UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form
|
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended
OR
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number
(Exact name of registrant as specified in its charter)
|
|
2834 |
|
|
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
(
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
|
|
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
☒ |
|
Accelerated filer |
|
☐ |
||||
|
Non-accelerated filer |
|
☐ |
|
Smaller reporting company |
|
|
||||
|
Emerging growth company |
|
|
|
|
|
|
||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
As of January 31, 2021, the registrant had
ENANTA PHARMACEUTICALS, INC.
FORM 10-Q — Quarterly Report
For the Quarterly Period Ended December 31, 2020
TABLE OF CONTENTS
|
|
|
|
Page |
|
|
|
||
|
Item 1. |
|
3 |
|
|
|
|
3 |
|
|
|
|
4 |
|
|
|
Unaudited Consolidated Statements of Comprehensive Income (Loss) |
|
5 |
|
|
|
6 |
|
|
|
|
7 |
|
|
|
|
8 |
|
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
17 |
|
Item 3. |
|
25 |
|
|
Item 4. |
|
25 |
|
|
|
|
||
|
Item 1A. |
|
26 |
|
|
Item 6. |
|
51 |
|
|
|
52 |
||
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q, or Form 10-Q, contains forward-looking statements concerning our business, operations and financial performance and condition, as well as our plans, objectives and expectations for our business operations and financial performance and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about overall trends, royalty revenue trends, research and clinical development plans, liquidity and capital needs and other statements of expectations, beliefs, future plans and strategies, anticipated events or trends and similar expressions. These forward-looking statements are based on our management’s current expectations, estimates, forecasts and projections about our business and the industry in which we operate and our management’s beliefs and assumptions. These forward-looking statements are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this Form 10-Q may turn out to be inaccurate. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under “Risk Factors” and discussed elsewhere in this Form 10-Q. These forward-looking statements speak only as of the date of this Form 10-Q. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future. You should, however, review the factors and risks we describe in the reports we will file from time to time with the SEC after the date of this Form 10-Q.
2
PART I—FINANCIAL INFORMATION
|
ITEM 1. |
CONSOLIDATED FINANCIAL STATEMENTS |
ENANTA PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(unaudited)
(in thousands, except per share amounts)
|
|
|
December 31, |
|
|
September 30, |
|
||
|
|
|
2020 |
|
|
2020 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
|
|
|
$ |
|
|
|
Short-term marketable securities |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
|
|
|
|
|
|
|
Prepaid expenses and other current assets |
|
|
|
|
|
|
|
|
|
Total current assets |
|
|
|
|
|
|
|
|
|
Long-term marketable securities |
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
|
|
|
|
|
|
|
Deferred tax assets |
|
|
|
|
|
|
|
|
|
Operating lease, right-of-use assets |
|
|
|
|
|
|
|
|
|
Restricted cash |
|
|
|
|
|
|
|
|
|
Other long-term assets |
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
|
|
|
$ |
|
|
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
|
|
|
$ |
|
|
|
Accrued expenses and other current liabilities |
|
|
|
|
|
|
|
|
|
Operating lease liabilities |
|
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
|
|
|
|
|
|
|
Operating lease liabilities, net of current portion |
|
|
|
|
|
|
|
|
|
Series 1 nonconvertible preferred stock |
|
|
|
|
|
|
|
|
|
Other long-term liabilities |
|
|
|
|
|
|
|
|
|
Total liabilities |
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 11) |
|
|
|
|
|
|
|
|
|
Stockholders' equity: |
|
|
|
|
|
|
|
|
|
Common stock; $ September 30, 2020, respectively |
|
|
|
|
|
|
|
|
|
Additional paid-in capital |
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income |
|
|
|
|
|
|
|
|
|
Retained earnings |
|
|
|
|
|
|
|
|
|
Total stockholders' equity |
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders' equity |
|
$ |
|
|
|
$ |
|
|
The accompanying notes are an integral part of these consolidated financial statements.
3
ENANTA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
(in thousands, except per share amounts)
|
|
|
Three Months Ended |
|
|
|||||
|
|
|
December 31, |
|
|
|||||
|
|
|
2020 |
|
|
2019 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
Royalty revenue |
|
$ |
|
|
|
$ |
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
|
|
|
|
|
|
|
|
|
Income (loss) from operations |
|
|
( |
) |
|
|
|
|
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
Other income (expense), net |
|
|
|
|
|
|
|
|
|
|
Total other income (expense), net |
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
( |
) |
|
|
|
|
|
|
Income tax (expense) benefit |
|
|
|
|
|
|
( |
) |
|
|
Net income (loss) |
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share: |
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
( |
) |
|
$ |
|
|
|
|
Diluted |
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding: |
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
|
|
|
|
|
|
|
|
Diluted |
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
4
ENANTA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(unaudited)
(in thousands)
|
|
|
Three Months Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Net income (loss) |
|
$ |
( |
) |
|
$ |
|
|
|
Other comprehensive income: |
|
|
|
|
|
|
|
|
|
Net unrealized gains (losses) on marketable securities, net of tax of $ |
|
|
( |
) |
|
|
|
|
|
Total other comprehensive income (loss), net of tax |
|
|
( |
) |
|
|
|
|
|
Comprehensive income (loss) |
|
$ |
( |
) |
|
$ |
|
|
The accompanying notes are an integral part of these consolidated financial statements.
5
ENANTA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(unaudited)
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Other |
|
|
|
|
|
|
Total |
|
|||
|
|
|
Common Stock |
|
|
Paid-In |
|
|
Comprehensive |
|
|
Retained |
|
|
Stockholders' |
|
|||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income |
|
|
Earnings |
|
|
Equity |
|
||||||
|
Balances at September 30, 2019 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Exercise of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Vesting of restricted stock units, net of withholding |
|
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Other comprehensive income, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Balances at December 31, 2019 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Other |
|
|
|
|
|
|
Total |
|
|||
|
|
|
Common Stock |
|
|
Paid-In |
|
|
Comprehensive |
|
|
Retained |
|
|
Stockholders' |
|
|||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income (Loss) |
|
|
Earnings |
|
|
Equity |
|
||||||
|
Balances at September 30, 2020 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Exercise of stock options |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Vesting of restricted stock units, net of withholding |
|
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Balances at December 31, 2020 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
The accompanying notes are an integral part of these consolidated financial statements
6
ENANTA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
(in thousands)
|
|
|
Three Months Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
( |
) |
|
$ |
|
|
|
Adjustments to reconcile net income to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
Depreciation and amortization expense |
|
|
|
|
|
|
|
|
|
Deferred income taxes |
|
|
— |
|
|
|
|
|
|
Premium paid on marketable securities |
|
|
( |
) |
|
|
( |
) |
|
(Accretion) amortization of (discount) premium on marketable securities |
|
|
|
|
|
|
( |
) |
|
Other non-cash items |
|
|
— |
|
|
|
( |
) |
|
Change in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
( |
) |
|
|
( |
) |
|
Prepaid expenses and other current assets |
|
|
( |
) |
|
|
|
|
|
Operating lease, right-of-use assets |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
|
|
|
|
|
( |
) |
|
Accrued expenses |
|
|
( |
) |
|
|
( |
) |
|
Operating lease liabilities |
|
|
( |
) |
|
|
( |
) |
|
Other long-term liabilities |
|
|
( |
) |
|
|
( |
) |
|
Net cash provided by (used in) operating activities |
|
|
( |
) |
|
|
|
|
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Purchase of marketable securities |
|
|
( |
) |
|
|
( |
) |
|
Proceeds from maturities and sale of marketable securities |
|
|
|
|
|
|
|
|
|
Purchase of property and equipment |
|
|
( |
) |
|
|
( |
) |
|
Net cash used in investing activities |
|
|
( |
) |
|
|
( |
) |
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from exercise of stock options |
|
|
|
|
|
|
|
|
|
Payments for settlement of share-based awards |
|
|
( |
) |
|
|
( |
) |
|
Net cash provided by financing activities |
|
|
|
|
|
|
|
|
|
Net decrease in cash, cash equivalents and restricted cash |
|
|
( |
) |
|
|
( |
) |
|
Cash, cash equivalents and restricted cash at beginning of period |
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and restricted cash at end of period |
|
$ |
|
|
|
$ |
|
|
|
Supplemental disclosure of non-cash operating and investing information: |
|
|
|
|
|
|
|
|
|
Purchases of fixed assets included in accounts payable and accrued expenses |
|
$ |
|
|
|
$ |
|
|
|
Operating lease liabilities arising from obtaining right-of-use assets |
|
$ |
|
|
|
$ |
|
|
The accompanying notes are an integral part of these consolidated financial statements.
7
ENANTA PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(unaudited)
(Amounts in thousands, except per share data)
|
1. |
Nature of the Business and Basis of Presentation |
Enanta Pharmaceuticals, Inc. (the “Company”), incorporated in Delaware in
The Company is subject to many of the risks common to companies in the biotechnology industry including, but not limited to, the uncertainties of research and development, competition from technological innovations of others, dependence on collaborative arrangements, protection of proprietary technology, dependence on key personnel and compliance with government regulation. Product candidates currently under development will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approvals, prior to commercialization. These efforts require significant amounts of capital, adequate personnel infrastructure, and extensive compliance reporting capabilities.
COVID-19
In March 2020, the World Health Organization declared COVID-19 a global pandemic and countries worldwide implemented various measures to contain the spread of the virus. National, state and local governments in affected regions have implemented and may continue to implement safety precautions, including quarantines, border closures, increased border controls, travel restrictions, shelter-in-place orders and shutdowns, business closures, cancellations of public gatherings and other measures. The extent and severity of the impact on the Company’s business and clinical trials will be determined largely by the extent to which there are disruptions in the supply chains for its research and product candidates, delays in the conduct of ongoing and future clinical trials, or reductions in the number of patients accessing AbbVie’s HCV regimens, or any combination of those events. During the second half of fiscal 2020 and into fiscal 2021, AbbVie experienced a decline in HCV sales compared to prior years as a result of a decline in patients accessing AbbVie’s HCV regimens due to the COVID-19 pandemic.
The full extent to which the COVID-19 pandemic will continue to directly or indirectly impact the Company’s business, results of operations and financial condition will depend on future developments that are highly uncertain and cannot be accurately predicted, including new information that may emerge concerning COVID-19 and its variants, public health actions taken to contain the pandemic and mitigate its economic effects and to roll out vaccinations worldwide, as well as the cumulative economic impact of all of these factors.
Unaudited Interim Financial Information
The consolidated balance sheet at September 30, 2020 was derived from audited financial statements but does not include all disclosures required by accounting principles generally accepted in the United States of America (“GAAP”). The accompanying unaudited consolidated financial statements as of December 31, 2020 and for the three months ended December 31, 2020 and 2019 have been prepared by the Company pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim financial statements. Certain information and footnote disclosures normally included in financial statements prepared in accordance with GAAP have been condensed or omitted pursuant to such rules and regulations. These financial statements should be read in conjunction with the Company’s audited financial statements and the notes thereto included in the Company’s Annual Report on Form 10-K for the year ended September 30, 2020.
In the opinion of management, all adjustments, consisting of normal recurring adjustments necessary for a fair statement of the Company’s financial position as of December 31, 2020 and results of operations for the three months ended December 31, 2020 and 2019 and cash flows for the three months ended December 31, 2020 and 2019, have been made. The results of operations for the three months ended December 31, 2020 are not necessarily indicative of the results of operations that may be expected for subsequent quarters or the year ending September 30, 2021.
8
The accompanying consolidated financial statements have been prepared in conformity with GAAP. All amounts in the consolidated financial statements and in the notes to the consolidated financial statements, except per share amounts, are in thousands unless otherwise indicated.
|
2. |
Summary of Significant Accounting Policies |
For the Company’s Significant Accounting Policies, please refer to its Annual Report on Form 10-K for the fiscal year ended September 30, 2020. Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Significant estimates and assumptions reflected in these consolidated financial statements include, but are not limited to, management’s judgments with respect to its revenue arrangements; valuation of Series 1 nonconvertible preferred stock and stock-based awards; the accrual of research and development expenses, and the accounting for income taxes, including uncertain tax positions and the valuation of net deferred tax assets. Estimates are periodically reviewed in light of changes in circumstances and other facts. The future developments of the COVID-19 pandemic which may directly or indirectly impact the Company’s business include quarantines, border closures, increased border controls, travel restrictions, shelter-in-place orders and shutdowns, business closures, cancellations of public gatherings and other measures. The Company has made estimates of the impact of COVID-19 in the Company’s consolidated financial statements as of December 31, 2020. Actual results could differ from the Company’s estimates.
Recently Adopted Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses (Topic 326) (“ASU 2016-13”), which introduces a new methodology for accounting for credit losses on financial instruments, including available-for-sale debt securities. The guidance establishes a new “expected loss model” that requires entities to estimate current expected credit losses on financial instruments by using all practical and relevant information. Any expected credit losses are to be reflected as allowances rather than reductions in the amortized cost of available-for-sale debt securities. The Company adopted ASU 2016-13 during the three months ended December 31, 2020. For available-for-sale debt securities with unrealized losses, the Company measures credit losses in a manner similar to previous U.S. GAAP, except that losses will be recognized as allowances instead of reductions in the amortized cost of the debt securities. The adoption of ASU 2016-13 did not have a material impact on the consolidated financial statements.
Recently Issued Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2019-12”). This standard simplifies various aspects of the income tax accounting guidance in ASC 740, including requirements related to hybrid tax regimes, the tax basis step-up in goodwill obtained in a transaction that is not a business combination, separate financial statements of entities not subject to tax, the intra-period tax allocation exception to the incremental approach, ownership changes in investments, changes from a subsidiary to an equity method investment, interim-period accounting for enacted changes in tax law, and the year-to-date loss limitation in interim-period tax accounting. This amendment is effective for the Company in the fiscal year beginning October 1, 2021; however, early adoption is permitted. The Company is currently in the process of evaluating the impact that ASU 2019-12 may have on its consolidated financial statements.
9
|
3. |
Fair Value of Financial Assets and Liabilities |
The following tables present information about the Company’s financial assets and liabilities that were subject to fair value measurement on a recurring basis as of December 31, 2020 and September 30, 2020, and indicate the fair value hierarchy of the valuation inputs utilized to determine such fair value:
|
|
|
Fair Value Measurements at December 31, 2020 Using: |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
|
|
(in thousands) |
|
|||||||||||||
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
|
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Treasury notes |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Commercial paper |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Corporate bonds |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series 1 nonconvertible preferred stock |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
$ |
|
|
|
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
$ |
|
|
|
|
|
Fair Value Measurements at September 30, 2020 Using: |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
|
|
(in thousands) |
|
|||||||||||||
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
|
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Treasury notes |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Commercial paper |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Corporate bonds |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series 1 nonconvertible preferred stock |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
|
|
|
|
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
$ |
|
|
During the three months ended December 31, 2020 and 2019, there were
The outstanding shares of Series 1 nonconvertible preferred stock are measured at fair value. The fair value of these instruments was based on significant inputs not observable in the market, which represented a Level 3 measurement within the fair value hierarchy. The Company utilized a probability-weighted valuation model which takes into consideration various outcomes that may require the Company to transfer assets upon exercise. Changes in the fair value of the Series 1 nonconvertible preferred stock are recognized in other income (expense), net in the consolidated statements of operations.
The recurring Level 3 fair value measurements of the Company’s outstanding Series 1 nonconvertible preferred stock using probability-weighted discounted cash flow include the following significant unobservable inputs:
|
|
|
Range |
||
|
|
|
December 31, |
|
September 30, |
|
Unobservable Input |
|
2020 |
|
2020 |
|
Probabilities of payout |
|
|
|
|
|
Discount rate |
|
|
|
|
10
The following table provides a rollforward of the aggregate fair values of the Company’s outstanding Series 1 nonconvertible preferred stock for which fair value is determined by Level 3 inputs:
|
|
|
Series 1 Nonconvertible Preferred Stock |
|
|
|
Balance, September 30, 2020 |
|
$ |
|
|
|
Change in fair value of nonconvertible preferred stock |
|
|
— |
|
|
Balance, December 31, 2020 |
|
$ |
|
|
|
4. |
Marketable Securities |
As of December 31, 2020 and September 30, 2020, the fair value of available-for-sale marketable securities, by type of security, was as follows:
|
|
|
December 31, 2020 |
|
|||||||||||||||||
|
|
|
Amortized Cost |
|
|
Gross Unrealized Gains |
|
|
Gross Unrealized Losses |
|
|
Credit Losses |
|
|
Fair Value |
|
|||||
|
|
|
(in thousands) |
|
|||||||||||||||||
|
Corporate bonds |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
— |
|
|
$ |
|
|
|
Commercial Paper |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
U.S. Treasury notes |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
— |
|
|
$ |
|
|
|
|
|
September 30, 2020 |
|
|||||||||||||||||
|
|
|
Amortized Cost |
|
|
Gross Unrealized Gains |
|
|
Gross Unrealized Losses |
|
|
Credit Losses |
|
|
Fair Value |
|
|||||
|
|
|
(in thousands) |
|
|||||||||||||||||
|
Corporate bonds |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
— |
|
|
$ |
|
|
|
Commercial Paper |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
U.S. Treasury notes |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
— |
|
|
$ |
|
|
As of December 31, 2020 and September 30, 2020 marketable securities consisted of investments that mature within
11
|
5. |
Accrued Expenses and Other Long-Term Liabilities |
Accrued expenses and other current liabilities, as well as other long-term liabilities, consisted of the following as of December 31, 2020 and September 30, 2020:
|
|
|
December 31, |
|
|
September 30, |
|
||
|
|
|
2020 |
|
|
2020 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Accrued expenses: |
|
|
|
|
|
|
|
|
|
Accrued research and development expenses |
|
$ |
|
|
|
$ |
|
|
|
Accrued pharmaceutical drug manufacturing |
|
|
|
|
|
|
|
|
|
Accrued payroll and related expenses |
|
|
|
|
|
|
|
|
|
Accrued professional fees |
|
|
|
|
|
|
|
|
|
Accrued other |
|
|
|
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other long-term liabilities: |
|
|
|
|
|
|
|
|
|
Uncertain tax positions |
|
$ |
|
|
|
$ |
|
|
|
Asset retirement obligation |
|
|
|
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
6. |
AbbVie Collaboration |
The Company has a Collaborative Development and License Agreement (as amended, the “AbbVie Agreement”), with AbbVie to identify, develop and commercialize HCV NS3 and NS3/4A protease inhibitor compounds, including paritaprevir and glecaprevir, under which the Company has received license payments, proceeds from a sale of preferred stock, research funding payments, milestone payments and royalties totaling approximately $
The Company is receiving annually tiered royalties per Company protease product ranging
|
7. |
Series 1 Nonconvertible Preferred Stock |
As of December 31, 2020,
|
8. |
Stock-Based Awards |
The Company grants stock-based awards, including stock options and unit awards under its 2019 Equity Incentive Plan (the “2019 Plan”), which was approved by its stockholders on February 28, 2019. The Company also has outstanding unit awards, stock options and restricted stock unit awards under its 2012 Equity Incentive Plan (the “2012 Plan”) and its amended and restated 1995 Equity Incentive Plan (the “1995 Plan”), but is no longer granting awards under these plans.
12
|
|
|
Shares Issuable Under Options |
|
|
Weighted Average Exercise Price |
|
|
Weighted Average Remaining Contractual Term |
|
|
Aggregate Intrinsic Value |
|
||||
|
|
|
(in thousands) |
|
|
|
|
|
|
(in years) |
|
|
(in thousands) |
|
|||
|
Outstanding as of September 30, 2020 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding as of December 31, 2020 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
Options vested and expected to vest as of December 31, 2020 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
Options exercisable as of December 31, 2020 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
Market and Performance-Based Stock Unit Awards
The Company awards both performance share units, or PSUs, and relative total stockholder return units, or rTSRUs, to its executive officers. The number of units granted represents the target number of shares of common stock that may be earned; however, the actual number of shares that may be earned ranges from
|
|
|
PSUs |
|
|
rTSRUs |
|
||||||||||
|
|
|
Shares |
|
|
Weighted Average Grant Date Fair Value |
|
|
Shares |
|
|
Weighted Average Grant Date Fair Value |
|
||||
|
|
|
(in thousands, except per share data) |
|
|||||||||||||
|
Unvested at September 30, 2020 |
|
|
|
|
|
$ |
|
|
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Cancelled |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Unvested at December 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
|
|
13
Restricted Stock Units
During the three months ended December 31, 2016, the Company awarded restricted stock units to its employees, which vest
|
|
|
Restricted Stock Units |
|
|
Weighted Average Grant Date Fair Value |
|
||
|
|
|
(in thousands, except per share data) |
|
|||||
|
Unvested at September 30, 2020 |
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
Vested |
|
|
( |
) |
|
|
|
|
|
Cancelled |
|
|
( |
) |
|
|
|
|
|
Unvested at December 31, 2020 |
|
|
|
|
|
$ |
|
|
Stock-Based Compensation Expense
During the three months ended December 31, 2020 and 2019, the Company recognized the following stock-based compensation expense:
|
|
|
Three Months ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Research and development |
|
$ |
|
|
|
$ |
|
|
|
General and administrative |
|
|
|
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
Three Months ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Stock options |
|
$ |
|
|
|
$ |
|
|
|
Performance stock units |
|
|
— |
|
|
|
|
|
|
rTSRUs |
|
|
|
|
|
|
|
|
|
Restricted stock units |
|
|
|
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
During the three months ended December 31, 2019, the Company recognized stock-based compensation expense for performance-based stock units for which vesting became probable upon achievement of performance-based targets that occurred during the performance period. During the three months ended December 31, 2020 no such units vested.
As of December 31, 2020, the Company had an aggregate of $
14
|
9. |
Net Income (Loss) Per Share |
Basic and diluted net income (loss) per share attributable to common stockholders was calculated as follows for the three months ended December 31, 2020 and 2019:
|
|
|
Three Months Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(in thousands, except per share data) |
|
|||||
|
Basic net income (loss) per share: |
|
|
|
|
|
|
|
|
|
Numerator: |
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
( |
) |
|
$ |
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding —basic |
|
|
|
|
|
|
|
|
|
Net income (loss) per share common share—basic |
|
$ |
( |
) |
|
$ |
|
|
|
Diluted net income (loss) per share: |
|
|
|
|
|
|
|
|
|
Numerator: |
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
( |
) |
|
$ |
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding —basic |
|
|
|
|
|
|
|
|
|
Dilutive effect of common stock equivalents |
|
|
— |
|
|
|
|
|
|
Weighted average common shares outstanding —diluted |
|
|
|
|
|
|
|
|
|
Net income (loss) per share common share—diluted |
|
$ |
( |
) |
|
$ |
|
|
|
Anti-dilutive common stock equivalents excluded from above |
|
|
|
|
|
|
|
|
The impact of certain common stock equivalents was excluded from the computation of diluted net loss per share for the periods in which the Company incurred a net loss since the impact of such common stock equivalents would have been anti-dilutive.
|
10. |
Income Taxes |
For the three months ended December 31, 2020 and 2019, the Company recorded an income tax benefit of $
The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. In the normal course of business, the Company is subject to examination by federal and state jurisdictions, where applicable. The Company’s tax years are still open under statute for the year ended September 30, 2017 to the present. Earlier years may be examined to the extent that tax credit or net operating loss carryforwards are used in future or prior periods. The Company is not currently under examination by any jurisdiction for any tax year open under statute.
The Company had unrecognized tax benefits totaling $
15
As of December 31, 2020, the Company recorded a valuation allowance against the majority of its net deferred tax assets. The Company continues to believe it more likely that it will not have sufficient taxable income in the future that will allow it to realize all of its existing deferred tax assets. This is due to the fact the Company continues to progress its wholly-owned research and development programs and its declining royalty revenues from its Collaboration Agreement with AbbVie.
|
11. |
Commitments and Contingencies |
Litigation and Contingencies Related to Use of Intellectual Property
From time to time, the Company may become subject to legal proceedings, claims and litigation arising in the ordinary course of business. The Company currently is not a party to any litigation. However, third parties might allege that the Company or its collaborators are infringing their patent rights or that the Company is otherwise violating their intellectual property rights. Such third parties may resort to litigation against the Company or its collaborators, which the Company has agreed to indemnify. With respect to some of these patents, the Company expects that it will be required to obtain licenses and could be required to pay license fees or royalties, or both. These licenses may not be available on acceptable terms, or at all. A costly license, or inability to obtain a necessary license, would have a material adverse effect on the Company’s financial condition, results of operations or cash flows. The Company accrues contingent liabilities when it is probable that future expenditures will be made and such expenditures can be reasonably estimated.
Indemnification Agreements
In the ordinary course of business, the Company may provide indemnifications of varying scope and terms to customers, vendors, lessors, business partners, and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements or from services to be provided to the Company, or from intellectual property infringement claims made by third parties. In addition, the Company has entered into indemnification agreements with members of its board of directors and its executive officers that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors or officers. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is, in many cases, unlimited. To date, the Company has not incurred any material costs as a result of such indemnifications. In addition, the Company maintains officers and directors insurance coverage. The Company does not believe that the outcome of any claims under indemnification arrangements will have a material effect on its financial position, results of operations or cash flows, and it has not accrued any liabilities related to such obligations in its financial statements as of December 31, 2020.
16
|
ITEM 2. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the unaudited consolidated financial statements and notes thereto included elsewhere in this Quarterly Report on Form 10-Q and the audited consolidated financial statements and notes thereto for our fiscal year ended September 30, 2020 included in our Annual Report on Form 10-K for that fiscal year which is referred to as our 2020 Form 10-K. Please refer to our note regarding forward-looking statements on page 2 of this Form 10-Q, which is incorporated herein by this reference.
The Enanta name and logo are our trademarks. This Quarterly Report also includes trademarks, trade names and service marks of other persons. All other trademarks, trade names and service marks appearing in this Quarterly Report are the property of their respective owners.
Overview
We are a biotechnology company that uses our robust, chemistry-driven approach and drug discovery capabilities to become a leader in the discovery and development of small molecule drugs for the treatment of viral infections and liver diseases. We discovered glecaprevir, the second of two protease inhibitors discovered and developed through our collaboration with AbbVie for the treatment of chronic hepatitis C virus, or HCV. Glecaprevir is co-formulated as part of AbbVie’s leading brand of direct-acting antiviral, or DAA, combination treatment for HCV, which is marketed under the tradenames MAVYRET® (U.S.) and MAVIRET® (ex-U.S.) (glecaprevir/pibrentasvir). Our royalties from our AbbVie collaboration provide us funding to support our wholly-owned research and development programs, which are primarily focused on the following disease targets:
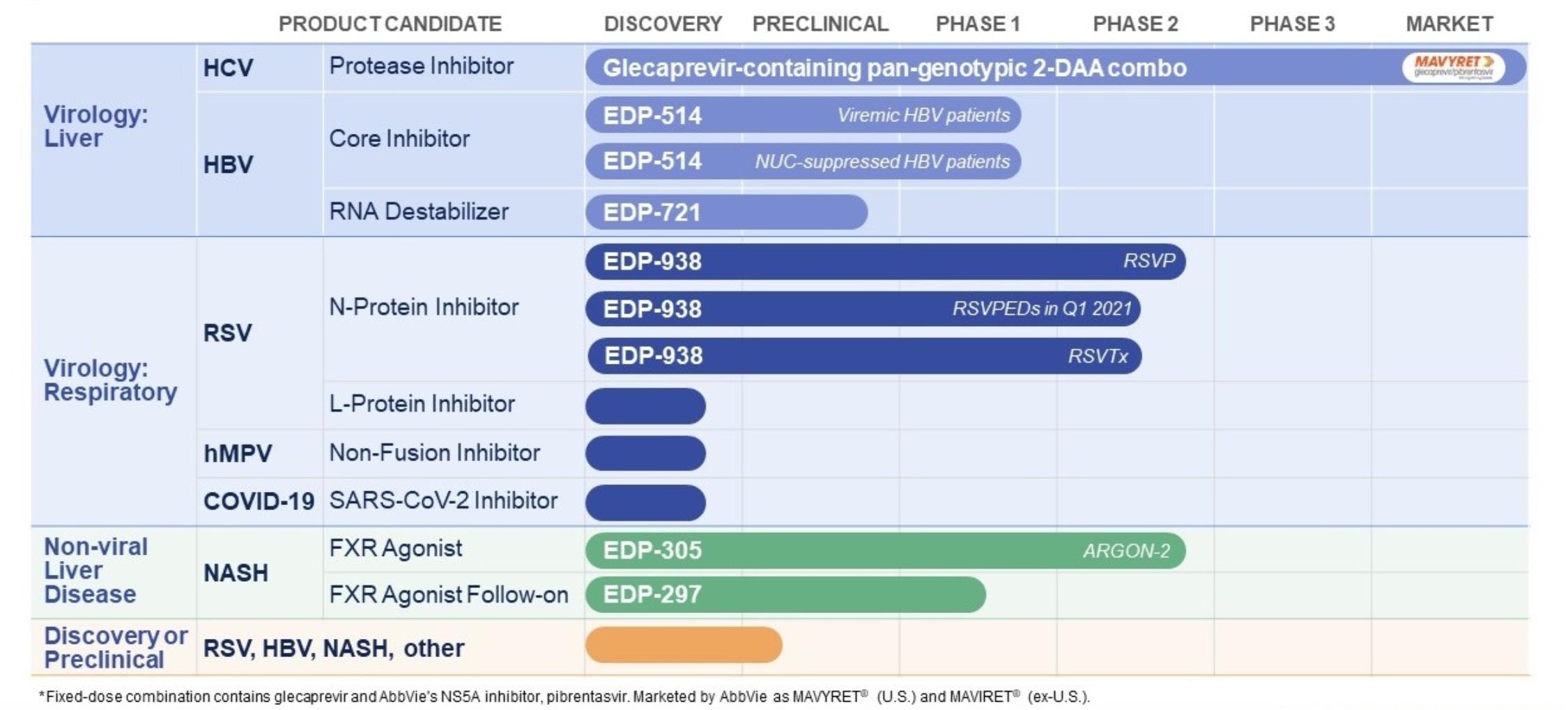
|
|
• |
Respiratory syncytial virus, or RSV, the most common cause of bronchiolitis and pneumonia in young children and a significant cause of respiratory illness in older adults, with estimates suggesting that approximately 200,000 hospitalizations in the U.S. and EU occur each year in children under the age of two and approximately 170,000 hospitalizations in these regions occur each year in adults over the age of 65; |
|
|
• |
Hepatitis B virus, or HBV, the most prevalent chronic hepatitis, which is estimated by the World Health Organization to affect more than 250 million individuals worldwide; |
|
|
• |
SARS-CoV-2, the virus that causes COVID-19; |
|
|
• |
Human metapneumovirus, or hMPV, a virus that causes respiratory infection with symptoms similar to RSV; and |
|
|
• |
Non-alcoholic steatohepatitis, or NASH, a liver disease estimated to affect approximately 1.5% to 6.5% of the population in the developed world (which translates to approximately 5 to 20 million individuals in the U.S. alone). |
We had $404.7 million in cash, cash equivalents and short-term and long-term marketable securities at December 31, 2020. In fiscal 2020, we earned $122.5 million in product royalties on AbbVie’s net sales of its HCV regimens. We expect cash flows from our continuing HCV royalties and our existing financial resources will allow us to continue to fund our wholly-owned research and development programs for the foreseeable future.
Our Wholly-Owned Programs
Our wholly-owned research and development programs are in virology, namely RSV, HBV, SARS-CoV-2 and hMPV, and in NASH, a non-viral liver disease:
|
|
• |
RSV: We have a clinical stage program for RSV, for which the lead asset is EDP-938. |
|
|
o |
In June 2019, we announced positive topline results from our Phase 2a human challenge study of EDP-938 in healthy adults infected with a specific strain of RSV. Data from this study demonstrated that EDP-938 achieved highly statistically significant reductions (p=<0.001) in RSV viral load (by both qRT-PCR and plaque assays), total symptom scores and mucus weights compared to placebo. In addition, the study showed that mean trough levels of drug achieved in study subjects were 20-40x higher than the amount of EDP-938 that has been shown in previously reported in vitro studies to reduce 90% of the viral RNA in RSV-infected human cells. |
|
|
o |
Based on the results of the challenge study, we now have an ongoing Phase 2b study of EDP-938, which is in adult outpatients with community-acquired RSV infection. This study, named RSVP, is designed to help us better understand the feasibility of this direct-acting antiviral therapy. While many of the sites for the Northern Hemisphere missed an unusually early RSV season in 2019-2020 and COVID-19 delayed or prevented activation of some trial sites in the Southern Hemisphere, the mitigation steps to manage the COVID-19 pandemic significantly reduced the incidence of respiratory illnesses (other than COVID-19) at the activated sites in that region’s RSV |
17
|
|
season. We reactivated sites in North America and added sites in several countries in Europe and Asia in preparation for the 2020-2021 winter RSV season, which has yet to begin due to COVID-19 mitigation measures. |
|
|
o |
We also have planned to initiate two additional Phase 2 studies of EDP-938, the first of which is a Phase 2b study called RSVTx in adult hematopoietic cell transplant recipients with acute RSV infection and symptoms of upper respiratory tract infection, which we initiated in December 2020. We plan to enroll, within 72 hours of symptom onset, approximately 200 adult subjects 18 to 75 years of age, who will receive EDP-938 or placebo for 21 days. The primary endpoint is the incidence of lower respiratory tract complications within 28 days of enrollment, while secondary endpoints include change from baseline in RSV RNA viral load, safety and pharmacokinetics. In addition, we plan to initiate a Phase 2 RSV study called RSVPEDs in pediatric patients. The double-blind, placebo-controlled study in hospitalized and non-hospitalized pediatric RSV patients age 28 days to 24 months, is expected to initiate in the first calendar quarter of 2021. |
|
|
o |
It is not possible to know when RSV will be prevalent again, but we have established trial sites in many regions of the world in order to be ready when it re-emerges. |
|
|
o |
We also have initiated an RSV L-protein inhibitor discovery effort centered around potent nanomolar leads active against both RSV-A and RSV-B, for potential use alone or in combination with agents targeting other RSV mechanisms, such as our lead RSV asset, EDP-938. |
|
|
• |
HBV: We have two candidates for the treatment of chronic infection with hepatitis B virus, or HBV: EDP-514, a novel core inhibitor that displays potent anti-HBV activity in vitro at multiple points in the HBV lifecycle, and EDP-721, our newest clinical candidate, which is a potent and selective oral HBV RNA destabilizer. |
|
|
o |
Our initial study of EDP-514 was a randomized, double-blind, placebo-controlled Phase 1a/1b study designed in two parts. In February 2020, we announced positive results from Part 1 of the Phase 1a/b clinical study in healthy subjects, which demonstrated that EDP-514 was well-tolerated with a favorable safety profile, and demonstrated a pharmacokinetic profile supportive of once daily dosing. These data enabled us to move on to Part 2 of the study, where we are studying EDP-514 in chronic HBV patients already being treated with a marketed nucleoside reverse transcriptase inhibitor, which we refer to as NUC-suppressed patients. In addition, we have a second Phase 1b study, which we initiated in July 2020, to evaluate EDP 514 in chronic HBV patients with high viral load not currently on treatment, which we refer to as viremic patients. Subject to any future adverse impact of the COVID-19 pandemic, we expect to have topline results for both of these studies in the second calendar quarter of 2021. |
|
|
o |
EDP-721 is a potent and selective oral HBV RNA destabilizer, which has demonstrated a robust reduction of viral transcripts in preclinical models, leading to reduced production of multiple HBV proteins, including HBV surface antigen (HBsAg) and e-antigen (HBeAg). EDP-721 is expected to reduce HBsAg derived from both integrated and covalently-closed circular DNA (cccDNA). Since high levels of HBsAg suppress immune responses through multiple mechanisms, a sustained reduction of HBsAg is regarded as a key component of a functional cure for HBV. We plan to develop EDP-721 for use alone or in combination with other mechanisms, such as EDP-514, with the ultimate goal of achieving a functional cure for chronic HBV infection. We expect to initiate a Phase 1 clinical study of EDP-721 in mid calendar 2021. |
|
|
• |
COVID-19: Since we announced our newest discovery program for the treatment of COVID-19, we have been leveraging our expertise in direct-acting antiviral mechanisms to discover new compounds to treat COVID-19, using a combination of screening and targeted drug design. |
|
|
• |
hMPV: Since announcing our new drug discovery effort for hMPV in January 2020, we have been optimizing nanomolar inhibitor leads against this virus and are working toward identifying our first clinical candidate for this indication. |
|
|
• |
NASH: We currently have two compounds in clinical development for NASH: EDP-305 and EDP-297, referred to as FXR agonists. These compounds, which selectively bind to and activate the Farnesoid X receptor, or FXR, represent a class of FXR agonists designed to take advantage of increased binding interactions with this receptor. |
|
|
o |
In July 2020 we initiated ARGON-2, a 72-week, Phase 2b, randomized, double-blind, placebo-controlled study, with histological endpoints, including fibrosis in 340 biopsy-confirmed NASH patients treated with EDP-305 or placebo. We are using EDP-305 doses of 1.5 mg and 2.0 mg, which we believe will favorably balance strong, efficacious target engagement with tolerability. |
|
|
o |
In September 2020, we initiated a Phase 1 clinical study of EDP-297, a highly potent and targeted follow-on FXR agonist, being developed for the treatment of NASH. The Phase 1, randomized, double-blind, placebo-controlled, first-in-human study is designed to assess the safety, tolerability, and pharmacokinetics, including the effect of food intake, of orally administered EDP-297 in approximately 74 healthy adult subjects. Two phases are planned: a single ascending dose phase enrolling six cohorts, including a two-part food effect cohort, and a multiple ascending |
18
|
|
dose phase enrolling three cohorts. Subject to any future adverse impact of the COVID-19 pandemic, we expect to have preliminary data from this study in mid-2021. |
|
|
o |
In mid-2021 we also expect that ARGON-2 will provide us an internal interim analysis at 12 weeks of treatment on a subset of patients, at which point we expect to have more information to enhance our ability to determine what dose or doses we move forward through partnering for potential combinations with other mechanisms for NASH with fibrosis. |
|
|
o |
Accordingly, in mid-2021 we expect to be positioned to prioritize our NASH clinical assets and define our optimal go-forward strategy. |
|
|
o |
In addition, we have been pursuing research in other mechanisms that may provide therapeutic benefit in NASH, any of which might be used in combination with an FXR agonist or other therapies for NASH. |
We have utilized our internal chemistry and drug discovery capabilities to generate all of our development-stage programs. We continue to invest substantial resources in research programs to discover back-up compounds as well as new compounds targeting different mechanisms of action, both in our disease areas of focus as well as potentially in other disease areas.
The following table summarizes our product development pipeline in our liver disease and virology programs:

Our Royalty Revenue Collaboration
Our royalty revenue is generated through our Collaborative Development and License Agreement with AbbVie, under which we have discovered and out-licensed to AbbVie two protease inhibitor compounds that have been clinically tested, manufactured, and commercialized by AbbVie as part of its combination regimens for HCV.
Glecaprevir is the protease inhibitor we discovered that was developed by AbbVie in a fixed-dose combination with its NS5A inhibitor, pibrentasvir, for the treatment of HCV. This patented combination, currently marketed under the brand names MAVYRET® (U.S.) and MAVIRET® (ex-U.S.), is referred to in this report as MAVYRET/MAVIRET. Since August 2017, substantially all of our royalty revenue has been derived from AbbVie’s net sales of MAVYRET/MAVIRET. Our ongoing royalty revenues from this regimen consist of annually tiered, double-digit, per-product royalties on 50% of the calendar year net sales of the 2-DAA glecaprevir/pibrentasvir combination in MAVYRET/MAVIRET. The annual royalty tiers return to the lowest tier for sales on and after each January 1.
COVID-19 Update
The current COVID-19 pandemic has presented substantial challenges for public health and economies around the world, and it is affecting our clinical trials, our royalty revenues received from AbbVie, and our business operations. The full extent to which the COVID-19 pandemic will directly or indirectly impact our business, results of operations and financial condition will depend on
19
future developments that are highly uncertain and cannot be accurately predicted, including new information that may emerge concerning COVID-19 and public health actions taken to contain it, the roll out of vaccinations worldwide, as well as the cumulative economic impact of all of these factors. Additionally, as new, more infectious variants emerge, it is possible that the impact of the pandemic on our business may increase or lengthen in duration.
We are continuing to assess the potential impact of the COVID-19 pandemic on our business and operations, including our expenses, clinical trials and royalty revenues. The majority of our employees have continued to work from home since March 2020, while scientific personnel have been working in our laboratories, on a shift basis, since May 2020.
Our third-party contract manufacturing partners continue to operate at or near normal levels producing drug substance and drug product for our research and clinical development programs, so we currently do not anticipate any material interruptions in our supply chain, but it is possible that may change. We paused enrollment in two of our ongoing clinical trials, ARGON-2 and our HBV nuc-suppressed study, in March 2020 and resumed recruiting in these studies in July 2020. Also, there has been no RSV season yet in the Northern Hemisphere this winter due to continuing COVID-19 mitigation measures, which has adversely affected enrollment in our RSVP study, and we continue to experience a variety of more minor interruptions and complications in our other clinical trials, such as limitations in clinical trial supplies other than drug product, as well as local changes in COVID-19 impacts at individual trial sites. While our ongoing trials are proceeding and we are continuing with plans to initiate additional clinical trials in calendar 2021, it is unclear what impact, if any, the COVID-19 pandemic may have on the timeline for initiation and/or completion of all or any of our clinical trials.
With regard to our royalty revenue, we continued to report lower royalty revenue reported during calendar 2020 as compared to the prior year due to the worldwide impact of COVID-19 on HCV sales driven by a decline in patient volumes, HCV diagnoses and HCV prescriptions of MAVYRET/MAVIRET. While the evolving impact of COVID-19 will likely continue to affect aspects of our business, including those described above, we remain capable of funding our research and development programs for the foreseeable future with the current level of royalty revenue and our existing cash and short-term and long-term investments, which totaled $404.7 million at December 31, 2020.
Please see Item 1A “Risk Factors” in this quarterly report for additional discussion of risks and potential risks of the COVID-19 pandemic on our business, results of operations and financial condition.
Financial Operations Overview
We are currently funding all research and development for our wholly-owned programs, which are targeted toward the discovery and development of novel compounds for the treatment of viral infections and liver diseases. We have three Phase 2 studies ongoing for our wholly-owned programs in RSV and NASH and three Phase 1 studies in our HBV and NASH programs. We also plan to initiate clinical investigation of our new HBV RNA destabilizer compound, EDP-721, by mid-calendar 2021. In addition, we are also progressing other compounds in preclinical development, with the goal of having two new clinical candidates identified out of our three respiratory discovery efforts by the end of calendar 2021.
During 2021, we experienced disruptions in our business due to the spread of COVID-19. Specifically, the rate of RSV infection in both the Northern and Southern Hemispheres has been suppressed due to COVID-19 mitigation measures. In order to offset this as the RSV season swings back to the Northern Hemisphere, we reactivated sites in North America and are adding sites in several countries in Europe and Asia in preparation for the re-emergence of RSV. The Northern Hemisphere RSV season has yet to begin due to these COVID-19 mitigation measures and the timing of its return is currently unknown.
As a result of our clinical development programs, as well as efforts to advance other compounds into preclinical development, we expect to incur greater expenses in fiscal 2021 than in 2020 as we continue to advance our RSV, NASH, HBV, hMPV, and SARS CoV-2 programs. However, if the COVID-19 pandemic slows down our research and development programs, it will reduce our spending in those areas in the near term.
We are funding our operations primarily through royalty payments received under our collaboration agreement with AbbVie and our existing cash, cash equivalents, and short-term and long-term marketable securities. Our revenue is currently dependent on royalty payments we receive from AbbVie on its sales of MAVYRET/MAVIRET. During 2020 and into 2021, royalty revenues declined as compared to the prior year due primarily to the worldwide impact of COVID-19 on treated patient volumes as well as competitive pricing pressures in select markets. Given the uncertainty regarding the level of AbbVie’s future MAVYRET/MAVIRET sales that will generate our royalty revenue as well as increases in our future expenditures for the advancement of our internally developed compounds, we expect our expenses will exceed our revenues in fiscal 2021.
20
Internal Programs
As our internal product candidates are currently in Phase 1 or Phase 2 clinical development, we have not generated any revenue from our own product sales and do not expect to generate any revenue from product sales derived from these product candidates for at least the next several years.
Operating Expenses
The following table summarizes our operating expenses for the three months ended December 31, 2020 and 2019:
|
|
|
Three Months Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Research and development |
|
$ |
36,665 |
|
|
$ |
32,778 |
|
|
General and administrative |
|
|
7,377 |
|
|
$ |
6,921 |
|
|
Total operating expenses |
|
$ |
44,042 |
|
|
$ |
39,699 |
|
Research and Development Expenses
Research and development expenses consist of costs incurred to conduct basic research, such as the discovery and development of novel small molecules as therapeutics, as well as any external expenses of preclinical and clinical development activities. We expense all costs of research and development as incurred. These expenses consist primarily of:
|
|
• |
personnel costs, including salaries, related benefits and stock-based compensation for employees engaged in scientific research and development functions; |
|
|
• |
third-party contract costs relating to research, formulation, manufacturing, preclinical study and clinical trial activities; |
|
|
• |
laboratory consumables; |
|
|
• |
allocated facility-related costs; and |
|
|
• |
third-party license fees. |
Project-specific expenses reflect costs directly attributable to our clinical development candidates and preclinical candidates nominated and selected for further development. Remaining research and development expenses are reflected in research and drug discovery, which represents early-stage drug discovery programs. At any given time, we typically have several active early stage research and drug discovery projects. Our internal resources, employees and infrastructure are not directly tied to any individual research or drug discovery project and are typically deployed across multiple projects. As such, we do not report information regarding costs incurred for our early-stage research and drug discovery programs on a project-specific basis. We expect that our research and development expenses will continue to increase in the future as we advance our research and development programs.
Our research and drug discovery programs are at early stages; therefore, the successful development of our product candidates is highly uncertain and may not result in approved products. Completion dates and completion costs can vary significantly for each product candidate and are difficult to predict. Given the uncertainty associated with clinical trial enrollments, particularly in the context of the COVID-19 pandemic, and the risks inherent in the development process, we are unable to determine the duration and completion costs of the current or future clinical trials of our product candidates or if, or to what extent, we will generate revenue from the commercialization and sale of any of our product candidates. We anticipate that we will make determinations as to which development programs to pursue and how much funding to direct to each program on an ongoing basis in response to the preclinical and clinical success and prospects of each product candidate, as well as ongoing assessments of the commercial potential of each product candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of personnel costs, which include salaries, related benefits and stock-based compensation, of our executive, finance, business and corporate development and other administrative functions. General and administrative expenses also include travel expenses, allocated facility-related costs not otherwise included in research and development expenses, directors and officers liability insurance premiums, and professional fees for auditing, tax, and legal services and patent expenses.
21
We expect that general and administrative expenses will increase in the future primarily due to the ongoing expansion of our operating activities in support of our own research and development programs, as well as potential additional costs associated with operating a growing publicly traded company.
Other Income (Expense), Net
Other income (expense), net consists of interest income, interest expense and the change in fair value of our outstanding Series 1 nonconvertible preferred stock. Interest income consists of interest earned on our cash equivalents and short-term and long-term marketable securities balances as well as interest earned for any refunds received from tax authorities.
Income Tax (Expense) Benefit
Income tax (expense) benefit is based on our best estimate of applicable income tax rates, net research and development tax credits, net operating loss carryforwards and deferred income taxes, for the entire fiscal year applied to pre-tax profit or loss reported for the year-to-date period, except when a reliable estimate of the annual effective tax rate cannot be made and we instead use the year-to-date effective tax rate.
Results of Operations
Comparison of the Three Months Ended December 31, 2020 and 2019
|
|
|
Three Months Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Royalty revenue |
|
$ |
31,743 |
|
|
$ |
52,570 |
|
|
Research and development |
|
|
36,665 |
|
|
|
32,778 |
|
|
General and administrative |
|
|
7,377 |
|
|
|
6,921 |
|
|
Other income (expense), net |
|
|
677 |
|
|
|
2,076 |
|
|
Income tax (expense) benefit |
|
|
3,294 |
|
|
|
(1,504 |
) |
Royalty Revenue
Our revenue consists of royalties received under our collaboration agreement with AbbVie, substantially all of which are now derived from sales of MAVYRET/MAVIRET. We are entitled to annually tiered, double-digit, per-product royalties on 50% of all net sales of MAVYRET/MAVIRET. Our royalty revenues eligible to be earned in the future will potentially fluctuate depending on AbbVie’s HCV market share, the pricing of the MAVYRET/MAVIRET regimen and the number of patients treated with that regimen. Beginning with each January 1, the cumulative net sales of each royalty-bearing product start at zero for purposes of calculating the tiered royalties on a product-by-product basis.
We recognized royalty revenue of $31.7 million during the three months ended December 31, 2020 as compared to $52.6 million during the three months ended December 31, 2019. Royalty revenue for the three months ended December 31, 2020 decreased by $20.8 million compared to the comparable period in 2019 due to lower patient volumes as a result of COVID-19 impacting new patient starts. AbbVie reported worldwide net sales of $481 million for MAVRYET/MAVIRET during the three months ended December 31, 2020 compared to $628 million in the prior year period.
Research and development expenses
|
|
|
Three Months Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(in thousands) |
|
|||||
|
R&D programs: |
|
|
|
|
|
|
|
|
|
Virology |
|
$ |
24,588 |
|
|
$ |
19,236 |
|
|
Liver disease (non-viral) |
|
|
11,197 |
|
|
|
13,433 |
|
|
Other |
|
|
880 |
|
|
|
109 |
|
|
Total research and development expenses |
|
$ |
36,665 |
|
|
$ |
32,778 |
|
22
The level of research and development expenses for the three months ended December 31, 2020 increased by $3.9 million compared to the same period in 2019. The increase was primarily driven by timing of our clinical studies in our virology programs and offset by a decrease in clinical trial expenses in our liver disease program. For our virology program, we had two Phase 1 studies of EDP-514 and two ongoing Phase 2 studies of EDP-938 in our RSV program during the three months ended December 31, 2020. In the prior year, we had initiated our Phase 1a/1b clinical study of EDP-514 and had our ongoing Phase 2 RSVP study of EDP-938 ongoing in 2019. For our liver disease program during the three months ended December 31, 2020, we had one Phase 2 study (ARGON-2) ongoing and our Phase 1 study of our FXR follow-on candidate, EDP-297, whereas in 2019 we had one Phase 2 study of EDP-305 ongoing (ARGON-1) and were in the process of concluding our Phase 2 INTREPID study.
In the near term, our clinical trial expenses could fluctuate if the impact of the COVID-19 pandemic continues through calendar 2021 and negatively impacts patient recruitment and monitoring. Furthermore, COVID-19 could also cause delays in our other studies we plan to initiate later in 2021. In the coming years, we expect our research and development expenses to increase as our wholly-owned programs advance, subject to any long-term impact of the COVID-19 pandemic.
General and administrative expenses
General and administrative expenses increased slightly by $0.5 million for the three months ended December 31, 2020 compared to the same period in the prior year due to increases in headcount and compensation expense.
Other income (expense), net
Other income (expense), net, decreased $1.4 million for the three months ended December 31, 2020 as compared to the same period in 2019 due to a net decrease in investment income primarily driven by lower interest rates year over year and purchases of marketable securities at a premium.
Income tax (expense) benefit
For the three months ended December 31, 2020 and 2019, we recorded an income tax benefit of $3.3 million and income tax expense of ($1.5) million, respectively. The income tax benefit for the three months ended December 31, 2020 was driven primarily by our pre-tax loss and a federal net operating loss carryback under the CARES Act. We continue to record a valuation allowance against the majority of our deferred tax assets as it is more likely than not that those tax benefits will not be realized in the future. The income tax expense for the three months ended December 31, 2019 was driven by our pre-tax income which was partially offset by research and development tax credits and the federal income tax benefit from foreign-derived royalty income.
Liquidity and Capital Resources
At December 31, 2020, our principal sources of liquidity were cash, cash equivalents and short-term and long-term marketable securities totaling $404.7 million. We believe, despite the ongoing global impact of COVID-19, that our principal sources of liquidity will be sufficient to meet our anticipated cash requirements for the foreseeable future.
The following table shows a summary of our cash flows for the three months ended December 31, 2020 and 2019:
|
|
|
Three Months Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Cash provided by (used in): |
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(14,890 |
) |
|
$ |
12,363 |
|
|
Investing activities |
|
|
(17,445 |
) |
|
|
(37,201 |
) |
|
Financing activities |
|
|
299 |
|
|
|
1,166 |
|
|
Net decrease in cash, cash equivalents and restricted cash |
|
$ |
(32,036 |
) |
|
$ |
(23,672 |
) |
23
Net cash provided by (used in) operating activities
The decrease in cash used in operating activities of $27.3 million for the three months ended December 31, 2020 as compared to the same period in 2019 was primarily driven by a decrease in net income year-over-year and net cash used by changes in our operating assets and liabilities of $11.7 million in the three months ended December 31, 2020 as compared to net cash used by changes in our operating assets and liabilities of $6.4 million for the same period in 2019. Changes in our operating assets and liabilities in both periods were generally due to a decrease in payments received under our collaboration with AbbVie, the advancement of our research programs and the timing of vendor invoicing and payments.
Net cash used in investing activities
The decrease of $19.8 million in cash used in investing activities for the three months ended December 31, 2020 as compared to the same period in 2019 was the result of reduced investment in debt securities, as well as timing of purchases, sales and maturities of marketable securities.
Net cash provided by financing activities
The decrease of $0.9 million in net cash provided by financing activities for the three months ended December 31, 2020 as compared to the same period in 2019 was driven by a decrease in proceeds from exercises of stock options of $1.5 million and offset by a decrease in withholding tax payments for the settlement of share-based awards of $0.6 million driven by a decline in our stock price year over year at the time of vesting of certain share-based awards.
Funding requirements
As of December 31, 2020, we had $404.7 million in cash, cash equivalents and short-term and long-term marketable securities. We believe that our existing cash, cash equivalents and marketable securities as of December 31, 2020 will be sufficient to meet our anticipated cash requirements for the foreseeable future. However, our forecast of the period for which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially.
Our future capital requirements are difficult to forecast and will depend on many factors, including:
|
|
• |
the amount of royalties generated from our existing collaboration with AbbVie; |
|
|
• |
any continuing impact of the COVID-19 pandemic on the numbers of treated HCV patients; |
|
|
• |
the number and characteristics of our research and development programs; |
|
|
• |
the scope, progress, results and costs of researching and developing any of our product candidates on our own, including conducting advanced clinical trials; |
|
|
• |
delays and additional expense in our clinical trials of our product candidates as a result of the COVID-19 pandemic; |
|
|
• |
the cost of manufacturing our product candidates for clinical development and any products we successfully commercialize independently; |
|
|
• |
opportunities to in-license or otherwise acquire new technologies, therapeutic candidates and therapies; |
|
|
• |
the timing of, and the costs involved in, obtaining regulatory approvals for any product candidates we develop independently; |
|
|
• |
the cost of commercialization activities, if any, of any product candidates we develop independently that are approved for sale, including marketing, sales and distribution costs; |
|
|
• |
the timing and amount of any sales of our product candidates, if any, or royalties thereon; |
|
|
• |
our ability to establish new collaborations, licensing or other arrangements, if any, and the financial terms of such arrangements; |
|
|
• |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patents, including any litigation costs and the outcomes of any such litigation; and |
|
|
• |
potential fluctuations in foreign currency exchange rates. |
24
Off-Balance Sheet Arrangements
We do not engage in any off-balance sheet financing activities. We do not have any interest in entities referred to as variable interest entities, which include special purpose entities and other structured finance entities.
Contractual Obligations and Commitments
In our 2020 Form 10-K Part II, Item 7, Management’s Discussion and Analysis of Financial Conditions and Results of Operations, under the heading “Contractual Obligations and Commitments”, we have described our commitments and contingencies. There were no material changes to our total contractual commitments and obligations during the three months ended December 31, 2020.
Critical Accounting Policies
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of our consolidated financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amount of assets, liabilities, revenue, costs and expenses, and related disclosures. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions and conditions. See our Annual Report on Form 10-K for the fiscal year ended September 30, 2020 (referred to as our 2020 Form 10-K) for information about critical accounting policies as well as a description of our other significant accounting policies.
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is set forth in Note 2 to the consolidated financial statements included in this Quarterly Report on Form 10-Q.
|
ITEM 3. |
QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK |
Interest Rate Sensitivity
We had cash, cash equivalents and short-term and long-term marketable securities of $404.7 million at December 31, 2020 consisting of cash, money market funds, agency securities, commercial paper, treasury notes and corporate bonds. Interest income is sensitive to changes in the general level of interest rates; however, due to the nature of these investments, a change in market interest rates of 100 basis points would not be expected to have a material impact on our financial condition or results of operations.
Foreign Exchange Risk
As we continue to progress our wholly-owned programs into clinical development we will conduct clinical trials outside of the U.S. and thus will face exposure to movements in foreign currency exchange rates, primarily the British Pound and Euro, against the U.S. Dollar, arising from our accounts payable and accrued expenses. During the three months ended December 31, 2020, the impact of foreign currency exposure was immaterial and thus did not have a significant impact on our consolidated financial statements. Our operations may become subject to more significant fluctuations in foreign currency exchange rates in the future if we continue to contract with vendors outside of the U.S.
|
ITEM 4. |
CONTROLS AND PROCEDURES |
|
a) |
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures. |
Our management, with the participation of the principal executive officer and principal financial officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) as of the end of the period covered by this quarterly report. Based on this evaluation, the principal executive officer and principal financial officer concluded that these disclosure controls and procedures are effective and designed to ensure that the information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the requisite time periods.
|
b) |
Changes in Internal Control Over Financial Reporting. |
There was no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) identified in connection with the evaluation of our internal control performed during the three months ended December 31, 2020 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
25
PART II —OTHER INFORMATION
ITEM 1A.RISK FACTORS
RISK FACTORS
Our business faces significant risks and uncertainties. Certain factors may have a material adverse effect on our business prospects, financial condition and results of operations, and you should carefully consider them. Accordingly, in evaluating our business, we encourage you to consider the following discussion of risk factors, in its entirety, in addition to other information contained in or incorporated by reference into this Annual Report on Form 10-K and our other public filings with the SEC. Other events that we do not currently anticipate or that we currently deem immaterial may also affect our business, prospects, financial condition and results of operations.
Risks Related to Our Business
Our financial prospects for the next several years are dependent upon AbbVie’s success selling MAVYRET/MAVIRET, which includes our protease inhibitor, glecaprevir, for the treatment of HCV. AbbVie may continue to experience lower sales volume in future quarters due to a reduction in diagnoses and treatment of HCV, which could adversely affect our business.
We rely on AbbVie to commercialize its regimen containing glecaprevir (our second protease inhibitor, which is one of the two DAAs in AbbVie’s MAVYRET/MAVIRET treatment), over which we have granted AbbVie complete control. Our ability to continue to generate revenue will depend primarily on the success of AbbVie’s efforts to maintain sales of MAVYRET/MAVIRET. Such success is subject to uncertainty, and we have no control over the resources, time and effort that AbbVie may devote to sales of this regimen. Any of several events or factors could have a material adverse effect on our ability to continue to generate revenue from AbbVie’s sales of MAVYRET/MAVIRET. For example, AbbVie:
|
|
• |
may continue to experience lower sales volume in future quarters due to a reduction in diagnoses and treatment of HCV if doctor visits and other routine healthcare activities remain at below normal levels as a result of the COVID-19 pandemic; |
|
|
• |
may not maintain satisfactory levels of prescriptions by physicians and reimbursement by third-party payors for the MAVYRET/MAVIRET regimen in the various markets of the world where it is being sold; |
|
|
• |
may not compete successfully with its MAVYRET/MAVIRET regimen against other products and therapies for HCV, including competition for exclusive arrangements with third-party payors and governmental entities as well as price competition; |
|
|
• |
may have to comply with additional requests and recommendations from the FDA, including label restrictions for its regimen containing glecaprevir; |
|
|
• |
may not obtain all commercially necessary reimbursement approvals; |
|
|
• |
may not commit sufficient resources to the marketing and distribution of MAVYRET/MAVIRET, whether for competitive or strategic reasons or otherwise due to a change in business priorities; |
|
|
• |
may cease to perform its obligations under the terms of our collaboration agreement; and |
|
|
• |
may unilaterally terminate our collaboration agreement on specified prior notice without any reason and without any further commitment. |
We do not have access to all information regarding AbbVie’s MAVYRET/MAVIRET, including certain information about spontaneous safety reports for any marketed product, regulatory affairs, process development, manufacturing, marketing, sales and other areas known by AbbVie. Thus, our ability to keep our stockholders informed about the status of the marketed products licensed under our collaboration is limited by the degree to which AbbVie keeps us informed. If AbbVie does not perform in the manner we expect or fulfill its responsibilities in a timely manner, or at all, the global commercialization of MAVYRET/MAVIRET could be terminated in selected jurisdictions or be commercially unsuccessful. In addition, AbbVie has the right to make decisions regarding the commercialization of licensed products without consulting us. For example, in 2018 AbbVie entered into a royalty-free licensing agreement with the Medicines Patent Pool to accelerate access to generic versions of MAVYRET/MAVIRET in 99 low- and middle-income countries and territories. AbbVie may also make decisions with which we do not agree. If AbbVie acts in a manner that is not in our best interest, then it could adversely affect our business and prospects.
26
Our royalty revenues are primarily derived from AbbVie’s net sales of its MAVYRET/MAVIRET regimen for HCV. If AbbVie is unable to maintain sales of this regimen at or above current levels of sales, our royalty revenues will be adversely affected.
AbbVie’s MAVYRET/MAVIRET regimen continues to be the leading HCV treatment in the U.S. and several market geographies in developed countries where it is approved. While commercialization of this regimen is exclusively in AbbVie’s control without any required input from us, we believe it is possible that prices will decline further due to payors obtaining additional discounts or competitive market dynamics. For example, the states of Louisiana and Washington have each announced efforts to negotiate a blanket price for one of the HCV drug companies to treat all patients in one or more state programs (e.g. Medicaid). Gilead was awarded the contract in Louisiana and AbbVie was awarded the contract in Washington. In addition, Gilead has been able to access the Medicaid market at a lower price point to build its market share by using an authorized generic version of its HCV regimen branded as Epclusa®. It is unknown whether these programs or other programs that states may adopt could have any further impact on MAVYRET/MAVIRET sales. There may also be fluctuations in AbbVie’s market share over time due to these and other competitive actions by Gilead.
In addition, in light of continued fiscal crises experienced by several countries in the European Union and Japan, governments have announced or implemented measures to manage and reduce healthcare expenditures. AbbVie may experience global pricing pressure for its MAVYRET/MAVIRET regimen from such measures, which may be reflected in larger discounts or rebates on its regimens or delayed reimbursement. Also, private and public payors may choose to exclude AbbVie’s MAVYRET/MAVIRET regimen from their formulary coverage lists or limit the types of patients for whom coverage will be provided. Any such change in formulary coverage, discounts or rebates or reimbursement for MAVYRET/MAVIRET would negatively affect the demand for this regimen and our royalty revenue derived from its sales.
Furthermore, we expect that the COVID-19 pandemic will continue to adversely affect AbbVie’s sales of MAVYRET/MAVIRET in the United States and the rest of the world if healthcare systems continue to experience varying levels of shut-down and diagnoses and treatment rates of HCV infections remain at below-normal levels. At this point in time we do not know the extent and duration of this adverse effect. We note, however, that the HCV patient pool will continue to carry the viral infection until treated.
We and AbbVie face substantial competition in the markets for HCV drugs, and there are many companies developing potential therapies for RSV, HBV, NASH, hMPV and SARS-CoV-2, which may result in others discovering, developing or commercializing products before we do or doing so more successfully than we do.
The pharmaceutical and biotechnology industries are intensely competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions, governmental agencies and other public and private research organizations are commercializing or pursuing the development of products that target HCV, RSV, HBV, NASH, hMPV and SARS-CoV-2 and other viral infections or diseases that we may target in the future. Many of our competitors have substantially greater commercial infrastructure and greater financial, technical and personnel resources than we have, as well as drug candidates in late-stage clinical development.
In all the disease areas currently under the focus of our research and development efforts, there are other companies with product candidates that are more advanced than ours. Our competitors may succeed in developing these product candidates or others and obtaining regulatory approval before we can do so with any of our product candidates. If we are not “first to market” with one of our product candidates in one or more of these disease indications, our competitive position could be compromised because it may be more difficult for us to obtain marketing approval for that product candidate and market acceptance of that product candidate as a follow-on competitor. In addition, any new product that competes with an approved product typically must demonstrate compelling advantages in efficacy, convenience, tolerability or safety, or some combination of these factors, in order to gain regulatory approvals, overcome price competition and be commercially successful.
We expect AbbVie’s MAVYRET/MAVIRET to continue to face intense competition due to existing approved products in the HCV market. AbbVie’s MAVYRET/MAVIRET regimen currently faces competition in various world markets and subpopulations of HCV from Gilead’s Epclusa® (a fixed dose combination of sofosbuvir and velpatasvir), Vosevi® (a triple combination therapy of sofosbuvir, velpatasvir and voxilaprevir approved by the FDA in July 2017 for specified sofosbuvir -treatment failures and NS5A-inhibitor treatment failures) and Harvoni® (a fixed-dose combination of sofosbuvir and ledipasvir); and to a lesser extent - Merck’s Zepatier® (a fixed-dose combination of grazoprevir and elbasvir). Gilead launched authorized generic versions of Epclusa and Harvoni in January 2019 through a newly created subsidiary, Asegua Therapeutics, LLC, which has had an impact on the competitive landscape. For example, in March 2019, the state of Louisiana announced the selection of Asegua Therapeutics as their HCV subscription model pharmaceutical partner to provide the state with unrestricted access to its direct-acting antiviral medication. Other competitive products in the form of other treatment methods or a vaccine for HCV may render MAVYRET/MAVIRET obsolete or noncompetitive. MAVYRET/MAVIRET will face competition based on its safety and effectiveness, reimbursement coverage, price, patent position, AbbVie’s marketing and sales capabilities, and other factors. If MAVYRET/MAVIRET faces competition from generic products other than authorized generic versions by the manufacturer of the branded product (i.e. Gilead and Asegua Therapeutics), our collaboration agreement provides that the royalty rate applicable to our protease product contained in the regimen is reduced significantly by a specified percentage on a product-by-product, country-by-country basis. If AbbVie is not able to compete effectively against its competitors in HCV, our business will not grow and our financial condition, operations and stock price will suffer.
27
We also expect our other product candidates to face intense and increasing competition. RSV and HBV represent competitive therapeutic areas.
For RSV, there are currently no safe and effective therapies for already established RSV infection. Several companies are seeking new antiviral treatments for RSV infection in adult and pediatric patients. Ark Biosciences, Johnson & Johnson and ReViral each have compounds in clinical development. A prophylactic, monoclonal-antibody-based treatment from MedImmune, which is commercialized by AbbVie outside the U.S., is approved for infants considered at high risk for RSV infection; however, studies have found that most young children with RSV infection were previously healthy, and thus would not normally be prescribed prophylactic treatment. AstraZeneca/Sanofi and Merck are developing long-acting versions of the monoclonal antibody for prophylaxis use in infants. In addition, a number of companies have RSV vaccines in development, primarily directed at prevention of RSV infection, and some companies are also evaluating vaccines in a therapeutic mode for treatment of established RSV infection.
While there are effective antiviral medications prescribed for HBV, they generally have low true cure rates. Many companies are seeking to develop new HBV drugs that alone or in combination with other mechanisms could lead to a functional cure of HBV. Assembly, Ascletis Pharma, Gilead, Green Cross, GSK/Ionis, HEC, Johnson & Johnson, MYR, Replicor, Roche and Vir Biotechnology have Phase 2 programs in progress, with many of these companies conducting earlier stage programs as well. In addition, a number of companies have Phase 1 or earlier stage HBV programs, including Aligos, Arbutus, ENYO, Fujian Cosunter, Sichuan Kelon, Qilu and Zhimeng Biopharma.
Though there is currently no approved treatment for NASH, we expect significant competition from other companies in the development of new treatments for NASH and related conditions. In February 2019, Intercept Pharmaceuticals announced positive Phase 3 trial results for OCA (brand name Ocaliva®) in NASH and submitted U.S. and European regulatory filings in the second half of calendar 2019 for approvals in this indication. In June 2020 Intercept received a complete response letter from the FDA and has announced that it is currently in ongoing discussions with the FDA and expects to refile an NDA for OCA in 2021 with additional safety and efficacy data. In addition to Intercept, we are aware of several other companies with NASH programs that are significantly more advanced than ours, including companies with compounds in Phase 3 clinical trials in NASH, namely AbbVie, Akero, CymaBay, Galmed, and Madrigal. In addition, a number of companies have NASH or related programs with compounds in Phase 2 clinical trials. These companies include 89bio, Afimmune, Akcea, AstraZeneca, BMS, Can-Fite BioPharma, Cirius, Corcept Therapeutics, Eli Lilly and Company, Enyo, Galectin, Gilead, Hanmi Pharma, HighTide Biopharma, Immuron, Inventiva, Kowa Company, Lipocine, Medicinova, Metacrine, Mitsubishi Tanabe Pharma, NGM Biopharmaceuticals, Northsea Therapeutics, Novartis, Novo Nordisk, NuSirt Biopharma, Oramed, Pfizer, Poxel SA, PharmaKing, Roche, Sagimet Biosciences, TaiwanJ Pharmaceuticals, Terns, Theratechnologies, Viking Therapeutics, and Zydus. A significant number of other companies are conducting earlier stage clinical trials that may be applicable in NASH. There are also additional companies conducting preclinical studies in these disease areas.
If we are not able to develop new products that can compete effectively against our current and future competitors, our business will not grow and our financial condition, operations and stock price will suffer.
The ongoing COVID-19 pandemic has had an impact on our business operations and clinical trials and could continue, directly or indirectly, to adversely affect our business, results of operations and financial condition and our stock price.
The COVID-19 pandemic has had an impact on our business operations and we continue to monitor applicable government recommendations. We have made modifications to our normal operations because of the COVID-19 pandemic, including allowing certain of our employees to work remotely and conducting our laboratory operations at reduced capacity. Notwithstanding these measures, the COVID-19 pandemic could affect the health and availability of our workforce as well as those of the third parties whom we are relying on to take similar measures. In addition, we have experienced and will continue to experience disruptions to our business operations resulting from shelter-in-place policies and other restrictions on the ability of our employees to perform their jobs.
Our business could continue to be materially adversely affected, directly or indirectly, by the ongoing COVID-19 pandemic, which has spread to the countries in which we, our contract manufacturers, our clinical research contractors and our collaborators in clinical research do business. National, state and local governments in affected regions have implemented and may continue to implement safety precautions, including quarantines, border closures, increased border controls, travel restrictions, shelter-in-place orders and shutdowns, business closures, cancellations of public gatherings and other measures. Organizations and individuals are taking additional steps to avoid or reduce infection, including limiting travel and staying home from work. These measures are disrupting normal business operations both inside and outside of affected areas and have had significant negative impacts on businesses and financial markets worldwide.
The extent and severity of the impact on our business and clinical trials will be determined largely by the extent of disruptions in the supply chains for our research and clinical trial materials, delays in the conduct and recruitment of current and future clinical trials and reductions in the number of patients accessing AbbVie’s HCV regimens. For example, we paused recruitment for our Phase 2b ARGON-2 study of EDP-305 in NASH patients and Part 2 of our Phase 1a/1b study of EDP-514 in nuc-suppressed HBV patients in March 2020, but we were able to resume recruitment of these studies in July 2020. In addition, the public health response to the
28
COVID-19 pandemic, including lock-downs, mask mandates and social distancing, began just before the start of the winter RSV season in the Southern Hemisphere which delayed or prevented activation of some trial sites in that region. Similarly, the mitigation steps to manage the COVID-19 pandemic significantly reduced the incidence of RSV and other respiratory illnesses at the activated sites in the Southern Hemisphere’s RSV season and the recruitment in our RSVP study. To date the 2021 RSV season in the Northern Hemisphere has not yet begun due to COVID-19 mitigation measures. We have made extensive efforts to expand our clinical sites beyond North America, including sites across Europe and Asia, to be ready when RSV infection re-emerges, but we cannot predict when that may occur. These impacts of COVID-19 could affect the future course of this study and our other ongoing clinical trials and delay their timelines.
During 2020 and 2021, COVID-19 has also impacted new HCV patient starts in both the United States and the rest of the world, resulting in a decline in sales of HCV treatments. While new HCV infections are continuing, at this time it is uncertain when and the extent to which treatment of new HCV patients and revenues will return to pre-COVID-19 levels.
In addition, the impact of the COVID-19 pandemic on the operations of the FDA and other health authorities may delay potential approvals of our clinical trial protocols.
Although it is not possible at this time to estimate the entirety of the impact that the COVID-19 pandemic will have on our business, operations and employees, our contract manufacturers, our clinical research contractors, and our collaborators in clinical research, any continued spread of COVID-19, measures taken by governments, actions taken to protect employees from this disease, and the broad impact of the pandemic on all business activities and financial markets, may materially and adversely affect our business, results of operations and financial condition and our stock price. Additionally, as new, more infectious variants emerge, it is possible that the impact of the pandemic on our business may increase or lengthen in duration.
We have not developed independently any approved products and we have limited clinical development experience, which makes it difficult to assess our ability to develop and commercialize our product candidates.
AbbVie has been responsible for all of the clinical development of our paritaprevir and glecaprevir protease inhibitor products. We have not yet demonstrated an ability to address successfully many of the risks and uncertainties associated with late stage clinical development, regulatory approval and commercialization of therapeutic products such as the ones we plan to develop independently. For example, to execute our business plan for development of our independent RSV, NASH, and HBV programs, we will need to successfully:
|
|
• |
execute clinical development of our product candidates and demonstrate acceptable safety and efficacy for them alone or in combination with other drugs or drug candidates; |
|
|
• |
obtain required regulatory approvals for the development and commercialization of our product candidates; |
|
|
• |
develop and maintain any future collaborations we may enter into for any of these programs; |
|
|
• |
obtain and maintain patent protection for our product candidates and freedom from infringement of intellectual property of others; |
|
|
• |
establish acceptable commercial manufacturing arrangements with third-party manufacturers; |
|
|
• |
build and maintain robust sales, distribution and marketing capabilities, either independently or in collaboration with future collaborators; |
|
|
• |
gain market acceptance for our product candidates among physicians, payors and patients; and |
|
|
• |
manage our spending as costs and expenses increase due to clinical trials, regulatory approvals and commercialization. |
If we are unsuccessful in accomplishing these objectives, we may not be able to successfully develop and commercialize our product candidates and expand our business or continue our operations.
29
If we are not successful in developing EDP-938, EDP-514, EDP-721, EDP-305, and/or EDP-297 or in discovering further product candidates, our ability to expand our business and achieve our strategic objectives will be impaired.
Much of our internal research is at preclinical stages. Research programs designed to identify product candidates require substantial technical, financial and human resources, whether or not any product candidates are ultimately identified. Our research programs may initially show promise in identifying additional potential product candidates, yet fail to yield product candidates for clinical development or commercialization for many reasons, including the following:
|
|
• |
the research methodology used may not be successful in identifying additional potential product candidates; |
|
|
• |
competitors may develop alternatives that render our product candidates less commercially viable or obsolete; |
|
|
• |
competitors may obtain intellectual property protection that effectively prevents us from developing a product candidate; |
|
|
• |
a product candidate may, on further study, be shown not to be an effective treatment in humans or to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria; and |
|
|
• |
a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all. |
Additional drug candidates that we may develop will require significant research, preclinical and clinical studies, regulatory approvals and commitments of resources before they can be commercialized. We cannot give assurance that our research will lead to the discovery of any additional drug candidates that will generate additional revenue for us. If we are unable to identify additional compounds suitable for preclinical and clinical development, we may not be able to obtain sufficient product revenue in future periods, which likely would result in significant harm to our financial position and adversely impact our stock price.
We reported a net loss for the fiscal year ended September 30, 2020 and the three months ended December 31, 2020 primarily due to the impact of COVID-19 on our royalty revenues. Continued changes in royalty revenue earned under our AbbVie agreement or changes in the level of expenses associated with development of our product candidates, or both, may cause our results of operations to fluctuate from period to period. Any continuation of the recent trend of reducing royalty revenue, combined with increasing research and development expenses in support of our advancing programs, would likely result in continued operating losses in the coming year and in future periods unless we develop other sources of revenue.
As discussed above, our principal source of revenue continues to be our royalty revenue earned under the AbbVie collaboration agreement. There is uncertainty regarding this future revenue stream given the competitive nature of the market for HCV therapies, which reflects price competition, the changing nature of payer contracts of AbbVie and others, the varying rates of reimbursement in different countries and the impact of the COVID-19 pandemic on HCV sales. Changes in royalty revenue earned under the AbbVie collaboration agreement, including those that occur from period to period due to the annually tiered structure of our royalties, may cause our revenues and operating results to fluctuate significantly from quarter to quarter and could have an adverse effect on our stock price.
Additionally, many of the preclinical and clinical development activities required for our product candidates must be contracted out to contract research organizations, or CROs, at significant expense. We expect these expenses to increase substantially in the coming years as we advance compounds and conduct more clinical studies. However, the global impact of the COVID-19 shut-down has had, and is likely to continue to have, an adverse effect on the ability of our CROs to conduct preclinical and clinical studies. At this point in time we do not know to what extent these studies will be impacted by the pandemic. Therefore, now more than ever it is difficult to accurately predict the timing and amounts of these expenses, and we expect that they will vary from quarter to quarter. In addition, the FDA or other regulatory agencies may require more preclinical or clinical testing than we originally anticipated for any of our product candidates. We may also be required to purchase expensive competitor drugs for use in our trials, either to demonstrate potential treatment combinations or as comparators to our product candidates. We also conduct clinical development activities outside the U.S. and are therefore exposed to foreign currency fluctuations for payments made to CROs in currencies other than the U.S. dollar. As a result, the expenses of our development programs and our operating results may fluctuate significantly from quarter to quarter. If combined with any continuation of the recent trend of reduced royalty revenue, such a trend would likely result in operating losses in the coming year and in future periods, unless we develop other sources of revenue.
If we fail to attract and keep senior management and key scientific personnel, we may be unable to successfully develop our product candidates, conduct our clinical trials and commercialize our product candidates.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel. We are highly dependent upon our senior management, particularly Jay R. Luly, Ph.D., our Chief Executive Officer and President, Yat Sun Or, Ph.D., our Senior Vice President, Research and Development and Chief Scientific Officer, and Nathalie Adda, M.D., our Senior Vice President and Chief Medical Officer, as well as other employees and consultants. Although
30
none of these individuals has informed us to date that he or she intends to retire or resign in the near future, the loss of the services of any of these individuals or one or more of our other members of senior management could delay or prevent the successful development of our product candidates.
Although we have not historically experienced unique difficulties attracting and retaining qualified employees, we could experience such problems in the future. For example, competition for qualified personnel in the biotechnology and pharmaceutical fields is intense. In addition, we will need to hire additional personnel as we expand our clinical development and ultimately seek regulatory approvals and prepare for commercial activities. We may not be able to attract and retain quality personnel on acceptable terms.
We may encounter difficulties in managing our growth and expanding our operations successfully.
As we expand our research efforts and seek to advance our product candidates through clinical trials, we will need to expand our development, regulatory, manufacturing, marketing and sales capabilities or contract with third parties to obtain these capabilities. As our operations expand, we expect that we will need to manage additional relationships with various strategic partners, suppliers and other third parties. Future growth will impose significant added responsibilities on members of management. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train and integrate additional management, administrative and sales and marketing personnel. We may not be able to accomplish these tasks, and our failure to accomplish any of them could prevent us from successfully growing our company.
We may require substantial additional financing in the longer term to achieve our goals if the sales of MAVYRET/MAVIRET decline substantially. A failure to obtain this necessary capital when needed could force us to delay, limit, reduce or terminate some or all of our product development efforts.
Since our inception, most of our resources have been dedicated to the discovery and preclinical development of our product candidates. In particular, we have expended, and believe that we will continue to expend for the foreseeable future, substantial resources discovering and developing our proprietary product candidates. These expenditures will include costs associated with research and development, preclinical manufacturing of product candidates, conducting preclinical experiments and clinical trials and obtaining regulatory approvals, as well as commercializing any products later approved for sale. For the foreseeable future, we expect to incur substantial additional costs associated with research and development for our internally developed programs. In addition, we may seek opportunities to in-license or otherwise acquire new therapeutic candidates and therapies.
Our future capital requirements depend on many factors, including:
|
|
• |
the amount of royalties generated from MAVYRET/MAVIRET sales under our existing collaboration with AbbVie; |
|
|
• |
any continuing impact of the COVID-19 pandemic on the numbers of treated HCV patients; |
|
|
• |
the number and characteristics of our research and development programs; |
|
|
• |
the scope, progress, results and costs of researching and developing any of our product candidates on our own, including conducting advanced clinical trials; |
|
|
• |
delays and additional expense in our clinical trials as a result of the COVID-19 pandemic; |
|
|
• |
the cost of manufacturing our product candidates for clinical development and any products we successfully commercialize independently; |
|
|
• |
opportunities to in-license or otherwise acquire new technologies, therapeutic candidates and therapies; |
|
|
• |
the timing of, and the costs involved in, obtaining regulatory approvals for any product candidates we develop independently; |
|
|
• |
the cost of commercialization activities, if any, of any product candidates we develop independently that are approved for sale, including marketing, sales and distribution costs; |
|
|
• |
the timing and amount of any sales of our product candidates, if any, or royalties thereon; |
|
|
• |
our ability to establish new collaborations, licensing or other arrangements, if any, and the financial terms of such arrangements; |
|
|
• |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patents, including any litigation costs and the outcomes of any such litigation; and |
|
|
• |
potential fluctuations in foreign currency exchange rates. |
31
Additional funds may not be available if and when we need them, on terms that are acceptable to us, or at all. Our ability to raise funds will depend on financial, economic and market conditions and other factors, many of which are beyond our control. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate preclinical studies, clinical trials or other research and development activities for one or more of our product candidates.
Risks Related to Development, Clinical Testing and Regulatory Approval of Our Product Candidates
Clinical drug development involves a lengthy and expensive process with uncertain timelines and uncertain outcomes. Any ongoing or future clinical trials of our product candidates may fail to demonstrate sufficient safety and efficacy. If clinical trials of any of our proprietary product candidates are prolonged or delayed or fail, we may be unable to commercialize our product candidates on a timely basis or ever.
Clinical testing is expensive and, depending on the stage of development, can take a substantial time period to complete. Its outcome is inherently uncertain, and failure can occur at any time during clinical development. None of our product candidates in our pipeline other than paritaprevir and glecaprevir, which were clinically developed by AbbVie, has yet to advance beyond completion of Phase 2 clinical trials. Any ongoing or future clinical trials of our product candidates may fail to demonstrate sufficient safety and efficacy. Moreover, regulatory and administrative delays, including those caused by the COVID-19 pandemic, for any product candidate in our pipeline may adversely affect our or any future collaborator’s clinical development plans and jeopardize our or any future collaborator’s ability to attain product approval, commence product sales and compete successfully against other therapies.
Clinical trials can be delayed for a variety of reasons, including delays related to:
|
|
• |
reaching an agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
|
|
• |
failure of third-party contractors, such as CROs, or investigators to comply with regulatory requirements; |
|
|
• |
failure to obtain on a timely basis, or at all, the necessary approvals from regulators or institutional review boards, or IRBs, in order to commence a clinical trial at a prospective trial site, or their suspension or termination of a clinical trial once commenced; |
|
|
• |
difficulty in recruiting suitable patients to participate in a trial; |
|
|
• |
the impact of the COVID-19 pandemic on the ability of CROs to conduct their own operations, resulting in, among other things, delays in recruitment or dosing of our clinical trials; |
|
|
• |
seasonality and variations in incidents of infection year to year (e.g. RSV) affecting enrollment in clinical trials; |
|
|
• |
difficulty in having patients complete a trial or return for post-treatment follow-up; |
|
|
• |
clinical sites deviating from trial protocol or dropping out of a trial; |
|
|
• |
problems with drug product or drug substance storage and distribution; |
|
|
• |
adding new clinical trial sites; |
|
|
• |
our inability to manufacture, or obtain from third parties, adequate supply of drug product sufficient to complete our preclinical studies and clinical trials; |
|
|
• |
changes in governmental or regulatory administration, including, for example, administrative delays due to the planned relocation of the EMA to the Netherlands; |
|
|
• |
changes in regulatory requirements, policy and guidelines, including guidelines specifically addressing requirements for the development of treatments for RSV, NASH, or HBV; |
|
|
• |
difficulty in obtaining and maintaining adequate insurance coverage; |
|
|
• |
program discontinuations or clinical holds for a program of a competitor, which could increase the level of regulatory scrutiny or delay data review or other response times by regulators with respect to one of our programs in the same class as the competitor’s program; or |
|
|
• |
varying interpretations of data by the FDA, the EMA and similar foreign regulatory agencies. |
We could also encounter delays if a clinical trial is suspended or terminated by us, by the IRBs of the institutions in which such trial is being conducted, by any Data Safety Monitoring Board, or DSMB, for such trial, or by the FDA, the EMA or other regulatory authorities. Such authorities may impose such a suspension or termination due to a number of factors, including failure to conduct the
32
clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. In addition, delays can occur due to safety concerns arising from trials or other clinical data regarding another company’s product candidate in the same compound class as one of ours. If we or any future collaborators experience delays in the completion of, or termination of, any clinical trial of one of our product candidates, the commercial prospects of the product candidate will be harmed, and our ability to commence product sales and generate product revenues from the product candidate will be delayed. In addition, any delays in completing our clinical trials will increase our costs in the long term and slow down our product candidate development and approval process. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
We may choose to test any of our clinical candidates preclinically and/or clinically in combination with other compounds with different mechanisms of action, and any adverse results from such testing may have adverse consequences for the further development potential of not only the combination but also the clinical candidate itself as a monotherapy or in combination with other mechanisms of action.
We expect that the further development of successful therapies in our principal disease areas of NASH and HBV may require combining one or more of our compounds with other compounds with different mechanisms of action. To advance our programs and achieve favorable opportunities for any such combinations we may conduct preclinical testing, as well as clinical testing, with one of our other compounds or with a compound of a third party, with or without a longer-term collaboration with any such party. We may choose to disclose such testing in advance, but we can anticipate that some of the testing would be done without any public disclosure. If any such testing produces adverse results, we may have to disclose it to regulatory authorities as part of the data available with respect to our product candidate and the data may have adverse consequences for the further development and the ultimate conditions attached to any approved use of the product candidate, whether in the combination tested or even as a monotherapy or in combination with other mechanisms.
EDP-938, EDP-514, EDP-721, EDP-305, EDP-297 or any other product candidate emerging from our current RSV, HBV, hMPV, SARS-Cov-2 and NASH programs may have undesirable side effects which may delay or prevent marketing approval, or, if approval is received, require our product candidate to be taken off the market, require us to include safety warnings or otherwise limit sales.
In our RSV program, we are developing inhibitors of the N protein. No inhibitor of the RSV N protein has progressed beyond a Phase 2 clinical trial, so we are not yet able to assess the potential liabilities of an N inhibitor in large scale studies or in the general population. In addition, in RSV the principal target populations, namely infants, the elderly, and the immunocompromised, represent sensitive patient populations that could be more prone to adverse effects of therapy.
In our HBV program, we are developing modulators of capsid assembly, also known as core inhibitors. This is a newer mechanism of action for potential HBV treatment, and no capsid assembly modulators have advanced beyond Phase 2 clinical studies. Thus, we are not able to predict what adverse effects may arise in longer term studies conducted in larger populations. In addition, long term consequences of an HBV infection can include hepatocellular carcinoma, liver failure, or liver transplant. It may be difficult to determine whether our drug candidates are playing a direct role in contributing to (or protecting from) these downstream effects of HBV infection.
In our NASH program, we are developing agonists of the farnesoid X receptor, or FXR, that are designed to bind to that receptor and then trigger a response from it. The adverse effects from long-term exposure to the FXR drug class are not well known since within this class only two drugs have been approved by the FDA—Ocaliva®, approved in May 2016 for PBC, and an older drug not commonly used but approved to treat cholesterol gallstones (by dissolving them) and a rare lipid storage disease. In the case of Ocaliva, which is also in development for NASH, the FDA issued a Complete Response Letter, or CRL, in June 2020, due at least in part to some still undisclosed safety signal identified by the FDA. Unforeseen side effects from any of our product candidates could arise either during clinical development or, if approved, after the approved product has been marketed. The range and potential severity of possible side effects from systemic therapies like FXR agonists could be significant.
In addition, our drug candidates for NASH are being developed as a potential treatment for a severe disease that commonly occurs in patients with other serious conditions, including metabolic syndrome and diabetes. Any clinical trials in NASH will necessarily be conducted in patient populations that may be more prone than the general population to exhibit certain disease states or adverse events. It may be difficult to discern whether certain events or symptoms observed during our trials were due to our drug candidates or placebo, resulting in our company and our development programs being negatively affected even if such events or symptoms are ultimately determined to be unlikely related to our drug candidates.
33
If any of our product candidates receives marketing approval and we or others later identify undesirable or unacceptable side effects caused by such products:
|
|
• |
regulatory authorities may require the addition of labeling statements, specific warnings, a contraindication or field alerts to physicians and pharmacies; |
|
|
• |
we may be required to change instructions regarding the way the product is administered, conduct additional clinical trials or change the labeling of the product; |
|
|
• |
we may be subject to limitations on how we may promote the product; |
|
|
• |
sales of the product may decrease significantly; |
|
|
• |
regulatory authorities may require us to take our approved product off the market; |
|
|
• |
we may be subject to litigation or product liability claims; and |
|
|
• |
our reputation and our stock price may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenue from the sale of any product we develop.
If we are required to suspend or discontinue clinical trials due to side effects or other safety risks associated with our product candidates, or if we are required to conduct studies on the long-term effects associated with the use of any of those product candidates, commercialization any of those product candidates could be delayed or halted.
Clinical trials involving our product candidates may be suspended or terminated at any time for a number of safety-related reasons. For example, we may voluntarily suspend or terminate clinical trials if at any time one of our product candidates, or a combination therapy including any of them, presents an unacceptable safety risk to the clinical trial patients. In addition, IRBs or regulatory agencies may order the temporary discontinuation or termination of clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements, including if they present an unacceptable safety risk to patients. Administering any product candidate to humans may produce undesirable side effects. The existence of undesirable side effects resulting from any of our product candidates, or a combination therapy including any of them, could cause us or regulatory authorities, such as the FDA or EMA, to interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or EMA or other regulatory agencies denying further development or approval of our product candidates for any or all targeted indications. This, in turn, could prevent us from commercializing our product candidates.
Results of earlier clinical trials may not be predictive of the results of later-stage clinical trials.
To date we have only tested our product candidates through initial Phase 2 studies. The results of preclinical studies and these early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials, if any. In addition, results of Phase 3 clinical trials in one or more ethnic groups are not necessarily indicative of results in other ethnic groups. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy results despite having progressed through preclinical studies and initial clinical trials. For example, several companies engaged in clinical development in the disease areas we are also engaged in have suffered significant setbacks in advanced clinical trials due to adverse safety profiles or lack of efficacy, notwithstanding promising results in earlier studies. Similarly, future clinical trial results may not be successful for these or other reasons.
Product candidate development risk is heightened by any changes in the planned clinical trials compared to the completed clinical trials. As product candidates are developed through preclinical early and late stage clinical trials towards approval and commercialization, it is customary that various aspects of the development program, such as manufacturing and formulation, are altered along the way in an effort to optimize processes and results. Such changes carry the risk that they will not achieve these intended objectives. Any of these changes could make the results of planned clinical trials or other future clinical trials we may initiate less predictable and could cause our product candidates to perform differently, which could delay completion of clinical trials, delay approval of our product candidates and/or jeopardize our ability to commence product sales and generate revenues.
34
The regulatory approval processes of the FDA, the EMA and other comparable foreign authorities are lengthy, time-consuming and inherently unpredictable, and if we are ultimately unable to obtain timely regulatory approval for our product candidates, our business will be substantially harmed.
The regulatory approval process is expensive and, while the time required to gain FDA and foreign regulatory approval is uncertain, it may take years. Regulatory approvals are unpredictable and depend upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of preclinical and clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We may be required to undertake and complete certain additional preclinical studies to generate toxicity and other data required to support the submission of a New Drug Application, or NDA, to the FDA or comparable application to other regulatory authorities. AbbVie obtained all regulatory approvals for its paritaprevir-containing regimens and for MAVYRET/MAVIRET, which contains glecaprevir. We have not obtained regulatory approval by ourselves for any of our wholly-owned product candidates and it is possible that none of our existing product candidates or any of our future product candidates will ever obtain regulatory approval. Furthermore, approval in the United States by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA.
Our product candidates could fail to receive regulatory approval for many reasons, including the following:
|
|
• |
the FDA, the EMA or other comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
|
|
• |
we may be unable to demonstrate to the satisfaction of the FDA, the EMA or other comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
|
|
• |
the results of clinical trials may not meet the level of statistical significance required by the FDA, the EMA or other comparable foreign regulatory authorities for approval; |
|
|
• |
we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
|
|
• |
the FDA, the EMA or other comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
|
|
• |
the data collected from clinical trials of our product candidates may not be sufficient to support the submission of an NDA or other submissions or to obtain regulatory approval in the United States or elsewhere; |
|
|
• |
the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies of any of our product candidates; and |
|
|
• |
the approval policies or regulations of the FDA, the EMA or other comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
We cannot be assured that after spending substantial time and resources, we will obtain regulatory approvals in any desired jurisdiction. Even if we were to obtain approval, regulatory authorities may grant approval contingent on the performance of costly post-marketing clinical trials or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Significant clinical trial delays could allow our competitors to obtain marketing approval before we do or could in effect shorten the patent protection period during which we may have the exclusive right to commercialize our product candidates. In addition, it may ultimately not be possible to achieve the prices intended for our products. In many foreign countries, including those in the European Union, a product candidate must be approved for reimbursement before it can be approved for sale in that country. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates and our business.
Even if we receive regulatory approval for any of our product candidates we develop independently, we will be subject to ongoing FDA obligations and continued regulatory review in other jurisdictions, which may result in significant additional expense. Additionally, our product candidates, if approved, could be subject to labeling and other restrictions and market withdrawal and we may be subject to penalties if we or our collaborators fail to comply with regulatory requirements or experience unanticipated problems with our products.
Any regulatory approvals that we receive for our product candidates we develop independently may be subject to limitations on the approved indicated uses for which the product may be marketed or subject to certain conditions of approval, or may contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate.
35
In addition, if the FDA approves any of our product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, as well as continued compliance with current good manufacturing practices, or cGMP, and good clinical practices, or GCP, for any clinical trials that we or our collaborators conduct post-approval. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
|
|
• |
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market or voluntary or mandatory product recalls; |
|
|
• |
fines, warning letters or holds on any post-approval clinical trials; |
|
|
• |
refusal by the FDA to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals; |
|
|
• |
product seizure or detention, or refusal to permit the import or export of products; and |
|
|
• |
injunctions or the imposition of civil or criminal penalties. |
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we, or AbbVie in the case of any licensed HCV product, are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or AbbVie are not able to maintain regulatory compliance, our product candidates or AbbVie’s licensed HCV products may lose any marketing approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
We may delay or terminate the development of a product candidate at any time if we believe the perceived market or commercial opportunity does not justify further investment, which could materially harm our business and adversely affect our stock price.
Even though the results of preclinical studies and clinical trials that we have conducted or may conduct in the future may support further development of one or more of our product candidates, we may delay, suspend or terminate the future development of a product candidate at any time for strategic, business, financial or other reasons, including the determination or belief that the emerging profile of the product candidate is such that it may not receive regulatory approvals in key markets, gain meaningful market acceptance, otherwise provide any competitive advantages in its intended indication or market or generate a significant return to stockholders. Such a delay, suspension or termination could materially harm our business, results of operations or financial condition.
Risks Related to Commercialization of Our Product Candidates
Unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives in the United States could harm our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. In the United States, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010, collectively referred to as the ACA, has significantly changed the way healthcare is financed by both governmental and private insurers. While we cannot predict what impact on federal reimbursement policies this law or any amendment to it will continue to have in general or specifically on MAVYRET/MAVIRET or any product or regimen that we may commercialize, the ACA or any such amendment may result in downward pressure on pharmaceutical reimbursement, which could negatively affect market acceptance of new products. In addition, several states have not implemented certain sections of the ACA, including 14 that have rejected the expansion of Medicaid eligibility for low income citizens, and some members of the U.S. Congress are still working to repeal the ACA. On December 14, 2018, a United States District Court judge in Texas ruled that the Affordable Care Act is unconstitutional in its entirety because the “individual mandate” was repealed by Congress as part of the Tax Cuts and Jobs Act of 2017. While this U.S. District Court judge, as well as the Trump administration and Centers for Medicare and Medicaid Services, have stated that the ruling will have no immediate effect pending appeal of the decision, on December 18, 2019, the Fifth Circuit U.S. Court of Appeals held that the individual mandate is unconstitutional and remanded the case to the District Court to reconsider its earlier invalidation of the entire ACA. This decision is currently on appeal in the United States Supreme Court, where briefs were argued in November 2020. Pending the decision on this appeal, it is unclear how this case will impact the ACA. We cannot predict what impact this case, or any legislation to replace the ACA if it is invalidated, may have on us or on AbbVie’s sales of MAVYRET/MAVIRET.
36
In addition, other legislative changes have been proposed since the Affordable Care Act was enacted. There has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices. Specifically, there have been several recent U.S. congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for drugs. We expect any healthcare reform measures that may be adopted in the future could result in more rigorous coverage criteria and an additional downward pressure on the price that AbbVie receives for MAVYRET/MAVIRET, which could seriously harm our future revenues, and the price of our common stock could be materially adversely affected.
Our ability to commercialize any product candidate successfully, as well as AbbVie’s continued commercialization of MAVYRET/MAVIRET, will also depend in part on the extent to which reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. In the case of HCV, limitations of coverage have recently been used to limit access to HCV treatments for only those patients with more advanced fibrosis. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and, in many cases involving HCV drugs, seeking discounts in exchange for greater patient access to a particular HCV drug. In addition, there are private and public payors challenging the prices charged for medical products. We cannot be sure that reimbursement will be available for any product that we may commercialize and, if reimbursement is available, the level of reimbursement. In addition, reimbursement may impact the demand for, or the price of, MAVYRET/MAVIRET or any product candidate for which we may obtain marketing approval. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we may seek marketing approval.
There may be significant delays in obtaining reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable authorities in other jurisdictions. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also be insufficient to cover our and any collaborator’s costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. AbbVie’s inability to continue to obtain coverage and profitable payment rates from both government-funded and private payors for MAVYRET/MAVIRET, or our inability to obtain the same for any product candidate that we develop, could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
In general, the United States and several other jurisdictions are considering a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our products profitably. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access to healthcare. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. We expect to experience pricing pressures in connection with the sale of any products that we develop or that are being commercialized under our collaboration with AbbVie. The implementation of cost containment measures or other healthcare reforms may limit our ability to generate revenue, maintain profitability or commercialize our product candidates.
Foreign governments tend to impose strict price controls, which may adversely affect our future profitability.
In most foreign countries, particularly in the European Union and Japan, prescription drug pricing and/or reimbursement is subject to governmental control. In those countries that impose price controls, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies.
Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we (or AbbVie in the case of MAVYRET/MAVIRET) might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues that are generated from the sale of the product in that country. If reimbursement of MAVYRET/MAVIRET or of any of our product candidates is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, or if there is competition from lower priced cross-border sales, our results of operations will be negatively affected.
37
If, in the future, we are unable to establish our own sales, marketing and distribution capabilities or enter into licensing or collaboration agreements for these purposes, we may not be successful in commercializing any product candidates.
We do not have a sales or marketing infrastructure and have no sales, marketing or distribution experience. We will seek to either build our own commercial infrastructure to commercialize any products if and when they are approved, or enter into licensing or collaboration agreements where our collaborator is responsible for commercialization, as in the case of our collaboration with AbbVie, or where we have the right to assist in the future development and commercialization of such products.
To develop internal sales, distribution and marketing capabilities, we will have to invest significant amounts of financial and management resources, some of which will be committed prior to any confirmation that any of our proprietary product candidates will be approved. For product candidates for which we decide to perform sales, marketing and distribution functions ourselves, we could face a number of additional risks, including:
|
|
• |
our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
|
|
• |
the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any products; |
|
|
• |
the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
|
|
• |
unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
Where and when appropriate, we may elect to utilize contract sales forces or distribution partners to assist in the commercialization of our product candidates. If we enter into arrangements with third parties to perform sales, marketing and distribution services for our products, the resulting revenues or the profitability from these revenues to us are likely to be lower than if we had sold, marketed and distributed our products ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell, market and distribute our product candidates or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of these third parties may fail to devote the necessary resources and attention to sell, market and distribute our products effectively.
If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
Commercial success of our product candidates depends upon significant market acceptance among physicians, patients and healthcare payors of any resulting approved drug.
MAVYRET/MAVIRET, as well as EDP-938, EDP-514, EDP-721, EDP-305, EDP-297 and any other product candidate that we may develop in the future, whether as part of a combination therapy or as a monotherapy, are subject to market acceptance among physicians, healthcare payors, patients and the medical community. The degree of market acceptance of any product candidate for which we obtain approval for commercial sale, will depend on a number of factors, including:
|
|
• |
the efficacy and safety of treatment regimens containing one of our product candidates, as demonstrated in clinical trials, and the degree to which these regimens represent a clinically meaningful improvement in care as compared with other available therapies; |
|
|
• |
the clinical indications for which any treatment regimen containing one of our product candidates become approved; |
|
|
• |
acceptance among physicians, major operators of clinics, payors and patients of any treatment regimen containing one of our product candidates; |
|
|
• |
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
|
|
• |
the potential and perceived advantages of treatment regimens containing one of our product candidates over alternative treatments; |
|
|
• |
the cost of treatment of regimens containing one of our product candidates in relation to the cost of alternative treatments; |
|
|
• |
the availability of adequate reimbursement and pricing by third parties and government authorities and successful negotiation of favorable agreements with payors by us or any collaborator of ours, as well as the impact of any agreements among any of the foregoing and one or more of our competitors limiting access to our product in favor of one or more competitive products; |
|
|
• |
the continued longevity of any market for which we develop a drug; |
38
|
|
• |
the levels of funding provided by government-funded healthcare for treatment of any disease for which we develop a drug; |
|
|
• |
the relative convenience and ease of administration of any treatment regimen containing one of our product candidates compared to competitive regimens; |
|
|
• |
the prevalence and severity of adverse side effects, whether involving the use of treatment regimens containing one of our products candidates or similar, competitive treatment regimens; and |
|
|
• |
the effectiveness of our sales and marketing efforts. |
If treatment regimens containing one of our product candidates are approved and then fail to achieve market acceptance, we may not be able to generate significant additional revenue. Further, if new, more favorably received therapies are introduced after any such regimen achieves market acceptance, then we may not be able to maintain that market acceptance over time.
Risks Related to Our Dependence on Third Parties
We may not be successful in establishing new product collaborations, which could adversely affect our ability to develop and commercialize one or more of our product candidates. If we are unsuccessful in maintaining or forming alliances on favorable terms, our business may not succeed.
We may seek to enter into additional product collaborations in the future, including alliances with other biotechnology or pharmaceutical companies, to enhance and accelerate the development and commercialization of one or more of our product candidates. We face significant competition in seeking appropriate collaborators and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish other product collaborations or other alternative arrangements for any product candidates and programs because our research and development pipeline may be insufficient, our product candidates and programs may be deemed to be at too early of a stage of development for collaborative effort and/or third parties may not view our product candidates and programs as having the requisite potential to demonstrate safety and efficacy. Even if we are successful in our efforts to establish product collaborations, the terms that we agree upon may not be favorable to us and we may not be able to maintain such product collaborations if, for example, development or approval of a product candidate is delayed or sales of an approved product are disappointing.
If our existing collaboration agreement with AbbVie is terminated, or if we determine that entering into other product collaborations is in our best interest but we either fail to enter into, experience a delay in entering into, or fail to maintain, such collaborations:
|
|
• |
the development of certain of our product candidates may be terminated or delayed; |
|
|
• |
our cash expenditures related to development of certain of our product candidates would increase significantly and we may need to seek additional financing; |
|
|
• |
we may be required to hire additional employees or otherwise develop expertise, such as clinical, regulatory, sales and marketing expertise, which we do not currently have; |
|
|
• |
we will bear all of the risk related to the development of any such product candidates; and |
|
|
• |
the competitiveness of any product candidate that is commercialized could be reduced. |
We intend to rely on third-party manufacturers to produce our development-stage product candidate supplies and any commercial supplies of any approved product candidates. Any failure by a third-party manufacturer to produce acceptable supplies for us may delay or impair our ability to initiate or complete our clinical trials or sell any resulting product.
We do not currently own or operate any manufacturing facilities. We plan to continue to work with third-party contract manufacturers to produce sufficient quantities of any product candidates for preclinical testing, clinical trials and commercialization. If we are unable to arrange for such a third-party manufacturing source for any of our product candidates, or fail to do so on commercially reasonable terms, we may not be able to successfully produce, develop and market one or more of our product candidates, or we may be delayed in doing so.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured product candidates ourselves, including reliance on the third party for regulatory compliance and quality control and assurance, volume production, the possibility of breach of the manufacturing agreement by the third party because of factors beyond our control (including a failure to synthesize and manufacture our product candidates in accordance with our product specifications), shutdowns of manufacturing sites as a result of the COVID-19 pandemic, and the possibility of termination or nonrenewal of the agreement by the third party at a time that is costly or damaging to us. In addition, the FDA and other regulatory authorities require that our product candidates be manufactured according to cGMP and similar foreign standards. Pharmaceutical manufacturers and their subcontractors are required
39
to register their facilities and/or products manufactured at the time of submission of the marketing application and then annually thereafter with the FDA and certain state and foreign agencies. They are also subject to periodic unannounced inspections by the FDA, state and other foreign authorities. Any subsequent discovery of problems with a product, or a manufacturing or laboratory facility used by us or our collaborators, may result in restrictions on the product or on the manufacturing or laboratory facility, including marketed product recall, suspension of manufacturing, product seizure, or a voluntary withdrawal of the drug from the market. Any failure by our third-party manufacturers to comply with cGMP or failure to scale up manufacturing processes, including any failure to deliver sufficient quantities of product candidates in a timely manner, could lead to a delay in, or failure to obtain, regulatory approval of any of our product candidates.
We plan to rely on third-party manufacturers to purchase from third-party suppliers the materials necessary to produce our product candidates for our clinical studies. There are a small number of suppliers for certain capital equipment and materials that we plan to use to manufacture our drugs. Such suppliers may not sell these materials to our manufacturers at the times we need them or on commercially reasonable terms. Moreover, we currently do not have any agreements for the production of these materials. Although we do not intend to begin a clinical trial unless we believe we have a sufficient supply of a product candidate to complete the clinical trial, any significant delay in the supply of a product candidate or the material components thereof for an ongoing clinical trial due to the need to replace a third-party manufacturer could considerably delay completion of our clinical studies, product testing and potential regulatory approval of our product candidates. If our manufacturers or we are unable to purchase these materials after regulatory approval has been obtained for our product candidates, the commercial launch of our product candidates would be delayed or there would be a shortage in supply, which would impair our ability to generate revenue from the sale of our product candidates.
Contract manufacturers may not be able to manufacture our product candidates at a cost or in quantities or in a timely manner necessary to develop and commercialize them. If we successfully commercialize any of our product candidates, to meet our projected needs we may need to find third parties that will increase their scale of production, or we may have to establish or access large-scale commercial manufacturing capabilities. We may require additional funds, personnel and other resources to build, lease or operate any manufacturing facility.
A portion of our research and a portion of our manufacturing of certain key intermediates used in the manufacture of the active pharmaceutical ingredients for our product candidates takes place in China through third-party researchers and manufacturers. A significant disruption in the operation of those researchers or manufacturers, or a trade war, political unrest or an epidemic in China, such as the COVID-19 pandemic, could materially adversely affect our business, financial condition and results of operations.
Although manufacturing for MAVYRET/MAVIRET is being conducted by AbbVie, we have relied on third parties located in China to manufacture and supply certain key intermediates used in the manufacture of our active pharmaceutical ingredients, or API, for our current product candidates, and we expect to continue to use such third party manufacturers for such intermediates for any product candidates we develop independently. Any disruption in production or inability of our manufacturers in China to produce adequate quantities to meet our needs, whether as a result of a natural disaster, pandemics or other causes, could impair our ability to operate our business on a day-to-day basis and to continue our research and development of our product candidates. We also use contract researchers in China to conduct a portion of our research for our early stage programs. Any disruption in the team conducting that research could cause delays in one or more of our research programs and could require us to curtail one or more programs, at least until we could contract for that research to be done elsewhere. For example, either of these risks could be triggered by an epidemic such as the outbreak of COVID-19 in the Wuhan region of China. To date our contract manufacturer in China, which is not located in the Wuhan region, has not had any material delays in its ability to deliver API and other services. Furthermore, since these researchers and manufacturers are located in China, we are exposed to the possibility of product supply disruption and increased costs in the event of changes in the policies of the United States or Chinese governments, political unrest or unstable economic conditions in China. For example, a trade war could lead to tariffs on the chemical intermediates we use that are manufactured in China. Any of these matters could materially and adversely affect our business and results of operations. Any recall of the manufacturing lots or similar action regarding our API used in clinical trials could delay the trials or detract from the integrity of the trial data and its potential use in future regulatory filings. In addition, manufacturing interruptions or failure to comply with regulatory requirements by any of these manufacturers could significantly delay clinical development of potential products and reduce third-party or clinical researcher interest and support of proposed trials. These interruptions or failures could also impede commercialization of our product candidates and impair our competitive position. Further, we may be exposed to fluctuations in the value of the local currency in China. Future appreciation of the local currency could increase our costs. In addition, our labor costs could continue to rise as wage rates increase due to increased demand for skilled laborers and the availability of skilled labor declines in China.
40
We will rely on third parties to monitor, support, conduct and/or oversee clinical trials of our product candidates that we develop independently and, in some cases, to maintain regulatory files for those product candidates. If we are not able to maintain or secure agreements with such third parties on acceptable terms, if these third parties do not perform their services as required, or if these third parties fail to timely transfer any regulatory information held by them to us, we may not be able to obtain regulatory approval for, or commercialize, our product candidates.
We will rely on CROs, hospitals, clinics, academic institutions and other third-party collaborators who are outside our control to monitor, support, conduct and/or oversee preclinical and clinical studies of our product candidates. We will also rely on third parties to perform clinical trials of our product candidates when they reach that stage. As a result, we have less control over the timing and cost of these studies and the ability to recruit trial subjects than if we conducted these trials wholly by ourselves. If we are unable to maintain or enter into agreements with these third parties on acceptable terms or engagement is terminated, we may be unable to enroll patients on a timely basis or otherwise conduct our trials in the manner we anticipate. In the case of our ongoing studies, we paused recruitment and dosing of the ARGON-2 Phase 2b NASH study and Part 2 of our Phase 1a/1b study of EDP-514 in nuc-suppressed HBV patients as a result of the COVID-19 pandemic in March 2020, but we were able to resume these studies in July 2020. The pause in these studies is expected to delay their completion, and it is uncertain whether they or any of our other ongoing studies may be subject to further disruptions due to the ongoing pandemic. In addition, there is no guarantee that these third parties will devote adequate time and resources to our studies or perform as required by a contract or in accordance with regulatory requirements, including maintenance of clinical trial information regarding our product candidates. If these third parties fail to meet expected deadlines, fail to timely transfer to us any regulatory information, fail to adhere to protocols or fail to act in accordance with regulatory requirements or our agreements with them, or if they otherwise perform in a substandard manner or in a way that compromises the quality or accuracy of their activities or the data they obtain, then clinical trials of our product candidates may be extended, delayed or terminated, or our data may be rejected by the FDA or regulatory agencies.
To the extent we elect to enter into additional licensing or collaboration agreements to partner our product candidates, our dependence on such relationships may adversely affect our business.
Our commercialization strategy for some of our product candidates may depend on our ability to enter into collaboration agreements with other companies to obtain access to other compounds for use in combination with any of our product candidates or for assistance and funding for the development and potential commercialization of any of these product candidates, similar to what we have done with AbbVie. Supporting diligence activities conducted by potential collaborators and negotiating the financial and other terms of a collaboration agreement are long and complex processes with uncertain results. Even if we are successful in entering into one or more additional collaboration agreements, collaborations can involve greater uncertainty for us, as we may have limited or no control over certain aspects of our collaborative programs. We may determine that continuing a collaboration under the terms provided is not in our best interest, and we may terminate the collaboration. Our collaborators could delay or terminate their agreements with us, and our product candidates subject to collaborative arrangements may never be successfully commercialized.
Further, our collaborators may develop alternative products or pursue alternative technologies either on their own or in collaboration with others, including our competitors, and the priorities or focus of our collaborators may shift such that our programs receive less attention or resources than we would like, or they may be terminated altogether. Any such actions by our collaborators may adversely affect our business prospects and ability to earn revenue. In addition, we could have disputes with our collaborators, such as the interpretation of terms in our agreements. Any such disagreements could lead to delays in the development or commercialization of any potential products or could result in time-consuming and expensive litigation or arbitration, which may not be resolved in our favor.
Even with respect to programs that we intend to commercialize ourselves, we may enter into agreements with collaborators to share in the burden of conducting clinical trials, manufacturing and marketing our product candidates or products. In addition, our ability to apply our proprietary technologies to develop proprietary compounds will depend on our ability to establish and maintain licensing arrangements or other collaborative arrangements with the holders of proprietary rights to such compounds. We may not be able to establish such arrangements on favorable terms or at all, and our collaborative arrangements may not be successful.
Risks Related to Our Intellectual Property Rights
We are competing to develop intellectual property in areas of small-molecule drug development that are highly competitive. We could be unsuccessful in obtaining or maintaining adequate patent protection for one or more of our product candidates.
Our commercial success will depend, in large part, on our ability to obtain and maintain patent and other intellectual property protection with respect to our product candidates. We cannot be certain that patents will be issued or granted with respect to our patent applications that are currently pending, or that issued or granted patents will not later be found to be invalid and/or unenforceable, be interpreted in a manner that does not adequately protect our products, or otherwise provide us with any competitive advantage. The patent position of biotechnology and pharmaceutical companies is generally uncertain because it involves complex legal and factual considerations. The standards applied by the United States Patent and Trademark Office and foreign patent offices in granting patents
41
are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in biotechnology and pharmaceutical patents. Consequently, patents may not issue from our pending patent applications. As such, we do not know the degree of future protection that we will have on our proprietary products and technology, if any, and a failure to obtain adequate intellectual property protection with respect to our product candidates and proprietary technology could have a material adverse impact on our business.
In addition, certain of our activities in the past have been funded, and others may in the future be funded, by the United States federal government. For example, the preclinical and early clinical development of the lead antibiotic product candidate in our former antibiotic program, which we are no longer developing, was funded under a contract with NIAID, an entity of the United States federal government. When new technologies are developed with United States federal government funding, the government obtains certain rights in any resulting patents, including a nonexclusive license authorizing the government to use the invention for non-commercial purposes. These rights may permit the government to disclose our confidential information to third parties and to exercise “march-in” rights to use or allow third parties to use our patented technology. The government can exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the United States government-funded technology, or because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations or to give preference to United States industry. In addition, United States government-funded inventions must be reported to the government and United States government funding must be disclosed in any resulting patent applications. In addition, our rights in such inventions are subject to certain requirements to manufacture products in the United States.
Claims that our product candidates or the sale or use of our products infringe the patent or other intellectual property rights of third parties could result in costly litigation or could require substantial time and money to resolve, even if litigation is avoided.
Our commercial success depends upon our ability to develop, manufacture, market and sell our product candidates and use our proprietary technology without infringing the intellectual property rights of others. We cannot guarantee that our product candidates or any uses of our product candidates do not and will not in the future infringe third-party patents or other intellectual property rights. Third parties might allege that we or our collaborators are infringing their patent rights or that we have misappropriated their trade secrets, or that we are otherwise violating their intellectual property rights, whether with respect to the manner in which we have conducted our research or to the composition, use or manufacture of the compounds we have developed or are developing with our collaborators. Such third parties might resort to litigation against us or other parties we have agreed to indemnify, which litigation could be based on either existing intellectual property or intellectual property that arises in the future.
It is also possible that we failed to identify, or may in the future fail to identify, relevant patents or patent applications held by third parties that cover our product candidates. For example, applications filed before November 29, 2000 and certain applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Other patent applications in the United States and several other jurisdictions are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Furthermore, publication of discoveries in the scientific or patent literature often lags behind actual discoveries. Therefore, we cannot be certain that we or our collaborators were the first to invent, or the first to file patent applications on, our product candidates or for their uses, or that our product candidates will not infringe patents that are currently issued or that are issued in the future. In the event that a third party has also filed a patent application covering one of our product candidates or a similar invention, we may have to participate in an adversarial proceeding, known as an interference, declared by the U.S. Patent and Trademark Office or its foreign counterpart to determine priority of invention. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our products or their use.
In order to avoid or settle potential claims with respect to any patent or other intellectual property rights of third parties, we may choose or be required to seek a license from a third party and be required to pay license fees or royalties or both, which could be substantial. These licenses may not be available on acceptable terms, or at all. Even if we were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced, by court order or otherwise, to cease some or all aspects of our business operations, if, as a result of actual or threatened patent or other intellectual property claims, we are unable to enter into licenses on acceptable terms. Further, we could be found liable for significant monetary damages as a result of claims of intellectual property infringement. For example, we have received, and may in the future receive, offers to license and demands to license from third parties claiming that we are infringing their intellectual property or owe license fees and, even if such claims are without merit, there can be no assurance that we will successfully avoid or settle such claims.
In addition, if AbbVie licenses or otherwise acquires rights to intellectual property controlled by a third party in various circumstances, for example, where a product could not be legally developed or commercialized in a country without the third-party intellectual property right, it is entitled under our collaboration agreement to decrease payments payable to us on a product-by-product basis and, in certain cases, on a country-by-country basis. Any of the foregoing events could harm our business significantly.
Defending against claims of patent infringement, misappropriation of trade secrets or other violations of intellectual property rights could be costly and time consuming, regardless of the outcome. Thus, even if we were to ultimately prevail, or to settle at an early
42
stage, such litigation could burden us with substantial unanticipated costs. In addition, litigation or threatened litigation could result in significant demands on the time and attention of our management team, distracting them from the pursuit of other company business. Claims that our product candidates or the sale or use of our future products infringe, misappropriate or otherwise violate third-party intellectual property rights could therefore have a material adverse impact on our business.
Confidentiality agreements with employees and third parties may not prevent unauthorized disclosure of trade secrets and other proprietary information.
In addition to patents, we rely on trade secrets, technical know-how and proprietary information concerning our business strategy and product candidates in order to protect our competitive position in the field of each of our antivirals and our NASH product candidates. In the course of our research and development activities and our business activities, we often rely on confidentiality agreements to protect our proprietary information. Such confidentiality agreements are used, for example, when we talk to vendors of laboratory or clinical development services or potential strategic partners. In addition, each of our employees is required to sign a confidentiality agreement and invention assignment agreement upon joining our company. We take steps to protect our proprietary information, and our confidentiality agreements and invention assignment agreements are carefully drafted to protect our proprietary interests. Nevertheless, there can be no guarantee that an employee or an outside party will not make an unauthorized disclosure of our proprietary confidential information. This might happen intentionally or inadvertently. It is possible that a competitor will make use of such information, and that our competitive position will be compromised, in spite of any legal action we might take against persons making such unauthorized disclosures. In addition, to the extent that our employees, consultants or contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, business partners or outside scientific collaborators might intentionally or inadvertently disclose our trade secret information to competitors or our trade secrets may otherwise be misappropriated. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States sometimes are less willing than United States courts to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
Our collaborators may have rights to publish data and other information to which we have rights. In addition, we sometimes engage individuals or entities to conduct research relevant to our business. The ability of these individuals or entities to publish or otherwise publicly disclose data and other information generated during the course of their research is subject to certain contractual limitations. These contractual provisions may be insufficient or inadequate to protect our confidential information. If we do not apply for patent protection prior to such publication, or if we cannot otherwise maintain the confidentiality of our proprietary technology and other confidential information, then our ability to obtain patent protection or to protect our trade secret information may be jeopardized, which could adversely affect our business.
Risks Related to Our Industry
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates, and we will face an even greater risk if we commercialize any product candidates. For example, we may be sued if any of our product candidates, including any that are developed in combination therapies, allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Even successful defense would require significant financial and management resources. There is also risk that third parties we have agreed to indemnify could incur liability.
Regardless of the merits or eventual outcome, liability claims may result in:
|
|
• |
decreased demand for our product candidates or any resulting products; |
|
|
• |
injury to our reputation; |
|
|
• |
withdrawal of clinical trial participants; |
|
|
• |
costs to defend the related litigation; |
|
|
• |
a diversion of management’s time and our resources; |
|
|
• |
substantial monetary awards to trial participants or patients; |
|
|
• |
product recalls, withdrawals or labeling, marketing or promotional restrictions; |
43
|
|
• |
loss of revenue; |
|
|
• |
the inability to commercialize our product candidates; and |
|
|
• |
a decline in our stock price. |
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. We currently carry product liability insurance covering our clinical studies in the amount of $15.0 million in the aggregate. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Our internal computer systems, or those of our collaborator, CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of development programs for our product candidates.
Despite the implementation of security measures, our internal computer systems and those of our collaborators, CROs, and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, pandemics, terrorism, war and telecommunication and electrical failures. Information security risks have significantly increased in recent years and during the COVID-19 pandemic in part due to the proliferation of new technologies, the increased sophistication and activities of organized crime, hackers, terrorists and other external parties, including foreign state actors as well as remote working for many businesses. As cyber threats continue to evolve, we may be required to expend significant additional resources to continue to modify or enhance our protective measures or to investigate and remediate any information security breaches.
While we have not experienced any such system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our independent drug development programs. For example, the loss of clinical trial data from ongoing or future clinical trials for any of our product candidates could result in delays in regulatory approval efforts and significantly increase costs to recover or reproduce the data. Our information security systems are also subject to laws and regulations requiring that we take measures to protect the privacy and security of certain information we gather and use in our business. For example, HIPAA and its implementing regulations impose, among other requirements, certain regulatory and contractual requirements regarding the privacy and security of personal health information. In the European Union the General Data Protection Regulation, or GDPR, is even more restrictive with respect to all personal information, including information masked by a coding system. In addition to HIPAA and GDPR, numerous other federal and state laws, including, without limitation, state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure and storage of personal information. To the extent that any disruption or security breach were to result in a loss of or damage to data or applications, or inappropriate disclosure of confidential or proprietary information or personal health information, we could incur substantial liability, our reputation would be damaged, and the further development of our product candidates could be delayed.
Our relationships with customers and third-party payors in the United States and elsewhere will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors in the United States and elsewhere play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal, state and foreign healthcare laws and regulations include the following:
|
|
• |
the federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
|
|
• |
the federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
44
|
|
• |
the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
|
|
• |
the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
|
|
• |
the federal transparency requirements under the Patient Protection and Affordable Care Act of 2010 requires manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; |
|
|
• |
analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures; and |
|
|
• |
analogous anti-kickback, fraud and abuse and healthcare laws and regulations in foreign countries. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other providers or entities with whom we expect to do business, including our collaborators, are found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs, which could also materially affect our business.
Risks Related to Our Common Stock
Our stock price has been, and is likely to continue to be, volatile, and thus our stockholders could incur substantial losses.
Our stock price has been volatile and could be subject to wide fluctuations in response to various factors, many of which are beyond our control. From September 30, 2014 through December 31, 2020, the daily closing price of our common stock on the NASDAQ Global Select Market has ranged from $21.00 to $126.37. The stock market in general and the market for biopharmaceutical companies, and for those developing potential therapies for viral infections and liver diseases in particular, have experienced extreme volatility that has often been unrelated to the operating performance of particular companies, which in some cases has been exacerbated by the COVID-19 pandemic. As a result of this volatility, you may not be able to sell your holdings of our common stock at or above your purchase price, if at all. The market price for our common stock may be influenced by many factors, including:
|
|
• |
results from or delays of clinical trials of our product candidates, as well as results of regulatory reviews relating to the approval of our product candidates; |
|
|
• |
actions by AbbVie regarding the MAVYRET/MAVIRET regimen, including announcements regarding regulatory or commercial developments; |
|
|
• |
market expectations about and response to the levels of sales or scripts achieved by, or the announced prices or discounts for, AbbVie’s MAVYRET/MAVIRET regimen or competitive HCV drugs; |
|
|
• |
failure of AbbVie’s MAVYRET/MAVIRET regimen to maintain its sales levels; |
|
|
• |
the results of our efforts to discover or develop additional product candidates; |
|
|
• |
new products, product candidates or new uses for existing products or technologies introduced or announced by our competitors and the timing of these introductions or announcements; |
|
|
• |
our dependence on third parties, including our collaborators, CROs, manufacturers, clinical trial sponsors and clinical investigators; |
|
|
• |
regulatory, political or legal developments in the United States or other countries; |
45
|
|
• |
developments or disputes concerning patent applications, issued patents or other proprietary rights; |
|
|
• |
the recruitment or departure of key scientific or management personnel; |
|
|
• |
our ability to commercialize our product candidates we develop independently, if approved; |
|
|
• |
the level of expenses related to any of our product candidates or clinical development programs; |
|
|
• |
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
|
|
• |
period-to-period variations in our financial results or those of companies that are perceived to be similar to us; |
|
|
• |
sales of common stock by us or our stockholders in the future, as well as the overall trading volume of our common stock; |
|
|
• |
changes in the structure of healthcare payment systems or other actions that affect the effective reimbursement rates for treatment regimens containing our products or for competitive regimens; |
|
|
• |
market conditions in the pharmaceutical and biotechnology sectors; |
|
|
• |
general economic, industry and market conditions and other factors that may be unrelated to our operating performance or the operating performance of our competitors, including changes in market valuations of similar companies; and |
|
|
• |
the other factors described in this “Risk Factors” section. |
Provisions in our corporate charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which they might otherwise receive a premium for their shares.
These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
|
|
• |
establish a classified or staggered board of directors such that not all members of the board are elected at one time; |
|
|
• |
allow the authorized number of our directors to be changed only by resolution of our board of directors; |
|
|
• |
limit the manner in which stockholders can remove directors from the board; |
|
|
• |
establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors; |
|
|
• |
require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent; |
|
|
• |
limit who may call stockholder meetings; |
|
|
• |
provide that the state courts or, in certain circumstances, the federal courts, in Delaware shall be the sole and exclusive forum for certain actions involving us, our directors, officers, employees and stockholders; |
|
|
• |
provide our board of directors with the authority to designate the terms of and issue a new series of preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and |
|
|
• |
require the approval of the holders of at least 66 2/3% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our charter or bylaws. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibit a person who owns 15% or more of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. Any provision in our corporate charter or our bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive
46
a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Our employment agreements with our executive officers may require us to pay severance benefits to any of those persons who are terminated in connection with a change of control of us, which could harm our financial condition or results.
Our executive officers are parties to employment agreements that provide for aggregate cash payments of up to approximately $5.0 million for severance and other non-equity-based benefits in the event of a termination of employment in connection with a change of control of our company. In addition, based on the closing price of our common stock of $42.10 per common share as of December 31, 2020, the aggregate intrinsic value of unvested stock options and other equity awards subject to accelerated vesting upon these events was $10.9 million. The accelerated vesting of awards options could result in dilution to our stockholders and harm the market price of our common stock. The payment of these severance benefits could harm our company’s financial condition and results. In addition, these potential severance payments may discourage or prevent third parties from seeking a business combination with us.
Because we do not anticipate paying cash dividends on our common stock for the foreseeable future, investors in our common stock may never receive a return on their investment.
You should not rely on an investment in our common stock to provide dividend income. We do not anticipate that we will pay any cash dividends to holders of our common stock for the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our existing operations.
Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any return on their investment. As a result, investors seeking cash dividends should not invest in our common stock.
A sale of a substantial number of shares of our common stock in the public market could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. As of December 31, 2020 we had 20.1 million shares of common stock outstanding. In addition, as of December 31, 2020, 3.8 million and 0.4 million shares of common stock that are subject to outstanding options or restricted stock unit awards, respectively, under our outstanding equity plans are eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, and Rule 144 under the Securities Act. If these additional shares of common stock are sold, or it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. If those analysts are unable to predict accurately the demand and net sales of AbbVie’s HCV regimens, that could result in our reported revenues and earnings being lower than the so-called “market consensus” of our projected revenues, which could negatively affect our stock price. In addition, if too few securities or industry analysts cover our company, the trading price for our stock would likely be negatively impacted. In the event that one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
General Risk Factors
Issued patents covering one or more of our product candidates could be found invalid or unenforceable if challenged in court.
Despite measures we take to obtain patent and other intellectual property protection with respect to our product candidates and proprietary technology, any of our intellectual property rights could be challenged or invalidated. For example, if we were to initiate legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the United States and in some other jurisdictions, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the United States Patent and Trademark Office, or the applicable foreign counterpart, or made a misleading statement, during
47
prosecution. Although we believe that we have conducted our patent prosecution in accordance with the duty of candor and in good faith, the outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on a product candidate. Even if a defendant does not prevail on a legal assertion of invalidity and/or unenforceability, our patent claims may be construed in a manner that would limit our ability to enforce such claims against the defendant and others. Any loss of patent protection could have a material adverse impact on one or more of our product candidates and our business.
Enforcing our intellectual property rights against third parties may also cause such third parties to file other counterclaims against us, which could be costly to defend and could require us to pay substantial damages, cease the sale of certain products or enter into a license agreement and pay royalties (which may not be possible on commercially reasonable terms or at all). Any efforts to enforce our intellectual property rights are also likely to be costly and may divert the efforts of our scientific and management personnel.
Unfavorable outcomes in intellectual property litigation could limit our research and development activities and/or our ability to commercialize certain products.
If third parties successfully assert their intellectual property rights against us, we might be barred from using certain aspects of our technology, or barred from developing and commercializing certain products. Prohibitions against using certain technologies, or prohibitions against commercializing certain products, could be imposed by a court or by a settlement agreement between us and a plaintiff. In addition, if we are unsuccessful in defending against allegations that we have infringed, misappropriated or otherwise violated patent or other intellectual property rights of others, we may be forced to pay substantial damage awards to the plaintiff. There is inevitable uncertainty in any litigation, including intellectual property litigation. There can be no assurance that we would prevail in any intellectual property litigation, even if the case against us is weak or flawed. If litigation leads to an outcome unfavorable to us, we may be required to obtain a license from the intellectual property owner in order to continue our research and development programs or to market any resulting product. It is possible that the necessary license will not be available to us on commercially acceptable terms, or at all. Alternatively, we may be required to modify or redesign our products in order to avoid infringing or otherwise violating third-party intellectual property rights. This may not be technically or commercially feasible, may render our products less competitive, or may delay or prevent the entry of our products to the market. Any of the foregoing could limit our research and development activities, our ability to commercialize one or more product candidates, or both.
Most of our competitors are larger than we are and have substantially greater resources. They are, therefore, likely to be able to sustain the costs of complex intellectual property litigation longer than we could. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to conduct our clinical trials, continue our internal research programs, in-license needed technology, or enter into strategic partnerships that would help us bring our product candidates to market.
In addition, any future intellectual property litigation, interference or other administrative proceedings will result in additional expense and distraction of our personnel. An adverse outcome in such litigation or proceedings may expose us or any future strategic partners to loss of our proprietary position, expose us to significant liabilities, or require us to seek licenses that may not be available on commercially acceptable terms, if at all, each of which could have a material adverse effect on our business.
Intellectual property litigation may lead to unfavorable publicity that harms our reputation and causes the market price of our common stock to decline.
During the course of any intellectual property litigation, there could be public announcements of the results of hearings, rulings on motions, and other interim proceedings in the litigation. If securities analysts or investors regard these announcements as negative, the perceived value of our products, programs or intellectual property could be diminished. Accordingly, the market price of our common stock may decline. Such announcements could also harm our reputation or the market for our future products, which could have a material adverse effect on our business.
Intellectual property rights do not necessarily protect us from all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
|
|
• |
others may be able to make compounds that are similar to our product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed; |
|
|
• |
we might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own or may in the future exclusively license, which could result in the patent applications not issuing or being invalidated after issuing; |
48
|
|
• |
we might not have been the first to file patent applications covering certain of our inventions, which could result in the patent applications not issuing or being invalidated after issuing; |
|
|
• |
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
|
|
• |
it is possible that our pending patent applications will not lead to issued patents; |
|
|
• |
issued patents that we own may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors; we may obtain patents for certain compounds many years before we obtain marketing approval for products containing such compounds, and because patents have a limited life, which may begin to run prior to the commercial sale of the related product, the commercial value of our patents may be limited; |
|
|
• |
our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
|
|
• |
we may fail to develop additional proprietary technologies that are patentable; |
|
|
• |
the laws of certain foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States, or we may fail to apply for or obtain adequate intellectual property protection in all the jurisdictions in which we operate; and |
|
|
• |
the patents of others may have an adverse effect on our business, for example by preventing us from marketing one or more of our product candidates for one or more indications. |
Any of the aforementioned threats to our competitive advantage could have a material adverse effect on our business.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with many other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining, maintaining and enforcing patents in the biopharmaceutical industry involves both technological complexity and legal complexity. Therefore, the process of obtaining, maintaining and enforcing biopharmaceutical patents is costly, time-consuming and inherently uncertain. In addition, recent legislative and judicial developments in the United States and elsewhere have in some cases narrowed the protection afforded to patent owners, made patents more difficult to obtain, or increased the uncertainty regarding the ability to obtain, maintain and enforce patents. For example, Congress recently passed patent reform legislation, and may pass patent reform legislation in the future. The United States Supreme Court has ruled on several patent cases in recent years, and in certain circumstances has narrowed the scope of patent protection available or otherwise weakened the rights of patent owners. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions and actions by the United States Congress, the federal courts, the United States Patent and Trademark Office, and their respective foreign counterparts, the laws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to maintain and enforce our existing patents and patents that we might obtain in the future.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials or our or third parties’ disposal of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
We may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
We maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials. This insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of hazardous or radioactive materials.
49
Our insurance policies are expensive and only protect us from specified business risks, which will leave us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. Some of the policies we currently maintain include general liability, employment practices liability, property, auto, workers’ compensation, products liability and directors’ and officers’ insurance. We do not know, however, if we have adequate levels of coverage for any liability we may incur, or whether we will always be able to continue to maintain such insurance. Any significant uninsured liability may require us to make substantial payments, which would adversely affect our financial position and results of operations. Furthermore, any increase in the volatility of our stock price, or changes in the insurance market generally, may result in us being required to pay substantially higher premiums for our directors’ and officers’ liability insurance than those to which we were previously subject and may even cause one or more of our underwriters to be unwilling to insure us.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement newly required or improved controls, or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations. In addition, any failure to maintain effective internal control as a result of shutdowns during the global COVID-19 pandemic could result in deficiencies in internal control. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act of 2002, or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
50
|
ITEM 6. |
EXHIBITS |
|
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
Date |
|
Exhibit Number |
|
File Number |
|
Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1 |
|
Restated Certificate of Incorporation of Enanta Pharmaceuticals, Inc. |
|
8-K |
|
03/28/2013 |
|
3.1 |
|
001-35839 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2
10.1†
10.2†
|
|
Amended and Restated Bylaws of Enanta Pharmaceuticals, Inc. (as amended and restated in August 2015)
|
|
8-K
8-K
8-K |
|
08/18/2015
02/05/2021
02/05/2021
|
|
3.2
10.1
10.2 |
|
001-35839
001-35839
001-35839
|
|
|
|
31.1 |
|
|
— |
|
— |
|
— |
|
— |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
|
— |
|
— |
|
— |
|
— |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1 |
|
|
— |
|
— |
|
— |
|
— |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101 |
|
The following financial statements from the Quarterly Report of Enanta Pharmaceuticals, Inc. on Form 10-Q for the quarter ended December 31, 2020, formatted in Inline XBRL: (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations, (iii) Consolidated Statements of Comprehensive Income, (iv) Consolidated Statements of Stockholders’ Equity, (v) Consolidated Statements of Cash Flows, (vi) and Notes to Consolidated Financial Statements, tagged as blocks of text and including detailed tags |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
104
|
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
|
|
|
|
|
|
|
|
|
X |
† Certain portions of this exhibit have been redacted pursuant to Item 601(b)(10(iv) of Regulation s-K. The Company has determined that such omitted information is (i) not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
51
ENANTA PHARMACEUTICALS, INC.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
ENANTA PHARMACEUTICALS, INC. |
|
|
|
|
|
Date: February 9, 2021 |
|
|
|
|
|
|
|
|
|
/s/ Paul J. Mellett |
|
|
|
Paul J. Mellett Chief Financial Officer (Principal Financial and Accounting Officer) |
52