UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Form
For the quarterly period ended:
For the transition period from ____________ to ______________
Commission File Number:
(Exact name of registrant as specified in its charter)
| (State of other jurisdiction of incorporation) | (IRS Employer ID No.) |
(Address of principal executive offices)
(Issuer’s Telephone Number)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol | Name of Each Exchange on Which Registered |
|
|
|
The The |
Indicate by check mark whether the registrant
(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months
(or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements
for the past 90 days:
Indicate by check mark whether the registrant
has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405
of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one)
| Large accelerated filer ☐ | Accelerated filer ☐ | |
| Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant
is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☑
The number of shares of the registrant’s common stock, par value $0.001, issued and outstanding as of May 6, 2022, was shares.
TABLE OF CONTENTS
|
OTHER INFORMATION |
||
| 18 | ||
| Item 1. | Legal Proceedings | 18 |
| Item 1A. | Risk Factors | 18 |
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds | 18 |
| Item 3. | Defaults Upon Senior Securities | 18 |
| Item 4. | Mine Safety Disclosures | 18 |
| Item 5. | Other Information | 18 |
| Item 6. | Exhibits | 18 |
| Signatures | 19 | |
| 2 |
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
Sunshine Biopharma, Inc.
Unaudited Consolidated Condensed Balance Sheets
| March 31, | December 31, | |||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Accounts receivable | ||||||||
| Inventory | ||||||||
| Prepaid expenses | ||||||||
| Deposits | ||||||||
| Total Current Assets | ||||||||
| Equipment (net of $ | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable and accrued expenses | $ | $ | ||||||
| Interest payable | ||||||||
| Total Current Liabilities | ||||||||
| Long-term portion of notes payable | ||||||||
| TOTAL LIABILITIES | ||||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| SHAREHOLDERS' EQUITY | ||||||||
Preferred stock, Series B $par value per share; Authorized shares; Issued and outstanding and shares as of March 31, 2022 and December 31, 2021, respectively. | ||||||||
Common Stock, $per share; Authorized Shares; Issued and outstanding and | ||||||||
| Capital paid in excess of par value | ||||||||
| Accumulated comprehensive income (loss) | ( | ) | ( | ) | ||||
| Accumulated (Deficit) | ( | ) | ( | ) | ||||
| TOTAL SHAREHOLDERS' EQUITY | ||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY | $ | $ | ||||||
See Accompanying Notes to These Financial Statements.
| 3 |
Sunshine Biopharma, Inc.
Unaudited Consolidated Condensed Statement of Operations and Comprehensive Loss
| 3 Months | 3 Months | |||||||
| Ended | Ended | |||||||
| March 31, | March 31, | |||||||
| 2022 | 2021 | |||||||
| . | ||||||||
| Revenue: | $ | $ | ||||||
| Cost of Sales | ||||||||
| Gross profit | ||||||||
| General and administrative expenses | ||||||||
| Accounting | ||||||||
| Advertising | ||||||||
| Consulting | ||||||||
| Legal | ||||||||
| Office | ||||||||
| Officer and director remuneration | ||||||||
| Patent fees | ||||||||
| R&D | ||||||||
| Depreciation | ||||||||
| Total general and administrative expenses | ||||||||
| (Loss) from operations | ( | ) | ( | ) | ||||
| Other Income (expense): | ||||||||
| Foreign exchange (loss) | ( | ) | ( | ) | ||||
| Interest income | ||||||||
| Interest expense | ( | ) | ( | ) | ||||
| Debt release | ||||||||
| Loss on debt conversions | ( | ) | ||||||
| Total Other (Expense) | ( | ) | ( | ) | ||||
| Net (loss) before income taxes | ( | ) | ( | ) | ||||
| Provision for income taxes | ||||||||
| Net (Loss) | $ | ( | ) | $ | ( | ) | ||
| Other comprehensive income: | ||||||||
| Gain (Loss) from foreign exchange translation | ( | ) | ||||||
| Comprehensive (Loss) | $ | ( | ) | $ | ( | ) | ||
| Basic (Loss) per common share | $ | ( | ) | $ | ( | ) | ||
| Weighted Average Common Shares Outstanding (Basic) | ||||||||
See Accompanying Notes to These Financial Statements.
| 4 |
Sunshine Biopharma, Inc.
Unaudited Consolidated Condensed Statement of Cash Flows
| 3 Months | 3 Months | |||||||
| Ended | Ended | |||||||
| March 31, | March 31, | |||||||
| 2022 | 2021 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net (Loss) | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | ||||||||
| Foreign exchange (gain) loss | ||||||||
| Stock issued for services | ||||||||
| Stock issued for payment interest | ||||||||
| Loss on debt conversion | ||||||||
| Debt release | ( | ) | ||||||
| (Increase) decrease in accounts receivable | ||||||||
| (Increase) decrease in inventory | ( | ) | ( | ) | ||||
| (Increase) in prepaid expenses | ( | ) | ( | ) | ||||
| Increase (decrease) in Accounts Payable and accrued expenses | ||||||||
| Increase (decrease) in interest payable | ( | ) | ||||||
| Net Cash Flows (used) in operations | ( | ) | ( | ) | ||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds public offering, net of offering costs | ||||||||
| Purchase of preferred shares | ( | ) | ||||||
| Payments of notes payable | ( | ) | ( | ) | ||||
| Net Cash Flows provided by financing activities | ||||||||
| Cash and Cash Equivalents at Beginning of Period | ||||||||
| Net Increase (Decrease) In Cash and cash equivalents | ||||||||
| Foreign currency translation adjustment | ( | ) | ||||||
| Cash and cash equivalents at end of period | $ | $ | ||||||
| Supplementary Disclosure of Cash Flow Information: | ||||||||
| Stock issued for note conversions including interest | $ | $ | ||||||
| Cash paid for interest | $ | $ | ||||||
See Accompanying Notes to These Financial Statements.
| 5 |
Sunshine Biopharma, Inc.
Unaudited Consolidated Statement of Shareholders' Equity
| Number Of | Capital Paid | Number Of | ||||||||||||||||||||||||||||||
| Common | Common | in Excess | Preferred | Preferred | Comprehensive | Accumulated | ||||||||||||||||||||||||||
| Shares Issued | Stock | of Par Value | Shares Issued | Stock | Income | Deficit | Total | |||||||||||||||||||||||||
| Three Months Period | ||||||||||||||||||||||||||||||||
| Balance December 31, 2020 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||
| Common stock issued for the reduction of note payable and payment of interest | – | |||||||||||||||||||||||||||||||
| Common stock issued for services | – | |||||||||||||||||||||||||||||||
| Net (loss) | – | – | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||
| Balance at March 31, 2021 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||
| Balance December 31, 2021 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||||||||
| Common stock and pre-funded warrants issued in an underwritten public offering, net of issuance costs | – | |||||||||||||||||||||||||||||||
| Exercise of warrants | – | |||||||||||||||||||||||||||||||
| Preferred stock purchased from related party | – | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||
| Net (loss) | – | – | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Balance at March 31, 2022 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||||||||
See Accompanying Notes to These Financial Statements.
| 6 |
Sunshine Biopharma, Inc.
Notes to Unaudited Consolidated Condensed Financial Statements
For the Three Month Interim Periods Ended March 31, 2022 and 2021
Note 1 – Nature of Business and Basis of Presentation
Sunshine Biopharma, Inc. (the "Company") was originally incorporated under the name Mountain West Business Solutions, Inc. on August 31, 2006, in the State of Colorado. Until October 2009, the Company was operating as a business consultancy firm.
Effective October 15, 2009, the Company acquired Sunshine Biopharma, Inc. in a transaction classified as a reverse acquisition. Sunshine Biopharma, Inc. held an exclusive license to a new anticancer drug bearing the laboratory name, Adva-27a (the “License Agreement”). Upon completion of the reverse acquisition transaction, the Company changed its name to Sunshine Biopharma, Inc. and began operating as a pharmaceutical company focusing on the development of the licensed Adva-27a anticancer drug.
In December 2015, the Company acquired all worldwide issued (US Patent Number 8,236,935, and 10,272,065) and pending patents under PCT/FR2007/000697 and PCT/CA2014/000029 for the Adva-27a anticancer compound from Advanomics Corporation, a related party, and terminated the License Agreement. In 2016, the remaining value of these patents was impaired. The Company is however continuing development of the Adva-27a anticancer drug covered by these patents.
In December 2018, the Company launched its first Science-Based Nutritional Supplements product, Essential 9™, an over-the-counter tablet comprised of the nine (9) essential amino acids that the human body cannot make. Essential 9™ has been authorized for marketing by Health Canada under NPN 80089663.
On May 22, 2020, the Company filed a provisional patent application in the United States for a new treatment for Coronavirus infections. The Company’s patent application covers composition subject matter pertaining to small molecules for inhibition of the main Coronavirus protease, Mpro, an enzyme that is essential for viral replication. The patent application has a priority date of May 22, 2020. On April 30, 2021, the Company filed a PCT application containing new research results and extending coverage to include the Coronavirus Papain-Like protease, PLpro. The priority date of May 22, 2020 has been maintained in the newly filed PCT application.
On January 26, 2021, the Company received a Notice of Allowances from the Canadian Intellectual Property Office for a new patent application covering Adva-27a. The newly issued patent contains new subject matter and extends the proprietary protection of Adva-27a in Canada until 2033.
On March 9, 2021, the Company received a Notice of Allowance from the European Patent Office for a new patent application covering Adva-27a. The newly issued patent contains new subject matter and extends the proprietary protection of Adva-27a in Europe until 2033. The equivalent patent in the United States was issued in 2019 (US Patent Number 10,272,065).
On October 1, 2021, the Company filed a patent application for a potential new treatment for neurodegenerative disorders. The patent application contains experimental results showing that certain mRNA molecules provide protective effects against oxidative stress in differentiated neuronal cells, a process that mimics neuronal degeneration. This new patent application has a priority date of October 1, 2021.
On February 15, 2022, the Company entered into an underwriting agreement with Aegis Capital Corp. as underwriter, for the issuance and sale in an underwritten public offering of 1,882,353 Units, each consisting of one share of common stock and two warrants (“Tradeable Warrants”) to purchase shares of common stock at a public offering price of $4.25 per Unit for total gross proceeds of $8,000,000 (“Offering”). We also granted the underwriter a 45-day option to purchase additional shares of common stock and/or Tradeable Warrants in an amount equal up to 15% of the number of shares and Tradeable Warrants, respectively, sold in the Offering solely to cover overallotments, if any.
Also on February 15, 2022, the Company’s shares of common Stock and Tradeable Warrants began trading on Nasdaq under the ticker symbol “SBFM” for the common stock and “SBFMW” for the Tradeable Warrants.
| 7 |
On February 17, 2022, the Offering closed
and the Company received net proceeds of $
On February 18, 2022, the Company entered into a research agreement with the Arizona Board of Regents on behalf of the University of Arizona (the “University of Arizona”). Pursuant to the research agreement, the University of Arizona agreed to use reasonable efforts to perform a research project focused on determining the in vivo safety, pharmacokinetics, and dose selection properties of three University of Arizona owned PLpro inhibitors, followed by efficacy testing in mice infected with SARS-CoV-2, in consideration for certain milestone payments to be made by the Company. Under the agreement, the University of Arizona granted the Company a first option to negotiate for a commercial, royalty-bearing license for all intellectual property invented or authored by University of Arizona personnel under the research project.
On February 22, 2022, the Company redeemed shares of the Series B Preferred Stock from the CEO of the Company at a redemption price equal to the stated value of $0.10 per share.
On March 14, 2022, the
Company completed a private placement wherein the Company sold (i) shares of its Common Stock together with Investor Warrants to purchase up to shares of Common Stock, and (ii) pre-funded warrants (“Pre-Funded Warrants”) with each Pre-Funded Warrant exercisable for one share of Common Stock,
together with Investor Warrants to purchase up to 1,302,251 shares of Common Stock. Each share of Common Stock and accompanying Investor
Warrant were sold together at a combined offering price of $2.22, and each Pre-Funded Warrant and accompanying Investor Warrant were
sold together at a combined offering price of $2.219. The Company received approximately $8 million in gross proceeds, and $
In March 2020, the World Health Organization declared Coronavirus and its associated disease, COVID-19, a global pandemic. Conditions surrounding the Coronavirus outbreak have been and are continuing to evolve rapidly. Government authorities in the U.S. and around the world have implemented emergency measures to mitigate the spread of the virus. The outbreak and related mitigation measures have had and will continue to have a material adverse impact on the world economies and the Company's business activities. It is not possible for the Company to predict the duration or magnitude of the adverse conditions of the outbreak and their effects on the Company’s business or ability to raise funds. No adjustments have been made to the amounts reported in the Company's financial statements as a result of this matter.
Basis of Presentation of Unaudited Financial Information
The unaudited financial statements of the Company for the three month periods ended March 31, 2022 and 2021 have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial information and pursuant to the requirements for reporting on Form 10-Q and Regulation S-X. Accordingly, they do not include all the information and footnotes required by accounting principles generally accepted in the United States of America for complete financial statements. However, such information reflects all adjustments (consisting solely of normal recurring adjustments), which are, in the opinion of management, necessary for the fair presentation of the financial position and the results of operations. Results shown for interim periods are not necessarily indicative of the results to be obtained for a full fiscal year. The balance sheet information as of December 31, 2021 was derived from the audited financial statements included in the Company's financial statements as of and for the year ended December 31, 2021 included in the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on March 21, 2022. These financial statements should be read in conjunction with that report.
Reverse Stock Splits
Effective February 1, 2019, the Company completed
a
Effective April 6, 2020, the Company completed
another
Effective February 9, 2022, the Company completed
a
The Company's financial statements reflect the First, Second, and Third Reverse Stock Split on a retroactive basis for all periods presented and for all references to common stock, unless specifically stated otherwise.
Recently Issued Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12 “Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes.” This guidance removes certain exceptions to the general principles in Topic 740 and provides consistent application of U.S. GAAP by clarifying and amending existing guidance. The effective date of the new guidance for public companies is for fiscal years beginning after December 15, 2020 and interim periods within those fiscal years. Early adoption is permitted. The Company is currently evaluating the timing of adoption and impact of the updated guidance on its financial statements.
| 8 |
Note 2 – Notes Payable
The Company’s Notes Payable at December 31, 2021 consisted of the following:
On April 20, 2021, the Company received monies
in exchange for a Note Payable having a Face Value of $
On July 6, 2021, the Company received monies in
exchange for a Note Payable having a Face Value of $
On August 18, 2021, the Company received monies
in exchange for a Note Payable having a Face Value of $
As of March 31, 2022, the Company had
At March 31, 2022 and December 31, 2021, total
accrued interest on Notes Payable was $-
Note 3 – Shareholders’ Equity
On February 17, 2022, the Company’s public
offering closed and the Company received net proceeds of $
On February 22, 2022, the Company redeemed shares of Series B Preferred Stock from the CEO of the Company at a redemption price equal to the stated value of $ per share.
On March 14, 2022, the Company completed a private
placement and received gross proceeds of approximately $8 million before deducting transaction related expenses payable by the Company.
The net proceeds to the Company from this private placement were $
The Company declared
| 9 |
Note 4 – Warrants
The Company accounts for issued warrants either as a liability or equity in accordance with ASC 480-10 or ASC 815-40. Under ASC 480-10, warrants are considered a liability if they are mandatorily redeemable and they require settlement in cash, other assets, or a variable number of shares. If warrants do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to determine whether the warrants should be classified as a liability or as equity. Under ASC 815-40, contracts that may require settlement for cash are liabilities, regardless of the probability of the occurrence of the triggering event. Liability-classified warrants are measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the warrants after the issuance date is recorded in the consolidated statements of operations as a gain or loss. If warrants do not require liability classification under ASC 815-40, in order to conclude warrants should be classified as equity, the Company assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP standard. Equity-classified warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
During the three months ended March 31, 2022, the Company completed two financing events, and in connection therewith, it issued warrants as follows:
Warrants issued with financing
| TYPE | NUMBER | EXERCISE PRICE | EXPIRY DATE | |||||
| Pre-Funded Warrants | $ | |||||||
| Tradeable Warrants | $ | |||||||
| Investor Warrants | $ | |||||||
*The Tradeable Warrants had an initial exercise price of $4.25, subject to adjustment. Upon the closing of the Company’s private placement on March 14, 2022, the exercise price of the Tradeable Warrants was reduced to $2.22, in accordance with the terms thereof.
During the three months ended March 31, 2022,
a total of Tradeable Warrants were exercised resulting in aggregate proceeds of $
The Company’s outstanding warrants at March 31, 2022 consisted of the following:
Schedule of outstanding warrants
| TYPE | NUMBER | EXERCISE PRICE | EXPIRY DATE | |||||
| Pre-Funded Warrants | $ | |||||||
| Tradeable Warrants | $ | |||||||
| Investor Warrants | $ | |||||||
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of shares of common stock outstanding during the period, without consideration for common stock equivalents.
Diluted net loss per share is calculated by dividing the net loss by the weighted-average number of shares of common stock outstanding during the period, considering common stock equivalents.
| 10 |
In March 2022, the Company issued and sold Pre-Funded
Warrants to purchase 1,302,251 shares of common stock at a nominal exercise price of $0.001 per share (see Note 3). During the three months
ended March 31, 2022, none of these warrants were exercised. As of March 31, 2021, there are
In February 2022, the Company issued
Tradeable Warrants pursuant to the Company’s public offering (see Note 3). In March 2022, the Company issued Investor Warrants
in a private placement (see Note 3). Tradeable Warrants were exercised as of March 31, 2022, leaving
Note 6 – Management Compensation
The Company paid its Officers cash
compensation totaling $
Note 7 – Subsequent Events
On April 28, 2022, the Company completed a private placement with certain accredited institutional investors for aggregate gross proceeds of approximately $19.5 million. The Company received net proceeds of $16,752,917 from this private placement. In connection with the private placement, the Company issued and sold (i) 2,472,820 shares of its common stock, (ii) non-tradeable warrants to purchase up to 9,725,690 shares of common stock, and (iii) 2,390,025 pre-funded warrants with each pre-funded warrant exercisable for one share of common stock. Each share of common stock and accompanying two warrants were sold together at a combined offering price of $4.01, and each pre-funded warrant and accompanying two warrants were sold together at a combined offering price of $4.009. The warrants have an exercise price of $3.76 and a term equal to five years from the issuance date.
During April 2022, a total of 1,302,251 Pre-Funded Warrants, 2,768,055 Tradeable Warrants, and 2,802,703 Investor Warrants were exercised resulting in aggregate net proceeds of $12,368,385 received by the Company.
| 11 |
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion should be read in conjunction with our consolidated financial statements and notes thereto included herein. This discussion includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. The statements regarding Sunshine Biopharma, Inc. contained in this Report that are not historical in nature, particularly those that utilize terminology such as “may,” “will,” “should,” “likely,” “expects,” “anticipates,” “estimates,” “believes” or “plans,” or comparable terminology, are forward-looking statements based on current expectations and assumptions, and entail various risks and uncertainties that could cause actual results to differ materially from those expressed in such forward-looking statements. Important factors known to us that could cause such material differences are identified in this report and in our annual report on Form 10-K for the year ended December 31, 2021. We undertake no obligation to correct or update any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable law. You are advised, however, to consult any future disclosures we make on related subjects in future reports to the SEC.
Overview
We are a pharmaceutical and nutritional supplement company focusing on the research and development of proprietary drugs including our anti-cancer compound Adva-27a, and anti-coronavirus lead compound, SBFM-PL4. In addition, we are engaged in the development of specific mRNA molecules for cancer therapy.
We also, through our wholly owned Canadian subsidiary, Sunshine Biopharma Canada Inc. (“Sunshine Canada”), develop science-based nutritional supplements, and currently sell one nutritional supplement product, Essential 9tm.
Proprietary Drug Development Operations
SBFM-PL4 Anti-Coronavirus Treatment
The following is a summary of the development to date of our coronavirus treatment project:
| · | On May 22, 2020, we filed a patent application in the United States for a new treatment for Coronavirus infections. Our patent application covers composition subject matter pertaining to small molecules for inhibition of the Coronavirus main protease (Mpro) and papain-like protease (PLpro). Both enzymes are essential for viral replication. In addition to being involved in maturation of specific viral proteins, PLpro is responsible for suppression of the human immune system making the virus more virulent. The small molecules covered by the patent application were designed by Dr. Steve N. Slilaty, our chief executive officer. The patent application has a priority date of May 22, 2020. |
| · | In August 2020, we completed the synthesis of four different potential inhibitors of PLpro. These compounds are based on the technology described in our patent application filed on May 22, 2020. |
| · | In September 2020, we completed the screening of our four compounds and subsequently identified a lead Anti-Coronavirus drug candidate (SBFM-PL4). The screening which pinpointed the lead compound was performed at the University of Georgia, College of Pharmacy under the leadership of Dr. Scott D. Pegan, Director of the Center for Drug Discovery and Interim Associate Head of Pharmaceutical and Biomedical Sciences. |
| · | The next steps in our SBFM-PL4 drug development plan will involve conducting in vitro studies followed by cell culture assays and assessment in Coronavirus infected mice before entering human clinical trials. | |
| · | In February 2022, we expanded our search for additional PLpro inhibitors by entering into a research agreement with the University of Arizona. Pursuant to the research agreement, the University of Arizona agreed to perform a research project focused on determining the in vivo safety, pharmacokinetics, and dose selection properties of three University of Arizona owned PLpro inhibitors, followed by efficacy testing in mice infected with SARS-CoV-2. Under the research agreement, the University of Arizona granted us a first option to negotiate for a commercial, royalty-bearing license for all intellectual property invented or authored by University of Arizona personnel under the research project. |
| 12 |
Adva-27a Anticancer Drug
In the area of oncology, our proprietary drug development activities have been focused on the development of a small molecule called Adva-27a for the treatment of aggressive forms of cancer. A Topoisomerase II inhibitor, Adva-27a has been shown to be effective at destroying Multidrug Resistant Cancer cells including Pancreatic Cancer cells, Breast Cancer cells, Small-Cell Lung Cancer cells and Uterine Sarcoma cells (Published in ANTICANCER RESEARCH, Volume 32, Pages 4423-4432, October 2012). Sunshine Biopharma is direct owner of all issued patents pertaining to Adva-27a including U.S. Patents Number 8,236,935 and 10,272,065.
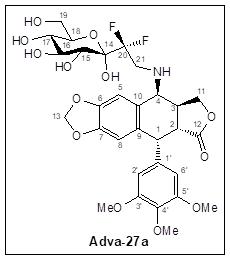
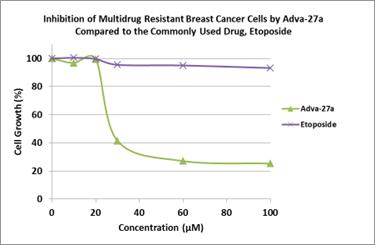
Adva-27a is a GEM-difluorinated C-glycoside derivative of Podophyllotoxin (see Figure 1). Another derivative of Podophyllotoxin called Etoposide is currently on the market and is used to treat various types of cancer including leukemia, lymphoma, testicular cancer, lung cancer, brain cancer, prostate cancer, bladder cancer, colon cancer, ovarian cancer, liver cancer and several other forms of cancer. Etoposide is one of the most widely used anticancer drugs. Adva-27a and Etoposide are similar in that they both attack the same target in cancer cells, namely the DNA unwinding enzyme, Topoisomerase II. Unlike Etoposide however, Adva-27a is able to penetrate and destroy Multidrug Resistant Cancer cells. In addition, Adva-27a has been shown to have distinct and more desirable biological and pharmacological properties compared to Etoposide. In side-by-side studies using Multidrug Resistant Breast Cancer cells and Etoposide as a reference, Adva-27a showed markedly greater cell killing activity (see Figure 2).
The next sequence of steps in our Adva-27a development program include:
| · | GMP Manufacturing of 2 kilograms for use in IND-Enabling Studies and Phase I Clinical Trials |
| · | IND-Enabling Studies |
| · | Regulatory Filing (Fast-Track status anticipated) |
| · | Phase I Clinical Trials (Pancreatic Cancer indication) |
| 13 |
Adva-27a’s initial indication will be pancreatic cancer for which there are currently little or no treatment options available. We are planning to conduct our clinical trials at McGill University’s Jewish General Hospital in Montreal, Canada. All aspects of the clinical trials in Canada will employ FDA standards at all levels.
According to the American Cancer Society, nearly 1.5 million new cases of cancer are diagnosed in the U.S. each year. While particularly effective against Multidrug Resistant Cancer, we believe Adva-27a can potentially treat all cancer types, particularly those in which Topoisomerase II has been amplified. We believe that upon successful completion of Phase I Clinical Trials we may receive one or more offers from large pharmaceutical companies to purchase or license our drug. However, there are no assurances that our Phase I Trials will be successful, or if successful, that any pharmaceutical companies will make an acceptable offer to us. In the event we do not consummate such a transaction, we will require significant capital in order to secure regulatory approval, manufacture and market our new drug on our own.
mRNA Molecules as Anti-Cancer Agents
In June 2021, we initiated a new research project in which we set out to determine if certain mRNA molecules can be used as anti-cancer agents. The data collected to date have shown that certain mRNA molecules are capable of destroying cancer cells in vitro including multidrug resistant breast cancer cells (MCF-7/MDR), ovarian adenocarcinoma cells (OVCAR-3), and pancreatic cancer cells (SUIT-2). Other studies using non-transformed (normal) human cells (HMEC cells) showed that these mRNA molecules had little cytotoxic effects. These new mRNA molecules are readily adaptable for delivery into patients using the mRNA vaccine technology. In April 2022, we filed a provisional patent application in the United States covering the subject mRNA molecules. We plan to commence mice xenograft studies within approximately the next twelve months.
Nutritional Supplements Operations
Our wholly owned Canadian subsidiary, Sunshine Canada, focuses on the development and marketing of science-based nutritional supplements. In December 2018, we completed the development of Essential 9™. On December 14, 2018, Health Canada issued NPN 80089663 through which it authorized us to manufacture and sell the Essential 9™ product. Our Essential 9™ nutritional supplement tablets contain a balanced formula of the 9 Essential Amino Acids that the human body cannot make. Essential Amino Acids are 9 out of the 20 amino acids required for protein synthesis. Proteins are involved in all body functions – From the musculature and immune system to hormones and neurotransmitters. Like vitamins, Essential Amino Acids cannot be made by the human body and must be obtained through diet. Deficiency in one or more of the 9 Essential Amino Acids can lead to loss of muscle mass, fatigue, weight gain and reduced ability to build muscle mass in athletes. Our Essential 9™ provides all 9 Essential Amino Acids in freeform and in the proportions recommended by Health Canada. Essential 9™ is currently available on Amazon.com and Amazon.ca. Figure 3 below shows our 60-Tablet Essential 9™ product.
In November 2019, we received Health Canada approval for another nutritional supplement, a new Calcium-Vitamin D tablet. Health Canada issued NPN 80093432 through which it authorized us to manufacture and sell the new Calcium-Vitamin D supplement under the brand name Essential Calcium-Vitamin D™. Vitamin D is a group of steroid-like molecules responsible for increasing intestinal absorption of calcium, magnesium, and phosphate. They are also involved in multiple other biological functions, including proper functioning of the immune system, promoting healthy growth of bone, and reduction of inflammation. The most important compounds in this group are ergocalciferol (Vitamin D2) and cholecalciferol (Vitamin D3). Sunshine Biopharma’s Essential Calcium-Vitamin D™ tablets contain both of these compounds as well as calcium for optimum health benefits. We are considering potentially launching this product in 2022.
| 14 |
We are also developing additional nutritional supplement products. We may launch additional nutritional supplement products within approximately 1-2 years.
Results of Operations
Comparison of results of operations for the three months ended March 31, 2022 and 2021
During the three months ended March 31, 20212, we generated $122,645 in revenues, compared to $40,058 for the three months ended March 31, 2021, an increase of $82,587. The increase is attributable to a slightly widened advertising program. All of these revenues were generated from our science-based nutritional supplements operations. The direct cost for generating these revenues was $59,845 for the three months ended March 31, 2022 (48.8%), compared to $18,520 (46.2%) for the three months ended March 31, 2021. The increase in the cost of goods sold in 2022 is due to increased manufacturing cost. Our gross profit increased to $62,800 for the three months ended March 31, 2022, compared to a gross profit of $21,538 for the same period in 2021.
General and administrative expenses during the three month period ended March 31, 2022 were $1,286,164 compared to $1,297,184 during the three month period ended March 31, 2021, a decrease of $10,420. Overall, we incurred a loss of $1,223,364 from our operations in the three month period ended March 31, 2022, compared to a loss from operations of $1,275,646 in the similar period of 2021.
In addition, we incurred $12,864 in interest expense during the three months ended March 31, 2022, compared to $49,711 in interest expense during the similar period in 2021. We incurred no losses related to debt conversion during the three months ended March 31, 2022, compared to $4,910,786 in losses arising from debt conversion during the three months ended March 31, 2021. This was due to the fact that all of our outstanding debt was paid during the quarter ended March 31, 2022 prior to the occurrence of any debt conversion events.
As a result, we incurred a net loss of $1,236,234 for the three month period ended March 31, 2022, compared to a net loss of $6,185,126 for the three month period ended March 31, 2021.
Liquidity and Capital Resources
As of March 31, 2022, we had cash or cash equivalents of $13,177,625.
Net cash used in operating activities was $1,304,208 during the three months ended March 31, 2022, compared to $297,355 during the three month period ended March 31, 2021. The increase was a result of increased business activities including expenses related to the two financing transactions completed during the quarter ended March 31, 2022.
Cash flows provided by financing activities were $12,437,673 for the three months ended March 31, 2022, compared to $1,102,000 for the three month periods ended March 31, 2021. The increase was a result of the two financing transactions completed on February 17 and March 14, 2022.
Cash flows used in investing activities were $0 for the three months ended March 31, 2022, compared to $0 for the three month period ended March 31, 2021.
We are not generating adequate revenues from our operations to fully implement our business plan as set forth herein. On February 17, 2022, we received net proceeds of approximately $6.8 million from the sale of common stock and warrants in an underwritten public offering. On March 14, 2022, we received net proceeds of approximately $6.8 million from the sale of common stock and warrants in a private placement. On April 28, 2022, we received net proceeds of approximately $16.8 million from the sale of common stock and warrants in a private placement. We believe our existing cash will be sufficient to fund our operations, including general and administrative expenses, expanded research and development activities, and nutritional supplement business, for the next 24 months. There is no assurance our estimates will be accurate. We have no committed sources of capital and we anticipate that we will need to raise additional capital in the future, including for further research and development activities and possibly clinical trials. Additional capital may not be available on terms acceptable to us, or at all.
| 15 |
Critical Accounting Policies and Estimates
Critical Accounting Estimates
The discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
For a detailed list of significant accounting policies, please see our annual report on Form 10-K for the fiscal year ended December 31, 2021, including our financial statements and notes thereto included therein as filed with the SEC on March 21, 2022,
Recently Adopted Accounting Standards
In February 2020, the FASB issued ASU 2020-02, Financial Instruments-Credit Losses (Topic 326) and Leases (Topic 842) - Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 119 and Update to SEC Section on Effective Date Related to Accounting Standards Update No. 2016-02, Leases (Topic 842) which amends the effective date of the original pronouncement for smaller reporting companies. ASU 2016-13 and its amendments will be effective for the Company for interim and annual periods in fiscal years beginning after December 15, 2022. The Company believes the adoption will modify the way the Company analyzes financial instruments, but it does not anticipate a material impact on results of operations. The Company is in the process of determining the effects adoption will have on its consolidated financial statements.
In August 2020, the FASB issued ASU 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815 – 40), (“ASU 2020-06”). ASU 2020-06 simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. The ASU2020-06 amendments are effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. The Company is evaluating the impact of this guidance on its unaudited consolidated financial statements.
Off Balance-Sheet Arrangements
None
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company and are not required to provide the information under this item.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act as of the end of the period covered by this report.
| 16 |
These controls are designed to ensure that information required to be disclosed in the reports we file or submit pursuant to the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission, and that such information is accumulated and communicated to our management, including our CEO and CFO, to allow timely decisions regarding required disclosure.
Based on this evaluation, our management, including our CEO and CFO, concluded that our disclosure controls and procedures were not effective as of March 31, 2022, at reasonable assurance level, for the following reasons:
| · | ineffective control environment and lack of qualified full-time CFO who has SEC experience to focus on our financial affairs; | |
| · | lack of qualified and sufficient personnel, and processes to adequately and timely identify making any and all required public disclosures; | |
| · | deficiencies in the period-end reporting process and accounting policies; | |
| · | inadequate internal controls over the application of new accounting principles or the application of existing accounting principles to new transactions; | |
| · | inadequate internal controls relating to the authorization, recognition, capture, and review of transactions, facts, circumstances, and events that could have a material impact on the company’s financial reporting process; | |
| · | deficient revenue recognition policies; | |
| · | inadequate internal controls with respect to inventory tracking and transactions; and | |
| · | improper and lack of timely accounting for accruals such as prepaid expenses, accounts payable and accrued liabilities. |
The Company is addressing the ineffective controls, including through the following steps:
| · | The Company added independent directors in the fourth quarter of 2021 and the first quarter of 2022. | |
| · | The Company has additional financial resources, including funds received through a public offering and a private placement completed in the first quarter of 2022, to enable the hiring of additional personnel that will result in a separation of duties going forward. | |
| · | The Company established an independent Audit Committee in the first quarter of 2022. |
Additionally, the Board of Directors will work with management to continuously review controls and procedures to identified deficiencies and implement remediation within our internal controls over financial reporting and our disclosure controls and procedures.
We believe that our financial statements presented in this report fairly present, in all material respects, our financial position, results of operations, and cash flows for all periods presented herein.
Changes in Internal Control Over Financial Reporting
Except as set forth above, there were no changes in our internal control over financial reporting during the quarter ended March 31, 2022, which were identified in conjunction with management’s evaluation required by paragraph (d) of Rules 13a-15 and 15d-15 under the Exchange Act, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| 17 |
PART II. OTHER INFORMATION
Item 1. Legal Proceedings.
We are not party to, and our property is not the subject of, any material legal proceedings.
Item 1A. Risk Factors.
We are a smaller reporting company and are not required to provide the information under this item.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not Applicable.
Item 5. Other Information.
None.
Item 6. Exhibits.
| Exhibit No. | Description | |
| 31.1 | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002* | |
| 31.2 | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2022* | |
| 32.1 | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002** | |
| 101 | Inline XBRL Document Set for the financial statements and accompanying notes in Part I, Item 1, of this Quarterly Report on Form 10-Q.* | |
| 104 | Inline XBRL for the cover page of this Quarterly Report on Form 10-Q, included in the Exhibit 101 Inline XBRL Document Set.* |
| * | Filed herewith. |
| ** | Furnished herewith. |
| 18 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized, on May 6, 2022.
| SUNSHINE BIOPHARMA, INC. | |||
| By: | /s/ Dr. Steve N. Slilaty | ||
| Dr. Steve N. Slilaty | |||
| Chief Executive Officer (principal executive officer) | |||
| By: | /s/ Camille Sebaaly | ||
|
Camille Sebaaly Chief Financial Officer (principal financial and accounting officer) |
|||
| 19 |