UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
|
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended
OR
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number
EVOKE PHARMA, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
(State or other jurisdiction of incorporation) |
|
(IRS Employer Identification No.) |
|
|
|
|
|
|
|
|
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading symbol |
Name of each exchange on which registered |
par value $0.0001 per share |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer, ” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
|
Emerging growth company ☐ |
||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
As of July 31, 2021, the registrant had
Evoke pharma, inc.
Form 10-Q
TABLE OF CONTENTS
i
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
Evoke Pharma, Inc.
Condensed Balance Sheets
|
|
|
June 30, 2021 |
|
|
December 31, 2020 |
|
||
|
|
|
(Unaudited) |
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
Current Assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
|
|
|
$ |
|
|
|
Accounts receivable, net |
|
|
|
|
|
|
|
|
|
Prepaid expenses |
|
|
|
|
|
|
|
|
|
Inventory |
|
|
|
|
|
|
|
|
|
Other current assets |
|
|
|
|
|
|
|
|
|
Total current assets |
|
|
|
|
|
|
|
|
|
Operating lease right-of-use asset |
|
|
|
|
|
|
|
|
|
Other assets |
|
— |
|
|
|
|
|
|
|
Total assets |
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and stockholders' equity (deficit) |
|
|
|
|
|
|
|
|
|
Current Liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses |
|
$ |
|
|
|
$ |
|
|
|
Accrued compensation |
|
|
|
|
|
|
|
|
|
Operating lease liability |
|
|
|
|
|
|
|
|
|
Paycheck protection program loan |
|
— |
|
|
|
|
|
|
|
Milestone payable |
|
|
|
|
|
|
|
|
|
Other current liabilities |
|
|
|
|
|
— |
|
|
|
Total current liabilities |
|
|
|
|
|
|
|
|
|
Long-term liabilities |
|
|
|
|
|
|
|
|
|
Note payable |
|
|
|
|
|
|
|
|
|
Accrued interest payable |
|
|
|
|
|
|
|
|
|
Total long-term liabilities |
|
|
|
|
|
|
|
|
|
Total liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' equity (deficit): |
|
|
|
|
|
|
|
|
|
Common stock, $ issued and outstanding shares - at June 30, 2021 and December 31, 2020, respectively |
|
|
|
|
|
|
|
|
|
Additional paid-in capital |
|
|
|
|
|
|
|
|
|
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
|
Total stockholders' equity (deficit) |
|
|
|
|
|
|
( |
) |
|
Total liabilities and stockholders' equity (deficit) |
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to these unaudited condensed financial statements.
1
Evoke Pharma, Inc.
Condensed Statements of Operations
(Unaudited)
|
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
|
|
2021 |
|
|
2020 |
|
|
2021 |
|
|
2020 |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net product sales |
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
$ |
— |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of goods sold |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
|
Research and development |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forgiveness of paycheck protection loan and accrued interest |
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|||
|
Interest income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Total other income (expense) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share of common stock, basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares used to compute basic and diluted net loss per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to these unaudited condensed financial statements.
2
Evoke Pharma, Inc.
Condensed Statements of Stockholders’ Equity (Deficit)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
Total |
|
||
|
|
|
Common Stock |
|
|
Paid-In |
|
|
Accumulated |
|
|
Stockholders' |
|
||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Equity (Deficit) |
|
|||||
|
Balance at January 1, 2021 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
( |
) |
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|||
|
Issuance of common stock, net of costs of $ |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
||
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|||
|
Balance at March 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|||
|
Issuance of common stock from stock option exercises |
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|||
|
Balance at June 30, 2021 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
Total |
|
||
|
|
|
Common Stock |
|
|
Paid-In |
|
|
Accumulated |
|
|
Stockholders' |
|
||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Equity (Deficit) |
|
|||||
|
Balance at January 1, 2020 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|||
|
Issuance of common stock from employee stock purchase plan |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
||
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|||
|
Balance at March 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|||
|
Issuance of common stock from At-the-Market offering, net of costs of $ |
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
Issuance of common stock from warrant exercises |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
— |
|
|
— |
|
||
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|||
|
Balance at June 30, 2020 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to these unaudited condensed financial statements.
3
Evoke Pharma, Inc.
Condensed Statements of Cash Flows
(Unaudited)
|
|
|
Six Months Ended June 30, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Forgiveness of paycheck protection loan and accrued interest |
|
|
( |
) |
|
— |
|
|
|
Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
Change in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Accounts receivable, net |
|
|
( |
) |
|
— |
|
|
|
Prepaid expenses, inventory and other assets |
|
|
|
|
|
|
|
|
|
Accounts payable and other current liabilities |
|
|
( |
) |
|
|
( |
) |
|
Accrued compensation |
|
|
( |
) |
|
|
|
|
|
Accrued interest expense |
|
|
|
|
|
— |
|
|
|
Milestone payable |
|
— |
|
|
|
|
|
|
|
Net cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
Financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock, net |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock from exercise of stock options |
|
|
|
|
|
— |
|
|
|
Proceeds from issuance of common stock from employee stock purchase plan |
|
— |
|
|
|
|
|
|
|
Proceeds from paycheck protection program |
|
— |
|
|
|
|
|
|
|
Proceeds from Eversana line of credit |
|
— |
|
|
|
|
|
|
|
Net cash provided by financing activities |
|
|
|
|
|
|
|
|
|
Net increase in cash and cash equivalents |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at beginning of period |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of period |
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash financing activities |
|
|
|
|
|
|
|
|
|
Forgiveness of paycheck protection loan and accrued interest |
|
$ |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to these unaudited condensed financial statements.
4
Evoke Pharma, Inc.
Notes to Condensed Financial Statements
(Unaudited)
1. Organization and Basis of Presentation
Evoke Pharma, Inc. (the “Company”) was incorporated in the state of Delaware in
Since its inception, the Company has devoted its efforts to developing its sole product, Gimoti™ (metoclopramide) nasal spray, the first and only nasally-administered product indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. On June 19, 2020, the Company received approval from the U.S. Food and Drug Administration (“FDA”) for its 505(b)(2) New Drug Application (“NDA”) for Gimoti. As discussed in Note 5, the Company launched U.S. commercial sales of Gimoti in October 2020 through its commercial partner Eversana Life Science Services, LLC (“Eversana”).
The Company’s activities are subject to the significant risks and uncertainties associated with any specialty pharmaceutical company that has launched its first commercial product, including market acceptance of the product and the potential need to obtain additional funding for its operations.
Going Concern
The financial statements have been prepared assuming the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred recurring losses and negative cash flows from operations since inception and expects to continue to incur net losses for the foreseeable future until such time, if ever, that it can generate significant revenues from the sale of Gimoti. As of June 30, 2021, the Company had approximately $
The Company’s net losses may fluctuate significantly from quarter to quarter and year to year. The Company believes, based on its current operating plan, that its cash and cash equivalents as of June 30, 2021, as well as cash flows from future net sales of Gimoti, will be sufficient to fund its operations into the third quarter of 2022, less than one year after the date these financial statements are issued. This period could be shortened if there are any significant increases in planned spending other than anticipated. The Company anticipates that it will be required to raise additional funds through debt, equity or other forms of financing, such as potential collaboration arrangements, to fund future operations and continue as a going concern.
There can be no assurance that additional financing will be available when needed or on acceptable terms. If the Company is not able to secure adequate additional funding, the Company may be forced to make reductions in spending, extend payment terms with suppliers, and/or suspend or curtail commercialization activities. Any of these actions could materially harm the Company’s business, results of operations, financial condition and future prospects. There can be no assurance that the Company will be able to successfully commercialize Gimoti. Because the Company’s business is entirely dependent on the success of Gimoti, if the Company is unable to secure additional financing, successfully commercialize Gimoti or identify and execute on strategic alternatives for Gimoti or the Company, the Company will be required to curtail all of its activities and may be required to liquidate, dissolve or otherwise wind down its operations.
Impact of COVID-19
Despite the COVID-19 pandemic, the Company began its commercial sales of Gimoti with Eversana in October 2020. The Company has experienced various disruptions to its sales activities, but has continued its efforts to reach physicians and customers. For example, Eversana’s commercialization efforts have been affected by operational restrictions imposed on its sales force from quarantines, travel restrictions and bans and other governmental restrictions related to COVID-19. As a result of these restrictions, Eversana’s sales force has been restricted from conducting in-person interactions with certain physicians and customers and has been restricted to conducting Gimoti educational and promotional activities virtually in certain circumstances, which has impacted Eversana’s ability to more actively market Gimoti. Third-party research stated that as a result of COVID-19, fewer patients are visiting physician offices resulting in lower patient volumes than normal. The Company anticipates that it and Eversana will continue to be impacted by the COVID-19 pandemic.
The COVID-19 pandemic has not significantly disrupted the operations of the Company’s third-party suppliers and manufacturers or delayed the Company’s manufacturing timelines of Gimoti, but may negatively impact the Company’s ability to successfully
5
commercialize Gimoti and generate product sales in the future. Further, the COVID-19 pandemic and mitigation measures have also had an adverse impact on global economic conditions which could have an adverse effect on the Company’s future business and financial condition, including impairing its ability to raise capital when needed.
In March 2020, the Coronavirus Aid, Relief, and Economic Security (“CARES”) Act was enacted in response to the COVID-19 pandemic. In April 2020, the Company applied for and was approved for a Small Business Administration (“SBA”) loan under the Paycheck Protection Program, established by the CARES Act. On May 1, 2020, the Company received the loan proceeds of approximately $
2. Summary of Significant Accounting Policies
The accompanying condensed balance sheet as of December 31, 2020, which has been derived from audited financial statements, and the unaudited interim condensed financial statements, have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) and follow the requirements of the U.S. Securities and Exchange Commission (“SEC”) for interim reporting. As permitted under those rules, certain footnotes or other financial information that are normally required by GAAP can be condensed or omitted. In management’s opinion, the unaudited interim financial statements have been prepared on the same basis as the audited financial statements and include all adjustments, which include only normal recurring adjustments, necessary for the fair statement of the Company’s financial position and its results of operations and its cash flows for the periods presented. These statements do not include all disclosures required by GAAP and should be read in conjunction with the Company’s financial statements and accompanying notes for the year ended December 31, 2020, which are contained in the Company’s Annual Report on Form 10-K filed with the SEC on March 11, 2021. The results for interim periods are not necessarily indicative of the results expected for the full fiscal year or any other interim period.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ materially from those estimates.
Contract Research Organizations and Consultants
The Company relies on contract research organizations (“CROs”) and consultants to assist with ongoing regulatory activities. If the CROs and consultants are unable to continue their support, this could adversely affect the Company’s operations.
In addition, the Company relies on third-party manufacturers for the production of Gimoti. If the third-party manufacturers are unable to continue manufacturing Gimoti, or if the Company loses one of its sole source suppliers used in its manufacturing processes, the Company may not be able to meet any development needs or commercial supply demand for Gimoti, and the development and/or commercialization of Gimoti could be materially and adversely affected.
The Company also relies on a dedicated third-party sales team to sell Gimoti. If such third-party organization is unable to continue serving as a dedicated sales team, the commercialization of Gimoti could be materially and adversely affected.
Accounts Receivable
Accounts receivable is recorded net of allowance for doubtful accounts. Estimates for allowances for doubtful accounts are determined based on existing contractual obligations and historical payment patterns. The allowance for doubtful accounts was
Inventory
The Company does not own or operate manufacturing facilities for the production of Gimoti, nor does it plan to develop its own manufacturing operations in the foreseeable future. The Company depends on third-party contract manufacturers for all of its required raw materials, drug substance and finished product for its commercial manufacturing. The Company has agreements with Cosma S.p.A. to supply metoclopramide for the manufacture of Gimoti, and with Thermo Fisher Scientific Inc., through its subsidiary Patheon UK Limited, for the manufacturing of Gimoti. The Company currently utilizes third-party consultants, which it engages on an as-needed, hourly basis, to manage the manufacturing contractors.
Prior to FDA approval of Gimoti in June 2020, the cost of materials and expenses associated with the manufacturing of Gimoti were recorded as research and development expense. Subsequent to FDA approval, the Company began manufacturing Gimoti for commercialization and began capitalizing inventory. The Company’s inventory consisted of approximately $
6
Revenue Recognition
The Company’s ability to generate revenue and become profitable depends on its ability to successfully commercialize Gimoti, which was launched in the United States through prescription in October 2020 through the Company’s commercial partner Eversana. If the Company or Eversana fail to successfully launch Gimoti and grow and maintain sales, the Company may never generate significant revenues and its results of operations and financial position will be adversely affected.
In accordance with Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers, the Company recognizes revenue when a customer obtains control of promised goods in an amount that reflects the consideration the Company expects to receive in exchange for the goods provided. Customer control is determined upon the customer’s physical receipt of the product. To determine revenue recognition for arrangements within the scope of ASC 606, the Company performs the following five steps: identify the contracts with the customer; identify the performance obligations in the contract; determine the transaction price; allocate the transaction price to the performance obligations in the contract; and recognize revenue when (or as) it satisfies a performance obligation. At contract inception, the Company assesses the goods promised within each contract and determines those that are performance obligations and assesses whether each promised good is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when the customer obtains control of the product.
Product sales are recorded at the transaction price, which includes variable considerations for co-payment assistance to commercially insured patients meeting certain eligibility requirements, as well as to uninsured patients. Co-payment assistance is recorded as an offset to gross revenue at the time revenue from the product sale is recognized based on expected and actual program participation. Co-pay liabilities are estimated using prescribing data available from customers. Actual amounts of consideration ultimately received may materially differ from the Company’s estimates. If actual results in the future vary from estimates, the Company will adjust these estimates, which would affect net product revenue and earnings in the period such variances become known.
Liabilities for co-pay assistance are classified as accounts payable and accrued expenses in the balance sheets.
Stock-Based Compensation
Stock-based compensation expense for stock option grants and employee stock purchases under the Company’s Employee Stock Purchase Plan (the “ESPP”) is recorded at the estimated fair value of the award as of the grant date and is recognized as expense on a straight-line basis over the employee’s requisite service period, except awards with a performance condition. Awards with a performance condition commence vesting when the satisfaction of the performance condition is probable. The estimation of stock option and ESPP fair value requires management to make estimates and judgments about, among other things, employee exercise behavior, forfeiture rates and volatility of the Company’s common stock. The judgments directly affect the amount of compensation expense that will be recognized.
The Company grants stock options to purchase common stock to employees and members of the board of directors with exercise prices equal to the Company’s closing market price on the date the stock options are granted. The risk-free interest rate assumption was based on the yield of an applicable rate for U.S. Treasury instruments with maturities similar to those of the expected term of the award being valued. The weighted average expected term of options and employee stock purchases was calculated using the simplified method as prescribed by accounting guidance for stock-based compensation. This decision was based on minimal historical data due to the Company’s limited number of stock option exercises. In addition, due to the Company’s limited historical data, the estimated volatility was calculated based upon the Company’s historical volatility, supplemented, as necessary, with historical volatility of comparable companies in the biotechnology industry whose share prices are publicly available for a sufficient period of time. The assumed dividend yield was based on the Company never paying cash dividends and having no expectation of paying cash dividends in the foreseeable future. The Company accounts for forfeitures as the forfeitures occur.
Research and Development Expenses
Research and development costs are expensed as incurred and primarily include compensation and related benefits, stock-based compensation expense, costs paid to third-party contractors for product development activities and drug product materials, and technology acquisition milestones. The Company has expensed costs relating to the purchase and production of pre-approval inventories as research and development expense in the period incurred prior to FDA approval received on June 19, 2020. The Company will expense the clinical, regulatory and manufacturing costs related to the post-marketing commitment to conduct a single dose pharmacokinetics clinical trial of Gimoti to characterize dose proportionality of a lower dose strength of Gimoti, as well
as other costs that may occur for any additional clinical trials the Company may pursue to expand the indication of Gimoti.
Net Loss Per Share
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of common stock outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share is calculated by dividing the net loss by the weighted-average number of common share equivalents outstanding for the period determined using the treasury-stock method.
7
Dilutive common stock equivalents are comprised of warrants to purchase common stock, options to purchase common stock under the Company’s equity incentive plans and potential shares to be purchased under the ESPP.
For the periods presented, the following table sets forth the outstanding potentially dilutive securities that have been excluded from the calculation of diluted net loss per share because to do so would be anti-dilutive:
|
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
|
|
2021 |
|
|
2020 |
|
|
2021 |
|
|
2020 |
|
||||
|
Warrants to purchase common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee stock purchase plan |
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
||
|
Total excluded securities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3. Technology Acquisition Agreement
In June 2007, the Company acquired all worldwide rights, data, patents and other related assets associated with Gimoti from Questcor Pharmaceuticals, Inc. (“Questcor”) pursuant to an Asset Purchase Agreement. The Company paid Questcor $
The remaining $
4. Stockholders’ Equity
Sale of Common Stock in Public Offering
In January 2021, the Company completed the sale of
At the Market Equity Offering Program
In November 2017, the Company filed a shelf registration statement with the SEC on Form S-3. The shelf registration statement included a prospectus for the at-the-market offering to sell up to an aggregate of $
In December 2020, the Company filed a new shelf registration statement with the SEC on Form S-3, or the replacement shelf registration statement. The replacement shelf registration statement replaced the registration statement on Form S-3 the Company originally filed with the SEC in November 2017, which registration statement expired in December 2020. The replacement shelf registration was declared effective by the SEC on January 6, 2021. In December 2020, the Company also entered into a new At Market Issuance Sales Agreement (the “ATM Sales Agreement”), with FBR and H.C. Wainwright & Co. (together with FBR, the “Sales Agents”), pursuant to which the Company may sell from time to time, at its option, up to an aggregate of $
8
Future sales under the ATM Sales Agreement will depend on a variety of factors including, but not limited to, market conditions, the trading price of the Company’s common stock and the Company’s capital needs. There can be no assurance that the Sales Agents will be successful in consummating future sales based on prevailing market conditions or in the quantities or at the prices that the Company deems appropriate.
In addition, the Company will not be able to make future sales of common stock pursuant to the ATM Sales Agreement unless certain conditions are met, which include the accuracy of representations and warranties made to the Sales Agents under the ATM Sales Agreement. Furthermore, each of the Sales Agents is permitted to terminate the ATM Sales Agreement with respect to itself in its sole discretion upon ten days’ notice, or at any time in certain circumstances, including the occurrence of an event that would be reasonably likely to have a material adverse effect on the Company’s assets, business, operations, earnings, properties, condition (financial or otherwise), prospects, stockholders’ equity or results of operations. The Company has no obligation to sell the shares available for sale pursuant to the ATM Sales Agreement.
Stock-Based Compensation
During the six months ended June 30, 2021 and 2020, the Company granted stock options to purchase
|
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
|
|
2021 |
|
|
2020 |
|
|
2021 |
|
|
2020 |
|
||||
|
Common Stock Options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Risk free interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Expected option term |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Expected volatility of common stock |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Expected dividend yield |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The estimated fair value of the shares to be acquired under the ESPP was determined on the initiation date of each six-month purchase period using the Black-Scholes option-pricing valuation model with the following weighted-average assumptions for ESPP shares to be purchased during the three and six months ended June 30, 2020:
|
|
|
Three and Six Months Ended June 30, 2021 |
|
|
Three and Six Months Ended June 30, 2020 |
|
||
|
Employee Stock Purchase Plan |
|
|
|
|
|
|
|
|
|
Risk free interest rate |
|
|
|
|
|
|
||
|
Expected term |
|
|
|
|
|
|
||
|
Expected volatility of common stock |
|
|
|
|
|
|
||
|
Expected dividend yield |
|
|
|
|
|
|
||
There were no employee withholdings to purchase shares during the six-month purchase period beginning March 1, 2021.
The Company recognized stock-based compensation expense to employees and directors in its research and development and its selling, general and administrative functions during the three and six months ended June 30, 2021 and 2020 as follows:
|
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
|
|
2021 |
|
|
2020 |
|
|
2021 |
|
|
2020 |
|
||||
|
Research and development |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Selling, general and administrative |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stock-based compensation expense |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of June 30, 2021, there was approximately $
9
5. Commercial Services and Loan Agreements with Eversana
On January 21, 2020, the Company entered into a commercial services agreement (the “Eversana Agreement”) with Eversana for the commercialization of Gimoti. Pursuant to the Eversana Agreement, Eversana commercializes and distributes Gimoti in the United States. Eversana also manages the marketing of Gimoti to targeted health care providers, as well as the sales and distribution of Gimoti in the United States.
Under the terms of the Eversana Agreement, the Company maintains ownership of the Gimoti NDA, as well as legal, regulatory, and manufacturing responsibilities for Gimoti. Eversana will utilize its internal sales organization, along with other commercial functions, for market access, marketing, distribution and other related patient support services. The Company will record sales for Gimoti and retain more than
The Eversana Agreement terminates on
In connection with the Eversana Agreement, the Company and Eversana have entered into the Eversana Credit Facility, pursuant to which Eversana agreed to provide a revolving Credit Facility of up to $
The Company may prepay any amounts borrowed under the Eversana Credit Facility at any time without penalty or premium. The maturity date of all amounts, including interest, borrowed under the Eversana Credit Facility will be 90 days after the expiration or earlier termination of the Eversana Agreement. The Eversana Credit Facility also includes events of default, the occurrence and continuation of which provide Eversana with the right to exercise remedies against the Company and the collateral securing the loans under the Eversana Credit Facility, including the Company’s cash. These events of default include, among other things, the Company’s failure to pay any amounts due under the Eversana Credit Facility, an uncured material breach of the representations, warranties and other obligations under the Eversana Credit Facility, the occurrence of insolvency events and the occurrence of a change in control.
10
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read in conjunction with our financial statements and accompanying notes included in this Quarterly Report on Form 10-Q and the financial statements and accompanying notes thereto for the fiscal year ended December 31, 2020 and the related Management’s Discussion and Analysis of Financial Condition and Results of Operations, both of which are contained in our Annual Report on Form 10-K filed with the Securities and Exchange Commission, or SEC, on March 11, 2021. Past operating results are not necessarily indicative of results that may occur in future periods.
Forward-Looking Statements
This Quarterly Report on Form 10-Q contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. All statements other than statements of historical facts contained in this Quarterly Report on Form 10-Q, including statements regarding our future results of operations and financial position, business strategy, commercial activities to be conducted by Eversana Life Science Services, LLC, or Eversana, the pricing and reimbursement for Gimoti, future regulatory developments, research and development costs, the timing and likelihood of success, plans and objectives of management for future operations, future results of current and anticipated products and the impact of the coronavirus, or COVID-19, pandemic, on us or on third parties on whom we rely, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statement. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. Although we believe the expectations reflected in these forward-looking statements are reasonable, such statements are inherently subject to risk and we can give no assurances that our expectations will prove to be correct. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, which speak only as of the date of this Quarterly Report on Form 10-Q. You should read this Quarterly Report on Form 10-Q completely. As a result of many factors, including without limitation those set forth under “Risk Factors” under Item 1A of Part II below, and elsewhere in this Quarterly Report on Form 10-Q, our actual results may differ materially from those anticipated in these forward-looking statements. Except as required by applicable law, we undertake no obligation to update these forward-looking statements to reflect events or circumstances after the date of this report or to reflect actual outcomes. For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
We use our registered trademark, EVOKE PHARMA, and other trademarks, including GIMOTI and EvokeAssist, in this Quarterly Report on Form 10-Q. This Quarterly Report on Form 10-Q also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks and tradenames referred to in this Quarterly Report on Form 10-Q appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert its rights, to these trademarks and tradenames.
Unless the context requires otherwise, references in this Quarterly Report on Form 10-Q to “Evoke,” “we,” “us” and “our” refer to Evoke Pharma, Inc.
Overview
We are a specialty pharmaceutical company focused primarily on the development and commercialization of drugs to treat gastrointestinal, or GI, disorders and diseases. Since our inception, we have devoted our efforts to developing our sole product, Gimoti (metoclopramide) nasal spray, the first and only nasally-administered product indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. On June 19, 2020, we received approval from the U.S. Food and Drug Administration, or FDA, for our 505(b)(2) New Drug Application, or NDA, for Gimoti. We launched commercial sales of Gimoti in the United States in October 2020 through our commercial partner Eversana.
Diabetic gastroparesis is a GI disorder affecting millions of patients worldwide, in which food in an individual’s stomach takes too long to empty resulting in a variety of serious GI symptoms and systemic metabolic complications. The gastric delay caused by gastroparesis can compromise absorption of orally administered medications.
On January 21, 2020, we entered into an agreement with Eversana, or the Eversana Agreement, for the commercialization of Gimoti. Pursuant to the Eversana Agreement, Eversana commercializes and distributes Gimoti in the United States. Eversana also manages the marketing of Gimoti to targeted health care providers, as well as the sales and distribution of Gimoti in the United States. Eversana also provided a $5 million revolving credit facility, or the Eversana Credit Facility, that became available upon FDA approval of the Gimoti NDA. In June 2020 we borrowed $2 million and in December 2020 we borrowed the remaining $3 million under the Eversana Credit Facility.
We have primarily funded our operations through the sale of our convertible preferred stock prior to our initial public offering in September 2013, borrowings under our bank loans and the sale of shares of our common stock on the Nasdaq Capital Market. We
11
launched commercial sales of Gimoti in late October 2020 with Eversana and, to date, have generated modest sales given the launch occurred during the COVID-19 pandemic.
We have incurred losses in each year since our inception. These operating losses resulted from expenses incurred in connection with advancing Gimoti through development activities and selling, general and administrative costs associated with our operations. We expect to continue to incur operating losses until revenues from sales of Gimoti exceed our expenses, if ever. We may never become profitable, or if we do, we may not be able to sustain profitability on a recurring basis.
As of June 30, 2021, we had cash and cash equivalents of approximately $16.7 million. Current cash on hand is intended to fund commercialization activities for Gimoti, including manufacturing Gimoti, conducting the post-marketing commitment single dose pharmacokinetics, or PK, clinical trial of Gimoti to characterize dose proportionality of a lower dose strength of Gimoti and any additional development activities should we seek additional indications, protecting our intellectual property portfolio and for selling, general and administrative costs to support operations. Our operations have consumed substantial amounts of cash since inception. We believe, based on our current operating plan, that our existing cash and cash equivalents as of June 30, 2021, as well as cash flows from future net sales of Gimoti, will be sufficient to fund our operations into the third quarter of 2022. This period could be shortened if there are any significant increases in planned spending other than anticipated. We anticipate that we will be required to raise additional funds in order to continue as a going concern. Because our business is entirely dependent on the success of Gimoti, if we are unable to secure additional financing or identify and execute on other development or strategic alternatives for Gimoti or our company, we will be required to curtail all of our activities and may be required to liquidate, dissolve or otherwise wind down our operations. Any of these events could result in a complete loss of your investment in our securities.
Impact of COVID-19
Despite the COVID-19 pandemic, we began our commercial sales of Gimoti with Eversana in October 2020. We have experienced various disruptions to our sales activities, but have continued our efforts to reach physicians and customers. For example, Eversana’s commercialization efforts have been adversely affected by operational restrictions imposed on its sales force from quarantines, travel restrictions and bans, and other governmental restrictions related to COVID-19. As a result of these restrictions, their sales force has been restricted from conducting in-person interactions with certain physicians and customers and has been restricted to conducting Gimoti educational and promotional activities virtually in certain circumstances, which has impacted Eversana’s ability to more actively market Gimoti. Research conducted by IOVIA stated that as a result of COVID-19, fewer patients are visiting physician offices resulting in lower patient volumes than normal, and the Centers for Disease Control and Prevention reported during 2020 that over 40% of patients were avoiding care due to COVID-19. We anticipate that we and Eversana will continue to be impacted by the COVID-19 pandemic.
The COVID-19 pandemic has not significantly disrupted the operations of our third-party suppliers and manufacturers or delayed our manufacturing timelines of Gimoti, but may negatively impact our ability to successfully commercialize Gimoti and generate product sales in the future. Further, the COVID-19 pandemic and mitigation measures have also had an adverse impact on global economic conditions which could have an adverse effect on our future business and financial condition, including impairing our ability to raise capital when needed.
In March 2020, the Coronavirus Aid, Relief, and Economic Security, or CARES, Act was enacted in response to the COVID-19 pandemic. In April 2020, we applied for and were approved for a Small Business Administration, or SBA, loan under the Paycheck Protection Program, or PPP, established by the CARES Act. On May 1, 2020, we received the loan proceeds of approximately $104,000. In January 2021, we received notice that our loan and accrued interest were forgiven by the SBA.
Technology Acquisition Agreement
In June 2007, we acquired all worldwide rights, data, patents and other related assets associated with Gimoti from Questcor Pharmaceuticals, Inc., or Questcor, pursuant to an asset purchase agreement. We paid Questcor $650,000 in the form of an upfront payment and $500,000 in May 2014 as a milestone payment based upon the initiation of the first patient dosing in our Phase 3 clinical trial for Gimoti. In August 2014, Mallinckrodt, plc, or Mallinckrodt, acquired Questcor. As a result of that acquisition, Questcor transferred its rights included in the asset purchase agreement with us to Mallinckrodt. In addition to the payments previously made to Questcor, we may be required to make additional milestone payments totaling up to $52 million. In March 2018, we amended the asset purchase agreement with Mallinckrodt to defer development and approval milestone payments, such that rather than paying two milestone payments based on FDA acceptance for review of the NDA and final product marketing approval, we would be required to make a single $5 million payment on the one-year anniversary after we receive FDA approval to market Gimoti. At the time of the Gimoti NDA approval by FDA, we recorded the $5 million payable owed to Mallinckrodt, along with a $5 million research and development expense. The $5 million milestone payment was paid in July 2021.
The remaining $47 million in milestone payments depend on Gimoti’s commercial success. We are also required to pay to Mallinckrodt a low single digit royalty on net sales of Gimoti. Our obligation to pay such royalties will terminate upon the expiration of the last patent right covering Gimoti, which is expected to occur in 2030, subject to possible extension should any additional, later expiring, licensed patents be granted.
12
Financial Operations Overview
Revenue Recognition
Our ability to generate revenue and become profitable depends on our ability to successfully commercialize Gimoti, which we launched in the United States through prescription in October 2020 through our commercial partner Eversana. If we or Eversana fail to successfully launch Gimoti and grow and maintain sales, we may never generate significant revenues and our results of operations and financial position will be adversely affected.
In accordance with Accounting Standards Codification, or ASC, 606, Revenue from Contracts with Customers, we recognize revenue when a customer obtains control of promised goods in an amount that reflects the consideration we expect to receive in exchange for the goods provided. Customer control is determined upon the customer’s physical receipt of the product. To determine revenue recognition for arrangements within the scope of ASC 606, we perform the following five steps: identify the contracts with the customer; identify the performance obligations in the contract; determine the transaction price; allocate the transaction price to the performance obligations in the contract; and recognize revenue when (or as) it satisfies a performance obligation. At contract inception, we assess the goods promised within each contract and determine those that are performance obligations and assess whether each promised good is distinct. We then recognize as revenue the amount of the transaction price that is allocated to the respective performance obligation when the customer obtains control of the product.
Product sales are recorded at the transaction price, which includes variable considerations for co-payment assistance to commercially insured patients meeting certain eligibility requirements, as well as to uninsured patients. Co-payment assistance is recorded as an offset to gross revenue at the time revenue from the product sale is recognized based on expected and actual program participation.
Co-pay liabilities are estimated using prescribing data available from customers. Actual amounts of consideration ultimately received may differ from our estimates. If actual results in the future vary from estimates, we will adjust these estimates, which would affect net product revenue and earnings in the period such variances become known.
Liabilities for co-pay assistance are classified as accounts payable and accrued expenses in the balance sheets.
Sales of Gimoti Metrics
Gimoti revenues continue to increase on several metrics. During the second quarter of 2021 compared to the first quarter of 2021, Gimoti experienced a nearly 162% growth of net product sales and 152% growth in the number of prescriptions. New prescribers of Gimoti increased from 84 during the first quarter of 2021 to 132 (57% increase) during the second quarter of 2021. Enrollments into the EvokeAssist reimbursement center have grown each month, with June 2021 having the largest number of monthly enrollments to date. Since product launch, patients that have an opportunity to refill the product (that is, patients who have completed their current supply and have additional refills on their prescription) have received a refill approximately 61% of the time. We believe some patients choose not to refill their prescriptions due to remission of symptoms.
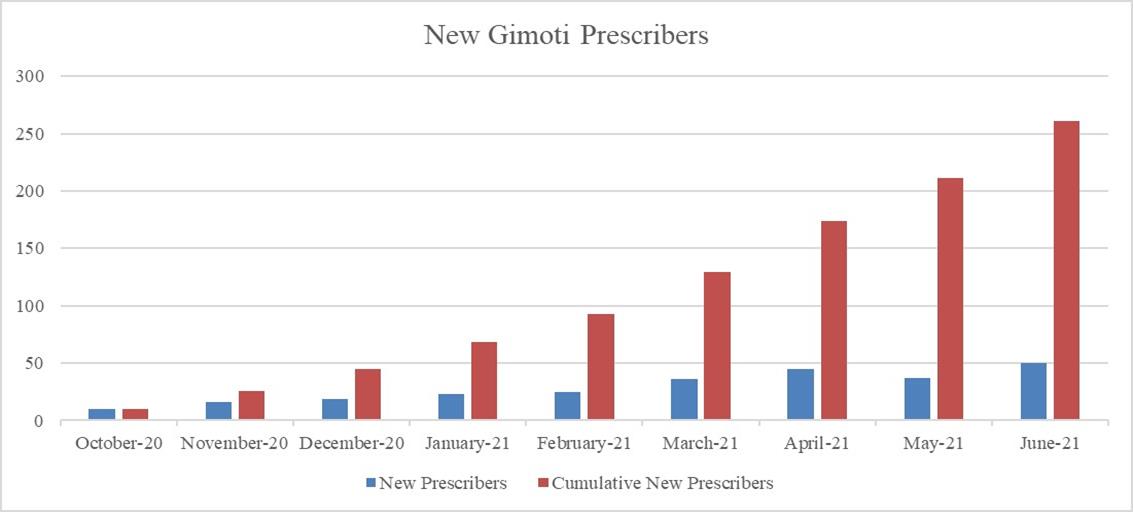
In December 2020 and January 2021, we began accessing the Medicare and Medicaid systems, respectively, to allow for reimbursement submission of products for patients seeking treatment. For the quarter ended June 30, 2021, these government programs
13
made up approximately 33% of the filled prescriptions for Gimoti. Through June 30, 2021, the patients have been mostly between the ages of 31-65. The vast majority are female and were being treated by a gastroenterologist.
The feedback from the Eversana sales organization continues to be positive with regard to physician interest. Although many target physician offices are only recently allowing face to face visits by sales team members, meetings with gastroenterology teams continue to generate positive enrollments and fills. Since product launch, it has taken an average of four to five physician calls before a physician writes their first prescription, which is lower than we initially expected and we believe congruent with the straight-forward non-oral benefit for route of delivery. Furthermore, we have detected a pattern within larger gastroenterology teams that the first physician adopting the use of Gimoti has led other physicians within the same practice to begin prescribing Gimoti as well. These market experiences follow the recently conducted market research announced in June 2021, which indicated, among other positive trends and benefits, that 90% of target gastroenterologists compared to 79% in a prior market research study, intend to prescribe Gimoti.
Research and Development Expenses
We expense all research and development expenses as they are incurred. Research and development expenses primarily include:
|
|
• |
clinical and regulatory-related costs; |
|
|
• |
expenses incurred under agreements with contract research organizations, or CROs; |
|
|
• |
manufacturing and stability testing costs and related supplies and materials; and |
|
|
• |
employee-related expenses, including salaries, benefits, travel and stock-based compensation expense. |
All of our research and development expenses to date have been incurred in connection with the development of Gimoti. With FDA approval of Gimoti, we expect research and development costs to decrease and shift to commercialization and selling costs. However, we have initiated planning for an FDA post-marketing commitment single dose PK clinical trial of Gimoti to characterize dose proportionality of a lower dose strength of Gimoti, as well as other costs that may occur for any additional clinical trials we may pursue to expand the indication of Gimoti. This trial will be designed to characterize dose proportionality of a lower dosage strength of Gimoti to accommodate patients that may require further dosage adjustments. We are unable to estimate with any certainty the costs we will incur related to this trial, or the regulatory review of such lower dosage of Gimoti, though such costs may be significant. Clinical development timelines, the probability of success and development costs can differ materially from expectations.
The costs of clinical trials may vary significantly over the life of a project owing to, but not limited to, the following:
|
|
• |
per subject trial costs; |
|
|
• |
the number of sites included in the trials; |
|
|
• |
the length of time required to enroll eligible subjects; |
|
|
• |
the number of subjects that participate in the trials; |
|
|
• |
the number of doses that subjects receive; |
|
|
• |
the cost of comparative agents used in trials; |
|
|
• |
the drop-out or discontinuation rates of subjects; |
|
|
• |
potential additional safety monitoring or other studies requested by regulatory agencies; and |
|
|
• |
the duration of patient follow-up. |
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist primarily of salaries and related benefits, including stock-based compensation. Other selling, general and administrative expenses include professional fees for accounting, tax, patent costs, legal services, insurance, facility costs and costs associated with being a publicly-traded company, including fees associated with investor relations and directors and officers liability insurance premiums. We expect that selling, general and administrative expenses will increase in the future as we continue to progress with the commercialization of Gimoti and we reimburse Eversana from the net profits attained from the sales of Gimoti.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses during the reporting periods. We evaluate these estimates and judgments on an ongoing basis. We base our estimates on historical experience and
14
on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Our actual results may differ materially from these estimates under different assumptions or conditions.
The critical accounting policies and estimates underlying the accompanying unaudited financial statements are those set forth in Part II, Item 7 included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, which was filed with the SEC on March 11, 2021.
Results of Operations
Comparison of Three Months Ended June 30, 2021 and 2020
The following table summarizes the results of our operations for the three months ended June 30, 2021 and 2020:
|
|
|
Three Months Ended June 30, |
|
|
Increase/ (Decrease) |
|
||||||
|
|
|
2021 |
|
|
2020 |
|
|
|
|
|||
|
Net product sales |
|
$ |
236,635 |
|
|
$ |
— |
|
|
$ |
236,635 |
|
|
Research and development expenses |
|
$ |
195,229 |
|
|
$ |
5,782,094 |
|
|
$ |
(5,586,865 |
) |
|
Selling, general and administrative expenses |
|
$ |
2,142,149 |
|
|
$ |
1,182,872 |
|
|
$ |
959,277 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Product Sales. Net product sales for the three months ended June 30, 2021 compared to the three months ended June 30, 2020 increased by approximately $237,000. We received FDA approval of our Gimoti NDA in June 2020 and began commercial sales in October 2020, so there were no commercial sales during the three months ended June 30, 2020.
Research and Development Expenses. Research and development expenses for the three months ended June 30, 2021 compared to the three months ended June 30, 2020 decreased by approximately $5.6 million. During 2021, we incurred expenses for ongoing stability testing of batches of Gimoti manufactured prior to receipt of FDA approval of the Gimoti NDA in June 2020, as well as preparing for a post-marketing commitment to conduct a single dose PK clinical trial of Gimoti to characterize dose proportionality of a lower dose strength of Gimoti, including the manufacture of clinical trial material.
Costs incurred in 2021 included approximately $65,000 for wages, taxes and employee insurance, including approximately $24,000 of stock-based compensation expense, and approximately $122,000 related to manufacturing. During 2020, we expensed $5 million upon achieving a technology acquisition milestone related to FDA’s approval of Gimoti. We also incurred expenses responding to requests for additional information from FDA related to our NDA and preparing for future manufacturing and potential commercial launch of Gimoti. In addition to the milestone expense, during the three months ended June 30, 2020, we incurred other costs including approximately $378,000 for wages, taxes and employee insurance, including approximately $134,000 of stock-based compensation expense, and approximately $347,000 related to manufacturing.
Selling, General and Administrative Expenses. Selling, general and administrative expenses for the three months ended June 30, 2021 compared to the three months ended June 30, 2020 increased by approximately $959,000. Costs incurred in 2021 primarily included approximately $1.0 million for wages, taxes and employee insurance, including approximately $376,000 of stock-based compensation expense, approximately $607,000 for legal, accounting, directors and officers liability insurance and other costs associated with being a public company, approximately $354,000 for marketing, royalties and Eversana profit sharing, and approximately $40,000 for facility-related expenses. Of the approximately $2.1 million of total selling, general and administrative expenses incurred during the three months ended June 30, 2021, approximately $1.0 million related to wages, taxes, employee insurance, stock-based compensation, and other commercialization activities. Costs incurred in 2020 primarily included approximately $610,000 for wages, taxes and employee insurance, including approximately $229,000 of stock-based compensation expense, and approximately $476,000 for legal, accounting, directors and officers liability insurance and other costs associated with being a public company. Of the total selling, general and administrative expenses incurred during the three months ended June 30, 2020, approximately $312,000 related to wages, taxes, employee insurance, stock-based compensation, and other costs related to pre-commercialization activities.
Comparison of Six Months Ended June 30, 2021 and 2020
The following table summarizes the results of our operations for the six months ended June 30, 2021 and 2020:
|
|
|
Six Months Ended June 30, |
|
|
Increase/ (Decrease) |
|
||||||
|
|
|
2021 |
|
|
2020 |
|
|
|
|
|||
|
Net product sales |
|
$ |
327,056 |
|
|
$ |
— |
|
|
$ |
327,056 |
|
|
Research and development expenses |
|
$ |
473,054 |
|
|
$ |
6,245,946 |
|
|
$ |
(5,772,892 |
) |
|
Selling, general and administrative expenses |
|
$ |
4,480,443 |
|
|
$ |
2,512,707 |
|
|
$ |
1,967,736 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15
Net Product Sales. Net product sales for the six months ended June 30, 2021 compared to the six months ended June 30, 2020 increased by approximately $327,000. We received FDA approval of our Gimoti NDA in June 2020 and began commercial sales in October 2020, so there were no commercial sales during the six months ended June 30, 2020
Research and Development Expenses. Research and development expenses for the six months ended June 30, 2021 compared to the six months ended June 30, 2020 decreased by approximately $5.8 million. During 2021, we incurred expenses for ongoing stability testing of batches of Gimoti manufactured prior to receipt of FDA approval of the Gimoti NDA in June 2020, as well as preparing for a post-marketing commitment to conduct a single dose PK clinical trial of Gimoti to characterize dose proportionality of a lower dose strength of Gimoti, including the manufacture of clinical trial material.
Costs incurred in 2021 included approximately $233,000 for wages, taxes and employee insurance, including approximately $92,000 of stock-based compensation expense, and approximately $199,000 related to manufacturing. During 2020, we expensed $5 million upon achieving a technology acquisition milestone related to FDA’s approval of Gimoti. We also incurred expenses responding to requests for additional information from FDA related to our NDA and preparing for future manufacturing and commercial launch of Gimoti. In addition to the milestone expense, during the six months ended June 30, 2020, we incurred other costs including approximately $749,000 for wages, taxes and employee insurance, including approximately $255,000 of stock-based compensation expense, and approximately $423,000 related to manufacturing.
Selling, General and Administrative Expenses. Selling, general and administrative expenses for the six months ended June 30, 2021 compared to the six months ended June 30, 2020 increased by approximately $2.0 million. Costs incurred in 2021 primarily included approximately $2.2 million for wages, taxes and employee insurance, including approximately $869,000 of stock-based compensation expense, and approximately $1.4 million for legal, accounting, directors and officers liability insurance and other costs associated with being a public company, approximately $567,000 for marketing, royalties and Eversana profit sharing, and approximately $79,000 for facility-related expenses. Of the approximately $4.5 million of total selling, general and administrative expenses incurred during the six months ended June 30, 2021, approximately $1.9 million related to wages, taxes, employee insurance, stock-based compensation, and other commercialization activities. Costs incurred in 2020 primarily included approximately $1.2 million for wages, taxes and employee insurance, including approximately $418,000 of stock-based compensation expense, and approximately $1.1 million for legal, accounting, directors and officers liability insurance and other costs associated with being a public company. Of the total selling, general and administrative expenses incurred during the six months ended June 30, 2020, approximately $412,000 related to wages, taxes, employee insurance, stock-based compensation, and other costs related to pre-commercialization activities.
Liquidity and Capital Resources
In November 2017, we filed a shelf registration statement with the SEC on Form S-3. The shelf registration statement included a prospectus for the at-the-market offering to sell up to an aggregate of $16.0 million of shares of our common stock through B. Riley FBR, Inc., or FBR, as a sales agent, or FBR Sales Agreement. During the six months ended June 30, 2020, we sold 1,395,855 shares of common stock at a weighted-average price per share of $2.42 pursuant to the FBR Sales Agreement and received proceeds of approximately $3.3 million, net of commission and fees. There were no shares sold under the FBR Sales Agreement during 2021. Effective January 6, 2021, we terminated the FBR Sales Agreement. As a result, there were no shares sold under the FBR Sales Agreement during 2021.
In December 2020, we filed a new shelf registration statement with the SEC on Form S-3, or the replacement shelf registration statement. The replacement shelf registration statement replaced the registration statement on Form S-3 we originally filed with the SEC in November 2017, which registration statement expired in December 2020. The replacement shelf registration was declared effective by the SEC on January 6, 2021. In December 2020, we also entered into the ATM Sales Agreement with FBR and H.C. Wainwright & Co., LLC pursuant to which we may sell from time to time, at our option, up to an aggregate of $30 million worth of shares of our common stock through the Sales Agents. The ATM Sales Agreement provides, among other things, that sales under the ATM Sales Agreement will be made pursuant to the replacement shelf registration statement, including the base prospectus filed as part of such registration statement. There were no shares sold under the ATM Sales Agreement during the six months ended June 30, 2021.
Under current SEC regulations, if at the time we file our Annual Report on Form 10-K our public float is less than $75 million, and for so long as our public float remains less than $75 million, the amount we can raise through primary public offerings of securities in any twelve-month period using shelf registration statements is limited to an aggregate of one-third of our public float, which is referred to as the baby shelf rules. As of March 11, 2021, the date we filed our Annual Report on Form 10-K, our public float exceeded $75 million, thereby allowing us to conduct primary offerings without being constrained by the baby shelf rules. We will remain unconstrained by the baby shelf rules under our Form S-3 shelf registration statement until the date we file a new registration statement or our Form 10-K for the fiscal year ending December 31, 2021, at which time if our public float is less than $75 million, the number of securities we may sell under a Form S-3 registration statement will again be limited by the baby shelf rules.
Future sales under the ATM Sales Agreement will depend on a variety of factors including, but not limited to, market conditions, the trading price of our common stock and our capital needs. There can be no assurance that the Sales Agents will be successful in consummating future sales based on prevailing market conditions or in the quantities or at the prices that we deem appropriate.
16
In addition, we will not be able to make future sales of common stock pursuant to the ATM Sales Agreement unless certain conditions are met, which include the accuracy of representations and warranties made to the Sales Agents under the ATM Sales Agreement. Furthermore, each of the Sales Agents is permitted to terminate the ATM Sales Agreement with respect to itself in its sole discretion upon ten days’ notice, or at any time in certain circumstances, including the occurrence of an event that would be reasonably likely to have a material adverse effect on our assets, business, operations, earnings, properties, condition (financial or otherwise), prospects, stockholders’ equity or results of operations. We have no obligation to sell the shares available for sale pursuant to the ATM Sales Agreement.
In connection with the Eversana Agreement, we entered into the Eversana Credit Facility, pursuant to which Eversana agreed to provide a revolving credit facility of up to $5 million to us upon FDA approval of the Gimoti NDA, as well as certain other customary conditions. The Eversana Credit Facility terminates on June 19, 2025, unless terminated earlier pursuant to its terms. The Eversana Credit Facility is secured by all of the Company’s personal property other than its intellectual property. Under the terms of the Eversana Credit Facility, we cannot grant an interest in our intellectual property to any other person. Each loan under the Eversana Credit Facility will bear interest at an annual rate equal to 10.0%, with such interest due at the end of the loan term. In June 2020 we borrowed $2 million and in December 2020 we borrowed $3 million from the Eversana Credit Facility.
In January 2021, we completed the sale of 5,750,000 shares of our common stock in an underwritten public offering led by Laidlaw & Company (UK) Ltd. The price to the public in this offering was $2.50 per share resulting in gross proceeds to us of approximately $14.4 million. After deducting underwriting discounts and commissions, and offering expenses paid by us, the net proceeds to us raised from this offering were approximately $13.1 million.
Management concluded that there is substantial doubt about our ability to continue as a going concern. Our independent registered public accounting firm also included an explanatory paragraph in their report on our financial statements as of and for the year ended December 31, 2020 with respect to our ability to continue as a going concern. This doubt about our ability to continue as a going concern for at least twelve months from the date of the financial statements could materially limit our ability to raise additional funds through the issuance of new debt or equity securities or otherwise. Future reports on our financial statements may also include an explanatory paragraph with respect to our ability to continue as a going concern. We have incurred significant losses since our inception and have never been profitable, and it is possible we will never achieve profitability. We believe, based on our current operating plan, that our existing cash and cash equivalents, as well as future cash flows from net sales of Gimoti, will be sufficient to fund our operations into the third quarter of 2022. This period could be shortened if there are any significant increases in planned spending other than anticipated. We anticipate that we will be required to raise additional funds in order to continue as a going concern. Because our business is entirely dependent on the success of Gimoti, if we are unable to secure additional financing or identify and execute on other development or strategic alternatives for Gimoti or our company, we will be required to curtail all of our activities and may be required to liquidate, dissolve or otherwise wind down our operations. Any of these events could result in a complete loss of your investment in our securities.
These estimates of cash runway could be shortened if there are any significant increases in planned spending on commercialization activities, including for marketing and manufacturing of Gimoti, and our selling, general and administrative costs to support operations. There is no assurance that other financing will be available when needed to allow us to continue as a going concern. The perception that we may not be able to continue as a going concern may cause others to choose not to deal with us due to concerns about our ability to meet our contractual obligations.
We expect to continue to incur expenses as we:
|
|
• |
continue the commercial activities for Gimoti; |
|
|
• |
manufacture Gimoti; |
|
|
• |
conduct the post-marketing commitment single dose PK trial of Gimoti and any additional development activities should we seek additional indications; |
|
|
• |
maintain, expand and protect our intellectual property portfolio; and |
|
|
• |
continue to fund the accounting, legal, insurance and other costs associated with being a public company. |
The following table summarizes our cash flows for the six months ended June 30, 2021 and 2020:
|
|
|
Six Months Ended June 30, |
|
|
Increase/ (Decrease) |
|
||||||
|
|
|
2021 |
|
|
2020 |
|
|
|
|
|||
|
Net cash used in operating activities |
|
$ |
(4,463,608 |
) |
|
$ |
(3,107,975 |
) |
|
$ |
1,355,633 |
|
|
Net cash provided by financing activities |
|
$ |
13,115,608 |
|
|
$ |
5,434,534 |
|
|
$ |
7,681,074 |
|
|
Net increase in cash and cash equivalents |
|
$ |
8,652,000 |
|
|
$ |
2,326,559 |
|
|
$ |
6,325,441 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
17
Operating Activities. The primary use of our cash has been to fund our clinical research, prepare our NDA, manufacture Gimoti, prepare for and begin commercial sales of Gimoti, and other general operations. The cash used in operating activities during the six months ended June 30, 2021 was primarily related to commercialization activities for Gimoti. The cash used in operating activities during the six months ended June 30, 2020 was primarily related to ongoing communication with FDA related to the resubmitted NDA, and pre-approval and pre-commercialization activities. We expect that cash used in operating activities will increase during the second half of 2021 due to commercialization activities, including manufacturing of Gimoti, the planned post-marketing commitment to conduct a single dose PK clinical trial of Gimoti to characterize dose proportionality of a lower dose strength of Gimoti and the payment of the $5 million royalty to Mallinckrodt.
Financing Activities. During the six months ended June 30, 2021, we received net proceeds of approximately $13.1 million from the sale of 5,750,000 shares of common stock pursuant to an underwritten public offering and approximately $45,000 from the exercise of stock options to purchase 67,426 shares of common stock. During the six months ended June 30, 2020, we received net proceeds of approximately $3.3 million from the sale of 1,395,855 shares of common stock pursuant to the FBR Sales Agreement, $2 million from borrowings under the Eversana Credit Facility, approximately $104,000 from the PPP loan, and $21,250 from the sale of 25,000 shares of common stock pursuant to our Employee Stock Purchase Plan.
The amount and timing of our future funding requirements will depend on many factors, including but not limited to:
|
|
• |
the costs of commercialization activities, including costs associated with commercial manufacturing; |
|
|
• |
the commercial success of Gimoti, including competition with well-established products approved earlier by FDA, including oral and intravenous forms of metoclopramide, the same active ingredient in the nasal spray for Gimoti; |
|
|
• |
the impact of the COVID-19 pandemic on us or on third parties on whom we rely; |
|
|
• |
our ability to manufacture sufficient quantities of Gimoti to meet demand, including whether our contract manufacturers, suppliers, and/or consultants are able to meet appropriate timelines; |
|
|
• |
the progress and costs of the post-marketing commitment to conduct a single dose PK clinical trial of Gimoti to characterize dose proportionality of a lower dose strength of Gimoti and the costs of any additional clinical trials we may pursue to expand the indication of Gimoti; |
|
|
• |
our ability to obtain, maintain and enforce our patents and other intellectual property rights, and the costs incurred to do so; |
|
|
• |
the terms and timing of any collaborative, licensing, co-promotion or other arrangements that we may establish; and |
|
|
• |
costs associated with any other product candidates that we may develop, in-license or acquire. |
Off-Balance Sheet Arrangements
Through June 30, 2021, we have not entered into and did not have any relationships with unconsolidated entities or financial collaborations, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purpose.
Contractual Obligations and Commitments
There were no material changes outside the ordinary course of our business during the six months ended June 30, 2021 to the information regarding our contractual obligations that was disclosed in Management’s Discussion and Analysis of Financial Condition and Results of Operations included in our Annual Report on Form 10-K for the year ended December 31, 2020, filed with the SEC on March 11, 2021.
Item 3. Quantitative and Qualitative Disclosure about Market Risk
As of June 30, 2021, there have been no material changes in our market risk from that described in “Item 7 – Management’s Discussion and Analysis of Financial Condition and Results of Operations – Quantitative and Qualitative Disclosures about Market Risk” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, filed with the SEC on March 11, 2021.
Item 4. Controls and Procedures
Conclusions Regarding the Effectiveness of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the timelines specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Business Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objectives, and in reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. In
18
addition, the design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, control may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
As required by SEC Rule 13a-15(b), as of June 30, 2021 we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Business Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as of the end of the period covered by this report. Based on the foregoing, our Chief Executive Officer and Chief Business Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of June 30, 2021.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the quarter ended June 30, 2021 that materially affect, or are reasonably likely to materially affect, our internal control over financial reporting.
19
PART II. OTHER INFORMATION
Item 1. Legal Proceedings
We are currently not a party to any material legal proceedings.
Item 1A. Risk Factors
There have been no material changes to the risk factors included in “Item 1A. Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, filed with the SEC on March 11, 2021 other than as follows:
We may require substantial additional funding and may be unable to raise capital when needed, which would force us to liquidate, dissolve or otherwise wind down our operations.
Our operations have consumed substantial amounts of cash since inception. We believe, based on our current operating plan, that our cash and cash equivalents as of June 30, 2021 of approximately $16.7 million, as well as cash flows from net sales of Gimoti, will be sufficient to fund our operations into the third quarter of 2022. This period could be shortened if there are any significant increases in planned spending other than anticipated. We anticipate that we will be required to raise additional funds through debt, equity or other forms of financing, such as potential collaboration arrangements, to fund future operations and continue as a going concern. There can be no assurance that we will be able to raise additional funds on acceptable terms, or at all. Because our business is entirely dependent on the success of Gimoti, if we are unable to secure additional financing, successfully commercialize Gimoti or identify and execute on other commercialization or strategic alternatives for Gimoti or our company, we will be required to curtail all of our activities and may be required to liquidate, dissolve or otherwise wind down our operations. Any of these events could result in a complete loss of your investment in our securities.
Our estimates of the amount of cash necessary to fund our activities may prove to be wrong and we could spend our available financial resources much faster than we currently expect. Our future funding requirements will depend on many factors, including, but not limited to:
|
|
• |
the commercial success of Gimoti; |
|
|
|
• |
the repayment of unreimbursed commercialization costs to Eversana, approximately $17 million as of June 30, 2021, to be payable only as net product profits are recognized; |
|
|
|
• |
the costs of commercialization activities, including costs associated with commercial manufacturing; |
|
|
|
• |
competition with well-established products approved earlier by FDA, including oral and intravenous forms of metoclopramide, the same active ingredient in the nasal spray for Gimoti; |
|
|
|
• |
the impact of the COVID-19 pandemic on us or on third parties on whom we rely; |
|
|
|
• |
our ability to manufacture sufficient quantities of Gimoti to meet demand, including whether our contract manufacturers, suppliers, and/or consultants are able to meet appropriate timelines; |
|
|
|
• |
the progress and costs of the post-marketing commitment PK trial of Gimoti to characterize dose proportionality of a lower dose strength of Gimoti and the costs of any additional clinical trials we may pursue to expand the indication of Gimoti; |
|
|
|
• |
our ability to obtain, maintain and enforce our patents and other intellectual property rights and the costs incurred in doing so; |
|
|
|
• |
the terms and timing of any collaborative, licensing, co-promotion or other arrangements that we may establish; and |
|
|
|
• |
costs associated with any other product candidates that we may develop, in-license or acquire. |
|
Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. We are authorized to issue up to 50,000,000 shares of common stock. As of June 30, 2021, we had 32,439,380 shares of common stock outstanding and have reserved an aggregate of 6,477,144 shares of common stock for issuance upon exercise of options and other awards granted under our stock option plan, 1,841,879 shares of common stock for issuance upon exercise of warrants, and 344,574 shares of common stock for issuance upon purchases under our employee stock purchase plan. As a result, we have a limited number of remaining unreserved and authorized shares available for issuance, which could impact our ability to raise additional funds in the future. If adequate funds are not available to us, we may not be able to make scheduled debt payments on a timely basis, or at all, and may be required to delay, limit, reduce or cease our operations.
Furthermore, the issuance of additional shares or other securities by us, or the possibility of such issuance, may cause the market price of our shares to decline and dilute the holdings of our existing stockholders. If we raise additional funds by incurring debt, the terms of the debt may involve significant cash payment obligations, as well as covenants and specific financial ratios that may restrict our ability
20
to operate our business. We cannot provide any assurance that our existing capital resources will be sufficient to enable us to identify or execute a viable plan for continued clinical development of Gimoti or to otherwise survive as a going concern.
Our business and operations would suffer in the event of system failures, including cyberattacks.
Despite the implementation of security measures, our internal computer systems and those of our current and any future CROs and other contractors and consultants and collaborators are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. For example, we have been the target of a cyber-attack which resulted in the misappropriation of an immaterial amount our funds and we may be subject to further cyber-attacks seeking to misappropriate our funds or otherwise disrupt our business. Although we have implemented certain additional procedures to reduce the risk of another successful cyber-attack, we cannot be sure that similar cyber-attacks or failures will not occur in the future. Attacks upon information technology systems are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. As a result of the COVID-19 pandemic, we may also face increased cybersecurity risks due to our reliance on internet technology and the number of our employees who are working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. Furthermore, because the techniques used to obtain unauthorized access to, or to sabotage, systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or implement adequate preventative measures. We may also experience security breaches that may remain undetected for an extended period. If another cyber-attack were to occur and cause interruptions in our operations, it could result in a material disruption of our development program for Gimoti and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture Gimoti and conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidate could be delayed, or otherwise adversely affected.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
21
Item 6. Exhibits
Index to Exhibits
|
Exhibit Number |
|
Description of Exhibit |
|
|
|
|
|
3.1 (1) |
|
Amended and Restated Certificate of Incorporation of the Company |
|
|
|
|
|
3.2 (1) |
|
|
|
|
|
|
|
4.1 (2) |
|
|
|
|
|
|
|
4.2 (3) |
|
Warrant dated June 1, 2012 issued by the Company to Silicon Valley Bank |
|
|
|
|
|
4.3 (4) |
|
|
|
|
|
|
|
4.4 (5) |
|
|
|
|
|
|
|
4.5 (6) |
|
|
|
|
|
|
|
4.6 (7) |
|
|
|
|
|
|
|
4.7 (8) |
|
|
|
|
|
|
|
4.8 (9) |
|
|
|
|
|
|
|
31.1* |
|
|
|
|
|
|
|
31.2* |
|
|
|
|
|
|
|
32.1* |
|
|
|
|
|
|
|
32.2* |
|
|
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedeed within the inline XBRL document |
|
|
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema |
|
|
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase |
|
|
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase |
|
|
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase |
|
|
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase |
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
|
(1) |
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on September 30, 2013. |
|
(2) |
Incorporated by reference to the Company’s Amendment No. 3 to Registration Statement on Form S-1 filed with the SEC on August 16, 2013. |
|
(3) |
Incorporated by reference to the Company’s Registration Statement on Form S-1 filed with the SEC on May 24, 2013. |
|
(4) |
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on July 20, 2016. |
|
(5) |
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on August 1, 2016. |
|
(6) |
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on December 16, 2016. |
|
(7) |
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on March 23, 2018. |
|
(8) |
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on April 4, 2018. |
|
(9) |
Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 12, 2020. |
22
|
* |
These certifications are being furnished solely to accompany this quarterly report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934 and are not to be incorporated by reference into any filing of Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing. |
23
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
Evoke Pharma, Inc. |
||
|
|
|
|
|
|
|
Date: August 12, 2021 |
|
By: |
|
/s/ David A. Gonyer |
|
|
|
|
|
David A. Gonyer President and Chief Executive Officer (Principal Executive Officer) |
|
|
|
|
|
|
|
Date: August 12, 2021 |
|
By: |
|
/s/ Matthew J. D’Onofrio |
|
|
|
|
|
Matthew J. D’Onofrio Executive Vice President, Chief Business Officer, Treasurer and Secretary (Principal Financial and Accounting Officer) |
24