UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition Period from to
Commission File Number: 001-34885
AMYRIS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 55-0856151 | |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
| 5885 Hollis Street, Suite 100, Emeryville, California | 94608 | |
| (Address of principal executive office) | (Zip Code) |
(510) 450-0761
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common Stock, $0.0001 par value per share |
The NASDAQ Stock Market LLC (NASDAQ Global Select Market) |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one.)
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☐ | |||
| Emerging growth company | ☐ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act.): Yes ☐ No ☒
As of June 30, 2016, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the voting stock held by non-affiliates of the registrant was approximately $37.0 million, based on the closing price of the registrant’s common stock on the NASDAQ Stock Market on such date.
278,320,194 shares of the registrant’s common stock, par value $0.0001 per share, were outstanding as of January 31, 2017.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s proxy statement to be delivered to stockholders in connection with the registrant’s 2017 Annual Meeting of Stockholders to be held on or about May 23, 2017 are incorporated by reference into Part III of this Form 10-K. The registrant intends to file its proxy statement within 120 days after its fiscal year end.
AMYRIS, INC.
ANNUAL REPORT ON FORM 10-K
For the Fiscal Year Ended December 31, 2015
INDEX
FORWARD-LOOKING STATEMENTS
This report on Form 10-K, including the sections entitled “Item 1. Business,” “Item 1A. Risk Factors,” and “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contains forward-looking statements reflecting our current expectations that involve risks and uncertainties and which are subject to safe harbors under the Securities Act of 1933, as amended, or the Securities Act, and the Securities Exchange Act of 1934. These forward-looking statements include, but are not limited to, statements concerning our strategy, future production capacity and other aspects of our future operations, ability to launch new products, improve our production efficiencies, future financial position, future revenues, projected costs, expectations regarding demand and acceptance for our technologies, growth opportunities and trends in the market in which we operate, prospects and plans and objectives of management. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause our actual results to differ materially from those in the forward-looking statements, including, without limitation, the risks set forth in Part I, Item 1A, “Risk Factors” in this Annual Report on Form 10-K and in our other filings with the Securities and Exchange Commission. The forward-looking statements contained in this report on Form 10-K are based on information available to us on the date of this report on Form 10-K and, except as required by law, we do not assume any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
TRADEMARKS
Amyris, the Amyris logo, Biofene, Biossance, Dial-A-Blend, Diesel de Cana, Evoshield, µPharm, Muck Daddy, Myralene, Neossance and No Compromise are trademarks or registered trademarks of Amyris, Inc. This report also contains trademarks and trade names of other businesses that are the property of their respective holders.
| 1 |
Overview
Amyris, Inc. (or the "Company," "Amyris," "we," "us," or "our") is a leading integrated industrial biotechnology company that is applying its technology platform to engineer, manufacture and sell high performance, low cost products into the Health and Nutrition, Personal Care and Performance Materials markets. Our proven technology platform allows us to rapidly engineer microbes and use them as catalysts to metabolize renewable, plant-sourced sugars into large volume, high-value ingredients. Our biotechnology platform and industrial fermentation process replaces existing complex and expensive chemical manufacturing processes. We believe industrial synthetic biology represents a third industrial revolution, bringing together biology and engineering to generate new, more sustainable materials to meet the growing global demand for bio-based replacements for petroleum, animal- or plant-derived ingredients. We continue to build demand for our current portfolio of products through a sales network comprised of direct sales and distributors, and are engaged in collaborations across each of our three market focus areas to drive additional product sales and partnership opportunities. Via our partnership model, we co-invest in the development of each molecule to bring it from the lab to commercial scale and then capture long term revenue either via the sale of the molecule to the partner and/or value sharing of end product sales.
Background
Amyris was founded in 2003 in the San Francisco Bay Area by a group of scientists from the University of California, Berkeley. Our first major milestone came in 2005 when, through a grant from the Bill & Melinda Gates Foundation, we developed technology capable of creating microbial strains that produce artemisinic acid - a precursor of artemisinin, an effective anti-malarial drug. In 2008, we granted royalty-free licenses to allow Sanofi-Aventis (Sanofi) to produce artemisinic acid using our technology. Building on our success with artemisinic acid, in 2007 we began applying our technology platform to develop, manufacture and sell sustainable alternatives to a broad range of markets.
We focused our initial development efforts primarily on the production of Biofene®, our brand of renewable farnesene, a long-chain, branched hydrocarbon molecule that we manufacture through fermentation using engineered microbes. Our farnesene derivatives are sold in hundreds of products as nutraceuticals, skin care, fragrances, solvents, polymers, and lubricants ingredients. The commercialization of farnesene pushed us to create a more cost efficient, faster and accurate development process in the lab and drive costs out of our Brotas, Brazil production facility. This investment has enabled our technology platform to rapidly develop microbial strains and commercialize target molecules. In 2014, we began manufacturing additional molecules for the flavors and fragrance (F&F) industry, in 2015 we began investing to expand our capabilities to other small molecule chemical classes beyond terpenes via our collaboration with the Defense Advanced Research Project Agency (DARPA), as discussed below, and in 2016 we expanded into proteins.
Since inception, we have received equity and debt financing from investors including affiliates of Total S.A. (collectively referred to as Total), the international energy company, and affiliates of Temasek Holdings (Private) Limited, the Singapore sovereign wealth fund (collectively referred to as Temasek), and various venture capital and private equity investors. Our common stock is traded on The NASDAQ Stock Market (NASDAQ) under the symbol AMRS.
| 2 |
Our Platform
Amyris has invested over $500 million in infrastructure and technology to create microbes that produce chemicals from sugar or other feedstocks at commercial scale. This platform has been used to design, build, optimize, and upscale strains producing 5 distinct molecules, leading to more than 15 commercial products used in 500 consumer products. Our time to market for molecules has decreased from 7 years to less than a year for our most recent molecule, mainly due to our ability to leverage the technology platform we have built.
Our technology platform has been in active use since 2008, and has been integrated with our commercial production since 2011, creating a seamless organism development process that we believe makes Amyris an industry leader in the successful scale-up of small molecules. The key performance characteristics of our platform that we believe differentiate Amyris include our proprietary computational tools, strain construction tools, screening and analytics tools, and advanced lab automation and data integration. Our state-of-the-art infrastructure includes industry leading strain engineering and lab automation located in Emeryville, CA, pilot scale production facilities in Emeryville, CA and Campinas, Brazil, a demonstration scale facility in Campinas, Brazil and a commercial scale production facility in Brotas, Brazil.
We are able to use a wide variety of feedstocks for production, but have focused on accessing Brazilian sugarcane for our large-scale production because of its renewability, low cost and relative price stability. We have also successfully used other feedstocks such as sugar beets, corn dextrose, sweet sorghum and cellulosic sugars at various manufacturing facilities.
We are currently producing three molecules at our Brotas, Brazil plant: farnesene and two fragrance molecules.
Corporate Information
We were founded in 2003 and completed our initial public offering in 2010. As of January 31, 2017, we had 440 full-time employees (including 280 in the United States and 160 in Brazil). Our corporate headquarters and pilot plant are located in Emeryville, California, and our Brazil headquarters and pilot plant are located in Campinas, Brazil. We have one operating subsidiary, Amyris Brasil Ltda. (Amyris Brasil) which oversees establishment and expansion of our production operations in Brazil.
Strategy and Business Model
Our mission is to apply innovative science to deliver sustainable solutions for a growing world. We seek to become the world's leading provider of renewable, high-performance alternatives to non-renewable and scarce products. In the past, choosing a renewable product often required producers to compromise on performance or price. With our technology, leading consumer brands can develop products made from renewable sources that offer equivalent or better performance and stable supply with competitive pricing. We call this our No Compromise® value proposition. We aim to improve the world one molecule at a time by providing the best alternatives to the products the world relies on every day.
We have developed and are operating our company under a business model that generates cash from collaborations, from product sales, and value share. We believe this combination will enable us to realize our vision of becoming the world’s leading renewable products company.
| 3 |
Collaborations
Collaborations provide us with funding to develop the solutions partners are looking for to gain a competitive advantage either through a lower cost, more reliable supply source or through access to a needed scarce ingredient. This results in our partners gaining a competitive performance and economic advantage while Amyris is able to further build upon our leading technology platform and gain access to upfront funding as well as a long-term revenue stream. These collaborative technology-based partnerships typically range from two to five years and have funded a substantial portion of our direct research and development expenses since 2012. These relationships have also provided us with a robust pipeline of molecules from which we expect to launch production of three to four products annually at our industrial scale facility in Brotas, Brazil. Our collaborations generate value in several ways, including:
| • | helping us identify and develop molecules that address critical supply or performance needs for our partners, while receiving collaboration payments for technology access and research and development; | |
| • | minimizing risk of product market entry and optimizing our resource prioritization; |
| • | using our integrated manufacturing capabilities to produce and sell collaboration target molecules to our partners; |
| • | participating in additional value-sharing arrangements based on the cost/benefits to our partners of using the molecules we develop; and |
| • | providing opportunities to develop products for markets outside the partner agreements from the research insights gained and intellectual property obtained during the development process. |
We believe this collaboration-based business model creates long-term relationships with aligned incentives for success, and allows us to access a portion of the capital and resources necessary to support large-scale production and global distribution of our products.
Product Sales
In addition to our collaborations (including product sales to and value sharing arrangements with our collaboration partners as described above), we have been developing, manufacturing and selling high-value farnesene derivatives that are branded as our Neossance® emollients for the cosmetics industry, and our BiossanceTM direct to consumer beauty brand. Both brands are based on our farnesene derivative, squalane. The Neossance brand is focused on business to business sales and is sold via our distributor network in order to accelerate commercialization. Selling squalane as a branded product has enabled us to differentiate ourselves from the other squalane sources available on the market which are either detrimental to the environment or of lower quality and subject to severe supply and pricing fluctuations. Since its launch in 2011, we have achieved worldwide reach for our Neossance emollients, with our initial high-performance emollient (Neossance squalane) serving as a key ingredient in personal care products for a growing list of cosmetics companies. In December 2016, we formed a joint venture for our Neossance business with Nikko Chemicals Co., Ltd. and Nippon Surfactant Industries Co., Ltd. (collectively referred to as Nikko), in which we hold a 50% interest. See below under “Business-Joint Ventures” for more information regarding our Neossance joint venture.
| 4 |
In 2015, we launched our direct to consumer beauty brand, Biossance. The brand was initially developed to capture a greater value share of the direct to consumer beauty brand market versus only selling squalane into this market as an ingredient. The first Biossance product launched, The Revitalizer, is made exclusively from Neossance squalane. Biossance products were initially sold solely through our ecommerce branded website and in 2016 we expanded the product line to include an expansive line of high-performance skin care products and began sales through the Home Shopping Network (HSN). In October 2016, we announced that our Biossance product line would begin to be carried at Sephora in 2017. In February 2017, we launched a full squalane based consumer cosmetic line at participating Sephora stores and Sephora online. All of the products are based on Amyris’s commitment to No CompromiseTM. Since the launch, sales have grown, and with Sephora’s partnership, we are looking to expand to more stores.
Via our collaboration partnership model, we also have several products we manufacture and sell to our partners in the F&F and Performance Materials industries. In 2014 we established sales of F&F ingredients to a collaboration partner, representing our first major product sales of a molecule other than farnesene and, in 2015, we established sales of a second F&F molecule.
With partners such as Kuraray Co., Ltd. (Kuraray) and Novvi LLC (Novvi), our joint-venture with Cosan US. Inc. (Cosan US and, together with its affiliates, Cosan), American Refining Group, Inc. (ARG) and Chevron Products Company, a division of Chevron U.S.A. Inc. (Chevron) we sell farnesene for the manufacture of performance materials such as polymers, lubricants and specialty adhesives. Kuraray uses our farnesene to produce liquid farnesene rubber, a highly effective tire additive with superior snow, ice and wet-grip properties. We also produce and sell a farnesene derivative, Myralene®, a solvent with very good cleaning, worker health and environmental benefits. We continue to produce and sell renewable diesel for niche markets in Brazil, and renewable jet fuel for early adoption of such jet fuel in specific routes selected by participating airlines. Though these products have not yet generated material net cash contributions to our business, we have maintained such sales as part of our industrial-scale manufacturing offtake and to support our ongoing development efforts toward a commercially-viable Biofene-based renewable fuel in collaboration with Total.
Manufacturing
We began industrial-scale production of our products at contract manufacturing facilities in 2011 and, in December 2012, commenced operations at our first purpose-built, large-scale production facility in southeastern Brazil. This multi-product production facility, located in Brotas, in the state of São Paulo, Brazil, is adjacent to an existing sugar and ethanol mill operated by Tonon Bioenergia SA (Tonon), formerly known as Paraíso Bioenergia. Through 2016, we produced farnesene and two ingredients for the F&F industry at commercial scale at such facility. Under our manufacturing agreement, Tonon supplies sugarcane syrup and certain utilities. Amyris is solely responsible for maintenance and operation of our plant. Our Brotas facility has six 200,000 liter production fermenters and was designed to process sugarcane juice and syrup, or their equivalent, from up to one million tons of raw sugarcane annually. In December 2012, we began production of farnesene at this facility. Our first shipment of farnesene produced at the Brotas facility occurred in February 2013, and our first shipment of a fragrance molecule from the facility occurred in August 2014. We began manufacturing our second fragrance molecule at the Brotas facility in September 2015 and commenced shipping the product in December 2015, with volume ramp up in 2016. In 2016, we made the first large scale shipments of Biofene to our partner who successfully produced and sold a high-value nutraceutical product to their customers. In February 2017, we broke ground on a second purpose-built, large-scale production facility adjacent to our current Brotas facility.
| 5 |
For many of our products, we perform additional distillation or chemical finishing steps to convert initial target molecules into other finished products, such as renewable squalane, F&F ingredients, lubricants, performance polymers and diesel. We have agreements with several facilities in the U.S. and Brazil to perform distillation, filtration, purification and hydrogenation steps for such products. We may enter into additional agreements with other facilities for finishing services and to access flexible capacity and an array of services as we develop additional products. In December 2016, we purchased a facility in Leland, North Carolina, which had been previously operated by Glycotech Inc. (Glycotech) to convert our Biofene into squalane and other final products. We subsequently contributed that facility to our Neossance joint venture discuss above. See below under “Business-Joint Ventures” for more information regarding our Neossance joint venture.
Technology
Synthetic biology uses engineering concepts to leverage the power of biology. We have developed innovative microbial engineering and screening technologies that allow us to transform the way microbes metabolize sugars. Specifically, we engineer microbes, such as yeast, and use them as catalysts to convert sugar, through fermentation, into high-value molecules. In 2015, we were awarded a DARPA investment to expand the capabilities of our technology platform beyond terpenoids. The investment has resulted in us developing an integrated platform with artificial intelligence that will speed up the development and commercialization of small molecules across 15 different chemical classes. In 2016, we entered into a partnership with Biogen. Inc. (Biogen) that is utilizing our strain engineering toolbox to develop and produce recombinant proteins, such as monoclonal antibodies, for pharmaceutical use.
Together with our collaboration partners, we use these molecules as building blocks for a wide range of products in our target markets. This is our foundation for providing high-performance, cost competitive and sustainable alternatives to a wide variety of markets.
Research and Development
Our ongoing technology development is focused primarily on developing microbial strains that produce targeted molecules and on improving the performance of our production microbial strains. As described in more detail below, our process consists of a series of steps including:
| • | identifying new target molecules; |
| • | creating new microbial strains capable of producing the target molecules; |
| • | increasing product yield and productivity from microbial strains through strain modification or fermentation process improvements; and |
| • | translating these steps from lab to commercial scale production consistently. |
| 6 |
We devote substantial resources to our research and development efforts. As of January 31, 2017, our research and development organization included approximately 147 employees, 45 of whom held Ph.D.s. Our research and development expenditures were approximately $51.4 million, $44.6 million, and $49.7 million for the fiscal years ended December 31, 2016, 2015 and 2014, respectively.
Strain Engineering and Scale-Up Process
The primary biological pathway within the microbe that we currently use to produce our commercial molecules is called the isoprenoid or terpenoid pathway. Isoprenoids constitute a large, diverse class of as many as 40,000 identified organic chemicals produced by this pathway in nature, with current product applications in a wide range of industries. Implementing the classical engineering cycle of “Design-Build-Test-Learn” with investments of more than $500 million to date for research and development, we have reduced strain engineering time to produce target isoprenoid molecules from years to months, opening up the possibility of quickly producing thousands of different target molecules from fermentation. Our platform has also allowed us to expand beyond terpenoids to other small molecule chemical classes and proteins.
We have developed a high-throughput strain engineering system that is currently capable of producing and screening more than 100,000 yeast strains per month, which allows us to achieve approximately a 95% lower cost per strain than we achieved in 2009. We generated more than 500,000 unique strains in 2016, surpassing 5.0 million unique strains created since our inception, with each strain testing for improved production of the target molecules. In addition, through our lab-scale and pilot-plant fermentation operations, and our proprietary analytical tools, we are now able to predict, with high reliability, the industrial performance of candidate strains in our 200,000-liter fermenters at our Brotas plant.
The following summarizes the key steps in our strain engineering and scale-up processes:
| 1. | Identifying target molecules. We start our process by identifying, usually based on input from collaborators, a commercial application for which we can deliver an attractive No Compromise® solution. We identify the key molecular properties that are essential to product performance in a specific commercial application and then analyze the chemical structures that drive those key performance characteristics. Finally, we identify target molecules or derivatives of molecules that contain these key chemical structures and that may be cost-effectively produced by our yeast strains. |
| 2. | Developing initial strains/proof of concept. We identify the enzyme-catalyzed chemical conversion steps required for the target molecule's production in a biological pathway. We then seek to design a pathway to produce the target molecule, either directly or by producing a molecule that can, through simple chemical steps, be synthesized, or converted, into the target. Once this pathway is identified, we undertake to engineer it into our yeast strains by employing the processes discussed below. |
| 3. | Improving strain performance and process development. To produce the target molecules at industrial scale in a cost-effective manner, a yeast strain must be improved to increase its level of efficiency of production. Initially, we focus primarily on yield, a measure of the amount of product produced from feeding the microbe a defined amount of sugar. As we advance in our scale-up and commercial scale process development, we also seek to improve production output through improvements in strain productivity, the rate at which our product is produced by a given strain, and titer, the concentration of product in the fermentation broth. In addition, we seek to develop processes to improve production cost and recovery efficiency, including optimizing cycle-time, which is the time needed to run a full fermentation cycle, fermentation process optimization to minimize cost, and separation efficiency, a measure of the amount of product that is recovered from a fermentation run. |
| 7 |
| 4. | Moving production from lab to commercial scale. Once we have established a pathway and verified that it can produce the target molecule, the yeast strain must be improved to increase the level of efficiency of production, and tested for performance in larger-volume facilities, before it is implemented at our larger-scale manufacturing facilities. Our infrastructure to support this scale-up process includes lab-scale fermenters (0.5 to 2 liter), operating pilot plants in our facilities in Emeryville, California and Campinas, Brazil (300 liters), and one 5,000-liter fermenters in our Campinas demonstration facility. Each of these stages mimic the conditions found in larger scale fermentation so that our findings may translate predictably from lab scale to pilot and ultimately to commercial scale. |
Products
We are expanding our range of products with our partners as well as with Amyris branded products. Our partner products are divided into three market areas: Health and Nutrition, Personal Care and Performance Materials. Independently, we have formulated end-user products such as our Biossance™ brand skin care products and products that are based on Biofene and Biofene derivatives that we sell into several markets.
Health and Nutrition
The Health and Nutrition markets include our pharmaceutical work in aiding new drug development and developing alternative cell lines to mammalian cell cultures, nutraceuticals, such as vitamins, and food ingredients. This is a fairly new area for Amyris and most of our work in these areas is still under development and have not been commercialized yet.
During 2016, we announced the signings of our first ingredient supply agreement and collaboration agreement for the global nutraceuticals market. Under the supply agreement, we source Biofene to our partner, which is then further processed into a nutraceutical product. In 2016, we made the first large scale shipments of Biofene to our partner, who successfully produced and sold a nutraceutical product to its customers. The availability of large volumes of Biofene as starting material enables a significant reduction in the production time, complexity and cost of producing end-use nutraceutical products. Under the collaboration agreement, we are working to create and develop additional nutraceutical compounds and, in the event we and our partner can achieve certain specified development targets, we and our partner would establish and implement a worldwide manufacturing and commercialization plan relating to such compounds.
Personal Care
The Personal Care markets include F&F ingredients, skin care ingredients and cosmetic actives. To date, we have successfully brought two F&F ingredients to market with a collaboration partner and have several other ingredients for all three areas under development.
| 8 |
Our technology allows us to cost-effectively produce natural oils and aroma chemicals that are commonly used in the F&F market. Many of the natural ingredients used in the F&F market are expensive because there is limited supply and the synthetic alternatives require complex chemical conversions. We offer F&F companies a natural route to procure these high-value ingredients without sacrificing cost or quality.
In late 2013, we commenced commercial production of our first F&F ingredient for a range of applications, from perfumes to laundry detergent, which is marketed by a collaboration partner which is a global F&F leader. In 2014, we completed our first production campaign of this ingredient at our Brotas biorefinery and shipped it to this collaboration partner. In late 2015, we commenced production and initial sales of our second F&F ingredient to the same collaboration partner.
We are currently working to develop and commercialize a variety of F&F ingredients that are either direct fermentation products or derivatives of fermentation products. Two of our Personal Care collaboration partners launched new products in 2016 using ingredients supplied by Amyris via our proprietary fermentation process. Both of these products have generated significant interest for use in consumer products. In addition, during 2016 we also completed R&D on two other ingredients and began scale-up work for commercialization.
Performance Materials
The Performance Materials markets consist consists of specialty chemicals used to produce products such as polymers, lubricants, solvents and transportation fuels.
Solvents
We have developed a best-in-class renewable solvent produced from farnesene. In addition to addressing regulatory and safety concerns over Volatile Organic Compounds (VOCs), our solvent product, which we market under the brand Myralene®, offers strong performance and environmental attributes. In 2015, we received approval from the Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA) to market and began commercializing Myralene®-based cleaning products as industrial cleaners for the auto service industry and other industrial applications.
Polymers
Our partners are developing applications for our farnesene that include high-performance polymers used in tires and other end-uses. In 2011, we began collaborating with Kuraray with an initial focus on using farnesene-based polymers to replace petroleum-derived additives in tires. During the collaboration, Kuraray developed farnesene-based liquid rubber (LFR), a tire additive that has superior cold and wet-grip properties for better performance. LFR reacts with tire rubber more easily than traditional materials and strengthens adhesion of rubber components to improve tire shape, stability and performance. In connection with our collaboration with Kuraray, multiple leading tire manufacturers have conducted and are continuing to conduct performance tests of this liquid rubber in tire formulations, and one such manufacturer, Sumitomo Rubber Industries, Ltd., has adopted LFR as a performance enhancing additive for use in the production of certain of its tires. Also, during this period, Kuraray produced and began customer sampling and product evaluation for a new category of elastomer, Hydrogenated Styrenic Farnesene Copolymer (HSFC), which has demonstrated performance attributes that open opportunities for vibration-dampening product applications.
| 9 |
Cray Valley, a division of Total, has developed a new farnesene-based addition to its line of Wingtack ® hydrocarbon resins. This product not only represents their first such product based on a renewable feedstock, rather than traditional petroleum based hydrocarbons, but also offers unique performance attributes. These attributes expand the scope of the product line’s applications as tackifying additives to enhance the properties of a broad range of elastomers, including SIS, SBS, polyisoprene, butyl rubber, EPDM and SBR materials, as well as hot melt and hot melt pressure sensitive adhesives.
Lubricants
Base oils are the building blocks of lubricating oils and are currently derived from the crude oil refining process. Additives are materials added to base oils to change their properties, characteristics and/or performance (e.g., anti-foam, anti-wear, corrosion inhibitor, detergent, dispersant, pour point depressant, anti-oxidant, or friction modifier). Lubricants are manufactured by combining a base oil with additives required by lubricant product applications, including engine oils, gear oils, hydraulic oils and turbine oils. Farnesene may be chemically modified to serve as a base oil, additive and/or lubricant. The high-purity synthetic base oil and additive molecules that can be made from Biofene enable lubricant products to perform in harsh environments under extremes of temperature, moisture, dirt and/or wear.
We are pursuing the base oils and lubricants market through our joint venture Novvi. Additional details regarding Novvi are provided below under “Business-Joint Ventures.”
Solvents
We have developed a best-in-class renewable solvent produced from farnesene. In addition to addressing regulatory and safety concerns over Volatile Organic Compounds (VOCs), our solvent product, which we market under the brand Myralene®, offers strong performance and environmental attributes. In 2015, we received approval from the Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA) to market and began commercializing Myralene®-based cleaning products as industrial cleaners for the auto service industry and other industrial applications.
Transportation Fuels
We have partnered with Total to develop renewable transportation fuels from farnesene. Under such partnership, we produce renewable diesel (a farnesene derivative referred to as farnesane) and jet fuel that delivers energy density, engine performance and storage properties comparable to the best petroleum fuels today.
| • | Jet Fuel. Our drop-in, renewable jet fuel is compliant with Jet A/A-1 fuel specifications and outperforms conventional petroleum-derived fuel in a range of performance metrics, including fit for purpose and greenhouse gas emission reduction potential, without compromising on performance or quality. Our renewable jet fuel is approved for use at a 10% blend level with petroleum jet fuel globally and for use by the US military. |
| • | Diesel. Our renewable diesel’s properties are superior to those of petroleum diesel, allowing it to be used as a drop-in replacement practically in any diesel engine today. Our renewable diesel is approved for use in the US at up to a 35% blend with petroleum diesel and in Brazil at up to a 30% blend. The US Maritime Division and U.S. Department of Transportation have validated our diesel as a renewable blend with maritime diesel fuel. |
| 10 |
In the future, as our development efforts with Total allow us to produce fuels at lower costs, we expect that our farnesane-based fuels business will be conducted through a joint venture we have established with Total (described in more detail below under “Business-Joint Ventures”). We have been limiting jet fuel and diesel sales in recent periods as sales of our fuels products have not been cost-effective given the current costs of producing farnesene and current market prices for petroleum fuels.
Amyris Branded Product Markets
Through basic chemical finishing steps, we are able to convert our farnesene molecule into squalane, which is used today as a premium emollient in cosmetics and other personal care products. We believe that our Neossance squalane offers performance attributes equal or superior to those of squalane derived from conventional sources. The ingredient traditionally has been manufactured from olive oil or extracted from deep-sea shark liver oil, which requires that the shark be killed in order to harvest its liver oil. The relatively high price and unstable supply of squalane in the past meant that its use was generally limited to luxury products or small quantities in mass-market product formulations. With our ability to produce a reliable supply of low-cost squalane that eliminates the need to harvest shark liver oil, we offer this ingredient at a price that we believe will drive increasing adoption by formulators. In addition to Neossance squalane, we offer a second, lower-cost emollient, Neossance hemisqualane, for the cosmetics market. In December 2016, we formed a joint venture for our Neossance business with Nikko, in which we hold a 50% interest. See below under “Business-Joint Ventures” for more information regarding our Neossance joint venture. The joint venture currently has Neossance emollient supply agreements with several regional distributors, including those with locations in Japan, South Korea, Europe, Brazil and North America, and, in some cases, directly with cosmetics formulators, which we transferred to the joint venture during the formation process.
In addition, in 2015 we launched our own consumer brand, BiossanceTM skin care products, featuring our Biofene-derived squalane. Under our BiossanceTM brand, we market and sell our products directly to retailers and consumers, initially in the United States. Biossance was initially sold solely through our ecommerce branded website and in 2016, we expanded the product line to include an expansive line of high-performance skin care products and opened up sales through Home Shopping Network (HSN). In October 2016, we announced our Biossance product line would begin to be carried at Sephora in 2017. In February 2017, we launched a full squalane based consumer cosmetic line at participating Sephora stores and Sephora online. All of the products are based on Amyris’s commitment to No CompromiseTM. Since the launch, sales have grown, and with Sephora’s partnership, we are looking to expand to more stores.
Collaborations
We believe that our leadership in the synthetic biology sector is demonstrated by collaboration partners who come to us to access our synthetic biology platform and industrial fermentation expertise. Together we seek to reduce environmental impact, enhance performance, reduce supply and price volatility, and improve profit margins. Our partners include Total, chemical companies such as Braskem S.A. (Braskem) and Kuraray, F&F companies such as Firmenich S.A. (Firmenich) and Givaudan International, SA (Givaudan), agricultural processing companies such as China National Cereals, Oils, and Foodstuff Corporation, nutraceutical companies such as Nenter & Co., Inc. (Nenter) and pharmaceutical companies such as Biogen, Inc. and Janssen Pharmaceutical (Janssen). Our work has also been funded by the U.S. government, including the Department of Energy (DOE) and DARPA, to develop technologies and processes capable of improving the ability to utilize biotechnology for the production of a broader range of molecules.
| 11 |
In 2016, Amyris entered into a partnership in the field of cosmetic actives and completed the engineering of a yeast strain that can produce the first target in this space at significantly reduced cost. This will enable our partner to expand the market for this molecule into new applications and products. The speed to market for this ingredient reinforces the value proposition and strength of the Amyris technology platform and Amyris’s ability to scale up products.
In addition to our collaborations for co-development of Health and Nutrition, Personal Care and Performance Material products, we have established collaborations and joint ventures for the development and commercialization of commodity products that will require larger investment of capital and longer lead times for commercialization than our existing portfolio. For example, we have established a collaboration and joint venture with Total to commercialize Biofene-based diesel and jet fuels, as described in more detail below. In connection with this arrangement, Total has provided substantial funding for Biofene research and development. In addition to this arrangement with Total, we have established our Novvi joint venture with Cosan, ARG and Chevron for the worldwide development, production and commercialization of renewable base oils for the automotive, industrial and commercial lubricants markets. In December 2016, we formed a joint venture for our Neossance business with Nikko, as discussed below. Additionally, Amyris's proprietary synthetic biology platform may be used to provide the pharmaceutical industry with an integrated discovery and production process for therapeutic compounds for which a natural source is scarce or unavailable, or for which chemical synthesis is not cost-effective.
In 2016, we established and developed collaboration relationships with pharmaceutical partners such as Biogen in order to develop an alternative cell line to mammalian cell cultures for the production of recombinant proteins, such as monoclonal antibodies. We also initiated two µPharmTM projects that utilize Amyris’s proprietary technology to develop customized libraries of natural and natural-like compounds for our partners’ selected targets.
Joint Ventures
Our business strategy is to generally focus our direct commercialization efforts on higher-value, lower-volume markets while establishing joint ventures to pursue our lower-margin, higher-volume commodity products, including for the commodity fuels and lubricants markets. We believe this approach will facilitate access to capital and resources necessary to support large-scale production and global distribution for our large-market commodity products as we continually improve our technology advantages and costs of production.
Total Amyris BioSolutions B.V.
We have entered into a series of agreements since 2011 to establish a research and development program and form a joint venture with Total to produce and commercialize Biofene-based diesel and jet fuels. We formed such joint venture, Total Amyris BioSolutions B.V. (TAB), in November 2013. With an exception for our fuels business in Brazil, the collaboration and joint venture established the exclusive means for us to develop, produce and commercialize fuels from Biofene. We granted TAB exclusive licenses under certain of our intellectual property to make and sell joint venture products. We also granted TAB, in the event of a buy-out of our interest in the joint venture by Total (which Total is entitled to do under certain circumstances described below), a non-exclusive license to optimize or engineer yeast strains used by us to produce farnesene for the joint venture’s diesel and jet fuels. As a result of these licenses, Amyris generally no longer had an independent right to make or sell Biofene fuels outside of Brazil without the approval of TAB.
| 12 |
Our agreements with Total relating to our fuels collaboration created a convertible debt financing structure for funding the research and development program. The collaboration agreements contemplated approximately $105.0 million in financing (or R&D Notes) for the collaboration, which as of January 27, 2015, had been completely funded by Total.
In July 2015, we entered into a Letter Agreement with Total (or, as amended in February 2016, the TAB Letter Agreement) regarding the restructuring of the ownership and rights of TAB (or the Restructuring), pursuant to which the parties agreed to enter into an Amended & Restated Jet Fuel License Agreement between us and TAB (or the Jet Fuel Agreement), a License Agreement regarding Diesel Fuel in the European Union (or the EU) between us and Total (or the EU Diesel Fuel Agreement, and together with the Jet Fuel Agreement, the Commercial Agreements), and an Amended and Restated Shareholders’ Agreement among us, Total and TAB (or, together with the Commercial Agreements, the Restructuring Agreements), and file a Deed of Amendment of Articles of Association of TAB, all in order to reflect certain changes to the ownership structure of TAB and license grants and related rights pertaining to TAB.
On March 21, 2016, we, Total and TAB closed the Restructuring and entered into the Restructuring Agreements.
Under the Jet Fuel Agreement, (a) we granted exclusive (co-exclusive in Brazil), world-wide, royalty-free rights to TAB for the production and commercialization of farnesene- or farnesane-based jet fuel, (b) we granted TAB the option, until March 1, 2018, to purchase our Brazil jet fuel business at a price based on the fair value of the commercial assets and on our investment in other related assets, (c) we granted TAB the right to purchase farnesene or farnesane for its jet fuel business from us on a “most-favored” pricing basis and (d) all rights to farnesene- or farnesane-based diesel fuel we previously granted to TAB reverted back to us. As a result of the Jet Fuel Agreement, we generally no longer have an independent right to make or sell, without the approval of TAB, farnesene- or farnesane-based jet fuels outside of Brazil.
Upon all farnesene-or farnesane-based diesel fuel rights reverting back to us, we granted to Total, pursuant to the EU Diesel Fuel Agreement, (a) an exclusive, royalty-free license to offer for sale and sell farnesene- or farnesane-based diesel fuel in the EU, (b) the non-exclusive right to make farnesene or farnesane anywhere in the world, but Total must (i) use such farnesene or farnesane to produce only diesel fuel to offer for sale or sell in the EU and (ii) pay us a to-be-negotiated, commercially reasonable, “most-favored” basis royalty and (c) the right to purchase farnesene or farnesane for its EU diesel fuel business from us on a “most-favored” pricing basis. As a result of the EU Diesel Fuel Agreement, we generally no longer have an independent right to make or sell, without the approval of Total, farnesene- or farnesane-based diesel fuels in the EU.
In addition, as part of the closing of the Restructuring and pursuant the TAB Letter Agreement, on March 21, 2016, we sold to Total one half of our ownership stake in TAB (giving Total an aggregate ownership stake of 75% of TAB and giving us an aggregate ownership stake of 25% of TAB) in exchange for Total cancelling (i) approximately $1.3 million of R&D Notes, plus all paid-in-kind and accrued interest under all outstanding R&D Notes (including all such interest that was outstanding as of July 29, 2015) and (ii) a note in the principal amount of Euro 50,000, plus accrued interest, issued to Total in connection with the original TAB capitalization. To satisfy its purchase obligation above, Total surrendered to us the remaining R&D Note of approximately $5 million in principal amount, and we executed and delivered to Total a new R&D Note containing substantially similar terms and conditions other than it is unsecured and its payment terms are severed from TAB’s business performance, in the principal amount of $3.7 million. See Note 16, “Subsequent Events” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for additional details regarding such R&D Note.
| 13 |
As a result of, and in order to reflect, the changes to the ownership structure of TAB described above, on March 21, 2016, (a) we, Total and TAB entered into an Amended and Restated Shareholders’ Agreement and filed a Deed of Amendment of Articles of Association of TAB and (b) we and Total terminated the Amended and Restated Master Framework Agreement, dated December 2, 2013 and amended on April 1, 2015, between us and Total.
Novvi LLC
In June 2011, we entered into joint venture agreements with Cosan related to the formation of a joint venture to focus on the worldwide development, production and commercialization of base oils made from Biofene for the automotive, commercial and industrial lubricants markets. In September 2011, we formed Novvi, an entity that was initially jointly owned by Cosan U.S. and us. In March 2013, we entered into additional agreements with Cosan U.S. to (i) expand our base oils joint venture with Cosan to also include additives and lubricants and (ii) operate the joint venture exclusively through Novvi. Under these agreements, Amyris and Cosan U.S. each owned 50% of Novvi, and each shared equally in any costs and any profits ultimately realized by the joint venture.
In July 2016, ARG agreed to make a capital contribution of up to $10.0 million in cash to Novvi, subject to certain conditions, in exchange for a one third ownership stake in Novvi. In connection with such investment, we and Cosan U.S. also agreed to make certain contributions to Novvi in exchange for receiving additional membership units in Novvi. Following the ARG investment, assuming it is made in full, and the capital contributions of us and Cosan U.S., each of Novvi’s three members (i.e., ARG, the Company and Cosan U.S.) will own one third of Novvi’s issued and outstanding membership units. In July 2016, the Novvi joint venture documents were amended in order to reflect the ARG investment in Novvi and related transactions.
In November 2016, Chevron made a capital contribution of $1.0 million in cash to Novvi in exchange for 20,000 membership units, representing an approximately 3% ownership stake in Novvi, which reduced the ownership interests of Amyris, Cosan U.S. and ARG pro rata.
SMA Indústria Química S.A.
In April 2010, we established SMA Indústria Química (SMA), a joint venture with São Martinho S.A. (SMSA), to build a production facility in Brazil.
We completed a significant portion of the construction of the new facility in 2012. We suspended construction of the facility in 2013 in order to focus on completing and operating our smaller production facility in Brotas, Brazil and in December 2015, we and SMSA entered into termination and a Share Purchase and Sale Agreement relating to the termination of the joint venture. December 2015, we and SMSA entered into a Termination Agreement and a Share Purchase and Sale Agreement relating to the termination of the joint venture. Under the Termination Agreement, the parties agreed that the joint venture would be terminated effective upon the closing of the purchase by Amyris Brasil of SMSA’s 50,000 shares of SMA (representing all of the shares of SMA held by SMSA) for R$50,000 (approximately US $15.342 based on the exchange rate as of December 31, 2016) pursuant to the Share Purchase and Sale Agreement, The purchase and sale of SMSA’s shares of SMA by Amyris Brasil was consummated on January 11, 2016. The Share Purchase and Sale Agreement also provided that Amyris and Amyris Brasil would have 12 months following the closing of the share purchase to remove assets from SMSA’s site, and would enter into an extension of the lease for such 12 month period for monthly rental payments of R$9,853 (approximately US$3,023 based on the exchange rate as of December 31, 2016. In September 2016, the parties entered into an addendum to the Share Purchase and Sale Agreement (and a corresponding amendment to the lease) which extended the deadline to remove assets from SMSA’s site to December 31, 2017.
| 14 |
Neossance, LLC
In December 2016, we entered into joint venture agreements with Nikko related to the formation of a joint venture to focus on the worldwide commercialization of our Neossance cosmetic ingredients business. In December 2016, we formed the joint venture under the name Neossance, LLC (Neossance), which is jointly owned by us and Nikko. Pursuant to the joint venture agreements relating to Neossance, we contributed certain assets to Neossance, including certain intellectual property and other commercial assets relating to our Neossance cosmetic ingredients business, as well as the production facility in Leland, North Carolina and related assets purchased by us from Glycotech in December 2016. We also agreed to provide Neossance with licenses to certain intellectual property necessary to make and sell products associated with the Neossance business. At the closing of the formation of the joint venture, Nikko purchased a 50% interest in Neossance in exchange for an initial payment of $10 million and the profits, if any, distributed from Neossance to Nikko as a member in cash during the three year period following December 12, 2016, up to a maximum of $10 million. In addition, as part of the formation of Neossance, we and Nikko agreed to make working capital loans to Neossance and we further agreed to execute, and cause Amyris Brasil to execute, a supply agreement to supply farnesene to Neossance, to guarantee a maximum production cost for certain products to be produced by Neossance and to bear any cost of production above such guaranteed costs.
Product Distribution and Sales
We distribute and sell (intend to distribute and sell) our products directly, to distributors or collaborators, or through joint ventures, depending on the market. For most of our products, we sell directly to our collaboration partners, except for our consumer care products, which we sell to distributors and formulators (other than our Biossance™ brand, which we sell directly to retailers and consumers in the United States). Generally, our collaboration agreements do not include any specific purchase obligations, and sales are contingent upon achievement of technical and commercial milestones.
For transportation fuels in Brazil, we sell our renewable diesel directly to fuels blenders and distributors. For transportation fuels outside of Brazil, we have typically sold our products to Total or to fuels blenders and distributors. Eventually, we expect to commercialize commodity products, including sales of fuels and base oils, through our joint ventures TAB and Novvi, respectively.
Renewable product sales to Firmenich and collaboration revenues from Firmenich, Ginkgo Bioworks, Inc. and DARPA each accounted for more than 10% of our reported revenues in 2016.
| 15 |
Intellectual Property
Our success depends in large part upon our ability to obtain and maintain proprietary protection for our products and technologies, and to operate without infringing in the proprietary rights of others. We seek to avoid the latter by monitoring patents and publications in our product areas and technologies to be aware of developments that may affect our business, and to the extent we identify such developments, evaluate and take appropriate courses of action. With respect to the former, our policy is to protect our proprietary position by, among other methods, filing for patent applications on inventions that are important to the development and conduct of our business with the U.S. Patent and Trademark Office (the USPTO), and its foreign counterparts.
As of January 31, 2017, we had approximately 500 issued U.S. and foreign patents and approximately 350 pending U.S. and foreign patent applications that are owned or co-owned by or licensed to us. We also use other forms of protection (such as trademark, copyright, and trade secret) to protect our intellectual property, particularly where we do not believe patent protection is appropriate or obtainable. We aim to take advantage of all of the intellectual property rights that are available to us and believe that this comprehensive approach provides us with a strong proprietary position.
Patents extend for varying periods according to the date of patent filing or grant and the legal term of patents in various countries where patent protection is obtained. The actual protection afforded by patents, which can vary from country to country, depends on the type of patent, the scope of its coverage and the availability of legal remedies in the country. See “Risk Factors - Risks Related to Our Business - Our proprietary rights may not adequately protect our technologies and product candidates.”
We also protect our proprietary information by requiring our employees, consultants, contractors and other advisers to execute nondisclosure and assignment of invention agreements upon commencement of their respective employment or engagement. Agreements with our employees also prevent them from bringing the proprietary rights of third parties to us. In addition, we also require confidentiality or material transfer agreements from third parties that receive our confidential data or materials.
Competition
We expect that our renewable products will compete with products produced from traditional sources as well as from alternative production methods that established enterprises and new companies are seeking to develop and commercialize.
Health and Nutrition
Many active ingredients in the pharmaceutical and nutraceutical markets are made via chemical synthesis by suppliers that have a deep chemistry knowhow and production facilities, including Active Pharmaceutical Ingredient (API) manufacturers and ingredient suppliers. We may compete directly with these companies with respect to specific ingredients or attempt to provide customers with more cost effective or higher performing alternatives. For food ingredients, we compete with companies that produce products from plant and animal derived sources as well as with companies that are also developing biotechnology production solutions to produce specific molecules.
| 16 |
Personal Care
The main competition for Personal Care ingredients, such as fragrances and cosmetic actives is from products derived from plant and animal sources as well as chemical synthesis. The products derived from plant and animal sources are typically produced at a higher cost and create a greater impact on the environment compared to our products. Products derived from chemical synthesis are often produced at a low cost but have ramifications on sustainability as well as non-natural sourcing. There are also companies that are working to develop products using similar technology to us.
Performance Materials
In the Performance Materials markets that we have entered or are seeking to enter, we compete primarily with the established providers of materials currently used in products in these markets. Producers of these incumbent products include global oil companies, large international chemical companies, independent and integrated oil refiners, advanced biofuels companies and biodiesel companies, and companies specializing in specific products that directly compete with our Biofene product and its derivatives. We may also compete in one or more of these markets with products that are offered as alternatives to the traditional petroleum-based or other traditional products being offered in these markets.
Competitive Factors
We believe the primary competitive factors in both the chemicals and fuels markets are:
| • | product price; |
| • | product performance and other measures of quality; |
| • | infrastructure compatibility of products; |
| • | sustainability; and |
| • | dependability of supply. |
We believe that, for our products to succeed in the market, we must demonstrate that our products are comparable or better alternatives to existing products and to any alternative products that are being developed for the same markets based on some combination of product cost, availability, performance, and consumer preference characteristics.
Environmental and Other Regulatory Matters
Our development and production processes involve the use, generation, handling, storage, transportation and disposal of hazardous chemicals and radioactive and biological materials. We are subject to a variety of federal, state, local and international laws, regulations and permit requirements governing the use, generation, manufacture, transportation, storage, handling and disposal of these materials in the United States, Brazil and other countries where we operate or may operate or sell our products in the future. These laws, regulations and permits can require expensive fees, pollution control equipment or operational changes to limit actual or potential impact of our technology on the environment and violation of these laws could result in significant fines, civil sanctions, permit revocation or costs from environmental remediation. We believe we are currently in substantial compliance with applicable environmental regulations and permitting. However, future developments including our commencement of commercial manufacturing of one or more of our products, more stringent environmental regulation, policies and enforcement, the implementation of new laws and regulations or the discovery of unknown environmental conditions may require expenditures that could have a material adverse effect on our business, results of operations or financial condition. See “Risk Factors - Risks Relating to Our Business - We may incur significant costs to comply with environmental laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities.”
| 17 |
GMM Regulations
The use of genetically-modified microorganisms (GMMs), such as our yeast strains, is subject to laws and regulations in many countries. In the United States, the Environmental Protection Agency (EPA) regulates the commercial use of GMMs as well as potential products produced from the GMMs. Various states within the United States could choose to regulate products made with GMMs as well. While the strain of genetically modified yeast that we use, S. cerevisiae, is eligible for exemption from EPA review because it is generally recognized as safe, we must satisfy certain criteria to achieve this exemption, including but not limited to, use of compliant containment structures and safety procedures. In Brazil, GMMs are regulated by the National Biosafety Technical Commission (CTNBio) under its Biosafety Law No. 11.105-2005. We have obtained approvals from CTNBio to use GMMs in a contained environment in our Brazil facilities for research and development purposes as well as at contract manufacturing facilities in Brazil. In addition, we have obtained initial commercial approvals from CTNBio for two of our yeast strains.
We expect to encounter GMM regulations in most if not all of the countries in which we may seek to make our products; however, the scope and nature of these regulations will likely vary from country to country. If we cannot meet the applicable requirements in countries in which we intend to produce our products using our yeast strains, then our business will be adversely affected. See “Risk Factors - Risks Related to Our Business - Our use of genetically-modified feedstocks and yeast strains to produce our products subjects us to risks of regulatory limitations and rejection of our products.”
Chemical Regulations
Our renewable products may be subject to government regulations in our target markets. In the United States, the EPA administers the requirements of the Toxic Substances Control Act (TSCA), which regulates the commercial registration, distribution and use of many chemicals. Before an entity can manufacture or distribute significant volumes of a chemical, it needs to determine whether that chemical is listed in the TSCA inventory. If the substance is listed, then manufacture or distribution can commence immediately. If not, then in most cases a “Chemical Abstracts Service” number registration and pre-manufacture notice must be filed with the EPA, which has 90 days to review the filing. A similar requirement exists in Europe under the Registration, Evaluation, Authorization and Restriction of Chemical Substances (REACH) regulation. See “Risk Factors - Risks Related to Our Business - We may not be able to obtain regulatory approval for the sale of our renewable products.” In 2013, the EPA registered farnesane as a new chemical substance under the TSCA, clearing the way for us to manufacture and sell farnesane without restriction in the United States.
| 18 |
Fuel Regulations
Our diesel and jet fuel is subject to regulation by various government agencies. In the United States, this includes the EPA and the California Air Resources Board (CARB). In Brazil, this includes Brazilian Agência Nacional do Petróleo, Gas Natural e Biocombustíveis (ANP).
We have completed significant steps to validate our ability to produce a market-accepted diesel product:
| • | Due to the similarity of its chemical composition to that of existing petroleum-sourced diesel, our diesel product has the properties required of diesel fuel and thereby satisfies the American Society for Testing and Materials International (ASTM International) D975 Table 1 specifications for petroleum-derived diesel fuel oils. The EPA has registered our diesel for use as a 35% blend rate with petroleum diesel in highway vehicles and non-road equipment. |
| • | In Europe, we obtained REACH registration for importing/manufacturing up to 1,000 metric tons of farnesane (our diesel fuel) per year and are pursuing data validation for greater volumes. REACH registration is required for the sale and use of our fuels within the applicable European jurisdictions. |
| • | We have received required approvals from ANP for specific uses of our diesel fuel in Brazil, have registered our diesel fuel with CARB and are pursuing registration or approvals with other relevant regulatory bodies. |
In 2013, the EPA registered farnesane as a new chemical substance under the TSCA, clearing the way for us to manufacture and sell farnesane without restrictions in the United States.
Jet fuel (aviation turbine fuel) validation and specifications are subject to the ASTM International industry consensus process and the ANP national adoption process. Our farnesane is generally approved for use in jet fuel for commercial flights at blends of up to 10%. This jet fuel blend was approved by ASTM International in June 2014. ASTM International approval is required by U.S. and international regulators before jet fuel can be used commercially. In December 2014, the same jet fuel was approved by ANP, which is an additional step required for Brazil commercialization.
For us to maximize our access to the U.S. fuels market for our fuel products, we will also need to obtain EPA and CARB (and potentially other state agencies) certifications for our feedstock pathway and production facilities, including certification of a feedstock lifecycle analysis relating to greenhouse gas emissions. Any delay in obtaining these additional certifications could impair our ability to sell our renewable fuels to refiners, importers, blenders and other parties that produce transportation fuels as they comply with federal and state requirements to include certified renewable fuels in their products. See “Risk Factors - Risks Related to Our Business - We may not be able to obtain regulatory approval for the sale of our renewable products.”
Employees
As of January 31, 2017, we had 440 full-time employees. Of these employees, 280 were in the United States and 160 were in Brazil. Except for labor union representation for Brazil-based employees based on labor code requirements in Brazil, none of our employees is represented by a labor union or is covered by a collective bargaining agreement. We have never experienced any employment-related work stoppages and consider relations with our employees to be good.
| 19 |
Financial Information by Geographic Areas
Financial information regarding revenues and long-lived assets by geographic area is included in Note 15, "Reportable Segments” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
Business Background and Available Information
We organized our business in July 2003 as a California corporation under the name Amyris Biotechnologies, Inc. and have maintained our headquarters and research facilities in the San Francisco Bay Area since that time. In April 2010, we reincorporated in Delaware and changed our name to Amyris, Inc. We commenced research activities in 2005, focusing on the development of an alternative source of artemisinic acid for the treatment of malaria, and launched research efforts for production of Biofene in 2006. In 2008, we began to sell third party ethanol to wholesale customers through our Amyris Fuels subsidiary, which generated revenue from the sale of ethanol and reformulated ethanol-blended gasoline to wholesale customers through a network of terminals in the eastern United States. We completed our planned transition out of the ethanol and ethanol-blended gasoline business in the third quarter of 2012, though we continue to maintain the Amyris Fuels subsidiary for activities related to renewable fuel sales. We first established a presence in Brazil in 2008 through the opening of offices and laboratories in Campinas. Our corporate headquarters are located at 5885 Hollis Street, Suite 100, Emeryville, California 94608, and our telephone number is (510) 450-0761. Our website address is www.amyris.com. The information contained in or accessible through our website or contained on other websites is not deemed to be part of this Annual Report on Form 10-K.
We are subject to the filing requirements of the Securities Exchange Act of 1934 (the Exchange Act). Therefore, we file periodic reports, proxy statements and other information with the Securities and Exchange Commission (the SEC). Such reports, proxy statements and other information may be obtained by visiting the Public Reference Room of the SEC at 100 F Street, NE, Washington, D.C. 20549. You may obtain information regarding the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically.
We make our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and all amendments to such reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act available free of charge through a link on the “Investors” section of our website located at www.amyris.com (under “Financial Information-SEC Filings”) as soon as reasonably practicable after they are filed with or furnished to the SEC.
| 20 |
Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information set forth in this Annual Report on Form 10-K, including the consolidated financial statements and related notes, which could materially affect our business, financial condition or future results. If any of the following risks actually occurs, our business, financial condition, results of operations and future prospects could be materially and adversely harmed. The trading price of our common stock could decline due to any of these risks, and, as a result, you may lose all or part of your investment.
Risks Related to Our Business
We have incurred losses to date, anticipate continuing to incur losses in the future, and may never achieve or sustain profitability.
We have incurred significant losses in each year since our inception and believe that we will continue to incur losses and negative cash flow from operations into at least 2018. As of December 31, 2016, we had an accumulated deficit of $1,134.4 million and had cash, cash equivalents and short term investments of $28.5 million. We have significant outstanding debt, a significant working capital deficit and contractual obligations related to capital and operating leases, as well as purchase commitments of $0.8 million. As of December 31, 2016, our debt totaled $227.0 million, net of discount of $42.5 million, of which $59.2 million is classified as current. Our debt service obligations over the next twelve months are significant, including approximately $18.3 million of anticipated interest payments (excluding interest paid in kind by adding to outstanding principal) and may include potential early conversion payments of up to approximately $15.8 million (assuming all note holders convert) under our outstanding 9.50% Convertible Senior Notes due 2019 (or the “2015 144A Notes”). Furthermore, our debt agreements contain various financial and operating covenants, including restrictions on business that could cause us to be at risk of defaults. We expect to incur additional costs and expenses related to the continued development and expansion of our business, including construction and operation of our manufacturing facilities, contract manufacturing, research and development operations, and operation of our pilot plants and demonstration facility. There can be no assurance that we will ever achieve or sustain profitability on a quarterly or annual basis.
Our audited consolidated financial statements as of and for the year ended December 31, 2016 have been prepared on the basis that we will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. We have incurred significant losses since our inception and we expect that we will continue to incur losses as we aim to successfully execute our business plan and will be dependent on additional public or private financings, collaborations or licensing arrangements with strategic partners, or additional credit lines or other debt financing sources to fund continuing operations. Based on our cash balances, recurring losses since inception and our existing capital resources to fund our planned operations for a twelve month period, there is substantial doubt about our ability to continue as a going concern. Our operating plan for 2017 contemplates a significant reduction in our net cash outflows resulting from (i) growth of sales of existing and new products with positive gross margins, (ii) reduced production costs as a result of manufacturing and technical developments, (iii) cash inflows from collaborations, (iv) access to various financing commitments and (v) strategic asset divestments. In addition, as noted below, for our 2017 operating plan, we are dependent on funding from sources that are not subject to existing commitments. We will need to obtain additional funding from equity or debt financings, which may require us to agree to burdensome covenants, grant further security interests in our assets, enter into collaboration and licensing arrangements that require us to relinquish commercial rights, or grant licenses on terms that are not favorable. No assurance can be given at this time as to whether we will be able to achieve our expense reduction or fundraising objectives, regardless of the terms. If we are unable to raise additional financing, or if other expected sources of funding are delayed or not received, our ability to continue as a going concern would be jeopardized and we may be forced to delay, scale back or eliminate some of our general and administrative, research and development, or production activities or other operations and reduce investment in new product and commercial development efforts in an effort to provide sufficient funds to continue our operations. If any of these events occurs, our ability to achieve our development and commercialization goals would be adversely affected. In addition, if we are unable to continue as a going concern, we may be unable to meet our obligations under our existing debt facilities, which could result in an acceleration of our obligation to repay all amounts outstanding under those facilities, and we may be forced to liquidate our assets. In such a scenario, the value we receive for our assets in liquidation or dissolution could be significantly lower than the value reflected in our financial statements.
| 21 |
Our audited consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty, which could have a material adverse effect on our financial condition and cause investors to suffer the loss of all or a substantial portion of their investment.
We have limited experience producing our products at commercial scale and may not be able to commercialize our products to the extent necessary to sustain and grow our current business.
To commercialize our products, we must be successful in using our yeast strains to produce target molecules at commercial scale and at a commercially viable cost. If we cannot achieve commercially-viable production economics for enough products to support our business plan, including through establishing and maintaining sufficient production scale and volume, we will be unable to achieve a sustainable integrated renewable products business. Virtually all of our production capacity is through a purpose-built, large-scale production plant in Brotas, Brazil. This plant commenced operations in 2013, and scaling and running the plant has been, and continues to be, a time-consuming, costly, uncertain and expensive process. Given our limited experience commissioning and operating our own manufacturing facilities and our limited financial resources, we cannot be sure that we will be successful in achieving production economics that allow us to meet our plans for commercialization of various products we intend to offer. In addition, our attempts to scale production of new molecules at the plant are subject to uncertainty and risk. For example, even to the extent we successfully complete product development in our laboratories and pilot and demonstration facilities, and at contract manufacturing facilities, we may be unable to translate such success to large-scale, purpose-built plants. If this occurs, our ability to commercialize our technology will be adversely affected and we may be unable to produce and sell any significant volumes of our products. Also, with respect to products that we are able to bring to market, we may not be able to lower the cost of production, which would adversely affect our ability to sell such products profitably. In addition, we will likely need to identify and secure access to additional production capacity to satisfy anticipated volume requirements in 2017. There can be no assurance that we will be able obtain such capacity on favorable or acceptable terms, if at all, and even if we are successful in obtaining such capacity, there can be no assurance that we will be able to scale and operate any additional plants to allow us to meet our operational goals, which could harm our ability to grow our business.
We will require significant inflows of cash from financings, product sales and collaborations to fund our anticipated operations and to service our debt obligations and may not be able to obtain such funding on favorable terms, if at all.
| 22 |
Our planned 2017 working capital needs, our planned operating and capital expenditures for 2017, and our ability to service our outstanding debt obligations are dependent on significant inflows of cash from financings, existing and new collaboration partners and renewable product sales. We will continue to need to fund our research and development and related activities and to provide working capital to fund production, storage, distribution and other aspects of our business. Some of our anticipated funding sources, such as research and development collaborations, are subject to the risk that we cannot meet milestones, that the collaborations may end prematurely for reasons that may be outside of our control (including technical infeasibility of the project or a collaborator’s right to terminate without cause), or the collaborations are not yet subject to definitive agreements or mandatory funding commitments and, if needed, we may not be able to secure additional types of funding in a timely manner or on reasonable terms, if at all. The inability to generate sufficient cash flow, as described above, could have an adverse effect on our ability to continue with our business plans and our status as a going concern.
If we are unable to raise additional funding, or if other expected sources of funding are delayed or not received, our ability to continue as a going concern would be jeopardized and we would take the following actions as early as the second quarter of 2017 to support our liquidity needs in 2017:
| • | Effect significant headcount reductions, particularly with respect to employees not connected to critical or contracted activities across all functions of the Company, including employees involved in general and administrative, research and development, and production activities. |
| • | Shift focus to existing products and customers with significantly reduced investment in new product and commercial development efforts. |
| • | Reduce production activity at our Brotas manufacturing facility to levels only sufficient to satisfy volumes required for product revenues forecast from existing products and customers. |
| • | Reduce expenditures for third party contractors, including consultants, professional advisors and other vendors. |
| • | Reduce or delay uncommitted capital expenditures, including those relating to proposed additional manufacturing capacity, non-essential facility and lab equipment, and information technology projects. |
| • | Closely monitor our working capital position with customers and suppliers, as well as suspend operations at pilot plants and demonstration facilities. |
Implementing this plan could have a negative impact on our ability to continue our business as currently contemplated, including, without limitation, delays or failures in our ability to:
| • | Achieve planned production levels; |
| • | Develop and commercialize products within planned timelines or at planned scales; and |
| • | Continue other core activities. |
| 23 |
Furthermore, any inability to scale-back operations as necessary, and any unexpected liquidity needs, could create pressure to implement more severe measures. Such measures could have an adverse effect on our ability to meet contractual requirements, including obligations to maintain manufacturing operations, and increase the severity of the consequences described above.
Future revenues are difficult to predict, and our failure to predict revenue accurately may cause our results to be below our expectations or those of analysts or investors and could result in our stock price declining.
Our revenues are comprised of product revenues and grants and collaborations revenues. We generate the substantial majority of our product revenues from sales to collaborators and distributors and only a small portion from direct sales. Our collaboration, supply and distribution agreements do not usually include any specific purchase obligations. The sales volume of our products in any given period has been difficult to predict. A significant portion of our product sales is dependent upon the interest and ability of third party distributors to create demand for, and generate sales of, such products to end-users. For example, if such distributors are unsuccessful in creating pull-through demand for our products with their customers, such distributors may purchase less of our products from us than we expect. In addition, many of our new and novel products are intended to be a component of other companies’ products; therefore, sales of our products may be contingent on our collaborators’ and/or customers’ timely and successful development and commercialization of end-use products that incorporate our products, and price volatility in the markets for such end-use products, which may include commodities, could adversely affect the demand for our products and the margin we receive for our product sales, which could harm our financial results. Furthermore, we have begun to market and sell some of our products directly to end-consumers, initially in the cosmetics market. Because we have little experience in marketing and selling directly to consumers, it is difficult to predict how successful our efforts will be and we may not achieve the product sales we expect to achieve on the timeline we anticipate, if at all.
In addition, we have in the past entered into, and expect in the future to enter into, research and development collaboration arrangements pursuant to which we receive payments from our collaborators. Some of such collaboration arrangements include advance payments in consideration for grants of exclusivity or research and development activities to be performed by us. It has in the past been difficult for us to know with certainty when we will sign a new collaboration arrangement and receive payments thereunder. As a result, achievement of our quarterly and annual financial goals has been difficult to predict with certainty. Once a collaboration agreement has been signed, receipt of cash payments and/or recognition of related revenues may depend on our achievement of research, development, production or cost milestones, which may be difficult to predict. In addition, a portion of the advance payments we receive under our collaboration agreements is typically classified as deferred revenue and recognized over multiple quarters or years. Since our business model depends in part on collaboration agreements with advance payments that we recognize over time, it may also be difficult for us to rapidly increase our revenues through additional collaborations in any period, as revenue from such new collaborations will often be recognized over multiple quarters or years.
A limited number of customers, collaboration partners and distributors account for a significant portion of our revenue, and the loss of major customers, collaboration partners or distributors could harm our operating results.
| 24 |
Our revenues have varied significantly from quarter to quarter and are dependent on sales to, and collaborations with, a limited number of customers, collaboration partners and/or distributors. We cannot be certain that customers, collaboration partners and/or distributors that have accounted for significant revenue in past periods, individually or as a group, will continue to generate similar revenue in any future period. If we fail to renew with, or if we lose a major customer, collaborator or distributor or group of customers, collaborators or distributors, our revenue could decline if we are unable to replace the lost revenue with revenue from other sources.
Our existing financing arrangements may cause significant risks to our stockholders and may impact our ability to pursue certain transactions and operate our business.
As of December 31, 2016, our debt totaled $227.0 million, net of discount of $42.5 million, of which $59.2 million is classified as current. Our cash balance is substantially less than the principal amount of our outstanding debt, and we will be required to generate cash from operations or raise additional working capital through future financings or sales of assets to enable us to repay this indebtedness as it becomes due. There can be no assurance that we will be able to do so.
In addition, we have agreed to significant covenants in connection with our debt financing transactions, including restrictions on our ability to incur future indebtedness, and customary events of default, including failure to pay amounts due, breaches of covenants and warranties, material adverse effect events, certain cross defaults and judgments, and insolvency. A failure to comply with the covenants and other provisions of our debt instruments, including any failure to make a payment when required would generally result in events of default under such instruments, which could permit acceleration of such indebtedness and could result in a material adverse effect on us. If such indebtedness is accelerated, it would generally also constitute an event of default under our other outstanding indebtedness, permitting acceleration of such other outstanding indebtedness. Any required repayment of our indebtedness as a result of acceleration or otherwise would lower our current cash on hand such that we would not have those funds available for use in our business or for payment of other outstanding indebtedness.
If we are at any time unable to generate sufficient cash flow from operations to service our indebtedness when payment is due, we may be required to attempt to renegotiate the terms of the instruments relating to the indebtedness, seek to refinance all or a portion of the indebtedness or obtain additional financing. There can be no assurance that we would be able to successfully renegotiate such terms, that any such refinancing would be possible or that any additional financing could be obtained on terms that are favorable or acceptable to us. Any debt financing that is available could cause us to incur substantial costs and subject us to covenants that significantly restrict our ability to conduct our business. If we seek to complete additional equity financings, the interests of existing equity holders may be diluted.
In addition, the covenants in our debt agreements materially limit our ability to take certain actions, including our ability to pay dividends, make certain investments and other payments, undertake certain mergers and consolidations, and encumber and dispose of assets. For example, the purchase agreement for convertible notes that we sold in separate closings in October 2013 and January 2014, which we refer to as the Tranche Notes, requires us to obtain the consent of the holders of a majority of these notes before completing any change of control transaction or purchasing assets in one transaction or a series of related transactions in an amount greater than $20.0 million, in each case while the Tranche Notes are outstanding. The holders of the Tranche Notes also have pro rata rights to invest in, and under which they could cancel up to the full amount of their outstanding Tranche Notes to pay for, equity securities that we issue in certain financings, which could delay or prevent us from completing such financings.
| 25 |
Furthermore, certain of our existing convertible notes, including the Tranche Notes, contain anti-dilution conversion price adjustment provisions, which may be triggered by future issuances of equity or equity-linked instruments in financing transactions. If such adjustment provisions are triggered, the conversion price of such convertible notes will decrease and the number of shares issuable upon conversion of such convertible notes will correspondingly increase. In such event, existing stockholders will be further diluted and the effective issuance price of such equity or equity-linked instruments will be reduced, which may harm our ability to engage in future financing transactions to fund our business.
Our substantial leverage could adversely affect our ability to fulfill our obligations under our existing indebtedness and may place us at a competitive disadvantage in our industry.
We continue to have substantial debt outstanding and we may incur additional indebtedness from time to time to finance working capital, product development efforts, strategic acquisitions, investments and partnerships, or capital expenditures, or for other general corporate purposes, subject to the restrictions contained in our debt agreements. Our significant indebtedness and debt service requirements could adversely affect our ability to operate our business and may limit our ability to take advantage of potential business opportunities. For example, our high level of indebtedness presents the following risks:
| • | we will be required to use a substantial portion of our cash flow from operations to pay principal and interest on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, product development efforts, acquisitions, investments and strategic alliances and other general corporate requirements; |
| • | our substantial leverage increases our vulnerability to economic downturns and adverse competitive and industry conditions and could place us at a competitive disadvantage compared to those of our competitors that are less leveraged; |
| • | our debt service obligations could limit our flexibility in planning for, or reacting to, changes in our business and our industry and could limit our ability to pursue other business opportunities, borrow more money for operations or capital in the future and implement our business strategies; |
| • | our level of indebtedness and the covenants within our debt instruments may restrict us from raising additional financing on satisfactory terms to fund working capital, capital expenditures, product development efforts, strategic acquisitions, investments and alliances, and other general corporate requirements; and |
| • | our substantial leverage may make it difficult for us to attract additional financing when needed. |
If we are at any time unable to generate sufficient cash flow from operations to service our indebtedness when payment is due, we may be required to attempt to renegotiate the terms of the instruments relating to the indebtedness, seek to refinance all or a portion of the indebtedness or obtain additional financing. There can be no assurance that we will be able to successfully renegotiate such terms, that any such refinancing would be possible or that any additional financing could be obtained on terms that are favorable or acceptable to us, if at all.
A failure to comply with the covenants and other provisions of our debt instruments, including any failure to make a payment when required, could result in events of default under such instruments, which could permit acceleration of such indebtedness. If such indebtedness is accelerated, it could also constitute an event of default under our other outstanding indebtedness, permitting acceleration of such other outstanding indebtedness. Any required repayment of our indebtedness as a result of acceleration or otherwise would lower our current cash on hand such that we would not have those funds available for use in our business or for payment of other outstanding indebtedness.
| 26 |
Our GAAP operating results could fluctuate substantially due to the accounting for the early conversion payment features of outstanding convertible promissory notes.
Several of our outstanding convertible debt instruments are accounted for under Accounting Standards Codification 815, Derivatives and Hedging, or ASC 815, as an embedded derivative. For instance, with respect to the 2015 144A Notes, if the holders elect convert their 2015 144A Notes, such converting holders will receive an early conversion payment equal to the present value of the remaining scheduled payments of interest that would have been made on the 2015 144A Notes being converted through April 15, 2019, the maturity date of the 2015 144A Notes. Our 6.50% Convertible Senior Notes due 2019, or the 2014 144A Notes, contain a similar early conversion payment feature, provided that the last reported sale price of our common stock for 20 or more trading days (whether or not consecutive) in a period of 30 consecutive trading days ending within five trading days immediately prior to the date we receive a notice of such election to convert exceeds the conversion price in effect on each such trading day. The early conversion payment features of the 2014 144A Notes and the 2015 144A Notes are accounted for under ASC 815 as embedded derivatives. ASC 815 requires companies to bifurcate conversion options from their host instruments and account for them as free standing derivative financial instruments according to certain criteria. The fair value of the derivative is remeasured to fair value at each balance sheet date, with a resulting non-cash gain or loss related to the change in the fair value of the derivative being charged to earnings (loss). We have determined that we must bifurcate and account for the early conversion payment features of the 2014 144A Notes and the 2015 144A Notes, as well as certain other features of our other convertible debt instruments, as embedded derivatives in accordance with ASC 815. We have recorded these embedded derivative liabilities as non-current liabilities on our consolidated balance sheet with a corresponding debt discount at the date of issuance that is netted against the principal amount of the 2014 144A Notes, the 2015 144A Notes or other convertible debt instrument, as applicable. The derivative liabilities are remeasured to fair value at each balance sheet date, with a resulting non-cash gain or loss related to the change in the fair value of the derivative liabilities being recorded in other income or loss. There is no current observable market for this type of derivative and, as such, we determine the fair value of the embedded derivatives using the binomial lattice model. The valuation model uses the stock price, conversion price, maturity date, risk-free interest rate, estimated stock volatility and estimated credit spread. Changes in the inputs for these valuation models may have a significant impact on the estimated fair value of the embedded derivative liabilities. For example, an increase in our stock price results in an increase in the estimated fair value of the embedded derivative liabilities. The embedded derivative liabilities may have, on a GAAP basis, a substantial effect on our balance sheet from quarter to quarter and it is difficult to predict the effect on our future GAAP financial results, since valuation of these embedded derivative liabilities are based on factors largely outside of our control and may have a negative impact on our earnings and balance sheet. The effects of these embedded derivatives may cause our GAAP operating results to be below expectations, which may cause our stock price to decline.
If we are not able to successfully commence, scale up or sustain operations at our existing and planned manufacturing facilities, our customer relationships, business and results of operations may be adversely affected.
| 27 |
A substantial component of our planned production capacity in the near and long term depends on successful operations at our existing and potential large-scale production plants. We are currently operating our first purpose-built, large-scale production plant in Brotas, Brazil and may complete construction of certain other facilities in the coming years. Delays or problems in the construction, start-up or operation of these facilities will cause delays in our ramp-up of production and hamper our ability to reduce our production costs. Delays in construction can occur due to a variety of factors, including regulatory requirements and our ability to fund construction and commissioning costs. For example, in 2012 we determined it was necessary to delay further construction of our large-scale manufacturing facility with São Martinho in order to focus on the construction and commissioning of our Brotas facility. We have since permanently ceased construction of the São Martinho facility. In 2016 we produced at capacity at our Brotas facility and will likely need to identify and secure access to additional production capacity in 2017 based on anticipated volume requirements, either by constructing a new custom-built facility, acquiring an existing facility from a third party, retrofitting an existing facility operated by a current or potential partner or increasing our use of contract manufacturing facilities. In December 2016, we acquired a production facility in Leland, North Carolina, which facility had been previously operated by our partner Glycotech to perform chemical conversion and production of our end-products, and which facility was subsequently transferred to our newly-formed joint venture with Nikko Chemicals Co., Ltd. and Nippon Surfactant Industries Co., Ltd. (or collectively “Nikko”), as further described in Note 7, “Joint Ventures and Noncontrolling Interest” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K. In addition, in February 2017 we broke ground on a second custom-built production facility adjacent to our existing Brotas facility. However, there can be no assurance that we will be able to complete such facility on our expected timeline, if at all.
Once our large-scale production facilities are built, acquired or retrofitted, we must successfully commission them, if necessary, and they must perform as we expect. If we encounter significant delays, cost overruns, engineering issues, contamination problems, equipment or raw material supply constraints, unexpected equipment maintenance requirements, safety issues, work stoppages or other serious challenges in bringing these facilities online and operating them at commercial scale, we may be unable to produce our renewable products in the time frame and at the cost we have planned. Industrial scale fermentation is an emerging field and it is difficult to predict the effects of scaling up production to commercial scale, which involves various risks to the quality and consistency of our molecules. In addition, in order to produce molecules at our existing and potential future plants, we have been and may in the future be required to perform thorough transition activities, and modify the design of the plant. Any modifications to the production plant could cause complications in the operations of the plant, which could result in delays or failures in production. If any of these risks occur, or if we are unable to create or obtain additional manufacturing capacity necessary to meet existing and potential customer demand, we may need to continue to use, or increase our use of, contract manufacturing sources, which generally entail greater cost to us to produce our products and would therefore reduce our anticipated gross margins and may also prevent us from accessing certain markets for our products. Further, if our efforts to increase (or commence, as the case may be) production at these facilities are not successful, our partners may decide not to work with us to develop additional production facilities, demand more favorable terms or delay their commitment to invest capital in our production. If we are unable to create and sustain manufacturing capacity and operations sufficient to satisfy the existing and potential demand of our customers and partners, our business and results of operations may be adversely affected.
Our reliance on the large-scale production plant in Brotas, Brazil subjects us to execution and economic risks.
Our decision to focus our efforts for production capacity on our manufacturing facility in Brotas, Brazil means that we have limited manufacturing sources for our products in 2017 and beyond. While we have undertaken efforts to identify and obtain additional manufacturing capacity for 2017 and beyond, including the manufacturing facility in Leland, North Carolina and the proposed second manufacturing facility at the Brotas site discussed above, there can be no assurance that such efforts will be successful on the timelines or at the cost we require, if at all. Any production delays could have a significant negative impact on our business, including our ability to achieve commercial viability for our products and meeting existing and potential customer demand. With the facility in Brotas, Brazil, we are, for the first time, operating a commercial fermentation and separation facility ourselves. We have in the past faced, and may in the future face, unexpected difficulties associated with the operation of our plants. For example, we have in the past, at certain contract manufacturing facilities and at the Brotas facility, encountered delays and difficulties in ramping up production based on contamination in the production process, problems with plant utilities, lack of automation and related human error, issues arising from process modifications to reduce costs and adjust product specifications or transition to producing new molecules, and other similar challenges. We cannot be certain that we will be able to remedy all of such challenges quickly or effectively enough to achieve commercially viable near-term production costs and volumes.
| 28 |
To the extent we secure collaboration arrangements with new or existing partners, we may be required to make significant capital investments at our existing or new facilities in order to produce molecules or other products for such collaborations. Any failure or difficulties in establishing, building up or retooling our operations for these new collaboration arrangements could have a significant negative impact on our business, including our ability to achieve commercial viability for our products, lead to the inability to meet our contractual obligations and could cause us to allocate capital, personnel and other resources from our organization which could adversely affect our business and reputation.
As part of our arrangement to build the plant in Brotas, Brazil we have an agreement with Tonon Bioenergia S.A., (Tonon), to purchase from Tonon sugarcane juice and syrup corresponding to a certain number of tons of sugarcane per year, along with specified water and vapor volumes. Until this annual volume is reached, we are restricted from purchasing sugarcane juice or syrup for processing in the facility from any third party, subject to limited exceptions, unless we pay the premium to Tonon that we would have paid if we bought the sugarcane juice from them. As such, we will be relying on Tonon to supply such juice and syrup and utilities on a timely basis, in the volumes we need, and at competitive prices. If a third party can offer superior prices and Tonon does not consent to our purchasing from such third party, we would be required to pay Tonon the applicable premium, which would have a negative impact on our production cost. Furthermore, we agreed to pay a price for the juice or syrup that is based on the lower of the cost of two other products produced by Tonon using such juice, plus a premium. Tonon may not want to sell sugarcane juice or syrup to us if the price of one of the other products is substantially higher than the one setting the price for the juice or syrup we purchase. While the agreement provides that Tonon would have to pay a penalty to us if it fails to supply the agreed-upon volume of syrup or juice for a given month, the penalty may not be enough to compensate us for the increased cost if third-party suppliers do not offer competitive prices. Also, if the prices of the other products produced by Tonon increase, we could be forced to pay those increased prices for production without a related increase in the price at which we can sell our products, reducing or eliminating any margins we can otherwise achieve. If in the future these supply terms no longer provide a viable economic structure for the operation in Brotas, Brazil we may be required to renegotiate our agreement, which could result in manufacturing disruptions and delays. In December 2015, Tonon filed for bankruptcy protection in Brazil. If Tonon is unable to supply sugarcane juice or syrup, water and steam in accordance with our agreement, we may not be able to obtain substitute supplies from third parties in necessary quantities or at favorable prices, or at all. In such event, our ability to manufacture our products in a timely or cost-effective manner, or at all, would be negatively affected, which would have a material adverse effect on our business.
Furthermore, as we continue to scale up production of our products, through contract manufacturers, at our existing and planned production plants in Brotas, Brazil and Leland, North Carolina and at any future manufacturing facility, we may be required to store increasing amounts of our products for varying periods of time and under differing temperatures or other conditions that cannot be easily controlled, which may lead to a decrease in the quality of our products and their utility profiles and could adversely affect their value. If our stored products degrade in quality, we may suffer losses in inventory and incur additional costs in order to further refine our stored products or we may need to make new capital investments in shipping, improved storage or sales channels and related logistics.
| 29 |
Loss or termination of contract manufacturing relationships could harm our ability to meet our production goals.
As we have focused on building and commissioning, acquiring or retrofitting our own plants or the plants of existing or potential partners, respectively, and improving our production economics, we have reduced our use of contract manufacturing and have terminated relationships with some of our contract manufacturing partners. The failure to have multiple available supply options for farnesene or other target molecules could create a risk for us if a single source or a limited number of sources of manufacturing runs into operational issues. In addition, if we are unable to secure the services of contract manufacturers when and as needed, we may lose customer opportunities and the growth of our business may be impaired. We cannot be sure that contract manufacturers will be available when we need their services, that they will be willing to dedicate a portion of their capacity to our projects, or that we will be able to reach acceptable price and other terms with them for the provision of their production services. If we shift priorities and adjust anticipated production levels (or cease production altogether) at contract manufacturing facilities, such adjustments or cessations could also result in disputes or otherwise harm our business relationships with contract manufacturers. In addition, reducing or stopping production at one facility while increasing or starting up production at another facility generally results in significant losses of production efficiency, which can persist for significant periods of time. Also, in order for production to commence under our contract manufacturing arrangements, we generally must provide equipment for such operations, and we cannot be assured that such equipment can be ordered or installed on a timely basis, at acceptable costs, or at all. Further, in order to establish new manufacturing facilities, we need to transfer our yeast strains and production processes from our labs to commercial plants controlled by third parties, which may pose technical or operational challenges that delay production or increase our costs.
Our use of contract manufacturers exposes us to risks relating to costs, contractual terms and logistics.
While we have commenced commercial production at our Brotas, Brazil and Leland, North Carolina plants, we continue to commercially produce, process and manufacture some specialty molecules through the use of contract manufacturers, and we anticipate that we will continue to use contract manufacturers for the foreseeable future for chemical conversion and production of end-products and, to mitigate cost and volume risks at our large-scale production facilities, for production of Biofene and other fermentation target compounds. Establishing and operating contract manufacturing facilities requires us to make significant capital expenditures, which reduces our cash and places such capital at risk. Also, contract manufacturing agreements may contain terms that commit us to pay for capital expenditures and other costs incurred or expected to be earned by the plant operators and owners, which can result in contractual liability and losses for us even if we terminate a particular contract manufacturing arrangement or decide to reduce or stop production under such an arrangement.
The locations of contract manufacturers can pose additional cost, logistics and feedstock challenges. If production capacity is available at a plant that is remote from usable chemical finishing or distribution facilities, or from customers, we will be required to incur additional expenses in shipping products to other locations. Such costs could include shipping costs, compliance with export and import controls, tariffs and additional taxes, among others. In addition, we may be required to use feedstock from a particular region for a given production facility. The feedstock available in such region may not be the least expensive or most effective feedstock for production, which could significantly raise our overall production cost or reduce our product’s quality until we are able to optimize the supply chain.
| 30 |
Our operations rely on sophisticated information technology and equipment systems and infrastructure, a disruption of which could harm our operations.
We rely on various information technology and equipment systems, some of which are dependent on services provided by third parties, to manage our technology platform and operations. These systems provide critical data and services for internal and external users, including procurement and inventory management, transaction processing, financial, commercial and operational data, human resources management, legal and tax compliance information and other information and processes necessary to operate and manage our business. These systems are complex and are frequently updated as technology improves, and include software and hardware that is licensed, leased or purchased from third parties. If our information technology and equipment systems experience breaches or other failures or disruptions, our systems and the information could be compromised. While we have implemented security measures and disaster recovery plans designed to mitigate the effects of any failures or disruption of these systems, such measures may not adequately prevent adverse events such as breaches or failures from occurring or mitigate their severity if they do occur. If our information technology or equipment systems are breached, damaged or fail to function properly due to internal errors or defects, implementation or integration issues, catastrophic events or power outages, we may experience a material disruption in our ability to manage our business operations. Failure or disruption of these systems could have an adverse effect on our operating results and financial condition.
If we are unable to reduce our production costs, we may not be able to produce our products at competitive prices and our ability to grow our business will be limited.
In order to be competitive in the markets we are targeting, our products must have superior qualities or be competitively priced relative to alternatives available in the market. Currently, our costs of production are not low enough to allow us to offer some of our planned products at competitive prices relative to alternatives available in the market. Our production costs depend on many factors that could have a negative effect on our ability to offer our planned products at competitive prices, including, in particular, our ability to establish and maintain sufficient production scale and volume, and feedstock cost. For example, see “We have limited experience producing our products at commercial scale and may not be able to commercialize our products to the extent necessary to sustain and grow our current business,” “Our manufacturing operations require sugar feedstock, energy and steam, and the inability to obtain such feedstock, energy and steam in sufficient quantities or in a timely manner, or at reasonable prices, may limit our ability to produce products profitably or at all,” and “The price of sugarcane and other feedstocks can be volatile as a result of changes in industry policy and may increase the cost of production of our products.”
We face financial risk associated with scaling up production to reduce our production costs. To reduce per-unit production costs, we must increase production to achieve economies of scale and to be able to sell our products with positive margins. However, if we do not sell production output in a timely manner or in sufficient volumes, our investment in production will harm our cash position and generate losses. Additionally, we may incur added costs in storage and we may face issues related to the decrease in quality of our stored products, which could adversely affect the value of such products. Since achieving competitive product prices generally requires increased production volumes and our manufacturing operations and cash flows from sales are in their early stages, we have had to produce and sell products at a loss in the past, and may continue to do so as we build our business. If we are unable to achieve adequate revenues from a combination of product sales and other sources, we may not be able to invest in production and we may not be able to pursue our business plans. In addition, in order to attract potential collaboration or joint venture partners, or to meet payment milestones under existing or future collaboration agreements, we have in the past and may in the future be required to guarantee or meet certain levels of production costs. If we are unable to reduce our production costs to meet such guarantees or milestones, our net cash flow will be further reduced.
| 31 |
Key factors beyond production scale and feedstock cost that impact our production costs include yield, productivity, separation efficiency and chemical process efficiency. Yield refers to the amount of the desired molecule that can be produced from a fixed amount of feedstock. Productivity represents the rate at which our product is produced by a given yeast strain. Separation efficiency refers to the amount of desired product produced in the fermentation process that we are able to extract and the time that it takes to do so. Chemical process efficiency refers to the cost and yield for the chemical finishing steps that convert our target molecule into a desired product. In order to compete successfully in our target markets, we must produce our products at significantly lower costs, which will require both substantially higher yields than we have achieved to date and other significant improvements in production efficiency, including in productivity and in separation and chemical process efficiencies. There can be no assurance that we will be able to make these improvements or reduce our production costs sufficiently to offer our planned products at competitive prices or to attract and maintain collaboration partners, and any such failure could have a material adverse impact on our business and prospects.
Our ability to establish substantial commercial sales of our products is subject to many risks, any of which could prevent or delay revenue growth and adversely impact our customer relationships, business and results of operations.
There can be no assurance that our products will be approved or accepted by customers, that customers will choose our products over competing products, or that we will be able to sell our products profitably at prices and with features sufficient to establish demand. The markets we have entered first are primarily those for specialty chemical products used by large consumer products or specialty chemical companies. In entering these markets, we have sold and we intend to sell our products as alternatives to chemicals currently in use, and in some cases the chemicals that we seek to replace have been used for many years. The potential customers for our molecules generally have well developed manufacturing processes and arrangements with suppliers of the chemical components of their products and may have a resistance to changing these processes and components. These potential customers frequently impose lengthy and complex product qualification procedures on their suppliers, influenced by consumer preference, manufacturing considerations such as process changes and capital and other costs associated with transitioning to alternative components, supplier operating history, established business relationships and agreements, regulatory issues, product liability and other factors, many of which are unknown to, or not well understood by, us. Satisfying these processes may take many months or years. Additionally, we may be subject to product safety testing and may be required to meet certain regulatory and/or product safety standards. Meeting these standards can be a time consuming and expensive process, and we may invest substantial time and resources into such qualification efforts without ultimately securing approval. If we are unable to convince these potential customers (and the consumers who purchase products containing such chemicals) that our products are comparable to the chemicals that they currently use or that the use of our products is otherwise to their benefit, we will not be successful in entering these markets and our business will be adversely affected.
| 32 |
We expect to face competition for our products from providers of petroleum-based products and from other companies seeking to provide alternatives to these products, and if we cannot compete effectively against these companies or products we may not be successful in bringing our products to market or further growing our business after we do so.
We expect that our renewable products will compete with both the traditional, largely petroleum-based products that are currently being used in our target markets and with the alternatives to these existing products that established enterprises and new companies are seeking to produce.
In the markets that we are initially entering, and in other markets that we may seek to enter in the future, we will compete primarily with the established providers of ingredients currently used in products in these markets. Producers of these incumbent products include global oil companies, large international chemical companies and companies specializing in specific products, such as squalane or essential oils. We may also compete in one or more of these markets with products that are offered as alternatives to the traditional petroleum-based or other traditional products being offered in these markets.
With the emergence of many new companies seeking to produce products from alternative sources, we may face increasing competition from such companies. As they emerge, some of these companies may be able to establish production capacity and commercial partnerships to compete with us. If we are unable to establish production and sales channels that allow us to offer comparable products at attractive prices, we may not be able to compete effectively with these companies.
We believe the primary competitive factors in our target markets are:
| • | product price; |
| • | product performance and other measures of quality; |
| • | infrastructure compatibility of products; |
| • | sustainability; and |
| • | dependability of supply. |
The oil companies, large chemical companies and well-established agricultural products companies with whom we compete are much larger than us, have, in many cases, well developed distribution systems and networks for their products, have valuable historical relationships with the potential customers we are seeking to serve and have much more extensive sales and marketing programs in place to promote their products. In order to be successful, we must convince customers that our products are at least as effective as the traditional products they are seeking to replace and we must provide our products on a cost basis that does not greatly exceed these traditional products and other available alternatives. Some of our competitors may use their influence to impede the development and acceptance of renewable products of the type that we are seeking to produce.
We believe that for our chemical products to succeed in the market, we must demonstrate that our products are comparable alternatives to existing products and to any alternative products that are being developed for the same markets based on some combination of product cost, availability, performance, and consumer preference characteristics. With respect to our diesel and other transportation fuels products, we believe that our product must perform as effectively as petroleum-based fuel, or alternative fuels, and be available on a cost basis that does not greatly exceed these traditional products and other available alternatives. In addition, with the wide range of renewable fuels products under development, we must be successful in reaching potential customers and convincing them that ours are effective and reliable alternatives.
| 33 |
Certain rights we have granted to Total and other existing stockholders, including in relation to our future securities offerings, could have substantial impacts on our company.
Under certain agreements between us and Total related to Total’s original investment in our capital stock, for as long as Total owns 10% of our voting securities, it has rights to an exclusive negotiation period if our Board of Directors decides to sell our company. Total also has the right to designate one director to serve on our Board of Directors. In addition, in connection with Total’s investments in Amyris, our certificate of incorporation includes a provision that excludes Total from prohibitions on business combinations between Amyris and an “interested stockholder.” These provisions could have the effect of discouraging potential acquirers from making offers to acquire us, and give Total more access to Amyris than other stockholders if Total decides to pursue an acquisition.
Additionally, in connection with subsequent investments by Total in Amyris, we granted Total, among other investors, a right of first investment if we propose to sell securities in a private placement financing transaction. With these rights, Total and other investors may subscribe for a portion of any new private placement financing and require us to comply with certain notice periods, which could discourage other investors from participating in, or cause delays in our ability to close, such a financing. Further, Total and such other investors have the right to pay for any securities purchased in connection with an exercise of their right of first investment by cancelling all or a portion of our debt held by them. To the extent Total or such other investors exercise these rights, it will reduce the cash proceeds we may realize from the relevant financing.
Our relationship with Ginkgo Bioworks, Inc. exposes us to financial and commercial risks.
In June 2016, we entered into an initial strategic partnership agreement with Ginkgo Bioworks, Inc., or Ginkgo, pursuant to which we licensed certain intellectual property to Ginkgo in exchange for a license fee and royalty, and agreed to pursue the negotiation and execution of a definitive partnership agreement setting forth the terms of a long-term commercial partnership and collaboration arrangement between us and Ginkgo, and in September 2016 we executed a definitive collaboration agreement with Ginkgo setting forth the terms of a commercial partnership under which the parties would collaborate to develop, manufacture and sell commercial products and would share in the value of such products. In connection with the entry into such commercial agreements, we received a waiver under, and subsequently entered into an amendment of, our senior secured credit facility, the agent and lender under which is an affiliate of Ginkgo, which amendment extended, subject to certain conditions which were satisfied in January 2017, the maturity of the loans under the senior secured credit facility, eliminated principal repayments under the facility prior to maturity, subject to the requirement that we apply certain monies received by us under the collaboration agreement with Ginkgo to repay the outstanding loans under the facility, and waived the covenant in the senior secured loan facility requiring the Company to maintain unrestricted, unencumbered cash in defined U.S. bank accounts in an amount equal to at least 50% of the principal amount outstanding under the facility until the maturity date. For more details on our transactions with Ginkgo, please see Note 5, “Debt” and Note 8, “Significant Agreements” to our consolidated financial statements contained in this Annual Report on Form 10-K.
| 34 |
There can be no assurance that our partnership with Ginkgo will be successful, and the partnership may prevent us from pursuing other business opportunities in the future. If the partnership is unsuccessful, our ability to continue with our business plans would be adversely affected. In addition, negative developments in our commercial partnership with Ginkgo could negatively affect our relationship with the agent and lender under our senior secured credit facility, an affiliate of Ginkgo, which could adversely impact our ability to incur additional indebtedness in the future or take other actions the consent for which would be required from the agent and lender under the facility. In such event, our financial condition and business operations could be adversely affected.
If we do not meet technical, development and commercial milestones in our collaboration agreements, our future revenue and financial results will be adversely impacted.
We have entered into a number of agreements regarding the further development of certain of our products and, in some cases, for ultimate sale of certain products to the customer under the agreement. None of these agreements affirmatively obligates the other party to purchase specific quantities of any products at this time, and most contain important conditions that must be satisfied before additional research and development funding or product purchases would occur. These conditions include research and development milestones and technical specifications that must be achieved to the satisfaction of our collaborators, which we cannot be certain we will achieve. If we do not achieve these contractual milestones, our revenues and financial results will be adversely affected.
We are subject to risks related to our reliance on collaboration arrangements to fund development and commercialization of our products and the success of such products is uncertain.
For most product markets we are seeking to enter, we either have or are seeking collaboration partners to fund the research and development, commercialization and production efforts required for the target products. Typically we provide limited exclusive rights and revenue sharing with respect to the production and sale of particular types of products in specific markets in exchange for such up-front funding. These exclusivity, revenue-sharing and other similar terms limit our ability to commercialize our products and technology, and may impact the size of our business or our profitability in ways that we do not currently envision. In addition, revenues from these types of relationships are a key part of our cash plan for 2017 and beyond. If we fail to collect expected collaboration revenues, or to identify and add sufficient additional collaborations to fund our planned operations, we may be unable to fund our operations or pursue development and commercialization of our planned products. To achieve our collaboration revenue targets from year to year, we may be forced to enter into agreements that contain less favorable terms. As part of our current and future collaboration arrangements, we may be required to make significant capital investments at our existing or new facilities in order to produce molecules or other products for such collaborations. Any failure or difficulties in establishing, building up or retooling our operations for these collaboration arrangements could have a significant negative impact on our business, including our ability to achieve commercial viability for our products, lead to the inability to meet our contractual obligations and could cause us to allocate capital, personnel and other resources from our organization which could adversely affect our business and reputation.
| 35 |
With respect to pharmaceutical collaborations, our experience in this industry is limited, so we may have difficulty identifying and securing collaboration partners and customers for pharmaceutical applications of our products and services. Furthermore, our success in the pharmaceutical market depends primarily upon our ability to identify and validate new small molecule compounds of pharmaceutical interest (including through the use of our discovery platform), and identify, test, develop and commercialize such compounds. Our research efforts may initially show promise in discovering potential new therapeutic candidates, yet fail to yield viable product candidates for clinical development for a number of reasons, including:
| • | because our research methodology, including our screening technology, may not successfully identify medically relevant product candidates; |
| • | we may identify and select from our discovery platform novel untested classes of product candidates for the particular disease indication we are pursuing, which may be challenging to validate because of the novelty of the product candidates or we may fail to validate at all after further research work; |
| • | our product candidates may cause adverse effects in patients or subjects, even after successful initial toxicology studies, which may make the product candidates unmarketable; |
| • | our product candidates may not demonstrate a meaningful benefit to patients or subjects; and |
| • | collaboration partners may change their development profiles or plans for potential product candidates or abandon a therapeutic area or the development of a partnered product. |
Research programs to identify new product targets and candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential discovery efforts, programs or product candidates that ultimately prove to be unsuccessful.
Our collaboration arrangements may restrict or prevent our future business activity in certain markets or industries, which could harm our ability to grow our business.
As part of our collaboration arrangements in the ordinary course of business, we may grant to our partners exclusive rights with respect to the development, production and/or commercialization of particular products or types of products in specific markets in exchange for up-front funding and/or downstream value sharing arrangements. These rights might inhibit potential collaboration or strategic partners or potential customers from entering into negotiations with us about further business opportunities, and we may be restricted or prevented from engaging with other partners or customers in those markets, which may limit our ability to grow our business.
For example, under our Amended and Restated Jet Fuel Agreement with TAB and our License Agreement regarding Diesel Fuel in the European Union with Total described above, we granted TAB and Total, respectively, certain exclusive rights to produce and commercialize farnesene- or farnesane-based jet and diesel fuel in certain jurisdictions, as well as certain purchase rights. As a result of these agreements, we generally no longer have an independent right to make or sell farnesene- or farnesane-based jet or diesel fuels in such jurisdictions without the approval of TAB or Total, as applicable. If, for any reason, we would like to pursue farnesene- or farnesane-based jet or diesel fuels in such jurisdictions independently or with a third party, these arrangements could impair our ability to develop, produce or commercialize such jet or diesel fuels, which could have a material adverse effect on our business and long term prospects.
In the past, we have had to grant concessions to existing partners in exchange for such partners waiving or modifying their exclusive rights with respect to a particular product, type of product or market so that we could engage with a third party with respect to such product, product type or market. There can be no assurance that existing partners will be willing to grant waivers or modify their exclusive rights in the future on favorable terms, if at all. If we are unable to engage other potential partners with respect to particular products, products types or markets for which we have previously granted exclusive rights, our ability to grow our business would be harmed and our results of operations may be adversely effected.
| 36 |
If our collaboration partners are not successful in commercializing products that incorporate our technology, our business and results of operations may be adversely affected.
We rely on our collaboration partners to create demand with end-users for products that incorporate our products and technologies. If such collaboration partners are unable to create such demand, we may not be able to successfully market or sell our products. In addition, while we maintain certain clawback rights to our technology in the event our collaboration partners are unable or unwilling to commercialize the products we create for them under the applicable collaboration arrangement, if our collaboration partners do not commercialize the products covered by our collaboration or supply arrangements, we may be restricted from or unable to market or sell such products or technologies to other potential collaboration partners, which could hinder the growth of our business. If we allocate resources to collaborations that do not lead to products that are commercially viable, our revenues, financial condition and results of operations could be adversely affected.
In addition, certain of our collaboration partners have the right to terminate their agreements with us if we undergo a change of control or a sale of our business, which could discourage a potential acquirer from making an offer to acquire us.
We have limited control over our joint ventures.
As a result of the restructuring of our joint ventures TAB and Novvi during 2016, as discussed above, we do not have the right or power to control the management of such entities, and our joint venture partners may take action with respect to such joint ventures which is contrary to our interests or objectives. In addition, with respect to the joint venture we formed in December 2016 with Nikko relating to our Neossance cosmetic ingredients business, while we hold a 50% equity interest in such joint venture and have a right to appoint one half of its board of directors, our joint venture partners acting together will have the right to designate the Chief Executive Officer and certain other officers, which would restrict our ability to control the operations of such joint venture. If our joint venture partners act contrary to our interest, it could harm our brand, business, results of operations and financial condition. In addition, operating a joint venture often requires additional organizational formalities as well as time-consuming procedures for sharing information and making decisions, which can divert management resources, and if a joint venture partner changes or relationships deteriorate, our success in the joint venture may be materially adversely affected, which could harm our business. Furthermore, with respect to TAB, if we were to experience a change of control or fail to make any required capital contribution to TAB, Total has a right to buy out our interest in TAB at fair market value. If Total were to exercise these rights, we would, in effect, relinquish our economic rights to the intellectual property we have exclusively licensed to TAB, and our ability to seek future revenue from farnesene-based jet fuel outside of Brazil would be adversely affected (or completely prevented). This could significantly reduce the value of our product offerings and have a material adverse effect on our ability to grow our business in the future.
Our manufacturing operations require sugar feedstock, energy and steam, and the inability to obtain such feedstock, energy and steam in sufficient quantities or in a timely manner, or at reasonable prices, may limit our ability to produce our products profitably, or at all.
| 37 |
We anticipate that the production of our products will require large volumes of feedstock. We have relied on a mixture of feedstock sources for use at our contract manufacturing operations, including cane sugar, corn-based dextrose and beet molasses. For our large-scale production facility in Brazil, we are relying primarily on Brazilian sugarcane. We cannot predict the future availability or price of these various feedstocks, nor can we be sure that our mill partners, which we expect to supply the sugarcane feedstock necessary to produce our products in Brazil, will be able to supply it in sufficient quantities or in a timely manner. For example, in December 2015, Tonon, one of our suppliers of sugarcane juice and syrup, filed for bankruptcy protection in Brazil, which may adversely affect its ability to supply us with sugarcane juice and syrup in the future. Furthermore, to the extent we are required to rely on sugar feedstock other than Brazilian sugarcane, the cost of such feedstock may be higher than we expect, increasing our anticipated production costs. Feedstock crop yields and sugar content depend on weather conditions, such as rainfall and temperature. Weather conditions have historically caused volatility in the ethanol and sugar industries by causing crop failures or reduced harvests. Excessive rainfall can adversely affect the supply of sugarcane and other sugar feedstock available for the production of our products by reducing the sucrose content and limiting growers' ability to harvest. Crop disease and pestilence can also occur from time to time and can adversely affect feedstock growth, potentially rendering useless or unusable all or a substantial portion of affected harvests. With respect to sugarcane, our initial primary feedstock, seasonal availability and price, the limited amount of time during which it keeps its sugar content after harvest, and the fact that sugarcane is not itself a traded commodity, increases these risks and limits our ability to substitute supply in the event of such an occurrence. If production of sugarcane or any other feedstock we may use to produce our products is adversely affected by these or other conditions, our production will be impaired, and our business will be adversely affected.
Additionally, our facility in Brotas, Brazil depends on large quantities of energy and steam to operate. We have a supply agreement with Cogeração de Energia Elétrica Rhodia Brotas S.A. pursuant to which we receive energy and steam in sufficient amounts to meet our current needs. However, we cannot predict the future availability or price of energy and steam. If, for whatever reason, we must purchase energy or steam from a different supplier, the cost of such energy and steam may be higher than we expect, increasing our anticipated production costs. Droughts or other weather conditions or natural disasters in Brazil may also affect energy and steam production, cost and availability and, therefore, may adversely affect our production. If our supply and access to energy or steam is adversely affected by these or other conditions, our production will be impaired, and our business will be adversely affected.
| 38 |
The price of sugarcane and other feedstocks can be volatile as a result of changes in industry policy and may increase the cost of production of our products.
In Brazil, Conselho dos Produtores de Cana, Açúcar e Álcool (Council of Sugarcane, Sugar and Ethanol Producers or Consecana), an industry association of producers of sugarcane, sugar and ethanol, sets market terms and prices for general supply, lease and partnership agreements for sugarcane. If Consecana makes changes to such terms and prices, it could result in higher sugarcane prices and/or a significant decrease in the volume of sugarcane available for the production of our products. Furthermore, if Consecana were to cease to be involved in this process, such prices and terms could become more volatile. Similar principles apply to the pricing of other feedstocks as well. Any of these events could adversely affect our business and results of operations.
Our large-scale commercial production capacity is centered in Brazil, and our business will be adversely affected if we do not operate effectively in that country.
For the foreseeable future, we will be subject to risks associated with the concentration of essential product sourcing and operations in Brazil. The Brazilian government has changed in the past, and may change in the future, monetary, taxation, credit, tariff, labor and other policies to influence the course of Brazil's economy. For example, the government's actions to control inflation have at times involved setting wage and price controls, adjusting interest rates, imposing taxes and exchange controls and limiting imports into Brazil. We have no control over, and cannot predict what policies or actions the Brazilian government may take in the future. Our business, financial performance and prospects may be adversely affected by, among others, the following factors:
| • | delays or failures in securing licenses, permits or other governmental approvals necessary to build and operate facilities and use our yeast strains to produce products; |
| • | rapid consolidation in the sugar and ethanol industries in Brazil, which could result in a decrease in competition; |
| • | political, economic, diplomatic or social instability in or affecting Brazil; |
| • | changing interest rates; |
| • | tax burden and policies; |
| • | effects of changes in currency exchange rates; |
| • | any changes in currency exchange policy that lead to the imposition of exchange controls or restrictions on remittances abroad; |
| • | inflation; |
| • | land reform or nationalization movements; |
| 39 |
| • | changes in labor related policies; |
| • | export or import restrictions that limit our ability to move our products out of Brazil or interfere with the import of essential materials into Brazil; |
| • | changes in, or interpretations of foreign regulations that may adversely affect our ability to sell our products or repatriate profits to the United States; |
| • | tariffs, trade protection measures and other regulatory requirements; |
| • | compliance with United States and foreign laws that regulate the conduct of business abroad; |
| • | compliance with anti-corruption laws recently enacted in Brazil; |
| • | an inability, or reduced ability, to protect our intellectual property in Brazil including any effect of compulsory licensing imposed by government action; and |
| • | difficulties and costs of staffing and managing foreign operations. |
We cannot predict whether the current or future Brazilian government will implement changes to existing policies on taxation, exchange controls, monetary strategy, labor relations, social security and the like, nor can we estimate the impact of any such changes on the Brazilian economy or our operations.
Brazil’s economy has recently experienced quarters of slow or negative gross domestic product growth and has experienced high inflation and a growing fiscal deficit of its federal government accounts. In addition, in recent months, major corruption scandals involving members of the executive, state-controlled enterprises and large private sector companies have been disclosed and are the subject of ongoing investigation by federal authorities. The final outcome of these investigations and their impact on the Brazilian economy is not yet known and cannot be predicted with certainty.
In addition, during the 2016 U.S. presidential election campaign, President Trump made comments suggesting that he was not supportive of certain existing international trade agreements as well as that he might take action to restrict or tax products imported into the U.S. from foreign jurisdictions. At this time, it remains unclear what President Trump will or will not do with respect to these international trade agreements or U.S. trade policy. If President Trump takes action to withdraw from or materially modify international trade agreements or place restrictions or tariffs on products imported from Brazil, our business, financial condition and results of operations could be adversely affected.
We maintain operations in foreign jurisdictions other than Brazil, and may in the future expand our operations to additional foreign jurisdictions. Many, if not all of the above-mentioned risks also apply to our operations in such jurisdictions. If any of these risks were to occur, our operations and business would be adversely affected.
Our international operations expose us to the risk of fluctuation in currency exchange rates and rates of foreign inflation, which could adversely affect our results of operations.
| 40 |
We currently incur significant costs and expenses in Brazilian real and may in the future incur additional expenses in foreign currencies and derive a portion of our revenues in the local currencies of customers throughout the world. As a result, our revenues and results of operations are subject to foreign exchange fluctuations, which we may not be able to manage successfully. During the past few decades, the Brazilian currency in particular has faced frequent and substantial exchange rate fluctuations in relation to the United States dollar and other foreign currencies. There can be no assurance that the Brazilian real will not significantly appreciate or depreciate against the United States dollar in the future. We also bear the risk that the rate of inflation in the foreign countries where we incur costs and expenses or the decline in value of the United States dollar compared to those foreign currencies will increase our costs as expressed in United States dollars. For example, future measures by the Central Bank of Brazil to control inflation, including interest rate adjustments, intervention in the foreign exchange market and actions to fix the value of the real, may weaken the United States dollar in Brazil. Whether in Brazil or elsewhere, we may not be able to adjust the prices of our products to offset the effects of inflation or foreign currency appreciation on our cost structure, which could increase our costs and reduce our net operating margins. If we do not successfully manage these risks through hedging or other mechanisms, our revenues and results of operations could be adversely affected.
Ethical, legal and social concerns about products using genetically modified microorganisms could limit or prevent the use of our products and technologies and could harm our business.
Our technologies and products involve the use of genetically modified microorganisms, or GMMs. Public perception about the safety of, and ethical, legal or social concerns over, genetically engineered products, including GMMs, could affect public acceptance of our products. If we are not able to overcome any such concerns relating to our products, our technologies may not be accepted by our customers or end-users. In addition, the use of GMMs has in the past received negative publicity, which could lead to greater regulation or restrictions on imports of our products. Further, there is a risk that products produced using our technologies could cause adverse health effects or other adverse events, which could also lead to negative publicity. If our technologies and products are not accepted by our customers or their end-users due to negative publicity or lack of public acceptance, our business could be significantly harmed.
Our use of genetically-modified feedstocks and yeast strains to produce our products subjects us to risks of regulatory limitations and rejection of our products.
The use of GMMs, such as our yeast strains, is subject to laws and regulations in many countries, some of which are new and some of which are still evolving. In the United States, the Environmental Protection Agency (EPA), regulates the commercial use of GMMs as well as potential products produced from GMMs. Various states or local governments within the United States could choose to regulate products made with GMMs as well. While the strain of genetically modified yeast that we currently use for the development and commercial production of our target molecules, S. cerevisiae, is eligible for exemption from EPA review because it is generally recognized as safe, we must satisfy certain criteria to achieve this exemption, including but not limited to use of compliant containment structures and safety procedures, and we cannot be sure that we will meet such criteria in a timely manner, or at all. If exemption of S. cerevisiae is not obtained, our business may be substantially harmed. In addition to S. cerevisiae, we may seek to use different GMMs in the future that will require EPA approval. If approval of different GMMs is not secured, our ability to grow our business could be adversely affected.
| 41 |
In Brazil, GMMs are regulated by the National Biosafety Technical Commission, or CTNBio. We have obtained approvals from CTNBio to use GMMs in a contained environment in our Brazil facilities for research and development purposes as well as at contract manufacturing facilities in Brazil. In addition, we have obtained initial commercial approvals from CTNBio for two of our yeast strains. As we continue to develop new yeast strains and deploy our technology at new production facilities in Brazil, we will be required to obtain further approvals from CTNBio in order to use these strains in commercial production in Brazil. We may not be able to obtain approvals from relevant Brazilian authorities on a timely basis, or at all, and if we do not, our ability to produce our products in Brazil would be impaired, which would adversely affect our results of operations and financial condition.
In addition to our production operations in the United States and Brazil, we have been party to contract manufacturing agreements with parties in other production locations around the world, including Europe. The use of GMM technology is strictly regulated in the European Union, which has established various directives for member states regarding regulation of the use of such technology, including notification processes for contained use of such technology. We expect to encounter GMM regulations in most, if not all, of the countries in which we may seek to establish production capabilities and/or conduct sales to customers or end-use consumers, and the scope and nature of these regulations will likely be different from country to country. If we cannot meet the applicable requirements in other countries in which we intend to produce or sell products using our yeast strains, or if it takes longer than anticipated to obtain such approvals, our business could be adversely affected. Furthermore, there are various non-governmental and quasi-governmental organizations that review and certify products with respect to the determination of whether products can be classified as “natural” or other similar classifications. While the certification from such non-governmental and quasi-governmental organizations is generally not mandatory, some of our current or prospective customers, collaborators or distributors may require that we meet the standards set by such organizations as a condition precedent to purchasing or distributing our products. We cannot be certain that we will be able to satisfy the standards of such organizations, and any delay or failure to do so could harm our ability to sell or distribute some or all of our products to certain customers and prospective customers, which could have a negative impact on our business.
We may not be able to obtain regulatory approval for the sale of our renewable products.
Our renewable chemical products may be subject to government regulation in our target markets. In the United States, the EPA administers the Toxic Substances Control Act, or the TSCA, which regulates the commercial registration, distribution, and use of many chemicals. Before an entity can manufacture or distribute a new chemical subject to the TSCA, it must file a Pre-Manufacture Notice, or PMN, to add the chemical to a product. The EPA has 90 days to review the filing but may request additional data, which could significantly extend the timeline for approval. As a result, we may not receive EPA approval to list future molecules on the TSCA registry as expeditiously as we would like, resulting in delays or significant increases in testing requirements. A similar program exists in the European Union, called REACH. Under this program, chemicals imported or manufactured in the European Union in certain quantities must be registered with the European Chemicals Agency, and this process could cause delays or entail significant costs. To the extent that other countries in which we are producing or selling (or seeking to produce or sell) our products, such as Brazil and various countries in Asia, rely on TSCA or REACH (or similar laws and programs) for chemical registration or regulation in their jurisdictions, delays with the United States or European authorities, or any relevant authorities in such other countries, may delay entry into these markets as well. In addition, some of our Biofene-derived products are sold for the cosmetics market, and some countries may impose additional regulatory requirements or permits for such uses, which could impair, delay or prevent sales of our products in those markets.
| 42 |
Our diesel and jet fuel is subject to regulation by various government agencies, including the EPA and the California Air Resources Board, or CARB, in the United States and Agência Nacional do Petróleo, Gas Natural e Biocombustíveis, or ANP, in Brazil. To date, we have obtained registration with the EPA for the use of our diesel fuel in the United States at a 35% blend rate with petroleum diesel. Farnesane is also listed on the TSCA registry. In addition, ANP has authorized the use of our diesel fuel at blend rates of 10% and 30% for specific transportation fleets. In Europe, we obtained REACH registration for importing/manufacturing less than 1,000 metric tons of farnesane (for use as diesel and jet fuel) per year and are pursuing data validation to maintain such registration. Registration with each of these bodies is required for the production, import, sale and use of our fuels within their respective jurisdictions. Jet fuel (aviation turbine fuel) validation and specifications are subject to the ASTM International industry consensus process and the Brazilian ANP national adoption process. Any failure to achieve required validation and certifications for our jet fuel could impair or delay future development, production or commercialization plans, which could have a material adverse impact on our renewable product revenues. In addition, for us to achieve full access to the United States fuels market for our fuel products, we will need to obtain EPA and CARB (and potentially other state agencies) certifications for our feedstock pathway and production facilities, including certification of a feedstock lifecycle analysis relating to greenhouse gas emissions. Any delay in obtaining these additional certifications could impair our ability to sell our renewable fuels to refiners, importers, blenders and other parties that produce transportation fuels as they comply with federal and state requirements to include certified renewable fuels in their products.
We expect to encounter regulations in most, if not all, of the countries in which we may seek to produce, import or sell our products (and our customers may encounter similar regulations in selling end-use products to consumers), and we cannot assure you that we (or our customers) will be able to obtain necessary approvals in a timely manner or at all. If our products do not meet applicable regulatory requirements in a particular country, then we (or our customers) may not be able to commercialize our products in such country and our business will be adversely affected.
In addition, many of our products are intended to be a component of our collaborators’ and/or customers’ (or their customers’) end-use products. Such end-use products may be subject to various regulations, including regulations promulgated by the EPA or the United States Food and Drug Administration. If our collaborators and customers (or their customers) are not successful in obtaining any required regulatory approval for their end-use products that incorporate our products, or fail to comply with any applicable regulations for such end-use products, whether due to our products or otherwise, demand for our products may decline and our revenues will be adversely affected.
Changes in government regulations, including subsidies and economic incentives, could have a material adverse effect on our business.
The market for renewable chemical products is heavily influenced by foreign, federal, state and local government regulations and policies. Changes to existing or adoption of new domestic or foreign federal, state and local legislative initiatives that impact the production, distribution or sale of renewable chemical products may harm our business. The uncertainty regarding future standards and policies may also affect our ability to develop new renewable products or to license our technologies to third parties and to sell products to our end customers. Any inability to address these requirements and any regulatory or policy changes could have a material adverse effect on our business, financial condition and results of operations.
| 43 |
Furthermore, the production of our products will depend on the availability of feedstock, especially sugarcane. Agricultural production and trade flows are subject to government policies and regulations. Governmental policies affecting the agricultural industry, such as taxes, tariffs, duties, subsidies, incentives and import and export restrictions on agricultural commodities and commodity products can influence the planting of certain crops, the location and size of crop production, whether unprocessed or processed commodity products are traded, the volume and types of imports and exports, and the availability and competitiveness of feedstocks as raw materials. Future government policies may adversely affect the supply of feedstocks, restrict our ability to use sugarcane or other feedstocks to produce our products, or encourage the use of feedstocks more advantageous to our competitors, which would put us at a commercial disadvantage and could negatively impact our future revenues and results of operations.
We may incur significant costs to comply with environmental laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities.
We use hazardous chemicals and radioactive and biological materials in our business, and such materials are subject to a variety of federal, state and local laws and regulations governing the use, generation, manufacture, storage, handling and disposal of these materials in the United States and in Brazil. Although we have implemented safety procedures for handling and disposing of these materials and related waste products in an effort to comply with these laws and regulations, we cannot be sure that our safety measures will prevent accidental injury or contamination from the use, storage, handling or disposal of hazardous materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our insurance coverage. There can be no assurance that violations of environmental, health and safety laws will not occur in the future as a result of human error, accident, equipment failure or other causes. Compliance with applicable environmental laws and regulations may be expensive, and the failure to comply with past, present, or future laws could result in the imposition of fines, third party property damage, product liability and personal injury claims, investigation and remediation costs, the suspension of production, or a cessation of operations, and our liability may exceed our total assets. Liability under environmental laws can be joint and several, without regard to comparative fault, and may be punitive in nature. Furthermore, environmental laws could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations, which could impair our research, development or production efforts and otherwise harm our business.
A decline in the price of petroleum and petroleum-based products has in the past and may in the future reduce demand for some of our renewable products and may otherwise adversely affect our business.
While many of our products do not compete with, and do not serve as alternatives to, petroleum-based products, we anticipate that some of our renewable products, and in particular our fuels, will be marketed as alternatives to corresponding petroleum-based products. The price of oil has fallen significantly in recent years, and accordingly, we may be unable to produce certain of our products as cost-effective alternatives to petroleum-based products. Declining oil prices, or the perception of a sustained or future decline in oil prices, has adversely affected the prices or demand for such products in the past and may do so in the future. During sustained periods of lower oil prices we may be unable to sell such products at anticipated levels, which could negatively impact our operating results.
Our financial results could vary significantly from quarter to quarter and are difficult to predict.
| 44 |
Our revenues and results of operations could vary significantly from quarter to quarter because of a variety of factors, many of which are outside of our control. As a result, comparing our results of operations on a period-to-period basis may not be meaningful. Factors that could cause our quarterly results of operations to fluctuate include:
| • | achievement, or failure, with respect to technology, product development or manufacturing milestones needed to allow us to enter identified markets on a cost effective basis; |
| • | delays or greater than anticipated expenses associated with the completion, commissioning, acquisition or retrofittting of new production facilities, or the time to ramp up and stabilize production following completion, acquisition or retrofittting of a new production facility or the transition to, and ramp up of, producing new molecules at our existing facilities; |
| • | impairment of assets based on shifting business priorities and working capital limitations; |
| • | disruptions in the production process at any manufacturing facility, including disruptions due to seasonal or unexpected downtime at our facilities as a result of feedstock availability, contamination, safety or other issues or other technical difficulties or the scheduled downtime at our facilities as a result of transitioning our equipment to the production of different molecules; |
| • | losses of, or the inability to secure new, major customers, collaboration partners, suppliers or distributors; |
| • | losses associated with producing our products as we ramp to commercial production levels; |
| • | failure to recover value added tax (VAT) that we currently reflect as recoverable in our financial statements (e.g., due to failure to meet conditions for reimbursement of VAT under local law); |
| • | the timing, size and mix of product sales to customers; |
| • | increases in price or decreases in availability of feedstock; |
| • | the unavailability of contract manufacturing capacity altogether or at reasonable cost; |
| • | exit costs associated with terminating contract manufacturing relationships; |
| • | fluctuations in foreign currency exchange rates; |
| • | gains or losses associated with our hedging activities; |
| • | change in the fair value of derivative instruments; |
| • | fluctuations in the price of and demand for sugar, ethanol, and petroleum-based and other products for which our products are alternatives; |
| • | seasonal variability in production and sales of our products; |
| • | competitive pricing pressures, including decreases in average selling prices of our products; |
| 45 |
| • | unanticipated expenses or delays associated with changes in governmental regulations and environmental, health, labor and safety requirements; |
| • | reductions or changes to existing fuel and chemical regulations and policies; |
| • | departure of executives or other key management employees resulting in transition and severance costs; |
| • | our ability to use our net operating loss carryforwards to offset future taxable income; |
| • | business interruptions such as earthquakes, tsunamis and other natural disasters; |
| • | our ability to integrate businesses that we may acquire; |
| • | our ability to successfully collaborate with business venture partners; |
| • | risks associated with the international aspects of our business; and |
| • | changes in general economic, industry and market conditions, both domestically and in our foreign markets. |
Due to the factors described above, among others, the results of any quarterly or annual period may not meet our expectations or the expectations of our investors and may not be meaningful indications of our future performance.
Loss of key personnel, including key management personnel, and/or failure to attract and retain additional personnel could delay our product development programs and harm our research and development efforts and our ability to meet our business objectives.
Our business involves complex, global operations across a variety of markets and requires a management team and employee workforce that is knowledgeable in the many areas in which we operate. As we continue to build our business, we will need to hire and retain qualified research and development, management and other personnel to succeed. The process of hiring, training and successfully integrating qualified personnel into our operations, in the United States, Brazil and other countries in which we may seek to operate, is a lengthy and expensive one. The market for qualified personnel is very competitive because of the limited number of people available who have the necessary technical skills and understanding of our technology and products, particularly in Brazil. Our failure to hire and retain qualified personnel could impair our ability to meet our research and development and business objectives and adversely affect our results of operations and financial condition.
| 46 |
The loss of any key member of our management or key technical and operational employees, or the failure to attract or retain such employees, could prevent us from developing and commercializing our products for our target markets and executing our business strategy. In addition, we may not be able to attract or retain qualified employees in the future due to the intense competition for qualified personnel among biotechnology and other technology-based businesses, particularly in the renewable chemicals and fuels area. Furthermore, reductions to our workforce as part of cost-saving measures, such as those discussed above with respect to our 2017 operating plan, may make it more difficult for us to attract and retain key employees. If we do not maintain the necessary personnel to accomplish our business objectives, we may experience staffing constraints that will adversely affect our ability to meet the demands of our collaborators and customers in a timely fashion or to support our internal research and development programs and operations. In particular, our product and process development programs depend on our ability to attract and retain highly skilled technical and operational personnel. Competition for such personnel from numerous companies and academic and other research institutions may limit our ability to do so on acceptable terms. All of our employees are “at-will” employees, which means that either the employee or we may terminate their employment at any time.
Growth may place significant demands on our management and our infrastructure.
We have experienced, and expect to continue to experience, expansion of our business as we continue to make efforts to develop and bring our products to market. We have grown from 18 employees at the end of 2005 to 440 full-time employees at January 31, 2017. Our growth and diversified operations have placed, and may continue to place, significant demands on our management and our operational and financial infrastructure. In particular, continued growth could strain our ability to:
| • | manage multiple research and development programs; |
| • | operate multiple manufacturing facilities around the world; |
| • | develop and improve our operational, financial and management controls; |
| • | enhance our reporting systems and procedures; |
| • | recruit, train and retain highly skilled personnel; |
| • | develop and maintain our relationships with existing and potential business partners; |
| • | maintain our quality standards; and |
| • | maintain customer satisfaction. |
Managing our growth will require significant expenditures and allocation of valuable management resources. If we fail to achieve the necessary level of efficiency in our organization as it grows, our business, results of operations and financial condition would be adversely impacted.
Our proprietary rights may not adequately protect our technologies and product candidates.
Our commercial success will depend substantially on our ability to obtain patents and maintain adequate legal protection for our technologies and product candidates in the United States and other countries. As of January 31, 2017, we had approximately 500 issued United States and foreign patents and approximately 350 pending United States and foreign patent applications that were owned or co-owned by or licensed to us. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies and future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
| 47 |
We apply for patents covering both our technologies and product candidates, as we deem appropriate. However, filing, prosecuting, maintaining and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States are less extensive than those in the United States. We may also fail to apply for patents on important technologies or product candidates in a timely fashion, or at all. Our existing and future patents may not be sufficiently broad to prevent others from practicing our technologies or from designing products around our patents or otherwise developing competing products or technologies. In addition, the patent positions of companies like ours are highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of patent claims has emerged to date in the United States and the landscape is expected to become even more uncertain in view of recent rule changes by the United States Patent Office, or USPTO. Additional uncertainty may result from legal decisions by the United States Federal Circuit and Supreme Court as they determine legal issues concerning the scope and construction of patent claims and inconsistent interpretation of patent laws or from legislation enacted by the U.S. Congress. The patent situation outside of the United States is even less predictable. As a result, the validity and enforceability of patents cannot be predicted with certainty. Moreover, we cannot be certain whether:
| • | we (or our licensors) were the first to make the inventions covered by each of our issued patents and pending patent applications; |
| • | we (or our licensors) were the first to file patent applications for these inventions; |
| • | others will independently develop similar or alternative technologies or duplicate any of our technologies; |
| • | any of our or our licensors' patents will be valid or enforceable; |
| • | any patents issued to us (or our licensors) will provide us with any competitive advantages, or will be challenged by third parties; |
| • | we will develop additional proprietary products or technologies that are patentable; or |
| • | the patents of others will have an adverse effect on our business. |
We do not know whether any of our pending patent applications or those pending patent applications that we license will result in the issuance of any patents. Even if patents are issued, they may not be sufficient to protect our technology or product candidates. The patents we own or license and those that may be issued in the future may be challenged, invalidated, rendered unenforceable, or circumvented, and the rights granted under any issued patents may not provide us with proprietary protection or competitive advantages. Moreover, third parties could practice our inventions in territories where we do not have patent protection or in territories where they could obtain a compulsory license to our technology where patented. Such third parties may then try to import products made using our inventions into the United States or other territories. Accordingly, we cannot ensure that any of our pending patent applications will result in issued patents, or even if issued, predict the breadth, validity and enforceability of the claims upheld in our and other companies' patents.
| 48 |
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries do not favor the enforcement of patents or other intellectual property rights, which could hinder us from preventing the infringement of our patents or other intellectual property rights. Proceedings to enforce our patent rights in the United States or foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert patent infringement or other claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license from third parties.
Unauthorized parties may attempt to copy or otherwise obtain and use our products or technology. Monitoring unauthorized use of our intellectual property is difficult, and we cannot be certain that the steps we have taken will prevent unauthorized use of our technology, particularly in certain foreign countries where the local laws may not protect our proprietary rights as fully as in the United States or may provide, today or in the future, for compulsory licenses. If competitors are able to use our technology, our ability to compete effectively could be harmed. Moreover, others may independently develop and obtain patents for technologies that are similar to, or superior to, our technologies. If that happens, we may need to license these technologies, and we may not be able to obtain licenses on reasonable terms, if at all, which could cause harm to our business.
We rely in part on trade secrets to protect our technology, and our failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
We rely on trade secrets to protect some of our technology, particularly where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to maintain and protect. Our strategy for contract manufacturing and scale-up of commercial production requires us to share confidential information with our international business partners and other parties. Our product development collaborations with third parties, including with Total and Ginkgo, require us to share confidential information, including with employees of Total and Ginkgo who are seconded to Amyris during the term of the collaboration. While we use reasonable efforts to protect our trade secrets, our or our business partners' employees, consultants, contractors or scientific and other advisors may unintentionally or willfully disclose our proprietary information to competitors. Enforcement of claims that a third party has illegally obtained and is using trade secrets is expensive, time consuming and uncertain. In addition, foreign courts are sometimes less willing than United States courts to protect trade secrets. If our competitors independently develop equivalent knowledge, methods and know-how, we would not be able to assert our trade secrets against them.
We require new employees and consultants to execute confidentiality agreements upon the commencement of an employment or consulting arrangement with us. We additionally require consultants, contractors, advisors, corporate collaborators, outside scientific collaborators and other third parties that may receive trade secret information to execute confidentiality agreements. These agreements generally require that all confidential information developed by the individual or made known to the individual by us during the course of the individual's relationship with us be kept confidential and not disclosed to third parties. These agreements also generally provide that inventions conceived by the individual in the course of rendering services to us shall be our exclusive property. Nevertheless, our proprietary information may be disclosed, or these agreements may be unenforceable or difficult to enforce. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such third party, or those to whom they communicate such technology or information, from using that technology or information to compete with us. Additionally, trade secret law in Brazil differs from that in the United States, which requires us to take a different approach to protecting our trade secrets in Brazil. Some of these approaches to trade secret protection may be novel and untested under Brazilian law and we cannot guarantee that we would prevail if our trade secrets are contested in Brazil. If any of the above risks materializes, our failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
| 49 |
We may not be able to fully enforce covenants not to compete with and not to solicit our employees, and therefore we may be unable to prevent our competitors from benefiting from the expertise of such employees.
Our proprietary information and inventions agreements with our employees contain non-compete and non-solicitation provisions. These provisions prohibit our employees from competing directly with our business or proposed business or working for our competitors during their term of employment, and from directly and indirectly soliciting our employees and consultants to leave our company for any purpose. Under applicable U.S. and Brazilian law, we may be unable to enforce these provisions. If we cannot enforce these provisions with our employees, we may be unable to prevent our competitors from benefiting from the expertise of such employees. Even if these provisions are enforceable, they may not adequately protect our interests. The defection of one or more of our employees to a competitor could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
Third parties may misappropriate our yeast strains.
Third parties, including collaborators, contract manufacturers, sugar and ethanol mill owners, other contractors and shipping agents, often have custody or control of our yeast strains. If our yeast strains were stolen, misappropriated or reverse engineered, they could be used by other parties who may be able to reproduce the yeast strains for their own commercial gain. If this were to occur, it would be difficult for us to challenge and prevent this type of use, especially in countries where we have limited intellectual property protection or that do not have robust intellectual property law regimes.
If we or one of our collaborators are sued for infringing intellectual property rights or other proprietary rights of third parties, litigation could be costly and time consuming and could prevent us from developing or commercializing our future products.
Our commercial success depends on our and our collaborators’ ability to operate without infringing the patents and proprietary rights of other parties and without breaching any agreements we have entered into with regard to our technologies and product candidates. We cannot determine with certainty whether patents or patent applications of other parties may materially affect our ability to conduct our business. Our industry spans several sectors, including biotechnology, renewable fuels, renewable specialty chemicals and other renewable compounds, and is characterized by the existence of a significant number of patents and disputes regarding patent and other intellectual property rights. Because patent applications can take several years to issue, there may currently be pending applications, unknown to us, that may result in issued patents that cover our technologies or product candidates. We are aware of a significant number of patents and patent applications relating to aspects of our technologies filed by, and issued to, third parties. The existence of third-party patent applications and patents could significantly reduce the coverage of patents owned by or licensed to us and our collaborators and limit our ability to obtain meaningful patent protection. If we wish to make, use, sell, offer to sell, or import the technology or compound claimed in issued and unexpired patents owned by others, we will need to obtain a license from the owner, enter into litigation to challenge the validity of the patents or incur the risk of litigation in the event that the owner asserts that we infringe its patents. If patents containing competitive or conflicting claims are issued to third parties and these claims are ultimately determined to be valid, we and our collaborators may be enjoined from pursing research, development, or commercialization of products, or be required to obtain licenses to these patents, or to develop or obtain alternative technologies.
| 50 |
If a third party asserts that we infringe upon its patents or other proprietary rights, we could face a number of issues that could seriously harm our competitive position, including:
| • | infringement and other intellectual property claims, which could be costly and time consuming to litigate, whether or not the claims have merit, and which could delay getting our products to market and divert management attention from our business; |
| • | substantial damages for past infringement, which we may have to pay if a court determines that our product candidates or technologies infringe a third party's patent or other proprietary rights; |
| • | a court prohibiting us from selling or licensing our technologies or future products unless the holder licenses the patent or other proprietary rights to us, which it is not required to do; and |
| • | if a license is available from a third party, such third party may require us to pay substantial royalties or grant cross licenses to our patents or proprietary rights. |
The industries in which we operate, and the biotechnology industry in particular, are characterized by frequent and extensive litigation regarding patents and other intellectual property rights. Many biotechnology companies have employed intellectual property litigation as a way to gain a competitive advantage. If any of our competitors have filed patent applications or obtained patents that claim inventions also claimed by us, we may have to participate in interference proceedings declared by the relevant patent regulatory agency to determine priority of invention and, thus, the right to the patents for these inventions in the United States. These proceedings could result in substantial cost to us even if the outcome is favorable. Even if successful, an interference proceeding may result in loss of certain claims. Our involvement in litigation, interferences, opposition proceedings or other intellectual property proceedings inside and outside of the United States, to defend our intellectual property rights, or as a result of alleged infringement of the rights of others, may divert management time from focusing on business operations and could cause us to spend significant resources, all of which could harm our business and results of operations.
Many of our employees were previously employed at universities, biotechnology, specialty chemical or oil companies, including our competitors or potential competitors. We may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel and be enjoined from certain activities. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize our product candidates, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and demand on management resources.
| 51 |
We may need to commence litigation to enforce our intellectual property rights, which would divert resources and management's time and attention and the results of which would be uncertain.
Enforcement of claims that a third party is using our proprietary rights without permission is expensive, time consuming and uncertain. Significant litigation would result in substantial costs, even if the eventual outcome is favorable to us and would divert management's attention from our business objectives. In addition, an adverse outcome in litigation could result in a substantial loss of our proprietary rights and we may lose our ability to exclude others from practicing our technology or producing our product candidates.
The laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology and/or bioindustrial technologies. This could make it difficult for us to stop the infringement of our patents or misappropriation of our other intellectual property rights. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Moreover, our efforts to protect our intellectual property rights in such countries may be inadequate.
We do not have exclusive rights to intellectual property we develop under U.S. federally funded research grants and contracts, including with DARPA and DOE, and we could ultimately share or lose the rights we do have under certain circumstances.
Some of our intellectual property rights have been or may be developed in the course of research funded by the U.S. government, including under our agreements with DARPA and DOE. As a result, the U.S. government may have certain rights to intellectual property embodied in our current or future products pursuant to the Bayh-Dole Act of 1980. Government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right to require us, or an assignee or exclusive licensee to such inventions, to grant licenses to any of these inventions to a third party if they determine that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; (iii) government action is necessary to meet requirements for public use under federal regulations; or (iv) the right to use or sell such inventions is exclusively licensed to an entity within the U.S. and substantially manufactured outside the U.S. without the U.S. government’s prior approval. Additionally, we may be restricted from granting exclusive licenses for the right to use or sell our inventions created pursuant to such agreements unless the licensee agrees to additional restrictions (e.g., manufacturing substantially all of the invention in the U.S.). The U.S. government also has the right to take title to these inventions if we fail to disclose the invention to the government and fail to file an application to register the intellectual property within specified time limits. In addition, the U.S. government may acquire title in any country in which a patent application is not filed within specified time limits. Additionally, certain inventions are subject to transfer restrictions during the term of these agreements and for a period thereafter, including sales of products or components, transfers to foreign subsidiaries for the purpose of the relevant agreements, and transfers to certain foreign third parties. If any of our intellectual property becomes subject to any of the rights or remedies available to the U.S. government or third parties pursuant to the Bayh-Dole Act of 1980, this could impair the value of our intellectual property and could adversely affect our business.
Our products subject us to product-safety risks, and we may be sued for product liability.
| 52 |
The design, development, production and sale of our products involve an inherent risk of product liability claims and the associated adverse publicity. Our potential products could be used by a wide variety of consumers with varying levels of sophistication. Although safety is a priority for us, we are not always in control of the final uses and formulations of the products we supply or their use as ingredients. Our products could have detrimental impacts or adverse impacts we cannot anticipate. Despite our efforts, negative publicity about Amyris, including product safety or similar concerns, whether real or perceived, could occur, and our products could face withdrawal, recall or other quality issues. In addition, we may be named directly in product liability suits relating to our products, even for defects resulting from errors of our commercial partners, contract manufacturers, chemical finishers or customers or end users of our products. These claims could be brought by various parties, including customers who are purchasing products directly from us or other users who purchase products from our customers. We could also be named as co-parties in product liability suits that are brought against the contract manufacturers or Brazilian sugar and ethanol mills with whom we partner to produce our products. Insurance coverage is expensive, may be difficult to obtain and may not be available in the future on acceptable terms. We cannot be certain that our contract manufacturers or the sugar and ethanol producers who partner with us to produce our products will have adequate insurance coverage to cover against potential claims. Any insurance we do maintain may not provide adequate coverage against potential losses, and if claims or losses exceed our liability insurance coverage, our business would be adversely impacted. In addition, insurance coverage may become more expensive, which would harm our results of operations.
We may become subject to lawsuits or indemnity claims in the ordinary course of business, which could materially and adversely affect our business and results of operations.
From time to time, we may in the ordinary course of business be named as a defendant in lawsuits, indemnity claims and other legal proceedings. These actions may seek, among other things, compensation for alleged personal injury, employment discrimination, breach of contract, property damage and other losses or injunctive or declaratory relief. In the event that such actions, claims or proceedings are ultimately resolved unfavorably to us at amounts exceeding our accrued liability, or at material amounts, the outcome could materially and adversely affect our reputation, business and results of operations. In addition, payments of significant amounts, even if reserved, could adversely affect our liquidity position.
If we fail to maintain an effective system of internal controls, we may not be able to report our financial results accurately or in a timely manner or prevent fraud; in that case, our stockholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our stock.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. In addition, Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, requires us to evaluate and report on our internal control over financial reporting. The process of implementing our internal controls and complying with Section 404 is expensive and time consuming, and requires significant attention of management. We cannot be certain that these measures will ensure that we maintain adequate controls over our financial processes and reporting in the future. In addition, to the extent we create joint ventures or have any variable interest entities and the financial statements of such entities are not prepared by us, we will not have direct control over their financial statement preparation. As a result, we will, for our financial reporting, depend on what these entities report to us, which could result in us adding monitoring and audit processes and increase the difficulty of implementing and maintaining adequate controls over our financial processes and reporting in the future and could lead to delays in our external reporting. In particular, this may occur where we are establishing such entities with commercial partners that do not have sophisticated financial accounting processes in place, or where we are entering into new relationships at a rapid pace, straining our integration capacity. Additionally, if we do not receive the information from the joint venture or variable interest entity on a timely basis, it could cause delays in our external reporting. Even if we conclude that our internal control over financial reporting provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our reporting obligations. If we or our independent registered public accounting firm discover a material weakness in our internal control over financial reporting, the disclosure of that fact, even if quickly remedied, could reduce the market’s confidence in our financial statements and harm our stock price. In addition, failure to comply with Section 404 could subject us to a variety of administrative sanctions, including SEC action, ineligibility for short form resale registration, the suspension or delisting of our common stock from the stock exchange on which it is listed, and the inability of registered broker-dealers to make a market in our common stock, which would further reduce our stock price and could harm our business.
| 53 |
If we fail to comply with our obligations as a public company, our business may be adversely affected.
As a public company, we incur significant legal, accounting and other expenses in connection with our obligations under applicable securities laws, including the internal and external costs of maintaining the system of internal controls discussed above as well as the costs of preparing and distributing periodic public reports, including financial statements and footnotes. In addition, changing laws, rules and regulations relating to corporate governance and public disclosure, including regulations implemented by the SEC and NASDAQ, increase our legal and financial costs, including costs relating to monitoring, evaluating and complying with such laws, rules and regulations. These laws, rules and regulations are subject to varying interpretations and may evolve over time as new guidance is provided by regulatory and governing bodies, which may result in increased compliance and governance costs and the diversion of management resources. If our efforts to comply with such laws, rules and regulations are not successful, we could be subject to fines, penalties or regulatory proceedings, which can be time consuming and costly to litigate and could lead to negative publicity about our company. These events could also make it more difficult for us to attract and retain qualified members of our board of directors, executive officers and other employees. If any of these risks occur, or if these requirements divert our management’s attention from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations.
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Section 382 of the Internal Revenue Code, or the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change net operating loss carryforwards, or NOLs, to offset future taxable income. If the Internal Revenue Service challenges our analysis that our existing NOLs are not subject to limitations arising from previous ownership changes, or if we undergo an ownership change in the future, our ability to utilize NOLs could be limited by Section 382 of the Code. Future changes in our stock ownership, some of which are outside of our control, could result in an ownership change under Section 382 of the Code. Furthermore, our ability to utilize NOLs of companies that we may acquire in the future may be subject to limitations under Section 382 of the Code. For these reasons, we may not be able to utilize a material portion of our NOLs as of December 31, 2016, even if we attain profitability, which could adversely affect our results of operations.
Loss of, or inability to secure government contract revenues could impair our business.
| 54 |
We have contracts or subcontracts with certain governmental agencies or their contractors. Generally, these agreements, as they may be amended or modified from time to time, have fixed terms and may be terminated, modified or be subject to recovery of payments by the government agency under certain conditions (such as failure to comply with detailed reporting and governance processes or failure to achieve milestones). Under these agreements, we are also subject to audits, which can result in corrective action plans and penalties up to and including termination. If these governmental agencies terminate these agreements with us, it could reduce our revenues which could harm our business. Additionally, we anticipate securing additional government contracts as part of our business plan for 2016 and beyond. If we are unable to secure such government contracts, it could harm our business.
Our headquarters and other facilities are located in an active earthquake and tsunami zone, and an earthquake or other type of natural disaster affecting us or our suppliers could cause resource shortages, disrupt our business and harm our results of operations.
We conduct our primary research and development operations in the San Francisco Bay Area in an active earthquake and tsunami zone, and certain of our suppliers conduct their operations in the same region or in other locations that are susceptible to natural disasters. In addition, California and some of the locations where certain of our suppliers are located have experienced shortages of water, electric power and natural gas from time to time. The occurrence of a natural disaster, such as an earthquake, drought or flood, or localized extended outages of critical utilities or transportation systems, or any critical resource shortages, affecting us or our suppliers could cause a significant interruption in our business, damage or destroy our facilities, production equipment or inventory or those of our suppliers and cause us to incur significant costs or result in limitations on the availability of our raw materials, any of which could harm our business, financial condition and results of operations. The insurance we maintain against fires, earthquakes and other natural disasters may not be adequate to cover our losses in any particular case.
Risks Related to Ownership of Our Common Stock
Our stock price may be volatile.
The market price of our common stock has been, and we expect it to continue to be, subject to significant volatility, and it has declined significantly from our initial public offering price. As of December 31, 2016, the reported closing price of our common stock on The NASDAQ Stock Market was $0.73 per share. Market prices for securities of early stage companies have historically been particularly volatile. Such fluctuations could be in response to, among other things, the factors described in this “Risk Factors” section, or other factors, some of which are beyond our control, such as:
| • | fluctuations in our financial results or outlook or those of companies perceived to be similar to us; |
| • | changes in estimates of our financial results or recommendations by securities analysts; |
| • | changes in market valuations of similar companies; |
| • | changes in the prices of commodities associated with our business such as sugar, ethanol and petroleum or changes in the prices of commodities that some of our products may replace, such as oil and other petroleum sourced products; |
| 55 |
| • | changes in our capital structure, such as future issuances of securities or the incurrence of debt; |
| • | announcements by us or our competitors of significant contracts, acquisitions or strategic alliances; |
| • | regulatory developments in the United States, Brazil, and/or other foreign countries; |
| • | litigation involving us, our general industry or both; |
| • | additions or departures of key personnel; |
| • | investors' general perception of us; and |
| • | changes in general economic, industry and market conditions. |
Furthermore, stock markets have experienced price and volume fluctuations that have affected, and continue to affect, the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market fluctuations, as well as general economic, political and market conditions, such as recessions, interest rate changes and international currency fluctuations, may negatively affect the market price of our common stock.
In the past, many companies that have experienced volatility and sustained declines in the market price of their stock have become subject to securities class action and derivative action litigation. We were involved in two such lawsuits, which were dismissed in 2014, and we may be the target of similar litigation in the future. Securities litigation against us could result in substantial costs and divert our management's attention from other business concerns, which could seriously harm our business.
If our common stock is delisted from The NASDAQ Stock Market, our business, financial condition, results of operations and stock price could be adversely affected, and the liquidity of our stock and our ability to obtain financing could be impaired.
On June 14, 2016, we received a notice from The NASDAQ Stock Market LLC, or NASDAQ, notifying us that we were not in compliance with the requirement of NASDAQ Listing Rule 5450(a)(1) for continued listing on The NASDAQ Global Market, or the Minimum Bid Price Listing Rule, as a result of the closing bid price of our common stock being below $1.00 per share for 30 consecutive business days. In accordance with NASDAQ Listing Rule 5810(c)(3)(A), we had 180 calendar days, or until December 12, 2016, to regain compliance with the Minimum Bid Price Listing Rule. To regain compliance, the closing bid price of our common stock had to be at least $1.00 per share for a minimum of 10 consecutive business days. On November 1, 2016, we received a notice from NASDAQ that we had regained compliance with the Minimum Bid Price Listing Rule. Subsequently, on December 19, 2016, we received a notice from NASDAQ notifying us that we were again not in compliance with the Minimum Bid Price Listing Rule as a result of the closing bid price of our common stock being below $1.00 per share for 30 consecutive business days. In accordance with NASDAQ Listing Rule 5810(c)(3)(A), we have 180 calendar days, or until June 19, 2017, to regain compliance with the Minimum Bid Price Listing Rule. If we do not regain compliance during such period, we may be eligible for an additional compliance period of 180 calendar days, provided that we meet NASDAQ’s continued listing requirement for market value of publicly held shares and all other initial listing standards for The NASDAQ Capital Market, other than the minimum bid price requirement, and provide written notice to NASDAQ of our intention to cure the deficiency during the second compliance period. If we do not regain compliance during the initial compliance period and are not eligible for an additional compliance period, NASDAQ will provide notice that our common stock will be subject to delisting from The NASDAQ Stock Market. In that event, we may appeal such determination to a hearings panel. There can be no assurance that we will satisfy these conditions and that our common stock will remain listed on The NASDAQ Stock Market.
| 56 |
Any delisting of our common stock from The NASDAQ Stock Market could adversely affect our ability to attract new investors, decrease the liquidity of our outstanding shares of common stock, reduce our flexibility to raise additional capital, reduce the price at which our common stock trades, and increase the transaction costs inherent in trading such shares with overall negative effects for our stockholders. In addition, the delisting of our common stock could deter broker-dealers from making a market in or otherwise seeking or generating interest in our common stock, and might deter certain institutions and persons from investing in our securities at all. Furthermore, the delisting of our common stock from The NASDAQ Stock Market would constitute a breach under certain of our financing agreements, including agreements governing our outstanding convertible indebtedness, which could result in an acceleration of such indebtedness. If such indebtedness is accelerated, it would generally also constitute an event of default under our other outstanding indebtedness, permitting acceleration of such other outstanding indebtedness as well. For these reasons and others, the delisting of our common stock from The NASDAQ Stock Market could materially adversely affect our business, financial condition and results of operations.
The concentration of our capital stock ownership with insiders will limit the ability of other stockholders to influence corporate matters and presents risks related to the operations of our significant stockholders.
As of January 31, 2017:
| • | our executive officers and directors and their affiliates together held approximately 9% of our outstanding common stock; |
| • | Maxwell (Mauritius) Pte Ltd, or Temasek (which has a designee on our Board of Directors), held approximately 21% of our outstanding common stock; and |
| • | Total (which has a designee on our Board of Directors) held approximately 23% of our outstanding common stock. |
Furthermore, Total and Temasek each hold certain of our convertible promissory notes, which are convertible into approximately 20,596,778 and 2,670,370 shares of our common stock, respectively, as of January 31, 2017. Total and Temasek also hold certain warrants pursuant to which they may purchase shares of our common stock. This significant concentration of share ownership may adversely affect the trading price of our common stock because investors often perceive disadvantages in owning stock in companies with stockholders with significant interests. Also, these stockholders, acting together, will be able to control our management and affairs and matters requiring stockholder approval, including the election of directors and the approval of significant corporate transactions, such as mergers, consolidations or the sale of all or substantially all of our assets, and may not act in the best interests of our other stockholders. Consequently, this concentration of ownership may have the effect of delaying or preventing a change of control, including a merger, consolidation or other business combination involving us, or a change in our management or Board of Directors, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of the company, even if such actions would benefit our other stockholders.
| 57 |
The concentration of our capital stock ownership also presents risks related to the operations of significant holders of our capital stock, including their international operations. For example, certain affiliates of Total that we do not control and that may be deemed to be our affiliates solely due to their control by Total may be deemed to have engaged in certain transactions or dealings with the government of Iran in 2016, for which Total has provided disclosure under Section 13(r) of the Exchange Act. Such disclosure is set forth in Exhibit 99.4 to this annual report on Form 10-K and is incorporated herein by reference. Disclosure of such activity, even if such activity is not subject to sanctions under applicable law, and any sanctions actually imposed on Total as a result of these activities or for other violations of applicable laws, such as anti-bribery laws, could harm our reputation and have a negative impact on our business.
In addition, our commercial partners, including Total, hold a significant portion of our capital stock and have various rights in connection with their security ownership in us. These stockholders may have interests that are different from those of our other stockholders, including commercial transactions between our company and such commercial partners or their affiliates. While we have a related-party transactions policy which requires certain approvals of any transaction between our company and a significant stockholder or its affiliates, there can be no assurance that such stockholders will act in the best interests of our other stockholders, which could harm our results of operations and cause our stock price to decline.
The market price of our common stock could be negatively affected by future sales of our common stock.
If our existing stockholders, particularly our largest stockholders, our directors, their affiliates, or our executive officers, sell a substantial number of shares of our common stock in the public market, the market price of our common stock could decrease significantly. The perception in the public market that these stockholders might sell our common stock could also depress the market price of our common stock and could impair our future ability to obtain capital, especially through an offering of equity securities.
We have in place a registration statement for the resale of certain shares of common stock held by, or issuable to, certain of our largest stockholders. All common stock sold pursuant to an offering covered by such registration statement will be freely transferable.
In addition, shares issued or issuable under our equity incentive plans have been registered on Form S-8 registration statements and may be freely sold in the public market upon issuance, except for shares held by affiliates who have certain restrictions on their ability to sell.
Conversion of our outstanding convertible promissory notes or the exercise of outstanding warrants to purchase our common stock will dilute the ownership interest of existing stockholders or may otherwise depress the market price of our common stock.
The conversion of some or all of our outstanding convertible promissory notes or the exercise of some or all of outstanding warrants to purchase our common stock will dilute the ownership interests of existing stockholders. In particular, the exercise of certain warrants which have a $0.01 per share exercise price may significantly dilute the economic ownership interest of our existing stockholders. In addition, any sales in the public market of the shares of our common stock issuable upon such conversion or exercise could adversely affect prevailing market prices of our common stock. Furthermore, the existence of our outstanding convertible promissory notes (including anti-dilution conversion price adjustment provisions contained therein which could lead to additional shares of common stock being issuable upon conversion) and warrants may encourage short selling by market participants because the anticipated conversion of such notes into, or exercise of such warrants for, shares of our common stock could depress the market price of our common stock.
| 58 |
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. If any of the analysts who cover us change their recommendation regarding our stock adversely, or provide more favorable relative recommendations about our competitors, our stock price would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
We do not expect to declare any dividends in the foreseeable future.
We do not anticipate declaring any cash dividends to holders of our common stock in the foreseeable future. In addition, certain of our equipment leases and credit facilities currently restrict our ability to pay dividends. Consequently, investors may need to rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investment. Investors seeking cash dividends should not purchase our common stock.
Anti-takeover provisions contained in our certificate of incorporation and bylaws, as well as provisions of Delaware law, could impair a takeover attempt.
Our certificate of incorporation and bylaws contain provisions that could delay or prevent a change in control of our company. These provisions could also make it more difficult for stockholders to elect directors and take other corporate actions. These provisions include:
| • | a staggered board of directors; |
| • | authorizing the board of directors to issue, without stockholder approval, preferred stock with rights senior to those of our common stock; |
| • | authorizing the board of directors to amend our bylaws, to increase the number of directors and to fill board vacancies until the end of the term of the applicable class of directors; |
| • | prohibiting stockholder action by written consent; |
| • | limiting the liability of, and providing indemnification to, our directors and officers; |
| • | eliminating the ability of our stockholders to call special meetings; and |
| • | requiring advance notification of stockholder nominations and proposals. |
| 59 |
Section 203 of the Delaware General Corporation Law prohibits, subject to some exceptions, “business combinations” between a Delaware corporation and an “interested stockholder,” which is generally defined as a stockholder who becomes a beneficial owner of 15% or more of a Delaware corporation's voting stock, for a three-year period following the date that the stockholder became an interested stockholder. We have agreed to opt out of Section 203 through our certificate of incorporation, but our certificate of incorporation contains substantially similar protections to our company and stockholders as those afforded under Section 203, except that we have agreed with Total that it and its affiliates will not be deemed to be “interested stockholders” under such protections.
In addition, we have an agreement with Total which provides that, so long as Total holds at least 10% of our voting securities, we must inform Total of any offer to acquire us or any decision of our Board of Directors to sell our company, and we must provide Total with information about the contemplated transaction. In such events, Total will have an exclusive negotiating period of fifteen business days in the event the Board of Directors authorizes us to solicit offers to buy Amyris, or five business days in the event that we receive an unsolicited offer to purchase us. This exclusive negotiation period will be followed by an additional restricted negotiation period of ten business days, during which we are obligated to continue to negotiate with Total and will be prohibited from entering into an agreement with any other potential acquirer.
These and other provisions in our certificate of incorporation and our bylaws that became effective upon the completion of our initial public offering under Delaware law and in our agreements with Total could discourage potential takeover attempts, reduce the price that investors might be willing to pay in the future for shares of our common stock and result in the market price of our common stock being lower than it would be without these provisions.
| 60 |
EXECUTIVE OFFICERS OF THE REGISTRANT
The following table provides the names, ages and offices of each of our executive officers as of April 17, 2017:
| Name | Age | Position | ||||||
| John Melo | 51 | Director, President and Chief Executive Officer | ||||||
| Kathleen Valiasek | 53 | Chief Financial Officer | ||||||
| Joel Cherry, Ph.D. | 56 | President of Research and Development | ||||||
John Melo
John Melo has nearly three decades of combined experience as an entrepreneur and thought leader in the global fuels industry and technology innovation. Mr. Melo has served as our Chief Executive Officer and a director since January 2007 and our President since January 2008. Before joining Amyris, Mr. Melo served in various senior executive positions at BP Plc (formerly British Petroleum), one of the world’s largest energy firms, from 1997 to 2006, most recently as President of U.S. Fuels Operations from 2004 until December 2006, and previously as Chief Information Officer of the refining and marketing segment from 2001 to 2003, Senior Advisor for e-business strategy to Lord Browne, BP Chief Executive, from 2000 to 2001, and Director of Global Brand Development from 1999 to 2000. Before joining BP, Mr. Melo was with Ernst & Young, an accounting firm, from 1996 to 1997, and a member of the management teams of several startup companies, including Computer Aided Services, a management systems integration company, and Alldata Corporation, a provider of automobile repair software to the automotive service industry. Mr. Melo currently serves on the board of directors of U.S. Venture, Inc. and Renmatix Inc., and also serves as Vice Chairman of the board of directors of BayBio. Mr. Melo was formerly an appointed member to the U.S. section of the U.S.-Brazil CEO Forum.
Kathleen Valiasek
Kathleen Valiasek has served as our Chief Financial Officer since January 2017. Prior to joining us, Ms. Valiasek served as Chief Executive Officer of a finance and strategic consulting firm she founded in 1994, in this capacity she worked closely with the senior management teams of fast-growing companies including start-ups, venture-backed and Fortune 500 companies. Prior to this, she served in key venture capital, real estate development and accounting roles. Ms. Valiasek holds a Bachelor of Business Administration degree from the University of Massachusetts, Amherst.
Joel Cherry, Ph.D.
Dr. Joel Cherry has served as our President of Research and Development since July 2011 and previously as our Senior Vice President of Research Programs and Operations since November 2008. Before joining Amyris, Dr. Cherry was Senior Director of Bioenergy Biotechnology at Novozymes, a biotechnology company focusing on development and manufacture of industrial enzymes from 1992 to November 2008. At Novozymes, he served in a variety of R&D scientific and management positions, including membership in Novozymes’ International R&D Management team, and as Principal Investigator and Director of the BioEnergy Project, a U.S. Department of Energy-funded $18 million effort initiated in 2000. Dr. Cherry holds a Bachelor of Arts degree in Chemistry from Carleton College and a Doctor of Philosophy degree in Biochemistry from the University of New Hampshire.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
| 61 |
We lease approximately 136,000 square feet of space in two adjacent buildings in Emeryville, California, pursuant to two leases. Of our space in Emeryville, we use approximately 113,000 square feet for general office purposes and lab space, and approximately 23,000 square feet comprise our pilot plant. In May 2014, pursuant to a sublease agreement and related documents, we agreed to provide Total with access to certain portions of our pilot plant facilities for a period of five years. Such subleased area is approximately 22,021 square feet and is composed of two areas, a dedicated area accessible only to Total, comprising approximately 3,671 square feet and a common area which is shared by the Company and Total, comprising approximately 18,350 square feet. Our master leases expire in May 2023 and we have an option to extend these leases for five years.
Amyris Brasil leases approximately 44,000 square feet of space in Campinas, Brazil, pursuant to two leases that will expire in November 2018 and October 2019. Of this space, approximately 36,000 square feet comprise a pilot plant and demonstration facility, and the remainder is general office and lab space. Amyris Brasil has a right of first refusal to purchase the space if the landlord elects to sell it and an option to extend the lease for five additional years.
Our first large-scale production plant commenced operations in December 2012 in Brotas in the state of São Paulo, Brazil and is adjacent to an existing sugar and ethanol mill, Tonon Bioenergia S.A. (Tonon). Amyris Brasil leases approximately 800,000 square feet of space for this plant, which has six 200,000 liter production fermenters and was designed to process sugarcane juice and syrup, or their equivalent, from up to one million tons of raw sugarcane annually; this lease expires in March 2026. Amyris Brasil also leases approximately 500,000 square feet of space for a future manufacturing site; this lease expires in January 2031. In February 2017, we broke ground on a second purpose-built, large-scale production facility adjacent to our current facility in Brotas.
We have also secured the use of a Biofene storage tank with an aggregate capacity of 3,000 barrels or 94,500 gallons in Philadelphia. This facility provides temporary storage of our renewable farnesene prior to further processing into one of our finished products. Our current agreement is under a month-to-month lease.
In December 2016, we purchased a manufacturing facility in Leland, North Carolina, which had been previously operated by Glycotech to convert our Biofene into squalane and other final products. We subsequently contributed that facility to our Neossance joint venture with Nikko in December 2016. See Note 7, “Joint Ventures and Noncontrolling Interest” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for additional details regarding our Neossance joint venture and the Leland manufacturing facility.
We believe that our current facilities are suitable and adequate to meet our needs and that suitable additional space will be available to accommodate the foreseeable expansion of our operations. Based on our anticipated volume requirement for 2017, we will likely need to identify and secure access to additional production capacity in 2017, either by constructing a new custom-built facility, acquiring an existing facility from a third party, retrofitting an existing facility operated by a current or potential partner or increasing our use of contract manufacturing facilities. We are currently in the process of identifying and securing such additional production capacity.
| 62 |
We may be involved, from time to time, in legal proceedings and claims arising in the ordinary course of our business. Such matters are subject to many uncertainties and there can be no assurance that legal proceedings arising in the ordinary course of business or otherwise will not have a material adverse effect on our business, results of operations, financial position or cash flows.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
| 63 |
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information for Common Stock
Our common stock commenced trading on the NASDAQ Global Market on September 28, 2010 under the symbol “AMRS” and currently trades on the NASDAQ Global Select Market under the same symbol. The following table sets forth the high and low per share sale prices of our common stock as reported on the NASDAQ Stock Market during each of the previous eight quarters.
| Price Range Per Share | ||||||||
| High | Low | |||||||
| Fiscal 2016 | ||||||||
| Fourth quarter | $ | 1.12 | $ | 0.58 | ||||
| Third quarter | $ | 0.58 | $ | 0.33 | ||||
| Second quarter | $ | 1.30 | $ | 0.35 | ||||
| First quarter | $ | 1.65 | $ | 1.11 | ||||
| Fiscal 2015 | ||||||||
| Fourth quarter | $ | 2.57 | $ | 1.46 | ||||
| Third quarter | $ | 2.62 | $ | 1.51 | ||||
| Second quarter | $ | 2.74 | $ | 1.55 | ||||
| First quarter | $ | 3.11 | $ | 1.56 | ||||
Holders
As of January 31, 2017, there were approximately 106 holders of record (not including beneficial holders of stock held in street names) of our common stock.
Dividend Policy
We have never declared or paid cash dividends on our capital stock. We currently intend to retain any future earnings and do not expect to declare or pay any dividends in the foreseeable future. Any further determination to pay dividends on our capital stock will be at the discretion of our Board of Directors and will depend on our financial condition, results of operations, capital requirements and other factors that our Board of Directors considers relevant.
Securities Authorized for Issuance Under Equity Compensation Plans
See Item 11 of Part III of this Report regarding information about securities authorized for issuance under our equity compensation plans.
| 64 |
Performance Graph(1)
The following graph shows a comparison from September 28, 2010 through December 31, 2016 of cumulative total return on an assumed investment of $100.00 in cash in our common stock, the S&P SmallCap 600 Index and the NASDAQ Clean Edge Green Energy Index. Such returns are based on historical results and are not intended to suggest future performance. Data for the S&P SmallCap 600 Index and the NASDAQ Clean Edge Green Energy Index assume reinvestment of dividends.
COMPARISON OF 75 MONTH CUMULATIVE TOTAL RETURN
Among Amyris, Inc., the S&P SmallCap 600 Index, and the NASDAQ Clean Edge Green Energy Index
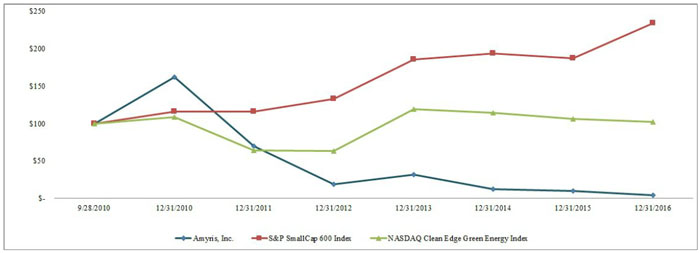
| 9/28/2010 | 12/31/2010 | 12/31/2011 | 12/31/2012 | 12/31/2013 | 12/31/2014 | 12/31/2015 | 12/31/2016 | |||||||||||||||||||||||||
| Amyris, Inc. | 100 | 162 | 70 | 19 | 32 | 12 | 10 | 4 | ||||||||||||||||||||||||
| S&P SmallCap 600 Index | 100 | 116 | 116 | 133 | 185 | 194 | 187 | 234 | ||||||||||||||||||||||||
| NASDAQ Clean Edge Green Energy Index | 100 | 109 | 64 | 63 | 119 | 115 | 106 | 102 | ||||||||||||||||||||||||
| (1) | This performance graph shall not be deemed “soliciting material” or to be “filed” with the SEC for purposes of Section 18 of the Securities Exchange Act of 1934, or otherwise subject to the liabilities under that Section, and shall not be deemed incorporated by reference into any filing of Amyris, Inc. under the Securities Act of 1933, as amended. |
Recent Sales of Unregistered Securities
Sales of Common Stock
On March 27, 2013, we sold 1,533,742 shares of common stock at a price of $3.26 per share for aggregate cash proceeds of $5.0 million.
On April 30, 2014, we sold 943,396 shares of common stock at a price of $4.24 per share for aggregate cash proceeds of $4.0 million.
On July 29, 2015, we sold 16,025,642 shares of common stock at a price of $1.56 per share for aggregate cash proceeds of $25.0 million. In addition, we issued warrants for the purchase, at an exercise price of $0.01 per share, of an aggregate of 1,602,562 shares of our common stock to the purchasers of shares in the offering. The exercisability of these warrants was subject to stockholder approval, which was obtained on September 17, 2015. As of December 31, 2016, 160,255 of such warrants had been exercised.
| 65 |
On May 10, 2016, we sold 4,385,964 shares of common stock at a price of $1.14 per share for aggregate cash proceeds of approximately $5 million.
On August 6, 2016, we issued a warrant to purchase 5,000,000 shares of our common stock, at an exercise price of $0.50 per share, to Ginkgo Bioworks, Inc. (“Ginkgo”) in exchange for the transfer of certain information technology from Ginkgo to Amyris.
On November 16, 2016, we issued a warrant to purchase 10,000,000 shares of our common stock, at an exercise price of $0.50 per share, to Nenter & Co., Inc. (“Nenter”) pursuant to the terms of, and as consideration for, that certain Cooperation Agreement, dated as of October 26, 2016, between Amyris and Nenter. As of December 31, 2016, such warrant had been exercised in full.
Sales of Promissory Notes
On June 6, 2013 and July 26, 2013, we issued an aggregate of $30.0 million of 1.5% Senior Unsecured Convertible Notes due 2017 (“Unsecured R&D Notes”) with an initial conversion price of $3.08 per share, subject to certain adjustments, to Total pursuant to our arrangement with Total for research and development-related funding for aggregate cash proceeds of $30.0 million. The conversion price of these notes is subject to adjustment for proportional adjustments to outstanding common stock and under anti-dilution provisions in case of certain dividends and distributions.
On October 4, 2013, we issued and sold a senior secured promissory note in the principal amount of $35.0 million (the “Bridge Note”) to Temasek for cash proceeds of $35.0 million. The Bridge Note was due on February 2, 2014 and accrued interest at a rate of 5.5% per quarter from October 4, 2013. The Bridge Note was cancelled as payment for Temasek's purchase of Tranche I Notes, as described below.
On October 16, 2013, we issued an aggregate of approximately $51.8 million of senior convertible promissory notes (“Tranche I Notes”) with an initial conversion price of $2.44 per share, subject to certain adjustments, for aggregate cash proceeds of approximately $7.6 million. The remaining approximately $44.2 million of notes was paid through the cancellation of the same amount of previously outstanding convertible promissory notes held by purchasers of the Tranche I Notes. The conversion price of the Tranche I Notes is subject to adjustment (a) according to proportional adjustments to our outstanding common stock in case of certain dividends and distributions, (b) according to anti-dilution provisions, and (c) with respect to Tranche I Notes held by any purchaser other than Total, in the event that Total exchanges existing convertible notes for new securities of the company in connection with future financing transactions in excess of its pro rata amount. The conversion price of the Tranche I Notes was reduced to approximately $1.42 per share upon the completion of a private placement of common stock and warrants to purchase common stock in July 2015, as described above. Following our private offering of unsecured promissory notes and warrants in February 2016, as described below, the conversion price of the Tranche I Notes was adjusted to $1.40 per share, and following our sale of shares of common stock in May 2016, as described above, the conversion price of the Tranche I Notes was further adjusted to $1.14 per share.
| 66 |
On December 2, 2013, in connection with our entry into agreements establishing our joint venture with Total, we exchanged the approximately $69.0 million of then-outstanding Unsecured R&D Notes held by Total for replacement 1.5% Senior Secured Convertible Notes due 2017 (“Secured R&D Notes” and, together with the Unsecured R&D Notes, “R&D Notes”), in principal amounts equal to the principal amount of the cancelled notes. The terms of the Secured R&D Notes were substantially similar to the terms of the Unsecured R&D Notes being exchanged, including conversion prices and terms, other than the security interest granted thereunder.
On January 15, 2014, we issued an aggregate of approximately $34.0 million of senior convertible promissory notes (“Tranche II Notes”) with an initial conversion price of $2.87 per share, subject to certain adjustments, for aggregate cash proceeds of approximately $28.0 million. The remaining approximately $6.0 million of notes was paid through the cancellation of the same amount of previously outstanding convertible promissory notes held by a purchaser of the Tranche II Notes. The conversion price of the Tranche II Notes is subject to adjustment (a) according to proportional adjustments to our outstanding common stock in case of certain dividends and distributions, (b) according to anti-dilution provisions, and (c) with respect to Tranche II Notes held by any purchaser other than Total, in the event that Total exchanges existing convertible notes for new securities of the company in connection with future financing transactions in excess of its pro rata amount. The conversion price of the Tranche II Notes was reduced to approximately $1.42 per share upon the completion of a private placement of common stock and warrants to purchase common stock in July 2015, as described above. Following our private offering of unsecured promissory notes and warrants in February 2016, as described below, the conversion price of the Tranche II Notes was adjusted to $1.40 per share, and following our sale of shares of common stock in May 2016, as described above, the conversion price of the Tranche II Notes was further adjusted to $1.14 per share.
On May 29, 2014, we issued an aggregate of $75.0 million of our 6.50% Convertible Senior Notes due 2019 (“2014 144A Notes”) with an initial conversion rate of 267.0370 shares of common stock per $1,000 principal amount of 2014 144A Notes (representing an initial effective conversion price of approximately $3.74 per share of common stock), subject to certain adjustments, for aggregate cash proceeds of approximately $71.5 million, after payment of the initial purchaser’s discount and offering expenses. The 2014 144A Notes are convertible into shares of the company's common stock at any time prior to the close of business on May 15, 2019. For any conversion on or after May 15, 2015, in the event that the last reported sale price of the company’s common stock for 20 or more trading days (whether or not consecutive) in a period of 30 consecutive trading days ending within five trading days immediately prior to the date the company receives a notice of conversion exceeds the conversion price of $3.74 per share on each such trading day, the holders, in addition to the shares deliverable upon conversion, will be entitled to receive a cash payment equal to the present value of the remaining scheduled payments of interest that would have been made on the 2014 144A Notes being converted from the conversion date to the earlier of the date that is three years after the date the company receives such notice of conversion and maturity (May 15, 2019), which will be computed using a discount rate of 0.75%. In addition, holders of the 2014 144A Notes who convert their 2014 144A Notes in connection with a make-whole fundamental change will, under certain circumstances, be entitled to an increase in the conversion rate. The conversion rate of the 2014 144A Notes is subject to adjustment according to proportional adjustments to our outstanding common stock in case of certain dividends and distributions.
On July 31, 2014 and January 27, 2015, we issued an aggregate of $21.7 million of additional Secured R&D Notes with an initial conversion price of $4.11 per share, subject to certain adjustments, to Total pursuant to our arrangement with Total for research and development funding, for aggregate cash proceeds of $21.7 million. The conversion price of these notes is subject to adjustment for proportional adjustments to outstanding common stock and under anti-dilution provisions in case of certain dividends and distributions.
| 67 |
On July 29, 2015, Temasek exchanged its Tranche I Notes and Tranche II Notes and Total exchanged $70 million in principal amount of R&D Notes for shares of the company's common stock (the “Exchange”). The exchange price was $2.30 per share (Exchange Price) and was paid by the exchange and cancellation of outstanding principal of Tranche I Notes, Tranche II Notes and R&D Notes, as the case may be, including paid-in-kind and accrued interest in the case of Temasek's Tranche I Notes and Tranche II Notes. Temasek exchanged and canceled all Tranche I Notes and Tranche II Notes held by it, having an aggregate principal amount of $71.0 million, in exchange for approximately 30.86 million shares of our common stock. Total exchanged and canceled all but $5.0 million of R&D Notes held by it, such cancelled notes having in an aggregate principal amount of $70 million, in exchange for approximately 30.4 million shares of our common stock. In addition, in connection with the Exchange, on July 29, 2015, Total received the following warrants: (i) a warrant to purchase 18,924,191 shares of the company's Common Stock (the “Total Funding Warrant”); and (ii) a warrant to purchase 2,000,000 shares of the company's common stock that would only be exercisable if the company failed, as of March 1, 2017, to achieve a target cost per liter to manufacture farnesene (Total R&D Warrant). The Total Funding Warrant and the Total R&D Warrant are collectively referred to as the "Total Warrants." Additionally, in connection with the Exchange, on July 29, 2015, Temasek received the following warrants: (i) a warrant to purchase 14,677,861 shares of the company's common stock (the “Temasek Exchange Warrant”); (ii) a warrant exercisable for that number of shares of the company's common stock equal to (1) (A) the number of shares for which Total exercises the Total Funding Warrant plus (B) the number of additional shares for which the Tranche I Notes and Tranche II Notes remaining outstanding following the completion of the Exchange may become convertible as a result of a reduction in the conversion price of such remaining notes as a result of and/or subsequent to the date of the Exchange plus (C) that number of additional shares in excess of 2,000,000, if any, for which the Total R&D Warrant becomes exercisable multiplied by a fraction equal to 30.6% divided by 69.4% plus (2) (A) the number of any additional shares for which the 2014 144A Notes may become convertible as a result of a reduction to the conversion price of the 2014 144A Notes multiplied by (B) a fraction equal to 13.3% divided by 86.7% (the “Temasek Funding Warrant”); and (iii) a warrant exercisable for that number of shares of the company's common stock equal to 880,339 multiplied by a fraction equal to the number of shares for which Total exercises the Total R&D Warrant divided by 2,000,000 (the “Temasek R&D Warrant”). As of December 31, 2016, the Total Funding Warrant and the Temasek Exchange Warrant had been fully exercised and Temasek had exercised the Temasek Funding Warrant with respect to 12,700,244 shares of common stock. Neither the Total R&D Warrant nor the Temasek R&D Warrant were exercisable as of December 31, 2016. See Note 16, “Subsequent Events” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for additional details regarding the Total R&D Warrant and Temasek R&D Warrant. Warrants to purchase 2,462,536 shares of common stock under the Temasek Funding Warrant were unexercised as of December 31, 2016.
| 68 |
On October 20, 2015, we issued an aggregate of $57.6 million of our 9.50% Convertible Senior Notes due 2019 (“2015 144A Notes”) with an initial conversion rate of 443.6557 shares of common stock per $1,000 principal amount of 2015 144A Notes (representing an initial effective conversion price of approximately $2.25 per share of common stock), subject to certain adjustments, for aggregate cash proceeds of approximately $54.4 million, after payment of offering expenses and placement agent fees. The 2015 144A Notes are convertible into shares of the Company's common stock at any time prior to the close of business on April 15, 2019. For any conversion on or after November 27, 2015, the holders, in addition to the shares deliverable upon conversion, will be entitled to receive a payment equal to the present value of the remaining scheduled payments of interest that would have been made on the 2015 144A Notes being converted from the conversion date (or, in the case of conversion between a record date and the following interest payment date, from such interest payment date) to the earlier of the date that is three years after the date the Company receives such notice of conversion and maturity (April 15, 2019), which will be computed using a discount rate of 0.75%. The Company may pay an Early Conversion Payment either in cash or in common stock, at its election. In addition, holders of the 2015 144A Notes who convert their 2015 144A Notes in connection with a make-whole fundamental change will, under certain circumstances, be entitled to an increase in the conversion rate. Following our issuance of warrants to purchase common stock in a private placement transaction in February 2016 and our issuance of convertible notes in May, September and October 2016, as described below, the conversion rate of the 2015 144A Notes was adjusted to 446.8707 shares of common stock per $1,000 principal amount of 2015 144A Notes. On January 11, 2017, we exchanged $15.3 million of our outstanding 3% Senior Unsecured Convertible Notes due 2017, originally issued in February 2012, together with accrued and unpaid interest thereon, for approximately $19.1 million in aggregate principal amount of additional 2015 144A Notes.
On February 12, 2016 and February 15, 2016, we issued an aggregate of $20.0 million of unsecured promissory notes and warrants for the purchase, at an exercise price of $0.01 per share, of an aggregate of 2,857,142 shares of our common stock, for aggregate cash proceeds of $20.0 million.
On March 21, 2016, we sold to Total one half of our ownership stake in our fuels joint venture with Total, Total Amyris BioSolutions B.V. (“TAB”) (giving Total an aggregate ownership stake of 75% of TAB and giving us an aggregate ownership stake of 25% of TAB) in exchange for Total cancelling (i) approximately $1.3 million of R&D Notes held by Total, plus all paid-in-kind and accrued interest under all outstanding R&D Notes (including all such interest that was outstanding as of July 29, 2015) and (ii) a note in the principal amount of Euro 50,000, plus accrued interest, issued by the Company to Total in connection with the original TAB capitalization. To satisfy its purchase obligation above, Total surrendered the remaining Secured R&D Note of approximately $5 million in principal amount, and we executed and delivered to Total a new Unsecured R&D Note (the “March 2016 R&D Note”) in the principal amount of $3.7 million. Other than it is unsecured and its payment terms are severed from TAB’s business performance, the March 2016 R&D Note contains substantially similar terms and conditions to the previous Secured R&D Notes. The March 2016 R&D Note upon issuance had a March 1, 2017 maturity date and an initial conversion price equal to $3.08 per share, which is subject to adjustment for proportional adjustments to outstanding common stock and under anti-dilution provisions in case of certain dividends and distributions. On February 27, 2017, we entered into an amendment of the March 2016 R&D Note with Total to extend the maturity of the March 2016 R&D Note to May 15, 2017.
| 69 |
A placement agent was used in connection with the sale of the Tranche II Notes to one of the purchasers in such financing and in connection with the sale of the 2015 144A Notes in October 2015. In connection with the sale of the 2014 144A Notes, Morgan Stanley & Co. LLC served as the initial purchaser. An exchange agent was used in connection with the issuance of the additional 2015 144A Notes in January 2017. In the other sales of securities described above, no underwriters were involved. Such securities were issued in private transactions pursuant to Section 4(2) of the Securities Act and Regulation D promulgated under Section 3(b) of the Securities Act. The recipients of these securities acquired the securities for investment purposes only and without intent to resell, were able to fend for themselves in these transactions, and were accredited investors as defined in Rule 501 of Regulation D promulgated under Section 3(b) of the Securities Act, and appropriate restrictions were set out in the agreements for, and stock certificates, notes and warrants issued in, these transactions. These security holders had adequate access, through their relationships with us, to information about us.
We may undertake further equity or debt offerings in the future in order to grow our business or fund operations. To the extent we issue further common stock, convertible promissory notes or other equity instruments, such issuances may cause further dilution to our existing stockholders.
| 70 |
ITEM 6. SELECTED FINANCIAL DATA
The selected consolidated statement of operations data for the years ended December 31, 2016, 2015 and 2014 and the selected consolidated balance sheet data as of December 31, 2016 and 2015 are derived from our audited Consolidated Financial Statements appearing elsewhere in this annual report on Form 10-K. The selected consolidated statement of operations data for the years ended December 31, 2013 and 2012 and the selected consolidated balance sheet data as of December 31, 2014, 2013 and 2012 are derived from our audited Consolidated Financial Statements not included in this annual report on Form 10-K. The historical results presented below are not necessarily indicative of financial results to be achieved in future periods. You should read the following selected financial data in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our Consolidated Financial Statements and related Notes included in Item 8 of this annual report on Form 10-K.
| Years Ended December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| Consolidated Statements of Operations Data: | ||||||||||||||||||||
| Total revenues | $ | 67,192 | $ | 34,153 | $ | 43,274 | $ | 41,119 | $ | 73,694 | ||||||||||
| Total cost and operating expenses | $ | 163,116 | $ | 182,686 | $ | 143,102 | $ | 160,735 | $ | 275,516 | ||||||||||
| Net loss from operations | $ | (95,924 | ) | $ | (148,533 | ) | $ | (99,828 | ) | $ | (119,616 | ) | $ | (201,822 | ) | |||||
| Net income (loss) before income taxes and loss from investment in affiliate | $ | (96,781 | ) | $ | (213,400 | ) | $ | 5,572 | $ | (235,754 | ) | $ | (205,052 | ) | ||||||
| Net income (loss) before loss from investment in affiliate | $ | (97,334 | ) | $ | (213,868 | ) | $ | 5,077 | $ | (234,907 | ) | $ | (206,033 | ) | ||||||
| Net income (loss) | $ | (97,334 | ) | $ | (218,052 | ) | $ | 2,167 | $ | (234,907 | ) | $ | (206,033 | ) | ||||||
| Net income (loss) attributable to Amyris, Inc. common stockholders | $ | (97,334 | ) | $ | (217,952 | ) | $ | 2,286 | $ | (235,111 | ) | $ | (205,139 | ) | ||||||
| Net income (loss) per share attributable to common stockholders: | ||||||||||||||||||||
| Basic | $ | (0.41 | ) | $ | (1.75 | ) | $ | 0.03 | $ | (3.12 | ) | $ | (3.62 | ) | ||||||
| Diluted | $ | (0.44 | ) | $ | (1.75 | ) | $ | (0.90 | ) | $ | (3.12 | ) | $ | (3.62 | ) | |||||
| Weighted-average shares of common stock outstanding used in computing net income/loss per share of common stock: | ||||||||||||||||||||
| Basic | 238,440,197 | 126,961,576 | 78,400,098 | 75,472,770 | 56,717,869 | |||||||||||||||
| Diluted | 264,644,449 | 126,961,576 | 121,859,441 | 75,472,770 | 56,717,869 | |||||||||||||||
| 71 |
| As of December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| Consolidated Balance Sheets Data: | ||||||||||||||||||||
| Cash, cash equivalents, investments and restricted cash | $ | 33,807 | $ | 14,685 | $ | 45,041 | $ | 9,944 | $ | 31,644 | ||||||||||
| Working capital (deficit)(2) | $ | (50,745 | ) | $ | (41,147 | ) | $ | 33,606 | $ | (382 | ) | $ | 3,668 | |||||||
| Property, plant and equipment, net | $ | 53,735 | $ | 59,797 | $ | 118,980 | $ | 140,591 | $ | 163,121 | ||||||||||
| Total assets | $ | 129,873 | $ | 110,198 | $ | 216,183 | $ | 198,864 | $ | 242,834 | ||||||||||
| Derivative liabilities | $ | 6,894 | $ | 51,439 | $ | 59,736 | $ | 134,717 | $ | 9,261 | ||||||||||
| Total indebtedness(1)(3) | $ | 228,299 | $ | 156,755 | $ | 233,277 | $ | 153,305 | $ | 106,774 | ||||||||||
| Total equity (deficit) | $ | (183,508 | ) | $ | (158,456 | ) | $ | (125,063 | ) | $ | (135,848 | ) | $ | 66,229 | ||||||
| (1) | Total indebtedness as of December 31, 2016, 2015, 2014, 2013 and 2012 includes $1.3 million, $0.7 million, $0.8 million, $1.2 million, and $2.6 million, respectively, in capital lease obligations, zero, zero, zero, zero, and $1.6 million, respectively, in notes payable, $43.8 million, $14.0 million, $21.1 million, $25.3 million and $26.2 million, respectively, in loans payable, and $49.1 million, $34.4 million, $35.7 million, $8.8 million, and $12.4 million, respectively, in credit facilities. Total indebtedness as of December 31, 2016, 2015, 2014 and 2013 also included $79.0 million, $64.6 million and $60.4 million and $28.5 million, respectively, in convertible notes and $42.8 million, $43.0 million and $115.2 million and $89.5 million, respectively, in related party convertible notes. |
| (2) | Including cash and cash equivalents, investments and restricted cash. |
| (3) | We adopted ASU 2015-03 Interest - Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs, in 2016 and applied the guidance to the December 31, 2016 and 2015 Consolidated Balance Sheets Data, thereby classifying debt issuance costs as a direct reduction of the carrying amount of debt. For the years ending December 31, 2014, 2013 and 2012, we did not reclassify debt issuance costs as such amounts were not material. |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
Amyris is a renewable products company focused on providing sustainable alternatives to a broad range of products. We have developed innovative microbial engineering and screening technologies that modify the way microorganisms process sugars. We are using our proprietary industrial bioscience technology to design microbes, primarily yeast, and use them as catalysts in established fermentation processes to convert plant-sourced sugars into renewable ingredients. We are developing, and, in some cases, already commercializing, products from these ingredients in the Health and Nutrition, Personal Care and Performance Materials markets. We call these No Compromise products because we design them to perform comparably to or better than currently available products.
We have been applying our industrial bioscience technology platform to provide alternatives to a broad range of petroleum-sourced and other traditional products. We have focused our initial development efforts on the production of Biofene, our brand of renewable farnesene, a long-chain, branched liquid hydrocarbon molecule. Using Biofene as a first commercial building block molecule, we are developing a wide range of renewable products for our target markets. In 2014, we began manufacturing additional molecules for the flavors and fragrance (F&F) industry, in 2015 we began investing to expand our capabilities to other small molecule chemical classes beyond terpenes via our collaboration with the Defense Advanced Research Project Agency (DARPA), as discussed below, and in 2016 we expanded into proteins.
| 72 |
While our platform is able to utilize a wide variety of feedstocks, we are focusing our large-scale production plans primarily on the use of Brazilian sugarcane as our feedstock because of its renewability, low cost and relative price stability. We have also been able to produce our ingredients through the use of other feedstocks such as sugar beets, corn dextrose, sweet sorghum and cellulosic sugars.
Our first purpose-built, large-scale production plant commenced operations in southeastern Brazil in December 2012. This plant is located in Brotas, in the state of São Paulo, Brazil, and is adjacent to an existing sugar and ethanol mill. In February 2017, we broke ground on a second purpose-built, large-scale production facility adjacent to our current Brotas facility.
Our business strategy is to generally focus our direct commercialization efforts on specialty products while moving commodity products, including our fuels and base oil lubricants products, into joint venture arrangements with established industry leaders. We believe this approach will permit access to the capital and resources necessary to support large-scale production and global distribution for our products. Our initial renewable products efforts have been focused on the Health and Nutrition, Personal Care and Performance Materials markets, including pharmaceutical products, nutraceuticals, food ingredients, F&F ingredients, skin care ingredients, cosmetic actives, polymers, lubricants, solvents and transportation fuels.
Sales and Revenues
Our revenues are comprised of product revenues and grants and collaborations (including license fees for intellectual property and value share) revenues. Our business model has been a key enabler for short and long-term revenue growth. The three components of our business model are: first, collaborations. Our partners fund the development of key ingredients to support their business strategy which allows us to maintain strong performance levels in our collaboration revenue. The second component, we produce and sell the products we develop to our partners. The third component, we have a value share mechanism where our partners share a portion of the value created from our products. We have entered into research and development collaboration arrangements pursuant to which we receive payments from our collaborators, which include Total, Manufacture Francaise de Pnematiques Michelin, DARPA, DOE, Firmenich, Givaudan and Cosan. Some of such collaboration arrangements include advance payments in consideration for grants of exclusivity or research efforts to be performed by us. Once a collaboration agreement has been signed, receipt of payments may depend on our achievement of milestones. See Note 8, “Significant Agreements” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for more details regarding certain of these agreements and arrangements.
| 73 |
Financing
In 2016 and 2015, we completed multiple financings involving loans, convertible debt, non-convertible debt, mezzanine equity and equity offerings.
In January 2015, we closed a second installment of the $21.7 million in convertible notes from Total under the Total Fuel Agreements, as described in more detail in Note 5, "Debt" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K, in the amount of $10.85 million.
In July 2015, we sold to certain purchasers 16,025,642 shares of our common stock at a price per share of $1.56, for aggregate proceeds to us of $25 million. We also granted to the purchasers warrants exercisable at an exercise price of $0.01 per share for the purchase of an aggregate of 1,602,562 shares of our common stock. The exercisability of these warrants was subject to stockholder approval, which was obtained on September 17, 2015. As of December 31, 2016, 160,255 of such warrants had been exercised.
In October 2015, we issued $57.6 million aggregate principal amount of 9.50% Convertible Senior Notes due 2019 to certain qualified institutional buyers, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In February 2016, we issued to certain purchasers an aggregate of $20.0 million of unsecured promissory notes and warrants for the purchase, at an exercise price of $0.01 per share, of an aggregate of 2,857,142 shares of our common stock, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K. The exercisability of these warrants was subject to stockholder approval, which was obtained on May 17, 2016. As of December 31, 2016, all of such warrants remained outstanding and unexercised.
In March 2016, we sold to Total one half of our ownership stake in TAB in exchange for Total cancelling $1.3 million of R&D Notes and certain other indebtedness, as described in more detail under “Relationship with Total” above and in Note 5, “Debt” and Note 7, “Joint Ventures and Noncontrolling Interest” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In May 2016, we sold and issued 4,385,964 shares of common stock to the Bill & Melinda Gates Foundation at a purchase price per share of $1.14, as described in more detail in Note 8, “Significant Agreements” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In May, September, October and December 2016, we sold and issued $25.0 million in aggregate principal amount of convertible promissory notes to a private investor, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In June and October 2016, we sold and issued $19.5 million in aggregate principal amount of secured promissory notes to Foris Ventures, LLC, an entity affiliated with director John Doerr of Kleiner Perkins Caufield & Byers, a current stockholder, and Ginkgo, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
| 74 |
In October 2016, we entered into a credit agreement with Guanfu Holding Co., Ltd. to make available to Amyris an unsecured credit facility with an aggregate principal amount of up to $25.0 million, which amount was fully drawn on December 31, 2016, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In December 2016, we sold and issued a purchase money promissory note in the principal amount of $3.5 million to Salisbury Partners, LLC in connection with our purchase of a production facility in Leland, North Carolina, as described in more detail in Note 5, “Debt” and Note 7, "Joint Ventures and Noncontrolling Interest" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In December 2016, we sold and issued a promissory note in the principal amount of $3.9 million to Nikko Chemicals Co., Ltd. in connection with the formation of our Neossance joint venture, as described in more detail in Note 5, “Debt” and Note 7, “Joint Ventures and Noncontrolling Interest” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
See Note 16, “Subsequent Events” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for details regarding financing transactions completed subsequent to December 31, 2016.
Exchange (Debt Conversion)
On July 29, 2015, we closed the "Exchange" pursuant to that certain Exchange Agreement, dated as of July 26, 2015 (or the “Exchange Agreement”), among us, Maxwell (Mauritius) Pte Ltd ( “Temasek”) and Total.
Under the Exchange Agreement, at the closing of the Exchange, Temasek exchanged approximately $71.0 million in principal of outstanding convertible promissory notes (including paid-in-kind and accrued interest through July 29, 2015) and Total exchanged $70.0 million in principal amount of outstanding convertible promissory notes for shares of the Company’s common stock. The exchange price was $2.30 per share (or the “Exchange Price”) and was paid by the exchange and cancellation of such outstanding convertible promissory notes, and Temasek and Total received 30,860,633 and 30,434,782 shares of the Company’s common stock, respectively, in the Exchange.
Under the Exchange Agreement, Total also received the following warrants, each with a five-year term, at the closing of the Exchange:
• A warrant to purchase 18,924,191 shares of our Common Stock (or the “Total Funding Warrant”).
• A warrant to purchase 2,000,000 shares of our common stock that would only be exercisable if we failed, as of March 1, 2017, to achieve a target cost per liter to manufacture farnesene (or the “Total R&D Warrant”). The Total Funding Warrant and the Total R&D Warrant are collectively referred to as the “Total Warrants.”
| 75 |
Additionally, under the Exchange Agreement, Temasek received the following warrants at the closing of the Exchange:
• A warrant to purchase 14,677,861 shares of our common stock (or the “Temasek Exchange Warrant”).
• A warrant exercisable for that number of shares of our common stock equal to (1) (A) the number of shares for which Total exercises the Total Funding Warrant plus (B) the number of additional shares for which the certain convertible notes remaining outstanding following the completion of the Exchange may become exercisable as a result of a reduction in the conversion price of such remaining notes as a result of and/or subsequent to the date of the Exchange plus (C) that number of additional shares in excess of 2,000,000, if any, for which the Total R&D Warrant becomes exercisable multiplied by a fraction equal to 30.6% divided by 69.4% plus (2) (A) the number of any additional shares for which certain other outstanding convertible promissory notes may become exercisable as a result of a reduction to the conversion price of such notes multiplied by (B) a fraction equal to 13.3% divided by 86.7% (or the “Temasek Funding Warrant”).
• A warrant exercisable for that number of shares of our common stock equal to 880,339 multiplied by a fraction equal to the number of shares for which Total exercises the Total R&D Warrant divided by 2,000,000 (or the “Temasek R&D Warrant”). If Total is entitled to, and does, exercise the Total R&D Warrant in full, the Temasek R&D Warrant would be exercisable for 880,339 shares.
The Temasek Exchange Warrant, the Temasek Funding Warrant and the Temasek R&D Warrant each have ten-year terms and are referred to herein as the “Temasek Warrants” and, the Temasek Warrants and Total Warrants are hereinafter collectively referred to as the “Exchange Warrants”. All of the Exchange Warrants have an exercise price of $0.01 per share.
In addition to the grant of the Exchange Warrants, a warrant issued by the Company to Temasek in October 2013 in conjunction with a prior convertible debt financing (or the “2013 Warrant”) became exercisable in full upon the completion of the Exchange. There were 1,000,000 shares underlying the 2013 Warrant, which was exercised in full at the exercise price of $0.01 per share.
The exercisability of all of the Exchange Warrants was subject to stockholder approval, which was obtained on September 17, 2015.
In February and May 2016, as a result of adjustments to the conversion price of our senior convertible notes issued in October 2013 (or the “Tranche I Notes”) and January 2014 (or the “Tranche II Notes”) discussed in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K, the Temasek Funding Warrant became exercisable for an additional 127,194 and 2,335,342 shares of common stock, respectively.
As of December 31, 2016, the Total Funding Warrant, the Temasek Exchange Warrant and the 2013 Warrant had been fully exercised, and Temasek had exercised the Temasek Funding Warrant with respect to 12,700,244 shares of our common stock. Neither the Total R&D Warrant nor the Temasek R&D Warrant were exercisable as of December 31, 2016. See Note 16, “Subsequent Events” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for additional details regarding the Total R&D Warrant and Temasek R&D Warrant. Warrants to purchase 2,462,536 shares of common stock under the Temasek Funding Warrant were unexercised as of December 31, 2016.
| 76 |
Maturity Treatment Agreement
At the closing of the Exchange, we, Total and Temasek also entered into a Maturity Treatment Agreement, dated as of July 29, 2015, pursuant to which Total and Temasek agreed to convert any Tranche I Notes, Tranche II Notes or 2014 144A Notes held by them that were not cancelled in the Exchange (or the “Remaining Notes”) into shares of our common stock in accordance with the terms of such Remaining Notes upon maturity, provided that certain events of default had not occurred with respect to the applicable Remaining Notes prior to such maturity. As of immediately following the closing of the Exchange and December 31, 2016, Temasek held $10.0 million in aggregate principal amount of Remaining Notes and Total held approximately $27.0 million and $29.5 million, respectively, in aggregate principal amount of Remaining Notes.
Liquidity
We have incurred significant losses since our inception and we believe that we will continue to incur losses and may have negative cash flow from operations through at least 2017. As of December 31, 2016, we had negative working capital of $50.7, an accumulated deficit of $1,134.4 million and had cash, cash equivalents and short term investments of $28.5 million. We have significant outstanding debt, working capital deficit and contractual obligations related to capital and operating leases, as well as purchase commitments. We will likely need additional financing as early as the second quarter of 2017 to support our liquidity needs. Our audited consolidated financial statements have been prepared on the basis that the Company will continue as a going concern. If we are unable to raise additional financing, our ability to continue as a going concern would be jeopardized and we may be unable to meet our obligations under our existing debt facilities, which could result in an acceleration of our obligations to repay all amounts outstanding under those facilities, and may be forced to liquidate our assets or we may be forced to delay, scale back or eliminate some of our activities to provide sufficient funds to continue our operations. In such a liquidation scenario, the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statement. The financial statements do not include any adjustments that might result from the outcome of this uncertainty, which could have a material adverse effect on our financial condition. Refer to "Liquidity and Capital Resources" for further details.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures. We base our estimates and assumptions on historical experience and on various other factors that we believe to be reasonable under the circumstances. We evaluate our estimates and assumptions on an ongoing basis. The results of our analysis form the basis for making assumptions about the carrying values of assets and liabilities that are not readily apparent from other sources. Our actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies involve significant areas of management’s judgments and estimates in the preparation of our financial statements.
Revenue Recognition
We recognize revenue from the sale of renewable products, from the delivery of collaborative research and development services, from licensing intellectual property, government grants and from value share. Revenue is recognized when all of the following criteria are met: persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the fee is fixed or determinable and collectability is reasonably assured.
| 77 |
If sales arrangements contain multiple elements, we evaluate whether the components of each arrangement represent separate units of accounting. Application of revenue recognition standards requires subjective determination and requires management to make judgments about the fair values of each individual element and whether it is separable from other aspects of the contractual relationship.
For each source of revenues, we apply the above revenue recognition criteria in the following manner:
Product Sales
Starting in the second quarter of 2011, we commenced sales of farnesene-derived products, and in the latter part of 2013 we initiated sales of flavors and fragrances and other products. Revenues are recognized, net of discounts and allowances, once passage of title and risk of loss have occurred, provided all other revenue recognition criteria have also been met.
Shipping and handling costs charged to customers are recorded as revenues. Shipping costs are included in cost of products sold. Such charges were not significant in any of the periods presented.
Grants, Collaborative Research Services and License Fees
Revenues from collaborative research services are recognized as the services are performed consistent with the performance requirements of the contract. In cases where the planned levels of research services fluctuate over the research term, we recognize revenues using the proportionate performance method based upon actual efforts to date relative to the amount of expected effort to be incurred by us. When up-front payments are received and the planned levels of research services do not fluctuate over the research term, revenues are recorded on a ratable basis over the arrangement term, up to the amount of cash received. When up-front payments are received and the planned levels of research services fluctuate over the research term, revenues are recorded using the proportionate performance method, up to the amount of cash received. Where arrangements include milestones that are determined to be substantive and at risk at the inception of the arrangement, revenues are recognized upon achievement of the milestone and is limited to those amounts whereby collectability is reasonably assured. License fees for intellectual property transferred to other parties, representing non-refundable payments received at the time of signature of license agreements, are recognized as revenue upon signature of the license agreements when the Company has no significant future performance obligations and collectability of the fees is assured. Upfront payments received at the beginning of licensing agreements are deferred and recognized as revenue on a systematic basis over the period during which the related services are rendered and all obligations are performed.
Government grants are made pursuant to agreements that generally provide cost reimbursement for certain types of expenditures in return for research and development activities over a contractually defined period. Revenues from government grants are recognized in the period during which the related costs are incurred, provided that the conditions under which the government grants were provided have been met and only perfunctory obligations are outstanding.
| 78 |
Variable Interest Entities
We have interests in certain joint venture entities that are variable interest entities or VIEs. Determining whether to consolidate a variable interest entity may require judgment in assessing (i) whether an entity is a variable interest entity and (ii) if we are the entity’s primary beneficiary and thus required to consolidate the entity. To determine if we are the primary beneficiary of a VIE, we evaluate whether we have (i) the power to direct the activities that most significantly impact the VIE’s economic performance and (ii) the obligation to absorb losses or the right to receive benefits of the VIE that could potentially be significant to the VIE. Our evaluation includes identification of significant activities and an assessment of our ability to direct those activities based on governance provisions and arrangements to provide or receive product and process technology, product supply, operations services, equity funding and financing and other applicable agreements and circumstances. Our assessment of whether we are the primary beneficiary of our VIEs requires significant assumptions and judgment.
Impairment of Long-Lived Assets
We assess impairment of long-lived assets, which include property, plant and equipment, and test long-lived assets for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to, significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; or expectations that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life.
Recoverability is assessed based on the fair value of the asset, which is calculated as the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset. An impairment loss is recognized in the consolidated statements of operations when the carrying amount is determined not to be recoverable and exceeds fair value, which is determined on a discounted cash flow basis.
We make estimates and judgments about future undiscounted cash flows and fair values. Although our cash flow forecasts are based on assumptions that are consistent with our plans, there is significant exercise of judgment involved in determining the cash flows attributable to a long-lived asset over its estimated remaining useful life. Although we believe that the assumptions and estimates that we have are reasonable and appropriate, different assumptions and estimates could materially impact our reported financial results.
| 79 |
Inventories
Inventories, which consist of farnesene-derived products and flavor and fragrances ingredients are stated at the lower of cost or market and categorized as finished goods, work-in-process or raw material inventories. We evaluate the recoverability of our inventories based on assumptions about expected demand and net realizable value. If we determine that the cost of inventories exceeds its estimated net realizable value, we record a write-down equal to the difference between the cost of inventories and the estimated net realizable value. If actual net realizable values are less favorable than those projected by management, additional inventory write-downs may be required that could negatively impact our operating results. If actual net realizable values are more favorable than those projected by management, we may have favorable operating results when products that have been previously written down are sold in the normal course of business. We also evaluate the terms of our agreements with our suppliers and establish accruals for estimated losses on adverse purchase commitments as necessary, applying the same lower of cost or market approach that is used to value inventory. Cost is computed on a first-in, first-out basis. Inventory costs are incurred in bringing inventory to its existing location.
Goodwill and Intangible Assets
Goodwill represents the excess of the cost over the fair value of net assets acquired from our business combinations. Intangible assets are comprised primarily of in-process research and development (IPR&D). We make significant judgments in relation to the valuation of goodwill and intangible assets resulting from business combinations and asset acquisitions. Goodwill and intangible assets with indefinite lives are assessed for impairment using fair value measurement techniques on an annual basis or more frequently if facts and circumstance warrant such a review. When required, a comparison of fair value to the carrying amount of assets is performed to determine the amount of any impairment.
There are several methods that can be used to determine the estimated fair value of the IPR&D acquired in a business combination. We have used the "income method," which applies a probability weighting that considers the risk of development and commercialization, to the estimated future net cash flows that are derived from projected sales revenues and estimated costs. These projections are based on factors such as relevant market size, pricing of similar products, and expected industry trends. The estimated future net cash flows are then discounted to the present value using an appropriate discount rate. These assets are treated as indefinite-lived intangible assets until completion or abandonment of the projects, at which time the assets will be amortized over the remaining useful life or written off, as appropriate.
Factors that could trigger an impairment review include significant under-performance relative to historical or projected future operating results, significant changes in the manner of our use of the acquired assets or the strategy for our overall business or significant negative industry or economic trends. If this evaluation indicates that the value of the intangible asset may be impaired, we make an assessment of the recoverability of the net carrying value of the asset over its remaining useful life. If this assessment indicates that the intangible asset is not recoverable, based on the estimated discounted future cash flows of the technology over the estimated useful life of the technology, we will reduce the net carrying value of the related intangible asset to fair value and may adjust the remaining amortization period. Any such impairment charge could be significant and could have a material adverse effect on our reported financial results. As of December 31, 2016, the Company's intangible assets had a carrying amount of zero.
| 80 |
Stock-Based Compensation
Stock-based compensation cost for restricted stock units (RSUs) is measured based on the closing fair market value of our common stock on the date of grant. Stock-based compensation cost for stock options and employee stock purchase plan rights is estimated at the grant date and offering date, respectively, based on the fair-value of our common stock using the Black-Scholes option pricing model. We amortize the fair value of the employee stock options on a straight-line basis over the requisite service period of the award, which is generally the vesting period. The measurement of nonemployee stock-based compensation is subject to periodic adjustments as the underlying equity instruments vest, and the resulting change in value, if any, is recognized in our consolidated statements of operations during the period the related services are rendered. There is inherent uncertainty in these estimates and if different assumptions had been used, the fair value of the equity instruments issued to nonemployee consultants could have been significantly different.
In future periods, our stock-based compensation expense is expected to change as a result of our existing unrecognized stock-based compensation still to be recognized and as we issue additional stock-based awards in order to attract and retain employees and nonemployee consultants.
See Note 11, "Stock-Based Compensation Plans" in “Notes to Consolidated Financial Statements” in Part II, Item 8 of this Report for a description of our stock-based compensation plans and more information on the assumptions used to calculate the fair value of stock-based compensation.
Income Taxes
We are subject to income taxes in the United States and foreign jurisdictions, and we use estimates in determining our provisions for income taxes. We use the liability method of accounting for income taxes, whereby deferred tax assets or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect for the year in which the differences are expected to affect taxable income.
Recognition of deferred tax assets is appropriate when realization of such assets is more likely than not. We recognize a valuation allowance against our net deferred tax assets unless it is more likely than not that they will be realized. This assessment requires judgment as to the likelihood and amounts of future taxable income by tax jurisdiction.
We apply the provisions of Financial Accounting Standards Board (FASB) guidance on accounting for uncertainty in income taxes. We assess all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position’s sustainability and the tax benefit to be recognized is measured at the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and we will determine whether (i) the factors underlying the sustainability assertion have changed and (ii) the amount of the recognized tax benefit is still appropriate. The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit might change as new information becomes available.
| 81 |
Embedded Derivatives Related to Convertible Notes
Embedded derivatives that are required to be bifurcated from the underlying debt instrument (i.e. host) are accounted for and valued as a separate financial instrument. We evaluated the terms and features of our convertible notes payable and identified compound embedded derivatives (conversion options that contain “make-whole interest” provisions or down round conversion price adjustment provisions) requiring bifurcation and accounting at fair value because the economic and contractual characteristics of the embedded derivatives met the criteria for bifurcation and separate accounting due to the conversion option containing a “make-whole interest” provision and down round conversion, that requires cash payment for forgone interest upon a change of control and down round conversion price adjustment provisions.
See Note 3, "Fair Value of Financial Instruments" in “Notes to Consolidated Financial Statements” in Part II, Item 8 of this Report for a description of our embedded derivatives related to convertible notes and information on the valuation models used to calculate the fair value of embedded derivatives. Changes in the inputs into these valuation models may have a significant impact on the estimated fair value of the embedded derivatives. For example, a decrease (increase) in the estimated credit spread for the Company results in an increase (decrease) in the estimated value of the embedded derivatives. Conversely, a decrease (increase) in the stock price results in a decrease (increase) in the estimated fair value of the embedded derivatives. The changes in the fair value of the bifurcated compound embedded derivatives are primarily related to the change in price of the underlying common stock of the Company and is reflected in our consolidated statements of operations as “Gain (loss) from change in fair value of derivative instruments.”
Results of Operations
Comparison of Year Ended December 31, 2016 to Year Ended December 31, 2015
Revenues
| Years Ended December 31, | Year to Year | Percentage | ||||||||||||||
| 2016 | 2015 | Change | Change | |||||||||||||
| (Dollars in thousands) | ||||||||||||||||
| Revenues | ||||||||||||||||
| Renewable product sales | $ | 24,788 | $ | 14,032 | $ | 10,756 | 77 | % | ||||||||
| Related party renewable product sales | 1,561 | 864 | 697 | 81 | % | |||||||||||
| Total product sales | 26,349 | 14,896 | 11,453 | 77 | % | |||||||||||
| Grants and collaborations revenue | 25,843 | 19,257 | 6,586 | 34 | % | |||||||||||
| License fees | 15,000 | — | 15,000 | nm | ||||||||||||
| Total grants, collaborations and license fee revenue | 40,843 | 19,257 | 21,586 | 112 | % | |||||||||||
| Total revenues | $ | 67,192 | $ | 34,153 | $ | 33,039 | 97 | % | ||||||||
______________
nm= not meaningful
Our total revenues increased by $33.0 million to $67.2 million in 2016 as compared to the prior year, primarily due to significant growth in product sales and grants, collaborations and license fee revenues.
| 82 |
Product sales increased by $11.5 million to $26.3 million in 2016 as compared to the prior year primarily due to increases in the personal care and health and nutrition segments.
Grants, collaborations and license fee revenue increased by $21.6 million to $40.8 million in 2016 compared to the prior year. This increase was due to new contracts with DARPA and Givaudan and license fee revenues resulting from the transfer of intellectual property to Ginkgo Bioworks for $15 million.
Cost and Operating Expenses
| Years Ended December 31, | Year-to Year | Percentage | ||||||||||||||
| 2016 | 2015 | Change | Change | |||||||||||||
| Costs and Operating Expenses | (Dollars in thousands) | |||||||||||||||
| Cost of products sold | $ | 56,678 | $ | 37,374 | $ | 19,304 | 52 | % | ||||||||
| Loss on purchase commitments, impairment of property, plant and equipment and other asset allowances | 7,305 | 34,166 | (26,861 | ) | (79 | )% | ||||||||||
| Withholding tax related to conversion of related party notes | — | 4,723 | (4,723 | ) | (100 | )% | ||||||||||
| Impairment of intangible assets | — | 5,525 | (5,525 | ) | (100 | )% | ||||||||||
| Research and development | 51,412 | 44,636 | 6,776 | 15 | % | |||||||||||
| Sales, general and administrative | 47,721 | 56,262 | (8,541 | ) | (15 | )% | ||||||||||
| Total cost and operating expenses | $ | 163,116 | $ | 182,686 | $ | (19,570 | ) | (11 | )% | |||||||
Cost of Products Sold
Our cost of products sold includes the cost of raw materials, labor and overhead, amounts paid to contract manufacturers, period costs related to inventory write-downs resulting from applying lower of cost or market inventory valuations, and costs related to scale-up in production of such products. Our cost of products sold increased by $19.3 million to $56.7 million in 2016 as compared to the prior year, primarily driven by product mix, higher volumes of products sold and production scale-up costs.
Loss on Purchase Commitments and Impairment of Property, Plant and Equipment and Other Asset Allowances
The loss on purchase commitments and impairment of property, plant and equipment and other asset allowances decreased by $26.9 million to $7.3 million in 2016 as compared to the prior year. This decline was primarily as a result of lower asset impairment charges.
| 83 |
Research and Development Expenses
Our research and development expenses increased by $6.8 million to $51.4 million in 2016 as compared to the prior year, primarily as a result of increases of $3.8 million in consulting and outside services, $1.5 million in salaries and benefits expense, $1.7 million in facilities costs and $0.1 million in lab supplies and equipment, offset by a decrease of $0.3 million in stock-based compensation expense.
Sales, General and Administrative Expenses
Our sales, general and administrative expenses decreased by $8.5 million to $47.7 million in 2016 as compared to the prior year, primarily due to decreases of $3.2 million in consulting and outside services expenses, $2.2 million in salaries and benefits, $1.7 million in facilities expenses and $1.4 million in stock-based compensation expense.
Other Income (Expense)
| Years Ended December 31, | Year-to Year | Percentage | ||||||||||||||
| 2016 | 2015 | Change | Change | |||||||||||||
| (Dollars in thousands) | ||||||||||||||||
| Other income (expense): | ||||||||||||||||
| Interest income | $ | 258 | $ | 264 | $ | (6 | ) | (2 | )% | |||||||
| Interest expense | (37,629 | ) | (78,854 | ) | 41,225 | (52 | )% | |||||||||
| Gain from change in fair value of derivative instruments | 41,355 | 16,287 | 25,068 | 154 | % | |||||||||||
| Loss from extinguishment of debt | (4,146 | ) | (1,141 | ) | (3,005 | ) | 263 | % | ||||||||
| Other income (expense), net | (695 | ) | (1,423 | ) | 728 | (51 | )% | |||||||||
| Total other income (expense) | $ | (857 | ) | $ | (64,867 | ) | $ | 64,010 | (99 | )% | ||||||
Total other expense was $0.9 million in 2016, compared to $64.9 million in 2015. The decrease in net expense of $64.0 million was primarily attributable to the decreases of $41.2 million in interest expense associated with our acceleration of accretion of debt discount related to debt extinguishments and conversions in 2015 and of $0.7 million in other expense, the increase in gain from change in fair value of derivative instruments of $25.1 million, attributed to the compound embedded derivative liabilities associated with certain of our convertible promissory notes, and the change in fair value of our interest rate swap derivative liability, which was partially offset by the increase in loss from extinguishment of debt of $3.0 million.
| 84 |
Comparison of Year Ended December 31, 2015 to Year Ended December 31, 2014
Revenues
| Years Ended December 31, | Year-to Year | Percentage | ||||||||||||||
| 2015 | 2014 | Change | Change | |||||||||||||
| (Dollars in thousands) | ||||||||||||||||
| Revenues | ||||||||||||||||
| Renewable product sales | $ | 14,032 | $ | 22,793 | $ | (8,761 | ) | (38 | )% | |||||||
| Related party renewable product sales | 864 | 646 | 218 | 34 | % | |||||||||||
| Total product sales | 14,896 | 23,439 | (8,543 | ) | (36 | )% | ||||||||||
| Grants and collaborations revenue | 19,257 | 19,835 | (578 | ) | (3 | )% | ||||||||||
| Total grants and collaborations revenue | 19,257 | 19,835 | (578 | ) | (3 | )% | ||||||||||
| Total revenues | $ | 34,153 | $ | 43,274 | $ | (9,121 | ) | (21 | )% | |||||||
Our total revenues decreased by $9.1 million to $34.2 million in 2015 as compared to the prior year, primarily due to lower product sales, the achievement of collaboration milestones in 2013 and 2014, which did not continue in 2015, and the recognizing of revenue in 2014 related to previous collaboration payments. This decrease was partly offset by the completion of several government grant contracts.
Product sales decreased by $8.5 million to $14.9 million in 2015 as compared to the prior year primarily due to the initial large shipment in 2014 of product to a collaboration partner, while our initial large shipment of a product to a collaboration partner expected for Q4 2015 was delayed.
Grants and collaborations revenue decreased by $0.6 million to $19.3 million in 2015 compared to the prior year. This was due to a $2.9 million decrease in government grants revenue offset by a $2.3 million increase in collaborations revenue. The decline in government grants by $2.9 million, includes a decrease of $2.0 million from the DARPA Technology Investment Agreement as a result of the project being completed during 2014. The decrease was reduced by the net increase in collaborations revenue of $2.4 million. The increase consists of new collaborations of $1.3 million and $1.1 million from collaborations that started later in 2014.
Cost and Operating Expenses
| Years Ended December 31, | Year-to Year | Percentage | ||||||||||||||
| 2015 | 2014 | Change | Change | |||||||||||||
| (Dollars in thousands) | ||||||||||||||||
| Cost of products sold | $ | 37,374 | $ | 33,202 | $ | 4,172 | 13 | % | ||||||||
| Loss on purchase commitments and write-off of property, plant and equipment | 34,166 | 1,769 | 32,397 | 1,831 | % | |||||||||||
| Withholding tax related to conversion of related party notes | 4,723 | — | 4,723 | nm | ||||||||||||
| Impairment of intangible assets | 5,525 | 3,035 | 2,490 | 82 | % | |||||||||||
| Research and development | 44,636 | 49,661 | (5,025 | ) | (10 | )% | ||||||||||
| Sales, general and administrative | 56,262 | 55,435 | 827 | 1 | % | |||||||||||
| Total cost and operating expenses | $ | 182,686 | $ | 143,102 | $ | 39,584 | 28 | % | ||||||||
______________
nm= not meaningful
| 85 |
Cost of Products Sold
Our cost of products sold includes cost of raw materials, labor and overhead, amounts paid to contract manufacturers, period costs related to inventory write-downs resulting from applying lower of cost or market inventory valuations, and costs related to scale-up in production of such products. Our cost of products sold increased by $4.2 million to $37.4 million in 2015 as compared to the prior year, primarily driven by an unfavorable product mix in 2015, with declining fuel average selling prices generating losses. This increase was partly offset by lower sales.
Loss on Purchase Commitments and Impairment of Property, Plant and Equipment and Other Asset Allowances
The loss on purchase commitments and impairment of property, plant and equipment and other asset allowances increased by $32.4 million to $34.2 million in 2015 as compared to the prior year. The increase was mainly due to an impairment charge associated with the termination of a production joint venture and indirect tax allowances. See Note 4 “Balance Sheet Components” to the financial statements for further details.
Impairment of Intangible Assets
The loss on impairment of intangible assets of $5.5 million was a result of the impairment of in-process research and development assets related to the 2011 acquisition of Draths Corporation (Draths).
Research and Development Expenses
Our research and development expenses decreased by $5.0 million to $44.6 million in 2015 as compared to the prior year, primarily as a result of decreases of $1.2 million in stock-based compensation, $1.2 million in salaries and benefits expense, $0.7 million from our overall cost reduction efforts and lower spending to manage our operating costs, $0.7 million from other expenses, $0.6 million in facilities and rent costs, and $0.6 million in consulting and outside services. Research and development expenses included stock-based compensation expense of $2.3 million and $3.5 million during the years 2015 and 2014, respectively.
Sales, General and Administrative Expenses
Our sales, general and administrative expenses increased by $0.8 million to $56.3 million in 2015 as compared to the prior year, primarily due to increases in consulting and outside services and personnel-related expenses from sales and marketing headcount to support the Company’s product commercialization plans, as well as a severance-related charge, offset in part by a decrease in stock-based compensation. Sales, general and administrative expenses included stock-based compensation expense of $6.8 million and $10.6 million during the years ended December 31, 2015 and 2014, respectively.
| 86 |
Other Income (Expense)
| Years Ended December 31, | Year-to Year | Percentage | ||||||||||||||
| 2015 | 2014 | Change | Change | |||||||||||||
| (Dollars in thousands) | ||||||||||||||||
| Other income (expense): | ||||||||||||||||
| Interest income | $ | 264 | $ | 387 | $ | (123 | ) | (32 | )% | |||||||
| Interest expense | (78,854 | ) | (28,949 | ) | (49,905 | ) | 172 | % | ||||||||
| Gain (loss) from change in fair value of derivative instruments | 16,287 | 144,138 | (127,851 | ) | (89 | )% | ||||||||||
| Loss from extinguishment of debt | (1,141 | ) | (10,512 | ) | 9,371 | (89 | )% | |||||||||
| Other income (expense), net | (1,423 | ) | 336 | (1,759 | ) | (524 | )% | |||||||||
| Total other income (expense) | $ | (64,867 | ) | $ | 105,400 | $ | (170,267 | ) | (162 | )% | ||||||
Total other income (expense) was $64.9 million net expense in 2015, compared to $105.4 million net income in 2014. The decrease in net income of $170.3 million was primarily attributable to the decrease in gain from change in fair value of derivative instruments of $127.9 million, attributed to the compound embedded derivative liabilities associated with our senior secured convertible promissory notes, the change in fair value of our interest rate swap derivative liability and the increases of $49.9 million in interest expense associated with our acceleration of accretion of debt discount related to debt extinguishments and conversions, which was offset by the decrease in losses from the extinguishment of debt of $9.4 million.
Liquidity and Capital Resources
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| (Dollars in thousands) | ||||||||
| Working capital (deficit), excluding cash and cash equivalents | $ | (77,895 | ) | $ | (53,139 | ) | ||
| Cash and cash equivalents and short-term investments | $ | 28,524 | $ | 13,512 | ||||
| Debt and capital lease obligations | $ | 228,299 | $ | 156,755 | ||||
| Accumulated deficit | $ | (1,134,438 | ) | $ | (1,037,104 | ) | ||
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (Dollars in thousands) | ||||||||||||
| Net cash used in operating activities | $ | (82,367 | ) | $ | (85,132 | ) | $ | (84,708 | ) | |||
| Net cash provided by (used in) investing activities | $ | 5,642 | $ | (5,144 | ) | $ | (9,831 | ) | ||||
| Net cash provided by financing activities | $ | 92,199 | $ | 61,424 | $ | 130,921 | ||||||
Working Capital. Our working capital deficit, excluding cash and cash equivalents was $77.9 million as of December 31, 2016, which represents an increase of $24.8 million compared to our working capital deficit of $53.1 million as of December 31, 2015. The increase of $24.8 million in working capital deficit for 2016 as compared to the prior year was due to increases of $4.9 million in accrued and other current liabilities, $7.4 million in accounts payable and $21.6 million in current portion of debt, partially offset by an increase of $9.1 million in accounts receivable.
| 87 |
To support production of our products in contract manufacturing and dedicated production facilities, we have incurred, and we expect to continue to incur, capital expenditures as we invest in these facilities. We plan to continue to seek external debt and equity financing from U.S. and Brazilian sources to help fund our investment in these contract manufacturing and dedicated production facilities.
We expect to fund our operations for the foreseeable future with cash and investments currently on hand, cash inflows from collaboration and grant funding, cash contributions from product sales, and proceeds from new investor debt and equity financings as well as strategic asset divestments. Some of our anticipated financing sources, such as research and development collaborations, debt and equity financings and strategic asset divestments, are subject to the risk that we cannot meet milestones, are not yet subject to definitive agreements or mandatory funding commitments and, if needed, we may not be able to secure additional types of financing in a timely manner or on reasonable terms, if at all. Our planned 2017 working capital needs and our planned operating and capital expenditures for 2017 are dependent on significant inflows of cash from renewable product sales and existing collaboration partners, as well as additional funding from new collaborations, new debt and equity financings and proceeds from strategic asset divestments. We will continue to need to fund our research and development and related activities and to provide working capital to fund production, storage, distribution and other aspects of our business.
Liquidity. We have incurred significant losses since our inception and we believe that we will continue to incur losses and may have negative cash flow from operations through at least 2017. As of December 31, 2016, we had an accumulated deficit of $1,134.4 million and had cash, cash equivalents and short term investments of $28.5 million. In March 2016, we entered into an At Market Issuance Sales Agreement with third party Agents under which we may issue and sell shares of our common stock through the Agents having an aggregate offering price of up to $50.0 million from time to time in “at the market” offerings under our Registration Statement on Form S-3 (File No. 333-203216). This agreement includes no commitment by other parties to purchase shares we offer for sale. See Note 8, “Significant Agreements” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for further details. As of the date hereof, $50.0 million remained available for future issuance under this facility. We have significant outstanding debt and contractual obligations related to capital and operating leases, as well as purchase commitments.
As of December 31, 2016, our debt, net of discount of $42.5 million, totaled $227.0 million, of which $59.2 million is classified as current. In addition to upcoming debt maturities, our debt service obligations over the next twelve months are significant, including $18.3 million of anticipated interest payments. Our debt agreements also contain various covenants, including restrictions on our business that could cause us to be at risk of defaults such as restrictions on additional indebtedness, material adverse effect and cross default clauses. A failure to comply with the covenants and other provisions of our debt instruments, including any failure to make a payment when required would generally result in events of default under such instruments, which could permit acceleration of such indebtedness. If such indebtedness is accelerated, it would generally also constitute an event of default under our other outstanding indebtedness, permitting acceleration of such other outstanding indebtedness. Any required repayment of our indebtedness as a result of acceleration or otherwise would lower our current cash on hand such that we would not have those funds available for use in our business or for payment of other outstanding indebtedness. Refer to Note 5, "Debt", Note 6, “Commitments and Contingencies” and Note 16, “Subsequent Events” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for further details of our debt arrangements.
| 88 |
The Company has incurred significant operating losses since its inception and believes that it will continue to incur losses and negative cash flow from operations into at least 2018. As of December 31, 2016, the 76 Company had negative working capital of $40.7 million, an accumulated deficit of $1,124.4 million and had cash, cash equivalents and short term investments of $28.5 million. The Company will likely need additional financing as early as the second quarter of 2017 to support its liquidity needs. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued. The financial statements do not include any adjustments that might result from the outcome of this uncertainty, which could have a material adverse effect on our financial condition. In addition, if we are unable to continue as a going concern, we may be unable to meet our obligations under our existing debt facilities, which could result in an acceleration of our obligation to repay all amounts outstanding under those facilities, and we may be forced to liquidate our assets. In such a scenario, the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements.
Our operating plan for 2017 contemplates a significant reduction in our net cash outflows, resulting from (i) revenue growth from sales of existing and new products with positive gross margins, (ii) reduced production costs as a result of manufacturing and technical developments, (iii) cash inflows from collaborations, (iv) access to various financing commitments, and (v) strategic asset divestments (see Note 5, “Debt” and Note 8, “Significant Agreements” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for details of financing commitments).
If we are unable to generate sufficient cash contributions from product sales, payments from existing and new collaboration partners and strategic asset divestments and draw sufficient funds from financing commitments due to contractual restrictions and covenants, we will need to obtain additional funding from equity or debt financings, which could require us to agree to burdensome covenants or grant further security interests in our assets, enter into collaboration and licensing arrangements that require us to relinquish commercial rights, or grant licenses on terms that are not favorable.
If we are unable to raise additional financing, or if other expected sources of funding are delayed or not received, our ability to continue as a going concern would be jeopardized and we would take the following actions as early as the second quarter of 2017 to support our liquidity needs through the remainder of 2017 and into 2018:
| • | Effect significant headcount reductions, particularly with respect to employees not connected to critical or contracted activities across all functions of the Company, including employees involved in general and administrative, research and development, and production activities. |
| • | Shift focus to existing products and customers with significantly reduced investment in new product and commercial development efforts. |
| • | Reduce production activity at our Brotas manufacturing facility to levels only sufficient to satisfy volumes required for product revenues forecast from existing products and customers. |
| • | Reduce expenditures for third party contractors, including consultants, professional advisors and other vendors. |
| • | Reduce or delay uncommitted capital expenditures, including those relating to proposed additional manufacturing capacity, non-essential facility and lab equipment, and information technology projects. |
| • | Closely monitor our working capital position with customers and suppliers, as well as suspend operations at pilot plants and demonstration facilities. |
| 89 |
Implementing this plan could have a negative impact on our ability to continue our business as currently contemplated, including, without limitation, delays or failures in our ability to:
| • | Achieve planned production levels; |
| • | Develop and commercialize products within planned timelines or at planned scales; and |
| • | Continue other core activities. |
Furthermore, any inability to scale-back operations as necessary, and any unexpected liquidity needs, could create pressure to implement more severe measures. Such measures could have an adverse effect on our ability to meet contractual requirements, including obligations to maintain manufacturing operations, and increase the severity of the consequences described above.
Collaboration Funding. For the year ended December 31, 2016, we received $30.6 million in cash from collaborations, including $13.2 million under flavors and fragrances collaboration agreements and $15.0 million for licensing of intellectual property.
We depend on collaboration funding to support our research and development and operating expenses. While part of this funding is committed based on existing collaboration agreements, we will be required to identify and obtain funding from additional collaborations. In addition, some of our existing collaboration funding is subject to our achievement of milestones or other funding conditions.
If we cannot secure sufficient collaboration funding to support our operating expenses in excess of cash contributions from product sales, existing debt and equity financings and strategic asset divestments, we may need to issue preferred and/or discounted equity, agree to onerous covenants, grant further security interests in our assets, and enter into collaboration and licensing arrangements that require us to relinquish commercial rights or grant licenses on terms that are not favorable to us. If we fail to secure such funding, we could be forced to curtail our operations, which would have a material adverse effect on our ability to continue with our business plans.
Government Contracts. In September 2015, we entered into a Technology Investment Agreement (as amended, the “TIA”) with The Defense Advanced Research Project Agency (or “DARPA”) under which we, with the assistance of five specialized subcontractors, will work to create new research and development tools and technologies for strain engineering and scale-up activities. The program that is the subject of the TIA is being performed and funded on a milestone basis. Under the TIA, we and our subcontractors could collectively receive DARPA funding of up to $35.0 million over the program’s four year term if all of the program’s milestones are achieved. In conjunction with DARPA’s funding, we and our subcontractors are obligated to collectively contribute approximately $15.5 million toward the program over its four year term (primarily by providing specified labor and/or purchasing certain equipment). We can elect to retain title to the patentable inventions we produce in the program, but DARPA receives certain data rights as well as a government purposes license to certain of such inventions. Either party may, upon written notice and subject to certain consultation obligations, terminate the TIA upon a reasonable determination that the program will not produce beneficial results commensurate with the expenditure of resources. We recognized $9.7 million in revenue under this agreement for the year ended December 31, 2016. Total cash received under this agreement as of December 31, 2016 was $8.1 million for the year ended December 31, 2016.
| 90 |
In October 2016, we entered into an assistance agreement with the United States Department of Energy (DOE) relating to a research grant award (Award) under which we, with the assistance of two specialized subcontractors, Total and Renmatix, which are related parties of the Company, will work to develop a manufacturing-ready process utilizing wood as the cellulosic feedstock to produce farnesene. The program that is the subject of the Award is being performed and funded on a milestone basis. Under the Award, we and our subcontractors could collectively receive reimbursement for up to $8.8 million in costs expended by us and our subcontractors over the program’s three year term if all of the program’s milestones are achieved. We can elect to retain title to the patentable inventions we produce in the program, but DOE receives certain data rights as well as a government purposes license to certain of such inventions. Either party may, upon written notice and subject to certain consultation obligations, terminate the Award upon a reasonable determination that the program will not produce beneficial results commensurate with the expenditure of resources. We recognized zero in revenue under this agreement for the year ended December 31, 2016. Total cash received under this agreement as of December 31, 2016 was zero for the year ended December 31, 2016.
Convertible Note Offerings. In February 2012, we sold $25.0 million in aggregate principal amount of senior unsecured convertible promissory notes due March 1, 2017, $9.7 million of which notes were repurchased in October 2015 with a portion of the proceeds from our sale of the 2015 144A Notes (as defined below), and the remainder of which were subsequently exchanged for $19.1 million of additional 2015 144A Notes in January 2017, as described in more detail in Note 5, "Debt" and Note 16, “Subsequent Events” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In July and September 2012, we issued $53.3 million in aggregate principal amount of 1.5% Senior Unsecured Convertible Notes to Total under the Total Fuel Agreements for an aggregate of $30.0 million in cash proceeds and our repayment of $23.3 million in previously-provided research and development funds pursuant to the Total Fuel Agreements, as described in more detail under "Related Party Convertible Notes" in Note 5, "Debt" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K. As part of our December 2012 private placement, we issued 1,677,852 shares of our common stock to Total in exchange for the cancellation of $5.0 million of an outstanding senior unsecured convertible promissory note held by Total.
In June and July 2013, we sold and issued $30.0 million in aggregate principal amount of 1.5% Senior Unsecured Convertible Notes to Total with a March 1, 2017 maturity date pursuant to the Total Fuel Agreements.
In August 2013, we entered into an agreement with Total and Temasek to issue up to $73.0 million in convertible promissory notes in private placements over a period of up to 24 months from the date of signing as described in more detail in Note 5, "Debt" in “Notes to audited Consolidated Financial Statements” included in this Annual Report on Form 10-K (such agreement referred to as the “August 2013 SPA” and such financing referred to as the “August 2013 Financing”). The August 2013 Financing was divided into two tranches (one for $42.6 million and one for $30.4 million). Of the total possible purchase price in the financing, $25.0 million was to be paid in the form of cash by Temasek ($25.0 million in the second tranche), $35.0 million was to be paid by the exchange and cancellation of the Temasek Bridge Note, as described below, and $13.0 million was to be paid by the exchange and cancellation of outstanding convertible promissory notes held by Total in connection with its exercise of pro rata rights ($7.6 million in the first tranche and $5.4 million in the second tranche).
| 91 |
On October 4, 2013, we issued a senior secured promissory note in the principal amount of $35.0 million (Temasek Bridge Note) to Temasek for cash proceeds of $35.0 million. The Temasek Bridge Note was due on February 2, 2014 and accrued interest at a rate of 5.5% per month from October 4, 2013. The Temasek Bridge Note was cancelled as payment for Temasek's purchase of a first tranche convertible note in the initial closing of the August 2013 Financing, as described below.
In October 2013, we amended the August 2013 SPA to include certain entities affiliated with FMR LLC (or the “Fidelity Entities”) in the first tranche closing (participating for a principal amount of $7.6 million), and to proportionally increase the amount acquired by exchange and cancellation of outstanding convertible promissory notes by Total to $14.6 million ($9.2 million in the first tranche and up to $5.4 million in the second tranche). Also in October 2013, we completed the closing of the issuance and sale of senior convertible notes under the first tranche of the August 2013 Financing (or the “Tranche I Notes”) for cash proceeds of $7.6 million and cancellation of outstanding convertible promissory notes of $44.2 million, of which $35.0 million resulted from the exchange and cancellation of the Temasek Bridge Note and the remaining $9.2 million from the exchange and cancellation of an outstanding convertible promissory notes held by Total. In December 2013, we further amended the August 2013 SPA to sell $3.0 million of senior convertible notes under the second tranche of the August 2013 Financing (or the “Tranche II Notes”) to funds affiliated with Wolverine Asset Management, LLC and we elected to call $25.0 million in additional funds from Temasek pursuant to its previous commitment to purchase such amount of Tranche II Notes. Additionally, pursuant to that amendment, we sold approximately $6.0 million of Tranche II Notes to Total through exchange and cancellation of the same amount of principal of previously outstanding convertible notes held by Total (in respect of Total’s preexisting contractual right to maintain its pro rata ownership position through such cancellation of indebtedness). The closing of the issuance and sale of such Tranche II Notes under the December amendment to the August 2013 SPA occurred in January 2014. The August 2013 Financing is more fully described in Note 5, "Debt" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In December 2013, in connection with our entry into agreements establishing our joint venture with Total, we exchanged the $69.0 million of then-outstanding unsecured convertible notes issued to Total pursuant to the Total Fuel Agreements for replacement 1.5% Senior Secured Convertible Notes, in principal amounts equal to the principal amounts of the cancelled notes.
In May 2014, we issued and sold $75.0 million in aggregate principal amount of 6.50% Convertible Senior Notes due 2019 (or the “2014 144A Notes”) to Morgan Stanley & Co. LLC as the Initial Purchaser in a private placement, and for initial resale by the Initial Purchaser to qualified institutional buyers pursuant to Rule 144A of the Securities Act (or the “2014 144A Offering”). The 2014 144A Offering is described in more detail in Note 5, "Debt" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K. In October 2015, we repurchased $22.9 million in aggregate principal amount of the 2014 144A Notes with a portion of the proceeds from our sale of the 2015 144A Notes (as defined below), as described in more detail in Note 5, "Debt" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
| 92 |
In July 2014 and January 2015, we issued and sold $21.7 million in aggregate principal amount of 1.5% Senior Secured Convertible Notes with a March 1, 2017 maturity date to Total pursuant to the Total Fuel Agreements.
In July 2015, Temasek exchanged approximately $71.0 million in principal amount of outstanding convertible promissory notes and Total exchanged $70.0 million in principal amount of outstanding convertible promissory notes for shares of our common stock, as further described above under “Exchange (debt conversion)”.
In October 2015, we issued and sold $57.6 million in aggregate principal amount of 9.50% Convertible Senior Notes due 2019 (2015 144A Notes), which were sold only to qualified institutional buyers and institutional accredited investors in a private placement (2015 144A Offering) under the Securities Act. The 2015 144A Offering is described in more detail in Note 5, "Debt" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K. In January 2017, we issued an additional $19.1 million in aggregate principal amount of 2015 144A Notes in exchange for the cancellation of $15.3 million in aggregate principal amount of outstanding senior unsecured convertible promissory notes issued in February 2012, as described in more detail in Note 16, "Subsequent Events" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In March 2016, we sold to Total one half of our ownership stake in TAB in exchange for Total cancelling $3.7 million in aggregate principal amount of outstanding 1.5% Senior Secured Convertible Notes and certain other indebtedness, as described in more detail under “Relationship with Total” above and in Note 5, “Debt” and Note 7, “Joint Ventures and Noncontrolling Interest” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K. In February 2017, we and Total agreed to extend the maturity of the remaining outstanding indebtedness under the Total Fuel Agreements from March 1, 2017 to May 15, 2017, as described in more detail in Note 16, "Subsequent Events" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
In May, September, October and December 2016, we issued $25.0 million in aggregate principal amount of convertible promissory notes to a private investor in offerings registered under the Securities Act, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
Export Financing with ABC Brasil. In March 2013, we entered into a one-year export financing agreement with Banco ABC Brasil S.A. (or “ABC Brasil”) for approximately $2.5 million to fund exports through March 2014. This loan was collateralized by future exports from our subsidiary in Brazil. As of December 31, 2016, the loan was fully paid.
In March 2014, we entered into an additional one-year-term export financing agreement with ABC Brasil for approximately $2.2 million to fund exports through March 2015. This loan is collateralized by future exports from our subsidiary in Brazil. As of December 31, 2016, the loan was fully paid.
In April 2015, we entered into an additional one-year-term export financing agreement with ABC Brasil for approximately $1.6 million to fund exports through April 2016. This loan is collateralized by future exports from our subsidiary in Brazil. As of December 31, 2016, the loan was fully paid.
| 93 |
Banco Pine/Nossa Caixa Financing. In July 2012, we entered into a Note of Bank Credit and a Fiduciary Conveyance of Movable Goods agreement with each of Nossa Caixa and Banco Pine. Under these instruments, we borrowed an aggregate of R$52.0 million (approximately US$16.0 million based on the exchange rate as of December 31, 2016) as financing for capital expenditures relating to our manufacturing facility in Brotas, Brazil. Under the loan agreements, Banco Pine agreed to lend R$22.0 million and Nossa Caixa agreed to lend R$30.0 million. The loans have a final maturity date of July 15, 2022 and bear a fixed interest rate of 5.5% per year. The loans are also subject to early maturity and delinquency charges upon occurrence of certain events including interruption of manufacturing activities at our manufacturing facility in Brotas, Brazil for more than 30 days, except during sugarcane off-season. The loans are secured by certain of our farnesene production assets at the manufacturing facility in Brotas, Brazil and we were required to provide parent guarantees to each of the lenders. As of December 31, 2016 and 2015, a principal amount of $11.1 million and $11.0 million, respectively, was outstanding under these loan agreements.
BNDES Credit Facility. In December 2011, we entered into a credit facility with Banco Nacional de Desenvolvimento Econômico e Social (or BNDES), a government-owned bank headquartered in Brazil (BNDES Credit Facility) to finance a production site in Brazil. The BNDES Credit Facility was for R$22.4 million (approximately US$6.9 million based on the exchange rate as of December 31, 2016). The credit line was divided into an initial tranche of up to approximately R$19.1 million and an additional tranche of approximately R$3.3 million that would become available upon delivery of additional guarantees. The credit line was cancelled in 2013. As of December 31, 2016 and 2015, the Company had R$3.8 million (approximately US$1.2 million based on the exchange rate as of December 31, 2016) and R$7.6 million (approximately US$1.9 million based on the exchange rate as of December 31, 2015), respectively, in outstanding advances under the BNDES Credit Facility.
The principal of loans under the BNDES Credit Facility is required to be repaid in 60 monthly installments, with the first installment due in January 2013 and the last due in December 2017. Interest was initially due on a quarterly basis with the first installment due in March 2012. From and after January 2013, interest payments are due on a monthly basis together with principal payments. The loaned amounts carry interest of 7% per year. Additionally, there is a credit reserve charge of 0.1% on the unused balance from each credit installment from the day immediately after it is made available through its date of use, when it is paid.
The BNDES Credit Facility is collateralized by first priority security interest in certain of our equipment and other tangible assets totaling R$24.9 million (approximately US$7.7 million based on the exchange rate as of December 31, 2016). We are a parent guarantor for the payment of the outstanding balance under the BNDES Credit Facility. Additionally, we were required to provide a bank guarantee equal to 10% of the total approved amount (R$22.4 million in total debt) available under the BNDES Credit Facility. For advances in the second tranche (above R$19.1 million), we are required to provide additional bank guarantees equal to 90% of each such advance, plus additional Amyris guarantees equal to at least 130% of such advance. The BNDES Credit Facility contains customary events of default, including payment failures, failure to satisfy other obligations under the credit facility or related documents, defaults in respect of other indebtedness, bankruptcy, insolvency and inability to pay debts when due, material judgments, and changes in control of Amyris Brasil. If any event of default occurs, BNDES may terminate its commitments and declare immediately due all borrowings under the facility.
FINEP Credit Facility. In November 2010, we entered into a credit facility with Financiadora de Estudos e Projetos (FINEP), a state-owned company subordinated to the Brazilian Ministry of Science and Technology (or the “FINEP Credit Facility”) to finance a research and development project on sugarcane-based biodiesel (or the “FINEP Project”) and provided for loans of up to an aggregate principal amount of R$6.4 million (approximately US$2.0 million based on the exchange rate as of December 31, 2016) which are secured by a chattel mortgage on certain equipment of Amyris as well as by bank letters of guarantee. All available credit under this facility was fully drawn. As of December 31, 2016 and 2015, the total outstanding loan balance under this credit facility was R$2.3 million (approximately US$0.7 million based on the exchange rate as of December 31, 2016) and R$3.4 million (approximately US$0.9 million based on the exchange rate as of December 31, 2015).
| 94 |
Interest on loans drawn under the FINEP Credit Facility is fixed at 5.0% per annum. In case of default under, or non-compliance with, the terms of the agreement, the interest on loans will be dependent on the long-term interest rate as published by the Central Bank of Brazil (such rate, the “TJLP”). If the TJLP at the time of default is greater than 6%, then the interest will be 5.0% plus a TJLP adjustment factor, otherwise the interest will be at 11.0% per annum. In addition, a fine of up to 10.0% will apply to the amount of any obligation in default. Additional interest on late balances will be 1.0% per month, levied on the overdue amount. Payment of the outstanding loan balance is being made in 81 monthly installments, which commenced in July 2012 and extends through March 2019. Interest on loans drawn and other charges are paid on a monthly basis and commenced in March 2011.
Senior Secured Loan Facility. In March 2014, we entered into a Loan and Security Agreement (LSA) with Hercules Technology Growth Capital, Inc. (Hercules) to make available a secured loan facility (Senior Secured Loan Facility) in the aggregate principal amount of up to $25.0 million, which loan facility was fully drawn at the closing. The initial loan of $25.0 million under the Senior Secured Loan Facility accrues interest at a rate per annum equal to the greater of either the prime rate reported in the Wall Street Journal plus 6.25% or 9.5%. We may repay the outstanding amounts under the Senior Secured Credit Facility before the maturity date (October 15, 2018) if we pay an additional fee of 1% of the outstanding amounts. We were also required to pay a 1% facility charge at the closing of the Senior Secured Credit Facility, and are required to pay a 10% end of term charge with respect to the initial loan of $25.0 million. In connection with the entry into the LSA, we agreed to certain customary representations and warranties and covenants, as well as certain covenants that were subsequently amended (as described below).
In June 2014, we entered into a first amendment of the LSA with Hercules. Pursuant to the first amendment, the parties agreed to extend the maturity date of the loans under the Senior Secured Loan Facility from May 31, 2015 to February 1, 2017 and remove (i) a requirement for us to pay a forbearance fee of $10.0 million in the event certain covenants were not satisfied, (ii) a covenant that we maintain positive cash flow commencing with the fiscal quarter beginning October 1, 2014, (iii) a covenant that, commencing with the fiscal quarter beginning July 1, 2014, we and our subsidiaries achieve certain projected cash product revenues and projected cash product gross profits, and (iv) an obligation for us to file a registration statement on Form S-3 with the SEC by no later than June 30, 2014 and complete an equity financing of more than $50.0 million by no later than September 30, 2014. We further agreed to include a new covenant in the LSA requiring us to maintain unrestricted, unencumbered cash in an amount equal to at least 50% of the principal amount of the loans then outstanding under the Senior Secured Loan Facility (Minimum Cash Covenant) and borrow an additional $5.0 million under the Senior Secured Loan Facility. The additional $5.0 million borrowing was completed in June 2014, and accrues interest at a rate per annum equal to the greater of (i) the prime rate reported in the Wall Street Journal plus 5.25% and (ii) 8.5%.
In March 2015, the Company and Hercules entered into a second amendment of the LSA. Pursuant to the second amendment, the parties agreed to, among other things, establish an additional credit facility in the principal amount of up to $15.0 million, which would be available to be drawn by the Company through the earlier of March 31, 2016 or such time as the Company raised an aggregate of at least $20.0 million through the sale of new equity securities. The additional facility was cancelled undrawn upon the completion of our private stock and warrant offering in July 2015.
| 95 |
In November 2015, the Company and Hercules entered into a third amendment of the LSA. Pursuant to the third amendment, the Company borrowed an additional $10,960,000 (Third Amendment Borrowed Amount) from Hercules on November 30, 2015. As of December 1, 2015, after the funding of the Third Amendment Borrowed Amount (and including repayment of $9.1 million of principal that had occurred prior to the third amendment), the aggregate principal amount outstanding under the Senior Secured Loan Facility was approximately $31.7 million. The Third Amendment Borrowed Amount accrues interest at a rate per annum equal to the greater of (i) 9.5% and (ii) the prime rate reported in the Wall Street Journal plus 6.25%, and, like the previous loans under the Senior Secured Loan Facility, has a maturity date of October 15, 2018. Upon the earlier of the maturity date, prepayment in full or such obligations otherwise becoming due and payable, in addition to repaying the outstanding Third Amendment Borrowed Amount (and all other amounts owed under the Senior Secured Loan Facility, as amended), the Company is also required to pay an end-of-term charge of $767,200. Pursuant to the third amendment, the Company also paid Hercules fees of $1.0 million, $750,000 of which was owed in connection with the expired $15.0 million facility under the second amendment and $250,000 of which was related to the Third Amendment Borrowed Amount. Under the third amendment, the parties agreed that the Company would, commencing on December 1, 2015, be required to pay only the interest accruing on all outstanding loans under the Senior Secured Loan Facility until February 29, 2016. Commencing on March 1, 2016, the Company would have been required to begin repaying principal of all loans under the Senior Secured Loan Facility, in addition to the applicable interest. However, pursuant to the third amendment, the Company could, by achieving certain cash inflow targets in 2016, extend the interest-only period to December 1, 2016. Upon the issuance and sale by the Company of $20.0 million of unsecured promissory notes and warrants in a private placement in February 2016 for aggregate cash proceeds of $20.0 million, the Company satisfied the conditions for extending the interest-only period to May 31, 2016. On June 1, 2016, the Company commenced the repayment of outstanding principal under the Senior Secured Loan Facility. In June 2016, the Company was notified by Hercules that it had transferred and assigned its rights and obligations under the Senior Secured Loan Facility to Stegodon Corporation (Stegodon), an affiliate of Ginkgo Bioworks, Inc. (Ginkgo). On June 29, 2016, in connection with the execution by the Company and Ginkgo of an initial strategic partnership agreement, the Company received a deferment of all scheduled principal repayments under the Senior Secured Loan Facility, as well as a waiver of the Minimum Cash Covenant, through October 31, 2016. Refer to Note 8, “Significant Agreements” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for additional details. On October 6, 2016, in connection with the execution by the Company and Ginkgo of a definitive collaboration agreement (or the “Ginkgo Collaboration Agreement”), the Company and Stegodon entered into a fourth amendment of the LSA, pursuant to which the parties agreed to (i) subject to the Company extending the maturity of certain of its other outstanding indebtedness (or the “Extension Condition”), extend the maturity date of the Senior Secured Loan Facility, (ii) make the Senior Secured Loan Facility interest-only until maturity, subject to the requirement that the Company apply certain monies received by it under the Ginkgo Collaboration Agreement to repay the amounts outstanding under the Senior Secured Loan Facility, up to a maximum amount of $1 million per month and (iii) waive the Minimum Cash Covenant until the maturity date of the Senior Secured Loan Facility. In January 2017, the Company satisfied the Extension Condition and the maturity date of the loans under the Senior Secured Credit Facility was extended to October 15, 2018. In December 2016, in connection with Stegodon granting certain waivers and releases under the LSA in connection with the Company’s formation of its Neossance joint venture with Nikko, as described in more detail in Note 7, "Joint Ventures and Noncontrolling Interest" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K, the Company agreed to pay to Stegodon (i) a fee of $425,000 on or prior to December 31, 2017 and (ii) a fee of $450,000 on or prior to the maturity date of the loans under the Senior Secured Credit Facility. Subsequently, in January 2017 the Company and Stegodon entered into a fifth amendment of the LSA, pursuant to which the Company agreed to apply additional monies received by it under the Ginkgo Collaboration Agreement towards repayment of the outstanding loans under the Senior Secured Loan Facility, up to a maximum amount of $3 million. Refer to Note 5, “Debt”, Note 8, “Significant Agreements” and Note 16, “Subsequent Events” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K for additional details regarding the LSA and Senior Secured Loan Facility.
| 96 |
As of December 31, 2016, $27.7 million was outstanding under the Senior Secured Loan Facility, net of discount and issuance cost of $0.9 million. The Senior Secured Loan Facility is secured by liens on our assets, including on certain of our intellectual property. The Senior Secured Loan Facility includes customary events of default, including failure to pay amounts due, breaches of covenants and warranties, material adverse effect events, certain cross defaults and judgments, and insolvency. If an event of default occurs, Stegodon may require immediate repayment of all amounts outstanding under the Senior Secured Loan Facility.
February 2016 Private Placement. In February 2016, we sold and issued to certain purchasers affiliated with members of our board of directors an aggregate of $20.0 million of unsecured promissory notes and warrants for the purchase, at an exercise price of $0.01 per share, of an aggregate of 2,857,142 shares of our common stock, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K. The exercisability of these warrants was subject to stockholder approval, which was obtained on May 17, 2016.
June 2016 Private Placement. In June 2016, we sold and issued $5.0 million in aggregate principal amount of secured promissory notes to Foris Ventures, LLC (or “Foris”), an entity affiliated with director John Doerr of Kleiner Perkins Caufield & Byers, a current stockholder, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
October 2016 Private Placements. In October 2016, we sold and issued to Foris and Ginkgo, respectively, $6.0 million and $8.5 million in principal amount of secured promissory notes, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
Guanfu Credit Facility. In October 2016, we entered into a credit agreement with Guanfu Holding Co., Ltd. to make available to the Company an unsecured credit facility with an aggregate principal amount of up to $25.0 million, which amount was fully drawn on December 31, 2016, as described in more detail in Note 5, “Debt” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
Salisbury Purchase Money Promissory Note. In December 2016, we sold and issued a purchase money promissory note in the principal amount of $3.5 million to Salisbury Partners, LLC in connection with our purchase of a production facility in Leland, North Carolina, as described in more detail in Note 5, “Debt” and Note 7, "Joint Ventures and Noncontrolling Interest" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
Nikko Promissory Note. In December 2016, we sold and issued a promissory note in the principal amount of $3.9 million to Nikko Chemicals Co., Ltd. in connection with the formation of our Neossance joint venture, as described in more detail in Note 5, “Debt” and Note 7, “Joint Ventures and Noncontrolling Interest” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
| 97 |
Common Stock Offerings. In December 2012, we completed a private placement of 14,177,849 shares of our common stock for aggregate cash proceeds of $37.2 million, of which $22.2 million was received in December 2012 and $15.0 million was received in January 2013. Of the 14,177,849 shares issued in the private placement, 1,677,852 of such shares were issued to Total in exchange for cancellation of $5.0 million of an outstanding convertible promissory note we previously issued to Total.
In March 2013, we completed a private placement of 1,533,742 of our common stock to Biolding SA (Biolding), which is affiliated with one of our directors, for aggregate proceeds of $5.0 million. This private placement represented the final tranche of Biolding's preexisting contractual obligation to fund $15.0 million upon satisfaction by us of certain criteria associated with the commissioning of our production plant in Brotas, Brazil.
In March 2014, we completed a private placement of 943,396 shares of our common stock to Kuraray for aggregate proceeds of $4.0 million.
In July 2015, we entered into a Securities Purchase Agreement with certain purchasers, including entities affiliated with members of our board of directors, under which we agreed to sell 16,025,642 shares of our common stock at a price of $1.56 per share, for aggregate proceeds to the Company of $25 million. The sale of common stock under the Securities Purchase Agreement was completed on July 29, 2015. Pursuant to the Securities Purchase Agreement, the Company granted to each of the purchasers a warrant exercisable at an exercise price of $0.01 per share for the purchase of a number of shares of the Company’s common stock equal to 10% of the shares purchased by such investor. The exercisability of the warrants was subject to stockholder approval, which was obtained on September 17, 2015. As of December 31, 2016, 160,255 of such warrants had been exercised.
In May 2016, we sold and issued 4,385,964 shares of our common stock to the Bill & Melinda Gates Foundation in a private placement at a purchase price per share equal to $1.14, for aggregate proceeds to the Company of approximately $5.0 million, as described in more detail in Note 8, “Significant Agreements” ” in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K.
Cash Flows during the Years Ended December 31, 2016, 2015, and 2014
Cash Flows from Operating Activities
Our primary uses of cash from operating activities are for costs related to production and sales of our products and personnel-related expenditures, offset by cash received from product sales, grants, collaborations and license fees. Cash used in operating activities was $82.4 million, $85.1 million and $84.7 million for the years ended December 31, 2016, 2015 and 2014, respectively.
| 98 |
Net cash used in operating activities of $82.4 million for the year ended December 31, 2016 was attributable to our net loss of $97.3 million, offset by net non-cash charges of $4.0 million and net change in our operating assets and liabilities of $10.9 million. Net non-cash charges of $4.0 million for the year ended December 31, 2016 consisted primarily of $14.4 million of amortization of debt discount and issuance costs, $11.4 million of depreciation and amortization expenses, $7.3 million of asset impairment charges, $7.3 million of stock-based compensation, $4.1 million of loss from extinguishment of debt, $0.9 million of loss on foreign currency exchange rates, partially offset by $41.4 million of gain from the change in the fair value of derivative instruments related to the embedded derivative liabilities associated with certain of our convertible promissory notes and currency interest rate swap derivative liability and $0.1 million of gain on disposition of property, plant and equipment. Net change in operating assets and liabilities of $10.9 million for the year ended December 31, 2016 primarily consisted of a $19.1 million increase in accounts payable and accrued other liabilities, a $5.7 million decrease in inventory and a $0.7 million increase in deferred revenue related to funds received under collaboration agreements, partially offset by a $5.7 million increase in prepaid expenses and other assets and deferred rent and a $8.9 million increase in accounts receivable and related party accounts receivable.
Net cash used in operating activities of $85.1 million for the year ended December 31, 2015 was attributable to our net loss of $218.1 million, offset by net non-cash charges of $113.8 million and net change in our operating assets and liabilities of $19.1 million. Net non-cash charges of $113.8 million for the year ended December 31, 2015 consisted primarily of a $58.6 million of amortization of debt discount and issuance costs, including a $36.6 million charge due to acceleration of accretion of debt discount on the Total and Temasek convertible notes converted to equity in July 2015, $16.3 million of loss from the change in the fair value of derivative instruments related to the embedded derivative liabilities associated with our senior convertible promissory notes and currency interest rate swap derivative liability, $12.9 million of depreciation and amortization expenses, $34.2 million of loss on purchase commitments and impairment of production assets, $9.1 million of stock-based compensation, $5.5 million of impairment of intangible assets, $4.7 million of withholding tax related to conversion of related party note, $4.2 million of loss from investment in affiliates, $1.1 million of loss from extinguishment of debt, $0.4 million of other non-cash expenses and $0.2 million on disposition of property, plant and equipment. Net change in operating assets and liabilities of $19.1 million for the year ended December 31, 2015 primarily consisted of a $15.3 million increase in accounts payable and accrued other liabilities and a $4.3 million decrease in accounts receivable and related party accounts receivable and a $4.5 million increase in inventory, partially offset by a $4.9 million decrease in prepaid expenses and other assets and deferred rent and $0.1 million decrease in deferred revenue related to the funds received under collaboration agreements.
Net cash used in operating activities of $84.7 million for the year ended December 31, 2014 was attributable to our net loss of $84.5 million excluding non-cash net income of $86.7 million, and a $0.2 million outflow from net changes in our operating assets and liabilities. Non-cash income of $86.7 million consisted primarily of a $144.1 million gain from change in the fair value of derivative instruments related to the embedded derivative liabilities associated with our senior secured convertible promissory notes and currency interest rate swap derivative liability, offset by $15.0 million of depreciation and amortization expenses, $14.1 million of stock-based compensation, $10.0 million of amortization of debt discount, $10.5 million loss associated with the extinguishment of convertible debt, $2.0 million loss on purchase commitments and write-off and disposal of property, plant and equipment, $2.9 million loss from investment in affiliate from our joint venture with Novvi and $3.0 million loss on impairment of IPR&D related to Draths. Net outflow from changes in operating assets and liabilities of $0.2 million primarily consisted of a $1.2 million increase in accounts receivable and related party accounts receivable, a $2.9 million increase in prepaid expenses and other assets, a $4.5 million increase in inventory as a result of the decrease in the allowance for lower of cost or market and a $3.2 million decrease in accounts payable, offset by a $6.8 million increase in accrued and other liabilities mainly due to an increase in accrued interest from new debt and a $4.8 million increase in deferred revenue from the collaboration agreement with Braskem and Michelin.
| 99 |
Cash Flows from Investing Activities
Our investing activities consist primarily of capital expenditures and investment activities.
Net cash provided from investing activities of $5.6 million for the year ended December 31, 2016, was a result of $10.0 million of proceeds on disposal of noncontrolling interest and $6.2 million of maturities of short-term investments, offset by $0.9 million of purchase of property, plant and equipment, a $4.0 million increase in restricted cash and $5.5 million of purchase of short-term investments.
Net cash used in investing activities of $5.1 million for the year ended December 31, 2015, was a result of $3.3 million of purchase of property, plant and equipment, $1.6 million of loans to an affiliate, $2.7 million of purchase of short-term investments, offset by $2.3 million of maturities of short-term investments and $0.2 million of change in restricted cash.
Net cash used in investing activities of $9.8 million for the year ended December 31, 2014, was a result of $5.0 million of capital expenditures mainly due to maintenance and upgrades of our facility in Brotas, Brazil and $4.9 million loans and investment in our joint venture with Novvi ($2.8 million in loans and $2.1 million in equity).
Cash Flows from Financing Activities
Net cash provided by financing activities of $92.2 million for the year ended December 31, 2016, was a result of the receipt of $63.9 million from debt financings, $29.7 million from notes payable issued to related parties, $5.0 million from proceeds from exercise of warrants and $5.0 million from proceeds from issuance of contingently redeemable equity, offset by $9.8 million of repayment of debt and $1.6 million of principal payments on capital leases.
Net cash provided by financing activities of $61.4 million for the year ended December 31, 2015, was a result of the receipt of $77.7 million from debt financings, of which $10.9 million was from debt issued to a related party, which related to the closing of the final installment of notes issued to Total under the Total Fuel Agreements and the receipt of $24.6 million from the issuance of common stock in private placements, offset by $40.8 million of repayment of debt.
Net cash provided by financing activities of $130.9 million for the year ended December 31, 2014, was a result of the net receipt of $139.5 million from debt and equity financing, which related to the closing of the second tranche of our convertible promissory note offering under the August 2013 SPA of $28.0 million, net of $6.0 million convertible promissory note issued to Total in exchange for cancellation of previously outstanding convertible promissory notes, borrowings under the Hercules Loan Facility of $29.8 million, the closing of our 2014 144A Offering for approximately $72.0 million proceeds (net of payments of discount and expenses of $3.0 million), the sale of $10.9 million convertible notes under the Total Fuel Agreements, $2.2 million from an export financing agreement with ABC Brasil and $4.7 million in proceeds from issuance of common stock, $4.0 million of which from issuance of common stock to Kuraray, offset by the $9.7 million settlement of convertible notes under the Total Fuel Agreements. These cash inflows were offset by other payments of debt principal and capital lease obligations of $6.8 million.
| 100 |
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any material off-balance sheet arrangements, as defined under SEC rules, such as relationships with unconsolidated entities or financial partnerships, which are often referred to as structured finance or special purpose entities, established for the purpose of facilitating financing transactions that are not required to be reflected on our consolidated financial statements.
Contractual Obligations
The following is a summary of our contractual obligations as of December 31, 2016 (in thousands):
| Total | 2017 | 2018 | 2019 | 2020 | 2021 | Thereafter | ||||||||||||||||||||||
| Principal payments on debt(1) | $ | 267,985 | $ | 61,657 | $ | 52,802 | $ | 120,504 | $ | 2,225 | $ | 27,237 | $ | 3,560 | ||||||||||||||
| Interest payments on long-term debt, fixed rate(2) | 50,562 | 18,257 | 18,344 | 7,936 | 2,867 | 2,642 | 516 | |||||||||||||||||||||
| Operating leases | 45,703 | 6,854 | 6,883 | 6,774 | 7,004 | 7,240 | 10,948 | |||||||||||||||||||||
| Principal payments on capital leases | 1,286 | 1,233 | 47 | 6 | — | — | — | |||||||||||||||||||||
| Interest payments on capital leases | 30 | 28 | 2 | — | — | — | — | |||||||||||||||||||||
Purchase obligations(3) | 837 | 808 | 29 | — | — | — | — | |||||||||||||||||||||
| Total | $ | 366,403 | $ | 88,837 | $ | 78,107 | $ | 135,220 | $ | 12,096 | $ | 37,119 | $ | 15,024 | ||||||||||||||
____________________
| (1) | The forecast payments assume no proceeds to be received by the Company under the Ginkgo Collaboration Agreement, which, if received, would need to be applied by the Company up to $1 million per month towards repayment of the debt due to Stegodon. Also including $11.8 million in 2017 related to Nomis Bay convertible note which, at the Company’s election, may be settled in shares or cash, and $46.8 million in 2018 and 2019 subject to Maturity Treatment Agreement, which will be converted to common stock at maturity, subject to there being no default under the terms of the debt. | |
| (2) | Does not include any obligations related to make-whole interest or downround provisions. The fixed interest rates are more fully described in Note 5, "Debt" in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K. | |
| (3) | Purchase obligations include noncancellable contractual obligations and construction commitments of $0.8 million, of which $0.6 million have been accrued as loss on purchase commitments. |
Recent Accounting Pronouncements
The information contained in Note 2 in “Notes to Consolidated Financial Statements” included in this Annual Report on Form 10-K under the heading "Recent Accounting Pronouncements" is hereby incorporated by reference into this Part II, Item 7.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The market risk inherent in our market risk sensitive instruments and positions is the potential loss arising from adverse changes in: commodity market prices, foreign currency exchange rates, and interest rates as described below.
| 101 |
Interest Rate Risk
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio and our outstanding debt obligations (including embedded derivatives therein). We generally invest our cash in investments with short maturities or with frequent interest reset terms. Accordingly, our interest income fluctuates with short-term market conditions. As of December 31, 2016, our investment portfolio consisted primarily of money market funds and certificates of deposit, all of which are highly liquid investments. Due to the short-term nature of our investment portfolio, we do not believe that an immediate 10% increase in interest rates would have a material effect on the fair value of our portfolio. Since we believe we have the ability to liquidate this portfolio, we do not expect our operating results or cash flows to be materially affected to any significant degree by a sudden change in market interest rates on our investment portfolio. Additionally, as of December 31, 2016, 100% of our outstanding debt is in fixed rate instruments or instruments which have capped rates. Therefore, our exposure to the impact of variable interest rates is limited. Changes in interest rates may significantly change the fair value of our embedded derivative liabilities.
Foreign Currency Risk
Most of our sales contracts are denominated in U.S. dollars and, therefore, our revenues are not currently subject to significant foreign currency risk. The functional currency of our consolidated subsidiaries in Brazil is the local currency (Brazilian real) in which recurring business transactions occur. We do not use currency exchange contracts as hedges against amounts permanently invested in our foreign subsidiary. The amount we consider permanently invested in our foreign subsidiaries and translated into U.S. dollars using the year end exchange rate is $119.4 million at December 31, 2016 and $99.5 million at December 31, 2015. The increase in the permanent investments in our foreign subsidiaries between 2015 and 2016 is due to the appreciation of the Brazilian real versus the U.S. dollar, offset by the additional capital contributions made. The potential loss in foreign exchange translation, which would be recognized in Other Comprehensive Income (Loss), resulting from a hypothetical 10% adverse change in quoted Brazilian real exchange rates is $2.7 million and $4.9 million for 2016 and 2015, respectively. Actual results may differ.
We make limited use of derivative instruments, which include currency interest rate swap agreements, to manage the Company's exposure to foreign currency exchange rate and interest rate fluctuations related to the Company's Banco Pine loan. In June 2012, we entered into a currency interest rate swap arrangement with Banco Pine for R$22.0 million (approximately US$6.8 million based on the exchange rate as of December 31, 2016). The swap arrangement exchanges the principal and interest payments under the Banco Pine loan entered into in July 2012 for alternative principal and interest payments that are subject to adjustment based on fluctuations in the foreign currency exchange rate between the U.S. dollar and Brazilian real. The swap has a fixed interest rate of 3.94%. This arrangement hedges the Company’s foreign currency exchange rate and interest rate exposure on the debt between the U.S. dollar and Brazilian real.
We analyzed our foreign currency exposure to identify assets and liabilities denominated in other currencies. For those assets and liabilities, we evaluated the effects of a 10% shift in exchange rates between those currencies and the U.S. dollar. We have determined that there would be an immaterial effect on our results of operations from such a shift.
Commodity Price Risk
Our primary exposure to market risk for changes in commodity prices currently relates to our purchases of sugar feedstocks. When possible, we manage our exposure to this risk primarily through the use of supplier pricing agreements.
| 102 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
AMYRIS, INC.
Index to Financial Statements and Financial Statement Schedules
| 103 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Amyris, Inc.:
In our opinion, the consolidated financial statements listed in the accompanying index present fairly, in all material respects, the financial position of Amyris, Inc. and its subsidiaries at December 31, 2016 and December 31, 2015, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. These financial statements and financial statement schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and on the financial statement schedule based on our audits. We conducted our audits of these financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and has a net stockholders’ deficit that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ PricewaterhouseCoopers LLP
San Jose, California
April 17, 2017
| 104 |
Consolidated Balance Sheets
(In Thousands, Except Share and Per Share Amounts)
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 27,150 | $ | 11,992 | ||||
| Restricted cash | 4,326 | 216 | ||||||
| Short-term investments | 1,374 | 1,520 | ||||||
| Accounts receivable, net of allowance of $478 and $479, respectively | 13,105 | 4,004 | ||||||
| Related party accounts receivable, net of allowance of $23 and $490, respectively | 872 | 1,176 | ||||||
| Inventories, net | 6,213 | 10,886 | ||||||
| Prepaid expenses and other current assets | 6,083 | 4,583 | ||||||
| Total current assets | 59,123 | 34,377 | ||||||
| Property, plant and equipment, net | 53,735 | 59,797 | ||||||
| Restricted cash | 957 | 957 | ||||||
| Equity and loans in affiliates | 34 | 68 | ||||||
| Other assets | 15,464 | 10,357 | ||||||
| Goodwill and intangible assets | 560 | 560 | ||||||
| Total assets | $ | 129,873 | $ | 106,116 | ||||
| Liabilities, Mezzanine Equity and Stockholders' Deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 15,315 | $ | 7,943 | ||||
| Deferred revenue | 5,288 | 6,509 | ||||||
| Accrued and other current liabilities | 29,188 | 24,268 | ||||||
| Capital lease obligation, current portion | 922 | 523 | ||||||
| Debt, current portion (Note 16) | 25,853 | 36,281 | ||||||
| Related party debt | 33,302 | — | ||||||
| Total current liabilities | 109,868 | 75,524 | ||||||
| Capital lease obligation, net of current portion | 334 | 176 | ||||||
| Long-term debt, net of current portion | 128,744 | 72,854 | ||||||
| Related party debt | 39,144 | 42,839 | ||||||
| Deferred rent, net of current portion | 8,906 | 9,682 | ||||||
| Deferred revenue, net of current portion | 6,650 | 4,469 | ||||||
| Derivative liabilities | 6,894 | 51,439 | ||||||
| Other liabilities | 7,841 | 7,589 | ||||||
| Total liabilities | 308,381 | 264,572 | ||||||
| Commitments and contingencies (Note 6) | ||||||||
| Mezzanine Equity | ||||||||
| Contingently redeemable common stock (Note 8) | 5,000 | — | ||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock - $0.0001 par value, 5,000,000 shares authorized, none issued and outstanding | — | — | ||||||
| Common stock - $0.0001 par value, 500,000,000 and 400,000,000 shares authorized as of December 31, 2016 and 2015; 274,108,808 and 206,130,282 shares issued and outstanding as of December 31, 2016 and 2015, respectively | 27 | 21 | ||||||
| Additional paid-in capital | 990,870 | 926,216 | ||||||
| Accumulated other comprehensive loss | (40,904 | ) | (47,198 | ) | ||||
| Accumulated deficit | (1,134,438 | ) | (1,037,104 | ) | ||||
| Total Amyris, Inc. stockholders’ deficit | (184,445 | ) | (158,065 | ) | ||||
| Noncontrolling interest | 937 | (391 | ) | |||||
| Total stockholders' deficit | (183,508 | ) | (158,456 | ) | ||||
| Total liabilities, mezzanine equity and stockholders' deficit | $ | 129,873 | $ | 106,116 | ||||
See the accompanying notes to the consolidated financial statements.
| 105 |
Consolidated Statements of Operations
(In Thousands, Except Share and Per Share Amounts)
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Revenues | ||||||||||||
| Renewable product sales | $ | 24,788 | $ | 14,032 | $ | 22,793 | ||||||
| Related party renewable product sales | 1,561 | 864 | 646 | |||||||||
| Total product sales | 26,349 | 14,896 | 23,439 | |||||||||
| Grants, collaborations and license fee revenue | 40,843 | 19,257 | 19,835 | |||||||||
| Total revenues | 67,192 | 34,153 | 43,274 | |||||||||
| Cost and operating expenses | ||||||||||||
| Cost of products sold | 56,678 | 37,374 | 33,202 | |||||||||
| Loss on purchase commitments, impairment of property, plant and equipment and other asset allowances | 7,305 | 34,166 | 1,769 | |||||||||
| Withholding tax related to conversion of related party notes | — | 4,723 | — | |||||||||
| Impairment of intangible assets | — | 5,525 | 3,035 | |||||||||
| Research and development | 51,412 | 44,636 | 49,661 | |||||||||
| Sales, general and administrative | 47,721 | 56,262 | 55,435 | |||||||||
| Total cost and operating expenses | 163,116 | 182,686 | 143,102 | |||||||||
| Loss from operations | (95,924 | ) | (148,533 | ) | (99,828 | ) | ||||||
| Other income (expense): | ||||||||||||
| Interest income | 258 | 264 | 387 | |||||||||
| Interest expense | (37,629 | ) | (78,854 | ) | (28,949 | ) | ||||||
| Gain from change in fair value of derivative instruments | 41,355 | 16,287 | 144,138 | |||||||||
| Loss upon extinguishment of debt | (4,146 | ) | (1,141 | ) | (10,512 | ) | ||||||
| Other income (expense), net | (695 | ) | (1,423 | ) | 336 | |||||||
| Total other income (expense) | (857 | ) | (64,867 | ) | 105,400 | |||||||
| Income (loss) before income taxes and loss from investments in affiliates | (96,781 | ) | (213,400 | ) | 5,572 | |||||||
| Provision for income taxes | (553 | ) | (468 | ) | (495 | ) | ||||||
| Net income (loss) before loss from investments in affiliates | (97,334 | ) | (213,868 | ) | 5,077 | |||||||
| Loss from investments in affiliates | — | (4,184 | ) | (2,910 | ) | |||||||
| Net income (loss) | (97,334 | ) | (218,052 | ) | 2,167 | |||||||
| Net loss attributable to noncontrolling interest | — | 100 | 119 | |||||||||
| Net income (loss) attributable to Amyris, Inc. common stockholders | $ | (97,334 | ) | $ | (217,952 | ) | $ | 2,286 | ||||
| Net income (loss) per share attributable to common stockholders: | ||||||||||||
| Basic | $ | (0.41 | ) | $ | (1.75 | ) | $ | 0.03 | ||||
| Diluted | $ | (0.44 | ) | $ | (1.75 | ) | $ | (0.90 | ) | |||
| Weighted-average shares of common stock outstanding used in computing net income (loss) per share of common stock: | ||||||||||||
| Basic | 238,440,197 | 126,961,576 | 78,400,098 | |||||||||
| Diluted | 264,644,449 | 126,961,576 | 121,859,441 | |||||||||
See the accompanying notes to the consolidated financial statements.
| 106 |
Consolidated Statements of Comprehensive Loss
(In Thousands)
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Comprehensive loss: | ||||||||||||
| Net income (loss) | $ | (97,334 | ) | $ | (218,052 | ) | $ | 2,167 | ||||
| Foreign currency translation adjustment, net of tax | 6,294 | (16,901 | ) | (9,798 | ) | |||||||
| Total comprehensive loss | (91,040 | ) | (234,953 | ) | (7,631 | ) | ||||||
| Income attributable to noncontrolling interest | — | 100 | 119 | |||||||||
| Foreign currency translation adjustment attributable to noncontrolling interest | — | (320 | ) | (92 | ) | |||||||
| Comprehensive loss attributable to Amyris, Inc. | $ | (91,040 | ) | $ | (235,173 | ) | $ | (7,604 | ) | |||
See the accompanying notes to the consolidated financial statements.
| 107 |
Consolidated Statements of Stockholders' Deficit
(In Thousands, Except Share and Per Share Amounts)
| Common Stock | ||||||||||||||||||||||||||||||||
| Shares | Amount | Additional Paid-in Capital | Accumulated Deficit | Accumulated Other Comprehensive Loss | Noncontrolling Interest | Total Deficit | Mezzanine Equity | |||||||||||||||||||||||||
| December 31, 2013 | 76,662,812 | $ | 8 | $ | 706,253 | $ | (821,438 | ) | $ | (20,087 | ) | $ | (584 | ) | $ | (135,848 | ) | $ | — | |||||||||||||
| Issuance of common stock upon exercise of stock options, net of restricted stock | 779,490 | — | 2,133 | — | — | — | 2,133 | — | ||||||||||||||||||||||||
| Issuance of common stock in a private placement | 943,396 | — | 4,000 | — | — | — | 4,000 | — | ||||||||||||||||||||||||
| Shares issued from restricted stock unit settlement | 836,185 | — | (1,822 | ) | — | — | — | (1,822 | ) | — | ||||||||||||||||||||||
| Stock-based compensation | — | — | 14,105 | — | — | — | 14,105 | — | ||||||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | (9,890 | ) | 92 | (9,798 | ) | — | ||||||||||||||||||||||
| Net income | — | — | — | 2,286 | — | (119 | ) | 2,167 | — | |||||||||||||||||||||||
| December 31, 2014 | 79,221,883 | $ | 8 | $ | 724,669 | $ | (819,152 | ) | $ | (29,977 | ) | $ | (611 | ) | $ | (125,063 | ) | $ | — | |||||||||||||
| Issuance of common stock upon exercise of stock options, net of restricted stock | 13,250 | — | 18 | — | — | — | 18 | — | ||||||||||||||||||||||||
| Issuance of common stock upon conversion of debt | 62,192,238 | 6 | 96,616 | — | — | — | 96,622 | — | ||||||||||||||||||||||||
| Issuance of warrants on conversion of debt | — | — | 51,704 | — | — | — | 51,704 | — | ||||||||||||||||||||||||
| Shares issued from restricted stock settlement | 908,877 | — | (333 | ) | — | — | — | (333 | ) | — | ||||||||||||||||||||||
| Shares issued upon ESPP purchase | 385,892 | — | 595 | — | — | — | 595 | — | ||||||||||||||||||||||||
| Issuance of common stock in a private placement, net of issuance costs | 16,025,642 | 2 | 24,624 | — | — | — | 24,626 | — | ||||||||||||||||||||||||
| Stock-based compensation | — | — | 9,134 | — | — | — | 9,134 | — | ||||||||||||||||||||||||
| Issuance of common stock upon exercise of warrants | 47,382,500 | 5 | 19,189 | — | — | — | 19,194 | — | ||||||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | (17,221 | ) | 320 | (16,901 | ) | — | ||||||||||||||||||||||
| Net loss | — | — | — | $ | (217,952 | ) | — | (100 | ) | (218,052 | ) | — | ||||||||||||||||||||
| December 31, 2015 | 206,130,282 | $ | 21 | $ | 926,216 | $ | (1,037,104 | ) | $ | (47,198 | ) | $ | (391 | ) | $ | (158,456 | ) | $ | — | |||||||||||||
| Issuance of common stock upon exercise of stock options, net of restricted stock | 134 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Issuance of common stock upon conversion of debt | 15,729,015 | 2 | 14,364 | — | — | — | 14,366 | — | ||||||||||||||||||||||||
| Issuance of common stock for settlement of debt principal payments | 35,723,842 | 4 | 17,410 | — | — | — | 17,414 | — | ||||||||||||||||||||||||
| Issuance of warrants with debt private placement and collaboration agreements | — | — | 4,387 | — | — | — | 4,387 | — | ||||||||||||||||||||||||
| Shares issued from restricted stock settlement | 1,803,496 | — | (254 | ) | — | — | — | (254 | ) | — | ||||||||||||||||||||||
| Stock-based compensation | — | — | 7,325 | — | — | — | 7,325 | — | ||||||||||||||||||||||||
| Disposal of noncontrolling interest in Neossance LLC | 9,063 | 937 | 10,000 | |||||||||||||||||||||||||||||
| Contribution upon restructuring of Fuels JV | — | — | 4,252 | — | — | — | 4,252 | — | ||||||||||||||||||||||||
| Issuance of contingently redeemable common stock | 4,385,964 | — | — | — | — | — | — | 5,000 | ||||||||||||||||||||||||
| Shares issued upon ESPP purchase | 336,075 | — | 180 | — | — | — | 180 | — | ||||||||||||||||||||||||
| Issuance of common stock upon exercise of warrants | 10,000,000 | — | 10,435 | — | — | — | 10,435 | — | ||||||||||||||||||||||||
| Acquisitions of noncontrolling interests | — | — | (2,508 | ) | — | — | 391 | (2,117 | ) | |||||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | 6,294 | — | 6,294 | — | ||||||||||||||||||||||||
| Net loss | — | — | — | (97,334 | ) | — | — | (97,334 | ) | — | ||||||||||||||||||||||
| December 31, 2016 | 274,108,808 | $ | 27 | $ | 990,870 | $ | (1,134,438 | ) | $ | (40,904 | ) | $ | 937 | $ | (183,508 | ) | $ | 5,000 | ||||||||||||||
See the accompanying notes to the consolidated financial statements.
| 108 |
Consolidated Statements of Cash Flows
(In Thousands)
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Operating activities | ||||||||||||
| Net income (loss) | $ | (97,334 | ) | $ | (218,052 | ) | $ | 2,167 | ||||
| Adjustments to reconcile net income (loss) to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization | 11,374 | 12,920 | 14,969 | |||||||||
| Loss (gain) on disposal of property, plant and equipment | (161 | ) | 154 | 263 | ||||||||
| Impairment of intangible assets | — | 5,525 | 3,035 | |||||||||
| Stock-based compensation | 7,325 | 9,134 | 14,105 | |||||||||
| Amortization of debt discount and issuance costs | 14,445 | 58,559 | 9,981 | |||||||||
| Loss upon extinguishment of debt | 4,146 | 1,141 | 10,512 | |||||||||
| Loss on purchase commitments, impairment of property, plant and equipment and other asset allowances | 7,305 | 34,166 | 1,769 | |||||||||
| Withholding tax related to conversion of related party debt | — | 4,723 | — | |||||||||
| Change in fair value of derivative instruments | (41,355 | ) | (16,287 | ) | (144,138 | ) | ||||||
| Loss from investments in affiliates | — | 4,184 | 2,910 | |||||||||
| Other non-cash expenses | 999 | (413 | ) | (113 | ) | |||||||
| Changes in assets and liabilities: | ||||||||||||
| Accounts receivable | (9,331 | ) | 4,920 | (1,217 | ) | |||||||
| Related party accounts receivable | 372 | (649 | ) | (4 | ) | |||||||
| Inventories, net | 5,686 | 4,470 | (4,481 | ) | ||||||||
| Prepaid expenses and other assets | (4,913 | ) | (4,297 | ) | (2,907 | ) | ||||||
| Accounts payable | 6,442 | 4,373 | (3,209 | ) | ||||||||
| Accrued and other liabilities | 12,696 | 10,954 | 6,830 | |||||||||
| Deferred revenue | 714 | (89 | ) | 4,760 | ||||||||
| Deferred rent | (777 | ) | (568 | ) | 60 | |||||||
| Net cash used in operating activities | (82,367 | ) | (85,132 | ) | (84,708 | ) | ||||||
| Investing activities | ||||||||||||
| Purchase of short-term investments | (5,559 | ) | (2,759 | ) | (1,371 | ) | ||||||
| Maturities of short-term investments | 6,187 | 2,321 | 1,409 | |||||||||
| Change in restricted cash | (4,040 | ) | 240 | — | ||||||||
| Proceeds on disposal of noncontrolling interest | 10,000 | — | (2,075 | ) | ||||||||
| Loan to affiliate | — | (1,579 | ) | (2,790 | ) | |||||||
| Purchase of property, plant and equipment, net of disposals | (922 | ) | (3,367 | ) | (5,004 | ) | ||||||
| Change in restricted stock | (24 | ) | — | — | ||||||||
| Net cash provided (used) in investing activities | 5,642 | (5,144 | ) | (9,831 | ) | |||||||
| Financing activities | ||||||||||||
| Proceeds from exercise of common stock, net of repurchases | 180 | 614 | 2,488 | |||||||||
| Employees' taxes paid upon vesting of restricted stock units | (253 | ) | (333 | ) | (1,822 | ) | ||||||
| Proceeds from issuance of common stock in private placements, net of issuance costs | — | 24,625 | 4,000 | |||||||||
| Proceeds from exercise of warrants | 5,000 | 285 | — | |||||||||
| Proceeds from issuance of contingently redeemable equity | 5,000 | — | — | |||||||||
| Principal payments on capital leases | (1,579 | ) | (729 | ) | (1,045 | ) | ||||||
| Proceeds from debt issued, net of discounts and issuance costs | 63,911 | 66,931 | 83,171 | |||||||||
| Proceeds from debt issued to related parties | 29,699 | 10,850 | 49,862 | |||||||||
| Principal payments on debt | (9,759 | ) | (40,819 | ) | (5,733 | ) | ||||||
| Net cash provided by financing activities | 92,199 | 61,424 | 130,921 | |||||||||
| Effect of exchange rate changes on cash and cash equivalents | (316 | ) | (1,203 | ) | (1,203 | ) | ||||||
| Net increase/(decrease) in cash and cash equivalents | 15,158 | (30,055 | ) | 35,179 | ||||||||
| Cash and cash equivalents at beginning of period | 11,992 | 42,047 | 6,868 | |||||||||
| Cash and cash equivalents at end of period | $ | 27,150 | $ | 11,992 | $ | 42,047 | ||||||
| 109 |
Amyris, Inc.
Consolidated Statements of Cash Flows—(Continued)
(In Thousands)
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Supplemental disclosures of cash flow information: | ||||||||||||
| Cash paid for interest | $ | 9,983 | $ | 9,425 | $ | 6,910 | ||||||
| Cash paid for income taxes, net of refunds | $ | — | $ | — | $ | — | ||||||
| Supplemental disclosures of non-cash investing and financing activities: | ||||||||||||
| Acquisitions of property, plant and equipment under accounts payable, accrued liabilities and notes payable | $ | (1,276 | ) | $ | (73 | ) | $ | 114 | ||||
| Financing of equipment | $ | 2,136 | $ | 613 | $ | 617 | ||||||
| Financing of insurance premium under notes payable | $ | (123 | ) | $ | 53 | $ | 166 | |||||
| Acquisition of noncontrolling interest in Glycotech via debt | $ | 3,906 | $ | — | $ | — | ||||||
| Purchase of property, plant and equipment via deposit | $ | 24 | $ | (392 | ) | $ | — | |||||
| Interest capitalized to debt | $ | 3,147 | $ | 6,354 | $ | 5,590 | ||||||
| Issuance of common stock upon conversion of debt | $ | 14,364 | $ | — | $ | — | ||||||
| Issuance of common stock for settlement of debt principal payments | $ | 17,410 | $ | — | $ | — | ||||||
| Cancellation of debt and accrued interest on disposal of interest in affiliate | $ | 4,252 | $ | — | $ | — | ||||||
| Non-cash investment in joint venture | $ | — | $ | — | $ | 1,281 | ||||||
See the accompanying notes to the consolidated financial statements.
| 110 |
Notes to Consolidated Financial Statements
1. The Company
Amyris, Inc. (or the Company) was incorporated in California on July 17, 2003 and reincorporated in Delaware on April 15, 2010 for the purpose of leveraging breakthroughs in bioscience technology to develop and provide renewable compounds for a variety of markets. The Company is currently applying its industrial synthetic biology platform to engineer, manufacture and sell high performance, low cost products into Health and Nutrition, Personal Care and Performance Material markets. The Company's first commercialization efforts have been focused on a renewable hydrocarbon molecule called farnesene (Biofene®), which forms the basis for a wide range of products including nutraceuticals, skin care, fragrances, solvents, polymers, and lubricants ingredients. In 2014, the Company began manufacturing additional molecules for the flavors and fragrance (F&F) industry, in 2015 the Company began investing to expand its capabilities to other small molecule chemical classes beyond terpenes via its collaboration with the Defense Advanced Research Project Agency, as discussed below, and in 2016 the Company expanded into proteins. While the Company's platform is able to use a wide variety of feedstocks, the Company has initially focused on Brazilian sugarcane because of its renewability, low cost and relative price stability. The Company has established one principal operating subsidiaries, Amyris Brasil Ltda. (formerly Amyris Brasil S.A., or Amyris Brasil) which oversees the Company’s production operations in Brazil.
The Company's renewable products business strategy is to generally focus on direct commercialization of specialty products while moving established commodity products into joint venture arrangements with leading industry partners. To commercialize its products, the Company must be successful in using its technology to manufacture its products at commercial scale and on an economically viable basis (i.e., low per unit production costs) and developing sufficient sales volume for those products to support its operations. The Company's prospects are subject to risks, expenses and uncertainties frequently encountered by companies in this stage of development.
Liquidity
The Company expects to fund its operations for the foreseeable future with cash and investments currently on hand, with cash inflows from collaborations and grants, with cash contributions from product sales, with new debt and equity financings and with proceeds from strategic asset divestments. The Company's planned 2017 and 2018 working capital needs and its planned operating and capital expenditures are dependent on significant inflows of cash from new and existing collaboration partners and from cash generated from renewable product sales and from strategic asset divestments, and will also require additional funding from debt or equity financings.
The Company has incurred significant operating losses since its inception and believes that it will continue to incur losses and negative cash flow from operations into at least 2018. As of December 31, 2016, the Company had negative working capital of $50.7 million, an accumulated deficit of $1,134.4 million and had cash, cash equivalents and short term investments of $28.5 million. The Company will likely need additional financing as early as the second quarter of 2017 to support its liquidity needs. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. If the Company is unable to continue as a going concern, it may be unable to meet its obligations under its existing debt facilities, which could result in an acceleration of its obligation to repay all amounts outstanding under those facilities, and it may be forced to liquidate its assets.
| 111 |
As of December 31, 2016, the Company's debt, net of discount of $42.5 million, totaled $227.0 million, of which $59.2 million is classified as current. In addition to upcoming debt maturities, the Company's debt service obligations over the next twelve months are significant, including $18.3 million of anticipated cash interest payments. The Company's debt agreements contain various covenants, including certain restrictions on the Company's business that could cause the Company to be at risk of defaults such as restrictions on additional indebtedness, material adverse effect and cross default clauses. A failure to comply with the covenants and other provisions of the Company’s debt instruments, including any failure to make a payment when required would generally result in events of default under such instruments, which could permit acceleration of such indebtedness. If such indebtedness is accelerated, it would generally also constitute an event of default under the Company’s other outstanding indebtedness, permitting acceleration of such other outstanding indebtedness. Any required repayment of such indebtedness as a result of acceleration or otherwise would consume current cash on hand such that the Company would not have those funds available for use in its business or for payment of other outstanding indebtedness. Please refer to Note 5, “Debt”, Note 6, “Commitments and Contingencies” and Note 16, “Subsequent Events” for further details regarding the Company's debt service obligations and commitments. The Company also has a significant working capital deficit and contractual obligations related to capital and operating leases, as well as purchase commitments.
In addition to the need for financing described above, the Company may take the following actions as early as the second quarter of 2017 to support its liquidity needs through the remainder of 2017 and into 2018:
| • | Effect significant headcount reductions, particularly with respect to employees not connected to critical or contracted activities across all functions of the Company, including employees involved in general and administrative, research and development, and production activities. |
| • | Shift focus to existing products and customers with significantly reduced investment in new product and commercial development efforts. |
| • | Reduce production activity at the Company’s Brotas manufacturing facility to levels only sufficient to satisfy volumes required for product revenues forecast from existing products and customers. |
| • | Reduce expenditures for third party contractors, including consultants, professional advisors and other vendors. |
| • | Reduce or delay uncommitted capital expenditures, including those relating to proposed additional manufacturing capacity, non-essential facility and lab equipment, and information technology projects. |
| • | Closely monitor the Company’s working capital position with customers and suppliers, as well as suspend operations at pilot plants and demonstration facilities. |
Implementing this plan could have a negative impact on the Company's ability to continue its business as currently contemplated, including, without limitation, delays or failures in its ability to:
| 112 |
| • | Achieve planned production levels; |
| • | Develop and commercialize products within planned timelines or at planned scales; and |
| • | Continue other core activities. |
Furthermore, any inability to scale-back operations as necessary, and any unexpected liquidity needs, could create pressure to implement more severe measures. Such measures could have an adverse effect on the Company's ability to meet contractual requirements, including obligations to maintain manufacturing operations, and increase the severity of the consequences described above.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with the accounting principles generally accepted in the United States of America (or GAAP) and with the instructions for Form 10-K and Regulations S-X. The consolidated financial statements include the accounts of the Company and its consolidated subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
The Company uses the equity method to account for investments in companies, if its investments provide it with the ability to exercise significant influence over operating and financial policies of the investee. Consolidated net income or loss includes the Company’s proportionate share of the net income or loss of these companies. Judgments made by the Company regarding the level of influence over each equity method investment include considering key factors such as the Company’s ownership interest, representation on the board of directors, participation in policy-making decisions and material intercompany transactions.
Principles of Consolidation
The consolidated financial statements of the Company include the accounts of Amyris, Inc., its subsidiaries and two consolidated variable interest entities (or “VIEs”), with respect to which the Company is considered the primary beneficiary, after elimination of intercompany accounts and transactions. Disclosure regarding the Company’s participation in the VIEs is included in Note 7, "Joint Ventures and Noncontrolling Interest."
Variable Interest Entities
The Company has interests in joint venture entities that are VIEs. Determining whether to consolidate a VIE requires judgment in assessing (i) whether an entity is a VIE and (ii) if the Company is the entity’s primary beneficiary and thus required to consolidate the entity. To determine if the Company is the primary beneficiary of a VIE, the Company evaluates whether it has (i) the power to direct the activities that most significantly impact the VIE’s economic performance and (ii) the obligation to absorb losses or the right to receive benefits of the VIE that could potentially be significant to the VIE. The Company’s evaluation includes identification of significant activities and an assessment of its ability to direct those activities based on governance provisions and arrangements to provide or receive product and process technology, product supply, operations services, equity funding and financing and other applicable agreements and circumstances. The Company’s assessment of whether it is the primary beneficiary of its VIEs requires significant assumptions and judgment.
| 113 |
Use of Estimates
In preparing the consolidated financial statements, management must make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents, short term investments and accounts receivable. The Company places its cash equivalents and investments (primarily certificates of deposits) with high credit quality financial institutions and, by policy, limits the amount of credit exposure with any one financial institution. Deposits held with banks may exceed the amount of insurance provided on such deposits. The Company has not experienced any losses on its deposits of cash and cash equivalents and short-term investments.
The Company performs ongoing credit evaluation of its customers, does not require collateral, and maintains allowances for potential credit losses on customer accounts when deemed necessary.
Customers representing 10% or greater of accounts receivable were as follows:
| December 31, | ||||||||
| Customers | 2016 | 2015 | ||||||
| Customer L | 33 | % | ** | |||||
| Customer K | 22 | % | * | |||||
| Customer H | ** | 23 | % | |||||
| Customer C | ** | 26 | % | |||||
| Customer G | ** | 10 | % | |||||
______________
* No outstanding balance
** Less than 10%
| 114 |
Customers representing 10% or greater of revenues were as follows:
| Years Ended December 31, | ||||||||||||
| Customers | 2016 | 2015 | 2014 | |||||||||
| Customer N | 14 | % | * | * | ||||||||
| Customer M | 22 | % | * | * | ||||||||
| Customer E | 27 | % | 37 | % | 47 | % | ||||||
| Customer J | ** | 10 | % | ** | ||||||||
| Customer C | ** | ** | 10 | % | ||||||||
______________
* Not a customer
** Less than 10%
Fair Value of Financial Instruments
The Company measures certain financial assets and liabilities at fair value based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. Where available, fair value is based on or derived from observable market prices or other observable inputs. Where observable prices or inputs are not available, valuation techniques are applied. These valuation techniques involve some level of management estimation and judgment, the degree of which is dependent on the price transparency for the instruments or market and the instruments’ complexity.
The carrying amounts of certain financial instruments, such as cash equivalents, short term investments, accounts receivable, accounts payable and accrued liabilities, approximate fair value due to their relatively short maturities. The fair values of the loans payable, convertible notes and credit facilities are based on the present value of expected future cash flows and assumptions about current interest rates and the creditworthiness of the Company. The loans payable, convertible notes and credit facilities are carried on the consolidated balance sheet on a historical cost basis, because the Company has not elected to recognize the fair value of these liabilities. However, the Remaining Notes subject to the Maturity Treatment Agreement were revalued to fair value on July 29, 2015 (see Note 5, “Debt” for details).
The Company estimates the fair value of the compound embedded derivatives for the convertible promissory notes issued to Total Energies Nouvelles Activités USA (formerly known as Total Gas & Power USA, SAS, or Total) under the Total Fuel Agreements (refer to Note 5, "Debt" for further details) using the Monte Carlo simulation valuation model that combines expected cash outflows with market-based assumptions regarding risk-adjusted yields, stock price volatility, probability of a change of control and the trading information of the Company's common stock into which the notes are or may become convertible.
The Company estimates the fair value of the compound embedded derivatives for the notes issued in the first and second tranches of the August 2013 Financing (or, Tranche I Notes and Tranche II Notes, respectively), the 2014 144A Notes and 2015 144A Notes (as defined in Note 5, "Debt" and collectively, Convertible Notes) using the binomial lattice model in order to estimate the fair value of the embedded derivatives. A binomial lattice model generates two probable outcomes - one up and another down - arising at each point in time, starting from the date of valuation until the maturity date. A lattice model was used to determine if the Convertible Notes would be converted, called or held at each decision point. Within the lattice model, the following assumptions are made: (i) the Convertible Notes will be converted early if the conversion value is greater than the holding value and (ii) the Convertible Notes will be called if the holding value is greater than both (a) redemption price and (b) the conversion value at the time. If the Convertible Notes are called, then the holder will maximize their value by finding the optimal decision between (1) redeeming at the redemption price and (2) converting the Convertible Notes. Using this lattice method, the Company valued the embedded derivatives using the "with-and-without method", where the fair value of the Convertible Notes including the embedded derivatives is defined as the "with", and the fair value of the Convertible Notes excluding the embedded derivatives is defined as the "without". This method estimates the fair value of the embedded derivatives by looking at the difference in the values between the Convertible Notes with the embedded derivatives and the fair value of the Convertible Notes without the embedded derivatives. The lattice model uses the stock price, conversion rate, conversion price, maturity date, risk-free interest rate, estimated stock volatility and estimated credit spread.
| 115 |
Changes in the inputs into these valuation models have a significant impact on the estimated fair value of the embedded derivatives. For example, a decrease (increase) in the estimated credit spread for the Company results in an increase (decrease) in the estimated fair value of the embedded derivatives. Conversely, a decrease (increase) in the stock price results in a decrease (increase) in the estimated fair value of the embedded derivatives. The changes during 2016, 2015 and 2014 in the fair values of the bifurcated compound embedded derivatives are primarily related to the change in price of the Company's common stock and are reflected in the consolidated statements of operations as “Gain (loss) from change in fair value of derivative instruments.”
Cash and Cash Equivalents
All highly liquid investments purchased with an original maturity date of 90 days or less at the date of purchase are considered to be cash equivalents. Cash and cash equivalents consist of money market funds and certificates of deposit.
Short Term Investments
Investments with original maturities greater than 90 days that mature less than 1 year from the consolidated balance sheet date are classified as short-term investments. The Company classifies investments as short-term or long-term based upon whether such assets are reasonably expected to be realized in cash or sold or consumed during the normal cycle of business. The Company invests its excess cash balances primarily in certificates of deposit. Certificates of deposits that have maturities greater than 90 days that mature less than one year from the consolidated balance sheet date are classified as short term investments. The Company classifies all of its investments as available-for-sale and records such assets at estimated fair value in the consolidated balance sheets, with unrealized gains and losses, if any, reported as a component of accumulated other comprehensive income (loss) in stockholders’ equity (deficit). Debt securities are adjusted for amortization of premiums and accretion of discounts and such amortization and accretion are reported as a component of interest income. Realized gains and losses and declines in value that are considered to be other-than-temporary are recognized in the statements of operations. The cost of securities sold is determined on the specific identification method. There were no material realized gains or losses from sales of debt securities during the years ended December 31, 2016, 2015 and 2014. As of December 31, 2016 and 2015, the Company did not have any other-than-temporary declines in the fair value of its debt securities.
| 116 |
Accounts Receivable
The Company maintains an allowance for doubtful accounts receivable for estimated losses resulting from the inability of its customers to make required payments. The Company determines this allowance based on specific doubtful account identification and management judgment on estimated exposure. The Company writes off accounts receivable against the allowance when it determines a balance is uncollectible and no longer actively pursues collection of the receivable.
Inventories
Inventories, which consist of farnesene-derived products and flavor and fragrances ingredients are stated at the lower of cost or market and categorized as finished goods, work-in-process or raw material inventories. The Company evaluates the recoverability of its inventories based on assumptions about expected demand and net realizable value. If the Company determines that the cost of inventories exceeds its estimated net realizable value, the Company records a write-down equal to the difference between the cost of inventories and the estimated net realizable value. If actual net realizable values are less favorable than those projected by management, additional inventory write-downs may be required that could negatively impact the Company's operating results. If actual net realizable values are more favorable, the Company may have favorable operating results when products that have been previously written down are sold in the normal course of business. The Company also evaluates the terms of its agreements with its suppliers and establishes accruals for estimated losses on adverse purchase commitments as necessary, applying the same lower of cost or market approach that is used to value inventory. Cost is computed on a first-in, first-out basis. Inventory costs include transportation costs incurred in bringing the inventory to its existing location.
Investments in Affiliates
We use the equity method to account for our investments in affiliates. We include our proportionate share of earnings and/or losses of our equity method investees in the loss from investments in affiliates in the consolidated statements of operations. The carrying value of our investments in affiliates includes loans to affiliates. Investments in affiliates are carried at cost less impairment, as adjusted for market rates of interest imputed to non-market interest rate loans advanced to affiliates.
Restricted Cash
Cash accounts that are restricted as to withdrawal or usage are presented as restricted cash. As of December 31, 2016 and 2015, the Company had $5.3 million and $1.2 million, respectively, of restricted cash for the purposes of lease terms, bank guarantees and other contractual commitments.
Derivative Instruments
The Company makes limited use of derivative instruments, which includes currency interest rate swap agreements, to manage the Company's exposure to foreign currency exchange rate fluctuations and interest rate fluctuations related to the Company's Banco Pine S.A. loan (discussed below under Note 5, "Debt"). Changes in the fair value of the derivative contracts are recognized immediately in the consolidated statements of operations.
| 117 |
Embedded derivatives that are required to be bifurcated from the underlying debt instrument (i.e., host) are accounted for and valued as separate financial instruments. The Company evaluated the terms and features of its convertible notes payable and identified compound embedded derivatives requiring bifurcation and accounting at fair value because the economic and contractual characteristics of the embedded derivatives met the criteria for bifurcation and separate accounting due to the conversion options containing “make-whole interest” provisions, down round conversion price adjustment provisions and conversion rate adjustments.
Property, Plant and Equipment, net
Property, plant and equipment, net are stated at cost less accumulated depreciation and amortization. Depreciation and amortization is computed using the straight-line method over the estimated useful lives of the related assets. Maintenance and repairs are charged to expense as incurred, and improvements and betterments are capitalized. When assets are retired or otherwise disposed of, the cost and accumulated depreciation are removed from the balance sheet and any resulting gain or loss is reflected in operations in the period realized.
Depreciation and amortization periods for the Company’s property, plant and equipment are as follows:
| Machinery and equipment (years) | 7 | - | 15 | |
| Buildings (years) | 15 | |||
| Computers and software (years) | 3 | - | 5 | |
| Furniture and office equipment (years) | 5 | |||
| Vehicles (years) | 5 |
Buildings and leasehold improvements are amortized on a straight-line basis over the term of the lease, or the useful life of the assets, whichever is shorter.
Computers and software includes internal-use software that is acquired to meet the Company’s needs. Amortization commences when the software is ready for its intended use and the amortization period is the estimated useful life of the software, generally 3 to 5 years. Capitalized costs primarily include contract labor costs of the individuals dedicated to the development and installation of internal-use software.
Impairment of Long-Lived Assets
Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable or the estimated useful life is no longer appropriate. If indicators of impairment exist and the undiscounted projected cash flows associated with such assets are less than the carrying amount of the asset, an impairment loss is recorded to write the assets down to their estimated fair values. Fair value is estimated based on discounted future cash flows. There were $7.3 million, $28.5 million, and $1.8 million of impairment charges recorded during the years ended December 31, 2016, 2015 and 2014, respectively.
Goodwill and Intangible Assets
Goodwill represents the excess of the cost over the fair value of net assets acquired from business combinations. Intangible assets are comprised primarily of in-process research and development (or IPR&D). Goodwill and IPR&D were recognized on an acquisition completed in 2011. Goodwill and intangible assets with indefinite useful lives are assessed for impairment using fair value measurement techniques on an annual basis or more frequently if facts and circumstances warrant such a review. When required, a comparison of fair value to the carrying amount of assets is performed to determine the amount of any impairment. The Company makes significant judgments in relation to the valuation of goodwill and intangible assets resulting from business combinations.
| 118 |
There are several methods that can be used to determine the estimated fair value of the IPR&D acquired in a business combination. We used the "income method," which applies a probability weighting that considers the risk of development and commercialization, to the estimated future net cash flows that are derived from projected sales revenues and estimated costs. These projections are based on factors such as relevant market size, pricing of similar products, and expected industry trends. The estimated future net cash flows are then discounted to the present value using an appropriate discount rate. These assets are treated as indefinite-lived intangible assets until completion or abandonment of the projects, at which time the assets will be amortized over the remaining useful life or written off, as appropriate. Amounts recorded as IPR&D will begin being amortized upon the completion of development activities over the estimated useful life of the technology. The development activities have not been completed, and therefore the amortization of the acquired IPR&D has not begun.
Factors that could trigger an impairment review include significant under-performance relative to expected historical or projected future operating results, significant changes in the manner of use of the acquired assets or the strategy for the Company's overall business or significant negative industry or economic trends. If this evaluation indicates that the value of the intangible asset may be impaired, we make an assessment of the recoverability of the net carrying value of the asset. If this assessment indicates that the intangible asset is not recoverable, based on the estimated discounted future cash flows of the technology over its expected life, we reduce the net carrying value of the related intangible asset to fair value. As a result of the impairment assessment of IPR&D, the Company recognized an impairment of its IPR&D asset of zero, $5.5 million and $3.0 million for the years ended December 31, 2016, 2015 and 2014, respectively. As of December 31, 2016, the Company's intangible assets had a carrying amount of zero.
Noncontrolling Interest
Changes in noncontrolling interest ownership that do not result in a change of control and where there is a difference between the fair value of the consideration paid/received and the amount by which the noncontrolling interest is adjusted are accounted for as equity transactions.
Revenue Recognition
The Company recognizes revenue from the sale of renewable products, delivery of research and development services, licensing of intellectual property, and from governmental grants. Revenue is recognized when all of the following criteria are met: persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the fee is fixed or determinable, and collectability is reasonably assured.
If sales arrangements contain multiple elements, the Company evaluates whether the components of each arrangement represent separate units of accounting.
| 119 |
Product Sales
The Company's renewable product sales do not include rights of return. Returns are only accepted if the product does not meet product specifications and such nonconformity is communicated to the Company within a set number of days of delivery. The Company offers a two year standard warranty provision for squalane products sold after March 31, 2012, if the products do not meet Company-established criteria as set forth in the Company's trade terms. The Company bases its return reserve on a historical rate of return for the Company's squalane products. Revenues are recognized, net of discounts and allowances, once passage of title and risk of loss has occurred and contractually specified acceptance criteria have been met, provided all other revenue recognition criteria have also been met.
Grants, Collaborative Research Services and License Fees
Revenues from collaborative research services are recognized as the services are performed consistent with the performance requirements of the contract. In cases where the planned levels of research services fluctuate over the research term, we recognize revenues using the proportionate performance method based upon actual efforts to date relative to the amount of expected effort to be incurred by us. When up-front payments are received and the planned levels of research services do not fluctuate over the research term, revenues are recorded on a ratable basis over the arrangement term, up to the amount of cash received. When up-front payments are received and the planned levels of research services fluctuate over the research term, revenues are recorded using the proportionate performance method, up to the amount of cash received. Where arrangements include milestones that are determined to be substantive and at risk at the inception of the arrangement, revenues are recognized upon achievement of the milestone and is limited to those amounts whereby collectability is reasonably assured. License fees for intellectual property transferred to other parties, representing non-refundable payments received at the time of signature of license agreements, are recognized as revenue upon signature of the license agreements when the Company has no significant future performance obligations and collectability of the fees is assured. Upfront payments received at the beginning of licensing agreements are deferred and recognized as revenue on a systematic basis over the period during which the related services are rendered and all obligations are performed.
Government grants are agreements that generally provide cost reimbursement for certain types of expenditures in return for research and development activities over a contractually defined period. Revenues from government grants are recognized in the period during which the related costs are incurred, provided that the conditions under which the government grants were provided have been met and only perfunctory obligations are outstanding.
Cost of Products Sold
Cost of products sold includes production costs of renewable products, which include cost of raw materials, amounts paid to contract manufacturers and period costs including inventory write-downs resulting from applying lower-of-cost-or-market inventory valuation. Cost of products sold also includes certain costs related to the scale-up in production of such products.
Shipping and handling costs charged to customers are recorded as revenues. Shipping costs are included in cost of products sold. Such charges were not material in any of the periods presented.
| 120 |
Research and Development
Research and development costs are expensed as incurred and include costs associated with research performed pursuant to collaborative agreements and government grants, including internal research. Research and development costs consist of direct and indirect internal costs related to specific projects as well as fees paid to others that conduct certain research activities on the Company’s behalf.
Debt Extinguishment
The Company accounts for the income or loss from extinguishment of debt in accordance with ASC 470, "Debt", which indicates that for all extinguishment of debt, the difference between the reacquisition price and the net carrying amount of the debt being extinguished should be recognized as gain or loss when the debt is extinguished. The gain or loss from debt extinguishment is recorded in the consolidated statements of operations under "other income (expense)" as "gain (loss) from extinguishment of debt".
Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires, among other things, that deferred income taxes be provided for temporary differences between the tax basis of the Company’s assets and liabilities and their financial statement reported amounts. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development credit carryforwards. A valuation allowance is provided against deferred tax assets unless it is more likely than not that they will be realized.
The Company recognizes and measures uncertain tax positions in accordance with Income Taxes subtopic 05-6 of ASC 740, which prescribes a recognition threshold and measurement process for recording uncertain tax positions taken, or expected to be taken in a tax return, in the consolidated financial statements. Additionally, the guidance also prescribes treatment for the derecognition, classification, accounting in interim periods and disclosure requirements for uncertain tax positions. The Company accrues for the estimated amount of taxes for uncertain tax positions if it is more likely than not that the Company would be required to pay such additional taxes. An uncertain tax position will not be recognized if it has a less than 50% likelihood of being sustained.
Currency Translation
The Company considers the local currency to be the functional currency of the Company’s wholly-owned subsidiary in Brazil and of the Company's consolidated joint venture in Brazil. Accordingly, asset and liability accounts of those operations are translated into United States dollars using the current exchange rate in effect at the balance sheet date and equity accounts are translated into United States dollars using historical rates. The revenues and expenses are translated using the exchange rates in effect when the transactions occur. Gains and losses from foreign currency translation adjustments are included as a component of accumulated other comprehensive income (loss) on the consolidated balance sheets.
Foreign currency differences arising from the translation of intercompany loans from a foreign currency into the functional currency of an entity, which are of a long-term investment nature (that is, settlement is not planned or anticipated in the foreseeable future) are recorded in “Accumulated other comprehensive income (loss)” on our Consolidated Balance Sheets. Foreign currency differences arising from the translation of other intercompany loans are recorded in “Other income (expense)” on our Consolidated Statements of Operations.
| 121 |
Stock-Based Compensation
The Company accounts for stock-based compensation arrangements with employees using a fair value method which requires the recognition of compensation expense for costs related to all stock-based payments including stock options. The fair value method requires the Company to estimate the fair value of stock-based payment awards on the date of grant. The Company uses the Black-Scholes option pricing model to estimate the fair value of options granted, which is expensed on a straight-line basis over the vesting period. The Company accounts for restricted stock unit awards issued to employees based on the fair market value of the Company’s common stock.
Comprehensive Income (Loss)
Comprehensive income (loss) represents all changes in stockholders’ deficit except those resulting from investments or contributions by stockholders. The Company’s foreign currency translation adjustments represent the components of comprehensive income (loss) excluded from the Company’s net income (loss) and have been disclosed in the consolidated statements of comprehensive loss for all periods presented.
The components of accumulated other comprehensive loss are as follows (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Foreign currency translation adjustment, net of tax | $ | (40,904 | ) | $ | (47,198 | ) | ||
| Total accumulated other comprehensive loss | $ | (40,904 | ) | $ | (47,198 | ) | ||
Net Loss Attributable to Common Stockholders and Net Loss per Share
The Company computes net loss per share in accordance with ASC 260, “Earnings per Share.” Basic net loss per share of common stock is computed by dividing the Company’s net loss attributable to Amyris, Inc. common stockholders (as adjusted in 2015 to remove the impact of the fair value adjustments for any currently exercisable warrants in which the number of shares are included in the weighted average number of shares of common stock outstanding) by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share of common stock is computed by giving effect to all potentially dilutive securities, including stock options, restricted stock units and common stock warrants, using the treasury stock method or the as converted method, as applicable. For the year ended December 31, 2015, basic net loss per share was the same as diluted net loss per share because the inclusion of all potentially dilutive securities outstanding was anti-dilutive. As such, the numerator and the denominator used in computing both basic and diluted net loss were the same for that year.
The following table presents the calculation of basic and diluted net loss per share of common stock attributable to Amyris, Inc. common stockholders (in thousands, except share and per share amounts):
| 122 |
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Numerator: | ||||||||||||
| Net income (loss) attributable to Amyris, Inc. common stockholders | $ | (97,334 | ) | $ | (217,952 | ) | $ | 2,286 | ||||
| Adjustment to exclude fair value gain on liability classified warrants(1) | — | (3,825 | ) | — | ||||||||
| Net income (loss) attributable to Amyris, Inc. common stockholders (for basic income (loss) per share) | (97,334 | ) | (221,777 | ) | 2,286 | |||||||
| Interest on convertible debt | 4,428 | — | 9,365 | |||||||||
| Accretion of debt discount | 2,889 | — | 5,597 | |||||||||
| Gain from change in fair value of derivative instruments | (25,630 | ) | — | (127,109 | ) | |||||||
| Net loss attributable to Amyris, Inc. common stockholders after assumed conversion | $ | (115,647 | ) | $ | (221,777 | ) | $ | (109,861 | ) | |||
| Denominator: | ||||||||||||
| Weighted-average shares of common stock outstanding used in computing net loss per share of common stock, basic | 238,440,197 | 126,961,576 | 78,400,098 | |||||||||
| Basic income (loss) per share | $ | (0.41 | ) | $ | (1.75 | ) | $ | 0.03 | ||||
| Weighted average shares of common stock outstanding | 238,440,197 | 126,961,576 | 78,400,098 | |||||||||
| Effect of dilutive securities: | ||||||||||||
| Convertible promissory notes | 26,204,252 | — | 43,459,343 | |||||||||
| Weighted common stock equivalents | 26,204,252 | — | 43,459,343 | |||||||||
| Diluted weighted-average common shares | 264,644,449 | 126,961,576 | 121,859,441 | |||||||||
| Diluted loss per share | $ | (0.44 | ) | $ | (1.75 | ) | $ | (0.9 | ) | |||
______________
| (1) | The amount represents a net gain related to a change in the fair value of a liability classified common stock warrant included in the Company’s consolidated statement of operations for the year ended December 31, 2015. The warrant has a nominal exercise price and shares issuable upon exercise of the warrant are considered equivalent to the Company’s common shares for the purpose of computation of basic earnings per share and consequently losses are adjusted to exclude the gain. The warrant was exercised in 2015. |
The following outstanding shares of potentially dilutive securities were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been anti-dilutive:
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Period-end stock options to purchase common stock | 13,487,685 | 12,930,112 | 10,539,978 | |||||||||
| Convertible promissory notes (1) | 35,933,931 | 72,537,306 | 26,887,005 | |||||||||
| Period-end common stock warrants | 5,021,087 | 2,901,926 | 1,021,087 | |||||||||
| Period-end restricted stock units | 6,991,133 | 5,554,844 | 1,975,503 | |||||||||
| Total | 61,433,836 | 93,924,188 | 40,423,573 | |||||||||
______________
| (1) | The potentially dilutive effect of convertible promissory notes was computed based on conversion ratios in effect as of December 31, 2016. A portion of the convertible promissory notes issued carries a provision for a reduction in conversion price under certain circumstances, which could potentially increase the dilutive shares outstanding. Another portion of the convertible promissory notes issued carries a provision for an increase in the conversion rate under certain circumstances, which could also potentially increase the dilutive shares outstanding. |
| 123 |
Recent Accounting Pronouncements
In May 2014, the FASB issued new guidance related to revenue recognition. In March, April, May and December 2016, the FASB issued additional amendments to the new revenue guidance relating to reporting revenue on a gross versus net basis, identifying performance obligations, licensing arrangements, collectability, noncash consideration, presentation of sales tax, and transition. This new standard will replace all current GAAP guidance on this topic and eliminate all industry-specific guidance. The new revenue recognition update guidance provides a unified model to determine how revenue is recognized. The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The FASB has issued several updates to the standard which i) clarify the application of the principal versus agent guidance (ASU 2016-08); ii) clarify the guidance on inconsequential and perfunctory promises and licensing (ASU 2016-10) and iii) narrow-scope improvements and practical expedients (ASU 2016-12). On July 9, 2015, the FASB voted to defer the effective date by one year to December 15, 2017 for interim and annual reporting periods beginning after that date and permitted early adoption of the standard, but not before the original effective date of December 15, 2016. Entities have the option of using either a full retrospective or a modified retrospective approach to adopt the new standard. Therefore, the new standard will be effective commencing with our quarter ending March 31, 2018. The Company is currently assessing the potential impact of this new standard on its consolidated financial statements and has not selected the transition method. While the Company continues to assess the impact of the new guidance on its revenue recognition policies, it is expected that a key area will be the assessment of contract modifications and collaboration contracts to determine if identification of performance obligations is impacted, which may impact the timing of revenue recognition. In addition, the Company expects additional disclosures related to revenue.
In August 2014, FASB issued new guidance related to the disclosure around going concern. The new standard provides guidance around management's responsibility to evaluate whether there is substantial doubt about an entity's ability to continue as a going concern and to provide related footnote disclosure if substantial doubt exists. The new standard is effective for annual periods ending after December 15, 2016 and for annual periods and interim periods thereafter. The Company adopted this standard in 2016 and this did not have a material impact on the financial statements.
In January 2015, FASB issued an update related to the presentation of extraordinary and unusual items. The update eliminates the concept of extraordinary items found in Subtopic 225-20, which required that an entity separately classify, present and disclose extraordinary events and transactions when the event or activity met both criteria of being unusual in nature and infrequent in occurrence. Although the concept of extraordinary items will be eliminated, the presentation and disclosure guidance for items that are unusual in nature or occur infrequently will be retained and will be expanded to include items that are both unusual in nature and infrequently occurring. The standard is effective for annual and interim periods within those annual years beginning after December 15, 2015. The Company adopted this standard in 2016 and this did not have a material impact on the financial statements.
In February 2015, FASB issued an amendment to ASC 810 Consolidation. The amendments affect reporting entities that are required to evaluate whether they should consolidate certain legal entities. The amendments are effective for the fiscal years and interim periods within those fiscal years, beginning after December 15, 2015. The Company adopted this standard in 2016 and this did not have a material impact on the financial statements.
| 124 |
In April 2015, FASB issued Accounting Standards Update 2015-3, Simplifying the Presentation of Debt Issuance Costs, which requires debt issuance costs to be presented in the balance sheet as a direct deduction from the carrying value of the associated debt liability, consistent with the presentation of a debt discount. The recognition and measurement guidance for debt issuance costs are not affected by this guidance. The guidance is effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. The Company adopted the guidance in 2016. As of December 31, 2015, this resulted in the reduction of noncurrent debt by $2.8 million, current debt by $1.4 million, other noncurrent assets by $2.8 million and prepaid expenses and other current assets by $1.4 million.
In July 2015, FASB issued Accounting Standards Update 2015-11, Simplifying the Measurement of Inventory, which requires that inventory within the scope of the guidance be measured at the lower of cost and net realizable value. The new standard is being issued as part of the simplification initiative. Prior to the issuance of the standard, inventory was measured at the lower of cost or market (where market was defined as replacement cost, with a ceiling of net realizable value and floor of net realizable value less a normal profit margin). The new guidance will be effective for fiscal years beginning after December 15, 2016, including interim periods within those years. Prospective application is required and early adoption is permitted. The Company is currently assessing the impact of adopting this new accounting standard on its financial statements.
In January 2016, FASB issued Accounting Standards Update 2016-01 Financial Instruments-Overall, which address certain aspects of recognition, measurement, presentation, and disclosure of financial instruments. The amendments in this Update are effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. Earlier application is permitted under specific circumstances. The Company expects the new standard to impact the extent of its disclosures of financial instruments, particularly in relation to fair value disclosures, but otherwise does not expect a significant impact from the new standard.
In February 2016, FASB issued Accounting Standards Update 2016-02-Leases with fundamental changes to how entities account for leases. Lessees will need to recognize a right-of-use asset and a lease liability for virtually all of their leases (other than leases that meet the definition of a short-term lease). The liability will be equal to the present value of lease payments. The asset will be based on the liability, subject to adjustment, such as for initial direct costs. Additional disclosures for leases will also be required. The standard is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. Early adoption is permitted. The new standard must be adopted using a modified retrospective transition, and provides for certain practical expedients. The new standard may materially impact the Company’s financial statements.
In March 2016, FASB issued ASU No. 2016-06, Contingent Put and Call Options in Debt Instruments. The amendments in this ASU clarify the requirements for assessing whether contingent call (put) options that can accelerate the payment of principal on debt instruments are clearly and closely related to their debt hosts. An entity performing the assessment under the amendments in this ASU is required to assess the embedded call (put) options solely in accordance with the four-step decision sequence. The ASU is effective for financial statements issued for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption is permitted. The Company does not expect this ASU to have a material impact on its financial statements.
| 125 |
In March 2016, FASB issued ASU No. 2016-09,Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. This ASU identifies areas for simplification involving several aspects of accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, an option to recognize gross stock compensation expense with actual forfeitures recognized as they occur, as well as certain classifications on the statement of cash flows. This ASU will be effective for fiscal years beginning after December 15, 2016, and interim periods within those annual periods. Early adoption is permitted. The Company does not expect this ASU to have a material impact on its financial statements.
In June 2016, FASB issued ASU No. 2016-13,Allowance for Loan and Lease Losses (Financial Instruments - Credit Losses Topic 326.). New impairment guidance for certain financial instruments (including trade receivables) will replace the current “incurred loss” model for estimating credit losses with a forward looking “expected loss” model. The ASU is effective for the Company for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. Early application is permitted as of the fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. The Company is evaluating the impact of this standard on its consolidated financial statements.
In August 2016, FASB issued ASU 2016-15Classification of Certain Cash Receipts and Cash Payments on the statement of cash flows. The new guidance clarifies classification of certain cash receipts and cash payments in the statement of cash flows. This guidance will be effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017 and interim periods within those fiscal years. Early adoption is permitted. The Company is evaluating the impact of adopting this accounting standard update on the financial statements and related disclosures.
In October 2016, FASB issued ASU 2016-16,Intra-Entity Transfers of Assets Other Than Inventory on simplifying the accounting for income taxes related to intra-entity asset transfers. The new guidance allows an entity to recognize the tax expense from the sale of an asset in the seller’s tax jurisdiction when the transfers occurs, even though the pre-tax effects of that transaction are eliminated in consolidation. This guidance will be effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. Early adoption is permitted only in the first quarter of 2017. The Company does not expect a material impact on its financial statements upon adoption of this standard.
In November 2016, FASB issued ASU 2016-18 Statement of Cash Flows - Restricted Cash. The amendments in this update require that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The amendments in this update are effective for public business entities for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted, including adoption in an interim period. The amendments in this update should be applied using a retrospective transition method to each period presented. The Company has not adopted the update. Upon adoption this would result in a change in the presentation of restricted cash in the statement of cash flows.
| 126 |
3. Fair Value of Financial Instruments
The inputs to the valuation techniques used to measure fair value are classified into the following categories:
Level 1: Quoted market prices in active markets for identical assets or liabilities.
Level 2: Observable market-based inputs or unobservable inputs that are corroborated by market data.
Level 3: Unobservable inputs that are not corroborated by market data.
There were no transfers between the levels during 2016, and as of December 31, 2016, the Company’s financial assets and financial liabilities at fair value were classified within the fair value hierarchy as follows (in thousands):
| Level 1 | Level 2 | Level 3 | Balance as of December 31, 2016 | |||||||||||||
| Financial Assets | ||||||||||||||||
| Money market funds | $ | 1,549 | $ | — | $ | — | $ | 1,549 | ||||||||
| Certificates of deposit | 1,373 | — | — | 1,373 | ||||||||||||
| Total financial assets | $ | 2,922 | $ | — | $ | — | $ | 2,922 | ||||||||
| Financial Liabilities | ||||||||||||||||
| Loans payable (1) | $ | — | $ | 53,579 | $ | — | $ | 53,579 | ||||||||
| Credit facilities (1) | — | 51,261 | — | 51,261 | ||||||||||||
| Convertible notes (1) | — | — | 117,767 | 117,767 | ||||||||||||
| Compound embedded derivative liabilities | — | — | 4,135 | 4,135 | ||||||||||||
| Currency interest rate swap derivative liability | — | 3,343 | — | 3,343 | ||||||||||||
| Total financial liabilities | $ | — | $ | 108,183 | $ | 121,902 | $ | 230,085 | ||||||||
_________
(1) These liabilities are carried on the consolidated balance sheet on a historical cost basis.
The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and consider factors specific to the asset or liability. The fair values of money market funds and certificates of deposit are based on fair values of identical assets. The fair values of the loans payable, convertible notes, credit facilities and currency interest rate swaps are based on the present value of expected future cash flows and assumptions about current interest rates and the creditworthiness of the Company. The method of determining the fair value of the compound embedded derivative liabilities is described subsequently in this note. Market risk associated with the fixed and variable rate long-term loans payable, credit facilities and convertible notes relates to the potential reduction in fair value and negative impact to future earnings, from an increase in interest rates. Market risk associated with the compound embedded derivative liabilities relates to the potential reduction in fair value and negative impact to future earnings from a decrease in interest rates.
The carrying amounts of certain financial instruments, such as cash equivalents, accounts receivable, accounts payable and accrued liabilities, approximate fair value due to their relatively short maturities and low market interest rates, if applicable.
| 127 |
As of December 31, 2015, the Company’s financial assets and financial liabilities are presented below at fair value and were classified within the fair value hierarchy as follows (in thousands):
| Level 1 | Level 2 | Level 3 | Balance as of December 31, 2015 | |||||||||||||
| Financial Assets | ||||||||||||||||
| Money market funds | $ | 2,078 | $ | — | $ | — | $ | 2,078 | ||||||||
| Certificates of deposit | 1,520 | — | — | 1,520 | ||||||||||||
| Total financial assets | $ | 3,598 | $ | — | $ | — | $ | 3,598 | ||||||||
| Financial Liabilities | ||||||||||||||||
| Loans payable (1) | $ | — | $ | 9,541 | $ | — | $ | 9,541 | ||||||||
| Credit facilities (1) | — | 34,893 | — | 34,893 | ||||||||||||
| Convertible notes (1) | — | — | 96,291 | 96,291 | ||||||||||||
| Compound embedded derivative liabilities | — | — | 46,430 | 46,430 | ||||||||||||
| Currency interest rate swap derivative liability | — | 5,009 | — | 5,009 | ||||||||||||
| Total financial liabilities | $ | — | $ | 49,443 | $ | 142,721 | $ | 192,164 | ||||||||
_________
(1) These liabilities are carried on the consolidated balance sheet on a historical cost basis (noting that the Remaining Notes subject to the Maturity Treatment Agreement were revalued to fair value on July 29, 2015, see Note 5 “Debt” for details).
The following table provides a reconciliation of the beginning and ending balances for the convertible notes disclosed at fair value using significant unobservable inputs (Level 3) (in thousands):
| 2016 | 2015 | |||||||
| Balance at January 1 | $ | 96,291 | $ | 222,031 | ||||
| Additions to convertible notes | 25,000 | 31,984 | ||||||
| Conversion/extinguishment of convertible notes | (28,310 | ) | (127,583 | ) | ||||
| Change in fair value of convertible notes | 24,786 | (30,141 | ) | |||||
| Balance at December 31 | $ | 117,767 | $ | 96,291 | ||||
Derivative Instruments
The following table provides a reconciliation of the beginning and ending balances for the compound embedded derivative liabilities measured at fair value using significant unobservable inputs (Level 3) (in thousands):
| 2016 | 2015 | |||||||
| Balance at January 1 | $ | 46,430 | $ | 56,026 | ||||
| Additions to Level 3 | 2,050 | 40,359 | ||||||
| Derecognition on conversion/extinguishment | (2,886 | ) | (30,806 | ) | ||||
| Gain from change in fair value of derivative liabilities | (41,459 | ) | (19,149 | ) | ||||
| Balance at December 31 | $ | 4,135 | $ | 46,430 | ||||
| 128 |
The compound embedded derivative liabilities represent the fair value of the equity conversion options and "make-whole" provisions, as well as the down round conversion price adjustment or conversion rate adjustment provisions of the R&D Notes, the Tranche I Notes, the Tranche II Notes, the 2014 144A Notes and the 2015 144A Notes (see Note 5, "Debt"). There is no current observable market for these types of derivatives and, as such, the Company determined the fair value of the embedded derivatives using a Monte Carlo simulation valuation model for the R&D Notes and the binomial lattice model for the Tranche I Notes, the Tranche II Notes, the 2014 144A Notes and the 2015 144A Notes (or, collectively, Convertible Notes). A Monte Carlo simulation valuation model combines expected cash outflows with market-based assumptions regarding risk-adjusted yields, stock price volatility, probability of a change of control and the trading information of the Company's common stock into which the notes are or may be convertible. A binomial lattice model generates two probable outcomes - one up and another down - arising at each point in time, starting from the date of valuation until the maturity date. A lattice model was used to determine if the Convertible Notes would be converted, called or held at each decision point. Within the lattice model, the following assumptions are made: (i) the Convertible Notes will be converted early if the conversion value is greater than the holding value and (ii) the Convertible Notes will be called if the holding value is greater than both (a) redemption price and (b) the conversion value at the time. If the Convertible Notes are called, then the holder will maximize their value by finding the optimal decision between (1) redeeming at the redemption price and (2) converting the Convertible Notes. Using this lattice method, the Company valued the embedded derivatives using the "with-and-without method", where the fair value of the Convertible Notes including the embedded derivative is defined as the "with", and the fair value of the Convertible Notes excluding the embedded derivatives is defined as the "without". This method estimates the fair value of the embedded derivatives by looking at the difference in the values between the Convertible Notes with the embedded derivatives and the fair value of the Convertible Notes without the embedded derivatives. The lattice model uses the stock price, conversion price, maturity date, risk-free interest rate, estimated stock volatility and estimated credit spread. The Company marks the compound embedded derivatives to market due to the conversion price not being indexed to the Company's own stock. As of December 31, 2016 and 2015, included in "Derivative Liabilities" on the consolidated balance sheet are the Company's compound embedded derivative liabilities of $4.1 million and $46.4 million, respectively.
The market-based assumptions and estimates used in valuing the compound embedded derivative liabilities include amounts in the following ranges/amounts:
| December 31, 2016 | December 31, 2015 | |||||||
| Risk-free interest rate | 0.55% | - | 1.31% | 1.26% | - | 1.40% | ||
| Risk-adjusted yields | 12.80% | - | 22.93% | 35.80% | - | 45.93% | ||
| Stock-price volatility | 45% | 45% | ||||||
| Probability of change in control | 5% | 5% | ||||||
| Stock price | $0.73 | $1.62 | ||||||
| Credit spread | 11.59% | - | 21.64% | 34.48% | - | 44.55% | ||
| Estimated conversion dates | 2017 | - | 2019 | 2016 | - | 2019 | ||
Changes in valuation assumptions can have a significant impact on the valuation of the embedded derivative liabilities. For example, all other things being equal, a decrease/increase in the Company’s stock price, probability of change of control, credit spread, term to maturity/conversion or stock price volatility decreases/increases the valuation of the liabilities, whereas a decrease/increase in risk adjusted yields or risk-free interest rates increases/decreases the valuation of the liabilities. Certain of the Convertible Notes also include conversion price adjustment features and, for example, issuances of common stock by the Company at prices lower than the current conversion price result in a reduction of the conversion price of such notes, which increases the value of the embedded derivative liabilities. See Note 5, "Debt" for further details of conversion price adjustment features in the Convertible Notes.
| 129 |
In June 2012, the Company entered into a loan agreement with Banco Pine S.A. (or Banco Pine) under which Banco Pine provided the Company with a loan (or the Banco Pine Bridge Loan) (see Note 5, "Debt"). At the time of the Banco Pine Bridge Loan, the Company also entered into a currency interest rate swap arrangement with Banco Pine with respect to the repayment of R$22.0 million (approximately US$6.8 million based on the exchange rate as of December 31, 2016) of the Banco Pine Bridge Loan. The swap arrangement exchanges the principal and interest payments under the Banco Pine Bridge Loan for alternative principal and interest payments that are subject to adjustment based on fluctuations in the foreign currency exchange rate between the U.S. dollar and Brazilian real. The swap has a fixed interest rate of 3.94%. Changes in the fair value of the swap are recognized in “Gain (loss) from change in fair value of derivative instruments" in the consolidated statements of operations are as follows (in thousands):
| Income | Years Ended December 31, | |||||||||||||
| Type of Derivative Contract | Statement Classification | 2016 | 2015 | 2014 | ||||||||||
| Gain (Loss) Recognized | ||||||||||||||
| Currency interest rate swap | Gain (loss) from change in fair value of derivative instruments | $ | 1,946 | $ | (3,367 | ) | $ | (480 | ) | |||||
The Company granted a warrant to Temasek to purchase the Company’s common stock (or the Temasek Funding Warrant), as part of the Exchange transaction completed on July 29, 2015. The terms of the Temasek Funding Warrant provide for an adjustment to the number of shares issuable in the future based on the number of any additional shares for which certain of the Company’s outstanding convertible promissory notes may become exercisable as a result of a reduction to the conversion price of such notes, including down-round provisions. As a result of the future adjustment feature (for reduction to the conversion price of outstanding convertible notes), the Company determined the Temasek Funding Warrant would not meet the conditions in ASC 815-40-15 to be considered indexed to the Company’s own equity. Consequently the Temasek Funding Warrant is a derivative and is marked to market each reporting period. The Temasek Funding Warrant is valued using a Black-Scholes valuation model with the following assumptions (in addition to the Company’s share price):
| Initial recognition (July 29, 2015) | ||||
| Expected dividend yield | — | % | ||
| Risk-free interest rate | 2 | % | ||
| Expected term (in years) | 10.0 | |||
| Expected volatility | 74 | % | ||
The Company recognized a derivative liability for the Temasek Funding Warrant of $19.4 million on July 29, 2015. On December 15, 2015, Temasek exercised the Temasek Funding Warrant for cash of $0.1 million. At the day of exercise, the Temasek Funding Warrant was valued at $18.9 million, being the fair value of the 12.7 million shares issued upon exercise of the warrant. In February and May 2016, as a result of adjustments to the conversion price of the Tranche I Notes and the Tranche II Notes (see Note 5, “Debt”), the Temasek Funding Warrant became exercisable for an additional 127,194 and 2,335,342 shares of common stock, respectively.
| 130 |
Derivative instruments measured at fair value as of December 31, 2016 and 2015, and their classification on the consolidated balance sheets are as follows (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Non-current fair market value of swap obligation | 3,343 | 5,009 | ||||||
| Fair value of compound embedded derivative liabilities | 4,135 | 46,430 | ||||||
| Total derivative liabilities (of which $584 is a current liability) | $ | 7,478 | $ | 51,439 | ||||
4. Balance Sheet Components
Inventories, net
Inventories are stated at the lower of cost or market and consist of the following (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Raw materials | $ | 3,159 | $ | 2,204 | ||||
| Work-in-process | 1,848 | 3,583 | ||||||
| Finished goods | 1,206 | 5,099 | ||||||
| Inventories, net | $ | 6,213 | $ | 10,886 | ||||
Property, Plant and Equipment, net
Property, plant and equipment, net is comprised of the following (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Leasehold improvements | $ | 38,785 | $ | 38,519 | ||||
| Machinery and equipment | 82,688 | 72,876 | ||||||
| Computers and software | 9,585 | 9,117 | ||||||
| Furniture and office equipment | 2,333 | 2,234 | ||||||
| Buildings | 4,699 | 3,922 | ||||||
| Vehicles | 164 | 215 | ||||||
| Land | 460 | — | ||||||
| Construction in progress | 2,216 | 5,736 | ||||||
| 140,930 | 132,619 | |||||||
| Less: accumulated depreciation and amortization | (87,195 | ) | (72,822 | ) | ||||
| Property, plant and equipment, net | $ | 53,735 | $ | 59,797 | ||||
The Company's first, purpose-built, large-scale Biofene production plant in southeastern Brazil commenced operations in December 2012. This plant is located at Brotas in the state of São Paulo, Brazil and is adjacent to an existing sugar and ethanol mill, Tonon Bioenergia S.A. (or “Tonon”) (formerly Paraíso Bioenergia) with which the Company has an agreement to purchase a certain number of tons of sugarcane per year, along with specified water and vapor volumes.
| 131 |
In July 2015, the Company announced that it was in discussions with São Martinho S.A. (“SMSA”) regarding the continuation of its joint venture with SMSA. In December 2015, the Company and SMSA agreed to terminate the joint venture. Pursuant to the Termination Agreement, the Company is required to remove the existing assets of the joint venture, which are currently situated on land owned by SMSA (the “SMSA site”). In December 2016, due to a lack of financing for the expansion of the Brotas plant, management recognized an additional impairment of assets intended to be used for such expansion.
As a result of the above developments, the Company recorded an impairment charge of $7.3 million (included in ‘Loss on purchase commitments, impairment of property, plant and equipment and other asset allowances’), related to the assets at the SMSA site of $4.2 million and the Biomin assets of $3.1 million in Brazil for the year ended December 31, 2016. The impairment is based on the estimated sales value of the remaining assets, which have a net book value of $1.9 million. If the Company's plans or estimate of the fair value of the remaining assets change, additional impairment charges may arise in future periods.
Property, plant and equipment, net includes $3.1 million and $2.7 million of machinery and equipment under capital leases as of December 31, 2016 and 2015, respectively. Accumulated amortization of assets under capital leases totaled $0.6 million and $0.5 million as of December 31, 2016 and 2015, respectively.
Depreciation and amortization expense, including amortization of assets under capital leases, was $11.4 million, $12.9 million and $15.0 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Other Assets
Other assets are comprised of the following (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Deposits on property and equipment, including taxes | $ | 291 | $ | 243 | ||||
| Recoverable taxes from Brazilian government entities, net | 13,723 | 8,887 | ||||||
| Other | 1,450 | 1,227 | ||||||
| Total other assets | $ | 15,464 | $ | 10,357 | ||||
Recoverable taxes from Brazilian government entities represents value added taxes paid on purchases in Brazil, which are reclaimable from the Brazilian tax authorities, net of reserves for amounts estimated not to be recoverable.
Accrued and Other Current Liabilities
Accrued and other current liabilities are comprised of the following (in thousands):
| 132 |
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Professional services | $ | 6,876 | $ | 4,017 | ||||
| Accrued vacation | 2,034 | 2,023 | ||||||
| Payroll and related expenses | 4,310 | 3,122 | ||||||
| Tax-related liabilities | 2,610 | 2,505 | ||||||
| Withholding tax related to conversion of related party notes | 1,370 | 4,723 | ||||||
| Deferred rent, current portion | 1,111 | 1,111 | ||||||
| Accrued interest | 4,847 | 1,984 | ||||||
| SMA relocation accrual | 3,641 | 3,641 | ||||||
| Other | 2,389 | 1,142 | ||||||
| Total accrued and other current liabilities | $ | 29,188 | $ | 24,268 | ||||
5. Debt and Mezzanine Equity
Debt is comprised of the following (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| FINEP credit facility | $ | 696 | $ | 840 | ||||
| BNDES credit facility | 1,172 | 1,956 | ||||||
| Guanfu credit facility | 19,564 | — | ||||||
| Senior secured loan facility | 27,658 | 31,590 | ||||||
| Total credit facilities | 49,090 | 34,386 | ||||||
| Convertible notes | 78,981 | 61,233 | ||||||
| Related party convertible notes | 42,754 | 42,749 | ||||||
| Related party loan payable | 29,691 | — | ||||||
| Loans payable | 26,527 | 13,606 | ||||||
| Total debt | 227,043 | 151,974 | ||||||
| Less: current portion | (59,155 | ) | (36,281 | ) | ||||
| Long-term debt | $ | 167,888 | $ | 115,693 | ||||
| Mezzanine equity(1) | $ | 5,000 | $ | — | ||||
____________
(1) See Note 8, "Significant Agreements" for details regarding the Bill & Melinda Gates Foundation Investment, classified as mezzanine equity.
| 133 |
Senior Secured Loan Facility
In March 2014, the Company entered into a Loan and Security Agreement (or the LSA) with Hercules Technology Growth Capital, Inc. (or Hercules) to make available to Amyris a secured loan facility (or the Senior Secured Loan Facility) in the aggregate principal amount of up to $25.0 million, which loan facility was fully drawn at the closing. The initial loan of $25.0 million under the Senior Secured Loan Facility accrues interest at a rate per annum equal to the greater of either the prime rate reported in the Wall Street Journal plus 6.25% or 9.50%. The Company may repay the outstanding amounts under the Senior Secured Credit Facility before the maturity date (October 15, 2018) if it pays an additional fee of 1% of the outstanding loans. The Company was also required to pay a 1% facility charge at the closing of the Senior Secured Credit Facility, and is required to pay a 10% end of term charge with respect to the initial loan of $25.0 million. In connection with the entry into the LSA, Amyris agreed to certain customary representations and warranties and covenants, as well as certain covenants that were subsequently amended (as described below).
In June 2014, the Company and Hercules entered into a first amendment of the LSA. Pursuant to the first amendment, the parties agreed to extend the maturity date of the loans under the Senior Secured Loan Facility from May 31, 2015 to February 1, 2017 and remove (i) a requirement for the Company to pay a forbearance fee of $10.0 million in the event certain covenants were not satisfied, (ii) a covenant that the Company maintain positive cash flow commencing with the fiscal quarter beginning October 1, 2014, (iii) a covenant that, commencing with the fiscal quarter beginning July 1, 2014, the Company and its subsidiaries achieve certain projected cash product revenues and projected cash product gross profits, and (iv) an obligation for the Company to file a registration statement on Form S-3 with the SEC by no later than June 30, 2014 and complete an equity financing of more than $50.0 million by no later than September 30, 2014. The Company further agreed to include a new covenant in the LSA requiring the Company to maintain unrestricted, unencumbered cash in defined U.S. bank accounts in an amount equal to at least 50% of the principal amount of the loans then outstanding under the Senior Secured Loan Facility (or the “Minimum Cash Covenant”) and borrow an additional $5.0 million. The additional $5.0 million borrowing under the Senior Secured Credit Facility was completed in June 2014, and accrues interest at a rate per annum equal to the greater of (i) the prime rate reported in the Wall Street Journal plus 5.25% and (ii) 8.5%.
In March 2015, the Company and Hercules entered into a second amendment of the LSA. Pursuant to the second amendment, the parties agreed to, among other things, establish an additional credit facility in the principal amount of up to $15.0 million, which would be available to be drawn by the Company through the earlier of March 31, 2016 or such time as the Company raised an aggregate of at least $20.0 million through the sale of new equity securities. Under the terms of the second amendment, the Company agreed to pay Hercules a 3.0% facility availability fee on April 1, 2015. The Company had the ability to cancel the additional facility at any time prior to June 30, 2015 at its own option, and the additional facility would terminate upon the Company securing a new equity financing of at least $20.0 million. If the facility was not canceled, and any outstanding borrowings were not repaid, before June 30, 2015, an additional 5.0% facility fee would become payable on June 30, 2015. The Company did not cancel the facility prior to June 30, 2015, and the 5.0% facility fee became payable as of June 30, 2015. The Company did not pay the additional facility fee and thereafter received a waiver from Hercules with respect thereto. The additional facility was cancelled undrawn upon the completion of the Company’s private offering of common stock and warrants in July 2015.
| 134 |
In November 2015, the Company and Hercules entered into a third amendment of the LSA. Pursuant to the third amendment, the Company borrowed $10,960,000 (or the Third Amendment Borrowed Amount) from Hercules on November 30, 2015. As of December 1, 2015, after the funding of the Third Amendment Borrowed Amount (and including repayment of $9.1 million of principal that had occurred prior to the third amendment), the aggregate principal amount outstanding under the Senior Secured Loan Facility was approximately $31.7 million. The Third Amendment Borrowed Amount accrues interest at a rate per annum equal to the greater of (i) 9.5% and (ii) the prime rate reported in the Wall Street Journal plus 6.25%, and, like the previous loans under the Senior Secured Loan Facility, has a maturity date of October 15, 2018. Upon the earlier of the maturity date, prepayment in full or such obligations otherwise becoming due and payable, in addition to repaying the outstanding Third Amendment Borrowed Amount (and all other amounts owed under the Senior Secured Loan Facility, as amended), the Company is also required to pay an end-of-term charge of $767,200. Pursuant to the third amendment, the Company also paid Hercules fees of $1.0 million, $750,000 of which was owed in connection with the expired $15.0 million facility under the second amendment and $250,000 of which was related to the Third Amendment Borrowed Amount. Under the third amendment, the parties agreed that the Company would, commencing on December 1, 2015, be required to pay only the interest accruing on all outstanding loans under the Senior Secured Loan Facility until February 29, 2016. Commencing on March 1, 2016, the Company would have been required to begin repaying principal of all loans under the Senior Secured Loan Facility, in addition to the applicable interest. However, pursuant to the third amendment, the Company could, by achieving certain cash inflow targets in 2016, extend the interest-only period to December 1, 2016. Upon the issuance and sale by the Company of $20.0 million of unsecured promissory notes and warrants in a private placement in February 2016 for aggregate cash proceeds of $20.0 million, the Company satisfied the conditions for extending the interest-only period to May 31, 2016. On June 1, 2016, the Company commenced the repayment of outstanding principal under the Senior Secured Loan Facility. In June 2016, the Company was notified by Hercules that it had transferred and assigned its rights and obligations under the Senior Secured Loan Facility to Stegodon Corporation (Stegodon), an affiliate of Ginkgo Bioworks, Inc. (Ginkgo). On June 29, 2016, in connection with the execution by the Company and Ginkgo of an initial strategic partnership agreement, the Company received a deferment from Stegodon of all scheduled principal repayments under the Senior Secured Loan Facility, as well as a waiver of the Minimum Cash Covenant, through October 31, 2016. Refer to Note 8, “Significant Agreements” for additional details. On October 6, 2016, in connection with the execution by the Company and Ginkgo of a definitive collaboration agreement (or the Ginkgo Collaboration Agreement), the Company and Stegodon entered into a fourth amendment of the LSA, pursuant to which the parties agreed to (i) subject to the Company extending the maturity of certain of its other outstanding indebtedness (or the Extension Condition), extend the maturity date of the Senior Secured Loan Facility, (ii) make the Senior Secured Loan Facility interest-only until maturity, subject to the requirement that the Company apply certain monies received by it under the Ginkgo Collaboration Agreement to repay the amounts outstanding under the Senior Secured Loan Facility, up to a maximum amount of $1 million per month and (iii) waive the Minimum Cash Covenant until the maturity date of the Senior Secured Loan Facility. In January 2017, the Company satisfied the Extension Condition and the maturity date of the loans under the Senior Secured Credit Facility was extended to October 15, 2018. Refer to Note 16, “Subsequent Events” for additional details. In December 2016, in connection with Stegodon granting certain waivers and releases under the LSA in connection with the Company’s formation of its Neossance joint venture with Nikko, as described in more detail in Note 7, “Joint Ventures and Noncontrolling Interest”, the Company agreed to pay to Stegodon (i) a fee of $425,000 on or prior to December 31, 2017 and (ii) a fee of $450,000 on or prior to the maturity date of the loans under the Senior Secured Credit Facility. Subsequently, in January 2017 the Company and Stegodon entered into a fifth amendment of the LSA, pursuant to which the Company agreed to apply additional monies received by it under the Ginkgo Collaboration Agreement towards repayment of the outstanding loans under the Senior Secured Loan Facility, up to a maximum amount of $3 million. Refer to Note 16, “Subsequent Events” for additional details.
| 135 |
As of December 31, 2016, $27.7million was outstanding under the Senior Secured Loan Facility, net of discount and issuance costs of $0.9 million. The amount outstanding is classified as noncurrent debt at December 31, 2016 because the Company had the intent and ability to extend the maturity of the debt beyond December 31, 2017, as evidenced by the completion of the amendment of the debt terms in January 2017. The Senior Secured Loan Facility is collateralized by liens on the Company's assets, including on certain Company intellectual property. The Senior Secured Loan Facility includes customary events of default, including failure to pay amounts due, breaches of covenants and warranties, material adverse effect events, certain cross defaults and judgments, and insolvency. If an event of default occurs, Stegodon may require immediate repayment of all amounts outstanding under the Senior Secured Loan Facility.
BNDES Credit Facility
In December 2011, the Company entered into a credit facility with the Brazilian Development Bank (or BNDES and such credit facility, the BNDES Credit Facility) in the amount of R$22.4 million (approximately US$6.9 million based on the exchange rate as of December 31, 2016). This BNDES Credit Facility was extended as project financing for a production site in Brazil. The credit line was divided into an initial tranche of up to approximately R$19.1 million and an additional tranche of approximately R$3.3 million that would become available upon delivery of additional guarantees. The credit line was cancelled in 2013.
The principal of the loans under the BNDES Credit Facility is required to be repaid in 60 monthly installments, with the first installment paid in January 2013 and the last due in December 2017. Interest was due initially on a quarterly basis with the first installment due in March 2012. From and after January 2013, interest payments are due on a monthly basis together with principal payments. The loaned amounts carry interest of 7% per annum. Additionally, there is a credit reserve charge of 0.1% on the unused balance from each credit installment from the day immediately after it is made available through its date of use, when it is paid.
The BNDES Credit Facility is collateralized by a first priority security interest in certain of the Company's equipment and other tangible assets totaling R$24.9 million (approximately US$7.7 million based on the exchange rate as of December 31, 2016). The Company is a parent guarantor for the payment of the outstanding balance under the BNDES Credit Facility. Additionally, the Company was required to provide a bank guarantee equal to 10% of the total approved amount (R$22.4 million in total debt) available under the BNDES Credit Facility. For advances of the second tranche (above R$19.1 million), the Company is required to provide additional bank guarantees equal to 90% of each such advance, plus additional Company guarantees equal to at least 130% of such advance. The BNDES Credit Facility contains customary events of default, including payment failures, failure to satisfy other obligations under this credit facility or related documents, defaults in respect of other indebtedness, bankruptcy, insolvency and inability to pay debts when due, material judgments, and changes in control of Amyris Brasil. If any event of default occurs, BNDES may terminate its commitments and declare immediately due all borrowings under the facility. As of December 31, 2016 and 2015, the Company had R$3.8 million (approximately US$1.2 million based on the exchange rate as of December 31, 2016) and R$7.6 million (approximately US$1.9 million based on the exchange rate as of December 31, 2015), respectively, in outstanding advances under the BNDES Credit Facility.
FINEP Credit Facility
In November 2010, the Company entered into a credit facility with Financiadora de Estudos e Projetos (or the FINEP Credit Facility). The FINEP Credit Facility was extended to partially fund expenses related to the Company’s research and development project on sugarcane-based biodiesel (or the FINEP Project) and provided for loans of up to an aggregate principal amount of R$6.4 million (approximately US$2.0 million based on the exchange rate as of December 31, 2016), which is secured by a chattel mortgage on certain equipment of Amyris Brasil as well as by bank letters of guarantee. All available credit under this facility is fully drawn.
| 136 |
Interest on loans drawn under the FINEP Credit Facility is fixed at 5% per annum. In case of default under or non-compliance with the terms of the agreement, the interest on loans will be dependent on the long-term interest rate as published by the Central Bank of Brazil (such rate, the “TJLP”). If the TJLP at the time of default is greater than 6%, then the interest will be 5% plus a TJLP adjustment factor, otherwise the interest will be 11% per annum. In addition, a fine of up to 10% shall apply to the amount of any obligation in default. Additional interest on late balances will be 1% per month, levied on the overdue amount. Payment of the outstanding loan balance is being made in 81 monthly installments, which commenced in July 2012 and extends through March 2019. Interest on loans drawn and other charges are paid on a monthly basis and commenced in March 2011. As of December 31, 2016 and December 31, 2015, the total outstanding loan balance under this credit facility was R$2.3 million (approximately US$0.7 million based on the exchange rate as of December 31, 2016) and R$3.4 million (approximately US$0.9 million based on exchange rate as of December 31, 2015), respectively.
Guanfu Credit Facility
On October 26, 2016, the Company and Guanfu Holding Co., Ltd. (or, together with its subsidiaries, Guanfu), an existing commercial partner of the Company, entered into a credit agreement (or the Guanfu Credit Agreement) to make available to the Company an unsecured credit facility (or the Guanfu Credit Facility) with an aggregate principal amount of up to $25.0 million, which the Company could borrow from time to time in up to three closings. The effectiveness of the Guanfu Credit Agreement was subject to the parties obtaining certain required approvals, which occurred on November 16, 2016, and upon the effectiveness of the Guanfu Credit Agreement, the Company granted to Guanfu the global exclusive purchase right with respect to the Company products subject to the parties’ pre-existing commercial relationship. The initial funding of the Guanfu Credit Facility was scheduled to occur on December 1, 2016, subject to Guanfu’s right to extend such initial funding to a date no later than December 31, 2016. Guanfu exercised its right to extend the initial funding to December 31, 2016, and on such date the Company borrowed the full amount under the Guanfu Credit Facility and issued to Guanfu a note in the principal amount of $25.0 million (or the Guanfu Note). The Guanfu Note has a term of five years and will accrue interest at a rate of 10% per annum, payable quarterly beginning March 31, 2017. The Company may, at its option, repay the Guanfu Note before its maturity date, in whole or in part, at a price equal to 100% of the amount being repaid plus accrued and unpaid interest on such amount to the date of repayment.
Upon the occurrence of certain specified events of default under the Guanfu Credit Facility, the Company will grant to Guanfu an exclusive, royalty-free, global license to certain intellectual property useful in connection with Guanfu’s existing commercial relationship with the Company. In addition, in the event the Company fails to pay interest or principal under the Guanfu Note within ten days of when due, the Company will also be required, subject to applicable laws and regulations, to repay the outstanding principal amount under the Guanfu Note, together with accrued and unpaid interest, in the form of shares of the Company’s common stock at a per share price equal to 90% of the volume weighted average closing sale price of the Company’s common stock for the 90 trading days ending on and including the trading day that is two trading days preceding such default.
| 137 |
Convertible Notes
Fidelity
In February 2012, the Company completed the sale of senior unsecured convertible promissory notes in an aggregate principal amount of $25.0 million pursuant to a securities purchase agreement between the Company and certain investment funds affiliated with FMR LLC (or the Fidelity Securities Purchase Agreement). The offering consisted of the sale of 3% senior unsecured convertible promissory notes with a March 1, 2017 maturity date and an initial conversion price equal to $7.0682 per share of the Company's common stock, subject to proportional adjustment for adjustments to outstanding common stock and anti-dilution provisions in case of dividends and distributions (or the Fidelity Notes). In October 2015, the Company issued and sold $57.6 million of convertible senior notes and used approximately $8.8 million of the proceeds therefrom to repurchase $9.7 million aggregate principal amount of outstanding Fidelity Notes. As of December 31, 2016, the Fidelity Notes were convertible into an aggregate of up to 2,165,898 shares of the Company's common stock. The holders of the Fidelity Notes have a right to require repayment of 101% of the principal amount of the Fidelity Notes in an acquisition of the Company, and the Fidelity Notes provide for payment of unpaid interest on conversion following such an acquisition if the note holders do not require such repayment. The Fidelity Securities Purchase Agreement and Fidelity Notes include covenants regarding payment of interest, maintaining the Company's listing status, limitations on debt, maintenance of corporate existence, and timely filing of SEC reports. The Fidelity Notes include standard events of default resulting in acceleration of indebtedness, including failure to pay, bankruptcy and insolvency, cross-defaults and breaches of the covenants in the Fidelity Securities Purchase Agreement and Fidelity Notes, with default interest rates and associated cure periods applicable to the covenant regarding SEC reporting. Furthermore, the Fidelity Notes include restrictions on the amount of debt the Company is permitted to incur. With exceptions for certain existing debt, refinancing of such debt and certain other exclusions and waivers, the Fidelity Notes provide that the Company's total outstanding debt at any time cannot exceed the greater of $200.0 million or 50% of its consolidated total assets and its secured debt cannot exceed the greater of $125.0 million or 30% of its consolidated total assets. In connection with the Company’s closing of a short-term bridge loan for $35.0 million in October 2013 (or the Temasek Bridge Note), holders of the Fidelity Notes waived compliance with the debt limitations outlined above as to the Temasek Bridge Note and the August 2013 Financing (as defined below). In consideration for such waiver, the Company granted to holders of the Fidelity Notes or their affiliates the right to purchase up to an aggregate of $7.6 million worth of convertible promissory notes in the first tranche of the August 2013 Financing (as defined below). See "Related Party Convertible Notes" in this Note 5, "Debt" for additional details. On December 28, 2016, Company entered into an Exchange Agreement (or the Fidelity Exchange Agreement) with the holders of the outstanding Fidelity Notes. Pursuant to the Fidelity Exchange Agreement, the Company and the holders agreed to exchange (or the Fidelity Exchange) all outstanding Fidelity Notes, together with accrued and unpaid interest thereon, for approximately $19.1 million in aggregate principal amount of additional 2015 144A Notes (as defined below), representing an exchange ratio of approximately 1:1.25 (i.e., each $1.00 of Fidelity Notes would be exchanged for approximately $1.25 of additional 2015 144A Notes), in a private placement exempt from registration under the Securities Act of 1933, as amended (or the Securities Act). In January 2017, we closed the Fidelity Exchange, as described in more detail in Note 16, “Subsequent Events”. The amount outstanding at December 31, 2016 is classified as noncurrent debt at December 31, 2016 because the Company had the intent and ability to extend the maturity of the debt beyond December 31, 2017, as evidenced by the completion of the Fidelity Exchange in January 2017.
| 138 |
2014 Rule 144A Convertible Note Offering
In May 2014, the Company entered into a Purchase Agreement with Morgan Stanley & Co. LLC, as the initial purchaser (or the Initial Purchaser), relating to the sale of $75.0 million aggregate in principal amount of 6.50% Convertible Senior Notes due 2019 (or the 2014 144A Notes) to the Initial Purchaser in a private placement, and for initial resale by the Initial Purchaser to certain qualified institutional buyers (or the 2014 144A Convertible Note Offering). In addition, the Company granted the Initial Purchaser an option to purchase up to an additional $15.0 million aggregate principal amount of 2014 144A Notes, which option expired unexercised according to its terms. Under the terms of the purchase agreement for the 2014 144A Notes, the Company agreed to customary indemnification of the Initial Purchaser against certain liabilities. The Notes were issued pursuant to an Indenture, dated as of May 29, 2014 (or the 2014 Indenture), between the Company and Wells Fargo Bank, National Association, as trustee. The net proceeds from the offering of the 2014 144A Notes were approximately $72.0 million after payment of the Initial Purchaser’s discounts and offering expenses. In addition, in connection with obtaining a waiver from Total of its preexisting contractual right to exchange certain senior secured convertible notes previously issued by the Company for new notes issued in the 2014 144A Convertible Note Offering, the Company used approximately $9.7 million of the net proceeds to repay previously issued notes (representing the amount of 2014 144A Notes purchased by Total from the Initial Purchaser). Certain of the Company's affiliated entities purchased $24.7 million in aggregate principal amount of 2014 144A Notes from the Initial Purchaser (described further below under "Related Party Convertible Notes"). In October 2015, as discussed below, the Company issued $57.6 million of convertible senior notes and used approximately $18.3 million of the net proceeds therefrom to repurchase $22.9 million aggregate principal amount of outstanding 2014 144A Notes. The 2014 144A Notes bear interest at a rate of 6.50% per year, payable semiannually in arrears on May 15 and November 15 of each year, beginning November 15, 2014. The 2014 144A Notes mature on May 15, 2019, unless earlier converted or repurchased. The 2014 144A Notes are convertible into shares of the Company's common stock at any time prior to the close of business on the business day immediately preceding the maturity date of the 2014 144A Notes, at the initial conversion rate of 267.037 shares of Common Stock per $1,000 principal amount of 2014 144A Notes (subject to adjustment in certain circumstances). This represents an effective conversion price of approximately $3.74 per share of common stock. For any conversion on or after May 15, 2015, in the event that the last reported sale price of the Company’s common stock for 20 or more trading days (whether or not consecutive) in a period of 30 consecutive trading days ending within five trading days immediately prior to the date the Company receives a notice of conversion exceeds the then-applicable conversion price per share on each such trading day, the holders, in addition to the shares deliverable upon conversion, noteholders will be entitled to receive a cash payment equal to the present value of the remaining scheduled payments of interest that would have been made on the 2014 144A Notes being converted from the conversion date to the earlier of the date that is three years after the date the Company receives such notice of conversion and maturity (May 15, 2019), which will be computed using a discount rate of 0.75%. In the event of a fundamental change, as defined in the 2014 Indenture, holders of the 2014 144A Notes may require the Company to purchase all or a portion of the 2014 144A Notes at a price equal to 100% of the principal amount of the 2014 144A Notes, plus any accrued and unpaid interest to, but excluding, the fundamental change repurchase date. In addition, holders of the 2014 144A Notes who convert their 2014 144A Notes in connection with a make-whole fundamental change will, under certain circumstances, be entitled to an increase in the conversion rate of such notes. Refer to the “Exchange” and “Maturity Treatment Agreement” sections of this Note 5, "Debt", for details of the impact of the Maturity Treatment and Exchange Agreements on the 2014 144A Notes.
2015 Rule 144A Convertible Note Offering
In October 2015, the Company entered into a purchase agreement with certain qualified institutional buyers relating to the sale of $57.6 million aggregate principal amount of 9.50% Convertible Senior Notes due 2019 (or the 2015 144A Notes) to the purchasers in a private placement (or the 2015 144A Offering). The 2015 144A Notes were issued pursuant to an Indenture, dated as of October 20, 2015 (or the 2015 Indenture), between the Company and Wells Fargo Bank, National Association, as trustee. The net proceeds from the offering of the 2015 144A Notes were approximately $54.4 million after payment of the offering expenses and placement agent fees. The Company used approximately $18.3 million of the net proceeds to repurchase $22.9 million aggregate principal amount of outstanding 2014 144A Notes and approximately $8.8 million to repurchase $9.7 million aggregate principal amount of outstanding Fidelity Notes, in each case held by purchasers of the 2015 144A Notes. The 2015 144A Notes bear interest at a rate of 9.50% per year, payable semiannually in arrears on April 15 and October 15 of each year, beginning April 15, 2016. Interest on the 2015 144A Notes is payable, at the Company’s option, entirely in cash or entirely in common stock. The Company elected to make the April 15, 2016 interest payment in shares of common stock and the October 15, 2016 interest payment in cash. The 2015 144A Notes will mature on April 15, 2019 unless earlier converted or repurchased.
| 139 |
The 2015 144A Notes are convertible into shares of the Company's common stock at any time prior to the close of business on April 15, 2019. The 2015 144A Notes had an initial conversion rate of 443.6557 shares of Common Stock per $1,000 principal amount of 2015 144A Notes (subject to adjustment in certain circumstances). This represented an initial effective conversion price of approximately $2.25 per share of common stock. Following the issuance by the Company of warrants to purchase common stock in a private placement transaction in February 2016 and the issuance by the Company of convertible notes in May and September 2016, as described below, the conversion rate of the 2015 144A Notes was 446.6719 shares of Common Stock per $1,000 principal amount of 2015 144A Notes as of December 31, 2016. Furthermore, following the issuance by the Company of additional convertible notes in October 2016, the conversion rate of the 2015 144A Notes is 446.8707 shares of Common Stock per $1,000 principal amount of 2015 144A Notes as of the date hereof, representing an effective conversion price of approximately $2.24 per share of common stock. For any conversion on or after November 27, 2015, in addition to the shares deliverable upon conversion, noteholders will be entitled to receive a payment equal to the present value of the remaining scheduled payments of interest that would have been made on the 2015 144A Notes being converted from the conversion date to the earlier of the date that is three years after the date the Company receives such notice of conversion and maturity (April 15, 2019), which will be computed using a discount rate of 0.75%. The Company may make such payment (the “Early Conversion Payment”) either in cash or in common stock, at its election, provided that it may only make such payment in common stock if such common stock is not subject to restrictions on transfer under the Securities Act by persons other than the Company’s affiliates. If the Company elects to pay an Early Conversion Payment in common stock, then the stock will be valued at 92.5% of the simple average of the daily volume-weighted average price per share for the 10 trading days ending on and including the trading day immediately preceding the conversion date. Through December 31, 2016, the Company has elected to make each Early Conversion Payment in shares of common stock. In the event of a fundamental change, as defined in the 2015 Indenture, holders of the 2015 144A Notes may require the Company to purchase all or a portion of the 2015 144A Notes at a price equal to 100% of the principal amount of the 2015 144A Notes, plus any accrued and unpaid interest to, but excluding, the fundamental change repurchase date. In addition, holders of the 2015 144A Notes who convert their 2015 144A Notes in connection with a make-whole fundamental change will, under certain circumstances, be entitled to an increase in the conversion rate. The issuance of shares of common stock upon conversion of the 2015 144A Notes, upon the Company’s election to pay interest on the 2015 144A Notes in shares of common stock and upon the Company’s election to pay the Early Conversion Payment in shares of common stock in an aggregate amount in excess of 38,415,626 shares of the Company’s common stock was subject to stockholder approval, which was obtained on May 17, 2016. With exceptions for certain existing debt, refinancing of such debt and certain other exclusions and waivers, the 2015 144A Notes provide that, as long as the aggregate outstanding principal amount of the 2015 144A Notes exceeds $25.0 million, the Company's outstanding unsecured debt at any time cannot exceed $200.0 million and its secured debt cannot exceed the greater of $65.0 million or 30% of its consolidated total assets. In January 2017, the Company issued an additional $19.1 million in aggregate principal amount of 2015 144A Notes in exchange for the cancellation of $15.3 million in aggregate principal amount of outstanding Fidelity Notes, as described in more detail in Note 16, “Subsequent Events”.
| 140 |
May 2016 Convertible Note Offering
In May 2016, the Company entered into a securities purchase agreement (or the May 2016 Purchase Agreement) between the Company and a private investor relating to the sale of up to $15.0 million aggregate principal amount of convertible notes (or the May 2016 Convertible Notes) that are convertible into shares of the Company’s common stock at an initial conversion price of $1.90 per share. The conversion price will be subject to adjustment in the event of any stock split, reverse stock split, recapitalization, reorganization or similar transaction. The May 2016 Purchase Agreement includes customary representations, warranties and covenants by the Company. The May 2016 Purchase Agreement also provides the purchaser with a right of first refusal with respect to any variable rate transaction on the same terms and conditions as are offered to a third-party purchaser for as long as the purchaser holds any May 2016 Convertible Notes or shares of the Company’s common stock underlying the May 2016 Convertible Notes.
Pursuant to the May 2016 Purchase Agreement, the May 2016 Convertible Notes were to be issued and sold in two separate closings. The initial closing occurred on May 10, 2016. At the initial closing, the Company issued and sold a May 2016 Convertible Note in a principal amount of $10.0 million to the purchaser, resulting in net proceeds to the Company of approximately $9.9 million. The second closing was to occur on the first trading day following the completion of the first three installment periods under the May 2016 Convertible Notes and the satisfaction or waiver of certain other closing conditions, including certain equity conditions, such as that no Triggering Event (as defined below) had occurred. At the second closing, the Company was to issue and sell a May 2016 Convertible Note in a principal amount of $5.0 million to the purchaser, resulting in expected net proceeds to the Company of approximately $5.0 million. On September 2, 2016, in connection with the Company and the purchaser waiving certain conditions to the second closing under the May 2016 Purchase Agreement, the Company issued and sold an additional May 2016 Convertible Note in the principal amount of $3.0 million to the purchaser, for proceeds to the Company of approximately $3.0 million, and granted the purchaser the option to purchase a further May 2016 Convertible Note in the principal amount of $2.0 million (or the Remaining May 2016 Note), representing the remaining May 2016 Convertible Notes provided for in the May 2016 Purchase Agreement, on or before December 31, 2016. On October 13, 2016, the Company issued and sold the Remaining May 2016 Note to the purchaser for proceeds to the Company of $2.0 million.
The May 2016 Convertible Notes are general unsecured obligations of the Company. Unless earlier converted or redeemed, the May 2016 Convertible Notes will mature on the 18-month anniversary of their respective issuance, subject to the rights of the holders to extend the maturity date in certain circumstances.
The May 2016 Convertible Notes are payable in monthly installments, in either cash at 118% of such installment amount or, at the Company’s option, subject to the satisfaction of certain equity conditions, shares of common stock at a discount to the then-current market price, subject to a price floor. In addition, in the event that the Company elects to pay all or any portion of a monthly installment in common stock, the holders of the May 2016 Convertible Notes shall have the right to require that the Company repay in common stock an additional amount of the May 2016 Convertible Notes not to exceed 50% of the cumulative sum of the aggregate amounts by which the dollar-weighted trading volume of the Company’s common stock for all trading days during the applicable installment period exceeds $200,000. The Company elected to make the May, June, July, August, September and October 2016 installment payments on the May 2016 Convertible Notes in shares of common stock. As of October 31, 2016, all May 2016 Convertible Notes had been repaid in full.
| 141 |
The May 2016 Convertible Notes contain customary terms and covenants, including certain events of default, including failure to pay amounts due, breaches of warranties, material adverse effect events, certain cross defaults and judgments, and insolvency, after which the holders may require the Company to redeem all or any portion of their May 2016 Convertible Notes in cash at a price equal to the greater of (i) 118% of the amount being redeemed and (ii) the intrinsic value of the shares of common stock issuable upon an installment payment of the amount being redeemed in shares.
In the event of a Fundamental Transaction (as defined in the May 2016 Convertible Notes), holders of the May 2016 Convertible Notes may require the Company to redeem all or any portion of their May 2016 Convertible Notes at a price equal to the greater of (i) 118% of the amount being redeemed and (ii) the intrinsic value of the shares of common stock issuable upon an installment payment of the amount being redeemed in shares.
The Company has the right to redeem the May 2016 Convertible Notes for cash, in whole, at any time, or in part, from time to time, at a redemption price equal to 118% of the principal amount of the May 2016 Convertible Notes being redeemed. In addition, if the volume-weighted average price of the Company’s common stock is (i) less than $1.00 for 30 consecutive trading days or (ii) less than $0.50 for five consecutive trading days (each, a “Triggering Event”) within four months of the issuance of any 2016 Convertible Notes, the Company will have the option to redeem such May 2016 Convertible Notes in whole for cash at a redemption price equal to 112% of the principal amount of such May 2016 Convertible Notes.
Notwithstanding the foregoing, the holders will not have the right to convert any portion of a May 2016 Convertible Note, and the Company may not issue shares of common stock upon conversion or repayment of the May 2016 Convertible Notes, if (a) the holder, together with its affiliates, would beneficially own in excess of 4.99% (or such other percentage as determined by the holder and notified to the Company in writing, not to exceed 9.99%, provided that any increase of such percentage will not be effective until 61 days after notice thereof) of the number of shares of the Company’s common stock outstanding immediately after giving effect to such conversion or payment, as applicable, or (b) the aggregate number of shares issued with respect to the May 2016 Convertible Notes after giving effect to such conversion or payment, as applicable, would exceed 19.99% of the number of shares of the Company’s common stock outstanding as of May 10, 2016. In the event that the Company is prohibited from issuing any shares of common stock in respect of the May 2016 Convertible Notes as a result of such limits, the Company will pay cash in lieu of any shares that would otherwise be deliverable in excess thereof.
For as long as they hold the May 2016 Convertible Notes or shares of common stock issued under the May 2016 Convertible Notes, the holders may not sell any shares of the Company’s common stock at a price less than $1.05 per share; provided, that with respect to any shares of common stock issued under the May 2016 Convertible Notes at a price less than $1.00, the holders may sell such shares at a price not less than the price floor applicable to the installment period with respect to which such shares were issued.
| 142 |
December 2016 Convertible Note Offering
On December 1, 2016, the Company entered into a securities purchase agreement (or the December 2016 Purchase Agreement) with a private investor relating to the sale of a convertible note in the original principal amount $10.0 million (or the December 2016 Convertible Note) that is convertible into shares of the Company’s common stock at an initial conversion price of $1.90 per share. The conversion price will be subject to adjustment in the event of any stock split, reverse stock split, recapitalization, reorganization or similar transaction. The December 2016 Purchase Agreement includes customary representations, warranties and covenants by the Company. The Purchase Agreement also provides the purchaser with a right of first refusal with respect to any variable rate transaction, subject to certain exceptions, on the same terms and conditions as are offered to a third-party purchaser for as long as the purchaser holds the December 2016 Convertible Note or shares of common stock underlying the December 2016 Convertible Note.
The December 2016 Convertible Note was issued on December 2, 2016, resulting in net proceeds to the Company of approximately $9.9 million.
The December 2016 Convertible Note is a general unsecured obligation of the Company. Unless earlier converted or redeemed, the December 2016 Convertible Note will mature on May 1, 2018, subject to the rights of the holder to extend the maturity date in certain circumstances.
The December 2016 Convertible Note is payable in monthly installments, beginning January 1, 2017, in either cash at 118% of such installment amount or, at the Company’s option, subject to the satisfaction of certain equity conditions, shares of common stock at a discount to the then-current market price, subject to a price floor. In addition, between December 1, 2016 and December 31, 2016, or in the event that the Company elects to pay all or any portion of a monthly installment in common stock, the holder of the December 2016 Convertible Note shall have the right to require that the Company repay in common stock an additional amount of the December 2016 Convertible Note not to exceed 50% of the cumulative sum of the aggregate amounts by which the dollar-weighted trading volume of the Company’s common stock for all trading days during the applicable period exceeds $200,000.
The December 2016 Convertible Note contains customary terms and covenants, including certain events of default, including failure to pay amounts due, breaches of warranties, material adverse effect events, certain cross defaults and judgments, and insolvency, after which the holders may require the Company to redeem all or any portion of their December 2016 Convertible Note in cash at a price equal to the greater of (i) 118% of the amount being redeemed and (ii) the intrinsic value of the shares of common stock issuable upon an installment payment of the amount being redeemed in shares.
In the event of a Fundamental Transaction (as defined in the December 2016 Convertible Note), the holder of the December 2016 Convertible Note may require the Company to purchase all or any portion of its December 2016 Convertible Note at a price equal to the greater of (i) 118% of the amount being redeemed and (ii) the intrinsic value of the shares of Common Stock issuable upon an installment payment of the amount being redeemed in shares.
The Company has the right to redeem the December 2016 Convertible Note for cash, in whole, at any time, or in part, from time to time, at a redemption price equal to 118% of the principal amount of the December 2016 Convertible Note to be redeemed.
| 143 |
Notwithstanding the foregoing, the holder will not have the right to convert any portion of the December 2016 Convertible Note, and the Company will not have the option to pay any amount in shares of common stock, if (a) the holder, together with its affiliates, would beneficially own in excess of 4.99% (or such other percentage as determined by the holder and notified to the Company in writing, not to exceed 9.99%, provided that any increase of such percentage will not be effective until 61 days after notice thereof) of the number of shares of the Company’s common stock outstanding immediately after giving effect to such conversion or payment, as applicable, or (b) the aggregate number of shares issued with respect to the December 2016 Convertible Note (and any other transaction aggregated for such purpose) after giving effect to such conversion or payment, as applicable, would exceed 19.99% of the number of shares of the Company’s common stock outstanding as of December 1, 2016. In the event that the Company is prohibited from issuing any shares of common stock in respect of the December 2016 Convertible Note as a result of such limits, the Company will pay cash in lieu of any shares that would otherwise be deliverable in excess thereof.
For as long as it holds the December 2016 Convertible Note or shares of common stock issued under the December 2016 Convertible Note, the holder may not sell any shares of the Company’s common stock at a price less than $1.05 per share; provided, that with respect to any shares of common stock issued under the December 2016 Convertible Note at a price less than $1.00, the holder may sell such shares at a price not less than the price floor applicable to the period with respect to which such shares were issued. The embedded derivative features in this instrument are separately accounted for and the fair value of such features was not material at issuance of the instrument and at December 31, 2016.
Related Party Convertible Notes
Total R&D Convertible Notes
In July 2012 and December 2013, the Company entered into a series of agreements (or the Total Fuel Agreements) with Total Energies Nouvelles Activités USA (formerly known as Total Gas & Power USA, SAS, and referred to as Total) to establish a research and development program (or the Program) and form a joint venture (or the Fuels JV) with Total to produce and commercialize farnesene- or farnesane-based diesel and jet fuels, and established a convertible debt structure for the collaboration funding from Total.
The purchase agreement for the notes related to the funding from Total (or the Total Purchase Agreement) provided for the sale of an aggregate of $105.0 million in 1.5% Senior Unsecured Convertible Notes due March 2017 (or the Unsecured R&D Notes) as follows:
| • | As part of an initial closing under the purchase agreement (which was completed in two installments), (i) on July 30, 2012, the Company sold an Unsecured R&D Note with a principal amount of $38.3 million, including $15.0 million in new funds and $23.3 million in previously-provided diesel research and development funding by Total, and (ii) on September 14, 2012, the Company sold another Unsecured R&D Note for $15.0 million in new funds from Total. These Unsecured R&D Notes had an initial conversion price of $7.0682 per share. |
| • | At a second closing under the Total Purchase Agreement (also completed in two installments) the Company sold additional Unsecured R&D Notes for an aggregate of $30.0 million in new funds from Total ($10.0 million in June 2013 and $20.0 million in July 2013). These Unsecured R&D Notes had an initial conversion price of $3.08 per share, as described below. |
| 144 |
| • | At a third closing under the Total Purchase Agreement (also completed in two installments) the Company sold additional Unsecured R&D Notes for an aggregate of $21.7 million in new funds from Total ($10.85 million in July 2014 and $10.85 million in January 2015). These Unsecured R&D Notes had an initial conversion price of $4.11 per share, as described below. |
In March 2013, the Company entered into a letter agreement with Total (or the March 2013 Letter Agreement) under which Total agreed to waive its right to cease its participation in the parties' fuels collaboration at the July 2013 decision point and committed to proceed with the July 2013 funding tranche of $30.0 million (subject to the Company's satisfaction of the relevant closing conditions for such funding in the Total Purchase Agreement). As consideration for this waiver and commitment, the Company agreed to:
| • | Reduce the conversion price for the $30.0 million in principal amount of Unsecured R&D Notes to be issued in connection with the second closing of the Unsecured R&D Notes (as described above) from $7.0682 per share to a price per share equal to the greater of (i) the consolidated closing bid price of the Company's common stock on the date of the March 2013 Letter Agreement, plus $0.01, and (ii) $3.08 per share, provided that the conversion price would not be reduced by more than the maximum possible amount permitted under the rules of The NASDAQ Stock Market (or NASDAQ) such that the new conversion price would require the Company to obtain stockholder consent; and |
| • | Grant Total a senior security interest in the Company's intellectual property, subject to certain exclusions and subject to release by Total when the Company and Total enter into final documentation regarding the establishment of the Fuels JV. |
In addition to the waiver by Total described above, Total also agreed that, at the Company's request and contingent upon the Company meeting its obligations described above, it would pay advance installments of the amounts otherwise payable at the second closing.
In June 2013, the Company sold and issued $10.0 million in principal amount of Unsecured R&D Notes to Total pursuant to the second closing of the Unsecured R&D Notes as discussed above. In accordance with the March 2013 Letter Agreement, this Unsecured R&D Note had an initial conversion price equal to $3.08 per share of the Company's common stock.
In July 2013, the Company sold and issued $20.0 million in principal amount of Unsecured R&D Notes to Total pursuant to the Total second closing of the Unsecured R&D Notes as discussed above. This purchase and sale completed Total's commitment to purchase $30.0 million of the Unsecured R&D Notes in the second closing by July 2013. In accordance with the March 2013 Letter Agreement, this Unsecured R&D Note has an initial conversion price equal to $3.08 per share of the Company's common stock.
In December 2013, in connection with the Company's entry into a Shareholders Agreement dated December 2, 2013 and License Agreement dated December 2, 2013 (or, collectively, the JV Documents) with Total and Total Amyris BioSolutions B.V. (or TAB) relating to the establishment of TAB (see Note 7, "Joint Ventures and Noncontrolling Interest"), the Company (i) exchanged the $69.0 million of the then-outstanding Unsecured R&D Notes issued pursuant to the Total Purchase Agreement for replacement 1.5% Senior Secured Convertible Notes due March 2017 (or the Secured R&D Notes, and together with the Unsecured R&D Notes, the R&D Notes), in principal amounts equal to the principal amount of each cancelled note and with substantially similar terms except that such replacement notes were secured, (ii) in connection therewith, granted to Total a security interest in and lien on all of the Company’s rights, title and interest in and to the Company’s shares in the capital of TAB as security for such Secured R&D Notes and (iii) agreed that any securities to be purchased and sold at the third closing under the Total Purchase Agreement by Total would be Secured R&D Notes instead of Unsecured R&D Notes. As a consequence of executing the JV Documents and forming TAB, the security interest in all of the Company's intellectual property, granted by the Company in favor of Total, Maxwell (Mauritius) Pte Ltd (or Temasek), and certain entities affiliated with FMR LLC (or the Fidelity Entities) pursuant to the Restated Intellectual Property Security Agreement dated as of October 16, 2013, was automatically terminated effective as of December 2, 2013 upon Total’s and the Company’s joint written notice to Temasek and the Fidelity Entities.
| 145 |
In April 2014, the Company and Total entered into a letter agreement dated as of March 29, 2014 (or the March 2014 Total Letter Agreement) to amend the Amended and Restated Master Framework Agreement entered into as of December 2, 2013 (included as part of JV Documents) and the Total Purchase Agreement. Under the March 2014 Total Letter Agreement, the Company agreed to (i) amend the conversion price of the Secured R&D Notes to be issued in the third closing under the Total Purchase Agreement from $7.0682 per share to $4.11 per share subject to stockholder approval at the Company's 2014 annual meeting (which was obtained in May 2014), (ii) extend the period during which Total may exchange for other Company securities Secured R&D Notes issued under the Total Fuel Agreements from June 30, 2014 to the later of December 31, 2014 and the date on which the Company shall have raised $75.0 million of equity and/or convertible debt financing (excluding any convertible promissory notes issued pursuant to the Total Purchase Agreement), (iii) eliminate the Company’s ability to qualify, in a disclosure letter to Total, certain of the representations and warranties that the Company must make at the closing of any third closing sale, and (iv) beginning on March 31, 2014, provide Total with monthly reporting on the Company’s cash, cash equivalents and short-term investments. In consideration of these agreements, Total agreed to waive its right not to consummate the third closing under the Total Purchase Agreement if it had decided not to proceed with the collaboration and had made a "No-Go" decision with respect thereto.
In July 2014, the Company sold and issued a Secured R&D Note to Total with a principal amount of $10.85 million with a March 1, 2017 maturity date pursuant to the Total Purchase Agreement. This purchase and sale constituted the initial installment of the $21.7 million third closing described above. In accordance with the March 2014 Total Letter Agreement, this Secured R&D Note had an initial conversion price equal to $4.11 per share of the Company's common stock.
In January 2015, the Company sold and issued a Secured R&D Note to Total with a principal amount of $10.85 million with a March 1, 2017 maturity date pursuant to the Total Purchase Agreement. This purchase and sale constituted the final installment of the $21.7 million third closing described above. In accordance with the March 2014 Total Letter Agreement, this Secured R&D Note had an initial conversion price equal to $4.11 per share of the Company's common stock.
In July 2015, Total exchanged all but $5.0 million of Secured R&D Notes then held by Total, such cancelled notes having an aggregate principal amount of $70 million, in exchange for approximately 30.4 million shares of the Company’s common stock in connection with the Exchange. Refer to the “Exchange” section of this Note 5, "Debt", for additional details of the impact of the Exchange on the R&D Notes.
| 146 |
In March 2016, in connection with the restructuring of TAB (see Note 7, "Joint Ventures and Noncontrolling Interest"), the Company sold to Total one half of the Company’s ownership stake in TAB (giving Total an aggregate ownership stake of 75% of TAB and giving the Company an aggregate ownership stake of 25% of TAB) in exchange for Total cancelling (i) approximately $1.3 million of Secured R&D Notes, plus all paid-in-kind and accrued interest under all outstanding Secured R&D Notes ($2.8 million, including all such interest that was outstanding as of July 29, 2015) and (ii) a note in the principal amount of Euro 50,000, plus accrued interest, issued to Total in connection with the original capitalization of TAB. To satisfy its purchase obligation above, Total surrendered to the Company the remaining Secured R&D Note of approximately $5.0 million in principal amount, and the Company executed and delivered to Total a new Unsecured R&D Note in the principal amount of $3.7 million. The disposal of the 25% ownership stake in the Fuels JV resulted in a gain to the Company of $4.2 million, which was recognized as a capital contribution from Total within equity.
As of December 31, 2016 and December 31, 2015, $3.7 million and $5.0 million, respectively, of R&D Notes were outstanding. As of December 31, 2016, the outstanding R&D Notes had a maturity date of March 1, 2017 and a conversion price equal to $3.08 per share, subject to certain adjustments as described below. In February 2017, the Company and Total agreed to extend the maturity of the outstanding R&D Notes from March 1, 2017 to May 15, 2017. See Note 16, “Subsequent Events” for additional details. The R&D Notes bear interest of 1.5% per annum (with a default rate of 2.5%), accruing from the date of issuance and payable at maturity or on conversion or a change of control where Total exercises the right to require the Company to repay the notes, as described below.
The R&D Notes become convertible into the Company's common stock (i) within 10 trading days prior to maturity, (ii) on a change of control of the Company, and (iii) on a default by the Company. The conversion price of the R&D Notes are subject to adjustment for proportional adjustments to outstanding common stock and under anti-dilution provisions in case of certain dividends and distributions. Total has a right to require repayment of 101% of the principal amount of the R&D Notes in the event of a change of control of the Company and the R&D Notes provide for payment of unpaid future interest through the maturity date on conversion following such a change of control, subject to a cap, if Total does not require such repayment. The Total Purchase Agreement and R&D Notes include covenants regarding payment of interest, maintenance of the Company's listing status, limitations on debt, maintenance of corporate existence, and filing of SEC reports. The R&D Notes include standard events of default resulting in acceleration of indebtedness, including failure to pay, bankruptcy and insolvency, cross-defaults, and breaches of the covenants in the Total Purchase Agreement and R&D Notes, with added default interest rates and associated cure periods applicable to the covenant regarding SEC reporting. Furthermore, with exceptions for certain existing debt, refinancing of such debt and certain other exclusions and waivers, the R&D Notes provide that the Company's total outstanding debt at any time may not exceed the greater of $200.0 million or 50% of its consolidated total assets and its secured debt may not exceed the greater of $125.0 million or 30% of its consolidated total assets.
| 147 |
August 2013 Financing Convertible Notes and Temasek Bridge Note
In August 2013, the Company entered into a Securities Purchase Agreement (or the August 2013 SPA) with Total and Temasek to sell up to $73.0 million in convertible promissory notes in private placements (or the August 2013 Financing), with such notes to be sold and issued over a period of up to 24 months from the date of signing. The August 2013 SPA provided for the August 2013 Financing to be divided into two tranches (the first tranche for $42.6 million and the second tranche for $30.4 million), each with differing closing conditions. Of the total possible purchase price in the financing, $25.0 million was to be paid in the form of cash by Temasek ($25.0 million in the second tranche), $35.0 million was to be paid by the exchange and cancellation of the Temasek Bridge Note, as described below, and $13.0 million was to be paid by the exchange and cancellation of outstanding R&D Notes held by Total in connection with its exercise of pro rata rights ($7.6 million in the first tranche and $5.4 million in the second tranche). The August 2013 SPA included requirements that the Company meet certain production milestones before the second tranche would become available, obtain stockholder approval prior to completing any closing of the transaction, and issue a warrant to Temasek to purchase 1,000,000 shares of the Company's common stock at an exercise price of $0.01 per share, initially exercisable only if Total converted R&D Notes previously issued to Total in the second closing under the Total Purchase Agreement. In September 2013, prior to the initial closing of the August 2013 Financing, the Company's stockholders approved the issuance in a private placement of up to $110.0 million aggregate principal amount of senior convertible promissory notes, the issuance of a warrant to purchase 1,000,000 shares of the Company's common stock and the issuance of the common stock issuable upon conversion or exercise of such notes and warrant, which approval included the transactions contemplated by the August 2013 Financing.
In October 2013, the Company sold and issued a senior secured promissory note to Temasek (or the Temasek Bridge Note) in exchange for a bridge loan of $35.0 million. The Temasek Bridge Note was due on February 2, 2014 and accrued interest at a rate of 5.5% quarterly from the October 4, 2013 date of issuance. The Temasek Bridge Note was cancelled on October 16, 2013 as payment for Temasek’s purchase of Tranche I Notes in the first tranche of the August 2013 Financing, as further described below.
In October 2013, the Company amended the August 2013 SPA to include the investment by the Fidelity Entities in the first tranche of the August 2013 Financing of $7.6 million, and to proportionally increase the amount of first tranche notes acquired by exchange and cancellation of outstanding R&D Notes held by Total in connection with its exercise of pro rata rights up to $9.2 million in the first tranche. Also in October 2013, the Company completed the closing of the first tranche of senior convertible notes provided for in the August 2013 Financing (or the Tranche I Notes), issuing a total of $51.8 million in Tranche I Notes for cash proceeds of $7.6 million and exchange and cancellation of outstanding convertible promissory notes of $44.2 million, of which $35.0 million resulted from the exchange and cancellation of the Temasek Bridge Note and the remaining $9.2 million from the exchange and cancellation of an outstanding R&D Note held by Total. As a result of the exchange and cancellation of the $35.0 million Temasek Bridge Note and the $9.2 million R&D Note held by Total for the Tranche I Notes, the Company recorded a loss from extinguishment of debt of $19.9 million. The Tranche I Notes are due sixty months from the date of issuance and were initially convertible into the Company’s common stock at a conversion price equal to $2.44 per share, which represents a 15% discount to the trailing 60-day weighted-average closing price of the common stock on The NASDAQ Stock Market (or NASDAQ) through August 7, 2013, subject to certain adjustments as described below. The Tranche I Notes are convertible at the option of the holder: (i) at any time after 18 months from the date of the August 2013 SPA, (ii) on a change of control of the Company and (iii) upon the occurrence of an event of default. Each Tranche I Note accrues interest from the date of issuance until the earlier of the date that such Tranche I Note is converted into the Company’s common stock or is repaid in full. Interest accrues on the Tranche I Notes at a rate of 5% per six months, compounded semiannually (with graduated interest rates of 6.5% applicable to the first 180 days and 8% applicable thereafter as the sole remedy should the Company fail to maintain NASDAQ listing status or of 6.5% for all other defaults). Interest for the first 30 months is payable in kind and added to the principal every six months and thereafter, the Company may continue to pay interest in kind by adding to the principal every six months or may elect to pay interest in cash. Through December 31, 2016, the Company has elected to pay interest on the Tranche I Notes in kind. The Tranche I Notes may be prepaid by the Company on the 30-month anniversary of the issuance date, and thereafter every six months at the date of payment of the semi-annual coupon.
| 148 |
In December 2013, the Company further amended the August 2013 SPA to sell $3.0 million of senior convertible notes under the second tranche of the August 2013 Financing (or the Tranche II Notes) to funds affiliated with Wolverine Asset Management, LLC (or Wolverine) and the Company elected to call $25.0 million in additional funds from Temasek pursuant to its previous commitment to purchase such amount of Tranche II Notes. In January 2014, the Company sold and issued, for face value, approximately $34.0 million of Tranche II Notes in the second tranche of the August 2013 Financing. At the closing, Temasek purchased $25.0 million of the Tranche II Notes and funds affiliated with Wolverine purchased $3.0 million of the Tranche II Notes, each for cash. Total purchased approximately $6.0 million of the Tranche II Notes through exchange and cancellation of the same amount of principal of previously outstanding R&D Notes held by Total. As a result of the exchange and cancellation of the $6.0 million R&D Note held by Total for the Tranche II Notes, the Company recorded a loss from extinguishment of debt of $9.4 million. The Tranche II Notes will be due sixty months from the date of issuance and were initially convertible into shares of common stock at a conversion price equal to $2.87 per share, which represents the trailing 60-day weighted-average closing price of the common stock on NASDAQ through August 7, 2013, subject to certain adjustments as described below. The Tranche II Notes are convertible at the option of the holder (i) at any time after the 12 month anniversary of the issue date, (ii) on a change of control of the Company and (iii) upon the occurrence of an event of default. Each Tranche II Note will accrue interest from the date of issuance until the earlier of the date that such Tranche II Note is converted into common stock or repaid in full. Interest will accrue on the Tranche II Notes at a rate per annum equal to 10%, compounded annually (with graduated interest rates of 13% applicable to the first 180 days and 16% applicable thereafter as the sole remedy should the Company fail to maintain NASDAQ listing status or of 12% for all other defaults). Interest for the first 36 months shall be payable in kind and added to principal every year following the issue date and thereafter, the Company may continue to pay interest in kind by adding to the principal on every year anniversary of the issue date or may elect to pay interest in cash.
The conversion prices of the Tranche I Notes and Tranche II Notes are subject to adjustment (i) according to proportional adjustments to outstanding common stock of the Company in case of certain dividends and distributions, (ii) according to anti-dilution provisions, and (iii) with respect to notes held by any purchaser other than Total, in the event that Total exchanges existing convertible notes for new securities of the Company in connection with future financing transactions in excess of its pro rata amount. The holders have a right to require repayment of 101% of the principal amount of the notes in the event of a change of control of the Company and the notes provide for payment of unpaid interest on conversion following such a change of control if the purchasers do not require such repayment. The August 2013 SPA, Tranche I Notes and Tranche II Notes include covenants regarding payment of interest, maintenance of the Company’s listing status, limitations on debt and on certain liens, maintenance of corporate existence, and filing of SEC reports. The Tranche I Notes and Tranche II Notes include standard events of default including failure to pay, bankruptcy and insolvency, cross-defaults, and breaches of the covenants in the August 2013 SPA, Tranche I Notes and Tranche II Notes, after which such notes may be due and payable immediately, as well as associated default interest rates as set forth above.
| 149 |
In July 2015, Temasek exchanged all of the Tranche I and Tranche II Notes then held by Temasek, such notes having an aggregate principal amount of approximately $71.0 million, in exchange for approximately 30.86 million shares of the Company’s common stock in connection with the Exchange. Refer to the “Exchange” section of this Note 5, "Debt", for additional details of the impact of the Exchange on the Tranche I Notes and Tranche II Notes.
The conversion price of the Tranche I Notes and Tranche II Notes was reduced to $1.42 per share upon the completion of a private placement of common stock and warrants to purchase common stock in July 2015, as described below. Following the issuance by the Company of warrants to purchase common stock in a private placement transaction in February 2016, as described below, the conversion price of the Tranche I Notes and Tranche II Notes was further adjusted to $1.40 per share, and following the sale by the Company of shares of common stock to the Bill & Melinda Gates Foundation in May 2016, as described below, the conversion price of the Tranche I Notes and Tranche II Notes was further adjusted to $1.14 per share.
As of December 31, 2016 and December 31, 2015, the related party convertible notes outstanding under the Tranche I Notes and Tranche II Notes were $21.8 million and $23.3 million, respectively, net of debt discount of $0.0 million and $0.0 million, respectively. Refer to the “Exchange” and “Maturity Treatment Agreement” sections of this Note 5, "Debt", for details of the impact of the Maturity Treatment and Exchange Agreements on the Tranche I and Tranche II Notes.
2014 144A Notes Sold to Related Parties
As of December 31, 2016 and December 31, 2015, the related party convertible notes outstanding under the 2014 144A Offering were $17.3 million and $14.6 million, respectively, net of discount and issuance costs of $7.4 million and $10.1 million, respectively.
As of December 31, 2016 and December 31, 2015, the total related party convertible notes outstanding were $42.8 million and $42.8 million, respectively, net of discount and issuance costs of $5.4 million and $4.9 million, respectively.
Loans Payable
In July 2012, the Company entered into a Note of Bank Credit and a Fiduciary Conveyance of Movable Goods Agreement (together, the July 2012 Bank Agreements) with each of Nossa Caixa Desenvolvimento (or Nossa Caixa) and Banco Pine S.A. (or Banco Pine). Under the July 2012 Bank Agreements, the Company pledged certain farnesene production assets as collateral for the loans of R$52.0 million. The Company's total acquisition cost for such pledged assets was approximately R$68.0 million (approximately US$20.9 million based on the exchange rate as of December 31, 2016). The Company is also a parent guarantor for the payment of the outstanding balance under these loan agreements. Under the July 2012 Bank Agreements, the Company could borrow an aggregate of R$52.0 million (approximately US$16.0 million based on the exchange rate as of December 31, 2016) as financing for capital expenditures relating to the Company's manufacturing facility located in Brotas, Brazil. Specifically, Banco Pine, agreed to lend R$22.0 million and Nossa Caixa agreed to lend R$30.0 million. The funds for the loans are provided by BNDES, but are guaranteed by the lenders. The loans have a final maturity date of July 15, 2022 and bear a fixed interest rate of 5.5% per year. The loans are also subject to early maturity and delinquency charges upon occurrence of certain events including interruption of manufacturing activities at the Company's manufacturing facility in Brotas, Brazil for more than 30 days, except during the sugarcane off-season. For the first two years that the loans are outstanding, the Company is required to pay interest only on a quarterly basis. Since August 15, 2014, the Company has been required to pay equal monthly installments of both principal and interest for the remainder of the term of the loans. As of December 31, 2016 and December 31, 2015, a principal amount of R$36.3 million (approximately US$11.1 million based on the exchange rate as of December 31, 2016) and R$43.0 million (approximately US$11.0 million based on the exchange rate as of December 30, 2015), respectively, was outstanding under these loan agreements.
| 150 |
In March 2014, the Company entered into an export financing agreement with Banco ABC Brasil S.A. (or ABC Brasil) for approximately $2.2 million to fund exports through March 2015. This loan is collateralized by future exports from the Company's subsidiary in Brazil. In April 2015, we entered into an additional export financing agreement with ABC Brasil for approximately $1.6 million to fund exports through March 2016. This loan is collateralized by future exports from the Company's subsidiary in Brazil. As of December 31, 2016, the aggregate principal amount outstanding under the ABC Brasil financing agreements was zero ($1.6 million at December 31, 2015). The Company was also a parent guarantor for the payment of the outstanding balance under these loan agreements.
Exchange (Debt Conversion - Related Party Transaction)
On July 29, 2015, the Company closed the "Exchange" pursuant to that certain Exchange Agreement, dated as of July 26, 2015 (or the Exchange Agreement), among the Company, Temasek and Total.
Under the Exchange Agreement, at the closing of the Exchange, Temasek exchanged $71.0 million in principal amount of outstanding Tranche I and Tranche II Notes (including paid-in-kind and accrued interest through July 29, 2015) and Total exchanged $70.0 million in principal amount of outstanding R&D Notes for shares of the Company’s common stock. The exchange price was $2.30 per share (or the Exchange Price) and was paid by the exchange and cancellation of such outstanding convertible promissory notes, and Temasek and Total received 30,860,633 and 30,434,782 shares of the Company’s common stock, respectively, in the Exchange. As a result of the Exchange, accretion of debt discount was accelerated based on the Company’s estimate of the expected conversion date, resulting in an additional interest expense of $39.2 million for the year ended December 31, 2015.
Under the Exchange Agreement, Total also received the following warrants, each with a five-year term, at the closing of the Exchange:
• A warrant to purchase 18,924,191 shares of the Company’s Common Stock (or the Total Funding Warrant).
• A warrant to purchase 2,000,000 shares of the Company’s common stock that would only be exercisable if the Company failed, as of March 1, 2017, to achieve a target cost per liter to manufacture farnesene (or the Total R&D Warrant). The Total Funding Warrant and the Total R&D Warrant are collectively referred to as the “Total Warrants.”
Additionally, under the Exchange Agreement, Temasek received the following warrants at the closing of the Exchange:
• A warrant to purchase 14,677,861 shares of the Company’s common stock (or the Temasek Exchange Warrant).
| 151 |
• A warrant exercisable for that number of shares of the Company’s common stock equal to (1) (A) the number of shares for which Total exercises the Total Funding Warrant plus (B) the number of additional shares for which the certain convertible notes remaining outstanding following the completion of the Exchange may become exercisable as a result of a reduction in the conversion price of such remaining notes as of a result of and/or subsequent to the date of the Exchange plus (C) that number of additional shares in excess of 2,000,000, if any, for which the Total R&D Warrant becomes exercisable multiplied by a fraction equal to 30.6% divided by 69.4% plus (2) (A) the number of any additional shares for which certain other outstanding convertible promissory notes may become exercisable as a result of a reduction to the conversion price of such notes multiplied by (B) a fraction equal to 13.3% divided by 86.7% (or the Temasek Funding Warrant).
• A warrant exercisable for that number of shares of the Company’s common stock equal to 880,339 multiplied by a fraction equal to the number of shares for which Total exercises the Total R&D Warrant divided by 2,000,000 (or the Temasek R&D Warrant). If Total is entitled to, and does, exercise the Total R&D Warrant in full, the Temasek R&D Warrant would be exercisable for 880,339 shares.
The Temasek Exchange Warrant, the Temasek Funding Warrant and the Temasek R&D Warrant each have ten-year terms and are referred to herein as the “Temasek Warrants” and, the Temasek Warrants and Total Warrants are hereinafter collectively referred to as the “Exchange Warrants”. All of the Exchange Warrants have an exercise price of $0.01 per share.
In addition to the grant of the Exchange Warrants, a warrant issued by the Company to Temasek in October 2013 in conjunction with a prior convertible debt financing (or the 2013 Warrant) became exercisable in full upon the completion of the Exchange. There were 1,000,000 shares underlying the 2013 Warrant, with an exercise price of $0.01 per share.
The exercisability of all of the Exchange Warrants was subject to stockholder approval, which was obtained on September 17, 2015.
As of December 31, 2016, the Total Funding Warrant, the Temasek Exchange Warrant, and the 2013 Warrant had been fully exercised and Temasek had exercised the Temasek Funding Warrant with respect to 12,700,244 shares of common stock. Neither the Total R&D Warrant nor the Temasek R&D Warrant were exercisable as of December 31, 2016. See Note 16, “Subsequent Events” for additional details regarding the Total R&D Warrant and Temasek R&D Warrant. Warrants to purchase 2,462,536 shares of common stock under the Temasek Funding Warrant were unexercised as of December 31, 2016.
Maturity Treatment Agreement - Related Party Transaction
At the closing of the Exchange, the Company, Total and Temasek also entered into a Maturity Treatment Agreement, dated as of July 29, 2015, pursuant to which Total and Temasek agreed to convert any Tranche I Notes, Tranche II Notes or 2014 144A Notes held by them that were not cancelled in the Exchange (or the Remaining Notes) into shares of the Company’s common stock in accordance with the terms of such Remaining Notes upon maturity, provided that certain events of default had not occurred with respect to the applicable Remaining Notes prior to such maturity. As of immediately following the closing of the Exchange, Temasek held $10.0 million in aggregate principal amount of Remaining Notes (consisting of 2014 144A Notes) and Total held approximately $27.0 million in aggregate principal amount of Remaining Notes (consisting of $9.7 million of 2014 144A Notes and $15.3 million of Tranche I and II Notes).
| 152 |
February 2016 Private Placement - Related Party Transaction
On February 12, 2016, the Company entered into a Note and Warrant Purchase Agreement (or the February 2016 Purchase Agreement) with the purchasers named therein for the sale of $18.0 million in aggregate principal amount of unsecured promissory notes (or the February 2016 Notes) to the purchasers, as well as warrants to purchase 2,571,428 shares of the Company’s common stock at an exercise price of $0.01 per share, representing aggregate proceeds to the Company of $18 million (or the Initial Sale). On February 15, 2016, an additional purchaser joined the February 2016 Purchase Agreement and purchased $2.0 million in aggregate principal amount of the February 2016 Notes, as well as warrants to purchase 285,714 shares of the Company’s common stock at an exercise price of $0.01 per share, representing aggregate proceeds to the Company of $2 million (or the Subsequent Sale and together with the Initial Sale, the February 2016 Private Placement). The February 2016 Notes and the warrants were issued in a private placement exempt from registration under the Securities Act. The purchasers are existing stockholders of the Company and affiliated with certain members of the Company’s Board of Directors: Foris Ventures, LLC (or Foris, an entity affiliated with director John Doerr of Kleiner Perkins Caufield & Byers, a current stockholder), which purchased $16.0 million aggregate principal amount of the February 2016 Notes and warrants to purchase 2,285,714 shares of the Company’s common stock; Naxyris S.A. (an investment vehicle owned by Naxos Capital Partners SCA Sicar; director Carole Piwnica is Director of NAXOS UK, which is affiliated with Naxos Capital Partners SCA Sicar), which purchased $2.0 million aggregate principal amount of the February 2016 Notes and warrants to purchase 285,714 shares of the Company’s common stock; and Biolding Investment SA, a fund affiliated with director HH Sheikh Abdullah bin Khalifa Al Thani, which purchased $2.0 million aggregate principal amount of the February 2016 Notes and warrants to purchase 285,714 shares of the Company’s common stock. The Initial Sale closed on February 12, 2016, and the Subsequent Sale closed on February 15, 2016.
The February 2016 Notes are unsecured obligations of the Company and are subordinate to the Company’s obligations under the Senior Secured Loan Facility pursuant to a Subordination Agreement, dated as of February 12, 2016, by and among the Company, the purchasers and the administrative agent under the Senior Secured Loan Facility. Interest will accrue on the February 2016 Notes from and including, with respect to the Initial Sale, February 12, 2016, and with respect to the Subsequent Sale, February 15, 2016, at a rate of 13.50% per annum and is payable on May 15, 2017, the maturity date of the February 2016 Notes, unless the February 2016 Notes are prepaid in accordance with their terms prior to such date. The February 2016 Purchase Agreement and the February 2016 Notes contain customary terms, provisions, representations and warranties, including certain events of default after which the February 2016 Notes may be due and payable immediately, as set forth in the February 2016 Notes.
The exercisability of the warrants issued in the February 2016 Private Placement, which each have a term of five years, was subject to stockholder approval, which was obtained on May 17, 2016. As of December 31, 2016, the carrying amount of the February 2016 Notes was $18.7 million.
| 153 |
June 2016 Private Placement - Related Party Transaction
On June 24, 2016, the Company entered into a Note Purchase Agreement (or the June 2016 Purchase Agreement) with Foris for the sale of $5.0 million in aggregate principal amount of secured promissory notes (or the June 2016 Notes) to Foris in exchange for aggregate proceeds to the Company of $5.0 million (or the June 2016 Private Placement). The June 2016 Notes were issued in a private placement exempt from registration under the Securities Act. The June 2016 Private Placement closed on June 24, 2016.
The June 2016 Notes are collateralized by a second priority lien on the assets securing the Company’s obligations under the Senior Secured Loan Facility, and are subordinate to the Company’s obligations under the Senior Secured Loan Facility pursuant to a Subordination Agreement, dated as of June 24, 2016, by and among the Company, Foris and the administrative agent under the Company’s Senior Secured Loan Facility. Interest will accrue on the June 2016 Notes from and including June 24, 2016 at a rate of 13.50% per annum and is payable in full on May 15, 2017, the maturity date of the June 2016 Notes, unless the June 2016 Notes are prepaid in accordance with their terms prior to such date. The June 2016 Purchase Agreement and the June 2016 Notes contain customary terms, provisions, representations and warranties, including certain events of default after which the June 2016 Notes may be due and payable immediately, as set forth in the June 2016 Notes.
October 2016 Private Placements
On October 21 and October 27, 2016, the Company entered into separate Note Purchase Agreements (or the October 2016 Purchase Agreements) with Foris and Ginkgo, respectively, for the sale of $6.0 million and $8.5 million, respectively, in aggregate principal amount of secured promissory notes (or the October 2016 Notes) in exchange for aggregate proceeds to the Company of $6.0 million and $8.5 million, respectively (or the October 2016 Private Placements). The October 2016 Notes were issued in private placements exempt from registration under the Securities Act. The October 2016 Private Placements closed on October 21 and October 27, 2016, respectively.
The October 2016 Notes are collateralized by a second priority lien on the assets securing the Company’s obligations under the Senior Secured Loan Facility, and are subordinate to the Company’s obligations under the Senior Secured Loan Facility pursuant to Subordination Agreements, dated as of the respective dates of the October 2016 Purchase Agreements, by and among the Company, the applicable purchaser and the administrative agent under the Company’s Senior Secured Loan Facility. Interest will accrue on the October 2016 Notes from and including October 21 and 27, 2016, respectively, at a rate of 13.50% per annum and is payable in full on May 15, 2017, the maturity date of the October 2016 Notes, unless the October 2016 Notes are prepaid in accordance with their terms prior to such date. The October 2016 Purchase Agreements and the October 2016 Notes contain customary terms, provisions, representations and warranties, including certain events of default after which the October 2016 Notes may be due and payable immediately, as set forth in the October 2016 Notes.
| 154 |
Salisbury Note
In December 2016, in connection with the Company’s purchase of a manufacturing facility in Leland, North Carolina and related assets (Glycotech Assets), as discussed in more detail in Note 7, “Joint Venture and Noncontrolling Interests,” the Company issued a purchase money promissory note in the principal amount of $3.5 million (Salisbury Note) in favor of Salisbury Partners, LLC (Salisbury). The Salisbury Note (i) bears interest at a rate of 5% per year, (ii) has a term of 13 years, (iii) is payable in equal monthly installments of principal and interest beginning on January 1, 2017 (which payments are subject to a penalty of 5% if delinquent more than 5 days) and (iv) is secured by a purchase money lien on the Glycotech Assets. The Salisbury Note contains customary terms and provisions, including certain events of default after which the Salisbury Note may become immediately due and payable. In addition, the Salisbury Note may be prepaid in full or in part at any time without penalty or premium. In January 2017, the Salisbury Note was repaid with proceeds from the Nikko Note (as defined below).
Nikko Note
In December 2016, in connection with the Company’s formation of its cosmetics joint venture (or the Neossance JV) with Nikko Chemicals Co., Ltd. (or Nikko), an existing commercial partner of the Company, and Nippon Surfactant Industries Co., Ltd., an affiliate of Nikko, as discussed in more detail in Note 7, “Joint Venture and Noncontrolling Interests,” Nikko made a loan to the Company in the principal amount of $3.9 million, and the Company in consideration therefor issued a promissory note (or the Nikko Note) to Nikko in an equal principal amount. The proceeds of the Nikko Note were used to satisfy the Company’s remaining liabilities relating to the Company’s purchase of the Glycotech Assets, including liabilities under the Salisbury Note. The Nikko Note (i) bears interest at a rate of 5% per year, (ii) has a term of 13 years, (iii) is payable in equal monthly installments of principal and interest beginning on January 1, 2017 (which payments are subject to a penalty of 5% if delinquent more than 5 days) and (iv) is collateralized by a first-priority lien on 10% of the Neossance JV interests owned by the Company. In addition to the payments under the Nikko Note set forth in the preceding sentence, the Company is required to (i) repay $400,000 of the Nikko Note in equal monthly installments of $100,000 on January 1, 2017, February 1, 2017, March 1, 2017 and April 1, 2017 and (ii) commencing with the distributions from the Neossance JV to its members relating to the fourth fiscal year of the Neossance JV and continuing for each fiscal year thereafter until the Nikko Note is fully repaid, repay the Nikko Note in an amount equal to the profits, if any, distributed to the Company by the Neossance JV. The Note contains customary terms and provisions, including certain events of default after which the Note may become immediately due and payable. In addition, the Nikko Note may be prepaid in full or in part at any time without penalty or premium.
Letters of Credit
In June 2012, the Company entered into a letter of credit agreement for $1.0 million under which it provided a letter of credit to the landlord for its headquarters in Emeryville, California in order to cover the security deposit on the lease. This letter of credit is secured by a certificate of deposit. Accordingly, the Company has $1.0 million as restricted cash under this arrangement as of December 31, 2016 and 2015.
| 155 |
Future minimum payments under the debt agreements as of December 31, 2016 are as follows (in thousands):
| Years ending December 31: | Related Party Convertible Debt | Convertible Debt | Loans Payable | Related Party Loans Payable | Credit Facility | Total | ||||||||||||||||||
| 2017 | $ | 4,837 | $ | 18,883 | $ | 16,628 | $ | 32,551 | $ | 7,014 | $ | 79,913 | ||||||||||||
| 2018 | 16,550 | 18,142 | 2,808 | — | 33,648 | 71,148 | ||||||||||||||||||
| 2019 | 34,260 | 88,902 | 2,699 | — | 2,578 | 128,439 | ||||||||||||||||||
| 2020 | — | — | 2,592 | — | 2,500 | 5,092 | ||||||||||||||||||
| 2021 | — | — | 2,483 | — | 27,395 | 29,878 | ||||||||||||||||||
| Thereafter | — | — | 4,075 | — | — | 4,075 | ||||||||||||||||||
| Total future minimum payments(1) | 55,647 | 125,927 | 31,285 | 32,551 | 73,135 | 318,545 | ||||||||||||||||||
| Less: amount representing interest(2) | (12,893 | ) | (46,946 | ) | (4,758 | ) | (2,860 | ) | (24,045 | ) | (91,502 | ) | ||||||||||||
| Present value of minimum debt payments | 42,754 | 78,981 | 26,527 | 29,691 | 49,090 | 227,043 | ||||||||||||||||||
| Less: current portion | (3,610 | ) | (8,957 | ) | (15,448 | ) | (29,691 | ) | (1,449 | ) | (59,155 | ) | ||||||||||||
| Noncurrent portion of debt | $ | 39,144 | $ | 70,024 | $ | 11,079 | $ | — | $ | 47,641 | $ | 167,888 | ||||||||||||
______________
| (1) | Including $11.8 million in 2017 related to Nomis Bay convertible note which , at the Company’s election, may be settled in shares or cash, be settled in shares and $46.8 million in 2018 and 2019 subject to Maturity Treatment Agreement, which will be converted to common stock at maturity, subject to there being no default under the terms of the debt. |
| (2) | Including debt discount and issuance cost of $42.5 million associated with the related party and non-related party debt which will be accreted to interest expense under the effective interest method over the term of the debt. |
During the year ended December 31, 2016, the Company adopted ASU 2015-03, Interest - Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs, which requires debt issuance costs previously reported as a deferred charge within other noncurrent assets and prepaid expenses and other current assets to be presented as a direct reduction from the carrying amount of debt, consistent with debt discounts, applied retrospectively for all periods presented. As of December 31, 2015, this resulted in the reduction of noncurrent debt by $2.8 million, current debt by $1.4 million, other noncurrent assets by $2.8 million and prepaid expenses and other current assets by $1.4 million.
| 156 |
6. Commitments and Contingencies
Lease Obligations
The Company leases certain facilities and finances certain equipment under operating and capital leases, respectively. Operating leases include leased facilities and capital leases include leased equipment (see Note 4, "Balance Sheet Components"). The Company recognizes rent expense on a straight-line basis over the non-cancellable lease term and records the difference between cash rent payments and the recognition of rent expense as a deferred rent liability. Where leases contain escalation clauses, rent abatements, and/or concessions, such as rent holidays and landlord or tenant incentives or allowances, the Company applies them as straight-line rent expense over the lease term. The Company has non-cancellable operating lease agreements for office, research and development, and manufacturing space that expire at various dates, with the latest expiration in February 2031. Rent expense under operating leases was $5.3 million, $5.5 million and $5.4 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Future minimum payments under the Company's lease obligations as of December 31, 2016, are as follows (in thousands):
| Years ending December 31: | Capital Leases | Operating Leases | Total Lease Obligations | |||||||||
| 2017 | $ | 1,233 | $ | 6,854 | $ | 8,087 | ||||||
| 2018 | 47 | 6,883 | 6,930 | |||||||||
| 2019 | 6 | 6,774 | 6,780 | |||||||||
| 2020 | — | 7,004 | 7,004 | |||||||||
| 2021 | — | 7,240 | 7,240 | |||||||||
| Thereafter | — | 10,948 | 10,948 | |||||||||
| Total future minimum lease payments | 1,286 | $ | 45,703 | $ | 46,989 | |||||||
| Less: amount representing interest | (30 | ) | ||||||||||
| Present value of minimum lease payments | 1,256 | |||||||||||
| Less: current portion | (922 | ) | ||||||||||
| Long-term portion | $ | 334 | ||||||||||
Guarantor Arrangements
The Company has agreements whereby it indemnifies its officers and directors for certain events or occurrences while the officer or director is serving in his or her official capacity. The indemnification period remains enforceable for the officer's or director’s lifetime. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited; however, the Company has a director and officer insurance policy that limits its exposure and enables the Company to recover a portion of any future payments. As a result of its insurance policy coverage, the Company believes the estimated fair value of these indemnification agreements is minimal. Accordingly, the Company had no liabilities recorded for these agreements as of December 31, 2016 and 2015.
The Company entered into the FINEP Credit Facility to finance a research and development project on sugarcane-based biodiesel (see Note 5, "Debt"). The FINEP Credit Facility is guaranteed by a chattel mortgage on certain equipment of the Company. The Company's total acquisition cost for the equipment under this guarantee is approximately R$6.0 million (approximately US$1.8 million based on the exchange rate as of December 31, 2016).
| 157 |
The Company entered into the BNDES Credit Facility to finance a production site in Brazil (see Note 5, "Debt").The BNDES Credit Facility is collateralized by a first priority security interest in certain of the Company's equipment and other tangible assets with a total acquisition cost of R$24.9 million (approximately US$7.7 million based on the exchange rate as of December 31, 2016). The Company is a parent guarantor for the payment of the outstanding balance under the BNDES Credit Facility. Additionally, the Company is required to provide certain bank guarantees under the BNDES Credit Facility. Accordingly, the Company had zero of restricted cash as of each of December 31, 2016 and 2015 related to the BNDES Credit Facility.
The Company entered into loan agreements and a security agreement whereby the Company pledged certain farnesene production assets as collateral (the fiduciary conveyance of movable goods) with each of Nossa Caixa and Banco Pine (see Note 5, "Debt"). The Company's total acquisition cost for the farnesene production assets pledged as collateral under these agreements is approximately R$68.0 million (approximately US$20.9 million based on the exchange rate as of December 31, 2016). The Company is also a parent guarantor for the payment of the outstanding balance under these loan agreements.
The Company had an export financing agreement with ABC Brasil for approximately $2.2 million for a one-year term to fund exports through March 2015. As of December 31, 2015, the loan was fully paid. On April 8, 2015, the Company entered into another export financing agreement with the same bank for approximately $1.6 million for a one year term to fund exports through March 2016. This loan is collateralized by future exports from Amyris Brasil. The Company is also a parent guarantor for the payment of the outstanding balance under these loan agreements.
In December 2013, in connection with the execution of the JV Documents entered into by and among the Company, Total and TAB relating to the establishment of TAB (see Note 5, "Debt" and Note 7, “Joint Venture and Noncontrolling Interests”), the Company agreed to exchange the $69.0 million of outstanding Unsecured R&D Notes issued pursuant to the Total Purchase Agreement for replacement 1.5% Senior Secured Convertible Notes due March 2017, and grant a security interest to Total in and lien on all the Company’s rights, title and interest in and to the Company’s shares in the capital of TAB. Following execution of the JV Documents, all Unsecured R&D Notes that had been issued were exchanged for Secured R&D Notes. Further, the $10.85 million in principal amount of such notes issued in the initial tranche of the third closing under the Total Purchase Agreement in July 2014 and the $10.85 million in principal amount of such notes issued in the second tranche of the third closing in January 2015 were Secured R&D Notes instead of Unsecured R&D Notes. See Note 5,"Debt" for details regarding the impact of the Exchange and Maturity Treatment Agreement on the R&D Notes. In March 2016, as a result of the restructuring of TAB discussed under Note 5, “Debt” and Note 7, “Joint Venture and Noncontrolling Interests,” the remaining Secured R&D Notes were exchanged for an Unsecured R&D Note in the principal amount of $3.7 million. Further, in February 2017, the Company and Total agreed to extend the maturity of the outstanding R&D Notes from March 1, 2017 to May 15, 2017, as discussed under Note 16, “Subsequent Events.”
The Senior Secured Loan Facility and the June 2016 Notes and October 2016 Notes (see Note 5, "Debt") are collateralized by first- and second- priority liens, respectively, on substantially all of the Company's assets, including Company intellectual property. In addition, as discussed above, the Nikko Note is collateralized by a first-priority lien on 10% of the Neossance JV interests owned by the Company.
| 158 |
Purchase Obligations
As of December 31, 2016, the Company had $0.8 million in purchase obligations which included $0.6 million in non-cancellable contractual obligations and construction commitments.
Production Cost Commitment
As of December 31, 2016, the Company committed to manufacture Squalane and Hemisqualane supplied to our Neossance JV at specified cost targets. The Company is obligated to pay all manufacturing costs above the production cost target but is not obligated to produce squalane and hemisqualane at a loss and no liability has been accrued for the Company’s commitment to produce at the specified cost target.
Other Matters
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but will only be recorded when one or more future events occur or fail to occur. The Company's management assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against and by the Company or unasserted claims that may result in such proceedings, the Company's management evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company's financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be reasonably estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed. Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed. The Company has levied indirect taxes on sugarcane-based biodiesel sales by Amyris Brasil to customers in Brazil based on advice from external legal counsel. In the absence of definitive rulings from the Brazilian tax authorities on the appropriate indirect tax rate to be applied to such product sales, the actual indirect rate to be applied to such sales could differ from the rate we levied.
The Company is subject to disputes and claims that arise or have arisen in the ordinary course of business and that have not resulted in legal proceedings or have not been fully adjudicated. Such matters that may arise in the ordinary course of business are subject to many uncertainties and outcomes are not predictable with reasonable assurance and therefore an estimate of all the reasonably possible losses cannot be determined at this time. Therefore, if one or more of these legal disputes or claims resulted in settlements or legal proceedings that were resolved against the Company for amounts in excess of management’s expectations, the Company’s consolidated financial statements for the relevant reporting period could be materially adversely affected.
| 159 |
7. Joint Ventures and Noncontrolling Interest
Novvi LLC
In September 2011, the Company and Cosan US, Inc. (Cosan U.S.) formed Novvi LLC (or Novvi), a U.S. entity that was initially jointly owned by the Company and Cosan U.S. In March 2013, the Company and Cosan U.S. entered into agreements to (i) expand their base oils joint venture to also include additives and lubricants and (ii) operate their joint venture exclusively through Novvi. Specifically, the parties entered into an Amended and Restated Operating Agreement for Novvi (or the Novvi Operating Agreement), which sets forth the governance procedures for Novvi and the parties' initial contribution. The Company also entered into an IP License Agreement with Novvi (as amended, the Novvi IP License Agreement) under which the Company granted Novvi (i) an exclusive (subject to certain limited exceptions for the Company), worldwide, royalty-free license to develop, produce and commercialize base oils, additives, and lubricants derived from Biofene for use in automotive, commercial, and industrial lubricants markets, and (ii) a non-exclusive, royalty free license, subject to certain conditions, to manufacture Biofene solely for its own products. In addition, both the Company and Cosan U.S. granted Novvi certain rights of first refusal with respect to alternative base oil and additive technologies that may be acquired by the Company or Cosan U.S. during the term of the IP License Agreement. Under these agreements, through December 31, 2015 the Company and Cosan U.S. each owned 50% of Novvi and each party shared equally in any costs and any profits ultimately realized by the joint venture. Novvi is governed by a six member Board of Managers (or the Board of Managers). The Board of Managers appoints the officers of Novvi, who are responsible for carrying out the daily operating activities of Novvi as directed by the Board of Managers. The Novvi IP License Agreement has an initial term of 20 years from the date of the agreement, subject to standard early termination provisions such as uncured material breach or a party's insolvency. Under the terms of the Novvi Operating Agreement, Cosan U.S. was obligated to fund its initial 50% ownership share of Novvi in cash in the amount of $10.0 million and the Company was obligated to fund its initial 50% ownership share of Novvi through the granting of an intellectual property license to develop, produce and commercialize base oils, additives, and lubricants derived from Biofene for use in the automotive, commercial and industrial lubricants markets, which Cosan U.S. and Amyris agreed was valued at $10.0 million. In March 2013, the Company measured its initial contribution of intellectual property to Novvi at the Company's carrying value of the licenses granted under the Novvi IP License Agreement, which was zero.
In April 2014, the Company, via its forgiveness of existing receivables due from Novvi related to rent and other services performed by the Company, purchased additional membership units of Novvi for a purchase price of $0.2 million. Concurrently, Cosan U.S. purchased an equal amount of additional membership units of Novvi. Also in April 2014, the Company and Cosan U.S. each contributed $2.1 million in cash in exchange for receiving additional membership units in Novvi. Following such transactions, the Company and Cosan U.S. continued to each own 50% of Novvi's issued and outstanding membership units.
In September 2014, the Company and Cosan U.S. entered into a member senior loan agreement to grant Novvi a loan amounting to approximately $3.7 million. The loan is due on September 1, 2017 and bears interest at a rate of 0.36% per annum. Interest accrues daily and is due and payable in arrears on September 1, 2017. The Company and Cosan U.S. each agreed to provide 50% of the loan. The Company's share of approximately $1.8 million was disbursed in two installments. The first installment of $1.2 million was made in September 2014 and the second installment of $0.6 million was made in October 2014. In November 2014, the Company and Cosan U.S. entered into a second member senior loan agreement to grant Novvi a loan of approximately $1.9 million on the same terms as the loan issued in September 2014, except that the due date is November 10, 2017. The Company and Cosan U.S. each agreed to provide 50% of the loan. The Company disbursed its share of the loan (i.e., approximately $1.0 million) in November 2014. In May 2015, the Company and Cosan U.S. entered into a third member senior loan agreement to grant Novvi a loan of approximately $1.1 million on the same terms as the loan issued in September 2014, except that the due date is May 14, 2018. The Company and Cosan U.S. each agreed to provide 50% of the loan.
| 160 |
In the fourth quarter of 2015, the Company and Cosan U.S. entered into four additional member senior loan agreements to grant Novvi an aggregate loan of approximately $1.6 million on the same terms as the loan issued in September 2014, except that the respective due dates are August 19, 2018, October 15, 2018, November 12, 2018 and December 17, 2018. The Company and Cosan U.S. each agreed to provide 50% of each of these four loans. In July 2016, the Company contributed all outstanding amounts owing by Novvi to the Company under the seven member senior loan agreements in exchange for receiving additional membership units in Novvi.
In February 2016, the Company purchased additional membership units of Novvi for an aggregate purchase price of approximately $0.6 million in the form of forgiveness of existing receivables due from Novvi related to rent and other services performed by the Company, and Cosan U.S. purchased an equal number of additional membership units in Novvi for approximately $0.6 million in cash. Following such transactions, each member continued to own 50% of Novvi's issued and outstanding membership units.
On July 19, 2016, American Refining Group, Inc. (ARG) agreed to make a capital contribution of up to $10.0 million in cash to Novvi, subject to certain conditions, in exchange for a one third ownership stake in Novvi. In connection with such investment, the Company agreed to contribute all outstanding amounts owed by Novvi to the Company under the seven existing member senior loan agreements between the Company and Novvi, as well as certain existing receivables due from Novvi to the Company related to rent and other services performance by the Company, in exchange for receiving additional membership units in Novvi. Likewise, Cosan U.S. contributed an equal amount to Novvi as the Company in exchange for receiving an equal amount of additional membership interests in Novvi. Following the ARG investment, assuming it is made in full, and the capital contributions of the Company and Cosan U.S., each of Novvi’s three members (i.e., ARG, the Company and Cosan U.S.) would own one third of Novvi’s issued and outstanding membership units and would each be represented by two members of the Board of Managers. In order to reflect the ARG investment in Novvi and related transactions, the Novvi Operating Agreement was amended and restated on July 19, 2016. In addition, the Novvi IP License Agreement was also amended on July 19, 2016. As of December 31, 2016, $9.0 million of ARG's capital contribution to Novvi had been funded. See Note 16, “Subsequent Events” for additional details regarding ARG’s investment in Novvi.
In November 2016, Chevron U.S.A. Inc. (Chevron) made a capital contribution of $1.0 million in cash to Novvi in exchange for 20,000 membership units, representing an approximately 3% ownership stake in Novvi, which reduced the ownership interests of the Company, Cosan U.S. and ARG pro rata. In connection with its investment in Novvi, for so long as Chevron or its affiliates owns any membership units in Novvi, Chevron shall have the right to purchase up to such additional membership units as would result in Chevron owning the greater of (i) 25% of the aggregate membership units then outstanding held by Chevron, the Company, Cosan U.S. and ARG (including their affiliates and successors-in-interest) following such purchase and (ii) the highest percentage of such membership units held by the Company, Cosan U.S. and ARG (including their affiliates and successors-in-interest) following such purchase. In addition, Chevron shall have the right to purchase up to its pro rata share (as determined by the then issued and outstanding membership units, excluding any such units beneficially owned by Novvi) of all additional membership units that Novvi may, from time to time, propose to sell or issue.
| 161 |
Additional funding requirements to finance the ongoing operations of Novvi are expected to happen through revolving credit or other loan facilities provided by unrelated parties (i.e., such as financial institutions); cash advances or other credit or loan facilities provided by Novvi’s members or their affiliates; or additional capital contributions by the existing Novvi members or new investors.
The Company has identified Novvi as a VIE and determined that the power to direct activities which most significantly impact the economic success of the joint venture (i.e., continuing research and development, marketing, sales, distribution and manufacturing of Novvi products) are shared among the Company, Cosan U.S. and ARG. Accordingly, the Company is not the primary beneficiary and therefore accounts for its investment in Novvi under the equity method of accounting. The Company will continue to reassess its primary beneficiary analysis of Novvi if there are changes in events and circumstances impacting the power to direct activities that most significantly affect Novvi's economic success. Under the equity method, the Company's share of profits and losses and impairment charges on investments in affiliates are included in “Loss from investments in affiliates” in the consolidated statements of operations. The carrying amount of the Company's equity investment in Novvi was zero as of each of December 31, 2016 and 2015.
Total Amyris BioSolutions B.V.
In November 2013, the Company and Total formed Total Amyris BioSolutions B.V. (TAB), a joint venture to produce and commercialize farnesene- or farnesane-based jet and diesel fuels. Prior to the restructuring of TAB in March 2016 as described below, the common equity of TAB was owned equally by the Company and Total, and TAB’s purpose was limited to executing the License Agreement dated December 2, 2013 between the Company, Total and TAB and maintaining such licenses under it, unless and until either (i) Total elected to go forward with either the full (diesel and jet fuel) TAB commercialization program (R&D Program) or the jet fuel component of the R&D Program (or a Go Decision), (ii) Total elected to not continue its participation in the R&D Program and TAB (or a No-Go Decision), or (iii) Total exercised any of its rights to buy out the Company’s interest in TAB. Following a Go Decision, the articles and shareholders’ agreement of TAB would be amended and restated to be consistent with the shareholders’ agreement contemplated by the Total Fuel Agreements (see Note 5, "Debt" and Note 8, "Significant Agreements").
In July 2015, the Company and Total entered into a Letter Agreement (or, as amended in February 2016, the TAB Letter Agreement) regarding the restructuring of the ownership and rights of TAB (Restructuring), pursuant to which the parties agreed to, among other things, enter into an Amended & Restated Jet Fuel License Agreement between the Company and TAB (Jet Fuel Agreement), a License Agreement regarding Diesel Fuel in the European Union (EU) between the Company and Total (EU Diesel Fuel Agreement), and an Amended and Restated Shareholders’ Agreement among the Company, Total and TAB, and file a Deed of Amendment of Articles of Association of TAB, all in order to reflect certain changes to the ownership structure of TAB and license grants and related rights pertaining to TAB.
On February 12, 2016, the Company and Total entered into an amendment to the TAB Letter Agreement, pursuant to which the parties agreed that, upon the closing of the Restructuring, Total would cancel R&D Notes in an aggregate principal amount of approximately $1.3 million, plus all paid-in-kind and accrued interest as of the closing of the Restructuring under all outstanding R&D Notes (including all such interest that was outstanding as of July 29, 2015), and a note in the principal amount of Euro 50,000, plus accrued interest, issued by the Company to Total in connection with the existing TAB capitalization, in exchange for an additional 25% ownership interest of TAB (giving Total an aggregate ownership stake of 75% of TAB and giving the Company an aggregate ownership stake of 25% of TAB). In connection therewith, Total would surrender to the Company the remaining R&D Notes and the Company would provide to Total a new R&D Note containing substantially similar terms and conditions to the outstanding R&D Notes other than it would be unsecured and its payment terms would be severed from TAB’s business performance, in the principal amount of $3.7 million (collectively, the “TAB Share Purchase”).
| 162 |
On March 21, 2016, the Company, Total and TAB closed the Restructuring and the TAB Share Purchase. See Note 5, “Debt” and Note 16, “Subsequent Events” for further details of these transactions and the impact of these transaction on the Company’s consolidated financial statements.
Under the Jet Fuel Agreement, (a) the Company granted exclusive (co-exclusive in Brazil), world-wide, royalty-free rights to TAB for the production and commercialization of farnesene- or farnesane-based jet fuel, (b) the Company granted TAB the option, until March 1, 2018, to purchase the Company’s Brazil jet fuel business at a price based on the fair value of the commercial assets and on the Company’s investment in other related assets, (c) the Company granted TAB the right to purchase farnesene or farnesane for its jet fuel business from us on a “most-favored” pricing basis and (d) all rights to farnesene- or farnesane-based diesel fuel the Company previously granted to TAB reverted back to the Company. As a result of the Jet Fuel Agreement, the Company generally no longer has an independent right to make or sell, without the approval of TAB, farnesene- or farnesane-based jet fuels outside of Brazil.
Upon all farnesene-or farnesane-based diesel fuel rights reverting back to the Company, the Company granted to Total, pursuant to the EU Diesel Fuel Agreement, (a) an exclusive, royalty-free license to offer for sale and sell farnesene- or farnesane-based diesel fuel in the EU, (b) the non-exclusive right to make farnesene or farnesane anywhere in the world, but Total must (i) use such farnesene or farnesane to produce only diesel fuel to offer for sale or sell in the EU and (ii) pay the Company a to-be-negotiated, commercially reasonable, “most-favored” basis royalty and (c) the right to purchase farnesene or farnesane for its EU diesel fuel business from the Company on a “most-favored” pricing basis. As a result of the EU Diesel Fuel Agreement, the Company generally no longer has an independent right to make or sell, without the approval of Total, farnesene- or farnesane-based diesel fuels in the EU.
As a result of, and in order to reflect, the changes to the ownership structure of TAB described above, on March 21, 2016, (a) the Company, Total and TAB entered into an Amended and Restated Shareholders’ Agreement and filed a Deed of Amendment of Articles of Association of TAB and (b) the Company and Total terminated the Amended and Restated Master Framework Agreement, dated December 2, 2013 and amended on April 1, 2015, between the Company and Total.
As of December 31, 2016, the common equity of TAB was owned 25% by the Company and 75% by Total. TAB has a capitalization as of December 31, 2016 of €0.1 million (approximately US$0.1 million based on the exchange rate as of December 31, 2016). The Company has identified TAB as a VIE and determined that the Company is not the primary beneficiary and therefore accounts for its investment in TAB under the equity method of accounting. Under the equity method, the Company's share of profits and losses are included in “Loss from investment in affiliate” in the consolidated statements of operations.
| 163 |
SMA Indústria Química S.A.
In April 2010, the Company established SMA Indústria Química (or SMA), a joint venture with São Martinho S.A. (or SMSA), to build a production facility in Brazil. SMA is located at the SMSA mill in Pradópolis, São Paulo state. The joint venture agreements establishing SMA had a 20 year initial term.
SMA was initially managed by a three member executive committee, of which the Company appointed two members, one of whom is the plant manager who is the most senior executive responsible for managing the construction and operation of the facility. SMA was initially governed by a four member board of directors, of which the Company and SMSA each appointed two members. The board of directors had certain protective rights which include final approval of the engineering designs and project work plan developed and recommended by the executive committee.
The joint venture agreements required the Company to fund the construction costs of the new facility and SMSA would reimburse the Company up to R$61.8 million (approximately US$19.0 million based on the exchange rate as of December 31, 2016) of the construction costs after SMA commences production. After commercialization, the Company would market and distribute Amyris renewable products produced by SMA and SMSA would sell feedstock and provide certain other services to SMA. The cost of the feedstock to SMA would be a price that is based on the average return that SMSA could receive from the production of its current products, sugar and ethanol. The Company would be required to purchase the output of SMA for the first four years at a price that guarantees the return of SMSA’s investment plus a fixed interest rate. After this four year period, the price would be set to guarantee a break-even price to SMA plus an agreed upon return.
Under the terms of the joint venture agreements, if the Company became controlled, directly or indirectly, by a competitor of SMSA, then SMSA would have the right to acquire the Company’s interest in SMA. If SMSA became controlled, directly or indirectly, by a competitor of the Company, then the Company would have the right to sell its interest in SMA to SMSA. In either case, the purchase price would be determined in accordance with the joint venture agreements, and the Company would continue to have the obligation to acquire products produced by SMA for the remainder of the term of the supply agreement then in effect even though the Company would no longer be involved in SMA’s management.
The Company initially had a 50% ownership interest in SMA. The Company had identified SMA as a VIE pursuant to the accounting guidance for consolidating VIEs because the amount of total equity investment at risk was not sufficient to permit SMA to finance its activities without additional subordinated financial support, as well as because the related commercialization agreement provides a substantive minimum price guarantee. Under the terms of the joint venture agreement, the Company directed the design and construction activities, as well as production and distribution. In addition, the Company had the obligation to fund the design and construction activities until commercialization was achieved. Subsequent to the construction phase, both parties equally would fund SMA for the term of the joint venture. Based on those factors, the Company was determined to have the power to direct the activities that most significantly impact SMA’s economic performance and the obligation to absorb losses and the right to receive benefits. As of December 31, 2016, the Company indirectly owned 100% of the equity interest in SMA and as a wholly owned subsidiary its financial results are included in the Company’s consolidated financial statements.
| 164 |
The Company completed a significant portion of the construction of the new facility in 2012. The Company suspended construction of the facility in 2013 in order to focus on completing and operating the Company's smaller production facility in Brotas, Brazil. In February 2014, the Company entered into an amendment to the joint venture agreement with SMSA which updated and documented certain preexisting business plan requirements related to the recommencement of construction at the joint venture operated plant and sets forth, among other things, (i) the extension of the deadline for the commencement of operations at the joint venture operated plant to no later than 18 months following the construction of the plant no later than March 31, 2017, and (ii) the extension of an option held by SMSA to build a second large-scale farnesene production facility to no later than December 31, 2018 with the commencement of operations at such second facility to occur no later than April 1, 2019. On July 1, 2015 SMSA filed a material fact document with CVM, the Brazilian securities regulator, that announced that certain contractual targets undertaken by the Company have not been achieved, which affects the feasibility of the project. Therefore, SMSA decided not to approve continuing construction of the plant for the joint venture with the Company and its Brazilian subsidiary Amyris Brasil. In July 2015, the Company announced that it was in discussions with SMSA regarding the continuation of the joint venture. In December 2015, the Company and SMSA entered into a Termination Agreement and a Share Purchase and Sale Agreement relating to the termination of the joint venture. Under the Termination Agreement, the parties agreed that the joint venture would be terminated effective upon the closing of a purchase by Amyris Brasil of SMSA’s shares of SMA. Under the Share Purchase and Sale Agreement, Amyris Brasil agreed to purchase, for R$50,000 (approximately US$15,342 based on the exchange rate as of December 31, 2016), 50,000 shares of SMA (representing all the outstanding shares of SMA held by SMSA), which purchase and sale was consummated on January 11, 2016. The Share Purchase and Sale Agreement also provided that the Company and Amyris Brasil would have 12 months following the closing of the share purchase to remove assets from SMSA’s site, and enter into an extension of the lease for such 12 month period for monthly rental payments of R$9,853 (approximately US$3,023 based on the exchange rate as of December 31, 2016). The Share Purchase and Sale Agreement also clarified that the Company and Amyris Brasil would not be required to demolish or remove the foundations of the plant at the SMSA site. On September 1, 2016, the parties entered into an addendum to the Share Purchase and Sale Agreement (and a corresponding amendment to the lease) which extended the deadline for the Company and Amyris Brasil to remove assets from SMSA’s site until December 31, 2017.
Salisbury transaction
In January 2011, the Company entered into a production service agreement (Glycotech Agreement) with Glycotech, Inc. (or Glycotech), under which Glycotech provides process development and production services for the manufacturing of various Company products at its leased facility in Leland, North Carolina (Glycotech Facility). The Company products manufactured by Glycotech are owned and distributed by the Company. Pursuant to the terms of the Glycotech Agreement, the Company is required to pay the manufacturing and operating costs of the Glycotech facility, which is dedicated solely to the manufacture of Amyris products. The initial term of the Glycotech Agreement was for a two year period commencing on February 1, 2011 and the Glycotech Agreement renews automatically for successive one-year terms, unless terminated by the Company. Concurrent with the Glycotech Agreement, the Company also entered into a Right of First Refusal Agreement with Salisbury Partners, LLC (or Salisbury), the lessor of the facility and site leased by Glycotech (ROFR Agreement). Per conditions of the ROFR Agreement, Salisbury agreed not to sell the facility and site leased by Glycotech during the term of the Glycotech Agreement. In the event that Salisbury was presented with an offer to sell or decides to sell an adjacent parcel, the Company had the right of first refusal to acquire it.
| 165 |
On November 10, 2016, the Company, Glycotech and Salisbury entered into a Purchase and Sale Agreement (PSA) for the purchase and sale of the Glycotech Facility, the real property on which the Glycotech Facility is located and the fixtures, equipment, materials and supplies and other tangible assets located at or used in connection with the Facility (collectively, the Glycotech Assets). Pursuant to the PSA, on December 5, 2016, the Company purchased the Glycotech Assets from Glycotech and Salisbury for an aggregate purchase price of $4.35 million, of which $3.5 million was paid in the form of a purchase money promissory note in favor of Salisbury, as described in more detail in Note 5, “Debt.” In connection with the closing of the purchase and sale of the Glycotech Assets under the PSA, the Company, Glycotech and Salisbury terminated the current lease of the Glycotech Facility and the Glycotech Agreement and modified the ROFR Agreement such that the Company’s right of first refusal with respect to certain parcels of real property owned by Salisbury adjacent to the Glycotech Facility would be an appurtenant right running with the ownership of the real property on which the Glycotech Facility is located. The Glycotech Assets were subsequently transferred to the Company’s cosmetics joint venture with Nikko Chemicals Co., Ltd. and Nippon Surfactant Industries Co., Ltd. in connection with the formation of such joint venture, as described below under “Neossance JV.”
Neossance JV
On December 12, 2016, the Company, Nikko Chemicals Co., Ltd. an existing commercial partner of the Company, and Nippon Surfactant Industries Co., Ltd., an affiliate of Nikko (collectively, Nikko) entered into a Joint Venture Agreement ( Neossance JV Agreement), pursuant to which the Company and Nikko agreed to form a joint venture under the name Neossance, LLC, a Delaware limited liability company (Neossance JV). Pursuant to the Neossance JV Agreement, the Company agreed to initially form the Neossance JV and contribute certain assets of the Company, including certain intellectual property and other commercial assets relating to its Neossance cosmetic ingredients business (Neossance JV Business), as well as the Glycotech Assets. The Company also agreed to provide the Neossance JV with exclusive (to the extent not already granted to a third party), royalty-free licenses to certain intellectual property of the Company necessary to make and sell products associated with the Neossance JV Business (Neossance JV Products), and, in the event the Company is unable to meet its supply commitments under the Neossance JV Supply Agreement (as defined below), or Nikko terminates the Neossance JV Supply Agreement due to a material breach or default thereunder by the Company, the Company would be required to grant to the Neossance JV and Nikko additional non-exclusive, royalty-free licenses to certain intellectual property rights of the Company related to the production of farnesene in connection with the manufacture, production and sale of the Neossance JV Products.
At the closing of the formation of the Neossance JV, which occurred on December 19, 2016, Nikko purchased a 50% interest in the Neossance JV in exchange for the following payments to the Company: (i) an initial payment of $10 million and (ii) the profits, if any, distributed to Nikko in cash as members of the Neossance JV during the three year period following the date of the Joint Venture Agreement, up to a maximum of $10 million.
| 166 |
Pursuant to the Joint Venture Agreement, the Company and Nikko agreed to make working capital loans to the Neossance JV in the amounts of $500,000 and $1,500,000, respectively. In addition, the Company agreed to execute, and cause Amyris Brasil to execute, a supply agreement (Neossance JV Supply Agreement) to supply farnesene to the Neossance JV, and further agreed to conduct its business in the Neossance JV Products through the Neossance JV, to purchase all of its requirements for the Neossance JV Products from the Neossance JV and to transfer all of its customers for the Neossance JV Products to the Neossance JV. In addition, the Company agreed to guarantee a maximum production cost for certain Neossance JV Products to be produced by the Neossance JV and to bear any cost of production above such guaranteed costs.
Under the Neossance JV Agreement, in the event of a merger, acquisition, sale or other similar reorganization, or a bankruptcy, dissolution, insolvency or other similar event, of the Company, on the one hand, or Nikko, on the other hand, the other member will have a right of first purchase with respect to such member’s interest in the Neossance JV, at the fair market value of such interest, in the case of a merger, acquisition, sale or other similar reorganization, and at the lower of the fair market value or book value of such interest, in the case of a bankruptcy, dissolution, insolvency or other similar event.
In connection with the formation of the Neossance JV, the members entered into a First Amended and Restated LLC Operating Agreement of the Neossance JV (Operating Agreement). Pursuant to the Operating Agreement, the Neossance JV will be managed by a Board of Directors, which shall initially consist of four directors, two of which will be appointed by the Company and two of which will be appointed by Nikko. In addition, Nikko will have the right to designate the Chief Executive Officer of the Neossance JV from among the directors and the Company will have the right to designate the Chief Financial Officer. Pursuant to the Joint Venture Agreement, Nikko designated John Melo, the President and CEO of the Company, to serve as the initial CEO of the Neossance JV for a period of one year and the Company designated Shizuo Ukaji, the President and CEO of Nikko, to serve as the initial CFO of the Neossance JV for a period of one year. The Company has determined that it controls the Neossance JV because of its significant ongoing involvement in operational decision making and its guarantee of production costs for squalane/hemisqualane.
Under the Operating Agreement, profits from the operations of the Neossance JV, if any, will be distributed as follows: (i) first, to the members in proportion to their respective unreturned capital contribution balances, until each member’s unreturned capital contribution balance equals zero and (ii) second, to the members in proportion to their respective interests. In addition, future capital contributions will be made from time to time as the members shall determine, in each case on an equal (50%/50%) basis between the Company, on the one hand, and Nikko, on the other hand, unless otherwise mutually agreed by the members.
In connection with the contribution of the Glycotech Assets by the Company to the Neossance JV, at the closing of the formation of the Neossance JV, Nikko made a loan to the Company in the principal amount of $3.9 million, and the Company in consideration therefor issued a promissory note to Nikko in an equal principal amount, as described in more detail in Note 5, “Debt.”
The table table below reflects the carrying amount of the assets and liabilities of the consolidated VIE for which the Company is the primary beneficiary at December 31, 2016 (two at December 31, 2015). The creditors of each consolidated VIE have recourse only to the assets of that VIE.
| 167 |
| December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Assets | $ | 2,277 | $ | 6,993 | ||||
| Liabilities | $ | 135 | $ | 1,221 | ||||
The change in noncontrolling interest for the years ended December 31, 2016 and 2015 is summarized below (in thousands):
| 2016 | 2015 | |||||||
| Balance at January 1 | $ | 391 | $ | 611 | ||||
| Foreign currency translation adjustment | — | (320 | ) | |||||
| Income attributable to noncontrolling interest | (1,328 | ) | 100 | |||||
| Balance at December 31 | $ | (937 | ) | $ | 391 | |||
8. Significant Agreements
Research and Development Activities
Firmenich Collaboration Agreement
In March 2013, the Company entered into a Collaboration Agreement (or, as amended, the Firmenich Collaboration Agreement) with Firmenich SA (or Firmenich), a global flavors and fragrances company, to establish a collaboration arrangement for the development and commercialization of multiple renewable flavors and fragrances compounds. Under the Firmenich Collaboration Agreement, except for rights granted under pre-existing collaboration relationships, the Company granted Firmenich exclusive access to specified Company intellectual property for the development and commercialization of flavors and fragrances compounds in exchange for research and development funding and a profit sharing arrangement. The Firmenich Collaboration Agreement superseded and expanded the November 2010 Master Collaboration and Joint Development Agreement between the Company and Firmenich. Unless sooner terminated in accordance with its terms, the Firmenich Collaboration Agreements has an initial term of 10 years and will automatically renew at the end of such term (and at the end of any extension) for an additional 3-year term unless and until a party provides the other party written notice, at least twelve months before the end of the then-current term, of its desire to terminate the agreement at the end of the then-current term.
The Firmenich Collaboration Agreement provided for annual, up-front funding to the Company by Firmenich of $10.0 million for each of the first three years of the collaboration. Payments of $10.0 million were received by the Company in each of March 2013, 2014 and 2015. The Firmenich Collaboration Agreement contemplates additional funding by Firmenich of up to $5.0 million under four potential milestone payments, as well as additional funding by the collaboration partner on a discretionary basis. Through December 2016, the Company had achieved the third performance milestone under the Firmenich Collaboration Agreement and recognized collaboration revenues of $7.5 million and $11.0 million for the years ended December 31, 2016 and 2015, respectively. The Firmenich Collaboration Agreement does not impose any specific research and development obligations on either party after year six, but provides that if the parties mutually agree to perform research and development activities after year six, the parties will fund such activities equally.
| 168 |
Under the Firmenich Collaboration Agreement, the parties agreed to jointly select target compounds, subject to final approval of compound specifications by Firmenich. During the development phase, the Company would be required to provide labor, intellectual property and technology infrastructure and Firmenich would be required to contribute downstream polishing expertise and market access. The Firmenich Collaboration Agreement provides that the Company will own research and development and strain engineering intellectual property, and Firmenich will own blending and, if applicable, chemical conversion intellectual property. Under certain circumstances, such as the Company’s insolvency, Firmenich would gain expanded access to the Company’s intellectual property. The Firmenich Collaboration Agreement contemplates that, following development of flavors and fragrances compounds, the Company will manufacture the initial target molecules for the compounds and Firmenich will perform any required downstream polishing and distribution, sales and marketing. The Firmenich Collaboration Agreement provides that the parties will mutually agree on a supply price for each compound developed under the agreement and, subject to certain exceptions, will share product margins from sales of each such compound on a 70/30 basis (70% for Firmenich) until Firmenich receives $15.0 million more than the Company in the aggregate from such sales, after which time the parties will share the product margins 50/50. The Company also agreed to pay a one-time success bonus to Firmenich of up to $2.5 million if certain commercialization targets are met.
In September 2014, the Company entered into a supply agreement with the collaboration partner for compounds developed under the Firmenich Collaboration Agreement. The Company recognized $9.7 million and $1.4 million of revenues from product sales under this agreement for the years ended December 31, 2016 and 2015, respectively.
In December 2016, the Company and Firmenich entered into an amendment of the Firmenich Collaboration Agreement, pursuant to which the parties agreed to exclude certain compounds from the scope of the agreement and to permit the Company to engage in certain activities relating to such excluded compounds with a third party, in exchange for a ten percent royalty on net sales by the Company to such third party of products related to such excluded compounds, as well as (i) the transfer of certain technical materials relating to the Firmenich Collaboration Agreement, previously held in escrow, to Firmenich, (ii) a credit to Firmenich against products previously ordered from the Company under the parties’ existing supply agreement, (iii) a reduced price for the sale of additional products to Firmenich under such supply agreement, and (iv) training for the employees of Firmenich at the Company’s manufacturing plant located in Brotas, Brazil.
DARPA Technology Investment Agreement
In September 2015, the Company entered into a Technology Investment Agreement (or, as amended, the 2015 TIA) with The Defense Advanced Research Projects Agency (or DARPA), under which the Company, with the assistance of five specialized subcontractors, will work to create new research and development tools and technologies for strain engineering and scale-up activities. The program that is the subject of the 2015 TIA will be performed and funded on a milestone basis, where DARPA, upon the Company’s successful completion of each milestone event in the 2015 TIA, will pay the Company the amount set forth in the 2015 TIA corresponding to such milestone event. Under the 2015 TIA, the Company and its subcontractors could collectively receive DARPA funding of up to $35.0 million over the program’s four year term if all of the program’s milestones are achieved. In conjunction with DARPA’s funding, the Company and its subcontractors are obligated to collectively contribute approximately $15.5 million toward the program over its four year term (primarily by providing specified labor and/or purchasing certain equipment). The Company can elect to retain title to the patentable inventions it produces under the program, but DARPA receives certain data rights as well as a government purposes license to certain of such inventions. Either party may, upon written notice and subject to certain consultation obligations, terminate the 2015 TIA upon a reasonable determination that the program will not produce beneficial results commensurate with the expenditure of resources.
| 169 |
The Company recognized collaboration revenues of $9.7 million and zero under this agreement for the years ended December 31, 2016 and 2015, respectively.
Ginkgo Initial Strategic Partnership Agreement and Collaboration Agreement
In June 2016, the Company entered into an Initial Strategic Partnership Agreement (Initial Ginkgo Agreement) with Ginkgo Bioworks, Inc. (or “Ginkgo”), pursuant to which the Company licensed certain intellectual property to Ginkgo in exchange for a fee of $20.0 million, to be paid by Ginkgo to the Company in two installments, and a ten percent royalty on net revenue, including without limitation net sales, royalties, fees and any other amounts received by Ginkgo related directly to such license. The first installment of $15.0 million was received on July 25, 2016. The second installment, in the amount of $5.0 million may be received, based upon the satisfaction of certain conditions as set forth below, by March 31, 2017. As of March 31, 2017, the Company had not received such second installment payment.
In addition, pursuant to the Initial Ginkgo Agreement, (i) the Company and Ginkgo agreed to pursue the negotiation and execution of a detailed definitive partnership and license agreement setting forth the terms of a commercial partnership and collaboration arrangement between the parties (Ginkgo Collaboration), (ii) the Company agreed to issue to Ginkgo an option to purchase five million shares of the Company’s common stock at an exercise price of $0.50, exercisable for one year from the date of issuance, in connection with the execution of such definitive agreement for the Ginkgo Collaboration, (iii) the Company received a deferment of all scheduled principal repayments under the Senior Secured Loan Facility, the lender and administrative agent under which is an affiliate of Ginkgo, as well as a waiver of the Minimum Cash Covenant, through October 31, 2016 and (iv) in connection with the execution of the definitive agreement for the Ginkgo Collaboration, the parties would effect an amendment of the LSA to (x) extend the maturity date of all outstanding loans under the Senior Secured Loan Facility, (y) waive any required amortization payments under the Senior Secured Loan Facility until maturity and (z) eliminate the Minimum Cash Covenant under the Senior Secured Loan Facility. See Note 5, “Debt” and Note 16, “Subsequent Events” for details regarding the amendments to the LSA entered into in connection with the Initial Ginkgo Agreement and Ginkgo Collaboration Agreement (as defined below).
On August 6, 2016, the Company issued to Ginkgo a warrant to purchase five million shares of the Company’s common stock at an exercise price of $0.50 per share, exercisable for one year from the date of issuance. The warrant was issued prior to the execution of the definitive agreement for the Ginkgo Collaboration in connection with the transfer of certain information technology from Ginkgo to the Company.
On September 30, 2016, the Company and Ginkgo entered into a Collaboration Agreement (Ginkgo Collaboration Agreement) setting forth the terms of the Ginkgo Collaboration, under which the parties will collaborate to develop, manufacture and sell commercial products and will share in the value created thereby. The Ginkgo Collaboration Agreement provides that, subject to certain exceptions, all third party contracts for the development of chemical small molecule compounds whose manufacture is enabled by the use of microbial strains and fermentation technologies that are entered into by the Company or Ginkgo during the term of the Ginkgo Collaboration Agreement will be subject to the Ginkgo Collaboration and the approval of the other party (not to be unreasonably withheld). Responsibility for the engineering and small-scale process development of the newly developed products will be allocated between the parties on a project-by-project basis, and the Company will be principally responsible for the commercial scale-up and production of such products, with each party generally bearing their own respective costs and expenses relating to the Ginkgo Collaboration, including capital expenditures. Notwithstanding the foregoing, subject to the Company sourcing funding and breaking ground on a new production facility by March 30, 2017, Ginkgo will pay the Company a fee of $5 million on or before March 31, 2017. As of March 31, 2017, the Company had not received such second installment payment.
| 170 |
Under the Ginkgo Collaboration Agreement, subject to certain exceptions, including excluded or refused products and cost savings initiatives, the profit on the sale of products subject to the Ginkgo Collaboration Agreement as well as cost-sharing, milestone and “value-creation” payments associated with the development and production of such products will be shared equally between the parties. The parties also agreed to provide each other with a license and other rights to certain intellectual property necessary to support the development and manufacture of the products under the Ginkgo Collaboration, and also to provide each other with access to certain other intellectual property useful in connection with the activities to be undertaken under the Ginkgo Collaboration Agreement, subject to certain carve-outs.
The initial term of the Ginkgo Collaboration Agreement is three years, and will automatically renew for successive one-year terms unless either party provides written notice of termination not less than 90 days prior to the expiration of the then-current term. In addition, the Ginkgo Collaboration Agreement provides that the parties will evaluate the performance of the Ginkgo Collaboration as of the 18-month anniversary of the Ginkgo Collaboration Agreement, and if either party has been repeatedly unable to perform or meet its commitments under the Ginkgo Collaboration Agreement, the other party will have the right to terminate the Ginkgo Collaboration Agreement on 30 days written notice.
The Company recognized $15.0 million and zero of collaboration revenue under the Initial Ginkgo Agreement and the Ginkgo Collaboration Agreement, respectively, for the year ended December 31, 2016. As of December 31, 2016, $1.6 million is payable by the Company to Ginkgo for its share of collaboration fees.
Intellectual Property License and Strain Access Agreement
In December 2016, the Company entered into an Intellectual Property License and Strain Access Agreement with a food ingredients and nutraceuticals company. Pursuant to the agreement, the Company granted the company a royalty-free, non-exclusive, worldwide, license to access and use certain Company intellectual property for the purpose of research and development, scale-up, manufacturing and commercialization activities. In exchange for such license, the company agreed to pay the Company a fee of $10 million in cash. The terms of the agreement are being renegotiated such that non-cash consideration may be received, instead of cash. The financial impact of the agreement will be recognized when the renegotiation is completed.
Financing Agreements
2016 Private Placements
| 171 |
See Note 5, “Debt” for details regarding the February, June and October 2016 Private Placements.
At Market Issuance Sales Agreement
On March 8, 2016, the Company entered into an At Market Issuance Sales Agreement (ATM Sales Agreement) with FBR Capital Markets & Co. and MLV & Co. LLC (Agents) under which the Company may issue and sell shares of its common stock having an aggregate offering price of up to $50.0 million (ATM Shares) from time to time through the Agents, acting as its sales agents, under the Company’s Registration Statement on Form S-3 (File No. 333-203216), effective April 15, 2015. Sales of the ATM Shares through the Agents, if any, will be made by any method that is deemed an “at the market offering” as defined in Rule 415 under the Securities Act, including by means of ordinary brokers’ transactions at market prices, in block transactions, or as otherwise agreed by the Company and the Agents. Each time that the Company wishes to issue and sell ATM Shares under the ATM Sales Agreement, the Company will notify one of the Agents of the number of ATM Shares to be issued, the dates on which such sales are anticipated to be made, any minimum price below which sales may not be made and other sales parameters as the Company deems appropriate. The Company will pay the designated Agent a commission rate of up to 3.0% of the gross proceeds from the sale of any ATM Shares sold through such Agent as agent under the ATM Sales Agreement. The ATM Sales Agreement contains customary terms, provisions, representations and warranties. The ATM Sales Agreement includes no commitment by other parties to purchase shares the Company offers for sale.
During the year ended December 31, 2016, the Company did not sell any shares of common stock under the ATM Sales Agreement. As of the date hereof, $50.0 million remained available for future sales under the ATM Sales Agreement.
March 2016 R&D Note
See Note 7, “Joint Ventures and Noncontrolling Interest” for details regarding the March 2016 R&D Note.
Bill & Melinda Gates Foundation Investment
On April 8, 2016, the Company entered into a Securities Purchase Agreement with the Bill & Melinda Gates Foundation (Gates Foundation), pursuant to which the Company agreed to sell and issue 4,385,964 shares of its common stock to the Gates Foundation in a private placement at a purchase price per share equal to $1.14, the average of the daily closing price per share of the Company’s common stock on the NASDAQ Stock Market for the twenty consecutive trading days ending on April 7, 2016, for aggregate proceeds to the Company of approximately $5 million (Gates Foundation Investment). The Securities Purchase Agreement includes customary representations, warranties and covenants of the parties. The closing of the Gates Foundation Investment occurred on May 10, 2016.
In connection with the entry into the Securities Purchase Agreement, on April 8, 2016, the Company and the Gates Foundation entered into a Charitable Purposes Letter Agreement, pursuant to which the Company agreed to expend an aggregate amount not less than the amount of the Gates Foundation Investment to develop a yeast strain that produces artemisinic acid and/or amorphadiene at a low cost and to supply such artemisinic acid and amorphadiene to companies qualified to convert artemisinic acid and amorphadiene to artemisinin for inclusion in artemisinin combination therapies used to treat malaria commencing in 2017. If the Company defaults in its obligation to use the proceeds from the Gates Foundation Investment as set forth above or defaults under certain other commitments in the Charitable Purposes Letter Agreement, the Gates Foundation will have the right to request that the Company redeem, or facilitate the purchase by a third party of, the Gates Foundation Investment shares then held by the Gates Foundation at a price per share equal to the greater of (i) the closing price of the Company’s common stock on the trading day prior to the redemption or purchase, as applicable, or (ii) an amount equal to $1.14 plus a compounded annual return of 10%. As of December 31, 2016, $5.0 million of the funding received was classified as mezzanine equity.
| 172 |
2016 Convertible Note Offerings
See Note 5, “Debt” for details regarding the May and December 2016 Convertible Note Offerings.
Fourth Amendment to LSA
See Note 5, “Debt” for details regarding the fourth amendment to the LSA.
Guanfu Credit Facility
See Note 5, “Debt” for details regarding the Guanfu Credit Facility.
Glycotech Acquisition and Salisbury Note
See Note 5, “Debt” and Note 7, “Joint Ventures and Noncontrolling Interest” for details regarding the acquisition of the Glycotech Assets and related Salisbury Note.
Neossance JV and Nikko Note
See Note 5, “Debt” and Note 7, “Joint Ventures and Noncontrolling Interest” for details regarding the formation of the Neossance JV and related Nikko Note.
Fidelity Exchange
See Note 5, “Debt” and Note 16, “Subsequent Events” for details regarding the Fidelity Exchange.
9. Goodwill and Intangible Assets
The following table presents the components of the Company's goodwill and intangible assets (in thousands):
| December 31, 2016 | December 31, 2015 | |||||||||||||||||||||||||
| Useful Life in Years | Gross Carrying Amount | Accumulated Amortization/ Impairment | Net Carrying Value | Gross Carrying Amount | Accumulated Amortization/ Impairment | Net Carrying Value | ||||||||||||||||||||
| In-process research and development | Indefinite | $ | 8,560 | $ | (8,560 | ) | $ | — | $ | 8,560 | $ | (8,560 | ) | $ | — | |||||||||||
| Acquired licenses and permits | 2 | 772 | (772 | ) | — | 772 | (772 | ) | — | |||||||||||||||||
| Goodwill | Indefinite | 560 | — | 560 | 560 | — | 560 | |||||||||||||||||||
| $ | 9,892 | $ | (9,332 | ) | $ | 560 | $ | 9,892 | $ | (9,332 | ) | $ | 560 | |||||||||||||
| 173 |
The in-process research and development (IPR&D) of $8.6 million was acquired through the acquisition of Draths in October 2011 and was treated as indefinite lived intangible assets pending completion or abandonment of the projects to which the IPR&D related. The IPR&D was fully impaired in 2015.
The Company has a single reportable segment (see Note 15, “Reporting Segments” for further details). Consequently, all of the Company's goodwill is attributable to that single reportable segment.
10. Stockholders’ Deficit
Private Placement
December 2012 Private Placement
In December 2012, the Company completed a private placement of 14,177,849 shares of its common stock at a price of $2.98 per share for aggregate proceeds of $37.2 million and the cancellation of $5.0 million worth of outstanding senior unsecured convertible promissory notes previously issued to Total by the Company. The Company issued 1,677,852 shares to Total in exchange for this note cancellation. Net cash received for this private placement as of December 31, 2012 was $22.2 million and the remaining $15.0 million of proceeds was received in January 2013. In connection with this, the Company entered into a letter of agreement with an investor under which the Company acknowledged that the investor's initial investment of $10.0 million in December 2012 represented partial satisfaction of the investor's preexisting contractual obligation to fund $15.0 million by March 31, 2013 upon satisfaction by the Company of criteria associated with the commissioning of the Company's production plant in Brotas, Brazil.
In January 2013, the Company received $15.0 million in proceeds from the private placement offering that closed in December 2012. Consequently, the Company issued 5,033,557 shares of the 14,177,849 shares of the Company's common stock.
March 2013 Private Placement
In March 2013, the Company completed a private placement of 1,533,742 shares of its common stock at a price of $3.26 per share for aggregate proceeds of $5.0 million. This private placement represented the final tranche of an investor’s preexisting contractual obligation to fund $15.0 million upon satisfaction by the Company of certain criteria associated with the commissioning of a production plant in Brotas, Brazil.
April 2014 Private Placement
In April 2014, the Company completed a private placement of 943,396 shares of its common stock at a price of $4.24 per share for aggregate proceeds of $4.0 million.
| 174 |
July 2015 Private Placement
In July 2015, the Company completed a private placement of 16,025,642 shares of its common stock at a price of $1.56 per share and warrants for the purchase of 1,602,562 shares of its common stock, exercisable at an exercise price of $0.01 per share, for aggregate proceeds to the Company of $25 million.
May 2016 Private Placement
In May 2016, the Company completed a private placement of 4,385,964 shares of its common stock at a price of $1.14 per share, for aggregate proceeds to the Company of approximately $5.0 million (see Note 8, “Significant Agreements”).
Evergreen Shares for 2010 Equity Plan and 2010 ESPP
In January 2016, the Company's Board of Directors (or Board) approved an increase to the number of shares available for issuance under the Company's 2010 Equity Incentive Plan (or Equity Plan) and the 2010 Employee Stock Purchase Plan (or ESPP). These shares represent an automatic annual increase in the number of shares available for issuance under the Equity Plan and the ESPP of 10,301,709 and 1,030,170, respectively. These increases are equal to 5% and 0.5%, respectively, of 206,034,184 shares, the total outstanding shares of the Company’s common stock as of December 31, 2015. This automatic increase was effective as of January 1, 2016. Shares available for issuance under the Equity Plan and ESPP were initially registered on a registration statement on Form S-8 filed with the Securities and Exchange Commission on October 1, 2010 (Registration No. 333-169715). The Company filed a registration statement on Form S-8 on April 1, 2016 (Registration No. 333-210569) with respect to the shares added by the automatic increase on January 1, 2016.
Common Stock
As of December 31, 2016 and 2015, the Company was authorized to issue 500,000,000 and 400,000,000 shares of common stock, respectively, pursuant to the Company’s certificate of incorporation, as amended and restated (in May 2016, the Company filed an amendment to its restated certificate of incorporation to increase the shares of common stock authorized by the Company from 400,000,000 to 500,000,000 in connection with the approval thereof by the Company's stockholders at the annual meeting of the Company’s stockholders held in May 2016). Holders of the Company’s common stock are entitled to dividends as and when declared by the Board, subject to the rights of holders of all classes of stock outstanding having priority rights as to dividends. There have been no dividends declared to date. The holder of each share of common stock is entitled to one vote.
Preferred Stock
Pursuant to the Company’s amended and restated certificate of incorporation, the Company is authorized to issue 5,000,000 shares of preferred stock. The Board has the authority, without action by its stockholders, to designate and issue shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. As of December 31, 2016 and December 31, 2015, the Company had zero shares of convertible preferred stock outstanding.
| 175 |
Common Stock Warrants
In December 2011, in connection with a capital lease agreement, the Company issued warrants to purchase 21,087 shares of the Company's common stock at an exercise price of $10.67 per share. The Company estimated the fair value of these warrants as of the issuance date to be $0.2 million and recorded these warrants as other assets, amortizing them subsequently over the term of the lease. The fair value was based on the contractual term of the warrants of 10 years, risk free interest rate of 2%, expected volatility of 86% and zero expected dividend yield. These warrants remain unexercised and outstanding as of December 31, 2016.
In October 2013, in connection with the issuance of the Tranche I Notes (see Note 5, "Debt"), the Company issued to Temasek contingently exercisable warrants to purchase 1,000,000 shares of the Company's common stock at an exercise price of $0.01 per share. The Company estimated the fair value of these warrants as of the issuance date at $1.3 million and recorded these warrants as debt issuance cost to be amortized over the term of the Tranche I Notes. The fair-value was calculated using a Monte Carlo simulation valuation model based on the contractual term of the warrants of 3.4 years, risk free interest rate of 0.77%, expected volatility of 45% and zero expected dividend yield. These warrants have been exercised in full as of December 31, 2016.
Each of these warrants includes a cashless exercise provision which permits the holder of the warrant to elect to exercise the warrant without paying the cash exercise price, and receive a number of shares determined by multiplying (i) the number of shares for which the warrant is being exercised by (ii) the difference between the fair market value of the stock on the date of exercise and the warrant exercise price, and dividing such by (iii) the fair market value of the stock on the date of exercise. During the years ended December 31, 2016 and 2015, no warrants were exercised through the cashless exercise provision.
The Temasek Exchange Warrant and Total Funding Warrant issued under the Exchange Agreement were recognized in equity at fair value on July 29, 2015. The fair value was calculated using a Black-Scholes valuation model based on the contractual term of the warrants, risk free interest rate of 2%, expected volatility of 74% and zero expected dividend yield. See Note 5, "Debt," for further details.
In July 2015, in connection with a private offering of the Company’s common stock, the Company issued warrants to purchase a total of 1,602,562 of shares of common stock to the investors in the private offering. The warrants have an exercise price of $0.01 per share and, as of December 31, 2016, 1,442,307 of these warrants remain unexercised and outstanding.
In February 2016, in connection with a private offering of promissory notes, the Company issued warrants for the purchase, at an exercise price of $0.01 per share, of an aggregate of 2,857,142 shares of its common stock. See Note 5, "Debt," for further details. As of December 31, 2016, these warrants remain unexercised and outstanding.
In August 2016, the Company issued warrants to purchase 5,000,000 shares of our common stock, at an exercise price of $0.50 per share, to Ginkgo in exchange for the transfer of certain information technology from Ginkgo to the Company. See Note 8, "Significant Agreements," for further details. As of December 31, 2016, these warrants remain unexercised and outstanding.
| 176 |
In November 2016, the Company issued warrants to purchase 10,000,000 shares of our common stock, at an exercise price of $0.50 per share, to Nenter & Co., Inc. (Nenter) pursuant to the terms of, and as consideration for, that certain Cooperation Agreement, dated as of October 26, 2016, between the Company and Nenter. As of December 31, 2016, such warrant had been exercised in full.
As of December 31, 2016 and 2015, the Company had 14,663,411 and 4,343,733 of unexercised common stock warrants, respectively.
11. Stock-Based Compensation Plans
2010 Equity Incentive Plan
The Company's 2010 Equity Incentive Plan (or 2010 Equity Plan) became effective on September 28, 2010 and will terminate in 2020. Pursuant to the 2010 Equity Plan, any shares of the Company’s common stock (i) issued upon exercise of stock options granted under the Company's 2005 Stock Option/Stock Issuance Plan (2005 Plan) that cease to be subject to such option and (ii) issued under the 2005 Plan that are forfeited or repurchased by the Company at the original purchase price will become part of the 2010 Equity Plan. Subsequent to the effective date of the 2010 Equity Plan, an additional 2,193,704 shares that were forfeited under the 2005 Plan were added to the shares reserved for issuance under the 2010 Equity Plan.
The number of shares reserved for issuance under the 2010 Equity Plan increase automatically on January 1st of each year starting with January 1, 2011, by a number of shares equal to 5% percent of the Company’s total outstanding shares as of the immediately preceding December 31st. However, the Company’s Board of Directors or the Leadership Development and Compensation Committee of the Board of Directors retains the discretion to reduce the amount of the increase in any particular year. The 2010 Equity Plan provides for the granting of common stock options, restricted stock awards, stock bonuses, stock appreciation rights, restricted stock units and performance awards. It allows for time-based or performance-based vesting for the awards. Options granted under the 2010 Equity Plan may be either incentive stock options (or ISOs) or non-statutory stock options (or NSOs). ISOs may be granted only to Company employees (including officers and directors who are also employees). NSOs may be granted to Company employees, non-employee directors and consultants. The Company will be able to issue no more than 30,000,000 shares pursuant to the grant of ISOs under the 2010 Equity Plan. Options under the 2010 Equity Plan may be granted for periods of up to ten years. All options issued to date have had a ten year life. Under the plan, the exercise price of any ISOs and NSOs may not be less than 100% of the fair market value of the shares on the date of grant. The exercise price of any ISOs and NSOs granted to a 10% stockholder may not be less than 110% of the fair value of the underlying stock on the date of grant. The options granted to date generally vest over four to five years.
As of December 31, 2016 and 2015, options to purchase 11,924,020 and 11,321,194 shares, respectively, of the Company's common stock granted from the 2010 Equity Plan were outstanding. As of December 31, 2016 and 2015, 8,285,891 and 1,833,004 shares, respectively, of the Company’s common stock remained available for future awards that may be granted from the 2010 Equity Plan. The options outstanding as of December 31, 2016 and 2015 had a weighted-average exercise price of approximately $3.01 per share and $4.27 per share, respectively.
| 177 |
2005 Stock Option/Stock Issuance Plan
In 2005, the Company established its 2005 Stock Option/Stock Issuance Plan (or 2005 Plan) which provided for the granting of common stock options, restricted stock units, restricted stock and stock purchase rights awards to employees and consultants of the Company. The 2005 Plan allowed for time-based or performance-based vesting for the awards. Options granted under the 2005 Plan were ISOs or NSOs. ISOs were granted only to Company employees (including officers and directors who are also employees). NSOs were granted to Company employees, non-employee directors, and consultants.
All options issued under the 2005 Plan had a ten year life. The exercise prices of ISOs and NSOs granted under the 2005 Plan were not less than 100% of the estimated fair value of the shares on the date of grant, as determined by the Board of Directors. The exercise price of an ISO and NSO granted to a 10% stockholder could not be less than 110% of the estimated fair value of the underlying stock on the date of grant as determined by the Board. The options generally vested over 5 years.
As of December 31, 2016 and 2015, options to purchase 1,503,665 and 1,548,918 shares, respectively, of the Company’s common stock granted from the 2005 Plan remained outstanding and as a result of the adoption of the 2010 Equity Plan discussed above, zero shares of the Company’s common stock remained available for future awards issuance under the 2005 Plan. The options outstanding under the 2005 Plan as of December 31, 2016 and 2015 had a weighted-average exercise price of approximately $8.51 per share and $8.46 per share, respectively.
2010 Employee Stock Purchase Plan
The 2010 Employee Stock Purchase Plan (2010 ESPP) became effective on September 28, 2010. The 2010 ESPP is designed to enable eligible employees to purchase shares of the Company’s common stock at a discount. Each offering period is for one year and consists of two six-month purchase periods. Each twelve-month offering period generally commences on May 16th and November 16th, each consisting of two six-month purchase periods. The purchase price for shares of common stock under the 2010 ESPP is the lesser of 85% of the fair market value of the Company’s common stock on the first day of the applicable offering period or the last day of each purchase period. A total of 168,627 shares of common stock were initially reserved for future issuance under the 2010 Employee Stock Purchase Plan. During the life of the 2010 ESPP, the number of shares reserved for issuance increases automatically on January 1st of each year, starting with January 1, 2011, by a number of shares equal to 1% of the Company’s total outstanding shares as of the immediately preceding December 31st. However, the Company’s Board of Directors or the Leadership Development and Compensation Committee of the Board of Directors retains the discretion to reduce the amount of the increase in any particular year. No more than 10,000,000 shares of the Company’s common stock may be issued under the 2010 ESPP and no other shares may be added to this plan without the approval of the Company’s stockholders.
During the years ended December 31, 2016 and 2015, 336,075 and 385,892 shares, respectively, of the Company's common stock were purchased under the 2010 ESPP. At December 31, 2016 and 2015, 885,821 and 1,221,896 shares, respectively, of the Company’s common stock remained available for issuance under the 2010 ESPP.
| 178 |
Stock Option Activity
The Company’s stock option activity and related information for the year ended December 31, 2016 was as follows:
| Number Outstanding | Weighted- Average Exercise Price | Weighted-Average Remaining Contractual Life (Years) | Aggregate Intrinsic Value | |||||||||||||
| (in thousands) | ||||||||||||||||
| Outstanding - December 31, 2015 | 12,930,112 | $ | 4.77 | 7.39 | $ | 22 | ||||||||||
| Options granted | 3,585,175 | $ | 0.59 | — | — | |||||||||||
| Options exercised | (134 | ) | $ | 0.28 | — | — | ||||||||||
| Options cancelled | (3,087,468 | ) | $ | 4.92 | — | — | ||||||||||
| Outstanding - December 31, 2016 | 13,427,685 | $ | 3.63 | 6.70 | $ | 443 | ||||||||||
| Vested and expected to vest after December 31, 2016 | 12,387,314 | $ | 3.84 | 6.54 | $ | 361 | ||||||||||
| Exercisable at December 31, 2016 | 7,357,308 | $ | 5.48 | 5.32 | $ | 1 | ||||||||||
The aggregate intrinsic value of options exercised under all option plans was $0.0 million, $0.0 million and $0.6 million for the years ended December 31, 2016, 2015 and 2014, respectively, determined as of the date of option exercise.
The Company’s restricted stock units (or RSUs) and restricted stock activity and related information for the year ended December 31, 2016 was as follows:
| RSUs | Weighted-Average Grant-Date Fair Value | Weighted Average Remaining Contractual Life (Years) | ||||||||||
| Outstanding - December 31, 2015 | 5,554,844 | $ | 2.03 | 1.61 | ||||||||
| Awarded | 4,897,840 | $ | 0.61 | — | ||||||||
| Vested | (2,064,881 | ) | $ | 1.98 | — | |||||||
| Forfeited | (1,390,719 | ) | $ | 1.39 | — | |||||||
| Outstanding - December 31, 2016 | 6,997,084 | $ | 1.18 | 1.44 | ||||||||
| Expected to vest after December 31, 2016 | 5,642,538 | $ | 1.20 | 1.28 | ||||||||
| 179 |
The following table summarizes information about stock options outstanding as of December 31, 2016:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Exercise Price | Number of Options | Weighted- Average Remaining Contractual Life (Years) | Weighted-Average Exercise Price | Number of Options | Weighted-Average Exercise Price | |||||||||||||||
| $0.28—$0.57 | 346,234 | 9.47 | $ | 0.43 | 2,734 | $ | 0.28 | |||||||||||||
| $0.59—$0.59 | 2,424,375 | 8.62 | $ | 0.59 | — | $ | — | |||||||||||||
| $0.78—$1.67 | 1,461,082 | 8.53 | $ | 1.46 | 396,916 | $ | 1.61 | |||||||||||||
| $1.69—$1.96 | 2,218,041 | 7.30 | $ | 1.84 | 863,045 | $ | 1.85 | |||||||||||||
| $1.98—$2.85 | 1,371,168 | 5.82 | $ | 2.61 | 1,129,021 | $ | 2.67 | |||||||||||||
| $2.87—$3.44 | 1,255,796 | 6.52 | $ | 3.02 | 1,095,271 | $ | 3.01 | |||||||||||||
| $3.51—$3.51 | 1,503,891 | 7.06 | $ | 3.51 | 1,020,549 | $ | 3.51 | |||||||||||||
| $3.55—$4.08 | 1,354,152 | 3.90 | $ | 3.88 | 1,296,826 | $ | 3.88 | |||||||||||||
| $4.31—$24.20 | 1,350,946 | 3.40 | $ | 13.05 | 1,350,946 | $ | 12.05 | |||||||||||||
| 24.50—$30.17 | 202,000 | 4.31 | $ | 27.52 | 202,000 | $ | 27.52 | |||||||||||||
| $0.28—$30.17 | 13,487,685 | 6.70 | $ | 3.63 | 7,357,308 | $ | 5.48 | |||||||||||||
Stock-Based Compensation Expense
Stock-based compensation expense related to options and restricted stock units granted to employees was allocated to research and development expense and sales, general and administrative expense as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Research and development | $ | 1,948 | $ | 2,306 | $ | 3,508 | ||||||
| Sales, general and administrative | 5,377 | 6,828 | 10,597 | |||||||||
| Total stock-based compensation expense | $ | 7,325 | $ | 9,134 | $ | 14,105 | ||||||
During the years ended December 31, 2016, 2015 and 2014, the Company granted options to purchase 3,585,175 shares, 4,720,278 shares, and 3,683,791 shares of its common stock, respectively, with weighted-average grant date fair values of $0.59, $1.21, and $2.31 per share, respectively. Compensation expense of $3.5 million, $6.0 million, and $10.1 million was recorded for the years ended December 31, 2016, 2015 and 2014, respectively, for stock-based options granted. As of December 31, 2016, 2015 and 2014, there were unrecognized compensation costs of $4.4 million, $8.0 million, and $11.4 million, respectively, related to these stock options. The Company expects to recognize those costs over a weighted-average period of 2.7 years and 3.0 years as of December 31, 2016 and 2015, respectively. Future option grants will increase the amount of compensation expense to be recorded in these periods.
During the years ended December 31, 2016, 2015 and 2014, 4,897,840, 4,988,539 and 1,083,300 of restricted stock units, respectively, were granted with a weighted-average service-inception date fair value of $0.61, $1.82 and $3.51 per unit, respectively. The Company recognized a total of $3.6 million, $2.8 million and $3.3 million, respectively, for the years ended December 31, 2016, 2015 and 2014 in stock-based compensation expense for restricted stock units granted. As of December 31, 2016, 2015 and 2014, there were unrecognized compensation costs of $5.4 million, $7.7 million and $3.6 million, respectively, related to these restricted stock units.
| 180 |
During the years ended December 31, 2016, 2015 and 2014, the Company also recognized stock-based compensation expense related to its 2010 ESPP of $0.1 million, $0.3 million, and $0.5 million, respectively.
Employee stock-based compensation expense recognized for the years ended December 31, 2016, 2015 and 2014 included zero, zero and $0.1 million, respectively, related to option modifications. As part of separation agreements with certain former senior employees, the Company agreed to accelerate the vesting of options for zero, zero and zero shares of common stock and extend the exercise period for certain grants in the years ended December 31, 2016, 2015 and 2014, respectively.
Stock-based compensation cost for RSUs is measured based on the closing fair market value of the Company's common stock on the date of grant. Stock-based compensation expense for stock options and employee stock purchase plan rights is estimated at the grant date and offering date, respectively, based on the fair-value using the Black-Scholes option pricing model. The fair value of employee stock options is being amortized on a straight-line basis over the requisite service period of the awards. The fair value of employee stock options was estimated using the following weighted-average assumptions:
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Expected dividend yield | — | % | — | % | — | % | ||||||
| Risk-free interest rate | 1.4 | % | 1.8 | % | 1.9 | % | ||||||
| Expected term (in years) | 6.16 | 6.08 | 6.10 | |||||||||
| Expected volatility | 73 | % | 74 | % | 75 | % | ||||||
Expected Dividend Yield - The Company has never paid dividends and does not expect to pay dividends.
Risk-Free Interest Rate - The risk-free interest rate was based on the market yield currently available on United States Treasury securities with maturities approximately equal to the options' expected terms.
Expected Term—Expected term represents the period that the Company’s stock-based awards are expected to be outstanding. The Company’s assumption about the expected term has been based on historical data on employee exercises.
Expected Volatility—The expected volatility is based on historical volatility for the Company's stock.
Forfeiture Rate—The Company estimates its forfeiture rate based on an analysis of its actual forfeitures and will continue to evaluate the adequacy of the forfeiture rate based on actual forfeiture experience, analysis of employee turnover behavior, and other factors. The impact from a forfeiture rate adjustment will be recognized in full in the period of adjustment, and if the actual number of future forfeitures differs from that estimated by the Company, the Company may be required to record adjustments to stock-based compensation expense in future periods.
Each of the inputs discussed above is subjective and generally requires significant management and director judgment.
| 181 |
12. Employee Benefit Plan
The Company established a 401(k) Plan to provide tax deferred salary deductions for all eligible employees. Participants may make voluntary contributions to the 401(k) Plan up to 90% of their eligible compensation, limited by certain Internal Revenue Service (or IRS) restrictions. Effective January 2014, the Company implemented a discretionary employer match plan whereby the Company matches employee contributions for the year ended December 31, 2014 onwards up to the IRS limit or 90% of compensation, with a minimum one year of service required for vesting. The total matching amount for each of the years ended December 31, 2016 and 2015 was $0.5 million.
13. Related Party Transactions
Related Party Financings
See Note 5, “Debt” for a description of the February 2016 Private Placement transaction with Foris Ventures, LLC (or Foris), Naxyris S.A. and Biolding SA, each a related party of the Company, the March 2016 R&D Note transaction with Total and the June 2016 and October 2016 Private Placement transactions with Foris. See Note 16, “Subsequent Events” for additional details regarding the March 2016 R&D Note.
As of December 31, 2016 and December 31, 2015, convertible notes and loans with related parties were outstanding in an aggregate amount of $72.4 million and $42.7 million, respectively, net of debt discount and issuance costs of $6.7 million and $4.9 million, respectively.
The fair value of the derivative liabilities related to the related party convertible notes as of December 31, 2016 and December 31, 2015 was $0.8 million and $7.9 million, respectively. The Company recognized a gain from change in fair value of these derivative liabilities of $7.6 million, $10.5 million and $141.2 million for the years ended December 31, 2016, 2015 and 2014, respectively, (see Note 3, "Fair Value of Financial Instruments" for further details).
Related Party Revenues
The Company recognized related party revenues from product sales to Total of $0.2 million, $0.9 million and $0.6 million for the years ended December 31, 2016, 2015 and 2014, respectively. Related party accounts receivable from Total as of December 31, 2016 and 2015, were $0.8 million and $1.2 million, respectively. The Company recognized related party revenues from product sales to Novvi of $1.4 million, zero and $0.1 million for the years ended December 31, 2016, 2015 and 2014, respectively. Related party accounts receivable from Novvi were zero as of each of December 31, 2016 and 2015. In addition, in October 2016 the Company entered into a collaboration contract with the Department of Energy, in which Total and Renmatix participate as subcontractors. There were no revenues, receivables or payables related to the Department of Energy contract in fiscal year 2016.
Loans to Related Parties
See Note 7, "Joint Ventures and Noncontrolling Interest" for details of the Company's transactions with its affiliate, Novvi LLC.
| 182 |
Joint Venture with Total
In November 2013, the Company and Total formed TAB as discussed above under Note 7, "Joint Ventures and Noncontrolling Interest."
Pilot Plant and Secondee Agreements
In May 2014, the Company received the final consents necessary for the Pilot Plant Services Agreement (Pilot Plant Services Agreement) and a Sublease Agreement (Sublease Agreement), each dated as of April 4, 2014 (collectively the Pilot Plant Agreements), between the Company and Total. The Pilot Plant Agreements generally have a term of five years. Under the terms of the Pilot Plant Services Agreement, the Company agreed to provide certain fermentation and downstream separations scale-up services and training to Total and receives an aggregate annual fee payable by Total for all services in the amount of up to approximately $0.9 million per annum. In July 2015, Total and the Company entered into Amendment #1 (Pilot Plant Agreement Amendment) to the Pilot Plant Services Agreement whereby the Company agreed to waive a portion of these fees, up to approximately $2.0 million, over the term of the Pilot Plant Services Agreement in connection with the restructuring of TAB discussed above. Under the Sublease Agreement, the Company receives an annual base rent payable by Total of approximately $0.1 million per annum.
As of December 31, 2016, the Company had received $1.7 million in cash under the Pilot Plant Agreements from Total. In connection with these arrangements, sublease payments and service fees of $0.4 million and $0.9 million for the years ended December 31, 2016 and 2015, respectively, were offset against costs and operating expenses. Total charges its secondees to the Company for research and development services. The amount charged to the Company by Total in 2016 and 2015 was $0.8 million and $0.9 million, respectively. The payable to Total under these arrangements was $2.2 million and $1.4 million as of December 31, 2016 and 2015, respectively.
Office Sublease
As of December 31, 2016, the Company recharged office rental expenses to Novvi of $0.4 million (net of $0.4 million forgiven) for 2016 and $0.7 million for 2015. As of December 31, 2016, 2015, $0.2 million and zero, respectively, was receivable from Novvi.
14. Income Taxes
For each of the years ended December 31, 2016, 2015 and 2014, the Company recorded a provision for income taxes of $0.5 million. The provision for income taxes for the years ended December 31, 2016, 2015 and 2014, primarily relates to accrued withholding taxes that would be due in connection with the payment of interest on intercompany loans. Other than the above mentioned provision for income taxes, no additional provision for income taxes has been made, net of the valuation allowance, due to cumulative losses since the commencement of operations.
The components of income (loss) before income taxes, loss from investments in affiliates and noncontrolling interest are as follows for the years ended December 31, 2016, 2015 and 2014 (in thousands):
| 183 |
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| United States | $ | (101,210 | ) | $ | (188,943 | ) | $ | 10,847 | ||||
| Foreign | 4,429 | (24,457 | ) | (5,275 | ) | |||||||
| Income (loss) before income taxes and loss from investments in affiliates | $ | (96,781 | ) | $ | (213,400 | ) | $ | 5,572 | ||||
The components of the provision for income taxes are as follows for the years ended December 31, 2016, 2015 and 2014 (in thousands):
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Current: | ||||||||||||
| Federal | $ | — | $ | — | $ | — | ||||||
| State | — | — | — | |||||||||
| Foreign | 553 | 468 | 495 | |||||||||
| Total current provision | 553 | 468 | 495 | |||||||||
| Deferred: | ||||||||||||
| Federal | — | — | — | |||||||||
| State | — | — | — | |||||||||
| Foreign | — | — | — | |||||||||
| Total deferred provision | — | — | — | |||||||||
| Total provision for income taxes | $ | 553 | $ | 468 | $ | 495 | ||||||
A reconciliation between the statutory federal income tax and the Company’s effective tax rates as a percentage of income (loss) before income taxes is as follows:
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Statutory tax rate | (34.0 | )% | (34.0 | )% | (34.0 | )% | ||||||
| State tax rate, net of federal benefit | 0.0 | % | (0.3 | )% | 23.3 | % | ||||||
| Stock-based compensation | — | % | 0.1 | % | (2.8 | )% | ||||||
| Federal R&D credit | (0.8 | )% | (0.6 | )% | 31.0 | % | ||||||
| Derivative liabilities | 1.4 | % | 3.6 | % | 541.5 | % | ||||||
| Non-Deductible Interest | 5.0 | % | 5.5 | % | 0.0 | % | ||||||
| Other | (3.2 | )% | 0.1 | % | (7.8 | )% | ||||||
| Foreign losses | 0.5 | % | (1.2 | )% | 32.3 | % | ||||||
| Change in valuation allowance | 31.7 | % | 27.1 | % | (592.4 | )% | ||||||
| Effective income tax rate | 0.6 | % | 0.3 | % | (8.9 | )% | ||||||
| 184 |
Temporary differences and carryforwards that gave rise to significant portions of deferred taxes are as follows (in thousands):
| December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Net operating loss carryforwards | $ | 236,741 | $ | 207,241 | $ | 195,536 | ||||||
| Fixed assets | 12,917 | 10,519 | 1,299 | |||||||||
| Research and development credits | 17,348 | 16,612 | 14,701 | |||||||||
| Foreign Tax Credit | 2,452 | 1,899 | 1,431 | |||||||||
| Accruals and reserves | 30,303 | 26,366 | 16,425 | |||||||||
| Stock-based compensation | 17,184 | 19,048 | 18,773 | |||||||||
| Capitalized start-up costs | 9,182 | 9,568 | 13,095 | |||||||||
| Capitalized research and development costs | 65,962 | 63,339 | 56,880 | |||||||||
| Other | 6,714 | 9,999 | 6,700 | |||||||||
| Total deferred tax assets | 398,803 | 364,591 | 324,840 | |||||||||
| Fixed assets | — | — | — | |||||||||
| Debt discount and derivative | (11,936 | ) | (4,402 | ) | (12,517 | ) | ||||||
| Total deferred tax liabilities | (11,936 | ) | (4,402 | ) | (12,517 | ) | ||||||
| Net deferred tax asset prior to valuation allowance | 386,867 | 360,189 | 312,323 | |||||||||
| Less: Valuation allowance | (386,867 | ) | (360,189 | ) | (312,323 | ) | ||||||
| Net deferred tax assets (liabilities) | $ | — | $ | — | $ | — | ||||||
Recognition of deferred tax assets is appropriate when realization of such assets is more likely than not. Based upon the weight of available evidence, especially the uncertainties surrounding the realization of deferred tax assets through future taxable income, the Company believes it is more likely than not that the net deferred tax assets will not be fully realizable. Accordingly, the Company has provided a full valuation allowance against its net deferred tax assets as of December 31, 2016, 2015 and 2014. The valuation allowance increased by $26.7 million, $47.9 million, and $28.3 million, during the years ended December 31, 2016, 2015 and 2014, respectively.
As of December 31, 2016, the Company had federal net operating loss carryforwards of approximately $645.3 million, and state net operating loss carryforwards $208.7 million, available to reduce future taxable income, if any. As of December 31, 2016 approximately $27.2 million, of the federal loss carryforwards and $12.9 million of state net operating loss carryforwards, respectively, related to excess stock-based compensation, and accordingly no deferred tax asset is recognized for such amounts prior to the adoption of ASU 2016-09, discussed above in the Recent Accounting Pronouncements section of Note 2, “Summary of Significant Accounting Policies.” Any deferred tax asset recognized on adoption of ASU 2016-09 related to excess stock-based compensation, net of any applicable valuation allowance, will be recorded to retained earnings as of the date of adoption.
During the year ended December 31, 2015, unrecognized tax benefits of $8.5 million were resolved in connection with the outcome of a California Supreme Court case, involving another taxpayer, that concluded on a methodology which follows that certain of the Company’s net operating losses cannot be sustained. The decision had no impact on the Company’s gross deferred tax assets as presented, as the Company’s deferred tax asset for net operating losses was previously reported, net of a reserve for this same item.
| 185 |
As of December 31, 2016, the Company had federal research and development credits of $9.8 million and California research and development credit carryforwards of $11.4 million.
The Tax Reform Act of 1986 (TRA) and similar state provisions limit the use of net operating loss and credit carryforwards in certain situations where equity transactions result in a change of ownership as defined by Internal Revenue Code Section 382. In the event the Company has experienced an ownership change, as defined in the TRA, utilization of its federal and state net operating loss and credit carryforwards could be limited. If not utilized, the federal net operating loss carryforward begins expiring in 2025, and the California net operating loss carryforward begins expiring in 2016. The federal research and development credit carryforwards will expire starting in 2024 if not utilized. The California tax credits can be carried forward indefinitely.
A reconciliation of the beginning and ending amounts of unrecognized tax benefits is as follows:
| Balance at December 31, 2013 | $ | 6,080 | ||
| Increases in tax positions for prior period | 4,736 | |||
| Increases in tax positions during current period | 6,265 | |||
| Balance at December 31, 2014 | $ | 17,081 | ||
| Decreases in tax positions for prior period | (9,404 | ) | ||
| Increases in tax positions during current period | 957 | |||
| Balance at December 31, 2015 | $ | 8,634 | ||
| Decreases in tax positions for prior period | (314 | ) | ||
| Increases in tax positions during current period | 781 | |||
| Balance at December 31, 2016 | 9,101 |
The Company’s policy is to include interest and penalties related to unrecognized tax benefits within the provision for taxes. The Company determined that no accrual for interest and penalties was required as of December 31, 2016, December 31, 2015 or December 31, 2014.
None of the tax benefits, if recognized, would affect the effective income tax rate for any of the above years due to the valuation allowance that currently offsets deferred tax assets. The Company does not anticipate the total amount of unrecognized income tax benefits will significantly increase or decrease in the next 12 months.
The Company’s primary tax jurisdiction is the United States. For United States federal and state tax purposes, returns for tax years 2004 and forward remain open and subject to tax examination by the appropriate federal or state taxing authorities. Brazil tax years 2009 through the current remain open and subject to examination.
As of December 31, 2016, the US Internal Revenue Service (IRS) has completed its audit of the Company for tax year 2008 which concluded that there were no adjustments resulting from the audit. While the statutes are closed for tax year 2008, the US federal tax carryforwards (net operating losses and tax credits) may be adjusted by the IRS in the year in which the carryforward is utilized.
| 186 |
15. Reportable Segments
The chief operating decision maker for the Company is the chief executive officer. The chief executive officer reviews financial information presented on a consolidated basis, accompanied by information about revenues by geographic region, for purposes of allocating resources and evaluating financial performance. The Company has one business activity comprised of research and development and sales of fuels and farnesene-derived products and there are no segment managers who are held accountable for operations, operating results or plans for levels or components below the consolidated unit level. Accordingly, the Company has determined that it has a single reportable segment and operating segment structure.
Revenues by geography are based on the location of the customer. The following tables set forth revenues and long-lived assets by geographic area (in thousands):
Revenues
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| United States | $ | 30,944 | $ | 20,897 | $ | 21,331 | ||||||
| Brazil | 489 | 5,070 | 5,961 | |||||||||
| Europe | 23,612 | 3,557 | 9,738 | |||||||||
| Asia | 12,068 | 4,629 | 6,244 | |||||||||
| Other | 79 | — | — | |||||||||
| Total | $ | 67,192 | $ | 34,153 | $ | 43,274 | ||||||
Long-Lived Assets
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| United States | $ | 9,342 | $ | 18,401 | ||||
| Brazil | 44,153 | 41,093 | ||||||
| Europe | 240 | 303 | ||||||
| Total | $ | 53,735 | $ | 59,797 | ||||
| 187 |
16. Subsequent Events
LSA Amendment
On January 10, 2017, in connection with Stegodon granting certain waivers of the debt and transfer covenants under the LSA, the Company, certain of its subsidiaries and Stegodon entered into a fifth amendment of the LSA. Pursuant to the fifth amendment, the Company agreed to apply additional monies received by the Company under the Ginkgo Collaboration Agreement towards repayment of the outstanding loans under the Senior Secured Loan Facility, up to a maximum amount of $3 million, to the extent any such monies are received by the Company.
As described in more detail below under “Fidelity Notes Exchange,” on January 11, 2017, the Stegodon debt was extended from a maturity of February 1, 2017 to October 15, 2018 due to conditions being met as a result of the Fidelity Exchange (as defined below). The extension of the Stegodon debt met the requirements for a troubled debt restructuring with the future undiscounted cash flows being greater than the carrying value of the debt prior to extension. No gain will be recorded and a new effective interest rate will be established based on the carrying value of the debt and the revised cash flows.
Fidelity Notes Exchange
As described in more detail in Note 5, “Debt”, on December 28, 2016, the Company entered into an Exchange Agreement with the holders of its outstanding Fidelity Notes, pursuant to which the Company and the holders agreed to exchange such Fidelity Notes, together with accrued and unpaid interest thereon, for approximately $19.1 million in aggregate principal amount of the Company’s 2015 144A Notes, representing an exchange ratio of approximately 1:1.25, in a private placement exempt from registration under the Securities Act (Fidelity Exchange).
The closing of the Fidelity Exchange occurred on January 11, 2017. At the closing, the Company issued approximately $19.1 million in aggregate principal amount of its 2015 144A Notes (Additional 2015 144A Notes) to the holders in exchange for the cancellation of the outstanding Fidelity Notes. The Company did not receive any cash proceeds from the Fidelity Exchange. The exchange will be accounted for as an extinguishment of the Fidelity Notes and issuance of the Additional 2015 144A Notes. A gain of approximately $0.1 million will be recognized in the first quarter to recognize excess of the fair value of the Additional 2015 144A Notes compared to the carrying value of the Fidelity Notes at the time of extinguishment.
The Additional 2015 144A Notes were issued pursuant to the 2015 Indenture, as amended by the First Supplemental Indenture thereto, dated as of January 11, 2017, which amended the 2015 Indenture to clarify the Exchange Cap (as defined below) for the Additional 2015 144A Notes. See Note 5, “Debt” for details regarding the terms of the 2015 144A Notes and the 2015 Indenture. Unless and until the Company obtains stockholder approval to issue a number of shares of the Company’s common Stock in excess of the Exchange Cap (as defined below), holders of the Additional 2015 144A Notes will not have the right to receive shares of the Company’s common stock upon conversion of the Additional 2015 144A Notes, and the Company will not have the right to issue shares of common stock as payment of interest on the Additional 2015 144A Notes, including any Early Conversion Payment, if the aggregate number of shares issued with respect to the Additional 2015 144A Notes (and any other transaction aggregated for such purpose) after giving effect to such conversion or payment, as applicable, would exceed 19.99% of the number of shares of the Company’s common stock outstanding as of December 28, 2016 (Exchange Cap). The Company will pay cash in lieu of any shares that would otherwise be deliverable in excess of the Exchange Cap.
Pursuant to the Exchange Agreement, upon the closing of the Fidelity Exchange, the Fidelity Securities Purchase Agreement terminated. In addition, upon the closing of the Fidelity Exchange, in accordance with the terms of the fourth amendment to the Senior Secured Loan Facility, the maturity date of all outstanding loans under the Senior Secured Loan Facility was extended to October 15, 2018. See Note 5, “Debt” for details regarding the fourth amendment to the Senior Secured Loan Facility.
| 188 |
Novvi Investment
As discussed above in Note 7, “Joint Ventures and Noncontrolling Interest,” in July 2016, ARG agreed to make a capital contribution of up to $10.0 million in cash to Novvi, subject to certain conditions, in exchange for a one third ownership stake in Novvi, which capital contribution had been funded in the amount of $9.0 million as of December 31, 2016. On February 15, 2017, ARG funded the remaining $1.0 million capital contribution to Novvi.
Amendment to March 2016 R&D Note
On February 27, 2017, the Company and Total entered into an amendment to the R&D Note issued by the Company to Total on March 21, 2016 in the principal amount of $3.7 million to extend the maturity date of such R&D Note from March 1, 2017 to May 15, 2017. See Note 5, “Debt” for additional details regarding the R&D Notes.
Total and Temasek R&D Warrants
As discussed above under Note 5, “Debt”, in connection with the Exchange in July 2015, the Company issued (i) a warrant to Total to purchase 2,000,000 shares of the Company's common stock that would only be exercisable if the Company failed, as of March 1, 2017, to achieve a target cost per liter to manufacture farnesene (Total R&D Warrant) and (ii) a warrant to Temasek exercisable for that number of shares of the Company's common stock equal to 880,339 multiplied by a fraction equal to the number of shares for which Total exercises the Total R&D Warrant divided by 2,000,000 (Temasek R&D Warrant). As of March 1, 2017, the Company had not achieved the target cost per liter to manufacture farnesene provided in the Total R&D Warrant, and as a result, on March 1, 2017 the Total R&D Warrant became exercisable in accordance with its terms. In addition, upon any exercise by Total of the Total R&D Warrant, the Temasek R&D Warrant will become exercisable for that number of shares of the Company's common stock equal to 880,339 multiplied by a fraction equal to the number of shares for which Total exercises the Total R&D Warrant divided by 2,000,000.
Ginkgo Collaboration Note
On April 13, 2017, the Company issued a secured promissory note to Ginkgo, in the principal amount of $3 million dollars (the “Ginkgo Collaboration Note”), in satisfaction of certain payments owed by the Company to Ginkgo under the Ginkgo Collaboration Agreement (see Note 8, “Significant Agreements” above for details regarding the Ginkgo Collaboration Agreement). The Ginkgo Collaboration Note is collateralized by a second priority lien on the assets securing the Company’s obligations under the Senior Secured Loan Facility, and is subordinate to the Company’s obligations under the Senior Secured Loan Facility pursuant to a Subordination Agreement, dated as of October 27, 2016 and ratified on April 13, 2017, by and among the Company, Ginkgo and Stegodon. Interest will accrue on the Ginkgo Collaboration Note from and including April 13, 2017 at a rate of 13.50% per annum and is payable in full on May 15, 2017, the maturity date of the Ginkgo Collaboration Note, unless the Ginkgo Collaboration Note is prepaid in accordance with their terms prior to such date. The Ginkgo Collaboration Note contains customary terms, provisions, representations and warranties, including certain events of default after which the Ginkgo Collaboration Note may be due and payable immediately, as set forth in the Ginkgo Collaboration Note.
| 189 |
SUPPLEMENTARY FINANCIAL DATA
Selected Quarterly Financial Data (unaudited)
The following table presents selected unaudited consolidated financial data for each of the eight quarters in the two-year periods ended December 31, 2016. In the Company’s opinion, this unaudited information has been prepared on the same basis as the audited information and includes all adjustments (consisting of only normal recurring adjustments) necessary for a fair statement of the financial information for the periods presented. Net income (loss) per share—basic and diluted, for the four quarters of each fiscal year may not sum to the total for the fiscal year because of the different number of shares outstanding during each period.
| Quarter | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| (In thousands, except share and per share amounts) | ||||||||||||||||
| Year Ended December 31, 2016 | ||||||||||||||||
| Total revenues | $ | 8,811 | $ | 9,599 | $ | 26,544 | $ | 22,238 | ||||||||
| Product sales | $ | 3,140 | $ | 4,922 | $ | 6,820 | $ | 11,467 | ||||||||
| Gross loss from product sales | $ | (8,038 | ) | $ | (2,969 | ) | $ | (8,056 | ) | $ | (11,290 | ) | ||||
| Net loss attributable to common stockholders (for basic loss per share)(1) | $ | (15,308 | ) | $ | (13,566 | ) | $ | (19,704 | ) | $ | (48,756 | ) | ||||
| Net loss attributable to common stockholders (for diluted loss per share) | $ | (30,273 | ) | $ | (29,245 | ) | $ | (19,704 | ) | $ | (48,756 | ) | ||||
| Net loss per share: | ||||||||||||||||
| Basic(1) | $ | (0.07 | ) | $ | (0.06 | ) | $ | (0.08 | ) | $ | (0.18 | ) | ||||
| Diluted | $ | (0.12 | ) | $ | (0.11 | ) | $ | (0.08 | ) | $ | (0.18 | ) | ||||
| Shares used in calculation: | ||||||||||||||||
| Basic | 207,199,563 | 223,112,019 | 249,190,339 | 273,406,492 | ||||||||||||
| Diluted | 260,932,085 | 262,896,140 | 249,190,339 | 273,406,492 | ||||||||||||
| Year Ended December 31, 2015 | ||||||||||||||||
| Total revenues | $ | 7,872 | $ | 7,843 | $ | 8,591 | $ | 9,847 | ||||||||
| Product sales | $ | 2,095 | $ | 3,340 | $ | 4,228 | $ | 5,233 | ||||||||
| Gross loss from product sales | $ | (4,548 | ) | $ | (7,619 | ) | $ | (4,227 | ) | $ | (6,084 | ) | ||||
| Net loss attributable to common stockholders (for basic loss per share)(1) | $ | (52,240 | ) | $ | (47,130 | ) | $ | (76,664 | ) | $ | (48,352 | ) | ||||
| Net loss attributable to common stockholders (for diluted loss per share) | $ | (52,240 | ) | $ | (54,527 | ) | $ | (76,664 | ) | $ | (68,316 | ) | ||||
| Net loss per share: | ||||||||||||||||
| Basic(1) | $ | (0.66 | ) | $ | (0.59 | ) | $ | (0.55 | ) | $ | (0.23 | ) | ||||
| Diluted | $ | (0.66 | ) | $ | (0.62 | ) | $ | (0.55 | ) | $ | (0.30 | ) | ||||
| Shares used in calculation: | ||||||||||||||||
| Basic | 79,222,051 | 80,041,152 | 140,374,297 | 206,661,506 | ||||||||||||
| Diluted | 79,222,051 | 87,421,439 | 140,374,297 | 231,014,248 | ||||||||||||
_________
(1) Basic loss per share for the fourth quarter of 2015 is calculated by excluding from net income (loss) attributable to common stockholders a gain of $6,424 (thousand) related to a change in the fair value of a liability classified common stock warrant included in the Company’s consolidated statement of operations. The warrant has a nominal exercise price and shares issuable upon exercise of the warrant are considered equivalent to the Company’s common shares for the purpose of computation of basic earnings per share and consequently losses are adjusted to exclude the gain.
| 190 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (CEO) and Chief Financial Officer (CFO), evaluated the effectiveness of our disclosure controls and procedures pursuant to Rule 13a-15 under the Securities Exchange Act of 1934 (Exchange Act), as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our CEO and CFO concluded that, as of December 31, 2016, our disclosure controls and procedures are designed and are effective to provide reasonable assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC's rules and forms, and that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure.
Our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed by, or under the supervision of, our CEO and CFO, and effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| • | Pertain to the maintenance of records that accurately and fairly reflect in reasonable detail the transactions and dispositions of the assets of our company; |
| • | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| • | Provide reasonable assurances regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material adverse effect on our financial statements. |
| 191 |
Our management assessed our internal control over financial reporting as of December 31, 2016, the end of our fiscal year. Management based its assessment on criteria established in "Internal Control-Integrated Framework" (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on management's assessment of our internal control over financial reporting, management concluded that, as of December 31, 2016, our internal control over financial reporting was effective.
Internal control over financial reporting has inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of such limitations, there is a risk that material misstatements will not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during our fourth fiscal quarter ended December 31, 2016 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| 192 |
Not applicable.
| 193 |
Certain information required by Part III is omitted from this Annual Report on Form 10-K and is incorporated herein by reference from our definitive proxy statement relating to our 2017 annual meeting of stockholders, pursuant to Regulation 14A of the Exchange Act, also referred to in this Form 10-K as our 2017 Proxy Statement, which we expect to file with the SEC no later than 120 days from the end of fiscal year 2016.
| 194 |
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information appearing in our 2017 Proxy Statement under the following headings is incorporated herein by reference:
| • | Proposal 1—Election of Directors |
| • | Corporate Governance |
| • | Section 16(a) Beneficial Ownership Reporting Compliance |
The information under the heading “Executive Officers of the Registrant” in Item 1(a) of this Annual Report on Form 10-K is also incorporated by reference in this section.
We have adopted a Code of Business Conduct and Ethics that applies to all directors, officers and employees of Amyris as required by NASDAQ governance rules and as defined by applicable SEC rules. Our Code of Business Conduct and Ethics includes a section entitled “Code of Ethics for Chief Executive Officer and Senior Financial Officers,” providing additional principles for ethical leadership and a requirement that such individuals foster a culture throughout Amyris that helps ensure the fair and timely reporting of our financial results and condition. Our Code of Business Conduct and Ethics is available on the corporate governance section of our website at “http://investors.amyris.com/corporate-governance.cfm.” Stockholders may also obtain a print copy of our Code of Business Conduct and Ethics and our Corporate Governance Guidelines by writing to the Secretary of Amyris at 5885 Hollis Street, Suite 100, Emeryville, California 94608. If we make any substantive amendments to our Code of Business Conduct and Ethics or grant any waiver from a provision of the Internal Revenue Code to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on the corporate governance section of our website at “http://investors.amyris.com/corporate-governance.cfm.”
ITEM 11. EXECUTIVE COMPENSATION
The information appearing in our 2017 Proxy Statement under the following headings is incorporated herein by reference:
| • | Executive Compensation |
| • | Director Compensation |
| • | Compensation Committee Interlocks and Insider Participation |
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information appearing in our 2017 Proxy Statement under the following heading is incorporated herein by reference:
| • | Security Ownership of Certain Beneficial Owners and Management |
| 195 |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information appearing in our 2017 Proxy Statement under the following headings is incorporated herein by reference:
| • | Transactions with Related Persons |
| • | Proposal 1—Election of Directors—Independence of Directors |
| • | Proposal 1—Election of Directors—Committees of the Board |
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information appearing in our 2017 Proxy Statement under the proposal entitled “Ratification of Appointment of Independent Registered Public Accounting Firm” is incorporated herein by reference.
| 196 |
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as part of this Annual Report on Form 10-K:
(1) Financial Statements. Reference is made to the Index to the registrant’s Financial Statements under Item 8 in Part II of this Annual Report on Form 10-K.
(2) Financial Statement Schedules. The following consolidated financial statement schedule of the registrant is filed as part of this Annual Report on Form 10-K and should be read in conjunction with the Consolidated Financial Statements of Amyris, Inc.
| 197 |
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2015 and 2014
(in thousands)
| Balance at Beginning of Period | Additions | Write-off Adjustments | Balance at End of Period | |||||||||||||
| Deferred Tax Assets Valuation Allowance: | ||||||||||||||||
| Year ended December 31, 2016 | $ | 360,189 | $ | 26,678 | $ | — | $ | 386,867 | ||||||||
| Year ended December 31, 2015 | $ | 312,323 | $ | 47,866 | $ | — | $ | 360,189 | ||||||||
| Year ended December 31, 2014 | $ | 284,021 | $ | 28,302 | $ | — | $ | 312,323 | ||||||||
| Balance at Beginning of Period | Additions | Write-off Adjustments | Balance at End of Period | |||||||||||||
| Allowance for Doubtful Accounts: | ||||||||||||||||
| Year ended December 31, 2016 | $ | 969 | $ | — | $ | (468 | ) | $ | 501 | |||||||
| Year ended December 31, 2015 | $ | 479 | $ | 490 | $ | — | $ | 969 | ||||||||
| Year ended December 31, 2014 | $ | 479 | $ | — | $ | — | $ | 479 | ||||||||
Schedules not listed above are omitted because they are not required, they are not applicable or the information is already included in the consolidated financial statements or notes thereto.
(3) Exhibits. The exhibits filed as a part of this report are listed in the exhibit index included herein at page 201.
| (b) | Exhibits. |
Reference is made to Item 15(a) above.
| (c) | Financial statements and schedules. |
Reference is made to Item 15(a) above.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized in the city of Emeryville, County of Alameda, State of California on April 17, 2017.
| Dated: April 17, 2017 | Amyris, Inc. |
| /s/ JOHN G. MELO | |
| John G. Melo | |
| President and Chief Executive Officer | |
| (Principal Executive Officer) |
| 198 |
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints John Melo and Kathleen Valiasek as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| 199 |
| Signature | Title | Date | ||
|
/s/ JOHN MELO John Melo |
Director, President and Chief Executive Officer (Principal Executive Officer) |
April 17, 2017 | ||
|
/s/ KATHLEEN VALIASEK Kathleen Valiasek |
Chief Financial Officer (Principal Financial Officer) |
April 17, 2017 | ||
|
/s/ KAREN WEAVER Karen Weaver |
Vice President Finance (Principal Accounting Officer) |
April 17, 2017 | ||
|
/s/ CHRISTOPHE VUILLEZ Christophe Vuillez |
Director | April 17, 2017 | ||
|
/s/ JOHN DOERR John Doerr |
Director | April 17, 2017 | ||
|
/s/ GEOFFREY DUYK Geoffrey Duyk |
Director | April 17, 2017 | ||
/s/ ABRAHAM KLAEIJSEN Abraham Klaeijsen |
Director | April 17, 2017 | ||
/s/ CAROLE PIWNICA Carole Piwnica |
Director | April 17, 2017 | ||
|
/s/ FERNANDO REINACH Fernando Reinach |
Director | April 17, 2017 | ||
/s/ HH SHEIKH ABDULLAH BIN KHALIFA AL THANI HH Sheikh Abdullah bin Khalifa Al Thani |
Director | April 17, 2017 | ||
/s/ R. NEIL WILLIAMS R. Neil Williams |
Director | April 17, 2017 | ||
/s/ PATRICK YANG Patrick Yang |
Director | April 17, 2017 | ||
| 200 |
EXHIBIT INDEX
The following table lists the exhibits filed as part of this report on Form 10-K. In some cases, these exhibits are incorporated into this report by reference to exhibits to our other filings with the Securities and Exchange Commission. Where an exhibit is incorporated by reference, we have noted the type of form filed with the Securities and Exchange Commission, the file number of that form, the date of the filing, and the number of the exhibit referenced in that filing.
| Exhibit | Previously Filed | Filed | |||||||||
| No. | Description | Form | File No. | Filing Date | Exhibit | Herewith | |||||
| 3.01 | Restated Certificate of Incorporation | 10-Q | 001-34885 | November 10, 2010 | 3.01 | ||||||
| 3.02 | Certificate of Amendment, dated May 9, 2013, to Restated Certificate of Incorporation | S-8 | 333-188711 | May 20, 2013 | 4.02 | ||||||
| 3.03 | Certificate of Amendment, dated May 12, 2014, to Restated Certificate of Incorporation | 10-Q | 001-34887 | August 8, 2014 | 3.02 | ||||||
| 3.04 | Certificate of Amendment, dated September 18, 2015, to Restated Certificate of Incorporation | S-3/A | 333-206331 | November 4, 2015 | 3.03 | ||||||
| 3.05 | Certificate of Amendment, dated May 18, 2016, to Restated Certificate of Incorporation | 10-Q | 001-34885 | August 9, 2016 | 3.05 | ||||||
| 3.06 | Restated Bylaws | 10-Q | 001-34885 | November 10, 2010 | 3.02 | ||||||
| 4.01 | Specimen of Common Stock Certificate | S-1 | 333-166135 | July 6, 2010 | 4.01 | ||||||
| 4.02 | Amended and Restated Investors’ Rights Agreement, dated June 21, 2010, among registrant and its security holders listed therein | S-1 | 333-166135 | June 23, 2010 | 4.02 | ||||||
| 4.03 | First Amendment to Amended and Restated Investors' Rights Agreement, dated February 23, 2012, among registrant and registrant's security holders listed therein | S-3 | 333-180005 | March 9, 2012 | 4.06 | ||||||
| 4.04 | Amendment No. 2 to Amended and Restated Investors' Rights Agreement, dated December 24, 2012, among registrant and registrant's security holders listed therein | 10-K | 001-34885 | March 28, 2013 | 4.04 | ||||||
| 4.05 | Amendment No. 3 to Amended and Restated Investors' Rights Agreement, dated March 27, 2013, among registrant and registrant's security holders listed therein | 10-Q | 001-34885 | May 9, 2013 | 4.02 | ||||||
| 4.06 | Amendment No. 4 to Amended and Restated Investors' Rights Agreement, dated October 16, 2013, among registrant and registrant's security holders listed therein | 10-K | 001-34885 | April 2, 2014 | 4.06 | ||||||
| 4.07 | Amendment No. 5 to Amended and Restated Investors' Rights Agreement, dated December 24, 2013, among registrant and registrant's security holders listed therein | 10-K | 001-34885 | April 2, 2014 | 4.07 | ||||||
| 4.08 | Amendment No. 6 to Amended and Restated Investors’ Rights Agreement dated July 29, 2015 among registrant and registrant’s security holders listed therein | S-3 | 333-206331 | August 12, 2015 | 4.17 | ||||||
| 4.09 a | Amended and Restated Letter Agreement re: Certain Registration Rights dated May 8, 2014 between registrant and the purchasers listed therein | 10-Q | 001-34885 | August 8, 2014 | 4.01 | ||||||
| 4.10 | Warrant to Purchase Stock, dated December 23, 2011, issued to ATEL Ventures, Inc. | 10-K | 001-34885 | February 28, 2012 | 4.07 | ||||||
| 4.11 | Side Letter, dated June 21, 2010, between registrant and Total Gas & Power USA, SAS | S-1 | 333-166135 | June 23, 2010 | 4.19 | ||||||
| 4.12 | Agreement, dated February 23, 2012, among registrant, Maxwell (Mauritius) Pte Ltd, Naxyris SA, Biolding Investment SA and Sualk Capital Ltd. | 10-Q | 001-34885 | May 9, 2012 | 4.02 | ||||||
| 4.13 | Securities Purchase Agreement, dated February 24, 2012, among registrant and certain investment funds affiliated with Fidelity Investments Institutional Services Company, Inc. listed therein (each, a Fidelity Purchaser) | S-3 | 333-180005 | March 9, 2012 | 4.02 | ||||||
| 201 |
| 4.14 | Form of Unsecured Senior Convertible Promissory Note issued by registrant to the Fidelity Purchasers in the amounts set forth next to each Fidelity Purchaser's name on Schedule I of Exhibit 4.13 hereof | S-3 | 333-180005 | March 9, 2012 | 4.03 | ||||||
| 4.15 | Registration Rights Agreement, dated February 27, 2012, among registrant and the Fidelity Purchasers | S-3 | 333-180005 | March 9, 2012 | 4.04 | ||||||
| 4.16 | Exchange Agreement, dated December 28, 2016, among registrant and certain Fidelity Purchasers | X | |||||||||
| 4.17 c | Form of Common Stock Purchase Agreement among registrant and certain investors | 10-Q | 001-34885 | August 8, 2012 | 4.01 | ||||||
| 4.18 | Securities Purchase Agreement, dated July 30, 2012, between registrant and Total Gas & Power USA, SAS | 10-Q | 001-34885 | November 9, 2012 | 4.01 | ||||||
| 4.19 | Registration Rights Agreement, dated July 30, 2012, between registrant and Total Gas & Power USA, SAS | 10-Q | 001-34885 | November 9, 2012 | 4.03 | ||||||
| 4.20 | 1.5% Senior Secured Convertible Note dated July 29, 2015 (RS-9) issued by registrant to Total Energies Nouvelles Activités USA (RS-9) | 10-Q | 001-34885 | November 9, 2015 | 4.21 | ||||||
| 4.21 | 1.5% Senior Convertible Note, dated March 21, 2016 (RS-10) issued by registrant to Total Energies Nouvelles Activités USA | 10-Q | 001-34885 | May 10, 2016 | 4.19 | ||||||
| 4.22 a | Securities Purchase Agreement, dated December 24, 2012, between registrant and certain investors listed therein | 10-K | 001-34885 | March 28, 2013 | 4.16 | ||||||
| 4.23 a | Follow-On Investment Agreement, dated December 24, 2012, between registrant and Biolding Investment SA | 10-K | 001-34885 | March 28, 2013 | 4.17 | ||||||
| 4.24 | Securities Purchase Agreement, dated March 27, 2013, between registrant and Biolding Investment SA | 10-Q | 001-34885 | May 9, 2013 | 4.01 | ||||||
| 4.25 | Securities Purchase Agreement (including Form of Tranche I Senior Convertible Note and Form of Tranche II Senior Convertible Note) , dated August 8, 2013, between registrant, Maxwell (Mauritius) Pte Ltd and Total Energies Nouvelles Activités USA (f.k.a. Total Gas & Power USA, SAS) | 10-Q | 001-34885 | November 5, 2013 | 4.01 | ||||||
| 4.26 a | Amendment No. 1 dated October 16, 2013, to the Securities Purchase Agreement, dated August 8, 2013, between registrant and other parties named therein | 10-K | 001-34885 | April 2, 2014 | 4.24 | ||||||
| 4.27 | Tranche I Note Amendment and Amendment No. 2 dated December 24, 2013, to the Securities Purchase Agreement, dated August 8, 2013, between registrant and other parties named therein | 10-K | 001-34885 | April 2, 2014 | 4.25 | ||||||
| 4.28 ad | 5% Unsecured Convertible Note dated October 16, 2013 issued to Total Energies Nouvelles Activités USA | 10-Q | 001-34885 | May 9, 2014 | 4.04 | ||||||
| 4.29 ae | 10% Unsecured Convertible Note dated January 15, 2014 issued to Total Energies Nouvelles Activités USA | 10-Q | 001-34885 | May 9, 2014 | 4.06 | ||||||
| 4.30 | Securities Purchase Agreement, dated September 20, 2013, between registrant and Naxyris S.A. | 10-Q | 001-34885 | November 5, 2013 | 4.03 | ||||||
| 4.31 | Securities Purchase Agreement, dated March 28, 2014 between registrant and Kuraray Co. Ltd. | 10-Q | 001-34885 | May 9, 2014 | 4.01 | ||||||
| 4.32 | Loan and Security Agreement, dated March 29, 2014 between registrant and Hercules Technology Growth Capital, Inc. | 10-Q | 001-34885 | May 9, 2014 | 4.02 | ||||||
| 4.33 | First Amendment, dated June 12, 2014, to Loan and Security Agreement dated March 29, 2014 between registrant and Hercules Technology Growth Capital, Inc. | 10-Q | 001-34885 | August 8, 2014 | 4.06 | ||||||
| 4.34 | Second Amendment, dated March 31, 2015, to Loan and Security Agreement dated March 29, 2014 between registrant and Hercules Technology Growth Capital, Inc. | 10-Q | 001-34885 | May 7, 2015 | 10.05 |
| 202 |
| 4.35 | Third Amendment, dated November 30, 2015, to Loan and Security Agreement dated March 29, 2014 between registrant and Hercules Technology Growth Capital, Inc. | 10-K | 001-34885 | March 30, 2016 | 4.37 | ||||||
| 4.36 | Fourth Amendment, dated October 6, 2016, to Loan and Security Agreement dated March 29, 2014 between registrant and Stegodon Corporation, as assignee of Hercules Capital, Inc. | X | |||||||||
| 4.37 | Indenture dated May 29, 2014 between registrant and Wells Fargo Bank, National Association, as Trustee | 8-K | 001-34885 | May 29, 2014 | 4.01 | ||||||
| 4.38 | 6.5% Convertible Senior Note due 2019 dated May 29, 2014 issued by registrant to Morgan Stanley & Co. LLC | 10-Q | 001-34885 | August 8, 2014 | 4.02 | ||||||
| 4.39 f | 6.5% Convertible Senior Note due 2019 dated May 29, 2014 issued by registrant to Maxwell (Mauritius) Pte Ltd. | 10-Q | 001-34885 | August 8, 2014 | 4.03 | ||||||
| 4.40 | Registration Rights Agreement dated February 24, 2015, between the registrant and Nomis Bay Ltd | 8-K | 001-34885 | February 26, 2015 | 4.01 | ||||||
| 4.41 g | Voting Agreement, dated July 24, 2015, between registrant and Foris Ventures, LLC | 10-Q | 001-34885 | November 9, 2015 | 4.43 | ||||||
| 4.42 | Securities Purchase Agreement, dated July 24, 2015, between registrant and the Purchasers listed therein | 10-Q | 001-34885 | November 9, 2015 | 4.44 | ||||||
| 4.43 h | Warrant to Purchase Stock issued on July 24, 2015 | S-3 | 333-206331 | August 12, 2015 | 4.21 | ||||||
| 4.44 | Exchange Agreement, dated July 29, 2015, between registrant and the Investors therein | 10-Q | 001-34885 | November 9, 2015 | 4.46 | ||||||
| 4.45 | Maturity Treatment Agreement dated July 29, 2015, between registrant and the Investors listed therein | 10-Q | 001-34885 | November 9, 2015 | 4.47 | ||||||
| 4.46 | Letter Agreement dated as of July 29, 2015 among registrant and registrant’s security holders listed therein | S-3 | 333-206331 | August 12, 2015 | 4.20 | ||||||
| 4.47 | Warrant to Purchase Stock issued July 29, 2015 by the registrant to Total Energies Nouvelles Activités USA | S-3 | 333-206331 | August 12, 2015 | 4.22 | ||||||
| 4.48 | Warrant to Purchase Stock issued July 29, 2015 by the registrant to Total Energies Nouvelles Activités USA | S-3 | 333-206331 | August 12, 2015 | 4.23 | ||||||
| 4.49 | Warrant to Purchase Stock issued July 29, 2015 by the registrant to Maxwell (Mauritius) PTE Ltd | S-3 | 333-206331 | August 12, 2015 | 4.24 | ||||||
| 4.50 | Warrant to Purchase Stock issued July 29, 2015 by the registrant to Maxwell (Mauritius) PTE Ltd | S-3 | 333-206331 | August 12, 2015 | 4.25 | ||||||
| 4.51 | Warrant to Purchase Stock issued July 29, 2015 by the registrant to Maxwell (Mauritius) PTE Ltd | S-3 | 333-206331 | August 12, 2015 | 4.26 | ||||||
| 4.52 | Indenture dated October 20, 2015 between registrant and Wells Fargo Bank, National Association, as Trustee | 8-K | 001-34885 | October 20, 2015 | 4.01 | ||||||
| 4.53 | 9.50% Convertible Senior Note due 2019 dated October 20, 2015 issued by registrant to Cede & Co. | 10-K | 001-34885 | March 30, 2016 | 4.56 | ||||||
| 4.54 | Registration Rights Agreement dated October 20, 2015 between the registrant and the registrant’s security holders listed therein | 8-K | 001-34885 | October 20, 2015 | 4.02 | ||||||
| 4.55 | Note and Warrant Purchase Agreement, dated February 12, 2016, between registrant and the purchasers listed therein | 10-Q | 001-34885 | May 10, 2016 | 4.50 | ||||||
| 4.56 i | Unsecured Promissory Note, dated February 12, 2016, between registrant and Foris Ventures, LLC | 10-Q | 001-34885 | May 10, 2016 | 4.51 | ||||||
| 4.57 j | Warrant to Purchase Stock, dated February 12, 2016, between registrant and Foris Ventures, LLC | 10-Q | 001-34885 | May 10, 2016 | 4.52j | ||||||
| 4.58 | Form of Convertible Note | 8-K | 001-34885 | May 10, 2016 | 4.1 | ||||||
| 4.59 | Note Purchase Agreement, dated June 24, 2016, between registrant and Foris Ventures, LLC | 10-Q | 001-34885 | August 9, 2016 | 4.50 | ||||||
| 4.60 | Secured Promissory Note, dated June 24, 2016 issued by registrant to Foris Ventures, LLC | 10-Q | 001-34885 | August 9, 2016 | 4.51 | ||||||
| 4.61 | Form of Additional Note | 8-K | 001-34885 | September 9, 2016 | 4.1 |
| 203 |
| 4.62 | Warrant to Purchase Common Stock, dated August 6, 2016, between registrant and Ginkgo Bioworks, Inc. | 10-Q | 001-34885 | November 9, 2016 | 4.58 | ||||||
| 4.63 | Note Purchase Agreement, dated October 21, 2016, between registant and Foris Ventures, LLC | X | |||||||||
| 4.64 | Secured Promissory Note, dated October 21, 2016, issued by registrant to Foris Ventures, LLC | X | |||||||||
| 4.65 b | Credit Agreement, dated October 26, 2016, between registrant and Guanfu Holding Co., Ltd. | X | |||||||||
| 4.66 | Note, dated December 31, 2016, issued by registrant to Wutian Supply Chain Corporation Limited | X | |||||||||
| 4.67 | Note Purchase Agreement, dated October 27, 2016, between registrant and Ginkgo Bioworks, Inc. | X | |||||||||
| 4.68 | Secured Promissory Note , dated October 27, 2016, issued by registrant to Ginkgo Bioworks, Inc. | X | |||||||||
| 4.69 | Warrant to Purchase Stock, issued November 16, 2016, by the registrant to Nenter & Co., Inc. | S-3 | 333-215318 | December 23, 2016 | 4.15 | ||||||
| 4.70 | Form of Convertible Note | 8-K | 001-34885 | December 2, 2016 | 4.1 | ||||||
| 4.71 | Purchase Money Promissory Note, issued December 5, 2016, by registrant to Salisbury Partners, LLC | X | |||||||||
| 4.72 | Purchase Money Promissory Note, issued December 19, 2016, by registrant to Nikko Chemicals Co. Ltd. | X | |||||||||
| 10.01 a | Technology Investment Agreement, dated September 22, 2015, between registrant and The Defense Advanced Research Projects Agency (DARPA) | 10-Q | 001-34885 | November 9, 2015 | 10.03 | ||||||
| 10.02 b | Modification No. 1, dated October 22, 2015, to the Technology Investment Agreement, dated September 22, 2015, between registrant and The Defense Advanced Research Projects Agency (DARPA) | X | |||||||||
| 10.03 b | Modification No. 2, dated February 29, 2016, to the Technology Investment Agreement, dated September 22, 2015, between registrant and The Defense Advanced Research Projects Agency (DARPA) | X | |||||||||
| 10.04 ak | Agreement for Credit Opening, dated November 16, 2011, between Amyris Brasil Ltda. and Banco Nacional de Desenvolvimento Econômico e Social - BNDES | 10-K | 001-34885 | February 28, 2012 | 10.11 | ||||||
| 10.05 ak | Amendment No. 1, dated June 28, 2013 to Agreement for Credit Opening, dated November 16, 2011, between Amyris Brasil Ltda. and Banco Nacional de Desenvolvimento Econômico e Social - BNDES | 10-Q | 001-34885 | November 9, 2015 | 10.04 | ||||||
| 10.06 ak | Amendment No. 2, dated September 16, 2015, to Agreement for Credit Opening, dated November 16, 2011, between Amyris Brasil Ltda. and Banco Nacional de Desenvolvimento Econômico e Social - BNDES | 10-Q | 001-34885 | November 9, 2015 | 10.05 | ||||||
| 10.07 a | Corporate Guarantee, dated November 28, 2011, issued by registrant to Banco Nacional de Desenvolvimento Econômico e Social - BNDES | 10-K | 001-34885 | February 28, 2012 | 10.12 | ||||||
| 10.08 k | Bank Credit Agreement, dated December 21, 2011, between Amyris Brasil Ltda. and Banco Pine S.A. | 10-K | 001-34885 | February 28, 2012 | 10.13 | ||||||
| 10.09 k | Addendum to the Banking Credit Form, dated February 17, 2012, between Amyris Brasil Ltda. and Banco Pine S.A. | 10-Q | 001-34885 | May 9, 2012 | 10.02 | ||||||
| 10.10 k | Addendum to the Banking Credit Form, dated May 17, 2012, between Amyris Brasil Ltda. and Banco Pine S.A. | 10-Q | 001-34885 | August 8, 2012 | 10.02 | ||||||
| 10.11 k | Note of Bank Credit, dated June 21, 2012, between Amyris Brasil Ltda. and Banco Pine S.A. | 10-Q | 001-34885 | August 8, 2012 | 10.03 |
| 204 |
| 10.12 ak | Global Derivatives Contract (swap agreement), dated June 15, 2012, between Amyris Brasil Ltda. and Banco Pine S.A. | 10-Q | 001-34885 | August 8, 2012 | 10.04 | ||||||
| 10.13 k | Global Derivatives Contract Annex, dated October 8, 2015, between Amyris Brasil Ltda. and Banco Pine S.A. (AB as purchaser) | 10-K | 001-34885 | March 30, 2016 | 10.15 | ||||||
| 10.14 k | Global Derivatives Contract Annex, dated October 8, 2015, between Amyris Brasil Ltda. and Banco Pine S.A. (Pine as purchaser) | 10-K | 001-34885 | March 30, 2016 | 10.16 | ||||||
| 10.15 ak | Note of Bank Credit, dated July 13, 2012, between Amyris Brasil Ltda. and Nossa Caixa Desenvolvimento | 10-Q | 001-34885 | November 9, 2012 | 10.01 | ||||||
| 10.16 ak | Note of Bank Credit, dated July 13, 2012, between Amyris Brasil Ltda. and Banco Pine S.A. | 10-Q | 001-34885 | November 9, 2012 | 10.02 | ||||||
| 10.17 k | Fiduciary Conveyance of Movable Goods Agreement, dated July 13, 2012, among Amyris Brasil Ltda., Nossa Caixa Desenvolvimento and Banco Pine S.A. | 10-Q | 001-34885 | November 9, 2012 | 10.03 | ||||||
| 10.18 | Corporate Guarantee, dated July 13, 2012, issued by registrant to Nossa Caixa Desenvolvimento | 10-Q | 001-34885 | November 9, 2012 | 10.04 | ||||||
| 10.19 | Corporate Guarantee, dated July 13, 2012, issued by registrant to Banco Pine S.A. | 10-Q | 001-34885 | November 9, 2012 | 10.05 | ||||||
| 10.20 a | Joint Venture Agreement dated April 14, 2010 among registrant, Amyris Brasil S.A. and Usina São Martinho S.A. | S-1 | 333-166135 | August 31, 2010 | 10.14 | ||||||
| 10.21 a | First Amendment dated January 27, 2014 to the Joint Venture Agreement dated April 14, 2010, among registrant, Amyris Brasil, Ltda, and Usina São Martinho S.A. | 10-Q | 001-34885 | May 9, 2014 | 10.01 | ||||||
| 10.22 a | Shareholders’ Agreement dated April 14, 2010 among registrant, Amyris Brasil S.A. and Usina São Martinho S.A. | S-1 | 333-166135 | May 25, 2010 | 10.17 | ||||||
| 10.23 | Termination Agreement, dated August 31, 2015, among registrant, Amyris Brasil, Ltda, and São Martinho S.A., and SMA Industria Quimica S.A. | 10-K | 001-34885 | March 30, 2016 | 10.25 | ||||||
| 10.24 | Share Purchase and Sale Agreement, dated August 31, 2015, among registrant, Amyris Brasil, Ltda, and São Martinho S.A., and SMA Industria Quimica S.A. | 10-K | 001-34885 | March 30, 2016 | 10.26 | ||||||
| 10.25 | First Addendum to the Share Purchase and Sale Agreement, dated September 1, 2016, between registrant and SMA Industria Quimica Ltda | 10-Q | 001-34885 | November 9, 2016 | 10.01 | ||||||
| 10.26 a | Amended and Restated Master Framework Agreement, dated December 2, 2013, between Amyris and Total Gas & Power USA, SAS | 10-K | 001-34885 | April 2, 2014 | 10.29 | ||||||
| 10.27 | Amendment #1, dated April 1, 2015, to the Amended and Restated Master Framework Agreement between registrant and Total Energies Nouvelles Activités USA SAS | 10-Q | 001-34885 | August 10, 2015 | 10.01 | ||||||
| 10.28 | Termination Agreement, dated March 21, 2016, regarding the Amended and Restated Master Framework Agreement, dated December 2, 2013, as amended | X | |||||||||
| 10.29 ak | Articles of Association of Total Amyris BioSolutions B.V. | 10-K | 001-34885 | April 2, 2014 | 10.22 | ||||||
| 10.30 k | Amendment, dated March 21, 2016, to Articles of Association of Total Amyris BioSolutions B.V. | 10-Q | 001-34885 | May 10, 2016 | 10.03 | ||||||
| 10.31 a | Shareholders Agreement dated December 2, 2013 | 10-K | 001-34885 | April 2, 2014 | 10.23 | ||||||
| 10.32 | Amended and Restated Shareholders’ Agreement, dated March 21, 2016, between registrant, Total Energies Nouvelles Activités USA, and Total Amyris BioSolutions B.V. | 10-Q | 001-34885 | May 10, 2016 | 10.02 | ||||||
| 10.33 a | License Agreement dated December 2, 2013 between registrant and Total Amyris BioSolutions B.V. | 10-K | 001-34885 | April 2, 2014 | 10.24 |
| 205 |
| 10.34 | Amended & Restated Jet Fuel License Agreement, dated March 21, 2016, between registrant and Total Amyris BioSolutions B.V. | 10-Q | 001-34885 | May 10, 2016 | 10.04 | ||||||
| 10.35 | License Agreement regarding Diesel Fuel in the EU, dated March 21, 2016, between registrant and Total Energies Nouvelles Activités USA | 10-Q | 001-34885 | May 10, 2016 | 10.05 | ||||||
| 10.36 a | Pledge of Shares dated December 2, 2013 among registrant, Total Energies Nouvelles Activités USA and Total Amyris BioSolutions B.V. | 10-K | 001-34885 | April 2, 2014 | 10.25 | ||||||
| 10.37 a | Escrow Agreement dated December 2, 2013 among registrant, Total Energies Nouvelles Activités USA and Stichting Total Amyris BioSolutions | 10-K | 001-34885 | April 2, 2014 | 10.26 | ||||||
| 10.38 | Letter Agreement re: Waiver of Debt Covenants dated December 24, 2013 between registrant and Total Energies Nouvelles Activités USA | 10-K | 001-34885 | April 2, 2014 | 10.28 | ||||||
| 10.39 | Letter Agreement dated December 2, 2013 relating to the Senior Secured Convertible Notes and the 1.5% Senior Unsecured Convertible Notes due 2017 between the registrant and Total Energies Nouvelles Activités USA | 10-K | 001-34885 | April 2, 2014 | 10.30 | ||||||
| 10.40 a | Letter Agreement re Joint Venture Restructuring, dated July 24, 2015, between registrant and Total Energies Nouvelles Activités USA | 10-Q | 001-34885 | November 9, 2015 | 10.01 | ||||||
| 10.41 | Amendment, dated February 12, 2016, to Letter Agreement re Joint Venture Restructuring, dated July 24, 2015, between registrant and Total Energies Nouvelles Activités USA | 10-Q | 001-34885 | May 10, 2016 | 10.01 | ||||||
| 10.42 | Securities Purchase Agreement, dated as of March 30, 2015, by and between registrant and Naxyris, S.A. | 10-Q | 001-34885 | May 7, 2015 | 10.04 | ||||||
| 10.43 | Technology License, Development, Research and Collaboration Agreement, dated June 21, 2010, between registrant and Total Gas & Power USA Biotech, Inc. | S-1 | 333-16135 | September 20, 2010 | 10.46 | ||||||
| 10.44 | Letter agreement, dated January 11, 2011, between registrant and Total Gas & Power USA Biotech, Inc. regarding assignment of Collaboration Agreement | 10-Q | 001-34885 | May 11, 2011 | 10.01 | ||||||
| 10.45 a | First Amendment to Technology License, Development, Research and Collaboration Agreement, dated November 23, 2011, between Amyris and Total Gas & Power USA SAS | 10-K/A | 001-34885 | May 2, 2012 | 10.19 | ||||||
| 10.46 a | Second Amendment to the Technology License, Development, Research and Collaboration Agreement, dated July 30, 2012, between registrant and Total Gas & Power USA, SAS | 10-Q | 001-34885 | November 9, 2012 | 10.07 | ||||||
| 10.47 | Amendment #1, dated April 1, 2015, to the Second Amendment to the Technology, License, Development, Research and Collaboration Agreement between registrant and Total Energies Nouvelles Activités USA SAS | 10-Q | 001-34885 | August 10, 2015 | 10.02 | ||||||
| 10.48 ak | Agreement for the Supply of Sugarcane Juice and Other Utilities, dated March 18, 2011, between Amyris Brasil Ltda. and Paraíso Bioenergia S.A. | 10-Q | 001-34885 | May 9, 2012 | 10.06 | ||||||
| 10.49 ak | First Amendment, dated as of May 3, 2013, to the Agreement for the Supply of Sugar Cane Juice and Other Utilities, by and between Amyris Brasil Ltda. and Tonon Bioenergia S.A. | 10-K | 001-34885 | March 30, 2016 | 10.46 | ||||||
| 10.50 ak | Second Amendment, dated as of November 7, 2013, to the Agreement for the Supply of Sugar Cane Juice and Other Utilities, by and between Amyris Brasil Ltda. and Tonon Bioenergia S.A. | 10-K | 001-34885 | March 30, 2016 | 10.47 | ||||||
| 10.51 ak | Third Amendment, dated as of January 27, 2015, to the Agreement for the Supply of Sugar Cane Juice and Other Utilities, by and between Amyris Brasil Ltda. and Tonon Bioenergia S.A. | 10-Q | 001-34885 | May 7, 2015 | 10.06 | ||||||
| 10.52 ak | Lease Agreement, dated March 18, 2011, between Amyris Brasil Ltda. and Paraíso Bioenergia S.A. | 10-K | 001-34885 | March 28, 2013 | 10.37 |
| 206 |
| 10.53 ak | Addendum to Lease Agreement, dated April 28, 2011, between Amyris Brasil Ltda. and Paraíso Bioenergia S.A. | 10-K | 001-34885 | March 28, 2013 | 10.38 | ||||||
| 10.54 | Lease, dated August 22, 2007, between registrant and ES East Associates, LLC | S-1 | 333-166135 | April 16, 2010 | 10.17 | ||||||
| 10.55 | First Amendment, dated March 10, 2008, to Lease between registrant and ES East Associates, LLC | S-1 | 333-166135 | April 16, 2010 | 10.18 | ||||||
| 10.56 | Second Amendment, dated April 25, 2008, to Lease between registrant and ES East Associates, LLC | S-1 | 333-166135 | April 16, 2010 | 10.19 | ||||||
| 10.57 | Third Amendment, dated July 31, 2008, to Lease between registrant and ES East Associates, LLC | S-1 | 333-166135 | April 16, 2010 | 10.20 | ||||||
| 10.58 | Fourth Amendment, dated November 14, 2009, to Lease between registrant and ES East Associates, LLC | S-1 | 333-166135 | April 16, 2010 | 10.21 | ||||||
| 10.59 | Fifth Amendment, dated October 15, 2010, to Lease between registrant and ES East, LLC | 10-K | 001-34885 | March 14, 2011 | 10.17 | ||||||
| 10.60 | Sixth Amendment, dated April 30, 2013, to Lease between registrant and ES East, LLC (as successor-in-interest to ES East Associates, LLC) | 10-Q | 001-34885 | August 9, 2013 | 10.02 | ||||||
| 10.61 | Lease dated April 25, 2008 between registrant and EmeryStation Triangle, LLC | S-1 | 333-166135 | April 16, 2010 | 10.22 | ||||||
| 10.62 | Letter, dated April 25, 2008, amending Lease between registrant and EmeryStation Triangle, LLC | S-1 | 333-166135 | April 16, 2010 | 10.23 | ||||||
| 10.63 | Second Amendment, dated February 5, 2010, to Lease between registrant and EmeryStation Triangle, LLC | S-1 | 333-166135 | April 16, 2010 | 10.24 | ||||||
| 10.64 | Third Amendment, dated May 1, 2013, to Lease between registrant and EmeryStation Triangle, LLC | 10-Q | 001-34885 | August 9, 2013 | 10.03 | ||||||
| 10.65 | Pilot Plant Expansion Right Letter dated December 22, 2008 between registrant and EmeryStation Triangle, LLC | S-1 | 333-166135 | April 16, 2010 | 10.25 | ||||||
| 10.66 | Lease Agreement, dated August 10, 2011, between Amyris Brasil Ltda. and Techno Park Empreendimentos e Administração Imobiliária Ltda. | 10-K | 001-34885 | February 28, 2012 | 10.32 | ||||||
| 10.67 | First Amendment to Lease Agreement, dated July 31, 2013, between Amyris Brasil Ltda. and Techno Park Empreendimentos e Administração Imobiliária Ltda. | 10-Q | 001-34885 | November 5, 2013 | 10.01 | ||||||
| 10.68 | Second Amendment to Lease Agreement, dated October 31, 2015, between Amyris Brasil Ltda. and Techno Park Empreendimentos e Administração Imobiliária Ltda. | 10-Q | 001-34885 | May 10, 2016 | 10.07 | ||||||
| 10.69 | Third Amendment to Lease Agreement, dated March 30, 2016, between Amyris Brasil Ltda. and Techno Park Empreendimentos e Administração Imobiliária Ltda. | 10-Q | 001-34885 | May 10, 2016 | 10.08 | ||||||
| 10.70 | Private Instrument of Non-Residential Real Estate Lease Agreement, dated March 31, 2008, between Lucio Tomasiello and Amyris Brasil S.A. (including Amendment No. 1, dated July 5, 2008, and Amendment No. 2, dated October 30, 2008) | S-1 | 333-166135 | April 16, 2010 | 10.26 | ||||||
| 10.71 ak | Third Amendment, dated October 1, 2012, to the Private Instrument of Non Residential Real Estate Lease Agreement between Lucio Tomasiello and Amyris Brasil Ltda. | 10-K | 001-34885 | March 28, 2013 | 10.51 | ||||||
| 10.72 ak | Fourth Amendment, dated March 3, 2015, to the Private Instrument of Non-Residential Real Estate Lease Agreement, by and among Amyris Brasil Ltda., Lucius Tomasiello and Mauricio Tomasiello | 10-Q | 001-34885 | May 7, 2015 | 10.07 | ||||||
| 10.73 k | Fifth Amendment, dated September 22, 2015, to the Private Instrument of Non-Residential Real Estate Lease Agreement, by and among Amyris Brasil Ltda., Lucius Tomasiello and Mauricio Tomasiello | X | |||||||||
| 10.74 k | Sixth Amendment, dated October 17, 2016, to the Private Instrument of Non-Residential Real Estate Lease Agreement, by and among Amyris Brasil Ltda., Lucius Tomasiello and Mauricio Tomasiello | X |
| 207 |
| 10.75 | Master Collaboration Agreement, dated March 13, 2013, between registrant and Firmenich SA | 10-Q | 001-34885 | May 8, 2013 | 10.02 | ||||||
| 10.76 b | Amendment #1, dated July 1, 2015, to the Collaboration Agreement between registrant and Firmenich SA | X | |||||||||
| 10.77 b | Amendment #2, dated November, 28, 2016, to the Collaboration Agreement between registrant and Firmenich SA | X | |||||||||
| 10.78 | Amended and Restated Operating Agreement, dated March 26, 2013, among registrant, Cosan US, Inc. and Novvi LLC | 10-Q | 001-34885 | May 8, 2013 | 10.04 | ||||||
| 10.79 | Second Amended and Restated Operating Agreement, dated July 19, 2016, between registrant, Cosan US, Inc., American Refining Group, Inc. and Novvi LLC | 10-Q | 001-34885 | November 9, 2016 | 10.02 | ||||||
| 10.80 | IP License Agreement, dated as of March 26, 2013, between registrant and Novvi LLC | 10-Q | 001-34885 | May 8, 2013 | 10.06 | ||||||
| 10.81 | Amendment No.1, dated March 21, 2016, to IP License Agreement, dated March 26, 2013, between registrant and Novvi LLC | 10-Q | 001-34885 | May 10, 2016 | 10.06 | ||||||
| 10.82 | Amended & Restated IP License Agreement, dated July 19, 2016, between registrant and Novvi LLC | 10-Q | 001-34885 | November 9, 2016 | 10.03 | ||||||
| 10.83 a | Pilot Plant Sublease, dated April 4, 2014, between registrant and Total New Energies USA, Inc. | 10-Q | 001-34885 | August 8, 2014 | 10.03 | ||||||
| 10.84 a | Pilot Plant Services Agreement, dated April 4, 2014, between registrant and Total New Energies USA, Inc. | 10-Q | 001-34885 | August 8, 2014 | 10.04 | ||||||
| 10.85 | Amendment No. 1, dated July 26, 2015, to the Pilot Plant Services Agreement, dated April 4, 2014, between registrant and Total New Energies USA, Inc. | 10-Q | 001-34885 | November 9, 2015 | 10.02 | ||||||
| 10.86 | Repurchase Agreement, dated October 19, 2015, between the registrant and registrant’s security holders listed therein | 10-K | 001-34885 | March 30, 2016 | 10.75 | ||||||
| 10.87 | Common Stock Purchase Agreement, dated as of February 24, 2015, by and between registrant and Nomis Bay Ltd. | 8-K | 001-34885 | February 26, 2015 | 10.01 | ||||||
| 10.88 | Engagement Letter, dated as of February 24, 2015, by and between registrant and Financial West Group | 8-K | 001-34885 | February 26, 2015 | 10.02 | ||||||
| 10.89 | At Market Issuance Sales Agreement, dated March 8, 2016, among Amyris, Inc., FBR Capital Markets & Co. and MLV & Co. LLC | 8-K | 001-34885 | March 9, 2016 | 10.01 | ||||||
| 10.90 | Securities Purchase Agreement, dated April 8, 2016, between registrant and Bill & Melinda Gates Foundation | 10-Q | 001-34885 | August 9, 2016 | 10.01 | ||||||
| 10.91 | Letter Agreement re Charitable Purposes and Use of Funds, dated April 8, 2016, between registrant and Bill & Melinda Gates Foundation | 10-Q | 001-34885 | August 9, 2016 | 10.02 | ||||||
| 10.92 | Form of Securities Purchase Agreement | 8-K | 001-34885 | May 10, 2016 | 10.01 | ||||||
| 10.93 a | Initial Strategic Partnership Agreement, dated June 28, 2016, between registrant and Gingko Bioworks, Inc. | 10-Q | 001-34885 | August 9, 2016 | 10.04 | ||||||
| 10.94 | Collaboration Agreement, dated September 30, 2016, between registrant and Ginkgo Bioworks, Inc. | 10-Q | 001-34885 | November 9, 2016 | 10.04 | ||||||
| 10.95 b | Purchase and Sale Agreement, dated November 10, 2016, between registrant, Glycotech, Inc., and Salisbury Partners, LLC | X | |||||||||
| 10.96 b | Cooperation Agreement, dated October 26, 2016, between registrant and Nenter & Co., Inc. | X | |||||||||
| 10.97 | Form of Securities Purchase Agreement | 8-K | 001-34885 | December 2, 2016 | 10.1 | ||||||
| 10.98 b | Joint Venture Agreement, dated December 12, 2016, among registrant, Nikko Chemicals Co. Ltd., and Nippon Surfactant Industries Co., Ltd. | X |
| 208 |
| 10.99 b | First Amended and Restated LLC Operating Agreement of Neossance, LLC dated December 6, 2016 | X | |||||||||
| 10.100 m | Offer Letter dated September 27, 2006 between registrant and John Melo | S-1 | 333-16135 | April 16, 2010 | 10.27 | ||||||
| 10.101 m | Amendment, dated December 18, 2008, between registrant and John Melo | S-1 | 333-16135 | April 16, 2010 | 10.28 | ||||||
| 10.102 m | Consulting Agreement, dated September 2, 2015, between registrant and Paulo Diniz | 10-K | 001-34885 | March 30, 2016 | 10.81 | ||||||
| 10.103 m | Offer letter, dated September 30, 2008, between registrant and Joel Cherry | S-1 | 333-16135 | April 16, 2010 | 10.29 | ||||||
| 10.104 m | Offer letter, dated September 20, 2010, between registrant and Nicholas Khadder | 10-Q | 001-34885 | August 10, 2015 | 10.04 | ||||||
| 10.105 m | Revised employment letter, dated December 3, 2013, between registrant and Nicholas Khadder | 10-Q | 001-34885 | August 10, 2015 | 10.05 | ||||||
| 10.106 m | Offer letter, dated October 23, 2014, between registrant and Raffi Asadorian | 10-K | 001-34885 | March 31, 3015 | 10.68 | ||||||
| 10.107 m | Offer letter, dated November 23, 2016, between registrant and Kathleen Valiasek | X | |||||||||
| 10.108 m | 2005 Stock Option/Stock Issuance Plan | 10-Q | 001-34885 | November 9, 2011 | 10.02 | ||||||
| 10.109 m | Form of Notice of Grant of Stock Option under registrant’s 2005 Stock Option/Stock Issuance Plan | S-1 | 333-16135 | April 16, 2010 | 10.38 | ||||||
| 10.110 m | Form of Notice of Grant of Stock Option (non-Exempt) under registrant’s 2005 Stock Option/Stock Issuance Plan | S-1 | 333-16135 | April 16, 2010 | 10.39 | ||||||
| 10.111 m | Form of Notice of Grant of Stock Option (non-US) under registrant’s 2005 Stock Option/Stock Issuance Plan | S-1 | 333-16135 | April 16, 2010 | 10.40 | ||||||
| 10.112 m | Form of Stock Option Agreement under registrant’s 2005 Stock Option/Stock Issuance Plan | S-1 | 333-16135 | April 16, 2010 | 10.41 | ||||||
| 10.113 m | Form of Stock Option Agreement (non-US) under registrant’s 2005 Stock Option/Stock Issuance Plan | S-1 | 333-16135 | April 16, 2010 | 10.42 | ||||||
| 10.114 m | Form of Stock Purchase Agreement under registrant’s 2005 Stock Option/Stock Issuance Plan | S-1 | 333-16135 | April 16, 2010 | 10.43 | ||||||
| 10.115 m | Form of Stock Purchase Agreement (non-US) under registrant’s 2005 Stock Option/Stock Issuance Plan | S-1 | 333-16135 | April 16, 2010 | 10.44 | ||||||
| 10.116 m | 2010 Equity Incentive Plan and forms of award agreements thereunder | S-1 | 333-16135 | June 23, 2010 | 10.46 | ||||||
| 10.117 m | 2010 Employee Stock Purchase Plan and form of subscription agreements thereunder | S-1 | 333-16135 | September 20, 2010 | 10.45 | ||||||
| 10.118 m | Amyris, Inc. Executive Severance Plan, effective November 6, 2013 | 10-K | 001-34885 | April 2, 2014 | 10.94 | ||||||
| 10.119 m | Compensation arrangements between registrant and its non-employee directors | X | |||||||||
| 10.120 m | Compensation arrangements between registrant and its executive officers | X | |||||||||
| 10.121 m | Form of Indemnity Agreement between registrant and its directors and officers | S-1 | 333-166135 | June 23, 2010 | 10.01 | ||||||
| 21.01 | List of subsidiaries | X | |||||||||
| 23.01 | Consent of PricewaterhouseCoopers LLP, independent registered public accounting firm | X | |||||||||
| 24.01 | Power of Attorney (see signature page to this Form 10-K) | X | |||||||||
| 31.01 | Certification of Chief Executive Officer pursuant to Securities Exchange Act Rules 13a-14(c) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | |||||||||
| 31.02 | Certification of Chief Financial Officer pursuant to Securities Exchange Act Rules 13a-14(c) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X |
| 209 |
| 32.01 n | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | |||||||||
| 32.02 n | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | |||||||||
| 99.1 | Novvi LLC Financial Statements December 31, 2014 | 10-K | 001-34885 | March 31, 2015 | 99.1 | ||||||
| 99.2 | Novvi LLC Financial Statements December 31, 2015 (Unaudited) | 10-K | 001-34885 | March 30, 2016 | 99.2 | ||||||
| 99.3 | Novvi LLC Financial Statements December 31, 2016 (Unaudited) | X | |||||||||
| 99.4 | Section 13(r) Disclosure | X | |||||||||
| 101 p | The following materials from registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2016, formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statements of Operations; (ii) the Consolidated Balance Sheets; (iii) the Consolidated Statements of Comprehensive Income; (iv) the Consolidated Statements of Convertible Preferred Stock, Redeemable Noncontrolling Interest and Equity (Deficit); (v) the Consolidated Statements of Cash Flows; and (vi) Notes to Consolidated Financial Statements | X |
| 210 |
| a | Portions of this exhibit, which have been granted confidential treatment by the Securities and Exchange Commission, have been omitted. |
| b | Portions of this exhibit have been omitted pending a determination by the Securities and Exchange Commission as to whether these portions should be granted confidential treatment. |
| c | Substantially identical Common Stock Purchase Agreements, each dated May 18, 2012, were entered into with five separate investors. Registrant has filed the form of such Common Stock Purchase Agreements, which is substantially identical in all material respects to all of such Common Stock Purchase Agreements, except as to the parties thereto and the number of shares being purchased. |
| d | Registrant issued substantially identical 5% Unsecured Convertible Notes (the "5% Notes") to Total Gas & Power USA, SAS (“Total’), FIAM Target Date Large Cap Stock Commingled Pool (formerly known as Fidelity Pyramis Lifecycle Large Cap Stock Commingled Pool. Fidelity Variable Insurance Products Fund Ill: Growth & Income Portfolio, Fidelity Hastings Street Trust: Fidelity Advisor Series Growth & Income Fund. Fidelity Securities Fund: Fidelity Growth & Income Portfolio Fidelity Hastings Street Trust: Fidelity Series Growth & Income Fund. Fidelity Commonwealth Trust: Fidelity Large Cap Stock Fund, and Maxwell (Mauritius) Pte Ltd on October 16. 2013. Registrant has filed the 5% Note issued to Total. and has included with Exhibit 4.04 a schedule (Schedule A to Exhibit 4.04 of registrant’s Form 10-Q filed on May 9, 2014) identifying each of the 5% Notes and setting forth the material detail in which the other 5% Note(s) differ from the filed 5% Note (i.e. the Purchasers. the amounts of the 5% Notes. and the conversion price). |
| e | Registrant issued substantially identical 10% Unsecured Convertible Notes (the" 10% Notes") to Total Wolverine Flagship Fund Trading Limited and Maxwell (Mauritius) Pte Ltd on January 15 2014. Registrant has filed the 10% Note issued to Total and has included with Exhibit 4.06. a schedule (Schedule A to Exhibit 4.06 of registrant’s Form 10-Q filed on May 9, 2014) identifying each of the 10% Notes and setting forth the material details in which the other 10% Note(s) differ from the filed 10% Note (i.e. the purchasers and the amounts of the 10% Notes). |
| f | Registrant issued substantially identical 6.5% Senior Convertible Notes due 2019 (the “6.5% Notes”) to Maxwell (Mauritius) Pte Ltd. (“Temasek”), Total Energies Nouvelles Activités USA, and Foris Ventures, LLC on May 29, 2014. Registrant has filed the 6.5% Note issued to Temasek, and has included, with such exhibit, a schedule (Schedule A to Exhibit 4.03 of registrant's Form 10-Q filed August 8, 2014) identifying each of the 6.5% Notes and setting forth the material details in which the other 6.5% Notes differ from the filed 6.5% Note (i.e., the note number, the purchasers, and the amounts of the 6.5% Notes). |
| g | Substantially identical Voting Agreements, each dated July 31, 2015, were entered into with five separate investors. Registrant has filed Voting Agreement entered into by registrant and Foris Ventures LLC, which is substantially identical in all material respects to all of such Voting Agreements, except as to the parties thereto. |
| h | Registrant issued substantially identical warrants to the purchasers under that certain Securities Purchase Agreement entered into on July 24. 2015~ Registrant has filed the warrant issued to Total Energies Nouvelles Activites USA and has included with such Exhibit a schedule (Schedule A to Exhibit 4.03 of registrants Form 10-Q filed on August 8, 2015) identifying each of the warrants and setting forth the material details in which the other warrants differ from the filed form of warrant (i.e. the names of the purchasers. the certificate numbers and the respective amounts of shares underlying the warrants). |
| i | Substantially identical Unsecured Promissory Notes, each dated February 15, 2016 (the “Notes”), were entered into with three separate investors. Registrant has filed the Note entered into by registrant and Foris Ventures LLC, which is substantially identical in all material respects to all of such Notes except as to the parties thereto and the value of the Notes. |
| j | Substantially identical Warrants to Purchase Stock, each dated February 15, 2016 (the “Warrants”), were entered into with three separate investors. Registrant has filed the Warrant entered into by registrant and Foris Ventures LLC, which is substantially identical in all material respects to all of such Warrants, except as to the parties thereto and the amount of underlying shares. |
| k | Translation to English from Portuguese or Dutch, as applicable, in accordance with Rule 12b-12(d) of the regulations promulgated by the Securities and Exchange Commission under the Securities Exchange Act of 1934, as amended (or the Exchange Act). |
| m | Indicates management contract or compensatory plan or arrangement. |
| n | This certification shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liability of that Section, nor shall it be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act. |
| p | Pursuant to applicable securities laws and regulations, registrant is deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and is not subject to liability under any anti-fraud provisions of the federal securities laws as long as registrant has made a good faith attempt to comply with the submission requirements and promptly amends the interactive data files after becoming aware that the interactive data files fails to comply with the submission requirements. These interactive data files are deemed not filed or part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act, are deemed not filed for purposes of section 18 of the Exchange Act and otherwise are not subject to liability under these sections. |
211