UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the fiscal year ended December 31, 2018
For the transition period from __________to __________
Commission file number 001-35887
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) | |
(Address of principal executive offices)
(Zip Code)
(770 ) 651-9100
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None.
Title of each class | Trading Symbol | Name of each exchange on which registered |
N/A | N/A | N/A |
Securities registered pursuant to Section 12(g) of the Act:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ¨ No þ
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§223.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ¨ No þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | ☑ | |
Non-accelerated filer | ☐ | Smaller reporting company | |
Emerging growth company | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No þ
The aggregate market value of the registrant’s voting common equity held by non-affiliates of the registrant as of June 28, 2019 (the last business day of the registrant’s most recently completed second quarter) was approximately $425.1 million based upon the last sale price ($4.05) of the shares as reported on the OTC Pink Market on such date.
There were 110,545,275 shares of the registrant’s common stock, par value $0.001 per share, outstanding as of March 3, 2020.
Documents Incorporated By Reference
None.
Table of Contents
Item | Description | Page |
Explanatory Note | ||
Part I | ||
Item 1. | Business | |
Item 1A. | Risk Factors | |
Item 2. | Properties | |
Item 3. | Legal Proceedings | |
Item 4. | Mine Safety Disclosures | |
Part II | ||
Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | |
Item 6. | Selected Financial Data | |
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | |
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | |
Item 8. | Financial Statements and Supplementary Data | |
Item 9. | Changes in Disagreements with Accountants on Accounting and Financial Disclosure | |
Item 9A. | Controls and Procedures | |
Item 9B. | Other Information | |
Part III | ||
Item 10. | Directors, Executive Officers and Corporate Governance | |
Item 11. | Executive Compensation | |
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |
Item 13. | Certain Relationships and Related Transactions, and Director Independence | |
Item 14. | Principal Accounting Fees and Services | |
Part IV | ||
Item 15. | Exhibits, Financial Statement Schedules | |
Item 16. | Form 10-K Summary | |
Signatures | ||
EXPLANATORY NOTE
As used herein, the terms “MiMedx,” “the Company,” “we,” “our” and “us” refer to MiMedx Group, Inc., a Florida corporation, and its consolidated subsidiaries, except where it is clear that the terms mean only MiMedx Group, Inc.
Financial Information Included in This Form 10-K
This Annual Report on Form 10-K for the year ended December 31, 2018 (this “Form 10-K”) is the first periodic report that MiMedx has filed since June 2018, when the Audit Committee, with the concurrence of management, concluded that the Company’s previously issued consolidated financial statements and financial information relating to each of the fiscal years ended December 31, 2016, 2015, 2014, 2013 and 2012 and each of the interim periods within such years, along with the unaudited condensed consolidated financial statements included in the Company’s Quarterly Reports on Form 10-Q for the quarters ended March 31, 2017, June 30, 2017 and September 30, 2017 (collectively, the “Non-Reliance Periods”), would need to be restated and could no longer be relied upon due to accounting irregularities regarding the recognition of revenue under generally accepted accounting principles in the United States of America (“GAAP”).
This Form 10-K contains our audited consolidated statements of operations, stockholders’ equity and cash flows for the years ended December 31, 2018 and 2017, which have not previously been filed, and for the year ended December 31, 2016, which have been restated from the consolidated financial statements previously filed in our Annual Report on Form 10-K for the year ended December 31, 2016. This Form 10-K also includes our audited consolidated balance sheets as of December 31, 2018 and 2017.
In addition, this Form 10-K includes selected unaudited condensed consolidated financial data as of, and for the years ended, December 31, 2015 (Restated) and 2014 (Restated), which reflect adjustments to our previously filed consolidated financial statements as of and for the years ended December 31, 2015 and 2014. Refer to Item 6, “Selected Financial Data” for information regarding the applicable adjustments or restatements of our financial results for 2016, 2015 and 2014. Refer to Note 4, “Restatement of Consolidated Financial Statements” for information regarding the applicable adjustments and restatement of our consolidated stockholders’ equity as of January 1, 2016.
In addition, this Form 10-K includes certain unaudited information related to fiscal year 2019 to provide necessary context for readers regarding the direction of the business.
We have not filed and do not intend to file amendments to any periodic reports filed for any of the Non-Reliance Periods. Instead, we are restating and correcting only the consolidated statements of operations and cash flows for the year ended December 31, 2016, the consolidated balance sheet as of December 31, 2016 and the selected financial data for the years ended December 31, 2015 and 2014 that are included in this Form 10-K in Item 6, “Selected Financial Data.” In addition, we have not filed, and do not intend to file, a separate Annual Report on Form 10-K for the year ended December 31, 2017 or Quarterly Reports on Form 10-Q for the periods ended March 31, June 30 or September 30, 2018 and 2019, respectively. We intend to include quarterly financial statements for the periods ended March 31, June 30 and September 30, 2019 and 2018 in our Annual Report on Form 10-K for the year ending December 31, 2019, but not file Quarterly Reports on Form 10-Q for the periods ended March 31, June 30 or September 30, 2019.
Audit Committee Investigation
In February 2018, the Audit Committee (the “Audit Committee”) of the Company’s Board of Directors (the “Board”) retained King & Spalding LLP (“King & Spalding”) as counsel to the Audit Committee to assist in conducting an independent investigation into current and prior-period matters relating to allegations regarding certain sales and distribution practices at the Company and certain other matters (the “Investigation” or the “Audit Committee Investigation”). Following its engagement by the Audit Committee, King & Spalding retained KPMG LLP (“KPMG”) to assist with the Investigation.
The Investigation focused primarily on the following areas: (1) the Company’s revenue recognition practices; (2) revenue management activities; (3) actions taken against whistleblowers; (4) tone set by former senior management and (5) Anti-Kickback Statute and related allegations.
In connection with the Investigation, King & Spalding and KPMG reviewed over 1.5 million documents including, but not limited to, emails, text exchanges and other electronic and hard-copy records. In addition, they reviewed significant amounts of data housed in the Company’s accounting, customer relationship management, inventory and other systems. They also reviewed over 2,750 hours of video derived from a secret video surveillance system installed at the direction of Parker H. Petit, the Company’s former Chairman and Chief Executive Officer, as well as telephonic recordings captured without the consent of all conversation
1
participants. King & Spalding and KPMG interviewed over 85 witnesses, many of them multiple times. The Audit Committee held 84 meetings during the course of the Investigation.
In a Form 8-K dated June 6, 2018, the Audit Committee, with concurrence from management, concluded that the Company’s previously-issued consolidated financial statements and financial information relating to the Non-Reliance Periods should be restated (the “Restatement”), and therefore, such consolidated financial statements and other financial information, any press releases, investor presentations, or other communications thereto should no longer be relied upon.
Findings of the Investigation
As a result of the Investigation and based upon their review and assessment of the evidence, King & Spalding and KPMG made a number of findings, which were presented to and accepted and adopted by the Audit Committee. The evidence includes, but is not limited to, the following:
Non-Reliance on Financial Statements
First, the Investigation revealed accounting irregularities regarding the recognition of revenue under GAAP. The Audit Committee, with the concurrence of management, concluded that the Company’s previously issued consolidated financial statements and financial information relating to each of the fiscal years ended December 31, 2016, 2015, 2014, 2013 and 2012 and each of the interim periods within such years, along with the unaudited condensed consolidated financial statements included in the Company’s Quarterly Reports on Form 10-Q for the quarters ended March 31, 2017, June 30, 2017 and September 30, 2017, would need to be restated. The determination of the need to restate was based on the findings as of June 2018 presented to the Audit Committee, which were primarily focused on the accounting treatment afforded to the sales and distribution practices with respect to two distributors. The evidence demonstrated that former members of senior management employed certain implicit arrangements, which resulted in a course of dealing that superseded the explicit terms of the contracts, and that the Company improperly recognized revenue from these two distributors.
Former Members of Management Disregarded Revenue Recognition Rules under GAAP
Second, the Investigation found evidence that demonstrated, among other things, that former members of senior management, including Mr. Petit, the Company’s former Chief Operating Officer, William C. Taylor, the Company’s former Chief Financial Officer, Michael J. Senken, and the Company’s former Controller, John Cranston, were aware of the Company’s course of dealing with its largest distributor and that this course of dealing was inconsistent with the explicit terms of the contract. Former members of senior management were also aware that this course of dealing included detailed procedures, established as early as 2012, to determine when the distributor would pay for the Company’s products.
In connection with these procedures, the distributor sent the Company a daily written report listing each tissue that the distributor’s customer had just purchased from the distributor and for which the customer would soon be paying the distributor. Each week the distributor would remit scheduled payments to the Company for only those tissues that the distributor’s customer had previously purchased. The Company tracked and monitored these daily reports and reconciled the payments that the Company received from the distributor to the tissues purchased by the distributor’s customers (compiled from the daily reports).
Weekly summaries of this reconciliation process were distributed to various Company personnel, including members of the Finance and Accounting group. This reconciliation process demonstrated that payment by the distributor to the Company was predicated on purchases made by the distributor’s customer. This payment process, which was housed outside the Company’s Finance and Accounting group and not disclosed to the Company’s financial statement auditors, was a key fact in determining that the Company’s revenue recognition was improper under GAAP and that the Company needed to restate its financials, as described above.
The evidence further demonstrated that these executives were aware of the proper revenue recognition rules not later than January 2016 and were likewise aware that the course of dealing affected the way in which the Company should have properly recognized revenue.
Other Revenue Management Activities at the Company
Third, the Investigation uncovered other conduct that appears to have been designed to manipulate the timing and recognition of revenue. This conduct included:
• | a distributor was given a lucrative consulting agreement simultaneous with a large purchase near the end of a reporting period; |
2
• | instances of intentionally shipping types and volumes of product that were not needed by the customer and recording revenue, typically near the end of a reporting period, and facilitating such sales by agreeing at the time of shipment to allow customers to return or exchange these products in subsequent accounting periods without recording specific provisions for such return or exchanges; |
• | the booking of a large end of quarter sale to a distributor that the Company was in the process of acquiring and for which the Company never received payment; |
• | several “side deals” with distributors and other customers, whereby the purchasers agreed to take product but were not required to pay for the product until the purchasers were successful in re-selling the product; however, the Company recorded revenue at the time of shipment rather than when the purchasers were obligated to pay, which was inconsistent with GAAP; and |
• | in at least one instance, Mr. Taylor concealed such a side deal from the Company’s Finance and Accounting group. In late 2015, Mr. Taylor forwarded to Messrs. Senken and Cranston a significant purchase order from an international distributor that provided for 180-day payment terms. Shortly after doing so, Mr. Taylor sent the distributor an email stating that if the distributor was unable to resell the product as expected, MiMedx would grant extended payment terms, assist the distributor with reselling the product or repurchase the product from the distributor. Mr. Taylor did not inform Messrs. Senken or Cranston about this side deal, and as a result MiMedx improperly recognized $2.5 million in revenue from this sale near the end of the fourth quarter of 2015. |
As a result of these and related activities, the Company recognized revenues in the wrong accounting periods, and in certain instances, improperly recognized revenue altogether. In certain of the situations outlined above, the timing and improper recognition of revenue allowed the Company to meet its published guidance. Absent these apparent revenue management activities, the Company’s results would have fallen short of guidance in these periods.
Material Misstatements and Omissions to Several Key Stakeholders and Regulators
Fourth, the Investigation found that the evidence demonstrated that after questions began to be raised regarding the Company’s accounting practices, Messrs. Petit, Taylor, Senken and Cranston made material misstatements and omissions about the Company’s course of dealing with its largest distributor, as well as the Company’s corresponding revenue recognition practices, to a number of key stakeholders and regulators, including the Division of Corporation Finance of the U.S. Securities and Exchange Commission (the “SEC”), the Board, the Audit Committee and the Company’s outside auditors. These included:
• | After Mr. Cranston’s predecessor questioned the Company’s accounting for revenue from its largest distributor, Messrs. Petit, Taylor, Senken and Cranston did not disclose to the Audit Committee or the Company’s outside auditors that the Company routinely issued credits to the distributor for lost, damaged or missing tissues, nor did they disclose that the distributor only paid the Company for a tissue after it had sold that tissue to its customer. |
• | On multiple occasions, Messrs. Petit, Senken and Cranston signed letters to the Company’s outside auditors misrepresenting that the Company had no side deals or other arrangements that had not been disclosed to the outside auditors. |
• | In November 2016, after two former employees alleged that the Company had engaged in channel stuffing and improper revenue recognition practices, Messrs. Petit and Senken signed a letter to the Company’s outside auditors misrepresenting that they had no knowledge of any allegations of fraud affecting the Company made by current or former employees. |
• | In early 2017, after the Audit Committee had retained counsel to investigate the allegations made by these former employees, Mr. Petit forwarded to the Board a set of written responses in which counsel for the Company’s largest distributor explicitly stated that it only paid the Company for tissues after receiving payment from the distributor’s customer. Mr. Petit misled the Board about the accuracy of the information provided by the distributor’s counsel. |
• | Also in early 2017, the Company retained an outside expert to opine on the appropriateness of the Company’s recognition of revenue from sales to its largest distributor. Messrs. Petit, Senken and Cranston made misrepresentations to the expert concerning the actual course of dealing between the Company and its largest distributor. |
• | In early 2017, in letters signed by Mr. Senken, the Company responded to comment letters received from the SEC’s Division of Corporation Finance by misrepresenting that the Company’s largest distributor was obligated to pay the Company, regardless of whether the distributor resold the product. As noted above, the Company routinely issued credits |
3
to the distributor for lost, damaged and missing tissues and received payments from the distributor based on the tissues purchased by the distributor’s customer.
• | In early 2018, the Company’s former senior management prepared a misleading memorandum to the Company’s outside auditors that misrepresented key facts regarding the Company’s historical relationship with its largest distributor, which were relevant to determining the appropriate revenue recognition under GAAP. |
• | During a deposition, Mr. Petit falsely testified under oath that it was not true that the Company’s largest distributor only paid the Company after the distributor had received a purchase order from its customer. |
Actions Taken Against Whistleblowers
Further, the Investigation determined that the evidence demonstrated that Messrs. Petit and Taylor engaged in a pattern of taking action against employees who raised concerns about the Company’s practices, without conducting a thorough investigation of those concerns. Instead, Messrs. Petit and Taylor focused on disputing the employees’ allegations and on seeking to discredit or find wrongdoing by the persons raising the concerns that would justify re-assignment, discipline or termination. For example, after certain employees made allegations of improper accounting practices in late 2016, Mr. Petit directed and oversaw an internal investigation dubbed “Project Snow White” that focused on potential wrongdoing by these employees, rather than the merits of their allegations. As part of Project Snow White, the secret video surveillance system referenced above was installed at Mr. Petit’s direction to record interviews that he, Mr. Taylor and other former members of management conducted of certain employees and those employee’s discussions amongst themselves without those employees’ knowledge or consent. The evidence showed that Mr. Petit directed that certain employees, whom he and other former members of senior management perceived to hold loyalty to an employee who had raised concerns about the Company’s practices, be terminated.
Tone Set by Former Senior Management
Finally, the Investigation found that based on former members of senior management’s involvement in the findings outlined above, the evidence demonstrated that these individuals set an inappropriate “tone at the top.” The evidence identified a recurrent trend in which former senior management emphasized short-term business goals over compliance and ethics, was not receptive to employee concerns and failed to respond appropriately to compliance issues. In particular, the Investigation’s findings on poor tone set by former senior management included evidence demonstrating:
• | Former senior management disregarded revenue recognition rules under GAAP and directed others to take actions that caused the Company to take actions that caused the Company to improperly recognize revenue under GAAP, which was a key factor in the Audit Committee concluding it was necessary to restate the Company’s financials, as described above. |
• | Former senior management was involved in conduct that appears to have been designed to manipulate the timing and recognition of revenue - in some instances where the improper recognition of revenue allowed the Company to meet its published guidance. |
• | After questions began to be raised regarding the Company’s accounting practices, former senior management made material misstatements and omissions to a number of key stakeholders and regulators, including the SEC’s Division of Corporation Finance, the Board, the Audit Committee and the Company’s outside auditors. |
• | Former senior management engaged in a pattern of taking action against employees who raised concerns about the Company’s practices. |
• | Former senior management overrode internal controls that otherwise might have mitigated certain issues identified in the Investigation. These included former senior management personally overseeing, outside of the Company’s normal control processes, the Company’s relationships with certain health care providers. |
• | Former senior management marginalized the Company’s legal and accounting departments and outside legal and accounting advisors, by dismissing or ignoring professional advice, withholding information from legal and accounting advisors necessary to appropriately exercise professional judgments and determinations and excluding senior legal and accounting personnel from regular senior management meetings. |
4
Anti-Kickback Statute and Related Allegations
From September 2018 through May 2019, the Audit Committee devoted significant time to investigating, with the assistance of King & Spalding and KPMG, allegations that the Anti-Kickback Statute may have been violated by the Company in its relationships with various physicians, customers and distributors. These efforts included the analysis of certain specific customer relationships, the review of the conduct of the Company’s sales team’s management and the evaluation of the adequacy and effectiveness of the Company’s compliance controls.
As part of these efforts, King & Spalding and KPMG performed targeted data analytics of financial and other data related to the Company’s customer base, reviewed email and other records and conducted numerous interviews. Among other things, King & Spalding and KPMG examined more than 80 physician and customer relationships in detail and conducted over 40 interviews of current and former Company personnel in connection with these relationships, some on multiple occasions.
Through this process, the Investigation identified certain customer accounts that presented potential compliance risks and warranted additional review. This additional review was completed by Company counsel in consultation with management to determine the Company’s legal risk, and among other things confirmed that no loss contingencies should be recognized or disclosed under GAAP, and is now complete.
Weaknesses in Internal Control
The Audit Committee Investigation and our review and assessment also identified various material weaknesses in internal control, including in our entity level controls and in certain accounting practices, all as described under Item 9A, “Controls and Procedures” in this Form 10-K. For further information regarding the specific adjustments resulting from the Investigation, refer to Item 6, “Selected Financial Data” in this Form 10-K.
SEC Investigation
In November 2019, the SEC brought charges against the Company and the Company’s former officers Parker H. Petit, Michael J. Senken, and William C. Taylor. The Company cooperated with the SEC’s investigation. The Company agreed to settle with the SEC, without admitting or denying the SEC’s allegations, by consenting to the entry of a final judgment that permanently restrains and enjoins the Company from violating certain provisions of the federal securities laws. As part of the resolution, the Company also paid a penalty of $1.5 million. The settlement concluded, as to the Company, the matters alleged by the SEC in its complaint. See Item 3, “Legal Proceedings–Investigations.”
PART I
As used herein, the terms “MiMedx,” “the Company,” “we,” “our” and “us” refer to MiMedx Group, Inc., a Florida corporation, and its consolidated subsidiaries as a combined entity, except where it is clear that the terms mean only MiMedx Group, Inc.
Forward-Looking Statements
This Form 10-K contains forward-looking statements. All statements relating to events or results that may occur in the future are forward-looking statements, including, without limitation, statements regarding the following:
• | our strategic focus, as illustrated by our strategic priorities and our ability to implement these priorities; |
• | our ability to access capital sufficient to implement our strategic priorities; |
• | our expectations regarding our ability to fund our ongoing and future operating costs; |
• | our expectations regarding future income tax liability; |
• | the advantages of our products and development of new products; |
• | market opportunities for our products; |
• | the regulatory pathway for our products, including the design and success of our clinical trials and pursuit of BLAs (as defined below) for certain products; |
• | our expectations regarding our ability to manufacture certain of our products in compliance with current Good Manufacturing Practices (“cGMP”); |
5
• | our expectations regarding costs relating to compliance with regulatory standards, including those arising from our clinical trials, pursuit of BLAs, and cGMP compliance; |
• | our ability to continue marketing our micronized, injectable products and certain other products during and following the end of the period of enforcement discretion announced by the United States Food and Drug Administration (“FDA”); |
• | expectations regarding government and other third-party coverage and reimbursement for our products; |
• | expectations regarding future revenue growth; |
• | our belief in the sufficiency of our intellectual property rights in our technology; |
• | our ability to procure sufficient supplies of human tissue to manufacture and process our products; |
• | the outcome of pending litigation and investigations; |
• | our ability to complete remedial actions to address all observations in the Forms FDA 483 issued to us by the FDA; |
• | our ability to regain and remain in compliance with SEC reporting obligations; |
• | our ability to relist our Common Stock on The Nasdaq Capital Market in connection with becoming current in our SEC reporting obligations; |
• | ongoing and future effects arising from the Audit Committee Investigation, the Restatement, the SEC investigation, and related litigation; |
• | demographic and market trends; |
• | our expectations regarding future compliance with our debt obligations, including under the Term Loan Agreement (as described below); |
• | our plans to remediate the identified material weaknesses in our internal control environment and to strengthen our internal control environment; and |
• | our ability to compete effectively. |
Forward-looking statements generally can be identified by words such as “expect,” “will,” “change,” “intend,” “seek,” “target,” “future,” “plan,” “continue,” “potential,” “possible,” “could,” “estimate,” “may,” “anticipate,” “to be” and similar expressions. These statements are based on numerous assumptions and involve known and unknown risks, uncertainties and other factors that could significantly affect the Company’s operations and may cause the Company’s actual actions, results, financial condition, performance or achievements to differ materially from any future actions, results, financial condition, performance or achievements expressed or implied by any such forward-looking statements. Factors that may cause such a difference include, without limitation, those discussed under the heading “Risk Factors” in this Form 10-K.
Unless required by law, the Company does not intend, and undertakes no obligation, to update or publicly release any revision to any forward-looking statements, whether as a result of the receipt of new information, the occurrence of subsequent events, a change in circumstances or otherwise. Each forward-looking statement contained in this Form 10-K is specifically qualified in its entirety by the aforementioned factors. Readers are advised to carefully read this Form 10-K in conjunction with the important disclaimers set forth above prior to reaching any conclusions or making any investment decisions and not to place undue reliance on forward-looking statements, which apply only as of the date of the filing of this Form 10-K with the SEC.
6
Item 1. Business
Overview
MiMedx is an industry leader in advanced wound care and an emerging therapeutic biologics company, developing and distributing placental tissue allografts with patent-protected processes for multiple sectors of healthcare. We derive our products from human placental tissues processed using our proprietary processing methodologies, including the PURION® process. We employ aseptic processing techniques in addition to terminal sterilization to produce our allografts. MiMedx provides products in the wound care, burn, surgical, orthopedic, spine, sports medicine, ophthalmic, and dental sectors of healthcare. Our mission is to offer physicians products and tissues to help the body heal itself. All of our products are regulated by the FDA.
MiMedx is the leading supplier of human placental allografts, which are human tissues that are transplanted from one person (a donor) to another person (a recipient). MiMedx has supplied over 1.8 million allografts, through both direct and consignment shipments. Our biomaterial platform technologies include AmnioFix®, EpiFix®, EpiCord®, AmnioCord® and AmnioFill®. AmnioFix and EpiFix are our tissue allografts derived from the amnion and chorion layers of the human placental membrane. EpiCord and AmnioCord are tissue allografts derived from umbilical cord tissue. AmnioFill is a placental connective tissue matrix, derived from the placental disc and other placental tissue.
Our EpiFix and EpiCord product lines are promoted for external use, such as in advanced wound care applications, while our AmnioFix, AmnioCord and AmnioFill products are positioned for use in surgical applications, including lower extremity repair, plastic surgery, vascular surgery and multiple orthopedic repairs and reconstructions. We describe these in greater detail below under the heading “Our Product Portfolio.”
2017 FDA Guidance. The products we sell are regulated by the FDA. Historically, we marketed our products as Human Cells, Tissues and Cellular and Tissue – Based Products (“HCT/Ps”), which do not require pre-market clearance or approval by the FDA and are subject solely to Section 361 of the Public Health Service Act (“Section 361”) and related regulations. However, in November 2017 the FDA published a series of related guidances, including one entitled “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue–Based Products: Minimal Manipulation and Homologous Use – Guidance for Industry and Food and Drug Administration Staff” (the “Guidance”), that established an updated framework for the FDA’s regulation of cellular and tissue-based products. Among other things, the guidances clarified the FDA’s views about the criteria that differentiate those products subject to regulation solely under Section 361 (“Section 361 HCT/Ps”) from those cellular and tissue-based products that are considered to be drugs and biological products (“Section 351 HCT/Ps”) subject to licensure under Section 351 of the Public Health Service Act (“Section 351”) and related regulations. As described below and elsewhere in this Form 10-K, the guidances clarified the FDA’s expectation that certain products such as those that MiMedx has long marketed as Section 361 HCT/Ps will be treated as Section 351 HCT/Ps moving forward. The Guidance also confirmed that amniotic membrane in sheet form generally can be characterized as “minimally manipulated” and therefore regulated solely under Section 361.
Effect on Our Products. Under the FDA Guidance, we expect that the FDA will continue to regulate our amniotic membrane sheet products (AmnioFix, EpiFix, EpiBurn and EpiXL) and umbilical cord products (EpiCord and AmnioCord) as Section 361 HCT/Ps so long as the claims we make for them are consistent with the Section 361 framework. We expect, however, that the FDA will regulate certain of our other products, such as our micronized, injectable products (AmnioFix Injectable and EpiFix Micronized) under Section 351 as biological products. Other products, like AmnioFill, could also be regulated as biological products under the Section 351 regulations.
Enforcement Discretion. The Guidance stated that the FDA intends to exercise enforcement discretion under limited conditions with respect to the investigative new drug (“IND”) application and pre-market approval requirements for certain HCT/Ps through November 2020. This means that, through November 2020, the FDA does not intend to enforce certain provisions as they currently apply to certain entities or activities. The FDA intended this period of enforcement discretion to give sponsors time to evaluate their products, have a dialogue with the agency and, if necessary, begin clinical trials and file the appropriate pre-market applications to transition products that had been marketed as Section 361 HCT/Ps into compliance with Section 351. The FDA’s approach is risk-based, and the Guidance clarified that high-risk products and uses might be subject to immediate enforcement action.
During the Period of Enforcement Discretion. We have continued to market our micronized, injectable products under this policy of enforcement discretion, while at the same time pursuing a Biologics License Application (“BLA”) for certain of our micronized products.
We have already filed INDs for three indications for our micronized, injectable product: plantar fasciitis, osteoarthritis knee pain, and Achilles tendonitis. We may file additional INDs, but our near-term plan is to focus on these indications. Further, as we previously announced, we will need more time than we originally anticipated to file our BLAs with the FDA, and clinical trial
7
protocol amendments and enhancements, further resources, and additional capabilities and expertise will be required. See “Clinical Trials” below for information regarding the revised timelines.
We have also begun investing in additional plant and equipment and compliance personnel to allow us to manufacture and market in accordance with Section 351 requirements at scale. Among other things, this has required us to make capital expenditures in 2019, which will continue in 2020. See discussion below – “Risk Factors” under the heading “If any of the BLAs are approved, the Company would be subject to additional regulation which will increase costs and results in adverse sanctions for non-compliance.”
Efforts to Seek Extension of Enforcement Discretion Period. MiMedx may request an extension of the enforcement discretion from the FDA prior to November 2020 to allow for the continued marketing of the impacted products in accordance with an agreed upon transition plan. However, there is no guarantee that the FDA will grant an extension, and even if issued, such an extension may be limited to the products and indications that are subject to clinical trials. See discussion below – “Risk Factors” under the heading “To the extent our products do not qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act, this could result in removal of the applicable products from the market, would make the introduction of new tissue products more expensive, and would significantly delay the expansion of our tissue product offerings and subject us to additional post-market regulatory requirements.”
Following the Period of Enforcement Discretion. Following the period of enforcement discretion, we may need to cease selling our micronized, injectable products and other products regulated under Section 351 until the FDA approves a BLA, and then we will only be able to market such products for indications that have been approved in a BLA. The loss of our ability to market and sell our micronized, injectable products would have a material adverse impact on our revenues, earnings and financial position. In addition, we expect the cost to manufacture our products will increase due to the costs to comply with the requirements that apply to Section 351 biological products such as cGMPs and ongoing product testing costs. See discussion below – “Risk Factors” under the heading “To the extent our products do not qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act, this could result in removal of the applicable products from the market, would make the introduction of new tissue products more expensive, and would significantly delay the expansion of our tissue product offerings and subject us to additional post-market regulatory requirements.”
The majority of our revenues are generated by wound care applications. We intend to sharpen our focus in advanced wound care, continue developing and expanding our product pipeline, and work toward continued operational excellence to support future growth and sustained productivity. This includes focusing on effective and efficient execution in our core advanced wound care business and maximizing clinical adoption. In 2020, we plan to continue executing our commercial strategy, bring our manufacturing and quality systems toward compliance with the requirements that apply to Section 351 biological products, and continue pursuing a dialogue with the FDA in advance of the end of the period of enforcement discretion. The Company is advancing its therapeutic biologics pipeline targeting specific FDA-approved clinical indications for the treatment of musculoskeletal degeneration. See the discussion below – “Clinical Trials” for more information.
Our History
Our current business began on February 8, 2008 when Alynx, Co., our predecessor company, acquired MiMedx, Inc., a development-stage medical device company, the assets of which included licenses to two development-stage medical device technology platforms that we do not currently market. On March 31, 2008, Alynx, Co. merged into MiMedx Group, Inc., a Florida corporation and wholly-owned subsidiary that had been formed for purposes of the merger, with MiMedx Group, Inc. (the “Company”) as the surviving corporation in the merger. In January 2011, the Company acquired all of the outstanding equity interests of Surgical Biologics, LLC (n/k/a MiMedx Tissue Services, LLC). In January 2016, we acquired all of the outstanding common stock of Stability Inc. d/b/a Stability Biologics and n/k/a Stability Biologics LLC (“Stability”), a company that developed and processed bioactive bone graft products and tissue allografts. In September 2017, we sold Stability. See Note 5, “Divestiture of Stability Biologics, LLC.”
8
Recent Developments
Audit Committee Investigation, Delisting of Common Stock, and Related Matters
As described above in the Explanatory Note and in Item 6, “Selected Financial Data–Restatement” of this Form 10-K, the Audit Committee completed its Investigation into matters related to our prior revenue recognition practices, revenue management activities, other accounting matters and internal controls in May 2019. As a result, we have adjusted or restated certain previously reported consolidated financial information for the Non-Reliance Periods. See Note 4, “Restatement of the Consolidated Financial Statements” in the consolidated financial statements and the notes thereto included in this Form 10-K (the “Consolidated Financial Statements”). Due to our inability to remain current in our reporting obligations under SEC requirements, The Nasdaq Stock Market LLC (“Nasdaq”) suspended our common stock (“Common Stock”) from trading on The Nasdaq Capital Market on November 8, 2018, and subsequently delisted our Common Stock effective March 8, 2019. We intend to seek relisting of our Common Stock after we become current with respect to our SEC reporting obligations. In connection with our announcement of the Audit Committee Investigation, we became subject to litigation as discussed in Item 3, “Legal Proceedings” of this Form 10-K. We also identified various material weaknesses in our internal controls over financial reporting, as discussed in Item 9A, “Controls and Procedures” of this Form 10-K.
Leadership Changes to Our Management and Board of Directors
Since June 2018, most of our executive leadership team has changed. Michael J. Senken, the Company’s former Chief Financial Officer and principal accounting officer, and John E. Cranston, the Company’s former Vice President, Corporate Controller and Treasurer, stepped down from their positions on June 6, 2018. On July 2, 2018, the Company announced the resignation of Parker H. “Pete” Petit as Chief Executive Officer and Chairman of the Board effective as of June 30, 2018. Mr. Petit also resigned as a member of the Board, effective September 20, 2018. On July 2, 2018 the Company also announced the resignation of William C. Taylor as President and Chief Operating Officer of the Company and as a member of the Board, effective as of June 30, 2018. These resignations were based on the Board’s business judgment regarding the Company’s leadership and direction and arose, in part, from information the Audit Committee identified through its independent investigation. In September 2018, the Board determined that all of the foregoing separations were “for cause.”
The Board appointed Edward J. Borkowski as Interim Chief Financial Officer, effective as of June 6, 2018. The Board appointed David Coles as Interim Chief Executive Officer, effective as of July 2, 2018, and Timothy R. Wright as Chief Executive Officer, effective as of May 13, 2019. Effective July 09, 2018, Mark Graves joined the Company as the Chief Compliance Officer, reporting directly to the Ethics and Compliance Committee of the Board of Directors. In August 2019, Alexandra O. Haden resigned from her position as General Counsel and Secretary of the Company to pursue another position, and in September 2019, Dr. I. Mark Landy’s position of Chief Strategy Officer was eliminated. In December 2019, William “Butch” Hulse joined the Company as General Counsel and Secretary.
In November 2019, Mr. Borkowski resigned as Executive Vice President and Interim Chief Financial Officer of the Company but agreed to perform the duties of the Interim Chief Financial Officer with respect to this Form 10-K and to assist with the transition of his duties. In December 2019, the Company hired Peter M. Carlson to serve as Executive Vice President, Finance, to assist the Company in the transition of financial duties with the departure of Mr. Borkowski. Mr. Carlson has significant executive and accounting experience, working as a senior Finance Executive at several large companies and previously serving as a Big 5 audit partner.
Charles R. Evans, the Company’s lead director, was appointed Chairman of the Board on July 2, 2018. In June 2019, Dr. M. Kathleen Behrens succeeded him as Chair of the Board.
The Board is in the process of executing a plan to refresh the composition of the Board while providing important business oversight and leadership continuity. The Board is currently comprised of nine directors, five of whom have joined the Board since June 2019. In addition, the Company has agreed with Prescience Partners, LP, a Delaware limited partnership (“Prescience Partners”) that the Board will nominate a mutually-agreed candidate for election as a Class III director at the upcoming 2019 annual meeting of shareholders (the “2019 Annual Meeting”). As a result, following the 2019 Annual Meeting, six of our nine directors will be new to the Board since June 2019.
Strategic Priorities
In the second half of 2018, the Company initiated a process to further define its business priorities. Following management’s initial review, the Company retained a leading strategic advisory firm to validate market dynamics, including its pipeline products, assess product adjacencies for acquisition or investment and provide a framework to determine the appropriate capital allocation strategy to support its current and future business opportunities.
9
Advanced wound care includes products or procedures used in the treatment of acute and chronic wounds, used when standard wound care has failed, or after 4 weeks of non-healing. The advanced wound care category is expected to continue growing due to certain demographic trends, including an aging population, increasing incidence of obesity and diabetes and the associated higher susceptibility to non-healing chronic wounds. Furthermore, the increasing number of patients requiring advanced treatment represents a significant cost burden on the healthcare system. After evaluating the potential impact of this data on the Company’s wound care franchise, we incorporated a strategy not only to participate in this market growth but also to increase the Company’s market share by demonstrating the positive health economics of our products.
In June 2019, the Company secured $75 million of debt financing to facilitate implementation of its strategic priorities which includes accelerating the Company’s timeline to achieve its long-term growth objectives, including the BLA pipeline. The additional financing was also intended to provide liquidity to fund the costs associated with the Audit Committee Investigation, the Restatement and the near-term efforts by the Company to address certain contingent liabilities relating to pending and threatened lawsuits, pending governmental investigations and other legal proceedings. See discussion below – “Risk Factors” under the heading “If we do not successfully execute our priorities, our business, operating results and financial condition could be adversely affected.”
Our priorities include sharpening our focus in advanced wound care, developing and expanding our portfolio pipeline and driving continued operational excellence to support future growth and sustained productivity, with the following elements:
Focus on effective and efficient execution in our core advanced wound care business, maximizing clinical adoption and health economics value.
We have identified and are aligning sales territories to focus our sales force and drive efficiencies, enabling the MiMedx field personnel and sales infrastructure to enhance productivity and better serve our customers and patients. We are advancing additional health economics outcomes data to further support the use of EpiFix and have expanded efforts to best position EpiCord within the treatment paradigm, capitalizing on expanded product coverage throughout our leading technology portfolio.
Enhance business development efforts, driving growth throughout the Company’s existing product portfolio pipeline and strategic adjacencies to create a long-term competitive advantage.
Our long-range planning identified opportunities for innovative pipeline growth and international regulatory and coverage expansion within targeted high growth geographies. Additionally, an ongoing assessment of the Company’s development programs has highlighted the need for greater cross-functional collaboration and increased investment. We continue to evaluate these opportunities in alignment with our focus on advanced wound care. We remain focused on advancing our BLA programs and are therefore aligning voice-of-customer input, industry expertise and additional resources toward seeking FDA approval for micronized dehydrated human amnion/chorion membrane (“dHACM”) for a potential indication to treat musculoskeletal degeneration across multiple indications.
Enable operational and organizational excellence to support future growth and sustained productivity.
In December 2018, we announced the launch of a broad-based organizational realignment, cost reduction and efficiency program to better ensure the Company’s cost structure was appropriate given its overall lower revenue expectations. This program included management changes, a strategic realignment of the Company’s sales force, reductions in non-employee expenses and certain changes to our business practices in response to the Audit Committee Investigation. The program has resulted in business efficiencies supportive of sustained, achievable and independent growth. Since enactment through December 31, 2019, the Company has realized cost savings of approximately $37 million associated with the realignment program. Additionally, management has continued its efforts to position the business for long-term success. As part of our effort to continue to improve our sales force effectiveness, the Company has prioritized the alignment of various market access functions across the organization under one business functional area. This is aimed toward aligning with providers and patients where our payer coverage, reimbursement and Group Purchasing Organization (“GPO”) and Integrated Delivery Network (“IDN”) contract opportunities exist.
We have re-focused our priorities on refining our near-term approach for our business and our products following the end of the enforcement discretion period, bringing our manufacturing and quality systems toward compliance with the requirements that apply to Section 351 biological products, and continuing the advancement of our BLA pipeline.
10
Our Product Portfolio
We sell our amniotic membrane products under our own brands and, on a limited basis, through a private label or original equipment manufacturer (“OEM”) basis. We maintain strict controls on quality at each step of the process beginning at the time of procurement. Our Quality Management System has long been focused on compliance with the American Association of Tissue Banks’ (“AATB”) standards and the FDA’s current Good Tissue Practices (“cGTPs”), and we are seeking to strengthen our controls now for future BLA products through development of our current Good Manufacturing Practices (“cGMP”) program.
EpiFix
Our EpiFix allograft is configured in a variety of sizes, appropriate for varying sizes of wounds for external use. It is composed of human amnion and chorion tissues for use as a barrier membrane. The EpiFix platform has been used as a barrier or covering to protect chronic wounds, including diabetic foot ulcers (“DFUs”), venous leg ulcers (“VLUs”), arterial ulcers, pressure ulcers, burns and surgical wounds.
MiMedx also has a micronized version of this product. As further discussed below under the heading “Government Regulation–Recent FDA Guidance and Transition Policy for HCT/Ps,” the FDA clarified in its 2017 guidance that it regards micronized amniotic membrane products as being subject to FDA licensure as biological products under Section 351. We are evaluating whether to pursue a BLA for the micronized EpiFix product for potential application in DFUs or other areas of advanced wound care.
AmnioFix
Our AmnioFix allografts are configured in a variety of sizes, appropriate for various applications of internal use. AmnioFix is composed of human amnion and chorion tissues. Currently, our AmnioFix product line consists of two main configurations, AmnioFix sheets and AmnioFix Injectable:
• | AmnioFix is provided in sheet form for homologous use as a barrier membrane. (“Homologous use” is when a tissue is intended to have the same basic function or functions in the recipient as it performed in the donor.) It has been used in spine, orthopedic, sports medicine, lower extremity repair, urology and general surgery applications. |
• | AmnioFix Injectable is supplied in micronized powder form and is reconstituted with 0.9% sterile saline for injection. This product is our lead BLA candidate. We are studying the product’s potential to address musculoskeletal degeneration across multiple indications. We have three IND studies underway: plantar fasciitis, Achilles tendonitis and knee osteoarthritis. We currently are in Phase 3 trial for the plantar fasciitis, Phase 2B for knee osteoarthritis, and Phase 3 for Achilles tendonitis. |
EpiCord and AmnioCord
EpiCord and AmnioCord are dehydrated, human umbilical cord allografts intended for homologous use. Their purpose is to provide a protective environment for the healing process. These are thicker allografts that can be sutured in place as needed.
AmnioFill
AmnioFill is a placental connective tissue matrix allograft that is used to replace or supplement damaged integumental tissue. It has been used in the treatment of acute and chronic wounds. We are evaluating our current regulatory pathway for AmnioFill, and we may pursue other regulatory pathways, including a BLA, for AmnioFill, as we are doing with AmnioFix Injectable. However, we have not yet initiated any clinical trials under an IND in furtherance of any regulatory approvals for AmnioFill.
OEM Products
We sell a selection of allografts for dental applications on an OEM basis pursuant to an agreement under which we have granted a third party an exclusive license to some of our technology for a specific field of use in dental applications. We also sell our amnion/chorion and umbilical tissue products through a variety of OEM partners on a non-exclusive basis.
We continue to research new opportunities for amniotic and other placental tissue, and we have several additional offerings in various stages of conceptualization and development.
11
Placental Donation Program
We partner with physicians and hospitals to recover donated placental tissue. Through our donor program, a mother who delivers a healthy baby via a Caesarean section can donate her placental and umbilical cord tissue in lieu of having it discarded as medical waste. After consent for donation is obtained, a blood sample from each donor is tested for communicable diseases, and the donor is screened for risk factors in order to determine eligibility in compliance with federal regulations and AATB standards. We operate a licensed tissue bank that is registered as a tissue establishment with the FDA, and we are an accredited member of the AATB. All donor records and test results are reviewed by our Medical Director and staff prior to the release of the tissue for distribution.
We have developed a large network of hospitals that participate in our placental donation program, and we employ a dedicated staff that work with these hospitals. We believe that we will be able to obtain an adequate supply of tissue to meet anticipated demand. However, see discussion below “Risk Factors” under the heading “Our products depend on the availability of tissue from human donors, and any disruption in supply could adversely affect our business.”
Processing (Manufacturing)
Over several years, we have developed and patented a unique and proprietary technique (PURION) for processing allografts from the donated placental tissue. This technique specifically focuses on preserving the tissue’s natural growth factor content and maintaining the structure and collagen matrix of the tissue. Our patented and proprietary processing method employs aseptic processing techniques in addition to terminal sterilization for increased patient safety. We believe that our process preserves more of the natural characteristics of the tissue than the processes used by many of our competitors.
The PURION process produces an allograft that retains the tissue’s inherent biological properties (cytokines, chemokines, growth factors, etc.) found in the placental tissue and produces an allograft that is easy for doctors to use. The allograft can be stored at ambient temperature and has a five-year shelf life. Each sheet allograft incorporates specialized visual embossments that assist the health care practitioner with proper allograft placement and orientation.
To ensure the safety of human tissue products, the FDA enforces Good Tissue Practice (“GTP”) manufacturing regulations. We believe that MiMedx has developed mature systems to comply with, and is in compliance with, these regulations. As an important part of the Company’s product safety compliance, MiMedx products are terminally sterilized to an internationally recognized industry standard in addition to having been processed via the PURION process.
Our facilities are subject to periodic unannounced inspections by regulatory authorities and may undergo compliance inspections conducted by the FDA and corresponding state and foreign agencies. We are registered with the FDA as a tissue establishment and are subject to the FDA’s cGTPs quality program regulations, state regulations and regulations promulgated by various regulatory authorities outside the United States. The Company’s most recent FDA inspection for compliance with GTP regulations, which took place in September 2018, resulted in no observations and a no action indicated (NAI) rating, which is the most favorable designation the FDA provides after an inspection.
In recent years, the FDA has clarified through inspection activity, letters to industry, and guidance documents its expectation that certain human tissue products, including product types manufactured by MiMedx, meet additional requirements that apply to traditional biological products, such as BLA approval and cGMP compliance beginning in November 2020. The guidance documents apply to products offered by many companies, not just MiMedx, and the guidance has implications for manufacturing processes. For example, the FDA generally requires products subject to Section 351 to be manufactured in compliance with cGMPs. After the end of the enforcement discretion period, these products will be subject to cGMP compliance. The Company is developing and enhancing systems to meet these requirements, and expects to complete those efforts by November 2020, although there is no guarantee that the Company will be able to meet the requirements by such date, or at all. In December 2019, the FDA conducted cGMP inspections at our Marietta, Georgia and Kennesaw, Georgia processing facilities. The FDA issued a Form FDA 483 (“483”), which is a list of inspectional observations, at the conclusion of each inspection. Specifically, the FDA issued a 483 consisting of 9 observations at our Marietta, Georgia processing facility, and a 483 consisting of 14 observations at our Kennesaw, Georgia processing facility. MiMedx timely responded to the FDA regarding each observation, providing substantive responses to all of the observations. The Company’s response included completed and planned actions to address each observation. As of March 6, 2020, approximately half of these remedial actions are complete. As we communicated to the FDA in our 483 responses, we expect that we will complete the remaining actions over the course of 2020 and prior to the expiration of enforcement discretion in November, 2020, although there is no guarantee that the Company will be able to meet the requirements by such date, if at all.
12
Intellectual Property
Our intellectual property includes owned and licensed patents, owned and licensed patent applications and patents pending, proprietary manufacturing processes and trade secrets, and trademarks associated with our technology. We believe that our patents, proprietary manufacturing processes, trade secrets, trademarks, and technology licensing rights provide us with important competitive advantages.
Patents and Patent Applications
Due to the substantial expertise and investment of time, effort and financial resources required to bring new regenerative biomaterial products and implants to the market, the importance of obtaining and maintaining patent protection for significant new technologies, products and processes cannot be underestimated. As of the date of the filing of this Form 10-K, in addition to international patents and patent applications, we own 48 U.S. patents related to our amniotic tissue technology and products, and 31 additional patent applications covering aspects of this technology are pending at the United States Patent and Trademark Office. The vast majority of our domestic patents covering our core amniotic tissue technology and products will not begin to expire until August 2027. See discussion below – “Risk Factors” under the heading “Risks Related to Our Intellectual Property.”
Market Overview
Domestic sales currently account for most of our revenue, and we are considering international expansion, primarily targeting Europe and Asia Pacific. In the United States, advanced wound care, including burns and lower extremity surgical applications, are our primary applications.
Wound Care
The broad wound care category includes traditional dressings such as bandages, gauzes and ointments, which are used to treat non-severe or non-chronic wounds, and advanced wound care products such as mechanical devices, advanced dressings, biological products, and HCT/Ps, which are used to treat severe wounds or chronic wounds that have not appropriately closed after four weeks of treatment with traditional dressings.
In the United States in 2018, third-party estimates indicate that there were 8.2 million total reported wounds, with 2.9 million of these wounds classified as chronic wounds. Of these chronic wounds, we estimate that 35% are candidates for skin substitute product treatment regimens, providing for a total addressable opportunity of approximately $3.3 billion. The overall cost of treating chronic wounds is rising sharply, and the current annual estimated cost in the United States exceeds $28 billion.
MiMedx is a leader in the advanced wound care category. This category is expected to continue growing due to certain demographic trends, including an aging population, increasing incidence of obesity and diabetes and the associated higher susceptibility to non-healing chronic wounds. Furthermore, the increasing number of patients requiring advanced treatment represents a significant cost burden on the healthcare system.
Traditional dressings such as bandages, gauzes and ointments, along with treatment of active infection and debridement, currently represent the “standard of care” for treating chronic wounds such as DFUs, VLUs, pressure ulcers and arterial ulcers. If after four weeks of use, the wound has not responded appropriately to “standard of care” therapy, clinical research has shown that advanced therapy such as a skin and dermal substitute can be beneficial as part of the patient’s treatment plan. According to data provided by BioMedGPS, MiMedx’s EpiFix is the current product of choice for physicians choosing to use a skin and dermal substitute product as a barrier or cover. EpiFix stores at ambient conditions for up to five years compared to certain cultured skin substitutes currently on the market that require cryogenic freezer storage and expire within days to months from the time of processing. In addition, we market multiple sizes of EpiFix sheets for use as protective barriers which enables a healthcare provider to select an appropriate size graft based on the size of the wound to reduce product waste.
Our AmnioFix tissue allografts have been used in a variety of surgical applications including, but not limited to, plastic surgery, general surgery, gynecology, urology, orthopedics, spinal surgery, lower extremity repair and sports medicine. AmnioFix can be used as a barrier membrane in procedures where a second surgery may be required and scar tissue formation may be problematic.
Biologics License Application (BLA) Programs
The FDA clarified its expectations in late 2017 that certain cellular and tissue-based products, including types of products marketed by MiMedx, are considered drugs and biological products subject to Section 351 requirements under the federal Food, Drug and Cosmetic Act (the “FD&C Act”). In order to conform to this regulatory guidance, MiMedx is pursuing several indications under the BLA pathway, although there can be no assurance that we will obtain a BLA and may ultimately decide not to pursue a BLA for certain products or indications. See Risk Factors - “Obtaining and maintaining the necessary regulatory approvals for certain
13
of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies.” AmnioFix Injectable is our lead BLA product candidate, and we are studying its potential to address a number of musculoskeletal conditions. In this regard, we have three ongoing IND programs: plantar fasciitis, Achilles tendonitis and knee osteoarthritis. We are currently in Phase 3 of a plantar fasciitis study and Phase 2B of a knee osteoarthritis study. Results of blinded, interim analyses of these studies revealed separation between treatment and control groups, but indicated that the power to observe a result with statistical and clinical significance could be increased by increasing the sample size. We have since amended the protocols and have taken other steps to improve on these trials. We are also completing a Phase 3 IND study for Achilles tendonitis, and we plan to review our options for this program after we have assessed the results of this study. However, an interim analysis of this study has indicated that the sample size needs to be increased to provide sufficient statistical and clinical significance. We have decided to continue the study to completion with the original sample size as we evaluate the endpoints for appropriateness, including appropriateness of the measures and the time required to measure differences between the treatment groups (e.g., three months, six months, etc.).
We are studying AmnioFix Injectable for a variety of uses other than wound care, and the applications described above (plantar fasciitis, osteoarthritis knee pain, and Achilles tendonitis) address unmet needs outside of traditional wound care. After oral non-habit forming pain medication fails to adequately relieve a patient’s joint, ligament or tendon pain, market available injections such as corticosteroids are a commonly available treatment option. However, a number of patients still do not get adequate relief from corticosteroid injections, or do not want to use corticosteroids given their potential to damage human tissue. (See McAlindon TE, LaValley MP, Harvey WF, et al. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial, JAMA. 2017;317(19):1967-1975. doi:10.1001/jama.2017.5283.) Additionally, in light of the current crisis with opioid abuse, non-surgical treatments and alternative approaches to musculoskeletal pain management are under consideration. Patients and physicians are searching for new products that are safe and effective for the management of chronic musculoskeletal conditions. According to data from the National Health Interview Survey, it was recently estimated that 14 million people in the U.S. have symptomatic knee osteoarthritis, with more than half under the age of 65. (See for example https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5832048/pdf/nihms940925.pdf and https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5319385/pdf/nihms-775448.pdf.) We are studying AmnioFix Injectable as a potential product candidate to address this unmet need, as well as in other degenerative musculoskeletal applications. As of the date of the filing of this Form 10-K, it has not been cleared by the FDA for any such use.
Marketing and Sales
As of December 31, 2019 our direct sales team was comprised of 285 sales professionals, including field sales representatives and field sales management, who call on hospitals, wound care clinics, physician offices, and federal health care facilities such as the Department of Veterans Affairs (the “VA”) and Department of Defense hospitals. Our direct sales force focuses on the advanced wound care category through multiple sites of service. We also maintain a network of independent sales agents that focus on musculoskeletal applications because of the complementary products that they carry, access to certain customers, and to provide sales coverage for areas where we do not have a full time sales representative.
We also sell our products through distributors. Distributors purchase products from us at wholesale prices and resell products to end users. Sales through distributors comprised a smaller percentage of our total sales in 2018 than in prior years. See Note 17, “Revenue Data by Customer Type.” As discussed above, we sell allografts for dental applications on an OEM basis pursuant to an agreement under which we granted a third party an exclusive license to some of our technology for use in certain fields in a specified field of use. We also sell our amnion/chorion and umbilical tissue products through a variety of OEM partners for use in additional musculoskeletal applications on a non-exclusive basis.
Coverage and Reimbursement
A significant portion of our products are purchased by U.S. government accounts (e.g., the VA, the Public Health Service (including the Indian Health Service)), which do not depend on reimbursement from third parties. Federal law requires that for a company to be eligible to have its products purchased by such federal agencies, as well as to be paid for with federal funds under the Medicaid and Medicare Part B programs, it also must participate in the VA Federal Supply Schedule (“FSS”) pricing program. To participate, we are required to enter into an FSS contract with the VA for our products and agree to certain prices.
With the exception of government accounts, most purchasers of our products are physicians, hospitals or ambulatory surgery centers (“ASCs”) that rely on reimbursement by third-party payers. Accordingly, our growth substantially depends on adequate levels of third-party reimbursement for our products from these payers. Third-party payers are sensitive to the cost of products and services and are increasingly seeking to implement cost containment measures to control, restrict access to, or influence the purchase of health care products and services. In the U.S., such payers include U.S. federal healthcare programs (e.g., Medicare and Medicaid), private insurance plans, managed care programs and workers’ compensation plans. Federal healthcare programs have prescribed coverage criteria and reimbursement rates for medical products, services and procedures. Similarly, private, third-party payers have their own coverage criteria and negotiate reimbursement amounts for medical products, services and procedures
14
with providers. In addition, in the U.S., an increasing percentage of insured individuals are receiving their medical care through managed care programs (including managed federal healthcare programs) which monitor and may require pre-approval of the products and services that a member receives. Ultimately, however, each third-party payer determines whether and on what conditions they will provide coverage for our products, and such decisions often include each payer’s assessment of the science and efficacy of the applicable product.
EpiFix Sheet Products and EpiCord
Medicare Coverage
By far, the largest third-party payer in the United States is the Medicare program, which is a federally-funded program that provides healthcare coverage for senior citizens and certain disabled individuals. The Medicare program is administered by the Centers for Medicare and Medicaid Services (“CMS”), an agency within the U.S. Department of Health and Human Services (“HHS”). Medicare Administrative Contractors (“MACs”) are private insurance companies that serve as agents of CMS in the administration of the Medicare program and are responsible for making coverage decisions and paying claims for the designated Medicare jurisdiction. There are seven Part A/B MACs in the U.S., each with its own geographical jurisdiction, and each has its own standards and process for determining coverage and reimbursement for a procedure or product. Private payers often follow the lead of governmental payers in making coverage and reimbursement determinations. Therefore, achieving favorable Medicare coverage and reimbursement is usually a significant gating factor for successful coverage and reimbursement for a new product by private payers.
The coverage and reimbursement framework for products under Medicare is determined in accordance with the Social Security Act and pursuant to regulations promulgated by CMS, as well as the agency’s regulatory coverage and reimbursement determinations. Ultimately, however, each of the MACs determines whether and on what conditions they will provide coverage for the product. Such decisions are based on each MAC’s assessments of the science and efficacy of the applicable product. As noted below under the heading “Research and Development,” we have devoted significant resources to clinical studies to provide data to the MACs, as well as other payers, in order to demonstrate the efficacy and clinical effectiveness of our tissue technologies. As of the date of this report, both EpiFix sheets and EpiCord allografts are eligible for coverage by all MACs. In January 2019, EpiFix and EpiCord received separate CMS HCPCS Codes, Q4186 and Q4187, distinguishing each product in coverage and reimbursement policies.
For Medicare reimbursement purposes, our EpiFix and EpiCord allografts are classified as “skin substitutes.” Current reimbursement methodology varies between the hospital outpatient department (“HOPD”) and ASCs setting versus the physician office. Currently, skin substitutes are reimbursed under a “packaged” or “bundled” methodology along with the related application procedure under a two-tier payment system. In the HOPD and ASCs setting, providers receive a single payment that reimburses for the application of the product as well as the product itself. CMS classifies skin substitutes into low cost or high cost groups, based on a geometric mean unit cost and per day cost. For 2019, the geometric mean unit cost threshold applicable to both our EpiFix and EpiCord allograft products is $48 per square centimeter, and the per day cost threshold is $790. The national HOPD average packaged (“bundled”) rate for our EpiFix and EpiCord allograft products was $1,427 in 2017, was $1,568 in 2018 and is $1,549 in 2019. All skin substitute products administered in the HOPD and ASCs setting are bundled except for those that have been approved by CMS for pass-through status. EpiFix was approved by CMS for pass-through status but that status expired on December 31, 2014, and EpiCord has not been approved by CMS for pass-through status. This “bundled” payment structure applies only to the HOPD and ASCs settings.
Currently, providers that administer EpiFix or EpiCord allografts and other skin substitutes in the physician office setting are reimbursed based on the size of the graft, computed on a per square centimeter basis. The payment rate is calculated using the manufacturer’s reported average sales price (“ASP”) submitted quarterly to CMS. This payment methodology applies only to physician offices. The Medicare payment rates are updated quarterly based on this ASP information for many skin substitute products but not all. EpiFix is included on the Medicare national ASP Drug Pricing File, but EpiCord is not. The published skin substitute Medicare payment rate established by statute is ASPs plus 6%. Reimbursement for products not included on the Medicare national ASP Drug Pricing File are at the discretion of each MAC, which typically is invoice cost or wholesale acquisition cost (“WAC”) plus 3%.
Since April 2013, Medicare payments for all items and services, including EpiFix sheet products and EpiCord, have been reduced by 2% under the sequestration required by the Budget Control Act of 2011, as amended by the American Taxpayer Relief Act of 2012. Subsequent legislation extended the 2% reduction, on average, to 2027. This 2% reduction in Medicare payments affects all parts of the Medicare program. The law allows for additional sequestration orders, potentially resulting in up to a 4% reduction in Medicare payments under a statutory PAYGO sequestration order.
15
Private Payers
We have devoted considerable resources to clinical trials to support coverage and reimbursement of our products and have confirmed an increasing number of private payers that reimburse for EpiFix in the physician office, the HOPD and the ASCs settings. Coverage and reimbursement vary according to the patient’s health plan and related benefits. The majority of health plans currently provide coverage for EpiFix for the treatment of DFUs, and many include treatment of VLUs. In 2019, numerous health plans have added EpiCord coverage for the treatment of DFUs. MiMedx has secured payer coverage for over 286 million covered lives allowing a significant number of patients access to our products.
We have established and continue to grow a reimbursement support group to educate providers and patients with regard to accurate coverage and reimbursement information regarding our products. See discussion below – “Risk Factors” under the heading “Our revenues depend on adequate reimbursement from public and private insurers and health systems.”
Hospital Use
Products administered in the hospital inpatient setting are bundled when submitted as part of the hospital’s claim under a diagnosis-related group (“DRG”). In these cases, we continue to educate the hospital that our products are cost-effective, and have the potential to improve patient outcomes and reduce the length of stay. We are working to develop additional health economic data to support this effort. As noted above, the ability to sell products in a hospital is dependent upon demonstrating to the hospital the product’s efficacy and cost effectiveness.
Micronized and Other Products
Currently, our micronized products are available for coverage by only a limited number of Medicare, commercial and state Medicaid plans. EpiFix Micronized is listed on the Medicare national ASP Drug Pricing File and, similar to most Medicare Part B drugs, is reimbursed at ASP plus 6%, effective July 2019. There is currently no specific third-party reimbursement available for AmnioCord or AmnioFill, except to the extent such products are bundled as part of a hospital’s claim under a DRG. See discussion below – “Risk Factors” under the heading “Our revenues depend on adequate reimbursement from public and private insurers and health systems.”
Customer Concentration
A significant portion of our products are purchased by U.S. government accounts (e.g., the VA, the Public Health Service (including the Indian Health Service)). For the years ended December 31, 2018, 2017 and 2016, our net sales to all U.S. government accounts comprised approximately 15%, 9% and 8%, respectively, of our net sales. Previously, some of the Company’s sales to government accounts, including the VA, were made through a distributor relationship with AvKARE Inc., which is a veteran-owned General Services Administration Federal Supply Schedule (FSS) contractor. The Company’s agreement with AvKARE expired on June 30, 2017. Upon termination of the agreement, the Company had an obligation to repurchase AvKARE’s remaining inventory within ninety 90 days in accordance with the terms of the agreement. As of September 30, 2017, the Company had satisfied the repurchase obligation. See discussion below – “Risk Factors” under the heading “A significant portion of our revenues and accounts receivable come from government accounts.”
Competition
Competition in the placental-based and allograft tissue field is intense and subject to new entrants and evolving market dynamics. Companies within the industry compete on the basis of product efficacy, pricing, ease of product handling, and product logistics. Another important factor is third-party reimbursement, which is difficult to obtain as it is a time-consuming and expensive process. We believe our success in obtaining third-party reimbursement for our products is a significant competitive advantage.
Advanced wound care therapies employ technologies to aid in wound healing in cases where the wound is chronic and healing progress has stalled or stopped. The primary competitive products in the skin and dermal substitutes category include, among others, amniotic membrane allografts, tissue-engineered living skin equivalents, porcine-, bovine- and fish skin-derived xenografts and collagen matrix products. Our main competitor within the advanced wound care category is Integra LifeSciences Holdings Corporation (“Integra”), a company that markets skin substitute products for wound reconstruction and surgical reconstruction. Integra’s range of skin substitutes includes its dermal regeneration template products and other xenografts, an amnion-only allograft resulting from Integra’s acquisition of Derma Sciences Inc., as well as an amnion/chorion/amnion allograft. Xenografts, or tissue transplants from non-human species, serve mainly as an extracellular matrix and have to undergo aggressive processing to remove immunogenic animal products from the tissue. In addition, challenges with xenografts include limited clinical published data, and some products may require suturing or stapling to the wound bed, making handling more difficult.
16
Another competitor within the advanced wound care category is Organogenesis, Inc. (“Organogenesis”), the manufacturer of tissue-engineered living skin equivalents that require special shipping and/or storage in freezers, and purified native collagen matrix dressing which has pass-through reimbursement status through 2020. Organogenesis also markets amniotic allografts.
Other competitors include Smith & Nephew plc (“Smith & Nephew”), which acquired Osiris Therapeutics, Inc. in April 2019. Smith & Nephew’s combined biologics assortment includes single-layer amnion products and porcine- or bovine- derived collagen matrix products.
The primary competitive products in the surgical, orthopedic or sports medicine categories are other amniotic membrane allografts and injectable solutions, such as platelet-rich plasma, evolving cellular alternatives, or steroids.
See discussion below – “Risk Factors” under the heading “We are in a highly competitive and evolving field and face competition from well-established tissue processors and medical device manufacturers, as well as new market entrants.”
Government Regulation
The products manufactured and processed by the Company are derived from human tissue. As discussed below, Section 361 HCT/Ps are tissue-based products that are regulated solely under Section 361 and do not require pre-market clearance or approval by the FDA. Section 351 HCT/Ps are also tissue products but are regulated as biological products, medical devices or drugs and, in order to be lawfully marketed in the United States, require FDA pre-market clearance or approval. See discussion below – “Risk Factors” under the heading “Risks Related to Regulatory Approval of Our Products and Other Government Regulations.”
Tissue Products
In 1997, the FDA proposed a new regulatory framework for cells and tissues. This framework was intended to provide adequate protection of public health while enabling the development of new therapies and products with as little regulatory burden as possible. A key innovation in the system is that covered HCT/Ps would be regulated solely under Section 361 and would not be subject to pre-market clearance. The registration and listing rules were finalized in January 2001 in 21 CFR Part 1271. Additional rules regarding donor eligibility and good tissue practices were soon adopted. Together, these rules form a comprehensive system intended to encourage significant innovation.
The FDA requires each HCT/P establishment to register and establish that its product meets the requirements to qualify for regulation solely under Section 361. To be a Section 361 HCT/P, a cellular or tissue-based product generally must meet all four of the following criteria (fully set forth in 21 CFR Part 1271):
• | it must be minimally manipulated; |
• | it must be intended for homologous use; |
• | its manufacture must not involve combination with another article, except for water, crystalloids or a sterilizing, preserving or storage agent; and |
• | it must not have a systemic effect and must not be dependent upon the metabolic activity of living cells for its primary function. |
Amniotic and other birth tissue are considered cellular and tissue-based articles and are therefore eligible for regulation solely as a Section 361 HCT/P depending on whether the specific product at issue and the claims made for it are consistent with the criteria set forth above. HCT/Ps that do not meet these criteria are subject to more extensive regulation as drugs, medical devices, biological products or combination products.
Products Regulated Solely as HCT/Ps
The FDA has specific regulations governing HCT/Ps, including some regulations specific to Section 361 HCT/Ps, which are set forth in 21 CFR Part 1271. All establishments that manufacture Section 361 HCT/Ps must register and list their HCT/Ps with the FDA’s Center for Biologics Evaluation and Research within five days after commencing operations. In addition, establishments are required to update their registration annually in December or within 30 days of certain changes and submit changes in HCT/P listing at the time of or within six months of such change.
The regulations in 21 CFR Part 1271 also require establishments to comply with donor screening, eligibility and testing requirements and cGTPs to prevent the introduction, transmission and spread of communicable diseases. The cGTPs govern, as may be applicable, the facilities, controls and methods used in the manufacture of all HCT/Ps, including processing, storage, recovery, labeling,
17
packaging and distribution of Section 361 HCT/Ps. cGTPs require us, among other things, to maintain a quality program, train personnel, control and monitor environmental conditions as appropriate, control and validate processes, properly store, handle and test our products and raw materials, maintain our facilities and equipment, keep records and comply with standards regarding recovery, pre-distribution, distribution, tracking and labeling of our products and complaint handling. 21 CFR Part 1271 also mandates compliance with adverse reaction and cGTP deviation reporting and labeling requirements.
The FDA conducts periodic inspections of HCT/P manufacturing facilities, and contract manufacturers’ facilities, to assess compliance with cGTP. Such inspections can occur at any time with or without written notice at such frequency as determined by the FDA in its sole discretion. To determine compliance with the applicable provisions, the inspection may include, but is not limited to, an assessment of the establishment’s facilities, equipment, finished and unfinished materials, containers, processes, HCT/Ps, procedures, labeling, records, files, papers and controls required to be maintained under 21 CFR Part 1271. If the FDA were to find serious non-compliant manufacturing or processing practices during such an inspection, it could take regulatory actions that could adversely affect our business, results of operations, financial condition and cash flows.
FDA Letter Regarding AmnioFix Injectable and Other Micronized Products
In August 2013, the Company received an untitled letter from the Office of Compliance and Biologics Quality (“OCBQ”) within the FDA’s Center for Biologics Evaluation and Research concerning AmnioFix Injectable and other micronized products (the “Untitled Letter”). The Untitled Letter asserted that our micronized products, including AmnioFix Injectable, are not properly regulated solely under Section 361 because they are more than “minimally manipulated” as that term is defined in FDA regulations. Accordingly, the Untitled Letter asserted that the products at issue are drugs and biological products that require valid biologics licenses to be in effect in order to be lawfully marketed.
The Company disagreed at the time, taking the position that micronization was allowed for Section 361 HCT/Ps under the then applicable guidance. Because the Untitled Letter seemed to be contrary to existing guidance, the Company attempted to engage with OCBQ and ultimately pursued two levels of supervisory review. As part of that process, the Company agreed to pursue a biologics license for AmnioFix Injectable, and has since filed IND applications with the FDA covering clinical studies for AmnioFix Injectable that are discussed in greater detail below. In November 2016, following this supervisory review process, the Acting Chief Scientist of the FDA informed the Company that additional agency review of the Untitled Letter was not warranted.
Recent FDA Guidance and Transition Policy for HCT/Ps
In November 2017, the FDA released four guidance documents that, collectively, the agency described as a “comprehensive policy framework” for applying existing laws and regulations governing regenerative medicine products, including HCT/Ps. One guidance document in particular, “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue – Based Products: Minimal Manipulation and Homologous Use – Guidance for Industry and Food and Drug Administration Staff,” offered important clarity on some of the issues that the Company raised on appeal to the Untitled Letter.
The guidance documents confirmed that sheet forms of amniotic tissue are appropriately regulated as solely Section 361 HCT/Ps when intended for use as a barrier or covering. We are in the process of evaluating our marketing materials for each of our products to align with the FDA’s guidance.
Second, the guidance documents confirmed the FDA’s stance that all micronized amniotic membrane products require a biologics license to be lawfully marketed in the United States. However, the guidance documents also stated that the FDA intends to exercise enforcement discretion under limited conditions with respect to the IND application and pre-market approval requirements for certain HCT/Ps through November 2020. This 36-month period of enforcement discretion was intended to give sponsors time to evaluate their products, have a dialogue with the agency and, if necessary, begin clinical trials and file the appropriate pre-market applications. The FDA’s approach is risk-based, and the guidance documents clarified that high-risk products and uses could be subject to immediate enforcement action.
This enforcement discretion applies across our industry, and the Company has continued to market its products under the policy of enforcement discretion. At the same time, we are pursuing the BLA pre-market approval process for certain uses of AmnioFix Injectable. There is no assurance that the FDA will grant these approvals on a timely basis, or at all, or that we will not discontinue our pursuit of a BLA for certain products or indications. In April 2019, we announced that we will need more time to file our BLAs with the FDA and that clinical trial protocol amendments and enhancements, further resources and additional capabilities and expertise will be required. See “Clinical Trials” below for information regarding the revised timelines.
During the remainder of the 36-month enforcement discretion period, the Company will also continue to explore possible options for extending this enforcement discretion period. To this end, the Company hopes to find support for a further transition plan with the FDA to allow for continued marketing of the impacted products while the Company transitions to compliance with Section
18
351, the applicable sections of the FD&C Act, the cGMP regulations in 21 CFR Part 210 and 211, and other applicable FDA regulations. This would be an extension of the current policy, and there is no guarantee that the FDA will provide more time, either for MiMedx or the industry at large.
Products Regulated as Biologics – The BLA Pathway
The typical steps for obtaining FDA approval of a BLA to market a biological product in the United States include:
• | Completion of preclinical laboratory tests, animal studies and formulations studies under the FDA’s Good Laboratory Practice regulations; |
• | Submission to the FDA of an IND application for human clinical testing, which must become effective before human clinical trials may begin and which must include independent Institutional Review Board approval at each clinical site before the trials may be initiated; |
• | Performance of adequate and well-controlled clinical trials in accordance with Good Clinical Practices to establish the safety and efficacy of the product for each indication; |
• | Development of purity, potency and identity tests to demonstrate consistency and reliability of the manufacturing process through a chemistry, manufacturing and control program; |
• | Submission to the FDA of a BLA for marketing the product, which includes, among other things, reports of the outcomes and full data sets of the clinical trials, and proposed labeling and packaging for the product; |
• | Satisfactory review of the contents of the BLA by the FDA, including the satisfactory resolution of any questions raised during the review; |
• | Satisfactory completion of an FDA Advisory Committee review, if applicable; |
• | Satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with FDA’s cGMP regulations, to assure that the facilities, methods and controls are adequate to ensure the product’s identity, strength, quality and purity; and |
• | FDA approval of the BLA, including agreement on post-marketing commitments, if applicable. |
Generally, clinical trials are conducted in three phases, though the phases may overlap or be combined. Phase 1 trials typically involve a small number of healthy volunteers and are designed to provide information about the product safety and to evaluate the pattern of drug distribution and metabolism within the body. Phase 2 trials are conducted in a larger but limited group of patients afflicted with a particular disease or condition in order to determine preliminary efficacy, dosage tolerance and optimal dosing, and to identify possible adverse effects and safety risks. Dosage studies are typically designated as Phase 2A, and efficacy studies are designated as Phase 2B. Phase 3 clinical trials are generally large-scale, multi-center, comparative trials conducted with patients who have a particular disease or condition in order to provide statistically valid proof of efficacy, as well as safety and potency. In some cases, the FDA will require Phase 4, or post-marketing trials, to collect additional data after a product is on the market. All phases of clinical trials are subject to extensive record keeping, monitoring, auditing and reporting requirements.
The FDA has broad regulatory compliance and enforcement powers. If the FDA determines that MiMedx has failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement actions, such as issuing an FDA Form 483 notice of inspectional observations; sending a warning letter or untitled letter; issuing an order of retention, destruction, or cessation of marketing; imposing civil money penalties; suspending or delaying issuance of approvals; requiring product recalls; imposing a total or partial shutdown of production; withdrawing approvals or clearances already granted; pursuing product seizures, consent decrees or other injunctive relief; and criminal prosecution through the Department of Justice.
Clinical Trials
Trial Overview
The Company is currently conducting three IND programs investigating the use of AmnioFix Injectable to reduce pain and increase function in patients with plantar fasciitis, Achilles tendonitis, or osteoarthritis of the knee. Based on a review of the studies and interim results, the Company has several actions underway with respect to its ongoing and anticipated clinical trials to address both resources, capabilities and expertise needed for commercial launch, including our strategy around an increased dialogue with the FDA regarding our BLA progress. The trials were developed and initially overseen by senior managers who are no longer with
19
the Company and, as previously disclosed, we have concluded that the trials must be improved if they are to support BLA applications and approvals. However, there can be no assurance that we will obtain a BLA and may ultimately decide not to pursue a BLA for certain products or indications. See Risk Factors - “Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies.”
Plantar Fasciitis
In March 2015, we initiated a Phase 2B prospective, single-blinded, randomized, controlled trial (“RCT”) investigating a single injection of 40 mg of AmnioFix Injectable as compared to a single intra-plantar injection of saline (placebo control) in the treatment of patients with recalcitrant plantar fasciitis pain and foot dysfunction. This trial enrolled 145 patients at 15 study sites. In September 2017, we announced the trial had met its efficacy endpoints, and final data were published in 2018. Based on the Phase 2B interim data, in January 2018 we initiated a Phase 3 prospective, double-blinded, RCT to assess the safety and efficacy of a single 40 mg intra-plantar injection of AmnioFix Injectable to treat patients with recalcitrant plantar fasciitis pain. This trial initially enrolled 164 patients, but in July 2019, we expanded it to 276 patients.
The need to increase enrollment was based on a blinded review conducted on 50% of enrolled patients who had reached the study endpoints. The purpose of the blinded analysis was to determine if the planned sample size was adequate to assess the differences between the treatment and control groups. We determined that increasing the sample size to 276 patients would provide sufficient power to observe a result with statistical and clinical significance to determine efficacy. We have instituted these changes and amendments and expect enrollment to complete in mid June 2020.
If the plantar fasciitis trials are not only successful, but also determined to be adequate proof of efficacy and safety, we expect to file a BLA for AmnioFix Injectable to treat patients with plantar fasciitis in the future. However, we now expect that FDA approval to market AmnioFix Injectable for this indication will take longer than previously expected and may take several years, and there can be no assurance that we will receive FDA approval. Approval may be delayed due to a variety of factors, including failure of the studies to achieve their endpoints, the extra effort and cost required to improve our clinical trials as described above, the potential that we reevaluate our commercialization strategy, and the work required to achieve commercial and manufacturing readiness. See discussion below – “Risk Factors” under the heading “Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time-consuming and may impede our ability to fully exploit our technologies.”
Knee Osteoarthritis
In March 2018, the FDA granted AmnioFix Injectable the Regenerative Medicine Advanced Therapy (“RMAT”) designation for use in the treatment of osteoarthritis of the knee. RMAT-designated products are eligible for increased and earlier interactions with the FDA, similar to those interactions available to fast track and breakthrough-designated therapies. In addition, these products may be eligible for rolling review and accelerated approval. The meetings with sponsors of RMAT-designated products may include discussions of whether accelerated approval would be appropriate based on surrogate or intermediate endpoints reasonably likely to predict long-term clinical benefit or reliance upon data obtained from a meaningful number of sites.
In March 2018, we initiated a Phase 2B prospective, double-blinded RCT investigating a single intra-articular injection of 40 mg of AmnioFix Injectable as compared to a single injection of saline (placebo control) in the treatment of pain and functional impairment in patients with osteoarthritis of the knee. This trial was expected to enroll 318 patients. However, a blinded interim analysis performed in August 2019 revealed that while differences in the treatment groups were being observed, the power to observe a statistically significant result would be increased by increasing the sample size to 466.
We have also concluded that we will update the protocol in this trial to enable subjects to receive the active treatment at six months if their pain has not resolved or responded, regardless of treatment arm. We have begun these changes along with other amendments to enhance the pain and function outcomes. If these trials are successful and determined to be adequate support for safety and efficacy observations, we expect to file a BLA for AmnioFix Injectable for this indication. However, we now expect that FDA approval to market AmnioFix Injectable for this indication will take longer than previously expected and may take several years, and there can be no assurance that we will receive FDA approval. Approval may be delayed due to a variety of factors, including failure of the studies to achieve their endpoints, the extra effort and cost required to improve our clinical trials as described above and the work required to achieve commercial and manufacturing readiness. See discussion below – “Risk Factors” under the heading “Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time-consuming and may impede our ability to fully exploit our technologies.”
20
Achilles Tendonitis
In January 2018, we initiated a Phase 3 prospective, double-blinded RCT investigating a single intra-tendon injection of 40 mg of AmnioFix Injectable as compared to a single injection of saline (placebo control) in the treatment of Achilles tendonitis. This trial was intended to enroll 158 patients. We have analyzed data received from a sample size analysis that was conducted on patients representing 50% of total enrollment that had reached the primary efficacy endpoint. This indicated that a substantial increase in sample size would be required to observe improved clinically and statistically significant improvement and separation between treatment and control groups. With this in mind, we have concluded that the most reasonable approach is to continue the current study to completion and analyze the results to determine the adequacy of the measures employed and time points of observation to show meaningful clinical and statistical analyses. Given current enrollment rates, we anticipate that this study will end in late 2020.
BLA Process
If any of the study results support potential product approval, we intend to file BLAs as described above. The process of obtaining an approved BLA requires the expenditure of substantial time, effort and financial resources and may take years to complete. The fee for filing a BLA and the annual user fees payable with respect to any establishment that manufactures biologics and with respect to each approved product are substantial. While there can be no assurance that we will ultimately obtain regulatory approval for our micronized products, we have already completed substantial work towards multiple BLAs, and we believe we have a multi-year advantage over our competitors.
FDA Post – Market Regulation
Tissue processors regulated solely under Section 361 are still required to register as an establishment with the FDA. As a registered establishment, we are required to comply with regulations regarding labeling, record keeping, donor eligibility, screening and testing. We are also required to process the tissue in accordance with established cGTP, as well as report any adverse reactions caused by a possible transmission of an infectious disease attributed to our tissue. Our facilities are also subject to periodic inspections to assess our compliance with the regulations.
Products covered by a BLA, New Drug Application, 510(k) clearance or a pre-market approval are subject to numerous additional regulatory requirements, which include, among others, compliance with cGMP (or, in the case of devices, with FDA’s Quality System Regulation), which imposes certain procedural, substantive and record keeping requirements, and labeling regulations to ensure a product’s identity, strength, quality, and purity. These products are also subject to the FDA’s general prohibition against promoting products for unapproved or “off-label” uses, and additional adverse reaction reporting.
As part of our BLA development effort, we are updating our manufacturing establishments into compliance with cGMP for production for our injectable product. We are also evaluating opportunities to partner with a contract manufacturing organization. The transition process includes development and enhancement of production processes, procedures, test and assays, and it requires extensive validation work. It can also involve the procurement and installation of new production or lab equipment. These efforts require human capital, expertise and resources. We have made significant improvements in this transition over the last year. We have engaged industry experts to assess our state of compliance and to provide guidance on the additional activities needed to meet cGMPs. Our goal is to achieve compliance with cGMP for our injectable commercial production systems by the time the FDA’s current period of enforcement discretion is complete in November 2020. See discussion below – “Risk Factors” under the heading “To the extent our products do not qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act, this could result in removal of the applicable products from the market, would make the introduction of new tissue products more expensive and would significantly delay the expansion of our tissue product offerings and subject us to additional post-market regulatory requirements,” and “We may be subject to fines, penalties, injunctions and even criminal sanctions if we are deemed to have made a misstatement of compliance to a federal agency.”
Other Regulation Specific to Tissue Products
National Organ Transplant Act
Procurement of certain human organs and tissue for transplantation is subject to the restrictions of the National Organ Transplant Act (“NOTA”), which prohibits the transfer of certain human organs, including skin and related tissue, for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. Our wholly-owned subsidiary, MiMedx Tissue Services, LLC, is registered with the FDA as an establishment that manufactures human cells, tissues and cellular and tissue-based productions and is involved with the recovery and storage of donated human amniotic tissue. We reimburse tissue banks, hospitals and physicians for their services associated with the recovery and storage of donated human tissue.
21
Tissue Bank Laws, Regulations, and Related Accreditation
As discussed above, we are required to register with the FDA as an establishment that manufactures human cells, tissues and cellular and tissue-based products. We also maintain state licensure as a human tissue bank in California, Georgia, Illinois, Maryland and New York. Additionally, we received and actively maintain AATB accreditation. The AATB has issued operating standards for tissue banking. Compliance with these standards is required in order to become an AATB-accredited tissue establishment. AATB standards include specific requirements for recovery, screening, testing, labeling and processing of placental tissue. We believe we are compliant in all material respects with AATB standards and our state licensure requirements.
To the extent we sell our products outside of the United States, we also are subject to laws and regulations of foreign countries.
Other Healthcare Laws and Compliance Requirements
In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including CMS, other divisions of the HHS (e.g., the Office of Inspector General), the DOJ and individual United States Attorney offices within the Department of Justice, and state and local governments. These regulations include those described below.
• | The federal Anti-Kickback Statute, which is a criminal law that prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward referrals, purchases or orders, or arranging for or recommending the purchase, order or referral of any item or service for which payment may be made in whole or in part by a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value. The Patient Protection and Affordable Care Act amended the intent requirement of the federal Anti-Kickback Statute, so that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. A conviction for violation of the Anti-Kickback Statute results in criminal fines and requires mandatory exclusion from participation in federal health care programs. Although there are a number of statutory exceptions and regulatory safe harbors to the federal Anti-Kickback Statute that protect certain common industry practices from prosecution, the exceptions and safe harbors are drawn narrowly, and arrangements may be subject to scrutiny or penalty if they do not fully satisfy all elements of an available exception or safe harbor. See discussion below under “Risk Factors–We and our sales representatives, whether employees or independent contractors, must comply with various federal and state anti-kickback, self-referral, false claims and similar laws, any breach of which could cause an adverse effect on our business, results of operations and financial condition.” |
• | The federal False Claims Act (“FCA”) imposes significant civil liability on any person or entity that knowingly presents, or causes to be presented, a claim for payment to the U.S. government, including the Medicare and Medicaid programs, that is false or fraudulent. The FCA also allows a private individual or entity as a whistleblower to sue on behalf of the government to recover civil penalties and treble damages. FCA liability is potentially significant in the healthcare industry because the statute provides for treble damages and mandatory penalties of between $11,181 and $22,363 per false claim or statement for penalties assessed after January 29, 2018, with respect to violations occurring after November 2, 2015. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government. |
• | The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) fraud and abuse provisions prohibit executing a scheme to defraud any healthcare benefit program, willfully obstructing a criminal investigation of a health care offense, or making false statements or concealing a material fact relating to payment for healthcare benefits, items or services. |
• | While manufacturers of human cell and tissue products regulated solely under Section 361 are not subject to the federal Physician Payments Sunshine Act and its implementing regulations (together with the Act, the “Sunshine Act”), in the future, if we receive a BLA, this law will require us (with certain exceptions) to report information to CMS related to certain payments or other transfers of value we make to U.S.-licensed physicians and teaching hospitals, and for reports submitted on or after January 1, 2022, physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists and certified nurse-midwives. If we receive a BLA, the Sunshine Act would also require us to report annually certain ownership and investment interests held by U.S.-licensed physicians and their immediate family members. Such information will subsequently be made publicly available by CMS on the Open Payments website. |
• | Federal conflicts of interest laws, the Standards of Ethical Conduct for Employees of the Executive Branch, and local site policies for each federal institution we call upon govern our interactions federal employees at our various government accounts (e.g., Department of Defense (“DoD”), VA, etc.) and impose a number of limitations on such interactions. |
22
• | There are state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payer, including commercial insurers, many of which differ from each other in significant ways and often are not preempted by federal laws, thus complicating compliance efforts. |
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”) and its implementing regulations, imposes certain requirements relating to the privacy, security and transmission of protected health information. Among other things, HITECH made HIPAA’s privacy and security standards directly applicable to “business associates,” independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates and possibly other persons and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Seasonality
We typically experience seasonality, with lower shipments in the first quarter of each year compared to the immediately preceding fourth quarter. This seasonal shipments pattern relates to U.S. annual insurance deductible resets and unfunded flexible spending accounts.
Research and Development
Our research and development group has extensive experience in developing products related to our field of interest, and works to design products that are intended to improve patient outcomes, simplify techniques, shorten procedures, reduce hospitalization and rehabilitation times and, as a result, reduce costs. Our research and development group also works to establish scientific evidence in support of the use of our products. Clinical trials that demonstrate the safety, efficacy and cost effectiveness of our products are key to obtaining broader reimbursement for our products. In addition to our internal staff, we contract with outside labs and physicians who aid us in our research and development process. See Part II, Item 7, below, for information regarding expenditures for research and development in each of the last three fiscal years.
Environmental Matters
Our tissue preservation activities generate a small amount of chemical and biomedical waste, consisting primarily of diluted alcohols and acids and human biological waste, including human tissue and body fluids removed during laboratory procedures. The biomedical waste generated by our tissue processing operations are placed in appropriately constructed and labeled containers and are segregated from other waste. We contract with third parties for transport, treatment, and disposal of our biomedical waste.
Employees
As of December 31, 2018, we had 753 employees, and as of December 31, 2019 we had 698 employees. We consider our relationships with our employees to be satisfactory. None of our employees are covered by a collective bargaining agreement.
Available Information; Unresolved Staff Comments
We are required to file proxy statements, annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K with the SEC. The SEC maintains an internet site, www.sec.gov, where these reports are available free of charge.
We also make these reports available free of charge on our website, www.mimedx.com, under the heading “Investors–SEC Filings.” In addition, our Audit Committee, Compensation Committee, Ethics and Compliance Committee, and Nominating and Corporate Governance Committee Charters as well as our Code of Business Conduct and Ethics, are on our website under the heading “Investors–Corporate Governance.” The reference to our website does not constitute incorporation by reference of any information contained on that site.
There are no unresolved SEC Staff comments with respect to our SEC filings.
23
Item 1A. Risk Factors
An investment in our Common Stock involves a substantial risk of loss. Set forth below are descriptions of those risks and uncertainties that we currently believe to be material, but the risks and uncertainties described below are not the only risks and uncertainties that could materially adversely affect our business, financial condition and operating results. If any of these risks materialize, our business, financial condition or operating results could suffer. In this case, the trading price of our Common Stock could decline, and you may lose part or all of your investment.
Risks Related to the Audit Committee Investigation, Consolidated Financial Statements, Internal Controls and Related Matters
We have identified deficiencies in our internal control over financial reporting which resulted in material weaknesses in our internal control over financial reporting, and we have concluded that our internal control over financial reporting and our disclosure controls and procedures were not effective as of December 31, 2018. If we fail to properly remediate these or any future material weaknesses or deficiencies or to maintain proper and effective internal controls, further material misstatements in our financial statements could occur and impair our ability to produce accurate and timely financial statements, preclude us from relisting our stock on a securities exchange, require significant expenditure of financial and other resources, give rise to litigation against us and otherwise affect our business, financial condition and operating results.
On December 4, 2018, Ernst & Young LLP (“EY”) notified the Audit Committee that EY was resigning from the engagement to audit the Company’s consolidated financial statements for the years ended December 31, 2018 and 2017, effective immediately. On the same date, EY also advised the Company that the internal controls necessary for the Company to develop reliable financial statements did not exist and identified additional matters involving operations that EY considered to be material weaknesses.
As discussed in the Explanatory Note to this Form 10-K, the Audit Committee Investigation concluded in May 2019 and found that the Company’s previously issued consolidated financial statements and financial information relating to the Non-Reliance Periods would need to be restated and could no longer be relied upon due to accounting irregularities regarding the recognition of revenue under GAAP. The Investigation also identified additional material weaknesses arising out of the Company’s revenue recognition practices and revenue management activities, material misstatements made by former members of Company management, actions taken against whistleblowers and an inappropriate tone set by former senior management.
We have concluded that our internal control over financial reporting was not effective as of December 31, 2018 due to the existence of material weaknesses in such controls, and we have also concluded that our disclosure controls and procedures were not effective as of December 31, 2018 due to material weaknesses in our control over financial reporting, all as described in the Explanatory Note and Item 9A, “Controls and Procedures,” of this Form 10-K.While we initiated meaningful remediation efforts during 2018 to address the identified weaknesses, we were not able to fully remediate our material weaknesses in internal controls as of December 31, 2018. Furthermore, while a substantial volume of additional control remediation measures were implemented between December 31, 2018 and the filing of this Form 10-K, we expect to conclude that these remediation efforts were not adequate to allow us to conclude that our control environment was effective and void of any material weaknesses as of December 31, 2019. One or more additional material weaknesses in our internal control over financial reporting might arise or be identified in the future. We intend to continue our control remediation activities and, in doing so, we will continue to incur expenses and expend management time on compliance-related issues.
If our remediation measures are insufficient to address the identified deficiencies, or if additional deficiencies in our internal control over financial reporting are discovered or occur in the future, our consolidated financial statements may contain material misstatements and we could be required to restate our financial results. Moreover, because of the inherent limitations of any control system, material misstatements due to error or fraud may not be prevented or detected on a timely basis, or at all. If we are unable to provide reliable and timely financial reports in the future, our business and reputation may be further harmed. Restated financial statements and failures in internal controls may also cause us to fail to meet reporting obligations, negatively affect investor confidence in our management and the accuracy of our financial statements and disclosures, or result in adverse publicity and concerns from investors, any of which could have a negative effect on the price of our Common Stock, subject us to further regulatory investigations and penalties or shareholder litigation, and adversely impact our business, results of operations and financial condition.
Matters relating to and arising out of the Audit Committee Investigation, including the accounting review of our previously issued consolidated financial statements and the audits of fiscal years 2018, 2017 and 2016, have been time consuming and expensive, and may result in additional expense.
We incurred significant expenses in connection with the Audit Committee Investigation, and we are continuing to incur significant expenses, including audit, legal, consulting and other professional fees, in connection with the ongoing review of our accounting
24
practices and systems, the audit of our financial statements and the remediation of deficiencies in our internal control over financial reporting. Specifically, in connection with the Audit Committee Investigation, audit and compliance efforts and related litigation, the Company incurred Investigation, Restatement and related expenses in the aggregate amount of approximately $68.3 million and $51.3 million for the years ended December 31, 2019 and 2018, respectively. To the extent our remediation efforts are unsuccessful or incomplete, or we identify additional problems requiring remediation, our management may be required to devote significant additional time to such efforts and we may be forced to incur significant additional expenses, including legal and accounting expenses. The incurrence of significant additional expense, or the requirement that management devote significant time that could reduce the time available to execute on our business strategies, could have an adverse effect on our business, results of operations and financial condition.
We will need to raise additional capital in the future, and our ability to raise capital on acceptable terms or at all is uncertain.
We require capital to execute our strategic priorities, fund the costs associated with the Restatement and the near-term efforts by the Company to address certain contingent liabilities relating to pending and threatened lawsuits, pending governmental investigations and other legal proceedings.
Our capital requirements will depend on many factors, including:
• | the revenues generated by sales of our products; |
• | the costs associated with expanding our sales and marketing efforts; |
• | the expenses we incur in manufacturing and selling our products; |
• | the costs of developing and commercializing new products or technologies; |
• | the cost of obtaining and maintaining regulatory approval or clearance of certain products and products in development; |
• | the costs associated with capital expenditures, including those required in connection with our efforts to become cGMP compliant; and |
• | unanticipated general and administrative expenses. |
We do not have current financial statements and are not current in our SEC filings. Additionally, our Common Stock was delisted from trading on The Nasdaq Capital Market in March 2019. As a result, we are significantly limited in our ability to access the capital markets to raise debt or equity capital. If we remain unable to access the capital markets on acceptable terms, or at all, our liquidity may be limited in a manner that has an adverse effect on our business, results of operations and financial condition.
If, in the future, we are able to issue equity or debt securities to raise capital, our existing shareholders may experience dilution, and any new equity or debt securities may have rights, preferences and privileges senior to those of our existing shareholders. In addition, if we raise capital through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our products, potential products or proprietary technologies, or grant licenses on terms that are not favorable to us.
If we cannot raise capital on acceptable terms, or at all, we may not be able to develop and expand our portfolio pipeline, advance our commercial strategy, enhance our business development efforts, advance our BLA programs, take advantage of future opportunities or respond to competitive pressure, changes in our supplier relationships or unanticipated customer requirements. Any of these events could adversely affect our ability to achieve our long-range goals, which could have an adverse effect on our business, results of operations and financial condition.
We are currently, and may in the future be, subject to substantial litigation and ongoing investigations that could cause us to incur significant legal expenses and result in harm to our business.
We are exposed to potential liabilities and reputational risk associated with litigation, regulatory proceedings and government enforcement actions. See Item 3, “Legal Proceedings” and Note 16, “Commitments and Contingencies” in the Consolidated Financial Statements for information regarding proceedings that we believe may be material to the Company as of the date of the filing of this Form 10-K. In addition, we are obligated to indemnify and advance expenses to certain individuals involved in certain of these proceedings. Further, volatility in our stock price may also make us vulnerable to future class action litigation.
Any adverse judgment in or settlement of any pending or any future litigation could result in payments, fines and penalties that could adversely affect our business, results of operations and financial condition. Regardless of the outcome, legal proceedings
25
have resulted in, and may continue to result in, significant legal fees and expenses, diversion of management’s time and other resources, and adverse publicity. Such proceedings could also adversely affect our business, results of operations and financial condition.
Our Common Stock might not be relisted, or once relisted, it might not remain listed.
Because we are not current in filing our periodic reports with the SEC, we were unable to comply with the listing standards of Nasdaq, and our Common Stock was suspended from trading on The Nasdaq Capital Market effective November 8, 2018 and was subsequently delisted effective March 8, 2019. After we have completed the Restatement and become current in our SEC reporting, we intend to apply to relist our Common Stock. However, we may not be able to do so in an expeditious manner or at all. We may be unable to relist our Common Stock, and even if our Common Stock is relisted, an active trading market may not develop or, if one develops, may not continue. The lack of an active trading market may limit the liquidity of an investment in our Common Stock, meaning you may not be able to sell any shares of Common Stock you own at times, or at prices, attractive to you. Any of these factors may adversely affect the price of our Common Stock.
Matters relating to or arising from the Restatement and the Audit Committee Investigation have had and could continue to have an adverse effect on our business, results of operations and financial condition.
We have been and could continue to be the subject of negative publicity focusing on the Restatement and the results of the Investigation. As a result, our customers or others with whom we do business have voiced concerns regarding the effort required to address our accounting and control environment and the ability for us to be a long-term provider to our customers. The continued occurrence of any of the foregoing could adversely affect our business, financial condition and results of operations.
Risks Related to Our Business and Industry
Our substantial indebtedness may adversely affect our ability to raise additional capital and our financial health.
As of December 31, 2019, we had approximately $73 million of debt outstanding, including $73 million aggregate principal amount of variable rate debt pursuant to our Loan Agreement, dated as of June 10, 2019 (the “Loan Agreement”), by and among the Company, the guarantors and lenders party thereto, and Blue Torch Finance LLC, as administrative agent and collateral agent. For more information, see Note 21, “Subsequent Events” in the Consolidated Financial Statements and Item 7, “Management’s Discussion and Analysis–Liquidity and Capital Resources.” Our substantial outstanding debt may limit our ability to borrow additional funds or may adversely affect the terms on which such additional funds may be available. Additionally, a default under certain other indebtedness constitutes an event of default under the Loan Agreement. Consequently, the effects of a default under other debt may be amplified by the lender exercising the remedies available to them in the Loan Agreement for events of default, including foreclosure on the collateral securing our obligations and the declaration that all amounts outstanding under the Loan Agreement are immediately due and payable. The limitations on our ability to access additional borrowing and the potential effects of a cross-default under the Loan Agreement may limit our liquidity and have an adverse effect on our business, financial condition, and results of operations.
Our variable rate indebtedness under the Loan Agreement subjects us to interest rate risk, which could result in higher expense in the event of increases in interest rates and adversely affect our business, financial condition, and results of operations.
Borrowings under the Loan Agreement bear interest at a rate equal to London Interbank Offered Rate (“LIBOR”) plus a margin of 8.00% per annum or (if LIBOR is not available) a prime rate plus a margin of 7.00% per annum. As a result, we are exposed to interest rate risk, which we do not hedge. If LIBOR or the applicable prime rate rises, the interest rate on outstanding borrowings under the Loan Agreement will increase. Therefore, an increase in LIBOR or the applicable prime rate will increase our interest payment obligations under the Loan Agreement and have a negative effect on our cash flows and liquidity, and could have a negative effect on our ability to make payments due under the Loan Agreement.
The restrictive covenants in the Loan Agreement and the Company’s obligation to make debt payments under the Loan Agreement may limit our operating and financial flexibility and may adversely affect our business, results of operations and financial condition.
The Loan Agreement imposes operating and financial restrictions and covenants, which may limit or prohibit our ability to, among other things:
• | incur indebtedness; |
• | make investments; |
26
• | incur liens; |
• | pay dividends; and |
• | engage in mergers and consolidations, sale and leasebacks and asset dispositions. |
In addition, we are required to comply with certain financial covenants under the Loan Agreement, including financial covenants that limit our maximum total leverage and require adherence to a minimum liquidity requirement. For more information, refer to Item 7, “Management’s Discussion and Analysis–Liquidity and Capital Resources.” We are also subject to certain reporting and performance obligations.
Such restrictive covenants in the Loan Agreement and the Company’s repayment obligations under the Loan Agreement could have adverse consequences to the Company, including:
• | limiting our flexibility in operating our business and planning for, or reacting to, changes in our business and our industry; |
• | limiting our ability to withstand a future downturn in our business or the economy in general; |
• | requiring the dedication of a portion of any cash flow from operations to the payment of principal of, and interest on, the indebtedness, thereby reducing the availability of such cash flow to fund our operations, working capital, capital expenditures, future business opportunities and other general corporate purposes; |
• | restricting us from making acquisitions or causing us to make divestitures; |
• | limiting our ability to obtain additional financing; |
• | limiting our ability to adjust to changing market conditions; and |
• | placing us at a competitive disadvantage relative to our competitors who are less highly leveraged. |
If we fail to comply with the terms of the Loan Agreement and there is an event of default, the lender may foreclose upon the collateral securing our obligations under the Loan Agreement. To secure the performance of our obligations under the Loan Agreement, the Company and its subsidiaries granted the lender a security interest in substantially all of its assets. The foreclosure on the collateral assets could adversely impact our business, financial condition, and results of operations.
Additionally, if we fail to comply with the covenants contained in the Loan Agreement, it could result in an event of default under the Loan Agreement, which could result in the lender declaring all amounts outstanding thereunder to be immediately due and payable. For example, if the Company’s net revenue decreases by more than 5% in 2020, the Company might exceed the applicable maximum leverage ratio permitted by the Loan Agreement during the second half of 2020. There can be no assurances that we will be able to repay all such amounts or able to find alternative financing in an event of a default. Even if alternative financing is available in an event of a default under the Loan Agreement, it may be on unfavorable terms, and the interest rate charged on any new borrowings could be substantially higher than the interest rate under the Loan Agreement, thus adversely affecting our cash flows, liquidity, and results of operations. Acceleration of the repayment of the loan pursuant to the terms of the Loan Agreement, in combination with the Company’s current commitments and contingent liabilities, could also cast doubt on the Company’s ability to continue as a going concern.
If we do not successfully execute our priorities, our business, operating results and financial condition could be adversely affected.
Our priorities are to participate in the growth in the advanced wound care category, increase the Company’s market share by demonstrating the positive health economics of our products, and accelerate the timeline to achieve our long-range growth objectives, including our BLA pipeline. We have sought and intend to continue to seek capital to implement our priorities, which include advancing our BLA programs and seeking FDA approval for micronized dHACM to treat musculoskeletal degeneration across multiple indications.
In developing our priorities, we evaluated many factors including, without limitation, those related to developments in our industry, customer demand, competition, regulatory developments, the ability of the Company to execute a capital raise and general economic conditions. Actual conditions may be different from our assumptions, and we may not be able to successfully execute our priorities or obtain capital on acceptable terms, if at all. If we do not successfully execute our priorities, or if actual results vary significantly from our assumptions, our business, operating results and financial condition could be adversely impacted.
27
In addition, managing our growth may be more difficult than we expect. We anticipate that a period of significant expansion will be required to penetrate and service the market for our existing and anticipated future products and to continue to develop new products. This expansion will place a significant strain on management and operational and financial resources. To manage the expected growth of our operations and personnel, we must both modify our existing operational and financial systems, procedures and controls and implement new systems, procedures and controls. We must also expand our finance, administrative and operations staff. Management may be unable to hire, train, retain, motivate and manage necessary personnel or to identify, manage and exploit existing and potential relationships and market opportunities.
We are in a highly competitive and evolving field and face competition from well-established tissue processors and medical device manufacturers, as well as new market entrants.
Our business is in a very competitive and evolving field. Competition from other tissue processors, medical device companies, and biotherapeutic companies, and from research and academic institutions, is intense, expected to increase and subject to rapid change and could be significantly affected by new product introductions. Established competitors and newer market entrants are investing in additional clinical research that may allow them to gain further clinician usage, adoption and payer coverage of their products. In addition, consolidation in the healthcare industry continues to give rise to demands for price concessions, which could have an adverse effect on our business, results of operations and financial condition. Further, competitors may introduce amniotic membrane products in the future at lower prices, adding new features or gaining additional reimbursement coverage. Further, they may copy our products outside the United States. The presence of this competition may lead to pricing pressure, which could have an adverse effect on our business, results of operations and financial condition.
Rapid technological change could cause our products to become obsolete and, if we do not enhance our product offerings through our research and development efforts, we may be unable to compete effectively.
The technologies underlying our products are subject to rapid and profound technological change. Competition intensifies as technical advances in each field are made and become more widely known. Others may develop services, products or processes with significant advantages over the products, services and processes that we offer or are seeking to develop. Any such occurrence could have an adverse effect on our business, results of operations and financial condition.
We plan to enhance and broaden our product offerings in response to changing customer demands and competitive pressure and technologies. The success of any new product offering or enhancement to an existing product will depend on numerous factors, including our ability to:
• | properly identify and anticipate physician and patient needs; |
• | acquire, through licensing, co-development or outright purchase, new technology developed outside of MiMedx; |
• | develop and introduce new products or product enhancements in a timely manner; |
• | adequately protect our intellectual property and avoid infringing upon the intellectual property rights of third parties; |
• | demonstrate the safety and efficacy of new products; and |
• | obtain the necessary regulatory clearances or approvals for new products or product enhancements. |
If we do not develop and, when necessary, obtain regulatory clearance or approval for new products or product enhancements in time to meet market demand, or if there is insufficient demand for these products or enhancements, our results of operations and financial condition will suffer. Our research and development efforts may require a substantial investment of time and resources before we are adequately able to determine the commercial viability of a new product, technology, material or other innovation. In addition, even if we are able to successfully develop enhancements or new generations of our products, these enhancements or new generations of products may not produce sales in excess of the costs of development, or they may never receive required regulatory approval and they may be quickly rendered obsolete by changing customer preferences or the introduction by our competitors of products embodying new technologies or features.
28
Our products depend on the availability of tissue from human donors, and any disruption in supply could adversely affect our business.
The success of our human tissue products depends upon, among other factors, the availability of tissue from human donors. Any failure to obtain tissue from our sources will interfere with our ability to effectively meet demand for our products incorporating human tissue. The availability of donated tissue could also be adversely impacted by regulatory changes, public opinion of the donor process and our own reputation in the industry. Obtaining adequate supplies of human tissue involves several risks, including limited control over availability, quality and delivery schedules. In addition, any interruption in the supply of any human tissue component could harm our ability to manufacture our products until a new source of supply, if any, could be found. We may be unable to find a sufficient alternative supply channel in a reasonable time period or on commercially reasonable terms, if at all, which would have an adverse effect on our business, results of operations and financial condition.
Health epidemics in regions where we have operations, sales and marketing teams, manufacturing facilities or other business operations could harm our business, results of operations and financial condition.
Significant epidemics or other disruptions to public health, including the novel coronavirus or COVID-19, could harm our operations and increase our costs and expenses in numerous ways. If our leadership, employees, sales agents, suppliers, medical professionals, or users of our products are impacted by an epidemic by illness or through social distancing, quarantine or other precautionary measures, our manufacturing operations, clinical trials, sales, and demand for our product may be adversely affected. Additionally, if we experience shortages of donated placentas because donors or our recovery specialists are excluded from hospitals, or because donated tissues are screened as ineligible under AATB or other standards, our results of operations may be adversely affected. Disruptions to the health care system also may adversely affect our business if health care providers restrict access to their facilities by our sales personnel for a material amount of time (and we have begun to receive notices from some of our hospital customers who are restricting access to only essential personnel, if patients are unable or unwilling to visit health care providers, or if health care providers prioritize treatment of acute or communicable illnesses over wound care. If the COVID-19 outbreak continues to spread domestically or internationally, the risks described herein could be elevated significantly. The ultimate impact of the COVID-19 outbreak or a similar health epidemic is highly uncertain and subject to change. We do not yet know the full extent of potential delays or impacts on our business, our clinical trials, healthcare systems or the global economy as a whole. However, the effects could have an adverse impact on our business, results of operations and financial condition.
We depend on our senior leadership team and may not be able to retain or replace these employees or recruit additional qualified personnel, which would harm our business, results of operations and financial condition.
Our business and success are materially dependent on attracting and retaining members of our senior leadership team to formulate and execute the Company’s business plans. Since June 2018, we have needed to replace a number of our senior leadership team members including our Chief Executive Officer, Chief Financial Officer, Chief Operating Officer, Controller, and General Counsel and Secretary. We had an interim CEO from July 2018 until Mr. Wright was appointed CEO effective May 13, 2019, and we had an interim CFO from June 2018 to November 2019. As of the date of the filing of this Form 10-K, our interim CFO continues to serve as acting CFO. See discussion above under “Business–Recent Developments.” Since early 2018, we have experienced difficulties in recruiting due to legal and business uncertainties resulting from the issues which were the subject of the Audit Committee Investigation.
Leadership changes can be inherently difficult to manage and may cause material disruption to our business or management team. Changes in senior management could also lead to an environment that presents additional challenges in recruiting and retaining employees, which could have an adverse effect on our business, results of operations and financial condition. Our success will depend, in part, upon our ability to attract and retain skilled personnel, including sales, managerial and technical personnel. There can be no assurance that we will be able to find and attract additional qualified employees to support our expected growth or retain any such personnel. We have experienced higher than normal attrition in our general workforce since June 2018. Our inability to hire and retain qualified personnel or the loss of services of our key personnel may have an adverse effect on our business, results of operations and financial condition.
A significant portion of our revenues and accounts receivable come from government accounts.
We have significant sales to the government (whether we are selling our products directly to government accounts or through a distributor). Any disruption of our products on the Federal Supply Schedule (“FSS”), or of the use of Indefinite Delivery, Indefinite Quantity contracts, or any change in the way the government purchases products like ours or the price it is willing to pay for our products, could adversely affect our business, results of operations and financial condition. Similarly, competitive pricing pressures and any non-compliance with applicable guidelines could cause the Company to lose existing or future contracts with the VA, which may result in an overall decline in revenue.
29
During 2018 and 2019, the Company conducted a comprehensive review of its pre- and post-award VA sales under its FSS contract and, at December 31, 2018, had accrued an obligation of $6.9 million in connection with a potential issue that it self-disclosed to the VA concerning the eligibility of one of its products for inclusion in the Company’s FSS contract. See Note 16, “Commitments and Contingencies,” below. As discussed below in “Item 3 - Legal Proceedings,” the Company has reached an agreement in principle to settle this matter for an amount within the Company’s reserve. However, any resulting negative impact to our contractual relationship with the VA going forward may adversely affect our business, results of operations and financial condition.
Our revenues depend on adequate reimbursement from public and private insurers and health systems.
Our success depends on the extent to which our customers receive adequate reimbursement for the costs of our products and related treatments from third-party payers, including government healthcare programs, such as Medicare and Medicaid, as well as private insurers and health systems. Government and other third-party payers attempt to contain healthcare costs by limiting both coverage and the level of reimbursement of medical products, particularly new products. Therefore, significant uncertainty usually exists as to the reimbursement status of new healthcare products by third-party payers. Although EpiFix has coverage with the majority of payers, a significant number of public and private insurers and health systems currently do not cover or reimburse our other products. If we are not successful in obtaining adequate coverage and reimbursement for our products from these third-party payers, it could have an adverse effect on market acceptance of our products. Inadequate reimbursement levels would likely also create downward price pressure on our products. Even if we do succeed in obtaining widespread coverage and reimbursement rates or policies for our products, future changes in coverage or reimbursement rates or policies could have a negative impact on our business, financial condition and results of operations. For example, through its rule-making process, CMS has requested stakeholder comments on the reimbursement methodology under the Medicare Hospital Outpatient Prospective Payment System for an episode of wound care for future years. In other words, the Medicare reimbursement payment methodology may change after 2020 in the hospital outpatient setting from the current reimbursement methodology, which is based on a bundled payment amount per wound care application (i.e. per skin substitute application), to a fixed, global payment to treat the wound until it is healed (i.e. a lump sum payment that covers the entire wound care episode). We are unable to assess the potential effects of these reimbursement changes on our business at this time, as it is not clear if any changes will take effect and CMS has not disclosed specific reimbursement details for a wound episode model. We are and will continue to participate in discussions with CMS on potential solutions for future wound episode reimbursement models.
Further, we have experienced some reluctance by payers to cover products for applications other than those we have published clinical trials. For example, Noridian, the MAC for 13 states, published a Local Coverage Article effective November 8, 2018 that limits coverage for amniotic membrane derived skin substitute products to diabetic foot ulcers and venous stasis ulcers only. Prior to the published article, Noridian did not have a written policy on the matter, which provided a pathway for physicians to utilize amniotic membrane derived skin substitute products, such as ours, based on medical necessity in a wide variety of wounds. Currently, there are three MACs that do not have a written medical policy in the form of a Local Coverage Determination (“LCD”) or article. If the three MACs created written medical policy criteria, this could limit providers to the use of products that have published clinical evidence for a specific wound type. As a result of the Noridian published article, our revenues for 2019 declined significantly compared to 2018. Our future revenues could experience additional declines if other MACs or other payers further limit their coverage of our products. This decline would adversely affect our business, financial condition and results of operations.
Our revenue, results of operations and cash flows may suffer upon the loss of a GPO or IDN.
As with many manufacturers in the healthcare space, the Company contracts with GPOs and IDNs to establish contracted pricing and terms and conditions for the members of GPOs and IDNs. Approximately two-thirds of our sales in the fiscal year ended December 31, 2018 came from customers that are members of our main GPOs or IDNs.
Our agreements with GPOs and IDNs allow us to sell our products efficiently to large groups of customers. Our agreements with GPOs and IDNs typically provide their members with favorable ordering terms and conditions and access to favorable product pricing. These customers purchase our product through GPO and IDN arrangements in part because of favorable pricing and terms and conditions. If our agreement with any GPO or IDN is terminated or expires without being extended, renewed or renegotiated this could adversely affect our revenue, results of operations and cash flows.
We contract with independent sales agents and distributors.
In 2018, we derived approximately 15% of our sales through our relationships with independent agents and distributors. (Sales agents act directly on behalf of MiMedx to arrange sales, while distributors take title to product and may set their own prices.) See Note 17, “ Revenue Date by Customer Type.”
Because our agents and distributors are not employees, there is a risk we will be unable to ensure that our sales processes, compliance safeguards, and related policies will be adhered to despite our communication of these requirements. If we fail to maintain
30
relationships with our key independent agents, or fail to ensure that our independent agents adhere to our sales processes, compliance safeguards and related policies, there could be an adverse effect on our business, results of operations, and financial condition.
Also, if our relationships with our independent sales agents or distributors were terminated for any reason, it could materially and adversely affect our revenues and profits. Because the independent agent often controls the customer relationships within its territory, there is a risk that if our relationship with the agent ends, our relationship with the customer will be lost.
We may obtain the assistance of additional distributors and independent sales representatives to sell products in certain sales channels, particularly in territories and fields where agents are commonly used. Our success is partially dependent upon our ability to retain and motivate our independent sales agencies, distributors, and their representatives to appropriately and compliantly sell our products in certain territories or fields. They may not be successful in implementing our marketing plans or compliance safeguards. Some of our independent sales agencies and distributors do not sell our products exclusively and may offer similar products from other companies. Our independent sales agencies and distributors may terminate their contracts with us, may devote insufficient sales efforts to our products or may focus their sales efforts on other products that produce greater commissions for them, which could have an adverse effect on our business, results of operations and financial condition. We also may not be able to find additional independent sales agencies and distributors who will agree to appropriately and compliantly market or distribute our products on commercially reasonable terms, if at all. If we are unable to establish new independent sales representative and distribution relationships or renew current sales agency and distribution agreements on commercially acceptable terms, our business, financial condition, and results of operations could be materially and adversely affected.
Disruption of our processing could adversely affect our business, financial condition and results of operations.
Our business depends upon the continued operation of our processing facilities in Marietta, Georgia and Kennesaw, Georgia. Risks that could impact our ability to use these facilities include the occurrence of natural and other disasters, and the need to comply with the requirements of directives from government agencies, including the FDA. Either of our processing facilities can serve as a redundant processing facility for our Section 361 products in the event the other facility experiences a disaster event. We have made efforts to transition manufacturing into compliance with cGMPs for commercial production for our injectable product. These efforts are concentrated at our Kennesaw, Georgia facility. However, the unavailability of our processing facilities could have a material adverse effect on our business, financial condition and results of operations during the period of such unavailability.
To be commercially successful, we must convince physicians, where appropriate, that our products are proper alternatives to existing treatments and that our products should be used in their procedures.
We believe physicians will only use our products if they determine, based on their independent medical judgment and experience, clinical data, and published peer reviewed journal articles, that the use of our products in a particular procedure is a favorable alternative to other treatments. Physicians may be hesitant to change their existing medical treatment practices for the following reasons, among others:
• | their lack of experience with prior procedures in the field using our products; |
• | lack of evidence supporting additional patient benefits of our products over conventional methods in certain therapeutic applications; |
• | perceived liability risks generally associated with the use of new products and procedures; |
• | limited availability of reimbursement from third-party payers; and |
• | the time that must be dedicated to physician training in the use of our products. |
If we cannot successfully address quality issues that may arise with our products, our brand and reputation could suffer, and our business, financial condition, and results of operations could be adversely impacted.
In the course of conducting our business, we must adequately address quality issues that may arise with our products, as well as defects in third-party components included in our products, as any quality issues or defects may negatively impact physician use of our products. Although we have established internal procedures to minimize risks that may arise from quality issues, we may not be able to eliminate or mitigate occurrences of these issues and associated liabilities. If the quality of our products does not meet the expectations of physicians or patients, then our brand and reputation could suffer and our business could be adversely impacted. We must also ensure any promotional claims made for our products comport with government regulations.
The formation of physician-owned distributorships (“PODs”) could result in increased pricing pressure on our products or harm our ability to sell our products to physicians who own or are affiliated with those distributorships.
31
PODs are medical product distributors that are owned, directly or indirectly, by physicians. These physicians derive a proportion of their revenue from selling or arranging for the sale of medical products for use in procedures they perform on their own patients at hospitals that agree to purchase from or through the POD, or that otherwise furnish ordering physicians with income that is based directly or indirectly on those orders of medical products. The Office of Inspector General (“OIG”) of the Department of Health & Human Services has issued a Special Fraud Alert on PODs, indicating that they are inherently suspect under the federal Anti-Kickback Statute.
Our commercial strategy emphasizes selling directly to healthcare providers and, to a limited extent, through distributors. To our knowledge, we do not directly sell to or distribute any of our products through PODs. The number of PODs in the industry may continue to grow as economic pressures increase throughout the industry and hospitals, insurers and physicians search for ways to reduce costs, and, in the case of the physicians, search for ways to increase their incomes. These companies and the physicians who own, or partially own, PODs have significant market knowledge and access to the physicians who use our products and the hospitals that purchase our products, and we may not be able to compete effectively for business from physicians who own PODs.
We face the risk of product liability claims and may not be able to obtain or maintain adequate product liability insurance.
Our business exposes us to the risk of product liability claims that are inherent in the manufacturing, processing and marketing of human tissue products. We may be subject to such claims if our products cause, or appear to have caused, an injury. Claims may be made by patients, healthcare providers or others selling our products. Defending a lawsuit, regardless of merit, could be costly, divert management attention and result in adverse publicity, which could result in the withdrawal of, or reduced acceptance of, our products in the market.
Although we have product liability insurance that we believe is adequate, this insurance is subject to deductibles and coverage limitations, and we may not be able to maintain this insurance. Also, it is possible that claims could exceed the limits of our coverage. If we are unable to maintain product liability insurance at an acceptable cost or on acceptable terms with adequate coverage or otherwise protect ourselves against potential product liability claims or we underestimate the amount of insurance we need, we could be exposed to significant liabilities, which may harm our business. A product liability claim or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could result in significant costs and significant harm to our business.
The products we manufacture and process are derived from human tissue and therefore have the potential for disease transmission.
The utilization of human tissue creates the potential for transmission of communicable disease, including, without limitation, human immunodeficiency virus, viral hepatitis, syphilis and other viral, fungal or bacterial pathogens. We are required to comply with federal and state regulations intended to prevent communicable disease transmission.
We maintain strict quality controls designed in accordance with cGTP to ensure the safe procurement and processing of our tissue. These controls are intended to prevent the transmission of communicable disease. However, risks exist with any human tissue implantation. We are also in the process of attempting to develop and enhance cGMP systems to comply with the regulations that will apply to our Section 351 HCT/Ps following the end of the FDA’s enforcement discretion period under the Guidance. In addition, negative publicity concerning disease transmission from other companies’ improperly processed donated tissue could have a negative impact on the demand for our products and adversely affect our business, financial condition and results of operations.
We may implement a product recall or voluntary market withdrawal, which could significantly increase our costs, damage our reputation, disrupt our business and adversely affect our business, results of operations and financial condition.
The processing and marketing of our tissue products involves an inherent risk that our tissue products or processes do not meet applicable quality standards and requirements. In that event, we may voluntarily implement a recall or market withdrawal or may be required to do so by a regulatory authority. A recall or market withdrawal of one of our products would be costly and would divert management resources. A recall or withdrawal of one of our products, or a similar product processed by another entity, also could impair sales of our products as a result of confusion concerning the scope of the recall or withdrawal, or as a result of the damage to our reputation for quality and safety.
Significant disruptions of information technology systems or breaches of information security could adversely affect our business, results of operation and financial condition.
A breach of cybersecurity, a disruption in availability, or the unauthorized alteration of systems or data could adversely affect our business, results of operations and financial condition. We rely on technology for day-to-day operations as well as positioning to
32
enhance our stance in the market. We generate intellectual property that is central to the future success of the business and transmit large amounts of confidential information. Additionally, we collect, store and transmit confidential information of customers, patients, employees and third parties. We also have outsourced significant elements of our operations to third parties, including significant elements of our information technology infrastructure, and, as a result, we are managing many independent vendor relationships with third parties who may or could have access to our confidential information. The continually changing threat landscape of cybersecurity today makes our systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees, partners, and vendors, and from attacks by malicious third parties, including supply chain attacks originating at our third-party partners. Such attacks are of ever-increasing levels of sophistication. Attacks are made by individuals or groups that have varying levels of expertise, some of which are technologically advanced and well-funded including, without limitation, nation states, organized criminal groups and hacktivists organizations.
To ensure protection of our information, we have invested in cybersecurity and have implemented processes and procedural controls to maintain the confidentiality and integrity of such information. We measure these controls and their success through a cybersecurity framework that is based on industry standards. While we have invested in the protection of our data and technology, there can be no guarantees that our efforts will prevent all service interruptions or security breaches. Any such interruption or breach of our systems could adversely affect our business operations and result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal and reputational harm to our business, including legal claims and proceedings, liability under laws that protect the privacy of personal information, government enforcement actions and regulatory penalties, as well as remediation costs. We maintain cyber liability insurance. However, this insurance may not be sufficient to cover the financial, legal or reputational losses that may result from an interruption or breach of our systems.
We may expand or contract our business through acquisitions, divestitures, licenses, investments, and other commercial arrangements in other companies or technologies, which may adversely affect our business, results of operations and financial condition.
We periodically evaluate opportunities to acquire or divest companies, divisions, technologies, products, and rights through licenses, distribution agreements, investments, and outright acquisitions to grow our business. In connection with one or more of those transactions, we may:
• | issue additional equity securities that would dilute the value of equity currently held by our shareholders; |
• | divest or license existing products or technology; |
• | use cash that we may need in the future to operate our business; |
• | incur debt that could have terms unfavorable to us or that we might be unable to repay; |
• | structure the transaction in a manner that has unfavorable tax consequences, such as a stock purchase that does not permit a step-up in the tax basis for the assets acquired; |
• | be unable to realize the anticipated benefits, such as increased revenues, cost savings, or synergies from additional sales; |
• | be unable to secure the services of key employees related to the acquisition; and |
• | be unable to succeed in the marketplace with the acquisition. |
Any of these items could adversely affect our revenues, results of operations and financial condition. Business acquisitions also involve the risk of unknown liabilities associated with the acquired business, which could be material. Incurring unknown liabilities or the failure to realize the anticipated benefits of an acquisition could adversely affect our business if we are unable to recover our initial investment. Inability to recover our investment, or any write off of such investment, associated goodwill or assets could have an adverse effect on our business, results of operations and financial condition.
Our international expansion and operations outside the U.S. expose us to risks associated with international sales and operations.
We may consider further expansion outside the U.S. Managing a global organization is difficult, time consuming and expensive. Conducting international operations subjects us to risks that could be different than those faced by us in the United States. The sale and shipment of our products across international borders, as well as the purchase of components and products from international sources, subject us to extensive U.S. and foreign governmental trade, import and export and customs regulations and laws, including, without limitation, the Export Administration Regulations and trade sanctions against embargoed countries, which are administered
33
by the Office of Foreign Assets Control within the Department of the Treasury, as well as the laws and regulations administered by the Department of Commerce. These regulations limit our ability to market, sell, distribute or otherwise transfer our products or technology to prohibited countries or persons. Additionally, MiMedx and our international distributors are subject to the Foreign Corrupt Practices Act and the UK Anti-Bribery statures. Additionally, international regulations on allowable promotional claims make the promotion of our products more difficult.
Compliance with these regulations and law is costly, and failure to comply with applicable legal and regulatory obligations could adversely affect us in a variety of ways that include, without limitation, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments and restrictions on certain business activities. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our distribution and sales activities.
These risks may limit or disrupt our expansion, restrict the movement of funds or result in the deprivation of contractual rights or the taking of property by nationalization or expropriation without fair compensation. Operating outside of the U.S. also requires significant management attention and financial resources.
If any of the BLAs are approved, the Company would be subject to additional regulation which will increase costs and could result in adverse sanctions for non-compliance.
Products subject to the FDA’s BLA requirements must comply with a range of pre- and post-market provisions. Pre-market compliance includes the conduct of clinical trials in support of BLA approval, the development and submission of a BLA, and the production of product for use in the clinical trials that meets FDA’s quality expectations. Post-approval requirements for BLA products include compliance with cGMPs, which will require us to make enhancements in our fixed plant as well as incur regular costs and reduced product yields from testing products to ensure identity, strength, quality and purity; compliance with promotional and labeling requirements, which limit our ability to make claims about regulated products; submission of annual reports in appropriate circumstances; compliance with the FDA’s “Biological Product Deviation Reporting System,” when applicable; “submission of adverse events;” reporting and correcting product problems within established timeframes; recalling or stopping the manufacture of a product if a significant problem is detected; complying with the appropriate laws and regulations relevant to the biologics license; and identifying any changes needed to help ensure product quality. In some instances, the FDA can also require that applicants conduct post-market studies or trials of the product. This additional compliance burden may increase costs, and failure to comply with such requirements may subject the Company to sanctions that would have an adverse impact on our business, results of operations and financial condition.
New lines of business or new products and services may subject us to additional risks.
From time to time, we may implement or may acquire new lines of business or offer new products and services within existing lines of business. There are risks and uncertainties associated with these efforts, particularly in instances where the markets are not fully developed or are evolving. In developing and marketing new lines of business and new products and services, we may invest significant time and resources. External factors, such as regulatory compliance obligations, competitive alternatives, and shifting market preferences, may also impact the successful implementation of a new line of business or a new product or service. Failure to successfully manage these risks in the development and implementation of new lines of business or new products or services could have an adverse effect on our business, results of operations and financial condition.
Risks Related to Our Intellectual Property
Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain and may be inadequate, which could have an adverse effect on our business, results of operations and financial condition.
Our success depends significantly on our ability to protect our proprietary rights to the technologies used in our products. We rely on patent protection, as well as a combination of copyright, trade secret and trademark laws and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology, including our licensed technology. These legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. In addition, our pending patent applications include claims to material aspects of our products and procedures that are not currently protected by issued patents. The patent application process can be time consuming and expensive. Our pending patent applications might not result in issued patents. Competitors may be able to design around our patents or develop products that provide outcomes that are comparable or even superior to ours. Although we have taken steps to protect our intellectual property and proprietary technology, including entering into confidentiality agreements and intellectual property assignment agreements with some of our officers, employees, consultants and advisors, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements.
34
The failure to obtain and maintain patents or protect our intellectual property rights could have an adverse effect on our business, results of operations, and financial condition. Whether a patent claim is valid is a complex matter of science, facts and law, and therefore we cannot be certain that, if challenged, our patent claims would be upheld. If any of those patent claims are invalidated, our competitive advantage may be reduced or eliminated.
In the event a competitor infringes upon our licensed or pending patent or other intellectual property rights, enforcing those rights may be costly, uncertain, difficult and time consuming. Even if successful, litigation to enforce or defend our intellectual property rights could be expensive and time consuming and could divert our management’s attention. Further, bringing litigation to enforce our patents subjects us to the potential for counterclaims. Other companies or entities also have commenced, and may again commence, actions seeking to establish the invalidity of our patents and certain related claims. In the event that any of our patents claims are challenged, a court, the United States Patent and Trademark Office (“USPTO”), or the Patent Trial and Appeal Board (“PTAB”) of the USPTO may invalidate one or more challenged patent claims or determine that the patent is unenforceable, which could harm our competitive position. If the USPTO or the PTAB ultimately cancels or narrows the claim scope of any of our patents through these proceedings, it could prevent or hinder us from being able to enforce them against competitors. Such adverse decisions could negatively impact our business, results of operations and financial condition. See Item 3, “Legal Proceedings” for information regarding our ongoing patent infringement lawsuits and related inter partes review proceedings.
In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in enforcing and defending intellectual property rights in certain foreign jurisdictions. This could make it difficult for us to stop infringement of our foreign patents, if obtained, or the misappropriation of our other intellectual property rights. For example, some foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, some countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in some countries may be inadequate.
We may become subject to claims of infringement of the intellectual property rights of others, which could prohibit us from developing our products, require us to obtain licenses from third parties or to develop non-infringing alternatives, and subject us to substantial monetary damages.
Third parties could assert that our products infringe their patents or other intellectual property rights. Whether a product infringes a patent or other intellectual property involves complex legal and factual issues, the determination of which is often uncertain. Therefore, we cannot be certain that we have not infringed the intellectual property rights of others. Because patent applications may take years to issue, there also may be applications now pending of which we are unaware that may later result in issued patents that our products or processes infringe. There also may be existing patents or pending patent applications of which we are unaware that our products or processes may inadvertently infringe.
Any infringement claim could cause us to incur significant costs, place significant strain on our financial resources, divert management’s attention from our business and harm our reputation. If the relevant patent claims at issue in such a dispute were upheld as valid and enforceable and we were found to infringe, we could be prohibited from selling any product that is found to infringe those claims unless we could obtain licenses to use the technology covered by the asserted patent claims or other intellectual property, or are able to design around the patent claim or claims at issue or other intellectual property. We may be unable to obtain such a license on terms acceptable to us, if at all, and we may not be able to redesign our products to avoid infringement. A court could also order us to pay compensatory damages for such infringement, plus prejudgment interest and could, in addition, treble the compensatory damages and award attorney fees. These damages could be substantial and could harm our reputation, business, financial condition and operating results. A court also could enter orders that temporarily, preliminarily or permanently enjoin us and our customers from making, using, or selling products, and could enter an order mandating that we undertake certain remedial measures. Depending on the nature of the relief ordered by the court, we could become liable for additional damages to third parties.
We may be subject to damages resulting from claims that we, our employees, or our independent contractors have wrongfully used or disclosed alleged trade secrets, proprietary or confidential information of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
Some of our employees were previously employed at other medical device, pharmaceutical or tissue companies. We may also hire additional employees who are currently employed at other medical device, pharmaceutical or tissue companies, including our competitors. Additionally, consultants or other independent agents with which we may contract may be or have been in a contractual
35
arrangement with one or more of our competitors. Although no claims are currently pending, we may be subject to claims that we, our employees, or our independent contractors have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of these former employers or competitors. In addition, we have been and may in the future be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail to defend such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Any future litigation or the threat thereof may adversely affect our ability to hire additional direct sales representatives. A loss of key personnel or their work product could hamper or prevent our ability to market existing or new products, which could severely harm our business, financial condition and operating results.
Risks Related to Regulatory Approval of Our Products and Other Government Regulations
To the extent our products do not qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act, this could result in removal of the applicable products from the market, would make the introduction of new tissue products more expensive and would significantly delay the expansion of our tissue product offerings and subject us to additional post-market regulatory requirements.
The products we manufacture and process are derived from human tissue. Amniotic and other birth tissue is generally regulated as an HCT/P and is therefore eligible for regulation solely as a Section 361 HCT/P depending on whether the specific product at issue and the claims made for it are consistent with the applicable criteria. HCT/Ps that do not meet these criteria are subject to more extensive regulation as drugs, medical devices, biological products, or combination products. These HCT/Ps must comply with both the FDA’s requirements for HCT/Ps and the requirements applicable to biologics, devices or drugs, including pre-market clearance or approval from the FDA. Obtaining FDA pre-market clearance or approval involves significant time and investment by the Company.
In November 2017, the FDA released a guidance document entitled “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue – Based Products: Minimal Manipulation and Homologous Use – Guidance for Industry and Food and Drug Administration Staff.” The document confirmed the FDA’s stance that all micronized amniotic products require a biologics license to be lawfully marketed in the United States. It also indicated that sheet forms of amniotic tissue are appropriately regulated as solely Section 361 HCT/Ps when manufactured in accordance with 21 CFR Part 1271 and intended for use as a barrier or covering. The final guidance also stated that the FDA intends to exercise enforcement discretion under limited conditions with respect to the IND application and pre-market approval requirements for certain HCT/Ps for a period of 36 months from the date of the guidance. The FDA’s approach is risk-based, and the guidance clarified that high-risk products and uses could be subject to immediate enforcement action. MiMedx continues to market AmnioFix Injectable and other micronized products under the policy of enforcement discretion as it works on the transition from Section 361 products to Section 351 products. Our sales of micronized products for all uses was $23.0 million, $45.0 million and $68.4 million, respectively, in 2016, 2017, and 2018. At the same time, we are pursuing the BLA pre-market approval process for certain of our micronized products, as more fully discussed under “Business – Government Regulation.” It is unclear whether all of our micronized products will be deemed to be Section 351 products following the end of the enforcement discretion period.
Following the period of enforcement discretion under the Guidance, we may need to cease selling our micronized, injectable products and other products regulated under Section 351 until the FDA approves a BLA, and then we will only be able to market such products for indications that have been approved in a BLA. The loss of our ability to market and sell our micronized, injectable products would have an adverse impact on our revenues, business, financial condition and results of operations. In addition, we expect the cost to manufacture our products will increase due to the costs to comply with the requirements that apply to Section 351 biological products such as current cGMP and ongoing product testing costs. Increased costs relating to regulatory compliance could have an adverse impact on our business, financial condition and results of operations.
In addition, the FDA might, at some future point, modify the scope of its enforcement discretion or change its position on which current or future products qualify as Section 361 HCT/Ps, or determine that some or all of our micronized products may not be lawfully marketed under the FDA’s policy of enforcement discretion. Any regulatory changes could have adverse consequences for us and make it more difficult or expensive for us to conduct our business by requiring pre-market clearance or approval and compliance with additional post-market regulatory requirements with respect to those products. It is also possible that the FDA could decide it will not allow the Company to market any form of a micronized product during the rest of the 36-month enforcement discretion period without a biologics license, and it could even require the Company to recall its micronized products. Further, under the November 2017 guidance, the FDA expressed its expectation that following the expiration of its 36-month enforcement discretion period, sales of micronized amniotic tissue will be limited to those products and indications for which applicants have received a BLA. In April 2019, we announced that we will need more time to file and commercialize our BLAs with the FDA and that clinical trial protocol enhancements, further resources and additional capabilities and expertise will be required for commercial
36
launch. While we do not track all uses of our micronized products by physicians, we believe that our micronized product is being used by physicians for more indications than those for which we presently intend to pursue BLAs, as well as in additional sizes (e.g. 100 mg). If the FDA does allow the Company to continue to market a micronized form of its sheet allografts without a biologics license, the FDA may impose conditions, such as labeling restrictions and the requirement that the product be manufactured in compliance with cGMP. Although the Company is preparing for these requirements in connection with its pursuit of a BLA for certain of its micronized products, earlier compliance with these conditions would require significant additional time and cost investments by the Company.
Moreover, increased regulatory scrutiny within the industry in which we operate could lead to increased regulation of HCT/Ps, including Section 361 HCT/Ps, which could ultimately increase our costs and adversely impact our business, results of operations and financial condition.
If the FDA approves the BLAs we seek, we will incur increased compliance costs on an ongoing basis. See “If any of the BLAs are approved, the Company would be subject to additional regulation which will increase costs and could result in adverse sanctions for non-compliance.”
Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies.
The process of obtaining regulatory clearances or approvals to market a biological product or medical device from the FDA or similar regulatory authorities outside of the USA may be costly and time consuming, and such clearances or approvals may not be granted on a timely basis, or at all. We are pursuing approval of BLAs for certain of our micronized products, but have not yet submitted a BLA for review. Additionally, the FDA may take the position that some of the other products that we currently market require a BLA as well. Some of the future products and enhancements to our current products that we expect to develop and market may require marketing clearance or approval from the FDA. However, clearance or approval may not be granted with respect to any of our products or enhancements and FDA review will involve delays that may adversely affect our ability to market such products or enhancements.
The process of obtaining an approved BLA requires the expenditure of substantial time, effort and financial resources and may take years to complete. The fee for filing a BLA and program fees payable with respect to any establishment that manufactures biologics are substantial. Additionally, there are significant costs associated with clinical trials that can be difficult to accurately estimate until a BLA is approved. Clinical trials may not be successful or may return results that do not support approval. Moreover, data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all, or we may decide not to pursue a BLA for certain products or indications. Additionally, the FDA may limit the indications for use or place other conditions on any approvals that could restrict the commercial application of the products. If we do receive approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval. Our revenues will be adversely affected if we fail to obtain BLA approvals on a timely basis or at all, if the FDA requires us to stop marketing our products until a BLA is approved, or if the FDA limits the indications for use or places other conditions that restrict the commercial application of our products.
Further, in April 2019, we announced that we will need more time to develop and file our BLAs with the FDA and that clinical trial protocol enhancements, further resources, and additional capabilities and expertise will be required for commercial launch. We expect that we will have to increase enrollment in our current clinical trials, or initiate new ones, which will add expense, time, and additional uncertainty to the overall BLA approval process.
If the BLAs we seek are approved, we will incur increased compliance costs on an ongoing basis. See “If any of the BLAs are approved, the Company would be subject to additional regulation which will increase costs and could result in adverse sanctions for non-compliance.”
Our business is subject to continuing regulatory compliance by the FDA and other authorities, which is costly, and our failure to comply could result in negative effects on our business, results of operations and financial condition.
As discussed above, the FDA has specific regulations governing our tissue-based products, or HCT/Ps. The FDA has broad post-market and regulatory and enforcement powers, even for Section 361 HCT/Ps. The FDA’s regulation of HCT/Ps includes requirements for registration and listing of products, donor screening and testing, processing and distribution, labeling, record keeping and adverse-reaction reporting, and inspection and enforcement.
HCT/Ps that are regulated as drugs, biological products or medical devices are subject to even more stringent regulation by the FDA. Even if pre-market clearance or approval is obtained, the approval or clearance may place substantial restrictions on the
37
indications for which the product may be marketed or to whom it may be marketed, may require warnings to accompany the product or impose additional restrictions on the sale or use of the product. In addition, regulatory approval is subject to continuing compliance with regulatory standards, including the FDA’s quality system regulations.
If we fail to comply with the FDA regulations regarding our tissue products or medical devices, the FDA could take enforcement action, including, without limitation, any of the following sanctions and the manufacture of our products or processing of our tissue could be delayed or terminated:
• | untitled letters, warning letters, cease and desist orders, fines, injunctions, and civil penalties; |
• | recall or seizure of our products; |
• | operating restrictions, partial suspension or total shutdown of production; |
• | refusing our requests for clearance or approval of new products; |
• | withdrawing or suspending current applications for approval or approvals already granted; |
• | refusal to grant export approval for our products; and |
• | criminal prosecution. |
The FDA’s regulation of HCT/Ps may continue to evolve. Complying with any such new regulatory requirements may entail significant time delays and expense, which could have an adverse effect on our business, results of operations and financial condition.
The American Association of Tissue Banks (“AATB”) has issued operating standards for tissue banking. Compliance with these standards is a requirement in order to become an accredited tissue bank. In addition, some states have their own tissue banking regulations.
In addition, procurement of certain human organs and tissue for transplantation is subject to the restrictions of the National Organ Transplant Act (“NOTA”), which prohibits the transfer of certain human organs, including skin and related tissue for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. We reimburse tissue banks, hospitals and physicians for their services associated with the recovery and storage of donated human tissue. Although we have independent third party appraisals that confirm the reasonableness of the service fees we pay, if we were to be found to have violated NOTA’s prohibition on the sale or transfer of human tissue for valuable consideration, we potentially would be subject to criminal enforcement sanctions, which could adversely affect our results of operations.
Finally, we and other manufacturers of skin substitutes are required to provide average selling price (“ASP”) information to CMS on a quarterly basis. The Medicare payment rates are updated quarterly based on this ASP information. If a manufacturer is found to have made a misrepresentation in the reporting of ASP, such manufacturer is subject to civil monetary penalties of up to $10,000 for each misrepresentation for each day in which the misrepresentation was applied, and potential False Claims Act liability, including treble damages and significant per-claim penalties, currently set at between $11,181 and $22,363 per false claim or statement for penalties assessed after January 29, 2018, with respect to violations occurring after November 2, 2015.
We may be subject to fines, penalties, injunctions and other sanctions if we are deemed to be promoting the use of our products for unapproved, or off-label, uses.
As a general rule, we can only market our 361 HCT/Ps for appropriate homologous uses and we can only promote pre-approved biological products or devices for FDA-approved indications. Generally, unless the products are approved or cleared by the FDA for alternative uses, the FDA contends that we may not make claims about the safety or effectiveness of our products, or promote them, for such uses. Such limitations present a risk that the FDA or other federal or state law enforcement authorities could determine that the nature and scope of our sales, marketing and support activities, though designed to comply with all FDA requirements, constitute the promotion of our products for an unapproved use in violation of the federal Food, Drug, and Cosmetic Act. We also face the risk that the FDA or other governmental authorities might pursue enforcement based on past activities that we have discontinued or changed, including sales activities, arrangements with institutions and doctors, educational and training programs and other activities.
Investigations concerning the promotion of unapproved uses and related issues are typically expensive, disruptive and burdensome and generate negative publicity. If our promotional activities are found to be in violation of the law, we may face significant legal
38
action, fines, penalties, and even criminal liability and may be required to substantially change our sales, promotion, grant and educational activities. There is also a possibility that we could be enjoined from selling some or all of our products for any unapproved use. In addition, as a result of an enforcement action against us or any of our executive officers, we could be excluded from participation in government healthcare programs such as Medicare and Medicaid.
However, the FDA’s Guidance stated that the FDA intends to exercise enforcement discretion under limited conditions with respect to IND application and pre-market approval requirements for certain HCT/Ps through November 2020. This means that, through November 2020, the FDA does not intend to enforce certain provisions as they currently apply to certain entities or activities. During the period of enforcement discretion, we have marketed, and intend to continue to market, our micronized, injectable products while at the same time pursuing a BLA for certain of our micronized products. We have already filed INDs for three indications for our micronized, injectable product: plantar fasciitis, osteoarthritis knee pain, and Achilles tendonitis.
Nevertheless, while we believe we are in compliance with the FDA's Guidance on HCT/Ps and enforcement discretion regarding products that do not meet some or all of the HCT/P requirements, there can be no assurance that we are correct or that the FDA will not suspend its enforcement discretion and, in such cases, we may need to discontinue marketing a product and/or may be subject to fines, penalties, injunctions, and other sanctions if we are deemed to be promoting the use of our products for unapproved uses.
We and our sales representatives, whether employees or independent contractors, must comply with various federal and state anti-kickback, self-referral, false claims and similar laws, any breach of which could cause an adverse effect on our business, results of operations and financial condition.
Our relationships with physicians, hospitals and other healthcare providers are subject to scrutiny under various federal and state healthcare fraud and abuse laws. Healthcare fraud and abuse laws are complex and, in some instances, even minor or inadvertent violations can give rise to liability. Possible sanctions for violation of the healthcare fraud and abuse laws include monetary fines, civil and criminal penalties, exclusion from participating in the federal and state healthcare programs, including, without limitation, Medicare, Medicaid, VA health programs and TRICARE (the healthcare program administered by or on behalf of the U.S. Department of Defense for uniformed service members, including both those in active duty and retirees, as well as their dependents), and forfeiture of amounts collected in violation of such prohibitions. Certain states have similar fraud and abuse laws, imposing substantial penalties for violations. A finding of a violation of one or more of these laws, or even a government investigation or inquiry into the same, would likely result in a material adverse effect on the market price of our Common Stock, as well as on our business, results of operations, and financial condition.
The federal Anti-Kickback Statute is a criminal law that prohibits, among other things, any person from knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward referrals, purchases or orders or arranging for or recommending the purchase, order or referral of any item or service for which payment may be made in whole or in part by a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value. The Patient Protection and Affordable Care Act (the “PPACA”) amended the intent requirement of the federal Anti-Kickback Statute, so that a person or entity need not have actual knowledge of this statute or specific intent to violate it. A conviction for violation of the Anti-Kickback Statute results in criminal fines and requires mandatory exclusion from participation in federal health care programs. Although there are a number of statutory exceptions and regulatory safe harbors to the federal Anti-Kickback Statute that protect certain common industry practices from prosecution, the exceptions and safe harbors are drawn narrowly, and arrangements may be subject to scrutiny or penalty if they do not fully satisfy all elements of an available exception or safe harbor. We have entered into consulting agreements, speaker agreements, research agreements and product development agreements with physicians, including some who may order our products or make decisions to use them. In addition, some of these physicians own our stock, which they purchased in arm’s-length transactions on terms identical to those offered to non-physicians, or received stock awards from us in the past as consideration for services performed by them. While we believe these transactions generally met the requirements of applicable laws, including the federal Anti-Kickback Statute and analogous state laws, it is possible that our arrangements with physicians and other providers may be questioned by regulatory or enforcement authorities under such laws, which could lead us to redesign the arrangements and subject us to significant civil or criminal penalties. We have designed our policies and procedures to comply with the Anti-Kickback Statute, the FCA, and industry norms. In addition, we have conducted training sessions on these principles. If, however, regulatory or enforcement authorities were to view these arrangements as non-compliant with applicable laws, there would be risk of government investigations or penalties. There is also risk that one or more of our employees or agents will disregard the rules we have established. Because our strategy relies on the involvement of physicians who consult with us on the design of our products, perform clinical research on our behalf or educate other health care professionals about the efficacy and uses of our products, we could be materially impacted if regulatory or enforcement agencies or courts interpret our financial relationships with physicians who refer or order or promote our products to be in violation of applicable laws. This could harm our reputation and the reputations of the physicians we engage to provide services on our behalf. In addition, the cost of noncompliance with
39
these laws could be substantial since we could be subject to monetary fines and civil or criminal penalties, and we could also be excluded from federally-funded healthcare programs, including Medicare, Medicaid, VA and TRICARE.
The federal False Claims Act (“FCA”) imposes civil liability on any person or entity that knowingly submits, or causes the submission of, a false or fraudulent claim to the U.S. government. Damages under the FCA can be significant and consist of the imposition of fines and penalties. The FCA also allows a private individual or entity to sue on behalf of the government to recover civil penalties and treble damages as a whistleblower. FCA liability is potentially significant in the healthcare industry because the statute provides for treble damages and mandatory penalties of between $11,181 and $22,363 per false claim or statement for penalties assessed after January 29, 2018, with respect to violations occurring after November 2, 2015.
Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payers if they are deemed to “cause” the submission of false or fraudulent claims. The Department of Justice (the “DOJ”) on behalf of the government has previously alleged that the marketing and promotional practices of pharmaceutical and medical device manufacturers, including the off-label promotion of products or the payment of prohibited kickbacks to doctors, violated the FCA, resulting in the submission of improper claims to federal and state healthcare programs such as Medicare and Medicaid. In certain cases, manufacturers have entered into criminal and civil settlements with the federal government under which they entered into plea agreements, paid substantial monetary amounts and entered into onerous corporate integrity agreements that require, among other things, substantial reporting and remedial actions, as well as oversight and review by an outside entity, an Independent Review Organization (“IRO”), at substantial expense to the Company.
Under the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) criminal federal healthcare fraud statute, it is a crime to knowingly and willfully execute, or attempt to execute, a scheme or artifice to defraud any health care benefit program or to obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any health care benefit program, in connection with the delivery of or payment for health care benefits, items or services.
There are federal and state laws requiring detailed reporting of manufacturer interactions with and payments to healthcare providers, such as the Sunshine Act. The Sunshine Act requires, among others, “applicable manufacturers” of drugs, devices, biological products, and medical supplies reimbursed under Medicare, Medicaid or the Children’s Health Insurance Program to annually report to CMS information related to payments and other transfers of value provided to “covered recipients.” The term covered recipients includes U.S.-licensed physicians and teaching hospitals, and, for reports submitted on or after January 1, 2022, physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists, and certified nurse-midwives. While manufacturers of human cell and tissue products regulated solely under Section 361 are not subject to the Sunshine Act, in the future, if we receive a BLA, we will be subject to this law. There is also risk that CMS or another government agency may take the position that our products are not human cell and tissue products regulated solely under Section 361, and thereby assert that we are currently subject to the Sunshine Act, which could subject us to civil penalties and the administrative burden of having to comply with the law.
There are state law equivalents of each of the above federal laws, such as the Anti-Kickback Statute and FCA, which may apply to items or services reimbursed by any third-party payer, including commercial insurers (i.e., so called “all-payer” anti-kickback laws).
The enforcement of all of these laws is uncertain and subject to rapid change. Federal or state regulatory or enforcement authorities may investigate or challenge our current or future activities under these laws. Any investigation or challenge could have a material adverse effect on our business, financial condition and results of operations. Any state or federal regulatory or enforcement review of us, regardless of the outcome, would be costly and time consuming. Additionally, we cannot predict the impact of any changes in these laws, whether these changes are retroactive or will have effect on a going-forward basis only.
We may be subject to fines, penalties, injunctions and even criminal sanctions if we are deemed to have made a misstatement of compliance to a federal agency.
Products that are subject to pre-approval as biologicals must also be manufactured in accord with cGMP. In August 2013, the FDA sent the Company an Untitled Letter asserting that its micronized amniotic allografts were unapproved biologics. The Company disputed the FDA’s position at the time and filed various appeals but ultimately agreed during the appeals process to pursue BLAs for certain products, but the transition to cGMP compliance for micronized, injectable products sold commercially was a larger task. In February 2016, the FDA inspected the Company’s Marietta facility against cGMP requirements for the commercially available product. The transition to cGMP compliance was underway, but the work was in its initial stages. At the close of the inspection, the FDA issued a Form 483 that included 13 observations. In response, the Company developed an action plan (the “Action Plan”). The Action Plan, which was shared with FDA, called for a systematic approach to the work and provided a vehicle
40
to update the FDA on progress. Over the course of the next year, the site did substantial work to transition to cGMP for the commercially available, micronized, injectable product and filed several updates with the FDA.
In February 2017, the Company sent a close-out letter to the FDA that indicated the work under the Action Plan had been completed. That letter overstated our state of compliance in regard to the commercially available product. The goal of the letter was to communicate the substantial progress to the FDA and to indicate that the work under the Action Plan had been completed. The site continues to transition to cGMP compliance for its micronized products, and we expect to complete the work by November 2020, when the FDA’s industrywide exercise of enforcement discretion for products like our micronized amnion expires. Exaggeration or misstatement of compliance to a federal agency creates regulatory risk. If the government were to take issue with the letter, it could take any number of actions adverse to the Company. These include issuing a warning letter, terminating the current exercise of enforcement discretion with respect to the sale of micronized products and initiating a civil judicial action against the Company and opening a criminal investigation. Each of these potential actions would be disruptive to the Company’s operations, consume considerable resources and potentially prohibit sales of certain products and adversely affect our business, financial condition and results of operations.
In July 2019, the Company formally notified the FDA that its February 2017 correspondence overstated the Company’s state of cGMP compliance.
In December 2019, the FDA conducted a cGMP audit of each of the Company’s two manufacturing facilities. At the close of the inspection the FDA issued two Form 483s (one for each facility). The Company timely responded to the Form 483s. See the discussion under “Item 1. Business - Processing (Manufacturing).”
Our results of operations may be adversely affected by current and potential future healthcare reforms.
In response to perceived increases in healthcare costs in recent years, there have been and continue to be proposals by the U.S. federal government, state governments, regulators and third-party payers to control these costs and, more generally, to reform the U.S. healthcare system. In the U.S., the PPACA was enacted in 2010 with a goal of reducing the cost of healthcare and substantially changing the way healthcare is financed by both government and private insurers.
In addition, other legislative changes have been proposed and adopted in the U.S. since the PPACA was enacted. The Budget Control Act of 2011 created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This included aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013. In January 2013, the American Taxpayer Relief Act was signed into law, which, among other things, further reduced Medicare payments to several provider types, including hospitals.
The current U.S. Presidential Administration and certain members of the U.S. Congress have stated that they will seek to modify, repeal or otherwise invalidate all, or certain provisions of, the PPACA. In 2017, the U.S. President signed an executive order which stated that it is the policy of his Administration to seek the prompt repeal of the PPACA and directed executive departments and federal agencies to waive, defer, grant exemptions from or delay the implementation of the provisions of the PPACA to the maximum extent permitted by law. Additionally, the House and Senate attempted, but failed, to pass legislation to repeal all or portions of the PPACA, and these efforts may be resumed. In December 2017, the U.S. President signed the Tax Cuts and Jobs Act, which, among numerous other actions, repealed the individual mandate of the PPACA, effective on January 1, 2019. In December 2018, a federal district court in Texas ruled the individual mandate was unconstitutional and could not be severed from the PPACA. As a result, the court ruled the remaining provisions of the PPACA were also invalid, though the court declined to issue a preliminary injunction with respect to the PPACA. The court’s ruling was appealed to the U.S. Court of Appeals for the Fifth Circuit. On March 25, 2019, the DOJ reversed its prior position and stated in a legal filing with the Fifth Circuit that the district court’s ruling that the PPACA was invalid should be upheld. In December 2019, the Fifth Circuit agreed that the individual mandate was unconstitutional, but remanded the case back to the district court to reassess how much of the PPACA would be damaged without the individual mandate provision, and if the individual mandate could indeed be severed. In January 2020, 21 state Attorneys General urged the Supreme Court of the United States to decide whether or not the PPACA should be struck down as unconstitutional, claiming that the Fifth Circuit erroneously remanded the case to the district court. The House of Representatives filed a similar petition and motion. The state Attorneys General and the House of Representatives also filed motions to expedite the Supreme Court’s decision to review the case, which the Supreme Court subsequently denied. This litigation is still ongoing, and places great uncertainty upon the longevity and nature of the PPACA moving forward. In addition, further legislative changes to and regulatory changes under PPACA remain possible.
There is uncertainty with respect to the impact the U.S. Administration, the executive order, and the attempted legislation may have, if any, and any changes will likely take time to unfold and could have an impact on coverage and reimbursement for healthcare
41
items and services, including our products. We believe that substantial uncertainty remains regarding the net effect of the PPACA, or its repeal and potential replacement, on our business, including uncertainty over how benefit plans purchased on exchanges will cover our products, how the expansion or contraction of the Medicaid program will affect access to our products, the effect of risk-sharing payment models such as Accountable Care Organizations and other value-based purchasing programs on coverage for our product, and the effect of the general increase or decrease in federal oversight of healthcare payers. The taxes imposed and the expansion in government’s role in the U.S. healthcare industry under the PPACA, if unchanged, may result in decreased revenues, lower reimbursements by payers for our products and reduced medical procedure volumes, all of which could have a material adverse effect on our business, results of operations and financial condition.
We may fail to obtain or maintain foreign regulatory approvals to market our products in other countries.
We currently market our products internationally and intend to consider expansion of our international marketing. International jurisdictions require separate regulatory approvals and compliance with numerous and varying regulatory requirements. The approval procedures vary among countries and may involve requirements for additional testing. Certain of our products require clearance or approval by the FDA. However, such clearance or approval does not ensure approval or certification by regulatory authorities in other countries or jurisdictions, and approval or certification by one foreign regulatory authority does not ensure approval or certification by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval or certification process may include all of the risks associated with obtaining FDA clearance or approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals or certifications and may not receive necessary approvals to commercialize our products in any foreign jurisdiction. Furthermore, many foreign jurisdictions operate under socialized medical care, and obtaining reimbursement for our products under that construct may also prove difficult. If we fail to receive necessary approvals, certifications, or reimbursements necessary to commercialize our products in foreign jurisdictions on a timely basis, or at all, our business, results of operations and financial condition could be adversely affected.
Federal and state laws that protect the privacy and security of personal information may increase our costs and limit our ability to collect and use that information and subject us to liability if we are unable to fully comply with such laws.
Numerous federal and state laws, rules and regulations govern the collection, dissemination, use, security and confidentiality of personal information, including individually identifiable health information. These laws include:
• | provisions of HIPAA that limit how covered entities and business associates may use and disclose protected health information, provide certain rights to individuals with respect to that information and impose certain security requirements; |
• | HITECH, which strengthened and expanded the HIPAA Privacy Rule and Security Rules, imposed data breach notification obligations, created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates and gave state attorneys general new authority to file civil actions for damages or injunctions in U.S. federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions; |
• | other federal and state laws restricting the use and protecting the privacy and security of personal information, including health information, many of which are not preempted by HIPAA; |
• | federal and state consumer protection laws; and |
• | federal and state laws regulating the conduct of research with human subjects. |
One relevant state law is the California Consumer Protection Act (“CCPA”), which became effective on January 1, 2020. The CCPA is a privacy bill that requires certain companies doing business in California to disclose information regarding the collection and use of a consumer’s personal data and to delete a consumer’s data upon request. The Act also permits the imposition of civil penalties and expands existing state security laws by providing a private right of action for consumers in certain circumstances where consumer data is subject to a breach. We are still evaluating whether and how this rule will impact our U.S. operations and /or limit the ways in which we can provide services or use personal data collected while providing services.
As part of our business operations, including our medical record keeping, third-party billing and reimbursement and research and development activities, we collect and maintain protected health information in paper and electronic format. Standards related to health information, whether implemented pursuant to HIPAA, HITECH, state laws, federal or state action or otherwise, could have a significant effect on the manner in which we handle personal information, including healthcare-related data, and communicate with payers, providers, patients, donors and others, and compliance with these standards could impose significant costs on us or limit our ability to offer services, thereby negatively impacting the business opportunities available to us.
42
If we are alleged not to comply with existing or new laws, rules and regulations related to personal information, we could be subject to litigation and to sanctions that include monetary fines, civil or administrative penalties, civil damage awards or criminal penalties.
Risks Related to the Securities Markets and Ownership of Our Common Stock
Our Common Stock has been delisted from The Nasdaq Capital Market, which may negatively impact the trading price of our Common Stock and the levels of liquidity available to our shareholders.
The trading of our Common Stock was suspended from the Nasdaq Capital Market in November 2018 and delisted in March 2019. It is currently quoted on the “over the counter” market operated by the OTC Markets Group, Inc. under the symbol “MDXG,” which may negatively impact the trading price of our Common Stock and the liquidity available to our shareholders.
Our Common Stock is subject to SEC rules and regulations relating to the market for penny stocks. A penny stock is any equity security not traded on a national securities exchange that has a market price of less than $5.00 per share. On March 12, 2020, the last sale price per share of our Common Stock as reported on the OTC Markets was $4.95. If our Common Stock is or becomes subject to regulation as a penny stock, such regulations may severely affect the market liquidity for our Common Stock and could limit the ability of shareholders to sell securities in the secondary market. Accordingly, investors in our Common Stock may find it more difficult to dispose of or obtain accurate quotations as to the market value of our Common Stock, and there can be no assurance that our Common Stock will continue to be eligible for trading or quotation on the over the counter market or any other alternative exchanges or markets.
Further, the delisting of our Common Stock from The Nasdaq Capital Market may adversely affect our ability to raise additional capital through public or private sales of equity securities, may significantly affect the ability of investors to trade our securities and may negatively affect the value and liquidity of our Common Stock. Such delisting may also have other negative effects, including the potential loss of confidence of employees, the loss of institutional investor interest, and fewer business development opportunities. Furthermore, because of the limited market and low volume of trading in the our Common Stock that could occur, the share price of our Common Stock could be disproportionately affected by broad market fluctuations, general market conditions, fluctuations in our operating results, changes in the market’s perception of our business and announcements made by us, our competitors, parties with whom we have business relationships or third parties.
The price of our Common Stock has been, and will likely continue to be, volatile.
The market price of our Common Stock, like that of the securities of many other healthcare companies that are engaged in research, development, and commercialization, has fluctuated over a wide range, and it is likely that the price of our common stock will fluctuate in the future. The market price of our Common Stock could be impacted by a variety of factors, including:
• | Fluctuations in stock market prices and trading volumes of similar companies or of the markets generally; |
• | Our ability to successfully launch, market and earn significant revenue from our products; |
• | Our ability to obtain additional financing to support our continuing operations; |
• | Disclosure of the details and results of regulatory applications and proceedings; |
• | Developments in and disclosure or publicity regarding existing or new litigation or contingent liabilities; |
• | Changes in government regulations or our failure to comply with any such regulations; |
• | Additions or departures of key personnel; |
• | Our investments in research and development or other corporate resources; |
• | Announcements of technological innovations or new commercial products by us or our competitors; |
• | Developments in the patents or other proprietary rights owned or licensed by us or our competitors; |
• | The timing of new product introductions; |
• | Actual or anticipated fluctuations in our operating results, including any restatements of previously reported results; |
43
• | Our ability to effectively and consistently manufacture our products and avoid costs associated with the recall of defective or potentially defective products; |
• | Our ability and the ability of our distribution partners to market and sell our products; |
• | Changes in reimbursement for our products or the price for our products to our customers; |
• | Removal of our products from the Federal Supply Schedule, or changes in how government accounts purchase products such as ours or in the price for our products to government accounts; |
• | Material amounts of short-selling of our Common Stock; and |
• | The other risks detailed in this Item 1A. |
Price volatility or a decrease in the market price of our Common Stock could have an adverse effect on our ability to raise capital, liquidity, business, financial condition and results of operations.
Fluctuations in revenue or results of operations could cause additional volatility in our stock price.
Any unanticipated shortfall in our revenue in any fiscal quarter could have an adverse effect on our results of operations in that quarter. The effect on our net income of such a shortfall could be exacerbated by the relatively fixed nature of most of our costs, which primarily include personnel costs as well as facilities costs. These fluctuations could cause the trading price of our stock to be negatively affected. Our quarterly operating results have varied substantially in the past and may vary substantially in the future.
We do not intend to pay cash dividends.
We have never declared or paid cash dividends on our capital stock. We currently expect to use available funds and any future earnings in the development, operation and expansion of our business and, to the extent authorized by our Board, repurchasing our Common Stock. We do not anticipate paying any cash dividends in the foreseeable future. As a result, capital appreciation, if any, of our Common Stock will be an investor’s only source of potential gain from our Common Stock for the foreseeable future.
Certain provisions of Florida law and anti-takeover provisions in our organizational documents may discourage or prevent a change of control, even if an acquisition would be beneficial to shareholders, which could affect our share price adversely and prevent attempts by shareholders to remove current management.
The Florida Business Corporation Act (the “FBCA”) includes several provisions applicable to the Company that may discourage potential acquirors. Such provisions include provisions that allow directors to take other stakeholders into account in discharging their duties, a requirement that certain transactions with a shareholder of 10% or more ownership must be approved by the affirmative vote of two-thirds of the other shareholders unless approved by a majority of the disinterested directors or certain fair price requirements are met and voting rights acquired by a shareholder at ownership levels at or above one-fifth, one-third and a majority of voting power are denied unless authorized by the Board prior to such acquisition or by a majority of the other shareholders (excluding interested shares (as defined in the FBCA)).
Additionally, our organizational documents contain provisions: authorizing the issuance of blank check preferred stock; restricting persons who may call shareholder meetings; providing for a classified Board; permitting shareholders to remove directors only “for cause” and only by super-majority vote; and providing the Board with the exclusive right to fill vacancies and to fix the number of directors. These provisions of Florida law and our articles of incorporation and bylaws could negatively affect our share price, prevent attempts by shareholders to remove current management, prohibit or delay mergers or other takeovers or changes of control of the Company and discourage attempts by other companies to acquire us, even if such a transaction would be beneficial to our shareholders.
Item 2. Properties
Our corporate headquarters are located in Marietta, Georgia, where we lease office, laboratory, tissue processing and warehouse space. We also lease a facility in Kennesaw, Georgia, which primarily consists of laboratory, tissue processing and warehouse space, and additional warehouse space in Marietta, Georgia. All of our properties are used by our one business segment, Regenerative Biomaterials, which includes the design, manufacture and marketing of products and tissue processing services for the wound care, burn, surgical, orthopedic, spine, sports medicine, ophthalmic and dental sectors of healthcare.
The Company’s properties are suitable and adequate for current business operations.
44
Item 3. Legal Proceedings
Shareholder Derivative Suits
On December 6, 2018, the United States District Court for the Northern District of Georgia entered an order consolidating three shareholder derivative actions (Evans v. Petit, et al. filed September 25, 2018, Georgalas v. Petit, et al. filed September 27, 2018, and Roloson v. Petit, et al. filed October 22, 2018) that had been filed in the Northern District of Georgia. On January 22, 2019, plaintiffs filed a Verified Consolidated Shareholder Derivative Complaint. The consolidated action sets forth claims of breach of fiduciary duty, corporate waste and unjust enrichment against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Joseph G. Bleser, J. Terry Dewberry, Charles R. Evans, Larry W. Papasan, Luis A. Aguilar, Bruce L. Hack, Charles E. Koob, Neil S. Yeston and Christopher M. Cashman. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Company filed a motion to stay on February 18, 2019, pending the completion of the investigation by the Company’s Special Litigation Committee. The Special Litigation Committee completed its investigation relating to this action and filed an executive summary of its findings with the Court on July 1, 2019. The parties held a mediation on February 11, 2020 and discussions continue.
On October 29, 2018, the City of Hialeah Employees Retirement System (“Hialeah”) filed a shareholder derivative complaint in the Circuit Court for the Second Judicial Circuit in and for Leon County, Florida (the “Florida Court”). The complaint alleges claims for breaches of fiduciary duty and unjust enrichment against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Joseph G. Bleser, J. Terry Dewberry, Charles R. Evans, Bruce L. Hack, Charles E. Koob, Larry W. Papasan, and Neil S. Yeston. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Company moved to stay the action on February 7, 2019, to allow the prior-filed consolidated derivative action in the Northern District of Georgia to be resolved first and to allow the Company’s Special Litigation Committee time to complete its investigation. The Company also filed a motion to dismiss on April 8, 2019. No hearing has been scheduled on the Company’s motion to stay or motion to dismiss. The plaintiff participated in the mediation that took place in connection with the prior-filed consolidated derivative action in the Northern District of Georgia.
On May 15, 2019, two individuals purporting to be shareholders of the Company filed a shareholder derivative complaint in the Superior Court for Cobb County, Georgia. (Nix and Demaio v. Evans, et al.) The complaint alleges claims for breaches of fiduciary duty, corporate waste and unjust enrichment against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Chris Cashman, Lou Roselli, Mark Diaz, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Court has ordered this matter stayed pending the resolution of the consolidated derivative suit pending in the Northern District of Georgia. The plaintiff participated in the mediation that took place in connection with the prior-filed consolidated derivative action in the Northern District of Georgia.
On August 12, 2019, John Murphy filed a shareholder derivative complaint in the United States District Court for the Southern District of Florida (Murphy v. Petit, et al.). The complaint alleged claims for breaches of fiduciary duty and unjust enrichment against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Company filed a motion to transfer this action to the Northern District of Georgia. Prior to resolution of that motion, the plaintiff voluntarily dismissed this action without prejudice. The plaintiff participated in the mediation that took place in connection with the prior-filed consolidated derivative action in the Northern District of Georgia.
On February 10, 2020, Charles Pike filed a shareholder derivative complaint in the United States District Court for the Southern District of Florida (Pike v. Petit, et al.). The complaint alleges claims for breaches of fiduciary duty against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. Similar to the prior-filed actions discussed above, the allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition.
On February 18, 2020, Bruce Cassamajor filed a shareholder derivative complaint in the United States District Court for the Northern District of Florida (Cassamajor v. Petit, et al.). The complaint alleges claims for breaches of fiduciary duty against certain
45
current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. Similar to the prior-filed actions discussed above, the allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. As of the date of the filing of this annual report on Form 10-K, MiMedx has not yet been served with the complaint.
Securities Class Action
On January 16, 2019, the United States District Court for the Northern District of Georgia entered an order consolidating two purported securities class actions (MacPhee v. MiMedx Group, Inc., et al. filed February 23, 2018 and Kline v. MiMedx Group, Inc., et al. filed February 26, 2018). The order also appointed Carpenters Pension Fund of Illinois as lead plaintiff. On May 1, 2019, the lead plaintiff filed a consolidated amended complaint, naming as defendants the Company, Michael J. Senken, Parker H. Petit, William C. Taylor, Christopher M. Cashman and Cherry Bekaert & Holland LLP. The amended complaint (the “Securities Class Action Complaint”) alleged violations of Section 10(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), Rule 10b-5 promulgated thereunder and Section 20(a) of the Exchange Act. It asserted a class period of March 7, 2013 through June 29, 2018. Following the filing of motions to dismiss by the various defendants, the lead plaintiff was granted leave to file an amended complaint. The lead plaintiff has until March 30, 2020 to file its amended complaint.
Annual Meeting Matters
On December 12, 2018, Hialeah filed an action against the Company in the Florida Court seeking to compel the Company to hold a shareholder meeting. Hialeah requested that the court enter an order compelling two annual meetings (for 2018 and 2019) to be held on the same date, when six of the Company’s ten directors would be elected. The Company answered the complaint on January 1, 2019, and Hialeah moved for summary judgment on January 30, 2019. After a hearing held on April 3, 2019, the Florida Court ordered a meeting to take place on June 17, 2019, where a single class of directors would be elected, and memorialized that order in a final declaratory judgment on April 26, 2019. The annual meeting took place on June 17, 2019. The action was dismissed on November 6, 2019.
On April 18, 2019, Hialeah filed an action against the Company in the Florida Court asking the Florida Court to enter a final declaratory judgment for the election of Class III directors at either the June 17, 2019 meeting or within 30 days of the June 17, 2019 meeting. Hialeah filed a motion for summary judgment and declaratory judgment on May 13, 2019. The Company filed a motion to dismiss the action on May 23, 2019. On August 5, 2019, the parties entered into a stipulation under which, among other things, MiMedx agreed to work in good faith to complete its 2018 audited financial statements by December 16, 2019, hold an annual meeting for the election of Class III directors by January 15, 2020, and hold an annual meeting for the election of Class I directors by June 15, 2020. The parties settled this matter, and the action was dismissed on November 6, 2019.
Investigations
SEC Investigation
On April 4, 2017, the Company received a subpoena from the SEC requesting information related to, among other things, the Company’s recognition of revenue, practices with certain distributors and customers, its internal accounting controls and certain employment actions. The Company cooperated with the SEC in its investigation (the “SEC Investigation”). In November 2019, the SEC brought claims against the Company and the Company’s former officers Parker H. Petit, Michael J. Senken, and William C. Taylor. The SEC alleged that from 2013 to 2017, the Company prematurely recognized revenue from sales to its distributors and exaggerated its revenue growth. The SEC’s complaint also alleged that the Company improperly recognized revenue because its former CEO and COO entered into undisclosed side arrangements with certain distributors. These side arrangements allowed distributors to return product to the Company or conditioned distributors’ payment obligations on sales to end users. The SEC complaint further alleged that the Company’s former CEO, COO, and CFO allegedly covered up their scheme for years, including after the Company’s former controller raised concerns about the Company’s accounting for specific distributor transactions. The SEC also alleged that the Company’s former CEO, COO, and CFO all misled the Company’s outside auditors, members of the Company’s Audit Committee, and outside lawyers who inquired about these transactions. The SEC brought claims against the Company and its former CEO, COO, and CFO for violating the antifraud, reporting, books and records, and internal controls provisions of the federal securities laws. The SEC also brought claims against the Company’s former CEO, COO, and CFO for lying to the Company’s outside auditors.
46
Without admitting or denying the SEC’s allegations, the Company settled with the SEC by consenting to the entry of a final judgment that permanently restrains and enjoins the Company from violating certain provisions of the federal securities laws. As part of the resolution, the Company paid a civil penalty of $1.5 million. The settlement concluded, as to the Company, the matters alleged by the SEC in its complaint. The SEC’s litigation continues against the Company’s former officers.
United States Attorney’s Office for the Southern District of New York (“USAO-SDNY”) Investigation
The USAO-SDNY conducted an investigation into topics similar to those at issue in the SEC Investigation. The USAO-SDNY requested that the Company provide it with copies of all information the Company furnished to the SEC and made additional requests for information. The USAO-SDNY conducted interviews of various individuals, including employees and former employees of the Company. The USAO-SDNY issued indictments in November 2019 against former executives Messrs. Petit and Taylor for securities fraud and conspiracy to commit securities fraud, to make false filings with the SEC, and improperly influence the conduct of audits relating to alleged misconduct that resulted in inflated revenue figures for fiscal 2015. The Company is cooperating with the USAO-SDNY.
Department of Veterans’ Affairs Office of Inspector General (“VA-OIG”) and Civil Division of the Department of Justice (“DOJ-Civil”) Subpoenas and/or Investigations
VA-OIG has issued subpoenas to the Company seeking, among other things, information concerning the Company’s financial relationships with VA clinicians. DOJ-Civil requested similar information. The Company has cooperated fully and produced responsive information to VA-OIG and DOJ-Civil. VA-OIG has periodically requested additional documents and information regarding payments to individual VA clinicians, The Company has continued to cooperate and responded to these requests.
As part of its cooperation, the Company provided documents in response to subpoenas concerning its relationship with three now former VA employees in South Carolina, who were ultimately indicted in May 2018. Among other things, the indictment referenced speaker fees paid by the Company to the former VA employees and other interactions between now former Company employees and the former VA employees. In January 2019, prosecution was deferred for 18 months to allow the three former VA employees to enter and complete a Pretrial Diversion Program, the completion of which would result in the dismissal of the indictment. Two of the former VA employees have completed the program early and the indictment has been dismissed with respect to them. To date, no actions have been taken against the Company with respect to this matter.
United States Attorney’s Office for the Southern District of Georgia (“USAO-SDGA”) Grand Jury Investigation
The USAO-SDGA is investigating the relationships of a Department of Defense physician with various vendors, including the Company. On August 20, 2018, a Company employee testified before the grand jury. The USAO-SDGA has not taken further action since this testimony was provided. We are not aware of the status of this matter.
Qui Tam Actions
On January 19, 2017, a former employee of the Company filed a qui tam False Claims Act complaint in the United States District Court for the District of South Carolina (United States of America, ex rel. Jon Vitale v. MiMedx Group, Inc.) alleging that the Company’s donations to the patient assistance program, Patient Access Network Foundation, violated the Anti-Kickback Statute and resulted in submission of false claims to the government. The government declined to intervene and the complaint was unsealed on August 10, 2018. The Company filed a motion to dismiss on October 1, 2018. The Company’s motion to dismiss was granted in part and denied in part on May 15, 2019. The case is currently in discovery.
On January 20, 2017, two former employees of the Company, filed a qui tam False Claims Act complaint in the United States District Court for the District of Minnesota (Kruchoski et. al. v. MiMedx Group, Inc.). An amended complaint was filed on January 27, 2017. The operative complaint alleges that the Company failed to provide truthful, complete and accurate information about the pricing offered to commercial customers in connection with the Company’s FSS contract. On May 7, 2019, the DOJ declined to intervene, and the case was unsealed. The parties have reached a settlement in principle and are working to finalize the same.
47
Former Employee Litigation
On December 13, 2016, the Company filed a complaint in the Circuit Court for Palm Beach County, Florida (MiMedx Group, Inc. v. Academy Medical, LLC et. al.) alleging several claims against a former employee, primarily based on his alleged competitive activities while he was employed by the Company (breach of contract, breach of fiduciary duty and breach of duty of loyalty). The former employee countersued for monetary damages and injunctive relief, alleging whistleblower retaliation in violation of the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Dodd-Frank Act”), unlawful discharge and defamation. The Court dismissed the Dodd-Frank Act whistleblower counterclaim, and in response, the former employee filed an amended complaint on September 11, 2018, adding allegations of post-termination retaliation in violation of the Dodd-Frank Act. The court dismissed the former employee’s retaliation counterclaim on January 24, 2019. After this dismissal, only the former employee’s claims of unlawful discharge and defamation remained pending. The parties resolved this matter, and the case was dismissed on September 5, 2019.
On December 29, 2016, the Company filed a complaint in the United States District Court for the Northern District of Illinois (MiMedx Group, Inc. v. Michael Fox) alleging several claims against a former employee of the Company, primarily based on his alleged competitive activities while he was employed by the Company (breach of contract, breach of fiduciary duty and breach of duty of loyalty). The former employee countersued the Company for monetary damages and injunctive relief, alleging improper wage rate adjustment, interference with the former employee’s job after his termination from the Company and retaliation. The parties resolved this matter, and the case was dismissed on November 4, 2019.
On July 13, 2018, a former employee filed a complaint against the Company in the United States District Court for the Northern District of Texas (Jennifer R. Scott v. MiMedx Group, Inc.), alleging sex discrimination and retaliation. The parties resolved this matter, and the case was dismissed on November 6, 2019.
On November 19, 2018, the Company’s former Chief Financial Officer filed a complaint in the Superior Court for Cobb County, Georgia (Michael J. Senken v. MiMedx Group, Inc.) in which he claims that the Company has breached its obligations under the Company’s charter and bylaws to advance to him, and indemnify him for, his legal fees and costs that he incurred in connection with certain Company internal investigations and litigation. The Company filed its answer denying the plaintiff’s claims on April 19, 2019. To date, no deadlines have been established by the court.
On January 21, 2019, a former employee filed a complaint in the Fifth Judicial Circuit, Richland County, South Carolina (Jon Michael Vitale v. MiMedx Group, Inc. et. al.) against the Company alleging retaliation, defamation and unjust enrichment and seeking monetary damages. The former employee claims he was retaliated against after raising concerns related to insurance fraud and later defamed by comments concerning the indictments of three South Carolina VA employees. On February 19, 2019, the case was removed to the U.S. District Court for the District of South Carolina. The Company filed a motion to dismiss on April 8, 2019, which was denied by the Court. This case is currently in discovery.
Defamation Claims
On June 4, 2018, Sparrow Fund Management, LP (“Sparrow”) filed a complaint against the Company and Mr. Petit, including claims for defamation and civil conspiracy in the United States District Court for the Southern District of New York (Sparrow Fund Management, L.P. v. MiMedx Group, Inc. et. al.). The complaint seeks monetary damages and injunctive relief and alleges the defendants commenced a campaign to publicly discredit Sparrow by falsely claiming it was a short seller who engaged in illegal and criminal behavior by spreading false information in an attempt to manipulate the price of our Common Stock. On March 31, 2019, a judge granted defendants’ motions to dismiss in full, but allowed Sparrow the ability to file an amended complaint. The Magistrate has recommended Sparrow’s motion for leave to amend be granted in part and denied in part. Both parties have filed objections to the Magistrate’s recommendation.
On June 17, 2019, the principals of Viceroy Research (“Viceroy”), filed suit in the Circuit Court for the Seventeenth Judicial Circuit in Broward County, Florida (Fraser John Perring et. al. v. MiMedx Group, Inc. et. al.) against the Company and Mr. Petit, alleging defamation and malicious prosecution based on the defendants’ alleged campaign to publicly discredit Viceroy and the lawsuit the Company previously filed against the plaintiffs, but which the Company subsequently dismissed without prejudice. On November 1, 2019, the Court granted Mr. Petit’s motion to dismiss on jurisdictional grounds, denied the Company’s motion to dismiss, and granted plaintiffs leave to file an amended complaint to address the deficiencies in its claims against Mr. Petit, which they did on November 21, 2019. The Company filed its answer on December 20, 2019.
48
Intellectual Property Litigation
The Bone Bank Action
On May 16, 2014, the Company filed a patent infringement lawsuit against Transplant Technology, Inc. d/b/a Bone Bank Allografts (“Bone Bank”) and Texas Human Biologics, Ltd. (“Biologics”) in the United States District Court for the Western District of Texas (MiMedx Group, Inc. v. Tissue Transplant Technology, LTD. d/b/a/ Bone Bank Allografts et. al.). The Company has asserted that Bone Bank and Biologics infringed certain of the Company’s patents through the manufacturing and sale of their placental-derived tissue graft products, and the Company is seeking permanent injunctive relief and unspecified damages. On July 10, 2014, Bone Bank and Biologics filed an answer to the complaint, denying the allegations in the complaint, and filed counterclaims seeking declaratory judgments of non-infringement and invalidity. The matter settled in 2019 prior to trial, and the case was dismissed on April 4, 2019.
The NuTech Action
On March 2, 2015, the Company filed a patent infringement lawsuit against NuTech Medical, Inc. (“NuTech”) and DCI Donor Services, Inc. (“DCI”) in the United States District Court for the Northern District of Alabama (MiMedx Group, Inc. v. NuTech Medical, Inc. et. al.). The Company has alleged that NuTech and DCI infringed and continue to infringe the Company’s patents through the manufacture, use, sale and/or offering of their tissue graft product. The Company has also asserted that NuTech knowingly and willfully made false and misleading representations about its products to customers and prospective customers. The Company is seeking permanent injunctive relief and unspecified damages. The case was stayed pending the restatement of the Company’s financial statements.
The Osiris Action
On February 20, 2019, Osiris Therapeutics, Inc. (“Osiris”) refiled its trade secret and breach of contract action against the Company (which had been dismissed in a different forum) in the United States District Court for the Northern District of Georgia (Osiris Therapeutics, Inc. v. MiMedx Group, Inc.). Osiris has alleged that the Company acquired Stability Biologics, LLC, a former distributor of Osiris, in order to illegally obtain trade secrets. On February 24, 2020, the Court issued an order granting in part and denying in party MiMedx’s motion to dismiss. The Court dismissed Osiris’s claims for tortious interference, conspiracy to breach contract, unfair competition, and conspiracy to commit unfair competition. The Court denied MiMedx’s motion to dismiss with respect to the claim for breach of the contract between Osiris and Stability Biologics, finding that there is a question as to whether Osiris can maintain such a claim by piercing the corporate veil between MiMedx and its former subsidiary. If Osiris cannot pierce the corporate veil, the claim against MiMedx fails; if Osiris can pierce the corporate veil, the breach of contract claim must be brought in an arbitration proceeding. MiMedx did not move to dismiss Osiris’s claims for misappropriation of trade secrets and conspiracy to misappropriate trade secrets. MiMedx plans to defend against all remaining claims.
Other Matters
In addition to the matters described above, the Company is a party to a variety of other legal matters that arise in the normal course of the Company’s business, none of which is deemed to be individually material at this time. Due to the inherent uncertainty of litigation, there can be no assurance that the resolution of any particular claim or proceeding would not have a material adverse effect on the Company’s business, results of operations, financial position or liquidity.
Item 4. Mine Safety Disclosures
Not applicable.
49
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market for Common Stock
Our Common Stock currently trades on the “over the counter” market operated by the OTC Markets Group Inc. (the “OTC Market”) under the symbol “MDXG.” The OTC Market quotations reflect inter-dealer prices, without retail markup, mark-down or commission and may not represent actual transactions. Previously, our Common Stock traded on Nasdaq under the symbol “MDXG.” Due to our inability to file periodic reports with the SEC, we were not able to comply with Nasdaq listing standards, and our Common Stock was suspended from trading on Nasdaq and subsequently delisted, effective on March 8, 2019.
Based upon information supplied from our transfer agent, there were approximately 1,185 shareholders of record of our Common Stock as of March 3, 2020.
We have not paid any cash dividends and do not anticipate paying any cash dividends on our common stock in the foreseeable future.
Information required by this Item regarding equity compensation plans is contained in our Proxy Statement under the caption “Equity Compensation Plan Information,” and is incorporated herein by reference.
Stock Performance Graph
The following graph compares the cumulative total stockholder return on our Common Stock with the cumulative total stockholder return of the Nasdaq Composite Index and the Nasdaq Biotechnology Index, assuming an investment of $100.00 on December 31, 2014, in each of our Common Stock, the stocks comprising the Nasdaq Composite Index, and the stocks comprising the Nasdaq Biotechnology Index.
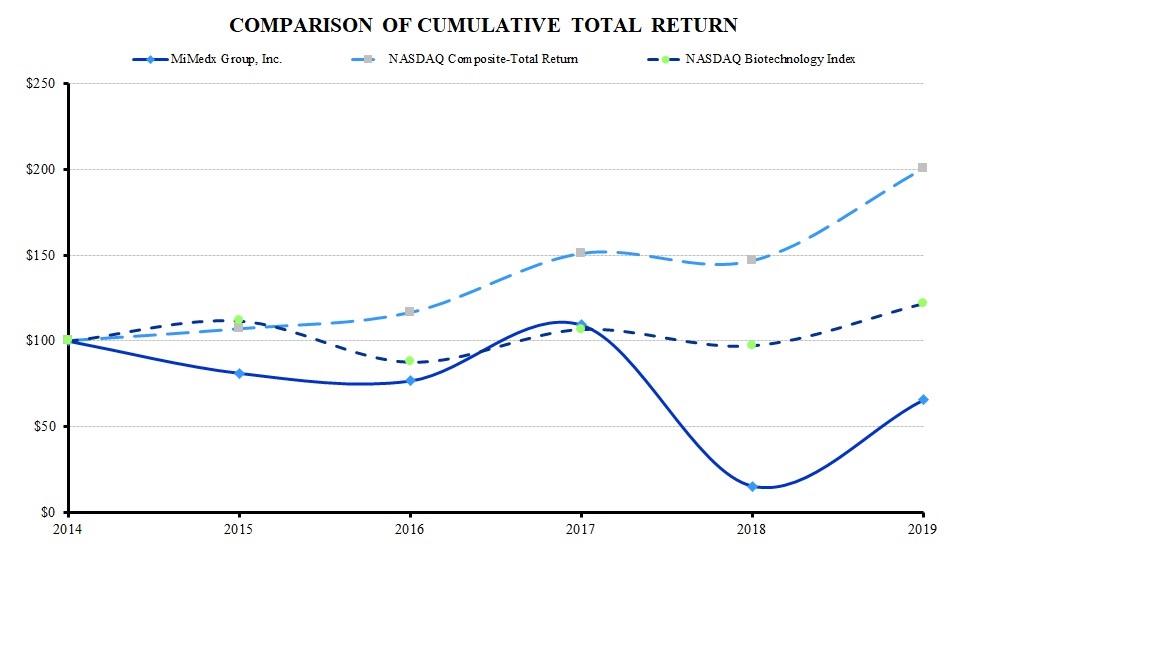
ASSUMES $100 INVESTED ON DEC. 31, 2014
ASSUMES NO DIVIDENDS
FISCAL YEAR ENDED DEC. 31, 2019
50
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
The following table sets forth information regarding the purchases of the Company’s equity securities made by or on behalf of the Company or any affiliated purchaser (as defined in Rule 10b-18 under the Exchange Act) during the three-month period ended December 31, 2017 and during the 12 month-period ending December 31, 2018.
Period | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Approximate Dollar Value of Shares that May Yet Be Purchased Under Plans or Programs (4) | ||||||
Total amount remaining October 1, 2017 | $ | 12,740 | ||||||||
October 2017 increased spending authorization | $ | 20,000,000 | ||||||||
October 1, 2017 - October 31, 2017 | 227,626 | $ | 13.00 | 188,500 | $ | 17,562,241 | ||||
November 1, 2017 - November 30, 2017 | 1,471,986 | $ | 11.90 | 1,460,227 | $ | 184,057 | ||||
December 2017 increased spending authorization | $ | 10,000,000 | ||||||||
December 1, 2017 - December 31, 2017 | 352,205 | $ | 12.69 | 342,023 | $ | 5,842,079 | ||||
Total for the quarter (1) | 2,051,817 | $ | 12.14 | 1,990,750 | ||||||
January 2018 increased spending authorization | $ | 10,000,000 | ||||||||
January 1, 2018 - January 31, 2018 | 379,535 | $ | 14.11 | 366,550 | $ | 10,668,339 | ||||
February 1, 2018 - February 28, 2018 | 589,968 | $ | 16.89 | 141,050 | $ | 8,285,732 | ||||
March 1, 2018 - March 31, 2018 | 2,898 | — | — | $ | 8,285,732 | |||||
Total for the quarter (2) | 972,401 | 507,600 | ||||||||
April 1, 2018 - April 30, 2018 | 28,571 | — | — | $ | 8,285,732 | |||||
May 1, 2018 - May 31, 2018 | 11,749 | — | — | $ | 8,285,732 | |||||
June 1, 2018 - June 30, 2018 | 1,939 | — | — | $ | 8,285,732 | |||||
Total for the quarter (3) | 42,259 | — | ||||||||
July 1, 2018 - July 31, 2018 | 43,956 | — | — | $ | 8,285,732 | |||||
August 1, 2018 - August 31, 2018 | 3,665 | — | — | $ | 8,285,732 | |||||
September 1, 2018 - September 30, 2018 | 2,567 | — | — | $ | 8,285,732 | |||||
Total for the quarter (3) | 50,188 | — | ||||||||
October 1, 2018 - October 31, 2018 | 51,516 | — | — | $ | 8,285,732 | |||||
November 1, 2018 - November 31, 2018 | 648 | — | — | $ | 8,285,732 | |||||
December 1, 2018 - December 31, 2018 | 4,711 | — | — | $ | 8,285,732 | |||||
Total for the quarter (3) | 56,875 | — | ||||||||
(1) Shares repurchased during the quarter include 61,067 shares surrendered by employees to satisfy tax withholding obligations upon vesting of restricted stock.
(2) Shares repurchased during the quarter include 464,801 shares surrendered by employees to satisfy tax withholding obligations upon vesting of restricted stock.
(3) Shares repurchased during the quarter include only shares surrendered by employees to satisfy tax withholding obligations upon vesting of restricted stock.
(4) On May 8, 2014, the Board authorized the repurchase of up to $10 million of shares of our Common Stock from time to time through December 31, 2014. The Board subsequently increased the amount authorized and extended the program through December 31, 2018. In the periods above, the Board increased the amount authorized for repurchase by $10 million on October 6, 2017, by $10 million on October 26, 2017, by $10 million on December 12, 2017, and by $10 million on January 24, 2018. On December 31, 2018, the repurchase authorization expired.
51
Item 6. Selected Financial Data
The selected consolidated financial data displayed below for the years ended December 31, 2018, 2017, and 2016 was derived from our audited consolidated financial statements for the three-year period ended December 31, 2018. As described below, the selected financial data as of and for the years ended December 31, 2015 (Restated) and 2014 (Restated) are unaudited, have been derived from our unaudited consolidated financial statements, which were prepared on the same basis as our audited consolidated financial statements, and reflect the impact of adjustments to, or restatement of, our previously filed financial information, including a January 1, 2014 cumulative effect adjustment to stockholders’ equity to correct for accounting errors in periods prior to January 1, 2014. The selected financial data set forth below is not necessarily indicative of results of future operations, and should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements.
Year Ended December 31, in thousands | |||||||||||||||||||||
2018 | 2017 | 2016 | 2015 | 2014 | |||||||||||||||||
(Restated) | (Restated) | (Restated) | |||||||||||||||||||
Statement of Operations Data: | (Unaudited) | (Unaudited) | |||||||||||||||||||
Net sales (1) | $ | 359,111 | $ | 321,139 | $ | 221,712 | $ | 153,131 | $ | 105,257 | |||||||||||
Gross profit | 322,725 | 285,920 | 190,774 | 137,579 | 92,835 | ||||||||||||||||
Operating income (loss) (2) | (3,924 | ) | 46,223 | 884 | (5,880 | ) | (3,644 | ) | |||||||||||||
Net income (loss) (3) | (29,979 | ) | 64,727 | 390 | (16,354 | ) | (4,743 | ) | |||||||||||||
Net income (loss) per common share - basic | $ | (0.28 | ) | $ | 0.61 | $ | — | $ | (0.15 | ) | $ | (0.04 | ) | ||||||||
Net income (loss) per common share - diluted | $ | (0.28 | ) | $ | 0.56 | $ | — | $ | (0.14 | ) | $ | (0.04 | ) | ||||||||
(1) Includes the following:
• | Sales to external customers by Stability Biologics, LLC, our wholly-owned subsidiary acquired on January 13, 2016 and sold on September 30, 2017, were $7.0 million and $11.7 million during the years ended December 31, 2017 and 2016, respectively. |
(2) Includes legal fees, forensic audit fees, and consulting fees relating to the Restatement; and legal fees relating to the SEC Investigation, shareholder derivative lawsuits, and other litigation, as well as settlements made with former employees.
• | Investigation, restatement and related expenses were $51.3 million in 2018 as compared with $0.0 million in 2017; |
• | As a result of the December 2018 broad-based organizational realignment, cost reduction and efficiency program, the Company incurred pre-tax charges of $6.1 million during 2018. |
(3) Includes the following:
• | Loss on sale of Stability Biologics, LLC of $1.0 million recognized during the year ended December 31, 2017 and further discussed in Item 8, Note 5 “Stability Biologics, LLC.” |
For further information regarding the comparability of the financial data presented in the tables above and factors that may impact comparability of future results, see Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as the Consolidated Financial Statements.
52
As of December 31, in thousands | |||||||||||||||||||||
2018 | 2017 | 2016 | 2015 | 2014 | |||||||||||||||||
(Restated) | (Restated) | (Restated) | |||||||||||||||||||
Balance Sheet Data: | (Unaudited) | (Unaudited) | |||||||||||||||||||
Cash and cash equivalents | $ | 45,118 | $ | 27,476 | $ | 30,321 | $ | 26,301 | $ | 46,337 | |||||||||||
Short term investments | — | — | — | 3,000 | 5,750 | ||||||||||||||||
Accounts receivable, net | — | — | 1,927 | — | — | ||||||||||||||||
Inventory, net | 15,986 | 9,467 | 15,872 | 7,460 | 5,133 | ||||||||||||||||
Prepaid expenses | 6,673 | 2,125 | 1,838 | 945 | 1,132 | ||||||||||||||||
Income tax receivable | 454 | 656 | — | — | — | ||||||||||||||||
Other current assets | 5,818 | 9,023 | 9,516 | 7,260 | 2,527 | ||||||||||||||||
Total current assets | 74,049 | 48,747 | 59,474 | 44,966 | 60,879 | ||||||||||||||||
Total assets | $ | 122,844 | $ | 121,255 | $ | 117,274 | $ | 69,560 | $ | 84,349 | |||||||||||
Accounts payable | $ | 14,864 | $ | 8,454 | $ | 12,412 | $ | 6,987 | $ | 3,908 | |||||||||||
Accrued compensation | 23,024 | 20,941 | 12,691 | 15,276 | 11,464 | ||||||||||||||||
Accrued expenses | 31,842 | 15,768 | 19,207 | 9,679 | 4,793 | ||||||||||||||||
Current portion of earn out liability | — | — | 8,260 | — | — | ||||||||||||||||
Deferred tax liability | — | — | 1,129 | 803 | 493 | ||||||||||||||||
Income taxes | — | — | 5,611 | 410 | 452 | ||||||||||||||||
Other current liabilities | 1,817 | 647 | 1,482 | 533 | 264 | ||||||||||||||||
Total current liabilities | 71,547 | 45,810 | 60,792 | 33,688 | 21,374 | ||||||||||||||||
Long term liabilities | 1,642 | 1,648 | 8,415 | 1,148 | 1,526 | ||||||||||||||||
Additional paid in capital | 164,744 | 164,649 | 161,481 | 163,438 | 162,323 | ||||||||||||||||
Accumulated deficit | (76,560 | ) | (46,581 | ) | (111,308 | ) | (111,698 | ) | (95,345 | ) | |||||||||||
Total stockholders' equity | 49,655 | 73,797 | 48,067 | 34,724 | 61,449 | ||||||||||||||||
Total liabilities and stockholders' equity | $ | 122,844 | $ | 121,255 | $ | 117,274 | $ | 69,560 | $ | 84,349 | |||||||||||
Working capital | 2,502 | 2,937 | (1,318 | ) | 11,278 | 39,505 | |||||||||||||||
Restatement
As a result of the issues identified in the Audit Committee Investigation and the related review of our accounting policies and our significant accounting transactions, as discussed in the Explanatory Note to this Form 10-K, the Company determined that the Restatement was needed. The impact of the Restatement on the Company’s consolidated statement of operations includes, but is not limited to, the following:
• | the timing of revenue recognition for sales through distributors and direct sales to customers, except for the sales recognized by our wholly-owned subsidiary, Stability, for the period from January 13, 2016 to September 30, 2017, which were not restated and continued to be recognized at the time of physical delivery of the product; |
• | the presentation of net revenue instead of gross revenue for administrative fees paid to GPOs; |
• | the impact of changes in revenue recognition on cost of goods sold; |
• | the timing of recognizing certain general and administrative expenses; |
• | the impact on losses associated with contingency exposures; |
• | the impact of other miscellaneous adjustments, such as patent cost and share-based compensation, and |
• | the impact of the above on income tax. |
53
The impact of the Restatement on the Company’s consolidated balance sheet includes, but is not limited to, the following:
• | changes in the amount of reported cash, due to the timing of certain cash collections; |
• | changes to reported accounts receivable and other current assets and the related reserves on each, due to the restatement of revenue recognition; |
• | accrual balances that are impacted by the expense and contingency determinations discussed above; and |
• | the related income tax effects of the above. |
Information relating to the Restatement as it affected the consolidated statements of stockholders’ equity and consolidated statements of cash flows and the causes of those effects can be found in Note 4, “Restatement of the Consolidated Financial Statements” in the consolidated financial statements, below.
The Audit Committee Investigation and our review and assessment also identified various material weaknesses in internal control, including in our entity level controls and in certain accounting practices, all as described under Item 9A, “Controls and Procedures” in this Form 10-K. We have taken steps to define, remediate and enhance our internal control environment, our tone at the top, and internal controls over financial reporting. These include:
• | improved processes and controls to monitor sales practices and recognize revenue; |
• | a restructured and bolstered pricing committee; |
• | tightened policies, procedures, and governance of credit and returns; |
• | revised criteria for granting credit and periodic credit limit and terms reviews; |
• | improved cash collection procedures and efforts; |
• | the enhancement of the cash forecast process; |
• | the establishment of an independent compliance department reporting to the Board; |
• | the assessment and initial implementation of remediation of controls required under the Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley”); |
• | the hiring of a Vice President of Internal Audit to develop and implement an internal audit function for the Company; and |
• | executing on the realignment program announced in December 2018. |
54
The following tables summarize the effects of the restatement adjustments on our previously issued, audited consolidated financial statements for the years ended December 31, 2016, 2015 and 2014 previously filed on Annual Reports on Form 10-K.
Year Ended December 31, 2016 (in thousands, except for per share information) | |||||||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||||||
Previously | Cash | Revenue | Total | ||||||||||||||||||||||||
Statement of Operations Data: | Reported | Revenue | GPO Fees | Related | Other | Adjustments | Restated | ||||||||||||||||||||
Net sales | $ | 245,015 | $ | (14,725 | ) | $ | (4,487 | ) | $ | (4,091 | ) | $ | — | $ | (23,303 | ) | $ | 221,712 | |||||||||
Gross profit | 212,608 | (14,725 | ) | (4,487 | ) | (2,622 | ) | — | (21,834 | ) | 190,774 | ||||||||||||||||
Operating income | 18,446 | (14,725 | ) | — | (878 | ) | (1,959 | ) | (17,562 | ) | 884 | ||||||||||||||||
Net income (loss) | 11,974 | (14,725 | ) | — | (878 | ) | 4,019 | (11,584 | ) | 390 | |||||||||||||||||
Net income (loss) per common share - basic | $ | 0.11 | $ | 0.00 | |||||||||||||||||||||||
Net income (loss) per common share - diluted | $ | 0.11 | $ | 0.00 | |||||||||||||||||||||||
Year Ended December 31, 2015 (in thousands, except for per share information) | |||||||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||||||
Previously | Cash | Revenue | Total | ||||||||||||||||||||||||
Statement of Operations Data: | Reported | Revenue | GPO Fees | Related | Other | Adjustments | Restated | ||||||||||||||||||||
Net sales | $ | 187,296 | $ | (32,708 | ) | $ | (1,457 | ) | $ | — | $ | — | $ | (34,165 | ) | $ | 153,131 | ||||||||||
Gross profit | 167,094 | (32,708 | ) | (1,457 | ) | 4,650 | — | (29,515 | ) | 137,579 | |||||||||||||||||
Operating income (loss) | 24,364 | (32,708 | ) | — | 6,057 | (3,593 | ) | (30,244 | ) | (5,880 | ) | ||||||||||||||||
Net income (loss) | 29,446 | (32,708 | ) | — | 6,057 | (19,149 | ) | (45,800 | ) | (16,354 | ) | ||||||||||||||||
Net income (loss) per common share - basic | $ | 0.28 | $ | (0.15 | ) | ||||||||||||||||||||||
Net income (loss) per common share - diluted | $ | 0.26 | $ | (0.14 | ) | ||||||||||||||||||||||
Year Ended December 31, 2014 (in thousands, except for per share information) | |||||||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||||||
Previously | Cash | Revenue | Total | ||||||||||||||||||||||||
Statement of Operations Data: | Reported | Revenue | GPO Fees | Related | Other | Adjustments | Restated | ||||||||||||||||||||
Net sales | $ | 118,223 | $ | (12,654 | ) | $ | (312 | ) | $ | — | $ | — | $ | (12,966 | ) | $ | 105,257 | ||||||||||
Gross profit | 105,558 | (12,654 | ) | (312 | ) | 243 | — | (12,723 | ) | 92,835 | |||||||||||||||||
Operating income (loss) | 7,100 | (12,654 | ) | — | 1,500 | 410 | (10,744 | ) | (3,644 | ) | |||||||||||||||||
Net income (loss) | 6,220 | (12,654 | ) | — | 1,500 | 191 | (10,963 | ) | (4,743 | ) | |||||||||||||||||
Net income (loss) per common share - basic | $ | 0.06 | $ | (0.04 | ) | ||||||||||||||||||||||
Net income (loss) per common share - diluted | $ | 0.05 | $ | (0.04 | ) | ||||||||||||||||||||||
55
As of December 31, 2016 (in thousands) | |||||||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||||||
Previously | Cash | Revenue | Deposits in | Total | |||||||||||||||||||||||
Balance Sheet Data | Reported | Revenue | Related | Transit | Other | Adjustments | Restated | ||||||||||||||||||||
Cash and cash equivalents | $ | 34,391 | $ | — | $ | — | $ | (4,070 | ) | $ | — | $ | (4,070 | ) | $ | 30,321 | |||||||||||
Accounts receivable, net | 67,151 | (69,400 | ) | 106 | 4,070 | — | (65,224 | ) | 1,927 | ||||||||||||||||||
Inventory, net | 17,814 | — | (1,942 | ) | — | — | (1,942 | ) | 15,872 | ||||||||||||||||||
Prepaid expenses | 5,894 | — | — | — | (4,056 | ) | (4,056 | ) | 1,838 | ||||||||||||||||||
Other current assets | 1,288 | 805 | 7,423 | — | — | 8,228 | 9,516 | ||||||||||||||||||||
Total current assets | 126,538 | (68,595 | ) | 5,587 | — | (4,056 | ) | (67,064 | ) | 59,474 | |||||||||||||||||
Total assets | $ | 193,263 | $ | (68,595 | ) | $ | 5,587 | $ | — | $ | (12,981 | ) | $ | (75,989 | ) | $ | 117,274 | ||||||||||
Accounts payable | $ | 11,436 | $ | — | $ | — | $ | — | $ | 976 | $ | 976 | $ | 12,412 | |||||||||||||
Accrued compensation | 12,365 | — | — | — | 326 | 326 | 12,691 | ||||||||||||||||||||
Accrued expenses | 10,941 | 6,194 | (204 | ) | — | 2,276 | 8,266 | 19,207 | |||||||||||||||||||
Current portion of earn out liability | 8,740 | — | — | — | (480 | ) | (480 | ) | 8,260 | ||||||||||||||||||
Deferred tax liability | — | — | — | — | 1,129 | 1,129 | 1,129 | ||||||||||||||||||||
Income taxes | 5,768 | — | — | — | (157 | ) | (157 | ) | 5,611 | ||||||||||||||||||
Other current liabilities | 1,482 | — | — | — | — | — | 1,482 | ||||||||||||||||||||
Total current liabilities | 50,732 | 6,194 | (204 | ) | — | 4,070 | 10,060 | 60,792 | |||||||||||||||||||
Long term liabilities | 9,531 | — | — | — | (1,116 | ) | (1,116 | ) | 8,415 | ||||||||||||||||||
Additional paid in capital | 161,261 | — | — | — | 220 | 220 | 161,481 | ||||||||||||||||||||
Accumulated deficit | (26,155 | ) | (74,789 | ) | 5,791 | — | (16,155 | ) | (85,153 | ) | (111,308 | ) | |||||||||||||||
Total stockholders' equity | 133,000 | (74,789 | ) | 5,791 | — | (15,935 | ) | (84,933 | ) | 48,067 | |||||||||||||||||
Total liabilities and stockholders' equity | $ | 193,263 | $ | (68,595 | ) | $ | 5,587 | $ | — | $ | (12,981 | ) | $ | (75,989 | ) | $ | 117,274 | ||||||||||
Working capital | $ | 75,806 | $ | (74,789 | ) | $ | 5,791 | $ | — | $ | (8,126 | ) | $ | (77,124 | ) | $ | (1,318 | ) | |||||||||
56
As of December 31, 2015 (in thousands) | |||||||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||||||
Previously | Cash | Revenue | Deposits in | Total | |||||||||||||||||||||||
Balance Sheet Data | Reported | Revenue | Related | Transit | Other | Adjustments | Restated | ||||||||||||||||||||
Cash and cash equivalents | $ | 28,486 | $ | — | $ | — | $ | (2,185 | ) | $ | — | $ | (2,185 | ) | $ | 26,301 | |||||||||||
Short term investments | 3,000 | — | — | — | — | — | 3,000 | ||||||||||||||||||||
Accounts receivable, net | 53,755 | (55,940 | ) | — | 2,185 | — | (53,755 | ) | — | ||||||||||||||||||
Inventory, net | 7,460 | — | — | — | — | — | 7,460 | ||||||||||||||||||||
Prepaid expenses | 3,609 | — | — | — | (2,664 | ) | (2,664 | ) | 945 | ||||||||||||||||||
Other current assets | — | 376 | 6,669 | — | 215 | 7,260 | 7,260 | ||||||||||||||||||||
Total current assets | 96,310 | (55,564 | ) | 6,669 | — | (2,449 | ) | (51,344 | ) | 44,966 | |||||||||||||||||
Total assets | $ | 135,913 | $ | (55,564 | ) | $ | 6,669 | $ | — | $ | (17,458 | ) | $ | (66,353 | ) | $ | 69,560 | ||||||||||
Accounts payable | $ | 6,633 | $ | — | $ | — | $ | — | $ | 354 | $ | 354 | $ | 6,987 | |||||||||||||
Accrued compensation | 15,034 | — | — | — | 242 | 242 | 15,276 | ||||||||||||||||||||
Accrued expenses | 4,644 | 4,500 | — | — | 535 | 5,035 | 9,679 | ||||||||||||||||||||
Deferred tax liability | — | — | — | — | 803 | 803 | 803 | ||||||||||||||||||||
Income taxes | (67 | ) | — | — | — | 477 | 477 | 410 | |||||||||||||||||||
Other current liabilities | 533 | — | — | — | — | — | 533 | ||||||||||||||||||||
Total current liabilities | 26,777 | 4,500 | — | — | 2,411 | 6,911 | 33,688 | ||||||||||||||||||||
Long term liabilities | 1,148 | — | — | — | — | — | 1,148 | ||||||||||||||||||||
Additional paid in capital | 163,133 | — | — | — | 305 | 305 | 163,438 | ||||||||||||||||||||
Accumulated deficit | (38,129 | ) | (60,064 | ) | 6,669 | — | (20,174 | ) | (73,569 | ) | (111,698 | ) | |||||||||||||||
Total stockholders' equity | 107,988 | (60,064 | ) | 6,669 | — | (19,869 | ) | (73,264 | ) | 34,724 | |||||||||||||||||
Total liabilities and stockholders' equity | $ | 135,913 | $ | (55,564 | ) | $ | 6,669 | $ | — | $ | (17,458 | ) | $ | (66,353 | ) | $ | 69,560 | ||||||||||
Working capital | $ | 69,533 | $ | (60,064 | ) | $ | 6,669 | $ | — | $ | (4,860 | ) | $ | (58,255 | ) | $ | 11,278 | ||||||||||
57
As of December 31, 2014 (in thousands) | |||||||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||||||
Previously | Cash | Revenue | Deposits in | Total | |||||||||||||||||||||||
Balance Sheet Data | Reported | Revenue | Related | Transit | Other | Adjustments | Restated | ||||||||||||||||||||
Cash and cash equivalents | $ | 46,582 | $ | — | $ | — | $ | (245 | ) | $ | — | $ | (245 | ) | $ | 46,337 | |||||||||||
Short term investments | 5,750 | — | — | — | — | — | 5,750 | ||||||||||||||||||||
Accounts receivable, net | 26,672 | (26,917 | ) | — | 245 | — | (26,672 | ) | — | ||||||||||||||||||
Inventory, net | 5,133 | — | — | — | — | — | 5,133 | ||||||||||||||||||||
Prepaid expenses | 1,540 | — | — | — | (408 | ) | (408 | ) | 1,132 | ||||||||||||||||||
Other current assets | — | 244 | 2,283 | — | — | 2,527 | 2,527 | ||||||||||||||||||||
Total current assets | 85,677 | (26,673 | ) | 2,283 | — | (408 | ) | (24,798 | ) | 60,879 | |||||||||||||||||
Total assets | $ | 109,259 | $ | (26,673 | ) | $ | 2,283 | $ | — | $ | (520 | ) | $ | (24,910 | ) | $ | 84,349 | ||||||||||
Accounts payable | $ | 3,661 | $ | — | $ | — | $ | — | $ | 247 | $ | 247 | $ | 3,908 | |||||||||||||
Accrued compensation | 11,523 | — | — | — | (59 | ) | (59 | ) | 11,464 | ||||||||||||||||||
Accrued expenses | 2,504 | 2,355 | — | — | (66 | ) | 2,289 | 4,793 | |||||||||||||||||||
Deferred tax liability | — | — | — | — | 493 | 493 | 493 | ||||||||||||||||||||
Income taxes | 452 | — | — | — | — | — | 452 | ||||||||||||||||||||
Other current liabilities | 264 | — | — | — | — | — | 264 | ||||||||||||||||||||
Total current liabilities | 18,404 | 2,355 | — | — | 615 | 2,970 | 21,374 | ||||||||||||||||||||
Long term liabilities | 1,526 | — | — | — | — | — | 1,526 | ||||||||||||||||||||
Additional paid in capital | 162,433 | — | — | — | (110 | ) | (110 | ) | 162,323 | ||||||||||||||||||
Accumulated deficit | (67,575 | ) | (29,028 | ) | 2,283 | — | (1,025 | ) | (27,770 | ) | (95,345 | ) | |||||||||||||||
Total stockholders' equity | 89,329 | (29,028 | ) | 2,283 | — | (1,135 | ) | (27,880 | ) | 61,449 | |||||||||||||||||
Total liabilities and stockholders' equity | $ | 109,259 | $ | (26,673 | ) | $ | 2,283 | $ | — | $ | (520 | ) | $ | (24,910 | ) | $ | 84,349 | ||||||||||
Working capital | $ | 67,273 | $ | (29,028 | ) | $ | 2,283 | $ | — | $ | (1,023 | ) | $ | (27,768 | ) | $ | 39,505 | ||||||||||
In its consolidated balance sheets as provided in the consolidated financial statements in the Company’s annual report on Form 10-K for 2015 and 2014, the Company presented income taxes as a component of Other current liabilities. Here, the Company presents income taxes separately to conform to current period presentation.
Certain errors impacted years prior to 2014. These errors are aggregated to adjust the December 31, 2013 opening balance of stockholders’ equity. The components of the cumulative effect of the restatement adjustments that were made as of December 31, 2013, to the opening balance of accumulated deficit to our consolidated statements of stockholders’ equity are also detailed in the table below:
As of December 31, 2013 | |||||||||||||||||||||||
(in thousands) | |||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||
Previously Reported | Cash Revenue | Revenue Related | Other | Total Adjustments | Restated | ||||||||||||||||||
Common stock | $ | 104 | $ | — | $ | — | $ | — | $ | — | $ | 104 | |||||||||||
Additional paid-in capital | 147,284 | — | — | — | — | 147,284 | |||||||||||||||||
Treasury stock | (25 | ) | — | — | — | — | (25 | ) | |||||||||||||||
Accumulated deficit | (73,795 | ) | (17,655 | ) | 2,064 | (1,216 | ) | (16,807 | ) | (90,602 | ) | ||||||||||||
Total stockholders' equity | $ | 73,568 | $ | (17,655 | ) | $ | 2,064 | $ | (1,216 | ) | $ | (16,807 | ) | $ | 56,761 | ||||||||
58
The following is a discussion of the significant adjustments that were made to our audited consolidated financial statements for the years ended December 31, 2016, 2015 and 2014 that were previously filed with the SEC.
Revenue Recognition
Under our previous revenue recognition policy utilized in the preparation of the financial statements for each of the years ended December 31, 2016, 2015, 2014, 2013 and 2012 and each of the quarters ended March 31, 2017, June 30, 2017, and September 30, 2017, revenue was recorded as follows:
• | For sales to distributors, revenue was recorded upon shipment to the distributor; |
• | Certain sales to direct customers were treated as consignment sales as the customer could return the product at any time and was not required to pay until the product was used, despite no formal consignment agreement being in place. Therefore, the Company did not record revenue until the product was sold to an end-user (i.e., the recipient of the product); |
• | For other sales to direct customers, revenue was recorded upon shipment to the customer. |
Under Accounting Standards Codification (“ASC”) 605, revenue should not be recognized until it is realized or realizable and earned. SEC Staff Accounting Bulletin (“SAB”) 13.A.1 (as codified in ASC 605-10-S99-1) outlines four criteria that generally indicate when revenue is realized or realizable and earned. If any of these criteria are not met, revenue recognition should be deferred until all criteria have been met. Therefore, we assessed these four criteria as follows:
1. | Persuasive evidence of an arrangement exists - The Company’s sales are driven either by contracts or purchase orders. These documents are typically used to establish persuasive evidence of an arrangement. The Company’s customary business practices, however, must be taken into account as a contract can be written, oral, or based on customary business practices. Throughout 2012-2017, although the Company may have created a legal contract upon the execution of a contract and/or fulfillment of a purchase order, the lack of clarity around the final terms of the arrangement due to the pervasive side agreements with customers preclude the Company’s sales transactions from meeting this criterion upon shipment of the product. Therefore, even though there may have been a legal contract governing the arrangement (which typically would indicate persuasive evidence of an arrangement), the Company’s selling and collection practices amended the stated contract terms. After considering these factors, the Company concluded that persuasive evidence of an arrangement did not exist upon shipment of the product. |
2. | Delivery has occurred or services have been rendered - For sales to customers, physical possession and title transferred upon shipment to the customer. However, the Company concluded that it did not pass the risks of ownership to the customer upon shipment because customers were allowed to return product for multiple reasons, which included being unable to sell the product, damages which may have occurred subsequent to delivery, and dropped product. See below for additional discussion of the Company’s rationale for concluding that delivery had not yet occurred upon shipment to the customer. |
3. | The seller’s price to the buyer is fixed or determinable - At certain quarter-ends, the Company was significantly increasing sales to customers without having visibility into the level of product remaining unsold at the customer’s location. This practice made it difficult to develop an appropriate estimate of future credits to be issued to customers at the time of sale, which, in turn, impacted whether the price at the time of transfer of physical possession to the customer was fixed or determinable. This previous practice in combination with the following actions of the Company preclude the price of the Company’s sales transactions from being fixed or determinable upon shipment of product: |
a. | Offering customers an unconditional right of return with many items being returned over a year after the initial sale. |
b. | Offering extended payment terms to customers with days sales outstanding averaging almost 3 months, and |
c. | A history of exceeding established credit limits for customers. |
4. | Collectability is reasonably assured - At the time of transfer of physical possession to the customer, collectability of the sales was questionable. As noted in the Investigation and described further below, the customers’ intention to pay amounts when due was uncertain in light of the conflicting messages customers received with respect to the payment terms, rights of return and lack of adherence to credit limits. Although the Company did have a process in place to establish credit |
59
limits, the evidence indicates that those credit limits were overridden by certain sales personnel and members of management. Despite these overrides, the Company recovered the majority of its billings made between 2012 and 2017 with insignificant write-offs recorded; however, a significant amount of these billings were collected well after payment was due under the contractual terms. Furthermore, the quantitative and qualitative evidence gathered by the Company raised considerable doubt as to the collectability of its billings at the time of shipment, but this evidence was not persuasive enough for the Company to reach a conclusion as to whether collectability was reasonably assured.
In the Company’s evaluation of the point at which delivery has occurred (the second criterion discussed above), the Company further considered the fact that there are instances under ASC 605 where the transfer of title of the product does not coincide with revenue recognition. Based on its review of all facts and circumstances the Company has now determined that it did not meet all of the criteria to recognize revenue at the time of shipment of product to the customer. Specifically, the Company determined that the Company did not transfer the risks of ownership upon the transfer of physical possession because the Company’s customers were routinely granted an extended return period with very limited restrictions on the right of return and extended payment terms which raise doubt as to the intent or ability of customers to use and pay for the product delivered. Customers were allowed to return product for multiple reasons which included being unable to sell the product, damages which may have occurred subsequent to delivery, and dropped product (i.e., product that becomes contaminated and unusable).
Accordingly, the Company has concluded that, from 2012 to 2017, its previous decision to recognize revenue at the time of shipment of the product to the customer was not appropriate. The Company has determined that the aforementioned revenue recognition criteria were met only when both of the following events had occurred: (1) the Company has fulfilled the customer’s purchase order by delivering product ordered; and (2) the Company has collected payment for the product delivered. Furthermore, the Company has determined that the amount of revenue to be recognized should be limited to the amount of payment received in a given period less the amount expected to be refunded or credited to customers for sales returns made after payment.
GPO Fees (Net Revenue Presentation)
We sell our products to GPO members who transact directly with the Company at GPO-agreed pricing. GPOs are funded by administrative fees that are paid by the Company. These fees are set as a percentage of the purchase volume, which is typically 3% of sales made to the GPO. In prior years, the Company concluded that these fees should be accounted for consistent with purchases of services from other suppliers within General and Administrative expenses and not as a reduction in transaction price. Based on analysis performed as part of the Restatement discussed above, the Company determined that the administrative fees paid to GPOs should be presented as a reduction of revenues, as the benefit received by the Company in exchange for the GPO fees was not sufficiently separable from the GPO member’s purchase of the Company’s products.
Revenue-Related Adjustments
The Company considered the accounting treatment for the related cost of sales when revenue is recognized at the time cash is collected from customers. Previously, cost of sales was recognized upon shipment of the Company’s products, which was consistent with the previous revenue recognition policy. However, the Company believes the matching of the recognition of costs of sales with the recognition of revenue is preferred, and the Company’s product is such that upon return of the product, the Company could sell the product if it is not damaged. Therefore, the Company determined that such costs should be deferred until revenue is recognized. The capitalized costs associated with delivered products are classified as deferred costs and reported within current assets, separately from inventory. The adjustment to cost of sales in the consolidated statement of operations and to other currents assets in the consolidated balance sheet reflects this change.
The Company also considers the financial viability of its customers based on their creditworthiness to determine if collectability of amounts sufficient to recover the costs of the products shipped is reasonably assured. In cases where the Company has concluded that collectability is not reasonably assured, the condition in paragraph ASC 450-20-25-2(a) is met and a loss contingency should be accrued. The Company therefore estimates this loss in each period and records a reserve against its deferred cost balance and charges income for the estimated loss. The adjustment to selling, general and administrative expenses in the consolidated statement of operations and to other current assets in the consolidated balance sheet reflects this change.
Deposits in Transit
The Company reduced the amount of reported cash for incorrectly reflected deposits in transit, due to the timing of certain cash collections.
60
Other Adjustments
In addition to the adjustments recorded to address the Company’s errors in accounting for revenue recognition, deposits in transit and gross versus net revenue presentation, the Company has identified other errors that have been recorded in connection with the Restatement, as follows:
• | timing adjustments for prepaid expenses and expense accruals for research and development expenses related to clinical trials, employee compensation and other employee-related costs, legal costs and other accruals; |
• | adjustments to stock-based compensation, primarily to reflect share-based awards granted to consultants as non-employee instead of as employee awards; and |
• | an adjustment for a change in fair value of $1.7 million for earn-out. See Note 5. “Stability Biologics, LLC” in the Consolidated Financial Statements. |
61
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Overview
MiMedx is an industry leader in advanced wound care and an emerging therapeutic biologics company, developing and distributing placental tissue allografts with patent-protected processes for multiple sectors of healthcare. We derive our products from human placental tissues processed using our proprietary processing methodologies, including the PURION® process. We employ aseptic processing techniques in addition to terminal sterilization to produce our allografts. MiMedx provides products in the wound care, burn, surgical, orthopedic, spine, sports medicine, ophthalmic, and dental sectors of healthcare. Our mission is to offer physicians products and tissues to help the body heal itself. All of our products are regulated by the FDA.
MiMedx is the leading supplier of human placental allografts, which are human tissues that are transplanted from one person (a donor) to another person (a recipient). MiMedx has supplied over 1.8 million allografts, through both direct sales and consignment shipments. Our biomaterial platform technologies include AmnioFix®, EpiFix®, EpiCord®, AmnioCord® and AmnioFill®. AmnioFix and EpiFix are our tissue allografts derived from the amnion and chorion layers of the human placental membrane. EpiCord and AmnioCord are tissue allografts derived from umbilical cord tissue. AmnioFill is a placental connective tissue matrix derived from the placental disc and other placental tissue.
Our EpiFix and EpiCord product lines are promoted for external use, such as in advanced wound care applications, while our AmnioFix, AmnioCord and AmnioFill products are positioned for use in surgical applications, including lower extremity repair, plastic surgery, vascular surgery and multiple orthopedic repairs and reconstructions.
MiMedx has two primary distribution channels: (1) direct to customers (healthcare professionals and/or facilities); and (2) sales through distributors. Prior to 2015, we did not sell directly to any federal customers (with only minor exceptions). Substantially all sales to federal customers went through one distributor, AvKARE, until 2015 when the Company began selling directly to federal customers rather than exclusively selling through AvKARE.
Trends in Our Business
Certain areas of our business suffered as a result of the issues identified in the Audit Committee Investigation
The results of the Investigation have caused us to incur significant legal fees, fines, and penalties. Additionally, the Company has incurred significant costs in connection with the associated Restatement. Negative publicity in the marketplace has created challenges for the Company in selling product to customers and retaining talented employees. All of these matters have caused the Company to incur significant costs and have negatively impacted our financial performance.
Demographic shifts are creating opportunities in the wound care space
The advanced wound care category is expected to continue growing due to certain demographic trends, including an aging population, increasing incidence of obesity and diabetes, and the associated higher susceptibility to non-healing chronic wounds. Furthermore, the increasing number of patients requiring advanced treatment represents a significant cost burden on the healthcare system. We expect that these shifts will benefit our business.
As we look for ways to achieve long-term competitive advantages, we plan to continue to invest in research & development
We continue to evaluate these opportunities in alignment with our focus on advanced wound care. We remain focused on advancing our BLA programs and are therefore aligning customer input, industry expertise, and additional resourcing toward seeking FDA approval for micronized dehydrated human amnion/chorion membrane (“dHACM”) for the potential indication to treat musculoskeletal degeneration across multiple indications. In addition, we expect to incur additional costs to achieve compliance with evolving regulatory standards.
Recent Events
Restatement and Remediation
As a result of the issues identified in the Audit Committee Investigation and the related review of our accounting policies and our significant accounting transactions, the Company determined that the Restatement was needed. As part of the Restatement, certain potential related party transactions, and other financial, internal control, and disclosure matters were analyzed to determine the impact on the Company’s financial statements. Refer to the Explanatory Note and Item 6, “Selected Financial Data–Restatement” in this Form 10-K for more information concerning the Audit Committee Investigation, the Restatement, and the impacts of the Restatement on our consolidated financial statements.
62
FDA Guidance and Enforcement Discretion
In November 2017, the FDA published a series of related guidances, including one entitled “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use–Guidance for Industry and Food and Drug Administration Staff” that established an updated framework for the FDA’s regulation of cellular and tissue-based products. Among other things, the guidances clarified the FDA’s views about the criteria that differentiate Section 361 HCT/Ps from Section 351 HCT/Ps. As described elsewhere in this Form 10-K, the guidances clarified the FDA’s expectation that certain products, such as micronized products that MiMedx has long marketed as Section 361 HCT/Ps, will be treated as Section 351 HCT/Ps moving forward. The guidances also confirmed that amniotic membrane in sheet form generally can be characterized as “minimally manipulated” and therefore regulated solely under Section 361.
The guidances stated that the FDA intends to exercise enforcement discretion under limited conditions with respect to the IND application and pre-market approval requirements for certain HCT/Ps through November 2020. This means that, through November 2020, the FDA does not intend to enforce certain provisions as they currently apply to certain entities or activities. The FDA intended this period of enforcement discretion to give sponsors time to evaluate their products, have a dialogue with the agency and, if necessary, begin clinical trials and file the appropriate pre-market applications to transition products that had been marketed as Section 361 HCT/Ps into compliance with Section 351. The FDA’s approach is risk-based, and the guidances clarified that high-risk products and uses might be subject to immediate enforcement action. We have continued to market our micronized, injectable products under this policy of enforcement discretion while at the same time pursuing BLAs for certain of our micronized products. For more information, refer to Item 1, “Business–Overview” and “Government Regulation,” and Item 1A, “Risk Factors,” under the heading “To the extent our products do not qualify for regulation as human cells, tissues and cellular and tissue-based products under Section 361 of the Public Health Service Act, this could result in removal of the applicable products from the market, would make the introduction of new tissue products more expensive and significantly delay the expansion of our tissue product offerings and subject us to additional post-market regulatory requirements.”
Critical Accounting Policies
We believe that of our significant accounting policies, which are described in Note 3 “Significant Accounting Policies” to our consolidated financial statements appearing elsewhere in this report, the following accounting policies involve a greater degree of judgment and complexity.
Revenue Recognition
We sell our products primarily to individual customers and independent distributors (collectively referred to as “customers”). Prior to 2015, substantially all federal healthcare providers, including the Department of Veterans Affairs, purchased our products from one distributor customer, AvKARE. In 2015, we also began selling product directly to federal customers rather than exclusively allowing federal healthcare providers to purchase Company product from AvKARE. Upon expiration of our agreement with AvKARE on June 30, 2017, we had an obligation to repurchase AvKARE’s remaining inventory within 90 days in accordance with the terms of the agreement. As of September 30, 2017, we had satisfied the repurchase obligation.
For sales of our products to customers for periods presented prior to January 1, 2018, the Company has determined that the revenue recognition criteria were met only when both of the following events had occurred: (1) the Company fulfilled the customer’s purchase order by delivering product ordered; and (2) the Company collected payment for the product delivered. Furthermore, the Company has determined that the amount of revenue to be recognized should be limited to the amount of payment received in a given period less the amount expected to be refunded or credited to customers for sales returns made after payment. Furthermore, the amount of revenue recognized was limited to the amount of payment received in a given period less any subsequent credits (includes credit memos, refunds, and rebates) issued for previously delivered product. The existence of extra-contractual or undocumented terms or arrangements initiated by our former executives at the onset of the transactions, such as unconditional acceptances of returns, lack of adherence to credit limits, and concessions agreed to by our former executives subsequent to the initial transaction, such as significantly extended payment terms, granting return or exchange rights, and contingent payment obligations caused the related sales transactions to fail the ASC 605 criteria required to be met under then applicable U.S. GAAP to recognize revenue.
We adopted ASC 606 on January 1, 2018 by using the modified retrospective method. ASC 606 establishes principles for reporting information about the nature, amount, timing and uncertainty of revenue and cash flows arising from the entity’s contracts to provide goods or services to customers. The core principle requires an entity to recognize revenue to depict the transfer of goods or services to customers in an amount that reflects the consideration that it expects to be entitled to receive in exchange for those goods or services recognized as performance obligations are satisfied. We have assessed the impact of the ASC 606 guidance by reviewing existing customer contracts and current accounting policies and practices to identify differences
63
that would result from applying the new requirements, including identification of the contract and the evaluation of our performance obligations, transaction price, customer payments, transfer of control and principal versus agent considerations.
When evaluating our customer relationships, agreements and purchase orders, we concluded that for 2018, although a contract may have existed from a legal perspective upon our fulfillment of a purchase order, a contract did not exist from an accounting perspective at that time because certain implicit arrangements (e.g. rights of return or exchange, extended payment terms and sales exceeding established credit limits) modified the explicit terms of the contract.
We therefore determined that, in 2018, we did not meet the criteria necessary for our revenue arrangements to qualify as “contracts” under the requirements of ASC 606 upon transfer of control of the product (i.e., upon physical delivery). Subsequent to the delivery of product, uncertainties surrounding contractual adjustment were not resolved until either: (1) the customer returned the product; or (2) we received payment from the customer. At that point, we determined that an accounting contract existed under ASC 606-10-25-1 and the performance obligations of the Company to deliver product and the customer payment for the product were satisfied. We determined the transaction price of our contracts to equal the amount of consideration received from customers less the amount expected to be refunded or credited to customers, which is recognized as a refund liability that is updated at the end of each reporting period for changes in circumstances. See discussion below – “Results of Operations” under the heading “Recent Developments.”
Based on the assessment, we concluded that during the year ended December 31, 2018 there was no substantial change to the timing and pattern of revenue recognition for our current revenue streams, and therefore there was no change to our consolidated financial statements upon adoption of ASC 606, as we continued deferring revenue recognition until the time we received cash consideration subsequent to the control of the product being transferred.
We sell to GPO members who transact directly with us at GPO-agreed pricing. GPOs are funded by administrative fees that we pay. These fees are set as a percentage of the purchase volume, which is typically 3% of sales made to the GPO members. Prior to adoption of ASC 606, for all periods presented prior to January 1, 2018, we presented the administrative fees paid to GPOs as a reduction of revenues as the benefit received in exchange for the GPO fees was not sufficiently separable from the GPO member’s purchase of our products. As part of the restatement, these fees were reclassified from expenses to a reduction of product revenues. Upon adoption of ASC 606, we concluded that although we benefited from the access that a GPO provides to its members, this benefit was neither distinct from other promises in our contracts with GPOs nor was the benefit separable from the sale of goods to the customer. Therefore, we continued presenting fees paid to GPOs as a reduction of product revenues.
Additionally, we considered how to account for costs associated with the delivered products of the contract for which revenue has been deferred, which is whether to match the related cost of sales expense with revenue or to recognize expense upon shipment. In making this assessment, we considered the financial viability of our distributors and customers based on their creditworthiness to determine if collectability of amounts sufficient to realize the costs of the products shipped was reasonably assured at the time of shipment. As we determined that there was a probable future economic benefit associated with the sales transactions, we deferred the costs of sales until the revenue was recognized.
Goodwill and Impairment of Long-Lived Assets
Goodwill represents the excess of purchase price over the fair value of net assets of acquired businesses. Goodwill is tested for impairment annually on September 30, or whenever an event occurs or circumstances change that would indicate that the carrying amount may be impaired. At each reporting date, we evaluate qualitative factors to determine whether additional analysis of goodwill is required. Goodwill is evaluated for impairment by comparing the fair value of the reporting unit to the carrying value. If the carrying value exceeds the fair value of the reporting unit, goodwill impairment is recorded for the amount that the reporting unit’s carrying value exceeds its fair value, not to exceed the total amount of goodwill allocated to the reporting unit. As of the date of the filing of this Form 10-K, we have only one reporting unit. We determine the fair value utilizing the income and market approaches. Under the income approach, the fair value of the reporting unit is the present value of its future economic benefits. These benefits can include revenue, cost savings, tax deductions, and proceeds from its disposition. Value indications are developed by discounting expected cash flows to their present value at a rate of return that incorporates the risk-free rate for the use of funds, trends within the industry, and risks associated with particular investments of similar type and quality as of the goodwill impairment testing date. Under the market approach, we use our market capitalization, which is calculated by taking our share price times the number of outstanding shares. Our estimates associated with the goodwill impairment test are considered critical due to the amount of goodwill recorded on our consolidated balance sheets and the judgment required in determining fair value, including projected future cash flows.
Acquired intangible assets are tested for impairment annually or whenever events or changes in circumstances indicate that the carrying amount of an intangible asset may not be recoverable. Refer to Note 8 to the Consolidated Financial Statements for additional information. Our impairment reviews are based on an estimated future cash flow approach that requires significant
64
judgment with respect to future revenue and expense growth estimates. We use estimates consistent with business plans and a market participant view of the assets being evaluated. Actual results may differ from the estimates used in these analyses.
There were no recorded impairment losses related to goodwill in 2018, 2017, or 2016. We recorded impairment losses of $0, $0.6 million and $0 related to the abandonment of patents in process during 2018, 2017, and 2016, respectively.
Share-based Compensation
Our share-based compensation cost for equity awards granted to employees and members of the Board is measured at the grant date based on the fair value of the award, is adjusted by the estimated forfeitures and is recognized as an expense over the requisite service period in accordance with Financial Accounting Standards Board (“FASB”) ASC Topic 718 “Compensation–Stock Compensation.”
Determining the appropriate fair value model and calculating the fair value of employee and non-employee stock option and restricted common stock awards requires estimates and judgments. Our share‐based compensation is a “critical accounting estimate” because changes in the assumptions used to develop estimates of fair value, the requisite service period, or estimated forfeitures could materially affect key financial measures, including results of operations.
The fair value of restricted common stock is a value of common stock on a grant date. The fair value of stock option grants is estimated using the Black-Scholes option pricing model. Use of the valuation model requires management to make certain assumptions with respect to selected model inputs. We use the simplified method for share-based compensation to estimate the expected term. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for the estimated option expected term. Historically, we did not have enough history to establish volatility based upon our own stock trading. Therefore, the expected volatility was based on that of similar publicly traded peer companies. We routinely review our calculation of volatility for potential changes in future volatility, our life cycle, our peer group, and other factors. In addition, an expected dividend yield of zero is used in the option valuation model because we do not pay cash dividends and does not expect to pay any cash dividends in the foreseeable future. For awards with service conditions only, we recognize stock-based compensation expense on a straight-line basis over the requisite service period.
For restricted common stock and stock options granted as consideration for services rendered by non-employees, we recognize compensation expense in accordance with the requirements of FASB ASC Topic 505-50, “Equity Based Payments to Non- Employees.” Non-employee restricted common stock and stock option grants that do not vest immediately upon grant, and whose terms are known, are recorded as an expense over the vesting period of the underlying instrument granted. At the end of each financial reporting period prior to vesting, the value of the instruments granted, is re-measured using the fair value of the Common Stock and the stock-based compensation recognized during the period is adjusted accordingly.
Income Taxes
Our income tax expense, deferred tax assets and liabilities, and liabilities for unrecognized tax benefits reflect management’s best assessment of estimated current and future taxes to be paid. We are subject to income taxes in the United States, including numerous state jurisdictions. Significant judgments and estimates are required in determining the consolidated income tax expense.
Deferred income taxes arise from temporary differences between the tax basis of assets and liabilities and their reported amounts in the financial statements, which will result in taxable or deductible amounts in the future. In evaluating our ability to recover our deferred tax assets within the jurisdiction from which they arise, we consider all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax-planning strategies, and results of recent operations. In projecting future taxable income, we begin with historical results adjusted for the results of discontinued operations and incorporate assumptions about the amount of future state, federal, and foreign pretax operating income adjusted for items that do not have tax consequences. The assumptions about future taxable income require significant judgment and are consistent with the plans and estimates we are using to manage the underlying businesses. In evaluating the objective evidence that historical results provide, we consider three years of cumulative operating income (loss). We account for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined on the basis of the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
We recognize deferred tax assets to the extent that we believe these assets are more likely than not to be realized. In making such a determination, we consider all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and results of recent operations. If we determine that we
65
would be able to realize our deferred tax assets in the future in excess of their net recorded amount, we would make an adjustment to the deferred tax asset valuation allowance, which would reduce the provision for income taxes.
The calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax laws and regulations both for U.S. federal income tax purposes and across numerous state jurisdictions. ASC Topic 740 (“ASC 740”) states that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, on the basis of the technical merits. We (1) record unrecognized tax benefits as liabilities in accordance with ASC 740, and (2) adjust these liabilities when our judgment changes as a result of the evaluation of new information not previously available. Because of the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from our current estimate of the unrecognized tax benefit liabilities. These differences will be reflected as increases or decreases to income tax expense in the period in which new information is available.
We record uncertain tax positions in accordance with ASC 740 on the basis of a two-step process whereby (1) we determine whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position, and (2) for those tax positions that meet the more-likely-than-not recognition threshold, we recognize the largest amount of tax benefit that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority.
We recognize interest and penalties related to unrecognized tax benefits within the income tax expense line in the accompanying Consolidated Statement of Operations. Accrued interest and penalties, if any, are included within the related tax liability line in the consolidated balance sheet.
As a result of the Restatement, we re-evaluated the valuation allowance determinations made in prior years. Our analysis was updated to consider the changes to our historical operating results following the Investigation and subsequent review by management. In that process, we evaluated the weight of all evidence, including the ability or inability to project future income to utilize our deferred tax assets, and we concluded that as of December 31, 2015, our U.S. federal and state net deferred tax assets were no longer more-likely-than-not to be realized and that a valuation allowance was required.
As of December 31, 2018, 2017, and 2016, we had a valuation allowance recorded of $27.3 million, $0.6 million, and $38.1 million, respectively, against our net deferred tax assets. The decrease in the valuation allowance during 2017 is primarily related to the weight of available evidence which resulted in a determination to release our valuation allowance and recognize an income tax benefit as of September 30, 2017. The increase in valuation allowance during 2018 is primarily related to the weight of available evidence which resulted in the determination to increase our valuation allowance and recognize income tax expense as of December 31, 2018.
To the extent we determine that, based on the weight of available evidence, all or a portion of our valuation allowance is no longer necessary, we will recognize an income tax benefit in the period such determination is made for the reversal of the valuation allowance. If management determines that, based on the weight of available evidence, it is more-likely-than-not that all or a portion of the net deferred tax assets will not be realized, the Company may recognize income tax expense in the period such determination is made to increase the valuation allowance.
U.S. Tax Reform
On December 22, 2017, the United States enacted into law the Tax Cuts and Jobs Act (“Tax Act”). The Tax Act made broad and complex changes to the U.S. tax code, including a permanent corporate rate reduction to 21%. The Tax Act includes provisions that affected 2017, including: (1) requiring a remeasurement of all U.S. deferred tax assets and liabilities to the newly enacted corporate tax rate of 21%; (2) providing for additional first-year depreciation that allows full expensing of qualified property placed into service after September 27, 2017; (3) repealing the domestic production activities deduction and (4) modifying the deductibility of certain meals & entertainment expenses incurred.
In late December 2017, the SEC staff issued Staff Accounting Bulletin 118 (“SAB 118”), which provided guidance on accounting for the tax effects of the Tax Act. SAB 118 provides a measurement period that should not extend beyond one year from the Tax Act enactment date for companies to complete the related accounting under U.S. GAAP. The most significant impact of the Tax Act on the Company was a one-time reduction in net deferred income tax assets of approximately $12 million, due primarily to the re-measurement of deferred tax assets at the lower 21% U.S. federal corporate income tax rate. The Company’s accounting for the tax implications of the Tax Cuts and Jobs Act is complete as of December 31, 2018.
66
Contingencies
We are subject to various patent challenges, product liability claims, government investigations and other legal proceedings in the ordinary course of business. Material legal proceedings are discussed in Note 16, “Commitments and Contingencies” in the Consolidated Financial Statements. Contingent accruals and legal settlements are recorded in the consolidated statements of operations as litigation-related and other contingencies when we determine that a loss is both probable and reasonably estimable. Legal fees and other expenses related to litigation are expensed as incurred and included in selling, general and administrative expenses in the consolidated statements of operations.
Due to the fact that legal proceedings and other contingencies are inherently unpredictable, our estimates of the probability and amount of any such liabilities involve significant judgment regarding future events. The factors we consider in developing our liabilities for legal proceedings include the merits and jurisdiction of the proceeding, the nature and the number of other similar current and past proceedings, the nature of the product and the current assessment of the science subject to the proceeding, if applicable, and the likelihood of the conditions of settlement being met.
In order to evaluate whether a claim is probable of loss, we may rely on certain information about the claim. Without access to and review of such information, we may not be in a position to determine whether a loss is probable. Further, the timing and extent to which we obtain any such information, and our evaluation thereof, is often impacted by items outside of our control including, without limitation, the normal cadence of the litigation process and the provision of claim information to us by opposing counsel. The amount of our liabilities for legal proceedings may change as we receive additional information and/or become aware of additional asserted or unasserted claims. Additionally, there is a possibility that we will suffer adverse decisions or verdicts of substantial amounts or that we will enter into additional monetary settlements, either of which could be in excess of amounts previously accrued for. Any changes to our liabilities for legal proceedings could have a material adverse effect on our business, financial condition, results of operations and cash flows.
As of December 31, 2018, our reserve for loss contingencies totaled $15.6 million, of which $6.9 million relates to our liability accrual for our self-disclosure to the VA concerning the eligibility of one of our products for inclusion in our FSS contract, and $8.7 million relates to other employee related matters. Although we believe there is a reasonable possibility that a loss in excess of the amount recognized exists, we are unable to estimate the possible loss or range of loss in excess of the amount recognized at this time.
Recently Adopted Accounting Pronouncements
See Note 3, “Significant Accounting Policies,” in the Consolidated Financial Statements for recently adopted accounting pronouncements.
Components of and Key Factors Influencing Our Results of Operations
In assessing the performance of our business, we consider a variety of performance and financial measures. We believe the items discussed below provide insight into the factors that affect these key measures.
Revenue
The majority of our revenues are generated by wound care applications. We have two distribution channels: (1) direct to customers and (2) sales through distributors. Each distribution channel can be further disaggregated between sales to federal customers and non-federal customers.
Several factors affect our reported revenue in any period, including product, payer and geographic sales mix, operational effectiveness, pricing realization, marketing and promotional efforts, timing of orders and shipments, regulatory actions including healthcare reimbursement scenarios, competition, and business acquisitions that involve our customers or competitors.
In connection with the Restatement, we revised our revenue recognition practices resulting in the restatement of previously issued financial statements. Refer to Item 6, “Selected Financial Data–Restatement” in this Form 10-K for more information concerning the impacts of the Restatement on our consolidated financial statements.
Cost of goods sold and gross profit
Cost of goods sold includes product testing costs, quality assurance costs, personnel costs, manufacturing costs, raw materials and product costs, and facility costs associated with our manufacturing and warehouse facilities. Fluctuations in our cost of goods sold correspond with the fluctuations in sales units driven by the changes in our sales force and sales territories, product portfolio offerings and the number of facilities that offer our products.
67
Gross profit is calculated as net revenue less cost of goods sold. Our gross profit is affected by product and geographic sales mix, realized pricing of our products, the efficiency of our manufacturing operations and the costs of materials used to make our products. Regulatory actions, including with respect to reimbursement for our products, may require costly expenditures or result in pricing pressure, and may decrease our gross profit and gross profit margin.
Selling, general and administrative expenses
Selling, general and administrative expenses include personnel costs, commissions, incentive compensation, customer support, administrative and labor costs, insurance, professional fees, depreciation and bad debt expense. We expect our selling, general and administrative expenses to fluctuate based on revenue fluctuations, geographic changes and any changes to the size of our sales and marketing forces.
Research and development expenses
Research and development expenses relate to our investments in improvements to our manufacturing processes (including additional costs to transition our manufacturing establishments into compliance with cGMP for commercial production), product enhancements, and additional investments in our product pipeline and platforms. Our research and development costs also include expenses such as clinical trial and regulatory costs.
We expense research and development costs as incurred. Our research and development expenses fluctuate from period to period primarily based on the ongoing improvement to our manufacturing processes and product enhancements. We expect that these costs will increase in the near term as we continue to transition our manufacturing facilities into compliance with cGMP, advance our IND applications, and pursue BLAs for certain of our micronized products.
Results of Operations for 2018 Compared to 2017
Year Ended December 31, | ||||||||||||||
(in thousands) | ||||||||||||||
2018 | 2017 | $ Change | % Change | |||||||||||
Revenue | $ | 359,111 | $ | 321,139 | $ | 37,972 | 11.8 | % | ||||||
Gross profit | 322,725 | 285,920 | 36,805 | 12.9 | % | |||||||||
Selling, general and administrative | 258,528 | 220,119 | 38,409 | 17.4 | % | |||||||||
Investigation, restatement and related | 51,322 | — | 51,322 | 100.0 | % | |||||||||
Research and development | 15,765 | 17,900 | (2,135 | ) | (11.9 | )% | ||||||||
Amortization of intangible assets | 1,034 | 1,678 | (644 | ) | (38.4 | )% | ||||||||
Loss on divestiture | — | (1,048 | ) | 1,048 | n/a | |||||||||
Other income (expense), net | 527 | (87 | ) | 614 | 705.7 | % | ||||||||
Income tax provision (expense) benefit | (26,582 | ) | 19,639 | (46,221 | ) | 235.4 | % | |||||||
Net income (loss) | $ | (29,979 | ) | $ | 64,727 | $ | (94,706 | ) | (146.3 | )% | ||||
Revenue
We recorded revenue for the year ended December 31, 2018 of $359.1 million, an increase of $38.0 million or 11.8% over 2017 revenue of $321.1 million. The increase primarily resulted from favorable insurance coverage developments, which resulted in an increase in the number of units sold. Additionally, we increased our direct sales force through the first three quarters of 2018 which resulted in the addition of new customers. Further, revenues benefited from sales made in prior periods and collected during the current period. These effects were partially offset by unfavorable insurance coverage developments, negative publicity resulting from the Investigation, increased turnover of experienced sales personnel and related events in the fourth quarter of 2018.
Gross Profit
Gross profit in 2018 was 89.9%, as compared to 89.0% in 2017. Gross profit increased due to the mix of products sold in 2018, including as a result of the divestiture of Stability on September 30, 2017 (whose products were relatively lower-margin). During 2018, we also saw an improvement in yield as a result of improved manufacturing efficiency in our wound care line.
68
Research and Development Expenses
Our research and development expenses decreased approximately $2.1 million, or 11.9%, to $15.8 million in 2018, compared to approximately $17.9 million in the prior year. The decrease is primarily due to year-over-year decreases in clinical trial activities as well as the decision to significantly reduce animal studies in 2018. In 2017, research and development expenses were driven by several multiple-site clinical trials related to our EpiCord and EpiFix products. These projects were completed during 2018.
Selling, General and Administrative Expenses
Selling, General and Administrative (“SG&A”) expense for 2018 increased approximately $38.4 million, or 17.4%, to $258.5 million (or 72.0% of revenues), compared to $220.1 million (or 68.5% of revenues) for 2017.
Sales and Marketing expense included in SG&A increased by $13.4 million, or 8.7%, to $167.3 million for 2018 compared to $153.9 million for 2017. The increase was primarily due to an increase in compensation related to the additional headcount of 72 employees from December 2017 through August 2018, as well as an increase in sales commissions based on shipments during the year.
General and administrative expense included in SG&A increased by $25.0 million, or 37.7%, to $91.2 million for 2018 compared to $66.2 million for 2017. Legal fees increased by $4.8 million, or 35.7%, to $18.4 million compared to $13.6 million for 2017. Consulting fees included in SG&A were $1.6 million for 2018 as compared with $0.3 million for 2017. The increase was primarily due to an increase in compensation related to the additional headcount of 34 employees from December 2017 through August 2018, prior to the reduction in force in December 2018, as well as an increase in accounting fees due to the change in audit firms in mid-2018.
In December 2018, we announced a reduction of our workforce by approximately 240 full-time employees, or 24% of our total workforce, of which about half were sales force personnel as part of previously announced plans to implement a broad-based organizational realignment, cost reduction and efficiency program to better ensure our cost structure is appropriate given our revenue expectations. As a result of the December 2018 broad-based organizational realignment, cost reduction and efficiency program, we incurred pre-tax charges of $6.1 million during the year ended December 31, 2018.
Investigation, restatement, and related expenses was $51.3 million in 2018 compared to $0 for 2017. The increase in legal, accounting, and other professional consulting fees incurred in 2018 as compared to 2017 primarily resulted from the Investigation, including legal fees, forensic audit fees, and consulting fees relating to the Restatement; and legal fees relating to the SEC Investigation, shareholder derivative lawsuits, and other litigation, as well as settlements made with former employees.
Share-based compensation included in SG&A for the years ended December 31, 2018 and 2017, was approximately $13.5 million and $20.1 million, respectively, a decrease of approximately $6.6 million, or 32.8%. The decrease was primarily due to our reduction in workforce in 2018, forfeitures of outstanding equity awards in connection with the reduction in workforce, and a reduction in the size of new equity-based awards to employees in 2018.
Amortization of Intangible Assets
Amortization expense related to intangible assets decreased approximately $0.7 million, or 38.4%, to $1.0 million for the year ended December 31, 2018, compared to $1.7 million in the prior year. Amortization decreased primarily due to the divestiture of Stability during the third quarter of 2017. We amortize our intangible assets over a period of 4 to 20 years, which we believe represents the remaining useful lives of the patents underlying the licensing rights and intellectual property. We do not amortize goodwill, but we test our goodwill at least annually for impairment and periodically evaluate other intangibles for impairment based on events or changes in circumstances as they occur.
Other Income (Expense), Net
Other income (expense), net increased to $0.5 million during the year ended December 31, 2018 from $(0.1) million during the year ended December 31, 2017. This increase was due to a decision in late 2017 to begin charging interest on past due customer balances, partially offset by the amortization of deferred financing costs incurred during 2018 related to our $50 million revolving credit facility and commitments and undrawn fees connected to our line of credit. See Note 10, “Long-Term Debt,” in the Consolidated Financial Statements for further details.
Income Taxes
The effective tax rate for 2018 was (782.6)% on pre-tax book loss of $3.4 million. This compares to an effective tax rate of (43.6)% based on pre-tax book income of $45.1 million in 2017.
69
Our 2018 effective tax rate was driven largely by increases in our valuation allowance, causing $1.2 million of incremental income tax expense, an effective tax rate impact of (788.3)%. Other offsetting tax adjustments yielded an effective tax rate of 5.7%. The decrease in the valuation allowance during 2017 is primarily related to the weight of available evidence which resulted in a determination to release our valuation allowance and recognize an income tax benefit as of September 30, 2017.
In 2017, the Company derived significant tax benefits associated with the disposition of the Company’s interest in Stability (total benefit of approximately $5.3 million; effective tax rate impact of (8.9)% along with significant tax benefits associated with stock compensation-related deductions (total benefit of approximately $4.8 million; effective tax rate benefit of (9.9)%.
As a result of the Restatement, we re-evaluated the valuation allowance determinations made in prior years. Our analysis was updated to consider the changes to our historical operating results following the Investigation and subsequent review by management. In that process, we evaluated the weight of all evidence, including the ability or inability to project future income to utilize our deferred tax assets, and we concluded that as of December 31, 2015, our U.S. federal and state net deferred tax assets were no longer more-likely-than-not to be realized and that a valuation allowance was required. The decrease in the valuation allowance during 2017 is primarily related to the weight of available evidence which resulted in a determination to release the Company’s valuation allowance and recognize an income tax benefit as of September 30, 2017. The increase in valuation allowance during 2018 is primarily related to the weight of available evidence which resulted in the determination to increase the Company’s valuation allowance and recognize income tax expense as of December 31, 2018.
Important Cautionary Statement
We caution the reader that actual results may differ materially from our expectations, including those described under the subheadings “Results of Operations - Recent Developments” below. Among the factors that could cause actual results to differ are: variances from our expectations or assumptions; changes in reimbursement policy from public and private insurers and health systems; the loss of a GPO or IDN; changes in purchasing behavior by government accounts; the loss of independent sales agents or distributors; the removal of any of our products from the market as a result of regulatory actions; the success of our marketing efforts; the fact that obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies; rapid technological change could cause our products to become obsolete and, if we do not enhance our product offerings through our research and development efforts, we may be unable to compete effectively; our ability to transition our manufacturing facilities into compliance with cGMP, advance our IND applications, complete our clinical trials and pursue BLAs for certain of our micronized products; the fact that our business is subject to continuing regulatory compliance by the FDA and other authorities, which is costly, and our failure to comply could result in negative effects on our business, results of operations and financial condition; the fact that litigation and other matters relating to and arising out of the Investigation, including the accounting review of our previously issued consolidated financial statements and the audits of fiscal years 2018, 2017 and 2016, have been time consuming and expensive, and may result in additional expense; and the fact that our variable rate indebtedness under the Loan Agreement subjects us to interest rate risk, which could result in higher expense in the event of increases in interest rates and adversely affect our business, financial condition, and results of operations. See Item 1A, “Risk Factors,” for more information.
Recent Developments
We expect that during 2019, the uncertainties of contractual adjustments with our customers will no longer be present such that we would be in a position to determine that an accounting contract exists at the time of physical delivery of our product to the customer. As of the date of this Form 10-K, the effective date of such transition is not certain. In light of this uncertainty and in the interests of providing additional information to investors regarding our results of operations for 2019, the estimates provided below regarding our 2019 performance were prepared for the entire year ended December 31, 2019 assuming that the Company continued to recognize revenue when payment was received after being adjusted for the amount expected to be refunded or credited to customers for sales returns made after payment. We expect that, once we determine the timing of the transition to a basis upon which revenue will be recognized at the time of physical delivery of the product to the customer, the final audited financial information will differ from that presented below to reflect additional revenue in 2019 in connection with such transition. We expect the amounts shipped and billed but not recorded as revenue at that date to be recognized as revenue over the following 60-90 days, consistent with our normal collection periods.
We expect 2019 revenue to decline from 2018 revenue between 25% to 28%, due to the continuation of trends which began in late 2018, including unfavorable insurance coverage developments, negative publicity resulting from the Investigation, increased turnover of experienced sales personnel, and related events, as well as the discontinuation of certain products in 2019. We expect our 2019 gross profit percentage to be down 5% to 6% as compared to 2018 due to higher manufacturing costs and the impact of lower volumes. We expect research and development expenses for 2019 to decline as compared to 2018 due to the completion of certain research initiatives and a reduction in headcount. For 2019, we expect Investigation, restatement, and related expenses to increase as compared to 2018 by $15 to $25 million due to costs incurred in connection with the completion of the Investigation,
70
the Restatement, and the resolution of various related matters. We expect other expense to increase in 2019 as compared to 2018 by approximately $5 million due to interest expense related to our $75 million Term Loan Agreement, which was funded on June 10, 2019 (and which was not outstanding in 2018).
Our expectations for 2019 results are based on our results in the year, but these results are unaudited and subject to period-end adjustments, along with uncertainty regarding when, and if, we will return to accrual accounting for revenue recognition. We caution the reader that actual results may differ materially from those described above.
In the second half of 2019 and the beginning of 2020, we believe our revenues stabilized. However, given the uncertainty regarding the impact on the economy from the COVID-19 virus, we are unable to provide any commentary regarding 2020 financial metrics. See Item 1A. Risk Factors - “Health epidemics in regions where we have operations, sales and marketing teams, manufacturing facilities or other business operations could harm our business, results of operations and financial condition.”
Results of Operations for 2017 Compared to 2016
Year Ended December 31, | ||||||||||||||
(in thousands) | ||||||||||||||
2017 | 2016 | $ Change | % Change | |||||||||||
(Restated) | ||||||||||||||
Revenue | $ | 321,139 | $ | 221,712 | $ | 99,427 | 44.8 | % | ||||||
Gross profit | 285,920 | 190,774 | 95,146 | 49.9 | % | |||||||||
Selling, general and administrative | 220,119 | 173,412 | 46,707 | 26.9 | % | |||||||||
Research and development | 17,900 | 14,341 | 3,559 | 24.8 | % | |||||||||
Amortization of intangible assets | 1,678 | 2,137 | (459 | ) | (21.5 | )% | ||||||||
Loss on divestiture | (1,048 | ) | — | (1,048 | ) | n/a | ||||||||
Other expense, net | (87 | ) | (339 | ) | 252 | 74.3 | % | |||||||
Income taxes | 19,639 | (155 | ) | 19,794 | (12,770.3 | )% | ||||||||
Net income (loss) | $ | 64,727 | $ | 390 | $ | 64,337 | 16,496.7 | % | ||||||
Revenue
We recorded revenue for the year ended December 31, 2017 of $321.1 million, a $99.4 million or 44.8% increase over 2016 revenue of $221.7 million. The increase is primarily due to an increase in the number of units sold. Additionally, we increased our direct sales force throughout 2017, which resulted in the addition of new customers and consequently supported the continued growth for our direct sales and agency channels. The comparison also benefited from insurance recoveries in 2017.
Gross Profit
Gross profit in 2017 was 89.0% as compared to 86.0% in 2016. Gross profit increased due to the impact of lower one-time inventory costs incurred in connection with the Stability acquisition in 2016 as well as lower overall revenue on lower margin Stability products related to the divestiture of Stability during the third quarter of 2017. Also contributing favorably to overall gross profit improvement were volume driven efficiencies and yield improvements resulting from manufacturing efficiencies, specifically in our wound care line.
Research and Development Expenses
Our research and development expenses increased approximately $3.6 million, or 24.8%, to $17.9 million in 2017, compared to approximately $14.3 million in the prior year. The increase is primarily related by a large, multiple-site clinical trial related to our EpiCord product, which began during 2016 and reached its apex during 2017.
Selling, General and Administrative Expenses
Selling, General and Administrative expenses for 2017 increased approximately $46.7 million, or 26.9%, to $220.1 million compared to $173.4 million for 2016.
Sales and Marketing expense included in SG&A increased by $25.3 million, or 19.6%, to $153.9 million compared to $128.6 million for 2016. This increase was driven primarily by costs associated with the continued build out of our direct sales organization.
71
Total sales and marketing head count was at 479 at December 31, 2017, an increase of 89 employees since December 31, 2016 with a significant portion of the additions dedicated to our direct sales activity. Related expense for sales commissions, travel, and GPO fees were also higher due to sales volume and head count increases.
General and administrative expense included in SG&A increased by $21.4 million, or 47.9%, to $66.2 million compared to $44.8 million for 2016. Total general and administrative head count was at 173 at December 31, 2017 as compared to 122 at December 31, 2016. The increase was driven primarily by costs associated with adding personnel to support and maintain the continued growth including reimbursement staff and other support areas as well as legal fees, bonus, and share-based compensation expenses.
Legal fees included in general and administrative expense increased by $5.8 million, or 75.7%, to $13.6 million compared to $7.7 million for 2016. The increase was primarily due to costs tied to general and patent litigation as well as litigation involving former employees.
Share-based compensation included in SG&A for the years ended December 31, 2017 and 2016, was approximately $20.1 million and $16.7 million, respectively, an increase of approximately $3.4 million, or 20.4%. The increase was primarily related to the increase in head count in 2017.
Amortization of Intangible Assets
Amortization expense related to intangible assets decreased approximately $0.5 million, or 21.5%, to $1.7 million for the year ended December 31, 2017, compared to $2.1 million in 2016. Amortization decreased primarily due to the divestiture of Stability during the third quarter of 2017. We amortize our intangible assets over a period of 4 to 20 years, which we believe represents the remaining useful lives of the patents underlying the licensing rights and intellectual property. We do not amortize goodwill, but we test our goodwill at least annually for impairment and periodically evaluate other intangibles for impairment based on events or changes in circumstances as they occur.
Other Expense, net
Other expense, net increased approximately $0.2 million, or 74.3%, to $0.1 million compared to $0.3 million for 2016. The increase is due an increase in interest income.
Income Taxes
The effective tax rate for 2017 was (43.6)% based on pre-tax book income of approximately $45.1 million. This compares to an effective tax rate of 28.46% based on pre-tax book income of $0.5 million in 2016.
In 2017, the Company derived significant tax benefits associated with disposition of the Company’s interest in Stability (total benefit of approximately $5.3 million; effective tax rate impact of (8.9)% along with significant tax benefits associated with stock compensation-related deductions (total benefit of approximately $4.8 million; effective tax rate benefit of (9.9)%.
As a result of the Restatement, we re-evaluated the valuation allowance determinations made in prior years. Our analysis was updated to consider the changes to our historical operating results following the Investigation and subsequent review by management. In that process, we evaluated the weight of all evidence, including the ability or inability to project future income to utilize our deferred tax assets, and we concluded that as of December 31, 2015, our U.S. federal and state net deferred tax assets were no longer more-likely-than-not to be realized and that a valuation allowance was required. The decrease in the valuation allowance during 2017 is primarily related to the weight of available evidence which resulted in a determination to release the Company’s valuation allowance and recognize an income tax benefit as of September 30, 2017.
72
Contractual Obligations
Contractual obligations associated with ongoing business activities are expected to result in cash payments in future periods. The table below summarizes the amounts and estimated timing of these future cash payments as of December 31, 2018 (in thousands):
Less than | |||||||||||||||||||
Contractual Obligations | Total | 1 year | 1-3 years | 3-5 years | Thereafter | ||||||||||||||
Operating lease obligations | $ | 6,722 | $ | 1,640 | $ | 4,877 | $ | 205 | $ | — | |||||||||
Meeting space commitments | 1,756 | 965 | 791 | — | — | ||||||||||||||
$ | 8,478 | $ | 2,605 | $ | 5,668 | $ | 205 | $ | — | ||||||||||
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements.
Liquidity and Capital Resources
Our business requires capital for its operating activities, including costs associated with the sale of product through direct and indirect sales channels, the conduct of research and development activities, compliance costs, and legal and consulting fees in connection with ongoing litigation and other matters. We generally fund our operating capital requirements through our operating activities and cash reserves. We expect to require additional capital in the near and medium term to implement our strategic priorities, including for capital investments, steps to achieve cGMP compliance, advancement of our IND applications, pursuit of BLAs for certain of our micronized products, and settlements of certain legal matters.
As of December 31, 2018, the Company had approximately $45.1 million of cash and cash equivalents.
Our net working capital at December 31, 2018, decreased $0.4 million to $2.5 million from $2.9 million at December 31, 2017. The decrease in working capital was primarily due the change in net income, as discussed above, partially offset by cash used and liabilities recognized for fees incurred in connection with the Investigation, including legal fees, forensic audit fees, and consulting fees relating to the Restatement; and legal fees relating to the SEC Investigation, shareholder derivative lawsuits, and other litigation, as well as settlements made with former employees. We also paid fees for an executive recruiting firm for its searches for senior executives and individuals to fill other management positions. Our current ratio (current assets divided by current liabilities) was 1.0 to 1 as of December 31, 2018 and 2017.
We have funded our cash requirements, including for our operating activities and for the Investigation and Restatement, through existing cash reserves and from operating activities. In addition, we entered into a $75 million term loan as described below under “Term Loan” for working capital and general corporate purposes. The Company is currently paying its obligations in the normal course of business. We believe that our anticipated cash from operating activities, existing cash, and cash equivalents will enable us to meet our operational liquidity needs due to the restructuring. See Note 19, “Restructuring,” in the Consolidated Financial Statements.
We do not expect to be required to make any income tax payments during the year ended December 31, 2019.
We expect to incur additional costs in connection with the execution of our strategic priorities, including efforts to become cGMP compliant and toward the completion of the BLA process. This includes development and enhancement of production processes, procedures, tests and assays, and it requires extensive validation work. It can also involve the procurement and installation of new production or lab equipment. In addition to additional costs, these efforts require human capital, expertise and resources.
To fund these costs, we are pursuing sources of capital to ensure sufficient liquidity in the near and long term. See discussion above “Risk Factors” under the heading “We will need to raise additional capital in the future, and our ability to raise capital on acceptable terms or at all is uncertain” in this Form 10-K.
Additionally, as discussed in Note 16, “Commitments and Contingencies,” and further addressed in Note 21, “Subsequent Events,” of the Consolidated Financial Statements, we anticipate cash requirements related to the following items within one year from the date of the filing of this Form 10-K:
• | shareholder derivative lawsuits, for which we are not able to estimate a loss; |
73
• | private securities lawsuits, for which we are unable to estimate a loss and for which it is unclear whether we would be indemnified under various insurance policies; |
• | the Company’s self-disclosure to the VA concerning the eligibility of one of its products for inclusion in the Company’s FSS contract, for which we recorded a liability of $6.9 million as of December 31, 2018, representing our best estimate for a potential loss. As explained below, the Company has reached an agreement in principle with the Department of Justice to resolve a qui tam matter relating to that issue. The settlement amount is within the Company’s previously-recorded liability; |
• | litigation involving former employees of the Company; we have settled with one former employee for $4.8 million, but we have potential exposure in other cases brought by former employees for which a loss is not currently estimable; |
• | $2.3 million in payments owed to Mr. Borkowski under the terms of the Separation and Transition Services Agreement dated November 18, 2019; |
• | payments in connection with our operating and capital lease agreements; and |
• | investments and other expenditures required in order to bring the Company’s facilities into compliance with cGMPs. |
We have analyzed our ability to address the aforementioned commitments and potential liabilities while remaining compliant with the financial covenants set forth in the Term Loan Agreement discussed below for the 12 months extending from the date of the filing of this Form 10-K. Based on this analysis, and in combination with existing cash on hand, we reasonably expect to meet all obligations as they come due without violating the financial covenants set forth by the Term Loan Agreement, as discussed below. Therefore, we concluded that there is no substantial doubt surrounding our ability to continue as a going concern.
Term Loan
On June 10, 2019, we entered into a Term Loan Agreement (the “Term Loan Agreement”) with the subsidiaries of the Company as guarantors party thereto from time to time, the lenders party thereto from time to time, and Blue Torch Finance LLC as administrative agent and collateral agent, to borrow funds with a face value of $75 million (the “Term Loan”), of which the full amount has been borrowed and funded. The proceeds of the Term Loan have been and will be used (i) for working capital and general corporate purposes and (ii) to pay transaction fees, costs and expenses incurred in connection with the Term Loan and the related transactions. The Term Loan matures on June 20, 2022 and is repayable in quarterly installments of $0.9 million; the balance is due on June 20, 2022. The Term Loan was issued net of the original issue discount of $2.3 million. The Company also incurred $6.7 million of deferred financing costs.
The interest rate applicable to any borrowings under the Term Loan will accrue at a rate equal to LIBOR plus a margin of 8.00% per annum or, if LIBOR is not available, a prime rate plus a margin of 7.00% per annum. The Term Loan had an interest rate equal to 10.46% at the time the Loan Agreement was executed.
The Term Loan Agreement includes events of default customary for facilities of this type, and upon the occurrence of such events of default, subject to customary cure rights, all outstanding loans under the Term Loan Agreement may be accelerated and/or the lenders’ commitments terminated.
The Term Loan Agreement also contains customary affirmative and negative covenants, including limitations on our ability to incur additional debt, grant or permit additional liens, make investments and acquisitions, merge or consolidate with others, dispose of assets, pay dividends and distributions, repay subordinated indebtedness, enter into sale and leaseback and affiliate transactions. In addition, the Term Loan Agreement contains financial covenants requiring us to maintain the following:
• | Maximum total leverage ratio, defined as funded debt divided by consolidated adjusted EBITDA, of not more than 3.0 to 1.0 as of the last day of the four consecutive fiscal quarters. We estimate our total leverage ratio to have been 2.0 times at December 31, 2019, a cushion of 1.0 times. |
• | Minimum liquidity of the Company, defined as unrestricted cash and cash equivalents, is not permitted, as of the last business day of each fiscal month following the term loan closing date through and including the fiscal month ending May 31, 2020, to be less than $40 million, and (b) beginning with the fiscal month ending June 30, 2020 and for each month ending thereafter, to be less than $30 million; provided that, beginning with fiscal month ending December 31, 2020, the total leverage ratio is less than 2.50 to 1.00 as of the last business day of any fiscal month, the liquidity of the Company shall not be less than $20 million. Our cash on hand was $69.1 million at December 31, 2019, exceeding the $40 million minimum liquidity by $29.1 million. |
74
The Term Loan Agreement also specifies that any prepayment of the loan, voluntary or mandatory, as defined in the Term Loan Agreement, subjects MiMedx to a prepayment penalty as of the date of the prepayment with respect to the Term Loan of:
• | During the period from June 10, 2019 through June 10, 2020, an amount equal to 3% of the principal amount of the Term Loan prepaid on such date; and |
• | During the period from June 11, 2020 through June 10, 2021, an amount equal to 2% of the principal amount of the Term Loan prepaid on such date. |
Principal prepayments after June 10, 2021 are not subject to a prepayment penalty.
As of the date of the filing of this Form 10-K, the Company is in compliance with the financial covenants in the Term Loan Agreement. The Company is monitoring the covenant compliance and in the event we determine that noncompliance with financial covenants is likely, we plan to seek to re-negotiate financial covenants with lenders, seek waivers if necessary, or seek alternative financing. See discussion above, “Risk Factors” under the heading “The restrictive covenants in the Loan Agreement and the Company’s obligation to make debt payments under the Loan Agreement may limit our operating and financial flexibility and may adversely affect our business, results of operations and financial condition.”
BLA Development Effort
As part of our BLA development effort, we have also made efforts to transition our manufacturing establishments into compliance with cGMP. During the enforcement discretion period, the FDA is permitting products that will become Section 351 HCT/Ps to be manufactured in compliance with GTP regulation. However, after the end of the enforcement discretion period, these products will be subject to cGMP compliance. The transition from GTP to cGMP compliance includes development and enhancement of production processes, procedures, test and assays, and it requires extensive validation work. It can also involve the procurement and installation of new production or lab equipment. These efforts require human capital, expertise and resources. The Company is developing and enhancing systems to meet these requirements, and expects to complete those efforts by November 2020, though there is no guarantee that the Company will be able to meet the requirements on that timeline or at all. For more information on our clinical trials, BLA development effort, and FDA enforcement discretion, see the section entitled “Business–Government Regulation.”
Share Repurchase
During 2018, the Company repurchased 507,600 shares of our Common Stock under our Board-approved share repurchase program for a purchase price of approximately $7.6 million. As of December 31, 2018, the repurchase program expired.
In addition, during 2018, the Company repurchased 614,123 shares of Common Stock surrendered by employees to satisfy tax withholding obligations upon vesting of restricted stock.
Discussion of Cash Flows
During the year ended December 31, 2018, net cash provided by operations decreased approximately $27.1 million to $35.8 million, compared to $62.9 million for the year ended December 31, 2017. This decrease was primarily attributable to decrease in net income (as a result of higher selling, general and administrative expenses primarily as a result of the Investigation and Restatement), partially offset by a $6.6 million increase in accounts payable due to working capital management efforts.
During the year ended December 31, 2017, net cash provided by operations increased approximately $39.1 million to $62.9 million, compared to $23.8 million for the year ended December 31, 2016. This increase was primarily attributable to increases in net income and accrued compensation, partially offset by an increase income tax payment as compared to the prior year.
During the year ended December 31, 2018, net cash used for investing activities increased approximately $3.8 million to $9.2 million compared to $5.4 million for the year ended December 31, 2017 due to the higher equipment purchases in 2018 as compared with 2017.
During the year ended December 31, 2017, net cash used for investing activities decreased approximately $6.3 million to $5.4 million compared to $11.7 million for the year ended December 31, 2016. This decrease was primarily due to $7.6 million paid for the acquisition of Stability in 2016.
During the year ended December 31, 2018, net cash flows used for financing activities was approximately $8.9 million compared to $60.4 million during the year ended December 31, 2017. The decrease was primarily due to lower share repurchases and lower proceeds from stock option exercises.
75
During the year ended December 31, 2017, net cash flows used for financing activities was approximately $60.4 million compared to $8.2 million during the year ended December 31, 2016. The increase was primarily due to increases in share repurchases and proceeds from option exercise.
Non-GAAP Financial Measures
In addition to our GAAP results, we provide certain Non-GAAP metrics including EBITDA, Adjusted EBITDA and Adjusted Gross Profit. We believe that the presentation of these measures provides important supplemental information to management and investors regarding our performance. These measurements are not a substitute for GAAP measurements. Company management uses these Non-GAAP measurements as aids in monitoring our on-going financial performance from quarter-to-quarter and year-to-year on a regular basis and for benchmarking against comparable companies.
EBITDA is intended to provide a measure of the Company’s operating performance as it eliminates the effects of financing and capital expenditures. EBITDA consists of GAAP net income (loss) excluding: (i) depreciation, (ii) amortization of intangibles and (iii) income tax provision.
Adjusted EBITDA is intended to provide a proxy for cash flows from operations generated by the enterprise as a whole, prior to the consideration of debt and tax effects. Additionally, Adjusted EBITDA provides a view of our business operations that we expect to endure on an ongoing basis by removing items which may be irregular, one-time, or non-recurring from EBITDA.
Adjusted EBITDA consists of GAAP net income (loss) excluding: (i) depreciation and amortization, (ii) income tax provision, (iii) loss on divestiture, (iv) one-time acquisition-related costs, (v) one-time fair value adjustments related to an acquisition, (vi) investigation and restatements costs, (vii) interest (income) expense, (vii) impairment of intangibles and (viii) share-based compensation. Beginning in 2016, we have reported Adjusted Gross Profit to assist in comparing results across periods. Adjusted Gross Profit consists of GAAP gross profit excluding amortization of inventory fair value step-up.
A reconciliation of GAAP Net Income (Loss) to EBITDA and Adjusted EBITDA appears in the table below (in thousands):
Years Ended December 31 | |||||||||||
2018 | 2017 | 2016 | |||||||||
(Restated) | |||||||||||
Net (loss) income | $ | (29,979 | ) | $ | 64,727 | $ | 390 | ||||
Non-GAAP Adjustments: | |||||||||||
Depreciation expense | 5,882 | 4,087 | 3,333 | ||||||||
Amortization of intangible assets | 1,034 | 1,678 | 2,137 | ||||||||
Income tax provision | 26,582 | (19,639 | ) | 155 | |||||||
EBITDA | 3,519 | 50,853 | 6,015 | ||||||||
Additional Non-GAAP Adjustments: | |||||||||||
Loss on divestiture | — | 1,048 | — | ||||||||
One-time costs incurred in connection with acquisition | — | — | 1,088 | ||||||||
One-time inventory fair value adjustments in connection with acquisition | — | 203 | 1,485 | ||||||||
Costs incurred in connection with investigation and restatement | 51,322 | — | — | ||||||||
Interest (income) expense, net | (527 | ) | 87 | 339 | |||||||
Impairment of intangible assets | — | 590 | — | ||||||||
Share-based compensation | 14,768 | 21,195 | 17,732 | ||||||||
Adjusted EBITDA | $ | 69,082 | $ | 73,976 | $ | 26,659 | |||||
76
“Adjusted Gross Profit” consists of GAAP gross profit excluding amortization of inventory fair value step-up. A reconciliation of Adjusted Gross Profit to GAAP gross profit appears in the table below (in thousands):
Years Ended December 31, | |||||||||||
2018 | 2017 | 2016 | |||||||||
(Restated) | |||||||||||
Gross profit (Per GAAP) | $ | 322,725 | $ | 285,920 | $ | 190,774 | |||||
Non-GAAP Adjustments: | |||||||||||
One time inventory costs incurred in connection with acquisition | — | 203 | 1,485 | ||||||||
Gross profit before amortization of inventory fair value step-up | $ | 322,725 | $ | 286,123 | $ | 192,259 | |||||
Adjusted gross profit | 89.9 | % | 89.1 | % | 86.7 | % | |||||
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Based on our lack of market risk sensitive instruments outstanding at December 31, 2018, we have determined that we had no material market risk exposure as of such date.
77
Item 8. Financial Statements and Supplementary Data
Index to Financial Statements | |
F-2 | |
Consolidated Balance Sheets – As of December 31, 2018 and December 31, 2017 | F-5 |
Consolidated Statements of Operations – For the years ended December 31, 2018, 2017 and 2016 (Restated) | F-6 |
Consolidated Statements of Stockholders’ Equity – For the years ended December 31, 2018, 2017 and 2016 (Restated) | F-7 |
Consolidated Statements of Cash Flows – For the years ended December 31, 2018, 2017 and 2016 (Restated) | F-8 |
F-10 | |
Schedule II - Valuation and Qualifying Accounts | F-51 |
F-1
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
MiMedx Group, Inc.
Marietta, Georgia
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of MiMedx Group, Inc. (the “Company”) as of December 31, 2018 and 2017, the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2018, and the related notes and schedule (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2018, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) and our report dated March 17, 2020 expressed an adverse opinion thereon.
Restatement of 2016 Consolidated Financial Statements
As discussed in Note 4 to the consolidated financial statements, the 2016 financial statements have been restated.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ BDO USA, LLP
We have served as the Company's auditor since 2019.
Atlanta, Georgia
March 17, 2020
F-2
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
MiMedx Group, Inc.
Marietta, Georgia
Opinion on Internal Control over Financial Reporting
We have audited MiMedx Group, Inc.’s (the “Company’s”) internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO criteria”). In our opinion, the Company did not maintain, in all material respects, effective internal control over financial reporting as of December 31, 2018, based on the COSO criteria.
We do not express an opinion or any other form of assurance on management’s statements referring to any corrective actions taken by the Company after the date of management’s assessment.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated balance sheets of the Company as of December 31, 2018 and 2017, the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2018, and the related notes and schedule (collectively referred to as “the financial statements”) and our report dated March 17, 2020 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Item 9A, Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit of internal control over financial reporting in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. A material weakness regarding management’s failure to maintain an effective control environment to identify and enable the identification of risks of material accounting errors in the control environment has been identified and described in management’s assessment. A material weakness regarding management’s failure to design and implement an effective risk assessment process based on criteria established in the COSO framework has been identified and described in management’s assessment. A material weakness regarding the design and implementation of effective control activities based on criteria established in the COSO framework has been identified and described in management’s assessment. A material weakness regarding a failure to generate and provide quality information and communication based on the criteria established in the COSO framework has been identified and described in management’s assessment. A material weakness regarding a failure to design and implement effective monitoring activities based on criteria established in the COSO framework has been identified and described in management’s assessment. These material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2018 financial statements, and this report does not affect our report dated March 17, 2020 on those financial statements.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets
F-3
of the Company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ BDO USA, LLP
Atlanta, Georgia
March 17, 2020
F-4
MIMEDX GROUP, INC. AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS (in thousands, except share data) | ||||||||
December 31, | ||||||||
2018 | 2017 | |||||||
ASSETS | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | $ | ||||||
Inventory, net | ||||||||
Prepaid expenses | ||||||||
Income tax receivable | ||||||||
Other current assets | ||||||||
Total current assets | ||||||||
Property and equipment, net | ||||||||
Goodwill | ||||||||
Intangible assets, net | ||||||||
Deferred tax asset, net | ||||||||
Other assets | ||||||||
Total assets | $ | $ | ||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | $ | ||||||
Accrued compensation | ||||||||
Accrued expenses | ||||||||
Other current liabilities | ||||||||
Total current liabilities | ||||||||
Other liabilities | ||||||||
Total liabilities | ||||||||
Commitments and contingencies (Note 16) | ||||||||
Stockholders’ equity: | ||||||||
Preferred stock; $0.001 par value; 5,000,000 shares authorized and 0 shares issued and outstanding | ||||||||
Common stock; $0.001 par value; 150,000,000 shares authorized; 112,703,926 issued and 109,098,663 outstanding at December 31, 2018 and 112,703,926 issued and 109,347,517 outstanding at December 31, 2017 | ||||||||
Additional paid-in capital | ||||||||
Treasury stock at cost: 3,605,263 shares at December 31, 2018 and 3,356,409 shares at December 31, 2017 | ( | ) | ( | ) | ||||
Accumulated deficit | ( | ) | 2 | ( | ) | |||
Total stockholders’ equity | ||||||||
Total liabilities and stockholders’ equity | $ | $ | ||||||
See notes to the consolidated financial statements.
F-5
MIMEDX GROUP, INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF OPERATIONS (in thousands, except share and per share data) | |||||||||||
Years Ended December 31, | |||||||||||
2018 | 2017 | 2016 | |||||||||
(Restated) | |||||||||||
Net sales | $ | $ | $ | ||||||||
Cost of sales | |||||||||||
Gross profit | |||||||||||
Operating expenses: | |||||||||||
Selling, general and administrative | |||||||||||
Investigation, restatement and related | |||||||||||
Research and development | |||||||||||
Amortization of intangible assets | |||||||||||
Operating (loss) income | ( | ) | |||||||||
Other income (expense) | |||||||||||
Loss on divestiture of Stability | ( | ) | |||||||||
Other income (expense), net | ( | ) | ( | ) | |||||||
(Loss) income before income tax provision | ( | ) | |||||||||
Income tax provision (expense) benefit | ( | ) | ( | ) | |||||||
Net (loss) income | $ | ( | ) | $ | $ | ||||||
Net (loss) income per common share - basic | $ | ( | ) | $ | $ | ||||||
Net (loss) income per common share - diluted | $ | ( | ) | $ | $ | ||||||
Weighted average shares outstanding - basic | |||||||||||
Weighted average shares outstanding - diluted | |||||||||||
See notes to the consolidated financial statements.
F-6
MIMEDX GROUP, INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (in thousands, except share data) | |||||||||||||||||||||||||
Common Stock | Additional Paid-in | Treasury Stock | Accumulated | ||||||||||||||||||||||
Shares | Amount | Capital | Shares | Amount | Deficit | Total | |||||||||||||||||||
Balances, December 31, 2015 (Restated) | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||
Share-based compensation (Restated) | — | — | — | — | — | ||||||||||||||||||||
Tax benefit of share-based compensation | — | — | ( | ) | — | — | — | ( | ) | ||||||||||||||||
Exercise of stock options | ( | ) | ( | ) | — | ||||||||||||||||||||
Issuance of restricted stock | ( | ) | ( | ) | — | ||||||||||||||||||||
Restricted stock cancellation / forfeited | — | — | ( | ) | — | ||||||||||||||||||||
Shares issued for services performed | — | — | ( | ) | — | ||||||||||||||||||||
Shares issued in conjunction with acquisition of Stability | — | — | ( | ) | ( | ) | — | ||||||||||||||||||
Shares repurchased | — | — | — | ( | ) | — | ( | ) | |||||||||||||||||
Shares repurchased for tax withholding on vesting of restricted stock units | — | — | — | ( | ) | — | ( | ) | |||||||||||||||||
Net income (Restated) | — | — | — | — | — | ||||||||||||||||||||
Balances, December 31, 2016 (Restated) | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||
Share-based compensation | — | — | — | — | — | ||||||||||||||||||||
Exercise of stock options | ( | ) | ( | ) | — | ||||||||||||||||||||
Issuance of restricted stock | ( | ) | ( | ) | — | ||||||||||||||||||||
Restricted stock cancellation / forfeited | — | — | ( | ) | — | ||||||||||||||||||||
Shares issued for services performed | — | — | ( | ) | — | ||||||||||||||||||||
Shares repurchased | — | — | — | ( | ) | — | ( | ) | |||||||||||||||||
Shares repurchased for tax withholding on vesting of restricted stock units | — | — | — | ( | ) | — | ( | ) | |||||||||||||||||
Net income | — | — | — | — | — | ||||||||||||||||||||
Balances, December 31, 2017 | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||
Share-based compensation | — | — | — | — | — | ||||||||||||||||||||
Exercise of stock options | — | — | ( | ) | ( | ) | — | ||||||||||||||||||
Issuance of restricted stock | — | — | ( | ) | ( | ) | — | ||||||||||||||||||
Restricted stock cancellation / forfeited | — | — | ( | ) | — | ||||||||||||||||||||
Shares repurchased | — | — | — | ( | ) | — | ( | ) | |||||||||||||||||
Shares repurchased for tax withholding on vesting of restricted stock units | — | — | — | ( | ) | — | ( | ) | |||||||||||||||||
Net income | — | — | — | — | — | ( | ) | ( | ) | ||||||||||||||||
Balances, December 31, 2018 | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||
See notes to the consolidated financial statements.
F-7
MIMEDX GROUP, INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF CASH FLOWS (in thousands) | |||||||||||
Years Ended December 31, | |||||||||||
2018 | 2017 | 2016 | |||||||||
(Restated) | |||||||||||
Cash flows from operating activities: | |||||||||||
Net (loss) income | $ | ( | ) | $ | $ | ||||||
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | |||||||||||
Depreciation | |||||||||||
Amortization of intangible assets | |||||||||||
Amortization of inventory fair value step-up | |||||||||||
Amortization of deferred financing costs | |||||||||||
Amortization of discount on notes receivable | ( | ) | ( | ) | |||||||
Change in fair value of earn-out consideration | ( | ) | ( | ) | |||||||
Intangible asset impairment | |||||||||||
Share-based compensation | |||||||||||
Change in deferred income taxes | ( | ) | ( | ) | |||||||
Loss on divestiture of Stability | |||||||||||
Increase (decrease) in cash, net of effects of acquisition and divestiture, resulting from changes in: | |||||||||||
Accounts receivable | ( | ) | |||||||||
Inventory | ( | ) | ( | ) | |||||||
Prepaid expenses | ( | ) | ( | ) | ( | ) | |||||
Income tax receivable | ( | ) | |||||||||
Other assets | ( | ) | |||||||||
Accounts payable | ( | ) | ( | ) | |||||||
Accrued compensation | ( | ) | |||||||||
Accrued expenses | ( | ) | |||||||||
Income taxes | ( | ) | |||||||||
Other liabilities | |||||||||||
Net cash flows provided by operating activities | |||||||||||
Cash flows from investing activities: | |||||||||||
Purchases of property and equipment | ( | ) | ( | ) | ( | ) | |||||
Proceeds from property and equipment sale | |||||||||||
Principal payments from note receivable | |||||||||||
Stability acquisition | ( | ) | |||||||||
Fixed maturity securities redemption | |||||||||||
Patent application costs | ( | ) | ( | ) | ( | ) | |||||
Net cash flows used in investing activities | ( | ) | ( | ) | ( | ) | |||||
Cash flows from financing activities: | |||||||||||
Proceeds from exercise of stock options | |||||||||||
Shares repurchased under repurchase plan | ( | ) | ( | ) | ( | ) | |||||
Shares repurchased for tax withholdings on vesting of restricted stock | ( | ) | ( | ) | ( | ) | |||||
Payments under capital lease obligations | ( | ) | ( | ) | ( | ) | |||||
Net cash flows used in financing activities | ( | ) | ( | ) | ( | ) | |||||
F-8
MIMEDX GROUP, INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF CASH FLOWS (in thousands) | |||||||||||
Net change in cash | ( | ) | |||||||||
Cash and cash equivalents, beginning of year | |||||||||||
Cash and cash equivalents, end of year | $ | $ | $ | ||||||||
See notes to the consolidated financial statements.
F-9
MIMEDX GROUP, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. |
MiMedx Group, Inc. (together with its subsidiaries except where the context otherwise requires “MiMedx,” or the “Company”) is an advanced wound care and emerging therapeutic biologics company, developing and distributing human placental tissue allografts with patent-protected processes for multiple sectors of healthcare. The Company derives its products from human placental tissues processed using proprietary processing methodologies. The Company’s mission is to offer physicians products and tissues to help the body heal itself. MiMedx provides products in the wound care, burn, surgical, orthopedic, spine, sports medicine, ophthalmic and dental sectors of healthcare. All of the Company’s products are regulated by the United States Food and Drug Administration (“FDA”).
MiMedx is the leading supplier of human placental allografts, which are human tissues that are transplanted from one person (a donor) to another person (a recipient). The Company operates in one business segment, Regenerative Biomaterials, which includes the design, manufacture, and marketing of products and tissue processing services for the Wound Care, Surgical, Sports Medicine, Ophthalmic and Dental market categories. The Company’s allograft product families include: dHACM family with AmnioFix® and EpiFix® brands; Umbilical family with EpiCord® and AmnioCord® brands; and Placental Collagen family with AmnioFill™ brands. AmnioFix and EpiFix are tissue allografts derived from amnion and chorion layers of human placental membrane; EpiCord and AmnioCord are tissue allografts derived from umbilical cord tissue. AmnioFill is a placental connective tissue matrix, derived from the placental disc and other placental tissue.
The Company’s business model is focused primarily on the United States of America but is exploring international expansion opportunities in the future.
2. | Liquidity and Capital Resources |
Net Working Capital
As of December 31, 2018, the Company had $45.1 million of cash and cash equivalents. The Company reported total current assets of $74.0 million and current liabilities of $71.5 million and had net working capital of $2.5 million as of December 31, 2018.
Overall Liquidity and Capital Resources
The Company’s largest cash requirement for the twelve months ended December 31, 2018 was cash for general working capital needs. In addition, the Company’s other cash requirements included capital expenditures, and repurchases of the Company’s common stock. The Company funded its cash requirements for 2018 through its existing cash reserves, and its operating activities which generated $35.8 million during the period. The Company believes that its anticipated cash from operating and financing activities and existing cash and cash equivalents, as well as the proceeds under the Term Loan Agreement (as defined below) will enable the Company to meet its operational liquidity needs and fund its planned investing activities for the twelve months from issuance of the consolidated financial statements.
As discussed in Note 16, “Commitments and Contingencies,” and further addressed in Note 21, “Subsequent Events,” of the consolidated financial statements, the Company anticipates additional cash requirements related to the following items within one year from the date in which the 2018 Form 10-K was available to be issued:
• | shareholder derivative lawsuits or potential settlements, for which the Company is not able to estimate a loss; |
• | operating loss of $ |
• | private securities lawsuits, for which the Company is currently not able to estimate a loss and for which it is unclear whether the Company would be indemnified under various insurance policies; |
• | the Company’s self-disclosure to the Department of Veterans’ Affairs (“VA”) concerning the eligibility of one of its products for inclusion in the Company’s FSS contract. The Company recorded a liability of $ |
F-10
• | litigation involving former employees of the Company. The Company has settled with one former employee for $ |
• | payments owed to the Company’s former Interim Chief Financial Officer, under a Separation and Transition Services Agreement dated November 18, 2019; |
• | payments in connection with the Company’s operating and capital lease agreements; and |
• | investments and other expenditures required in order to bring the Company’s facilities into compliance with current Good Manufacturing Practices (“cGMPs”). |
In addition, on June 10, 2019, the Company entered into a Term Loan Agreement (the “Term Loan Agreement”) with Blue Torch Finance LLC, as administrative agent and collateral agent, to borrow funds with a face value of $75.0 million (the “Term Loan”), of which the full amount has been borrowed and funded. The proceeds from the Term Loan have been and will continue to be used (i) for working capital and general corporate purposes and (ii) to pay transaction fees, costs and expenses incurred in connection with the Term Loan and the related transactions. The Term Loan matures on June 20, 2022 and is repayable in quarterly installments of $0.9 million with the balance due on June 20, 2022.
The Term Loan Agreement contains financial covenants requiring the Company, on a consolidated basis, to maintain the following:
• | Maximum Total Leverage Ratio, defined as funded debt divided by consolidated adjusted EBITDA, of not more than |
• | Minimum Liquidity, defined as unrestricted cash and cash equivalents, of not less than $ |
The Term Loan Agreement also specifies that any prepayment of the loan, voluntary or mandatory, as defined in the Term Loan Agreement, subjects MiMedx to a prepayment penalty as of the date of the prepayment with respect to the Term Loan of:
• | During the period from June 10, 2019 through June 10, 2020, an amount equal to |
• | During the period from June 11, 2020 through June 10, 2021, an amount equal to |
Principal prepayments after June 10, 2021 are not subject to a prepayment penalty.
The Term Loan Agreement also includes events of default customary for facilities of this type, and upon the occurrence of such events of default, subject to customary cure rights, all outstanding loans under the Term Loan Agreement may be accelerated and/or the lenders’ commitments terminated.
As of the date of the issuance of the consolidated financial statements, the Company is currently in compliance with the financial covenants in the Term Loan Agreement. However, the violation of these covenants in the 12 months from such date could result in an acceleration of the repayment of the loan which, in combination with the Company’s current commitments and contingent liabilities, could cast doubt on the Company’s ability to continue as a going concern.
The Company has analyzed its ability to address the aforementioned commitments and potential liabilities while remaining compliant with the financial covenants set forth in the Term Loan Agreement for the 12 months from the date of the issuance of the consolidated financial statements, consistent with the guidance prescribed by Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) 2014-05, “Going Concern: Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern.”
F-11
3. Significant Accounting Policies
Use of Estimates
The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles (“GAAP”) in the United States of America (“U.S.”). Conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported consolidated statements of operations during the reporting period. Actual results could differ from those estimates. Significant estimates include estimated useful lives and potential impairment of property and equipment and intangible assets, estimate for contingent liabilities, management’s assessment of the Company’s ability to continue as a going concern, estimate of fair value of share-based payments and valuation of deferred tax assets.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of MiMedx Group, Inc. and its wholly-owned subsidiaries, including, for the periods prior to its divestiture further discussed in Note 5, Stability Biologics, LLC (formerly known as Stability, Inc.). All intercompany balances and transactions have been eliminated upon consolidation.
Segment Reporting
Accounting Standards Codification (“ASC”) 280, “Segment Reporting” requires use of the “management approach” model for segment reporting. The management approach model is based on the way a company’s chief operating decision-maker (“CODM”) organizes segments within the Company for which separate discrete financial information is available regarding resource allocation and assessing performance. The Company has determined it has one operating segment.
Market Concentrations and Credit Risk
Cash and Cash Equivalents
Cash and cash equivalents include cash and FDIC insured certificates of deposit held at various banks with an original maturity of three months or less.
Notes Receivable
Notes receivable represent formal payment agreements with customers which generally arise in situations where amounts shipped and billed have aged significantly as well as the promissory note issued by Stability Biologics, LLC as part of the divestiture discussed in Note 5. The Company’s notes receivable are included in other current and long-term assets in the accompanying consolidated balance sheets and were valued taking into consideration cost of the market participant inputs, market conditions, liquidity, operating results and other qualitative factors.
Inventories
Inventories are valued at the lower of cost or net realizable value, using the first–in, first-out (“FIFO”) method. Inventory is tracked through raw material, work-in-progress, and finished goods stages as the product progresses through various production steps and stocking locations. Labor and overhead costs are absorbed through the various production processes up to when the work order closes. Historical yields and normal capacities are utilized in the calculation of production overhead rates. Reserves for inventory obsolescence are utilized to account for slow-moving inventory as well as inventory no longer needed due to diminished demand.
F-12
Property and Equipment
Property and equipment are recorded at cost and depreciated on a straight-line method over their estimated useful lives, principally three years to seven years . Leasehold improvements are depreciated on a straight-line method over the shorter of the estimated useful lives or the lease term. The Company is party to various lease arrangements for its facility space and equipment. These arrangements include interest, scheduled rent increases and rent holidays which are included in the determination of minimum lease payments when assessing lease classification, and are included in rent expense on a straight line basis over the lease term. See Notes 7, “Property and Equipment,” and 16, “Commitments and Contingencies,” for further information regarding capital leases, operating leases and rent expense.
Impairment of Long-lived Assets
The Company evaluates the recoverability of its long-lived assets (property and equipment) whenever adverse events or changes in business climate indicate that the expected undiscounted future cash flows from the related assets may be less than previously anticipated. If the net book value of the related assets exceeds the expected undiscounted future cash flows of the assets, the carrying amount would be reduced to the present value of their expected future cash flows and an impairment loss would be recognized.
Goodwill and Indefinite-lived Intangible Assets
Goodwill represents the excess of purchase price over the fair value of net assets of acquired businesses. The Company assesses the recoverability of its goodwill at least annually on September 30, or more frequently whenever events or substantive changes in circumstances indicate that the asset may be impaired. The Company may first choose to assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If the Company determines that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, then the Company performs a quantitative analysis. The Company may also choose to bypass the qualitative assessment and proceed directly to the quantitative analysis.
If the carrying value exceeds the fair value of the reporting unit, goodwill impairment is recorded for the amount that the reporting unit’s carrying value exceeds its fair value, not to exceed the total amount of goodwill allocated to the reporting unit. At present, the Company has only one reporting unit. The Company determines the fair value utilizing the income and market approaches. Under the income approach, the fair value of the Company is the present value of its future economic benefits. These benefits can include revenue, cost savings, tax deductions, and proceeds from its disposition. Value indications are developed by discounting expected cash flows to their present value at a rate of return that incorporates the risk-free rate for the use of funds, trends within the industry, and risks associated with particular investments of similar type and quality as of the goodwill impairment testing date. Under the market approach, the Company uses its market capitalization which is calculated by taking the Company’s share price times the number of outstanding shares. The Company’s estimates associated with the goodwill impairment test are considered critical due to the amount of goodwill recorded on its consolidated balance sheets and the judgment required in determining fair value, including projected future cash flows.
Acquired intangible assets are tested for impairment annually on September 30 or whenever events or changes in circumstances indicate that the carrying amount of an intangible asset may not be recoverable. The Company’s impairment reviews are based on an estimated future cash flow approach that requires significant judgment with respect to future revenue and expense growth estimates. The Company uses estimates consistent with business plans and a market participant view of the assets being evaluated. Actual results may differ from the estimates used in these analyses.
Impairment of Intangible Assets with Finite Lives
The Company reviews purchased intangible assets with finite lives for impairment whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable using a two-step impairment test. In step one, the Company determines the sum of the undiscounted future cash flows of the assets based on management’s estimates and compare it to the carrying value of the assets. If the carrying amount is greater than the sum of the undiscounted cash flows, then the asset is impaired and step two is required. In step two, the impairment loss is calculated as the difference between the fair value of the assets and the carrying value of the assets.
Impairment reviews are based on an estimated future cash flow approach that requires significant judgment with respect to future revenue and expense growth rates, selection of appropriate discount rate, asset groupings, and other assumptions and estimates.
F-13
There were no impairment losses recognized with respect to intangible assets with finite lives in 2018, 2017, or 2016.
Patent Costs
Contingencies
The Company is subject to various patent challenges, product liability claims, government investigations, shareholder derivative suits, former employee matters and other legal proceedings, see Note 16 “Commitments and Contingencies.” Legal fees and other expenses related to litigation are expensed as incurred and included in selling, general and administrative expenses in the accompanying consolidated statements of operations. The Company records an accrual for legal settlements and other contingencies in the consolidated financial statements when the Company determines that a loss is both probable and reasonably estimable. The Company discloses all ongoing legal matters for which a loss is probable, regardless of whether an estimate can be reasonably determined.
Revenue Recognition
The Company sells its products primarily to individual customers and independent distributors (collectively referred to as “customers”). Prior to 2015, substantially all federal healthcare providers, including the Department of Veterans Affairs, purchased Company product from one distributor customer of the Company, AvKARE Inc. (“AvKARE”), which is a veteran-owned General Services Administration Federal Supply Schedule contractor. In 2015, the Company began selling product directly to federal customers rather than exclusively allowing federal healthcare providers to purchase Company product from AvKARE. Upon expiration of the Company’s agreement with AvKARE on June 30, 2017, the Company had an obligation to repurchase AvKARE’s remaining inventory within 90 days in accordance with the terms of the agreement. As of September 30, 2017, the Company had satisfied the repurchase obligation.
For sales of the Company’s products to customers for all periods presented prior to January 1, 2018 (except sales from the Company’s wholly owned subsidiary Stability Biologics, LLC discussed below), the Company recognized revenue under ASC 605 only when both of the following events had occurred: (1) the Company has fulfilled the customer’s purchase order by delivering all product ordered; and (2) the Company has collected payment for the product delivered. Furthermore, the amount of revenue recognized was limited to the amount of payment received in a given period less the amount expected to be refunded or credited to customers for sales returns made after payment. The existence of extra-contractual or undocumented terms or arrangements initiated by former executives and other members of former Company management at the onset of the transactions, such as sales above established customer credit limits, concessions agreed to by former executives of the Company and other members of former Company management subsequent to the initial transaction (e.g., significantly extended payment terms, granting return or exchange rights) and contingent payment obligations caused the related sales transactions to fail the criteria required to be met under then applicable GAAP to recognize revenue.
F-14
On January 13, 2016, the Company completed the acquisition of Stability, a provider of human tissue products to surgeons, facilities, and distributors serving the surgical, spine, and orthopedic sectors of the healthcare industry. For sales of the Company’s products through Stability the Company recognized revenue under ASC 605 only when both of the following events had occurred: (1) the Company has fulfilled the customer’s purchase order by delivering all product ordered; and (2) the product has been delivered to the customer. Total sales from Stability were $7.0 million and $11.7 million for December 31, 2017 and 2016, respectively. Stability was divested on September 30, 2017.
The Company adopted ASC 606 on January 1, 2018 by using the modified retrospective method. ASC 606 establishes principles for reporting information about the nature, amount, timing and uncertainty of revenue and cash flows arising from the entity's contracts to provide goods or services to customers. The core principle requires an entity to recognize revenue to depict the transfer of goods or services to customers in an amount that reflects the consideration that it expects to be entitled to receive in exchange for those goods or services recognized as performance obligations are satisfied. The Company has assessed the impact of the ASC 606 guidance by reviewing existing customer contracts and accounting policies and practices to identify differences, including identification of the contract and the evaluation of the Company’s performance obligations, transaction price, customer payments, transfer of control and principal versus agent considerations.
When evaluating its customer relationships and agreements with respect to customer sales, the Company concluded that although a contract may exist from a legal perspective upon fulfillment of a purchase order by the Company a contract does not exist from an accounting perspective at the time because certain implicit arrangements (e.g. rights of return or exchange, extended payment terms and sales exceeding established credit limits) modified the explicit terms of the contract.
The Company therefore determined that it did not meet the criteria necessary for its revenue arrangements to qualify as “contracts” under the requirements of ASC 606 upon physical delivery of the product. Subsequent to the delivery of product, uncertainties surrounding contractual adjustment are not resolved until either: (1) the customer returns the product prior to payment; or (2) the Company receives payment from the customer. At that point, the Company has determined that an accounting contract exists and the performance obligations of the Company to deliver product and the customer to pay for the product are satisfied. The Company has determined the transaction price of its contracts to equal the amount of consideration received from customers less the amount expected to be refunded or credited to customers, which is recognized as a refund liability that is updated at the end of each reporting period for changes in circumstances. The refund liability is included within accrued expenses in our consolidated balance sheet and is presented as estimated returns in Note 9, “Accrued Expenses.” The change in the balance of this reserve between December 31, 2017 and 2018 increased revenue by $0.6 million for the year ended December 31, 2018.
Based on the assessment, the Company concluded that during the year ended December 31, 2018 there was no substantial change to the timing and pattern of revenue recognition for the Company’s current revenue streams, and therefore there was no change to the Company's consolidated financial statements upon adoption of ASC 606, as the Company continued deferring revenue recognition until the time the Company receives cash consideration subsequent to the control of the product being transferred.
The Company sells to Group Purchasing Organization (“GPO”) members who transact directly with the Company at GPO-agreed pricing. GPOs are funded by administrative fees that are paid by the Company. These fees are set as a percentage of the purchase volume, which is typically 3 % of sales made to the GPO members. Prior to adoption of ASC 606, for all periods presented prior to January 1, 2018, the Company presented the administrative fees paid to GPOs as a reduction of revenues as the benefit received by the Company in exchange for the GPO fees was not sufficiently separable from the GPO member’s purchase of the Company’s products. Upon adoption of ASC 606, the Company concluded that although it benefited from the access that a GPO provides to its members, this benefit was neither distinct from other promises in the Company’s contracts with GPOs nor was the benefit separable from the sale of goods by the Company to the end customer. Therefore, the Company continued presenting fees paid to GPOs as a reduction of product revenues.
Additionally, the Company considered how to account for costs associated with the delivered products of the contract for which revenue has been deferred, which is whether to match the related cost of sales expense with revenue or to recognize expense upon shipment. In making this assessment, the Company considered the financial viability of its distributors and customers based on their creditworthiness to determine if collectability of amounts sufficient to realize the costs of the products shipped was reasonably assured at the time of shipment. As the Company determined that there was a probable future economic benefit associated with the sales transactions, the Company deferred the costs of sales until the revenue was recognized. See section “Cost of Sales” below for the policy.
The Company offsets deferred revenue with the associated accounts receivable obligations in connection with the sales of products to its customers. The Company believes that because the conditions for revenue recognition have not yet been met and payment has not been received, neither party has fulfilled its obligations under the contract. Amounts shipped and billed but not recorded as revenue were $51.0 million and $64.8 million, for the years ended December 31, 2018 and 2017, respectively.
F-15
Cost of Sales
Cost of sales includes all costs directly related to bringing the Company’s products to their final selling destination. Amounts include direct and indirect costs to manufacture products including raw materials, personnel costs and direct overhead expenses necessary to convert collected tissues into finished goods, product testing costs, quality assurance costs, facility costs associated with the Company’s manufacturing and warehouse facilities, including depreciation, freight charges, costs to operate equipment and other shipping and handling costs for products shipped to customers.
Research and Development Costs
Research and development costs consist of direct and indirect costs associated with the development of the Company’s technologies. These costs are expensed as incurred.
Advertising expense
Income Taxes
Income tax expense (benefit), deferred tax assets and liabilities, and liabilities for unrecognized tax benefits reflect management’s best assessment of estimated current and future taxes to be paid. The Company is subject to income taxes in the United States, including numerous state jurisdictions. Significant judgments and estimates are required in determining the consolidated income tax expense.
Deferred income taxes arise from temporary differences between the tax basis of assets and liabilities and their reported amounts in the financial statements, which will result in taxable or deductible amounts in the future. In evaluating the Company’s ability to recover its deferred tax assets within the jurisdiction from which they arise, management considers all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax-planning strategies, and results of recent operations. In projecting future taxable income, the Company begins with historical results adjusted for the results of discontinued operations and incorporate assumptions about the amount of future state and federal pretax operating income adjusted for items that do not have tax consequences. The assumptions about future taxable income require significant judgment and are consistent with the plans and estimates the Company uses to manage the underlying businesses. In evaluating the objective evidence that historical results provide, management considers three years of cumulative income (loss). The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined on the basis of the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in the tax provision (benefit) in the period that includes the enactment date.
The Company recognizes deferred tax assets to the extent that it believes these assets are more likely than not to be realized. In making such a determination, management considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income exclusive of temporary differences, tax-planning strategies, and results of recent operations. If the Company determines that it would be able to realize its deferred tax assets in the future in excess of their net recorded amount, the Company would make an adjustment to the deferred tax asset valuation allowance, which would reduce the provision for income taxes.
The calculation of income tax liabilities involves dealing with uncertainties in the application of complex tax laws and regulations both for U.S. federal income tax purposes and across numerous state jurisdictions. ASC Topic 740 (“ASC 740”) states that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, on the basis of the technical merits. The Company (1) records unrecognized tax benefits as liabilities in accordance with ASC 740, and (2) adjusts these liabilities when management’s judgment changes as a result of the evaluation of new information not previously available. Because of the complexity of some
F-16
of these uncertainties, the ultimate resolution may result in a payment that is materially different from management’s current estimate of the unrecognized tax benefit liabilities. These differences will be reflected as increases or decreases to income tax expense in the period in which new information is available.
The Company records uncertain tax positions in accordance with ASC 740 on the basis of a two-step process whereby (1) it determines whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position, and (2) for those tax positions that meet the more-likely-than-not recognition threshold, it recognizes the largest amount of tax benefit that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority.
The Company recognizes interest and penalties related to unrecognized tax benefits within the income tax expense line in the accompanying consolidated statements of operations. Accrued interest and penalties, if any, are included within the related tax liability line in the consolidated balance sheet and recorded as a component of income tax expense.
The Company grants share-based awards to employees and members of the Company’s Board of Directors (the “Board”). Such awards are recognized as share-based payment expense over the requisite service period, to the extent such awards are expected to vest in accordance with Financial Accounting Standards Board (“FASB”) ASC Topic 718 “Compensation—Stock Compensation.” The amount of expense to be recognized is determined by the fair value of the award using inputs available as of the grant date.
The fair value of restricted common stock is the value of common stock on the grant date. The fair value of stock option grants is estimated using the Black-Scholes option pricing model. Use of the valuation model requires management to make certain assumptions with respect to selected model inputs. The Company uses the simplified method for share-based compensation to estimate the expected term. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for the estimated option expected term. Historically, the Company did not have enough history to establish volatility based upon its own stock trading. Therefore, the expected volatility was based on similar publicly traded peer companies. The Company routinely reviews its calculation of volatility for potential changes in future volatility, the Company’s life cycle, its peer group, and other factors. In addition, an expected dividend yield of zero is used in the option valuation model because the Company does not pay cash dividends and does not expect to pay any cash dividends in the foreseeable future. For awards with service conditions only, the Company recognizes share-based compensation expense on a straight-line basis over the requisite service period.
For restricted common stock and stock options granted as consideration for services rendered by non-employees, the Company recognizes compensation expense in accordance with the requirements of FASB ASC Topic 505-50, “Equity Based Payments to Non-Employees.” Non-employee restricted common stock and stock option grants that do not vest immediately upon grant, and whose terms are known, are recorded as an expense over the vesting period of the underlying instrument granted. At the end of each financial reporting period prior to vesting, the value of the instruments granted, is re-measured using the fair value of the Company’s common stock and the stock-based compensation recognized during the period is adjusted accordingly.
Basic and Diluted Net (Loss) Income per Share
Basic net (loss) income per share is determined by dividing net (loss) income by the weighted average ordinary shares outstanding during the period. Diluted net income per ordinary share is based on the weighted average number of ordinary shares outstanding and potentially dilutive ordinary shares outstanding determined by using the treasury stock method. For all periods presented with a net loss, the shares underlying the common share options, warrants and restricted stock have been excluded from the calculation because their effect would have been anti‑dilutive. Therefore, the weighted average shares outstanding used to calculate both basic and diluted loss per share are the same for periods with a net loss.
Fair Value of Financial Instruments and Fair Value Measurements
The respective carrying value of certain on-balance sheet financial instruments approximated their fair values due to the short-term nature and type of these instruments. These financial instruments include cash and cash equivalents, accounts receivable, notes receivable, and certain current financial liabilities. The carrying cost of the Company’s investments also reflects their fair values due to the type of these investments, and the fair value of capital leases approximates their carrying value based upon current rates available to the Company.
F-17
Fair value financial instruments are recorded in accordance with the fair value measurement framework. The fair value measurement framework includes a fair value hierarchy that prioritizes observable and unobservable inputs used to measure fair values in their broad levels. These levels from highest to lowest priority are as follows:
• | Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities; |
• | Level 2: Quoted prices in active markets for similar assets or liabilities or observable prices that are based on inputs not quoted on active markets, but corroborated by market data. |
• | Level 3: Unobservable inputs or valuation techniques that are used when little or no market data is available. |
The determination of fair value and the assessment of a measurement’s placement within the hierarchy require judgment. Level 3 valuations often involve a higher degree of judgment and complexity. Level 3 valuations may require the use of various cost, market, or income valuation methodologies applied to unobservable management estimates and assumptions. Management’s assumptions could vary depending on the asset or liability valued and the valuation method used. Such assumptions could include: estimates of prices, earnings, costs, actions of market participants, market factors, or the weighting of various valuation methods. The Company may also engage external advisors to assist it in determining fair value, as appropriate.
Although the Company believes that the recorded fair value of its financial instruments is appropriate, these fair values may not be indicative of net realizable value or reflective of future fair values.
Recently Adopted Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update (“ASU”) 2014-09, “Revenue from Contracts with Customers (Topic 606)” (“ASC 606”) that requires companies to recognize revenue when a customer obtains control rather than when companies have transferred substantially all risks and rewards of a good or service. The underlying principle of the new standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. ASC 606 defines a five-step process to achieve this core principle and more judgment and estimates are required within the revenue recognition process than are required under existing guidance, including identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation, among others. This update is effective for annual reporting periods beginning on or after December 15, 2017 and interim periods therein and requires expanded disclosures.
The Company’s analysis entailed a full review of existing revenue streams upon which the Company applied the core principles of the standard. Through this analysis, the Company identified one revenue stream from contracts with customers: product sales. The Company concluded that the timing and amount of revenue recognized for the year ended December 31, 2018 should not change following our adoption of the standard and the Company should continue deferring revenue recognition until the time the Company realized non-refundable cash receipts subsequent to the control of the product being transferred. Adoption of the standard had no impact on the Company’s consolidated financial statements. See “Revenue Recognition” accounting policy above for further information.
In July 2015, the FASB issued ASU 2015-11, “Inventory (Topic 330): Simplifying the Measurement of Inventory,” which requires companies to measure inventory within the scope of this Update at the lower of cost and net realizable value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. Subsequent measurement is unchanged for inventory measured using LIFO or the retail inventory method. The Company adopted this standard as on January 1, 2017 and this standard did not have a material impact on the Company’s consolidated financial statements.
In March 2016, the FASB issued ASU 2016-09, “Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting,” which simplifies several aspects of the accounting for share-based payment award transactions including (a) income tax consequences; (b) classification of awards as either debt or equity liabilities; and (c) classification on the statement of cash flows. The amendments are effective for public business entities for annual periods beginning after December 15, 2016, and interim periods within those annual periods. The Company adopted this ASU as of January 1, 2017. The primary amendment impacting the Company’s financial statements is the requirement for excess tax benefits or shortfalls on the exercise of share-based compensation awards to be presented in income tax expense in the consolidated statements of operations during the period the award is exercised as opposed to being recorded in additional paid-in capital on the consolidated balance sheets. The excess tax benefit or shortfall is calculated as the difference between the fair value of the award on the date of exercise and the fair value of the award used to measure the expense to be recognized over the service period. Changes are required to be applied
F-18
prospectively to all excess tax benefits and deficiencies resulting from the exercise of awards after the date of adoption. The ASU requires a “modified retrospective” approach application for excess tax benefits that were not previously recognized in situations where the tax deduction did not reduce current taxes payable. For the twelve months ended December 31, 2017, the Company recorded an income tax benefit of $4.8 million related to the excess tax benefit of exercised awards during the period that would have been recorded in additional paid-in capital during prior years. As the end result is dependent on the future value of the Company’s stock as well as the timing of employee exercises, the amount of future impact cannot be quantified at this time. The Company has elected to continue to estimate forfeitures expected to occur to determine the share-based compensation expense.
In August 2016, the FASB issued ASU 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments.” The primary purpose of ASU 2016-15 is to reduce the diversity in practice that has resulted from the lack of consistent principles on this topic. The ASU 2016-15 addresses the following eight specific cash flow issues: Debt prepayment or debt extinguishment costs; settlement of zero-coupon debt instruments or other debt instruments with coupon interest rates that are insignificant in relation to the effective interest rate of the borrowing; contingent consideration payments made after a business combination; proceeds from the settlement of insurance claims; proceeds from the settlement of corporate-owned life insurance policies; distributions received from equity method investees; beneficial interests in securitization transactions; and separately identifiable cash flows and application of the predominance principle. ASU 2016-15 is effective for the Company for annual periods beginning after December 15, 2017, and early adoption is permitted for all entities. Entities must apply the guidance retrospectively to all periods presented but may apply it prospectively from the earliest date practicable if retrospective application would be impracticable. The Company adopted this standard as of January 1, 2018 and applied the ASU 2016-15 retrospectively for all periods presented.
In January 2017, the FASB issued ASU 2017‐01, “Business Combinations (Topic 805): Clarifying the Definition of a Business” which clarifies the definition of a business. This ASU provides a screen to determine when a set is not a business by stating that when substantially all of the fair value of gross assets acquired is concentrated in a single identifiable asset or a group of similar identifiable assets, that the set of assets acquired is not a business. If the screen is not met, it (1) requires that to be considered a business, a set must include, at a minimum, an input and a substantive process that together significantly contribute to the ability to create output and (2) removes the evaluation of whether a market participant could replace the missing elements. The Company adopted this guidance on January 1, 2018 and this standard did not have a material impact on the Company’s consolidated financial statements.
In January 2017, the FASB issued ASU 2017-04, “Intangibles - Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment.” ASC 350 currently requires an entity to perform a two-step test to determine the amount, if any, of goodwill impairment. ASU 2017-04 removes the second step of the test. An entity will apply a one-step quantitative test and record the amount of goodwill impairment as the excess of a reporting unit’s carrying amount over its fair value, not to exceed the total amount of goodwill allocated to the reporting unit. The new guidance does not amend the optional qualitative assessment of goodwill impairment. The ASC amendments are effective for annual or any interim goodwill impairment tests in fiscal years beginning after December 15, 2020 with early adoption permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. The Company adopted this standard as of January 1, 2017.
In March 2018, the FASB issued ASU 2018-05, “Income Taxes (Topic 740): Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 118,” which adds Securities and Exchange Commission (“SEC”) paragraphs pursuant to the SEC Staff Accounting Bulletin No. 118, which expresses the view of the SEC staff regarding application of Topic 740, Income Taxes, in the reporting period that includes December 22, 2017 - the date on which the Tax Cuts and Jobs Act was signed into law. ASU 2018-05 was effective immediately upon issuance. The Company considered this additional guidance in determining the impact of the Tax Cuts and Jobs Act as of and for the year ended December 31, 2018. The Company’s accounting for the tax implications of the Tax Cuts and Jobs Act is complete as of December 31, 2018.
F-19
Recently Issued Accounting Pronouncements Not Yet Adopted
In February 2016, the FASB issued ASU 2016-02, “Leases (Topic 842).” This ASU will require lessees to recognize most leases on their balance sheets as lease liabilities with corresponding right-of-use (“ROU”) assets. Recognition, measurement and presentation of expenses will depend on classification as a finance or operating lease. The Company adopted the standard as of January 1, 2019 using a modified retrospective approach and applying the standard’s transition provisions at January 1, 2019, the effective date. The Company elected the package of practical expedients permitted under the transition guidance which, among other things, allows the Company to carryforward the historical lease classification. In addition, the Company elected to combine the lease and non-lease components for the asset categories comprising existing leases and is making an accounting policy election to exclude from balance sheet reporting those leases with initial terms of 12 months or less. The Company has implemented new controls and processes to enable the preparation of financial information as necessitated by the new standard. The Company estimates that adoption of this standard will result in recognition of additional lease ROU assets and lease liabilities of approximately$4.6 million and $5.2 million, respectively. The Company does not expect adoption of the standard to materially affect its consolidated financial statements.
In June 2016, the FASB issued ASU 2016-13, “Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” that introduces a new model for recognizing credit losses on financial instruments based on an estimate of current expected credit losses. This includes accounts receivable, trade receivables, loans, held-to-maturity debt securities, net investments in leases and certain off-balance sheet credit exposures. The guidance also modifies the impairment model for available-for-sale debt securities. The update is effective for fiscal years beginning after December 15, 2020. The Company is currently assessing the potential effects this update may have on its consolidated financial statements.
In June 2018, the FASB issued ASU 2018-07, “Improvements to Non-employee Share-Based Payment Accounting” (“ASU 2018-07”), which simplifies the accounting for share-based payments to non-employees by aligning it with the accounting for share-based payments to employees, with certain exceptions. The Company adopted ASU 2018-07 prospectively as of January 1, 2019. The cumulative effect of the adoption of ASU 2018-07 on retained earnings as of January 1, 2019 was a $0.1 million reduction of previously recognized share-based compensation expense.
In August 2018, the FASB issued ASU 2018-13, “Fair Value Measurement (Topic 820): Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement” (“ASU 2018-13”), which changes the fair value measurement disclosure requirements of ASC 820 “Fair Value Measurement,” based on the concepts in the FASB Concepts Statement, Conceptual Framework for Financial Reporting-Chapter 8: “Notes to Financial Statements,” including the consideration of costs and benefits. The ASU 2018-13 is effective for all entities for fiscal years beginning after December 15, 2019. Early adoption is permitted for any eliminated or modified disclosures upon issuance of ASU 2018-13. The Company is currently evaluating the impact the adoption of ASU 2018-13 will have on its consolidated financial statements.
All other ASUs issued and not yet effective for the twelve months ended December 31, 2018, and through the date of this report, were assessed and determined to be either not applicable or are expected to have minimal impact on the Company’s financial position or results of operations.
4. | Restatement of the Consolidated Financial Statements |
In February 2018, the Audit Committee (the “Audit Committee”) of the Board retained King & Spalding LLP (“King & Spalding”) as counsel to the Audit Committee to assist in conducting an independent investigation into current and prior-period matters relating to allegations regarding certain sales and distribution practices at the Company and certain other matters (the “Investigation” or the “Audit Committee Investigation”). Following its engagement by the Audit Committee, King & Spalding retained KPMG LLP (“KPMG”) to assist with the Investigation.
As a result of the Investigation, the Company determined that the financial statements as of period ended December 31, 2016, 2015, 2014, 2013, and 2012 should no longer be relied upon and accordingly would need to be restated. A summary of the impact of the Restatement on the Company’s consolidated statement of operations includes, but is not limited to, the following:
• | the timing of revenue recognition for sales through all distributors and direct sales to all customers, as discussed above; |
• | the impact of bad debt expense as a result of the timing of revenue recognition; |
• | the presentation of net revenue instead of gross revenue for administrative fees paid to GPOs; |
• | the impact of changes in revenue recognition on cost of goods sold; |
• | the timing of recognizing certain general and administrative expenses; |
F-20
• | the impact on losses associated with contingency exposures; |
• | the impact of other miscellaneous adjustments, such as patent cost and share-based compensation, and |
• | the impact of the above on income tax. |
The impact of the Restatement on the Company’s consolidated balance sheet includes, but is not limited to, the following:
• | changes in the amount of reported cash, due to the timing of certain cash collections; |
• | changes to reported accounts receivable and other current assets and the related reserves on each, due to the restatement of revenue recognition; |
• | accrual balances that are impacted by the expense and contingency determinations discussed above; and |
• | the related income tax effects of the above. |
The effect of adjustments made to the Company’s previously filed consolidated statements of operations as a result of these matters are shown in the table below. The tax effect of the adjustments is estimated based on the Company’s effective tax rate of 28.46 %.
Year Ended December 31, 2016 | |||||||||||
(in thousands, except share and per share data) | |||||||||||
Previously Reported | Adjustments | Restated | |||||||||
Net sales | $ | $ | ( | ) | $ | ||||||
Cost of sales | ( | ) | |||||||||
Gross profit | ( | ) | |||||||||
Operating expenses: | |||||||||||
Selling, general and administrative | ( | ) | |||||||||
Research and development | |||||||||||
Amortization of intangible assets | |||||||||||
Operating income | ( | ) | |||||||||
Other expense, net | ( | ) | ( | ) | |||||||
Income before income tax provision | ( | ) | |||||||||
Income tax provision (expense) | ( | ) | ( | ) | |||||||
Net income (loss) | $ | $ | ( | ) | $ | ||||||
Net income (loss) per common share - basic | $ | $ | — | $ | |||||||
Net income (loss) per common share - diluted | $ | $ | — | $ | |||||||
Weighted average shares outstanding - basic | — | ||||||||||
Weighted average shares outstanding - diluted | — | ||||||||||
F-21
The effects of the restatements on the Company’s consolidated statement of operations by category for the year ended December 31, 2016 are shown in the table below. The tax effect of the adjustments is estimated based on the Company’s effective tax rate of 28.46 %.
Year Ended December 31, 2016 | |||||||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||||||
(in thousands) | Previously | Cash | Revenue | Total | |||||||||||||||||||||||
Reported | Revenue | GPO Fees | Related | Other | Adjustments | Restated | |||||||||||||||||||||
Net sales | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | $ | ( | ) | $ | ||||||||||||
Cost of sales | ( | ) | ( | ) | |||||||||||||||||||||||
Gross profit | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||
Operating expenses: | |||||||||||||||||||||||||||
Selling, general and administrative expenses | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||
Research and development expenses | |||||||||||||||||||||||||||
Amortization of intangible assets | |||||||||||||||||||||||||||
Operating income | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||
Other expense, net | ( | ) | ( | ) | |||||||||||||||||||||||
Income before income tax provision | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||
Income tax provision (expense) benefit | ( | ) | ( | ) | |||||||||||||||||||||||
Net income (loss) | $ | $ | ( | ) | $ | $ | ( | ) | $ | $ | ( | ) | $ | ||||||||||||||
F-22
The effects of the restatements on the Company’s consolidated statement of stockholders’ equity for the year ended December 31, 2016 are as follows:
Year-ended December 31, 2016 | |||||||||||||||||||||||
(in thousands) | |||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||
Previously Reported | Cash Revenue | Revenue Related | Other | Total Adjustments | Restated | ||||||||||||||||||
Total stockholders' equity, December 31, 2015 | $ | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||
Share-based compensation | ( | ) | ( | ) | |||||||||||||||||||
Tax benefit of share-based compensation | ( | ) | ( | ) | |||||||||||||||||||
Exercise of stock options | |||||||||||||||||||||||
Issuance of restricted stock | ( | ) | ( | ) | |||||||||||||||||||
Shares issued for services performed | |||||||||||||||||||||||
Shares repurchased | ( | ) | ( | ) | |||||||||||||||||||
Shares repurchased for tax withholding | ( | ) | ( | ) | |||||||||||||||||||
Shares issued in conjunction with acquisition of Stability | |||||||||||||||||||||||
Net income (loss) | ( | ) | ( | ) | ( | ) | |||||||||||||||||
Total stockholders' equity, December 31, 2016 | $ | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||
The effects of the restatements on the Company’s consolidated statement of stockholders’ equity as of December 31, 2016 are as follows:
As of December 31, 2016 | |||||||||||||||||||||||
(in thousands) | |||||||||||||||||||||||
Adjustments by Category | |||||||||||||||||||||||
Previously Reported | Cash Revenue | Revenue Related | Other | Total Adjustments | Restated | ||||||||||||||||||
Common stock | $ | $ | $ | $ | $ | $ | |||||||||||||||||
Additional paid-in capital | |||||||||||||||||||||||
Treasury stock | ( | ) | ( | ) | |||||||||||||||||||
Accumulated deficit | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||
Total stockholders' equity | $ | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||
F-23
The effects of the restatements on the Company’s consolidated balance sheet as of December 31, 2016 are as follows:
Year Ended December 31, 2016 | |||||||||||
(in thousands) | |||||||||||
Previously Reported | Adjustments | Restated | |||||||||
ASSETS | |||||||||||
Current assets: | |||||||||||
Cash and cash equivalents | $ | $ | ( | ) | $ | ||||||
Accounts receivable, net | ( | ) | |||||||||
Inventory, net | ( | ) | |||||||||
Prepaid expenses | ( | ) | |||||||||
Other current assets | |||||||||||
Total current assets | ( | ) | |||||||||
Property and equipment, net | |||||||||||
Goodwill | |||||||||||
Intangible assets, net | ( | ) | |||||||||
Deferred tax asset, net | ( | ) | |||||||||
Other assets | |||||||||||
Total assets | $ | $ | ( | ) | $ | ||||||
LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||||||
Current liabilities: | |||||||||||
Accounts payable | $ | $ | $ | ||||||||
Accrued compensation | |||||||||||
Accrued expenses | |||||||||||
Current portion of earn out liability | ( | ) | |||||||||
Deferred tax liability, net | |||||||||||
Income taxes | ( | ) | |||||||||
Other current liabilities | |||||||||||
Total current liabilities | |||||||||||
Earn out liability | ( | ) | |||||||||
Other liabilities | |||||||||||
Total liabilities | |||||||||||
Stockholders' equity: | |||||||||||
Preferred stock; $.001 par value; 5,000,000 shares authorized and 0 shares issued and outstanding | |||||||||||
Common stock; $0.001 par value; 150,000,000 shares authorized; 110,212,547 issued and 109,862,787 outstanding at December 31, 2016 | |||||||||||
Additional paid-in capital | |||||||||||
Treasury stock at cost: 349,760 shares at December 31, 2016 | ( | ) | ( | ) | |||||||
Accumulated deficit | ( | ) | ( | ) | ( | ) | |||||
Total stockholders' equity | ( | ) | |||||||||
Total liabilities and stockholders' equity | $ | $ | ( | ) | $ | ||||||
F-24
The effects of the restatements on the Company’s consolidated statement of cash flows for the year ended December 31, 2016 are as follows:
Year Ended December 31, 2016 | |||||||||||
(in thousands) | |||||||||||
Previously Reported | Adjustments | Restated | |||||||||
Cash flows provided by operating activities: | |||||||||||
Net (loss) income | $ | $ | ( | ) | $ | ||||||
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | |||||||||||
Depreciation | |||||||||||
Amortization of intangible assets | |||||||||||
Amortization of inventory fair value step-up | |||||||||||
Amortization of deferred financing costs | ( | ) | |||||||||
Change in fair value of earn-out consideration | ( | ) | ( | ) | |||||||
Share-based compensation | ( | ) | |||||||||
Change in deferred income taxes | ( | ) | ( | ) | ( | ) | |||||
Increase (decrease) in cash, net of effects of acquisition and divestiture, resulting from changes in: | |||||||||||
Accounts receivable | ( | ) | |||||||||
Inventory | ( | ) | ( | ) | |||||||
Prepaid expenses | ( | ) | ( | ) | |||||||
Other assets | ( | ) | ( | ) | ( | ) | |||||
Accounts payable | ( | ) | ( | ) | |||||||
Accrued compensation | ( | ) | ( | ) | |||||||
Accrued expenses | |||||||||||
Income taxes | ( | ) | |||||||||
Other liabilities | |||||||||||
Net cash flows provided by operating activities | ( | ) | |||||||||
Cash flows used in investing activities: | |||||||||||
Purchases of property and equipment | ( | ) | ( | ) | |||||||
Stability acquisition | ( | ) | ( | ) | |||||||
Fixed maturity securities redemption | |||||||||||
Patent application costs | ( | ) | ( | ) | |||||||
Net cash flows used in investing activities | ( | ) | ( | ) | |||||||
Cash flows used in financing activities: | |||||||||||
Proceeds from exercise of stock options | |||||||||||
Shares repurchased under repurchase plan | ( | ) | ( | ) | |||||||
Shares repurchased for tax withholdings on vesting of restricted stock | ( | ) | ( | ) | |||||||
Deferred financing costs | ( | ) | |||||||||
Payments under capital lease obligations | ( | ) | ( | ) | |||||||
Net cash flows used in financing activities | ( | ) | ( | ) | |||||||
Net change in cash | ( | ) | |||||||||
Cash and cash equivalents, beginning of year | ( | ) | |||||||||
Cash and cash equivalents, end of year | $ | $ | ( | ) | $ | ||||||
F-25
The following include descriptions of the significant adjustments to the Company’s financial position and results of operations from the previously reported consolidated financial statements.
Revenue Recognition
Under the Company’s previous revenue recognition policy utilized in the preparation of the financial statements for each of the years ended December 31, 2016, 2015, 2014, 2013 and 2012 and each of the quarters ended March 31, 2017, June 30, 2017 and September 30, 2017, revenue was recorded as follows:
• | For sales to distributors, revenue was recorded upon shipment to the distributor; |
• | Certain sales to direct customers were treated as consignment sales as the customer could return the product at any time and was not required to pay until the product was used, despite no formal consignment agreement being in place. Therefore, the Company did not record revenue until the product was sold to an end-user (i.e., the recipient of the product); and |
• | For other sales to direct customers, revenue was recorded upon shipment to the customer. |
Under ASC 605, revenue should not be recognized until it is realized or realizable and earned. SEC Staff Accounting Bulletin (“SAB”) Topic 13.A.1 (as codified in ASC 605-10-S99-1) outlines four criteria that generally indicate when revenue is realized or realizable and earned. If any of these criteria are not met, revenue recognition should be deferred until all criteria have been met. Therefore, the Company assessed these four criteria as follows:
1. | Persuasive evidence of an arrangement exists - The Company’s sales are driven either by contracts or purchase orders. These types of documents are typically used to establish persuasive evidence of an arrangement. The Company’s customary business practices, however, must be taken into account as a contract can be written, oral, or modified based on customary business practices. Throughout 2012-2017, although the Company may have created a legal contract upon the execution of a contract and/or fulfillment of a purchase order, the lack of clarity around the final terms of the arrangement due to the pervasive side agreements with customers preclude the Company’s sales transactions from meeting this criterion upon shipment of product. Therefore, even though there may have been a legal contract governing the arrangement (which typically would indicate persuasive evidence of an arrangement), the Company’s selling and collection practices amended the stated contract terms. After considering these factors, the Company concluded that persuasive evidence of an arrangement did not exist upon shipment of product. |
2. | Delivery has occurred or services have been rendered - For sales to customers, physical possession and title transferred upon shipment to the customer. However, the Company concluded that it did not pass the risks of ownership to the customer upon shipment because customers were allowed to return product for multiple reasons, which included being unable to sell the product, damages which may have occurred subsequent to delivery, and dropped product. See below for additional discussion of the Company’s rationale for concluding that delivery had not yet occurred upon shipment to the customer. |
3. | The seller’s price to the buyer is fixed or determinable - At certain quarter-ends, the Company was significantly increasing sales to customers without having visibility into the level of product remaining unsold at the customer’s location. This practice made it difficult to develop an appropriate estimate of future credits to be issued to customers at the time of sale, which, in turn, impacted whether the price at the time of transfer of physical possession to the customer was fixed or determinable. This previous practice in combination with the following actions of the Company preclude the price of the Company’s sales transactions from being fixed or determinable upon shipment of product: |
a. | Offering customers an unconditional right of return with many items being returned over a year after the initial sale, |
b. | Offering extended payment terms to customers with days sales outstanding averaging almost 3 months, and |
c. | A history of exceeding established credit limits for customers. |
4. | Collectability is reasonably assured - At the time of transfer of physical possession to the customer, collectability of the sales was questionable. As determined in the Investigation and described further below, the customers’ intention to pay amounts when due was uncertain in light of the conflicting messages customers received with respect to the payment |
F-26
terms, rights of return and lack of adherence to credit limits. Although the Company did have a process in place to establish credit limits, the evidence indicates that those credit limits were overridden by certain sales personnel and members of management. Despite these overrides, the Company recovered the majority of its billings made between 2012 and 2017 with insignificant write-offs recorded; however, a significant amount of these billings were collected well after payment was due under the contractual terms. Furthermore, the quantitative and qualitative evidence gathered by the Company raised considerable doubt as to the collectability of its billings at the time of shipment, but this evidence was not persuasive enough for the Company to reach a conclusion as to whether collectability was reasonably assured.
In the Company’s evaluation of the point at which delivery occurred (the second criterion discussed above), the Company further considered the fact that there are instances under ASC 605 where the transfer of title of the product did not coincide with revenue recognition. Based on its review of all facts and circumstances, the Company determined that it did not meet all of the criteria to recognize revenue at the time of shipment of product to the customer. Specifically, the Company determined that the Company did not transfer the risks of ownership upon the transfer of physical possession because the Company’s customers were routinely granted an extended return period with very limited restrictions on the right of return and extended payment terms which raised doubt as to the intent or ability of customers to use and pay for the product delivered. Customers were allowed to return product for multiple reasons which included being unable to sell the product, damages which may have occurred subsequent to delivery, and dropped product (i.e., product that becomes contaminated and unusable). In other words, only upon use of the product in a surgical application (whether by the customer or by the ultimate end user in the case of distributors) would the customer no longer have the ability to return the product.
Accordingly, the Company concluded that, from 2012 to 2017, its previous decision to recognize revenue at the time of shipment of product to the customer was not appropriate. The Company determined that the aforementioned revenue recognition criteria were met only when both of the following events had occurred: (1) the Company fulfilled the customer's purchase order by delivering product ordered, and (2) the Company collected payment for the product delivered. Furthermore, the Company determined that the amount of revenue to be recognized should be limited to the amount of payment received in a given period less the amount expected to be refunded or credited to customers for sales returns made after payment.
GPO Fees (Net Revenue Presentation)
The Company sells its products to GPO members who transact directly with the Company at GPO agreed pricing. GPOs are funded by administrative fees that are paid by the Company. These fees are set as a percentage of the purchase volume, which is typically 3 % of sales made to the GPO members. In prior years, Company concluded that these fees should be accounted for consistent with purchases of services from other suppliers within General and Administrative expenses and not as a reduction in transaction price. Based on analysis performed as part of the Restatement discussed above, the Company determined that the administrative fees paid to GPOs should be presented as a reduction of revenues, as the benefit received by the Company in exchange for the GPO fees was not sufficiently separable from the GPO member’s purchase of the Company’s products.
Revenue-Related Adjustments
As discussed above under “Revenue Recognition,” based on the results of the Audit Committee Investigation and subsequent management review, the Company determined that all distributor and customer transactions should be transitioned to the cash collection method of accounting as of January 1, 2012.
The Company considered the accounting treatment for the related cost of sales when distributor revenue is recognized on a cash collection basis. Previously, cost of sales were recognized upon shipment of the Company’s products, which was consistent with the previous revenue recognition policy. However, the Company believes the matching of the recognition of costs of sales with the recognition of revenue is preferred, and the Company’s product is such that upon return the Company could resell the product if it is not damaged. Therefore, the Company determined that such costs should be deferred until revenue is recognized. The capitalized costs associated with delivered products are classified as deferred costs and reported within current assets, separately from inventory. The adjustment to Cost of sales in the consolidated statement of operations and to Other current assets in the consolidated balance sheet reflects this change.
The Company also considers the financial viability of its customers based on their creditworthiness to determine if collectability of amounts sufficient to recover the costs of the products shipped is reasonably assured. In cases where the Company has concluded that collectability is not reasonably assured, the condition in paragraph ASC 450-20-25-2(a) is met and a loss contingency should be accrued. The Company therefore estimates this loss in each period and records a reserve against its deferred cost balance and charges income for the estimated loss. The adjustment to Selling, general and administrative expenses in the consolidated statement of operations and to Other current assets in the consolidated balance sheet reflects this change.
F-27
Deposits in Transit
The Company reduced the amount of reported cash and decreased revenue for incorrectly reflected deposits in transit, due to the timing of certain cash collections. The adjustments to net (loss) income and net change in cash in the consolidated statement of cash flows reflect this change.
Other Adjustments
In addition to the adjustments recorded to address the Company’s errors in accounting for revenue recognition, deposits in transit and gross versus net revenue presentation, the Company has identified other errors that have been recorded in connection with the Restatement, as follows:
• | timing adjustments for prepaid expenses and expense accruals for research and development expenses related to clinical trials, employee compensation and other employee-related costs, legal costs and other accruals; |
• | adjustments to stock-based compensation, primarily to reflect share-based awards granted to consultants as non-employee instead of as employee awards; and |
• |
5. | Stability Biologics, LLC |
On January 13, 2016, the Company completed the acquisition of Stability Inc., a provider of human tissue products to surgeons, facilities, and distributors serving the surgical, spine, and orthopedic sectors of the healthcare industry. As a result of this transaction, the Company acquired all of the outstanding shares of Stability in exchange for $6.0 million cash, $3.3 million (or 441,009 shares) of the Company’s common stock, and assumed debt of $1.8 million. Additional one-time costs incurred in connection with the transaction totaled $1.1 million and were included within selling, general and administrative expenses on the consolidated statements of operations. Contingent consideration might have been payable based on a formula determined by sales less certain expenses for the years 2016 and 2017. The contingent consideration was valued at $17.5 million as of January 13, 2016 and is shown in the schedule below as fair value of earn-out. The contingent consideration was classified as a liability.
The Company evaluated the contingent consideration for accounting purposes under GAAP and determined that the classification of the contingent consideration is within the scope of ASC 480 “Distinguishing Liabilities from Equity” whereby a financial instrument, other than an outstanding share, that embodies a conditional obligation that the issuer may settle by issuing a variable number of its equity shares shall be classified as a liability if, at inception, the monetary value of the obligation is based solely or predominantly on variations in something other than the fair value of the issuer’s equity shares.
The actual purchase price was based on cash paid, the fair value of the Company’s stock on the date of the acquisition, and direct costs associated with the acquisition. The fair value of stock consideration was determined as set forth below:
Common Share Price at Closing on 1/13/2016 | $ | |||
Multiplied by: Number of Common Shares Transferred to the Sellers | ||||
Indicated Value of Equity Consideration (on a Freely Tradable Interest Basis) | $ | |||
Less: Marketability Discount @ 10% | [a] | ( | ) | |
Fair Value of Equity Consideration Transferred | $ | |||
[a] Shares transferred to the Sellers were restricted securities pursuant to Rule 144. As such, the Sellers were prevented from selling the shares until July 13, 2016. In addition, they were subject to contractual lockups which restricted sales for up to twelve months following the closing of the transaction. | ||||
F-28
The actual purchase price has been allocated as follows (in thousands):
Cash paid at closing | $ | |||
Working capital adjustment | ( | ) | ||
Common stock issued (441,009 shares) | ||||
Assumed debt | ||||
Fair value of earn-out | ||||
Total fair value of purchase price | $ | |||
Net assets acquired: | ||||
Debt-free working capital | $ | |||
Other long-term assets | ||||
Property, plant and equipment | ||||
Deferred tax liability | ( | ) | ||
Subtotal | ( | ) | ||
Intangible assets: | ||||
Customer relationships | ||||
Patents and know-how | ||||
Trade names and trademarks | ||||
Non compete agreements | ||||
Licenses and permits | ||||
Subtotal | ||||
Goodwill | ||||
Total assets purchased | $ | |||
Working capital and other assets were composed of the following (in thousands): | ||||
Working capital | ||||
Cash | $ | |||
Prepaid Expenses and other current assets | ||||
Accounts receivable | ||||
Federal and state taxes receivable | ||||
Inventory | ||||
Accounts payable and accrued expenses | ( | ) | ||
Debt-free working capital | $ | |||
Current portion of long-term debt | $ | ( | ) | |
Long-term debt | ( | ) | ||
Line of Credit | ( | ) | ||
Shareholder loan | ( | ) | ||
Assumed debt | $ | ( | ) | |
Net working capital | $ | |||
The acquisition was accounted for as a purchase business combination as defined by ASC 805, “Business Combinations.” The fair value of the contingent consideration is measured as a Level 3 instrument. The contingent consideration liability was recorded at fair value on the acquisition date. Increases or decreases in the fair value of contingent consideration can result from changes in anticipated revenue levels and changes in assumed discount periods and rates. As the fair value measured is based on significant
F-29
inputs that are not observable in the market, they are categorized as Level 3. The income valuation approach was applied in determining the fair value of the contingent consideration using a discounted cash flow valuation technique with significant unobservable inputs comprised of projected sales and certain expenses. The values assigned to intangible assets are subject to amortization. The intangible assets were assigned the following lives for amortization purposes:
Estimated useful life (in years) | ||
Intangible asset: | ||
Customer relationships | ||
Patents and know-how | ||
Trade name and trademarks | Indefinite | |
Non compete agreements | ||
Licenses and permits | ||
Goodwill consists of the excess of the purchase price paid over the identifiable net assets and liabilities acquired at fair value. Goodwill is attributable to the assembled workforce of Stability and the synergies expected to arise following the acquisition. Goodwill acquired is not deductible for tax purposes. Goodwill was determined using the residual method based on an independent appraisal of the assets and liabilities acquired in the transaction. Goodwill is tested for impairment on an annual basis as defined by ASC 350, “Intangibles - Goodwill and Other.”
Pursuant to the terms of the earn-out arrangement, the Company was obligated to pay, for each of the years ended December 31, 2016 and 2017, an amount equal to one times the gross profit margin from (a) the net sales of Stability products sold by Stability's or the Company's sales personnel and (b) the net sales of Company products sold by Stability's sales personnel; provided, however, if the amount of such net sales for either earn-out period was less than $12 million, the earn-out amount would decrease to 0.5 times the gross profit margin for such earn-out period.
The following unaudited pro forma summary financial information presents the consolidated results of operations for the Company as if the acquisition had occurred on January 1, 2016, as required by ASC 805, “Business Combinations.” The Company does not present the consolidated financial statements as of and for the year ended December 31, 2015 and did not provide pro forma financial information for the year then ended. The pro forma results are shown for illustrative purposes only and do not purport to be indicative of the results that would have been reported if the acquisition had occurred on the date indicated.
Unaudited pro forma information for the twelve months ended December 31, 2016 (in thousands) is as follows:
Year Ended December 31, | ||||
2016 | ||||
Revenue | $ | |||
Net income | $ | |||
Income per share, fully diluted | $ | |||
The 2016 supplemental pro forma earnings were adjusted to exclude $1.1 million of acquisition-related legal, audit and other costs, net of tax. The number of shares outstanding used in calculating the income per share for 2016 was adjusted to include 441,009 shares issued as part of the purchase price.
On September 30, 2017, the Company completed its divestiture of Stability pursuant to the Membership Interest Purchase Agreement by and among the Company, Stability LLC, each person that, as of January 13, 2016, was a stockholder of Stability Inc., a Florida corporation and a predecessor-in-interest to Stability LLC (“Stability, Inc.”), and Brian Martin, as stockholder representative. Under the agreement the Company was released from its obligations with respect to the contingent consideration.
F-30
A summary of the assets divested and consideration received follows (in thousands):
Year ended | ||||
December 31, 2017 | ||||
Assets divested | ||||
Trade receivables | $ | |||
Inventories | ||||
Prepaid expenses and other assets | ||||
Goodwill (a) | ||||
Intangible assets | ||||
Property and equipment, net | ||||
Total assets divested | ||||
Liabilities divested | ||||
Accounts payable and accrued liabilities | ||||
Total liabilities divested | ||||
Total net assets divested | $ | |||
Transaction costs | ||||
Consideration received | ||||
Non-trade receivable (b) | ||||
Note receivable (c) | ||||
Intangible assets (d) | ||||
Extinguishment of earn out liability (e) | ||||
Total consideration received | $ | |||
Loss on sale | $ | ( | ) | |
(a) In accordance with ASC 350-20-35-52 when a portion of a reporting unit is disposed of, goodwill associated with that business shall be included in the carrying amount of the business in determining the gain on disposal. In accordance with ASC 350-20-35-53, the amount of goodwill to be included in that carrying amount shall be based on the relative fair values of the business to be disposed of and the portion of the reporting unit that will be retained. Based on an estimated fair value of Stability LLC of $16.2 million representing a consideration received for the business compared to the fair value of business retained determined based on the market approach, approximately $0.2 million of the total goodwill of $20.2 million residing in the reporting unit was included in the carrying amount of the business sold.
(b) non-trade receivable represents a cash payment due within 60 days of closing.
(c) a promissory note issued by Stability LLC in the principal amount of $3.5 million in favor of the Company recognized at a discounted value of $3.2 million.
(d) a fair value of $0.5 million for the distributor agreements with Stability LLC and a fair value of $0.1 million for the non-compete agreements with the former stockholders of Stability Inc.
(e) a waiver by the former stockholders of Stability Inc. of all claims and rights to earn-out consideration, which was recorded as a liability at a fair value of $12.2 million immediately prior to the divestiture. The fair value of the earn-out liability was determined based on the income approach and includes the actual realized results of operations and expected future performance over the remaining earn-out period.
The total loss on the Stability Divestiture of $(0.5 ) million is comprised of a pretax book loss of $(1.0 ) million and an associated tax benefit of $0.5 million.
The earn-out arrangement was classified as a liability on the Stability LLC acquisition date of January 13, 2016 and remeasured at fair value each reporting period until the Stability LLC was divested on September 30, 2017. A decrease in fair value of $1.7 million and $3.6 million for the years ended December 31, 2016 and 2017, respectively, are included in selling, general and administration expenses on the Consolidated Statements of Operations.
F-31
6. Inventories
Inventories consisted of the following items as of (in thousands):
December 31, | |||||||
2018 | 2017 | ||||||
Raw materials | $ | $ | |||||
Work in process | |||||||
Finished goods | |||||||
Inventory, gross | |||||||
Reserve for obsolescence | ( | ) | ( | ) | |||
Inventory, net | $ | $ | |||||
7. Property and Equipment
Property and equipment consist of the following (in thousands):
December 31, | |||||||
2018 | 2017 | ||||||
Leasehold improvements | $ | $ | |||||
Laboratory and clean room equipment | |||||||
Furniture and equipment | |||||||
Construction in progress | |||||||
Property and equipment, gross | |||||||
Less accumulated depreciation and amortization | ( | ) | ( | ) | |||
Property and equipment, net | $ | $ | |||||
Included in property and equipment is $1.0 million in leasehold improvements paid for by the landlord of the Company’s main operating facility with a corresponding liability included in other liabilities in the consolidated financial statements, which is amortized over the term of the lease or its useful life, whichever is shorter.
Assets recorded under capital leases were as follows (in thousands):
December 31, | |||||||
2018 | 2017 | ||||||
Leasehold improvements | $ | $ | |||||
Less accumulated amortization | ( | ) | ( | ) | |||
Net leasehold improvements | $ | $ | |||||
Obligations under capitalized leases | $ | $ | |||||
Depreciation expense, included in selling, general and administrative expenses in the accompanying consolidated statements of operations, for the years ended December 31, 2018, 2017, and 2016 was $5.9 million, $4.1 million, and $3.3 million, respectively.
F-32
8. Goodwill and Intangible Assets
Intangible assets are summarized as follows (in thousands):
December 31, 2018 | December 31, 2017 | |||||||||||||||||||
Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | |||||||||||||||
Amortized intangible assets | ||||||||||||||||||||
Licenses | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||
Patents and know how | ( | ) | ( | ) | ||||||||||||||||
Customer and supplier relationships | ( | ) | ( | ) | ||||||||||||||||
Non-compete agreements | ( | ) | ( | ) | ||||||||||||||||
Total amortized intangible assets | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||
Unamortized intangible assets | ||||||||||||||||||||
Trade names and trademarks | $ | $ | $ | $ | ||||||||||||||||
Patents in process | ||||||||||||||||||||
Total intangible assets | $ | $ | $ | $ | ||||||||||||||||
Expected future amortization of intangible assets as of December 31, 2018, is as follows (in thousands):
Estimated | |||
Amortization | |||
Year ending December 31, | Expense | ||
2019 | $ | ||
2020 | |||
2021 | |||
2022 | |||
2023 | |||
Thereafter | |||
$ | |||
Goodwill is evaluated for impairment on an annual basis on September 30 and in interim periods when events or changes indicate the carrying value may not be recoverable. The Company operates under one reporting unit. For the years ended December 31, 2018 and 2017, the Company performed a quantitative analysis to determine if there was any impairment. As a result of this assessment, the Company determined that there was no impairment for the years ended December 31, 2018 and 2017.
The following represents the changes in the carrying amount of goodwill 2018 and 2017 (in thousands):
Goodwill | |||
Balance as of January 1, 2017 | $ | ||
Divestment of Stability | ( | ) | |
Balance as of December 31, 2017 | |||
Activity | — | ||
Balance as of December 31, 2018 | $ | ||
F-33
9. | Accrued Expenses |
Accrued expenses include the following at December 31, 2018 and December 31, 2017 (in thousands):
December 31, | |||||||
2018 | 2017 | ||||||
Legal costs | $ | $ | |||||
Settlement costs | |||||||
Pricing adjustment settlement with Veterans Affairs | |||||||
Estimated returns | |||||||
Accrued clinical trials | |||||||
External commissions | |||||||
Other | |||||||
Total | $ | $ | |||||
10. | Long-Term Debt |
Credit Facility
On October 12, 2015, the Company and its subsidiaries entered into a Credit Agreement (the “Credit Agreement”) with certain lenders and Bank of America, N.A., as administrative agent. The Credit Agreement established a senior secured revolving credit facility in favor of the Company with a maturity date of October 12, 2018 and an aggregate lender commitment of up to $50 million. In September 2017, the expiration date of the Credit Agreement was extended to October 12, 2019. The Credit Agreement also provided for an uncommitted incremental facility of up to $35 million, which could be exercised as one or more revolving commitment increases or new term loans, all subject to certain customary terms and conditions set forth in the Credit Agreement. The obligations of the Company under the Credit Agreement were guaranteed by the Company’s subsidiaries. The obligations of the loan parties under the Credit Agreement and the other credit documents were secured by liens on and security interests in substantially all of the assets of each of the loan parties and a pledge of the equity interests of each subsidiary owned by a loan party, subject to certain customary exclusions. Borrowings under the facility had an interest at LIBOR plus 1.5 % to 2.25 %. Fees paid in connection with the initiation of the credit facility totaled approximately $0.5 million. These deferred financing costs were being amortized to interest expense over the three-year life of the facility. The Credit Agreement contained customary representations, warranties, covenants, and events of default, including restrictions on certain payments of dividends by the Company.
On August 31, 2018, the lending parties’ terminated their commitments to make loans and issue letters of credit under the Credit Agreement due to the Company’s failure to timely file its periodic reports with the SEC. Accordingly, since then, the Company has not had the ability to borrow under the Credit Agreement. There were no outstanding borrowings or letters of credit issued under the Credit Agreement at the time of termination, and the Company never drew down any amounts under the credit facility during the entire term of the Credit Agreement. No termination penalties were paid as a result of the termination.
11. Net (Loss) Income Per Share
Basic net (loss) income per common share is computed using the weighted-average number of common shares outstanding during the period. Diluted net income per common share is computed using the weighted-average number of common and dilutive common equivalent shares from stock options, warrants and restricted stock using the treasury stock method.
F-34
The following table sets forth the computation of basic and diluted net income per share (in thousands except for share and per share data):
Year Ended December 31, | |||||||||||
2018 | 2017 | 2016 | |||||||||
Net (loss) income | $ | ( | ) | $ | $ | ||||||
Denominator for basic earnings (loss) per share - weighted average shares | |||||||||||
Effect of dilutive securities: Stock options, warrants, and restricted stock (a) | |||||||||||
Denominator for diluted earnings (loss) per share - weighted average shares adjusted for dilutive securities | |||||||||||
(Loss) income per common share - basic | $ | ( | ) | $ | $ | ||||||
(Loss) income per common share - diluted | $ | ( | ) | $ | $ | ||||||
(a)Securities that are included in the computation of the denominator above, utilizing the treasury stock method for the years ended December 31, 2018, 2017 and 2016 are as follows:
Effect of dilutive securities: | 2018 | 2017 | 2016 | ||||||||
Stock options | $ | $ | $ | ||||||||
Restricted stock awards | |||||||||||
$ | $ | $ | |||||||||
12. Equity
Stock Incentive Plans
The Company has two share-based compensation plans which provide for the granting of equity awards, including qualified incentive and non-qualified stock options, stock appreciation awards and restricted common stock awards: the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan (the “2016 Plan”), which was approved by shareholders on May 18, 2016 and the MiMedx Group, Inc. Assumed 2006 Stock Incentive Plan (the “Prior Incentive Plan”). During the years ended December 31, 2018, 2017 and 2016 the Company used and intends to use only the 2016 Plan to make future grants.
The 2016 Plan permits the grant of equity awards to the Company’s employees, directors, consultants and advisors for up to 5,000,000 shares of the Company’s common stock plus (i) the number of shares of the Company’s common stock that remain available for issuance under the Prior Incentive Plan, and (ii) the number of shares that are represented by outstanding awards that later become available because of the expiration or forfeiture of the award without the issuance of the underlying shares. The awards are subject to a vesting schedule as set forth in each individual agreement. Option awards are generally granted with an exercise price equal to the market price of the Company’s stock at the date of grant, and those option awards generally vest based on three years of continuous service and have 10 -year contractual terms. Restricted common stock awards generally vest over three years . Certain option and restricted stock awards provide for accelerated vesting if there is a change in control and upon death or disability.
F-35
A summary of stock option activity as of December 31, 2018, and changes during the year then ended are presented below:
Number of Shares | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value | |||||||||
Outstanding at January 1, 2018 | $ | |||||||||||
Granted | ||||||||||||
Exercised | ( | ) | ||||||||||
Unvested options forfeited | ||||||||||||
Vested options expired | ( | ) | ||||||||||
Outstanding at December 31, 2018 | $ | |||||||||||
Exercisable at December 31, 2018 | $ | $ | ||||||||||
The fair value of options vested during the years ended December 31, 2018, 2017 and 2016 were $0.1 million, $3.7 million, and $7.8 million, respectively. There were no options granted during the years ended December 31, 2018, 2017 and 2016 and no unrecognized compensation expense at December 31, 2018.
The fair value of the options granted in prior years was estimated on the date of grant using the Black-Scholes-Merton option-pricing model that uses assumptions for expected volatility, expected dividends, expected term, and the risk-free interest rate. Expected volatilities were based on historical volatility of peer companies and other factors estimated over the expected term of the options. The term of employee options granted was derived using the “simplified method” which computes expected term as the midpoint between the weighted average time to vesting and the contractual maturity of ten years . The simplified method was used due to the Company’s lack of sufficient historical data to provide a reasonable basis upon which to estimate the expected term due to the limited period of time its equity shares had been publicly traded. The term for non-employee options was generally based upon the contractual term of the option. The risk-free rate was based on the U.S. Treasury yield curve in effect at the time of grant for the period of the expected term or contractual term as described.
Restricted Stock Awards
Following is summary information for restricted stock awards for the year ended December 31, 2018. Shares vest over a one to three year period in equal annual increments and require continuous service. For the time-based awards with service vesting conditions, the Company recognizes stock-based compensation expense using the straight-line expense attribution method.
As of December 31, 2018, there was approximately $17.0 million of total unrecognized stock-based compensation related to non-vested restricted stock. That expense is expected to be recognized over a weighted-average period of 1.7 years, which approximates the remaining vesting period of these grants. All shares noted below as unvested are considered issued and outstanding at December 31, 2018.
Number of Shares | Weighted-Average Grant Date Fair Value | ||||||
Unvested at January 1, 2018 | $ | ||||||
Granted | |||||||
Vested | ( | ) | |||||
Forfeited | ( | ) | |||||
Unvested at December 31, 2018 | $ | ||||||
F-36
The total fair value of restricted stock awards vested during the years ended December 31, 2018, 2017, and 2016, was $17.9 million, $17.3 million, and $9.5 million, respectively.
For the years ended December 31, 2018, 2017, and 2016 the Company recognized share-based compensation as follows (in thousands):
Years Ended December 31, | |||||||||||
2018 | 2017 | 2016 | |||||||||
(Restated) | |||||||||||
Cost of sales | $ | $ | $ | ||||||||
Research and development | |||||||||||
Selling, general and administrative | |||||||||||
Total share-based compensation | $ | $ | $ | ||||||||
Income tax benefit | ( | ) | ( | ) | ( | ) | |||||
Total share-based compensation, net of tax benefit | $ | $ | $ | ||||||||
Treasury Stock
On May 8, 2014, the Board authorized the repurchase of up to $10 million of shares of Company common stock from time to time through December 31, 2014. The Board increased the authorization during the year ended December 31, 2015 to $60 million, during the year ended December 31, 2016 to $66 million, and during the year ended December 31, 2017 to $130 million. In January 2018 the Board announced that it had increased the total authorization to $140 million. The share repurchase program subsequently expired during the year ended December 31, 2018.
For the years ended December 31, 2018, 2017 and 2016 the Company purchased 507,600 , 5,635,077 , and 1,338,616 shares of its common stock, respectively, for an aggregate purchase price of approximately $7.6 million, $68.3 million and $10.4 million, respectively, exclusive of commissions of approximately $0.0 million, $0.2 million and $0.0 million, respectively.
13. Income Taxes
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
F-37
Significant components of the Company’s deferred tax assets and liabilities are as follows (in thousands):
December 31, | ||||||||
2018 | 2017 | |||||||
Deferred Tax Assets: | ||||||||
Accrued expenses | $ | $ | ||||||
Deferred revenue | ||||||||
Sales return and allowances | ||||||||
Accrued settlement costs | ||||||||
Research and development and other tax credits | ||||||||
Net operating loss | ||||||||
Share-based compensation | ||||||||
Other | ||||||||
Deferred Tax Liabilities: | ||||||||
Prepaid expenses | ( | ) | ( | ) | ||||
Property and equipment | ( | ) | ( | ) | ||||
Intangible assets | ( | ) | ( | ) | ||||
Net Deferred Tax Assets | ||||||||
Less: Valuation allowance | ( | ) | ( | ) | ||||
Net Deferred Tax Assets after Valuation Allowance | $ | $ | ||||||
In December 2017 the President signed into law what is commonly referred to as The Tax Cuts and Jobs Act (the “TCJA”). The TCJA changed existing United States tax law and included numerous provisions that affect the Company including a corporate rate reduction, changes to §162(m), changes to the deduction for meals and entertainment, and an increase in capital expensing. Specifically, the reduction of the U.S. federal tax rate from 35% to 21% effective on January 1, 2018 reduced the Company’s net deferred tax asset by $12.0 million with the benefit recognized in the 2018.
F-38
The reconciliation of the federal statutory income tax rate of 35 % (21% for the tax year ended December 31, 2018) to the effective rate is as follows:
December 31, | ||||||||
2018 | 2017 | 2016 | ||||||
(Restated) | ||||||||
Federal statutory rate | % | % | % | |||||
State taxes, net of federal benefit | % | % | % | |||||
Nondeductible compensation | ( | )% | % | % | ||||
Meals and entertainment | ( | )% | % | % | ||||
Keyman life insurance | ( | )% | % | % | ||||
Transaction costs | % | % | % | |||||
Inventory contribution deduction | % | ( | )% | ( | )% | |||
Domestic production activities deduction | % | ( | )% | ( | )% | |||
Fair value adjustment | % | ( | )% | ( | )% | |||
Share-based compensation | % | ( | )% | % | ||||
Tax credits | % | ( | )% | ( | )% | |||
Uncertain tax position | ( | )% | % | % | ||||
Write-off of net operating losses | ( | )% | % | % | ||||
Payable true-up | ( | )% | % | ( | )% | |||
Sale of Stability | % | ( | )% | % | ||||
Fixed asset true-up | % | % | % | |||||
Federal provision to return | % | % | % | |||||
Impact of federal rate change | % | % | % | |||||
Other | ( | )% | ( | )% | ( | )% | ||
Valuation allowance | ( | )% | ( | )% | % | |||
( | )% | ( | )% | % | ||||
Stock based compensation had a significant impact on the Company’s effective tax rate as of December 31, 2017 due to the Company’s adoption of ASU 2016-09. Additionally, on September 30, 2017, the Company completed the Stability Divestiture, which resulted in a significant reduction in the Company’s effective tax rate. See Note 5 for details regarding the transaction.
The domestic production activities deduction had a significant impact on the Company's effective tax rate as of December 31, 2016. As part of the TCJA previously mentioned, this deduction ceased to exist for tax years beginning on or after December 1, 2018. Additionally, federal and state tax credits, mostly related to the Company's research and development activities, had a significant impact on the Company's effective rate.
F-39
Current and deferred income tax expense (benefit) is as follows (in thousands):
December 31, | |||||||||||
2018 | 2017 | 2016 | |||||||||
Current: | (Restated) | ||||||||||
Federal | $ | $ | $ | ||||||||
State | |||||||||||
Total current | |||||||||||
Deferred: | |||||||||||
Federal | ( | ) | ( | ) | |||||||
State | ( | ) | ( | ) | |||||||
Total deferred | ( | ) | ( | ) | |||||||
Total expense (benefit) | $ | $ | ( | ) | $ | ||||||
A valuation allowance of $27.3 million and $0.6 million was recorded against our deferred tax asset balance as of December 31, 2018 and December 31, 2017, respectively. The decrease in the valuation allowance during 2017 is primarily related to the weight of available evidence which resulted in a determination to release the Company’s valuation allowance and recognize an income tax benefit as of December 31, 2017. The increase in valuation allowance during 2018 is primarily related to the weight of available evidence which resulted in the determination to increase the Company’s valuation allowance and recognize income tax expense as of December 31, 2018.
To the extent the Company determines that, based on the weight of available evidence, all or a portion of its valuation allowance is no longer necessary, the Company will recognize an income tax benefit in the period such determination is made for the reversal of the valuation allowance. If management determines that, based on the weight of available evidence, it is more-likely-than-not that all or a portion of the net deferred tax assets will not be realized, the Company may recognize income tax expense in the period such determination is made to increase the valuation allowance.
At December 31, 2018 and December 31, 2017 the Company had income tax net operating loss (“NOL”) carryforwards for federal and state purposes of $11.4 million and $15.6 million and $0.2 million and $21.9 million, respectively. A portion of the Company’s NOLs and tax credits are subject to annual limitations due to ownership change limitations provided by Internal Revenue Code Section 382. If not utilized, the federal and state tax NOL carryforwards will expire between 2028 and 2036. As of December 31, 2017, the Company has recorded a deferred tax asset for federal and state NOL carryforwards of $0.0 million and approximately $1.3 million, respectively. As of December 31, 2018, the Company has recorded a deferred tax asset for both federal and state NOL carryforwards of approximately $2.4 million and $0.9 million, respectively.
F-40
The following is a tabular reconciliation of the total amounts of unrecognized tax benefits (in thousands) included in other liabilities in the consolidated balance sheets:
2018 | 2017 | 2016 | |||||||||
Unrecognized tax benefits - January 1 | $ | $ | $ | ||||||||
Gross increases - tax positions in current period | |||||||||||
Gross increases - tax positions in prior period | |||||||||||
Unrecognized tax benefits - December 31 | $ | $ | $ | ||||||||
The Company recognizes accrued interest related to unrecognized tax benefits and penalties as income tax expense. Related to the unrecognized tax benefits noted above, the Company accrued $0.1 million of interest during 2018, and, in total, as of December 31, 2018 has recognized $0.1 million of interest. The Company accrued $0.1 million of interest during 2017, and, in total, as of December 31, 2017 had recognized $0.1 million of interest. During 2016 the Company did no t accrue any penalties or interest and, in total, as of December 31, 2016, had no t recognized any liability for penalties or interest.
Certain positions included in the tabular reconciliation above will be reduced as a result of the expiration of the applicable statutes of limitations within the 12 months following the issuance of the consolidated financial statements. The reserve would be reduced by approximately $0.4 million.
14. Supplemental Disclosure of Cash Flow and Non-Cash Investing and Financing Activities
Selected cash payments, receipts, and noncash activities are as follows (in thousands):
Years Ended December 31, | |||||||||||
2018 | 2017 | 2016 | |||||||||
Cash paid for interest | $ | $ | $ | ||||||||
Income taxes paid | |||||||||||
Purchases of equipment financed through accounts payable | |||||||||||
Deferred financing costs | |||||||||||
Additional paid-in capital related tax adjustments | ( | ) | |||||||||
Stock issuance of 441,009 shares in connection with acquisition of Stability | |||||||||||
Stock issuance of 17,539 and 43,344 shares in exchange for services performed in 2017 and 2016, respectively | |||||||||||
15. 401(k) Plan
The Company has a 401(k) plan (the “401(k) Plan”) covering all employees who have completed one month of service. Under the 401(k) Plan, participants could defer up to 90 % of their eligible wages to a maximum of $18,500 per year (annual limit for 2018). Employees age 50 or over in 2018 could make additional pre-tax contributions up to $6,000 . Annually, the Company could elect to match employee contributions up to 5 % of the employee’s eligible compensation. Additionally, the Company could elect to make a discretionary contribution to the 401(k) Plan. The Company did not provide matching contributions for the years ended December 31, 2017, and 2016. The matching contribution for the year ended December 31, 2018 was $1.9 million.
F-41
16. Commitments and Contingencies
Contractual Commitments
In addition to the capital leases noted under Note 7 “Property and Equipment,” the Company has entered into operating lease agreements for facility space and equipment. These leases expire over 4 to 4.5 years following December 31, 2018, and generally contain renewal options. The Company anticipates that most of these leases will be renewed or replaced upon expiration. The Company also has commitments for meeting space.
The estimated annual lease payment and meeting space commitments are as follows (in thousands):
Years Ended December 31, | |||
2019 | $ | ||
2020 | |||
2021 | |||
2022 | |||
2023 | |||
Thereafter | |||
$ | |||
Rent expense for the years ended December 31, 2018, 2017 and 2016, was approximately $1.5 million, $1.6 million and $1.8 million, respectively, and is allocated among cost of sales, research and development, and selling, general and administrative expenses.
Letters of Credit
Previously, as a condition of the leases for the Company’s facilities, we were obligated under standby letters of credit in the amount of approximately $0.1 million. The Company amended its lease during 2018 to eliminate this obligation.
Legal Proceedings
Shareholder Derivative Suits
On December 6, 2018, the United States District Court for the Northern District of Georgia entered an order consolidating three shareholder derivative actions (Evans v. Petit, et al. filed September 25, 2018, Georgalas v. Petit, et al. filed September 27, 2018, and Roloson v. Petit, et al., filed October 22, 2018) that had been filed in the Northern District of Georgia. On January 22, 2019, plaintiffs filed a Verified Consolidated Shareholder Derivative Complaint. The consolidated action sets forth claims of breach of fiduciary duty, corporate waste and unjust enrichment against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Joseph G. Bleser, J. Terry Dewberry, Charles R. Evans, Larry W. Papasan, Luis A. Aguilar, Bruce L. Hack, Charles E. Koob, Neil S. Yeston and Christopher M. Cashman. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Company filed a motion to stay on February 18, 2019, pending the completion of the investigation by the Company’s Special Litigation Committee. The Special Litigation Committee completed its investigation relating to this action and filed an executive summary of its findings with the Court on July 1, 2019. The parties held a mediation on February 11, 2020 and discussions continue.
On October 29, 2018, the City of Hialeah Employees Retirement System (“Hialeah”) filed a shareholder derivative complaint in the Circuit Court for the Second Judicial Circuit in and for Leon County, Florida (the “Florida Court”). The complaint alleges claims for breaches of fiduciary duty and unjust enrichment against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Joseph G. Bleser, J. Terry Dewberry, Charles R. Evans, Bruce L. Hack, Charles E. Koob, Larry W. Papasan, and Neil S. Yeston. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Company moved to stay the action on February 7, 2019, to allow the prior-filed consolidated derivative action in the Northern District of Georgia to be resolved first and to allow the Company’s Special Litigation Committee time to complete its investigation. The Company also filed a motion to dismiss on April 8, 2019. No hearing has been scheduled on the Company’s motion to stay or motion to dismiss. The plaintiff participated in the mediation that took place in connection with the prior-filed consolidated derivative action in the Northern District of Georgia.
F-42
On May 15, 2019, two individuals purporting to be shareholders of the Company filed a shareholder derivative complaint in the Superior Court for Cobb County, Georgia. (Nix and Demaio v. Evans, et al.) The complaint alleges claims for breaches of fiduciary duty, corporate waste and unjust enrichment against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Chris Cashman, Lou Roselli, Mark Diaz, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Court has ordered this matter stayed pending the resolution of the consolidated derivative suit pending in the Northern District of Georgia. The plaintiff participated in the mediation that took place in connection with the prior-filed consolidated derivative action in the Northern District of Georgia.
On August 12, 2019, John Murphy filed a shareholder derivative complaint in the United States District Court for the Southern District of Florida (Murphy v. Petit, et al.). The complaint alleged claims for breaches of fiduciary duty and unjust enrichment against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Company filed a motion to transfer this action to the Northern District of Georgia. Prior to resolution of that motion, the plaintiff voluntarily dismissed this action without prejudice. The plaintiff participated in the mediation that took place in connection with the prior-filed consolidated derivative action in the Northern District of Georgia.
On February 10, 2020, Charles Pike filed a shareholder derivative complaint in the United States District Court for the Southern District of Florida (Pike v. Petit, et al.). The complaint alleges claims for breaches of fiduciary duty against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. Similar to the prior-filed actions discussed above, the allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition.
On February 18, 2020, Bruce Cassamajor filed a shareholder derivative complaint in the United States District Court for the Northern District of Florida (Cassamajor v. Petit, et al.). The complaint alleges claims for breaches of fiduciary duty against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. Similar to the prior-filed actions discussed above, the allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. As of the date of the filing of this annual report on Form 10-K, MiMedx has not yet been served with the complaint.
Securities Class Action
On January 16, 2019, the United States District Court for the Northern District of Georgia entered an order consolidating two purported securities class actions (MacPhee v. MiMedx Group, Inc., et al. filed February 23, 2018 and Kline v. MiMedx Group, Inc., et al. filed February 26, 2018). The order also appointed Carpenters Pension Fund of Illinois as lead plaintiff. On May 1, 2019, the lead plaintiff filed a consolidated amended complaint, naming as defendants the Company, Michael J. Senken, Parker H. Petit, William C. Taylor, Christopher M. Cashman and Cherry Bekaert & Holland LLP. The amended complaint (the “Securities Class Action Complaint”) alleged violations of Section 10(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), Rule 10b-5 promulgated thereunder and Section 20(a) of the Exchange Act. It asserted a class period of March 7, 2013 through June 29, 2018. Following the filing of motions to dismiss by the various defendants, the lead plaintiff was granted leave to file an amended complaint. The lead plaintiff has until March 30, 2020 to file its amended complaint.
Annual Meeting Matters
On December 12, 2018, Hialeah filed an action against the Company in the Florida Court seeking to compel the Company to hold a shareholder meeting. Hialeah requested that the court enter an order compelling two annual meetings (for 2018 and 2019) to be held on the same date, when six of the Company’s ten directors would be elected. The Company answered the complaint on January 1, 2019, and Hialeah moved for summary judgment on January 30, 2019. After a hearing held on April 3, 2019, the Florida Court ordered a meeting to take place on June 17, 2019, where a single class of directors would be elected, and memorialized that order in a final declaratory judgment on April 26, 2019. The annual meeting took place on June 17, 2019. The action was dismissed on November 6, 2019.
F-43
On April 18, 2019, Hialeah filed an action against the Company in the Florida Court asking the Florida Court to enter a final declaratory judgment for the election of Class III directors at either the June 17, 2019 meeting or within 30 days of the June 17, 2019 meeting. Hialeah filed a motion for summary judgment and declaratory judgment on May 13, 2019. The Company filed a motion to dismiss the action on May 23, 2019. On August 5, 2019, the parties entered into a stipulation under which, among other things, MiMedx agreed to work in good faith to complete its 2018 audited financial statements by December 16, 2019, hold an annual meeting for the election of Class III directors by January 15, 2020, and hold an annual meeting for the election of Class I directors by June 15, 2020. The parties settled this matter, and the action was dismissed on November 6, 2019.
Investigations
SEC Investigation
On April 4, 2017, the Company received a subpoena from the SEC requesting information related to, among other things, the Company’s recognition of revenue, practices with certain distributors and customers, its internal accounting controls and certain employment actions. The Company cooperated with the SEC in its investigation (the “SEC Investigation”). In November 2019, the SEC brought claims against the Company and the Company’s former officers Parker H. Petit, Michael J. Senken, and William C. Taylor. The SEC alleged that from 2013 to 2017, the Company prematurely recognized revenue from sales to its distributors and exaggerated its revenue growth. The SEC’s complaint also alleged that the Company improperly recognized revenue because its former CEO and COO entered into undisclosed side arrangements with certain distributors. These side arrangements allowed distributors to return product to the Company or conditioned distributors’ payment obligations on sales to end users. The SEC complaint further alleged that the Company’s former CEO, COO, and CFO allegedly covered up their scheme for years, including after the Company’s former controller raised concerns about the Company’s accounting for specific distributor transactions. The SEC also alleged that the Company’s former CEO, COO, and CFO all misled the Company’s outside auditors, members of the Company’s Audit Committee, and outside lawyers who inquired about these transactions. The SEC brought claims against the Company and its former CEO, COO, and CFO for violating the antifraud, reporting, books and records, and internal controls provisions of the federal securities laws. The SEC also brought claims against the Company’s former CEO, COO, and CFO for lying to the Company’s outside auditors.
Without admitting or denying the SEC’s allegations, the Company settled with the SEC by consenting to the entry of a final judgment that permanently restrains and enjoins the Company from violating certain provisions of the federal securities laws. As part of the resolution, the Company paid a civil penalty of $1.5 million. The settlement concluded, as to the Company, the matters alleged by the SEC in its complaint. The SEC’s litigation continues against the Company’s former officers.
United States Attorney’s Office for the Southern District of New York (“USAO-SDNY”) Investigation
The USAO-SDNY conducted an investigation into topics similar to those at issue in the SEC Investigation. The USAO-SDNY requested that the Company provide it with copies of all information the Company furnished to the SEC and made additional requests for information. The USAO-SDNY conducted interviews of various individuals, including employees and former employees of the Company. The USAO-SDNY issued indictments in November 2019 against former executives, Messrs. Petit and Taylor for securities fraud and conspiracy to commit securities fraud, to make false filings with the SEC, and improperly influence the conduct of audits relating to alleged misconduct that resulted in inflated revenue figures for fiscal 2015. The Company is cooperating with the USAO-SDNY.
Department of Veterans’ Affairs Office of Inspector General (“VA-OIG”) and Civil Division of the Department of Justice (“DOJ-Civil”) Subpoenas and/or Investigations
VA-OIG has issued subpoenas to the Company seeking, among other things, information concerning the Company’s financial relationships with VA clinicians. DOJ-Civil requested similar information. The Company has cooperated fully and produced responsive information to VA-OIG and DOJ-Civil. VA-OIG has periodically requested additional documents and information regarding payments to individual VA clinicians, The Company has continued to cooperate and responded to these requests.
As part of its cooperation, the Company provided documents in response to subpoenas concerning its relationship with three now former VA employees in South Carolina, who were ultimately indicted in May 2018. Among other things, the indictment referenced speaker fees paid by the Company to the former VA employees and other interactions between now former Company employees and the former VA employees. In January 2019, prosecution was deferred for 18 months to allow the three former VA employees to enter and complete a Pretrial Diversion Program, the completion of which would result in the dismissal of the indictment. Two of the former VA employees have completed the program early and the indictment has been dismissed with respect to them. To date, no actions have been taken against the Company with respect to this matter.
F-44
United States Attorney’s Office for the Southern District of Georgia (“USAO-SDGA”) Grand Jury Investigation
The USAO-SDGA is investigating the relationships of a Department of Defense physician with various vendors, including the Company. On August 20, 2018, a Company employee testified before the grand jury. The USAO-SDGA has not taken further action since this testimony was provided. We are not aware of the status of this matter.
Qui Tam Actions
On January 19, 2017, a former employee of the Company, filed a qui tam False Claims Act complaint in the United States District Court for the District of South Carolina (United States of America, ex rel. Jon Vitale v. MiMedx Group, Inc.) alleging that the Company’s donations to the patient assistance program, Patient Access Network Foundation, violated the Anti-Kickback Statute and resulted in submission of false claims to the government. The government declined to intervene and the complaint was unsealed on August 10, 2018. The Company filed a motion to dismiss on October 1, 2018. The Company’s motion to dismiss was granted in part and denied in part on May 15, 2019. The case is currently in discovery.
On January 20, 2017, two former employees of the Company, filed a qui tam False Claims Act complaint in the United States District Court for the District of Minnesota (Kruchoski et. al. v. MiMedx Group, Inc.). An amended complaint was filed on January 27, 2017. The operative complaint alleges that the Company failed to provide truthful, complete and accurate information about the pricing offered to commercial customers in connection with the Company’s FSS contract. On May 7, 2019, the DOJ declined to intervene, and the case was unsealed. At December 31, 2018 and 2017 the Company had accrued $6.9 million and $5.6 million in connection with expected pricing adjustments. The parties have reached a settlement in principle and are working to finalize the same.
Former Employee Litigation
On December 13, 2016, the Company filed a complaint in the Circuit Court for Palm Beach County, Florida (MiMedx Group, Inc. v. Academy Medical, LLC et. al.) alleging several claims against a former employee, primarily based on his alleged competitive activities while he was employed by the Company (breach of contract, breach of fiduciary duty and breach of duty of loyalty). The former employee countersued for monetary damages and injunctive relief, alleging whistleblower retaliation in violation of the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Dodd-Frank Act”), unlawful discharge and defamation. The Court dismissed the Dodd-Frank Act whistleblower counterclaim, and in response, the former employee filed an amended complaint on September 11, 2018, adding allegations of post-termination retaliation in violation of the Dodd-Frank Act. At December 31, 2018, the Company reserved $0.3 million for this case. The court dismissed the former employee’s retaliation counterclaim on January 24, 2019. After this dismissal, only the former employee’s claims of unlawful discharge and defamation remained pending. The parties resolved this matter and the case was dismissed on September 5, 2019.
On December 29, 2016, the Company filed a complaint in the United States District Court for the Northern District of Illinois (MiMedx Group, Inc. v. Michael Fox) alleging several claims against a former employee of the Company, primarily based on his alleged competitive activities while he was employed by the Company (breach of contract, breach of fiduciary duty and breach of duty of loyalty). The former employee countersued the Company for monetary damages and injunctive relief, alleging improper wage rate adjustment, interference with the former employee’s job after his termination from the Company and retaliation. The parties resolved this matter and the case was dismissed on November 4, 2019.
On July 13, 2018, a former employee filed a complaint against the Company in the United States District Court for the Northern District of Texas (Jennifer R. Scott v. MiMedx Group, Inc.), alleging sex discrimination and retaliation. The parties resolved this matter, and the case was dismissed on November 6, 2019.
On November 19, 2018, the Company’s former Chief Financial Officer filed a complaint in the Superior Court for Cobb County, Georgia (Michael J. Senken v. MiMedx Group, Inc.) in which he claims that the Company has breached its obligations under the Company’s charter and bylaws to advance to him, and indemnify him for, his legal fees and costs that he incurred in connection with certain Company internal investigations and litigation. The Company filed its answer denying the plaintiff’s claims on April 19, 2019. To date, no deadlines have been established by the court.
On January 21, 2019, a former employee filed a complaint in the Fifth Judicial Circuit, Richland County, South Carolina, (Jon Michael Vitale v. MiMedx Group, Inc. et. al.) against the Company alleging retaliation, defamation and unjust enrichment and seeking monetary damages. The former employee claims he was retaliated against after raising concerns related to insurance fraud and later defamed by comments concerning the indictments of three South Carolina VA employees. On February 19, 2019, the case was removed to the U.S. District Court for the District of South Carolina. The Company filed a motion to dismiss on April 8, 2019 which was denied by the Court. This case is currently in discovery.
F-45
Defamation Claims
On June 4, 2018, Sparrow Fund Management, LP (“Sparrow”) filed a complaint against the Company and Mr. Petit, including claims for defamation and civil conspiracy in the United States District Court for the Southern District of New York (Sparrow Fund Management, L.P. v. MiMedx Group, Inc. et. al.). The complaint seeks monetary damages and injunctive relief and alleges the defendants commenced a campaign to publicly discredit Sparrow by falsely claiming it was a short seller who engaged in illegal and criminal behavior by spreading false information in an attempt to manipulate the price of the Company common stock. On March 31, 2019, a judge granted defendants’ motions to dismiss in full, but allowed Sparrow the ability to file an amended complaint. The Magistrate has recommended Sparrow’s motion for leave to amend be granted in part and denied in part. Both parties have filed objections to the Magistrate’s recommendation.
On June 17, 2019, the principals of Viceroy Research (“Viceroy”), filed suit in the Circuit Court for the Seventeenth Judicial Circuit in Broward County, Florida (Fraser John Perring et. al. v. MiMedx Group, Inc. et. al.) against the Company and Mr. Petit, alleging defamation and malicious prosecution based on the defendants’ alleged campaign to publicly discredit Viceroy and the lawsuit the Company previously filed against the plaintiffs, but which the Company subsequently dismissed without prejudice. On November 1, 2019, the Court granted Mr. Petit’s motion to dismiss on jurisdictional grounds, denied the Company’s motion to dismiss, and granted plaintiffs leave to file an amended complaint to address the deficiencies in its claims against Mr. Petit, which they did on November 21, 2019. The Company filed its answer on December 20, 2019.
Intellectual Property Litigation
The Bone Bank Action
On May 16, 2014, the Company filed a patent infringement lawsuit against Transplant Technology, Inc. d/b/a Bone Bank Allografts (“Bone Bank”) and Texas Human Biologics, Ltd. (“Biologics”) in the United States District Court for the Western District of Texas (MiMedx Group, Inc. v. Tissue Transplant Technology, LTD. d/b/a Bone Bank Allografts et. al.). The Company has asserted that Bone Bank and Biologics infringed certain of the Company’s patents through the manufacturing and sale of their placental-derived tissue graft products, and the Company is seeking permanent injunctive relief and unspecified damages. On July 10, 2014, Bone Bank and Biologics filed an answer to the complaint, denying the allegations in the complaint, and filed counterclaims seeking declaratory judgments of non-infringement and invalidity. The matter settled in 2019 prior to trial, and the case was dismissed on April 4, 2019.
The NuTech Action
On March 2, 2015, the Company filed a patent infringement lawsuit against NuTech Medical, Inc. (“NuTech”) and DCI Donor Services, Inc. (“DCI”) in the United States District Court for the Northern District of Alabama (MiMedx Group, Inc. v. NuTech Medical, Inc. et. al.). The Company has alleged that NuTech and DCI infringed and continue to infringe the Company’s patents through the manufacture, use, sale and/or offering of their tissue graft product. The Company has also asserted that NuTech knowingly and willfully made false and misleading representations about its products to customers and prospective customers. The Company is seeking permanent injunctive relief and unspecified damages. The case was stayed pending the restatement of the Company’s financial statements.
The Osiris Action
On February 20, 2019, Osiris Therapeutics, Inc. (“Osiris”) refiled its trade secret and breach of contract action against the Company (which had been dismissed in a different forum) in the United States District Court for the Northern District of Georgia (Osiris Therapeutics, Inc. v. MiMedx Group, Inc.). Osiris has alleged that the Company acquired Stability Biologics, LLC, a former distributor of Osiris, in order to illegally obtain trade secrets. On February 24, 2020, the Court issued an order granting in part and denying in party MiMedx’s motion to dismiss. The Court dismissed Osiris’s claims for tortious interference, conspiracy to breach contract, unfair competition, and conspiracy to commit unfair competition. The Court denied MiMedx’s motion to dismiss with respect to the claim for breach of the contract between Osiris and Stability Biologics, finding that there is a question as to whether Osiris can maintain such a claim by piercing the corporate veil between MiMedx and its former subsidiary. If Osiris cannot pierce the corporate veil, the claim against MiMedx fails; if Osiris can pierce the corporate veil, the breach of contract claim must be brought in an arbitration proceeding. MiMedx did not move to dismiss Osiris’s claims for misappropriation of trade secrets and conspiracy to misappropriate trade secrets. MiMedx plans to defend against all remaining claims.
F-46
Other Matters
Under the Florida Business Corporation Act and agreements with its current and former officers and directors, the Company is obligated to indemnify its current and former officers and directors who are made party to a proceeding, including a proceeding brought by or in the right of the corporation, with certain exceptions, and to advance expenses to defend such matters. The Company has already borne substantial costs to satisfy these indemnification and expense advance obligations and expects to continue to do so in the future.
In addition to the matters described above, the Company is a party to a variety of other legal matters that arise in the normal course of the Company’s business, none of which is deemed to be individually material at this time. Due to the inherent uncertainty of litigation, there can be no assurance that the resolution of any particular claim or proceeding would not have a material adverse effect on the Company’s business, results of operations, financial position or liquidity.
17. | Revenue Data by Customer Type |
MiMedx has two primary distribution channels: (1) direct to customers (healthcare professionals and/or facilities) (“Direct Customers”); and (2) sales through distributors (“Distributors”). For purposes of the required disclosure under ASC 606-10-50-5, the Company groups its customers into these two groups. This grouping by customer types does not constitute a basis for resource allocation but is information intended to provide the reader with ability to better understand how the nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factors applicable to each customer type. These groupings also do not meet the criteria under ASC 280-10-50-1 to qualify as separate operating segments. The Company did not have significant foreign operations or a single external customer from which 10% or more of revenues were derived during the years ended December 31, 2018, 2017 and 2016.
Below is a summary of net sales by each customer type (in thousands):
Years Ended December 31, | |||||||||||
2018 | 2017 | 2016 | |||||||||
(Restated) | |||||||||||
Direct Customers | $ | $ | $ | ||||||||
Distributors | |||||||||||
Other(1) | |||||||||||
Total | $ | $ | $ | ||||||||
18. | Related Party Transactions |
The Company employs Simon Ryan, the brother-in-law of Alexandra O. Haden, the Company’s former General Counsel and Secretary, as a sales representative. Ms. Haden resigned from her position as General Counsel and Secretary in August 2019. In 2017, the Company paid Mr. Ryan total compensation of $0.2 million, consisting of a salary of $0.1 million and sales commissions, equity and other compensation of $0.1 million. In 2018, the Company paid Mr. Ryan total compensation of $0.2 million, consisting of a salary of $0.1 million and sales commissions, equity and other compensation of $0.1 million.
The Company has employed Thomas Koob as its Chief Scientific Officer (a non-executive officer) since 2006. Thomas Koob is the brother of a director, Charles Koob. Subsequent to the Company’s employment of Thomas Koob, Charles Koob was appointed as a director of the Company in March 2008. In 2017, the Company paid Thomas Koob a salary of $0.2 million and provided equity, incentive compensation and other compensation of $0.2 million. In 2018, the Company paid Thomas Koob a salary of $0.2 million and provided equity, incentive compensation and other compensation of $0.3 million.
The Company recorded sales of $2.3 million, $3.5 million, and $2.7 million for the years ended December 31, 2018, 2017, and 2016, respectively, to a distributor in which the family of the former CEO, at that time, had a financial interest. Product pricing, payment terms, rights of return, and other conditions of sale to this distributor were similar to those available to distributors of the Company.
F-47
19. | Restructuring |
Set forth below are disclosures relating to restructuring initiatives that resulted in material expenses or cash expenditures during the year ended December 31, 2018, and had material restructuring liabilities at December 31, 2018. Employee retention and certain other employee benefit-related costs related to the Company’s restructuring are expensed ratably over an agreed-upon service period. One-time employee separation and related employee benefit costs are generally expensed as incurred.
In December 2018, the Company announced a reduction of the Company’s workforce by approximately 240 full-time employees, or 24 % of its total workforce, of which approximately half were sales personnel as part of the plans to implement a broad-based organizational realignment, cost reduction and efficiency program to better ensure the Company’s cost structure was appropriate given its revenue expectations.
As a result of the December 2018 broad-based organizational realignment, cost reduction and efficiency program, the Company incurred pre-tax charges of $6.1 million during the year ended December 31, 2018. The 2018 charges related to employee retention and other one-time employee separation benefit-related costs. These charges are included in the cost of sales, research and development, and selling, general and administrative expenses in the consolidated statements of operations.
The liability related to the December 2018 restructuring initiative is included in Accrued compensation in the consolidated balance sheets. Changes to this liability during the year ended December 31, 2018 were as follows (in thousands):
Liability balance as of January 1, 2018 | $ | ||
Expenses | |||
Cash distributions | ( | ) | |
Liability balance as of December 31, 2018 | $ | ||
20. | Quarterly Financial Data (Unaudited) (in thousands except per share data) |
Restatement of the 2017 Unaudited Quarterly Financial Statements
As previously described in the Company’s Current Reports on Form 8-K filed on February 20, 2018 and on June 6, 2018, the Audit Committee, in consultation with outside advisors and management, concluded that the Company’s interim financial statements previously issued for the quarterly and year-to-date periods ended March 31, 2017, June 30, 2017 and September 30, 2017 should not be relied upon due to errors identified in such financial statements related to the timing of revenue recognition, gross vs. net presentation of administrative fees paid to GPOs, the related impacts on cost of goods sold and bad debt expense due to changes in revenue recognition practices, the timing of certain general and administrative expenses and cash collections, impacts of any losses associated with contingency exposures, share-based compensation expense and the related income tax impacts, as well disclosures and internal controls.
The corrections contained in the below restated unaudited quarterly financial statement information were prepared following the Audit Committee Investigation as described in the Note 4 “Restatement of the Consolidated Financial Statements,” and a review by management of other accounting matters not specifically addressed by the Audit Committee Investigation. The unaudited quarterly information for the quarter ended December 31, 2018 is presented below for the first time.
F-48
The following tables summarize the impacts of the restatement on our previously reported condensed consolidated statements of operations included in our Quarterly Reports on Form 10-Q for each respective period. Information for the first, second, and third quarters of 2017 are restated.
First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||||||
Net sales | 2018 | $ | $ | $ | $ | |||||||||||
2017 | ||||||||||||||||
Gross profit | 2018 | $ | $ | $ | $ | |||||||||||
2017 | ||||||||||||||||
Income tax (provision) benefit | 2018 | $ | $ | $ | ( | ) | $ | ( | ) | |||||||
2017 | ( | ) | ( | ) | ( | ) | ||||||||||
Net income (loss) | 2018 | $ | $ | $ | ( | ) | $ | ( | ) | |||||||
2017 | ||||||||||||||||
Net income (loss) per common share - basic | 2018 | $ | $ | $ | $ | ( | ) | |||||||||
2017 | ||||||||||||||||
Net income (loss) per common share - diluted | 2018 | $ | $ | $ | $ | ( | ) | |||||||||
2017 | ||||||||||||||||
The Company’s previously reported selected quarterly financial data for the First, Second, and Third Quarter of 2017 is as follows:
First Quarter | Second Quarter | Third Quarter | ||||||||||
Net sales | 2017 | $ | $ | $ | ||||||||
Gross profit | 2017 | $ | $ | $ | ||||||||
Income tax provision (benefit) | 2017 | $ | ( | ) | $ | $ | ||||||
Net income | 2017 | $ | $ | $ | ||||||||
Net income per share - basic | 2017 | $ | $ | $ | ||||||||
Net income per share - diluted | 2017 | $ | $ | $ | ||||||||
F-49
21. | Subsequent Events |
Term Loan
On June 10, 2019, the Company entered into a Term Loan Agreement (the “Term Loan Agreement”) Blue Torch Finance LLC, as administrative agent and collateral agent, to borrow funds with a face value of $75.0 million (the “Term Loan”), of which the full amount has been borrowed and funded. The proceeds from the Term Loan have been and will continue to be used (i) for working capital and general corporate purposes and (ii) to pay transaction fees, costs and expenses incurred in connection with the Term Loan and the related transactions. The Term Loan matures on June 20, 2022 and is repayable in quarterly installments of $0.9 million; the balance is due on June 20, 2022. The Term Loan was issued net of the original issue discount of $2.3 million. The Company also incurred $6.7 million of deferred financing costs.
The interest rate applicable to any borrowings under the Term Loan accrues at a rate equal to LIBOR plus a margin of 8.00 % per annum or (if LIBOR is not available) a prime rate plus a margin of 7.00 % per annum. The Term Loan had an interest rate equal to 10.46 % at the time the Loan Agreement was executed.
The Term Loan Agreement contains financial covenants requiring the Company, on a consolidated basis, to maintain the following:
• | Maximum Total Leverage Ratio, defined as funded debt divided by consolidated adjusted EBITDA, of not more than |
• | Minimum Liquidity, defined as unrestricted cash and cash equivalents, of less than $ |
The Term Loan Agreement also specifies that any prepayment of the loan, voluntary or mandatory, as defined in the Term Loan Agreement, subjects MiMedx to a prepayment penalty as of the date of the prepayment with respect to the Term Loan of:
• | During the period from June 10, 2019 through June 10, 2020, an amount equal to |
• | During the period from June 11, 2020 through June 10, 2021, an amount equal to |
Principal prepayments after June 10, 2021 are not subject to a prepayment penalty.
The Term Loan Agreement also includes events of default customary for facilities of this type, and upon the occurrence of such events of default, subject to customary cure rights, all outstanding loans under the Term Loan Agreement may be accelerated and/or the lenders’ commitments terminated.
Separation and Transition Services Agreement of Edward J. Borkowski
On November 18, 2019, the Company entered into a Separation and Transition Services Agreement (“Separation Agreement”) with Edward J. Borkowski, under which Mr. Borkowski resigned as Executive Vice President and Interim Chief Financial Officer of the Company, as well as from any and all officer, director or other positions that he held with the Company and its affiliates, effective November 15, 2019. Pursuant to the Separation Agreement, Mr. Borkowski agreed to perform the duties of the Interim Chief Financial Officer with respect the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2018 (the “2018 Form 10-K”) and assist with the transition of his duties as described in the Separation Agreement from November 15, 2019 through the earlier of the first business day following the Company’s filing of its 2018 Form 10-K with the SEC or December 31, 2019 (the “Transition Period”). Commencing on the date the Transition Period ends and until March 31, 2020, Mr. Borkowski agreed to provide services as may be requested by the Company with respect to matters related to the 2018 Form 10-K and the Company’s Annual Report on Form 10-K for the fiscal year ending December 31, 2019.
The Separation Agreement provides that the Company will pay Mr. Borkowski a special payment in installments as follows: (i)$1.7 million to be paid within seven business days following November 15, 2019, (ii) $1.8 million to be paid within seven business days following the filing of the 2018 Form 10-K with the SEC; and (iii) after March 31, 2020, $0.5 million to be paid within seven business days following the execution and delivery of a supplemental release by Mr. Borkowski.
See Note 16 “Commitments and Contingencies” for discussion on legal proceedings.
F-50
Schedule II Valuation and Qualifying Accounts
MIMEDX GROUP, INC. AND SUBSIDIARIES SCHEDULE II VALUATION AND QUALIFYING ACCOUNTS | ||||||||||||
Years ended December 31, 2018, 2017 and 2016 (in thousands) | ||||||||||||
Balance at Beginning of Year | Additions charged to Expense or Revenue | Deductions and write-offs | Balance at End of Year | |||||||||
For the Year ended December 31, 2018 | ||||||||||||
Allowance for product returns | ||||||||||||
Allowance for obsolescence | ( | ) | ||||||||||
For the Year ended December 31, 2017 | ||||||||||||
Allowance for product returns | ( | ) | ||||||||||
Allowance for obsolescence | ( | ) | ||||||||||
For the Year ended December 31, 2016 | ||||||||||||
Allowance for product returns (restated) | ||||||||||||
Allowance for obsolescence | ( | ) | ||||||||||
F-51
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
The independent registered public accounting firm of the Company for the fiscal year ended December 31, 2016 was Cherry Bekaert LLP (“CB”). The Audit Committee conducted a competitive selection process to determine the Company’s independent registered public accounting firm for the fiscal year ended December 31, 2017. The Audit Committee invited several public accounting firms to participate in this process. As a result of this process, the Audit Committee approved the appointment of Ernst & Young LLP (“EY”) as the Company’s independent registered public accounting firm for the fiscal year ended December 31, 2017, effective August 4, 2017. This action effectively dismissed CB as the Company’s independent registered public accounting firm as of August 4, 2017.
In connection with the audits of the Company’s consolidated financial statements for the fiscal years ended December 31, 2015 and 2016, and in the subsequent interim period through August 4, 2017, there were no disagreements with CB on any matters of accounting principles or practices, financial statement disclosure or auditing scope and procedures which, if not resolved to the satisfaction of CB, would have caused CB to make reference to the matter in its report. Except as provided in the succeeding sentence, there were no reportable events (as that term is described in Item 304(a)(1)(v) of Regulation S-K) during the two fiscal years ended December 31, 2015 and 2016, or in the subsequent period through August 4, 2017. The reports of CB on the Company’s consolidated financial statements for the fiscal years ended December 31, 2015 and 2016 did not contain an adverse opinion or disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles, except that CB’s report on internal controls over financial reporting expressed its opinion that the Company had not maintained effective internal control over financial reporting as of December 31, 2016 because of the effect of a material weakness identified by Company management in the design of the Company’s controls over tax accounting related to not having adequate supervision and review of certain technical tax accounting performed by a third-party tax specialist in 2016.
On December 4, 2018, EY informed the Audit Committee that EY was resigning from the engagement to audit the Company’s consolidated financial statements for the years ended December 31, 2017 and 2018, effective immediately. As noted above, EY was engaged on August 4, 2017 to audit the Company’s consolidated financial statements as of and for the year ended December 31, 2017. The 2017 audit was still in process at the time of EY’s resignation, and EY did not issue any audit reports on the Company’s consolidated financial statements for this or any other period. During the engagement period, EY had one “disagreement,” as that term is defined in Item 304(a)(1)(iv) of Regulation S-K, with certain members of the Company’s prior senior management who were subsequently separated from the Company, which separations were later determined to be “for cause” as disclosed in a Form 8-K filed by the Company on September 20, 2018, regarding revenue recognition under certain distributor contracts. However, this disagreement was not the cause of EY’s resignation and was in any event resolved in June 2018 when the Audit Committee, after discussing the disagreement with EY and based on interim findings of its Investigation, concluded that the Company’s previously issued consolidated financial statements could no longer be relied upon, as disclosed in a Form 8-K filed by the Company on June 7, 2018. This disagreement was only between EY and the separated officers.
Except as noted above, during the period from August 4, 2017 through December 4, 2018, there were no disagreements with EY on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedures which, if not resolved to the satisfaction of EY, would have caused EY to make reference to the subject matter of the disagreements in connection with its audit report. During this same period, there were the following “reportable events,” as that term is defined in Item 304(a)(1)(v) of Regulation S-K:
• | EY advised the Company that the internal controls necessary for the Company to develop reliable financial statements did not exist; |
• | Although EY could accept representations from the Company’s Interim CEO and Interim CFO based on their knowledge, EY advised the Company that EY was unable to rely on representations from them because, as of the date of the resignation, the current Company’s CEO and Interim CFO, in turn, would have needed to rely on representations from certain legacy management personnel still in positions that could affect what is reflected in the Company’s books and records. At the time of EY’s resignation, the Audit Committee Investigation was still ongoing; |
• | EY advised the Company of the need to significantly expand the scope of the Audit Committee Investigation, due to material allegations of inappropriate financial reporting, material allegations of noncompliance with laws and regulations, the findings to date from the Audit Committee Investigation into these allegations, and the lack of internal controls necessary for the Company to develop reliable financial statements. EY had not completed the necessary work in connection with this expanded audit scope at the time of its resignation; and |
• | EY advised the Company that information had come to EY’s attention that EY had concluded materially impacts the reliability of previously issued financial statements, and the issues raised by this information had not been resolved to EY’s satisfaction prior to its resignation. |
79
On May 23, 2019, the Company announced that the Audit Committee Investigation was complete and, as a result of the Investigation, the Audit Committee, with the concurrence of management, concluded that the Company’s previously issued consolidated financial statements and financial information relating to the Non-Reliance Periods would need to be restated. For more information, refer to the disclosure in the Explanatory Note to this Form 10-K, which is incorporated by reference in this Item.
On May 24, 2019, the Audit Committee approved the engagement of and executed an agreement with BDO USA, LLP as the Company’s new independent registered public accounting firm for the fiscal years ended December 31, 2018, 2017, and 2016.
This Form 10-K contains the Company’s audited consolidated statements of operations, stockholders’ equity and cash flows for the years ended December 31, 2018 and 2017, which have not previously been filed, and for the year ended December 31, 2016, which have been restated from the consolidated financial statements previously filed in its Annual Report on Form 10-K for the year ended December 31, 2016. This Form 10-K also includes the Company’s audited consolidated balance sheets as of December 31, 2018 and 2017.
Item 9A. Controls and Procedures
Background
On June 7, 2018, the Company announced that its previously issued consolidated financial statements and financial information relating to each of the fiscal years ended December 31, 2016, 2015, 2014, 2013 and 2012 and each of the interim periods within such years, along with the unaudited condensed consolidated financial statements included in the Company’s Quarterly Reports on Form 10-Q for the quarters ended March 31, June 30 and September 30, 2017 (collectively, the “Non-Reliance Periods”), should be restated (the “Restatement”), and therefore, such consolidated financial statements and other financial information, any press releases, investor presentations or other communications related thereto should no longer be relied upon. The Company also stated that, as a result of the material weaknesses relating to the Restatement, it concluded that its internal control over financial reporting was not effective for all of the Non-Reliance Periods.
Four members of senior management during the Non-Reliance Periods, including the Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), were separated from the Company in June and July 2018. The Company appointed Edward Borkowski as interim CFO on June 6, 2018. The Company appointed David Coles as the interim CEO on July 2, 2018. Both individuals served in these roles through the remainder of 2018. Mr. Coles was succeeded by Mr. Wright as CEO of the Company, effective May 13, 2019.
Mr. Borkowski separated from the Company in November 2019. Under the terms of a Separation and Transition Services Agreement, Mr. Borkowski agreed to continue as acting CFO of the Company through the filing of this Form 10-K.
Ernst & Young LLP (“EY”) was engaged on August 4, 2017 to serve as the Company’s independent registered public accountant. On December 4, 2018, EY informed the Audit Committee that it was resigning from the engagement to audit the Company’s consolidated financial statements for the years ended December 31, 2017 and 2018. In connection with its resignation, EY informed the Audit Committee that, in EY’s view, the internal controls necessary for the Company to develop reliable financial statements did not exist.
As a result of the Audit Committee Investigation, the Company became aware of material weaknesses in its internal control over financial reporting during the first half of 2018. The process of remediating these material weaknesses began in June 2018, and these remediation efforts included the senior management changes which occurred during June and July of 2018. Due to these material weaknesses within our control environment, our internal controls failed to prevent or were overridden by management in certain instances to allow recording of accounting entries without appropriate support, recording of accounting entries that were inconsistent with information known by management at the time, inadequate communication of relevant information within our organization and, in some cases, withholding information from our independent directors, our Audit Committee, and our independent registered public accountant, which resulted in material accounting errors. Although some remediation progress was achieved during the latter half of 2018, our material weaknesses were not remediated as of December 31, 2018, and these remediation efforts continued throughout 2019 with varying effective dates. Since control effective dates extended into late third quarter and the fourth quarter of 2019, internal controls will not have been in operation for a sufficient period of time to definitively opine on their operating effectiveness as of December 31, 2019. Therefore, it is likely that we will conclude that our internal control over financial reporting was not effective as of December 31, 2019.
80
Evaluation of Disclosure Controls and Procedures
Management maintains a set of disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) designed to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to management, including our CEO and principal financial officer (“PFO”), to allow for timely decisions regarding required disclosure.
An evaluation of the effectiveness of the design and operation of our disclosure controls and procedures was performed under the supervision and with the participation of our management, including our CEO and PFO. As a result of this evaluation, our current CEO and PFO concluded that our disclosure controls and procedures were not effective as of December 31, 2018 because of the material weaknesses in internal control over financial reporting described below.
Management’s Report on Internal Control Over Financial Reporting
Management, including our CEO and PFO, is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act and based upon the criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO framework”). The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external purposes in accordance with U.S. generally accepted accounting principles (“GAAP”).
An effective internal control system, no matter how well designed, has inherent limitations, including the possibility of human error or overriding of controls, and therefore can provide only reasonable assurance with respect to reliable financial reporting. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may demonstrate.
In connection with the Audit Committee Investigation and management’s review of financial records, management, with the assistance of internal audit personnel and outside consultants, conducted an evaluation of the effectiveness of our internal control over financial reporting based on the COSO framework. As a result of this evaluation, management determined, based upon the existence of the material weaknesses described below, that we did not maintain effective internal control over financial reporting as of December 31, 2018.
A material weakness (as defined in Rule 12b-2 under the Exchange Act) is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that a reasonable possibility exists that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
The following identified material weaknesses correspond to each of the five components of internal control as defined by COSO (Control Environment, Risk Assessment, Control Activities, Information and Communication, and Monitoring):
Control Environment
We did not maintain an effective control environment to enable the identification and mitigation of risks of material accounting errors based on the contributing factors to material weakness in the control environment, including:
• | The Company failed to establish a tone at the top that demonstrated a commitment to integrity and ethical values, resulting in activity by senior management, members of the sales group and others that was inconsistent with both the accounting applied and applicable regulatory requirements. |
• | Both the Board and senior management failed to appropriately respond to allegations of improper accounting activities, improper sales practices, and activities consistent with retaliation against employees who raised concerns of such inappropriate accounting and sales activity. |
• | There were not adequate policies and procedures for review, authorization and approval of certain transactions (such as contracts with vendors and customers) by the appropriate internal resources. |
• | The Company did not have a sufficient complement of personnel with an appropriate level of knowledge, experience, and oversight commensurate with its financial reporting requirements to ensure proper selection and application of U.S. GAAP. |
81
• | There was not a mechanism in place to regularly educate and communicate to management and employees the importance of internal controls, and to raise their level of understanding of controls. |
Risk Assessment
We did not design and implement an effective risk assessment based on the criteria established in the COSO framework, specifically relating to the following:
• | The organization did not have an effective process to evaluate the range of its activities to assess whether all material activities were appropriately reflected in the financial statements. |
• | There were not adequate processes in place to communicate changes in the operating environment to the accounting department so they could review the changes and determine what, if any, effect the change may have on the Company’s accounting policies. |
• | There were not adequate processes in place to ensure that the accounting department (and/or Audit Committee) was aware of significant transactions with related parties so it could determine whether such transactions are appropriately approved, accounted for, and disclosed. |
• | The Company did not have an effective, documented and continuous risk assessment process and related controls to properly monitor, identify and analyze regulatory compliance risks, including compliance with applicable regulations around product pricing, payments to medical professionals, and related activities, and related risks of financial misstatement due to error and / or fraud, including management override of controls. |
Control Activities
We did not design and implement effective control activities based on the criteria established in the COSO framework, contributing to material accounting errors or the potential for there to have been material accounting errors in substantially all financial statement account balances and disclosures. In part, management identified the following:
• | The Company had inadequate or ineffective senior accounting leadership and corresponding process level and monitoring controls in the area of accounting close and financial reporting around the accounting for and disclosure of material transactions and business activities. These ineffective processes and controls impacted the Company’s ability to meet a variety of its financial reporting objectives, including (but not limited to) the following: proper cutoff for cash receipts, appropriate application of cash receipts to the correct corresponding receivables, accurate calculation of stock based compensation expense, the development of quality estimates related to accrued expenses, and the expensing of prepaid expenditures (such as clinical trial costs) within the correct periods. |
• | The Company did not properly design or maintain effective controls to prevent unauthorized access to certain systems, programs and data, or provide for periodic review and monitoring of access, including analysis of segregation of duties conflicts. |
• | There was a lack of robust, established and documented accounting policies and insufficiently detailed Company procedures to put these policies into effective action. |
• | The Company did not have adequate management oversight around completeness and accuracy of data material to financial reporting. |
• | The Company’s revenue recognition methodology was not aligned with the Company’s customary business practices, resulting in certain revenue events being recorded prior to the time at which all of the sales recognition criteria were met. Such misalignment was frequently due to the existence of extra-contractual or undocumented terms or arrangements initiated by former executives of the Company at the onset of sales transactions, such as sales above established distributor and customer credit limits, and concessions agreed to by former executives of the Company subsequent to the initial transaction, such as extended unusually long payment terms, granting return or exchange rights, and contingent payment obligations. |
Information and Communication
We did not generate and provide quality information and communication based on the criteria established in the COSO framework. More specifically, the organization did not implement policies and procedures that facilitate effective internal communication, including individual internal control authorities and responsibilities and standards of conduct across the organization.
82
Monitoring Activities
We did not design and implement effective monitoring activities based on the criteria established in the COSO framework, specifically relating to the following:
• | Management did not have processes in place to assess whether controls within each of the five components of internal control were present and functioning as intended. |
• | The level of staffing, training and specialized skills of the people performing the monitoring were not adequate given the environment. |
• | There were not adequate procedures in place to monitor when controls were overridden and to determine whether the override was appropriate. |
BDO USA, LLP, our independent registered public accounting firm, has audited the effectiveness of our internal control over financial reporting as of December 31, 2018. BDO USA, LLP has expressed an adverse report on internal control over financial reporting which appears on page F-2 of this Form 10-K.
Remediation Plan and Status
Remediation of the identified material weaknesses and strengthening our internal control environment was an identified priority for us throughout 2019 and will continue to be a priority in 2020. We will test the design and ongoing operating effectiveness of the new and existing controls in future periods. The material weaknesses cannot be considered completely remediated until the applicable controls have operated for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively. With oversight from the Audit Committee, the Company’s management has designed and begun implementing changes in processes and controls to remediate the material weaknesses described above and enhanced the Company’s internal control over financial reporting as follows:
Control Environment
• | The Company underwent a leadership transition during the second and third quarters of 2018, during which the former CEO, CFO, COO, Controller, and VP of IT were removed from the organization. During this same period, each of these positions was filled by interim resources with appropriate technical expertise. |
• | The Board created an Ethics and Compliance Committee consisting solely of independent directors. This committee is responsible for reviewing the status of the Company’s ethics and compliance program, reviewing and advising the Board regarding any open cases and trends that may impact the business, and recommending future initiatives to improve compliance performance and effectiveness. |
• | Management conducted internal training courses over Sarbanes-Oxley regulations and the Company’s internal control over financial reporting program for Company personnel involved in the execution of the program. |
• | Management reinforced the importance of integrity, accountability, and adherence to established internal controls, policies, and procedures through the adoption of a revised Code of Business Conduct Policy. Each newly hired employee or agent (including executives and Board members) will be required to certify that they read and understood the policy upon hire, and then re-certify their reading and understanding of the policy on an annual basis thereafter. |
• | The Company enhanced the onboarding training provided to newly hired salespeople to emphasize the importance of compliance with the various regulations specific to the Life Sciences industry to which the Company is subject. |
• | The Chief Compliance Officer intends to facilitate ethics and compliance training for all members of the Company’s Board. |
• | The purpose of the whistleblower hotline and the mechanics of its use were formally communicated by the Chief Compliance Officer during numerous meetings with all levels of the sales department during the last two quarters of 2018, with an emphasis on the following: (a) each employee’s responsibility to report any actual or apparent violations of law or ethical standards and any questionable accounting or auditing matters so that they may be investigated and dealt with appropriately, and (b) management’s commitment to ensuring that any employees communicating such an issue via the hotline will not be subject to retaliation. |
83
• | In addition to enhancing processes and controls over adoption of new accounting standards and the proper application of existing accounting standards, the Company enhanced the technical capabilities of its accounting department by leveraging third party consultants with expertise in U.S. GAAP. In December 2019, the Company hired an experienced EVP of Finance and, as of the date of the filing of this Form 10-K, the Company is searching for a full-time Controller. Furthermore, the Company intends to lessen its reliance on third-party consultants for its technical accounting needs during 2020 by transitioning roles currently assigned to outside consultants to full-time employees with similar technical accounting competencies. |
• | Management plans to develop and implement a contract management policy that defines who is required to review new, extended, or amended contracts (including those with distributors and agents). |
Risk Assessment
• | The Company hired a new Chief Compliance Officer during the second quarter of 2018 to manage compliance and regulatory risk. This person reports directly to the Board’s Ethics and Compliance Committee. |
• | Management hired consultants to evaluate the Company’s compliance with regulations specific to the Life Sciences industry. Going forward, similar periodic assessments will be performed by the Chief Compliance Officer. |
• | Management is designing an Anti-Fraud Program to assess (and subsequently mitigate) the organization’s susceptibility to fraud, including management override of controls. The first phase of this Anti-Fraud Program to be implemented will in an annual fraud risk assessment. |
• | Management has developed a set of procedures to identify and define its population of related parties, identify transactions with those related parties, and analyze such transactions to determine whether additional approval or financial statement disclosure is required. |
• | Prior to the filing of this Form 10-K, the Company established a Disclosure Committee comprised of senior management representatives from all relevant departments within the organization. Members of this committee are responsible for reviewing all quarterly and annual SEC filings, and meet prior to each filing to discuss the completeness and accuracy of the document being filed. The Company is designing and implementing a variety of new procedures, such as monthly operational meetings amongst senior management that are attended by members of the accounting department, to confirm that the accounting department is aware of operational changes that may affect the Company’s accounting policies. |
• | On an annual basis (or more frequently, should a significant triggering event occur), the Company now performs a risk assessment designed to ensure that the scope of its Sarbanes-Oxley compliance program adequately reflects changes to the business and its operations. |
Control Activities
• | Management collaborated with outside consultants possessing significant financial reporting and internal control expertise to perform an extensive review of the design of the Company’s internal controls over financial reporting. This review included the identification of internal control deficiencies and the development of remediation plans for each identified deficiency. These internal control deficiencies identified included (but were not limited to) the following: improper cutoff for cash receipts, application of cash receipts to the incorrect receivables, inaccurate calculation of stock-based compensation expense, the use of suboptimal methodologies in the development of estimates related to accrued expenses, and the expensing of prepaid expenditures (such as clinical trial costs) within the incorrect periods. |
• | The Company is enhancing its financial close process by formalizing its accounting policies, introducing additional layers of independent reviews by appropriately qualified individuals, improving the precision applied to various financial result analyses, and providing education and training to the members of the finance department. |
• | The Company has enhanced its review of salesperson activity which may indicate noncompliance with the Company’s sales policies, such as a quarterly review of data by the PFO, Interim Controller, and SVP of Sales which quantifies no charge evaluations, sales returns, and other key metrics both by region and at the individual salesperson level. |
• | Management has gained a better understanding of system functionality through a comprehensive review of permissions and profiles within each IT application that is significant to the Company’s financial reporting objectives, and subsequently reconfigured profiles with appropriate permissions to better align with job responsibilities and enforce segregation of duties. |
84
• | Once user profiles and their associated permissions were reconfigured, management employed procedures to ensure the continued appropriateness of all applicable system and network access. This objective was achieved through the performance of periodic user access reviews and the enhancement of procedures related to the granting and removing of system and network access. |
• | Management implemented additional procedures (such as the evaluation of report query parameters and the sampling of data within reports) to validate the completeness and accuracy of system generated reports and other data deemed to be significant to the Company’s financial reporting objectives. |
Information and Communication
• | Management implemented quarterly required communications amongst relevant members of senior management in the form of certification surveys. A control certification survey is distributed to obtain information regarding any internal control related issues or concerns that control owners may have, and additional certification surveys are distributed to Disclosure Committee members and key members of the sales department which address (to the best of their knowledge) whether the period’s financial statements are free from either material misstatements, material misclassifications, or material omissions. |
Monitoring Activities
• | The Company further developed its Internal Audit Department, led by a VP of Internal Audit, comprised of internal resources who are tasked with continually evaluating and monitoring the effectiveness of the Company’s internal controls over financial reporting. |
• | Management modified its sales procedures to enhance the Finance Department’s awareness and oversight of sales activities in order to verify the validity and proper accounting treatment of sales transactions. This has been achieved via the participation of the Finance Department in various sales and operations meetings, as well as through new requirements that all credit limit increases, credit memos related to out of policy returns, and bulk sales orders exceeding a defined threshold be reviewed and approved by the Interim Controller prior to being processed. |
• | Management began, and will continue, to schedule training sessions with the Company’s Sales Department to ensure that they are familiar with the Company’s current sales related policies and procedures, including those which are significant to the Company’s financial reporting objectives. Portions of these training sessions are facilitated by the Interim Controller, who presents on topics such as the Company’s current sales return policy, acceptable credit terms for customers, events that would trigger commission claw-backs, customer credit limit modification approval protocol, and the importance of proper revenue recognition. |
• | The Company modified the composition of its Pricing Committee to include representatives from the Legal and Finance departments. This committee meets monthly to discuss any requested deviations from the Company’s standard price list, and its approval is required in order for any such deviation to be applied to a sales transaction. |
While we believe the steps taken to date and those planned for implementation will improve the effectiveness of our internal control over financial reporting, we have not completed all remediation efforts identified above. Accordingly, as we continue to monitor the effectiveness of our internal control over financial reporting in the areas affected by the material weaknesses described above, we have and will continue to perform additional procedures prescribed by management, including the use of manual mitigating control procedures and employing any additional tools and resources deemed necessary, to ensure that our consolidated financial statements are fairly stated in all material respects. We will continue to monitor the effectiveness of these remediation measures and will make changes and take other actions that are appropriate given the circumstances.
Changes in Internal Control Over Financial Reporting
Other than the changes described above in “Remediation Plan and Status,” there were no changes during the year ended December 31, 2018 in our internal control over financial reporting (as such term is defined in the Exchange Act) that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
85
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Board of Directors
Set forth below is certain information regarding our current directors. There are no family relationships among any of our directors or executive officers.
Name | Age | Since | Tenure | Independent | Committees | |||||
Richard J. Barry | 61 | 2019 | — | ü | CC* | |||||
M. Kathleen Behrens | 67 | 2019 | — | ü | COB, EC | |||||
James L. Bierman | 67 | 2019 | — | ü | AC, CC | |||||
J. Terry Dewberry | 76 | 2009 | 10 | ü | AC*, NCG | |||||
Charles R. Evans | 72 | 2012 | 7 | ü | AC, NCG* | |||||
Charles E. Koob | 75 | 2008 | 12 | — | ||||||
K. Todd Newton | 57 | 2019 | — | ü | AC, EC | |||||
Timothy R. Wright | 62 | 2019 | — | — | ||||||
Neil S. Yeston | 76 | 2012 | 7 | ü | CC, EC*, SL | |||||
* = Chair; AC = Audit Committee; CC = Compensation Committee; COB = Chairperson of the Board; EC = Ethics & Compliance Committee; NCG = Nominating and Corporate Governance Committee; SL = Science and Research Liaison
Richard J. Barry, age 61. Mr. Barry has served as a director of Sarepta Therapeutics, Inc. (SRPT), a genetic medicine company, since June 2015, and he has been a Partner and Advisory Board member of the San Diego Padres since 2009. Earlier in his career, he was a founding member of Eastbourne Capital Management LLC, a large equity hedge fund investing in a variety of industries, including health care, and served as the Managing General Partner and Portfolio Manager from 1999 to its close in 2010. Prior to that, he was a Portfolio Manager and Managing Director of Robertson Stephens Investment Management, an investment company, from 1995 until 1999. Before that, Mr. Barry spent over 13 years in various roles in institutional equity and investment management firms, including Lazard Frères, Legg Mason and Merrill Lynch. Mr. Barry has served as a director of Elcelyx Therapeutics, Inc., a private pharmaceutical company, since February 2013 and has served as a Managing Member of GSM Fund, LLC, a fund established for the sole purpose of investing in Elcelyx Therapeutics, since February 2013. Mr. Barry previously served as a director of Cluster Wireless, LLC, a software company, from 2011 until 2014, and of BlackLight Power, Inc. (n/k/a Brilliant Light Power, Inc.), an energy research company, from 2009 until 2010. Mr. Barry holds a B.A. from Pennsylvania State University and is a member of its Shreyer’s Honors College Advisory Board. Mr. Barry was nominated as a director because of his substantial experience, including in the healthcare and biotechnology sectors.
M. Kathleen Behrens, Ph.D., age 67. Dr. Behrens has worked as an independent life sciences consultant and investor since December 2009. Dr. Behrens served as the Co-Founder, President and Chief Executive Officer, and as a director, of the KEW Group Inc., a private oncology services company, from January 2012 until June 2014. Earlier in her career, Dr. Behrens served as a general partner for selected venture funds for RS Investments, a mutual fund firm, from 1996 until December 2009. While Dr. Behrens worked at RS Investments, from 1996 to 2002, she served as a managing director at the firm and, from 2003 to December 2009, she served as a consultant to the firm. During that time, Dr. Behrens also served as a member of the President’s Council of Advisors on Science and Technology (PCAST) from 2001 to 2009 and as chairwoman of PCAST’s Subcommittee on Personalized Medicine, as well as the President, director and chairwoman of the National Venture Capital Association, an organization that advocates for public policy that supports the American entrepreneurial ecosystem, from 1993 until 2000. Prior to that, she served as a general partner and managing director for Robertson Stephens & Co., an investment company, from 1983 through 1996. Dr. Behrens has served as a member of the board of directors of each of Sarepta Therapeutics, Inc. (NASDAQ: SRPT), a medical research and drug development company, since March 2009 (Chairwoman of the Board since April 2015) and IGM Biosciences, Inc. (NASDAQ: IGMS) since January 2019. She served as a director of Amylin Pharmaceuticals, Inc. (formerly NASDAQ: AMLN), a biopharmaceutical company, from 2009 until its sale in 2012 to Bristol-Myers Squibb Co. Prior to that, she served on the board of directors of Abgenix, Inc. (formerly NASDAQ: ABGX), a biopharmaceutical company, from 2001 until the company was sold to Amgen, Inc. in 2006. From 1997 to 2005, Dr. Behrens was a director of the Board on Science, Technology and Economic Policy for the National Research Council. Dr. Behrens was also a Co-Founder of the Coalition for 21st Century Medicine, a trade association for new generation diagnostics companies. Dr. Behrens holds a B.S. in biology and a Ph.D. in microbiology from the University of California, Davis. Dr. Behrens was nominated as a director because of her substantial experience in the financial services and biotechnology sectors, as well as in healthcare policy.
86
James L. Bierman, age 67. Mr. Bierman served as President and Chief Executive Officer and as a member of the board of directors of Owens & Minor, Inc. (NYSE: OMI), a Fortune 500 company and a leading distributor of medical and surgical supplies, from September 2014 to June 2015. Previously, he served in various other senior roles at Owens & Minor, including President and Chief Operating Officer from August 2013 to September 2014, Executive Vice President and Chief Operating Officer from March 2012 to August 2013, Executive Vice President and Chief Financial Officer from April 2011 to March 2012, and Senior Vice President and Chief Financial Officer from June 2007 to April 2011. Earlier in his career Mr. Bierman served as Executive Vice President and Chief Financial Officer at Quintiles Transnational Corp. (formerly NASDAQ: QTRN). Quintiles was a market leader in providing product development and commercialization solutions to the pharmaceutical, biotech, and medical device industries. As a member of management he helped lead the successful privatization of the company in 2004. Before joining Quintiles, Mr. Bierman was a partner with Arthur Andersen LLP from 1988 to 1998. Mr. Bierman currently serves on the board of directors of Tenet Healthcare Corporation (NYSE: THC), a Fortune 100 company and a diversified healthcare services company operating more than 500 facilities, acute care hospitals and outpatient centers, throughout the United States. He previously served on the board of directors of Team Health Holdings, Inc. (formerly NYSE: TMH) where as Independent Lead Director, he helped lead the successful privatization of the company in 2017. Team Health is one of the largest suppliers of outsourced healthcare professional staffing and administrative services to hospitals and other healthcare providers in the United States. Mr. Bierman earned his B.A. from Dickinson College and his M.B.A. at Cornell University’s Johnson Graduate School of Management. Mr. Bierman has served on the Board since June 2019 and was nominated as a director because of his substantial operational and financial experience in the healthcare sector.
J. Terry Dewberry, age 76. Mr. Dewberry is a private investor with significant experience at both the management and board levels in the healthcare industry. He has extensive experience in corporate mergers and takeovers on both the buy and sell sides for consideration up to $5 billion. Mr. Dewberry has served on the boards of directors of several publicly traded healthcare products and services companies, including Respironics, Inc. (Nasdaq: RESP) (1998-2008), Matria Healthcare, Inc. (Nasdaq: MATR) (2006-2008), Healthdyne Information Enterprises, Inc. (1996-2002), Healthdyne Technologies, Inc. (1993-1997), Home Nutritional Services, Inc. (1989-1994) and Healthdyne, Inc. (1981-1996). From March 1992 until March 1996, Mr. Dewberry was Vice Chairman of Healthdyne, Inc. From 1984 to 1992, he served as President and Chief Operating Officer, and Executive Vice President of Healthdyne, Inc. Mr. Dewberry received a Bachelor of Electrical Engineering from Georgia Institute of Technology in 1967 and a Master of Professional Accountancy from Georgia State University in 1972. Mr. Dewberry has served on the Board since 2009 and was nominated as a director due to his extensive business and financial background and experience as a member of the boards of directors of other publicly traded companies and a member of the audit committee of at least one other public company.
Charles R. Evans, age 72. The Board named Mr. Evans Lead Director on March 9, 2018, and he served as Chairman from July 2, 2018 through June 2019. Mr. Evans has over 40 years' of experience in the healthcare industry. He is currently President of the International Health Services Group, an organization he founded to support health services development in underserved areas of the world. Since 2009, he has served as a senior adviser with Jackson Healthcare, a consortium of companies that provide physician and clinical staffing, anesthesia management and information technology solutions for hospitals, health systems and physician groups. In addition, Mr. Evans is a Fellow in the American College of Healthcare Executives having previously served as Governor of the College from 2004 to 2007 and as Chairman Officer from 2008 to 2011. In 2012, he attained the Board Leadership Fellow credential of the National Association of Corporate Directors. Previously, Mr. Evans was a senior officer with Hospital Corporation of America (HCA), having managed various HCA divisions and completing his service with the responsibility for operations in the Eastern half of the country. Mr. Evans currently serves on the board of directors of Jackson Healthcare and WellStreet Urgent Care. Mr. Evans also serves on the boards of nonprofit organizations including American International Health Alliance and FaithBridge Foster Care. Mr. Evans has served on the Board since 2012 and was nominated as a director due to his healthcare management expertise.
Charles E. (“Chuck”) Koob, age 75. In 2007, Mr. Koob retired as a partner in the law firm of Simpson Thacher & Bartlett, LLP. While at that firm, Mr. Koob was the co-head of the Litigation Department and served on the firm’s Executive Committee. Mr. Koob specialized in competition, trade regulation and antitrust issues. Throughout his 37-year tenure, he represented clients before the Federal Trade Commission, the Antitrust Division of the Department of Justice, and numerous state and foreign competition authorities. He received his B.A. from Rockhurst College in 1966 and his J.D. from Stanford Law School in 1969. Mr. Koob serves on the board of Stanford Hospital and Clinics. He previously served on the board of a private drug development company and MRI Interventions (OTCBB: MRIC), a publicly traded medical device company. Mr. Koob has served on our Board since 2008 and was nominated as a director due to his 37 years of legal expertise in representing both publicly traded and privately held businesses.
K. Todd Newton, age 57. Mr. Newton has served as Chief Executive Officer and as a member of the board of directors of Apollo Endosurgery, Inc. (NASDAQ: APEN), a medical device company, since July 2014. Earlier in his career, Mr. Newton served as Executive Vice President, Chief Financial Officer and Chief Operating Officer at ArthroCare Corporation (formerly NASDAQ:
87
ARTC), a medical device company, from 2009 to June 2014. Prior to that, Mr. Newton served in a number of executive officer roles, including President and Chief Executive Officer and as a director, at Synenco Energy, Inc., a Canadian oil sands company, from 2004 until 2008. Mr. Newton was a Partner at Deloitte & Touche LLP, a professional services network and accounting organization, from 1994 to 2004. Mr. Newton holds a B.B.A. in accounting from The University of Texas at San Antonio. Mr. Newton has served on the Board since June 2019 and was nominated as a director because of his significant experience in the medical device sector as well as strong executive leadership experience.
Timothy R. Wright, age 62, joined the Company as its Chief Executive Officer on May 13, 2019. Mr. Wright has more than 30 years of experience in the pharmaceutical, biotech and medical devices industries. Most recently, Mr. Wright served as a Partner at Signal Hill Advisors, LLC, a consulting practice, since February 2011. Mr. Wright served as President and Chief Executive Officer of M2Gen Corp., a privately held cancer and health informatics company, between July 2017 and September 2018. Prior to M2Gen Corp., Mr. Wright served as Executive Vice President, Mergers and Acquisitions, Strategy and Innovation for Teva Pharmaceutical Industries Ltd. (“Teva”), a pharmaceutical company specializing in generic medicines, from April 2015 until August 2017. Before Teva, Mr. Wright was the founding partner of The Ohio State University Comprehensive Cancer Drug Development Institute. Mr. Wright also served as Chairman, Interim Chief Executive Officer and a director of Curaxis Pharmaceutical Corporation (“Curaxis”), a pharmaceutical company specializing in the development of drugs for the treatment of Alzheimer’s disease and various cancers, from July 2011 to July 2012. Curaxis had been experiencing financial difficulties prior to Mr. Wright’s tenure and, as a result, the company filed for Chapter 11 bankruptcy in July 2012. Mr. Wright has been a director of Agenus, Inc. (NASDAQ: AGEN), an immune oncology company, since 2006 and its lead director since 2009. Mr. Wright also serves as Chairperson of The Ohio State University Comprehensive Cancer Center Drug Development Institute, serves as director of The Ohio State Innovation Foundation and sits on The Ohio State University College of Pharmacy Dean’s Corporate Council. Over his career, Mr. Wright has served on boards of directors in North America, Europe and Asia. Mr. Wright earned a Bachelor’s of Science in Marketing from The Ohio State University. Mr. Wright has served on the Board since June 2019 and was nominated as a director to bring the perspective of the Chief Executive Officer on the Board and also for the benefit of his many years of experience in the healthcare and pharmaceutical industry.
Neil S. Yeston, M.D., age 76. Dr. Yeston is the Past President of the New England Surgical Society and currently serves as Active Senior Staff, Department of Surgery at Hartford Hospital. During his association with Hartford Hospital, Dr. Yeston previously served in various roles including Vice President of Academic Affairs, Director of Corporate Compliance, Vice President of Quality Management and Director of the Section on Critical Care Medicine, Department of Surgery. In addition, Dr. Yeston was responsible for the enterprise wide acquisition of all biomedical engineering technology. Dr. Yeston has formerly served as Professor of Surgery at the University of Connecticut and the Assistant Dean, Medical Education at the University of Connecticut School of Medicine. Prior to his associations with Hartford Hospital and the University of Connecticut, Dr. Yeston served with Boston University Medical Center, in various positions including the Vice Chairman Department of Surgery, Associate Professor of Anesthesiology, Director Progressive Care Unit and Associate Professor of Surgery. Dr. Yeston has served on the Board since 2012 and was nominated as a director because of his in-depth understanding of healthcare issues from the perspective of the practitioner, academician, administrator and executive.
Audit Committee and Audit Committee Financial Expert
The following directors serve on the Audit Committee: J. Terry Dewberry (Chair), James L. Bierman, Charles R. Evans and K. Todd Newton, each of whom satisfies NASDAQ’s independence standards for audit committee members. The Board has determined that each of Messrs. Bierman, Dewberry, and Newton is an “audit committee financial expert” as that term is defined by the SEC in Item 407(d)(5)(ii) of Regulation S-K.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all of our employees, officers and directors, a copy of which is on our website at https://mimedx.gcs-web.com/corporate-governance/highlights. Any amendments to or waivers of the Code of Business Conduct and Ethics that require disclosure under applicable law or listing standards will be disclosed on our website at www.mimedx.com. We undertake to provide a copy to any person, without charge, upon written request to Secretary, MiMedx Group, Inc., 1775 West Oak Commons Court, NE Marietta, Georgia 30062.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires the Company’s executive officers and directors, and any beneficial owner of more than ten percent of a registered class of the Company’s equity securities, to file reports (Forms 3, 4 and 5) of stock ownership and changes in ownership with the SEC. Officers, directors and beneficial owners of more than ten percent of the outstanding shares of Company common stock are required by SEC regulations to furnish the Company with copies of all such forms that they file.
88
Based solely on the Company’s review of the copies of Forms 3, 4 and 5, the Company believes that during the year ended December 31, 2018 all filing requirements were complied with by its executive officers, directors and beneficial owners of more than ten percent of the outstanding shares of Company common stock.
Material Changes to the Procedures by which Security Holders May Nominate Individuals for Election to the Board
On October 3, 2018, the Company’s Board of Directors (the “Board”) amended and restated the Company’s bylaws to, among other things, clarify certain corporate procedures and make certain other enhancements and technical changes. The changes effected by the amendment and restatement of the Company’s bylaws (the “Amended and Restated Bylaws”) include, without limitation, the following:
• | Adding procedural mechanics for shareholders to call special meetings of shareholders or act by written consent; |
• | Enhancing procedural mechanics in connection with shareholder nominations of directors and submission of shareholder proposals at shareholder meetings; |
• | Specifying powers of the chair of a shareholder meeting to establish and enforce rules of conduct and the order of business at the meeting; |
• | Enhancing provisions related to the adjournment and postponement of, and establishment of record dates for, shareholder meetings; |
• | Clarifying that the Chair of the Board shall be chosen from among Board members and may, but need not, be the Chief Executive Officer of the Company; and |
• | Allowing emergency special Board meetings to be held with less than 24 hours’ notice. |
All of the amendments were effective October 3, 2018.
Procedures by which Security Holders May Nominate Individuals for Election to the Board
Our Amended and Restated Bylaws include a procedure that shareholders must follow in order to nominate a person for election as a director at an annual meeting of shareholders. The Amended and Restated Bylaws require that timely notice of the nomination in proper written form, including all required information as specified in the Amended and Restated Bylaws, be mailed to the Secretary, at 1775 West Oak Commons Court, NE Marietta, Georgia 30062.
In accordance with our Amended and Restated Bylaws, the Nominating and Corporate Governance Committee will consider for nomination candidates recommended by shareholders if the shareholders comply with the requirements described above. The Nominating and Corporate Governance Committee will review and evaluate the qualifications of such candidates in compliance with procedures established from time to time by the Nominating and Corporate Governance Committee and will conduct such inquiries as it deems appropriate. The Nominating and Corporate Governance Committee will consider for nomination any proposed director candidate who is deemed qualified by the Nominating and Corporate Governance Committee in light of criteria for Board membership set forth in the charter of the Nominating and Corporate Governance Committee or otherwise approved by the Nominating and Corporate Governance Committee and the Board from time to time.
Cooperation Agreement
The Company entered into a Cooperation Agreement, dated as of May 29, 2019 (the “Cooperation Agreement”), with M. Kathleen Behrens, K. Todd Newton, Richard J. Barry, Prescience Partners, LP, a Delaware limited partnership (“Prescience Partners”), its affiliates and Eiad Asbahi (Prescience Partners, together with Prescience Point Special Opportunity LP, Prescience Capital, LLC, Prescience Investment Group, LLC d/b/a Prescience Point Capital Management LLC and Mr. Asbahi, “Prescience Point”; Prescience Point, Dr. Behrens, Mr. Barry and Mr. Newton collectively, the “Investor Group”). With certain exceptions relating to breaches of the Cooperation Agreement, the Cooperation Agreement terminates at least five business days after the Company or the Investor Group delivers notice of termination (the “Termination Date”) following the date of the 2020 Annual Meeting. Pursuant to the Cooperation Agreement, the Company nominated Dr. Behrens, Mr. Newton and Mr. Wright as three Class II director candidates for election to the Board at the 2018 Annual Meeting. The 2018 Annual Meeting was duly held on June 17, 2019, and Dr. Behrens, Mr. Newton, and Mr. Wright were elected to the Board. The Board also appointed Mr. Barry and Mr. Bierman as Class III directors pursuant to the Cooperation Agreement. The Cooperation Agreement further provides for the Company and Prescience Point to identify and mutually agree upon an individual (the “Mutual Designee”) to stand for election as a Class III director at the 2019 Annual Meeting. At this time, the Board and Prescience Point have yet to identify the Mutual Designee for election as Class III directors at the 2019 Annual Meeting.
89
The Cooperation Agreement provides Prescience Point with certain other rights with respect to designating replacement Board nominees and with respect to the designated directors’ service on certain Board committees, as long as Prescience Point holds more than 5.0% of the outstanding shares of Company common stock. The Cooperation Agreement also contains standstill restrictions on Prescience Point’s ownership of Company common stock. Through the Termination Date and subject to certain qualifications, Prescience Point is required to vote all of its shares of Company common stock at any annual or special meeting, and any consent solicitation of the Company’s shareholders, in accordance with the recommendations of the Board. Pursuant to the Cooperation Agreement, the Company reimbursed Prescience Point for $500,000 of its reasonable, documented out-of-pocket fees and expenses incurred in connection with the matters related to the 2018 Annual Meeting.
Executive Officers
The following persons currently serve as our executive officers:
Timothy R. Wright, 62, became the Company’s Chief Executive Officer, effective as of May 13, 2019. The biography for Mr. Wright can be found under the heading “Board of Directors” above.
Edward J. Borkowski, age 60, is our acting Chief Financial Officer, principal financial officer, and principal accounting officer. Mr. Borkowski joined the Company as Executive Vice President in April, 2018. He was appointed Executive Vice President and Interim Chief Financial Officer effective June 6, 2018, and since such time has served as our principal financial officer and principal accounting officer. He resigned as an employee effective November 15, 2019 but has agreed to provide certain transitional services related to the completion and filing of our annual report on Form 10-K for the period ended December 31, 2018. Prior to joining the Company, Mr. Borkowski served as the Chief Financial Officer of ACETO Corporation, an international company engaged in the development, marketing, sales and distribution of pharmaceutical products, from February 2018 until April 2018, and prior to that, he held several executive level positions with Concordia International Corp., an international specialty pharmaceutical company, from May 2015 to February 2018, including as Chief Financial Officer and as Executive Vice President. Previously, Mr. Borkowski served as Chief Financial Officer at Amerigen Pharmaceuticals, a pharmaceutical company focused on generic products, from 2013 to 2016 and ConvaTec Group plc, an international medical products and technologies company, from 2012 to 2013. He is a Member of the American Institute of Certified Public Accountants and the New York State Society of CPAs. He currently serves on the boards of AzurRx BioPharma, Inc. (Nasdaq: AZRX), Co-Diagnostics, Inc. (Nasdaq: CODX), and Acacia Pharma Group, Plc (EPA: ACPH), and during the previous five years he also served on the board of WhereverTV Inc. (OTCMKTS: TVTV). Mr. Borkowski holds a Bachelor of Science in Economics and Political Science from Allegheny College and a Master in Business Administration in Finance and Accounting from Rutgers University.
Peter M. Carlson, age 55, was appointed Executive Vice President - Finance in December 2019. From 2017 to 2018, Mr. Carlson served as Chief Operating Officer at Brighthouse Financial, Inc., where he helped establish the $200 billion (assets) U.S. life and annuity insurance company as a separate entity following its August 2017 spin-off from MetLife, Inc. He was the Chief Accounting Officer at MetLife, Inc. from 2009 to 2017 where his global responsibilities included accounting, financial planning, tax, and investment finance. Prior to joining MetLife in 2009, Carlson was the Corporate Controller at Wachovia Corporation. He currently serves as a director of White Mountains Insurance Company (NYSE: WTM). Mr. Carlson holds a Bachelor of Science from Wake Forest University and is a trustee of the university. He is licensed as a certified public accountant in North Carolina and New York.
Mark P. Graves, age 55, was appointed Chief Compliance Officer in July 2018. Prior to joining the Company, he served as the U.S. leader for the global Patient Experience & Value function in the neurology division of UCB, Inc., a biopharmaceutical company. From 2011 to 2015, he was UCB’s Deputy Compliance Officer involved in all aspects of compliance including the implementation and management of the company’s corporate integrity agreement. Prior to that, Graves was Senior Director in the Office of Ethics and Compliance for the Pharmaceutical Products Division of Abbott Laboratories, as well as Deputy Ethics & Compliance Officer for Takeda Pharmaceuticals North America, Inc. and TAP Pharmaceutical Products, Inc. Prior to his pharmaceutical and biotech career, he practiced labor and employment law. Mr. Graves holds a B.A. in Criminology and Law, and a J.D., from the University of Florida as well as an MBA from the University of Chicago Booth School of Business.
William F. “Butch” Hulse IV, age 46, has served as General Counsel since December, 2019. Prior to joining the Company, Mr. Hulse was a member of Dykema Gossett, PLLC, a national law firm since 2017. Prior thereto, he was with Acelity, LP, Inc. (formerly Kinetic Concepts, Inc.), a global medical technology company with leadership positions in advanced wound care, surgical solutions and regenerative medicine, from 2008 to 2017 in a variety of roles of increasing responsibility. From 2013 to 2017, he served as Acelity’s Chief Compliance Officer and Senior Vice President for Enterprise Risk Management, Quality, and Regulatory. Prior to that, he served as Division General Counsel for Acelity’s advanced wound care business unit and as Associate General Counsel for litigation matters. Mr. Hulse holds a Bachelor of Arts from Angelo State University and a J.D. from the Baylor University School of Law.
90
Scott M. Turner, age 54, has served as Senior Vice President, Operations and Procurement since April 2017. Mr. Turner oversees supply chain including donor recovery services, procurement, processing, and facilities. Mr. Turner joined the Company in April 2016 as Vice President, Procurement. Prior to joining the Company, Mr. Turner served as a director with Alvarez & Marsal North America, LLC in their Corporate Performance Improvement group from October 2015 until March 2016. Prior thereto, Mr. Turner served as Vice President, Supply Chain, with Larson-Juhl, a Berkshire Hathaway company, from June 2013 until September 2015. Additionally, Mr. Turner has more than 20 years' of Supply Chain and Procurement leadership in life sciences at Shionogi and Johnson & Johnson, spanning the consumer, medical device, and pharmaceutical sectors domestically and overseas. Mr. Turner holds a Bachelor of Science in Commerce & Engineering from Drexel University and a President / Key Executives MBA from Pepperdine University.
91
Item 11. Executive Compensation
COMPENSATION DISCUSSION AND ANALYSIS
The Compensation Committee is responsible for evaluating and determining the compensation paid to the executive officers who are listed in the Summary Compensation Table (the “NEOs”). All components of compensation for the NEOs are then recommended by the Compensation Committee for approval by the Board. This Compensation Discussion and Analysis (“CD&A”) pertains to 2018 compensation.
For 2018, the Company’s NEOs were:
• | Parker H. “Pete” Petit. Mr. Petit served as Chairman and Chief Executive Officer from February 2009 until June 30, 2018. On September 20, 2018, the Board of Directors determined that his termination of employment was “for cause.” |
• | David Coles. Mr. Coles served as Interim Chief Executive Officer from July 2, 2018 until May 13, 2019. He was an employee of Alvarez & Marsal North America, LLC. |
• | Michael J. Senken. Mr. Senken served as Chief Financial Officer from January 2010 until June 6, 2018. On September 20, 2018, the Board of Directors determined that his termination of employment was “for cause.” |
• | Edward Borkowski. Mr. Borkowski served as Executive Vice President and Interim Chief Financial Officer from June 7, 2018 until his resignation on November 15, 2019. He continued to serve as Acting Chief Financial Officer through the date of this filing. |
• | William C. Taylor. Mr. Taylor served as President and Chief Operating Officer from September 2009 until June 30, 2018. On September 20, 2018, the Board of Directors determined that his termination of employment was “for cause.” |
• | Alexandra O. Haden. Ms. Haden served as General Counsel & Secretary from March 2015 until her resignation on August 12, 2019. |
• | I. Mark Landy. Mr. Landy served as Executive Vice President and Chief Strategy Officer from December 5, 2018 until the Company eliminated this role effective September 16, 2019. |
• | Scott M. Turner. Mr. Turner has served as Senior Vice President—Operations & Procurement since December 5, 2018 and continues to serve in such role. |
Audit Committee Investigation’s Impact on 2018 Compensation
In February 2018, the Company announced that the Audit Committee had engaged independent legal and accounting advisors to conduct an independent investigation into current and prior-period reporting matters relating to allegations of certain sales and distribution practices at the Company (the “Audit Committee Investigation” or the “Investigation”). In June 2018, the Audit Committee determined that there were material accounting irregularities regarding the recognition of revenue under GAAP and that the Company’s financial statements dating back to and including the year ended December 31, 2012 should not be relied upon by our investors and stakeholders and would need to be restated. The Audit Committee subsequently found, among other things, that the Company’s former senior management, including Messrs. Petit, Taylor and Senken, were aware that the Company’s course of dealing with its largest distributor was inconsistent with the terms of the parties’ contract and this course of dealing affected the way in which the Company should have properly recognized revenue; these individuals also made material misstatements and omissions about the Company’s course of dealing with its largest distributor as well as the Company’s corresponding revenue recognition practices to a number of key stakeholders, including the SEC, the Board, the Audit Committee and the Company’s independent registered public accounting firm; and in at least one instance, Mr. Taylor concealed a side agreement with a customer from the Company’s finance and accounting group. In addition, the Audit Committee found that Messrs. Petit and Taylor engaged in a pattern of taking action against employees who raised concerns about these practices, without conducting a thorough investigation of those concerns. Instead, Messrs. Petit and Taylor focused on disputing the employees’ allegations and on seeking to discredit or find wrongdoing by the persons raising the concerns that would justify re-assignment, discipline or termination.
Messrs. Petit, Taylor and Senken resigned from their respective executive officer positions in June 2018. Mr. Taylor also resigned from the Board. Mr. Petit resigned from the Board in September 2018. In September 2018, following a review of evidence
92
uncovered in the Audit Committee Investigation, the Board retroactively determined that these individuals’ termination of employment should be considered “for cause” and all restricted shares and stock options held by these executives were forfeited as a result of the “for cause” determination.
In February 2018, at or around the time that the Audit Committee decided to engage an independent legal adviser to conduct its Investigation, the Compensation Committee was making decisions about 2018 compensation, including increasing base salaries, determining the amount of the non-equity incentive awards and granting the 2018 equity awards. The fact that the Audit Committee was beginning to conduct the Investigation generally did not affect the Compensation Committee's 2018 actions.
Prior Say-on-Pay Proposals and Shareholder Support
The Company conducted an advisory say-on-pay vote at the 2016 annual meeting of shareholders, where approximately 95% of the votes cast were in favor of the proposal. The Board and Compensation Committee reviewed these final vote results together with the other factors and data discussed in this Compensation Discussion and Analysis and determined that, given the significant level of support of the Company’s approach to compensation by its shareholders, no changes to its executive compensation policies and related decisions were necessary.
Compensation Philosophy
MiMedx’s executive compensation philosophy is based on the belief that competitive compensation is essential to attract and retain highly-qualified executives and motivate them to achieve the Company’s operational and financial goals. In line with this philosophy, the Company’s practice is to provide total compensation that is competitive with comparable positions at peer organizations. The compensation program is based on individual and organizational performance and includes components that reinforce the Company’s motivational and retention-related compensation objectives.
The principal components of compensation for MiMedx’s NEOs are base salary, annual cash incentives and long-term equity incentives. Cash incentives are included to encourage and reward effective performance relative to the Company’s near-term plans and objectives. Equity incentives are included to promote longer-term focus, to help retain key contributors and to align the interests of the Company’s executives and shareholders.
Pay-Setting Process
Compensation Consultant
Beginning in mid-2018, the Compensation Committee engaged an independent executive compensation consulting firm, Meridian Compensation Partners, LLC (“Meridian”), to provide compensation consulting services relating to (1) NEO compensation, (2) peer group composition and practices, (3) incentives design, (4) compensation governance, (5) amount and form of director compensation and (6) alternatives to equity compensation. Meridian’s services were provided only to the Compensation Committee, and the Compensation Committee determined that Meridian’s work did not raise any conflict of interest.
Use of a Peer Group
In making compensation decisions, the Compensation Committee has considered the recommendations of the CEO and of a senior HR executive, which, in turn, have been informed by a compensation analysis of the practices of peer group companies, which are publicly-traded companies in the medical device, pharmaceuticals, biotechnology and life sciences sectors of the healthcare industry. The peer group selection and comparability are determined primarily using organizational criteria, revenue, market capitalization, and industry sector. The data from the peer group companies for the NEOs provides the Compensation Committee with a benchmark that it views as a point of reference, but not as a determining factor, for the compensation of the NEOs.
93
In 2018, the Company’s peer group was as follows:
Abiomed, Inc. | Geron Corporation | Momenta Pharmaceuticals, Inc. | ||
Acorda Therapeutics, Inc. | Halozyme Therapeutics, Inc. | Newlink Genetics Corp. | ||
AMAG Pharmaceuticals, Inc. | ImmunoGen, Inc. | OPKO Health, Inc. | ||
Array BioPharma, Inc. | Infinity Pharmaceuticals, Inc. | Seattle Genetics, Inc. | ||
CryoLife, Inc. | Insulet Corporation | Spectrum Pharmaceuticals, Inc. | ||
DexCom, Inc. | Insys Therapeutics, Inc. | Vanda Pharmaceuticals, Inc. | ||
Exelixis, Inc | Ionis Pharmaceuticals, Inc. | Wright Medical Group, Inc. | ||
Genomic Health, Inc. | Ironwood Pharmaceuticals, Inc. | |||
In order to compete effectively for top executive-level talent, the Compensation Committee generally targets cash compensation for the NEOs between the 50th and 60th percentile, and long-term equity compensation between the 60th and 75th percentile, of compensation paid to similarly-situated executives of the companies comprising the peer group; however, in practice and in the case of 2018, actual compensation awarded by the Compensation Committee has generally lagged these targets. Although compensation survey data are useful guides for comparative purposes, the Compensation Committee believes that a successful compensation program also requires the application of judgment and subjective determinations of individual performance. In that regard, the Compensation Committee applies its judgment in reconciling the program’s objectives with the realities of retaining valued employees.
2018 Compensation Components
Base Salaries
MiMedx employees, including its NEOs, are paid a base salary commensurate with the responsibilities of their positions, the skills and experience required for the position, their individual performance, business performance, labor market conditions, and with reference to peer company salary levels. Base salaries may be increased depending on the compensation of comparable positions within the peer group companies and published compensation surveys, the executive’s responsibilities, skills, expertise, experience and performance, the executive’s contributions to the Company’s results, and the overall performance of the Company compared to its peer group and other participants within the industry. In determining the increases, the Compensation Committee relies on judgment about each individual, as well as on recommendations from senior management, rather than applying a stated formula.
Annual Non-Equity Incentive Awards
In 2018, annual non-equity incentive awards for the NEOs were determined under the Company’s Management Incentive Plan (the “MIP”), which is an annual cash incentive plan that is designed to incentivize and reward achievement of the current year’s financial and operational goals. The MIP targets a base bonus equal to a specified percentage of the NEO’s base salary as follows: Mr. Petit, 75%; Mr. Coles, N/A; Mr. Senken, 50%; Mr. Borkowski, 60%; Mr. Taylor, 65%; Ms. Haden, 45%; Mr. Landy, 50% and Mr. Turner, 40%.
The Committee approved the 2018 MIP in February 2018 structured with two performance conditions:
•Revenue (75% weight), and
•Adjusted EBITDA (25% weight).
(The bonus opportunity for performance above target, which we call the excess bonus, was based 100% on revenue.)
However, after the resignations of Messrs. Petit, Taylor and Senken in June 2018, the Committee re-evaluated the structure of the MIP and ultimately determined, by October 2018, to revise its structure to deemphasize revenue, to introduce individual performance goals, and to weight each performance condition equally:
•Revenue (1/3 weight), and
•Adjusted EBITDA (1/3 weight);
•Individual performance goals (1/3 weight).
In addition, in order to retain and motivate employees, over the course of the year, the Compensation Committee and the Board approved further downward revisions to the revenue and Adjusted EBITDA performance targets initially set in February
94
as the Company’s performance worsened. For the same reason, the Compensation Committee also determined to adjust revenue and to make further adjustments to Adjusted EBITDA for the purpose of 2018 MIP determinations. Specifically, the Committee adjusted revenue to add back certain fees that historically had been recorded as expenses but which had been reclassified as reductions in revenue. The Committee also adjusted revenue by adding back the amount accrued for a potential regulatory liability. The Committee further adjusted Adjusted EBITDA by excluding certain fees and expenses which were not contemplated by the Company's budget and forecast when the financial metrics were set. These included certain legal, consulting, and accounting expenses associated with the Audit Committee Investigation and the Restatement, as well as certain expenses related to litigation matters and severance paid or accrued in connection with a reduction in force. These adjustments had the net effect of increasing the values of adjusted revenue and Adjusted EBITDA upon which the 2018 MIP determinations were based such that they achieved target base bonus thresholds.
As finally approved by the Committee, the 2018 MIP was structured as follows and included the following individual performance objectives:
2018 Management Incentive Plan Structure | |||||||||
Minimum | Target | Maximum | |||||||
Revenue (1/3 weight) | $308,400,000 | $350,500,000 | $375,000,000 | ||||||
Payout as % of Target Incentive | 15 | % | 100 | % | 200 | % | |||
Adjusted EBITDA (1/3 weight) | $46,795,000 | $66,850,000 | $66,850,000 | ||||||
Payout as % of Target Incentive | 10 | % | 100 | % | n/a | ||||
Individual Objectives (1/3 weight) | — | — | — | ||||||
Payout as % of Target Incentive | — | 100 | % | n/a | |||||
The NEOs’ individual performance goals were as follows:
Borkowski:
• | develop financial assumptions and components of five-year strategic plan; |
• | develop 2019 budget; |
• | restate financial statements; |
• | work with external firms to identify, assess, interview and select qualified candidates for senior finance and accounting positions; and |
• | remediate control deficiencies. |
Haden:
• | hit forecast legal spend through end of year, decrease legal spend overall and develop a framework for managing legal needs going forward; |
• | lead insurance renewal process; |
• | support transition to biologics via intellectual property program; and |
• | support upgrade of sales practices/polices/procedures. |
Landy:
• | form and lead Internal BLA Launch Team; |
• | define commercialization plans for BLA products; |
• | identify specific pipeline projects for product development; and |
• | lead execution of aspects of five-year strategic plan. |
Turner:
• | achieve operational metrics; |
• | align monthly sales and operations plan process with financial plan; |
• | define facility plan and costs; and |
• | implement facility plan to support business. |
Based on the Compensation Committee’s understanding in February 2019 of the Company’s 2018 revenues and Adjusted EBITDA, the Compensation Committee and the Board determined that a payout for the target base bonus at the 100% level for each of the NEOs was warranted. Despite modestly exceeding the revised goals, the Compensation Committee exercised downward discretion due to the lack of audited financial statements, the ongoing Audit Committee Investigation, and the fact that the performance targets had been decreased from the levels set at the beginning of 2018 and, therefore, and did not recommend the
95
payment of excess bonuses. The Committee and the Board approved 2018 non-equity incentive awards to the NEOs as follows: Borkowski—$330,000; Haden—$191,250; Landy—$117,250; and Turner—$108,500. However, after completion of the Restatement, the Committee determined that the revenue and Adjusted EBITDA portions of the 2018 non-equity incentive awards were based on unaudited financial information which was subsequently revised as part of the restatement and therefore portions of these amounts are subject to clawback. See “Compensation Discussion and Analysis—Company Policies—Recoupment of Compensation,” below.
Long-Term Equity Incentives
All equity incentive awards are granted under the Company’s 2016 Equity and Cash Incentive Plan (the “2016 Plan”), which was approved by shareholders in 2016. The 2016 Plan is designed to align the interests of the Company’s Named Executive Officers and other MiMedx officers, members of management and key employees with the interests of the Company’s shareholders, and serve as a key retention tool. Stock options and restricted stock vest over a period of time, generally pro rata annually over three years. The Committee believes that a vesting period is a positive motivator for the Company’s officers, management and key employees to focus their strategy and efforts on the Company’s long-term goals. Working toward the long term growth of the price of the Company’s stock produces the ultimate financial gain for the executives’ equity awards and increase in value for the Company’s shareholders.
In recent years, the Compensation Committee has granted only restricted stock awards, rather than a mix of stock and stock options, based on its review of market conditions and peer practices and to conserve the number of shares available under the 2016 Plan. The Compensation Committee believes that restricted stock awards are an effective form of equity compensation because they are a strong retention tool for NEOs and other key executives. Restricted stock awards increase in value as the Company’s stock price increases over time, but they also continue to have value in the event of a stock price decline. Thus, unlike stock options, restricted stock does not lose its retention value in the event of a decline in stock price. Additionally, the Compensation Committee recognizes that restricted stock awards are becoming an increasingly prevalent tool in the incentive compensation reported by our peers.
All awards of stock options and/or restricted stock to Named Executive Officers were approved by the Compensation Committee for recommendation to the full Board for approval. All awards of stock options and/or restricted stock to all other eligible participants in the 2016 Plan were determined and approved by the Compensation Committee.
In determining the approved level of equity grants, the Compensation Committee considers the individual’s target annual long term incentive value, the Company’s overall option “overhang,” the employee’s level of responsibility and performance, prior equity awards, comparative compensation information and the anticipated expense to the Company.
For 2018, all awards of stock options and restricted stock were dated and priced as follows:
• | All awards of stock options and awards of restricted stock to current employees were granted and priced as of the close of the business day on which the Committee approved the grant. |
• | All awards of stock options and awards of restricted stock granted to newly-hired employees were granted and priced as of the later of the business day on which the Committee approved such grants or the date of employment. |
The Committee establishes vesting schedules for awards under the 2016 Plan at the time of the grant. To optimize the retention value of the awards and to orient recipients to the achievement of longer-term goals, objectives and success, awards typically vest in three equal installments on the first, second and third anniversaries of the Grant Date
Historically, the Company made an annual grant of stock options and/or restricted stock awards to a broad group of its management employees, including the Named Executive Officers, in February or March of each year. It is the Compensation Committee’s intention to continue the practice of granting annual awards at the time of the Compensation Committee’s February or March meeting. In addition to the Company’s annual grant to Named Executive Officers and certain other management, professional and key employees, the Company made additional equity grants throughout the year to certain management, professional and other key employees to reward specific performance, in connection with promotions or other achievements and to address specific retention concerns, and to certain newly hired employees to attract management, professional and other key employee talent to join the Company.
In 2018, all equity-based awards were issued under plans previously approved by the Company’s shareholders.
96
2018 Restricted Stock Grants to Named Executive Officers
The Compensation Committee’s philosophy is to benchmark long-term equity incentive awards at the 60th to 75th percentile of awards to similarly-situated executives of companies in the peer group. However, the actual amount of equity awards granted to the NEOs in 2018 was less than the benchmark target grant value in order to preserve shares in the 2016 Plan.
In general, in determining the level of equity grants, the Compensation Committee considers the individual’s target annual long term incentive value, the Company’s unexercised and unvested grants, the employee’s level of responsibility and performance, prior equity awards, comparative compensation information and the anticipated expense to the Company. On February 22, 2018, each of Messrs. Petit, Taylor, Senken, Landy, and Turner, and Ms. Haden were granted 14,500; 90,900; 46,200; 17,300, 15,900, and 37,300 shares of restricted stock, respectively. Upon their promotions on December 5, 2018, the Board awarded Messrs. Landy and Turner 30,000 and 10,000 shares, respectively. All restricted shares vest in equal amounts over three years from the date of the grant.
Agreements with our Executive Officers
Agreement with Alvarez & Marsal to employ Mr. Coles
The Board appointed Mr. Coles as Interim Chief Executive Officer of the Company, effective as of July 2, 2018. In connection with his appointment, the Company entered into an engagement letter with Alvarez & Marsal North America, LLC (“A&M”), where Mr. Coles has been employed since 1997, providing for Mr. Coles’ services and the services of additional A&M employees as needed to assist Mr. Coles in the execution of his duties. Under the terms of the engagement letter, during his service at the Company, Mr. Coles would continue to be employed by A&M and will not receive any compensation directly from the Company or participate in any of the Company’s employee benefit plans. The Company will instead pay A&M an hourly rate of $975 per hour for Mr. Coles’ services, with an option to change the fee arrangement for Mr. Coles’ services to a fixed monthly fee of $200,000 per calendar month after the first 60 days of the engagement. In 2018, the Company paid A&M $1,147,751 for Mr. Coles’ services. Mr. Coles resigned on May 13, 2019 upon the hiring of our permanent Chief Executive Officer.
Agreement with Mr. Borkowski
The Board appointed Mr. Borkowski, an Executive Vice President of the Company, as interim Chief Financial Officer, effective June 6, 2018. In 2018, Mr. Borkowski received an annual salary of $550,000 and a target annual performance bonus of 60% of his base salary. He also received a $150,000 signing bonus on the 90th day following the commencement of his employment.
The Company awarded Mr. Borkowski two restricted stock grants on February 21, 2019: one for 100,000 shares, one-third of which vested immediately and the other two-thirds vest ratably over a two-year period from the date of grant; and the other for 103,305 shares, which vest ratably over a two-year period from the date of grant. These awards were contemplated, but not granted, at the time Mr. Borkowski joined the Company.
In addition, the Company agreed to provide Mr. Borkowski severance, both in connection with a change in control and other than in connection with a change in control. The Company entered into a double-trigger Change in Control Severance Agreement with Mr. Borkowski, which provides for severance payments equal to 1.75 times his base salary and target bonus on the date of the change in control; and continuation of benefits for the period for which the severance is computed. The Company also entered into a severance agreement with Mr. Borkowski that is not conditioned upon a change in control, which provides for severance payments equal to 1.0 times his annual base salary plus target bonus, plus continuation of benefits for the period for which the severance is computed, if his employment was terminated for qualifying reasons. Mr. Borkowski is also eligible for relocation benefits.
On November 18, 2019, the Company entered into a Separation and Transition Services Agreement (the “Transition Agreement”) with Mr. Borkowski pursuant to which (i) he resigned as Executive Vice President and Interim Chief Financial Officer of the Company, as well as from any and all officer, director or other positions that he held with the Company and its affiliates, effective November 15, 2019, (ii) he agreed to perform the duties of the Interim Chief Financial Officer with respect to filing the 2018 Form 10-K and assist with the transition of his duties, and (iii) until March 31, 2020, he agreed to provide services as may be requested by the Company with respect to matters related to the 2018 Form 10-K and the Company’s Annual Report on Form 10-K for the fiscal year ending December 31, 2019. The Agreement provides that the Company will pay Mr. Borkowski a special payment in installments as follows: (i) $1,700,000, which was paid within seven business days following the Transition Agreement, (ii) $1,750,000 to be paid within seven business days following the filing of the 2018 Form 10-K with the SEC; and (iii) after March 31, 2020, $500,000 to be paid within seven business days following the execution and delivery of a supplemental
97
release by Mr. Borkowski. Mr. Borkowski forfeited all stock owned by him which had not already vested, and all other claims to stock and other benefits. The Agreement also includes terms and conditions governing the Company’s and Mr. Borkowski’s general release of claims and other customary provisions.
Agreement with Ms. Haden
Ms. Haden resigned as General Counsel and Secretary effective August 12, 2019. The Company subsequently entered into a consulting agreement with her pursuant to which she will provide up to 40 hours of consulting services per month through February 29, 2020 and has executed a release of claims in favor of the Company and its affiliates. The Company will compensate Ms. Haden at the rate of $8,000 per month and will provide nine months' severance ($476,250).
Additional Compensation Practices and Policies
Perquisites
The Company generally does not provide executive officers with perquisites and other personal benefits beyond the Company benefits offered to similarly situated employees, with the following exception: when the Company hosts performance incentive trips, it pays for executives to bring their spouses at the Company’s expense. Also, Mr. Borkowski’s agreement provided for commuting and transportation expenses between his home and corporate headquarters, temporary lodging, relocation and rental car expenses.
Stock Ownership Guidelines
The Board has adopted stock ownership guidelines that apply to the NEOs. Under the guidelines, covered persons are required to own stock, including unvested time-based restricted stock, equal to certain multiples of their annual cash compensation:
Person Subject to Policy | Requirement | ||||
CEO | 3.0X | ||||
President & COO | 2.5X | ||||
CFO | 2.0X | ||||
General Counsel | 1.5X | ||||
Until such time as the NEO reaches his or her applicable threshold and subject to certain exceptions, the NEOs are required to hold 100% of the shares of Company common stock awarded to him/her from the Company or received upon vesting of restricted stock and upon exercise of stock options (net of any shares utilized to pay for tax withholding and any exercise price).
The Board has suspended the stock ownership guidelines until the Company becomes current in its SEC reporting obligations since subject persons may be prohibited by applicable insider trading laws from buying or selling Company securities.
Recoupment of Compensation
The Board adopted a recoupment (clawback) policy, effective April 1, 2016, covering executive officers of the Company. The policy provides that if the Company is required to restate its financial results due to material noncompliance with financial reporting requirements under the securities laws, the Compensation Committee may seek reimbursement of any cash or equity-based bonus or other incentive compensation paid or awarded to the officer or effect cancellation of previously granted equity awards to the extent the bonus or incentive compensation was based on erroneous financial data and was in excess of what would have been paid to the officer under the restatement.
With the completion of the restatement of Company’s previously issued consolidated financial statements and financial information, the Compensation Committee has reviewed the annual non-equity incentive awards paid to executive officers based on financial performance for the years 2015 and 2016, and the amounts that would have been paid to such officers under the corrected, restated financial statements. In addition, the Compensation Committee has reviewed the annual non-equity incentive awards paid to executive officers for 2017 and 2018, and the amounts that would have been paid to such officers under the corrected financial statements (which had never been published and therefore technically not restated). The Company did not grant any equity awards based on incorrect financial metrics. This review determined that the Company paid annual non-equity incentive awards between 2015 and 2018 to the following NEOs in excess of what would have been paid to such executive officers under the restated or revised financial metrics, by the following, aggregate amounts: Mr. Petit - $468,504; Mr. Senken - $215,550; Mr. Taylor - $356,555; Ms. Haden - $183,725; Mr. Borkowski - $88,000; Mr. Landy - $31,267; and Mr. Turner - $28,933. This review
98
also determined that the Company did not grant any equity awards based on incorrect financial metrics. The Compensation Committee has not yet reached a final determination as to whether or how to recoup the amounts stated above.
The Committee notes that the Company effectively recovered $26.3 million of vested, unexercised options and unvested restricted stock as a result of the Board’s determination that the terminations of employment of Messrs. Petit, Senken and Taylor were “for cause,” which resulted in the forfeiture of those awards. On September 20, 2018, the Company announced that the Board and the Compensation Committee had each determined that the previously announced separations of four senior Company executives, including Messrs. Petit, Taylor and Senken (collectively, the “Separated Officers”), be treated as “for cause.” The Company announced that, as a result of findings related to the conduct of these individuals in addition to one non-executive officer, the Board and the Compensation Committee, as the administrators of the Plans, had taken all required action to cause all equity and incentive awards outstanding under the Plans held by the Separated Officers to be forfeited. Under the Plans, all unvested restricted stock awards and vested and unvested stock option awards were forfeited, as follows:
Former NEO | Options Forfeited | Value on 9/20/2018 at $6.20 per share | Unvested Restricted Stock Forfeited | Value on 9/20/2018 at $6.20 per share | Total Value of Equity Forfeited | |||||||
Petit | 2,867,820 | $12.1 million | 361,667 | $2.2 million | $14.3 million | |||||||
Senken | 887,107 | $3.7 million | 120,368 | $0.7 million | $4.4 million | |||||||
Taylor | 1,558,221 | $6.2 million | 229,234 | $1.4 million | $7.6 million | |||||||
TOTAL | 5,313,148 | $22.0 million | 711,269 | $4.3 million | $26.3 million | |||||||
(The value of forfeited options is based on market price at close of business on date of forfeiture, which was September 20, 2018 and $6.20 per share, less the exercise price. The value of forfeited restricted stock based on market price at close of business on date of forfeiture.)
The Committee also notes that on November 26, 2019, the SEC filed suit against Messrs. Petit, Senken and Taylor in the U.S. District Court for the Southern District of New York, including claims for relief as to Messrs. Petit and Senken for the disgorgement of all bonuses, incentive-based and equity-based compensation pursuant to Section 304 of the Sarbanes-Oxley Act of 2002, among other claims for relief. The Committee further notes that Messrs. Landy and Turner only became executive officers in December 2018 and therefore were subject to the policy for less than one month.
Anti-Hedging and Pledging
The Company prohibits directors, officers and employees from engaging in hedging transactions, subject to exceptions granted in the sole discretion of the General Counsel in limited circumstances. Hedging transactions may permit one to continue to own Company securities without the full risks and rewards of ownership. When that occurs, the director, officer or employee may no longer have the same objectives as the Company’s other shareholders.
The Company prohibits directors, officers and other employees from holding Company securities in a margin account or otherwise pledging Company securities as collateral for a loan. Securities held in a margin account as collateral for a margin loan may be sold by the broker without the customer’s consent if the customer fails to meet a margin call. Similarly, securities pledged as collateral for a loan may be sold if the borrower defaults on the loan, including at a time when the pledgor is aware of material nonpublic information or otherwise is not permitted to trade in Company securities,
Compensation Risk Assessment
On an ongoing basis, the Compensation Committee considers the risks inherent in the Company’s compensation programs. With the change in the structure of the annual non-equity incentive compensation awards in late 2018, which de-emphasizes revenue, the Compensation Committee believes that our compensation policies and practices do not encourage excessive and unnecessary risk-taking, and that the level of risk that they do encourage is not reasonably likely to have a material adverse effect on the Company. The design of our compensation policies and practices encourages our employees to remain focused on both our short and long-term goals.
99
COMPENSATION COMMITTEE REPORT
The Compensation Committee has reviewed the Compensation Discussion and Analysis in this Annual Report and discussed it with management. Based on its review and discussions with management, the Compensation Committee recommended to the Board that the Compensation Discussion and Analysis be included in this Annual Report. This report is provided by the following independent directors, who comprise the Compensation Committee:
Richard J. Barry, Chair (member of the Committee since June 19, 2019) |
James L. Bierman (member of the Committee since July 23, 2019) |
Neil S. Yeston (member of the Committee since September 17, 2012) |
March 17, 2020
100
SUMMARY COMPENSATION TABLE
Executive Officers as of December 31, 2018
Name and Principal Position | Period | Salary | Bonus(6) | Stock(7) Awards | Option Awards | Non-Equity Incentive Plan Compensation Awards | All Other(8) Compensation | Total | ||||||||
David Coles,(1) Former Interim Chief Executive Officer | 2018 | $0 | $0 | $0 | $0 | $0 | $0 | $0 | ||||||||
Edward Borkowski,(2) EVP and Interim Chief Financial Officer | 2018 | $363,846 | $150,000 | $0 | $0 | $330,000 | $47,294 | $891,140 | ||||||||
Alexandra O. Haden(3) General Counsel and Secretary | 2018 | $418,365 | $0 | $331,224 | $0 | $191,250 | $9,496 | $950,335 | ||||||||
2017 | $382,673 | $153,231 | $580,300 | $0 | $271,350 | $5,761 | $1,393,315 | |||||||||
2016 | $327,884 | $0 | $267,960 | $0 | $0 | $3,786 | $599,630 | |||||||||
I. Mark Landy,(4) EVP and Chief Strategy Officer | 2018 | $327,788 | $100,000 | $199,824 | $0 | $117,250 | $0 | $744,862 | ||||||||
Scott M. Turner,(5) SVP, Operations & Procurement | 2018 | $302,788 | $0 | $156,592 | $0 | $108,500 | $9,978 | $577,858 | ||||||||
(1) | The Board appointed Mr. Coles as Interim Chief Executive Officer effective July 2, 2018. The Company paid his employer, A&M, $1,147,751 for Mr. Coles’ services in 2018. Mr. Coles stepped down from his position as of May 13, 2019, when Mr. Wright became the Company’s new Chief Executive Officer. |
(2) | The Board appointed Mr. Borkowski as Interim Chief Financial Officer effective June 6, 2018. He resigned effective November 15, 2019. |
(3) | On August 12, 2019, Ms. Haden resigned from her position as General Counsel and Secretary of the Company. |
(4) | The Board appointed Mr. Landy as Executive Vice President and Chief Strategy Officer effective December 5, 2018. On September 16, 2019, the Company notified Mr. Landy that the position of Chief Strategy Officer was eliminated on such date. |
(5) | The Board designated Mr. Turner as an executive officer on December 5, 2018. |
(6) | The bonus for Mr. Borkowski reflects a one-time cash signing bonus. Mr. Landy received a one-time cash bonus for his outstanding performance. |
(7) | Represents the aggregate grant date fair value of awards of restricted stock made to the executive officer in accordance with FASB ASC Topic 718. The restricted stock awards vest pro rata annually over a three-year period. In addition to their February 2018 grants, Messrs. Landy and Turner received additional equity awards in December when their roles were enlarged as part of a management restructuring. |
(8) | Represents the following amounts: (a) reimbursement for travel expenses for their spouses to attend certain work-related events: Haden—$2,621; Turner—$3,103; (b) 401(k) match: Borkowski, Haden and Turner—each, $6,875; (c) commuting expense between Atlanta and personal residence: Borkowski—$6,040; (d) lodging in Atlanta: Borkowski—$22,099; and (e) automobile lease, Borkowski—$12,280. |
101
Former Executive Officers
Name and Principal Position | Period | Salary | Bonus(4) | Stock(5) Awards | Option Awards | Non-Equity Incentive Plan Compensation Awards | All Other(6) Compensation | Total | ||||||||
Parker H. “Pete” Petit,(1) former Chairman and Chief Executive Officer | 2018 | $457,163 | $0 | $1,283,160 | $0 | $0 | $4,651 | $1,744,974 | ||||||||
2017 | $639,904 | $318,625 | $1,989,600 | $0 | $731,250 | $0 | $3,679,379 | |||||||||
2016 | $602,904 | $0 | $1,088,080 | $0 | $0 | $2,796 | $1,693,780 | |||||||||
Michael J. Senken,(2) former Chief Financial Officer | 2018 | $281,813 | $0 | $410,256 | $0 | $0 | $6,875 | $698,944 | ||||||||
2017 | $403,462 | $162,275 | $663,200 | $0 | $311,250 | $3,584 | $1,543,771 | |||||||||
2016 | $365,039 | $0 | $324,800 | $0 | $0 | $0 | $689,839 | |||||||||
William C. Taylor,(3) former President and Chief Operating Officer | 2018 | $376,442 | $0 | $807,192 | $0 | $0 | $11,726 | $1,195,360 | ||||||||
2017 | $527,962 | $242,745 | $1,243,500 | $0 | $531,375 | $4,825 | $2,550,407 | |||||||||
2016 | $502,170 | $0 | $690,200 | $0 | $0 | $4,086 | $1,196,456 | |||||||||
(1) | Mr. Petit resigned as Chief Executive Officer effective June 30, 2018. On September 20, 2018, the Company announced that the Compensation Committee and the Board determined that his termination would be treated as “for cause.” |
(2) | Mr. Senken resigned as Chief Financial Officer effective June 6, 2018 and continued in a transitional role through June 30, 2018. On September 20, 2018, the Company announced that the Compensation Committee and the Board determined that his termination would be treated as “for cause.” |
(3) | Mr. Taylor resigned as President and Chief Operating Officer effective June 30, 2018. On September 20, 2018, the Company announced that the Compensation Committee and the Board determined that his termination would be treated as “for cause.” |
(4) | Represents a one-time cash bonus, paid in recognition of outstanding performance, and a one-time bonus of restricted share awards. Amount reported for restricted share awards is grant date fair value. |
(5) | Represents the aggregate grant date fair value of awards of restricted stock made to the executive officer in accordance with FASB ASC Topic 718. The restricted stock awards vest pro rata annually over a three-year period. See the “Forfeited Awards Table” as all of the restricted stock awards granted in 2018 were forfeited. |
(6) | Represents the following amounts: for 2018: (a) reimbursement for travel expenses for their spouses to attend certain work-related events: Petit—$4,651; Taylor—$4,851; and (b) 401(k) match: Senken—$6,875; Taylor—$6,875. |
102
GRANTS OF PLAN-BASED AWARDS FOR 2018
The following table provides information regarding grants of plan-based awards to the Company’s NEOs during 2018.
Executive Officers as of December 31, 2018
Estimated Future Payouts Under Non-Equity Incentive Plan Awards(1) | All Other Stock Awards: Number of Shares of Stock or Units(2) | All Other Option Awards: Number of Securities Underlying Options | Exercise or Base Price of Option Awards | Grant Date Fair Value of Stock and Option Awards(3) | ||||||||||||||||
Name | Grant Date | Threshold | Target | Maximum | ||||||||||||||||
Coles | — | $0 | $0 | $0 | — | — | — | $0 | ||||||||||||
Borkowski | 4/3/2018 | $27,500 | $330,000 | $660,000 | — | — | — | $0 | ||||||||||||
Haden | 2/22/2018 | $15,938 | $191,250 | $382,500 | 37,300 | — | — | $331,224 | ||||||||||||
Landy | 2/22/2018 | $9,771 | $117,250 | $234,500 | 17,300 | — | — | $153,624 | ||||||||||||
12/11/2018 | $0 | $0 | $0 | 30,000 | — | — | $46,200 | |||||||||||||
Turner | 2/22/2018 | $9,042 | $108,500 | $217,000 | 15,900 | — | — | $141,192 | ||||||||||||
12/11/2018 | $0 | $0 | $0 | 10,000 | — | — | $15,400 | |||||||||||||
(1) | For Non-Equity Incentive Plan Awards, these columns show the range of possible cash payouts that could have been earned by each of the NEOs under the 2018 MIP. “Threshold” represents the lowest possible payout if there is a payout and “Maximum” reflects the highest possible payout. In 2018, threshold performance would have resulted in a 15% payout of the revenue portion, a 10% payout of the Adjusted EBITDA portion and a 0% payout of the individual performance portion of the award. |
(2) | Represents restricted stock awards granted under the 2016 Plan. The restricted shares vest ratably over three years from the grant date. |
(3) | The amounts shown reflect the grant date fair market values of the awards computed in accordance with FASB ASC Topic 718—“Compensation-Stock compensation.” |
Former Executive Officers
Estimated Future Payouts Under Non-Equity Incentive Plan Awards(1) | All Other Stock Awards: Number of Shares of Stock or Units(2) | All Other Option Awards: Number of Securities Underlying Options | Exercise or Base Price of Option Awards | Grant Date Fair Value of Stock and Option Awards(3) | |||||||||||||||
Name | Grant Date | Threshold | Target | Maximum | |||||||||||||||
Petit | 2/22/2018 | $73,219 | $532,500 | $1,065,000 | 144,500 | — | — | $1,283,160 | |||||||||||
Senken | 2/22/2018 | $30,250 | $220,000 | $440,000 | 46,200 | — | — | $410,256 | |||||||||||
Taylor | 2/22/2018 | $53,625 | $390,000 | $780,000 | 90,900 | — | — | $807,192 | |||||||||||
(1) | For Non-Equity Incentive Plan Awards, these columns show the range of possible cash payouts that could have been earned by each of the NEOs under the 2018 MIP. “Threshold” represents the lowest possible payout if there is a payout and “Maximum” reflects the highest possible payout. In 2018, threshold performance would have resulted in a 15% payout of the revenue portion and a 10% payout of the Adjusted EBITDA portion of the award. Importantly, although the non-equity incentive plan awards were granted to Messrs. Petit, Senken and Taylor in February 2018, none of them actually received these awards for 2018 given the “for cause” termination finding in September 2018. |
(2) | Represents restricted stock awards granted under the 2016 Plan. The restricted shares vest ratably over three years from the grant date. See the “Forfeited Awards Table” as the 2018 awards have been forfeited. |
(3) | The amounts shown reflect the grant date fair market values of the awards computed in accordance with FASB ASC Topic 718—“Compensation-Stock compensation.” |
103
OUTSTANDING EQUITY AWARDS ON DECEMBER 31, 2018
The following table shows the number of shares covered by exercisable and un-exercisable options and unvested restricted stock awards held by the Company’s NEOs on December 31, 2018. As discussed in the CD&A, Messrs. Petit, Senken, and Taylor forfeited all unvested restricted stock and all vested and unvested stock options held by Petit, Senken, and Taylor during 2018.
Option Awards | Stock Awards | ||||||||||||||||
Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price | Option Expiration Date | Number of Securities Unvested | Market Value of Unvested Securities(11) | |||||||||||
Coles | — | — | — | ||||||||||||||
Borkowski | — | — | — | ||||||||||||||
Haden | 60,000 | $6.02 | 7/16/2023 | ||||||||||||||
20,350 | $7.24 | 2/25/2024 | |||||||||||||||
20,000 | $5.84 | 4/24/2024 | |||||||||||||||
11,000 | (1) | $19,690 | |||||||||||||||
46,667 | (6) | $83,534 | |||||||||||||||
5,000 | (8) | $8,950 | |||||||||||||||
37,300 | (9) | $66,767 | |||||||||||||||
Landy | — | — | 9,334 | (3) | $16,708 | ||||||||||||
2,334 | (5) | $4,178 | |||||||||||||||
16,667 | (6) | $29,834 | |||||||||||||||
4,000 | (7) | $7,160 | |||||||||||||||
5,000 | (8) | $8,950 | |||||||||||||||
17,300 | (9) | $30,967 | |||||||||||||||
30,000 | (10) | $53,700 | |||||||||||||||
Turner | — | — | 5,000 | (2) | $8,950 | ||||||||||||
1,167 | (4) | $2,089 | |||||||||||||||
20,000 | (6) | $35,800 | |||||||||||||||
2,000 | (8) | $3,580 | |||||||||||||||
15,900 | (9) | $28,461 | |||||||||||||||
10,000 | (10) | $17,900 | |||||||||||||||
(1) | The remaining balance vested on February 22, 2019. |
(2) | The remaining balance vested on April 25, 2019. |
(3) | The remaining balance vested on July 25, 2019. |
(4) | The remaining balance vested on October 26, 2019. |
(5) | The remaining balance vested and on December 14, 2019. |
(6) | An installment vested on February 22, 2019 and the remaining balance vested on February 22, 2020. |
(7) | An installment vested on July 26, 2019 and the remaining balance was scheduled to vest on July 26, 2020. |
(8) | An installment vested on October 26, 2019 and the remaining balance was scheduled to vest on October 26, 2020. |
(9) | Installments vested on February 22, 2019 and February 22, 2020, and the remaining balance was scheduled to vest on 2021. |
(10) | An installment vested on December 11, 2019, and the remaining balance was scheduled to vest December 11, 2020 and 2021. |
(11) | Calculated based on a closing stock price of $1.79 per share on December 31, 2018. |
104
2018 OPTION EXERCISES AND STOCK VESTED TABLE
The following table provides information concerning each exercise of stock options and each vesting of restricted stock during 2018, on an aggregated basis with respect to each of the Company’s NEOs.
Executive Officers as of December 31, 2018
Option Awards | Stock Awards | ||||||||||||
Name | Number of Securities Acquired on Exercise | Value Realized on Exercise | Number of Securities Acquired on Vesting | Value Realized on Vesting(1) | |||||||||
Coles | — | — | — | — | |||||||||
Borkowski | — | — | — | — | |||||||||
Haden | — | — | 43,509 | $366,016 | |||||||||
Landy | — | — | 24,499 | $140,775 | |||||||||
Turner | — | — | 17,167 | $141,398 | |||||||||
(1) | Represents the number of shares acquired on vesting multiplied by the closing price of Company common stock on the vesting date. |
Former Executive Officers
Option Awards | Stock Awards | |||||||||||
Name | Number of Securities Acquired on Exercise | Value Realized on Exercise | Number of Securities Acquired on Vesting | Value Realized on Vesting(1) | ||||||||
Petit | — | — | 162,183 | $1,400,793 | ||||||||
Senken | — | — | 50,507 | $437,469 | ||||||||
Taylor | 20,000 | $112,400 | 102,345 | $883,611 | ||||||||
(1) | Represents the number of shares acquired on vesting multiplied by the closing price of Company common stock on the vesting date. Because the vesting date of the restricted stock awards is the anniversary of the date of grant, which is in the first quarter, restricted stock awards vested in 2018 prior to the September 20, 2018 “for cause” termination. |
105
2018 POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
This section describes additional payments that the Company would make to the NEOs assuming a hypothetical termination of employment occurs on December 31, 2018 under various scenarios. We did not include Messrs. Petit, Taylor and Senken in the table below because they were not employed by the Company on December 31, 2018.
The Company entered into an agreement with A&M related to the employment of Mr. Coles on an hourly basis. The Company was not obligated to pay either A&M or Mr. Coles any amount in connection with his termination of employment for any reason.
The Company entered into a letter agreement with Mr. Borkowski that obligated the Company to make two stock grants to him and, if such stock grants were not made prior to his termination without cause and for good reason, or the consummation of a change in control, the Company would make a lump sum payment of $750,000 with respect to each grant not made. On December 31, 2018, the Company had not made either grant.
The Company has entered into severance agreements with Messrs. Borkowski, Landy and Turner. The agreements provide for compensation to the executive in the event the executive’s employment with the Company is terminated involuntarily without “Cause” (as defined in the respective agreements), or if the executive voluntarily terminates employment for “Good Reason” (as defined in the respective agreements). The compensation payable under the agreements is a lump sum severance payment equal to a multiple of the executive’s annual base salary and targeted base bonus as of the date of termination. The multiples are 1.0, 1.0, and 0.5 for Messrs. Borkowski, Landy and Turner, respectively. In addition, following termination of employment, these executives are entitled to receive life, health insurance coverage (subject to a COBRA election), and certain other fringe benefits equivalent to those in effect at the date of termination for period of 12 months, 12 months, and 6 months for each of Messrs. Borkowski, Landy and Turner, respectively.
The Company has entered into change-in-control severance agreements with Mr. Borkowski, Ms. Haden and Mr. Landy. The agreements provide for compensation to the executive in the event the executive’s employment with the Company is terminated following the consummation of a “change-in-control” for reasons other than the executive’s death, disability or for “Cause” (as defined in the respective agreements), or if the executive voluntarily terminates employment for “Good Reason” (as defined in the respective agreements). The compensation payable under the agreements is a lump sum severance payment equal to a multiple of the executive’s annual base salary and targeted base bonus as of the date of the change-in-control. The multiples are 1.75, 1.0, and 1.5 for Mr. Borkowski, Ms. Haden and Mr. Landy, respectively. In addition, following termination of employment, these executives are entitled to receive life, health insurance coverage (subject to a COBRA election), and certain other fringe benefits equivalent to those in effect at the date of termination for periods of 21 months, 12 months, and 18 months for Mr. Borkowski, Ms. Haden and Mr. Landy, respectively. The agreements require the executive to comply with certain covenants that preclude the executive from competing with the Company or soliciting customers or employees of the Company for a period following termination of employment equal to the period for which fringe benefits are continued under the applicable agreement. The agreements expire three years after a change in control of the Company or any successor to the Company.
Upon a “change in control,” as defined in the 2006 Plan and subject to any requirements of Section 409A of the Internal Revenue Code of 1986, as amended, all outstanding awards vest and become exercisable. The Compensation Committee has discretion whether to provide that awards granted under the 2016 Plan will vest upon a “change in control.” Thus far, the Committee has exercised such discretion and provided for full vesting upon a change in control for all awards granted under the 2016 Plan to NEOs to date.
106
Executive | Involuntary Without Cause or for Good Reason | Involuntary or for Good Reason with Change in Control | Death or Disability | |||||||||
Coles | ||||||||||||
cash severance | $0 | $0 | $0 | |||||||||
estimated benefits | $0 | $0 | $0 | |||||||||
estimated value of accelerated equity awards | $0 | $0 | $0 | |||||||||
Borkowski | ||||||||||||
cash severance | $2,380,000 | (1)(5) | $3,040,000 | (1)(2)(5) | $0 | |||||||
estimated benefits | $15,583 | (3) | $27,270 | (2)(3) | $0 | |||||||
estimated value of accelerated equity awards | $0 | $0 | $0 | |||||||||
Haden | ||||||||||||
cash severance | $0 | $616,250 | (1)(2) | $0 | ||||||||
estimated benefits | $0 | $5,194 | (2)(3) | $0 | ||||||||
estimated value of accelerated equity awards | $0 | $178,941 | (4) | $178,941 | (4) | |||||||
Landy | ||||||||||||
cash severance | $616,250 | (1) | $924,375 | (1)(2) | $0 | |||||||
estimated benefits | $42 | (3) | $63 | (2)(3) | $0 | |||||||
estimated value of accelerated equity awards | $0 | $151,497 | (4) | $151,497 | (4) | |||||||
Turner | ||||||||||||
cash severance | $238,000 | (1) | $0 | $0 | ||||||||
estimated benefits | $7,791 | (3) | $0 | $0 | ||||||||
estimated value of accelerated equity awards | $0 | $96,780 | (4) | $96,780 | (4) | |||||||
(1) | Includes (a) annual base salary as of December 31, 2018, plus (b) annual targeted bonus for the year ended December 31, 2018, times the multiple applicable to the NEO. |
(2) | Payable only in the event the executive’s employment is terminated without cause or for “good reason” within three years following a change in control. |
(3) | Includes (a) the estimated value of medical, dental, vision and life insurance, plus (b) the employer’s cost of FICA for the duration of the severance period. |
(4) | Includes the value of (a) unvested stock options as of December 31, 2018 that are in-the-money based on the December 31, 2018 closing stock price of $1.79, plus (b) unvested restricted stock based on the December 31, 2018 stock price, the vesting of which is deemed accelerated to December 31, 2018. |
(5) | Also includes $1.5 million pursuant to a letter agreement with Mr. Borkowski when he first joined the Company. With respect to two promised restricted stock grants, the agreement provided that he would receive a lump sum payment of $750,000 if one grant was not made before his termination, and another lump sum payment of $750,000 if one-third of the other grant did not vest before his termination. As of December 31, 2018, the Company had not made either grant. |
(6) | On August 12, 2019, Ms. Haden resigned from her position as General Counsel and Secretary of the Company. On August 27, 2019, the Company and Ms. Haden entered into a consulting agreement, pursuant to which Ms. Haden will provide up to 40 hours of consulting services per month through February 29, 2020 and has executed a release of claims in favor of the Company and its affiliates. The Company will compensate Ms. Haden at the rate of $8,000 per month and will provide nine months’ severance ($476,250). |
(7) | On September 16, 2019, the Company notified Mr. Landy that the position of Chief Strategy Officer was eliminated on such date. |
107
2018 DIRECTOR COMPENSATION
The Company compensates non-employee directors with a mix of equity and cash. Directors who are full-time Company employees do not receive any compensation for their service as directors or as members of Board committees. The Company attempts to compensate non-employee directors at approximately the median of peer practices. The 2016 Plan imposes limits on awards to directors for their service as directors of (i) 125,000 shares granted during any calendar year and (ii) a maximum of $300,000 for any consecutive 12-month period for awards stated with reference to a specific dollar amount.
Upon being first elected or appointed to the Board, each non-employee director receives a one-time grant of restricted shares of Company common stock valued at $50,000, plus a prorated portion of the prior year’s annual grant (based on the number of months between the date of appointment to the Board and targeted date for the next annual meeting of shareholders). This grant vests on the first anniversary of the grant date. In addition, each non-employee director receives an annual grant of restricted shares of Company common stock valued at $175,000. The Board usually makes this grant on the date of the annual meeting of shareholders and vests on the first anniversary of the grant date. However, the Board did not make the annual equity grant to directors in 2018 in light of the then-pending Audit Committee investigation and related restatements of the Company’s consolidated financial statements and financial information.
The Company also pays the following cash amounts to non-employee directors:
Chairman | Non-Chair Member | |||
Board | $71,000 | $42,000 | ||
Audit Committee | $21,000 | $11,000 | ||
Compensation Committee | $16,000 | $8,500 | ||
Nominating and Corporate Governance | $11,000 | $6,000 | ||
Science and Research Liaison | $15,000 | n/a | ||
Ethics and Compliance Committee | $12,500 | $6,500 | ||
Special Litigation Committee | $15,000 | $7,500 | ||
The following table provides information concerning 2018 compensation of the Company’s non-employee directors who served in 2018.
Name | Year | Fees Earned or Paid in Cash | Stock Awards(1) | Total | ||||
Luis A. Aguilar(2) | 2018 | $64,042 | — | $64,042 | ||||
Joseph G. Bleser(3) | 2018 | $70,875 | — | $70,875 | ||||
J. Terry Dewberry | 2018 | $69,000 | — | $69,000 | ||||
Charles R. Evans | 2018 | $97,167 | — | $97,167 | ||||
Bruce L. Hack(3) | 2018 | $48,000 | — | $48,000 | ||||
Charles E. Koob | 2018 | $42,000 | — | $42,000 | ||||
Larry W. Papasan(4) | 2018 | $61,500 | — | $61,500 | ||||
Neil S. Yeston | 2018 | $66,167 | — | $66,167 | ||||
(1) | The following directors had stock options outstanding as of December 31, 2018: Bleser, Dewberry and Hack—each with 110,000; Papasan—87,000; Koob—75,000; and Evans and Yeston—each with 60,000. The following directors had stock unvested restricted stock outstanding as of December 31, 2018: Aguilar—14,574. |
(2) | Mr. Aguilar resigned from the Board on September 19, 2019. |
(3) | Mr. Bleser's and Mr. Hack's terms expired at the 2018 Annual Meeting. |
(4) | Mr. Papasan resigned from the Board following the 2018 Annual Meeting on June 17, 2019. |
108
Director Stock Ownership Guidelines
The Nominating and Corporate Governance Committee adopted stock ownership guidelines for the Company’s non-employee directors to better align the interests of non-employee directors with shareholders. The guidelines require non-employee directors to own shares of Company common stock with a value equal to or greater than three times their annual gross cash compensation. Newly elected directors have three years from the date of election to the Board to comply with the ownership guidelines. Shares must be owned directly by the director or the director’s immediate family members residing in the same household, held in trust for the benefit of the non-employee director or the director’s immediate family or owned by a partnership, limited liability company or other entity to the extent of the director’s interest therein (including the interests of the director’s immediate family members residing in the same household) provided that the individual has the power to vote or dispose of the shares. Unvested shares of restricted stock and unexercised stock options (vested or unvested) do not count toward satisfaction of the guidelines. The Board has suspended application of these stock ownership guidelines because the Company is not current in its SEC reporting obligations and the Company’s insider trading policy prevents the non-employee directors from buying or selling shares of Company common stock at this time.
Compensation Committee Interlocks and Insider Participation
During 2018, the following persons served on the Compensation Committee: Joseph G. Bleser, Larry W. Papasan, and Neil S. Yeston. No member of the Compensation Committee is or has been an officer or employee of the Company. During 2018, none of the Company’s executive officers served on the board of directors or compensation committee of any other entity that had an executive officer that serves on the Company’s Board or Compensation Committee.
CEO Pay Ratio
In 2018, we paid total annual compensation to our median employee of $104,702. Because we did not pay compensation to our CEO in 2018, we do not have a CEO Pay Ratio for 2018. However, if we use the amounts we paid to A&M for Mr. Coles’ services, which was $1,147,751, then the pay ratio would be 11:1. We determined our median employee using “gross pay” from our payroll system, which is essentially all W-2 income other than equity compensation, for all employees other than our CEO, based on information as of December 31, 2018. We excluded our two non-U.S. employees in determining the median employee. The total number of U.S. and non-U.S. employees as of December 31, 2018 was 749.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
EQUITY COMPENSATION PLAN INFORMATION
The following table provides information about the Company’s equity compensation plans as of December 31, 2018.
Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans | |||||
Equity compensation plans approved by security holders | 3,697,147 | $4.59 | 7,671,401 | |||||
Equity compensation plans not approved by security holders | — | $0 | — | |||||
Total | 3,697,147 | $4.59 | 7,671,401 | |||||
109
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information as of March 3, 2020 regarding the Company’s capital stock, beneficially owned by each person known to the Company to beneficially own more than 5% of the outstanding shares of Company common stock, each NEO, each director, and all directors and executive officers as a group. Unless otherwise indicated below the address of those identified in the table is c/o MiMedx Group, Inc., 1775 West Oak Commons Court, NE, Marietta, Georgia 30062.
Name of Beneficial Owner | Number of Shares(1) | Percentage Ownership(1) | |||||
Prescience Investment Group, LLC(2) | 7,618,335 | 6.7% | |||||
Group One Trading, LP(3) | 6,379,103 | 5.6% | |||||
NEOs, Executive Officers, and Directors(1) | Number of Shares(1) | Percentage Ownership(1) | |||||
Richard J. Barry(4) | 3,300,000 | 2.8% | |||||
M. Kathleen Behrens, Ph.D.(4) | — | * | |||||
James L. Bierman(4) | — | * | |||||
Edward J. Borkowski(5) | 21,194 | * | |||||
Peter M. Carlson(6) | 49,295 | * | |||||
David Coles(7) | — | * | |||||
J. Terry Dewberry(8)(9) | 187,126 | * | |||||
Charles R. Evans(9)(10) | 125,460 | * | |||||
Mark P. Graves(11) | 48,098 | * | |||||
Alexandra O. Haden(12) | 254,854 | * | |||||
William F. Hulse IV(13) | — | * | |||||
Charles E. Koob(9)(14) | 1,520,628 | 1.3% | |||||
I. Mark Landy(15) | 33,529 | * | |||||
K. Todd Newton(4) | — | * | |||||
Parker H. Petit(16) | 4,325,595 | 3.7% | |||||
Michael J. Senken(17) | 150,162 | * | |||||
Scott M. Turner | 109,910 | * | |||||
Timothy R. Wright | 681,818 | * | |||||
Neil S. Yeston(9)(18) | 130,460 | * | |||||
Total Directors and Executive Officers(19) (14 persons) | 6,173,989 | 5.3% | |||||
* | Less than 1% |
110
(1) | The beneficial ownership set forth in the table is determined in accordance with SEC rules. The percentage of beneficial ownership is based on 110,545,275 shares of Company common stock outstanding on March 3, 2020, plus 2,643,882 share deemed outstanding pursuant to Rule 13d-3 under the Exchange Act. |
(2) | On May 30, 2019, Prescience Investment Group, LLC filed an amendment to its Schedule 13D indicating shared voting power and dispositive power over 7,618,335 shares, shared voting power and dispositive power over 4,888,652 shares by Prescience Partners, LP, shared voting power and dispositive power over 1,845,539 shares by Prescience Point Special Opportunity LP, and shared voting power and dispositive power over 6,734,191 shares by Prescience Capital, LLC. The address for Prescience Investment Group, LLC is 1670 Lobdell Avenue, Suite 200, Baton Rouge, LA 70806. |
(3) | According to the most recent Schedule 13G filed with the SEC on January 31, 2019, Group One Trading, LP had sole voting and dispositive power with respect to 6,379,103 shares. The address for Group One Trading, LP is 440 South LaSalle St, Ste. 3232, Chicago, IL 60605. |
(4) | Does not includes restricted stock units granted on October 22, 2019 with a value of $225,000 which will be settled in common stock based on a stock price determined after the 2019 annual meeting of shareholders and after the Company becomes current in its reporting obligations. |
(5) | Mr. Borkowski resigned as Executive Vice President and Interim Chief Financial Officer effective November 15, 2019. He continues to serve as principal financial officer and principal accounting officer. |
(6) | Mr. Carlson joined the Company as Executive Vice President, Finance, on December 16, 2019. |
(7) | Mr. Coles served as Interim Chief Executive Officer until May 13, 2019. |
(8) | Includes 60,000 shares issuable upon the exercise of options. |
(9) | Does not includes restricted stock units granted on October 22, 2019 with a value of $175,000 which will be settled in common stock based on a stock price determined after the 2019 annual meeting of shareholders and after the Company becomes current in its reporting obligations. |
(10) | Includes 60,000 shares issuable upon the exercise of options. |
(11) | Includes 44,558 shares of restricted stock subject to forfeiture. |
(12) | On July 31, 2019, Alexandra O. Haden resigned as General Counsel and Secretary effective August 12, 2019. Includes 53,201 shares of unvested restricted stock, 3,300 shares owned by Ms. Haden’s spouse and 100,350 shares issuable upon the exercise of options. |
(13) | Mr. Hulse joined the Company as General Counsel on December 2, 2019. |
(14) | Includes 1,375,627 shares held by a trust and 60,000 shares issuable upon the exercise of options. |
(15) | The Company eliminated Mr. Landy's position of Chief Strategy Officer effective September 16, 2019. |
(16) | Based on the Schedule 14A filed by the Petit Group on April 11, 2019. Mr. Petit resigned as Chief Executive Officer effective June 30, 2018. Mr. Petit’s address is 1650 Cox Road, Roswell, Georgia 30075. |
(17) | Mr. Senken resigned as Chief Financial Officer effective June 6, 2018. Number of shares based solely on record ownership, and includes 50,000 shares jointly owned by Mr. Senken's spouse. Mr. Senken’s address is 145 Inwood Terrace, Roswell, GA 30075. |
(18) | Includes 60,000 shares issuable upon the exercise of options. |
(19) | Represents the ownership of only those persons currently serving as a director or executive officer of the Company. |
111
Item 13. Certain Relationships and Related Transactions, and Director Independence
Policies and Procedures for Approval of Related Party Transactions
Under its charter, the Audit Committee is responsible for reviewing and approving all transactions or arrangements between the Company and Section 16 reporting persons and any of their respective affiliates, associates or related parties. In determining whether to approve or ratify a related party transaction, the Audit Committee considers all relevant facts and circumstances available to it, such as:
• | Whether the terms of the transaction are fair to the Company and at least as favorable to the Company as would apply if the transaction did not involve a related party; |
• | Whether there are demonstrable business reasons for the Company to enter into the transaction; |
• | Whether the transaction would impair the independence of an outside director; and |
• | Whether the transaction would present an improper conflict of interest for any director or executive officer, taking into account the size of the transaction, the direct or indirect nature of the related party’s interest in the transaction and the ongoing nature of any proposed relationship, and any other factors the Audit Committee deems relevant. |
Related Party Transactions
The Company has employed Thomas Koob as its Chief Scientific Officer (a non-executive officer) since 2006. Thomas Koob is the brother of a director, Charles Koob. Subsequent to the Company’s employment of Thomas Koob, Charles Koob was appointed as a director of the Company in March 2008. In 2018, the Company paid Thomas Koob a salary of $233,003 and provided equity, incentive compensation and other compensation of $306,326. In 2019, the Company paid Thomas Koob an annual salary of $235,210 and provided equity, incentive compensation and other compensation of $155,957.
The Company employs Simon Ryan, the brother-in-law of our former General Counsel, Alexandra O. Haden, as a sales representative. In 2018, the Company paid Mr. Ryan total compensation of $183,659, consisting of a salary of $95,000 and sales commissions, equity and other compensation of $88,659. In 2019, the Company paid Mr. Ryan total compensation of $152,126, consisting of a salary of $95,000 and sales commissions, equity and other compensation of $57,126.
See also Note 18, “Related Party Transactions.”
Director Independence
Although the Company common stock is no longer listed on NASDAQ due to the Company’s inability to file periodic reports, the Board continues to comply with NASDAQ’s listing standards with respect to Board independence. NASDAQ listing standards require that a majority of the members of the Board be independent, which means that they are not officers or employees of the Company and are free of any relationship that would interfere with the exercise of their independent judgment. The Board has determined that Dr. Behrens and Messrs. Barry, Bierman, Dewberry, Evans, Newton, and Yeston are “independent” under NASDAQ listing standards.
112
Item 14. Principal Accounting Fees and Services
Audit Firm Fees
The Audit Committee’s duties include pre-approving audit and non-audit services provided to the Company by the Company’s independent registered public accounting firm. All of the services in respect of 2018, 2017, and 2016 under the Audit Fees, Audit-Related Fees, Tax Fees and All Other Fees categories below were pre-approved by the Audit Committee.
Type of Fee | Year Ended(1) December 31, 2018 | Year Ended(1) December 31, 2017 | Year Ended(1) December 31, 2016 | ||
Audit Fees(2) | $2,433,333 | $2,433,333 | $2,433,333 | ||
Audit-Related Fees(3) | $21,400 | $0 | $0 | ||
Tax Fees | $0 | $0 | $0 | ||
All Other Fees | $0 | $0 | $0 | ||
(1) | The Company engaged BDO in 2019 to audit its financial statements for years ended December 31, 2018, 2017, and 2016. Total fees incurred by BDO are estimated to be $7.3 million and were and apportioned equally to each of the three years. The Company paid or incurred these fees in 2019. |
(2) | This category includes fees for the audit of the Company’s annual financial statements and review of financial statements included in its quarterly reports on Form 10-Q. |
(3) | This relates to BDO’s audit of the Company’s 401(k) plan. |
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a) | Documents filed as part of this report: |
(1) | Financial Statements |
(2) | Financial Statement Schedule: |
The following Financial Statement Schedule is filed as part of this Report:
Schedule II Valuation and Qualifying Accounts for the years ended December 31, 2018, 2017 and 2016
(3) | Exhibits |
See Item 15(b) below. Each management contract or compensation plan has been identified with an asterisk.
(b) | Exhibits |
113
Exhibit Number | Description | |
2.1## | Agreement and Plan of Merger dated January 10, 2016 by and among MiMedx Group, Inc., Titan Acquisition Sub I, Inc., Titan Acquisition Sub II, LLC, Stability Inc., certain stockholders of Stability Inc. and Brian Martin as representative of the Stability stockholders (incorporated by reference to Exhibit 2.1 to the Registrant’s Form 8-K filed on January 13, 2016). | |
2.2## | Membership Interest Purchase Agreement dated August 18, 2017 by and among MiMedx Group, Inc. Stability Biologics, LLC, each person that, as of January 13, 2016, was a stockholder of Stability Inc. and Brian Martin as stockholder representative (incorporated by reference to Exhibit 2.1 to the Registrant’s 8-K filed on August 18, 2017). | |
3.1 | Articles of Incorporation, together with Articles of Amendment effective each of May 14, 2010; August 8, 2012, November 8, 2012; and May 15, 2015 (incorporated by reference to Exhibit 3.1 to the Registrant’s Form 10-K filed on March 1, 2017). | |
3.2 | Articles of Amendment to the Articles of Incorporation effective November 6, 2018 (incorporated by reference to Exhibit 3.1 to the Registrant’s Form 8-A filed on November 7, 2018). | |
3.3 | Bylaws of MiMedx Group, Inc., as amended and restated as of October 3, 2018 (incorporated by reference to Exhibit 3.1 to the Registrant’s Form 8-K filed on October 4, 2018). | |
4.1 | The description of the Registrant’s Common Stock, $0.001 par value per share (incorporated by reference to the Registration Statement on Form 8-A (Registration No. 001-35887) filed on April 22, 2013). | |
10.1 | Technology License Agreement dated January 29, 2007 between MiMedx, Inc., Shriners Hospitals for Children and University of South Florida Research Foundation (incorporated by reference to Exhibit 10.32 to the Registrant’s Form 8-K filed on February 8, 2008). | |
10.2 | Lease effective May 1, 2013 between Hub Properties of GA, LLC and MiMedx Group, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 10-Q filed on May 10, 2013). | |
10.3 | First Amendment to Lease dated March 7, 2017 between CPVF II West Oak LLC (as successor in interest to HUB Properties of GA, LLC) and MiMedx Group, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K filed on March 13, 2017). | |
10.4# | Second Amendment to Lease made as of August 29, 2018 for real property and improvements located at 1775 West Oak Commons Court, Marietta, Georgia between RE Fields, LLC, successor in interest to HUB Properties GA, LLC, and CPVF II West Oak LLC, and MiMedx Group, Inc., dated January 25, 2013, as amended March 7, 2017. | |
10.5* | MiMedx Group, Inc. Assumed 2006 Stock Incentive Plan, as amended and restated effective February 25, 2014 (incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K filed on March 3, 2014). | |
10.6* | Form of Incentive Stock Option Agreement under the MiMedx Group, Inc. Assumed 2006 Stock Incentive Plan (incorporated by reference to Exhibit 10.4 to the Registrant’s Form 10-K filed on March 4, 2014). | |
10.7* | Form of Nonqualified Stock Option Agreement under the MiMedx Group, Inc. Assumed 2006 Stock Incentive Plan (incorporated by reference to Exhibit 10.5 to the Registrant’s Form 10-K filed on March 4, 2014). | |
10.8* | Form of Restricted Stock Agreement for Non-Employee Directors under the MiMedx Group, Inc. 2006 Assumed Stock Incentive Plan (incorporated by reference to Exhibit 10.66 to the Registrant’s Form 10-Q filed on August 8, 2013). | |
10.9* | Form of Restricted Stock Agreement under the MiMedx Group, Inc. 2006 Assumed Stock Incentive Plan (incorporated by reference to Exhibit 10.3 to the Registrant’s Form 10-K filed on March 4, 2014). | |
10.10* | 2016 Equity and Cash Incentive Plan (incorporated by reference to Appendix A to the Registrant’s Definitive Proxy Statement on Schedule 14A filed on April 12, 2016). | |
10.11* | Form of Incentive Stock Option Agreement under the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan (incorporated by reference to Exhibit 10.2 to the Registrant’s Form 10-Q filed on August 2, 2016). | |
10.12* | Form of Restricted Stock Agreement under the MiMedx Group, Inc 2016 Equity and Cash Incentive Plan (for shares not registered under the Securities Act of 1933) (incorporated by reference to Exhibit 10.9 to the Registrant’s Form 8-K filed on May 30, 2019). | |
10.13* | Form of Restricted Stock Agreement under the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan (incorporated by reference to Exhibit 10.3 to the Registrant’s Form 10-Q filed on August 2, 2016). | |
10.14* | Form of Restricted Stock Agreement for Non-Employee Directors under the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan (incorporated by reference to Exhibit 10.11 to the Registrant’s Form 8-K filed on May 30, 2019). | |
10.15* | Form of Nonqualified Stock Option Agreement under the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan (incorporated by reference to Exhibit 10.4 to the Registrant’s Form 10-Q filed on August 2, 2016). | |
10.16*# | ||
10.17* | 2016 Management Incentive Plan (incorporated by reference to Exhibit 10.13 to the Registrant’s Form 8-K filed on May 30, 2019). | |
10.18* | 2017 Management Incentive Plan (incorporated by reference to Exhibit 10.14 to the Registrant’s Form 8-K filed on May 30, 2019). | |
114
Exhibit Number | Description | |
10.19*# | ||
10.20* | Change in Control Severance Compensation and Restrictive Covenant Agreement dated November 11, 2011 between MiMedx Group, Inc. and Parker H. Petit (incorporated by reference to Exhibit 10.91 to the Registrant’s Form 10-Q filed on November 14, 2011). | |
10.21* | Change in Control Severance Compensation and Restrictive Covenant Agreement dated November 11, 2011 between MiMedx Group, Inc. and with William C. Taylor (incorporated by reference to Exhibit 10.92 to the Registrant’s Form 10-Q filed on November 14, 2011). | |
10.22* | First Amendment to Change in Control Severance Compensation and Restrictive Covenant Agreement dated May 9, 2013 between MiMedx Group, Inc. and William C. Taylor (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K filed on May 15, 2013). | |
10.23* | Change in Control Severance Compensation and Restrictive Covenant Agreement dated November 11, 2011 between MiMedx Group, Inc. and Michael J. Senken (incorporated by reference to Exhibit 10.93 to the Registrant’s Form 10-Q filed on November 14, 2011). | |
10.24* | First Amendment to Change in Control Severance Compensation and Restrictive Covenant Agreement dated May 9, 2013 between MiMedx Group, Inc. and Michael J. Senken (incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K filed on May 15, 2013). | |
10.25* | Change in Control Severance Compensation and Restrictive Covenant Agreement dated May 20, 2016 between MiMedx Group, Inc. and Alexandra O. Haden (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K filed on May 25, 2016). | |
10.26*# | Consulting Agreement with Alexandra O. Haden dated August 27, 2019. | |
10.27* | Employment Offer Letter dated April 3, 2018 between MiMedx Group, Inc. and Edward Borkowski (incorporated by reference to Exhibit 10.22 to the Registrant’s Form 8-K filed on May 30, 2019). | |
10.28* | Change in Control Severance Compensation and Restrictive Covenant Agreement between MiMedx Group, Inc. and Edward J. Borkowski (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K/A filed on June 25, 2018). | |
10.29* | Form of Change in Control Severance Compensation and Restrictive Covenant Agreement (incorporated by reference to Exhibit 10.24 to the Registrant’s Form 8-K filed on May 30, 2019). | |
10.30* | Form of (Non-change in Control) Executive Severance Agreement (incorporated by reference to Exhibit 10.25 to the Registrant’s Form 8-K filed on May 30, 2019). | |
10.31* | Separation and Transition Services Agreement, dated as of November 15, 2019, between MiMedx Group, Inc. and Edward J. Borkowski (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed November 20, 2019). | |
10.32* | Form of Indemnification Agreement (incorporated by reference to Exhibit 10.65 to the Registrant’s Form 8-K filed July 15, 2008). | |
10.33* | Form of Employee Inventions and Assignment Agreement (incorporated by reference to Exhibit 10.4 to the Registrant’s Form 8-K/A filed on June 12, 2018). | |
10.34* | Form of Confidentiality and Non-Solicitation Agreement (incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K/A filed on June 12, 2018). | |
10.35* | Form of Non-Competition Agreement (incorporated by reference to Exhibit 10.3 to the Registrant’s Form 8-K/A filed on June 12, 2018). | |
10.36* | Engagement Letter dated July 2, 2018 between MiMedx Group, Inc. and Alvarez & Marsal North America, LLC (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K/A filed on July 11, 2018). | |
10.37* | Letter Agreement dated April 10, 2019 between MiMedx Group, Inc. and Timothy R. Wright (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K filed on May 9, 2019). | |
10.38* | Cooperation Agreement dated as of May 29, 2019 among MiMedx Group, Inc., M. Kathleen Behrens Wilsey, K. Todd Newton, Richard J. Barry, Prescience Partners, LP, Prescience Point Special Opportunity LP, Prescience Capital LLC, Prescience Investment Group, LLC d/b/a Prescience Point Capital Management LLC and Eiad Asbahi (incorporated by reference to Exhibit 10.32 to the Registrant’s Form 8-K filed on May 30, 2019). | |
10.39## | Loan Agreement, dated June 10, 2019, by and between MiMedx Group, Inc., the other guarantors party thereto, the lenders party thereto and Blue Torch Finance LLC, as administrative agent and collateral agent (incorporated by reference to Exhibit 10.1 to Form 8-K Filed June 11, 2019). | |
16.1 | Letter from Cherry Bekaert LLP dated August 9, 2017 (incorporated by reference to Exhibit 16.1 to Current Report on Form 8-K filed August 10, 2017). | |
16.2 | Letter from Ernst & Young LLP dated December 7, 2018 (incorporated by reference to Exhibit 16.1 to Current Report on Form 8-K filed December 7, 2018). | |
21.1# | ||
23.1# | ||
115
Exhibit Number | Description | |
24.1# | Power of Attorney (included on the signature page to this Report). | |
31.1# | ||
31.2# | ||
32.1# | ||
32.2# | ||
101.INS# | XBRL Instance Document | |
101.SCH# | XBRL Taxonomy Extension Schema Document | |
101.CAL# | XBRL Taxonomy Extension Calculation Linkbase Document | |
101.DEF# | XBRL Taxonomy Extension Definition Linkbase Document | |
101.LAB# | XBRL Taxonomy Extension Label Linkbase Document | |
101.PRE# | XBRL Taxonomy Extension Presentation Linkbase Document | |
Notes
* | Indicates a management contract or compensatory plan or arrangement |
# | Filed herewith |
## | Certain exhibits and schedules have been omitted pursuant to Item 601(b)(2) of Regulation S-K, but a copy will be furnished supplementally to the Securities and Exchange Commission upon request. |
Item 16. Form 10-K Summary
Not applicable.
116
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
March 17, 2020 | MIMEDX GROUP, INC. | |
By: | /s/ Edward J. Borkowski | |
Edward J. Borkowski | ||
Acting Chief Financial Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints William F. Hulse IV and David A. Wisniewski and each of them acting individually, as his or her true and lawful attorneys-in-fact and agents, each with full power of substitution and resubstitution, for him or her in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K for the year ended December 31, 2018, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming our signatures as they may be signed by our said attorney to any and all amendments to said Annual Report on Form 10-K.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
117
Signature / Name | Title | Date | ||
/s/ Timothy R. Wright | Chief Executive Officer and Director | March 17, 2020 | ||
Timothy R. Wright | (Principal Executive Officer) | |||
/s/ Edward J. Borkowski | Acting Chief Financial Officer | March 17, 2020 | ||
Edward J. Borkowski | (Principal Financial and Accounting Officer) | |||
/s/ M. Kathleen Behrens | Chair of the Board (Director) | March 17, 2020 | ||
M. Kathleen Behrens | ||||
/s/ Richard J. Barry | Director | March 17, 2020 | ||
Richard J. Barry | ||||
/s/ James L. Bierman | Director | March 17, 2020 | ||
James L. Bierman | ||||
/s/ J. Terry Dewberry | Director | March 17, 2020 | ||
J. Terry Dewberry | ||||
/s/ Charles R. Evans | Director | March 17, 2020 | ||
Charles R. Evans | ||||
/s/ Charles E. Koob | Director | March 17, 2020 | ||
Charles E. Koob | ||||
/s/ K. Todd Newton | Director | March 17, 2020 | ||
K. Todd Newton | ||||
/s/ Neil Yeston | Director | March 17, 2020 | ||
Neil Yeston | ||||
118