Table of Contents
As filed with the Securities and Exchange Commission on June 4, 2014
Registration No. 333-194606
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 3
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
GlobeImmune, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 84-1353925 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial |
(I.R.S. Employer |
1450 Infinite Drive
Louisville, CO 80027
(303) 625-2700
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Timothy C. Rodell, M.D.
Chief Executive Officer and President
GlobeImmune, Inc.
1450 Infinite Drive
Louisville, CO 80027
(303) 625-2700
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| Copies to: | ||
| Brent D. Fassett Francis R. Wheeler Matthew P. Dubofsky Cooley LLP 380 Interlocken Crescent, Suite 900 Broomfield, Colorado 80021 Tel: (720) 566-4000 Fax: (720) 566-4099 |
Michael J. Lerner John D. Hogoboom Lowenstein Sandler LLP 1251 Avenue of the Americas New York, New York 10020 Tel: (212) 262-6700 Fax: (973) 597-2300 | |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended (the “Securities Act”) check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 under the Securities Exchange Act of 1934, as amended. (Check one):
| Large Accelerated Filer | ¨ | Accelerated Filer | ¨ | |||
| Non-accelerated Filer | x (Do not check if a smaller reporting company) | Smaller Reporting Company | ¨ | |||
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price(1) |
Amount of Registration Fee(2) | ||
| Common Stock, $0.001 par value per share |
$30,546,875 | $3,935 | ||
| Underwriter’s Warrant to Purchase Common Stock(3) |
— | — | ||
| Common Stock Underlying Underwriter’s Warrant(4) |
$531,250 | $69 | ||
| Total |
$31,078,125 | $4,004(5) | ||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act. Includes the offering price of additional shares that the underwriter has the option to purchase. |
| (2) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price. |
| (3) | No registration fee pursuant to Rule 457(g) under the Securities Act. |
| (4) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act. The warrants are exercisable at a per share exercise price equal to 150% of the public offering price. As estimated solely for the purpose of recalculating the registration fee pursuant to Rule 457(g) under the Securities Act, the proposed maximum aggregate offering price of the underwriter’s warrant is $531,250 (which is equal to 2% of $26,562,500). |
| (5) | A registration fee $5,605 has been previously paid in connection with this Registration Statement based on an estimate of the aggregate offering price. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
| PROSPECTUS | SUBJECT TO COMPLETION, DATED JUNE 4, 2014 | |||
1,562,500 Shares
Common Stock

This is an initial public offering of GlobeImmune, Inc. We are offering 1,562,500 shares of our common stock. We currently estimate that the initial public offering price of our common stock will be between $15.00 and $17.00 per share.
Prior to this offering there has been no public market for our common stock. We have filed an application for our common stock to be listed on The NASDAQ Global Market under the symbol “GBIM”.
We are an “emerging growth company” under the federal securities laws and will be subject to reduced public company reporting requirements. Investing in our common stock involves risks. See “Risk Factors” beginning on page 14.
| Per Share |
Total |
|||||||
| Initial price to public |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds, before expenses, to GlobeImmune, Inc. |
$ | $ | ||||||
| (1) | The underwriter will receive compensation in addition to the underwriting discount. See the section entitled “Underwriting” beginning on page 163 of this prospectus for a description of compensation payable to the underwriter. |
Certain of our existing stockholders including Celgene Corporation, HealthCare Ventures VII, L.P., Lilly Ventures Fund I, LLC, Morgenthaler Partners, VII, L.P., and certain other affiliates of our directors, have indicated an interest in purchasing an aggregate of up to $10 million of shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriter may determine to sell more, less or no shares in this offering to any of these stockholders, or any of these stockholders may determine to purchase more, less or no shares in this offering. The underwriter will receive an underwriting discount of $ per share on any sales of shares to such stockholders.
We have granted the underwriter an option to purchase up to 234,375 additional shares of our common stock to cover over-allotments, if any, exercisable at any time until 45 days after the date of this prospectus. The underwriter will receive compensation in addition to the underwriting discounts and commissions. See “Underwriting” for a description of compensation payable to the underwriter.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriter expects to deliver the shares on or about , 2014.
Aegis Capital Corp.
Prospectus dated , 2014.
Table of Contents
| 1 | ||||
| 14 | ||||
| 53 | ||||
| 54 | ||||
| 54 | ||||
| 55 | ||||
| 58 | ||||
| 61 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
63 | |||
| 91 | ||||
| 121 | ||||
| 127 | ||||
| 145 | ||||
| 147 | ||||
| 151 | ||||
| 157 | ||||
| MATERIAL U.S. FEDERAL TAX CONSIDERATIONS FOR NON-U.S. HOLDERS |
160 | |||
| 164 | ||||
| 171 | ||||
| 171 | ||||
| 171 | ||||
| F-1 |
You should rely only on the information contained in this prospectus and any related free writing prospectus we may authorize to be delivered to you. We have not, and the underwriter has not, authorized any person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. Neither this prospectus nor any related free writing prospectus is an offer to sell, nor are they seeking an offer to buy, these securities in any jurisdiction where the offer or solicitation is not permitted. The information contained in this prospectus is accurate only as of the date on the front cover of this prospectus and the information in any free writing prospectus that we may provide you in connection with this offering is accurate only as of the date of that free writing prospectus, and information may have changed since those dates.
For investors outside the United States: neither we nor the underwriter have done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus and any such free writing prospectus outside of the United States.
i
Table of Contents
The items in the following summary are described in more detail later in this prospectus. This summary does not contain all of the information you should consider. Before investing in our common stock, you should read the entire prospectus carefully, including the “Risk Factors” beginning on page 14 and the financial statements and related notes beginning on page F-1. Unless the context indicates otherwise, as used in this prospectus, the terms “GlobeImmune”, “the Company”, “we”, “us” and “our” refer to GlobeImmune, Inc.
Overview
We are a biopharmaceutical company established in 1995 focused on developing products for the treatment of cancer and infectious diseases based on our proprietary Tarmogen® platform. Tarmogens activate the immune system by stimulating a subset of white blood cells called T cells that destroy infected or malignant cells, in contrast to traditional vaccines which predominately stimulate antibody production. We believe that our Tarmogen platform has applicability to a number of diseases, and may enable us to develop a broad portfolio of products. We have four Tarmogen product candidates in clinical evaluation for infectious disease and multiple cancer indications.
Our Tarmogen platform technology has characteristics that we believe will enable us, in collaboration with our strategic collaborators and independently, to develop and commercialize a portfolio of products. Highlights of the technology include:
| • | Tarmogens activate the cellular immune response: Each Tarmogen product candidate consists of intact, heat-inactivated yeast containing the target protein. We believe our data demonstrate that immunization with a Tarmogen results in T cell immune responses against the target protein and reduction in the number of abnormal cells containing the same target protein. |
| • | Broad applicability: We have five clinical trials evaluating Tarmogen product candidates in oncology and infectious diseases in randomized, controlled Phase 2 clinical trials. We have successfully created Tarmogens that express over 100 different proteins. In eleven Phase 1 and 2 clinical trials, we have administered Tarmogen product candidates to more than 500 patients and healthy volunteers, including some who have received monthly dosing for over five years, with a tolerability profile that we believe will allow our product candidates to be added to other therapeutic regimens without leading to additional toxicity. |
| • | Proven development capability: We have advanced five Tarmogen programs from concept into human clinical trials in approximately six to 18 months. |
| • | Efficient manufacturing: We manufacture Tarmogens through a process that yields a stable, off-the-shelf product that is disease- or protein-specific. We have an approximately 22,000 square foot manufacturing facility that we believe has the capacity for commercial-scale production. |
Tarmogens target the molecular profile that distinguishes a diseased cell from a normal cell. We have designed Tarmogens to target specific intracellular and extracellular proteins, or antigens, that play a role in oncology and infectious diseases which represent unmet medical needs. Collaborations with biopharmaceutical companies and research institutions have allowed us to advance the development of a number of our product candidates while managing our own research and development expenses relating to these product candidates.
We have two strategic collaborations with leading biotechnology companies. In October 2011, Gilead Sciences, Inc., or Gilead, exclusively licensed product candidates intended to treat chronic hepatitis B virus, or HBV, infection. Celgene Corporation, or Celgene, entered into a collaboration and option agreement for certain oncology product candidates in May 2009. Under this agreement, in July 2013 Celgene exercised its option for a
1
Table of Contents
worldwide, exclusive license to the GI-6300 program, which is a Tarmogen program targeting the brachyury protein. Brachyury plays a role in the metastatic progression of certain cancers and is believed to be fundamental in the formation of chordomas, rare bone tumors of the spine. Through March 31, 2014, we have received over $60 million from these collaborations.
The following tables summarize the status of our pipeline of product candidates:
| INFECTIOUS DISEASE PRODUCT CANDIDATES | ||||||||||
| Indication | Target | Stage of Development |
Worldwide Commercial |
Next Development Milestone | ||||||
|
GS-4774 |
Chronic hepatitis B infection |
HBV antigens | Phase 2 | Gilead | Complete Phase 2 | |||||
|
GI-19000 |
Tuberculosis | TB antigens | Preclinical | GlobeImmune | IND | |||||
|
GI-2010 |
Human immunodeficiency virus |
HIV antigens | Preclinical | GlobeImmune | IND | |||||
|
GI-18000 |
Chronic hepatitis D infection |
Delta virus antigens | Preclinical | GlobeImmune | IND | |||||
| ONCOLOGY PRODUCT CANDIDATES | ||||||||||
| Product Candidate |
Indication | Target | Stage of Development |
Worldwide Commercial Rights |
Next Development Milestone | |||||
|
GI-6207 |
Medullary thyroid cancer |
Carcinoembryonic Antigen |
Phase 2 | Celgene Option | Complete Phase 2 | |||||
|
GI-6301 |
Chordoma, breast cancer |
Brachyury | Phase 1 | Celgene License | Complete Phase 1 | |||||
|
GI-4000 |
Resected pancreas cancer |
Mutated Ras | Phase 2b | GlobeImmune | Validate companion diagnostic | |||||
|
GI-4000 |
Non-small cell lung cancer |
Mutated Ras | Phase 2 | GlobeImmune | Initiate Phase 2b | |||||
|
GI-4000 |
Colorectal cancer |
Mutated Ras | Phase 2 | GlobeImmune |
Complete Phase 2a | |||||
Infectious Disease Programs
GI-5005, a Tarmogen product candidate intended to treat chronic hepatitis C infection was our first program to demonstrate clinical proof of concept for the treatment of chronic infectious disease. GI-5005 was evaluated in a Phase 1 trial followed by a randomized Phase 2b trial comparing GI-5005 plus pegylated interferon and ribavirin, or GI-5005 Triple Therapy, vs. the then standard of care regimen, pegylated interferon and ribavirin, or
2
Table of Contents
SOC. Subjects receiving GI-5005 Triple Therapy had statistically significant improved end of treatment viral clearance (43 of 68 subjects, or 63%, vs. 29 of 65 subjects, or 45%; p=0.037) and normalization of alanine aminotransferase, or ALT level, a measure of liver damage, (29 of 61 subjects, or 48%, vs. nine of 44 subjects, or 21%; p=0.007) compared to SOC alone. In treatment naïve subjects, GI-5005 Triple Therapy appeared to improve end of treatment viral clearance rates (37 of 50 subjects, or 74%, vs. 27 of 46 subjects, or 59%; p=0.133, which is not statistically significant), and improved sustained virologic clearance rates six months after completion of therapy (29 of 50 subjects, or 58%, vs. 22 of 46 subjects, or 48%; p=0.413, which is not statistically significant) compared to SOC alone, with a comparably favorable profile observed in patients who had previously failed treatment with SOC. We believe that GI-5005 was the first therapeutic vaccine to demonstrate a clinically meaningful outcome in patients with a chronic infectious disease. While we are no longer actively developing GI-5005 for commercial reasons, we believe that the clinical results generated have validated the Tarmogen platform’s application in infectious diseases.
Our world-wide collaboration with Gilead is focused on developing a lead product candidate, GS-4774, to target patients chronically infected with HBV who are also on, or are candidates for, oral antiviral suppressive therapy. Under this collaboration, in 2011 we received a $10 million upfront payment and Gilead agreed to fund a Phase 1 trial. As a result of our activities under this agreement, we have received an additional $5 million in milestone payments. Gilead is responsible for all future clinical, regulatory and commercial activities. We are eligible to receive up to an additional $130 million in development and regulatory milestones under this collaboration. If products are commercialized, we will be entitled to receive tiered royalty rates based on net sales of GS-4774 from the high single digits to the mid-teens, and up to $40 million of sales milestone payments.
Chronic HBV infection affects approximately 400 million people worldwide. While antiviral drugs have been used effectively to control this disease, cure rates are very low, with less than eight percent cured after four years of daily oral antiviral therapy. GS-4774 is being developed as a therapeutic vaccine designed to generate T cell immune responses against cells containing HBV antigens in combination with antiviral therapy with the goal of increasing the cure rate in patients with chronic HBV infection.
In August 2013, we completed a Phase 1 clinical trial of GS-4774 in 60 healthy volunteers. Twenty subjects were enrolled to one of three arms in the study, receiving either 10YU, 40YU, or 80YU of GS-4774 (one YU, or yeast unit, equals 10 million yeast cells). Within each of the three 20 subject arms, ten subjects were randomized to weekly dosing, and ten to monthly only dosing, each for three months. The Phase 1 results indicated that GS-4774 elicited HBV specific T cell immune responses. Subjects in all three dose groups displayed immune responses, and there was little difference between the weekly versus the monthly-only immunization regimens in the ability to generate T cell immune responses. Eighty-eight percent of subjects across all three dose groups responded to receiving GS-4774 by at least one measure of T cell immune response.
Gilead initiated a Phase 2 clinical trial in September 2013 investigating GS-4774 in combination with ongoing oral antiviral treatment in patients with chronic HBV infection. This Phase 2 clinical trial is designed to enroll 175 patients in a randomized, open-label design comparing different doses of GS-4774, administered in combination with oral antiviral therapy vs. antiviral treatment alone. The primary endpoint for this trial is decline in serum HBV surface antigen, or HBsAg.
We have multiple additional preclinical infectious disease programs in various stages of development. In August 2013, we received a $4 million Research Project Grant from the National Institute of Allergy and Infectious Diseases, or NIAID, of the National Institutes of Health, or NIH, to support the development of Tarmogen immunotherapy product candidates intended to treat or prevent tuberculosis infection. The work for this grant will be performed and reimbursed over four years.
3
Table of Contents
Oncology Programs
In May 2009, we entered into a worldwide strategic collaboration and option agreement with Celgene focused on the discovery, development and commercialization of certain product candidates intended to treat cancer. Under the terms of this agreement we have received $31.3 million. Celgene also made a $10 million equity investment in us. Under this agreement, the GI-6301 and GI-6207 programs may result in up to $290 million in milestone payments from Celgene to us. For product candidates subject to option by Celgene, we are responsible for initial development under the agreement, and Celgene has the option to license each of them at specific points in the development plan. Upon the achievement of certain development, regulatory and commercial milestones, we would be eligible to receive milestone payments and tiered royalties based on net sales of each licensed product.
Pursuant to the agreement, in July 2013 Celgene exercised its option to obtain an exclusive license to our GI-6300 program, including GI-6301, upon payment of a $9 million option exercise milestone. We are eligible to receive a total of $85 million in additional development and regulatory milestone payments for GI-6301. If GI-6301 is commercialized, we may receive up to $60 million in sales milestone payments and tiered royalty rates on net sales ranging from single digits to low double digits. GI-6301 targets cancers expressing the brachyury protein, which is believed to play a role in the metastatic progression of certain cancers and also in the initiation of chordomas. The National Cancer Institute, or NCI, is currently conducting a dose escalation Phase 1 trial of GI-6301 with a planned enrollment of 34 subjects with metastatic cancers and chordomas who have failed previous therapy or have no further therapeutic options. In seven chordoma patients evaluated to date, one subject was determined to be a confirmed partial responder at previously irradiated sites, one subject who had progressive disease at study entry was diagnosed with stable disease and two subjects who were determined to have stable disease at study entry continue to have stable disease. The other three chordoma subjects in the study have been diagnosed with progressive disease. We expect to complete enrollment and report data from the GI-6301 Phase 1 trial in the first half of 2014.
A second oncology product candidate, GI-6207, is being evaluated in a 34 subject Phase 2 clinical trial at the NCI. GI-6207 targets carcinoembryonic antigen, or CEA, a protein that is over-expressed in a large number of epithelial cancers, which we estimate represent approximately 500,000 new cancer cases in the United States each year. This Phase 2 trial is being conducted under an Investigational New Drug Application, or IND, filed by us on December 27, 2012. The NCI has completed a dose escalation Phase 1 clinical trial of GI-6207 in 25 subjects with Stage IV cancers expressing CEA, and initiated a randomized Phase 2 trial in 34 subjects with medullary thyroid cancer, or MTC, in 2013. The Phase 1 trial of G1-6301 is being conducted under an IND filed by us on October 24, 2011. Development and commercialization rights to the GI-6200 program, including GI-6207, remain subject to option by Celgene. Celgene’s decision to option GI-6207 will be after the data from the Phase 2 trial in MTC are available.
We have a third, wholly-owned, clinical stage oncology program, GI-4000, that targets tumors with mutations in a protein called Ras. In March 2013, Celgene declined to exercise its option to GI-4000 and returned all rights and development responsibility to us. We have Phase 2 survival data in pancreas and non-small cell lung cancer, or NSCLC, for GI-4000. We conducted a multicenter, placebo controlled Phase 2b pancreas cancer study. While we did not see an improvement in survival in the overall study population, we did see a non-statistically significant three-month improvement in survival in a pre-specified subgroup. We also performed a retrospective analysis of 90 pre-administration blood samples using an analytic technique called proteomics. The goal of the analysis was to identify a pre-administration companion diagnostic test that could predict which subjects are likely to respond to GI-4000 to assist in subject selection for future clinical trials. BDX-001, the resulting potential proteomic companion diagnostic test, appeared to predict whether a subject administered GI-4000 and the chemotherapy drug gemcitabine in this trial would have improved recurrence free and overall survival compared to gemcitabine alone. We believe BDX-001 differentiates between subject blood samples using the relationship of 100 different proteins and protein fragments. Overall, 21 of the 44 (48%) of studied
4
Table of Contents
subjects administered GI-4000 and gemcitabine were classified as BDX-001 positive. In BDX-001 positive subjects administered GI-4000 and gemcitabine, there was an 11.7 month improvement in median recurrence free survival, or RFS, and a 16.6 month improvement in median overall survival, or OS, compared with BDX-001 positive subjects administered placebo and gemcitabine. There was no difference in RFS or OS in the gemcitabine-alone arm based on BDX-001 selection. The proportion of BDX-001 positive patients may vary in any future studies. This study was not powered for, and these results did not reach, statistical significance. If BDX-001 is prospectively validated in a second pancreas cancer trial, this companion diagnostic could be used to select the patients appropriate for GI-4000 therapy. The BDX-001 test is controlled by Biodesix, Inc. We intend to negotiate a development and commercialization agreement regarding this test with them. However, we may not be able to obtain the rights to use the test on commercially reasonable terms, if at all.
Investigators at Memorial Sloan Kettering Cancer Center, or MSKCC, also conducted a Phase 2a trial in non-small cell lung cancer, or NSCLC, in 24 subjects. Based on the updated survival analysis from December 2013, this study shows a 43% reduction in the risk of mortality for patients administered GI-4000 compared to a matched set of controls (p=0.24, which is not statistically significant). This is an investigator sponsored study that was funded by MSKCC, and we supplied the study drug. We retained all rights to GI-4000 under our agreement with MSKCC for this trial. We also have an ongoing Phase 2a clinical trial studying GI-4000 in colon cancer, which is being conducted at the Lombardi Cancer Center at Georgetown University. This is an investigator sponsored study that was funded by the Lombardi Cancer Center, and we supplied the study drug. We retained all rights to GI-4000 under our agreement with the Lombardi Cancer Center for this trial.
Our Strategy
Our strategy is to develop and commercialize Tarmogen products targeting diseases where lack of effective treatment represents significant unmet medical needs, while leveraging our collaborations to manage our expenses. The key components of our strategy include managing the HBV collaboration with Gilead, advancing our product candidates targeting infectious diseases, advancing the clinical development of oncology product candidates in collaboration with Celgene and the NCI, financing continued development of GI-4000, and retaining certain development and commercialization rights to proprietary product candidates.
We seek to manage our expenses by limiting corporate overhead spending and outsourcing appropriate functions. We intend to continue to use corporate and research collaborations to advance the development of our product candidates. We intend to use the proceeds of this financing to advance our product candidates and increase our working capital.
Expected Milestones
We expect that the following milestones will occur:
| • | Potential milestone payments from Gilead, if Gilead proceeds with a Phase 2b or Phase 3 clinical study of GS-4774; |
| • | One or more IND filings for infectious disease Tarmogens; |
| • | Celgene option decision point data from the GI-6207 Phase 2 trial for MTC; |
| • | Complete enrollment and data from GI-6301 Phase 1 trial by the first half of 2014 (results from Phase 1 trial may support initiation of Phase 2 program); and |
| • | Phase 2 development of GI-4000 in pancreas cancer and NSCLC, subject to entering into a collaboration or receiving additional funding other than this transaction. |
5
Table of Contents
Our Risks
An investment in our common stock involves a high degree of risk. These risks and uncertainties are discussed more fully in the “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” sections of this prospectus. You should carefully consider all of the information set forth in this prospectus and, in particular, should evaluate the specific factors set forth under “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in deciding whether to invest in our common stock. Among these important risks and uncertainties that could adversely affect our results of operations and business condition are the following:
| • | We have not completed clinical development of any of our product candidates and do not have any products approved for sale by the United States Federal Drug Administration, or FDA, or any other regulatory bodies. Regulatory authorities may not approve our product candidates even if they meet safety and efficacy endpoints in clinical trials. |
| • | If we, or our collaborators, Celgene Corporation and Gilead Sciences, Inc., fail to successfully complete clinical trials, fail to obtain regulatory approval or fail to successfully commercialize our Tarmogen product candidates, our business would be harmed and the value of our securities would decline. |
| • | We have incurred net operating losses throughout our history. We expect to continue to incur increasing net losses for the foreseeable future, and we may never achieve or maintain profitability. |
| • | We, or our collaborators, may face delays in completing our clinical trials, and we may not be able to complete them at all. |
| • | Results of earlier studies and clinical trials may not be predictive of future trial results. |
| • | Our Tarmogen product candidates are based on a novel technology, which may raise development issues we may not be able to resolve, regulatory issues that could delay or prevent approval, or personnel issues that may keep us from being able to develop our product candidates. |
| • | If we are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience significant delays in doing so, we may not realize the full commercial potential of our product candidates. |
| • | Competitive products for treatment of pancreas cancer, non-small cell lung cancer, colorectal cancer, MTC, chordoma and chronic hepatitis B infection may reduce or eliminate the commercial opportunity for our Tarmogen product candidates. |
| • | We expect to depend on existing and future collaborations with third parties for the development of some of our product candidates. If those collaborations are not successful, we may not be able to complete the development of these product candidates. |
| • | We will require substantial additional capital in the future. If additional capital is not available, we will have to delay, reduce or cease operations. |
| • | We have limited experience manufacturing our product candidates at commercial scale, and there can be no assurance that our product candidates can be manufactured in compliance with regulations at a cost or in quantities necessary to make them commercially viable. Our manufacturing facility has not been inspected by regulatory agencies and there can be no assurance that it will be acceptable for licensure by regulatory authorities or that we can contract or build acceptable facilities. |
| • | We rely on relationships with third-party contract manufacturers, which limits our ability to control the availability of, and manufacturing costs for, our product candidates. |
| • | If we are unable to protect our proprietary rights or to defend against infringement claims, we may not be able to compete effectively or operate profitably. |
| • | Other factors identified elsewhere in this prospectus, including those set forth under “Risk Factors”. |
6
Table of Contents
Corporate Information
We were incorporated as Ceres Pharmaceuticals, Ltd. in Colorado on February 10, 1995. Our principal offices are located at 1450 Infinite Dr., Louisville CO 80027. We changed our name to GlobeImmune, Inc. on May 26, 2001, and reincorporated in Delaware on June 5, 2002. Our principal executive offices are located at 1450 Infinite Drive, Louisville, CO 80027, and our telephone number is (303) 625-2700. Our website address is www.globeimmune.com. Our website and the information contained on, or that can be accessed through, our website will not be deemed to be incorporated by reference in, and are not considered part of, this prospectus. You should not rely on our website or any such information in making your decision whether to purchase our common stock.
GlobeImmune® , Tarmogen® , Targeted Molecular Immunotherapy® and the GlobeImmune logo are our registered trademarks. This prospectus also includes references to trademarks and tradenames for other entities, and those trademarks and tradenames are the property of their respective owners. Except as set forth above and solely for convenience, the trademarks and tradenames in this prospectus are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
7
Table of Contents
The Offering
| Common stock offered |
1,562,500 shares (1,796,875 shares if the underwriter’s over-allotment option is exercised in full) |
| Common stock to be outstanding after this offering |
5,136,400 shares (5,370,775 shares if the underwriter’s over-allotment option is exercised in full) |
| Use of proceeds |
We intend to use the net proceeds from this offering to advance an additional infectious disease product into clinical trials and through clinical proof of concept; to prepare our manufacturing facility for clinical trial and commercial scale manufacturing; for additional development on our own internal programs and filing of one or more additional IND(s); for completion of ongoing clinical trials for GI-6207, GI-6301 and GI-4000, including manufacturing of drug supply for such trials; and for working capital and other general corporate purposes. See the section entitled “Use of Proceeds”. |
| Risk factors |
An investment in our common stock involves a high degree of risk. You should read the “Risk Factors” section of this prospectus beginning on page for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. |
| Proposed NASDAQ Global Market symbol |
GBIM |
| Proposed purchase by certain current stockholders |
Certain of our existing stockholders including Celgene Corporation, HealthCare Ventures VII, L.P., Lilly Ventures Fund I, LLC, Morgenthaler Partners, VII, L.P., and certain other affiliates of our directors, have indicated an interest in purchasing an aggregate of up to $10 million of shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriter may determine to sell more, less or no shares in this offering to any of these stockholders, or any of these stockholders may determine to purchase more, less or no shares in this offering. The underwriter will receive an underwriting discount of $ per share on any sales of shares to such stockholders. |
Any shares purchased by such stockholders will be subject to lock-up restrictions described in the section entitled “Underwriting—Lock-up Agreements.”
The number of shares of common stock to be outstanding after this offering is based on 3,573,900 shares of common stock outstanding as of March 31, 2014, after giving effect to the conversion of all of our outstanding preferred stock and convertible promissory notes into shares of common stock upon completion of this offering, and excludes:
| • | 235,342 shares of common stock issuable upon the exercise of outstanding options under our 2002 stock incentive plan, at a weighted average exercise price of $8.14 per share; |
8
Table of Contents
| • | 104,696 shares of common stock issuable upon the exercise of outstanding warrants to purchase preferred stock, which will convert into warrants to purchase common stock upon completion of this offering, at a weighted average exercise price of $44.76 per share; |
| • | 592,524 shares of common stock reserved for future issuance under our 2014 equity incentive plan, plus annual increases in the number of shares of common stock reserved for future issuance pursuant to the “evergreen provision” of such plan, as more fully described in “Executive and Director Compensation—Employee Benefit Plans—2014 Equity Incentive Plan”; |
| • | 201,163 shares of common stock reserved for future issuance under our 2014 employee stock purchase plan, plus annual increases in the number of shares of common stock reserved for future issuance pursuant to the “evergreen provision” of such plan, as more fully described in “Executive and Director Compensation—Employee Benefit Plans—2014 Employee Stock Purchase Plan”; |
| • | 46,877 shares of common stock issuable upon the exercise of outstanding warrants to purchase common stock of the Company at an assumed exercise price of $16.00 per share held by the underwriter, and its designees, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; |
| • | 67,175 shares of common stock issuable upon the exercise of outstanding warrants to purchase common stock of the Company at an assumed exercise price of $16.80 per share held by the underwriter, and its designees, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; |
| • | 31,250 shares of our common stock issuable upon exercise of warrants to purchase our common stock at an assumed exercise price of $24.00 per share to be issued to the underwriter upon completion of this public offering assuming an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus; |
| • | 468,769 shares of common stock issuable upon the exercise of outstanding warrants to purchase capital stock of the Company issued in January and February 2014, or the 2014 Warrants, which will convert into warrants to purchase common stock at an assumed exercise price of $16.00 per share upon completion of this offering, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; and |
| • | 7,671 shares common stock issuable upon the exercise of a warrant to purchase capital stock of the Company to be issued to Cooley LLP, or the Cooley Warrant, upon completion of this offering at an assumed exercise price of $16.00 per share assuming an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus. |
In addition, unless otherwise indicated, the information in this prospectus assumes:
| • | the conversion of all our outstanding shares of preferred stock into an aggregate of 2,757,825 shares of common stock automatically upon the completion of this offering; |
| • | the issuance of 690,663 shares of common stock in connection with the closing of this offering as a result of the automatic conversion of the $7,500,000 aggregate principal amount of the convertible promissory notes issued in January and February 2014, or the 2014 Notes, plus accrued interest thereon, assuming an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, and based on an assumed conversion date of May 31, 2014; |
| • | the issuance of 31,964 shares of common stock in connection with the closing of this offering as a result of the automatic conversion of the $391,730 principal amount of a convertible promissory notes issued in November 2013 to Cooley LLP, or the Cooley Note, plus accrued interest thereon, assuming |
9
Table of Contents
| an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, and based on an assumed conversion date of May 31, 2014; |
| • | the filing of our amended and restated certificate of incorporation and the adoption of our amended and restated bylaws effective upon completion of this offering; |
| • | no exercise of the underwriter’s over-allotment option to purchase additional shares; and |
| • | a 4.3-for-1 reverse split of our common stock effected in April 2014. |
10
Table of Contents
Summary Financial Data
The following tables summarize certain of our financial data. The summary statement of operations data for the years ended December 31, 2011, 2012 and 2013 are derived from our audited financial statements included elsewhere in this prospectus. The summary statement of operations data for the three months ended March 31, 2013 and 2014 and the balance sheet data as of March 31, 2014 are derived from our unaudited interim financial statements included elsewhere in this prospectus. The unaudited interim financial information has been prepared on a basis consistent with our audited financial statements included elsewhere in this prospectus and includes all adjustments, consisting only of normal recurring adjustments, that, in the opinion of management, are necessary for a fair presentation of such financial information. Our historical results are not necessarily indicative of our future results. The summary financial data set forth below should be read together with our financial statements and related notes, “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
11
Table of Contents
The pro forma net loss attributable to common stockholders per share and pro forma weighted average common shares outstanding assume the conversion of all our outstanding preferred stock into an aggregate of 2,757,825 shares of common stock as of December 31, 2013 and March 31, 2014. The pro forma balance sheet data as of March 31, 2014 gives effect to such conversion as well as the reclassification of our preferred stock warrant liability to additional paid-in capital, which will occur upon completion of this offering. The pro forma as adjusted balance sheet data as of March 31, 2014 gives further effect to our receipt of the estimated net proceeds from the sale of shares of common stock by us in this offering at an assumed initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||||||
| 2011 | 2012 | 2013 | 2013 | 2014 | ||||||||||||||||
| (in thousands, except share data) | (unaudited) | |||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||
| Revenue |
||||||||||||||||||||
| Collaboration license and services |
$ | 5,108 | 12,642 | 16,350 | 1,192 | 1,283 | ||||||||||||||
| Milestones |
— | 2,000 | 3,000 | — | — | |||||||||||||||
| Manufacturing services |
— | — | 3,168 | 940 | 135 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
5,108 | 14,642 | 22,518 | 2,132 | 1,418 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research and development expenses: |
||||||||||||||||||||
| Costs of collaboration license and services |
8,380 | 10,033 | 5,856 | 2,113 | 833 | |||||||||||||||
| Costs of manufacturing services |
— | — | 3,168 | 940 | 135 | |||||||||||||||
| Research and development for proprietary programs |
3,683 | 1,702 | 1,861 | 160 | 571 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total research and development |
12,063 | 11,735 | 10,885 | 3,213 | 1,539 | |||||||||||||||
| General and administrative |
4,686 | 5,948 | 3,175 | 737 | 1,014 | |||||||||||||||
| Depreciation and amortization |
952 | 925 | 771 | 220 | 70 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
17,701 | 18,608 | 14,831 | 4,170 | 2,623 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
(12,593 | ) | (3,966 | ) | 7,687 | (2,038 | ) | (1,205 | ) | |||||||||||
| Change in value of warrants and put and call options, income (expense) |
(1,135 | ) | 1,951 | 1,843 | 486 | 247 | ||||||||||||||
| Interest expense |
— | — | — | — | (1,487 | ) | ||||||||||||||
| Other income (expense) |
3 | — | 62 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before taxes |
(13,725 | ) | (2,015 | ) | 9,592 | (1,552 | ) | (2,445 | ) | |||||||||||
| Income taxes |
— | — | 116 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
(13,725 | ) | (2,015 | ) | 9,476 | (1,552 | ) | (2,445 | ) | |||||||||||
| Preferred stock dividends and accretion of offering costs to redemption value |
(11,316 | ) | (12,104 | ) | (12,885 | ) | (3,221 | ) | (3,437 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss applicable to common stockholders |
$ | (25,041 | ) | (14,119 | ) | (3,409 | ) | $ | (4,773 | ) | (5,882 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted net loss attributable to common stockholders per share(1) |
||||||||||||||||||||
| Historical |
$ | (285.04 | ) | (157.97 | ) | (36.84 | ) | (51.64 | ) | (63.11 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Pro Forma (unaudited) |
$ | 2.60 | (0.94 | ) | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Weighted-average common shares outstanding(1) |
||||||||||||||||||||
| Historical |
87,853 | 89,377 | 92,522 | 92,430 | 93,201 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Pro Forma (unaudited) |
2,945,047 | 2,851,026 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| (1) | See Note 3 to our financial statements appearing elsewhere in this prospectus for an explanation of the method used to calculate the historical and pro forma net income or loss attributable to common stockholders per share and the number of common shares used in the computation of historical and pro forma per share amounts. |
12
Table of Contents
| As of March 31, 2014 | ||||||||||||
| (Unaudited) | ||||||||||||
| Actual | Pro Forma (1) | Pro Forma as Adjusted (2) |
||||||||||
| (in thousands) |
||||||||||||
| Balance Sheet Data: |
||||||||||||
| Cash and cash equivalents |
$ | 9,216 | 9,216 | 30,941 | ||||||||
| Working capital |
(2,829 | ) | 4,938 | 26,363 | ||||||||
| Total assets |
12,087 | 10,480 | 31,905 | |||||||||
| Total liabilities |
26,155 | 15,008 | 15,008 | |||||||||
| Redeemable convertible preferred stock |
191,120 | — | — | |||||||||
| Accumulated deficit |
(205,188 | ) | (213,731 | ) | (213,731 | ) | ||||||
| Total stockholders’ equity (deficit) |
(205,188 | ) | (4,528 | ) | 16,897 | |||||||
| (1) | The unaudited pro forma balance sheet as of March 31, 2014 reflects the automatic conversion of all outstanding shares of redeemable, convertible preferred stock as of that date into 2,757,825 shares of common stock, the automatic conversion of the Cooley Note and accrued interest (carrying value of $222,813) and extinguishment of the related put option (carrying value of $101,222) into 31,964 shares of common stock, which results in a loss upon conversion of $187,389, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, the automatic conversion of the 2014 Notes and accrued interest (carrying value of $2,506,682) and extinguishment of the related put option ($1,795,372) into 690,663 shares of our common stock, which results in a loss upon conversion of $8,355,442, including the write off of the remaining debt issuance costs ($1,606,888), assuming an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, and the reclassification of our preferred stock warrants ($6,779,876) into additional paid-in capital, which will occur upon completion of this offering. |
| (2) | The pro forma as adjusted balance sheet data as of December 31, 2013 gives further effect to our receipt of the estimated net proceeds from the sale of shares of common stock by us in this offering at an assumed initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
13
Table of Contents
Investing in our common stock involves a high degree of risk. In evaluating our business, investors should carefully consider the following risk factors. These risk factors contain, in addition to historical information, forward-looking statements that involve risks and uncertainties. Our actual results could differ significantly from the results discussed in the forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed below. The order in which the following risks are presented is not intended to reflect the magnitude of the risks described. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations and prospects. In that case, the trading price of our common stock could decline, and you may lose part or all of your investment.
FINANCIAL RISKS
We have incurred net operating losses throughout our history. We expect to continue to incur increasing net losses for the foreseeable future, and we may never achieve or maintain profitability.
We are not profitable and have incurred significant net losses in each year since our inception in February 1995 except for 2013, including net losses of $2.4 million, $2.0 million and $13.7 million for the three months ended March 31, 2014, and the years ended December 31, 2012 and 2011, respectively. As of March 31, 2014, we had an accumulated deficit of $205.2 million. Our losses have resulted principally from costs incurred in our discovery and development activities. We anticipate that our operating losses will substantially increase over the next several years as we expand our discovery, research and development activities, including the clinical development of our Tarmogen product candidates. While we reported net income in 2013, we do not anticipate that we will have net income again in the foreseeable future.
Because of the numerous risks and uncertainties associated with biopharmaceutical product development and commercialization, we are unable to accurately predict the timing or amount of future expenses or when, or if, we will be able to achieve or maintain profitability. Currently, we have no products approved for commercial sale, and to date we have not generated any product revenue. We have financed our operations primarily through the sale of equity securities, upfront payments pursuant to our collaboration agreements, government grants and capital lease and equipment financing. The size of our future net losses will depend, in part, on the rate of growth or contraction of our expenses and the level and rate of growth, if any, of our revenues. Our ability to achieve profitability is dependent on our ability, alone or with others, to complete the development of our products successfully, obtain the required regulatory approvals, manufacture and market our proposed products successfully or have such products manufactured and marketed by others, and gain market acceptance for such products. There can be no assurance as to whether or when we will achieve profitability.
We will require substantial additional capital in the future. If additional capital is not available, we will have to delay, reduce or cease operations.
Development of our Tarmogen product candidates will require substantial additional funds to conduct research, development and clinical trials necessary to bring such product candidates to market and to establish manufacturing, marketing and distribution capabilities. Our future capital requirements will depend on many factors, including, among others:
| • | the scope, rate of progress, results and costs of our preclinical and non-clinical studies, clinical trials and other research and development activities; |
| • | the scope, rate of progress and costs of our manufacturing development and commercial manufacturing activities; |
| • | the cost, timing and outcomes of regulatory proceedings, including U.S. Food and Drug Administration, or FDA, review of any Biologics License Application, or BLA, that we submit; |
14
Table of Contents
| • | payments required with respect to development milestones we achieve under our in-licensing agreements, including any such payments to University of Colorado pursuant to our license agreement with them; |
| • | the costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims; |
| • | the costs associated with commercializing our product candidates, if they receive regulatory approval; |
| • | the cost and timing of establishing sales and marketing capabilities; |
| • | competing technological efforts and market developments; |
| • | changes in our existing research relationships; |
| • | our ability to establish collaborative arrangements to the extent necessary; |
| • | revenues received from any future products; |
| • | the ability to achieve and receive milestone payments for products licensed to collaborators; and |
| • | payments received under any future strategic collaborations. |
We anticipate that we will continue to generate significant losses for the next several years as we incur expenses to complete our clinical trial programs for our product candidates, build commercial capabilities, develop our pipeline and expand our corporate infrastructure. We believe that the net proceeds from this Offering, together with our existing cash and cash equivalents will allow us to fund our operating plan into 2016. However, our operating plan may change as a result of factors currently unknown to us. Changing circumstances may cause us to consume capital faster or slower than we currently anticipate or to alter our operations. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available financial resources sooner than we currently expect.
There can be no assurance that our revenue and expense forecasts will prove to be accurate, and any change in the foregoing assumptions could require us to obtain additional financing earlier than anticipated. There is a risk of delay or failure at any stage of developing a product candidate, and the time required and costs involved in successfully accomplishing our objectives cannot be accurately predicted. Actual drug research and development costs could substantially exceed budgeted amounts, which could force us to delay, reduce the scope of or eliminate one or more of our research or development programs.
We may never be able to generate a sufficient amount of product revenue to cover our expenses. Until we do, we expect to seek additional funding through public or private equity or debt financings, collaborative relationships, or other available financing transactions. However, there can be no assurance that additional financing will be available on acceptable terms, if at all, and such financings could be dilutive to existing security holders. Moreover, in the event that additional funds are obtained through arrangements with collaborators, such arrangements may require us to relinquish rights to certain of our technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves.
If adequate funds are not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or development programs. Our failure to obtain adequate financing when needed and on acceptable terms would have a material adverse effect on our business, financial condition and results of operations.
15
Table of Contents
BUSINESS RISKS
Risks Relating to Clinical Development and Commercialization of Our Product Candidates
If we, or our collaborators, Celgene Corporation and Gilead Sciences, Inc., fail to successfully complete clinical trials, fail to obtain regulatory approval or fail to successfully commercialize our Tarmogen product candidates, our business would be harmed and the value of our securities would decline.
Investors should evaluate us in light of the uncertainties and complexities affecting a pre-commercial biopharmaceutical company. We have not completed clinical development for any of our product candidates. Our lead Tarmogen product candidate is GS-4774, which has been exclusively licensed to Gilead Sciences, Inc., or Gilead, and which has begun Phase 2 clinical testing.
Gilead is responsible for the clinical development and any future commercialization activities for GS-4774. The GI-6300 program, including GI-6301, which is being developed for the treatment of brachyury-expressing cancers, was exclusively licensed to Celgene Corporation, or Celgene, and is in Phase 1 clinical testing. We anticipate Celgene will lead future clinical development and any future commercialization activities for GI-6301. As a result, we are completely dependent on their ability and willingness to fund and execute clinical development, regulatory approvals and commercialization activities. We have limited control over the amount and timing of resources that Celgene and Gilead dedicate to the development of our product candidates, potentially negatively impacting the likelihood of clinical or commercial success for these products candidates.
Regulatory agencies, including the FDA, must approve GS-4774, GI-6301, GI-4000 and any of our other product candidates before they can be marketed or sold. The approval process is lengthy, requires significant capital expenditures, and the outcome is uncertain. Our, or our collaborator’s, ability to obtain regulatory approval of any Tarmogen product candidate depends on, among other things, completion of additional clinical trials, whether such clinical trials demonstrate statistically significant efficacy with safety issues that do not potentially outweigh the therapeutic benefit of the product candidates, and whether the regulatory agencies agree that the data from our future clinical trials are sufficient to support approval for any of our product candidates. The final results of our current and future clinical trials may not meet FDA or other regulatory agencies’ requirements to approve a product candidate for marketing, and the regulatory agencies may otherwise determine that our manufacturing processes or facilities are insufficient to support approval. We or our collaborators may need to conduct more clinical trials than we currently anticipate. Even if we do receive FDA or other regulatory agency approval, we or our collaborators may not be successful in commercializing approved product candidates. If any of these events occur, our business could be materially harmed and the value of our securities would decline.
We, or our collaborators, may face delays in completing our clinical trials, and may not be able to complete them at all.
Clinical trials necessary to support an application for approval to market any Tarmogen product candidates have not been completed. Our, or our collaborators’, current and future clinical trials may be delayed, unsuccessful, or terminated as a result of many factors, including:
| • | delays in designing an appropriate clinical trial protocol and reaching agreement on trial design with investigators and regulatory authorities; |
| • | governmental or regulatory delays, failure to obtain regulatory approval or changes in regulatory requirements, policy or guidelines; |
| • | delays in establishing necessary clinical trial sites or the need to establish new clinical trial sites; |
| • | reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
16
Table of Contents
| • | the actual performance of CROs and clinical trial sites in ensuring the proper and timely conduct of our clinical trials; |
| • | developing and validating companion diagnostics on a timely basis; |
| • | adverse effects experienced by subjects in clinical trials; |
| • | manufacturing sufficient quantities of product candidates for use in clinical trials; and |
| • | delays in achieving study endpoints and completing data analysis for a trial. |
In addition to these factors, our trials may be delayed, unsuccessful or terminated because:
| • | regulators or institutional review boards, or IRBs, may not authorize us to commence or continue a clinical trial; |
| • | regulators or IRBs may suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or concerns about patient safety; |
| • | we may suspend or terminate our clinical trials if we believe that they expose the participating patients to unacceptable health risks; |
| • | patients may not complete clinical trials due to safety issues, side effects, such as injection site discomfort, a belief that they are receiving placebo instead of our product candidates, the length of follow-up periods, or other reasons; |
| • | patients with serious diseases included in our clinical trials may die or suffer other adverse medical events for reasons that may not be related to our product candidates; |
| • | in those trials where our product candidate is being tested in combination with one or more other therapies, deaths may occur that may be attributable to the other therapies; |
| • | we may have difficulty in maintaining contact with patients after administration of our Tarmogen product candidates, preventing us from collecting the data required by our study protocol; |
| • | product candidates may demonstrate a lack of efficacy during clinical trials; |
| • | diagnostic tests we or our collaborators develop, such as the BDX-001 diagnostic for a potential future GI-4000 clinical trial in pancreas cancer patients, may not effectively identify patients that respond to our product candidates; |
| • | we may have difficulty in finding suitable patients to participate in our, or our collaborators’, clinical trials due to a number of factors, including competing clinical trials, a limited number of clinical trial sites, or the inability to identify a sufficient number of patients that meet trial eligibility criteria, including any diagnostic tests being developed by us or our collaborators, including the BDX-001 diagnostic; |
| • | personnel conducting clinical trials may fail to properly administer our product candidates; and |
| • | our collaborators may decide not to pursue further clinical trials, or may allocate resources to other clinical trials, including clinical trials of competitor product candidates. |
We could encounter delays if our clinical trials are suspended or terminated by us, by IRBs of the institutions in which such trials are being conducted, by the Data Safety Monitoring Boards for such trials or by the FDA or other regulatory authorities. Such authorities may impose a suspension or termination due to a number of factors, including potential for unacceptable safety risks to patients, inspection of the clinical trial operation or trial site, changes in government regulations or administrative actions.
In addition, we rely on academic institutions, physician practices and CROs to conduct, supervise or monitor some or all aspects of clinical trials involving our product candidates. For example several of our other trials are being conducted by the NCI, including GI-6207-02 for MTC and the GI-6301-01 Phase 1 trial. We have
17
Table of Contents
less control over the timing and other aspects of these clinical trials than if we conducted the monitoring and supervision entirely on our own. Third parties may not perform their responsibilities for our clinical trials on our anticipated schedule or consistent with a clinical trial protocol or applicable regulations. We also may rely on CROs to perform our data management and analysis. They may not provide these services as required or in a timely or compliant manner, and we may be held legally responsible for any or all of their performance failures or inadequacies.
Moreover, our development costs will increase because we will be required to complete additional or larger clinical trials for our Tarmogen product candidates prior to FDA or other regulatory approval. If we or our collaborators experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed or eliminated. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process, and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also lead to the denial of regulatory approval of our product candidates.
If we encounter difficulties enrolling patients in our clinical trials, our clinical trials could be delayed or otherwise adversely affected.
Clinical trials for our product candidates require us to identify and enroll a large number of patients with the disease under investigation. We may not be able to enroll a sufficient number of patients, or those with required or desired characteristics, in a timely manner. Patient enrollment is affected by factors including:
| • | severity of the disease under investigation; |
| • | design of the trial protocol; |
| • | the size and nature of the patient population; |
| • | eligibility criteria for the study in question; |
| • | lack of a sufficient number of patients who meet the enrollment criteria for our clinical trials; |
| • | delays required to characterize tumor types to allow us to select the proper product candidate, which may lead patients to seek to enroll in other clinical trials or seek alternative treatments; |
| • | perceived risks and benefits of the product candidate under study; |
| • | availability of competing therapies and clinical trials; |
| • | efforts to facilitate timely enrollment in clinical trials; |
| • | scheduling conflicts with participating clinicians; |
| • | patient referral practices of physicians; |
| • | the ability to monitor patients adequately during and after administration of our Tarmogen product candidates; |
| • | diagnostic tests we or our collaborators develop may not effectively identify patients that respond to our product candidates; and |
| • | proximity and availability of clinical trial sites for prospective patients. |
We have experienced difficulties enrolling patients in our smaller clinical trials due to lack of referrals and may experience similar difficulties in the future. For example, the GI-4000-05 trial conducted at the Lombardi Cancer Center at Georgetown University commenced enrollment in August 2010. Eleven subjects were enrolled as of December 31, 2013. This enrollment rate was slower than we had anticipated and enrollment has been discontinued.
18
Table of Contents
If we have difficulty enrolling a sufficient number or diversity of patients to conduct our clinical trials as planned, we may need to delay or terminate ongoing or planned clinical trials, either of which would have an adverse effect on our business.
Our Tarmogen product candidates are based on a novel technology, which may raise development issues we may not be able to resolve, regulatory issues that could delay or prevent approval, or personnel issues that may keep us from being able to develop our product candidates.
Our product candidates are based on our novel Tarmogen technology platform. There can be no assurance that development problems related to our novel technology will not arise in the future that cause significant delays or that we are not able to resolve.
Regulatory approval of novel product candidates such as ours can be more expensive and take longer than for other, more well-known or extensively studied pharmaceutical or biopharmaceutical product candidates due to our and regulatory agencies’ lack of experience with them. The novelty of our platform may lengthen the regulatory review process, require us to conduct additional studies or clinical trials, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of our product candidates or lead to significant post-approval limitations or restrictions. For example, the FDA could require additional studies or characterization related to our use of heat-inactivated yeast that may be difficult to perform.
The novel nature of our product candidates also means that fewer people are trained in or experienced with product candidates of this type, which may make it difficult to find, hire and retain capable personnel, particularly for research, development, commercial and manufacturing positions. For example, study personnel may administer the wrong version of our product candidates or assign study therapy to the wrong study group, resulting in potential disqualification of subjects from data analysis. These factors could potentially cause a trial to fail for a reason unrelated to the efficacy of our product candidates. If we are unable to hire and retain the necessary personnel, the rate and success at which we can develop and commercialize product candidates will be limited. Any such events would increase our costs and could delay or prevent our ability to commercialize our product candidates, which could adversely impact our business, financial condition and results of operations.
If we are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience significant delays in doing so, we may not realize the full commercial potential of our product candidates.
An important component of our business strategy is to use molecular profiling of patients to target our Tarmogen product candidates to those patients we believe may be most likely to benefit from them, including profiling necessary to determine which version of our GI-4000 series should be given to a patient with Ras mutated cancer and determining which patients should be enrolled in our GI-4000 pancreas cancer trials based on the BDX-001 proteomic signature. The BDX-001 test is controlled by Biodesix, Inc. and we may not be able to obtain rights to use the test on commercially reasonable terms, if at all. If we do not obtain rights to BDX-001, we will not be able to use it in clinical development or commercialization. If we do not have access to BDX-001, our GI-4000 trials may not be successful and we may never obtain approval to market GI-4000. There has been limited experience in our industry in prospective development of companion diagnostics required to perform the required testing. To be successful, we will need to address a number of scientific, technical and logistical challenges related to the use of companion diagnostics in the development and regulatory approval of our product candidates.
The FDA and similar regulatory authorities outside the United States regulate companion diagnostics. Companion diagnostics require separate or coordinated regulatory approval prior to commercialization of the therapeutic product. The regulatory pathway for co-development of therapeutics and companion diagnostics is uncertain. Changes to regulatory advice could delay our development programs or delay or prevent eventual
19
Table of Contents
marketing approval for our product candidates that may otherwise be approvable. For our oncology product candidate, GI-4000 we will require in vitro companion diagnostics that will help identify patients we believe may be likely to benefit from our product candidates. In vitro diagnostics are tests used on specimens taken from the patient being tested.
The FDA’s evolving position on companion diagnostics could affect our clinical development programs that utilize companion diagnostics. In particular, the FDA may limit our ability to use retrospective data, otherwise disagree with our approaches to trial design, biomarker qualification, clinical and analytical validity, and clinical utility, or make us repeat aspects of a trial or initiate new trials.
Assays that can be used as companion diagnostics for detecting Ras mutations are commercially available, but in some cases they do not yet have regulatory approval for use as companion diagnostics. In future clinical trials, we may use commercially available companion diagnostics or we may co-develop companion diagnostics ourselves or with collaborators. We have limited experience in the development of diagnostics and may not be successful in developing necessary diagnostics to pair with those product candidates that require a companion diagnostic.
Certain proteomic analyses have been performed on plasma samples from 90 of the subjects from the GI-4000-02 trial, potentially identifying a patient selection test named BDX-001 for future trials. Proteomic analyses were not specified in the original trial protocol for GI-4000-02 and the predictive value of the proteomic analyses will need to be validated in another clinical trial. We were only able to review a limited number of samples from the subjects in the trial and future analysis may not confirm the analytical results we have observed to date. Future testing with a companion diagnostic designed to detect the specific protein pattern identified by the initial proteomic testing may show a different proportion of the specific protein signatures which were correlated with later recurrence of the cancer and it may also fail to show the specific protein signature is predictive of the response to GI-4000.
Given our limited experience in developing diagnostics, we expect to rely in part on third parties for their design and manufacture. If we, or any third parties that we engage to assist us, are unable to successfully develop companion diagnostics for our product candidates that require such diagnostics, or experience delays in doing so, the development of our product candidates may be adversely affected, our product candidates may not receive marketing approval and we may not realize the full commercial potential of any products that receive marketing approval. As a result, our business could be materially harmed.
Results of earlier studies and clinical trials may not be predictive of future trial results.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of our product candidates may not be predictive of the design or results of later-stage clinical trials. The positive results generated to date in clinical trials for our Tarmogen product candidates do not ensure that later clinical trials will demonstrate similar results. While we have observed statistically significant improvements in the outcomes of some of our clinical trials, many of the improvements we have seen have not reached statistical significance. The test for the specific proteomic signature, called BDX-001, which we have identified through proteomic testing of subjects from our GI-4000-02 trial, may fail to predict which subjects respond to GI-4000 in our future pancreas cancer clinical trials. Statistical significance is a statistical term that means that an effect is unlikely to have occurred by chance. In order to be approved, product candidates must demonstrate that their effect on patients’ diseases in the trial is statistically significant. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical trials. Early clinical trials frequently enroll patient populations that are different from the patient populations in later trials, resulting in different outcomes in later clinical trials from those in earlier stage clinical trials. In addition, adverse events may not occur in early clinical trials and only emerge in larger, late-stage clinical trials or after commercialization. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier clinical trials. If later stage clinical trials do not demonstrate efficacy and safety of our product candidates we will not be able to market them and our business will be materially harmed.
20
Table of Contents
We have not completed clinical development of any of our product candidates and do not have any products approved for sale by the United States Federal Drug Administration, or FDA, or any other regulatory bodies. Regulatory authorities may not approve our product candidates even if they meet safety and efficacy endpoints in clinical trials.
We have discussions with and obtain guidance from regulatory authorities regarding certain aspects of our clinical development activities. These discussions are not binding commitments on the part of regulatory authorities. Under certain circumstances, regulatory authorities may revise or retract previous guidance during the course of our clinical activities or after the completion of our clinical trials. A regulatory authority may also disqualify a clinical trial in whole or in part from consideration in support of approval of a potential product for commercial sale or otherwise deny approval of that product. Prior to regulatory approval, a regulatory authority may elect to obtain advice from outside experts regarding scientific issues and/or marketing applications under a regulatory authority review. In the United States, these outside experts are convened through the FDA’s Advisory Committee process, which would report to the FDA and make recommendations that may ultimately differ from the views of the FDA. Should an Advisory Committee be convened, it would be expected to lengthen the time for obtaining regulatory approval, if such approval is obtained at all.
The FDA and foreign regulatory agencies may delay, limit or deny marketing approval for many reasons, including:
| • | a product candidate may not be considered safe or effective; |
| • | our manufacturing processes or facilities may not meet the applicable requirements; |
| • | changes in the agencies’ approval policies or adoption of new regulations may require additional work on our part, for example, the FDA may require us to submit a separate BLA for each product version of GI-4000; |
| • | different divisions of the FDA are reviewing different product candidates and those divisions may have different requirements for approval; and |
| • | changes in regulatory law, FDA or foreign regulatory agency organization, or personnel may result in different requirements for approval than anticipated. |
Our product candidates may not be approved even if they achieve their endpoints in clinical trials. Regulatory agencies, including the FDA, or their advisors may disagree with our trial design and our interpretations of data from preclinical studies and clinical trials. Regulatory agencies also may approve a product candidate for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates.
Any delay in or failure to receive or maintain approval for any of our product candidates could prevent us from ever generating revenues or achieving profitability.
We may be required to suspend, repeat or terminate our clinical trials if they are not conducted in accordance with regulatory requirements, the results are negative or inconclusive, or the trials are not well designed.
Clinical trials must be conducted in accordance with FDA regulations governing clinical studies, or other applicable foreign government guidelines, and are subject to oversight by the FDA, other foreign governmental agencies and IRBs at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with product candidates produced under current Good Manufacturing Practices, or cGMP, and may require large numbers of test subjects. Clinical trials may be suspended by the FDA, other foreign governmental agencies or us for various reasons, including:
| • | deficiencies in the conduct of the clinical trials, including failure to conduct the clinical trial in accordance with regulatory requirements or clinical protocols; |
| • | deficiencies in the clinical trial operations or trial sites; |
21
Table of Contents
| • | the product candidate may have unforeseen adverse side effects; |
| • | the time required to determine whether the product candidate is effective may be longer than expected; |
| • | deaths or other adverse events arising during a clinical trial due to medical problems that may not be related to clinical trial administration of our Tarmogen product candidates; |
| • | the product candidate may not appear to be more effective than current therapies; |
| • | the quality or stability of the product candidate may fall below acceptable standards; and |
| • | insufficient quantities of the product candidate might be available to complete the trials. |
In addition, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols to reflect these changes. Amendments to our clinical trial protocols may require resubmission to IRBs for reexamination, which may impact the costs, timing or successful completion of a clinical trial. Due to these and other factors, our Tarmogen product candidates could take longer to gain regulatory approval than we expect or we may never gain approval for any product candidates, which could reduce or eliminate our revenue by delaying or terminating the commercialization of our Tarmogen product candidates.
Certain of our product candidates are being and will be studied in clinical trials conducted by the NCI, and in investigator-initiated clinical trials, the conduct of which we do not control.
Early clinical studies of our GI-6207 and GI-6301 product candidates are being conducted in collaboration with and under the direction of the NCI. These include an ongoing Phase 1 trial of GI-6301 in patients with metastatic cancer and chordoma, and a Phase 2 clinical trial of GI-6207 in patients with medullary thyroid cancer, or MTC. We expect to continue to supply drugs for these and otherwise support similar trials in the future. In addition, there is an investigator-initiated clinical trial ongoing with our GI-4000 product candidate, a Phase 2a clinical study in colorectal cancer at the Lombardi Cancer Center at Georgetown University. We do not control the design protocols, administration or conduct of these trials and, as a result, we are subject to risks associated with the way these types of trials are conducted, in particular should any problems arise. These risks include difficulties or delays in communicating with investigators or administrators, procedural delays and other timing issues, inappropriate trial design or trial protocols and difficulties or differences in interpreting data. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials or the trials not being conducted according to protocols may ultimately lead to the denial of regulatory approval of our product candidates, which would adversely affect our business and lead to a decline in the trading price of our securities.
We do not control the conduct of certain clinical trials conducted by our collaborators, Gilead and Celgene.
We have exclusively licensed two of our Tarmogen product candidates to collaborators for further clinical development and commercialization. GS-4774 is licensed to Gilead and the GI-6300 program, including GI-6301, is licensed to Celgene. Control for the GS-4774 program, including but not limited to future clinical development, regulatory strategy and any commercialization activities, has been transferred to Gilead under the terms of our collaboration agreement. Control of the GI-6300 program, including but not limited to future clinical development, regulatory strategy, and any commercialization activities, is currently being transferred to Celgene. We anticipate that these responsibilities will be completed by the third quarter of 2014. As a result, we do not have control over the design protocols, administration or conduct for any future trials and we are therefore subject to risks associated with the way these types of trials are conducted, in particular should any problems arise. These risks include difficulties or delays in communicating with investigators or administrators, procedural delays and other timing issues, inappropriate trial design or trial protocols and difficulties or differences in interpreting data. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials or the trials not being conducted according to protocols may ultimately lead to the denial of regulatory approval of these product candidates, which would adversely affect our business and lead to a decline in the trading price of our securities.
22
Table of Contents
Any product candidate for which we, or our collaborators, obtain marketing approval could be subject to restrictions or withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products, when and if any of them are approved.
Any product candidate that we, or our collaborators obtain marketing approval for, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to ongoing requirements of the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information, reports, registration and listing and annual payment requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval of a product candidate is granted, the approval will be subject to limitations on the indicated uses for which the product may be marketed or to conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use. If we market our products outside of their approved indications, we will be subject to enforcement action for off-label marketing.
In addition, later discovery of previously unknown problems with these products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| • | restrictions on such products, manufacturers or manufacturing processes; |
| • | restrictions on the labeling or marketing of a product; |
| • | restrictions on product distribution or use; |
| • | requirements to conduct post-marketing clinical trials; |
| • | warning or untitled letters; |
| • | withdrawal of the products from the market; |
| • | refusal to approve pending applications or supplements to approved applications that we submit; |
| • | recall of products, fines, restitution or disgorgement of profits or revenue; |
| • | suspension or withdrawal of marketing approvals; |
| • | refusal to permit the import or export of our products; |
| • | product seizure; and |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA’s policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we, or our collaborators, are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we, or our collaborators, are not able to maintain regulatory compliance, any marketing approval that was obtained could be lost, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
If we, or our collaborators, are unable to comply with foreign regulatory requirements or obtain foreign regulatory approvals, our ability to develop foreign markets for our products could be impaired.
Sales of our products outside the United States will be subject to foreign regulatory requirements governing clinical trials, marketing approval, manufacturing, product licensing, pricing and reimbursement. These regulatory requirements vary greatly from country to country. As a result, the time required to obtain approvals
23
Table of Contents
outside the United States may differ from that required to obtain FDA approval and we may not be able to obtain foreign regulatory approvals on a timely basis or at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other countries or by the FDA and foreign regulatory authorities could require additional testing. Failure to comply with these regulatory requirements or obtain required approvals could impair our ability to develop foreign markets for our products.
Developing product candidates in combination with other therapies may lead to unforeseen side effects or failures in our clinical trials.
We, and our collaborators, are studying our product candidates in clinical trials in combination with approved therapies, including chemotherapies and antivirals, and we anticipate that if any Tarmogen product candidates are approved for marketing, they will be approved to be used only in combination with other therapies. Our, and our collaborators’, development programs and planned studies carry all the risks inherent in drug development activities, including the risk that they will fail to demonstrate meaningful efficacy or acceptable safety. In addition, our development programs are subject to additional regulatory, commercial, manufacturing and other risks because of the use of other therapies in combination with our product candidates. For example, the other therapies may lead to toxicities that are improperly attributed to our product candidates or the combination of our product candidates with other therapies may result in toxicities that the product candidate or other therapy does not produce when used alone. The other therapies we are using in combination may be removed from the market and thus be unavailable for testing or commercial use with any of our approved products. The other therapies we are using in combination may be replaced with newer therapies that may be effective without our product candidates or that are not compatible with our product candidates. Testing product candidates in combination with other therapies may increase the risk of significant adverse effects or test failures. The timing, outcome and cost of developing products to be used in combination with other therapies is difficult to predict and dependent on a number of factors that are outside our reasonable control. If any safety or toxicity issues arise in these clinical trials or with any approved products, the products may not be approved, which could prevent us from ever generating revenues or achieving profitability.
Competitive products for treatment of pancreas cancer, non-small cell lung cancer, colorectal cancer, MTC, chordoma and chronic hepatitis B infection may reduce or eliminate the commercial opportunity for our Tarmogen product candidates.
The clinical and commercial landscape for pancreas cancer, non-small cell lung cancer, colorectal cancer, MTC, chordoma and chronic hepatitis B infection is rapidly changing. New data from commercial and clinical-stage products continue to emerge. It is possible that these data may alter current standards of care, completely precluding us from further developing our Tarmogen product candidates, or getting them approved by regulatory agencies. Further, it is possible that we may initiate a clinical trial or trials for these product candidates, only to find that data from competing products make it impossible for us to complete enrollment in these trials, resulting in our inability to file for marketing approval with regulatory agencies. Even if these products are approved for marketing in a particular indication or indications, they may have limited sales due to particularly intense competition in these markets.
We expect to depend on existing and future collaborations with third parties for the development of some of our product candidates. If those collaborations are not successful, we may not be able to complete the development of these product candidates.
We currently have a collaboration and option agreement, or Option Agreement, with Celgene for the development of our oncology product candidates. Under the Option Agreement, Celgene has a worldwide exclusive license to the GI-6300 program, including GI-6301. Celgene did not exercise its option to GI-4000 and returned all rights and development responsibility, including any future expenses, to us. Gilead has taken a worldwide exclusive license to all of our hepatitis B virus, or HBV, Tarmogen product candidates. Celgene and Gilead can terminate their respective collaborations with us at any time, subject to certain notice provisions. We plan to seek third-party collaborators for the development of certain other Tarmogen product candidates.
24
Table of Contents
Under our current arrangements with Celgene and Gilead, we have limited control over the amount and timing of resources that our collaborators dedicate to the development of our product candidates. This is also likely to be true for any future collaborations with third parties. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
Collaborations involving our product candidates pose the following risks to us:
| • | Celgene may not exercise any more options to any of our oncology product candidates, for example, Celgene did not elect to exercise their option to license GI-4000; |
| • | Celgene may return all rights to GI-6301 to us; |
| • | Gilead may return all rights to HBV product candidates to us; |
| • | collaborators have significant discretion in determining the efforts and resources, if any, that they will apply to these collaborations; |
| • | collaborators may not pursue development and commercialization of our product candidates, such as Celgene not exercising more of its option to any oncology programs other than GI-6301, or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator’s strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| • | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| • | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| • | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
| • | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our product candidates or that result in costly litigation or arbitration that diverts management attention and resources; |
| • | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates; |
| • | collaborators may elect to take over manufacturing rather than retain us as manufacturers and may encounter problems in starting up or gaining approval for their manufacturing facility and so be unable to continue development of product candidates; |
| • | we may be required to undertake the expenditure of substantial operational, financial and management resources in connection with any collaboration; |
| • | we may be required to issue equity securities to collaborators that would dilute our existing security holders’ percentage ownership; |
| • | we may be required to assume substantial actual or contingent liabilities; |
| • | collaborators may not commit adequate resources to the marketing and distribution of our product candidates, limiting our potential revenues from these products; and |
| • | collaborators may experience financial difficulties. |
25
Table of Contents
We face a number of challenges in seeking additional collaborations. Collaborations are complex and any potential discussions may not result in a definitive agreement for many reasons. For example, whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration, and the proposed collaborator’s evaluation of a number of factors, such as the design or results of our clinical trials, the potential market for our product candidates, the costs and complexities of manufacturing and delivering our product candidates to patients, the potential of competing products, the existence of uncertainty with respect to ownership or the coverage of our intellectual property, and industry and market conditions generally. If we were to determine that additional collaborations for our Tarmogen product candidates are necessary and were unable to enter into such collaborations on acceptable terms, we might elect to delay or scale back the development or commercialization of our product candidates in order to preserve our financial resources or to allow us adequate time to develop the required physical resources and systems and expertise ourselves.
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner, or at all. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators. If a present or future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated.
We will need to develop or acquire additional manufacturing and distribution capabilities in order to commercialize any product candidates that obtain marketing approval, and we may encounter unexpected costs or difficulties in doing so.
If we independently develop and commercialize one or more of our product candidates, we will need to invest in acquiring or building additional capabilities and effectively manage our operations and facilities to successfully pursue and complete future research, development and commercialization efforts. We will require additional investment and validation process development in order to qualify our commercial-scale manufacturing process to manufacture clinical trial materials and commercial material if any of our products are approved for marketing. This investment and validation process development may be expensive and time-consuming. We will require additional personnel with experience in commercial-scale manufacturing, managing of large-scale information technology systems and managing a large-scale distribution system. We will need to add personnel and expand our capabilities, which may strain our existing managerial, operational, regulatory compliance, financial and other resources. To do this effectively, we must:
| • | recruit, hire, train, manage and motivate a growing employee base; |
| • | accurately forecast demand for our products; |
| • | assemble and manage the supply chain to ensure our ability to meet demand; and |
| • | expand existing operational, manufacturing, financial and management information systems. |
We may seek FDA approval for our production process and facilities simultaneously with seeking approval for sale of our product candidates. Should we not complete the development of adequate capabilities, including manufacturing capacity, or fail to receive timely approval of our manufacturing process and facilities, our ability to supply clinical trial materials for planned clinical trials or supply products following regulatory approval for sale could be delayed, which would further delay our clinical trials or the period of time when we would be able to generate revenues from the sale of such products, if we are even able to obtain approval or generate revenues at all.
Additionally, we may decide to outsource some or all of our manufacturing activities to a third party CMO. Under any agreement with a CMO, we would have less control over the timing and quality of manufacturing than if we were to perform such manufacturing ourselves. A CMO would be manufacturing other pharmaceutical products in the same facilities as our Tarmogen product candidates, increasing the risk of cross product contamination. Further, there is no guarantee that any CMO will continue ongoing operations, causing potential delays in product supply, reduced revenues and other liabilities for us.
26
Table of Contents
Any such events would increase our costs and could delay or prevent our ability to commercialize our product candidates, which could adversely impact our business, financial condition and results of operations.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
Undesirable side effects caused by our product candidates could cause us, our collaborators, or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authorities. To date, patients who have received Tarmogens have experienced drug-related side effects, including local skin reactions and systemic and constitutional symptoms including muscle aches, fever and fatigue. Subjects have also reported developing a taste in their mouths similar to yeast following injection of our product candidates. In one instance, a patient in the GI-6207 clinical trial who had pleural and pericardial metastases, or cancer in the spaces surrounding the heart and lungs, experienced pleural and pericardial effusions, or fluid buildup in those areas of the body, following immunization with GI-6207. GI-6207 had to be discontinued in this patient because of this adverse event. For this reason, we have excluded patients with large pericardial metastases from current and future clinical trials.
Our Tarmogen product candidates are intended to stimulate the immune system. As such, results of our clinical trials could reveal an unacceptable severity and prevalence of side effects, including, but not limited to, adverse immune responses that lead to previously unobserved autoimmune complications or yeast allergies. As a result of any side effects, our clinical trials could be suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development, or deny approval, of our product candidates for any or all targeted indications. The drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition and prospects significantly.
Additionally if one or more of our product candidates receives marketing approval, and we, our collaborators, or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including:
| • | regulatory authorities may withdraw approvals of such product; |
| • | regulatory authorities may require additional warnings on the label; |
| • | we may be required to create a medication guide outlining the risks of such side effects for distribution to patients; |
| • | we may be sued and held liable for harm caused to patients; and |
| • | our reputation may suffer. |
Any such event noted in this risk factor could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results of operations and prospects.
If we cannot demonstrate an acceptable toxicity profile for our product candidates in non-clinical studies, we will not be able to initiate or continue clinical trials or obtain approval for our product candidates.
In order to move a product candidate into human clinical trials, we must first demonstrate an acceptable toxicity profile in preclinical testing. Furthermore, in order to obtain approval, we must also demonstrate safety in various non-clinical tests. For example, we are conducting preclinical testing for GI-19000 in anticipation of filing an investigational new drug application, or IND, subject to obtaining additional funding. We may not have
27
Table of Contents
conducted or may not conduct the types of non-clinical testing required by regulatory authorities, or future non-clinical tests may indicate that our product candidates are not safe for use. Preclinical and non-clinical testing is expensive, time-consuming and has an uncertain outcome. In addition, success in initial non-clinical testing does not ensure that later non-clinical testing will be successful. We may experience numerous unforeseen events during, or as a result of, the non-clinical testing process, which could delay or prevent our ability to develop or commercialize our product candidates, including:
| • | our preclinical and non-clinical testing may produce inconclusive or negative safety results, which may require us to conduct additional non-clinical testing or to abandon product candidates; |
| • | our product candidates may have unfavorable pharmacology or toxicity characteristics; |
| • | our product candidates may cause undesirable side effects such as negative immune responses that lead to autoimmune complications; |
| • | our enrolled patients may have yeast allergies that lead to complications after administration of Tarmogen product candidates; and |
| • | the FDA or other regulatory authorities may determine that additional safety testing is required. |
Any such events would increase our costs and could delay or prevent our ability to commercialize our product candidates, which could adversely impact our business, financial condition and results of operations.
If we are unable to establish sales and marketing capabilities or enter into additional agreements with third parties to sell and market our product candidates, we may not be successful in commercializing our product candidates if and when they are approved.
We do not have a sales and marketing infrastructure or any experience in the sales, marketing or distribution of pharmaceutical products. We currently have collaboration agreements with Celgene for the development and commercialization of our oncology product candidates, other than GI-4000, and with Gilead for HBV Tarmogens. We may seek additional third-party collaborators for the commercialization of our other product candidates. In the future, we may choose to build a focused sales and marketing infrastructure to market or co-promote some of our product candidates if and when they are approved, which would be expensive and time-consuming. Alternatively, we may elect to outsource these functions to third parties. Either approach carries significant risks. For example, recruiting and training a sales force is expensive and time-consuming and, if done improperly, could delay a product launch and result in limited sales. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our products on our own include:
| • | our inability to recruit, manage and retain adequate numbers of effective sales and marketing personnel; |
| • | the inability of marketing personnel to develop effective marketing materials; |
| • | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe any future products; |
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| • | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
As a result of our arrangements with Celgene and Gilead, as well as any other arrangements with third parties we may enter into to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues are likely to be lower than if we were to market and sell any products that
28
Table of Contents
we develop ourselves. In addition, we may not be successful in entering into additional arrangements with third parties to sell and market our product candidates or doing so on terms that are favorable to us. We likely will have limited control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we may not be successful in commercializing our product candidates.
The availability and amount of reimbursement for our product candidates, if approved, and the manner in which government and private payors may reimburse for any potential Tarmogen products, are uncertain.
In both U.S. and foreign markets, sales of any Tarmogen products will depend in part on the availability of reimbursement from third-party payors such as government health administration authorities, private health insurers and other organizations. The future magnitude of our revenues and profitability may be affected by the continuing efforts of governmental and third-party payors to contain or reduce the costs of health care. We cannot predict the effect that private sector or governmental health care reforms may have on our business, and there can be no assurance that any such reforms will not have a material adverse effect on our business, financial condition and results of operations.
In addition, in both the United States and elsewhere, sales of prescription drugs are dependent in part on the availability of reimbursement to the consumer from third-party payors, such as government and private insurance plans. The ability to obtain reimbursement of our products from these parties is a critical factor in the commercial success for any of our products. Failure to obtain reimbursement could result in reduced or no sales of our products.
Third-party payors are increasingly challenging the price and cost-effectiveness of medical products and services. Significant uncertainty exists as to the reimbursement status of newly-approved health care products. There can be no assurance that our products will be considered cost-effective or that adequate third-party reimbursement will be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. Legislation and regulations affecting the pricing of pharmaceuticals may change before any of our products are approved for marketing. Adoption of such legislation could further limit reimbursement for medical products and services.
Current and future legislation may increase the difficulty and cost of commercializing our product candidates, affect the prices we may obtain and limit reimbursement amounts.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could restrict or regulate post-approval activities and affect our revenues from future sales of our products.
The Medicare Modernization Act, or MMA, enacted in December 2003, has altered the way in which some physician-administered drugs and biologics, such as our product candidates, are reimbursed by Medicare Part B. Under this reimbursement methodology, physicians are reimbursed based on a product’s “average sales price.” This reimbursement methodology has generally led to lower reimbursement levels. This legislation also added an outpatient prescription drug benefit to Medicare, which went into effect in January 2006. These benefits are provided primarily through private entities, which we expect will attempt to negotiate price concessions from pharmaceutical manufacturers.
The Patient Protection and Affordable Care Act of 2010, or the PPACA, may have a significant impact on the healthcare system. As part of this legislative initiative, Congress enacted a number of provisions that are intended to reduce or limit the growth of healthcare costs, which could significantly change the market for pharmaceuticals and biological products. The provisions of the PPACA could, among other things, increase pressure on drug pricing or make it more costly for patients to gain access to prescription drugs like our product
29
Table of Contents
candidates at affordable prices. This could ultimately lead to fewer prescriptions for our product candidates and could force individuals who are prescribed our products to pay significant out-of-pocket costs or pay for the prescription entirely by themselves. As a result of such initiatives, market acceptance and commercial success of our products, once approved, may be limited and our business may be harmed.
Failure to attract and retain key personnel could impede our ability to develop our products and to obtain new collaborations or other sources of funding.
Because of the specialized scientific nature of our business and the unique properties of our Tarmogen platform, our success is highly dependent upon our ability to attract and retain qualified scientific and technical personnel, consultants and advisors. We are dependent on the principal members of our scientific and management staff, particularly Dr. Timothy C. Rodell. The loss of Dr. Rodell’s services might significantly delay or prevent the achievement of our research, development and business objectives. We currently maintain key-man life insurance on Dr. Rodell. However, we may not continue to maintain such insurance in the future or the proceeds of such insurance may not be adequate.
We will need to recruit a significant number of additional personnel in order to achieve our operating goals. In order to pursue our product development and marketing and sales plans, we will need to hire additional qualified scientific personnel to perform research and development, as well as personnel with expertise in clinical testing, government regulation, manufacturing, marketing and sales, which may strain our existing managerial, operational, regulatory compliance, financial and other resources. We also rely on consultants and advisors to assist in formulating our research and development strategy and adhering to complex regulatory requirements. We face competition for qualified individuals from numerous pharmaceutical and biotechnology companies, universities and other research institutions. There can be no assurance that we will be able to attract and retain such individuals on acceptable terms, if at all. Additionally, our facilities are located in Colorado, which may make attracting and retaining qualified scientific and technical personnel from outside of Colorado difficult. The failure to attract and retain qualified personnel, consultants and advisors could have a material adverse effect on our business, financial condition and results of operations.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and product candidates for the indications that we believe are the most scientifically and commercially promising. Our resource allocation decisions may cause us to fail to capitalize on viable scientific or commercial products or profitable market opportunities. In addition, we may spend valuable time and managerial and financial resources on research programs and product candidates for specific indications that ultimately do not yield any scientifically or commercially viable products. If we do not accurately evaluate the scientific and commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in situations where it would have been more advantageous for us to retain sole rights to development and commercialization.
RISKS RELATING TO MANUFACTURING ACTIVITIES
We have limited experience manufacturing our product candidates at commercial scale, and there can be no assurance that our product candidates can be manufactured in compliance with regulations at a cost or in quantities necessary to make them commercially viable. Our manufacturing facility has not been inspected by regulatory agencies and there can be no assurance that it will be acceptable for licensure by regulatory authorities or that we can contract to build acceptable facilities.
We have limited experience in commercial-scale manufacturing of Tarmogens. We may develop our manufacturing capacity in part by expanding our current facility or building additional facilities. This activity would require substantial additional funds and we would need to hire and train significant numbers of qualified
30
Table of Contents
employees to staff these facilities. We may not be able to develop commercial-scale manufacturing facilities that are adequate to produce materials for additional later-stage clinical trials or commercial use. Since our product candidates are produced by a biological process, we may find that our recombinant yeast strains will not grow at sites other than our facility, or do not result in a comparable product. All of our manufacturing of Tarmogen product candidates is currently performed in our Colorado facility. Damage to, or other impairment of, this facility could limit or eliminate our ability to manufacture Tarmogens.
We currently rely on CMOs for sterile fill and finish of our products, and these contractors currently fill our product candidates at a scale that is not adequate for commercial supply. Failure to find and maintain satisfactory commercial-scale fill and finish contractors could impair our ability to supply product for clinical and commercial needs. Additionally, we may decide to outsource some or all of our bulk product manufacturing activities to a third party CMO. Failure of any of these contractors to maintain compliance with cGMPs and other regulatory and legal requirements could result in a clinical hold on our clinical trials or other government actions that would limit or eliminate clinical trial and commercial product supply. Under any agreement with a CMO, we would have less control over the timing and quality of manufacturing than if we were perform such manufacturing ourselves. A CMO would be manufacturing other pharmaceutical products in the same facilities as our Tarmogen product candidates, increasing the risk of cross product contamination. Further, there is no guarantee that any CMO will continue ongoing operations, causing potential delays in product supply, reduced revenues and other liabilities for us.
The equipment and facilities employed in the manufacture of pharmaceuticals are subject to stringent qualification requirements by regulatory agencies, including validation of equipment, systems and processes. We may be subject to lengthy delays and expense in conducting validation studies, if we can meet the requirements at all. Our manufacturing facility has not been inspected by regulatory agencies and there can be no assurance that it will be acceptable for licensure by regulatory authorities.
If we are unable to manufacture or contract for a sufficient supply of our product candidates on acceptable terms, or if we encounter delays or difficulties in our manufacturing processes or our relationships with other manufacturers, our preclinical and clinical testing schedule would be delayed. This in turn would delay the submission of product candidates for regulatory approval and thereby delay the market introduction and subsequent sales of any products that receive regulatory approval, which would have a material adverse effect on our business, financial condition and results of operations. Furthermore, we or our contract manufacturers must supply all necessary documentation in support of our regulatory approval applications on a timely basis and must adhere to cGMP regulations enforced by the FDA and other regulatory bodies through their facilities inspection programs. If these facilities cannot pass a pre-approval plant inspection, the approval by the FDA or other regulatory bodies of the products will not be granted. If the FDA or a comparable foreign regulatory authority does not approve our facilities and processes for the manufacture of our product candidates or if they withdraw any such approval in the future, we may need to correct the issues or find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved.
We and our contract manufacturers are subject to significant regulation with respect to manufacturing of our products.
All entities involved in the preparation of a product candidate for clinical trials or commercial sale, including our manufacturing facility and our CMOs used for filling and finishing of our bulk product, are subject to extensive regulation. Components of a finished product approved for commercial sale or used in late-stage clinical trials must be manufactured in accordance with cGMP. These regulations govern manufacturing processes and procedures, including record keeping, and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Our facilities and quality systems and the facilities and quality systems of some or all of our third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of any regulatory approval
31
Table of Contents
of our product candidates. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our product candidates or the associated quality systems for compliance with the regulations applicable to the activities being conducted. The regulatory authorities also may, at any time following approval of a product for sale, audit our manufacturing facilities or those of our third-party contractors or raw material suppliers. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and time-consuming for us or a third party to implement and that may include the temporary or permanent suspension of a clinical trial or commercial sales or the temporary or permanent closure of a facility. Our third-party contractors or raw material suppliers may refuse to implement remedial measures required by regulatory authorities. Any failure to comply with applicable manufacturing regulations or failure to implement required remedial measures imposed upon us or third parties with whom we contract could materially harm our business.
We rely on relationships with third-party CMOs, which limits our ability to control the availability of, and manufacturing costs for, our product candidates.
Problems with any of our CMOs’ or raw material suppliers’ facilities or processes, could prevent or delay the production of adequate supplies of finished Tarmogens. This could delay clinical trials or delay and reduce commercial sales and materially harm our business. Any prolonged delay or interruption in the operations of our collaborators’ facilities or CMOs’ facilities could result in cancellation of shipments, loss of components in the process of being manufactured or a shortfall in availability of a product candidate or products. A number of factors could cause interruptions, including:
| • | the inability of a supplier to provide raw materials; |
| • | equipment malfunctions or failures at the facilities of our collaborators or suppliers; |
| • | high process failure rates; |
| • | damage to facilities due to natural or man-made disasters; |
| • | changes in regulatory requirements or standards that require modifications to our or our collaborators’ and suppliers’ manufacturing processes; |
| • | action by regulatory authorities or by us that results in the halting or slowdown of production of components or finished product at our facilities or the facilities of our collaborators or suppliers; |
| • | problems that delay or prevent manufacturing technology transfer to another facility, contract manufacturer or collaborator with subsequent delay or inability to start up a commercial facility; |
| • | a contract manufacturer or supplier going out of business, undergoing a capacity shortfall or otherwise failing to produce product as contractually required; |
| • | employee or contractor misconduct or negligence; |
| • | shipping delays, losses or interruptions; and |
| • | other similar factors. |
Because manufacturing processes are complex and are subject to a lengthy regulatory approval process, alternative qualified production capacity and sufficiently trained or qualified personnel may not be available on a timely or cost-effective basis or at all. Difficulties or delays in our CMOs’ production of drug substances could delay our clinical trials, increase our costs, damage our reputation and cause us to lose revenue and market share if we are unable to timely meet market demand for any products that are approved for sale.
The manufacturing process for our Tarmogen product candidates has several components that are sourced from a single manufacturer. If we utilize an alternative manufacturer or alternative component, we may be required to demonstrate comparability of the drug product before releasing the product for clinical use and we may not be to find an alternative supplier. For example, the stoppers used to seal the vials of our products are
32
Table of Contents
made by a single supplier using a proprietary formula and process. Any change to the stopper would require us to carry out lengthy studies to verify that our product remains stable with the replacement stopper. The loss of any of our current suppliers could result in manufacturing delays for the component substitution, and we may need to accept changes in terms or price from our existing supplier in order to avoid such delays.
Further, if our CMOs are not in compliance with regulatory requirements at any stage, including post-marketing approval, we may be fined, forced to remove a product from the market and/or experience other adverse consequences, including delays, which could materially harm our business.
We use and generate hazardous materials in our business and must comply with environmental laws and regulations, which can be expensive.
Our research, development and manufacturing involves the controlled use of hazardous materials, chemicals, various active microorganisms and volatile organic compounds, and we may incur significant costs as a result of the need to comply with numerous laws and regulations. For example, as a pharmacologically-active material, any residual Tarmogen in process-waste streams must be disposed of as hazardous waste. We are subject to laws and regulations enforced by the FDA, the Drug Enforcement Agency, foreign health authorities and other regulatory requirements, including the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act, and other current and potential federal, state, local and foreign laws and regulations governing the use, manufacture, storage, handling and disposal of our products, materials used to develop and manufacture our product candidates, and resulting waste products. Although we believe that our safety procedures for handling and disposing of such materials, and for killing any unused microorganisms before disposing of them, comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and any such liability could exceed our resources.
We replicate all yeast cells for our products internally and utilize a single manufacturing site to manufacture our clinical product candidates. Any disruption in the operations of our manufacturing facility would have a significant negative impact on our ability to manufacture products for clinical testing and would result in increased costs and losses.
We grow all yeast cells for our products internally using a complex process. Any disruption of our operations could result in manufacturing delays due to the inability to purchase the cell lines from outside sources. We have only one manufacturing facility in which we can manufacture clinical products. In the event of a physical catastrophe at our manufacturing or laboratory facilities, we could experience costly delays in reestablishing manufacturing capacity due to a lack of redundancy in manufacturing capability.
Consistent manufacture of our products relies on maintenance of a master yeast bank, or MYB, as an essential starting material for all production. We currently store our MYB in ultra-low temperature freezers at two geographically distinct locations. We may discover storage stability problems that prevent use of the MYB. We may also suffer catastrophic events at the two storage locations that would destroy all available stocks of the MYB. Should we lose the MYB of any of our products we would experience significant delays in producing and gaining regulatory approval to use a replacement MYB. We may not be able to replicate the original MYB with sufficient fidelity to assure regulatory authorities that we are able to produce a comparable product, which could require us to perform clinical trials to gain approval of product made with the replacement MYB. This could result in a lengthy period in which we are unable to manufacture product candidates for clinical trials or, if any of our product candidates are approved, for sale.
Our manufacturing facility contains specialized equipment and utilizes complicated production processes developed over a number of years, which would be difficult, time-consuming and costly to replace. Any prolonged disruption in the operations of our manufacturing facility would have a significant negative impact on our ability to manufacture products for clinical testing on our own and would cause us to seek additional third-party manufacturing contracts, thereby increasing our development costs. We may suffer losses as a result of
33
Table of Contents
business interruptions that exceed the coverage available under our insurance policies or any losses may be excluded under our insurance policies. Certain events, such as natural disasters, fire, political disturbances, sabotage or business accidents, which could impact our current or future facilities, could have a significant negative impact on our operations by disrupting our product development efforts until such time as we are able to repair our facility or put in place third-party CMOs to assume this manufacturing role.
During the course of the product life cycle we will make process changes to scale up manufacturing to commercial manufacture or transfer the production to alternate sites or CMOs. Our ability to successfully implement these changes will depend on our ability to demonstrate, to the satisfaction of the FDA and other regulatory agencies that the product made by the new process or at the new site is comparable to the original product.
In the event that manufacturing process changes are necessary for the further development of a product candidate, we may not be able to reach agreement with regulatory agencies on the criteria for demonstrating comparability to the original product, which would require us to repeat clinical studies performed with the original product. This could result in lengthy delays in implementing the new process or site and substantial lost sales as a result of our inability to meet commercial demand. If we reach agreement with regulatory agencies on the criteria for establishing comparability, we may not be able to meet these criteria or may suffer lengthy delays in meeting these criteria. This may result in significant lost sales due to inability to meet commercial demand with the original product. Furthermore, studies to demonstrate comparability, or any other studies on the new process or site such as validation studies, may uncover findings that result in regulatory agencies delaying or refusing to approve the new process or site.
RISKS RELATING TO REGULATION OF OUR INDUSTRY
The biopharmaceutical industry is subject to significant regulation and oversight in the United States, in addition to approval of products for sale and marketing.
In addition to FDA restrictions on marketing of biopharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the biopharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes.
The federal health care program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any health care item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Several pharmaceutical and other health care companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of marketing of the product for unapproved, and thus non-reimbursable, uses. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment.
34
Table of Contents
Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of these laws, which could have a material adverse effect on our business, financial condition and results of operations.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
If we obtain FDA approval for any of our product candidates and begin commercializing those products in the United States, our operations may be directly, or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal anti-kickback statute. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology and Clinical Health Act and its implementing regulations, which impose certain requirements relating to the privacy, security and transmission of individually identifiable health information; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing standards we have established, comply with federal and state health-care fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent misconduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
35
Table of Contents
Health care reform measures could adversely affect our business.
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs. Most recently, in March 2010 the Patient Protection and Affordable Health Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively the PPACA, was enacted, which includes measures to significantly change the way health care is financed by both governmental and private insurers. Among the provisions of the PPACA of greatest importance to the pharmaceutical and biotechnology industry are the following:
| • | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, that began in 2011; |
| • | new requirements to report certain financial arrangements with physicians and others, including reporting any “transfer of value” made or distributed to prescribers and other healthcare providers and reporting any investment interests held by physicians and their immediate family members; |
| • | a licensure framework for follow-on biologic products; |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
| • | creation of the Independent Payment Advisory Board which, beginning in 2014, will have authority to recommend certain changes to the Medicare program that could result in reduced payments for prescription drugs and those recommendations could have the effect of law even if Congress does not act on the recommendations; and |
| • | establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending that began on January 1, 2011. |
Many of the details regarding the implementation of the PPACA are yet to be determined, and at this time, the full effect that the PPACA would have on our business remains unclear. In particular, there is uncertainty surrounding the applicability of the biosimilars provisions under the PPACA to our Tarmogen product candidates. The PPACA allows applicants seeking approval of biosimilar or interchangeable versions of biological products to initiate a process for challenging some or all of the patents covering the innovator biological product used as the reference product. This process is complicated and could result in the limitation or loss of certain patent rights. The FDA has issued several guidance documents, but no implementing regulations, on biosimilars and no biosimilar applications have yet been approved. It is not certain that we will receive 12 years of biologics marketing exclusivity for any of our products. The regulations that are ultimately promulgated and their implementation are likely to have considerable impact on the way we conduct our business and may require us to change current strategies. A biosimilar is a biological product that is highly similar to an approved drug notwithstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences between the biological product and the approved drug in terms of the safety, purity, and potency of the product.
Individual states have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and marketing cost disclosure and transparency measures, and designed to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce ultimate demand for our products or put pressure on our product pricing, which could negatively affect our business, results of operations, financial condition and prospects.
36
Table of Contents
In addition, given recent federal and state government initiatives directed at lowering the total cost of healthcare, Congress and state legislatures will likely continue to focus on healthcare reform, the cost of prescription drugs and biologics and the reform of the Medicare and Medicaid programs. While we cannot predict the full outcome of any such legislation, it may result in decreased reimbursement for drugs and biologics, which may further exacerbate industry-wide pressure to reduce prescription drug prices. This could harm our ability to generate revenues. Increases in importation or re-importation of pharmaceutical products from foreign countries into the United States could put competitive pressure on our ability to profitably price our products, which, in turn, could adversely affect our business, results of operations, financial condition and prospects. We might elect not to seek approval for or market our products in foreign jurisdictions in order to minimize the risk of re-importation, which could also reduce the revenue we generate from our product sales. It is also possible that other legislative proposals having similar effects will be adopted.
Furthermore, regulatory authorities’ assessment of the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information, including on other products, changing policies and agency funding, staffing and leadership. We cannot be sure whether future changes to the regulatory environment will be favorable or unfavorable to our business prospects. For example, average review times at the FDA for marketing approval applications can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes.
RISKS RELATING TO COMPETITIVE FACTORS
We compete in an industry characterized by extensive research and development efforts and rapid technological progress. New discoveries or commercial developments by our competitors could render our potential products obsolete or non-competitive.
New developments occur and are expected to continue to occur at a rapid pace in our industry, and there can be no assurance that discoveries or commercial developments by our competitors will not render some or all of our potential products obsolete or non-competitive, which could have a material adverse effect on our business, financial condition and results of operations.
We expect to compete with fully integrated and well-established pharmaceutical and biotechnology companies in the near- and long-term. Most of these companies have substantially greater financial, research and development, manufacturing and marketing experience and resources than we do and represent substantial long-term competition for us. Such companies may succeed in discovering and developing pharmaceutical products more rapidly than we do or pharmaceutical products that are safer, more effective or less costly than any that we may develop. Such companies also may be more successful than we are in manufacturing, sales and marketing. Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical and established biotechnology companies. Academic institutions, governmental agencies and other public and private research organizations also conduct clinical trials, seek patent protection and establish collaborative arrangements for the development of product candidates.
We expect competition among products will be based on product efficacy and safety, the timing and scope of regulatory approvals, availability of supply, marketing and sales capabilities, reimbursement coverage, price and patent position. There can be no assurance that our competitors will not develop safer and more effective products, commercialize products earlier than we do, or obtain patent protection or intellectual property rights that limit our ability to commercialize our products.
There can be no assurance that our issued patents or pending patent applications, if issued, will not be challenged, invalidated or circumvented or that the rights granted thereunder will provide us with proprietary protection or a competitive advantage.
37
Table of Contents
Our competitors may develop and market products that are less expensive, more effective, safer or reach the market sooner than our product candidates, which may diminish or eliminate the commercial success of any products we may commercialize.
The biopharmaceutical industry is highly competitive. There are many public and private biopharmaceutical companies, public and private universities and research organizations actively engaged in the discovery and research and development of products for cancer and infectious disease. Given the significant unmet patient need for new therapies, oncology is an area of focus for large and small companies as well as research institutions. As a result, there are and will likely continue to be extensive research and substantial financial resources invested in the discovery and development of new oncology products. In addition, there are a number of multinational pharmaceutical companies and large biotechnology companies currently marketing or pursuing the development of products or product candidates targeting the same cancer indications as our product candidates, and several large public biopharmaceutical companies have approved or are developing cancer immunotherapy products, including Dendreon Corporation, Bristol-Myers Squibb Company, GlaxoSmithKline plc, Merck & Co. and Merck KGaA.
There are several marketed products indicated for pancreas cancer, including Astellas Pharma Inc.’s erlotinib, Celgene Corporation’s protein bound paclitaxel protein-bound particles for injectable suspension (albumin-bound), Teva Pharmaceutical Industries Limited’s streptozocin, and gemcitabine, fluorouracil, or 5-FU, and mitomycin that are marketed by several generic pharmaceutical firms. In addition, there are multiple companies or institutions conducting clinical trials of immunotherapy products in pancreas cancer including NCI, Bavarian Nordic, NewLink Genetics Corporation, Aduro BioTech Inc., Sidney Kimmel Comprehensive Cancer Center, Providence Health & Services, Duke University, Advantagene, Inc. and AlphaVax, Inc.
There are numerous marketed therapeutics indicated for NSCLC, including Roche Holding AG’s bevacizumab, Eli Lilly’s pemetrexed, Astellas Pharma’s erlotinib, AstraZeneca PLC’s gefitinib, as well as generically available gemcitabine, platinum-based chemotherapeutics (cisplatin, oxaliplatin and carboplatin) and mitotic inhibitors (paclitaxel and vinorelbine), which are marketed by several generic pharmaceutical firms. In addition, there are multiple companies or institutions with clinical trials of immunotherapy products in late stage lung cancer, including NIH/NCI, UbiVac, University of Pittsburgh, Oslo University, Lee Moffitt Cancer Center, Shiga University, Kael-GemVax Co., Recombio SL, Bioven Sdn., Vaxn Biotech and New Link Genetics.
There are numerous marketed therapeutics indicated for colorectal cancer, including Roche Holding AG’s bevacizumab, Bristol Myers-Squibb’s cetuximab, Amgen’s panitumumab, as well as irinotecan, oxaliplatin, leucovorin and 5-FU, which are marketed by several generic pharmaceutical firms. In addition, there are multiple companies or institutions with clinical trials of immunotherapy products in colorectal cancer including National Cancer Institute, Instituto Cientifico y Tecnological de Navara, Stanford University, Radboud University, University of Michigan Cancer Center, AlphaVax Inc., Duke University, Immunovative Therapies Ltd. and Ohio State University Comprehensive Cancer Center.
AstraZeneca’s vandetanib and Exelixis’ cabozantinib are FDA approved for late-stage, or metastatic, MTC in adult patients who are ineligible for resection. Further, there are several companies or institutions with clinical trials of immunotherapy products generally targeting carcinoembryonic antigen, or CEA, including Bavarian Nordic, NCI and Radboud University.
There are several marketed therapeutics indicated for the treatment of chronic HBV infection, including Roche Holding AG’s pegylated interferon 2a, Gilead’s tenofovir and adefovir, Bristol Myers-Squibb’s entecavir, Novartis’ telbivudine, and lamivudine, which is marketed by several generic pharmaceutical firms. In addition, there are several companies or institutions with clinical trials of immunotherapy products for the treatment of chronic HBV infection including Transgene, Inc, Chongqing Jiachen Biotechnology, Emergent Biosolutions, Shanghai Medical University, Dynavax Technologies Corporation, Institut Pasteur, Pohang University of Science and Technology and Vaxine Pty. Ltd. Additionally, Arrowhead Research Corp. is investigating an RNAi therapeutic for the treatment of HBV.
38
Table of Contents
Many of our competitors, either alone or with their strategic collaborators, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of drugs, obtaining FDA and other regulatory approvals, and the commercialization of those products. Accordingly, our competitors may be more successful in obtaining approval for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive before we can recover the significant expenses of developing and commercializing any of our product candidates. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available.
There are many different approaches to using immunotherapies to treat cancer, including anti-idiotype, whole cell, DNA, peptide/antigen, viral, tumor lysate, immune check-point inhibitors, shed antigens, and dendritic cells. Cancer immunotherapies are also distinguished by whether or not they are derived from autologous or allogeneic sources. Each of the various approaches to cancer immunotherapy has potential advantages and disadvantages based on factors such as its immunostimulatory mechanisms, formulation characteristics and manufacturing requirements.
We also compete with other clinical-stage companies and institutions for clinical trial participants, which could reduce our ability to recruit participants for our clinical trials. Delay in recruiting clinical trial participants could adversely affect our ability to bring a product to market prior to our competitors. Further, research and discoveries by others may result in breakthroughs that render our product candidates obsolete even before they begin to generate any revenue.
In addition, our competitors may obtain patent protection or FDA approval and commercialize products more rapidly than we do, which may impact future sales of any of our product candidates that receive marketing approval. If the FDA approves the commercial sale of any of our product candidates, we will also be competing with respect to marketing capabilities and manufacturing efficiency, areas in which we have limited or no experience. We expect competition among products will be based on product efficacy and safety, the timing and scope of regulatory approvals, availability of supply, marketing and sales capabilities, product price, reimbursement coverage by government and private third-party payors, and patent position. Our profitability and financial position will suffer if our products receive regulatory approval, but cannot compete effectively in the marketplace.
If any of our product candidates are approved and commercialized, we may face competition from biosimilars. The route to market for biosimilars was established with the passage of the BPCIA in March 2010, providing 12 years of marketing exclusivity for reference products and an additional six months of exclusivity if pediatric studies are conducted. In Europe, the European Medicines Agency has issued guidelines for approving products through an abbreviated pathway, and biosimilars have been approved in Europe. If a biosimilar version of one of our potential products were approved in the United States or Europe, it could have a negative effect on sales and gross profits of the potential product and our financial condition.
Even if we achieve market acceptance for our products, we may experience downward pricing pressure on the price of our drugs because of generic and biosimilar competition and social pressure to lower the cost of drugs.
Several of the FDA approved products for HBV face patent expiration in the next several years. As a result, generic versions and biosimilars of these drugs and biologicals may become available. We expect to face competition from these products, including price-based competition. Pressure from government and private reimbursement groups, plus patient awareness and other social activist groups to reduce drug prices may also put downward pressure on the prices of drugs, including our product candidates, if they are commercialized. Also, if a biosimilar to any of our product candidates is approved by regulatory agencies, there will be significant pricing pressure on our products, causing us or our collaborators to reduce the sales price of our products.
39
Table of Contents
Our product candidates may not be accepted in the marketplace; therefore, we may not be able to generate significant revenue, if any.
Even if our Tarmogen product candidates are approved for sale, physicians and the medical community may not ultimately use them or may use them only in applications more restricted than we expect. Our product candidates, if successfully developed, will compete with a number of traditional products and immunotherapies manufactured and marketed by major pharmaceutical and other biotechnology companies. Our product candidates will also compete with new products currently under development by such companies and others. Physicians will prescribe a product only if they determine, based on experience, clinical data, side effect profiles, reimbursement for their patients and other factors, that it is beneficial as compared to other products currently in use. Many other factors influence the adoption of new products, including marketing and distribution restrictions, course of treatment, adverse publicity, product pricing, the views of thought leaders in the medical community and reimbursement by government and private third-party payors.
For our products that are developed in combination with other therapies, changes in standard of care or use patterns could make those combinations obsolete. For example, we are developing GI-4000 for pancreas cancer in combination with gemcitabine. If GI-4000 is approved for marketing in combination with gemcitabine and use of another therapy becomes more prevalent than gemcitabine, sales of the combination of GI-4000 with gemcitabine could be negatively impacted and our financial results and the value of our securities would be adversely affected.
RISKS RELATING TO OUR ARRANGEMENTS WITH THIRD PARTIES
We rely on third parties to conduct our non-clinical studies and some of our clinical trials. If these third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for our product candidates, or we may be delayed in doing so.
We often rely on third parties, such as CROs, medical institutions, academic institutions, clinical investigators and contract laboratories, to conduct our non-clinical studies and clinical trials. For example, the NCI is conducting clinical trials for GI-6207 and GI-6301 and we are supporting the investigator-initiated GI-4000-03 clinical trial in NSCLC at MSKCC. We are responsible for confirming that our preclinical studies are conducted in accordance with applicable regulations and that each of our clinical trials is conducted in accordance with its general investigational plan and protocol. The FDA requires us to comply with Good Laboratory Practice for conducting and recording the results of our preclinical studies and Good Clinical Practices, or GCP, for conducting, monitoring, recording and reporting the results of clinical trials, to ensure that data and reported results are accurate and that the clinical trial participants are adequately protected. Our reliance on third parties does not relieve us of these responsibilities. If the third parties conducting our clinical trials do not perform their contractual duties or obligations, do not meet expected deadlines, fail to comply with GCP, do not adhere to our clinical trial protocols or otherwise fail to generate reliable clinical data, we may need to enter into new arrangements with alternative third parties and our clinical trials may be more costly than expected or budgeted, extended, delayed or terminated or may need to be repeated, and we may not be able to obtain regulatory approval for or commercialize the product candidate being tested in such trials.
Further, if our CMOs are not in compliance with regulatory requirements at any stage, including post-marketing approval, we may be fined, forced to remove a product from the market and/or experience other adverse consequences, including delays, which could materially harm our business.
We may explore strategic collaborations that may never materialize or may fail.
We may, in the future, periodically explore a variety of possible strategic collaborations in an effort to gain access to additional product candidates or resources. At the current time, we cannot predict what form such a strategic collaboration might take. We are likely to face significant competition in seeking appropriate strategic collaborators, and these strategic collaborations can be complicated and time-consuming to negotiate and document. We may not be able to negotiate strategic collaborations on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional strategic collaborations because of the numerous risks and uncertainties associated with establishing strategic collaborations.
40
Table of Contents
RISKS RELATING TO PROTECTING OUR INTELLECTUAL PROPERTY
If we are unable to protect our proprietary rights or to defend against infringement claims, we may not be able to compete effectively or operate profitably.
Our success will depend, in part, on our ability to obtain patents, operate without infringing the proprietary rights of others and maintain trade secrets, both in the United States and other countries. Patent matters in the biotechnology and pharmaceutical industries can be highly uncertain and involve complex legal and factual questions. Accordingly, the validity, breadth and enforceability of our patents and the existence of potentially blocking patent rights of others cannot be predicted, either in the United States or in other countries.
There can be no assurance that we will discover or develop patentable products or processes or that patents will issue from any of the currently pending patent applications or that claims granted on issued patents will be sufficient to protect our technology or adequately cover the actual products we may actually sell. Potential competitors or other researchers in the field may have filed patent applications, been issued patents, published articles or otherwise created prior art that could restrict or block our efforts to obtain additional patents. There also can be no assurance that our issued patents or pending patent applications, if issued, will not be challenged, invalidated, rendered unenforceable or circumvented or that the rights granted hereunder will provide us with proprietary protection or competitive advantages. Our patent rights also depend on our compliance with technology and patent licenses upon which our patent rights are based and upon the validity of assignments of patent rights from consultants and other inventors that were, or are, not employed by us.
In addition, competitors may manufacture and sell our potential products in those foreign countries where we have not filed for patent protection or where patent protection may be unavailable, not obtainable or ultimately not enforceable. In addition, even where patent protection is obtained, third-party competitors may challenge our patent claims in the various patent offices, for example via opposition in the European Patent Office or reexamination, interparties review, post-grant review or interference proceedings in the United States Patent and Trademark Office, or USPTO. The ability of such competitors to sell such products in the United States or in foreign countries where we have obtained patents is usually governed by the patent laws of the countries in which the product is sold.
We will incur significant ongoing expenses in maintaining our patent portfolio. Should we lack the funds to maintain our patent portfolio or to enforce our rights against infringers, we could be adversely impacted. Even if claims of infringement are without merit, any such action could divert the time and attention of management and impair our ability to access additional capital and/or cost us significant funds to defend.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| • | Others may be able to make compounds that are similar to our product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed. |
| • | We or our licensors or strategic collaborators might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed. |
| • | We or our licensors or strategic collaborators might not have been the first to file patent applications covering certain of our inventions. |
| • | Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights. |
| • | It is possible that our pending patent applications will not lead to issued patents. |
41
Table of Contents
| • | Issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors. |
| • | Our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets. |
| • | We may not develop additional proprietary technologies that are patentable. |
| • | The patents of others may have an adverse effect on our business. |
Should any of these events occur, they could significantly harm our business, results of operations and prospects.
Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. Many of the substantive changes to patent law associated with the Leahy-Smith Act have only become effective within the last year. Accordingly, it is yet not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business, our current and pending patent portfolio and future intellectual property strategy. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
We may be subject to litigation with respect to the ownership and use of intellectual property that will be costly to defend or pursue and uncertain in its outcome.
Our success also will depend, in part, on our refraining from infringing patents or otherwise violating intellectual property owned or controlled by others. Pharmaceutical companies, biotechnology companies, universities, research institutions and others may have filed patent applications or have received, or may obtain, issued patents in the United States or elsewhere relating to aspects of our technology. It is uncertain whether the issuance of any third-party patents will require us to alter our products or processes, obtain licenses, or cease certain activities. Some third-party applications or patents may conflict with our issued patents or pending applications. Any such conflict could result in a significant reduction of the scope or value of our issued or licensed patents.
In addition, if patents issued to other companies contain blocking, dominating or conflicting claims and such claims are ultimately determined to be valid, we may be required to obtain licenses to these patents or to develop or obtain alternative non-infringing technology and cease practicing those activities, including potentially manufacturing or selling any products deemed to infringe those patents. If any licenses are required, there can be no assurance that we will be able to obtain any such licenses on commercially favorable terms, if at all, and if these licenses are not obtained, we might be prevented from pursuing the development and commercialization of certain of our potential products. Our failure to obtain a license to any technology that we may require to commercialize our products on favorable terms may have a material adverse impact on our business, financial condition and results of operations.
Litigation, which could result in substantial costs to us (even if determined in our favor), may also be necessary to enforce any patents issued or licensed to us or to determine the scope and validity of the proprietary rights of others. The FDA has published draft guidance documents for implementation of the Biologics Price Competition and Innovation Act (BPCIA) under the PPACA, related to the development of follow-on biologics (biosimilars), although detailed guidance for patent litigation procedures under this act has not yet been provided.
42
Table of Contents
If another company files for approval to market a competing follow-on biologic, and/or if such approval is given to such a company, we may be required to promptly initiate patent litigation to prevent the marketing of such biosimilar version of our product prior to the normal expiration of the patent. There can be no assurance that our issued or licensed patents would be held valid by a court of competent jurisdiction or that any follow-on biologic would be found to infringe our patents.
In addition, if our competitors file or have filed patent applications in the United States that claim technology also claimed by us, we may have to participate in interference proceedings to determine priority of invention. These proceedings, if initiated by the USPTO, could result in substantial costs to us, even if the eventual outcome is favorable to us. Such proceedings can be lengthy, are costly to defend and involve complex questions of law and fact, the outcomes of which are difficult to predict. Moreover, we may have to participate in post-grant review proceedings or third-party ex parte reexamination or inter partes review proceedings under the USPTO. An adverse outcome with respect to a third-party claim or in an interference proceeding could subject us to significant liabilities, require us to license disputed rights from third parties, or require us to cease using such technology, any of which could have a material adverse effect on our business, financial condition and results of operations.
We also rely on trade secrets to protect technology, especially where patent protection is not believed to be appropriate or obtainable or where patents have not issued. For example, our manufacturing process involves a number of trade secret steps, processes, and conditions. We attempt to protect our proprietary technology and processes, in part, with confidentiality agreements and assignment of invention agreements with our employees and confidentiality agreements with our consultants and certain contractors. There can be no assurance that these agreements will not be breached, that we would have adequate remedies for any breach, or that our trade secrets will not otherwise become known or be independently discovered by competitors. We may fail in certain circumstances to obtain the necessary confidentiality agreements, or their scope or term may not be sufficiently broad to protect our interests.
If our trade secrets or other intellectual property become known to our competitors, it could result in a material adverse effect on our business, financial condition and results of operations. To the extent that we or our consultants or research collaborators use intellectual property owned by others in work for us, disputes may also arise as to the rights to related or resulting know-how and inventions.
The patent protection and patent prosecution for some of our product candidates is dependent or may be dependent in the future on third parties.
While we normally seek and gain the right to fully prosecute the patents relating to our product candidates, there may be times when platform technology patents or product-specific patents that relate to our product candidates are controlled by our licensors. This is the case with our license of patents related to CEA from the National Institutes of Health. In addition, our licensors and/or licensees may have back-up rights to prosecute patent applications in the event that we do not do so or choose not to do so, and our licensees may have the right to assume patent prosecution rights after certain milestones are reached. If any of our licensing collaborators fails to appropriately prosecute and maintain patent protection for patents covering any of our product candidates, our ability to develop and commercialize those product candidates may be adversely affected and we may not be able to prevent competitors from making, using and selling competing products.
43
Table of Contents
RISKS RELATING TO OUR EXPOSURE TO LITIGATION
We are exposed to potential product liability or similar claims, and insurance against these claims may not be available to us at a reasonable rate in the future.
Our business exposes us to potential liability risks that are inherent in the testing, manufacturing and marketing of human therapeutic products. Clinical trials involve the testing of product candidates on human subjects or volunteers under a research plan, and carry a risk of liability for personal injury or death to patients due to unforeseen adverse side effects, improper administration of the product candidate, or other factors. Many of these patients are already seriously ill and are therefore particularly vulnerable to further illness or death.
We currently carry clinical trial liability insurance in the amount of $5 million in the aggregate, but there can be no assurance that we will be able to maintain such insurance or that the amount of such insurance will be adequate to cover claims. We could be materially and adversely affected if we were required to pay damages or incur defense costs in connection with a claim outside the scope of indemnity or insurance coverage, if the indemnity is not performed or enforced in accordance with its terms, or if our liability exceeds the amount of applicable insurance. In addition, there can be no assurance that insurance will continue to be available on terms acceptable to us, if at all, or that if obtained, the insurance coverage will be sufficient to cover any potential claims or liabilities. Similar risks would exist upon the commercialization or marketing of any products by us or our collaborators.
Regardless of their merit or eventual outcome, product liability claims may result in:
| • | decreased demand for our product; |
| • | injury to our reputation and significant negative media attention; |
| • | withdrawal of clinical trial volunteers; |
| • | costs of litigation; |
| • | distraction of management; and |
| • | substantial monetary awards to plaintiffs. |
Should any of these events occur, it could have a material adverse effect on our business and financial condition.
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to the Company.
Our amended and restated certificate of incorporation provides that we will indemnify our directors to the fullest extent permitted by Delaware law.
In addition, as permitted by Section 145 of the Delaware General Corporation Law, our amended and restated bylaws and the indemnification agreements that we have entered into with our directors and executive officers provide that:
| • | We will indemnify our directors and executive officers for serving us in those capacities or for serving other business enterprises at our request, to the fullest extent permitted by Delaware law. Delaware law provides that a corporation may indemnify such person if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal proceeding, had no reasonable cause to believe such person’s conduct was unlawful. |
| • | We may, in our discretion, indemnify other officers, employees and agents in those circumstances where indemnification is permitted by applicable law. |
44
Table of Contents
| • | We are required to advance expenses, as incurred, to our directors and executive officers in connection with defending a proceeding, except that such directors or executive officers shall undertake to repay such advances if it is ultimately determined that such person is not entitled to indemnification. |
| • | We will not be obligated pursuant to our amended and restated bylaws to indemnify any director or executive officer in connection with any proceeding (or part thereof) initiated by such person unless (i) such indemnification is expressly required to be made by law, (ii) the proceeding was authorized by our Board of Directors, (iii) such indemnification is provided by us, in our sole discretion, pursuant to the powers vested in the corporation under applicable law or (iv) such indemnification is required to be made pursuant to our amended and restated bylaws. |
| • | The rights conferred in our amended and restated bylaws are not exclusive, and we are authorized to enter into indemnification agreements with our directors, officers, employees and agents and to obtain insurance to indemnify such persons. |
| • | We may not retroactively amend our amended and restated bylaw provisions to reduce our indemnification obligations to directors, officers, employees and agents. |
As a result, if we are required to indemnify one or more of our directors or executive officers, it may reduce our available funds to satisfy successful third-party claims against us, may reduce the amount of money available to us and may have a material adverse effect on our business and financial condition.
OFFERING RISKS
We do not know whether a market will develop for our common stock or what the market price of our common stock will be and, as a result, it may be difficult for you to sell your shares of our common stock.
Before this offering, there was no public trading market for our common stock and there can be no assurance that a regular trading market will develop and continue after this offering or that the market price of our common stock will not decline, perhaps substantially, below the initial public offering price. The initial public offering price has been determined through negotiations between us and the underwriter and may not be indicative of the market price of our common stock following this offering. Among the factors considered in such negotiations were prevailing market conditions; our results of operations and financial condition; financial and operating information and market valuations with respect to other companies that we and the underwriter believe to be comparable or similar to us; the present state of our development; and our future prospects. See the “Underwriting” section of this prospectus for additional information. If you purchase shares of our common stock, you may not be able to resell those shares at or above the initial public offering price. We cannot predict the extent to which investor interest in our company will lead to the development of an active trading market on The NASDAQ Global Market or otherwise or how liquid that market might become. If a market for our common stock does not develop or is not sustained, it may be difficult for you to sell your shares of common stock at an attractive price or at all. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic collaborations or acquire companies or products by using our shares of common stock as consideration. We cannot predict the prices at which our common stock will trade. It is possible that in one or more future periods our results of operations may be below the expectations of public market analysts and investors and, as a result of these and other factors, the price of our common stock may fall.
The market price of our common stock may be highly volatile.
The trading price of our common stock is likely to be highly volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including those described elsewhere in this “Risk Factors” section in this prospectus and the following:
| • | new products, product candidates or new uses for existing products introduced or announced by our competitors or our collaborators, and the timing of these introductions or announcements; |
| • | actual or anticipated results from and any delays in our clinical trials as well as results of regulatory reviews relating to the approval of our product candidates; |
45
Table of Contents
| • | variations in the level of expenses related to any of our product candidates or clinical development programs, including relating to the timing of invoices from, and other billing practices of, our CROs and clinical trial sites; |
| • | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| • | announcements by us or our competitors of significant acquisitions, strategic collaborations, joint ventures and capital commitments; |
| • | additions or departures of key scientific or management personnel; |
| • | changes in the status of our relationships with Celgene, Gilead, NCI and other collaborators; |
| • | conditions or trends in the biotechnology and biopharmaceutical industries; |
| • | actual or anticipated changes in earnings estimates, development timelines or recommendations by securities analysts; |
| • | actual and anticipated fluctuations in our quarterly operating results; |
| • | financial projections we may provide to the public, any changes in these projections or our failure to meet these projections; |
| • | deviations from securities analysts’ estimates or the impact of other analyst ratings downgrades by any securities analysts who follow our common stock; |
| • | the results of our efforts to discover, develop, acquire or in-license additional product candidates or products; |
| • | other events or factors, including those resulting from war, incidents of terrorism, natural disasters or responses to these events; |
| • | changes in accounting principles or accounting judgments; |
| • | discussion of us or our stock price by the financial and scientific press and in online investor communities; |
| • | general economic and market conditions and other factors that may be unrelated to our operating performance or the operating performance of our competitors, including changes in market valuations of similar companies; and |
| • | sales of common stock by us or our stockholders in the future, as well as the overall trading volume of our common stock. |
In addition, the stock market in general and the market for biotechnology and biopharmaceutical companies in particular have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market, securities class action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could materially and adversely affect our business and financial condition.
Our management has broad discretion in using the net proceeds from this offering.
We have stated, in only a general manner, how we intend to use the net proceeds from this offering. See “Use of Proceeds.” We cannot, with any assurance, be more specific at this time. We will have broad discretion in the timing of the expenditures and application of proceeds received in this offering. If we fail to apply the net proceeds effectively, we may not be successful in bringing our proposed products to market. You will not have the opportunity to evaluate all of the economic, financial or other information upon which we may base our decisions to use the net proceeds from this offering.
46
Table of Contents
After this offering, our principal stockholders and management own a significant percentage of our stock and will be able to exercise significant influence over matters subject to stockholder approval.
After this offering, our executive officers, directors and principal security holders, together with their respective affiliates, owned approximately 43.5% of our securities, including shares issuable upon conversion of our preferred securities and shares subject to outstanding options and warrants that are exercisable within 60 days of the date hereof and excluding any shares of common stock that these stockholders may purchase in this offering. Accordingly, these security holders will be able to exert a significant degree of influence over our management and affairs and over matters requiring security holder approval, including the election of our Board of Directors, future issuances of our securities, declaration of dividends and approval of other significant corporate transactions. This concentration of ownership could have the effect of delaying or preventing a change-of-control of the Company or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material and adverse effect on the fair market value of our securities.
A significant portion of our total outstanding shares may be sold into the public market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time after the expiration of the lock-up agreements described in the “Underwriting” section of this prospectus. These sales, or the market perception that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. Upon completion of this offering, we will have outstanding a total of 5,136,400 shares of common stock, assuming no exercise of the underwriter’s over-allotment option. Except for the shares sold to the public in this offering and any shares sold upon exercise of the underwriter’s over-allotment option, none of these shares will be freely tradable, without restriction, in the public market immediately following this offering. In addition, the underwriter, may, in its sole discretion, permit our officers, directors and other stockholders who are subject to lock-up agreements to sell shares prior to the expiration of the lock-up agreements.
As of March 31, 2014, there were 235,342 shares subject to outstanding options and an additional 793,687 shares reserved for future issuance under our employee benefit plans. All of the foregoing shares will become eligible for sale in the public market to the extent permitted by any applicable vesting requirements, the lock-up agreements and Rules 144 and 701 under the Securities Act of 1933, as amended, or the Securities Act. Upon completion of this offering, and assuming an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, there will be an additional 104,696 shares of common stock issuable upon exercise of outstanding warrants to purchase preferred stock, which will convert into warrants to purchase common stock, 31,250 shares of common stock issuable upon the exercise of a warrant to purchase common stock to be issued to the underwriter or its designees, 7,671 shares of common stock issuable upon the exercise of a warrant to purchase common stock to be issued to Cooley LLP, 468,769 shares of common stock issuable upon the exercise of the 2014 Warrants by the holders thereof, and 115,940 shares of common stock issuable upon the exercise of outstanding warrants to purchase our capital stock, which will convert into warrants to purchase common stock, held by the underwriter and its designees, all of which will be eligible for sale in the public market to the extent permitted by the same restrictions, except for the warrant to be issued to the underwriter, and the common stock issuable upon exercise thereof, which are registered hereby. Moreover, upon completion of this offering, holders of an aggregate of 2,851,273 shares of our common stock will have rights, subject to some conditions, to require us to file a registration statement covering their shares or to include their shares in a registration statement that we may file for ourselves or other stockholders. If such holders, by exercising their registration rights, cause a large number of securities to be registered and sold into the public market, these sales could have an adverse effect on the market price for our common stock. We also intend to register all shares of common stock that we may issue under our employee benefit plans. Shares so registered may be freely sold in the public market upon issuance, subject to the lock-up agreements and the restrictions imposed on our affiliates under Rule 144.
47
Table of Contents
If you purchase common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
If you purchase common stock in this offering, you will pay more for your shares than our pro forma as adjusted net tangible book value per share. Based upon an assumed initial public offering price of $16.00 per share, the midpoint of the range on the cover page of this prospectus, you will incur immediate and substantial dilution of approximately $12.71 per share, representing the difference between our assumed initial public offering price and our pro forma as adjusted net tangible book value per share. Based upon an assumed initial public offering price of $16.00 per share, the midpoint of the range on the cover page of this prospectus, purchasers of common stock in this offering will have contributed approximately 17.3% of the aggregate purchase price paid by all purchasers of our stock but will own only approximately 30.4% of our common stock outstanding after this offering.
We do not expect to pay any cash dividends for the foreseeable future. Investors in this Offering may never obtain a return on their investment.
You should not rely on an investment in our securities to provide dividend income. We do not anticipate we will pay any cash dividends to holders of our securities in the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our existing operations. In addition, any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our securities. Accordingly, investors must rely on sales of their securities after price appreciation, which may never occur, as the only way to realize any return on their investment. As a result, investors seeking cash dividends should not purchase our securities.
Our security holders may be diluted by future issuances of securities by us.
We may issue additional securities or security units convertible into or exchangeable for our securities. The issuance of additional securities or security units convertible into or exchangeable for our securities would dilute the ownership of us by existing investors and could adversely affect the value of our securities. In addition, we may issue securities in the future with rights senior to the rights of the securities acquired in this Offering.
Participation in this offering by certain of our existing stockholders would reduce the public float for our shares.
Certain of our existing stockholders, including Celgene, HealthCare Ventures VII, L.P., Lilly Ventures Fund I, LLC, and Morgenthaler Partners, VII, L.P., and certain other affiliates of our directors, have indicated an interest in purchasing an aggregate of approximately $10 million of shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriter may determine to sell more, less or no shares in this offering to any of these stockholders, or any of these stockholders may determine to purchase more, less or no shares in this offering. If such stockholders were to purchase all of such shares, assuming no exercise by the underwriter of its over-allotment option, our executive officers, directors and 5% or greater stockholders will beneficially own, in the aggregate, approximately 55.1% of our outstanding capital stock upon completion of this offering.
If our stockholders are allocated all or a portion of the shares in which they have indicated an interest in this offering and purchase any such shares, such purchase would reduce the available public float for our shares because such stockholders would be restricted from selling the shares by a lock-up agreement they have entered into with our underwriter and by restrictions under applicable securities laws. As a result, any purchase of shares by such stockholders in this offering may reduce the liquidity of our common stock relative to what it would have been had these shares been purchased by investors that were not affiliated with us.
48
Table of Contents
The requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain executive management and qualified board members.
As a public company, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act the listing requirements of The NASDAQ Stock Market and other applicable securities rules and regulations. Compliance with these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an emerging growth company. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to maintain and, if required, improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight may be required. As a result, management’s attention may be diverted from other business concerns, which could adversely affect our business and operating results. We will need to hire additional employees in the future or engage outside consultants to help us comply with these requirements, which will increase our costs and expenses. For example, during the year ended December 31, 2013, we identified a material weakness in our internal controls due to the fact that we only have one employee in our accounting and finance department. As a result, we were unable to allow for proper segregation of duties and reviews of transactions prior to being entered into our books and records.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure create uncertainty for public companies, increase legal and financial compliance costs and make some activities more time-consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from our business activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies, regulatory authorities may initiate legal proceedings against us and our business may be adversely affected.
We also expect that being a public company and the associated public company rules and regulations will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
As a result of disclosure of information in this prospectus and in filings required of a public company, our business and financial condition will become more visible, which we believe may result in threatened or actual litigation. If such claims are successful, our business and operating results would be adversely affected, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and adversely affect our business and operating results.
As a result of becoming a public company, we will be obligated to develop and maintain proper and effective internal control over financial reporting. We may not complete our analysis of our internal control over financial reporting in a timely manner, or these internal controls may not be determined to be effective, which may adversely affect investor confidence in our company and, as a result, the value of our common stock.
We will be required pursuant to Section 404 of the Sarbanes-Oxley Act to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting for the first fiscal year
49
Table of Contents
beginning after the effective date of this offering. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. We will also be required to disclose changes made in our internal control and procedures on a quarterly basis. Eventually, after we are no longer an emerging growth company, we may be required to obtain a statement that our independent registered public accounting firm has issued an opinion on our internal control over financial reporting.
We are in the very early stages of the costly and challenging process of compiling the system and processing documentation necessary to perform the evaluation needed to comply with Section 404. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. For example, during the year ended December 31, 2013, we identified a material weakness in our internal controls due to the fact that we only have one employee in our accounting and finance department. As a result, we were unable to allow for proper segregation of duties and reviews of transactions prior to being entered into our books and records. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are effective.
If we are unable to assert that our internal control over financial reporting is effective, or, if when required, our independent registered public accounting firm is unable to express an opinion on the effectiveness of our internal controls, we could lose investor confidence in the accuracy and completeness of our financial reports, which would cause the price of our common stock to decline, and we may be subject to investigation or sanctions by the Securities and Exchange Commission, or SEC.
As an emerging growth company, we are subject to reduced reporting obligations and eligible for various exemptions from public reporting obligations which may reduce demand for our common stock.
For as long as we remain an emerging growth company as defined in the Jumpstart our Business Startups Act of 2012, or the JOBS Act, we intend to take advantage of certain exemptions from various reporting requirements, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our public filings, periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We intend to take advantage of these reporting exemptions until we are no longer an emerging growth company.
We will remain an emerging growth company for up to five years, although if the market value of our common stock that is held by non-affiliates exceeds $700 million as of any date before that time, we would cease to be an emerging growth company as of the following December 31 or if our annual gross revenues equal or exceed $1 billion, we would cease to be an emerging growth company on the last day of the year in which that occurs. We cannot predict if investors will find our common stock less attractive because we may rely on the exemptions from certain reporting standards as an emerging growth company. If some investors find our common stock less attractive, there may be a less active trading market for our common stock, and our stock price may be more volatile or decline.
New accounting pronouncements may impact our reported results of operations and financial position.
The JOBS Act provides that an emerging growth company can take advantage of an extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to opt out of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable. U.S. generally accepted accounting principles, or GAAP, and related implementation guidelines and interpretations can be highly complex and involve subjective judgments. Changes in these rules or their interpretation, the adoption of new pronouncements or the application of existing pronouncements to changes in our business could significantly alter our reported financial statements and results of operations.
50
Table of Contents
Provisions in our amended and restated certificate of incorporation, our amended and restated bylaws or Delaware law might discourage, delay or prevent a change-of-control of our company or changes in our management and, therefore, depress the trading price of our common stock.
Provisions of our amended and restated certificate of incorporation, our amended and restated bylaws or Delaware law may have the effect of deterring unsolicited takeovers or delaying or preventing a change-of-control of our company or changes in our management, including transactions in which our stockholders might otherwise receive a premium for their shares over then current market prices. Our amended and restated certificate of incorporation and amended and restated bylaws will be effective upon completion of this offering. In addition, these provisions may limit the ability of stockholders to approve transactions that they may deem to be in their best interest. These provisions include:
| • | advance notice requirements for stockholder proposals and nominations of directors; |
| • | the inability of stockholders to act by written consent or to call special meetings; |
| • | limitations on the ability of stockholders to remove directors or amend our bylaws; and |
| • | the ability of our Board of Directors to designate the terms of and issue new series of preferred stock without stockholder approval, which could include the right to approve an acquisition or other change-of-control or could be used to institute a rights plan, also known as a poison pill, that would work to dilute the stock ownership of a potential hostile acquirer, likely preventing acquisitions that have not been approved by our Board of Directors. |
In addition, Section 203 of the Delaware General Corporation Law prohibits a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person that together with its affiliates owns, or within the last three years has owned, 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner.
The existence of the foregoing provisions and anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of the Company, thereby reducing the likelihood that you could receive a premium for your common stock in an acquisition.
Our ability to use our net operating loss carry-forwards and certain other tax attributes is limited by Sections 382 and 383 of the Internal Revenue Code.
Subject to certain limitations, a corporation may offset a net operating loss carryforward against profit earned in a future year to determine its U.S. federal income tax expenses for such year. Sections 382 and 383 of the Internal Revenue Code of 1986 limit a corporation’s ability to utilize its net operating loss carryforwards and certain other tax attributes (including research credits) to offset future federal taxable income or tax if, in general, the corporation experiences a cumulative ownership change of more than 50% over any rolling three year period. State net operating loss carryforwards (and certain other tax attributes) may be similarly limited. For the year ended December 31, 2013, we recorded a current state tax liability of $115,765 due to statutory limitations in the use of state net operating loss carryforwards. An ownership change can therefore result in significantly greater tax liabilities than a corporation would incur in the absence of such a change and any increased liabilities could adversely affect the corporation’s business, results of operations, financial condition and cash flow.
As of December 31, 2013, we had available total federal and state net operating loss carryforwards of approximately $106.9 million, which expire in the years 2022 through 2033, and federal research credit carryforwards of $6.7 million, which expire in the years 2022 through 2033. Based on an analysis from our inception through December 31, 2013, we have experienced Section 382 ownership changes in June 2003 and August 2007. These two ownership changes limit our ability to utilize our federal net operating loss carryforwards (and certain other tax attributes) in future years. Additional analysis will be required after this
51
Table of Contents
offering to determine whether a change in our ownership resulting from this offering caused another ownership change to occur that further limits our ability to use our net operating loss carryforwards and other tax attributes. Any such change could result in significant limitations on all of our net operating loss carryforwards and other tax attributes.
Even if another ownership change has not occurred and does not occur as a result of this offering, additional ownership changes may occur in the future as a result of additional equity offerings or events over which we will have little or no control, including purchases and sales of our equity by our five-percent security holders, the emergence of new five-percent security holders, redemptions of our securities or certain changes in the ownership of any of our five percent security holders.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of our company, the trading price for our stock would be negatively impacted. If we obtain securities or industry analyst coverage and if one or more of the analysts who covers us downgrades our stock, or publishes unfavorable research about our business, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our stock could decrease, which could cause our stock price and trading volume to decline.
52
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this prospectus, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate”, “believe”, “estimate”, “expect”, “intend”, “may”, “plan”, “predict”, “project”, “target”, “potential”, “will”, “would”, “could”, “should”, “continue”, “contemplate”, or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
The forward-looking statements in this prospectus include, among other things, statements about our intentions, beliefs, projections, outlook, analyses or expectations concerning, among other things, our plans to develop and commercialize our product candidates, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our relationships with our collaborators and the potential for additional future collaborations, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, the applicability of our Tarmogen platform to address new indications, our manufacturing requirements and capabilities, our ability to obtain regulatory approval for our manufacturing facilities, our sales and marketing plans, our results of operations, financial condition, liquidity, need for financing, prospects, growth and strategies, the industry in which we operate and the trends that may affect the industry or us.
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this prospectus, particularly in the “Risk Factors” section, that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from what we expect.
The forward-looking statements in this prospectus represent our views as of the date of this prospectus. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this prospectus.
We obtained the industry, market and competitive position data in this prospectus from our own internal estimates and research as well as from industry and general publications and research surveys and studies conducted by third parties. Industry publications, studies and surveys generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe that each of these studies and publications is reliable, we have not independently verified market and industry data from third-party sources. While we believe our internal company research is reliable and the market definitions we use are appropriate, neither such research nor these definitions have been verified by any independent source.
53
Table of Contents
We estimate that the net proceeds from our issuance and sale of 1,562,500 shares of common stock in this offering will be approximately $21.4 million (or approximately $24.9 million if the underwriter’s over-allotment option is exercised in full), assuming an initial public offering price of $16.00 per share, which is the midpoint of the price range listed on the cover page of this prospectus, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
Each $1.00 increase (decrease) in the assumed initial public offering price of $16.00 per share would increase (decrease) our net proceeds from this offering by approximately $1.5 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 250,000 shares in the number of shares of common stock offered by us would increase (decrease) our net proceeds from this offering by approximately $3.7 million, assuming that the assumed initial public offering price remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The principal purposes of this offering are to obtain additional capital to support our operations, to create a public market for our common stock and to facilitate our future access to the public equity markets. We currently expect to use the net proceeds of this offering as follows:
| • | Approximately $10.0 million to advance an additional infectious disease product into clinical trials and through clinical proof of concept, which means evidence of biological activity in the target patient population, and could be a Phase 1 or Phase 2 study; |
| • | Approximately $5.0 million to prepare our manufacturing facility for clinical trial and commercial scale manufacturing; |
| • | Approximately $5.0 million for additional development on our own internal programs and filing of one or more additional IND(s); |
| • | Approximately $1.0 million for completion of ongoing clinical trials for GI-6207, GI-6301 and GI-4000, including manufacturing of drug supply for such trials; and |
| • | The remainder for working capital and other general corporate purposes, including hiring of additional personnel and expenses associated with being a public company. |
Although it is difficult to predict future liquidity requirements, we believe that the net proceeds from this offering, together with our existing cash and cash equivalents, and contingent, future milestone payments under our collaboration agreements, will allow us to fund our operations into 2016.
This expected use of net proceeds from this offering represents our intention based upon our current plans and business conditions. The amounts and timing of our actual expenditures depend on numerous factors, including the ongoing status of, and results from, clinical trials and other studies, achievement of milestones under our existing collaborations, any additional collaborations that we may enter into with third parties for our product candidates and any unforeseen cash needs. Changing circumstances may cause us to consume capital significantly faster or slower than we currently anticipate or to alter our operations. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available financial resources sooner than we currently expect. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
Pending use of the proceeds from this offering, we intend to invest the proceeds in a variety of capital preservation investments, including short-term, interest-bearing investment grade securities, certificates of deposit or government securities.
We have never declared or paid any dividends on our common stock. We anticipate that we will retain all of our future earnings, if any, to fund the development and expansion of our business, and we do not anticipate paying cash dividends in the foreseeable future.
54
Table of Contents
The following table sets forth our capitalization as of March 31, 2014 on:
| • | an actual basis; |
| • | a pro forma basis to give effect to the conversion of all of our outstanding preferred stock into 2,757,825 shares of common stock, which will take place automatically upon completion of this offering in accordance with the terms of our preferred stock, the reclassification of our preferred stock warrants of $6,520,835 to additional paid-in capital upon the completion of the offering, the conversion of $409,140 aggregate principal amount of the Cooley Note plus accrued interest thereon into 31,964 shares of our common stock, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, and based on a conversion date of May 31, 2014, upon the completion of the offering and the conversion of $7,500,000 aggregate principal amount of the 2014 Notes plus accrued interest thereon into 690,663 shares of our common stock, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, and based on an assumed conversion date of May 31, 2014; and |
| • | a pro forma as adjusted basis to give further effect to the issuance and sale of 1,562,500 shares of common stock in this offering at an assumed initial public offering price of $16.00 per share, which is the midpoint of the price range listed on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, and the receipt by us of the proceeds of such sale. |
You should read this information together with our financial statements and the related notes appearing elsewhere in this prospectus, and the information set forth under the headings “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other financial information contained in this prospectus.
55
Table of Contents
| As of March 31, 2014 (unaudited) | ||||||||||||
| (Unaudited) | ||||||||||||
| Actual | Pro Forma (1) | Pro Forma As Adjusted (2) |
||||||||||
| (in thousands, except share data) | ||||||||||||
| Cash and cash equivalents |
$ | 9,216 | 9,216 | 30,941 | ||||||||
|
|
|
|
|
|
|
|||||||
| Convertible preferred stock, $0.001 par value: authorized 93,980,000 shares, issued and outstanding 86,570,158 shares, actual; authorized, issued and outstanding no shares, pro forma, and pro forma as adjusted |
191,120 | — | — | |||||||||
| Convertible promissory notes |
2,730 | — | — | |||||||||
| Stockholder’s equity (deficit) |
||||||||||||
| Preferred stock, par value $0.001 per share; no shares authorized, issued or outstanding, actual; 5,000,000 shares authorized, and no shares issued and outstanding, pro forma, and pro forma as adjusted |
— | — | — | |||||||||
| Common stock, $0.001 par value: authorized 112,500,000 shares, issued and outstanding 93,448 shares, actual; 100,000,000 shares authorized, 3,573,900 shares issued and outstanding pro forma; 100,000,000 shares authorized, 5,136,400 shares issued and outstanding pro forma as adjusted |
— | 4 | 6 | |||||||||
| Additional paid-in capital |
— | 209,199 | 230,622 | |||||||||
| Accumulated deficit |
(205,188 | ) | (213,731 | ) | (213,731 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity (deficit) |
(205,188 | ) | (4,528 | ) | 16,897 | |||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | (11,338 | ) | (4,528 | ) | 16,897 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | The unaudited pro forma balance sheet as of March 31, 2014 reflects the automatic conversion of all outstanding shares of redeemable, convertible preferred stock as of that date into 2,757,825 shares of common stock, the automatic conversion of the Cooley Note and accrued interest (carrying value of $222,813) and extinguishment of the related put option (carrying value of $101,222) into 31,964 shares of common stock, which results in a loss upon conversion of $187,389, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, the automatic conversion of the 2014 Notes and accrued interest (carrying value of $2,506,682) and extinguishment of the related put option ($1,795,372) into 690,663 shares of our common stock, which results in a loss upon conversion of $8,355,442, including the write off of the remaining debt issuance costs ($1,606,888), assuming an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, and the reclassification of our preferred stock warrants ($6,779,876) into additional paid-in capital, which will occur upon completion of this offering. |
| (2) | The pro forma as adjusted balance sheet data as of December 31, 2013 gives further effect to our receipt of the estimated net proceeds from the sale of shares of common stock by us in this offering at an assumed initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
56
Table of Contents
The number of shares of our common stock that will be outstanding upon completion of this offering is based on 3,573,900 shares of common stock outstanding as of March 31, 2014 after giving effect to the conversion of our outstanding preferred stock and convertible promissory notes into shares of common stock upon completion of this offering, and excludes:
| • | 235,342 shares of common stock issuable upon the exercise of outstanding options under our 2002 stock incentive plan, at a weighted average exercise price of $8.14 per share; |
| • | 104,696 shares of common stock issuable upon the exercise of outstanding warrants to purchase preferred stock, which will convert into warrants to purchase common stock upon completion of this offering, at a weighted average exercise price of $44.76 per share; |
| • | 592,524 shares of common stock reserved for future issuance under our 2014 equity incentive plan, plus annual increases in the number of shares of common stock reserved for future issuance pursuant to the “evergreen provision” of such plan, as more fully described in “Executive and Director Compensation—Employee Benefit Plans—2014 Equity Incentive Plan”; |
| • | 201,163 shares of common stock reserved for future issuance under our 2014 employee stock purchase plan, plus annual increases in the number of shares of common stock reserved for future issuance pursuant to the “evergreen provision” of such plan, as more fully described in “Executive and Director Compensation—Employee Benefit Plans—2014 Employee Stock Purchase Plan”; |
| • | 46,877 shares of common stock issuable upon the exercise of outstanding warrants to purchase common stock at an assumed exercise price of $16.00 per share of the Company held by the underwriter, and its designees, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; |
| • | 69,063 shares of common stock issuable upon the exercise of outstanding warrants to purchase common stock at an assumed exercise price of $16.80 per share of the Company held by the underwriter, and its designees, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; |
| • | 31,250 shares of our common stock issuable upon exercise of a warrant to purchase our common stock at an assumed exercise price of $24.00 per share to be issued to the underwriter upon completion of this public offering assuming an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus; |
| • | 468,769 shares of common stock issuable upon the exercise of outstanding warrants to purchase capital stock of the Company issued in January and February 2014, which will convert into warrants to purchase common stock at an assumed exercise price of $16.00 per share upon completion of this offering assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; and |
| • | 7,671 shares common stock issuable upon the exercise of a warrant to purchase capital stock of the Company to be issued to Cooley LLP upon completion of this offering at an assumed exercise price of $16.00 per share assuming an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus. |
The pro forma as adjusted information is illustrative only, and we will adjust this information based on the actual initial public offering price, number of shares offered and other terms of this offering determined at pricing.
57
Table of Contents
If you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the initial public offering price per share of our common stock and the net tangible book value per share of our common stock immediately after this offering. Net tangible book value (deficit) per share of our common stock is determined at any date by subtracting our total liabilities and preferred stock from the amount of our total tangible assets (total assets less intangible assets) and dividing the difference by the number of shares of our common stock deemed to be outstanding at that date.
Our historical net tangible book value (deficit) as of March 31, 2014 (unaudited) was $(205.2) million or $(2,195.75) per share of our common stock. On a pro forma basis, after giving effect to the conversion of all of our outstanding preferred stock and convertible promissory notes into an aggregate of 2,757,825 and 722,627 shares of common stock, respectively, as well as the reclassification of our preferred stock warrants to additional paid-in capital upon completion of this offering, our pro forma net tangible book value (deficit) as of March 31, 2014 (unaudited) would have been $(4.5) million or $(1.27) per share of our pro forma outstanding common stock.
Investors participating in this offering will incur substantial dilution. After giving effect to the issuance and sale by us of shares of common stock in this offering at an assumed initial public offering price of $16.00 per share, which is the midpoint of the price range listed on the cover page of this prospectus, less underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of March 31, 2014 (unaudited) would have been approximately $16.9 million, or $3.29 per share. This represents an immediate increase in pro forma net tangible book value per share of $4.56 to existing stockholders and immediate dilution of $12.71 in pro forma net tangible book value per share to new investors purchasing common stock in this offering. Dilution per share to new investors is determined by subtracting pro forma as adjusted net tangible book value per share after this offering from the initial public offering price per share paid by new investors.
The following table illustrates this dilution on a per share basis.
| Assumed initial public offering price per share |
$ | 16.00 | ||||||
| Historical net tangible book value (deficit) per share as of March 31, 2014 (unaudited) |
(2,195.75 | ) | ||||||
|
|
|
|||||||
| Pro forma increase in net tangible book value per share attributable to pro forma transactions described in preceding paragraphs |
2,194.48 | |||||||
|
|
|
|||||||
| Pro forma net tangible book value (deficit) per share as of March 31, 2014 (unaudited) |
(1.27 | ) | ||||||
|
|
|
|||||||
| Pro forma increase in net tangible book value per share attributable to investors participating in this offering |
4.56 | |||||||
|
|
|
|||||||
| Pro forma as adjusted net tangible book value per share after this offering |
3.29 | |||||||
|
|
|
|||||||
| Dilution of pro forma as adjusted net tangible book value per share to new investors |
$ | 12.71 | ||||||
|
|
|
Each $1.00 increase (decrease) in the assumed initial public offering price of $16.00 per share, which is the midpoint of the price range listed on the cover page of this prospectus, would increase (decrease) our pro forma as adjusted net tangible book value per share by approximately $0.28 and our dilution per share to new investors in this offering by approximately $0.72, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriter’s over-allotment option is exercised in full, the pro forma as adjusted net tangible book value per share after giving effect to this offering would be $3.80 per share, which amount represents an immediate increase in net tangible book value of $5.07 per share of our common stock to existing stockholders and an immediate dilution in net tangible book value of $12.20 per share of our common stock to new investors purchasing shares of common stock in this offering.
58
Table of Contents
The following table summarizes, on the pro forma as adjusted basis described above as of March 31, 2014 (unaudited), the difference between the number of shares of common stock purchased from us, the total consideration paid to us and the average price per share paid to us by our existing stockholders and by investors purchasing shares in this offering at an assumed initial public offering price of $ 16.00 per share, which is the midpoint of the price range listed on the cover page of this prospectus, before deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| Shares Purchased | Total Consideration | Average Price Per Share |
||||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||||
| Existing stockholders before this offering |
3,573,900 | 69.6 | % | $ | 119,106,746 | 82.7 | % | $ | 33.33 | |||||||||||
| New investors participating in this offering |
1,562,500 | 30.4 | % | 25,000,000 | 17.3 | % | 16.00 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
5,136,400 | 100.0 | % | 144,106,746 | 100.0 | % | 28.06 | |||||||||||||
If the underwriter’s over-allotment option is exercised in full, the percentage of shares of our common stock held by existing stockholders will be reduced to 66.5% of the total number of shares of our common stock outstanding after this offering, and the number of shares held by new investors will increase to 1,796,875 shares, or 33.5% of the total number of shares of our common stock outstanding after this offering.
Each $1.00 increase (decrease) in the assumed initial public offering price of $ 16.00 per share would increase (decrease) the total consideration paid by new investors by $ 1.6 million and increase (decrease) the percentage of total consideration paid by new investors by approximately 0.9%, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. Similarly, each increase (decrease) of 250,000 in the number of shares of common stock offered by us would increase (decrease) the total consideration paid by new investors by $4.0 million and increase (decrease) the percentage of total consideration paid by new investors by approximately 2.2%, assuming that the assumed initial public offering price remains the same. We may also increase or decrease the number of shares we are offering.
The foregoing calculations exclude the following shares as of March 31, 2014 (unaudited):
| • | 235,342 shares of common stock issuable upon the exercise of outstanding options under our 2002 stock incentive plan, at a weighted average exercise price of $ 8.14 per share; |
| • | 104,696 shares of common stock issuable upon the exercise of outstanding warrants to purchase preferred stock, which will convert into warrants to purchase common stock upon completion of this offering, at a weighted average exercise price of $ 44.76 per share; |
| • | 592,524 shares of common stock reserved for future issuance under our 2014 equity incentive plan, plus annual increases in the number of shares of common stock reserved for future issuance pursuant to the “evergreen provision” of such plan, as more fully described in “Executive and Director Compensation—Employee Benefit Plans—2014 Equity Incentive Plan”; |
| • | 201,163 shares of common stock reserved for future issuance under our 2014 employee stock purchase plan, plus annual increases in the number of shares of common stock reserved for future issuance pursuant to the “evergreen provision” of such plan, as more fully described in “Executive and Director Compensation—Employee Benefit Plans—2014 Employee Stock Purchase Plan”; |
| • | 46,877 shares of common stock issuable upon the exercise of outstanding warrants to purchase our common stock at an assumed exercise price of $16.00 per share held by the underwriter, and its designees, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; |
| • | 69,063 shares of common stock issuable upon the exercise of outstanding warrants to purchase our common stock at an assumed exercise price of $16.80 per share held by the underwriter, and its designees, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; |
59
Table of Contents
| • | 31,250 shares of common stock issuable upon the exercise of a warrant to purchase our common stock at an assumed exercise price of $24.00 per share to be issued to the underwriter upon completion of this offering, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; |
| • | 468,769 shares of common stock issuable upon the exercise of outstanding warrants to purchase capital stock of the Company issued in January and February 2014, which will convert into warrants to purchase common stock at an assumed exercise price of $16.00 per share upon completion of this offering, assuming an initial public offering price of $16.00 per share, the midpoint of price range set forth on the cover page of this prospectus. |
| • | 7,671 shares of common stock issuable upon the exercise of a warrant to purchase common stock of the Company to be issued to Cooley LLP upon completion of this offering at an assumed exercise price of $16.00 per share assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus; |
In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital by issuing equity securities or convertible debt, your ownership will be further diluted.
60
Table of Contents
The following selected financial data should be read together with our financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
We derived the statements of operations data for the years ended December 31, 2011, 2012 and 2013 and the balance sheet data as of December 31, 2012 and 2013 from our audited financial statements included elsewhere in this prospectus. We derived the summary statement of operations data for the years ended December 31, 2009 and 2010 and the balance sheet data as of December 31, 2009, 2010 and 2011 from our audited financial statements not included in this prospectus. The selected statement of operations data for the three months ended March 31, 2013 and 2014 and the selected balance sheet data as of March 31, 2014 are derived from our unaudited financial statements included elsewhere in this prospectus. The unaudited interim financial information has been prepared on a basis consistent with our audited financial statements included in this prospectus and includes all adjustments, consisting only of normal recurring adjustments, that, in the opinion of management, are necessary for a fair presentation of such financial information. Our historical results are not necessarily indicative of our future results.
| Year Ended December 31, 2013 | Three Months Ended March 31, |
|||||||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2012 | 2013 | 2013 | 2014 | ||||||||||||||||||||||
| (in thousands, except for share date) | (unaudited) | |||||||||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||||||
| Revenue |
||||||||||||||||||||||||||||
| Collaboration license and services |
$ | 2,542 | 4,068 | 5,108 | 12,642 | 16,350 | 1,192 | 1,283 | ||||||||||||||||||||
| Milestones |
— | — | — | 2,000 | 3,000 | — | — | |||||||||||||||||||||
| Manufacturing services |
— | — | — | — | 3,168 | 940 | 135 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total revenue |
2,542 | 4,068 | 5,108 | 14,642 | 22,518 | 2,132 | 1,418 | |||||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||
| Research and development expenses: |
||||||||||||||||||||||||||||
| Costs of collaboration license and services |
5,114 | 8,119 | 8,380 | 10,033 | 5,856 | 2,113 | 833 | |||||||||||||||||||||
| Costs of manufacturing services |
— | — | — | — | 3,168 | 940 | 135 | |||||||||||||||||||||
| Research and development for proprietary programs |
10,902 | 6,011 | 3,683 | 1,702 | 1,861 | 160 | 571 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total research and development |
16,016 | 14,130 | 12,063 | 11,735 | 10,885 | 3,213 | 1,539 | |||||||||||||||||||||
| General and administrative |
5,048 | 4,362 | 4,686 | 5,948 | 3,175 | 737 | 1,014 | |||||||||||||||||||||
| Depreciation and amortization |
1,853 | 1,331 | 952 | 925 | 771 | 220 | 70 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total operating expenses |
22,917 | 19,823 | 17,701 | 18,608 | 14,831 | 4,170 | 2,623 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Income (loss) from operations |
(20,375 | ) | (15,755 | ) | (12,593 | ) | (3,966 | ) | 7,687 | (2,038 | ) | (1,205 | ) | |||||||||||||||
| Change in value of warrants and put and call options, income (expense) |
(920 | ) | 206 | (1,135 | ) | 1,951 | 1,843 | 486 | 247 | |||||||||||||||||||
| Interest Expense |
(312 | ) | — | — | — | — | — | (1,487 | ) | |||||||||||||||||||
| Other income (expense) |
(530 | ) | 757 | 3 | — | 62 | — | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Income (loss) before taxes |
(22,137 | ) | (14,792 | ) | (13,725 | ) | (2,015 | ) | 9,592 | (1,552 | ) | (2,445 | ) | |||||||||||||||
| Income taxes |
— | — | — | — | 116 | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net income (loss) |
(22,137 | ) | (14,792 | ) | (13,725 | ) | (2,015 | ) | 9,476 | (1,552 | ) | (2,445 | ) | |||||||||||||||
| Preferred stock dividends and accretion of offering costs to redemption value |
(8,405 | ) | (10,564 | ) | (11,316 | ) | (12,104 | ) | (12,885 | ) | (3,221 | ) | (3,437 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss applicable to common stockholders |
$ | (30,542 | ) | (25,356 | ) | (25,041 | ) | (14,119 | ) | (3,409 | ) | (4,773 | ) | (5,882 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Basic and diluted net loss per common share(1) |
$ | (364.27 | ) | (295.93 | ) | (285.04 | ) | (157.97 | ) | (36.84 | ) | (51.64 | ) | (63.11 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Common shares used in the computation of basic and diluted net loss per common share(1) |
83,845 | 85,682 | 87,853 | 89,377 | 92,522 | 92,430 | 93,201 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Pro forma basic and diluted net income per common share (unaudited)(2) |
$ | 2.60 | (0.94 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||
| Pro forma diluted weighted average common shares outstanding (unaudited)(2) |
2,945,047 | 2,851,026 | ||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||
61
Table of Contents
| (1) | See Note 3 to our financial statements appearing elsewhere in this prospectus for an explanation of the method used to calculate the historical net loss attributable to common stockholders per share and the number of common shares used in the computation of historical per share amounts. |
| (2) | See Note 3 to our financial statements appearing elsewhere in this prospectus for an explanation of the method used to calculate the pro forma net loss per common share and pro forma weighted average common shares outstanding. The pro forma net loss per common share and pro forma weighted average common shares outstanding assume the conversion of our outstanding preferred stock and convertible promissory note into shares of common stock and incremental stock options based on the treasury stock method as of December 31, 2013 and March 31, 2014. |
| As of December 31 | As of March 31, 2014 |
|||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2012 | 2013 | ||||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||||||
| Cash and cash equivalents |
$ | 22,524 | 20,291 | 15,155 | 2,003 | 5,924 | 9,216 | |||||||||||||||||
| Working capital |
16,020 | 14,281 | 2,134 | (5,462 | ) | 411 | (2,829 | ) | ||||||||||||||||
| Total assets |
24,911 | 23,672 | 17,745 | 4,241 | 7,418 | 12,087 | ||||||||||||||||||
| Total liabilities |
34,784 | 30,067 | 37,504 | 25,569 | 19,058 | 26,155 | ||||||||||||||||||
| Redeemable convertible preferred stock |
122,886 | 151,321 | 162,638 | 174,797 | 187,682 | 191,120 | ||||||||||||||||||
| Accumulated deficit |
(132,759 | ) | (157,717 | ) | (182,397 | ) | (196,125 | ) | (199,322 | ) | (205,188 | ) | ||||||||||||
| Total stockholders’ deficit |
(132,759 | ) | (157,716 | ) | (182,397 | ) | (196,125 | ) | (199,322 | ) | (205,188 | ) | ||||||||||||
62
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes that appear elsewhere in this prospectus. In addition to historical financial information, the following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly in “Risk Factors”.
Overview
We are a biopharmaceutical company established in 1995 focused on developing products for the treatment of cancer and infectious diseases based on our proprietary Tarmogen® platform. Tarmogens activate the immune system by stimulating a subset of white blood cells called T cells that destroy infected or malignant cells, in contrast to traditional vaccines which predominately stimulate antibody production. We believe that our Tarmogen platform has applicability to a number of diseases, and may enable us to develop a broad portfolio of products. We have four Tarmogen product candidates in clinical evaluation for infectious disease and multiple cancer indications.
Our Tarmogen platform technology has characteristics that we believe will enable us, in collaboration with our strategic collaborators and independently, to develop and commercialize a portfolio of products. Highlights of the technology include:
Tarmogens target the molecular profile that distinguishes a diseased cell from a normal cell. We have designed Tarmogens to target specific intracellular and extracellular proteins, or antigens, that play a role in oncology and infectious diseases which represent unmet medical needs. Collaborations with biopharmaceutical companies and research institutions have allowed us to advance the development of a number of our product candidates while managing our own research and development expenses relating to these product candidates.
We have two strategic collaborations with leading biotechnology companies. In October 2011, Gilead Sciences, Inc., or Gilead, exclusively licensed product candidates intended to treat chronic hepatitis B virus, or HBV, infection. Celgene Corporation, or Celgene, entered into a collaboration and option agreement for certain oncology product candidates in May 2009. Under this agreement, in July 2013 Celgene exercised its option for a worldwide, exclusive license to the GI-6300 program, which is a Tarmogen program targeting the brachyury protein. Brachyury plays a role in the metastatic spread of certain cancers and is believed to be fundamental in the formation of chordomas, rare bone tumors of the spine. Through March 31, 2014, we have received over $60 million from these collaborations.
We have incurred operating losses and have an accumulated deficit as a result of ongoing research and development spending. As of March 31, 2014, the Company had an accumulated deficit of $(205.2) million. The Company had net losses of $13.7 million, $2.0 million, net income of $9.5 million and net loss of $2.4 million for the years ended December 31, 2011, 2012 and 2013, and the three months ended March 31, 2014, respectively. Our losses, other than in 2013, have resulted principally from costs incurred in our discovery and development activities. We anticipate that operating losses will occur and substantially increase over the next several years as we expand discovery, research and development activities, including clinical development of our Tarmogen product candidates. Because of the numerous risks and uncertainties associated with biopharmaceutical product development and commercialization, we are unable to accurately predict the timing or amount of future expenses or when, or if, we will be able to achieve or maintain profitability. We have no products approved for commercial sale, and to date we have not generated any product revenue. We have financed our operations primarily through the sale of equity and convertible debt securities, upfront and milestone payments pursuant to our collaboration agreements, government grants and capital lease and equipment financing. The size of our future net losses will depend, in part, on the magnitude and timing of changes in our expenses, as well as the level and rate of growth, if any, of our revenues. Our ability to achieve
63
Table of Contents
profitability is dependent on our ability, alone or with others, to complete the development of our product candidates successfully, obtain required regulatory approvals, manufacture and market our potential products successfully or have such products manufactured and marketed by others, and gain market acceptance for such products. There can be no assurance as to whether or when we will achieve profitability.
We were incorporated as Ceres Pharmaceuticals, Ltd. in Colorado on February 10, 1995. We changed our name to GlobeImmune, Inc. on May 26, 2001, and reincorporated in Delaware on June 5, 2002.
Financial Operations
Revenue
Infectious Disease Programs
GI-5005, a Tarmogen product candidate intended to treat chronic hepatitis C infection was our first program to demonstrate clinical proof of concept for the treatment of chronic infectious disease. GI-5005 was evaluated in a Phase 1 trial followed by a randomized Phase 2b trial comparing GI-5005 plus pegylated interferon and ribavirin, or GI-5005 Triple Therapy, vs. the then standard of care regimen, pegylated interferon and ribavirin, or SOC. Subjects receiving GI-5005 Triple Therapy had statistically significant improved end of treatment viral clearance (43 of 68 subjects, or 63%, vs. 29 of 65 subjects, or 45%; p=0.037) and normalization of alanine aminotransferase, or ALT level, a measure of liver damage, (29 of 61 subjects, or 48%, vs. nine of 44 subjects, or 21%; p=0.007) compared to SOC alone. In treatment naïve subjects, GI-5005 Triple Therapy appeared to improve end of treatment viral clearance rates (37 of 50 subjects, or 74%, vs. 27 of 46 subjects, or 59%; p=0.133, which is not statistically significant), and improve sustained virologic clearance rates six months after completion of therapy (29 of 50 subjects, or 58%, vs. 22 of 46 subjects, or 48%; p=0.413, which is not statistically significant) compared to SOC alone, with a comparably favorable profile observed in patients who had previously failed treatment with SOC. We believe that GI-5005 was the first therapeutic vaccine to demonstrate a clinically meaningful outcome in patients with a chronic infectious disease. While we are no longer actively developing GI-5005 for commercial reasons, we believe that the clinical results generated have validated the Tarmogen platform’s application in infectious diseases.
Our world-wide collaboration with Gilead is focused on developing a lead product candidate, GS-4774, to target patients chronically infected with HBV who are also on, or are candidates for, oral antiviral suppressive therapy. Under this collaboration, in 2011 we received a $10 million upfront payment and Gilead agreed to fund a Phase 1 trial. As a result of our activities under this agreement, we have received an additional $5 million in milestone payments. Gilead is responsible for all future clinical, regulatory and commercial activities. We are eligible to receive up to an additional $130 million in development and regulatory milestones under this collaboration. If products are commercialized, we will be entitled to receive tiered royalty rates based on net sales of GS-4774 from the high single digits to the mid-teens, and up to $40 million of sales milestone payments.
Chronic HBV infection affects approximately 400 million people worldwide. While antiviral drugs have been used effectively to control this disease, cure rates are very low, with less than eight percent cured after four years of daily oral antiviral therapy. GS-4774 is being developed as a therapeutic vaccine designed to generate T cell immune responses against cells containing HBV antigens in combination with antiviral therapy with the goal of increasing the cure rate in patients with chronic HBV infection.
In August 2013, we completed a Phase 1 clinical trial of GS-4774 in 60 healthy volunteers. Twenty subjects were enrolled to one of three arms in the study, receiving either 10YU, 40YU, or 80YU of GS-4774 (one YU, or yeast unit, equals 10 million yeast cells). Within each of the three 20 subject arms, ten subjects were randomized to weekly dosing, and ten to monthly only dosing, each for three months. The Phase 1 results indicated that GS-4774 elicited HBV specific T cell immune responses. Subjects in all three dose groups displayed immune responses, and there was little difference between the weekly versus the monthly-only immunization regimens in the ability to generate T cell immune responses. Eighty-eight percent of subjects across all three dose groups responded to receiving GS-4774 by at least one measure of T cell immune response.
64
Table of Contents
Gilead initiated a Phase 2 clinical trial in September 2013 investigating GS-4774 in combination with ongoing oral antiviral treatment in patients with chronic HBV infection. This Phase 2 clinical trial is designed to enroll 175 patients in a randomized, open-label design comparing different doses of GS-4774, administered in combination with oral antiviral therapy vs. antiviral treatment alone. The primary endpoint for this trial is decline in serum HBV surface antigen, or HBsAg.
We have multiple additional preclinical infectious disease programs in various stages of development. In August 2013, we received a $4 million Research Project Grant from the National Institute of Allergy and Infectious Diseases, or NIAID, of the National Institutes of Health, or NIH, to support the development of Tarmogen immunotherapy product candidates intended to treat or prevent tuberculosis infection. The work for this grant will be performed and reimbursed over four years.
Oncology Programs
In May 2009, we entered into a worldwide strategic collaboration and option agreement with Celgene focused on the discovery, development and commercialization of certain product candidates intended to treat cancer. Under the terms of this agreement we have received $31.3 million. Celgene also made a $10 million equity investment in us. Under this agreement, the GI-6301 and GI-6207 programs may result in up to $290 million in milestone payments from Celgene to us. For product candidates subject to option by Celgene, we are responsible for initial development under the agreement, and Celgene has the option to license each of them at specific points in the development plan. Upon the achievement of certain development, regulatory and commercial milestones, we would be eligible to receive milestone payments and tiered royalties based on net sales of each licensed product.
Pursuant to the agreement, in July 2013 Celgene exercised its option to obtain an exclusive license to our GI-6300 program, including GI-6301, upon payment of a $9 million option exercise milestone. We are eligible to receive a total of $85 million in additional development and regulatory milestone payments for GI-6301. If GI-6301 is commercialized, we may receive up to $60 million in sales milestone payments and tiered royalty rates on net sales ranging from single digits to low double digits. GI-6301 targets cancers expressing the brachyury protein, which is believed to play a role in the metastatic progression of certain cancers and also in the initiation of chordomas. The National Cancer Institute, or NCI, is currently conducting a dose escalation Phase 1 trial of GI-6301 with a planned enrollment of 34 subjects with metastatic cancers and chordomas who have failed previous therapy or have no further therapeutic options. In seven chordoma patients evaluated to date, one subject was determined to be a confirmed partial responder at previously irradiated sites, one subject who had progressive disease at study entry was diagnosed with stable disease and two subjects who were determined to have stable disease at study entry continue to have stable disease. The other three chordoma subjects in the study have been diagnosed with progressive disease. We expect to complete enrollment and report data from the GI-6301 Phase 1 trial in the first half of 2014.
A second oncology product candidate, GI-6207, is being evaluated in a 34 subject Phase 2 clinical trial at the NCI. GI-6207 targets carcinoembryonic antigen, or CEA, a protein that is over-expressed in a large number of epithelial cancers, which we estimate represent approximately 500,000 new cancer cases in the United States each year. This Phase 2 trial is being conducted under an IND filed by us on December 27, 2012. The NCI has completed a dose escalation Phase 1 clinical trial of GI-6207 in 25 subjects with Stage IV cancers expressing CEA, and initiated a randomized Phase 2 trial in 34 subjects with medullary thyroid cancer, or MTC, in 2013. The Phase 1 trial of GI-6301 is being conducted under an IND filed by us on October 24, 2011. Development and commercialization rights to the GI-6200 program, including GI-6207, remain subject to option by Celgene. Celgene’s decision to option GI-6207 will be after the data from the Phase 2 trial in MTC are available.
We have a third, wholly-owned, clinical stage oncology program, GI-4000, that targets tumors with mutations in a protein called Ras. In March 2013, Celgene declined to exercise its option to GI-4000 and returned all rights and development responsibility to us. We have Phase 2 survival data in pancreas and non-small cell lung cancer, or NSCLC, for GI-4000. We conducted a multicenter, placebo controlled Phase 2b pancreas cancer study. While we did not see an improvement in survival in the overall study population, we did see a non-statistically significant three month improvement in survival in a pre-specified subgroup. We also performed a retrospective analysis of 90
65
Table of Contents
pre-adminstration blood samples using an analytic technique called proteomics. The goal of the analysis was to identify a pre-administration companion diagnostic test that could predict which subjects are likely to respond to GI-4000 to assist in subject selection for future clinical trials. BDX-001, the resulting potential proteomic companion diagnostic test, appeared to predict whether a subject administered GI-4000 and the chemotherapy drug gemcitabine in this trial would have improved recurrence free and overall survival compared to gemcitabine alone. We believe BDX-001 differentiates between subject blood samples using the relationship of 100 different proteins and protein fragments. Overall, 21 of the 44 (48%) of studied subjects administered GI-4000 and gemcitabine were classified as BDX-001 positive. In BDX-001 positive subjects administered GI-4000 and gemcitabine, there was an 11.7 month improvement in median recurrence free survival, or RFS, and a 16.6 month improvement in median overall survival, or OS, compared with BDX-001 positive subjects samples administered placebo and gemcitabine. There was no difference in RFS or OS in the gemcitabine-alone arm based on BDX-001 selection. The proportion of BDX-001 positive patients may vary in any future studies. This study was not powered for, and these results did not reach, statistical significance. If BDX-001 is prospectively validated in a second pancreas cancer trial, this companion diagnostic could be used to select the patients appropriate for GI-4000 therapy. The BDX-001 test is controlled by Biodesix, Inc. We intend to negotiate a development and commercialization agreement regarding this test with them. However, we may not be able to obtain the rights to use the test on commercially reasonable terms, if at all.
Investigators at Memorial Sloan Kettering Cancer Center, or MSKCC, also conducted a Phase 2a trial in non-small cell lung cancer, or NSCLC, in 24 subjects. Based on the updated survival analysis from December 2013, this study shows a 43% reduction in the risk of mortality for patients administered GI-4000 compared to a matched set of controls (p=0.24 which is not statistically significant). This was an investigator sponsored study that was funded by MSKCC, and we supplied the study drug. We retained all rights to GI-4000 under our agreement with MSKCC for this trial. We also have an ongoing Phase 2a clinical trial studying GI-4000 in colon cancer, which is being conducted at the Lombardi Cancer Center at Georgetown University. This is an investigator sponsored study that was funded by the Lombardi Cancer Center, and we supplied the study drug. We retained all rights to GI-4000 under our agreement with the Lombardi Cancer Center for this trial.
Research and Development Expense
Research and development expenses, which include costs of collaboration license and services, cost of manufacturing services and research and development for proprietary programs, in our statement of operations and comprehensive income and loss, consists of:
| • | personnel related expenses, including salaries, benefits, stock-based compensation, travel, and related costs for the personnel involved in drug discovery and development; |
| • | payments we make to third-party contract research organizations, contract manufacturers, investigative sites, consultants and other clinical trial costs; |
| • | technology and intellectual property license costs; |
| • | manufacturing costs; |
| • | activities relating to regulatory filings and the advancement of our product candidates through preclinical studies and clinical trials; and |
| • | facilities and other allocated expenses, which include direct and allocated expenses for rent and facility maintenance, as well as laboratory and other supplies. |
We have multiple research and development projects ongoing at any one time. We utilize our internal resources, employees and infrastructure across multiple projects. We do not believe that allocating internal costs on the basis of estimates of time spent by our employees accurately reflects the actual costs of a project. We record and maintain information regarding hours spent on specific projects when needed for our collaboration agreements and for external, out-of-pocket research and development expenses on a project-specific basis.
66
Table of Contents
The following table summarizes our principal product development programs and the expenses incurred with respect to each product candidate (in thousands).
| Year ended December 31, | Three Months Ended March 31, | |||||||||||||||||||
| 2011 | 2012 | 2013 | 2013 | 2014 | ||||||||||||||||
| GI-4000 |
$ | 3,614 | 3,941 | 1,659 | 501 | 311 | ||||||||||||||
| GI-5005 |
1,846 | 841 | 293 | 87 | 67 | |||||||||||||||
| GI-6207 |
482 | 711 | 611 | 130 | 155 | |||||||||||||||
| GI-6301 |
701 | 748 | 632 | 129 | 216 | |||||||||||||||
| GI-13000 |
682 | 1,434 | 5,201 | 1,533 | 200 | |||||||||||||||
| Other research and development |
565 | 139 | 89 | 20 | 87 | |||||||||||||||
| Compensation and benefits |
4,173 | 3,921 | 2,400 | 813 | 503 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 12,063 | 11,735 | 10,885 | 3,213 | 1,539 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
We expense research and development costs as incurred, including payments made to date under our in-licensing agreements. We believe that significant investment in product development is a competitive necessity and plan to continue these investments in order to realize the potential of our product candidates; therefore, we expect our research and development expense to increase as we continue to develop our product candidates.
The successful development of our product candidates is uncertain. We cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the remainder of the development of, or the period, if any, in which material net cash inflows may commence from any of our clinical or preclinical product candidates. This uncertainty is due to the numerous risks and uncertainties associated with the duration and cost of clinical trials which vary significantly over the life of a project as a result of differences arising during clinical development, including:
| • | the number of clinical sites included in the trials; |
| • | the length of time required to enroll suitable patients; |
| • | the number of patients that ultimately participate in the trials; and |
| • | the results of our clinical trials. |
Our expenditures are subject to additional uncertainties, including the terms and timing of collaboration agreements, clinical trial expenses, regulatory approvals, and the expense of filing, prosecuting, defending and enforcing any patent claims or other intellectual property rights. We may obtain unexpected results from our clinical trials. We may elect to discontinue, delay or modify clinical trials of some product candidates or focus on others. A change in the outcome of any of the variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. For example, if the FDA or other regulatory authorities were to require us to conduct clinical trials beyond those which we anticipate, or if we experience significant delays in enrollment in any of our clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development.
General and Administrative Expense
General and administrative expense primarily consists of salaries and other related costs, including stock-based compensation expense, for employees and consultants in our executive, finance, accounting, legal, information technology and human resource departments. Other general and administrative expenses include facility-related costs not otherwise included in research and development expense, promotional expenses, costs associated with industry and trade shows, and professional fees for legal services, including patent-related expense, insurance and accounting services.
67
Table of Contents
We anticipate that our general and administrative expense will increase over the next several years for the following reasons, among others:
| • | increased payroll, expanded infrastructure and higher consulting, legal, auditing and tax services and investor relations costs, and director and officer insurance premiums associated with being a public company; |
| • | increased expenses to support our research and development activities, which we expect to expand as we continue to advance the clinical development of our product candidates; and |
| • | we may also begin to incur expenses related to the planned sales and marketing of our product candidates in anticipation of commercial launch before we receive regulatory approval, if any, of a product candidate. |
Depreciation and Amortization Expense
Depreciation and amortization primarily consists of the depreciation of property and equipment using the straight-line method over the respective estimated useful lives of such property and equipment, or in the case of leasehold improvements, the shorter of the related lease term or the estimated useful life.
Change in Value of Warrants
Change in value of warrants consists of the gain or loss due to the change in the fair value of preferred stock warrants. The future gains or losses, prior to the completion of this offering, on the change in the fair value of preferred stock warrants will fluctuate based on the future fair market value of our preferred stock, the prevailing interest rates at the date of valuation, the remaining term of the warrants and the volatility of the future fair market value of our preferred stock. Subsequent to the completion of this offering, the change in fair value of the preferred stock warrants will no longer be applicable, and as such the requirements for liability classification and mark-to-market adjustments will cease.
Preferred Stock Accretion
Preferred stock accretion consists of the accretion of the preferred stock issuance price to its redemption price and accretion of issuance costs on preferred stock. The issuance costs on the shares of our preferred stock were recorded as a reduction to the carrying amount of the stock when issued, and are accreted to preferred stock ratably through January 8, 2015, the date the preferred stock may first be redeemed in part, by a charge to additional paid-in capital and loss attributable to common stockholders. All of our preferred stock will convert into common stock upon completion of this offering and, as a result, we will no longer record accretion on the preferred stock.
Tax Loss Carryforwards
As of December 31, 2013, we had net operating loss carryforwards of $106.9 million and federal research credit carryforwards of $6.7 million that expire at various dates from 2022 through 2033. Sections 382 and 383 of the Internal Revenue Code of 1986 limit a corporation’s ability to utilize its net operating loss carryforwards and certain other tax attributes (including research credits) to offset any future taxable income or tax if, in general, the corporation experiences a cumulative ownership change of more than 50% over any rolling three year period. An ownership change can therefore result in significantly greater tax liabilities than a corporation would incur in the absence of such a change and any increased liabilities could adversely affect the corporation’s business, results of operations, financial condition and cash flow.
Based on an analysis, as defined by Section 382, from our inception in February 1995 through December 31, 2013, we have experienced Section 382 ownership changes in June 2003 and August 2007. These two ownership changes limit our ability to utilize our federal net operating loss carryforwards (and certain other tax attributes) that accrued prior to the 2003 and 2007 ownership changes.
68
Table of Contents
Additional analysis will be required to determine whether changes in our ownership that will result from this offering have caused or will cause another ownership change to occur, and the conclusions will depend on the terms of this offering and other information that may not be available to us until after this offering has occurred. Any such change could result in significant limitations on all of our net operating loss carryforwards and other tax attributes.
Even if another ownership change has not occurred and does not occur as a result of this offering, additional ownership changes may occur in the future as a result of additional equity offerings or events over which we will have little or no control, including purchases and sales of our equity by our five percent stockholders, the emergence of new five percent stockholders, redemptions of our stock or certain changes in the ownership of any of our five percent stockholders.
Results of Operations
Comparison of the Three Months Ended March 31, 2013 and 2014
The following table sets forth our results for the periods shown.
| Three months ended March 31, | Increase (Decrease) |
% Increase (Decrease) |
||||||||||||||
| 2013 | 2014 | |||||||||||||||
| (unaudited) |
||||||||||||||||
| Revenue |
||||||||||||||||
| Collaboration license and services |
$ | 1,192 | 1,283 | 91 | 8 | % | ||||||||||
| Manufacturing services |
940 | 135 | (805 | ) | (86 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
2,132 | 1,418 | (714 | ) | (33 | %) | ||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development expenses: |
||||||||||||||||
| Costs of collaboration license and services |
2,113 | 833 | (1,280 | ) | (61 | %) | ||||||||||
| Costs of manufacturing services |
940 | 135 | (805 | ) | (86 | %) | ||||||||||
| Research and development for proprietary programs |
160 | 571 | 411 | 257 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total research and development |
3,213 | 1,539 | (1,674 | ) | (52 | %) | ||||||||||
| General and administrative |
737 | 1,014 | 277 | 38 | % | |||||||||||
| Depreciation and amortization |
220 | 70 | (150 | ) | (68 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
4,170 | 2,623 | (1,547 | ) | (37 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(2,038 | ) | (1,205 | ) | 833 | (41 | %) | |||||||||
| Change in value of warrants and put and call options, income |
486 | 247 | (239 | ) | (49 | %) | ||||||||||
| Interest expense |
— | (1,487 | ) | (1,487 | ) | 100 | % | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Net Loss |
(1,552 | ) | (2,445 | ) | (893 | ) | 58 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
Collaboration license and services revenues. Collaboration license and services revenues for the three months ended March 31, 2014 were $1.3 million compared to $1.2 million for the three months ended March 31, 2013, an increase of $0.1 million. The increase was due to $0.1 million of revenue recognized under the July 2013 GI-6300 license agreement with Celgene.
Manufacturing services revenues. Manufacturing services revenues for the three months ended March 31, 2014 were $0.1 million compared to $0.9 million for the three months ended March 31, 2013, a decrease of $0.8 million. The decrease was due to a decrease in revenue relating to manufacturing services for Gilead for the Phase 2 HBV trial of $0.8 million.
Costs of Collaboration License and Services. Costs of collaboration license and services expense for the three months ended March 31, 2014 was $0.8 million compared to $2.1 million for the three months ended March 31, 2013, a decrease of $1.3 million. The decrease was primarily due to a $1.3 million decrease in the expenses related to the completion of the GS-4774 Phase 1 clinical trial in the third quarter of 2013.
69
Table of Contents
Costs of Manufacturing Services. Costs of manufacturing services for the three months ended March 31, 2014 were $0.1 million compared to $0.9 million for the three months ended March 31, 2013, a decrease of $0.8 million. The decrease was due to a decrease in expenses relating to manufacturing services for Gilead for the Phase 2 HBV trial of $0.8 million.
Research and Development for Proprietary Programs Expense. Research and development for proprietary programs expense for the three months ended March 31, 2014 was $0.6 million compared to $0.2 million for the three months ended March 31, 2013, an increase of $0.4 million. The increase was due to $0.4 million of GI-4000 expense included in research and development for proprietary programs expense for the three months ended March 31, 2014 that was included in costs of collaboration of license and services for the three months ended March 31, 2013 due to Celgene declining their option on GI-4000 in March 2013 and returning all rights and development responsibility to us.
General and Administrative Expense. General and administrative expense for the three months ended March 31, 2014 was $1.0 million compared to $0.7 million for the three months ended March 31, 2013, an increase of $0.3 million. The increase was due to a $0.2 million increase in patent costs related to our intellectual property and a $0.1 million increase in other costs.
Depreciation and Amortization Expense. Depreciation and amortization expense for the three months ended March 31, 2014 was $0.1 million compared to $0.2 million for the three months ended March 31, 2013, a decrease of $0.1 million. This decrease was primarily due to leasehold improvements becoming fully depreciated in October 2013 as a result of the lease term at our principal executive offices ending.
Change in Value of Warrants and Put and Call Options. Change in value of warrants and put and call options for the three months ended March 31, 2014 was $0.2 million compared to $0.5 million for the three months ended March 31, 2013. The income recorded was due to the decrease in the estimated fair value of the outstanding preferred stock warrants and put and call options.
Interest Expense. Interest expense for the three months ended March 31, 2014 was $1.5 million compared to $0 for the three months ended March 31, 2013. Interest expense in the three months ended March 31, 2014 was due to the $7.5 million of the 2014 Notes and the related amortization of debt discount and debt issuance costs.
70
Table of Contents
Comparison of the Years Ended December 31, 2012 and 2013
The following table sets forth our results for the periods shown.
| Year Ended December 31, | Increase (Decrease) |
% Increase (Decrease) |
||||||||||||||
| 2012 | 2013 | |||||||||||||||
| (in thousands, except percentages) |
||||||||||||||||
| Revenue |
||||||||||||||||
| Collaboration license and services |
$ | 12,642 | 16,350 | 3,708 | 29 | % | ||||||||||
| Milestones |
2,000 | 3,000 | 1,000 | 50 | % | |||||||||||
| Manufacturing services |
— | 3,168 | 3,168 | 100 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
14,642 | 22,518 | 7,876 | 54 | % | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development expenses: |
||||||||||||||||
| Costs of collaboration license and services |
10,033 | 5,856 | (4,177 | ) | (42 | %) | ||||||||||
| Costs of manufacturing services |
— | 3,168 | 3,168 | 100 | % | |||||||||||
| Research and development for proprietary programs |
1,702 | 1,861 | 159 | 9 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total research and development |
11,735 | 10,885 | (850 | ) | (7 | %) | ||||||||||
| General and administrative |
5,948 | 3,175 | (2,773 | ) | (47 | )% | ||||||||||
| Depreciation and amortization |
925 | 771 | (154 | ) | (17 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
18,608 | 14,831 | (3,777 | ) | (20 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
(3,966 | ) | 7,687 | 11,653 | (294 | )% | ||||||||||
| Change in value of warrants, income |
1,951 | 1,843 | (108 | ) | (6 | )% | ||||||||||
| Other income |
— | 62 | 62 | 100 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before taxes |
(2,015 | ) | 9,592 | 11,607 | (576 | )% | ||||||||||
| Income Taxes |
— | 116 | 116 | 100 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
$ | (2,015 | ) | 9,476 | 11,491 | (570 | %) | |||||||||
|
|
|
|
|
|
|
|||||||||||
Collaboration license and services revenues. Collaboration license and services revenues for the year ended December 31, 2013 were $16.4 million compared to $12.6 million for the year ended December 31, 2012, an increase of $3.7 million. The increase was due to $8.8 million of revenue recognized under the July 2013 GI-6300 license agreement with Celgene offset by a decrease of $5.0 million of revenue recognized under the collaboration agreement with Gilead and other decreases of $0.1 million.
Milestones revenues. Milestones revenues for the year ended December 31, 2013 were $3.0 million compared to $2.0 million for the year ended December 31, 2012, an increase of $1.0 million. In the year ended December 31, 2012 we received a $2.0 million milestone upon filing an IND for HBV from Gilead and a $3.0 million milestone at the point of commencement of the Phase 1b/2a clinical trial from Gilead in the year ended December 31, 2013.
Manufacturing services revenues. Manufacturing services revenues for the year ended December 31, 2013 were $3.2 million compared to $0 for the year ended December 31, 2012, an increase of $3.2 million. In the year ended December 31, 2013 we recorded $3.2 million of revenue relating to manufacturing services for Gilead for the Phase 2 HBV trial. In the year ended December 31, 2012 we did not recorded any revenue relating to manufacturing services for Gilead for the Phase 2 HBV trial as the Phase 1 trial was ongoing.
Costs of Collaboration License and Services. Costs of collaboration license and services expense for the year ended December 31, 2013 was $5.8 million compared to $10.0 million for the year ended December 31, 2012, a decrease of $4.2 million. The decrease was primarily due to a $1.4 million decrease in compensation due to employee attrition in 2013 and a $2.1 million decrease in the GI-4000 program Phase 2b clinical trial expenses
71
Table of Contents
and immunology work as the number of patients being followed decreased and Celgene declining their option on GI-4000 in March 2013 and returning all rights and development responsibility to us and other decreases of $0.7 million.
Manufacturing Services. Manufacturing services for the year ended December 31, 2013 were $3.2 million compared to $0 for the year ended December 31, 2012, an increase of $3.2 million. In the year ended December 31, 2013 we recorded $3.2 million of expense relating to manufacturing services for Gilead for the Phase 2 HBV trial and $0 in the year ended December 31, 2012 as no manufacturing services were provided.
Research and Development for Proprietary Programs Expense. Research and development for proprietary programs expense for the year ended December 31, 2013 was $1.9 million compared to $1.7 million for the year ended December 31, 2012, an increase of $0.2 million. The increase was primarily due to $1.3 million of costs related to GI-4000 in the year ended December 31, 2013 due to Celgene declining their option on GI-4000 in March 2013 and returning all rights and development responsibility to us offset by a $1.1 million decrease in the expenses related to the completion of the GI-5005 Phase 2b clinical in 2011.
General and Administrative Expense. General and administrative expense for the year ended December 31, 2013 was $3.2 million compared to $5.9 million for the year ended December 31, 2012, a decrease of $2.8 million. The decrease was due to writing off $2.0 million of initial public offering costs in 2012 as a result of the termination of our proposed 2012 initial public offering, a $0.3 million decrease in personnel costs due to lower headcount, and a $0.5 million decrease in travel and other costs.
Depreciation and Amortization Expense. Depreciation and amortization expense for the year ended December 31, 2013 was $0.8 million compared to $0.9 million for the year ended December 31, 2012, a decrease of $0.1 million. This decrease was primarily due to leasehold improvements becoming fully depreciated in October 2013 as a result of the lease term at our principal executive offices ending. Beginning in November 2013, we lease our existing facility on a month to month basis.
Change in Value of Warrants. Change in value of warrants for the year ended December 31, 2013 was $1.8 million compared to $2.0 million for the year ended December 31, 2012. The income recorded was due to the decrease in the estimated fair value of the outstanding preferred stock warrants.
Other Income. Other income for the year ended December 31, 2013 was $0.1 million compared to $0 for the year ended December 31, 2012. In the year ended December 31, 2013, we received $0.1 million from miscellaneous non-recurring transactions.
Income taxes. Income taxes for the year ended December 31, 2013 were $0.1 million compared to $0 for the year ended December 31, 2012, an increase of $0.1 million. In the year ended December 31, 2013, we recorded $0.1 million of state income tax expense due to statutory limitations in the use of state net operating loss carryforwards. In the year ended December 31, 2012, we did not have income tax expense due to our net loss position.
72
Table of Contents
Comparison of the Years Ended December 31, 2011 and 2012
The following table sets forth our results for the periods shown.
| Year Ended December 31, | Increase (Decrease) |
% Increase (Decrease) |
||||||||||||||
| 2011 | 2012 | |||||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
| Revenue |
||||||||||||||||
| Collaboration license and services |
$ | 5,108 | 12,642 | 7,534 | 147 | % | ||||||||||
| Milestones |
— | 2,000 | 2,000 | 100 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
5,108 | 14,642 | 9,534 | 187 | % | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development expenses: |
||||||||||||||||
| Costs of collaboration license and services |
8,380 | 10,033 | 1,653 | 20 | % | |||||||||||
| Research and development for proprietary programs |
3,683 | 1,702 | (1,981 | ) | (54 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total research and development |
12,063 | 11,735 | (328 | ) | (3 | %) | ||||||||||
| General and administrative |
4,686 | 5,948 | 1,262 | 27 | % | |||||||||||
| Depreciation and amortization |
952 | 925 | (27 | ) | (3 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
17,701 | 18,608 | 907 | 5 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
(12,593 | ) | (3,966 | ) | 8,627 | (69 | %) | |||||||||
| Change in value of warrants, income (expense) |
(1,135 | ) | 1,951 | 3,086 | (272 | %) | ||||||||||
| Other income |
3 | — | (3 | ) | (100 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (13,725 | ) | (2,015 | ) | 11,710 | (85 | %) | ||||||||
|
|
|
|
|
|
|
|||||||||||
Collaboration license and services revenues. Collaboration license and services revenues for the year ended December 31, 2012 were $12.6 million compared to $5.1 million for the year ended December 31, 2011, an increase of $7.5 million. The increase was due to a $7.7 million increase due to recognizing a full year of license and services revenue in 2012 under the October 2011 collaboration agreement with Gilead and other decreases of $0.2 million.
Milestones revenues. Milestones revenues for the year ended December 31, 2012 were $2.0 million compared to $0 for the year ended December 31, 2011, an increase of $2.0 million. The increase was due to $2.0 million milestone from Gilead for the filing of an IND application for GS-4774.
Costs of Collaboration License and Services. Costs of collaboration license and services expense for the year ended December 31, 2012 was $10.0 million compared to $8.4 million for the year ended December 31, 2011, an increase of $1.6 million. The increase was primarily due to a $1.3 million increase in costs related to the initiation of the Phase 1 clinical trial for GS-4774 and other increases of $0.3 million.
Research and Development for Proprietary Programs Expense. Research and development for proprietary programs expense for the year ended December 31, 2012 was $1.7 million compared to $3.7 million for the year ended December 31, 2011, a decrease of $2.0 million. The decrease was primarily due to a $1.1 million decrease in expenses related to the completion of the GI-5005 program, a decrease in personnel costs of $0.3 million due to lower bonus costs, a decrease in GS-4774 costs in research and development for proprietary programs expense of $0.3 million due to Gilead signing a collaboration agreement in October 2011 and other increases of $0.3 million.
General and Administrative Expense. General and administrative expense for the year ended December 31, 2012 was $5.9 million compared to $4.7 million for the year ended December 31, 2011, an increase of $1.2 million. The increase was due to writing off $2.0 million of initial public offering costs in 2012 as a result of the termination of our proposed 2012 initial public offering, offset by a $0.6 million decrease in personnel costs due to lower bonus and severance costs and a $0.2 million decrease other costs.
73
Table of Contents
Depreciation and Amortization Expense. Depreciation and amortization expense for the year ended December 31, 2012 was $0.9 million compared to $1.0 million for the year ended December 31, 2011, a decrease of $0.1 million. The decrease in depreciation expense was due to assets reaching the end of their estimated useful life for depreciation purposes.
Change in Value of Warrants. Change in value of warrants for the year ended December 31, 2012 was $2.0 million compared to $(1.1) million for the year ended December 31, 2011, an increase of $3.1 million. In the year ended December 31, 2012, we recorded $2.0 million of income from the decrease in the fair value of our outstanding preferred stock warrants. In the year ended December 31, 2011, we recorded $1.1 million of expense due to the increase in the estimated fair value of the outstanding preferred stock warrants.
Liquidity and Capital Resources
Since our inception through March 31, 2014, we have funded our operations principally through the receipt of $191.1 million, in proceeds, consisting of: $108.2 million of net proceeds from the private placement of preferred equity securities; $0.5 million from the sale of common stock; $13.4 million of net proceeds from the private placement of convertible notes; $40.4 million received under the Celgene collaboration and license agreements; $22.9 million received under the Gilead collaboration agreement and additional supply services to Gilead; and receipt of $5.7 million from research grants. We had cash and cash equivalents of $9.2 million as of March 31, 2014. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Our funds are currently held in cash and money market funds that are invested in securities issued by the U.S. Treasury. Holders of preferred stock are entitled to present a redemption request to the Company in January 2015 for redemption of 25% of their total cumulative holdings per year, in the amount of the original purchase price plus 7% per annum compounded annually plus accrued but unpaid dividends. This preferred stock will convert into common stock upon the closing of this offering and all rights to such redemption will terminate upon such conversion.
Based on our current level of operations, we believe that the net proceeds from this offering, together with our existing cash and cash equivalents will provide adequate funds for ongoing operations, planned capital expenditures and working capital requirements into 2016. Successful completion of our research and development programs, and ultimately, the attainment of profitable operations are dependent upon future events, including completion of our development activities resulting in commercial products and/or technology, obtaining adequate financing to complete our development activities, progress of collaboration arrangements, market acceptance and demand for our products, and attracting and retaining qualified personnel. Changing circumstances may cause us to consume capital significantly faster or slower than we currently anticipate or to alter our operations. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available financial resources sooner than we currently expect.
We have incurred operating losses and have an accumulated deficit as a result of ongoing research and development spending. As of March 31, 2014, we have an accumulated deficit of approximately $205.2 million. We have net losses of $13.7 million and $2.0 million, net income of $9.5 million and net loss of $2.4 million for the years ended December 31, 2011, 2012, 2013 and three months ended March 31, 2014, respectively, and net cash used in operating activities of $5.0 million and $13.1 million and net cash provided by operating activities of $3.9 million, and net cash used in operating activities of $3.3 million for the years ended December 31, 2011, 2012, 2013 and three months ended March 31, 2014, respectively. Our losses and net cash used in operating activities, other than in 2013, have resulted principally from costs incurred in our discovery and development activities. In 2013, we recorded net income and generated cash flows from operations. This was primarily the result of recognizing $8.8 million in revenue from the license of GI-6300 to Celgene. As this revenue is not recurring, we do not believe these results will necessarily continue in future periods. We anticipate that operating losses and net cash used in operating activities will occur and substantially increase over the next several years as we expand discovery, research and development activities, including clinical development of our Tarmogen product candidates.
74
Table of Contents
We have historically financed our operations primarily through the sale of equity and convertible debt securities, payments pursuant to collaboration agreements, government grants and capital lease and equipment financing. We will continue to be dependent upon such sources of funds until we are able to generate positive cash flows from our operations.
We will be required to fund future operations through the sale of our equity securities, issuance of convertible debt, potential milestone payments if achieved and possible future collaboration partnerships. There can be no assurance that sufficient funds will be available to us when needed from equity or convertible debt financings, that milestone payments will be earned or that future collaboration partnerships will be entered into. If we are unable to obtain additional funding from these or other sources when needed, or to the extent needed, it may be necessary to significantly reduce our current rate of spending through reductions in staff and delaying, scaling back, or stopping certain research and development programs. Insufficient liquidity may also require us to relinquish greater rights to product candidates at an earlier stage of development or on less favorable terms to it or its stockholders than we would otherwise choose. These events could prevent us from successfully executing on its operating plan and could raise substantial doubt about our ability to continue as a going concern in future periods.
The following table sets forth the primary sources and uses of cash for each of the periods set forth below.
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||||||
| 2011 | 2012 | 2013 | 2013 | 2014 | ||||||||||||||||
| Net cash provided by (used in) operating activities |
$ | (5,005 | ) | (13,068 | ) | 3,931 | (1,540 | ) | (3,281 | ) | ||||||||||
| Net cash provided by (used in) investing activities |
(133 | ) | (109 | ) | (12 | ) | (10 | ) | 121 | |||||||||||
| Net cash provided by financing activities |
1 | 25 | 2 | — | 6,452 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net increase (decrease) in cash and cash equivalents |
$ | (5,137 | ) | (13,152 | ) | 3,921 | (1,550 | ) | 3,292 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Operating Activities
For the years ended December 31, 2011, 2012 and 2013 and the three months ended March 31, 2014, our operating activities provided (used) cash of $(5.0) million, $(13.1) million, $3.9 million, and $(3.2) million, respectively. The cash used in operating activities of $(5.0) million for the year ended December 31, 2011 was primarily due to a net loss of $13.7 million primarily attributed to research and development activities offset by an increase in deferred revenue of $5.9 million primarily due to the proceeds from the Gilead collaboration agreement. The cash used in operating activities of $(13.1) million for the year ended December 31, 2012 was primarily due to a net loss of $2.0 million primarily attributed to research and development activities and by a decrease in deferred revenue of $11.7 million, which primarily resulted from the recognition of revenue under the Gilead collaboration agreement. The cash provided by operating activities of $3.9 million for the year ended December 31, 2013 was primarily due to net income of $9.5 million primarily attributed to $8.8 million of revenue from the license agreement with Celgene for GI-6300 offset by a decrease in deferred revenue of $3.1 million. The cash used in operating activities of $(3.2) million for the three months ended March 31, 2014 was primarily due to a net loss of $2.4 million primarily attributed to research and development activities, a decrease in deferred revenue of $1.2 million, a decrease in accounts payable of $0.8 million offset by a noncash interest expense of $1.5 million.
Investing Activities
For the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2014, our investing activities provided (used) cash of $(0.1) million, $(0.1) million, $0 million and $0.1 million respectively, and was primarily attributable to the purchase of fixed assets for the years ended December 31, 2011, 2012 and 2013 and sale of fixed assets in the three months ended March 31, 2014.
75
Table of Contents
Financing Activities
For the years ended December 31, 2011, 2012, and 2013, we did not engage in any significant financing activities. For the three months ended March 31, 2014, our financing activities provided cash of $6.5 million from the issuance of the 2014 Notes.
Operating Capital Requirements
We anticipate that we will continue to generate significant operating losses for the next several years as we incur expenses related to the research and development of our product candidates, expand our corporate infrastructure and potentially build out our commercial manufacturing capabilities. We believe that the net proceeds from this offering, together with our existing cash and cash equivalents and contingent, future milestone payments under our collaboration agreements will allow us to fund our operations into 2016. The amounts and timing of our actual expenditures depend on numerous factors, including the ongoing status of, and results from, clinical trials and other studies, achievement of milestones under our existing collaborations, any additional collaborations that we may enter into with third parties for our product candidates and any unforeseen cash needs. Changing circumstances may cause us to consume capital significantly faster or slower than we currently anticipate or to alter our operations. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available financial resources sooner than we currently expect.
We may seek to sell additional equity or debt securities or obtain a credit facility if our available cash and cash equivalents are insufficient to satisfy our liquidity requirements or if we develop additional opportunities to do so. The sale of additional equity and debt securities may result in additional dilution to our stockholders. If we raise additional funds through the issuance of debt securities or preferred stock, these securities could have rights senior to those of our common stock and could contain covenants that would restrict our operations. We may require additional capital beyond our currently forecasted amounts. Any such required additional capital may not be available on reasonable terms, if at all. If we were unable to obtain additional financing, we may be required to reduce the scope of, delay or eliminate some or all of our planned research, development and commercialization activities, which could harm our business.
Because of the numerous risks and uncertainties associated with research, development and commercialization of biopharmaceutical products, we are unable to estimate the exact amounts of our working capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| • | the scope, rate of progress, results and costs of our preclinical studies, clinical trials and other research and development activities; |
| • | the scope, rate of progress and costs of our manufacturing development and commercial manufacturing activities; |
| • | the cost, timing and outcomes of regulatory proceedings (including FDA review of any BLA that we file); |
| • | payments required with respect to development milestones we achieve under our in-licensing agreements, including any such payments to University of Colorado, or CU, pursuant to our license agreement with them; |
| • | the costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims; |
| • | the costs associated with commercializing our product candidates, if they receive regulatory approval; |
| • | the cost and timing of developing our ability to establish sales and marketing capabilities; |
| • | competing technological efforts and market developments; |
| • | changes in our existing research relationships; |
| • | our ability to establish collaborative arrangements to the extent necessary; |
| • | revenues received from any existing or future products; and |
| • | payments received under any future strategic collaborations. |
76
Table of Contents
Critical Accounting Policies and Significant Judgments and Estimates
The discussion and analysis of our financial condition and results of operations are based on our financial statements, which we have prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those described in greater detail below. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 3 to our financial statements included elsewhere in this prospectus, we believe the following items to be the most important accounting policies, including those that affect our more significant judgments and estimates used in the preparation of our financial statements.
Revenue Recognition
We currently derive our revenue from the amortization of the payments received in 2009, 2011, 2012 and 2013 for research funding through our collaboration and license agreements with Celgene and Gilead. Our collaboration agreements also provide opportunities to derive revenue from milestone payments, license fees, reimbursement of costs, and fees paid for the manufacturing of drug candidates. Our agreements with Celgene and Gilead include fees based on a nonrefundable upfront fee, nonrefundable milestone payments that are triggered upon achievement of specific development or regulatory goals, and future royalties on sales of products that result from the collaboration.
We recognize revenue in accordance with ASC Topic 605 Revenue Recognition (ASC 605). ASC 605 establishes four criteria, each of which must be met, in order to recognize revenue related to the performance of services or the shipment of products. Revenue is recognized when (a) persuasive evidence of an arrangement exists, (b) products are delivered or services are rendered, (c) the sales price is fixed or determinable, and (d) collectability is reasonably assured.
Our collaboration and license agreements provide for a combination of nonrefundable upfront fees, nonrefundable potential milestone payments based on achievement of specific goals, reimbursement of costs incurred for clinical trials, payment received for manufacturing of drug candidates, and future license and royalty fees and are evaluated to determine whether each deliverable under the agreement has value to the customer on a stand-alone basis and whether reliable evidence of fair value for the deliverable exists. Deliverables in an arrangement that do not meet the separation criteria are treated as a single unit of accounting, generally applying applicable revenue recognition guidance for the final deliverable to the combined unit of accounting in accordance with ASC 605.
We recognize revenue from nonrefundable upfront payments over the estimated term of performance under the agreement. Since the term is not specifically identifiable in the agreements, we have estimated the performance term based on the likelihood and forecasted achievement of development commitments, and other significant commitments we must achieve. These advance payments are deferred and recorded as deferred revenue upon receipt, pending recognition, and are classified as a short-term or long-term liability in the accompanying balance sheets. We evaluate the likely performance period under our collaboration agreements on a periodic basis. If there are changes to the estimated performance period as a result of the outcome of certain events, the period over which the nonrefundable upfront payments are recognized will be adjusted prospectively. The events that will impact the estimation of the performance period include the progress of the product candidate programs and changes in the terms of our collaboration agreements.
77
Table of Contents
Each milestone payment is recognized as revenue when the specific milestone is achieved. To date, we have recognized $5.0 million revenue in connection with milestone payments.
Under our agreement with Celgene entered into in May 2009, we recorded the initial $30.0 million upfront payment received in May 2009 as deferred revenue and began recognizing this amount into revenue ratably over a 7.3 year period, which represented the initial expected performance period. We review the expected performance period quarterly and adjust the revenue recognition term if the period changes. In 2013, we reassessed our performance period under the agreement and revised the expected performance period to 8.9 years due to a longer research and development forecast for GI-6207.
Pursuant to the agreement, in July 2013 Celgene exercised its option to obtain an exclusive license to the GI-6300 program and we recorded the $9.0 million upfront option exercise milestone received in July 2013 as deferred revenue. We allocated the upfront payment using the relative selling price method between the license, $8.8 million, and services to be performed, $0.2 million, and recognized the license portion in the fourth quarter of 2013 upon delivery of all intellectual property, reports and documentation for the license to Celgene. The current estimated service period for the remaining services is through December 2014.
Under our agreement with Gilead entered into in October 2011, we recorded the initial $10.0 million upfront payment received in November 2011 as deferred revenue and we are recognizing these proceeds along with amounts we will receive as reimbursement from Gilead of costs to perform the initial Phase 1a trial on a proportional performance basis over the substantive period of performance to complete the preclinical development and Phase 1a trial, Joint Research and Development Committee, or JRC, and consultation services, which is estimated to be from October 2011 through 2020. We will measure its progress under the proportional performance method based on hours incurred in proportion to total estimated hours. However, the cumulative revenue recognized under this agreement will be limited to the cumulative cash received from Gilead. We have substantially incurred all of the hours required for preclinical development and the Phase 1a trial period.
In addition, we may continue to receive nonrefundable milestone payments based on achievement of specific goals, reimbursement of costs incurred for clinical trials, payments received for the transfer of certain of our manufacturing technologies and development support thereof, and future license and royalty fees. In assessing the milestone payments contemplated in our agreements, we have reviewed the criteria for achievement of future milestones. Based on this review, we believe that achievement is uncertain and dependent upon a number of factors which will involve substantial effort. A separate earnings process has been identified for each of the remaining development and commercial milestones. As such, the amounts received will be fixed and determinable and, therefore, we intend to recognize revenue related to each of these milestones upon achievement. To date, we have recognized revenue of $5.0 million in milestone payments under this agreement.
Revenue recognition related to upfront payments and to milestone payments could be accelerated in the event of early termination of drug programs or alternatively, decelerated, if programs are extended. As such, while changes to such estimates have no impact on our reported cash flows, our reported revenue is significantly influenced by our estimates of the period over which our obligations are expected to be performed.
Accrued Liabilities
As part of the process of preparing our financial statements, we are required to estimate accrued expenses. This process involves identifying services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. The majority of our service providers invoice us monthly in arrears for services performed. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us. Examples of estimated accrued expenses include:
| • | fees owed to contract research organizations in connection with preclinical and toxicology studies and clinical trials; |
78
Table of Contents
| • | fees owed to investigative sites in connection with clinical trials; |
| • | fees owed to contract manufacturers in connection with the production of clinical trial materials; |
| • | fees owed for professional services; |
| • | property taxes; and |
| • | unpaid salaries, wages, and benefits. |
We have not had any material adjustments to estimated amounts recorded in previous periods as a result of subsequent actual activity.
Impairment of Long-Lived Assets
The long-lived assets held and used by us are reviewed for impairment no less frequently than annually or whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. In the event that facts and circumstances indicate that the cost of any long-lived assets may be impaired, we perform an evaluation of recoverability. No asset impairments were recorded during 2011, 2012, 2013 or the three months ended March 31, 2014.
Fair Value of Preferred Stock Warrants
We have issued warrants for the purchase of preferred stock in connection with equipment financings, convertible promissory notes and preferred stock financings. Since the warrants can be exercised for redeemable preferred stock, they have been deemed to be derivative instruments that require liability classification and mark-to-market accounting at each balance sheet date. Subsequent to the completion of this offering, the provisions described above will no longer be applicable and the requirements for liability classification and mark-to-market adjustments will cease.
Upon issuance, we estimated the fair values of these warrants using the Black-Scholes option pricing model with the following assumptions: the estimated fair value of the underlying stock at the valuation measurement date; the risk-free interest rates; the expected dividend rates; the remaining contractual terms of the warrants; and the expected volatility of the underlying preferred stock. Our estimates are based, in part, on subjective assumptions and could differ materially in the future.
At the end of each reporting period, we determine the fair values of the warrants using the Black-Scholes option-pricing model using the following assumptions: the estimated fair value of the underlying preferred stock at the valuation measurement date; the risk-free interest rates; the expected dividend rates; the remaining contractual terms of the warrants; and the expected volatility of the price of the underlying preferred stock with any changes in value during the period recorded as a component of other income (expense). The valuations for the underlying preferred stock are based on valuations performed by an independent third-party valuation specialist that allocate the total equity value of the Company to all classes of stock. We will continue to adjust the fair values of the warrants at each period end for changes in fair value until the earlier of the exercise or expiration of the applicable warrants or the completion of this offering.
For the 2014 Warrants, we used a third-party valuation firm to value the warrants. A combination of Black-Scholes and Monte Carlo simulation methods were used to value these warrants, due to these warrants including non-standard terms and features such as multiple exercise options based on future events.
There is inherent uncertainty in these estimates and if we had made different assumptions than those described above, the amount of our (gain) loss on change in fair value of preferred stock warrants, net income or loss and net loss per share amounts could have been significantly different.
Stock-Based Compensation
To date, stock-based compensation expense has not been material to our financial results. Nevertheless, we expect to make additional equity incentive grants, which will result in additional stock-based compensation
79
Table of Contents
expense. Accordingly, described below is the methodology we have employed to date in measuring such expenses.
Stock Option Valuation. We are required to estimate the grant-date fair value of stock options issued to employees and recognize this cost over the period these awards vest. We estimate the fair value of each option granted using the Black-Scholes option-pricing model. Generally, we have issued employee awards that vest over time. For these awards, we record compensation cost on a straight-line basis over the vesting period. We issue awards that typically vest 25% on the first anniversary of the date of issuance with the remaining options vesting ratably over the next 36 months.
We have issued awards to nonemployee consultants and advisers. All grants to nonemployees are valued using the same fair value method that we use for grants to employees. The compensation cost on these awards is on a straight-line basis over the vesting period. We issue awards which typically vest ratably over 24 to 36 months following the date of grant.
The following table summarizes our assumptions used in the Black-Scholes model for option grants during the last three years.
Black-Scholes Model Assumptions
| Year Ended December 31, | Three Months Ended March 31, 2014 | |||||||
| 2011 | 2012 | 2013(*) | ||||||
| Exercise price |
$10.07 - $12.56 | $18.24 | — | $15.10 | ||||
| Risk-free interest rate |
1.6% -3.5% | 1.3% -2.2% | — | 1.82% | ||||
| Expected term, in years |
5.9 - 10.0 | 5.8 -10.0 | — | 6.0 | ||||
| Expected volatility |
82.9% - 87.1% | 83.3% - 91.2% | — | 95.7% | ||||
| Expected dividend yield |
0.0% | 0.0% | — | 0.0% | ||||
| * | There were no stock option grants in 2013. |
Exercise Price. Our stock options are granted with an exercise price at or above the then current fair value of our common stock as determined by the Board of Directors. As an input to making these determinations, the Board of Directors obtained multiple third-party valuations. See “—Common Stock Fair Value” below.
Risk-Free Interest Rate. We use the average yield on current U.S. Treasury instruments with terms that approximate the expected term of the stock options being valued.
Expected Term (in Years). The expected term of a stock option is the period of time for which the option is expected to be outstanding. We have a large number of options outstanding. There is no secondary market for our options and they contain only basic terms. Therefore, we used a simplified method of determining expected term by selecting the midpoint between the date upon which they would be fully vested in accordance with their terms and the anticipated forfeiture date as the expected term for grants. For certain non-employee grants, the contractual life of the option was used.
Expected Volatility. Since prior to this offering we were a privately-held company, the estimated future expected volatility for each stock option valuation utilizes volatility rates of similar publicly-traded companies considered to be in the same peer group. The volatility is calculated over a period of time commensurate with the expected term for the options granted.
Expected Dividend Yield. The expected dividend yield for all of our stock option grants is 0%, as we have not declared a cash dividend since inception, and do not expect to do so in the foreseeable future.
Forfeitures. The stock-based compensation expense recognized has been reduced for estimated forfeitures. The estimated forfeiture rate is based on historical experience of our option plan, which we expect to continue at
80
Table of Contents
the current level, and any adjustments in the forfeiture rate in the future will result in a cumulative adjustment in the period that this estimate is changed. Ultimately, the total compensation expense recognized for any given stock-based award over its vesting period will only be for those shares that actually vest.
Common Stock Fair Value. Due to the absence of an active market for our common stock, the fair value of our common stock for purposes of determining the exercise price for stock option grants was determined by our Board of Directors, with the assistance of an independent third-party valuation specialist, in good faith based on a number of objective and subjective factors including:
| • | the prices of our preferred stock sold to outside investors in arms-length transactions, and the rights, preferences and privileges of our preferred stock as compared to those of our common stock, including the liquidation preference of our preferred stock; |
| • | our results of operations, financial position and the status of our research and development efforts; |
| • | our stage of development and business strategy; |
| • | the lack of liquidity of our private stock as a private company; |
| • | valuations performed by an independent third-party valuation specialist prepared in accordance with methodologies outlined in the American Institute of Certified Public Accountants Practice Aid, “Valuation of Privately-Held-Company Equity Securities Issued as Compensation”, or the AICPA Practice Aid; |
| • | the likelihood of achieving a liquidity event for the shares of our common stock and underlying stock options, such as an initial public offering, given prevailing market conditions; |
| • | the material risks related to our business; and |
| • | the composition of and changes to our management team. |
Common Stock Valuations
After taking into account management’s recommendations based on the valuation reports prepared by an independent third-party valuation specialist, our Board of Directors adopted valuations of our common stock as of October 31, 2011, January 19, 2012, June 30, 2012, December 31, 2012 and December 31, 2013.
Valuation of Our Common Stock
The valuations of our common stock were determined in accordance with the guidelines outlined in the AICPA Practice Aid. There are several approaches for allocating enterprise value of a privately-held company among the securities held in a complex capital structure. The possible methodologies include the probability-weighted expected return method, the option-pricing method and the current value method.
October 31, 2011 and January 19, 2012
In 2009 and thereafter, we achieved several operating milestones, including the commencement of two Phase 2 clinical trials and entering into collaboration agreements in 2009 and 2011. The option-pricing model was determined to be the most appropriate method to be utilized in the valuations beginning in 2009 and through January 2012. The option-pricing method treats common stock and preferred stock as call options on a subject company’s equity value, with exercise prices based on the liquidation preference of the preferred stock. The model estimates the fair value of each class of securities as a function of the current estimated fair value of the company.
To implement this method, we examined the characteristics of each class of stock, including: (1) the conversion ratio of preferred stock; (2) the liquidation preferences assigned to the preferred stock; and (3) the
81
Table of Contents
exercise price for all outstanding options and warrants. Under this method we allocated value to common shares only in circumstances where the total equity value exceeded the liquidation rights associated with the preferred shares. The option-pricing method provides the stockholder the right, but not the obligation, to buy the underlying net assets at a predetermined price or “strike” price. The strike price is determined by analyzing the break points at which value would be allocated to each class of stock, based on the distribution characteristics associated with the equity. There are five key inputs integral to the implementation of the option pricing method. These include: (1) the market value of equity; (2) the time to expiration; (3) the risk free rate; (4) the volatility of the underlying equity; and (5) the break points for each class of stock.
The Market Value of Equity. We used the market approach, which considers transactions in a subject company’s stock or transactions in similar companies. Our market value of equity input to the option-pricing method relied on indications resulting from this market approach.
Time to Expiration. In assessing the appropriate time to expiration, we relied on our estimation of the timing for a liquidity event at the time of each valuation. 1.5 years as of October 31, 2011 and 1.3 years as of January 19, 2012.
Risk Free Rate. A risk free rate over the interval corresponding to the time to expiration was considered. We used the average yield on current U.S. Treasury instruments with terms that were commensurate with our outlook for a liquidity event.
Volatility. Since prior to this offering we were a privately-held company, the volatility of the underlying equity for each valuation utilizes volatility rates of similar publicly-traded companies considered to be in the same peer group. The selected volatility is commensurate with our estimation of timing for a liquidity event.
Breakpoints for Each Class of Stock. As different stockholders in the company have differing rights and preferences, per share values must take into account those rights and preferences. The capitalization table is combined with the liquidation priority, liquidation preference, dividend policy, participation rights, and conversion ratio to determine payout breakpoint and each class of stockholder’s claim on the assets of the company upon liquidation. Accounting for the breakpoints and characteristics of each class of shares allows for the determination of per share value, which takes into account all rights and preferences of our outstanding capital stock.
Valuation as of June 30, 2012
The valuation analysis that was conducted as of this date determined the total value available to equity holders by applying a probability-weighted expected return model as we had commenced an initial public offering process. We estimated the fair value of our common stock using the probability-weighted analysis of the present value of the returns afforded to our stockholders under two possible scenarios. The two scenarios were an initial public offering and a continued operations scenario.
For each scenario, future and present values for the common shares were calculated, taking into consideration the preferred share liquidation and conversion rights. The present values were determined by discounting the future values by the common stock’s cost of equity. Finally, the likelihood of occurrence of each scenario was probability weighted.
Continued Operations Scenario. The option-pricing model was determined to be the most appropriate method to be utilized for the continued operations scenario. The model estimates the fair value of each class of securities as a function of the current estimated fair value of the company. To implement this method, we examined the characteristics of each class of stock, including: (1) the conversion ratio of preferred stock; (2) the liquidation preferences assigned to the preferred stock; and (3) the exercise price for all outstanding options and warrants. Under this method we allocated value to common shares only in circumstances where the total equity
82
Table of Contents
value exceeded the liquidation rights associated with the preferred shares. The option-pricing method provides the stockholder the right, but not the obligation, to buy the underlying net assets at a predetermined price or “strike” price. The strike price is determined by analyzing the break points at which value would be allocated to each class of stock, based on the distribution characteristics associated with the equity. There are five key inputs integral to the implementation of the option pricing method. These include: (1) the market value of equity; (2) the time to expiration; (3) the risk free rate; (4) the volatility of the underlying equity; and (5) the break points for each class of stock.
Initial Public Offering Scenario. The probability-weighted expected return analysis was determined to be the most appropriate method to be utilized for the initial public offering scenario. We used market multiples to estimate liquidation values. Specifically, we identified publicly-traded companies using specific criteria as it pertained to our business model. Companies operating within the biotechnology or biopharmaceutical industry that were actively traded on a national stock exchange in the United States were identified. We identified guideline companies with similar business descriptions and business models. From our analysis of these companies, we calculated the enterprise value for each company as of a particular valuation date as well as when the company became a publicly-traded company. In selecting multiples to apply to us, we compared our business to the guideline companies based on business description, size and financial performance.
Valuation as of December 31, 2012
Since we achieved several operating milestones, including the commencement of two Phase 2 clinical trials, entering into collaboration agreements in 2009 and 2011 and terminating our initial public offering in 2012, the option-pricing model was determined to be the most appropriate method to be utilized for the December 31, 2012 valuation. The option-pricing method treats common stock and preferred stock as call options on a subject company’s equity value, with exercise prices based on the liquidation preference of the preferred stock. The model estimates the fair value of each class of securities as a function of the current estimated fair value of the company.
To implement this method, we examined the characteristics of each class of stock, including: (1) the conversion ratio of preferred stock; (2) the liquidation preferences assigned to the preferred stock; and (3) the exercise price for all outstanding options and warrants. Under this method we allocated value to common shares only in circumstances where the total equity value exceeded the liquidation rights associated with the preferred shares. The option-pricing method provides the stockholder the right, but not the obligation, to buy the underlying net assets at a predetermined price or “strike” price. The strike price is determined by analyzing the break points at which value would be allocated to each class of stock, based on the distribution characteristics associated with the equity. There are five key inputs integral to the implementation of the option pricing method. These include: (1) the market value of equity; (2) the time to expiration; (3) the risk free rate; (4) the volatility of the underlying equity; and (5) the break points for each class of stock.
The Market Value of Equity. We used the market approach, which considers transactions in a subject company’s stock or transactions in similar companies. Our market value of equity input to the option-pricing method relied on indications resulting from this market approach.
Time to Expiration. In assessing the appropriate time to expiration, we relied on our estimation of the timing for a liquidity event at the time of the valuation. The expected exit was 1.5 years as of December 31, 2012.
Risk Free Rate. A risk free rate over the interval corresponding to the time to expiration was considered. We used the average yield on current U.S. Treasury instruments with terms that were commensurate with our outlook for a liquidity event.
Volatility. Since prior to this offering we were a privately-held company, the volatility of the underlying equity for each valuation utilizes volatility rates of similar publicly-traded companies considered to be in the same peer group. The selected volatility is commensurate with our estimation of timing for a liquidity event.
83
Table of Contents
Breakpoints for Each Class of Stock. As different stockholders in the company have differing rights and preferences, per share values must take into account those rights and preferences. The capitalization table is combined with the liquidation priority, liquidation preference, dividend policy, participation rights, and conversion ratio to determine payout breakpoint and each class of stockholder’s claim on the assets of the company upon liquidation. Accounting for the breakpoints and characteristics of each class of shares allows for the determination of per share value, which takes into account all rights and preferences of our outstanding capital stock.
Valuation as of December 31, 2013
The valuation analysis that was conducted as of this date determined the total value available to equity holders by applying a probability-weighted expected return model. We estimated the fair value of our common stock using the probability-weighted analysis of the present value of the returns afforded to our stockholders under each of four possible scenarios. The four scenarios were an initial public offering high value, an initial public offering low value, a continued operations scenario and a dissolution scenario.
For each scenario, future and present values for the common shares were calculated, taking into consideration the preferred share liquidation and conversion rights. The present values were determined by discounting the future values by the common stock’s cost of equity. Finally, the likelihood of occurrence of each scenario was probability weighted.
Continued Operations and Dissolution Scenarios. The option-pricing model was determined to be the most appropriate method to be utilized for the continued operations and dissolution scenarios. The model estimates the fair value of each class of securities as a function of the current estimated fair value of the company. To implement this method, we examined the characteristics of each class of stock, including: (1) the conversion ratio of preferred stock; (2) the liquidation preferences assigned to the preferred stock; and (3) the exercise price for all outstanding options and warrants. Under this method we allocated value to common shares only in circumstances where the total equity value exceeded the liquidation rights associated with the preferred shares. The option-pricing method provides the stockholder the right, but not the obligation, to buy the underlying net assets at a predetermined price or “strike” price. The strike price is determined by analyzing the break points at which value would be allocated to each class of stock, based on the distribution characteristics associated with the equity. There are five key inputs integral to the implementation of the option pricing method. These include: (1) the market value of equity; (2) the time to expiration; (3) the risk free rate; (4) the volatility of the underlying equity; and (5) the break points for each class of stock.
Initial Public Offering Scenarios. The common stock equivalent method was determined to be the most appropriate method to be utilized for the initial public offering scenarios. We used market capitalization to estimate high and low market values to be used in the analysis. Specifically, we identified publicly-traded companies using specific criteria as it pertained to our business model. Companies operating within the biotechnology or biopharmaceutical industry that had recently completed initial public offerings and that were actively traded on a national stock exchange in the United States were identified. We identified guideline companies with similar business descriptions, business models, size and financial performance. From our analysis of these companies, we determined a high and low market capitalization.
Fair Value Estimates
After taking into account all of the assumptions and estimates described in our application of the option-pricing method, we determined the fair value of our common stock to be as follows:
| • | approximately $10.07 per share at November 30, 2010; |
| • | approximately $14.45 per share as of October 31, 2011; |
| • | approximately $18.24 per share as of January 19, 2012; |
84
Table of Contents
| • | approximately $57.15 per share as of June 30, 2012; |
| • | approximately $8.82 per share as of December 31, 2012; and |
| • | approximately $15.10 per share as of December 31, 2013. |
The following table lists grants of options to purchase shares of common stock with grant dates in 2011, 2012 and 2014.
| Grant Date |
Number of Shares Underlying Options Granted |
Exercise Price per Share |
Common Stock Fair Value per Share on Grant Date |
|||||||||
| January 26, 2011(1) |
16,876 | $ | 12.56 | $ | 10.07 | |||||||
| March 17, 2011 |
3,893 | 10.07 | 10.07 | |||||||||
| March 28, 2012 |
27,105 | 18.24 | 18.24 | |||||||||
| March 13, 2014 |
7,279 | 15.10 | 15.10 | |||||||||
| (1) | The January 26, 2011 grants was made prior to the completion and approval of the third-party valuation report of our common stock as of November 30, 2010, and therefore we used the prior common stock fair value as the exercise price. |
From November 30, 2010 to October 31, 2011, the estimated per share fair value of our common stock increased from $10.07 to $14.45. This increase in estimated fair value was due primarily to entering into the collaboration agreement with Gilead and an amendment to our collaboration agreement with Celgene.
From October 31, 2011 to January 19, 2012, the estimated per share fair value of our common stock increased from $14.45 to $18.24. This increase in estimated fair value was due primarily to data from the GI-4000-02 trial as well as our Board of Directors’ decision to explore an initial public offering in January 2012.
From January 19, 2012 to June 30, 2012, the estimated per share fair value of our common stock increased from $18.24 to $57.15. This increase in estimated fair value was due primarily to the filing of a registration statement for an initial public offering in May 2012 taking into account the reduced rights and preferences for the preferred stock upon an initial public offering.
From June 30, 2012 to December 31, 2012, the estimated per share fair value of our common stock decreased from $57.15 to $8.82. This decrease in estimated fair value was due primarily to the termination of our initial public offering process.
From December 31, 2012 to December 31, 2013, the estimated per share fair value of our common stock increased from $8.82 to $15.10. This increase in estimated fair value was due primarily to the completion of the phase 1 and the initiation of a phase 2 clinical trial in GS-4774, Celgene’s licensing of GI-6300 and the anticipated filing of this registration statement of which this prospectus is a part and the related amendments taking into account the reduced rights and preferences for the preferred stock upon an initial public offering.
Financial Obligations Related to Licensing and Development
In-Licensing Agreements
We are party to a number of licensing agreements with respect to certain of the technologies that underlie our intellectual property. Unless otherwise noted, these agreements typically provide that we have exclusive rights to the use and sublicensing of the technologies in question for the duration of the intellectual property patent protection in question, subject to us meeting our financial and other contractual obligations under the agreements. Certain of the key licensing agreements with significant financial obligations include the following:
University of Colorado. We are a party to a license agreement, or the CU Agreement, dated September 18, 1997, with CU, which was amended March 18, 1998, June 1, 2001 and October 16, 2003. The CU Agreement
85
Table of Contents
grants to us an exclusive, worldwide license to make, have made, use and sell licensed products that are covered by CU patent rights, proprietary information and know-how relating to Tarmogens. In partial consideration of the license under the CU Agreement, we entered into a stock purchase agreement with CU, under which we issued to CU shares of our common stock and granted CU certain rights related to ownership of such shares. The agreement requires us to make certain advance royalty payments, which are offset against future royalties, make development milestone payments, make royalty payments based on sales of approved products, if any, and pay a portion of any consideration received by us in exchange for granting a sublicense. Development milestone payments payable to CU may total up to $150,000 per product candidate beginning upon filing of an IND and continuing through approval from the FDA and after the first commercial sale of the licensed products. Each milestone payment shall be credited against future royalties, until the full amount of such milestone payment has been credited in full.
National Institutes of Health. We are a party to a series of license agreements with the National Institutes of Health, or NIH, consisting of the NIH MUC1 license agreement dated March 12, 2012, the NIH Brachyury Agreement, dated January 3, 2012, NIH VirusPlus Agreement, dated August 23, 2011 and the NIH CEA Agreement, dated June 12, 2007. Collectively we refer to these agreements as the NIH license agreements. The NIH license agreements grant us worldwide, exclusive licenses to make and have made, to use and have used, to sell and have sold, to offer to sell and have offered for sale, and to import and have imported products relating to the use of the Tarmogen immunotherapy platform with certain antigens, other immunotherapy platforms and other intellectual property intended to treat cancer that are covered by licensed patent rights and to practice and have practiced any licensed processes in the licensed fields of use. These license agreements required us to make certain noncreditable and nonrefundable initial royalty payments upon signing of each license agreement, make certain milestone payments upon achievement of specified development and commercial milestones, make royalty payments based on sales of approved products, if any, and pay a portion of any consideration we receive in exchange for granting a sublicense.
| • | The NIH has the right to terminate for our uncured material breach of our obligations under the NIH license agreements, or if we become insolvent or bankrupt. NIH also has the right to terminate or modify the NIH Agreements if it determines that certain specific events have occurred, such as our failure to achieve any development benchmarks or failure to satisfy unmet health and safety needs, provided that such right is subject to appeal by us. |
| • | Under the NIH MUC1 Agreement, we are required to make royalty advances totaling $500,000 beginning upon the acceptance of the first filing of an application for marketing approval with the FDA through the first commercial sale, and low single digit percentage royalty payments once we begin selling products developed under the agreement. |
| • | Under the NIH Brachyury Agreement, we are required to make royalty advances totaling $650,000 beginning upon the successful completion of the first Phase 3 clinical study through the first commercial sale, and low single digit percentage royalty payments once we begin selling products developed under the agreement. |
| • | Under the NIH VirusPlus Agreement, we are required to make royalty advances totaling $500,000 beginning upon the first filing of an application for marketing approval through the first commercial sale, and low single digit percentage royalty payments once we begin selling products developed under the agreement. |
| • | Under the NIH CEA Agreement, we are required to make royalty advances totaling $745,000 beginning upon the filing of an IND through FDA approval, and low single digit percentage royalty payments once we begin selling products developed under the agreement. |
Collaboration Agreements
NIH — Cooperative Research and Development Agreement. We are a party to a collaboration agreement, or the CRADA, dated May 8, 2008, with the NIH. The CRADA is for the preclinical and clinical development of
86
Table of Contents
our proprietary yeast-based Tarmogens expressing tumor-associated antigens as potential vaccines for the prevention and/or therapy of a range of human cancers. The CRADA provides that the producing party will own all inventions invented solely by its employees. For any invention made by the NIH under the CRADA, we have an exclusive option to negotiate for commercialization rights. We must pay an annual fee to the NIH based on the clinical trial phase of Tarmogens and supply product for any clinical trials the NIH conducts. The CRADA requires us to make annual payments of up to $0.3 million, depending on the stage of development of a covered product candidate.
Celgene Collaboration and Option Agreement. We are party to a Collaboration and Option Agreement, or the Celgene Agreement, with Celgene, dated May 14, 2009, as amended November 6, 2009, February 9, 2010, June 16, 2011, October 24, 2011 and July 2013, pursuant to the GI-6300 Program License Agreement between us and Celgene. Under the Celgene Agreement, we granted Celgene the exclusive option, on a program-by-program basis, to certain specified programs and all of our future oncology programs. Celgene declined to exercise its option to the GI-4000 program and we own worldwide rights to this program. For each such program subject to Celgene’s option, we have agreed to conduct early development of the product candidates in such oncology program through certain predefined endpoints, at which time Celgene will have the right to exercise its option and obtain the exclusive license to develop and commercialize the product candidates in such program. After the exercise of the option, Celgene will be solely responsible for the development and commercialization of the applicable products. We are responsible for the manufacture and supply of the products for both development and commercial use, for which we will be paid a fee, unless Celgene exercises its option to manufacture the products. Celgene has assumed manufacturing responsibility for clinical trial supplies in the GI-6300 program, except for clinical trial supplies used by us in clinical trials conducted by us.
Under the Celgene Agreement, Celgene paid us $31.3 million. If certain development, regulatory, and sales milestones are achieved, we would be eligible to receive up to $290 million in milestone payments for GI-6301 and GI-6207. Other future oncology programs have potential development, regulatory and sales milestones per program of up to approximately $100 million per program if Celgene exercises its option to license a program. If future products are commercialized, we are eligible to receive tiered royalty rates in the teens based on net sales of each licensed product candidate. In July 2013, Celgene paid us $9 million in connection with the exercise of its option to obtain an exclusive license to the GI-6300 program, including GI-6301. We anticipate that GI-6301 will be investigated in chordoma, and other brachyury-expressing cancers. Under the GI-6300 license, we are eligible to receive a total of $85 million in future development and regulatory milestone payments. If a product from the GI-6300 program is commercialized, we may receive up to $60 million in sales milestone payments and tiered royalties ranging from high single digits to low double digits.
Each party has the right to terminate the Celgene Agreement for the other party’s uncured material breach or insolvency, and Celgene has the right to terminate the Celgene Agreement for convenience at any time upon prior notice. Following termination of the Celgene Agreement by Celgene for our uncured material breach or insolvency, all licenses granted to Celgene will continue with respect to the programs for which Celgene has exercised the option, subject to certain continuing obligations. If not terminated earlier, the Celgene Agreement will remain in effect, for a particular product in a particular country, until the expiration of all payment obligations for such product in such country. The payment obligations for a particular product will expire in such country at the later of (i) the date of the last to expire claim on a U.S. patent for such product, (ii) the date upon which the regulatory exclusivity for such product expires, or (iii) the tenth anniversary of the first commercial sale of such product in such country.
Gilead License and Collaboration Agreement. We are party to a License and Collaboration Agreement, or the Gilead Agreement, dated October 24, 2011 with Gilead Sciences. Under the agreement, we granted Gilead exclusive worldwide rights to use our platform technology on Tarmogens to research, develop, and commercialize vaccine products directed at HBV. Under the agreement, we also granted Gilead licenses under certain trademarks owned or controlled by us, solely for use with respect to such HBV vaccine product.
87
Table of Contents
Under the Gilead Agreement, Gilead paid us an upfront payment of $10 million and agreed to fund a Phase 1a clinical trial of GS-4774. Since signing the agreement, we have received $5 million in milestone payments in association with certain events connected with the Phase 1a and Phase 1b/2a clinical trial. Gilead is responsible for clinical development beyond the Phase 1a clinical trial. We are eligible to receive up to an additional $130 million in development and regulatory milestones, and if products are commercialized, tiered royalty rates in the upper single digits to mid-teens and up to $40 million of sales milestone payments based on net sales of the licensed product candidates.
The term of the Gilead Agreement continues on a product-by-product and country-by-country basis until the expiration of Gilead’s obligation to pay royalties for such product in such country, or until the agreement is earlier terminated. The payment obligations, and therefore the term of the Gilead Agreement, with respect to a particular product will expire in such country on the later of (i) the date of the last to expire claim on a U.S. patent for such product and (ii) the tenth anniversary of the first commercial sale of such product in such country. Gilead can terminate the agreement at will on prior written notice to us. Each party has the right to terminate the agreement for the other party’s uncured material breach of the agreement, or if such other party becomes insolvent or bankrupt. Under certain circumstances of termination of the agreement, Gilead will negotiate in good faith with us the terms under which Gilead will grant to us an exclusive, royalty-bearing license to a terminated product in the terminated country.
Contractual Obligations and Commitments
The following table summarizes our contractual obligations at March 31, 2014.
| Total | Less than 1 year |
1-3 years | 3-5 years | Over 5 years |
||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating lease obligations |
$ | 10 | 7 | 3 | — | — | ||||||||||||||
| Licensing obligations |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 10 | 7 | 3 | — | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
In April 2014, we extended the lease agreement for our office and research facility in Louisville, Colorado into 2018 with an automatic extension into 2019 upon completion of this offering.
The following table summarizes our contractual obligations at March 31, 2014, including the lease amendment signed in April 2014.
| Total | Less than 1 year |
1-3 years | 3-5 years | Over 5 years |
||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating lease obligations |
$ | 2,430 | 585 | 1,212 | 632 | — | ||||||||||||||
| Licensing obligations |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 2,430 | 585 | 1,212 | 632 | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
In November 2013, we entered into an unsecured convertible promissory note, or Note, with Cooley LLP, in settlement of $391,730 of accounts payable. The Note bears an interest rate of 8.0%, has a term of three years and can be prepaid at any time. The Note and unpaid accrued interest will convert, upon the consummation of this offering, into common stock at a 20% discount to the initial public offering price.
In the first quarter 2014, we entered into $7,500,000 of unsecured convertible promissory notes, or 2014 Notes. The Notes bear an interest rate of 10.0%, have a term of one year and cannot be prepaid. The 2014 Notes and unpaid accrued interest will convert into common stock upon the closing of this offering at a 30% discount to the initial public offering price. At maturity, at our option, the 2014 Notes can be repaid in cash or can be settled
88
Table of Contents
through the issuance convertible preferred stock price at the lower of a pre-determined valuation or 50% of the price of the lowest round of financing during the term of the 2014 Notes. The holders of the 2014 Notes will ultimately receive a five year warrant, equal to the principal balance of the 2014 Notes, to purchase common stock upon the closing of this offering, with an exercise equal to the initial public offering price.
Under the license agreements described above in “—Financial Obligations Related to Licensing and Development”, we are obligated to make potential milestone and royalty payments. These obligations are contingent upon achieving applicable milestone and revenue events, the timing of which cannot presently be determined.
Patents and Trademarks
We presently have a portfolio of patents and patent applications (and certain trademark registrations) with the United States Patent and Trademark Office. During the three months ended March 31, 2013 and March 31, 2014, we incurred expenses related to the filing, maintenance, and initiation of our patent portfolio of $0.2 million and $0.3 million, respectively. During the years ending December 31, 2011, 2012 and 2013, these expenses totaled $0.6 million, $0.6 million and $0.6 million. We anticipate these expenses will increase in 2014.
Related Party Transactions
In September 2007, Celgene purchased $3.0 million of our Series C preferred stock. In March 2009, we issued Celgene (i) a convertible promissory note for approximately $0.1 million and (ii) the right to receive a warrant to purchase shares of our Series C preferred stock upon certain pre-specified conditions. In May 2009, we issued 8,650,519 shares of our Series D preferred stock at a purchase price of $1.156 per share, and a warrant to purchase 2,076,125 shares of our Series C preferred stock with an exercise price of $1.445 per share, to Celgene for an aggregate purchase price of approximately $10 million. In addition, in May 2009, in connection with our Series D preferred stock financing, (i) all principal and unpaid interest accrued under the convertible promissory note converted into 84,557 shares of our Series C preferred stock at a price per share of $1.156 and (ii) we issued a warrant to purchase 20,203 shares of our Series C preferred stock at an exercise price of $1.445 per share. In connection with our collaboration agreement with Celgene, we received an upfront payment of $30.0 million in May 2009. In January 2010, Celgene purchased approximately $2.7 million of our Series E preferred stock. In October 2011, we signed an amendment to the collaboration agreement with Celgene to perform additional immunology work for $1.3 million, of which we received $1.0 million in October 2011 and the remaining $0.3 million in April 2012. In July 2013, Celgene exercised its option to obtain an exclusive license to the GI-6300 program, including GI-6301, upon payment of a $9 million option exercise milestone.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules.
Recent Accounting Pronouncements
In July 2013, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update “Income Taxes (Topic 740): Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists” (ASU 2013-11). ASU 2013-11 requires an entity to present an unrecognized tax benefit as a reduction of a deferred tax asset for a net operating loss (NOL) carryforward, or similar tax loss or tax credit carryforward, rather than as a liability when (1) the uncertain tax position would reduce the NOL or other carryforward under the tax law of the applicable jurisdiction and (2) the entity intends to use the deferred tax asset for that purpose. ASU 2013-11 is effective prospectively for fiscal years and interim periods within those years, beginning after December 15, 2013 for
89
Table of Contents
public entities. Early adoption and retrospective application are permitted. The adoption of ASU 2013-11 did not have a material impact on our financial position or results of operations.
As an emerging growth company under the Jumpstart Our Business Startups Act of 2012, or JOBS Act, the Company has elected to opt out of the extended transition period for complying with new or revised accounting standards pursuant to Section 107(b) of the JOBS Act. This election is irrevocable.
Internal Control Over Financial Reporting
Assessing our staffing and training procedures to improve our internal control over financial reporting is an ongoing process. We are not currently required to comply with Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and are therefore not required to make an assessment of the effectiveness of our internal control over financial reporting. As a result, our management did not perform an evaluation of our internal control over financial reporting as of December 31, 2013. Further, our independent registered public accounting firm has not been engaged to express, nor have they expressed, an opinion on the effectiveness of our internal control over financial reporting.
For the year ending December 31, 2015, pursuant to Section 404 of the Sarbanes-Oxley Act, management will be required to deliver a report that assesses the effectiveness of our internal control over financial reporting. Under current SEC rules, our independent registered public accounting firm may also eventually be required to deliver an attestation report on the effectiveness of our internal control over financial reporting when we no longer qualify as an emerging growth company. During the year ended December 31, 2013, we identified a material weakness in our internal controls due to the fact that we only have one employee in our accounting and finance department. As a result, we were unable to allow for proper segregation of duties and reviews of transactions prior to being entered into our books and records. We may qualify as an emerging growth company for as long as five years, although if the market value of our common stock that is held by non-affiliates exceeds $700 million as of any June 30 before that time or if our annual gross revenues equal or exceed $1 billion, we would cease to be an emerging growth company as of the following December 31.
Quantitative and Qualitative Disclosures about Market Risks
Interest Rate Risk
We are exposed to market risk related to changes in interest rates. As of December 31, 2011, 2012, 2013 and March 31, 2014, we had cash and cash equivalents of $15.2 million, $2.0 million, $5.9 million, and $9.2 million, respectively, consisting of money market funds. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of United States interest rates, particularly because our investments are in short-term marketable securities. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate 10% change in interest rates would not have a material effect on the fair market value of our portfolio.
Foreign Currency Risk
All of our employees are located, and all of our major operations are currently performed, in the United States. Accordingly, we believe we do not have a material exposure to foreign currency risk. We occasionally pay for contractor or research services in a currency other than the U.S. dollar. Today, we have minimal exposure to fluctuations in foreign currency exchange rates as the difference from the time period for any services performed which require payment in a foreign currency and the date of payment is short.
90
Table of Contents
Overview
We are a biopharmaceutical company established in 1995 focused on developing products for the treatment of cancer and infectious diseases based on our proprietary Tarmogen® platform. Tarmogens activate the immune system by stimulating a subset of white blood cells called T cells that destroy infected or malignant cells, in contrast to traditional vaccines which predominately stimulate antibody production. We believe that our Tarmogen platform has applicability to a number of diseases, and may enable us to develop a broad portfolio of products. We have four Tarmogen product candidates in clinical evaluation for infectious disease and multiple cancer indications.
Our Tarmogen platform technology has characteristics that we believe will enable us, in collaboration with our strategic collaborators and independently, to develop and commercialize a portfolio of products. Highlights of the technology include:
| • | Tarmogens activate the cellular immune response: Each Tarmogen product candidate consists of intact, heat-inactivated yeast containing the target protein. We believe our data demonstrate that immunization with a Tarmogen results in T cell immune responses against the target protein and reduction in the number of abnormal cells containing the same target protein. |
| • | Broad applicability: We have five clinical trials evaluating Tarmogen product candidates in oncology and infectious diseases in randomized, controlled Phase 2 clinical trials. We have successfully created Tarmogens that express over 100 different proteins. In eleven Phase 1 and 2 clinical trials, we have administered Tarmogen product candidates to more than 500 patients and healthy volunteers, including some who have received monthly dosing for over five years, with a tolerability profile that we believe will allow our product candidates to be added to other therapeutic regimens without leading to additional toxicity. |
| • | Proven development capability: We have advanced five Tarmogen programs from concept into human clinical trials in approximately six to 18 months. |
| • | Efficient manufacturing: We manufacture Tarmogens through a process that yields a stable, off-the-shelf product that is disease- or protein-specific. We have an approximately 22,000 square foot manufacturing facility that we believe has the capacity for commercial-scale production. |
Tarmogens target the molecular profile that distinguishes a diseased cell from a normal cell. We have designed Tarmogens to target specific intracellular and extracellular proteins, or antigens, that play a role in oncology and infectious diseases which represent unmet medical needs. Collaborations with biopharmaceutical companies and research institutions have allowed us to advance the development of a number of our product candidates while managing our own research and development expenses relating to these product candidates.
We have two strategic collaborations with leading biotechnology companies. In October 2011, Gilead Sciences, Inc., or Gilead, exclusively licensed product candidates intended to treat chronic hepatitis B virus, or HBV, infection. Celgene Corporation, or Celgene, entered into a collaboration and option agreement for certain oncology product candidates in May 2009. Under this agreement, in July 2013 Celgene exercised its option for a worldwide, exclusive license to the GI-6300 program, which is a Tarmogen program targeting the brachyury protein. Brachyury plays a role in the metastatic progression of certain cancers and is believed to be fundamental in the formation of chordomas, rare bone tumors of the spine. Through March 31, 2014, we have received over $60 million from these collaborations.
91
Table of Contents
The following tables summarize the status of our pipeline of product candidates:
| INFECTIOUS DISEASE PRODUCT CANDIDATES | ||||||||||
| Indication | Target | Stage of Development |
Worldwide Commercial |
Next Development Milestone | ||||||
|
GS-4774 |
Chronic hepatitis B infection |
HBV antigens | Phase 2 | Gilead | Complete Phase 2 | |||||
|
GI-19000 |
Tuberculosis | TB antigens | Preclinical | GlobeImmune | IND | |||||
|
GI-2010 |
Human immunodeficiency virus |
HIV antigens | Preclinical | GlobeImmune | IND | |||||
|
GI-18000 |
Chronic hepatitis D infection |
Delta virus antigens | Preclinical | GlobeImmune | IND | |||||
| ONCOLOGY PRODUCT CANDIDATES | ||||||||||
| Product Candidate |
Indication | Target | Stage of Development |
Worldwide Commercial Rights |
Next Development Milestone | |||||
|
GI-6207 |
Medullary thyroid cancer |
Carcinoembryonic Antigen |
Phase 2 | Celgene Option | Complete Phase 2 | |||||
|
GI-6301 |
Chordoma, breast cancer |
Brachyury | Phase 1 | Celgene License | Complete Phase 1 | |||||
|
GI-4000 |
Resected pancreas cancer |
Mutated Ras | Phase 2b | GlobeImmune | Validate companion diagnostic | |||||
|
GI-4000 |
Non-small cell lung cancer |
Mutated Ras | Phase 2 | GlobeImmune | Initiate Phase 2b | |||||
|
GI-4000 |
Colorectal cancer |
Mutated Ras | Phase 2 | GlobeImmune |
Complete Phase 2a | |||||
Infectious Disease Programs
GI-5005, a Tarmogen product candidate intended to treat chronic hepatitis C infection was our first program to demonstrate clinical proof of concept for the treatment of chronic infectious disease. GI-5005 was evaluated in a Phase 1 trial followed by a randomized Phase 2b trial comparing GI-5005 plus pegylated interferon and ribavirin, or GI-5005 Triple Therapy, vs. the then standard of care regimen, pegylated interferon and ribavirin, or SOC. Subjects receiving GI-5005 Triple Therapy had statistically significant improved end of treatment viral clearance (43 of 68 subjects, or 63%, vs. 29 of 65 subjects, or 45%; p=0.037) and normalization of alanine aminotransferase, or ALT level, a measure of liver damage, (29 of 61 subjects, or 48%, vs. nine of 44 subjects, or 21%; p=0.007) compared to SOC alone. In treatment naïve subjects, GI-5005 Triple Therapy appeared to improve end of treatment viral clearance rates (37 of 50 subjects, or 74%, vs. 27 of 46 subjects, or 59%; p=0.133, which is not statistically significant), and improve sustained virologic clearance rates six months after completion of therapy (29 of 50 subjects, or 58%, vs. 22 of 46 subjects, or 48%; p=0.413, which is not statistically
92
Table of Contents
significant) compared to SOC alone, with a comparably favorable profile observed in patients who had previously failed treatment with SOC. We believe that GI-5005 was the first therapeutic vaccine to demonstrate a clinically meaningful outcome in patients with a chronic infectious disease. While we are no longer actively developing GI-5005 for commercial reasons, we believe that the clinical results generated have validated the Tarmogen platform’s application in infectious diseases.
Our world-wide collaboration with Gilead is focused on developing a lead product candidate, GS-4774, to target patients chronically infected with HBV who are also on, or are candidates for, oral antiviral suppressive therapy. Under this collaboration, in 2011 we received a $10 million upfront payment and Gilead agreed to fund a Phase 1 trial. As a result of our activities under this agreement, we have received an additional $5 million in milestone payments. Gilead is responsible for all future clinical, regulatory and commercial activities. We are eligible to receive up to an additional $130 million in development and regulatory milestones under this collaboration. If products are commercialized, we will be entitled to receive tiered royalty rates based on net sales of GS-4774 from the high single digits to the mid-teens, and up to $40 million of sales milestone payments.
Chronic HBV infection affects approximately 400 million people worldwide. While antiviral drugs have been used effectively to control this disease, cure rates are very low, with less than eight percent cured after four years of daily oral antiviral therapy. GS-4774 is being developed as a therapeutic vaccine designed to generate T cell immune responses against cells containing HBV antigens in combination with antiviral therapy with the goal of increasing the cure rate in patients with chronic HBV infection.
In August 2013, we completed a Phase 1 clinical trial of GS-4774 in 60 healthy volunteers. Clinical studies of GS-4774 are being conducted under an IND filed by us on December 14, 2012. This IND was subsequently transferred to Gilead on April 17, 2013. Twenty subjects were enrolled to one of three arms in the study, receiving either 10YU, 40YU, or 80YU of GS-4774 (one YU, or yeast unit, equals 10 million yeast cells). Within each of the three 20 subject arms, ten subjects were randomized to weekly dosing, and ten to monthly only dosing, each for three months. The Phase 1 results indicated that GS-4774 elicited HBV specific T cell immune responses. Subjects in all three dose groups displayed immune responses, and there was little difference between the weekly versus the monthly-only immunization regimens in the ability to generate T cell immune responses. Eighty-eight percent of subjects across all three dose groups responded to receiving GS-4774 by at least one measure of T cell immune response.
Gilead initiated a Phase 2 clinical trial in September 2013 investigating GS-4774 in combination with ongoing oral antiviral treatment in patients with chronic HBV infection. This Phase 2 clinical trial is designed to enroll 175 patients in a randomized, open-label design comparing different doses of GS-4774, administered in combination with oral antiviral therapy vs. antiviral treatment alone. The primary endpoint for this trial is decline in serum HBV surface antigen, or HBsAg.
We have multiple additional preclinical infectious disease programs in various stages of development. In August 2013, we received a $4 million Research Project Grant from the National Institute of Allergy and Infectious Diseases, or NIAID, of the National Institutes of Health, or NIH, to support the development of Tarmogen immunotherapy product candidates intended to treat and or prevent tuberculosis infection. The work for this grant will be performed and reimbursed over four years.
Oncology Programs
In May 2009, we entered into a worldwide strategic collaboration and option agreement with Celgene focused on the discovery, development and commercialization of certain product candidates intended to treat cancer. Under the terms of this agreement we have received $31.3 million. Celgene also made a $10 million equity investment in us. Under this agreement, the GI-6301 and GI-6207 programs may result in up to $290 million in milestone payments from Celgene to us. For product candidates subject to option by Celgene, we are responsible for initial development under the agreement, and Celgene has the option to license each of them at specific points in the development plan. Upon the achievement of certain development, regulatory and commercial milestones, we would be eligible to receive milestone payments and tiered royalties based on net sales of each licensed product.
93
Table of Contents
Pursuant to the agreement, in July 2013 Celgene exercised its option to obtain an exclusive license to our GI-6300 program, including GI-6301, upon payment of a $9 million option exercise milestone. We are eligible to receive a total of $85 million in additional development and regulatory milestone payments for GI-6301. If GI-6301 is commercialized, we may receive up to $60 million in sales milestone payments and tiered royalty rates on net sales ranging from single digits to low double digits. GI-6301 targets cancers expressing the brachyury protein, which is believed to play a role in the metastatic progression of certain cancers and also in the initiation of chordomas. The National Cancer Institute, or NCI, is currently conducting a dose escalation Phase 1 trial of GI-6301 with a planned enrollment of 34 subjects with metastatic cancers and chordomas who have failed previous therapy or have no further therapeutic options. In seven chordoma patients evaluated to date, one subject was determined to be a confirmed partial responder at previously irradiated sites, one subject who had progressive disease at study entry was diagnosed with stable disease and two subjects who were determined to have stable disease at study entry continue to have stable disease. The other three chordoma subjects in the study have been diagnosed with progressive disease. We expect to complete enrollment and report data from the GI-6301 Phase 1 trial in the first half of 2014.
A second oncology product candidate, GI-6207, is being evaluated in a 34 subject Phase 2 clinical trial at the NCI. GI-6207 targets carcinoembryonic antigen, or CEA, a protein that is over-expressed in a large number of epithelial cancers, which we estimate represent approximately 500,000 new cancer cases in the United States each year. This Phase 2 trial is being conducted under an Investigational New Drug Application, or IND, filed by us on December 27, 2012. The NCI has completed a dose escalation Phase 1 clinical trial of GI-6207 in 25 subjects with Stage IV cancers expressing CEA, and initiated a randomized Phase 2 trial in 34 subjects with medullary thyroid cancer, or MTC, in 2013. This Phase 1 trial of GI-6301 is being conducted under an IND, filed by us on October 24, 2011. Development and commercialization rights to the GI-6200 program, including GI-6207, remain subject to option by Celgene. Celgene’s decision to option GI-6207 will be after the data from the Phase 2 trial in MTC are available.
We have a third, wholly-owned, clinical stage oncology program, GI-4000, that targets tumors with mutations in a protein called Ras. In March 2013, Celgene declined to exercise its option to GI-4000 and returned all rights and development responsibility to us. We have Phase 2 survival data in pancreas and non-small cell lung cancer, or NSCLC, for GI-4000. We conducted a multicenter, placebo controlled Phase 2b pancreas cancer study. While we did not see an improvement in survival in the overall study population, we did see a non-statistically significant three month improvement in survival in a pre-specified subgroup. We also performed a retrospective analysis of 90 pre-administration blood samples using an analytic technique called proteomics. The goal of the analysis was to identify a pre-administration companion diagnostic test that could predict which subjects are likely to respond to GI-4000 to assist in subject selection for future clinical trials. BDX-001, the resulting potential proteomic companion diagnostic test, appeared to predict whether a subject administered GI-4000 and the chemotherapy drug gemcitabine in this trial would have improved recurrence free and overall survival compared to gemcitabine alone. We believe BDX-001 differentiates between subject blood samples using the relationship of 100 different proteins and protein fragments. Overall, 21 of the 44 (48%) of studied subjects administered GI-4000 and gemcitabine were classified as BDX-001 positive. In BDX-001 positive subjects administered GI-4000 and gemcitabine, there was an 11.7 month improvement in median recurrence free survival, or RFS, and a 16.6 month improvement in median overall survival, or OS, compared with BDX-001 positive subjects administered placebo and gemcitabine. There was no difference in RFS or OS in the gemcitabine-alone arm based on BDX-001 selection. The proportion of BDX-001 positive patients may vary in any future studies. This study was not powered for, and these results did not reach, statistical significance. If BDX-001 is prospectively validated in a second pancreas cancer trial, this companion diagnostic could be used to select the patients appropriate for GI-4000 therapy. The BDX-001 test is controlled by Biodesix, Inc. We intend to negotiate a development and commercialization agreement regarding this test with them. However, we may not be able to obtain the rights to use the test on commercially reasonable terms, if at all.
Investigators at Memorial Sloan Kettering Cancer Center, or MSKCC, also conducted a Phase 2a trial in non-small cell lung cancer, or NSCLC, in 24 subjects. Based on the updated survival analysis from December 2013, this study shows a 43% reduction in the risk of mortality for patients administered GI-4000 compared to a matched
94
Table of Contents
set of controls (p=0.24, which is not statistically significant). This is an investigator sponsored study that was funded by MSKCC, and we supplied the study drug. We also have an ongoing Phase 2a clinical trial studying GI-4000 in colon cancer, which is being conducted at the Lombardi Cancer Center at Georgetown University. This is an investigator sponsored study that was funded by Lombardi Cancer Center, and we supplied the study drug.
Our Strategy
Our strategy is to develop and commercialize Tarmogen products targeting diseases where lack of effective treatment represents significant unmet medical needs, while leveraging our collaborations to manage our expenses. The key components of our strategy include managing the HBV collaboration with Gilead, advancing our product candidates targeting infectious diseases, advancing the clinical development of oncology product candidates in collaboration with Celgene and the NCI, financing continued development of GI-4000, and retaining certain development and commercialization rights to proprietary product candidates.
We seek to manage our expenses by limiting corporate overhead spending and outsourcing appropriate functions. We intend to continue to use corporate and research collaborations to advance the development of our product candidates. We intend to use the proceeds of this financing to advance our product candidates and increase our working capital.
Expected Milestones
We expect that the following milestones will occur:
| • | Potential milestone payments from Gilead, if Gilead proceeds with a Phase 2b or Phase 3 clinical study of GS-4774; |
| • | One or more IND filings for infectious disease Tarmogens; |
| • | Celgene option decision point data from the GI-6207 Phase 2 trial for MTC; |
| • | Complete enrollment and data from GI-6301 Phase 1 trial by the first half of 2014 (results from Phase 1 trial may support initiation of Phase 2 program); and |
| • | Phase 2 development of GI-4000 in pancreas cancer and NSCLC, subject to entering into a collaboration or receiving additional funding other than this transaction. |
Infectious Disease Programs
GS-4774
GS-4774 is a therapeutic vaccine engineered to activate an HBV-specific T cell immune response to reduce the number of cells containing HBV. The GS-4774 Tarmogen expresses a fusion protein utilizing sequences of the hepatitis B virus contained in the four major HBV genotypes worldwide, in order to ensure applicability for this product across multiple markets. HBV specific T cell responses have been shown to have a positive association with infection status in patients with chronic HBV, with the weakest T cell responses being seen in patients with untreated chronic active infection and the strongest T cell responses being seen in patients who have achieved seroconversion or cure. GS-4774 is being developed to increase the HBVsAg seroconversion rate or cure, when used in combination with oral antiviral therapy.
Market Opportunity
Chronic HBV is the most common serious liver infection in the world affecting approximately 400 million people. National and regional prevalence ranges of approximately 10% in Asia to under 1% in North America and Europe. In the United States, chronic HBV infection affects up to 1.4 million people. Vaccination has dramatically reduced the prevalence rates among children in western nations. However, mother-to-newborn transmission,
95
Table of Contents
especially in Asia, remains a common source of new infection. While approximately 80% of acutely infected patients clear the virus without treatment predominantly through a T cell immune response, there is currently no cure for the vast majority of chronically-infected patients. Untreated chronic HBV infection is associated with significant increase in related diseases, including liver cirrhosis, hepatic decompensation and liver cancer. Mortality is also increased for patients with chronic HBV infection, with 25-40% of patients dying from complications of liver disease.
There are currently several agents that are used for the treatment of chronic HBV infection, including two immunomodulatory drugs and five antiviral agents. Antiviral drugs have been effectively used to suppress the virus from replicating, effectively controlling the disease. However, most patients do not achieve HBsAg seroconversion on treatment and require long term therapy. Tenofovir and entecavir have emerged as the standard of care in this disease with very low rates of resistance over five years. Even with effective antiviral therapy, HBVsAg seroconversion rates are less than 8% after four years of antiviral therapy.
Development Status
Preclinical efficacy data
GS-4774 has been shown to elicit HBV antigen-specific T cell responses in mice.
Phase 1 clinical data
GI-13020-01 was a randomized, open-label, multi-arm, dose escalation, Phase 1a trial completed in August 2013 assessing the safety, tolerability, and immunogenicity of GS-4774 in 60 healthy adults. Three doses were evaluated, 10 YU, 40YU and 80YU, each in 20 subjects. Within each dose group of 20 subjects, two separate dosing regimens were evaluated. Subjects in all three dose groups displayed treatment-emergent immune responses. Overall, 88% of all subjects responded to GS-4774 as measured by at least one measure of a T cell immune response. GS-4774 was generally well tolerated at the three doses studied in this trial. There were no deaths or serious adverse events. Most of the adverse events observed during the study were mild in severity.
Phase 2
Gilead initiated a Phase 2 clinical trial in September 2013 investigating GS-4774 in combination with ongoing oral antiviral treatment in patients with chronic HBV infection. This Phase 2 clinical trial is designed to enroll 175 subjects in a randomized, open-label design comparing different doses of GS-4774, administered once per month for six months in combination with oral antiviral therapy vs. antiviral treatment alone. The primary endpoint for this trial is decline in HBsAg.
Other Infectious Disease Product Candidates
Several other product candidates targeting a variety of infectious diseases are being explored in preclinical programs, including tuberculosis, HIV/AIDS, hepatitis delta virus, and certain pathogenic fungi.
| • | GI-19000: Tuberculosis, or TB, once considered mostly eliminated, now is a common, and in many cases lethal, infectious disease caused by Mycobacterium TB. In the United States, the estimated prevalence of latent tuberculosis infection is 4.2%, or 11.2 million people. Only 25.5% of persons with latent TB infection have been diagnosed, and only 13.2% have been prescribed treatment. TB typically attacks the lungs, but can also affect other parts of the body. It is spread through the air when people who have an active TB infection cough, sneeze, or otherwise transmit respiratory fluids through the air. Most infections are asymptomatic and latent, but about one in ten latent infections eventually progresses to active disease which, if left untreated, kills more than 50% of infected individuals. In 2013, we received a $4 million Research Project Grant by the NIAID of the NIH to support the development of Tarmogen immunotherapy product candidates intended to treat or prevent tuberculosis infection. The work for this grant will be performed and reimbursed over four years. |
96
Table of Contents
| • | GI-2010: HIV/AIDS is a chronic infection that is generally well managed by a daily combination of small molecule medicines known as highly active anti-retroviral therapy, or HAART. However, cures in this disease are rare, and HAART therapy must be given for the rest of a patient’s life. We believe that we have the potential to develop GI-2010 or related Tarmogens to cause a cellular immune response against cells infected with HIV to help clear the virus. |
| • | GI-18000: Hepatitis D virus, or HDV, is a superinfection of HBV, meaning that only patients already infected with HBV can be infected with HDV. HDV/HBV co-infected patients progress more rapidly to cirrhosis, liver failure, transplant, and death than HBV mono-infected patients. We believe that we have the potential to develop our GI-18000 Tarmogen to cause a T cell immune response against cells infected with HDV and thereby improve outcomes. |
Oncology Programs
In oncology, our strategy is to identify molecular targets that distinguish diseased cells from normal cells and activate the immune system to selectively target and eliminate only the diseased cells. The GI-6200 program, including GI-6207 is subject to option by Celgene and is being investigated for the treatment of MTC. The GI-6300 program, including GI-6301, is exclusively licensed to Celgene and is being investigated for the treatment of brachyury-expressing cancers such as chordoma. GI-4000 is our wholly-owned oncology program for the treatment of pancreas, NSCLC and colon cancers.
GI-6207
GI-6207 is a Tarmogen that expresses a modified version of the human CEA protein as the target cancer antigen. The NCI has conducted and funded preclinical efficacy and safety studies, as well as the early clinical development of the product candidate, and we are supplying study drug.
We believe that CEA represents an attractive target antigen for immunotherapy since it is over-expressed in many cancers. Because CEA is minimally or not expressed in normal cells, we believe GI-6207 can be used for targeted reduction of cancer cells with little or no effect on normal tissues.
Market Opportunity
CEA is over-expressed in a number of human epithelial cancers, including NSCLC, colorectal, pancreas, breast, gastric and MTC. We estimate CEA is over-expressed in approximately 500,000 new cancer cases in the United States each year.
The American Cancer Society estimates that there were approximately 60,220 new thyroid cancer cases in the United States in 2013. We believe MTC accounts for approximately 8% of thyroid cancer cases annually in the United States. Studies show that CEA is over expressed in over 60% of MTC. Surgery is currently the only curative treatment for MTC. Patients who develop metastatic MTC have a poor prognosis, with approximately 25% and 10% alive at five and ten years, respectively. Furthermore, metastatic MTC is largely unresponsive to conventional chemotherapy and radiotherapy. The only approved drugs for metastatic disease have demonstrated limited clinical benefit and substantial toxicity.
Development Status
NCI completed a Phase 1 dose escalation clinical trial of GI-6207. Twenty-five subjects with Stage IV cancers expressing CEA were enrolled into three dose groups, 4YU, 16YU and 40YU. GI-6207 doses were evenly divided and administered subcutaneously at four injection sites. Five subjects have had stable or decreased CEA levels and stable disease after receiving GI-6207. One subject experienced a grade 3 toxicity, defined as an event severe enough to interfere with daily living activities. This subject had pleural and pericardial metastases, or cancer in the spaces
97
Table of Contents
surrounding the heart and lungs, at the beginning of the study. The trial investigator and sponsor believed the event was likely caused by a therapeutic immune response directed at the metastatic lesions. The grade 3 toxicity resolved with cessation of GI-6207 therapy and treatment with corticosteroids. The patient resumed treatment with chemotherapy.
GI-6207-02 is a randomized Phase 2 study, being performed at the NCI that is planned to enroll a total of 34 subjects in a cross-over trial design. Subjects will be administered either GI-6207 for one year or be observed for six months and then administered GI-6207 for one year. The primary endpoint for the trial will be the effect of GI-6207 on changes in calcitonin levels. Calcitonin is a tumor marker that can be measured in a patient’s circulating blood that correlates with tumor burden in MTC. Elevated calcitonin values after surgery indicate persistent or recurrent disease. We initiated this trial with the NCI in February 2013. We believe that this trial could be fully enrolled in 2014 with results available by the end of the second quarter 2015 if the trial is fully enrolled on this schedule. Celgene has the option to exclusively license GI-6207 after the data from the Phase 2 trial in MTC are available.
GI-6301
The GI-6301 Tarmogen is designed to target cancers expressing the brachyury protein, which plays a role in metastatic progression of certain cancers and the initiation of chordoma. The frequency of brachyury expression increases with stage of disease. In lung cancer, approximately 60% of later stage lung cancer biopsies showed expression of brachyury, compared to approximately 40% of earlier stage lung cancers. In July 2013, Celgene paid us $9 million in connection with the exercise of its option to obtain an exclusive license to the GI-6300 program, including GI-6301. We anticipate that GI-6301 will be investigated in chordoma and other brachyury-expressing cancers. Under the GI-6300 license, we are eligible to receive a total of $85 million in future development and regulatory milestone payments. If a product from the GI-6300 program is commercialized, we may receive up to $60 million in sales milestone payments and tiered royalties ranging from high single digits to low double digits.
Market Opportunity
A variety of cancers express the brachyury protein, including lung, breast, colon, bladder, kidney, ovary, uterus and prostate. The brachyury protein increases the ability of a tumor cell to spread to other parts of the body in a number of tumors. Brachyury is also expressed in all chordomas, a rare and difficult-to-treat bone tumor. We believe that targeting this protein could potentially interfere with the metastatic process, potentially controlling and eliminating cancer cells containing this protein. We are not aware of any other product candidates in U.S. clinical trials targeting brachyury.
Chordoma is a rare bone cancer occurring in the spine and base of the skull that is aggressive, locally invasive, and has a poor prognosis. It occurs more frequently in men than women, with a median age at diagnosis of 59 years with a generally progressive increase in incidence with age. Median overall survival for patients with this disease is 6.3 years with 5-year, 10-year and 20-year survival rates of 68%, 40% and 13% respectively.
Chordomas can be indolent and slow growing, therefore they are often clinically silent until later stages of disease. Chordomas are not typically metastatic on presentation, with only five percent showing metastasis to the lungs, bone, skin and brain at the time of initial presentation. Patient survival appears to be less affected by distant metastasis than by local progression of the disease. Local progression has emerged as the most important predictor of mortality, and the extent of initial resection has become the most important factor in affording an opportunity for cure.
Aggressive surgical resection with an emphasis on neurological preservation, followed by adjuvant radiation therapy is the standard of care for this disease. Aggressive surgical resection with wide surgical margins has substantially improved local control of disease recurrence. However, preservation of a patient’s neurological function and quality of life are important when assessing surgical outcomes. Any tumor that remains after surgery, particularly when small in volume, is managed with radiotherapy. Currently, complete resection is attainable in approximately half of sacral chordomas, with much lower rates for spinal and skull base chordomas. While stand-alone radiotherapy has proven to be ineffective, Hadron-based adjuvant radiotherapy may offer an added advantage over surgery alone, with five year local control rates of 50-60%. Despite efforts at initial
98
Table of Contents
treatment, most chordomas will recur or progress. While many patients elect to undergo complex reoperations, despite a high rate of associated secondary complications for all lesion locations, there are few reports of treatment protocols and outcomes for recurrent lesions. Previous radiation treatment often limits the ability to safely re-irradiate, as well as causing increased morbidity for subsequent surgeries. There are no U.S. Food and Drug Administration, or FDA, approved treatments for chordoma.
The U.S. incidence of chordoma is 0.08 per 100,000 resulting in approximately 250 new US cases annually. We estimate the incidence in the European Union, or EU is similar to the U.S., resulting in approximately 400 new EU cases annually. With an average overall survival of approximately 10 years, the prevalence of chordoma is approximately 2,400 in the US and 3,600 in EU. We believe that GI-6301 in chordoma has the potential to be designated as an Orphan Drug and receive Breakthrough Therapy designation from the FDA.
Development Status
In January 2012, in collaboration with the NCI, we initiated a Phase 1 clinical trial with GI-6301 in subjects with metastatic cancers who have failed previous therapy or have no further therapeutic options. The trial is an open label, single agent, sequential dose escalation trial, with three to six subjects per dose group. GI-6301 doses are evenly divided and administered subcutaneously at four injection sites, every other week for seven visits, then monthly. The endpoints of this study are safety, tolerability, brachyury specific immune responses and clinical benefit.
Three dose levels were planned under the original protocol: 4YU, 16YU and 40YU. Ten additional subjects were enrolled in an expansion group at the 40YU dose. The protocol was amended in November 2013 to add a fourth, 80YU dose group of ten subjects to the trial. Enrollment of the 80YU group is ongoing. We anticipate the 80YU dose group will be fully enrolled by the end of the first half of 2014. Initial data from this study demonstrates that GI-6301 is generally well tolerated at all dose levels. In seven chordoma patients from the 40YU group evaluated to date, one subject was determined to be a confirmed partial responder at previously irradiated sites, one subject who had progressive disease at study entry was diagnosed with stable disease and two subjects who were determined to have stable disease at study entry continue to have stable disease. The other three chordoma subjects in the study have been diagnosed with progressive disease.
GI-4000 Overview
Our GI-4000 product candidates are designed to stimulate immune responses against the mutated Ras protein, a protein that is implicated in difficult to treat cancers, as further described below. We own worldwide development, manufacturing and commercialization rights to GI-4000. GI-4000 is a product series of four Tarmogens; each Tarmogen is a heat-inactivated S. cerevisiae yeast expressing a unique combination of three Ras mutations, collectively targeting seven of the most common Ras mutations observed in human cancers. In the GI-4000 clinical trials, each patient’s tumor is sequenced to identify the specific Ras mutation contained in the patient’s tumor and the corresponding, off-the-shelf Tarmogen containing the identified mutated protein is then administered. Each Tarmogen in the GI-4000 series is manufactured and vialed separately.
Mutated Ras in Cancer
We estimate that Ras mutations are found in approximately 200,000 new cancer cases each year in the United States across a spectrum of tumor types, including colorectal, NSCLC, pancreas, endometrial and ovarian cancers, as well as melanoma and multiple myeloma. Studies have shown that tumors with Ras mutations may be less responsive than tumors with normal Ras to conventional chemotherapy as well as targeted agents. For some cancers, such as NSCLC or colorectal cancer, therapies that target epidermal growth factor receptor, or EGFR, have improved clinical outcomes. However, the presence of a Ras mutation in the tumor has been associated with poor prognosis despite use of EGFR targeted therapies. For example, studies have shown that NSCLC with a Ras mutation is associated with a lack of response to tyrosine kinase inhibitors, such as erlotinib and gefitinib, while these therapies result in better survival rates for patients without a Ras mutation. Similarly, other studies have
99
Table of Contents
shown that patients with Ras mutated colorectal tumors do not benefit from cetuximab therapy, another EGFR targeted agent, compared to patients with normal Ras, who have improved survival rates when treated with the same therapy. As a result, patients with Ras mutations have fewer available effective treatment options. We believe these patients could benefit from GI-4000.
We believe that targeted reduction of cells containing Ras mutations could result in improved clinical outcomes for patients with a number of human cancers due to the role mutated Ras plays in tumor growth. In all of our GI-4000 clinical trials, we obtained a sample of tumor tissue from each subject during the screening period and evaluated the tumor for the presence of a Ras mutation. If a subject has a product-related mutation, we then administer the GI-4000 Tarmogen version that matches the specific Ras mutation in the subject’s tumor.
We believe that GI-4000’s unique mechanism of action may allow targeting of these tumors.
GI-4000 for Pancreas Cancer
GI-4000 has been tested in subjects with resected pancreas cancer in combination with gemcitabine. We begin administering GI-4000 to the patient when the overall tumor burden in the body is relatively low after resection, allowing enough time before disease progression for GI-4000 to induce an immune response against residual tumor.
Market Opportunity
The American Cancer Society predicts that in the United States in 2013 there will be 45,220 new cases of pancreas cancer diagnosed and 38,460 deaths from pancreas cancer. Pancreas cancer is rarely curable, with a median survival of 9 to 12 months and an overall five-year survival rate of three percent. A patient’s eligibility to undergo resection is an important factor in the patient’s prognosis. Only 15% to 20% of patients with pancreas cancer are candidates for resection. Pancreas cancer is particularly aggressive with non-specific initial symptoms, which frequently results in a delayed diagnosis. Therefore, the majority of patients are frequently not aware they have the disease until the cancer has metastasized.
Development Status
GI-4000-02 is a fully-enrolled Phase 2b randomized, double-blind, placebo-controlled, multi-center, adjuvant clinical trial in 176 subjects, with initial results reported in November 2012, evaluating GI-4000 plus gemcitabine or placebo plus gemcitabine in patients with R0 or R1 resected pancreas cancer. An R0 resection is defined by the absence of microscopic residual disease at the surgical margin. An R1 resection is defined by the presence of microscopic residual disease at the surgical margin. Since R0 and R1 patients have different expected survival rates, with R0 patients living longer on average, this clinical trial was stratified to ensure similar numbers of R0 and R1 patients between the different arms of the study.
Thirty-nine R1 subjects were enrolled, of whom 19 were assigned to the GI-4000 plus gemcitabine group and 20 were assigned to the placebo plus gemcitabine group. 137 R0 subjects were enrolled, of whom 69 were assigned to the GI-4000 plus gemcitabine group and 68 were assigned to the placebo plus gemcitabine group. The primary endpoint for this clinical trial was recurrence-free survival. Secondary endpoints included overall survival, immune responses and biomarkers of disease burden, such as CA19-9. The R1 subjects in the GI-4000 and placebo groups were comparable for general baseline features such as gender, age and race. In the R1 group, we observed the following results, which were not powered for, and did not reach, statistical significance:
| • | A one month advantage in median recurrence free survival for the 19 subjects in the GI-4000 group compared to the 20 subjects in the placebo group (9.4 months compared to 8.4 months, respectively, representing a 13% relative advantage); |
| • | A 2.6 month advantage in median overall survival for the 19 subjects in the GI-4000 group compared to the 20 subjects in the placebo group (17.2 months compared to 14.6 months, respectively), representing an 18% relative advantage; |
100
Table of Contents
| • | A 5.0 month survival advantage in the seven subjects with immune response in the GI-4000 group compared to the 20 subjects in the placebo group (19.6 months compared to 14.6 months, respectively, representing a 34% relative improvement); and |
| • | A safety profile for GI-4000 consistent with what is generally observed in patients with early stage resected pancreas cancer treated with gemcitabine alone. |
The R0 subjects in the GI-4000 and placebo groups were comparable based on demographics and baseline characteristics. Unlike the R1 subjects, there was no difference for R0 subjects in recurrence free or overall survival between the GI-4000 and placebo groups.
Companion Diagnostic BDX-001 – Proteomic Analysis of GI-4000-02
We performed a retrospective proteomic analysis of 90 pre-administration blood samples remaining after all pre-specified analyses under the clinical trial protocol for GI-4000-02 were completed. The demographics and baseline characteristics for the 90 subjects included in this analysis were generally balanced between arms and were representative of the 176 subjects in the trial. The goal of the analysis was to identify a pre-administration companion diagnostic test that could predict which subjects are likely to respond to GI-4000 regardless of their resection status to assist in subject selection for future clinical trials. Proteomic analysis is a testing method intended to measure the levels of specific proteins or protein fragments in blood or other body fluid. The intent of this type of analysis is to identify a pattern of proteins or fragments in a patient’s blood which predicts a patient’s response to a treatment. The analysis was performed on half of the samples to identify the proteomic companion diagnostic; the remaining half of the samples were retained for prospective testing of the final diagnostic test.
BDX-001, the resulting potential proteomic companion diagnostic test, appeared to predict whether a subject administered GI-4000 and the chemotherapy drug gemcitabine in this trial would have improved recurrence free and overall survival compared to gemcitabine alone. We believe BDX-001 differentiates between subject blood samples using the relationship of 100 different proteins and protein fragments. Overall, 21 of the 44 (48%) studied subjects administered GI-4000 and gemcitabine were classified as BDX-001 positive. In BDX-001 positive subjects administered GI-4000 and gemcitabine, there was an 11.7 month improvement in median recurrence free survival, or RFS, and a 16.6 month improvement in median overall survival, or OS, compared with BDX-001 positive subjects administered placebo and gemcitabine. There was no difference in RFS or OS in the gemcitabine-alone arm based on BDX-001 selection. The proportion of BDX-001 positive patients may vary in any future studies. This study was not powered for, and these results did not reach, statistical significance. If BDX-001 is prospectively validated in a second pancreas cancer trial, this companion diagnostic could be used to select the patients appropriate for GI-4000 therapy. The BDX-001 test is controlled by Biodesix, Inc. We intend to negotiate a development and commercialization agreement regarding this test with them. However, we may not be able to obtain the rights to use the test on commercially reasonable terms, if at all.
GI-4000 for Non-Small Cell Lung Cancer
Market Opportunity
There are an estimated 420,000 new cases of NSCLC annually in the United States, Western Europe and Japan. Studies suggest approximately 25% of NSCLCs have Ras mutations. There are numerous treatments for NSCLC, including multiple chemotherapies, EGFR targeted molecular therapies and immunotherapeutics and immunomodulatory therapies. A significant unmet medical need for targeted therapy of Ras mutations continues to exist for patients with NSCLCs containing a Ras mutation. Studies have shown that NSCLCs with Ras mutations are associated with poorer recurrence free survival and overall survival with adjuvant chemotherapy, as well as a lack of recurrence and survival benefit with EGFR targeted tyrosine kinase inhibitors, such as erlotinib and gefitinib. The five-year survival rate for patients with NSCLC is approximately 16%.
101
Table of Contents
Development Status
GI-4000-03 was a single arm, open label, Phase 2a clinical trial in 24 subjects at MSKCC designed to evaluate GI-4000 following successful first-line treatment for non-metastatic, or Stage I to III, Ras-mutated NSCLC. Subjects were enrolled between February 2008 and July 2010. Patients having no evidence of active cancer one to four months following completion of first-line therapy consisting of resection and/or radiation therapy and/or chemotherapy were eligible for this study. This study was funded by MSKCC, and we supplied study drug.
Subjects received 40 YU of GI-4000 given in three weekly doses, followed by six monthly doses, followed by supplemental doses every three months for up to three years. The 40YU dose was administered as four separate 10YU subcutaneous injections, one in each arm and leg, at each dosing visit. Administration of study drug continues according to this schedule until subjects withdrew from the study, experienced disease recurrence or died.
The objectives for the study were to evaluate immune response and safety. The study met its primary efficacy endpoint with 50% of the subjects who received GI-4000 showing Ras-specific T cell responses. Of the 20 subjects with immune samples sufficient for analysis, ten subjects developed a Ras-specific T cell response. The study also evaluated RFS and OS. Twenty-four subjects were enrolled; 17 were female and seven were male. Twelve subjects had Stage I disease, five subjects had Stage II disease and seven subjects had Stage III disease. Survival results from the Stage I – III who received GI-4000 subjects were compared to 64 Stage I – III Ras mutation-positive NSCLC patients not enrolled in the trial but treated and followed at MSKCC over the same period of time as the GI-4000-03 study. We believe these 64 patients are useful as a comparison group because they were treated at the same institution over the same time period. We refer to these 64 patients as case-matched controls. We used a statistical adjustment to account for differences in baseline characteristics when comparing the results of the subjects who received GI-4000 to the case-matched control group using Kaplan Meier estimates of survival. Subjects who received GI-4000 demonstrated a trend for improved 1-, 2- and 3-year overall survival relative to the case-matched control group. At Year 1, all subjects who received GI-4000 were alive vs. 93% of control subjects. At Year 2, all subjects who received GI-4000 were alive vs. 88% of control subjects. At Year 3, 92% of subjects who received GI-4000 were alive vs. 83% of control subjects. These data were not statistically significant.
There were no serious adverse events related to GI-4000 in the study. Further development of GI-4000 in NSCLC would require randomized, controlled trials that demonstrate statistically significant efficacy before the product candidate could be approved for commercialization.
The Tarmogen Platform
A Tarmogen consists of intact, heat-inactivated yeast containing a target protein. Immunization with a Tarmogen results in antigen-specific cellular immune responses against the target protein and reduction in the number of abnormal cells containing the same target antigen. Tarmogens also reduce the number and function of regulatory T cells, thus further enabling the antigen-specific cellular immune response. Tarmogens target the molecular profile that distinguishes a diseased cell from a normal cell but are not required to be custom manufactured for each individual patient. Tarmogens are manufactured by a process that yields a stable, off-the-shelf product candidate that is disease- or antigen-specific. While some antibody may be generated against the yeast, the antibody does not block the activity of the yeast, allowing for repeated administration and boosting of the immune response with additional administrations. The following graphics and corresponding text describe the mechanism by which we believe Tarmogens work.
102
Table of Contents
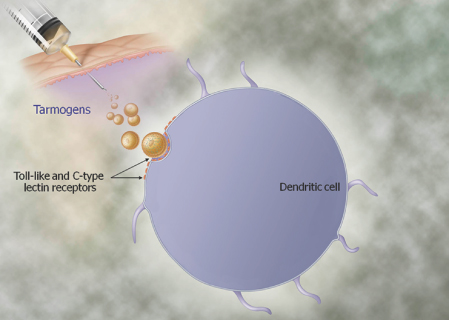
Administration of Tarmogens initially results in binding of the yeast to white blood cells called antigen-presenting cells, the most important of which are known as dendritic cells, near the injection site. The dendritic cells are activated as a result of the Tarmogens binding to molecules called Toll-like receptors and other receptor molecules on the surface of the dendritic cell, resulting in the activation of immune signaling molecules called cytokines. The dendritic cell then engulfs the Tarmogen. Multiple Tarmogens may be taken up by the same dendritic cell.
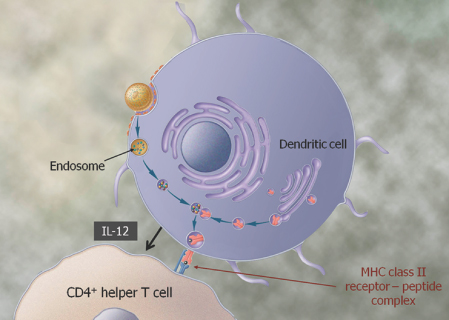
The Tarmogen is processed by the dendritic cell in two ways. First, the Tarmogen is engulfed by subcellular bodies known as endosomes and the protein inside the endosome is cut into shorter fragments called peptides. These peptides are presented by Class II MHC molecules on the surface of the dendritic cell. In combination with IL-12, a cytokine that is produced by the dendritic cell, these MHC-peptide complexes on the surface of the dendritic cell are recognized by and activate cells involved called CD4 + helper T cells.
103
Table of Contents
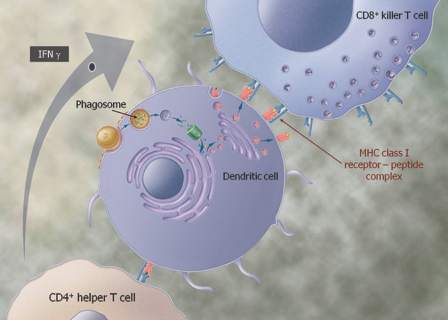
Dendritic cells also process Tarmogens by engulfing them with different subcellular bodies called phagosomes. This results in presentation of peptides, including the antigen from inside the Tarmogen, to cells, known as CD8 + killer T cells, via Class I MHC molecules on the surface of the dendritic cell, resulting in proliferation of identical antigen specific CD8 + T cells. CD4 + helper T cells are so named because one of their roles is to “help” activate killer T cells by expressing a cytokine called interferon gamma, IFN g .
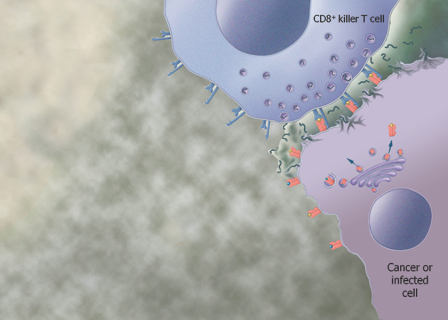
The newly activated CD8 + killer T cells move throughout the body and identify any other cell that expresses the same disease protein as the one recognized by the CD8 + killer T cells. Once the CD8 + killer T cell finds another cell in the body containing the target protein, it can kill the cell using multiple mechanisms.
104
Table of Contents
In addition to generating these antigen-specific T cell immune responses, Tarmogens also reduce the number and function of regulatory T cells, the component of the immune system that suppresses immune responses of other cells. Regulatory T cells represent an important mechanism built into the immune system to prevent excessive reactions. We believe that suppression of regulatory T cells could further enhance the ability of antigen-specific T cells to eliminate diseased cells.
Manufacturing
We commissioned an approximately 22,000 square foot facility in 2006 in Louisville, Colorado that incorporates current Good Manufacturing Practices, or cGMP, for the manufacture of clinical supplies of our product candidates. The manufacturing facility includes clean room space, laboratories, support areas, a warehouse and a loading dock, and has enabled us to meet clinical demand in a cost-effective and timely manner. Controlling our own manufacturing facility has allowed us to produce Tarmogens at relatively small scale to meet Phase 1 and 2 clinical trial demands while avoiding the need to transfer technology and schedule production at contractors. We have invested $11.0 million since 2005 in our facility to support both small-scale early clinical production and commercial-scale production for a pivotal trial and inventory build for potential product launch. Our small-scale production process is in routine operation. We use this facility to produce bulk product candidate that is then shipped to CMOs for filling and finishing. Currently, we are working with two qualified filling contractors.
The Tarmogen manufacturing process yields an off-the-shelf vialed product candidate with multi-year stability that can be distributed through conventional pharmaceutical channels. We believe the projected yields using a 250 liter fermentor that will allow Phase 3 clinical trial and commercial manufacturing will result in productivity estimates that compare favorably to those reported by biotechnology companies for their products. We have designed the process using scalable unit operations implemented on portable, disposable equipment, which gives us the flexibility to scale up when needed and facilitates technology transfer to a contract manufacturer or a partner, should this be desirable.
Vials of live yeast cells for each of our product candidates are stored frozen at two different locations. We believe the storage conditions and available number of vials ensure adequate availability of cells through the life-cycle of each of our product candidates. To make a batch of a product candidate, a vial containing live cells is thawed and used to inoculate a series of fermentors of increasing size until a sufficient mass of cells is produced for further processing. The cells are then collected, heat treated so that no live cells remain, washed to remove impurities and vialed at the appropriate volume and concentration for dosing patients.
All production activities are conducted under cGMP, the global standards established by the FDA and other regulatory agencies for pharmaceutical production. The equipment used in the manufacturing process is based on designs typically encountered in the production of other biotechnology products, and has been customized to tailor their use to Tarmogen production. Tarmogens are tested according to standards reviewed by the FDA and other applicable regulatory bodies before the Tarmogens can be released for use in humans.
We currently supply clinical trial materials under our collaboration agreements with Celgene. Celgene has the option to take over manufacturing under specified circumstances and has assumed responsibility for manufacturing for activities conducted by Celgene under the GI-6300 program. If Celgene assumes manufacturing responsibility, we will transfer the manufacturing process to them. We may also serve as a primary or secondary source of manufacturing for Celgene. Gilead has assumed manufacturing responsibility for GS-4774.
Sales and Marketing
We do not currently have any internal sales and marketing capabilities.
105
Table of Contents
Oncology Products
All of our oncology product candidates, including GI-6207, are subject to our collaboration and option agreement signed in May 2009 with Celgene, other than GI-4000. The GI-6300 program, including GI-6301, has been exclusively licensed by Celgene. If Celgene exercises its option to a specific oncology product candidate, then Celgene will have an exclusive license to develop, market and sell that product in all markets worldwide. If Celgene does not exercise its option to a particular oncology product candidate, we would evaluate the opportunity and may elect to further pursue development and commercialization independently or find a new corporate partner to support that product candidate.
Infectious Disease Products
GS-4774. Our Tarmogen product candidates targeting HBV are subject to our license and collaboration agreement with Gilead. As a result, Gilead has an exclusive license to develop, market and sell any approved HBV products worldwide.
Other infectious disease product candidates. Other than our GS-4774 program, we retain worldwide rights to all infectious disease Tarmogen product candidates. We intend to develop and commercialize these product candidates through a combination of collaborations and internal sales and marketing teams, appropriate to each situation.
Competition
The biopharmaceutical industry is highly competitive. We face competition from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. Given the significant unmet medical need for novel therapies to treat cancer and chronic infection, these are areas of specialty for many companies, public and private universities and research organizations that are actively engaged in the discovery and research and development of products. As a result, there are and will likely continue to be extensive research and substantial financial resources invested in the discovery and development of new products to treat cancer and chronic infection. We anticipate facing intense and increasing competition as new products enter the market and advanced technologies become available. We expect competition among products will be based on product efficacy and safety, the timing and scope of regulatory approvals, availability of supply, marketing and sales capabilities, reimbursement coverage, price and patent position.
In addition, there are numerous multinational pharmaceutical companies and large biotechnology companies currently marketing or pursuing the development of products or product candidates targeting the same indications as our product candidates. Many of our competitors will have substantially greater financial, technical and human resources. Accordingly, our competitors may be more successful in developing or marketing products and technologies that are more effective, safer or less costly. Additionally, our competitors may obtain regulatory approval for their products more rapidly and may achieve more widespread market acceptance.
Competing Immunotherapy Technologies
There are numerous immunotherapy products in clinical development, with over 215 targeting cancer, over 90 targeting infectious diseases and over 90 targeting chronic and other conditions. These products are generally based on one of several different competing platform technologies. These include peptide-based vaccines, whole autologous-cell based vaccines, whole allogeneic-cell based vaccines, DNA vaccines, viral-vector based vaccines, tumor lysate vaccines and dendritic cell vaccines. We are not aware of any other companies specifically utilizing recombinant yeast vectors. However, as research progresses, it is possible that competing approaches may prove superior to our Tarmogen technology, as each approach has the potential to confer different advantages and disadvantages based on its immunostimulatory mechanisms, formulation characteristics, manufacturing requirements, and logistical demands.
There is only one FDA-approved therapeutic vaccine, Dendreon Corporation’s sipuleucel-T, active cellular immunotherapy for the treatment of asymptomatic or minimally symptomatic metastatic castrate resistant hormone refractory prostate cancer. In addition, ipilimumab is an immunomodulatory product marketed by
106
Table of Contents
Bristol-Myers Squibb Company for the treatment of patients with late-stage melanoma. The cancer immunotherapy landscape is broad but still in the earlier stages of development with several public biopharmaceutical companies having clinical stage cancer immunotherapy products, including Dendreon Corporation, Bristol-Myers Squibb Company, GlaxoSmithKline plc, Merck & Co., Inc., NewLink Genetics Corporation, Merck KGaA and Sanofi. The immunotherapy landscape for the treatment of chronic infection is equally broad, with competing programs from large public biopharmaceutical companies such as Dynavax Technologies Corporation, Transgene S.A., Emergent BioSolutions Inc., and Arrowhead Research Corporation.
GI-6301
We believe there are no approved products targeting brachyury, and no immunotherapy products in clinical development targeting brachyury other than GI-6301.
GI-6207
AstraZeneca’s vandetanib and Exelixis’ cabozantinib are the only FDA-approved treatments for late-stage, or metastatic MTC in adult patients who are ineligible for resection. We believe there are no immunotherapy products in late stage clinical development other than GI-6207 for MTC. There are companies or institutions with clinical trials of immunotherapy products generally targeting CEA, including Bavarian Nordic, Etubics Corporation, Duke University, NIH/NCI, AlphaVax, Georgetown, Karolinska University; Cyto Pulse Sciences, Herbert Irving CCC, University of Virginia, University of Texas Galveston, Mayo Clinic, Radboud University, University of Chicago, GlaxoSmithKline and Merck.
GI-4000
GI-4000 is the only late-stage product candidate targeting Ras mutated cancer. There are several marketed products indicated for pancreas cancer, including Astellas Pharma Inc.’s erlotinib, Teva Pharmaceutical Industries Limited’s streptozocin, and gemcitabine, fluorouracil, or 5-FU, and mitomycin, which are marketed by several generic pharmaceutical firms. In addition, there are multiple companies or institutions conducting clinical trials of immunotherapy products in pancreas cancer, including NCI, Bavarian Nordic, NewLink Genetics Corporation, Aduro BioTech Inc., Sidney Kimmel Comprehensive Cancer Center, Providence Health & Services, Duke University, Advantagene, Inc. and AlphaVax, Inc.
There are numerous marketed therapeutics indicated for NSCLC, including Roche Holding AG’s bevacizumab, Eli Lilly’s pemetrexed, Astellas Pharma’s erlotinib and AstraZeneca PLC’s gefitinib, as well as generically available gemcitabine, platinum-based chemotherapeutics (cisplatin and carboplatin) and mitotic inhibitors (paclitaxel and vinorelbine), which are marketed by several generic pharmaceutical firms. In addition, there are multiple companies or institutions with clinical trials of immunotherapy products in late stage lung cancer, including NIH/NCI, UbiVac, University of Pittsburgh, Oslo University, Lee Moffitt Cancer Center, Shiga University, Kael-GemVax Co., Recombio SL, Bioven Sdn., Vaxn Biotech and NewLink Genetics.
There are numerous marketed therapeutics indicated for colorectal cancer, including Roche Holding AG’s bevacizumab, Bristol Myers-Squibb’s cetuximab, and Amgen’s panitumumab, as well as irinotecan, oxaliplatin, leucovorin and 5-FU, which are marketed by several generic pharmaceutical firms. In addition, there are multiple companies or institutions with clinical trials of immunotherapy products in colorectal cancer, including NIH/NCI, Universidad de Navarra, Duke University, AlphaVAx, Inc., Stanford University, Radboud University, Immunovative Therapies, Ltd., Etubics Corporation, Ohio State University, Roswell Park Cancer Institute and Mankind Coproration.
GS-4774
There are several marketed therapeutics indicated for the treatment of chronic HBV infection, including Roche Holding AG’s pegylated interferon 2a, Gilead’s tenofovir and adefovir, Bristol Myers-Squibb’s entecavir, Novartis’ telbivudine, and lamivudine, which is marketed by several generic pharmaceutical firms. In addition,
107
Table of Contents
there are several companies or institutions with clinical trials of immunotherapy products for the treatment of chronic HBV infection, including Arrowhead Research Corporation, GlaxoSmithKline, Institut National de la Santé Et de la Recherche Médicale, PowderMed, Beijing 302 Hospital, Dynavax, Genexine and NIAID.
Intellectual Property
It is important to our business to maintain the proprietary nature of and to protect our technology and know-how, including our proprietary Tarmogen product candidates, methods of making and using Tarmogen products, manufacturing processes, trade secrets, and know-how related to our Tarmogen products, processes and technology. Our success depends in part on our ability to protect our proprietary Tarmogen product candidates, technology and know-how, to operate without infringing the proprietary rights of others, and to prevent others from infringing our proprietary rights. We seek patent protection in the United States and internationally for our Tarmogen technology, Tarmogen product candidates, and other technology we develop. Our policy is to patent or in-license the technology, inventions and improvements that we consider important to the development of our business. We also rely on trade secrets, know-how and continuing innovation to develop and maintain our competitive position. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents granted to us in the future will be commercially useful in protecting our technology.
The Tarmogen technology platform is covered by seven families of patents and patent applications of which 11 are issued U.S. patents and 30 are issued foreign patents in Australia, Canada, China, Hong Kong, India, Japan, Mexico, Singapore, South Korea and multiple European countries, and of which 67 are patent applications in the U.S. and foreign jurisdictions, covering the basic Tarmogen platform, as well as various improvements and modifications to the Tarmogen platform, technology and manufacturing processes. These seven patent families are owned and/or co-owned by us and/or exclusively licensed from The Regents of the University of Colorado or The United States of America as represented by the Department of Health and Human Services, or HHS. One of the patent families that cover the basic Tarmogen compositions of matter and method of use extends until November 2015 in the U.S. and abroad. Following expiration of the 2015 patents, all product candidates will still be covered by issued or pending patents, including but not limited to those within the other six patent families with claims that continue in effect through November 2021, December 2023, February 2027, February 2028, April 2030, and September 2030, respectively, in the U.S. and abroad. The patent family expiring in November 2021 includes issued claims in the United States for compositions, methods of use of compositions, and methods to produce compositions. The patent family expiring in December 2023 includes issued claims in the United States for compositions and methods of use of compositions. This family also includes issued claims, pending claims or the option to pursue claims for compositions and methods of use of compositions in foreign countries. The patent family expiring in February 2027 includes issued claims for compositions and pending claims or the option to pursue claims for methods of use of compositions and methods to produce compositions in the United States. This family also includes issued claims, pending claims or the option to pursue claims for compositions, methods of use of compositions and methods to produce compositions in foreign countries. The patent family expiring in February 2028 includes pending claims in the United States for compositions, methods of use of compositions and methods to produce compositions and issued and pending claims for compositions, methods of use of compositions and methods to produce compositions in foreign countries. The patent families expiring in April 2030 and September 2030 include pending claims or the option to pursue claims for compositions, methods of use of compositions, and methods to produce compositions in the United States and foreign countries.
In addition to the protection provided by the existing patent families that we currently own, co-own and/or license, our exclusive, worldwide license from The Regents of the University of Colorado provides intellectual property rights related to the Tarmogen platform including various improvements and specific technologies related to the Tarmogen platform that have been or may be discovered in the future by University of Colorado researchers during the term of the license.
108
Table of Contents
Our oncology product candidate, GI-4000, targeting cancers expressing mutated Ras, is specifically covered under two patent families that we currently own and/or co-own and/or license under our exclusive, worldwide license from The Regents of the University of Colorado, of which eight are issued U.S. patents and 12 are issued foreign patents in Australia, China, Hong Kong, India, Mexico, South Korea and Japan, and of which 27 are patent applications in the U.S. and foreign jurisdiction. These issued patents and any patent applications that issue as patents in the United States and/or abroad will expire in December 2023 or March 2027, unless the patent term is extended by patent term adjustment or patent term extension. An additional patent family consists of one pending U.S. patent application, one pending Patent Cooperation Treaty, or PCT, application, and one pending Taiwan application containing subject matter related to GI-4000 that we co-own with Biodesix, Inc. and/or for which we have the first right to negotiate an exclusive license with Biodesix, Inc. These U.S. and Taiwan patent applications and any patent applications claiming priority to the PCT application, if issued as patents in the U.S. and/or abroad, will expire June 2033, unless the patent term is extended by patent term adjustment or patent term extension. The patent families covering GI-4000 include issued claims for compositions, methods of use of compositions, and methods of detecting Ras mutants in the United States, as well as pending claims or the option to pursue claims for compositions, methods of use of compositions, and methods to identify subjects for treatment in the United States. This family also includes issued claims, pending claims or the option to pursue claims for compositions, methods of use of compositions, methods to identify subjects for treatment, and methods to produce compositions in foreign countries.
GI-6207, our oncology product candidate targeting cancers expressing CEA, is specifically covered by a patent family that we currently own and/or co-own and/or license under our exclusive, worldwide license from The Regents of the University of Colorado, of which six are issued U.S. patents and eight are issued foreign patent applications in Australia, China, Hong Kong, India, South Korea and Japan, and of which 16 are patent applications in the United States and foreign jurisdictions. These issued patents and any patent applications that issue as patents in the United States and/or abroad will expire in December 2023, unless the patent term is extended by patent term adjustment or patent term extension. The antigen expressed by GI-6207 is also covered under a patent family that we have in-licensed from the Public Health and Human Services of the United States Government. The last of the 29 issued patents covered by the license from the Public Health and Human Services of The United States Government expires in September 2018. The patent family expiring in 2023 and covering GI-6207 includes issued claims, pending claims or the option to pursue claims for compositions and methods of use of compositions in the United States and foreign countries.
GI-6301, our oncology product candidate targeting cancers expressing brachyury, is covered by three patent families. One patent family, of which six are issued U.S. patents and eight are issued foreign patent applications in Australia, China, Hong Kong, India, South Korea and Japan, and of which 16 are patent applications in the United States and foreign jurisdictions, is owned and/or co-owned by us and/or licensed under our exclusive, worldwide license from The Regents of the University of Colorado. These issued patents and any patent applications that issue as patents in the United States and/or abroad will expire December 2023, unless the patent term is extended by patent term adjustment or patent term extension. A second patent family covering GI-6301, of which 14 are pending U.S. or foreign patent applications, is co-owned by us and the United States of America as represented by HHS. Any of these patent applications that issue as patents in the United States and/or abroad will expire in March 2032, unless the patent term is extended by patent term adjustment or patent term extension. We also have an exclusive, worldwide license from the Public Health Service to the U.S. Government’s rights in this patent family. A third patent family covering GI-6301, currently consisting of one international (PCT) application and one Taiwanese application, is co-owned by us and by the United States of America as represented by HHS, and if patent applications claiming priority to this patent application issue as patents in the United States and/or abroad, such issued patents will expire in March 2034, unless the patent term is extended by patent term adjustment or patent term extension. The patent families covering GI-6301 include issued claims, pending claims or the option to pursue claims for compositions, methods of use of compositions, and methods to produce compositions in the United States and foreign countries.
109
Table of Contents
Our lead product candidates in HBV infection, known collectively as GI-13000 and including GI-13020 also known as GS-4774, are primarily covered by one patent family that is owned by us and exclusively licensed to Gilead. The family currently contains 38 pending patent applications, and one issued United States patent. The issued United States patent, as well as any of these patent applications, or patent applications claiming priority to these patent applications, if issued as patents in the United States and/or abroad, will expire February 2032, unless the patent term is extended by patent term adjustment or patent term extension. The patent family covering GI-13000 includes allowed compensation claims in the United States and pending claims or the option to pursue claims for compositions and methods of use of compositions in the United States and foreign countries.
We are also actively pursuing additional patent applications in the United States and foreign patent jurisdictions for other preclinical Tarmogen product candidates and methods of use, including additional Tarmogen product candidates targeting oncology antigens, and additional Tarmogen product candidates for infectious disease. In addition, we intend to seek patent protection whenever available for any products or product candidates and related technology we develop and/or acquire in the future.
The patent positions of biotechnology companies like ours are generally uncertain and involve complex legal, scientific and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued. Consequently, we do not know whether the Tarmogen product candidates we are developing will gain patent protection or, if patents are issued, whether they will provide significant proprietary protection or will be challenged, circumvented or invalidated. Because patent applications in the United States and certain other jurisdictions are maintained in secrecy for 18 months, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in post-grant review proceedings, interference proceedings, third-party ex parte reexamination proceedings or inter partes review proceedings under the U.S. Patent and Trademark Office, or in opposition proceedings in a foreign patent office, any of which could result in substantial cost to us, even if the eventual outcome is favorable to us. There can be no assurance that the patents, if issued, would be held valid by a court of competent jurisdiction. An adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using specific compounds or technology. To the extent prudent, we intend to bring litigation against third parties that we believe are infringing one or more of our patents.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office in granting a patent, or may be shortened if a patent is terminally disclaimed over another patent. Certain of our patents currently benefit from patent term adjustment and some of our patents issuing in the future may benefit from patent term adjustment.
The patent term of a patent that covers an FDA-approved product may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the product is under regulatory review. Patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved product may be extended. Similar provisions are available in Europe and other non-U.S. jurisdictions to extend the term of a patent that covers an approved product. In the future, if and when our Tarmogen product candidates receive FDA approval, we expect to apply for patent-term extensions on patents covering those products.
To protect our rights to any of our issued patents and proprietary information, we may need to litigate against infringing third parties, or avail ourselves of the courts or participate in hearings to determine the scope
110
Table of Contents
and validity of those patents or other proprietary rights. These types of proceedings are often costly and could be very time-consuming to us, and there can be no assurance that the deciding authorities will rule in our favor. An unfavorable decision could allow third parties to use our technology without being required to pay us licensing fees or may compel us to license needed technologies to avoid infringing third-party patent and proprietary rights. Such a decision could even result in the invalidation or a limitation in the scope of our patents or forfeiture of the rights associated with our patents or pending patent applications.
We intend to seek orphan drug status whenever it is available. If a product which has an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, meaning that the applicable regulatory authority may not approve any other applications to market the same drug for the same indication, except in certain very limited circumstances, for a period of seven years in the United States and 10 years in the European Union. Orphan drug designation does not prevent competitors from developing or marketing different drugs for an indication.
We also rely on trademark registration to protect our intellectual property. We currently have trademarks registered on the Principal Register in the United States for “GLOBEIMMUNE”, “TARMOGEN”, and for our company logo, and on the Principal and the Supplemental Register for “TARGETED MOLECULAR IMMUNOTHERAPY.” We also have a trademark application pending for “GBIM”.
We also rely on trade secret protection for our confidential and proprietary information. No assurance can be given that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technology, or that we can meaningfully protect our trade secrets. However, we believe that the substantial costs and resources required to develop technological innovations will help us to protect the competitive advantage of our products.
It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting or collaborative relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual shall be and are our exclusive property. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for our trade secrets in the event of unauthorized use or disclosure of such information.
Licensing Agreements
Celgene Corporation – Collaboration and Option Agreement
We are party to a Collaboration and Option Agreement, or the Celgene Agreement, with Celgene, dated May 14, 2009, as amended November 6, 2009, February 9, 2010, June 16, 2011, October 24, 2011 and July 2013, pursuant to the GI-6300 Program License Agreement between us and Celgene. Under the Celgene Agreement, we granted Celgene the exclusive option, on a program-by-program basis, to certain specified programs and all of our future oncology programs. Celgene declined to exercise its option to the GI-4000 program and we own worldwide rights to this program. For each such program subject to Celgene’s option, we have agreed to conduct early development of the product candidates in such oncology program through certain predefined endpoints, at which time Celgene will have the right to exercise its option and obtain the exclusive license to develop and commercialize the product candidates in such program. However, Celgene has assumed manufacturing responsibility for product in the GI-6300 program, except for product used by the Company in clinical trials conducted by the Company.
Under the Celgene Agreement, Celgene has paid us a $31.3 million. If certain development, regulatory, and sales milestones are achieved, we would be eligible to receive up to $290 million in milestone payments for GI-
111
Table of Contents
6301 and GI-6207. Other future oncology programs have potential development, regulatory and sales milestones per program of up to approximately $100 million if Celgene exercises its option to license a program. If future products are commercialized, we are eligible to receive tiered royalty rates in the teens based on net sales of each licensed product candidate. In July 2013, Celgene paid us $9 million in connection with the exercise of its option to obtain an exclusive license to the GI-6300 program, including GI-6301. We anticipate that GI-6301 will be investigated in chordoma, and other brachyury-expressing cancers. Under the GI-6300 license, we are eligible to receive a total of $85 million in future development and regulatory milestone payments. If a product from the GI-6300 program is commercialized, we may receive up to $60 million in sales milestone payments and tiered royalties ranging from high single digits to low double digits.
Each party has the right to terminate the Celgene Agreement for the other party’s uncured material breach or insolvency, and Celgene has the right to terminate the Celgene Agreement for convenience at any time upon prior notice. Following termination of the Celgene Agreement by Celgene for our uncured material breach or insolvency, all licenses granted to Celgene will continue with respect to the programs for which Celgene has exercised the option, subject to certain continuing obligations. If not terminated earlier, the Celgene Agreement will remain in effect, for a particular product in a particular country, until the expiration of all payment obligations for such product in such country. The payment obligations for a particular product will expire in such country at the later of (i) the date of the last to expire claim on a U.S. patent for such product, (ii) the date upon which the regulatory exclusivity for such product expires, or (iii) the tenth anniversary of the first commercial sale of such product in such country.
Gilead Sciences, Inc. – License and Collaboration Agreement
We are party to a License and Collaboration Agreement, or the Gilead Agreement, dated October 24, 2011 with Gilead Sciences. Under the agreement, we granted Gilead exclusive worldwide rights to use our platform technology on Tarmogens to research, develop, and commercialize vaccine products directed at HBV. Under the agreement, we also granted Gilead licenses under certain trademarks owned or controlled by us, solely for use with respect to such HBV vaccine product.
Under the Gilead Agreement, Gilead paid us an upfront payment of $10 million and agreed to fund a Phase 1a clinical trial of GS-4774. Since signing the agreement, we have received $5 million in milestone payments in association with certain milestones connected with the Phase 1a and Phase 1b/2a clinical trial. Gilead is responsible for clinical development beyond the Phase 1a clinical trial. We are eligible to receive up to an additional $130 million in development and regulatory milestones, and if products are commercialized, tiered royalty rates in the upper single digits to mid-teens and up to $40 million of sales milestone payments based on net sales of the licensed product candidates.
The term of the Gilead Agreement continues on a product-by-product and country-by-country basis until the expiration of Gilead’s obligation to pay royalties for such product in such country, or until the agreement is earlier terminated. The payment obligations, and therefore the term of the Gilead Agreement, with respect to a particular product will expire in such country on the later of (i) the date of the last to expire claim on a U.S. patent for such product and (ii) the tenth anniversary of the first commercial sale of such product in such country. Gilead can terminate the agreement at will on prior written notice to us. Each party has the right to terminate the agreement for the other party’s uncured material breach of the agreement, or if such other party becomes insolvent or bankrupt. Under certain circumstances of termination of the agreement, Gilead will negotiate in good faith with us the terms under which Gilead will grant to us an exclusive, royalty-bearing license to a terminated product in the terminated country.
The Regents of the University of Colorado – Restated Intellectual Property License Agreement
We are party to a Restated Intellectual Property License Agreement, or the CU Agreement, with the Regents of the University of Colorado, or CU, dated May 30, 2006 and originally effective as of September 18, 1997, as
112
Table of Contents
amended May 5, 2009 and March 12, 2010. The CU Agreement granted to us an exclusive, worldwide, sublicenseable license under specified CU patent rights relating to yeast-based immunotherapy, to make, use and sell products and processes that are covered by such patent rights. CU retains a non-exclusive, non-transferrable right to use such patent rights for academic and research purposes, and also to certain pre-existing rights of the U.S. government. The CU Agreement also granted to us an option to obtain rights to any future inventions or discoveries created or developed at CU by one or more of the original inventors of the licensed CU patents.
In consideration of our license under the CU Agreement, we issued to CU shares of our Common Stock. We also are obligated to pay CU low single digit percentage royalties on net revenues from our and our sublicensee’s sale of any commercialized licensed product or process, and certain other payments. We are responsible for diligently prosecuting and maintaining the licensed CU patent rights, at our sole cost and expense.
Under the CU Agreement, we have certain obligations to obtain funding or financing to conduct further research and development, and are obligated to commercialize, either directly or through a sublicensee, the licensed CU patent rights as licensed products or processes.
The term of the CU Agreement continues until the expiration of the last patent included within the licensed CU patents, or until the agreement is earlier terminated. We may terminate the agreement on prior written notice to CU. Each party has the right to terminate the agreement for the other party’s uncured material breach of obligations under the agreement. We are obligated to pay low single digit royalties on net sales of licensed products through expiration of the licensed intellectual property.
National Cancer Institute – Cooperative Research and Development Agreement
We are party to a Cooperative Research and Development Agreement, or CRADA, with NCI, entered into in 2008, as amended on August 8, 2011 and on July 30, 2013. We previously carried out a series of collaborative research studies with NCI on the generation and analysis of yeast-based vaccines in preclinical models. Under the CRADA, the parties will jointly develop products intended to treat a variety of cancers, through collaborative research and development activities set forth in a research plan. We will utilize our proprietary Tarmogen technology to develop multiple immunotherapy products expressing various cancer antigens provided by the NCI, and the NCI will conduct and fund preclinical and early clinical development of the product candidates.
Under the CRADA, NCI will be the sponsor of, and will prepare and submit an IND covering the applicable product candidate or candidates. We may sponsor our own clinical trials for Tarmogens developed within or outside the scope of the CRADA and hold our own IND for studies performed outside the scope of the CRADA, or for studies within the scope of the CRADA if mutually agreed by the parties.
The party that produces an invention or develops materials under the CRADA retains sole ownership of such invention and materials. The parties will jointly own all data created under the CRADA. Subject to certain rights retained by the U.S. government, (i) with respect to any inventions made solely by NCI, NCI has granted us an irrevocable, perpetual, paid-up, nonexclusive, nontransferable, royalty-free, world-wide license for internal research and development purposes only, and (ii) with respect to any inventions made jointly between the parties under the CRADA, NCI has granted us an exclusive option to elect an exclusive or nonexclusive commercialization license. Further, we have granted to the U.S. government, for research or other U.S. government purposes, a non-exclusive, worldwide, nontransferable, irrevocable, paid-up license to practice any inventions made solely by us under the CRADA.
The term of the CRADA is for ten years, as amended in July 2013. A party may unilaterally terminate the CRADA by providing prior written notice. In the event we terminate the CRADA, or otherwise suspend development of the product candidates during the term of the CRADA without transferring our development
113
Table of Contents
efforts, assets and obligations to a third party within ninety days of such discontinuation, we have agreed to continue supplying the product candidates with respect to all patients enrolled under any active or approved protocols, subject to certain restrictions.
Public Health Service – Patent License Agreements
We are party to a Patent License Agreement, or the PHS Agreement, with the U.S. Public Health Service, or PHS of which the National Institute of Health, or NIH, is an agency, dated June 12, 2007, as amended April 5, 2010 and October 31, 2011. Under the PHS Agreement, we are granted a worldwide exclusive license under certain PHS patent rights to develop and commercialize licensed products and licensed processes for oncology indications related to over-expressed carcinoembryonic antigen, or CEA,. The Company has the right to grant sublicenses, through multiple tiers, upon written approval of PHS, such approval not to be unreasonably delayed or withheld, and subject to certain additional conditions and obligations.
Our license under the PHS Agreement is subject to the U.S. government’s retained rights under a non-exclusive, worldwide, royalty-free license for the practice of all inventions licensed under the PHS patent rights, by or on behalf of the U.S. government and on behalf of any foreign government or international organization pursuant to any existing or future treaty or agreement to which the U.S. government is a signatory. For purposes of encouraging basic research, the U.S. government also reserves the right to grant or require us to grant to a third party on reasonable terms a non-exclusive, non-transferable license to make and use the licensed products or licensed processes for research purpose only, but subject to PHS consulting with us in the event such third party is a commercial entity. Under certain exceptional and enumerated circumstances, the U.S. government may require us to grant a sublicense to a responsible third party applicant, on terms that are reasonable under the circumstances. The PHS takes responsibility for all aspects of the preparation, filing, prosecution and maintenance of any and all patent applications or patents included in the licensed PHS patent rights, subject to our payment of certain patent-related expenses.
In consideration of our license under the PHS Agreement, we have agreed to pay to PHS certain low single digit percentage royalties, including as a percentage of net sales of any licensed products or licensed processes, an annual, minimum royalty, and benchmark royalties in the aggregate amount of approximately $750,000 upon the achievement of certain benchmark events. Under the agreement, we agreed to use reasonable commercial efforts to bring the licensed products and licensed processes to practical application, including meeting certain development benchmarks as agreed to by the parties, and making such licensed products and licensed processes commercially available to the public in accordance with an agreed-to commercial development plan. Upon the first commercial sale of any licensed product or licensed process, we have agreed to use reasonable commercial efforts to make reasonable quantities of such licensed product available on a compassionate use basis to patients, and to develop educational materials directed to patients and physicians.
The term of the PHS Agreement continues until the expiration or termination of the last to expire of the patents under the PHS patent rights, or until the agreement is earlier terminated. We have the unilateral right to terminate the agreement, or our license in any country, on written notice to PHS. PHS has the right to terminate for our uncured material breach of our obligations under the agreement, or if we become insolvent or bankrupt. PHS also has the right to terminate or modify the PHS Agreement if it determines that certain specific events have occurred, such as our failure to achieve any development benchmarks or failure to satisfy unmet health and safety needs, provided such right is subject to appeal by us. Upon termination of the PHS Agreement, a sublicensee of ours under the PHS patent rights may elect for termination of its sublicense, or the conversion to a license directly between it and PHS. Such conversion is subject to PHS approval and contingent upon the sublicensee’s acceptance of the remaining provisions of the PHS Agreement.
We are also party to three other Patent License Agreements, or the Additional PHS Agreements, with PHS dated August 23, 2011, March 12, 2012, and January 3, 2012. Under the Additional PHS Agreements, we are granted worldwide exclusive licenses under certain PHS patent rights covering “subject inventions” that arose
114
Table of Contents
under the CRADA between us and NCI, to make, have made, use, have use, sell, have sold, offer to sell, and import licensed products and licensed processes. We have the right to grant sublicenses, through multiple tiers, upon written approval of PHS, such approval not to be unreasonably delayed or withheld, and subject to certain additional conditions and obligations.
Our licenses under the Additional PHS Agreements are subject to the U.S. government’s retained rights under a non-exclusive, worldwide, royalty-free license for the practice of all inventions licensed under the PHS patent rights, by or on behalf of the U.S. government and on behalf of any foreign government or international organization pursuant to any existing or future treaty or agreement to which the U.S. government is a signatory. For purposes of encouraging basic research, the U.S. government also reserves the right to grant or require us to grant to a third party on reasonable terms a non-exclusive license to make and use the licensed products or licensed processes for research purpose only, but subject to PHS consulting with us in the event such third party is a commercial entity. Under certain exceptional and enumerated circumstances, the U.S. government may require us to grant a sublicense to a responsible third party applicant, on terms that are reasonable under the circumstances.
In consideration of our licenses under the Additional PHS Agreements, we have agreed to pay to PHS certain low single digit percentage royalties, including as a percentage of net sales of any licensed products or licensed processes, annual minimum royalty payments, and benchmark royalties in the aggregate amount of $1.65 million upon the achievement of certain benchmark events. We are also responsible for the preparation, filing, prosecution and maintenance of any and all patent applications or patents included in the licensed PHS patent rights, subject to consultation with PHS. In addition, under the agreement, we agreed to use reasonable commercial efforts to bring the licensed products and licensed processes to practical application, including meeting certain development benchmarks as agreed to by the parties, and making such licensed products and licensed processes commercially available to the public in accordance with an agreed-to commercial development plan. Upon the first commercial sale of any licensed product or licensed process, we have agreed to use reasonable commercial efforts to make reasonable quantities of such licensed product available on a compassionate use basis to patients, and to develop educational materials directed to patients and physicians.
The terms of the Additional PHS Agreements continue until the expiration or termination of the last to expire of the patents under the applicable PHS patent rights, or until the agreements are earlier terminated. For each agreement within the Additional PHS Agreements, we have the unilateral right to terminate the agreement, or our license in any country, on written notice to PHS. PHS has the right to terminate for our uncured material breach of our obligations under the agreement, or if we become insolvent or bankrupt. PHS also has the right to terminate or modify the Additional PHS Agreements if it determines that certain specific events have occurred, such as our failure to achieve any development benchmarks or failure to satisfy unmet health and safety needs, provided that such right is subject to appeal by us. Upon termination of any of the agreements within the Additional PHS Agreements, a sublicensee of ours under the applicable PHS patent rights may elect for termination of its sublicense, or the conversion to a license directly between it and PHS. Such conversion is subject to PHS approval and contingent upon the sublicensee’s acceptance of the remaining provisions of the applicable Additional PHS Agreement.
Government Regulation
We operate in a highly regulated industry that is subject to significant federal, state, local and foreign regulation. Our present and future business has been, and will continue to be, subject to a variety of laws including, the Federal Food, Drug, and Cosmetic Act, or FDC Act”) and the Public Health Service Act, among others.
The FDC Act and other federal and state statutes and regulations govern the testing, manufacture, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion of our products. As a result of these laws and regulations, product development and product approval processes are very expensive and time-consuming.
115
Table of Contents
In July 2011, the FDA issued draft guidance that stated that if safe and effective use of a therapeutic depends on an in vitro diagnostic, then the FDA generally will not approve the therapeutic unless the FDA approves or clears this “in vitro companion diagnostic device” at the same time that the FDA approves the therapeutic. The approval or clearance of the companion diagnostic would occur through the FDA’s Center for Devices and Radiological Health Office of In Vitro Diagnostic Device Evaluation and Safety. It is unclear whether the FDA will finalize this guidance in its current form, or when it will do so. Even if the FDA does finalize the guidance, it is difficult to predict how it will implement the guidance. For example, the draft guidance allows for flexibility by the FDA in the case of disease indications with serious unmet medical needs, but it is unclear how this discretion will be applied by the agency. Therefore, even with the issuance of the draft guidance, the FDA’s expectations for companion diagnostics remain unclear.
FDA Approval Process
In the United States, pharmaceutical products, including biologics, are subject to extensive regulation by the FDA. The FDC Act and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending biologic license applications, or BLAs, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution.
Pharmaceutical product development in the United States typically involves preclinical laboratory and animal tests, the submission to the FDA of an IND, which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the biologic for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation as well as animal trials to assess the characteristics and potential pharmacology and toxicity of the product. The conduct of the preclinical tests must comply with federal regulations and requirements including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has not objected to the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational product to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted in compliance with federal regulations and good clinical practices, or GCP, as well as under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The clinical trial protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
116
Table of Contents
Clinical trials to support a BLA submission for marketing approval, are typically conducted in three sequential Phases, but the Phases may overlap. In Phase 1, the initial introduction of the investigational product candidate into healthy human subjects or patients, the investigational product is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses and, if possible, early evidence on effectiveness. Phase 2 usually involves trials in a limited patient population, to determine the effectiveness of the investigational product for a particular indication or indications, dosage tolerance and optimum dosage, and identify common adverse effects and safety risks. In the case of product candidates for severe or life-threatening diseases such as cancer, the initial human testing is often conducted in patients rather than in healthy volunteers.
If an investigational product demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 clinical trials are undertaken to obtain additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the investigational product and to provide adequate information for its labeling.
After completion of the required clinical testing a BLA, is prepared and submitted to the FDA. FDA approval of the marketing application is required before marketing of the product may begin in the United States. The marketing application must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture, and controls.
The FDA has 60 days from its receipt of a BLA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of marketing applications. Most such applications for non-priority drug products are reviewed within ten months after the FDA’s filing of the application. The review process may be extended by the FDA for three additional months to consider new information submitted during the review or clarification regarding information already provided in the submission. The FDA may also refer applications for novel drug products or drug products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving a marketing application, the FDA will typically inspect one or more clinical sites to assure compliance with GCP.
Additionally, the FDA will inspect the facility or the facilities at which the product is manufactured. The FDA will not approve the BLA unless compliance with cGMPs is satisfactory and the marketing application contains data that provide substantial evidence that the product is safe, pure and potent, or effective in the indication studied. Manufacturers of biologics also must comply with FDA’s general biological product standards.
After the FDA evaluates the BLA and the manufacturing facilities, it issues an approval letter or a complete response letter. A complete response letter outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed in a resubmission of the marketing application, the FDA will re-initiate review. If the FDA is satisfied that the deficiencies have been addressed, the agency will issue an approval letter. It is not unusual for the FDA to issue a complete response letter because it believes that the product is not safe enough or effective enough or because it does not believe that the data submitted are reliable or conclusive.
An approval letter authorizes commercial marketing of the drug product with specific prescribing information for specific indications. As a condition of approval of the marketing application, the FDA may require substantial post-approval testing and surveillance to monitor the product’s safety or efficacy and may impose other conditions, including labeling restrictions, which can materially affect the product’s potential market and profitability. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
117
Table of Contents
Biosimilars
The Biologics Price Competition and Innovation Act, or BPCIA, was passed on March 23, 2010 as part of the Patient Protection and Affordable Care Act, or PPACA. The law provides for an abbreviated approval pathway for biological products that demonstrate biosimilarity to a previously-approved biological product. Biosimilarity means a product has been shown to be biosimilar. The BPCIA provides 12 years of exclusivity for innovator biological products. The BPCIA may be applied to our product candidates in the future and could be applied to allow approval of biosimilars to our products. Although it has issued some draft guidance, the FDA is still drafting regulations under the BPCIA. It is not certain that we will receive 12 years of biologics marketing exclusivity for any of our products. Many of the details regarding the implementation of the BPCIA are yet to be determined.
Other Regulatory Requirements
Once a BLA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of therapeutic products, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet.
Biologics may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new BLA or BLA supplement, before the change can be implemented. A BLA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing BLA supplements as it does in reviewing BLAs. We cannot be certain that the FDA or any other regulatory agency will grant approval for our product candidates for any other indications or any other product candidate for any indication on a timely basis, if at all.
Adverse event reporting and submission of periodic reports is required following FDA approval of a BLA. The FDA also may require post-marketing testing, known as Phase 4 testing, risk evaluation and mitigation strategies, and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control as well as product manufacturing, packaging, and labeling procedures must continue to conform to cGMPs after approval. Manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Companion Diagnostic Review and Approval
Some of our product candidates currently rely upon the use of a companion diagnostic test to select patients with the appropriate mutation and in the future we may utilize other biomarkers as companion diagnostic tests for our other product candidates. Presently, these mutation tests are available only as Laboratory Developed Tests that are commercialized by laboratories certified under the Clinical Laboratory Improvement Amendments. Approval of our product candidates will likely require FDA approval of a Premarket Approval Application, or PMA, for a reproducible, validated diagnostic test to be used with our Tarmogens.
The PMA process is costly, lengthy, and uncertain, although the PMA review for a mutation test is currently planned to occur concurrently with the development and review of a BLA for our product candidates. The receipt and timing of PMA approval may have a significant effect on the receipt and timing of commercial approval for
118
Table of Contents
our product candidates. Human diagnostic products are subject to pervasive and ongoing regulatory obligations, including the submission of medical device reports, adherence to the Quality Systems Regulation, recordkeeping and product labeling, as enforced by the FDA and comparable state authorities.
U.S. Foreign Corrupt Practices Act
The U.S. Foreign Corrupt Practices Act, to which we are subject, prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity.
Federal and State Fraud and Abuse Laws
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the biopharmaceutical and medical device industries in recent years. These laws include anti-kickback statutes and false claims statutes.
The federal health care program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce or in return for purchasing, leasing, ordering, or arranging for the purchase, lease, or order of any health care item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Recently, several pharmaceutical and other health care companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the company’s marketing of the product for unapproved, and thus non-reimbursable, uses. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines, and imprisonment.
Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Such a challenge could have a material adverse effect on our business, financial condition and results of operations.
Regulation in the European Union
Biologics are also subject to extensive regulation outside of the United States. In the European Union, for example, there is a centralized approval procedure that authorizes marketing of a product in all countries of the European Union, which includes most major countries in Europe. If this procedure is not used, approval in one country of the European Union can be used to obtain approval in another country of the European Union under two simplified application processes, the mutual recognition procedure or the decentralized procedure, both of
119
Table of Contents
which rely on the principle of mutual recognition. After receiving regulatory approval through any of the European registration procedures, pricing and reimbursement approvals are also required in most countries.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances and biological materials. We may incur significant costs to comply with such laws and regulations now or in the future.
Research and Development
We incurred $1.5 million, $3.2 million, $10.9 million, $11.7 million, and $12.1 million in research and development, which includes costs of collaboration license and services, costs of manufacturing services and research and development for proprietary programs in the three months ending March 31, 2014 and 2013 and in the years ended December 31, 2013, 2012 and 2011, respectively.
Legal Proceedings
We are not currently a party to any material pending legal proceedings.
Employees
As of March 31, 2014, we had 25 employees. 19 of our employees were engaged in research and development and manufacturing activities and six were engaged in support administration, including business development, finance, information systems and human resources. None of our employees is subject to a collective bargaining agreement. We consider our relationship with our employees to be good.
Facilities
Our corporate headquarters are located in Louisville, Colorado, where we lease approximately 40,000 gross square feet of office, laboratory and manufacturing space under a lease expiring March 31, 2018 which automatically extends to March 31, 2019 upon completion of this offering.
120
Table of Contents
The following table sets forth the name, age and position of each of our executive officers and directors as of March 31, 2014.
| Name |
Age | Position | ||||
| Executive Officers |
||||||
| Timothy C. Rodell, M.D. |
63 | Chief Executive Officer, President and Director | ||||
| C. Jeffrey Dekker, C.P.A. |
49 | Vice President, Finance, Treasurer and Secretary | ||||
| Kirk A. Christoffersen, M.B.A. |
46 | Vice President, Corporate Development | ||||
| Non-Employee Directors |
||||||
| J. William Freytag, Ph.D. |
62 | Chairman of the Board, Director | ||||
| Ralph E. Christoffersen, Ph.D.(1) |
76 | Director | ||||
| Augustine J. Lawlor |
57 | Director | ||||
| Paul A. Mieyal, Ph.D.(2) |
44 | Director | ||||
| Dan J. Mitchell |
57 | Director | ||||
| Pennina Safer, Ph.D. |
57 | Director | ||||
| S. Edward Torres |
51 | Director | ||||
| (1) | Dr. Christoffersen has informed the Company that he will resign from our Board of Directors one day prior to the effectiveness of the registration statement of which this prospectus is a part. |
| (2) | Dr. Mieyal has informed the Company that he will resign from our Board of Directors one day prior to the effectiveness of the registration statement of which this prospectus is a part. |
Executive Officers
Timothy C. Rodell, M.D. has been our Chief Executive Officer and a member of our Board of Directors since April 2003 and has been our President since June 2005. From March 2002 until April 2003, Dr. Rodell worked with SMG, Inc., a pharmaceutical consulting firm. From November 1999 until February 2002, Dr. Rodell was President and Chief Executive Officer of RxKinetix, Inc., a private drug delivery company. From March 1996 until October 2000, he held a number of positions at OXIS International, Inc., a publicly-traded developer of biotech and pharmaceutical technologies and products, including Chief Technology Officer and Chairman and President of OXIS International, SA, the company’s French subsidiary. From 1985 until 1995, Dr. Rodell was at Cortech, Inc., a publicly-traded biopharmaceutical company, where he was most recently Executive Vice President of Operations and Product Development. He currently serves on the board of directors of the Biotechnology Industry Organization. Dr. Rodell earned his M.D. from the University of North Carolina School of Medicine and is board certified in internal medicine and pulmonary medicine. He also completed post-doctoral fellowships in molecular biology and cell biology at the Eleanor Roosevelt Cancer Institute and the Webb Waring Institute, respectively. We believe that Dr. Rodell possesses specific attributes that qualify him to serve as a member of our board of directors, including his experience as a medical doctor, his operational and management expertise, and his years of leadership experience.
C. Jeffrey Dekker, C.P.A. has been our Vice President, Finance and Treasurer since October 2011 and Secretary since March 2012. Mr. Dekker joined us as Senior Director, Finance in February 2006. Prior to joining us, Mr. Dekker held leadership positions in finance and accounting at three private software companies, including at Webroot Software Inc. from October 2004 to February 2006, where he was Controller, at Requisite Technology Inc. from August 1999 to October 2004, where he was most recently Vice President and Controller, and at NxTrend Technology, Inc. from July 1993 to August 1999, where he was most recently Vice President, Corporate Controller. Mr. Dekker was at ITT Rayonier Port Angeles Pulp Division, Port Angeles, Washington from July 1989 to July 1993, where he was most recently Manager, General Accounting. From September 1986 until July 1989, he was at KPMG Peat Marwick, where he was most recently a Senior Accountant. Mr. Dekker earned a B.S. in accounting from Utah State University and is a certified public accountant.
121
Table of Contents
Kirk A. Christoffersen, M.B.A. has been our Vice President, Corporate Development since December 2013. He joined us as Senior Director Corporate development in November 2004. Prior to GlobeImmune, Mr. Christoffersen held leadership positions in corporate development and marketing at three biotechnology companies, including OSI pharmaceuticals where he was Brand Manager for Gelclair from 2003 to 2004, and Associate Director Commercial Planning from 2002 to 2003. He held positions with increasing levels of responsibility at Gilead Sciences from 1998 to 2002 where he was Manager, Senior Manager, and then Associate Director Corporate Development. He was Business Development Consultant for Ribozyme Pharmaceuticals during 1996 and 1997. Prior to working exclusively in the biopharmaceutical industry, from 1990 to 1997 he worked for Vail Resorts Inc., holding a management position for the last three years of his tenure there. Mr. Christoffersen earned a B.G.S. from the University of Michigan in 1990, and an M.B.A. from the Daniels College of Business at the University of Denver in 1993.
Non-Employee Directors
J. William Freytag, Ph.D. has served as Chairman of our Board of Directors since January 2011 and has served as a member of our Board of Directors since March 2008. Dr. Freytag served as a member of the Board of Directors of ARCA biopharma, Inc., a publicly-traded biopharmaceutical company, from January 2009 to March 2011, serving as chair and a member of its compensation committee and its Lead Director from January 2009 to March 2011. Dr. Freytag served as a director of Immunicon Corp., a publicly-traded developer of diagnostic products, as well as a member of its compensation committee, from May 1998 until its merger with Veridex, LLC in June 2008. Dr. Freytag was Chairman and Chief Executive Officer of Aspreva Pharmaceuticals Corp., a publicly-traded pharmaceutical company, from July 2007 until its merger with Galenica AG in January 2008. Prior to Aspreva, Dr. Freytag was President, Chief Executive Officer and Chairman of the Board of Directors of Myogen, Inc., a publicly-traded pharmaceutical company, from July 1998 until Myogen was acquired by Gilead Sciences, Inc. in November 2006. From November 2006 through June 2007, Dr. Freytag served as Senior Advisor to Gilead. From October 1994 to May 1998, Dr. Freytag was a Senior Vice President at Somatogen, Inc., a publicly-traded biotechnology company. Prior to Somatogen, he was President of Research and Development at Boehringer Mannheim Corporation, an international healthcare company, from May 1990 to September 1994. Previously, Dr. Freytag spent ten years with DuPont in various research and business positions in the Medical Products Department. Dr. Freytag received a B.S. from Purdue University and a Ph.D. in biochemistry from the University of Kansas Medical Center. We believe that Dr. Freytag possesses specific attributes that qualify him to serve as a member of our board of directors, including his business and leadership experience in the pharmaceutical industry.
Ralph E. Christoffersen, Ph.D. has served as a member of our Board of Directors since June 2003. Dr. Christoffersen has been a Partner of Morgenthaler Ventures, a private equity firm, since July 2001. From July 2001 to May 2002, he was Chairman of the Board of Directors of Ribozyme Pharmaceuticals, Inc., a publicly-traded company involved in developing ribozyme-based therapeutic agents, and from June 1992 to July 2001, he was Chief Executive Officer and President of Ribozyme. Prior to joining Ribozyme, he was the Senior Vice President of Research at SmithKline Beecham Corporation from August 1989 until June 1992, Vice President of Discovery Research at The Upjohn Company from September 1983 until August 1989 and President and a Professor at Colorado State University from 1981 until 1983. Dr. Christoffersen also serves as a director of a number of private biotechnology companies. He received his Ph.D. from Indiana University and did his post-doctorate work at Nottingham University, United Kingdom and Iowa State University, and received a B.S. from Cornell College. Dr. Christoffersen has informed the Company he will resign from our Board of Directors one day prior to the effectiveness of the registration statement of which this prospectus is a part due to Morgenthaler Ventures’ policy.
Augustine J. Lawlor has been a member of our Board of Directors since June 2003. Mr. Lawlor has been a managing director of HealthCare Ventures LLC since June 2000. Prior to joining HealthCare Ventures, Mr. Lawlor served as Chief Operating Officer of LeukoSite Inc., a biotechnology company, from June 1997 to
122
Table of Contents
June 2000. Before joining LeukoSite, Mr. Lawlor served as Chief Financial Officer and Vice President of Corporate Development of Alpha-Beta Technology, Inc., a biotechnology company. He was also previously Chief Financial Officer and Vice President, Business Development, of BioSurface Technologies Corporation, a biofilm company. Mr. Lawlor serves on the Board of Directors of Cardiovascular Systems, Inc., a publicly-traded biopharmaceutical company. From May 2004 to July 2012 Mr. Lawlor served on the Board of Directors of Human Genome Sciences, Inc., a publicly-traded biopharmaceutical company, and a number of private companies. He received a B.A. from the University of New Hampshire and a master’s degree in management from Yale University. We believe that Mr. Lawlor possesses specific attributes that qualify him to serve as a member of our board of directors, including his experience in the venture capital industry, his years of business and leadership experience and his financial sophistication and expertise.
Paul A. Mieyal, Ph.D., CFA has been a member of our Board of Directors since July 2007. Since October 2006, Dr. Mieyal has served as a Vice President of Wexford Capital LP, a Securities and Exchange Commission, or SEC, registered investment advisor. Prior to that, from January 2000 to September 2006, he was Vice President in charge of healthcare investments for Wechsler & Co., Inc., a private investment firm and registered broker-dealer. Dr. Mieyal has served as a director of Nephros, Inc., a publicly-traded medical device company, since September 2007 and served as its acting CEO from April 2010 to April 2012. Dr. Mieyal served as a director of Nile Therapeutics, Inc., a publicly-traded biopharmaceutical company, from September 2007 until November 2013, and also served as a member of its audit and compensation committees. Between March 2009 and November 2010, Dr. Mieyal served as a director of OncoVista Innovative Therapies, Inc., a publicly-traded biopharmaceutical company. Dr. Mieyal received his Ph.D. in pharmacology from New York Medical College and a B.A. in chemistry and psychology from Case Western Reserve University, and he is a chartered financial analyst. We believe that Mr. Mieyal possesses specific attributes that qualify him to serve as a member of our board of directors, including his experience in the venture capital industry, his years of business and leadership experience and his financial sophistication and expertise. Dr. Mieyal has informed the Company he will resign from our Board of Directors one day prior to the effectiveness of the registration statement of which this prospectus is a part.
Dan J. Mitchell has been a member of our Board of Directors since June 2003. Mr. Mitchell founded and is a Manager of Sequel Venture Partners, L.L.C., a venture capital firm formed in January 1997. Prior to founding Sequel Venture Partners, Mr. Mitchell was a founder of Capital Health Venture Partners, a health care focused venture capital firm, in October 1986 where he was a General Partner until 2006. From 2002 to 2009, he served on the board of directors of Replidyne, Inc., a publicly-traded pharmaceutical company acquired by Cardiovascular Systems, Inc. In February 2014, Mr. Mitchell joined the board of directors of ARCA biopharma, Inc., a publicly-traded pharmaceutical company, where Mr. Mitchell serves as a member of the board of directors’ Audit Committee and Nominating and Corporate Governance Committee. Mr. Mitchell currently serves on the board of directors of several private companies. Mr. Mitchell holds a B.S. from the University of Illinois and an M.B.A. from the University of California at Berkeley. We believe that Mr. Mitchell possesses specific attributes that qualify him to serve as a member of our board of directors, including his experience in the venture capital industry, his years of business and leadership experience and his financial sophistication and expertise.
Pennina Safer, Ph.D. has been a member of our Board of Directors since October 2011. Dr. Safer is the Executive Vice President of Operations and Product Development at ImmunArray Ltd. Dr. Safer has also been a Partner with Medica Venture Partners, a venture capital fund, since November 2002. Prior to joining Medica, Dr. Safer was a Principal at Concord Ventures, a venture capital fund, from October 2000 to October 2002. From September 1998 until September 2000, she was Vice President of Research and Development at CBD Technologies, now a wholly-owned subsidiary of FuturaGene PLC, an agricultural biotechnology company that is publicly-traded on the London Stock Exchange. From September 1984 until August 1998, Dr. Safer held various positions including Director, Technology Acquisition at Genetics Institute, a biotechnology company, now a wholly-owned subsidiary of Pfizer. Dr. Safer received a B.S. from Brooklyn College of the City of New York and a Ph.D. in human genetics from Yale University, and did a post-doctoral fellowship at the Roche
123
Table of Contents
Institute of Molecular Biology. We believe that Dr. Safer possesses specific attributes that qualify her to serve as a member of our board of directors, including her experience in the venture capital industry, and her years of business and leadership experience in the pharmaceutical industry.
S. Edward Torres, has been a member of our Board of Directors since November 2010. Mr. Torres has been a Managing Director of Lilly Ventures Fund I, LLC, a venture capital fund since March 2009. From January 2006 until February 2009, he was a Managing Director of Lilly Ventures while Lilly Ventures was wholly-owned by Eli Lilly and Company. From December 2001 until December 2005, Mr. Torres was a Principal with Lilly Ventures. Prior to his various roles with Lilly Ventures, Mr. Torres held a range of positions with Eli Lilly and Company from 1989 through 2001, which included operational finance, planning, mergers and acquisitions, business development and global marketing roles. He currently serves on the board of Receptos, Inc., a publicly traded biopharmaceutical company, where Mr. Torres serves as the chairman of the Audit Committee and as a member of Compensation Committee. Mr. Torres received a B.A. from Creighton University and an M.B.A. from the University of Michigan Business School. We believe that Mr. Torres possesses specific attributes that qualify him to serve as a member of our board of directors, including his experience in the venture capital industry, his years of business and leadership experience in the pharmaceutical industry and his financial sophistication. We believe that Mr. Torres possesses specific attributes that qualify him to serve as a member of our board of directors, including his experience in the venture capital industry, his years of business and leadership experience in the pharmaceutical industry and his financial sophistication.
Our executive officers are appointed by and serve at the discretion of our board of directors. Mr. Kirk A. Christoffersen, our Vice President of Corporate Development, is the son of Dr. Ralph E. Christoffersen, our director. Dr. Christoffersen has indicated he will resign upon the effectiveness of this registration statement of which this prospectus is a part.
Board Composition and Election of Directors
Our amended and restated certificate of incorporation that is to become effective upon completion of this offering, or the amended and restated certificate of incorporation, will permit our Board of Directors to establish by resolution the authorized number of directors. Our Board of Directors currently consists of eight members, including seven non-employee directors and our Chief Executive Officer and President, Timothy C. Rodell, M.D. Our Board of Directors has determined that all of our directors, other than Dr. Rodell, are independent within the meaning of applicable NASDAQ listing standards. Drs. Christoffersen and Mieyal have indicated that they intend to resign from our Board of Directors one day prior to the effectiveness of the registration statement of which this prospectus is a part, and effective upon the closing of the offering our Board of Directors will consist of six members.
Each director serves until the expiration of the term for which such director was elected or appointed, or until such director’s death, resignation or removal. At each annual meeting of stockholders, successors to directors will be elected to serve from the time of election and qualification until the next annual meeting following election. Our amended and restated certificate of incorporation provides that the authorized number of directors may be changed only by resolution of the board of directors.
We believe that the composition of our Board of Directors meets the requirements for independence under the rules of The NASDAQ Global Market. As required by such rules, we anticipate that our independent directors will meet in regularly scheduled executive sessions at which only independent directors are present. We intend to comply with future independence requirements to the extent they become applicable to us.
Board Committees
Upon completion of this offering, our Board of Directors will have an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee. Our Board of Directors may establish other committees to facilitate management of our business. The composition and primary responsibilities of each committee are described below.
124
Table of Contents
Audit Committee
Upon completion of this offering, the members of our Audit Committee will be Mr. Torres, Mr. Lawlor and Mr. Mitchell. Mr. Torres will serve as chairman of the Audit Committee. Our Board of Directors has determined that each member of the Audit Committee meets the independence requirements of Rule 10A-3 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the NASDAQ listing standards. Our Board of Directors has also determined that Mr. Torres qualifies as an audit committee financial expert within the meaning of SEC regulations.
The primary purpose of the Audit Committee is to discharge the responsibilities of our Board of Directors with respect to our accounting, financial and other reporting and internal control practices and to oversee our independent registered public accounting firm. Specific responsibilities of our Audit Committee include:
| • | evaluating the performance of our independent registered public accounting firm and determining whether to retain or terminate its services; |
| • | determining and pre-approving the engagement of our independent registered public accounting firm to perform audit services and any permissible non-audit services, other than immaterial aggregate amounts of non-audit services as excepted under applicable laws and rules; |
| • | reviewing and discussing with management and our independent registered public accounting firm the results of the annual audit and the independent registered public accounting firm’s review of our annual and quarterly financial statements and reports; |
| • | reviewing with management and our independent registered public accounting firm significant issues that arise regarding accounting principles and financial statement presentation; |
| • | conferring with management and our independent registered public accounting firm regarding the scope, adequacy and effectiveness of our internal control over financial reporting; and |
| • | establishing procedures for the receipt, retention and treatment of any complaints we receive regarding accounting, internal control or auditing matters. |
Compensation Committee
Upon completion of this offering, the members of our Compensation Committee will be Dr. Freytag and Mr. Mitchell. Dr. Freytag will serve as chairman of the Compensation Committee. Our Board has determined that each member of the Compensation Committee is independent within the meaning of the applicable NASDAQ listing standards, is a non-employee director as defined in Rule 16b-3 under the Exchange Act and is an outside director as that term is defined in Section 162(m) of the Internal Revenue Code of 1986. The purpose of our Compensation Committee is to discharge the responsibilities of our Board of Directors to oversee our compensation policies, plans and programs and to review and determine the compensation to be paid to our executive officers and other senior management. Specific responsibilities of our Compensation Committee include:
| • | determining the compensation and other terms of employment of our executive officers and reviewing and approving corporate performance goals and objectives relevant to such compensation; |
| • | evaluating and recommending to our Board of Directors the compensation plans and programs advisable for us, and evaluating and recommending the modification or termination of existing plans and programs; and |
| • | reviewing and approving the terms of any employment agreements, severance arrangements, change-of-control protections and any other compensatory arrangements for our executive officers. |
125
Table of Contents
Nominating and Corporate Governance Committee
Upon completion of this offering, the members of our Nominating and Corporate Governance Committee will be Dr. Safer and Mr. Mitchell. Mr. Mitchell will serve as chairman of the Nominating and Corporate Governance Committee. Each member of the Nominating and Corporate Governance Committee is independent within the meaning of applicable NASDAQ listing standards. The specific responsibilities of our Nominating and Corporate Governance Committee include:
| • | identifying, reviewing, evaluating and recommending for selection candidates for membership to our Board of Directors; |
| • | reviewing, evaluating and considering the recommendation for nomination of incumbent members of our Board of Directors for reelection to our Board of Directors and monitoring the size of our Board of Directors; |
| • | evaluating nominations by stockholders of candidates for election to our Board of Directors; and |
| • | reviewing, discussing and reporting to our Board of Directors an assessment of the performance of the Board of Directors. |
Compensation Committee Interlocks and Insider Participation
For the fiscal year ended December 31, 2013, members of the Compensation Committee consisted of Drs. Freytag, Christoffersen and Mieyal. None of the members of the Compensation Committee is currently, or has ever been at any time since the Company’s formation, one of the Company’s officers or employees. None of our officers currently serve, nor have they served during the last completed fiscal year, as a member of the board of directors or compensation committee of any entity that has one or more officers serving as a member of our Board of Directors or Compensation Committee. In December 2013, we promoted Kirk A. Christoffersen to the position of Vice President, Corporate Development. This promotion and his annual salary of $191,475 were recommended by our Compensation Committee, of which his father, Dr. Christoffersen, is a member.
Code of Business Conduct and Ethics
We have adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer and controller, or persons performing similar functions. Following the completion of this offering, the code of business conduct and ethics will be posted on the Corporate Governance section of our website, www.globeimmune.com. We expect that any amendments to the code of business conduct and ethics will be disclosed on our website. The reference to our web address does not constitute incorporation by reference of the information contained at or available through our website. You should not rely on our website or any such information in making your decision whether to purchase our common stock.
126
Table of Contents
EXECUTIVE AND DIRECTOR COMPENSATION
Summary Compensation Table
The following table sets forth information regarding compensation earned during the year ended December 31, 2013 by our principal executive officer and our two other most highly compensated executive officers serving as executive officers at December 31, 2013 whose total compensation exceeded $100,000 for the year ended December 31, 2013. We refer to these individuals as our named executive officers.
| Name and Principal Position | Year | Salary ($) |
Non-equity Incentive Plan Compensation ($) |
All Other Compensation ($)(1) |
Total ($) |
|||||||||||||||
| Timothy C. Rodell, M.D. |
2013 | 388,125 | — | 54 | 388,179 | |||||||||||||||
| C. Jeff Dekker, C.P.A. |
2013 | 191,475 | — | 54 | 191,529 | |||||||||||||||
| Kirk A. Christoffersen |
2013 | 188,756 | — | 54 | 188,810 | |||||||||||||||
| (1) | The amounts in this column represent life insurance premiums paid by the Company for the benefit of the named executive officer. |
Narrative Disclosure to Summary Compensation Table
Employment Agreements
The Company entered into employment agreements with each of the named executive officers, in each case, effective upon the date of effectiveness of the registration statement of which this prospectus is a part. The material terms of the agreements, including severance and change-of-control terms, are summarized below.
Employment Agreement with Dr. Timothy Rodell
The Company has negotiated the principal terms of an employment agreement with Dr. Rodell, which agreement and terms will be effective upon the effective date of a registration statement for the initial underwritten public offering of shares of our Common Stock. The initial term of the agreement will be for three years, and the agreement will renew automatically at the end of the term unless either party notifies the other within 90 days of the agreement’s expiration of its or his desire to not renew the agreement or to renew the agreement on different terms. The agreement will provide for an initial annual base salary of $388,125, which will be subject to increase once every 12 months upon review by our Board of Directors, and subject to then-current market data for similar positions. Dr. Rodell will be eligible to participate on the same basis as similarly situated executives in the Company’s benefit plans in effect from time to time during his employment. In addition, Dr. Rodell will be eligible to receive an annual bonus of up to 40% of his base salary if he meets targets established by our Board of Directors, subject to the Company’s financial performance. Dr. Rodell will also be eligible for equity incentive compensation, subject to the terms of the Company’s 2014 Equity Incentive Plan. Any such grants of equity-based compensation will be made at the discretion of the Board of Directors.
Under the terms of the employment agreement, in the event that the Company terminates Dr. Rodell’s employment without cause, or if Dr. Rodell resigns for good reason (other than in connection with a change-of-control of the Company), and provided that Dr. Rodell executes a general release in favor of the Company, he will be entitled to receive certain payments and other benefits, which are as follows:
| • | an amount equal to 12 months of his base salary then in effect, payable on our standard payroll dates; and |
127
Table of Contents
| • | if Dr. Rodell elects to continue coverage under our group health insurance plan, reimbursement of his insurance premiums (or in certain cases a taxable cash payment) for a period of 12 months or until he qualifies for health insurance benefits from a new employer, whichever occurs first. |
The agreement will further provide that, upon termination of Dr. Rodell’s employment by us within two months prior or 12 months after the date on which the Company experiences a change-of-control (as defined in the agreement), and provided that Dr. Rodell executes a general release in favor of the Company, Dr. Rodell would receive an amount equal to 18 months of his base salary then in effect, payable on our standard payroll dates and, if Dr. Rodell elects to continue coverage under our group health insurance plan, reimbursement of his insurance premiums (or in certain cases a taxable cash payment) for a period of 18 months or until he qualifies for health insurance benefits from a new employer, whichever occurs first. In addition, the unvested, unexpired portion of Dr. Rodell’s stock options and/or equity awards, as applicable, will be accelerated in full and the term and period during which Dr. Rodell’s stock options may be exercised will be extended to the earlier of 12 months after the date his employment ended, or the expiration date of the option as set forth in the applicable stock option grant notice and/or agreement. For the purpose of Dr. Rodell’s employment agreement, “good reason” will be defined as (i) a material reduction of Dr. Rodell’s salary or bonus target by more than ten percent; (ii) any request by us that Dr. Rodell relocate a distance of more than thirty-five miles; or (iii) following a change-of-control, Dr. Rodell’s benefits and responsibilities are materially reduced, or his base compensation or annual bonus target are reduced by more than ten percent.
Dr. Rodell has entered into an Employee Proprietary Information and Inventions Agreement with the Company. His agreement imposes certain confidentiality, non-compete and/or non-solicitation obligations on him. In the event that Dr. Rodell violates his confidentiality, non-compete and/or non-solicitation obligations, or the terms of his proprietary and inventions assignment agreement with the Company, Dr. Rodell’s right to the severance benefits that he would have otherwise been entitled to receive pursuant to his employment agreement will cease on the date of such violation.
Employment Agreement with Mr. C. Jeffrey Dekker, C.P.A.
The Company has negotiated the principal terms of an employment agreement with Mr. Dekker, which agreement and terms will be effective upon the effective date of a registration statement for the initial underwritten public offering of shares of our Common Stock. The initial term of the agreement will be for three years, and the agreement will renew automatically at the end of the term unless either party notifies the other within 90 days of the agreement’s expiration of its or his desire to not renew the agreement or to renew the agreement on different terms. The agreement will provide for an initial annual base salary of $191,475, which is subject to increase once every 12 months upon review by our Board of Directors and subject to then-current market data for similar positions. Mr. Dekker will be eligible to participate on the same basis as similarly situated executives in the Company’s benefit plans in effect from time to time during his employment. In addition, Mr. Dekker will be eligible to receive an annual bonus of up to 25% of his base salary if he meets targets established by our Board of Directors, subject to the Company’s financial performance. Mr. Dekker will also be eligible for equity incentive compensation, subject to the terms of the Company’s 2014 Equity Incentive Plan. Any such grants of equity-based compensation will be made at the discretion of the Board of Directors.
Under the terms of the employment agreement, in the event that the Company terminates Mr. Dekker’s employment without cause, or if Mr. Dekker resigns for good reason (other than in connection with a change-of-control of the Company), and provided that Mr. Dekker executes a general release in favor of the Company, he will be entitled to receive certain payments and other benefits, which are as follows:
| • | an amount equal to six months of his base salary then in effect, payable on our standard payroll dates; and |
| • | if Mr. Dekker elects to continue coverage under our group health insurance plan, reimbursement of his insurance premiums (or in certain cases a taxable cash payment) for a period of six months or until he qualifies for health insurance benefits from a new employer, whichever occurs first. |
128
Table of Contents
The agreement will further provide that, upon termination of Mr. Dekker’s employment by us within two months prior or 12 months after the date on which the Company experiences a change-of-control (as defined in the agreement), and provided that Mr. Dekker executes a general release in favor of the Company, Mr. Dekker would receive an amount equal to 12 months of his base salary then in effect, payable on our standard payroll dates and, if Mr. Dekker elects to continue coverage under our group health insurance plan, reimbursement of his insurance premiums (or in certain cases a taxable cash payment) for a period of 12 months or until he qualifies for health insurance benefits from a new employer, whichever occurs first. In addition, the unvested, unexpired portion of Mr. Dekker’s stock options and/or equity awards, as applicable, will be accelerated in full and the term and period during which Mr. Dekker’s stock options may be exercised will be extended to the earlier of 12 months after the date his employment ended, or the expiration date of the option as set forth in the applicable stock option grant notice and/or agreement. For the purpose of Mr. Dekker’s employment agreement, “good reason” will be defined as (i) a material reduction of Mr. Dekker’s salary or bonus target by more than ten percent; (ii) any request by us that Mr. Dekker relocate a distance of more than thirty-five miles; or (iii) following a change-of-control, Mr. Dekker’s benefits and responsibilities are materially reduced, or his base compensation or annual bonus target are reduced by more than ten percent.
Mr. Dekker has entered into an Employee Proprietary Information and Inventions Agreement with the Company. His agreement imposes certain confidentiality, non-compete and/or non-solicitation obligations on him. In the event that Mr. Dekker violates his confidentiality, non-compete and/or non-solicitation obligations, or the terms of his proprietary and inventions assignment agreement with the Company, Mr. Dekker’s right to the severance benefits that he would have otherwise been entitled to receive pursuant to his employment agreement will cease on the date of such violation.
Employment Agreement with Mr. Kirk A. Christoffersen
The Company has negotiated the principal terms of an employment agreement with Mr. Christoffersen, which agreement and terms will be effective upon the effective date of a registration statement for the initial underwritten public offering of shares of our Common Stock. The initial term of the agreement will be for three years, and the agreement will renew automatically at the end of the term unless either party notifies the other within 90 days of the agreement’s expiration of its or his desire to not renew the agreement or to renew the agreement on different terms. The agreement will provide for an initial annual base salary of $191,475, which is subject to increase once every 12 months upon review by our Board of Directors and subject to then-current market data for similar positions. Mr. Christoffersen will be eligible to participate on the same basis as similarly situated executives in the Company’s benefit plans in effect from time to time during his employment. In addition, Mr. Christoffersen will be eligible to receive an annual bonus of up to 25% of his base salary if he meets targets established by our Board of Directors, subject to the Company’s financial performance. Mr. Christoffersen will also be eligible for equity incentive compensation, subject to the terms of the Company’s 2014 Equity Incentive Plan. Any such grants of equity-based compensation will be made at the discretion of the Board of Directors.
Under the terms of the employment agreement, in the event that the Company terminates Mr. Christoffersen’s employment without cause, or if Mr. Christoffersen resigns for good reason (other than in connection with a change-of-control of the Company), and provided that Mr. Christoffersen executes a general release in favor of the Company, he will be entitled to receive certain payments and other benefits, which are as follows:
| • | an amount equal to six months of his base salary then in effect, payable on our standard payroll dates; and |
| • | if Mr. Christoffersen elects to continue coverage under our group health insurance plan, reimbursement of his insurance premiums (or in certain cases a taxable cash payment) for a period of six months or until he qualifies for health insurance benefits from a new employer, whichever occurs first. |
129
Table of Contents
The agreement will further provide that, upon termination of Mr. Christoffersen’s employment by us within two months prior or 12 months after the date on which the Company experiences a change-of-control (as defined in the agreement), and provided that Mr. Christoffersen executes a general release in favor of the Company, Mr. Christoffersen would receive an amount equal to 12 months of his base salary then in effect, payable on our standard payroll dates and, if Mr. Christoffersen elects to continue coverage under our group health insurance plan, reimbursement of his insurance premiums (or in certain cases a taxable cash payment) for a period of 12 months or until he qualifies for health insurance benefits from a new employer, whichever occurs first. In addition, the unvested, unexpired portion of Mr. Christoffersen’s stock options and/or equity awards, as applicable, will be accelerated in full and the term and period during which Mr. Christoffersen’s stock options may be exercised will be extended to the earlier of 12 months after the date his employment ended, or the expiration date of the option as set forth in the applicable stock option grant notice and/or agreement. For the purpose of Mr. Christoffersen’s employment agreement, “good reason” will be defined as (i) a material reduction of Mr. Christoffersen’s salary or bonus target by more than ten percent; (ii) any request by us that Mr. Christoffersen relocate a distance of more than thirty-five miles; or (iii) following a change-of-control, Mr. Christoffersen’s benefits and responsibilities are materially reduced, or his base compensation or annual bonus target are reduced by more than ten percent.
Mr. Christoffersen has entered into an Employee Proprietary Information and Inventions Agreement with the Company. His agreement imposes certain confidentiality, non-compete and/or non-solicitation obligations on him. In the event that Mr. Christoffersen violates his confidentiality, non-compete and/or non-solicitation obligations, or the terms of his proprietary and inventions assignment agreement with the Company, Mr. Christoffersen’s right to the severance benefits that he would have otherwise been entitled to receive pursuant to his employment agreement will cease on the date of such violation.
Non-Equity Incentive Plan Compensation
Under our performance-based non-equity incentive plan, each executive officer is eligible for a discretionary annual cash incentive payment up to a specified target percentage of the executive officer’s salary. These annual cash bonuses are based upon the achievement of pre-specified corporate and individual performance objectives. Based on the recommendation of our Compensation Committee, the Board of Directors sets the target percentages at levels that, upon achievement of the target percentage, are likely to result in cash bonus payments that the Board of Directors believes to be approximately the level paid to high-performing executives of comparable companies in the biopharmaceutical industry. These bonuses are ordinarily paid in a single installment in the first quarter of each year for performance in the prior year.
For 2013, due to the cash position of the Company, no performance-based non-equity incentive plan was established and no bonuses were awarded.
At the end of each year, our Chief Executive Officer develops bonus recommendations for all executive officers other than himself based on the Company’s corporate accomplishments. These recommendations are subjective determinations that may vary, from time to time, depending on our overall strategic objectives, but relate generally to accomplishment of the established corporate goals, as well as factors such as development and progression of our existing product candidates, achievement of clinical and regulatory milestones, operational goals such as the expansion of our manufacturing capabilities, and financial factors such as raising and maintaining capital. The Compensation Committee assesses the Chief Executive Officer’s bonus recommendations and makes its bonus recommendations to the full Board of Directors. Based on its consideration of the recommendations of the Compensation Committee, the full Board of Directors then makes a final decision regarding cash bonus payments, if any, for the year. Whether or not a cash bonus is paid for any year is solely within the discretion of the Board of Directors.
For 2014, based upon recommendations of the Compensation Committee, the Board of Directors again determined that the target annual bonus for Dr. Rodell should equal 40% of his annual base salary and the target
130
Table of Contents
annual bonuses for Mr. Dekker and Mr. Christoffersen should equal 25% of their respective annual base salaries. As a basis for these target performance bonuses, our Board of Directors established corporate and individual performance objectives in 2014, which were communicated to the named executive officers at that time. The corporate goals for the year include:
| • | obtain financing sufficient to carry us into 2016; |
| • | complete enrollment of GI-6207-02 MTC trial; |
| • | complete enrollment and analysis of GI-6301-01 Phase 1 trial; |
| • | development support as needed for partnered programs; |
| • | select new infectious disease target and initiate development program; and |
| • | initiate manufacturing of one or more product candidates at large scale. |
For 2014, each named executive officer’s individual goals consist of the aforementioned corporate goals.
We have not determined whether we would seek to recover cash bonus payments paid to our executive officers if the performance objectives that led to the determination of such payments were to be restated or found not to have been met to the extent that we originally believed.
Equity Incentive Compensation
We did not grant any stock options to our named executive officers in 2013. In March 2012, we granted Dr. Rodell a stock option for 4,267 shares, Mr. Dekker a stock option for 8,816 shares, and Mr. Christoffersen a stock option for 269 shares, each at an exercise price of $18.24 per share. These options were granted under the Company’s 2002 Stock Incentive Plan. These options vest as to 25% of the shares subject to the option on the first anniversary of the vesting commencement date and as to the remainder in equal monthly increments over the following 36 months, subject to the recipient’s continued employment with the Company through such vesting dates. These options expire on March 27, 2022. In March 2014, we granted Mr. Christoffersen a stock option for 7,279 shares, at an exercise price of $15.10 per share. This option was granted under the Company’s 2002 Stock Incentive Plan. This option vests as to 25% of the shares subject to the option on the first anniversary of the vesting commencement date and as to the remainder in equal monthly increments over the following 36 months, subject to the recipient’s continued employment with the Company through such vesting dates. This option expires on March 12, 2024. All of these grants were recommended by our Compensation Committee to the Board of Directors. In recommending these grants, the Compensation Committee considered the executives’ roles and responsibilities within the Company and their ownership positions in relation to similarly-situated companies as determined by our Compensation Committee.
Other Elements of Executive Compensation Program
Other Benefits and Perquisites. We pay a portion of the premiums for medical insurance, dental insurance, vision insurance, life insurance and accidental death and dismemberment insurance benefits to all full-time employees, including our named executive officers. These benefits are available to all employees, subject to applicable laws. Our named executive officers have not historically received perquisites valued in aggregate at more than $10,000 per year per person. The Compensation Committee will evaluate perquisites annually as an element of overall compensation. From time to time, we have provided relocation expenses in connection with the relocation of executive officers to the geographic area of our corporate headquarters in Louisville, Colorado. We intend to continue to provide relocation expenses in the future, as necessary, to obtain the services of qualified individuals.
Other Compensation. We intend to continue to maintain the current benefits for our named executive officers, which are also available to all of our other employees. However, our Compensation Committee, in its discretion, may in the future revise, amend or add to the benefits of any named executive officer if it deems it advisable.
131
Table of Contents
Emerging Growth Company. As an emerging growth company we will not be required to provide information relating to the ratio of total compensation of our Chief Executive Officer to the median of the annual total compensation of all of our employees, as required by the Investor Protection and Securities Reform Act of 2010, which is part of the Dodd-Frank Wall Street Reform and Consumer Protection Act.
Outstanding Equity Awards at Fiscal Year-End
The following table provides information about outstanding stock options held by each of our named executive officers at December 31, 2013. All of these options were granted under our 2002 Stock Incentive Plan. Our named executive officers did not hold any restricted stock or other stock awards at the end of 2013.
| Number of Shares Underlying Unexercised Options (1) |
Option Exercise Price ($) |
Option Expiration Date |
||||||||||||||
| Name |
(#) Exercisable | (#) Unexercisable (2) | ||||||||||||||
| Timothy C. Rodell, M.D. |
41,413 | — | $ | 4.73 | 5/8/2016 | |||||||||||
| 3,380 | — | 5.04 | 5/2/2017 | |||||||||||||
| 47,785 | — | 5.98 | 3/19/2018 | |||||||||||||
| 12,240 | — | 6.93 | 4/21/2019 | |||||||||||||
| 13,095 | 278 | 12.56 | 2/1/2020 | |||||||||||||
| 3,683 | 1,094 | 12.56 | 11/29/2020 | |||||||||||||
| 2,133 | 2,133 | 18.24 | 3/27/2022 | |||||||||||||
| C. Jeffrey A. Dekker, C.P.A. |
3,185 | — | 4.73 | 5/8/2016 | ||||||||||||
| 359 | — | 5.04 | 5/2/2017 | |||||||||||||
| 2,134 | — | 5.98 | 3/19/2018 | |||||||||||||
| 1,051 | — | 8.52 | 9/23/2019 | |||||||||||||
| 996 | 21 | 12.56 | 2/1/2020 | |||||||||||||
| 316 | 93 | 10.07 | 3/16/2021 | |||||||||||||
| 4,408 | 4,408 | 18.24 | 3/27/2022 | |||||||||||||
| Kirk A. Christoffersen, M.B.A. |
2,389 | — | 4.73 | 12/15/2014 | ||||||||||||
| 1,592 | — | 4.73 | 5/8/2016 | |||||||||||||
| 410 | — | 5.04 | 5/2/2017 | |||||||||||||
| 2,561 | — | 5.98 | 3/19/2018 | |||||||||||||
| 860 | — | 8.52 | 9/23/2019 | |||||||||||||
| 1,116 | 23 | 12.56 | 2/1/2020 | |||||||||||||
| 364 | 108 | 10.07 | 3/16/2021 | |||||||||||||
| 134 | 134 | 18.24 | 3/27/2022 | |||||||||||||
| (1) | These options have a 10-year term and vest over a four-year period, with 25% of the shares subject to the option vesting on the first anniversary of the vesting commencement date and the remaining 75% of the shares subject to the option vesting in equal monthly installments thereafter over the next three years, subject to the recipient’s continued employment with the Company through such vesting dates. |
| (2) | This column shows options that were unvested as of December 31, 2013. |
Employee Benefit Plans
We believe that our ability to grant equity-based awards is a valuable and necessary compensation tool that aligns the long-term financial interests of our employees, consultants and directors with the financial interests of our stockholders. In addition, we believe that our ability to grant options and other equity-based awards helps us to attract, retain and motivate qualified personnel and service providers, and encourages them to devote their best efforts to our business and financial success. The material terms of our equity incentive plans are described below.
132
Table of Contents
2002 Stock Incentive Plan
On June 5, 2002, our Board of Directors adopted and our stockholders approved the Company’s 2002 Stock Incentive Plan, or the 2002 Plan. The Board and stockholders approved an extension of the 2002 Plan to December 31, 2017 on December 4, 2012 and January 24, 2013, respectively. As described below, following adoption of the Company’s 2014 Equity Incentive Plan the 2002 Plan will be superseded by the Company’s 2014 Equity Incentive Plan. From and after the effective date of the Company’s 2014 Equity Incentive Plan, no further grants will be made under the 2002 Plan and all outstanding stock awards granted under the 2002 Plan will continue to be governed by the terms of our 2002 Plan.
Our 2002 Plan provides for the grant of incentive stock options (within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended, or the Code) to our employees or employees of any of our subsidiaries, and for the grant of nonstatutory stock options and restricted stock awards to employees, directors, consultants and advisors of the Company and any of our affiliates. We have granted only stock options under our 2002 Plan.
Authorized Shares. The maximum number of shares of our common stock that may be issued under our 2002 Plan is 448,222 shares. As of March 31, 2014, options to purchase 235,342 shares of common stock at a weighted average exercise price per share of $8.14 were outstanding under the 2002 Plan, and 199,166 shares remained available for future grant under our 2002 Plan.
Administration. Our Board of Directors, or a duly authorized committee thereof, has the authority to administer our 2002 Plan. It is the intent of our Board of Directors to delegate its authority to administer the 2002 Plan to our Compensation Committee. Subject to the terms of the 2002 Plan, the plan administrator has the authority to determine the terms of awards, including recipients, the exercise or purchase price of stock awards, the number of shares subject to each stock award, the fair market value of a share of our common stock, the vesting schedule applicable to the awards, together with any vesting acceleration and the form of consideration, if any, payable upon exercise or purchase of the award and the terms of the award agreements for use under our 2002 Plan. In addition, the plan administrator has the authority to amend outstanding awards under the 2002 Plan, to interpret the provisions of the 2002 Plan and any outstanding awards, and to amend, suspend, or terminate the 2002 Plan, provided that no amendment, suspension, or termination of the 2002 Plan will, without the consent of the award holder, alter or impair rights or obligations under any outstanding award under the 2002 Plan.
Acquisitions. In the event of change-of-control of the Company where there will be no assumption, continuation, or substitution of options granted under the 2002 Plan, either of the following two actions will be taken: (a) fifteen days prior to the scheduled consummation of the transaction, all outstanding options under the 2002 Plan held by an optionholder whose service has not yet terminated will become immediately exercisable and will remain exercisable for a period of fifteen days, or (b) our Board of Directors may elect, in its sole discretion, to cancel any outstanding option grants that are vested (including vesting that occurred as a result of the transaction) and pay or deliver, or cause to be paid or delivered, to the holder thereof an amount in cash or securities having a value (as determined by our Board of Directors acting in good faith) equal to the product of the number of shares of stock subject to the option multiplied by the amount, if any, by which (i) the formula or fixed price per share paid to holders of shares of Company common stock pursuant to such transaction exceeds (ii) the per share exercise price. With respect to our establishment of an exercise window, any exercise of an option granted under the 2002 Plan during such fifteen-day period shall be conditioned upon the consummation of the transaction and shall be effective only immediately before the consummation of the transaction, and upon consummation of the transaction all outstanding but unexercised options shall terminate. Our Board of Directors will send written notice of an event that will result in such a termination to all individuals who hold options under the 2002 Plan not later than the time at which the Company gives notice of the event to its stockholders.
133
Table of Contents
For purposes of the 2002 Plan, a “change-of-control” means (i) the dissolution or liquidation of the Company or a merger, consolidation, or reorganization of the Company with one or more other entities in which the Company is not the surviving entity, (ii) a sale of substantially all of the assets of the Company to another person or entity, or (iii) any transaction (including without limitation a merger or reorganization in which the Company is the surviving entity) which results in any person or entity (other than persons who are stockholders or affiliates immediately prior to the transaction) owning 50% or more of the combined voting power of all classes of stock of the Company.
The 2002 Plan also contains a “better after-tax” provision, which provides that if any of the award holder’s stock awards, payments or benefits under the 2002 Plan constitutes a parachute payment under Section 280G of the Internal Revenue Code of 1986, as amended (the “Code”), the awards, payments or benefits will either (x) not become exercisable or vested, or (y) be provided in full to the award holder, whichever results in the award holder receiving the greater after-tax amount after taking into consideration the payment of all taxes, including the excise tax under Code Section 4999.
2014 Equity Incentive Plan
Our Board of Directors adopted the Company’s 2014 Equity Incentive Plan, or the 2014 Plan on April 25, 2014 and our stockholders approved the 2014 Plan on April 25, 2014. The 2014 Plan is the successor to and continuation of the 2002 Plan. The options still outstanding under the 2002 Plan will continue to be governed by their existing terms, but any shares subject to outstanding options granted under the 2002 Plan that would for any reason subsequently return to the share reserve of the 2002 Plan under its terms, will not return to the share reserve of the 2002 Plan but will become available for issuance pursuant to awards granted under the 2014 Plan. We do not expect to utilize our 2014 Plan until after completion of this offering.
Available Awards. The 2014 Plan provides for the discretionary grant of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance stock awards, performance cash awards and other stock awards to our employees, directors and consultants. Incentive stock options may be granted only to employees of the Company or certain affiliates.
Administration. Our Board of Directors has delegated its authority to administer the 2014 Plan to our Compensation Committee. Subject to the terms of the 2014 Plan, our Board of Directors, our Compensation Committee or another authorized committee, referred to as the “plan administrator”, determines: grant recipients, when and how each award will be granted, what type of award will be granted, the provisions of each award granted (including the time or times when a person will be permitted to receive cash or common stock pursuant to the award), the number of shares of common stock subject to, or the cash value of, an award, and the fair market value of a share of our common stock.
The plan administrator also has the authority, under appropriate circumstances, to engage in any action that is treated as a repricing under United States generally accepted accounting principles, to reduce the exercise, purchase or strike price of any outstanding stock award, and to cancel any outstanding stock award and to grant in exchange a new stock award, cash or other valuable consideration, with any such substituted award being granted under the 2014 Plan or another equity or compensatory plan of the Company and covering the same or a different number of shares of common stock as the cancelled stock award.
Amendment and Termination. The plan administrator has the authority to amend, suspend, or terminate our 2014 Plan at any time, provided that such action does not impair the existing rights of any participant without such participant’s written consent. No incentive stock options may be granted after the tenth anniversary of the date our Board of Directors adopts the 2014 Plan.
Share Reserve. Subject to the provisions of the 2014 Plan relating to adjustments upon changes in stock, the aggregate number of shares of common stock that are available for issuance pursuant to stock awards under the
134
Table of Contents
2014 Plan will not exceed 827,866 shares, which number is the sum of (i) 393,358 new shares, plus (ii) the 199,166 shares reserved for issuance under our 2002 Plan at the time our 2014 Plan becomes effective, plus (iii) 235,342 shares subject to outstanding options granted under the 2002 Plan that subsequently become available for issuance as described above. This amount will be increased pursuant to an “evergreen provision” on January 1 of each year, from 2015 to (and including) 2024, in an amount equal to 4.0% of the total number of shares of common stock outstanding on December 31 of the preceding calendar year. However, our Board of Directors will have the authority to designate a lesser number of shares by which the share reserve will be increased. Subject to the share reserve, the aggregate maximum number of shares of common stock that may be issued pursuant to the exercise of incentive stock options under the 2014 Plan is 2,700,000 shares. In addition, a maximum of the greater of 100,000 shares of our common stock or such number of shares of our common stock that has a total value on the grant date equal to $500,000 may be granted to any one non-employee director during any one calendar year pursuant to stock awards.
No person may be granted stock awards covering more than 900,000 shares of our common stock under our 2014 Plan during any calendar year pursuant to stock options, stock appreciation rights and other stock awards whose value is determined by reference to an increase over an exercise or strike price of at least 100% of the fair market value on the date the stock award is granted. Additionally, no person may be granted a performance stock award covering more than 900,000 shares or a performance cash award having a maximum value in excess of $6,500,000 in any calendar year. Such limitations are designed to help assure that any deductions to which we would otherwise be entitled with respect to such awards will not be subject to the $1,000,000 limitation on the income tax deductibility of compensation paid to any covered executive officer imposed by Section 162(m) of the Code.
If a stock award granted under the 2014 Plan, or any portion thereof, expires or otherwise terminates without all of the shares covered by the stock award having been issued or is settled in cash rather than in shares, such expiration, termination or settlement will not reduce or otherwise offset the number of shares available for issuance under the 2014 Plan. Additionally, shares issued pursuant to stock awards granted under the 2014 Plan that are forfeited back to or repurchased by the Company because of the failure to vest, as well as shares reacquired by us as consideration for the exercise or purchase price of a stock award or to satisfy tax withholding obligations related to a stock award, will become available again for issuance under our 2014 Plan.
The stock issuable under the 2014 Plan may be shares of authorized but unissued or reacquired common stock, including shares repurchased by the Company on the open market or otherwise.
Stock Options. Incentive and nonstatutory stock options are granted pursuant to incentive and nonstatutory stock option agreements adopted by the plan administrator. The plan administrator determines the exercise price for a stock option, within the terms and conditions of the 2014 Plan, provided that the exercise price of an incentive stock option and nonstatutory stock option cannot be less than 100% of the fair market value of our common stock on the date of grant. Options granted under the 2014 Plan vest at the rate specified by the plan administrator.
Generally, the plan administrator determines the term of stock options granted under the 2014 Plan, up to a maximum of 10 years, except in the case of certain incentive stock options, as described below.
Unless the terms of an optionholder’s stock option agreement provides otherwise, if an optionholder’s service relationship with us, or any of our affiliates, ceases for any reason other than a termination for cause or a termination because of disability or death, the optionholder may exercise the vested portion of any options for a period of three months following the cessation of service. If an optionholder’s service relationship with us, or any of our affiliates, ceases due to disability or death (or an optionholder dies within a specified period following cessation of service), the optionholder or a beneficiary may exercise the vested portion of any options for a period of 12 months or 18 months, respectively. If an optionholder’s service relationship with us is terminated for cause, then the unexercised portion of any outstanding stock option held by the optionholder terminates
135
Table of Contents
immediately and may not be exercised. The option term may be extended in the event that exercise of the option following termination of service is prohibited by applicable securities laws. In no event, however, may an option be exercised beyond the expiration of its term.
Acceptable consideration for the purchase of common stock issued upon the exercise of a stock option will be determined by the plan administrator and may include cash, check, bank draft or money order, a broker-assisted cashless exercise, the tender of common stock previously owned by the optionholder, a net exercise of the option, and other legal consideration approved by the plan administrator.
Unless the plan administrator provides otherwise, options generally are not transferable except by will, the laws of descent and distribution, or pursuant to a domestic relations order. An optionholder may designate a beneficiary, however, who may exercise the option following the optionholder’s death.
Limitations on Incentive Stock Options. Incentive stock options may be granted only to our employees or employees of certain affiliates. The aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to incentive stock options that are exercisable for the first time by an optionholder during any calendar year under all of our equity incentive plans may not exceed $100,000. No incentive stock option may be granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than ten percent of our total combined voting power or that of any of our affiliates unless the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant, and the term of the incentive stock option does not exceed five years from the date of grant.
Restricted Stock Awards. Restricted stock awards are granted pursuant to restricted stock award agreements adopted by the plan administrator. Restricted stock awards may be granted in consideration for cash, check, bank draft or money order, past or future services rendered to us or our affiliates, or any other form of legal consideration. Shares of common stock acquired under a restricted stock award may, but need not, be subject to a share repurchase option or forfeiture restriction in our favor in accordance with a vesting schedule to be determined by the plan administrator. Rights to acquire shares under a restricted stock award may be transferred only upon such terms and conditions as set by the plan administrator. Except as otherwise provided in the applicable award agreement, restricted stock awards that have not vested will be forfeited or subject to repurchase upon the participant’s cessation of continuous service for any reason.
Restricted Stock Unit Awards. Restricted stock unit awards are granted pursuant to restricted stock unit award agreements adopted by the plan administrator. Restricted stock unit awards may be granted in consideration for any form of legal consideration. A restricted stock unit award may be settled by cash, delivery of stock, a combination of cash and stock as deemed appropriate by the plan administrator, or in any other form of consideration set forth in the restricted stock unit award agreement. Additionally, dividend equivalents may be credited in respect of shares covered by a restricted stock unit award. Except as otherwise provided in the applicable award agreement, restricted stock units that have not vested will be forfeited upon the participant’s cessation of continuous service for any reason.
Stock Appreciation Rights. Stock appreciation rights may be granted pursuant to stock appreciation rights agreements adopted by the plan administrator. The plan administrator determines the strike price for a stock appreciation right, which cannot be less than 100% of the fair market value of our common stock on the date of grant. Upon the exercise of a stock appreciation right, we will pay the participant an amount not to exceed the product of (a) the excess of the per share fair market value of our common stock on the date of exercise over the strike price, multiplied by (b) the number of shares of common stock with respect to which the stock appreciation right is exercised. Such payment may be made in shares of our common stock, cash, a combination of shares and cash, or in any other form of consideration set forth in the award agreement. Stock appreciation rights granted under the 2014 Plan vest at the rate specified by the plan administrator. Stock appreciation rights will be subject to the same conditions upon termination and restrictions on transfer as stock options under the 2014 Plan.
136
Table of Contents
Performance Stock Awards and Performance Cash Awards. Our 2014 Plan will permit the grant of performance-based stock and cash awards that may qualify as performance-based compensation that is not subject to the $1,000,000 limitation on the income tax deductibility of compensation paid per covered executive officer imposed by Section 162(m) of the Code. To help assure that the compensation attributable to performance-based awards will so qualify, our Compensation Committee can structure such awards so that the stock or cash will be issued or paid pursuant to such awards only following the achievement of certain pre-established performance goals during a designated performance period.
Our Compensation Committee may establish performance goals by selecting from one or more of the following performance criteria: (1) earnings (including earnings per share and net earnings); (2) earnings before interest, taxes and depreciation; (3) earnings before interest, taxes, depreciation and amortization; (4) total stockholder return; (5) return on equity or average stockholders’ equity; (6) return on assets, investment, or capital employed; (7) stock price; (8) margin (including gross margin); (9) income (before or after taxes); (10) operating income; (11) operating income after taxes; (12) pre-tax profit; (13) operating cash flow; (14) sales or revenue targets; (15) increases in revenue or product revenue; (16) expenses and cost reduction goals; (17) improvement in or attainment of working capital levels; (18) economic value added (or an equivalent metric); (19) market share; (20) cash flow; (21) cash flow per share; (22) share price performance; (23) debt reduction; (24) implementation or completion of projects or processes; (25) customer satisfaction; (26) stockholders’ equity; (27) capital expenditures; (28) debt levels; (29) operating profit or net operating profit; (30) workforce diversity; (31) growth of net income or operating income; (32) billings; (33) pre-clinical development related compound goals; (34) financing; (35) regulatory milestones, including approval of a compound; (36) stockholder liquidity; (37) corporate governance and compliance; (38) product commercialization; (39) intellectual property; (40) personnel matters; (41) progress of internal research or clinical programs; (42) progress of partnered programs; (43) implementation or completion of projects and processes; (44) partner satisfaction; (45) budget management; (46) clinical achievements; (47) completing phases of a clinical study (including the treatment phase); (48) or announcing or presenting preliminary or final data from clinical studies; in each case, whether on particular timelines or generally); (49) timely completion of clinical trials; (50) submission of INDs and NDAs and other regulatory achievements; (51) partner or collaborator achievements; (52) internal controls, including those related to the Sarbanes-Oxley Act of 2002; (53) research progress, including the development of programs; (54) investor relations, analysts and communication; (55) manufacturing achievements (including obtaining particular yields from manufacturing runs and other measurable objectives related to process development activities); (56) strategic collaborations or transactions (including in-licensing and out-licensing of intellectual property); (57) establishing relationships with commercial entities with respect to the marketing, distribution and sale of the Company’s products (including with group purchasing organizations, distributors and other vendors); (58) supply chain achievements (including establishing relationships with manufacturers or suppliers of active pharmaceutical ingredients and other component materials and manufacturers of our products); (59) co-development, co-marketing, profit sharing, joint venture or other similar arrangements; and (60) to the extent that an award is not intended to comply with Section 162(m) of the Code, other measures of performance selected by our Board of Directors or Compensation Committee.
Our Compensation Committee may establish performance goals on a company-wide basis, with respect to one or more business units, divisions, affiliates, or business segments, and in either absolute terms or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. Unless specified otherwise (a) in the award agreement at the time the award is granted or (b) in such other document setting forth the performance goals at the time the goals are established, our Compensation Committee will appropriately make adjustments in the method of calculating the attainment of the performance goals as follows: (1) to exclude restructuring and/or other nonrecurring charges; (2) to exclude exchange rate effects; (3) to exclude the effects of changes to U.S. generally accepted accounting principles, or GAAP; (4) to exclude the effects of any statutory adjustments to corporate tax rates; (5) to exclude the effects of any “extraordinary items” as determined under GAAP; (6) to exclude the dilutive effects of acquisitions or joint ventures; (7) to assume that any business divested by the Company achieved performance objectives at targeted levels during the balance of a performance period
137
Table of Contents
following such divestiture; (8) to exclude the effect of any change in the outstanding shares of common stock of the Company by reason of any stock dividend or split, stock repurchase, reorganization, recapitalization, merger, consolidation, spin-off, combination or exchange of shares or other similar corporate change, or any distributions to common stockholders other than regular cash dividends; (9) to exclude the effects of stock based compensation and the award of bonuses under the Company’s bonus plans; (10) to exclude costs incurred in connection with potential acquisitions or divestitures that are required to expensed under generally accepted accounting principles; (11) to exclude the goodwill and intangible asset impairment charges that are required to be recorded under generally accepted accounting principles; and (12) to exclude the effect of any other unusual, non-recurring gain or loss or other extraordinary item. In addition, our Compensation Committee retains the discretion to reduce or eliminate the compensation or economic benefit due upon attainment performance goals and to define the manner of calculating the performance criteria it selects to use for such performance period. Partial achievement of the specified criteria may result in the payment or vesting corresponding to the degree of achievement as specified in the stock award agreement or the written terms of a performance cash award. The performance goals may differ from participant to participant and from award to award.
Other Stock Awards. The plan administrator may grant other awards based in whole or in part by reference to our common stock. The plan administrator will set the number of shares under the award and all other terms and conditions of such awards.
Clawback Policy. All awards granted under the 2014 Plan will be subject to recoupment in accordance with any clawback policy that we are required to adopt pursuant to the listing standards of any national securities exchange or association on which our securities are listed or as is otherwise required by the Dodd-Frank Wall Street Reform and Consumer Protection Act or other applicable law. In addition, our Board of Directors may impose such other clawback, recovery or recoupment provisions in an award agreement as our Board of Directors determines necessary or appropriate.
Changes to Capital Structure. In the event of certain capitalization adjustments, such as a stock split, appropriate adjustments will be made to (a) the class and maximum number of shares reserved under the 2014 Plan, (b) the class and maximum number of shares by which the share reserve may increase automatically each year, (c) the class and maximum number of shares subject to options, stock appreciation rights and performance stock awards that can be granted in a calendar year, (d) the class and maximum number of shares that may be issued pursuant to the exercise of incentive stock options, and (e) the class and number of shares and price per share of all outstanding stock awards.
Transactions. In the event of certain specified transactions, our Board of Directors will determine how to treat each outstanding stock award. The plan administrator may take one or more of the following actions with respect to outstanding stock awards, contingent upon the closing or completion of such transaction: (a) arrange for the assumption, continuation or substitution of a stock award by the surviving or acquiring corporation (or its parent company); (b) arrange for the assignment of any reacquisition or repurchase rights held by us to the surviving or acquiring corporation (or its parent company); (c) accelerate the vesting of the stock award and provide for its termination if the award is not exercised, if applicable, at or prior to the effective time of the transaction; (d) arrange for the lapse, in whole or in part, of any reacquisition or repurchase right held by us; (e) cancel or arrange for the cancellation of a stock award, to the extent not vested or exercised prior to the transaction, in exchange for such cash consideration, if any, as the Board of Directors may consider appropriate; and (f) make a payment equal to the excess, if any, of (i) the value of the property that the participant would have received upon the exercise of the stock award over (ii) any exercise price payable in connection with such exercise.
The Board of Directors will not be obligated to treat all stock awards or portions of stock awards, even those that are of the same type, in the same manner. The Board of Directors may take different actions with respect to the vested and unvested portions of a stock award.
138
Table of Contents
For purposes of the 2014 Plan, a transaction will be deemed to occur in the event of a corporate transaction or a change in control.
For purposes of the 2014 Plan, a corporate transaction will be the consummation of a sale or other disposition of all or substantially all of our assets, a sale or other disposition of at least 90% of our outstanding securities, a merger, consolidation or similar transaction in which we are not the surviving corporation, or a merger, consolidation or similar transaction in which we are the surviving corporation but the shares of our common stock outstanding immediately preceding such transaction are converted by virtue of such transaction into other property.
For purposes of the 2014 Plan, a change-of-control is the occurrence of one or more of the following events: (a) a transaction in which one person or a group acquires stock that, combined with stock previously owned, controls more than 50% of our value or voting power; (b) a merger, consolidation or similar transaction involving us (directly or indirectly) in which our stockholders immediately before the transaction do not own more than 50% of the outstanding securities following such transaction; (c) a sale, lease, license or other disposition of all or substantially all of our assets, other than to an entity in which more than 50% of the voting power is owned by our stockholders in substantially the same proportions as their ownership of our voting securities immediately prior to such transaction; or (d) a majority of our Board of Directors is replaced by persons whose appointment or election is not endorsed by a majority of our Board of Directors.
Change-of-Control. Our Board of Directors may provide, in an individual award agreement or in any other written agreement between us and a participant, that a stock award will be subject to additional acceleration of vesting and exercisability in the event of a change-of-control. In the absence of such a provision, no such acceleration of the stock award will occur. Award agreements may also contain a “better after-tax” provision, which provides that if any of the award holder’s payments or benefits constitutes a parachute payment under Section 280G of the Code, the payments or benefits will be either (x) reduced, or (y) provided in full to the participant, whichever results in the participant receiving the greater amount after taking into consideration the payment of all taxes, including the excise tax under Code Section 4999, in each case based upon the highest marginal rate for the applicable tax.
Section 162(m) of the Code. In general, under Section 162(m) of the Code, income tax deductions of publicly held corporations may be limited to the extent total compensation (including, but not limited to, base salary, annual bonus, and income attributable to stock option exercises and other non-qualified benefits) for certain executive officers exceeds $1,000,000 (less the amount of any “excess parachute payments” as defined in Section 280G of the Code) in any taxable year of the corporation. However, under Section 162(m), the deduction limit does not apply to certain “performance-based compensation” established by an independent compensation committee that is adequately disclosed to, and approved by, stockholders. In particular, stock options and stock appreciation rights will satisfy the “performance-based compensation” exception if the awards are made by a qualifying compensation committee, the 2014 Plan sets the maximum number of shares that can be granted to any person within a specified period and the compensation is based solely on an increase in the stock price after the grant date. Specifically, the option exercise price must be equal to or greater than the fair market value of the stock subject to the award on the grant date.
We have attempted to structure the 2014 Plan in such a manner that the compensation attributable to stock options, stock appreciation rights and other performance-based awards which meet the other requirements of Section 162(m) will not be subject to the $1,000,000 limitation. We have not, however, requested a ruling from the Internal Revenue Service or an opinion of counsel regarding this issue.
2014 Employee Stock Purchase Plan
Our Board of Directors adopted our 2014 Employee Stock Purchase Plan, or the Purchase Plan on April 25, 2014 and our stockholders approved the Purchase Plan on April 25, 2014. The Purchase Plan will become effective upon the execution of the underwriting agreement related to this offering.
139
Table of Contents
Share Reserve. Subject to the provisions of the Purchase Plan relating to capitalization adjustments, the shares of common stock that may be sold pursuant to purchase rights will not exceed in the aggregate 201,163 shares of common stock. The number of shares of our common stock reserved for issuance under our Purchase Plan will automatically increase on January 1 of each year, for a period of up to ten years, from January 1, 2015 continuing through (and including) January 1, 2024, by the lesser of (a) 1.0% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year, (b) 402,326 shares, or (c) a lower number of shares determined by our Board of Directors.
If any purchase right granted under the Purchase Plan terminates without having been exercised, the shares of common stock not purchased under such purchase right will again become available for issuance under the Purchase Plan. Shares purchasable under our Purchase Plan will be authorized but unissued or reacquired common stock, including shares repurchased by the Company on the open market or otherwise. The Purchase Plan is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Code.
Administration. Our Board of Directors has delegated its authority to administer the Purchase Plan to our Compensation Committee. Subject to the terms of the Purchase Plan, our Board of Directors or an authorized Committee, referred to as the “plan administrator”, determines the provisions of each offering of rights to purchase our common stock and whether employees of any of our parent or subsidiary companies will be eligible to participate in the Purchase Plan. The plan administrator also has the authority to construe and interpret the Purchase Plan and the authority to suspend, terminate and amend the Purchase Plan as described in more detail in the paragraph titled Termination and Amendment below.
Offerings and Purchase Rights. The Purchase Plan will be implemented through a series of offerings of such duration as determined by the plan administrator to eligible employees, provided that in no event may an offering exceed 27 months. The plan administrator will establish one or more purchase dates on which shares of our common stock will be purchased for the employees who are participating in each offering. The plan administrator has the authority to alter the duration of subsequent offerings or change the number of purchase dates within each such offering. The provisions of separate offerings need not be identical. The plan administrator has not yet established the terms of any offering.
Payroll Deductions. When an eligible employee elects to join an offering, he or she will be granted a purchase right to acquire shares of common stock on each purchase date within the offering. Generally, all regular employees, including executive officers, employed by us or by any of our parent or subsidiary companies designated by the plan administrator may contribute, normally through payroll deductions, up to 15% of their eligible compensation (or such lesser amount set by the plan administrator for a specific offering) for the purchase of common stock under the Purchase Plan. Amounts deducted and accumulated for a participant are used to purchase shares of our common stock on the purchase dates established by the plan administrator. All payroll deductions made for a participant are credited to his or her account under the Purchase Plan and deposited with our general funds unless a law requires that those funds be deposited with a third party. A participant may make additional payments into such account only as specifically provided for in the offering or as required by law and only if the participant has not exceeded certain limitations under the Purchase Plan or under the terms of such offering.
Purchase of Stock. The Purchase Plan permits our common stock to be purchased at a price per share no less than the lower of (i) 85% of the fair market value of a share of our common stock on the offering date, or (ii) 85% of the fair market value of a share of our common stock on the applicable purchase date. An eligible employee must sign and return an agreement in order to participate in the Purchase Plan. On the purchase date, all payroll deductions collected from the participant are automatically applied to the purchase of common stock, subject to certain limitations. In connection with offerings made under the Purchase Plan, the plan administrator may specify a maximum number of shares of common stock a participant may purchase and the maximum aggregate number of shares of common stock that may be purchased by all participants in such offering. In addition, in connection with each offering that contains more than one purchase date, the plan administrator may
140
Table of Contents
specify a maximum aggregate number of shares of common stock that may be purchased by all participants on any purchase date under the offering. If the aggregate number of shares to be purchased upon exercise of outstanding purchase rights in the offering would exceed the maximum aggregate number of shares of common stock available, in the absence of any action by the plan administrator otherwise, a pro rata allocation of available shares will be made in a uniform and equitable manner. Unless the employee’s participation is discontinued, his or her right to purchase shares using the employee’s accumulated contributions is exercised automatically at the next purchase date at the applicable price, provided, however, that at the end of an offering, the employee’s excess contributions will be returned.
Withdrawal. During an offering, a participant may cease making contributions and withdraw from the offering by delivering a notice of withdrawal and terminating his or her payroll deductions in such form as we may require. We may impose a deadline for withdrawing before a particular purchase date but absent any violation of such deadline, withdrawal may occur at any time prior to the end of an offering. Upon such withdrawal, we will refund accumulated payroll deductions without interest to the employee, and such employee’s right to participate in that offering will terminate. However, an employee’s withdrawal from an offering does not generally affect such employee’s eligibility to participate in subsequent offerings under the Purchase Plan.
Reset Feature. The plan administrator has the authority to provide that if the fair market value of the shares of our common stock on the first day of a new purchase period within a particular offering is less than the fair market value of the shares of common stock on the start date of that offering, then the participants in that offering will automatically be transferred and enrolled in a new offering which will begin on the first day of that purchase period and the participant’s purchase rights in the original offering will terminate.
Limitations. The plan administrator may limit participation in the Purchase Plan to those persons who are customarily employed more than 20 hours per week and five months per calendar year by us (or by any of our parent or subsidiary companies designated by the plan administrator) on the first day of an offering. The plan administrator may also provide that a person must have been employed for such continuous period preceding the first day of the offering as the plan administrator may require, but in no event may the required period of continuous employment be greater than two years. In addition, the plan administrator may provide in any offering that certain of our employees who are “highly compensated” as defined in the Code are not eligible to participate in the Purchase Plan. The plan administrator may also provide that each person who, during the course of an offering, first becomes an eligible employee will, on or after the day that the person becomes eligible, receive a purchase right under that offering, which purchase right will be deemed to be a part of that offering, and such purchase right will generally have the same characteristics as any purchase rights originally granted under that offering. No employee is eligible to participate in the Purchase Plan if, immediately after the grant of purchase rights, the employee would own, directly or indirectly, stock possessing five percent or more of the total combined voting power or value of all classes of our stock or of any of our parent or subsidiary companies (including any stock which such employee may purchase under all outstanding purchase rights and stock options). In addition, no employee may purchase more than $25,000 worth of our common stock (valued at the time each purchase right is granted) for each calendar year during which those purchase rights are outstanding.
Termination of Employment. Purchase rights granted pursuant to any offering under the Purchase Plan terminate upon cessation of employment for any reason, and we will refund all accumulated payroll deductions to the terminated employee without interest, unless otherwise required by law.
Restrictions on Transfer. A participant may not transfer rights granted under the Purchase Plan other than by will, the laws of descent and distribution, or by a beneficiary designation as provided in the Purchase Plan. During a participant’s lifetime, purchase rights will be exercisable only by such participant.
Changes to Capital Structure. In the event that there is any change to the outstanding common stock (whether by reason of merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend,
141
Table of Contents
dividend in property other than cash, large nonrecurring cash dividend, stock split, liquidating dividend, combination of shares, exchange of shares, change in corporate structure, or other transaction not involving the receipt of consideration by the Company), the plan administrator will appropriately and proportionately adjust (a) the class and maximum number of securities subject to the Purchase Plan, (b) the class and maximum number of securities by which the share reserve is to increase automatically each year, (c) the class and number of securities subject to outstanding purchase rights, and (d) the class and number of securities imposed by purchase limits under each ongoing offering.
Corporate Transactions. In the event of certain significant corporate transactions, any surviving or acquiring corporation (or the surviving or acquiring corporation’s parent company) may assume or continue outstanding purchase rights or substitute similar purchase rights for those outstanding under the Purchase Plan. If the surviving or acquiring corporation (or its parent company) does not assume or continue such purchase rights or substitute similar rights, then the participants’ accumulated payroll deductions will be used to purchase shares of common stock within ten business days prior to the corporate transaction under any ongoing offerings, and such purchase rights will terminate immediately thereafter.
For purposes of the Purchase Plan, a corporate transaction will be the consummation of (a) a sale or other disposition of all or substantially all of our assets, (b) a sale or other disposition of at least 90% of our outstanding securities, (c) a merger, consolidation or similar transaction in which we are not the surviving corporation, or (d) a merger, consolidation or similar transaction in which we are the surviving corporation but the shares of our common stock outstanding immediately preceding such transaction are converted by virtue of such transaction into other property.
Termination and Amendment. The plan administrator may amend, suspend or terminate the Purchase Plan at any time. Any amendment of the Purchase Plan must be approved by our stockholders to the extent stockholder approval is necessary under the terms of the Purchase Plan or for the Purchase Plan to satisfy Section 423 of the Code or other applicable laws and regulations. Purchase rights granted before amendment, suspension or termination of the Purchase Plan generally may not be altered or impaired by any amendment, suspension or termination of the Purchase Plan without consent of the employee to whom such purchase rights were granted unless an amendment is necessary to ensure the Purchase Plan complies with the requirements of Section 423 of the Code or other law or listing requirements. No purchase rights may be granted under the Purchase Plan while the Purchase Plan is suspended or after it is terminated. Our Purchase Plan will remain in effect until terminated by the plan administrator in accordance with the terms of the Purchase Plan.
Indebtedness of Management and Related Agreements
No named executive officer or director is indebted to the Company.
Limitation of Liability and Indemnification
Our amended and restated certificate of incorporation, which will become effective upon completion of this offering, limits the liability of directors to the maximum extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
| • | breach of their duty of loyalty to the corporation or its stockholders; |
| • | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| • | transaction from which the directors derived an improper personal benefit. |
142
Table of Contents
Our amended and restated certificate of incorporation, which will become effective upon completion of this offering, does not eliminate a director’s duty of care and, in appropriate circumstances, equitable remedies, such as injunctive or other forms of non-monetary relief, which remain available under Delaware law. These limitations also do not affect a director’s responsibilities under any other laws, such as the federal securities laws or other state or federal laws. Our amended and restated bylaws, which will become effective upon completion of this offering, provide that we will indemnify our directors and executive officers, and may indemnify other officers, employees and other agents, to the fullest extent permitted by law. Our amended and restated bylaws, which will become effective upon completion of this offering, also provide that we are obligated to advance expenses incurred by a director or executive officer in advance of the final disposition of any action or proceeding for which indemnification is provided and also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with his or her services to us, regardless of whether our amended and restated bylaws permit such indemnification. We have obtained a policy of directors’ and officers’ liability insurance.
We have entered into amended and restated indemnification agreements with each of our directors and executive officers that require us to indemnify such persons against any and all expenses, including attorneys’ fees, witness fees, judgments, fines, settlements and other amounts incurred, including expenses of a derivative action, in connection with any action, suit or proceeding or alternative dispute resolution mechanism, inquiry hearing or investigation, whether threatened, pending or completed, to which any such person may be made a party by reason of the fact that such person is or was a director, an officer or an employee of our company, provided that such person’s conduct did not constitute a breach of his or her duty of loyalty to us or our stockholders, and was not an act or omission not in good faith or that involved intentional misconduct or a knowing violation of laws. The indemnification agreements also set forth procedures that will apply in the event of a claim for indemnification thereunder. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and executive officers of our company.
At present, there is no pending litigation or proceeding involving a director or officer of our company for which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, or the Securities Act, may be permitted for directors, executive officers or persons controlling us, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Non-Employee Director Compensation
The following table shows certain information with respect to the compensation of all non-employee directors of the Company for the fiscal year ended December 31, 2013.
| Name |
Fees Earned or Paid in Cash ($) |
Option Awards ($) | All Other Compensation ($)(1) |
Total ($) | ||||||||||||
| J. William Freytag, Ph.D. |
$ | — | — | — | — | |||||||||||
| Ralph E. Christoffersen, Ph.D. |
— | — | 7 | 7 | ||||||||||||
| Augustine J. Lawlor |
— | — | 2,932 | 2,932 | ||||||||||||
| Paul A. Mieyal, Ph.D. |
— | — | 1,186 | 1,186 | ||||||||||||
| Dan J. Mitchell |
— | — | 661 | 661 | ||||||||||||
| Pennina Safer, Ph.D. |
— | — | 4,795 | 4,795 | ||||||||||||
| S. Edward Torres |
— | — | — | — | ||||||||||||
| (1) | Represents reimbursement of expenses incurred in attending meetings of our Board of Directors. |
143
Table of Contents
Narrative Disclosure to Director Compensation Table
Cash Compensation
No cash compensation was paid to directors in 2013. We reimburse our non-employee directors for travel, lodging and other reasonable expenses incurred in attending meetings of our Board of Directors and committees of our Board of Directors.
Equity Incentive Compensation
In connection with Dr. Freytag’s election to the Board of Directors in March 2008 and in consideration for his service as a director, we granted to him an option to acquire 3,982 shares of common stock, with an exercise price of $5.98 per share. In connection with Dr. Freytag’s appointment as Chairman of our Board of Directors in January 2011 and in consideration for his service as Chairman, we granted to him an option to acquire 16,876 shares of common stock, with an exercise price of $12.56 per share. The shares subject to each grant vest in equal monthly installments over a 36-month period, until fully vested, subject to Dr. Freytag’s continued service through each vesting date. As of March 31, 2014, we have not issued stock options or other stock awards to any of our other directors in consideration for service on our Board of Directors.
Non-Employee Director Compensation Policy
Our Board of Directors has adopted a Non-Employee Director Compensation Policy, or the Policy, to be effective as of the effectiveness of the registration statement of which this prospectus is a part. A summary of the terms of the Policy is set forth below.
Annual Cash Retainer. Each non-employee director will receive an annual cash retainer of $35,000, as well as reimbursement for travel, lodging and other reasonable expenses incurred in attending meetings of our Board of Directors and committees of our Board of Directors.
Initial Option Grant. Each non-employee director initially elected to our Board of Directors after effectiveness of the Policy will receive an initial grant of a stock option to purchase 3,418 shares of our common stock, which will vest as to 1/48th of the shares subject to the option on an equal monthly basis over a four-year period and subject to such director’s continued service through each vesting date.
Annual Option Grant. Each non-employee director will receive a grant of a stock option to purchase 1,697 shares of our common stock on the date of each annual meeting of stockholders, vesting in full one year following the date of such grant and subject to such director’s continued service through such vesting date. If an individual first becomes a non-employee director other than at the annual meeting, we will grant such non-employee director, on the date that he or she is first elected or appointed to our Board of Directors, a stock option to purchase that number of shares of our common stock equal to the pro rata portion of the annual option grant such person would have received at the prior annual meeting. All options granted under the Policy will be granted under the 2014 Plan with an exercise price equal to the fair market value of our common stock on the date of the grant, and will be entitled to full vesting in the event of a change-of-control.
Compensation for Committee Service. Directors serving on committees of the Board of Directors shall receive additional compensation in recognition of the additional time commitment and responsibility required for such committee service. Each director serving as a member of our Audit Committee will receive a cash retainer of $7,500 per year, except that the Chairman of our Audit Committee will receive $15,000 per year. Each director serving as a member of our Compensation Committee will receive a cash retainer of $5,000 per year, except that the Chairman of our Compensation Committee will receive $10,000 per year. Each director serving as a member of our Nominating and Governance Committee will receive a cash retainer of $3,500 per year, except that the Chairman of our Nominating and Governance Committee will receive $7,000 per year.
Compensation for Service as Chairman of the Board of Directors . In addition to the $35,000 annual cash retainer received as a member of our Board of Directors, Dr. Freytag will receive an additional $37,000 for his service as Chairman of our Board of Directors.
144
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a description of transactions since January 1, 2013 to which we have been a party, in which the amount involved in the transaction exceeds $120,000, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than five percent of our capital stock had or will have a direct or indirect material interest, other than compensation, termination and change-of-control arrangements, which are described under “Executive and Director Compensation”. We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions. Upon completion of this offering, each 31.39 shares of preferred stock will convert into one share of our common stock.
Stockholders Agreement
On January 14, 2010, we entered into a Fifth Amended and Restated Stockholders Agreement, or the Stockholders Agreement, with the holders of our outstanding preferred stock, including entities with which certain of our directors are affiliated. As of March 31, 2014, the holders of 3,448,488 shares of our common stock, including the common stock issuable upon the conversion of our preferred stock and common stock issuable upon conversion of the 2014 Notes based on an assumed initial public offering price of $16.00 per share, and based on a conversion date of May 31, 2014, are entitled to rights with respect to the registration of their shares following completion of this offering under the Securities Act. For a more detailed description of these registration rights, see “Description of Capital Stock—Registration Rights”. Pursuant to the Stockholders Agreement, holders of our preferred stock, including entities with which certain of our directors are affiliated, have agreed to vote in a certain way on certain matters, including with respect to the election of directors. Upon completion of this offering, the board election voting provisions contained in the Stockholders Agreement will terminate and none of our stockholders will have any special rights regarding the nomination or election of members of our Board of Directors.
Appointment of Executive Officer
In December 2013, we promoted Kirk A. Christoffersen to the position of Vice President, Corporate Development. This promotion and his annual salary of $191,475 were approved by our Board and recommended by our Compensation Committee, both of which his father, Dr. Christoffersen, is a member.
Policies and Procedures for Related Party Transactions
In order to avoid actual or potential conflicts of interest, our Board of Directors has adopted a policy that our executive officers, directors, director nominees and 5% stockholders, their immediate family members and any entity in which such person is an executive, partner or principal or in which such person has a 5% or greater beneficial ownership interest are not permitted to enter into a transaction with us in which the amount involved exceeds $120,000 without the prior consent of our Audit Committee. In approving or rejecting any such transaction, our Audit Committee is to consider the relevant facts of the transaction, including, but not limited to, the terms available to or from, as the case may be, unrelated third parties. Our Board of Directors has determined that transactions involving compensation for services provided to us as an employee, consultant or a director and transactions in which a related party’s participation is solely due to his or her position as a director of an entity that is participating such transaction will not be subject to this policy.
Participation in our Initial Public Offering
Certain of our existing stockholders including Celgene Corporation, HealthCare Ventures VII, L.P., Lilly Ventures Fund I, LLC, Morgenthaler Partners, VII, L.P., and certain other affiliates of our directors, have indicated an interest in purchasing an aggregate of up to $10 million of shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriter may determine to sell more, less or no shares in this offering to any
145
Table of Contents
of these stockholders, or any of these stockholders may determine to purchase more, less or no shares in this offering. The underwriter will receive an underwriting discount of $ per share on any sales of shares to such stockholders.
Any shares purchased by such stockholders will be subject to lock-up restrictions described in the section entitled “Underwriting – Lock-up Agreements.”
146
Table of Contents
The following table sets forth the beneficial ownership of our common stock as of March 31, 2014 by:
| • | each person, or group of affiliated persons, who is known by us to beneficially own more than five percent of our common stock; |
| • | each of our named executive officers; |
| • | each of our directors; and |
| • | all of our executive officers and directors as a group. |
The percentage of shares beneficially owned before the offering shown in the table is based upon 2,851,273 shares of common stock outstanding as of March 31, 2014, after giving effect to the conversion of all of our outstanding preferred stock into 2,757,825 shares of common stock and giving effect to the conversion of the 2014 Notes, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, and based on a conversion date of May 31, 2014, into 722,627 shares of common stock, which will occur automatically upon completion of this offering. The information relating to numbers and percentages of shares beneficially owned after the offering gives effect to the issuance of shares of common stock in this offering. The percentage ownership information assumes no exercise of the underwriter’s over-allotment option.
We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable on or before May 30, 2014, which is 60 days after March 31, 2014. These shares are deemed to be outstanding and beneficially owned by the person holding the applicable options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
147
Table of Contents
Except as otherwise noted below, the address for persons listed in the table is c/o GlobeImmune, Inc., 1450 Infinite Drive, Louisville, CO 80027.
| Shares Beneficially Owned Before Offering |
Shares Beneficially Owned After Offering |
|||||||||||||||
| Name of Beneficial Owner |
Number | Percentage | Number | Percentage | ||||||||||||
| Five Percent Stockholders: |
||||||||||||||||
| Celgene Corporation(1)(13) |
467,452 | 12.8 | % | 467,452 | 9.0 | % | ||||||||||
| HealthCare Ventures VII, L.P.(2)(13) |
403,153 | 11.3 | % | 403,153 | 7.8 | % | ||||||||||
| Morgenthaler Partners VII, L.P.(3)(13) |
409,347 | 11.4 | % | 409,347 | 8.0 | % | ||||||||||
| Entities affiliated with Wexford-Kappa Investors LLC(4) |
266,789 | 7.5 | % | 266,789 | 5.2 | % | ||||||||||
| Entities affiliated with Sequel Limited Partnership III(5) |
262,547 | 7.3 | % | 262,547 | 5.1 | % | ||||||||||
| Lilly Ventures Fund I, LLC(6) |
184,508 | 5.2 | % | 184,508 | 3.6 | % | ||||||||||
| Entities affiliated with Medica Venture Partners(7) |
183,589 | 5.1 | % | 183,589 | 3.6 | % | ||||||||||
| Named Executive Officers and Directors: |
||||||||||||||||
| Timothy C. Rodell, M.D.(8) |
126,853 | 3.4 | % | 126,853 | 2.4 | % | ||||||||||
| C. Jeffrey Dekker, C.P.A. (9) |
13,432 | * | 13,432 | * | ||||||||||||
| Kirk A. Christoffersen, M.B.A. (10) |
9,527 | * | 9,527 | * | ||||||||||||
| J. William Freytag, Ph.D.(11) |
20,858 | * | 20,858 | * | ||||||||||||
| Ralph E. Christoffersen, Ph.D.(3) |
409,347 | 11.4 | % | 409,347 | 8.0 | % | ||||||||||
| Augustine J. Lawlor(2) |
403,153 | 11.3 | % | 403,153 | 7.8 | % | ||||||||||
| Paul A. Mieyal, Ph.D.(4) |
266,789 | 7.5 | % | 266,789 | 5.2 | % | ||||||||||
| Dan J. Mitchell(5) |
262,547 | 7.3 | % | 262,547 | 5.1 | % | ||||||||||
| Pennina Safer, Ph.D.(7) |
— | — | — | — | ||||||||||||
| S. Edward Torres(6) |
184,508 | 5.2 | % | 184,508 | 3.6 | % | ||||||||||
| All current directors and executive officers as a group (10 persons)(12) |
1,697,014 | 45.0 | % | 1,697,014 | 31.8 | % | ||||||||||
| * | Represents beneficial ownership of less than 1% of the outstanding common stock. |
| (1) | Includes 66,782 shares issuable upon exercise of an outstanding warrant. The address for Celgene Corporation is 86 Morris Avenue, Summit, NJ 07901. |
| (2) | Includes 8,886 shares issuable upon exercise of outstanding warrants. The General Partner of HealthCare Ventures VII, L.P. (“HealthCare Ventures”) is HealthCare Partners VII, L.P. (“HealthCare GP”). HealthCare GP may be deemed to indirectly beneficially own the shares owned by HealthCare Ventures. Mr. Lawlor is a general partner of HealthCare GP and may be deemed to be the indirect beneficial owner of the shares owned by HealthCare Ventures. Mr. Lawlor disclaims beneficial ownership of the shares held by HealthCare Ventures, except to the extent of his pecuniary interest arising therein. The address for HealthCare Ventures is 47 Thorndike Street, Suite B1-1, Cambridge, MA 02141. |
| (3) | Includes 8,886 shares issuable upon exercise of outstanding warrants. The general partner of Morgenthaler Partners VII, L.P. (“Morgenthaler”) is Morgenthaler Management Partners, VII, L.L.C. (“MMP VII”). MMP VII may be deemed to indirectly beneficially own the shares owned by Morgenthaler. Dr. Christoffersen is a manager of MMP VII and may be deemed to be the indirect beneficial owner of the shares owned by Morgenthaler. Dr. Christoffersen disclaims beneficial ownership of the shares held by Morgenthaler, except to the extent of his pecuniary interest arising therein. The address for Morgenthaler Partners VII, L.P. is Terminal Tower, 50 Public Square, Suite 2700, Cleveland, OH 44113. |
| (4) | Consists of 231,692 shares, which includes 2,145 shares issuable upon exercise of an outstanding warrant, held by Kappa Investors LLC and 35,097 shares held by Wex SP LLC (collectively, the “Wexford Securities”). Wexford Capital LP (“Wexford”) is the manager of Kappa Investors LLC and Wex SP LLC (collectively, the “Wexford Investment Entities”). Wexford, as manager of the Wexford Investment Entities, may be deemed to own beneficially the interest in the Wexford Securities of which the Wexford Investment Entities possess beneficial ownership. Wexford GP LLC (“Wexford GP”) may, as the general partner of Wexford, be deemed to own beneficially the Wexford Securities of which the Wexford Investment Entities |
148
Table of Contents
| possess beneficial ownership. Each of Charles E. Davidson (“Davidson”) and Joseph M. Jacobs (“Jacobs”) may, by reason of his status as a controlling person of Wexford GP, be deemed to own beneficially the interests in the Wexford Securities of which the Wexford Investment Entities possess beneficial ownership. Each of Davidson, Jacobs, Wexford GP and Wexford shares the power to vote and to dispose of the interests in the Wexford Securities beneficially owned by the Wexford Investment Entities. Each of Davidson, Jacobs, Wexford GP and Wexford disclaims beneficial ownership of the Wexford Securities owned by the Wexford Investment Entities and this prospectus shall not be deemed as an admission that they are the beneficial owners of such Wexford Securities except, in the case of Davidson and Jacobs, to the extent of their interests in each member of the Wexford Investment Entities. Dr. Mieyal is an employee of Wexford, and as an employee of Wexford, Dr. Mieyal does not direct the voting of the Wexford Securities and disclaims beneficial ownership of the Wexford Securities held by the Wexford Investment Entities. The address for the Wexford Investment Entities is Wexford Plaza, 411 West Putman Avenue, Greenwich, CT 06830. |
| (5) | Consists of 250,645 shares (which includes 4,808 shares issuable upon exercise of outstanding warrants) held by Sequel Limited Partnership III and 6,961 shares (which includes 133 shares issuable upon exercise of outstanding warrants) held by Sequel Entrepreneurs Fund III, L.P. (collectively, the “Sequel Funds”). The general partner of the Sequel Funds is Sequel Venture Partners III, L.L.C. (“SVP III”). SVP III may be deemed to indirectly beneficially own the shares owned by the Sequel Funds. Mr. Mitchell is a manager of SVP III and may be deemed to be the indirect beneficial owner of the shares owned by the Sequel Funds. Mr. Mitchell disclaims beneficial ownership of the shares held by the Sequel Funds, except to the extent of his pecuniary interest arising therein. The address for the Sequel Funds is 4430 Arapahoe Avenue, Suite 220, Boulder, CO 80303. |
| (6) | Includes 1,614 shares issuable upon exercise of an outstanding warrant. Mr. Torres is a managing director of Lilly Ventures Fund I LLC (“Lilly Ventures”) and may be deemed to be the indirect beneficial owner of the shares owned by Lilly Ventures. Mr. Torres disclaims beneficial ownership of the shares held by Lilly Ventures, except to the extent of his pecuniary interest arising therein. The address for Lilly Ventures is 115 West Washington Street, South Tower, Suite 1680, Indianapolis, IN 46204. |
| (7) | Consists of 61,398 shares (which includes 646 shares issuable upon exercise of an outstanding warrant) held by Medica III Investments (International) L.P., 31,557 shares (which includes 332 shares issuable upon exercise of an outstanding warrant) held by Medica III Investments (Israel) (B) L.P., 24,286 shares (which includes 255 shares issuable upon exercise of an outstanding warrant) held by Medica III Investments (S.F.) L.P., 13,042 shares (which includes 137 shares issuable upon exercise of an outstanding warrant) held by Medica III Investments (P.F.) L.P., 22,316 shares (which includes 234 shares issuable upon exercise of an outstanding warrant) held by Medica III Investments (Israel) L.P., and 29,080 shares (which includes 306 shares issuable upon exercise of an outstanding warrant) held by Poalim Medica Investments L.P. The general partner of Medica III Investments (International) L.P., Medica III Investments (Israel) L.P., Medica III Investments (S.F.) L.P., Medica III Investments (P.F.) L.P., Medica III Investments (Israel) (B) L.P., and Poalim Medica Investments L.P. (collectively, the “Medica Entities”) is Medica III Management L.P. (“Medica Management LP”). The general partner of Medica Management L.P. is Medica III Management Co. (“Medica Management Co.”). Medica Management LP and Medica Management Co. may be deemed to indirectly beneficially own the shares owned by the Medica Entities. The Medica Entities have the right to appoint one representative to the Board of Directors and have appointed Dr. Pennina Safer to hold such position. Such right shall terminate upon completion of this offering. The address for the Medica Entities is 4 HaSadnaot Street, Herzliya, 46728 Israel. |
| (8) | Consists of 124,949 shares Dr. Rodell has the right to acquire through the exercise of options exercisable within 60 days of March 31, 2014. |
| (9) | Consists of 13,432 shares Mr. Dekker has the right to acquire through the exercise of options exercisable within 60 days of March 31, 2014. |
| (10) | Consists of 9,527 shares Mr. Christoffersen has the right to acquire through the exercise of options exercisable within 60 days of March 31, 2014. |
| (11) | Consists of 20,858 shares Dr. Freytag has the right to acquire through the exercise of options exercisable within 60 days of March 31, 2014. |
149
Table of Contents
| (12) | Includes 26,472 shares issuable upon exercise of outstanding warrants and 168,766 shares issuable upon exercise of stock options by all executive officers and directors exercisable within 60 days of March 31, 2014. See Notes (2) through (11) above. |
| (13) | Certain of our existing stockholders, including Celgene, HealthCare Ventures VII, L.P., Lilly Ventures Fund I, LLC, and Morgenthaler Partners, VII, L.P., and certain other affiliates of our directors, have indicated an interest in purchasing an aggregate of approximately $10 million of shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriter may determine to sell more, less or no shares in this offering to any of these stockholders, or any of these stockholders may determine to purchase more, less or no shares in this offering. The information in the table above does not reflect any potential purchases by these potential investors. If such stockholders were to purchase all of such shares, assuming no exercise by the underwriter of its over-allotment option, our executive officers, directors and 5% or greater stockholders will beneficially own, in the aggregate, approximately 55.1% of our outstanding capital stock upon completion of this offering. |
150
Table of Contents
Upon completion of this offering and the filing of our amended and restated certificate of incorporation, our authorized capital stock will consist of 100,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, par value $0.001 per share. The following is a summary of the rights of our common and preferred stock and some of the provisions of our amended and restated certificate of incorporation and amended and restated bylaws, which will become effective upon completion of this offering, our outstanding warrants, the Stockholders Agreement and of the Delaware General Corporation Law. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description you should refer to our amended and restated certificate of incorporation, amended and restated bylaws, warrants and Stockholders Agreement, copies of which have been filed as exhibits to the registration statement of which the prospectus is a part, as well as the relevant provisions of the Delaware General Corporation Law.
Common Stock
As of March 31, 2014, there were 93,448 shares of common stock outstanding, and 86,570,158 shares of Preferred Stock outstanding. After giving effect to the conversion of all outstanding shares of our preferred stock into shares of common stock upon completion of this offering, there would have been 2,851,273 shares of our common stock outstanding on that date held by 72 stockholders of record without giving effect to the conversion of the 2014 Notes or the Cooley Note. Assuming the conversion of the 2014 Notes and the Cooley Note into common stock upon completion of this offering, assuming an initial public offering price of $16.00, the midpoint on the cover page of this prospectus, there would have been 3,573,900 shares of our common stock outstanding as of March 31, 2014 held by 163 stockholders of record. As of March 31, 2014, there were outstanding options to purchase 235,342 shares of common stock. Upon completion of this offering, and assuming an initial public offering price of $16.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, there will be an additional 104,696 shares of common stock issuable upon exercise of outstanding warrants to purchase preferred stock, which will convert into warrants to purchase common stock, 31,250 shares of common stock issuable upon the exercise of a warrant to purchase common stock to be issued to the underwriter or its designees, 7,671 shares of common stock issuable upon the exercise of a warrant to purchase common stock to be issued to Cooley LLP, 468,769 shares of common stock issuable upon the exercise of the 2014 Warrants by the holders thereof, and 115,940 shares of common stock issuable upon the exercise of outstanding warrants to purchase our capital stock, which will convert into warrants to purchase common stock, held by the underwriter and its designees.
Voting Rights. Each holder of common stock is entitled to one vote for each share of common stock on all matters submitted to a vote of the stockholders, including the election of directors. Our amended and restated certificate of incorporation and amended and restated bylaws do not provide for cumulative voting. Because of this, the holders of a majority of the shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, subject to the rights of holders of any then outstanding shares of preferred stock. The holders a majority of voting power of the common stock, voting as a separate class, have the exclusive right to elect one member of our Board of Directors.
Dividends. Subject to preferences that may be applicable to any then outstanding preferred stock, the holders of common stock are entitled to receive dividends, if any, as may be declared from time to time by our Board of Directors out of legally available funds.
Liquidation. In the event of our liquidation, dissolution or winding up or an Event of Sale (as defined in our certificate of incorporation), holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction of any liquidation preference granted to the holders of any then outstanding shares of preferred stock.
Rights and Preferences. Holders of common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences
151
Table of Contents
and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Fully Paid and Nonassessable. All of our outstanding shares of common stock are, and the shares of common stock that may become issuable upon the exercise or conversion of the Securities will be, duly authorized, validly issued, fully paid and nonassessable.
Preferred Stock
Upon completion of this offering, our Board of Directors will have the authority under our amended and restated certificate of incorporation, without further action by our stockholders, to issue up to 5,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences, privileges and restrictions of the shares of each wholly-unissued series, including dividend rights, conversion rights, voting rights, terms of redemption, liquidation preference and sinking fund terms, and to increase or decrease the number of shares of any such series (but not below the number of shares of such series then outstanding).
Our Board of Directors may authorize the issuance of preferred stock with voting or conversion rights that could have the effect of restricting dividends on our common stock, diluting the voting power of our common stock, impairing the liquidation rights of our common stock or otherwise adversely affect the rights of holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change-of-control and may adversely affect the market price of our common stock. Upon completion of this offering, no shares of preferred stock will be outstanding, and we have no current plans to issue any shares of preferred stock.
Warrants
As of March 31, 2014, there were outstanding warrants to purchase the number and type of shares of our capital stock set forth in column “Number of Shares of Capital Stock” below. Upon completion of this offering, the outstanding warrants to purchase shares of our preferred stock will convert into warrants to purchase the number of shares of our common stock set forth in column “Number of Shares of Common Stock After this Offering” below.
| Description |
Number of Shares of Capital Stock |
Number of Shares Of Capital Stock After this Offering |
Weighted Average Exercise Price After this Offering($) |
|||||||||
| Series B preferred stock |
590,432 | 18,807 | $ | 42.00 | ||||||||
| Series C preferred stock |
2,696,419 | 85,889 | $ | 45.36 | ||||||||
Between February 2005 and June 2005, in connection with the issuance of an aggregate amount of $2,750,000 of convertible promissory notes, we issued warrants exercisable for an aggregate of 411,060 shares of our Series B preferred stock at an exercise price of $1.338 per share, or the 2005 warrants. These 2005 warrants have a net exercise provision and contain provisions for the adjustment of the exercise price and the number of shares issuable upon exercise in the event of certain stock dividends, stock splits, recapitalizations, reclassifications and consolidations. These 2005 warrants will become exercisable for an aggregate of 13,093 shares of our common stock at an exercise price of $42.00 per share upon completion of this offering and are exercisable until their expiration on the later of June 30, 2015 or five years after completion of this offering. The convertible promissory notes are no longer outstanding and were converted into shares of our Series B preferred stock.
In April 2006, in connection with a loan and security agreement entered into with Silicon Valley Bank and Oxford Finance Corporation, or Oxford, we issued to each of SVB and Oxford a warrant to purchase 89,686
152
Table of Contents
shares of our Series B preferred stock at an exercise price of $1.338 per share. These warrants have a net exercise provision and contain provisions for the adjustment of the exercise price and the number of shares issuable upon exercise in the event of certain stock dividends, stock splits, recapitalizations, reclassifications and consolidations. These warrants will become exercisable for an aggregate of 5,714 shares of our common stock at an exercise price of $42.00 per share upon completion of this offering and are exercisable until their expiration on April 14, 2016. The loan and security agreement terminated in December 2009.
In May 2009, we issued the 2009 warrants for the purchase of an aggregate of 622,826 shares of our Series C preferred stock at an exercise price of $1.445 per share, of which 620,294 are outstanding as of December 31, 2013. The 2009 warrants have a net exercise provision and contain provisions for the adjustment of the exercise price and the number of shares issuable upon exercise in the event of certain stock dividends, stock splits, recapitalizations, reclassifications and consolidations. The 2009 warrants will become exercisable for an aggregate of 19,750 shares of our common stock at an exercise price of $45.36 per share upon completion of this offering and are exercisable until their expiration on the later of May 14, 2019 or five years after completion of this offering.
In May 2009, we also issued a warrant to purchase 2,076,125 shares of our Series C preferred stock with an exercise price of $1.445 per share to Celgene in connection with its purchase of shares of our Series D preferred stock. The warrant has a net exercise provision and contains provisions for the adjustment of the exercise price and the number of shares issuable upon exercise in the event of certain stock dividends, stock splits, recapitalizations, reclassifications and consolidations. The warrant will become exercisable for an aggregate of 66,139 shares of our common stock at an exercise price of $45.36 per share upon completion of this offering and is exercisable until its expiration on the later of May 14, 2019 or five years after completion of this offering.
In January 2014, we engaged the underwriter to act as placement agent for a private placement of convertible debt and, in connection therewith, issued it, and certain of its designees, two forms of warrant to purchase shares of our capital stock. The warrants have a net exercise provision and contain provisions for the adjustment of the exercise price and the number of shares issuable upon the exercise of the warrants in the event of certain stock dividends, stock splits, recapitalizations, reclassifications and consolidations. The warrants will become exercisable for an aggregate of 115,940 shares of our common stock at an assumed exercise price of $16.80 per share and $16.00 per share, respectively, assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, upon completion of this offering and are exercisable until their expiration on the five year anniversary of the effectiveness of the registration statement of which this prospectus is a part.
In January and February 2014, in connection with the private placement of the 2014 Notes, we issued the 2014 warrants, for the purchase of our capital stock to investors in the 2014 Notes. The 2014 warrants have a net exercise provision and contain provisions for the adjustment of the exercise price and the number of shares issuable upon the exercise of the warrant in the event of certain stock dividends, stock splits, recapitalizations, reclassifications and consolidations. The warrants will become exercisable for an aggregate of 468,769 shares of our common stock at an assumed exercise price of $16.00 per share assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, upon completion of this offering and are exercisable until their expiration on January 31, 2019.
Upon the completion of this offering and automatic conversion of the promissory note with Cooley LLP into common stock, we will issue a warrant exercisable for an aggregate of 7,671 shares of our common stock at an assumed exercise price of $16.00 per share based upon an assumed initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, upon completion of this offering and is exercisable until its expiration on the five year anniversary of the close of this offering.
The holders of certain of these warrants are entitled to registration rights under the Stockholders Agreement, as described in “Description of Capital Stock—Registration Rights” below.
153
Table of Contents
We have agreed to issue to the underwriter or its designees warrants to purchase 31,250 shares of common stock of the Company at an assumed exercise price of $24.00 per share based on the assumed initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus. In addition, the warrants provide for registration rights upon request, in certain cases. The demand registration right provided will not be greater than five years from the effective date of the registration statement of which this prospectus is a part in compliance with FINRA Rule 5110(f)(2)(H)(iv). The piggyback registration right provided will not be greater than seven years from the effective date of the registration statement of which this prospectus is a part in compliance with FINRA Rule 5110(f)(2)(H)(v). See “Underwriting—Underwriter’s Warrants” section of this prospectus for a description of these warrants.
Registration Rights
In addition to the registration rights granted with respect to the underwriter warrants described above, under the Stockholders Agreement, 180 days after the public offering date set forth on the cover page of this prospectus, the holders of 2,757,825 shares of our common stock, including stock issued upon conversion of our preferred stock, or their transferees, are entitled to demand, “piggyback” and Form S-3 registration rights. In addition, 180 days after the public offering date set forth on the cover page of this prospectus, the following warrant holders have the demand, “piggyback” and Form S-3 registration rights described below: (i) the holders of warrants exercisable for Series B preferred stock described above, or their transferees, with respect to 18,807 shares of common stock issuable upon exercise of their warrants, and (ii) the holders of warrants exercisable for Series C preferred stock described above, or their transferees, with respect to 85,889 shares of common stock issuable upon exercise of their warrants. In addition, the holders of 2,976 shares of our common stock have “tag along” registration rights.
Demand Registration Rights. At any time after 180 days after the public offering date set forth on the cover page of this prospectus, the holders of (x) at least 33 1/3% of the shares having demand registration rights or (y) shares having demand registration rights that were issued upon conversion of shares representing at least $15 million in aggregate original purchase price of shares of Series C preferred stock, Series D preferred stock and Series E preferred stock have the right to make up to two demands that we file a registration statement, subject to specified exceptions, conditions and limitations, including the right of the underwriter to limit the number of shares included in any such registration under certain circumstances.
Form S-3 Registration Rights. If we are eligible to file a registration statement on Form S-3, holders of registration rights have the right to demand that we file a registration statement on Form S-3 so long as the aggregate amount of shares to be sold under the registration statement on Form S-3 is at least $1 million, subject to specified exceptions, conditions and limitations.
“Piggyback” Registration Rights. If we register any securities for public sale, holders of registration rights will have the right to include their shares in the registration statement. The underwriters of any underwritten offering will have the right to limit the number of shares having registration rights to be included in the registration statement.
“Tag-Along” Registration Rights. If we file a registration statement for certain shares held by our founders, then the holders of these registration rights shall have the right that we use our best efforts to register such holders’ shares at the same time and on the same terms and conditions as the registration of such founders’ shares.
Expenses of Registration. Generally, we are required to bear all registration and selling expenses incurred in connection with the demand, Form S-3 and piggyback registrations described above.
154
Table of Contents
Anti-Takeover Effects of Provisions of Delaware Law and Our Charter Documents
Delaware Anti-Takeover Law. We are subject to Section 203 of the Delaware General Corporation Law, or Section 203. Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
| • | prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| • | the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (a) shares owned by persons who are directors and also officers and (b) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66-2/3% of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| • | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; |
| • | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; and |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Section 203 could depress our stock price and delay, discourage or prohibit transactions not approved in advance by our Board of Directors, such as takeover attempts that might otherwise involve the payment to our stockholders of a premium over the market price of our common stock.
Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws. Provisions of our amended and restated certificate of incorporation and amended and restated bylaws, which will become effective upon completion of this offering, may delay or discourage transactions involving an actual or potential change-of-control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests.
155
Table of Contents
Therefore, these provisions could adversely affect the price of our common stock. Among other things, our amended and restated certificate of incorporation and amended and restated bylaws:
| • | permit our Board of Directors to issue up to 5,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate (including the right to approve an acquisition or other change-of-control); |
| • | provide that the authorized number of directors may be changed only by resolution of the Board of Directors; |
| • | provide that, subject to the rights of any series of preferred stock to elect directors, directors may only be removed for cause, which removal may be effected subject to any limitation imposed by law, by the holders of at least 66 2/3 % of the voting power of our then outstanding capital stock entitled to vote generally at an election of directors; |
| • | provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
| • | require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent; |
| • | provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a stockholder’s notice; |
| • | do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); and |
| • | provide that special meetings of our stockholders may be called only by the Chairman of the Board of Directors, our chief executive officer or by the Board of Directors pursuant to a resolution adopted by a majority of the total number of authorized directors. |
The amendment of any of these provisions would require approval by the holders of at least 66 2/3% of our then outstanding common stock.
Transfer agent and registrar
The transfer agent and registrar for the common stock is Computershare Trust Company, N.A. The transfer agent and registrar’s address is 250 Royall Street, Canton, Massachusetts 02021.
NASDAQ Global Market listing
We have applied to have our common stock listed on The NASDAQ Global Market under the symbol “GBIM”.
156
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, no public market has existed for our common stock. Market sales of shares of our common stock after this offering and from time to time, and the availability of shares for future sale, may reduce the market price of our common stock. Sales of substantial amounts of our common stock, or the perception that these sales could occur, could adversely affect prevailing market prices for our common stock and could impair our future ability to obtain capital, especially through an offering of equity securities. As described below, only a limited number of shares of our common stock will be available for sale in the public market for a period of several months after completion of this offering due to contractual and legal restrictions on resale described below. Nevertheless, sales of a substantial number of shares of our common stock in the public market after such restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price of our common stock. Although we have applied to list our common stock on The NASDAQ Global Market, we cannot assure you that there will be an active market for our common stock.
Based upon the number of shares outstanding as of March 31, 2014, upon completion of this offering, we will have outstanding an aggregate of 5,136,400 shares of our common stock, assuming no exercise of the underwriter’s over-allotment option, no exercise of outstanding options or warrants, after giving effect to the conversion of all outstanding shares of our preferred stock into 2,757,825 shares of common stock immediately prior to completion of this offering and the issuance of 722,627 shares of our Common Stock pursuant to the 2014 Notes and the Cooley Note. All of the shares sold in this offering, including those shares of common stock issuable upon exercise of the warrant to be issued to the underwriter upon completion of this offering, will be freely tradable without restrictions or further registration under the Securities Act, unless held by our affiliates as that term is defined under Rule 144 under the Securities Act or subject to lock-up agreements. Other than the shares sold in this offering, the shares of common stock outstanding upon completion of this offering are restricted securities as defined in Rule 144. Restricted securities may be sold in the U.S. public market only if registered or if they qualify for an exemption from registration, including by reason of Rule 144 or Rule 701 under the Securities Act, which rules are summarized below. These shares will generally become available for sale in the public market as follows:
| • | none of the restricted shares will be eligible for sale upon completion of this offering; and |
| • | approximately 3,573,900 restricted shares will be eligible for sale in the public market upon expiration of lock-up agreements 180 days after the date of this prospectus, which date may be extended in specified circumstances, subject in certain circumstances to the volume, manner of sale and other limitations of Rule 144 and Rule 701. |
Additionally, of the 235,342 shares of common stock issuable upon exercise of options outstanding as of March 31, 2014 approximately 219,850 shares will be vested and eligible for sale 180 days after the date of this prospectus.
Rule 144
In general, under Rule 144 under the Securities Act of 1933, as in effect on the date of this prospectus, beginning 90 days after the date of this prospectus, a person who is not one of our affiliates at any time during the three months preceding a sale, and who has beneficially owned shares of our common stock to be sold for at least six months, would be entitled to sell an unlimited number of shares of our common stock, provided current public information about us is available. In addition, under Rule 144, a person who is not one of our affiliates at any time during the three months preceding a sale, and who has beneficially owned the shares of our common stock to be sold for at least one year, would be entitled to sell an unlimited number of shares immediately upon completion of this offering without regard to whether current public information about us is available. Beginning 90 days after the date of this prospectus, our affiliates who have beneficially owned shares of our common stock
157
Table of Contents
for at least six months are entitled to sell within any three-month period a number of shares that does not exceed the greater of:
| • | 1% of the number of shares of our common stock then outstanding, which will equal approximately 51,364 shares upon completion this offering, or 53,708 shares if the underwriter exercises its over-allotment option in full; and |
| • | the average weekly trading volume of our common stock on The NASDAQ Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales of restricted shares under Rule 144 by our affiliates are also subject to requirements regarding the manner of sale, notice and the availability of current public information about us. Rule 144 also provides that affiliates relying on Rule 144 to sell shares of our common stock that are not restricted shares must nonetheless comply with the same restrictions applicable to restricted shares, other than the holding period requirement.
Rule 701
In general, under Rule 701 as currently in effect, any of our employees, directors, officers, consultants or advisors who acquired common stock from us in connection with a written compensatory stock or option plan or other written agreement in compliance with Rule 701 under the Securities Act before the effective date of the registration statement of which this prospectus is a part (to the extent such common stock is not subject to a lock-up agreement) is entitled to rely on Rule 701 to resell such shares beginning 90 days after we become subject to the public company reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, in reliance on Rule 144, but without compliance with the holding period requirements contained in Rule 144. Accordingly, subject to any applicable lock-up agreements, beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act, under Rule 701 persons who are not our “affiliates”, as defined in Rule 144, may resell those shares without complying with the minimum holding period or public information requirements of Rule 144, and persons who are our “affiliates” may resell those shares without compliance with Rule 144’s minimum holding period requirements (subject to the terms of the lock-up agreement referred to below, if applicable).
Lock-Up Agreements
As described under the section entitled “Underwriting—Lock-Up Agreements” below, we, all of our directors and officers, the holders of substantially all of the other shares of our common stock outstanding prior to this offering, and the holders of substantially all of our warrants and substantially all of our options outstanding prior to this offering, have agreed, subject to certain exceptions, that, without the prior written consent of the underwriter, we and they will not, during the period including the date of this prospectus through and including the date that is the 180th day after the date of this prospectus, directly or indirectly issue (in the case of us), offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of any shares of our common stock or other capital stock or any securities convertible into or exercisable or exchangeable for our common stock or other capital stock or take certain related actions.
The underwriter may, in its sole discretion and at any time or from time to time, release all or any portion of the shares or other securities subject to the lock-up agreements with them.
Registration Rights
Upon completion of this offering, the holders of an aggregate of 2,757,825 shares of our common stock, including the common stock issuable upon conversion of our preferred stock, will have the right to require us to register their shares for resale under the Securities Act, beginning 180 days after the date of this prospectus. Registration of these shares for resale under the Securities Act would result in the shares becoming freely
158
Table of Contents
tradable without restriction under the Securities Act immediately upon the effectiveness of such registration. Any sales of securities by these stockholders could adversely affect the trading price of our common stock. These registration rights are described in more detail under the caption “Description of Capital Stock—Registration Rights”.
Equity Incentive Plans
Following completion of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act to register the shares of common stock issued or reserved for issuance under our equity incentive plans, including the equity incentive plans we adopted in connection with this offering. Accordingly, shares registered under such registration statements will be available for sale in the open market, unless such shares are subject to vesting restrictions with us or the lock-up restrictions described above.
159
Table of Contents
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
The following is a general discussion of the material U.S. federal income and estate tax considerations relating to the ownership and disposition of our common stock acquired in this offering by a non-U.S. holder. For purposes of this discussion, the term “non-U.S. holder” means a beneficial owner of our common stock that is not, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation, or other entity treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States or of any political subdivision of the United States; |
| • | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust, if a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust or if the trust has a valid election in effect to be treated as a U.S. person under applicable U.S. Treasury Regulations. |
An individual may be a resident alien for U.S. federal income tax purposes in any calendar year if the individual was present in the United States for at least 31 days in that calendar year and for an aggregate of at least 183 days during the three-year period ending with the current calendar year. For purposes of this calculation, all of the days present in the current year, one-third of the days present in the immediately preceding year and one-sixth of the days present in the second preceding year are counted. Residents are taxed for U.S. federal income tax purposes as if they were U.S. citizens.
This discussion is based on current provisions of the Internal Revenue Code of 1986, or the Code, existing and proposed U.S. Treasury Regulations promulgated thereunder, current administrative rulings and judicial decisions, all as in effect as of the date of this prospectus and all of which are subject to change or to differing interpretation, possibly with retroactive effect. Any change could alter the tax consequences to non-U.S. holders described in this prospectus. In addition, the Internal Revenue Service, or the IRS, could challenge one or more of the tax consequences described in this prospectus.
We assume in this discussion that each non-U.S. holder holds shares of our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all aspects of U.S. federal income and estate taxation that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder’s individual circumstances, the potential application of the Medicare contribution tax, or any aspects of state, local or non-U.S. taxes, or U.S. federal taxes other than income and estate taxes. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax rules applicable to particular non-U.S. holders, such as:
| • | insurance companies; |
| • | tax-exempt organizations; |
| • | integral parts or controlled entities of a foreign sovereign; |
| • | financial institutions; |
| • | banks; |
| • | brokers, dealers or traders in securities or currencies; |
| • | regulated investment companies; |
| • | real estate investment trusts; |
| • | pension plans;individual retirement or tax-deferred accounts; |
| • | controlled foreign corporations; |
160
Table of Contents
| • | passive foreign investment companies; |
| • | corporations that accumulated earnings to avoid U.S. federal income tax; |
| • | hybrid entities; |
| • | persons who own more than 5% of our common stock; |
| • | owners that hold our common stock as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment; |
| • | persons subject to the alternative minumum tax or the unearned income Medicare contribution tax; and |
| • | certain U.S. expatriates. In addition, this discussion does not address the tax treatment of partnerships or persons who hold their common stock through partnerships or other entities which are transparent for U.S. federal income tax purposes. A partner in a partnership or other transparent entity that will hold our common stock should consult his, her or its own tax advisor regarding the tax consequences of the ownership and disposition of our common stock through a partnership or other transparent entity. |
Prospective investors should consult their own tax advisors regarding the U.S. federal, state, local and non-U.S. income and other tax considerations of acquiring, holding and disposing of our common stock.
Dividends
If we pay distributions on our common stock, those distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder’s investment, up to such holder’s tax basis in the holder’s common stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below under the heading “—Gain on Disposition of Common Stock”.
Dividends paid to a non-U.S. holder generally will be subject to withholding of U.S. federal tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence. A non-U.S. holder of our common stock who claims the benefit of an applicable income tax treaty generally will be required to provide us with a properly executed IRS Form W-8BEN (or successor form) and satisfy certain other requirements. Non-U.S. holders are urged to consult their own tax advisors regarding their entitlement to benefits under an income tax treaty. A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS.
Dividends that are effectively connected with a trade or business conducted by a non-U.S. holder within the United States, and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by the non-U.S. holder within the United States, are generally exempt from the 30% withholding tax. To obtain this exemption, a non-U.S. holder must provide us with a properly executed original IRS Form W-8ECI (or successor form) and satisfy certain other requirements. However, such U.S. effectively connected income, or if an income tax treaty applies, such income that is attributable to a permanent establishment, net of specified deductions and credits, is taxed at the same graduated U.S. federal income tax rates applicable to U.S. persons (as defined in the Code). Any U.S. effectively connected income or income that is attributable to a permanent establishment that is received by a non-U.S. holder that is a corporation may also be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence.
161
Table of Contents
Gain on Disposition of Common Stock
Subject to the discussion below regarding FATCA, a non-U.S. holder generally will not be subject to U.S. federal income tax on gain recognized on a disposition of our common stock unless:
| • | the gain is effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States, and, if an applicable income tax treaty so provides, the gain is attributable to a permanent establishment maintained by the non-U.S. holder in the United States; in these cases, the non-U.S. holder will be taxed on a net income basis at graduated rates and in the manner applicable to U.S. persons, and, if the non-U.S. holder is a foreign corporation, an additional branch profits tax at a rate of 30%, or a lower rate as may be specified by an applicable income tax treaty, may also apply; |
| • | the non-U.S. holder is an individual present in the United States for 183 days or more in the taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty) on the net gain derived from the disposition; or |
| • | we are or have been, at any time during the five-year period preceding such disposition (or the non-U.S. holder’s holding period, if shorter) a “U.S. real property holding corporation” unless our common stock is regularly traded on an established securities market and the non-U.S. holder held no more than five percent of our outstanding common stock, directly indirectly or constructively, during the shorter of the five-year period ending on the date of the disposition or the period that the non-U.S. holder held our common stock. Generally, a corporation is a “U.S. real property holding corporation” if the fair market value of its “U.S. real property interests” equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. We believe that we are not currently, and we do not anticipate becoming, a “U.S. real property holding corporation”. |
Information Reporting and Backup Withholding Tax
We must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions on our common stock paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. holders may have to comply with specific certification procedures to establish that the holder is not a U.S. person (as defined in the Code) in order to avoid backup withholding at the applicable rate, currently 28%, with respect to dividends on our common stock. Generally, a holder will comply with such procedures if it provides a properly executed IRS Form W-8BEN or otherwise meets documentary evidence requirements for establishing that it is a non-U.S. holder, or otherwise establishes an exemption.
Information reporting and backup withholding generally will apply to the proceeds of a disposition of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder can be refunded or credited against the non-U.S. holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
162
Table of Contents
Foreign Account Tax Compliance Act
The Foreign Account Tax Compliance Act, or FATCA, will impose a 30% withholding tax on any “withholdable payment” to (i) a “foreign financial institution” (as specifically defined for this purpose), unless such institution (A) enters into an agreement with the U.S. government to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which would include certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with United States owners) or (B) complies with legislation or other rules promulgated pursuant to the intergovernmental agreement between the foreign financial institution’s home country and the U.S. (ii) a foreign entity that is not a foreign financial institution, unless such entity provides the withholding agent with a certification identifying the substantial U.S. owners of the entity, if any, which generally includes any U.S. person who directly or indirectly owns more than 10% of the entity. Under certain limited circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes.
“Withholdable payments” will include U.S.-source payments otherwise subject to nonresident withholding tax, and also include the entire gross proceeds from the sale of any equity of U.S. issuers. The withholding tax will apply regardless of whether the payment would otherwise be exempt from withholding under the existing U.S. nonresident withholding tax rules. The IRS is authorized to provide rules for implementing the FATCA withholding regime with the existing nonresident withholding tax rules.
Withholding under the FATCA will apply to U.S.-source dividend payments made on or after July 1, 2014 and to the payment of gross proceeds from the sale of any equity of U.S. issuers made on or after January 1, 2017.
Federal Estate Tax
Common stock owned or treated as owned by an individual who is a non-U.S. holder (as specially defined for U.S. federal estate tax purposes) at the time of death will be included in the individual’s gross estate for U.S. federal estate tax purposes and, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax or other treaty provides otherwise.
The preceding discussion of material U.S. federal tax considerations is for general information only. It is not tax advice. Prospective investors should consult their own tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing of our common stock, including the consequences of any proposed changes in applicable laws.
163
Table of Contents
Aegis Capital Corp., or the underwriter, is acting as the sole underwriter of this offering. We have entered into an underwriting agreement, dated , 2014, with the underwriter. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriter and the underwriter has agreed to purchase from us, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of shares of common stock listed next to its name in the following table.
| Name of Underwriter |
Number of Shares |
|||
| Aegis Capital Corp. |
1,562,500 | |||
|
|
|
|||
| Total |
1,562,500 | |||
|
|
|
|||
The underwriter is committed to purchase all the shares of common stock offered by us other than those covered by the option to purchase additional shares described below, if it purchases any shares. The obligations of the underwriter may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriter’s obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriter of officers’ certificates and legal opinions.
We have agreed to indemnify the underwriter against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriter may be required to make in respect thereof.
The underwriter is offering the shares, subject to prior sale, when, as and if issued to and accepted by it, subject to approval of legal matters by its counsel and other conditions specified in the underwriting agreement. The underwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
We have granted the underwriter an over-allotment option. This option, which is exercisable for up to 45 days after the date of this prospectus, permits the underwriter to purchase a maximum of 234,375 additional shares (15% of the shares sold in this offering) from us to cover over-allotments, if any. If the underwriter exercises all or part of this option, it will purchase shares covered by the option at the public offering price that appears on the cover page of this prospectus, less the underwriting discount. If this option is exercised in full, the total price to the public will be $ and the total net proceeds, before expenses, to us will be $ .
Discount. The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriter of its over-allotment option.
| Per Share |
Total Without Over- Allotment Option |
Total With Over- Allotment Option |
||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discount (7%)(1) |
$ | $ | $ | |||||||||
| Non-accountable expense allowance(1%) |
||||||||||||
| Proceeds, before expenses, to us(2) |
$ | $ | $ | |||||||||
| (1) | We have paid a $25,000 advance to the underwriter to be applied against the accountable expenses that will be paid by us to the underwriter in connection with this offering. |
| (2) | In addition to the underwriting discounts and commissions and non-accountable expense allowance, we agreed to pay or reimburse the underwriter to cover certain out of pocket expenses of the underwriter in connection with this offering in an amount of up to $146,775. |
164
Table of Contents
The underwriter proposes to offer the shares offered by us to the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriter may offer some of the shares to other securities dealers at such price less a concession of $ per share. If all of the shares offered by us are not sold at the public offering price, the underwriter may change the offering price and other selling terms by means of a supplement to this prospectus.
We have paid an advance of $25,000 to the underwriter, which will be applied against the accountable expenses that will be paid by us to the underwriter in connection with this offering. The underwriting agreement provides that in the event the offering is terminated, the $25,000 expense deposit paid to the underwriter will be returned to us to the extent that offering expenses are not actually incurred by the underwriter.
We have also agreed to pay the underwriter’s expenses relating to the offering, including (a) all fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed $25,000 in the aggregate; (b) all filing fees incurred in clearing this offering with FINRA; (c) all fees, expenses and disbursements relating to the registration, qualification or exemption of our shares offered under the “blue sky” securities laws of such states and other jurisdictions as we and the underwriter may reasonably agree (including the reasonable fees and disbursements of “blue sky counsel” counsel); (d) the costs associated with advertising this offering in the national editions of the Wall Street Journal and New York Times in an amount not to exceed $5,000 without our prior written consent; (f) upon successful completion of this offering the $21,775 cost for the underwriter’s use of Ipreo’s book-building, prospectus tracking and compliance software for this offering; (g) upon successful completion of this offering, the fees and expenses of the underwriter’s legal counsel in an amount not to exceed $75,000; and (h) upon successful completion of this offering, up to $20,000 of the underwriter’s actual accountable road show expenses for the offering.
We estimate that the total expenses of the offering payable by us, excluding the total underwriting discount and non-accountable expense allowance, will be approximately $1.9 million.
Discretionary Accounts. The underwriter does not intend to confirm sales of the securities offered hereby to any accounts over which it has discretionary authority.
Lock-Up Agreements. Pursuant to certain “lock-up” agreements, we, our executive officers and directors, and each holder of our voting securities have agreed, subject to certain exceptions, not to offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic risk of ownership of, directly or indirectly, engage in any short selling of any of our common stock or securities convertible into or exchangeable or exercisable for our common stock, whether currently owned or subsequently acquired, during a period ending 180 days after the effectiveness of the registration statement of which this prospectus is a part, without first obtaining the written consent of the underwriter.
Underwriter’s Warrants. We have agreed to issue to the underwriter or its designees warrants to purchase up to a total of 31,250 shares of common stock (2% of the shares of our common stock sold in this offering, excluding the underwriter’s over-allotment option). We are registering hereby the warrants and the shares of common stock issuable upon exercise of the warrants. The warrants will be exercisable commencing one year and a day from the closing date of this offering, and from time to time, in whole or in part, until the fifth anniversary of the effective date of the registration statement of which this prospectus is a part in compliance with FINRA Rule 5110(f)(2)(H)(i). The warrants are exercisable at a per share price equal to 150% of the public offering price per share in the offering. The warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The holders of these warrants (or permitted assignees under Rule 5110(g)(1)) will not sell, transfer, assign, pledge, or hypothecate these warrants or the securities underlying these warrants, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the warrants or the underlying securities for a period of 180 days from the effective date of the registration statement of which this prospectus is a part. In addition, the warrants provide for registration rights upon request, in certain cases. The demand registration right
165
Table of Contents
provided will not be greater than five years from the effective date of the registration statement of which this prospectus is a part in compliance with FINRA Rule 5110(f)(2)(H)(iv). The piggyback registration right provided will not be greater than seven years from the effective date of the registration statement of which this prospectus is a part in compliance with FINRA Rule 5110(f)(2)(H)(v). We will bear all fees and expenses attendant to registering the securities issuable on exercise of the warrants other than underwriting commissions incurred and payable by the holders. The exercise price and number of shares issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend or our recapitalization, reorganization, merger or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
Right of First Refusal. Subject to certain limited exceptions, until twelve months after the effective date of this registration statement, the underwriter has a right of first refusal to act as a book-runner, for economics of no less than 42.5% of the aggregate compensation paid in connection with any public or private equity or public debt offering of the Company during such twelve-month period.
Electronic Offer, Sale and Distribution of Shares. A prospectus in electronic format may be made available on the websites maintained by the underwriter or selling group members, if any, participating in this offering and the underwriter may distribute prospectuses electronically. The underwriter may agree to allocate a number of shares to selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriter and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or the underwriter in its capacity as underwriter, and should not be relied upon by investors.
Stabilization. In connection with this offering, the underwriter may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
| • | Stabilizing transactions permit bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the shares while the offering is in progress. |
| • | Over-allotment transactions involve sales by the underwriter of shares in excess of the number of shares the underwriter is obligated to purchase. This creates a short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriter is not greater than the number of shares that it may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriter may close out any short position by exercising its over-allotment option and/or purchasing shares in the open market. |
| • | Syndicate covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriter will consider, among other things, the price of shares available for purchase in the open market as compared with the price at which they may purchase shares through exercise of the over- allotment option. If the underwriter sells more shares than could be covered by exercise of the over-allotment option and, therefore, have a naked short position, the position can be closed out only by buying shares in the open market. A naked short position is more likely to be created if the underwriter is concerned that after pricing there could be downward pressure on the price of the shares in the open market that could adversely affect investors who purchase in the offering. |
| • | Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the shares originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions. |
166
Table of Contents
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our shares or common stock or preventing or retarding a decline in the market price of our shares or common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on The NASDAQ Global Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive market making. In connection with this offering, the underwriter and selling group members may engage in passive market making transactions in our common stock on The NASDAQ Global Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
Other Relationships. As consideration for services provided as our placement agent in connection with our issuance of 10% convertible promissory notes from January 31, 2014 to February 11, 2014, we paid the underwriter a cash fee in the aggregate amount of $749,250 and reimbursed certain of the underwriter’s expenses in the aggregate amount of $50,000. Additionally, as compensation for services provided in such offering, we issued to the underwriter (i) a warrant to purchase 46,877 shares of common stock of the Company at an exercise price of $16.00 per share assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, and (ii) a warrant to purchase 69,063 shares of common stock of the Company at an assumed exercise price of $16.00 per share assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus. Three persons or entities affiliated with the underwriter purchased an aggregate of $300,000 of our 10% convertible promissory notes between January 31, 2014 and February 11, 2014.
The underwriter and its affiliates have provided, and may in the future provide, various investment banking, commercial banking and other financial services for us and our affiliates for which they have received, and may in the future receive, customary fees. However, except as disclosed in this prospectus, we have no present arrangements with the underwriter for any further services.
Offer restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more
167
Table of Contents
exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer for the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area—Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
(a) to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements);
(c) to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or
(d) in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive.
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
168
Table of Contents
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority, or the ISA, nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Societ—$$—Aga e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| • | to Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
| • | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
| • | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
169
Table of Contents
| • | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA.
This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be
170
Table of Contents
communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to Kips Bay.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
The validity of the shares of common stock being offered by this prospectus will be passed upon for us by Cooley LLP , Broomfield, Colorado. As of the date of this prospectus, GC&H Investments, LLC, an entity that includes current and former partners and associates of Cooley LLP , beneficially owns 73,434 shares of our preferred stock, which will be converted into 2,337 shares of our outstanding common stock upon completion of this offering. Additionally, Cooley LLP beneficially holds the Cooley Note in the principal amount of $391,730, which will convert into 31,964 shares of common stock assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, and based on a conversion date of May 31, 2014. We will also issue the Cooley Warrant exercisable for 7,671 shares of common stock at an assumed exercise price of $16.00 per share assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus. Lowenstein Sandler LLP , New York, New York has acted as counsel for the underwriter in connection with certain legal matters related to this offering.
Each $1.00 increase (decrease) in the assumed initial public offering price of $16.00 per share, which is the midpoint of the price range listed on the cover page of this prospectus, would increase (decrease) our the number of shares of common stock issued to Cooley LLP pursuant to the Cooley Note by approximately 2,006 shares, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
The financial statements of GlobeImmune, Inc. as of December 31, 2013 and 2012 and for each of the years in the three-year period ended December 31, 2013, have been included herein and in the registration statement in reliance upon the report of KPMG LLP, independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the shares of common stock being offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further information with respect to GlobeImmune, Inc. and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at http://www.sec.gov. You may also read and copy any document we file with the SEC at its public reference
171
Table of Contents
facilities at 100 F Street, N.E., Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
Upon completion of this offering, we will be subject to the information reporting requirements of the Exchange Act, and we will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for inspection and copying at the public reference room and web site of the SEC referred to above. We also maintain a website at www.globeimmune.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of, and is not incorporated into, this prospectus.
172
Table of Contents
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| Statements of Redeemable, Convertible Preferred Stock, and Stockholders’ Deficit |
F-5 | |||
| F-9 | ||||
| F-10 |
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
GlobeImmune, Inc.:
We have audited the accompanying balance sheets of GlobeImmune, Inc. (the Company) as of December 31, 2013 and 2012, and the related statements of operations and comprehensive income and loss, redeemable, convertible preferred stock, and stockholders’ deficit, and cash flows for each of the years in the three-year period ended December 31, 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of GlobeImmune, Inc. as of December 31, 2013 and 2012, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2013, in conformity with U.S. generally accepted accounting principles.
/s/ KPMG LLP
Boulder, Colorado
March 14, 2014, except as to note 3(c), which is as of April 28, 2014
F-2
Table of Contents
Balance Sheets
| December 31, | March 31, 2014 | |||||||||||
| 2012 | 2013 | |||||||||||
| (unaudited) | ||||||||||||
| Assets | ||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 2,002,948 | 5,924,241 | 9,215,907 | ||||||||
| Debt issuance costs |
— | — | 1,606,888 | |||||||||
| Other current assets |
886,395 | 900,896 | 770,869 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
2,889,343 | 6,825,137 | 11,593,664 | |||||||||
| Property and equipment, net |
1,251,848 | 492,802 | 392,891 | |||||||||
| Other assets |
100,000 | 100,000 | 100,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
$ | 4,241,191 | 7,417,939 | 12,086,555 | ||||||||
|
|
|
|
|
|
|
|||||||
| Liabilities, Redeemable, Convertible Preferred Stock and Stockholders’ Deficit | ||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 2,957,234 | 1,736,621 | 918,615 | ||||||||
| Accrued liabilities |
1,631,782 | 920,962 | 719,908 | |||||||||
| Convertible notes |
— | — | 2,506,682 | |||||||||
| Fair value of warrants |
— | 13 | 5,072,134 | |||||||||
| Fair value of put and call options |
— | — | 1,795,372 | |||||||||
| Deferred revenue |
3,761,908 | 3,756,899 | 3,410,282 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
8,350,924 | 6,414,495 | 14,422,993 | |||||||||
| Other long-term liabilities |
121,807 | 223,029 | 223,029 | |||||||||
| Deferred revenue |
13,794,707 | 10,656,976 | 9,837,209 | |||||||||
| Fair value of warrants, net of current portion |
3,301,411 | 1,564,928 | 1,448,701 | |||||||||
| Convertible promissory note |
— | 197,955 | 222,813 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
25,568,849 | 19,057,383 | 26,154,745 | |||||||||
|
|
|
|
|
|
|
|||||||
| Commitments and contingencies |
||||||||||||
| Redeemable, convertible preferred stock – Series C, $0.001 par value. |
||||||||||||
| Authorized 37,600,000 shares; issued and outstanding 31,147,071 |
||||||||||||
| (liquidation preference of $63,503,744, $67,948,149 and $69,137,027 respectively) |
62,933,146 | 67,548,103 | 68,779,619 | |||||||||
| Redeemable, convertible preferred stock – Series D, $0.001 par value. |
||||||||||||
| Authorized 8,900,000 shares; issued and outstanding 8,650,519 shares |
||||||||||||
| (liquidation preference of $12,795,434, $13,691,114 and $13,930,709 respectively) |
11,758,907 | 12,964,405 | 13,281,454 | |||||||||
| Redeemable, convertible preferred stock – Series E, $0.001 par value. |
||||||||||||
| Authorized 11,750,000 shares; issued and outstanding 11,665,019 shares |
||||||||||||
| (liquidation preference of $21,995,486, $23,535,170 and $23,947,035 respectively) |
21,920,758 | 23,482,780 | 23,900,229 | |||||||||
| Redeemable, convertible preferred stock – Series A, $0.001 par value. |
||||||||||||
| Authorized 6,430,000 shares; issued and outstanding 6,407,998 shares |
||||||||||||
| (liquidation preference of $14,661,229, $15,687,341 and $15,961,826 respectively) |
14,637,381 | 15,670,621 | 15,946,888 | |||||||||
| Redeemable, convertible preferred stock – Series B, $0.001 par value. |
||||||||||||
| Authorized 29,300,000 shares; issued and outstanding 28,699,551 shares |
||||||||||||
| (liquidation preference of $63,604,338, $68,056,641 and $69,247,632 respectively) |
63,547,057 | 68,016,481 | 69,211,752 | |||||||||
| Stockholders’ deficit: |
||||||||||||
| Common stock, $0.001 par value. Authorized 112,500,000 shares; issued and outstanding 92,430, 92,812 and 93,448 shares, respectively |
92 | 93 | 93 | |||||||||
| Additional paid-in capital |
— | — | — | |||||||||
| Accumulated deficit |
(196,124,999 | ) | (199,321,927 | ) | (205,188,225 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ deficit |
(196,124,907 | ) | (199,321,834 | ) | (205,188,132 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities, redeemable, convertible preferred stock and stockholders’ deficit |
$ | 4,241,191 | 7,417,939 | 12,086,555 | ||||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes to financial statements.
F-3
Table of Contents
Statements of Operations and Comprehensive Income and Loss
| Year ended December 31, | Three months ended March 31, | |||||||||||||||||||
| 2011 | 2012 | 2013 | 2013 | 2014 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Revenue |
||||||||||||||||||||
| Collaboration license and services |
$ | 5,107,842 | 12,641,606 | 16,350,132 | 1,192,267 | 1,283,521 | ||||||||||||||
| Milestones |
2,000,000 | 3,000,000 | — | — | ||||||||||||||||
| Manufacturing services |
— | — | 3,168,237 | 940,000 | 134,825 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
5,107,842 | 14,641,606 | 22,518,369 | 2,132,267 | 1,418,346 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research and development expenses: |
||||||||||||||||||||
| Costs of collaboration license and services |
8,380,250 | 10,033,040 | 5,856,013 | 2,112,916 | 832,854 | |||||||||||||||
| Costs of manufacturing services |
— | — | 3,168,237 | 940,000 | 134,825 | |||||||||||||||
| Research and development for proprietary programs |
3,683,153 | 1,701,511 | 1,860,378 | 160,491 | 571,100 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total research and development |
12,063,403 | 11,734,551 | 10,884,628 | 3,213,407 | 1,538,779 | |||||||||||||||
| General and administrative |
4,685,825 | 5,948,066 | 3,174,769 | 737,145 | 1,014,632 | |||||||||||||||
| Depreciation and amortization |
952,074 | 925,473 | 771,280 | 219,930 | 69,866 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
17,701,302 | 18,608,090 | 14,830,677 | 4,170,482 | 2,623,277 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
(12,593,460 | ) | (3,966,484 | ) | 7,687,692 | (2,038,215 | ) | (1,204,931 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Change in value of warrants and put and call options, income (expense) |
(1,135,032 | ) | 1,951,490 | 1,842,557 | 486,581 | 247,045 | ||||||||||||||
| Interest expense |
— | — | — | — | (1,486,686 | ) | ||||||||||||||
| Other income (expense) |
3,679 | 250 | 62,215 | 62 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before taxes |
(13,724,813 | ) | (2,014,744 | ) | 9,592,464 | (1,551,572 | ) | (2,444,572 | ) | |||||||||||
| Income taxes |
— | — | 115,765 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
(13,724,813 | ) | (2,014,744 | ) | 9,476,699 | (1,551,572 | ) | (2,444,572 | ) | |||||||||||
| Preferred stock dividends and accretion of offering costs to redemption value |
(11,316,453 | ) | (12,104,251 | ) | (12,885,141 | ) | (3,221,285 | ) | (3,437,552 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss applicable to common stockholders |
$ | (25,041,266 | ) | (14,118,995 | ) | (3,408,442 | ) | (4,772,857 | ) | (5,882,124 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average shares outstanding—basic and diluted |
87,853 | 89,377 | 92,522 | 92,430 | 93,201 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per share attributable to common stockholders—basic and diluted |
$ | (285.04 | ) | (157.97 | ) | (36.84 | ) | (51.64 | ) | (63.11 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Pro forma net income per share of common stock attributable to GlobeImmune, Inc. stockholders -diluted (unaudited) (note 3(q)) |
$ | 2.60 | (0.94 | ) | ||||||||||||||||
| Pro forma weighted-average common shares outstanding—diluted (unaudited) (note 3(q)) |
2,945,047 | 2,851,026 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
See accompanying notes to financial statements.
F-4
Table of Contents
Statements of Redeemable, Convertible Preferred Stock, and Stockholders’ Deficit
| Redeemable, convertible Series C preferred stock |
Redeemable, convertible Series D preferred stock |
Redeemable, convertible Series E preferred stock |
Redeemable, convertible Series A preferred stock |
Redeemable, convertible Series B preferred stock |
Stockholders’ deficit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock |
Additional paid-in capital |
Accumulated Deficit |
Total stockholders’ deficit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2010 |
31,115,107 | $ | 54,495,917 | 8,650,519 | $ | 9,517,862 | 11,665,019 | $ | 19,088,862 | 6,407,998 | $ | 12,765,602 | 28,699,551 | $ | 55,453,014 | 87,701 | $ | 88 | — | (157,716,259 | ) | (157,716,171 | ) | |||||||||||||||||||||||||||||||||||||
| Accretion of Series A offering costs |
— | — | — | — | — | — | — | 7,128 | — | — | — | — | — | (7,128 | ) | (7,128 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B offering costs |
— | — | — | — | — | — | — | — | — | 17,121 | — | — | — | (17,121 | ) | (17,121 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C offering costs |
— | 170,552 | — | — | — | — | — | — | — | — | — | — | — | (170,552 | ) | (170,552 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series D offering costs |
— | — | — | 309,818 | — | — | — | — | — | — | — | — | — | (309,818 | ) | (309,818 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series E offering costs |
— | — | — | — | — | 22,338 | — | — | — | — | — | — | — | (22,338 | ) | (22,338 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Estimated fair value of options for common stock issued for |
— | — | — | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||
| services |
— | — | — | — | — | — | — | — | — | — | — | — | 29,850 | — | 29,850 | |||||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series A preferred stock to redemption value |
— | — | — | — | — | — | — | 896,085 | — | — | — | — | — | (896,085 | ) | (896,085 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B preferred stock to redemption value |
— | — | — | — | — | — | — | — | — | 3,888,117 | — | — | — | (3,888,117 | ) | (3,888,117 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C preferred stock to redemption value |
— | 3,878,533 | — | — | — | — | — | — | — | — | — | — | — | (3,878,533 | ) | (3,878,533 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series D preferred stock to redemption value |
— | — | — | 782,182 | — | — | — | — | — | — | — | — | — | (782,182 | ) | (782,182 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series E preferred stock to redemption value |
— | — | — | — | — | 1,344,579 | — | — | — | — | — | — | (360,799 | ) | (983,780 | ) | (1,344,579 | ) | ||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | — | — | — | — | — | — | — | — | 329,933 | — | 329,933 | |||||||||||||||||||||||||||||||||||||||||||||
| Exercise of options |
— | — | — | — | — | — | — | — | — | — | 163 | — | 1,016 | — | 1,016 | |||||||||||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | — | — | — | — | (13,724,813 | ) | (13,724,813 | ) | |||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Balance, December 31, 2011 |
31,115,107 | $ | 58,545,002 | 8,650,519 | $ | 10,609,862 | 11,665,019 | $ | 20,455,779 | 6,407,998 | $ | 13,668,815 | 28,699,551 | $ | 59,358,252 | 87,864 | $ | 88 | — | (182,396,726 | ) | (182,396,638 | ) | |||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
F-5
Table of Contents
| Redeemable, convertible Series C preferred stock |
Redeemable, convertible Series D preferred stock |
Redeemable, convertible Series E preferred stock |
Redeemable, convertible Series A preferred stock |
Redeemable, convertible Series B preferred stock |
Stockholders’ deficit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock |
Additional paid-in capital |
Accumulated Deficit |
Total stockholders’ deficit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2011 |
31,115,107 | $ | 58,545,002 | 8,650,519 | $ | 10,609,862 | 11,665,019 | $ | 20,455,779 | 6,407,998 | $ | 13,668,815 | 28,699,551 | $ | 59,358,252 | 87,864 | $ | 88 | — | (182,396,726 | ) | (182,396,638 | ) | |||||||||||||||||||||||||||||||||||||
| Accretion of Series A offering costs |
— | — | — | — | — | — | — | 7,128 | — | — | — | — | — | (7,128 | ) | (7,128 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B offering costs |
— | — | — | — | — | — | — | — | — | 17,121 | — | — | — | (17,121 | ) | (17,121 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C offering costs |
— | 170,552 | — | — | — | — | — | — | — | — | — | — | — | (170,552 | ) | (170,552 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series D offering costs |
— | — | — | 309,818 | — | — | — | — | — | — | — | — | — | (309,818 | ) | (309,818 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series E offering costs |
— | — | — | — | — | 22,338 | — | — | — | — | — | — | — | (22,338 | ) | (22,338 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Estimated fair value of options for common stock issued for services |
— | — | — | — | — | — | — | — | — | — | — | — | 66,731 | — | 66,731 | |||||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series A preferred stock to redemption value |
— | — | — | — | — | — | — | 961,438 | — | — | — | — | — | (961,438 | ) | (961,438 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B preferred stock to redemption value |
— | — | — | — | — | — | — | — | — | 4,171,684 | — | — | — | (4,171,684 | ) | (4,171,684 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C preferred stock to redemption value |
— | 4,162,304 | — | — | — | — | — | — | — | — | — | — | — | (4,162,304 | ) | (4,162,304 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series D preferred stock to redemption value |
— | — | — | 839,227 | — | — | — | — | — | — | — | — | — | (839,227 | ) | (839,227 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series E preferred stock to redemption value |
— | — | — | — | — | 1,442,641 | — | — | — | — | — | — | (390,722 | ) | (1,051,919 | ) | (1,442,641 | ) | ||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | — | — | — | — | — | — | — | — | 302,449 | — | 302,449 | |||||||||||||||||||||||||||||||||||||||||||||
| Exercise of warrants |
31,964 | 55,288 | — | — | — | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Exercise of options |
— | — | — | — | — | — | — | — | — | — | 4,566 | 4 | 21,542 | — | 21,546 | |||||||||||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | — | — | — | — | (2,014,744 | ) | (2,014,744 | ) | |||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Balance, December 31, 2012 |
31,147,071 | $ | 62,933,146 | 8,650,519 | $ | 11,758,907 | 11,665,019 | $ | 21,920,758 | 6,407,998 | $ | 14,637,381 | 28,699,551 | $ | 63,547,057 | 92,430 | $ | 92 | — | (196,124,999 | ) | (196,124,907 | ) | |||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
F-6
Table of Contents
| Redeemable, convertible Series C preferred stock |
Redeemable, convertible Series D preferred stock |
Redeemable, convertible Series E preferred stock |
Redeemable, convertible Series A preferred stock |
Redeemable, convertible Series B preferred stock |
Stockholders’ deficit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock |
Additional paid-in capital |
Accumulated Deficit |
Total stockholders’ deficit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2012 |
31,147,071 | $ | 62,933,146 | 8,650,519 | $ | 11,758,907 | 11,665,019 | $ | 21,920,758 | 6,407,998 | $ | 14,637,381 | 28,699,551 | $ | 63,547,057 | 92,430 | $ | 92 | — | (196,124,999 | ) | (196,124,907 | ) | |||||||||||||||||||||||||||||||||||||
| Accretion of Series A offering costs |
— | — | — | — | — | — | — | 7,128 | — | — | — | — | — | (7,128 | ) | (7,128 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B offering costs |
— | — | — | — | — | — | — | — | — | 17,121 | — | — | — | (17,121 | ) | (17,121 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C offering costs |
— | 170,552 | — | — | — | — | — | — | — | — | — | — | — | (170,552 | ) | (170,552 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series D offering costs |
— | — | — | 309,818 | — | — | — | — | — | — | — | — | — | (309,818 | ) | (309,818 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series E offering costs |
— | — | — | — | — | 22,338 | — | — | — | — | — | — | — | (22,338 | ) | (22,338 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Estimated fair value of options for common stock issued for services |
— | — | — | — | — | — | — | — | — | — | — | — | 27,165 | — | 27,165 | |||||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series A preferred stock to redemption value |
— | — | — | — | — | — | — | 1,026,112 | — | — | — | — | — | (1,026,112 | ) | (1,026,112 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B preferred stock to redemption value |
— | — | — | — | — | — | — | — | — | 4,452,303 | — | — | — | (4,452,303 | ) | (4,452,303 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C preferred stock to redemption value |
— | 4,444,405 | — | — | — | — | — | — | — | — | — | — | — | (4,444,405 | ) | (4,444,405 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series D preferred stock to redemption value |
— | — | — | 895,680 | — | — | — | — | — | — | — | — | — | (895,680 | ) | (895,680 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series E preferred stock to redemption value |
— | — | — | — | — | 1,539,684 | — | — | — | — | — | — | (211,514 | ) | (1,328,170 | ) | (1,539,684 | ) | ||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | — | — | — | — | — | — | — | — | 182,550 | — | 182,550 | |||||||||||||||||||||||||||||||||||||||||||||
| Exercise of options |
— | — | — | — | — | — | — | — | — | — | 382 | 1 | 1,799 | — | 1,800 | |||||||||||||||||||||||||||||||||||||||||||||
| Net income |
— | — | — | — | — | — | — | — | — | — | — | — | — | 9,476,699 | 9,476,699 | |||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Balance, December 31, 2013 |
31,147,071 | $ | 67,548,103 | 8,650,519 | $ | 12,964,405 | 11,665,019 | $ | 23,482,780 | 6,407,998 | $ | 15,670,621 | 28,699,551 | $ | 68,016,481 | 92,812 | $ | 93 | — | (199,321,927 | ) | (199,321,834 | ) | |||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
F-7
Table of Contents
| Redeemable, convertible Series C preferred stock |
Redeemable, convertible Series D preferred stock |
Redeemable, convertible Series E preferred stock |
Redeemable, convertible Series A preferred stock |
Redeemable, convertible Series B preferred stock |
Stockholders’ deficit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock |
Additional paid-in capital |
Accumulated Deficit |
Total stockholders’ deficit |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2013 |
31,147,071 | $ | 67,548,103 | 8,650,519 | $ | 12,964,405 | 11,665,019 | $ | 23,482,780 | 6,407,998 | $ | 15,670,621 | 28,699,551 | $ | 68,016,481 | 92,812 | $ | 93 | — | (199,321,927 | ) | (199,321,834 | ) | |||||||||||||||||||||||||||||||||||||
| Accretion of Series A offering costs (unaudited) |
— | — | — | — | — | — | — | 1,782 | — | — | — | — | — | (1,782 | ) | (1,782 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B offering costs (unaudited) |
— | — | — | — | — | — | — | — | — | 4,280 | — | — | — | (4,280 | ) | (4,280 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C offering costs (unaudited) |
— | 42,638 | — | — | — | — | — | — | — | — | — | — | — | (42,638 | ) | (42,638 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series D offering costs (unaudited) |
— | — | — | 77,454 | — | — | — | — | — | — | — | — | — | (77,454 | ) | (77,454 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series E offering costs (unaudited) |
— | — | — | — | — | 5,584 | — | — | — | — | — | — | — | (5,584 | ) | (5,584 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Estimated fair value of options for common stock issued for services (unaudited) |
— | — | — | — | — | — | — | — | — | — | — | — | (10,000 | ) | — | (10,000 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series A preferred stock to redemption value (unaudited) |
— | — | — | — | — | — | — | 274,485 | — | — | — | — | — | (274,485 | ) | (274,485 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B preferred stock to redemption value (unaudited) |
— | — | — | — | — | — | — | — | — | 1,190,991 | — | — | — | (1,190,991 | ) | (1,190,991 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C preferred stock to redemption value (unaudited) |
— | 1,188,878 | — | — | — | — | — | — | — | — | — | — | — | (1,188,878 | ) | (1,188,878 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series D preferred stock to redemption value (unaudited) |
— | — | — | 239,595 | — | — | — | — | — | — | — | — | — | (239,595 | ) | (239,595 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series E preferred stock to redemption value (unaudited) |
— | — | — | — | — | 411,865 | — | — | — | — | — | — | (15,826 | ) | (396,039 | ) | (411,865 | ) | ||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation (unaudited) |
— | — | — | — | — | — | — | — | — | — | — | — | 22,813 | — | 22,813 | |||||||||||||||||||||||||||||||||||||||||||||
| Exercise of options (unaudited) |
— | — | — | — | — | — | — | — | — | — | 636 | — | 3,013 | — | 3,013 | |||||||||||||||||||||||||||||||||||||||||||||
| Net loss (unaudited) |
— | — | — | — | — | — | — | — | — | — | — | — | — | (2,444,572 | ) | (2,444,572 | ) | |||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Balance, March 31, 2014 (unaudited) |
31,147,071 | $ | 68,779,619 | 8,650,519 | $ | 13,281,454 | 11,665,019 | $ | 23,900,229 | 6,407,998 | $ | 15,946,888 | 28,699,551 | $ | 69,211,752 | 93,448 | $ | 93 | — | (205,188,225 | ) | (205,188,132 | ) | |||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
See accompanying notes to financial statements.
F-8
Table of Contents
Statements of Cash Flows
| Year ended December 31, | Three Months Ended March 31 |
|||||||||||||||||||
| 2011 | 2012 | 2013 | 2013 | 2014 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Cash flows from operating activities: |
||||||||||||||||||||
| Net income (loss) |
$ | (13,724,813 | ) | (2,014,744 | ) | 9,476,699 | (1,551,572 | ) | (2,444,572 | ) | ||||||||||
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
||||||||||||||||||||
| Depreciation and amortization |
952,074 | 925,473 | 771,280 | 219,930 | 69,866 | |||||||||||||||
| Share-based compensation |
329,933 | 302,449 | 182,550 | 63,098 | 22,813 | |||||||||||||||
| Stock-based payments for services |
29,850 | 66,731 | 27,165 | 8,873 | (10,000 | ) | ||||||||||||||
| Noncash interest expense from amortization of debt discount and amortization of debt issuance costs |
— | — | 9,268 | — | 1,358,656 | |||||||||||||||
| Noncash interest expense on convertible notes |
— | — | — | — | 115,794 | |||||||||||||||
| Noncash expense (income) from change in valuation of warrants and put and call options |
1,135,032 | (1,951,490 | ) | (1,842,557 | ) | (486,581 | ) | (247,045 | ) | |||||||||||
| Gain on sale of property and equipment |
— | — | — | — | (91,284 | ) | ||||||||||||||
| Proceeds from tenant improvement reimbursement |
369,297 | — | — | — | — | |||||||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||||||||
| Increase (decrease) in other current assets |
(29,108 | ) | (464,418 | ) | (14,501 | ) | (727,352 | ) | 130,027 | |||||||||||
| Increase (decrease) in accounts payable |
182,331 | 1,642,319 | (828,883 | ) | 1,293,509 | (818,006 | ) | |||||||||||||
| Increase (decrease) in accrued liabilities |
141,984 | 287,256 | (710,820 | ) | (349,558 | ) | (201,054 | ) | ||||||||||||
| Increase (decrease) in deferred revenue |
5,892,158 | (11,725,373 | ) | (3,142,740 | ) | (10,057 | ) | (1,166,384 | ) | |||||||||||
| Increase (decrease) in other long-term liabilities |
(283,766 | ) | (136,243 | ) | 4,266 | — | — | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by (used in) operating activities |
(5,005,028 | ) | (13,068,040 | ) | 3,931,727 | (1,539,710 | ) | (3,281,189 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash flows from investing activities: |
||||||||||||||||||||
| Purchase of property and equipment |
(132,751 | ) | (108,828 | ) | (12,234 | ) | (10,235 | ) | (6,025 | ) | ||||||||||
| Sale of property and equipment |
— | — | — | — | 127,354 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by (used in) investing activities |
(132,751 | ) | (108,828 | ) | (12,234 | ) | (10,235 | ) | 121,329 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash flows from financing activities: |
||||||||||||||||||||
| Proceeds from issuance of common stock |
1,016 | 21,546 | 1,800 | — | 3,013 | |||||||||||||||
| Proceeds from exercise of warrants |
— | 3,659 | — | — | — | |||||||||||||||
| Proceeds from issuance of convertible notes payable |
— | — | — | — | 7,500,000 | |||||||||||||||
| Convertible promissory notes issuance costs |
— | — | — | — | (1,051,487 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by financing activities |
1,016 | 25,205 | 1,800 | — | 6,451,526 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net increase (decrease) in cash and cash equivalents |
(5,136,763 | ) | (13,151,663 | ) | 3,921,293 | (1,549,945 | ) | 3,291,666 | ||||||||||||
| Cash and cash equivalents, beginning of period |
20,291,374 | 15,154,611 | 2,002,948 | 2,002,948 | 5,924,241 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents, end of period |
$ | 15,154,611 | 2,002,948 | 5,924,241 | 453,003 | 9,215,907 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Supplemental disclosures of noncash investing and financing activities: |
||||||||||||||||||||
| Accretion of preferred stock (dividends) |
$ | 10,789,496 | 11,577,294 | 12,358,184 | 3,089,546 | 3,305,814 | ||||||||||||||
| Accretion of preferred stock (offering costs) |
526,957 | 526,957 | 526,957 | 131,739 | 131,738 | |||||||||||||||
| Transfer of fair value of warrants to preferred stock upon exercise |
— | 51,629 | — | — | — | |||||||||||||||
| Settlement of accounts payable through issuance of convertible note payable |
— | — | 391,730 | — | — | |||||||||||||||
| Fair value of warrants and put option issued in connection with convertible note payable |
— | — | 207,309 | — | 6,121,533 | |||||||||||||||
| Fair value of warrants issued for debt issuance costs |
— | — | — | — | 876,778 | |||||||||||||||
See accompanying notes to financial statements.
F-9
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| (1) | Organization and Nature of Business |
GlobeImmune, Inc. (the Company) was incorporated as Ceres Pharmaceuticals, Ltd. in Colorado on February 10, 1995. The Company changed its name to GlobeImmune, Inc. on May 26, 2001, and reincorporated in Delaware on June 5, 2002. The Company is a biopharmaceutical company focused on developing therapeutic products for cancer and infectious diseases based on our proprietary Tarmogen® platform. The Company has two strategic collaborations with leading biotechnology companies. In October 2011, Gilead Sciences, Inc., or Gilead, exclusively licensed product candidates to treat chronic hepatitis B virus, or HBV, infection. Celgene Corporation, or Celgene, entered into a collaboration and option agreement for certain oncology product candidates in May 2009. Under this agreement, in July 2013 Celgene exercised its option for a worldwide, exclusive license to the GI-6300 program, which is a Tarmogens program targeting the brachyury protein. The Company has four product candidates in five ongoing clinical trials.
The Company’s operations are subject to certain risks and uncertainties. The risks include negative outcome of clinical trials, inability or delay in completing clinical trials or obtaining regulatory approvals, changing market conditions for products being developed by the Company, more stringent regulatory environment, the need to retain key personnel and protect intellectual property, product liability, and the availability of additional capital financing on terms acceptable to the Company. Because of the numerous risks and uncertainties associated with biopharmaceutical product development and commercialization, the Company is unable to accurately predict the timing or amount of future expenses or when, or if, it will be able to achieve or maintain profitability. Currently, the Company has no products approved for commercial sale, and to date it has not generated any product revenue. The Company has financed its operations primarily through the sale of equity securities, upfront payments pursuant to collaboration agreements, government grants and equipment financing. The size of the Company’s future net losses will depend, in part, on the rate of growth or contraction of expenses and the level and rate of growth, if any, of revenues. The Company’s ability to achieve profitability is dependent on its ability, alone or with others, to complete the development of its product candidates successfully, obtain the required regulatory approvals, manufacture and market its proposed products successfully or have such products manufactured and marketed by others and gain market acceptance for such products. There can be no assurance as to whether or when the Company will achieve profitability.
| (2) | Liquidity Risks |
The Company has incurred operating losses and has an accumulated deficit as a result of ongoing research and development spending. As of December 31, 2013 and March 31, 2014 (unaudited), the Company had an accumulated deficit of $199,321,927 and $205,188,225, respectively. The Company had net losses of $13,724,813 and $2,014,744, net income of $9,476,699 and net losses of $1,551,572 and $2,444,572 for the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited), respectively, and net cash used in operating activities of $5,005,028 and $13,068,040, net cash provided by operating activities of $3,931,727 and net cash used in operating activities of $1,539,710 and $3,281,189 for the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited), respectively. The Company anticipates that operating losses and net cash used in operating activities will occur and substantially increase over the next several years as it expands discovery, research and development activities, including clinical development of its Tarmogen product candidates.
The Company has historically financed its operations primarily through the sale of equity securities, payments pursuant to collaboration agreements, government grants and equipment financing. The Company will continue to be dependent upon such sources of funds until it is able to generate positive cash flows from its operations. The Company believes that its existing cash and cash equivalents as of December 31, 2013 as well as
F-10
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
amounts raised subsequent to December 31, 2013 through the issuance of convertible promissory notes will be sufficient to fund operations through at least January 1, 2015.
The Company will be required to fund future operations through the sale of its equity securities, issuance of convertible debt, potential milestone payments, if achieved. and possible future collaboration. There can be no assurance that sufficient funds will be available to the Company when needed from equity or convertible debt financings, that milestone payments will be earned or that future collaboration partnerships will be entered into. If the Company is unable to obtain additional funding from these or other sources when needed, or to the extent needed, it may be necessary to significantly reduce its current rate of spending through reductions in staff and delaying, scaling back, or stopping certain research and development programs. Insufficient liquidity may also require the Company to relinquish greater rights to product candidates at an earlier stage of development or on less favorable terms to it or its stockholders than the Company would otherwise choose. These events could prevent the Company from successfully executing on its operating plan and could raise substantial doubt about the Company’s ability to continue as a going concern in future periods.
Further, the holders of the various series of redeemable, convertible preferred stock have certain redemption rights. As described in note 7, these holders have the rights beginning in January 2015 to require redemption in cash of 25% of the then outstanding shares of preferred stock, which would be approximately $47 million if calculated as of December 31, 2013. These rights exist only if the various shares of preferred stock are not converted prior to January 2015 and redemption will only be required if the holders exercise such voluntary rights.
| (3) | Summary of Significant Accounting Policies |
| (a) | Use of Estimates in the Preparation of Financial Statements |
The preparation of the financial statements in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP) requires management of the Company to make estimates and assumptions relating to the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. The most significant estimates in the accompanying financial statements relate to the estimated useful lives of property and equipment; the terms of performance under collaboration agreements; the estimated fair values of warrants for redeemable, convertible preferred stock; and the estimated fair values of share-based awards, including the estimated fair value of the underlying common stock.
(b) Unaudited Interim Financial Data
The accompanying balance sheet as of March 31, 2014, statements of operations and comprehensive income and loss and cash flows for the three months ended March 31, 2013 and 2014 are unaudited. The unaudited interim financial statements have been prepared on a basis consistent with the audited financial statements and, in the opinion of management, reflect all adjustments (consisting of normal recurring adjustments) considered necessary to state fairly the Company’s financial position as of March 31, 2014 and the results of operations and cash flows for the three months ended March 31, 2013 and 2014. The financial data and other information disclosed in these notes to the financial statements related to the three months ended March 31, 2013 and 2014 and the unaudited statement of redeemable convertible preferred stock and stockholders’ deficit for the three months ended March 31, 2014 are unaudited. The results for the three months ended March 31, 2014 are not necessarily indicative of the results to be expected for the year ending December 31, 2014 or for any other interim period.
F-11
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| (c) | Reverse Stock Split |
On April 25, 2014, the Company affected a 1-for-4.3 reverse stock split of the Company’s common stock after approval by the Company’s stockholders. In connection with the reverse stock split, the Company filed a Certificate of Amendment of its Restated Certificate of Incorporation with the Secretary of State of Delaware on April 25, 2014 affecting the reverse stock split. This reverse stock split has been reflected retroactively for all periods presented in the financial statements.
| (d) | Cash and Cash Equivalents |
The Company considers all highly liquid instruments purchased with original maturity dates of 90 days or less to be cash equivalents. The Company places its temporary cash investments on deposit with financial institutions it believes to be of high quality. Cash equivalents have maturities of 90 days or less, and their carrying amounts approximate fair value.
| (e) | Concentrations of Credit Risk |
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents. The Company has established guidelines to limit its exposure to credit risk by placing investments with high credit quality financial institutions, diversifying its investment portfolio, and making investments with maturities that maintain safety and liquidity. At December 31, 2012 and 2013 and March 31, 2014 (unaudited), the Company’s cash equivalents were with money market funds that invest in securities issued by the U.S. Treasury.
| (f) | Property and Equipment |
Property and equipment are recorded at cost. Maintenance and repairs are charged to expense as incurred, and major additions, replacements, and improvements are capitalized. Leasehold improvements are amortized over the shorter of the related lease term or the estimated useful life.
Depreciation of property and equipment is provided using the straight-line method over the estimated useful lives as follows:
| Furniture and fixtures | 7 years | |
| Leasehold improvements | Lesser of useful life or life of the lease | |
| Laboratory machinery and equipment | 5 years | |
| Computer equipment | 3 years |
The cost of assets sold and the related accumulated depreciation are removed from the accounts, and the resulting gain or loss is reflected in the statements of operations and comprehensive loss in the period in which such sale or disposition occurs. Depreciation and amortization expense for 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited) was $952,074, $925,473, $771,280, $219,930 and $69,866, respectively.
F-12
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| (g) | Impairment of Long-Lived Assets |
The Company reviews its long-lived assets, consisting of property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable from future undiscounted cash flows. Impairment losses are recorded for the difference between the carrying value and the estimated fair value of the long-lived assets. The Company has not yet generated consistent positive cash flows on an annual basis, and such positive cash flows may not materialize for a significant period in the future. As a result, it is reasonably possible that future evaluations of long-lived assets may result in a conclusion that such assets have been impaired.
| (h) | Other Assets |
As of December 31, 2012, other assets consisted of restricted cash collateralizing a $100,000, irrevocable standby letter of credit as security on a lease agreement for the corporate headquarters building. The letter of credit expired on October 31, 2013. As of December 31, 2013 and March 31, 2014 (unaudited), other assets consisted of a security deposit for the lease agreement for the corporate headquarters building.
| (i) | Accrued Liabilities |
The Company makes estimates of its accrued expenses by identifying services that have been performed on its behalf and estimating the level of service performed and the associated cost incurred for the service when it has not yet been invoiced or otherwise notified of actual cost. The majority of the Company’s service providers invoice it monthly in arrears for services performed. Examples of estimated accrued expenses include:
| • | fees owed to contract research organizations in connection with preclinical and toxicology studies and clinical trials; |
| • | fees owed to investigative sites in connection with clinical trials; |
| • | fees owed to contract manufacturers in connection with the production of clinical trial materials; |
| • | fees owed for professional services; |
| • | property taxes; and |
| • | unpaid salaries, wages, and benefits. |
Accrued liabilities consisted of the following as of:
| December 31, | March 31, 2014 |
|||||||||||
| 2012 | 2013 | |||||||||||
| (unaudited) | ||||||||||||
| Accrued compensation |
$ | 420,703 | 278,108 | 299,724 | ||||||||
| Accrued clinical trial holdbacks |
151,255 | 126,455 | 111,186 | |||||||||
| Deferred rent-short term |
136,243 | — | — | |||||||||
| Deposits from customer |
279,000 | — | — | |||||||||
| Income taxes |
— | 115,765 | — | |||||||||
| Other |
644,581 | 400,634 | 308,998 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total accrued liabilities |
$ | 1,631,782 | 920,962 | 719,908 | ||||||||
|
|
|
|
|
|
|
|||||||
F-13
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
As of December 31, 2012 and 2013 and March 31, 2014 (unaudited), accrued liabilities included $151,255, $126,455 and $111,186 respectively, of holdbacks representing five percent of payments due to entities conducting clinical trials for patient-related fees. The holdbacks will be paid upon completion of the studies and when all data has been received and validated by the Company.
| (j) | Deferred Revenue |
The Company records amounts received but not earned under its collaboration agreements as deferred revenue, which are then classified as either current or long-term in the accompanying balance sheets based on the period during which they are expected to be recognized as revenue.
| (k) | Stock-Based Compensation |
The Company accounts for stock compensation pursuant to ASC Topic 718, Compensation-Stock Compensation. The Company applies the straight-line attribution method and, accordingly, amortizes the fair value of each option over the requisite service period through the last separately vesting portion of the award. Employee stock options granted by the Company are generally structured to qualify as “incentive stock options” (ISOs). Under current tax regulations, the Company does not receive a tax deduction for the issuance, exercise, or disposition of ISOs if the employee meets certain holding requirements. If the employee does not meet the holding requirements, a disqualifying disposition occurs, at which time the Company may receive a tax deduction. The Company does not record tax benefits related to ISOs unless and until a disqualifying disposition occurs.
The Company accounts for stock options issued to nonemployees in accordance with the provisions of ASC Topic 718 and ASC Subtopic 505-50, Equity-Based Payments to Nonemployees , which requires valuing the stock options using a Black-Scholes option pricing model and remeasuring such stock options to their current fair value until the performance date has been reached.
| (l) | Income Taxes |
The Company accounts for income taxes pursuant to ASC Topic 740, Income Taxes, which requires the use of the asset and liability method of accounting for deferred income taxes. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and for operating loss and tax credit carryforwards. A valuation allowance is recorded to the extent it is more likely than not that a deferred tax asset will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. As of December 31, 2012 and 2013 and March 31, 2014 (unaudited), the Company has no unrecognized uncertain tax positions.
| (m) | Fair Value of Financial Instruments |
The carrying amounts of financial instruments, including cash and cash equivalents, restricted cash, accrued compensation, accrued clinical holdbacks and accounts payable, approximate fair value due to their short-term maturities. The carrying amount of the convertible note payable approximates its fair value as its terms are comparable to what would be included in similar debt instruments.
F-14
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
The Company accounts for its preferred stock warrants pursuant to ASC Topic 480, Distinguishing Liabilities from Equity, and classifies warrants for redeemable preferred stock as liabilities. The warrants are reported as short-term or long-term liabilities, depending on their remaining term, at their estimated fair value at December 31, 2012 and 2013 and March 31, 2014 (unaudited), and any changes in fair value are reflected in changes in value of warrants.
The fair value of all the outstanding warrants at December 31, 2012 and 2013 and March 31, 2014 (unaudited) was $3,301,411, $1,564,941 and $6,520,835, respectively (see note 7).
| (n) | Segment Information |
The Company operates in one segment, which is the business of developing and commercializing various biopharmaceutical products.
| (o) | Research and Development Expenses |
During 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited), the Company incurred $12,063,403, $11,734,551, $10,884,628, $3,213,407 and $1,538,779, respectively, in expenses relating to research and development. Research and development costs are expensed as incurred and include costs of collaboration license and services, cost of manufacturing services and research and development for proprietary programs. These costs consist primarily of salaries, supplies, and contract services relating to the development of new products and technologies.
The Company contracts with third parties to perform a range of clinical trial activities in the ongoing development of its product candidates. The terms of these agreements vary and may result in uneven payments. Payments under these contracts depend on factors such as the progress toward achievement of certain defined milestones, the successful enrollment of patients, and other events.
| (p) | Comprehensive Income (Loss) |
Comprehensive income (loss), as defined, includes all changes in equity during a period from nonowner sources. Net income or loss is the Company’s only component of comprehensive income or loss for the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited).
| (q) | Revenue Recognition |
In 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited), the Company’s revenue was in the form of upfront fees derived from the discovery, development and commercialization of multiple product candidates based on targeted molecular immunotherapy for the treatment of cancer and infectious diseases and fees for manufacturing services for the Company’s collaborators. The Company’s agreements with its collaborators include fees based on a nonrefundable upfront fee, nonrefundable milestone payments that are triggered upon achievement of specific development or regulatory goals, and future royalties on sales of products that result from the collaboration (see note 8(a) and 8(b)) and fees for manufacturing services.
The Company recognizes revenue in accordance with ASC Topic 605 Revenue Recognition (ASC 605). ASC 605 establishes four criteria, each of which must be met, in order to recognize revenue related to the performance of services or the shipment of products. Revenue is recognized when (a) persuasive evidence of an arrangement exists, (b) products are delivered or services are rendered, (c) the sales price is fixed or determinable, and (d) collectability is reasonably assured.
F-15
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
The Company evaluates the deliverables under our multiple-element arrangements to determine if they meet the separation criteria in ASC 605-25 and have stand-alone value. The Company allocates revenue to each identified deliverable based on its estimated stand-alone value in relation to the combined estimated stand-alone value of all deliverables, otherwise known as the relative selling price method. The allocated consideration for each deliverable is then recognized over the related obligation period for that deliverable. The Company treats deliverables in an arrangement that do not meet the separation criteria as a single unit of accounting, generally applying applicable revenue recognition guidance for the final deliverable to the combined unit of accounting.
The Company recognizes revenue from nonrefundable upfront payments over the estimated term of performance under the agreements. Since the term is not specifically identifiable in the agreements, management has estimated the performance terms based on the likelihood and forecasted achievement of development commitments, and other significant commitments of the Company. These advance payments are deferred and recorded as deferred revenue upon receipt, pending recognition, and are classified as a short-term or long-term liability in the accompanying balance sheets.
Management evaluates the likely performance period under its collaboration agreements on a periodic basis. If there are changes to the estimated performance periods as a result of the outcome of certain events, the period over which the nonrefundable upfront payments are recognized will be adjusted prospectively. The events that will impact the estimation of the performance period include, among others, the success of the drug candidate programs and the likelihood of the collaborator exercising their options under the collaboration agreement.
The Company’s collaboration agreements provide for milestone payments and royalties on future sales. In accordance with the milestone method, each substantive milestone payment is recognized as revenue when the specific milestone is achieved and royalties are recorded when earned.
Revenue recognition related to upfront payments and to milestone payments could be accelerated in the event of early termination of drug programs or, alternatively, decelerated if programs are extended. As such, while changes to such estimates have no impact on its reported cash flows, the Company’s reported revenue is significantly influenced by its estimates of the period over which its obligations are expected to be performed.
The Company categorizes its revenues into collaboration license and services, milestones and manufacturing services, substantially all of which come from its collaboration partners.
In 2013, the Company received a $4 million Research Project Grant by the National Institute of Allergy and Infectious Diseases, or NIAID, of the National Institute of Health, or NIH, to support the development of Tarmogen immunotherapy product candidates to treat and prevent tuberculosis infection. This work for this grant will be performed and reimbursed over four years. The Company recognizes revenue from this grant as work is performed.
| (r) | Net Loss per Share |
Basic net loss per share is computed by dividing net loss applicable to common stockholders for the period by the weighted average number of common shares outstanding during the period. Diluted net loss per share reflects the additional dilution from potential issuances of common stock, such as stock issuable pursuant to the exercise of stock options and warrants. The treasury stock method is used to calculate the potential dilutive effect of these common stock equivalents. Potentially dilutive shares are excluded from the computation of diluted net loss per share when their effect is anti-dilutive. In the periods with net losses, all potentially dilutive securities were anti-dilutive and therefore have been excluded from the computation of diluted net loss per share.
F-16
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| Years ended December 31, | Three Months Ended March 31, | |||||||||||||||||||
| 2011 | 2012 | 2013 | 2013 | 2014 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Historical net loss per share: |
||||||||||||||||||||
| Numerator: |
||||||||||||||||||||
| Net loss attributable to GlobeImmune, Inc. common stockholders |
$ | (25,041,266 | ) | (14,118,995 | ) | (3,408,442 | ) | (4,772,857 | ) | (5,882,124 | ) | |||||||||
| Denominator: |
||||||||||||||||||||
| Weighted-average common shares used in computing net loss per share of common stock - basic and diluted |
87,853 | 89,377 | 92,522 | 92,430 | 93,201 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per share of common stock attributable to GlobeImmune, Inc. stockholders - basic and diluted |
$ | (285.04 | ) | (157.97 | ) | (36.84 | ) | (51.64 | ) | (63.11 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Pro forma net income per share (unaudited): |
||||||||||||||||||||
| Numerator: |
||||||||||||||||||||
| Net loss attributable to GlobeImmune, Inc. common stockholders |
$ | (3,408,442 | ) | $ | (5,882,124 | ) | ||||||||||||||
| Deduct: income on change in fair value of warrant and put and call option liability |
(1,842,557 | ) | (247,045 | ) | ||||||||||||||||
| Add back: interest expense on convertible promissory notes |
13,534 | (*) | ||||||||||||||||||
| Add back: Preferred stock dividends and accretion of offering costs to redemption value |
12,885,141 | 3,437,552 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Net income (loss) used in computing pro forma net income per share of common stock attributable to GlobeImmune, Inc. stockholders—diluted |
$ | 7,647,676 | $ | (2,691,617 | ) | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Denominator: |
||||||||||||||||||||
| Basic and diluted weighted-average common shares, as used above |
92,522 | 93,201 | ||||||||||||||||||
| Add back: pro forma adjustment to reflect weighted-average of assumed conversion of convertible preferred stock (1) |
2,757,825 | |
2,757,825 |
| ||||||||||||||||
| Add back: pro forma adjustment to reflect weighted-average convertible promissory note on an if-converted basis |
5,570 | (*) | ||||||||||||||||||
| Add back: incremental stock options on a treasury stock method |
89,130 | (*) | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Weighted-average shares used in computing pro forma diluted net income per common share |
2,945,047 | 2,851,026 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Pro forma net income per share of common stock attributable to GlobeImmune, Inc. stockholders -diluted |
$ | 2.60 | $ | (0.94 | ) | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
| (*) | Balance and shares were excluded for the three months ended March 31, 2014 calculation as they were anti-dilutive. |
F-17
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| (1) | The pro forma net income per share reflects the assumed conversion of all outstanding shares of redeemable, convertible preferred stock as of December 31, 2013 and March 31, 2014 into 2,757,825 shares, respectively, of common stock which will occur upon the closing of the Company’s proposed initial public offering. The numerator in the pro forma diluted net income per share calculation has been adjusted to remove the preferred stock dividends and accretion of offering costs to redemption value as this charge will be eliminated upon the automatic conversion of all outstanding shares of redeemable, convertible preferred stock. In addition, the numerator in the pro forma diluted net income per share calculation has been adjusted for income resulting from remeasurement of the preferred stock warrant liability as these measurements would no longer be required when the preferred stock warrants become warrants to purchase shares of the Company’s common stock upon the closing of the Company’s proposed initial public offering. |
The following potentially dilutive securities were excluded from the calculation of diluted net loss per share during each period as the effect was anti-dilutive:
| Years Ended December 31, | Three Months Ended March 31, | |||||||||||||||||||
| 2011 | 2012 | 2013 | 2013 | 2014 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Weighted-average convertible preferred stock upon conversion to common stock (as converted basis) * |
2,756,808 | 2,757,113 | 2,757,825 | 2,757,825 | 2,757,825 | |||||||||||||||
| Weighted-average warrants to purchase convertible preferred stock (as converted basis) * |
110,513 | 108,986 | 106,426 | 105,396 | 496,096 | |||||||||||||||
| Outstanding stock options to purchase common stock at period-end |
362,274 | 376,880 | 229,494 | 367,758 | 235,342 | |||||||||||||||
| Weighted-average convertible promissory note upon conversion to common stock (as converted basis) |
— | — | 4,291 | — | 484,732 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
3,229,595 | 3,242,979 | 3,098,036 | 3,230,979 | 3,973,995 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| * | The convertible preferred stock and convertible preferred stock warrants were computed on an as converted basis using a 31.39-to-one conversion ratio for all periods presented. See note 7 for conversion ratio adjustments that may be applicable upon future events. |
| (s) | Recently Issued Accounting Standards |
In July 2013, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update “Income Taxes (Topic 740): Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists” (ASU 2013-11). ASU 2013-11 requires an entity to present an unrecognized tax benefit as a reduction of a deferred tax asset for a net operating loss (NOL) carryforward, or similar tax loss or tax credit carryforward, rather than as a liability when (1) the uncertain tax position would reduce the NOL or other carryforward under the tax law of the applicable jurisdiction and (2) the entity intends to use the deferred tax asset for that purpose. ASU 2013-11 is effective prospectively for fiscal years and interim periods within those years, beginning after December 15, 2013 for public entities. Early adoption and retrospective application are permitted. The adoption of ASU 2013-11 did not have a material impact on our financial position or results of operations.
F-18
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
As an emerging growth company under the Jumpstart Our Business Startups Act of 2012, or JOBS Act, the Company has elected to opt out of the extended transition period for complying with new or revised accounting standards pursuant to Section 107(b) of the JOBS Act. This election is irrevocable.
| (t) | Fair Value Measurements |
In general, asset and liability fair values are determined using the following categories:
Level 1 – inputs utilize quoted prices in active markets for identical assets or liabilities.
Level 2 – inputs include quoted prices for similar assets or liabilities in active markets, and inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly.
Level 3 – inputs are unobservable inputs and include situations where there is little, if any, market activity for the balance sheet items at period end. Pricing inputs are unobservable for the terms and are based on the Company’s own assumptions about the assumptions that a market participant would use.
The Company’s financial instruments, including money market investments, warrants, and put options are measured at fair value on a recurring basis. The carrying amount of money market investments as of December 31, 2012 and 2013 approximates fair value based on quoted prices in active markets, or Level 1 inputs. The carrying amount of outstanding warrants and put options as of December 31, 2012 and 2013 and March 31, 2014 (unaudited) approximates fair value based on unobservable inputs, or Level 3 inputs, using assumptions made by the Company, including pricing, volatility, and expected term. There were no transfers between levels for the years ended December 31, 2011, 2012 and 2013 and the three months ended March 31, 2013 and 2014 (unaudited).
Assets and liabilities measured at fair value on a recurring basis consisted of the following types of instruments as of December 31, 2012 and 2013 and March 31, 2014 (unaudited):
| Description |
December 31, 2012 |
Quoted prices in active markets for identical assets (Level 1) |
Significant unobservable inputs (Level 3) |
December 31, 2013 |
Quoted prices in active markets for identical assets (Level 1) |
Significant unobservable inputs (Level 3) |
March 31 2014 |
Quoted prices in active markets for identical assets (Level 1) |
Significant unobservable inputs (Level 3) |
|||||||||||||||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||||||||||||||||||||||||||||||
| Assets measured at fair value: |
||||||||||||||||||||||||||||||||||||
| Money market investments (included in cash and cash equivalents) |
$ | 21,172 | 21,172 | — | 20,672 | 20,672 | — | 20,547 | 20,547 | — | ||||||||||||||||||||||||||
| Liabilities measured at fair value: |
||||||||||||||||||||||||||||||||||||
| Warrants (included in fair value of warrants) |
3,301,411 | — | 3,301,411 | 1,564,941 | — | 1,564,941 | 6,520,835 | — | 6,520,835 | |||||||||||||||||||||||||||
| Put and call options (included in fair value of put and call options and other long-term liabilities) |
— | — | — | 101,222 | — | 101,222 | 1,896,594 | — | 1,896,594 | |||||||||||||||||||||||||||
F-19
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
A reconciliation of the beginning and ending balances of the Company’s assets and liabilities measured at fair value using significant unobservable, or Level 3, inputs is as follows:
| Warrants | Put and call options | |||||||
| Balance of liability at December 31, 2010 |
$ | (4,169,498 | ) | — | ||||
| Loss included in net loss: |
||||||||
| Loss due to change in fair value |
(1,135,032 | ) | — | |||||
|
|
|
|
|
|||||
| Balance of liability at December 31, 2011 |
(5,304,530 | ) | — | |||||
| Transfer of fair value of warrants to preferred stock upon exercise |
51,629 | — | ||||||
| Income included in net loss: |
||||||||
| Income due to change in fair value |
1,951,490 | — | ||||||
|
|
|
|
|
|||||
| Balance of liability at December 31, 2012 |
(3,301,411 | ) | — | |||||
| Issuance of warrants in connection with convertible promissory notes |
(106,087 | ) | — | |||||
| Issuance of put options in connection with convertible promissory notes |
— | (101,222 | ) | |||||
| Income included in net income: |
||||||||
| Income due to change in fair value |
1,842,557 | — | ||||||
|
|
|
|
|
|||||
| Balance of liability at December 31, 2013 |
(1,564,941 | ) | (101,222 | ) | ||||
| Issuance of warrants in connection with convertible promissory notes (unaudited) |
(5,331,175 | ) | — | |||||
| Issuance of put and call options in connection with convertible promissory notes (unaudited) |
— | (1,667,136 | ) | |||||
| Income (loss) included in net loss: |
||||||||
| Income (loss) due to change in fair value (unaudited) |
375,281 | (128,236 | ) | |||||
|
|
|
|
|
|||||
| Balance of liability at March 31, 2014 (unaudited) |
$ | (6,520,835 | ) | (1,896,594 | ) | |||
|
|
|
|
|
|||||
Gains (losses) included in net income and loss for the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited) are reported in change in value of warrants.
| (4) | Cash and Cash Equivalents |
The following is a summary of cash and cash equivalents and their fair values at:
| Amortized cost |
Unrealized gains |
Unrealized losses |
Fair market value |
|||||||||||||
| December 31, 2012: |
||||||||||||||||
| Cash |
$ | 1,981,776 | — | — | 1,981,776 | |||||||||||
| Money market funds |
21,172 | — | — | 21,172 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total for December 31, 2012 |
$ | 2,002,948 | — | — | 2,002,948 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| December 31, 2013: |
||||||||||||||||
| Cash |
$ | 5,903,569 | — | — | 5,903,569 | |||||||||||
| Money market funds |
20,672 | — | — | 20,672 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total for December 31, 2013 |
$ | 5,924,241 | — | — | 5,924,241 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| March 31, 2014 |
||||||||||||||||
| Cash (unaudited) |
$ | 9,195,360 | — | — | 9,195,360 | |||||||||||
| Money market funds (unaudited) |
20,547 | — | — | 20,547 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total for March 31, 2014 (unaudited) |
$ | 9,215,907 | — | — | 9,215,907 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-20
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| (5) | Property and Equipment |
Property and equipment consist of the following at:
| December 31, | March 31 2014 |
|||||||||||
| 2012 | 2013 | |||||||||||
| (unaudited) | ||||||||||||
| Furniture and fixtures |
$ | 212,471 | 212,471 | 212,471 | ||||||||
| Leasehold improvements |
7,562,560 | 7,563,511 | 7,563,511 | |||||||||
| Laboratory equipment |
3,716,670 | 3,726,953 | 3,607,380 | |||||||||
| Computer and office equipment |
594,872 | 595,872 | 595,872 | |||||||||
|
|
|
|
|
|
|
|||||||
| 12,086,573 | 12,098,807 | 11,979,234 | ||||||||||
| Less accumulated depreciation |
(10,834,725 | ) | (11,606,005 | ) | (11,586,343 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,251,848 | 492,802 | 392,891 | |||||||||
|
|
|
|
|
|
|
|||||||
| (6) | Convertible Notes |
2013 Notes
In November 2013, the Company entered into an unsecured convertible promissory note (Note) with a service provider (Holder) in settlement of $391,730 of accounts payable. The Note bears an interest rate of 8.0%, has a term of three years and can be prepaid at any time. The Note and unpaid accrued interest will convert, upon the occurrence of certain events described in the Note, into common stock, Series E preferred stock or a newly issued series of preferred stock at a 20% discount. At maturity, if the Note and accrued interest is still outstanding, the Note will convert into Series E Preferred Stock at a 20% discount of the then fair value as determined by a independent, third party valuation firm. The Holder will ultimately receive a warrant, equal to 30% of the principal balance of the Note, to purchase common stock, Series E preferred stock or a newly issued series of preferred stock, for 10 years, depending upon the event causing the warrant issuance, with an exercise equal to the conversion price. Due to the warrants required to be issued at a future date, they have been accounted for as if they were issued as of the date of the Note.
The Company recorded the proceeds from the Note based on the fair value of the warrants ($106,087), put option embedded in the Note ($101,222) and the Note and, as such, recorded a debt discount of $207,309 for the allocated value of the warrants and put option. This debt discount is being amortized to interest expense over the term of the Note. Amortization of $9,268 and $17,024 was recorded in the year ended December 31, 2013 and the three months ended March 31, 2014 (unaudited), respectively, and the unamortized balance of the debt discount was $198,041 and $181,017 as of December 31, 2013 and March 31, 2014 (unaudited), respectively. As of December 31, 2013 and March 31, 2014 (unaudited), the Note balance includes accrued interest of $4,266 and $12,100, respectively.
The estimated fair value of the Note as of December 31, 2013 and March 31, 2014 (unaudited) approximated its carrying value.
2014 Convertible Notes (unaudited)
In January and February 2014, the Company entered into unsecured convertible notes (2014 Notes) with various holders for a total of $7,500,000. The 2014 Notes bear an interest rate of 10.0% and have a maturity date of January 31, 2015. The Company cannot make any payments of principal and interest without the consent of a
F-21
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
majority of the holders. The 2014 Notes are not convertible at the option of the holders. The 2014 Notes and unpaid accrued interest will convert upon the occurrence of an initial public offering into common stock at a price equal to 70% of the price at which the Company’s common stock is first offered to the public. At maturity, if the 2014 Notes and accrued interest are still outstanding, the Company has the option of paying the outstanding principal and accrued interest. If the Company chooses not to make payment on the 2014 Notes at maturity, the 2014 Notes and accrued interest will automatically convert into a newly issued series of redeemable convertible preferred stock at a conversion price that will be based on various factors including a $45 million pre-money valuation of the Company and the issuance price of subsequent rounds of financing, if any.
The holders of the 2014 Notes received warrants, equal to 100 % of the principal amount of the 2014 Notes to purchase equity securities of the Company for a five-year period. In the event of an initial public offering, the warrants will be exercisable into common stock with an exercise price equal to the price at which the Company’s common stock is first offered to the public. If an initial public offering does not occur prior to maturity, the principal balance of the 2014 Notes will be exercisable into a newly issued series of redeemable convertible preferred stock at an exercise price equal to either the issuance price of a subsequent round of financing, if any, or at an exercise price based on a $45 million pre-money valuation of the Company.
The Company recorded the proceeds from the 2014 Notes based on the fair value of the warrants ($4,454,397), the net of the put and call option embedded in the 2014 Notes ($1,667,136) and the 2014 Notes. As such, the Company recorded a debt discount of $6,121,533 from the allocated value of the warrants and the put and call options. This debt discount is being amortized to interest expense over the term of the 2014 Notes. Amortization of $1,020,255 was recorded in the three-month period ended March 31, 2014 and the unamortized balance of the debt discount was $5,101,278 as of March 31, 2014. As of March 31, 2014, the 2014 Notes balance includes accrued interest of $107,960.
The Company incurred $1,928,265 of debt issuance costs related to the 2014 Notes. Included in the debt issuance costs were warrants issued to the placement agent that had an estimated value of $876,778 at issuance. These costs are included in debt issuance costs and are being amortized to interest expense over the term of the 2014 Notes. Amortization of $321,378 was recorded in the three-month period ended March 31, 2014 and the unamortized balance of the debt issuance costs was $1,606,888 as of March 31, 2014.
The estimated fair value of the 2014 Notes as of March 31, 2014 approximated its carrying value.
| (7) | Redeemable, Convertible Preferred Stock and Stockholders’ Equity |
| (a) | Stockholders Agreement |
All stockholders are party to a stockholders agreement that significantly restricts the transferability of shares of the Company’s capital stock and provides for other corporate governance matters. The stockholders agreement also gives the Company right of first offer on the purchase of shares from stockholders.
| (b) | Series C Redeemable, Convertible Preferred Stock |
In the third quarter of 2007, the Company issued 28,482,897 shares of Series C redeemable, convertible (Series C) preferred stock for cash of $1.445 per share. Each share is currently convertible into shares of common stock on approximately a 31.39-for-one basis. In addition, the Series C preferred stock will automatically convert to common stock upon the completion of a qualified financing transaction. Holders of Series C preferred stock possess
F-22
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
certain rights, including, among others, preference in liquidation (including a sale of the Company), antidilution protection, and preemptive rights relative to holders of common stock, Series A redeemable, convertible (Series A) preferred stock and Series B redeemable, convertible (Series B) preferred stock. In the event of a liquidation event, holders of Series C preferred stock shall be entitled to receive the amount of the original purchase price, plus 7% per annum compounded annually plus accrued but unpaid dividends. Each holder of Series C preferred stock is entitled to receive, if and when declared, payment of an equivalent per share dividend based on the number of common shares into which each share of preferred stock is convertible, as of the date of declaration. The rate of conversion of Series C preferred stock into common stock shall be adjusted in the event the Company issues dilutive shares of common stock according to a formula defined in the Company’s Restated Certificate of Incorporation. Holders of Series C preferred stock are entitled to vote as though the preferred stock was converted into common stock. Holders of Series C preferred stock are entitled to present a redemption request to the Company in January 2015 for redemption of 25% of their total cumulative holdings per year, in the amount of the original purchase price plus 7% per annum compounded annually plus accrued but unpaid dividends.
During March and April 2009, the Company issued $2,999,989 of unsecured convertible promissory notes at an interest rate of 8.0%. Upon the closing of the sale of Series D redeemable, convertible (Series D) preferred stock, these notes and the accrued interest converted to 2,632,210 shares of Series C preferred stock at $1.156 per share. The existing stockholders waived their antidilution rights as part of the issuance of the notes. In connection with the issuance and conversion of these unsecured convertible promissory notes, the Company issued warrants to purchase 622,826 shares of Series C preferred stock for $1.445 per share. At the date of issuance, the Company estimated the value of the warrants at $603,767 using the Black-Scholes option pricing model, and the following assumptions: 10-year term, 87.5% volatility, and a risk-free interest rate of 2.87%.
During 2012, a warrant for 158,129 shares was net exercised for 29,432 shares of Series C preferred stock. The fair value of the warrant at the exercise date was reclassified to redeemable convertible preferred stock Series C.
During 2012, a warrant for 2,532 shares was exercised for Series C preferred stock at an exercise price of $1.445 per warrant. The total proceeds of $3,659 were recorded as redeemable convertible preferred stock Series C and the fair value of the warrant at the exercise date was reclassified to redeemable convertible preferred stock Series C.
| (c) | Series D Redeemable, Convertible Preferred Stock |
In May 2009, the Company issued 8,650,519 shares of Series D preferred stock for $10,000,000 at $1.156 per share with Celgene. In connection with the closing, the existing stockholders waived their antidilution rights. Each share is currently convertible into shares of common stock on approximately a 31.39-for-one basis. In addition, the Series D preferred stock will automatically convert to common stock upon the completion of a qualified financing transaction. Holders of Series D preferred stock possess certain rights, including, among others, preference in liquidation (including a sale of the Company), antidilution protection, and preemptive rights relative to holders of common stock, Series A preferred stock and Series B preferred stock. In the event of a liquidation event, holders of Series D preferred stock shall be entitled to receive the amount of the original purchase price, plus 7% per annum compounded annually plus accrued but unpaid dividends. Each holder of Series D preferred stock is entitled to receive, if and when declared, payment of an equivalent per share dividend based on the number of common shares into which each share of preferred stock is convertible, as of the date of declaration. The rate of conversion of Series D preferred stock into common stock shall be adjusted in the event the Company issues dilutive shares of common stock according to a formula defined in the Company’s Restated Certificate of Incorporation. Holders of Series D preferred stock are entitled to vote as though the preferred stock was converted into common stock.
F-23
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
Holders of Series D preferred stock are entitled to present a redemption request to the Company in January 2015 for redemption of 25% of their total cumulative holdings per year, in the amount of the original purchase price plus 7% per annum compounded annually plus accrued but unpaid dividends.
In connection with the closing, the Company also issued warrants to purchase 2,076,125 shares of Series C preferred stock for $1.445 per share. The warrants were accounted for as a preferred stock issuance cost, with the estimated fair value of $2,052,662 determined using the Black-Scholes option pricing model, and the following assumptions: 10-year term, 90.5% volatility, and a risk-free interest rate of 3.29%.
| (d) | Series E Redeemable, Convertible Preferred Stock |
In January 2010, the Company issued 11,665,019 shares of Series E redeemable, convertible (Series E) preferred stock for approximately $17,999,124 at $1.543 per share. Each share is currently convertible into shares of common stock on approximately a 31.39-for-one basis. In addition, the Series E preferred stock will automatically convert to common stock upon the completion of a qualified financing transaction. Holders of Series E preferred stock possess certain rights, including, among others, preference in liquidation (including a sale of the Company), antidilution protection, and preemptive rights relative to holders of common stock, Series A preferred stock and Series B preferred stock. In the event of a liquidation event, holders of Series E preferred stock shall be entitled to receive the amount of the original purchase price, plus 7% per annum compounded annually plus accrued but unpaid dividends. Each holder of Series E preferred stock is entitled to receive, if and when declared, payment of an equivalent per share dividend based on the number of common shares into which each share of preferred stock is convertible, as of the date of declaration. The rate of conversion of Series E preferred stock into common stock shall be adjusted in the event the Company issues dilutive shares of common stock according to a formula defined in the Company’s Restated Certificate of Incorporation. Holders of Series E preferred stock are entitled to vote as though the preferred stock was converted into common stock. Holders of Series E preferred stock are entitled to present a redemption request to the Company in January 2015 for redemption of 25% of their total cumulative holdings per year, in the amount of the original purchase price plus 7% per annum compounded annually plus accrued but unpaid dividends.
| (e) | Series A Redeemable, Convertible Preferred Stock |
In June and October 2003, the Company issued 3,999,999 shares of Series A preferred stock for cash of $1.25 per share and the conversion of $100,000 in aggregate principal amount of convertible promissory notes and $1,532 in aggregate accrued interest thereon. In September 2004, the Company issued 2,399,999 shares of Series A preferred stock for cash of $1.25 per share. Each share is currently convertible into shares of common stock on approximately a 31.39-for-one basis. In addition, the Series A preferred stock will automatically convert to common stock upon the completion of a qualified financing transaction. Holders of Series A preferred stock possess certain rights including, among others, preference in liquidation (including a sale of the Company), antidilution protection, and preemptive rights relative to holders of common stock. In the event of a liquidation event, holders of Series A preferred stock shall be entitled to receive the amount of the original purchase price, plus 7% per annum compounded annually plus accrued but unpaid dividends. Each holder of Series A preferred stock is entitled to receive, if and when declared, payment of an equivalent per share dividend based on the number of common shares into which each share of preferred stock is convertible, as of the date of declaration. The rate of conversion of Series A preferred stock into common stock shall be adjusted in the event the Company issues dilutive shares of common stock according to a formula defined in the Company’s Restated Certificate of Incorporation. Holders of Series A preferred stock are entitled to vote as though the preferred stock was converted into common stock. Holders of Series A preferred stock are entitled to present a redemption request to
F-24
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
the Company in January 2015 for redemption of 25% of their total cumulative holdings per year, in the amount of the original purchase price plus 7% per annum compounded annually plus accrued but unpaid dividends.
During 2010, a warrant for 8,000 shares was exercised for Series A preferred stock at an exercise price of $1.25 per warrant. The total proceeds of $10,000 was recorded as redeemable convertible preferred stock Series A and the fair value of the warrant at the exercise date was reclassified to redeemable convertible preferred stock Series A.
| (f) | Series B Redeemable, Convertible Preferred Stock |
During 2005, the Company closed on several issuances of Series B preferred stock for cash and convertible promissory notes payable at $1.338 per share as follows:
| Amount | Shares | |||||||||
| Initial closing |
June 2005 | 25,050,000 | 18,721,973 | |||||||
| Second closing |
July 2005 | 3,500,001 | 2,615,845 | |||||||
| Third closing |
August 2005 | 4,750,000 | 3,550,073 | |||||||
| Fourth closing |
September 2005 | 1,000,000 | 747,384 | |||||||
| Fifth closing |
September 2005 | 2,000,000 | 1,494,768 | |||||||
| Sixth closing |
October 2005 | 1,000,001 | 747,385 | |||||||
| Final closing |
December 2005 | 1,100,001 | 822,123 | |||||||
|
|
|
|
|
|||||||
| 38,400,003 | 28,699,551 | |||||||||
|
|
|
|
|
|||||||
The initial closing in June 2005 included the conversion of $2,750,000 in aggregate principal amount of convertible promissory notes and $50,342 of aggregate accrued interest thereon. Each share of Series B is currently convertible into shares of common stock on approximately a 31.39-for-one basis. In addition, the Series B preferred stock will automatically convert to common stock upon the completion of a qualified financing transaction. Holders of Series B preferred stock possess certain rights, including, among others, preference in liquidation (including a sale of the Company), antidilution protection, and preemptive rights relative to holders of common stock. In the event of a liquidation event, holders of Series B preferred stock shall be entitled to receive the amount of the original purchase price, plus 7% per annum compounded annually plus accrued but unpaid dividends. Each holder of Series B preferred stock is entitled to receive, if and when declared, payment of an equivalent per share dividend based on the number of common shares into which each share of preferred stock is convertible, as of the date of declaration. The rate of conversion of Series B preferred stock into common stock shall be adjusted in the event the Company issues dilutive shares of common stock according to a formula defined in the Company’s Restated Certificate of Incorporation. Holders of Series B preferred stock are entitled to vote as though the preferred stock was converted into common stock. Holders of Series B preferred stock are entitled to present a redemption request to the Company in January 2015 for redemption of 25% of their total cumulative holdings per year, in the amount of the original purchase price plus 7% per annum compounded annually plus accrued but unpaid dividends.
| (g) | Stock Option Plan |
During 2002, the Company established a stock option plan (the 2002 Option Plan) providing for the grant of options to purchase common shares to outside directors, executives, certain key employees, and consultants. The board of directors administers the plan and approves stock option grants. Stock options granted under the plan are exercisable at a price equal to the price determined by the committee on the date of the grant. The options are exercisable under the vesting terms as determined by the board and generally expire 10 years from the date of grant.
F-25
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
As of December 31, 2013 and March 31, 2014 (unaudited), there were 448,222 shares authorized, of which 205,650 and 199,166, respectively, are available for future issuance under the 2002 Option Plan.
The 2002 Option Plan is administered by the Board of Directors, which has the authority to select the individuals to whom awards will be granted, the number of shares, vesting exercise price and term of each option grant. Generally, options have a four-year annual vesting term, an exercise price equal to the market value of the underlying shares at the grant date and a ten-year life from the date of grant.
In April 2014 (unaudited), the Company adopted the 2014 Employee Stock Purchase Plan and the 2014 Equity Incentive Plan and reserved 201,163 and 393,358 shares of common stock, respectively, for issuance under the plans. Both plans include annual increases in the number of shares of common stock reserved for future issuances pursuant to the evergreen provisions of each plan. These plans are inactive until the Company completes a successful initial public offering.
The Company uses the Black-Scholes option pricing model to estimate the fair value of stock options for employee grants and used the following assumptions to obtain the weighted average grant date fair values:
| Year Ended December 31, | Three Months Ended March 31, 2014 | |||||||
| 2011 | 2012 | 2013 (a) | ||||||
| (unaudited) | ||||||||
| Risk-free interest rate |
2.2% | 1.3% | 1.82% | |||||
| Expected life (in years) |
5.9 -6.0 | 5.8 -6.0 | 6.0 | |||||
| Expected volatility |
86.9% -87.1% | 90.9% -91.2% | 95.7% | |||||
| (a) | There were no stock options granted in 2013. |
The Company uses the Black-Scholes option pricing model to estimate the fair value of stock options for nonemployee grants and used the following assumptions to estimate fair value each respective year:
| Year Ended December 31, | Three Months Ended March 31, 2014 | |||||||
| 2011 | 2012 | 2013 | ||||||
| (unaudited) | ||||||||
| Risk-free interest rate |
1.6% -3.5% | 1.4% -2.2% | 1.8% -2.7% | 2.8% -3.0% | ||||
| Expected life (in years) |
10.0 | 10.0 | 10.0 | 10.0 | ||||
| Expected volatility |
82.9% -84.8% | 83.3% -85.2% | 82.6% -85.1% | 85.2% -85.4% | ||||
The Black-Scholes model requires inputs for risk-free interest rate, dividend yield, volatility, and expected lives of the options. Since the Company has a limited history of stock activity, expected volatility is based on historical data from several public companies similar in size and nature of operations to the Company. The risk-free rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant for a period commensurate with the expected term of the grant. The expected term (without regard to forfeitures) for employee options granted represents the period of time that options granted are expected to be outstanding and is derived from the contractual terms of the options granted. Since the Company has limited employee share option exercises, the expected term was determined using the average of the vesting periods and expirations.
F-26
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
To value its common stock for measuring equity based awards, the Company used a combination of the option pricing method and the Probability-Weighted Expected Return Method (“PWERM”) as outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation (AICPA Practice Aid). The option pricing method treats common stock and preferred stock as call options on a subject company’s equity value, with exercise prices based on the liquidation preference of the preferred stock. The model estimates the fair value of each class of securities as a function of the current estimated fair value of the company. The characteristics of each class of stock are examined, including: (1) the conversion ratio; (2) the liquidation preferences assigned to the preferred classes of stock; and (3) the exercise price for all outstanding options and warrants. Under this method, value is allocated to common shares only in circumstances where the total equity value exceeds the liquidation rights associated with the preferred shares. The option-pricing method provides the stockholder the right, but not the obligation, to buy the underlying net assets at a predetermined price or “strike” price. The strike price is determined by analyzing the break points at which value would be allocated to each class of stock, based on the distribution characteristics associated with the equity. The PWERM analyzes the returns afforded to common equity holders under multiple future scenarios. Under the PWERM, share value is based upon the probability-weighted present value of expected future net cash flows (distributions to shareholders), considering each of the possible future events and giving consideration to the rights and preferences of each share class. While this method relies on certain key assumptions, it is best used when the range of possible future outcomes and the corresponding time frames are highly uncertain.
Share-based compensation expense is recognized net of estimated pre-vesting forfeitures, which results in recognition of expense on options that are ultimately expected to vest over the expected option term. Forfeitures are estimated at the time of grant using actual historical forfeiture experience and are revised in subsequent periods if actual forfeitures differ from those estimates.
F-27
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
The following table summarizes common stock option and warrant activities for common stock options and warrants issued to employees, directors, and consultants:
| Number of common stock options |
Weighted average exercise price |
Weighted average remaining contractual term (years) |
||||||||||
| Outstanding at December 31, 2010 |
396,740 | $ | 6.79 | |||||||||
| Granted |
20,769 | 12.09 | ||||||||||
| Exercised |
(163 | ) | 6.21 | |||||||||
| Canceled |
(55,072 | ) | 6.91 | |||||||||
|
|
|
|||||||||||
| Outstanding at December 31, 2011 |
362,274 | 7.08 | ||||||||||
| Granted |
27,105 | 18.24 | ||||||||||
| Exercised |
(4,566 | ) | 4.73 | |||||||||
| Canceled |
(7,933 | ) | 9.15 | |||||||||
|
|
|
|||||||||||
| Outstanding at December 31, 2012 |
376,880 | 7.87 | ||||||||||
| Exercised |
(382 | ) | 4.73 | |||||||||
| Canceled |
|
(147,004 |
) |
7.82 | ||||||||
|
|
|
|||||||||||
| Outstanding at December 31, 2013 |
229,494 | 7.90 | 4.5 | |||||||||
| Granted (unaudited) |
7,279 | 15.10 | ||||||||||
| Exercised (unaudited) |
(636 | ) | 4.73 | |||||||||
| Canceled (unaudited) |
(795 | ) | 4.73 | |||||||||
|
|
|
|||||||||||
| Outstanding at March 31, 2014 (unaudited) |
235,342 | 8.14 | 4.5 | |||||||||
|
|
|
|||||||||||
| Exercisable at December 31, 2013 |
218,947 | 7.48 | 4.3 | |||||||||
| Exercisable at March 31, 2014 (unaudited) |
219,856 | 7.57 | 4.1 | |||||||||
The following table summarizes information about stock options issued to employees, directors, and consultants that is outstanding at December 31, 2013:
| Common stock options outstanding |
Common stock options exercisable |
|||||||||||||||
| Exercise price |
Number outstanding |
Weighted average remaining contractual life (years) |
Number exercisable |
Weighted average exercise price |
||||||||||||
| $ 4.73 |
65,280 | 2.23 | 65,280 | $ | 4.73 | |||||||||||
| 5.04 |
7,184 | 3.34 | 7,184 | 5.04 | ||||||||||||
| 5.98 |
72,355 | 4.18 | 72,355 | 5.98 | ||||||||||||
| 6.93 |
16,104 | 5.25 | 16,104 | 6.93 | ||||||||||||
| 8.52 |
6,971 | 5.75 | 6,971 | 8.52 | ||||||||||||
| 10.07 |
2,837 | 7.21 | 2,346 | 10.07 | ||||||||||||
| 12.56 |
41,464 | 6.61 | 39,454 | 12.56 | ||||||||||||
| 18.24 |
17,299 | 8.24 | 9,257 | 18.24 | ||||||||||||
|
|
|
|
|
|||||||||||||
| 229,494 | 218,947 | |||||||||||||||
|
|
|
|
|
|||||||||||||
F-28
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
The following table summarizes information about stock options issued to employees, directors, and consultants that is outstanding at March 31, 2014 (unaudited):
| Common stock options outstanding |
Common stock options exercisable |
|||||||||||||||
| Exercise price |
Number outstanding |
Weighted average remaining contractual life (years) |
Number exercisable |
Weighted average exercise price |
||||||||||||
| (unaudited) | (unaudited) | (unaudited) | (unaudited) | (unaudited) | ||||||||||||
| $ 4.73 |
63,849 | 2.03 | 63,849 | $ | 4.73 | |||||||||||
| 5.04 |
7,184 | 3.09 | 7,184 | 5.04 | ||||||||||||
| 5.98 |
72,355 | 3.93 | 72,355 | 5.98 | ||||||||||||
| 6.93 |
16,104 | 5.00 | 16,104 | 6.93 | ||||||||||||
| 8.52 |
6,971 | 5.50 | 6,971 | 8.52 | ||||||||||||
| 10.07 |
2,837 | 6.96 | 2,483 | 10.07 | ||||||||||||
| 12.56 |
41,464 | 6.36 | 40,628 | 12.56 | ||||||||||||
| 15.10 |
7,279 | 9.96 | — | 15.10 | ||||||||||||
| 18.24 |
17,299 | 7.99 | 10,282 | 18.24 | ||||||||||||
|
|
|
|
|
|||||||||||||
| 235,342 | 219,856 | |||||||||||||||
|
|
|
|
|
|||||||||||||
The weighted average grant date fair value of options granted during the years ended December 31, 2011, and 2012 and the three months ended March 31, 2014 (unaudited) was $6.92, $13.55 and $11.66 per share, respectively. The total grant date fair value of options that vested during 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited) was $386,254, $308,803, $193,828, $65,531 and $23,522, respectively. The total intrinsic value, or the difference between the aggregate exercise price and the aggregate fair market price on the day of exercise, of options exercised during the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited) was $630, $139,620, $1,560, $0 and $6,600, respectively. The total intrinsic value of options outstanding as of December 31, 2012, 2013 and March 31, 2014 (unaudited) was $881,163, $1,703,515 and $1,668,665, respectively. The total intrinsic value of options exercisable as of December 31, 2012, 2013 and March 31, 2014 (unaudited) was $879,170, $1,696,012 and $1,684,778, respectively. As of December 31, 2013 and March 31, 2014 (unaudited), the Company had unrecognized stock-based compensation of $60,510 and $91,412, respectively, related to nonvested stock options, which is expected to be recognized over an estimated weighted average period of 1.8 and 2.7 years, respectively.
The Company’s net loss for the years ended December 31, 2011, 2012, net income for 2013 and net loss for the three months ended March 31, 2013 and 2014 (unaudited) includes $359,783, $369,180, $209,715, $71,971 and $12,813, respectively, of stock-based compensation costs. Stock-based compensation included in the Company’s statements of operations and comprehensive loss for the years ended December 31, 2011, 2012, 2013 and for the three months ended March 31, 2013 and 2014 (unaudited) was $139,338, $143,169, $47,082, $26,431 and $2,548 in research and development expenses and $220,445, $226,011, $162,633, $45,540 and $10,265 in general and administrative expenses, respectively.
The Company did not recognize a tax benefit from share-based compensation expense because the Company has concluded that it is not more likely than not that the related deferred tax assets, which have been reduced by a full valuation allowance, will be realized.
F-29
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| (h) | Warrants |
The Company has issued the following warrants that vested upon issuance. Also included below are the Black-Scholes assumptions used to estimate the fair value of the warrants:
| Warrants issued in connection with |
2004 Financing agreement |
Convertible promissory notes |
2006 Financing agreement |
Convertible promissory notes |
Issuance of Series D preferred stock |
Total Long- term |
Total Short- term |
|||||||||||||||||||||
| Issue date |
March 2004 | June 2005 | April 2006 | May 2009 | May 2009 | |||||||||||||||||||||||
| Redeemable, convertible preferred stock underlying the warrant |
Series A | Series B | Series B | Series C | Series C | |||||||||||||||||||||||
| Number of warrants |
22,000 | 411,060 | 179,372 | 622,826 | 2,076,125 | |||||||||||||||||||||||
| Exercise price |
$ | 1.250 | 1.338 | 1.338 | 1.445 | 1.445 | ||||||||||||||||||||||
| Black-Scholes assumptions as of December 31, 2012 |
||||||||||||||||||||||||||||
| Remaining term (years) |
1.2 | 2.5 | 3.3 | 6.4 | 6.4 | |||||||||||||||||||||||
| Estimated volatility |
83.0 | % | 86.5 | % | 85.5 | % | 92.0 | % | 92.0 | % | ||||||||||||||||||
| Risk-free interest rate |
0.16 | % | 0.31 | % | 0.35 | % | 0.92 | % | 0.92 | % | ||||||||||||||||||
| Number of warrants remaining |
22,000 | 411,060 | 179,372 | 620,294 | 2,076,125 | |||||||||||||||||||||||
| Estimated fair value at December 31, 2012 |
$ | 2,837 | 100,270 | 52,680 | 723,631 | 2,421,993 | 3,301,411 | — | ||||||||||||||||||||
| Black-Scholes assumptions as of December 31, 2013 |
||||||||||||||||||||||||||||
| Remaining term (years) |
0.2 | 1.5 | 2.3 | 5.4 | 5.4 | |||||||||||||||||||||||
| Estimated volatility |
61.0 | % | 66.5 | % | 84.5 | % | 97.0 | % | 97.0 | % | ||||||||||||||||||
| Risk-free interest rate |
0.07 | % | 0.24 | % | 0.52 | % | 1.58 | % | 1.58 | % | ||||||||||||||||||
| Number of warrants remaining |
22,000 | 411,060 | 179,372 | 620,294 | 2,076,125 | |||||||||||||||||||||||
| Estimated fair value at December 31, 2013 |
$ | 13 | 28,098 | 32,735 | 321,603 | 1,076,405 | 1,458,841 | 13 | ||||||||||||||||||||
| Black-Scholes assumptions as of at March 31, 2014 (unaudited) |
||||||||||||||||||||||||||||
| Remaining term (years) (unaudited) |
1.2 | 2.0 | 5.1 | 5.1 | ||||||||||||||||||||||||
| Estimated volatility (unaudited) |
71.5 | % | 77.5 | % | 91.5 | % | 91.5 | % | ||||||||||||||||||||
| Risk-free interest rate (unaudited) |
0.06 | % | 0.17 | % | 1.52 | % | 1.52 | % | ||||||||||||||||||||
| Number of warrants remaining (unaudited) |
411,060 | 179,372 | 620,294 | 2,076,125 | ||||||||||||||||||||||||
| Estimated fair value at March 31, 2014 (unaudited) |
$ | ( | *) | 26,688 | 24,825 | 296,941 | 993,862 | 1,342,316 | — | |||||||||||||||||||
| (*) | This warrant expired unexercised on March 18, 2014 |
In addition to the warrants above, the Company has recorded the estimated value of the warrants related to the Note discussed in Note 6 that will ultimately be issued in the future. The estimated value of these warrants as of December 31, 2013 and March 31, 2014 (unaudited) was $106,087 and $106,385, respectively, and is included
F-30
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
in the long-term portion of fair value of warrants. The warrants expire 10 years from the date of issuance with an estimated fair value of $106,087 as of December 31, 2013 determined using the Black-Scholes option pricing model, and the following assumptions: 10-year term, 85.17% volatility, and a risk-free interest rate of 2.67%. The estimated fair value as of March 31, 2014 (unaudited) of $106,385 was determined using the Black-Scholes option pricing model, and the following assumptions: 9.6 year remaining term, 87.5% volatility, and a risk-free interest rate of 2.71%.
2014 Notes Warrants (Unaudited)
As part of the issuance of the 2014 Notes (see Note 6), the Company issued warrants to the holders of the 2014 Notes with the following terms:
| • | Five year exercise period. |
| • | Exercisable into a newly issued series of preferred stock if an initial public offering or subsequent round of equity financing is not completed prior to January 31, 2015. If an initial public offering occurs prior to January 31, 2015, the warrants will be exercisable into common stock. If an initial public offering does not occur and a subsequent round of financing occurs, then the warrants will be exercisable into the equity securities issued in the subsequent round of financing. |
| • | The exercise price will be equal to the issuance price of the securities that the warrants are ultimately exercisable into. |
| • | The number of shares received upon exercise will be equal to the face value of the 2014 Notes ($7,500,000) plus interest accrued divided by the applicable exercise price. |
The Company engaged a third-party valuation firm to value the warrants as of the commitment date of the 2014 Notes. Due to the various exercise prices and securities that the warrants are exercisable into, the methodology to value the warrants included a combination of Black-Scholes and Monte Carlo Simulation models that take into consideration probability factors of the various outcomes related to the exercise terms of the warrants. This valuation resulted in an initial valuation of these warrants of $4,454,397. The estimated value of these warrants, using the same valuation methodology, as of March 31, 2014 was $4,238,256 and is included in the short term portion of fair value of warrants.
The placement agent of the 2014 Notes also received warrants as compensation for the placement of the 2014 notes. The warrant coverage received by the placement agent is equal to 10% of the total face value of the 2014 Notes ($750,000) and 10% of the warrant coverage received by the holders of the 2014 Notes ($750,000). The terms of the placement agent warrants are the same as the warrants held by the 2014 Notes holders except for modified warrant coverage and exercise prices on warrants issued related to the face value of the 2014 Notes. The valuation of these warrants was consistent with the above methodology and resulted in an initial valuation of $876,778. The estimated value of these warrants, using the same valuation methodology, as of March 31, 2014 was $833,878 and is included in the short term portion of fair value of warrants.
These warrants have been classified as liabilities as they will be exercisable into redeemable preferred stock unless the contingent events described above occur. If and when these contingent events occur, the warrants will be classified as equity or a liability based on the nature and terms of the securities that the warrants are exercisable into.
Pursuant to ASC Topic 480, the estimated fair value of these warrants are reported as liabilities at their estimated fair value at December 31, 2012 and 2013 and March 31, 2014 (unaudited), and any changes in fair value during the period are reflected in change in value of warrants.
F-31
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| (8) | Deferred Revenue |
Deferred revenue consisted of the following as of:
| 2012 | 2013 | March 31, 2014 |
||||||||||
| (unaudited) | ||||||||||||
| Celgene |
$ | 17,243,383 | 14,169,164 | 13,210,422 | ||||||||
| Gilead |
313,232 | 244,711 | 37,069 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total deferred revenue |
17,556,615 | 14,413,875 | 13,247,491 | |||||||||
| Less: current portion |
(3,761,908 | ) | (3,756,899 | ) | (3,410,282 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Deferred revenue, long-term |
$ | 13,794,707 | 10,656,976 | 9,837,209 | ||||||||
|
|
|
|
|
|
|
|||||||
| (a) | Celgene Agreements |
In May 2009, the Company entered into a Collaboration and Option Agreement with Celgene for the early development of four oncology products and all future oncology drug candidates (which options to future oncology drug candidates is subject to expiration if Celgene did not license one of the initial four named products). Celgene is also a holder of Series C, D and E preferred stock. Under the collaboration agreement, Celgene has the option to obtain an exclusive worldwide license to develop and commercialize the products subject to diligence requirements, an up-front development funding fee, milestone payments and royalties. This agreement was amended in June 2011 to replace one of the four named products with another oncology Tarmogen product. The terms of the amendment did not materially modify the agreement as the financial terms and the length of the agreement remained substantially the same. Celgene’s options with respect to the GI-6200 and GI-3000 oncology drug candidate programs will terminate if Celgene does not exercise its options for such programs after the Company delivers certain reports on predefined clinical trials with respect to such drug programs. In March 2013, Celgene declined to exercise its option to the GI-4000 program and returned all rights and development responsibility to the Company. In July 2013, Celgene exercised its option to license the GI-6300 program. As a result of the election to license the GI-6300 program, Celgene has an option to license all future oncology drug candidates developed by the Company on a product by product basis.
In July 2013, Celgene exercised its option to license the GI-6300 program, including GI- 6301, in exchange for an upfront payment of $9,000,000. As part of that exercise, the Collaboration and Option Agreement was amended as it related to the GI-6300 program. The agreement, as amended, includes (1) a license granted to Celgene as of the date of the exercise of the option to develop and commercialize the GI-6300 product candidates using all of the Company’s related patents, intellectual property and know-how related to these product candidates that existed at the inception of the Collaboration and Option Agreement or any time during the term, (2) the Company supplying drug product for the Phase 2 clinical trial and (3) the Company’s option to perform Phase 2 clinical trials, subject to Celgene’s right to assume performance of those trials. As part of this exercise, certain milestones were modified and adjustments to the royalty rates on net sales were reduced. The modification to the milestones did not materially impact the deliverables that existed at the time of the modification.
The Collaboration and Option agreement, as amended including the amendment relating to the GI-6300 program contain the following provisions:
| • | The Company received a $30,000,000 upfront payment to perform research and development and for the option to license products based on the GI-4000, GI-6200, GI-6300 and GI-3000 programs. This payment was made by Celgene in May 2009. |
F-32
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| • | The Company received $1,000,000 in October 2011 and $300,000 in April 2012 from Celgene for additional immunology work for the GI-4000 program. |
| • | The Company may receive a total of $85,000,000 in development and regulatory milestones for the GI-6200 and GI-3000 programs; activities for which the Company is not responsible for completing. |
| • | If Celgene exercises its option to the GI-6200 or GI-3000 program and these products are commercialized, the Company may also receive up to $60,000,000 in net sales milestones and tiered royalty rates on net sales in the teens on worldwide net sales; activities for which the Company is not responsible for completing. |
| • | The Company is eligible to receive a total of $85,000,000 in development and regulatory milestone payments for GI-6301; activities for which the Company is not responsible for completing. |
| • | If GI-6301 is commercialized by Celgene, the Company may receive up to $60,000,000 in sales milestone payments for which the Company is not responsible for completing. and tiered royalty rates on net sales ranging from single digits to low double digits |
| • | For programs other than GI-6200, GI-3000 and GI-6300, the Company may be eligible to receive up to $101,000,000 in development and regulatory milestone payments for Celgene’s clinical trials, NDA filing and regulatory approvals, up to $60,000,000 in net sales milestone payments for such programs, and tiered royalty rates on net sales in the teens on worldwide net sales; activities for which the Company is not responsible for completing. |
Upon execution of the May 2009 agreement, the Company estimated that its obligations to perform research and development under the agreement would continue through September 30, 2016 and accordingly was recognizing as revenue the upfront fees received of $31,300,000 from the date of receipt through September 30, 2016. The Company reviews the term of performance on a quarterly basis and adjusts the revenue recognition period if there are any changes. As of December 31, 2012, 2013 and March 31, 2014 (unaudited), the unamortized balance was amortized on a straight line basis through December 31, 2017, March 31, 2018 and March 31, 2018, respectively.
The Company determined that there were two units of accounting under the July 2013 GI-6300 License Agreement with Celgene: the license to further develop and commercialize GI-6300 and undelivered items related to supplying drug product for the Phase 1 clinical trial and the option to perform the Phase 2 clinical trial (subject to Celgene’s right to assume performance of those trials). The Company determined that the license had standalone value based on the fact that the drug candidate has been developed and is currently in a clinical trial, Celgene possesses the knowledge, technology, skills, experience and background necessary for all further development of the drug through commercialization, the Company is not required to perform any additional development work related to GI-6300 and Celgene has the right to sublicense the product. The Company allocated the $9,000,000 of proceeds to the two units of accounting using the relative selling price method. The Company determined the estimated selling price for the license based upon a third party valuation and vendor specific objective evidence for the undelivered items. The allocation resulted in $8,766,881 being allocated to the license and the remaining amount of $233,119 allocated to the undelivered items. The Company recognized $8,766,881 in revenue related to the license in the fourth quarter of 2013 upon the delivery of all intellectual property, reports and documentation for the license to Celgene. Revenue related to the undelivered items will be recognized as the services are performed. The current estimated service period for the undelivered items under the GI-6300 License Agreement is through December 2014.
F-33
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
The Company recognized $3,823,684, $3,622,764, $3,307,338, $848,035 and $958,742 in revenue related to research and development services and other services during the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited), respectively, and license revenue of $8,766,881 during the year ended December 31, 2013. Costs incurred under these agreements, included in costs of collaboration licenses and services in the Company’s statement of operations and comprehensive income and loss, for the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited) were $7,833,184, $8,184,806, $2,515,055, $1,079,284 and $613,955, respectively.
To date, the Company has not recognized any revenue in connection with milestone payments, other than the license election noted above, or royalties under this agreement.
| (b) | Gilead Agreement |
In October 2011, the Company entered into a License and Collaboration Agreement with Gilead, granting Gilead an exclusive license to all hepatitis B Tarmogen product candidates to be developed and commercialized under the collaboration, which includes a license granted at contract outset to develop and commercialize the HBV Tarmogen product candidate GS-4774 using all of the Company’s related patents, intellectual property and know-how related to these product candidates. Under the terms of the agreement, in November 2011 Gilead made a $10,000,000 initial nonrefundable payment to the Company. The Company conducted preclinical development, filed the Investigational New Drug application (IND) and performed the initial Phase 1a trial in healthy volunteers for the selected HBV Tarmogen. Gilead reimbursed the Company on a periodic basis for the costs and expenses of the Phase 1a clinical trial. Gilead will perform any future clinical development, regulatory and commercialization activities. Gilead activities are subject to commercially reasonable diligence, milestone payments and royalties. Upon satisfaction of certain substantive milestone events for which the Company was partially responsible for completing, the Company received $2,000,000 upon filing an IND for HBV in 2012 and $3,000,000 at the point of commencement of the Phase 1b/2a clinical trial in 2013. The Company is also eligible to receive additional proceeds of up to $130,000,000 in development and regulatory milestones based upon achievement of such milestones by Gilead. If products are commercialized, the Company is eligible to receive tiered royalty rates in the upper single digit to mid-teens and up to $40,000,000 of sales milestone payments based on net sales of the licensed product candidates. The Company is not responsible for the sales efforts.
The Company determined there was one unit of accounting under the agreement with Gilead. The non-contingent deliverables under the agreement include: the license to all intellectual property and know-how related to hepatitis B Tarmogen products, the services to be performed in preclinical development (including the filing of an IND) and in conducting the Phase 1a clinical trial, participation on the Joint Research and Development Committee (JRC), and the requirement to provide consultation to Gilead after Gilead assumes control of development activities. The Company has estimated the performance period for these deliverables to be from October 2011 through 2020.
When the agreement was signed, the Company determined that its obligation to supply drug product to Gilead after Gilead assumed control of the development was a contingent deliverable, as the obligation to supply product was contingent on the successful development of the Hepatitis B Tarmogen product candidate and the related approval of the IND among other items. Subsequently, Gilead has assumed control of manufacturing. However, a services agreement between the parties also continues to exist. As a result, the services agreement deliverables and the potential incremental fees to be received by the Company will be accounted for only if and when delivery takes place. The Company has determined that the consideration to be received is an appropriate incremental fee and, therefore there is not a significant incremental discount associated with the selling price of the services agreement.
F-34
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
The Company determined that the license did not have standalone value at the inception of the agreement. This determination is based on the fact that the license is not sold on a standalone basis, nor could it be resold by Gilead on a standalone basis because the Company has proprietary knowledge, technology, skills, experience and background that no other third party, including Gilead, currently possesses and could not readily obtain at contract inception. Such knowledge, technology, skills, experience and background would be necessary for further development of the Hepatitis B Tarmogen product candidate as required under the agreement.
The Company is recognizing the initial consideration of $10,000,000 and the amounts it will receive as reimbursement from Gilead of costs to perform the initial Phase 1a trial on a proportional performance basis over the estimated period of performance to complete the preclinical development and Phase 1a trial services, JRC and consultation services, which is estimated to be from October 2011 through 2020. The Company will measure its progress under the proportional performance method based on hours incurred in proportion to total estimated hours. However, the cumulative revenue recognized under this agreement will be limited to the cumulative cash received to date from Gilead. The Company incurred substantially all of the Company hours during the preclinical development and Phase 1a trial period, which ended in February 2014.
The contractual term of the license is on a product and country basis that begins on the effective date of the contract, October 2011, and runs through the expiration of Gilead’s obligation to pay royalties for such product in such country, or until the agreement is terminated. The JRC term is from the effective date of the agreement and terminates at the end of the Research Term, which is the period of time commencing on the date the agreement was signed and ending upon completion of the final clinical study report for the Gilead Phase 1b/2a trial. The consulting term begins upon the conclusion of the Research Term, and the Company estimates the term would end upon commercialization, currently estimated in 2020.
The Company recognized $1,284,158, $9,018,842, $4,039,538, $344,232 and $217,273 in license and services revenue under the arrangement during the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited), respectively. The Company also recognized milestone payments of $2,000,000 in 2012 and $3,000,000 in 2013. In addition, the Company recognized revenue of $3,168,237, $940,000 and $134,825 during the year ended December 31, 2013 and the three months ended March 31, 2013 and 2014 (unaudited), respectively, related to manufacturing supply for Phase 2 trials for which Gilead is responsible for performing. Collaboration license and service costs incurred under these agreements, included in costs of collaboration licenses and services in the Company’s statement of operations and comprehensive income and loss, for the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited) were $547,066, $1,848,234, $3,340,958, $1,033,632 and $218,899, respectively. Manufacturing services costs incurred under agreements with Gilead, included in costs of manufacturing services in the Company’s statement of operations and comprehensive income and loss, for the years ended December 31, 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited) were $0, $0, $3,168,237, $940,000 and $134,825, respectively.
| (9) | Commitments and Contingencies |
| (a) | Contract Commitments |
The Company has an exclusive license with the University of Colorado (CU) that is used in its research and development activities. This agreement requires the Company make certain development milestone payments, make royalty payments based on sales of approved products, if any, and pay a portion of any consideration received by the Company in exchange for granting a sublicense. Under this agreement, the Company is required
to pay to CU certain development milestone payments totaling $150,000 per product candidate beginning upon
F-35
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
the filing of an IND through the approval from the FDA and royalties on sales of any products and pay a portion of any consideration the Company receives in exchange for granting a sublicense. After the first commercial sale of the licensed products, each milestone payment shall be credited against future royalties, until the full amount of such milestone payment has been credited in full. In 2011, 2012 and 2013, CU was due $62,500, $325,000 and $450,000, respectively, under this agreement.
The Company has entered into a collaboration agreement with the NIH for the preclinical and clinical development of the Company’s proprietary yeast-based Tarmogens expressing tumor-associated antigens as potential vaccines for the prevention and/or therapy of a range of human cancers. The Company has the right to terminate this agreement with 60 days notice. The agreement requires the Company to make annual payments of up to $300,000 based on the clinical trial stage. The Company made payments of $250,000, $250,000 and $300,000 in years ended December 31, 2011, 2012 and 2013, respectively.
The Company is a party to two license agreements with the NIH as of December 31, 2013, consisting of the NIH VirusPlus Agreement, dated August 23, 2011 and the NIH CEA Agreement, dated June 12, 2007, collectively referred to as the NIH license agreements. The NIH license agreements grant the Company worldwide, exclusive licenses to make and have made, to use and have used, to sell and have sold, to offer to sell and have offered for sale, and to import and have imported products relating to the use of the Tarmogen immunotherapy platform with certain antigens, other immunotherapy platforms and other intellectual property to treat cancer that are covered by licensed patent rights and to practice and have practiced any licensed processes in the licensed fields of use. These license agreements required the Company to make certain noncreditable and nonrefundable initial royalty payments upon signing of each license agreement, make certain milestone payments upon achievement of specified development and commercial milestones, make royalty payments based on sales of approved products, if any and pay a portion of any consideration received by the Company in exchange for granting a sublicense. The NIH license agreements contain the following provisions:
| • | Under the NIH VirusPlus agreement, the Company is required to make royalty advances totaling $500,000 beginning upon the first filing of an application for marketing approval through the first commercial sale, and royalty payments once it begins selling products developed under the agreement. |
| • | Under the NIH CEA agreement, the Company is required to make royalty advances totaling $745,000 beginning upon the filing of an IND through FDA approval, and royalty payments once it begins selling products developed under the agreement. |
In January and March 2012 the Company signed exclusive license agreements with the NIH, consisting of the NIH Brachyury Agreement and the NIH MUC1 Agreement, collectively referred to as the 2012 NIH license agreements. The 2012 NIH license agreements grant the Company worldwide, exclusive licenses to make and have made, to use and have used, to sell and have sold, to offer to sell and have offered for sale, and to import and have imported products relating to the use of the Tarmogen immunotherapy platform with certain antigens, other immunotherapy platforms and other intellectual property to treat cancer that are covered by licensed patent rights and to practice and have practiced any licensed processes in the licensed fields of use. These license agreements require the Company to make certain royalty payments based on sales of approved products, if any, and pay a portion of any consideration the Company receives in exchange for granting a sublicense. In addition, the 2012 NIH license agreements contain the following provisions:
| • | Under the NIH Brachyury Agreement the Company is required to make royalty advances totaling $650,000 beginning upon the successful completion of the first Phase 3 clinical study through the first commercial sale, and royalty payments once it begins selling products developed under the agreement. |
F-36
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
| • | Under the NIH MUC1 Agreement the Company is required to make royalty advances totaling $500,000 beginning upon the acceptance of the first filing of an application for marketing approval with the FDA through the first commercial sale, and royalty payments once it begins selling products developed under the agreement. |
| (b) | Lease Commitments |
In October 2013, the Company amended the lease agreement for its office and research facility in Louisville, Colorado to a month to month lease that can be cancelled with 30 days notice.
At December 31, 2013, future minimum lease payments under the Company’s noncancelable operating leases are as follows:
| 2014 |
$ | 6,612 | ||
| 2015 |
4,261 | |||
|
|
|
|||
| Total |
$ | 10,873 | ||
|
|
|
In April 2014, the Company amended the lease agreement for its office and research facility in Louisville, Colorado. The amendment extended the term four years and extends the term one additional year upon completion of a public offering. The amendment includes escalating rent payments throughout the term. The rent expense related to this lease is recorded monthly on a straight-line basis.
Including the lease amendment, future minimum lease payments under the Company’s noncancelable operating leases are as follows as of March 31, 2014 (unaudited):
| 2014 |
$ | 440,536 | ||
| 2015 |
595,747 | |||
| 2016 |
609,339 | |||
| 2017 |
627,702 | |||
| 2018 |
158,080 | |||
|
|
|
|||
| $ | 2,431,404 | |||
|
|
|
During 2011, 2012, 2013 and the three months ended March 31, 2013 and 2014 (unaudited), the Company incurred rental expense of $398,069, $398,069, $448,664 $99,517 and $140,390, respectively.
| (10) | Benefit Plan |
The Company has adopted a 401(k) plan that covers substantially all employees who are at least 21 years of age. The plan is a defined contribution plan to which the employees may contribute up to 60% of their compensation. The Company does not match employee contributions.
| (11) | Income Taxes |
The Company has incurred net losses every year, except for 2013, and incurred a net loss during the three months ended March 31, 2014 (unaudited) except 2013 and expects to incur losses in the future. As a result, the Company did not record a federal income tax provision or benefit during 2011 and 2012 and the three months
F-37
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
ended March 31, 2014 (unaudited). In 2013, the Company recorded a current state tax provision of $115,765 which was recorded due to statutory limitations in the use of state net operating loss carryforwards.
A reconciliation of income taxes at the statutory federal income tax rate to net income taxes included in the accompanying statements of operations is as follows:
| Year Ended December 31, | Three Months ended March 31, |
|||||||||||||||||||
| 2011 | 2012 | 2013 | 2013 | 2014 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| U.S. federal income tax expense at the statutory rate |
(34.0 | )% | (34.0 | )% | 34.0 | % | 34.0 | % | 34.0 | % | ||||||||||
| State income taxes, net of federal taxes |
(3.6 | ) | (7.2 | ) | 4.1 | 5.1 | 2.0 | |||||||||||||
| Available research credits |
(4.0 | ) | — | (9.3 | ) | 42.8 | — | |||||||||||||
| Effect of permanent differences |
3.8 | (27.2 | ) | (5.8 | ) | 9.2 | (17.4 | ) | ||||||||||||
| Change in valuation allowance |
37.8 | 68.4 | (21.8 | ) | (91.1 | ) | (18.6 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
— | % | — | % | 1.2 | % | — | % | — | % | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Deferred tax assets and liabilities reflect the net tax effects of net operating loss carryforwards, credit carryforwards and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and amounts used for income tax purposes. The components of the Company’s deferred tax assets and liabilities are as follows:
| December 31, | March 31, 2014 |
|||||||||||
| 2012 | 2013 | |||||||||||
| (Unaudited) | ||||||||||||
| Accrued benefits |
$ | 146,094 | 99,494 | 105,789 | ||||||||
| Net operating loss carryforwards |
41,637,864 | 40,636,733 | 41,378,974 | |||||||||
| Research credit carryforwards |
5,767,824 | 6,661,348 | 6,661,348 | |||||||||
| Deferred revenue |
6,438,485 | 5,295,697 | 5,072,771 | |||||||||
| Depreciation of property and equipment |
1,515,316 | 1,532,398 | 1,464,142 | |||||||||
| Other |
921,901 | 116,537 | 112,737 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total deferred tax assets |
56,427,484 | 54,342,207 | 54,795,761 | |||||||||
| Valuation allowance |
(56,427,484 | ) | (54,342,207 | ) | (54,795,761 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Deferred tax assets, net of valuation allowance |
$ | — | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
Based upon the level of historical taxable losses and projections of future taxable losses over the periods in which the deferred tax assets are deductible, management believes it is more likely than not that the Company will not realize the benefits of these deductible differences and accordingly has established a full valuation allowance as of December 31, 2012 and 2013 and March 31, 2014 (unaudited). The increase in valuation allowance was $5,235,644, $1,374,216, $1,413,084 and $453,554 in 2011 and 2012 and the three months ended March 31, 2013 and 2014 (unaudited), respectively. In 2013, the valuation allowance decreased by $2,085,277.
Future realization depends on the future earnings of the Company, if any, the timing and amount of which are uncertain as of December 31, 2013 and March 31, 2014 (unaudited). In the future, should management conclude that it is more likely than not that the deferred tax assets are, in fact, at least in part, realizable, the
F-38
Table of Contents
GLOBEIMMUNE, INC.
Notes to Financial Statements — (Continued)
December 31, 2011, 2012 and 2013 and March 31, 2014
(Information as of March 31, 2014 and for the three months ended March 31, 2013 and 2014 is unaudited)
valuation allowance would be reduced to the extent of such realization and recognized as a deferred income tax benefit in the Company’s Statements of Operations and Comprehensive Loss.
As of December 31, 2013, the Company had available total federal and state net operating loss carryforwards of approximately $106,900,000, which expire in the years 2022 through 2033, and federal research credit carryforwards of $6,700,000, which expire in the years 2022 through 2033. The utilization of the net operating loss carryforwards and research credits may be limited due to the provisions of Sections 382 and 383 of the Internal Revenue Code if there are significant changes in ownership. Due to the nature of changes of ownership during 2003 and 2007, there will be limitations in the Company’s ability to utilize existing net operating loss carryforwards in future periods.
The Company has adopted the authoritative guidance under U.S. GAAP related to the accounting for uncertainty in income taxes, including derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. The Company has evaluated tax positions taken or expected to be taken in the course of preparing the financial statements to determine if the tax positions are “more likely than not” of being sustained by the applicable tax authority. Tax positions not deemed to meet the “more likely than not” threshold would be recorded as a tax benefit or expense in the current year. The Company has concluded that there was no impact related to uncertain tax positions on the results of its operations for the years ended December 31, 2011, 2012 and 2013 and the three months ended March 31, 2013 and 2014 (unaudited). The Company classifies interest and penalties arising from the underpayment of income taxes in the statements of operations as income tax expense. As of December 31, 2013 and March 31, 2014 (unaudited), the Company has no accrued interest or penalties related to uncertain tax positions. The Company’s conclusions regarding tax positions will be subject to review and may be adjusted at a later date based on factors including, but not limited to, ongoing analyses of tax laws, regulations, and interpretations thereof. The United States is the major tax jurisdiction for the Company, and the earliest tax year subject to examination is 2002, which includes the earliest year for which net operating loss carryforwards are available.
| (12) | Subsequent Events |
The Company has evaluated subsequent events after December 31, 2013 and up to the date these financial statements were issued.
F-39
Table of Contents
1,562,500 Shares
Common Stock

PRELIMINARY PROSPECTUS
June 4, 2014
Aegis Capital Corp.
Through and including 2014 (25 days after commencement of this offering), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery is in addition to the dealers’ obligation to deliver a prospectus when acting as an underwriter and with respect to such dealer’s unsold allotments or subscriptions.
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other expenses of issuance and distribution. |
The following table sets forth all costs and expenses, other than underwriting discounts and commissions, paid or payable by us in connection with the sale of the common stock being registered. All amounts shown are estimates except for except for the Securities and Exchange Commission, or SEC, registration fee, the Financial Industry Regulatory Authority, Inc., or FINRA, filing fee and the initial listing fee for The NASDAQ Global Market.
| Amount Paid or to be Paid |
||||
| SEC registration fee |
$ | 4,003 | ||
| FINRA filing fee |
5,162 | |||
| NASDAQ Global Market listing fee |
125,000 | |||
| Blue sky fees and expenses |
5,000 | |||
| Printing expenses |
225,000 | |||
| Legal fees and expenses |
725,000 | |||
| Accounting fees and expenses |
150,000 | |||
| Transfer agent and registrar fees and expenses |
10,000 | |||
| Miscellaneous fees and expenses |
52,000 | |||
|
|
|
|||
| Total |
$ | 1,301,170 | ||
|
|
|
|||
| Item 14. | Indemnification of directors and officers. |
Our amended and restated certificate of incorporation, which will become effective upon completion of this offering, limits the liability of directors to the maximum extent permitted by Delaware law. Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act of 1933, as amended. Our amended and restated certificate of incorporation, which will become effective upon completion of this offering, does not eliminate a director’s duty of care and, in appropriate circumstances, equitable remedies, such as injunctive or other forms of non-monetary relief, which remain available under Delaware law. These limitations also do not affect a director’s responsibilities under any other laws, such as the federal securities laws or other state or federal laws. Our amended and restated bylaws, which will become effective upon completion of this offering, provide that we will indemnify our directors and executive officers, and may indemnify other officers, employees and other agents, to the fullest extent permitted by law.
We have entered into amended and restated indemnification agreements with our directors and executive officers, whereby we agree to indemnify our directors and executive officers to the fullest extent permitted by law, including indemnification against expenses and liabilities incurred in legal proceedings to which the director or executive officer was, or is threatened to be made, a party by reason of the fact that such director is or was a director, officer, employee or agent of GlobeImmune, Inc., provided that such director or executive officer acted in good faith and in a manner that the director or executive officer reasonably believed to be in, or not opposed to, the best interest of GlobeImmune, Inc. At present, there is no pending litigation or proceeding involving a director or executive officer of GlobeImmune, Inc. regarding which indemnification is sought, nor are we aware of any threatened litigation that may result in claims for indemnification.
We maintain insurance policies that indemnify our directors and officers against various liabilities arising under the Securities Act of 1933, as amended, and the Securities Exchange Act of 1934, as amended, that might be incurred by any director or officer in his capacity as such.
The underwriter is obligated, under certain circumstances, pursuant to the underwriting agreement filed as Exhibit 1.1 hereto, to indemnify us, our officers and directors against liabilities under the Securities Act of 1933, as amended.
Table of Contents
Reference is made to the following documents filed as exhibits to this registration statement regarding relevant indemnification provisions described above and elsewhere herein:
| Exhibit Document |
Number | |||
| Form of Underwriting Agreement |
1.1 | |||
| Form of Amended and Restated Certificate of Incorporation to be effective upon completion of this offering |
3.3 | |||
| Form of Amended and Restated Bylaws to be effective upon completion of this offering |
3.5 | |||
| Form of Amended and Restated Indemnification Agreement |
10.4 | |||
| Item 15. | Recent sales of unregistered securities. |
Set forth below is information regarding shares of common stock, preferred stock, warrants and convertible promissory notes that we issued and options that we granted within the past three years that were not registered under the Securities Act. Also included is the consideration, if any, that we received for such shares, warrants, notes and options and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
| (a) | Issuances of Capital Stock, Warrants and Convertible Promissory Notes. |
| 1. | On August 17, 2012, we sold 1,904 shares of our common stock pursuant to the exercise of a warrant at a price of $34.37 per share to one accredited investor for cash consideration of $8,969. |
| 2. | On September 12, 2012, we issued 29,432 shares of our Series C Preferred Stock upon the net exercise of a warrant to purchase Series C Preferred Stock at an exercise price of $1.644 per share. We received no additional consideration in connection with this exercise. |
| 3. | On September 12, 2012, we issued 2,532 shares of our Series C Preferred Stock pursuant to the exercise of a warrant as a price of $1.445 per share to one accredited investor for cash consideration of $3,659. |
| 4. | On November 12, 2013, we issued a 8% convertible promissory note due on November 12, 2016 with an aggregate principal amount of $391,730. |
| 5. | From January 31, 2014 to February 11, 2014 we issued 10% convertible promissory notes, or the 2014 Notes, due on January 31, 2015 with an aggregate principal amount of $7,500,000 to accredited investors. At the close of this offering the aggregate principal and accrued interest of the 2014 Notes will convert into 690,663 shares of common stock assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus, and based on a conversion date of May 31, 2014, and (ii) we issued warrants to purchase our capital stock, which, upon completion of this offering, will convert into warrants to purchase an aggregate of 468,769 shares of our common stock with an assumed exercise price of $16.00 per share assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus. These warrants are exercisable until their expiration on January 31, 2019. |
| 6. | In connection with the issuance of the 2014 Notes and the related warrants we issued warrants to purchase an aggregate of 46,877 shares of our common stock at an assumed exercise price of $16.00 per share assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus. This warrant is exercisable until its expiration on the five year anniversary of the effective date of this registration statement. |
| 7. | In connection with the issuance of the 2014 Notes and the related warrants we issued a warrant to purchase an aggregate of 69,063 shares of our common stock at an assumed exercise price of $16.80 per share assuming an initial public offering price of $16.00 per share, the midpoint of the range set forth on the cover page of this prospectus. This warrant is exercisable until its expiration on the five year anniversary of the effective date of this registration statement. |
Table of Contents
| (b) | Grants of Stock Options. |
| 1. | From January 1, 2011 through December 31, 2013, we granted stock options to purchase an aggregate of 47,874 shares of common stock with exercise prices ranging from $10.07 to $18.24 per share to certain of our employees, consultants, advisors and directors under our 2002 Stock Incentive Plan, or the 2002 Plan, in connection with services provided to us by such parties. Of these options, no shares have been exercised. 10,862 options have been cancelled without being exercised and 37,012 options remain outstanding. |
| 2. | On March 13, 2014, we granted stock options to purchase an aggregate of 7,279 shares of common stock with exercise prices of $15.10 per share to certain of our employees, consultants, advisors and directors under the 2002 Plan, in connection with services provided to us by such parties. None of these options have been exercised. None of these options have been cancelled without being exercised and 7,279 options remain outstanding. |
No underwriters were involved in the foregoing issuances of securities.
The offers, sales and issuances of the securities described in Item 15(a) were deemed to be exempt from registration under the Securities Act in reliance on Rule 506 of Regulation D in that the issuance of securities to the accredited investors did not involve a public offering. The recipients of securities in each of these transactions acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited investor under Rule 501 of Regulation D.
The offers, sales and issuances of the securities described in Item 15(b) were deemed to be exempt from registration under the Securities Act in reliance on Rule 701 in that the transactions were under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of such securities were our employees, directors or bona-fide consultants and received the securities under the 2002 Plan. Appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions had adequate access, through employment, business or other relationships, to information about the Company.
| Item 16. | Exhibits and Financial Statement Schedules |
| (a) | Exhibits |
| Exhibit |
Description of Exhibit | |
| 1.1# | Form of Underwriting Agreement | |
| 3.1# | Restated Certificate of Incorporation of Registrant filed on June 14, 2012 | |
| 3.2# | Certificate of Amendment to the Restated Certificate of Incorporation filed on August 31, 2012 | |
| 3.2.1# | Certificate of Amendment to the Restated Certificate of Incorporation filed on April 25, 2014 | |
| 3.3# | Form of Amended and Restated Certificate of Incorporation of Registrant to be effective upon completion of this offering | |
| 3.4# | Amended and Restated Bylaws of Registrant, as currently in effect | |
| 3.5# | Form of Amended and Restated Bylaws of Registrant to be effective upon completion of this offering | |
| 4.1# | Form of Registrant’s Common Stock Certificate | |
| 4.2# | Form of Warrants to purchase Series B Preferred Stock and a Schedule of Warrantholders | |
| 4.3# | Warrant to purchase Series B Preferred Stock, dated April 14, 2006, issued to SVB Financial Group | |
Table of Contents
| Exhibit |
Description of Exhibit | |
| 4.4# | Warrant to purchase Series B Preferred Stock, dated April 14, 2006, issued to Oxford Finance Corporation | |
| 4.5# | Form of Warrants to purchase Series C Preferred Stock and a Schedule of Warrantholders | |
| 4.6# | Convertible Promissory Note issued by Registrant to Cooley LLP, dated as of November 12, 2013 | |
| 4.7# | Form of Warrant Certificate and a Schedule of Warrantholders | |
| 4.8# | Form of Convertible Term Note and a Schedule of Noteholders | |
| 4.9# | Form of Warrant to purchase capital stock issued to Aegis Capital Corp. or its designees | |
| 4.10# | Form of Warrant to purchase capital stock issued to Aegis Capital Corp. or its designees | |
| 4.11# | Form of Warrant to purchase Common Stock to be issued to Aegis Capital Corp. or its designees upon completion of this offering | |
| 4.12# | Fifth Amended and Restated Stockholders Agreement between Registrant and certain holders of Common and Preferred Stock dated January 14, 2010 | |
| 4.12.1# | Amendment No. 1 to Fifth Amended and Restated Stockholders Agreement between Registrant and certain holders of Common and Preferred Stock dated August 31, 2012 | |
| 4.13# | Form of Warrant to be issued to Cooley LLP upon completion of this offering | |
| 5.1# | Opinion of Cooley LLP | |
| 10.1†# | 2002 Stock Incentive Plan | |
| 10.1.1†# | Form of Incentive Stock Option Agreement under 2002 Stock Incentive Plan | |
| 10.1.2†# | Form of Non-Qualified Stock Option Agreement under 2002 Stock Incentive Plan | |
| 10.2†# | 2014 Equity Incentive Plan | |
| 10.2.1†# | Form of Stock Option Grant Notice and Stock Option Agreement under 2014 Equity Incentive Plan | |
| 10.3†# | 2014 Employee Stock Purchase Plan | |
| 10.4†# | Form of Indemnification Agreement between Registrant and its directors and executive officers | |
| 10.5†# | Executive Employment Agreement between the Registrant and Timothy C. Rodell | |
| 10.6†# | Executive Employment Agreement between Registrant and C. Jeffrey Dekker | |
| 10.6.1†# | Executive Employment Agreement between Registrant and Kirk A. Christoffersen | |
| 10.7# | Lease between Registrant and Triumph 1450 LLC, dated October 25, 2005 | |
| 10.7.1# | Lease Amendment between Registrant and Triumph 1450 LLC, dated August 25, 2006 | |
| 10.7.2# | Second Lease Amendment between Registrant and SF Infinite Drive, LLC, dated June 3, 2010 | |
| 10.7.3# | Third Lease Amendment between Registrant and SF Infinite Drive, LLC, dated October 31, 2013 | |
| 10.7.4# | Fourth Amendment to Lease Agreement between Registrant and SF Infinite Drive, LLC, dated April 14, 2014 | |
| 10.8* | Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of May 14, 2009 | |
| 10.8.1# | Amendment #1 to the Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of November 6, 2009 | |
Table of Contents
| Exhibit |
Description of Exhibit | |
| 10.8.2# | Amendment #2 to the Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of February 9, 2010 | |
| 10.8.3*# | Amendment #3 to the Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of June 16, 2011 | |
| 10.8.4*# | Amendment #4 to the Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of October 24, 2011 | |
| 10.9*# | GI-6300 Program License Agreement by and among the Registrant, Celgene Corporation, and Celgene Alpine Investment Co., LLC, dated July 26, 2013 | |
| 10.10*# | License and Collaboration Agreement between Registrant and Gilead Sciences, Inc., dated as of October 24, 2011 | |
| 10.10.1# | First Amendment to License and Collaboration Agreement between Registrant and Gilead Sciences, Inc. dated as of December 14, 2012 | |
| 10.11*# | Agreement between Registrant and The Regents of the University of Colorado, dated as of May 30, 2006 | |
| 10.11.1*# | Amendment (1) to Agreement and Restated Intellectual Property License Agreement among Registrant, The Regents of the University of Colorado and University License Equity Holdings, Inc., effective as of May 5, 2009 | |
| 10.11.2*# | Second Amendment to Agreement and Restated Intellectual Property License Agreement among Registrant, The Regents of the University of Colorado and University License Equity Holdings, Inc., effective as of March 12, 2010 | |
| 10.11.3*# | Stock Purchase Agreement between the Registrant and University License Equity Holding, Inc., dated the 8th day of August, 2003 | |
| 10.11.4*# | Stock Purchase Agreement between the Registrant and University License Equity Holding, Inc. dated the 15th day of October, 2003 | |
| 10.11.5*# | Stock Purchase Agreement between the Registrant and University License Equity Holding, Inc. dated the 7th day of September, 2004 | |
| 10.11.6*# | Stock Purchase Agreement between the Registrant and University License Equity Holding, Inc. dated the 25th day of August, 2005 | |
| 10.12*# | Cooperative Research and Development Agreement (CRADA #2264) between Registrant and National Cancer Institute, dated January 23, 2008 | |
| 10.12.1*# | Amendment No. 1 to CRADA #2264 between Registrant and National Cancer Institute, dated August 8, 2011 | |
| 10.12.2*# | Amendment No. 2 to CRADA #2264 between Registrant and National Cancer Institute, dated July 30, 2013 | |
| 10.13*# | Public Health Service Patent License Agreement – Exclusive (License Number: L127-2007/0) (CEA) between Registrant and the National Institutes of Health, or NIH, dated as of June 11, 2007 | |
| 10.13.1*# | First Amendment to Public Health Service Patent License Agreement – Exclusive (License Number: L127-2007/1) (CEA) between Registrant and the NIH, dated as of April 5, 2010 | |
| 10.13.2*# | Second Amendment to Public Health Service Patent License Agreement – Exclusive (License Number: L127-2007/2) (CEA) between Registrant and the NIH, dated as of October 31, 2011 | |
| 10.14*# | Public Health Service Patent License Agreement – Exclusive (License Number L-121-2011/0) (VirusPlus) between Registrant and the NIH, dated as of August 23, 2011 | |
Table of Contents
| Exhibit |
Description of Exhibit | |
| 10.15*# | Public Health Service Patent License Agreement – Exclusive (License Number: L-036-2012/0) (Brachyury) between Registrant and the NIH, dated as of January 3, 2012 | |
| 10.16*# | Public Health Service Patent License Agreement – Exclusive (License Number: L-067-2012/0) (MUC1) between Registrant and the NIH, dated as of March 12, 2012 | |
| 10.17†# | 2014 Performance Based Non-Equity Incentive Plan | |
| 10.18# | Form of Employee Proprietary Information and Inventions Agreement | |
| 10.19# | Engagement Letter between Registrant and Aegis Capital Corp., dated as of December 17, 2013 | |
| 10.20# | Placement Agency Agreement between Registrant and Aegis Capital Corp., dated as of January 27, 2014 | |
| 23.1 | Consent of KPMG LLP, independent registered public accounting firm | |
| 23.2# | Consent of Cooley LLP (included in Exhibit 5.1) | |
| 24.1# | Power of Attorney (see signature page of this registration statement) | |
| † | Indicates management contract or compensatory plan. |
| * | Indicates confidential treatment has been requested with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and Exchange Commission. |
| # | Previously filed. |
| (b) | Financial statement schedule. |
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or notes.
| Item 17. | Undertakings. |
The undersigned Registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective. |
Table of Contents
| (2) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona-fide offering thereof. |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Amendment No. 3 to the registration statement on From S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Louisville, State of Colorado, on June 4, 2014.
| GLOBEIMMUNE, INC. | ||
| By: | /s/ Timothy C. Rodell | |
| Timothy C. Rodell, M.D. Chief Executive Officer and President | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this Amendment No. 3 to the Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Timothy C. Rodell Timothy C. Rodell, M.D. |
Chief Executive Officer, President and Director (Principal Executive Officer) |
June 4, 2014 | ||
| /s/ C. Jeffrey Dekker C. Jeffrey Dekker |
Vice President, Finance and Treasurer (Principal Financial and Accounting Officer) |
June 4, 2014 | ||
| * J. William Freytag, Ph.D. |
Chairman of the Board of Directors and Director |
June 4, 2014 | ||
| * Ralph E. Christoffersen, Ph.D. |
Director | June 4, 2014 | ||
| * Augustine J. Lawlor |
Director | June 4, 2014 | ||
| * Paul A. Mieyal, Ph.D. |
Director | June 4, 2014 | ||
| * Dan J. Mitchell |
Director | June 4, 2014 | ||
| * Pennina Safer, Ph.D. |
Director | June 4, 2014 | ||
| * S. Edward Torres |
Director | June 4, 2014 | ||
| * By | /s/ Timothy C. Rodell | |
| Timothy C. Rodell | ||
| Attorney-in-Fact |
Table of Contents
EXHIBIT INDEX
| Exhibit |
Description of Exhibit | |
| 1.1# | Form of Underwriting Agreement | |
| 3.1# | Restated Certificate of Incorporation of Registrant filed on June 14, 2012 | |
| 3.2# | Certificate of Amendment to the Restated Certificate of Incorporation filed on August 31, 2012 | |
| 3.2.1# | Certificate of Amendment to the Restated Certificate of Incorporation filed on April 25, 2014 | |
| 3.3# | Form of Amended and Restated Certificate of Incorporation of Registrant to be effective upon completion of this offering | |
| 3.4# | Amended and Restated Bylaws of Registrant, as currently in effect | |
| 3.5# | Form of Amended and Restated Bylaws of Registrant to be effective upon completion of this offering | |
| 4.1# | Form of Registrant’s Common Stock Certificate | |
| 4.2# | Form of Warrants to purchase Series B Preferred Stock and a Schedule of Warrantholders | |
| 4.3# | Warrant to purchase Series B Preferred Stock, dated April 14, 2006, issued to SVB Financial Group | |
| 4.4# | Warrant to purchase Series B Preferred Stock, dated April 14, 2006, issued to Oxford Finance Corporation | |
| 4.5# | Form of Warrants to purchase Series C Preferred Stock and a Schedule of Warrantholders | |
| 4.6# | Convertible Promissory Note issued by Registrant to Cooley LLP, dated as of November 12, 2013 | |
| 4.7# | Form of Warrant Certificate and a Schedule of Warrantholders | |
| 4.8# | Form of Convertible Term Notes and a Schedule of Noteholders | |
| 4.9# | Form of Warrant to purchase capital stock issued to Aegis Capital Corp. or its designees | |
| 4.10# | Form of Warrant to purchase capital stock issued to Aegis Capital Corp. or its designees | |
| 4.11# | Form of Warrant to purchase Common Stock to be issued to Aegis Capital Corp. or its designees upon completion of this offering | |
| 4.12# | Fifth Amended and Restated Stockholders Agreement between Registrant and certain holders of Common and Preferred Stock dated January 14, 2010 | |
| 4.12.1# | Amendment No. 1 to Fifth Amended and Restated Stockholders Agreement between Registrant and certain holders of Common and Preferred Stock dated August 31, 2012 | |
| 4.13# | Form of Warrant to be issued to Cooley LLP upon completion of this offering | |
| 5.1# | Opinion of Cooley LLP | |
| 10.1†# | 2002 Stock Incentive Plan | |
| 10.1.1†# | Form of Incentive Stock Option Agreement under 2002 Stock Incentive Plan | |
| 10.1.2†# | Form of Non-Qualified Stock Option Agreement under 2002 Stock Incentive Plan | |
| 10.2†# | 2014 Equity Incentive Plan | |
| 10.2.1†# | Form of Stock Option Grant Notice and Stock Option Agreement under 2014 Equity Incentive Plan | |
| 10.3†# | 2014 Employee Stock Purchase Plan | |
Table of Contents
| Exhibit |
Description of Exhibit | |
| 10.4†# | Form of Indemnification Agreement between Registrant and its directors and executive officers | |
| 10.5†# | Executive Employment Agreement between the Registrant and Timothy C. Rodell | |
| 10.6†# | Executive Employment Agreement between Registrant and C. Jeffrey Dekker | |
| 10.6.1†# | Executive Employment Agreement between Registrant and Kirk A. Christoffersen | |
| 10.7# | Lease between Registrant and Triumph 1450 LLC, dated October 25, 2005 | |
| 10.7.1# | Lease Amendment between Registrant and Triumph 1450 LLC, dated August 25, 2006 | |
| 10.7.2# | Second Lease Amendment between Registrant and SF Infinite Drive, LLC, dated June 3, 2010 | |
| 10.7.3# | Third Lease Amendment between Registrant and SF Infinite Drive, LLC, dated October 31, 2013 | |
| 10.7.4# | Fourth Amendment to Lease Agreement between Registrant and SF Infinite Drive, LLC, dated April 14, 2014 | |
| 10.8* | Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of May 14, 2009 | |
| 10.8.1# | Amendment #1 to the Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of November 6, 2009 | |
| 10.8.2# | Amendment #2 to the Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of February 9, 2010 | |
| 10.8.3*# | Amendment #3 to the Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of June 16, 2011 | |
| 10.8.4*# | Amendment #4 to the Collaboration and Option Agreement between Registrant and Celgene Corporation, dated as of October 24, 2011 | |
| 10.9*# | GI-6300 Program License Agreement by and among the Registrant, Celgene Corporation, and Celgene Alpine Investment Co., LLC, dated July 26, 2013 | |
| 10.10*# | License and Collaboration Agreement between Registrant and Gilead Sciences, Inc., dated as of October 24, 2011 | |
| 10.10.1# | First Amendment to License and Collaboration Agreement between Registrant and Gilead Sciences, Inc, dated as of December 14, 2012 | |
| 10.11*# | Agreement between Registrant and The Regents of the University of Colorado, dated as of May 30, 2006 | |
| 10.11.1*# | Amendment (1) to Agreement and Restated Intellectual Property License Agreement among Registrant, The Regents of the University of Colorado and University License Equity Holdings, Inc., effective as of May 5, 2009 | |
| 10.11.2*# | Second Amendment to Agreement and Restated Intellectual Property License Agreement among Registrant, The Regents of the University of Colorado and University License Equity Holdings, Inc., effective as of March 12, 2010 | |
| 10.11.3*# | Stock Purchase Agreement between the Registrant and University License Equity Holding, Inc., dated the 8th day of August, 2003 | |
| 10.11.4*# | Stock Purchase Agreement between the Registrant and University License Equity Holding, Inc. dated the 15th day of October, 2003 | |
| 10.11.5*# | Stock Purchase Agreement between the Registrant and University License Equity Holding, Inc. dated the 7th day of September, 2004 | |
Table of Contents
| Exhibit |
Description of Exhibit | |
| 10.11.6*# | Stock Purchase Agreement between the Registrant and University License Equity Holding, Inc. dated the 25th day of August, 2005 | |
| 10.12*# | Cooperative Research and Development Agreement (CRADA #2264) between Registrant and National Cancer Institute, dated January 23, 2008 | |
| 10.12.1*# | Amendment No. 1 to CRADA #2264 between Registrant and National Cancer Institute, dated August 8, 2011 | |
| 10.12.2*# | Amendment No. 2 to CRADA #2264 between Registrant and National Cancer Institute, dated July 30, 2013 | |
| 10.13*# | Public Health Service Patent License Agreement – Exclusive (License Number: L127-2007/0) (CEA) between Registrant and the National Institutes of Health, or NIH, dated as of June 11, 2007 | |
| 10.13.1*# | First Amendment to Public Health Service Patent License Agreement – Exclusive (License Number: L127-2007/1) (CEA) between Registrant and the NIH, dated as of April 5, 2010 | |
| 10.13.2*# | Second Amendment to Public Health Service Patent License Agreement – Exclusive (License Number: L127-2007/2) (CEA) between Registrant and the NIH, dated as of October 31, 2011 | |
| 10.14*# | Public Health Service Patent License Agreement – Exclusive (License Number L-121-2011/0) (VirusPlus) between Registrant and the NIH, dated as of August 23, 2011 | |
| 10.15*# | Public Health Service Patent License Agreement – Exclusive (License Number: L-036-2012/0) (Brachyury) between Registrant and the NIH, dated as of January 3, 2012 | |
| 10.16*# | Public Health Service Patent License Agreement – Exclusive (License Number: L-067-2012/0) (MUC1) between Registrant and the NIH, dated as of March 12, 2012 | |
| 10.17†# | 2014 Performance-Based Non-Equity Incentive Plan | |
| 10.18# | Form of Employee Proprietary Information and Inventions Agreement | |
| 10.19# | Engagement Letter between Registrant and Aegis Capital Corp., dated as of December 17, 2013 | |
| 10.20# | Placement Agency Agreement between Registrant and Aegis Capital Corp., dated as of January 27, 2014 | |
| 23.1 | Consent of KPMG LLP, independent registered public accounting firm | |
| 23.2# | Consent of Cooley LLP (included in Exhibit 5.1) | |
| 24.1# | Power of Attorney (see signature page of this registration statement) | |
| † | Indicates management contract or compensatory plan. |
| * | Indicates confidential treatment has been requested with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and Exchange Commission. |
| # | Previously filed. |
