UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
xQUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended March 31, 2021
OR
oTRANSITION REPORT UNDER SECTION 13 OF 15(d) OF THE EXCHANGE ACT OF 1934
From the transition period from to .
Commission File Number 001-35798
Humanigen, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 77-0557236 | |
| (State or other jurisdiction of | (IRS Employer | |
| incorporation) | Identification No.) |
533 Airport Boulevard, Suite 400 Burlingame, CA 94010
(Address of principal executive offices)
(Zip Code)
Registrant’s telephone number, including area code: (650) 243-3100
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on with registered |
| Common Stock | HGEN | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
| Large accelerated filer o | Accelerated filer o | |
| Non-accelerated filer x | Smaller reporting company x | |
| Emerging growth company o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes x No o
As of May 5, 2021, there were 59,083,706 shares of common stock of the issuer outstanding.
HUMANIGEN, INC.
FORM 10-Q
| 2 |
| Item 1. | Financial Statements |
Humanigen, Inc.
Condensed Consolidated Balance Sheets
(in thousands, except share data)
(Unaudited)
| March 31, 2021 | December 31, 2020 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 92,892 | $ | 67,737 | ||||
| Other receivable | 5,010 | - | ||||||
| Prepaid expenses and other current assets | 856 | 475 | ||||||
| Total current assets | 98,758 | 68,212 | ||||||
| Other assets | 90 | 90 | ||||||
| Total assets | $ | 98,848 | $ | 68,302 | ||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 43,412 | $ | 15,366 | ||||
| Accrued expenses | 5,698 | 3,175 | ||||||
| Deferred revenue | 4,145 | 1,874 | ||||||
| Total current liabilities | 53,255 | 20,415 | ||||||
| Non-current liabilities: | ||||||||
| Deferred revenue | 4,126 | 2,342 | ||||||
| Long-term debt | 24,444 | - | ||||||
| Total liabilities | 81,825 | 22,757 | ||||||
| Stockholders’ equity: | ||||||||
| Common stock, $0.001 par value: 225,000,000 shares authorized at March 31, 2021 and December 31, 2020; 53,656,689 and 51,626,508 shares issued and outstanding at March 31, 2021 and December 31, 2020, respectively | 54 | 52 | ||||||
| Additional paid-in capital | 456,966 | 419,923 | ||||||
| Accumulated deficit | (439,997 | ) | (374,430 | ) | ||||
| Total stockholders’ equity | 17,023 | 45,545 | ||||||
| Total liabilities and stockholders’ equity | $ | 98,848 | $ | 68,302 | ||||
See accompanying notes.
| 3 |
Humanigen, Inc.
Condensed Consolidated Statements of Operations
(in thousands, except share and per share data)
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenue: | ||||||||
| License revenue | $ | 486 | $ | - | ||||
| Total revenue | 486 | - | ||||||
| Operating expenses: | ||||||||
| Research and development | $ | 59,934 | $ | 659 | ||||
| General and administrative | 4,948 | 1,398 | ||||||
| Total operating expenses | 64,882 | 2,057 | ||||||
| Loss from operations | (64,396 | ) | (2,057 | ) | ||||
| Other expense: | ||||||||
| Interest expense | (19 | ) | (410 | ) | ||||
| Other expense | (1,152 | ) | - | |||||
| Net loss | $ | (65,567 | ) | $ | (2,467 | ) | ||
| Basic and diluted net loss per common share | $ | (1.25 | ) | $ | (0.11 | ) | ||
| Weighted average common shares outstanding used to calculate basic and diluted net loss per common share | 52,655,756 | 22,854,106 | ||||||
See accompanying notes.
| 4 |
Humanigen, Inc.
Condensed Consolidated Statements of Cash Flows
(in thousands)
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Operating activities: | ||||||||
| Net loss | $ | (65,567 | ) | $ | (2,467 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock based compensation expense | 510 | 265 | ||||||
| Issuance of common stock for payment of compensation | - | 19 | ||||||
| Issuance of common stock in exchange for services | - | 13 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Other receivable | (5,010 | ) | - | |||||
| Prepaid expenses and other assets | (381 | ) | 16 | |||||
| Accounts payable | 28,046 | 714 | ||||||
| Accrued expenses | 2,523 | 833 | ||||||
| Deferred revenue | 4,055 | - | ||||||
| Net cash used in operating activities | (35,824 | ) | (607 | ) | ||||
| Financing activities: | ||||||||
| Net proceeds from issuance of common stock | 36,106 | 65 | ||||||
| Proceeds from exercise of stock options | 429 | - | ||||||
| Net proceeds from issuance of long-term debt | 24,444 | 467 | ||||||
| Net cash provided by financing activities | 60,979 | 532 | ||||||
| Net increase (decrease) in cash and cash equivalents | 25,155 | (75 | ) | |||||
| Cash and cash equivalents, beginning of period | 67,737 | 143 | ||||||
| Cash and cash equivalents, end of period | $ | 92,892 | $ | 68 | ||||
| Supplemental cash flow disclosure: | ||||||||
| Cash paid for interest | $ | 4 | $ | 2 | ||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||
| Issuance of stock options in lieu of cash compensation | $ | - | $ | 133 | ||||
| Issuance of warrants for services | $ | - | $ | 1 | ||||
See accompanying notes.
| 5 |
Humanigen, Inc.
Condensed Consolidated Statements of Stockholders’ Equity (Deficit)
(in thousands, except share data)
(Unaudited)
| Three Months Ended March 31, 2021 | ||||||||||||||||||||
| Additional | Total | |||||||||||||||||||
| Common Stock | Paid-In | Accumulated | Stockholders’ | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Equity | ||||||||||||||||
| Balances at January 1, 2021 | 51,626,508 | $ | 52 | $ | 419,923 | $ | (374,430 | ) | $ | 45,545 | ||||||||||
| Issuance of common stock, net of expenses | 1,796,858 | 2 | 36,104 | - | 36,106 | |||||||||||||||
| Issuance of common stock upon option exercise | 233,323 | - | 429 | - | 429 | |||||||||||||||
| Stock-based compensation expense | - | - | 510 | - | 510 | |||||||||||||||
| Net loss | - | - | - | (65,567 | ) | (65,567 | ) | |||||||||||||
| Balances at March 31, 2021 | 53,656,689 | $ | 54 | $ | 456,966 | $ | (439,997 | ) | $ | 17,023 | ||||||||||
| Three Months Ended March 31, 2020 | ||||||||||||||||||||
| Total | ||||||||||||||||||||
| Additional | Stockholders’ | |||||||||||||||||||
| Common Stock | Paid-In | Accumulated | Equity | |||||||||||||||||
| Shares | Amount | Capital | Deficit | (Deficit) | ||||||||||||||||
| Balances at January 1, 2020 | 22,806,890 | $ | 22 | $ | 270,555 | $ | (284,895 | ) | $ | (14,318 | ) | |||||||||
| Issuance of common stock, net of expenses | 40,000 | - | 65 | - | 65 | |||||||||||||||
| Issuance of common stock in exchange for services | 5,868 | - | 13 | - | 13 | |||||||||||||||
| Issuance of stock options for payment of compensation | - | - | 133 | - | 133 | |||||||||||||||
| Issuance of common stock for payment of compensation | 9,599 | - | 19 | - | 19 | |||||||||||||||
| Issuance of warrant for services | - | - | 1 | - | 1 | |||||||||||||||
| Stock-based compensation expense | - | - | 265 | - | 265 | |||||||||||||||
| Net loss | - | - | - | (2,467 | ) | (2,467 | ) | |||||||||||||
| Balances at March 31, 2020 | 22,862,357 | $ | 22 | $ | 271,051 | $ | (287,362 | ) | $ | (16,289 | ) | |||||||||
See accompanying notes.
| 6 |
Humanigen, Inc.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1. Nature of Operations
Description of the Business
Humanigen, Inc. (the “Company”) was incorporated on March 15, 2000 in California, reincorporated as a Delaware corporation in September 2001 as KaloBios Pharmaceuticals, Inc., and in August 2017, the Company changed its name to Humanigen, Inc.
The Company is a clinical stage biopharmaceutical company, developing its portfolio of anti-inflammatory immunology and immuno-oncology monoclonal antibodies. The Company is focusing its efforts on the development of its lead product candidate, lenzilumab™, its proprietary Humaneered® (“Humaneered”) anti-human granulocyte-macrophage colony-stimulating factor (“GM-CSF”) monoclonal antibody. Lenzilumab is a monoclonal antibody that has been demonstrated to neutralize GM-CSF, a cytokine that the Company believes is of critical importance in the hyperinflammatory cascade, sometimes referred to as cytokine release syndrome (“CRS”) or cytokine storm, associated with COVID-19, chimeric antigen receptor T-cell (“CAR-T”) therapy and acute Graft versus Host Disease (“GvHD”) associated with bone marrow transplants. The Company believes the results from its Phase 3 study in COVID-19 and its Phase 1b study in CAR-T support the mechanism of action of lenzilumab.
On March 29, 2021, the Company announced top-line data on the primary endpoint and one secondary endpoint from a Phase 3, multi-center, double-blind, placebo-controlled potential registrational trial of lenzilumab (the “LIVE-AIR” study) as a potential therapeutic for newly hospitalized, hypoxic patients with COVID-19 pneumonia. Additional data from the trial, subsequently published on MedRxiv, a non-peer reviewed journal, on May 5, 2021, support the previously reported primary endpoint that showed lenzilumab improved the likelihood of survival without ventilation, (“SWOV”), sometimes referred to as “ventilator-free survival”, by 54% in the modified intent-to-treat (“mITT”) population (Hazard Ratio, (“HR”): 1.54; 95%CI: 1.02-2.31, p=0.041). New analysis included in the publication showed that lenzilumab improved the likelihood of SWOV by 90% in the intent-to-treat (“ITT”) population (HR: 1.90; 1.02-3.52, nominal p=0.043) compared to placebo. SWOV also relatively improved by 92% in subjects who received both corticosteroids and remdesivir (1.92; 1.20-3.07, nominal p=0.0067); by 2.96-fold in subjects with C-reactive protein (“CRP”) levels <150 mg/L and age <85 years (2.96; 1.63–5.37, nominal p=0.0003); and by 88% in subjects hospitalized ≤2 days prior to randomization (1.88; 1.13-3.12, nominal p=0.015). Survival was improved by 2.17-fold in subjects with CRP<150 mg/L and age <85 years (2.17; 1.04-4.54, nominal p=0.040). The Company believes that the data published on MedRxiv, generated from the LIVE-AIR study support the conclusion that lenzilumab significantly improved SWOV in hospitalized, hypoxic subjects with COVID-19 pneumonia over and above treatment with remdesivir and/or corticosteroids. Subjects with CRP<150 mg/L and age <85 years demonstrated an improvement in survival and appeared to derive the greatest benefit from lenzilumab.
The Company shared the topline data on the primary endpoint and one secondary endpoint (the only data then available while the remaining analysis was pending) with the U.S. Food and Drug Administration (“FDA”) in a Pre-Emergency Use Authorization (“EUA”) Type B meeting in mid-April 2021. The Company is preparing to submit an EUA application at the end of May 2021. As requested by the FDA, the EUA application will include secondary endpoints and supplemental data analysis from LIVE-AIR, including those referenced in the MedRxiv publication, as well as additional stability and compatibility information required for the Chemistry Manufacturing and Control (“CMC”) section of the EUA application. There can be no assurance that the data published on MedRxiv will be sufficient for an EUA or that the FDA will not require additional information in order to grant an EUA. If the EUA is granted, the Company could begin to commercialize lenzilumab for the treatment of newly hospitalized COVID-19 pneumonia patients. COVID-19 infections are widespread and as of April 30, 2021 in the U.S., there were over 32 million cases, over 2.1 million hospitalizations and over 570 thousand deaths. Several vaccines have been developed which show efficacy in excess of 90%; even with the improvements in the rollout of vaccinations in the U.S., given the emergence of numerous COVID-19 variants, the Company believes the need for therapeutics to treat hospitalized COVID-19 patients will continue to exist as COVID-19 becomes endemic.
The Company has been in discussion with the Medicines and Healthcare Products Regulatory Agency (“MHRA”) for the use of lenzilumab in COVID-19 patients in the United Kingdom and plans to initiate a rolling submission for Conditional Marketing Authorization (“CMA”) before the end of the second quarter of 2021. The Company also plans to submit for CMA to the European Medicines Agency (“EMA”) for the use of lenzilumab in the European Union. Further, the Company is reviewing the possibility of similar submissions for approval or compassionate use in other territories or countries worldwide.
| 7 |
As previously reported, lenzilumab has been selected to be part of the ongoing Accelerating COVID-19 Therapeutic Interventions and Vaccines (“ACTIV”)-5 and Big Effect Trial (“BET-B”), referred to as ACTIV-5/BET-B, study sponsored by the National Institutes of Health (“NIH”). ACTIV-5/BET-B is designed to determine whether certain approved therapies or investigational drugs in late-stage clinical development show promise against COVID-19 and, therefore, merit advancement into larger clinical trials. ACTIV-5/BET-B currently has 30 active U.S. sites enrolling and will evaluate lenzilumab with remdesivir, compared to placebo and remdesivir, in hospitalized COVID-19 patients, with approximately 100 patients expected to be assigned to each study arm. The Company is providing lenzilumab for the study, which is fully funded by NIH. The ACTIV-5/BET-B Data and Safety Monitoring Board (“DSMB”) met on May 7th and determined that the study should continue per protocol.
The Company intends to submit a Biologics License Application (“BLA”) to FDA in 2022, for the use of lenzilumab in hospitalized, hypoxic COVID-19 patients. Since BLAs typically require more than one study, the Company is currently evaluating the extent to which ACTIV-5/BET-B may serve as a basis for a BLA-confirmatory study for lenzilumab.
Lenzilumab has been studied in a multi-center Phase 1b trial as a sequenced therapy with Yescarta® (axicabtagene ciloleucel) to reduce CRS and neurotoxicity in patients with relapsed or refractory diffuse large B-cell lymphoma (“DLBCL”) (NCT04314843). This study was a standard 3+3 design with three patients administered 600 mg lenzilumab (“cohort 1”) and three patients administered 1,800 mg lenzilumab (“cohort 2”) just prior to CAR-T. The recommended Phase 2 dose was determined to be 1,800 mg. On April 19, 2021, the Company announced positive data from the ZUMA-19 study. At the recommended Phase 2 dose of lenzilumab, the Objective Response Rate (“ORR”) was 100% and no patient experienced severe cytokine release syndrome (“CRS”) or severe neurotoxicity (“NT”). In the six study patients, the ORR reported was 83% (n=5) which included four complete responses (“CR”). In cohort 1, there was no severe CRS (≥ grade 3). One patient experienced grade 3 NT with a two-day duration. At the recommended Phase 2 dose (cohort 2), ORR was 100% (n=3) and the toxicity-free CR (CRS and NT < grade 2) was 66% (n = 2). There was no severe CRS or severe NT at the recommended Phase 2 dose. There were no adverse events attributed to lenzilumab across the study. Inflammatory markers were correlated with reduced rates of CRS and NT. Lenzilumab dose-dependently reduced myeloid cytokines IL-6, IL-8, MCP-1, and IP-10 (CXCL-10) and systemic inflammatory markers CRP, ferritin, and SAA in the patients studied. As a result of this positive data and the conclusion of the Phase 1b portion of the study, the Company elected to terminate the clinical collaboration with Kite Pharmaceuticals, Inc., a Gilead company (“Kite”), which markets Yescarta. The Company intends to initiate a randomized, multi-center, potentially registrational, Phase 2 study to evaluate the efficacy and safety of lenzilumab combined with all commercially available CD19 CAR-T therapies in DLBCL. The study plans to enroll approximately 150 patients and the protocol is being submitted to FDA.
The Company is also in the final planning stages for a Phase 2/3 trial for lenzilumab to treat patients who have undergone allogeneic hematopoietic stem cell therapy (“HSCT”) who are at high and intermediate risk for acute GvHD (the “Risk Adapted Therapy in Acute GvHD” or the “RATinG” study). The trial is expected to be conducted by the IMPACT Partnership, a collection of 22 stem cell transplant centers located in the United Kingdom. It is anticipated the RATinG study will begin in the fourth quarter of 2021. The Company will provide lenzilumab for this study and support certain laboratory tests related to the study. The majority of the study costs will be borne by the partner.
In addition, the Company is in partnership with the South Australian Health & Medical Research Institute (“SAHMRI”) and the University of Adelaide to conduct a Phase 2 Trial studying the efficacy of lenzilumab in combination with azacitadine. The study, (“PREcision Approach to Chronic Myelomonocytic Leukemia” or “PREACH-M”) is anticipated to begin enrollment in the third quarter of 2021 and to include four sites. The Company will provide lenzilumab for this study and the majority of the study costs will be borne by the partner.
The Company’s proprietary, patented Humaneered technology platform is a method for converting existing antibodies (typically murine) into engineered, high-affinity human antibodies designed for therapeutic use, particularly with acute and chronic conditions. The Company has developed or in-licensed targets or research antibodies, typically from academic institutions, and then applied our Humaneered technology to produce them. Lenzilumab and the Company’s other two product candidates, ifabotuzumab and HGEN005, are Humaneered monoclonal antibodies. The Company’s Humaneered antibodies are closer to human antibodies than chimeric or conventionally humanized antibodies and have a high affinity for their target but low immunogenicity. The Company believes its Humaneered antibodies offer additional advantages, such as high potency, a slow off-rate and a lower likelihood to induce an inappropriate immune response or infusion related reactions.
| 8 |
See Management’s Discussion and Analysis of Financial Condition and Results of Operations in Item 2 of this Quarterly Report on Form 10-Q for additional information regarding the business.
Liquidity
The Company has been continuing to advance its efforts to develop lenzilumab for use in hospitalized COVID-19 pneumonia patients. As of March 31, 2021, the Company had cash and cash equivalents of $92.9 million, which included a $25.0 million draw under the Company’s secured term loan with Hercules Capital, Inc. (“Hercules”) or the (“Term Loan”) (see Note 5 below). Together with approximately $94.1 million of net proceeds from the underwritten public offering completed in the second quarter of 2021, potential additional draws under the Term Loan, and potential revenues from the commercial sale of lenzilumab (if an EUA is granted), the Company expects to be able to fund its planned operations and capital expenditure requirements, although it may pursue other funding options on an opportunistic basis to support its liquidity.
As discussed in further detail in Note 6 below, the Company has entered into agreements with several contract manufacturing organizations (“CMOs”) to provide manufacturing, fill/finish and packaging services for lenzilumab, and is actively working with its existing and additional CMOs on additional agreements to bolster its ability to supply lenzilumab in the event of receipt of EUA or other conditional marketing authorization (“CMA”). Most of these additional manufacturing agreements, such as those currently in place, are expected to require payment of upfront fees upon execution and further payments against performance of the manufacturing services to be provided, often over a lengthy performance period. Given the competitive environment, it is not possible for the Company to predict when it will be in position to execute any additional manufacturing agreements, or to estimate the aggregate amount of potential future payments under such new agreements or the timing in which they may be made. If an EUA or other CMA were granted, the Company expects to be able to satisfy the bulk of the cash requirements associated with its manufacturing commitments from revenues from the commercial sale of lenzilumab, supplemented as necessary with proceeds from the sale of its equity securities; potential additional draws under the Term Loan; upfront and milestone payments from licensees; and government funding or financial support, if offered.
In the first quarter of 2021 the amounts included in Research and Development for lenzilumab COVID-19 clinical studies were $7.6 million and $51.7 million for production of lenzilumab finished good. The Company believes that its cash and cash equivalents will be sufficient to fund its planned operational requirements for at least the next 12 months. This evaluation is based on relevant conditions and events that are currently known or reasonably knowable. As a result, the Company could deplete its available capital resources sooner than it currently expects, and a delay in obtaining or failure to obtain an EUA could further constrain its cash resources. The Company has based these estimates on assumptions that may prove to be wrong, and its operating projections, including its projected net revenue following the potential receipt of an EUA for lenzilumab in COVID-19 patients, may change as a result of many factors currently unknown to it. If the Company is unable to raise additional capital when needed or on acceptable terms, it would be forced to delay, reduce or eliminate its research and development programs and commercialization efforts. Alternatively, the Company might raise funds through strategic collaborations, public or private financings or other arrangements. Such funding, if needed, may not be available on favorable terms, or at all. The financial statements included herein do not include any adjustments that might result from the outcome of these uncertainties.
Reclassifications
Certain prior year amounts in the Condensed Consolidated Financial Statements have been reclassified to conform to the current year's presentation. Such reclassifications had no effect on prior years’ Net loss or Stockholders’ equity.
Basis of Presentation
The accompanying interim unaudited Condensed Consolidated Financial Statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) for interim financial information and on a basis consistent with the annual consolidated financial statements and include all adjustments necessary for the presentation of the Company’s condensed consolidated financial position, results of operations and cash flows for the periods presented.
| 9 |
The Condensed Consolidated Financial Statements include the accounts of the Company and its wholly-owned subsidiaries. These financial statements have been prepared on a basis that assumes that the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The December 31, 2020 Condensed Consolidated Balance Sheet was derived from the audited financial statements but does not include all disclosures required by U.S. GAAP. These interim financial results are not necessarily indicative of the results to be expected for the year ending December 31, 2021, or for any other future annual or interim period. The accompanying unaudited Condensed Consolidated Financial Statements should be read in conjunction with the audited consolidated financial statements and the related notes thereto included in the Company’s 2020 Annual Report on Form 10-K.
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts and disclosures reported in the Condensed Consolidated Financial Statements and accompanying notes. Actual results could differ materially from those estimates. The Company believes judgment is involved in accounting for the determination of revenue recognition, fair value-based measurement of stock-based compensation, accruals and warrants. The Company evaluates its estimates and assumptions as facts and circumstances dictate. As future events and their effects cannot be determined with precision, actual results could differ from these estimates and assumptions, and those differences could be material to the Condensed Consolidated Financial Statements.
2. Summary of Significant Accounting Policies
The Company’s significant accounting policies are detailed in its Annual Report on Form 10-K for the year ended December 31, 2020. There have been no significant changes to the Company’s significant accounting policies during the three months ended March 31, 2021, from those previously disclosed in its 2020 Annual Report on Form 10-K.
In December 2019, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2019-12, Simplifying the Accounting for Income Taxes. ASU No. 2019-12 eliminates certain exceptions related to the approach for intra-period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. It also clarifies and simplifies other aspects of the accounting for income taxes. ASU 2019-12 is effective for fiscal years beginning after December 15, 2020, and interim periods within those fiscal years. The Company adopted No. ASU 2019-12 on January 1, 2021 which did not have any impact to the Company’s Condensed Consolidated Financial Statements.
In August 2020, the FASB issued ASU No. 2020-06, “Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity”, as part of its overall simplification initiative to reduce costs and complexity of applying accounting standards while maintaining or improving the usefulness of the information provided to users of financial statements. Among other changes, the new guidance removes from U.S. GAAP, separation models for convertible debt that require the convertible debt to be separated into a debt and equity component, unless the conversion feature is required to be bifurcated and accounted for as a derivative or the debt is issued at a substantial premium. As a result, after adopting the guidance, entities will no longer separately present such embedded conversion features in equity, and will instead account for the convertible debt wholly as debt. The new guidance also requires use of the “if-converted” method when calculating the dilutive impact of convertible debt on earnings per share, which is consistent with the Company’s current accounting treatment under the current guidance. The guidance is effective for the Company’s financial statements issued for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years, with early adoption permitted, but only at the beginning of the fiscal year. The Company early adopted the new guidance on January 1, 2021, using the modified retrospective approach. The adoption did not have any impact to the Company’s Condensed Consolidated Financial Statements.
3. Potentially Dilutive Securities
The Company’s potentially dilutive securities, which include stock options and warrants and shares of common stock issuable upon conversion of convertible debt, have been excluded from the computation of diluted net loss per common share as the effect of including those securities would be to reduce the net loss per common share and be antidilutive. Therefore, the denominator used to calculate both basic and diluted net loss per common share is the same in each period presented.
| 10 |
The following outstanding potentially dilutive securities have been excluded from the computations of diluted net loss per common share:
| As of March 31, | ||||||||
| 2021 | 2020 | |||||||
| Options to purchase common stock | 4,224,111 | 3,420,930 | ||||||
| Warrants to purchase common stock | 51,238 | 66,239 | ||||||
| Convertible debt | 255,493 | 2,295,545 | ||||||
| 4,530,842 | 5,782,714 | |||||||
4. License Revenue
On November 3, 2020, the Company entered into a License Agreement (the “South Korea Agreement”) with KPM Tech Co., Ltd. (“KPM”) and its affiliate, Telcon RF Pharmaceutical, Inc. (together with KPM, the “Licensee”). Pursuant to the South Korea Agreement, among other things, the Company granted the Licensee a license under certain patents and other intellectual property to develop and commercialize lenzilumab for treatment of COVID-19 pneumonia, in South Korea and the Philippines (the “Territory”), subject to certain reservations and limitations. The Licensee will be responsible for gaining regulatory approval for, and subsequent commercialization of, lenzilumab in the Territory.
As consideration for the license, the Licensee has agreed to pay the Company (i) an up-front license fee of $6.0 million, payable promptly following the execution of the License Agreement, which was received in the fourth quarter of 2020, (ii) up to an aggregate of $14.0 million in two payments based on achievement by the Company of two specified milestones in the U.S., of which the first milestone was met in the first quarter of 2021 and $6.0 million (or $4.5 million net of withholding taxes and other fees and royalties) was received in the second quarter of 2021, and (iii) subsequent to the receipt by the Licensee of the requisite regulatory approvals, double-digit royalties on the net sales of lenzilumab in South Korea and the Philippines. The Licensee has agreed to certain development and commercial performance obligations. It is expected that the Company will supply lenzilumab to the Licensee for a minimum of 7.5 years at a cost-plus basis from an existing or future manufacturer. The Licensee has agreed to certain minimum purchases of lenzilumab on an annual basis.
The Company assessed the South Korea Agreement in accordance with Accounting Standards Codification (“ASC”) 606 – Revenue from Contracts with Customers and ASC 808 – Collaborative Arrangements and determined that its performance obligations under the South Korea agreement include (i) the exclusive, royalty-bearing, sublicensable license to lenzilumab, (ii) the manufacturing supply services to be provided by the Company, (iii) cooperation and assistance to be provided by the Company to the Licensee with regulatory authorities in the Territory and (iv) its obligation to serve on a joint steering committee (Items (iii) and (iv) above collectively, the “Research and Development Services” or the “Services”). The Company concluded that in the initial period leading up to regulatory approval in the Territory (the “Initial Period”), the license was not distinct since it was of no benefit to Licensee without the aforementioned Services and that, as such, the license and the Services should be bundled as a single performance obligation.
The Company has concluded that the nature of its promise is to stand ready to provide Research and Development Services as needed during the Performance Period (as defined below). The Company has further concluded that for all of the increments of time during the Performance Period its promise of standing ready to provide the Services is substantially the same. While the specific tasks performed during each increment of time will vary, the nature of the overall promise to provide the Services remains the same throughout the Performance Period.
Since the provision of the license and the Services are considered a single performance obligation, the $6.0 million upfront payment (or $4.5 million net of withholding taxes and other fees and royalties) and the first milestone payment of $6.0 million (or $4.5 million net of withholding taxes and other fees and royalties) which was met in the first quarter of 2021 and received in the second quarter of 2021, are being recognized as revenue ratably over the performance period through March 2023 (the “Performance Period”), the expected period over which the Company conservatively expects the Services to be performed with approval in the Territory expected by the end of March 2023. Therefore, in the three months ended March 31, 2021, the Company recognized license revenue totaling approximately $0.5 million.
Licensee’s purchases of lenzilumab for development purposes or for commercial requirements, represent options under the agreement and revenues will therefore be recognized when control of the product is transferred to Licensee.
| 11 |
5. Long-Term Debt
Secured Term Loan Facility
On March 10, 2021, the Company executed a Loan and Security Agreement with Hercules as agent for its affiliates serving as lenders thereunder. The Term Loan provides a loan in the aggregate principal amount of $80 million. On March 29, 2021, the Company drew the initial $25.0 million tranche under the Term Loan. After giving effect to payment of fees and expenses associated with the draw, the Company received net proceeds of approximately $24.4 million.
In addition to the initial amount of $25.0 million, the Term Loan provides the Company with the potential for two additional tranches: a second tranche in the amount of $35.0 million or $25.0 million, which it may become entitled to draw through September 15, 2021 subject to the Company’s receipt of the EUA for lenzilumab for the treatment of hospitalized patients with COVID-19 pneumonia; and a third tranche of $20.0 million which the Company may become entitled to draw through June 15, 2022, at the discretion of Hercules if the Company requests additional funding in support of the Company’s strategic initiatives.
The Company will be required to repay amounts borrowed by March 1, 2025, subject to a one-year extension option that it may exercise if it has received FDA approval of a BLA for the use of lenzilumab for the treatment of hospitalized patients with COVID-19 pneumonia, and the FDA-authorized label for lenzilumab is generally consistent with that sought in the Company’s BLA filing, and the Company has paid Hercules certain fees and expenses associated with the extension.
Amounts drawn bear interest at a floating rate equal to the greater of either (i) 8.75% plus the prime rate as reported in The Wall Street Journal minus 3.25%, or (ii) 8.75% (such greater amount, the “Base Interest Rate”). Subject to there not having occurred any default or event of default under the loan agreement, the Base Interest Rate will be reduced by 25 basis points upon the occurrence of each of the first three of the four following events to occur:
| · | if the Company achieves the protocol-specified primary efficacy endpoint for the pivotal Phase 3 study of lenzilumab for COVID-19, (clinicaltrials.gov identifier NCT04351152), and receives EUA for the use of lenzilumab for the treatment of hospitalized patients with COVID-19 pneumonia; |
| · | if the Company achieves product revenue from lenzilumab that is invoiced and/or recognized as revenue (as determined in accordance with GAAP) solely from the sale of lenzilumab (“Net Lenzilumab Product Revenue”) of at least $100.0 million; |
| · | if the Company achieves Net Lenzilumab Product Revenue of at least $250.0 million; and |
| · | if the Company achieves Net Lenzilumab Product Revenue of at least $350.0 million. |
No principal payments will be due during an interest-only period, commencing on the initial borrowing date and continuing to April 1, 2023, subject to extension to April 1, 2024, and potentially October 1, 2024, under certain conditions. Following the interest-only period, the outstanding balance of the loan will be required to be repaid monthly, continuing through the maturity date.
The Company may prepay amounts drawn under the agreement in full prior to the maturity date then in effect, subject to payment of prepayment charges equal to:
| · | 2.0% of the amounts borrowed, if the prepayment occurs on or prior to March 29, 2022; |
| · | 1.5% of the amounts borrowed, if the prepayment occurs after March 29, 2022 and before March 29, 2023; and |
| · | 1.0% of the amounts borrowed, if the prepayment occurs after March 29, 2023 and before March 29, 2024. |
In addition, on the earliest to occur of (i) the maturity date, (ii) the date the Company prepays the outstanding principal amount of the Term Loan, or (iii) the date the outstanding principal amount of the Term Loan otherwise becomes due, the Company will owe Hercules an end of term (“EOT”) charge equal to 6.75% of the aggregate amount of the Term Loan funded by Hercules.
As a condition to obtaining the term loan facility, the Company granted Hercules a security interest in substantially all of its assets and personal property not otherwise subject to existing or permitted liens. In addition, the loan agreement contains customary representations and warranties and events of default for a term loan facility of this size and type. Under the Term Loan, the Company also agreed to comply with certain customary affirmative and negative covenants that become effective upon the initial draw of the first tranche, including covenants to:
| 12 |
| · | maintain $10.0 million unrestricted cash, which amount may be increased to $20.0 million if the Company borrows the second tranche of the Term Loan; |
| · | comply with certain requirements to provide Hercules with financial information and other rights to inspect the Company’s books and records and the collateral for the Term Loan; |
| · | refrain from incurring debt that is not expressly subordinated to the Term Loan; “Rule 144A-style” convertible notes in aggregate principal amount up to $250.0 million; and other permitted indebtedness; |
| · | refrain from granting (or permitting to exist) liens on the Company’s assets and properties, other than certain permitted liens, including in respect of its patents and other intellectual property; |
| · | refrain from making certain investments, other than permitted acquisitions and certain other permitted investments; |
| · | refrain from repurchasing the Company’s stock or paying dividends, subject to limited exceptions; |
| · | refrain from transferring any material portion of its assets; and |
| · | refrain from entering into any merger or consolidation in which the Company is not the surviving entity. |
All of these covenants will not apply upon repayment of any borrowings under the Term Loan.
Certain of the baskets for permitted investments and other exceptions to the covenants described above will increase in the event the Company raises more than $100.0 million in unrestricted net cash proceeds from one or more bona fide equity financings prior to March 31, 2022. Further, the covenants in the loan agreement will not prohibit the Company from pursuing its strategy of entering into out-bound license agreements for lenzilumab that may be exclusive as to specific geographic regions outside the U.S., nor will the covenants prohibit the Company from entering into co-development or co-promotion or commercialization agreements relating to lenzilumab, so long as such agreements generally are negotiated on arm’s length and commercially reasonable terms.
While the Term Loan is outstanding, the lenders will have the right to convert a portion of the principal amount outstanding under the Term Loan (ranging from $5.0 million to $10.0 million in the aggregate) into shares of the Company’s common stock at a conversion price equal to $19.57 per share, subject to customary anti-dilution adjustments.
The following table summarizes the outstanding future payments of principal and interest associated with the Company’s Term Loan as of March 31, 2021 (in thousands):
| 2021 | $ | 1,501 | ||
| 2022 | 2,218 | |||
| 2023 | 10,800 | |||
| 2024 | 13,671 | |||
| 2025 | 5,153 | |||
| Total payments | 33,343 | |||
| Less amount representing interest | (6,655 | ) | ||
| Notes payable, gross | 26,688 | |||
| Less: Unamortized portion of EOT charge | (1,688 | ) | ||
| Less: Unamortized discount on notes payable | (213 | ) | ||
| Less: Unamortized debt issuance costs | (343 | ) | ||
| Long-term debt | 24,444 | |||
| Less current portion | - | |||
| Long-term debt, net of current portion | $ | 24,444 |
Interest expense related to the Term Loan, recorded during the three months ended March 31, 2021, was approximately $18 thousand and the effective interest rate was 9.0%.
| 13 |
6. Commitments and Contingencies
Eversana Agreement
On January 10, 2021, the Company announced that it had entered into a master services agreement (the “Eversana Agreement”) with Eversana Life Science Services, LLC (“Eversana”) pursuant to which Eversana will provide the Company multiple services from its integrated commercial platform in preparation for the potential commercialization of lenzilumab.
Under the Eversana Agreement, Eversana will serve as the Company’s end-to-end commercial partner, providing the Company with a full suite of services in connection with the potential launch of lenzilumab. Eversana services initially will comprise marketing, market access, consulting, field solutions, field operations, health economics and medical affairs. Additional services may be negotiated by the parties and set forth in statements of work delivered in accordance with the Eversana Agreement.
The Company will pay Eversana fees and reimburse it for expenses in performing the services as established in applicable statements of work. Eversana has agreed to defer its fees pending the Company’s receipt of an EUA from the FDA for lenzilumab for newly hospitalized and hypoxic COVID-19 patients. The Company has accrued $0.9 million for Eversana activities performed in the first quarter of 2021, which are included in Accrued expenses in the Condensed Consolidated Balance Sheets, with the anticipated payment upon receipt of the EUA.
The Eversana Agreement provides for a one-year term and will renew for subsequent one-year terms unless either party provides a notice of non-renewal. After the first year, the Company may terminate the Eversana Agreement upon advance written notice to Eversana. The Eversana Agreement contains customary provisions allowing either party to terminate the Eversana Agreement as a result of certain changes in law and material breaches and certain insolvency events by or relating to the other party.
The Eversana Agreement imposes customary mutual obligations on the parties to protect and not disclose the confidential information and intellectual property of the other, and contains insurance, non-solicitation, indemnification and limitation of liability provisions customary for service contracts of this type.
Manufacturing Agreements
The Company has entered into agreements with several contract manufacturing organizations (“CMOs”) to manufacture bulk drug substance (“BDS”) and fill/finish/drug product (“DP”) for our lenzilumab clinical trial activities related to COVID-19 as well as to manufacture BDS and DP for a potential launch of lenzilumab in anticipation of an EUA in 2021. The Company has also entered into agreements for packaging of the drug. These agreements represent large commitments, including upfront amounts prior to commencement of manufacturing and progress payments through the course of the manufacturing process and include payments for technology transfer. As of March 31, 2021, the Company estimates that its commitments remaining to be incurred during 2021 will amount to approximately $169 million. The agreements contain customary cancellation clauses.
7. Stockholders’ Equity
Reverse Stock Split
Effective September 11, 2020 (the “Effective Date”), the Company amended its charter to effect a reverse stock split at a ratio of 1-for-5 (the “Split Ratio”). No fractional shares were issued in connection with the reverse stock split. Stockholders of record otherwise entitled to receive fractional shares of common stock received cash (without interest or deduction) in lieu of such fractional share interests.
The reverse stock split reduced the total number of shares of the Company’s common stock outstanding as of the Effective Date from approximately 210.9 million shares to approximately 42.2 million shares. The par value per share and other terms of the Company’s common stock were not affected by the reverse stock split, and the number of authorized shares of the Company’s common stock remains at 225,000,000.
The reverse stock split resulted in a proportionate adjustment in the number of shares reserved for issuance under the 2020 Equity Plan, such that a total of 7,000,000 shares of the Company’s common stock were reserved for issuance under the 2020 Equity Plan following the Effective Date. In addition, proportionate adjustments were made to the number of shares covered by, and the exercise price applicable to, each outstanding stock option award under the 2012 Equity Plan and outstanding warrants issued by the Company, in each case to give effect to the Split Ratio and the reverse stock split.
The reverse stock split was accounted for retroactively and is reflected in our common stock, stock option and warrant activity as of and during the period ended December 31, 2020 and the periods ended March 31, 2021 and 2020. Unless stated otherwise, all share data in this Quarterly Report on Form 10-Q have been adjusted, as appropriate, to reflect the reverse stock split.
| 14 |
Controlled Equity Offering
On December 31, 2020, the Company entered into a Controlled Equity OfferingSM Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. (“Cantor”), under which the Company could issue and sell from time-to-time shares of the Company’s common stock, having an aggregate gross sales price of up to $100 million through Cantor, as sales agent. Through March 31, 2021, the Company issued and sold 1,796,858 shares of its common stock under the Sales Agreement for net proceeds of $36.1 million.
2021 Underwritten Public Offering
On March 30, 2021, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with Jefferies LLC, Credit Suisse Securities (USA) LLC and Cantor, as representatives of the several` underwriters (the “Underwriters”), in connection with the public offering of 5,000,000 shares of the Company’s common stock. Pursuant to the Underwriting Agreement, the Company granted the underwriters a 30-day option to purchase an additional 750,000 shares of common stock. See Note 11 for additional information.
8. Stock-Based Compensation
A summary of stock option activity for the three months ended March 31, 2021 under all the Company’s options plans is as follows:
| Options | Weighted Average Exercise Price | |||||||
| Outstanding at January 1, 2021 | 3,728,149 | $ | 5.57 | |||||
| Granted | 758,240 | 16.13 | ||||||
| Exercised | (250,242 | ) | (3.13 | ) | ||||
| Cancelled (forfeited) | (11,932 | ) | (3.33 | ) | ||||
| Cancelled (expired) | (104 | ) | (162.09 | ) | ||||
| Outstanding at March 31, 2021 | 4,224,111 | $ | 7.61 | |||||
The weighted average fair value of options granted during the three months ended March 31, 2021 was $13.30 per share.
The Company valued the options granted using the Black-Scholes options pricing model and the following weighted-average assumption terms for the three months ended March 31, 2021:
| Three Months Ended | ||
| March 31, 2021 | ||
| Exercise price | $16.07 - $17.79 | |
| Market value | $16.07 - $17.79 | |
| Expected term | 6 years | |
| Expected volatility | 109% | |
| Risk-free interest rate | 1.06% - 1.08% | |
| Expected dividend yield | -% |
The Company recorded stock-based compensation expense in the Condensed Consolidated Statements of Operations as follows (in thousands):
| 15 |
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| General and administrative | $ | 364 | $ | 211 | ||||
| Research and development | $ | 146 | $ | 54 | ||||
| Total stock-based compensation | $ | 510 | $ | 265 | ||||
At March 31, 2021, the Company had $12.8 million of total unrecognized stock-based compensation expense, net of estimated forfeitures, related to outstanding stock options that will be recognized over a weighted-average period of 2.7 years.
9. License and Collaboration Agreements
Kite Agreement
On May 30, 2019, the Company entered into a clinical collaboration agreement with Kite, providing in part for conduct of a multi-center Phase 1b study of lenzilumab with Kite’s Yescarta in patients with relapsed or refractory B-cell lymphoma, including DLBCL. On April 19, 2021, the Company announced positive data from this study. As a result of this positive data and the conclusion of the Phase 1b portion of the study, the Company elected to terminate the clinical collaboration agreement with Kite (see Note 11). The Company intends to initiate a randomized, multi-center, potentially registrational, Phase 2 study to evaluate the efficacy and safety of lenzilumab combined with all commercially available CD19 CAR-T therapies in DLBCL. The study plans to enroll approximately 150 patients and the protocol is being submitted to FDA.
Mayo Agreement
On June 19, 2019, the Company entered into an exclusive worldwide license agreement (the “Mayo Agreement”) with the Mayo Foundation for Medical Education and Research (“Mayo”) for certain technologies used to create CAR-T cells lacking GM-CSF expression through various gene-editing tools including CRISPR-Cas9 (GM-CSF knock-out). The license covers various patent applications and know-how developed by Mayo in collaboration with the Company. These licensed technologies complement and broaden the Company’s position in the GM-CSF neutralization space and expand the Company’s discovery platform aimed at improving CAR-T to include gene-edited CAR-T cells.
Pursuant to the Mayo Agreement, the Company paid $0.2 million to Mayo in June 2020, which payment was accrued in Research and development expense in June 2019. The Mayo Agreement also requires the payment of milestones and royalties upon the achievement of certain regulatory and commercialization milestones.
Zurich Agreement
On July 19, 2019, the Company entered into an exclusive worldwide license agreement (the “Zurich Agreement”) with the University of Zurich (“UZH”) for technology used to prevent or treat Graft versus Host Disease (“GvHD”) through GM-CSF neutralization. The Zurich Agreement covers various patent applications filed by UZH which complement and broaden the Company’s position in the application of GM-CSF and expands the Company’s development platform to include improving allogeneic Hematopoietic Stem Cell Transplantation (“HSCT”).
Pursuant to the Zurich Agreement, the Company paid $0.1 million to UZH in July 2019. The Zurich Agreement also requires the payment of annual maintenance fees and milestones and royalties upon the achievement of certain regulatory and commercialization milestones. The license payment of $0.1 million was recorded as Research and development expense in July 2019.
Clinical Trial Agreement with the National Institute of Allergy and Infectious Diseases
On July 24, 2020, the Company entered into a clinical trial agreement (the “ACTIV-5 Clinical Trial Agreement”) with the National Institute of Allergy and Infectious Diseases (“NIAID”), part of NIH, which is part of the U.S. Government Department of Health and Human Services, as represented by the Division of Microbiology and Infectious Diseases. Pursuant to the ACTIV-5 Clinical Trial Agreement, lenzilumab is being evaluated in the NIAID-sponsored ACTIV-5/BET-B in hospitalized patients with COVID-19.
| 16 |
The NIH created the ACTIV public-private partnership and selected lenzilumab to be evaluated in its ACTIV-5/BET-B, which is comparing lenzilumab in combination with Gilead’s investigational antiviral, remdesivir, versus placebo plus remdesivir in hospitalized COVID-19 patients. The trial is expected to enroll 100 patients in each arm of the study.
Pursuant to the ACTIV-5 Clinical Trial Agreement, NIAID will serve as sponsor and will be responsible for funding, supervising and overseeing ACTIV-5/BET-B. The Company will be responsible for providing lenzilumab to NIAID without charge and in quantities to ensure a sufficient supply of lenzilumab. The ACTIV-5 Clinical Trial Agreement imposes additional obligations on the Company that are reasonable and customary for clinical trial agreements of this nature, including in respect of compliance with data privacy laws and potential indemnification obligations. The Company will have access to data from ACTIV-5/BET-B once concluded.
CRADA
On November 5, 2020, the Company and the Department of Defense (“DoD”) Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (“JPEO-CBRND” or “JPEO”) entered into a Cooperative Research and Development Agreement (“CRADA”) in collaboration with the Biomedical Advanced Research and Development Authority (“BARDA”), part of the Office of the Assistant Secretary for Preparedness and Response (“ASPR”) at the U.S. Department of Health and Human Services (“HHS”), in support of Operation Warp Speed (“OWS”), to assist in the development of lenzilumab, in connection with a potential EUA for COVID-19.
On January 22, 2021, the Company announced an expansion of the CRADA that it had previously entered with JPEO on November 5, 2020, was subsequently co-signed by BARDA. This provides the Company with access to manufacturing capacity reserved by BARDA for fill-finish product to accelerate the drug product manufacturing of lenzilumab.
Pursuant to the CRADA, the Company has been provided access to a full-scale, integrated team of OWS manufacturing, and regulatory subject matter experts, leading decision makers and statistical support in anticipation of applying for EUA and subsequently a BLA for lenzilumab as a potential treatment for COVID-19. The CRADA also provides that OWS regulatory experts will work with the Company on FDA communications, meetings and regulatory filings. The CRADA aims to support the lenzilumab Phase 3 clinical trial output, focusing on efficiently generating an EUA and BLA submission. In addition to providing access under EUA, a goal of the CRADA is to ensure lenzilumab receives the benefits provided by Public Law 115-92. The Company may receive advance purchase contracts or other benefits under Public Law 115-92, if FDA issues an EUA or BLA.
10. Litigation
Savant Litigation
On February 29, 2016, the Company entered into a binding letter of intent (the “LOI”) with Savant Neglected Diseases, LLC (“Savant”). The LOI provided that the Company would acquire certain worldwide rights relating to benznidazole from Savant. On June 30, 2016, the Company and Savant entered into an Agreement for the Manufacture, Development and Commercialization of Benznidazole for Human Use (the “MDC Agreement”), pursuant to which the Company acquired certain worldwide rights relating to benznidazole. The MDC Agreement consummates the transactions contemplated by the LOI.
In addition, on June 30, 2016, the Company and Savant also entered into a Security Agreement (the “Security Agreement”), pursuant to which the Company granted Savant a continuing senior security interest in the assets and rights acquired by the Company pursuant to the MDC Agreement and certain future assets developed from those acquired assets.
On June 30, 2016, in connection with the MDC Agreement, the Company issued to Savant a five-year warrant to purchase 40,000 shares of the Company’s Common Stock, at an exercise price of $11.25 per share, subject to adjustment.
On May 26, 2017, the Company submitted its benznidazole Investigational New Drug Application (“IND”) to the FDA which became effective on June 26, 2017. The Company recorded expense of $1.0 million during the year ended December 31, 2017 as Research and development expense related to the milestone achievement associated with the IND being declared effective.
| 17 |
On July 10, 2017, FDA notified the Company that it granted Orphan Drug Designation to benznidazole for the treatment of Chagas disease. The Company recorded expense of $1.0 million during the year ended December 31, 2017 as Research and development expense related to the milestone achievement associated with Orphan Drug Designation.
The $2.0 million in milestone payments due Savant are included in Accrued expenses in the accompanying Condensed Consolidated Balance Sheets as of March 31, 2021 and December 31, 2020.
On July 10, 2017, the Company filed a complaint against Savant in the Superior Court for the State of Delaware, New Castle County (the “Delaware Court”). KaloBios Pharmaceuticals, Inc. v. Savant Neglected Diseases, LLC, No. N17C-07-068 PRW-CCLD. The Company asserted breach of contract and declaratory judgment claims against Savant arising under the MDC Agreement. The Company alleges that Savant has breached its MDC Agreement obligations to pay cost overages that exceed a budgetary threshold as well as other related MDC Agreement representations and obligations. In the litigation, the Company has alleged that as of June 30, 2017, Savant was responsible for aggregate cost overages of approximately $3.4 million, net of a $0.5 million deductible under the MDC. The Company asserts that it is entitled to offset $2.0 million in milestone payments due Savant against the cost overages.
On July 12, 2017, Savant removed the case to the Bankruptcy Court, claiming that the action is related to or arises under the Bankruptcy Case from which the Company emerged in July 2016. In re KaloBios Pharmaceuticals, Inc., No. 15-12628 (LSS) (Bankr. D. Del.). On July 27, 2017, Savant filed an Answer and Counterclaims. Savant’s filing alleges breaches of contracts under the MDC Agreement and the Security Agreement, claiming that the Company breached its obligations to pay the milestone payments and other related representations and obligations. On August 1, 2017, the Company moved to remand the case back to the Delaware Court (the “Motion to Remand”).
On August 2, 2017, Savant sent a foreclosure notice to the Company, demanding that it provide the Collateral as defined in the Security Agreement for inspection and possession on August 9, 2017, with a public sale to be held on September 1, 2017. The Company moved for a Temporary Restraining Order (the “TRO”) and Preliminary Injunction in the Bankruptcy Court on August 4, 2017. Savant responded on August 7, 2017. On August 7, 2017, the Bankruptcy Court granted the Company’s motion for a TRO, entering an order prohibiting Savant from collecting on or selling the Collateral, entering our premises, issuing any default notices to us, or attempting to exercise any other remedies under the MDC Agreement or the Security Agreement. On August 9, 2017, the parties have stipulated to continue the provisions of the TRO in full force and effect until further order of the appropriate court, which the Bankruptcy Court signed that same day (the “Stipulated Order”).
On January 22, 2018, Savant wrote to the Bankruptcy Court requesting dissolution of the TRO and the Stipulated Order. On January 29, 2018, the Bankruptcy Court granted the Motion to Remand and denied Savant’s request to dissolve the TRO and Stipulated Order, ordering that any request to dissolve the TRO and Stipulated Order be made to the Delaware Court.
On February 13, 2018 Savant made a letter request to the Delaware Court to dissolve the TRO and Stipulated Order. Also, on February 13, 2018, the Company filed its Answer and Affirmative defenses to Savant’s Counterclaims. On February 15, 2018 the Company filed a letter of opposition to Savant’s request to dissolve the TRO and Stipulated Order and requesting a status conference. A hearing on Savant’s request to dissolve the TRO and Stipulated Order was held before the Delaware Court on March 19, 2018. The Delaware Court denied Savant’s request to dissolve the TRO and Stipulated order, which remain in effect.
On April 11, 2018, the Company advised the Delaware Court that it would meet and confer with Savant regarding a proposed case management order and date for trial. On April 26, 2018 the Delaware Court so-ordered a proposed case management order submitted by the Company and Savant. The schedule in the case management order was modified by stipulation on August 24, 2018.
On April 8, 2019, the Company moved to compel Savant to produce documents in response to the Company’s document requests. The parties thereafter agreed to a discovery schedule through June 30, 2019, which the Superior Court so ordered, and the parties produced documents to each other.
| 18 |
On June 4, 2019, Savant filed a complaint against the Company and Madison Joint Venture LLC (“Madison”) in the Delaware Court of Chancery (the “Chancery Action”) seeking to “recover as damages that amounts owed to it under the MDC Agreement, and to reclaim Savant’s intellectual property,” among other things. Savant also requested leave to move to dismiss the Company’s complaint on the grounds that the Company’s transfer of assets to Madison was champertous. On June 10, 2019, the Company requested by letter that the Superior Court hold a contempt hearing because the Chancery Action violated the TRO entered by the Bankruptcy Court, the terms of which have been extended by stipulation of the parties. On June 18, 2019, the Superior Court held a telephonic status conference. The parties agreed that the Chancery Action should be consolidated with the Superior Court action, after which the Superior Court would address the parties’ motions.
On July 22, 2019, the Company moved for contempt against Savant. Savant filed its opposition on July 29, 2019. On August 12, 2019, the Superior Court denied the Company’s motion for contempt.
On July 23, 2019, Savant moved for summary judgment on the issue of champerty. The Company filed its response and cross-motion for summary judgment on August 27, 2019. Savant filed its reply on September 10, 2019 and the Company filed its cross-reply on September 20, 2019. The motion is fully briefed and was argued at a hearing on February 3, 2020. On August 17, 2020 the Superior Court issued a memorandum opinion denying Savant’s motion for summary judgment on the issue of champerty.
On July 26, 2019, the Company moved to modify the previously agreed-upon discovery schedule to extend discovery through December 31, 2019, which the Superior Court granted. In subsequent orders, the discovery schedule was further extended until the end of June 2020.
On July 30, 2019, the Company filed a motion to dismiss Savant’s Chancery Action. Savant filed an amended complaint on September 4, 2019, and the Company filed its opening brief in support of its motion to dismiss on October 11, 2019. That motion is fully briefed and was argued at a hearing on February 3, 2020. On August 17, 2020 the Superior Court issued a memorandum opinion denying the Company’s motion to dismiss Savant’s Chancery Action.
On August 19, 2019, Savant moved to dismiss the Company’s amended Superior Court complaint. On September 27, 2019, the Company filed an opposition to Savant’s motion and, in the alternative, requested leave to file a second amended complaint against Savant. Savant consented to the filing of the second amended complaint and withdrew their motion to dismiss. Savant filed a partial motion to dismiss against a co-defendant on October 30, 2019. That motion is fully briefed and was argued at a hearing on February 3, 2020. At the February 3, 2020 hearing, the Court reserved judgment on the parties’ reciprocal motions. On August 17, 2020, the Superior Court issued a memorandum opinion granting in part and denying in part Savant’s motion to dismiss the Company’s amended Superior Court complaint. The Superior Court found the Company lacked standing because it assigned its interest to its co-plaintiff Madison. However, the Superior Court found that Madison had standing to proceed. Although the Company was dismissed from all counts of the Superior Court Action it continues to possess an interest in the Superior Court Action because of its ownership of Madison and remains a litigant because of Savant’s Chancery Action. Savant’s motion to dismiss the Company’s amended Superior Court complaint was denied in all other respects.
On May 22, 2020, upon the request of the parties, the Superior Court stayed both Delaware actions until July 29, 2020.
On June 30, 2020, Savant exercised 20,000 warrants in a cashless exercise resulting in 10,909 shares being issued to Savant.
On July 24, 2020, the parties submitted a joint status report in the Delaware actions. The parties also requested a status conference with the Court to discuss moving the trial from October 2020 to some later time.
On August 20, 2020, the Court held the requested status conference and ordered that a consolidated trial for the Superior Court Action and Chancery Action would be held in April 2021. The parties subsequently agreed to a five-day trial starting on April 12, 2021.
On September 29, 2020, upon the request of the parties, the Superior Court revised the existing discovery schedule. Under the revised discovery schedule fact discovery closed on November 9, 2020 and expert discovery closed on December 18, 2020.
| 19 |
On October 7, 2020, Savant moved for leave to amend its complaint in the Chancery Action to add a claim for breach of contract related to its exercise of 20,000 warrants on June 30, 2020. Savant claims that the Company’s issuance of 10,909 shares was insufficient under the warrant. On November 23, 2020, the Superior Court granted Savant’s motion for leave to amend its complaint in the Chancery Action. The Company answered the second amended complaint in the Chancery Action on December 11, 2020.
On November 30, 2020, the Superior Court revised the discovery schedule to permit the Company to take additional discovery regarding Savant’s second amended complaint in the Chancery Action. Under this revised schedule, the Company had until December 18, 2020 to take additional discovery. Further, the deadline to complete expert discovery was moved to January 5, 2021. A consolidated trial for the Superior Court Action and Chancery Action remained scheduled for April 2021.
On December 18, 2020, fact discovery closed.
On January 5, 2021, the Company moved for leave to amend the Superior Court complaint to add Scott Freeman as a defendant. Savant opposed the motion for leave to amend on February 5, 2021. Briefing was completed on March 5, 2021. Further, the deadline for expert discovery closed.
On January 19, 2021, the Company and Savant filed competing motions for summary judgment on their respective claims in the Superior Court Action and Chancery Action. Oppositions to the motions for summary judgment were filed on February 5, 2021 and replies were filed on February 24, 2021.
On March 18, 2021, the Court held a hearing on the summary judgment motions and the Company’s motion for leave to amend. The Court reserved decision on the summary judgment motions, denied the Company’s motion for leave to amend and postponed the April 2021 trial. The Company is awaiting the Court’s availability to proceed with trial.
Private Placement Litigation
On June 15, 2020, a complaint was filed against the Company and Dr. Durrant in the Commercial Division of the Supreme Court of the State of New York. The case caption is Alliance Texas Holdings, LLC et al. v. Humanigen, Inc. et al., Index No. 652490/2020 (“Alliance Texas Holdings Case”). The plaintiffs in the Alliance Texas Holdings Case comprise a group of 17 prospective investors introduced to Humanigen by Noble Capital Markets, Inc. (“Noble”), which had been engaged by the Company as a non-exclusive placement agent in connection with a private placement of its common stock (the “Private Placement”). The plaintiffs had indicated interest in purchasing shares of common stock in the Private Placement but, due to the strength of demand for shares from other prospective investors, the plaintiffs were not allocated any investment amount. The plaintiffs allege that the Company and Dr. Durrant breached a contractual obligation to deliver shares of common stock to the plaintiffs. The plaintiffs seek to recover for losses due to alleged fraudulent misstatements and the Company’s failure to deliver shares to them and seek equitable relief in the form of specific performance. A motion to dismiss the complaint in the Alliance Texas Holdings Case has been fully briefed and is currently being considered by the court.
The Company believes that the claims made in the Alliance Texas Holdings Case are without merit, and it is prepared to defend itself vigorously.
On April 19, 2021, the Company and Noble entered into a confidential settlement agreement in respect of a separate lawsuit brought by Noble related to the Private Placement (the “Noble Case”) captioned Noble Capital Markets, Inc. v. Humanigen, Inc., Case No. 9:20-CV-81131-WPD, pursuant to which the Noble Case was dismissed with prejudice.
11. Subsequent Events
On March 30, 2021, the Company entered into the Underwriting Agreement providing for the public offering of 5,000,000 shares of the Company’s common stock. In addition, the Company granted the underwriters a 30-day option to purchase an additional 750,000 shares of its common stock. The initial sale of 5,000,000 shares closed on April 5, 2021. On May 3, 2021, the Company closed on the sale of an additional 427,017 shares of its common stock related to the exercise of the underwriters’ 30-day option.
The net proceeds from this offering, after deducting underwriting discounts and estimated offering costs, are estimated to be approximately $94.1 million. The Company expects to use the net proceeds for manufacturing and inventory costs in connection with its efforts to prepare for potential commercialization of lenzilumab in the event of receipt of an EUA or other CMA for use in COVID-19 patients, as well as for working capital and other general corporate purposes.
| 20 |
On April 19, 2021, the Company announced positive data with lenzilumab in the Phase 1b study evaluating the efficacy and safety of lenzilumab with Yescarta, the CAR-T therapy developed by Kite in patients with DLBCL. In addition, the Company announced its plans to initiate a randomized, multicenter, potentially registrational, Phase 2 study to evaluate the efficacy and safety of lenzilumab combined with all commercially available CD19 CAR-T therapies in DLBCL. In connection with its announcement of its decision to pursue the Phase 2 study, the Company also announced the termination of the clinical collaboration agreement with Kite effective on May 18, 2021, the 30th day following the Company’s notice of termination. The parties are expected to collaborate on the wind down of activities related to the Phase 1b study as the Company prepares to initiate a Company-sponsored Phase 2 study with all commercially available CD19 CAR-T therapies in DLBCL.
| 21 |
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
You should read the following discussion and analysis together with our financial statements and the notes to those statements included elsewhere in this Quarterly Report on Form 10-Q and our Annual Report on Form 10-K for the fiscal year ended December 31, 2020. This Quarterly Report on Form 10-Q contains statements that discuss future events or expectations, projections of results of operations or financial condition, trends in our business, business prospects and strategies and other “forward-looking” information. In some cases, you can identify “forward-looking statements” by words like “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “intends,” “potential” or “continue” or the negative of those words and other comparable words. These statements may relate to, among other things, our expectations regarding the scope, progress, timing, expansion, and costs of researching, developing and commercializing our product candidates; our expectations relating to regulatory pathways to emergency use or other conditional marketing authorizations and the opportunity to benefit from various regulatory incentives; expectations for our financial results, revenue, operating expenses and other financial measures in future periods; and the adequacy of our sources of liquidity to satisfy our working capital needs, capital expenditures, and other liquidity requirements. Among the factors that could cause actual results to differ materially are the factors discussed under “Risk Factors” in this Quarterly Report on Form 10-Q, and each subsequently filed Quarterly Report on Form 10-Q. Some additional factors that could cause actual results to differ include:
| · | the timing of the initiation, enrollment and completion and results of ongoing or planned clinical trials; |
| · | the evolution of scientific discovery around the coronavirus, COVID-19 and the lung and other organ and systems dysfunction resulting in some patients may indicate that cytokine release syndrome (“CRS”) or cytokine storm is caused by or results from something other than elevated granulocyte-macrophage colony-stimulating factor (“GM-CSF”) levels; |
| · | the possibility that FDA might not grant an Emergency Use Authorization (“EUA”) for lenzilumab in COVID-19 patients or, if one were granted, that its duration might be shorter than anticipated or the EUA might be conditioned to a greater degree than anticipated; |
| · | the duration and impact of the COVID-19 pandemic and the potential that the National Emergency concerning the COVID-19 pandemic declared in March 2020 and currently set to expire in July 2021 without extension, may end at any time and without advanced notice; |
| · | our ability to timely source adequate supply of bulk drug substance and bulk drug product for our development and if approved, commercial products from third-party manufacturers on which we depend; |
| · | the negative impact that may result from manufacturing delays due to displacement of scheduled production at certain of our Contract Manufacturing Organizations (“CMOs”) as mandated from Rated Orders issued under the Defense Production Act (“DPA”) or the shortage of raw materials and critical components we are experiencing and expect to continue to experience relating to Rated Orders and increased demand for production at our CMOs; |
| · | our ability to attain conditional marketing approvals for lenzilumab in COVID-19 patients in markets outside the U.S.; |
| · | if an EUA or other conditional marketing approval is granted for lenzilumab, our ability to accurately forecast and predict future revenues in the U.S. and outside the U.S. coupled with our ability to produce sufficient quantities on a timely basis to meet demand; |
| · | our ability to research, develop and commercialize our product candidates, including our ability to do so before our competitors develop and commercialize competing products or alternative therapies or vaccines that may reduce the demand for our product candidates; |
| · | our ability to initiate a study of lenzilumab with commercially available chimeric antigen receptor T-cell (“CAR-T”) therapies in diffuse large B-cell lymphoma (“DLBCL”); |
| · | our ability to execute our strategy and business plan focused on developing our proprietary monoclonal antibody portfolio and our GM-CSF knockout gene-editing CAR-T platform; |
| · | our ability to enter into partnerships with potential collaborators with development, regulatory and commercialization expertise to enable us to pursue the other initiatives in our development pipeline; |
| 22 |
| · | our ability to attain the additional financing we may need to pursue our development initiatives, manufacturing requirements and commercialize our product candidates on favorable terms or at all; |
| · | the potential, if any, for future development of any of our present or future products; |
| · | increasing levels of market acceptance of CAR-T therapies and stem cell transplants and the development of a market for lenzilumab in these therapies; |
| · | our ability to identify and develop additional uses for our products; |
| · | our ability to maintain licenses with third parties; |
| · | our ability to attain market exclusivity and/or to obtain, maintain, protect and enforce our intellectual property and to operate our business without infringing, misappropriating or otherwise violating, the intellectual property rights of others; |
| · | the outcome of pending, threatened or future litigation; |
| · | acquisitions or in-licensing or out-licensing transactions that we may pursue may fail to perform as expected; |
| · | our ability to obtain and maintain regulatory approval of our product candidates, and any related restrictions; |
| · | limitations and/or warnings in the label of an approved product candidate or one that is granted an EUA or other conditional marketing authorization (“CMA”) outside the U.S.; |
| · | changes in the regulatory landscape that may prevent us from pursuing or realizing any of the expected benefits from the various regulatory incentives, or the imposition of regulations that affect our products; and |
| · | the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing. |
These are only some of the factors that may affect the forward-looking statements contained in this Form 10-Q. For a discussion identifying additional important factors that could cause actual results to vary materially from those anticipated in the forward-looking statements, see “Risk Factors” in Item 1A of Part II below. You should review these risk factors for a more complete understanding of the risks associated with an investment in our securities. However, we operate in a competitive and rapidly changing environment and new risks and uncertainties emerge, are identified or become apparent from time-to-time. It is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this Form 10-Q. You should be aware that the forward-looking statements contained in this Form 10-Q are based on our current views and assumptions. We undertake no obligation to revise or update any forward-looking statements made in this Form 10-Q to reflect events or circumstances after the date hereof or to reflect new information or the occurrence of unanticipated events, except as required by law.
Overview
Humanigen, Inc. is a clinical stage biopharmaceutical company, developing its portfolio of anti-inflammatory immunology and immuno-oncology monoclonal antibodies. We are focusing our efforts on the development of our lead product candidate, lenzilumab™, our proprietary Humaneered® (“Humaneered”) anti-human GM-CSF monoclonal antibody. Lenzilumab is a monoclonal antibody that has been demonstrated to neutralize GM-CSF, a cytokine that we believe is of critical importance in the hyperinflammatory cascade, sometimes referred to CRS or cytokine storm, associated with COVID-19, CAR-T therapy and acute GvHD associated with bone marrow transplants. We believe the results from our Phase 3 study in COVID-19 and our Phase 1b study in CAR-T support the mechanism of action of lenzilumab.
We believe that we have built a strong intellectual property position in the area of GM-CSF neutralization through multiple approaches and mechanisms, as they pertain to COVID-19, CAR-T, GvHD and multiple other oncology/transplantation, inflammation, fibrosis and autoimmune conditions which may be driven by GM-CSF.
On March 29, 2021, we announced top-line data on the primary endpoint and one secondary endpoint from a Phase 3, multi-center, double-blind, placebo-controlled potential registrational trial of lenzilumab (the “LIVE-AIR” study) as a potential therapeutic for newly hospitalized, hypoxic patients with COVID-19 pneumonia. Additional data from the trial, subsequently published on MedRxiv, a non-peer reviewed journal, on May 5, 2021, support the previously reported primary endpoint, which showed that patients who received lenzilumab and other treatments, including steroids and/or remdesivir, had a 54% greater relative likelihood of survival without ventilation, (“SWOV”), sometimes referred to as “ventilator-free survival” compared with patients receiving placebo and other treatments. Further information about the data generated in the trial and our subsequent meeting with the FDA is presented below under “—Recent Developments.”
| 23 |
Lenzilumab is part of the Accelerating COVID-19 Therapeutic Interventions and Vaccines (“ACTIV”)-5 and Big Effect Trial (“BET-B”), referred to as ACTIV-5/BET-B. This study is sponsored by the National Institutes of Health (“NIH”). ACTIV-5/BET-B is designed to determine whether certain approved therapies or investigational drugs in late-stage clinical development show promise against COVID-19 and, therefore, merit advancement into larger clinical trials. ACTIV-5/BET-B currently has 27 active U.S. sites enrolling and is evaluating lenzilumab in combination with remdesivir, compared to placebo and remdesivir, in hospitalized COVID-19 patients, with approximately 100 patients expected to be assigned to each study arm. We are providing lenzilumab for the study, which is fully funded by NIH. The ACTIV-5/BET-B Data and Safety Monitoring Board (“DSMB”) met on May 7th and determined that the study should proceed per protocol.
Lenzilumab has also been studied in a multi-center Phase 1b trial as a sequenced therapy with Yescarta® (axicabtagene ciloleucel) to reduce CRS and neurotoxicity in patients with relapsed or refractory DLBCL (NCT04314843), for which we recently announced positive results. Further information about the data generated in the trial is presented below under “—Recent Developments.” This trial was conducted in partnership with Kite Pharmaceuticals, Inc., a Gilead company (“Kite”), which markets Yescarta. As a result of this positive data and the conclusion of the Phase 1b portion of the study, we elected to terminate the clinical collaboration with Kite. We intend to initiate a randomized, multi-center, potentially registrational, Phase 2 study to evaluate the efficacy and safety of lenzilumab combined with all commercially available CD19 CAR-T therapies in DLBCL. The study plans to enroll approximately 150 patients and the protocol is being submitted to FDA.
We are also in the final planning stages for a Phase 2/3 trial for lenzilumab to treat patients who have undergone allogeneic hematopoietic stem cell therapy (“HSCT”) who are at high and intermediate risk for acute GvHD known as the RATinG study. The trial is expected to be conducted by the IMPACT Partnership, a collection of 22 stem cell transplant centers located in the United Kingdom, and to begin enrollment in the fourth quarter of 2021. We will provide lenzilumab for this study and support certain laboratory tests related to the study. The majority of the study costs will be borne by the partner.
In addition, we are in partnership with SAHMRI and the University of Adelaide to conduct a Phase 2 Trial studying the efficacy of lenzilumab in combination with azacitadine. The study, PREACH-M, is anticipated to begin enrollment in the third quarter of 2021 and to include four sites. We will provide lenzilumab for this study and the majority of the study costs will be borne by the partner.
Our proprietary, patented Humaneered technology platform is a method for converting existing antibodies (typically murine) into engineered, high-affinity human antibodies designed for therapeutic use, particularly with acute and chronic conditions. We have developed or in-licensed targets or research antibodies, typically from academic institutions, and then applied our Humaneered technology to produce them. Lenzilumab and our other two product candidates, ifabotuzumab and HGEN005, are Humaneered monoclonal antibodies. Our Humaneered antibodies are closer to human antibodies than chimeric or conventionally humanized antibodies and have a high affinity for their target but low immunogenicity. We believe our Humaneered antibodies offer additional advantages, such as high potency, a slow off-rate and a lower likelihood to induce an inappropriate immune response or infusion related reactions.
Our Pipeline
In addition to our ongoing work with lenzilumab described above, our pipeline also includes two other monoclonal antibodies, ifabotuzumab and HGEN005.
Ifabotuzumab
Ifabotuzumab, a Humaneered monoclonal antibody, formerly referred to as KB004, is a non-fucosylated IgG1κ antibody targeting the EphA3 receptor. EphA3 is a tumor-restricted antigen expressed in the tumor vasculature and tumor stroma of various solid tumors including breast, colon, lung, prostate, melanoma, and glioblastoma multiforme (“GBM”), a highly aggressive type of cancer that begins within the brain. On April 10, 2021, we announced positive results from a Phase 1 study of ifabotuzumab in GBM at the American Association for Cancer Research (“AACR”) 2021 Annual Meeting, as described below. We believe that ifabotuzumab, as part of an antibody drug conjugate (“ADC”), has the potential for treating solid tumors, hematologic malignancies and serious pulmonary conditions.
| 24 |
HGEN005
HGEN005 is a Humaneered monoclonal antibody which targets the EMR1, and in which the antibody carbohydrate chains lack fucose, thereby enhancing the targeted cell-killing activity of the antibody. A major limitation of current eosinophil-targeted therapies is incomplete depletion of tissue eosinophils and/or lack of cell selectivity. In preclinical work, HGEN005’s anti-EMR1 activity resulted in dramatically enhanced NK killing of eosinophils from normal and eosinophilic donors and also induced a rapid and sustained depletion of eosinophils in a non-human primate model without any clinically significant adverse events. In 2020, we engaged with NIH to discuss expanding the initial work they have conducted utilizing HGEN005. Given the COVID-19 pandemic the development of HGEN005 and discussions with a leading center in the U.S. to perform the IND- enabling work are on hold.
Except for the potential of lenzilumab for COVID-19 under an EUA or other CMA outside the U.S., our product candidates are in the clinical stage of development and will require substantial time, resources, research and development, and regulatory approval prior to commercialization. Furthermore, it may be years before any of our products are approved for use, if at all. Our current pipeline is depicted below:
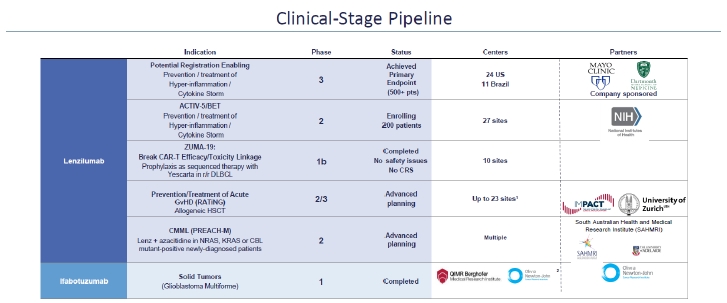
1 UK
2 Australia
Recent Developments
On March 29, 2021, we announced top-line data on the primary endpoint and one secondary endpoint from the LIVE-AIR study. Additional data from the trial, subsequently published on MedRxiv, a non-peer reviewed journal, on May 5, 2021, support the previously reported primary endpoint that showed lenzilumab improved the likelihood of SWOV, sometimes referred to as ”ventilator-free survival”, by 54% in the modified intent-to-treat (“mITT”) population (Hazard Ratio, (“HR”): 1.54; 95%CI: 1.02-2.31, p=0.041) ). New analysis included in the publication showed that lenzilumab improved the likelihood of SWOV aby 90% in the intent-to-treat (“ITT”) population (HR: 1.90; 1.02-3.52, nominal p=0.043) compared to placebo. SWOV also relatively improved by 92% in subjects who received both corticosteroids and remdesivir (1.92; 1.20-3.07, nominal p=0.0067); by 2.96-fold in subjects with C-reactive protein (CRP) levels <150 mg/L and age <85 years (2.96; 1.63–5.37, nominal p=0.0003); and by 88% in subjects hospitalized ≤2 days prior to randomization (1.88; 1.13-3.12, nominal p=0.015). Survival was improved by 2.17-fold in subjects with CRP<150 mg/L and age <85 years (2.17; 1.04-4.54, nominal p=0.040). We believe the data published on MedRxiv, generated from the LIVE-AIR study, support the conclusion that lenzilumab significantly improved SWOV in hospitalized, hypoxic subjects with COVID-19 pneumonia over and above treatment with remdesivir and/or corticosteroids. Subjects with CRP<150 mg/L and age <85 years demonstrated an improvement in survival and appeared to derive the greatest benefit from lenzilumab.
| 25 |
We have shared the top-line data on just the primary endpoint and one secondary endpoint (the only data then available while the remaining analysis was pending) with the FDA in a Pre-EUA Type B meeting in mid-April 2021. We are preparing to submit an EUA application at the end of May 2021. As requested by the FDA, the EUA application will include secondary endpoints and supplemental data analysis from LIVE-AIR, including those referenced in the MedRxiv publication, as well as additional stability and compatibility information required for the CMC section of the EUA application. There can be no assurance that the data published on MedRxiv will be sufficient for an EUA or that the FDA will not require additional information in order to grant an EUA. If the EUA is granted, we could begin to commercialize lenzilumab for the treatment of newly hospitalized COVID-19 pneumonia patients.
We have also been in discussion with the Medicines and Healthcare Products Regulatory Agency (“MHRA”) for the use of lenzilumab in COVID-19 patients in the United Kingdom and we plan to initiate a rolling submission for CMA before the end of the second quarter of 2021. We also plan to submit for CMA to the European Medicines Agency (“EMA”) for the use of lenzilumab in the European Union. Further, we are reviewing the possibility of similar submissions for approval or compassionate use in other territories or countries worldwide.
We intend to submit a Biologics License Application (“BLA”) to FDA in 2022, for the use of lenzilumab in hospitalized, hypoxic COVID-19 patients. Since BLAs typically require more than one study, we are currently evaluating the extent to which ACTIV-5/BET-B may serve as a basis for a BLA-confirmatory study for lenzilumab.
On March 30, 2021, we entered into an underwriting agreement (the “Underwriting Agreement”) with Jefferies LLC, Credit Suisse Securities (USA) LLC and Cantor, as representatives of the several underwriters, in connection with the public offering of 5,000,000 shares of our common stock. In addition, we granted the underwriters a 30-day option to purchase an additional 750,000 shares of our common stock. The initial offering closed on April 5, 2021. On May 3, 2021, we closed on the sale of an additional 427,017 shares of our common stock related to the exercise of the underwriters’ 30-day option. The aggregate gross proceeds from the sale of the 5,427,017 shares in the offering, inclusive of the additional shares purchased by the underwriters, were approximately $100.4 million. The net proceeds from this offering, after deducting underwriting discounts and estimated offering costs, will be approximately $94.1 million.
On April 10, 2021, we presented top-line results from the Phase 1 safety and bioimaging trial of ifabotuzumab in patients with GBM at the AACR 2021 Annual Meeting. The Phase 1 study primarily sought to determine the safety and recommended Phase 2 dose of ifabotuzumab in patients with GBM, the most frequent and lethal primary brain neoplasm, with 5-year survival rates of 10%. It is estimated there are more than 18,000 deaths from brain cancer annually in the U.S. The results of the study show in all patients for both doses, that ifabotuzumab demonstrates highly sensitive, specific, and reproducible targeting of the tumor and tumor microenvironment. There were no dose-limiting toxicities observed and all adverse events were readily manageable. Additional studies are being planned to evaluate ifabotuzumab as an antibody-drug conjugate in patients with solid tumors, such as lung, colon, breast, prostate, melanoma and pancreatic cancer.
On April 19, 2021, we announced positive data from the ZUMA-19 study. At the recommended Phase 2 dose of lenzilumab, the Objective Response Rate (“ORR”) was 100% and no patient experienced severe cytokine release syndrome (“CRS”) or severe neurotoxicity (“NT”). This study was a standard 3+3 design with three patients administered 600 mg lenzilumab (“cohort 1”) and three patients administered 1,800 mg lenzilumab (“cohort 2”) just prior to CAR-T. The recommended Phase 2 dose was determined to be 1,800 mg. In the six study patients, the ORR reported was 83% (n=5) which included four complete responses (“CR”). In cohort 1, there was no severe CRS (≥ grade 3). One patient experienced grade 3 NT with a two-day duration. At the recommended Phase 2 dose (cohort 2), ORR was 100% (n=3) and the toxicity-free CR (CRS and NT < grade 2) was 66% (n = 2). There was no severe CRS or severe NT at the recommended Phase 2 dose. There were no adverse events attributed to lenzilumab across the study. Inflammatory markers were correlated with reduced rates of CRS and NT. Lenzilumab dose-dependently reduced myeloid cytokines IL-6, IL-8, MCP-1, and IP-10 (CXCL-10) and systemic inflammatory markers CRP, ferritin, and SAA in the patients studied. As a result of this positive data and the conclusion of the Phase 1b portion of the study, we elected to terminate the clinical collaboration with Kite. We intend to initiate a randomized, multi-center, potentially registrational, Phase 2 study to evaluate the efficacy and safety of lenzilumab combined with all commercially available CD19 CAR-T therapies in DLBCL. The study plans to enroll approximately 150 patients and the protocol is being submitted to FDA.
| 26 |
Critical Accounting Policies and Use of Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our Condensed Consolidated Financial Statements, which have been prepared in accordance with accounting principles generally accepted in the U.S., or GAAP. The preparation of our financial statements in conformity with GAAP requires our management to make estimates and assumptions that affect the amounts and disclosures reported in the financial statements and accompanying notes. Actual results could differ materially from those estimates. Our management believes judgment is involved in determining revenue recognition, the fair value-based measurement of stock-based compensation, and accruals. Our management evaluates estimates and assumptions as facts and circumstances dictate. As future events and their effects cannot be determined with precision, actual results could differ from these estimates and assumptions, and those differences could be material to the Condensed Consolidated Financial Statements. If our assumptions change, we may need to revise our estimates, or take other corrective actions, either of which may also have a material adverse effect on our statements of operations, liquidity and financial condition.
There were no significant and material changes in our critical accounting policies and use of estimates during the three months ended March 31, 2021, as compared to those disclosed in “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Use of Estimates” in our 2020 Annual Report on Form 10-K, filed with the SEC on March 10, 2021.
Results of Operations
At March 31, 2021, we had an accumulated deficit of $440.0 million primarily as a result of research and development and general and administrative expenses. While we may in the future generate revenue from a variety of sources, including license fees, milestone payments, and research and development payments in connection with strategic partnerships, our product candidates may never be successfully developed or commercialized and we may therefore never realize revenue from any product sales, particularly because most of our product candidates are at an early stage of development. Accordingly, we expect to continue to incur substantial losses from operations for the foreseeable future, and there can be no assurance that we will ever generate significant revenue or profits.
Comparison of Three Months Ended March 31, 2021 and 2020
The following table summarizes the results of our operations for the periods indicated (amounts in thousands, except percentages):
| Three Months Ended March 31, | Increase/ (Decrease) | |||||||||||||||
| (in thousands) | 2021 | 2020 | Amount | % | ||||||||||||
| Revenue: | ||||||||||||||||
| License revenue | $ | 486 | $ | - | $ | 486 | 100 | |||||||||
| Total revenue | 486 | - | 486 | |||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 59,934 | 659 | 59,275 | 8,995 | ||||||||||||
| General and administrative | 4,948 | 1,398 | 3,550 | 254 | ||||||||||||
| Total operating expenses | 64,882 | 2,057 | 62,825 | 3,054 | ||||||||||||
| Loss from operations | (64,396 | ) | (2,057 | ) | 62,339 | 3,031 | ||||||||||
| Other expense: | ||||||||||||||||
| Interest expense | (19 | ) | (410 | ) | (391 | ) | (95 | ) | ||||||||
| Other expense | (1,152 | ) | - | 1,152 | 100 | |||||||||||
| Net loss | $ | (65,567 | ) | $ | (2,467 | ) | $ | 63,100 | 2,558 | |||||||
| 27 |
Revenue
Revenue represents license revenue under the license agreement (the “South Korea Agreement”) with KPM Tech Co., Ltd. (“KPM”) and its affiliate, Telcon RF Pharmaceutical, Inc. (together with KPM, the “Licensee”).
Research and Development Expenses
Conducting research and development is central to our business model. We expense both internal and external research and development costs as incurred. We track external research and development costs incurred by project for each of our clinical programs. Our external research and development costs consist primarily of:
| · | expenses incurred under agreements with contract research organizations, investigative sites, and consultants that conduct our clinical trials and our pre-clinical activities; |
| · | the cost of acquiring and manufacturing clinical trial, pre-commercial and other materials, the cost to transfer the manufacturing process for bulk drug substance and fill/finish production, development of and periodic performance of a variety of tests and assays for stability, release, comparability and product characterization, costs associated with quality management, the preparation of documents and information necessary to file with regulatory authorities; and |
| · | other costs associated with development activities, including additional studies. |
Other research and development costs consist primarily of internal research and development costs such as salaries and related fringe benefit costs for our employees, stock-based compensation charges, and travel costs not allocated to one of our clinical programs. Internal research and development costs generally benefit multiple projects and are not separately tracked per project.
Research and development expenses increased by $59.2 million from $0.7 million for the three months ended March 31, 2020 to $59.9 million for the three months ended March 31, 2021. The increase is primarily due to a $7.5 million increase in clinical trial expenses and a $51.4 million increase in lenzilumab manufacturing costs related to the trial in COVID-19, which began in May 2020. We expect our development costs will continue to increase throughout 2021 as a result of securing additional manufacturing capacity, the production of lenzilumab for commercial use and preparation of the materials necessary to file for an EUA in the U.S. and for conditional approval in the UK and Europe. In addition, we anticipate clinical trial costs to increase due to the enrollment of patients in the planned clinical trials.
The following table shows our total research and development expenses for the three months ended March 31, 2021 and 2020:
| Three Months Ended March 31, | ||||||||
| (in thousands) | 2021 | 2020 | ||||||
| External Costs | ||||||||
| Lenzilumab | $ | 59,332 | $ | 483 | ||||
| Ifabotuzumab | 25 | 25 | ||||||
| Internal costs | 577 | 151 | ||||||
| Total research and development | $ | 59,934 | $ | 659 | ||||
General and Administrative Expenses
General and administrative expenses consist principally of personnel-related costs (including stock-based compensation), professional fees for legal and patent expenses, consulting, audit and tax services, public, governmental and investor relations costs, and other general operating expenses not otherwise included in research and development.
General and administrative expenses increased by $3.5 million from $1.4 million for the three months ended March 31, 2020 to $4.9 million for the three months ended March 31, 2021. The increase for the three months ended March 31, 2021, is primarily due to increases in consulting and other professional services fees of $1.8 million, personnel-related expenses of $1.0 million attributable to five new hires since the first quarter of 2020, including our Chief Operating Officer and Chief Financial Officer, and our Chief Commercial Officer, public and investor relations expenses of $0.5 million, and business insurance-related expenses of $0.2 million, all in support of our increased operating activities to support the trial for COVID-19 and prepare for potential commercialization of lenzilumab. We expect our overall general and administrative costs to increase in 2021 as we continue to scale our support operations in connection with additional clinical development activities, and increase our commercial operations spending in preparation for potential commercialization under an EUA or other CMA outside the U.S.
| 28 |
Interest Expense
Interest expense decreased $0.4 million to $19 thousand for the three months ended March 31, 2021, reflecting the payoff of substantially all of our debt with proceeds from the private placement of our common stock in June 2020. On March 10, 2021, we executed a Loan and Security Agreement with Hercules Capital, Inc., (“Hercules”), as agent for its affiliates serving as lenders thereunder. The agreement provides a loan in the aggregate principal amount of $80 million (the “Term Loan”). On March 29, 2021, the Company drew the initial $25.0 million under the Term Loan. After giving effect to payment of fees and expenses associated with the draw, the Company received net proceeds of approximately $24.4 million. See Note 5 to the Condensed Consolidated Financial Statements included in Item 1 of this Quarterly Report on Form 10-Q for additional information on the Term Loan.
Other Expense
Other expense increased by $1.2 million for the three months ended March 31, 2021, primarily due to litigation settlement costs.
Liquidity and Capital Resources
Since our inception, we have financed our operations primarily through proceeds from the public offerings of our common stock, private placements of our common and preferred stock, debt financings, interest income earned on cash, and cash equivalents, and marketable securities, and borrowings against lines of credit, and more recently, with the proceeds under the South Korea Agreement. Specifically, under the South Korea Agreement, we received a $6.0 million upfront payment (or $4.5 million, net of withholding taxes and other fees and royalties) in the fourth quarter of 2020 and the first milestone payment of $6.0 million (or $4.5 million, net of withholding taxes and other fees and royalties) which was met in the first quarter of 2021 and received in the second quarter of 2021. At March 31, 2021, we had cash and cash equivalents of $92.9 million. In the second quarter of 2021, we sold an aggregate of 5,427,017 shares of our common stock in connection with an underwritten public offering, raising net proceeds of approximately $94.1 million after deducting underwriting discounts and estimated offering costs.
Primary Sources of and Uses of Cash
The following table sets forth the primary sources and uses of cash and cash equivalents for each of the periods presented below:
| Three Months Ended March 31, | ||||||||
| (In thousands) | 2021 | 2020 | ||||||
| Net cash (used in) provided by: | ||||||||
| Operating activities | $ | (35,824 | ) | $ | (607 | ) | ||
| Financing activities | 60,979 | 532 | ||||||
| Net increase (decrease) in cash and cash equivalents | $ | 25,155 | $ | (75 | ) | |||
Net cash used in operating activities was $35.8 million and $0.6 million for the three months ended March 31, 2021 and 2020, respectively. Cash used in operating activities of $35.8 million for the three months ended March 31, 2021, primarily related to our net loss of $65.6 million, adjusted for non-cash items, such as $0.5 million in stock-based compensation, and a net increase in operating assets and liabilities of $29.2 million.
Cash used in operating activities of $0.6 million for the three months ended March 31, 2020 primarily related to our net loss of $2.5 million, adjusted for non-cash items, such as $0.3 million in stock-based compensation, and a net increase in operating assets and liabilities of $1.6 million.
Net cash provided by financing activities was $61.0 million for the three months ended March 31, 2021 and consists primarily of $36.1 million received from the issuance of common stock in connection with the Controlled Equity OfferingSM Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. (“Cantor”), $24.4 million in net proceeds received from the Term Loan, and $0.4 million received from the exercise of stock options.
| 29 |
Net cash provided by financing activities was $0.5 million for the three months ended March 31, 2020 and consisted primarily of $0.4 million received from the March 2020 issuance of two convertible redeemable promissory notes which we subsequently paid off in June 2020, and $0.1 million received from the issuance of common stock under an equity line of credit, which we terminated in June 2020.
Recent Financings
Controlled Equity Offering
On December 31, 2020, the Company entered into the Sales Agreement (the “Sales Agreement”) with Cantor, under which the Company could issue and sell shares of the Company’s common stock, having an aggregate gross sales price of up to $100 million through Cantor, as sales agent. During the period from January 1, 2021 through March 31, 2021, the Company issued and sold 1,796,858 shares of its common stock under the Sales Agreement, raising net proceeds of $36.1 million.
2021 Underwritten Public Offering
On March 30, 2021, we entered into the Underwriting Agreement in connection with the public offering of 5,000,000 shares of our common stock. In addition, we granted the underwriters a 30-day option to purchase an additional 750,000 shares of our common stock. The initial offering closed on April 5, 2021. On May 3, 2021, closed on the sale of an additional 427,017 shares of our common stock related to the exercise of the underwriters’ 30-day option.
The aggregate gross proceeds from the sale of the 5,427,017 shares in the offering, inclusive of the additional shares purchased by the underwriters, were approximately $100.4 million. The net proceeds from this offering, after deducting underwriting discounts and estimated offering costs, will be approximately $94.1 million. We expect to use the net proceeds from the offering for manufacturing and inventory costs in connection with our efforts to prepare for commercialization of lenzilumab in the event of receipt of an EUA for use in COVID-19 patients, as well as for working capital and other general corporate purposes.
Term Loan with Hercules
On March 10, 2021, we entered into the Term Loan with Hercules which provided us with the ability to draw an initial amount of $25.0 million, which we drew on March 29, 2021, and with up to an additional $55.0 million available for future draws subject to achievement of future milestones and satisfaction of other conditions. See Note 5 to the Condensed Consolidated Financial Statements included in Item 1 of this Quarterly Report on Form 10-Q for additional information on the Term Loan.
Liquidity
We continue to advance our efforts in support of the development of lenzilumab as a therapy for hospitalized COVID-19 pneumonia patients. As of March 31, 2021, we had cash and cash equivalents of $92.9 million, which included a $25 million draw under the Term Loan. Together with approximately $94.1 million of net proceeds from the underwritten public offering completed in the second quarter of 2021, potential additional draws under the Term Loan, and potential revenues from the commercial sale of lenzilumab (if an EUA or other CMA is granted), we expect to be able to fund our planned operations and capital expenditure requirements in the near-term, although we may pursue other funding options on an opportunistic basis to support our liquidity.
In the long-term, we expect we will need to obtain additional financing to fund our future operations, including for the development, manufacturing, distribution and potential commercialization of lenzilumab for patients with COVID-19 pneumonia and other indications and our other product candidates. We may need to obtain additional financing to conduct additional trials for the approval of our product candidates if requested by regulatory authorities in the U.S. and other countries, and to complete the development of any additional product candidates we own or might acquire. Moreover, our fixed expenses such as salaries, committed payments to our contract manufacturers, and other contractual commitments, including those for our other research and clinical collaborations, are substantial and are expected to increase in the future. Our need to raise funds will depend on a number of factors, including our ability to establish additional relationships for the manufacture of lenzilumab and our ability to commence commercialization and begin generating revenues from product sales if lenzilumab were to receive any applicable authorization or approval.
| 30 |
Until we can generate a sufficient amount of revenue, we may finance future cash needs through public or private equity offerings, license agreements, grant financing and support from governmental agencies, convertible debt, borrowings under our Term Loan with Hercules and other debt financings, collaborations, strategic alliances and marketing, supply, distribution, or licensing arrangements. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available, we may be required to delay or reduce the scope of or eliminate one or more of our research or development programs, our commercialization efforts or our manufacturing commitments and capacity. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time. In addition, if we raise additional funds through collaborations, strategic alliances or marketing, supply, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to us.
Capital Commitments and Capital Resources
We intend to submit an application for EUA for lenzilumab at the end of May 2021. If the EUA is granted, we could begin to commercialize lenzilumab, subject to the terms and conditions of such EUA, for the treatment of newly hospitalized COVID-19 pneumonia patients. We also plan to submit for Conditional Marketing Authorization in the UK and in the European Union.
To support our application for EUA and the potential launch of lenzilumab under an EUA, we have entered into agreements with several organizations for clinical trial services, contract manufacturing services and commercialization services, as more fully described in the Company’s Annual Report on Form 10-K for the year ended December 31, 2020 under “Item 1. Business—Manufacturing and Raw Materials.” We expect to be able to fund the cash commitments related to these agreements from our cash and cash equivalents of $92.9 million as of March 31, 2021, together with approximately $94.1 million of net proceeds from the underwritten public offering completed in the second quarter of 2021, potential additional draws under our Term Loan, and revenues from the commercial sale of lenzilumab (if an EUA is granted), although we may pursue other funding options on an opportunistic basis to support our liquidity.
As discussed in further detail in Note 6 to the Condensed Consolidated Financial Statements included in Item 1 of this Quarterly Report on Form 10-Q, we have entered into agreements with several CMOs to provide manufacturing, fill/finish and packaging services for lenzilumab, and we are actively working with our existing and additional CMOs on additional agreements to bolster our ability to supply lenzilumab in the event of receipt of EUA or other CMA. Most of these additional manufacturing agreements, such as those currently in place, are expected to require payment of upfront fees upon execution and further payments against performance of the manufacturing services to be provided, often over a lengthy performance period. Given the competitive environment, it is not possible for us to predict when we will be in position to execute any additional manufacturing agreements, or to estimate the aggregate amount of potential future payments under such new agreements or the timing in which they may be made. If an EUA or other CMA were granted, we expect to be able to satisfy the bulk of the cash requirements associated with our manufacturing commitments from revenues from the commercial sale of lenzilumab, supplemented as necessary with proceeds from the sale of our equity securities; the incurrence of debt; upfront and milestone payments from licensees; and government funding or financial support, if offered.
While our clinical manufacturing agreements specify maximum payment terms, they also generally contain standard terms that provide us the right to cancel pending orders prior to the initiation of manufacturing and, if the manufacturer is able to fill the manufacturing slots with other products, to do so with limited further payment obligations to the manufacturers. Accordingly, in the event we do not receive an EUA or other CMA, we would seek to terminate these agreements and related commitments. Given our sense of the demand for biologic manufacturing, sterile fill/finish capacity and the materials and components required, in the event of cancellation of production orders, we believe, the spending under these agreements would be limited.
See “Contracts” below for additional information regarding our agreements with Eversana and our contract manufacturers.
| 31 |
Contracts
Eversana Agreement
On January 10, 2021, we announced that we had entered into a master services agreement (the “Eversana Agreement”) with Eversana Life Science Services, LLC (“Eversana”) pursuant to which Eversana will serve as our end-to-end commercial partner, providing us with a full suite of services in connection with the potential launch of lenzilumab.
We will pay Eversana fees and reimburse it for expenses in performing the services as established in applicable statements of work. Eversana has agreed to defer its fees pending our receipt of an EUA from the FDA for lenzilumab for hospitalized and hypoxic COVID-19 patients. We accrued $0.9 million for Eversana activities performed in the first quarter of 2021, which is included in Accrued expenses in the Condensed Consolidated Balance Sheets, with the anticipated payment upon receipt of the EUA.
Manufacturing Agreements
We have entered into agreements with several CMOs to manufacture bulk drug substance (“BDS”) and fill/finish/drug product (“DP”) for our lenzilumab clinical trial activities in COVID-19 as well as to manufacture BDS and DP for a potential launch of lenzilumab in anticipation of an EUA in 2021. We have also entered into agreements for packaging of the drug. These agreements represent large commitments, including upfront amounts prior to commencement of manufacturing and progress payments through the course of the manufacturing process and include payments for technology transfer. As of March 31, 2021, we estimate that our commitments remaining to be incurred during 2021 will amount to approximately $169 million. The agreements contain customary cancellation clauses.
Off-Balance Sheet Arrangements
We currently have no off-balance sheet arrangements, such as structured finance, special purpose entities or variable interest entities.
| Item 3. | Quantitative and Qualitative Disclosures About Market Risk |
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and are not required to provide the information specified under this item.
| Item 4. | Controls and Procedures |
We maintain disclosure controls and procedures (as defined in Exchange Act Rule 13a–15(e) and 15d-15(e)) that are designed to ensure that information required to be disclosed in our reports under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the rules and regulations thereunder, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Evaluation of disclosure controls and procedures. As required by Rule 13a-15(b) under the Exchange Act, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this Quarterly Report on Form 10-Q. Based on the foregoing, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in internal control over financial reporting. There have been no changes in our internal control over financial reporting during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| 32 |
| Item 1. | Legal Proceedings. |
Please see Note 10 to the Condensed Consolidated Financial Statements included in Item 1 of this Quarterly Report on Form 10-Q for a summary of legal proceedings and developments during the quarter ended March 31, 2021.
| Item 1A. | Risk Factors. |
Risk Factor Summary
The following summary highlights the material risks that may affect our business, operating results, financial condition and prospects, as more fully described in the pages that follow this summary.
| · | If our competitors receive FDA approval for treatments or vaccines for COVID-19, our commercial opportunity may be reduced or eliminated, if such treatments directly compete for the same segment of patients that lenzilumab targets or indirectly compete for the same patients by preventing hospitalization. |
| · | The scientific rationale behind the hypothesis that GM-CSF is a cause, or the main cause, of the cytokine storm that leads to adverse results in COVID-19 patients is still being tested and may not prove accurate. |
| · | We face risks related to the development, manufacturing and distribution of lenzilumab as a treatment for COVID-19, which has not been granted an EUA or approved by FDA. We cannot provide any assurance that lenzilumab will receive an EUA or be approved by FDA. |
| · | Manufacturing problems at our third-party manufacturers or their requirements to displace our reserved manufacturing slots to comply with government orders could cause inventory shortages and delay or impair our ability to obtain an EUA or BLA or other regulatory approval or delay shipments of lenzilumab for commercial use, which may adversely affect our results of operations. |
| · | We may not be able to obtain materials or supplies necessary to conduct clinical trials or, following requisite regulatory authorizations or approvals, to manufacture and sell our products, which could limit our ability to generate revenues. |
| · | If an EUA or other conditional marketing approval is granted for lenzilumab, we may be unable to accurately predict demand for lenzilumab or to produce sufficient quantities on a timely basis to meet demand. |
| · | There can be no assurance that lenzilumab, even if approved, would ever become profitable, due to government or healthcare provider or payer interest and public perception regarding vaccines and treatments for COVID-19 related complications. Competing products that are subsequently developed also may diminish demand for lenzilumab or impair its profitability. |
| · | The regulatory pathway for lenzilumab is highly dynamic and continues to evolve and may result in unexpected or unforeseen challenges. It may also be affected by the duration and the impact of the COVID-19 pandemic. |
| · | We currently have no internal sales and marketing capabilities and will rely on partners such as Eversana and other third parties to market and sell lenzilumab if we attain an EUA or other regulatory approval for its commercialization, and any product candidates we may successfully develop. We or they may not be able to effectively market and sell any such product candidates. |
| · | We may experience significant volatility in the market price of our common stock following announcements and releases regarding our ongoing development of lenzilumab as a potential COVID-19 therapy. |
| · | We have a history of operating losses and we may never become profitable. |
| · | We will need to obtain additional financing to fund our operations and, if we are unable to obtain such financing, we may be unable to complete the development and commercialization of our product candidates. |
| · | Our business depends on the success of our current product candidates. We cannot be certain that we will be able to obtain Emergency Authorization, Conditional Marketing Authorization or regulatory approval for, or successfully commercialize, any of our product candidates. |
| · | The adoption of CAR-T therapies as the potential standard of care for treatment of certain cancers is uncertain and, and dependent on the efforts of a limited number of market entrants, and if not adopted as anticipated, the market for lenzilumab or next-generation gene-edited CAR-T therapies may be limited or not develop. |
| 33 |
| · | Our business could target benefits from various regulatory incentives, such as EUA, orphan drug exclusivity, breakthrough therapy designation, fast track designation, and priority review, but we may not ultimately qualify for or benefit from these arrangements. |
| · | We may experience delays in commencing or conducting our clinical trials, in receiving data from third parties or in the continuation or completion of clinical testing, which could result in increased costs to us, delay our ability to generate product candidate revenue or, ultimately, render us unable to complete the development and commercialization of our product candidates. |
| · | We face risks associated with clinical operations abroad and we may not be able to receive conditional marketing approvals for lenzilumab in COVID-19 patients in markets outside the U.S. |
| · | If our competitors develop similar or comparable treatments for the target indications of our product candidates that are approved more quickly, marketed more successfully or are demonstrated to be safer or more effective than our product candidates, or if FDA approves generic or biosimilar competitors to our products post-approval, our commercial opportunity will be reduced or eliminated. |
| · | We may encounter difficulties in managing our growth and expanding our operations successfully. |
| · | Even if we are able to obtain regulatory approval for our product candidates, we will continue to be subject to ongoing and extensive regulatory requirements, and our failure to comply with these requirements could substantially harm our business. |
| · | Currently pending, threatened or future litigation or governmental proceedings or inquiries could result in material adverse consequences, including judgments or settlements. |
| · | We may not be able to protect our intellectual property rights in the U.S. and throughout the world. |
| · | Our stock price is volatile and purchasers of our common stock could incur substantial losses. |
| · | Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities. |
In addition to the other information set forth in this Quarterly Report on Form 10-Q and other filings we have made and will make in the future with the SEC, you should carefully consider the following risk factors and uncertainties, which could materially affect our business, financial condition or results of operations in future periods. Additional risks not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition or results of operations in future periods.
Risks Related to Our Business and Industry
Risks Related to COVID-19 and Lenzilumab
The fluid and unpredictable nature of the COVID-19 pandemic, which began in late 2019 and has spread worldwide, may affect our ability to conduct the clinical trial with NIH, the IMPACT Partnership and SAHMRI and the University of Adelaide, delay the initiation of planned and future clinical trials, disrupt regulatory activities, or have other adverse effects on our business and operations. In addition, this pandemic has caused substantial volatility in the financial markets and may adversely impact economies worldwide, both of which could result in adverse effects on our business and operations.
In December 2019, an outbreak of the respiratory illness COVID-19 caused by a strain of novel coronavirus, SARS-Cov-2, was first reported in China. The COVID-19 outbreak has spread worldwide, causing many governments to implement measures to slow the spread of the outbreak through quarantines, strict travel restrictions, heightened border scrutiny, and other measures. COVID-19 infections are widespread and as of April 30, 2021, in the U.S., there were over 32 million cases, over 2.1 million hospitalizations and over 570 thousand deaths. Several vaccines have been developed which show efficacy in excess of 90%, however the rollout of inoculations and the emergence of numerous COVID-19 variants lead us to believe the need for therapeutics to treat COVID-19 currently exists and will continue to exist for the next several years. The outbreak and government measures taken in response have had a significant impact, both direct and indirect, on businesses and commerce, as worker shortages have occurred; supply chains have been disrupted; facilities and production have been suspended; and demand for certain goods and services, such as medical services and supplies, has spiked, while demand for other goods and services, such as travel, has fallen. The future progression of the outbreak and its effects on our business and operations are uncertain.
We and our third-party CRO clinical sites and contract manufacturing organizations (“CMOs”) may experience disruptions in supply of product candidates and/or procuring items that are essential for our research, development and manufacturing activities, including raw materials and components used in the manufacturing of our product candidates, medical and laboratory supplies used in our clinical trials or preclinical studies, in each case, for which there may be shortages because of ongoing efforts to address the outbreak. These delays have impacted our overall manufacturing supply chain operations to date, and we continue to explore back up or alternative sources of supply, any future disruption in the supply chain from the COVID-19 outbreak, or any continued outbreak could have a material adverse impact on our clinical trial plans and business operations.
| 34 |
Additionally, we will seek to enroll patients in several planned studies. Patients in our clinical trials are at sites located in many areas affected by COVID-19 and, as a result, our trials may be impacted. In addition, even if sites are actively recruiting, we may face difficulties recruiting or retaining patients in our ongoing and planned clinical trials if patients are affected by the virus or are fearful of visiting or traveling to our clinical trial sites because of the outbreak. Prolonged delays or closure to enrollment in our trials or patient discontinuations could have a material adverse impact on our clinical trial plans and timelines.
In addition, our ability to collect all data requested of patients enrolled in our clinical trials during this pandemic is being impacted to varying degrees by COVID-19. Clinical trial data collection generally continues for each of our clinical trials, but at a slower pace, and in some instances, we encounter disruption of collection of complete study data. This could have a material adverse impact on our data analysis.
The response to the COVID-19 pandemic may redirect resources with respect to regulatory and intellectual property matters in a way that would adversely impact our ability to progress regulatory approvals and protect our intellectual property. In addition, we may face impediments to regulatory meetings and approvals due to measures intended to limit in-person interactions.
Any negative impact that the COVID-19 pandemic has on the ability of our suppliers to provide materials for our product candidates or on recruiting or retaining patients in our clinical trials or our ability to collect patient data could cause delays to clinical trial activities including collection of the final results from LIVE-AIR study, which could adversely affect our ability to obtain regulatory approval for and to commercialize our product candidates, increase our operating expenses, and have a material adverse effect on our financial results. Furthermore, any negative impact that the outbreak has on the ability of our CROs to deliver data sets and execute on experimentation has caused and could cause substantial delays for our discovery activities and materially impact our ability to fuel our pipeline with new product candidates.
Any negative impact that the COVID-19 pandemic has on recruiting or retaining patients in our clinical trials, obtaining complete clinical trial data or on the ability of our suppliers to provide materials for our product candidates could cause additional delays to clinical trial and developmental activities, which could materially and adversely affect our ability to obtain regulatory approval for and to commercialize our product candidates, increase our operating expenses, affect our ability to raise additional capital, and have a material adverse effect on our financial results.
The COVID-19 pandemic has caused significant volatility in the financial markets, and may continue to cause such disruptions, which could impact our ability to raise additional funds through public offerings and may also impact the volatility of our stock price and trading in our stock. Moreover, the pandemic has significantly impacted economies worldwide, which could result in adverse effects on our business and operations. We cannot be certain what the overall impact of the COVID-19 pandemic will be on our business. It has the potential to adversely affect our business, financial condition, results of operations, and prospects.
If our competitors develop and receive FDA approval for treatments or vaccines for COVID-19, our commercial opportunity may be reduced or eliminated, if such treatments directly compete for the same segment of patients that lenzilumab targets or indirectly compete for the same patients by preventing hospitalization.
On October 22, 2020, FDA approved remdesivir for use in adult and certain pediatric patients for the treatment of COVID-19 requiring hospitalization. Baricitinib has also been approved for use with remdesivir for the treatment of COVID-19. Baricitinib may not be used with corticosteroids. Several neutralizing antibodies have been approved to prevent progression in the early stages of infection. Three vaccines have been authorized by the FDA with efficacy rates ranging from less than 50% in certain populations in excess of 90%. There are numerous other companies working on therapies to treat COVID-19 and/or vaccines to prevent or treat COVID-19, including oral antiviral compounds. If additional competing therapies are approved, such approval could have a material adverse impact on our ability to commercialize lenzilumab as a therapy for COVID-19. The speed at which all parties are acting to create and test many treatments and vaccines for COVID-19 is unusual and evolving or changing plans or priorities within the FDA and other branches of the U.S. government involved in the fight against the pandemic, including changes based on new knowledge of COVID-19 and new COVID-19 variants This can all impact how the disease affects the human body and may significantly affect the regulatory timeline for lenzilumab. In addition, there are numerous clinical trial programs in progress for COVID-19 therapies which are all competing for largely the same patient population for enrollment. This problem is exacerbated by changing rates of infection and spread, which make it challenging to ensure there are sufficient centers and patients to be enrolled in additional studies. Results from clinical testing may raise new questions and require us to redesign proposed clinical trials. Many of our competitors and potential competitors have substantially greater scientific, research, and product development capabilities, as well as greater financial, marketing, sales and human resources capabilities than we do. In addition, many specialized biotechnology firms have formed collaborations with large, established companies to support the research, development and commercialization of products that may be competitive with ours.
| 35 |
The scientific rationale behind the hypothesis that GM-CSF is a cause, or the main cause, of the cytokine storm that leads to adverse results in COVID-19 patients is still being tested and may not prove accurate.
The hypothesis that elevated GM-CSF levels may contribute to cytokine storm-induced immune mechanisms that places patients at greater risk of ICU admission and mortality with the current pandemic strain of coronavirus is unproven. However, a paper in Science Immunology attributed GM-CSF to be a key factor in COVID-19. The data for this paper were drawn from the largest study conducted to date and the study was supported financially by the UK government. While supportive of our hypothesis, additional clinical data will be needed to confirm the role of GM-CSF in patients with COVID-19. Certain additional data on the role of GM-CSF are also the subject of publications as well as pre-publication papers that have not been peer-reviewed and may not be substantiated. If this hypothesis is not ultimately proven through clinical trials which are underway, the potential for lenzilumab to play a meaningful role in a COVID-19 therapy likely would decrease or be eliminated. We cannot assure you that our exploratory efforts in this respect will be fruitful.
We face risks related to the development, manufacturing and distribution of lenzilumab as a treatment for COVID-19, which has not been granted an EUA or approved by FDA. We cannot provide any assurance that lenzilumab will receive an EUA or be approved by FDA.
While we achieved favorable results in our Phase 3 multi-center, randomized, placebo-controlled, double-blinded, clinical trial of lenzilumab as a potential treatment for COVID-19 , there is no assurance of favorable results from future clinical trials, including the NIH sponsored ACTIV-5/BET-B or any other studies we may initiate.
Based on a meeting, the FDA has requested that the EUA application for lenzilumab include secondary endpoints and supplemental data analysis from the first arm of the Phase 3 study (also known as LIVE-AIR), including those referenced in the MedRxiv publication, as well as additional stability and compatibility information required for the CMC section of the EUA application. There can be no assurance that the data published on MedRxiv will be sufficient for an EUA or that the FDA will not require additional information in order to grant an EUA. It is also possible that FDA and other regulatory authorities may not grant an EUA or subsequently approve lenzilumab for the treatment of COVID-19, or that any such EUA or approval, if granted, may have significant limitations on its use. Widespread uptake of a safe, effective, scalable and affordable vaccine may have a negative impact on the demand for lenzilumab over the long term, even if an EUA or approval were issued, although substantial numbers of patients may continue to be hospitalized until there is widespread vaccination/herd immunity, notwithstanding the impact of current and future variants. As a result, we may never successfully commercialize lenzilumab in COVID-19 or realize a return on our significant investment in the development, supply, and commercialization of lenzilumab for this purpose.
Furthermore, even if an EUA were granted to permit lenzilumab to be commercialized for use in the treatment of COVID-19, the authorization is only in effect while it is determined that a “Public Health Emergency” is still underway and might be expressly conditioned or limited by FDA. An EUA does not take the place of the formal BLA submission, review and approval process. We intend to submit a BLA to FDA in 2022, for the use of lenzilumab in hospitalized, hypoxic COVID-19 patients. Since BLAs typically require more than one study, we are currently evaluating the extent to which ACTIV-5/BET-B may serve as a basis for a BLA-confirmatory study for lenzilumab. There can be no assurance that the ACTIV-5/BET-B will be sufficient for a BLA or that the FDA will not require additional information in order to grant a BLA. Furthermore, there is no assurance of favorable results from the ongoing ACTIV-5/BET-B clinical trial, or that this trial will be completed in anticipated timelines or at all. It is also possible that FDA and other regulatory authorities may not approve lenzilumab for the treatment of COVID-19, or that any marketing approvals, if granted, may have significant limitations on its use. Further, we may make a strategic decision to discontinue development of lenzilumab in this indication if other parties are successful in developing a more effective treatment for COVID-19. As a result, we may never successfully commercialize lenzilumab for use in COVID-19 patients.
| 36 |
Manufacturing problems at our third-party manufacturers or their requirements to displace our reserved manufacturing slots to comply with government orders could cause inventory shortages and delay or impair our ability to obtain an EUA or BLA or other regulatory approval or delay shipments of lenzilumab for commercial use, which may adversely affect our results of operations.
We believe that our ability to obtain an EUA and, ultimately, a BLA to permit lenzilumab to be used commercially for patients with COVID-19 depends at least in part on our ability to demonstrate to FDA that we will be able to scale the manufacturing to produce a sufficient quantity of dosages to address the potential demand for the product. We have contracted and expect to continue to contract with third-party manufacturers to produce lenzilumab. We depend on these third parties to perform the manufacturing of lenzilumab effectively, timely, and in compliance with Good Manufacturing Practices (“GMP”), which are extensive regulations governing manufacturing processes, stability testing, record-keeping and quality standards as defined by FDA. Similar regulations are in effect in other jurisdictions.
Our complete reliance on third-party manufacturers to produce lenzilumab subjects us to additional risks, including the possible breach of the manufacturing agreement by the third party, the possible cancellation, delay or modification of contracted manufacturing slots by the third party, or the termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. In addition, in an effort to increase the availability of needed medical products, vaccinations or related therapies in response to the COVID-19 pandemic, governments have required and may continue require our third-party manufacturers or our suppliers to allocate manufacturing capacity (for example pursuant to the U.S Defense Production Act, “DPA”) in a way that adversely affects our ability to obtain regulatory approval for and to commercialize lenzilumab . During the latter half of 2020, and continuing into 2021, it has become more challenging and expensive to obtain these slots due to increased demand for production at our CMOs, many of which also manufacture vaccines and other therapeutics. Even after being reserved and paid for, the manufacturing slots for which we contract are subject to risk of displacement as mandated from Rated Orders issued under the DPA. This displacement risk has caused us to expend significant efforts to manage our supply chain and add additional CMOs. Despite those efforts, we have experienced and expect to continue to experience shortages of raw materials and critical components we necessary for our manufacturing processes. Accordingly, the inability to manage our manufacturing capacity could delay our ability to gain approval and ultimately generate revenues from sales of lenzilumab.
Our third-party manufacturers are independent entities subject to their own unique operational and financial risks that are out of our control. If we or any of these third-party manufacturers fail to perform as required, this could cause delays in our clinical trials and applications for regulatory approval. Further, we may have to pay the costs of manufacturing any batch that fails to pass quality inspection or meet regulatory approval. In addition, we, our third-party manufacturers may only be able to produce some of our products at one or a limited number of facilities and, therefore, we have limited manufacturing capacity for certain products, and we may not be able to locate additional or replacement facilities on a reasonable basis or at all. Our sales of such products could also be adversely impacted by our reliance on such limited number of facilities. To the extent these risks materialize and affect their performance obligations to us, our financial results may be adversely affected.
In addition, the process of manufacturing biologics is extremely susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, or vendor or operator error. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral, foreign substances or other contaminations are discovered in our products or in the manufacturing facilities in which our products are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. Lenzilumab produced in these facilities may be quarantined or recalled and not available for clinical or commercial use. We have encountered and may continue to encounter these difficulties, our ability to manufacture and sell any products that may be authorized or approved for commercial use could be impaired, which could have an adverse effect on our business.
We may not be able to obtain materials or supplies necessary to conduct clinical trials or, following requisite regulatory authorizations or approvals, to manufacture and sell our products, which could limit our ability to generate revenues.
We need access to certain supplies and products to conduct our clinical trials and, if an EUA or BLA or other approval were to be received, to manufacture and sell our products. If we are unable to purchase sufficient quantities of these materials or find suitable alternative materials in a timely manner, our development efforts for our product candidates may be delayed or our ability to manufacture our products could be limited, which could limit our ability to generate revenues.
| 37 |
Suppliers of key components and materials must be named in the EUA, BLA or other marketing authorization application filed with the regulatory authority for any product candidate for which we are seeking marketing approval, and significant delays can occur if the qualification of a new supplier is required. Even after a manufacturer is qualified by the regulatory authority, the manufacturer must continue to expend time, money and effort in the area of production and quality control to ensure full compliance with GMP. Manufacturers are subject to regular periodic inspections by regulatory authorities following initial approval. If, as a result of these inspections, a regulatory authority determines that the equipment, facilities, laboratories or processes do not comply with applicable regulations and conditions of product approval, the regulatory authority may suspend the manufacturing operations. If the manufacturing operations of any of the single suppliers for our products are suspended, we may be unable to generate sufficient quantities of commercial or clinical supplies of product to meet market demand, which could in turn decrease our revenues and harm our business. In addition, if deliveries of materials from our suppliers were interrupted for any reason, we may be unable to supply our product candidates in development for clinical trials or ship them to customers, if authorized or approved for commercial use. In addition, some of our products and the materials that we utilize in our operations are manufactured at only one facility or from a single vendor, which we may not be able to replace in a timely manner and on commercially reasonable terms, or at all. Problems with any of the single suppliers we depend on, including in the event of a natural disaster, power or equipment failure or other difficulty, may negatively impact our development and commercialization efforts.
A significant portion of the raw materials and intermediates used to manufacture our product candidates are supplied by third-party manufacturers and corporate partners outside of the U.S. As a result, any political or economic factors in a specific country or region, including any changes in or interpretations of trade regulations, compliance requirements or tax legislation, that would limit or prevent third parties outside of the U.S. from supplying these materials could adversely affect our ability conduct our pending or contemplated clinical trials.
If we were to encounter any of these difficulties, our ability to conduct clinical trials on product candidates and to manufacture and sell any products that may be authorized or approved for commercial use could be impaired, which could have an adverse effect on our business.
Interim, topline or preliminary data from our clinical trials that we announce or publish from time-to-time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time-to-time, we may publicly disclose preliminary, “topline” or other data from our clinical trials, which is based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a full analysis of all data related to the particular trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, such results that we report may differ from future results of the same trials, or different conclusions or considerations may qualify such results once additional data have been received and fully evaluated. Such data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim, topline, or preliminary data should be viewed with caution until the final data are available and such data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Adverse differences between such data and final data could significantly harm our business prospects. Further, disclosure of interim, topline or preliminary data by us or by our competitors could result in volatility in the price of shares of our common stock.
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our business in general. In addition, the information we choose to publicly disclose or to publish via a preprint publication regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure, and any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular drug, product candidate or our business. If the top-line data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for and commercialize in our current or any our future product candidate, our business, operating results, prospects or financial condition may be harmed.
| 38 |
If an EUA or other conditional marketing approval is granted for lenzilumab, we may be unable to accurately predict demand for lenzilumab or to produce sufficient quantities on a timely basis to meet demand.
We may be unable to accurately predict demand for lenzilumab, or other approved or unapproved products for COVID-19, as demand depends on a number of factors, including rollout and effectiveness of approved vaccines, competing drug products and other treatments that may be developed for COVID-19 and other illnesses and conditions and federal and state social measures design to slow the spread of COVID-19. If lenzilumab is commercialized, we may be unsuccessful in estimating user demand and may not be effective in matching inventory levels and locations to actual end user demand, in particular if the COVID-19 pandemic, including emerging variants, is effectively contained or the risk of patients being hospitalized as a result of coronavirus infection is significantly diminished. In addition, adverse changes in economic conditions, increased competition, including through approved vaccines or other therapeutic, or other factors may cause hospitals or other users or distributors of lenzilumab to reduce inventories of our products, which would reduce their orders for lenzilumab, even if end user demand has not changed. As a result, even if lenzilumab receives an EUA or BLA approval for use in COVID-19 patients, our revenues may be difficult to predict and vulnerable to extreme fluctuations.
There can be no assurance that lenzilumab, even if approved, would ever become profitable, due to government or healthcare provider or payer interest and public perception regarding vaccines and treatments for COVID-19 related complications.
As a result of the emergency situations in many countries, there is a heightened risk that a COVID-19 therapy or other treatments for COVID-19 pneumonia and symptoms may be subject to adverse governmental actions in certain countries, including intellectual property expropriation, compulsory licenses, strict price controls or other actions. Additionally, we may need to, or we may be required by governmental or non-governmental authorities to, set aside specific quantities of doses of lenzilumab for designated purposes or geographic areas. We may face challenges related to the allocation of supply of lenzilumab, particularly with respect to geographic distribution. Thus, even if lenzilumab is approved, such governmental actions may limit our ability to recoup our current and future expenses incurred to develop and commercialize lenzilumab.
Furthermore, public sentiment regarding commercialization of a COVID-19 therapy or other treatment may limit or negate our ability to generate revenues from sales of lenzilumab. Given that COVID-19 has been designated as a National Emergency and represents an urgent public health crisis, we are likely to face significant public attention and scrutiny over any future business models and pricing decisions with respect to lenzilumab. If we are unable to successfully manage these risks, we could face significant reputational harm, which could negatively affect the price of our common stock.
The regulatory pathway for lenzilumab is highly dynamic and continues to evolve and may result in unexpected or unforeseen challenges. It may also be affected by the duration and the impact of the COVID-19 pandemic.
To date, lenzilumab has moved rapidly through the regulatory review process of FDA. The speed at which all parties are acting to create and test many therapeutics and vaccines for COVID-19 is unusual and evolving or changing plans or priorities within FDA and foreign regulatory authorities, including changes based on new knowledge of COVID-19 and how the disease affects the human body, may significantly affect the regulatory timeline for lenzilumab. Results from clinical testing may raise new questions and require us to redesign proposed clinical trials, including revising proposed endpoints or adding new clinical trial sites or cohorts of subjects.
Although we intend to design any future clinical trials for lenzilumab in accordance with FDA and other applicable regulatory guidance, we cannot be certain that, as the regulatory pathway continues to evolve, we will be able to complete a clinical trial in accordance with FDA’s guidance and regulations then in effect. A failure to complete a clinical trial in accordance with guidance and regulations then in effect could impair our ability to obtain approval for lenzilumab, which may adversely affect our operating results, reputation and ability to raise capital and enter into or maintain collaborations to advance lenzilumab.
Additionally, FDA has the authority to grant an EUA to allow unapproved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when there are no adequate, approved, and available alternatives. If we are granted an EUA for lenzilumab, we would be able to commercialize lenzilumab prior to FDA approval. However, because EUA authorization is only in effect while it is determined that a Public Health Emergency is underway, FDA may revoke an EUA where it is determined that the underlying Public Health Emergency no longer exists or warrants such authorization. Widespread vaccination programs and potential “herd immunity” may result in the public perception that the medical emergency posed by COVID-19 has diminished to the point of no longer necessitating EUA for therapies for COVID-19. Accordingly, even if granted, we cannot predict how long, if ever, an EUA would remain in place. Such revocation could adversely impact our business in a variety of ways, including if lenzilumab is not yet approved by FDA and if we and our manufacturing partners have invested in the supply chain to provide lenzilumab under an EUA.
| 39 |
Even if EUA or regulatory approval is received for lenzilumab, the later discovery of previously unknown problems associated with the use of lenzilumab may result in restrictions, including withdrawal of the product from the market, and lead to significant liabilities and reputational damage.
Because the path to marketing approval of any vaccine for COVID-19, or drug used to treat COVID-19 pneumonia or other symptoms is unclear, lenzilumab may be widely used and in circulation in the U.S. or another country prior to our receipt of marketing approval. Unexpected safety issues, including any that we have not yet observed in our Phase 3 clinical trial for lenzilumab, could lead to patient harm and significant reputational damage to us and our platforms going forward and other issues, including delays in our other programs, the need for re-design of our clinical trial and the need for significant additional financial resources.
We also may be restricted or prohibited from marketing or manufacturing lenzilumab, even after obtaining product approval, if previously unknown problems with lenzilumab or its manufacture are subsequently discovered. We cannot provide assurance that newly discovered or developed safety issues will not arise following regulatory approval. With the use of any drug product or treatment by a wide patient population, serious adverse events may occur from time-to-time that did not arise in the clinical trials of the product or that initially appeared to be unrelated to the vaccine itself and only with the collection of subsequent information were found to be causally related to the product. Any such safety issues could cause us to suspend or cease marketing of our approved products, possibly subject us to substantial liabilities, and adversely affect our ability to generate revenue and our financial condition.
We currently have no internal sales and marketing capabilities and will rely on partners such as Eversana and other third parties to market and sell lenzilumab if we attain an EUA or other regulatory approval for its commercialization, and any product candidates we may successfully develop. We or they may not be able to effectively market and sell any such product candidates.
While we have entered into several agreements with commercial partners who will provide sales and marketing support as well as logistic and distribution services, we currently do not have the internal sales and marketing infrastructure in place that would be necessary to sell and market products. As is the case with many, if not most, small companies seeking to commercialize their products and who have not yet partnered with a larger biotech or pharmaceutical company, we have engaged a contract commercial organization, Eversana, to serve as our end-to-end commercial partner in marketing and selling lenzilumab, if authorized and/or approved, and have entered into other arrangements with distribution partners as described in our 2020 Annual Report on Form 10-K under “Item 1. Business—Sales and Marketing.” There can be no assurance that our partners will be effective in marketing and selling lenzilumab.
If we or Eversana fail to hire, train, retain and manage qualified sales personnel, market our product successfully or on a cost-effective basis or otherwise terminate our agreement, our ability to generate revenue will be limited and we will need to identify and retain an alternative third-party, or develop our own sales and marketing capability. The establishment of an in-house sales and marketing operation can be expensive and time consuming and could delay any product candidate launch.
We may experience significant volatility in the market price of our common stock following announcements and releases regarding our ongoing development of lenzilumab as a potential COVID-19 therapy.
Biopharmaceutical companies that are developing potential therapeutics and vaccines to combat COVID-19, including our company, have experienced significant volatility in the price of their securities upon publication of preclinical and clinical data as well as news about their development programs. Since our initial announcement of our plans to develop lenzilumab as a therapy for COVID-19 patients, our common stock has experienced wide variations in daily trading volume and price movements. The volatility in the price per share is further exacerbated by the large percentage of retail shareholders. We expect that over the coming months we may make public several additional data, and clinical and regulatory updates with respect to lenzilumab and our commercialization efforts for lenzilumab. We cannot predict public reaction or the impact on the market price of our common stock once further announcements regarding lenzilumab or developments from our on-going clinical trial are announced. Given the attention being paid to the COVID-19 pandemic and the public scrutiny of COVID-19 development announcements and data releases to date, we expect that any public announcements we make in the coming months regarding the ongoing development and commercialization of lenzilumab will attract significant attention and scrutiny and that, as a result, the price of our common stock may be particularly volatile during this time.
| 40 |
Other Risks Related to Our Business and Industry
We have a history of operating losses, we expect to continue to incur losses, and we may never become profitable.
We have incurred net losses in nearly every year since our inception. For the three months ended March 31, 2021, we incurred a net loss of $65.6 million, and we have an accumulated deficit of $440.0 million as of March 31, 2021. Since inception, we have recognized a nominal amount of revenue from payments for license or collaboration fees, $0.5 million of which was recognized in the first quarter of 2021. We expect to make substantial expenditures and incur additional operating losses in the future to further develop and commercialize our product candidates. Our accumulated deficit is expected to increase significantly as we continue our development and clinical trial efforts. Our ability to achieve and sustain profitability depends on obtaining regulatory approvals for and successfully commercializing our product candidates, either alone or with third parties. We do not currently have the required approvals to market any of our product candidates and we may never receive them. We may not be profitable even if we or any future development partners succeed in commercializing any of our product candidates. Because of the numerous risks and uncertainties associated with developing and commercializing our product candidates, we are unable to predict the extent of any future losses or when we will become profitable, if at all.
Our ability to execute on all of the initiatives in our development pipeline is substantially dependent on third parties to plan and conduct the referenced studies and clinical trials.
Our success depends on our ability to negotiate agreements with third parties with resources to plan and conduct the initiatives, studies and clinical trials we are pursuing. If we are not able to reach agreements with current or future partners for it, we will not be able to execute on each of these particular initiatives, studies and trials. Our inability to identify and complete negotiations with any such third party therefore could have a material and adverse impact on our ability to pursue our business plan in respect of the applicable element of our pipeline, which in turn could have a material adverse effect on our business.
We will need to obtain additional financing to fund our operations and, if we are unable to obtain such financing, we may be unable to complete the development and commercialization of our product candidates.
We believe that our cash and cash equivalents will be sufficient to fund our planned operations and capital expenditure requirements for at least the next 12 months. This evaluation is based on relevant conditions and events that are currently known or reasonably knowable. As a result, we could deplete our available capital resources sooner than we currently expect, and a delay in obtaining or failure to obtain an EUA could further constrain our cash resources. We have based these estimates on assumptions that may prove to be wrong, and our operating projections, including our projected net revenue following the potential receipt of an EUA for lenzilumab in COVID-19 patients, may change as a result of many factors currently unknown to us.
We may need to obtain additional financing to fund our future operations, including for clinical development, manufacturing, distribution and commercialization of lenzilumab for patients with COVID-19 and other indications including CAR-T, GvHD and CMML and our other product candidates. We may need to obtain additional financing to conduct additional trials for the approval of our product candidates if requested by regulatory authorities in the U.S. and other countries, and to complete the development of any additional product candidates we own or might acquire. Moreover, our fixed expenses such as salaries, committed payments to our contract manufacturers, and other contractual commitments, are substantial and are expected to increase in the future. Our need to raise funds will depend on a number of factors, including our ability to establish additional relationships for the manufacture of lenzilumab and our ability to commence commercialization and begin generating revenues from product sales if lenzilumab were to be issued an EUA and eventually approved under a BLA.
Until we can generate a sufficient amount of revenue, we may finance future cash needs through public or private equity offerings, license agreements, grant financing and support from governmental agencies, convertible debt, other debt financings including the Term Loan from Hercules, collaborations, strategic alliances and marketing, supply, distribution or licensing arrangements. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available, we may be required to delay or reduce the scope of or eliminate one or more of our research or development programs, our commercialization efforts or our manufacturing commitments and capacity. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time. In addition, if we raise additional funds through collaborations, strategic alliances or marketing, supply, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to us.
| 41 |
Our business depends on the success of our current product candidates. We cannot be certain that we will be able to obtain regulatory approval for, or successfully commercialize, any of our product candidates.
We have a limited pipeline of product candidates and we do not plan to conduct active research at this time for discovery of new molecules or antibodies. We depend on the successful continued development and regulatory approval of our current product candidates for our future business success. Since the fall of 2017, our primary focus has been the clinical development of lenzilumab. We are also working to create next-generation gene-edited CAR-T therapies using GM-CSF gene knockout technologies, as well as working to develop ifabotuzumab and related products. We will need to successfully enroll and complete clinical trials of lenzilumab and ifabotuzumab, and potentially obtain regulatory approval to market these products. The future clinical, regulatory and commercial success of our product candidates is subject to a number of risks, including the following:
| · | we may not be able to enroll adequate numbers of eligible patients in the clinical trials we propose to conduct, whether alone or through collaborations, including the collaboration with NIH, the IMPACT Partnership, SAHMRI and the University of Adelaide and other partnerships; |
| · | we may not have sufficient financial and other resources to fund our clinical trials or collaborations; |
| · | we may not be able to provide acceptable evidence of safety and efficacy for our product candidates; |
| · | the results of our clinical trials or collaborations may not meet the level of statistical or clinical significance, or product safety, required to move to the next stage of development or, ultimately, obtain marketing approval from the FDA; |
| · | we may not be able to obtain, maintain and enforce our patents and other intellectual property rights; and |
| · | we may not be able to obtain and maintain commercial manufacturing arrangements with third-party manufacturers or establish commercial-scale manufacturing capabilities. |
Furthermore, even if we do receive regulatory approval to market any of our product candidates, any such approval may be subject to limitations on the indicated uses for which we may market the product. If any of our product candidates are unsuccessful, that could have a substantial negative impact on our business.
Accordingly, we cannot assure you that our product candidates will be successfully developed or commercialized. If we or any future development partners are unable to develop, or obtain regulatory approval for or, if approved, successfully commercialize, one or more of our product candidates, we may not be able to generate sufficient revenue to continue our business.
Our product candidates are at an early stage of development and may not be successfully developed or commercialized.
Our product candidates are in the early stages of development and will require substantial clinical development, testing, and regulatory approval prior to commercialization. Of the large number of drugs in development, only a small percentage successfully completes the FDA regulatory approval process and are commercialized. Accordingly, we cannot assure you that our product candidates will be successfully developed or commercialized. If we or any future development partners are unable to develop, or obtain regulatory approval for or, if approved, successfully commercialize, one or more of our product candidates, we may not be able to generate sufficient revenue to continue our business.
The adoption of CAR-T therapies as the potential standard of care for treatment of certain cancers is uncertain, and dependent on the efforts of a limited number of market entrants, and if not adopted as anticipated, the market for lenzilumab or next-generation gene-edited CAR-T therapies may be limited or not develop.
We are seeking to advance the development of lenzilumab to address, among other things, the serious and potentially fatal side effects associated with CAR-T therapies and to improve the efficacy of these treatments. We are also working to create next-generation gene-edited CAR-T therapies using GM-CSF gene knockout technologies. Although five CAR-T therapies have been approved by FDA to date, the use of engineered T cells as a potential cancer treatment is a recent development and may not be broadly accepted by physicians, patients, hospitals, cancer treatment centers, payers and others in the medical community. The degree of market acceptance of any approved product candidates will depend on a number of factors, including:
| · | the efficacy and safety as demonstrated in clinical trials; |
| · | the clinical indications for which the product candidate is approved; |
| · | acceptance by physicians, major operators of hospitals and clinics, and patients of the product candidate as a safe and effective treatment; |
| · | the potential and perceived advantages of product candidates over alternative treatments; |
| · | the safety of product candidates seen in a broader patient group, including its use outside the approved indications; |
| 42 |
| · | competitive approaches to tackle similar issues; |
| · | the cost of treatment in relation to alternative treatments; |
| · | the availability of adequate reimbursement and pricing by payers; |
| · | relative convenience and ease of administration; |
| · | the prevalence and severity of adverse events; |
| · | the effectiveness of sales and marketing efforts; and |
| · | the ability to manage any unfavorable publicity relating to the product candidate. |
If the medical and payer community is not sufficiently persuaded of the safety, efficacy and cost-effectiveness of CAR-T therapy and the potential advantages of using lenzilumab compared to existing and future therapeutics, and there is not significant market acceptance of CAR-T therapy as the standard of care for treatment of certain cancers, the market for lenzilumab or next-generation gene-edited CAR-T therapies may be limited or not develop, and our stock price could be adversely affected.
CAR-T therapies currently in early development purport to incorporate technology that may minimize or eliminate the adverse side-effects and improve on efficacy, that we believe have impaired the uptake of the approved CAR-T therapies. If these developing therapies are proven safer and equally or more efficacious in their proposed indications and approved for use by FDA and other regulatory agencies, the market growth for the currently approved CAR-T therapies may be limited, impairing demand for lenzilumab.
In recent years, several biotechnology companies describing business plans focusing on development of CAR-T therapies have completed or announced they are pursuing initial public offerings (“IPOs”). Several of these companies have described their belief that their therapies will not result in the same level of CRS or NT as has been experienced in use of previously FDA-approved CAR-T therapies. While these products are in early-stage development, the data is limited and these products have not yet been approved for use by FDA, if any such product were also proven equally efficacious and subsequently approved, the market for lenzilumab may not develop or grow as anticipated. Recently the FDA approved Breyanzi™, (liso-cel), a new CAR-T cell therapy for adults with relapsed or refractory Large B-cell Lymphoma. Grade ≥3 cytokine release syndrome and Grade ≥3 neurologic toxicities following Breyanzi treatment occurred in 4% and 12% of patients, respectively. If new CAR-T therapies with lower occurrences of CRS and NT are approved, the market for lenzilumab in conjunction with CAR-T therapy may be diminished. Any such failure of a market for lenzilumab to develop could adversely affect our stock price. In addition, if new CAR-T therapies that offer improved efficacy, either with or without improved safety, the market for lenzilumab in conjunction with CAR-T therapy may be diminished.
Our business could target benefits from various regulatory incentives, such as EUA, orphan drug exclusivity, breakthrough therapy designation, fast track designation, and priority review, but we may not ultimately qualify for or benefit from these arrangements.
We may seek various regulatory incentives, such as EUA, orphan drug exclusivity, breakthrough therapy designation, fast track designation, accelerated approval, priority review and Priority Review Vouchers (“PRVs”), where available, that provide for certain periods of exclusivity, expedited review and/or other benefits, and we may also seek similar designations elsewhere in the world. Often, regulatory agencies have broad discretion in determining whether or not products qualify for such regulatory incentives and benefits. We cannot guarantee that we will be able to receive orphan drug status from FDA or equivalent regulatory designations elsewhere. We also cannot guarantee that we will obtain breakthrough therapy or fast track designation, which may provide certain potential benefits such as more frequent meetings with FDA to discuss the development plan, intensive guidance on an efficient drug development program, and potential eligibility for rolling review or priority review. Legislative developments in the U.S., including proposed legislation that would restrict eligibility for PRVs, may affect our ability to qualify for these programs in the future.
Even if we are successful in obtaining beneficial regulatory designations by FDA or other regulatory agency for our product candidates, such designations may not lead to faster development or regulatory review or approval, and it does not increase the likelihood that our product candidates will receive marketing approval. We may not be able to obtain or maintain such designations for our product candidates, and our competitors may obtain these designations for their product candidates, which could impact our ability to develop and commercialize our product candidates or compete with such competitors, which would adversely impact our business, financial condition or results of operations.
| 43 |
There is a limited amount of information about us upon which investors can evaluate our product candidates and business prospects, including because we have a limited operating history developing product candidates, have not yet successfully commercialized any products, and have a relatively small management team.
Our primary focus is developing our proprietary monoclonal antibody portfolio, which comprises lenzilumab, ifabotuzumab and HGEN005. We are also currently developing our GM-CSF knockout gene-editing CAR-T platform to create next-generation CAR-T therapies that preserve the benefits of CAR-T therapy while altogether avoiding its serious and potentially life-threatening side-effects. Our limited operating history developing clinical-stage product candidates may make it more difficult for us to succeed or for investors to be able to evaluate our business and prospects. In addition, as an early-stage clinical development company, we have limited experience in development activities, including conducting clinical trials, or seeking and obtaining regulatory approvals, even though our executives have had relevant experience at other companies. We currently have eleven full-time employees and therefore are heavily dependent on external consultants and expert vendors for scientific, clinical manufacturing and regulatory expertise. To execute our business plan we will need to successfully:
| · | execute our product candidate development activities, including successfully completing our clinical trial programs, including our collaborations and partnerships; |
| · | obtain required regulatory approvals or authorizations for the development and commercialization of our product candidates; |
| · | manage our costs and expenses related to clinical trials, regulatory approvals, manufacturing and commercialization; |
| · | secure substantial additional funding; |
| · | develop and maintain successful strategic relationships; |
| · | build and maintain a strong intellectual property portfolio; |
| · | build and maintain appropriate clinical, sales, manufacturing, distribution, and marketing capabilities on our own or through third parties; and |
| · | gain market acceptance and favorable reimbursement status for our product candidates. |
If we are unsuccessful in accomplishing these objectives, we may not be able to develop product candidates, raise capital, expand our business, or continue our operations.
In addition to our collaboration with the NIH, IMPACT Partnership and SAHMRI and the University of Adelaide, we may, in the future, seek to enter into collaborations with other third parties for the discovery, development and commercialization of our product candidates. If our collaborators cease development efforts under our collaboration agreements, or if any of those agreements are terminated, these collaborations may fail to lead to commercial products, and we may never receive milestone payments or future royalties under these agreements.
We may in the future seek to enter into agreements with other third-party collaborators for research, development and commercialization of other therapeutic technologies or product candidates. Biopharmaceutical companies are our likely future collaborators for any marketing, distribution, development, licensing, or broader collaboration arrangements. If we fail to enter into future collaborations on commercially reasonable terms, or at all, or such collaborations are not successful, we may not be able to execute our strategy to develop our product candidates or therapies that we believe could benefit from the resources of either larger biopharmaceutical companies or those specialized in a particular area of relevance.
With respect to our existing collaboration agreements, we have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Moreover, our ability to generate revenues from these arrangements will depend on our collaborators' abilities to successfully perform the functions assigned to them in these arrangements.
Collaborations involving our product candidates pose the following risks to us:
| · | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| · | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on preclinical studies or clinical trial results, changes in the collaborators' strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| · | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| 44 |
| · | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| · | collaborators with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; |
| · | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to litigation or potential liability; |
| · | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; |
| · | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and |
| · | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. |
As a result of the foregoing, our current and any future collaboration agreements may not lead to development or commercialization of our product candidates in the most efficient manner or at all. If a collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated. Any failure to successfully develop or commercialize our product candidates pursuant to our current or any future collaboration agreements could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Moreover, to the extent that any of our existing or future collaborators were to terminate a collaboration agreement, we may be forced to independently develop these product candidates, including funding preclinical studies or clinical trials, assuming marketing and distribution costs and defending intellectual property rights, or, in certain instances, abandon product candidates altogether, any of which could result in a change to our business plan and have a material adverse effect on our business, financial condition, results of operations and prospects.
We have relied and may in the future rely on third parties to conduct ISTs of our products, which is cost-effective for us but affords the investigators the ability to retain significant control over the design and conduct of the trials, as well as the use of the data generated from their efforts.
We have relied and may in the future rely on third parties to conduct and sponsor clinical trials relating to lenzilumab, our GM-CSF gene knockout platform and our other immunotherapies, ifabotuzumab and HGEN005. Such ISTs may provide us with valuable clinical data that can inform our future development strategy in a cost-efficient manner, but we do not control the design or conduct of the ISTs, and it is possible that the FDA or non-U.S. regulatory authorities will not view these ISTs as providing adequate support for future clinical trials, whether controlled by us or third parties, for any one or more reasons, including elements of the design or execution of the trials or safety concerns or other trial results.
These arrangements provide us limited information rights with respect to the ISTs, including access to and the ability to use and reference the data, including for our own regulatory filings, resulting from the ISTs. However, we would not have control over the timing and reporting of the data from ISTs, nor would we own the data from the ISTs. If we are unable to confirm or replicate the results from the ISTs or if negative results are obtained, we would likely be further delayed or prevented from advancing further clinical development. Further, if investigators or institutions breach their obligations with respect to the clinical development of our product candidates, or if the data proves to be inadequate compared to the first-hand knowledge, we might have gained had the ISTs been sponsored and conducted by us, then our ability to design and conduct any future clinical trials ourselves may be adversely affected.
If the third parties conducting our clinical trials do not conduct the trials in accordance with our agreements with them, our ability to pursue our clinical development programs could be delayed or unsuccessful and we may not be able to obtain regulatory approval for or commercialize our product candidates when expected or at all.
We do not have the ability to conduct all aspects of our preclinical testing or clinical trials ourselves. Therefore, the timing of the initiation and completion of these trials is uncertain and may occur on substantially different timing from our estimates. We also use CROs to conduct our clinical trials and rely on medical institutions, clinical investigators, CROs, and consultants to conduct our trials in accordance with our clinical protocols and regulatory requirements. Our CROs, investigators, and other third parties play a significant role in the conduct of these trials and subsequent collection and analysis of data.
| 45 |
There is no guarantee that any CROs, investigators, or other third parties on which we rely for administration and conduct of our clinical trials will devote adequate time and resources to such trials or perform as contractually required. If any of these third parties fails to meet expected deadlines, fails to adhere to our clinical protocols, or otherwise performs in a substandard manner, our clinical trials may be extended, delayed, or terminated. If any of our clinical trial sites terminates for any reason, we may experience the loss of follow-up information on subjects enrolled in our ongoing clinical trials unless we are able to transfer those subjects to another qualified clinical trial site. In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time-to-time and may receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, the integrity of the data generated at the applicable clinical trial site may be jeopardized.
We may experience delays in commencing or conducting our clinical trials, in receiving data from third parties or in the continuation or completion of clinical testing, which could result in increased costs to us, delay our ability to generate product candidate revenue or, ultimately, render us unable to complete the development and commercialization of our product candidates.
We have product candidates in clinical development and preclinical development. As with most small biotech companies, the risk of failure for our product candidates is high. It is impossible to predict when or if any of our product candidates will prove effective or safe in humans or will receive regulatory approval. Before obtaining marketing approval from regulatory authorities for the sale of any product candidate, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans.
Before we can initiate clinical trials in the U.S. for any new product candidates, we are required to submit the results of preclinical testing to FDA as part of an IND application, along with other information including information about product candidate chemistry, manufacturing, and controls and our proposed clinical trial protocol. For our programs already underway, we are required to report or provide information to appropriate regulatory authorities in order to continue with our testing programs. If we are unable to make timely regulatory submissions for any of our programs, it will delay our plans for our clinical trials. If those third parties do not make the required data available to us, we will likely have to identify and contract with another third party, and/or develop all necessary preclinical and clinical data on our own, which will lead to significant delays and increase development costs of the product candidate. In addition, FDA may require us to conduct additional preclinical testing for any product candidate before it allows us to initiate clinical testing under any IND application, which may lead to additional delays and increase the costs of our preclinical development. Moreover, despite the presence of an active IND application for a product candidate, clinical trials can be delayed for a variety of reasons, including delays in:
| · | identifying, recruiting, and enrolling qualified subjects to participate in a clinical trial; |
| · | identifying, recruiting, and training suitable clinical investigators; |
| · | reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation, may be subject to modification from time-to-time, and may vary significantly among different CROs and trial sites; |
| · | obtaining and maintaining sufficient quantities of a product candidate for use in clinical trials, either as a result of transferring the manufacturing of a product candidate to another site or manufacturer, deferring ordering or production of product in order to conserve resources or mitigate risk, having product in inventory become no longer suitable for use in humans, or other reasons that reduce or delay availability of drug supply; |
| · | obtaining and maintaining Institutional Review Board (“IRB”) or ethics committee approval to conduct a clinical trial at an existing or prospective site; |
| · | retaining or replacing participants who have initiated a clinical trial but may withdraw due to adverse events from the therapy, insufficient efficacy, fatigue with the clinical trial process, or personal issues; |
| · | developing any companion diagnostic necessary to ensure the study enrolls the target population; |
| · | being required by the FDA to add more patients or sites or to conduct additional trials; or |
| · | the FDA placing a clinical trial on hold. |
Once a clinical trial has begun, recruitment and enrollment of subjects may be slower than we anticipate. Numerous companies and institutions are conducting clinical studies in similar patient populations which can result in competition for qualified patients. In addition, clinical trials will take longer than we anticipate if we are required, or believe it is necessary, to enroll additional subjects than originally planned. Clinical trials may also be delayed as a result of ambiguous or negative interim results. Further, a clinical trial may be suspended or terminated by us, an IRB, an ethics committee, or a data safety monitoring committee overseeing the clinical trial, any of our clinical trial sites with respect to that site, or FDA or other regulatory authorities, due to several factors, including:
| 46 |
| · | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| · | inspection of the clinical trial operations or clinical trial site by the FDA or other regulatory authorities; |
| · | inability to provide timely supply of drug product due to shortages or other issues with the drug product; |
| · | unforeseen safety issues, known safety issues that occur at a greater frequency or severity than we anticipate, or any determination that the clinical trial presents unacceptable health risks; or |
| · | lack of adequate funding to continue the clinical trial or unforeseen significant incremental costs related to the trial. |
Additionally, if any future development partners do not develop the licensed product candidates in the time and manner that we expect, or at all, the clinical development efforts related to these licensed product candidates could be delayed or terminated. In addition, our ability to enforce our partners’ obligations under any future collaboration efforts may be limited due to time and resource constraints, competing corporate priorities of our future partners, and other factors.
Any delays in the commencement of our clinical trials may delay or preclude our ability to further develop or pursue regulatory approval for our product candidates. Changes in U.S. and foreign regulatory requirements and guidance also may occur, and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to IRBs for re-examination, which may affect the costs, timing, and likelihood of a successful completion of a clinical trial.
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these trials or tests are not positive or do not meet the standard for acceptability by regulatory authorities or if there are safety concerns, we may:
| · | be delayed in obtaining marketing approval for our product candidates; |
| · | not obtain marketing approval or EUA at all; |
| · | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| · | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| · | be subject to additional post-marketing testing requirements; or |
| · | have the product removed from the market after obtaining marketing approval. |
Our product development costs will also increase if we experience delays in testing or in obtaining marketing approvals. We do not know whether any of our preclinical studies or clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. We may also determine to change the design or protocol of one or more of our clinical trials, including to add additional arms or patient populations, which could result in increased costs and expenses and/or delays. If we or any future development partners experience delays in the completion of, or if we or any future development partners must terminate, any clinical trial of any product candidate our ability to obtain regulatory approval for that product candidate will be delayed and the commercial prospects, if any, for the product candidate may suffer as a result. Significant preclinical study or clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do and impair our ability to successfully commercialize our product candidates and may harm our business and results of operations. In addition, many of these factors may also ultimately lead to the denial of regulatory approval of a product candidate.
Our product candidates are subject to extensive regulation, compliance with which is costly and time consuming, may cause unanticipated delays, or may prevent the receipt of the required approvals to commercialize our product candidates.
The clinical development, approval, manufacturing, labeling, storage, record-keeping, advertising, promotion, import, export, marketing, and distribution of our product candidates are subject to extensive regulation by FDA in the U.S. and by comparable authorities in foreign markets. In the U.S., we are not permitted to market our product candidates until we receive regulatory approval from FDA. The process of obtaining regulatory approval is expensive, often takes many years, and can vary substantially based upon the type, complexity, and novelty of the products involved, as well as the target indications. Approval policies or regulations may change, and FDA has substantial discretion in the drug approval process, including the ability to delay, limit, or deny approval of a product candidate for many reasons. Despite the time and expense invested in clinical development of product candidates, regulatory approval is never guaranteed. FDA or other comparable foreign regulatory authorities can delay, limit, or deny approval of a product candidate for many reasons, including:
| 47 |
| · | such authorities may disagree with the design or implementation of our or any future development partners’ clinical trials; |
| · | such authorities may not accept clinical data from trials that are conducted at clinical facilities or in countries where the standard of care is potentially different from the U.S.; |
| · | the results of clinical trials may not demonstrate the safety or efficacy required by such authorities for approval; |
| · | we or any future development partners may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| · | such authorities may disagree with our interpretation of data from preclinical studies or clinical; |
| · | such authorities may find deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we or any future development partners contract for clinical and commercial supplies; or |
| · | the approval policies or regulations of such authorities may significantly change in a manner rendering our or any future development partners’ clinical data insufficient for approval. |
With respect to foreign markets, approval procedures vary widely among countries and, in addition to the aforementioned risks, can involve additional product testing, administrative review periods, and agreements with pricing authorities. In addition, events raising questions about the safety of certain marketed pharmaceuticals may result in increased caution by FDA and comparable foreign regulatory authorities in reviewing new drugs based on safety, efficacy or other regulatory considerations and may result in significant delays in obtaining regulatory approvals. Any delay in obtaining, or inability to obtain, applicable regulatory approvals may delay or prevent us or any future development partners from commercializing our product candidates.
The results of preclinical studies and early clinical trials are not always predictive of future results. Any product candidate we or any future development partners advance into clinical trials may not have favorable results in later clinical trials, if any, or receive regulatory approval.
Drug development has substantial inherent risk. We or any future development partners will be required to demonstrate through adequate and well-controlled clinical trials that our product candidates are effective, with a favorable benefit-risk profile, for use in their target populations for their intended indications before we can seek regulatory approvals for their commercial sale. Drug development is a long, expensive and uncertain process, and delay or failure can occur at any stage of development, including after commencement of any of our clinical trials. Success in early clinical trials does not mean that later clinical trials will be successful because product candidates in later-stage clinical trials may fail to demonstrate sufficient safety or efficacy despite having progressed through initial clinical testing. Furthermore, our future trials will need to demonstrate sufficient safety and efficacy for approval by regulatory authorities in larger patient populations. Companies frequently suffer significant setbacks in advanced clinical trials, even after earlier clinical trials have shown promising results. In addition, only a small percentage of drugs under development result in the submission of an NDA or BLA to FDA, and even fewer are approved for commercialization.
In addition, serious adverse or undesirable side effects may emerge or be identified during later stages of development that were not observed in earlier stages. If our product candidates, either alone or in combination with other therapeutics, are associated with serious adverse events or undesirable side effects or unacceptable drug-drug interactions in clinical trials or have characteristics that are unexpected in clinical trials or preclinical testing, we may need to abandon their development or limit development to more narrow uses or subpopulations in which the serious adverse events, undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. In pharmaceutical development, many compounds that initially show promise in early-stage or clinical testing are later found to cause side effects that prevent further development of the compound. In addition, if third parties manufacture or use our product candidates without our permission, and generate adverse events or unacceptable side effects, this could also have an adverse impact on our development efforts.
Unacceptable adverse events caused by any of our product candidates that we advance into clinical trials could cause us or regulatory authorities to interrupt, delay, or halt clinical trials and could result in the denial of regulatory approval by FDA or other regulatory authorities for any or all targeted indications and markets. This in turn could prevent us from completing development or commercializing the affected product candidate and generating revenue from its sale. We have not yet successfully completed testing of any of our product candidates for the treatment of the indications for which we intend to seek approval in humans, and we currently do not know the extent of adverse events, if any, that will be observed in individuals who receive any of our product candidates. If any of our product candidates cause unacceptable adverse events in clinical trials, we may not be able to obtain regulatory approval or commercialize such product candidates.
| 48 |
We face risks associated with clinical operations abroad, which may adversely affect our financial condition and results of operations, and we may not be able to receive conditional marketing approvals for lenzilumab in COVID-19 patients in markets outside the U.S.
The Mexican regulatory agency, COFEPRIS, granted us permission to expand the Phase 3 study of lenzilumab in patients with COVID-19 in Mexico. We did not open any sites in Mexico or enroll any patients in that country; however, we may do so in the future. Partners also are conducting or planning to conduct clinical trials involving lenzilumab in Korea, Australia and the United Kingdom, as well as potentially in other countries in patients with COVID-19 and other indications. In addition, we are working to seek conditional marketing authorization (“CMA”) for lenzilumab in hospitalized COVID-19 patients in the European Union and the United Kingdom. We have not received any authorization or approval to sell lenzilumab in any country but would expect to commence commercial operations, through a partner or on our own, only after such authorization or approval were received. As with our development programs in the U.S., our product candidates must be approved for marketing and sale by regulatory authorities in each jurisdiction to which we may apply for approval and, once approved, are subject to extensive regulation by regulatory agencies in other countries. We anticipate that we may file for marketing approval in additional countries and for additional indications and products over the next several years. These and any future marketing applications we file may not be approved by the regulatory authorities on a timely basis, or at all. Even if marketing approval is granted for these products, there may be significant limitations on their use. We cannot state with certainty when or whether any of our product candidates under development will be approved by any foreign regulators or launched; whether we will be able to develop, license or acquire additional product candidates or products; or whether any products, once launched, will be commercially successful.
In addition, operations abroad are accompanied by certain financial, political, economic and other risks, including those listed below:
| · | Foreign Currency Exchange: Operations internationally may subject us to risks related to foreign currency exchange risks as we make payments, or incur obligations, denominated in foreign currencies. We cannot predict future fluctuations in the foreign currency exchange rates of the U.S. dollar. If the U.S. dollar appreciates significantly against certain currencies and our practices do not sufficiently offset the effects of such appreciation, our results of operations would be adversely affected, and our stock price may decline. |
| · | Anti-Bribery: We are subject to the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws that will govern our international operations with respect to payments to government officials. Our international operations would be heavily regulated and require significant interaction with foreign officials. In certain circumstances, strict compliance with anti-bribery laws may conflict with local customs and practices or may require us to interact with doctors and hospitals, some of which may be state controlled, in a manner that is different than local custom. In addition, despite our efforts, our policies and procedures may not protect us from reckless or criminal acts committed by persons who act on our behalf. Enforcement activities under anti-bribery laws could subject us to administrative and legal proceedings and actions, which could result in civil and criminal sanctions, including monetary penalties and exclusion from health care programs. |
| · | Other risks inherent in conducting foreign operations include: |
| o | International operations, including any use of third-party manufacturers, distributors, CROs and collaboration arrangements outside the U.S., expose us to increased risk of theft of our intellectual property and other proprietary technology, particularly in jurisdictions with less robust intellectual property protections than the U.S., as well as restrictive government actions against our intellectual property and other foreign assets such as nationalization, expropriation or the imposition of compulsory licenses. |
| o | We may be subject to protective economic policies taken by foreign governments, such as trade protection measures and import and export licensing requirements, which may result in the imposition of trade sanctions or similar restrictions by the U.S. or other governments. |
| o | Our foreign operations, third-party manufacturers, CROs or strategic partners could be subject to business interruptions for which we or they may be uninsured or inadequately insured. |
| o | Our operations may also be adversely affected if there is political instability or disruption in any other geographic region where we may have operations, which could impact our ability to do business in those areas. |
If we were to encounter any of these risks, our foreign operations may be adversely affected, which could have an adverse effect on our overall business and results of operations.
| 49 |
If we fail to attract and retain key management and clinical development personnel, or if the attention of such personnel is diverted, we may be unable to successfully manage our business and develop or commercialize our product candidates.
We will need to effectively manage our managerial, operational, financial, and other resources in order to successfully pursue our clinical development and commercialization efforts. As a company with a limited number of personnel, we are heavily affected by turnover and highly dependent on the expertise of the members of our senior management, in particular our Chief Executive Officer, Dr. Cameron Durrant, and Dr. Dale Chappell, the controlling owner of the Black Horse Entities (as defined below), our current Chief Scientific Officer and a director. Furthermore, we rely on third party consultants for a variety of services. We cannot predict the impact of the loss of such individuals or the loss of services of any of our other senior management, should they occur, or the difficulty in replacing such individuals. Such losses could delay or prevent the further development and potential commercialization of our product candidates and, if we are not successful in finding suitable replacements, could harm our business.
If our competitors develop similar or comparable treatments for the target indications of our product candidates that are approved more quickly, marketed more successfully or are demonstrated to be safer or more effective than our product candidates, or if FDA approves generic or biosimilar competitors to our products post-approval, our commercial opportunity will be reduced or eliminated.
We compete in an industry characterized by rapidly advancing technologies, intense competition, a changing regulatory and legislative landscape and a strong emphasis on the benefits of intellectual property protection and regulatory exclusivities. Our competitors include pharmaceutical companies, other biotechnology companies, academic institutions, government agencies and other private and public research organizations. We compete with these parties in immunotherapy and oncology treatments and in recruiting highly qualified personnel. Our product candidates, if successfully developed and approved, may compete with established therapies, with new treatments that may be introduced by our competitors, including competitors relying on our biologics approvals under section 351(k) of the Public Health Service Act, or with generic copies of our products approved by FDA under an abbreviated new drug application (“ANDA”), referencing our drug products. We believe that competitors are actively developing competing products to our product candidates. See “Competition” in the “Business” section of our 2020 Annual Report on Form 10-K for a discussion of competition with respect to our current product candidates.
Many of our competitors and potential competitors have substantially greater scientific, research, and product development capabilities, as well as greater financial, marketing, sales and human resources capabilities than we do. In addition, many specialized biotechnology firms have formed collaborations with large, established companies to support the research, development and commercialization of products that may be competitive with ours. Accordingly, our competitors may be more successful with respect to their products than we may be in developing, commercializing, and achieving widespread market acceptance for our products. If a competitor obtains approval for an orphan drug that is the same drug or the same biologic as one of our candidates before we do, we will be blocked from obtaining FDA approval for seven years from the date of the competitor’s product, unless we can establish that our product is clinically superior to the previously-approved competitor’s product or we can meet another exception, such as by showing that the competitor has failed to provide an adequate supply of its product to patients after approval. In addition, our competitors’ products may be more effective or more effectively marketed and sold than any treatment we or our development partners may commercialize and may render our product candidates obsolete or non-competitive before we can recover the expenses related to developing and supporting the commercialization of any of our product candidates. Developments by competitors may render our product candidates obsolete or noncompetitive. After one of our product candidates is approved, FDA may also approve a generic version with the same dosage form, safety, strength, route of administration, quality, performance characteristics and intended use as our product. These generic equivalents would be less costly to bring to market and could generally be offered at lower prices, thereby limiting our ability to gain or retain market share.
The acquisition or licensing of pharmaceutical products is also very competitive, and a number of more established companies, which have acknowledged strategies to in-license or acquire products, may have competitive advantages as may other emerging companies taking similar or different approaches to product acquisitions. The more established companies may have a competitive advantage over us due to their size, cash flows, institutional experience and historical corporate reputation.
| 50 |
We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs and limit supply of our products.
We are wholly dependent on third party contract manufacturers for the timely supply of adequate quantities of our products which meet or exceed requisite quality and production standards for use in clinical and nonclinical studies. Given the extensive risks, scope, complexity, cost, regulatory requirements, and commitment of resources associated with developing the capabilities to manufacture one or more of our products, we have no present plan or intention of developing in-house manufacturing capabilities for nonclinical, clinical or commercial scale production, beyond our current supervision and management of our third-party contract manufacturers. In addition, in order to balance risk and conserve financial and human resources, we have and may continue from time-to-time to defer commitment to production of product, which could result in delays to the continued progress of our clinical and nonclinical testing.
In addition to the foregoing, the process of manufacturing our products is complex, highly regulated, and subject to several risks, including but not limited to the following:
| · | We, and our contract manufacturers, must comply with FDA’s current Good Manufacturing Practice, (“cGMP”), regulations and guidance. We, and our contract manufacturers, may encounter difficulties in achieving quality control and quality assurance and may experience shortages in qualified personnel. We, and our contract manufacturers, are subject to inspections by FDA and comparable agencies in other jurisdictions to confirm compliance with applicable regulatory requirements. Any failure to follow cGMP or other regulatory requirements or any delay, interruption or other issues that arise in the manufacture, fill-finish, packaging, or storage of our products as a result of a failure of our facilities or the facilities or operations of third parties to comply with regulatory requirements, or a failure to pass any regulatory authority inspection, could significantly impair our ability to develop and commercialize our products, including leading to significant delays in the availability of products for our clinical studies or the termination or hold on a clinical study, or the delay or prevention of a filing or approval of marketing applications for our product candidates. Significant noncompliance could also result in the imposition of sanctions, including injunctions, civil penalties, failure of regulatory authorities to grant marketing approvals for our product candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions, adverse publicity, and criminal prosecutions, any of which could damage our reputation. If we are not able to maintain regulatory compliance, we may not be permitted to market our products and/or may be subject to product recalls, seizures, injunctions, or criminal prosecution. Any adverse developments affecting manufacturing operations for our products may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls, or other interruptions in the supply of our products. Once our product candidates are approved, we may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives. |
| · | The manufacturing facilities in which our products are made could be adversely affected by equipment failures, plant closures, capacity constraints, competing customer priorities or changes in corporate strategy or priorities, process changes or failures, changes in business models or operations, materials or labor shortages, natural disasters, power failures and numerous other factors. |
| · | We are wholly dependent upon third party CMOs for the timely supply of adequate quantities of requisite quality product for our nonclinical, clinical and, if approved by regulatory authorities, commercial scale production. |
If any product candidate that we successfully develop does not achieve broad market acceptance among physicians, patients, healthcare payers and the medical community, the revenue that it generates may be limited.
Even if our product candidates receive regulatory approval, they may not gain market acceptance among physicians, patients, healthcare payers, and the medical community. Coverage and reimbursement of our product candidates by third-party payers, including government payers, generally is also necessary for commercial success. The degree of market acceptance of any approved product candidates will depend on several factors, including:
| · | the efficacy and safety as demonstrated in clinical trials; |
| · | the clinical indications for which the product candidate is approved; |
| · | acceptance by physicians, major operators of hospitals and clinics, and patients of the product candidate as a safe and effective treatment; |
| · | the potential and perceived advantages of product candidates over alternative treatments; |
| · | the safety of product candidates seen in a broader patient group, including its use outside the approved indications; |
| · | the cost of treatment in relation to alternative treatments; |
| · | the availability of adequate reimbursement and pricing by payers; |
| · | relative convenience and ease of administration; |
| · | the prevalence and severity of adverse events; |
| · | the effectiveness of our sales and marketing efforts; and |
| · | the ability to manage any unfavorable publicity relating to the product candidate. |
| 51 |
If any product candidate is approved but does not achieve an adequate level of acceptance by physicians, hospitals, healthcare payers, and patients, we may not generate sufficient revenue from that product candidate and may not become or remain commercially attractive as a standalone indication for that product.
Reimbursement may be limited or unavailable in certain market segments for our product candidates, which could make it difficult for us to sell our product candidates profitably.
Market acceptance and sales of our product candidates will depend significantly on the availability of adequate insurance coverage and reimbursement from third-party payers for any of our product candidates and may be affected by existing and future health care reform measures. Government authorities and third-party payers, such as private health insurers and health maintenance organizations, decide which drugs they will pay for and establish reimbursement levels. Reimbursement by a third-party payer may depend upon a number of factors including the third-party payer’s determination that use of a product candidate is:
| · | a covered benefit under its health plan; |
| · | safe, effective, and medically necessary; |
| · | appropriate for the specific patient; |
| · | cost-effective; and |
| · | neither experimental nor investigational. |
Obtaining coverage and reimbursement approval for a product candidate from a government or other third-party payer is a time-consuming and costly process that could require us to provide supporting scientific, clinical, and cost effectiveness data for the use of our product candidates to the payer. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. We cannot be sure that coverage or adequate reimbursement will be available for any of our product candidates. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our product candidates. If reimbursement is not available or is available only to limited levels or with restrictions, we may not be able to commercialize certain of our product candidates profitably, or at all, even if approved.
In the U.S. and in certain foreign jurisdictions, there have been a number of legislative and regulatory changes to the health care system that could affect our ability to sell our product candidates profitably. In particular, the Medicare Modernization Act of 2003 revised the payment methods for many product candidates under Medicare. This has resulted in lower rates of reimbursement. There have been numerous other federal and state initiatives designed to reduce payment for pharmaceuticals.
As a result of legislative proposals and the trend toward managed health care in the U.S., third-party payers are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new drugs. They may also refuse to provide coverage of approved product candidates for medical indications other than those for which FDA has granted market approvals. As a result, significant uncertainty exists as to whether and how much third-party payers will reimburse patients for their use of newly approved drugs, which in turn will put pressure on the pricing of drugs. We could be subject to pricing pressures in connection with the sale of our product candidates due to the trend toward managed health care, the increasing influence of health maintenance organizations, and additional legislative proposals as well as country, regional, or local healthcare budget limitations. Similar concerns about the costs of treatment have been raised in Europe and the United Kingdom.
Governments may impose price controls, which may adversely affect our future profitability.
We intend to seek approval to market our future product candidates in the U.S. and potentially in foreign jurisdictions. If we obtain approval in one or more foreign jurisdictions, we will be subject to rules and regulations in those jurisdictions relating to our product candidates. In some foreign countries, particularly in the European Union and the United Kingdom, the pricing of prescription pharmaceuticals and biologics is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. If reimbursement of our future products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
| 52 |
We face potential product liability exposure and, if successful claims are brought against us, we may incur substantial liability for a product candidate and may have to limit its commercialization.
The use of our product candidates in clinical trials and the sale of any product candidates for which we may obtain marketing approval expose us to the risk of product liability claims. Product liability claims may be brought against us or any future development partners by participants enrolled in our clinical trials, patients, health care providers, or others using, administering, or selling our product candidates. If we cannot successfully defend ourselves against any such claims, or have insufficient insurance protection, we would incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in:
| · | withdrawal of clinical trial participants; |
| · | termination of clinical trial sites or entire trial programs; |
| · | costs of related litigation; |
| · | substantial monetary awards to trial participants or other claimants; |
| · | decreased demand for our product candidates and loss of revenue; |
| · | impairment of our business reputation; |
| · | diversion of management and scientific resources from our business operations; and |
| · | the inability to commercialize our product candidates. |
We have obtained limited product liability insurance coverage for our clinical trials domestically and in selected foreign countries where we are conducting clinical trials. As such, our insurance coverage may not reimburse us or may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to product liability. We intend to expand our insurance coverage for product candidates to include the sale of commercial products if we obtain marketing approval for our product candidates in development; however, we may be unable to obtain commercially reasonable product liability insurance for any product candidates approved for marketing. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our working capital and adversely affect our business.
Our employees and consultants may engage in misconduct or other improper activities, including noncompliance with regulatory standards, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees or consultants could include intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, failure to provide accurate information to FDA or comparable foreign regulatory authorities, failure to comply with manufacturing standards, failure to comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, failure to report financial information or data accurately, violations of anti-bribery laws, or failure to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee or consultant misconduct could also involve the improper use of confidential information obtained in the course of our business, which could result in civil or criminal legal actions, regulatory sanctions, or serious harm to our reputation. We have adopted a Code of Business Conduct and Ethics and other corporate policies, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
We may encounter difficulties in managing our growth and expanding our operations successfully.
As we seek to advance our product candidates through clinical trials, we will need to expand our development, regulatory, manufacturing, marketing, and sales capabilities, and contract with third parties to provide these capabilities for us. As our operations expand, we expect that we will need to manage additional relationships with various development partners, suppliers, and other third parties. Future growth will impose significant added responsibilities on members of management. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend in part on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively. We may not be able to accomplish these tasks and our failure to accomplish any of them could prevent us from successfully growing our company.
| 53 |
We and any future development partners, third-party manufacturers and suppliers use hazardous materials, and any claims relating to improper handling, storage, or disposal of these materials could be time consuming or costly.
We and any future development partners, third-party manufacturers and suppliers may use hazardous materials, including chemicals and biological agents and compounds that could be dangerous to human health and safety or the environment. Our operations and the operations of our development partner, third-party manufacturers and suppliers also produce hazardous waste products. Federal, state, and local laws and regulations govern the use, generation, manufacture, storage, handling, and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive and current or future environmental laws and regulations may impair our product development efforts. In addition, we cannot eliminate the risk of accidental injury or contamination from these materials or wastes. We do not carry specific biological or hazardous waste insurance coverage and our property, casualty, and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury we could be held liable for damages or be penalized with fines in an amount exceeding our resources, and our clinical trials or regulatory approvals could be suspended.
Healthcare reform measures, when implemented, could hinder or prevent our commercial success.
There have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at broadening the availability of health care and containing or lowering the overall cost of health care. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations, and other payers of healthcare services to contain or reduce costs of health care may adversely affect:
| · | the demand for any drug products for which we may obtain regulatory approval; |
| · | our ability to set a price that we believe is fair for our product candidates; |
| · | our ability to gain reimbursement at commercially acceptable levels; |
| · | our ability to generate revenue and achieve or maintain profitability; |
| · | the level of taxes that we are required to pay; and |
| · | the availability of capital. |
We and any of our future development partners will be required to report to regulatory authorities if any of our approved products cause or contribute to adverse medical events, and any failure to do so would result in sanctions that would materially harm our business.
If we and any future development partners are successful in commercializing our products, FDA and foreign regulatory authorities would require that we and any future development partners report certain information about adverse medical events if those products may have caused or contributed to those adverse events. The timing of our obligation to report would be triggered by the date we become aware of the adverse event as well as the nature of the event. We and any future development partners may fail to report adverse events we become aware of within the prescribed timeframe. We and any future development partners may also fail to appreciate that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our products. If we and any future development partners fail to comply with our reporting obligations, FDA or a foreign regulatory authority could take action including criminal prosecution, the imposition of civil monetary penalties, seizure of our products, or delay in approval or clearance of future products.
Our product candidates for which we intend to seek approval as biologic products may face competition sooner than anticipated.
With the enactment of the Biologics Price Competition and Innovation Act of 2009, or the BPCIA, as part of the Affordable Care Act, an abbreviated pathway for the approval of biosimilar and interchangeable biological products was created. The abbreviated regulatory pathway establishes legal authority for FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as ‘‘interchangeable’’ based on its similarity to an existing brand product. Under the BPCIA, an application for a biosimilar product cannot be approved by FDA until 12 years after the original branded product was approved under a BLA. The law is complex and is still being interpreted and implemented by FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty. While it is uncertain when such processes intended to implement BPCIA may be fully adopted by FDA, any such processes could have a material adverse effect on the future commercial prospects for our biological products.
| 54 |
We believe that any of our product candidates, such as lenzilumab, ifabotuzumab and/or HGEN005, if approved as biological products under a BLA, should qualify for the 12-year period of exclusivity. However, there is a risk that FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for biosimilar competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing. Finally, there is a risk that the 12-year exclusivity period could be reduced which could negatively affect our products.
In addition, foreign regulatory authorities may also provide for exclusivity periods for approved biological products. For example, biological products in Europe may be eligible for a 10-year period of exclusivity. However, biosimilar products have been approved under a sub-pathway of the centralized procedure since 2006. The pathway allows sponsors of a biosimilar product to seek and obtain regulatory approval based in part on the clinical trial data of an originator product to which the biosimilar product has been demonstrated to be ‘‘similar.’’ In many cases, this allows biosimilar products to be brought to market without conducting the full suite of clinical trials typically required of originators. It is unclear whether we and our development partner would face competition to our products in European markets sooner than anticipated.
We may in the future be subject to various U.S. federal and state laws pertaining to health care fraud and abuse, including anti-kickback, self-referral, false claims and fraud laws, and any violations by us of such laws could result in fines or other penalties.
If one or more of our product candidates is approved, we will likely be subject to the various U.S. federal and state laws intended to prevent health care fraud and abuse. The federal anti-kickback statute prohibits the offer, receipt, or payment of remuneration in exchange for or to induce the referral of patients or the use of products or services that would be paid for in whole or part by Medicare, Medicaid, or other federal health care programs. Remuneration has been broadly defined to include anything of value, including cash, improper discounts, and free or reduced-price items and services. Many states have similar laws that apply to their state health care programs as well as private payers. Violations of the anti-kickback laws can result in exclusion from federal health care programs and substantial civil and criminal penalties.
The False Claims Act imposes liability on persons who, among other things, present or cause to be presented false or fraudulent claims for payment by a federal health care program. The False Claims Act has been used to prosecute persons submitting claims for payment that are inaccurate or fraudulent, that are for services not provided as claimed, or for services that are not medically necessary. The False Claims Act includes a whistleblower provision that allows individuals to bring actions on behalf of the federal government and share a portion of the recovery of successful claims. If our marketing or other arrangements were determined to violate the False Claims Act or anti-kickback or related laws, then our revenue could be adversely affected, which would likely harm our business, financial condition, and results of operations.
State and federal authorities have aggressively targeted medical technology companies for alleged violations of these anti-fraud statutes, based on improper research or consulting contracts with doctors, certain marketing arrangements that rely on volume-based pricing, off-label marketing schemes, and other improper promotional practices. Companies targeted in such prosecutions have paid substantial fines in the hundreds of millions of dollars or more, have been forced to implement extensive corrective action plans or corporate integrity agreements, and have often become subject to consent decrees severely restricting the manner in which they conduct their business. If we become the target of such an investigation or prosecution based on our contractual relationships with providers or institutions, or our marketing and promotional practices, we could face similar sanctions, which would materially harm our business.
Also, the Foreign Corrupt Practices Act and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. We cannot assure you that our internal control policies and procedures will protect us from reckless or negligent acts committed by our employees, future distributors, partners, collaborators, or agents. Violations of these laws, or allegations of such violations, could result in fines, penalties, or prosecution and have a negative impact on our business, results of operations and reputation.
| 55 |
Legislative or regulatory healthcare reforms in the U.S. may make it more difficult and costly for us to obtain regulatory approval of our product candidates and to produce, market, and distribute our products after approval is obtained.
From time-to-time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulatory approval, manufacture, and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of our current product candidates or any future product candidates. We cannot determine what effect changes in regulations, statutes, legal interpretation, or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
| · | changes to manufacturing methods; |
| · | additional studies, including clinical studies; |
| · | recall, replacement, or discontinuance of one or more of our products; and |
| · | additional record-keeping. |
Each of these would likely entail substantial time and cost and could materially harm our business and our financial results. In addition, delays in receipt of or failure to receive regulatory approvals for any future products would harm our business, financial condition, and results of operations.
Even if we are able to obtain regulatory approval for our product candidates, we will continue to be subject to ongoing and extensive regulatory requirements, and our failure to comply with these requirements could substantially harm our business.
If we receive regulatory approval for our product candidates, we will be subject to ongoing FDA obligations and continued regulatory oversight and review, such as continued safety reporting requirements, and we may also be subject to additional FDA post-marketing obligations. If we are not able to maintain regulatory compliance, we may not be permitted to market our product candidates and/or may be subject to product recalls or seizures.
If the FDA approves any of our product candidates, the labeling, manufacturing, packaging, storage, distribution, export, adverse event reporting, advertising, promotion and record-keeping for our products will be subject to extensive regulatory requirements. Violations of these regulatory requirements or the subsequent discovery of previously unknown problems with the products, including adverse events of unanticipated severity or frequency, may result in:
| · | the issuance of warning or untitled letters; |
| · | requirements to conduct post-marking clinical trials; |
| · | restrictions on the marketing and distribution of the product, including potential withdrawal of the product from the market; |
| · | suspension of ongoing clinical trials; |
| · | the issuance of product recalls, import and export restrictions, seizures, and detentions; and |
| · | the issuance of injunctions, or imposition of other civil and/or criminal penalties. |
Our ability to utilize our net operating loss carryforwards and certain other tax attributes may be subject to certain limitations.
We have incurred substantial losses during our history and do not expect to become profitable in the foreseeable future and may never achieve profitability. To the extent that we continue to generate taxable losses, unused losses will carry forward to offset future taxable income, if any, until such unused losses expire. We may be unable to use these losses to offset income before such unused losses expire. The Tax Cuts and Jobs Act, enacted in 2017, limited the use of net operating loss carryforwards for periods beginning after 2017 to eighty percent of taxable income in the period to which the losses were carried. However, this limitation on the use of the carryforwards was eliminated by the Coronavirus Aid, Relief and Economic Security Act (the “CARES” Act) for tax years beginning before January 1, 2021. In addition, Section 382 of the Internal Revenue Code of 1986, as amended, may limit the utilization of net operating loss carryforwards. Under Section 382, if a corporation undergoes an ‘‘ownership change’’ (generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. We have recently and in the past experienced ownership changes that have resulted in limitations on the use of a portion of our net operating loss carryforwards. If we experience further ownership changes our ability to utilize our net operating loss carryforwards could be further limited.
| 56 |
We rely completely on third parties to supply drug substance and manufacture drug product for our clinical trials and preclinical studies and intend to rely on other third parties to produce commercial supplies of product candidates, and our dependence on third parties could adversely impact our business.
We are dependent on third-party suppliers. We regularly evaluate potential alternate sources of supply of raw materials, various components used in production, drug substance and drug product, but there can be no assurance that any such suppliers would be available, acceptable, or successful. The costs of manufacturing our drug candidates are high, and we will require additional capital to ensure that we can maintain an adequate supply to conduct our contemplated development programs.
If our third-party suppliers do not supply sufficient quantities for product candidates to us on a timely basis and in accordance with applicable specifications and other regulatory requirements, there could be a significant interruption of our supplies, which would adversely affect clinical development of the product candidate, including affecting our ability to enroll in and timely progress clinical trials. Furthermore, if any of our contract manufacturers cannot successfully manufacture material that conforms to our specifications and with regulatory requirements, we will not be able to secure and/or maintain regulatory approval, if any, for our product candidates.
We will also rely on our contract manufacturers to purchase from third-party suppliers the materials necessary to produce our product candidates for our anticipated clinical trials. There are a small number of suppliers for certain capital equipment and raw materials used to manufacture our product candidates. We do not have any control over the process or timing of the acquisition of these raw materials by our contract manufacturers. Moreover, we currently do not have agreements in place for the commercial production of these raw materials. Any significant delay in the supply of a product candidate or the raw material components thereof for an ongoing clinical trial could considerably delay completion of that clinical trial, product candidate testing, and potential regulatory approval of that product candidate.
We do not expect to have the resources or capacity to commercially manufacture any of our proposed product candidates, if approved, and will likely continue to be dependent on third-party manufacturers. Our dependence on third parties to manufacture and supply us with clinical trial materials and any approved product candidates may adversely affect our ability to develop and commercialize our product candidates on a timely basis.
We may not be successful in establishing and maintaining development partnerships and licensing agreements, which could adversely affect our ability to develop and commercialize product candidates.
Part of our strategy is to enter into development partnerships and licensing agreements. We face significant competition in seeking appropriate partners and the negotiation process is time consuming and complex. Even if we are successful in securing a development partnership, we may not be able to continue it. Moreover, we may not be successful in our efforts to establish a development partnership or other alternative arrangements for any of our other existing or future product candidates and programs because, among other reasons, our research and development pipeline may be insufficient, our product candidates and programs may be deemed to be at too early a stage of development for collaborative effort and/or third parties may not view our product candidates and programs as having the requisite potential to demonstrate safety and efficacy. Even if we are successful in our efforts to establish new development partnerships, the terms that we agree upon may not be favorable to us and we may not be able to maintain such development partnerships if, for example, development or approval of a product candidate is delayed or sales of an approved product candidate are disappointing. Any delay in entering into new development partnership agreements related to our product candidates could delay the development and commercialization of our product candidates and reduce their competitiveness if they reach the market.
Moreover, if we fail to establish and maintain additional development partnerships related to our product candidates:
| · | the development of our current or future product candidates may be terminated or delayed; |
| · | our cash expenditures related to development of certain of our current or future product candidates would increase significantly and we may need to seek additional financing; |
| · | we may be required to hire additional employees or otherwise develop expertise, such as sales and marketing expertise, for which we have not budgeted; and |
| · | we will bear all of the risk related to the development of any such product candidates. |
Our or any new partner’s failure to develop, manufacture or effectively commercialize our product would result in a material adverse effect on our business and results of operations and would likely cause our stock price to decline.
| 57 |
Currently pending, threatened or future litigation or governmental proceedings or inquiries could result in material adverse consequences, including judgments or settlements.
We are, or may from time-to-time become, involved in lawsuits and other legal or governmental proceedings. See Note 10 to the Condensed Consolidated Financial Statements included in this Quarterly Report on Form 10-Q for information regarding currently pending litigation that could have a material impact on the Company. Many of these matters raise complicated factual and legal issues and are subject to uncertainties and complexities, all of which make the matters costly to address. The timing of the final resolutions to any such lawsuits, inquiries, and other legal proceedings is uncertain.
Additionally, the possible outcomes or resolutions to these matters could include adverse judgments or settlements, either of which could require substantial payments, adversely affecting our consolidated financial condition, results of operations and cash flows. Any judgment against us, the entry into any settlement agreement, or the imposition of any fine could have a material adverse effect on our consolidated financial condition, results of operations and cash flows.
The terms of our loan agreement with Hercules may restrict our current and future operations, particularly our ability to respond to changes in business or to take certain actions, including to pay dividends to our stockholders.
On March 10, 2021, we entered into the Loan and Security Agreement with Hercules Capital, Inc. (the “Term Loan”). As of March 31, 2021, we had approximately $24.4 million of accrued debt under the Term Loan. The Term Loan has a scheduled maturity date of March 1, 2025 and is secured by a first priority security interest in substantially all of our assets. The Term Loan contains, and any future indebtedness we incur will likely contain, a number of restrictive covenants that impose operating restrictions, including restrictions on our ability to engage in acts that may be in our best long-term interests. The Term Loan includes covenants that, among other things, restrict our ability to (i) declare dividends or redeem or repurchase equity interests; (ii) incur additional liens; (iii) make loans and investments; (iv) incur additional indebtedness; (v) engage in mergers, acquisitions, and asset sales; (vi) transact with affiliates; (vii) undergo a change in control; (viii) add or change business locations; and (ix) engage in businesses that are not related to our existing business. The Term Loan also requires that we at all times maintain unrestricted cash of not less than $10.0 million, which amount may be increased to $20.0 million if we borrow the second tranche of the Term Loan.
A breach of any of these covenants could result in an event of default under the Loan Agreement. Upon the occurrence of such an event of default, Hercules may, at its discretion, accelerate and demand payment of all or any part of the outstanding advances, together with a prepayment charge, end of term charge, and all interest due and payable under the Term Loan. Hercules also may seek to realize on the collateral we have pledged as security for the Term Loan. If our indebtedness is accelerated, we cannot assure you that we will have sufficient assets to repay the indebtedness. The restrictions and covenants in the Term Loan and any future financing agreements may adversely affect our ability to finance future operations or capital needs or to engage in other business activities.
Future acquisitions of and investments in new businesses could impact our business and financial condition.
From time-to-time, we may acquire or invest in businesses or partnerships that we believe could complement our business and drug candidates. The pursuit of such acquisitions or investments may divert the attention of management and cause us to incur various expenses, regardless of whether the acquisition or investment is ultimately completed. In addition, acquisitions and investments may not perform as expected and we may be unable to realize the expected benefits, synergies, or developments that we may initially anticipate. Further, if we are able to successfully identify and acquire additional businesses, we may not be able to successfully integrate the acquired personnel or operations, or effectively manage the combined business following the acquisition, any of which could harm our business and financial condition.
In addition, to the extent we finance any acquisition or investment in cash, it would reduce our cash reserves, and to the extent the purchase price is paid with shares of our common or preferred stock, it could be dilutive to our current stockholders. To the extent we finance any acquisition or investment with the proceeds from the incurrence of debt, this would increase our level of indebtedness and could negatively affect our liquidity, credit rating and restrict our operations. Moreover, we may face contingent liabilities in connection with any acquisitions or investments.
| 58 |
Risks Related to Intellectual Property
If we fail to adequately protect or enforce our intellectual property rights or secure rights to patents of others, the value of our intellectual property rights would diminish, and our business and competitive position would suffer.
Our success, competitive position and future revenues will depend in part on our ability and the abilities of our licensors and licensees to obtain and maintain patent protection for our products, methods, processes and other technologies, to preserve our trade secrets, to prevent third parties from infringing on our proprietary rights and to operate without infringing the proprietary rights of third parties. We have an active patent protection program that includes filing patent applications on new compounds, formulations, delivery systems and methods of making and using products and prosecuting these patent applications in the U.S. and abroad. As patents issue, we also file continuation applications as appropriate. Although we have taken steps to build what we believe to be a strong patent portfolio, we cannot predict:
| · | the degree and range of protection any patents will afford us against competitors, including whether third parties find ways to invalidate or otherwise circumvent our licensed patents; |
| · | if and when patents will issue in the U.S. or any other country; |
| · | whether or not others will obtain patents claiming aspects similar to those covered by our licensed patents and patent applications; |
| · | whether we will need to initiate litigation or administrative proceedings to protect our intellectual property rights, which may be costly whether we win or lose; |
| · | whether any of our patents will be challenged by our competitors alleging invalidity or unenforceability and, if opposed or litigated, the outcome of any administrative or court action as to patent validity, enforceability or scope; |
| · | whether a competitor will develop a similar compound that is outside the scope of protection afforded by a patent or whether the patent scope is inherent in the claims modified due to interpretation of claim scope by a court; |
| · | whether there were activities previously undertaken by a licensor that could limit the scope, validity or enforceability of licensed patents and intellectual property; or |
| · | whether a competitor will assert infringement of its patents or intellectual property, whether or not meritorious, and what the outcome of any related litigation or challenge may be. |
Our success also depends upon the skills, knowledge and experience of our scientific and technical personnel, our consultants and advisors as well as our licensors, sublicensees and contractors. To help protect our proprietary know-how and our inventions for which patents may be unobtainable or difficult to obtain, we rely on trade secret protection and confidentiality agreements. To this end, we require all employees, consultants and board members to enter into agreements that prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business. These agreements may not provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information. If any of our trade secrets, know-how or other proprietary information is disclosed, the value of our trade secrets, know-how and other proprietary rights would be significantly impaired, and our business and competitive position would suffer.
Due to legal and factual uncertainties regarding the scope and protection afforded by patents and other proprietary rights, we may not have meaningful protection from competition.
Our long-term success will substantially depend upon our ability to protect our proprietary technologies from infringement, misappropriation, discovery and duplication and avoid infringing the proprietary rights of others. Our patent rights, and the patent rights of biopharmaceutical companies in general, are highly uncertain and include complex legal and factual issues. These uncertainties also mean that any patents that we own or may obtain in the future could be subject to challenge, and even if not challenged, may not provide us with meaningful protection from competition. Patents already issued to us or our pending applications may become subject to dispute, and any dispute could be resolved against us.
If some or all of our or any licensor’s patents expire or are invalidated or are found to be unenforceable, or if some or all of our patent applications do not result in issued patents or result in patents with narrow, overbroad, or unenforceable claims, or claims that are not supported in regard to written description or enablement by the specification, or if we are prevented from asserting that the claims of an issued patent cover a product of a third party, we may be subject to competition from third parties with products in the same class of products as our product candidates or products with the same active pharmaceutical ingredients as our product candidates, including in those jurisdictions in which we have no patent protection.
Our commercial success will depend in part on obtaining and maintaining patent and trade secret protection for our product candidates, as well as the methods for treating patients in the product indications using these product candidates. We will be able to protect our product candidates and the methods for treating patients in the applicable product indications using these product candidates from unauthorized use by third parties only to the extent that we or our exclusive licensor owns or controls such valid and enforceable patents or trade secrets.
| 59 |
Even if our product candidates and the methods for treating patients for prescribed indications using these product candidates are covered by valid and enforceable patents and have claims with sufficient scope, disclosure and support in the specification, the patents will provide protection only for a limited amount of time. Our and any licensor’s ability to obtain patents can be highly uncertain and involve complex and in some cases unsettled legal issues and factual questions. Furthermore, different countries have different procedures for obtaining patents, and patents issued in different countries provide different degrees of protection against the use of a patented invention by others. Therefore, if the issuance to us or any licensor, in a given country, of a patent covering an invention is not followed by the issuance, in other countries, of patents covering the same invention, or if any judicial interpretation of the validity, enforceability, or scope of the claims in, or the utility, written description or enablement in, a patent issued in one country is not similar to the interpretation given to the corresponding patent issued in another country, our ability to protect our intellectual property in those countries may be limited. Changes in either patent laws or in interpretations of patent laws in the U.S. and other countries may materially diminish the value of our intellectual property or narrow the scope of our patent protection.
We may be subject to competition from third parties with products in the same class of products as our product candidates, or products with the same active pharmaceutical ingredients as our product candidates in those jurisdictions in which we have no patent protection. Even if patents are issued to us or any licensor regarding our product or methods of using them, those patents can be challenged by our competitors who can argue such patents are invalid or unenforceable on a variety of grounds, including lack of utility, lack sufficient written description or enablement, utility, or that the claims of the issued patents should be limited or narrowly construed. Patents also will not protect our product candidates if competitors devise ways of making or using these products without legally infringing our patents. The current U.S. regulatory environment may have the effect of encouraging companies to challenge branded drug patents or to create non-infringing versions of a patented product in order to facilitate the approval of ANDAs for generic substitutes. These same types of incentives encourage competitors to submit NDAs that rely on literature and clinical data not prepared for or by the drug sponsor, providing another less burdensome pathway to approval.
If we infringe the rights of third parties, we could be prevented from selling products and be forced to defend against litigation and pay damages.
There is a risk that we may be inadvertently infringing the proprietary rights of third parties because numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields that are the focus of our development and manufacturing efforts. Others might have been the first to make the inventions covered by each of our or any licensor’s pending patent applications and issued patents and/or might have been the first to file patent applications for these inventions. In addition, because patent applications take many months to publish and patent applications can take many years to issue, there may be currently pending applications, unknown to us or any licensor, which may later result in issued patents that cover the production, manufacture, synthesis, commercialization, formulation or use of our product candidates. In addition, the production, manufacture, synthesis, commercialization, formulation or use of our product candidates may infringe existing patents of which we are not aware. Defending ourselves against third-party claims, including litigation in particular, would be costly and time consuming and would divert management’s attention from our business, which could lead to delays in our development or commercialization efforts. If third parties are successful in their claims, we might have to pay substantial damages or take other actions that are adverse to our business.
If our products, methods, processes and other technologies infringe the proprietary rights of other parties, we could incur substantial costs and may have to:
| · | obtain licenses, which may not be available on commercially reasonable terms, if at all; |
| · | redesign our products or processes to avoid infringement, which may not be possible or could require substantial funds and time; |
| · | stop using the subject matter claimed in patents held by others, which could cause us to lose the use of one or more of our drug candidates; |
| · | pay damages royalties, or other amounts; or |
| · | grant a cross license to our patents to another patent holder. |
We expect that, as our drug candidates move further into clinical trials and commercialization and our public profile is raised, we will be more likely to be subject to such claims.
| 60 |
We may fail to comply with any of our obligations under existing agreements pursuant to which we license or have otherwise acquired rights or technology, which could result in the loss of rights or technology that are material to our business.
We are a party to technology licenses and have acquired certain assets and rights that are important to our business and we may enter into additional licenses or acquire additional assets and rights in the future. We currently hold licenses from Ludwig Institute for Cancer Research (“LICR”), BioWa, Inc. (“BioWa”), Lonza Sales AG (“Lonza”) Mayo Foundation (“Mayo”) and the University of Zurich (“UZH”). These licenses impose various commercial, contingent payments, royalty, insurance, indemnification, and other obligations on us. If we fail to comply with these obligations, the licensor may have the right to terminate the license or take back rights or assets, in which event we would lose valuable rights under our collaboration agreements, potential claims and our ability to develop product candidates.
We may be subject to claims that our consultants or independent contractors have wrongfully used or disclosed alleged trade secrets of their other clients or former employers to us.
As is common in the biotechnology and pharmaceutical industry, we engage the services of consultants to assist us in the development of our product candidates. Many of these consultants were previously employed at or may have previously or may be currently providing consulting services to, other biotechnology or pharmaceutical companies including our competitors or potential competitors. We may become subject to claims that our company or a consultant inadvertently or otherwise used or disclosed trade secrets or other information proprietary to their former employers or their former or current clients. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management team.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and we intend to seek patent protection only in selected countries. Our intellectual property rights in some countries outside the U.S. can be less extensive than those in the U.S. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the U.S. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S., or from selling or importing products made using our inventions in and into the U.S. or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the U.S. These products may compete with our product candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Our Common Stock
A significant portion of our total outstanding shares are eligible to be sold into the market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market or the perception in the market that the holders of a large number of shares intend to sell shares, could depress the market price of our common stock and could impair our future ability to obtain capital, especially through an offering of equity securities. In addition, holders of a substantial number of shares of our common stock have rights, subject to certain conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders.
| 61 |
We also have registered all shares of common stock that we may issue under our equity compensation plans or that are issuable upon exercise of outstanding options. These shares can be freely sold in the public market upon issuance and once vested, subject to volume limitations applicable to affiliates. If any of these additional shares are sold, or if it is perceived that they will be sold, in the public market, the market price of our common stock could decline.
We have also entered into a Controlled Equity OfferingSM Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co., under which we may issue and sell from time-to-time shares of common stock having an aggregate gross sales price of up to $100 million through Cantor Fitzgerald, as sales agent. Through March 31, 2021, we issued and sold 1,796,858 shares of its common stock under the Sales Agreement for net proceeds of $36.1 million. While we have committed that we will not make any sales of our securities pursuant to the Sales Agreement, unless and until a new prospectus supplement or a new registration statement is filed, the Sales Agreement remains in full force and effect. Sales of a substantial number of shares under the Sales Agreement, or the perception that those sales may occur, could cause the market price of our common stock to decline.
Further, certain shares of our common stock that are currently outstanding but have not been registered for resale may currently be sold under Rule 144 under the Securities Act or the Securities Act. Sales of a substantial number of these shares in the public market, or the perception that those sales may occur, could cause the market price of our common stock to decline.
Our executive officers, directors and principal stockholders, if they choose to act together, will continue to have the ability to significantly influence all matters submitted to stockholders for approval, and this concentration of ownership may contribute to volatility in our stock price.
We have a relatively small public float due to the ownership percentage of our executive officers and directors, and greater than 5% stockholders. Our directors, executive officers, and the other holders of more than 5% of our common stock together with their affiliates beneficially owned approximately 27.3% of our common stock as of April 16, 2021.
Some of these persons or entities may have interests that are different from our other stockholders, which could prevent or discourage unsolicited acquisition proposals or offers for our common stock that may be in the best interest of our other stockholders. This may also adversely affect the trading price of our common stock because investors may perceive disadvantages in owning stock in companies with a significant concentration of ownership.
As a result of our small public float, our common stock may be less liquid, experience reduced daily trading volume and have greater stock price volatility than the common stock of companies with broader public ownership. In addition, the trading of a relatively small volume of shares of our common stock may result in significant volatility in our stock price. If and to the extent ownership of our common stock becomes more concentrated, whether due to increased ownership by our directors and executive officers or other principal stockholders, or other factors, our public float would further decrease, which in turn would likely result in increased stock price volatility.
Additionally, because a large amount of our stock is closely held, we may experience low trading volume or large fluctuations in share price and volume due to sales by our principal stockholders. If our existing stockholders, particularly our directors, executive officers and the holders of more than 5% of our common stock, or their affiliates or associates, sell substantial amounts of our common stock in the public market, or are perceived by the public market as intending to sell substantial amounts of our common stock, the trading price of our common stock could decline significantly.
Despite our listing on the Nasdaq Capital Market, there can be no assurance that an active trading market for our common stock will develop or be sustained, and the Nasdaq Capital Market may subsequently delist our common stock if we fail to comply with ongoing listing standards.
On September 18, 2020, our common stock commenced trading on the Nasdaq Capital Market under the symbol “HGEN.” The Nasdaq Capital Market’s rules for listed companies will require us to meet certain financial, public float, bid price and liquidity standards on an ongoing basis in order to continue the listing of our common stock. To satisfy the initial listing standards for the Nasdaq Capital Market, we have had to execute a reverse stock split. In addition to specific listing and maintenance standards, the Nasdaq Capital Market has broad discretionary authority over the continued listing of securities, which it could exercise with respect to the listing of our common stock.
| 62 |
As a listed company, we are required to meet the continued listing requirements applicable to all Nasdaq Capital Market companies. If we fail to meet those standards, as applied by Nasdaq in its discretion, our common stock may be subject to delisting. We intend to take all commercially reasonable actions to maintain our Nasdaq listing. If our common stock is delisted in the future, it is not likely that we will be able to list our common stock on another national securities exchange and, as a result, we expect our securities would be quoted on an over-the-counter market; however, if this were to occur, our stockholders could face significant material adverse consequences, including limited availability of market quotations for our common stock and reduced liquidity for the trading of our securities. In addition, in the event of such delisting, we could experience a decreased ability to issue additional securities and obtain additional financing in the future.
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Because our common stock is listed on Nasdaq, shares of our common stock qualify as covered securities under the statute. Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. Further, if we were no longer listed on Nasdaq, our securities would not qualify as covered securities under the statute, and we would be subject to regulation in each state in which we offer our securities.
Further, there can be no assurance that an active trading market for our common stock will be sustained despite our listing on the Nasdaq Capital Market.
Raising additional funds by issuing securities or through licensing or lending arrangements may cause dilution to our existing stockholders, restrict our operations or require us to relinquish proprietary rights.
To the extent that we raise additional capital by issuing equity securities, the share ownership of existing stockholders will be diluted. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance could result in further dilution to our stockholders by causing a reduction in their proportionate ownership and voting power.
Any future debt financing may involve covenants that restrict our operations, including, among other restrictions, limitations on our ability to incur liens or additional debt, pay dividends, redeem our stock, make certain investments, and engage in certain merger, consolidation, or asset sale transactions. In addition, if we raise additional funds through licensing arrangements, it may be necessary to grant potentially valuable rights to our product candidates or grant licenses on terms that are not favorable to us.
Any material weaknesses in our internal control over financing reporting that we may identify in the future could adversely affect investor confidence, impair the value of our common stock and increase our cost of raising capital.
If we were to identify any material weaknesses or significant deficiencies in our internal controls over financial reporting in the future, our operating results might be harmed, we may fail to meet our reporting obligations or fail to prevent or detect material misstatements in our financial statements. Any such failure could, in turn, affect the future ability of our management to certify that internal control over our financial reporting is effective. Inferior internal control over financial reporting could also subject us to the scrutiny of the SEC and other regulatory bodies which could cause investors to lose confidence in our reported financial information and could subject us to civil or criminal penalties or stockholder litigation, which could have an adverse effect on our results of operations and the market price of our common stock.
In addition, if we or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting, the disclosure of that fact, even if quickly remedied, could reduce the market’s confidence in our financial statements and harm our share price. Furthermore, deficiencies could result in future non-compliance with Section 404 of the Sarbanes-Oxley Act of 2002. Such non-compliance could subject us to a variety of administrative sanctions, including review by the SEC or other regulatory authorities.
Our stock price is volatile and purchasers of our common stock could incur substantial losses.
The market price of our common stock may fluctuate significantly in response to a number of factors. These factors include those discussed in this “Risk Factors” section of this Quarterly Report and others such as:
| · | the U.S. Government ending the National Emergency declared for the COVID-19 pandemic; |
| · | delay or failure in initiating or completing preclinical studies or clinical trials, or unsatisfactory results of these trials and the resulting impact on ongoing product development; |
| · | the success, progress, timing and costs of our efforts to evaluate or consummate various strategic alternatives if in the best interests of our stockholders; |
| 63 |
| · | our ability to maintain the listing of our common stock on the Nasdaq Capital Market; |
| · | announcements regarding equity or debt financing transactions; |
| · | sales or potential sales of substantial amounts of our common stock or securities convertible into our common stock; |
| · | announcements or social media comments about us or about our competitors including clinical trial results, regulatory approvals, or new product candidate introductions; |
| · | developments concerning our development partner, licensors or product candidate manufacturers; |
| · | litigation and other developments relating to our patents or other proprietary rights or those of our competitors; |
| · | conditions in the pharmaceutical or biotechnology industries and the economy as a whole; |
| · | governmental regulation and legislation; |
| · | recruitment or departure of members of our board of directors, management team or other key personnel; |
| · | changes in our operating results; |
| · | any financial projections we may provide to the public, any changes in these projections, our failure to meet these projections, or changes in recommendations by any securities analysts that elect to follow our common stock; |
| · | change in securities analysts’ estimates of our performance, or our failure to meet analysts’ expectations; and |
| · | price and volume fluctuations in the overall stock market or resulting from inconsistent trading volume levels of our shares. |
In recent years, the stock market in general, and the market for pharmaceutical and biotechnological companies in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to changes in the operating performance of the companies whose stock is experiencing those price and volume fluctuations. Broad market and industry factors may seriously affect the market price of our common stock, regardless of our actual operating performance.
In addition, public statements by us, government agencies, traditional and social media or others relating to the COVID-19 pandemic (including currently authorized or approved vaccines and therapeutics and efforts to develop additional COVID-19 vaccines, treatments or therapies) have in the past resulted, and may in the future result, in significant fluctuations in our stock price. Given the global focus on the COVID-19 pandemic, any information in the public arena on this topic, whether or not accurate, could have an outsized impact (either positive or negative) on our stock price. Information related to progress towards our EUA or BLA, the ACTIV-5/BET-B trial or any other studies, or our development, manufacturing and distribution efforts with respect to lenzilumab, or information regarding such efforts by competitors, may also impact our stock price.
We have never paid and do not intend to pay cash dividends and, consequently, the ability to achieve a return on any investment in our common stock will depend on appreciation in the price of our common stock.
We have never paid cash dividends on any of our capital stock, and we currently intend to retain future earnings, if any, to fund the development and growth of our business. Therefore, a holder of our stock is not likely to receive any dividends on our common stock for the foreseeable future. Since we do not intend to pay dividends, the ability to receive a return on an investment in our common stock will depend on any future appreciation in the market value of our common stock. There is no guarantee that our common stock will appreciate or even maintain the price at which it was purchased.
Anti-takeover provisions in our charter documents and Delaware law, could discourage, delay, or prevent a change in control of our company and may affect the trading price of our common stock.
We are a Delaware corporation, and the anti-takeover provisions of the Delaware General Corporation Law may discourage, delay, or prevent a change in control by prohibiting us from engaging in a business combination with an interested stockholder for a period of three years after the person becomes an interested stockholder, even if a change in control would be beneficial to our existing stockholders.
Our Amended and Restated Certificate of Incorporation, as amended (the “Charter”), and our Second Amended and Restated Bylaws (the “Bylaws”) may discourage, delay, or prevent a change in our management or control over us that stockholders may consider favorable. Our Charter and Bylaws:
| · | provide that vacancies on our board of directors, including newly created directorships, may be filled only by a majority vote of directors then in office; |
| · | do not provide stockholders with the ability to cumulate their votes; and |
| · | require advance notification of stockholder nominations and proposals. |
| 64 |
In addition, our Charter permits the Board to issue up to 25 million shares of preferred stock with such powers, rights, terms and conditions as may be designated by the Board upon the issuance of shares of preferred stock at one or more times in the future. Specifically, the Charter permits the Board to approve the future issuance of all or any shares of the preferred stock in one or more series, to determine the number of shares constituting any series and to determine any voting powers, conversion rights, dividend rights, and other designations, preferences, limitations, restrictions and rights relating to such shares without any further authorization by our stockholders. The Board’s power to issue preferred stock could have the effect of delaying, deterring or preventing a transaction or a change in control of our company that might otherwise be in the best interest of our stockholders.
General Risk Factors
Our internal computer systems, or those of our third-party vendors, collaborators or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs, compromise sensitive information related to our business or prevent us from accessing critical information, potentially exposing us to liability or otherwise adversely affecting our business.
Our internal computer systems and those of our current and any future third-party vendors, collaborators and other contractors or consultants are vulnerable to damage or interruption from computer viruses, computer hackers, malicious code, employee theft or misuse, denial-of-service attacks, sophisticated nation-state and nation-state-supported actors, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we seek to protect our information technology systems from system failure, accident and security breach, if such an event were to occur and cause interruptions in our operations, it could result in a disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other proprietary information or other disruptions. For example, the loss of clinical trial data from clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. If we were to experience a significant cybersecurity breach of our information systems or data, the costs associated with the investigation, remediation and potential notification of the breach to counterparties and data subjects could be material. In addition, our remediation efforts may not be successful. If we do not allocate and effectively manage the resources necessary to build and sustain the proper technology and cybersecurity infrastructure, we could suffer significant business disruption, including transaction errors, supply chain or manufacturing interruptions, processing inefficiencies, data loss or the loss of or damage to intellectual property or other proprietary information.
To the extent that any disruption or security breach were to result in a loss of, or damage to, our or our third-party vendors’, collaborators’ or other contractors’ or consultants’ data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability including litigation exposure, penalties and fines, we could become the subject of regulatory action or investigation, our competitive position could be harmed and the further development and commercialization of our product candidates could be delayed. Any of the above could have a material adverse effect on our business, financial condition, results of operations or prospects.
Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. Some of the policies we currently maintain include general liability, employment practices liability, property, inventory and cargo, auto, workers’ compensation, products liability, and directors’ and officers’ insurance. We do not know, however, if we will be able to maintain existing insurance with adequate levels of coverage. Any significant, uninsured liability may require us to pay substantial amounts, which would adversely affect our working capital and results of operations.
If securities analysts do not publish research or publish unfavorable research about our business, our stock price and trading volume could decline.
The trading market for a company’s common stock often is based in part on the research and reports that securities and industry analysts publish about the company. If additional analyst coverage does develop, and an analyst downgrades our stock or publishes unfavorable research about our business, or if our clinical trials or operating results fail to meet the analysts’ expectations, our stock price would likely decline.
| 65 |
Changes in laws or regulations relating to data privacy and security, or any actual or perceived failure by us to comply with such laws and regulations, or contractual or other obligations relating to data privacy and security, could have a material adverse effect on our reputation, results of operations, financial condition and cash flows.
We are, and may increasingly become, subject to various laws and regulations, as well as contractual obligations, relating to data privacy and security in the jurisdictions in which we operate. The regulatory environment related to data privacy and security is increasingly rigorous, with new and constantly changing requirements applicable to our business, and enforcement practices are likely to remain uncertain for the foreseeable future. These laws and regulations may be interpreted and applied differently over time and from jurisdiction to jurisdiction, and it is possible that they will be interpreted and applied in ways that may have a material adverse effect on our business, financial condition, results of operations or prospects.
In the U.S., various federal and state regulators, including governmental agencies like the Consumer Financial Protection Bureau and the Federal Trade Commission, have adopted, or are considering adopting, laws and regulations concerning personal information and data security. In particular, regulations promulgated pursuant to the Health Insurance Portability and accountability Act of 1996 (“HIPAA”) establish privacy and security standards that limit the use and disclosure of protected health information and require the implementation of safeguards to protect the privacy, integrity and availability of protected health information. Determining whether protected health information has been handled in compliance with applicable privacy standards and our contractual obligations can be complex and may be subject to changing interpretation. If we fail to comply with applicable HIPAA privacy and security standards, we could face civil and criminal penalties. In addition, state attorneys general are authorized to bring civil actions seeking either injunctions or damages in response to violations that threaten the privacy of state residents. We cannot be sure how these regulations will be interpreted, enforced or applied to our operations.
Certain state laws may be more stringent or broader in scope, or offer greater individual rights, with respect to personal information than federal, international, or other state laws, and such laws may differ from each other, all of which may complicate compliance efforts. For example, the California Consumer Privacy Act (“CCPA”), which increases privacy rights for California residents and imposes obligations on companies that process their personal information, came into effect on January 1, 2020. Among other things, the CCPA requires covered companies to provide new disclosures to California consumers and provide such consumers new data protection and privacy rights, including the ability to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the loss of personal information. This private right of action may increase the likelihood of, and risks associated with, data breach litigation.
Internationally, laws, regulations and standards in many jurisdictions apply broadly to the collection, use, retention, security, disclosure, transfer and other processing of personal information. For example, the E.U. General Data Protection Regulation (“GDPR”), which became effective in May 2018, greatly increased the European Commission’s jurisdictional reach of its laws and adds a broad array of requirements for handling personal data. EU member states are tasked under the GDPR to enact, and have enacted, certain implementing legislation that adds to and/or further interprets the GDPR requirements and potentially extends our obligations and potential liability for failing to meet such obligations. The GDPR, together with national legislation, regulations and guidelines of the EU member states governing the processing of personal data, impose strict obligations and restrictions on the ability to collect, use, retain, protect, disclose, transfer and otherwise process personal data. In particular, the GDPR includes obligations and restrictions concerning the consent and rights of individuals to whom the personal data relates, the transfer of personal data out of the European Economic Area, security breach notifications and the security and confidentiality of personal data. The GDPR authorizes fines for certain violations of up to 4% of global annual revenue or €20 million, whichever is greater.
All of these evolving compliance and operational requirements impose significant costs, such as costs related to organizational changes, implementing additional protection technologies, training associates and engaging consultants, which are likely to increase over time. In addition, such requirements may require us to modify our data processing practices and policies, distract management or divert resources from other initiatives and projects, all of which could have a material adverse effect on our results of operations, financial condition and cash flows. Any failure or perceived failure by us to comply with any applicable federal, state or similar foreign laws and regulations relating to data privacy and security could result in damage to our reputation and our relationship with our customers, as well as proceedings or litigation by governmental agencies or customers, including class action privacy litigation in certain jurisdictions, which would subject us to significant fines, sanctions, awards, penalties or judgments, all of which could have a material adverse effect on our business, financial condition, results of operations or prospects.
| 66 |
We review and explore strategic alternatives on an on-going basis, but there can be no assurance that we will be successful in identifying or completing any strategic alternative or that any such strategic alternative will yield additional value for our stockholders.
We regularly review strategic alternatives to ensure our current structure optimizes our ability to execute our strategic plan and to maximize stockholder value. The review of strategic alternatives could result in, among other things, a sale, merger, consolidation or business combination, asset divestiture, partnering, licensing or other collaboration agreements, or potential acquisitions or recapitalizations, in one or more transactions, or continuing to operate with our current business plan and strategy. There can be no assurance that the exploration of strategic alternatives will result in the identification or consummation of any transaction.
In addition, we may incur substantial expenses associated with identifying and evaluating potential strategic alternatives. The process of exploring strategic alternatives may be time consuming and disruptive to our business operations and if we are unable to effectively manage the process, our business, financial condition and results of operations could be adversely affected. We also cannot assure that any potential transaction or other strategic alternative, if identified, evaluated and consummated, will provide greater value to our stockholders than that reflected in our current stock price. Any potential transaction would be dependent upon a number of factors that may be beyond our control, including, among other factors, market conditions, industry trends, the interest of third parties in our business or product candidates and the availability of financing to potential buyers on reasonable terms.
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds |
Reference is made to the disclosures in Note 5 to the Condensed Consolidated Financial Statements appearing in Part 1, Item 1, of this Quarterly Report on Form 10-Q regarding the portion of the principal amount of the Term Loan with Hercules that may be converted into shares of our common stock. The transaction with Hercules was exempt from registration under the Securities Act by virtue of Section 4(a)(2) thereof. Any shares of common stock that may be issued upon such conversion also would be exempt under Section 4(a)(2) or Section 3(a)(9) of the Securities Act.
| Item 3. | Defaults Upon Senior Securities |
None.
| Item 4. | Mine Safety Disclosures |
Not applicable.
| Item 5. | Other Information |
None.
| 67 |
| Item 6. | Exhibits. |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
§ Schedules omitted pursuant to Item 601(a)(5) of Regulation S-K. The Registrant agrees to furnish supplementally a copy of any omitted schedule upon request by the SEC.
† Certain portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K. The Company will furnish supplementally an unredacted copy of such exhibit to the U.S. Securities and Exchange Commission or its staff upon request.
***The certifications attached as Exhibits 32.1 and 32.2 that accompanies this Quarterly Report on Form 10-Q are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Quarterly Report on Form 10-Q, irrespective of any general incorporation language contained in such filing.
| 68 |
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| HUMANIGEN, INC. | |||
| Date: May 13, 2021 | By: | /s/ Cameron Durrant | |
| Cameron Durrant | |||
| Chief Executive Officer | |||
| (Principal Executive Officer) | |||
| Date: May 13, 2021 | By: | /s/ Timothy Morris | |
| Timothy Morris | |||
| Chief Operating Officer and Chief Financial Officer | |||
| (Principal Accounting and Financial Officer) | |||
69