
Bentracimab Program Update MARCH 15, 2021

This presentation includes forward-looking statements. All statements contained in this presentation other than statements of historical facts, including statements regarding future results of operations and financial position of PhaseBio Pharmaceuticals, Inc. (“we,” “us” or “our”), our business strategy and plans, the preclinical and clinical development of our product candidates and our objectives for future operations, are forward-looking statements. The words “anticipate,” believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “will” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, clinical development, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Annual Report on Form 10-K for the year ended December 31, 2020. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. Legal Disclaimer 2

Introduction and corporate update Jonathan Mow Chief Executive Officer, PhaseBio

Program Pre-Clinical Phase 1 Phase 2 Phase 3 Commercial Rights Upcoming Milestone Target2 Bentracimab Reversal of Ticagrelor Antiplatelet Activity Q2 2021 Publication of Phase 2A trial results Pemziviptadil Pulmonary Arterial Hypertension (PAH) 2H 2021 Report Phase 2B data PB6440 Resistant Hypertension 20223 Submit IND and initiate first-in-human clinical trial A Clinical Stage, Cardiopulmonary Focused Biopharmaceutical Company 4 REVERSE-IT1 Phase 3 ongoing Targeting to submit BLA in Mid 20222 Phase 2B ongoing2 1. REVERSE-IT: Rapid and SustainEd ReVERSal of TicagrElor – Intervention Trial 2. Targeted timeline could be impacted by the continued scope and duration of the COVID-19 pandemic. 3. Timing changed from FY:21 to FY:22 due to COVID-19-related delays Pre-Clinical Recent Achievements & Upcoming Milestone Targets2 ✓ Bentracimab Q1 2020 Executed bentracimab funding and co-development agreement with SFJ Pharmaceuticals Bentracimab Q2 2021 Publication of Phase 2A trial results ✓ Bentracimab Q1 2020 Granted PRIority MEdicines (PRIME) designation by European Medicines Agency (EMA) Bentracimab Mid 2021 First 100 patients in REVERSE-IT phase 3 trial ✓ Bentracimab Q1 2020 Initiated Phase 3 trial based on plan to pursue accelerated approval pathway Pemziviptadil 2H 2021 Report Phase 2B data from PAH trial

PRECLINICAL STUDIES Support Clinical Development for: • Pulmonary Arterial Hypertension • DMD Cardiomyopathy • Heart Failure (Chemotherapy-Induced HF or HFpEF) • Cystic Fibrosis MECHANISM OF ACTION VIA VPAC2 RECEPTOR • Potent vasodilator and immunomodulator • Anti-inflammatory and anti-fibrotic • Cardiac support through increased inotropy and lusitropy Pemziviptadil: Harnessing VIP to Create a Stable, Long-Acting Drug 5 VIP as a THERAPEUTIC AGENT PemziviptadilEndogenous VIP Pemziviptadil

High Unmet Need in an Orphan Disease Primary Pulmonary Arterial Hypertension (PAH, WHO Group 1 PH) • High unmet need for novel disease-modifying PAH therapies for greater efficacy All 3 approved drug classes in PAH are vasodilators: prostacyclin, endothelin, and nitric oxide pathways Patients inevitably continue to decline and die on current standard of care • VIP addresses PAH vasoconstriction, progressive vascular remodeling, and right heart failure 6

Pemziviptadil Clinical Development Activities to Date Have Supported Advancement into Phase 2 Efficacy Studies 7 PHASE 1 Studies Completed PHASE 2 PAH Studies • Well tolerated for a week over broad range of exposure; no drug-related SAEs reported • Prolonged PK/PD profile over 1 week • VIP activity confirmed (Systolic and Diastolic BP lowering) • Well tolerated across dose range; no drug-related SAEs reported • Replicated PK/PD from SAD over 4 weekly SC injections • VIP activity reproduced in HFrEF patients on SOC CardioMEMS Open-Label PAH Study • N = 3 patients, dosed weekly for 8 wks, followed by extension – No longer enrolling patients • Real-time PA pressure and other hemodynamic monitoring • Initial data show improved hemodynamics • One drug-related SAE reported in extension portion of open-label pilot study Phase 2B PAH 16 wk randomized, controlled study • N = ~60 NYHA class II/III PAH patients, dosed weekly x 16 weeks – Individual dose titration to MTD • Efficacy endpoints PVR via RHC, 6MWD • Extension study to follow COMPLETED COMPLETED COMPLETED Phase 2B ongoing SAD study in hypertensive patients washed off meds 4-week MAD study in HFrEF patients on SOC Open-Label Phase 2A CardioMEMS in PAH study: Safety and Hemodynamics Phase 2B PAH Efficacy 16-Wk Randomized, Controlled Study

Why We Are Here Today 8 What is Bentracimab • Highlight features of the P2Y12 class • Describe unmet need for reversal • Outline potential future directions Current Situation • Review clinical data and progress • Global regulatory path forward • Manufacturing at commercial scale Future Potential • Global scale of P2Y12 class • Commercial strategy overview • Hospital pharmacy perspective Understanding Bentracimab

Key Take-Aways 9 • Bentracimab clinical development program is enrolling ahead of schedule Global regulatory path for bentracimab is clear and supported by the REVERSE-IT trial • Need for P2Y12-inhibitor reversal is completely unmet in today’s medical practice Ticagrelor has a best-in-class efficacy profile in large, global outcomes trials Recent ticagrelor label expansion and future patent expiration among multiple potential growth drivers Data in Phase 1 and Phase 2A trials highlight immediate and sustained restoration of platelet function Bentracimab has the potential to change the way P2Y12 inhibitors are utilized • We are planning for commercial success Commercial-scale manufacturing process is operational; capable of global supply at launch, if bentracimab is approved • Commercial opportunity is significant and, we believe, underappreciated
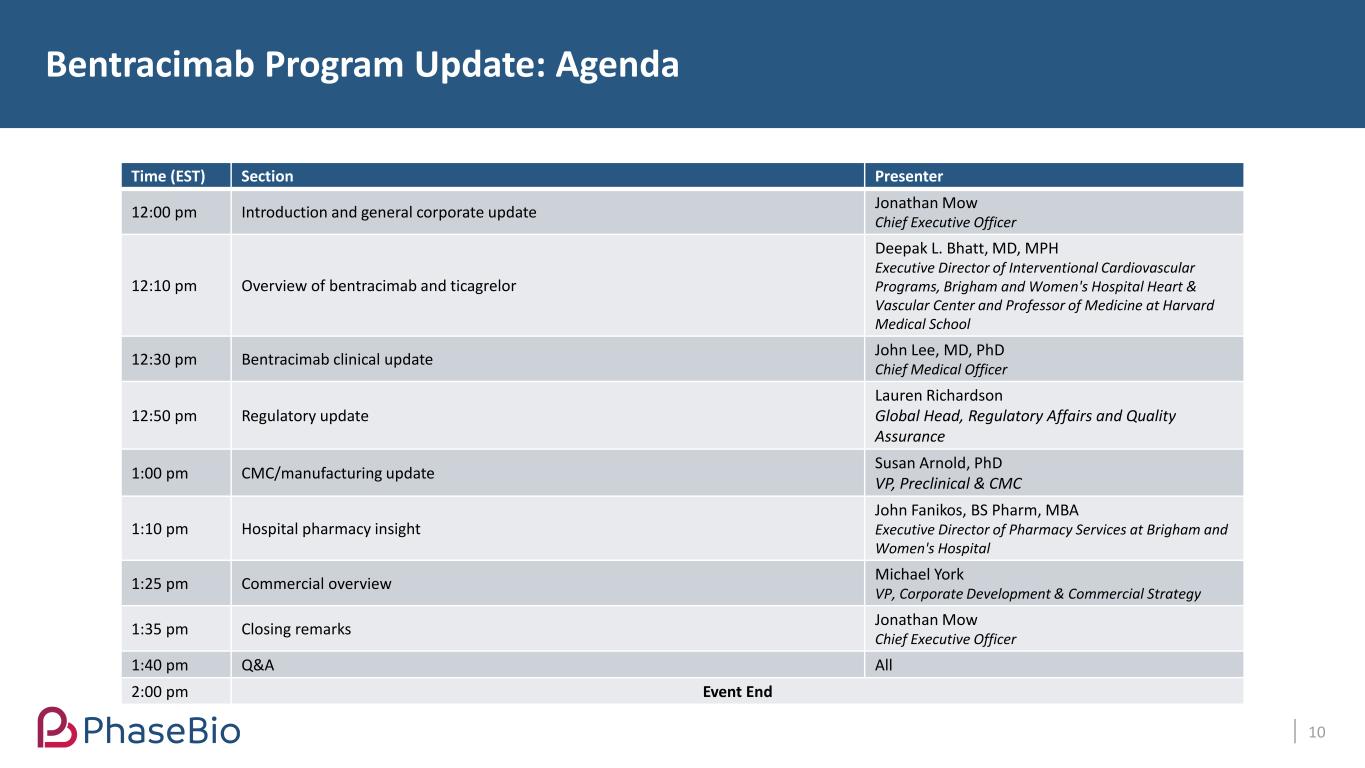
Time (EST) Section Presenter 12:00 pm Introduction and general corporate update Jonathan Mow Chief Executive Officer 12:10 pm Overview of bentracimab and ticagrelor Deepak L. Bhatt, MD, MPH Executive Director of Interventional Cardiovascular Programs, Brigham and Women's Hospital Heart & Vascular Center and Professor of Medicine at Harvard Medical School 12:30 pm Bentracimab clinical update John Lee, MD, PhD Chief Medical Officer 12:50 pm Regulatory update Lauren Richardson Global Head, Regulatory Affairs and Quality Assurance 1:00 pm CMC/manufacturing update Susan Arnold, PhD VP, Preclinical & CMC 1:10 pm Hospital pharmacy insight John Fanikos, BS Pharm, MBA Executive Director of Pharmacy Services at Brigham and Women's Hospital 1:25 pm Commercial overview Michael York VP, Corporate Development & Commercial Strategy 1:35 pm Closing remarks Jonathan Mow Chief Executive Officer 1:40 pm Q&A All 2:00 pm Event End Bentracimab Program Update: Agenda 10
Overview of Bentracimab and Ticagrelor Deepak L. Bhatt, MD, MPH Executive Director of Interventional Cardiovascular Programs, Brigham and Women's Hospital Heart & Vascular Center and Professor of Medicine at Harvard Medical School

12 URGENT SURGERY OR INTERVENTION • Currently oral P2Y12 agents, including ticagrelor, require a 5-day washout prior to surgery1,2 Urgent surgery often cannot wait 5 days Higher thrombotic risk during washout • In Phase 1 and Phase 2A studies, bentracimab observed to immediately and sustainably reverse ticagrelor inhibition of platelet activation Enables immediate surgery • Within the oral P2Y12 inhibitor class, ticagrelor has proven superiority vs. clopidogrel, and a unique reversible binding profile Clopidogrel and prasugrel, the other members of the oral P2Y12 antagonist class, both permanently bind to the receptor and cannot be reversed • Uncontrolled major bleeding is the main adverse event associated with the P2Y12 inhibitor class of therapies • Bentracimab is the only specific reversal agent in development for ticagrelor for both surgical and active bleed indications • Approval would differentiate ticagrelor on safety vs. other oral antiplatelet agents Bentracimab: Novel Reversal Agent for Brilinta (Ticagrelor) 1. Plavix/clopidogrel Prescribing Information: https://packageinserts.bms.com/pi/pi_plavix.pdf, https://www.ema.europa.eu/en/documents/product-information/plavix-epar-product-information_en.pdf 2. Brilinta/Brilique/ticagrelor Prescribing Information: https://www.azpicentral.com/brilinta/brilinta.pdf#page=1, https://www.ema.europa.eu/en/documents/product-information/brilique-epar-product-information_en.pdf MAJOR BLEEDING • Intracranial Hemorrhage (ICH), GI, Trauma • All oral antiplatelet agents have the potential to cause major bleeding, which can be severe or even fatal • Bentracimab designed to immediately and sustainably reverse the antiplatelet effects of ticagrelor Significant unmet need for antiplatelet agent reversal
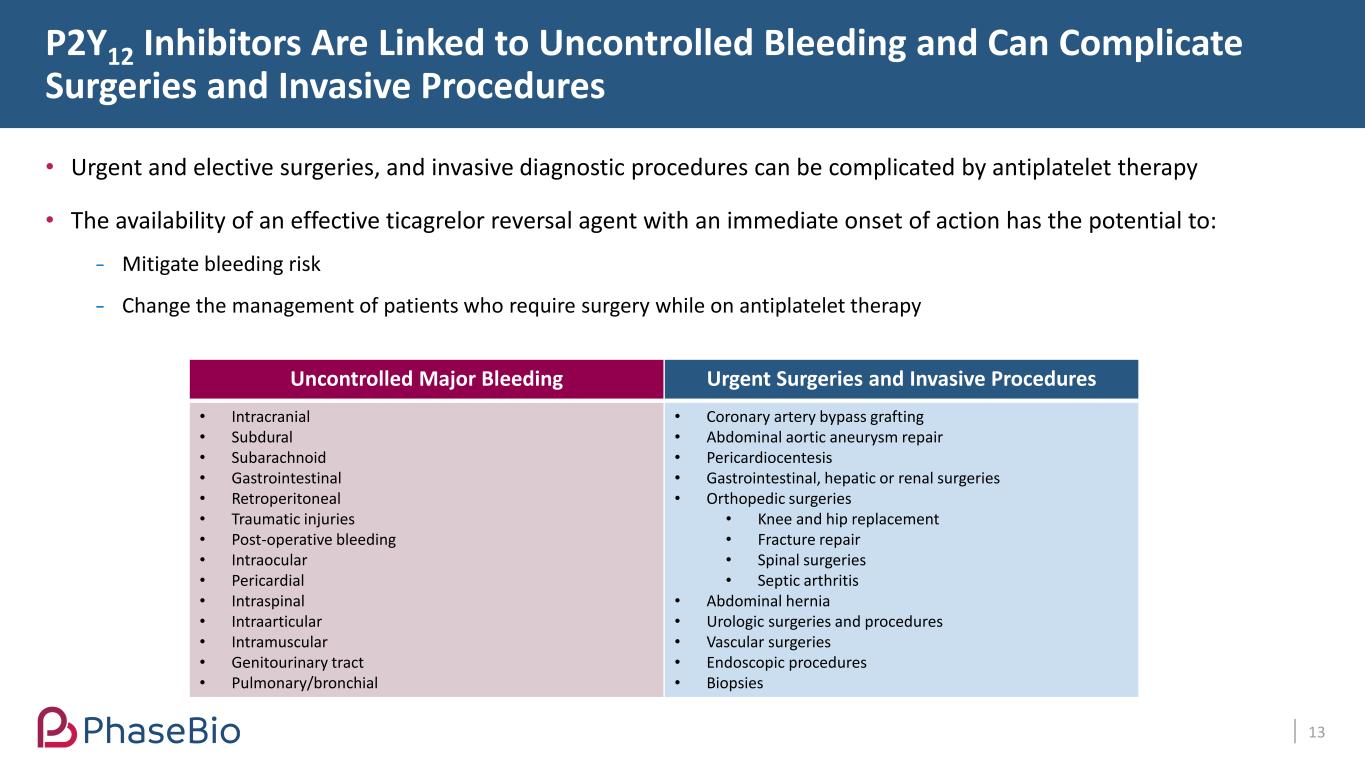
P2Y12 Inhibitors Are Linked to Uncontrolled Bleeding and Can Complicate Surgeries and Invasive Procedures 13 Uncontrolled Major Bleeding Urgent Surgeries and Invasive Procedures • Intracranial • Subdural • Subarachnoid • Gastrointestinal • Retroperitoneal • Traumatic injuries • Post-operative bleeding • Intraocular • Pericardial • Intraspinal • Intraarticular • Intramuscular • Genitourinary tract • Pulmonary/bronchial • Coronary artery bypass grafting • Abdominal aortic aneurysm repair • Pericardiocentesis • Gastrointestinal, hepatic or renal surgeries • Orthopedic surgeries • Knee and hip replacement • Fracture repair • Spinal surgeries • Septic arthritis • Abdominal hernia • Urologic surgeries and procedures • Vascular surgeries • Endoscopic procedures • Biopsies • Urgent and elective surgeries, and invasive diagnostic procedures can be complicated by antiplatelet therapy • The availability of an effective ticagrelor reversal agent with an immediate onset of action has the potential to: Mitigate bleeding risk Change the management of patients who require surgery while on antiplatelet therapy

Intracranial Hemorrhage Case Study1 Highlights the Delicate Balance Between Managing the Bleed and Protecting the Stent • A 59-year old male with a history of coronary artery disease, hypertension and hyperlipidemia reported sudden onset headache, mild dysmetria and severe nausea. Medical history included a PCI performed 2 weeks ago for symptomatic coronary artery disease (CAD) with placement of two platinum-chromium everolimus-eluting (PROMUS Element™; Boston Scientific, Natick, MA) drug eluting stents (DES) in the left anterior descending and circumflex artery. Left ventricular ejection fraction was sixty percent. DAT with aspirin and clopidogrel was initiated post-procedure. • On admission to the Intensive Care Unit (ICU) the patient was normotensive with a 1Glasgow Coma Scale of 15. Computed tomography (CT) scan demonstrated a left para- median cerebellar hemorrhage measuring 29 mm × 18 mm with partial effacement of the fourth ventricle (Figure 1). The lateral and third ventricles were within normal limits with no evidence of hydrocephalus. • After consultation with the cardiologist, neurosurgeon and neuro-intensivist, a decision was made to hold clopidogrel and continue aspirin. Due to the very recent placement of the DES, the cardiologist felt that stopping all anti-platelet therapy would significantly increase the risk of a major cardiovascular event. No platelets were administered to reverse the effects of the anti-platelet drugs. Hourly clinical neurological evaluations and a repeat CT scan was performed 12 hours after the admission CT. This scan demonstrated no enlargement of the intracranial hemorrhage (ICH) or ventricular dilation. Both clinical and biochemical indicators of cardiac ischemia were absent during aspirin monotherapy. • After one week, with the absence of any clinical or radiological deterioration, the cardiologist and neurosurgeon felt that the risk of stent thrombosis was greater than the risk of worsening intracranial hemorrhage. Clopidogrel was restarted and a repeat CT scan following re-commencement demonstrated a stable ICH with no radiological features of enlargement. The patient was discharged home without any further complications. 141. Naik BI, Keeley EC, Gress DR, Zuo Z. Case scenario: a patient on dual antiplatelet therapy with an intracranial hemorrhage after percutaneous coronary intervention. Anesthesiology. 2014;121(3):644-653. doi:10.1097/ALN.0000000000000350 Figure 1

Oral P2Y12 Inhibitor Class: Only Ticagrelor Can Be Reversed 15 Clopidogrel Prasugrel Ticagrelor Receptor blockade Irreversible Irreversible Reversible Prodrug Yes Yes No Half-life of parent drug ≈6 h <5 min 6–12 h Half-life of active metabolite 30 mins Distribution half-life, 30–60 mins 8–12 h Elimination half-life, 2–15 h Binding site ADP-binding site ADP-binding site Allosteric binding site Administration route Oral Oral Oral Frequency Once daily Once daily Twice daily Onset of action† 2–8 h 30 min–4 h 30 min–4 h Offset of action 5–10 d 7–10 d 3–5 d CYP drug interaction‡ CYP2C19 No CYP3A Approved settings ACS (invasive and noninvasively managed), PAD, and ischemic stroke ACS undergoing PCI ACS (invasive or noninvasively managed), history of MI, stable CAD and acute stroke ACS indicates acute coronary syndrome; CAD, coronary artery disease; CYP, cytochrome P450; MI, myocardial infarction; PAD, peripheral arterial disease; and PCI, percutaneous coronary intervention. †Indicates times after loading dose for oral agents. Times for oral agents refer to clinically stable subjects and may be pro longed in patients with ST-segment–elevation myocardial infarction or treated with opioids. ‡Indicates clinically significant drug interactions. 1. Adapted from: Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation. 2017;136(20):1955–1975. doi: 10.1161/CIRCULATIONAHA.117.031164. - DOI - PubMed Intravenous cangrelor data from original publication not shown Ticagrelor indications updated to reflect recent label expansions following THEMIS (stable CAD) and THALES (acute stroke) trials
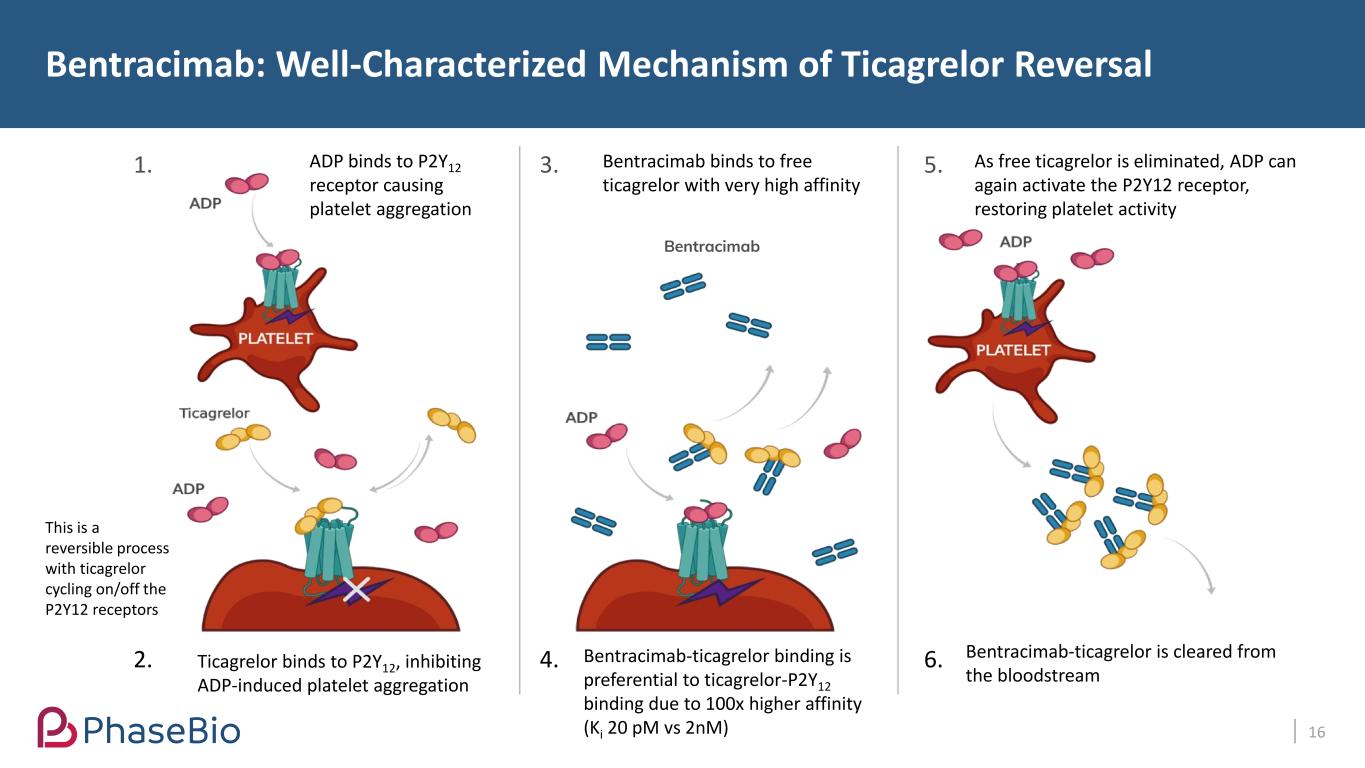
Bentracimab: Well-Characterized Mechanism of Ticagrelor Reversal 16 1. 2. 3. 4. 5. 6. ADP binds to P2Y12 receptor causing platelet aggregation Ticagrelor binds to P2Y12, inhibiting ADP-induced platelet aggregation Bentracimab binds to free ticagrelor with very high affinity This is a reversible process with ticagrelor cycling on/off the P2Y12 receptors Bentracimab-ticagrelor binding is preferential to ticagrelor-P2Y12 binding due to 100x higher affinity (Ki 20 pM vs 2nM) As free ticagrelor is eliminated, ADP can again activate the P2Y12 receptor, restoring platelet activity Bentracimab-ticagrelor is cleared from the bloodstream Bentracimab

Ticagrelor Clinical Evidence Supports Broad Use • Ticagrelor clinical development program encompassed six major outcomes trials enrolling nearly 100,000 patients PLATO, PEGASUS-TIMI-54, SOCRATES, EUCLID, THALES, and THEMIS The four trials linked to the current ticagrelor prescribing label are highlighted above • Broad label for the reduction of risk of cardiovascular death, myocardial infarction, and stroke in the following patient populations: Acute coronary syndrome Patients with a history of myocardial infarction Patients with coronary artery disease at high risk for myocardial infarction or stroke Acute ischemic stroke or transient ischemic attack 17 PLATO PEGASUS-TIMI-54 THEMIS THALES
THEMIS Trial and Recent Ticagrelor Label Expansion 18 • Patients with stable coronary artery disease (CAD) and diabetes mellitus (T2D) are at high risk for cardiovascular events • Data from the THEMIS1 trial highlights the potential for ticagrelor to reduce the risk of ischemic cardiovascular events in CAD patients with T2D A higher risk of major bleeding in the ticagrelor-treated population was also observed • According to the CDC2, there are over 30 million T2D patients in the United States Approximately 25% of T2D patients have CAD3 • Approval of bentracimab could help mitigate bleeding risk in ticagrelor in patients with established CAD and T2D 1. Steg PG, Bhatt DL, Simon T, et al.; THEMIS Steering Committee and Investigators. Ticagrelor in Patients with Stable Coronary Disease and Diabetes. N Engl J Med. 2019 Oct 3;381(14):1309-1320. doi: 10.1056/NEJMoa1908077. Epub 2019 Sep 1. PMID: 31475798. 2. https://www.cdc.gov/diabetes/basics/type2.html 3. Budoff MJ, Raggi P, Beller GA, et al.; Imaging Council of the American College of Cardiology Noninvasive cardiovascular risk assessment of the asymptomatic diabetic patient: the Imaging Council of the American College of Cardiology. JACC Cardiovasc Imaging 2016;9:176–192

THEMIS PCI: Subgroup Analysis Highlights Ticagrelor Net Clinical Benefit • Prespecified subgroup of patients • Fulfill major THEMIS inclusion criterion of having a history of previous percutaneous coronary intervention (PCI) • Data suggest significantly favorable effect on primary efficacy endpoint, and greater net clinical benefit • Important cohort of patients: Appeared to benefit from ticagrelor Significant number of patients; easy to identify Logical group in whom to consider extended duration DAPT • Individual risks of future ischemic events and bleeding need to be weighed carefully TIMI major bleeding was significantly increased • Ticagrelor is approved for use in patients with CAD in the United States and for CAD patients with prior PCI in Canada 19Bhatt DL, Steg PG, et al. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019 Sep 28;394(10204):1169-1180. doi: 10.1016/S0140-6736(19)31887-2. Epub 2019 Sep 1. PMID: 31484629.
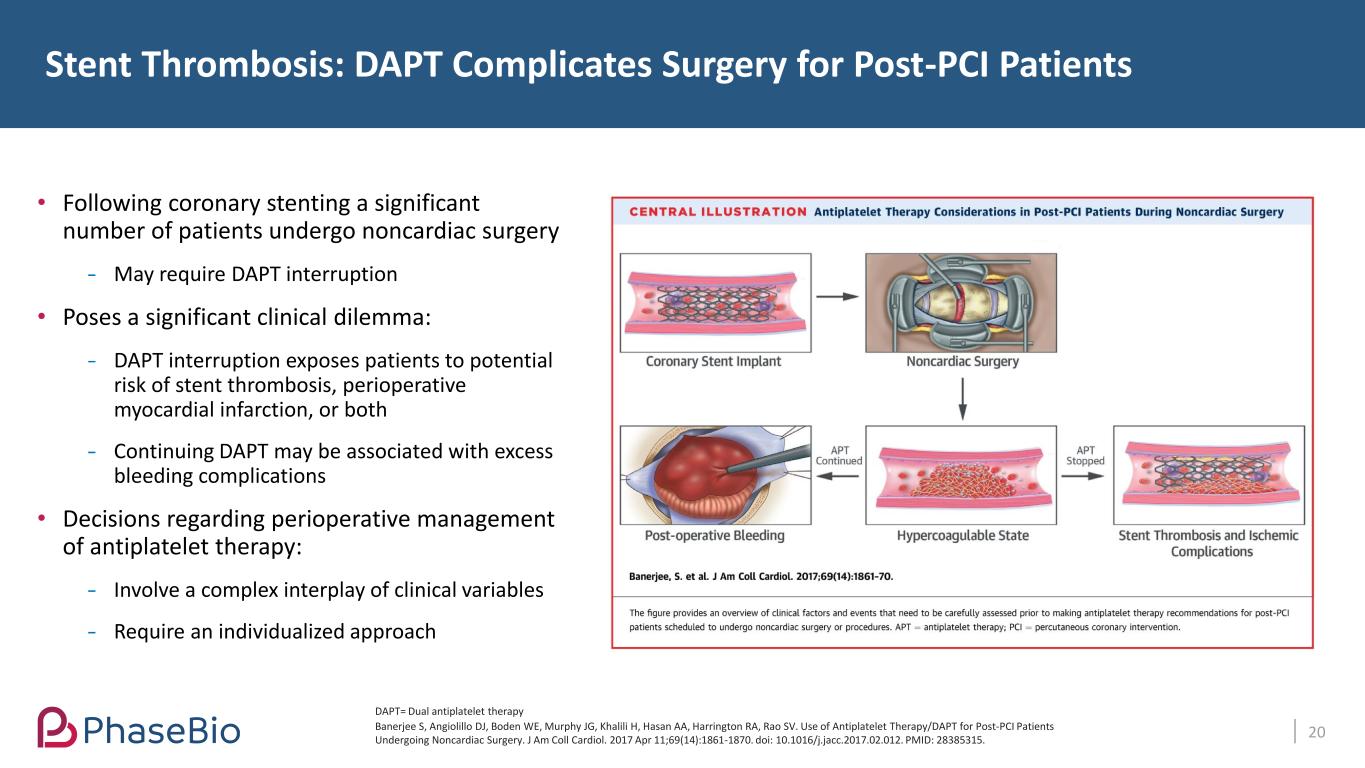
• Following coronary stenting a significant number of patients undergo noncardiac surgery May require DAPT interruption • Poses a significant clinical dilemma: DAPT interruption exposes patients to potential risk of stent thrombosis, perioperative myocardial infarction, or both Continuing DAPT may be associated with excess bleeding complications • Decisions regarding perioperative management of antiplatelet therapy: Involve a complex interplay of clinical variables Require an individualized approach Stent Thrombosis: DAPT Complicates Surgery for Post-PCI Patients 20Banerjee S, Angiolillo DJ, Boden WE, Murphy JG, Khalili H, Hasan AA, Harrington RA, Rao SV. Use of Antiplatelet Therapy/DAPT for Post-PCI Patients Undergoing Noncardiac Surgery. J Am Coll Cardiol. 2017 Apr 11;69(14):1861-1870. doi: 10.1016/j.jacc.2017.02.012. PMID: 28385315. DAPT= Dual antiplatelet therapy

Current Paradigm for Bridging Oral P2Y12 Patients Ahead of Surgery 1 211. Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation. 2017;136(20):1955–1975. doi: 10.1161/CIRCULATIONAHA.117.031164. - DOI - PubMed Low dose aspirin continued throughout Surgery STOP prasugrel STOP clopidogrel ticagrelor WASH OUT oral P2Y12 inhibitors -7 -6 -5 -4 -3 -2 -1 -1-6 h STOP cangrelor* RESUME cangrelor** 0 START clopidogrel*** ***With 300-600 mg loading dose, as soon as oral administration possible. Prasugrel or ticagrelor discouraged +1-6 h Follow-up until discharge **If oral administration not possible START cangrelor* *Initiate within 72 hours from P2Y12 inhibitor discontinuation at a dose of 0.75 µ/kg/min (no bolus) for a minimum of 48 hours and a maximum of 7 days • Current pre-operative paradigm for washing out and bridging patients to surgery can be costly and is potentially risky - Oral antiplatelet therapy should ideally be stopped 5-7 days ahead of procedures - Risk of stent thrombosis is a critical concern - Inpatient stays to facilitate safe wash-out periods can quickly become expensive - Resumption of ticagrelor or prasugrel is discouraged at discharge due to perception of higher bleeding risk Bentracimab could allow ticagrelor patients to remain on oral antiplatelet therapy up to the day of surgery and potentially provide a strategy to mitigate post-operative bleeding complications if patients are discharged on ticagrelor

Hypothetical Future Paradigm for Bridging Oral P2Y12 Patients Ahead of Surgery Following Potential Bentracimab Launch 22 • Bentracimab could potentially change the way surgeries and invasive procedures are managed in P2Y12 patients - Switching from clopidogrel or prasugrel to ticagrelor is well defined in international treatment guidelines1 - Maintaining platelet inhibition, especially in recently-stented patients, should be a top priority to minimize the risk of stent thrombosis - Elimination of inpatient stays ahead of surgeries has the potential to drive down costs and free up hospital beds - Post-op resumption of ticagrelor instead of clopidogrel provides a safety net that could help mitigate the downstream impact of subsequent bleeds or surgical needs Bentracimab could allow ticagrelor patients to remain on oral antiplatelet therapy up to the day of surgery and potentially provide a strategy to mitigate post-operative bleeding complications if patients are discharged on ticagrelor 1. Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation. 2017;136(20):1955–1975. doi: 10.1161/CIRCULATIONAHA.117.031164. - DOI - PubMed Low dose aspirin continued throughout Surgery SWITCH from prasugrel to ticagrelor SWITCH from clopidogrel to ticagrelor SWITCH to ticagrelor instead of traditional wash out -7 -6 -5 -4 -3 -2 -1 ≥ -0.08 h START bentracimab START cangrelor** 0 RESUME ticagrelor*** +1-6 h Follow-up until discharge **If oral administration not possible ***With 180mg loading dose, as soon as oral administration possible Prasugrel or clopidogrel discouraged

23 First Randomized Human Experience with a Ticagrelor Reversal Agent Deepak L. Bhatt, MD, MPH, Charles V. Pollack, MD, Jeffrey I. Weitz, MD, Lisa K. Jennings, PhD, Sherry Xu, PhD, Susan E. Arnold, PhD, Bret R. Umstead, MS, Michael C. Mays, BS, John S. Lee MD, PhD
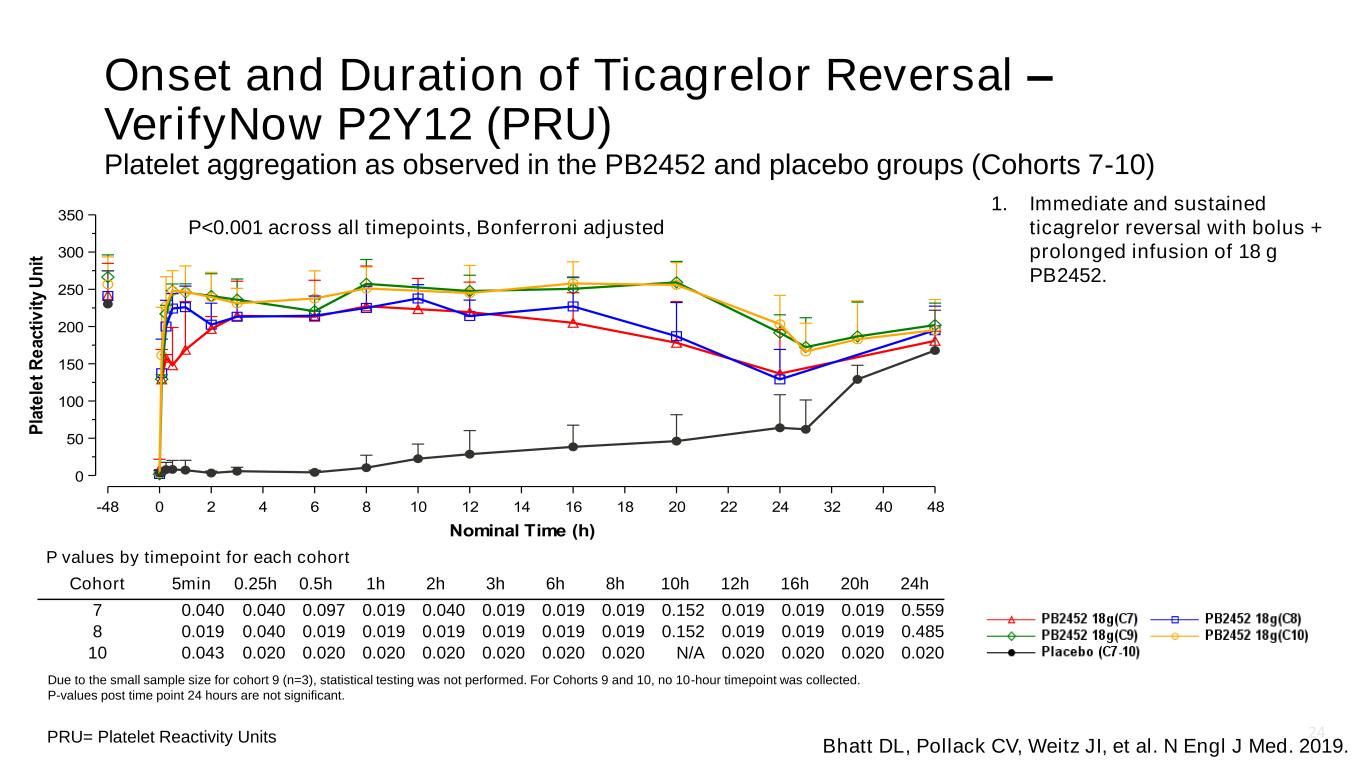
24 Onset and Duration of Ticagrelor Reversal – VerifyNow P2Y12 (PRU) Platelet aggregation as observed in the PB2452 and placebo groups (Cohorts 7-10) PRU= Platelet Reactivity Units Due to the small sample size for cohort 9 (n=3), statistical testing was not performed. For Cohorts 9 and 10, no 10-hour timepoint was collected. P-values post time point 24 hours are not significant. P values by timepoint for each cohort Cohort 5min 0.25h 0.5h 1h 2h 3h 6h 8h 10h 12h 16h 20h 24h 7 0.040 0.040 0.097 0.019 0.040 0.019 0.019 0.019 0.152 0.019 0.019 0.019 0.559 8 0.019 0.040 0.019 0.019 0.019 0.019 0.019 0.019 0.152 0.019 0.019 0.019 0.485 10 0.043 0.020 0.020 0.020 0.020 0.020 0.020 0.020 N/A 0.020 0.020 0.020 0.020 P<0.001 across all timepoints, Bonferroni adjusted Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019. 1. Immediate and sustained ticagrelor reversal with bolus + prolonged infusion of 18 g PB2452.

25 Onset and Duration of Ticagrelor Reversal – VerifyNow P2Y12 (PRU) Platelet aggregation as observed in the PB2452 and placebo groups (Cohorts 7-10) PRU= Platelet Reactivity Units Due to the small sample size for cohort 9 (n=3), statistical testing was not performed. For Cohorts 9 and 10, no 10-hour timepoint was collected. P-values post time point 24 hours are not significant. P values by timepoint for each cohort Cohort 5min 0.25h 0.5h 1h 2h 3h 6h 8h 10h 12h 16h 20h 24h 7 0.040 0.040 0.097 0.019 0.040 0.019 0.019 0.019 0.152 0.019 0.019 0.019 0.559 8 0.019 0.040 0.019 0.019 0.019 0.019 0.019 0.019 0.152 0.019 0.019 0.019 0.485 10 0.043 0.020 0.020 0.020 0.020 0.020 0.020 0.020 N/A 0.020 0.020 0.020 0.020 P<0.001 across all timepoints, Bonferroni adjusted Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019. 1. Immediate and sustained ticagrelor reversal with bolus + prolonged infusion of 18 g PB2452. 2. Significant reversal was observed 5 minutes after initiation of PB2452 infusion.
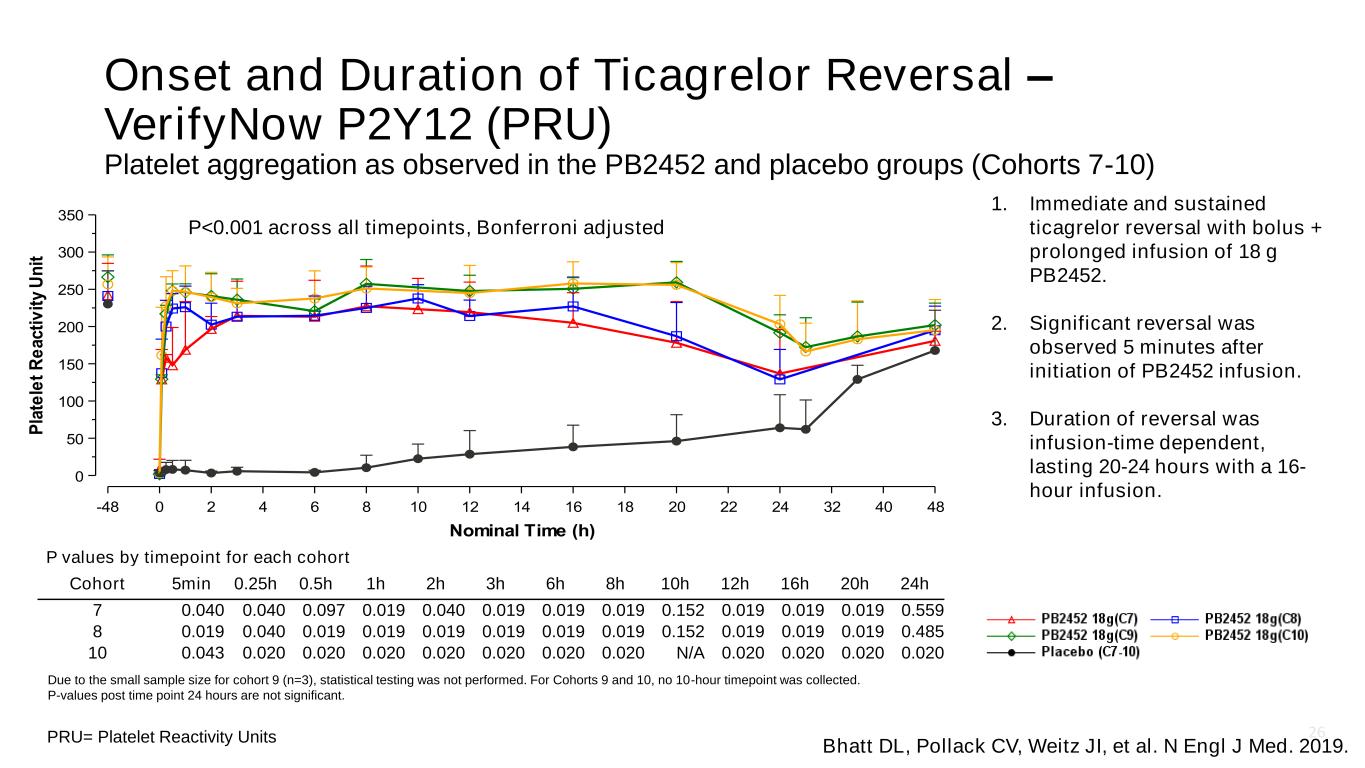
26 Onset and Duration of Ticagrelor Reversal – VerifyNow P2Y12 (PRU) Platelet aggregation as observed in the PB2452 and placebo groups (Cohorts 7-10) PRU= Platelet Reactivity Units Due to the small sample size for cohort 9 (n=3), statistical testing was not performed. For Cohorts 9 and 10, no 10-hour timepoint was collected. P-values post time point 24 hours are not significant. P values by timepoint for each cohort Cohort 5min 0.25h 0.5h 1h 2h 3h 6h 8h 10h 12h 16h 20h 24h 7 0.040 0.040 0.097 0.019 0.040 0.019 0.019 0.019 0.152 0.019 0.019 0.019 0.559 8 0.019 0.040 0.019 0.019 0.019 0.019 0.019 0.019 0.152 0.019 0.019 0.019 0.485 10 0.043 0.020 0.020 0.020 0.020 0.020 0.020 0.020 N/A 0.020 0.020 0.020 0.020 P<0.001 across all timepoints, Bonferroni adjusted Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019. 1. Immediate and sustained ticagrelor reversal with bolus + prolonged infusion of 18 g PB2452. 2. Significant reversal was observed 5 minutes after initiation of PB2452 infusion. 3. Duration of reversal was infusion-time dependent, lasting 20-24 hours with a 16- hour infusion.

27 Platelet Aggregation with Low- and High-Dose ADP Mean platelet aggregation by LTA using 5 µM ADP (top) or 20 µM ADP (bottom) as agonist LTA: light transmission aggregometry; ADP: adenosine diphosphate Platelet hyper-reactivity, or rebound, between 5 minutes and 48 hours was ruled out by the response to low-dose ADP versus high-dose ADP. Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019.
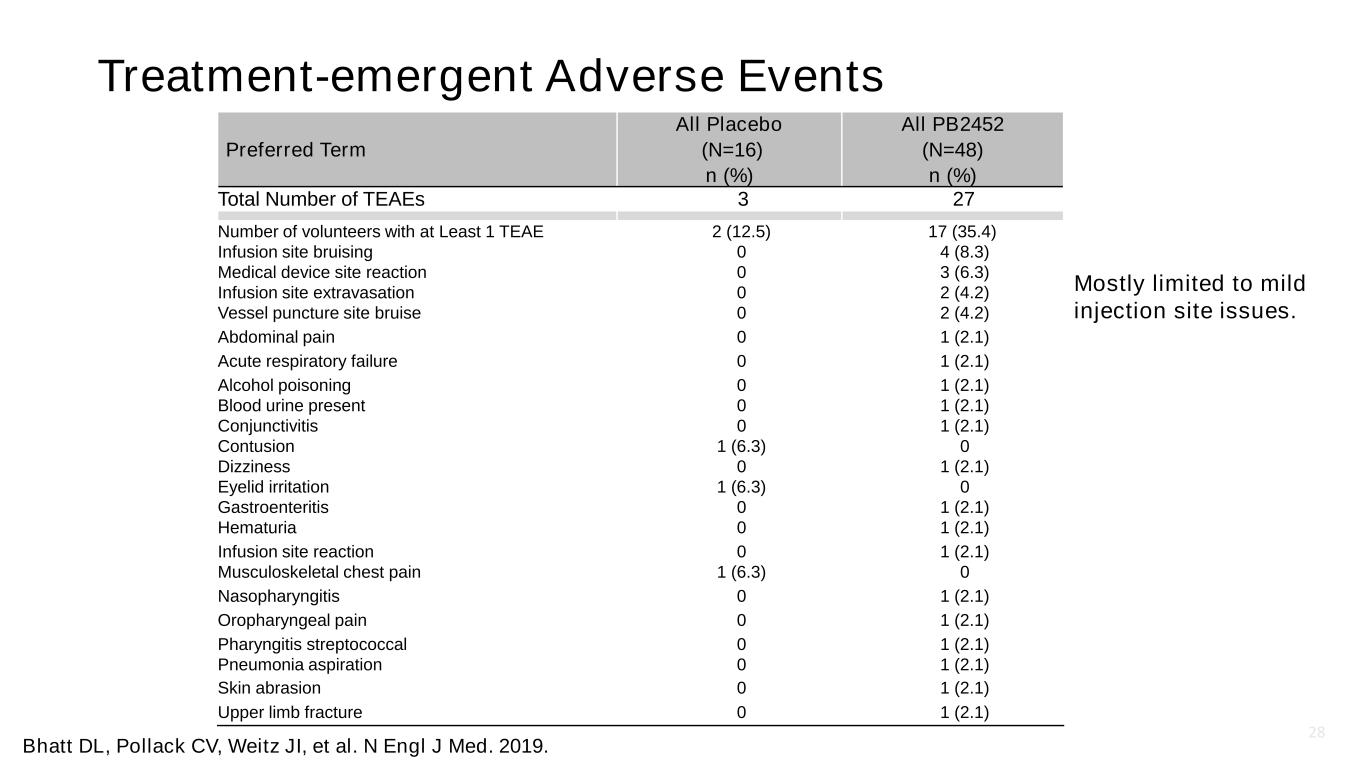
28 Treatment-emergent Adverse Events Preferred Term All Placebo (N=16) n (%) All PB2452 (N=48) n (%) Total Number of TEAEs 3 27 Number of volunteers with at Least 1 TEAE 2 (12.5) 17 (35.4) Infusion site bruising 0 4 (8.3) Medical device site reaction 0 3 (6.3) Infusion site extravasation 0 2 (4.2) Vessel puncture site bruise 0 2 (4.2) Abdominal pain 0 1 (2.1) Acute respiratory failure 0 1 (2.1) Alcohol poisoning 0 1 (2.1) Blood urine present 0 1 (2.1) Conjunctivitis 0 1 (2.1) Contusion 1 (6.3) 0 Dizziness 0 1 (2.1) Eyelid irritation 1 (6.3) 0 Gastroenteritis 0 1 (2.1) Hematuria 0 1 (2.1) Infusion site reaction 0 1 (2.1) Musculoskeletal chest pain 1 (6.3) 0 Nasopharyngitis 0 1 (2.1) Oropharyngeal pain 0 1 (2.1) Pharyngitis streptococcal 0 1 (2.1) Pneumonia aspiration 0 1 (2.1) Skin abrasion 0 1 (2.1) Upper limb fracture 0 1 (2.1) Mostly limited to mild injection site issues. Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019.

29 Conclusion • Using multiple assays in healthy volunteers, PB2452, a specific reversal agent for ticagrelor, provided immediate and sustained reversal of ticagrelor’s antiplatelet effects. Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019.

30 Conclusion • Using multiple assays in healthy volunteers, PB2452, a specific reversal agent for ticagrelor, provided immediate and sustained reversal of ticagrelor’s antiplatelet effects. • The ability to reverse ticagrelor’s antiplatelet effects rapidly could distinguish it from other antiplatelet drugs. Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019.

31 Conclusion • Using multiple assays in healthy volunteers, PB2452, a specific reversal agent for ticagrelor, provided immediate and sustained reversal of ticagrelor’s antiplatelet effects. • The ability to reverse ticagrelor’s antiplatelet effects rapidly could distinguish it from other antiplatelet drugs. • PB2452 may be a useful way to treat or prevent bleeding complications due to ticagrelor. Bhatt DL, Pollack CV, Weitz JI, et al. N Engl J Med. 2019.

Clinical Update John Lee, MD, PhD Chief Medical Officer, PhaseBio Phase 1 and 2A Studies

Bentracimab Profile from Phase 1 Study 33 Article available at https://www.nejm.org Slides available for download at https://www.ACC.org • A specific reversal agent for ticagrelor, provided immediate and sustained reversal of ticagrelor’s antiplatelet effects • Breakthrough Therapy designation from FDA and PRIME designation from EMA • Phase 2A dose-translation study completed and enabled start-up of pivotal trials • Potential to transform treatment and prevention of P2Y12 inhibitor-related bleeding complications
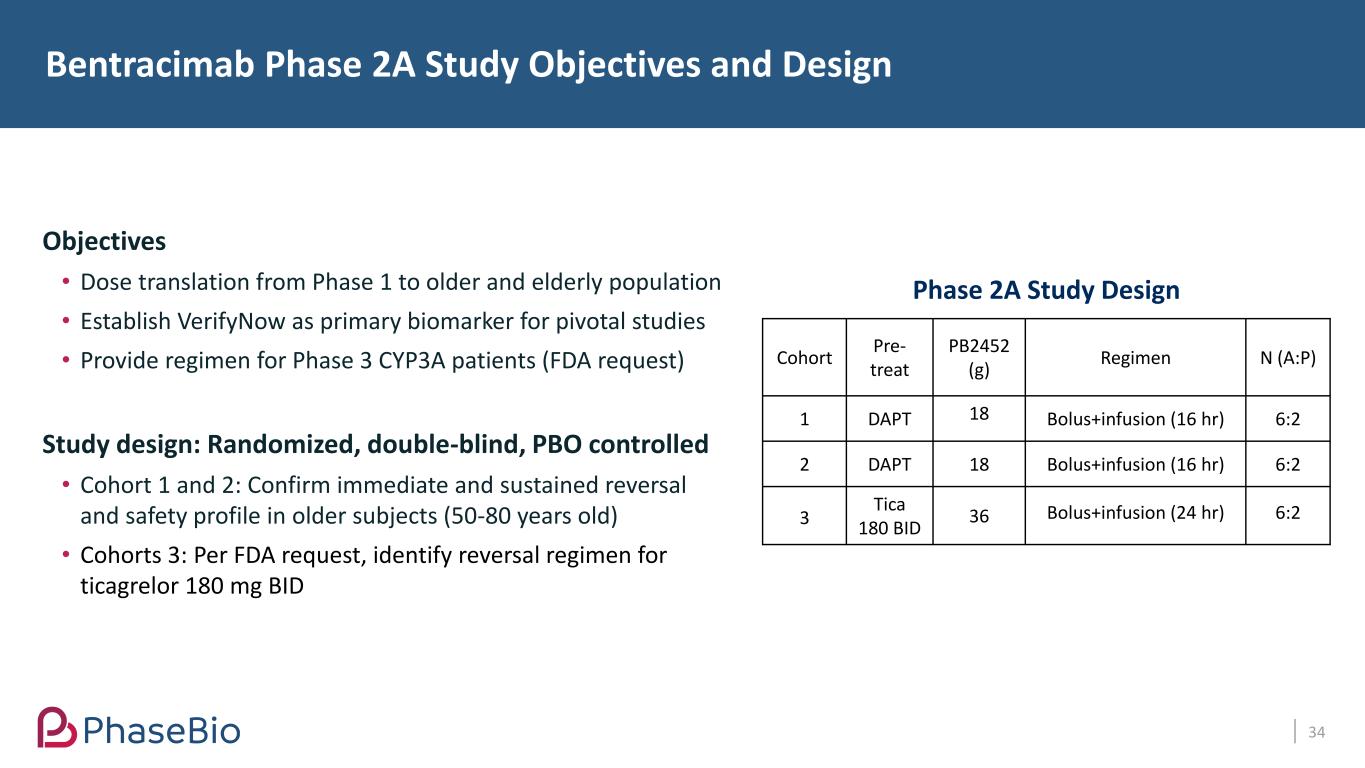
Bentracimab Phase 2A Study Objectives and Design 34 Objectives • Dose translation from Phase 1 to older and elderly population • Establish VerifyNow as primary biomarker for pivotal studies • Provide regimen for Phase 3 CYP3A patients (FDA request) Study design: Randomized, double-blind, PBO controlled • Cohort 1 and 2: Confirm immediate and sustained reversal and safety profile in older subjects (50-80 years old) • Cohorts 3: Per FDA request, identify reversal regimen for ticagrelor 180 mg BID Cohort Pre- treat PB2452 (g) Regimen N (A:P) 1 DAPT 18 Bolus+infusion (16 hr) 6:2 2 DAPT 18 Bolus+infusion (16 hr) 6:2 3 Tica 180 BID 36 Bolus+infusion (24 hr) 6:2 Phase 2A Study Design

Bentracimab Phase 2A Study Results 35 In 50-80 year olds, nearly identical reversal profile as observed in Phase 1 study • Immediate reversal within 5 minutes of initiation of PB2452, sustained 20-24 hour reversal • No evidence of prothrombotic rebound effect post-reversal • Per FDA request, we identified and tested regimen for patients with high ticagrelor drug levels Favorable safety profile with consistent with the Phase 1 • Mostly minor, unrelated AEs • No thrombotic events Phase 2A data presented to FDA and EMA enabled Phase 2B and Phase 3 REVERSE-IT trial start-up • Confirmed 18 gram regimen in both pivotal studies • Provided alternative dose for ticagrelor patients with CYP3A drug interaction • Critical for granting of EMA PRIME designation

bentracimab Pivotal Program John Lee, MD, PhD Chief Medical Officer, PhaseBio Phase 2B and Phase 3 REVERSE-IT

bentracimab Bentracimab (PB2452) Pivotal Trials: Prior Publicly Guided Timelines 37 Phase 2B trial: randomized, double-blind, placebo-controlled trial in volunteers 50-80 years old (N=200) • Enrollment ongoing in US and Canada, 3:1 randomization (active:placebo) • Primary endpoint is reversal measured by VerifyNow; secondary endpoints: safety, PK, immunogenicity Phase 3 REVERSE-IT trial: open-label, single-arm study, 100 sites in US, Canada, EU, China • Enrolling ticagrelor patients with uncontrolled major bleeding or who need urgent surgery • FDA and EMA agreement that Interim analysis (N=~100 patients) sufficient for BLA submission for approval Targeted timelines could be impacted by the continued scope and duration of the COVID-19 pandemic NEJM= New England Journal of Medicine, EOP1=End-of-Phase 1 Meeting, BLA=Biologics License Application 2019 2020 2021 2022 2023 Phase 2B: 50-80 year old volunteers Post-approval completion of Phase 3 Phase 3 REVERSE-IT trial in ticagrelor patients Phase 2APhase 1 NEJM FDA Breakthrough Therapy FDA EOP1 EMA PRIME Designation N=200, 150 randomized to receive PB2452 N=100, major bleeding + urgent surgery patients 2H’2022 BLA Submission 2H’2021 Last Patient Final N=200 Potential BLA Approval

bentracimab REVERSE-IT Phase 3 Study and Primary Endpoints 38 REVERSE-IT trial in ticagrelor patients with major bleeding or who need urgent surgery (N=100 for Interim Analysis, N=200 total) Screening (pre-dose) Informed consent Day 1 • VerifyNow • PB2452/tica PK • Central lab (hgb) • Hemostasis Inclusion/Exclusion (Major bleeding or urgent surgery) PB2452 infusion Day 3 • VerifyNow • PB2452/tica PK • Central lab (hgb) Discharge or Day 7 • VerifyNow • PB2452/tica PK • Central lab (hgb) • ECG, ADA Day 35 End of Study • VerifyNow • Central labs (safety) • PB2452/tica PK • ECG, ADA Central Adjudication • Inclusion criteria • Hemostasis • Thrombotic Events In-patient safety follow-up Out-patient safety follow-up Primary Reversal Endpoint: Minimum %inhibition of PRU within 4 hours of PB2452 initiation Primary Hemostasis Endpoint (Adjudicated): Effective hemostasis achieved within 24 hrs after start of infusion • Uncontrolled major bleeding: Effective hemostasis for major bleeding adapted from (Connolly, 2016) • Urgent surgery or invasive procedure: Effective hemostasis based on GUSTO clinical bleeding scale (GUSTO, 1993)

bentracimab REVERSE-IT Trial Global Footprint 39 REVERSE-IT trial in ticagrelor patients with major bleeding or who need urgent surgery (N=100 for Interim Analysis, N=200 total) Screening (pre-dose) Central Adjudication • Inclusion criteria • Hemostasis • Thrombotic Events REVERSE-IT sites are enrolling in all open regions (US, Canada, Europe) • US sites include high-volume cardiovascular, stroke/neurosurgery, and Emergency centers • Canadian sites include large metropolitan hospitals with CT surgery, critical care, invasive cardiology • European sites include high-volume cardiovascular, critical care, and emergency centers Total REVERSE-IT footprint expected to be >100 sites globally in 2021, equally distributed • US and Canadian site enrollment has been robust in regions with lower pandemic impact • Open regions in Europe achieving similar enrollment metrics as North America - Expect to open sites in 10 European countries • China regulatory filing and study recruitment expected 2H2021

bentracimab REVERSE-IT Enrollment Ahead of Original Projection 40 REVERSE-IT has enrolled 60 of the first 100 patients for the Interim Analysis • Enrollment is robust and ahead of initial projections despite COVID pandemic • Subjects include both major bleeding and urgent surgery/procedure patients • Pace of current enrollment supports acceleration of BLA submission by 3-6 months REVERSE-IT patients enrolled from all recruitment regions (US, Canada, Europe) • Surgical patient population includes cardiac surgery, orthopedic surgery, and urgent procedure patients • Smaller pool of bleeding patients observed includes ICH, GI bleed, and procedure-related bleeds • Total enrollment significantly weighted towards surgical patients REVERSE-IT trial in ticagrelor patients with major bleeding or who need urgent surgery (N=100 for Interim Analysis, N=200 total) Screening (pre-dose) Central Adjudication • Inclusion criteria • Hemostasis • Thrombotic Events

bentracimab REVERSE-IT Clinical Data Objectives 41 REVERSE-IT Data Safety Monitoring Board (DSMB) concluded first meeting in January 2021 • No concerning safety signals identified in first 25 enrolled patients • Unanimous recommendation to continue REVERSE-IT as currently designed REVERSE-IT efficacy objectives: Immediate and sustained reversal as observed in previous studies • If reproduced in Phase 3, would dramatically improve hemostasis in urgent surgery and bleeding populations - No observed non-responders from previous studies - Target fewer procedure-related bleeding events, fewer transfusions, shorter ICU and hospital LOS - Target low thrombotic event rate confirming absent pro-thrombotic rebound effect as in previous studies REVERSE-IT trial in ticagrelor patients with major bleeding or who need urgent surgery (N=100 for Interim Analysis, N=200 total) Screening (pre-dose) Central Adjudication • Inclusion criteria • Hemostasis • Thrombotic Events
bentracimab REVERSE-IT Predominance of Surgical Patients 42 Pandemic impact on bleeding patient admissions • Reduction in hospital admissions for major adverse spontaneous events like bleeding • Broad, global moratorium on clinical research and furloughs of research staff • Smaller REVERSE-IT footprint significantly reduced size of “safety net” to catch random events Pandemic impact on urgent surgery patient enrollment • Urgent surgical cases benefit from physician-managed patient flow (active vs passive recruitment) • Patients with progressive disease waited longer for medical follow-up, creating backlog of urgent cases • Major health centers re-opened to urgent or emergent cases first, creating robust environment to enroll REVERSE-IT trial in ticagrelor patients with major bleeding or who need urgent surgery (N=100 for Interim Analysis, N=200 total) Screening (pre-dose) Central Adjudication • Inclusion criteria • Hemostasis • Thrombotic Events

bentracimab REVERSE-IT Plan to Accelerate Bleeding Patient Recruitment 43 Internal resources re-allocated to bleeding site activation to widen catchment area • Prioritizing sites to lean towards bleeding recruitment – neurosurgery, stroke centers, trauma • Supplement surgical footprint with expanded bleeding site network – enabled by robust surgical enrollment • Utilize boutique vendors and technology platforms to reduce time-to-activation metrics Shift site phenotype away from ticagrelor volume and focus on total bleeding volume • Identify large stroke, neurosurgery, and trauma centers in US rather than largest ticagrelor prescribers - Some percentage of these cases will be in ticagrelor patients • Outreach by our cardiovascular and ticagrelor KOLs to engage stroke and trauma KOLs REVERSE-IT trial in ticagrelor patients with major bleeding or who need urgent surgery (N=100 for Interim Analysis, N=200 total) Screening (pre-dose) Central Adjudication • Inclusion criteria • Hemostasis • Thrombotic Events

bentracimab Revised Timelines of Bentracimab Program 44 Targeted timelines could be impacted by the continued scope and duration of the COVID-19 pandemic NEJM= New England Journal of Medicine, EOP1=End-of-Phase 1 Meeting, BLA=Biologics License Application 2019 2020 2021 2022 2023 Phase 2B: 50-80 year old volunteers Post-approval completion of Phase 3 Phase 3 REVERSE-IT trial in ticagrelor patients Phase 2APhase 1 NEJM FDA Breakthrough Therapy FDA EOP1 EMA PRIME Designation N=200, 150 randomized to receive PB2452 N=100, major bleeding + urgent surgery patients 2H’2022 BLA Submission 2H’2021 Last Patient Final N=200 Original Target BLA Approval Phase 2B study on track to complete enrollment mid-2021 • Plateauing of pandemic provides window to expand footprint of study in US and Canada • Pandemic-related economic pressures enhance recruitment of volunteers for Phase 2B study Rapid Phase 3 recruitment accelerates completion of 100 patient Interim Analysis cohort • Completion of Interim Analysis enrollment expected mid-2021 with top-line data expected by year end • Accelerated Interim Analysis enrollment expected to accelerate time to BLA submission
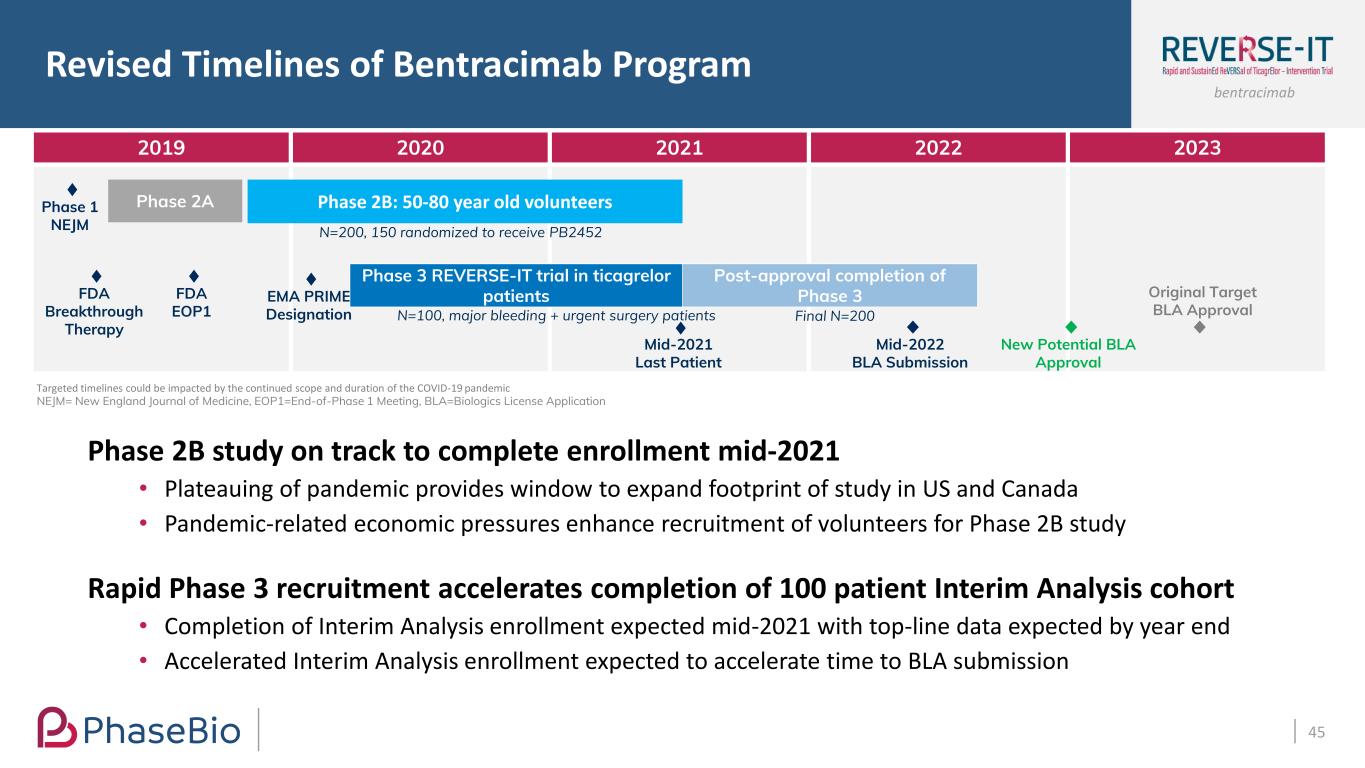
bentracimab Revised Timelines of Bentracimab Program 45 2019 2020 2021 2022 2023 Phase 2B: 50-80 year old volunteersPhase 2APhase 1 NEJM FDA Breakthrough Therapy FDA EOP1 EMA PRIME Designation N=200, 150 randomized to receive PB2452 N=100, major bleeding + urgent surgery patients Final N=200 Post-approval completion of Phase 3 Phase 3 REVERSE-IT trial in ticagrelor patients Mid-2022 BLA Submission New Potential BLA Approval Mid-2021 Last Patient Targeted timelines could be impacted by the continued scope and duration of the COVID-19 pandemic NEJM= New England Journal of Medicine, EOP1=End-of-Phase 1 Meeting, BLA=Biologics License Application Phase 2B study on track to complete enrollment mid-2021 • Plateauing of pandemic provides window to expand footprint of study in US and Canada • Pandemic-related economic pressures enhance recruitment of volunteers for Phase 2B study Rapid Phase 3 recruitment accelerates completion of 100 patient Interim Analysis cohort • Completion of Interim Analysis enrollment expected mid-2021 with top-line data expected by year end • Accelerated Interim Analysis enrollment expected to accelerate time to BLA submission Original Target BLA Approval

Regulatory Lauren Richardson Global Head of Regulatory Affairs and Quality Assurance, PhaseBio

EMA: • Scientific Advice Face-to-face interaction and written guidance in general agreement with proposed development plan for bentracimab • PRIME designation1 Granted to support medicines that demonstrate the potential to address substantial unmet medical need Potentially expedites the review and approval process • Multi-disciplinary PRIME Meeting Ongoing interactions to meet MAA expectations FDA: • End-of-Phase 1 meeting Alignment on development plan and Accelerated approval regulatory path • Breakthrough Therapy designation2 Granted to drugs intended to treat a serious condition with potential to address substantial improvement over currently available therapies Potentially expedites the development and review of promising new drugs • Breakthrough Therapy Multi-disciplinary Meeting Final Phase 3 protocol agreement of study design, endpoints and statistical analysis Bentracimab Regulatory Correspondence Development plan for bentracimab designed with objective to broadly support global regulatory filings 471. The U.S. Food and Drug Administration. “Expedited Programs for Serious Conditions – Drugs and Biologics.” Available at: https://www.fda.gov/downloads/Drugs/Guidances/UCM358301.pdf. Accessed February 20202. The European Medicines Agency. “PRIME: Priority Medicines” Available at: https://www.ema.europa.eu/en/human-regulatory/research-development/prime-priority-medicines#. Accessed February 2020 Separate written guidance from FDA and EMA indicates that REVERSE-IT, a single, non-randomized, open-label Phase 3 trial of bentracimab in both surgical and major bleeding populations, has the potential to support regulatory filings in the United States and the European Union

Bentracimab: Regulatory Highlights • Numerous regulatory interactions and planned future interactions Acceptance of clinical development program and study design Agreement with toxicity and CMC strategy Clarification of expectations for approval packages (multi-disciplinary) • Future interactions* Clarification of content and expectations for filing packages Review of top line data (Pre-BLA Meeting, Pre-Submission Meeting) 48 Region 2019 2020 2021 2022 US (FDA) Europe (EMA) Breakthrough Therapy FDA EOP1 PRIME Designation Scientific Advice PRIME Mtg Breakthrough Mtg Clinical Study Conduct and Commercial Preparation Regulatory Interactions* and Preparation for Filing BLA MAA

Revised Clinical Timeline Provides Opportunity for Accelerated Approval 49 Accelerated and Conditional Approval Packages • Initial BLA/MAA filing package based on a minimum safety requirement of at least 100 Phase 3 patients • Current Phase 3 protocol - No requirement for minimum number of bleeding patients - Interim Analysis triggered by first 100 enrolled patients irrespective of bleed vs surgery category - FDA and EMA agreed the PRU biomarker endpoint at interim likely predictive of clinical benefit in all patients Clinical confirmation of interim biomarker endpoint in both bleeding and surgical patients • Phase 3 completion and registry cohorts submitted for full approval • Opportunity to supplement interim cohort with bleeding patients after approval with broad label • Current study activities designed to enrich for bleeding patients in time for final study analysis
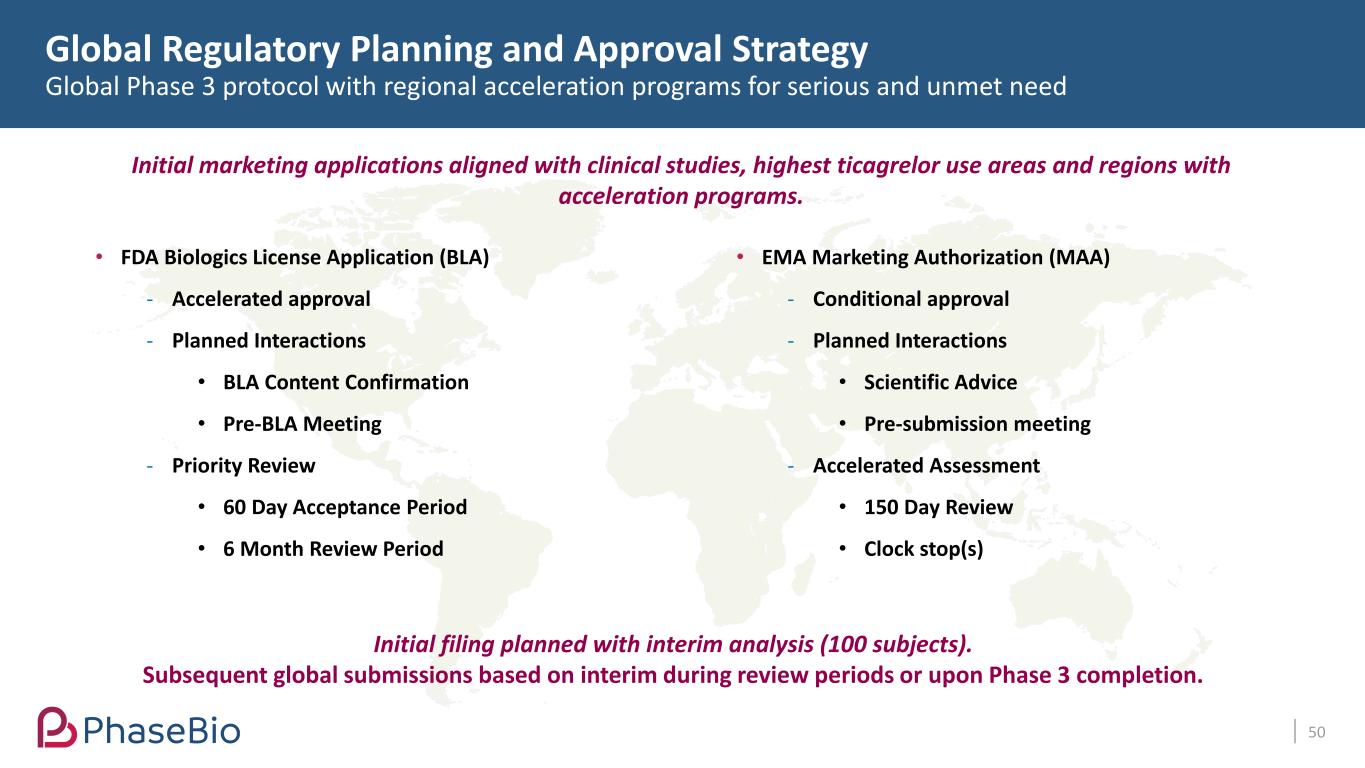
Global Regulatory Planning and Approval Strategy Global Phase 3 protocol with regional acceleration programs for serious and unmet need 50 Initial marketing applications aligned with clinical studies, highest ticagrelor use areas and regions with acceleration programs. Initial filing planned with interim analysis (100 subjects). Subsequent global submissions based on interim during review periods or upon Phase 3 completion. • FDA Biologics License Application (BLA) - Accelerated approval - Planned Interactions • BLA Content Confirmation • Pre-BLA Meeting - Priority Review • 60 Day Acceptance Period • 6 Month Review Period • EMA Marketing Authorization (MAA) - Conditional approval - Planned Interactions • Scientific Advice • Pre-submission meeting - Accelerated Assessment • 150 Day Review • Clock stop(s)

Manufacturing & Product Presentation Susan Arnold, PhD Vice President of Preclinical & CMC, PhaseBio

Drug Substance/API • Bentracimab drug substance is manufactured in E. coli Simple straight-forward four column purification process (no specialized resins) • Successful scale up of the FIH manufacturing process to a 17,000L bioreactor scale at BioVectra (Canada) No change in strain No major changes introduced in the manufacturing process during scale up • Currently manufacturing material for use in the Phase 2B and Phase 3 REVERSE-IT trial 4 drug substance lots completed to date • Validation/PPQ batches are planned for second half 2021 Manufacturing 52

• On March 10, 2021, PhaseBio entered into a 10-year Commercial Supply Agreement for Drug Substance/API manufacturing of bentracimab • 5 manufacturing facilities located in eastern Canada Supporting multiple commercial products Customers include top pharma and biotech companies • Facility located in Windsor, Nova Scotia • Dedicated microbial facility supporting BSL-1 products • Commercial Manufacturing planned to initiate in 2022 to support launch BioVectra – Commercial Supply Agreement 53 BioVectra

Manufacturing Drug Product • Drug product manufacturing consists of thawing, pooling, filtration/fill • No further formulation or extensive manipulation required • Validation of the commercial process is planned for late 2021 We plan to file a complete CMC manufacturing package at BLA filing 54

Launch Presentation • 100 mg/mL bentracimab Liquid stable Filled at 6 g/vial (3 vials per dose) • Ready to use refrigerated formulation No dilution or manipulation of the drug product required Does not require preparation in a hospital pharmacy • 2-year shelf-life Real time stability currently supports storage at 2-8°C for 15 months and room temperature for 12 months Intend to have 24 months of 2-8°C stability once on- site in a hospital setting with a goal of 30-36 months to support distribution activities Phase 3 and Commercial Launch Presentation 55

Hospital Pharmacy Perspective John Fanikos, BS Pharm, MBA Executive Director of Pharmacy Services at Brigham and Women's Hospital

57 Antiplatelet Therapy, the Landscape, the Need for Bentracimab: the Pharmacist’s View John Fanikos, BS Pharm, MBA Brigham and Women’s Hospital Monday, March 15, 2021

58 Outline •Antiplatelet agents and patterns of use •Guidelines and Expert Opinion •Case examples-The need for a reversal agent • Bleeding • Unplanned surgeries •Current Hospital Support Structure • Summary

59 Prasugrel Ticagrelor Clopidogrel Fatani N, et al. J Thromb and Thrombolysis, 2019;48:539-41. 131,431 123,825 121,093 118,752 107,253 101,466 0 5 10 15 20 25 30 0 20,000 40,000 60,000 80,000 100,000 120,000 140,000 C u m u la ti v e P e rc e n t O cc u rr e n ce s (0 0 0 ) CDC. See https://www.cdc.gov/nchs/hus/index.htm National Ambulatory Medical Care Survey Top Medications Discussed with Physician Antiplatelet Therapy: An Ongoing Concern

60BWH Internal data FY19-20 Zekery-Saad SA, et al. J Thromb Thrombolysis 2021; 51 (2):405-419. BWH Monthly P2Y12 Use Antiplatelet Therapy: An Ongoing Concern 0 500 1,000 1,500 2,000 2,500 Clopidogrel Prasugrel Ticagrelor Covid Pandemic Peak Monthly Average AC, 70% SAPT+AC, 29% DAPT+AC, 1% Single and Combination Antithrombotic Therapy
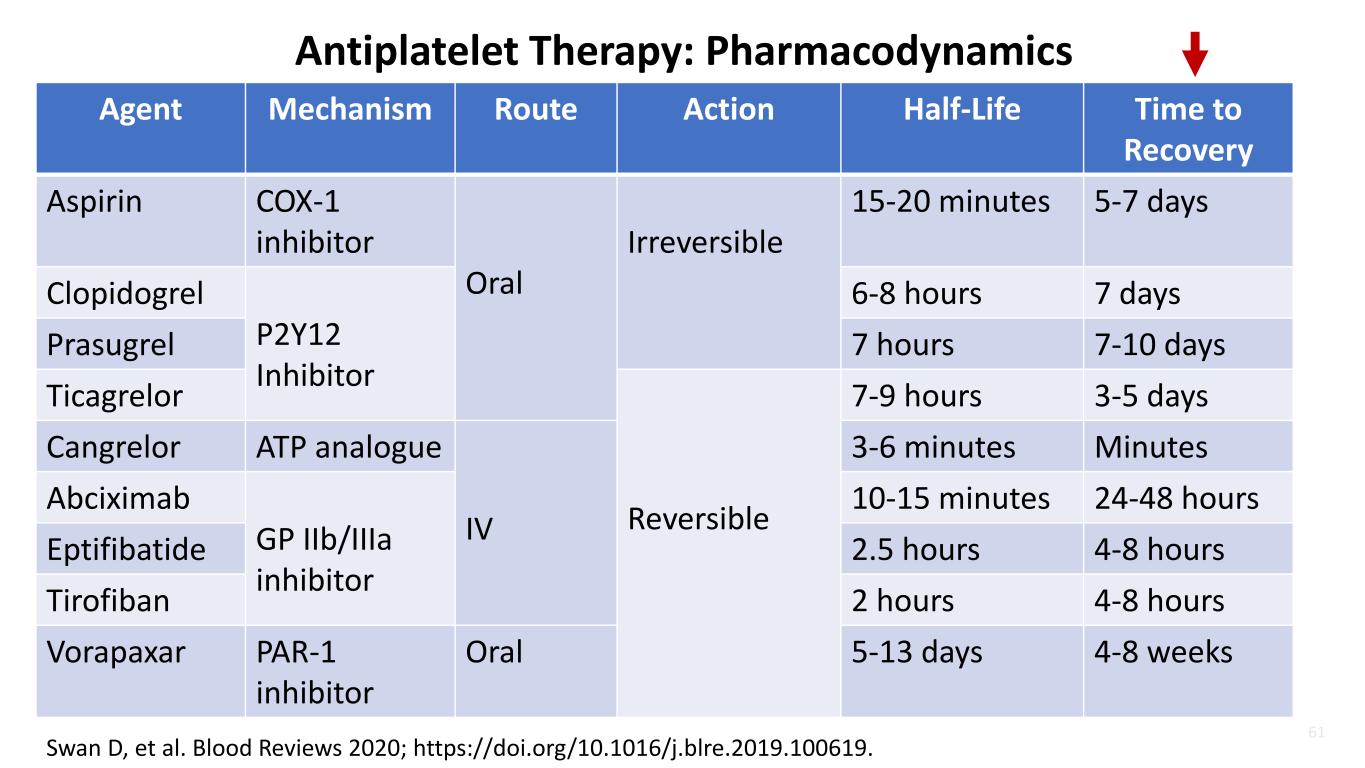
61 Swan D, et al. Blood Reviews 2020; https://doi.org/10.1016/j.blre.2019.100619. Antiplatelet Therapy: Pharmacodynamics Agent Mechanism Route Action Half-Life Time to Recovery Aspirin COX-1 inhibitor Oral Irreversible 15-20 minutes 5-7 days Clopidogrel P2Y12 Inhibitor 6-8 hours 7 days Prasugrel 7 hours 7-10 days Ticagrelor Reversible 7-9 hours 3-5 days Cangrelor ATP analogue IV 3-6 minutes Minutes Abciximab GP IIb/IIIa inhibitor 10-15 minutes 24-48 hours Eptifibatide 2.5 hours 4-8 hours Tirofiban 2 hours 4-8 hours Vorapaxar PAR-1 inhibitor Oral 5-13 days 4-8 weeks

62 Surgery Recommendations Recommendations for Bleeding Tafur A, et al. Interrupt 2-3 days, Cangrelor bridge, Platelet transfusion. None.Patel IJ, et al Hold 5 days before surgery, Consult Cardiology. Filipescu DC, et al. Interrupt for minimum amount of time, Cangrelor bridge. Desmopressin, Factor VIIa, Fibrinogen, Tranexamic acid Dastur CK, et al. Platelet transfusion, Platelet function testing, Desmopressin. Desmopressin. Kaufman RM, et al. Cannot recommend for or against platelet transfusion. Frontera JA, et al Platelet transfusion, Platelet function testing, Desmopressin. No platelet transfusion, Desmopressin. Swan D, et al Interrupt several days before surgery, ICH and surgery-Transfuse platelets, GIB and intervention–No platelets, Emergency surgery-Consider IV tranexamic. None. Valgimigli M, et al Non-cardiac surgery interrupt, Cangrelor, GPIIb/IIIa. Sever/Life-threatening bleed-Platelet transfusion. Tomaselli GF, et al None. No routine platelet administration for bleeding. Tafur A, et al. Heart 2018;104:1461-67. Filapescu DC, et al. Curr Opin Anesthesiol 2020;33;454-62. Patel IJ, et al. J Vasc Interv Radiol 2019;30;1168-1184. Dastur CK, et al. Stroke Vasc Neurol 2017;2.e000047. doi:10.1136/svn-2016-000047. Washington CW, at al. J Trauma 2011;71(2):358-63. Kaufman RM, et al. Ann Intern Med 2015;162:205-213. Frontera JA, et al. Neurocrit Care 2016;24:6-46. Swan D, et al. Blood Reviews 2020;39:1-12. Valgimigli M, et al. Eur Heart J 2018;;39:213-54. Tomaselli GF, et all JACC 2020;76;5:594-621. Professional Guidelines and Expert Opinion Vary

63 Case Presentation: 63 year old male with Headache Chief Complaint: • New onset headache, N+V, dizziness, new arm paralysis. Past Medical History: • COPD, lung cancer in remission (s/p resection in 2012), HFrEF (EF 30%), DM, CAD, PAD-s/p bypass graft. • Taking ASA + Clopidogrel. ED Course: • CT Head ordered. • Consults: Neurology, Neurosurgery, Hematology. Imagining: • Hemorrhage in the cerebellum measuring 2.7 x 1.7 cm, with intraventricular extension. Treatment & Hospital Course • 2 units of platelets ordered. • Neuro ICU Admission. • Neurosurgery for Craniotomy. Category Amount Hospital stay 23 days Neuro ICU 13 days Operating Room Charge $40,344 Imaging $32,393 Total Charges $309,156
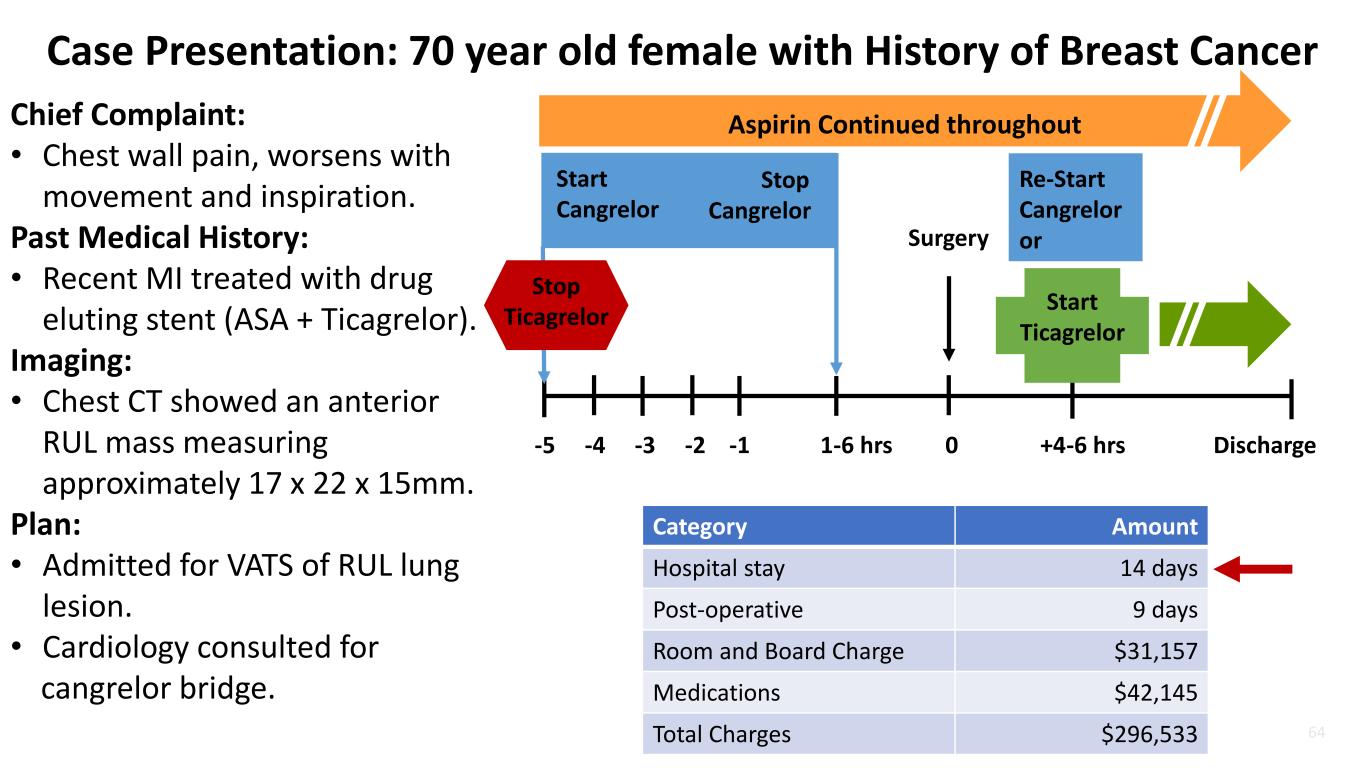
64 Case Presentation: 70 year old female with History of Breast Cancer Chief Complaint: • Chest wall pain, worsens with movement and inspiration. Past Medical History: • Recent MI treated with drug eluting stent (ASA + Ticagrelor). Imaging: • Chest CT showed an anterior RUL mass measuring approximately 17 x 22 x 15mm. Plan: • Admitted for VATS of RUL lung lesion. • Cardiology consulted for cangrelor bridge. Aspirin Continued throughout Start Cangrelor -5 -4 -3 -2 -1 1-6 hrs 0 +4-6 hrs Discharge Stop Cangrelor Surgery Re-Start Cangrelor or Stop Ticagrelor Start Ticagrelor Category Amount Hospital stay 14 days Post-operative 9 days Room and Board Charge $31,157 Medications $42,145 Total Charges $296,533
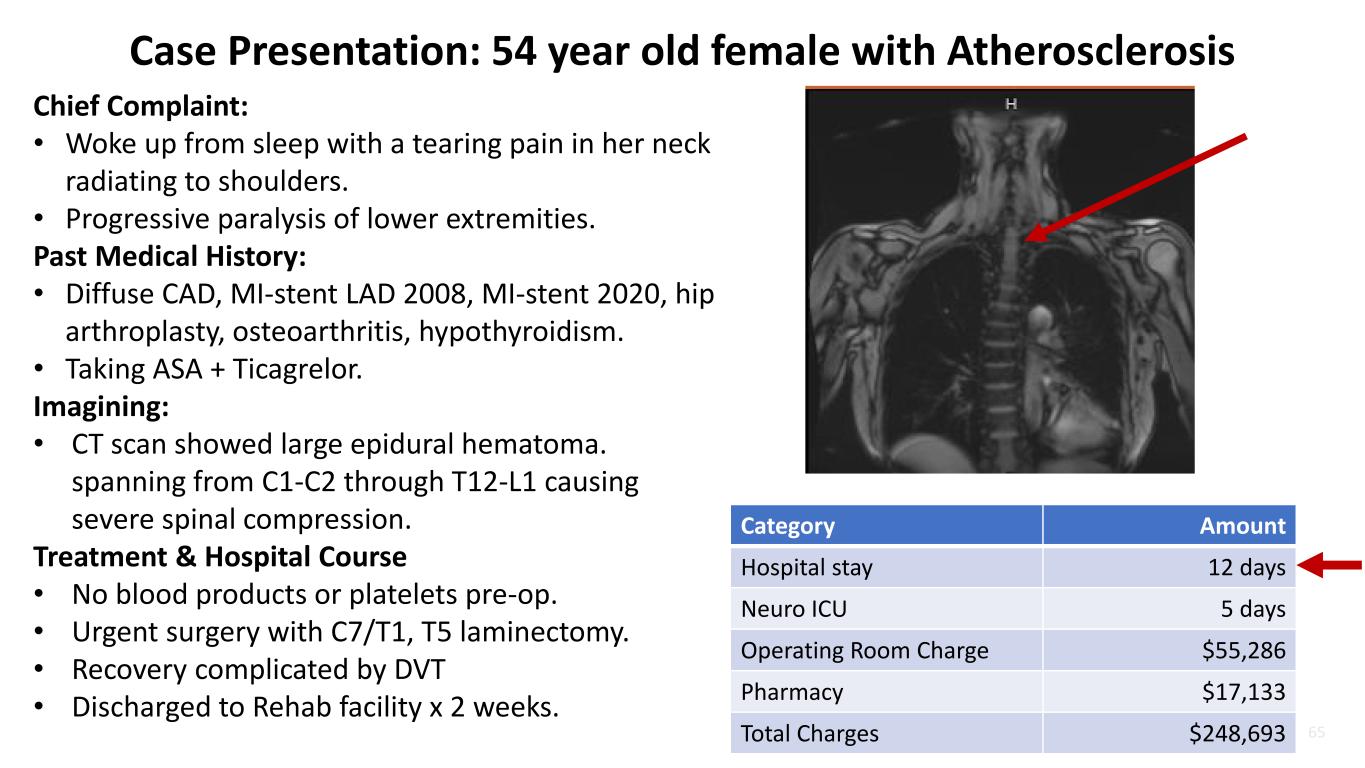
65 Case Presentation: 54 year old female with Atherosclerosis Chief Complaint: • Woke up from sleep with a tearing pain in her neck radiating to shoulders. • Progressive paralysis of lower extremities. Past Medical History: • Diffuse CAD, MI-stent LAD 2008, MI-stent 2020, hip arthroplasty, osteoarthritis, hypothyroidism. • Taking ASA + Ticagrelor. Imagining: • CT scan showed large epidural hematoma. spanning from C1-C2 through T12-L1 causing severe spinal compression. Treatment & Hospital Course • No blood products or platelets pre-op. • Urgent surgery with C7/T1, T5 laminectomy. • Recovery complicated by DVT • Discharged to Rehab facility x 2 weeks. Category Amount Hospital stay 12 days Neuro ICU 5 days Operating Room Charge $55,286 Pharmacy $17,133 Total Charges $248,693

66 Stewardship Structure Pharmacy Hematology Cardiology Hemostatic and Antithrombotic Stewardship Hospital Administration Pharmacy and Therapeutics Committee • Antithrombotic Reversal and Bleeding Management • Andexanet, Idarucizumab • Clotting Factor Management • Ensure appropriate use of agents (i.e. Platelets, aPCC, PCC, VIIa, VIII, IX, etc) • Antithrombotic Management of Mechanical Circulatory Support Devices • ECMO, TAH, VAD • Antithrombotic Pre-op Management • Warfarin, DOACS, P2Y12 Agents • Dual and Triple Therapies • Heparin-Induced ThrombocytopeniaBM Ritchie. J Thromb Thrombolysis 2016.42;616-622. Amerine LB. Am J Health-Syst Pharm 2015;72:1579-1584. Padron M, J Pharm Practice 2015; 23:93-98. Burnett A. J Thromb Thrombolysis 2016:42:471-78. Wychowski MK. J Thromb Thrombolysis 2017:43:380-86. Antithrombotic Stewardship: Value-Added Service Prescriber Patient

67 Antithrombotic Stewardship: Value-Added Service Lewin AR, et al. Crit Pathways in Cardiol 2020, 19(4):178-86. Filapescu DC, et al. Curr Opin Anesthesiol 2020;33;454-62.

68 Trauma, ICH patients on anticoagulation (AC) Continuous Quality Improvement Minutes • Hold anticoagulant & give a Reversal Agent • Send laboratory analysis for baseline values and if necessary to modify therapy • Initiate prior to lab being drawn or resulted Bleeding with Hemodynamic Changes Hours • Hold anticoagulant & consider a Reversal Agent if expedited reversal is necessary • Topical hemostatic agents may be used in select situations • Supportive care: IV fluids, compression • Send laboratory analysis for baseline values and if necessary to modify therapy, initiate prior to lab being drawn or resulted Days • Hold the anticoagulant • Lab tests & reassess bleeding risks • Supportive care Bleeding Time Verified to Delivery (Minutes) 30.9 Minutes

69 Summary • Antiplatelet therapy is common. • Antiplatelet therapy in combination (ASA, Anticoagulants) • Recommendations for how to stop antiplatelet activity vary. • There is no “ideal” approach. • Complications with patient are common. • A tool (bentracimab) to halt antiplatelet activity is needed. • May improve patient outcomes. • May improve efficiency. • May help contain healthcare costs. • Hospital support structure is present to optimize use. • We will figure out who, why, and when.

Commercial Opportunity Michael York Vice President of Corporate Development & Commercial Strategy, PhaseBio

~42M Global Patients Estimated on P2Y12 Inhibitors: The Foundation of ACS Therapy 71 2019 P2Y12 Class: Top 20 Countries by Patient Count ~3.3M Ticagrelor Patients By Key Markets Iqvia®, PhaseBio Analysis of MIDAS Data – Full Year 2019 Country Clopidogrel Prasugrel Ticagrelor Country Total CHINA 7,353,753 0 642,522 7,996,275 INDIA 3,695,849 255,051 442,060 4,392,960 US 3,845,640 175,491 439,163 4,460,294 GERMANY 1,416,325 126,840 278,783 1,821,949 FRANCE 775,972 18,769 212,432 1,007,173 SPAIN 721,112 18,710 125,400 865,222 UK 1,222,631 9,045 120,260 1,351,937 ITALY 1,301,775 11,448 113,186 1,426,409 TURKEY 1,100,541 10,197 93,039 1,203,777 RUSSIA 833,037 2,507 82,846 918,390 NETHERLANDS 957,768 8,443 67,935 1,034,146 CANADA 341,260 2,275 63,386 406,921 SWEDEN 255,967 2,076 51,940 309,983 BRAZIL 1,610,178 16,326 50,311 1,676,815 ROMANIA 722,705 51 43,300 766,057 POLAND 214,993 2,034 42,818 259,846 AUSTRALIA 242,325 4,378 34,574 281,277 INDONESIA 1,047,602 0 28,002 1,075,605 KOREA 929,254 8,743 27,083 965,080 SAUDI ARABIA 205,389 0 24,465 229,855 EU 38% China 19% United States 13% APAC 5% ROW 25%

Introduction of Bentracimab May Fundamentally Change Prescribing in the P2Y12 Class 72 Ticagrelor Differentiation vs. clopidogrel Now Post Bentracimab Launch Post LOE of ticagrelor Efficacy ✓ ✓ ✓ Safety ≈ (no reversal agent) ✓ ✓ Price ✕ (branded vs. generic) ✕ (branded vs. generic) ✓

PhaseBio Strategy to Drive Hospital Focused Launch 73 Pre-Launch Imperatives Establish Burden of Bleeds Identify and Remove Barriers to Adoption Segment and Target Hospitals and IDNs Establish Launch Capabilities Build Brand Awareness Stocked • Inclusion in order sets at targeted institutions • Enable “claw-back” by securing NTAP* program Reimbursement Clinical and Economic Story • Positive clinical data and compelling economic value proposition • Position and messages demonstrate efficacy and address clear needs Guidelines • Targeted associations include Bentracimab in guidelines • Awareness of guidelines with targeted health care professionals Mobilize KOLs and Hospitals • Experienced and trained specialty team • Product agreements in place and reviews sequenced * Medicare New Technology Add-on Payment Launch Critical Success Factors
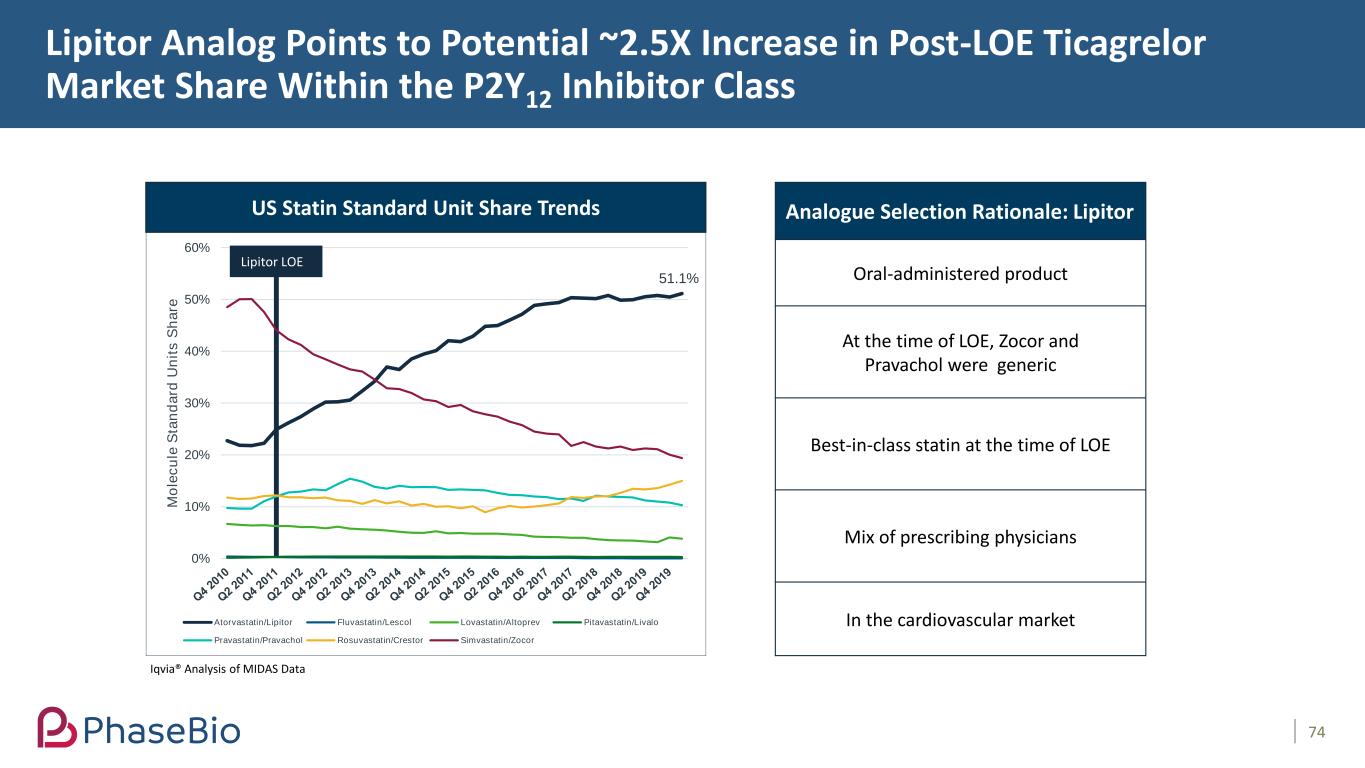
Lipitor Analog Points to Potential ~2.5X Increase in Post-LOE Ticagrelor Market Share Within the P2Y12 Inhibitor Class 74 51.1% 0% 10% 20% 30% 40% 50% 60% M o le c u le S ta n d a rd U n it s S h a re Atorvastatin/Lipitor Fluvastatin/Lescol Lovastatin/Altoprev Pitavastatin/Livalo Pravastatin/Pravachol Rosuvastatin/Crestor Simvastatin/Zocor Lipitor LOE Analogue Selection Rationale: Lipitor Oral-administered product At the time of LOE, Zocor and Pravachol were generic Best-in-class statin at the time of LOE Mix of prescribing physicians In the cardiovascular market Iqvia® Analysis of MIDAS Data US Statin Standard Unit Share Trends
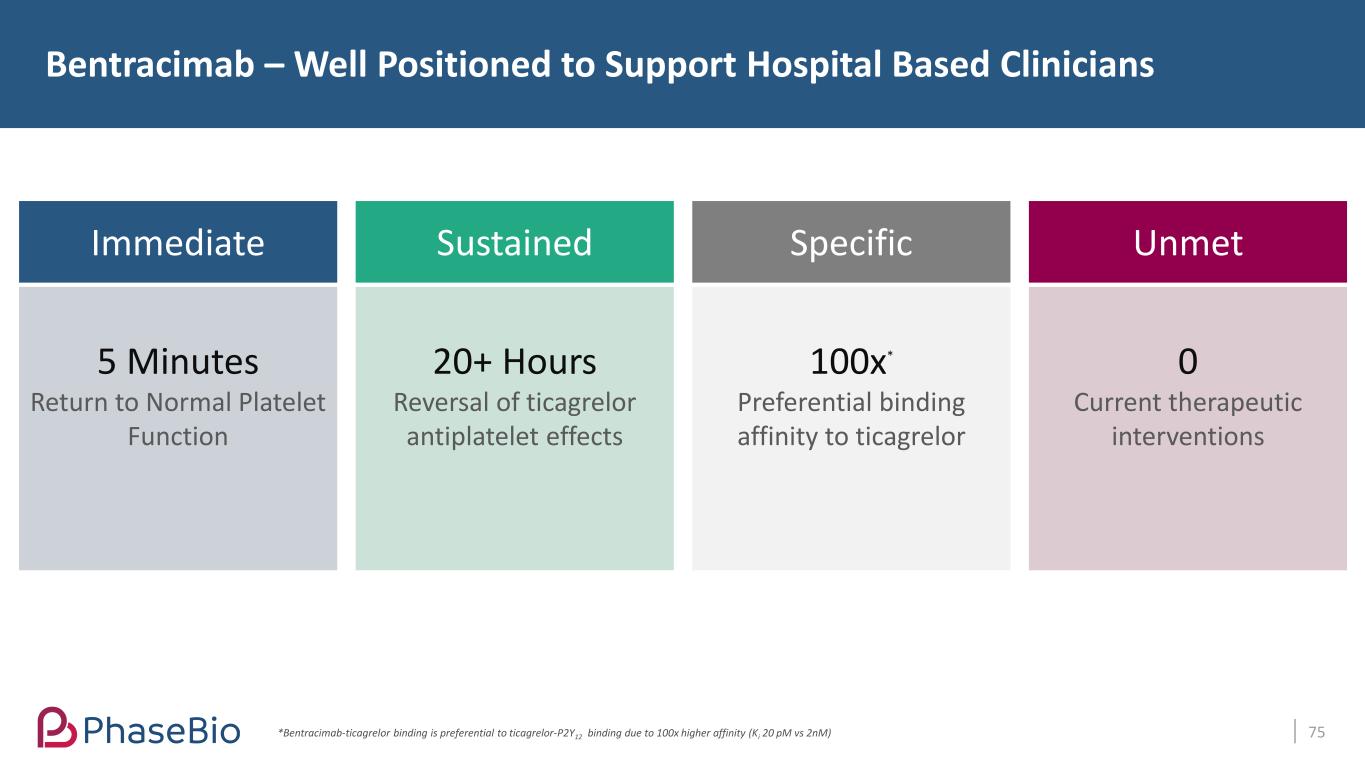
Bentracimab – Well Positioned to Support Hospital Based Clinicians 75 Immediate 5 Minutes Return to Normal Platelet Function Sustained 20+ Hours Reversal of ticagrelor antiplatelet effects Specific 100x* Preferential binding affinity to ticagrelor Unmet 0 Current therapeutic interventions *Bentracimab-ticagrelor binding is preferential to ticagrelor-P2Y12 binding due to 100x higher affinity (Ki 20 pM vs 2nM)

Closing Remarks Jonathan Mow Chief Executive Officer, PhaseBio

Key Take-Aways 77 • Bentracimab clinical development program is enrolling ahead of schedule Global regulatory path for bentracimab is clear and supported by the REVERSE-IT trial • Need for P2Y12-inhibitor reversal is completely unmet in today’s medical practice Ticagrelor has a best-in-class efficacy profile in large, global outcomes trials Recent ticagrelor label expansion and future patent expiration among multiple potential growth drivers Data in Phase 1 and Phase 2A trials highlight immediate and sustained restoration of platelet function Bentracimab has the potential to change the way P2Y12 inhibitors are utilized • We are planning for commercial success Commercial-scale manufacturing process is operational; capable of global supply at launch, if bentracimab is approved • Commercial opportunity is significant and, we believe, underappreciated













































































