
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________________
FORM
(Mark One)
For the fiscal year ended
or
_____________________________________________
Commission file number:

(Exact name of registrant as specified in its charter)
|
||||
State or other jurisdiction of incorporation or organization |
|
I.R.S. Employer Identification No. |
||
|
|
|
||
|
||||
Address of principal executive offices |
|
Zip Code |
||
Registrant’s telephone number, including area code: (
_____________________________________________
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Securities registered pursuant to Section 12(g) of the Act:
None
_____________________________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
|
Accelerated filer |
|
☐ |
|
☒ |
|
|
Smaller reporting company |
|
||
|
|
|
|
|
Emerging growth company |
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☐ No
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b).
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No
As of June 30, 2023, the aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $
As of February 21, 2024, there were
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s proxy statement in connection with the registrant’s annual meeting of stockholders, scheduled to be held in June 2024, are incorporated by reference in Part III of this report. Except as expressly incorporated by reference, such proxy statement shall not be deemed to be part of this report.
STANDARD BIOTOOLS INC.
FISCAL YEAR 2023
FORM 10-K
ANNUAL REPORT
TABLE OF CONTENTS
|
|
|
|
Page |
PART I |
|
|
|
|
ITEM 1. |
|
|
1 |
|
ITEM 1A. |
|
|
24 |
|
ITEM 1B. |
|
|
58 |
|
ITEM 1C. |
|
|
58 |
|
ITEM 2. |
|
|
59 |
|
ITEM 3. |
|
|
60 |
|
ITEM 4. |
|
|
60 |
|
|
|
|
|
|
PART II |
|
|
|
|
|
|
|
|
|
ITEM 5. |
|
|
61 |
|
ITEM 6. |
|
|
61 |
|
ITEM 7. |
|
Management's Discussion and Analysis of Financial Condition and Results of Operations |
|
62 |
ITEM 7A. |
|
|
71 |
|
ITEM 8. |
|
|
72 |
|
ITEM 9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosures |
|
105 |
ITEM 9A. |
|
|
105 |
|
ITEM 9B. |
|
|
105 |
|
ITEM 9C |
|
Disclosures Regarding Foreign Jurisdictions that Prevent Inspections |
|
105 |
|
|
|
|
|
PART III |
|
|
|
|
|
|
|
|
|
ITEM 10. |
|
|
106 |
|
ITEM 11. |
|
|
106 |
|
ITEM 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
106 |
ITEM 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
106 |
ITEM 14. |
|
|
106 |
|
|
|
|
|
|
PART IV |
|
|
|
|
|
|
|
|
|
ITEM 15. |
|
|
107 |
|
ITEM 16. |
|
|
107 |
|
|
|
|
|
|
|
108 |
|||
|
117 |
|||
i
Special Note Regarding Forward-looking Statements and Industry Data
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that are based on our management’s beliefs and assumptions and on information currently available to our management. The forward-looking statements are contained principally in the sections entitled “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Forward-looking statements include information concerning our possible or assumed future cash flow, revenue, sources of revenue and results of operations, cost of product revenue and product margin, operating and other expenses, unit sales and the selling prices of our products, business strategies, financing plans, expansion of our business, investments to expand our customer base, plans for our products, competitive position, industry environment, potential growth opportunities, market growth expectations, the effects of competition, our planned use of the proceeds from the Bridge Loans and Private Placement Issuance described herein, cost structure optimization, acceleration of growth, potential merger and acquisition (M&A) activity and restructuring plans (including expense reduction activities involving potential subleasing and talent relocation plans, modifications to the scope of the company’s proteomic and genomics businesses and discontinuing of certain product lines) and our expectations regarding the benefits and integration of acquired businesses and/or products (including in connection with our merger with SomaLogic, Inc. in January 2024). Forward-looking statements include statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “seeks,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts, “projects,” “should,” “will,” “would,” or similar expressions and the negatives of those terms.
Forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. We discuss these risks in greater detail in the section entitled “Risk Factors” and elsewhere in this Annual Report on Form 10-K. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
Forward-looking statements represent our management’s beliefs and assumptions only as of the date of this Annual Report on Form 10-K. Except as required by law, we assume no obligation to update these forward-looking statements, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future. You should read this Annual Report on Form 10-K completely and with the understanding that our actual future results may be materially different from what we expect.
This Annual Report on Form 10-K also contains estimates, projections and other information concerning our industry, our business, and the markets for certain of our products, including data regarding the estimated size of those markets. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events, or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market, and other data from reports, research surveys, studies, and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data, and similar sources.
Standard BioTools, the Standard BioTools logo, Fluidigm®, the Fluidigm logo, 48.Atlas™, Access Array™, Advanta™, Advanta EASE™, Atlas™, Biomark™, “Bringing new insights to life”™, C1™, Callisto™, Cell-ID™, CyTOF®, CyTOF XT™, the CyTOF XT logo, D3™, Delta Gene™, Direct™, Digital Array™, Dynamic Array™, EP1™, EQ™, FC1™, Flex Six™, Flow Conductor™, FluiDesign™, Helios™, High-Precision 96.96 Genotyping™, HTI™, Hyperion™, Hyperion+™, IMC™, Imaging Mass Cytometry™, Immune Profiling Assay™, Juno™, Maxpar®, MCD™, MSL®, Nanoflex™, Open App™, Pathsetter™, Polaris™, qdPCR 37K™, Script Builder™, Script Hub™, Singular™, SNP Trace™, SNP Type™, “Unleashing tools to accelerate breakthroughs in human health”™, X9™ Real Time PCR System, Xgrade™, SomaLogic®, SomaScan®, SOMAmer®, SomaSignal®, Power by SomaLogic™, DataDelve™, and CardioDM are trademarks or registered trademarks of Standard BioTools Inc. or its affiliates in the United States and/or other countries. Other service marks, trademarks and trade names referred to in this Annual Report on Form 10-K are the property of their respective owners.
Unless the context requires otherwise, references in this Annual Report on Form 10-K to “Standard BioTools,” the “Company,” “we,” “us,” and “our” refer to Standard BioTools Inc. and its subsidiaries.
PART I
ITEM 1. BUSINESS
Overview
Standard BioTools Inc. is driven by a bold purpose – unleashing tools to accelerate breakthroughs in human health. We develop, manufacture and sell technologies that help biomedical researchers in their search for developing medicines faster and better. Our tools provide insights in health and disease using our proprietary mass cytometry and microfluidics technologies, which serve applications in proteomics and genomics, respectively.
Within proteomics our mass cytometry technology is embodied in two analytical platforms: flow cytometry and tissue imaging or spatial biology. Our flow cytometry systems (Helios™ and CyTOF XT) deeply profile cell phenotype and function. Referenced by more than 2,200 peer-reviewed publications around the world, our CyTOF technology has set a new standard in human immune profiling with our proprietary digital readout. Our spatial biology systems (Hyperion™ Imaging System and Hyperion+TM Imaging System) enable highly multiplexed protein biomarker detection at a single cellular level in tissues and tumors while still preserving tissue architecture and cellular morphology information and without any autofluorescence artifacts by using our Imaging Mass Cytometry™ (IMC™) technology.
Within genomics, our microfluidics technology with our proprietary Integrated Fluidic Circuits (IFCs) provides high throughput and automated workflows for quantitative polymerase chain reaction (PCR), gene expression, copy number variation analysis, and next-generation sequencing (NGS) library preparation. These automated systems are used to detect somatic and genomic variations from a range of different sample types which provide cost efficiencies, flexibility and proven analytical performance that customers need to meet the increasing demands of molecular biomarker analysis for diagnostics and research applications.
On January 5, 2024, we completed the merger (the Merger) with SomaLogic, Inc. (SomaLogic), creating a leading provider of differentiated multi-omics tools for research.
Strategic Investment Transaction and Business Restructuring
On April 1, 2022, our stockholders approved the closing of a private placement of preferred stock to Casdin Private Growth Equity Fund II, L.P. and Casdin Partners Master Fund, L.P. (collectively, Casdin) and Viking Global Opportunities Illiquid Investments Sub-Master LP and Viking Global Opportunities Drawdown (Aggregator) LP (collectively, Viking and together with Casdin, the Purchasers). On the closing date, April 4, 2022, we sold an aggregate of $225.0 million worth, and converted $25.0 million of previously issued bridge loans (the Bridge Loans), into shares of Series B-1 Convertible Preferred Stock and Series B-2 Convertible Preferred Stock pursuant to separate Series B Convertible Preferred Stock Purchase Agreements, dated and effective as of January 23, 2022, with each of the Purchasers (the Private Placement Issuance). The $250.0 million proceeds from the Bridge Loans and Private Placement Issuance are being used for working capital and general corporate purposes. On the closing date, the stockholders also approved the name change of our company to “Standard BioTools Inc.”
In connection with the closing of the private placement, Dr. Michael Egholm was appointed President and Chief Executive Officer and Alex Kim as Chief Operating Officer of the Company. Between April 2022 and today, Mr. Egholm has rebuilt our entire executive leadership team, assembling a team of disciplined operators that we believe can execute our vision to become a diversified leader in life sciences tools. Collectively, the Standard BioTools executive leadership team has over 140 years of combined life sciences experience. We have also reconstituted our board of directors since early 2022, adding significant life sciences operating, technology and capital markets expertise. In only seven quarters since the initiation of our restructuring plans in April 2022, our new management has significantly improved our legacy business performance, as demonstrated by the results of the fiscal year ended December 31, 2023 during which revenues increased 9% year-to-year (including over 40% growth in instrument revenue), gross margins expanded by more than 25% year-to-year, operating expenses declined by approximately 17% year-to-year, and operating cash burn declined by 53% year-to-year.
Merger With SomaLogic, Inc.
On January 5, 2024, we completed the Merger with SomaLogic and Martis Merger Sub, Inc., a Delaware corporation and direct wholly owned subsidiary of the Company (Merger Sub), pursuant to which, at the effective time of the Merger (the Effective Time), among other matters, Merger Sub merged with and into SomaLogic, with SomaLogic surviving as a wholly owned subsidiary of the Company. Upon the terms and subject to the conditions set forth in that certain Agreement and Plan of Merger, dated as of October 4,
1
2023, by and between the Company, SomaLogic and Merger Sub (the Merger Agreement), at the Effective Time, each share of SomaLogic common stock, par value $0.0001 per share (SomaLogic Common Stock), converted into the right to receive 1.11 shares (the Exchange Ratio) of the Company’s common stock, par value $0.001 per share.
SomaLogic is catalyzing drug research and development and biomarker identification as a global leader in proteomics technology. With a single 55 microliter plasma or serum sample, SomaLogic can run 11,000 protein measurements, covering more than a third of the approximately 20,000 proteins in the human body and twice as many as other proteomic platforms. For more than 20 years SomaLogic has supported pharmaceutical companies, and academic and contract research organizations who rely on SomaLogic’s protein detection and analysis technologies to fuel drug, disease, and treatment discoveries in such areas as oncology, diabetes, and cardiovascular, liver and metabolic diseases.
The combination of Standard BioTools and SomaLogic represents an opportunity for the combined company to leverage this operational expertise and discipline to achieve cost synergies for the combined company and to create new revenue opportunities with the combined platform, which we believe will inure to the benefit of our customers and create long-term value for the combined company’s stockholders.
Including expected acquired cash and investments, the combined company had a balance of approximately $565.5 million of cash, cash equivalents, short-term investments and restricted cash at December 31, 2023, which amount includes SomaLogic’s unaudited cash, cash equivalents and short-term investments of $449.8 million as of December 31, 2023. For the year ended December 31, 2023, the combined company had pro forma revenue of $192.4 million, which amount includes SomaLogic’s unaudited 2023 revenue of $86.1 million. The preliminary combined company cash and revenue included in this Form 10-K has been prepared by, and is the responsibility of, Standard BioTools’ management. PricewaterhouseCoopers LLP has not audited, reviewed, examined, compiled, nor applied agreed-upon procedures with respect to the preliminary financial data. Accordingly, PricewaterhouseCoopers LLP does not express an opinion or any other form of assurance with respect thereto.
The disclosures in this Item 1 of this Annual Report on Form 10-K (the Annual Report) under the heading “Business” speak to the combined company subsequent to the Merger unless otherwise noted. However, the financial results described herein relate, except as otherwise expressly noted herein, to Standard BioTools on a standalone basis without to giving effect to Merger and, accordingly, do not include the results of SomaLogic. Future filings, beginning with our Quarterly Report on Form 10-Q for the fiscal quarter ending March 31, 2024, will reflect the results of the combined company.
Strategy
Our new leadership team has identified three strategic priorities: revenue growth, improving operating discipline through the Standard BioTools Business Systems (SBS) and strategic capital allocation.
Revenue Growth
One of our top priorities is to grow our instrument, consumables and service revenue. We have established new growth strategies for our product lines, returning our business to growth in 2023. This growth has been driven by over 40% increase in instrument revenue, led by new placements of our Hyperion XTi Imaging System, which we launched in April 2023. Importantly, we believe growth in instrument placements is a leading indicator. And while we expect to see continued variability in quarter-to-quarter instrument placements, the growing installed base expands future consumables and service pull-through, which are significant drivers of both revenue and margin growth.
We continue to invest in research and development (R&D) to create and launch new products and have adopted best practice approaches to improve our lead generation and funnel management growth, among other things.
Improving Operating Discipline Through SBS
Our second priority is to improve our operating discipline through the implementation of SBS. We are leveraging SBS with a set of organizing principles, rigorous standard work processes, and a continuous improvement mindset to build more efficient operations and commercial execution and reduce costs.
Following the closing of our private placement described above, we implemented a phased restructuring plan designed to improve efficiencies, expand gross margins, reduce operating costs and better align our workforce with the current needs of our business.
Key highlights of our restructuring initiatives have included:
2
Strategic Capital Allocation
Our third priority is strategic capital allocation. We are actively pursuing business development opportunities in the life sciences industry with consolidation and synergies expected to be a key growth driver to sustain the longer-term value proposition of the Company. The merger with SomaLogic is our latest step forward toward unlocking value in a highly fragmented market through scale, diversification and operational expertise.
Market Opportunity
We participate in growing and emerging market segments within the broader proteomics and genomics markets.
Proteomics
The market for proteomics is broadly defined as instruments, consumables and reagents, software, and services for all technologies used in the identification of proteins. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, replicating DNA, signaling response to stimuli and transporting molecules from one location to another. The proteome varies and is dynamic. Every cell in an individual organism has the same set of genes, but the set of proteins produced in different tissues differ from one another and are dependent on gene expression. Protein analysis is required to profile and understand cellular function as well as the interaction in tissues and other complex microenvironments. Within the proteomics market, we focus on Flow Cytometry and Spatial Biology.
Flow Cytometry is a method to detect and measure physical and chemical characteristics of cells or particles. With our CyTOF technology, we focus in a smaller sub-segment of Flow Cytometry for high-parameter analysis defined as greater than 20 parameters.
Spatial Biology, which itself is a sub-segment of the broader Tissue Image Analysis market that includes immunohistochemistry and in-situ hybridization, is the study of single cells in its spatial context to understand the role of heterogeneity in cell function and assess complex phenotypes and tumor-immune interactions in the tissue and tumor microenvironment.
3
Genomics
The market for genomics is broadly defined as instruments, consumables and reagents, software, and services for all technologies used in the identification of genes (DNA, RNA) and their function. The hereditary material or nucleic acid of an organism is often referred to as its genome, the protein-encoding regions of which are commonly known as genes. Analysis of variations in genomes, genes and gene activity in and between organisms can provide insights into their health and functioning.
Within the genomics market, we focus on two sub-segments: qPCR analysis and NGS library preparation.
There are several forms of genetic analysis in use today, including genotyping, gene expression analysis and NGS:
OEM Markets
We also utilize our proprietary microfluidics technology to collaborate with OEM providers to pursue market opportunities outside our core markets. These OEM markets are highly varied, and we believe represent significant expansion opportunities for our technology.
Products
We market life science tools, including preparatory and analytical instruments, consumables, and software for single cell proteomics analysis via mass cytometry and tissue imaging, and protein detection and analysis technologies and services for genomics analysis via real-time PCR and NGS library preparation.
4
Throughout 2022 and 2023, we have greatly enhanced our proteomics offerings through continuous quality improvements and targeting of our flagship flow cytometry instrument, the CyTOF XT™ System, and the commercial release of our third-generation imaging solution for spatial biology, the Hyperion XTi™ Imaging System.
We have begun to more clearly distinguish the technological and workflow advantages of our flow cytometry solution. The CyTOF XT is the only technology that can do a high number of both extra cellular markers and intra cellular markers. The ability to do both at the same time allows our customers to gain biological insights that would otherwise go unnoticed using competing technologies.
Since the commercial launch of the Hyperion XTi™ Imaging System in the second quarter of 2023, the system’s market-leading data quality and throughput continue to be very well-received, and we believe early customer feedback suggests that it is the strongest contender in the emerging field of spatial biology for translational research.
In addition, at the end of 2022, we completed the strategic repositioning of our microfluidics, or genomics, business, consolidating the portfolio to a single commercially available instrument, the Biomark X9, which combines the functionality of the Juno and the Biomark HD with improved workflow and performance. The Juno and Biomark HD are still available to legacy customers under certain circumstances. We continued to deliver the Signature Q100 microfluidics platform to our OEM collaborator, Olink Holding AB.
As discussed above, on January 5, 2024, we completed the Merger with SomaLogic, a global leader in proteomics technology. With a single 55 microliter plasma or serum sample, SomaLogic can run 11,000 protein measurements, covering more than a third of the approximately 20,000 proteins in the human body and twice as many as other proteomic platforms. For more than 20 years, SomaLogic has supported pharmaceutical companies, and academic and contract research organizations who rely on SomaLogic’s protein detection and analysis technologies to fuel drug, disease and treatment discoveries in such areas as oncology, diabetes, and cardiovascular, liver and metabolic diseases.
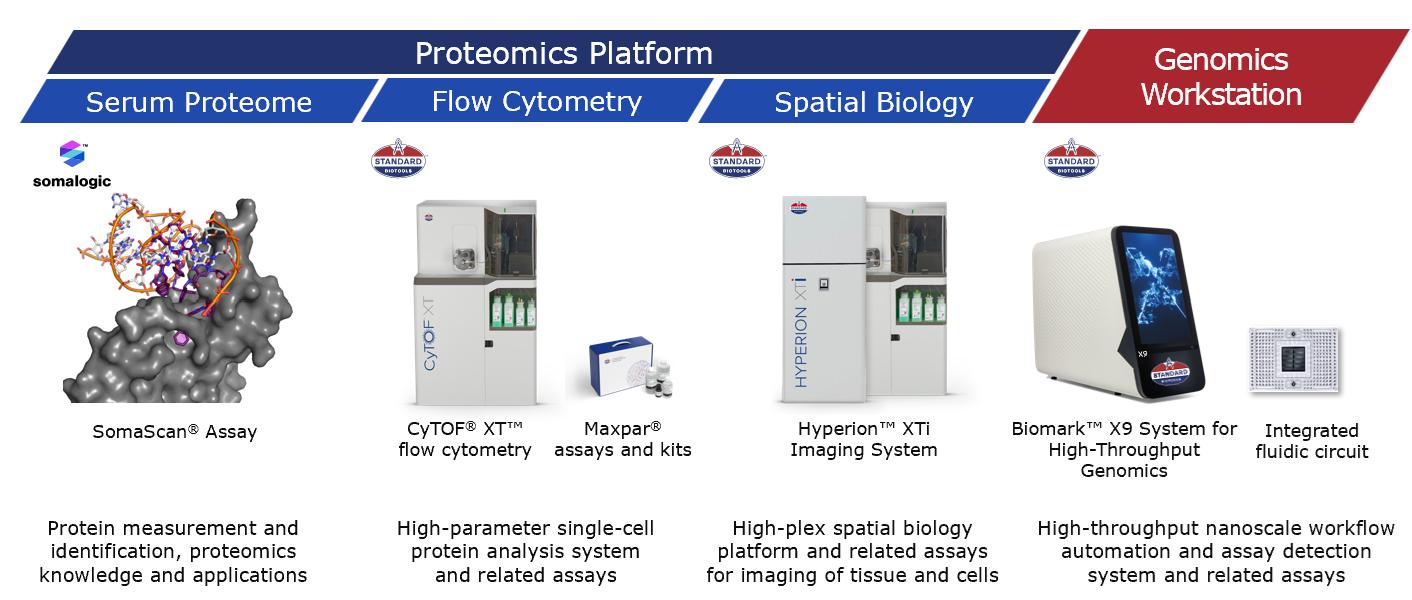
Standard BioTools offers a comprehensive suite of multi-omics technologies.
Our primary instrument offerings are summarized in the table below:
Product |
|
Product Description |
|
Applications |
Single Cell Proteomics |
|
|
|
|
|
|
|
|
|
Analytical Systems: |
|
|
|
|
|
|
|
|
|
CyTOF XT™ System |
|
The CyTOF XT mass cytometry system performs highly automated high-parameter (>50) single-cell analysis using antibodies conjugated to metal isotopes. |
|
Flow Cytometry |
|
|
|
|
|
Hyperion XTi™ Imaging System |
|
Our third-generation spatial biology instrument, the Hyperion XTi™ Imaging System brings together |
|
Tissue Imaging |
5
Product |
|
Product Description |
|
Applications |
|
|
imaging capability with proven high-parameter mass cytometry technology to enable the simultaneous detection of up to 40 protein markers in the spatial context of the tissue microenvironment. |
|
|
|
|
|
|
|
Genomics |
|
|
|
|
|
|
|
|
|
Analytical and Preparatory Instruments: |
|
|
||
|
|
|
|
|
X9TM Real-Time PCR System |
|
Real-time PCR analytical instrument, including pre-processing steps for microfluidics-based workflows using IFCs. |
|
Real-time PCR analysis |
|
|
|
|
|
BiomarkTM HD System |
|
Real-time PCR analytical instrument for microfluidics-based workflows using prepared IFCs. |
|
Real-time PCR analysis |
|
|
|
|
|
IFC Controllers (HX, MX, and RX) |
|
Each controller is designed to work with specific IFC formats: (i) IFC Controller MX- for priming and loading the 48.48 Dynamic ArrayTM IFC, the 12.765 Digital ArrayTM, IFC, the 48.770 Digital Array IFC, and qdPCR 37KTM , (ii) IFC Controller HX- for priming and loading the Flex SixTM Gene Expression IFC and Flex Six Genotyping IFC, 96.96 Dynamic Array IFC, (iii) IFC Controller RX- for loading the 192.24 Gene Expression IFC, 192.24 Genotyping IFC, and the 24.192 Dynamic Array IFC for gene expression. |
|
Real-time PCR analysis |
|
|
|
|
|
Integrated Fluidic Circuits
Our IFCs incorporate several different types of technology that together enable us to use multi-layer soft lithography (MSL) technology to rapidly design and deploy new microfluidic applications with state-of-the-art commercial manufacturing processes. The first level of our IFC technology is a library of components that perform basic microfluidic functions, such as pumps, mixers, single-cell capture chambers, separation columns, control logic, and reaction chambers. The second level of our IFC technology comprises the architectures we have designed to exploit our ability to conduct thousands of reactions on a single IFC. The third level of our IFC technology involves the interaction of our IFCs with the actual laboratory environment.
Instrumentation and Software
Our mass cytometry instrumentation technology includes a custom-designed inductively coupled plasma ion source, ion-optical and vacuum systems, and instrument control electronics. With our CyTOF systems, individual cells are atomized, ionized, and extracted. A time-of-flight mass analyzer separates atomic ions of different mass-to-charge ratios, providing information on temporal distribution of ions. Our Imaging Mass Cytometry systems combine mass cytometry technology with imaging capability to enable simultaneous interrogation of up to 50 protein markers in the spatial context of the tissue microenvironment. Our systems have the ability to utilize up to 135 channels to detect additional parameters to meet future market needs.
Our microfluidics-based X9 Real-Time PCR system includes our custom thermal cycler, a sophisticated fluorescence imaging system, and on-board scripting and protocol control software, and utilizes our IFC technology for a wide range automated genomics applications.
We also offer specialized software to manage and analyze the unusually large amounts of data produced by our systems. We offer Cytobank, our cloud-based platform of analytical tools, FCS Express7 Flow, and Maxpar Pathsetter data analysis packages for use with the CyTOF systems. For our Imaging Mass Cytometry platform, Hyperion, we offer various state of the art software packages to enable data analysis from basic to translational research: CyTOF Software 7.0, MCD Viewer, histoCAT, Visiopharm Phenomap and Indica Lab Halo. Our bioinformatic toolset, the Singular software, facilitates the analysis and visualization of single-cell gene expression data. More recently, we extended the scope of the toolset to include DNA analysis tools.
6
Assays and Reagents
We manufacture over 800 metal-conjugated antibodies for use with our mass cytometry and Imaging Mass Cytometry instruments to allow detection of up to 48 protein targets simultaneously in a single cell for a total of more than 50 detected cellular parameters. Our metal-conjugated antibodies are manufactured using metal-chelating polymers, which are produced using proprietary polymerization processes and subsequent post-polymerization modifications.
Our genotyping and single nucleotide polymorphism type (SNP Type) assay products consist of assay design and custom content delivery systems for gene expression and genotyping, respectively. These offerings provide low-cost alternatives to other available chemistries and allow customers to use IFCs in more flexible ways with validated assays for their targets of interest.
SomaLogic® Offerings and Applications
Effective with the closing of our Merger on January 5, 2024, our SomaLogic offering consists primarily of a service model whereby we receive samples (including from pharmaceutical, biotechnology or academic clients), perform the SomaScan® assay, and subsequently use bioinformatics and analytics to further refine the collected data and deliver the results back to the customer.
SomaScan® Assay
As of January 5, 2024, the SomaScan® assay measures approximately 11,000 protein targets in a single sample. The SomaScan® assay is designed to have breadth (or number of proteins measured), precision, specificity, dynamic range, depth (or lower limits of detection), and throughput. SomaScan® assay customers can also gain access to individual SOMAmer® reagents for a wide range of follow-up studies, which is a feature we consider to be a unique addition to our research services in comparison to the offerings of other proteomic platforms. The SomaScan 11K Platform is the largest proteomics offering available on the market.
SomaScan® Certified Sites
The SomaScan Certified Sites program allows global pharmaceutical and biotech companies, academic and core labs, and government research institutions to run our industry leading proteomics platform on site with the same precision, robustness, and reproducibility seen in our assay services facility. Certified Sites run the assay on the same equipment as in the SomaLogic Assay Service facility utilizing a reagent based kit. As of January 4, 2024, we had 17 Certified Sites, utilizing the SomaScan Assay kits.
SomaSignal™ Tests
As of January 5, 2024, we had 15 SomaSignal™ tests currently available for use as laboratory developed tests (LDTs) under our CLIA certification, with several more in various stages of development. As of January 5, 2024, we had 29 RUO tests primarily targeting clinical trial applications, such as characterizing and monitoring patients through the clinical trial cycle. We believe our SomaSignal™ tests will provide health systems and national health services with a leading-edge scientific tool set to allocate resources, risk stratify both populations and individual patients and personalize therapy.
SOMAmer® Reagents
Customers have been granted licenses to use a limited subset of our SOMAmer® reagents and to access to our Systematic Evolution of Ligands by Exponential enrichment (“SELEX”) technology in three ways: (i) through the transfer of individual reagents that have been developed by SomaLogic, (ii) through the transfer of custom reagents developed by SomaLogic on behalf of a customer, and (iii) through the development of custom reagents by a customer after receiving a license to perform the SELEX process and to use the subsequently developed reagents. An example of the commercial use of SOMAmer® reagents by a licensee of SomaLogic is the inclusion of SOMAmer® reagent-based inhibitors of thermophilic enzymes, such as polymerases used in a product category offered by certain biotechnology companies referred to as “hot-start” PCR amplification.
7
Customers
We sell our instruments and consumables for research use only (RUO) to leading academic research institutions, translational research and medicine centers, cancer centers, clinical research laboratories, and biopharmaceutical, biotechnology, and plant and animal research companies. One genomics customer accounted for 10% and 11% of our total revenue for the years ended December 31, 2023 and 2022, respectively.
Marketing, Sales, Service and Support
We distribute our systems through our direct sales force and support organizations located in North America, Europe, and Asia-Pacific, and through distributors or sales agents in European, Latin American, Middle Eastern, and Asia-Pacific countries. Our sales and marketing efforts are targeted at laboratory directors and principal investigators at leading academic, translational research, healthcare consortiums, and biopharmaceutical companies who need reliable life science automation solutions to power their disease research with the goal of providing actionable insights.
Our sales process often involves numerous interactions and demonstrations with multiple people within an organization. Some potential customers conduct in-depth evaluations of the system, including running experiments on our system and competing systems. In addition, in most countries, sales to academic or governmental institutions require participation in a tender process involving preparation of extensive documentation and a lengthy review process. As a result of these factors and the budget cycles of our customers, our sales cycle, the time from initial contact with a customer to our receipt of a purchase order, can often be 12 months or longer.
Manufacturing
Our manufacturing operations are located in Singapore and Canada. Our facility in Singapore manufactures IFCs and assemblies of microfluidics instruments. In 2022, assembly of microfluidics instruments was insourced to our Singapore facility to reduce cost and improve product quality. All of our IFCs for commercial sale and some IFCs for our research and development purposes are also fabricated at our Singapore facility. Our mass cytometry instruments and reagents for commercial sale, as well as for internal research and development purposes, are manufactured at our facility in Canada. Our genomics reagent manufacturing was transferred from South San Francisco to Markham, Canada in late 2022.
Additionally, in connection with the Merger we acquired additional manufacturing operations in Boulder, Colorado. Our facility in Boulder manufactures reagents, SomaScan® assay kits, and other consumables used to run SomaScan® assays.
We rely on a limited number of suppliers for certain components and materials used in our products. Key components in Standard BioTools legacy products and SomaLogic products are supplied by sole or limited source suppliers. The loss of a single or sole source supplier would require significant time and effort to locate and qualify an alternative source of supply, if at all, and could adversely impact our business. For additional information, please refer to “Item 1A. Risk Factors.”
Laboratory Operations
We perform all of our SomaSignalTM tests in our laboratory facility located in Boulder, Colorado. Our laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists (CAP). Our laboratory is certified for performance of high-complexity testing by the Centers for Medicare & Medicaid Services (CMS) in accordance with the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and is licensed by certain other states requiring out-of-state licensure including California, Maryland, Pennsylvania and Rhode Island.
We believe that our existing laboratory facility is adequate to meet our business needs for at least the next 12 months and that additional laboratory space will be available on commercially reasonable terms, if required.
Quality Assurance
Our quality assurance function oversees the quality of our laboratory operations. We have established oversight for systems implementation and maintenance procedures, document control processes, supplier qualification, preventive or corrective actions and employee training processes that we believe achieves excellence in operations. We continuously monitor and improve our processes and procedures and believe this high-quality service leads to customer satisfaction and retention.
8
Research and Development
We have assembled experienced research and development teams at our South San Francisco, California, Markham, Ontario, Canada, and Singapore locations and have the scientific, engineering, software, bioinformatic, and process talent that we believe is required to grow our business.
The largest components of our current research and development efforts are in the areas of new products, new applications and new content. We launched our Hyperion Imaging System in October 2017. The Hyperion Imaging System provides spatial resolution of protein expression in complex tissue samples at the single-cell level, quantitative measurement using metal isotope tags, and analysis of up to 40 proteins, while having 135 channels available. We also developed metal-labeled antibodies compatible with formalin fixed paraffin embedded tissue samples, to be used with the Hyperion Imaging System. In 2022 we launched the Hyperion+ Imaging System, our second-generation imaging platform.
In 2019, we launched the Maxpar Direct Immune Profiling Assay, a sample-to-answer workflow for comprehensive human immune profiling for use with our CyTOF systems, which puts pre-titrated antibodies in dry format in a single tube, with automated software that provides data analysis in as few as five minutes. This assay is reproducible from site-to-site and lot-to-lot, which is important for translational and pharma/biotech research work. We have collaborated with industry partners to enable workflows and software for the Hyperion and CyTOF systems. Also in 2019, we added seven new metal antibody labels, becoming the first company to enable 50-plex cytometry panels, and launched three Imaging Mass Cytometry panel kits as well as CyTOF Software v7.0, an updated CyTOF software application.
In May 2021, we launched the new, fourth generation cell suspension mass cytometry system, CyTOF XT. Its main features include automation of sample introduction and acquisition, and lower cost of ownership and enhanced performance in resolution of cell populations. The system enables storage of pelleted samples in the cooled autosampler, automated resuspension of pellets, and addition of beads standards.
We also invest significantly in research and development efforts to expand our microfluidics applications. For example, we continue to develop and commercialize various panel sets for use with our systems. In 2017, we successfully launched the Advanta™ Immuno-Oncology Gene Expression Assay, which is a 170-gene expression qPCR assay that enables profiling of tumor immunobiology and new biomarker identification. In 2019, we launched the Advanta™ RNA-Seq NGS Library Prep Kit. Designed to drive significant improvement in the RNA-seq workflow, the Advanta RNA-Seq NGS Library Prep Kit together with the Juno™ system delivers an integrated solution for automated, cost-efficient NGS library prep. In 2020, we expanded our microfluidics franchise to develop products for the COVID-19 testing marketplace and we launched the AdvantaDx SARS-CoV-2 RT-PCR assay. These COVID-19 related products were discontinued in 2022.
In 2022, we launched the X9 Real Time PCR System: a next-generation system platform that integrates all the features of all our legacy platforms into one ultra-compact footprint with a simple-to-operate user interface. In addition, we secured significant development collaborations, including for development of OEM systems using our microfluidics technology.
The second component of our research and development effort is to continuously develop new manufacturing processes and test methods to drive down manufacturing costs, increase manufacturing throughput, widen fabrication process capability, and support new microfluidic devices and designs.
Additionally, in connection with the Merger we acquired research and development facilities in Boulder, Colorado and La Jolla, California. These facilities are focused on the discovery of protein biomarkers and the development of slow off-rate modified aptamers (SOMAmers®), which are modified nucleic acid-based protein binding reagents that are specific for their cognate proteins. The facility in La Jolla is specifically focused on creating small-mid plex solutions for SOMAmer® technology that will enable SomaLogic products to serve additional market segments.
We expect some synergies to be achieved in research and development activities as the combined company shares certain resources; however, the research and development efforts of SomaLogic and Standard BioTools will continue to operate independently for the foreseeable future, with primarily the same teams that were in place prior to the Merger.
Competition
The life science markets are highly competitive and expected to grow more competitive with the increasing knowledge gained from ongoing research and development. We believe that the principal competitive factors in our target markets include quality of product, cost of capital equipment and supplies; reputation among customers; innovation in product offerings; flexibility and ease of use; accuracy
9
and reproducibility of results; competition for human resources; and compatibility with existing laboratory processes, tools, and methods.
We compete with both established and development stage life science companies that design, manufacture, and market instruments for gene expression analysis, genotyping, other nucleic acid detection, protein expression analysis, imaging, and additional applications. In addition, a number of other companies and academic groups are in the process of developing novel technologies for life science markets. Many of our competitors enjoy several competitive advantages over us, including significantly greater name recognition; greater financial and human resources; broader product lines and product packages; larger sales forces and e-commerce channels; larger and more geographically dispersed customer support organizations; substantial intellectual property portfolios; larger and more established customer bases and relationships; greater resources dedicated to marketing efforts; better established and larger scale manufacturing capability; and greater resources and longer experience in research and development. For additional information, please refer to “Item 1A. Risk Factors.”
To successfully compete with existing products and future technologies, we need to demonstrate to potential customers that the performance of our technologies and products, the solutions we provide our customers, as well as our customer support capabilities, are superior to those of our competitors.
Intellectual Property
Patents
We have developed a portfolio of issued patents and patent applications directed towards commercial products and technologies in development. As of December 31, 2023, we owned or licensed approximately 400 patents and had over 175 pending patent applications worldwide. Our utility patents have expiration dates ranging up to year 2039, and our design patents have expiration dates ranging up to year 2047.
As of December 31, 2023, SomaLogic owned or licensed more than 660 patents and had approximately 435 pending patent applications worldwide. SomaLogic's utility patents have expiration dates ranging up to year 2043.
License Agreements
We have entered into licenses for technologies from various companies and academic institutions.
Genomics Technologies. Our core genomics technology originated at the California Institute of Technology (Caltech) in the laboratory of Professor Stephen Quake, who is a co-founder of Fluidigm (now Standard BioTools Inc.). We license genomics technology from Caltech, Harvard University, and Caliper Life Sciences, Inc. (Caliper), now a PerkinElmer company.
Proteomics. Some of the intellectual property rights covering our mass cytometry products were subject to a license agreement (the Original License Agreement) between Fluidigm Canada Inc. (now Standard BioTools Canada Inc.), and PerkinElmer Health Sciences, Inc. (PerkinElmer). Under the Original License Agreement, Fluidigm Canada Inc, received an exclusive, royalty bearing, worldwide license to certain patents owned by PerkinElmer in the field of inductively coupled plasma (ICP)-based proteomics, including the analysis of elemental tagged materials in connection therewith (the Patents), and a non-exclusive license for reagents outside the field of ICP-based mass cytometry. In November 2015, we entered into a patent purchase agreement with PerkinElmer pursuant to which we purchased the Patents for a purchase price of $6.5 million and a patent assignment agreement pursuant to which PerkinElmer transferred and assigned to us all rights, title, privileges, and interest in and to the Patents and the Original License Agreement. Accordingly, we have no further financial obligations to PerkinElmer under the Original License Agreement. Contemporaneously with the purchase of the Patents, we entered into a license agreement with PerkinElmer pursuant to which we granted PerkinElmer a worldwide, non-exclusive, fully paid-up license to the Patents in fields other than (i) ICP-based mass analysis of atomic elements associated with a
10
biological material, including any elements that are unnaturally bound, directly or indirectly, to such biological material (Mass Analysis) and (ii) the development, design, manufacture, and use of equipment or associated reagents for such Mass Analysis. The license will terminate on the last expiration date of the Patents, currently expected to be in November 2026, unless earlier terminated pursuant to the terms of the license agreement.
InstruNor AS. In January 2020, we completed the acquisition of InstruNor AS (InstruNor) for $7.2 million, including $5.2 million in cash and $2.0 million in stock. InstruNor provided automated sample preparation solutions for proteomics and flow cytometry instrument markets and became part of Standard BioTools Inc.’s proteomics business. Included in this acquisition were certain intellectual property portfolio assets comprised of patents and/or patent applications directed to various aspects of automated cell pretreatment instruments. The expiration dates for the issued patents in this patent portfolio extended to March 2033. We recognized a $3.5 million impairment charge on InstruNor’s developed technology intangible asset in the second quarter of 2022 related to our discontinued Flow Conductor product line.
Any loss, termination, or adverse modification of our licensed intellectual property rights could have a material adverse effect on our business, operating results, and financial condition. For additional information, please refer to “Item 1A. Risk Factors.”
Other
In addition to pursuing patents and licenses on key technologies, we have taken steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, OEM counterparties and collaborators and, when needed, our advisers.
Government Regulation
We are subject to a variety of laws and regulations in the United States, the European Union and other countries. The level and scope of the regulation varies depending on the country or defined economic region, but may include, among other things, the research, development, testing, clinical trials, manufacture, storage, recordkeeping, marketing authorization, labeling, safety, efficacy, packaging, advertising, promotion and commercial sales and distribution, of many of our products.
Clinical Laboratory Improvement Amendments of 1988
We are required to hold certain federal, state and local licenses, certifications and permits to operate our clinical laboratory facility in Boulder, Colorado, including the performance of certain diagnostic assays. Under CLIA, we are required to hold a certificate applicable to the categories of laboratory tests we perform and to comply with standards applicable to our operations, including test processes, personnel, facilities administration, equipment maintenance, recordkeeping, quality systems and proficiency testing. We must maintain CLIA certification to be eligible to bill for diagnostic services provided to Medicare beneficiaries. Many commercial third-party payors also require CLIA certification as a condition of payment.
Our Boulder facility holds a current CLIA certificate. To renew our CLIA certificate, we are subject to survey and inspection every two years to assess compliance with program standards. We elect to participate in the accreditation program of CAP. CMS has deemed CAP standards to be equally or more stringent than CLIA regulations and has approved CAP as a recognized accrediting organization. Inspection by CAP is performed in lieu of inspection by CMS for CAP-accredited laboratories. Because we are accredited by the CAP Laboratory Accreditation Program, we are deemed to also comply with CLIA. The regulatory and compliance standards applicable to the testing we perform may change over time, and any such changes could have a material effect on our business.
Penalties for non-compliance with CLIA or CAP requirements include suspension, limitation or revocation of the laboratory’s CLIA or CAP certificate, as well as a directed plan of correction, state on-site monitoring, civil money penalties, civil injunctive suit or criminal penalties, as applicable.
State Laboratory Licensing
Our Boulder facility also holds a state license issued by the Colorado Department of Public Health and Environment (CDPHE). Colorado law and regulations establish standards for the day-to-day operation of a clinical laboratory, including the training and skills required of laboratory personnel and quality control.
Federal Oversight of Laboratory Developed Tests and Certain Devices
11
The laws and regulations governing the marketing of diagnostic products are evolving, extremely complex, and in many instances, there are no significant regulatory or judicial interpretations of these laws and regulations. We perform our diagnostic tests like the SomaSignal™ assays in our Boulder, Colorado CLIA-certified and CAP-accredited clinical laboratory, and we believe such tests are primarily regulated under CLIA, as administered by CMS, as well as by applicable state laws, as described above. The FDA regulates any diagnostic tests that meet the definition of a medical device, except under specific, narrow circumstances. The Federal Food, Drug and Cosmetic Act (FDCA) defines a medical device as "an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory, which is, among other things: intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes." By this definition, in vitro reagents and diagnostic tests are considered medical devices. Specifically, the FDA defines an in vitro diagnostic test (IVD), as "reagents, instruments, and systems intended for use in the diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae." Therefore, the FDA generally considers diagnostic testing products like ours to be IVDs subject to the agency's regulatory requirements.
Among other things, pursuant to the FDCA and its implementing regulations, the FDA regulates the research, testing, manufacturing, safety, labeling, storage, recordkeeping, pre-market clearance or approval, marketing and promotion and sales and distribution of medical devices, including IVDs, in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. In addition, the FDA regulates the export of medical devices manufactured in the United States to international markets. Many of the instruments, reagents, kits or other consumable products used within our laboratory facility are regulated as medical devices and therefore must comply with FDA quality system regulations and certain other device requirements. We have policies and procedures in place to ensure that we source such materials from suppliers that are in compliance with any applicable medical device regulatory requirements.
The FDCA classifies medical devices into one of three categories based on the risks associated with the device and the level of control necessary to provide reasonable assurance of safety and effectiveness. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices or devices deemed not substantially equivalent to a previously 510(k) cleared device, are categorized as class III. These devices typically require submission and approval of a premarket approval application (PMA). Devices deemed to pose lower risk are categorized as either class I or II. For most class II devices, a manufacturer must submit to the FDA a 510(k) premarket notification submission requesting clearance of the device for commercial distribution in the United States. However, some low-risk class II devices are exempted from this requirement. When a 510(k) premarket notification submission is required, the manufacturer must submit to the FDA a premarket notification submission demonstrating that the device is "substantially equivalent" to a predicate device, which is: (i) a device that was legally marketed prior to May 28, 1976, for which PMA approval is not required, (ii) a legally marketed device that has been reclassified from class III to class II or class I, or (iii) another legally marketed, similar device that has been cleared through the 510(k) clearance process. Class II devices may also be subject to special controls such as performance standards, post-market surveillance, FDA guidelines, or particularized labeling. Most class I devices are exempt from 510(k) premarket notification requirements, but like class II and III devices, are subject to general controls, such as registration and listing, quality system, labeling, and reporting requirements.
After the FDA permits a device to enter commercial distribution, numerous regulatory requirements apply. These include: the Quality System Regulation, which requires manufacturers to follow elaborate design, testing, control, documentation and other quality assurance procedures during the manufacturing process; labeling regulations; the FDA’s general prohibition against promoting products for unapproved or "off-label" uses; and the medical device reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur. The FDA has broad post-market and regulatory and enforcement powers. Failure to comply with the applicable U.S. medical device regulatory requirements could result in, among other things, warning letters, fines, injunctions, consent decrees, civil penalties, repairs, replacements, refunds, recalls or seizures of products, total or partial suspension of production, the FDA’s refusal to grant future premarket clearances or approvals, withdrawals or suspensions of current product applications, and criminal prosecution.
In February 2022, we were granted an Emergency Use Authorization (EUA) for our the Advanta Dx COVID-19 EASE Assay, which was authorized for the qualitative detection of nucleic acid from SARS-CoV-2 in nasopharyngeal swab, oropharyngeal swab, mid-turbinate nasal swab, and anterior nasal swab specimens from individuals suspected of COVID-19 by their healthcare provider. Subsequently, in February 2023, the FDA granted our request to withdraw the EUA for our Advanta Dx SARS-CoV-2 RT-PCR Assay. We submitted our request to withdraw such EUA as we had discontinued commercial distribution of the product.
Although the FDA has statutory authority to assure that medical devices, including IVDs, are safe and effective for their intended uses, the FDA has generally exercised its enforcement discretion and not enforced applicable device regulations with respect to IVDs that are
12
designed, manufactured and used within a single high-complexity CLIA-certified laboratory. Such tests are referred to as LDTs. We believe that the SomaSignalTMassays we offer for clinical diagnostic use are LDTs, as are our near-term pipeline candidate tests intended for clinical diagnostic use. However, in October 2023, the FDA issued a proposed rule aimed at regulating LDTs under the current medical device framework and proposing to phase out its existing enforcement discretion policy for this category of diagnostic tests; the public comment period ended in early December 2023. The proposal envisions that the LDT enforcement policy phase-out process would occur in gradual stages over a total period of four years, with pre-market approval applications for high-risk tests to be submitted by the 3.5-year mark, although more details are expected to be provided with the upcoming final rule. The likelihood of the FDA finalizing the proposed rule in April 2024 (as is currently projected), as well as potential litigation challenging the agency’s authority to take such action, is uncertain at this time. Affected stakeholders continue to press for a comprehensive legislative solution to create a harmonized paradigm for oversight of LDTs by both the FDA and CMS, instead of implementation of the proposed FDA administrative action, which may be disruptive to the industry and to patient access to certain diagnostic tests.
Even though we presently commercialize some of our SomaSignalTM tests as LDTs, the FDA may disagree that such tests are within the scope of its current enforcement discretion criteria for LDTs, or our SomaSignalTM tests may in the future become subject to more onerous regulation by the FDA. For several years, members of Congress have been working with stakeholders on a possible bill to regulate in vitro clinical tests including LDTs. For example, as drafted and re-introduced for consideration in the 118th Congress, legislation called the Verifying Accurate, Leading-edge IVCT Development (VALID Act), has been garnering bipartisan and bicameral support. The VALID Act would codify into law the term "in vitro clinical test" (IVCT) to create a new medical product category separate from medical devices that includes products currently regulated as IVDs as well as LDTs. The VALID Act would also create a new system for labs and hospitals to use to submit their tests electronically to the FDA for approval, which is aimed at reducing the amount of time it takes for the agency to approve such tests, and establish a new program to expedite the development of diagnostic tests that can be used to address a current unmet need for patients.
It is unclear whether the VALID Act would be passed by Congress in its current form or signed into law by the President. If the FDA finalizes its position on regulation of LDTs through the ongoing notice-and-comment rulemaking process or the VALID Act or other federal legislation is passed reforming the government’s regulation of LDTs, or alternatively, if the FDA disagrees with our assessment that our SomaSignalTMassays intended for clinical diagnostic use fall within the definition of an LDT, we could, for the first time, be subject to enforcement of regulatory requirements such as registration and listing requirements, medical device reporting requirements and quality control requirements. Any new legislation or FDA regulations affecting LDTs may result in increased regulatory burdens on our ability to continue marketing our tests and to develop and introduce new tests in the future. Additionally, if and when the FDA begins to actively enforce its premarket submission regulations with respect to LDTs generally or our SomaSignalTM tests in particular, whether as a result of new legislative authority or culmination of the current notice-and-comment rulemaking process, we may be required to obtain premarket clearance for our SomaSignalTM assays intended for clinical diagnostic use under Section 510(k) of the FDCA or approval of a PMA. The process for submitting a 510(k) premarket notification and receiving FDA clearance usually takes from three to 12 months, but it can take significantly longer and clearance is never guaranteed. The process for submitting and obtaining FDA approval of a PMA generally takes from one to three years or even longer and approval is not guaranteed. PMA approval typically requires extensive clinical data and can be significantly longer, more expensive and more uncertain than the 510(k) clearance process. If premarket review is required for some or all of our tests, the FDA could require that we stop selling our products pending clearance or approval and conduct clinical testing prior to making submissions to the FDA to obtain premarket clearance or approval. The FDA could also require that we label our SomaSignalTM tests as investigational or limit the labeling claims we are permitted to make.
Regulation of Clinical Trials
We may in the future conduct research studies for our SomaSignalTMtests intended for clinical diagnostic use and our other assays in development that involve clinical investigators and human subjects (or stored specimens from human subjects) at sites in the United States. We may need to conduct additional clinical trials for the SomaSignalTM tests for clinical use, as well as other tests we may offer in the future, to drive test adoption in the marketplace and reimbursement. Should we not be able to perform these studies, or should their results not provide clinically meaningful data and value for clinicians, adoption of our tests could be impaired and we may not be able to obtain reimbursement for them.
The conduct of clinical trials is also subject to extensive federal and institutional regulations intended to assure that the data and reported results are credible and accurate and that the rights, safety, and welfare of study participants are protected. Most studies involving human participants must be reviewed and approved by, and conducted under the auspices of, a duly-constituted institutional review board (IRB), which is a multi-disciplinary committee responsible for reviewing and evaluating the risks and benefits of a clinical trial for participating subjects and monitoring the trial on an ongoing basis. Companies sponsoring the clinical trials and investigators also must comply with, as applicable, regulations, guidelines and IRB requirements for obtaining informed consent from the study subjects, following the protocol and investigational plan, adequately monitoring the clinical trial, and timely reporting of adverse events. The sponsoring
13
company or the IRB may suspend or terminate a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk. In addition, trials involving human subjects often require significant time and cash resources to complete and are subject to a high degree of risk, including risks of experiencing delays, failing to complete the trial or obtaining unexpected or negative results.
Laboratory Technology for Research Use Only
Our proteomics, genomics, and analytical instruments, reagents, and other consumables are currently intended for, labeled and sold for research use only (RUO) applications, and we sell them to academic institutions, life sciences and clinical research laboratories that conduct research, and biopharmaceutical and biotechnology companies for non-clinical and non-diagnostic purposes. In addition, the SomaLogic offerings, other than the SomaSignalTM assays intended for clinical diagnostic use, are intended and offered for RUO applications. Such products are not intended or promoted for use in clinical practice in the diagnosis of disease or other conditions. Accordingly, they are not subject to pre- and post-market controls for medical devices by the FDA, with the exception that we must comply with the agency’s regulations relating to the labeling of IVDs intended for RUO applications. In accordance with such regulations, our RUO products are labeled, “For Research Use Only. Not for use in diagnostic procedures.”
The FDA’s final guidance document “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only” (the RUO/IUO Guidance), provides the FDA’s thinking on when IVDs are properly labeled for RUO or for IUO. The RUO/IUO Guidance explains that merely including a labeling statement that the product is for research purposes only will not necessarily render the device exempt from the FDA’s clearance, approval, or other regulatory requirements if the totality of circumstances surrounding the distribution of the product indicate that the manufacturer knows its product is being used by customers for clinical diagnostic uses or that the manufacturer intends such uses. These circumstances may include, among other things, written or verbal marketing claims regarding a product’s performance in clinical diagnostic applications, a manufacturer’s provision of technical support for clinical validation or clinical applications of the product, or solicitation of business from clinical laboratories, all of which FDA may consider evidence of intended uses that conflict with RUO/IUO labeling. In the future, certain of our products or related applications could become subject to regulation as medical devices by the FDA. If we are required to submit our products for pre-market review by the FDA, we may be required to delay marketing and commercialization while we obtain pre-market clearance or approval from the FDA. There would be no assurance that we could ever obtain such clearance or approval.
In some cases, our customers may, on their own initiative and without consulting us, use our RUO-labeled products in their own LDTs or in other FDA-regulated products for clinical diagnostic use.
Advertising of Laboratory Technologies and Services
Whether our proteomics or genomics technologies or our laboratory assays are not regulated by FDA, regulated as class I or class II devices, or subject to enforcement discretion with respect to FDA’s device requirements, advertising for such services and products is subject to federal truth-in-advertising laws enforced by the Federal Trade Commission (FTC), as well as comparable state consumer protection laws. Under the Federal Trade Commission Act (FTC Act), the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution.
Federal and State Anti-Kickback Laws
The Federal Anti-Kickback Statute makes it a felony for a person or entity, including a clinical laboratory, to knowingly and willfully offer, pay, solicit or receive any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, in order to induce or in return for the referral of an individual for the furnishing of, or the recommending or arranging for the furnishing of, purchasing, leasing, ordering or arranging for or recommending purchasing, leasing or ordering of any item or service that is reimbursable in whole or in part, under any federal healthcare program. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Courts have broadly interpreted the scope of the Anti-Kickback Statute and generally have held that the statute may be violated if merely one purpose of a payment arrangement is to induce referrals.
14
In addition to statutory exceptions to the Anti-Kickback Statute, regulations provide for a number of safe harbors. If an arrangement meets the provisions of a safe harbor or exception, it is deemed not to violate the Anti-Kickback Statute, and the parties are immune from prosecution. An arrangement must fully comply with each element of an applicable safe harbor in order to qualify for protection.
Failure to meet the requirements of an exception or a safe harbor does not render an arrangement illegal. Rather, the government may evaluate such arrangements on a case-by-case basis, taking into account all facts and circumstances.
A violation of the Anti-Kickback Statute may result in imprisonment for up to ten years and significant fines for each violation and additional administrative civil money penalties, plus up to three times the amount of the remuneration paid. Convictions under the Anti-Kickback Statute result in mandatory exclusion from federal healthcare programs for a minimum of five years. In addition, a violation of the Anti-Kickback Statute can serve as the basis of liability under the federal False Claims Act, which is discussed in greater detail below.
Although the Anti-Kickback Statute applies only to items and services reimbursable under any federal healthcare program, a number of states, including California, have passed statutes substantially similar to the Anti-Kickback Statute that apply to all third-party payors, including commercial insurers, and, in some states, to patients without insurance. The California Attorney General and courts have interpreted the California anti-kickback and fee-splitting laws in substantially the same way as the courts have interpreted the Anti-Kickback Statute. Penalties under such state laws include imprisonment and significant monetary fines.
In addition, in October 2018, the Eliminating Kickbacks in Recovery Act of 2018 (EKRA) was enacted as part of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act. EKRA is an all-payor anti-kickback law that makes it a criminal offense to pay any remuneration to induce referrals to, or in exchange for, patients using the services of a recovery home, a substance use clinical treatment facility, or laboratory. However, unlike the Anti-Kickback Statute, EKRA is not limited to services covered by federal healthcare programs but applies more broadly to services covered by “healthcare benefit programs,” including commercial third-party payors. Although EKRA apparently was intended to reach patient brokering and similar arrangements to induce patronage of substance use recovery and treatment, the language in EKRA is broadly written. Further, certain of EKRA’s exceptions are inconsistent with the Anti-Kickback Statute and regulations. EKRA permits the U.S. Department of Justice to issue regulations clarifying EKRA’s exceptions or adding additional exceptions, but such regulations have not yet been issued.
Other Federal and State Healthcare Laws
In addition to the requirements discussed above, several other healthcare fraud and abuse laws could have an effect on our business. For example, federal law permits the Office of Inspector General for the Department of Health and Human Services (HHS-OIG) to exclude an individual or entity from Medicare or Medicaid for charging federal healthcare programs, including Medicare or Medicaid, substantially in excess of its usual charges for its items or services absent a finding of good cause. The terms “usual charge” and “substantially in excess” are subject to varying interpretations, and the HHS OIG has withdrawn multiple versions of a proposed rule intended to implement the statute.
The federal False Claims Act prohibits, among other things, a person from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to the federal government. In addition to actions initiated by the government itself, the statute authorizes actions to be brought on behalf of the federal government by a private party having knowledge of the alleged fraud pursuant to its qui tam provisions. Because the complaint in a qui tam action is initially filed under seal, the action may be pending for some time before the defendant is even aware of the action. Regardless of whether the government intervenes in the action, the relator, if successful, will receive a percentage of the recovery. In addition, providers and suppliers must report and return any overpayments received from the Medicare and Medicaid programs within 60 days of identification, and failure to identify and return such overpayments exposes the provider or supplier to federal False Claims Act liability. Violation of the federal False Claims Act may result payment of up to three times the actual damages sustained by the government, plus significant per-claim civil penalties, as well as mandatory exclusion from government healthcare programs. Several states, including California, have enacted comparable false claims laws that may apply regardless of payor.
The federal civil monetary penalties law (the CMP Law) prohibits, among other things, (1) the offering or transfer of remuneration (including a waiver of copayments and deductible amounts) to a Medicare or Medicaid beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or Medicaid, unless an exception applies; (2) employing or contracting with an individual or entity that the provider knows or should know is excluded from participation in a federal healthcare program; (3) billing for services requested by an unlicensed physician or an excluded provider; (4) billing for medically unnecessary services; and (5) presenting or causing to be presented a claim to a federal healthcare program that the person knows or should know is for an item or service that was not provided as claimed or is false or
15
fraudulent. The penalties for violating the CMP Law may include exclusion, substantial fines, and payment of up to three times the amount billed, depending on the nature of the offense.
Federal criminal statutes prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including those administered by commercial payors, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the Anti-Kickback Statute, this federal criminal statute requires a showing of intent, but a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
The Physician Payments Sunshine Act imposes annual reporting requirements on manufacturers of certain devices, drugs and biologics for certain payments and transfers of value by them and in some cases their distributors to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other healthcare providers (such as nurse practitioners), and teaching hospitals, as well as ownership and investment interests held by physicians (as defined under the statute) and their immediate family members. It applies to manufacturers when their products become eligible for reimbursement under a federal healthcare program such as Medicare or Medicaid. Any failure to comply with these reporting requirements could result in significant fines and penalties. Because we manufacture our own IVD products solely for use by or within our Boulder laboratory facility, we believe that we are exempt from these reporting requirements. We may become subject to such reporting requirements under the terms of current CMS regulations, however, if enacted federal legislation renders our tests regulated by FDA, or if FDA finalizes its recently initiated notice-and-comment rulemaking to exercise authority over LDTs as medical devices or otherwise requires us to obtain premarket clearance or approval for one or more of our tests. A determination that we have violated these laws and regulations, or a public announcement that we are being investigated for possible violations, could adversely affect our business, prospects, results of operations or financial condition.
We are also subject to applicable state restrictions on laboratory billing. These laws vary from state to state but generally are intended to prevent a provider who ordered but did not perform the service from billing for that service at a markup. For example, California has an anti-markup statute with which we must comply, which prohibits a provider from charging for any laboratory test that it did not perform unless the provider (a) notifies the patient, client or customer of the name, address and charges of the laboratory performing the test, and (b) charges no more than what the provider was charged by the clinical laboratory that performed the test except for any other service actually rendered to the patient by the provider (for example, specimen collection, processing and handling). This provision applies, with certain limited exceptions, to licensed persons such as physicians and clinical laboratories regulated under California’s Business and Professions Code. A violation of this provision can lead to imprisonment and/or a fine of up to $10,000. Other states have similar anti-markup and other client billing restrictions with which we must comply. Many states also have "direct-bill" laws, which require the party that performed the service to bill for the service, with certain exceptions.
If our operations are found to be in violation of any of the fraud and abuse laws described above or any other healthcare regulatory laws that apply to us, we may be subject to penalties, including potentially significant criminal and civil and/or administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
U.S. Data Privacy and Security Laws
The Health Insurance Portability and Accountability Act of 1996 (HIPAA) established comprehensive federal standards for the privacy and security of health information. In 2009, Congress enacted Subtitle D of the Health Information Technology for Economic and Clinical Health Act (HITECH) provisions of the American Recovery and Reinvestment Act of 2009. HITECH amended HIPAA and, among other things, expanded and strengthened HIPAA, created new targets for enforcement, imposed new penalties for noncompliance and established new breach notification requirements. HIPAA applies to health plans, healthcare clearing houses and healthcare providers that conduct certain healthcare transactions electronically (collectively, Covered Entities), as well as individuals or entities that perform services for them involving the use, or disclosure of, individually identifiable health information or "protected health information" (PHI) under HIPAA (Business Associates). Under HIPAA, as amended by the HITECH Act, the U.S. Department of Health and Human Services (HHS) has issued regulations to protect the privacy and security of PHI used or disclosed by Covered Entities and Business Associates. HIPAA also regulates and standardizes the codes, formats and identifiers used in certain healthcare transactions and standardization of identifiers for health plans and providers, for example insurance billing. Any non-compliance with HIPAA and HITECH and related penalties, could adversely impact our business.
The HIPAA security standards require the adoption of administrative, physical and technical safeguards and the adoption of written security policies and procedures to maintain the security of protected health information.
16
The HIPAA privacy regulations address the privacy of PHI by limiting the use and release of such information. They also set forth certain rights that an individual has with respect to his or her PHI maintained by a Covered Entity, including the right to access or amend certain records containing PHI, request an accounting of disclosures of PHI or to request restrictions on the use or disclosure of PHI. The HIPAA breach notification regulations impose certain reporting requirements on Covered Entities and their Business Associates in the event of a breach of PHI.
Covered Entities must report breaches of PHI that has not been encrypted or otherwise secured in accordance with guidance from the Secretary of HHS (the Secretary). Breaches must be reported as soon as reasonably practicable, but no later than 60 days following discovery of the breach. Reports must be made to affected individuals, the HHS Secretary, and depending on the size of the breach, the local and national media. Covered Entities are also subject to the HHS HIPAA audit program and may be investigated in connection with a privacy or data security complaint.
Significant civil and criminal fines and other penalties may be imposed for violating HIPAA directly, and in connection with acts or omissions of any agents, including downstream business associates, as determined according to the federal common law of agency. Civil penalties are adjusted for inflation on an annual basis and can exceed $1.0 million per year for failure to comply with a HIPAA requirement. A single breach incident can violate multiple requirements. Additionally, a person who knowingly obtains or discloses PHI in violation of HIPAA may face criminal penalties (including fines and imprisonment), which increase if the wrongful conduct involves false pretenses or the intent to sell, transfer or use PHI for commercial advantage, personal gain or malicious harm. Covered Entities are also subject to enforcement by state Attorneys General who were given authority to enforce HIPAA.
Additionally, while HIPAA does not create a private right of action allowing individuals to file suit against us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care cases in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
Even when HIPAA does not apply, according to the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the FTC Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Individually identifiable health information is considered sensitive data that merits stronger safeguards. The FTC and state Attorneys General have also brought enforcement actions and prosecuted some data breach cases as unfair and/or deceptive acts or practices under the FTC Act and comparable state laws.
The HIPAA privacy and security regulations establish a uniform federal "floor" and do not preempt state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing PHI or insofar as such state laws apply to personal information that is broader in scope than PHI. Certain state laws govern the privacy and security of health-related and other personal information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. The State of California, for example, has implemented comprehensive laws and regulations. The California Confidentiality of Medical Information Act (CMIA), imposes restrictive requirements regulating the use and disclosure of health information and other personally identifiable information. California has also recently adopted the California Consumer Privacy Act of 2018 (CCPA), which went into effect January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information. It also creates individual privacy rights for California consumers and increases the privacy and security obligations of entities handling certain personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. Although the law includes limited exceptions, including for PHI maintained by a Covered Entity or Business Associate under HIPAA and medical information maintained by healthcare providers under the CMIA, it may regulate or impact our processing of personal information depending on the context. Further, the California Privacy Rights Act (CPRA) went into effect January 1, 2023 amending and strengthening the CCPA. The CPRA imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data and expands the application of the CCPA to all human resources personal information of our California-based employees. It also created a new California data protection agency authorized to issue substantive regulations and is expected to result in increased privacy and information security enforcement. Various states such as Colorado, Connecticut, Delaware, Florida, Indiana, Iowa, Montana, Oregon, Tennessee, Texas, Utah and Virginia have enacted their own privacy laws similar to the CCPA, and other states are considering proposals for such laws, all of which increases the complexity of compliance and the risk of failures to comply.
17
Numerous other federal and state laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of patient health information. In addition, Congress and some states are considering new laws and regulations that further protect the privacy and security of medical records or medical information. With the recent increase in publicity regarding data breaches resulting in improper dissemination of consumer information, all 50 states have passed laws regulating the actions that a business must take if it experiences a data breach, as defined by state law, including prompt disclosure within a specified amount of time to affected individuals. In addition to data breach notification laws, some states have enacted statutes and rules requiring businesses to reasonably protect certain types of personal information they hold or to otherwise comply with certain specified data security requirements for personal information. Congress has also been considering similar federal legislation relating to data privacy and data protection.
Many states, such as Massachusetts, have also implemented genetic testing and privacy laws imposing specific patient consent requirements and requirements for protecting test results. The interplay of federal and state laws regulating genetic information may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and potentially exposing us to additional expense, adverse publicity and liability. Further, as regulatory focus on genetic privacy issues continues to increase and laws and regulations concerning the protection of personal information expand and become more complex, these potential risks to our business could intensify.
Information Blocking Rules
The Office of the National Coordinator for Health Information Technology (ONC), coordinates the ongoing development of standards to enable interoperable health information technology infrastructure nationwide in the healthcare sector. In May 2020, ONC released the final Information Blocking Rule to implement the interoperability and patient access provisions of the 21st Century Cures Act. We will need to continually review our practices for conduct that could be considered as likely to interfere with access, exchange or use of electronic health information, as those practices are prohibited by the Information Blocking Rule, unless one of the exceptions outlined in the Information Blocking Rule applies. Among other things, the Information Blocking Rule requires us to provide patients with on-demand access to laboratory test results. These requirements can be inconsistent with our obligations as a laboratory under state law and/or medical or ethical standards. It is currently unclear how the ONC will approach delays in providing patient access in these situations. Healthcare providers including laboratories will be subject to civil monetary penalties for violations of the Information Blocking Rule once the penalty regulations are finalized. The amount of such penalties is unknown, but the regulations for health industry networks (HINs), health information exchanges (HIEs), and certified developers of health information technology allow for up to $1.0 million in penalties per violation.
International Laws and Regulations
Many countries in which we may offer any of our testing products in the future have anti-kickback regulations prohibiting providers from offering, paying, soliciting or receiving remuneration, directly or indirectly, in order to induce business that is reimbursable under any national healthcare program. In situations involving physicians employed by state-funded institutions or national healthcare agencies, violation of the local anti-kickback law may also constitute a violation of the U.S. Foreign Corrupt Practices Act (FCPA), and/or other applicable anti-corruption laws.
The FCPA prohibits any U.S. individual, business entity or employee of a U.S. business entity from offering or providing, directly or through a third party, including any potential distributors we may rely on in certain markets, anything of value to a foreign official with corrupt intent to influence an award or continuation of business or to gain an unfair advantage, whether or not such conduct violates local laws. In addition, it is illegal for a company that reports to the SEC to have false or inaccurate books or records or to fail to maintain a system of internal accounting controls. We will also be required to maintain accurate information and control over sales and distributors’ activities that may fall within the purview of the FCPA, including its books and records provisions and its anti-bribery provisions.
The standard of intent and knowledge under the FCPA’s anti-bribery provisions is minimal intent and knowledge are usually inferred from the fact that bribery took place. The FCPA’s accounting provisions do not require intent. Violations of the FCPA’s anti-bribery provisions for corporations and other business entities are subject to a fine of up to $2.0 million and officers, directors, stockholders, employees and agents are subject to a fine of up to $100,000 and imprisonment for up to five years. Other countries, including the United Kingdom and other Organisation for Economic Co-Operation and Development Anti-Bribery Convention members, have similar anti-corruption regulations, such as the U.K. Bribery Act.
18
When marketing our testing products outside of the United States, we may be subject to foreign regulatory requirements governing human clinical testing, prohibitions on the import of tissue necessary for us to perform our testing products or restrictions on the export of tissue imposed by countries outside of the United States or the import of tissue into the United States, and marketing approval. These requirements vary by jurisdiction, differ from those in the United States and may in some cases require us to perform additional pre-clinical or clinical testing. In many countries outside of the United States, coverage, pricing and reimbursement approvals are also required.
European Union IVD Laws and Regulations
Whether or not we are required to comply with requirements for marketing clinical diagnostic products in the United States, we may be required to obtain marketing authorizations from regulatory authorities in non-United States countries prior to the marketing of any product for clinical diagnostic use in such countries. The laws and regulations relating to laboratory equipment, reagents and assays in other jurisdictions vary from those in the United States and may be easier or more difficult to satisfy and are subject to change. For example, in the European Union (EU), IVDs had been regulated under EU-Directive 98/79/EC (IVD Directive) and corresponding national provisions prior to May 2022. The IVD Directive required that medical devices, including IVDs, meet the essential requirements, including those relating to device safety and efficacy, set out in an annex of the Directive. According to the IVD Directive, EU Member States have presumed compliance with these essential requirements for devices that are in conformity with the relevant national standards transposing the harmonized standards, such as ISO 13485:2016, the quality system standard for medical device manufacturers.
IVDs, other than devices for performance evaluation, must bear the CE marking of conformity when they are placed on the European market. The CE mark is a declaration by the manufacturer that the product meets all the appropriate provisions of the applicable legislation implementing the relevant European Directive. As a general rule, the manufacturer must follow the EU declaration of conformity procedure to obtain or apply a CE mark.
In May 2022, the Directive was replaced by the In Vitro Diagnostic Device Regulation (IVDR) (EU) 2017/746 that was published in May 2017 and given a 5-year transition period until its full implementation on May 26, 2022. Unlike the IVD Directive, the IVDR has binding legal force throughout every Member State. The major goal of the IVDR was to standardize diagnostic procedures within the EU, increase reliability of diagnostic analysis and enhance patient safety. Under the IVDR as enacted by the European Commission (EC), IVDs are subject to additional legal regulatory requirements. Among other things, the IVDR introduces a new risk-based classification system and requirements for conformity assessments. Under the IVDR and subsequent amendments, IVDs already certified by a Notified Body under the IVD Directive may remain on the market until May 26, 2025, and IVDs certified without the involvement of a Notified Body may be placed on, or remain in, the market for up to three additional years (until May 26, 2028) depending on the classification of the IVD. The manufacturers of such devices remaining on the market must comply with specific requirements in the IVDR, but ultimately, such products, as with all new IVDs, will have to undergo the IVDR’s conformity assessment procedures. In addition, the IVDR imposes additional requirements relating to post-market surveillance and submission of post-market performance follow-up reports.
The EC has designated 12 Notified Bodies to perform conformity assessments under the IVDR. MedTech Europe has issued guidance relating to the IVDR in several areas, e.g., clinical benefit, technical documentation, state of art, accessories, and EUDAMED. On December 5, 2023, the European Commission adopted Implementing Regulation (EU) 2023/2713 designating five EU Reference Laboratories covering the following types of high risk, class D IVDs: hepatitis and retroviruses; herpesviruses; bacterial agents; respiratory viruses that cause life-threatening diseases. The designated EU Reference Laboratories are responsible for verifying performance of IVDs in accordance with common specifications, batch testing of class D IVDs, collaborating with Notified Bodies to develop best practices for IVD conformity assessments, and providing scientific and technical assistance on the implementation of the IVDR.
International Data Privacy and Security Laws
The collection and use of personal health data in the EU is governed by the General Data Protection Regulation, or GDPR. The GDPR applies to any company established in the European Economic Area, or EEA, (which includes the EU Member States plus Iceland, Liechtenstein, and Norway) and to companies established outside the EEA that process personal data in connection with the offering of goods or services to data subjects in the EEA or the monitoring of the behavior of data subjects in the EEA. The GDPR establishes stringent requirements applicable to the processing of personal data, including strict requirements relating to the validity of consent of data subjects, expanded disclosures about how personal data is used, requirements to conduct data protection impact assessments for “high risk” processing, limitations on retention of personal data, special provisions affording greater protection to and requiring additional compliance measures for “special categories of personal data” including health and genetic information of data subjects,
19
mandatory data breach notification (in certain circumstances), “privacy by design” requirements, and direct obligations on service providers acting as processors. The GDPR also prohibits the international transfer of personal data from the EEA to countries outside of the EEA unless made to a country deemed to have adequate data privacy laws by the European Commission or a data transfer mechanism has been put in place. Failure to comply with the GDPR requirements may subject an entity to litigation, regulatory investigations, enforcement notices and/or fines of up to 20 million Euros or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, as well as compensation claims by affected individuals, negative publicity, reputational harm and a potential loss of business and goodwill.
Among other requirements, the GDPR also regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data. For example, in 2016, the EU and the United States agreed to a transfer framework for data transferred from the EU to the United States, called the Privacy Shield, but the Court of Justice of the EU invalidated the Privacy Shield when it decided the case Maximilian Schrems vs. Facebook (Case C-311-18), known as Schrems II. However, on July 10, 2023, the European Commission adopted an adequacy decision for a new mechanism for transferring data from the EU to the United States – the EU-US Data Privacy Framework, which provides EU individuals with several new rights, including the right to obtain access to their data, or obtain correction or deletion of incorrect or unlawfully handled data. The adequacy decision followed the signing of an executive order introducing new binding safeguards to address the points raised in the Schrems II decision. Notably, the new obligations were geared to ensure that data can be accessed by U.S. intelligence agencies only to the extent necessary and proportionate and to establish an independent and impartial redress mechanism to handle complaints from Europeans concerning the collection of their data for national security purposes. The European Commission will continually review developments in the United States along with its adequacy decision. Adequacy decisions can be adapted or even withdrawn in the event of developments affecting the level of protection in the applicable jurisdiction. Future actions of EU data protection authorities are difficult to predict. Some customers or other service providers may respond to these evolving laws and regulations by asking us to make certain privacy or data-related contractual commitments that we are unable or unwilling to make. This could lead to the loss of current or prospective customers or other business relationships.
Relatedly, following the United Kingdom’s withdrawal from the EU, the GDPR was implemented in the United Kingdom as the U.K. GDPR. which sits alongside the amended U.K. Data Protection Act 2018, which implements certain derogations in the EU GDPR into UK law. Under the U.K. GDPR, companies not established in the United Kingdom but who process personal data in relation to the offering of goods or services to individuals in the United Kingdom, or to monitor their behavior will be subject to the U.K. GDPR – the requirements of which are (at this time) largely aligned with those under the EU GDPR and as such, may lead to similar compliance and operational costs with potential fines of up to £17.5 million or 4% of global turnover. In June of 2021, the European Commission issued a decision, which will sunset on June 27, 2025 without further action, that the United Kingdom ensures an adequate level of protection for personal data transferred under the EU GDPR from the EU to the United Kingdom. The U.K. Parliament is currently considering the Data Protection and Digital Information Bill to harmonize the 2018 Data Protection Act, U.K. GDPR, and the Privacy and Electronic Communications Regulations under one legislative framework.
In China, rules relating to personal data protection and data security are part of a complex framework and are found across various laws and regulations. The three main pillars of the personal data protection framework in China are the Personal Information Protection Law (PIPL), the Cybersecurity Law (CSL) and the Data Security Law (DSL). The CSL, which became effective on June 1, 2017, and the Cybersecurity Review Measures promulgated by the Cyberspace Administration of China (CAC), provide that personal information and important data collected and generated by a critical information infrastructure operator in the course of its operations in mainland China must be stored in mainland China, and if a critical information infrastructure operator purchases internet products and services that affect or may affect national security, it should be subject to national security review by the CAC together with competent departments of the State Council. The DSL came into force on September 1, 2021, and requires that data (not limited to personal data) shall not be collected by theft or other illegal means, and it also provides for a data classification and hierarchical protection system, which protects data according to its importance in economic and social development and the potential damage to national security, public interests, or the legitimate rights and interests of individuals and organizations if the data is falsified, damaged, disclosed, illegally obtained or illegally used. Most significantly, the PIPL came into effect on November 1, 2021. The PIPL is the first comprehensive, national–level personal data protection law in China. The PIPL mirrors certain provisions found under the GDPR such as the purpose limitation principle, the concept of a data protection officer, data subject rights, the requirement to conduct data protection impact assessments, and restrictions on data exports. With respect to data exports, China has adopted its own standard contractual clauses which qualifying businesses can use to legitimize their data exports.
Other countries, such as Brazil and Japan, have enacted or amended omnibus laws, and others, such as Russia, have also passed laws that require personal data relating to their citizens to be maintained in the country under certain circumstances and impose additional data transfer restrictions. In addition, India enacted new privacy legislation, the Digital Personal Data Protection Act, 2023, which applies to the processing of personally identifiable digital data about an individual whether the data is processed in India or outside of the country in connection with the offering of goods or services to data subjects who are residents of India. Complying with these
20
numerous, complex and often changing regulations is expensive and difficult, and failure to comply with any privacy laws or data security laws or any security incident or breach involving the misappropriation, loss or other unauthorized use or disclosure of personal data (including sensitive or confidential patient or consumer information), whether by us or a third-party, could have a material adverse effect on our business, reputation, financial condition and results of operations, including but not limited to: material fines and penalties; damages; litigation; consent orders; extensive audits and inspections; bans on all or some processing of personal data carried out by noncompliant actors; and injunctive relief.
Environmental Matters
We are subject to many federal, state, local, and foreign environmental regulations. To comply with applicable regulations, we have and will continue to incur significant expenses and allocate internal resources to manage compliance-related issues. In addition, such regulations could restrict our ability to expand or equip our facilities or could require us to acquire costly equipment or to incur other significant expenses to comply with the regulations. For example, the Restriction on the Use of Certain Hazardous Substances in Electrical and Electronic Equipment Directive (RoHS), the Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH) and the Waste Electrical and Electronic Equipment Directive (WEEE), enacted in the European Union, regulate the use of certain hazardous substances, notification of customers of the presence of any substances of very high concern in products, and require the collection, reuse, and recycling of waste from, products we manufacture. Certain products sold in these countries are subject to RoHS, REACH and WEEE requirements. If we fail to comply with any present and future regulations, we could be subject to future fines, penalties, and restrictions, such as the suspension of manufacturing of our products or a prohibition on the sale of products we manufacture. For additional information, please refer to “Item 1A. Risk Factors.”
Our research and development and manufacturing processes also involve the controlled use of hazardous materials, including flammables, toxics, corrosives, and biologics. Our research and manufacturing operations produce hazardous biological and chemical waste products. We seek to comply with applicable laws regarding the handling and disposal of such materials. The volume of such materials used or generated at our facilities is small. However, we cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We do not currently maintain separate environmental liability coverage and any such contamination or discharge could result in significant cost to us in penalties, damages, and suspension of our operations.
Geographic Area Information
During the last two years, a significant portion of our revenue was generated outside of the United States. Total revenue received from customers outside the United States was $62.2 million, or 59% of our total revenue, in 2023, compared to $56.9 million, or 58% of our total revenue, in 2022. The majority of our long-lived assets are located within the United States, Singapore and Canada. Refer to Note 3 to our consolidated financial statements for additional information regarding geographic areas.
Seasonality
Our fourth quarter revenues are often the highest, primarily due to seasonality since many of our customers tend to spend budgeted money before the end of their calendar fiscal year-end. Our revenue in the first quarter is generally sequentially lower than the prior year’s revenue in the fourth quarter.
Raw Materials
Certain raw materials used in our Delta Gene and SNP Type assays and Access Array target-specific primers are available from a limited number of sources. Additionally, certain metals used in our Maxpar reagents are available from a sole source. Currently, we do not have supply agreements with these suppliers. While we generally attempt to keep our inventory at minimal levels, we purchase incremental inventory as circumstances warrant to protect our supply chain.
Backlog
We manufacture products based on forecasts of our customers’ demand and advance non-binding commitments from customers as to future purchases. Our customers generally do not place purchase orders far in advance. A substantial portion of our products are sold on the basis of standard purchase orders that are cancellable prior to shipment without penalty. Accordingly, backlog at any given time is not a meaningful indicator of future sales.
21
Human Resource Capital
Our team members share our commitment to improving the human condition and, in turn, Standard BioTools strives to create an environment where our people can do their best work. We know that our employees, who supply the ideas, energy, and innovation that powers our business, are amongst some of Standard BioTools’ valued assets.
We are a values-driven organization. We believe strong shared values are essential for Standard BioTools to evolve and grow and to be successful for the long-term. Our values form our relationships with customers, suppliers, investors and each other. They help us to model respect and inclusiveness in our words and actions. Our core values conceived and developed by our employees are:
A Diverse Global Workforce
As of December 31, 2023, Standard BioTools had a total of 539 employees worldwide of which 534 were full-time employees and 130 were located in the United States. Additionally, 47% of our employees worldwide were female and 35% of our employees in the United States were female as of December 31, 2023. None of our employees are represented by a labor union nor are they subject to a collective bargaining agreement. Subsequent to the Merger with SomaLogic, which was completed on January 5, 2023, we had approximately 928 employees worldwide, with 510 employed in the United States and 418 employed at other non-U.S. locations.
Information About Our Executive Officers and Directors
The following persons were our executive officers and directors as of February 21, 2024:
Name |
Position |
Executive Officers |
|
Michael Egholm, Ph.D. |
President, Chief Executive Officer, and Director |
Jeffrey Black |
Chief Financial Officer |
Hanjoon Alex Kim |
Chief Operating Officer |
Non-Employee Directors |
|
Tom Carey |
Chairperson of the Board of Directors |
Fenel M. Eloi |
Managing Partner of P&M Capital Partners, LLC |
Eli Casdin |
Founder and Chief Investment Officer of Casdin Capital, LLC and its affiliates |
Troy Cox |
Director and Chairperson of the Board of Directors of SOPHiA GENETICS SA, Director and Vice Chairperson of the Board of Directors of LetsGetChecked Inc., and Director at Zymeworks Inc. |
Kathy Hibbs |
Chief Administrative Officer of 23andMe |
Frank Witney, Ph.D. |
Operating Partner at Ampersand Capital Partners |
Compensation and Benefits
The primary goal of our compensation program is to ensure that we attract, hire, and retain talented and highly skilled team members who are motivated to achieve or exceed our corporate goals.
22
We offer competitive total reward packages comprising various elements including market-driven base pay, short- and long-term incentives in the form of performance-based cash and equity, as well as comprehensive health and welfare benefits that include medical, dental, vision, group life, disability, and accidental death and dismemberment insurance, as well as our 401(k) or comparable non-U.S. retirement plans, subject to applicable law. We also provide vacation and other paid holidays to all employees at levels that we believe are comparable to those provided at peer companies.
Our intention is to align our compensation practices with the changing marketplace. By doing so, we strive to provide incentives to our team members to achieve short-term and long-term business goals, ensuring they feel rewarded for their performance and contributions.
Professional Development
In addition to providing attractive and competitive total rewards packages, Standard BioTools believes in fostering individual and organizational effectiveness by offering our team members a variety of professional development programs. These programs are designed to:
Diversity and Inclusion
At Standard BioTools, our commitment to diversity, inclusion and equity is reflective of our values. We believe that we are strongest when we embrace all forms of diversity, and that it is essential to seek out diverse, innovative ideas and foster an inclusive culture where all colleagues are respected and engaged. We endeavor to apply this commitment to diversity to every aspect of the employee experience, from recruitment to development, training and advancement.
Corporate and Available Information
We were incorporated in California in May 1999 as Mycometrix Corporation, changed our name to Fluidigm Corporation in April 2001, and reincorporated in Delaware in July 2007. On April 1, 2022, the Company changed its name from Fluidigm Corporation to Standard BioTools Inc. Our principal executive offices are located at Two Tower Place, South San Francisco, California 94080. Our telephone number is (650) 266-6000. Our website address is www.standardbio.com. We make available on our website, free of charge, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and any amendments to those reports, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission (SEC). Our SEC reports can be accessed through the investor relations page of our website located at http://investors.standardbio.com. The SEC also maintains an internet site at www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
The contents of our website are not a part of, and are not incorporated by reference into, this Annual Report or any other report or document we file with the SEC. Any reference to our website is intended to be an inactive textual reference only.
We intend to use our website, www.standardbio.com as a means of disclosing material non-public information and for complying with our disclosure obligations under SEC Regulation FD. Such disclosures will be included on our website under “About> Investors.” Accordingly, investors should monitor the “Investors” section of our website, in addition to following our press releases, SEC filings, and public conference calls and webcasts.
23
Item 1A. Risk Factors
We operate in a rapidly changing environment that involves numerous uncertainties and risks. The following risks and uncertainties may have a material and adverse effect on our business, financial condition, or results of operations. You should consider these risks and uncertainties carefully, together with all of the other information included or incorporated by reference in this Annual Report. The risks described below are not the only ones we face. Our business is also subject to the risks that affect many other companies, such as employment relations, general economic conditions, global sociopolitical events and international operations. Further, additional risks not currently known to us or that we currently believe are immaterial may in the future materially and adversely affect our business, operations, liquidity and stock price. If any of these risks occur, our business, results of operations, or financial condition could suffer, the trading price of our securities could decline, and you may lose all or part of your investment.
Summary of Risk Factors
Risks Related to our Business, Industry, and Strategy
Risks Related to Operations and Reliance on Third Parties
24
Risks Related to Quality and the Regulatory Environment
Risks Related to Economic Conditions and Operating a Global Business
Financial, Tax, and Accounting Risks
Risks Related to Intellectual Property
Risks Related to the Recently Completed Merger
Risks Related to our Capital Structure
25
RISKS RELATED TO OUR BUSINESS, INDUSTRY, AND STRATEGY
Our financial results and revenue growth rates have varied significantly from quarter-to-quarter and year-to-year due to a number of factors, and a significant variance in our operating results or rates of growth from our financial guidance or market expectations, if any, could lead to substantial volatility in our stock price.
Our revenue, results of operations, and revenue growth rates have varied in the past and may continue to vary significantly from quarter-to-quarter or year-to-year. We may experience substantial variability in our product mix from period-to-period as revenue from sales of our instruments relative to sales of our consumables may fluctuate or deviate significantly from expectations. We may be unable to achieve revenue growth in future periods similar to some past years. Variability in our quarterly or annual results of operations, mix of product revenue, or rates of revenue growth, if any, may lead to volatility in our stock price as research analysts and investors respond to these fluctuations. These fluctuations are due to numerous factors that are difficult to forecast, including:
Additionally, we have certain customers who have historically placed large orders in multiple quarters during a calendar year. A significant reduction in orders from one or more of these customers could adversely affect our revenue and operating results, and if these customers defer or cancel purchases or otherwise alter their purchasing patterns, our financial results and actual results of operations could be significantly impacted. Similarly, the loss of one or more key customers, or the inability of any such customer to pay amounts owing to us, could materially and adversely affect our business, financial performance and results of operations. Other unknown or unpredictable factors also could harm our results.
In addition, inflationary pressure, including as a result of supply shortages, has adversely impacted and could continue to adversely impact our financial results. Our operating costs have increased, and may continue to increase, due to the recent growth in inflation. We may not fully offset these cost increases by raising prices for our products and services, which could result in downward pressure on our margins. Further, our customers may choose to reduce their business with us if we increase our pricing.
The foregoing factors, as well as other factors, could materially and adversely affect our quarterly and annual results of operations and rates of revenue growth, if any. We have experienced significant revenue growth in the past but we may not achieve similar growth rates in future periods. You should not rely on our operating results for any prior quarterly or annual period as an indication of our future operating performance. If we are unable to achieve adequate revenue growth, our operating results could suffer and our stock price could
26
decline. In addition, a significant amount of our operating expenses is relatively fixed due to our manufacturing, research and development, and sales and general administrative efforts. Any failure to adjust spending quickly enough to compensate for a shortfall relative to our anticipated revenue could magnify the adverse impact of such shortfalls on our results of operations. We expect that our sales will continue to fluctuate on an annual and quarterly basis and that our financial results for some periods may be below market expectations or projections of securities analysts, which could significantly decrease the price of our common stock.
If we engage in future acquisitions or strategic collaborations, our capital requirements may increase, our stockholders may be diluted, we may incur debt or assume contingent liabilities, and we may be subject to other risks.
We may evaluate various acquisitions and strategic collaborations, including licensing or acquiring complementary products, intellectual property rights, technologies, or businesses. Any potential acquisition or strategic collaborations may entail numerous risks, including:
If we undertake acquisitions or pursue strategic mergers, such as the Merger with SomaLogic, we may issue dilutive securities, assume or incur debt obligations, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or products that may be important to the development of our business. In addition, the Merger was financed by the issuance of shares of our common stock to stockholders of SomaLogic. SomaLogic stockholders may decide not to hold the shares of our common stock they received in the Merger. Other SomaLogic stockholders, such as funds with limitations on the amount of stock they are permitted to hold in individual issuers, may be required to sell the shares of our common stock they received in the Merger. Such sales of our common stock could result in higher than average trading volume and may cause the market price for our common stock to decline. Any of the foregoing may materially harm our business, financial condition, results of operations, stock price and prospects.
We have incurred losses since inception, and we may continue to incur substantial losses for the foreseeable future.
We have incurred significant losses in each fiscal year since our inception, including net losses of $74.7 million and $190.1 million during the years 2023 and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of $1.0 billion. These losses have resulted principally from costs incurred in our research and development programs, and from our manufacturing costs and selling, general, and administrative (SG&A) expenses. To date, we have funded our operations primarily through equity offerings, the issuance of debt instruments, and from sales of our products. Until we are able to generate additional revenue to support our level of operating expenses, we will continue to incur operating and net losses and negative cash flow from operations and may have to seek additional financing.
While we plan to reduce our operating expenses as part of ongoing restructuring initiatives, our cost restructuring efforts may not result in the anticipated savings or other economic benefits, or could result in total costs and expenses that are greater than expected, and there is no guarantee that our post-restructuring focus will be sufficient for us to achieve success. Consequently, we may incur operating losses for the foreseeable future and may never achieve profitability.
We are subject to risks associated with natural disasters and global events.
Our activities, including manufacturing, R&D and administration and information technology management, can be adversely affected by natural disasters such as major earthquakes, hurricanes, floods, tsunamis, tornadoes, fires and epidemics or pandemics, such as the COVID-19 pandemic. Climate change may cause certain of these events to become more severe and therefore more damaging. In the event of a major natural disaster affecting one or more of our facilities, our operations, including manufacturing and R&D, could be significantly disrupted. Such events could delay or prevent product manufacturing for an extended period of time. Any extended inability
27
to continue our operations at affected facilities following such an event could reduce our revenue. Further, geopolitical events like the war in Ukraine and conflict in the Middle East may also impact our operations by affecting our supply chain or impacting our operations located in the region of instability.
Market opportunities may not develop as we expect, limiting our ability to successfully sell our products, or our product development and strategic plans may change and our entry into certain markets may be delayed, if it occurs at all.
The application of our technologies to high-throughput genomics, single-cell genomics and, particularly, mass cytometry applications are in many cases emerging market opportunities. We believe these opportunities will take several years to develop or mature and we cannot be certain that these market opportunities will develop as we expect. The future growth of our markets and the success of our products depend on many factors beyond our control, including recognition and acceptance by the scientific community, and the growth, prevalence, and costs of competing methods of genetic and protein analysis. Additionally, our success depends on the ability of our sales organization to successfully sell our products into these new markets. If we are not able to successfully market and sell our products, or to achieve the revenue or margins we expect, our operating results may be harmed and we may not recover our product development and marketing expenditures. In addition, our product development and strategic plans may change, which could delay or impede our entry into these markets.
The life science markets are highly competitive and subject to rapid technological change, and we may not be able to successfully compete.
The markets for our products are characterized by rapidly changing technology, evolving industry standards, changes in customer needs, emerging competition, new product introductions, and strong price competition. We compete with both established and development stage life science research companies that design, manufacture, and market instruments and consumables for gene expression analysis, single-cell targeted gene expression and protein expression analysis, single nucleotide polymorphism (SNP) genotyping, quantitative polymerase chain reaction (qPCR), digital PCR, flow cytometry, tissue imaging, and additional applications using well established laboratory techniques, as well as newer technologies such as bead encoded arrays, microfluidics, next-generation DNA sequencing (NGS), microdroplets, spatial protein expression, and photolithographic arrays. Most of our current competitors have significantly greater name recognition, greater financial and human resources, broader product lines and product packages, larger sales forces, larger existing installed bases, larger intellectual property portfolios, and greater experience and scale in research and development, manufacturing, and marketing than we do.
We consider Agilent Technologies, Inc., Thermo Fisher Scientific Inc. (Thermo), Bio-Rad Laboratories, Inc., and Mesa Laboratories, Inc. (formerly Agena Bioscience, Inc.) to be our principal competitors in the genomics space. We believe that Cytek Biosciences, Inc. and Becton, Dickinson and Company are currently our principal competitors in Flow Cytometry, and that Akoya Biosciences, Inc., NanoString Technologies, Inc., and 10x Genomics, Inc. are our principal competitors in Spatial Biology. While the aforementioned principal competitors are the largest and most prevalent in their representative technology areas, the combined markets in which we compete have an additional 10 to 20 smaller competitors with competing approaches and technologies that we routinely face in selling situations.
Competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards, or customer requirements. In light of these advantages, even if our technology is more effective than the product or service offerings of our competitors, current or potential customers might accept competitive products and services in lieu of purchasing our technology. We anticipate that we will continue to face increased competition in the future as existing companies and competitors develop new or improved products and as new companies enter the market with new technologies. Increased competition is likely to result in pricing pressures, which could reduce our profit margins and increase our sales and marketing expenses. In addition, mergers, consolidations, or other strategic transactions between two or more of our competitors, or between our competitor and one of our key customers, could change the competitive landscape and weaken our competitive position, adversely affecting our business.
If our research and product development efforts do not result in commercially viable products within anticipated timelines, if at all, our business and results of operations will be adversely affected.
Our business is dependent on the improvement of our existing products, our development of new products to serve existing markets, and our development of new products to create new markets and applications that were previously not practical with existing systems. We have developed design rules for the implementation of our technology that are frequently revised to reflect new insights we have gained about the technology. In addition, we have discovered that biological or chemical reactions sometimes behave differently when implemented on our systems rather than in a standard laboratory environment. Furthermore, many such reactions take place within the confines of single cells, which have also demonstrated unexpected behavior when grown and manipulated within microfluidic environments. As a result, research and development efforts may be required to transfer certain reactions and cell handling techniques
28
to our systems. In the past, product development projects have been significantly delayed when we encountered unanticipated difficulties in implementing a process on our systems. We may have similar delays in the future, and we may not obtain any benefits from our research and development activities. Any delay or failure by us to develop and release new products or product enhancements would have a substantial adverse effect on our business and results of operations.
Our future success is dependent upon our ability to expand our customer base and introduce new applications.
Our customer base is primarily composed of academic research institutions, translational research and medicine centers, cancer centers, clinical research laboratories, biopharmaceutical, biotechnology, and plant and animal research companies, and contract research organizations that perform analyses for research and commercial purposes. Our success will depend, in part, upon our ability to increase our market share among these customers, attract additional customers outside of these markets, and market new applications to existing and new customers as we develop such applications. Attracting new customers and introducing new applications require substantial time and expense. For example, it may be difficult to identify, engage, and market to customers who are unfamiliar with the current applications of our systems. Any failure to expand our existing customer base or launch new applications would adversely affect our ability to increase our revenue.
If our products fail to achieve and sustain sufficient market acceptance, our revenue will be adversely affected.
Our success depends on our ability to develop and market products that are recognized and accepted as reliable, enabling and cost-effective. Most of our potential customers already use expensive research systems in their laboratories and may be reluctant to replace those systems. Market acceptance of our systems will depend on many factors, including our ability to convince potential customers that our systems are an attractive alternative to existing technologies. Compared to some competing technologies, our technology is relatively new, and most potential customers have limited knowledge of, or experience with, our products. Prior to adopting our systems, some potential customers may need to devote time and effort to testing and validating our systems. Any failure of our systems to meet these customer benchmarks could result in customers choosing to retain their existing systems or to purchase systems other than ours, and revenue from the sale of legacy instruments that may have contributed significant revenue in prior periods may decrease.
In addition, it is important that our systems be perceived as accurate and reliable by the scientific and medical research community as a whole. Historically, a significant part of our sales and marketing efforts has been directed at convincing industry leaders of the advantages of our systems and encouraging such leaders to publish or present the results of their evaluation of our systems. If we are unable to continue to induce leading researchers to use our systems, or if such researchers are unable to achieve and publish or present significant experimental results using our systems, acceptance and adoption of our systems will be slowed and our ability to increase our revenue would be adversely affected.
We may not be able to develop new products or enhance the capabilities of our existing systems to keep pace with rapidly changing technology and customer requirements, which could have a material adverse effect on our business, revenue, financial condition, and operating results.
Our success depends on our ability to develop new products and applications for our technology in existing and new markets, while improving the performance and cost-effectiveness of our systems. New technologies, techniques, or products could emerge that might offer better combinations of price and performance than our current or future product lines and systems. Existing markets for our products, including high-throughput genomics, single-cell genomics and mass cytometry, as well as potential markets for our products such as high-throughput NGS and molecular applications, are characterized by rapid technological change and innovation. It is critical to our success for us to anticipate changes in technology and customer requirements and to successfully introduce new, enhanced, and competitive technology to meet our customers’ and prospective customers’ needs on a timely and cost-effective basis. Developing and implementing new technologies typically involve substantial development costs and we may not have adequate resources available to be able to successfully introduce new applications of, or enhancements to, our systems. We cannot guarantee that we will be able to maintain technological advantages over emerging technologies in the future. While we typically plan improvements to our systems, we may not be able to successfully implement these improvements. If we fail to keep pace with emerging technologies, demand for our systems will not grow and may decline, and our business, revenue, financial condition, and operating results could suffer materially. In addition, if we introduce enhanced systems but fail to manage product transitions effectively, customers may delay or forgo purchases of our systems and our operating results may be adversely affected by product obsolescence and excess inventory. Even if we successfully implement some or all of these planned improvements, we cannot guarantee that our current and potential customers will find our enhanced systems to be an attractive alternative to existing technologies, including our current products.
29
If we fail to achieve the expected financial and operational benefits of our previously announced or future restructuring plans and other strategic initiatives, our business and financial results may be harmed.
From time to time, we have implemented efficiency and cost-savings initiatives intended to stabilize our business operations. The purpose of the restructuring plans is to improve operational efficiency, reduce operating costs and better align our workforce with the current needs of our business. There is no guarantee that the restructuring plan will achieve its intended benefits and cost savings or that our post-restructuring focus will be sufficient for us to achieve success. For example, our cost restructuring efforts may not result in the anticipated savings or other economic benefits, or could result in total costs and expenses that are greater than expected, which could require us to seek potentially dilutive financing alternatives, disrupt or restrain the scope of our business activities, and would make it more difficult to attract and retain qualified personnel, each of which could have a material adverse effect on our business, financial condition, results of operations and prospects. Similarly, changes in our commercial and strategic focus and allocation of resources contemplated by the restructuring plan, including reductions in our levels of investment in microfluidics research and development and marketing, as well as implementation of our other strategic initiatives, may be unsuccessful or result in unanticipated risks or other unintended consequences for our business, any of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Risks associated with implementing a company-wide enterprise resource planning (ERP) system could adversely affect our business and results of operations or the effectiveness of internal control over financial reporting.
We are preparing to implement a new company-wide ERP system in 2024 to handle the business and financial processes within our operations and corporate functions. ERP implementations are complex and time-consuming projects that involve substantial expenditures on system software and implementation activities that can continue for several years. ERP implementations also require transformation of business and financial processes in order to reap the benefits of the ERP system. Our business and results of operations may be adversely affected if we experience operating problems and/or cost overruns during the ERP implementation process, or if the ERP system and the associated process changes do not give rise to the benefits that we expect. Additionally, if we do not effectively implement the ERP system as planned or if the system does not operate as intended, our business, results of operations, and internal controls over financial reporting could be adversely affected.
Our business growth strategy involves the potential for significant acquisitions, and our operating results and prospects could be harmed if we are unable to integrate future acquisitions successfully.
We may acquire other businesses to improve our product offerings or expand into new markets. Our future acquisition strategy will depend on our ability to identify, negotiate, complete, and integrate acquisitions and, if necessary, to obtain satisfactory debt or equity financing to fund those acquisitions. Mergers and acquisitions are inherently risky, and any transaction we complete may not be successful. Any merger or acquisition we may pursue would involve numerous risks, including but not limited to the following:
We may be unable to secure the equity or debt funding necessary to finance future acquisitions on terms that are acceptable to us. If we finance acquisitions by issuing equity or convertible debt securities, our existing stockholders will likely experience dilution, and if we
30
finance future acquisitions with debt funding, we will incur interest expense and may have to comply with financial covenants and secure that debt obligation with our assets.
Our future growth may depend, in part, on our ability to operate in foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future growth may depend, in part, on our ability to develop and commercialize our testing products in foreign markets. We may not be permitted to market or promote any of our products before we receive regulatory approval from applicable regulatory authorities in foreign markets, and we may never receive such regulatory approvals for any of our testing products. To obtain separate regulatory approval in many other countries, we and our collaborators and service providers must comply with numerous and varying regulatory requirements regarding safety and efficacy and governing, among other things, clinical trials, commercial sales, pricing and distribution of our products. If we obtain regulatory approval of our products and ultimately commercialize them in foreign markets, we would be subject to additional risks and uncertainties, including any or all of the following:
RISKS RELATED TO THE RECENTLY COMPLETED MERGER AND THE COMBINED COMPANY’S BUSINESS FOLLOWING THE MERGER
The market price for our common stock following completion of the Merger may be affected by factors different from those that historically have affected shares of our common stock.
SomaLogic’s business differs in important respects from that of Standard BioTools and the combined company’s business now differs from that of Standard BioTools and SomaLogic prior to the completion of the Merger, including but not limited to, a primarily service-based revenue model and more concentrated customer base associated with the SomaLogic business. Accordingly, the results of operations of the combined company and the market price of Standard BioTools Common Stock after the completion of the Merger may be affected by factors different from those which affected the independent results of operations of each of Standard BioTools and SomaLogic prior to the completion of the Merger.
Combining the two companies may be more difficult, costly or time consuming than expected, and Standard BioTools may not realize all of the anticipated benefits of the Merger.
The success of the Merger will depend on, among other things, the combined company’s ability to integrate the businesses of SomaLogic and Standard BioTools in a timely fashion. Additionally, the combined company may not be able to successfully achieve the level of cost savings, revenue enhancements and synergies that it expects. If the combined company is not able to successfully achieve these objectives, the anticipated benefits of the Merger may not be realized fully or at all or may take longer to realize than expected. In
31
addition, failure to successfully integrate the businesses in the expected timeframe may adversely affect the combined company’s business, financial condition, results of operations or cash flows.
In addition, the combined operation of two businesses may be a complex, costly and time-consuming process. The difficulties of combining the operations of the companies include, among others:
Many of these factors are outside the control of SomaLogic and Standard BioTools, and any one of them could result in increased costs, decreased expected revenues and diversion of management time and energy, which could materially impact the business, financial condition, results of operations and cash flows of the combined company. These factors could cause dilution to the earnings per share of the combined company, decrease or delay the expected accretive effect of the Merger and negatively impact the price of our common stock. As a result, it cannot be assured that the combined company will realize the full benefits anticipated from the Merger within the anticipated time frames, or at all.
In addition, following the Merger, Standard BioTools became responsible for SomaLogic’s liabilities and obligations, including with respect to legal, financial, regulatory, and compliance matters. These obligations will result in additional cost and investment by Standard BioTools and, if Standard BioTools has underestimated the amount of these costs and investments or if Standard BioTools fails to satisfy any such obligations, Standard BioTools may not realize the anticipated benefits of the Merger. Further, it is possible that there may be unknown, contingent or other liabilities or problems that may arise in the future, the existence and/or magnitude of which Standard BioTools was previously unaware. Any such liabilities or problems could have an adverse effect on the combined company’s business, financial condition, results of operations or cash flows.
Even if the businesses are successfully integrated, there can be no assurance that the Merger will result in the realization of the full benefit of the anticipated synergies and cost savings or that these benefits will be realized within the expected time frames or at all. Difficulties in integrating the businesses could harm the reputation of the combined company. In addition, by engaging in the Merger, Standard BioTools may forego or delay pursuit of other opportunities that may have proven to have greater commercial potential.
The future results of the combined company may be adversely impacted if the combined company does not effectively manage its complex operations following the completion of the merger.
Following the completion of the Merger, the size of the combined company’s business became significantly larger than the previous size of either Standard BioTools’ or SomaLogic’s business. The combined company’s ability to successfully manage this expanded business will depend, in part, upon management’s ability to design and implement strategic initiatives that address not only the integration of the Standard BioTools and SomaLogic businesses, but also the increased scale and scope of the combined business with its associated increased costs and complexity. There can be no assurances that the combined company will be successful in integrating
32
the businesses or that it will realize the expected operating efficiencies, cost savings and other benefits currently anticipated from the merger.
SomaLogic and Standard BioTools has and will continue to incur substantial direct and indirect costs as a result of the Merger and the combined company will continue to incur substantial direct and indirect costs in connection with combining the business of SomaLogic and Standard BioTools following the Merger.
SomaLogic and Standard BioTools has and will continue to incur substantial expenses in connection with and as a result of consummating the Merger. Standard BioTools also expects to incur substantial expenses as a combined company in connection with coordinating and, in certain cases, combining the businesses, operations, policies and procedures of SomaLogic and Standard BioTools. While SomaLogic and Standard BioTools have assumed that a certain level of transaction expenses will be incurred, factors beyond SomaLogic’s and Standard BioTools’ control could affect the total amount or the timing of these expenses. Although many of the expenses that will be incurred, by their nature, are difficult to estimate accurately, the current estimate of the aggregate transaction-related expenses that will be incurred by SomaLogic and Standard BioTools is approximately $34.8 million, which is subject to change. These expenses may exceed the costs historically borne by SomaLogic and Standard BioTools. These expenses could adversely affect the financial condition, results of operations and cash flows of the combined company going forward.
Uncertainties associated with the Merger may cause a loss of management personnel and other key employees, which could adversely affect the future business and operations of the combined company following completion of the Merger.
We are dependent on the experience and industry knowledge of our officers and other key employees to execute our business plans. The combined company’s success after the completion of the Merger will depend in part upon the ability of the combined company to retain certain key management personnel and employees of Standard BioTools and SomaLogic. As a result of the Merger, current and prospective employees may experience uncertainty about their roles following the completion of the transactions, which may have an adverse effect on our ability to attract or retain key management and other key personnel. In addition, no assurance can be given that the combined company will be able to attract or retain key management personnel and other key employees to the same extent that Standard BioTools and SomaLogic have previously been able to attract or retain their own employees.
We have been exposed to litigation related to the Merger and may in the future be exposed to increased litigation, including stockholder litigation, which could have an adverse effect on our business and operations.
We have been exposed to litigation related to the Merger and may in the future be exposed to increased litigation from stockholders, customers, suppliers and other third parties due to the combination of Standard BioTools’ business and SomaLogic’s business following the Merger. On November 28, 2023, a purported stockholder filed a complaint against us and the members of our Board in the United States District Court for the Northern District of California. The complaint has since been voluntarily dismissed. On December 12, 2023 two separate shareholder complaints were filed in the District of Delaware, The complaints asserted claims under Section 14(a) of the Exchange Act and Rule 14a-9 promulgated thereunder and Section 20(a) of the Exchange Act for allegedly causing the filing with the SEC on November 14, 2023 of a materially deficient registration statement on Form S-4. Among other remedies, the plaintiffs sought to enjoin a stockholder vote on the proposed Merger. We are reviewing the complaints and have not yet formally responded to them. On December 13, 2023, a complaint was filed in the Delaware Court of Chancery against SomaLogic and certain officers and directors alleging Breach of Fiduciary Duty and Aiding and Abetting Breach of Fiduciary Duty. This complaint also sought an injunction postponing the proposed transaction, which was denied by the Court on January 4, 2024. The non-injunctive claims, including breach of fiduciary duty, are still being litigated. Litigation is inherently uncertain and there can be no assurance regarding the outcome. Whether or not any plaintiffs’ claim is successful, this type of litigation may result in significant costs and divert management’s attention and resources, which could adversely affect the operation of our business.
Between October 24, 2023 and January 3, 2024, SomaLogic received 16 letters from purported shareholders demanding that SomaLogic allow the inspection of its books and records and/or make corrective disclosures to its registration statement.
Additional lawsuits against us and certain of our officers or directors may be filed in the future. If additional similar complaints are filed, absent new or different allegations that are material, we will not necessarily announce such additional filings.
Such litigation may have an adverse impact on our business and results of operations or may cause disruptions to our operations. In addition, in the past, stockholders have initiated class action lawsuits against biotechnology companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against us, could cause us to incur substantial costs and
33
divert management’s attention and resources, which could have a material adverse effect on our business, financial condition and results of operations.
SomaLogic previously identified material weaknesses over financial reporting and information systems for the year ended December 31, 2022 that had not been tested for remediation as of the closing of the Merger. If SomaLogic’s remediation measures are ineffective, or if we fail to successfully integrate the operations of SomaLogic into our internal controls over financial reporting, we may not be able to report our financial condition or results of operations accurately or on a timely basis, which could adversely affect our business and our stock price.
The requirement to evaluate and report on our internal control also applies to companies that we acquire. SomaLogic, our recently acquired wholly-owned subsidiary, previously identified material weaknesses surrounding its attestation of internal controls as of December 31, 2022. In 2023, SomaLogic commenced remediation actions which included the hiring of additional resources with significant accounting and financial reporting experience, devoting resources to improving its system of processes and internal controls and enhancing the design of its information technology general controls. If SomaLogic’s actions are not effective in correcting the material weaknesses and if we fail to successfully integrate the operations of SomaLogic into our internal controls over financial reporting, investors could lose confidence in the combined entity’s financial reporting, and our stock price could decline.
RISKS RELATED TO OPERATIONS AND RELIANCE ON THIRD PARTIES
We may experience development or manufacturing problems or delays that could limit the potential growth of our revenue or increase our losses.
We may encounter unforeseen situations in the manufacturing and assembly of our products that would result in delays or shortfalls in our production. For example, our production processes and assembly methods may have to change to accommodate any significant future expansion of our manufacturing capacity, which may increase our manufacturing costs, delay production of our products, reduce our product margin, and adversely impact our business. Conversely, if demand for our products shifts such that a manufacturing facility is operated below its capacity for an extended period, we may adjust our manufacturing operations to reduce fixed costs, which could lead to uncertainty and delays in manufacturing times and quality during any transition period.
Additionally, all of our IFCs for commercial sale are manufactured at our facility in Singapore. Production of the elastomeric block that is at the core of our IFCs is a complex process requiring advanced clean rooms, sophisticated equipment, and strict adherence to procedures. Any contamination of the clean room, equipment malfunction, or failure to strictly follow procedures can significantly reduce our yield in one or more batches. We have in the past experienced variations in yields due to such factors. A drop in yield can increase our cost to manufacture our IFCs or, in more severe cases, require us to halt the manufacture of our IFCs until the problem is resolved. Identifying and resolving the cause of a drop in yield can require substantial time and resources.
Furthermore, developing an IFC for a new application may require developing a specific production process for that type of IFC. While all of our IFCs are produced using the same basic processes, significant variations may be required to ensure adequate yield of any particular type of IFC. Developing such a process can be time consuming, and any unexpected difficulty in doing so can delay the introduction of a product.
If our manufacturing activities are adversely impacted, or if we are otherwise unable to keep up with demand for our products by successfully manufacturing, assembling, testing, and shipping our products in a timely manner, our revenue could be impaired, market acceptance for our products could be adversely affected and our customers might instead purchase our competitors’ products.
Our business depends on research and development spending levels of our customers, a reduction in which could limit our ability to sell our products and adversely affect our business.
We expect that our revenue in the foreseeable future will continue to be derived primarily from sales of our systems, IFCs, assays, and reagents to academic research institutions, translational research and medicine centers, cancer centers, clinical research laboratories, biopharmaceutical, biotechnology, and plant and animal research companies, and contract research organizations worldwide. Our success will depend upon their demand for and use of our products. Accordingly, the spending policies and practices of these customers—which have been impacted by the COVID-19 pandemic and may additionally be impacted by other factors, including a potential domestic and global recession—have had and will continue to have a significant effect on the demand for our technology. These policies may be based on a wide variety of factors, including concerns regarding any future federal government budget sequestrations, the availability of resources to make purchases, the spending priorities among various types of equipment, policies
34
regarding spending during recessionary periods, tariffs and trade restrictions, and changes in the political climate. In addition, academic, governmental, and other research institutions that fund research and development activities may be subject to stringent budgetary constraints that could result in spending reductions, reduced allocations, or budget cutbacks, which could jeopardize the ability of these customers to purchase our products. Our operating results have fluctuated and may continue to fluctuate substantially due to reductions and delays in research and development expenditures by our customers. For example, reductions in operating expenditures by global academic research facilities have resulted in lower than expected sales of our mass cytometry instruments. Additionally, the imposition of tariffs and delays in issuing VAT and import tax exemptions have adversely affected the sales of our products in China. Similar reductions and delays in customer spending have resulted and may continue to result from other factors that are not within our control, such as:
Any decrease in our customers’ budgets or expenditures or in the size, scope, or frequency of capital or operating expenditures, as well as any increase in local tariffs could materially and adversely affect our operations or financial condition.
If one or more of our manufacturing facilities become unavailable or inoperable, we will be unable to continue manufacturing our instruments, IFCs, assays and/or reagents and, as a result, our business will be harmed until we are able to secure a new facility.
We manufacture our microfluidics analytical and preparatory instruments and IFCs for commercial sale at our facility in Singapore and our mass cytometry instruments, assays, and reagents for commercial sale at our facility in Canada. No other manufacturing facilities are currently available to us, particularly facilities of the size and scope of our Singapore and Canada operations. Our facilities and the equipment we use to manufacture our instruments, IFCs, assays, and reagents would be costly to replace and could require substantial lead times to repair or replace. Our facilities may be harmed or rendered inoperable by natural or man-made disasters, which may render it difficult or impossible for us to manufacture our products for some period of time. If any of our facilities become unavailable to us, we cannot provide assurances that we will be able to secure a new manufacturing facility on acceptable terms, if at all. The inability to manufacture our products, combined with our limited inventory of manufactured supplies, may result in the loss of customers or harm our reputation, and we may be unable to reestablish relationships with those customers in the future. Although we possess insurance for damage to our property and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. If our manufacturing capabilities are impaired, we may not be able to manufacture and ship our products in a timely manner, which would adversely impact our business.
Disruption of our manufacturing facilities or other operations, or in the operations of our customers or business partners, due to earthquake, flood, other natural catastrophic events, public health crises, or terrorism could result in cancellation of orders, delays in deliveries or other business activities, or loss of customers and could seriously harm our business.
We have significant manufacturing operations in Singapore and Canada and operations in the United States. In addition, our business is international in nature, with our sales, service and administrative personnel and our customers located in numerous countries throughout the world. Operations at our manufacturing facilities and our subcontractors, as well as our other operations and those of our customers,
35
are subject to disruption for a variety of reasons, including work stoppages, acts of war, terrorism, public health crises, fire, earthquake, volcanic eruptions, energy shortages, flooding, or other natural disasters. Such disruption could cause delays in, among other things, shipments of products to our customers, our ability to perform services requested by our customers, or the installation of our products at customer sites.
We cannot provide any assurance that alternate means of conducting our operations (whether through alternate production capacity or service providers or otherwise) would be available if a major disruption were to occur or that, if such alternate means were available, they could be obtained on favorable terms.
We rely on a limited number of third-party suppliers for some of the components and materials used in our products, and the loss of any of these suppliers, or delays or problems in the supply of components and materials could harm our business.
We rely on a limited number of third-party suppliers for certain components and materials used in our products, including single and sole source suppliers. Additionally, certain of our instruments are assembled at the facilities of contract manufacturers in Singapore. We do not have long-term contracts with our suppliers of these components and materials or our assembly service providers. The loss of a single or sole source supplier of any of the following components and/or materials would require significant time and effort to locate and qualify an alternative source of supply, if at all:
Our reliance on single and sole source suppliers and assembly service providers also subjects us to other risks that could harm our business, including the following:
If, as a result of global economic or political instability, such as the ongoing escalation of the situation in Ukraine, or health pandemics, our suppliers experience shortages or delays for materials sourced or manufactured in the affected countries, their ability to supply us with instruments or product components may be affected. If any of these events occur, our business and operating results could be harmed. In connection with the global supply chain disruptions following the onset of the COVID-19 pandemic, we have experienced and are continuing to experience problems with some of our suppliers. In the third quarter of 2021, shortages of certain components caused a backlog and we were unable to fulfill all of the demand for our products during the quarter. We have in the past experienced supply issues, as well as quality control problems such as manufacturing errors, with some of our suppliers, and may again experience problems in the future. We may not be able to quickly establish additional or replacement suppliers, particularly for our single source components, or assembly service providers. Any continued or future interruption or delay in the supply of components or materials or assembly of our instruments, or our inability to obtain components, materials, or assembly services from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and cause them to cancel orders or switch to competitive products, which would harm our business. Additionally, in response to a surge in COVID-19 infections in the first half of 2022, the Chinese government imposed lockdowns in certain parts of the country that have negatively impacted and continue to negatively impact manufacturing and/or supply chains.
36
We may not be able to convert our orders in backlog into revenue.
Our backlog represents product orders from our customers that we have confirmed but have not been able to fulfill, and, accordingly, for which we have not yet recognized revenue. We may not receive revenue from these orders, and any order backlog we report may not be indicative of our future revenue.
Many events can cause an order to be delayed or not completed at all, some of which may be out of our control, including, for example, the impacts from the COVID-19 pandemic and our suppliers not being able to provide us with products or components. If we delay fulfilling customer orders or if customers reconsider their orders, those customers may seek to cancel or modify their orders with us. Customers may otherwise seek to cancel or delay their orders even if we are prepared to fulfill them. If our orders in backlog do not result in sales, our operating results may suffer.
Any disruption or delay in the shipping or off-loading of our products, whether domestically or internationally, may have an adverse effect on our financial condition and results of operations.
We rely on shipping providers to deliver products to our customers globally. Labor, tariff, or World Trade Organization-related disputes, piracy, physical damage to shipping facilities or equipment caused by severe weather or terrorist incidents, congestion at shipping facilities, complications related to public health crises (including the COVID-19 pandemic), inadequate equipment to load, dock, and offload our products, energy-related tie-ups, or other factors could disrupt or delay shipping or off-loading of our products domestically and internationally. Such disruptions or delays may have an adverse effect on our financial condition and results of operations.
Our business operations depend upon the continuing efforts of our management team and other skilled and experienced personnel, and if we are unable to retain them or to recruit and train new key executives, scientists, and technical support personnel, we may be unable to achieve our goals.
Our success depends largely on the skills, experience, and performance of our management team and scientific and technical support personnel. The loss of the services of certain members of our management team or our scientific or technical support staff might significantly delay or prevent the development of our products or achievement of other business objectives by diverting management’s attention to transition matters and identification of suitable replacements, and staffing shortages could also negatively impact our ability to expand and scale functions that are needed to support the development of our products and the growth of our business. Our research and product development efforts could also be delayed or curtailed if we are unable to attract, train, and retain highly skilled employees, particularly senior scientists and engineers. Competition for qualified senior management and key employees in our industry is intense. We have experienced increased turnover at all levels since the start of the COVID-19 pandemic and general labor shortages in various areas of our business, all of which could have a material adverse impact on our business. We may need to increase employee wages and benefits in order to attract and retain the personnel necessary to achieve our goals, and our business, operations, and financial results may suffer if we are unable to do so. Attrition and workforce reductions included in the August 2022 restructuring plan could adversely affect our reputation among job seekers. It may also cause our existing employees to experience distractions or a decrease in employee morale. It could result in a loss of institutional know-how, reduced productivity, slower customer service response, reduced effectiveness of internal compliance and risk-mitigation programs, and cancellations of or delays in completing new product developments and other strategic projects. We do not currently maintain key person life insurance covering any of our employees and all our employees, including our management team, may terminate employment without notice and without cause or good reason.
Additionally, in connection with our research and product development efforts, we need to retain and recruit scientists skilled in areas such as molecular and cellular biology, assay development, engineering physics, and manufacturing. We also need highly trained technical support personnel with the necessary scientific background and ability to understand our systems at a technical level to effectively support potential new customers and the expanding needs of current customers. Competition for these people is intense and we may face challenges in retaining and recruiting such individuals if, for example, our stock price declines, thereby reducing the retention value of equity awards, or our business or technology is no longer perceived as leading in our field. Because of the complex and technical nature of our systems and the dynamic market in which we compete, any failure to attract and retain a sufficient number of qualified employees could materially harm our ability to develop and commercialize our technology.
If our direct sales, field support, and marketing forces and distribution capabilities are not sufficient to adequately address our customers’ needs, our business will be adversely affected.
We may not be able to market, sell, and distribute our products effectively enough to support our planned growth. We sell our products primarily through our own sales force and through distributors in certain territories. Our future sales will depend on a number of factors including our ability to execute with our existing team, the scope of our marketing efforts and development of our direct sales force, field application specialists and service engineer teams. Our products are technically complex and used for highly specialized
37
applications. As a result, we believe it is necessary to continue to develop a direct sales force that includes people with specific scientific backgrounds and expertise, and a marketing group with technical sophistication.
In the past year, we have experienced significant changes and increased turnover in our sales and marketing organizations, and we face considerable challenges in recruiting and training qualified replacements. Our future success will depend largely on our ability to recruit, retain, and motivate the skilled sales and marketing force necessary to support our business activities, and any failure to maintain competitive levels of compensation will negatively impact our ability to so.
Because competition for such employees is intense, we can provide no assurance that we will be able to retain them on favorable or commercially reasonable terms, if at all. Failure to attract and retain our current personnel or to build an efficient and effective sales and marketing force would negatively impact sales of our products and reduce our revenue and profitability.
In addition, we may continue to enlist one or more sales representatives and distributors to assist with sales, distribution, and customer support globally or in certain regions of the world. If we do seek to enter into such arrangements, we may not be successful in attracting desirable sales representatives and distributors, or we may not be able to enter into such arrangements on favorable terms. If our sales and marketing efforts, or those of any third-party sales representatives and distributors, are not successful, our technologies and products may not gain market acceptance, which would materially and adversely impact our business operations.
To use our products—our X9, CyTOF, and Hyperion systems in particular—customers typically need to purchase specialized reagents. Any interruption in the availability of these reagents for use in our products could limit our ability to market them.
Our products, and our X9, CyTOF, and Hyperion systems in particular, must be used in conjunction with one or more reagents designed to produce or facilitate the particular biological or chemical reaction desired by the user. Many of these reagents are highly specialized and available to the user only from a single supplier or a limited number of suppliers. Although we sell reagents for use with certain of our products, our customers may purchase these reagents directly from third-party suppliers, and we have no control over the supply of those materials. In addition, our products are designed to work with these reagents as they are currently formulated. We have no control over the formulation of reagents sold by third-party suppliers, and the performance of our products might be adversely affected if the formulation of these reagents is changed. If one or more of these reagents were to become unavailable or were reformulated, our ability to market and sell our products could be materially and adversely affected.
In addition, the use of a reagent for a particular process may be covered by one or more patents relating to the reagent itself, the use of the reagent for the particular process, the performance of that process, or the equipment required to perform the process. Typically, reagent suppliers, who are either the patent holders or their authorized licensees, sell the reagents along with a license or covenant not to sue with respect to such patents. The license accompanying the sale of a reagent often purports to restrict the purposes for which the reagent may be used. If a patent holder or authorized licensee were to assert against us or our customers that the license or covenant relating to a reagent precluded its use with our systems, our ability to sell and market our products could be materially and adversely affected. For example, our X9 system involves real-time quantitative polymerase chain reaction (qPCR) technology. Leading suppliers of reagents for real-time qPCR reactions include Life Technologies Corporation (now part of Thermo) and Roche Diagnostics Corporation, who are our direct competitors, and their licensees. These real-time qPCR reagents are typically sold pursuant to limited licenses or covenants not to sue with respect to patents held by these companies. We do not have any contractual supply agreements for these real-time qPCR reagents, and we cannot assure you that these reagents will continue to be available to our customers for use with our systems, or that these patent holders will not seek to enforce their patents against us, our customers, or suppliers.
Security incidents, loss of data, cyberattacks, and other information technology failures could disrupt our operations, damage our reputation, and adversely affect our business, operations, and financial results.
We are dependent upon our data and information technology systems for the effective operation of our business and for the secure maintenance and storage of confidential data, personal data, and trade secret information relating to our business and third-party businesses. Our information technology systems may be damaged, disrupted or shut down due to cybersecurity attacks, which are often carried out by experienced programmers or hackers, which may be able to penetrate our security. Cyberattacks include deployment of harmful malware and key loggers, ransomware, a denial-of-service attack, a malicious website, the use of social engineering and other means to affect the confidentiality, integrity and availability of our technology systems and data. Cyberattacks may also be due to employee error or malfeasance, power outages, hardware failures, telecommunication or utility failures, catastrophes or other unforeseen events, and our system redundancy and other disaster recovery planning may be ineffective or inadequate in preventing or responding to any of these circumstances. Techniques used in cybersecurity attacks to obtain unauthorized access, disable or sabotage information technology systems are evolving rapidly with data breaches and other cybersecurity events becoming commonplace. Furthermore, there may be a heightened risk of potential cyberattacks by state actors or others since the escalation of the war in Ukraine. Any such compromise of our information technology systems could result in the unauthorized access to, or acquisition or publication of our
38
confidential business or proprietary information, customer, supplier or employee data, or other personal data or trade secrets information, any of which could expose us to a risk of legal claims or proceedings, liability under privacy or other laws, disruption of our operations and damage to our reputation, which could divert our management’s attention from the operation of our business and materially and adversely affect our business, revenues, and competitive position. In addition, our liability insurance may not be sufficient in type or amount to cover us against claims related to security incidents, cyberattacks, and other related cybersecurity incidents. The cost and operational consequences of implementing further data protection measures, either as a response to specific cybersecurity incidents or as a result of evolving risks, could be material. In addition, our inability to use or access our information systems at critical points in time could adversely affect the timely and efficient operation of our business. Any delayed sales, significant costs or lost customers resulting from these technology failures could adversely affect our business, operations, and financial results.
We have implemented security controls to protect our information technology infrastructure but, due to the ever-evolving nature of cybersecurity threats, however, there can be no assurance that cybersecurity incidents that impact our systems will not occur, which could adversely affect our business and operations, and could result in financial, legal, operational or reputational harm to us, loss of competitive advantage or loss of consumer confidence. For example, in early 2019, we experienced a ransomware attack that infiltrated and encrypted certain of our information technology systems, including systems containing critical business data. Immediately following the attack, actions were taken to recover the compromised systems and we were able to restore their operation without significant loss of business data within weeks. Based on the nature of the attack and its impact on our systems, we believe no confidential data was lost or disclosed. If, however, confidential or personal data were determined to have been accessed, acquired, or released in the course of any future event, it is possible that we could be the subject of actions by governmental authorities or claims from persons alleging they suffered damages from such access, acquisition, or release. We believe our mitigation measures and expanded information security program have reduced, but cannot eliminate, the risk of a similar attack, and we anticipate additional work and expense in the future as we continuously improve our security processes and initiatives in response to ever-changing information security challenges.
In addition to risks affecting our own systems, we could also be negatively impacted by a data breach or security incident impacting a third party’s network and affecting us, such as our third-party vendors and service providers. Third parties with which we conduct business have access to certain portions of our personal and sensitive data, including information pertaining to our customers and employees. In the event that these third parties do not adequately safeguard our data, cybersecurity incidents could result and negatively impact our business, operations, and financial results.
Since the beginning of the COVID-19 pandemic, a significant percentage of our employees has been working remotely. As a result, we may have increased cyber security and data security risks, due to increased use of home wi-fi networks and virtual private networks, as well as increased disbursement of physical machines. While we have implemented security controls, updated our policies, and augmented our information security training program to reduce the risk of cyberattacks and cybersecurity incidents, there is no guarantee that these measures will be adequate to safeguard all systems with the increased number of employees working remotely.
RISKS RELATED TO QUALITY AND THE REGULATORY ENVIRONMENT
Our products could have defects or errors, which may give rise to claims against us, adversely affect market adoption of our systems, and adversely affect our business, financial condition, and results of operations.
Our systems utilize novel and complex technology and such systems may develop or contain undetected defects or errors. We cannot assure you that material performance problems, defects, or errors will not arise, and as we increase the density and integration of our systems, these risks may increase. We generally provide warranties that our systems will meet performance expectations and will be free from defects. The costs incurred in correcting any defects or errors may be substantial and could adversely affect our operating margins. For example, we have experienced a performance issue with respect to certain IFCs used in our C1 systems due to the presence of more than one cell in an IFC chamber. Although we have redesigned such C1 IFCs, we may experience additional unexpected product defects or errors that could affect our ability to adequately address these performance issues.
In manufacturing our products, including our systems, IFCs, and assays, we depend upon third parties for the supply of various components, many of which require a significant degree of technical expertise to produce. In addition, we purchase certain products from third-party suppliers for resale. If our suppliers fail to produce components to specification or provide defective products to us for resale and our quality control tests and procedures fail to detect such errors or defects, or if we or our suppliers use defective materials or workmanship in the manufacturing process, the reliability and performance of our products will be compromised.
If our products contain defects, we may experience:
39
In addition, certain of our products are marketed for use with products sold by third parties. For example, certain of our systems are marketed as compatible with major NGS instruments. If such third-party products are not produced to specification, are produced in accordance with modified specifications, or are defective, they may not be compatible with our products. In such case, the reliability and performance of our products may be compromised.
The occurrence of any one or more of the foregoing could negatively affect our business, financial condition, and results of operations.
The healthcare industry is highly regulated and if we fail to comply with applicable healthcare laws and regulations, we could suffer fines and penalties or be required to make significant changes to our operations which could have a significant adverse effect on the results of our business operations.
We compete in markets in which we or our customers must comply with federal, state, local and foreign regulations, such as healthcare fraud and abuse, data privacy and medical product laws and regulations. The healthcare industry is subject to extensive and frequently changing international and United States federal, state and local laws and regulations. In addition, federal and state enforcement agencies have substantial powers and remedies to pursue suspected violations under broad laws and regulations relating to healthcare fraud and abuse, interactions and financial arrangements with healthcare professionals or entities, data privacy and misconduct involving government programs or contracts. If we, our employees, collaborators or contractors fail to comply with applicable laws and regulations, we could suffer civil and criminal damages, fines and penalties, exclusion from participation in governmental healthcare programs, and the loss of various licenses and authorizations necessary to operate our business, as well as incur liabilities from third-party claims, all of which could have a significant adverse effect on our business. The relevant laws and regulations include, among others:
40
Any future growth of our business, including, in particular, continued reliance on consultants, commercial partners and other third parties, may increase the potential for violating these laws. In some cases, our risk of violating these or other laws and regulations is further increased because of the lack of their complete interpretation by applicable regulatory authorities or courts, and their provisions are thus open to a variety of interpretations.
Given the complexity of these existing and changing rules and regulations, it is not always possible to identify and deter misconduct by employees, distributors, consultants and commercial partners and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from government investigations or other actions or lawsuits stemming from a failure to comply with applicable laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and harm our reputation. If our operations, including the conduct of our employees, consultants and commercial partners, are found to be in violation of any of these laws and regulations, we may be subject to applicable penalties associated with the violation, including administrative, civil and criminal penalties, damages, fines, individual imprisonment, exclusion from participation in federal healthcare programs, refunding of payments received by us and curtailment or cessation of our operations. Any of these consequences could seriously harm our business and our financial results.
It is possible that some of our business activities could be subject to challenge under one or more of such laws. Such a challenge, regardless of the outcome, could have a material adverse effect on our business, business relationships, reputation, financial condition and results of operations. Although an effective compliance program can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with these laws may prove costly. If we or our operations, or any of the rheumatologists or entities with whom we do business are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to significant penalties, including administrative, civil and/or criminal penalties, damages, fines, disgorgement, individual imprisonment, exclusion from participation in U.S. federal or state healthcare programs, such as Medicare and Medicaid, and similar programs outside the United States, a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, any of which could materially adversely affect our ability to operate our business and our financial results. To the extent that any of our products are sold in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals.
Complying with numerous regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
We are subject to CLIA, a federal law that regulates clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. CLIA regulations mandate specific standards in the areas of personnel qualifications, administration and participation in proficiency testing, patient test management, quality control,
41
quality assurance and inspections. We have a current certificate of accreditation under CLIA because we are accredited to perform testing by CAP. To renew this certificate, we are subject to survey and inspection every two years. Moreover, inspectors from CMS or CAP may make random inspections of our clinical reference laboratory.
Although we are required to hold a certificate of accreditation or compliance under CLIA that allows us to perform high complexity testing, we are not required to hold a certificate of accreditation through CAP. We could alternatively maintain a certificate of accreditation from another accrediting organization or a certificate of compliance through inspection by surveyors acting on behalf of the CLIA program. If our accreditation under CAP were to terminate, either voluntarily or involuntarily, we would need to convert our certification under CLIA to a certificate of compliance (or to a certificate of accreditation with another accreditation organization) in order to maintain our ability to perform clinical testing and to continue commercial operations. Whether we would be able to successfully maintain operations through either of these alternatives would depend upon the facts and circumstances surrounding termination of our CAP accreditation, such as whether any deficiencies were identified by CAP as the basis for termination and, if so, whether these were addressed to the satisfaction of the surveyors for the CLIA program (or another accrediting organization).
The failure to comply with CLIA requirements can result in enforcement actions, including the revocation, suspension, or limitation of our CLIA certificate of accreditation, as well as a directed plan of correction, state on-site monitoring, civil money penalties, civil injunctive suit and/or criminal penalties. We must maintain CLIA compliance and certification to be eligible to bill for tests provided to Medicare beneficiaries. If we were to be found out of compliance with CLIA program requirements and subjected to sanctions, our business and reputation could be harmed. Even if it were possible for us to bring our laboratory back into compliance, we could incur significant expenses and potentially lose revenue in doing so.
Moreover, several states require that we hold licenses to test specimens from patients in those states. Other states may have similar requirements or may adopt similar requirements in the future. Although we have obtained licenses from states where we believe we are required to be licensed, we may become aware of other states that require out-of-state laboratories to obtain licensure in order to accept specimens from the state, and it is possible that other states currently have such requirements or will have such requirements in the future.
If we were to lose our CLIA accreditation, whether as a result of a revocation, suspension or limitation, we would no longer be able to sell our testing products, which would limit our revenue and harm our business. If we were to lose our license in states where we are required to hold licenses, we would not be able to test specimens from those states, which would limit our revenue.
The FDA may disagree with our assessment that our SomaSignalTM test products and any other clinical diagnostic tests we may develop are LDTs and determine that such test products are medical devices subject to the FDCA and FDA regulations.
The FDA regulates any diagnostic test that meets the definition of a medical device, except under specific, narrow circumstances. The FDCA defines a medical device as “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is , among other things: intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.” By this definition, in vitro reagents and diagnostic tests are considered medical devices. Specifically, the FDA defines an IVD as “reagents, instruments, and systems intended for use in the diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae.” Therefore, the FDA generally considers diagnostic testing products to be IVDs subject to the agency’s regulatory requirements for IVDs. However, the FDA has generally exercised its enforcement discretion and not enforced applicable regulations with respect to LDTs, which are IVDs that are designed, manufactured, and used within a single high-complexity CLIA-certified laboratory. We believe that our SomaSignalTM test products intended for clinical diagnostic use are LDTs.
If the FDA were to disagree with our conclusion that our SomaSignalTM test products for clinical diagnostic use fall within the scope of the agency’s LDT definition and determines that such tests are thus subject to FDA’s medical device authorities and implementing regulations, we would become subject to extensive regulatory requirements and may be required to stop selling our existing tests or refrain from launching any other tests we may develop. In particular, the FDA may require us to obtain PMAs or another type of device marketing authorization in order for us to commercialize our SomaSignalTM tests for clinical diagnostic use. The premarket review process for diagnostic testing products can be lengthy, expensive, time-consuming, and unpredictable. As part of the process to prepare regulatory submissions for FDA review, we may be required to conduct formal clinical trials before applying for commercial marketing authorization. Performing additional, new nonclinical studies or clinical trials in order to obtain product approval from the FDA, if any
42
were to become necessary, would take a significant amount of time and would substantially delay our ability to commercialize our SomaSignalTM tests intended for clinical diagnostic use, all of which would adversely impact our business.
While we believe that we are currently in material compliance with applicable laws and regulations as historically enforced by the FDA with respect to LDTs, we cannot assure you that the FDA will agree with our determination. Any finding by the FDA or another regulatory authority that we have violated these laws and regulations, or a public announcement that we are being investigated for possible violations, could adversely affect our business, prospects, results of operations and financial condition.
The FDA may finalize its rulemaking to regulate LDTs or Congress may take action to reform the current legal requirements applicable to LDTs. In either case we may become subject to extensive regulatory requirements and may be required to conduct additional clinical trials prior to continuing to sell our existing tests intended for clinical diagnostic use or launching any other diagnostic tests we may develop, which may increase the cost of conducting, or otherwise harm, our business.
We currently market our SomaLogicTMtests intended for clinical diagnostic use as LDTs and may in the future market other diagnostic tests as LDTs. Although historically, the FDA has applied a policy of enforcement discretion with respect to LDTs whereby the FDA does not generally actively enforce its regulatory requirements for such tests, in October 2023, the FDA issued a proposed rule to regulate LDTs under the current medical device framework. The agency’s proposal also includes a plan to phase out its current enforcement discretion policy over several years. This FDA rulemaking was initiated after years of failed congressional attempts to harmonize the regulatory paradigms applicable to LDTs and other in vitro diagnostic tests, as discussed further below. The likelihood of the FDA finalizing the proposed rule following a public comment period, as well as potential litigation challenging its authority to take such action, is uncertain at this time as stakeholders continue to press for a comprehensive legislative solution instead of administrative agency action.
If there are changes in FDA regulations or legislative authorities such that the agency begins to exercise oversight over LDTs, or if the FDA disagrees that our marketed tests are within the scope of its criteria used for defining LDTs, we may become subject to extensive regulatory requirements and may be required to stop selling our existing diagnostic tests or launching any other similar tests we may develop and to conduct additional clinical trials or take other actions prior to continuing to market our tests. If the FDA allows our SomaSignalTM tests for clinical diagnostic use to remain on the market but there is uncertainty about our tests, if they are labeled investigational by the FDA or if labeling claims the FDA allows us to make do not include the claims necessary or desirable for successful commercialization, orders from healthcare providers or reimbursement for our diagnostic tests may decline.
In addition, as noted above, Congress had been working on legislation to create an LDT and IVD, regulatory framework that would be separate and distinct from the existing medical device regulatory framework. Reform legislation called the VALID Act garnered bipartisan and bicameral support in recent years but failed to move out of committee during the last congressional session. As drafted and re-introduced for consideration by the current Congress, the VALID Act would codify the term IVCT to create a new medical product category separate from medical devices to include products currently regulated as IVDs as well as LDTs, among other provisions. The VALID Act would also create a new system for laboratories to use to submit their tests electronically to the FDA for approval, which is aimed at reducing the amount of time it would take for the agency to approve such tests, and establish a new program to expedite the development of diagnostic tests that can be used to address a current unmet need for patients. The FDA’s October 2023 publication of an LDT proposed rule that would apply the existing medical device framework to laboratory-developed products has renewed stakeholder calls for a more targeted approach to modernizing the federal government’s oversight of clinical diagnostic tests. It remains possible that congressional action in this area could displace the need for the FDA to complete its recently proposed rulemaking.
If Congress were to pass the VALID Act or any other legislation applicable to the FDA’s regulation of LDTs, or if the FDA were to successfully promulgate new regulations for such products through the recently initiated notice-and-comment rulemaking or a future rulemaking proceeding, we will likely be subject to increased regulatory burdens such as registration and listing requirements, adverse event reporting requirements and quality control requirements. Any legislation or formal FDA regulatory framework affecting LDTs is also likely to have premarket application requirements prohibiting commercialization without FDA authorization and controls regarding modification to the tests that may require further FDA submissions. The premarket review process can be lengthy, expensive, time-consuming and unpredictable. Further, obtaining premarket clearance may involve, among other things, successfully completing clinical trials, which require significant time and cash resources and are subject to a high degree of risk, including risks of experiencing delays, failing to complete the trial or obtaining unexpected or negative results. If we are required to obtain premarket clearance or approval and/or conduct premarket clinical trials, our development costs could significantly increase, marketing of any new diagnostic tests we may develop may be delayed, and sales of our existing diagnostic tests could be interrupted or stopped. Any of these outcomes could
43
reduce our revenue or increase our costs and materially adversely affect our business, prospects, results of operations or financial condition.
The outcome and ultimate impact on our business of any changes to the federal government’s regulation of LDTs is difficult to predict. Failure to comply with any applicable FDA requirements could trigger a range of enforcement actions, including warning letters, fines, penalties, suspension of operations, product recalls or seizures, denial of applications for clearance or approval, injunctions and other civil or criminal sanctions, which could have a material and adverse effect upon our business, operating results and financial condition.
Furthermore, should it be required in the future, we cannot be sure that our SomaSignalTM tests intended for clinical diagnostic use, or any new diagnostic tests that we may develop, will be reviewed and authorized for marketing by the FDA in a timely or cost-effective manner, if authorized at all. Even if such tests are authorized for marketing by the FDA, the agency could limit the test’s indications for use, which may significantly limit the market for that product and may adversely affect our business and financial condition.
We are currently limited to RUO with respect to many of the materials and components used in our consumable products including our assays.
We sell our instruments and consumable products, and certain of our assays, with a restrictions that they be used for RUO applications. The sale of our RUO products for any clinical or diagnostic purposes may require that we obtain regulatory clearance or approval to market the products for such purposes and also that we acquire certain materials and components used in the products from suppliers without an RUO restriction. There can be no assurance that we will be able to acquire these materials and components for use in diagnostic products on acceptable terms, if at all. If we are unable to do so, we would not be able to expand our instrument, consumable and assay product offerings beyond RUO, and our business and prospects would suffer.
The RUO/IUO Labeling Guidance, emphasizes that the FDA will review the totality of the circumstances when evaluating whether equipment and testing components are properly labeled as RUO. It further states that merely including a labeling statement that a product is intended for RUO will not necessarily render the device exempt from the FDA’s 510(k) clearance, PMA, or other requirements, if the circumstances surrounding the distribution of the product indicate that the manufacturer intends for its product to be offered for clinical diagnostic use. These circumstances may include written or verbal marketing claims or links to articles regarding a product’s performance in clinical applications, a manufacturer’s provision of technical support for clinical validation or clinical applications, or solicitation of business from clinical laboratories, all of which could be considered evidence of intended uses that conflict with RUO labeling. If the FDA were to determine that our RUO products were intended for use in clinical investigation, diagnosis or treatment decisions, or that express or implied clinical or diagnostic claims were made for our RUO products, those products could be considered misbranded or adulterated under the FDCA. If the FDA determines that our RUO products are being marketed for clinical diagnostic use without the required PMA or 510(k) clearance, we may be required to cease marketing our products as planned, recall the products from customers, revise our marketing plans, and/or suspend or delay the commercialization of our products until we obtain the required authorization. We also may be subject to a range of enforcement actions by the FDA, including warning or untitled letters, injunctions, civil monetary penalties, criminal prosecution, and recall and/or seizure of products, as well as significant adverse publicity. For instance, some of our customers may, on their own initiative, use our RUO-labeled products in the development of their own LDTs or in other FDA-regulated products for clinical diagnostic use and may request our assistance in developing such uses or validating the instrument, consumable or assay for diagnostic use. If we provide such services or advice, FDA could determine that we intend such instruments, consumables, or assays for clinical or diagnostic uses in contradiction of the RUO labeling and require us to recall the products, prepare and submit applications for marketing authorization for the clinical or diagnostic uses or initiate enforcement actions against us. Any of these developments may adversely affect our business and financial condition.
If the FDA determines that our RUO products are medical devices or if we seek to market our RUO products for clinical diagnostic or health screening use, we will be required to obtain regulatory clearance(s) or approval(s), and may be required to cease or limit sales of our then marketed products, which could materially and adversely affect our business, financial condition and results of operations. Any such regulatory process would be expensive, time-consuming and uncertain both in timing and in outcome.
Our RUO products are focused on the life sciences research market. This includes laboratories associated with academic and governmental research institutions, as well as pharmaceutical, biotechnology and contract research companies. Accordingly, our products are labeled as RUO, and are not intended for diagnostic use. While our marketing for our RUO products is focused on the life sciences research market, we may decide to expand our product line to encompass products that are intended to be used for the diagnosis of disease or other medical purposes. Laboratory instruments, consumables and assays intended for clinical or diagnostic purposes are subject to regulation by the FDA as medical devices, or comparable international agencies, including requirements for regulatory clearance or approval of such products before they can be marketed. If the FDA were to determine that our products are intended for
44
clinical use or if we decided to market our products for such use, we would be required to obtain 510(k) clearance or premarket approval from the agency in order to sell our products in a manner consistent with applicable U.S. laws and regulations. Such regulatory authorization processes are expensive, time-consuming and uncertain; our efforts may never result in any marketing authorization for our products; and failure by us to obtain or comply with such authorizations could have an adverse effect on our business, financial condition or operating results. Even if we obtain approval of a PMA or 510(k) clearance, where required, such authorization may not be for the use or uses we believe are commercially attractive and/or are critical to the commercial success of our products. As a result, being subject to the FDA’s premarket review and/or post-market control requirements for our products could materially and adversely affect our business, financial condition and results of operations.
If we are required to obtain approval of a PMA or 510(k) clearance for our instruments, consumables or assay products, we or they would be subject to a substantial number of additional requirements applicable to medical devices, including establishment registration; device listing; Quality Systems Regulations which cover the design, testing, production, control, quality assurance, labeling, packaging, servicing, sterilization (if required), and storage and shipping of medical devices (among other activities); device labeling; advertising and promotion; recordkeeping; post-market surveillance; post-market studies; adverse event reporting; and device corrections, removals and recalls. One or more of our current or future products may also require clinical trials in order to generate the data required for approval of a PMA. Complying with these requirements may be time-consuming and expensive. We may be required to expend significant resources to ensure ongoing compliance with applicable regulations and implement satisfactory corrective or preventive actions in response to quality issues or enforcement action, which may have a material adverse effect on the ability to design, develop and commercialize products using our technology as planned. Failure to comply with these requirements may subject us to a range of enforcement actions, such as warning letters, injunctions, civil monetary penalties, criminal prosecution, recall and/or seizure of products, and revocation of marketing authorizations, as well as significant adverse publicity. If we or our collaborators fail to obtain, or experience significant delays in obtaining, regulatory approvals for our products, we may not be able to launch or successfully commercialize such products in a timely manner, or at all.
Failure to comply with applicable FDA requirements could subject us to misbranding or adulteration allegations under the Federal Food, Drug, and Cosmetic Act. We could be subject to a range of enforcement actions, including warning letters.
The FTC and/or state enforcement or regulatory agencies may object to the methods and materials we use to promote our products and services and initiate enforcement against us, which could adversely affect our business and financial condition.
The FTC and/or state enforcement or regulatory agencies (including but not limited to the offices of state attorneys general) may object to the materials and methods we use to promote our services and our currently marketed instruments, reagents, or assays, including diagnostic LDTs, or other products we may develop in the future, including with respect to the product claims in our promotional materials or advertising, and may initiate enforcement actions against us. Enforcement actions by the FTC may include, among others, injunctions, civil penalties and equitable monetary relief.
Actual or perceived failures to comply with applicable data protection, privacy and security laws, regulations, standards and other requirements could adversely affect our business, results of operations and financial condition.
Any failure or perceived failure by us to comply with federal or state laws or regulations, our internal policies and procedures or our contracts governing our use and disclosures of personal information could result in negative publicity, government investigations and enforcement actions including significant penalties, claims by third parties, and damage to our reputation, any of which could have a material adverse effect on our operations, financial performance and business.
Failure to comply with HIPAA, the HITECH Act, their implementing regulations and similar comparable state laws and regulations affecting the transmission, security and privacy of health information could result in significant penalties.
Numerous federal, state and foreign laws and regulations, including HIPAA and the HITECH Act, govern the collection, dissemination, disclosure, security, use and confidentiality of individually identifiable health information health-related and other personal information. HIPAA and the HITECH Act require us to comply with standards for the use and disclosure of PHI within our company and with third-parties. The privacy, security and breach notification rules promulgated under HIPAA, as amended by the HITECH Act, Standards for Privacy of Individually Identifiable Health Information (Privacy Standards) and the Security Standards for the Protection of Electronic Protected Health Information (Security Standards) under HIPAA establish a set of basic national privacy and security standards for the protection of individually identifiable health information by Covered Entities and their Business Associates. HIPAA requires Covered Entities to develop and maintain policies and procedures with respect to individually identifiable health information that is used or disclosed, including the adoption of administrative, physical and technical safeguards to protect the privacy and security of such
45
information. HIPAA also requires us to provide individuals with certain rights with respect to their PHI. Business Associates must have a written Business Associate contracts or other arrangements with a Covered Entity that establishes specifically what the Business Associate has been engaged to do and requires the Business Associate to comply with the requirements of HIPAA. Further, in the event of a breach of unsecured PHI we must notify each individual whose PHI is breached as well as federal regulators and in some cases, must publicize the breach in local or national media.
HIPAA also includes standards for common healthcare electronic transactions and code sets, such as claims information, plan eligibility, payment information and the use of electronic signatures, and privacy and electronic security of individually identifiable health information. Covered Entities, such as certain healthcare providers, are required to conform to such transaction set standards, known as the Standards for Electronic Transactions, pursuant to HIPAA. Submission of electronic healthcare claims and payment transactions that do not comply with the HIPAA electronic data transmission standards could result in delayed or denied payments.
In the conduct of our business, we process, maintain, and transmit sensitive data, including PHI. There can be no assurance that a breach of privacy or security will not occur. If there is a breach, we could be subject to various lawsuits, penalties and damages and may be required to incur costs to mitigate the impact of the breach on affected individuals.
Penalties for failure to comply with HIPAA requirements are substantial and could include corrective action plans and/or the imposition of civil or criminal penalties. HIPAA also authorizes state attorneys general to file suit under HIPAA on behalf of state residents. Courts can award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for HIPAA violations, its standards have been used as the basis for a duty of care claim in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
Additionally, certain states have adopted comparable privacy and security laws and regulations, some of which may apply more broadly or be more stringent than HIPAA. For example, the CCPA, which went into effect on January 1, 2020, gives California residents expanded rights to access and delete their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. Further, the CPRA went into effect in California amending the CCPA and may increase our compliance costs and potential liability, imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data and adds opt outs for certain uses of sensitive data. It also created a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. In the event that we are subject to or affected by HIPAA, the CCPA, the CPRA or other domestic privacy and data protection laws (for example, the Colorado Privacy Act and other similar laws that recently went into effect in other states, such as Utah, Virginia, Connecticut, Delaware, Florida, Indiana, Iowa, Montana, Oregon, Tennessee, and Texas), any liability from failure to comply with the requirements of these laws could adversely affect our financial condition.
In Europe, the GDPR went into effect in May 2018 and imposes strict requirements for processing the personal data of individuals within the EEA. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. Among other requirements, the GDPR regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States, and the efficacy and longevity of current transfer mechanisms between the EU and the United States remains uncertain. For example, in 2016, the EU and United States agreed to a transfer framework for data transferred from the EU to the United States, called the Privacy Shield, but the Privacy Shield was invalidated in July 2020 by the Court of Justice of the EU. In July 2023, however, the European Commission adopted an adequacy decision for a new mechanism for transferring data from the EU to the United States – the EU-US Data Privacy Framework, which provides EU individuals with several new rights, including the right to obtain access to their data, or obtain correction or deletion of incorrect or unlawfully handled data. The adequacy decision followed the signing of an executive order introducing new binding safeguards addressing the reasons behind the Court of Justice of the EU’s invalidation of the original Privacy Shield. The European Commission will continually review developments in the United States along with its adequacy decision. However, future actions of EU data protection authorities are difficult to predict.
Relatedly, following the United Kingdom’s withdrawal from the EU, the GDPR was implemented in the United Kingdom as the U.K. GDPR. which sits alongside the amended U.K. Data Protection Act 2018, which implements certain derogations in the EU GDPR into United Kingdom law. The U.K. GDPR mirrors the fines under the GDPR, i.e., fines up to the greater of €20 million (£17.5 million) or 4% of global turnover. In June of 2021, the European Commission issued a decision, which will sunset on June 27, 2025 without further action, that the United Kingdom ensures an adequate level of protection for personal data transferred under the EU GDPR from the EU
46
to the United Kingdom. The U.K. Parliament is currently considering the Data Protection and Digital Information Bill to harmonize the 2018 Data Protection Act, U.K. GDPR, and the Privacy and Electronic Communications Regulations under one legislative framework.
The regulatory framework governing the collection, storage, use and sharing of certain information, particularly financial and other personal information, is rapidly evolving and is likely to continue to be subject to uncertainty and varying interpretations. Additionally, increasing concerns about health information privacy have recently prompted the federal government to issue guidance taking a newly expansive view of the scope of the laws and regulations that they enforce. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our existing practices. Any failure or perceived failure by us, or any third parties with which we do business, to comply with our privacy policies, changing expectations, evolving laws, rules and regulations, industry standards or contractual obligations to which we or such third parties are or may become subject, may result in actions or other claims against us by governmental entities or private actors, the expenditure of substantial costs, time and other resources or the incurrence of significant fines, penalties or other liabilities. In addition, any such action, particularly to the extent we were found to be guilty of violations or otherwise liable for damages, would damage our reputation and adversely affect our business, financial condition and results of operations.
Although we work to comply with applicable laws, regulations and standards, our contractual obligations and other legal obligations, these requirements are evolving and may be modified, interpreted and applied in an inconsistent manner from one jurisdiction to another and may conflict with one another or other legal obligations with which we must comply. Any failure or perceived failure by us or our employees, representatives, contractors, consultants, collaborators, or other third parties to comply with such requirements or adequately address privacy and security concerns, even if unfounded, could result in additional cost and liability to us, damage our reputation and adversely affect our business and results of operations.
Our actual or perceived failure to comply with data protection laws and regulations could lead to government enforcement actions, private litigation and/or adverse publicity and could negatively affect our business.
We are subject to domestic and international data protection laws and regulations that address privacy and data security and may affect our collection, use, storage, and transfer of personal information. The legislative and regulatory landscape for data protection continues to evolve, and in recent years there has been an increasing focus on privacy and data security issues with the potential to affect our business. In the U.S., numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws and federal and state consumer protection laws govern the collection, use, disclosure and protection of health-related and other personal information. Failure to comply with data protection laws and regulations, where applicable, could result in government enforcement actions, which could include civil or criminal penalties, private litigation and/or adverse publicity and could negatively affect our operating results and business. For example, California has enacted the California Consumer Privacy Act (the “CCPA”), which went into effect in January of 2020. The CCPA established a new privacy framework for covered businesses by creating an expanded definition of personal information, establishing new data privacy rights for California residents, requiring covered businesses to provide new disclosures to California residents, and creating a new and potentially severe statutory damages framework for violations of the CCPA and for businesses that fail to implement reasonable security procedures and practices to prevent data breaches. Additionally in 2020, California voters passed the CPRA which went into effect on January 1, 2023. The CPRA significantly amends the CCPA, potentially resulting in further uncertainty, additional costs and expenses in an effort to comply and additional potential for harm and liability for failure to comply. Among other things, the CPRA established a new regulatory authority, the California Privacy Protection Agency, which is tasked with enacting new regulations under the CPRA and will have expanded enforcement authority. In addition to California, more U.S. states are enacting similar legislation, increasing compliance complexity and increasing risks of failures to comply. In 2023, comprehensive privacy laws in Virginia, Colorado, Connecticut, and Utah all took effect, and laws in Montana, Oregon, and Texas will take effect in 2024. In addition, laws in other U.S. states are set to take effect beyond 2024, and additional U.S. states have proposals under consideration, all of which are likely to increase our regulatory compliance costs and risks, exposure to regulatory enforcement action and other liabilities.
Numerous other countries have, or are developing, laws governing the collection, use and transmission of personal information as well. For example, the European Union’s General Data Protection Regulation (GDPR), became effective in 2018 and imposed a broad data protection framework that expanded the scope of EU data protection law, including to non-EU entities meeting the jurisdictional requirements that process, or control the processing of, personal data relating to individuals located in the EU, including clinical trial data. The GDPR sets out a number of requirements for controllers and/or processors, as applicable, that must be complied with when handling the personal data of EU based data subjects, including: providing expanded disclosures about how their personal data will be used; higher standards for organizations to demonstrate that they have obtained valid consent or have another legal basis in place to justify their data processing activities; the obligation to appoint data protection officers in certain circumstances; new rights for individuals to be “forgotten” and rights to data portability, as well as enhanced current rights (e.g., access requests); the principal of accountability and demonstrating compliance through policies, procedures, training and audit; and a new mandatory data breach regime.
47
In particular, medical or health data, genetic data and biometric data are all classified as “special category” data under the GDPR and afford greater protection and require additional compliance obligations. Further, EU member states have a broad right to impose additional conditions—including restrictions—on these data categories. This is because the GDPR allows EU member states to derogate from the requirements of the GDPR mainly in regard to specific processing situations (including special category data and processing for scientific or statistical purposes).
The GDPR is applicable to part of our business and has increased our responsibility and liability in relation to personal data that we process, and we may be required to put in place additional procedures to comply. The GDPR is complex and regulatory guidance continues to evolve. Furthermore, national GDPR variations, including the fields of clinical study and other health-related information may raise our costs of compliance and result in greater legal risks.
We are also subject to evolving GDPR requirements on data export, because we transfer data to third countries outside of the EU that are not deemed “adequate.” The GDPR only permits exports of personal data outside of the EU to “non-adequate” countries where there is a suitable data transfer mechanism in place to safeguard personal data (e.g., the EU Commission approved Standard Contractual Clauses or certification under the newly-adopted Data Privacy Framework). On July 16, 2020, the Court of Justice of the EU, or the CJEU, issued a landmark opinion in the case Maximilian Schrems vs. Facebook (Case C-311/18) (Schrems II). This decision calls into question certain data transfer mechanisms as between the EU member states and the U.S. The CJEU is the highest court in Europe and the Schrems II decision heightened the burden to assess U.S. national security laws on their business, and future actions of EU data protection authorities are difficult to predict at this time. While the newly-adopted Data Privacy Framework was meant to address the concerns raised by the CJEU in Schrems II, it will likely be subject to future legal challenges. Consequently, there is some risk of any data transfers from the EU being halted. If we have to rely on third parties to carry out services for us, including processing personal data on our behalf, we are required under GDPR to enter into contractual arrangements to flow down or help ensure that these third parties only process such data according to our instructions and have sufficient security measures in place. Any security breach or non-compliance with our contractual terms or breach of applicable law by such third parties could result in enforcement actions, litigation, fines and penalties or adverse publicity and could cause customers to lose trust in us, which would have an adverse impact on our reputation and business. Any contractual arrangements requiring the processing of personal data from the EU to us in the U.S. will require greater scrutiny and assessments as required under Schrems II and may have an adverse impact on cross-border transfers of personal data or increase costs of compliance. The GDPR provides an enforcement authority to impose large penalties for noncompliance, including the potential for fines of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater.
Applicable data privacy and data protection laws may conflict with each other, and by complying with the laws or regulations of one jurisdiction, we may find that we are violating the laws or regulations of another jurisdiction. Despite our efforts, we may not have fully complied in the past and may not in the future. That could require us to incur significant expenses, which could significantly affect our business. Failure to comply with data protection laws may expose us to risk of enforcement actions taken by data protection authorities or other regulatory agencies, private rights of action in some jurisdictions, and potential significant penalties if we are found to be non-compliant. Furthermore, the number of government investigations related to data security incidents and privacy violations continue to increase and government investigations typically require significant resources and generate negative publicity, which could harm our business and reputation.
RISKS RELATED TO ECONOMIC CONDITIONS AND OPERATING A GLOBAL BUSINESS
We generate a substantial portion of our revenue internationally and our international business exposes us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
During the years ended December 31, 2023 and 2022 approximately 59% of our product and service revenue was generated from sales to customers located outside of the United States. We believe that a significant percentage of our future revenue will continue to come from international sources as we expand our international operations and develop opportunities in other countries. Engaging in international business inherently involves a number of difficulties and risks, including:
48
During much of the COVID-19 pandemic, travel restrictions caused significant slowdowns in China, Japan, and other parts of the Asia-Pacific region. These slowdowns, in addition to shipment delays in China due to delays in obtaining VAT and import tax exemptions for our products, have caused our financial results to suffer. If these situations continue, or if other risks occur, we could be forced to dedicate significant resources to their resolution, and if we are unsuccessful in finding a solution, our financial condition and results will suffer.
In addition, political instability, civil unrest, the deterioration of the political situation in a country in which we have significant sales or operations, or the breakdown of trade relations between the United States and a foreign country in which we have significant operations, could adversely affect our business, financial condition, and results of operations. For example, a change in trade status between the United States and a foreign country could result in a substantial increase in the import duty applicable to products manufactured in that foreign country and imported into the United States. The United States has commenced certain trade actions, including imposing increased tariffs on certain goods imported into the United States from China, which has resulted in retaliatory tariffs by China. In addition, the United States has commenced certain trade actions as a result of the Russian invasion of Ukraine, which has resulted in retaliatory measures by Russia. Any increased trade barriers or restrictions on global trade imposed by the United States, or further retaliatory trade measures taken by China, Russia, or other countries in response, could adversely affect our business, financial condition, and results of operations.
Our business is subject to a variety of new U.S. and foreign export controls and economic sanctions regulations that were issued in response to Russia’s invasion of Ukraine; our failure to comply with these laws and regulations could harm our business.
Due to recent regulations, U.S. companies can no longer provide or receive services or conduct any business with, including selling, shipping, or otherwise transferring any U.S.-controlled products to, the Donetsk People’s Republic (DNR) and Luhansk People’s Republic (LNR) regions of Ukraine. Additionally, existing U.S. sanctions continue to extend these prohibitions to the Crimea region of Ukraine. Our business is also subject to the expansion of previously existing sanctions imposed by the Treasury Department’s Office of Foreign Assets Controls that now cover a significant number of individuals and entities located in Russia, Belarus, and surrounding regions as well as new U.S. export controls imposed by the U.S. Department of Commerce’s Export Administration Regulations on exports to Russia. These laws and regulations cover U.S. persons as well as U.S.-controlled products, software, and technologies wherever located. Failure to comply with U.S. and foreign export control and economic sanctions laws and regulations can result in criminal sanctions, civil fines, debarment from government contracting, the loss of export privileges, and, in some cases, imprisonment.
Any additional changes in export control laws, sanctions requirements, or our operations in the affected regions may require us to expend additional resources or to discontinue certain products or services, which would negatively affect our business, financial condition, and operating results. In addition, the increased attention focused upon liability issues as a result of lawsuits, regulatory proceedings, and legislative proposals could damage our brand or otherwise impact the growth of our business. Finally, our ability to receive payment from these regions has been significantly impacted. Any costs incurred or loss of business that occurs as a result of compliance or other liabilities under these laws or regulations could harm our business and operating results.
49
Adverse conditions in the domestic and global economy and disruption of financial markets may significantly harm our revenue, profitability, and results of operations.
Adverse economic conditions in the U.S. and international markets, including any worldwide economic disruption related to another or worsening global pandemic or a recession, could negatively impact our revenues and results of operations. The global credit and financial markets continue to experience volatility and disruptions, including diminished liquidity and credit availability, increased concerns about inflation, and uncertainty about economic stability. Geopolitical events including a potential recession, the Russian invasion of Ukraine, including any resulting adoption and expansion of trade restrictions by the United States, Russia, and/or China, and Brexit have caused significant economic, market, political and regulatory uncertainty in some of our markets. Volatility and disruption of financial markets could limit our customers’ ability to obtain adequate financing or credit to purchase and pay for our products in a timely manner or to maintain operations, which could result in a decrease in sales volume that could harm our results of operations. General concerns about the fundamental soundness of domestic and international economies may also cause our customers to reduce their purchases. Changes in governmental banking, monetary, and fiscal policies to address liquidity and increase credit availability may not be effective. Significant government investment and allocation of resources to assist the economic recovery of sectors that do not include our customers may reduce the resources available for government grants and related funding for life science, plant and animal research, and clinical research and development. Continuation or further deterioration of these financial and macroeconomic conditions could significantly harm our sales, profitability, and results of operations.
We are subject to fluctuations in the exchange rate of the U.S. dollar and foreign currencies.
Our revenue is generally denominated in the local currency of the contracting party. Historically, the majority of our revenue has been denominated in U.S. dollars and fluctuations in the value of the U.S. dollar relative to foreign currencies could decrease demand for our products and adversely impact our financial performance. For example, if the value of the U.S. dollar increases relative to foreign currencies, our products could become more costly to the international consumer and therefore less competitive in international markets, or if the value of the U.S. dollar decreases relative to the Singapore dollar or the Canadian dollar, it would become more costly in U.S. dollars for us to manufacture our products in Singapore and/or in Canada. Additionally, our expenses are generally denominated in the currencies where our operations are located, which is primarily in the United States, with a portion of expenses incurred in Singapore and Canada where our manufacturing facilities are located. Our results of operations and cash flows are, therefore, subject to fluctuations due to changes in foreign currency exchange rates. The volatility of exchange rates depends on many factors that we cannot forecast with reliable accuracy. We have experienced and will continue to experience fluctuations in our net income or loss as a result of transaction gains or losses related to revaluing certain current asset and current liability balances that are denominated in currencies other than the functional currency of the entities in which they are recorded. Fluctuations in currency exchange rates could have an adverse impact on our financial results in the future.
FINANCIAL, TAX, AND ACCOUNTING RISKS
Our future capital needs are uncertain and we may need to raise additional funds in the future, which may cause dilution to stockholders or may be upon terms that are not favorable to us.
We continue to experience losses and, if that trend continues, we may need to seek additional sources of financing. In addition, we may need to raise substantial additional capital for various purposes, including:
Our future funding requirements will depend on many factors, including:
50
To the extent we draw on our Revolving Credit Facility or otherwise incur additional indebtedness, the risks described above could increase. Further, if we increase our indebtedness, our actual cash requirements in the future may be greater than expected. Our cash flow from operations may not be sufficient to repay all of the outstanding debt as it becomes due, and we cannot assure you that we will be able to obtain additional funds on acceptable terms, or at all. In recent years, there has been significant volatility in the global capital markets, increasing the cost of—and adversely impacting access to—capital. If we raise additional funds by issuing equity securities, our stockholders will experience dilution. Debt financing in addition to the Credit Facility (as defined below), if available, may involve covenants restricting our operations or our ability to incur additional debt. Any additional debt or equity financing that we raise may contain terms that are not favorable to us or our stockholders, and our ability to raise additional capital may be adversely impacted by the impact of the COVID-19 pandemic on the economy.
If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us. If we do not have or are unable to raise adequate funds, we may have to liquidate some or all of our assets, delay development or commercialization of our products, or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to reduce marketing, customer support, research and development, or other resources devoted to our products, or cease operations. Any of these factors could harm our operating results.
If we fail to maintain effective internal control over financial reporting in the future, the accuracy and timing of our financial reporting may be impaired, which could adversely affect our business and our stock price.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our testing may reveal deficiencies in our internal control over financial reporting that are deemed to be material weaknesses.
Our compliance with Section 404 requires that we incur substantial accounting expense and expend significant management time on compliance-related issues. We currently do not have an internal audit group, and we continue to evaluate our need for additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. We also monitor our ability to retain and motivate our key existing workers with highly trained accounting and finance skills in a competitive market. Our restructuring activities could diminish our resource capacity and impact our control processes with changes implemented. Our planned enterprise resource planning (ERP) upgrade in 2023 will also result in changes to our processes and control procedures. If we do not comply with the requirements of Section 404, or if we or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by The Nasdaq Stock Market LLC, the Securities and Exchange Commission (SEC), or other regulatory authorities, which would require additional financial and management resources.
We may not realize the value of our goodwill or other intangible assets, which would be reflected in an impairment charge.
Our business acquisitions typically result in goodwill and other intangible assets, which affect the amount of future period amortization expense and possible impairment expense. We make estimates and assumptions in valuing such intangible assets that affect our consolidated financial statements. As of December 31, 2023, we had approximately $107.7 million of goodwill and net intangible assets, including approximately $106.3 million of goodwill and $1.4 million of net intangible assets. These assets represent a significant portion
51
of the assets recorded on our consolidated balance sheet. In addition, if in the future we acquire additional businesses, technologies, or other intangible assets, a substantial portion of the value of such assets may be recorded as goodwill or intangible assets.
We assess the realizability of goodwill and indefinite-lived intangible assets annually as well as whenever events or changes in circumstances indicate that these assets may be impaired. We also assess the realizability of definite-lived intangible assets whenever events or changes in circumstances indicate that these assets may be impaired. These events or circumstances may include a significant deterioration in overall economic conditions, a decline in our market capitalization, reorganizations of our business, the disposal of all or a portion of a reporting unit, operating losses or a significant decline in earnings associated with the acquired business or asset. Our ability to realize the value of the goodwill and intangible assets will depend on the future cash flows of these businesses, including our ability to realize revenue growth, cost savings, and other macro factors which impact the enterprise value. These cash flows in turn depend in part on how well we have integrated these businesses. If we are not able to realize the value of the goodwill and intangible assets, we may be required to incur material charges relating to the impairment of those assets.
In determining the fair value of our two operating segments, significant assumptions including forecasted cash flows (revenue growth rates), discount rates, earnings multiples and an implied control premium are utilized. As these assumptions are inherently judgmental and subject to uncertainty, future impairments that cannot be reasonably estimated, but could be material, may occur. We performed our annual goodwill assessment as of December 31, 2023 and concluded that we did not have a goodwill impairment as of December 31, 2023.
If we fail to comply with the covenants and other obligations under our Term Loan Facility, the lending bank may be able to accelerate amounts owed under the facility and may foreclose upon the assets securing our obligations.
The stated maturity of the Term Loan Facility is July 1, 2025. However, if the principal amount of our convertible debt exceeds $0.6 million as of June 1, 2024 or if the maturity of our 2019 Notes has not been extended beyond January 1, 2026 by June 1, 2024, then the maturity date of the Term Loan Facility will be June 1, 2024. The interest rate on the Term Loan Facility is the greater of 4.0% or a floating per annum rate equal to three quarters of one percentage point (0.75%) above the prime rate. Interest on any outstanding term loan advances is due and payable monthly. In addition to the monthly interest payments, a final payment equal to 6.5% of the original principal amount of each advance is due on the earlier of the maturity date or the date the advance is repaid. Principal balances are required to be repaid in twenty-four equal installments beginning on August 1, 2023. The Term Loan Facility is secured by substantially all of our assets, other than intellectual property. The Term Loan Facility contains customary affirmative and negative covenants which, unless waived by the bank, limit our ability to, among other things, incur additional indebtedness, grant liens, make investments, repurchase stock, pay dividends, transfer assets, enter into affiliate transactions, undergo a change of control, or engage in merger and acquisition activity, including merging or consolidating with a third party. Additionally, we are required to maintain a minimum Adjusted Quick Ratio (as defined in the Term Loan Facility) of at least 1.25 to 1.00. If we fail to comply with the covenants and our other obligations under the Term Loan Facility, the lending bank would be able to accelerate the required repayment of amounts due under the Term Loan Facility and, if they are not repaid, could foreclose upon the assets securing our obligations under the Term Loan Facility.
Our ability to use net operating loss carryforwards to offset future taxable income for U.S. federal income tax purposes and other tax benefits may be limited.
Section 382 of the Internal Revenue Code of 1986, as amended (the Code), imposes an annual limitation on the amount of taxable income that may be offset by net operating loss carryforwards (NOLs) if a corporation experiences an “ownership change.” As provided in Section 382 of the Code, an “ownership change” occurs when a company’s “five-percent shareholders” collectively increase their ownership in the company by more than 50 percentage points (by value) over a rolling three-year period. Various states also have limitations on the use of state NOLs following an ownership change.
Future changes in our stock ownership, some of which are outside our control, could result in an ownership change under Section 382 of the Code. In 2022, we experienced an ownership change, which substantially limited our ability to use our NOLs. There is no assurance that we will be able to fully utilize our future NOLs or other tax benefits, which could adversely impact our results of operations.
We believe that these tax benefits are a valuable asset for us and we monitor our stock ownership to determine whether our NOLs are at material risk of limitation based on an ownership change pursuant to Section 382. If our board of directors determines a potential risk exists that our NOLs could be limited, it could elect to adopt a tax benefit preservation plan in an effort to protect our tax benefits. Adoption of a tax benefit preservation plan could make it more difficult for a third party to acquire, or could discourage a third party from acquiring, us or a large block of our common stock.
52
We are subject to risks related to taxation in multiple jurisdictions and our effective income tax rate could be adversely affected and we could have additional tax liability if existing tax laws or regulations change or if taxing authorities disagree with our interpretations of tax laws or regulations.
We are subject to income taxes in both the United States and certain foreign jurisdictions. Significant judgments based on interpretations of existing tax laws or regulations are required in determining the provision for income taxes. For example, we have made certain interpretations of existing tax laws or regulations based upon the operations of our business internationally and we have implemented intercompany agreements based upon these interpretations and related transfer pricing analyses. If the U.S. Internal Revenue Service or other taxing authorities disagree with the positions, our effective income tax rate could be adversely affected and we could have additional tax liability, including interest and penalties. From time to time, we may review our corporate structure and tax positions in the various international jurisdictions in which we operate and such review may result in changes to how we structure our international business operations, which may adversely impact our effective income tax rate. Our effective income tax rate could also be adversely affected by changes in the mix of earnings in tax jurisdictions with different statutory tax rates, changes in the valuation of deferred tax assets and liabilities, changes in existing tax laws or tax rates, changes in the level of non-deductible expenses (including share-based compensation), changes in our future levels of research and development spending, mergers and acquisitions, or the result of examinations by various tax authorities. Payment of additional amounts as a result of changes in applicable tax law or upon final adjudication of any disputes could have a material impact on our results of operations and financial position.
Changes in accounting principles, or interpretations thereof, could have a significant impact on our financial position and results of operations.
We prepare our consolidated financial statements in accordance with U.S. GAAP. These principles are subject to interpretation by the SEC and various bodies formed to interpret and create appropriate accounting principles. A change in these principles can have a significant effect on our reported results and may even retroactively affect previously reported transactions. Additionally, the adoption of new or revised accounting principles may require that we make significant changes to our systems, processes and controls.
It is not clear if or when potential changes in accounting principles may become effective, whether we have the proper systems and controls in place to accommodate such changes and the impact that any such changes may have on our financial position and results of operations.
We have a significant amount of outstanding indebtedness, and our financial condition and results of operations could be adversely affected if we do not efficiently manage our liabilities.
We have significant outstanding convertible debt. As of December 31, 2023, we had outstanding $0.6 million aggregate principal amount of our 2.75% Convertible Senior Notes due 2034 that were issued in February 2014 (2014 Notes) and $55.0 million aggregate principal amount of our 5.25% Convertible Senior Notes due 2024 that were issued in November 2019 (2019 Notes and, together with the 2014 Notes, the Convertible Notes). The 2014 Notes will mature on February 1, 2034, unless earlier converted, redeemed, or repurchased in accordance with the terms of the 2014 Notes. Pursuant to the terms of the indenture governing the 2014 Notes, holders of the 2014 Notes may require us to repurchase all or a portion of their 2014 Notes at a repurchase price in cash equal to 100% of the principal amount of such 2014 Notes plus accrued and unpaid interest thereon, on each of February 6, 2024 and February 6, 2029. On February 6, 2024, one holder of the 2014 Notes exercised their repurchase right, and we repurchased an immaterial amount of principal and accrued interest. The 2019 Notes will mature on December 1, 2024, unless earlier converted or repurchased in accordance with the terms of the 2019 Notes.
If we undergo a fundamental change (as defined in the indenture governing the 2014 Notes or the 2019 Notes, as applicable), holders of the applicable series of Convertible Notes may require us to repurchase such Convertible Notes in whole or in part for cash at a repurchase price equal to 100% of the principal amount of the applicable series of Convertible Notes plus accrued and unpaid interest. If we refinance all or any portion of the Convertible Notes, we may issue additional convertible notes or other debt, which could include additional company obligations and represent more dilution to existing stockholders and noteholders.
This significant amount of debt has important risks to us and our investors, including:
53
In addition, to the extent we draw on our Revolving Credit Facility or otherwise incur additional indebtedness, the risks described above could increase. Further, if we increase our indebtedness, our actual cash requirements in the future may be greater than expected. Our cash flow from operations may not be sufficient to repay all of the outstanding debt as it becomes due, and we may not be able to borrow money, sell assets or otherwise raise funds on acceptable terms, or at all, to refinance our debt.
RISKS RELATED TO INTELLECTUAL PROPERTY
Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain.
Our commercial success depends in part on our ability to protect our intellectual property and proprietary technologies. We rely on patent protection, where appropriate and available, as well as a combination of copyright, trade secret, and trademark laws, and nondisclosure, confidentiality, and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. We apply for patents covering our products and technologies and uses thereof, as we deem appropriate. However, we may fail to apply for patents on important products and technologies in a timely fashion or at all. Our pending U.S. and foreign patent applications may not issue as patents or may not issue in a form that will be sufficient to protect our proprietary technology and gain or keep our competitive advantage. Any patents we have obtained or do obtain may be subject to re-examination, reissue, opposition, or other administrative proceeding, or may be challenged in litigation, and such challenges could result in a determination that the patent is invalid or unenforceable. In addition, competitors may be able to design alternative methods or devices that avoid infringement of our patents. Both the patent application process and the process of managing patent disputes can be time consuming and expensive.
Furthermore, the laws of some foreign countries may not protect our intellectual property rights to the same extent as do the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business. Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example:
To the extent our intellectual property, including licensed intellectual property, offers inadequate protection, or is found to be invalid or unenforceable, our competitive position and our business could be adversely affected.
We may be involved in lawsuits to protect or enforce our patents and proprietary rights, to determine the scope, coverage and validity of others’ proprietary rights, or to defend against third-party claims of intellectual property infringement, any of which could be time-intensive and costly and may adversely impact our business or stock price.
Litigation may be necessary for us to enforce our patent and proprietary rights, determine the scope, coverage, and validity of others’ proprietary rights, and/or defend against third-party claims of intellectual property infringement against us as well as against our suppliers, distributors, customers, and other entities with which we do business. Litigation could result in substantial legal fees and could adversely affect the scope of our patent protection. The outcome of any litigation or other proceeding is inherently uncertain and might not be favorable to us, and we might not be able to obtain licenses to technology that we require. Even if such licenses are obtainable, they may not be available at a reasonable cost. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our product margins or financial position. Further, we could encounter delays in product introductions, or interruptions in product sales, as we develop alternative methods or products.
As we move into new markets and applications for our products, incumbent participants in such markets may assert their patents and other proprietary rights against us as a means of impeding our entry into such markets or as a means to extract substantial license and royalty payments from us. Our commercial success may depend in part on our non-infringement of the patents or proprietary rights of third parties. Numerous significant intellectual property issues have been litigated, and will likely continue to be litigated, between existing and new participants in our existing and targeted markets. For example, some of our products provide for the testing and analysis of genetic material, and patent rights relating to genetic materials remain a developing area of patent law. A recent U.S. Supreme Court decision held, among other things, that claims to isolated genomic DNA occurring in nature are not patent eligible, while claims relating
54
to synthetic DNA may be patent eligible. We expect the ruling will result in additional litigation in our industry. In addition, third parties may assert that we are employing their proprietary technology without authorization, and if they are successful in making such claims, we may be forced to enter into license agreements, pay additional royalties or license fees, or enter into settlements that include monetary obligations or restrictions on our business.
Our customers have been sued for various claims of intellectual property infringement in the past, and we expect that our customers will be involved in additional litigation in the future. In particular, our customers may become subject to lawsuits claiming that their use of our products infringes third-party patent rights, and we could become subject to claims that we contributed to or induced our customer’s infringement. In addition, our agreements with some of our suppliers, distributors, customers, and other entities with which we do business may require us to defend or indemnify these parties to the extent they become involved in infringement claims against us, including the claims described above. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify any of these third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, operating results, or financial condition.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our employees’ former employers or other institutions or third parties with which such employees may have been previously affiliated.
Many of our employees were previously employed at universities or other life science or plant and animal research companies, including our competitors or potential competitors. In the future, we may become subject to claims that our employees, or we, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers or other third parties or institutions with which our employees may have been previously affiliated. Litigation may be necessary to defend against these claims. A resulting loss of key research personnel work product could hamper or prevent our ability to commercialize certain potential products or a loss of or inability to hire key marketing, sales or research and development personnel could adversely affect our future product development, sales and revenues, any of which could severely harm our business. Even if we are successful in defending against any such claims, litigation could result in substantial costs and be a distraction to management.
We depend on certain technologies that are licensed to us. We do not control these technologies and any loss of our rights to them could prevent us from selling our products, which would have an adverse effect on our business.
We rely on licenses in order to be able to use various proprietary technologies that are material to our business, including our core IFC, multi-layer soft lithography, and mass cytometry technologies. In some cases, we do not control the prosecution, maintenance, or filing of the patents to which we hold licenses, or the enforcement of these patents against third parties. Additionally, our business and product development plans anticipate and may substantially depend on future in-license agreements with additional third parties, some of which are currently in the early discussion phase. For example, our Canadian subsidiary (SB Canada) was party to an interim license agreement, now expired, under which the licensor granted SB Canada a worldwide, non-exclusive, RUO, royalty bearing license to certain cytometric reagents, instruments, and other products. While we were able to secure a license under a new license agreement with the licensor, we cannot provide assurances that we will always be able to obtain suitable license rights to technologies or intellectual property of other third parties on acceptable terms, if at all.
In December 2021, SomaLogic entered into the Collaboration Agreement with Illumina, Inc. (Illumina) to develop co-branded, distributable NGS-based proteomic products. As part of the Collaboration Agreement, Illumina will develop and deploy NGS-based protein identification and measurement tools into laboratories worldwide, and facilitate the development and use of high-plex protein pattern recognition tests.
There can be no assurance that any current contractual arrangements between us and third parties, such as Illumina, for example, or between our strategic partners and other third parties will be continued on materially similar terms and will not be breached or terminated early. Any failure to obtain or retain the rights to necessary technologies on acceptable commercial terms could require us to re-configure our products and services, which could negatively impact their commercial sale or increase the associated costs, either of which could materially harm our business and adversely affect our future revenues and ability to achieve sustained profitability.
In-licensed intellectual property rights that are fundamental to our business being operated present numerous risks and limitations. For example, all or a portion of the license rights granted may be limited for RUO, and in the event we attempt to expand into diagnostic applications, we would be required to negotiate additional rights, which may not be available to us on commercially reasonable terms, if at all.
55
Our rights to use the technology we license are also subject to the negotiation and continuation of those licenses. Certain of our licenses contain provisions that allow the licensor to terminate the license upon specific conditions. Our rights under the licenses are subject to our continued compliance with the terms of the license, including the payment of royalties due under the license. Because of the complexity of our products and the patents we have licensed, determining the scope of the license and related royalty obligation can be difficult and can lead to disputes between us and the licensor. An unfavorable resolution of such a dispute could lead to an increase in the royalties payable pursuant to the license. If a licensor believed we were not paying the royalties due under the license or were otherwise not in compliance with the terms of the license, the licensor might attempt to revoke the license. If such an attempt were successful and the license is terminated, we might be barred from marketing, producing, and selling some or all of our products, which would have an adverse effect on our business. Potential disputes between us and one of our existing licensors concerning the terms or conditions of the applicable license agreement could result, among other risks, in substantial management distraction; increased expenses associated with litigation or efforts to resolve disputes; substantial customer uncertainty concerning the direction of our product lines; potential infringement claims against us and/or our customers, which could include efforts by a licensor to enjoin sales of our products; customer requests for indemnification by us; and, in the event of an adverse determination, our inability to operate our business as currently operated. Termination of material license agreements could prevent us from manufacturing and selling our products unless we can negotiate new license terms or develop or acquire alternative intellectual property rights that cover or enable similar functionality. Any of these factors would be expected to have a material adverse effect on our business, operating results, and financial condition and could result in a substantial decline in our stock price.
We are subject to certain manufacturing restrictions related to licensed technologies that were developed with the financial assistance of U.S. governmental grants.
We are subject to certain U.S. government regulations because we have licensed technologies that were developed with U.S. government grants. In accordance with these regulations, these licenses provide that products embodying the technologies are subject to domestic manufacturing requirements. If this domestic manufacturing requirement is not met, the government agency that funded the relevant grant is entitled to exercise specified rights, referred to as “march-in rights,” which if exercised would allow the government agency to require the licensors or us to grant a non-exclusive, partially exclusive, or exclusive license in any field of use to a third party designated by such agency. All of our microfluidic systems revenue is dependent upon the availability of our IFCs, which incorporate technology developed with U.S. government grants. Our genomics instruments, including microfluidic systems and IFCs, are manufactured at our facility in Singapore. The federal regulations allow the funding government agency to grant, at the request of the licensors of such technology, a waiver of the domestic manufacturing requirement. Waivers may be requested prior to any government notification. We are not currently manufacturing instruments and IFCs in the United States that incorporate the relevant licensed technology. If our lack of compliance with any such provisions constituted a material breach of the license agreement, the license of the relevant patents could be terminated or we could be compelled to relocate our manufacturing of microfluidic systems and IFCs to the United States to avoid or cure a material breach of the license agreement. Any of the exercise of march-in rights, the termination of our license of the relevant patents or the relocation of our manufacturing of microfluidic systems and IFCs to the United States could materially adversely affect our business, operations and financial condition.
We are subject to certain obligations and restrictions relating to technologies developed in cooperation with Canadian government agencies.
Some of our Canadian research and development is funded in part through government grants and by government agencies. The intellectual property developed through these projects is subject to rights and restrictions in favor of government agencies and Canadians generally. In most cases the government agency retains the right to use intellectual property developed through the project for non-commercial purposes and to publish the results of research conducted in connection with the project. This may increase the risk of public disclosure of information relating to our intellectual property, including confidential information, and may reduce its competitive advantage in commercializing intellectual property developed through these projects. In certain projects, we have also agreed to use commercially reasonable efforts to commercialize intellectual property in Canada, or more specifically in the province of Ontario, for the economic benefit of Canada and the province of Ontario. These restrictions will limit our choice of business and manufacturing locations, business partners and corporate structure and may, in certain circumstances, restrict our ability to achieve maximum profitability and cost efficiency from the intellectual property generated by these projects. In one instance, a dispute with the applicable government funded entity may require mediation, which could lead to unanticipated delays in our commercialization efforts to that project. One of our Canadian government funded projects is also subject to certain limited “march-in” rights in favor of the government of the Province of Ontario, under which we may be required to grant a license to our intellectual property, including background intellectual property developed outside the scope of the project, to a responsible applicant on reasonable terms in circumstances where the government determines that such a license is necessary in order to alleviate emergency or extraordinary health or safety needs or for public use. In addition, we must provide reasonable assistance to the government in obtaining similar licenses from third parties required in connection with the use of its intellectual property. Instances in which the government of the Province of Ontario has exercised similar
56
“march-in” rights are rare; however, the exercise of such rights could materially adversely affect our business, operations and financial condition.
RISKS RELATED TO OUR CAPITAL STRUCTURE
The market value of our common stock could decline if the Purchasers sell their Series B Preferred Stock or common stock.
Pursuant to the Registration Rights Agreement that we entered into on January 23, 2022 with the Purchasers, we registered the resale of the shares of common stock issuable upon conversion of the Series B Preferred Stock with the SEC, which means that such shares would become eligible for resale in the public markets, subject to any applicable transfer restrictions. Any sale of such shares, or the anticipation of the possibility of such sales, could create downward pressure on the market price of our common stock.
Our Series B Preferred Stock has rights, preferences and privileges that are not held by, and are preferential to, the rights of our common stockholders, which could adversely affect our liquidity and financial condition, result in the interests of holders of our Series B Preferred Stock differing from those of our common stockholders and make an acquisition of us more difficult.
Holders of our Series B Preferred Stock have (i) a liquidation preference, (ii) rights to dividends, which are senior to all of our other equity securities, (iii) the right to require us to repurchase any or all of their Series B Preferred Stock in connection with certain change of control events, and (iv) conversion price adjustments upon the occurrence of certain events, each subject to the terms, conditions and exceptions contained in the applicable Certificate of Designations. These dividend and other rights and obligations could impact our liquidity and reduce the amount of cash flows available for working capital, capital expenditures, growth opportunities, acquisitions, and other general corporate purposes.
The terms of our Series B Preferred Stock could also limit our ability to obtain additional financing or increase our borrowing costs, which could have an adverse effect on our financial condition. The preferential rights could also result in divergent interests between the Purchasers and holders of our common stock. Furthermore, a sale of our Company, as a change of control event, may require us to repurchase the Series B Preferred Stock, which could have the effect of making an acquisition of our Company more expensive and potentially deterring proposed transactions that may otherwise be beneficial to our stockholders.
The holders of our Series B Preferred Stock are entitled to vote with the holders of our common stock with voting power measured in a manner related to the conversion ratio of the shares of Series B Preferred Stock, and the holders of our Series B Preferred Stock have rights to approve certain actions. The holders of our Series B Preferred Stock may exercise influence over us, including through the ability of the holders of the Series B-1 Preferred Stock and the holders of the Series B-2 Preferred Stock to each designate a member of our board of directors.
The holders of our Series B Preferred Stock are generally entitled to vote with the holders of our common stock on all matters submitted for a vote of holders of our common stock (voting together with the holders of common stock as one class) with voting power measured in a manner related to the conversion ratio of the shares of Series B Preferred Stock, subject to certain voting limitations as described in the applicable Certificate of Designations. Additionally, the consent of the holders of at least 60% of the shares of Series B Preferred Stock is required for, among other things, (i) amendments to our certificate of incorporation or bylaws that have an adverse effect on the rights, preferences, privileges or voting powers of the Series B Preferred Stock and (ii) issuances by us of securities that are senior to, or equal in priority with, the Series B Preferred Stock.
Additionally, pursuant to the Certificates of Designations for the Series B Preferred Stock, the holders of a majority of the outstanding Series B-1 Preferred Stock and the holders of a majority of the outstanding Series B-2 Preferred Stock each have the right to nominate and elect one member to our board of directors at each annual meeting of the stockholders of the Company or at any special meeting called for the purpose of electing directors, for so long as the Casdin Preferred Percentage or Viking Preferred Percentage (each as defined in the applicable Certificate of Designations), as applicable, is equal to or greater than 7.5%. Such directors are not subject to the classified board of directors provisions of our certificate of incorporation, and are entitled to serve on committees of our board of directors, subject to applicable law and Nasdaq rules. Notwithstanding the fact that all directors will be subject to fiduciary duties to us and to applicable law, the interests of the directors designated by the holders of Series B Preferred Stock may differ from the interests of our security holders as a whole or of our other directors.
These significant stockholders may be able to determine or significantly influence matters requiring stockholder approval. The interests of significant stockholders may not always coincide with our interests or the interests of other stockholders. The Certificates of Designations for the Series B Preferred Stock also provide that for so long as the Casdin Preferred Percentage or Viking Preferred Percentage, as applicable, is equal to or greater than 7.5%, the director designated by the holders of the Series B-1 Preferred Stock or the Series B-2 Preferred Stock, as applicable, will have certain consent rights over, among other things: (i) any increase in the number
57
of directors on our board of directors beyond seven; (ii) the hiring, promotion, demotion, or termination of the Company’s Chief Executive Officer; (iii) entering into or modifying (including by waiver) any transaction, agreement or arrangement with any Related Person (as defined in the Certificates of Designations for the Series B Preferred Stock), subject to certain exceptions; (iv) any voluntary petition under any applicable federal or state bankruptcy or insolvency law effected by the Company; (v) any change in the principal business of the Company or entry by the Company into any material new line of business; and (vi) for a period of three years after the closing date of the Private Placement Issuance, (A) any acquisition (including by merger, consolidation or acquisition of stock or assets) of any assets, securities or property of any other person or (B) any sale, lease, license, transfer or other disposition of any assets of the Company or any of its subsidiaries, in each case, other than acquisitions or dispositions of inventory or equipment in the ordinary course of business consistent with past practice, for consideration in excess of $50.0 million in the aggregate in any six month period.
As a result, the holders of our Series B Preferred Stock have the ability to influence the outcome of certain matters affecting our governance and capitalization. Our obligations to the holders of our Series B Preferred Stock could also limit our ability to obtain additional financing or increase our borrowing costs, which could have an adverse effect on our financial condition.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
Risk Management and Strategy
Standard BioTools regularly assesses risks from cybersecurity threats; monitors our information systems for potential vulnerabilities; and tests those systems pursuant to our cybersecurity policies, processes, and practices, which are integrated into our overall risk management program. To protect our information systems from cybersecurity threats, we use various security tools that are designed to protect against cyber security incidents, as well as to identify, escalate, investigate, resolve, and recover from security incidents in a timely manner. As part of this program, we conduct periodic assessments of our assets to evaluate the effectiveness of applicable security controls. These assessments are informed by industry standard frameworks (NIST, ISO) and include a review of our information security controls, policies and procedures to assess cybersecurity maturity against industry standards. In accordance with our IT Risk Management Program, we actively identify and assess risks based on the probability and potential impact to key business systems and processes. All risks identified will be assessed to identify the range of possible outcomes and risks will be prioritized by their level of importance. Each risk will be assigned to a risk owner who will track, monitor, and report on the status with a risk response aligned to the probability and impact of occurrence. Risks that are considered high are incorporated into our corporate risk management program overseen by the Audit Committee and our Board of Directors.
All employees receive cybersecurity training upon hire with at least annual training thereafter with job-specific topic considerations. Our Information Security team, consisting of the VP of Information Technology, Sr. Manager of Network Security and IT Security Manger, among others, engage third-party vendors to assist with providing timely cybersecurity threat alerts in addition to monitoring for cybersecurity threats and our defenses against cyberattacks. This monitoring includes the proactive identification of vulnerabilities in our systems through testing and threat intelligence awareness. The employees within our Information Security team and broader IT team who specialize in cybersecurity operations are responsible for coordinating and overseeing the activities of these third-party vendors.
Additionally, we require each third-party service provider with access to our internal systems, applications or data to certify that it has the ability to implement and maintain appropriate security measures, consistent with all applicable laws, to implement and maintain reasonable security measures in connection with their work with us, and to promptly report any suspected breach of its security measures that may affect our company. Our practice is to perform due diligence, including the completion of security questionnaires and risk assessments, as appropriate, on these third parties.
We describe whether and how risks from identified cybersecurity threats, including as a result of any previous cybersecurity incidents, have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations, or financial condition in our risk factor titled “Security incidents, loss of data, cyberattacks, and other information technology failures could disrupt our operations, damage our reputation, and adversely affect our business, operations, and financial results,” in Part I, Item 1A. "Risk Factors." Refer to this risk factor for additional description of cybersecurity risks and potential related impacts on our Company.
58
As previously disclosed, in early 2019, we became aware of a ransomware attack that infiltrated and encrypted certain information technology systems, including systems containing critical business data. The financial impact of this incident was not material, and there were no changes to the previously released financial results or financial statements. As previously disclosed, immediately following the discovery, we commenced an investigation and were able to recover access to the compromised systems and restore their operation without significant loss of business data within weeks of the incident. Following the incident, we implemented additional protective measures and internal control policies and procedures. We also retained a professional cybersecurity investigation firm to conduct a full forensic analysis of the incident, and concluded that there was no evidence of malware, persistence mechanisms or other compromised exchange on-premises accounts within the Company’s environment.
In early 2024, Standard BioTools completed a merger with SomaLogic Operating Co, Inc. Critical to integration activities has been a wholesale review of policies, procedures and tools relevant to the combined cybersecurity environment with the objective of deploying and maintaining those which serve to reinforce our security presence to the greatest extent. While these activities persist, it has been noted that the SomaLogic organization takes a comparable, if not more stringent, approach to their cyber and information security posture inclusive of their ongoing ISO27001 compliance certification.
Governance
While our management team is responsible for the day-to-day management of the risks Standard BioTools faces, our Board has the responsibility to oversee management’s processes for identifying, monitoring, and addressing enterprise risks, evaluate and discuss with management its assessments of matters relating to enterprise risks, and oversee and monitor management’s plans to address such risks. The Board takes an enterprise-wide approach to risk management designed to support the achievement of organizational objectives, including strategic objectives, to improve long-term organizational performance, and to enhance stockholder value. In order to understand the most significant risks faced by the Company and the steps being taken to manage those risks, Standard BioTools conducts quarterly enterprise risk management assessments, facilitated by the Company’s executive leadership team in collaboration with the internal audit function, which are presented by management at each quarterly Board meeting. The Board’s review of our business is an integral aspect of its assessment of management’s tolerance for risk and its determination as to the appropriate level of risk for our Company.
Although the Board has determined that enterprise risk management should be the responsibility of the Board as a whole, it has delegated responsibility to oversee specific areas of risk management to its committees. Our Audit Committee oversees and reviews the Company’s cybersecurity, data privacy, and other information technology risks, controls and procedures, including the Company’s plans to mitigate cybersecurity risks and respond to data breaches. At periodic meetings of the Board and its committees and in other meetings and discussions, management reports to the Board and its committees with respect to the most significant risks that could affect our business, including cybersecurity-related risks. Our Audit Committee also receives prompt and timely information regarding any cybersecurity incident to meet reporting thresholds, as well as ongoing updates regarding any such incident until it has been addressed.
Our cybersecurity risk management and strategy processes are led by our Chief Financial Officer and our Vice President of Information Technology. Our Vice President of Information Technology has over 18 years of work experience in various roles involving managing information security, developing cybersecurity strategy, implementing effective information and cybersecurity programs and has carried relevant degrees and certifications, including Certified Information Systems Auditor. These management team members are informed about and monitor the prevention, mitigation, detection, and remediation of cybersecurity incidents through their management of, and participation in, the cybersecurity risk management and strategy processes described above, including the operation of our incident response plan. As discussed above, these management team members report to the Audit Committee of our Board of Directors about cybersecurity threat risks, among other cybersecurity related matters, on an at least annual basis. Should a material breach be identified, as defined by the Board and the executive team, these management team members will notify the executive team and the Board and draft the required disclosure.
ITEM 2. PROPERTIES
We lease approximately 78,000 square feet of office and laboratory space at our headquarters in South San Francisco, California under a 10-year operating lease that commenced in March 2020. In Singapore, we lease approximately 40,000 square feet of office, laboratory and manufacturing space that expires in June 2027 and 5,000 square feet of similar mixed-use space that expires at the end of April 2024. In Ontario, Canada, we lease a 9,000 square foot property that expires in February 2025, a 44,500 square feet property that that expires in March 2026 and a 19,000 square feet property that expires in March 2027. As of December 31, 2023, we also lease office space in Japan, China, and France under short-term arrangements that expire through November 2026.
59
In August 2022, we entered into an operating agreement to sublease approximately 25% of our corporate headquarters facility in South San Francisco, California for $4.8 million over a 39-month term. On February 28, 2023, we entered into a separate agreement with an unrelated party to sublease an additional 25% of the headquarters facility. We expect to recognize $9.1 million in sublease income over the 77-month term of the agreement, which commenced in December 2023 and expires concurrent with the expiration of the head-lease in April 2030.
In connection with the Merger, on January 5, 2023, we assumed leases for office and laboratory space in Boulder, Colorado and La Jolla, California. We lease approximately 60,000 square feet of space under two leases in Boulder, Colorado, and approximately 10,000 square feet under one lease in La Jolla, California. All leases serve as both office and laboratory space.
We believe that all of our leased properties are in good condition and are adequate and suitable to use for their intended purpose, and that suitable additional space would be available on commercially reasonable terms if required. Refer to Note 7 of our consolidated financial statements for additional information about leased properties in this Annual Report.
ITEM 3. LEGAL PROCEEDINGS
Shareholder Litigation
On November 28, 2023, a purported stockholder filed a complaint against us and the members of our Board in the United States District Court for the Northern District of California. The complaint has since been voluntarily dismissed. On December 12, 2023 two separate shareholder complaints were filed in the District of Delaware, The complaints asserted claims under Section 14(a) of the Exchange Act and Rule 14a-9 promulgated thereunder and Section 20(a) of the Exchange Act for allegedly causing the filing with the SEC on November 14, 2023 of a materially deficient registration statement on Form S-4. Among other remedies, the plaintiffs sought to enjoin a stockholder vote on the proposed Merger. We are reviewing the complaints and have not yet formally responded to them. On December 13, 2023, a complaint was filed in the Delaware Court of Chancery against SomaLogic and certain officers and directors alleging Breach of Fiduciary Duty and Aiding and Abetting Breach of Fiduciary Duty. This complaint also sought an injunction postponing the proposed transaction, which was denied by the Court on January 4, 2024. The non-injunctive claims, including breach of fiduciary duty, are still being litigated. Litigation is inherently uncertain and there can be no assurance regarding the outcome. Whether or not any plaintiffs’ claim is successful, this type of litigation may result in significant costs and divert management’s attention and resources, which could adversely affect the operation of our business.
Between October 24, 2023 and January 3, 2024, SomaLogic received 16 letters from purported shareholders demanding that SomaLogic allow the inspection of its books and records and/or make corrective disclosures to its registration statement.
Additional lawsuits against us and certain of our officers or directors may be filed in the future. If additional similar complaints are filed, absent new or different allegations that are material, we will not necessarily announce such additional filings.
In the normal course of business, we are from time to time involved in legal proceedings or potential legal proceedings, including matters involving employment, intellectual property, or others. Although the results of litigation and claims cannot be predicted with certainty, we currently believe that the final outcome of any currently pending matters would not have a material adverse effect on our business, operating results, financial condition, or cash flows. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
60
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market for Our Common Stock; Dividends
Our common stock began trading on the Nasdaq Global Select Market under the symbol “FLDM” on February 10, 2011. As of April 2022, in connection with the closing of the Private Placement Issuance and the approval of our Eighth Amended and Restated Certificate of Incorporation, our common stock is listed on the Nasdaq Global Select Market under the symbol “LAB”.
We had 267 stockholders of record as of February 21, 2024; however, because many of our outstanding shares are held by brokers or other institutions on behalf of stockholders, we are unable to estimate the total number of beneficial owners represented by the holders of record.
We have never declared or paid cash dividends on our common stock and do not expect to pay dividends on our common stock for the foreseeable future. Instead, we anticipate that all of our earnings in the foreseeable future will be used for the operation and growth of our business.
Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
On November 23, 2022, our board of directors authorized a share repurchase program (the 2022 Share Repurchase Program) pursuant to which we may repurchase up to $20.0 million of our common stock through open market or privately negotiated transactions until December 31, 2023. The repurchases are contingent upon favorable market and business conditions and are funded by cash on hand. The program does not obligate us to acquire any specific number of shares. On October 4, 2023, we terminated the share repurchase program.
The following table provides information with respect to the shares of common stock repurchased by us during the year ended December 31, 2023:
Period |
|
Total Number of Shares Purchased |
|
|
Average Price Paid Per Share (1) |
|
|
Total Number of Shares Purchased as Part of Publicly Announced Program |
|
|
Approximate Dollar Value of Shares that May Yet Be Purchased Under the Program |
|
||||
January 1, 2023 - March 31, 2023 |
|
|
1,250,484 |
|
|
$ |
1.97 |
|
|
|
1,250,484 |
|
|
$17.0 million |
|
|
April 1, 2023 - June 30, 2023 |
|
|
1,208,200 |
|
|
$ |
1.96 |
|
|
|
1,208,200 |
|
|
$14.6 million |
|
|
July 1, 2023 - September 30, 2023 |
|
|
175,910 |
|
|
$ |
2.27 |
|
|
|
175,910 |
|
|
$14.2 million |
|
|
October 1, 2023 - December 31, 2023 |
|
|
75,109 |
|
|
$ |
2.32 |
|
|
|
75,109 |
|
|
|
— |
|
1 Average price paid per share includes related expenses.
On February 6, 2024, our board of directors authorized a new share repurchase program (the 2024 Share Repurchase Program) pursuant to which we may repurchase up to $50.0 million of shares of our common stock in the open market, in one or more Rule 10b5-1 trading plans, or in negotiated transactions through March 1, 2026. The repurchases are contingent upon favorable market and business conditions and are funded by cash on hand. The program does not obligate us to acquire any specific number of shares. As of the date of this Annual Report on Form 10-K, we have not repurchased any shares of our common stock under the 2024 Share Repurchase Program.
ITEM 6. RESERVED
61
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A) is intended to help the reader understand the results of operations and financial condition of Standard BioTools. This MD&A is provided as a supplement to, and should be read together with, our consolidated financial statements and the notes to those statements included elsewhere in this Annual Report. This Annual Report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended (the Exchange Act), that are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements include information concerning our possible or assumed future cash flow, revenue, sources of revenue and results of operations, cost of product revenue and product margin, operating and other income and expenses, unit sales and the selling prices of our products, business strategies and strategic priorities, changes in commercial and strategic focus, restructuring plan, reduction-in-force and real estate footprint reduction plans, microfluidics research and development and marketing investment reduction plans, other cost reduction initiatives, portfolio rationalization initiatives, operating discipline improvement plans, implementation of Standard BioTools Business Systems, expected costs and cost savings associated with such plans and initiatives, future product offerings, financing plans, capital allocation plans, expansion of our business, merger and acquisition opportunities, competitive position, industry environment, potential growth opportunities and drivers, market growth expectations, the effects of competition and public health crises on our business, the global supply chain, and our customers, suppliers and other business partners, and our expectations with respect to the Merger with SomaLogic , the anticipated financial impact and potential benefits to us related to the Merger, and integration of the businesses and other matters related to the Merger;. Forward-looking statements include statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “seeks,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms.
Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. We discuss these risks in greater detail in Part I, Item 1A, “Risk Factors” in this Annual Report. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, forward-looking statements represent our management’s beliefs and assumptions only as of the date of this Annual Report.
Except as required by law, we assume no obligation to update these forward-looking statements, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future. Unless otherwise stated, our forward-looking statements do not reflect the potential impact of the Merger or any other future acquisitions, mergers, dispositions, joint ventures or investments we may make. You should read this Annual Report completely and with the understanding that our actual future results may be materially different from what we expect.
Overview
Standard BioTools Inc. is driven by a bold purpose – unleashing tools to accelerate breakthroughs in human health. We have an established portfolio of essential, standardized next-generation high resolution technologies that assist biomedical researchers develop medicines faster and better. Our tools are designed to provide reliable and repeatable insights in health and disease using our proprietary mass cytometry and microfluidics technologies, which are useful in proteomics and genomics that help transform scientific discoveries into better patient outcomes. We work with leading academic, government, pharmaceutical, biotechnology, plant and animal research, and clinical laboratories worldwide, focusing on the most pressing needs in translational and clinical research, including oncology, immunology, and immunotherapy.
We distribute our systems through our direct sales force and support organizations located in North America, Europe, and Asia-Pacific, and through distributors or sales agents in several European, Latin American, Middle Eastern, and Asia-Pacific countries. Our manufacturing operations are located in Singapore and Canada.
On January 5, 2024, we completed the Merger with SomaLogic, creating a leading provider of differentiated multi-omics tools for research.
62
Recent Developments
Reductions in Headcount and Sub-Leases
We took additional actions in the year ended December 31, 2023 under our strategic initiative to improve operating discipline, including reductions to our headcount in Europe and additional reductions to our real estate footprint in the U.S. On February 28, 2023, we signed an agreement to sublease an additional 25% of our corporate headquarters for a period of 77 months. As a result, 50% of the space at our headquarters was subleased as of December 31, 2023. We expect to recognize $9.1 million of sublease income over the term of this new agreement, and payments commenced on December 1, 2023.
Merger
On January 5, 2024, we completed the Merger pursuant to the Merger Agreement by and among us, SomaLogic and Merger Sub, pursuant to which Merger Sub merged with and into SomaLogic, with SomaLogic surviving as a wholly owned subsidiary of Standard BioTools. Upon the terms and subject to the conditions set forth in the Merger Agreement, at the Effective Time, each share of SomaLogic common stock converted into the right to receive 1.11 shares of our common stock.
In addition, as of the Effective Time, we assumed each SomaLogic stock incentive plan, outstanding option to purchase shares of SomaLogic Stock and outstanding restricted stock units convertible into shares of SomaLogic common stock, whether vested or unvested. In addition, as of the Effective Time, each SomaLogic warrant was treated in accordance with its terms.
Financial Operations Overview
Revenue
We generate revenue primarily from sales of our products and services. Other revenue consists of revenue from product development and license agreements.
Our product revenue consists of sales of instruments and consumables. Consumables revenue is largely driven by the size of our active installed base of instruments and the level of usage per instrument. Service revenue is linked to the sales and active installed base of our instruments as our service revenue primarily consists of post-warranty service contracts, preventive maintenance plans, instrument parts, installation and training for our instruments. We expect the average selling prices of our products and services to fluctuate over time based on market conditions, product mix and currency fluctuations.
Cost of Revenue
Cost of product revenue includes manufacturing costs incurred in the production process, including component materials, labor and overhead, installation, packaging and delivery costs. In addition, cost of product revenue includes amortization of developed technology and intangibles, royalty costs for licensed technologies included in our products, warranty costs, provisions for slow-moving excess and obsolete inventory and stock-based compensation expense. Our cost of product revenue and related product margin may fluctuate depending on the capacity utilization of our manufacturing facilities in response to market conditions and the demand for our products.
Cost of service revenue includes direct labor hours, overhead and instrument parts. Our cost of service revenue and related service margin may fluctuate depending on the variability in material and labor costs of servicing.
Research and Development (R&D)
R&D expense consists primarily of compensation-related costs, product development and material expenses and other allocated facilities and information technology expenses. Our R&D efforts have focused primarily on enhancing our technologies and supporting development and commercialization of new and existing products and services. R&D expense also includes costs incurred in conjunction with research grants and product development arrangements.
Selling, General, and Administrative
SG&A expense consists primarily of personnel costs for our sales and marketing, business development, finance, legal, human resources, information technology and general management teams, as well as professional services, including legal and accounting services.
63
Restructuring and Related Charges
Restructuring and related charges primarily consist of severance costs and facilities costs for floors we have subleased or have the intent to sublease (net of sublease income) under our facility lease in South San Francisco. These costs, including a reduction in force, are incurred to improve operational efficiency, achieve cost savings and align our workforce to the future needs of the business. In addition to the reduction in force, we are reducing leased office space, optimizing our manufacturing footprint and streamlining support functions.
Transaction-related expenses
Transaction-related expenses consist of costs incurred during the year ended December 31, 2023 in connection with the Merger Agreement, including legal, advisory, accounting and other transaction-related costs. We expect to continue incurring these costs through the first quarter of 2024. The costs incurred during the year ended December 31, 2022 relate to the private placement whereby the Company issued and sold an aggregate of $225.0 million of convertible preferred stock in connection with the conversion of the bridge loans, which closed on April 4, 2022.
Results of Operations
The following table presents our consolidated statements of operations and as a percentage of total revenue for the years ended December 31, 2023 and 2022 ($ in thousands):
|
|
Year Ended December 31, |
|
|||||||||||||
|
|
2023 |
|
|
2022 |
|
||||||||||
Revenue |
|
$ |
106,340 |
|
|
|
100 |
% |
|
$ |
97,948 |
|
|
|
100 |
% |
Cost of revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cost of product revenue |
|
|
44,942 |
|
|
|
42 |
% |
|
|
52,555 |
|
|
|
54 |
% |
Cost of service and other revenue |
|
|
10,948 |
|
|
|
11 |
% |
|
|
8,342 |
|
|
|
9 |
% |
Total cost of revenue |
|
|
55,890 |
|
|
|
53 |
% |
|
|
60,897 |
|
|
|
63 |
% |
Gross profit |
|
|
50,450 |
|
|
|
47 |
% |
|
|
37,051 |
|
|
|
37 |
% |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
|
25,948 |
|
|
|
24 |
% |
|
|
37,382 |
|
|
|
38 |
% |
Selling, general and |
|
|
87,541 |
|
|
|
82 |
% |
|
|
102,285 |
|
|
|
104 |
% |
Restructuring and related charges |
|
|
7,076 |
|
|
|
7 |
% |
|
|
9,732 |
|
|
|
10 |
% |
Transaction-related expenses |
|
|
6,485 |
|
|
|
6 |
% |
|
|
3,857 |
|
|
|
4 |
% |
Total operating expenses |
|
|
127,050 |
|
|
|
119 |
% |
|
|
153,256 |
|
|
|
156 |
% |
Loss from operations |
|
|
(76,600 |
) |
|
|
(72 |
)% |
|
|
(116,205 |
) |
|
|
(119 |
)% |
Interest expense |
|
|
(4,567 |
) |
|
|
(4 |
)% |
|
|
(4,331 |
) |
|
|
(4 |
)% |
Loss on forward sale of Series B |
|
|
— |
|
|
|
— |
% |
|
|
(60,081 |
) |
|
|
(61 |
)% |
Loss on Bridge Loans |
|
|
— |
|
|
|
— |
% |
|
|
(13,719 |
) |
|
|
(14 |
)% |
Other income (expense), net |
|
|
6,963 |
|
|
|
6 |
% |
|
|
1,408 |
|
|
|
1 |
% |
Loss before income taxes |
|
|
(74,204 |
) |
|
|
(70 |
)% |
|
|
(192,928 |
) |
|
|
(197 |
)% |
Income tax benefit (expense) |
|
|
(452 |
) |
|
|
— |
% |
|
|
2,830 |
|
|
|
3 |
% |
Net loss |
|
$ |
(74,656 |
) |
|
|
(70 |
)% |
|
$ |
(190,098 |
) |
|
|
(194 |
)% |
Revenue
Revenue by product type and as a percentage of total revenue were as follows ($ in thousands):
|
|
Year Ended December 31, |
|
|
Year-over- |
|
||||||||||||||
|
|
2023 |
|
|
2022 |
|
|
Year Change |
|
|||||||||||
Product revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Instruments |
|
$ |
37,459 |
|
|
|
36 |
% |
|
$ |
25,664 |
|
|
|
26 |
% |
|
|
46 |
% |
Consumables |
|
|
41,739 |
|
|
|
39 |
% |
|
|
46,790 |
|
|
|
48 |
% |
|
|
(11 |
)% |
Total product revenue |
|
|
79,198 |
|
|
|
75 |
% |
|
|
72,454 |
|
|
|
74 |
% |
|
|
9 |
% |
Service revenue |
|
|
25,980 |
|
|
|
24 |
% |
|
|
23,712 |
|
|
|
24 |
% |
|
|
10 |
% |
Other revenue |
|
|
1,162 |
|
|
|
1 |
% |
|
|
1,782 |
|
|
|
2 |
% |
|
|
(35 |
)% |
Total revenue |
|
$ |
106,340 |
|
|
|
100 |
% |
|
$ |
97,948 |
|
|
|
100 |
% |
|
|
9 |
% |
64
Total revenue grew 9% to $106.3 million for the year ended December 31, 2023, compared to 2022. The growth was primarily attributable to increased instrument placements, primarily in our proteomics end user markets, offset by declines in our genomics end user markets as the result of our decision to reorganize, simplify and reposition our business over the past year. Revenue reported for the year ended December 31, 2022 includes a $1.6 million instrument revenue reduction for a one-time reserve recorded for our discontinued LCM product line in the genomics segment.
Instrument revenue grew 46% to $37.5 million for the year ended December 31, 2023, compared to 2022. Combined consumables and services revenue has experienced a flat to moderate decrease in 2023; however, the increase in instrument revenue is expected to drive increased consumables and services pull-through based on an expanded installed base, particularly in our proteomics end user markets.
Revenue by segment and as a percentage of total revenue were as follows ($ in thousands):
|
|
Year Ended December 31, |
|
|
Year-over- |
|
||||||||||||||
|
|
2023 |
|
|
2022 |
|
|
Year Change |
|
|||||||||||
Proteomics revenue |
|
$ |
63,883 |
|
|
|
60 |
% |
|
$ |
52,502 |
|
|
|
54 |
% |
|
|
22 |
% |
Genomics revenue |
|
|
42,457 |
|
|
|
40 |
% |
|
|
45,446 |
|
|
|
46 |
% |
|
|
(7 |
)% |
Total revenue |
|
$ |
106,340 |
|
|
|
100 |
% |
|
$ |
97,948 |
|
|
|
100 |
% |
|
|
9 |
% |
Total proteomics revenue grew 22% for the year ended December 31, 2023, compared to 2022, primarily due to the timing of customer orders. Our growth in proteomics was driven by expanded adoption of our flow symmetry solution, the CYTOF XT and early traction from the April 2023 release of Hyperion XTi, our next-generation imaging solution.
Total genomics revenue decreased 7% for the year ended December 31, 2023, compared to 2022, with instrument growth offsetting declines in consumables, service, and development revenues during the year. Consumables revenue in genomics was down over 2022, driven by the impact of initial consumables purchases by our OEM partner in 2022. The anticipated decline in the genomics segment was a primary driver of our decision to reorganize, simplify and reposition this business over the past year. We have implemented our strategy to focus on growing the OEM business and manage this segment to sustainable positive contribution margin in the near-term.
Cost of Revenue
Product and service cost, gross profit, and gross margin were as follows ($ in thousands):
|
|
Year Ended December 31, |
|
|
Year-over- |
|
||||||
|
|
2023 |
|
|
2022 |
|
|
Year Change |
|
|||
Cost of product revenue |
|
$ |
44,942 |
|
|
$ |
52,555 |
|
|
|
(14 |
)% |
Cost of service and other revenue |
|
|
10,948 |
|
|
|
8,342 |
|
|
|
31 |
% |
Total cost of revenue |
|
$ |
55,890 |
|
|
$ |
60,897 |
|
|
|
(8 |
)% |
Gross profit |
|
$ |
50,450 |
|
|
$ |
37,051 |
|
|
|
36 |
% |
Gross margin |
|
|
47.4 |
% |
|
|
37.8 |
% |
|
|
9.6 |
% |
Gross profit increased by $13.4 million, or 36%, for the year ended December 31, 2023, compared to 2022. The increases in gross profit were primarily attributable to certain one-time reductions to gross profit recognized during 2022, including a $7.9 million provision for excess and obsolete inventory, a $1.6 million revenue reserve related to the discontinuation of our laser capture microdissection products during the second quarter of 2022, and cost reductions driven by the relocation of operations to lower cost regions completed at the end of 2022.
Gross profit by segment was as follows ($ in thousands):
|
|
Year Ended December 31, |
Year-over- |
|
||||||||
|
|
2023 |
|
|
2022 |
Year Change |
|
|||||
Proteomics gross profit |
|
$ |
26,239 |
|
|
$ |
20,041 |
|
|
|
31 |
% |
Genomics gross profit |
|
|
24,211 |
|
|
|
17,010 |
|
|
|
42 |
% |
Total gross profit |
|
$ |
50,450 |
|
|
$ |
37,051 |
|
|
|
36 |
% |
65
During 2023, the proteomics business returned to growth with a gross profit improvement of 31% for the year ended December 31, 2023, compared to 2022. The increase was primarily attributable to increased proteomics revenue of $11.4 million as well as improved manufacturing efficiencies driven by higher unit sales of instruments. Genomics gross profit improved by 42% for the year ended December 31, 2023, compared to 2022. The year over year increase was primarily attributable to one certain one-time reductions to gross profit recognized during 2022, including $7.2 million of inventory write-offs involving discontinued products and other slow-moving inventory. We expect profitable growth in the genomics business in future years. These impacts resulted in an overall increase of 36% to gross profit for the year ended December 31, 2023.
Operating Expenses
Operating expenses were as follows ($ in thousands):
|
|
Year Ended December 31, |
|
|
Year-over- |
|
||||||
|
|
2023 |
|
|
2022 |
|
|
Year Change |
|
|||
Research and development |
|
$ |
25,948 |
|
|
$ |
37,382 |
|
|
|
(31 |
)% |
Selling, general and administrative |
|
|
87,541 |
|
|
|
102,285 |
|
|
|
(14 |
)% |
Restructuring and related charges |
|
|
7,076 |
|
|
|
9,732 |
|
|
|
(27 |
)% |
Transaction-related expenses |
|
|
6,485 |
|
|
|
3,857 |
|
|
|
68 |
% |
Total operating expenses |
|
$ |
127,050 |
|
|
$ |
153,256 |
|
|
|
(17 |
)% |
Research and Development
R&D expense decreased by $11.4 million, or 31%, for the year ended December 31, 2023, compared to 2022. The decreases were primarily due to a $3.5 million impairment charge related to our acquisition of InstruNor AS (InstruNor) recognized during the year December 31, 2022, as well as lower compensation and consulting costs and reduced spending on laboratory supplies. These reductions were related to our strategic initiatives to reduce headcount and improve operating efficiencies by engaging in lower-cost and more focused R&D projects and activities.
Selling, General and Administrative
SG&A expense decreased by $14.7 million, or 14%, for the year ended December 31, 2023 compared to 2022. The decreases were primarily attributable to decreased salaries and benefits expense and stock-based compensation expense as a result of the restructuring plan that downsized our global workforce.
Restructuring and Related Charges
Restructuring and related charges consisted of the following (in thousands):
|
|
Year Ended December 31, |
|
|
Year-over- |
|
||||||
|
|
2023 |
|
|
2022 |
|
|
Year Change |
|
|||
Severance and other termination benefits |
|
$ |
2,379 |
|
|
$ |
5,849 |
|
|
|
(59 |
)% |
Facilities and other |
|
|
4,697 |
|
|
|
3,883 |
|
|
|
21 |
% |
Total restructuring and related charges |
|
$ |
7,076 |
|
|
$ |
9,732 |
|
|
|
(27 |
)% |
Restructuring and related charges decreased by $2.7 million for the year ended December 31, 2023 compared to 2022, due to decreased severance costs and decreased facilities expenses (net of sublease income) as a result of the subleases that commenced in October 2022 and December 2023 as part of our restructuring plan. During the year ended December 31, 2023, we recognized $2.7 million in sublease income which offset operating lease expense within restructuring and related charges on our consolidated statements of operations. As of December 31, 2023, we expect to recognize an additional $11.9 million in sublease income over the remaining lease terms to further offset operating lease expense.
Transaction-related expenses
Transaction-related expenses increased by $2.6 million for the year ended December 31, 2023 compared to 2022. The increase was due to legal, advisory, and accounting costs incurred in connection with the Merger Agreement offset by $3.9 million in costs related to our Private Placement which closed in April 2022. The Company expects to incur additional amounts in future periods for the Merger Agreement.
66
Other Non-Operating Income (Expense)
The increase in other income (expense), net of $5.6 million for the year ended December 31, 2023 compared to 2022, was primarily due to the interest earned on money market funds and short-term investments. We previously had no such investments until the second half of 2022.
The improvement in non-operating income (expense) in the year ended December 31, 2023, compared to 2022, primarily reflects losses related to the Private Placement. The Series B Convertible Preferred Stock Purchase Agreements entered into with various investors were accounted for as forward sales contracts and recorded at fair value. The loan agreements (collectively, the Bridge Loans) that we entered into on January 23, 2022 with various investors for a $25.0 million term loan were also recorded at fair value. In the year ended December 31, 2022 the $60.1 million loss on the forward sales of Series B Preferred Stock and the loss on the Bridge Loans of $13.7 million reflected the increase in the price of our common stock from January 23, 2022 (the date of the Purchase Agreements and the Bridge Loan agreements) to the Private Placement Closing Date.
Income Tax Benefit (Expense)
We recorded income tax expense of $0.5 million for the year ended December 31, 2023 and an income tax benefit of $2.8 million for the year ended December 31, 2022. The increase in our tax provision reflects the effect of our foreign operations, which reported pre-tax income in the year ended December 31, 2023 and pre-tax loss in the year ended December 31, 2022.
Our effective tax rates for both periods differ from the 21% U.S. Federal statutory tax rate primarily due to valuation allowances recorded against deferred tax assets on domestic losses and the tax rate differences between the U.S. and foreign countries.
Liquidity and Capital Resources
We have experienced operating losses since inception and have an accumulated deficit of $1.0 billion as of December 31, 2023. To date, we have funded our operating losses primarily through equity offerings, term loans, convertible notes and redeemable preferred stock. Our ability to fund future operations and meet debt covenant requirements will depend upon our level of future revenue and operating cash flow and our ability to access additional funding through either equity offerings, issuances of debt instruments or both.
Our liquidity and capital requirements depend upon many factors, including market acceptance of our products and services; effectiveness of our business improvement initiatives and restructuring programs; costs of supporting sales growth, product quality, R&D and capital expenditures, including our ERP upgrade; and costs and timing of acquiring other businesses, assets or technologies.
We continually evaluate our liquidity requirements considering our operating needs, growth initiatives and capital resources. We expect that our existing liquidity and sources of capital will be sufficient to support our operations for at least the next 12 months from the filing date of this Annual Report.
Sources of Liquidity
Our principal sources of liquidity are cash, cash equivalents and short-term investments. Our collective balances of cash, cash equivalents and short-term investments were $114.9 million at December 31, 2023 and $165.8 million at December 31, 2022. Our working capital was $48.9 million at December 31, 2023.
Giving effect to our Merger with SomaLogic on January 5, 2024, our balance of cash, cash equivalents, short-term investments and restricted cash on a pro forma basis was $565.5 million at December 31, 2023.
Capital Resources and Commitments
We enter into arrangements that serve as sources of capital and the associated contractual agreements may result in firm or contingent obligations of us. In addition to our common stockholders’ equity, our sources of capital primarily include debt, mezzanine equity and operating leases. Our Series B Preferred Stock, which is classified as mezzanine equity, contains rights that may result in their conversion to our common stock or their redemption in cash. Our term loan and operating lease arrangements require cash repayment and our convertible debt that matures on December 1, 2024 contains rights that may result in their conversion to our common stock prior to maturity. We also enter into contractual and legally binding commitments to purchase goods.
A summary of our significant future capital requirements include:
67
Purchase Obligations and Commitments
Purchase obligations consist of contractual and legally binding commitments to purchase goods and services. Our purchase obligations with suppliers specify all significant terms, including fixed, minimum or variable price provisions, and the approximate timing of the transaction The majority of our contracts are cancellable with little or no notice or penalty. However, once a vendor has incurred costs to fulfill a contract with us, and which costs cannot be otherwise deployed, we are liable for those costs upon cancellation. As of December 31, 2023, these purchase commitments totaled $9.7 million. Capital expenditure commitments as of December 31, 2023 were immaterial. In addition, we have certain non-cancellable commitments with service providers that are not material in the aggregate.
In connection with the Merger, on January 5, 2024, we assumed a purchase commitment of $6.9 million to a contract manufacturer. Under the contract manufacturing agreement, we are required to spend $2.3 million per year for three years.
We have additional obligations beyond the purchase of goods and services, including the following:
The expected timing of payments of our obligations is estimated based on current information. Timing of payments and actual amounts paid may be different, depending on the timing of receipt of goods or services, or changes to agreed-upon amounts for some obligations. In addition, some of our future purchasing needs are not current contractual obligations and are therefore not included in the commitment amounts above as they are not handled through binding contracts or are not fulfilled by vendors on a purchase order basis within short time horizons.
Cash Flow Activity
Our cash flow summary was as follows ($ in thousands):
68
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Cash flow summary: |
|
|
|
|
|
|
||
Net cash used in operating activities |
|
$ |
(43,287 |
) |
|
$ |
(89,370 |
) |
Net cash provided by (used in) investing activities |
|
|
20,237 |
|
|
|
(88,127 |
) |
Net cash provided by (used in) financing activities |
|
|
(6,809 |
) |
|
|
230,758 |
|
Effect of foreign exchange rate fluctuations on cash |
|
|
34 |
|
|
|
(404 |
) |
Net increase (decrease) in cash, cash equivalents and restricted cash |
|
$ |
(29,825 |
) |
|
$ |
52,857 |
|
We derive cash flows from operations primarily by collecting amounts due from sales of our products and services, and fees earned under our product development and license agreements. Our cash flows from operating activities are also significantly influenced by our use of cash for operating expenses and working capital to support the business. We have historically experienced negative cash flows from operating activities as we have expanded our business and built our infrastructure, domestically and internationally.
In the year ended December 31, 2023, we used $23.1 million of net proceeds from the sales and maturities of short-term investments to help fund $43.3 million of net cash used in operating activities, $5.4 million of common stock repurchases and $2.1 million of term loan repayments.
In the year ended December 31, 2022, we used $230.7 million of net debt and Series B Preferred Stock proceeds in part to fund $89.4 million used in operating activities, the purchase of short-term investments of $137.3 million and a $52.9 million increase in cash, cash equivalents and restricted cash.
Operating Activities
Net cash used in operating activities for the year ended December 31, 2023 decreased by $46.1 million compared to the same period in 2022. The decrease reflects a lower net loss and adjustments for non-cash items, which collectively used $45.1 million in the year ended December 31, 2023 compared to $73.9 million used in the same period of 2022, and changes in net operating assets and liabilities which provided $1.8 million and used $15.5 million in the years ended December 31, 2023 and 2022, respectively.
Investing Activities
Net cash provided by investing activities for the year ended December 31, 2023 was $20.2 million compared to $88.1 million used in the year ended December 31, 2022. The year ended December 31, 2023 primarily reflects $23.1 million of proceeds from sales and maturities of short-term investments, net of purchases. Net proceeds from the Private Placement issuance and the Bridge Loans were used to purchase short-term investments of $137.3 million during the year ended December 31, 2022.
Financing Activities
Financing activities used cash of $6.8 million for the year ended December 31, 2023 and provided cash of $230.8 million in the same period of 2022. These changes in cash from financing activities primarily reflect $5.4 million of common stock share repurchases and $2.1 million of term loan repayments in the year ended December 31, 2023, and $25.0 million of borrowings under the Bridge Loans and the repayment of $6.8 million borrowed under our Revolving Credit Facility as well as $225.0 million proceeds received from the issuance of Series B Preferred Sock less payments of $12.5 million in equity issuance costs in the year ended December 31, 2022.
Critical Accounting Estimates
The consolidated financial statements and related notes included in this Annual Report are prepared in accordance with U.S. GAAP. Preparing U.S. GAAP financial statements requires the use of estimates and assumptions to determine the value of the assets, liabilities, revenues and expenses reported on the consolidated balance sheets and statements of operations. We develop these estimates after considering historical transactions, the current economic environment and various other assumptions considered reasonable under the circumstances. Actual results may differ materially from these estimates and judgments. Accounts that rely heavily on estimated information to determine their values include revenue, trade receivables, inventories, right-of-use assets, goodwill, long-lived intangible assets, lease liabilities, and preferred equity. Refer to Note 2 to our consolidated financial statements for further information on our most significant accounting policies.
Revenue
We recognize revenue based on the amount of consideration we expect to receive in exchange for the goods and services we transfer to the customer. Our commercial arrangements typically include multiple, distinct products and services, and we allocate purchase
69
consideration to the products and services based on each item’s relative standalone selling price. Standalone selling prices (SSP) are generally determined using observable data from recent transactions. In cases where sufficient data is not available, we estimate a product’s SSP using a cost plus margin approach or by applying a discount to the product’s list price.
We have entered and may continue to enter into development agreements with customers that require us to recognize revenue using an input method that determines the extent of our progress toward completion by comparing the actual costs incurred to the total expected cost. As part of the accounting for these arrangements, we develop estimates and assumptions that require judgment to determine the transaction price and progress towards completion. We review these estimates at the end of each reporting period using the best available information, revise the estimates as necessary, and recognize revenue commensurate with our progress toward completion.
Inventories
Inventories are stated at the lower of cost (on a first-in, first-out basis) or net realizable value. Inventory costs include direct materials, direct labor, and normal manufacturing overhead. We regularly review inventory for excess and obsolete products and components. Significant judgment is required in determining provisions for slow-moving, excess, and obsolete inventories which are recorded when required to reduce inventory values to their estimated net realizable values based on product life cycle, development plans, product expiration, and quality issues.
Goodwill and Long-Lived Assets
Assessing goodwill and long-lived assets for impairment requires significant judgment as it involves selecting an appropriate valuation method, identifying reporting units, assigning assets and liabilities to the reporting units, and estimating future cash flows, remaining service lives, revenue growth rates, terminal values and discount rates. Refer to Note 4 to the consolidated financial statements for additional information.
Series B Redeemable Preferred Stock
The Purchase Agreements (as defined in Note 9 to the consolidated financial statements) for the issuance of shares of Series B Preferred Stock were accounted for as forward sales contracts at fair value in accordance with ASC 480, Distinguishing Liabilities from Equities. The Series B Preferred Stock was treated as mezzanine equity and recorded at its fair value upon issuance, net of issuance costs due to its redemption features, such as change of control and liquidation preference, which are outside of the Company’s control. Subsequent remeasurement of the Series B Redeemable Preferred Stock amount presented within mezzanine equity to its redemption amount is not required since it is not probable that the instrument will become redeemable. Mezzanine equity which has characteristics of both liabilities and shareholders’ equity (deficit) is presented separately on the consolidated balance sheets between these two items because it has some characteristics of both. Refer to Note 9 to the consolidated financial statements for additional information.
Stock-Based Compensation
The Company recognizes compensation costs for all stock-based awards, including stock options, Restricted Share Units (RSUs), Performance Share Units (PSUs) and stock purchased under the Company's Employee Share Purchase Plan (ESPP), based on the grant date fair value of the award. The Company recognizes stock-based compensation expense on a straight-line basis over the requisite service periods for non-performance-based awards. For RSUs, fair value is measured based on the closing fair market value of the Company's common stock on the date of grant. For PSUs with a market condition, the Company uses a Monte Carlo simulation pricing model to incorporate the market condition effects at the grant date. The Monte Carlo pricing model requires inputs which are subjective and generally requires judgment. For PSUs with performance conditions, stock-based compensation expense is recognized over the requisite service period when the achievement of each individual performance goal becomes probable.
The fair value of options and stock purchases under ESPP on the grant date is estimated using the Black-Scholes option-pricing model, which requires the use of certain subjective assumptions, including expected term, volatility, risk-free interest rate and the fair value of the Company's common stock. These assumptions generally require judgment. Refer to Note 11 to the consolidated financial statements for additional information.
70
Recent Accounting Changes and Accounting Pronouncements
Adoption of New Accounting Guidance
None.
Recent Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position, results of operations or cash flows is disclosed in Note 2 to our consolidated financial statements included in this Annual Report.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
71
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Index to Consolidated Financial Statements
72
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Standard BioTools Inc.
Opinion on the Financial Statements
We have audited the consolidated financial statements, including the related notes, of Standard BioTools Inc. and its subsidiaries (the “Company”) as listed in the accompanying index (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Revenue recognition – product revenues
As described in Notes 2 and 3 to the consolidated financial statements, the Company’s product revenue was $79.2 million for the year ended December 31, 2023. The Company generates revenue primarily from the sale of products and services. Product revenue is derived from the sale of instruments and consumables and is recognized once control of goods passes to the customer and the Company has an enforceable right to payment. The Company recognizes revenue based on the amount of consideration the Company expects to receive in exchange for the goods transferred to the customer.
The principal consideration for our determination that performing procedures relating to revenue recognition for product revenues is a critical audit matter is a high degree of auditor effort in performing procedures related to the Company’s revenue recognition.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included, among others, (i) testing the completeness, accuracy and occurrence of revenue recognized for a sample of revenue transactions by obtaining and inspecting source documents, such as sales contracts,
73
purchase orders, customer invoices, and proof of delivery and (ii) confirming a sample of outstanding customer invoice balances as of December 31, 2023 and, for confirmations not returned, obtaining and inspecting source documents, such as invoices, proof of delivery, and subsequent cash receipts.
/s/
March 1, 2024
We have served as the Company’s auditor since 2015.
74
STANDARD BIOTOOLS INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except par value)
|
|
December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Short-term investments |
|
|
|
|
|
|
||
Accounts receivable (net of allowances of $ |
|
|
|
|
|
|
||
Inventories, net |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
||
Property and equipment, net |
|
|
|
|
|
|
||
Operating lease right-of-use asset, net |
|
|
|
|
|
|
||
Other non-current assets |
|
|
|
|
|
|
||
Developed technology, net |
|
|
|
|
|
|
||
Goodwill |
|
|
|
|
|
|
||
Total assets |
|
$ |
|
|
$ |
|
||
LIABILITIES, MEZZANINE EQUITY AND STOCKHOLDERS’ DEFICIT |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
|
|
$ |
|
||
Accrued compensation and related benefits |
|
|
|
|
|
|
||
Operating lease liabilities, current |
|
|
|
|
|
|
||
Deferred revenue, current |
|
|
|
|
|
|
||
Deferred grant income, current |
|
|
|
|
|
|
||
Other accrued liabilities |
|
|
|
|
|
|
||
Term loan, current |
|
|
|
|
|
|
||
Convertible notes, current |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
Convertible notes, non-current |
|
|
|
|
|
|
||
Term loan, non-current |
|
|
|
|
|
|
||
Deferred tax liability |
|
|
|
|
|
|
||
Operating lease liabilities, non-current |
|
|
|
|
|
|
||
Deferred revenue, non-current |
|
|
|
|
|
|
||
Deferred grant income, non-current |
|
|
|
|
|
|
||
Other non-current liabilities |
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
Mezzanine equity: |
|
|
|
|
|
|
||
Redeemable preferred stock: $ |
|
|
|
|
|
|
||
Stockholders’ deficit: |
|
|
|
|
|
|
||
Preferred stock: $ |
|
|
|
|
|
|
||
Common stock: $ |
|
|
|
|
|
|
||
Additional paid-in capital |
|
|
|
|
|
|
||
Accumulated other comprehensive loss |
|
|
( |
) |
|
|
( |
) |
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Treasury stock at cost: |
|
|
( |
) |
|
|
( |
) |
Total stockholders’ deficit |
|
|
( |
) |
|
|
( |
) |
Total liabilities, mezzanine equity and stockholders’ deficit |
|
$ |
|
|
$ |
|
||
See accompanying notes
75
STANDARD BIOTOOLS INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Revenue: |
|
|
|
|
|
|
||
Product revenue |
|
$ |
|
|
$ |
|
||
Service and other revenue |
|
|
|
|
|
|
||
Total revenue |
|
|
|
|
|
|
||
Cost of revenue: |
|
|
|
|
|
|
||
Cost of product revenue |
|
|
|
|
|
|
||
Cost of service and other revenue |
|
|
|
|
|
|
||
Total cost of revenue |
|
|
|
|
|
|
||
Gross profit |
|
|
|
|
|
|
||
Operating expenses: |
|
|
|
|
|
|
||
Research and development |
|
|
|
|
|
|
||
Selling, general and administrative |
|
|
|
|
|
|
||
Restructuring and related charges |
|
|
|
|
|
|
||
Transaction-related expenses |
|
|
|
|
|
|
||
Total operating expenses |
|
|
|
|
|
|
||
Loss from operations |
|
|
( |
) |
|
|
( |
) |
Interest expense |
|
|
( |
) |
|
|
( |
) |
Loss on forward sale of Series B Preferred Stock |
|
|
|
|
|
( |
) |
|
Loss on Bridge Loans |
|
|
|
|
|
( |
) |
|
Other income (expense), net |
|
|
|
|
|
|
||
Loss before income taxes |
|
|
( |
) |
|
|
( |
) |
Income tax benefit (expense) |
|
|
( |
) |
|
|
|
|
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Net loss per share, basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
Shares used in computing net loss per share, basic and diluted |
|
|
|
|
|
|
||
See accompanying notes
76
STANDARD BIOTOOLS INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Other comprehensive income (loss), net of tax: |
|
|
|
|
|
|
||
Foreign currency translation adjustment |
|
|
( |
) |
|
|
( |
) |
Net change in unrealized gain (loss) on investments |
|
|
|
|
|
( |
) |
|
Other comprehensive income (loss), net of tax |
|
|
( |
) |
|
|
( |
) |
Comprehensive loss |
|
$ |
( |
) |
|
$ |
( |
) |
See accompanying notes
77
STANDARD BIOTOOLS INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands)
|
|
Common Stock |
|
|
Additional |
|
|
Accum. |
|
|
Accum. |
|
|
Treasury Stock |
|
|
Total Stockholders’ |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Comp. Loss |
|
|
Deficit |
|
|
Shares |
|
|
Amount |
|
|
(Deficit) |
|
||||||||
Balance as of December 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
|
||||
Issuance of restricted stock, net of shares withheld |
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
||
Issuance of common stock under ESPP |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Issuance of common stock from option exercises |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Repurchase of common stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Other comprehensive loss, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Balance as of December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|||
Issuance of restricted stock, net of shares withheld |
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
||
Issuance of common stock from option exercises |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Issuance of common stock under ESPP |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Repurchase of common stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Other comprehensive loss net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Balance as of December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|||
See accompanying notes
78
STANDARD BIOTOOLS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Operating activities |
|
|
|
|
|
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Loss on forward sale of Series B Preferred Stock |
|
|
|
|
|
|
||
Loss on bridge loans |
|
|
|
|
|
|
||
Stock-based compensation expense |
|
|
|
|
|
|
||
Amortization of developed technology |
|
|
|
|
|
|
||
Depreciation and amortization |
|
|
|
|
|
|
||
Provision for excess and obsolete inventory |
|
|
|
|
|
|
||
Impairment of InstruNor developed technology intangible |
|
|
|
|
|
|
||
Amortization of debt discounts, premiums and issuance costs |
|
|
|
|
|
|
||
Other non-cash items |
|
|
( |
) |
|
|
|
|
Changes in assets and liabilities: |
|
|
|
|
|
|
||
Accounts receivable, net |
|
|
( |
) |
|
|
|
|
Inventories, net |
|
|
( |
) |
|
|
( |
) |
Prepaid expenses and other assets |
|
|
|
|
|
|
||
Accounts payable |
|
|
|
|
|
( |
) |
|
Accrued compensation and related benefits |
|
|
|
|
|
|
||
Deferred revenue |
|
|
|
|
|
( |
) |
|
Other liabilities |
|
|
|
|
|
( |
) |
|
Net cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
Investing activities |
|
|
|
|
|
|
||
Purchases of short-term investments |
|
|
( |
) |
|
|
( |
) |
Proceeds from sales and maturities of investments |
|
|
|
|
|
|
||
Purchases of property and equipment |
|
|
( |
) |
|
|
( |
) |
Net cash provided by (used in) investing activities |
|
|
|
|
|
( |
) |
|
Financing activities |
|
|
|
|
|
|
||
Proceeds from bridge loans |
|
|
|
|
|
|
||
Proceeds from issuance of Series B Preferred Stock |
|
|
|
|
|
|
||
Repayment of term loan and advances under revolving credit facility |
|
|
( |
) |
|
|
( |
) |
Payment of debt and equity issuance costs |
|
|
|
|
|
( |
) |
|
Repurchase of common stock |
|
|
( |
) |
|
|
( |
) |
Proceeds from ESPP stock issuance and exercise of stock options |
|
|
|
|
|
|
||
Payments for taxes related to net share settlement of equity awards and other |
|
|
( |
) |
|
|
( |
) |
Net cash provided by (used in) financing activities |
|
|
( |
) |
|
|
|
|
Effect of foreign exchange rate fluctuations on cash and cash equivalents |
|
|
|
|
|
( |
) |
|
Net increase (decrease) in cash, cash equivalents and restricted cash |
|
|
( |
) |
|
|
|
|
Cash, cash equivalents and restricted cash at beginning of period |
|
|
|
|
|
|
||
Cash, cash equivalents and restricted cash at end of period |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Supplemental disclosures of cash flow information |
|
|
|
|
|
|
||
Cash paid for interest |
|
$ |
|
|
$ |
|
||
Cash paid for income taxes, net of refunds |
|
$ |
|
|
$ |
|
||
Non-cash right-of-use assets and lease liabilities |
|
$ |
|
|
$ |
|
||
Asset retirement obligations |
|
$ |
|
|
$ |
|
||
See accompanying notes
79
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2023
1. Description of Business
Standard BioTools Inc. (Standard BioTools or the Company), formerly known as Fluidigm Corporation, is a Delaware corporation headquartered in South San Francisco, California.
The Company has an established portfolio of essential, standardized next-generation technologies that help biomedical researchers develop medicines faster and better. As a leading solutions provider, the Company endeavors to provide reliable and repeatable insights in health and disease using its proprietary mass cytometry and microfluidics technologies that help transform scientific discoveries into better patient outcomes. Standard BioTools works with leading academic, government, pharmaceutical, biotechnology, plant and animal research and clinical laboratories worldwide, focusing on the most pressing needs in translational and clinical research, including oncology, immunology and immunotherapy.
On January 5, 2024, the Company completed its previously announced merger (the Merger) with SomaLogic, Inc. (SomaLogic) a protein biomarker discovery company enabling researchers to analyze various types of biological samples for protein biomarker signatures, which can be utilized in drug discovery and development. See Note 16 – Subsequent Events, for more information.
2. Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
The accompanying consolidated financial statements have been prepared in conformity with U.S. generally accepted accounting principles (U.S. GAAP) and include the accounts of the Company's wholly owned subsidiaries. As of December 31, 2023, the Company had wholly owned subsidiaries in Singapore, Canada, the Netherlands, Japan, France, Italy, the United Kingdom, China, Germany and Norway. All subsidiaries, except for Singapore, use their local currency as their functional currency. The Singapore subsidiary uses the U.S. dollar as its functional currency. All intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. The Company bases its estimates on historical experience, the current economic environment and on various other assumptions believed to be reasonable, which together form the basis for making judgments about the carrying values of assets and liabilities. These accounting matters included but were not limited to inventory and related reserves, the carrying value of goodwill and other long-lived assets, and the potential outcome of uncertain tax positions that have been recognized in the Company's financial statements or tax returns. The Company also uses significant judgment in determining the fair value of financial instruments, including debt and equity instruments. Actual results could differ materially from these estimates and could have a material adverse effect on the Company's consolidated financial statements.
Foreign Currency
Assets and liabilities of non-U.S. subsidiaries that use their local currency as their functional currency are translated into U.S. dollars at exchange rates in effect on the balance sheet date. Income and expense accounts are translated at monthly average exchange rates during the year. The adjustments resulting from the foreign currency translations are recorded in accumulated other comprehensive loss, a separate component of stockholders’ equity (deficit).
Revenue Recognition
The Company generates revenue primarily from the sale of its products and services. Product revenue is derived from the sale of instruments and consumables, including IFCs, assays and reagents. Service revenue is derived from the sale of instrument service contracts, repairs, installation, training and other specialized product support services. The Company also generates revenue from product development agreements, license and royalty agreements, and grants. Revenue is reported net of any sales, use and value-added taxes the Company collects from customers as required by government authorities. Research and development cost includes costs associated with development and grant revenue.
The Company recognizes revenue based on the amount of consideration it expects to receive in exchange for the goods and services it transfers to the customer. The Company's commercial arrangements typically include multiple distinct products and services, and the Company allocates revenue to these performance obligations based on their relative standalone selling prices. Standalone selling prices
80
(SSP) are generally determined using observable data from recent transactions. In cases where sufficient data is not available, the Company estimates a product’s SSP using a cost plus a margin approach or by applying a discount to the product’s list price.
Product Revenue
The Company recognizes product revenue at the point in time when control of the goods passes to the customer, and the Company has an enforceable right to payment. This generally occurs either when the product is shipped from one of the Company's facilities or when it arrives at the customer’s facility, based on the contractual terms. Customers do not have a unilateral right to return products after delivery. Invoices are generally issued at shipment or in advance of service and become due in
The Company sometimes perform shipping and handling activities after control of the product passes to the customer. The Company has made an accounting policy election to account for these activities as product fulfillment activities rather than as separate performance obligations.
Service and Other Revenue
The Company recognizes revenue from repairs, maintenance, installation, training and other specialized product support services at the point in time the work is completed. Installation and training services are generally billed in advance of service. Repairs and other services are generally billed at the point the work is completed.
Revenue associated with instrument service contracts is recognized on a straight-line basis over the life of the agreement, which is generally to
Other revenue consists of license and royalty revenue and grant revenue. The Company recognizes revenue from license agreements when the license is transferred to the customer and the customer is able to use and benefit from the license. For contracts that include sales-based royalties, the Company recognizes revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied.
The Company receives grants from various entities to perform research and development activities over contractually defined periods. Grant revenue is not accounted for under ASC Topic 606, Revenue from Contracts with Customers, as the grant agreement is not with a customer. As there is no authoritative U.S. GAAP guidance for grants awarded to for-profit entities, the Company has applied the guidance in ASC Topic 958, Not-for-Profit Entities by analogy. Revenue is generally recognized provided that the conditions under which the grants were provided have been met and any remaining performance obligations are perfunctory.
Significant Judgments
Applying the revenue recognition practices discussed above often requires significant judgment. Significant judgment is required when interpreting commercial terms in sales agreements and determining when control of goods and services passes to the customer. Judgment is also required when identifying performance obligations, estimating SSP and allocating purchase consideration in agreements that include multiple performance obligations. Any material changes created by errors in judgment could have a material effect on the Company's operating results and overall financial condition.
Cash and Cash Equivalents
The Company considers all highly liquid financial instruments with maturities at the time of purchase of three months or less to be cash equivalents. Cash and cash equivalents balance at December 31, 2023, and 2022 represent cash on deposit with banks and money market funds.
Short-term Investments
Short-term investments are comprised of U.S treasury securities that mature within one year. The Company classifies its short-term investments as available-for-sale and records such assets at estimated fair value in the consolidated balance sheets. Any unrealized gains and losses from short-term investments are reported as a component of other comprehensive income (loss) within the consolidated statements of comprehensive loss and as a separate component of stockholders’ equity (deficit). The Company evaluates its short-term investments to assess whether investments with unrealized loss positions are other-than-temporarily impaired. An investment is
81
considered to be other-than-temporarily impaired if the impairment is related to deterioration in credit risk or if it is likely that the Company will sell the securities before the recovery of their cost basis. No investment has been assessed as other than temporarily impaired. Realized gains and losses are calculated on the specific identification method and are recorded as interest income (loss). There were
The Company excludes accrued interest from the fair value and amortized cost basis of its short-term investments.
Accounts Receivable, net
Trade accounts receivable are recorded at net invoice value. The Company reviews its exposure to accounts receivable and provides allowances of specific amounts if collectability is no longer reasonably assured based on historical experience and specific customer collection issues. The Company evaluates such allowances on a regular basis and adjust them as needed.
Concentrations of Business and Credit Risk
Financial instruments that potentially subject the Company to credit risk consist of cash, cash equivalents, short-term investments, and accounts receivable. The Company's cash, cash equivalents, and short-term investments may consist of deposits held with banks, money market funds, and other highly liquid investments that may at times exceed federally insured limits. Cash equivalents and short-term investments are financial instruments that potentially subject the Company to concentrations of risk. Under the Company's investment policy, the Company invests exclusively in securities issued by the U.S. government or U.S. government agencies, or in government money-market funds. The goals of the Company's investment policy, in order of priority, are to: preserve capital, meet liquidity needs, and optimize returns. For these reasons, management believes that the Company is not exposed to significant credit risk.
The Company generally does not require collateral to support credit sales. To reduce credit risk, the Company performs credit evaluations of its customers.
The Company's products include components that are currently procured from a single source or a limited number of sources. The Company believes that other vendors would be able to provide similar components; however, the qualification of such vendors may require start-up time. In order to mitigate any adverse impacts from a disruption of supply, the Company attempts to maintain an adequate supply of critical limited-source components.
Inventories, net
Inventories are stated at the lower of cost (on a first-in, first-out basis) or net realizable value. Inventory costs include direct materials, direct labor, and normal manufacturing overhead. The Company regularly reviews inventory for excess and obsolete products and components. Significant judgment is required in determining provisions for slow-moving, excess, and obsolete inventories which are recorded when required to reduce inventory values to their estimated net realizable values based on product life cycle, development plans, product expiration, discontinuance of product lines, and quality issues.
Property and Equipment, net
Property and equipment, including leasehold improvements, are stated at cost less accumulated depreciation. Accumulated depreciation is calculated using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are amortized over the estimated useful lives of the assets or the remaining term of the lease, whichever is shorter. The estimated useful lives of the Company's property and equipment are generally as follows: computer equipment and software, to
Leases
At the inception of a contractual arrangement, the Company determines whether the contract contains a lease by assessing whether there is an identified asset and whether the contract conveys the right to control the use of the identified asset in exchange for consideration over a period of time. Lease terms are determined at the commencement date by considering whether renewal options and termination options are reasonably assured of exercise. For its long-term operating leases, the Company recognizes a lease liability and a right-of-use asset (ROU) on its consolidated balance sheets. ROU assets represent the Company's right-to-use an underlying asset for the lease term and lease liabilities represent the Company's obligation to make lease payments arising from the lease. The lease liability is determined at the lease commencement date based on the present value of lease payments over the lease term. As most of the Company's leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate based on the estimated rate of interest for collateralized borrowing over a term similar to the lease arrangement. Significant judgment is required in determining the incremental
82
collateralized borrowing rate. The ROU asset is based on the lease liability, adjusted for any prepaid or deferred rent. Lease expense is recognized on a straight-line basis over the lease term. Sublease income from an operating lease is recognized on a straight-line basis over the sublease term. The Company does not have any finance leases.
The Company elected the short-term lease recognition exemption for all leases that qualify. For those leases that qualify, the Company will not recognize ROU assets or lease liabilities for leases with an initial lease term of one year or less. The Company also elected not to separate lease and nonlease components for the Company's building leases. The nonlease components are generally variable in nature and are expected to represent most of the Company's variable lease costs. Variable costs are expensed as incurred. The Company has taken a portfolio approach for its vehicle leases by country.
Business Combinations, Goodwill, Intangible Assets and Other Long-Lived Assets
The Company has completed acquisitions of businesses in the past and may acquire additional businesses or technologies in the future. The results of businesses acquired in a business combination are included in the Company's consolidated financial statements from the date of acquisition. The Company allocates the purchase price, which is the sum of the consideration provided in a business combination, to the identifiable assets and liabilities of the acquired business at their acquisition date fair values. Determining the fair value of assets acquired and liabilities assumed requires management to use significant judgment and estimates, including the selection of valuation methodologies and estimates of future revenue.
Goodwill, which has an indefinite useful life, represents the excess of cost over fair value of net assets acquired. The Company's intangible assets include developed technology, patents and licenses. The cost of identifiable intangible assets with finite lives is generally amortized on a straight-line basis over the assets’ respective estimated useful lives. Judgment is needed to assess the factors that could indicate an impairment of intangible assets.
Goodwill and intangible assets with indefinite lives are not subject to amortization but are tested for impairment on an annual basis during the fourth quarter or whenever events or changes in circumstances indicate the carrying amount of these assets may not be recoverable. Events or changes in circumstances that could affect the likelihood that the Company will be required to recognize an impairment charge include, but are not limited to, declines in the Company's stock price or market capitalization, economic downturns and other macroeconomic events, declines in the Company's market share or revenues, or significant litigation. Any impairment charges could have a material adverse effect on the Company's operating results and net asset value in the period in which the Company recognizes the impairment charge.
In evaluating goodwill and intangible assets with indefinite lives for indications of impairment, the Company first conducts an assessment of qualitative factors to determine whether it is more likely than not that the fair value of each of the Company's reporting units is less than its carrying amount. If the Company determines that it is more likely than not that the fair value of each of its reporting units is less than its carrying amount, the Company compares the fair value of each of its reporting units to its carrying value. If the fair value of each of the Company's reporting units exceeds its carrying value, goodwill is not considered impaired, and no further analysis is required. If the carrying value of each of the Company's reporting units exceeds its fair value, then an impairment loss equal to the difference would be recorded to goodwill.
The Company evaluates its long-lived assets, including finite-lived intangibles, for indicators of possible impairment when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. If any indicator of impairment exists, the Company assesses the recoverability of the affected long-lived assets by determining whether the carrying value of the asset can be recovered through undiscounted future operating cash flows. If impairment is indicated, the Company estimates the asset’s fair value using future discounted cash flows associated with the use of the asset and adjust the carrying value of the asset accordingly.
Series B Redeemable Preferred Stock
The Purchase Agreements (as described in Note 9) for the issuance of shares of Series B Redeemable Preferred Stock were accounted for as forward sales contracts at fair value in accordance with ASC Topic 480, Distinguishing Liabilities from Equities. The Series B Redeemable Preferred Stock was classified as mezzanine equity and recorded at fair value upon issuance, net of issuance costs, due to its redemption features that are outside of the Company’s control. Mezzanine equity is presented separately on the consolidated balance sheets between liabilities and shareholders’ equity because it shares characteristics of both. In the year ended December 31, 2022, the Company recognized a $
83
Restructuring and Related Charges
The Company records liabilities for costs associated with exit or disposal activities in the period in which the liability is incurred. Costs for involuntary separation programs are recorded when management has approved the plan for separation, the employees are identified and made aware of the benefits they are entitled to, it is unlikely that the plan will change significantly, and if applicable, any required governmental notification is made. Costs associated with benefits that are contingent on the employee continuing to provide service are recognized over the required service period. Costs associated with leased facilities (net of sublease income, if applicable) that the Company has vacated as part of a restructuring plan are also included.
Transaction-related Expenses
The Company expensed certain costs incurred related to the merger agreement with SomaLogic, described further in Note 16, including legal, advisory, accounting and other transaction-related costs. The expenses in the prior period relate to the private placement whereby the Company issued and sold an aggregate of $
Deferred Grant Income
Proceeds from the NIH Contract have been principally recorded as capital expenditures and to offset applicable operating costs. The non-operating income recognized from the grant proceeds received in excess of the amounts spent for capital expenditures and operating expenses is reflected on the consolidated statement of operations as surplus funding from the NIH contract.
The NIH Contract met the definition of grants related to assets as the primary purpose for the payments was to fund the purchase and construction of capital assets to scale up production capacity. The Company elected to record the grants received as deferred income in accordance with International Accounting Standards (IAS) 20.
Deferred grant income related to production capacity expansion is being amortized for the related assets as a reduction of depreciation expense.
Term Loan, net
The term loan is recorded at its carrying value, which includes the outstanding principal amount and the cumulative accreted final payment, less unamortized debt issuance costs. Amortization of the debt issuance costs and accretion of the final payment are reflected in interest expense. The final payment is being accreted to the carrying value of the term loan through the expected maturity of July 1, 2025 using the effective interest method. Debt issuance costs were recorded as an offset to the carrying value of the loan and are amortized over the expected term also using the effective interest method.
Convertible Notes, net
The Company records the 2014 Notes and 2019 Notes (as described in Note 6) at their carrying values, which includes their principal amounts plus accrued and unpaid interest. Offering-related costs, including underwriting costs, on the 2014 Notes and 2019 Notes were capitalized as debt issuance costs, recorded as an offset to the carrying value of the related Notes, and are amortized over the expected term of the related Notes using the effective interest method.
Treasury Stock
The Company uses the cost method to account for the repurchases of its common stock in accordance with ASC 505-30, Equity-Treasury Stock. The direct costs associated with settled share repurchases, including trading commissions, are reported as treasury stock in the shareholders’ equity (deficit) section of the Company's consolidated balance sheets.
Fair Value of Financial Instruments
The Company's financial instruments consist primarily of cash and cash equivalents, restricted cash, short-term investments, accounts receivable, accounts payable, term loan and convertible notes. The Company's cash equivalents, restricted cash, accounts receivable and accounts payable generally have short maturity or payment periods. Accordingly, their carrying values approximated their fair values at December 31, 2023 and 2022. The Company's short-term investments consist of U.S. treasury securities that are classified as available-for-sale and reported at fair value on the Company's consolidated balance sheets. The convertible notes and term loan are presented at their net carrying values.
84
As a basis for computing fair value, the Company follows a three-tier value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level I: observable inputs such as quoted prices in active markets;
Level II: inputs other than quoted prices in active markets that are observable either directly or indirectly; and
Level III: unobservable inputs for which there is little or no market data, which requires the Company to develop its own assumptions.
This hierarchy requires the Company to use observable market data, when available, and to minimize the use of unobservable inputs when determining fair value. The Company's cash equivalents, which include money market funds are classified as Level I because they are valued using quoted market prices. The Company's short-term investments, which include U.S. treasuries, are classified as Level II because they are valued using non-binding market consensus prices that were corroborated with observable market data, quoted market prices for similar instruments, or pricing models.
The Company's convertible notes are not regularly traded, and it is difficult to estimate a reliable and accurate market price for these securities. The estimated fair values of these securities represent Level III valuations since a fair value for these securities cannot be determined by using readily observable inputs or measures, such as market prices. Fair values were estimated using pricing models and risk-adjusted value ranges. The estimated fair value of the Company's term loan also represents a Level III valuation since the value cannot be determined by using readily observable inputs or measures, such as market prices. The fair value of the Company's term loan was estimated using a discounted cash flows approach and current market interest rate data for similar loans. The carrying value of the Company's lines of term loan approximates fair value as the interest rate and terms are reflective of the rate the Company could obtain on debt with similar terms and conditions.
Research and Development
The Company recognizes research and development expenses in the period incurred. Research and development (R&D) expenses generally consist of personnel costs, independent contractor costs, prototype and materials expenses, allocated facilities and information technology expenses, and related overhead expenses.
Advertising Costs
The Company expenses advertising costs as incurred. The Company incurred advertising costs of $
Stock-Based Compensation
The Company recognizes compensation costs for all stock-based awards, including stock options, Restricted Share Units (RSUs), Performance Share Units (PSUs) and stock purchased under the Company's Employee Share Purchase Plan (ESPP), based on the grant date fair value of the award. The Company recognizes stock-based compensation expense on a straight-line basis over the requisite service periods for non-performance-based awards. For RSUs, fair value is measured based on the closing fair market value of the Company's common stock on the date of grant. For PSUs with a market condition, the Company uses a Monte Carlo simulation pricing model to incorporate the market condition effects at the grant date. The Monte Carlo pricing model requires inputs which are subjective and generally requires judgment. For PSUs with performance conditions, stock-based compensation expense is recognized over the requisite service period when the achievement of each individual performance goal becomes probable.
The fair value of options and stock purchases under ESPP on the grant date is estimated using the Black-Scholes option-pricing model, which requires the use of certain subjective assumptions, including expected term, volatility, risk-free interest rate and the fair value of the Company's common stock. These assumptions generally require judgment. The Company determines the expected volatility based on the Company's historical stock price volatility generally commensurate with the estimated expected term of the stock awards. The expected term of an award is based on historical forfeiture experience, exercise activity, and the terms and conditions of the stock awards. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to each grant’s expected term. The Company accounts for forfeitures as they occur.
Income Taxes
The Company uses the asset and liability method to account for income taxes. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and
85
liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Valuation allowances are provided when the expected realization of deferred tax assets does not meet a “more likely than not” criterion. The Company makes estimates and judgments about its future taxable income that are based on assumptions that are consistent with its plans and estimates. Should the actual amounts differ from the Company's estimates, the amount of the valuation allowance could be materially impacted. Changes in these estimates may result in significant increases or decreases to the Company's tax provision in a period in which such estimates are changed, which in turn would affect net income or loss.
The Company recognizes the financial statement effects of a tax position when it is more likely than not, based on the technical merits, that the position will be sustained upon examination. Any interest and penalties related to uncertain tax positions are reflected in the income tax provision.
Segment Reporting
The Company operates in
The Company's CEO, who is its Chief Operating Decision Maker (CODM), measures segment performance using gross profit which is determined by subtracting cost of product and service revenues from segment revenues. Depreciation and amortization expense is included in each segment’s gross profit.
Comprehensive Loss
Comprehensive loss is comprised of net loss and other comprehensive income (loss). Other comprehensive income (loss) consists of unrealized gains and losses on the Company's short-term investments and foreign currency translation adjustments. Total comprehensive loss for all periods presented has been disclosed in the consolidated statements of comprehensive loss.
Net Loss per Share
The Company's basic and diluted net loss per share is calculated by dividing net loss by the weighted-average number of shares of common stock outstanding for the period. RSUs, PSUs, stock options to purchase the Company's common stock, ESPP shares pending issuance, Series B Preferred Stock and convertible notes are considered to be potentially dilutive common shares but have been excluded from the calculation of diluted net loss per share as their effect is anti-dilutive for all periods presented.
The following potentially dilutive common shares were excluded from the computations of diluted net loss per share for the periods presented because including them would have been anti-dilutive (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
RSUs, PSUs, stock options and ESPP shares |
|
|
|
|
|
|
||
Series B Preferred Stock |
|
|
|
|
|
|
||
2019 Notes(1) |
|
|
|
|
|
|
||
2014 Notes |
|
|
|
|
|
|
||
Total |
|
|
|
|
|
|
||
The
86
Recent Accounting Changes and Accounting Pronouncements
Adoption of New Accounting Guidance
From time to time, new accounting standards are issued by the Financial Accounting Standards Board (FASB) or other standard setting bodies and adopted by the Company as of the specified effective date. Unless otherwise discussed, the impact of recently issued standards that are not yet effective will not have a material impact on the Company’s financial position or results of operations upon adoption.
Recent Accounting Pronouncements
In November 2023, the FASB issued ASU 2023-07, Segment Reporting - Improvements to Reportable Segment Disclosures, which requires disclosure of more detailed information about a reportable segment’s expenses. The new standard is effective for fiscal years beginning after December 15, 2023 and interim periods beginning after December 15, 2024. The amendments must be applied retrospectively, and early adoption is permitted. The Company is currently assessing the effects of adoption on its consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, Improvements to Income Tax Disclosures, which requires disaggregation of rate reconciliation categories and income taxes paid by jurisdiction. The new standard is effective for fiscal years beginning after December 15, 2024. The amendments may be applied prospectively or retrospectively, and early adoption is permitted. The Company is currently assessing the effects of adoption on its consolidated financial statements.
Reclassification
Certain amounts in the consolidated financial statements have been reclassified from their original presentation to conform to current year presentation.
3. Revenue and Geographic Area
Disaggregation of Revenue by Product Type and Geographic Area
The following tables present the Company's revenue for the years ended December 31, 2023 and 2022, respectively, based on product type and the geographic location of customers’ facilities (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Instruments |
|
$ |
|
|
$ |
|
||
Consumables |
|
|
|
|
|
|
||
Total product revenue |
|
|
|
|
|
|
||
Service revenue |
|
|
|
|
|
|
||
Product and service revenue |
|
|
|
|
|
|
||
Other revenue |
|
|
|
|
|
|
||
Total revenue |
|
$ |
|
|
$ |
|
||
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Americas |
|
$ |
|
|
$ |
|
||
Europe, Middle East and Africa (EMEA) |
|
|
|
|
|
|
||
Asia-Pacific |
|
|
|
|
|
|
||
Total revenue |
|
$ |
|
|
$ |
|
||
Most of the Company's principal operations, other than manufacturing, are located at its corporate headquarters in the United States. Revenue from customers in the United States represented $
87
2023, and $
Revenue from customers in China represented $
One genomics customer accounted for
Long-lived Assets by Geographical Area
The Company had long-lived assets consisting of property and equipment, net of accumulated depreciation, and operating lease ROU assets, net of accumulated amortization, in the following geographic areas for each year presented (in thousands):
|
|
December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
United States |
|
$ |
|
|
$ |
|
||
Singapore |
|
|
|
|
|
|
||
Canada |
|
|
|
|
|
|
||
Other Asia-Pacific |
|
|
|
|
|
|
||
EMEA |
|
|
|
|
|
|
||
Total |
|
$ |
|
|
$ |
|
||
Unfulfilled Performance Obligations
The consolidated balance sheets as of December 31, 2023 and 2022 included total deferred revenue of $
The Company expects to recognize revenue from unfulfilled performance obligations associated with service contracts that were partially completed as of December 31, 2023 in the following periods (in thousands):
Fiscal Year |
|
Expected Revenue (1) |
|
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
Total |
|
$ |
|
|
The Company also has unsatisfied performance obligations for service contracts with an expected term of one year or less not included in the amounts above.
4. Goodwill and Intangible Assets, net
During the second quarter of 2022, the Company discontinued the sale of products that utilized the developed technology acquired from InstruNor and recorded a $
The Company assessed goodwill for impairment when it performed its annual testing at the end of the fourth quarter of 2023. A qualitative approach was employed which included assessing significant events and circumstances such as the Company's current results,
88
assumptions regarding future performance, strategic initiatives and overall macroeconomic factors to determine the existence of potential indicators of impairment and assess if it is more likely than not that the fair value of each of the Company's reporting units is less than their carrying value. The Company determined there was
The changes in the carrying value of goodwill by segment are as follows (in thousands):
|
|
Proteomics |
|
|
Genomics |
|
|
Total |
|
|||
Balance as of December 31, 2022 |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Foreign currency translation |
|
|
|
|
|
|
|
|
|
|||
Balance as of December 31, 2023 |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Intangible assets with finite lives include developed technology, patents and licenses. In the consolidated balance sheets, developed technology is reported separately while patents and licenses are reported in other non-current assets. Intangible assets, net, were as follows (in thousands):
|
|
December 31, 2023 |
||||||||||||
|
|
Gross Amount |
|
|
Accumulated |
|
|
Net |
|
|
Weighted- |
|||
Developed technology |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
|||
Patents and licenses |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
|||
|
|
December 31, 2022 |
||||||||||||
|
|
Gross Amount |
|
|
Accumulated |
|
|
Net |
|
|
Weighted- |
|||
Developed technology |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
|||
Patents and licenses |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
|||
Total amortization expense of the Company's intangible assets was $
Based on the net carrying value of intangible assets at December 31, 2023, the Company expects annual amortization expense to be as follows (in thousands):
Fiscal Year |
|
Developed |
|
|
Patents and |
|
|
Total |
|
|||
2024 |
|
|
|
|
|
|
|
|
|
|||
Total |
|
$ |
|
|
$ |
|
|
$ |
|
|||
5. Balance Sheet Details
Cash, Cash Equivalents and Restricted Cash
Cash, cash equivalents and restricted cash consisted of the following (in thousands):
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Restricted cash |
|
|
|
|
|
|
||
Total cash, cash equivalents and restricted cash |
|
$ |
|
|
$ |
|
||
89
Restricted cash of $
Inventories, net
Inventories, net consisted of the following (in thousands):
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Raw materials |
|
$ |
|
|
$ |
|
||
Work-in-process |
|
|
|
|
|
|
||
Finished goods |
|
|
|
|
|
|
||
Total inventory, gross |
|
|
|
|
|
|
||
Allowance for excess and obsolete inventory |
|
|
( |
) |
|
|
( |
) |
Total inventories, net |
|
$ |
|
|
$ |
|
||
Property and Equipment, net
Property and equipment, net consisted of the following (in thousands):
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Laboratory and manufacturing equipment |
|
$ |
|
|
$ |
|
||
Leasehold improvements |
|
|
|
|
|
|
||
Computer equipment and software |
|
|
|
|
|
|
||
Office furniture and fixtures |
|
|
|
|
|
|
||
Property and equipment, gross |
|
|
|
|
|
|
||
Less accumulated depreciation and amortization |
|
|
( |
) |
|
|
( |
) |
Construction-in-progress |
|
|
|
|
|
|
||
Property and equipment, net |
|
$ |
|
|
$ |
|
||
Depreciation expense was $
Accrued Compensation and Related Benefits
Accrued compensation and related benefits, which are included in current liabilities on the consolidated balance sheets consisted of the following (in thousands):
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Accrued incentive compensation |
|
$ |
|
|
$ |
|
||
Accrued vacation |
|
|
|
|
|
|
||
Accrued payroll taxes and other |
|
|
|
|
|
|
||
Accrued restructuring |
|
|
|
|
|
|
||
Accrued compensation and related benefits |
|
$ |
|
|
$ |
|
||
Refer to Note 14 for additional information on restructuring.
90
Other Accrued Liabilities
Other accrued liabilities, which are included in current liabilities on the consolidated balance sheets consisted of the following (in thousands):
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Accrued commissions |
|
$ |
|
|
$ |
|
||
Accrued interest |
|
|
|
|
|
|
||
Accrued legal expenses |
|
|
|
|
|
|
||
Accrued taxes |
|
|
|
|
|
|
||
Uninvoiced receipts |
|
|
|
|
|
|
||
Accrued warranties |
|
|
|
|
|
|
||
Customer advances |
|
|
|
|
|
|
||
Accrued restructuring |
|
|
|
|
|
|
||
Accrued other |
|
|
|
|
|
|
||
Other accrued liabilities |
|
$ |
|
|
$ |
|
||
Deferred Grant Income
In September 2020, the Company executed a contract with the National Institutes of Health (NIH) under NIH’s Rapid Acceleration of Diagnostics program to support the expansion of the Company’s production capacity for its COVID-19 test products. Under the now-completed contract, the Company received $
The current portion of deferred grant income on the Company’s consolidated balance sheets represents amounts expected to be offset against depreciation expense over the next twelve months. The non-current portion of deferred grant income includes amounts expected to be offset against depreciation expense in later periods.
6. Debt
The carrying value of debt consists of the following (in thousands):
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Convertible notes: |
|
|
|
|
|
|
||
2014 Notes |
|
$ |
|
|
$ |
|
||
2019 Notes, non-current |
|
|
|
|
|
|
||
2019 Notes, current |
|
|
|
|
|
|
||
Total convertible notes, net |
|
|
|
|
|
|
||
Term loan, non-current |
|
|
|
|
|
|
||
Term loan, current |
|
|
|
|
|
|
||
Total debt |
|
$ |
|
|
$ |
|
||
Convertible Notes
In February 2014, the Company closed an underwritten public offering of 2014 Senior Convertible Notes (2014 Notes), which will mature on February 1, 2034, unless earlier converted, redeemed or repurchased in accordance with the terms of the 2014 Notes. Holders may require the Company to repurchase all or a portion of their 2014 Notes on each of February 6, 2024 and February 6, 2029, at a repurchase price in cash equal to
In November 2019, the Company issued $
91
earlier repurchased or converted pursuant to their terms. The 2019 Notes will be convertible at the option of the holder at any point prior to the close of business on the second scheduled trading day preceding the maturity date. The initial conversion rate of the 2019 Notes is
Offering-related costs related to both notes were capitalized as debt issuance costs and are recorded as an offset to the carrying value of the 2019 Notes.
Revolving Credit Facility
On August 2, 2018, the Company entered into a revolving credit facility with Silicon Valley Bank (as amended, the Revolving Credit Facility) in an aggregate principal amount of up to the lesser of (i) $
Term Loan Facility, net
On August 2, 2021, the Company amended its Revolving Credit Facility to, amongst other things, provide for a new $
On October 26, 2023, the Company entered into an amendment to the Term Loan Facility agreement which removed certain collateral covenants related to the Revolving Credit Facility due to its expiration on August 2, 2023.
Future minimum payments under the Term Loan Facility including the end of term fee payment as of December 31, 2023, are as follows (in thousands):
2024 |
|
$ |
|
|
2025 |
|
|
|
|
|
|
|
|
|
End of term fee and debt issuance costs |
|
|
|
|
Total Term Loan Facility |
|
$ |
|
Bridge Loans
On January 23, 2022, the Company entered into separate loan agreements (collectively, the Bridge Loan Agreements) with various investors for a $
Applying the guidance in ASC 825 Financial Instruments, the Company elected to record the Bridge Loans at their fair value using a probability‐weighted expected return method for the valuation analysis of the Bridge Loans. This resulted in a $
92
in fair value of the Bridge Loans from $
7. Commitments and Contingencies
Leases
The Company has operating leases for buildings, equipment and vehicles. Existing leases have remaining terms ranging from less than
As part of the Company’s restructuring plan discussed further in Note 14, in August 2022, the Company entered into an agreement to sublease approximately
Rent expense, net of sublease income, is reported within restructuring and related charges for the year ended December 31, 2023, in the consolidated statements of operations. The Company is currently in the process of fully vacating and potentially subleasing an additional floor (20th floor).
Information about the Company's operating leases is as follows:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Weighted average remaining lease term (in years) |
|
|
|
|
||||
Weighted average discount rate per annum |
|
|
% |
|
|
% |
||
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Operating lease cost (including variable costs) |
|
$ |
|
|
$ |
|
||
Variable costs (including non-lease components) |
|
$ |
|
|
$ |
|
||
Sublease income |
|
$ |
|
|
$ |
|
||
Cash paid for amounts included in the measurement of |
|
$ |
|
|
$ |
|
||
Future minimum lease payments and sublease income as of December 31, 2023 under commenced non-cancelable operating leases are as follows (in thousands):
93
Fiscal Year |
|
Minimum Lease |
|
|
Sublease Income |
|
|
Net Minimum Lease Payments for Operating Leases |
|
|||
2024 |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||
2025 |
|
|
|
|
|
( |
) |
|
|
|
||
2026 |
|
|
|
|
|
( |
) |
|
|
|
||
2027 |
|
|
|
|
|
( |
) |
|
|
|
||
2028 |
|
|
|
|
|
( |
) |
|
|
|
||
Thereafter |
|
|
|
|
|
( |
) |
|
|
|
||
Total future minimum payments (receipts) |
|
|
|
|
$ |
( |
) |
|
$ |
|
||
Imputed interest |
|
|
( |
) |
|
|
|
|
|
|
||
Total operating lease liabilities |
|
|
|
|
|
|
|
|
|
|||
Less: current portion of operating lease liabilities |
|
|
|
|
|
|
|
|
|
|||
Operating lease liabilities, net of current portion |
|
$ |
|
|
|
|
|
|
|
|||
Other Commitments
In the normal course of business, the Company enters into various contractual and legally binding purchase commitments. As of December 31, 2023, the Company's open commitments totaled $
The Company has entered into several license and patent agreements. Under these agreements, the Company pays annual license maintenance fees, non-refundable license issuance fees, and royalties as a percentage of net sales for the sale or sublicense of products using the licensed technology. Future payments related to these license agreements have not been included in the open commitments above, as the period of time over which the future license payments will be required to be made, and the amount of such payments, are indeterminable. The Company does not expect the license payments to be material in any particular year.
Indemnification
From time to time, the Company has entered into indemnification provisions under certain of its agreements in the ordinary course of business, typically with business partners, customers and suppliers. Pursuant to these agreements, the Company may indemnify, hold harmless and agree to reimburse the indemnified parties on a case-by-case basis for losses suffered or incurred by the indemnified parties in connection with any patent or other intellectual property infringement claim by any third party with respect to the Company’s products. The term of these indemnification provisions is generally perpetual from the time of the execution of the agreement. The maximum potential amount of future payments the Company could be required to make under these indemnification provisions is typically not limited to a specific amount. In addition, the Company has entered into indemnification agreements with its officers, directors and certain other employees. With certain exceptions, these agreements provide for indemnification for related expenses including, among others, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding.
Litigation
On November 28, 2023, a purported stockholder filed a complaint against the Company and the members of the Company’s Board in the United States District Court for the Northern District of California. The complaint has since been voluntarily dismissed. On December 12, 2023 two separate shareholder complaints were filed in the District of Delaware, The complaints asserted claims under Section 14(a) of the Exchange Act and Rule 14a-9 promulgated thereunder and Section 20(a) of the Exchange Act for allegedly causing the filing with the SEC on November 14, 2023 of a materially deficient registration statement on Form S-4. Among other remedies, the plaintiffs sought to enjoin a stockholder vote on the proposed Merger. The Company is reviewing the complaints and has not yet formally responded to them. On December 13, 2023, a complaint was filed in the Delaware Court of Chancery against SomaLogic and certain officers and directors alleging Breach of Fiduciary Duty and Aiding and Abetting Breach of Fiduciary Duty. This complaint also sought an injunction postponing the proposed transaction, which was denied by the Court on January 4, 2024. The non-injunctive claims, including breach of fiduciary duty, are still being litigated. Litigation is inherently uncertain and there can be no assurance regarding the outcome. Whether or not any plaintiffs’ claim is successful, this type of litigation may result in significant costs and divert management’s attention and resources, which could adversely affect the operation of the Company.
94
Between October 24, 2023 and January 3, 2024, SomaLogic received 16 letters from purported shareholders demanding that SomaLogic allow the inspection of its books and records and/or make corrective disclosures to its registration statement.
Additional lawsuits against the Company and certain of our officers or directors may be filed in the future. If additional similar complaints are filed, absent new or different allegations that are material, the Company will not necessarily announce such additional filings.
In the normal course of business, the Company is from time to time involved in legal proceedings or potential legal proceedings, including matters involving employment, intellectual property, or others. Although the results of litigation and claims cannot be predicted with certainty, the Company currently believes that the final outcome of any currently pending matters would not have a material adverse effect on our business, operating results, financial condition, or cash flows. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
8. Fair Value of Financial Instruments
The following tables summarize the Company's financial instruments by significant investment category measured at fair value on a recurring basis within the fair value hierarchy (in thousands):
|
|
|
|
|
Fair Value Measurements At Reporting Date Using |
|
||||||||||
|
|
Total |
|
|
Quoted Prices in Active Markets For Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
||||
As of December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash and cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Total cash and cash equivalents |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
U.S. treasury securities |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Total short-term investments |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total assets measured at fair value |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
Fair Value Measurements At Reporting Date Using |
|
||||||||||
|
|
Total |
|
|
Quoted Prices in Active Markets For Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
||||
As of December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash and cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Total cash and cash equivalents |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
U.S. treasury securities |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Total short-term investments |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total assets measured at fair value |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
There were no transfers within the hierarchy and no changes in the valuation techniques used during the year ended December 31, 2023.
The following table summarizes available-for-sale-securities (in thousands):
95
|
|
|
|
|
|
|
As of December 31, 2023 |
|
||||||||||
|
|
Maturity (in years) |
|
Amortized Cost |
|
|
Unrealized Gains |
|
|
Unrealized Losses |
|
|
Estimated Fair Value |
|
||||
Cash and cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Total cash and cash equivalents |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
U.S. treasury securities |
|
1 or less |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Total short-term investments |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total available-for-sale securities |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
As of December 31, 2022 |
|
||||||||||
|
|
Maturity (in years) |
|
Amortized Cost |
|
|
Unrealized Gains |
|
|
Unrealized Losses |
|
|
Estimated Fair Value |
|
||||
Cash and cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Total cash and cash equivalents |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
U.S. treasury securities |
|
1 or less |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
Total short-term investments |
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total available-for-sale securities |
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
As of December 31, 2023,
Debt
The 2014 Notes and 2019 Notes (collectively, the Convertible Notes) are not regularly traded. The estimated fair values for these securities represent Level III valuations since a fair value for these securities cannot be determined by using readily observable inputs or measures, such as market prices. Fair values were estimated using pricing models and risk-adjusted value ranges. The estimated fair value of the 2019 Notes was $
The estimated fair value of the Term Loan Facility also represents a Level III valuation since the value cannot be determined by using readily observable inputs or measures, such as market prices. The fair value of the Company's Term Loan Facility was estimated using a discounted cash flow model and current market interest rate data for similar loans. The carrying value of the Company's lines of Term Loan Facility approximates fair value as interest rates applied to the underlying debt are adjusted quarterly to market interest rates.
9
Series B Redeemable Preferred Stock
On January 23, 2022, the Company entered into separate Series B Convertible Preferred Stock Purchase Agreements (collectively, the Purchase Agreements) with Casdin Private Growth Equity Fund II, L.P. and Casdin Partners Master Fund, L.P. (together, Casdin), and Viking Global Opportunities Illiquid Investments Sub Master LP and Viking Global Opportunities Drawdown LP (together, Viking, and together with Casdin, the Lenders), whereby the Company issued and sold an aggregate of $
96
converted into
The Purchase Agreements were accounted for as forward sales contracts at fair value in accordance with the authoritative accounting guidance as the Series B Preferred Stock included certain contingent redemption features that created an obligation for the Company to repurchase its shares. The fair value of the payable portion of the forward sales contracts was determined using a Monte Carlo Simulation, which relies on significant assumptions regarding the estimated yield and term of the Series B Preferred Stock.
The components of the carrying value of the Series B Preferred Stock as of December 31, 2023 and 2022 were as follows (in thousands):
Proceeds from Purchase Agreements |
|
$ |
|
|
Proceeds from Bridge Loans |
|
|
|
|
Change in fair value of Forward Purchase Agreements |
|
|
|
|
Change in the fair value of Bridge Loans |
|
|
|
|
Less equity issuance costs |
|
|
( |
) |
Total Series B Redeemable Preferred Stock |
|
$ |
|
The Series B Preferred Stock ranks senior to the Company's common stock with respect to dividend rights, redemption rights and rights on the distribution of assets on any voluntary or involuntary liquidation, dissolution or winding up of the affairs of the Company. The holders of Series B Preferred Stock are entitled to participate in all dividends declared on the Company's common stock on an as-converted basis.
10. Shareholders' Deficit
Stock Repurchase Program
On November 23, 2022, the Company’s board of directors authorized the repurchase of up to $
Common Shares Reserved
As of December 31, 2023, the Company had reserved shares of common stock for future issuance under equity compensation plans as follows (in thousands):
|
|
Securities To Be Issued Upon Exercise Of Options |
|
|
Securities To Be Issued Upon Release Of Restricted Stock |
|
|
Number Of Remaining Securities Available For Future Issuance |
|
|||
2022 Inducement Equity Incentive Plan |
|
|
|
|
|
|
|
|
|
|||
2011 Equity Incentive Plan |
|
|
|
|
|
|
|
|
|
|||
2017 Inducement Award Plan |
|
|
|
|
|
|
|
|
|
|||
2017 Employee Stock Purchase Plan |
|
|
|
|
|
|
|
|
|
|||
Total common stock reserved for future issuance |
|
|
|
|
|
|
|
|
|
|||
11. Stock-based Compensation
Equity Compensation Plans
2011 Equity Incentive Plan
In January 2011, the Company's board of directors adopted the 2011 Equity Incentive Plan (2011 Plan) under which incentive stock options, non-statutory stock options, RSUs, stock appreciation rights, PSUs, and performance shares may be granted to its employees, directors, and consultants.
97
2022 Inducement Equity Incentive Plan
In April 2022, the Company's board of directors adopted the 2022 Inducement Plan and reserved
The Company's board of directors sets the terms, conditions, and restrictions related to the grant of stock options, RSUs and performance-based awards under its stock-based plans, as well as employee participation in the 2017 Employee Stock Purchase Plan (ESPP). The Company's board of directors determines the number of awards to grant and also sets vesting criteria. In general, RSUs vest on a quarterly basis over a period of
Restricted Stock Units
|
|
Number of Units |
|
|
Weighted-Average |
|
||
Balance at December 31, 2022 |
|
|
|
|
$ |
|
||
RSU granted |
|
|
|
|
$ |
|
||
RSU released |
|
|
( |
) |
|
$ |
|
|
RSU forfeited |
|
|
( |
) |
|
$ |
|
|
Balance at December 31, 2023 |
|
|
|
|
$ |
|
||
As of December 31, 2023, the unrecognized compensation costs related to outstanding unvested RSUs under the Company’s equity incentive plans were $
Stock Options
|
|
Number of |
|
|
Weighted-Average |
|
|
Weighted- |
|
|
Aggregate |
|
||||
Balance at December 31, 2022 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Options granted |
|
|
|
|
$ |
|
|
|
|
|
|
|
||||
Options exercised |
|
|
( |
) |
|
$ |
|
|
|
|
|
|
|
|||
Options cancelled |
|
|
( |
) |
|
$ |
|
|
|
|
|
|
|
|||
Balance at December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Vested at December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Unvested awards at December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Aggregate intrinsic value as of December 31, 2023 was calculated as the difference between the closing price per share of the Company’s common stock on the last trading day of December, which was $
The total intrinsic value of options exercised during the years ended December 31, 2023 and 2022 was immaterial. The total intrinsic value of options vested during the years ended December 31, 2023 and 2022 was $
98
2023, the unrecognized compensation costs related to outstanding unvested options under the Company’s equity incentive plans were $
The weighted average assumptions used to estimate the fair value of options granted were as follows:
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Stock options |
|
|
|
|
|
|
||
Weighted average expected volatility |
|
|
% |
|
|
% |
||
Weighted average expected term |
|
|
|
|
||||
Weighted average risk-free interest rate |
|
|
% |
|
|
% |
||
Dividend yield |
|
|
|
|
|
|
||
Weighted-average fair value per share |
|
$ |
|
|
$ |
|
||
Expected Term—The expected term of options granted represents the period of time that the options are expected to be outstanding and is derived by analyzing historical exercise behavior.
Expected Volatility—The estimated volatility was based on the historical volatility of the common stock of the Company.
Risk-Free Interest Rate—The risk-free interest rate is the implied yield in effect at the time of the option grant based on U.S. Treasury securities with contract maturities similar to the expected term of the Company’s stock options.
Dividend Rate—The Company has not paid any cash dividends on common stock since inception and does not anticipate paying any dividends in the foreseeable future. Consequently, an expected dividend yield of zero was used.
Performance-based Awards
The Company previously granted PSUs to certain executive officers and senior-level employees with the number of PSUs ultimately earned under these awards being calculated by comparing the Total Shareholder Return (TSR) of the Company’s common stock over the applicable
In July 2023, the Company granted performance-based restricted stock units to certain executive officers that will vest in the first quarter of 2024 based upon the achievement of specified revenue and EBITDA targets for the twelve months ended December 31, 2023, and the executive’s continued employment with the Company. Stock-based compensation expense is being recognized over the requisite service period, as it is deemed probable the Company will satisfy the performance measures.
Activity under the performance-based awards was as follows:
|
|
Number of Units |
|
|
Weighted-Average |
|
||
Balance at December 31, 2022 |
|
|
|
|
$ |
|
||
PSU granted |
|
|
|
|
$ |
|
||
PSU released |
|
|
( |
) |
|
$ |
|
|
Performance adjustment for 2020 awards |
|
|
( |
) |
|
$ |
|
|
Balance at December 31, 2023 |
|
|
|
|
$ |
|
||
Stock-based Compensation Expense
Stock-based compensation expense is reported in the Company's consolidated statement of operations as follows (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Cost of product revenue |
|
$ |
|
|
$ |
|
||
Research and development expense |
|
|
|
|
|
|
||
Selling, general and administrative expense |
|
|
|
|
|
|
||
Total stock-based compensation expense |
|
$ |
|
|
$ |
|
||
99
12. Income Taxes
The Company'
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Domestic |
|
$ |
( |
) |
|
$ |
( |
) |
International |
|
|
( |
) |
|
|
( |
) |
Loss before income taxes |
|
$ |
( |
) |
|
$ |
( |
) |
Significant components of the Company's benefit (expense) from income taxes are as follows (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Current: |
|
|
|
|
|
|
||
Federal |
|
$ |
|
|
$ |
|
||
State |
|
|
( |
) |
|
|
( |
) |
Foreign |
|
|
( |
) |
|
|
( |
) |
Total current tax expense |
|
|
( |
) |
|
|
( |
) |
Deferred: |
|
|
|
|
|
|
||
Federal |
|
|
|
|
|
|
||
State |
|
|
|
|
|
|
||
Foreign |
|
|
|
|
|
|
||
Total deferred benefit |
|
|
|
|
|
|
||
Total benefit (expense) from income taxes |
|
$ |
( |
) |
|
$ |
|
|
Reconciliation of income taxes at the statutory rate to the benefit (expense) from income taxes recorded in the statements of operations is as follows:
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Tax benefit at federal statutory rate |
|
|
% |
|
|
% |
||
State tax expense, net of federal benefit |
|
|
|
|
|
|
||
Foreign tax expense |
|
|
|
|
|
|
||
NOL carryforwards expiring unutilized |
|
|
( |
) |
|
|
( |
) |
Change in valuation allowance |
|
|
( |
) |
|
|
|
|
Federal R&D credit |
|
|
|
|
|
|
||
Unrecognized tax benefit |
|
|
|
|
|
|
||
Non-deductible interest/premium |
|
|
|
|
|
( |
) |
|
Non-deductible loss on Forward Sale of Preferred Stock and |
|
|
|
|
|
( |
) |
|
R&D tax credits expiring unutilized |
|
|
|
|
|
( |
) |
|
Transaction costs |
|
|
( |
) |
|
|
|
|
Executive stock-based compensation |
|
|
( |
) |
|
|
( |
) |
Return to provision |
|
|
|
|
|
|
||
Other, net |
|
|
( |
) |
|
|
( |
) |
Effective tax rate |
|
|
( |
)% |
|
|
% |
|
100
Significant components of the Company's deferred tax assets and liabilities are as follows (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Deferred tax assets: |
|
|
|
|
|
|
||
Net operating loss carryforward |
|
$ |
|
|
$ |
|
||
Reserves and accruals |
|
|
|
|
|
|
||
Depreciation and amortization |
|
|
|
|
|
|
||
Capitalized R&D costs |
|
|
|
|
|
|
||
Tax credit carryforwards |
|
|
|
|
|
|
||
Stock-based compensation |
|
|
|
|
|
|
||
Right-of-use lease liabilities |
|
|
|
|
|
|
||
Total gross deferred tax assets |
|
|
|
|
|
|
||
Valuation allowance on deferred tax assets |
|
|
( |
) |
|
|
( |
) |
Total deferred tax assets, net of valuation allowance |
|
|
|
|
|
|
||
Deferred tax liabilities: |
|
|
|
|
|
|
||
Fixed assets and intangibles |
|
|
( |
) |
|
|
( |
) |
Right-of-use assets |
|
|
( |
) |
|
|
( |
) |
Total deferred tax liabilities |
|
|
( |
) |
|
|
( |
) |
Net deferred tax liability |
|
$ |
( |
) |
|
$ |
( |
) |
|
|
|
|
|
|
|
||
Deferred tax liability per balance sheet |
|
$ |
( |
) |
|
$ |
( |
) |
Less deferred tax assets included in other long-term assets |
|
|
|
|
|
|
||
Net deferred tax liability |
|
$ |
( |
) |
|
$ |
( |
) |
Utilization of the net operating loss carryforwards and credits may be subject to a substantial annual limitation due to the ownership change limitations provided by Section 382 of the Internal Revenue Code of 1986, as amended, and similar state provisions. The Company completed its Section 382 Study through December 31, 2022 and determined that an ownership change occurred on April 4, 2022 due to the issuance of preferred equity. As a result of this ownership change, a portion of net operating loss (NOL) carryforwards and all R&D credits will expire unutilized. Further limitations are also expected as a result of the merger with SomaLogic that occurred on January 5, 2024.
The Company establishes a valuation allowance for deferred tax assets if the Company determines it is more likely than not the related tax benefit will not be realized. The Company relies on several factors when assessing the realizability of deferred tax assets, including historical financial results, the Company's ability to recover net operating loss carry-forwards, the projected future operating results, and the Company's ability to use tax planning strategies.
The valuation allowances of $
A reconciliation of the beginning and ending amounts of the valuation allowance for the years ended December 31, 2023 and 2022 is as follows (in thousands):
|
|
Valuation Allowance |
|
|
December 31, 2021 |
|
$ |
|
|
Charges to earnings |
|
|
|
|
Charges to other accounts |
|
|
( |
) |
December 31, 2022 |
|
|
|
|
Charges to earnings |
|
|
|
|
Charges to other accounts |
|
|
|
|
December 31, 2023 |
|
$ |
|
|
As of December 31, 2023, the Company had net operating loss carryforwards for U.S. federal income tax purposes of $
101
Company had net operating loss carryforwards for state income tax purposes of $
The aggregate changes in the balance of the Company's gross unrecognized tax benefits during 2023, and 2022 were as follows (in thousands):
December 31, 2021 |
|
$ |
|
|
Increases in balances related to tax positions during a prior |
|
|
|
|
Increases in balances related to tax positions taken during |
|
|
|
|
Decreases in balances related to tax positions taken during prior |
|
|
( |
) |
December 31, 2022 |
|
|
|
|
Increases in balances related to tax positions during a prior |
|
|
|
|
Decreases in balances related to tax positions taken during |
|
|
( |
) |
December 31, 2023 |
|
$ |
|
As of December 31, 2023, there were
Accrued interest and penalties related to unrecognized tax benefits was included in the income tax provision. The amount was immaterial as of December 31, 2023 and 2022.
The Company files income tax returns in the United States, its various states, and in certain foreign jurisdictions. As a consequence of having operating loss carryforwards, all tax years are open to federal and state examination in the United States. Tax years from 2012 are open to examination in various foreign countries.
13. Segment Reporting
The Company operates in
During 2023, the CODM began using gross profit to measure the operating performance of the segments. The Company determines each segment’s gross profit by subtracting cost of product and service revenues from segment revenues.
The Company does not prepare or report segmented balance sheet information as the CODM does not use the information to assess segment operating performance. The segments adhere to the same accounting policies as the Company as a whole.
102
The Company's business segment information was as follows (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Revenue: |
|
|
|
|
|
|
||
Proteomics |
|
$ |
|
|
$ |
|
||
Genomics |
|
|
|
|
|
|
||
Total revenue |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Gross profit: |
|
|
|
|
|
|
||
Proteomics |
|
$ |
|
|
$ |
|
||
Genomics |
|
|
|
|
|
|
||
Total gross profit |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Depreciation & amortization: |
|
|
|
|
|
|
||
Proteomics |
|
$ |
|
|
$ |
|
||
Genomics |
|
|
|
|
|
|
||
Total depreciation & amortization |
|
$ |
|
|
$ |
|
||
14. Restructuring and Related Charges
Beginning with the appointment of the Company’s new management team in April 2022 and as further announced in August 2022, the Company has implemented a restructuring plan, including a reduction in force, to improve operational efficiency, achieve cost savings and align the Company’s workforce to the future needs of the business. In addition to the reduction in force, the Company is reducing leased office space, optimizing its manufacturing footprint and streamlining support functions. The Company is developing a more disciplined cost management culture throughout its organization by investing in training and advanced information systems.
The Company records restructuring and related charges as incurred. These items are classified within restructuring and related charges in the consolidated statements of operations for the year ended December 31, 2023, and primarily include severance costs as well as facility costs (net of sublease income) for leased space in South San Francisco that the Company has vacated as part of the restructuring plan. The Company recognized restructuring and related charges of $
The Company expects to relieve the majority of the existing liability for restructuring charges primarily related to employee severance in 2024. Ongoing restructuring charges will continue to be incurred for facility related costs through the termination of the facility leases. These estimates are subject to a number of assumptions, and actual results may differ.
The following table summarizes the change in the Company’s restructuring and other related liabilities for the years ended December 31, 2023 and 2022 (in thousands):
|
|
Severance |
|
|
Facility |
|
|
Other(2) |
|
|
Total |
|
||||
Balance at December 31, 2021 |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Restructuring and related charges |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash payments |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Balance at December 31, 2022 |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Restructuring and related charges |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash payments |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Balance at December 31, 2023 |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
103
The Company’s restructuring and related charges by segment and corporate were as follows (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Restructuring: |
|
|
|
|
|
|
||
Proteomics |
|
$ |
|
|
$ |
|
||
Genomics |
|
|
|
|
|
|
||
Corporate expenses |
|
|
|
|
|
|
||
Total restructuring and related charges |
|
$ |
|
|
$ |
|
||
15. 401(k) Plan
The Company sponsors a 401(k) savings plan for its employees in the United States that stipulates that eligible employees may elect to contribute to the plan, subject to certain limitations, up to the lesser of
16. Subsequent Event
SomaLogic Merger
On January 5, 2024, the Company completed the previously announced Merger pursuant to the Agreement and Plan of Merger, dated as of October 4, 2023 (the “Merger Agreement”), by and among the Company, SomaLogic, and Martis Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of the Company (Merger Sub). Pursuant to the Merger Agreement, Merger Sub merged with and into SomaLogic, with SomaLogic surviving as a wholly owned subsidiary of Standard BioTools. At the consummation of the Merger, each issued and outstanding share of common stock of SomaLogic, was converted into the right to receive
In addition, as of the effective time of the Merger, the Company assumed each SomaLogic stock incentive plan, outstanding option to purchase shares of SomaLogic common stock and outstanding restricted stock units convertible into shares of SomaLogic common stock, whether vested or unvested. In addition, as of the Effective Time, each SomaLogic warrant was treated in accordance with its terms.
Due to the limited time between the Merger date and the filing of this Annual Report, it is not practicable for the Company to disclose the preliminary allocation of the purchase price to assets acquired and liabilities assumed.
104
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2023, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Exchange Act Rule 13a-15(f)) to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Management assessed our internal control over financial reporting as of December 31, 2023. Management based its assessment on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework). Based on that evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2023.
This Annual Report does not include an attestation report of our registered public accounting firm regarding internal controls over financial reporting due to the Company's status as a non-accelerated filer and smaller reporting company.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the three months ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on the Effectiveness of Controls
Control systems, no matter how well conceived and operated, are designed to provide a reasonable, but not an absolute, level of assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Because of the inherent limitations in any control system, misstatements due to error or fraud may occur and not be detected.
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURES REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
None.
105
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information called for by this item will be set forth in our proxy statement for the 2024 annual meeting of stockholders (the Proxy Statement) to be filed with the SEC within 120 days of the fiscal year ended December 31, 2023 and is incorporated herein by reference.
Our board of directors has adopted a Code of Ethics and Conduct that applies to all of our employees, officers and directors, including our Chief Executive Officer, Chief Financial Officer and other executive and senior financial officers. The full text of our code of business conduct and ethics is posted on the investor relations page on our website, which is located at www.standardbio.com. We will post any amendments to our code of business conduct and ethics, or waivers of its requirements, on our website.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item will be set forth in our Proxy Statement and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information, if any, required by this item will be set forth in our Proxy Statement and is incorporated herein by reference.
The information, if any, required by this item will be set forth in our Proxy Statement and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item will be set forth in our Proxy Statement and is incorporated herein by reference.
106
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
ITEM 16. FORM 10-K SUMMARY
None.
107
INDEX TO EXHIBITS
Exhibit Number |
|
Description |
|
Incorporated by Reference From Form |
|
File Number |
|
Incorporated by Reference From Exhibit Number |
|
Date Filed |
2.1†† |
|
|
8-K |
|
001-34180 |
|
2.1 |
|
1/29/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
2.2†† |
|
|
S-4/A |
|
333-256127 |
|
2.1 |
|
8/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
2.3† |
|
|
8-K |
|
001-40090 |
|
2.1 |
|
7/27/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
2.4†† |
|
|
8-K |
|
001-34180 |
|
2.1 |
|
10/4/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
3.1 |
|
|
10-K |
|
001-34180 |
|
3.1 |
|
3/28/2011 |
|
|
|
|
|
|
|
|
|
|
|
|
3.2 |
|
Amended and Restated Bylaws of Standard BioTools Inc. (formerly Fluidigm Corporation). |
|
S-8 |
|
333-264086 |
|
4.8 |
|
4/1/2022 |
|
|
|
|
|
|
|
|
|
|
|
3.3 |
|
|
S-8 |
|
333-264086 |
|
4.3 |
|
4/1/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
3.4 |
|
|
8-K |
|
001-34180 |
|
3.1 |
|
1/5/2024 |
|
|
|
|
|
|
|
|
|
|
|
|
3.5 |
|
|
8-K |
|
001-34180 |
|
3.1 |
|
11/22/2016 |
|
|
|
|
|
|
|
|
|
|
|
|
3.6 |
|
Certificate of Elimination of Series A Participating Preferred Stock of Fluidigm Corporation. |
|
8-K |
|
001-34180 |
|
3.1 |
|
8/2/2017 |
|
|
|
|
|
|
|
|
|
|
|
3.7 |
|
|
8-K |
|
001-34180 |
|
3.6 |
|
4/5/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
3.8 |
|
|
8-K |
|
001-34180 |
|
3.7 |
|
4/5/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
4.1 |
|
|
S-8 |
|
333-264086 |
|
4.1 |
|
4/1/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
4.2 |
|
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.3 |
|
|
8-K |
|
001-34180 |
|
4.1 |
|
2/4/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
108
Exhibit Number |
|
Description |
|
Incorporated by Reference From Form |
|
File Number |
|
Incorporated by Reference From Exhibit Number |
|
Date Filed |
4.4 |
|
|
8-K |
|
001-34180 |
|
4.2 |
|
2/4/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
4.5 |
|
|
8-K |
|
001-34180 |
|
4.3 |
|
2/4/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
4.6 |
|
|
8-K |
|
001-34180 |
|
4.1 |
|
11/22/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
4.7 |
|
Form of 5.25% Convertible Senior Note due 2024 (included in Exhibit 4.6). |
|
8-K |
|
001-34180 |
|
4.2 |
|
11/22/2019 |
|
|
|
|
|
|
|
|
|
|
|
4.8 |
|
|
8-K |
|
001-40090 |
|
10.1 |
|
2/26/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
4.9 |
|
|
8-K |
|
001-40090 |
|
10.1 |
|
3/29/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.1# |
|
|
S-1/A |
|
333-170965 |
|
10.1 |
|
1/28/2011 |
|
|
|
|
|
|
|
|
|
|
|
|
10.2# |
|
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3 |
|
|
10-Q |
|
001-34180 |
|
10.1 |
|
5/7/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
10.4 |
|
|
10-Q |
|
001-34180 |
|
10.2 |
|
5/7/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
10.5 |
|
|
10-K |
|
001-34180 |
|
10.2B |
|
2/25/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.6† |
|
|
10-Q |
|
001-34180 |
|
10.1 |
|
11/9/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
10.7 |
|
|
S-1 |
|
333-170965 |
|
10.20 |
|
12/3/2010 |
|
|
|
|
|
|
|
|
|
|
|
|
10.8 |
|
|
10-K |
|
001-34180 |
|
10.21 |
|
3/12/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
10.9 |
|
|
10-Q |
|
001-34180 |
|
10.1 |
|
8/10/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
109
Exhibit Number |
|
Description |
|
Incorporated by Reference From Form |
|
File Number |
|
Incorporated by Reference From Exhibit Number |
|
Date Filed |
10.10 |
|
|
10-Q |
|
001-34180 |
|
10.2 |
|
8/6/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.11 |
|
|
10-Q |
|
001-34180 |
|
10.3 |
|
8/6/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.12 |
|
|
10-K |
|
001-34180 |
|
10.5D |
|
3/8/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.13 |
|
Sublease, dated as of August 30, 2022, by and between Standard BioTools Inc. and CIRC Bio, Inc. |
|
10-Q |
|
001-34180 |
|
10.1 |
|
11/9/2022 |
|
|
|
|
|
|
|
|
|
|
|
10.14 |
|
|
10-Q |
|
001-34180 |
|
10.1 |
|
5/9/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.15†† |
|
|
8-K |
|
001-40090 |
|
10.1 |
|
2/16/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.16†† |
|
|
8-K |
|
001-40090 |
|
10.2 |
|
2/16/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.17† |
|
|
10-Q |
|
001-34180 |
|
10.2 |
|
11/9/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
10.18† |
|
|
10-Q |
|
001-34180 |
|
10.2A |
|
11/9/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
10.19† |
|
|
10-Q |
|
001-34180 |
|
10.3 |
|
11/9/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
10.20† |
|
|
10-Q |
|
001-34180 |
|
10.3A |
|
11/9/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
10.21† |
|
|
10-Q |
|
001-34180 |
|
10.4 |
|
11/9/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
110
Exhibit Number |
|
Description |
|
Incorporated by Reference From Form |
|
File Number |
|
Incorporated by Reference From Exhibit Number |
|
Date Filed |
10.22† |
|
|
10-Q |
|
001-34180 |
|
10.5 |
|
11/9/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
10.23† |
|
|
10-Q |
|
001-34180 |
|
10.6 |
|
11/9/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
10.24† |
|
|
10-Q/A |
|
001-34180 |
|
10.3 |
|
9/15/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
10.25† |
|
|
10-Q/A |
|
001-34180 |
|
10.4 |
|
9/15/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
10.26† |
|
|
8-K |
|
001-34180 |
|
10.1 |
|
8/2/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
10.27 |
|
|
10-K |
|
001-34180 |
|
10.13A |
|
2/27/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
10.28 |
|
|
8-K |
|
001-34180 |
|
10.2 |
|
11/22/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
10.29 |
|
|
8-K |
|
001-34180 |
|
10.1 |
|
4/22/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
10.30 |
|
|
8-K |
|
001-34180 |
|
10.1 |
|
8/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.31 |
|
|
10-K |
|
001-34180 |
|
10.19E |
|
3/8/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.32 |
|
|
10-K |
|
001-34180 |
|
10.19F |
|
3/8/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.33 |
|
|
8-K |
|
001-34180 |
|
10.1 |
|
11/22/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
10.34† |
|
|
10-Q |
|
001-34180 |
|
10.1 |
|
11/9/2020 |
111
Exhibit Number |
|
Description |
|
Incorporated by Reference From Form |
|
File Number |
|
Incorporated by Reference From Exhibit Number |
|
Date Filed |
|
|
(formerly Fluidigm Corporation) and National Institutes of Health, as amended on September 28, 2020. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.35† |
|
|
10-Q |
|
001-34180 |
|
10.1 |
|
8/6/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.36† |
|
|
10-Q |
|
001-34180 |
|
10.1 |
|
11/9/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.37 |
|
|
8-K/A |
|
001-34180 |
|
10.1 |
|
2/11/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.38 |
|
|
8-K |
|
001-34180 |
|
10.2 |
|
1/24/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.39 |
|
|
DEF 14A |
|
001-34180 |
|
Anx. B |
|
2/24/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.40 |
|
|
DEF 14A |
|
001-34180 |
|
Anx. C |
|
2/24/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.41 |
|
|
8-K |
|
001-40090 |
|
10.4 |
|
2/26/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.42 |
|
|
8-K |
|
001-34180 |
|
10.5 |
|
1/24/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.43 |
|
|
8-K |
|
001-40090 |
|
10.6 |
|
3/29/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.44 |
|
|
8-K |
|
001-34180 |
|
10.1 |
|
3/28/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.45 |
|
Letter Agreement, dated as of May 10, 2023, by and between Standard BioTools Inc. and Vikram Jog. |
|
8-K |
|
001-34180 |
|
10.3 |
|
5/15/2023 |
112
Exhibit Number |
|
Description |
|
Incorporated by Reference From Form |
|
File Number |
|
Incorporated by Reference From Exhibit Number |
|
Date Filed |
|
|
|
|
|
|
|
|
|
|
|
10.46 |
|
|
8-K |
|
001-34180 |
|
10.1 |
|
3/29/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.47# |
|
|
10-Q |
|
001-34180 |
|
10.5 |
|
11/7/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
10.48# |
|
Standard BioTools Inc. (formerly Fluidigm Corporation) Executive Bonus Plan. |
|
10-Q |
|
001-34180 |
|
10.25 |
|
3/28/2011 |
|
|
|
|
|
|
|
|
|
|
|
10.49# |
|
|
8-K |
|
001-34180 |
|
10.14 |
|
12/11/2012 |
|
|
|
|
|
|
|
|
|
|
|
|
10.50# |
|
Standard BioTools Inc. (formerly Fluidigm Corporation) Form of Retention Letter. |
|
8-K |
|
001-34180 |
|
10.10 |
|
1/24/2022 |
|
|
|
|
|
|
|
|
|
|
|
10.51# |
|
|
8-K |
|
001-34180 |
|
10.7 |
|
1/24/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.52# |
|
|
8-K |
|
001-34180 |
|
10.9 |
|
1/24/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.53# |
|
|
8-K |
|
001-34180 |
|
10.1 |
|
5/15/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.54# |
|
Standard BioTools Inc. (formerly Fluidigm Corporation) 2020 Change of Control and Severance Plan. |
|
10-Q |
|
001-34180 |
|
10.5 |
|
8/7/2020 |
|
|
|
|
|
|
|
|
|
|
|
10.55# |
|
|
8-K |
|
001-34180 |
|
10.2 |
|
5/15/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.56# |
|
Standard BioTools Inc. 2023 Change of Control and Severance Plan. |
|
8-K |
|
001-34180 |
|
10.1 |
|
7/28/2023 |
|
|
|
|
|
|
|
|
|
|
|
10.57# |
|
|
8-K |
|
001-34180 |
|
10.2 |
|
7/28/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.58# |
|
|
10-Q |
|
001-34180 |
|
10.9 |
|
8/8/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.59# |
|
Standard BioTools Inc. (formerly Fluidigm Corporation) 2009 Equity Incentive Plan, as amended. |
|
S-1 |
|
333-170965 |
|
10.3 |
|
12/3/2010 |
|
|
|
|
|
|
|
|
|
|
|
10.60# |
|
|
S-1 |
|
333-170965 |
|
10.3A |
|
12/3/2010 |
|
|
|
|
|
|
|
|
|
|
|
|
10.61# |
|
|
8-K |
|
001-34180 |
|
10.2 |
|
8/2/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
113
Exhibit Number |
|
Description |
|
Incorporated by Reference From Form |
|
File Number |
|
Incorporated by Reference From Exhibit Number |
|
Date Filed |
10.62# |
|
Standard BioTools Inc. Amended and Restated 2011 Equity Incentive Plan. |
|
8-K |
|
001-34180 |
|
10.1 |
|
1/5/2024 |
|
|
|
|
|
|
|
|
|
|
|
10.63# |
|
|
SC TO-I |
|
005-86635 |
|
(d)(2) |
|
8/23/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
10.64# |
|
|
SC TO-I |
|
005-86635 |
|
(d)(3) |
|
8/23/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
10.65# |
|
|
SC TO-I |
|
005-86635 |
|
(d)(4) |
|
8/23/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
10.66# |
|
|
SC TO-I |
|
005-86635 |
|
(d)(5) |
|
8/23/20217 |
|
|
|
|
|
|
|
|
|
|
|
|
10.67# |
|
|
SC TO-I |
|
005-86635 |
|
(d)(6) |
|
8/23/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
10.68# |
|
|
SC TO-I |
|
005-86635 |
|
(d)(7) |
|
8/23/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
10.69# |
|
Standard BioTools Inc. 2011 Equity Incentive Plan Form of PSU Award Agreement. |
|
8-K |
|
001-34180 |
|
10.3 |
|
7/28/2023 |
|
|
|
|
|
|
|
|
|
|
|
10.70# |
|
|
8-K |
|
001-34180 |
|
10.1 |
|
1/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
10.71# |
|
|
8-K |
|
001-34180 |
|
10.1 |
|
6/24/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
10.72# |
|
Standard BioTools Inc. 2022 Inducement Equity Incentive Plan. |
|
S-8 |
|
333-264086 |
|
4.9 |
|
4/1/2022 |
|
|
|
|
|
|
|
|
|
|
|
10.73# |
|
|
S-8 |
|
333-264086 |
|
99.1 |
|
4/1/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.74# |
|
|
S-8 |
|
333-264086 |
|
99.2 |
|
4/1/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.75# |
|
|
S-4 |
|
333-256127 |
|
10.7 |
|
5/14/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.76# |
|
Form of Non-Statutory Stock Option Agreement under the SomaLogic, Inc. 2009 Equity Incentive Plan. |
|
S-4 |
|
333-256127 |
|
10.8 |
|
5/14/2021 |
|
|
|
|
|
|
|
|
|
|
|
10.77# |
|
Form of Incentive Stock Option Agreement under the SomaLogic, Inc. 2009 Equity Incentive Plan. |
|
S-4 |
|
333-256127 |
|
10.9 |
|
5/14/2021 |
|
|
|
|
|
|
|
|
|
|
|
10.78# |
|
|
S-4 |
|
333-256127 |
|
10.10 |
|
5/14/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.79# |
|
|
S-4 |
|
333-256127 |
|
10.11 |
|
5/14/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.80# |
|
|
S-4/A |
|
333-256127 |
|
10.1 |
|
8/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
114
Exhibit Number |
|
Description |
|
Incorporated by Reference From Form |
|
File Number |
|
Incorporated by Reference From Exhibit Number |
|
Date Filed |
10.81# |
|
|
S-4/A |
|
333-256127 |
|
10.2 |
|
8/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.82# |
|
|
S-4/A |
|
333-256127 |
|
10.3 |
|
6/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.83# |
|
|
S-4/A |
|
333-256127 |
|
10.4 |
|
6/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.84# |
|
Form of Restricted Stock Unit Award Agreement under the SomaLogic, Inc. 2021 Omnibus Incentive Plan. |
|
S-4/A |
|
333-256127 |
|
10.5 |
|
6/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
10.85# |
|
Form of Restricted Stock Award Agreement under the SomaLogic, Inc. 2021 Omnibus Incentive Plan. |
|
S-4/A |
|
333-256127 |
|
10.6 |
|
6/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
10.86# |
|
|
S-4/A |
|
333-256127 |
|
10.7 |
|
6/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.87 |
|
|
10-Q |
|
001-40090 |
|
10.1 |
|
11/8/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.88 |
|
|
10-Q |
|
001-40090 |
|
10.4 |
|
8/14/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.89 |
|
|
10-K |
|
001-39796 |
|
10.38 |
|
3/28/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.90† †† |
|
|
10-K |
|
001-40090 |
|
10.36 |
|
3/29/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.91† |
|
|
8-K |
|
001-40090 |
|
10.1 |
|
1/10/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.92† |
|
|
S-4/A |
|
001-39796 |
|
10.33 |
|
6/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.93† |
|
|
S-4/A |
|
001-39796 |
|
10.34 |
|
6/5/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
10.94 |
|
|
10-Q |
|
001-40090 |
|
10.1 |
|
8/14/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
10.95† |
|
|
10-K |
|
001-40090 |
|
10.34 |
|
3/29/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
10.96# |
|
Standard BioTools Inc. Amended and Restated Outside Director Equity Compensation Policy. |
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
115
Exhibit Number |
|
Description |
|
Incorporated by Reference From Form |
|
File Number |
|
Incorporated by Reference From Exhibit Number |
|
Date Filed |
21.1 |
|
|
Filed herewith |
|
|
|
|
|
|
|
23.1 |
|
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. |
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24.1 |
|
Power of Attorney (contained in the signature page to this Form 10-K). |
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1~ |
|
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.2~ |
|
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
97.1# |
|
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.INS |
|
XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema with Embedded Linkbases Document |
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
Filed herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
# Management contracts or compensation plans or arrangements in which directors or executive officers are eligible to participate.
† Portions of this exhibit have been redacted in compliance with Regulation S-K Item 601(b)(10)(iv) or pursuant to an order granted by the Securities and Exchange Commission for confidential treatment.
†† The schedules and exhibits to this exhibit have been omitted pursuant to Item 601(b)(2) of Regulation S-K.
~ In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release No. 33-8238 and 34-47986, Final Rule: Management’s Reports on Internal Control Over Financial Reporting and Certification of Disclosure in Exchange Act Periodic Reports, the certifications furnished in Exhibits 32.1 and 32.2 hereto are deemed to accompany this Annual Report on Form 10-K and will not be deemed “filed” for purposes of Section 18 of the Exchange Act. Such certifications will not be deemed to be incorporated by reference into any filings under the Securities Act or the Exchange Act, except to the extent that Standard BioTools Inc. specifically incorporates it by reference.
116
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
STANDARD BIOTOOLS INC. |
||
|
|
|
|
Dated: March 1, 2024 |
By: |
|
/s/ Michael Egholm, Ph.D. |
|
|
|
Michael Egholm, Ph.D. |
|
|
|
President and Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Michael Egholm and Jeffrey Black, jointly and severally, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign this Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises hereby ratifying and confirming all that said attorneys-in-fact and agents, or his or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature |
|
Title |
|
Date |
|
|
|
|
|
/s/Michael Egholm, Ph.D. |
|
President and Chief Executive Officer (Principal Executive Officer); Director |
|
March 1, 2024 |
Michael Egholm, Ph.D. |
|
|
|
|
|
|
|
|
|
/s/ Jeffrey Black |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
March 1, 2024 |
Jeffrey Black |
|
|
|
|
|
|
|
|
|
/s/ Tom Carey |
|
Chairman of the Board of Directors |
|
March 1, 2024 |
Tom Carey |
|
|
|
|
|
|
|
|
|
/s/ Fenel M. Eloi |
|
Director |
|
March 1, 2024 |
Fenel M. Eloi |
|
|
|
|
|
|
|
|
|
/s/ Eli Casdin |
|
Director |
|
March 1, 2024 |
Eli Casdin |
|
|
|
|
|
|
|
|
|
/s/ Kathy Hibbs |
|
Director |
|
March 1, 2024 |
Kathy Hibbs |
|
|
|
|
|
|
|
|
|
/s/ Troy Cox |
|
Director |
|
March 1, 2024 |
Troy Cox |
|
|
|
|
|
|
|
|
|
/s/ Frank Witney, Ph.D. |
|
Director |
|
March 1, 2024 |
Frank Witney, Ph.D. |
|
|
|
|