Exhibit 99.1
| Universal Biosensors Inc ARBN 121 559 993
1 Corporate Avenue Rowville VIC 3178 Australia
Telephone +61 3 9213 9000 Facsimile +61 3 9213 9099 Email info@universalbiosensors.com www.universalbiosensors.com |

|
8 March 2019
Universal Biosensors, Inc. (UBI) is pleased to provide an update on its business activities and a review of its fiscal year ended 31 December 2018 (FY18) results. All figures contained in this announcement are reported in A$, unless otherwise stated.
| 1. | Coagulation Activities |
UBI is undertaking several initiatives to maximise the value of its coagulation intellectual property.
Siemens Term Sheet Agreement and Negotiations
On 8 February 2019, UBI and Siemens Healthcare Diagnostics Inc. entered into a term sheet agreement, to negotiate possible modifications to the parties’ commercial relationship in good faith1. The term sheet provides for a negotiation period ending on 8 June 2019. UBI is unable to determine the likely outcome of the negotiations and will provide an update once these are concluded.
UBI continues to provide in-market support of Siemen’s Xprecia Stride™ Coagulation Analyser.
Scaling back of Research and Development Spending
All proprietary coagulation product research and development spending was suspended in 4Q FY18. Research and development obligations relating to Siemens have been scaled back.
Partner Discussions
Discussions have commenced with parties to explore coagulation product development and manufacturing outsourcing opportunities.
| 2. | Receipt of Lump Sum Service Fee |
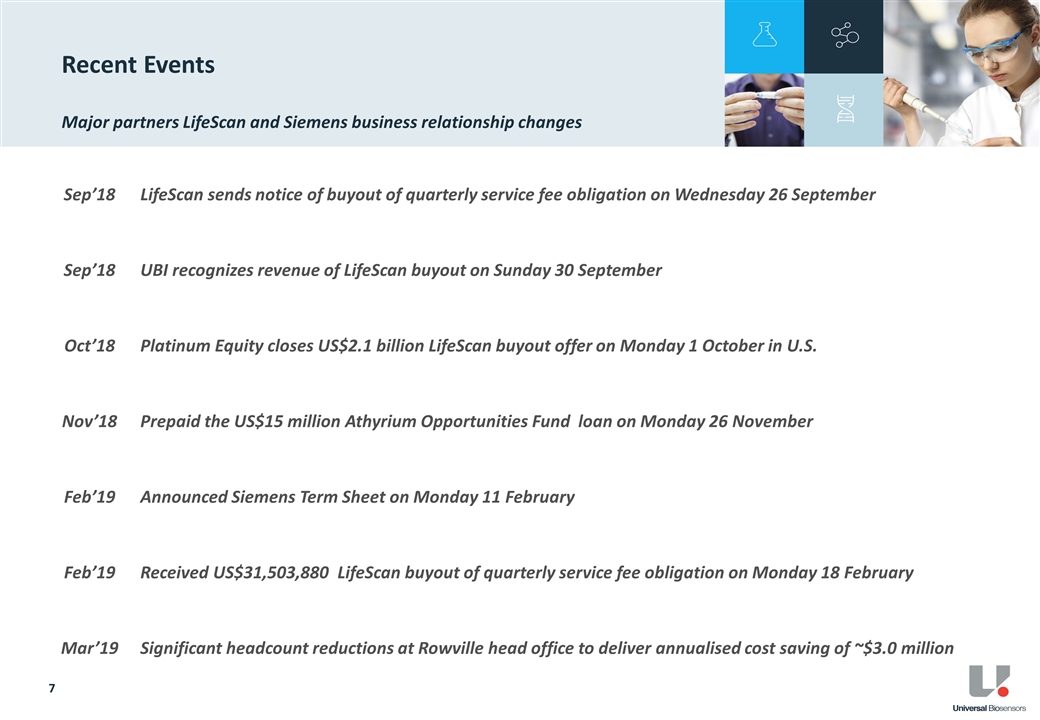
On 18 February 2019, UBI received a US$31,503,880 lump sum service fee from LifeScan. UBI will no longer receive any quarterly service fees from LifeScan beyond 2018.
| 3. | Cost Reductions |
On 6 March 2019, UBI ceased employment of approximately one third of its workforce in Rowville, Melbourne. This cost reduction initiative is expected to deliver an annualised saving of approximately $3.0 million.
| 1 | Please refer to the company’s Term Sheet Agreement and Appendix 4E and Annual Report on Form 10-K FY2018 announcements released to the ASX platform on 11 and 22 February 2019 respectively for further details |
| Universal Biosensors Inc ARBN 121 559 993
1 Corporate Avenue Rowville VIC 3178 Australia
Telephone +61 3 9213 9000 Facsimile +61 3 9213 9099 Email info@universalbiosensors.com www.universalbiosensors.com |
|
| 4. | Hemostasis Reference Laboratory (HRL) |
HRL continues to provide internal and third-party laboratory testing services for quality assurance and accreditation purposes. Management has successfully transitioned HRL from a ‘cost center’ to near-breakeven in FY18. HRL is expected to remain at breakeven operating performance during FY19, as a minimum.
| 5. | Cash Position |
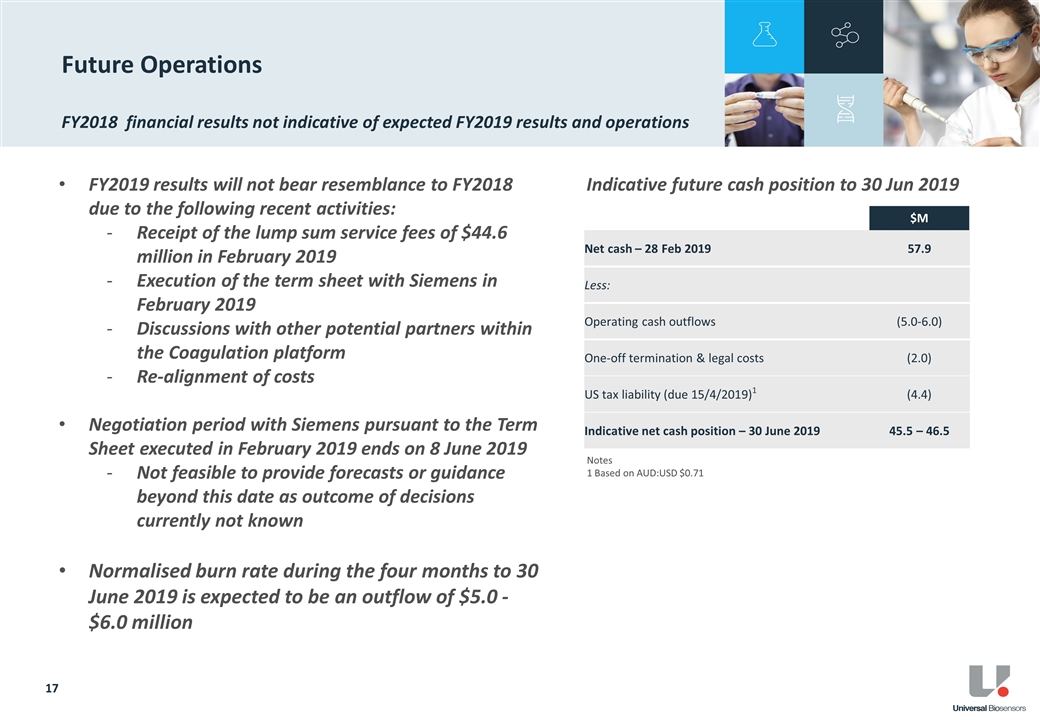
UBI’s net cash position as at 28 February 2019 was $57.9 million. An estimate of key cash flow movements to 30 June 2019 is provided below and excludes any outcome of the Siemens negotiations.
| Net Cash Position - 28 February 2019 |
$ | 57.9m | ||
| Less: |
||||
| Operating Cash Outflows |
$ | 5.0-6.0m | ||
| One-off Termination and Legal Costs |
$ | 2.0m | ||
| US Tax Liability due 15 April 2019 |
$ | 4.4m | 2 | |
|
|
|
|||
| Indicative Net Cash Position - 30 June 2019 |
$ | 45.5-46.5m | ||
|
|
|
UBI intends to provide an update on its cash deployment plan following conclusion of the Siemens negotiations.
| 6. | FY18 Result Commentary |
A review of the FY18 result is provided below. Management cautions against extrapolation of FY18 results into future periods due to the significant initiatives implemented during and since FY18 (outlined prior).
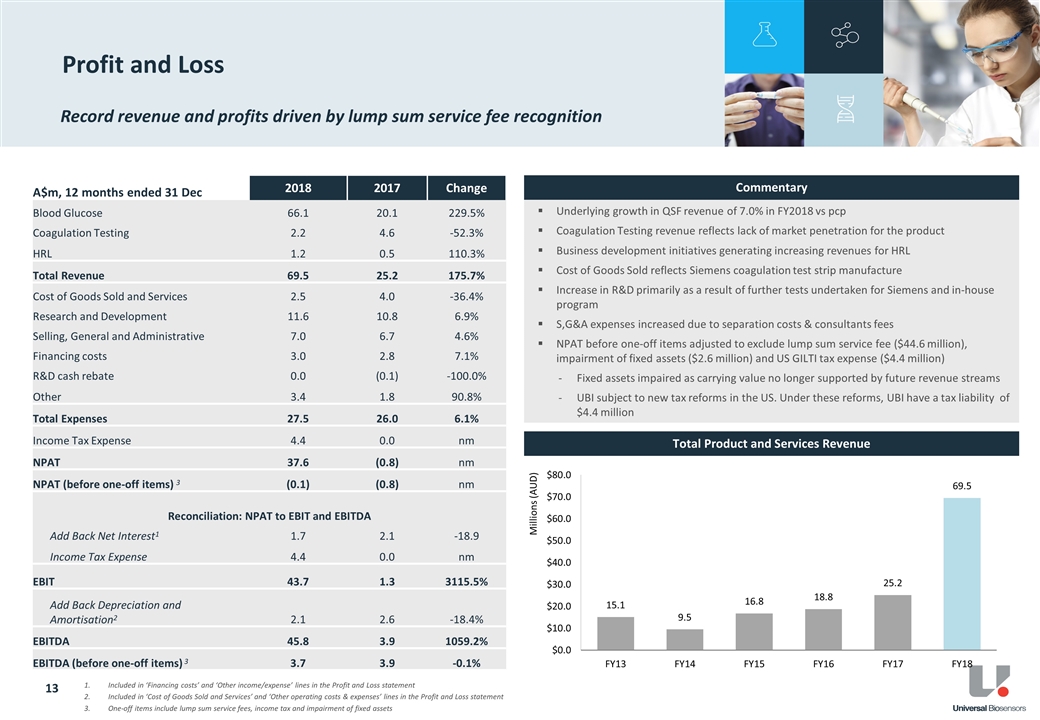
Revenue
Total revenue for FY18 was $69.5 million, an increase of 176% from $25.2 million in the prior comparable period (pcp). Total revenue for the period included the recognition of one-off revenue of $44.6 million from the lump sum service fee payable to UBI under the terms of the Master Services & Supply Agreement with LifeScan3. During 2018, LifeScan gave notice and exercised its right to convert its obligation to pay future Quarterly Service Fees (QSF) to UBI. No further QSF will be received by UBI beyond FY18.
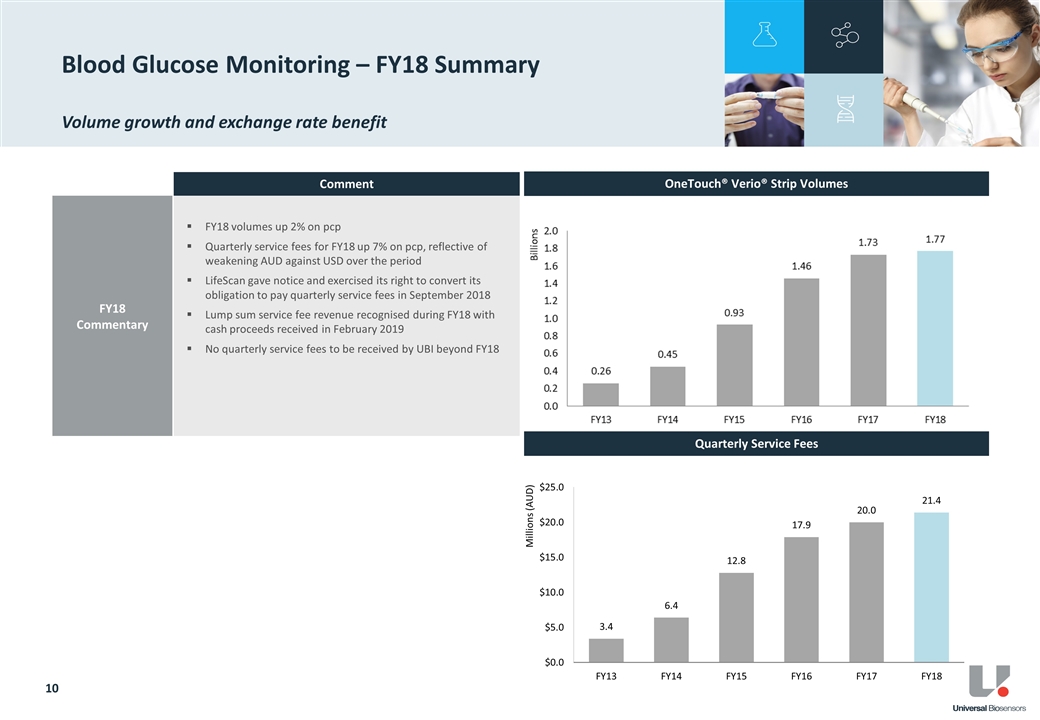
QSF for the supply of blood glucose test strips to LifeScan in FY18 were $21.4 million, a 7% increase above the pcp. This was in-line with management’s previous guidance and growth expectations and driven by both volume growth and the weakening of the AUD against the USD.
| 2 | Based on AUD:USD $0.71. Please refer to the company’s Business Update and Appendix 4E and Annual Report on Form 10-K FY2018 announcements released to the ASX platform on 20 December 2018 and 22 February 2019 respectively for further details |
| 3 | The Master Service & Supply Agreement, executed between UBI and LifeScan (a division of Cilag GmbH International) on 14 May 2009, specifies that the Lump Sum Service Fee is calculated by multiplying the total QSFs for the 2018 LifeScan financial year by two. |
| Universal Biosensors Inc ARBN 121 559 993
1 Corporate Avenue Rowville VIC 3178 Australia
Telephone +61 3 9213 9000 Facsimile +61 3 9213 9099 Email info@universalbiosensors.com www.universalbiosensors.com |
|
Revenue from the sale of Xprecia Stride™ Coagulation Analyser test strips were $1.7 million in FY18 compared to $4.1 million in the pcp, and is reflective of inventory build-up by Siemens during 2017 as it commenced its full commercial launch of the Xprecia Stride™ in various markets, including the US. As previously reported, UBI Management expects that Xprecia Stride™ test strip volume and revenues will remain low and volatile unless and until the Xprecia Stride™ product gains meaningful global market share.
Research & Development
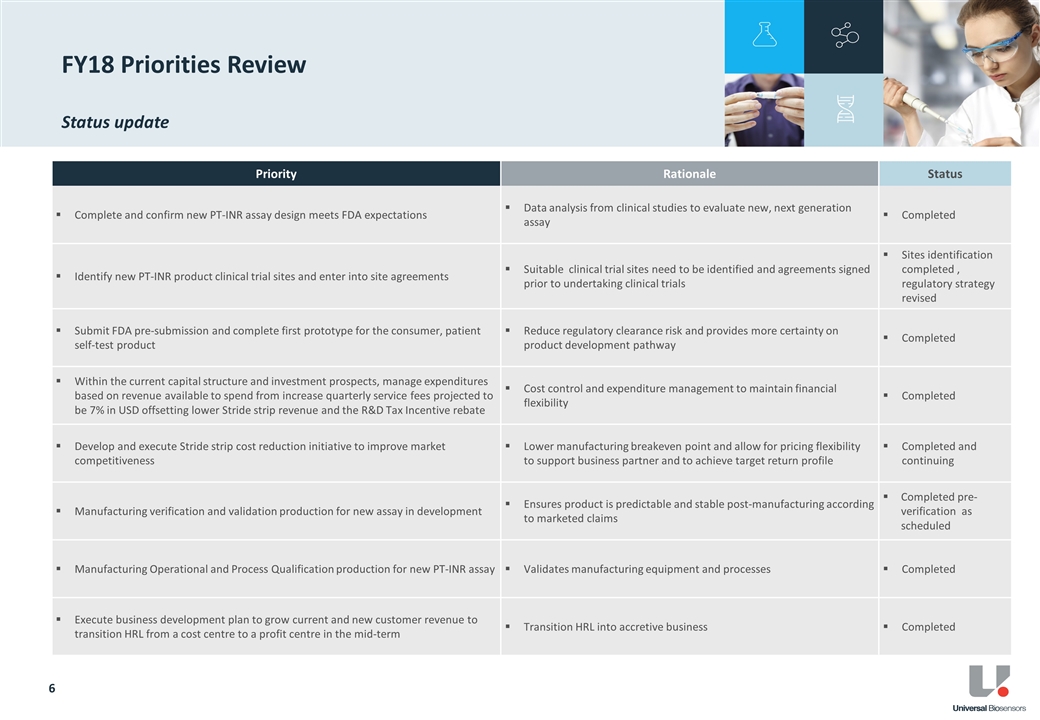
During FY18, UBI continued its research and development program focused on blood coagulation testing technologies. Research and development expense in FY18 was $11.6 million, a 7% increase on pcp ($10.8 million). The increase primarily reflects ramp up of further tests in connection with our agreement with our collaboration partner, Siemens Healthcare Diagnostics Inc. (Siemens) in relation to an alternative coagulation product that is being designed to expand PT-INR functionality and penetration in the Point-of-Care coagulation market. These tests are required as this product progresses toward regulatory clinical trials.
All proprietary coagulation product research and development spending was suspended in 4Q FY18. Research and development obligations relating to Siemens have been scaled back.
Other Expenses and Income
General & Administrative expenses of $7.0 million for FY18 increased 5% compared to the pcp despite management’s on-going cost containment focus and headcount reductions. The increase was primarily due to one-off costs including separation costs related to certain staff departures during the year and legal and specialist consultant fees incurred as part of contract negotiations supporting customer relationship management and partner development.
Management remains committed to controlling expenditures.
EBITDA
FY18 EBITDA was $45.8 million, a significant uplift from $3.9 million in the pcp given the recognition of the one-off LifeScan lump sum service fee. EBITDA in FY2018 excluding the one-off item is $3.7 million (essentially flat compared to pcp).
HRL contributed a small loss of $44k on a turnover of $1.2 million. Management has successfully transitioned HRL from a ‘cost center’ to near-breakeven performance.
Net income for FY2018 was $37.6 million. Excluding one-off items, including LifeScan lump sum service fees ($44.6 million), income tax expense ($4.4 million) and fixed assets impairment ($2.6 million), UBI’s net loss for FY2018 was $0.1 million, an improvement compared to the net loss of $0.8 million in the pcp.
| Universal Biosensors Inc ARBN 121 559 993
1 Corporate Avenue Rowville VIC 3178 Australia
Telephone +61 3 9213 9000 Facsimile +61 3 9213 9099 Email info@universalbiosensors.com www.universalbiosensors.com |
|
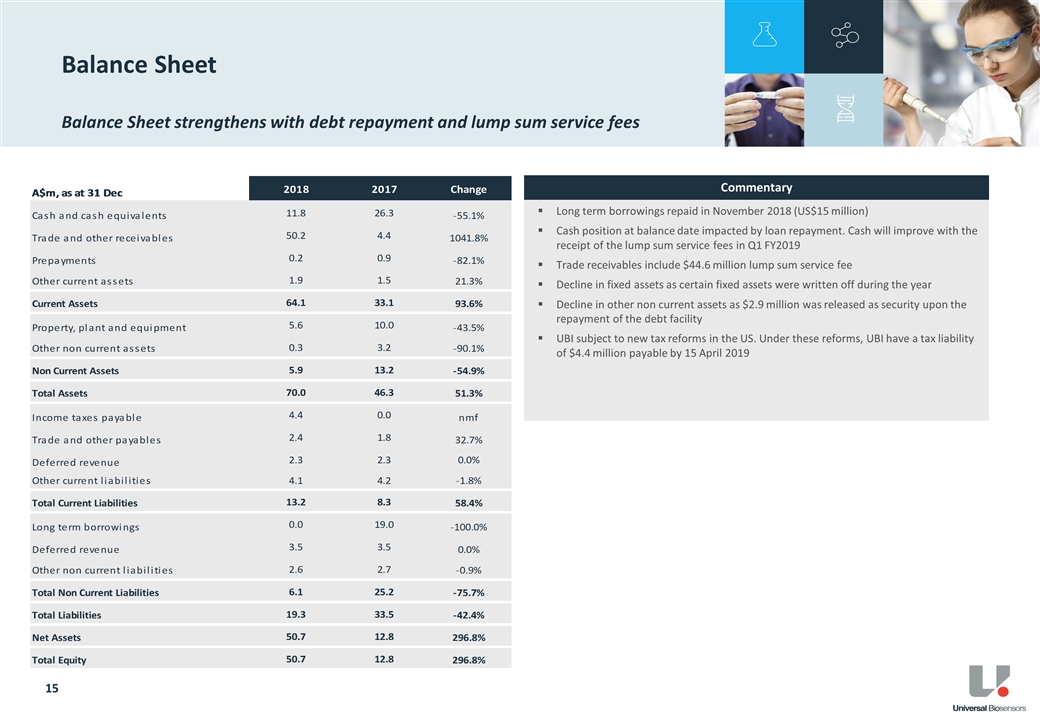
Balance Sheet
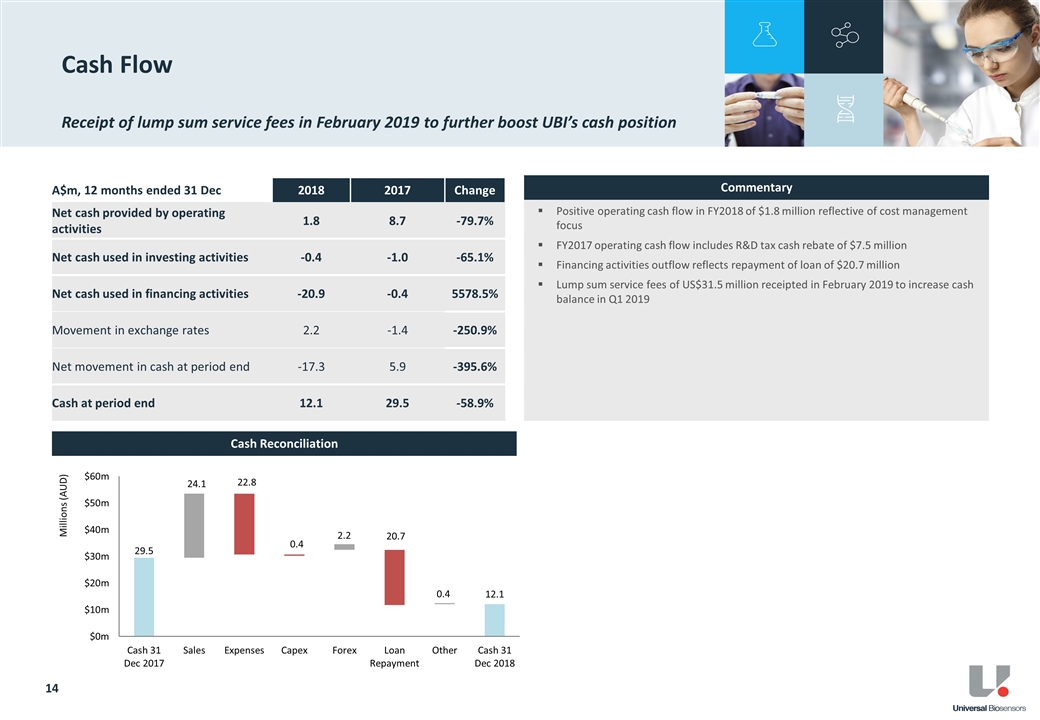
Net cash4 as at 31 December 2018 was $12.1 million, an improvement of $1.7 million from the pcp. UBI extinguished all outstanding secured debt obligations via the repayment of the Athyrium loan of US$15 million in November 2018.
On 18 February 2019, UBI received a US$31,503,880 lump sum service fee from LifeScan. UBI will no longer receive any quarterly service fees from LifeScan beyond 2018.
As previously disclosed5, UBI has become subject to new US tax reform rules addressing Global Intangible Low-Taxed Income. Under these reforms UBI have a US tax liability of US$3.1 million payable by 15 April 2019.
| 7. | Outlook |
UBI intends to provide an update on its continuing business activities (including progress on coagulation product partner discussions) and future cash deployment plan following conclusion of the Siemens negotiations.
—Ends—
| Enquiries: Rick Legleiter Salesh Balak +61 3 9213 9000 |
Investor: Kyahn Williamson +61 3 8866 1214 |
About Universal Biosensors
For additional information in relation to Universal Biosensors, refer to
http://www.universalbiosensors.com/announcements.html.
Universal Biosensors is a specialist medical diagnostics company, founded in 2001, that is focused on the development, manufacture and commercialisation of a range of in vitro diagnostic tests for point-of-care use. These tests capitalise on a technology platform which uses a novel electrochemical cell that can be adapted for multiple analytes and provide for enhanced measurements in whole blood.
| 4 | Cash and restricted cash less debt |
| 5 | Please refer to the company’s Business Update and Appendix 4E and Annual Report on Form 10-K FY2018 announcements released to the ASX platform on 20 December 2018 and 22 February 2019 respectively for further details |
| Universal Biosensors Inc ARBN 121 559 993
1 Corporate Avenue Rowville VIC 3178 Australia
Telephone +61 3 9213 9000 Facsimile +61 3 9213 9099 Email info@universalbiosensors.com www.universalbiosensors.com |
|
Forward-Looking Statements
The statements contained in this release that are not purely historical are forward-looking statements within the meaning of the Exchange Act. Forward-looking statements in this release include statements regarding our expectations, beliefs, hopes, intentions or strategies regarding the proposed offering. All forward-looking statements included in this release are based upon information available to us as of the date hereof, and we assume no obligation to update any such forward-looking statement as a result of new information, future events or otherwise. Our actual results could differ materially from our current expectations. We cannot assure you when, if at all, the proposed offering will occur, and the terms of any such offering are subject to change. Factors that could cause or contribute to such differences include, but are not limited to, factors and risks disclosed from time to time in reports filed with the SEC.