located in Oceanside, CA. The amendment extends the expiration date to April 30, 2023 with escalating monthly payments ranging from $2,024 to $2,153.
On March 2, 2020, we issued 8,333,333 shares of common stock for the partial conversion of $10,000 for the convertible note dated August 28, 2019.
On March 5, 2020, we filed with the Nevada Secretary of State a Certificate of Amendment to Articles of Incorporation to effect an amendment (the “Amendment”) changing the number of authorized shares of our common stock to 3,500,000,000 (and changing the total number of authorized shares of stock to 3,505,000,000).
On March 3, 2020, our stockholders acted by way of nonunanimous majority written consent action (pursuant to a solicitation of consents commenced on February 27, 2020, and in lieu of a special meeting of stockholders) to approve the Amendment. The number of shares giving written consent (i.e., voting) in favor of such matter was 927,629,005 (57.45%); no shares were overtly “voted against” the Amendment; and 686,998,806 shares did not participate in the nonunanimous majority written consent action (42.55%).
On March 12, 2020, we issued 11,764,706 shares of common stock for the partial conversion of $10,000 for the convertible note dated August 28, 2019.
On March 26, 2020, we issued 21,818,182 shares of common stock for the partial conversion of $12,000 for the convertible note dated August 28, 2019.
In accordance with ASC 855, the Company has analyzed its operations subsequent to December 31, 2019 through the date these financial statements were issued, and has determined that it does not have any other material subsequent events to disclose in these financial statements.
F-20