
U.S. FDA Approval of EKTERLY® (sebetralstat) July 7, 2025 Exhibit 99.2

Forward-looking statements This presentation and the accompanying oral commentary contain forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available to our management. For this purpose, any statements that are not statements of historical fact may be deemed forward-looking statements. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expect,” “plan,” anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential,” “would,” “continue,” “ongoing”, "seek", "future", "likely", "goal", "strategy", "project", or the negative of these terms or other comparable terminology. These forward-looking statements include statements contained in this presentation, including, among others, those relating to: information regarding the potential commercial success and growth of EKTERLY, including market size, acceptance, demand, and adoption rate for EKTERLY (sebetralstat), our ability to successfully implement our patient and provider outreach campaign, whether EKTERLY will receive foreign approval when expected or at all, information relating to our general business plans and objectives, the timing and success of our planned nonclinical and clinical development activities, the timing and results of nonclinical studies and clinical trials, the efficacy and safety profiles of our product candidates, any expectations about safety, the efficacy of EKTERLY, the ability of EKTERLY to treat hereditary angioedema (HAE), the potential therapeutic benefits and economic value of our product candidates, statements regarding potential market and growth opportunities, our competitive position, the industry environment as a whole, our ability to protect intellectual property and the impact of global business or macroeconomic conditions, including as a result of inflation, rising interest rates, instability in the global banking system, and geopolitical conflicts, including the conflicts in Ukraine and the Middle East, on our business and operations. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that are described under the heading “Risk Factors” contained in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission (“SEC”) on July 11, 2024, as updated by our subsequent filings with the SEC, including our Quarterly Reports on Form 10-Q, as well as other documents we file from time to time with the SEC, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this presentation, and although we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted a thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely upon these statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.

EKTERLY® now approved by U.S. FDA What is EKTERLY? EKTERLY (sebetralstat) is indicated for the treatment of acute attacks of hereditary angioedema (HAE) in adult and pediatric patients aged 12 years and older. Please see Prescribing Information at KalVista.com.

EKTERLY U.S. Prescribing Information & Clinical Data

Hereditary angioedema: a rare disease defined by unpredictable swelling attacks 1 in 35,000 - 1 in 50,000 people worldwide1,2 Lifelong attacks of debilitating swelling in the face, extremities, abdomen and genitals2 Life threatening if the upper airway is involved3 Attack severity may increase rapidly over 24 hours4 Symptoms take 2 to 5 days to resolve2 1.Castaldo, A. J., et al. (2025). Establishing a hereditary angioedema prevalence for the United States using a large administrative claims database. Annals of Allergy, Asthma & Immunology. 2. HAEi. Available at: www.haei.org. 3. Hereditary Angioedema Deaths: A Review from the Romanian Registry Moldovan, D. et al. Journal of Allergy and Clinical Immunology, Volume 135, Issue 2, AB196 4. Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008;359(10):1027–1036. Prevalence: Approximately
All Patients Treatment goals: achieve total disease control and normalize patients' lives1,2 Management of HAE 1. Busse PJ, et al. J Allergy Clin Immunol Pract. 2021;9(1):132-150.e3. 2. Maurer M, et al. Allergy. 2022;77(7):1961-1990 Long-term prophylaxis in appropriate patients: Effective On-Demand Treatment High disease activity Poor patient quality of life Inadequate control with on-demand therapy2 Should ideally offer: Rapid symptom relief Easy self-administration enabling early treatment Favorable safety profile

Four key recommendations in global guidelines for the treatment of HAE attacks1-3 Treat attacks as early as possible after recognition of onset Treatment should be considered for all attacks, regardless of anatomic location or severity Train all patients in the self-administration of on-demand treatment Ensure all patients have ready access to, and carry, sufficient on-demand medication to treat at least two attacks 1 2 3 4 Injectable on-demand therapies come with challenges: 3.8 hours average time people with HAE waited to treat an attack4 of patients carry on-demand treatment outside the home all the time7 1/3 to 1/2 < 40% 1. Betschel S, et al. Abstract presented at: 13th C1-inhibitor Deficiency and Angiodema Workshop; May 4-7, 2023. 2. Soteres DF et al. Abstract presented at: AAAAI Annual Meeting; Feb 23-26, 2024; Washington D.C. 3. Betschel S, et al. Abstract presented at: EAACI 2023 Hybrid Congress’ June 9-11, 2023; Hamburg, Germany 4. Results from a 2023 HAE Association survey of 94 people taking either on-demand treatment or both on-demand and preventative treatment. 5. Christiansen S, et al. Ann Allergy Asthma Immunol. 2024.doi:10.1016/j.anai.2024.12.012. 6. Squeglia V. Orphanet J Rare Dis. 2016;11(1):133. 7. Lumry et al. Management of hereditary angioedema attacks by patients on long-term prophylaxis versus on-demand therapy only. Allergy Asthma Proc 46:000–000, 2025. 7.7 hours average time adolescents waited to treat an attack5 of attacks go untreated, including among patients on long-term prophylaxis6

Management of HAE today Long-term prophylaxis in majority of patients Injectable or Intravenous On-demand treatment is underutilized Complex logistics and painful Delays and denial of treatment Inadequate control High treatment burden High cost Less benefit than anticipated in many cases Inadequate control with parenteral on-demand treatment and disproportionate use of LTP
Management of HAE with EKTERLY 1. Busse PJ, et al. J Allergy Clin Immunol Pract. 2021;9(1):132-150.e3. 2. Maurer M, et al. Allergy. 2022;77(7):1961-1990. Long-term prophylaxis in appropriate patients1,2 EKTERLY On-Demand Treatment First and only oral on-demand HAE treatment Poised to Become the Foundational HAE Treatment
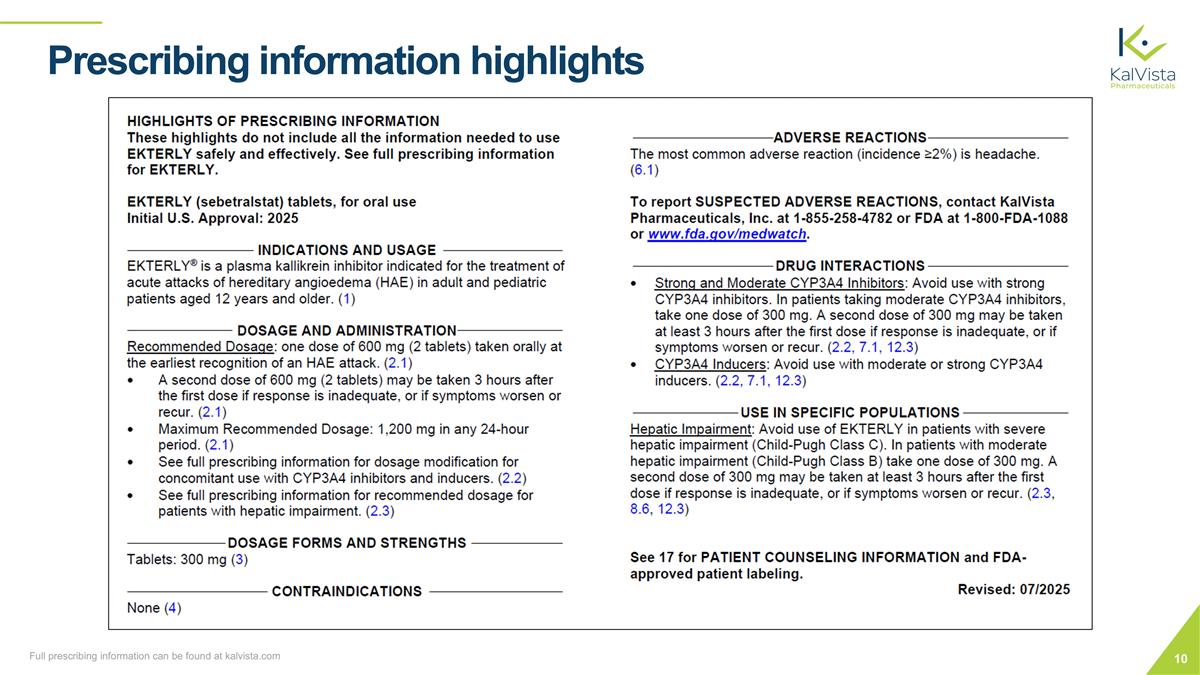
Prescribing information highlights Full prescribing information can be found at kalvista.com
Key label takeaways Dosing & Administration Only label to include "at the earliest recognition of an acute HAE attack" 600 mg with second dose in 3 hours up to 1200 mg max daily dose Contraindications, Warnings & Precautions No contraindications, warnings, or precautions Indications and Usage Adolescents and adults All types of HAE All severities and attack locations, including laryngeal attacks No limitations related to LTP usage
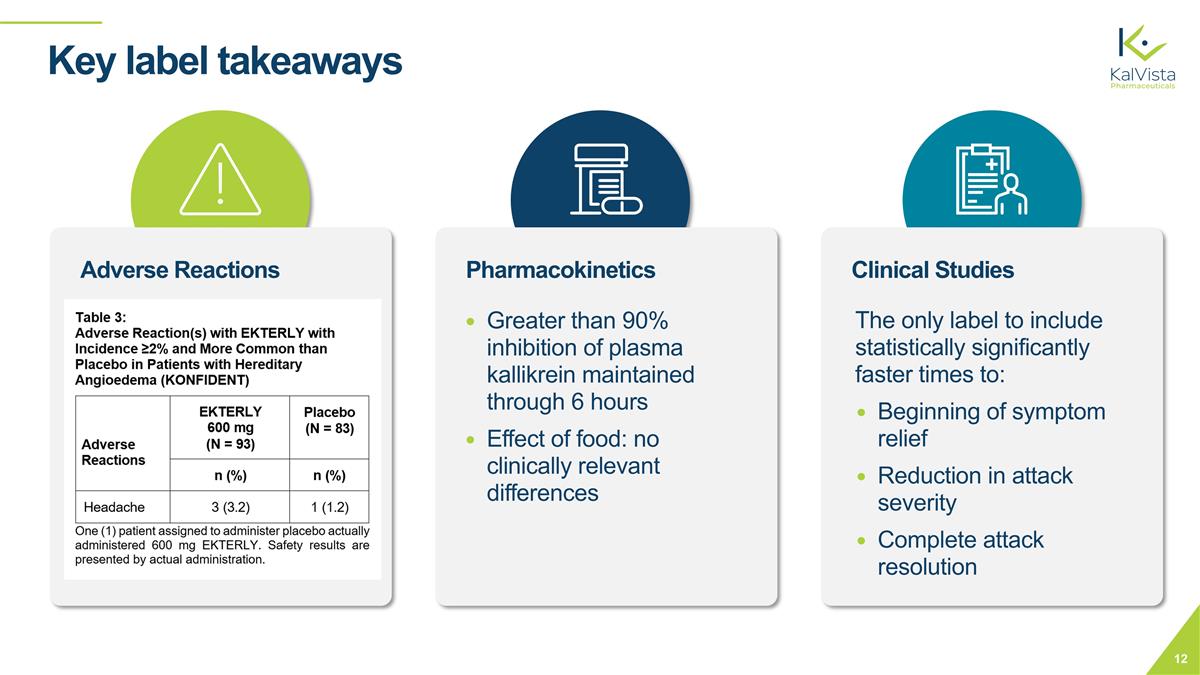
Key label takeaways Pharmacokinetics Clinical Studies Adverse Reactions Greater than 90% inhibition of plasma kallikrein maintained through 6 hours Effect of food: no clinically relevant differences The only label to include statistically significantly faster times to: Beginning of symptom relief Reduction in attack severity Complete attack resolution
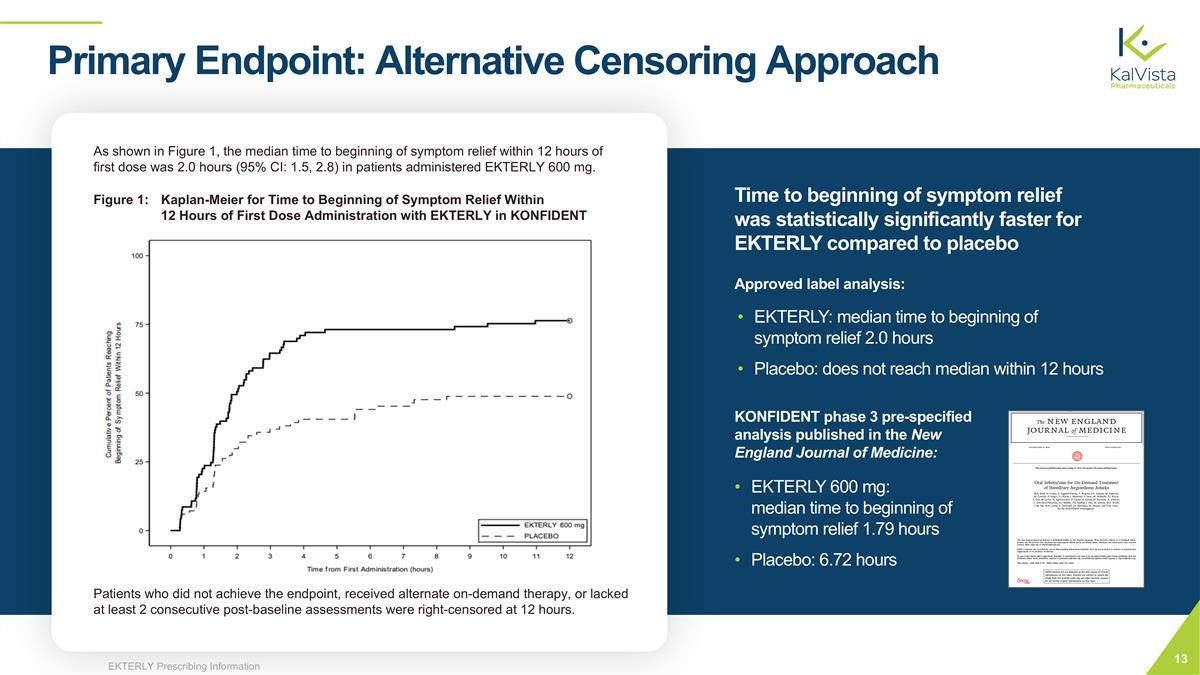
As shown in Figure 1, the median time to beginning of symptom relief within 12 hours of first dose was 2.0 hours (95% CI: 1.5, 2.8) in patients administered EKTERLY 600 mg. Patients who did not achieve the endpoint, received alternate on-demand therapy, or lacked at least 2 consecutive post-baseline assessments were right-censored at 12 hours. EKTERLY: median time to beginning of symptom relief 2.0 hours Placebo: does not reach median within 12 hours KONFIDENT phase 3 pre-specified analysis published in the New England Journal of Medicine: EKTERLY 600 mg: median time to beginning of symptom relief 1.79 hours Placebo: 6.72 hours Primary Endpoint: Alternative Censoring Approach Approved label analysis: Time to beginning of symptom relief was statistically significantly faster for EKTERLY compared to placebo Figure 1: Kaplan-Meier for Time to Beginning of Symptom Relief Within 12 Hours of First Dose Administration with EKTERLY in KONFIDENT EKTERLY Prescribing Information
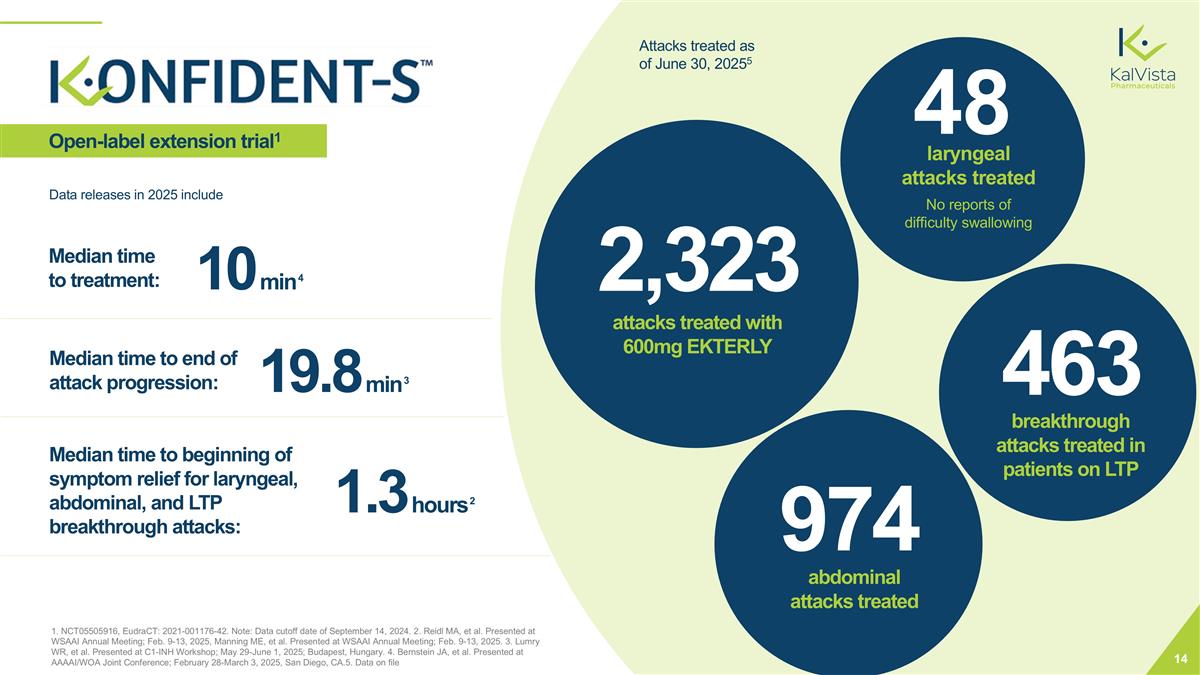
1. NCT05505916, EudraCT: 2021-001176-42. Note: Data cutoff date of September 14, 2024. 2. Reidl MA, et al. Presented at WSAAI Annual Meeting; Feb. 9-13, 2025, Manning ME, et al. Presented at WSAAI Annual Meeting; Feb. 9-13, 2025. 3. Lumry WR, et al. Presented at C1-INH Workshop; May 29-June 1, 2025; Budapest, Hungary. 4. Bernstein JA, et al. Presented at AAAAI/WOA Joint Conference; February 28-March 3, 2025, San Diego, CA.5. Data on file Open-label extension trial1 1.3 hours 2 Median time to beginning of symptom relief for laryngeal, abdominal, and LTP breakthrough attacks: 19.8 min 3 Median time to end of attack progression: 10 min 4 Median time to treatment: Data releases in 2025 include No reports of difficulty swallowing 48 laryngeal attacks treated X breakthrough attacks treated in patients on LTP 463 breakthrough attacks treated in patients on LTP X breakthrough attacks treated in patients on LTP 974 abdominal attacks treated ~2,300 attacks treated with 600mg EKTERLY Attacks treated as of June 30, 20255 2,323 attacks treated with 600mg EKTERLY

Commercial Strategy & Launch Plans

EKTERLY: The first and only oral on-demand HAE treatment Early Treatment Can be taken immediately upon attack recognition Injection-like Efficacy Proven to halt attack progression quickly and safely without any needles or pain All Attacks Effective against all types of HAE attacks, regardless of prophylactic therapy Poised to be the foundational HAE treatment that enables early treatment of all attacks to improve outcomes
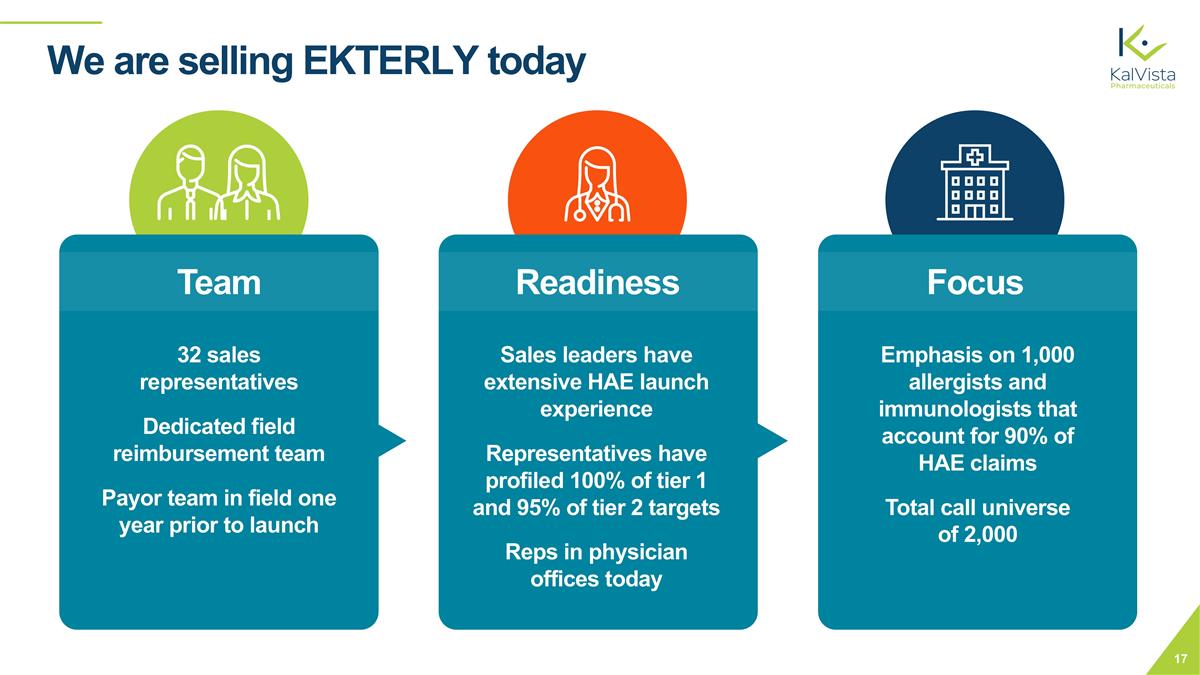
We are selling EKTERLY today Team Sales leaders have extensive HAE launch experience Representatives have profiled 100% of tier 1 and 95% of tier 2 targets Reps in physician offices today Readiness Focus Emphasis on 1,000 allergists and immunologists that account for 90% of HAE claims Total call universe of 2,000 32 sales representatives Dedicated field reimbursement team Payor team in field one year prior to launch
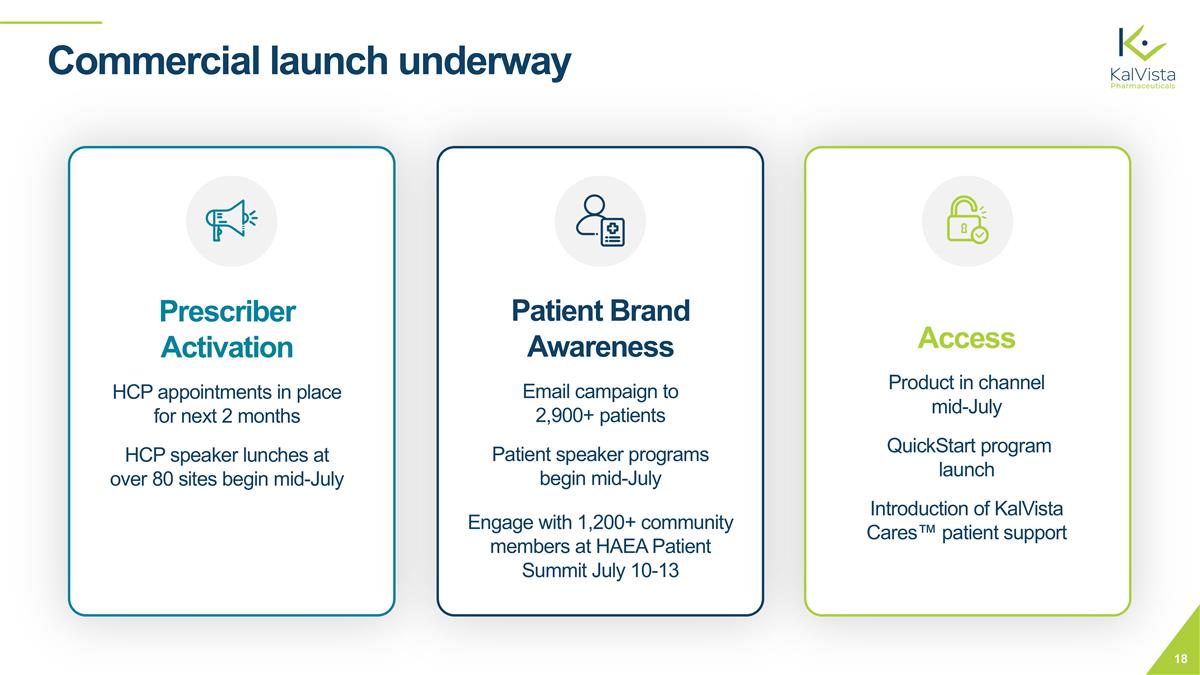
Prescriber Activation HCP appointments in place for next 2 months HCP speaker lunches at over 80 sites begin mid-July Patient Brand Awareness Email campaign to 2,900+ patients Patient speaker programs begin mid-July Engage with 1,200+ community members at HAEA Patient Summit July 10-13 Commercial launch underway Access Product in channel mid-July QuickStart program launch Introduction of KalVista Cares™ patient support

Access and product support Patient Support Services Reimbursement support and insurance navigation Patient education and advocacy Work with the specialty pharmacy Navigate insurance coverage Each EKTERLY pack includes two doses (4 tablets) WAC price $16,720 per dose Comparable to current branded products $11K-17K per dose Access Services Quick Start Program Patient Assistance Program Bridge Program Commercial Drug Access Co-pay Assistance Program KalVista Cares offers access and patient support services:
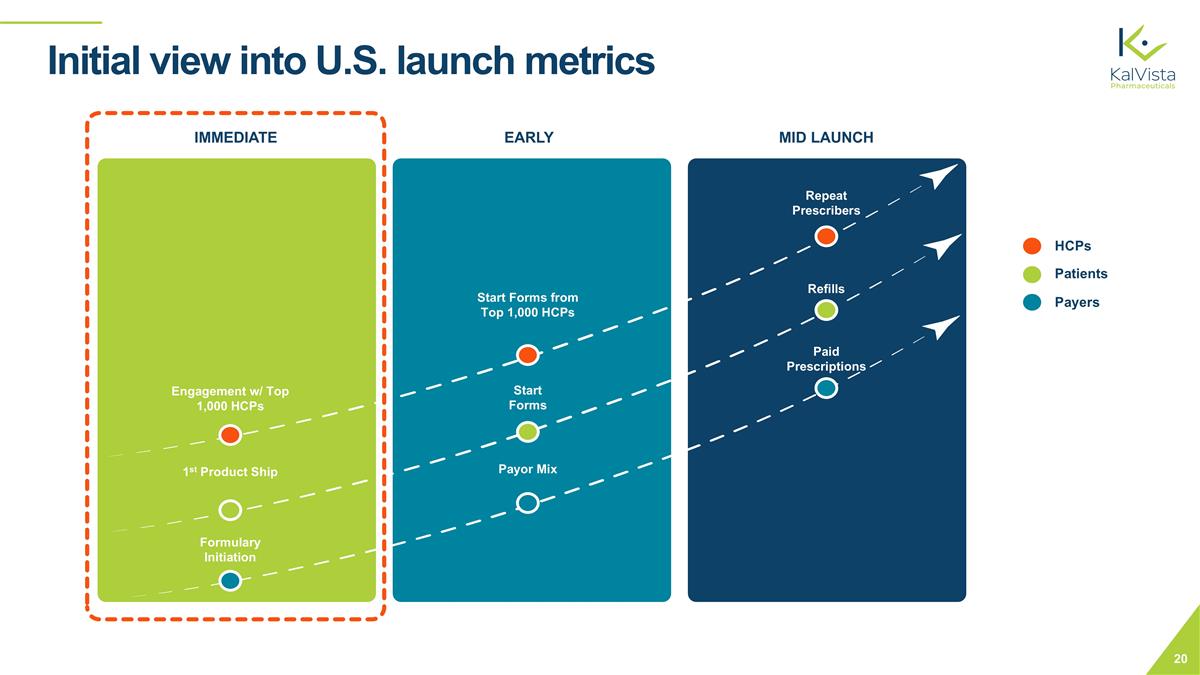
Initial view into U.S. launch metrics HCPs Patients Payers Start Forms from Top 1,000 HCPs Start Forms Payor Mix IMMEDIATE EARLY MID LAUNCH Formulary Initiation Engagement w/ Top 1,000 HCPs 1st Product Ship Paid Prescriptions Repeat Prescribers Refills
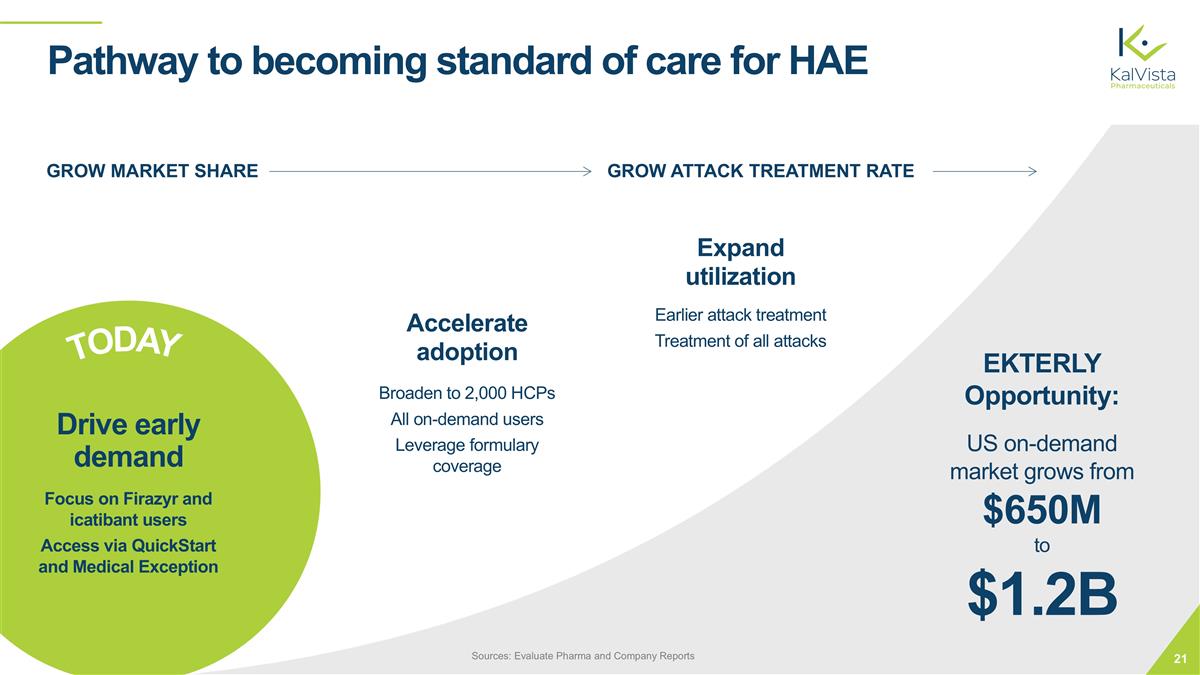
Pathway to becoming standard of care for HAE GROW ATTACK TREATMENT RATE GROW MARKET SHARE EKTERLY Opportunity: US on-demand market grows from $650M to $1.2B Sources: Evaluate Pharma and Company Reports TODAY Drive early demand Focus on Firazyr and icatibant users Access via QuickStart and Medical Exception Accelerate adoption Broaden to 2,000 HCPs All on-demand users Leverage formulary coverage Expand utilization Earlier attack treatment Treatment of all attacks
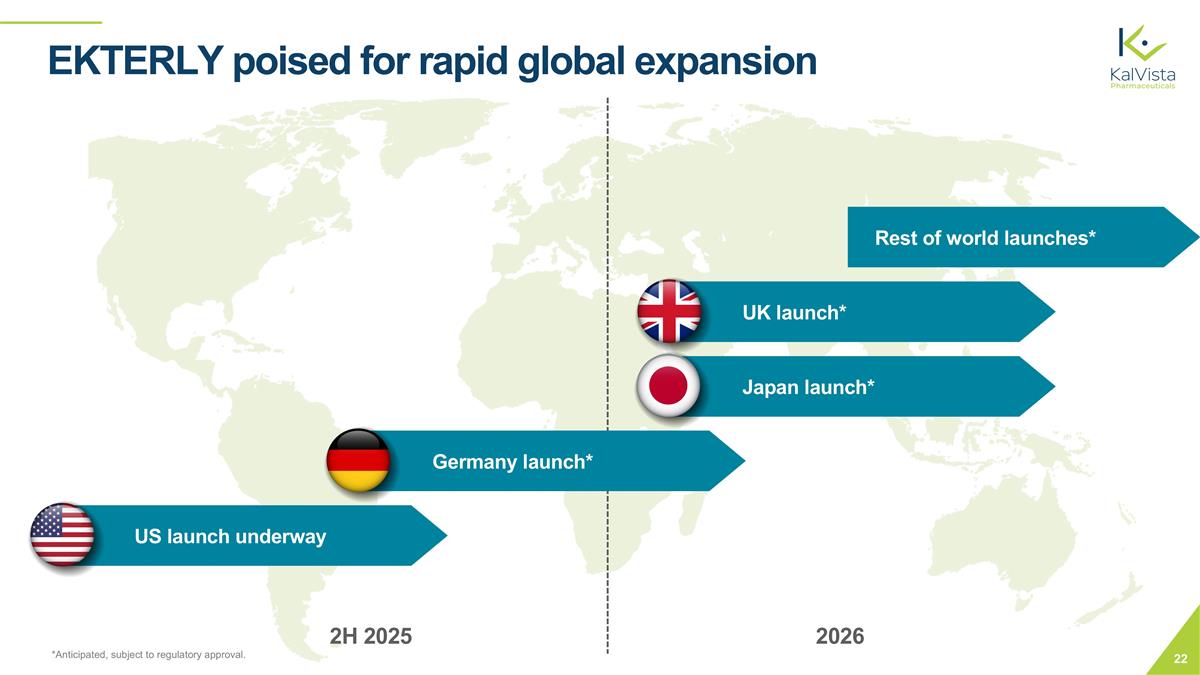
EKTERLY poised for rapid global expansion US launch underway Germany launch* Japan launch* Rest of world launches* UK launch* *Anticipated, subject to regulatory approval. 2H 2025 2026

Poised to become the foundational therapy for HAE

Q&A