Use these links to rapidly review the document
TABLE OF CONTENTS
PART III
NOVARTIS GROUP INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
As filed with the Securities and Exchange Commission on January 27, 2016
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM 20-F
o |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
OR |
||
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 for the fiscal year ended December 31, 2015 |
|
OR |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
OR |
||
o |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
Commission file number 1-15024
NOVARTIS AG
(Exact name of Registrant as specified in its charter)
NOVARTIS Inc.
(Translation of Registrant's name into English)
Switzerland
(Jurisdiction of incorporation or organization)
Lichtstrasse 35
4056 Basel, Switzerland
(Address of principal executive offices)
Felix R. Ehrat
Group General Counsel
Novartis AG
CH-4056 Basel
Switzerland
Tel.: 011-41-61-324-1111
Fax: 011-41-61-324-7826
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered pursuant to Section 12(b) of the Act:
Title of class
|
Name of each exchange on which registered
|
|
|---|---|---|
| American Depositary Shares each representing 1 share Ordinary shares, nominal value CHF 0.50 per share* |
New York Stock Exchange New York Stock Exchange* |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
Indicate the number of outstanding shares of each of the issuer's classes of capital or common stock as of the close of the period covered by the annual report:
2,373,894,817 shares
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ý No o
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ý No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of "accelerated filer and large accelerated filer" in Rule 12b-2 of the Exchange Act (Check one):
Large accelerated filer ý Accelerated filer o Non-accelerated filer o
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| |
|
|
||
|---|---|---|---|---|
| o U.S. GAAP | ý International Financial Reporting Standards as issued by the International Accounting Standards Board | o Other |
If "Other" has been checked in response to the previous question indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 o Item 18 o
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No ý
- *
- Not for trading but only in connection with the registration of American Depositary Shares representing such ordinary shares.
2
3
INTRODUCTION AND USE OF CERTAIN TERMS
Novartis AG and its consolidated affiliates publish consolidated financial statements expressed in US dollars. Our consolidated financial statements found in Item 18 of this annual report on Form 20-F (Form 20-F) are prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB).
Unless the context requires otherwise, the words "we," "our," "us," "Novartis," "Group," "Company," and similar words or phrases in this Form 20-F refer to Novartis AG and its consolidated affiliates. However, each Group company is legally separate from all other Group companies and manages its business independently through its respective board of directors or other top local management body. No Group company operates the business of another Group company. Each executive identified in this Form 20-F reports directly to other executives of the Group company which employs the executive, or to that Group company's board of directors.
In this Form 20-F, references to "US dollars" or "$" are to the lawful currency of the United States of America, and references to "CHF" are to Swiss francs; references to the "United States" or to "US" are to the United States of America, references to the "European Union" or to "EU" are to the European Union and its 28 member states, references to "Latin America" are to Central and South America, including the Caribbean, and references to "Australasia" are to Australia, New Zealand, Melanesia, Micronesia and Polynesia, unless the context otherwise requires; references to the "EC" are to the European Commission; references to "associates" are to employees of our affiliates; references to the "FDA" are to the US Food and Drug Administration, references to "EMA" are to the European Medicines Agency, an agency of the EU, and references to the "CHMP" are to the Committee for Medicinal Products for Human Use of the EMA; references to "ADR" or "ADRs" are to Novartis American Depositary Receipts, and references to "ADS" or "ADSs" are to Novartis American Depositary Shares; references to the "NYSE" are to the New York Stock Exchange, and references to the "SIX" are to the SIX Swiss Exchange; references to "GSK" are to GlaxoSmithKline plc, references to "Lilly" are to Eli Lilly and Company, and references to "CSL" are to CSL Limited.
All product names appearing in italics are trademarks owned by or licensed to Group companies. Product names identified by a "®" or a "™" are trademarks that are not owned by or licensed to Group companies.
This Form 20-F contains certain "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Other written materials filed with or furnished to the US Securities and Exchange Commission (SEC) by Novartis, as well as other written and oral statements made to the public, may also contain forward-looking statements. Forward-looking statements can be identified by words such as "potential," "expected," "will," "planned," "pipeline," "outlook," or similar terms, or by express or implied discussions regarding potential new products, potential new indications for existing products, or regarding potential future revenues from any such products; potential shareholder returns or credit ratings; or regarding the potential financial or other impact on Novartis or any of our divisions of the strategic actions announced in January 2016 to focus our divisions, integrate certain functions and leverage our scale; or regarding any potential financial or other impact on Novartis as a result of the creation and operation of NBS; or regarding the potential financial or other impact on Novartis of the transactions with GSK, Lilly or CSL; or regarding potential future sales or earnings of the Novartis Group or any of its divisions; or by discussions of strategy, plans, expectations or intentions. You should not place undue reliance on these statements.
4
Such forward-looking statements are based on the current beliefs and expectations of management regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that any new products will be approved for sale in any market, or that any new indications will be approved for any existing products in any market, or that any approvals which are obtained will be obtained at any particular time, or that any such products will achieve any particular revenue levels. Nor can there be any guarantee that Novartis will be able to realize any of the potential strategic benefits, synergies or opportunities as a result of the strategic actions announced in January 2016, the creation and operation of NBS, or the transactions with GSK, Lilly or CSL. Neither can there be any guarantee that Novartis will achieve any particular financial results in the future. Nor can there be any guarantee that shareholders will achieve any particular level of shareholder returns. Neither can there be any guarantee that the Group, or any of its divisions, will be commercially successful in the future, or achieve any particular credit rating.
In particular, management's expectations could be affected by, among other things:
- •
- unexpected regulatory actions or delays or government regulation generally;
- •
- the potential that the strategic benefits, synergies or opportunities expected from the strategic actions announced in January 2016,
the creation and operation of NBS, or the transactions with GSK, Lilly or CSL may not be realized or may take longer to realize than expected;
- •
- the inherent uncertainties involved in predicting shareholder returns or credit ratings;
- •
- the uncertainties inherent in research and development, including unexpected clinical trial results and additional analysis of
existing clinical data;
- •
- our ability to obtain or maintain proprietary intellectual property protection, including the ultimate extent of the impact on
Novartis of the loss of patent protection and exclusivity on key products which commenced in prior years and will continue this year;
- •
- unexpected safety, quality or manufacturing issues;
- •
- global trends toward health care cost containment, including ongoing pricing pressures, in particular from increased publicity on
pharmaceuticals pricing;
- •
- uncertainties regarding actual or potential legal proceedings, including, among others, actual or potential product liability
litigation, litigation and investigations regarding sales and marketing practices, government investigations and intellectual property disputes;
- •
- general economic and industry conditions, including uncertainties regarding the effects of the persistently weak economic and
financial environment in many countries;
- •
- uncertainties regarding future global exchange rates, including the continued significant increase in value of the US dollar, our
reporting currency, against a number of currencies;
- •
- uncertainties regarding future demand for our products;
- •
- uncertainties involved in the development of new healthcare products; and
- •
- uncertainties regarding potential significant breaches of data security or disruptions of our information technology systems.
Some of these factors are discussed in more detail in this Form 20-F, including under "Item 3. Key Information—3.D. Risk factors," "Item 4. Information on the Company," and "Item 5. Operating and Financial Review and Prospects." Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in this Form 20-F as anticipated, believed, estimated or expected. We provide the information in this Form 20-F as of the date of its filing. We do not intend, and do not assume any obligation, to update any information or forward-looking statements set out in this Form 20-F as a result of new information, future events or otherwise.
5
Item 1. Identity of Directors, Senior Management and Advisers
Not applicable.
Item 2. Offer Statistics and Expected Timetable
Not applicable.
The selected financial information set out below has been extracted from our consolidated financial statements prepared in accordance with IFRS as issued by the IASB. Our consolidated financial statements for the years ended December 31, 2015, 2014 and 2013 are included in "Item 18. Financial Statements" in this Form 20-F.
All financial data should be read in conjunction with "Item 5. Operating and Financial Review and Prospects". All financial data presented in this Form 20-F are qualified in their entirety by reference to the consolidated financial statements and their notes.
| |
Year Ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | 2012 | 2011 | |||||||||||
| |
($ millions, except per share information) |
|||||||||||||||
INCOME STATEMENT DATA |
||||||||||||||||
Net sales to third parties from continuing operations |
49,414 | 52,180 | 51,869 | 51,080 | 51,939 | |||||||||||
| | | | | | | | | | | | | | | | | |
Operating income from continuing operations |
8,977 | 11,089 | 10,983 | 11,507 | 10,293 | |||||||||||
Income from associated companies |
266 | 1,918 | 599 | 549 | 526 | |||||||||||
Interest expense |
(655 | ) | (704 | ) | (683 | ) | (724 | ) | (751 | ) | ||||||
Other financial income and expense |
(454 | ) | (31 | ) | (92 | ) | (96 | ) | (2 | ) | ||||||
| | | | | | | | | | | | | | | | | |
Income before taxes from continuing operations |
8,134 | 12,272 | 10,807 | 11,236 | 10,066 | |||||||||||
Taxes |
(1,106 | ) | (1,545 | ) | (1,498 | ) | (1,706 | ) | (1,381 | ) | ||||||
| | | | | | | | | | | | | | | | | |
Net income from continuing operations |
7,028 | 10,727 | 9,309 | 9,530 | 8,685 | |||||||||||
Net income/(loss) from discontinued operations |
10,766 | (447 | ) | (17 | ) | (147 | ) | 387 | ||||||||
| | | | | | | | | | | | | | | | | |
Group net income |
17,794 | 10,280 | 9,292 | 9,383 | 9,072 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Attributable to: |
||||||||||||||||
Shareholders of Novartis AG |
17,783 | 10,210 | 9,175 | 9,270 | 8,940 | |||||||||||
Non-controlling interests |
11 | 70 | 117 | 113 | 132 | |||||||||||
Basic earnings per share ($) |
||||||||||||||||
Continuing operations |
2.92 | 4.39 | 3.76 | 3.89 | 3.59 | |||||||||||
Discontinued operations |
4.48 | (0.18 | ) | 0.00 | (0.06 | ) | 0.16 | |||||||||
Total |
7.40 | 4.21 | 3.76 | 3.83 | 3.75 | |||||||||||
Diluted earnings per share ($) |
||||||||||||||||
Continuing operations |
2.88 | 4.31 | 3.70 | 3.85 | 3.54 | |||||||||||
Discontinued operations |
4.41 | (0.18 | ) | 0.00 | (0.06 | ) | 0.16 | |||||||||
Total |
7.29 | 4.13 | 3.70 | 3.79 | 3.70 | |||||||||||
Cash dividends(1) |
6,643 | 6,810 | 6,100 | 6,030 | 5,368 | |||||||||||
Cash dividends per share in CHF(2) |
2.70 | 2.60 | 2.45 | 2.30 | 2.25 | |||||||||||
- (1)
- Cash
dividends represent cash payments in the applicable year that generally relates to earnings of the previous year.
- (2)
- Cash dividends per share represent dividends proposed that relate to earnings of the current year. Dividends for 2011 through 2014 were approved at the respective AGMs and dividends for 2015 will be proposed to the Annual General Meeting on February 23, 2016 for approval.
6
| |
Year Ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | 2012 | 2011 | |||||||||||
| |
($ millions) |
|||||||||||||||
BALANCE SHEET DATA |
||||||||||||||||
Cash, cash equivalents and marketable securities & derivative financial instruments |
5,447 | 13,862 | 9,222 | 8,119 | 5,075 | |||||||||||
Inventories |
6,226 | 6,093 | 7,267 | 6,744 | 5,930 | |||||||||||
Other current assets |
11,172 | 10,805 | 13,294 | 13,141 | 13,079 | |||||||||||
Non-current assets |
108,711 | 87,826 | 95,712 | 96,187 | 93,384 | |||||||||||
Assets related to discontinued operations |
6,801 | 759 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Total assets |
131,556 | 125,387 | 126,254 | 124,191 | 117,468 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Trade accounts payable |
5,668 | 5,419 | 6,148 | 5,593 | 4,989 | |||||||||||
Other current liabilities |
18,040 | 19,136 | 20,170 | 18,458 | 18,159 | |||||||||||
Non-current liabilities |
30,726 | 27,570 | 25,414 | 30,877 | 28,331 | |||||||||||
Liabilities related to discontinued operations |
2,418 | 50 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Total liabilities |
54,434 | 54,543 | 51,782 | 54,928 | 51,479 | |||||||||||
| | | | | | | | | | | | | | | | | |
Issued share capital and reserves attributable to shareholders of Novartis AG |
77,046 | 70,766 | 74,343 | 69,137 | 65,893 | |||||||||||
Non-controlling interests |
76 | 78 | 129 | 126 | 96 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total equity |
77,122 | 70,844 | 74,472 | 69,263 | 65,989 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total liabilities and equity |
131,556 | 125,387 | 126,254 | 124,191 | 117,468 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Net assets |
77,122 | 70,844 | 74,472 | 69,263 | 65,989 | |||||||||||
Outstanding share capital |
890 | 898 | 912 | 909 | 895 | |||||||||||
Total outstanding shares (millions) |
2,374 | 2,399 | 2,426 | 2,421 | 2,407 | |||||||||||
Cash dividends are translated into US dollars at the Bloomberg Market System Rate on the payment date. Because we pay dividends in Swiss francs, exchange rate fluctuations will affect the US dollar amounts received by holders of ADRs.
Year Earned
|
Month and Year Paid |
Total Dividend per share (CHF) |
Total Dividend per share ($) |
||||||
|---|---|---|---|---|---|---|---|---|---|
2011 |
March 2012 | 2.25 | 2.48 | ||||||
2012 |
March 2013 | 2.30 | 2.44 | ||||||
2013 |
March 2014 | 2.45 | 2.76 | ||||||
2014 |
March 2015 | 2.60 | 2.67 | ||||||
2015(1) |
March 2016 | 2.70 | 2.73 | (2) | |||||
- (1)
- Dividend
to be proposed by the Board of Directors to the Annual General Meeting on February 23, 2016 and to be distributed
February 29, 2016
- (2)
- Translated into US dollars at the Bloomberg Market System December 31, 2015 rate of $1.011 to the Swiss franc. This translation is an example only, and should not be construed as a representation that the Swiss franc amount represents, or has been or could be converted into US dollars at that or any other rate.
7
The following table shows, for the years and dates indicated, certain information concerning the rate of exchange of US dollar per Swiss franc based on exchange rate information found on Bloomberg Market System. The exchange rate in effect on January 20, 2016, as found on Bloomberg Market System, was CHF 1.00 = $0.998.
Year ended December 31, ($ per CHF) |
Period End | Average(1) | Low(2) | High(2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
2011 |
1.06 | 1.13 | 1.06 | 1.25 | |||||||||
2012 |
1.09 | 1.07 | 1.02 | 1.12 | |||||||||
2013 |
1.12 | 1.08 | 1.05 | 1.12 | |||||||||
2014 |
1.01 | 1.09 | 1.01 | 1.13 | |||||||||
2015 |
1.01 | 1.04 | 0.97 | 1.08 | |||||||||
Month |
|||||||||||||
August 2015 |
1.02 | 1.07 | |||||||||||
September 2015 |
1.02 | 1.04 | |||||||||||
October 2015 |
1.01 | 1.05 | |||||||||||
November 2015 |
0.97 | 1.01 | |||||||||||
December 2015 |
0.97 | 1.02 | |||||||||||
January 2016 (through January 20, 2016) |
0.99 | 1.01 | |||||||||||
- (1)
- Represents
the average of the exchange rates on the last day of each month during the year.
- (2)
- Represents the lowest, respectively highest, of the exchange rates on the last day of each month during the year.
3.B Capitalization and Indebtedness
Not applicable.
3.C Reasons for the offer and use of proceeds
Not applicable.
Our businesses face significant risks and uncertainties. You should carefully consider all of the information set forth in this annual report on Form 20-F and in other documents we file with or furnish to the SEC, including the following risk factors, before deciding to invest in or to maintain an investment in any Novartis securities. Our business, as well as our financial condition or results of operations could be materially adversely affected by any of these risks, as well as other risks and uncertainties not currently known to us or not currently considered material.
Our products face important patent expirations and significant competition.
The products of our Pharmaceuticals and Alcon Divisions, as well as certain key products of our Sandoz Division, are generally protected by patent and other intellectual property rights, which are intended to provide us with exclusive rights to market the products. However, those intellectual property rights have varying strengths and durations. Loss of market exclusivity for one or more important products has had, and can be expected to continue to have a material adverse effect on our results of operations.
8
The introduction of generic competition for a patented medicine typically results in a significant and rapid reduction in net sales and net income for the patented product because generic manufacturers typically offer their unpatented versions at sharply lower prices. Such competition can occur after successful challenges to intellectual property rights or the regular expiration of the term of the patent or other intellectual property rights. Such competition can also result from the entry of generic versions of another medicine in the same therapeutic class as one of our drugs, or in another competing therapeutic class, from the compulsory licensing of our drugs by governments, or from a general weakening of intellectual property laws in certain countries around the world. In addition, generic manufacturers sometimes take an aggressive approach to challenging patents, conducting so-called "launches at risk" of products that are still under legal challenge for patent infringement, before final resolution of legal proceedings.
We also rely in all aspects of our businesses on unpatented proprietary technology, know-how, trade secrets and other confidential information, which we seek to protect through various measures including confidentiality agreements with licensees, employees, third-party collaborators, and consultants who may have access to such information. If these agreements are breached, our contractual or other remedies may not be adequate to cover our losses.
Some of our best-selling products have begun or are about to face significant competition due to the end of market exclusivity resulting from the expiry of patent or other intellectual property protection.
- •
- We already face generic competition in Japan and some EU countries for our best-selling product Gleevec/Glivec (cancer). In the US, we have resolved
patent litigation with certain generic manufacturers. We have licensed one generic manufacturer to
market a generic version of Gleevec in the US as of February 1, 2016. In the EU, our Glivec
intellectual property rights also are being challenged by generic manufacturers.
- •
- Diovan and Co-Diovan/Diovan HCT (high
blood pressure), which had long been our best-selling product, has generic competitors for Diovan in the US, EU and Japan and for Co-Diovan/Diovan HCT in
the US and EU. In Japan, Novartis has resolved patent litigation with a generic manufacturer. Patent protection for Co-Diovan will expire in Japan in 2016. In addition, valsartan, the active ingredient
in Diovan, is also
used in the single-pill combination therapies Exforge/Exforge HCT (high blood pressure), and despite the existence of separate patents covering the
product, Exforge faces generic competition in the US. Our Exforge patents also face challenges in the
EU.
- •
- Patent protection for octreotide acetate, the active ingredient in Sandostatin, has
expired. Generic versions of Sandostatin SC are available in the US, EU and Japan. A US patent protects Sandostatin
LAR, the long-acting version of Sandostatin which represents a majority of our Sandostatin US sales. This patent is expected to
expire in 2017. Patents protecting the Sandostatin LAR
formulation in the EU and Japan have expired. There is currently no generic competition for Sandostatin LAR in the US, EU or Japan.
- •
- Patent protection on rivastigmine, the active ingredient in Exelon, has expired and Exelon capsules are subject to generic competition in major markets, including the US, Japan and all of Europe. We hold additional patents with respect
to Exelon Patch, which makes up a substantial portion of our Exelon sales, but generic versions of Exelon
Patch are on the market in the US and most EU countries.
- •
- Certain patents and extensions protecting our top-selling products, Afinitor and Gilenya will begin to expire in 2018 and 2019, and some of the patents protecting these products are being challenged in the US.
For more information on the patent status of our Pharmaceuticals Division's products see "Item 4. Information on the Company—Item 4.B Business Overview—Pharmaceuticals—Intellectual Property" and "Item 18. Financial Statements—Note 20".
9
In 2016, we expect an impact on our net sales of approximately $3.2 billion as a result of the loss of intellectual property protection for our products, including Gleevec/Glivec. Because we typically have substantially reduced marketing and research and development expenses related to products that are in their final year of exclusivity, we expect that this loss of intellectual property protection also will have an impact on our 2016 operating income in an amount corresponding to a significant portion of the products' lost sales. The magnitude of the impact of generic competition could depend on a number of factors, including the time of year at which such exclusivity would be lost; the ease or difficulty of manufacturing a competitor product and obtaining regulatory approval to market it; the number of generic competitor products approved, including whether, in the US, a single competitor is granted an exclusive marketing period, and whether an authorized generic is launched; and the geographies in which generic competitor products are approved, including the strength of the market for generic pharmaceutical products in such geographies and the comparative profitability of branded pharmaceutical products in such geographies.
Clearly, with respect to major products for which the patent terms are expiring, the loss of exclusivity of these products can be expected to have a material adverse effect on our business, financial condition and results of operations. In addition, should we unexpectedly lose exclusivity on additional products as a result of patent litigation or other reasons, this could also have a material adverse effect on our business, financial condition and results of operations, both due to the loss of revenue and earnings, and the difficulties in planning for such losses.
Similarly, all of our businesses are faced with intense competition from new products and technological advances from competitors, including new competitors from other industries such as Google and IBM that are entering the healthcare field. Physicians, patients and third-party payors may choose our competitors' products instead of ours if they perceive them to be safer, more effective, easier to administer, less expensive, more convenient, or more cost-effective.
Products that compete with ours, including products competing against some of our best-selling products, are launched from time to time. We cannot predict with accuracy the timing of the introduction of such competitive products or their possible effect on our sales. However, products significantly competitive to our major products Lucentis, Gilenya and Afinitor have been launched. Such products, and other competitive products, could significantly affect the revenues from our products and our results of operations.
Similarly, our Alcon Division, a leader in the eye care industry, has recently suffered declining growth rates due in part to increased competition for its products, across all of its business franchises. To counter this, we are taking steps to accelerate growth to improve the division's sales and profits. Our efforts under this plan are expected to take time to succeed. As a result, such competition and other factors can be expected to affect Alcon's business, financial condition or results of operations in the near term. In addition, despite the implementation of the growth acceleration plan, our efforts to improve Alcon's performance may prove insufficient. Should our growth acceleration efforts fail to accomplish its goals, or fail to do so in a timely manner, it could have a material adverse impact on our business, financial condition or results of operations beyond the near term, as well. See also the discussion of Alcon's new product development efforts in "—Our research and development efforts may not succeed in bringing new products to market, or may fail to do so cost efficiently enough, or in a manner sufficient to grow our business, replace lost revenues and income and take advantage of new technologies" below.
Our research and development efforts may not succeed in bringing new products to market, or may fail to do so in a cost-efficient manner, or in a manner sufficient to grow our business, replace lost revenues and income and take advantage of new technologies.
Our ability to continue to maintain and grow our business, to replace sales lost due to competition, entry of generics or other reasons, and to bring to market products and medical advances that take advantage of new, and potentially disruptive technologies depends in significant part upon the success of our research and development activities in identifying, and successfully and cost-effectively developing
10
new products that address unmet medical needs, are accepted by patients and physicians, and are reimbursed by payors. To accomplish this, we commit substantial effort, funds and other resources across our divisions to research and development, both through our own dedicated resources and through collaborations with third parties. Developing new healthcare products and bringing them to market, however, is a highly costly, lengthy and uncertain process. In spite of our significant investments, there can be no guarantee that our research and development activities will produce commercially viable new products that will enable us to grow our business and replace revenues and income lost to generic and other competition.
Using the products of our Pharmaceuticals Division as an example, the research and development process for a new product can take up to 15 years, or even longer, from discovery to commercial product launch—and with limited available intellectual property protections, the longer it takes to develop a product, the less time there will be for us to recoup our research and development costs. New products must undergo intensive preclinical and clinical testing, and must be approved by means of highly complex, lengthy and expensive approval processes which can vary from country to country. During each stage, there is a substantial risk that we will encounter serious obstacles that will further delay us and add substantial expense, that we will develop a product with limited potential for commercial success, or that we will be forced to abandon a product in which we have invested substantial amounts of time and money. These risks may include failure of the product candidate in preclinical studies, difficulty enrolling patients in clinical trials, clinical trial holds or other delays in completing clinical trials, delays in completing formulation and other testing and work necessary to support an application for regulatory approval, adverse reactions to the product candidate or other safety concerns, insufficient clinical trial data to support the safety or efficacy of the product candidate, an inability to manufacture sufficient quantities of the product candidate for development or commercialization activities in a timely and cost-effective manner, and failure to obtain, or delays in obtaining, the required regulatory approvals for the product candidate or the facilities in which it is manufactured.
In addition, following a series of widely publicized issues, health regulators have increased their focus on product safety. Governmental authorities and payors around the world have also paid increased attention to whether new products offer a significant benefit over other products in the same therapeutic class. These developments have led to requests for more clinical trial data, for the inclusion of a significantly higher number of patients in clinical trials, and for more detailed analyses of the trials. As a result, the already lengthy and expensive process of obtaining regulatory approvals and reimbursement for pharmaceutical products has become even more challenging.
For the same reason, the post-approval regulatory burden has also increased. Approved drugs are subject to various requirements such as risk evaluation and mitigation strategies (REMS), risk management plans, comparative effectiveness studies, health technology assessments and requirements to conduct post-approval Phase IV clinical trials to gather additional safety and other data on products. These requirements have the effect of making the maintenance of regulatory approvals and of achieving reimbursement for our products increasingly expensive, and further heightening the risk of recalls, product withdrawals, loss of revenues or loss of market share.
Our Alcon Division faces similar challenges in developing new products and bringing them to market. Alcon's Ophthalmic Pharmaceuticals products must be developed and approved in accordance with essentially the same processes as our Pharmaceuticals Division. Alcon's Surgical and Vision Care products face medical device development and approval processes that are often similarly difficult. Alcon is taking steps to accelerate its growth, and this can be expected to be costly and to require extensive efforts over time. There can be no certainty that Alcon will be successful in these efforts, in either the short- or the long-term, and if Alcon is not successful, there could be a material adverse effect on the success of the Alcon Division, and on the Group as a whole. See also the discussion of Alcon in "—Our products face important patent expirations and significant competition" above.
11
In addition, our Sandoz Division has made, and expects to continue to make, significant investments in the development of differentiated, "difficult-to-make" generic products, including biotechnology-based, "biologic" medicines intended for sale as bioequivalent or "biosimilar" generic versions of currently-marketed biotechnology products. While the development of such products typically is significantly less costly and complex than the development of the equivalent originator medicines, it is nonetheless significantly more costly and complex than for non-differentiated generic products. In addition, despite significant efforts by us and others, to date many countries do not yet have a fully-developed legislative or regulatory pathway which would facilitate the development of biosimilars and permit biosimilars to be sold in a manner in which the biosimilar product would be readily substitutable for the originator product. Further delays in the development and completion of such regulatory pathways, or any significant impediments that may ultimately be built into such pathways, or any other significant difficulties that may arise in the development or marketing of biosimilars or other differentiated products, could put at risk the significant investments that Sandoz has made, and will continue to make, in the development of differentiated products in general, and in its biopharmaceuticals business in particular, and could have a material adverse effect on the long-term success of the Sandoz Division and the Group as a whole.
Further, in all of our divisions, our research and development activities must be conducted in an ethical and compliant manner. Among other things, we must be concerned with patient safety, Good Clinical Practices requirements, data integrity requirements, the fair treatment of patients in developing countries, and animal welfare requirements. Should we fail to properly manage such issues, we risk injury to third parties, damage to our reputation, negative financial consequences as a result of potential claims for damages, sanctions and fines, and the potential that our investments in research and development activities could have no benefit to the Group.
If we are unable to cost-effectively maintain an adequate flow of successful new products and new indications for existing products sufficient to maintain and grow our business, cover our substantial research and development costs and the decline in sales of older products that become subject to generic or other competition, and take advantage of technological and medical advances, then this could have a material adverse effect on our business, financial condition or results of operations. For a description of the approval processes which must be followed to market our products, see the sections headed "Regulation" included in the descriptions of our operating divisions under "Item 4. Information on the Company—Item 4.B Business Overview."
Our business is affected by pressures on pricing for our products.
The growth of overall healthcare costs as a percentage of gross domestic product in many countries means that governments and payors are under intense pressure to control healthcare spending even more tightly. These pressures are particularly strong given the persistently weak economic and financial environment in many countries and the increasing demand for healthcare resulting from the aging of the global population and the prevalence of behaviors that increase the risk of obesity and other chronic diseases. In addition, in certain countries, governments, patients, healthcare providers and the media are increasingly raising questions about healthcare pricing issues. As a result, our businesses and the healthcare industry in general are operating in an ever more challenging environment with very significant pricing pressures. These ongoing pressures affect all of our divisions, and involve a number of cost-containment measures, such as government-imposed industry-wide price reductions, mandatory pricing systems, reference pricing systems, payors limiting access to treatments based on cost-benefit analyses, an increase in imports of drugs from lower-cost countries to higher-cost countries, shifting of the payment burden to patients through higher co-payments, limiting physicians' ability to choose among competing medicines, mandatory substitution of generic drugs for the patented equivalent, and growing pressure on physicians to reduce the prescribing of patented prescription medicines. For more information on such price controls see "Item 4. Information on the Company—Item 4.B Business Overview—Pharmaceuticals—Price Controls."
12
As a result of such measures, we faced downward pricing pressures on our patented and generic drugs in countries around the world in 2015. These pressures ranged from efforts by many governments and proposals by politicians to reduce the amounts we would be paid for our medicines, intense publicity regarding the pricing of pharmaceuticals, including publicity and pressure resulting from prices charged by competitors and peer companies for new products as well as price increases by competitors and peer companies on older products that the public deemed excessive, and government investigations into pharmaceutical pricing practices.
We expect these challenges to continue and possibly increase in 2016 as political pressures mount, and healthcare payors around the globe, including government-controlled health authorities, insurance companies and managed care organizations, step up initiatives to reduce the overall cost of healthcare, restrict access to higher-priced new medicines, increase the use of generics and impose overall price cuts. Such pressures could have a material adverse impact on our business, financial condition or results of operations, as well as on our reputation.
Failure to comply with law, legal proceedings and government investigations may have a significant negative effect on our results of operations.
We are obligated to comply with the laws of all of the countries around the world in which we operate and sell products with respect to an extremely wide and growing range of activities. Such legal requirements can vary from country to country and new requirements may be imposed on us from time to time as government and public expectations regarding acceptable corporate behavior change. For example, there are new laws in the US and in other countries around the world that require us to be more transparent with respect to our interactions with healthcare professionals. To help us in our efforts to comply with the many requirements that impact us, we have a significant global ethics and compliance program in place, and we devote substantial time and resources to efforts to ensure that our business is conducted in a lawful and publicly acceptable manner. Nonetheless, despite our efforts, any actual or alleged failure to comply with law or with heightened public expectations could lead to substantial liabilities that may not be covered by insurance, or to other significant losses, and could affect our business and reputation.
In particular, in recent years, there has been a trend of increasing civil and criminal government investigations, litigations and law enforcement activities against companies operating in our industry, both in the US and in an increasing number of countries around the world. A number of our subsidiaries across each of our divisions are, or may in the future be subject to various investigations and legal proceedings that arise or may arise from time to time, such as proceedings regarding sales and marketing practices, pricing, corruption, trade regulation and embargo legislation, product liability, commercial disputes, employment and wrongful discharge, antitrust, securities, insider trading, health and safety, environmental, tax, cybersecurity and data privacy, and intellectual property matters, and are increasingly challenging practices previously considered to be legal.
Such proceedings are inherently unpredictable, and large judgments sometimes occur. As a consequence, we may in the future incur judgments or enter into settlements of claims that could involve large cash payments, including the potential repayment of amounts allegedly obtained improperly, and other penalties, including treble damages. In addition, such proceedings may affect our reputation, create a risk of potential exclusion from government reimbursement programs in the US and other countries, may lead to civil litigation and otherwise subject us to monetary penalties. Further, judgments and settlements sometimes require companies to enter into corporate integrity or similar agreements, which are intended to regulate company behavior for a period of years. Any such resolutions could have a material adverse impact on our business, financial condition or results of operations, as well as on our reputation.
13
Our businesses are and have been subject to a number of these types of cases and governmental investigations, including the following:
- •
- In 2014, the Tokyo District Public Prosecutor Office indicted our Japanese affiliate, Novartis Pharma K.K. (NPKK), as well as a former
NPKK employee on certain charges relating to the alleged manipulation of data in certain clinical trials. The charges against NPKK are subject to a maximum total fine of JPY 4 million. Trial in
this matter commenced in December 2015. In addition, in February 2015, the Japanese Ministry of Health, Labor and Welfare (MHLW) issued a business suspension order for failure to report adverse
events, which required NPKK to halt manufacturing and sales in Japan for the period from March 5 to 19, 2015. NPKK is implementing a corrective and preventive action plan in response to a
business improvement order and instruction issued by the MHLW in the fourth quarter of 2015 regarding additional instances of delayed adverse events reporting.
- •
- In 2013 and 2014, the US government and certain states filed civil charges against our US affiliate, Novartis Pharmaceuticals Corporation (NPC) in federal court in the Southern District of New York, asserting federal False Claims Act and state law claims related to alleged unlawful contractual discounts and rebates to specialty pharmacies in connection with certain of our products. The US government alleged substantial damages, including treble damages and civil penalties of up to $11,000 per claim, which according to the government could exceed $2 billion. In the second half of 2015, NPC reached a settlement with all plaintiffs, including the United States Department of Justice, 45 states (made up of the eleven intervening states, as well as all the other states which were either part of the relator's complaint, or which reimbursed prescriptions of Myfortic and Exjade during the relevant time period), the District of Columbia and the qui tam relator. This resolves all the above-described claims related to Myfortic, Exjade, Tasigna, Gleevec and TOBI. As part of the settlement, NPC agreed to pay $390 million plus additional legal expenses to plaintiffs, and agreed with the Office of Inspector General of the US Department of Health & Human Services on an amendment and extension of its current Corporate Integrity Agreement until 2020.
A number of significant legal matters remain pending against us. For more detail see "Item 18. Financial Statements—Note 20." See also "—Our reliance on outsourcing and third parties for the performance of key business functions heightens the risks faced by our businesses" below.
In addition, our Sandoz Division may from time to time seek approval to market a generic version of a product before the expiration of patents claimed by the marketer of the patented product. We do this in cases where we believe that the relevant patents are invalid, unenforceable, or would not be infringed by our generic product. As a result, affiliates of our Sandoz Division frequently face patent litigation, and in certain circumstances, we may elect to market a generic product even though patent infringement actions are still pending. Should we elect to proceed in this manner and conduct a "launch at risk," we could face substantial damages if the final court decision is adverse to us.
Adverse judgments or settlements in any of the significant investigations or cases against us could have a material adverse effect on our business, financial condition, results of operations and reputation.
The manufacture of our products is highly regulated and complex, and may result in a variety of issues that could lead to extended supply disruptions and significant liability.
The manufacture of our products is heavily regulated by governmental health authorities around the world, including the FDA. Whether our products are manufactured at our own dedicated manufacturing facilities or by third parties, we must ensure that all manufacturing processes comply with current Good Manufacturing Practices (cGMP) and other applicable regulations, as well as with our own high quality standards. In recent years, health authorities have substantially intensified their scrutiny of manufacturers' compliance with such requirements. If we or our third-party suppliers fail to comply fully with these
14
requirements and the health authorities' expectations, then we could be required to shut down our production facilities or production lines, or could be prevented from importing our products from one country to another. This could lead to product shortages, or to our being entirely unable to supply products to patients for an extended period of time. And such shortages or shut downs have led to and could continue to lead to significant losses of sales revenue and to potential third-party litigation. In addition, health authorities have in some cases imposed significant penalties for such failures to comply with cGMP. A failure to comply fully with cGMP could also lead to a delay in the approval of new products to be manufactured at the impacted site.
Like many of our competitors, we have faced significant manufacturing issues in recent years. As a result of such issues, we were unable to supply certain products to the market for significant periods of time, and suffered significant losses in sales and market share. In October 2015, the FDA issued a warning letter to our Sandoz Division concerning their sites in Kalwe and Turbhe, India, relating to documentation practices in Kalwe and sterile manufacturing practices in Turbhe that were identified during an inspection in August 2014. Though we have taken steps to respond to the warning letter, there can be no guarantee that FDA's concerns will be met.
In order to meet increasing health authority expectations and our own high quality standards, we are devoting substantial time and resources to remediate issues, improve quality and assure consistency of product supply at our manufacturing sites around the world. Ultimately, there can be no guarantee of the outcome of these efforts. Nor can there be any guarantee that we will not again face significant manufacturing issues, or that we will successfully manage such issues when they arise.
In addition to regulatory requirements, many of our products involve technically complex manufacturing processes or require a supply of highly specialized raw materials. For some products and raw materials, we may rely on a single source of supply. In particular, a significant portion of our portfolio are "biologic" products. Unlike traditional "small-molecule" drugs, biologic drugs or other biologic-based products cannot be manufactured synthetically, but typically must be produced from living plant or animal micro-organisms. As a result, the production of biologic-based products which meet all regulatory requirements is especially complex. Even slight deviations at any point in the production process may lead to production failures or recalls. In addition, because the production process is based on living plant or animal micro-organisms, the process could be affected by contaminants that could impact those micro-organisms. As a result, the inherent fragility of certain of our raw material supplies and production processes may cause the production of one or more of our products to be disrupted, potentially for extended periods of time.
Also as part of the Group's portfolio of products, we have a number of sterile products, including oncology products, which are technically complex to manufacture, and require sophisticated environmental controls. Because the production process for such products is so complex and sensitive, the chance of production failures and lengthy supply interruptions is increased.
Finally, in addition to potential liability for government penalties, because our products are intended to promote the health of patients, for some of our products, any supply disruption or other production issue could endanger our reputation and subject us to lawsuits or to allegations that the public health, or the health of individuals, has been harmed.
In sum, a disruption in the supply of certain key products—whether as a result of a failure to comply with applicable regulations or health authority expectations, the fragility of the production process, inability to obtain product or raw materials from a sole source of supply, natural or man-made disasters at one of our facilities or at a critical supplier or vendor, or our failure to accurately predict demand—could have a material adverse effect on our business, financial condition or results of operations, as well as our reputation. See also "—Earthquakes and other natural disasters could adversely affect our business," below.
15
The persistently weak global economic and financial environment in many countries and increasing political and social instability may have a material adverse effect on our results.
Many of the world's largest economies and financial institutions continue to be impacted by a weak ongoing global economic and financial environment, with some continuing to face financial difficulty, liquidity problems and limited availability of credit. In addition, we continue to see weak economic growth or a slowing of economic growth rates in certain emerging growth markets, such as China, Russia, Brazil and India. It is uncertain how long these effects will last, or whether economic and financial trends will worsen or improve. In addition, these issues may be further impacted by the unsettled political conditions currently existing in the US and Europe, as well as the difficult conditions existing in parts of the Middle East and places such as Ukraine, as well as the ongoing refugee crisis, anti-immigrant activities, social unrest and fears of terrorism that have followed in many countries. Such uncertain times may have a material adverse effect on our revenues, results of operations, financial condition and, if circumstances worsen, our ability to raise capital at reasonable rates. For example, financial weakness in certain countries has increased pressures on those countries, and on payors in those countries, to force healthcare companies to decrease the prices at which we may sell them our products. See also "Item 4. Information on the Company—Item 4.B Business Overview—Pharmaceuticals—Price Controls."
Concerns continue that payors in some countries, including Greece, Italy, Portugal and Spain, may not be able to pay us in a timely manner. Certain other countries are experiencing high inflation rates and have taken steps to introduce exchange controls and limit companies from distributing retained earnings or paying intercompany payables due from those countries. The most significant country in this respect is Venezuela, where we are exposed to a potential devaluation loss in the income statement on our total intercompany balances with our subsidiaries there, which at December 31, 2015 amounted to $0.3 billion. In November 2015, one of our Venezuelan subsidiaries agreed with Venezuelan authorities to settle a substantial part of our existing intercompany trade payables dated on or before December 31, 2014 in a transaction that, in turn, required us to use a significantly devalued US dollar/Venezuela bolivar exchange rate for consolidation of the financial statements of our Venezuela subsidiaries. The use of the new exchange rate resulted in a $211 million loss from the re-measurement of the intra-Group and third party liabilities. Ongoing conditions in Venezuela and other such countries could continue to lead to further devaluations of their currencies, which could in turn result in significant additional financial losses to the Group in the future. See also "Item 5. Operating and Financial Review and Prospects—Item 5.B Liquidity and Capital Resources—Effects of Currency Fluctuations" and "—Condensed Consolidated Balance Sheets," and "Item 18. Financial Statements—Notes 15 and 29."
Current economic conditions may adversely affect the ability of our distributors, customers, suppliers and service providers to obtain the liquidity required to pay for our products, or otherwise to buy necessary inventory or raw materials, and to perform their obligations under agreements with us, which could disrupt our operations, and could negatively impact our business and cash flow. Although we make efforts to monitor these third parties' financial condition and their liquidity, our ability to do so is limited, and some of them may become unable to pay their bills in a timely manner, or may even become insolvent, which could negatively impact our business and results of operations. These risks may be elevated with respect to our interactions with third parties with substantial operations in countries where current economic conditions are the most severe, particularly where such third parties are themselves exposed to payment risks from business interactions directly with fiscally-challenged government payers. See also "—Our reliance on outsourcing and third parties for the performance of key business functions heightens the risks faced by our businesses" below.
In addition, the varying effects of difficult economic times on the economies, currencies and financial markets of different countries has impacted, and may continue to unpredictably impact, our business and results of operations including the conversion of our operating results into our reporting currency, the US dollar, as well as the value of our investments in our pension plans. See "—Foreign exchange fluctuations may adversely affect our earnings and the value of some of our assets," below, and "—If any of numerous
16
key assumptions and estimates in calculating our pension plan obligations turn out to be different from our actual experience, we may be required to increase substantially our contributions to pension plans as well as our pension-related costs in the future," below. In addition, the financial situation may also result in a lower return on our financial investments, and a lower value on some of our assets. Alternately, inflation could accelerate, which could lead to higher interest rates, which would increase our costs of raising capital.
To the extent that the economic and financial conditions directly affect consumers, some of our businesses, including the elective surgical business of our Alcon Division, may be particularly sensitive to declines in consumer spending. In addition, our Pharmaceuticals and Sandoz Divisions, and the remaining businesses of our Alcon Division, may not be immune to consumer cutbacks, particularly given the increasing requirements in certain countries that patients pay a larger contribution toward their own healthcare costs. As a result, there is a risk that consumers may cut back on prescription drugs and medical devices to help cope with rising costs and difficult economic times.
At the same time, significant changes and volatility in the financial markets, in the consumer and business environment, in the competitive landscape and in the global political and security landscape make it increasingly difficult for us to predict our revenues and earnings into the future. As a result, any revenue or earnings guidance or outlook which we have given or might give may be overtaken by events, or may otherwise turn out to be inaccurate. Though we endeavor to give reasonable estimates of future revenues and earnings at the time we give such guidance, based on then-current knowledge and conditions, there is a significant risk that such guidance or outlook will turn out to be, or to have been, incorrect.
Similarly, increased scrutiny of corporate taxes and executive pay may lead to significant business disruptions or other adverse business conditions, and may interfere with our ability to attract and retain qualified personnel. See "—Changes in tax laws or their application could adversely affect our results of operation" and "—An inability to attract and retain qualified personnel could adversely affect our business" below.
Foreign exchange fluctuations may adversely affect our earnings and the value of some of our assets.
Changes in exchange rates between the US dollar, our reporting currency, and other currencies can result in significant increases or decreases in our reported sales, costs and earnings as expressed in US dollars, and in the reported value of our assets, liabilities and cash flows.
In 2015, the US dollar continued its significant increase in value against most currencies. In particular, the average value of the euro, the Japanese yen and emerging market currencies (especially the ruble) decreased in 2015 against the US dollar. However, in January 2015, following an announcement by the Swiss National Bank that it was discontinuing its minimum exchange rate with the euro, the value of the Swiss franc increased substantially. In addition, in 2015, China took steps to devalue its currency, and the value of its currency against the US dollar has continued to decline.
There is a risk that other countries could also take steps that could significantly impact the value of their currencies. Such steps could include "quantitative easing" measures and potential withdrawals by countries from common currencies. In addition, certain countries are or may experience periods of high inflation. This could lead these countries to devalue their currencies, and to set exchange controls, as, for example, Venezuela has done. Such steps taken by Venezuela have impacted our financial results. See "—The persistently weak global economic and financial environment in many countries and increasing political and social instability may have a material adverse effect on our results" above. Ongoing conditions in Venezuela and other such countries could continue to lead to further devaluations of their currencies, which could in turn result in significant additional financial losses to the Group in the future.
Despite measures undertaken to reduce, or hedge against, foreign currency exchange risks, because a significant portion of our earnings and expenditures are in currencies other than the US dollar, including
17
expenditures in Swiss francs that are significantly higher than our revenues in Swiss francs, such exchange rate volatility may negatively and materially impact the Group's business, results of operations and financial condition, and may impact the reported value of our net sales, earnings, assets and liabilities. In addition, the timing and extent of such volatility can be difficult to predict. Further, depending on the movements of particular foreign exchange rates, the Group may be materially adversely affected at a time when the same currency movements are benefiting some of our competitors.
For more information on the effects of currency fluctuations on our consolidated financial statements and on how we manage currency risk, see "Item 5. Operating and Financial Review and Prospects—Item 5.B Liquidity and Capital Resources—Effects of Currency Fluctuations" "Item 11. Quantitative and Qualitative Disclosures about Market Risk", and "Item 18. Financial Statements—Note 29."
We may not successfully achieve our goals in strategic transactions or reorganizations, including the portfolio transformation transactions, the strategic reorganizations we announced in January 2016, and the formation of Novartis Business Services.
As part of our strategy, from time to time we evaluate and pursue potential strategic business acquisitions and divestitures to expand or complement our existing businesses, or to enable us to focus more sharply on our strategic businesses. We cannot ensure that suitable acquisition candidates will be identified. Acquisition activities can be thwarted by overtures from competitors for the targeted assets, potentially increasing prices demanded by sellers, governmental regulation (including market concentration limitations) and replacement product developments in our industry. Once an acquisition is agreed upon with a third party, we may not be able to complete the acquisition in the expected form or within the expected time frame, or at all, due to a failure to obtain required regulatory approvals or a failure to achieve contractual or other required closing conditions. Further, after an acquisition, efforts to integrate the business may not meet expectations, or may otherwise not be successful, as a result of corporate cultural differences, difficulties in retention of key personnel, customers and suppliers, coordination with other products and processes, or other reasons. Also, acquisitions and divestments could divert management's attention from our existing businesses, and could result in the existing businesses failing to achieve expected results, or in liabilities being incurred that were not known at the time of acquisition, or the creation of tax or accounting issues.
Similarly, we cannot ensure that suitable buyers will be identified for businesses or other assets that we might want to divest. Neither can we ensure that we will correctly select businesses or assets as candidates for divestiture, that we will be able to successfully complete any agreed upon divestments, or that any expected strategic benefits, synergies or opportunities will arise as a result of any divestiture.
In 2015, we completed a series of transactions intended to transform our portfolio of businesses. In these transactions, we acquired GSK oncology products and certain related assets; created a joint venture with GSK in consumer healthcare of which Novartis owns 36.5%; divested our vaccines business (excluding the influenza vaccines business) to GSK; divested our Animal Health business to Lilly; and divested our influenza vaccines business to CSL. In 2014, we had also divested the blood transfusion diagnostics unit to Grifols S.A. that had been part of our former Vaccines and Diagnostics Division. In agreeing to these transactions, we expect to achieve certain strategic benefits, synergies and opportunities, including certain financial results, but there can be no certainty that such expected benefits will be fully realized or that they will be realized at any particular time.
In addition, as part of our strategy, from time to time we reassess the optimal organization of our business, including the allocation of products by division and the level of centralization and simplification of certain functions across the Group, to better align those products and functions with the capabilities and expertise required for competitive advantage. As an example of this, in January 2016 we announced a series of strategic actions intended to further focus our divisions, including focusing our Alcon Division on its Surgical and Vision Care franchises, strengthening our ophthalmic medicines business by transferring Alcon's Ophthalmic Pharmaceuticals products to our Pharmaceuticals Division, and shifting selected
18
mature pharmaceutical products from our Pharmaceuticals Division into Sandoz. We also announced steps to increase Group-wide coordination of drug development, and to improve efficiency with an integrated manufacturing operation and more shared commercial and medical services at the country level. We expect these actions to further strengthen our competitive position, enable us to maintain our leading position in research and development, and free resources for our growth priorities. But the expected benefits of this reorganization may never be fully realized or may take longer to realize than expected. There can be no certainty that the numerous businesses and functions involved will be successfully integrated into the new organizations and that key personnel will be retained. Disruption from the reorganizations may make it more difficult to maintain relationships with customers, employees or suppliers, and may result in the Group not achieving the expected productivity and financial benefits, including potential sales declines and lost profits.
Similarly, in 2014 we created a shared services organization, Novartis Business Services (NBS). NBS consolidates a number of business support services previously spread across divisions, including Information Technology, Financial Reporting and Accounting Operations, Real Estate & Facility Services, Procurement, Payroll and Personnel Administration and the Pharmaceuticals Global Business Services. This reorganization was designed to improve profitability and free up resources that could be reinvested in growth and innovation, and to allow our divisions to focus more on customer-facing activities. But the expected benefits of this reorganization may never be fully realized or may take longer to realize than expected. There can be no certainty that the numerous business functions involved will be successfully integrated into a single organization and that key personnel will be retained. Disruption from the reorganization may make it more difficult to maintain relationships with customers, employees or suppliers, and may result in the Group not achieving the expected productivity and financial benefits.
Both with respect to the transactions and reorganizations previously announced, and to potential future transactions and reorganizations, if we fail to timely recognize or address these risks, or to devote adequate resources to them, we may fail to achieve our strategic objectives, including our growth strategy, or otherwise may not realize the intended benefits of the acquisition, divestiture or reorganization.
Intangible assets and goodwill on our books may lead to significant impairment charges in the future.
We carry a significant amount of goodwill and other intangible assets on our consolidated balance sheet, primarily due to acquisitions. As a result, significant impairment charges may result in the future if the expected fair value of the goodwill and other intangible assets would be less than their carrying value on the Group's consolidated balance sheet at any point in time.
We regularly review our long-lived intangible and tangible assets, including identifiable intangible assets, investments in associated companies and goodwill, for impairment. Goodwill, acquired research and development, and acquired development projects not yet ready for use are subject to impairment review at least annually. Other long-lived assets are reviewed for impairment when there is an indication that an impairment may have occurred. Impairment testing under IFRS may lead to impairment charges in the future. Any significant impairment charges could have a material adverse effect on our results of operations and financial condition. In 2015, for example, we recorded intangible asset impairment charges of $347 million. For a detailed discussion of how we determine whether an impairment has occurred, what factors could result in an impairment and the increasing impact of impairment charges on our results of operations, see "Item 5. Operating and Financial Review and Prospects—Item 5.A Operating Results—Critical Accounting Policies and Estimates—Impairment of Goodwill, Intangible Assets and Property, Plant and Equipment" and "Item 18. Financial Statements—Notes 1 and 11."
Our indebtedness could adversely affect our operations.
As of December 31, 2015 we had $16.3 billion of non-current financial debt and $5.6 billion of current financial debt. Our current and long-term debt requires us to dedicate a portion of our cash flow to service interest and principal payments and, if interest rates rise, this amount may increase. In addition, our
19
existing debt may limit our ability to engage in transactions or otherwise may place us at a competitive disadvantage relative to competitors that have less debt. We may also have difficulty refinancing our existing debt or incurring new debt on terms that we would consider to be commercially reasonable, if at all.
Our reliance on outsourcing and third parties for the performance of key business functions heightens the risks faced by our businesses.
We invest a significant amount of effort and resources into outsourcing and offshoring certain key business functions with third parties, including research and development collaborations, manufacturing operations, warehousing, distribution activities, certain finance functions, marketing activities, data management and others. Our reliance on outsourcing and third parties for certain functions, such as the research and development or manufacturing of products, may limit the potential profitability of such products. In addition, despite contractual relationships with the third parties to whom we outsource these functions, we cannot ultimately control how they perform their contracts. Nonetheless, we depend on these third parties to achieve results which may be significant to us. If the third parties fail to meet their obligations or to comply with the law, we may lose our investment in the collaborations and fail to receive the expected benefits. In addition, should any of these third parties fail to comply with the law in the course of their performance of services for us, there is a risk that we could be held responsible for such violations of law, as well and that our reputation may suffer. Any such failures by third parties could have a material adverse effect on our business, financial condition, results of operations or reputation.
In particular, in many countries, including many developing markets, we rely heavily on third party distributors and other agents for the marketing and distribution of our products. Many of these third parties do not have internal compliance resources comparable to those within our organization. Some of these countries are plagued by corruption. If our efforts to screen our third party agents and detect cases of potential misconduct fail, we could be held responsible for the noncompliance of these third parties with applicable laws and regulations, which may have a material adverse effect on our reputation and on our business, financial condition or results of operations.
We may not be able to realize the expected benefits of our significant investments in Emerging Growth Markets.
At a time of slowing growth in sales of healthcare products in industrialized countries, many emerging markets have in recent years experienced proportionately higher sales growth and an increasing contribution to the industry's global performance. In 2015, our Continuing Operations generated $12.4 billion, or approximately 25% (2014: 26%) of our net sales from Emerging Growth Markets—which comprise all markets other than the Established Markets of the US, Canada, Western Europe, Japan, Australia and New Zealand—as compared with $37.0 billion, or approximately 75% (2014: 74%) of our net sales, in the Established Markets. However, combined net sales in the Emerging Growth Markets grew 7% in constant currencies in 2015, compared to 4% sales growth in constant currencies in the Established Markets during the same period. As a result of this trend, we continue to take steps to increase our activities in the Emerging Growth Markets, and have been making significant investments in our businesses in those countries.
In the past year, however, certain of these Emerging Growth Market countries, including Brazil, India, China and Russia, have experienced economic slowdowns. As a result, there can be no guarantee that our efforts to expand our sales in these countries will succeed, or that these countries will once again experience growth rates significantly in excess of the world's largest markets. In particular, some Emerging Growth Market countries may be especially vulnerable to the effects of the persistently weak global financial environment, may have very limited resources to spend on healthcare or may be susceptible to political and social instability. See "—The persistently weak global economic and financial environment in many countries and increasing political and social instability may have a material adverse effect on our results" above. Many of these countries are subject to increasing political and social pressures, including
20
from a growing middle class seeking increased access to healthcare. Such pressures on local government may in turn result in an increased focus by the governments on our pricing, and may put at risk our intellectual property. See "—Our business is increasingly affected by pressures on pricing for our products," and "Our products face important patent expirations and significant competition" above.
These countries also may have a relatively limited number of persons with the skills and training suitable for employment at an enterprise such as ours. See "—An inability to attract and retain qualified personnel could adversely affect our business" below. In some Emerging Growth Market countries, a culture of compliance with law may not be as fully developed as in the Established Markets—China's investigations of the activities of multinational healthcare companies, for example, have been well publicized—standards of acceptable behavior may be lower than such standards in Established Markets, or we may be required to rely on third-party agents, in each case putting us at risk of liability and reputational damage. See "—Failure to comply with law, and resulting investigations and legal proceedings may have a significant negative effect on our results of operations," and "—Our reliance on outsourcing and third parties for the performance of key business functions heightens the risks faced by our businesses," above.
In addition, many of these countries have currencies that may fluctuate substantially. If these currencies devalue significantly against the US dollar—as happened in China and Russia, among others, in the past year—and we cannot offset the devaluations with price increases, then our products may become less profitable, or may otherwise impact our reported financial results. Currency devaluation risk may also exist in countries with high inflation economies. Should these countries take steps that cause their currencies to be devalued, we may realize a significant financial loss. See "—The persistently weak global economic and financial environment in many countries and increasing political and social instability may have a material adverse effect on our results" and "—Foreign exchange fluctuations may adversely affect our earnings and the value of some of our assets," above. Ongoing conditions in such high inflation countries could continue to lead to further devaluations of their currencies, which could in turn result in significant additional financial losses to the Group in the future.
For all these reasons, our sales to Emerging Growth Markets carry significant risks. A failure to continue to expand our business in Emerging Growth Markets could have a material adverse effect on our business, financial condition or results of operations.
Failure to obtain marketing exclusivity periods for new generic products, or to develop biosimilars and other differentiated products, as well as intense competition from patented and generic pharmaceuticals companies, may have an adverse effect on the success of our Sandoz Division.
Our Sandoz Division achieves significant revenue opportunities when it secures and maintains exclusivity periods granted for generic products in certain markets—particularly the 180-day exclusivity period granted in the US by the Hatch-Waxman Act for first-to-file generics—and when it is able to develop biosimilars and other differentiated products with few, if any, generic competitors. Failure to obtain and maintain these market opportunities could have an adverse effect on the success of Sandoz.
In addition, the division faces intense competition from companies that market patented pharmaceutical products, which sometimes take aggressive steps to prevent or delay the introduction of generic medicines, to limit the availability of exclusivity periods or to reduce their value, and from other generic pharmaceuticals companies, which aggressively compete for exclusivity periods and for market share of generic products that may be identical to certain of our generic products. These activities may increase the costs and risks associated with our efforts to introduce generic products and may delay or entirely prevent their introduction.
Sandoz has also invested heavily in the development of biosimilar drugs, despite the fact that regulations concerning their marketing and sale in certain countries, including in the US, are still under development or not entirely clear. If, despite ongoing efforts by us and others to encourage the
21
development of such regulations, such regulations do not ultimately favor the development and sale of biosimilar products, then we may fail to achieve expected returns on the investments by Sandoz in the development of biosimilars. See also "—Our research and development efforts may not succeed in bringing new products to market, or may fail to do so cost-efficiently enough, or in a manner sufficient to grow our business and replace lost revenues and income" above, with regard to the risks involved in our efforts to develop differentiated generic products.
If any of numerous key assumptions and estimates in calculating our pension plan obligations turn out to be different from our actual experience, we may be required to increase substantially our contributions to pension plans as well as the amount we pay toward pension-related expenses in the future.
We sponsor pension and other post-employment benefit plans in various forms. These plans cover a significant portion of our current and former associates. While most of our plans are now defined contribution plans, certain of our associates remain under defined benefits plans. For these defined benefits plans, we are required to make significant assumptions and estimates about future events in calculating the present value of expected future expenses and liabilities related to these plans. These include assumptions used to determine the discount rates we apply to estimated future liabilities and rates of future compensation increases. In addition, our actuarial consultants provide our management with historical statistical information such as withdrawal and mortality rates in connection with these estimates. Assumptions and estimates used by Novartis may differ materially from the actual results we experience due to changing market and economic conditions (including the effects of the persistently weak global financial environment, which, to date, have resulted in extremely low or negative interest rates in many countries), higher or lower withdrawal rates, or longer or shorter life spans of participants, among other variables. For example, a decrease in the interest rate we apply in determining the present value of expected future defined benefit obligations of one-quarter of one percent would have increased our year-end defined benefit pension obligation for plans in Switzerland, US, UK, Germany and Japan, which represent about 95% of the Group total defined benefit pension obligation, by $0.8 billion. Any differences between our assumptions and estimates and our actual experience could have a material effect on our results of operations and financial condition. Further, additional employer contributions might be required if plan funding falls below the levels required by local rules. For more information on obligations under retirement and other post-employment benefit plans and underlying actuarial assumptions, see "Item 5. Operating and Financial Review and Prospects—Item 5.A Operating Results—Critical Accounting Policies and Estimates—Retirement and other post-employment benefit plans" and "Item 18. Financial Statements—Note 25". See also "—The persistently weak global economic and financial environment in many countries and increasing political and social instability may have a material adverse effect on our results" above.
Changes in tax laws or their application could adversely affect our results of operations.
The integrated nature of our worldwide operations enables us to achieve an attractive effective tax rate on our earnings because a portion of our earnings are earned in jurisdictions that tax profits at more favorable rates. In recent years, tax authorities around the world have increased their scrutiny of company tax structures, and have become more rigid in exercising any discretion they may have. As a result, companies' flexibility to optimally structure their organizations for business and tax purposes may be significantly reduced. In addition, the public is increasingly taking an interest in what the tax burden of multinational companies should be. Any changes in tax laws or in the laws' application that may result from this, including with respect to tax base or rate, transfer pricing, intercompany dividends and cross-border transactions, controlled corporations, and limitations on tax relief allowed on the interest on intercompany debt, could increase our effective tax rate and adversely affect our financial results.
22
Counterfeit versions of our products could harm our patients and reputation.
Our industry continues to be challenged by the vulnerability of distribution channels to illegal counterfeiting and the presence of counterfeit products in a growing number of markets and over the Internet. Counterfeit products are frequently unsafe or ineffective, and can potentially be life-threatening. To distributors and patients, counterfeit products may be visually indistinguishable from the authentic version. Reports of adverse reactions to counterfeit drugs or increased levels of counterfeiting could materially affect patient confidence in the authentic product, and harm the business of companies such as ours or lead to litigation. In addition, it is possible that adverse events caused by unsafe counterfeit products could mistakenly be attributed to the authentic product. If a product of ours was the subject of counterfeits, we could incur substantial reputational and financial harm.
Ongoing consolidation among our distributors and retailers is increasing both the purchasing leverage of key customers and the concentration of credit risk.
Increasingly, a significant portion of our global sales are made to a relatively small number of drug wholesalers, retail chains and other purchasing organizations. For example, our three most important customers globally are all in the US, and accounted for approximately 14%, 11% and 5%, respectively, of Group net sales in 2015. The largest trade receivables outstanding were for these three customers, amounting to 13%, 9% and 6%, respectively, of the Group's trade receivables at December 31, 2015. The trend has been toward further consolidation among distributors and retailers, both in the US and internationally. As a result, our customers are gaining additional purchasing leverage, which increases the pricing pressures facing our businesses. Moreover, we are exposed to a concentration of credit risk as a result of this concentration among our customers. If one or more of our major customers experienced financial difficulties, the effect on us would be substantially greater than in the past. This could have a material adverse effect on our business, financial condition and results of operations.
An inability to attract and retain qualified personnel could adversely affect our business.
We highly depend upon skilled personnel in key parts of our organization, and we invest heavily in recruiting, training and retaining qualified individuals. The loss of the service of key members of our organization—including senior members of our scientific and management teams, high-quality researchers and development specialists, and skilled personnel in emerging markets—could delay or prevent the achievement of major business objectives.
Future economic growth will demand talented associates and leaders, yet the market for talent has become increasingly competitive. In particular, emerging markets are expected to be a driving force in global growth, but in countries like Russia and China there is a limited pool of executives with the training and international experience needed to work successfully in a global organization like Novartis.
In addition, shifting demographic trends are expected to result in fewer students, fewer graduates and fewer people entering the workforce in the Western world in the next 10 years. Moreover, many members of younger generations around the world have changing expectations toward careers, engagement and the integration of work in their overall lifestyles.
The supply of talent for certain key functional and leadership positions is decreasing, and a talent gap is visible for some professions and geographies—engineers in Germany, for example. Recruitment is increasingly regional or global in specialized fields such as clinical development, biosciences, chemistry and information technology. In addition, the geographic mobility of talent is expected to decrease in the future, with talented individuals in developed and emerging countries anticipating ample career opportunities closer to home than in the past. This decrease in mobility may be worsened by anti-immigrant sentiments in many countries, and laws discouraging immigration.
In addition, our ability to hire qualified personnel also depends on the flexibility to reward superior performance and to pay competitive compensation. Laws and regulations on executive compensation,
23
including legislation in our home country, Switzerland, may restrict our ability to attract, motivate and retain the required level of qualified personnel.
We face intense competition for an increasingly limited pool of qualified individuals from numerous pharmaceutical and biotechnology companies, universities, governmental entities, other research institutions, other companies seeking to enter the healthcare space, and companies in other industries. As a result, despite significant efforts on our part, we may be unable to attract and retain qualified individuals in sufficient numbers, which could have an adverse effect on our business, financial condition and results of operations.
Significant breaches of data security or disruptions of information technology systems, including by cyber-attack or other security breach, and breaches of the privacy rights of third parties could adversely affect our business.
Our business is heavily dependent on critical, complex and interdependent information technology systems, including Internet-based systems, to support business processes. In addition, Novartis and our employees rely on internet and social media tools and mobile technologies as a means of communications, and to gather information. We are also increasingly seeking to develop technology-based products such as mobile applications that go "beyond the pill" to improve patient welfare in a variety of ways, which could result in us gathering information about patients and others electronically.
The size and complexity of our information technology systems, and, in some instances, their age, make them potentially vulnerable to external or internal security breaches, breakdowns, malicious intrusions malware, misplaced or lost data, programming or human errors, or other similar events. Although we have devoted and continue to devote significant resources and management attention to the protection of our data and information technology, like many companies, we have experienced such events and expect to continue to experience them in the future. We believe that the data security breaches we have experienced to date have not resulted in significant disruptions to our operations, and will not have a significant adverse effect on our current or future results of operations. However, we may not be able to prevent breakdowns or breaches in our systems that could have a material adverse effect on our business, financial condition, results of operation or reputation.
Any such events could negatively impact important business processes, such as the conduct of scientific research and clinical trials, the submission of the results of such efforts to health authorities in support of requests for product approvals, the functioning of our manufacturing and supply chain processes, our compliance with legal obligations and other key business activities. Such potential information technology issues could lead to the loss of important information such as trade secrets or other intellectual property and could accelerate development or manufacturing of competing products by third parties. In addition, malfunctions in software or devices that make significant use of information technology, including our Alcon surgical equipment, could lead to a risk of harm to patients.
Our use of information technologies, including internet, social media, mobile technologies, and technology-based medical devices, as well as other routine business operations, sometimes involve our gathering personal information (including sensitive personal information) regarding our patients, vendors, customers, employees, collaborators and others. Breaches of our systems or other failures to protect such information could expose the personal information of third parties to unauthorized persons. Any such information or other privacy breaches could give rise to significant potential liability and reputational harm. In addition, we make substantial efforts to ensure that any international transfers of personal data are done in compliance with applicable law. Any restrictions that may be placed on our ability to transfer such data could have a material adverse effect on our business, financial condition, results of operations and reputation.
In addition, to the extent that we seek as a company to use internet, social media and mobile tools as a means to communicate with the public about our products or about the diseases our products are intended to treat, there continue to be significant uncertainties as to the rules that apply to such
24
communications, and as to the interpretations that health authorities will apply in this context to the rules that do exist. As a result, despite our efforts to comply with applicable rules, there is a significant risk that our use of social media and mobile technologies for such purposes may cause us to nonetheless be found in violation of them.
Any such breaches of data security or information technology disruptions or privacy violations could give rise to the loss of trade secrets or other intellectual property, to the public exposure of personal information, and to interruptions to our operations, and could result in liability or enforcement actions, which could require us to expend significant resources to continue to modify or enhance our protective measures and to remediate any damage. Such events could have a material adverse effect on our business, financial condition, results of operations and reputation.
Environmental liabilities may adversely impact our results of operations.
The environmental laws of various jurisdictions impose actual and potential obligations on us to remediate contaminated sites, in some cases over many years. While we have set aside substantial provisions for worldwide environmental liabilities, there is no guarantee that additional costs will not be incurred beyond the amounts for which we have provided in the Group consolidated financial statements. If environmental contamination caused by us adversely impact third parties, if we fail to properly manage the safety of our facilities and the environmental risks, or if we are required to further increase our provisions for environmental liabilities in the future, this could have a material adverse effect on our business, financial condition, results of operations, and on our reputation. See also "Item 4.D Property, Plants and Equipment—Environmental Matters" and "Item 18. Financial Statements—Note 20."
Extreme weather events, earthquakes and other natural disasters could adversely affect our business.
In recent years, extreme weather events and changing weather patterns such as storms, flooding, drought, and temperature changes, appear to have become more common. We operate in countries around the world. As a result, we are potentially exposed to varying natural disaster or extreme weather risks like hurricanes, tornadoes or floods, or other events that may result from the impact of climate change on the environment. As a result of such events, we could experience business interruptions, destruction of facilities and loss of life, all of which could have a material adverse effect on our business, financial condition and results of operations.
In addition, our corporate headquarters, the headquarters of our Pharmaceuticals Division, and certain of our major Pharmaceuticals Division production and research facilities are located near earthquake fault lines in Basel, Switzerland. Other major facilities are located near major earthquake fault lines in various locations around the world. In the event of a major earthquake, we could experience business interruptions, destruction of facilities and loss of life, all of which could have a material adverse effect on our business, financial condition and results of operations. See also "—The manufacture of our products is highly regulated and complex, and may result in a variety of issues that could lead to extended supply disruptions and significant liability," above.
The price of our ADRs and the US dollar value of any dividends may be negatively affected by fluctuations in the US dollar/Swiss franc exchange rate.
Our American Depositary Shares (ADSs) each representing one Novartis share and evidenced by American Depositary Receipts (ADRs) trade on the NYSE in US dollars. Since the shares underlying the ADRs are listed in Switzerland on the SIX Swiss Exchange (SIX) and trade in Swiss francs, the value of the ADRs may be affected by fluctuations in the US dollar/Swiss franc exchange rate. In addition, since dividends that we may declare will be denominated in Swiss francs, exchange rate fluctuations will affect the US dollar equivalent of dividends received by holders of ADRs. If the value of the Swiss franc
25
decreases against the US dollar, the price at which our ADRs trade may—and the value of the US dollar equivalent of any dividend will—decrease accordingly.
Holders of ADRs may not be able to exercise preemptive rights attached to shares underlying ADRs.
Under Swiss law, shareholders have preemptive rights to subscribe for issuances of new shares on a pro rata basis. Shareholders may waive their preemptive rights in respect of any offering at a general meeting of shareholders. Preemptive rights, if not previously waived, are transferable during the subscription period relating to a particular offering of shares and may be quoted on the SIX. US holders of ADRs may not be able to exercise the preemptive rights attached to the shares underlying their ADRs unless a registration statement under the US Securities Act of 1933 is effective with respect to such rights and the related shares, or an exemption from this registration requirement is available. In deciding whether to file such a registration statement, we would evaluate the related costs and potential liabilities, as well as the benefits of enabling the exercise by ADR holders of the preemptive rights associated with the shares underlying their ADRs. We cannot guarantee that a registration statement would be filed, or, if filed, that it would be declared effective. If preemptive rights could not be exercised by an ADR holder, JPMorgan Chase Bank, N.A., as depositary, would, if possible, sell the holder's preemptive rights and distribute the net proceeds of the sale to the holder. If the depositary determines, in its discretion, that the rights could not be sold, the depositary might allow such rights to lapse. In either case, the interest of ADR holders in Novartis would be diluted and, if the depositary allowed rights to lapse, holders of ADRs would not realize any value from the preemptive rights.
26
Item 4. Information on the Company
4.A History and Development of Novartis
Novartis AG was incorporated on February 29, 1996 under the laws of Switzerland as a stock corporation (Aktiengesellschaft) with an indefinite duration. On December 20, 1996, our predecessor companies, Ciba-Geigy AG and Sandoz AG, merged into this new entity, creating Novartis. We are domiciled in and governed by the laws of Switzerland. Our registered office is located at the following address:
Novartis
AG
Lichtstrasse 35
CH-4056 Basel, Switzerland
Telephone: 011-41-61-324-1111
Web: www.novartis.com
Novartis is a multinational group of companies specializing in the research, development, manufacturing and marketing of a broad range of healthcare products led by innovative pharmaceuticals. Novartis AG, our Swiss holding company, owns, directly or indirectly, all of our significant operating companies. For a list of our significant operating subsidiaries, see "Item 18. Financial Statements—Note 32."
Important Corporate Developments 2013-January 2016
| |
|
|
|---|---|---|
| 2016 | ||
January |
Novartis announces leadership changes effective February 1, 2016. Mike Ball has been appointed Division Head and CEO Alcon, succeeding Jeff George; Dr. Vas Narasimhan has been appointed Global Head Drug Development and Chief Medical Officer; and André Wyss has been appointed President, Novartis Operations. |
|
Novartis announces that it is taking a number of steps to further build on its strategy, including focusing the Alcon Division on its Surgical and Vision Care franchises, with specific actions identified to accelerate growth, and strengthening the ophthalmic medicines business by transferring Alcon's Ophthalmic Pharmaceuticals products to the Pharmaceuticals Division; centralizing manufacturing operations across divisions within a single technical operations unit; increasing Group-wide coordination of drug development by establishing a single Global Head of Drug Development and centralizing certain common functions such as the Chief Medical Office; and shifting selected mature, non-promoted pharmaceutical products from the Pharmaceuticals Division into the Sandoz Division. |
||
Novartis announces a collaboration and licensing agreement with Surface Oncology, which gives Novartis access to four pre-clinical programs in immuno-oncology. |
||
2015 |
||
November |
Novartis completes a $3 billion bond offering under its US SEC Registration Statement on Form F-3. |
|
October |
Novartis announces the acquisition of Admune Therapeutics to broaden its portfolio of cancer immunotherapies. |
27
| September | Novartis announces the appointment of Dr. James E. Bradner as President of the Novartis Institutes for BioMedical Research and a member of the Executive Committee of Novartis, to be effective March 1, 2016, concurrent with the retirement of Dr. Mark C. Fishman, who will reach his contractual retirement age in March 2016. | |
Novartis announces the launch of Novartis Access, a portfolio of affordable medicines to treat chronic diseases in lower-income countries offered to governments, non-governmental organizations and other public-sector healthcare providers for $1 per treatment, per month. |
||
Novartis announces that it has entered into a global collaboration with Amgen to commercialize and develop neuroscience treatments. |
||
August |
Novartis announces an agreement to acquire all remaining rights to GSK's ofatumumab to develop treatments for multiple sclerosis and other autoimmune indications. This transaction was completed on December 21, 2015. |
|
July |
Novartis announces a swap of three mid-stage clinical assets for equity and a share of milestones and royalties on future commercial sales with Mereo BioPharma Group Limited. |
|
June |
Novartis announces that it has entered into an agreement to acquire Spinifex Pharmaceuticals, Inc., a US and Australian-based, privately held development stage company focused on developing a peripheral approach to treat neuropathic pain such as EMA401, a novel angiotensin II Type 2 receptor (AT2R) antagonist. This acquisition was completed on July 24, 2015. |
|
March |
Novartis announces entry into an alliance with Aduro Biotech focused on discovery and development of next-generation cancer immunotherapies targeting the STING signaling pathway, and the launch of a new immuno-oncology research group. |
|
February |
Novartis completes a CHF 1.375 billion bond offering listed on the SIX Swiss Exchange. |
|
2014 |
||
October |
Novartis announces a definitive agreement with CSL of Australia to divest its influenza vaccines business for $275 million. This divestment was completed effective July 31, 2015. |
|
Novartis announces changes to the Novartis Executive Committee. Three members of the Executive Committee of Novartis, George Gunn, Brian MacNamara and Andrin Oswald, would leave the Company following the completion of the relevant portfolio transactions announced in April 2014. |
||
Novartis announces that it has entered into a collaboration with Bristol-Myers Squibb Company to evaluate three molecularly targeted compounds in combination with Bristol-Myers Squibb's investigational PD-1 immune checkpoint inhibitor, Opdivo® (nivolumab), in Phase I/II trials of patients with non-small cell lung cancer. |
||
August |
Novartis appoints a Chief Ethics, Compliance and Policy Officer reporting directly to the CEO. |
|
July |
Novartis announces that its Alcon Division has entered into an agreement with a division of Google Inc., to in-license its "smart lens" technology for all ocular medical uses. |
|
June |
Novartis announces that the FDA licensed its manufacturing facility in Holly Springs, North Carolina for the commercial production of cell-culture influenza vaccines, with the capacity to significantly increase production in the event of an influenza pandemic. |
28
| May | Novartis enters into a licensing and commercialization agreement with Ophthotech Corporation for the exclusive rights to market Fovista (pegpleranib; OAP030, anti-PDGF aptamer) outside the US. In November 2015, Genentech entered into an agreement with Novartis to participate in certain rights related to the Novartis licensing and commercialization agreement with Ophthotech Corporation for OAP030. | |
April |
Novartis announces a set of definitive inter-conditional agreements with GSK. Under these agreements, Novartis would acquire GSK oncology products and certain related assets, would be granted a right of first negotiation over the co-development or commercialization of GSK's current and future oncology R&D pipeline (excluding oncology vaccines) and would divest the Vaccines Division (excluding its influenza vaccines business) to GSK. The two companies would also create a joint venture in consumer healthcare, of which Novartis would own 36.5%. These transactions were completed on March 2, 2015. |
|
Novartis also announces a definitive agreement with Lilly to divest the Company's Animal Health Division. This divestment was completed on January 1, 2015. |
||
Novartis announces the creation of a shared services organization, Novartis Business Services (NBS). NBS consolidates a number of business support services previously spread across divisions, including Information Technology, Financial Reporting and Accounting Operations, Real Estate & Facility Services, Procurement, Payroll and Personnel Administration and the Pharmaceuticals Global Business Services. This reorganization was designed to improve profitability and free up resources that could be reinvested in growth and innovation, and to allow our divisions to focus more on customer-facing activities. NBS became effective on July 1, 2014. |
||
February |
Novartis announces the acquisition of CoStim Pharmaceuticals Inc., a Cambridge, Massachusetts-based, privately held biotechnology company focused on cancer immunotherapy. The acquisition brings to Novartis late discovery stage immunotherapy programs directed to several targets, including PD-1. |
|
Novartis appoints a Global Head, Corporate Responsibility reporting directly to the CEO. |
||
January |
Novartis implements several changes to its governance structure. These include elimination of the Chairman's Committee of the Novartis AG Board of Directors; transfer of operational responsibilities that previously rested with the Chairman or the Chairman's Committee, such as approval authority for management compensation, to the CEO or the Executive Committee; and establishment of the Research and Development Committee of the Novartis AG Board of Directors to oversee Novartis research and development strategy and advise the Board on scientific trends and activities. |
|
2013 |
||
November |
Novartis announces a $5.0 billion share buyback. The buyback begins on the date of the announcement and will be executed over two years on the second trading line. |
|
Novartis announces a definitive agreement to divest its blood transfusion diagnostics unit to Grifols S.A. of Spain, for $1.7 billion. This transaction was completed on January 9, 2014. |
||
Novartis announces that it will co-locate certain scientific resources in order to improve the efficiency and effectiveness of its global research organization. Changes include establishing a respiratory research group in Cambridge, Massachusetts, a proposal to close the Horsham, UK, research site, a plan to exit from the Vienna, Austria research site, consolidation of the US-based component of oncology research from Emeryville, California to Cambridge, Massachusetts, closure of the biotherapeutics development unit in La Jolla, California, and a plan to exit research in topical applications for dermatology. |
29
| September | Novartis announces that it has entered into an exclusive global licensing and research collaboration agreement with Regenerex LLC, a biopharmaceutical company based in Louisville, Kentucky, for use of the company's novel Facilitating Cell Therapy (FCRx) platform. | |
August |
Joerg Reinhardt, Ph.D., assumes role of Chairman of the Board of Directors of Novartis AG on August 1. |
|
July |
The Novartis Board of Directors announces a final agreement with its former Chairman, Dr. Daniel Vasella. From the date of the Annual General Meeting held on February 22, 2013, until October 31, 2013, Dr. Vasella was to provide certain transitional services, including select Board mandates with subsidiaries of Novartis and support of the ad-interim Chairman and the new Chairman. For his transitional services during such period, Dr. Vasella would receive cash of CHF 2.7 million, and 31,724 unrestricted shares as of October 31, 2013 (the market value of the shares as of the date of the announcement was approximately CHF 2.2 million). In addition, from November 1, 2013, to December 31, 2016, Dr. Vasella will receive a minimum of $250,000 per annum in exchange for making himself available to Novartis, at Novartis' request and discretion, to provide specific consulting services, such as the coaching of high-potential associates of Novartis and speeches at key Novartis events at a daily fee rate of $25,000, which will be offset against the $250,000 minimum annual payment. During November and December 2013, Dr. Vasella did not provide any coaching to associates and did not receive any compensation for this period. |
|
Novartis announces that it has entered into a development and licensing agreement with Biological E Limited (BioE), a biopharmaceutical company based in India, for two vaccines to protect against typhoid and paratyphoid fevers. The agreement advances the Novartis goal to deliver accessible and affordable vaccines that address unmet medical need in endemic regions. |
||
April |
Novartis and Malaria No More, a leading global charity determined to end malaria deaths, announce that they are joining forces on the Power of One campaign to help close the treatment gap and accelerate progress in the fight against malaria. Over the next three years, Novartis will support the campaign financially and also donate up to three million full courses of its pediatric antimalarial drug to match the treatments donated by the public, doubling the impact of these donations. |
|
February |
Novartis announces that the Novartis AG Board of Directors and Dr. Vasella agreed to cancel his non-competition agreement and all related conditional compensation. The agreement was to take effect after Dr. Vasella stepped down as Chairman of the Board at the Novartis Annual General Meeting on February 22, 2013. |
|
January |
Novartis announces that, at his own wish, Novartis AG Chairman of the Board of Directors Dr. Daniel Vasella will not stand for re-election as a member of the Board of Directors at the Annual General Meeting to be held on February 22, 2013. The Board of Directors proposed the election of, among others, Joerg Reinhardt, Ph.D., as a member of the Board for a term of office beginning on August 1, 2013, and ending on the day of the Annual General Meeting in 2016. The Board announced its intention to elect Joerg Reinhardt as Chairman of the Board of Directors as from August 1, 2013. The Board of Directors further announced its intention to elect its current Vice-Chairman, Ulrich Lehner, Ph.D., as Chairman of the Board of Directors for the period from February 22, 2013, until the new Chairman took office. |
For information on our principal expenditures on property, plants and equipment, see "Item 4. Information on the Company—4.D Property, Plants and Equipment." For information on our significant expenditures in research and development, see the sections headed "Research and Development" included in the descriptions of our Pharmaceuticals Division and Alcon Division, and the section headed "Development and Registration" included in the description of our Sandoz Division under "Item 4.
30
Information on the Company—4.B Business Overview." For information on other principal capital expenditures and divestitures, see "Item 5. Operating and Financial Review and Prospects—Item 5.A Operating Results—Factors Affecting Comparability of the Year-On-Year Results of Operations." For more information on the transactions with GSK, Lilly or CSL, see "Item 4.B Business Overview—Overview" and "Item 10.C Material Contracts."
Novartis provides healthcare solutions that address the evolving needs of patients and societies worldwide. Our broad portfolio includes innovative medicines, eye care products and cost-saving generic pharmaceuticals.
Following the completion of a series of transactions in 2014 and 2015, the Group's portfolio is organized into three global operating divisions. In addition, we separately report the results of Corporate activities. The disclosure in this Item focuses on these continuing operations, which are made up of Pharmaceuticals, Alcon, Sandoz and Corporate activities. In addition, from March 2, 2015, the date of the completion of a series of transactions with GSK, continuing operations also includes the results from the oncology assets acquired from GSK and the 36.5% interest in the GSK consumer healthcare joint venture (the latter reported as an investment in associated companies). We sold our Vaccines Division, excluding our influenza business, to GSK. Our influenza vaccines business was sold to CSL and our Animal Health Division was sold to Lilly. For more detail on these transactions see, "Item 10.C Material Contracts."
Continuing Operations:
- •
- Pharmaceuticals: Innovative patent-protected prescription medicines
- •
- Alcon: Surgical, ophthalmic pharmaceutical and vision care products
- •
- Sandoz: Generic pharmaceuticals and biosimilars
- •
- Corporate activities
Discontinued Operations:
- •
- Vaccines and Diagnostics: Preventive human vaccines and blood-testing diagnostics
- •
- Consumer Health: OTC (over-the-counter medicines) and Animal Health
- •
- Corporate: certain transactional and other expenses related to the portfolio transformation
Novartis has leading positions globally in each of the three areas of our continuing operations. To maintain our competitive positioning across these growing segments of the healthcare industry, we place a strong focus on innovating to meet the evolving needs of patients around the world, growing our presence in new and emerging markets, and enhancing our productivity to invest for the future and increase returns to shareholders.
We separately report the financial results of our Corporate activities as part of our continuing operations. Income and expenses from Corporate activities include the costs of the Group headquarters and those of corporate coordination functions in major countries. In addition, Corporate includes other items of income and expense which are not attributable to specific segments such as certain expenses related to post-employment benefits, environmental remediation liabilities, charitable activities, donations and sponsorships.
31
Our continuing operations are supported by the Novartis Institutes for BioMedical Research and Novartis Business Services.
- •
- The Novartis Institutes for BioMedical Research (NIBR) is the innovation engine of Novartis, and is headquartered in Cambridge,
Massachusetts. More than 6,000 scientists and associates at NIBR conduct research into various disease areas at sites located in the US, Switzerland, Singapore and China. For more information about
NIBR, see "—Pharmaceuticals—Research and Development—Research program," below.
- •
- Novartis Business Services (NBS), our shared services organization, consolidates support services across Novartis divisions, helping to drive efficiency, standardization and simplification. NBS includes six service domains: human resources services, real estate and facility management, procurement, information technology, product lifecycle services and financial reporting and accounting operations. NBS has approximately 9,500 associates. Moving from division-specific services to a cross-divisional model, NBS continues to scale up the offshoring of transactional services to its five selected Global Service Centers in Mexico City, Mexico; Kuala Lumpur, Malaysia; Prague, Czech Republic; Hyderabad, India; and Dublin, Ireland.
Our continuing operations achieved net sales of $49.4 billion in 2015, while net income from continuing operations amounted to $7.0 billion. Research & Development expenditure in 2015 amounted to $8.9 billion ($8.7 billion excluding impairment and amortization charges). Of total net sales from continuing operations, $12.4 billion, or 25%, came from Emerging Growth Markets, and $37.0 billion, or 75%, came from Established Markets. Emerging Growth Markets comprise all markets other than the Established Markets of the US, Canada, Western Europe, Japan, Australia and New Zealand.
Headquartered in Basel, Switzerland, our Group companies employed 118,700 full-time equivalent associates as of December 31, 2015. Our products are available in approximately 180 countries around the world.
In September 2015, Novartis announced the launch of Novartis Access, a portfolio of 15 medicines to treat chronic diseases in low- and middle-income countries. The portfolio addresses cardiovascular diseases, diabetes, respiratory illnesses, and breast cancer and will be offered to governments, non-governmental organizations (NGOs) and other public-sector healthcare providers for $1 per treatment, per month.
In 2016, having completed our portfolio transformation and operationalized NBS, we are taking further steps to build on our strategy. We are focusing our Alcon Division on its Surgical and Vision Care franchises. Within these franchises, we have identified key actions to accelerate growth in 2016 and beyond. These include optimizing intraocular lens (IOL) innovation and commercial execution; prioritizing and investing in promising pipeline opportunities; ensuring best-in-class service, training and education for eye care professionals; improving sales force effectiveness; and investing in direct to consumer activities for key brands.
We are strengthening our ophthalmic medicines business by transferring Alcon's Ophthalmic Pharmaceuticals products to our Pharmaceuticals Division. This is expected to simplify our ophthalmic medicines business, leverage Alcon's strong brand with Pharmaceuticals Division development and marketing capabilities, and help us accelerate innovation and growth in eye care.
At the same time, we are shifting selected mature, non-promoted pharmaceutical products from our Pharmaceuticals Division into Sandoz, which has proven experience in managing mature products successfully.
To increase innovation even further, we are increasing Group-wide coordination of drug development. We are establishing a single Global Head of Drug Development to improve resource allocation and standards across our divisions. We are also centralizing certain common functions, such as the Chief Medical Office, which will cover safety and pharmacovigilance policy for the Group.
32
To further improve efficiency, we are centralizing our manufacturing operations across our divisions within a single technical operations unit. The new unit is expected to optimize capacity planning and lower costs through simplification, standardization and external spend optimization. Centralization is also expected to improve our ability to develop next-generation technologies, implement continuous manufacturing and share best practices across divisions.
We expect these changes to generate over $1.0 billion in annual cost savings from 2020, with the ramp-up starting in 2016. Associated with these changes we expect one-time restructuring costs of approximately $1.4 billion spread over five years. We plan to use the net savings to fund innovation and improve our profit margins.
In addition, we announced leadership changes effective February 1, 2016. Mike Ball has been appointed Division Head and CEO Alcon, and will be a member of the Executive Committee of Novartis (ECN). Mr. Ball joins Novartis from Hospira, where he was CEO from 2011 until recently. Mr. Ball succeeds Jeff George, who has decided to leave Novartis. Dr. Vas Narasimhan has been appointed Global Head Drug Development and Chief Medical Officer, a new position in the ECN. André Wyss, already a member of the ECN, Head NBS and Country President for Switzerland, has been appointed President, Novartis Operations. In his new role, he will assume responsibility for the integrated Technical Operations organization as well as for Global Public & Government Affairs, in addition to his current responsibilities.
Except as described above and as briefly described in "—Alcon" below, and "Item 5. Operating and Financial Review and Prospects—Item 5.A Operating Results—Results of Operations—Alcon," this Form 20-F reflects the organization of the Group prior to the changes described above.
Continuing Operations:
Pharmaceuticals researches, develops, manufactures, distributes and sells patented prescription medicines and is organized in the following franchises: Oncology, Cardio-metabolic, Immunology and Dermatology, Retina, Respiratory, Neuroscience and Established Medicines. Our Pharmaceuticals Division also includes a franchise focused on the development and commercialization of Cell and Gene Therapies.
On March 2, 2015, we completed the acquisition of the oncology products of GSK, together with certain related assets. In addition, we acquired a right of first negotiation over the co-development or commercialization of GSK's current and future oncology R&D pipeline, excluding oncology vaccines. The right of first negotiation is for a period of twelve and one half years from the acquisition closing date.
In 2015, the Pharmaceuticals Division accounted for $30.4 billion, or 62%, of Group net sales, and for $7.6 billion, or 81%, of Group operating income (excluding Corporate income and expense, net).
Our Alcon Division researches, develops, manufactures, distributes and sells eye care products and technologies to serve the full life cycle of eye care needs. Alcon offers a broad range of products to treat many eye diseases and conditions, and is organized into three franchises: Surgical, Ophthalmic Pharmaceuticals and Vision Care. The Surgical portfolio includes technologies and devices for cataract, retinal, glaucoma and refractive surgery, as well as intraocular lenses to treat cataracts and refractive errors, like presbyopia and astigmatism. Alcon also provides viscoelastics, surgical solutions, surgical packs, and other disposable products for cataract and vitreoretinal surgery. In Ophthalmic Pharmaceuticals, the portfolio includes treatment options for elevated intraocular pressure caused by glaucoma, anti-infectives to aid in the treatment of bacterial infections and bacterial conjunctivitis, and ophthalmic solutions to treat inflammation and pain associated with ocular surgery, as well as an intravitreal injection for vitreomacular traction including macular hole. The Ophthalmic Pharmaceuticals
33
portfolio also includes eye and nasal allergy treatments, as well as over-the-counter dry eye relief and ocular vitamins. The Vision Care portfolio comprises daily disposable, monthly replacement, and color-enhancing contact lenses, as well as a complete line of contact lens care products including multi-purpose and hydrogen-peroxide based solutions, rewetting drops and daily protein removers.
In 2015, Alcon accounted for $9.8 billion, or 20%, of Group net sales, and for $0.8 billion, or 9%, of Group operating income (excluding Corporate income and expense, net).
Our Sandoz Division focuses primarily on developing, manufacturing, distributing and selling prescription medicines that are not protected by valid and enforceable third-party patents, and intermediary products including active pharmaceutical ingredients. Sandoz is organized globally in three franchises: Retail Generics, Anti-Infectives, and Biopharmaceuticals & Oncology Injectables. In Retail Generics, Sandoz develops, manufactures and markets active ingredients and finished dosage forms of pharmaceuticals to third parties. Retail Generics includes the areas of dermatology, respiratory and ophthalmics, as well as the areas of cardiovascular, metabolism, central nervous system, pain, gastrointestinal, and hormonal therapies. Finished dosage form anti-infectives sold to third parties are also part of Retail Generics. In Anti-Infectives, Sandoz supplies generic antibiotics to a broad range of customers, as well as active pharmaceutical ingredients and intermediates to the pharmaceutical industry worldwide. In Biopharmaceuticals, Sandoz develops, manufactures and markets protein or other biotechnology based products known as biosimilars and provides biotechnology manufacturing services to other companies, and in Oncology Injectables, Sandoz develops, manufactures and markets cytotoxic products for the hospital market.
In 2015, Sandoz accounted for $9.2 billion, or 18%, of Group net sales, and for $1.0 billion, or 11%, of Group operating income (excluding Corporate income and expense, net).
Discontinued Operations:
Vaccines and Diagnostics Division
Prior to the completion of certain transactions in 2014 and 2015, our Vaccines and Diagnostics Division researched, developed, manufactured, distributed and sold human vaccines and blood-testing products worldwide. On January 9, 2014, we completed the divestment of our blood transfusion diagnostics unit to Grifols S.A. On March 2, 2015, we completed the divestment of our Vaccines Division (excluding its influenza vaccines business) to GSK. On July 31, 2015, we completed the divestment of our influenza vaccines business to CSL Limited.
Prior to the completion of certain transactions in 2015, Consumer Health consisted of our OTC (Over-the-Counter) and Animal Health Divisions. On January 1, 2015 we completed the divestment of our Animal Health Division to Lilly. On March 2, 2015, we completed the divestment of our OTC Division, which we contributed to a new consumer healthcare joint venture with GSK, of which we own 36.5%.
Our Pharmaceuticals Division is a world leader in offering innovation-driven, patent-protected medicines to patients and physicians.
34
The Pharmaceuticals Division researches, develops, manufactures, distributes and sells patented pharmaceuticals in the following therapeutic areas:
- •
- Oncology
- •
- Cardio-Metabolic
- •
- Immunology and Dermatology
- •
- Retina
- •
- Respiratory
- •
- Neuroscience
- •
- Established Medicines
The Pharmaceuticals Division is organized into global business franchises responsible for the commercialization of various products. Our Pharmaceuticals Division also includes a franchise focused on the development and commercialization of Cell and Gene Therapies.
On March 2, 2015, we completed the acquisition of the oncology products of GSK, together with certain related assets. In addition, we acquired a right of first negotiation over the co-development or commercialization of GSK's current and future oncology R&D pipeline, excluding oncology vaccines. The right of first negotiation is for a period of twelve and one half years from the acquisition closing date.
The Pharmaceuticals Division is the largest contributor among the divisions of Novartis and reported consolidated net sales of $30.4 billion in 2015, which represented 62% of the Group's net sales.
The product portfolio of the Pharmaceuticals Division includes more than 60 key marketed products, many of which are leaders in their respective therapeutic areas. In addition, the division's portfolio of development projects includes 135 potential new products and new indications or new formulations for existing products in various stages of clinical development.
Pharmaceuticals Division Products
The following table and summaries describe certain key marketed products in our Pharmaceuticals Division. While we intend to sell our marketed products throughout the world, not all products and indications are currently available in every country. Compounds and new indications in development are subject to required regulatory approvals and, in certain instances, contractual limitations. These compounds and indications are in various stages of development throughout the world. It may not be possible to obtain regulatory approval for any or all of the new compounds and new indications referred to in this Form 20-F in any country or in every country. See "—Regulation" for further information on the approval process. Some of the products listed below have lost patent protection or are otherwise subject to generic competition. Others are subject to patent challenges by potential generic competitors. Please see "—Intellectual Property" for general information on intellectual property and regulatory data protection, and for further information on the status of patents and exclusivity for Pharmaceuticals Division products.
35
Selected Marketed Products
Business franchise |
Product | Common name | Indications (vary by country and/or formulation) |
Formulation | ||||
|---|---|---|---|---|---|---|---|---|
Oncology |
Afinitor/Votubia and Afinitor Disperz/Votubia dispersible tablets |
everolimus |
Advanced renal cell carcinoma after failure of treatment with VEGF-targeted therapy Advanced pancreatic neuroendocrine tumors SEGA associated with tuberous sclerosis Renal angiomyolipoma associated with tuberous sclerosis Advanced breast cancer in post-menopausal HR+/HER2– women in combination with exemestane, after failure of anastrozole or letrozole |
Tablet Dispersible tablets for oral suspension | ||||
|
Arzerra | ofatumumab |
In combination with chlorambucil for first- line chronic lymphocytic leukemia (CLL) In combination with chlorambucil or bendamustine for first-line CLL CLL refractory to fludarabine and alemtuzumab Extended treatment of patients who are in complete or partial response after at least two lines of therapy for recurrent or progressive CLL |
Intravenous infusion | ||||
|
Atriance/Arranon | nelarabine | Relapsed and/or refractory T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma | Solution for infusion | ||||
|
Exjade and Jadenu | deferasirox | Chronic iron overload due to blood transfusions and non-transfusion dependent thalassemia | Dispersible tablet for oral suspension Oral film-coated tablet |
||||
|
Farydak | panobinostat | Relapsed and/or refractory multiple myeloma, in combination with bortezomib and dexamethasone, after at least two prior regimens including bortezomib and an immunomodulatory agent | Capsules | ||||
|
Femara | letrozole |
Hormone receptor-positive early breast cancer in postmenopausal women following surgery (upfront adjuvant therapy) Early breast cancer in post-menopausal women following standard tamoxifen therapy (extended adjuvant therapy) Advanced breast cancer in post-menopausal women (both as first- and second-line therapies) |
Tablet | ||||
|
Gleevec/Glivec | imatinib mesylate/imatinib |
Certain forms of Ph+ chronic myeloid leukemia Certain forms of KIT+ gastrointestinal stromal tumors Certain forms of acute lymphoblastic leukemia Dermatofibrosarcoma protuberans Hypereosinophilic syndrome Aggressive systemic mastocytosis Myelodysplastic/myeloproliferative diseases |
Tablet Capsules |
||||
36
Business franchise |
Product | Common name | Indications (vary by country and/or formulation) |
Formulation | ||||
|---|---|---|---|---|---|---|---|---|
|
Hycamtin | topotecan |
Relapsed small cell lung cancer Metastatic carcinoma of the ovary after failure of initial or subsequent chemotherapy |
Capsule Powder for infusion |
||||
|
Combination therapy with cisplatin for Stage IV-B, recurrent, or persistent carcinoma of the cervix which is not amenable to curative treatment with surgery and/or radiation therapy |
|
||||||
|
Jakavi | ruxolitnib |
Disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post-polycythemia vera myelofibrosis or post-essential thrombocythemia myelofibrosis Polycythemia vera in adult patients who are resistant to or intolerant of hydroxyurea |
Tablet | ||||
|
Odomzo | sonidegib | Locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or is not a candidate for surgery or radiation therapy | Capsule | ||||
|
Proleukin | aldesleukin |
Metastatic renal cell carcinoma Metastatic melanoma |
Powder for injection or infusion | ||||
|
Promacta/Revolade | eltrombopag |
Thrombocytopenia in adult and pediatric patients one year and older with chronic immune (idiopathic) thrombocytopenia who have had insufficient response to corticosteroids, immunoglobulins, or splenectomy Thrombocytopenia in patients with chronic hepatitis C to allow initiation and maintenance of interferon-based therapy Severe aplastic anemia in patients who have had an insufficient response to immunosuppressive therapy |
Tablet Eltrombopag for oral suspension | ||||
|
Sandostatin LAR and Sandostatin SC | octreotide acetate |
Acromegaly Symptom control for certain forms of neuroendocrine tumors Delay of tumor progression in patients with midgut tumors |
Vial Ampoule/pre-filled syringe |
||||
|
Signifor and Signifor LAR | pasireotide |
Cushing's disease Acromegaly |
Solution for subcutaneous injection in ampoule Powder and solvent for suspension for IM injection |
||||
|
Tafinlar + Mekinist | dabrafenib + trametinib | BRAF V600+ metastatic melanoma | Capsule (Tafinlar) Tablet (Mekinist) |
||||
|
Tasigna | nilotinib |
Certain forms of chronic myeloid leukemia in patients resistant or intolerant to prior treatment including Gleevec/Glivec First-line chronic myeloid leukemia |
Capsule | ||||
37
Business franchise |
Product | Common name | Indications (vary by country and/or formulation) |
Formulation | ||||
|---|---|---|---|---|---|---|---|---|
|
Tykerb | lapatinib |
In combination with capacitabine for the treatment of patients with HER2+ advanced or metastatic breast cancer who have progressed on prior trastuzumab therapy In combination with trastuzumab for patients with HR-negative metastatic disease that has progressed on prior trastuzumab therapy(ies) plus chemotherapy In combination with paclitaxel for first line treatment of patients with HER2+ metastatic breast cancer for whom trastuzumab is not appropriate In combination with an aromatase inhibitor for the treatment of patients with hormone sensitive metastatic breast cancer |
Tablet | ||||
|
Votrient | pazopanib |
Advanced renal cell carcinoma Certain types of advanced soft tissue sarcoma after prior chemotherapy |
Tablet | ||||
|
Zofran | ondansetron | Use in children and adults for the prevention of chemotherapy induced nausea and vomiting and prevention of post-operative nausea and vomiting, and in adults for the prevention of radiation-induced nausea and vomiting | Tablet Oral solution Orally disintegrating tablets Solution for injection/infusion |
||||
|
Zometa | zoledronic acid |
Skeletal-related events from bone metastases (cancer that has spread to the bones) Hypercalcemia of malignancy |
Vial/4mg Ready-to-use | ||||
|
Zykadia | ceritinib | Anaplastic lymphoma kinase-positive metastatic non-small cell lung cancer | Capsules | ||||
Cardio-Metabolic |
Entresto | sacubitril/valsartan | Chronic heart failure with reduced ejection fraction | Tablet | ||||
|
Galvus and Eucreas | Galvus: vildagliptin Eucreas: vildagliptin and metformin | Type 2 diabetes | Tablet | ||||
Immunology and Dermatology |
Cosentyx | secukinumab |
Active ankylosing spondylitis in adults who have responded inadequately to conventional therapy Active psoriatic arthritis in adult patients when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate Moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy Moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy Psoriasis vulgaris and psoriatic arthritis in adults who are not adequately responding to systemic therapies (except for biologics) |
Lyophilized pre-filled syringe; Auto-injector | ||||
|
Ilaris | canakinumab |
Cryopyrin-associated periodic syndromes Systemic juvenile idiopathic arthritis Gouty arthritis |
Lyophilized powder for reconstitution for subcutaneous injection | ||||
|
Myfortic | mycophenolic acid (as mycophenolate sodium) | Prophylaxis of organ rejection in patients receiving allogeneic renal transplants | Gastro-resistant tablet | ||||
38
Business franchise |
Product | Common name | Indications (vary by country and/or formulation) |
Formulation | ||||
|---|---|---|---|---|---|---|---|---|
|
Neoral and Sandimmune | cyclosporine, USP Modified |
Prevention of rejection following certain organ transplantation Non-transplantation autoimmune conditions such as severe psoriasis and severe rheumatoid arthritis |
Capsule Oral solution Intravenous (Sandimmune) |
||||
|
Simulect | basiliximab | Prevention of acute organ rejection in de novo renal transplantation | Vial for injection or infusion | ||||
|
Xolair | omalizumab |
Chronic spontaneous urticaria/ Chronic idiopathic urticaria See also, "Respiratory" |
Lyophilized powder in vial and liquid formulation in pre-filled syringes | ||||
|
Zortress/Certican | everolimus | Prevention of organ rejection (heart, liver and kidney) | Tablet Dispersible tablet | ||||
Retina |
Lucentis | ranibizumab |
Neovascular age-related macular degeneration Visual impairment due to diabetic macular edema Visual impairment due to macular edema secondary to central retinal vein occlusion Visual impairment due to macular edema secondary to branch retinal vein occlusion Visual impairment due to choroidal neovascularization secondary to pathologic myopia |
Intravitreal injection | ||||
Respiratory |
Arcapta Neohaler/ Onbrez Breezhaler | indacaterol | Chronic obstructive pulmonary disease | Inhalation powder hard capsules | ||||
|
Seebri Neohaler/ Seebri Breezhaler | glycopyrronium bromide (glycopyrrolate) | Chronic obstructive pulmonary disease | Inhalation powder hard capsules | ||||
|
TOBI and TOBI Podhaler | tobramycin | Pseudomonas aeruginosa infection in cystic fibrosis | Nebulizer solution (TOBI), Inhalation powder (TOBI Podhaler) | ||||
|
Utibron Neohaler/ Ultibro Breezhaler | indacaterol / glycopyrronium bromide (glycopyrrolate) | Chronic obstructive pulmonary disease | Inhalation powder hard capsules | ||||
|
Xolair | omalizumab |
Severe allergic asthma See also, "Immunology and Dermatology" |
Lyophilized powder in vial and liquid formulation in pre-filled syringes | ||||
Neuroscience |
Comtan | entacapone | Parkinson's disease patients who experience end-of-dose motor (or movement) fluctuations | Tablet | ||||
|
Exelon | rivastigmine |
Mild-to-moderate Alzheimer's disease dementia Severe Alzheimer's disease dementia |
Capsule Oral solution Transdermal patch |
||||
|
Extavia | interferon beta-1b | Relapsing remitting and/or relapsing forms of multiple sclerosis in adult patients | Subcutaneous injection | ||||
|
Gilenya | fingolimod | Relapsing forms of multiple sclerosis | Capsule | ||||
39
Business franchise |
Product | Common name | Indications (vary by country and/or formulation) |
Formulation | ||||
|---|---|---|---|---|---|---|---|---|
|
Stalevo | carbidopa, levodopa and entacapone | Parkinson's disease patients who experience end-of-dose motor (or movement) fluctuations | Tablet | ||||
Established Medicines |
Cibacen | benazepril hydrochloride |
Hypertension Adjunct therapy in congestive heart failure Progressive chronic renal insufficiency |
Tablet | ||||
|
Clozaril/Leponex | clozapine |
Treatment-resistant schizophrenia Prevention and treatment of recurrent suicidal behavior in patients with schizophrenia and psychotic disorders |
Tablet | ||||
|
Coartem/Riamet | artemether and lumefantrine |
Plasmodium falciparum malaria or mixed infections that include Plasmodium falciparum Standby emergency malaria treatment |
Tablet Dispersible tablet for oral suspension | ||||
|
Diovan | valsartan |
Hypertension Heart failure Post-myocardial infarction |
Tablets Capsules Oral solution |
||||
|
Diovan HCT and Co-Diovan | valsartan and hydrochlorothiazide | Hypertension | Tablet | ||||
|
Exforge and Exforge HCT |
valsartan and amlodipine besylate | Hypertension | Tablet | ||||
|
Focalin and Focalin XR |
dexmethylphenidate HCl and dexmethylphenidate extended release | Attention deficit hyperactivity disorder | Tablet Capsule |
||||
|
Foradil | formoterol |
Asthma Chronic obstructive pulmonary disease |
Aerolizer (capsules) Aerosol | ||||
|
Lamisil | terbinafine (terbinafine hydrochloride) |
Fungal infection of the skin and nails caused by dermatophyte fungi tinea capitis Fungal infections of the skin for the treatment of tinea corporis, tinea cruris, tinea pedis and yeast infections of the skin caused by the genus candida Onychomycosis of the toenail or fingernail due to dermatophytes |
Tablet | ||||
|
Lescol and Lescol XL | fluvastatin sodium |
Hypercholesterolemia and mixed dyslipidemia in adults Secondary prevention of major adverse cardiac events Slowing the progression of atherosclerosis Heterozygous familial hypercholesterolemia in children and adolescents |
Capsule (Lescol) Tablet (Lescol XL) |
||||
|
Reclast/Aclasta | zoledronic acid 5 mg |
Treatment of osteoporosis in postmenopausal women Treatment of osteoporosis in men Treatment and prevention of glucocorticoid-induced osteoporosis Prevention of postmenopausal osteoporosis Treatment of Paget's disease of the bone |
Intravenous—solution for infusion | ||||
40
Business franchise |
Product | Common name | Indications (vary by country and/or formulation) |
Formulation | ||||
|---|---|---|---|---|---|---|---|---|
|
Ritalin | methylphenidate HCl | Attention deficit hyperactivity disorder and narcolepsy | Tablet | ||||
|
Ritalin LA | methylphenidate HCl modified release | Attention deficit hyperactivity disorder | Capsule | ||||
|
Tegretol | carbamazepine |
Epilepsy Pain associated with trigeminal neuralgia Acute mania and bipolar affective disorders Alcohol withdrawal syndrome Painful diabetic neuropathy Diabetes insipidus centralis Polyuria and polydipsia of neurohormonal origin |
Tablet Chewable tablet Oral suspension Suppository |
||||
|
Tekamlo/Rasilamlo | aliskiren and amlodipine besylate | Hypertension | Tablet | ||||
|
Tekturna/Rasilez | aliskiren | Hypertension | Tablet | ||||
|
Tekturna HCT/ Rasilez HCT | aliskiren and hydrochlorothiazide | Hypertension | Tablet | ||||
|
Trileptal | oxcarbazepine | Epilepsy | Tablet Oral suspension |
||||
|
Tyzeka/Sebivo | telbivudine | Chronic hepatitis B | Tablet Oral solution |
||||
|
Vivelle-Dot/Estradot | estradiol hemihydrate |
Estrogen replacement therapy for the treatment of the symptoms of natural or surgically induced menopause Prevention of postmenopausal osteoporosis |
Transdermal patch | ||||
|
Voltaren/Cataflam | diclofenac sodium/potassium/resinate/free acid |
Inflammatory and degenerative forms of rheumatism Post traumatic and post-operative pain, inflammation and swelling Painful and/or inflammatory conditions in gynecology Other painful and/or inflammatory conditions such as renal and biliary colic, migraine attacks and as adjuvant in severe ear, nose and throat infections |
Tablet Capsule Oral drops/oral suspension Ampoule for injection Suppository Gel Powder for oral solution Transdermal patch |
||||
Key Marketed Products
- •
- Gleevec/Glivec (imatinib mesylate/imatinib) is a kinase inhibitor approved to treat patients with metastatic and/or unresectable KIT (CD117) positive (KIT+) gastrointestinal stromal tumors (GIST), as an adjuvant treatment for certain adult patients following resection of KIT+ GIST, and as a targeted therapy for Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML). First launched in 2001, Gleevec/Glivec is available in more than 120 countries. Gleevec/Glivec is also approved in the US, EU and Japan to treat Ph+ acute lymphoblastic leukemia, a rapidly progressive form of leukemia. Gleevec/Glivec is also approved in the US and EU to treat dermatofibrosarcoma protuberans, a rare solid tumor; hypereosinophilic syndrome; myelodysplastic/myeloproliferative diseases and other rare blood disorders. In the US, Gleevec is also approved for aggressive systemic mastocytosis. Gleevec/Glivec has received approvals in more than 65 countries as a post-surgery (adjuvant setting) therapy for certain adult patients with KIT+ GIST. Following approval by the FDA in 2013, the EMA approved Gleevec/Glivec in July 2013 for pediatric patients with newly diagnosed Ph+ acute lymphoblastic leukemia in combination with chemotherapy.
Oncology
41
- •
- Afinitor/Votubia and Afinitor
Disperz/Votubia dispersible tablets (everolimus) is an oral inhibitor of the mTOR pathway. Afinitor is approved in more than 120
countries including the US, EU member states and Japan for advanced renal cell carcinoma following vascular endothelial growth factor-targeted therapy (in the US, after failure of sunitinib or
sorafenib). Afinitor is also approved in more than 95 countries, including the US, EU member states and Japan for the treatment of advanced pancreatic
neuroendocrine tumors. In addition, Afinitor is approved in more than 100 countries for advanced hormone receptor-positive, HER2-negative
(HR+/HER2–) breast cancer in combination with the drug exemestane. Everolimus is also approved in more than 95 countries including in the US as Afinitor and in the EU as Votubia to treat patients with subependymal giant cell astrocytoma (SEGA)
associated with tuberous sclerosis complex (TSC) and in more than 90 countries for the treatment of adult patients with renal angiomyolipomas and TSC who do not require immediate surgery. Afinitor Disperz, the dispersible tablet for oral suspension formulation of Afinitor, is approved for
the TSC-SEGA population in several countries including the US and Japan. Votubia dispersible tablets are approved for the treatment of patients with
TSC-SEGA in the EU member states. Everolimus, the active ingredient in Afinitor, is also available under the trade names Zortress/Certican for use in
transplantation in the US and EU, respectively, and is exclusively licensed to Abbott and sublicensed to Boston Scientific
for use in drug-eluting stents.
- •
- Tasigna (nilotinib) is a signal transduction inhibitor of the BCR-ABL tyrosine kinase.
Since its launch in 2007, Tasigna has been approved in more than 110 countries to treat patients with Ph+ CML in the chronic and/or accelerated phase
who are resistant or intolerant to existing treatment, including Gleevec/Glivec. It is also approved in more than 85 markets, including the US, EU
member states, Switzerland and Japan, to treat newly diagnosed patients in the chronic phase.
- •
- Sandostatin SC and Sandostatin LAR
(octreotide acetate/octreotide acetate for injectable suspension) is a somatostatin analogue indicated for the treatment of patients with acromegaly, a chronic disease caused by over-secretion of
pituitary growth hormone in adults. Sandostatin is also indicated for the treatment of patients with certain symptoms associated with carcinoid tumors
and other types of gastrointestinal and pancreatic neuroendocrine tumors. Additionally, Sandostatin LAR is approved in more than 60 countries for
treatment of patients with advanced neuroendocrine tumors of the midgut or unknown primary tumor location. More than 65 countries have also approved an enhanced presentation of Sandostatin LAR, which
includes a diluent, safety needle and vial adapter. Sandostatin was first
launched in 1988 and is approved in more than 100 countries.
- •
- Exjade and Jadenu (deferasirox) is an
iron chelator approved for the treatment of chronic iron overload due to blood transfusions in patients two years of age and older as well as chronic iron overload in patients with
non-transfusion-dependent thalassemia. Exjade is a dispersible tablet for oral suspension. Jadenu is an
oral tablet formulation of Exjade that can be swallowed or crushed and was approved by the FDA in 2015. Patients with chronic anemia from diseases such
as thalassemia, sickle cell disease and myelodysplastic syndromes require repeated transfusions, which puts them at risk of iron overload. Exjade was
first approved in 2005 and is now approved in more than 100 countries, including the US, EU member states and Japan. Exjade is also approved in more
than 70 countries, including the US and EU member states, for the treatment of chronic iron overload in patients 10 years of age and older with non-transfusion-dependent thalassemia. Regulatory
applications for Jadenu have been submitted in the EU, Canada, Switzerland and other countries.
- •
- Votrient (pazopanib) is a small molecule tyrosine kinase inhibitor that targets a number of intracellular proteins to limit tumor growth and cell survival. Votrient is approved in the US for the treatment of patients with advanced renal cell carcinoma (aRCC), and in the EU for first-line treatment of adult patients with aRCC and for patients who have received prior cytokine therapy
42
- •
- Tafinlar + Mekinist (dabrafenib + trametinib) is the first
combination of its kind for the treatment of patients with BRAF V600 mutation positive unresectable or metastatic melanoma, as detected by a validated test, in the US, EU and several other markets. In
November 2015, the FDA granted regular approval for the combination of Tafinlar + Mekinist
for the treatment of patients with BRAF V600E/K mutation-positive unresectable or metastatic melanoma as detected by an FDA-approved test. In August 2015, the combination of Tafinlar and Mekinist was approved in Europe for the treatment of adult patients with unresectable or
metastatic melanoma with a BRAF V600 mutation. Tafinlar targets the serine/threonine kinase BRAF in the RAS/RAF/MEK/ERK pathway and Mekinist targets the
threonine/tyrosine kinases MEK1 and MEK2 in the MAP kinase pathway, resulting in dual blockade of this pathway. This is the first
combination of BRAF/MEK inhibitors to achieve a median overall survival of more than two years in two Phase III studies in BRAF V600 mutation positive unresectable or metastatic melanoma
patients. Tafinlar and Mekinist are each also approved as single agents for the treatment
of patients with unresectable or metastatic melanoma in more than 45 and 30 countries worldwide, respectively. Tafinlar and Mekinist were each acquired
from GSK. As part of our purchase of oncology products from GSK, we obtained the worldwide exclusive rights granted by Japan
Tobacco Inc. (JT) to develop, manufacture, and commercialize trametinib.
- •
- Jakavi (ruxolitinib) is an oral inhibitor of the JAK1 and JAK2 tyrosine kinases. It is
the first JAK inhibitor indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis, post-polycythemia vera myelofibrosis or post-essential
thrombocythemia myelofibrosis and adult patients with polycythemia vera who are resistant to or intolerant of hydroxyurea. Jakavi is currently approved
in more than 95 countries for patients with myelofibrosis, including EU member states, Japan, Canada, Australia, Mexico and Argentina. Jakavi is
approved for the polycythemia vera indication in more than 45 countries, including Switzerland, Japan and Canada. Worldwide regulatory filings are ongoing in different regions for myelofibrosis and
polycythemia vera. In the COMFORT-II Phase III study, five-year treatment with Jakavi demonstrated an overall survival advantage for
myelofibrosis patients, despite crossover from best available therapy after week 48. Novartis licensed ruxolitinib from Incyte Corporation for development and commercialization outside the US.
Ruxolitinib, marketed in the US as Jakafi® by Incyte Corporation, is approved by the FDA for the treatment of patients with polycythemia vera who have had an inadequate response to or are
intolerant of hydroxyurea. Jakafi® is also approved by the FDA for treatment of patients with intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia
vera myelofibrosis and post-essential thrombocythemia myelofibrosis.
- •
- Promacta/Revolade (eltrombopag) is a once-daily oral thrombopoietin (TPO) receptor agonist that works by stimulating bone marrow cells to produce platelets. It is the only approved once-daily oral thrombopoietin receptor agonist, and is marketed under the brand name Promacta in the US and Revolade in most countries outside the US. In the US, Promacta is approved for the treatment of thrombocytopenia in adult and pediatric patients one year and older with chronic immune (idiopathic) thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. In August 2015, the FDA approved an oral suspension formulation of Promacta that is designed for younger children with chronic ITP who may not be able to swallow tablets. Promacta is also approved for the treatment of thrombocytopenia in
for advanced disease. RCC is the most common type of kidney cancer in adults, and nearly one-fifth of patients have aRCC at the time of diagnosis. Votrient is also indicated for the treatment of patients with advanced soft tissue sarcoma (STS) who have received prior chemotherapy (efficacy in adipocytic STS or gastrointestinal stromal tumors has not been demonstrated). STS is a type of cancer which can arise from a wide variety of soft tissues including muscle, fat, blood vessel and nerves. Votrient is approved in more than 95 countries worldwide for aRCC and in more than 85 countries for aSTS. Votrient was acquired from GSK.
43
- •
- Farydak (panobinostat), previously known as LBH589, is a histone deacetylase (HDAC)
inhibitor indicated, in combination with bortezomib and dexamethasone, for the treatment of adult patients with relapsed and/or refractory multiple myeloma who have received at least two prior
regimens including bortezomib and an immunomodulatory agent. Farydak marks the first time an HDAC inhibitor with epigenetic activity is available to
patients with multiple myeloma and provides an additional treatment option for patients whose disease has progressed after standard-of-care therapy. Farydak in combination with bortezomib and
dexamethasone was approved in 2015 in the US, EU and Japan for certain patients with previously treated
multiple myeloma. The exact indication for Farydak varies by country. Additional regulatory submissions for Farydak are being reviewed by health
authorities worldwide. Results from the pivotal Phase III PANORAMA-1 study of Farydak in combination with bortezomib and dexamethasone in patients with multiple myeloma who have received at least
two prior regimens, including
bortezomib and an immunomodulatory agent, were published online in the journal Blood and showed a progression free survival benefit favoring the Farydak
combination.
- •
- Odomzo (sonidegib), previously known as LDE225, is a selective smoothened inhibitor approved in the US in July 2015 for the treatment of adult patients with locally advanced basal cell carcinoma (laBCC) that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy. In addition, the EC approved Odomzo in August 2015 for the treatment of adult patients with laBCC who are not amenable to curative surgery or radiation therapy.
patients with chronic hepatitis C to allow the initiation and maintenance of interferon-based therapy, and for the treatment of patients with severe aplastic anemia (SAA) who have had an insufficient response to immunosuppressive therapy. Revolade is approved in more than 100 countries worldwide for the treatment of adult chronic ITP splenectomised patients who are refractory to other treatments (e.g., corticosteroids, immunoglobulins). Revolade may be considered as second line treatment for adult non-splenectomised patients where surgery is contraindicated. In December 2015, the CHMP adopted a positive opinion recommending a change to the adult ITP indication to remove language which limited Revolade use only to splenectomised patients who are refractory to other treatments. The EC decision is expected in February 2016. Revolade is also indicated in more than 45 countries worldwide in adult patients with chronic hepatitis C virus infection for the treatment of thrombocytopenia, where the degree of thrombocytopenia is the main factor preventing the initiation or limiting the ability to maintain optimal interferon-based therapy. In September 2015, Revolade was approved by the EC for the treatment of adults with acquired SAA who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for hematopoietic stem cell transplant. Promacta/Revolade is marketed under a collaboration agreement between Ligand Pharmaceuticals, Inc., and Novartis. Promacta/Revolade was acquired from GSK.
- •
- Galvus (vildagliptin), an oral DPP-4 inhibitor, and Eucreas, a vildagliptin and metformin single-pill combination, are indicated for the treatment of type 2 diabetes. The products were first approved in 2007. Galvus is currently approved in more than 130 countries, including EU member states, Japan (as Equa) and countries in Latin America and Asia-Pacific. Eucreas was the first single-pill combination of a DPP-4 inhibitor and metformin approved in Europe, and also under the trade name Galvus Met, and is currently approved in more than 125 countries. In 2012, Galvus received EU approval for expanded use as a second-line monotherapy for type 2 diabetes patients who cannot take metformin. In 2012, the EC approved the use of Galvus and Eucreas in combination with other diabetes treatments. The first new approval was for the use of vildagliptin in combination with insulin, with or without metformin, for patients with type 2 diabetes when diet, exercise and a stable dose of insulin do not result in glycemic control. The second approval was for
Cardio-Metabolic
44
- •
- Entresto (sacubitril/valsartan), previously known as LCZ696, is a first-in-class angiotensin receptor/neprilysin inhibitor indicated for the treatment of chronic heart failure with reduced ejection fraction (HFrEF). It acts to enhance the protective neurohormonal systems of the heart (neprilysin system) while simultaneously suppressing the harmful system (the renin-angiotensin-aldosterone system, or RAAS). Entresto was approved and launched in the US in July 2015 as a treatment for HFrEF. In September 2015, the Swiss health authority approved Entresto to reduce the risk of cardiovascular mortality and morbidity in patients with HFrEF. In November 2015, Entresto was approved in the EU for the treatment of adult patients with symptomatic HFrEF. PARAGON-HF, a Phase III trial of Entresto in patients with chronic heart failure with preserved ejection fraction is underway.
the use of vildagliptin in triple combination with metformin and a sulphonylurea for the treatment of type 2 diabetes when diet and exercise plus dual therapy with these two agents do not provide adequate glycemic control. In 2013, a German agency, the Gemeinsamer Bundesausschuss (G-BA), initiated an analysis of the benefits of drugs approved prior to 2011. As part of that analysis, the G-BA concluded that Galvus and Eucreas did not provide an added benefit over certain other medicines indicated for the treatment of that disease. As a result, we were unable to reach agreement with the head organization of the German statutory health insurance funds, GKV-Spitzenverband, on an acceptable price for Galvus and Eucreas, and in 2014 we stopped distribution of these products in Germany. In 2014, Eucreas (850/50mg and 1000/50mg) was approved in China as the first high-dose single-pill combination metformin/DPP-4 inhibitor approved in that country. Galvus monotherapy indication was approved in China in April 2015. Eucreas was approved in Japan in September 2015 under the name EquMet as the first single-pill combination metformin/DPP-4 inhibitor approved in that country.
- •
- Neoral (cyclosporine, USP Modified) is an immunosuppressant to prevent organ rejection
following a kidney, liver, or heart transplant. Neoral is also approved for use in lung transplant in many countries outside of the US. This
micro-emulsion formulation of cyclosporine is also indicated for treating selected autoimmune disorders such as psoriasis and rheumatoid arthritis. First launched in 1995, Neoral is marketed in
approximately 100 countries.
- •
- Myfortic (enteric-coated formulation of mycophenolate sodium) is approved in more than
90 countries for the prevention of acute rejection of kidney allografts, and is indicated in combination with cyclosporine and corticosteroids. Myfortic was first approved in the US in 2004 and in
the EU in 2003.
- •
- Zortress/Certican (everolimus) is an oral inhibitor of the mTOR pathway, indicated to
prevent organ rejection following solid organ transplantation. Under the trade name Certican, it is approved in more than 90 countries to prevent organ
rejection for renal and heart transplant patients, and in addition, in more than 70 countries worldwide to prevent organ rejection for liver transplant patients. In the US, under the trade name Zortress,
the drug is approved for the prophylaxis of organ rejection in adult patients at low-moderate immunologic risk receiving a kidney transplant
as well as for the prophylaxis of allograft rejection in adult liver transplant recipients. Everolimus is also available from Novartis in different dosage strengths and for different uses in
non-transplant patient populations under the brand names Afinitor, Afinitor Disperz and Votubia.
Everolimus is also exclusively licensed to Abbott and sublicensed to Boston Scientific for use in drug-eluting stents.
- •
- Ilaris (canakinumab) is a human monoclonal antibody that selectively binds and neutralizes interleukin-1b (IL-1b), a pro-inflammatory cytokine. Since 2009, Ilaris has been approved in more than 70 countries for the treatment of children and adults suffering from cryopyrin-associated periodic syndromes, a group of rare disorders characterized by chronic recurrent fever, urticaria,
Immunology and Dermatology
45
- •
- Xolair (omalizumab) is currently approved in the EU, Switzerland and more than 40
other countries as a treatment for chronic spontaneous urticaria (CSU)/chronic idiopathic urticaria (CIU) including approvals in the EU as add-on therapy for the treatment of CSU in adult and
adolescent (12 years and above) patients with inadequate response to H1 antihistamine treatment, and, in the US, for the treatment of adults and adolescents (12 years of age and above)
with CIU who remain symptomatic despite H1 antihistamine treatment. See also, Xolair in "Respiratory" below. We co-promote Xolair with Genentech in the US
and share a portion of operating income, but we do not record any US sales. Novartis records all sales of Xolair outside the US. See "Item 18. Financial Statements—Note 27" for further
information.
- •
- Cosentyx (secukinumab) is a fully human monoclonal antibody that selectively neutralizes circulating interleukin 17A (IL-17A). In December 2014, Cosentyx was approved in Japan for the treatment of both psoriasis vulgaris and psoriatic arthritis in adults who are not adequately responding to systemic therapies (except for biologics). This approval marked the first country approval for Cosentyx in the world and made it the first IL-17A inhibitor to receive regulatory approval in either of these indications. In January 2015, Cosentyx was approved in the EU as a first-line systemic treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy, and in the US as a treatment for moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. In addition to the EU and US, Cosentyx has been approved and launched in Switzerland, Canada, Australia and various other markets for the treatment of moderate-to-severe plaque psoriasis. In November 2015, Cosentyx was approved in the EU for the treatment of adults with ankylosing spondylitis who have responded inadequately to conventional therapy, such as non-steroidal anti-inflammatory drugs, and for the treatment of active psoriatic arthritis in adults when the response to disease modifying anti-rheumatic drug therapy is unsatisfactory. In Japan, Cosentyx is approved for the treatment of moderate-to-severe plaque psoriasis as well as PsA. In December 2015, the Japanese MHLW approved Cosentyx for the treatment of patients with pustular psoriasis. In January 2016, Cosentyx was approved in the US for the treatment of adults with active ankylosing spondylitis and for the treatment of adults with active psoriatic arthritis.
occasional arthritis, deafness, and potentially life-threatening amyloidosis. In 2013, Ilaris was approved in the EU for the treatment of acute gouty arthritis in patients who cannot be managed with standard of care, and in the US, EU and other countries for the treatment of systemic juvenile idiopathic arthritis. Ilaris is also being developed for hereditary periodic fever syndromes.
- •
- Lucentis (ranibizumab) is a recombinant humanized high affinity antibody fragment that binds to vascular endothelial growth factors (VEGF). It is an anti-VEGF therapy licensed in many countries for five ocular indications: neovascular age-related macular degeneration (nAMD), visual impairment due to diabetic macular edema (DME), visual impairment due to macular edema secondary to branch retinal vein occlusion (BRVO), visual impairment due to macular edema secondary to central retinal vein occlusion (CRVO), and visual impairment due to choroidal neovascularization secondary to pathologic myopia (myopic CNV). Lucentis is approved in more than 100 countries to treat patients with nAMD, for the treatment of visual impairment due to DME and macular edema secondary to RVO. Also, Lucentis is licensed in more than 80 countries for the treatment of visual impairment due to myopic CNV. Lucentis is the only anti-VEGF treatment available in a pre-filled syringe. Since its launch in 2007, there have been more than 3.7 million patient-treatment years of exposure for Lucentis and more than 22 million injections. We licensed Lucentis from Genentech for development and commercialization outside of the US. See "Item 18. Financial Statements—Note 27" for further information.
Retina
46
- •
- Xolair (omalizumab) is the only humanized monoclonal antibody approved for the
treatment of moderate to severe persistent allergic asthma in the US in adolescents (aged 12 and above) and adults. Xolair is approved in more than 90
countries, including the US since 2003 and the EU since 2005. It is approved for severe persistent allergic asthma in the EU in children (aged six and above), adolescents, and adults. A liquid
formulation of Xolair in pre-filled syringes has been launched in most European countries. In Japan, Xolair was approved in January 2009 for the treatment
of severe persistent allergic asthma in adults (aged 15 and older) and was approved in August 2013
in pediatric patients aged 6 years or older for the same indication. Xolair was submitted to the FDA in December 2015 for pediatric allergic
asthma. See also, Xolair in "Immunology and Dermatology" above.
- •
- Ultibro Breezhaler (indacaterol/glycopyrronium bromide) / Utibron Neohaler (indacaterol/glycopyrrolate) is a fixed-dose combination of the long-acting beta2-adrenergic agonist (LABA) indacaterol and the long-acting muscarinic antagonist (LAMA) glycopyrronium bromide. Ultibro Breezhaler (indacaterol 85 mcg/glycopyrronium 43 mcg), inhalation powder, hard capsules was approved in the EU in 2013 as a once-daily maintenance bronchodilator treatment to relieve symptoms in adult patients with COPD, and in Japan the MHLW approved Ultibro Inhalation Capsules (glycopyrronium 50 mcg/indacaterol 110 mcg), delivered through the low resistance Breezhaler inhalation device, for relief of various symptoms due to airway obstruction in COPD (chronic bronchitis, emphysema). In October 2015 the combination was approved in the US under the name Utibron Neohaler (indacaterol 27.5 mcg/glycopyrrolate 15.6 mcg) as a twice-daily dual bronchodilator for the long-term maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and/or emphysema. The combination is approved in more than 80 countries and launched in more than 40 countries. The LAMA glycopyrronium bromide is approved individually as once-daily Seebri Breezhaler in the EU, Seebri (glycopyrronium) Inhalation Capsules 50 mcg administered through the Breezhaler device in Japan, and twice-daily Seebri Neohaler in the US, where the active ingredient is known as glycopyrrolate. It is now approved in more than 90 countries worldwide. Glycopyrronium bromide was exclusively licensed to Novartis in April 2005 by Vectura Group plc and its co-development partner Sosei. The LABA indacaterol is approved individually as once-daily Onbrez Breezhaler in the EU, Onbrez Inhalation Capsules delivered through the Breezhaler inhalation device in Japan, and Arcapta Neohaler in the US. It is now approved in more than 100 countries worldwide.
Respiratory
- •
- Gilenya (fingolimod) is the first oral therapy approved to treat relapsing forms of
multiple sclerosis (RMS) and the first in a new class of compounds called sphingosine 1-phosphate receptor modulators. In the US, Gilenya is indicated
for relapsing forms of MS. In the EU, Gilenya is indicated for adult patients with high disease activity despite treatment with at least one disease
modifying agent, or rapidly evolving severe relapsing-remitting MS. Gilenya is the only oral disease-modifying therapy to impact the course of RMS with
high efficacy across four key measures of disease activity: relapses, MRI lesions, brain shrinkage (brain volume loss) and disability progression. As of November 2015, more than 130,000 patients have
been treated in clinical trials and in a post-marketing setting, with more than 285,000 total patient-years of exposure. Gilenya is currently approved
in more than 80 countries around the world. Gilenya is licensed from Mitsubishi Tanabe Pharma Corporation.
- •
- Exelon (rivastigmine tartrate) and Exelon Patch (rivastigmine transdermal system) are cholinesterase inhibitors indicated for the treatment of Alzheimer's disease (AD) dementia and Parkinson's disease (PD) dementia. They are the oral and transdermal formulations, respectively, of the cholinesterase inhibitor rivastigmine. Exelon capsules have been available since 1997 to treat
Neuroscience
47
- •
- Diovan (valsartan), together with Diovan
HCT/Co-Diovan (valsartan and hydrochlorothiazide), is an angiotensin II receptor blocker (ARB) and is one of the top-selling branded anti-hypertensive medications worldwide
(IMS MAT October 2015; 58 countries audited). Diovan is the only agent in its class approved to treat all of the following: high blood pressure
(including children 6 to 18 years), high-risk heart attack survivors and patients with heart failure. First launched in 1996, Diovan is available
in more than 120 countries for treating high blood pressure, in more than 90 countries for heart failure, and in more than 70 countries for heart attack survivors. First launched in 1997, Diovan
HCT/Co-Diovan is approved in more than 100 countries worldwide. Diovan is subject to generic
competition in the US, EU and Japan. Diovan HCT/Co-Diovan is subject to generic competition in the US and EU.
- •
- Exforge (valsartan and amlodipine besylate) is a single-pill combination of the ARB Diovan and the
calcium channel blocker amlodipine besylate. First approved for the treatment of high blood pressure in Switzerland in 2006, and in the
US and EU in 2007, it is now available in more than 100 countries. Exforge HCT (valsartan, amlodipine besylate and hydrochlorothiazide) is a
single pill combining three widely prescribed high blood pressure treatments: an ARB, a calcium channel blocker and a diuretic (hydrochlorothiazide). Exforge
HCT was approved in the EU and the US in 2009, and is now available in more than 75 countries.
- •
- Voltaren/Cataflam (diclofenac sodium/potassium/resinate/free acid) is a leading
non-steroidal anti-inflammatory drug (NSAID) for the relief of symptoms in rheumatic diseases such as rheumatoid arthritis and osteoarthritis, and for various other inflammatory and pain conditions. Voltaren/Cataflam was first registered in 1973 and is available in more than 140 countries. This product, which is subject to generic competition, is
marketed by the Pharmaceuticals Division in a wide variety of dosage forms, including tablets, drops, suppositories, ampoules and topical therapy. In addition, in various countries, our Sandoz
Division markets generic versions of the product, and our Alcon Division markets Voltaren for ophthalmic indications. In addition, we have licensed the Voltaren trademarks to our consumer healthcare joint venture with GSK to be used in the marketing of low dose oral forms and the topical forms of Voltaren as over-the-counter products.
- •
- Ritalin, Ritalin LA, Focalin and Focalin XR (methylphenidate HCl, methylphenidate HCl extended release, dexmethylphenidate HCl and dexmethylphenidate HCl extended release) are indicated for the treatment of attention deficit hyperactivity disorder (ADHD) in children. Ritalin LA and Focalin XR are additionally indicated for ADHD in adults. Ritalin is also indicated for narcolepsy. Ritalin was first marketed during the 1950s and is available in more than 70 countries. Ritalin LA is available in more than 30 countries. Focalin comprises the active d-isomer of methylphenidate and therefore requires half the dose of Ritalin. Focalin and Focalin XR are available in the US.
mild to moderate AD dementia and are approved in more than 90 countries. In 2006, Exelon became the only cholinesterase inhibitor to be approved for mild to moderate PD dementia in addition to AD in both the US and EU. Exelon Patch was approved in 2007 in the US and EU and has been approved for the treatment of mild-to-moderate AD in more than 90 countries, including more than 20 countries where it is also approved for Parkinson's disease dementia. The once-daily Exelon Patch has shown comparable efficacy and superior tolerability to the highest recommended doses of Exelon capsules, with significant improvement in cognition and overall functioning compared to placebo. In 2013, the FDA expanded the approved indication for Exelon Patch to also include the treatment of patients with severe Alzheimer's disease. In 2013, European Marketing Authorization was obtained for the higher dose in mild-to-moderate AD. The higher dose has been approved in more than 50 countries. In 2013, the FDA expanded the approved indication for Exelon Patch to also include the treatment of patients with severe Alzheimer's disease. The severe indication has now been approved in more than 10 countries.
Established Medicines
48
The traditional model of development comprises three phases, which are defined as follows:
Phase I: First clinical trials of a new compound, generally performed in a small number of healthy human volunteers, to assess the clinical safety and tolerability as well as metabolic and pharmacologic properties of the compound.
Phase II: Clinical studies that are performed with patients who have the target disease, with the aim of continuing the Phase I safety assessment in a larger group, assessing the efficacy of the drug in the patient population, and determining the appropriate doses for further evaluation.
Phase III: Large-scale clinical studies with several hundred to several thousand patients, which are conducted to establish the safety and efficacy of the drug-specific indications for regulatory approval. Phase III trials may also be used to compare a new drug against a current standard of care to evaluate the overall benefit-risk relationship of the new medicine.
Though we use this traditional model as a platform, we have tailored the process to be simpler, more flexible and efficient. Our development paradigm consists of two parts: Exploratory Development and Confirmatory Development. Exploratory Development consists of clinical "proof of concept" (PoC) studies, which are small clinical trials (typically 5-15 patients) that combine elements of traditional Phase I/II testing. These customized trials are designed to give early insights into issues such as safety, efficacy and toxicity for a drug in a given indication. Once a positive proof of concept has been established, the drug moves to the Confirmatory Development stage. Confirmatory Development has elements of traditional Phase II/III testing and includes trials aimed at confirming the safety and efficacy of the drug in the given indication leading up to submission of a dossier to health authorities for approval. Like traditional Phase III testing, this stage can also include trials which compare the drug to the current standard of care for the disease, in order to evaluate the drug's overall risk/benefit profile.
The following table and paragraph summaries provide an overview of the key projects currently in the Confirmatory Development stage within our Pharmaceuticals Division, including projects seeking to develop potential uses of new molecular entities, as well as potential additional indications or new formulations for already marketed products. The year that each project entered the current phase of development disclosed below reflects the year in which the decision to enter the phase was made. This may be different from the year in which the first patient received the first treatment in the related clinical trial. A reference to a project being in registration means that an application has been filed with a health authority for marketing approval.
49
Selected Development Projects
Project/Product
|
Common name | Mechanism of action | Potential indication/ Disease area |
Business franchise |
Formulation/ Route of administration |
Year Project Entered Current Development Phase |
Planned filing dates/Current phase |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABL001 | TBD | BCR-ABL inhibitor | Chronic myeloid leukemia | Oncology | Oral | 2015 | ³2020/I | |||||||
| ACZ885 | canakinumab | Anti-interleukin-1b monoclonal antibody | Hereditary periodic fevers | Immunology and Dermatology | Subcutaneous injection | 2013 | 2016/III | |||||||
| Secondary prevention of cardiovascular events | Cardio-Metabolic | Subcutaneous injection | 2011 | 2017/III | ||||||||||
| Afinitor/Votubia (RAD001) | everolimus | mTOR inhibitor | Non-functioning GI and lung neuroendocrine tumors | Oncology | Oral | 2015 | US/EU (registration) | |||||||
| Tuberous sclerosis complex seizures | Oncology | Oral | 2013 | 2016/III | ||||||||||
| Diffuse large B-cell lymphoma | Oncology | Oral | 2009 | 2016/III | ||||||||||
| AMG 334 | TBD | Selective CGRP receptor antagonist | Migraine | Neuroscience | Subcutaneous injection | 2015 | III | |||||||
| Arzerra | ofatumumab | Anti-CD20 monoclonal antibody | Chronic lymphocytic leukemia (extended treatment) | Oncology | Intravenous infusion | 2015 | EU (registration) US (approved) |
|||||||
| Chronic lymphocytic leukemia (relapse) | Oncology | Intravenous infusion | 2009 | 2016/III | ||||||||||
| Refractory non-Hodgkin's lymphoma | Oncology | Intravenous infusion | 2010 | 2017/III | ||||||||||
| ASB183 | afuresertib | AKT inhibitor | Solid and hematologic tumors | Oncology | Oral | 2011 | ³ 2020/I | |||||||
| BAF312 | siponimod | Sphingosine-1-phosphate receptor modulator | Secondary progressive multiple sclerosis | Neuroscience | Oral | 2012 | 2019/III | |||||||
| BGJ398 | infigratinib | Pan-FGF receptor kinase inhibitor | Solid tumors | Oncology | Oral | 2012 | ³ 2020/II | |||||||
| BKM120 | buparlisib | PI3K inhibitor | Metastatic breast cancer, hormone receptor-positive, aromatase inhibitor resistant/mTOR naïve, 2nd line (+ fulvestrant) | Oncology | Oral | 2011 | 2016/III | |||||||
| Metastatic breast cancer, hormone receptor-positive, aromatase inhibitor and mTOR inhibitor resistant, 3rd line (+ fulvestrant) | Oncology | Oral | 2011 | 2016/III | ||||||||||
| Solid tumors | Oncology | Oral | 2011 | ³ 2020/I | ||||||||||
| BYL719 | alpelisib | PI3Ka inhibitor | Hormone receptor-positive, HER2 negative advanced breast cancer (postmenopausal women), 2nd line (+ fulvestrant) | Oncology | Oral | 2015 | 2019/III | |||||||
| Solid tumors | Oncology | Oral | 2010 | ³2020/I | ||||||||||
50
Project/Product
|
Common name | Mechanism of action | Potential indication/ Disease area |
Business franchise |
Formulation/ Route of administration |
Year Project Entered Current Development Phase |
Planned filing dates/Current phase |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BYM338 | bimagrumab | Inhibitor of activin receptor Type II | Sporadic inclusion body myositis | Neuroscience | Intravenous infusion | 2013 | 2016/III | |||||||
| Hip fracture | Neuroscience | Intravenous infusion | 2013 | ³2020/II | ||||||||||
| Sarcopenia | Neuroscience | Intravenous infusion | 2014 | ³2020/II | ||||||||||
| CAD106 | TBD | Beta-amyloid-protein therapy | Alzheimer's disease | Neuroscience | Intramuscular injection | 2008 | ³2020/ II/III | |||||||
| CJM112 | TBD | Anti-IL-17 monoclonal antibody | Immune disorders | Immunology and Dermatology | Subcutaneous injection | 2015 | ³2020/II | |||||||
| CNP520 | TBD | BACE inhibitor | Alzheimer's Disease | Neuroscience | Oral | 2015 | ³2020/ I/II | |||||||
| Cosentyx (AIN457) | secukinumab | Anti-IL-17 monoclonal antibody | Non-radiographic axial spondyloarthritis | Immunology and Dermatology | Subcutaneous injection | 2015 | 2018/III | |||||||
| CTL019 | tisagenlecleucel-T | CD19-targeted chimeric antigen receptor T-cell immunotherapy | Pediatric acute lymphoblastic leukemia | Cell and Gene Therapies | Intravenous | 2012 | 2017/II | |||||||
| Diffuse large B-cell lymphoma | Cell and Gene Therapies | Intravenous | 2014 | 2017/II | ||||||||||
| EGF816 | TBD | Epidermal growth factor receptor inhibitor | Solid tumors | Oncology | Oral | 2014 | 2018/ I/II | |||||||
| EMA401 | TBD | Angiotensin II receptor antagonist | Neuropathic Pain | Neuroscience | Oral | 2011 | ³2020/II | |||||||
| Entresto (LCZ696) | valsartan and sacubitril (as sodium salt complex) | Angiotensin receptor/ neprilysin inhibitor | Chronic heart failure with preserved ejection fraction | Cardio-Metabolic | Oral | 2013 | 2019/III | |||||||
| Post-acute myocardial infarction | Cardio-Metabolic | Oral | 2015 | ³ 2020/III | ||||||||||
| Exjade film-coated tablet (FCT) | deferasirox | Iron chelator | Iron overload | Oncology | Oral film-coated tablet | 2015 | EU (registration) US (approved as Jadenu) | |||||||
| FCR001 | TBD | Inducing stable donor chimerism and immunological tolerance | Renal transplant | Cell and Gene Therapies | Intravenous | 2009 | ³2020/II | |||||||
| Gilenya | fingolimod | Sphingosine-1-phosphate receptor modulator | Chronic inflammatory demyelinating polyradiculoneuropathy | Neuroscience | Oral | 2012 | 2017/III | |||||||
| HSC835 | TBD | Stem cell regeneration | Stem cell transplantation | Cell and Gene Therapies | Intravenous | 2012 | ³2020/II | |||||||
| INC280 | capmatinib | c-MET inhibitor | Non-small cell lung cancer | Oncology | Oral | 2013 | 2018/II | |||||||
| KAE609 | cipargamin | PfATP4 inhibitor | Malaria | Established Medicines | Oral | 2012 | ³2020/II | |||||||
| KAF156 | TBD | Imidazolopiperazines derivative | Malaria | Established Medicines | Oral | 2013 | 2019/II | |||||||
| LCI699 | osilodrostat | Aldosterone synthase inhibitor | Cushing's disease | Oncology | Oral | 2011 | 2017/III | |||||||
| LEE011 | ribociclib | CDK4/6 inhibitor | Hormone receptor-positive, HER2 negative advanced breast cancer (postmenopausal women), 1st line (+ letrozole) | Oncology | Oral | 2013 | 2016/III | |||||||
51
Project/Product
|
Common name | Mechanism of action | Potential indication/ Disease area |
Business franchise |
Formulation/ Route of administration |
Year Project Entered Current Development Phase |
Planned filing dates/Current phase |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormone receptor-positive, HER2 negative advanced breast cancer (postmenopausal women), 1st/2nd line (+ fulvestrant) | Oncology | Oral | 2015 | 2018/III | ||||||||||
| Hormone receptor-positive, HER2 negative advanced breast cancer (premenopausal women), 1st line, (+ tamoxifen + goserelin or NSAI + goserelin) | Oncology | Oral | 2014 | 2018/III | ||||||||||
| Solid tumors | Oncology | Oral | 2011 | ³2020/I | ||||||||||
| LJM716 | elgemtumab | HER3 monoclonal antibody | Solid tumors | Oncology | Intravenous infusion | 2012 | ³2020/I | |||||||
| LJN452 | TBD | FXR agonist | Non-alcoholic steatohepatitis | Immunology and Dermatology | Oral | 2015 | ³2020/II | |||||||
| Lucentis | ranibizumab | Anti-VEGF monoclonal antibody fragment | Choroidal neovascularization secondary to conditions other than age-related macular degeneration and pathologic myopia | Retina | Intravitreal injection | 2013 | 2016/III | |||||||
| Retinopathy of Prematurity | Retina | Intravitreal injection | 2014 | 2018/III | ||||||||||
| OAP030 (also known as Fovista / E10030) | pegpleranib | Aptamer anti-platelet-derived growth factor (PDGF) | Neovascular age-related macular degeneration | Retina | Solution | 2013 | 2017/III | |||||||
| OMB157 | ofatumumab | Anti-CD-20 monoclonal antibody | Relapsing multiple sclerosis | Neuroscience | Subcutaneous injection | 2008 | 2019/II | |||||||
| PIM447 | TBD | Pan-PIM inhibitor | Hematologic tumors | Oncology | Oral | 2015 | ³2020/I | |||||||
| PKC412 | midostaurin | Signal transduction inhibitor | Acute myeloid leukemia | Oncology | Oral | 2008 | 2016/III | |||||||
| Aggressive systemic mastocytosis | Oncology | Oral | 2008 | 2016/II | ||||||||||
| Promacta/ Revolade | eltrombopag | Thrombopoietin receptor agonist | Pediatric immune thrombocytopenia | Oncology | Oral and oral suspension | 2015 | EU (registration) US (approved) | |||||||
| QAW039 | fevipiprant | CRTH2 antagonist | Asthma | Respiratory | Oral | 2010 | 2019/III | |||||||
| Atopic dermatitis | Immunology and Dermatology | Oral | 2013 | ³2020/II | ||||||||||
| QAX576 | TBD | Anti-interleukin-13 monoclonal antibody | Allergic diseases | Immunology and Dermatology; Respiratory | Subcutaneous injection | 2013 | ³2020/II | |||||||
| QGE031 | ligelizumab | High affinity anti-IgE monoclonal antibody | Chronic spontaneous urticaria/ Inducible urticaria | Immunology and Dermatology | Subcutaneous injection | 2015 | ³2020/II | |||||||
| QMF149 | indacaterol, mometasone furoate (in fixed dose combination) | Long-acting beta2-adrenergic agonist and inhaled corticosteroid | Asthma | Respiratory | Inhalation | 2015 | 2018/III | |||||||
| QVM149 | indacaterol, mometasone furoate, glycopyrronium bromide (in fixed dose combination) | Long-acting beta2-adrenergic agonist, Long-acting muscarinic antagonist and inhaled corticosteroid | Asthma | Respiratory | Inhalation | 2015 | 2018/III | |||||||
| RLX030 | serelaxin | Recombinant form of human relaxin-2 hormone | Acute heart failure | Cardio-Metabolic | Intravenous infusion | 2009 | 2017/III | |||||||
52
Project/Product
|
Common name | Mechanism of action | Potential indication/ Disease area |
Business franchise |
Formulation/ Route of administration |
Year Project Entered Current Development Phase |
Planned filing dates/Current phase |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signifor LAR (SOM230) | pasireotide | Somatostatin analogue | Cushing's disease | Oncology | Long-acting release/ intramuscular injection | 2011 | 2016/III | |||||||
| Tafinlar+Mekinist | dabrafenib + trametinib | BRAF inhibitor + MEK inhibitor | BRAF V600+ non-small cell lung cancer | Oncology | Oral | 2011 | 2016/II | |||||||
| BRAF V600+ melanoma (adjuvant) | Oncology | Oral | 2013 | 2017/III | ||||||||||
| BRAF V600+ colorectal cancer | Oncology | Oral | 2012 | ³ 2020/ I/II | ||||||||||
| Tasigna | nilotinib | BCR-ABL inhibitor | Chronic myeloid leukemia treatment-free remission | Oncology | Oral | 2012 | 2016/III | |||||||
| VAY736 | TBD | Anti BAFF (B-cell activating factor) antibody | Primary Sjoegren's syndrome | Immunology and Dermatology | Subcutaneous injection | 2015 | ³2020/II | |||||||
| Votrient | pazopanib | Angiogenesis inhibitor | Renal cell carcinoma (adjuvant) | Oncology | Oral | 2010 | 2016/III | |||||||
| Zykadia (LDK378) | ceritinib | ALK inhibitor | ALK + advanced non-small cell lung cancer (first line, treatment naïve) | Oncology | Oral | 2013 | 2017/III | |||||||
| ALK + advanced non-small cell lung cancer (brain metastases) | Oncology | Oral | 2015 | 2019/II | ||||||||||
Key Development Projects
- •
- ACZ885 (canakinumab) was first approved in 2009 for cryopyrin associated periodic syndrome (CAPS) as Ilaris. Since then, Ilaris has been approved in the EU in 2013 for the treatment of acute attacks in
gouty arthritis and for the treatment of systemic juvenile idiopathic arthritis in the US, EU and other countries. Based on Phase II data of ACZ885 in TNF-receptor associated periodic syndrome
and Familial Mediterranean Fever showing substantial symptom relief in these two rare periodic fever syndromes, a Phase III study was initiated in June 2014. The goal of this pivotal
confirmatory study is to demonstrate efficacy and safety in TNF-receptor associated periodic syndrome, colchicine resistant Familial Mediterranean Fever and Hyper-IgD syndrome. This approach has been
agreed with FDA and CHMP. ACZ885 is also being investigated in the fully enrolled pivotal Phase III CANTOS study to determine whether ACZ885 can reduce the risk of recurrent cardiovascular
events (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke) in post-myocardial infarction patients with elevated inflammatory burden versus placebo when administered quarterly in
addition to standard of care.
- •
- Afinitor/Votubia and Afinitor Disperz (RAD001, everolimus) is an oral inhibitor of the mTOR pathway. Phase III studies are underway in patients with advanced breast cancer and diffuse large B-cell lymphoma (PILLAR-2). The EXIST-3 (EXamining everolimus In a Study of TSC) clinical trial is underway to evaluate the efficacy and safety of everolimus in patients with TSC who have refractory partial-onset seizures (uncontrollable seizures localized to a specific area of the brain). Results from the pivotal Phase III RADIANT-4 (RAD001 In Advanced Neuroendocrine Tumors) trial were presented in 2015 at a European medical congress and showed that Afinitor reduced the risk of progression by 52% versus placebo in patients with advanced, progressive, nonfunctional neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin. The safety results were in line with the known safety profile of Afinitor. In October 2015, the FDA granted priority review to Afinitor for use in advanced, progressive, non-functional neuroendocrine tumors of gastrointestinal or lung origin. Results from the RADIANT-4 trial were published in The Lancet in December 2015.
53
- •
- AMG 334 is a fully human monoclonal antibody which is part of a new class of compounds targeting Calcitonin-Gene-Related-Peptide
(CGRP) being investigated for the prevention of migraine. AMG 334 inhibits the activity of CGRP by targeting its receptor, which is believed to transmit signals to cause the pain associated with
migraine. Data announced in May 2015 showed that AMG 334 met its primary endpoint of reduction of monthly mean migraine days compared with placebo in a Phase II trial for the prevention of
episodic migraine. AMG 334 is currently being evaluated in a large global Phase II trial in the prevention of chronic migraine and in two large global Phase III studies to further assess
its safety and efficacy in the prevention of episodic migraine. Novartis and Amgen entered into a collaboration agreement in August 2015 with respect to the development and commercialization of
Amgen's proprietary monoclonal antibody AMG 334.
- •
- Arzerra (ofatumumab) is a human monoclonal antibody that is designed to target the
CD20 molecule found on the surface of chronic lymphocytic leukemia (CLL) cells and normal B lymphocytes. In 2015, results from the Phase III COMPLEMENT 2 study showed that treatment with
ofatumumab plus fludarabine and cyclophosphamide significantly improved median progression-free survival by 54% compared to treatment with fludarabine and cyclophosphamide alone in patients with
relapsed CLL. In addition, results from the Phase III PROLONG study evaluating ofatumumab maintenance therapy versus no further treatment (observation) in patients with relapsed CLL who
responded to induction treatment at relapse formed the basis for submissions made in 2015 to the EMA and FDA for this indication. In September 2015, the FDA granted Priority Review for ofatumumab as
maintenance therapy in relapsed CLL, and in January 2016 the FDA approved Arzerra for extended treatment of patients who are in complete or partial
response after at least two lines of therapy for recurrent or progressive CLL. A Phase III trial is also underway to investigate ofatumumab in refractory non-Hodgkin's lymphoma. In November
2015, Genmab announced that the Phase III study of single-agent ofatumumab compared to single-agent rituximab in patients with follicular non-Hodgkin's lymphoma that has relapsed at least six
months after completion of treatment with a rituximab-containing regimen will be stopped early. The decision to stop the study was made after a planned interim analysis performed by an independent
data monitoring committee showed that it was unlikely that ofatumumab would show superiority if the trial were to be completed as planned. Arzerra is
marketed under a license agreement between Genmab and Novartis. Arzerra for oncology indications was acquired from GSK as part of the
previously-announced portfolio transformation transactions. In December 2015, we acquired all remaining rights from GSK to develop ofatumumab for multiple sclerosis and other autoimmune indications,
disclosed as OMB157.
- •
- BAF312 (siponimod) is an oral, second-generation sphingosine 1-phosphate receptor modulator in Phase III development for
secondary progressive multiple sclerosis. BAF312 binds selectively to the sphingosine 1-phosphate receptor subtypes 1 and 5, distributes effectively to the brain where it may modulate central
S1P1,5 receptors to impact central nervous system inflammation and repair mechanisms. The results from the BOLD study, an adaptive dose-ranging Phase II study, were published in Lancet
Neurology in 2013. These results showed that compared to placebo, BAF312 reduced brain MRI lesions by up to 80% in relapsing-remitting multiple sclerosis and relapses were infrequent and significantly
reduced. BAF312 entered Phase III development in secondary progressive multiple sclerosis in 2012.
- •
- BKM120 (buparlisib) is an orally bioavailable pan-PI3K inhibitor. The PI3K/AKT/mTOR pathway is an important intracellular signaling network that regulates cellular metabolism, proliferation and survival. Abnormal activation of the PI3K/AKT/mTOR pathway has been identified as an important step in the initiation and maintenance of tumors and a key regulator of angiogenesis and upregulated metabolic activities in tumor cells. BKM120 has shown significant cell growth inhibition and induction of apoptosis in a variety of tumor cell lines as well as in animal models. BKM120 is currently being investigated in clinical trials in advanced solid tumors in combination
54
- •
- BYL719 (alpelisib) is an orally bioavailable, alpha isoform-specific PI3K inhibitor. In breast cancer cell lines harboring PIK3CA
mutations, BYL719 has been shown to inhibit the PI3K/AKT/mTOR pathway and have anti-proliferative effects. In addition, cancer cell lines with PIK3CA mutations were more sensitive to alpelisib than
those without the mutation across a broad range of different cancers. BYL719 is in a Phase III study in hormone receptor-positive advanced breast cancer.
- •
- BYM338 (bimagrumab) is a novel, human monoclonal antibody in development to treat sporadic inclusion body myositis (sIBM). In 2013,
FDA granted Breakthrough Therapy designation to BYM338 for sIBM, and we initiated a Phase II/III study of bimagrumab in patients with sIBM. This study showed that in sIBM patients, a single
dose of bimagrumab improved muscle volume at eight weeks (muscle volume for right leg increased 6.5% compared to placebo) and walking distance at 16 weeks. BYM338 binds with high affinity to
activin type II receptors, preventing natural ligands, including myostatin and activin, from binding. BYM338 stimulates muscle growth by blocking signaling from these inhibitory molecules. In
addition to sIBM, BYM338 is in clinical development for multiple acute and chronic muscle-wasting conditions, including recovery from hip fracture and sarcopenia. BYM338 was developed by Novartis, in
collaboration with MorphoSys.
- •
- Cosentyx (secukinumab) is a fully human monoclonal antibody that selectively
neutralizes circulating IL-17A. In January 2016, Cosentyx was approved by the FDA for the treatment of adults with ankylosing spondylitis (AS) and for
the treatment of adults with psoriatic arthritis (PsA). Results for Cosentyx presented at a US medical meeting showed up to 80% of patients with AS had
no radiographic progression in the spine as shown by x-ray assessment over two years. This is the first time that data on structural spinal progression in AS have been presented for an IL-17A
inhibitor. At the same meeting, new data was also presented showing no further progression in joint damage in 84% of patients with PsA over two years of treatment. In addition, the results of the
MEASURE 1 and MEASURE 2 Phase III studies for Cosentyx in AS were published in the New England Journal of Medicine in December 2015. These
pivotal studies demonstrated significant clinical improvements with Cosentyx versus placebo in the signs and symptoms of active AS, and collectively,
the studies form the largest clinical trial program ever conducted in AS, involving 590 patients. Secukinumab is also in Phase III development for non-radiographic axial spondyloarthritis.
- •
- CTL019 (tisagenlecleucel-T) is an investigational therapy that uses chimeric antigen receptors (CARs) to fight cancer. CARs are engineered proteins that transform a patient's own T cells into antigen-specific cells which seek out target proteins present on a patient's cancerous tumor. When these cells are re-introduced into the patient's blood, they demonstrate the potential to bind to the cancer cells and destroy them. CTL019 targets a protein called CD19 that is associated with a number of B-cell malignancies. The latest findings from two ongoing Phase II studies of CTL019 were presented in December 2015 at the American Society of Hematology annual meeting. In a study of relapsed/refractory pediatric acute lymphoblastic leukemia, 55 of 59 patients experienced complete remissions with CTL019. Overall survival was 79% at 12 months and relapse-free survival was 76% at six months and 55% at 12 months. Additionally, 52 of 59 patients developed Grade 1–4 cytokine-release syndrome. In a study of CTL019 in certain relapsed/refractory non-Hodgkin
with other agents, including two Phase III trials in hormone receptor-positive (HR+) advanced breast cancer. Results from the Phase III BELLE-2 trial of BKM120 in patients with HR+, HER2 negative advanced breast cancer were presented in December 2015 at a US breast cancer symposium. In this trial, BKM120 plus fulvestrant led to 6.9 months of progression free survival compared to 5.0 months for placebo plus fulvestrant, a statistically significant difference. The subpopulation of patients with ctDNA PIK3CA mutation experienced a 3.8 month progression-free survival improvement when adding BKM120 to fulvestrant compared to the placebo plus fulvestrant arm. The results of this trial are being discussed with regulatory authorities.
55
- •
- EMA401 is a novel angiotensin II Type 2 receptor (AT2R) antagonist in Phase II development. Targeting
AT2R is an emerging approach to neuropathic pain treatment. AT2R antagonists block the pain signaling pathways in the peripheral nervous system. Positive results from a
Phase II clinical trial of EMA401 in post-herpetic neuralgia, a painful condition that develops in some people following herpes zoster (shingles), were published in a major medical journal in
February 2014. In addition, thus far, EMA401 has not been associated with central nervous system side effects such as dizziness or confusion, which are typically associated with existing therapies.
- •
- Entresto (sacubitril/valsartan), previously known as LCZ696, is a first-in-class
angiotensin receptor/neprilysin inhibitor approved and marketed for the treatment of chronic heart failure with reduced ejection fraction (HFrEF). In addition, Novartis is conducting two large outcome
studies. The first, PARAGON-HF, a Phase III trial of Entresto in patients with chronic heart failure with preserved ejection fraction is
underway, and the second, PARADISE-HF, in patients at high risk for heart failure after a myocardial infarction (MI) is about to start.
- •
- Gilenya (fingolimod) is a sphingosine 1-phosphate receptor modulator approved for the
treatment of relapsing forms of multiple sclerosis. A Phase III study of fingolimod in patients with chronic inflammatory demyelinating polyradiculoneuropathy was initiated in 2012. Submissions
to health authorities in this indication are anticipated in 2017.
- •
- LEE011 (ribociclib) is an orally bioavailable, highly selective small molecule inhibitor of cyclin dependent kinase (CDK) 4 and 6. The
compound is in Phase III registration studies in hormone receptor-positive advanced breast cancer with results expected in 2016. LEE011 is also in Phase I and II investigation, with a
number of ongoing studies in solid tumors. LEE011 was discovered by Novartis as part of a drug discovery collaboration with Astex Pharmaceuticals.
- •
- Lucentis (ranibizumab) is an anti-VEGF monoclonal antibody fragment in
Phase III development for the treatment of visual impairment due to choroidal neovascularization secondary to conditions other than age-related macular degeneration and pathologic myopia.
Filings for this indication are expected in 2016.
- •
- OAP030 (pegpleranib; also known as Fovista and E10030) is an oligo-nucleotide aptamer
that inhibits the action of platelet-derived growth factor (PDGF), and has the potential to enhance the symptomatic treatment effect of anti-VEGFs to induce lesion regression, which may result in
vision gains, reduce vision loss and potentially modify the disease in the longer term. The OAP030 Phase III program consists of three clinical trials to evaluate the safety and efficacy of
OAP030 in combination with anti-VEGF drugs for the treatment of neovascular age-related macular degeneration (AMD). The second Phase III trial of pegpleranib in combination with Lucentis for the
treatment of neovascular age-related macular degeneration (nAMD) completed enrollment in October 2015. Initial top-line data from the
OAP030 Phase III clinical program are expected to be available in 2016. In November 2015, Genentech entered into an agreement with Novartis to participate in certain rights related to the
Novartis licensing and commercialization agreement with Ophthotech Corporation for OAP030. We continue to hold the license for the exclusive rights to develop and market OAP030 outside the US and will
remain responsible for the development and commercialization for OAP030 outside of the US. Genentech will share certain risks and benefits with Novartis.
- •
- OMB157 (ofatumumab) is a fully human monoclonal antibody administered by subcutaneous injection in development for MS. OMB157 works by binding to the CD20 molecule on the B cell surface and inducing B cell depletion. Positive phase IIb results in MS patients were presented in
lymphomas, an overall response rate of 73% (8/11) was observed in patients with follicular lymphoma and an overall response rate of 47% (7/15) in patients with diffuse large B-cell lymphoma. Four patients developed cytokine-release syndrome of grade 3 or higher at peak T cell expansion.
56
- •
- PKC412 (midostaurin) is an oral, multi-targeted kinase inhibitor in Phase III development for treatment of patients with
FLT3-mutated acute myeloid leukemia (AML) and in Phase II development for aggressive systemic mastocytosis (ASM) and mast cell leukemia (MCL). The pivotal Phase III RATIFY study of
newly-diagnosed AML patients aged 18 to under 60 who have a FLT3 mutation was presented at a major US congress in 2015. In the RATIFY study, patients who received PKC412 plus standard induction and
consolidation chemotherapy experienced a 23% improvement in overall survival compared to those treated with standard induction and consolidation chemotherapy alone. The median overall survival for
patients in the PKC412 treatment group was 74.7 months, versus 26.0 months for patients in the placebo group. This study evaluated the addition of either PKC412 or placebo to
daunorubicin/cytarabine in the induction phase, followed by high-dose cytarabine in the consolidation phase. Patients who achieved complete remission after consolidation chemotherapy continued
treatment with PKC412 or placebo as a single agent for up to one year. PKC412 is the first compound to illustrate an overall survival benefit targeting FLT3 in AML. These data are expected to form the
basis for regulatory filings for PKC412 in newly diagnosed FLT3 mutated AML. Filings are expected for newly diagnosed, FLT3-mutated AML and for ASM/MCL in 2016.
- •
- Promacta/Revolade (eltrombopag) is a once-daily oral thrombopoietin receptor agonist
that works by stimulating bone marrow cells to produce platelets. Promacta/Revolade is currently under review in the EU for pediatric immune
thrombocytopenia. Phase II and III studies to investigate eltrombopag in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) associated thrombocytopenia do not support registration
of Promacta/Revolade in intermediate-1, 2 and high risk MDS and/or AML. We are evaluating the data from both trials to assess whether ongoing
development of Promacta/Revolade in these patient populations is warranted.
- •
- RLX030 (serelaxin), the first in a new class of medicines, is a recombinant form of the human hormone relaxin-2, and is believed to
act through multiple mechanisms on the heart, kidneys and blood vessels. Results from the Phase III RELAX-AHF study show that RLX030 improved symptoms and reduced mortality in patients with
acute heart failure (AHF). Data from the study were presented at the American Heart Association congress in 2012 and published simultaneously in The Lancet showing that RLX030 significantly reduced
dyspnea (or shortness of breath), the most common symptom of AHF, which was the primary objective of the study based on pre-specified protocol criteria. In addition, RLX030 was associated with
reductions in worsening of heart failure and all-cause mortality (a safety endpoint) and in deaths due to cardiovascular causes (an additional pre-specified exploratory endpoint) at the end of six
months. In 2014, the FDA and CHMP each decided that further data would be required in order for marketing authorizations to be granted. A second Phase III study, RELAX-AHF-2, is underway and
aims to replicate the key findings of RELAX-AHF, with cardiovascular mortality as the primary endpoint. Following interim analysis, the Data Monitoring Committee of the RELAX-AHF-2 study recommended
continuing the serelaxin RELAX-AHF-2 trial without changes. Top-line results are expected in 2017, after study completion based on a pre-specified number of cardiovascular events. RLX030 received
regulatory approval from the Ministry of Health in Russia in 2014 and is launched there under the trade name Reasanz.
- •
- Signifor LAR (SOM230, pasireotide) is a somatostatin analogue in development as a
long-acting release formulation for patients with Cushing's disease, with a Phase III study underway.
- •
- Tafinlar (dabrafenib) targets the serine/threonine kinase BRAF in the RAS/RAF/MEK/ERK pathway and Mekinist (trametinib) targets the threonine/tyrosine kinases MEK1 and MEK2 in the
2014 and showed significant reduction in the number of new brain lesions in the first 24 weeks after ofatumumab administration. Novartis plans to initiate a Phase III program for OMB157 in MS in 2016. We expect to make regulatory filings in MS in 2019. Ofatumumab is marketed by Novartis for oncology indications under the brand name Arzerra.
57
- •
- Tasigna (nilotinib) is a selective tyrosine-kinase inhibitor that inhibits the BCR-ABL
protein produced by the Philadelphia chromosome, which is found in most people who have chronic myeloid leukemia (CML). Novartis has initiated a global clinical trial program to evaluate the potential
for Philadelphia chromosome positive (Ph+) CML patients to maintain deep molecular response after stopping nilotinib. ENESTfreedom, ENESTop, ENESTgoal, and ENESTpath will evaluate the feasibility of
stopping treatment, and achieving successful treatment free remission in patients with Ph+ CML in the chronic phase and deep molecular response on nilotinib. ENESTfreedom and ENESTop are pivotal
trials and have completed enrollment.
- •
- Votrient (pazopanib) is a small molecule tyrosine kinase inhibitor that targets a
number of intracellular proteins to limit tumor growth and cell survival. A phase III trial (PROTECT) is underway to evaluate Votrient for the
adjuvant treatment of patients with localized or locally advanced renal cell carcinoma following nephrectomy.
- •
- Zykadia (LDK378, ceritinib) is a potent and highly selective oral anaplastic lymphoma kinase (ALK) inhibitor that is in development for ALK positive (ALK+) cancers. Two Phase III clinical trials comparing ceritinib with chemotherapy in treatment-naïve and in previously-treated NSCLC patients are ongoing and actively recruiting patients worldwide.
MAP kinase pathway, resulting in dual blockade of this pathway, which is the main escape mechanism for resistance. Phase II studies are underway to evaluate the efficacy and safety of Tafinlar + Mekinist in patients with BRAF V600 mutation positive non-small cell lung cancer (NSCLC). Tafinlar has a Breakthrough Therapy designation from the FDA for treatment of NSCLC patients with BRAF V600E mutations who have received at least one prior line of platinum-containing chemotherapy. In July 2015, the combination therapy Tafinlar + Mekinist also received Breakthrough Therapy designation from the FDA for NSCLC patients with BRAF V600E mutations. A Phase III study is also underway for BRAF V600 mutation positive melanoma patients in the adjuvant setting. Results from a pooled data analysis showed that patients with BRAF V600E/K mutation-positive unresectable or metastatic melanoma treated with Tafinlar + Mekinist experienced longer progression-free survival and overall survival when baseline lactate dehydrogenase (LDH) levels were normal compared to those with elevated LDH levels, further validating the combination for BRAF positive patients with a better prognosis (indicated by a normal LDH level).
Projects Added To And Subtracted From The Development Table Since 2014
Project/Product
|
Potential indication/ Disease area |
Change | Reason | |||
|---|---|---|---|---|---|---|
| ABL001 | Chronic myeloid leukemia | Added | Entered Confirmatory Development | |||
| AMG 334 | Migraine | Added | Collaboration with Amgen announced in September 2015 | |||
| Arzerra | Chronic lymphocytic leukemia (extended treatment) | Added | Acquired from GSK | |||
| Chronic lymphocytic leukemia (relapse) | Added | Acquired from GSK | ||||
| Refractory non-Hodgkin's lymphoma | Added | Acquired from GSK | ||||
58
Project/Product
|
Potential indication/ Disease area |
Change | Reason | |||
|---|---|---|---|---|---|---|
| ASB183 | Solid and hematologic tumors | Added | Acquired from GSK | |||
| BCT197 | Chronic obstructive pulmonary disease | Removed | Transferred to Mereo BioPharma Group Limited | |||
| BGS649 | Obese hypogonadotropic hypogonadism | Removed | Transferred to Mereo BioPharma Group Limited | |||
| BKM120 | Metastatic breast cancer, hormone receptor-positive, aromatase inhibitor resistant, mTOR inhibitor naïve | Now disclosed as metastatic breast cancer, hormone receptor-positive, aromatase inhibitor resistant/mTOR naïve, 2nd line (+ fulvestrant) | ||||
| Metastatic breast cancer, hormone receptor-positive, aromatase inhibitor and mTOR inhibitor resistant | Now disclosed as metastatic breast cancer, hormone receptor-positive, aromatase inhibitor and mTOR inhibitor resistant, 3rd line (+ fulvestrant) | |||||
| BYL719 | Hormone receptor-positive, HER2 negative advanced breast cancer (postmenopausal women), 2nd line (+ fulvestrant) | Added | Entered Confirmatory Development | |||
| CNP520 | Alzheimer's disease | Added | Entered Confirmatory Development | |||
| CTL019 | Adult and pediatric acute lymphoblastic leukemia | Now disclosed as pediatric acute lymphoblastic leukemia | ||||
| Cosentyx (AIN457) | Non-radiographic axial spondyloarthritis | Added | Entered Confirmatory Development | |||
| Psoriatic arthritis | Commercialized | |||||
| Ankylosing spondylitis | Commercialized | |||||
| EMA401 | Neuropathic Pain | Added | Acquired in acquisition of Spinifex Pharmaceuticals, Inc. | |||
| Entresto (LCZ696) | Chronic heart failure with reduced ejection fraction | Commercialized | ||||
59
Project/Product
|
Potential indication/ Disease area |
Change | Reason | |||
|---|---|---|---|---|---|---|
| Post-acute myocardial infarction | Added | Entered Confirmatory Development | ||||
| Jakavi | Polycythemia vera | Commercialized | ||||
| LBH589 (Farydak) | Relapsed or relapsed-and-refractory multiple myeloma | Commercialized | ||||
| LCQ908 | Familial chylomicronemia syndrome | Removed | Development discontinued | |||
| LDE225 (Odomzo) | Advanced basal cell carcinoma | Commercialized | ||||
| LEE011 | Hormone receptor-positive, HER2 negative advanced breast cancer (postmenopausal women) | Now disclosed as hormone receptor-positive, HER2 negative advanced breast cancer (postmenopausal women), 1st line (+ letrozole) | ||||
| Hormone receptor-positive, HER2 negative advanced breast cancer (postmenopausal women), 1st/2nd line (+ fulvestrant) | Added | Entered Confirmatory Development | ||||
| Hormone receptor-positive, HER2 negative advanced breast cancer (premenopausal women) | Now disclosed as hormone receptor-positive, HER2 negative advanced breast cancer (premenopausal women), 1st line, (+ tamoxifen + goserelin or NSAI + goserelin) | |||||
| LGX818 | Solid tumors | Removed | Divested to Array BioPharma Inc. | |||
| LIK066 | Type 2 diabetes | Removed | Development discontinued | |||
| LJN452 | Non-alcoholic steatohepatitis | Added | Entered Confirmatory Development | |||
60
Project/Product
|
Potential indication/ Disease area |
Change | Reason | |||
|---|---|---|---|---|---|---|
| Lucentis | Choroidal neovascularization and macular edema secondary to conditions other than age-related macular degeneration, diabetic macular edema, retinal vein occlusion and pathologic myopia | Now disclosed as choroidal neovascularization secondary to conditions other than age-related macular degeneration and pathologic myopia | ||||
| MEK162 | NRAS mutant melanoma | Removed | Rights returned to Array BioPharma Inc. | |||
| Low-grade serous ovarian cancer | Removed | Rights returned to Array BioPharma Inc. | ||||
| Solid tumors | Removed | Rights returned to Array BioPharma Inc. | ||||
| MEK162 and LGX818 | BRAF mutant melanoma | Removed | MEK162 rights returned to Array BioPharma Inc. | |||
| LGX818 divested to Array BioPharma Inc. | ||||||
| OAP030 (also known as Fovista/E10030) | Wet age-related macular degeneration | Now disclosed as neovascular age-related macular degeneration | ||||
| OMB157 | Relapsing multiple sclerosis | Added | Acquired from GSK | |||
| PIM447 | Hematologic tumors | Added | Entered Confirmatory Development | |||
| Promacta/Revolade | Pediatric immune thrombocytopenia | Added | Acquired from GSK | |||
| QGE031 | Asthma | Removed | Development discontinued | |||
| Chronic spontaneous urticaria/ Inducible urticaria | Added | Entered Confirmatory Development | ||||
| QMF149 | Asthma | Added | Entered Confirmatory Development | |||
| QVM149 | Asthma | Added | Entered Confirmatory Development | |||
| Seebri (NVA237) | Chronic obstructive pulmonary disease | Commercialized | ||||
61
Project/Product
|
Potential indication/ Disease area |
Change | Reason | |||
|---|---|---|---|---|---|---|
| Tafinlar + Mekinist | BRAF V600+ non-small-cell lung cancer | Added | Acquired from GSK | |||
| BRAF V600+ melanoma (adjuvant) | Added | Acquired from GSK | ||||
| BRAF V600+ colorectal cancer | Added | Acquired from GSK | ||||
| Tekturna | Reduction of cardiovascular death/ hospitalizations in chronic heart failure patients | Removed | Development discontinued | |||
| Ultibro (QVA149) | Chronic obstructive pulmonary disease | Commercialized | ||||
| Votrient | Renal cell carcinoma (adjuvant) | Added | Acquired from GSK | |||
| VAY736 | Primary Sjoegren's syndrome | Added | Entered Confirmatory Development | |||
| Zykadia (LDK378) | ALK + advanced non-small cell lung cancer (brain metastases) | Added | Entered Confirmatory Development | |||
| ALK + advanced non-small cell lung cancer (post chemotherapy and post crizotinib) | Commercialized | |||||
| ALK + advanced non-small cell lung cancer (chemotherapy naïve, crizotinib naïve) | Now disclosed as ALK + advanced non-small cell lung cancer (first line, treatment naïve) | |||||
The Pharmaceuticals Division sells products in approximately 155 countries worldwide. Net sales are generally concentrated in the US, Europe and Japan. However, sales from expanding "emerging growth
62
markets" have become increasingly important to us. The following table sets forth the aggregate 2015 net sales of the Pharmaceuticals Division by region:
Pharmaceuticals
|
2015 Net sales to third parties |
||||||
|---|---|---|---|---|---|---|---|
| |
$ millions |
% |
|||||
Europe |
10,139 | 33 | |||||
United States |
10,279 | 34 | |||||
Asia, Africa, Australasia |
7,224 | 24 | |||||
Canada and Latin America |
2,803 | 9 | |||||
| | | | | | | | |
Total |
30,445 | 100 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Of which in Established Markets* |
22,615 | 74 | |||||
Of which in Emerging Growth Markets* |
7,830 | 26 | |||||
- *
- Emerging Growth Markets comprise all markets other than the Established Markets of the US, Canada, Western Europe, Japan, Australia and New Zealand.
Many of our Pharmaceuticals Division's products are used for chronic conditions that require patients to consume the product over long periods of time, ranging from months to years. Net sales of the vast majority of our products are not subject to material changes in seasonal demand.
The primary goal of our manufacturing and supply chain management program is to ensure the uninterrupted, timely and cost-effective supply of products that meet all product specifications. We manufacture our products at eleven pharmaceutical and four bulk chemical production facilities, as well as one biotechnology site. Bulk chemical production involves the manufacture of therapeutically active compounds, mainly by chemical synthesis or by biological processes such as fermentation. Pharmaceutical production involves the manufacture of "galenic" forms of pharmaceutical products such as tablets, capsules, liquids, ampoules, vials and creams. Major bulk chemical sites are located in Schweizerhalle, Switzerland; Grimsby, UK; Ringaskiddy, Ireland and Changshu, China. Significant pharmaceutical production facilities are located in Stein, Switzerland; Wehr, Germany; Singapore; Torre, Italy; Barbera, Spain and in various other locations. Operational responsibility for biologics manufacturing at our facilities in Huningue, France and Singapore, and at our Sandoz Division facilities in Kundl and Schaftenau, Austria, and Menges, Slovenia, has been brought together within our Pharmaceuticals Division. In addition, we own and operate a Good Manufacturing Practices quality cell processing site in Morris Plains, New Jersey. In 2015, we announced the closing of our site in Resende, Brazil and the downsizing of our site in Ringaskiddy, Ireland, and finalized the divestment of our manufacturing site in Taboão de Serra, Brazil.
During clinical trials, which can last several years, the manufacturing process for a particular product is rationalized and refined. By the time clinical trials are completed and products are launched, the manufacturing processes have been extensively tested and are considered stable. However, improvements to these manufacturing processes may continue over time.
Raw materials for the manufacturing process are either produced in-house or purchased from a number of third party suppliers. Where possible, we maintain multiple supply sources so that the business is not dependent on a single or limited number of suppliers. However, our ability to do so may at times be limited by regulatory or other requirements. We monitor market developments that could have an adverse effect on the supply of essential materials. Our suppliers of raw materials are required to comply with Novartis quality standards.
63
The manufacture of our products is complex and heavily regulated by governmental health authorities, which means that supply is never guaranteed. If we or our third party suppliers fail to comply with applicable regulations then there could be a product recall or other shutdown or disruption of our production activities. We have implemented a global manufacturing strategy to maximize business continuity in case of such events or other unforeseen catastrophic events. However, there can be no guarantee that we will be able to successfully manage such issues when they arise.
The Pharmaceuticals Division serves customers with nearly 2,000 field force representatives in the US, and an additional nearly 20,000 in the rest of the world, as of December 31, 2015, including supervisors and administrative personnel. These trained representatives, where permitted by law, present the therapeutic risks and benefits of our products to physicians, pharmacists, hospitals, insurance groups and managed care organizations. We continue to see increasing influence of customer groups beyond prescribers, and Novartis is responding by adapting our business practices to engage appropriately with such constituencies.
Although specific distribution patterns vary by country, Novartis generally sells its prescription drugs primarily to wholesale and retail drug distributors, hospitals, clinics, government agencies and managed healthcare providers. The growing number of so-called "specialty" drugs in our portfolio has resulted in increased engagement with specialty pharmacies. In the US, specialty pharmacies continue to grow as a distribution channel for specialty products, with an increasing number of health plans mandating use of specialty pharmacies to monitor specialty drug utilization and costs.
In the US, certain products can be advertised by way of television, newspaper and magazine advertising. Novartis also pursues co-promotion/co-marketing opportunities as well as licensing and distribution agreements with other companies when legally permitted and economically attractive.
The marketplace for healthcare is evolving with consumers becoming more informed stakeholders in their healthcare decisions and looking for solutions to meet their changing needs. Where permitted by law, Novartis seeks to assist the patient, delivering innovative solutions to drive education, access, and improved patient care.
As a result of continuing changes in healthcare economics and an aging population, the US Centers for Medicare & Medicaid Services (CMS) is now the largest single payor for healthcare services in the US. In addition, both commercial and government sponsored managed care organizations continue to be one of the largest groups of payors for healthcare services in the US. In other territories, national health services are often the only significant payor for healthcare services. In an effort to control prescription drug costs, almost all managed care organizations and national health services use formularies that list specific drugs that may be reimbursed, and/or the level of reimbursement for each drug. Managed care organizations and national health services also increasingly utilize various cost-benefit analyses to determine whether or not newly-approved drugs will be added to a formulary and/or the level of reimbursement for that drug, and whether or not to continue to reimburse existing drugs. We have dedicated teams that actively seek to optimize formulary positions for our products.
The global pharmaceutical market is highly competitive, and we compete against other major international corporations which sell patented prescription pharmaceutical products, and which have substantial financial and other resources. Competition within the industry is intense and extends across a wide range of commercial activities, including pricing, product characteristics, customer service, sales and marketing, and research and development.
In addition, as is the case with other pharmaceutical companies selling patented pharmaceuticals, Novartis faces ever-increasing challenges from companies selling products which compete with our
64
products, including competing patented products and generic forms of our products following the expiry of patent protection. Generic companies may also gain entry to the market through successfully challenging our patents, but we vigorously use legally permissible measures to defend our patent rights. We also face competition from over-the-counter (OTC) products that do not require a prescription from a physician. See also "—Regulation—Price Controls", below.
There is ongoing consolidation in the pharmaceutical industry. At the same time, new entrants are looking to use their expertise to establish or expand their presence in healthcare, including technology companies hoping to benefit as data and data management become increasingly important in our industry.
We are a leader in the pharmaceuticals industry in terms of research and development, including the level of our investment. In 2015, our Pharmaceuticals Division expensed $7.2 billion (on a core basis $7.1 billion) in research and development, which amounted to 24% of the division's net sales. For additional information about research and development expenditures by our Pharmaceuticals Division over the last three years, please see "Item 5. Operating and Financial Review and Prospects—5.A Operating Results—Results of Operations—Research and development."
The discovery and development of a new drug is a lengthy process, usually requiring approximately 10 to 15 years from the initial research to bringing a drug to market, including approximately six to eight years from Phase I clinical trials to market entry. At each of these steps, there is a substantial risk that a compound will not meet the requirements to progress further. In such an event, we may be required to abandon a compound in which we have made a substantial investment.
We manage our research and development expenditures across our entire portfolio in accordance with our strategic priorities. We make decisions about whether or not to proceed with development projects on a project-by-project basis. These decisions are based on the project's potential to meet a significant unmet medical need or to improve patient outcomes, the strength of the science underlying the project, and the potential of the project (subject to the risks inherent in pharmaceutical development) to generate significant positive financial results for the Company. Once a management decision has been made to proceed with the development of a particular molecule, the level of research and development investment required will be driven by many factors, including the medical indications for which it is being developed, the number of indications being pursued, whether the molecule is of a chemical or biological nature, the stage of development, and the level of evidence necessary to demonstrate clinical efficacy and safety.
Research program
Our Research program is conducted by the Novartis Institutes for BioMedical Research (NIBR), which is responsible for the discovery of new medicines. We established NIBR in 2003. The principal goal of our research program is to discover new medicines for diseases with unmet medical need. To do this we focus our work in areas where we have sufficient scientific understanding and believe we have the potential to change the practice of medicine. This requires the hiring and retention of the best talent, a focus on fundamental disease mechanisms that are relevant across different disease areas, continuous improvement in technologies for drug discovery and potential therapies, close alliance with clinical colleagues, and the establishment of appropriate external complementary alliances.
At NIBR's headquarters in Cambridge, Massachusetts, and at sites in Switzerland, Singapore, China and three other US locations, more than 6,000 scientists, physicians and business professionals contribute to research into disease areas such as cardiovascular and metabolism disease, neuroscience, oncology, muscle disorders, ophthalmology, autoimmune diseases, and gastrointestinal diseases. Research platforms such as the Center for Proteomic Chemistry are headquartered at the NIBR site in Basel, Switzerland. In addition, the Novartis Institute for Tropical Diseases, the Friedrich Miescher Institute, and the Genomics
65
Institute of the Novartis Research Foundation focus on basic genetic and genomic research as well as research into diseases of the developing world such as malaria, dengue and African sleeping sickness.
All drug candidates are taken to the clinic via "proof-of-concept" trials to enable rapid testing of the fundamental efficacy of the drug while collecting basic information on pharmacokinetics, safety and tolerability, and adhering to the guidance for early clinical testing set forth by health authorities. Following proof-of-concept, our Pharmaceuticals Division conducts confirmatory trials on the drug candidates.
In 2012, Novartis and the University of Pennsylvania (Penn) announced an exclusive global research and development collaboration to develop and commercialize targeted chimeric antigen receptor (CAR) immunotherapies for the treatment of cancers. The research component of this collaboration focuses on accelerating the discovery and development of additional therapies using CAR immunotherapy. In September 2014, as part of its alliance with Novartis, Penn announced plans for the construction of the Center for Advanced Cellular Therapeutics (CACT) on the Perelman School of Medicine campus in Philadelphia, Pennsylvania. The CACT is planned to be a first of its kind research and development center established specifically to develop and manufacture adoptive T cell immunotherapies under the research collaboration guided by scientists and clinicians from NIBR and Penn. Construction of the CACT is expected to be completed in 2016.
In February 2014, we acquired CoStim Pharmaceuticals Inc., a Cambridge, Massachusetts-based, privately held biotechnology company focused on harnessing the immune system to eliminate immune-blocking signals from cancer. This acquisition enhanced our late discovery stage immunotherapy programs directed to several targets, including PD-1.
In January 2015, we announced collaboration and licensing agreements with Intellia Therapeutics for the discovery and development of new medicines using CRISPR genome editing technology and Caribou Biosciences for the development of drug discovery tools. CRISPR, an acronym that stands for clustered regularly interspaced short palindromic repeats, is an approach that allows scientists to easily and precisely edit the genes of targeted cells. In a short period of time it has proven to be a powerful tool for creating very specific models of disease for use in drug discovery and has potential for use as a therapeutic modality for treating disease at the genetic level by deleting, repairing or replacing the genes that cause disease.
In March 2015, we entered into a collaboration with Aduro Biotech focused on the discovery and development of next generation cancer immunotherapies targeting the STING signaling pathway. STING is a signaling pathway that when activated is known to initiate broad innate and adaptive immune responses in tumors. Aduro's novel small molecule cyclic dinucleotides (CDNs) have proven to generate an immune response in preclinical models that specifically attacks tumor cells. In addition, we launched a new research group dedicated to immuno-oncology.
In September 2015, we announced that NIBR's President Dr. Mark Fishman will retire when he reaches his contractual retirement age in March 2016. Dr. James E. Bradner, a physician-scientist from Dana Farber Cancer Institute and Harvard Medical School has been named Dr. Fishman's successor.
In January 2016, we announced a collaboration and licensing agreement with Surface Oncology, which gives Novartis access to four pre-clinical programs in immuno-oncology.
Development program
The focus of our Development program is to determine the safety and efficacy of a potential new medicine in humans. As previously described (see "—Compounds in Development"), we view the development process as generally consisting of an Exploratory phase where "proof of concept" is established, and a Confirmatory phase where this concept is confirmed in large numbers of patients.
Within this paradigm, clinical trials of drug candidates generally proceed through the traditional three phases: I, II and III. In Phase I clinical trials, a drug is usually tested with about 5 to 15 subjects. The
66
tests study the drug's safety profile, including the safe dosage range. The studies also determine how a drug is absorbed, distributed, metabolized and excreted, and the duration of its action. In Phase II clinical trials, the drug is tested in controlled studies of approximately 100 to 300 volunteer patients to assess the drug's efficacy and safety, and to establish the appropriate therapeutic dose. In Phase III clinical trials, the drug is further tested in larger numbers of volunteer patients in clinics and hospitals. In each of these phases, physicians monitor volunteer patients closely to assess the potential new drug's safety and efficacy. The vast amount of data that must be collected and evaluated makes clinical testing the most time-consuming and expensive part of new drug development. The next stage in the drug development process is to seek registration for the new drug. See "—Regulation."
At each of these phases of clinical development, our activities are managed by our Innovation Management Board (IMB). The IMB is responsible for oversight over all major aspects of our development portfolio. In particular, the IMB is responsible for the endorsement of proposals to commence the first clinical trials of a development compound, and of major project phase transitions and milestones following a positive proof of concept outcome, including transitions to full development and the decision to submit a regulatory application to the health authorities. The IMB is also responsible for project discontinuations, for the endorsement of overall development strategy and the endorsement of development project priorities. The IMB is chaired by the Head of Development of our Pharmaceuticals Division and has representatives from Novartis senior management, as well as experts from a variety of fields among its core members and extended membership.
Cell and Gene Therapies
In 2014, Novartis Pharmaceuticals created a franchise focused on the development and commercialization of Cell and Gene Therapies. The Cell and Gene Therapies franchise aims to develop a new approach to treating or potentially curing some patients suffering from a variety of life-threatening diseases, including blood-borne cancers, sickle cell disease, thalassemias and other diseases of the blood by developing a portfolio of new treatments that replace, repopulate and/or reprogram cells, and potentially selectively regulate the immune system. The franchise will initially focus on novel cell therapies and cell-based gene therapies including: Chimeric Antigen Receptor T-Cell technology in immuno-oncotherapy with CTL019, Facilitated Cell Therapy Platform (FCRx) in renal transplantation with FCR001 and stem cell expansion and transplantation with HSC835.
Diagnostics
Recent advances in biology and bioinformatics have led to a much deeper understanding of the underlying genetic drivers of disease and the molecular pathways cancer uses to progress. Novartis is developing new therapies that specifically target the mechanisms responsible for disease. To support these advances, Novartis is developing innovative diagnostic tests that could potentially improve physicians' ability to administer the appropriate treatment to those patients who have the greatest potential to benefit from them. Our Pharmaceuticals Division has two units that support our commitment to advancing precision medicine.
Companion Diagnostics
Our Companion Diagnostics (CDx) function works as an integrated part of the drug development process. CDx brings internal capabilities and resources to bear in the development of new diagnostic tests to support our global program teams and efforts in various disease areas. Additionally, the CDx team forms strategic collaborations with third parties to secure access to technologies and capabilities that fit the requirements of our drug development programs. The CDx unit develops tests to meet high regulatory standards for the approval of companion diagnostics around the world.
67
Genoptix Medical Laboratory
In 2011, Novartis acquired Genoptix Medical Laboratory, located in Carlsbad, California. This organization provides comprehensive diagnostics and informatics services to community-based hematologists and oncologists in the US. As one of the largest hematopathology centers in the US, Genoptix offers comprehensive testing solutions in hematology and solid tumor molecular profiling. Their mission is to create value for the patient and the healthcare system by transforming diagnostic information into actionable clinical insights. Genoptix also provides services to support Novartis and third-party clinical trials.
Alliances and acquisitions
Our Pharmaceuticals Division enters into business development agreements with other pharmaceutical and biotechnology companies and with academic institutions in order to develop new products and access new markets. We license products that complement our current product line and are appropriate to our business strategy. Therapeutic area strategies have been established to focus on alliances and acquisition activities for key disease areas and indications that are expected to be growth drivers in the future. We review products and compounds we are considering licensing using the same criteria as we use for our own internally discovered drugs.
The international pharmaceutical industry is highly regulated. Regulatory authorities around the world administer numerous laws and regulations regarding the testing, approval, manufacturing, importing, labeling and marketing of drugs, and also review the safety and efficacy of pharmaceutical products. Extensive controls exist on the non-clinical and clinical development of pharmaceutical products. These regulatory requirements, and the implementation of them by local health authorities around the globe, are a major factor in determining whether a substance can be developed into a marketable product, and the amount of time and expense associated with that development.
Health authorities, including those in the US, EU and Japan, have high standards of technical evaluation. The introduction of new pharmaceutical products generally entails a lengthy approval process. In all major countries, products must be authorized or registered prior to marketing, and such authorization or registration must subsequently be maintained. In recent years, the registration process has required increased testing and documentation for the approval of new drugs, with a corresponding increase in the expense of product introduction.
To register a pharmaceutical product, a registration dossier containing evidence establishing the safety, efficacy and quality of the product must be submitted to regulatory authorities. Generally, a therapeutic product must be registered in each country in which it will be sold. In every country, the submission of an application to a regulatory authority does not guarantee that approval to market the product will be granted. Although the criteria for the registration of therapeutic drugs are similar in most countries, the formal structure of the necessary registration documents and the specific requirements, including risk tolerance, of the local health authorities can vary significantly from country to country. It is possible that a drug can be registered and marketed in one country while the registration authority in another country may, prior to registration, request additional information from the pharmaceutical company or even reject the product. It is also possible that a drug may be approved for different indications in different countries.
The registration process generally takes between six months and several years, depending on the country, the quality of the data submitted, the efficiency of the registration authority's procedures and the nature of the product. Many countries provide for accelerated processing of registration applications for innovative products of particular therapeutic interest. In recent years, intensive efforts have been made among the US, the EU and Japan to harmonize registration requirements in order to achieve shorter
68
development and registration times for medical products. However, the requirement in many countries to negotiate selling prices or reimbursement levels with government regulators and other payors can substantially extend the time until a product may finally be available to patients.
The following provides a summary of the regulatory processes in the principal markets served by Pharmaceuticals Division affiliates:
United States
In the US, applications for drug registration are submitted to and reviewed by the FDA. The FDA regulates the testing, manufacturing, labeling and approval for marketing of pharmaceutical products intended for commercialization in the US. The FDA continues to monitor the safety of pharmaceutical products after they have been approved for sale in the US market. The pharmaceutical development and registration process is typically intensive, lengthy and rigorous. When a pharmaceutical company has gathered data which it believes sufficiently demonstrates a drug's safety, efficacy and quality, then the company may file a New Drug Application (NDA) or Biologics License Application (BLA), as applicable, for the drug. The NDA or BLA must contain all the scientific information that has been gathered about the drug and typically includes information regarding the clinical experiences of patients tested in the drug's clinical trials. A Supplemental New Drug Application (sNDA) or BLA amendment must be filed for new indications for a previously approved drug.
Once an application is submitted, the FDA assigns reviewers from its staff in biopharmaceutics, chemistry, clinical microbiology, pharmacology/toxicology, and statistics. After a complete review, these content experts then provide written evaluations of the NDA or BLA. These recommendations are consolidated and are used by senior FDA staff in its final evaluation of the NDA or BLA. Based on that final evaluation, the FDA then provides to the NDA or BLA's sponsor an approval, or a "complete response" letter if the NDA or BLA application is not approved. If not approved, the letter will state the specific deficiencies in the NDA or BLA which need to be addressed. The sponsor must then submit an adequate response to the deficiencies in order to restart the review procedure.
Once the FDA has approved an NDA, BLA, sNDA or BLA amendment, the company can make the new drug available for physicians to prescribe. The drug owner must submit periodic reports to the FDA, including any cases of adverse reactions. For some medications, the FDA requires additional post-approval studies (Phase IV) to evaluate long-term effects or to gather information on the use of the product under special conditions.
Throughout the life cycle of a product, the FDA also requires compliance with standards relating to good laboratory, clinical, manufacturing and promotional practices.
European Union
In the EU, there are three main procedures for application for authorization to market pharmaceutical products in the EU Member States, the Centralized Procedure, the Mutual Recognition Procedure and the Decentralized Procedure. It is also possible to obtain a national authorization for products intended for commercialization in a single EU member state only, or for additional indications for licensed products.
Under the Centralized Procedure, applications are made to the EMA for an authorization which is valid for the European Community. The Centralized Procedure is mandatory for all biotechnology products and for new chemical entities in cancer, neurodegenerative disorders, diabetes and AIDS, autoimmune diseases or other immune dysfunctions and optional for other new chemical entities or innovative medicinal products or in the interest of public health. When a pharmaceutical company has gathered data which it believes sufficiently demonstrates a drug's safety, efficacy and quality, then the company may submit an application to the EMA. The EMA then receives and validates the application, and appoints a Rapporteur and Co-Rapporteur to review it. The entire review cycle must be completed
69
within 210 days, although there is a "clock stop" at day 120, to allow the company to respond to questions set forth in the Rapporteur and Co-Rapporteur's Assessment Report. When the company's complete response is received by the EMA, the clock restarts on day 121. If there are further aspects of the dossier requiring clarification, the EMA will then request an Oral Explanation on day 180, in which case the sponsor must appear before the CHMP to provide the requested additional information. On day 210, the CHMP will then take a vote to recommend the approval or non-approval of the application. The final decision under this Centralized Procedure is a European Community decision which is applicable to all Member States. This decision occurs on average 60 days after a positive CHMP recommendation.
Under the Mutual Recognition Procedure (MRP), the company first obtains a marketing authorization from a single EU member state, called the Reference Member State (RMS). In the Decentralized Procedure (DCP) the application is done simultaneously in selected or all Member States if a medicinal product has not yet been authorized in a Member State. During the DCP, the RMS drafts an Assessment Report within 120 days. Within an additional 90 days the Concerned Member States (CMS) review the application and can issue objections or requests for additional information. On Day 90, each CMS must be assured that the product is safe and effective, and that it will cause no risks to the public health. Once an agreement has been reached, each Member State grants national marketing authorizations for the product.
After the Marketing Authorizations have been granted, the company must submit periodic safety reports to the EMA (if approval was granted under the Centralized Procedure) or to the National Health Authorities (if approval was granted under the DCP or the MRP). In addition, several pharmacovigilance measures must be implemented and monitored including Adverse Event collection, evaluation and expedited reporting and implementation, as well as update Risk Management Plans. For some medications, post approval studies (Phase IV) may be required to complement available data with additional data to evaluate long term effects (called a Post Approval Safety Study, or PASS) or to gather additional efficacy data (called a Post Approval Efficacy Study, or PAES).
European Marketing Authorizations have an initial duration of five years. After this time, the Marketing Authorization may be renewed by the competent authority on the basis of re-evaluation of the risk/benefit balance. Once renewed the Marketing Authorization is valid for an unlimited period. Any Marketing Authorization which is not followed within three years of its granting by the actual placing on the market of the corresponding medicinal product ceases to be valid.
Japan
In Japan, applications for new products are made through the Pharmaceutical and Medical Devices Agency (PMDA). Once an NDA is submitted, a review team is formed consisting of specialized officials of the PMDA, including chemistry/manufacturing, non-clinical, clinical and biostatistics. While a team evaluation is carried out, a data reliability survey and Good Clinical Practice/Good Laboratory Practice/Good Manufacturing Practice inspection are carried out by the Office of Conformity Audit and Office of GMP/GQP Inspection of the PMDA. Team evaluation results are passed to the PMDA's external experts who then report back to the PMDA. After a further team evaluation, a report is provided to the Ministry of Health, Labor and Welfare (MHLW), which makes a final determination for approval and refers this to the Council on Drugs and Foods Sanitation which then advises the MHLW on final approvability. Marketing and distribution approvals require a review to determine whether or not the product in the application is suitable as a drug to be manufactured and distributed by a person who has obtained a manufacturing and distribution business license for the type of drug concerned and confirmation that the product has been manufactured in a plant compliant with Good Manufacturing Practices.
Once the MHLW has approved the application, the company can make the new drug available for physicians to prescribe. After that, the MHLW lists its national health insurance price within 60 days (or 90 days) from the approval, and physicians can obtain reimbursement. For some medications, the MHLW requires additional post-approval studies (Phase IV) to evaluate safety, effects and/or to gather information on the use of the product under special conditions. The MHLW also requires the drug's
70
sponsor to submit periodic safety update reports. Within three months from the specified re-examination period, which is designated at the time of the approval of the application for the new product, the company must submit a re-examination application to enable the drug's safety and efficacy to be reassessed against approved labeling by the PMDA.
In most of the markets where we operate, the prices of pharmaceutical products are subject to both direct and indirect price controls and to drug reimbursement programs with varying price control mechanisms. Due to increasing political pressure and governmental budget constraints, we expect these mechanisms to continue to remain robust—and to perhaps even be strengthened—and to have a negative influence on the prices we are able to charge for our products.
Direct efforts to control prices
United States. In the US, as a result of the Patient Protection and Affordable Care Act (ACA), the recurring focus on deficit reduction, and public pressure on elected officials based on recent price increases by certain pharmaceutical manufacturers, there is a significant likelihood of continued actions to control prices. Specifically, one proposal that has been repeatedly advanced would impose a government-mandated pricing formula on both patented and generic medications provided through the Medicare prescription drug benefit (Medicare Part D). In addition, the ACA mandated the creation of a new entity, the Independent Payment Advisory Board (IPAB), which has been granted unprecedented authority to implement broad actions to reduce future costs of the Medicare program. This could include required prescription drug discounts or rebates, which could limit net prices for our products. The Medicare Trustees' Report from July 2015 predicted that the projected 5-year average growth in per capita Medicare program spending could exceed a specified target level as early as 2017. If the Chief Actuary for CMS determines that the projected 5-year average growth rate exceeds the target, the IPAB would then develop savings proposals in 2018 based on a savings target set by the Chief Actuary, to be implemented in 2019. There is also a possibility that government officials will continue to search for additional ways to reduce or control prices.
Europe. In Europe, our operations are subject to significant price and marketing regulations. Many governments are introducing healthcare reforms in a further attempt to curb increasing healthcare costs. In the EU, governments influence the price of pharmaceutical products through their control of national healthcare systems that fund a large part of the cost of such products to consumers. The downward pressure on healthcare costs in general in the EU, particularly with regard to prescription drugs, has become very intense. Increasingly strict analyses are applied when evaluating the entry of new products, and, as a result, payors are more frequently limiting access to innovative medicines based on these strict cost-benefit analyses. In addition, prices for marketed products are referenced within Member States and across Member State borders, including new EU Member States, further impacting individual EU Member State pricing.
Japan. In Japan, the government generally introduces price cuts every other year, and the government additionally mandates price decreases for specific products. In 2014, the National Health Insurance price calculation method for new products and the price revision rule for existing products were reviewed, and the resulting new drug tariffs became effective beginning April 2014. The Japanese government is currently undertaking a healthcare reform initiative with a goal of sustaining the universal coverage of the National Health Insurance program, and is addressing the efficient use of drugs, including promotion of generic use. Meanwhile, the government tentatively initiated a premium system which basically maintains the price of patented drugs for unmet medical needs in order to promote innovative new drug creation and the solution of the unapproved indication issue. The continuance of this system will be reviewed as a part of price reforms in 2016. In addition, the MHLW has proposed extraordinary price cuts in 2016 for certain products the sales of which have increased substantially more than official forecasts.
71
Rest of World. Many other countries around the world are also taking steps to control prescription drug prices. As an example, China, one of our most important emerging growth markets, organized tendering in every province, with requested drug price reductions of up to 20% in 2015. Drug prices in China may further decline due to a stated national policy of reducing healthcare costs, including recent strategic initiatives implemented at the province level specifically designed to reduce drug prices. China has also been monitoring drug pricing for irregularities in the market. Although the ultimate impact of this monitoring on the regulatory and pricing framework is not yet clear, China is developing a new pricing framework in which price reductions remain a consistent national priority.
Regulations favoring generics and biosimilars
In response to rising healthcare costs, many governments and private medical care providers, such as Health Maintenance Organizations, have instituted reimbursement schemes that favor the substitution of generic pharmaceuticals for more expensive brand-name pharmaceuticals. In the US, generic substitution statutes have been enacted by virtually all states and permit or require the dispensing pharmacist to substitute a less expensive generic drug instead of an original patented drug. Other countries have similar laws, including numerous European countries. We expect that the pressure for generic substitution will continue to increase. In addition, the US, EU and other jurisdictions are increasingly developing laws and regulations encouraging the development of biosimilar versions of biologic drugs, which can also be expected to have an impact on pricing.
Cross-Border Sales
Price controls in one country can also have an impact in other countries as a result of cross-border sales. In the EU, products which we have sold to customers in countries with stringent price controls can in some instances legally be re-sold to customers in other EU countries with less stringent price controls at a lower price than the price at which the product is otherwise available in the importing country. In North America, products which we have sold to customers in Canada, which has relatively stringent price controls, are sometimes re-sold into the US, again at a lower price than the price at which the product is otherwise sold in the US. Such imports from Canada and other countries into the US are currently illegal.
We expect that pressures on pricing will continue worldwide, and will likely increase. Because of these pressures, there can be no certainty that, in every instance, we will be able to charge prices for a product that, in a particular country or in the aggregate, would enable us to earn an adequate return on our investment in that product.
We attach great importance to patents, trademarks, copyrights and know-how, including research data, in order to protect our investment in research and development, manufacturing and marketing. It is our policy to seek the broadest protection available under applicable laws for significant product developments in all major markets. Among other things, patents may cover the products themselves, including the product's active ingredient and its formulation. Patents may cover processes for manufacturing a product, including processes for manufacturing intermediate substances used in the manufacture of the products. Patents may also cover particular uses of a product, such as its use to treat a particular disease, or its dosage regimen. In addition, patents may cover assays or tests for certain diseases or biomarkers, which will improve patient outcomes when administered certain drugs, as well as assays, research tools and other techniques used to identify new drugs. The protection offered by such patents extends for varying periods depending on the grant and duration of patents in the various countries or region. The protection afforded, which may vary from country to country, depends upon the type of patent and its scope of coverage. Even though we may own, co-own or in-license patents protecting our products,
72
and conduct pre-launch freedom-to-operate analyses, a third party may nevertheless claim that one of our products infringes a third party patent for which we do not have a license.
In addition to patent protection, various countries offer data or marketing exclusivities for a prescribed period of time. Data exclusivity may be available which would preclude a potential competitor from filing a regulatory application for a set period of time that relies on the sponsor's clinical trial data, or the regulatory authority from approving the application. The data exclusivity period can vary depending upon the type of data included in the sponsor's application. When it is available, market exclusivity, unlike data exclusivity, precludes a competitor from obtaining marketing approval for a product even if a competitor's application relies on its own data. Data exclusivity and other regulatory exclusivity periods generally run from the date a product is approved, and so their expiration dates cannot be known with certainty until the product approval date is known.
In the US and other countries, pharmaceutical products are eligible for a patent term extension for patent periods lost during product development and regulatory review. The law recognizes that product development and review by the FDA and other health authorities can take an extended period, and permits an extension of the patent term for a period related to the time taken for the conduct of clinical trials and for the health authority's review. However, the length of this extension and the patents to which it applies cannot be known in advance, but can only be determined after the product is approved.
Patents, patent term extensions and marketing exclusivities can be challenged through various proceedings that depend on the country. For example, patents in the US can be challenged in the United States Patent and Trademark Office (USPTO) through various proceedings, including Inter Partes Review (IPR) proceedings. They may also be challenged through patent infringement litigation under the Hatch-Waxman Act. See generally, "—Sandoz—Intellectual Property" In the EU, EU patents may be challenged through oppositions in the European Patent Office (EPO) or national patents may be challenged in national courts or national patent offices. In Japan, patents may be challenged in the Japanese patent office and in national courts.
United States
Patents
In the US, a patent issued for an application filed today will receive a term of 20 years from the application filing date, subject to potential patent term adjustments for USPTO delay. A US pharmaceutical patent which claims a product, method of treatment using a product, or method of manufacturing a product, may also be eligible for a patent term extension based on the time the FDA took to approve the product. This type of extension may only extend the patent term for a maximum of 5 years, and may not extend the patent term beyond 14 years from regulatory approval. Only one patent may be extended for any product based on FDA delay.
In practice, however, it is not uncommon for significantly more than the 5 year maximum patent extension period to pass between the time that a patent application is filed for a product and the time that the product is approved by the FDA. As a result, it is rarely the case that, at the time a product is approved by FDA, it will have the full 20 years of remaining patent life. Rather, in our experience, it is not uncommon that, at the date of approval, a product will have from 13 to 16 years of patent life remaining, including all extensions available at that time.
Data and Market Exclusivity
In addition to patent exclusivities, the FDA may provide data or market exclusivity for a new chemical entity or an "orphan drug," each of which run in parallel to any patent protection. Regulatory data protection or exclusivity prevents a potential generic competitor from relying on clinical trial data which were generated by the sponsor when establishing the safety and efficacy of its competing product. Market exclusivity prohibits any marketing of the same drug for the same indication.
73
- •
- A new small-molecule active pharmaceutical ingredient shall have 5 years of regulatory data exclusivity, during which time a
competitor generally may not submit an application to the FDA based on a sponsor's clinical data.
- •
- Orphan drug exclusivity provides 7 years of market exclusivity for drugs designated by the FDA as "orphan drugs," meaning drugs
that treat rare diseases, as designated by the FDA. During this period, a potential competitor may not market the same drug for the same indication even if the competitor's application does not rely
on data from the sponsor.
- •
- A new biologic active pharmaceutical ingredient shall have 12 years of market exclusivity, during which time a competitor may
not market the same drug for the same indication.
- •
- The FDA may also request that a sponsor conduct pediatric studies, and in exchange will grant an additional 6-month period of pediatric market exclusivity, if the FDA accepts the data, the sponsor makes a timely application for approval for pediatric treatment, and the sponsor has either a patent-based or regulatory-based exclusivity period for the product which can be extended.
European Community
Patents
Patent applications in Europe may be filed in the EPO or in a particular country in Europe. The EPO system permits a single application to be granted for the EU, plus other non-EU countries, such as Switzerland and Turkey. When the EPO grants a patent, it is then validated in the countries that the patent owner designates. A patent granted by the EPO or a European country office will expire no later than 21 years from the earliest patent application on which the patent is based. Pharmaceutical patents can also be granted a further period of exclusivity under the Supplementary Protection Certificate (SPC) system. SPCs are designed to compensate the owner of the patent for the time it took to receive marketing authorization by the European Health Authorities. An SPC may be granted to provide, in combination with the patent, up to 15 years of exclusivity from the date of the first European marketing authorization. However, an SPC cannot last longer than 5 years. The SPC duration can additionally be extended by a further 6 months if the product is the subject of an agreed pediatric investigation plan. The post-grant phase of patents, including the SPC system, is currently administered on a country-by-country basis under national laws which, while differing, are intended to, but do not always, have the same effect.
As in the US, in practice, it is not uncommon for the granting of an SPC to not fully compensate the owner of a patent for the time it took to receive marketing authorization by the European health authorities. Rather, since it can often take from 5 to 10 years to obtain a granted patent in Europe after the filing of the application, and since it can commonly take longer than this to obtain a marketing authorization for a pharmaceutical product in Europe, it is not uncommon that a pharmaceutical product, at the date of approval, will have a patent lifetime of 10 to 15 years, including all extensions available at that time.
Data and Market Exclusivity
In addition to patent exclusivity, the EU also provides a system of regulatory data exclusivity for authorized human medicines, which runs in parallel to any patent protection. The system for drugs being approved today is usually referred to as "8+2+1" because it provides: an initial period of 8 years of data exclusivity, during which a competitor cannot rely on the relevant data; a further period of 2 years of market exclusivity, during which the data can be used to support applications for marketing authorization, but the competitive product cannot be launched; and a possible 1-year extension of the market exclusivity period if, during the initial 8-year data exclusivity period, the sponsor registered a new therapeutic indication with "significant clinical benefit." This system applies both to national and centralized authorizations. This system has been in force since 2005, therefore some medicines remain covered by the previous system in which EU member states provided either 6 or 10 years of data exclusivity.
74
The EU also has an orphan drug exclusivity system for medicines similar to the US system. If a medicine is designated as an "orphan drug," then it benefits from 10 years of market exclusivity after it is authorized, during which time a similar medicine for the same indication will not receive marketing authorization.
Japan
In Japan, a patent can be issued for active pharmaceutical ingredients. Although methods of treatment, such as dosage and administration, are not patentable in Japan, pharmaceutical compositions for a specific dosage or administration method are patentable. Processes to make a pharmaceutical composition are also patentable. The patent term granted is generally 20 years from the filing date of the patent application on which the patent is based. A patent term extension can be granted for up to 5 years under the Japanese Patent Act to compensate for erosion against the patent term caused by the time needed to obtain marketing authorization from the MHLW. Japan also has an 8-year regulatory data protection system called a "re-examination period" and a 10-year orphan drug exclusivity system.
Typically, it takes approximately 7 to 8 years to obtain marketing authorization in Japan. A patent application on a pharmaceutical substance is usually filed shortly before or at the time when clinical testing begins. Regarding compound patents, it commonly takes approximately 4 to 5 years or more from the patent application filing date to the date that the patent is ultimately granted. As a result, it is not uncommon for the effective term of patent protection for an active pharmaceutical ingredient in Japan to be approximately 10 to 15 years, if duly extended.
The following are certain additional details regarding intellectual property protection for selected Pharmaceuticals Division products and compounds in development. Administrative proceedings or litigation to obtain intellectual property, to enforce intellectual property or to resolve challenges to intellectual property are uncertain and unpredictable. In some circumstances a competitor may be able to market a generic version of one of our products despite the existence of our intellectual property by, for example, designing around our intellectual property or marketing the generic product for non-protected indications. Despite data exclusivity protections, a competitor could opt to incur the costs of conducting its own clinical trials and preparing its own regulatory application, and avoid our data exclusivity protection altogether. There is also a risk that some countries may seek to impose limitations on the availability of patent protection for pharmaceutical products, or on the extent to which such protections may be enforced. As a result, there can be no assurance that our intellectual property will protect our products or that we will be able to avoid adverse effects from the loss of intellectual property protection in the future.
For each selected product or compound in development, we identify certain issued, unexpired patents by general subject matter and, in parentheses, years of expiry in, if relevant, the US, EU and Japan that are owned, co-owned or exclusively in-licensed by Novartis and that relate to one or more forms of the product or methods of use. Novartis may own or control additional patents relating to compound forms, formulations, processes, synthesis, purification and detection. For additional information regarding commercial arrangements with respect to these products, see "—Key Marketed Products." Identification of an EU patent refers to national patents in EU countries and/or to the national patents that have been derived from a patent granted by the EPO. We identify unexpired regulatory data protection periods and, in parentheses, years of expiry for selected products and compounds in development if the relevant marketing authorizations have been authorized or granted. The term "RDP" refers to regulatory data protection, regulatory data exclusivity (which in the EU refers to the protections under "8+2+1" regulatory data exclusivity), and to data re-examination protection systems. We also identify certain unexpired patent term extensions, SPCs and marketing exclusivities and, in parentheses, years of expiry if they are granted; their subject matter scope may be limited, and is not specified. We designate them as "pending" if they have been applied for but not granted and years of expiry are estimable. Such pending applications may or may not ultimately be granted. In the case of the EU, grant or authorization of a patent term extension, marketing exclusivity or data protection means grant or authorization in at least
75
one country and possibly pending in others. Marketing exclusivities and patent term extensions include orphan drug exclusivity (ODE), pediatric exclusivity (PE), patent term extension (PTE) and SPC. For each selected product and compound in development, we indicate whether there is current generic competition for one or more product versions or approved indications in each of the major markets for which intellectual property is identified. We also identify ongoing challenges to the disclosed intellectual property that have not been finally resolved without indicating the likelihood of success in each individual case. Resolution of such challenges may include agreements under which Novartis grants licenses permitting marketing of generic versions of our products before expiration of the relevant intellectual property. We disclose certain material terms of certain settlement agreements relating to certain selected products and compounds in development where they could have a material adverse effect on our business. In other cases, certain settlement agreements may contain confidentiality obligations restricting what may be disclosed.
Oncology
- •
- Gleevec/Glivec. US: Patent on polymorphic compound form (2019), PE (2019); patent on GIST method of use (2021), PE (2022); patent on tablet formulation (2018). EU: Patent on compound (2013), SPC (2016), PE (2016); patent on polymorphic compound form (2018); patent on GIST method of use (2021); patent on tablet formulation (2023). Japan: Patent on polymorphic compound form (2019); patent on GIST method of use (2021); patent on tablet formulation (2023).
- •
- Afinitor/Votubia and Afinitor Disperz/Votubia dispersible tablets and Zortress/Certican.
There is currently no generic competition in the US. There is generic competition in Japan and some EU countries. In the US, Novartis has resolved patent litigation with certain generic manufacturers. Novartis has licensed a subsidiary of Sun Pharmaceutical Industries to market a generic version of Gleevec in the US as of February 1, 2016. Additional generic manufacturers have filed ANDAs challenging the US polymorphic compound form patent; the earliest automatic 30-month stay preventing FDA approval will expire in December 2016. Novartis is taking steps in some EU countries to enforce the EU compound patent, the EU polymorphic compound form patent and the EU GIST method of use patent against generic manufacturers. The EU compound patent PE and the EU GIST method of use patent are being challenged in the patent offices and courts of several EU countries.
Afinitor/Votubia and Afinitor Disperz/Votubia dispersible tablets: US: Patent on compound (2014), PTE (2019), PE (2020); patent on tablet formulation (2016), PE (2017); patent on dispersible tablet formulation (2022), PE (2023); patents on antioxidant (2019), PE (2020); patent on TSC/SEGA use (2022), PE 2022); patent on breast cancer use (2022), PE (2022); patent on renal cell carcinoma use (2025), PE (2026); patent on pancreatic neuroendocrine tumor use (2028). EU: Patent on compound (2013), SPC (2018); patent on tablet formulation (2016); patent on dispersible tablet formulation (2022); patent on antioxidant (2019); patent on breast cancer use (2022); patent on pancreatic neuroendocrine tumor use (2026); ODE (Votubia) (2021). Japan: Patent on compound (2013), PTE (2018); patent on tablet formulation (2016); patent on dispersible tablet formulation (2022); patent on antioxidant (2019); patent on breast cancer use (2022); patent on pancreatic neuroendocrine tumor use (2026); patent on renal cell carcinoma use (2022); ODE (tuberous sclerosis) (2022); RDP (2018).
There is currently no generic competition in the US, EU or Japan. In the US, generic manufacturers have filed ANDAs challenging several patents; the earliest automatic 30-month stay preventing FDA approval will expire in October 2017. The US compound patent and antioxidant patents are being challenged in IPR proceedings in the USPTO.
76
- •
- Tasigna. US: Patent on compound (2023); patents on salt forms (2026, 2027, 2028);
patent on polymorph compound form (2026). EU: Patent on compound (2023); patent on salt form (2026); patent on polymorph compound form (2026); ODE (2017). Japan: Patent on compound (2023), PTE (2024);
patent on salt form (2026); patent on polymorph compound form (2026); RDP (2017). There is currently no generic competition in the US, EU or Japan. The EU salt form patent and polymorph compound form
patent are being opposed in the EPO.
- •
- Sandostatin. Sandostatin SC: There is no patent protection in the US, EU or Japan.
There is generic competition in the US, EU and Japan. Sandostatin LAR: US: Patent on microparticles (2017). There is no patent protection in the EU or
Japan. There is currently no generic competition in the US, EU or Japan.
- •
- Exjade and Jadenu. Exjade: US: Patent on compound (2019); patent on method of use (2017). EU: Patent on compound (2017), SPC (2021); patent on tablet formulation (2023); ODE (2016). Japan: Patent on compound (2017), SPC (2021); RDP (2016). There is currently no generic competition in the US, EU or Japan. In the US, Novartis has resolved patent litigation with generic manufacturers relating to Exjade.
- •
- Votrient. US: Patent on compound (2021), PTE (2023), ODE (2019). EU: Patent on
compound (2021), SPC (2025); RDP (2020). Japan: patent on compound (2021), PTE (2025); RDP (2020). There is currently no generic competition in the US, EU or Japan.
- •
- Tafinlar and Mekinist. Tafinlar: US:
Patent on compound (2030); RDP (2018); ODE (2020). EU: RDP (2023). Japan: Patent on compound (2029). There is currently no generic competition in the US, EU or Japan. Mekinist: US: Patent on
compound (2025), pending PTE (2027); patent on method of use (2025); patent on formulation (2032); RDP (2018); ODE (2020). EU:
Patent on compound and method of use (2025), SPC (2029); RDP (2025). Japan: Patent on compound (2025); patent on method of use (2025); patent on formulation (2031). There is currently no generic
competition in the US, EU or Japan. Use of Mekinist with Taflinar or Taflinar with Mekinist: US: Patent on use of Tafinlar
and Mekinist (2030); RDP (2017); ODE 2021. EU: RDP (2025). Japan: Patent on use of Tafinlar and Mekinist
(2030). There is currently no generic competition in the US, EU or Japan.
- •
- Jakavi. EU: Patent on compound (2026), SPC (2027); patent on salt (2028); RDP (2023). Japan: Patent on compound (2026), PTE (2028), pending PTE (2030); patent on salt (2028), PTE (2028), pending PTE (2030); patent on compositions for medical uses (2026), pending PTE (2027); RDP
Zortress/Certican: US: Patent on compound (2014), PTE (2019), PE (2020); patent on tablet formulation (2016), PE (2017); patent on dispersible tablet formulation (2022), PE (2023); two patents on antioxidant (2019, 2019); patents on methods of use (2017, PE (2018)). EU: Patent on compound (2013), SPC (2018); patent on tablet formulation (2016); patent on dispersible tablet formulation (2022); patent on antioxidant (2019). Japan: Patent on compound (2013), PTE (2018); patent on tablet formulation (2016); patent on dispersible tablet formulation (2022); patent on antioxidant (2019).
There is currently no generic competition in the US, EU or Japan. In the US, generic manufacturers have filed ANDAs challenging several patents; the earliest automatic 30-month stay preventing FDA approval will expire in March 2017. The US compound patent and a method of use patent are being challenged in IPR proceedings in the USPTO.
Jadenu: The compound patents in the US, EU and Japan and the US method of use patent identified for Exjade also protect Jadenu. There is currently no generic competition in the US, EU or Japan. In the US, a generic manufacturer has filed an ANDA challenging the US compound patent; the earliest automatic 30-month stay preventing FDA approval will expire in May 2018.
77
- •
- Promacta/Revolade. US: Patent on compound (2021), PTE (2022), PE (2023); patent on
salt form (2025); patent on formulation (2027). EU: Patent on compound (2021), SPC (2025); patent on salt form (2023); patent on formulation (2027). Japan: Patent on compound (2021), PE (2025); patent
on salt form (2023); patent on formulation (2027). There is currently no generic competition in the US, EU or Japan.
- •
- Farydak. US: Patent on compound (2021), pending PTE (2026); patent on method of use
(2026); patent on crystalline salt (2028); RDP (2020). EU: Patent on compound (2021), pending SPC (2026); patent on method of use (2026); RDP (2025). Japan: Patent on compound (2021), pending PTE
(2026); patent on method of use (2026); RDP (2023). There is currently no generic competition in the US, EU or Japan.
- •
- Odomzo. US: Patent on compound (2029), pending PTE (2029); patent on salt form (2029);
RDP (2020). EU: Patent on compound (2027), pending SPC (2030); patent on salt form (2029); RDP (2025). Japan: Patent on compound (2027); patent on salt form (2029). There is currently no generic
competition in the US, EU or Japan.
- •
- Arzerra. US: Patent on compound (2031); RDP (2023). EU: Patent on compound (2023), SPC (2025); RDP (2021). Japan: Patent on compound (2023), PTE (2025); patent on formulation (2028); RDP (2019). There is currently no generic competition in the US, EU or Japan.
(2022). There is currently no generic competition in the EU or Japan. The EU salt patent is being opposed in the EPO.
Cardio-Metabolic
- •
- Galvus and Eucreas. EU: Patent on
compound (2019), SPC (2022); patent on combination (2021), SPC (2022); patent on Eucreas formulation (2026); RDP (2017). Japan: Patent on compound
(2019), PTE (2024), pending PTE (2024); patent on combination (2021); patent on Galvus formulation (2025), PTE (2025); patent on Eucreas formulation (2026),
pending PTE (2028); Galvus RDP (2018); Eucreas RDP (2019). Galvus/Eucreas is not marketed in the US. There
is currently no generic competition
in the EU or Japan. The EU Eucreas formulation patent is being opposed in the EPO.
- •
- Entresto. US: Patents on combination (2023); patent on complex (2027); RDP (2020). EU: Patent on combination (2023); patent on complex (2026); patent on formulation (2028); RDP (2025). Japan: Patent on combination (2023); patent on complex (2026); patent on formulation (2028). There is currently no generic competition in the US, EU or Japan. The EU complex patent and the EU formulation patent are being opposed in the EPO.
Immunology and Dermatology
- •
- Neoral. There is no patent protection for Neoral in the US, EU or Japan. There is generic competition in the US, EU and
Japan.
- •
- Myfortic. US: Patent on formulation (2017), PTE (2018); patent on particle size
(2024). EU: Patent on formulation (2017), SPC (2017); patent on formulation (2022); patent on particle size (2024). There is generic competition in the US. There is currently no generic competition in
the EU. In the EU, Novartis has resolved patent litigation with a generic manufacturer. The EU formulation patent and particle size patent are being opposed in the EPO.
- •
- Xolair. US: Patent on compound (2018); patent on lyophilized formulation (2016), PTE (2017); patents on syringe formulation (2021, 2024). EU: Patent on compound (2012), SPC (2017); patent on lyophilized formulation (2016); patents on syringe formulation (2021, 2024). Japan: Patent on compound (2012), PTE (2017); patent on lyophilized formulation (2016); patents on syringe formulation (2021, 2024); RDP (2017). There is currently no generic competition in the US, EU or
78
- •
- Cosentyx. US: Patent on compound (2027), pending PTE (2029); RDP (2027). EU: Patent on compound (2025), pending SPC (2030), pending PE (2030); RDP (2026). Japan: Patent on compound (2025), PTE (2029); patent on method of use (2031), PTE (2032); RDP (2022). There is currently no generic competition in the US, EU, or Japan.
Japan. The EU syringe formulation patent and lyophilized formulation patent are being opposed in the EPO.
Retina
- •
- Lucentis. EU: Patent on compound (2018), SPC (2022); patent on method of use (2016). Japan: Patent on compound (2018), PTE (AMD indication) (2019), PTE (other indications) (2023). There is currently no generic competition in the EU or Japan.
Neuroscience
- •
- Gilenya. US: Patent on compound (2014), PTE (2019); patent on formulation (2026);
patent on dose (2027). EU: Patent on compound (2013), SPC (2018); RDP (2021); patent on formulation (2024), SPC (2026). There is currently no generic competition in the US or EU. In the US, generic
manufacturers have filed ANDAs challenging the US compound patent and formulation patent; the earliest automatic 30-month stays preventing FDA approval will expire in March 2018. Generic manufacturers
have filed ANDAs challenging the US dose patent. The US formulation patent is being challenged in an IPR proceeding in the USPTO.
- •
- Exelon/Exelon Patch. Exelon: There is no patent protection for Exelon capsules in the US, EU or Japan. There is generic competition in the US, EU and Japan. Exelon Patch: US: Patents on formulations (2019). EU: Patent on formulation (2019); patent on transdermal dosage regime (2026). Japan: Patent on formulation (2019); RDP (2019). There is generic competition in the US and in most EU countries. There is currently no generic competition in Japan. We are taking steps in several countries to enforce our EU transdermal dosage regime patent against generic competitors. The EU transdermal dosage regime patent is being opposed in the EPO and several national patents are being challenged in national courts. In the US, generic manufacturers have filed ANDAs challenging the US formulation patents; the earliest automatic 30-month stays preventing FDA approval expires in 2017. The US formulation patents are being challenged in an IPR proceeding in the USPTO.
Established Medicines
- •
- Diovan and Co-Diovan/Diovan HCT.
Diovan: US: Patent on formulation (2017), PE (2017). There is generic competition in the US, EU and Japan. Co-Diovan/Diovan HCT:
US: Patent on formulation (2017), PE (2017). Japan: Patent on compound (2011), PTE for Co-Diovan (2016); patent on formulation (2017). In Japan,
Novartis has resolved patent litigation with a generic manufacturer. There is generic competition in the US and EU. There is currently no generic competition in Japan.
- •
- Exforge and Exforge HCT. Exforge: US: Patent on Exforge combination (2019). EU: Patent on Exforge combination/Exforge HCT combination (2019); RDP (2017). There is generic competition in the US and Japan. There is currently no generic competition in the EU. The EU Exforge combination/Exforge HCT combination patent is being challenged in the patent offices of some EU countries. We are taking steps to enforce the EU Exforge combination/Exforge HCT combination patent against generic manufacturers seeking to market Exforge. Exforge HCT: US: Patent on Exforge HCT combination (2023). EU: patent on Exforge combination/Exforge HCT combination (2019); RDP (2019). Japan: Patent on Exforge HCT combination (2023). There is generic
79
- •
- Voltaren/Cataflam. There is no patent protection in the US, EU or Japan. There is
generic competition in the US, EU and Japan.
- •
- Ritalin LA/Focalin XR. US: Patent on drug-delivery formulation (2019). EU: Patent on dose (2018); patent on drug-delivery formulations (2019). Japan: Patent on dose (2018); patent on drug-delivery formulation (2019). There is generic competition in the US for Ritalin LA and Focalin XR. There is currently no generic competition in the EU or Japan. The EU formulation patent is being opposed in the EPO.
competition in the US. There is currently no generic competition in the EU. Exforge HCT is not currently marketed in Japan.
Compounds in Development
We provide the following information regarding our compounds in Phase III clinical development, if any, that have been submitted for registration to the FDA or the EMA: As of the date of this 20-F, the only compounds that we have in Phase III clinical development that have been submitted for registration to the FDA or the EMA are compounds that have previously been approved by FDA or EMA, and have been submitted for the approval of one or more additional indications. See above for intellectual property information regarding our selected Pharmaceuticals Division products.
Our Alcon Division is a leader in the research, development, manufacturing and marketing of eye care products worldwide, and its products are available in more than 180 markets. In 2015, the Alcon Division had consolidated net sales of $9.8 billion representing 20% of total Group net sales.
Alcon offers an extensive breadth of products serving the full lifecycle of patient needs across eye diseases, vision conditions and refractive errors. To meet the needs of patients, ophthalmologists, surgeons, optometrists, opticians and physician specialists, Alcon operates with three franchises: Surgical, Ophthalmic Pharmaceuticals and Vision Care. Each franchise operates with specialized sales forces and marketing support.
To accelerate growth, we are taking concerted action on two fronts. For the Surgical and Vision Care franchises, we have identified key actions as part of a growth plan. They include steps to optimize innovation in intraocular lenses (IOLs) for cataract surgery, prioritizing and investing in the development of promising new products, and improving the effectiveness of our sales force.
In addition, we plan to strengthen our ophthalmic medicines business by transferring Ophthalmic Pharmaceuticals products from Alcon to our Pharmaceuticals Division, combining expertise in pharmaceuticals development and marketing with the strong Alcon brand.
Alcon's dedication to research and development is important to our growth plans. As part of our efforts, the Alcon Division works together with the Novartis Institutes for BioMedical Research (NIBR), our global pharmaceutical research organization. This collaboration allows our Alcon Division to leverage the resources of NIBR in an effort to discover and expand ophthalmic pharmaceutical research targets and to develop chemical and biologic compounds for the potential treatment of diseases of the eye, with a particular focus on diseases such as glaucoma and macular degeneration.
In July 2014, Alcon entered into an agreement with Verily (formerly Google Life Sciences) to license its "smart lens" technology with the potential to address ocular conditions. In October 2014, Alcon acquired WaveTec Vision. The acquisition provided Alcon with the ORA System, the first commercialized intra-operative guidance system for cataract surgeons implanting IOLs. Alcon has integrated the ORA System into its existing Cataract Refractive Suite by Alcon.
80
Surgical
Our Alcon Division's Surgical franchise is the market leader in global ophthalmic surgical product revenues, offering ophthalmic surgical equipment, instruments, disposable products and intraocular lenses for surgical procedures that address cataracts, vitreoretinal conditions, glaucoma and refractive errors.
Alcon's Surgical portfolio includes the Cataract Refractive Suite by Alcon, a suite of equipment to help plan and perform some of the most challenging steps of cataract surgery with automation and precision. It is comprised of the Centurion vision system phacoemulsification technology platform; the LenSx laser, a femtosecond laser for increased precision and reproducibility for the corneal incision, capsulorhexis and lens fragmentation steps of the procedure; the Verion image guided system, an ocular surgical planning, imaging and guidance technology; the ORA System, an intra-operative guidance system for IOL implantation during cataract surgery; and the LuxOR LX3 surgical microscope for greater visualization during surgery. The portfolio also includes Contoura vision, the latest vision system in the Wavelight refractive suite portfolio for refractive procedures and LASIK treatments, the Constellation vision system for retinal operations, and the Infiniti vision system to perform cataract surgeries, which is the phacoemulsification platform introduced prior to the Centurion vision system. Alcon also offers the AcrySof family of intraocular lenses (IOLs) to treat cataracts, including the AcrySof IQ, AcrySof IQ PanOptix, AcrySof IQ Toric and AcrySof IQ ReSTOR Toric IOLs. In addition, Alcon provides advanced viscoelastics, surgical solutions, surgical packs and other disposable products for cataract and vitreoretinal surgery.
Ophthalmic Pharmaceuticals
Our Alcon Division's Ophthalmic Pharmaceuticals franchise develops and markets a broad range of pharmaceuticals to treat chronic and acute conditions of the eye including glaucoma, elevated intraocular pressure (associated with glaucoma), eye infection and inflammation, eye allergies, dry eye and retinal diseases. Ophthalmic Pharmaceuticals also oversees the line of professionally driven over-the-counter brands that include artificial tears and ocular vitamins. Product highlights within the Ophthalmic Pharmaceuticals portfolio include Ilevro ophthalmic suspension for the treatment of pain and inflammation associated with cataract surgery; Simbrinza suspension to lower intraocular pressure as a fixed-dose combination; Azopt, Azarga, Travatan Z and DuoTrav, each ophthalmic solutions for the treatment of elevated intraocular pressure associated with open-angle glaucoma or ocular hypertension; Vigamox ophthalmic solution for bacterial conjunctivitis; Pazeo and Pataday ophthalmic solutions for ocular itching associated with allergic conjunctivitis; Nevanac ophthalmic suspension for eye pain and inflammation following cataract surgery and to reduce the risk of macular edema associated with cataract surgery in diabetic patients; the Systane family of over-the-counter products for dry eye relief; and Jetrea intravitreal injection for treating vitreomacular traction.
Vision Care
Our Alcon Division's Vision Care franchise develops and markets contact lenses and lens care products. Alcon's broad portfolio of silicone hydrogel, daily disposables and color contact lenses includes our Air Optix, Dailies and Freshlook brands. Our Dailies product line includes Dailies Total1 lenses, a first-of-its-kind water gradient contact lens. Our Air Optix monthly contact lens product line includes Air Optix Colors silicone hydrogel contact lenses. Our contact lens care solutions business includes the Opti-Free line of multi-purpose disinfecting solutions and drops, as well as the Clear Care and AOSept Plus hydrogen peroxide lens care solutions.
81
Alcon received a number of approvals and launched a number of products in 2015, including:
- •
- AcrySof IQ aspheric intraocular lens with UltraSert Pre-loaded Delivery System was approved in the US and EU to provide a
single-use system for cataract surgery.
- •
- AcrySof IQ PanOptix trifocal intraocular lens was launched in the EU for patients
seeking presbyopia-correction during cataract surgery.
- •
- AcrySof IQ ReSTOR multifocal +2.5D intraocular lens was approved in the US for
patients wanting near, intermediate and distance vision correction during cataract surgery.
- •
- Air Optix Colors contact lenses: silicone hydrogel, color cosmetic monthly contact
lenses were launched in Japan.
- •
- Air Optix Colors contact lenses in plus powers were launched in the US for patients
with hyperopia.
- •
- Air Optix with HydraGlyde contact
lenses received approval in the EU for longer-lasting surface wettability.
- •
- Clear Care Plus/AOSept Plus with HydraGlyde was launched in the US and EU to provide hydrogen
peroxide-based cleaning and disinfecting for contact lenses.
- •
- Contoura vision topography guided, refractive surgical system was launched in the US
for patients seeking myopic and astigmatic vision correction.
- •
- Dailies Total1 contact lenses with plus powers were launched in the US and EU for
patients with hyperopia seeking a daily disposable lens.
- •
- LuxOr ceiling-mounted ophthalmic microscope system was approved in the US for enhanced
visualization during cataract surgery.
- •
- ORA System with VerifEye+ was launched
in the US and EU for enhanced pre-operative planning during cataract surgery.
- •
- Pazeo Solution (olopatadine hydrochloride) for 24-hour ocular allergy itch relief was
approved and launched in the US.
- •
- Systane Hydration lubricant eye drops in unit-dose and multi-dose were launched in the EU for the palliative treatment of dry eye.
82
Key Marketed Products
The following tables set forth certain key marketed products in our Alcon Division. While we intend to sell our marketed products throughout the world, not all products and indications are currently available in every country.
Surgical
Cataract |
AcrySof family of intraocular lenses includes but is not limited to: | |
|
AcrySof IQ ReSTOR, AcrySof IQ PanOptix, AcrySof IQ Toric and | |
|
AcrySof IQ ReSTOR Toric advanced technology intraocular lenses that correct cataracts and distance vision with presbyopia and/or astigmatism | |
|
Cataract Refractive Suite by Alcon designed to streamline the cataract surgical procedure through surgical planning and execution | |
|
Centurion vision system intelligent phacoemulsification technology platform with cataract removal capabilities | |
|
Infiniti vision system with the OZil torsional hand piece for cataract procedures | |
|
LenSx laser used for specific steps in the cataract surgical procedure | |
|
LuxOR microscope used for ophthalmic surgical procedures | |
|
ORA System intra-operative guidance system for intraocular lens implant during cataract surgery | |
|
UltraSert pre-loaded delivery system for intraocular lenses that correct cataracts | |
|
Verion imaged-guided system for use during cataract surgery | |
Vitreoretinal |
Constellation vision system for vitreoretinal operations | |
|
Ultravit vitrectomy probes | |
|
23+, 25+ and 27+ vitrectomy packs | |
|
Purepoint laser system and probes | |
|
Finesse flex loop | |
|
Grieshaber surgical instruments | |
|
Edgeplus blade trocar cannula system | |
|
Ispan gas, Perfluron, Silkon oil: Retina stabilizing adjuncts | |
Refractive |
Allegretto Wave Eye-Q excimer laser for LASIK vision correction | |
|
Contoura vision for LASIK vision correction in patients with myopia and astigmatism | |
|
WaveLight FS200 laser for specific steps in LASIK surgical procedures | |
|
WaveLight EX500 laser for LASIK vision correction | |
Glaucoma |
Ex-press glaucoma filtration device |
In addition, Alcon provides advanced viscoelastics, surgical solutions, surgical packs and other disposable products for cataract and vitreoretinal surgery.
83
Ophthalmic Pharmaceuticals
Glaucoma |
Simbrinza suspension to lower intraocular pressure without a beta blocker | |
|
Izba, Travatan and Travatan Z ophthalmic solutions to lower intraocular pressure | |
|
Azopt ophthalmic suspension to lower intraocular pressure | |
|
DuoTrav ophthalmic solution to lower intraocular pressure (outside US markets) | |
|
Azarga/Azorga ophthalmic suspension to lower intraocular pressure (outside US markets) | |
Anti-Infectives |
Vigamox and Moxeza ophthalmic solution for treatment of bacterial conjunctivitis | |
Anti-Inflammation |
Ilevro suspension to treat pain and inflammation following cataract surgery | |
|
Nevanac ophthalmic suspension to treat pain and inflammation following cataract surgery, and to reduce the risk of macular edema associated with cataract surgery in diabetic patients | |
|
Durezol emulsion to treat pain and inflammation associated with eye surgery, and to treat endogenous anterior uveitis | |
|
TobraDex and TobraDex ST ophthalmic suspensions, combination anti-infective/anti-inflammatory products | |
|
Voltaren ophthalmic solution to treat post-operative inflammation after cataract surgery, and for temporary relief of pain and photophobia after refractive surgery | |
Dry Eye |
The Systane family of over-the-counter dry eye products: | |
|
Systane lubricant eye drops | |
|
Systane Balance lubricant eye drops | |
|
Systane Hydration lubricant eye drops | |
|
Systane Ultra lubricant eye drops | |
|
Systane gel drops | |
|
Systane lid wipes | |
|
Lubricants for eye dryness, discomfort or ocular fatigue: | |
|
GenTeal lubricant eye drops | |
|
Tears Naturale lubricant eye drops | |
Allergy |
Pazeo, Patanol and Pataday ophthalmic solutions for ocular itching associated with allergic conjunctivitis | |
|
Patanase nasal spray for seasonal nasal allergy symptoms | |
|
Zaditor antihistamine eye drops for temporary relief of itchy eyes associated with eye allergies (over-the-counter in the US) | |
|
Zaditen Ophtha an H1-antagonist to fight allergic conjunctivitis | |
|
Livostin an H1-antagonist to fight allergic conjunctivitis (Canada only) | |
Ear Infections |
Ciprodex* otic suspension to treat middle and outer ear infections |
84
Ocular Nutrition |
ICaps eye vitamin dietary supplements provide essential dietary ingredients to support eye health | |
|
Vitalux nutrient supplements to help patients with age-related macular degeneration maintain their vision (outside US markets) | |
Retinal |
Jetrea (ocriplasmin) intravitreal injection for the treatment of vitreomacular traction, including macular hole | |
|
Triesence suspension for visualization during vitrectomy |
- *
- Ciprodex is a registered trademark of Bayer Intellectual Property GmbH.
Vision Care
Contact Lenses |
Air Optix family of silicone hydrogel contact lenses (including Air Optix Colors lenses) | |
|
Dailies family of daily disposable contact lenses (including Dailies Total1 lenses) | |
|
FreshLook family of color contact lenses | |
Contact Lens Care |
Opti-Free PureMoist MPDS | |
|
Opti-Free RepleniSH MPDS | |
|
Opti-Free Express MPDS | |
|
Clear Care Plus cleaning and disinfecting solution (AOSept Plus outside of North America) |
Selected Development Projects
Surgical
Project/Product(1)
|
Mechanism of action |
Potential indication | Planned submission date/Current Phase |
|||
|---|---|---|---|---|---|---|
| AcrySof IQ ReSTOR Toric 2.5D IOL | Multifocal, aspheric and cylinder correcting intraocular lens | Cataractous lens replacement with or without presbyopia, and with astigmatism | 2016 US/Advanced development | |||
| AcrySof IQ ReSTOR Toric 3.0D IOL | Multifocal, aspheric and cylinder correcting intraocular lens | Cataractous lens replacement with or without presbyopia, and with astigmatism | US/Submitted(2) | |||
| AcrySof IQ Aspheric IOL with UltraSert | Pre-loaded intraocular lens delivery device | Cataractous lens replacement | Japan/Submitted(3) |
- (1)
- AcrySof IQ ReSTOR Toric 3.0D diopter range expansion IOL was terminated in July 2015, as
clinical data did not support submissions in the US or Japan.
- (2)
- Submitted
to the FDA in 2014. Additional information regarding clinical data has been requested by the FDA.
- (3)
- Submission pending acceptance by regulatory authority.
85
Ophthalmic Pharmaceuticals
Project/Product
|
Mechanism of action |
Potential indication | Route of Administration |
Planned submission date/Current Phase |
||||
|---|---|---|---|---|---|---|---|---|
| EXE844b (finafloxacin) | Anti-infective | Otitis media-tympanostomy tube surgery | Topical | 2016 US/III | ||||
| Jetrea Ready-Diluted Injection (ocriplasmin) | Alpha-2 antiplasmin reducer | Retina (vitreomacular traction) | Intravitreal injection | 2017 Japan/III | ||||
| Ilevro (nepafenac 0.3%) | Anti-inflammation | Postsurgical macular edema in patients with diabetes | Topical | EU Submitted† 2018 US/III | ||||
| RTH258 (brolucizumab) | Anti-VEGF single-chain antibody fragment | Wet age-related macular degeneration | Intravitreal injection | ³ 2018/III |
- †
- Submission pending acceptance by regulatory authority.
Vision Care
Project/Product
|
Mechanism of action |
Potential indication | Planned submission date/Current Phase |
|||
|---|---|---|---|---|---|---|
| AOSept Plus/Clear Care Plus with HydraGlyde | Disinfection and cleaning | Contact lens care | 2017 Japan/Advanced development |
The principal markets for our Alcon Division include the US, Canada and Latin America, Japan and Europe. The following table sets forth the aggregate 2015 net sales of the Alcon Division by region:
Alcon Division
|
2015 Net Sales to third parties |
||||||
|---|---|---|---|---|---|---|---|
| |
$ millions |
% |
|||||
Europe |
2,408 | 25 | |||||
United States |
4,275 | 44 | |||||
Asia, Africa, Australasia |
2,154 | 22 | |||||
Canada and Latin America |
975 | 9 | |||||
| | | | | | | | |
Total |
9,812 | 100 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Of which in Established Markets* |
7,423 | 76 | |||||
Of which in Emerging Growth Markets* |
2,389 | 24 | |||||
| | | | | | | | |
- *
- Emerging Growth Markets comprise all markets other than the Established Markets of the US, Canada, Western Europe, Japan, Australia and New Zealand.
Sales of certain ophthalmic pharmaceutical products, including those for allergies, anti-inflammatory and dry eye, are subject to seasonal variation. Sales of the majority of our other products are not subject to material changes in seasonal demand.
86
In 2015, our Alcon Division expensed $0.9 billion (on a core basis $0.9 billion) in research and development, which amounted to 9% of the Division's net sales. The Alcon Division expensed $0.9 billion (on a core basis $0.9 billion) and $1.0 billion (on a core basis $0.9 billion) in research and development in 2014 and 2013, respectively. Core results exclude impairments, amortization and certain exceptional items. For additional information, see "Item 5. Operating and Financial Review and Prospects—5.A Operating Results—Non-IFRS Measures as Defined by Novartis—Core Results."
Our Alcon Division associates in research and development work to address diseases and conditions that affect vision, such as cataracts, glaucoma, retina diseases, dry eye, infection, ocular allergies and refractive errors. Alcon's pipeline strategy is built around a proof-of-concept qualification process, which quickly identifies opportunities that have the best chance for technical success and advances those projects, while terminating others with a low probability of success.
In addition, the Novartis Institutes for BioMedical Research (NIBR) is the Novartis global pharmaceutical research organization that works to discover innovative medicines to treat disease and improve human health. See "—Pharmaceuticals—Research and Development." For Alcon's Ophthalmic Pharmaceuticals franchise, NIBR engages in research activities in an effort to discover and expand ophthalmic research targets, and to develop chemical and biologic compounds for the potential treatment of diseases of the eye, with a particular focus on diseases such as glaucoma and macular degeneration. The costs for these activities are allocated to Alcon.
Research and development activities for Alcon's Surgical franchise are focused on expanding intraocular lens capabilities to improve refractive outcomes and on developing instruments for cataract, vitreoretinal and corneal refractive surgeries. The focus for the Vision Care franchise is on the research and development of new contact lens materials, coatings and designs to improve patient comfort, and on lens care solutions that provide the safety, disinfecting and cleaning power needed to help maintain ocular health. As announced in 2014, Alcon is also collaborating with Verily (formerly Google Life Sciences), and has licensed its smart lens technology for ocular medical uses, including the potential to provide an accommodative contact lens/intraocular lens for patients living with presbyopia and to monitor glucose levels in diabetic patients. The Ophthalmic Pharmaceuticals franchise is focused on the development of products for the treatment of retinal diseases, glaucoma (intraocular pressure lowering) and dry eye.
We manufacture our Alcon Division's pharmaceutical products at six facilities in the United States, Belgium, Spain, Brazil and Singapore. Our Alcon Division's surgical equipment and other surgical medical devices are manufactured at nine facilities in the United States, Belgium, Switzerland, Ireland, Germany and Israel. Our Alcon Division's contact lens and certain lens care production facilities are in the US, Canada, Germany, Singapore, Malaysia and Indonesia.
The goal of our supply chain strategy is to efficiently produce and distribute high quality products. The manufacture of our products is heavily regulated by governmental health authorities around the world, including the FDA. In addition to regulatory requirements, many of our products involve technically complex manufacturing processes or require a supply of highly specialized raw materials. For some products and raw materials, we may also rely on a single source of supply.
The manufacture of our products is complex and heavily regulated, which means that supply is never guaranteed. Like some of our competitors, our Alcon Division has faced manufacturing issues and has received Warning Letters relating to such manufacturing issues. In particular, in December 2012, Alcon received an FDA Warning Letter following an inspection at the LenSx laser manufacturing site in Aliso Viejo, California. Alcon responded in writing to the FDA, and in February 2013, FDA responded to Alcon acknowledging that the corrective actions described in Alcon's written response appear to address the items identified in the Warning Letter. The Warning Letter was lifted in May 2014 after all corrective
87
actions were completed. The items noted in the Warning Letter did not affect the safety or effectiveness of the LenSx laser, or impact Alcon's ability to sell the product. If we or our third-party suppliers fail to comply fully with regulations then there could be a product recall or other shutdown or disruption of our production activities. We have implemented a global manufacturing strategy to maximize business continuity in case of such events or other unforeseen catastrophic events. However, there can be no guarantee that we will be able to successfully manage such issues when they arise.
Our Alcon Division conducts sales and marketing activities around the world organized under five operating regions (US, Europe/Middle East/Africa, Latin America/Caribbean/Canada, Asia and Japan). The Alcon Division's global commercial capability is organized around sales and marketing organizations dedicated to the Surgical, Ophthalmic Pharmaceuticals and Vision Care franchises.
Most of our global Alcon marketing efforts are supported by advertising in trade publications and by marketing and sales representatives attending regional and national medical conferences. Marketing efforts are reinforced by targeted and timely promotional materials and direct mail to eye care practitioners in the office, hospital or surgery center setting. Technical service after the sale is provided and an integrated customer relationship management system is in place in many markets. Where applicable in our Ophthalmic Pharmaceuticals and Vision Care franchises, direct-to-consumer marketing campaigns are executed to promote selected products.
While our Alcon Division markets all of its products by calling on medical professionals, direct customers and distribution methods differ across business lines. Although physicians write prescriptions, distributors, wholesalers, hospitals, government agencies and large retailers are the main direct customers for Alcon ophthalmic pharmaceutical products. Alcon surgical products are sold directly to hospitals and ambulatory surgical centers, although Alcon sells through distributors in certain markets outside the US. In most countries, contact lenses are available only by prescription. Our contact lenses can be purchased from eye care professionals, optical chains and large retailers, subject to country regulation. Lens care products can be found in major drugstores, food, mass merchandising and optical retail chains globally, subject to country regulations.
As a result of changes in healthcare economics, managed care organizations are now one of the largest groups of payors for healthcare services in the US. In an effort to control prescription drug costs, almost all managed care organizations use a formulary that lists specific drugs that can be prescribed and/or the amount of reimbursement for each drug. We have a dedicated managed care team that actively seeks to optimize formulary positions for our products.
The eye care industry is highly competitive and subject to rapid technological change and evolving industry requirements and standards. Our Alcon Division typically competes with different companies across its three respective franchises—Ophthalmic Pharmaceuticals, Surgical and Vision Care. Companies within this industry compete on technological leadership and innovation, quality and efficacy of their products, relationships with eye care professionals and healthcare providers, breadth and depth of product offering and pricing. The presence of these factors varies across our Alcon Division's product offerings, which provides a broad line of proprietary eye care products and competes in all major product categories in the eye care market, with the exception of eyeglasses. Our principal competitors also sometimes form strategic alliances and enter into co-marketing agreements in an effort to better compete with us.
88
Our Ophthalmic Pharmaceuticals products are subject to the same regulatory procedures as are the products of our Pharmaceuticals Division. See "—Pharmaceuticals—Regulation."
Our Surgical and Vision Care products are regulated as medical devices in the US and the EU. These jurisdictions each have risk-based classification systems that determine the type of information that must be provided to the local regulators in order to obtain the right to market a product. In the US, safety and effectiveness information for Class II and III devices must be reviewed by the FDA. There are two review procedures: a Pre-Market Approval (PMA) and a Pre-Market Notification (510(k)) submission. Under a PMA, the manufacturer must submit to the FDA supporting evidence sufficient to prove the safety and effectiveness of the device. The FDA review of a PMA usually takes 180 days from the date of filing of the application. Under Pre-Market Notification (510(k)), the manufacturer submits notification to the FDA that it intends to commence marketing the product, with data that establishes the product as substantially equivalent to another product already on the market. The FDA usually determines whether the device is substantially equivalent within 90 days.
In the EU, the CE marking is required for all medical devices sold. By affixing the CE marking, the manufacturer certifies that a product is in compliance with provisions of the EU's Medical Device Directive. Most such products are subject to a self-certification process by the manufacturer, which requires the manufacturer to confirm that the product performs to appropriate standards. This allows the manufacturer to issue a Declaration of Conformity and to notify competent authorities in the EU that the manufacturer intends to market the product. In order to comply with European regulations, our Alcon Division maintains a full Quality Assurance system and is subject to routine auditing by a certified third party (a "notified body") to ensure that this quality system is in compliance with the requirements of the EU's Medical Device Directive as well as the requirements of the ISO quality systems' standard ISO 13485.
We attach great importance to patents, trademarks, copyrights and know-how in order to protect our investment in research and development, manufacturing and marketing. It is our policy to seek the broadest possible protection for significant product developments in all major markets. Among other things, patents may cover the products themselves, including the product's active substance, its use and its formulation. Patents may also cover the processes for manufacturing a product, including processes for manufacturing intermediate substances used in the manufacture of the products. Patents may also cover particular uses of a product, such as its use to treat a particular disease, or its dosage regimen.
The protection offered by such patents extends for varying periods depending on the legal life of patents in the various countries. The protection afforded, which may also vary from country to country, depends upon the type of patent and its scope of coverage. We monitor our competitors and typically challenge infringements of our intellectual property. We also defend challenges, often by generic manufacturers, to the validity of our intellectual property. However, because the outcome of intellectual property litigation is uncertain and unpredictable, there can be no assurance that we will be able to successfully protect our intellectual property rights in all cases. See generally "—Pharmaceuticals—Intellectual Property."
We take reasonable steps to ensure that our products do not infringe valid intellectual property rights held by others. Nevertheless, third parties may assert patent and other intellectual property rights against our products. As a result, we can become involved in significant intellectual property litigation regarding our products. If we are unsuccessful in defending these suits, we could be subject to injunctions preventing us from selling our products and to damages, which may be substantial. Litigation or administrative proceedings challenging the validity of our intellectual property is similarly unpredictable. If we are
89
unsuccessful in such proceedings, we may face loss of exclusivity and increased competition in the affected territories.
Worldwide, all of our major products are sold under trademarks that we consider in the aggregate to be important to our business as a whole. We consider trademark protection to be particularly important in the protection of our investment in the sales and marketing of our Surgical, Ophthalmic Pharmaceuticals and Vision Care franchises. The scope and duration of trademark protection varies widely throughout the world. In some countries, trademark protection continues only as long as the mark is used. Other countries require registration of trademarks and the payment of registration fees. Trademark registrations are generally for fixed, but renewable, terms.
We rely on copyright protection in various jurisdictions to protect the exclusivity of the code for the software used in our surgical equipment. The scope of copyright protection for computer software varies throughout the world, although it is generally for a fixed term which begins on the date of copyright registration.
Our Sandoz Division is a leader in generic pharmaceuticals and biosimilars and sells products in products in more than 160 countries. In 2015, the Sandoz Division achieved consolidated net sales of $9.2 billion, representing 18% of the Group's total net sales.
Sandoz is organized globally in three franchises: Retail Generics, Anti-Infectives, and Biopharmaceuticals & Oncology Injectables. In Retail Generics, Sandoz develops, manufactures and markets active ingredients and finished dosage forms of pharmaceuticals to third parties. Retail Generics includes the areas of dermatology, respiratory and ophthalmics, as well as cardiovascular, metabolism, central nervous system, pain, gastrointestinal, and hormonal therapies. Finished dosage form anti-infectives sold to third parties are also a part of Retail Generics.
In Anti-Infectives, Sandoz manufactures active pharmaceutical ingredients and intermediates—mainly antibiotics—for internal use by Retail Generics and for sale to third-party customers. In Biopharmaceuticals, Sandoz develops, manufactures and markets protein- or other biotechnology-based products known as biosimilars and provides biotechnology manufacturing services to other companies, and in Oncology Injectables, Sandoz develops, manufactures and markets cytotoxic products for the hospital market.
Sandoz develops, produces and markets finished dosage form medicines as well as intermediary products including active pharmaceutical ingredients. Nearly half of the Sandoz portfolio, in terms of sales, is in differentiated products—products that are scientifically more difficult to develop and manufacture than standard generics. Examples of differentiated products in the Sandoz portfolio are the multiple sclerosis treatment Glatopa (glatiramer acetate injection), the cardiovascular polypill Sincronium (acetylsalicylic acid, atorvastatin and ramipril), and the pain medication fentanyl, which is difficult to manufacture because its delivery mechanism is a transdermal patch. Differentiated products also include biosimilars, which Sandoz began developing in 1996 and today sells in more than 60 countries. Sandoz is the market leader in biosimilars and all three of its biosimilars continue to demonstrate strong growth in their respective categories—Omnitrope, a human growth hormone; Binocrit, an erythropoiesis-stimulating agent used to treat anemia; and filgrastim for neutropenia under the brand names Zarzio outside the US and Zarxio in the US. According to IMS Health, Sandoz holds the global #1 position in terms of sales in biosimilars and generic anti-infectives, as well as in ophthalmics and transplanattion medicines. In addition, Sandoz holds leading global positions in key therapeutic areas ranging from generic injectables, dermatology and respiratory to cardiovascular, metabolism, central nervous system, pain and gastrointestinal.
Sandoz is focused on several key priorities, including investing in key markets and therapeutic areas, increasing the performance of its Development & Regulatory organization, optimizing its manufacturing network and maximizing opportunities in biosimilars.
90
In 2015, key product launches in the US included Glatopa, the first generic version of Teva's Copaxone® 20mg (glatiramer acetate injection), the biosimilar Zarxio (filgrastim-sndz), and budesonide inhalation suspension (Astra Zeneca's Pulmicort Respules®), as well as authorized generic versions of the The Medicine Company's Angiomax® (bivalirudin) and our Pharmaceutical Division's Exelon Patch (rivastigmine patch).
In 2015, key product launches in various European countries included aripiprazole TAB (Atsuka's Abilify®), duloxetine (Eli Lilly's Cymbalta®) pregabalin HGC (Pfizer's Lyrica®) and valganciclovir FCT (Roche's Valcyte®). In addition, the global rollout of AirFluSal Forspiro continued with launches across Europe. As of December 31, 2015, AirFluSal Forspiro was marketed in 24 countries.
In 2015, Sandoz continued to accelerate its efforts across Sub-Saharan Africa, supported by a strong product portfolio that comprises anti-infectives, tuberculosis treatments, maternal and child health products, and medicines to address non-communicable diseases.
Sandoz launched a number of important products in various countries in 2015, including:
- •
- Aripiprazole TAB (Atsuka's Abilify®)
- •
- Bivalirudin (authorized generic of The Medicine Company's Angiomax®)
- •
- Budesonide inhalation suspension (Astra Zeneca's Pulmicort Respules®)
- •
- Duloxetine (Eli Lilly's Cymbalta®)
- •
- Glatopa (Teva's Copaxone® 20mg; glatiramer acetate injection)
- •
- Rivastigmine patch (authorized generic of our Pharmaceutical Division's Exelon Patch)
- •
- Pregabalin HGC (Pfizer's Lyrica®)
- •
- Valganciclovir FCT (Roche's Valcyte®)
- •
- Zarxio (filgrastim-sndz)
The following tables describe key marketed products for Sandoz (availability varies by market):
Retail Generics
Product
|
Originator Drug | Description | ||
|---|---|---|---|---|
| Acetylcysteine | Fluimucil® | Respiratory system | ||
| Amoxicillin/clavulanic acid | Augmentin® | Anti-infective | ||
| Atorvastatin | Lipitor® | Blood cholesterol reduction | ||
| Diclofenac | Voltaren | Analgesic | ||
| Fentanyl | Duragesic® | Analgesic | ||
| Levothyroxine Sodium | Synthroid®; Levoxyl® | Hypothyroidism treatment | ||
| Omeprazole | Prilosec® | Ulcer and heartburn treatment | ||
| Pantoprazole | Protonix® | Gastrointestinal | ||
| Potassium | Klor-Con® | Hypokalemia | ||
| Tacrolimus | Prograf® | Transplantation |
91
Anti-Infectives
Active Ingredients
|
Description | |
|---|---|---|
Oral and sterile penicillins |
Anti-infectives | |
Oral and sterile cephalosporins |
Anti-infectives | |
Clavulanic acid and mixtures with clavulanic acid |
ß-lactam inhibitors | |
Classical and semisynthetic erythromycins |
Anti-infectives |
Intermediates
|
Description | |
|---|---|---|
Various cephalosporin intermediates |
Anti-infectives | |
Erythromycin base |
Anti-infectives | |
Various crude compounds produced by fermentation |
Cyclosporine, ascomysine, rapamycine, mycophenolic acid, etc. |
Biopharmaceuticals
Product
|
Originator Drug | Description | ||
|---|---|---|---|---|
| Binocrit and Epoetin alfa Hexal | Eprex®/Erypo® | Recombinant protein used for anemia | ||
| Omnitrope | Genotropin® | Recombinant human growth hormone | ||
| Zarzio, Zarxio and Filgrastim Hexal | Neupogen® | Recombinant protein used in oncology |
Oncology Injectables
Product
|
Originator Drug | Description | ||
|---|---|---|---|---|
| Azacitidine | Vidaza® | Bone marrow cancer, leukemia | ||
| Bortezomib | Velcade® | Multiple myeloma, lymphoma | ||
| Cyclophosphamide | Endoxan® | Breast, ovarian and non-small cell lung cancer | ||
| Decitabine | Dacogen® | Bone marrow cancer, leukemia | ||
| Docetaxel | Taxotere® | Breast, ovarian and non-small cell lung cancer | ||
| Gemcitabine | Gemzar® | Bladder, pancreas, lung, ovarian, and breast cancer | ||
| Leuprorelin | Lupron®, Eligard® | Prostate cancer | ||
| Levoleucovorin Calcium | Fusilev® | Rescue after methotrexate high-dose therapy | ||
| Methotrexate | Folex®, Rheumatrex® | Arthritis; breast, lung, cervix and ovarian cancer, and others | ||
| Paclitaxel | Taxol® | Breast, lung and ovarian cancer, Kaposi sarcoma |
92
Biosimilars in Phase III Development and Registration
The following table describes Sandoz biosimilar projects that are in Phase III clinical trials (including filing preparation) and registration:
Project/product
|
Common name | Mechanism of action | Potential indication/ indications |
Therapeutic areas |
Route of administration |
Current phase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
GP2013 |
rituximab | Anti-CD20 antibody | Non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis (also known as Wegener's granulomatosis), and microscopic polyangiitis and others (same as originator) | Oncology and Immunology | Intravenous | II and III | ||||||
GP2015 |
etanercept | TNF-a inhibitor | Arthritidies (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis), plaque psoriasis and others (same as originator) | Immunology | Subcutaneous | US/EU: Registration | ||||||
GP2017 |
adalimumab | TNF-a inhibitor | Arthritidies (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis), plaque psoriasis and others (same as originator) | Immunology | Subcutaneous | III | ||||||
HX575* |
epoetin alfa | Erythropoiesis-stimulating agent | Chronic kidney disease, chemotherapy-induced anemia and others (same as originator) | Oncology and Nephrology | Subcutaneous and intravenous | III | ||||||
HX575 s.c.** |
epoetin alfa | Erythropoiesis-stimulating agent | Chronic kidney disease | Nephrology | Subcutaneous | EU: Registration | ||||||
LA-EP2006 |
pegfilgrastim | Pegylated granulocyte colony-stimulating factor | Chemotherapy-induced neutropenia and others (same as originator) | Oncology | Subcutaneous | US: Registration EU: III | ||||||
- *
- Planned
submission for US.
- **
- Filing in the EU for the addition of the subcutaneous (s.c.) route of administration for Binocrit nephrology indications.
The two largest generics markets in the world—the US and Europe—are the principal markets for Sandoz, although Sandoz sells products in more than 160 countries. The following table sets forth the aggregate 2015 net sales of Sandoz by region:
Sandoz
|
2015 Net Sales to third parties |
||||||
|---|---|---|---|---|---|---|---|
| |
$ millions |
% |
|||||
Europe |
3,925 | 43 | |||||
United States |
3,525 | 38 | |||||
Asia, Africa, Australasia |
1,150 | 13 | |||||
Canada and Latin America |
557 | 6 | |||||
| | | | | | | | |
Total |
9,157 | 100 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Of which in Established Markets* |
6,972 | 76 | |||||
Of which in Emerging Growth Markets* |
2,185 | 24 | |||||
| | | | | | | | |
- *
- Emerging Growth Markets comprise all markets other than the Established Markets of the US, Canada, Western Europe, Japan, Australia and New Zealand.
Many Sandoz products are used for chronic conditions that require patients to consume the product over long periods of time, from months to years. Sales of our anti-infective products are subject to seasonal variation. Sales of the vast majority of our other products are not subject to material changes in seasonal demand.
93
The goal of our supply chain strategy is to produce and distribute high-quality products efficiently. The manufacture of our products is heavily regulated by governmental health authorities around the world, including the FDA and EMA. In addition to regulatory requirements, many of our products involve technically complex manufacturing processes or require a supply of highly specialized raw materials.
We manufacture and package our Sandoz products at 45 manufacturing sites across 19 countries, supplying more than 160 countries globally. Among these, our most significant production facilities are located in Barleben and Rudolstadt, Germany; Kundl, Schaftenau and Unterach, Austria; Ljubljana and Menges, Slovenia; Stryków, Poland. In 2015, we announced that we were exiting our manufacturing sites in Frankfurt and Gerlingen, Germany, as well as in Turbhe, India. We anticipate that these site exits will be completed by the end of 2016. Our global manufacturing strategy focuses on building a high-quality manufacturing network that optimizes cost, service, technology and geography.
Active pharmaceutical ingredients are manufactured in our own facilities or purchased from third-party suppliers. We maintain state-of-the-art and cost-competitive processes within our own production network. Those processes include fermentation, chemical syntheses and precipitation processes, such as sterile processing. Many biosimilars are manufactured using recombinant DNA derived technology, by which a gene is introduced into a host cell, which then produces a human protein. This manufacturing process requires sophisticated technical expertise. We are constantly working to improve current, and to develop new, manufacturing processes.
Where possible, we strive to maintain multiple supply sources so that the business is not dependent on a single or limited number of suppliers, and competitive material sourcing can be assured. However, our ability to do so may at times be limited by regulatory or other requirements. We monitor market developments that could have an adverse effect on the supply of essential active pharmaceutical ingredients. All active pharmaceutical ingredients we purchase must comply with high quality standards.
We obtain agricultural, chemical and other raw materials from suppliers around the world. The raw materials we purchase are generally subject to market price fluctuations. We seek to avoid these fluctuations, where possible, through the use of long-term supply contracts. We also proactively monitor markets and developments that could have an adverse effect on the supply of essential materials. All raw materials we purchase must comply with our quality standards. For some products and raw materials, we may also rely on a single source of supply.
In October 2015, we received a Warning Letter from the FDA with respect to our Kalwe and Turbhe, India manufacturing sites. The Warning Letter observations follow an FDA inspection at both sites in August 2014 and are related to deficiencies in current good manufacturing practice (cGMP) for finished pharmaceuticals. The Warning Letter did not contain any new issues in addition to the 483 observations issued following the August 2014 inspection. Sandoz plans to continue to collaborate with the FDA to resolve the Warning Letter observations.
In September 2015, the FDA confirmed that it closed out the May 2013 Warning Letter relating to our oncology injectables manufacturing facility in Unterach, Austria. That Warning Letter contained two observations which followed an FDA inspection at the site in October 2012, and were related to historical visual inspection practices for products manufactured at the site. A follow up inspection by the FDA in 2014 resulted in no observations.
In July 2014, the FDA confirmed that it had decided to close out the Warning Letter issued in November 2011 against three Sandoz North American facilities in Broomfield, Colorado; Wilson, North Carolina; and Boucherville, Canada. The Warning Letter, which followed inspections at all three sites in the course of 2011, had raised concerns regarding these facilities' compliance with FDA cGMP regulations. The FDA observations in the Warning Letter related primarily to general documentation,
94
validation and investigation practices. Novartis took steps in collaboration with the FDA to correct the observations in the Warning Letter with respect to all three sites.
Our Sandoz Division has experienced significant supply interruptions in the past, and there can be no assurance that supply will not be interrupted again in the future as a result of unforeseen circumstances. The manufacture of our products is complex and heavily regulated, making supply never an absolute certainty. If we or our third-party suppliers fail to comply fully with regulations or other unforeseen challenges occur, then there could be a product recall or other shutdown or disruption of our production activities. We have implemented a global manufacturing strategy to maximize business continuity in case of business interruptions or other unforeseen catastrophic events. However, there can be no guarantee that we will be able to successfully manage such issues and maintain continuous supply if such issues arise.
Sandoz sells a broad portfolio of generic pharmaceutical products and biosimilars to wholesalers, pharmacies, hospitals and other healthcare outlets. Sandoz adapts its marketing and sales approach to local decision making processes, depending on the structure of the market in each country.
In response to rising healthcare costs, many governments and private medical care providers, such as health maintenance organizations, have instituted reimbursement schemes that favor the substitution of bioequivalent generic products for patented pharmaceutical products. In the US, statutes have been enacted by virtually all states that permit or require pharmacists to substitute a less expensive generic product for the brand-name version of a drug that has been prescribed to a patient. Generic use is growing in Europe, but penetration rates in many EU countries (as a percentage of volume) remain well below those in the US. Legislative or regulatory changes can have a significant impact on our business in a country. In Germany, for example, the generic market is in transition and healthcare reforms have increasingly shifted decision making from physicians to insurance funds.
Our Anti-Infectives franchise supplies generic antibiotics to a broad range of customers, as well as active pharmaceutical ingredients and intermediates to the pharmaceutical industry worldwide.
Our Biopharmaceuticals franchise operates in an emerging business environment. Regulatory pathways for approving biosimilar products are either relatively new or still in development, and policies have not yet been fully defined or implemented for the automatic substitution and reimbursement of biosimilars in many markets, including the US (see "—Regulation"). As a result, in many of these markets, including the US, our biosimilar products are marketed as branded competitors to the originator products.
The market for generic products is characterized by increasing demand for high-quality pharmaceuticals that can be marketed at lower costs due to comparatively minimal initial research and development investments. Increasing pressure on healthcare expenditures and numerous patent and data exclusivity period expirations have created a favorable market environment for the generics industry. This positive market trend, however, brings increased competition among the companies selling generic pharmaceutical products, leading to ongoing price pressure on generic pharmaceuticals.
In addition, research-based pharmaceutical companies have responded to increased competition from generic products by licensing their patented products to generic companies (so-called "authorized generics"). By doing so, research-based pharmaceutical companies participate directly in the generic conversion process. Consequently, generic companies that were not in a position to compete on a specific product may enter the generic market using the innovator's product. In the US, the authorized generic is not subject to the US Hatch-Waxman Act rules regarding exclusivity (see "—Regulation"). The company that launches an authorized generic typically launches its product at the same time as the generic exclusivity holder. Authorized generics serve as a business opportunity for Sandoz when the product of a
95
research-based pharmaceutical company loses patent protection and Sandoz secures a license from the research-based pharmaceutical company to launch the authorized generic of that product. However, because they are not subject to the Hatch-Waxman Act rules on exclusivity, authorized generics also reduce the value of the exclusivity for the company that invested in creating the first generic medicine to compete with the originator product. Furthermore, certain research-based companies continually seek new ways to protect their market franchise and to decrease the impact of generic competition. For example, some research-based pharmaceutical companies have reacted to generic competition by decreasing the prices of their patented product, or engaging in other tactics to preserve the sales of their branded products, thus potentially limiting the profit that the generic companies can earn on the competing generic product.
Before a generic pharmaceutical may be marketed, intensive technical and clinical development work must be performed to demonstrate, in bioavailability studies, the bioequivalency of the generic product to the reference product. Nevertheless, research and development costs associated with generic pharmaceuticals generally are much lower than those of the originator pharmaceuticals, as no pre-clinical studies or clinical trials on dose finding, safety and efficacy must be performed by the generic company. As a result, pharmaceutical products for which the patent and data exclusivity period has expired can be offered for sale at prices often much lower than those of products protected by patents and data exclusivity, which must recoup substantial basic research and development costs through higher prices over the life of the product's patent and data exclusivity period.
While generic pharmaceuticals are follow-on versions of chemically synthesized molecules, "biosimilar" products contain a version of the active substance of an already approved original biological medicine. Due to the inherent variability of biologic products and their higher complexity, the development and the regulatory pathway of biosimilars differ significantly from that of generics.
Development of a biosimilar product is much more technically challenging than the development of a generic pharmaceutical. Unlike generic pharmaceuticals, development of biosimilars requires clinical studies in patients. Biosimilars are engineered to match the reference product in quality, safety and efficacy. This is achieved by systematically defining the target of the reference product and then comparing the biosimilar to the reference product at various development stages to confirm biosimilarity and to establish that there are no clinically meaningful differences between the proposed biosimilar and the reference biologic. Because the purpose of a biosimilar clinical development program is to confirm biosimilarity and not establish efficacy and safety de novo, the clinical studies required are less than those required for an originator biologic. Therefore, the cost of development for a biosimilar is usually less than that of an originator biologic.
The regulatory pathways for approval of biosimilar products are being developed and established in many countries of the world. A regulatory framework for the approval of biosimilars has been established in the EU, Japan, Canada and US, while the WHO issued guidance. Sandoz has successfully registered and launched the first biosimilar (or biosimilar type) product in Europe, the US, Canada, Japan, Taiwan, Australia and many countries in Latin American and Asia. Sandoz has three approved biosimilar products in more than 60 countries of the world, and is the first company to secure approval for a biosimilar under the US biosimilar pathway which was established as part of the Biologics Price Competition and Innovation Act (BPCIA).
The Sandoz Division explores alternative routes for the manufacture of known compounds and develops innovative dosage forms of well-established medicines. The Development and Registration staff employed by affiliates of the Sandoz Division are based worldwide, including facilities in Holzkirchen and Rudolstadt, Germany; Kundl, Schaftenau and Unterach, Austria; Ljubljana and Mengeš, Slovenia; Boucherville, Canada; and East Hanover, New Jersey. In 2015, Sandoz expensed $0.8 billion (on a core basis $0.8 billion) in product development, which amounted to 8% of the division's net sales. Sandoz
96
expensed $0.8 billion (on a core basis $0.8 billion) and $0.8 billion (on a core basis $0.8 billion) in 2014 and 2013, respectively. Core results exclude impairments, amortization and certain exceptional items. For additional information, see "Item 5. Operating and Financial Review and Prospects—5.A Operating Results—Non-IFRS Measures as Defined by Novartis—Core Results."
The Hatch-Waxman Act in the US (and similar legislation in the EU and in other countries) eliminated the requirement that manufacturers of generic pharmaceuticals repeat the extensive clinical trials required for originator products, so long as the generic version could be shown in bioavailability studies to be of identical quality and purity, and to be therapeutically equivalent to the reference product.
In the US, the decision whether a generic pharmaceutical is bioequivalent to the original patented product is made by the FDA based on an Abbreviated New Drug Application (ANDA) filed by the generic product's manufacturer. The process typically takes nearly two years from the filing of the ANDA until FDA approval. However, delays can occur if issues arise regarding the interpretation of bioequivalence study data, labeling requirements for the generic product, or qualifying the supply of active ingredients. In addition, the Hatch-Waxman Act requires a generic manufacturer to certify in certain situations that the generic product does not infringe on any current applicable patents on the product held by the innovator, or to certify that such patents are invalid or the product is non-infringing. This certification often results in a patent infringement lawsuit being brought by the patent holder against the generic company. In the event of such a lawsuit, the Hatch-Waxman Act imposes an automatic 30 month delay in the approval of the generic product in order to allow the parties to resolve the intellectual property issues. For generic applicants who are the first to file their ANDA containing a certification claiming non-infringement or patent invalidity, the Hatch-Waxman Act provides those applicants with 180 days of marketing exclusivity to recoup the expense of challenging the innovator patents. However, generic applicants must launch their products within certain time frames or risk losing the marketing exclusivity that they had gained by being a first to file applicant.
In the EU, decisions on the granting of a marketing authorization are made either by the European Commission based on a positive recommendation by the EMA under the Centralized Procedure, or by a single Member State under the national or decentralized procedure. See "—Pharmaceuticals—Regulation—European Union." Companies may submit Abridged Applications for approval of a generic medicinal product based upon its "essential similarity" to a medicinal product authorized and marketed in the EU following the expiration of the product's data exclusivity period. In such cases, the generic company is able to submit its Abridged Application based on the data submitted by the medicine's innovator, without the need to conduct extensive Phase III clinical trials of its own. For all products that received a marketing authorization in the EU after late 2005, the Abridged Application can be submitted throughout the EU. However, the data submitted by the innovator in support of its application for a marketing authorization for the reference product will be protected for ten years after the first grant of marketing authorization in all Member States, and can be extended for an additional year if a further innovative indication has been authorized for that product, based on pre-clinical and clinical trials filed by the innovator that show a significant clinical benefit in comparison to the existing therapies. Approval of biosimilars in Europe follows the same process. However, biosimilars usually have to be approved through the centralized procedure because they are manufactured using recombinant DNA technology. As part of the approval process in the EU, biosimilars have to demonstrate comparability to the originator product in terms of safety, efficacy and quality through an extensive comparability exercise, based on strict guidelines set by the authorities. Regulators will only approve a biosimilar based on data which allows the regulators to conclude that there are no clinically meaningful differences between the reference product and the biosimilar.
97
In the US, the regulatory pathway for the approval of a biosimilar product was established under the BPCIA, signed into law in March 2010. Under the BPCIA, a biosimilar must be highly similar with no clinically meaningful differences compared to the reference product. Approval of a biosimilar in the US requires the submission of a BLA to the FDA, including an assessment of immunogenicity, and pharmacokinetics or pharmacodynamics. The BLA for a biosimilar can be submitted as soon as four years after the initial approval of the reference biologic, but can only be approved 12 years after the initial approval of the reference biologic. This pathway is still relatively new and some aspects remain untried, controversial and subject to litigation.
We take all reasonable steps to ensure that our generic products do not infringe valid intellectual property rights held by others. Nevertheless, originator companies commonly assert patent and other intellectual property rights in an effort to delay or prevent the launch of competing generic products. As a result, we can become involved in significant litigation regarding our generic products. If we are unsuccessful in defending these suits, we could be subject to injunctions preventing us from selling our generic products and to damages, which may be substantial.
Wherever possible, our generic products are protected by our own patents. Among other things, patents may cover the products themselves, including the product's formulation, or the processes for manufacturing a product.
See "Item 4. Information on the Company—4.A History and Development of Novartis," and "Item 4. Information on the Company—4.B Business Overview—Overview."
4.D Property, Plants and Equipment
Our principal executive offices are located in Basel, Switzerland. Our divisions operate through a number of affiliates having offices, research facilities and production sites throughout the world.
We generally own our facilities. However, some sites are leased under long-term leases. Some of our principal facilities are subject to mortgages and other security interests granted to secure indebtedness to certain financial institutions.
For a discussion of our manufacturing facilities, see "—Item 4.B Business Overview—Pharmaceuticals—Production," "—Alcon—Production," and "—Sandoz—Production." The following table sets forth our major headquarters and most significant production and research and development
98
facilities by division. A number of the facilities associated with our former Vaccines, OTC and Animal Health Divisions were transferred as part of the portfolio transformation transactions completed in 2015.
| Location/Division |
Size of Site (in square meters) |
Major Activity |
||
|---|---|---|---|---|
Major facilities: |
||||
| Pharmaceuticals | ||||
East Hanover, New Jersey |
400,000 |
Division US headquarters, research and development |
||
| Changshu (Suzhou), China | 230,000 | Technical research, development and manufacturing of drug substances and drug intermediates | ||
| Cambridge, Massachusetts | 212,000 | Global NIBR headquarters, research and development | ||
| Basel, Switzerland—St. Johann | 200,000 | Global Group headquarters, global division headquarters, research and development, production of drug substances and drug intermediates | ||
| Ringaskiddy, Ireland | 85,000 | Production of drug substances and drug intermediates | ||
| Stein, Switzerland | 64,700 | Production of sterile vials, pre-filled syringes and ampoules, and of inhalation capsules, tablets and transdermals, and of active pharmaceutical ingredients | ||
| Grimsby, UK | 64,000 | Production of drug substances and drug intermediates | ||
| Huningue, France | 35,000 | Production of drug substances for clinical and commercial supply | ||
| Barbera, Spain | 33,000 | Production of tablets, capsules and inhalation products | ||
| Basel, Switzerland—Schweizerhalle | 31,700 | Production of drug substances and drug intermediates | ||
| Wehr, Germany | 31,700 | Production of tablets, creams and ointments | ||
99
| Location/Division |
Size of Site (in square meters) |
Major Activity |
||
|---|---|---|---|---|
| Shanghai, China | 14,200 | Research and development | ||
| Morris Plains, New Jersey | 14,000 | Production of personalized cell therapy | ||
| Alcon | ||||
Fort Worth, Texas |
252,800 |
Division headquarters, production, research and development for Ophthalmic Pharmaceuticals, Vision Care, Surgical |
||
| Grosswallstadt, Germany | 82,400 | Production, research and development for Vision Care | ||
| Johns Creek, Georgia | 73,400 | Production, research and development for Vision Care | ||
| Puurs, Belgium | 55,000 | Production for Ophthalmic Pharmaceuticals, Surgical | ||
| Houston, Texas | 36,300 | Production for Surgical | ||
| Huntington, West Virginia | 24,600 | Production for Surgical | ||
| Irvine, California | 20,700 | Production for Surgical | ||
| Sandoz | ||||
Kundl and Schaftenau, Austria |
480,000 |
Production of biotech products, anti-infectives, active drug substances, product development |
||
| Barleben, Germany | 340,000 | Production of broad range of finished dosage forms | ||
| Ljubljana, Slovenia | 83,000 | Production of broad range of finished solid and sterile dosage forms | ||
| Holzkirchen, Germany | 72,300 | Division headquarters, production of oral films, transdermal delivery systems, matrix patches, product development | ||
| Stryków, Poland | 45,000 | Production of broad range of bulk oral solid forms | ||
| Rudolstadt, Germany | 44,000 | Development and production of respiratory technologies and ophthalmics | ||
| Princeton, New Jersey | 14,300 | Division US headquarters | ||
In 2010, we announced a Group-wide review of our manufacturing footprint. In 2015, and continuing into 2016, we continued to optimize our manufacturing footprint, bringing the total number of production
100
sites that have been or are in the process of being restructured, exited or divested as part of these activities to 25 for our continuing operations. These steps help us balance production capacity and further increase efficiency. We have recorded exceptional charges of $375 million in 2015, bringing the total charges to $950 million since the program began for our continuing operations. As part of this initiative we announced in 2015 the closing of our Pharmaceuticals Division facility in Resende, Brazil and plans to exit our Sandoz Division plants in Gerlingen and Frankfurt, Germany, and Turbhe, India. We also announced downsizing at a Pharmaceutical Division site in Ringaskiddy, Ireland. In addition, we finalized the divestment of our Alcon Division manufacturing operations in Kaysersberg, France, and the divestment of our pharmaceutical manufacturing site in Taboão da Serra, Brazil.
Our St. Johann site in Basel, Switzerland, is our largest research and development site as well as the headquarters for the Group and for the Pharmaceuticals Division. A project was started in 2001, known as "Campus," with the aim of transforming the site into a center of knowledge, innovation and encounter with a primary emphasis on international corporate functions and research activities. At that time, changes needed to be made to the site, since it had originally been designed primarily for pharmaceuticals production, but research and development had come to account for a greater proportion of our activities there. The Campus project is progressing as planned. By the end of 2015, 17 new buildings had begun operations, eight of them laboratory buildings. The current phase of the long term redevelopment of our St. Johann site is expected to be completed in 2016. In addition, the Novartis Board of Directors has approved planning for the next phase of the campus extension after 2015 in line with the overall plan for the site. A large laboratory building is planned for the northern end of the site and construction is expected to begin in 2016. In October 2014, the Basel "Grand Council" approved the second part of a high-rise building zone at the St. Johann site, which will allow us to plan a third high-rise building on the site. Through December 31, 2015, the total amount paid and committed to be paid on the Campus project is equivalent to $2.2 billion. Novartis expects to have spent more than the equivalent of $2.2 billion on the Campus project and the relocation of production facilities to other sites in the Basel region through 2017. We intend to fund these expenditures from internally generated resources.
In 2007, NIBR opened a start-up facility for our new R&D center in Shanghai, China (CNIBR). In 2008, we broke ground on Phase 1 of a new facility that was originally to be home to approximately 400 R&D scientists and approximately 400 other Pharmaceuticals Division personnel. In 2009, we announced that we would expand the scope of the site and invest $1 billion over the next five years to increase the size of our operations in Shanghai. Based on a re-evaluation of the site conducted in 2010, the current Phase one has been extended by two buildings to fulfill the requirements for the cross-divisional Shanghai campus to house 800 offices and 400 laboratory work places. As of December 31, 2015, two laboratory buildings and two office buildings of the first phase of the project are completed. In addition, the other two office buildings which are part of phase one are nearly complete with testing, commissioning and resolution of punch list items in progress. Through December 31, 2015, the total amount paid and committed to be paid on the CNIBR Project is equivalent to $844 million.
In 2010, we announced that we would build new laboratory and office space for NIBR in Cambridge, Massachusetts on an area of land close to the existing NIBR research facilities on Massachusetts Avenue. In 2011 we finalized design plans for the new buildings, received necessary zoning changes from the City of Cambridge and began preparing the site for construction. Construction began on the site in April 2012, and as of the end of 2015, construction is complete and associates will begin moving into the new buildings. Through December 31, 2015, the total amount paid and committed to be paid on the NIBR Project is $743 million.
In 2012, Novartis announced the construction of a new state-of-the-art production facility to produce solid dosage form medicines for the Pharmaceuticals Division in Stein, Switzerland. We expect our investment in this facility to exceed $600 million. The new facility is planned to replace an older facility. In addition, Novartis plans to invest in new technologies and packaging facilities for pharmaceuticals at Stein. Stein is planned to be a technological competence center for both sterile and solid dosage form drugs,
101
while Novartis plans to expand the site's strategic role as a key platform for global launches of new pharmaceutical products. Through December 31, 2015, the total amount paid and committed to be paid on this project is equivalent to $554 million.
In 2012, we announced the planned construction of a new state-of-the-art biotechnology production site in Singapore with a planned investment of over $700 million. The new facility will focus on drug substance manufacturing based on cell culture technology. Ground was broken in February 2013 and construction was completed in the third quarter of 2015 for phase one of the project. We expect phase one of this project to be operational in 2017 and phase two in 2019. It will be co-located with the pharmaceutical production site based in Tuas, Singapore. In the future, Singapore is expected to be a technological competence center for both biotechnology and pharmaceutical manufacturing at Novartis. Through December 31, 2015, the total amount paid and committed to be paid on this project is equivalent to $452 million.
In 2012, we acquired a 16,000 square meter FDA-approved manufacturing facility in Morris Plains, New Jersey, from Dendreon Corporation for $43 million. In particular, we purchased all fixed assets at the site, including all equipment, machinery, utilities, and cell therapy related plant infrastructure, while the land and building will continue to be leased from a third party. The facility, and the former Dendreon personnel whom we retained, will support both clinical and commercial production of potential new products and therapies that emerge from the Novartis-University of Pennsylvania collaboration announced in August 2012, including CTL019. The facility space and infrastructure could also accommodate future chimeric antigen receptor production activities, in addition to CTL019. Through December 31, 2015, the total amount paid and committed to be paid on this project is $33 million.
A second expansion of the Johns Creek, Georgia facility was approved in the third quarter of 2014 to add nine production lines for Dailies and Dailies Total1 contact lenses. This project is expected to be completed by the third quarter of 2017. Through December 31, 2015, the total amount paid and committed to be paid on this project is $219 million.
The Alcon Division began an expansion of its Singapore facility in 2014 for contact lens manufacturing. The expansion is expected to add 16,000 square meters to the existing production lines. Through December 31, 2015, the total amount paid and committed to be paid on this project is equivalent to $95 million.
We integrate core values of environmental protection into our business strategy to add value to the business, manage risk and enhance our reputation.
We are subject to laws and regulations concerning the environment, safety matters, regulation of chemicals and product safety in the countries where we manufacture and sell our products or otherwise operate our business. These requirements include regulation of the handling, manufacture, transportation, use and disposal of materials, including the discharge of pollutants into the environment. In the normal course of our business, we are exposed to risks relating to possible releases of hazardous substances into the environment which could cause environmental or property damage or personal injuries, and which could require remediation of contaminated soil and groundwater, in some cases over many years, regardless of whether the contamination was caused by us, or by previous occupants of the property.
See also "Item 3. Key Information—Item 3.D Risk Factors—Environmental liabilities may adversely impact our results of operations" and "Item 18. Financial Statements—Note 20."
102
Item 4A. Unresolved Staff Comments
Not applicable.
Item 5. Operating and Financial Review and Prospects
This operating and financial review should be read together with the Group's consolidated financial statements in this Annual Report, which have been prepared in accordance with International Financial Reporting Standards (IFRS) as published by the International Accounting Standards Board.
Novartis provides healthcare solutions that address the evolving needs of patients and societies worldwide. Our broad portfolio includes innovative medicines, eye care products and cost-saving generic pharmaceuticals.
Following the completion of a series of transactions in 2014 and 2015, the Group's portfolio is organized into three global operating divisions. In addition, we separately report the results of Corporate activities. The disclosure in this Item focuses on these continuing operations, which are made up of Pharmaceuticals, Alcon, Sandoz and Corporate activities. In addition, from March 2, 2015, the date of the completion of a series of transactions with GSK, continuing operations also includes the results from the oncology assets acquired from GSK and the 36.5% interest in the GSK consumer healthcare joint venture (the latter reported as an investment in associated companies). We sold our Vaccines Division, excluding our influenza business, to GSK. Our influenza vaccines business was sold to CSL and our Animal Health Division was sold to Lilly. For more detail on these transactions see, "Item 10.C Material Contracts."
- •
- Pharmaceuticals: Innovative patent-protected prescription medicines
- •
- Alcon: Surgical, ophthalmic pharmaceutical and vision care products
- •
- Sandoz: Generic pharmaceuticals and biosimilars
- •
- Corporate activities
Continuing Operations:
- •
- Vaccines and Diagnostics: Preventive human vaccines and blood-testing diagnostics
- •
- Consumer Health: OTC (over-the-counter medicines) and Animal Health
- •
- Corporate: certain transactional and other expenses related to the portfolio transformation
Discontinued Operations:
Novartis has leading positions globally in each of the three areas of our continuing operations. To maintain our competitive positioning across these growing segments of the healthcare industry, we place a strong focus on innovating to meet the evolving needs of patients around the world, growing our presence in new and emerging markets, and enhancing our productivity to invest for the future and increase returns to shareholders.
We separately report the financial results of our Corporate activities as part of our continuing operations. Income and expenses from Corporate activities include the costs of the Group headquarters and those of corporate coordination functions in major countries. In addition, Corporate includes other items of income and expense which are not attributable to specific segments such as certain expenses
103
related to post-employment benefits, environmental remediation liabilities, charitable activities, donations and sponsorships.
Our continuing operations are supported by the Novartis Institutes for BioMedical Research and Novartis Business Services.
- •
- The Novartis Institutes for BioMedical Research (NIBR) is the innovation engine of Novartis, and is headquartered in Cambridge,
Massachusetts. More than 6,000 scientists and associates at NIBR conduct research into various disease areas at sites located in the US, Switzerland, Singapore and China. For more information about
NIBR, see "—Pharmaceuticals—Research and Development—Research program," below.
- •
- Novartis Business Services (NBS), our shared services organization, consolidates support services across Novartis divisions, helping to drive efficiency, standardization and simplification. NBS includes six service domains: human resources services, real estate and facility management, procurement, information technology, product lifecycle services and financial reporting and accounting operations. NBS has approximately 9,500 associates. Moving from division-specific services to a cross-divisional model, NBS continues to scale up the offshoring of transactional services to its five selected Global Service Centers in Mexico City, Mexico; Kuala Lumpur, Malaysia; Prague, Czech Republic; Hyderabad, India; and Dublin, Ireland.
Our continuing operations achieved net sales of $49.4 billion in 2015, while net income from continuing operations amounted to $7.0 billion. Research & Development expenditure in 2015 amounted to $8.9 billion ($8.7 billion excluding impairment and amortization charges). Of total net sales from continuing operations, $12.4 billion, or 25%, came from Emerging Growth Markets, and $37.0 billion, or 75%, came from Established Markets. Emerging Growth Markets comprise all markets other than the Established Markets of the US, Canada, Western Europe, Japan, Australia and New Zealand.
Headquartered in Basel, Switzerland, our Group companies employed 118,700 full-time equivalent associates as of December 31, 2015. Our products are available in approximately 180 countries around the world.
In September 2015, Novartis announced the launch of Novartis Access, a portfolio of 15 medicines to treat chronic diseases in low- and middle-income countries. The portfolio addresses cardiovascular diseases, diabetes, respiratory illnesses, and breast cancer and will be offered to governments, non-governmental organizations (NGOs) and other public-sector healthcare providers for $1 per treatment, per month.
Continuing Operations:
Pharmaceuticals researches, develops, manufactures, distributes and sells patented prescription medicines and is organized in the following franchises: Oncology, Cardio-Metabolic, Immunology and Dermatology, Retina, Respiratory, Neuroscience and Established Medicines. Our Pharmaceuticals Division also includes a franchise focused on the development and commercialization of Cell and Gene Therapies.
On March 2, 2015, we completed the acquisition of the oncology products of GSK, together with certain related assets. In addition, we acquired a right of first negotiation over the co-development or commercialization of GSK's current and future oncology R&D pipeline, excluding oncology vaccines. The right of first negotiation is for a period of twelve and one half years from the acquisition closing date.
In 2015, the Pharmaceuticals Division accounted for $30.4 billion, or 62%, of Group net sales, and for $7.6 billion, or 81%, of Group operating income (excluding Corporate income and expense, net).
104
Our Alcon Division researches, develops, manufactures, distributes and sells eye care products and technologies to serve the full life cycle of eye care needs. Alcon offers a broad range of products to treat many eye diseases and conditions, and is organized into three franchises: Surgical, Ophthalmic Pharmaceuticals and Vision Care. The Surgical portfolio includes technologies and devices for cataract, retinal, glaucoma and refractive surgery, as well as intraocular lenses to treat cataracts and refractive errors, like presbyopia and astigmatism. Alcon also provides viscoelastics, surgical solutions, surgical packs, and other disposable products for cataract and vitreoretinal surgery. In Ophthalmic Pharmaceuticals, the portfolio includes treatment options for elevated intraocular pressure caused by glaucoma, anti-infectives to aid in the treatment of bacterial infections and bacterial conjunctivitis, and ophthalmic solutions to treat inflammation and pain associated with ocular surgery, as well as an intravitreal injection for vitreomacular traction including macular hole. The Ophthalmic Pharmaceuticals portfolio also includes eye and nasal allergy treatments, as well as over-the-counter dry eye relief and ocular vitamins. The Vision Care portfolio comprises daily disposable, monthly replacement, and color-enhancing contact lenses, as well as a complete line of contact lens care products including multi-purpose and hydrogen-peroxide based solutions, rewetting drops and daily protein removers.
In 2015, Alcon accounted for $9.8 billion, or 20%, of Group net sales, and for $0.8 billion, or 8%, of Group operating income (excluding Corporate income and expense, net).
Our Sandoz Division focuses primarily on developing, manufacturing, distributing and selling prescription medicines that are not protected by valid and enforceable third-party patents, and intermediary products including active pharmaceutical ingredients. Sandoz is organized globally in three franchises: Retail Generics, Anti-Infectives, and Biopharmaceuticals & Oncology Injectables. In Retail Generics, Sandoz develops, manufactures and markets active ingredients and finished dosage forms of pharmaceuticals to third parties. Retail Generics includes the areas of dermatology, respiratory and ophthalmics, as well as the areas of cardiovascular, metabolism, central nervous system, pain, gastrointestinal, and hormonal therapies. Finished dosage form anti-infectives sold to third parties are also part of Retail Generics. In Anti-Infectives, Sandoz supplies generic antibiotics to a broad range of customers, as well as active pharmaceutical ingredients and intermediates to the pharmaceutical industry worldwide. In Biopharmaceuticals, Sandoz develops, manufactures and markets protein or other biotechnology based products known as biosimilars and provides biotechnology manufacturing services to other companies, and in Oncology Injectables, Sandoz develops, manufactures and markets cytotoxic products for the hospital market.
In 2015, Sandoz accounted for $9.2 billion, or 18%, of Group net sales, and for $1.0 billion, or 11%, of Group operating income (excluding Corporate income and expense, net).
Discontinued Operations:
Vaccines and Diagnostics Division
Prior to the completion of certain transactions in 2014 and 2015, our Vaccines and Diagnostics Division researched, developed, manufactured, distributed and sold human vaccines and blood-testing products worldwide. On January 9, 2014, we completed the divestment of our blood transfusion diagnostics unit to Grifols S.A. On March 2, 2015, we completed the divestment of our Vaccines Division (excluding its influenza vaccines business) to GSK. On July 31, 2015, we completed the divestment of our influenza vaccines business to CSL Limited.
105
Prior to the completion of certain transactions in 2015, Consumer Health consisted of our OTC (Over-the-Counter) and Animal Health Divisions. On January 1, 2015 we completed the divestment of our Animal Health Division to Lilly. On March 2, 2015, we completed the divestment of our OTC Division, which we contributed to a new consumer healthcare joint venture with GSK, of which we own 36.5%.
Our financial results are affected to varying degrees by external factors. The aging of the global population and rising rates of chronic diseases are driving demand for healthcare worldwide, as well as for treatments that Novartis provides. Continued growth in healthcare spending is contributing to increased scrutiny on drug pricing by governments, media and consumers, but also to increased demand for lower-cost treatment options, such as those produced by our generics division, Sandoz. Advances in science and technology are opening new opportunities to develop treatments tailored for individual patients.
At the same time, the loss of market exclusivity and the introduction of branded and generic competitors could significantly erode sales of our innovative products. Heightened regulatory requirements and the inherent complexity of our industry could lead to difficulties in bringing products to market, while increased pressure on pricing could impact our ability to generate returns and invest for the future. The growing trend of government investigations and litigations against healthcare companies, despite our best efforts to comply with local laws, could also have an adverse effect on our business and reputation.
For more detail on these trends and how they impact our results, see "Factors Affecting Results of Operations" below.
In evaluating the Group's performance, we consider not only the IFRS results, but also certain non-IFRS measures, including core results and constant currency results. These measures assist us in evaluating our ongoing performance from year to year and we believe this additional information is useful to investors in understanding our business.
The Group's core results exclude the amortization of intangible assets, impairment charges, expenses relating to divestments, the integration of acquisitions, restructuring charges that exceed a threshold of $25 million, as well as other income and expense items that management deems exceptional and that are or are expected to accumulate within the year to be over a $25 million threshold. For a reconciliation between IFRS results and core results see "—core results," below.
We present information about our net sales and other key figures relating to operating and net income in constant currencies (cc). We calculate constant currency net sales and operating income by applying the prior-year average exchange rates to current financial data expressed in local currencies in order to estimate an elimination of the impact of foreign exchange rate movements.
The core results, constant currencies and other non-IFRS measures are explained in more detail see "Non-IFRS Measures as Defined by Novartis," below and are not intended to be substitutes for the equivalent measures of financial performance prepared in accordance with IFRS. These measures may differ from similarly titled non-IFRS measures of other companies.
106
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Net sales to third parties from continuing operations |
49,414 | 52,180 | (5 | ) | 5 | ||||||||
Sales to discontinued segments |
26 | 239 | (89 | ) | (88 | ) | |||||||
| | | | | | | | | | | | | | |
Net sales from continuing operations |
49,440 | 52,419 | (6 | ) | 4 | ||||||||
Other revenues |
947 | 1,215 | (22 | ) | (22 | ) | |||||||
Cost of goods sold |
(17,404 | ) | (17,345 | ) | 0 | (8 | ) | ||||||
| | | | | | | | | | | | | | |
Gross profit from continuing operations |
32,983 | 36,289 | (9 | ) | 2 | ||||||||
Marketing & Sales |
(11,772 | ) | (12,377 | ) | 5 | (5 | ) | ||||||
Research & Development |
(8,935 | ) | (9,086 | ) | 2 | (3 | ) | ||||||
General & Administration |
(2,475 | ) | (2,616 | ) | 5 | (1 | ) | ||||||
Other income |
2,049 | 1,391 | 47 | 55 | |||||||||
Other expense |
(2,873 | ) | (2,512 | ) | (14 | ) | (24 | ) | |||||
| | | | | | | | | | | | | | |
Operating income from continuing operations |
8,977 | 11,089 | (19 | ) | (2 | ) | |||||||
Return on net sales (%) |
18.2 | 21.3 | |||||||||||
Income from associated companies |
266 | 1,918 | (86 | ) | (86 | ) | |||||||
Interest expense |
(655 | ) | (704 | ) | 7 | 2 | |||||||
Other financial income and expense |
(454 | ) | (31 | ) | nm | nm | |||||||
| | | | | | | | | | | | | | |
Income before taxes from continuing operations |
8,134 | 12,272 | (34 | ) | (17 | ) | |||||||
Taxes |
(1,106 | ) | (1,545 | ) | 28 | 10 | |||||||
| | | | | | | | | | | | | | |
Net income from continuing operations |
7,028 | 10,727 | (34 | ) | (18 | ) | |||||||
Net income/loss from discontinued operations |
10,766 | (447 | ) | nm | nm | ||||||||
| | | | | | | | | | | | | | |
Net income |
17,794 | 10,280 | 73 | 91 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Attributable to: |
|||||||||||||
Shareholders of Novartis AG |
17,783 | 10,210 | 74 | 92 | |||||||||
Non-controlling interests |
11 | 70 | (84 | ) | (84 | ) | |||||||
Basic earnings per share ($) from continuing operations |
2.92 | 4.39 | (33 | ) | (17 | ) | |||||||
Basic earnings per share ($) from discontinued operations |
4.48 | (0.18 | ) | nm | nm | ||||||||
| | | | | | | | | | | | | | |
Total basic earnings per share ($) |
7.40 | 4.21 | 76 | 94 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Free cash flow from continuing operations |
9,259 | 10,934 | (15 | ) | |||||||||
Free cash flow |
9,029 | 10,762 | (16 | ) | |||||||||
nm = not meaningful
Novartis delivered solid financial performance in 2015, driven by our continued success with growth products and expansion in emerging growth markets, which helped offset the effects of generic competition of approximately $2.2 billion. As a result, we achieved net sales to third parties from continuing operations of $49.4 billion (–5%, +5% cc). Growth in constant currencies has been more than
107
offset by negative currency impacts driven by the strengthening of the US dollar versus the euro, Japanese yen and major emerging market currencies.
Operating income decreased by 2% in constant currencies to $9.0 billion (–19%, –2% cc), mainly due to the amortization of the new oncology assets in Pharmaceuticals. In addition, an exceptional expense of $400 million for a settlement of the specialty pharmacies case in the Southern District of New York was recorded in 2015, whereas the prior-year benefitted from a one-time commercial settlement gain of $302 million and $248 million gain from selling a Novartis Venture Fund investment. Operating income margin was 18.2 percent of net sales.
Net income from continuing operations was $7.0 billion, declining more than operating income (–34%, –18% cc) mainly due to higher financial expense driven by $0.4 billion exceptional charges related to Venezuela and lower income from associated companies, which included in the prior year a gain of $0.8 billion from the sale of the shares of Idenix Pharmaceuticals, Inc., US (Idenix) to Merck & Co., US, and a gain of $0.4 billion from the divestment of the shareholding in LTS Lohmann Therapie-Systeme AG, Germany (LTS).
Basic earnings per share from continuing operations decreased 33% (–17% cc) to $2.92, declining less than net income from continuing operations due to the lower number of average outstanding shares.
Free Cash Flow from continuing operations decreased 15% to $9.3 billion, primarily due to negative currency impact on operations.
Net income from discontinued operations amounted to $10.8 billion in 2015, which included $12.7 billion of pre-tax divestment gains and the operational results of the divested businesses until the respective dates of completion of the transactions, compared to a net loss of $447 million in 2014. For more information on discontinued operations see "—Factors Affecting Comparability of Year-On-Year Results of Operations", below and "Item 18. Financial Statements—Note 30".
For the total Group, net income amounted to $17.8 billion in 2015 compared to $10.3 billion in 2014, impacted by the exceptional divestment gains included in net income from the discontinued operations. Basic earnings per share increased to $7.40 from $4.21 in the prior year and free cash flow for the total Group amounted to $9.0 billion.
Across our divisions, our portfolio of growth products continued to support performance in 2015. Sales of growth products increased 17% to $16.6 billion, or 34% of net sales, demonstrating our ability to renew our product portfolio and helping offset the impact of patent expirations. In our Pharmaceuticals Division, sales of growth products increased 33% (cc) and accounted for 44% of net sales, up from 36% in 2014.
Pharmaceutical growth products in 2015 included Gilenya ($2.8 billion, +21% cc), our oral therapy for multiple sclerosis; Tasigna ($1.6 billion, +16% cc), a treatment for chronic myeloid leukemia; and Afinitor ($1.6 billion, +10% cc), a treatment for several types of cancer.
Although overall Alcon performance lagged in 2015, some products continued to do well. Alcon saw continued growth in sales of its innovative Dailies Total1 contact lenses, as well as double-digit growth in glaucoma fixed-dose combination products and Systane for dry eye. Sales of disposable cataract and vitreoretinal surgical supplies also grew.
In the Sandoz Division, sales of biopharmaceuticals, including biosimilar follow-on versions of complex biologic drugs, rose 39% (cc) to $772 million globally.
108
Efforts to expand in emerging growth markets2 such as those in Asia, Africa and Latin America continued to deliver results, although growth moderated as overall economic activity slowed in China, Brazil, India and elsewhere. Net sales in emerging markets rose 7% (cc) to $12.4 billion, led by Turkey, up 14% (cc), and Brazil, up 12% (cc).
Last year Novartis continued to find synergies across divisions in our ongoing effort to improve productivity. Total productivity gains reached $3.2 billion in 2015, 6% of net sales. Novartis Business Services (NBS), the cross-divisional services organization that ramped up last year, played a key role in achieving this result. NBS continues to scale up the offshoring of services to global service centers, while outsourcing selected services to third parties.
The biggest savings came from our procurement efforts, through which we saved more than $1.7 billion on goods and services, or about 8% of the spending managed by Novartis procurement organizations.
An ongoing effort begun in 2010 to optimize our global manufacturing network continues to yield results. In 2015, we announced plans to exit Sandoz manufacturing sites in Frankfurt and Gerlingen, Germany, as well as in Turbhe, India. We also closed a Pharmaceuticals Division facility in Resende, Brazil, divested an Alcon site in Kaysersberg, France, as well as a pharmaceutical site in Taboão da Serra, Brazil, and announced the downsizing of a Pharmaceuticals Division site in Ringaskiddy, Ireland. To date, 25 sites in our continuing operations have been or are being restructured or divested. These steps help us balance production capacity and further increase efficiency.
The following table provides an overview of net sales to third parties by segment:
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Pharmaceuticals |
30,445 | 31,791 | (4 | ) | 6 | ||||||||
Alcon |
9,812 | 10,827 | (9 | ) | (1 | ) | |||||||
Sandoz |
9,157 | 9,562 | (4 | ) | 7 | ||||||||
| | | | | | | | | | | | | | |
Net sales to third parties from continuing operations |
49,414 | 52,180 | (5 | ) | 5 | ||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pharmaceuticals
Pharmaceuticals delivered net sales of $30.4 billion (–4%, +6% in constant currencies, or cc) as increased volumes, including from the oncology portfolio acquired from GlaxoSmithKline (GSK) in 2015, countered the impact of greater generic competition, which reduced sales by 7.0 percentage points.
Growth products generated $13.5 billion of division net sales, growing 33% (cc) compared to last year. These products—which include Gilenya, Tasigna, Ultibro, the combination of Tafinlar + Mekinist, Jakavi, Revolade and Cosentyx—contributed 44% of division net sales, compared to 36% in 2014.
Sales in emerging growth markets increased 9% (cc) to $7.8 billion.
- 2
- Growth products are products launched in 2010 or later, or products with exclusively until at least 2019 in key markets (EU, US, Japan), except Sandoz (launched in the last 24 months). Emerging growth markets are all markets except the US, Canada, Western Europe, Japan, Australia and New Zealand.
109
Highlights in 2015 included regulatory approval in the US and EU for Entresto (formerly LCZ696) for chronic heart failure; Farydak for multiple myeloma; and Tafinlar + Mekinist, the first combination therapy for metastatic melanoma. Cosentyx, which was successfully launched in the US and EU in 2015 to treat psoriasis, also received approval in Europe to treat psoriatic arthritis and ankylosing spondylitis.
Oncology
Oncology sales rose 15% (+24% cc) to $13.5 billion, boosted by the newly acquired portfolio from GSK and continued growth in our existing products. By brand, growth drivers included Afinitor, up 10% (cc) to $1.6 billion; Tasigna, up 16% (cc) to $1.6 billion; and Jakavi, up 71% (cc) to $410 million.
Neuroscience
Neuroscience sales were $3.9 billion (–4%, +5% cc), with Gilenya rising 12% (+21% cc) to $2.8 billion and more than offsetting declines in Exelon/Exelon Patch due to generic competition.
Retina
Sales in Retina were $2.1 billion (–16%, –3% cc), driven mainly by lower sales of Lucentis, which faced increased competitive pressure in Japan and some European markets.
Immunology and Dermatology
Sales in Immunology and Dermatology were $2.1 billion (0%, +11% cc). Cosentyx made a strong start after launching in February, reaching sales of $261 million. Additionally, Zortress/Certican rose 2% (+17% cc) to $335 million, and Ilaris increased 19% (+30% cc), helping offset declines in other products primarily stemming from generic competition.
Respiratory
Respiratory sales were $1.6 billion (+1%, +17% cc). We had sales of $0.6 billion (+19%, +40% cc) for our portfolio of drugs for chronic obstructive pulmonary disease (COPD), including Onbrez Breezhaler/Arcapta Neohaler, Seebri Breezhaler and Ultibro Breezhaler. Sales of Xolair reached $0.8 billion (–3%, +14% cc), including as a treatment for chronic hives.
Cardio-Metabolic
Entresto was launched in the US in the third quarter and full-year sales reached $21 million. Galvus sales were $1.1 billion (–7%, +8% cc).
Established Medicines
Established medicines such as Diovan ($1.3 billion, –40% cc) and Exforge ($1.0 billion, –15% cc) continued to see declines as a result of generic competition.
110
TOP 20 PHARMACEUTICALS DIVISION PRODUCT NET SALES—2015
| |
|
|
US | Rest of world | Total | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Brands
|
Business Franchise |
Indication | $ m | % change in constant currencies |
$ m | % change in constant currencies |
$ m | % change in $ |
% change in constant currencies |
|||||||||||||||||
Gleevec/Glivec |
Oncology | Chronic myeloid leukemia and GIST | 2,533 | 17 | 2,125 | (5 | ) | 4,658 | (2 | ) | 5 | |||||||||||||||
Gilenya |
Neuroscience | Relapsing multiple sclerosis | 1,497 | 26 | 1,279 | 17 | 2,776 | 12 | 21 | |||||||||||||||||
Lucentis |
Retina | Age-related macular degeneration | 2,060 | (2 | ) | 2,060 | (16 | ) | (2 | ) | ||||||||||||||||
Tasigna |
Oncology | Chronic myeloid leukemia | 661 | 22 | 971 | 12 | 1,632 | 7 | 16 | |||||||||||||||||
Sandostatin |
Oncology | Carcinoid tumors and Acromegaly | 823 | 10 | 807 | 5 | 1,630 | (1 | ) | 7 | ||||||||||||||||
Afinitor/Votubia |
Oncology | Breast cancer / TSC | 892 | 11 | 715 | 9 | 1,607 | 2 | 10 | |||||||||||||||||
Diovan/Co—Diovan |
Established Medicines | Hypertension | 254 | (74 | ) | 1,030 | (17 | ) | 1,284 | (45 | ) | (40 | ) | |||||||||||||
Galvus |
Cardio-Metabolic | Diabetes | 1,140 | 8 | 1,140 | (7 | ) | 8 | ||||||||||||||||||
Exforge |
Established Medicines | Hypertension | 67 | (76 | ) | 980 | 1 | 1,047 | (25 | ) | (15 | ) | ||||||||||||||
Exjade |
Oncology | Chronic iron overload | 365 | 19 | 552 | 3 | 917 | (1 | ) | 8 | ||||||||||||||||
Xolair(1) |
Respiratory | Asthma | 755 | 14 | 755 | (3 | ) | 14 | ||||||||||||||||||
Exelon/Exelon Patch |
Neuroscience | Alzheimer's disease | 340 | (30 | ) | 388 | (13 | ) | 728 | (28 | ) | (21 | ) | |||||||||||||
Neoral/Sandimmun(e) |
Immunology and Dermatology | Transplantation | 47 | (15 | ) | 523 | (5 | ) | 570 | (17 | ) | (6 | ) | |||||||||||||
Votrient |
Oncology | Renal cell carcinoma | 287 | nm | 278 | nm | 565 | nm | nm | |||||||||||||||||
Voltaren (excl. other divisions) |
Established Medicines | Inflammation/pain | 558 | 0 | 558 | (12 | ) | 0 | ||||||||||||||||||
Tafinlar/Mekinist |
Oncology | Melanoma | 267 | nm | 186 | nm | 453 | nm | nm | |||||||||||||||||
Myfortic |
Immunology and Dermatology | Transplantation | 109 | (27 | ) | 332 | 0 | 441 | (19 | ) | (8 | ) | ||||||||||||||
Jakavi |
Oncology | Myelofibrosis | 410 | 71 | 410 | 47 | 71 | |||||||||||||||||||
Promacta/Revolade |
Oncology | Immune thrombocytopenic purpura | 196 | nm | 206 | nm | 402 | nm | nm | |||||||||||||||||
Ritalin/Focalin |
Established Medicines | Attention deficit/ hyperactivity disorder | 226 | (31 | ) | 139 | 1 | 365 | (26 | ) | (20 | ) | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Top 20 products total |
8,564 | 7 | 15,434 | 7 | 23,998 | (3 | ) | 7 | ||||||||||||||||||
Rest of portfolio |
1,715 | (2 | ) | 4,732 | 4 | 6,447 | (9 | ) | 2 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total Division sales |
10,279 | 5 | 20,166 | 6 | 30,445 | (4 | ) | 6 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Net sales reflect Xolair sales for all indications (e.g. including Xolair SAA and Xolair CSU, which are managed by the Immunology and Dermatology franchise).
nm = not meaningful
Gleevec/Glivec ($4.7 billion, +5% cc) is a treatment for adult patients with metastatic and/or unresectable KIT+ gastrointestinal stromal tumors (GIST), as an adjuvant treatment for certain adult patients following resection of KIT+ GIST, and as a targeted therapy for Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML). Sales growth were driven mainly by the US, and more than compensated for the loss of patent exclusivity in some markets. In the US, Novartis Pharmaceuticals Corporation has settled its litigation with a subsidiary of Sun Pharmaceutical Industries Ltd. relating to Novartis patents covering the use of certain polymorphic forms of Gleevec/Glivec, which expire in 2019 (including pediatric exclusivity). The basic compound patent for Gleevec/Glivec expired in the US on July 4, 2015. As a result of the settlement, Novartis will permit Sun's subsidiary to market a generic version of Gleevec/Glivec in the US commencing on February 1, 2016.
111
Gilenya ($2.8 billion, +21% cc), the first once-daily oral therapy to treat relapsing forms of multiple sclerosis (RMS), continued to outgrow the market, achieving double-digit growth in 2015 in recognition of strong trends towards oral treatments with higher efficacy. Growth was also fueled by an increasing acceptance of the role of high-efficacy treatments when used earlier in the course of the disease. Gilenya continues to see volume growth through new patient initiations in both the US and non-US markets. In the US, Gilenya is indicated for relapsing forms of MS. In the EU, Gilenya is indicated for adult patients with high disease activity despite treatment with at least one disease modifying agent, or rapidly evolving severe relapsing remitting MS. In an expanding oral market with multiple options, Gilenya is the only oral disease-modifying therapy (DMT) to impact the course of RMS with high efficacy across four key measures of disease activity: relapses, MRI lesions, brain shrinkage (brain volume loss) and disability progression. Gilenya has an overall positive benefit-risk profile with over ten years of safety experience. As of November 30, 2015, Gilenya has been used to treat approximately 134,000 patients in clinical trials and in a post-marketing setting, with a total patient exposure of approximately 289,000 patient years. Gilenya is currently approved in over 80 countries around the world. Gilenya is licensed from Mitsubishi Tanabe Pharma.
Lucentis ($2.1 billion, –2% cc) sales were impacted by increased competition in Japan and in some European markets, which offset growth opportunities in Emerging Markets. Lucentis maintained a strong ex-US market position across indications but was impacted by competitive pressures in the neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME) indications, partially offset by continued growth in macular edema secondary to central and branch retinal vein occlusion (CRVO and BRVO), and choroidal neovascularization secondary to pathologic myopia (mCNV) indications. Lucentis is an anti-VEGF therapy licensed in many countries for the treatment of the following five ocular indications: nAMD, DME, CRVO, BRVO, and mCNV. Lucentis is approved in more than 100 countries to treat patients with the first four conditions, and in more than 80 countries for mCNV. In 2015, Lucentis obtained reimbursement for DME and RVO in Australia. It is the only anti-VEGF treatment delivered in a pre-filled syringe and approved for a treat & extend regimen across all indications in Europe. Since its launch in 2006, there have been more than 3.7 million patient-treatment years of exposure for Lucentis with more than 22 million injections. Lucentis is an anti-VEGF therapy specifically designed for the eye, minimizing systemic exposure, that has demonstrated significant efficacy with individualized dosing in its five licensed indications and has a well-established safety profile supported by extensive clinical studies and real-world experience. Lucentis is licensed from Genentech, and Novartis holds the rights to commercialize the product outside the US. Genentech holds the rights to commercialize Lucentis in the US.
Tasigna ($1.6 billion, +16% cc) performance was driven by strong growth in the US and other markets. Tasigna is currently approved as a first-line therapy for newly diagnosed patients with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in the chronic phase in more than 85 countries globally, including the US, EU, Japan and Switzerland, with additional submissions pending worldwide. Tasigna (nilotinib) is also approved in more than 110 countries as a second-line treatment for patients with Ph+ CML in chronic and/or accelerated phase who are resistant or intolerant to existing treatment, such as Gleevec/Glivec.
Sandostatin ($1.6 billion, +7% cc) continued to benefit from the increasing use of Sandostatin LAR (long acting release) in key markets and from the launch of the enhanced presentation (now approved in 69 countries) which includes a diluent, safety needle and vial adapter. Sandostatin is a somatostatin analogue used to treat patients with acromegaly as well as neuroendocrine tumors (NET). In NET, it is used for both the treatment of patients with symptoms of carcinoid syndrome and those with advanced NET of the midgut or unknown primary tumor location (currently approved in more than 60 countries).
Afinitor/Votubia ($1.6 billion, +10% cc) performance was driven by strong growth in the US, Japan and other markets. Afinitor is an oral inhibitor of the mTOR pathway approved in combination with exemestane for the treatment of patients with HR+/HER2– advanced breast cancer after failure with a
112
non-steroidal aromatase inhibitor (NSAI), for advanced renal cell carcinoma (RCC) following vascular endothelial growth factor-targeted therapy (after failure of sunitinib and sorafenib in the US) and for the treatment of advanced pancreatic neuroendocrine tumors (NET). Afinitor is also approved for treatment of patients with subependymal giant cell astrocytoma (SEGA) and renal angiomyolipoma associated with tuberous sclerosis complex (TSC), including as a dispersible tablet formulation in the US and EU for SEGA. Everolimus is also in Phase III development for patients with nonfunctional gastrointestinal and lung NET, HER2+ breast cancer, diffuse large B-cell lymphoma and TSC-related seizures. Everolimus, the active ingredient in Afinitor/Votubia, is available under the trade names Zortress/Certican for use in other non-oncology indications and is exclusively licensed to Abbott and sublicensed to Boston Scientific for use in drug-eluting stents.
Diovan Group ($1.3 billion, –40% cc), consisting of Diovan monotherapy and the combination product Co-Diovan/Diovan HCT, continues to retain a blockbuster status despite generic competition in most markets, including the US (following July 7, 2014 Diovan monotherapy generic entry), many EU countries and Japan (generic entry in June 2014). Sales continued to grow in Emerging Growth Markets, including China and selected countries in Latin America, Asia Pacific and Africa, partially compensating for loss of exclusivity in the US and the EU.
Galvus Group ($1.1 billion, +8% cc), includes Galvus, an oral treatment for type 2 diabetes, and Eucreas, a single-pill combination of vildagliptin (the active ingredient in Galvus) and metformin. Galvus delivered solid growth with major milestones including approval of the Galvus monotherapy indication in China in April 2015. In September 2015, the Japanese HA PMDA approved Eucreas (EquMet), the first single-pill combination of a DPP4 inhibitor and metformin approved in this market. The focus for Galvus remains on patients whose diabetes remains uncontrolled on metformin, earlier treatment intensification as well as on an expansion of usage in key segments such as elderly and renal-impaired patients. Galvus Group is currently approved in more than 125 countries.
Exforge Group ($1.0 billion, –15% cc) includes two medicines approved for the treatment of hypertension: Exforge, a single-pill combination of the angiotensin receptor blocker (ARB) valsartan and the calcium channel blocker amlodipine besylate; and ExforgeHCT, a single pill combining an ARB (valsartan), calcium channel blocker (amlodipine) and a diuretic (hydrochlorothiazide) three widely prescribed blood pressure treatments. Exforge lost exclusivity in October 2014 and ExforgeHCT in November 2014 in the US. Outside the US, Exforge HCT is growing across all regions, showing significantly high growth in emerging markets. Exforge continues to grow with double-digit growth in China and a number of emerging markets. Exforge is now available in more than 100 countries and ExforgeHCT is available in over 77 countries.
Exjade ($917 million, +8% cc), a once-daily dispersible tablet for chronic transfusional iron overload saw sales increases in the US and Asia augmented by the March 2015 approval in the US of Jadenu, an oral tablet formulation that can be swallowed or crushed, and was approved by the FDA in 2015. Regulatory applications for Jadenu have been submitted in the EU, Canada, Switzerland, and many other countries. Exjade, first approved in 2005 and now approved in more than 100 countries, is also approved for the treatment of chronic iron overload in patients with non-transfusion-dependent thalassemia in more than 70 countries, with additional regulatory reviews underway. Jadenu is also approved for the treatment of chronic iron overload in patients with non-transfusion-dependent thalassemia in the US.
Xolair ($755 million, +14% cc), a biologic drug for appropriate patients with severe persistent allergic asthma in Europe and moderate-to-severe persistent allergic asthma in the US, is currently approved in more than 90 countries. Its sales continued to grow strongly in Canada, Europe and Latin America. Xolair is also approved in the EU, Switzerland and over 40 other countries as a treatment for chronic spontaneous urticaria (CSU), also known as chronic idiopathic urticaria (CIU), for which it is approved in the US and now Canada and Australia. Novartis co-promotes Xolair with Genentech in the US and shares a portion of the operating income, but does not book US sales.
113
Exelon/Exelon Patch ($728 million, –21% cc) sales declined due to generic competition for ExelonPatch in the EU and now in the US. ExelonPatch is approved for the treatment of mild-to-moderate Alzheimer's disease dementia (AD) in more than 90 countries, including more than 20 countries where it is also approved for Parkinson's disease dementia. ExelonPatch is also indicated for the treatment of patients with severe AD in 14 countries, including the US.
Neoral/Sandimmun ($570 million, –6% cc), a micro-emulsion formulation of cyclosporine, is an immunosuppressant to prevent organ rejection following a kidney, liver or heart transplant. Neoral is also approved for use in lung transplant in many countries outside of the US. Additionally, it is indicated for treating selected autoimmune disorders such as psoriasis and rheumatoid arthritis. First launched in 1995, Neoral is marketed in approximately 100 countries. Although sales are declining due to generic competition and mandatory price reductions, most notably in Europe and Japan, the decrease is not as rapid as has been the case in other therapeutic areas, due to the special characteristics of the solid organ transplant market.
Votrient ($565 million) is a small molecule tyrosine kinase inhibitor that targets a number of intracellular proteins to limit tumor growth and cell survival. Acquired from GSK in 2015, Votrient is approved in the US for the treatment of patients with advanced renal cell carcinoma (aRCC), and in the EU for first-line treatment of adult patients with aRCC and for patients who have received prior cytokine therapy for advanced disease. RCC is the most common type of kidney cancer in adults, and nearly one-fifth of patients have aRCC at the time of diagnosis. Votrient is also indicated for patients with advanced soft tissue sarcoma (STS) who have received prior chemotherapy. The efficacy of Votrient for the treatment of patients with adipocytic STS or gastrointestinal stromal tumors has not been demonstrated. STS is a type of cancer which can arise from a wide variety of soft tissues including muscle, fat, blood vessel and nerves. Votrient is approved in 99 countries worldwide for aRCC and in 87 countries for aSTS.
Voltaren/Cataflam ($558 million, 0% cc), is a leading non-steroidal anti-inflammatory drug (NSAID) for the relief of symptoms in rheumatic diseases such as rheumatoid arthritis and osteoarthritis, and for various other inflammatory and pain conditions. Voltaren/Cataflam was first registered in 1973 and is available in more than 140 countries. This product, which is subject to generic competition, is marketed by the Pharmaceuticals Division in a wide variety of dosage forms, including tablets, drops, suppositories, ampoules and topical therapy. In addition, in various countries, our Sandoz Division markets generic versions of the product and our Alcon Division markets Voltaren for ophthalmic indications.
Tafinlar + Mekinist ($453 million) achieved strong growth in sales. Acquired from GSK in 2015, this combination is the first of its kind for the treatment of patients with BRAF V600 mutation-positive unresectable or metastatic melanoma, as detected by a validated test, in the US, EU, Canada and several other markets. In August, the combination of Tafinlar + Mekinist was approved in Europe for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation and in November, this combination received regular approval in the US based on the completion of two Phase III confirmatory trials. The combination was previously approved in the US under accelerated approval. Tafinlar targets the serine/threonine kinase BRAF in the RAS/RAF/MEK/ERK pathway and Mekinist targets the threonine/tyrosine kinases MEK1 and MEK2 in the MAP kinase pathway, resulting in dual blockade of this pathway, improving the clinical efficacy of the treatment. This is the first combination of BRAF/MEK inhibitors to achieve a median overall survival of more than two years in two Phase III studies in BRAF V600 mutation positive unresectable or metastatic melanoma patients. Tafinlar + Mekinist are also approved as single agents for the treatment of patients with unresectable or metastatic melanoma in more than 45 and 30 countries worldwide, respectively. In addition, Tafinlar also has Breakthrough Therapy designation from the FDA for treatment of non-small cell lung cancer (NSCLC) patients with BRAF V600E mutations who have received at least one prior line of platinum-containing chemotherapy. In July, the combination therapy Tafinlar + Mekinist also received Breakthrough Therapy designation from the FDA for NSCLC patients with BRAF V600E mutations.
114
Myfortic ($441 million, –8% cc), a transplantation medicine, is available in more than 90 countries to prevent organ rejection in adult kidney transplant patients. Although it has experienced declining sales after the expected launch of generic competition in the US in early 2014, the decrease is not as rapid as has been the case in other therapeutic areas, due to the special characteristics of the solid organ transplant market. Myfortic continued to grow in some geographies where generic competition has not yet begun. Marketing authorizations for generic competitors have been granted in European countries.
Jakavi ($410 million, +71% cc) performance was driven by strong volume growth across multiple markets. Jakavi is an oral inhibitor of the JAK 1 and JAK 2 tyrosine kinases. It is the first JAK inhibitor indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post-polycythemia vera myelofibrosis or post-essential thromboycythemia myelofibrosis. Jakavi is currently approved in more than 95 countries, including EU member states, Japan and Canada. In March 2015, the EC approved Jakavi for the treatment of adult patients with polycythemia vera (PV) who are resistant to or intolerant of hydroxyurea. Jakavi is the first targeted treatment approved by the EC for these patients. More than 45 countries have approved Jakavi in the PV indication, including Switzerland, Canada and Japan, and regulatory applications have been submitted in other countries. Novartis licensed ruxolitinib from Incyte Corporation for development and commercialization outside the US.
Promacta/Revolade ($402 million) performance was driven by strong growth in the US and other markets. Acquired from GSK in 2015, Promacta is marketed under the brand name Promacta in the US and Revolade in most countries outside the US. It is the only approved once-daily oral thrombopoietin receptor agonist. In August 2015, the US FDA approved an expanded use for Promacta to include children 1 year of age and older with chronic immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins or splenectomy. The updated label includes a new oral suspension formulation of Promacta that is designed for younger children who may not be able to swallow tablets. Revolade is currently under review for this same indication with the EMA. In December, Novartis received a positive CHMP opinion on a potential update to the adult chronic ITP indication with regards to the use of Revolade in non-splenectomised patients; the EMA decision is expected in February 2016. Revolade was approved by the European Commission in September 2015 for the treatment of adults with acquired severe aplastic anemia (SAA) who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for hematopoietic stem cell transplant.
Ritalin/Focalin ($365 million, –20% cc) is a treatment for attention deficit hyperactivity disorder (ADHD) in children. Ritalin and Ritalin LA are available in more than 70 and 30 countries, respectively, and are also indicated for narcolepsy. To date in 2015, Ritalin LA has been granted the adult ADHD indication in over 20 countries. Focalin and Focalin XR are available in the US and Focalin XR is additionally indicated for adults. Focalin XR is also approved in Switzerland. Ritalin Immediate Release has generic competition in most countries. Most strengths of Ritalin LA and Focalin are subject to generic competition in the US.
Alcon
Alcon net sales in 2015 were $9.8 billion (–9%, –1% in constant currencies, or cc). Regionally, sales were flat in Japan and rose in Latin America and the Caribbean. In Europe, the Middle East and Africa, sales rose 1% (cc), with strong sales of recently launched contact lenses, including Dailies Total1 and Air Optix Colors, offset by declines in surgical equipment.
Sales in North America declined 3%, mainly due to increased generic competition for some pharmaceutical products and soft surgical equipment sales. In Asia and Russia, sales declined 5% (cc), driven by a significant market slowdown, with weak performance in China, India and Southeast Asia.
To accelerate growth, we are taking concerted action on two fronts. For the Surgical and Vision Care businesses, we have identified key actions as part of a growth plan. They include steps to optimize
115
innovation in intraocular lenses (IOLs) for cataract surgery, prioritizing and investing in the development of promising new products, and improving the effectiveness of our sales force.
In addition, we plan to strengthen our ophthalmic medicines business by transferring pharmaceutical products from Alcon to our Pharmaceuticals Division, combining expertise in pharmaceuticals development and marketing with the strong Alcon brand.
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
Change in $ |
Constant currencies change |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Surgical |
|||||||||||||
Cataract products |
2,853 | 3,174 | (10 | ) | (2 | ) | |||||||
of which IOLs |
1,099 | 1,264 | (13 | ) | (4 | ) | |||||||
Vitreoretinal products |
594 | 615 | (3 | ) | 6 | ||||||||
Refractive/other |
251 | 284 | (12 | ) | (5 | ) | |||||||
| | | | | | | | | | | | | | |
Total |
3,698 | 4,073 | (9 | ) | (1 | ) | |||||||
| | | | | | | | | | | | | | |
Ophthalmic Pharmaceuticals |
|||||||||||||
Glaucoma |
1,196 | 1,319 | (9 | ) | 2 | ||||||||
Allergy/otic/nasal |
780 | 887 | (12 | ) | (8 | ) | |||||||
Infection/inflammation |
1,011 | 1,066 | (5 | ) | 2 | ||||||||
Dry eye |
583 | 608 | (4 | ) | 6 | ||||||||
Other |
243 | 331 | (27 | ) | (15 | ) | |||||||
| | | | | | | | | | | | | | |
Total |
3,813 | 4,211 | (9 | ) | 0 | ||||||||
| | | | | | | | | | | | | | |
Vision Care |
|||||||||||||
Contact lenses |
1,743 | 1,897 | (8 | ) | 1 | ||||||||
Contact lens care |
558 | 646 | (14 | ) | (8 | ) | |||||||
| | | | | | | | | | | | | | |
Total |
2,301 | 2,543 | (10 | ) | (2 | ) | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Total net sales |
9,812 | 10,827 | (9 | ) | (1 | ) | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Surgical
Surgical franchise sales were $3.7 billion (–9%, –1% cc). Solid sales of cataract and vitreoretinal disposable surgical supplies were offset by competitive pressure on IOL sales, as well as a slowdown in equipment purchases in the US and emerging markets, particularly Asia. Launches in 2015 of our UltraSert pre-loaded and PanOptix trifocal IOLs in Europe, as well as regulatory approval of UltraSert pre-loaded IOLs in the US, provide an opportunity to renew growth in this segment.
Ophthalmic Pharmaceuticals
Ophthalmic Pharmaceuticals sales were $3.8 billion (–9%, 0% cc). In glaucoma products, strong performance of fixed-dose combination products, including Azarga and Simbrinza, was offset by generic competition for monotherapies. Systane eye drops to treat the symptoms of dry eye saw sales grow in the US and Europe, the Middle East and Africa, with softer sales across emerging markets. Sales of allergy, nasal and ear medicines declined, driven by continued generic competition in the US.
Vision Care
Vision Care sales were $2.3 billion (–10%, –2% cc). Contact lens sales reached $1.7 billion (–8%, +1% cc), with strong sales of innovative lenses, particularly Dailies Total1 and Air Optix Colors, offset by
116
declines in older products. Sales of contact lens solutions were $0.6 billion (–14%, –8% cc), affected by ongoing market shifts to daily disposable lenses, as well as competitive pressure in the US.
Sandoz
In 2015, Sandoz had net sales of $9.2 billion (–4%, +7% in constant currencies, or cc, from the prior year), driven by a 15.0 percentage-point increase in volume, more than offsetting 8.0 percentage points of price erosion. Performance was driven by strong sales growth in the US (+10% cc), Asia Pacific (+13% cc), Latin America (+18% cc), and Middle East and Africa (+13% cc). Sales in Western Europe grew 3% (cc), with Germany growing 5% (cc).
Sandoz continued to strengthen its global leadership position in biopharmaceuticals, which include medicines that are difficult to develop and manufacture. In June, Sandoz launched Glatopa—the first generic competitor to Copaxone® 20 mg—in the US. And in September in the US, Sandoz also launched Zarxio, which is the first biosimilar approved by the US Food and Drug Administration (FDA) under new regulations.
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
Change in $ |
Constant currencies change |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Retail Generics |
7,199 | 7,933 | (9 | ) | 2 | ||||||||
Biopharmaceuticals & Oncology Injectables |
1,378 | 1,094 | 26 | 39 | |||||||||
Anti-Infectives |
580 | 535 | 9 | 18 | |||||||||
| | | | | | | | | | | | | | |
Total |
9,157 | 9,562 | (4 | ) | 7 | ||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Retail Generics
In Retail Generics, Sandoz develops, manufactures and markets active ingredients and finished dosage forms of pharmaceuticals. This franchise includes the specialty areas of dermatology, respiratory and ophthalmics, as well as finished dosage forms of anti-infective products sold under the Sandoz name. Retail Generics sales worldwide were $7.2 billion (–9%, +2% cc). New product launches included US-authorized generics of our Pharmaceuticals Division's Exelon Patch and Exforge, as well as bivalirudin, an injectable anticoagulant.
Biopharmaceuticals and Oncology Injectables
In Biopharmaceuticals, Sandoz develops, manufactures and markets protein- and biotechnology-based products known as biosimilars, as well as Glatopa. Sandoz also provides biotechnology manufacturing services to other companies. Sales of biopharmaceuticals rose 25% (+39% cc) to $772 million. Sandoz further strengthened its leadership in biosimilars in 2015 with the US approval of Zarxio (filgrastim), used to fight infection in cancer patients receiving chemotherapy.
Sandoz is the global market leader in biosimilars with three products that continue to see strong growth in their respective categories: Omnitrope, a human growth hormone; Binocrit, an erythropoiesis-stimulating agent; and filgrastim under the brand names Zarzio outside the US and Zarxio in the US. We continued in 2015 to build our portfolio of biosimilars. The FDA and European Medicines Agency confirmed acceptance of our applications for etanercept, a proposed biosimilar to Amgen's Enbrel®, which treats autoimmune diseases such as rheumatoid arthritis and psoriasis. The FDA also accepted our applications for pegfilgrastim, a proposed biosimilar to Amgen's Neulasta®, used to reduce the chance of infection in cancer patients receiving chemotherapy. Sandoz has five biosimilars in Phase III development or registration preparation.
117
Sandoz also develops, manufactures and markets cytotoxic products for traditional cancer chemotherapy. The Oncology Injectables business now includes a portfolio of more than 25 products.
Anti-Infectives
Sandoz manufactures pharmaceutical ingredients and intermediates—mainly antibiotics—for sale under the Sandoz name and to third-party customers. Total Anti-Infectives sales were $1.4 billion, up 9% (cc) driven by a strong flu season and restored production capacity after 2014 quality upgrades. Sales of finished dosage forms sold under the Sandoz name reached $860 million. Anti-Infectives sold to third parties for sale under their own name reached $580 million.
Operating Income from Continuing Operations
Operating income from continuing operations was $9.0 billion (–19%,–2% cc), mainly due to amortization of the new oncology assets in Pharmaceuticals. The current year includes an exceptional expense of $400 million for a settlement of the specialty pharmacies case in the Southern District of New York, whereas the prior-year benefitted from a one-time commercial settlement gain of $302 million and $248 million gain from selling a Novartis Venture Fund investment. The negative currency impact of 17 percentage points was mainly due to the strong $ versus the euro, Japanese yen and emerging market currencies. Operating income margin in constant currencies decreased 1.4 percentage points; currency had a negative impact of 1.7 percentage points resulting in a net decrease of 3.1 percentage points to 18.2 percent of net sales.
The following table provides an overview of operating income by segment:
| |
Year ended Dec 31, 2015 |
% of net sales |
Year ended Dec 31, 2014 |
% of net sales |
Change in $ |
Change in constant currencies |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
|
$ m |
|
% |
% |
|||||||||||||
Pharmaceuticals |
7,597 | 25.0 | 8,471 | 26.6 | (10 | ) | 5 | ||||||||||||
Alcon |
794 | 8.1 | 1,597 | 14.8 | (50 | ) | (20 | ) | |||||||||||
Sandoz |
1,005 | 11.0 | 1,088 | 11.4 | (8 | ) | 1 | ||||||||||||
Corporate |
(419 | ) | (67 | ) | nm | nm | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating income from continuing operations |
8,977 | 18.2 | 11,089 | 21.3 | (19 | ) | (2 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
nm = not meaningful
118
Core Operating Income key figures(1)
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Core gross profit from continuing operations |
36,900 | 38,821 | (5 | ) | 5 | ||||||||
Marketing & Sales |
(11,729 | ) | (12,355 | ) | 5 | (5 | ) | ||||||
Research & Development |
(8,738 | ) | (8,723 | ) | 0 | (6 | ) | ||||||
General & Administration |
(2,389 | ) | (2,552 | ) | 6 | 0 | |||||||
Other income |
823 | 563 | 46 | 59 | |||||||||
Other expense |
(1,077 | ) | (1,281 | ) | 16 | 7 | |||||||
| | | | | | | | | | | | | | |
Core operating income from continuing operations |
13,790 | 14,473 | (5 | ) | 10 | ||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
as % of net sales |
27.9 | % | 27.7 | % | |||||||||
- (1)
- For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS Measures as Defined by Novartis".
The adjustments made to operating income to arrive at core operating income from continuing operations amounted to $4.8 billion (2014: $3.4 billion). The increase was mainly driven by higher amortization of the new oncology assets in Pharmaceuticals, higher legal settlement expense and higher acquisition-related expense, whereas 2014 included a commercial settlement gain of $302 million, partially offset by the provision of $204 million for the US healthcare reform fee.
Excluding these items, core operating income from continuing operations decreased 5% (+10% cc) to $13.8 billion. Core operating income margin in constant currencies increased 1.3 percentage points mainly due to higher sales and productivity initiatives; currency had a negative impact of 1.1 percentage points, resulting in a margin of 27.9% of net sales, compared to 27.7% in 2014.
The following table provides an overview of core operating income by segment:
| |
Year ended Dec 31, 2015 |
% of net sales |
Year ended Dec 31, 2014 |
% of net sales |
Change in $ |
Change in constant currencies |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
|
$ m |
|
% |
% |
|||||||||||||
Pharmaceuticals |
9,420 | 30.9 | 9,514 | 29.9 | (1 | ) | 14 | ||||||||||||
Alcon |
3,063 | 31.2 | 3,811 | 35.2 | (20 | ) | (7 | ) | |||||||||||
Sandoz |
1,659 | 18.1 | 1,571 | 16.4 | 6 | 17 | |||||||||||||
Corporate |
(352 | ) | (423 | ) | 17 | 11 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Core operating income from continuing operations |
13,790 | 27.9 | 14,473 | 27.7 | (5 | ) | 10 | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Operating income was $7.6 billion (–10%, +5% cc) and included the effects of the acquisition of GSK's oncology portfolio, among other exceptional items.
Core operating income, which excludes certain exceptional items, was $9.4 billion (–1%, +14% cc), helped by our ongoing efforts to improve productivity and control costs. Core operating income margin improved by 2.4 percentage points in constant currencies. However, that was offset by 1.4 percentage points of negative impact from currency exchange rates, yielding a core margin of 30.9% of net sales.
119
Research and development
The following table provides an overview on the reported and core Research and Development expense of the Pharmaceuticals Division:
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Research and Exploratory Development |
(2,565 | ) | (2,724 | ) | 6 | 3 | |||||||
Confirmatory Development |
(4,667 | ) | (4,607 | ) | (1 | ) | (7 | ) | |||||
| | | | | | | | | | | | | | |
Total Pharmaceuticals Division Research and Development expense |
(7,232 | ) | (7,331 | ) | 1 | (3 | ) | ||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
as % of Pharmaceuticals net sales to third parties |
23.8 | % | 23.1 | % | |||||||||
Core Research and Exploratory Development(1) |
(2,493 | ) | (2,654 | ) | 6 | 3 | |||||||
Core Confirmatory Development(1) |
(4,560 | ) | (4,343 | ) | (5 | ) | (11 | ) | |||||
| | | | | | | | | | | | | | |
Total Core Pharmaceuticals Division Research and Development expense |
(7,053 | ) | (6,997 | ) | (1 | ) | (5 | ) | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
as % of Pharmaceuticals net sales to third parties |
23.2 | % | 22.0 | % | |||||||||
- (1)
- Core excludes impairments, amortization and certain exceptional items.
Pharmaceuticals Division Research and Exploratory Development expenditure amounted to $7.2 billion in 2015, a decrease of 1% (–3% cc) compared to 2014. Confirmatory Development expenditures increased by 1% (–7% cc) to $4.7 billion, compared to $4.6 billion in 2014, mainly driven by the additional development expense for the new oncology assets acquired from GSK.
Core R&D expense in the Pharmaceuticals Division as percent of sales decreased by 0.1 percentage points in constant currencies, which was offset by negative currency movements of 1.3 percentage points mainly from the sales base, as the Core R&D expenses are primarily denominated in US dollars and Swiss francs, which resulted in a net increase of 1.2 percentage points to 23.2% of net sales.
Operating income was $0.8 billion (–50%,–20% cc).
Core operating income, which excludes certain items, was $3.1 billion (–20%,–7% cc), impacted by lower sales, higher spending (primarily on marketing and sales), investments in product development, and increased provisions for bad debt in Asia. Core operating income margin declined 2.1 percentage points in constant currencies and currency exchange rates had a negative impact of 1.9 percentage points, yielding a core margin of 31.2% of net sales.
Operating income was $1.0 billion (–8%, +1% cc).
Core operating income, which excludes certain exceptional items, increased 6% (+17% cc) to $1.7 billion. Core operating income margin increased 1.5 percentage points in constant currencies and currency exchange rates had a positive impact of 0.2 percentage points, yielding a core margin of 18.1% of net sales.
120
Corporate Income and Expense, Net
Corporate income and expense amounted to a net expense of $419 million in 2015 compared to a net expense of $67 million in the prior year. The increased expense was mainly due to the $302 million commercial settlement gain and a $248 million gain from selling Novartis Venture Fund investments recorded in 2014, partially offset by the gain on the sale of real estate in Switzerland of $54 million, lower share-based compensation accruals and lower provisions in the captive insurance companies in 2015.
Non-Operating Income and Expense
The following table provides an overview of non-operating income and expense:
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Operating income from continuing operations |
8,977 | 11,089 | (19 | ) | (2 | ) | |||||||
Income from associated companies |
266 | 1,918 | (86 | ) | (86 | ) | |||||||
Interest expense |
(655 | ) | (704 | ) | 7 | 2 | |||||||
Other financial income and expense |
(454 | ) | (31 | ) | nm | nm | |||||||
| | | | | | | | | | | | | | |
Income before taxes from continuing operations |
8,134 | 12,272 | (34 | ) | (17 | ) | |||||||
Taxes |
(1,106 | ) | (1,545 | ) | 28 | 10 | |||||||
| | | | | | | | | | | | | | |
Net income from continuing operations |
7,028 | 10,727 | (34 | ) | (18 | ) | |||||||
Net income/loss from discontinued operations |
10,766 | (447 | ) | nm | nm | ||||||||
| | | | | | | | | | | | | | |
Net income |
17,794 | 10,280 | 73 | 91 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Basic EPS ($) from continuing operations |
2.92 | 4.39 | (33 | ) | (17 | ) | |||||||
Basic EPS ($) from discontinued operations |
4.48 | (0.18 | ) | nm | nm | ||||||||
| | | | | | | | | | | | | | |
Total basic EPS ($) |
7.40 | 4.21 | 76 | 94 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
nm = not meaningful
121
The following table provides an overview of core non-operating income and expense:
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Core operating income from continuing operations |
13,790 | 14,473 | (5 | ) | 10 | ||||||||
Income from associated companies |
981 | 943 | 4 | 4 | |||||||||
Interest expense |
(655 | ) | (704 | ) | 7 | 2 | |||||||
Other financial income and expense |
(24 | ) | (31 | ) | 23 | nm | |||||||
| | | | | | | | | | | | | | |
Core income before taxes from continuing operations |
14,092 | 14,681 | (4 | ) | 10 | ||||||||
Taxes |
(2,051 | ) | (2,028 | ) | (1 | ) | (16 | ) | |||||
| | | | | | | | | | | | | | |
Core net income from continuing operations |
12,041 | 12,653 | (5 | ) | 9 | ||||||||
Core net income/loss from discontinued operations |
(256 | ) | 102 | nm | nm | ||||||||
| | | | | | | | | | | | | | |
Core net income |
11,785 | 12,755 | (8 | ) | 6 | ||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Core basic EPS ($) from continuing operations |
5.01 | 5.19 | (3 | ) | 10 | ||||||||
Core basic EPS ($) from discontinued operations |
(0.11 | ) | 0.04 | nm | nm | ||||||||
| | | | | | | | | | | | | | |
Core basic EPS ($) |
4.90 | 5.23 | (6 | ) | 7 | ||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
nm = not meaningful
Income from associated companies
Income from associated companies from continuing operations amounted to $266 million in 2015, compared to $1.9 billion in 2014. The prior-year benefited from a pre-tax gain of $0.8 billion recognized on the sale of the shares of Idenix to Merck, a gain of $0.4 billion from the divestment of the shareholding in LTS and from the gain of $64 million recorded on the Novartis Venture Funds investments.
In addition, the estimated income from Roche Holding AG declined from $599 million in the prior-year period to $343 million in 2014, due to an adjustment of $157 million recognized in the first quarter of 2015 when Roche published full year results, as well as a lower estimated income contribution from Roche for 2015 due to an announced restructuring.
The estimated share in net results from the GSK Consumer Healthcare joint venture amounted to a loss of $17 million, as income from operations was more than offset by integration charges. This estimate will be adjusted based on actual results in the first quarter of 2016. In addition, in 2015, we finalized the purchase price allocation for the investment in the GSK Consumer Healthcare joint venture which is accounted for as associated company and recognized amortization of purchase price adjustments of $62 million, resulting in a total estimated loss of $79 million for our share in the net results from the GSK Consumer Healthcare joint venture for the year.
Core income from associated companies increased to $981 million compared to $943 million in 2014. Our estimated share in core results from the consumer healthcare joint venture with GSK, which amounted to $213 million in 2015, was offset by decreases in our estimated share of core results from Roche (from $856 million to $766 million) and prior-year income from associated companies of the Novartis Venture Fund.
122
Interest Expense and other financial income and expense
Interest expense from continuing operations decreased by 7% (–2% cc) to $655 million from $704 million in the prior year.
Other financial income and expense amounted to an expense of $454 million compared to $31 million in the prior-year period mainly on account of the exceptional charges of $410 million related to Venezuela due to foreign exchange losses of $211 million and monetary losses from hyperinflation accounting of $72 million and a loss of $127 million on the sale of PDVSA bonds received to settle a portion of intra-group payables.
Core other financial income and expense, which exclude the exceptional charges of $410 million related to Venezuela, amounted to a net expense of $24 million, compared to $31 million in 2014.
Taxes
The tax rate for continuing operations (taxes as percentage of pre-tax income) in 2015 increased to 13.6% from 12.6% in the prior year, as a result of a change in profit mix from lower to higher tax jurisdictions.
The core tax rate from continuing operations (core tax as a percentage of core pre-tax income) increased to 14.6% from 13.8% in 2014, mainly as a result of a change in profit mix from lower to higher tax jurisdictions.
Net Income
Net income from continuing operations of $7.0 billion was down 34% (–18% cc) declining more than operating income mainly due to the exceptional charges related to Venezuela in the current year and the prior-year gains of $0.8 billion from the sale of Idenix shares and $0.4 billion from the sale of LTS shares.
Core net income from continuing operations of $12.0 billion was down 5% (+9% cc), in line with core operating income.
EPS
Basic earnings per share (EPS) from continuing operations was $2.92 per share, down 33% (–17% cc), declining less than net income from continuing operations due to the lower number of outstanding shares.
Core basic EPS from continuing operations was $5.01 (–3%, +10% cc), growing ahead of core net income due to lower average outstanding shares and lower minority interests.
123
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
|||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Net sales to third parties from discontinued operations |
601 | 5,816 | |||||
Operating income/loss from discontinued operations |
12,477 | (353 | ) | ||||
Net income/loss from discontinued operations |
10,766 | (447 | ) | ||||
Attributable to: |
|||||||
Shareholders of Novartis AG |
10,758 | (444 | ) | ||||
Non-controlling interests |
8 | (3 | ) | ||||
Basic earnings per share ($) from discontinued operations |
4.48 | (0.18 | ) | ||||
Free cash flow from discontinued operations |
(230 | ) | (172 | ) | |||
Operational results for discontinued operations in 2015 include the results from the Vaccines influenza business, prior to its divestment to CSL Limited on July 31, 2015, as well as results from the Vaccines non-influenza business and OTC until March 2, 2015. Operational results from the Animal Health business, which was divested on January 1, 2015 include only the divestment gain. The prior year included the results of all divested units during the full year.
Discontinued operations also include the exceptional pre-tax gains of $12.7 billion from the divestment of Animal Health ($4.6 billion) and the transactions with GSK ($2.8 billion for the Vaccines non-influenza business and $5.9 billion arising from the contribution of Novartis OTC into the GSK Consumer Healthcare joint venture). In addition, the GSK transactions resulted in $0.6 billion of additional transaction-related costs that were expensed.
Net sales to third parties of the discontinued operations in 2015 amounted to $0.6 billion compared to $5.8 billion in 2014.
Operating income from discontinued operations in 2015 amounted to an income of $12.5 billion which was mainly driven by the exceptional pre-tax gains from the portfolio transformation. Excluding the divestment gains, the remaining operating loss from discontinued operations was $0.2 billion, representing the operating performance of the Vaccines influenza business up to July 31, 2015 as well as the Vaccines non-influenza business and OTC until their respective divestment dates, and is net of the partial reversal of $0.1 billion of the impairment of the assets of Vaccines influenza business recorded in 2014.
The prior year operating loss of $353 million included an exceptional impairment charge of $1.1 billion for the Vaccines influenza business which was partially offset by an exceptional pre-tax gain of $0.9 billion from the divestment of our blood transfusion diagnostics unit.
Net income from discontinued operations amounted to $10.8 billion in 2015 compared to a net loss $447 million in 2014. For more information on discontinued operations see "—Factors Affecting Comparability of Year-On-Year Results of Operations", below and "Item 18. Financial Statements—Note 30".
For the total Group, net income amounted to $17.8 billion compared to $10.3 billion in 2014, impacted by the exceptional divestment gains included in the net income from the discontinued operations. Basic earnings per share increased to $7.40 from $4.21.
124
Following the announcement of the transactions with GlaxoSmithKline plc (GSK) and Eli Lilly and Company (Lilly) on April 22, 2014 (and the subsequent announcement of the transaction with CSL Limited (CSL)), in which we agreed to divest our Vaccines, OTC and Animal Health businesses to those companies, the businesses to be divested were accounted for as discontinued operations and were not included in our results from continuing operations for 2013 and 2014. In addition, on January 9, 2014, Novartis completed the divestment to Grifols S.A. of our former blood transfusion diagnostics unit, which had been included in our former Vaccines and Diagnostics Division. The results of this divested business were also accounted for as discontinued operations and not included in our results from continuing operations. See "—Factors Affecting Comparability Of Year-On-Year Results Of Operations."
| |
Year ended Dec 31, 2014 |
Year ended Dec 31, 2013 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Net sales to third parties from continuing operations |
52,180 | 51,869 | 1 | 3 | |||||||||
Sales to discontinued segments |
239 | 221 | 8 | 8 | |||||||||
| | | | | | | | | | | | | | |
Net sales from continuing operations |
52,419 | 52,090 | 1 | 3 | |||||||||
Other revenues |
1,215 | 626 | 94 | 94 | |||||||||
Cost of goods sold |
(17,345 | ) | (16,579 | ) | (5 | ) | (6 | ) | |||||
| | | | | | | | | | | | | | |
Gross profit from continuing operations |
36,289 | 36,137 | 0 | 3 | |||||||||
Marketing & Sales |
(12,377 | ) | (12,638 | ) | 2 | 0 | |||||||
Research & Development |
(9,086 | ) | (9,071 | ) | 0 | 0 | |||||||
General & Administration |
(2,616 | ) | (2,603 | ) | 0 | (1 | ) | ||||||
Other income |
1,391 | 1,205 | 15 | 15 | |||||||||
Other expense |
(2,512 | ) | (2,047 | ) | (23 | ) | (23 | ) | |||||
| | | | | | | | | | | | | | |
Operating income from continuing operations |
11,089 | 10,983 | 1 | 7 | |||||||||
Return on net sales (%) |
21.3 | 21.2 | |||||||||||
Income from associated companies |
1,918 | 599 | 220 | 221 | |||||||||
Interest expense |
(704 | ) | (683 | ) | (3 | ) | (6 | ) | |||||
Other financial income and expense |
(31 | ) | (92 | ) | 66 | 31 | |||||||
| | | | | | | | | | | | | | |
Income before taxes from continuing operations |
12,272 | 10,807 | 14 | 19 | |||||||||
Taxes |
(1,545 | ) | (1,498 | ) | (3 | ) | (8 | ) | |||||
| | | | | | | | | | | | | | |
Net income from continuing operations |
10,727 | 9,309 | 15 | 21 | |||||||||
Net income/loss from discontinued operations |
(447 | ) | (17 | ) | nm | nm | |||||||
| | | | | | | | | | | | | | |
Net income |
10,280 | 9,292 | 11 | 17 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Attributable to: |
|||||||||||||
Shareholders of Novartis AG |
10,210 | 9,175 | 11 | 18 | |||||||||
Non-controlling interests |
70 | 117 | (40 | ) | (41 | ) | |||||||
Basic earnings per share ($) from continuing operations |
4.39 | 3.76 | 17 | 22 | |||||||||
Basic earnings per share ($) from discontinued operations |
(0.18 | ) | 0.00 | nm | nm | ||||||||
| | | | | | | | | | | | | | |
Total basic earnings per share ($) |
4.21 | 3.76 | 12 | 18 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Free cash flow from continuing operations |
10,934 | 9,521 | 15 | ||||||||||
Free cash flow |
10,762 | 9,945 | 8 | ||||||||||
nm = not meaningful
125
Novartis delivered solid financial performance in 2014, driven by our continued success with growth products and expansion in emerging growth markets, which helped offset the effects of generic competition of approximately $2.4 billion. As a result, we achieved net sales from continuing operations of $52.2 billion (+1%, +3% cc). Operating income from continuing operations amounted to $11.1 billion (+1%, +7% cc). Operating income margin was 21.3% of net sales. Net income from continuing operations rose 15% (+21% cc) to $10.7 billion. Earnings per share (EPS) from continuing operations rose 17% (+22% cc) to $4.39. In 2014, free cash flow from continuing operations increased by $1.4 billion to $10.9 billion, mainly due to higher cash flows from operating activities.
In addition, to help investors' understanding of the performance of our business, we present our core results, which exclude the exceptional impact of significant disposals and acquisitions, as well as other significant exceptional items. In 2014, our core operating income from continuing operations increased 2% (+7% cc) to $14.5 billion. Core operating income margin increased 0.3 percentage points to 27.7% of net sales, as our efforts to enhance productivity helped to offset 0.8 percentage points of negative impact from changing currency exchange rates. Core net income from continuing operations was $12.7 billion, up 3% (+8% cc), and core basic earnings per share from continuing operations rose 4% (+9% cc) to $5.19.
Across divisions, our portfolio of growth products and presence in emerging growth markets continued to fuel performance in 2014. Growth products comprise products launched in 2009 or later, or products with exclusivity until at least 2018 in key markets (EU, US, Japan) (except Sandoz, which includes only products launched in the last 24 months).
Sales of growth products increased 18% to $18.6 billion, or 36% of net sales. In the Pharmaceuticals Division, growth products accounted for 43% of net sales, up from 37% in 2013—demonstrating how we are rejuvenating our portfolio and mitigating the impact of patent expirations on key products.
Top-performing Pharmaceuticals products in 2014 included Gilenya ($2.5 billion, +30% cc), our oral therapy for multiple sclerosis; Afinitor ($1.6 billion, +22% cc), a treatment for several types of cancer including breast and kidney; and Tasigna ($1.5 billion, +24% cc), a treatment for chronic myeloid leukemia.
At Alcon, surgical equipment was a key growth driver, following the launch in late 2013 of the Centurion vision system and continued growth of the LenSx femtosecond laser for cataract surgery. Disposable products for cataract and vitreoretinal surgery also showed strong growth.
In the Sandoz Division, biosimilars—which are follow-on versions of complex biologic drugs—made a strong contribution to growth, with sales rising 23% (cc) to $514 million globally.
In addition, efforts to expand our presence in emerging growth markets such as Asia, Africa and Latin America continued to show good results. Emerging growth markets comprise all markets except the US, Canada, Western Europe, Japan, Australia and New Zealand. Net sales in those markets rose 11% (cc) to $15.3 billion, led by China, up 15% (cc), and by Brazil, up 18% (cc).
Novartis made solid progress in 2014 in generating synergies across divisions to improve productivity. Overall savings reached approximately $2.9 billion, exceeding our target. In 2014, we also created Novartis Business Services (NBS), a shared services organization designed to enhance profitability by harmonizing and simplifying the provision of services to the divisions. NBS is expected to play a key role in accelerating our productivity gains.
The most significant savings of $1.6 billion came from ongoing efforts in procurement to manage spending on goods and services across all our divisions. That represents 7% of the annual spending of $22 billion managed by the procurement organization.
126
An area where we made significant progress in 2014 was travel, where we reduced spending by about 23% across the company. We primarily achieved this by increasing the use of virtual meetings among Novartis colleagues, in lieu of travel. We aim to continue increasing the use of videoconferences and other technology for internal meetings to make these savings sustainable.
We also made strides in managing capital spending for equipment at manufacturing sites worldwide. In 2014, we began adopting standard technical requirements for machinery across our divisions. For instance, we now have uniform specifications for tablet presses, a common type of equipment previously purchased individually by each manufacturing site. This standardization enabled us to negotiate better prices from our supplier and will help reduce future costs related to such things as commissioning new equipment and maintenance.
Additionally, our multi-year plan begun in 2010 to optimize our global manufacturing network is on track. In 2014, we announced several further steps, including the closure of our pharmaceuticals manufacturing site in Suffern, New York, in the US and the planned sale of our pharmaceuticals manufacturing site in Taboão da Serra, Brazil—bringing the total number of production sites that have been or are being restructured or divested to 24. These changes are helping us balance capacity, reducing it where no longer needed and adding new capacity for the products and technologies of the future.
We continued to find synergies to increase sales through our Customers First program, which delivered $1.6 billion in revenues in 2014, generating 2.8% of total Group net sales. This program aims to serve our customers more effectively by ensuring they have access to a full range of Novartis products from all divisions.
| |
Year ended Dec 31, 2014 |
Year ended Dec 31, 2013 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Pharmaceuticals |
31,791 | 32,214 | (1 | ) | 1 | ||||||||
Alcon |
10,827 | 10,496 | 3 | 6 | |||||||||
Sandoz |
9,562 | 9,159 | 4 | 7 | |||||||||
| | | | | | | | | | | | | | |
Net sales to third parties from continuing operations |
52,180 | 51,869 | 1 | 3 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pharmaceuticals
Pharmaceuticals delivered net sales of $31.8 billion (–1%, +1% in constant currencies, or cc) as strong sales of growth products countered the impact of greater generic competition for Diovan and other products, particularly in the US and Japan. Generic competition reduced sales by seven percentage points.
Growth products generated $13.7 billion of division net sales, growing 17% (cc) compared to last year. These products—which include Gilenya, Afinitor, Tasigna, Galvus, Lucentis, Xolair, Jakavi and our portfolio of products for the treatment of chronic obstructive pulmonary disease (COPD)—contributed 43% of division net sales, compared to 37% in 2013.
Sales in emerging growth markets increased 11% (cc) to $8.1 billion.
Oncology
Oncology sales rose 4% (+6% cc) to $11.7 billion, despite increased generic competition for Zometa ($264 million,–55% cc). By brand, growth was driven mainly by Afinitor, up 22% (cc) to $1.6 billion; Tasigna, up 24% (cc) to $1.5 billion; and Jakavi, up 72% (cc) to $279 million.
127
Primary Care
Sales in Primary Care, which includes mainly cardiovascular, metabolic and respiratory products amounted to $8.0 billion in 2014, down 12% (–10% cc). Excluding older, established medicines such as Diovan ($2.3 billion,–32% cc), sales rose 13% (+16%) to $2.8 billion. The recently launched COPD portfolio, for example, which includes Onbrez Breezhaler/Arcapta Neohaler, Seebri Breezhaler, and Ultibro Breezhaler, grew 93% (cc) to $484 million. Other key products include the Galvus Group, up 6% (cc) to $1.2 billion; and Xolair, up 30% (cc) to $777 million.
Specialty Care
Sales in Specialty Care, which includes our Neuroscience, Integrated Hospital Care and Ophthalmics products, amounted to $10.1 billion. Gilenya, our oral multiple sclerosis therapy, grew 30% (cc) to $2.5 billion, with strong volume growth through new patient initiations in the US and elsewhere. Sales of Lucentis, for ocular conditions, rose 5% (cc) to $2.4 billion, driven by increased use in new indications beyond wet age-related macular degeneration.
TOP 20 PHARMACEUTICALS DIVISION PRODUCT NET SALES—2014
| |
|
|
US | Rest of world | Total | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Brands
|
Business Franchise |
Indication | $ m | % change in constant currencies |
$ m | % change in constant currencies |
$ m | % change in $ |
% change in constant currencies |
|||||||||||||||||
Gleevec/Glivec |
Oncology | Chronic myeloid leukemia | 2,170 | 12 | 2,576 | (5 | ) | 4,746 | 1 | 2 | ||||||||||||||||
Gilenya |
Neuroscience | Relapsing multiple sclerosis | 1,190 | 16 | 1,287 | 45 | 2,477 | 28 | 30 | |||||||||||||||||
Lucentis |
Ophthalmics | Age-related macular degeneration | 2,441 | 5 | 2,441 | 2 | 5 | |||||||||||||||||||
Diovan/Co-Diovan |
Primary Care | Hypertension | 960 | (43 | ) | 1,385 | (22 | ) | 2,345 | (33 | ) | (32 | ) | |||||||||||||
Sandostatin |
Oncology | Acromegaly | 751 | 6 | 899 | 6 | 1,650 | 4 | 6 | |||||||||||||||||
Afinitor/Votubia |
Oncology | Breast cancer | 805 | 16 | 770 | 29 | 1,575 | 20 | 22 | |||||||||||||||||
Tasigna |
Oncology | Chronic myeloid leukemia | 540 | 26 | 989 | 23 | 1,529 | 21 | 24 | |||||||||||||||||
Exforge |
Primary Care | Hypertension | 284 | (20 | ) | 1,112 | 4 | 1,396 | (4 | ) | (2 | ) | ||||||||||||||
Galvus |
Primary Care | Diabetes | 1,224 | 6 | 1,224 | 2 | 6 | |||||||||||||||||||
Exelon/Exelon Patch |
Neuroscience | Alzheimer's disease | 485 | 6 | 524 | (6 | ) | 1,009 | (2 | ) | (1 | ) | ||||||||||||||
Exjade |
Oncology | Iron chelator | 307 | 16 | 619 | 1 | 926 | 4 | 6 | |||||||||||||||||
Xolair(1) |
Primary Care | Asthma | 777 | 30 | 777 | 27 | 30 | |||||||||||||||||||
Neoral/Sandimmun |
Integrated Hospital Care | Transplantation | 55 | (2 | ) | 629 | (6 | ) | 684 | (9 | ) | (6 | ) | |||||||||||||
Voltaren (excl. other divisions) |
Established medicines | Inflammation/pain | 632 | (3 | ) | 632 | (6 | ) | (3 | ) | ||||||||||||||||
Myfortic |
Integrated Hospital Care | Transplantation | 149 | (45 | ) | 394 | 14 | 543 | (15 | ) | (11 | ) | ||||||||||||||
Ritalin/Focalin |
Established medicines | Attention deficit/ hyperactivity disorder | 327 | (25 | ) | 165 | 8 | 492 | (17 | ) | (16 | ) | ||||||||||||||
Femara |
Oncology | Breast cancer | 27 | 42 | 353 | 0 | 380 | (1 | ) | 2 | ||||||||||||||||
Comtan/Stalevo |
Neuroscience | Parkinson's disease | 19 | (42 | ) | 352 | (1 | ) | 371 | (7 | ) | (4 | ) | |||||||||||||
Tegretol |
Established medicines | Epilepsy | 82 | 19 | 264 | 1 | 346 | 1 | 4 | |||||||||||||||||
Zortress/Certican |
Integrated Hospital Care | Transplantation | 60 | 88 | 267 | 28 | 327 | 31 | 36 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Top 20 products total |
8,211 | (3 | ) | 17,659 | 4 | 25,870 | 0 | 2 | ||||||||||||||||||
Rest of portfolio |
1,561 | (13 | ) | 4,360 | 0 | 5,921 | (6 | ) | (4 | ) | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total Division sales |
9,772 | (5 | ) | 22,019 | 3 | 31,791 | (1 | ) | 1 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Net sales reflect Xolair sales for all indications (i.e. Xolair SAA and Xolair CSU, which are managed by the Integrated Hospital Care franchise).
128
Gleevec/Glivec ($4.7 billion, +2% cc) sales grew slightly in 2014. Gleevec/Glivec is a treatment for adult patients with metastatic and/or unresectable KIT+ gastrointestinal stromal tumors (GIST), as an adjuvant treatment for certain adult patients following resection of KIT+ GIST, and as a targeted therapy for Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML). Sales were driven mainly by the US, and more than compensated for the loss of patent exclusivity in some markets. In the US, Novartis Pharmaceuticals Corporation has settled its litigation with a subsidiary of Sun Pharmaceutical Industries Ltd. relating to Novartis patents covering the use of certain polymorphic forms of Gleevec/Glivec, which expire in 2019 (including pediatric exclusivity). The basic compound patent for Gleevec/Glivec expires in the US on July 4, 2015. As a result of the settlement, Novartis will permit Sun's subsidiary to market a generic version of Gleevec/Glivec in the US beginning on February 1, 2016.
Gilenya ($2.5 billion, +30% cc), the first once-daily oral therapy to treat relapsing forms of multiple sclerosis (MS), continued to outgrow the market, achieving double-digit growth in 2014 in recognition of strong trends towards oral treatments with higher efficacy. Growth was also fueled by an increasing acceptance of the role of high-efficacy treatments when used earlier in the course of the disease. Gilenya continues to see volume growth through new patient initiations in both the US and non-US markets. In the US, Gilenya is indicated for relapsing forms of MS. In the EU, Gilenya is indicated for adult patients with high disease activity despite treatment with at least one disease modifying agent, or rapidly evolving severe relapsing remitting MS. Gilenya is currently approved in over 80 countries around the world. Gilenya is licensed from Mitsubishi Tanabe Pharma.
Lucentis ($2.4 billion, +5% cc) saw volume growth driven by the uptake in non-Age-Related macular degeneration (AMD) indications (such as visual impairment due to diabetic macular edema; macular edema secondary to central and branch retinal vein occlusion; and choroidal neovascularization secondary to pathologic myopia). In addition, the Lucentis pre-filled syringe was successfully launched in all key European countries, as well as Japan and Australia. Non-AMD indications contributed 41% of Lucentis sales in 2014, compared to 27% for 2013, and became a blockbuster in Q4. Emerging growth markets contributed 18% of Lucentis sales versus 16% last year. Emerging growth markets comprise all markets except the US, Canada, Western Europe, Japan, Australia and New Zealand. At the same time, Lucentis sales in the wet AMD indication, impacted by competition, are stabilizing in some markets. Lucentis is the only anti-VEGF therapy licensed in most countries for the treatment of the following ocular indications: wet AMD, visual impairment due to diabetic macular edema, visual impairment due to macular edema secondary to retinal vein occlusion and secondary to branch retinal vein occlusion, and visual impairment due to choroidal neovascularization secondary to pathologic myopia (mCNV). Lucentis is approved in more than 100 countries to treat patients with the first four conditions, and in more than 70 countries for mCNV. Genentech/Roche holds the rights to Lucentis in the US.
Diovan Group ($2.3 billion, –32% cc), consisting of Diovan monotherapy and the combination product Co-Diovan/Diovan HCT, saw a continued sales decline worldwide due to generic competition in most markets, including the US (following July 7, 2014 Diovan monotherapy generic entry), many EU countries and Japan (generic entry in June 2014), compounded in Japan by the impact of issues related to investigator initiated trials. Sales continued to grow in Emerging Growth Markets, including China and selected countries in Latin America, Asia Pacific and Africa.
Sandostatin ($1.7 billion, +6% cc) continued to benefit from the increasing use of Sandostatin LAR (long acting release) in key markets. Sandostatin is a somatostatin analogue used to treat patients with acromegaly as well as neuroendocrine tumors (NET). In NET, it is used for both the treatment of patients with symptoms of carcinoid syndrome and those with advanced NET of the midgut or unknown primary tumor location (currently approved in 47 countries). An enhanced presentation of Sandostatin LAR, which includes an improved diluent, safety needle and vial adapter, has been approved in 58 countries, with additional filings underway.
Afinitor/Votubia ($1.6 billion, +22% cc) performance was driven by strong growth in the US, Japan and other markets. Afinitor is an oral inhibitor of the mTOR pathway approved for the treatment of
129
patients with HR+/HER2– advanced breast cancer after failure with a non-steroidal aromatase inhibitor, for advanced renal cell carcinoma following vascular endothelial growth factor-targeted therapy and for the treatment of advanced pancreatic neuroendocrine tumors. Afinitor is also approved for subependymal giant cell astrocytoma (SEGA) and renal angiomyolipoma associated with tuberous sclerosis complex (TSC). Everolimus, the active ingredient in Afinitor/Votubia, is also available in more than 60 countries for the treatment of renal angiomyolipomas and/or SEGA associated with TSC, including as a dispersible tablet formulation in the US and EU for SEGA. Everolimus, the active ingredient in Afinitor/Votubia, is available under the trade names Zortress/Certican for use in other non-oncology indications and is exclusively licensed to Abbott and sublicensed to Boston Scientific for use in drug-eluting stents.
Tasigna ($1.5 billion, +24% cc) performance was driven by strong growth in the US and other markets. Tasigna is a more effective, targeted therapy than Gleevec/Glivec for adult patients newly diagnosed with Ph+ CML in the chronic phase or for adult patients in the chronic or accelerated phase who are resistant or intolerant to at least one prior therapy including Gleevec/Glivec. It is currently approved as a first-line therapy for newly diagnosed patients with Ph+ CML in the chronic phase in more than 85 countries globally, including the US, EU, Japan and Switzerland, with additional submissions pending worldwide. Tasigna (nilotinib) is also approved in more than 110 countries as a second-line treatment for patients with Ph+ CML in chronic and/or accelerated phase who are resistant or intolerant to existing treatment, such as Gleevec/Glivec.
Exforge Group ($1.4 billion, –2% cc), includes two medicines approved for the treatment of hypertension: Exforge, a single-pill combination of the angiotensin receptor blocker (ARB) valsartan and the calcium channel blocker amlodipine besylate; and Exforge HCT, a single pill combining an ARB (valsartan), calcium channel blocker (amlodipine) and a diuretic (hydrochlorothiazide). Exforge lost exclusivity in October 2014 and Exforge HCT in November 2014 in the US. Outside the US, Exforge continues to grow, with double-digit growth in China and a number of emerging growth markets. Emerging growth markets comprise all markets except the US, Canada, Western Europe, Japan, Australia and New Zealand. Exforge is now available in more than 100 countries. Exforge HCT is available in over 60 countries.
Galvus Group ($1.2 billion, +6% cc), which includes Galvus, an oral treatment for type 2 diabetes, and Eucreas, a single-pill combination of vildagliptin (the active ingredient in Galvus) and metformin, continued to grow in 2014 despite the distribution stop in the German market on July 1, 2014. Sales for the first six months of 2014 in Germany were $57 million. Galvus delivered a solid performance with strong growth coming from emerging markets. The focus for Galvus remains on patients whose diabetes remains uncontrolled on metformin, as well as on an expansion of usage in new patient segments based on new indications. Galvus Group is currently approved in more than 120 countries.
Exelon/Exelon Patch ($1.0 billion, –1% cc) sales declined slightly, due to generic competition for Exelon Patch in the EU offsetting a solid performance for Exelon Patch in the US. Exelon Patch is approved for the treatment of mild-to-moderate Alzheimer's disease dementia (AD) in more than 90 countries, including more than 20 countries where it is also approved for Parkinson's disease dementia. Exelon Patch is also indicated for the treatment of patients with severe AD in 11 countries, including the US. In Europe, the high-dose patch (15 cm2) for mild-to-moderately severe AD was launched in several markets in 2013.
Exjade ($926 million, +6% cc), a once-daily oral therapy for chronic transfusional iron overload first approved in 2005 and now approved in more than 100 countries, saw sales increases in the US and Asia. Exjade is also approved for the treatment of chronic iron overload in patients with non-transfusion-dependent thalassemia in more than 70 countries.
Xolair ($777 million, +30% cc), a biologic drug for appropriate patients with severe persistent allergic asthma in Europe and moderate-to-severe persistent allergic asthma in the US, is currently approved in more than 90 countries as a treatment for moderate-to-severe or severe persistent allergic asthma. Its
130
sales continued to grow strongly in Canada, Europe and Latin America. Xolair is also approved in the EU, Switzerland and 35 other countries as a treatment for chronic spontaneous urticaria, also known as chronic idiopathic urticaria, for which it is approved in the US and now Canada and Australia. Novartis co-promotes Xolair with Genentech/Roche in the US and shares a portion of the operating income, but does not book US sales.
Neoral/Sandimmun ($684 million, –6% cc), a micro-emulsion formulation of cyclosporine, is an immunosuppressant to prevent organ rejection following a kidney, liver or heart transplant. Neoral is also approved for use in lung transplant in many countries outside of the US. Additionally, it is indicated for treating selected autoimmune disorders such as psoriasis and rheumatoid arthritis. First launched in 1995, Neoral is marketed in approximately 100 countries. This product is subject to generic competition.
Voltaren/Cataflam ($632 million, –3% cc), is a leading non-steroidal anti-inflammatory drug (NSAID) for the relief of symptoms in rheumatic diseases such as rheumatoid arthritis and osteoarthritis, and for various other inflammatory and pain conditions. Voltaren/Cataflam was first registered in 1973 and is available in more than 140 countries. This product, which is subject to generic competition, is marketed by the Pharmaceuticals Division in a wide variety of dosage forms, including tablets, drops, suppositories, ampoules and topical therapy. In addition, in various countries, our Sandoz Division markets generic versions of Voltaren, our Alcon Division markets Voltaren for ophthalmic indications, and our OTC Division markets low-dose oral forms and the topical therapy of Voltaren as over-the-counter products. Total sales across all divisions of Voltaren/Cataflam (diclofenac) amounted to $1.6 billion in 2014 and grew 7.5% in constant currencies against the prior year.
Myfortic ($543 million, –11% cc), a transplantation medicine, is available in more than 90 countries to prevent organ rejection in adult kidney transplant patients. It has experienced a sales decline after the expected launch of generic competition in the US in early 2014. Myfortic continues to grow in geographies without generic competition.
Ritalin/Focalin ($492 million, –16% cc) is a treatment for attention deficit hyperactivity disorder (ADHD) in children. Ritalin and Ritalin LA are available in more than 70 and 30 countries, respectively, and are also indicated for narcolepsy. Ritalin LA has been granted in 2014 the "adult ADHD indication" in several countries (16 to date). Focalin and Focalin XR are available in the US and Focalin XR is additionally indicated for adults. Focalin XR is also approved in Switzerland. Ritalin Immediate Release has generic competition in most countries. Some strengths of Ritalin and Focalin are subject to generic competition in the US.
Femara ($380 million, +2% cc), a treatment for early stage and advanced breast cancer in postmenopausal women, experienced steady sales despite multiple generic entries in the US, Europe and other key markets.
Comtan/Stalevo ($371 million, –4% cc), indicated for the treatment of Parkinson's disease, saw sales decline in 2014 due to generic competition in some markets. Stalevo (carbidopa, levodopa and entacapone) is indicated for certain Parkinson's disease patients who experience end-of-dose motor fluctuations, known as "wearing off." In July 2014, Stalevo was granted marketing authorization for the treatment of Parkinson's disease in Japan. Stalevo is available in more than 90 countries. Comtan (entacapone) is also indicated for the treatment of Parkinson's disease patients who experience end-of-dose wearing off and is marketed in 42 countries. Both products are marketed by Novartis under a licensing agreement with the Orion Corporation.
Tegretol ($346 million, +4% cc) a treatment for epilepsy (partial seizures and generalized tonic-clonic seizures) and for several other neuro-psychiatric diseases including bipolar disorders or neuropathic pain, was launched in 1962. It is marketed in approximately 129 countries and, although it faces generic competition in most of them, sales continue to be very stable due to its established position as a gold-standard, first-line treatment. Tegretol is also listed as an 'essential medicine' by the World Health Organization.
131
Zortress/Certican ($327 million, +36% cc), a transplantation medicine available in more than 90 countries to prevent organ rejection in adult heart and kidney transplant patients, continued to show strong growth in 2014. It is also approved in over 70 countries for liver transplant patients, including the US and EU countries. Everolimus, the active ingredient in Zortress/Certican, is marketed for other indications under the trade names Afinitor/Votubia. Everolimus is exclusively licensed to Abbott and sublicensed to Boston Scientific for use in drug-eluting stents.
Other Products of Significance
HRT Range ($297 million, –8% cc), encompasses Vivelle-Dot/Estradot, Estalis/CombiPatch, Sequidot and Estracomb MX. Vivelle-Dot/Estradot, which makes up the bulk of the HRT Range sales, is a transdermal patch formulation of estradiol hemihydrate. This estrogen replacement therapy is used for the treatment of the symptoms of natural or surgically induced menopause and the prevention of postmenopausal osteoporosis. First launched in May 1999, Vivelle-Dot/Estradot is marketed in approximately 29 countries. This product is subject to generic competition outside the US.
Jakavi ($279 million, +72% cc), is an oral inhibitor of the JAK 1 and JAK 2 tyrosine kinases. It is the first JAK inhibitor indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post-polycythemia vera myelofibrosis or post-essential thromboycythemia myelofibrosis. Jakavi is currently approved in more than 65 countries worldwide. Novartis licensed ruxolitinib from Incyte Corporation for development and commercialization outside the US.
Zometa ($264 million, –55% cc), which is used in an oncology setting to reduce or delay skeletal-related events in patients with bone metastases from solid tumors and multiple myeloma, continued to decline as anticipated in 2014 due to generic competition following patent expirations in 2013 on its active ingredient, zoledronic acid.
Trileptal ($265 million, +6% cc), a treatment for epilepsy partial seizures (and generalized tonic-clonic seizures in some countries) was launched in 1973. It is marketed in approximately 97 countries and, although it faces generic competition in most of them, sales are stable due to the continued sales growth outside the EU offsetting the price pressure from generics.
Alcon
Alcon net sales in 2014 grew 3% (+6% in constant currencies, or cc) to $10.8 billion. Growth was driven by key product launches, such as Centurion and LenSx for cataract surgery, Azarga and Simbrinza for the treatment of glaucoma, Ilevro to treat ocular inflammation, as well as AirOptix Colors and the continued rollout of Dailies Total1 contact lenses.
Regionally, sales were driven by strong performance in emerging growth markets, led by Asia (+13% cc), particularly in China (+23% cc) and Russia (+27% cc).
Latin America delivered robust growth (+17% cc), driven by the Surgical and Ophthalmic Pharmaceuticals franchises.
North America (+4% cc) accelerated its growth in the Surgical franchise, offset by softness in the Ophthalmic Pharmaceuticals franchise. Sales in Europe, the Middle East and Africa (+3% cc) were driven by moderate performance in the Surgical and Ophthalmic Pharmaceuticals franchises. Japan
132
sales (+3% cc) grew moderately in the Surgical franchise, offsetting weaker growth in Ophthalmic Pharmaceuticals and Vision Care.
| |
Year ended Dec 31, 2014 |
Year ended Dec 31, 2013 |
Change in $ |
Constant currencies change |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Surgical |
|||||||||||||
Cataract products |
3,174 | 3,037 | 5 | 7 | |||||||||
of which IOLs |
1,264 | 1,297 | (3 | ) | 0 | ||||||||
Vitreoretinal products |
615 | 592 | 4 | 7 | |||||||||
Refractive/other |
284 | 268 | 6 | 8 | |||||||||
| | | | | | | | | | | | | | |
Total |
4,073 | 3,897 | 5 | 7 | |||||||||
| | | | | | | | | | | | | | |
Ophthalmic Pharmaceuticals |
|||||||||||||
Glaucoma |
1,319 | 1,265 | 4 | 7 | |||||||||
Allergy/otic/nasal |
887 | 939 | (6 | ) | (4 | ) | |||||||
Infection/inflammation |
1,066 | 1,019 | 5 | 7 | |||||||||
Dry eye |
608 | 558 | 9 | 12 | |||||||||
Other |
331 | 327 | 1 | 6 | |||||||||
| | | | | | | | | | | | | | |
Total |
4,211 | 4,108 | 3 | 5 | |||||||||
| | | | | | | | | | | | | | |
Vision Care |
|||||||||||||
Contact lenses |
1,897 | 1,793 | 6 | 7 | |||||||||
Contact lens care |
646 | 698 | (7 | ) | (5 | ) | |||||||
| | | | | | | | | | | | | | |
Total |
2,543 | 2,491 | 2 | 4 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Total net sales |
10,827 | 10,496 | 3 | 6 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Surgical
Surgical franchise sales rose 5% (+7% cc) to $4.1 billion. The increase was driven by strong equipment sales, led by the Centurion vision system for phacoemulsification cataract surgery, the continued growth of the LenSx femtosecond laser for refractive cataract surgery, strong sales of vitreoretinal and cataract disposable surgical equipment, as well as the launch of the Verion image-guided system.
Alcon experienced a more modest increase in intraocular lens (IOL) sales, driven by strong competition in the US, Japan and EU.
Ophthalmic Pharmaceuticals
Ophthalmic Pharmaceuticals sales grew 3% (+5% cc) to $4.2 billion despite a weak allergy season in the US. Sales were led by glaucoma products such as DuoTrav, Azarga and the newly-launched Simbrinza. Systane eye drops to treat the symptoms of dry eye saw double-digit growth.
Within the Infection/Inflammation segment, sales growth (+7% cc) was driven by Ilevro and Durezol. Jetrea, a first-in-class treatment for symptomatic vitreomacular adhesion/traction, continued to gain regulatory approvals, notably in Latin America and Asia.
133
Vision Care
Vision Care sales increased 2% (+4% cc) to $2.5 billion. Contact lens sales rose 6% (+7% cc), driven by key launches of AirOptix Colors, Dailies AquaComfort Plus (DACP) Toric, and DACP Multifocal, as well as the continued rollout of Dailies Total1 worldwide.
At the same time, contact lens care solutions declined (–7% cc), driven by market shifts to daily disposable lenses, as well as competitive pressure in the US.
Sandoz
Sandoz had net sales of $9.6 billion in 2014, up 4% (+7% in constant currencies, or cc) from the prior year, driven by a 15 percentage points increase in volume, more than offsetting 8 percentage points of price erosion. Performance was driven by strong retail generics and biosimilars sales growth in Asia (excluding Japan) (+15% cc), the US (+14% cc), and Latin America (+10% cc). Sales growth in Western Europe (excluding Germany) was solid at 4% (cc).
Sandoz continued to strengthen its global leadership position in differentiated generics, including medicines that are difficult to develop and manufacture. Differentiated generics accounted for 45% of Sandoz sales.
| |
Year ended Dec 31, 2014 |
Year ended Dec 31, 2013 |
Change in $ |
Constant currencies change |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Retail Generics |
7,933 | 7,663 | 4 | 6 | |||||||||
Biopharmaceuticals & Oncology Injectables |
1,094 | 888 | 23 | 25 | |||||||||
Anti-Infectives |
535 | 608 | (12 | ) | (12 | ) | |||||||
| | | | | | | | | | | | | | |
Total |
9,562 | 9,159 | 4 | 7 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Retail Generics
In Retail Generics, Sandoz develops, manufactures and markets active ingredients and finished dosage forms of pharmaceuticals. It includes the specialty areas of Dermatology, Respiratory and Ophthalmics. Retail Generics sales worldwide rose 4% (+6% cc) to $7.9 billion. US sales grew 10% (cc), dampened by customer consolidation. Sales in Western Europe (excluding Germany) rose 3% (cc), driven by strong growth in Italy, Nordics and the United Kingdom. German sales were down 1% (cc) due to weak market demand. Emerging growth markets grew strongly, driven by Asia (excluding Japan), up 14% (cc); Central and Eastern Europe, up 4% (cc); and Latin America, up 8% (cc).
Biopharmaceuticals & Oncology Injectables
In Biopharmaceuticals, Sandoz develops, manufactures and markets protein- and other biotechnology-based products, which are known as biosimilars, or follow-on biologics. Sandoz also provides biotechnology manufacturing services to other companies. Sales of Biopharmaceuticals & Oncology Injectables rose 23% (+25% cc) to $1.1 billion. In 2014, Sandoz continued to strengthen its global leadership position in biosimilars. In May, Sandoz was the first to apply for approval of a biosimilar in the US under the new biosimilar pathway created in the Biologics Price Competition and Innovation Act of 2009, with filgrastim, which is used to decrease the incidence of infection among cancer patients receiving chemotherapy. In January 2015, a US Food and Drug Administration advisory body recommended approval. Sandoz leads the industry with six biosimilars in Phase III clinical trials or registration.
134
Three Sandoz biosimilar products occupy the number one position in market share in their respective categories—Omnitrope, a human growth hormone; Binocrit for anemia; and filgrastim under the brand name Zarzio. Biosimilars sales in 2014 amounted to $514 million, up 23% (cc) from the previous year, mainly due to continued strong growth across all our brands and regions.
Sandoz also develops, manufactures and markets cytotoxic products for traditional cancer chemotherapy. The Oncology Injectables business now includes a portfolio of more than 25 products. Oncology Injectables sales in 2014 amounted to $477 million, up 29% (cc) from the previous year, mainly due to recent launches in the US.
Anti-Infectives
In Anti-Infectives, Sandoz manufactures active pharmaceutical ingredients and intermediates—mainly antibiotics—for sale under the Sandoz name and by third-party customers. Anti-Infectives sales in 2014 amounted to $535 million, down 12% (cc) from the previous year, as production capacities were temporarily constrained due to quality upgrades.
Operating Income from Continuing Operations
Operating income from continuing operations amounted to $11.1 billion (+1%, +7% cc). The negative currency impact of 6 percentage points was mainly due to the weakening of emerging market currencies (especially the ruble) and the yen against the US dollar. Operating income margin was 21.3% of net sales, which was 0.1 percentage points higher than the prior year. A 0.9 percentage point increase (in constant currencies) from the prior year, was offset by a negative currency impact of 0.8 percentage points.
The following table provides an overview of operating income by segment:
| |
Year ended Dec 31, 2014 |
% of net sales |
Year ended Dec 31, 2013 |
% of net sales |
Change in $ |
Change in constant currencies |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
|
$ m |
|
% |
% |
|||||||||||||
Pharmaceuticals |
8,471 | 26.6 | 9,376 | 29.1 | (10 | ) | (5 | ) | |||||||||||
Alcon |
1,597 | 14.8 | 1,232 | 11.7 | 30 | 43 | |||||||||||||
Sandoz |
1,088 | 11.4 | 1,028 | 11.2 | 6 | 14 | |||||||||||||
Corporate |
(67 | ) | (653 | ) | nm | nm | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating income from continuing operations |
11,089 | 21.3 | 10,983 | 21.2 | 1 | 7 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
nm = not meaningful
135
Core Operating Income key figures(1)
| |
Year ended Dec 31, 2014 |
Year ended Dec 31, 2013 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Core gross profit from continuing operations |
38,821 | 38,792 | 0 | 3 | |||||||||
Marketing & Sales |
(12,355 | ) | (12,611 | ) | 2 | 0 | |||||||
Research & Development |
(8,723 | ) | (8,885 | ) | 2 | 2 | |||||||
General & Administration |
(2,552 | ) | (2,578 | ) | 1 | 0 | |||||||
Other income |
563 | 648 | (13 | ) | (13 | ) | |||||||
Other expense |
(1,281 | ) | (1,159 | ) | (11 | ) | (10 | ) | |||||
| | | | | | | | | | | | | | |
Core operating income from continuing operations |
14,473 | 14,207 | 2 | 7 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
as % of net sales |
27.7 | % | 27.4 | % | |||||||||
- (1)
- For an explanation of non-IFRS measures and reconciliation tables, see "—Non-IFRS Measures as Defined by Novartis".
The adjustments made to operating income to arrive at core operating income from continuing operations amounted to $3.4 billion (2013: $3.2 billion). These adjustments include amortization of intangible assets of $2.7 billion; the exceptional non tax deductible US Healthcare Fee levy of $204 million in the year due to a change in regulations; impairment charges of $0.4 billion and net restructuring charges of $0.7 billion. These were partly offset by a $302 million commercial settlement gain; and a $248 million gain from selling a Novartis Venture Fund investment.
Excluding these items, core operating income from continuing operations increased 2% (+7% cc) to $14.5 billion. Core operating income margin in constant currencies increased 1.1 percentage points; currency had a negative impact of 0.8 percentage points, resulting in a net increase of 0.3 percentage points to 27.7% of net sales. Additional comments on the changes in the core operating income by division, see "—Non IFRS Measures as Defined by Novartis".
The following table provides an overview of core operating income by segment:
| |
Year ended Dec 31, 2014 |
% of net sales |
Year ended Dec 31, 2013 |
% of net sales |
Change in $ |
Change in constant currencies |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
|
$ m |
|
% |
% |
|||||||||||||
Pharmaceuticals |
9,514 | 29.9 | 9,523 | 29.6 | 0 | 4 | |||||||||||||
Alcon |
3,811 | 35.2 | 3,694 | 35.2 | 3 | 8 | |||||||||||||
Sandoz |
1,571 | 16.4 | 1,541 | 16.8 | 2 | 7 | |||||||||||||
Corporate |
(423 | ) | (551 | ) | 23 | 25 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Core operating income from continuing operations |
14,473 | 27.7 | 14,207 | 27.4 | 2 | 7 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Operating income was $8.5 billion (–10%,–5% cc), with the decline mainly due to restructuring and other exceptional charges.
Core operating income, which excludes certain exceptional items, was $9.5 billion (0%, +4% cc). Core operating income margin improved by 0.3 percentage points to 29.9% of net sales, despite the negative effect of 0.8 percentage points of changing currency exchange rates.
136
Research and development
Research and development for continuing operations totaled $9.1 billion, in line with the prior-year level. As shown in the following table, in the Pharmaceuticals Division, Research and Exploratory Development expenditure amounted to $2.7 billion in 2014, up by 2% from 2013, and Confirmatory Development expenditures amounted to $4.6 billion, practically unchanged from 2013.
| |
Year ended Dec 31, 2014 |
Year ended Dec 31, 2013 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Research and Exploratory Development |
(2,724 | ) | (2,664 | ) | (2 | ) | (2 | ) | |||||
Confirmatory Development |
(4,607 | ) | (4,578 | ) | (1 | ) | (1 | ) | |||||
| | | | | | | | | | | | | | |
Total Pharmaceuticals Division Research and Development expense |
(7,331 | ) | (7,242 | ) | (1 | ) | (1 | ) | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
as % of Pharmaceuticals net sales to third parties |
23.1 | 22.5 | |||||||||||
| | | | | | | | | | | | | | |
Core Research and Exploratory Development(1) |
(2,654 | ) | (2,611 | ) | (2 | ) | (1 | ) | |||||
Core Confirmatory Development(1) |
(4,343 | ) | (4,550 | ) | 5 | 4 | |||||||
| | | | | | | | | | | | | | |
Total Core Pharmaceuticals Division Research and Development expense |
(6,997 | ) | (7,161 | ) | 2 | 2 | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
as % of Pharmaceuticals net sales to third parties |
22.0 | % | 22.2 | % | |||||||||
- (1)
- Core excludes impairments, amortization and certain exceptional items.
Operating income increased 30% (+43% cc) to $1.6 billion, driven by operational performance, as well as the ending in 2013 of charges related to the acquisition of Alcon.
Core operating income, which excludes certain items, rose +3% (+8% cc) to $3.8 billion. Core operating income margin increased 0.6 percentage points in constant currencies, however that was fully offset by a 0.6 percentage point negative currency effect, resulting in a stable core margin of 35.2% of sales.
Operating income increased 6% (+14% cc) to $1.1 billion. Core operating income, which excludes certain exceptional items, was $1.6 billion (+2%, +7% cc), impacted by high price erosion. Core operating income margin decreased by 0.4 percentage points to 16.4% of net sales, mainly due to a negative impact of 0.5 percentage points due to changing currency exchange rates.
Corporate Income and Expense, Net
Corporate income and expense of continuing operations amounted to a net expense of $67 million in 2014 compared to $653 million in the prior year, mainly due to a $456 million increase in other revenues principally related to the retained Vaccines intellectual property rights, including a $302 million commercial settlement gain and a $248 million gain from the sale of a Novartis Venture Fund investment.
137
Non-Operating Income and Expense
The following table provides an overview of non-operating income and expense:
| |
Year ended Dec 31, 2014 |
Year ended Dec 31, 2013 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Operating income from continuing operations |
11,089 | 10,983 | 1 | 7 | |||||||||
Income from associated companies |
1,918 | 599 | 220 | 221 | |||||||||
Interest expense |
(704 | ) | (683 | ) | (3 | ) | (6 | ) | |||||
Other financial income and expense |
(31 | ) | (92 | ) | 66 | 31 | |||||||
| | | | | | | | | | | | | | |
Income before taxes from continuing operations |
12,272 | 10,807 | 14 | 19 | |||||||||
Taxes |
(1,545 | ) | (1,498 | ) | (3 | ) | (8 | ) | |||||
| | | | | | | | | | | | | | |
Net income from continuing operations |
10,727 | 9,309 | 15 | 21 | |||||||||
Net loss from discontinued operations |
(447 | ) | (17 | ) | nm | nm | |||||||
| | | | | | | | | | | | | | |
Net income |
10,280 | 9,292 | 11 | 17 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Basic EPS ($) from continuing operations |
4.39 | 3.76 | 17 | 22 | |||||||||
Basic EPS ($) from discontinued operations |
(0.18 | ) | 0.00 | nm | nm | ||||||||
| | | | | | | | | | | | | | |
Total basic EPS ($) |
4.21 | 3.76 | 12 | 18 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The following table provides an overview of core non-operating income and expense:
| |
Year ended Dec 31, 2014 |
Year ended Dec 31, 2013 |
Change in $ |
Change in constant currencies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
% |
|||||||||
Core operating income from continuing operations |
14,473 | 14,207 | 2 | 7 | |||||||||
Income from associated companies |
943 | 876 | 8 | 8 | |||||||||
Interest expense |
(704 | ) | (683 | ) | (3 | ) | (6 | ) | |||||
Other financial income and expense |
(31 | ) | (48 | ) | 35 | 31 | |||||||
| | | | | | | | | | | | | | |
Core income before taxes from continuing operations |
14,681 | 14,352 | 2 | 7 | |||||||||
Taxes |
(2,028 | ) | (2,057 | ) | 1 | (3 | ) | ||||||
| | | | | | | | | | | | | | |
Core net income from continuing operations |
12,653 | 12,295 | 3 | 8 | |||||||||
Core net income from discontinued operations |
102 | 238 | (57 | ) | (34 | ) | |||||||
| | | | | | | | | | | | | | |
Core net income |
12,755 | 12,533 | 2 | 7 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Core basic EPS ($) from continuing operations |
5.19 | 4.99 | 4 | 9 | |||||||||
Core basic EPS ($) from discontinued operations |
0.04 | 0.10 | nm | nm | |||||||||
| | | | | | | | | | | | | | |
Core basic EPS ($) |
5.23 | 5.09 | 3 | 8 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
nm = not meaningful
138
Income from associated companies
Income from associated companies from continuing operations amounted to $1.9 billion in 2014, compared to $599 million in 2013. The increase was mainly due to the gains recognized on the sale of shares of LTS Lohmann Therapie-Systeme AG, Germany, (LTS) and on the sale of the shares of Idenix Pharmaceuticals, Inc., US, (Idenix) which amounted to $421 million and $812 million, respectively. An additional income of $64 million was recorded on investments in associated companies held by the Novartis Venture Funds, which have been accounted at fair value from January 1, 2014 onwards, consistent with other investments held by these Funds, instead of using the equity method of accounting. The contribution from the investment in Roche of $599 million was approximately in line with the prior-year level.
Core income from associated companies from continuing operations increased to $943 million from $876 million in the prior-year period.
Interest Expense and other financial income and expense
Interest expense from continuing operations increased slightly to $704 million from $683 million in the prior year. Other financial income and expense amounted to a net expense of $31 million, compared to $92 million in 2013, mainly as a result of hedging gains.
Taxes
The tax rate for continuing operations in the full year of 2014 decreased to 12.6% from 13.9% in the prior year, mainly due to the impact of taxes on the various exceptional gains which occurred during the year.
The core tax rate from continuing operations decreased slightly to 13.8% from 14.3% in 2013.
Net Income
Net income from continuing operations of $10.7 billion was up 15% (+21% cc), growing ahead of operating income mainly due to higher income from associated companies, which included a gain of $0.8 billion from the sale of the shares of Idenix to Merck & Co., and a gain of $0.4 billion from the divestment of the shareholding in LTS, partly offset by an increase in tax expense.
Core net income from continuing operations of $12.7 billion was up 3% (+8% cc), growing slightly ahead of core operating income (2%, 7% cc).
EPS
Earnings per share (EPS) from continuing operations was $4.39 per share, up 17% (+22% cc), growing ahead of net income due to lower average outstanding shares and lower minority interests.
Core EPS from continuing operations was $5.19 (+4%, +9% cc), growing ahead of core net income due to lower average outstanding shares and lower minority interests.
139
| |
Year ended Dec 31, 2014 |
Year ended Dec 31, 2013 |
|||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Net sales to third parties from discontinued operations |
5,816 | 6,051 | |||||
Operating loss from discontinued operations |
(353 | ) | (73 | ) | |||
Net loss from discontinued operations |
(447 | ) | (17 | ) | |||
Attributable to: |
|||||||
Shareholders of Novartis AG |
(444 | ) | (14 | ) | |||
Non-controlling interests |
(3 | ) | (3 | ) | |||
Basic earnings per share ($) from discontinued operations |
(0.18 | ) | 0 | ||||
Free cash flow from discontinued operations |
(172 | ) | 424 | ||||
Net sales to third parties of the discontinued operations in 2014 declined –4% (–1% in cc) to $5.8 billion from $6.1 billion in 2013.
Operating loss from discontinued operations amounted to $353 million in 2014 compared to $73 million in 2013. The operating loss of $353 million in 2014 included an exceptional impairment charge of $1.1 billion for the influenza vaccines business which was partially offset by an exceptional pre-tax gain of $0.9 billion from the divestment of our blood transfusion diagnostics unit.
Net loss from discontinued operations amounted to $447 million in 2014 compared to a net loss $17 million in 2013.
For the total Group, net income amounted to $10.3 billion compared to $9.3 billion in 2013., impacted by the exceptional divestment gains included in the net income. Basic earnings per share increased to $4.21 from $3.76.
FACTORS AFFECTING COMPARABILITY OF YEAR-ON-YEAR RESULTS OF OPERATIONS
Significant transactions in 2015
The comparability of the year-on-year results of our operations for the total Group can be significantly affected by acquisitions and divestments. The transactions of significance during 2015 and 2014 are mentioned below.
Acquisitions and Divestments in 2015
PORTFOLIO TRANSFORMATION TRANSACTIONS
Transaction with Eli Lilly and Company
On January 1, 2015, Novartis closed its transaction with Eli Lilly and Company, USA (Lilly) announced in April 2014 to divest its Animal Health business for $5.4 billion in cash. This resulted in a pre-tax gain of $4.6 billion which is recorded in operating income from discontinued operations.
140
Transactions with GlaxoSmithKline plc
On March 2, 2015, Novartis closed its transactions with GlaxoSmithKline plc, Great Britain (GSK) announced in April 2014 with the following consequences:
Pharmaceuticals—Acquisition of GSK oncology products
Novartis acquired GSK's oncology products and certain related assets for an aggregate cash consideration of $16.0 billion. Up to $1.5 billion of this cash consideration at the acquisition date is contingent on certain development milestones. The fair value of this potentially refundable consideration is $0.1 billion. In addition, under the terms of the agreement, Novartis is granted a right of first negotiation over the co-development or commercialization of GSK's current and future oncology R&D pipeline, excluding oncology vaccines. The right of first negotiation is for a period of 12.5 years from the acquisition closing date. The purchase price allocation of the fair value of the consideration of $15.9 billion resulted in net identified assets of $13.5 billion and goodwill of $2.4 billion. Since the acquisition the business generated net sales of $1.8 billion. Management estimates net sales for the entire year 2015 would have amounted to $2.1 billion had the Oncology products been acquired at the beginning of the 2015 reporting period. The net results from operations on a reported basis since the acquisition date were not significant, mainly due to amortization of intangible assets.
Vaccines—Divestment
Novartis has divested its Vaccines business (excluding its Vaccines influenza business) to GSK for up to $7.1 billion, plus royalties. The $7.1 billion consists of $5.25 billion paid at closing and up to $1.8 billion in future milestone payments. The fair value of the contingent future milestones and royalties is $1.0 billion, resulting in a fair value of consideration received of $6.25 billion. Included in this amount is a $450 million milestone payment received in late March 2015. The sale of this business resulted in a pre-tax gain of $2.8 billion which is recorded in operating income from discontinued operations.
Novartis's Vaccines influenza business is excluded from the GSK Vaccines business acquisition. However, GSK entered into a future option arrangement with Novartis in relation to the Vaccines influenza business, pursuant to which Novartis could have unilaterally required GSK to acquire the entire or certain parts of its Vaccines influenza business for consideration of up to $250 million (the Influenza Put Option) if the divestment to CSL Limited, Australia (CSL), discussed below, had not been completed. The option period was 18 months from the closing date of the GSK transaction, but terminated with the sale of the Vaccines influenza business to CSL on July 31, 2015. Novartis paid GSK a fee of $5 million in consideration for the grant of the Influenza Put Option.
Consumer Health—Combination of Novartis OTC with GSK Consumer Healthcare in a joint venture
Novartis and GSK agreed to create a combined consumer healthcare business through a joint venture between Novartis OTC and GSK Consumer Healthcare. On March 2, 2015, a new entity was formed via contribution of businesses from both Novartis and GSK. Novartis has a 36.5% interest in the newly created entity. Novartis has valued the contribution of 63.5% of its OTC Division in exchange for 36.5% of the GSK Consumer Healthcare business at fair value.
Based on the estimates of the fair values exchanged, an investment in an associated company of $7.6 billion was recorded. The resulting pre-tax gain, net of transaction-related costs, of $5.9 billion is recorded in operating income from discontinued operations.
Novartis has four of eleven seats on the joint venture entity's Board of Directors. Furthermore, Novartis has customary minority rights and also exit rights at a pre-defined, market-based pricing mechanism.
141
The investment is accounted for using the equity method of accounting using estimated results for the last quarter of the year. Any differences between this estimate and actual results, when available, will be adjusted in the Group's 2016 consolidated financial statements.
Additional GSK related cost
The GSK transaction resulted in $0.6 billion of additional transaction-related costs that were expensed.
On October 26, 2014, Novartis entered into an agreement with CSL to sell its Vaccines influenza business to CSL for $275 million. Entering into the separate divestment agreement with CSL resulted in the Vaccines influenza business being classified as a separate disposal group consisting of a group of cash generating units within the Vaccines Division, requiring the performance of a separate valuation of the Vaccines influenza business net assets. This triggered the recognition of an exceptional impairment charge in 2014 of $1.1 billion, as the estimated net book value of the Vaccines influenza business net assets was above the $275 million consideration.
The transaction with CSL was completed on July 31, 2015, resulting in a partial reversal of the impairment recorded in 2014 in the amount of $0.1 billion, which is included in operating income from discontinued operations.
Other significant Transactions in 2015
Pharmaceuticals—Acquisition of Spinifex Pharmaceuticals, Inc.
On June 29, 2015 Novartis entered into an agreement to acquire Spinifex Pharmaceuticals, Inc. (Spinifex), a US and Australian-based, privately held development stage company, focused on developing a peripheral approach to treat neuropathic pain. The transaction closed on July 24, 2015, and the total purchase consideration was $312 million. The amount consisted of an initial cash payment of $196 million and the net present value of the contingent consideration of $116 million due to previous Spinifex shareholders, which they are eligible to receive upon achievement of specified development and commercialization milestones. The purchase price allocation resulted in net identifiable assets of $263 million and goodwill of $49 million. Results of operations since the date of acquisition were not material.
Pharmaceuticals—Acquisition Admune Therapeutics LLC.
On October 16, 2015, Novartis acquired Admune Therapeutics LLC (Admune), a US-based, privately held company, broadening Novartis' pipeline of cancer immunotherapies. The total purchase consideration amounted to $258 million. This amount consists of an initial cash payment of $140 million and the net present value of the contingent consideration of $118 million due to Admune's previous owners, which they are eligible to receive upon the achievement of specified development and commercialization milestones. The purchase price allocation resulted in net identifiable assets of $258 million. No goodwill was recognized. Results of operations since the date of acquisition were not material.
Acquisitions and Divestments in 2014
Vaccines—Divestment of blood transfusion diagnostics unit
On January 9, 2014, Novartis completed the divestment of its blood transfusion diagnostics unit to the Spanish company Grifols S.A. for $1.7 billion in cash. The pre-tax gain on this transaction was approximately $0.9 billion and was recorded in operating income from discontinued operations.
142
Pharmaceuticals—Acquisition of CoStim Pharmaceuticals, Inc.
On February 17, 2014, Novartis acquired all of the outstanding shares of CoStim Pharmaceuticals Inc., a Cambridge, Massachusetts, US-based, privately held biotechnology company focused on harnessing the immune system to eliminate immune-blocking signals from cancer, for a total purchase consideration of $248 million (excluding cash acquired). This amount consists of an initial cash payment and the net present value of contingent consideration of $153 million due to previous CoStim shareholders, which they are eligible to receive upon the achievement of specified development and commercialization milestones. The purchase price allocation resulted in net identified assets of $152 million (excluding cash acquired) and goodwill of $96 million. Results of operations since the date of acquisition were not material.
Pharmaceuticals—Divestment of Idenix Pharmaceuticals, Inc. (Idenix) Shareholding
On August 5, 2014, Merck & Co., USA completed a tender offer for Idenix. As a result, Novartis divested its 22% shareholding in Idenix and realized a gain of approximately $0.8 billion which was recorded in income from associated companies.
Alcon—Acquisition of WaveTec Vision Systems, Inc. (WaveTec)
On October 16, 2014, Alcon acquired all of the outstanding shares of WaveTec, a privately held company, for $350 million in cash. The purchase price allocation resulted in net identified assets of $180 million and goodwill of $170 million. Results of operations since the date of acquisition were not material.
Corporate—Divestment of LTS Lohmann Therapie-Systeme AG (LTS) Shareholding
On November 5, 2014, Novartis divested its 43% shareholding in LTS and realized a gain of approximately $0.4 billion which was recorded in income from associated companies.
Classification as continuing operations and discontinued operations
Following the April 22, 2014 announcement of the portfolio transformation transactions with Lilly and GSK, as described above, Novartis reported the Group's financial statements for the current and prior years as "continuing operations" and "discontinued operations".
Continuing operations comprise the activities of the Pharmaceuticals, Alcon and Sandoz Divisions and the continuing Corporate activities. Continuing operations also include the results from Oncology assets acquired from GSK and the estimated results from the 36.5% interest in the GSK/Novartis consumer healthcare joint venture for the period from March 2, 2015 to December 31, 2015 (the latter reported as part of income from associated companies).
Discontinued operations include in 2015 the operational results from the Vaccines influenza business, prior to its divestment to CSL Limited on July 31, 2015, as well as results from the Vaccines non-influenza business and OTC business until March 2, 2015. Operational results from the Animal Health business, which was divested on January 1, 2015, include only the divestment gain.
Discontinued operations in 2015 also include the exceptional pre-tax gain of $12.7 billion from the divestment of Animal Health ($4.6 billion) and the transactions with GSK ($2.8 billion for the Vaccines non-influenza business and $5.9 billion arising from the contribution of Novartis OTC into the GSK Consumer Healthcare joint venture). In addition the GSK transactions resulted in $0.6 billion of additional transaction-related expenses reported in Corporate discontinued operations.
In 2014, discontinued operations include the results of the Vaccines influenza and non-influenza business, OTC and Animal Health for the full year. Results also included an exceptional impairment
143
charge of $1.1 billion for the Vaccines influenza business, which was reduced by $0.1 billion in 2015 upon closing of the CSL transaction and an exceptional pre-tax gain of $0.9 billion arising from the $1.7 billion divestment of the blood transfusion diagnostics unit to Grifols S.A., completed on January 9, 2014.
Excluded from discontinued operations are certain intellectual property rights and related other revenues of the Vaccines Division, which are retained by Novartis and are now reported under Corporate activities.
As required by IFRS, results of the discontinued operations exclude any further depreciation and amortization related to discontinued operations from the date of the portfolio transformation announcement of April 22, 2014.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our significant accounting policies are set out in "Item 18. Financial Statements—Note 1", which are prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB).
Given the uncertainties inherent in our business activities, we must make certain estimates and assumptions that require difficult, subjective and complex judgments. Because of uncertainties inherent in such judgments, actual outcomes and results may differ from our assumptions and estimates, which could materially affect the Group's consolidated financial statements. Application of the following accounting policies requires certain assumptions and estimates that have the potential for the most significant impact on our consolidated financial statements.
As is typical in the pharmaceuticals industry, our gross sales are subject to various deductions which are composed primarily of rebates and discounts to retail customers, government agencies, wholesalers, health insurance companies and managed healthcare organizations. These deductions represent estimates of the related obligations, requiring the use of judgment when estimating the effect of these sales deductions on gross sales for a reporting period. These adjustments are deducted from gross sales to arrive at net sales.
The following summarizes the nature of some of these deductions and how the deduction is estimated. After recording these, net sales represent our best estimate of the cash that we expect to ultimately collect. The US market has the most complex arrangements related to revenue deductions.
United States specific healthcare plans and program rebates
The United States Medicaid Drug Rebate Program is administered by State governments using State and Federal funds to provide assistance to certain vulnerable and needy individuals and families. Calculating the rebates to be paid related to this Program involves interpreting relevant regulations, which are subject to challenge or change in interpretative guidance by government authorities. Provisions for estimating Medicaid rebates are calculated using a combination of historical experience, product and population growth, product price increases and the mix of contracts and specific terms in the individual State agreements. These provisions are adjusted based on established processes and experiences from filing data with individual States.
The United States Federal Medicare Program, which funds healthcare benefits to individuals age 65 or older, provides prescription drug benefits under Part D of the program. This benefit is provided through private prescription drug plans. Provisions for estimating Medicare Part D rebates are calculated based on the terms of individual plan agreements, product sales and population growth, product price increases and the mix of contracts, and are adjusted periodically.
144
We offer rebates to key managed healthcare plans in an effort to sustain and increase sales of our products. These rebate programs provide payors a rebate after they have demonstrated they have met all terms and conditions set forth in their contract with us. These rebates are estimated based on the terms of individual agreements, historical experience and projected product growth rates. We adjust provisions related to these rebates periodically to reflect actual experience.
There is often a time lag of several months between us recording the revenue deductions and our final accounting for them.
Non-United States specific healthcare plans and program rebates
In certain countries other than the US, we provide rebates to governments and other entities. These rebates are often mandated by laws or government regulations.
In several countries we enter into innovative pay-for-performance arrangements with certain healthcare providers, especially in Europe and Australia. Under these agreements, we may be required to make refunds to the healthcare providers or to provide additional medicines free of charge if anticipated treatment outcomes do not meet predefined targets. Potential refunds and the delivery of additional medicines at no cost are estimated and recorded as a deduction of revenue at the time the related revenues are recorded. Estimates are based on historical experience and clinical data. In cases where historical experience and clinical data are not sufficient for a reliable estimation of the outcome, revenue recognition would be deferred until such history would be available. In addition, we offer global patient assistant programs.
There is often a time lag of several months between us recording the revenue deductions and our final accounting for them.
Non-healthcare plans and program rebates, returns and other deductions
Charge-backs occur where our subsidiaries have arrangements with indirect customers to sell products at prices that are lower than the price charged to wholesalers. A charge-back represents the difference between the invoice price to the wholesaler and the indirect customer's contract price. We account for vendor charge-backs by reducing revenue by an amount equal to our estimate of charge-backs attributable to a sale and they are generally settled within one to three months of incurring the liability. Provisions for estimated charge-backs are calculated using a combination of factors such as historical experience, product growth rates, payments, level of inventory in the distribution channel, the terms of individual agreements and our estimate of the claims processing time lag.
We offer rebates to purchasing organizations and other direct and indirect customers to sustain and increase market share for our products. Since rebates are contractually agreed upon, rebates are estimated based on the terms of the individual agreements, historical experience, and projected product growth rates.
When we sell a product providing a customer the right to return it, we record a provision for estimated sales returns based on our sales returns policy and historical rates. Other factors considered include actual product recalls, expected marketplace changes, the remaining shelf life of the product, and the expected entry of generic products. In 2015, sales returns amounted to approximately 1% of gross product sales. If sufficient experience is not available, sales are only recorded based on evidence of product consumption or when the right of return has expired.
We enter into distribution service agreements with major wholesalers, which provide a financial disincentive for the wholesalers to purchase product quantities in excess of current customer demand. Where possible, we adjust shipping patterns for our products to maintain wholesalers' inventories level consistent with underlying patient demand.
145
We offer cash discounts to customers to encourage prompt payment. Cash discounts are estimated and accrued at the time of invoicing and deducted from revenue.
Following a decrease in the price of a product, we generally grant customers a "shelf stock adjustment" for a customer's existing inventory for the relevant product. Provisions for shelf stock adjustments, which are primarily relevant within the Sandoz Division, are determined at the time of the price decline or at the point of sale, if the impact of a price decline on the products sold can be reasonably estimated based on the customer's inventory levels of the relevant product.
Other sales discounts, such as consumer coupons and co-pay discount cards, are offered in some markets. The estimated amount of these discounts are recorded at the time of sale, or when the coupon is issued, and are estimated utilizing historical experience and the specific terms for each program. If a discount for a probable future transaction is offered as part of a sales transaction then an appropriate portion of revenue is deferred to cover this estimated obligation.
We adjust provisions for revenue deductions periodically to reflect actual experience. To evaluate the adequacy of provision balances, we use internal and external estimates of the level of inventory in the distribution channel, actual claims data received and the time lag for processing rebate claims. Management also estimates the level of inventory of the relevant product held by retailers and in transit. External data sources include reports of wholesalers and third-party market data purchased by Novartis.
146
The following table shows the worldwide extent of our revenue deductions provisions and related payment experiences for the Pharmaceuticals, Alcon and Sandoz divisions:
PROVISIONS FOR REVENUE DEDUCTIONS
| |
|
|
|
Income statement charge |
|
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Effect of currency translation and business combinations |
|
Change in provisions offset against gross trade receivables |
|
|||||||||||||||||
| |
Revenue deductions provisions at January 1 |
|
Revenue deductions provisions at December 31 |
|||||||||||||||||||
| |
Payments/ utilizations |
Adjustments of prior years |
Current year |
|||||||||||||||||||
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||||
2015 |
||||||||||||||||||||||
US-specific healthcare plans and program rebates |
1,097 | (2,823 | ) | (90 | ) | 2,981 | 1,165 | |||||||||||||||
Non-US-specific healthcare plans and program rebates |
1,015 | (109 | ) | (1,716 | ) | (3 | ) | 1,846 | (9 | ) | 1,024 | |||||||||||
Non-healthcare plans and program-related rebates, returns and other deductions |
1,421 | (69 | ) | (10,679 | ) | (124 | ) | 10,993 | 59 | 1,601 | ||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Total continuing operations 2015 |
3,533 | (178 | ) | (15,218 | ) | (217 | ) | 15,820 | 50 | 3,790 | ||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
2014 |
||||||||||||||||||||||
US-specific healthcare plans and program rebates |
1,376 | (3,118 | ) | (186 | ) | 3,025 | 1,097 | |||||||||||||||
Non-US-specific healthcare plans and program rebates |
1,145 | (124 | ) | (1,743 | ) | (19 | ) | 1,787 | (31 | ) | 1,015 | |||||||||||
Non-healthcare plans and program-related rebates, returns and other deductions |
1,427 | (83 | ) | (9,046 | ) | (52 | ) | 9,564 | (389 | ) | 1,421 | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Total continuing operations 2014 |
3,948 | (207 | ) | (13,907 | ) | (257 | ) | 14,376 | (420 | ) | 3,533 | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
2013 |
||||||||||||||||||||||
US-specific healthcare plans and program rebates |
1,434 | (2,990 | ) | (74 | ) | 3,006 | 1,376 | |||||||||||||||
Non-US-specific healthcare plans and program rebates |
942 | 10 | (1,634 | ) | (45 | ) | 1,935 | (63 | ) | 1,145 | ||||||||||||
Non-healthcare plans and program-related rebates, returns and other deductions |
1,444 | (10 | ) | (7,745 | ) | (34 | ) | 7,934 | (162 | ) | 1,427 | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Total continuing operations 2013 |
3,820 | 0 | (12,369 | ) | (153 | ) | 12,875 | (225 | ) | 3,948 | ||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
147
The table below shows the gross to net sales reconciliation for our Pharmaceuticals Division:
GROSS TO NET SALES RECONCILIATION
| |
Income statement charge | |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Charged through revenue deduction provisions |
Charged directly without being recorded in revenue deduction provisions |
Total | In % of gross sales |
|||||||||
| |
$ m |
$ m |
$ m |
|
|||||||||
2015 |
|||||||||||||
Pharmaceuticals gross sales subject to deductions |
37,853 | 100.0 | |||||||||||
| | | | | | | | | | | | | | |
US-specific healthcare plans and program rebates |
(1,422 | ) | (1,422 | ) | (3.8 | ) | |||||||
Non-US-specific healthcare plans and program rebates |
(1,150 | ) | (779 | ) | (1,929 | ) | (5.1 | ) | |||||
Non-healthcare plans and program-related rebates, returns and other deductions |
(2,241 | ) | (1,816 | ) | (4,057 | ) | (10.7 | ) | |||||
| | | | | | | | | | | | | | |
Total Pharmaceuticals gross to net sales adjustments |
(4,813 | ) | (2,595 | ) | (7,408 | ) | (19.6 | ) | |||||
| | | | | | | | | | | | | | |
Pharmaceuticals net sales 2015 |
30,445 | 80.4 | |||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
2014 |
|||||||||||||
Pharmaceuticals gross sales subject to deductions |
39,529 | 100.0 | |||||||||||
| | | | | | | | | | | | | | |
US-specific healthcare plans and program rebates |
(1,800 | ) | (1,800 | ) | (4.6 | ) | |||||||
Non-US-specific healthcare plans and program rebates |
(1,200 | ) | (877 | ) | (2,077 | ) | (5.3 | ) | |||||
Non-healthcare plans and program-related rebates, returns and other deductions |
(1,873 | ) | (1,989 | ) | (3,862 | ) | (9.8 | ) | |||||
| | | | | | | | | | | | | | |
Total Pharmaceuticals gross to net sales adjustments |
(4,873 | ) | (2,866 | ) | (7,739 | ) | (19.6 | ) | |||||
| | | | | | | | | | | | | | |
Pharmaceuticals net sales 2014 |
31,790 | 80.4 | |||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
2013 |
|||||||||||||
Pharmaceuticals gross sales subject to deductions |
40,188 | 100.0 | |||||||||||
| | | | | | | | | | | | | | |
US-specific healthcare plans and program rebates |
(2,125 | ) | (2,125 | ) | (5.3 | ) | |||||||
Non-US-specific healthcare plans and program rebates |
(1,368 | ) | (802 | ) | (2,170 | ) | (5.4 | ) | |||||
Non-healthcare plans and program-related rebates, returns and other deductions |
(1,731 | ) | (1,948 | ) | (3,679 | ) | (9.2 | ) | |||||
| | | | | | | | | | | | | | |
Total Pharmaceuticals gross to net sales adjustments |
(5,224 | ) | (2,750 | ) | (7,974 | ) | (19.8 | ) | |||||
| | | | | | | | | | | | | | |
Pharmaceuticals net sales 2013 |
32,214 | 80.2 | |||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Impairment of Goodwill, Intangible Assets and Property, Plant and Equipment
We review long-lived intangible assets and property, plant and equipment for impairment whenever events or changes in circumstance indicate that the asset's balance sheet carrying amount may not be recoverable. Goodwill, the Alcon brand-name and other currently not amortized intangible assets are reviewed for impairment at least annually.
An asset is generally considered impaired when its balance sheet carrying amount exceeds its estimated recoverable amount, which is defined as the higher of its fair value less costs of disposal and its value in use. Usually, Novartis adopts the fair value less costs of disposal method for its impairment evaluation. In most cases no directly observable market inputs are available to measure the fair value less
148
costs of disposal. Therefore an estimate of fair value less costs of disposal is derived indirectly and is based on net present value techniques utilizing post-tax cash flows and discount rates. In the limited cases where the value in use method is applied, net present value techniques are utilized using pre-tax cash flows and discount rates.
Fair value reflects estimates of assumptions that market participants would be expected to use when pricing the asset and for this purpose management considers the range of economic conditions that are expected to exist over the remaining useful life of the asset. The estimates used in calculating net present values are highly sensitive, and depend on assumptions specific to the nature of the Group's activities with regard to:
- –
- amount and timing of projected future cash flows;
- –
- future tax rates;
- –
- behavior of competitors (launch of competing products, marketing initiatives, etc.); and
- –
- appropriate discount rate.
Due to the above factors and those further described in "Item 18. Financial Statements—Note 1", actual cash flows and values could vary significantly from forecasted future cash flows and related values derived using discounting techniques.
The recoverable amount of cash-generating units and related goodwill is usually based on the fair value less costs of disposal derived from applying discounted future cash flows based on the key assumptions in the following table:
| |
Pharmaceuticals | Alcon | Sandoz | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
% |
% |
% |
|||||||
Cash flows growth rate assumptions after forecast period |
1 | 3 | 0 to 2 | |||||||
Discount rate (post-tax) |
6 | 6 | 6 | |||||||
In 2015, intangible asset impairment charges for continuing operations of $206 million were recognized, of which $120 million were recorded in the Alcon Division and $86 million in total in the Pharmaceuticals and Sandoz divisions.
In 2014, intangible asset impairment charges of continuing operations amounted to $347 million ($302 million in the Pharmaceuticals Division and $45 million in total in the Sandoz and Alcon divisions).
In 2015, the reversal of impairment charges recorded in prior years amounted to $40 million (2014: $70 million).
Goodwill and other intangible assets represent a significant part of our consolidated balance sheet, primarily due to acquisitions. Although no significant additional impairments are currently anticipated, impairment evaluation could lead to material impairment charges in the future. For more information, see "Item 18. Financial Statements—Note 11".
Additionally, net impairment charges for property, plant and equipment from continuing operations during 2015 amounted to $68 million (2014: $44 million).
Trade receivables are initially recognized at their invoiced amounts including any related sales taxes less adjustments for estimated revenue deductions such as rebates, charge backs and cash discounts.
Provisions for doubtful trade receivables are established once there is an indication that it is likely that a loss will be incurred. These provisions represent the difference between the trade receivable's
149
carrying amount in the consolidated balance sheet and the estimated net collectible amount. Significant financial difficulties of a customer, such as probability of bankruptcy, financial reorganization, default or delinquency in payments are considered indicators that recovery of the trade receivable is doubtful. Trade receivable balances include sales to drug wholesalers, retailers, private health systems, government agencies, managed care providers, pharmacy benefit managers and government-supported healthcare systems. Novartis continues to monitor sovereign debt issues and economic conditions in Greece, Italy, Portugal, Spain and other countries, and evaluates trade receivables in these countries for potential collection risks. Substantially all of the trade receivables overdue from such countries are due directly from local governments or from government-funded entities. Deteriorating credit and economic conditions and other factors in these countries have resulted in, and may continue to result in an increase in the average length of time that it takes to collect these trade receivables and may require Novartis to re-evaluate the collectability of these trade receivables in future periods.
In a business combination or divestment of a business, it is necessary to recognize contingent future payments to previous or from new owners representing contractually defined potential amounts as a liability or asset. Usually for Novartis these are linked to milestone or royalty payments related to certain assets and are recognized as a financial liability or asset at their fair value which is then re-measured at each subsequent reporting date. These estimations typically depend on factors such as technical milestones or market performance and are adjusted for the probability of their likelihood of payment and if material, appropriately discounted to reflect the impact of time. Changes in the fair value of contingent liabilities in subsequent periods are recognized in the consolidated income statement in "Cost of goods sold" for currently marketed products and in "Research & Development" for IPR&D. Changes in contingent assets are recognized in "Other income and expense". The effect of unwinding the discount over time is recognized in "Interest expense" in the consolidated income statement. Novartis does not recognize contingent consideration associated with asset purchases outside of a business combination that are conditional upon future events which are within its control until such time as there is an unconditional obligation. If the contingent consideration is outside the control of Novartis, a liability is recognized once it becomes probable that the contingent consideration will become due. In both cases, if appropriate, a corresponding asset is recorded.
Impairment of Associated Companies Accounted for at Equity
Novartis considers investments in associated companies for impairment evaluation whenever there is a quoted share price indicating a fair value less than the per-share balance sheet carrying value for the investment. For unquoted investments in associated companies, recent financial information is taken into account to assess whether an impairment evaluation is necessary.
If the recoverable amount of the investment is estimated to be lower than the balance sheet carrying amount an impairment charge is recognized for the difference in the consolidated income statement under "Income from associated companies".
Retirement and Other Post-Employment Benefit Plans
We sponsor pension and other post-employment benefit plans in various forms that cover a significant portion of our current and former associates. For post-employment plans with defined benefit obligations, we are required to make significant assumptions and estimates about future events in calculating the expense and the present value of the liability related to these plans. These include assumptions about the interest rates we apply to estimate future defined benefit obligations and net periodic pension expense as well as rates of future pension increases. In addition, our actuarial consultants provide our management with historical statistical information such as withdrawal and mortality rates in connection with these estimates.
150
Assumptions and estimates used by the Group may differ materially from the actual results we experience due to changing market and economic conditions, higher or lower withdrawal rates, and longer or shorter life spans of participants among other factors. For example, in 2015, a decrease in the interest rate we apply in determining the present value of the defined benefit obligations of one quarter of one percent would have increased our year-end defined benefit pension obligation for plans in Switzerland, US, UK, Germany and Japan, which represent 95% of the Group total defined benefit pension obligation, by approximately $0.8 billion. Similarly, if the 2015 interest rate had been one quarter of one percentage point lower than actually assumed, net periodic pension cost for pension plans in these countries, which represent about 88% of the Group's total net periodic pension cost for pension plans, would have increased by approximately $22 million. Depending on events, such differences could have a material effect on our total equity. For more information on obligations under retirement and other post-employment benefit plans and underlying actuarial assumptions, see "Item 18. Financial Statements—Note 25".
A number of Group companies are involved in various government investigations and legal proceedings (intellectual property, sales and marketing practices, product liability, commercial, employment and wrongful discharge, environmental claims, etc.) arising out of the normal conduct of their businesses. For more information, see "Item 18. Financial Statements—Note 20".
We record accruals for contingencies when it is probable that a liability has been incurred and the amount can be reliably estimated. These accruals are adjusted periodically as assessments change or additional information becomes available. For significant product liability cases the accrual is actuarially determined based on factors such as past experience, amount and number of claims reported, and estimates of claims incurred but not yet reported. Expected legal defense costs are accrued when the amount can be reliably estimated.
In some instances, the inherent uncertainty of litigation, the resources required to defend against governmental actions, the potential impact on our reputation, and the potential for exclusion from government reimbursement programs in the US and other countries have contributed to decisions by Novartis and other companies in our industry to enter into settlement agreements with governmental authorities in the absence of an acknowledgement of legal liability. These settlements have had in the past, and may continue in the future, to involve large cash payments, including potential repayment of amounts that were allegedly improperly obtained and other penalties including treble damages. In addition, settlements of governmental healthcare fraud cases often require companies to enter into corporate integrity agreements, which are intended to regulate company behavior for a period of years. Our affiliate Novartis Pharmaceuticals Corporation is a party to such an agreement, which will expire in 2020. Also, matters underlying governmental investigations and settlements may be the subject of separate private litigation.
Provisions are recorded for environmental remediation costs when expenditure on remedial work is probable and the cost can be reliably estimated. Remediation costs are provided for under "Non-current liabilities" in the Group's consolidated balance sheet.
Provisions relating to estimated future expenditure for liabilities do not usually reflect any insurance or other claims or recoveries, since these are only recognized as assets when the amount is reasonably estimable and collection is virtually certain.
Internal Research & Development costs are fully charged to the consolidated income statement in the period in which they are incurred. We consider that regulatory and other uncertainties inherent in the development of new products preclude the capitalization of internal development expenses as an
151
intangible asset usually until marketing approval from the regulatory authority is obtained in a relevant major market, such as for the US, the EU, Switzerland or Japan.
In many countries our subsidiaries are required to make contributions to the countries' healthcare costs as part of programs other than the ones mentioned above under deductions from revenues. The amounts to be paid depend on various criteria such as the subsidiary's market share or sales volume compared to certain targets. Considerable judgment is required in estimating these contributions as not all data is available when the estimates need to be made.
The largest of these healthcare contributions relates to the US Healthcare Reform fee, which was introduced in 2011. This fee is an annual levy to be paid by US pharmaceutical companies, including various Novartis subsidiaries, based on each company's qualifying sales as a percentage of the prior year's government-funded program sales. This pharmaceutical fee levy is recognized in "Other expense".
On July 25, 2014, the US Department of the Treasury and the US Internal Revenue Service issued final guidance on this pharmaceutical fee levy which stipulated that instead of a liability being estimated and recognized immediately with the first qualifying sale in the following fee year, as had been industry practice, the levy is owed in the year in which the sales occur.
As a result of this final guidance, in 2014, "Other expense" includes the recurring non-tax deductible annual expense of approximately $200 million for the 2014 pharmaceutical fee levy, as well as the non-tax deductible expense of $204 million for the 2013 pharmaceutical fee levy. $204 million of this charge has been considered as an additional exceptional charge in 2014 since it results from the change in timing of recognition of the pharmaceutical fee levy as required by the final guidance.
In addition, effective 2013, the US government also implemented a medical device sales tax which is levied on the Alcon Division's US sales of products which are considered surgical devices under the law. This medical device tax is initially included in the cost of inventory as, for Alcon, the tax is usually levied on intercompany sales. It is expensed as cost of goods sold when the inventory is sold to third parties.
We prepare and file our tax returns based on an interpretation of tax laws and regulations, and record estimates based on these judgments and interpretations. Our tax returns are subject to examination by the competent taxing authorities, which may result in an assessment being made requiring payments of additional tax, interest or penalties. Inherent uncertainties exist in our estimates of our tax positions. We believe that our estimated amounts for current and deferred tax assets or liabilities, including any amounts related to any uncertain tax positions, are appropriate based on currently known facts and circumstances.
See "Item 18. Financial Statements—Note 1".
Internal Control Over Financial Reporting
The Group's management has assessed the effectiveness of internal control over financial reporting. The Group's independent statutory auditor also issued an opinion on the effectiveness of internal control over financial reporting. Both the Group's management and its external auditors concluded that the Group maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015.
152
FACTORS AFFECTING RESULTS OF OPERATIONS
Long-term demographic trends and changing lifestyles are driving increased demand for healthcare around the world, while advances in science and technology are opening new frontiers in patient treatments. In the coming years, these trends are expected to drive steady growth overall in the healthcare market and accelerate growth in key segments of our business. At the same time, the current business and regulatory environment poses significant risks and potential impediments to our growth and to the growth of the healthcare industry.
Transformational Changes Fueling Demand
Aging population and shifting behaviors
Scientific advances and increased access to healthcare are contributing to a rise in life expectancy, increasing the proportion of elderly people worldwide. According to United Nations projections, the number of people over the age of 60 is expected to rise by 500 million, reaching 1.4 billion, by 2030.
The aging of the world's population has contributed to an increase in chronic illnesses that are prevalent among the elderly, such as cancer, heart disease, respiratory ailments, diabetes and eye disease. A global shift toward more sedentary lifestyles is also increasing demand for healthcare. In the last 20 years, obesity rates have doubled among adults and tripled among children.
Novartis has developed new treatments to address some of these growing health threats and we plan to continue research and development activities in these areas.
In 2015, for example, Novartis received approval from the US Food and Drug Administration (FDA) and the European Commission for Entresto in chronic heart failure with reduced ejection fraction, which affects more than two million people in the United States and more than five million people in Europe. Regulatory decisions were based on the PARADIGM-HF study, which showed a 20% reduction in cardiovascular deaths versus an ACE inhibitor, the current standard of care in heart failure.
Global rise in healthcare spending
Increased demand for healthcare around the world has translated into rising healthcare costs. If growth in healthcare spending were to continue at the current pace, global outlays could more than double by 2025 to $15 trillion. At the same time, economic uncertainty and tight budgets are prompting many governments, healthcare insurers and consumers to look for ways to moderate spending.
In the context of these trends, we believe that our portfolio spanning pharmaceuticals, generics and eye care, is well-positioned to meet the evolving needs of patients and healthcare systems. For example, the use of generic medicines and biosimilars helps reduce healthcare costs and free up resources for new innovative medicines. Indeed, the global biosimilars market is expected to reach $35 billion by 2020 from an estimated $1.3 billion in 2013, according to a report by Allied Market Research. Our Sandoz Division is a global leader in biosimilars, with three products on the market in Europe and ten major filings (including etanercept and pegfilgrastim, which were submitted in 2015) planned in the next three years. In 2015, Sandoz became the first company to win approval for a biosimilar in the United States under the pathway created by the Biologics Price Competition and Innovation Act.
Scientific advances opening new opportunities
As scientific research has become more sophisticated, we have developed a better understanding of the genetic basis of diseases. This has given rise to a new generation of innovative therapies that could more effectively target the underlying causes of disease.
153
For example, our investigational therapy CTL019 works by reprogramming a patient's own T-cells to hunt cancer cells that express specific proteins. After they have been reprogrammed, the T cells are re-introduced into the patient's blood; they proliferate and bind to the targeted cancer cells and destroy them.
Therapies like these have the potential to transform the treatment of disease. We believe that our ability to leverage scientific advances to generate innovative new treatments will enable us to create value over the long-term for society, patients and shareholders.
Convergence of healthcare and technology
From molecular diagnostics to clinical trial recruitment to real world data and analytics, technology continues to play an increasingly important role in the pharmaceutical industry. This is attracting new entrants to the sector. For instance, venture funding grew 200% for digital health companies between 2012 and 2014. Established technology companies such as Google are also using their expertise to expand into healthcare.
While new entrants may shift the competitive landscape, the growing role of technology in healthcare presents an opportunity to pharmaceutical companies like Novartis. Google, for example, is collaborating with our Alcon Division to develop an accommodating contact or intraocular lens for people living with presbyopia. Through the collaboration, we are marrying Google's expertise in miniaturized electronics and microfabrication with Alcon's expertise in the physiology of the eye, as well as clinical development and commercialization of contact and intraocular lenses, to advance a product that has the potential to make reading glasses obsolete.
We also formed a joint investment company with Qualcomm Ventures to support early stage companies with technologies, products or services that "go beyond the pill" to benefit physicians and patients. We recognize the potential of technology to enhance our ability to deliver the right medicine to the right patient at the right time, and seek to partner with experts in emerging technologies to build our expertise in these areas.
Increasingly Challenging Business Environment
Patent expirations and product competition
It is common for pharmaceutical companies to face generic erosion when their products lose patent or other intellectual property protection, and Novartis is no exception. The products of our Pharmaceuticals and Alcon Divisions are generally protected by patent or other intellectual property rights, allowing us to exclusively market those products. The loss of exclusivity has had, and will continue to have, an adverse effect on our results. In 2015, the impact of generic competition on our net sales amounted to $2.2 billion.
Like other players in the pharmaceutical industry, some of our products have begun to face considerable competition due to the expiration of patent or other intellectual property protection. For example:
- –
- We already face generic competition in Japan and some EU countries for Gleevec/Glivec.
In the US, we have resolved patent litigation with certain generic manufacturers. We licensed to a subsidiary of Sun Pharmaceutical Industries the right to market a generic version of Gleevec in the US
as of February 1, 2016. In the EU, our Glivec intellectual property rights are
also being challenged by generic manufacturers.
- –
- Diovan and Co-Diovan/Diovan HCT, which had long been our best-selling product, has generic competitors for Diovan in the US, EU and Japan and for Co-Diovan/Diovan HCT in the US and EU. In Japan, Novartis resolved patent litigation with a generic manufacturer. Patent protection for Co-Diovan will expire in Japan in 2016.
154
To counter the impact of patent expirations, we continuously invest in research and development to rejuvenate our portfolio. For example, in 2015, we invested 18% of total net sales in research and development. One measure of the output of our efforts is the performance of our Growth Products—products launched in a key market (EU, US, Japan) in 2010 or later, or products with exclusivity in key markets until at least 2019 (except Sandoz, which includes only products launched in the last 24 months). These products accounted for 34% of total net sales in 2015, up 17% from the previous year.
Moreover, while patent expirations present a significant challenge to our Pharmaceuticals and Alcon divisions, they also create an opportunity for Sandoz, our generics business. With our global footprint and advanced technical expertise, we expect Sandoz to help offset the financial impact of generic competition on our branded portfolio.
Heightened regulatory and safety hurdles
Our ability to grow is dependent on our ability to bring new products to market. In recent years, health regulators have raised the bar on product innovation. They are increasingly focused on the benefit-risk profile of pharmaceutical products, emphasizing product safety and improvements over older products in the same therapeutic class. These developments have led to requests for more clinical trial data, the inclusion of significantly higher numbers of patients in those trials, and more detailed analyses of trial outcomes. As a result, the long and expensive process of obtaining regulatory approvals for pharmaceutical products has become even more challenging.
In addition, approved drugs have increasingly been subject to requirements such as risk management plans, comparative effectiveness studies, health technology assessments and post-approval Phase IV clinical trials, making the maintenance of regulatory approvals and achievement of reimbursement for our products increasingly expensive. In addition, these requirements further heighten the risk of recalls, product withdrawals, or loss of market share.
Despite this risk, however, we expect that our focus on accelerating innovation in areas of unmet medical need and demonstrating real improvement in patient outcomes will allow Novartis to continue to bring effective and safe medicines to market.
Increasing pressure on pricing
Against the backdrop of steadily rising healthcare costs, there has been increased scrutiny on drug pricing by governments, media and consumers. Following the launch of Gilead's Sovaldi® in hepatitis C, media focused on the price tag and lawsuits were filed against the company, alleging price-gouging. In 2015, the pricing debate reached a new level of intensity when Turing Pharmaceuticals acquired the rights to the decades-old medicine Daraprim® and raised the price by 5,000%.
We expect scrutiny on prices to continue in 2016 as political pressures mount and healthcare payors around the globe—including government-controlled health authorities, insurance companies and managed care organizations—step up initiatives to reduce the overall cost of healthcare, restrict access to higher-priced new medicines, increase the use of generics and impose overall price cuts.
In this environment, we believe that it is more important than ever to demonstrate the value that true innovation brings to the healthcare system. For example, with our psoriasis medicine Cosentyx, we demonstrated superiority to Stelara® in a head-to-head study, but still adopted a similar price for our product. Similarly, with Entresto, an independent organization called the Institute for Clinical and Economic Review found that its US list price was "well-aligned with the degree of benefit it brings to patients." Furthermore, we expressed a willingness to work with our customers on flexible, performance-based pricing models, where we would only be fully compensated if the drug succeeded in meeting certain targets, such as reducing heart failure hospitalizations and associated costs.
155
To manage pricing pressure, we aim to invest in access to real-world data and analytics, explore new technologies and patient management services, and partner with payors to develop and scale outcomes-based commercial models.
Potential liability arising from legal proceedings and government investigations
In recent years, there has been a trend of increasing government investigations and litigation against companies operating in our industry, including in the US and other countries. We are obligated to comply with the laws of all countries in which we operate, with new requirements imposed on us as government and public expectations of corporate behavior develop. We have a significant global compliance program in place, and devote substantial time and resources to ensure that our business is conducted in a legal and publicly acceptable manner. Despite our efforts, any failure to comply with the law could lead to substantial liabilities that may not be covered by insurance and could affect our business and reputation.
Governments and regulatory authorities worldwide are also increasingly challenging practices previously considered to be legal and responding to such challenges and new regulations is costly. Such investigations may affect our reputation, create a risk of potential exclusion from government reimbursement programs in the US and other countries, and may lead to costly litigation.
These factors have contributed to recent trends in the pharmaceutical industry to enter into settlement agreements with governmental authorities around the world prior to any formal decision by the authorities. For example, in 2015, our affiliate Novartis Pharmaceuticals Corporation settled litigation in the Southern District of New York related to interactions with specialty pharmacies from 2004 to 2013. The settlement included payments totalling $390 million plus additional legal expenses to plaintiffs, and an agreement to amend and extend for five years an existing corporate integrity agreement (CIA) with the Office of Inspector General of the US Department of Health and Human Services. This resolution and the new CIA obligations provide clear guidelines as we continue to work with independent specialty pharmacies in support of patient care.
Risk of liability and supply disruption from manufacturing issues
The manufacture of our products is both highly regulated and complex, which introduces a greater chance for disruptions and liabilities. Government authorities closely regulate our manufacturing processes, and if those processes fail to meet the necessary requirements, then there is a risk that our production facilities could be shut down. Disturbances in our supply chain can lead to product shortages, significant loss in sales revenue, and litigation. Furthermore, any manufacturing issue compromising supply or quality could have serious consequences for the health of our patients.
Beyond regulatory requirements, many of our products involve technically complex manufacturing processes or require a supply of highly specialized raw materials. For example, biologic products, produced from living plant or animal micro-organisms, comprise a significant portion of the portfolio across the Group. For biologic-based products, even slight deviations at any point in the production process could lead to production failures or recalls. The Group's portfolio also includes a number of sterile products, such as oncology treatments, which are technically complex to manufacture and require strict environmental controls. There is a greater chance of production failures and supply interruptions for these products.
Given the complexity of our manufacturing processes, we have had a multi-year effort in place to ensure adherence to a single high quality standard across the Group. This effort continued to yield steady improvement in 2015: regulatory agencies carried out 192 inspections of Novartis facilities worldwide last year, with 189 or 98.4% resulting in a good or acceptable outcome, in line with prior year. In addition, in September the FDA closed out the May 2013 Warning Letter issued for our Sandoz site in Unterach, Austria.
156
Despite this progress, more work remains to be done. In October 2015, the FDA issued a Warning Letter to our Sandoz Division concerning its Indian sites in Kalwe and Turbhe. The letter related to documentation practices in Kalwe and sterile manufacturing practices in Turbhe that were identified during an inspection in August 2014. Novartis took action immediately and has addressed a majority of the issues.
Risk assessment and disclosures
The Risk Committee of the Board ensures the Group has implemented an appropriate and effective risk management system and process. It reviews with management and internal audit the identification, prioritization and management of the risks, the accountabilities and roles of the functions involved with risk management, the risk portfolio and the related actions implemented by management. The Risk Committee informs the Board of Directors on a periodic basis.
The Group Risk Office coordinates and aligns the risk management processes, and reports to the Risk Committee on a regular basis on risk assessment and risk management. Organizational and process measures have been designed to identify and mitigate risks at an early stage. Organizationally, the responsibility for risk assessment and management is allocated to the divisions, with specialized Corporate functions—such as Group Finance, Group Quality Assurance, Corporate Health, Safety and Environment, Business Continuity Management, Integrity and Compliance, and the Business Practices Office—providing support and controlling the effectiveness of the risk management by the divisions and functions in these respective areas.
Financial risk management is described in more detail, see "Item 18. Financial Statements—Note 29".
NON-IFRS MEASURES AS DEFINED BY NOVARTIS
Novartis uses certain non-IFRS metrics when measuring performance, especially when measuring current year results against prior periods, including core results, constant currencies, free cash flow and net debt.
Despite the use of these measures by management in setting goals and measuring the Group's performance, these are non-IFRS measures that have no standardized meaning prescribed by IFRS. As a result, such measures have limits in their usefulness to investors.
Because of their non-standardized definitions, the non-IFRS measures (unlike IFRS measures) may not be comparable to the calculation of similar measures of other companies. These non-IFRS measures are presented solely to permit investors to more fully understand how the Group's management assesses underlying performance. These non-IFRS measures are not, and should not be viewed as, a substitute for IFRS measures.
As an internal measure of Group performance, these non-IFRS measures have limitations, and the Group's performance management process is not solely restricted to these metrics.
The Group's core results—including core operating income, core net income and core earnings per share—exclude the amortization of intangible assets, impairment charges, expenses relating to divestments, the integration of acquisitions and restructuring charges that exceed a threshold of $25 million, as well as other income and expense items that management deems exceptional and that are or are expected to accumulate within the year to be over a $25 million threshold.
Novartis believes that investor understanding of the Group's performance is enhanced by disclosing core measures of performance because, since they exclude items which can vary significantly from year to
157
year, the core measures enable better comparison of business performance across years. For this same reason, Novartis uses these core measures in addition to IFRS and other measures as important factors in assessing the Group's performance.
The following are examples of how these core measures are utilized:
- –
- In addition to monthly reports containing financial information prepared under IFRS, senior management receives a monthly analysis
incorporating these core measures.
- –
- Annual budgets are prepared for both IFRS and core measures.
A limitation of the core measures is that they provide a view of the Group's operations without including all events during a period, such as the effects of an acquisition or amortization of purchased intangible assets.
Changes in the relative values of non-US currencies to the US dollar can affect the Group's financial results and financial position. To provide additional information that may be useful to investors, including changes in sales volume, we present information about our net sales and various values relating to operating and net income that are adjusted for such foreign currency effects.
Constant currency calculations have the goal of eliminating two exchange rate effects so that an estimate can be made of underlying changes in the consolidated income statement excluding the impact of fluctuations in exchange rates:
- –
- the impact of translating the income statements of consolidated entities from their non-$ functional currencies to $; and
- –
- the impact of exchange rate movements on the major transactions of consolidated entities performed in currencies other than their functional currency.
We calculate constant currency measures by translating the current year's foreign currency values for sales and other income statement items into $ using the average exchange rates from the prior year and comparing them to the prior year values in $.
We use these constant currency measures in evaluating the Group's performance, since they may assist us in evaluating our ongoing performance from year to year. However, in performing our evaluation, we also consider equivalent measures of performance which are not affected by changes in the relative value of currencies.
For ease of understanding, Novartis uses a sign convention for its growth rates such that a reduction in operating expenses or losses compared to the prior year is shown as a positive growth.
Novartis defines free cash flow as cash flow from operating activities and cash flow associated with the purchase or sale of property, plant and equipment, intangible, other non-current and financial assets. Cash flows in connection with the acquisition or divestment of subsidiaries, associated companies and non-controlling interests in subsidiaries are not taken into account to determine free cash flow.
Free cash flow is presented as additional information because Novartis considers it to be a useful indicator of the Group's ability to operate without reliance on additional borrowing or use of existing cash. Free cash flow is a measure of the net cash generated that is available for debt repayment, investment in strategic opportunities and for returning to shareholders. Novartis uses free cash flow in internal
158
comparisons of results from the Group's divisions. Free cash flow is not intended to be a substitute measure for cash flow from operating activities (as determined under IFRS).
Novartis defines net debt as current and non-current financial debt less cash and cash equivalents, current investments and derivative financial instruments. Net debt is presented as additional information because management believes it is a useful supplemental indicator of the Group's ability to pay dividends, to meet financial commitments and to invest in new strategic opportunities, including strengthening its balance sheet.
The Novartis Cash Value Added (NCVA) is a metric that is based on what the company assesses to be its cash flow return less a capital charge on gross operating assets. NCVA is used as the primary internal financial measure for determining payouts under the new Long-Term Performance Plan (LTPP) introduced in 2014. More information on NCVA is presented as part of the Compensation report, see "Item 6.B Compensation".
Novartis utilizes its own definition for measuring Novartis Economic Value Added (NVA), which is utilized for determining payouts under the Old Long-Term Performance Plan (OLTPP). The following table shows NVA for 2015 and 2014:
| |
Year ended Dec 31, 2015 |
Year ended Dec 31, 2014 |
Change in $ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
% |
|||||||
Operating income from continuing operations |
8,977 | 11,089 | (19 | ) | ||||||
Income from associated companies |
266 | 1,918 | (86 | ) | ||||||
Operating interest |
(298 | ) | (306 | ) | 3 | |||||
Operating tax |
(1,937 | ) | (2,565 | ) | 24 | |||||
Capital charge |
(6,164 | ) | (5,938 | ) | (4 | ) | ||||
| | | | | | | | | | | |
Novartis Economic Value Added from continuing operations |
844 | 4,198 | (80 | ) | ||||||
Novartis Economic Value Added from discontinued operations |
10,808 | (678 | ) | nm | ||||||
| | | | | | | | | | | |
Total Novartis Economic Value Added |
11,652 | 3,520 | 231 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Operating interest is the internal charge on average working capital based on the short-term borrowing rules of the entity owning them.
Operating tax is the internal tax charge for each entity applying the applicable tax rate to the operational profit before tax unadjusted for tax-disallowed items or tax loss carryforwards.
The capital charge is the notional interest charge on the average non-current assets of operations based on an internally calculated weighted average cost of capital for the Group.
The NVA for continuing operations decreased to $844 million in 2015 from $4.2 billion in the prior-year, mainly on account of the negative currency effect on operating income and lower income from associated companies, which included in the prior year exceptional one-time gains from the sale of the shares of Idenix ($0.8 billion) and LTS ($0.4 billion).
159
The NVA for discontinued operations in 2015 was mainly driven by the $12.7 billion exceptional pre-tax gains form the portfolio transformation transactions with GSK and Lilly.
EBITDA
Novartis defines earnings before interest, tax, depreciation and amortization (EBITDA) as operating income from continuing operations excluding depreciation of property, plant and equipment (including any related impairment charges) and amortization of intangible assets (including any related impairment charges).
| |
2015 | 2014 | Change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Operating income from continuing operations |
8,977 | 11,089 | (2,112 | ) | ||||||
Depreciation of property, plant & equipment |
1,470 | 1,586 | (116 | ) | ||||||
Amortization of intangible assets |
3,755 | 2,775 | 980 | |||||||
Impairments of property, plant & equipment and intangible assets |
246 | 321 | (75 | ) | ||||||
| | | | | | | | | | | |
EBITDA from continuing operations |
14,448 | 15,771 | (1,323 | ) | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Enterprise Value
Enterprise value represents the total amount that shareholders and debt holders have invested in Novartis, less the Group's liquidity.
| |
Dec 31, 2015 | Dec 31, 2014 | Change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Market capitalization |
208,321 | 223,728 | (15,407 | ) | ||||||
Non-controlling interests |
76 | 78 | (2 | ) | ||||||
Financial debts and derivatives |
21,931 | 20,411 | 1,520 | |||||||
Liquidity |
(5,447 | ) | (13,862 | ) | 8,415 | |||||
| | | | | | | | | | | |
Enterprise value |
224,881 | 230,355 | (5,474 | ) | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Enterprise value/EBITDA |
16 | 15 | ||||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
160
2015, 2014 AND 2013 RECONCILIATION OF IFRS RESULTS TO CORE RESULTS—GROUP
2015
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Acquisition or divestment related items, including restructuring and integration charges(3) |
Other exceptional items(4) |
Core results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Gross profit from continuing operations |
32,983 | 3,666 | 126 | 125 | 36,900 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating income from continuing operations |
8,977 | 3,709 | 369 | 182 | 553 | 13,790 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Income before taxes from continuing operations |
8,134 | 4,132 | 369 | 182 | 1,275 | 14,092 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Taxes from continuing operations(5) |
(1,106 | ) | (2,051 | ) | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net income from continuing operations |
7,028 | 12,041 | |||||||||||||||||
Net income/loss from discontinued operations(6) |
10,766 | (256 | ) | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net income |
17,794 | 11,785 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Basic EPS from continuing operations ($)(7) |
2.92 | 5.01 | |||||||||||||||||
Basic EPS from discontinued operations ($)(7) |
4.48 | (0.11 | ) | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total basic EPS ($)(7) |
7.40 | 4.90 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit from continuing operations |
|||||||||||||||||||
Other revenues |
947 | (28 | ) | 919 | |||||||||||||||
Cost of goods sold |
(17,404 | ) | 3,666 | 126 | 153 | (13,459 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income from continuing operations |
|||||||||||||||||||
Marketing & Sales |
(11,772 | ) | 43 | (11,729 | ) | ||||||||||||||
Research & Development |
(8,935 | ) | 43 | 40 | 114 | (8,738 | ) | ||||||||||||
General & Administration |
(2,475 | ) | 86 | (2,389 | ) | ||||||||||||||
Other income |
2,049 | (56 | ) | (283 | ) | (887 | ) | 823 | |||||||||||
Other expense |
(2,873 | ) | 259 | 465 | 1,072 | (1,077 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Income before taxes from continuing operations |
|||||||||||||||||||
Income from associated companies |
266 | 423 | 292 | 981 | |||||||||||||||
Other financial income and expense |
(454 | ) | 430 | (24 | ) | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms; Income from associated companies includes
$423 million for the Novartis share of the estimated Roche core items.
- (2)
- Impairments:
Cost of goods sold, Research & Development and Other expense consist principally of net impairment charges or reversals
related to intangible assets, property, plant and equipment, and financial assets; Other income includes a reversal of an impairment related to property, plant and equipment.
- (3)
- Acquisition
or divestment related items, including restructuring and integration charges: Other income and Other expense include items
related to the portfolio transformation.
- (4)
- Other
exceptional items: Other revenues and Other income include additional gains from product divestments; Cost of goods sold and Other
expense include charges for the Group-wide rationalization of manufacturing sites; Cost of goods sold also includes an inventory write-off; Marketing & Sales, Research & Development and
Other expense include other restructuring charges; Research & Development also includes expenses related to product acquisitions; General & Administration includes charges for
transforming IT and finance processes and expenses related to setup costs for Novartis Business Services; Other income also includes a gain of $110 million from a Swiss pension plan amendment
and items related to portfolio transformation; Other expense also includes legal settlement provisions; Income from associated companies includes $292 million for the Novartis share of the
estimated OTC joint venture core items; Other financial income and expense includes a charge of $410 million related to Venezuela consisting of foreign exchange losses ($211 million),
the loss on the sale of PDVSA bonds ($127 million) and the monetary loss due to hyperinflation ($72 million).
- (5)
- Taxes on the adjustments between IFRS and core results take into account, for each individual item included in the adjustment, the tax rate that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax
161
impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on exceptional items although this is not always the case for items arising from legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total adjustments for continuing operations of $6.0 billion to arrive at the core results before tax amounts to $945 million. The average tax rate on the adjustments for continuing operations is 15.9%.
- (6)
- For
details on discontinued operations reconciliation from IFRS to core net income, see "—Non-IFRS Measures as Defined by
Novartis".
- (7)
- Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.
2014
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Acquisition or divestment related items, including restructuring and integration charges(3) |
Other exceptional items(4) |
Core results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Gross profit from continuing operations |
36,289 | 2,692 | (21 | ) | (139 | ) | 38,821 | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating income from continuing operations |
11,089 | 2,743 | 433 | 33 | 175 | 14,473 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Income before taxes from continuing operations |
12,272 | 3,000 | 434 | 33 | (1,058 | ) | 14,681 | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Taxes from continuing operations(5) |
(1,545 | ) | (2,028 | ) | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net income from continuing operations |
10,727 | 12,653 | |||||||||||||||||
Net income/loss from discontinued operations(6) |
(447 | ) | 102 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net income |
10,280 | 12,755 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Basic EPS from continuing operations ($)(7) |
4.39 | 5.19 | |||||||||||||||||
Basic EPS from discontinued operations ($)(7) |
(0.18 | ) | 0.04 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total basic EPS ($)(7) |
4.21 | 5.23 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit from continuing operations |
|||||||||||||||||||
Other revenues |
1,215 | (302 | ) | 913 | |||||||||||||||
Cost of goods sold |
(17,345 | ) | 2,692 | (21 | ) | 163 | (14,511 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income from continuing operations |
|||||||||||||||||||
Marketing & Sales |
(12,377 | ) | 22 | (12,355 | ) | ||||||||||||||
Research & Development |
(9,086 | ) | 48 | 298 | 17 | (8,723 | ) | ||||||||||||
General & Administration |
(2,616 | ) | 64 | (2,552 | ) | ||||||||||||||
Other income |
1,391 | (15 | ) | (813 | ) | 563 | |||||||||||||
Other expense |
(2,512 | ) | 3 | 171 | 33 | 1,024 | (1,281 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Income before taxes from continuing operations |
|||||||||||||||||||
Income from associated companies |
1,918 | 257 | 1 | (1,233 | ) | 943 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms; Other expense includes amortization of intangible
assets; Income from associated companies includes $257 million for the Novartis share of the estimated Roche core items.
- (2)
- Impairments:
Cost of goods sold, Research & Development, Other income and Other expense consist principally of net impairment charges
or reversals related to intangible assets, property, plant and equipment and financial assets.
- (3)
- Acquisition
or divestment related items, restructuring and integration charges: Other expense includes costs related to the portfolio
transformation.
- (4)
- Other exceptional items: Other revenues includes an amount for a commercial settlement; Cost of goods sold includes charges for the Group-wide rationalization of manufacturing sites; Marketing & Sales, Research & Development and General & Administration include charges for transforming IT and finance processes; Other income includes product related divestment gains and gains in the Novartis Venture Fund, an insurance recovery net of a deferred amount, a partial reversal of a legal expense provision, a reduction in restructuring provisions, and the impact from a post-retirement medical plan amendment;
162
Other expense includes restructuring provision charges, charges for transforming IT and finance processes, an expense related to Lucentis in Italy, the expense of $204 million related to the advancement of the timing of recording the US Healthcare Fee liability as a result of final regulations. Income from associated companies includes gains from the divestment of Idenix and LTS Lohmann Therapie-Systeme AG shareholdings.
- (5)
- Taxes
on the adjustments between IFRS and core results of continuing operations take into account, for each individual item included in the
adjustment, the tax rate that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and
impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on exceptional items although this is not always the
case for items arising from legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Due to these factors and the
differing effective tax rates in the various jurisdictions, the tax on the total adjustments of $2.4 billion to arrive at the core results before tax amounts to $483 million. This
results in the average tax rate on the adjustments being 20.0%.
- (6)
- For
details on discontinued operations reconciliation from IFRS to core net income, see "—Non-IFRS Measures as Defined by
Novartis".
- (7)
- Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.
2013
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Acquisition or divestment related items, including restructuring and integration charges(3) |
Other exceptional items(4) |
Core results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Gross profit from continuing operations |
36,137 | 2,615 | 20 | 20 | 38,792 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating income from continuing operations |
10,983 | 2,680 | 210 | 331 | 3 | 14,207 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Income before taxes from continuing operations |
10,807 | 2,939 | 210 | 349 | 47 | 14,352 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Taxes from continuing operations(5) |
(1,498 | ) | (2,057 | ) | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net income from continuing operations |
9,309 | 12,295 | |||||||||||||||||
Net income/loss from discontinued operations(6) |
(17 | ) | 238 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net income |
9,292 | 12,533 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
EPS from continuing operations ($)(7) |
3.76 | 4.99 | |||||||||||||||||
EPS from discontinued operations ($)(7) |
0.10 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
EPS ($)(7) |
3.76 | 5.09 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit from continuing operations |
|||||||||||||||||||
Cost of goods sold |
(16,579 | ) | 2,615 | 20 | 20 | (13,924 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income from continuing operations |
|||||||||||||||||||
Marketing & Sales |
(12,638 | ) | 27 | (12,611 | ) | ||||||||||||||
Research & Development |
(9,071 | ) | 61 | 86 | 39 | (8,885 | ) | ||||||||||||
General & Administration |
(2,603 | ) | 25 | (2,578 | ) | ||||||||||||||
Other income |
1,205 | (52 | ) | (505 | ) | 648 | |||||||||||||
Other expense |
(2,047 | ) | 4 | 156 | 331 | 397 | (1,159 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Income before taxes from continuing operations |
|||||||||||||||||||
Income from associated companies |
599 | 259 | 18 | 876 | |||||||||||||||
Other financial income and expense |
(92 | ) | 44 | (48 | ) | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms; Other expense includes amortization of intangible
assets; Income from associated companies includes $259 million for the Novartis share of the estimated Roche core items.
- (2)
- Impairments: Cost of goods sold, Research & Development, Other income and Other expense include principally net impairment charges or reversals related to intangible assets and property, plant and equipment, mainly related to the Group-wide rationalization of manufacturing sites.
163
- (3)
- Acquisition
or divestment related items, restructuring and integration charges: Other expense includes Alcon integration costs. Income from
associated companies includes restructuring charges related to Roche.
- (4)
- Other
exceptional items: Cost of goods sold, Other income and Other expense include restructuring charges related to the Group-wide
rationalization of manufacturing sites; Marketing & Sales includes charges related to termination of a co-promotional contract; Research & Development also includes a net increase of
contingent consideration liabilities related to acquisitions; General & Administration includes exceptional IT-related costs; Other income includes divestment gains, a reversal of a Corporate
provision, income from post-retirement medical plan amendments and reduction in restructuring charge provisions; Other expense includes a restructuring provision charge, provisions for legal matters,
and charges for transforming IT and finance processes; Other financial income and expense includes devaluation losses of $44 million related to Venezuela.
- (5)
- Taxes
on the adjustments between IFRS and core results take into account, for each individual item included in the adjustment, the tax rate
that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets
and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on exceptional items although this is not always the case for items arising from
legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Due to these factors and the differing effective tax
rates in the various jurisdictions, the tax on the total adjustments of $3.5 billion to arrive at the core results before tax amounts to $559 million. This results in the average tax
rate on the adjustments is 15.8%.
- (6)
- For
details on discontinued operations reconciliation from IFRS to core net income, see "—Non-IFRS Measures as Defined by
Novartis".
- (7)
- Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.
2015, 2014 AND 2013 RECONCILIATION OF IFRS RESULTS TO CORE RESULTS—PHARMACEUTICALS
2015
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Acquisition or divestment related items, including restructuring and integration charges(3) |
Other exceptional items(4) |
Core results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Gross profit |
23,993 | 1,262 | (20 | ) | 88 | 25,323 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating income |
7,597 | 1,290 | 12 | 192 | 329 | 9,420 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
|||||||||||||||||||
Other revenues |
790 | (28 | ) | 762 | |||||||||||||||
Cost of goods sold |
(7,379 | ) | 1,262 | (20 | ) | 116 | (6,021 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
|||||||||||||||||||
Marketing & Sales |
(7,789 | ) | 43 | (7,746 | ) | ||||||||||||||
Research & Development |
(7,232 | ) | 28 | 39 | 112 | (7,053 | ) | ||||||||||||
Other income |
1,145 | (56 | ) | (22 | ) | (743 | ) | 324 | |||||||||||
Other expense |
(1,583 | ) | 49 | 214 | 829 | (491 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms.
- (2)
- Impairments:
Cost of goods sold and Other income include a reversal of intangible asset impairments; Research & Development includes
impairment charges for in process projects and termination of collaboration and license agreements; Other expense includes impairment charges related to property, plant and equipment and financial
assets.
- (3)
- Acquisition
or divestment related items, including restructuring and integration charges: Other income and Other expense include income and
costs related to the portfolio transformation.
- (4)
- Other exceptional items: Other revenues and Other income include additional gains from product divestments; Cost of goods sold and Other expense include net restructuring charges related to the Group-wide rationalization of manufacturing sites; Cost of goods sold also includes an inventory write-off; Marketing & Sales, Research & Development and Other expense include other restructuring charges; Research & Development also includes expenses related to product acquisitions; Other income also includes a gain from a Swiss pension plan amendment; Other expense also includes legal settlement provisions.
164
2014
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Acquisition or divestment related items, including restructuring and integration charges(3) |
Other exceptional items(4) |
Core results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Gross profit |
25,793 | 238 | (58 | ) | 127 | 26,100 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating income |
8,471 | 276 | 266 | 33 | 468 | 9,514 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
|||||||||||||||||||
Cost of goods sold |
(6,889 | ) | 238 | (58 | ) | 127 | (6,582 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
|||||||||||||||||||
Marketing & Sales |
(8,178 | ) | 2 | (8,176 | ) | ||||||||||||||
Research & Development |
(7,331 | ) | 38 | 289 | 7 | (6,997 | ) | ||||||||||||
General & Administration |
(1,009 | ) | 1 | (1,008 | ) | ||||||||||||||
Other income |
734 | (13 | ) | (451 | ) | 270 | |||||||||||||
Other expense |
(1,538 | ) | 48 | 33 | 782 | (675 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms.
- (2)
- Impairments:
Cost of good sold includes partial reversal of previously impaired production assets, partly offset by the impairment of
intangible assets related to a marketed product; Research & Development includes impairment charges for in process projects and termination of collaboration and license agreements; Other income
relates to impairment reversals of property, plant and equipment; Other expense includes impairment charges related to property, plant and equipment and financial assets.
- (3)
- Acquisition
or divestment related items, including restructuring and integration charges: Other expense includes costs related to the
acquisition of GSK oncology assets.
- (4)
- Other exceptional items: Cost of goods sold, Research & Development and Marketing & Sales include net restructuring charges related to the Group-wide rationalization of manufacturing sites; Other income includes an insurance recovery from Corporate related to exchange risks, gains related to the rationalization of manufacturing sites, the impact from a post-retirement medical plan amendment, as well as additional gains from divestments announced in prior periods; Other expense include restructuring charges, an expense related to Lucentis in Italy and an expense of $157 million related to the advancement of the timing of recording the US Healthcare Fee liability as a result of final regulations.
165
2013
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Other exceptional items(3) |
Core results |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Gross profit |
26,258 | 228 | 6 | 26,492 | ||||||||||||
| | | | | | | | | | | | | | | | | |
Operating income |
9,376 | 278 | 74 | (205 | ) | 9,523 | ||||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
||||||||||||||||
Cost of goods sold |
(6,655 | ) | 228 | 6 | (6,421 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
||||||||||||||||
Marketing & Sales |
(8,514 | ) | 27 | (8,487 | ) | |||||||||||
Research & Development |
(7,242 | ) | 50 | 29 | 2 | (7,161 | ) | |||||||||
Other income |
699 | (46 | ) | (390 | ) | 263 | ||||||||||
Other expense |
(774 | ) | 91 | 150 | (533 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms.
- (2)
- Impairments:
Research & Development includes impairment charges for in process projects; Other income includes charges related to the
reversal of impairment charges related to aliskiren production equipment for which an alternative use has been found; Other expense includes impairment charges related to property, plant and
equipment.
- (3)
- Other exceptional items: Cost of goods sold includes principally restructuring charges related to the Group-wide rationalization of manufacturing sites offset by a provision reduction related to aliskiren; Marketing & Sales includes charges related to termination of a co-promotional contract; Research & Development includes restructuring charges; Other income includes principally divestment gains and a reduction in restructuring charge provisions; Other expense includes restructuring charges and provisions for legal matters.
166
2015, 2014 AND 2013 RECONCILIATION OF IFRS RESULTS TO CORE RESULTS—ALCON
2015
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Other exceptional items(3) |
Core results |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Gross profit |
4,729 | 2,049 | 119 | 4 | 6,901 | |||||||||||
| | | | | | | | | | | | | | | | | |
Operating income |
794 | 2,063 | 121 | 85 | 3,063 | |||||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
||||||||||||||||
Cost of goods sold |
(5,153 | ) | 2,049 | 119 | 4 | (2,981 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
||||||||||||||||
Research & Development |
(926 | ) | 14 | 1 | 2 | (909 | ) | |||||||||
General & Administration |
(544 | ) | 32 | (512 | ) | |||||||||||
Other income |
58 | (13 | ) | 45 | ||||||||||||
Other expense |
(125 | ) | 1 | 60 | (64 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms.
- (2)
- Impairments:
Cost of goods sold includes impairment charges related to intangible assets; Research & Development and Other expense
include impairment charges related to property, plant and equipment.
- (3)
- Other exceptional items: Cost of goods sold includes net restructuring charges related to the Group-wide rationalization of manufacturing sites; Research & Development includes non capitalized costs for the US; General & Administration includes charges for transforming IT and finance processes; Other income includes a gain from a Swiss pension plan amendment and a partial reversal of restructuring charges; Other expense includes other restructuring charges and a legal settlement.
167
2014
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Other exceptional items(3) |
Core results |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Gross profit |
5,717 | 2,056 | 26 | 7,799 | ||||||||||||
| | | | | | | | | | | | | | | | | |
Operating income |
1,597 | 2,064 | 6 | 144 | 3,811 | |||||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
||||||||||||||||
Cost of goods sold |
(5,193 | ) | 2,056 | 26 | (3,111 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
||||||||||||||||
Marketing & Sales |
(2,474 | ) | 20 | (2,454 | ) | |||||||||||
Research & Development |
(928 | ) | 8 | 7 | 10 | (903 | ) | |||||||||
General & Administration |
(613 | ) | 45 | (568 | ) | |||||||||||
Other income |
79 | (1 | ) | (52 | ) | 26 | ||||||||||
Other expense |
(184 | ) | 95 | (89 | ) | |||||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms.
- (2)
- Impairments:
Research & Development includes impairment charges for in process projects; Other income includes a reversal of
impairment charges related to property, plant and equipment.
- (3)
- Other exceptional items: Cost of goods sold and Other expense include net restructuring charges related to the Group-wide rationalization of manufacturing sites; Marketing & Sales and General & Administration include charges for transforming IT and finance processes; Research & Development includes a net increase of contingent consideration liabilities related to acquisitions; Other income includes the reversal of restructuring charges related to the Group-wide rationalization of manufacturing sites, as well as the impact from a post-retirement medical plan amendment; Other expense also includes an expense of $29 million related to the advancement of the timing of recording the US Healthcare Fee liability as a result of final regulations.
168
2013
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Acquisition or divestment related items, restructuring and integration charges(3) |
Other exceptional items(4) |
Core results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Gross profit |
5,673 | 1,980 | 12 | 7,665 | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating income |
1,232 | 1,989 | 61 | 330 | 82 | 3,694 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
|||||||||||||||||||
Cost of goods sold |
(4,900 | ) | 1,980 | 12 | (2,908 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
|||||||||||||||||||
Research & Development |
(1,042 | ) | 9 | 57 | 37 | (939 | ) | ||||||||||||
General & Administration |
(589 | ) | 25 | (564 | ) | ||||||||||||||
Other income |
79 | (40 | ) | 39 | |||||||||||||||
Other expense |
(437 | ) | 4 | 330 | 48 | (55 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms.
- (2)
- Impairments:
Research & Development includes impairment charges related to in process projects; Other expense includes impairment
charges related to property, plant and equipment.
- (3)
- Acquisition
or divestment related items, restructuring and integration charges: Other expense reflects acquisition-related Alcon integration
and restructuring charges.
- (4)
- Other exceptional items: Cost of goods sold includes net restructuring charges related to the Group-wide rationalization of manufacturing sites offset by the release of a contingent consideration liability related to recent acquisitions; Research & Development includes a net increase of contingent consideration liabilities related to acquisitions; General & Administration includes exceptional IT costs; Other income includes the impact of an income from a post-retirement medical plan amendment; Other expense includes net restructuring charges related to European commercial operations and the Group-wide rationalization of manufacturing sites.
169
2015, 2014 AND 2013 RECONCILIATION OF IFRS RESULTS TO CORE RESULTS—SANDOZ
2015
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Acquisition or divestment related items, including restructuring and integration charges(3) |
Other exceptional items(4) |
Core results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Gross profit |
3,985 | 355 | 27 | 33 | 4,400 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating income |
1,005 | 356 | 124 | 174 | 1,659 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
|||||||||||||||||||
Cost of goods sold |
(5,325 | ) | 355 | 27 | 33 | (4,910 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
|||||||||||||||||||
Research & Development |
(777 | ) | 1 | (776 | ) | ||||||||||||||
Other income |
109 | (1 | ) | (4 | ) | 104 | |||||||||||||
Other expense |
(381 | ) | 97 | 1 | 145 | (138 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold include recurring amortization of acquired rights to in-market products and other
production-related intangible assets.
- (2)
- Impairments:
Cost of goods sold includes impairments of intangible assets; Other expense includes impairment charges related to property,
plant and equipment.
- (3)
- Acquisition
or divestment related items, including restructuring and integration charges: Other income and Other expense include items
related to the portfolio transformation.
- (4)
- Other exceptional items: Cost of goods sold includes marketable intangible assets not capitalized; Cost of goods sold and Other expense include net restructuring charges related to the Group-wide rationalization of manufacturing sites; Other income includes a gain from a Swiss pension plan amendment; Other expense also includes a legal settlement.
170
2014
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Other exceptional items(3) |
Core results |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Gross profit |
4,109 | 398 | 37 | 10 | 4,554 | |||||||||||
| | | | | | | | | | | | | | | | | |
Operating income |
1,088 | 400 | 47 | 36 | 1,571 | |||||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
||||||||||||||||
Cost of goods sold |
(5,751 | ) | 398 | 37 | 10 | (5,306 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
||||||||||||||||
Research & Development |
(827 | ) | 2 | 2 | (823 | ) | ||||||||||
Other income |
97 | (1 | ) | (3 | ) | 93 | ||||||||||
Other expense |
(190 | ) | 9 | 29 | (152 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms.
- (2)
- Impairments:
Cost of goods sold and Research & Development include charges related to impairment of intangible assets; Other income
includes a reversal of impairment charges related to property, plant and equipment; Other expense includes impairment charges related to property, plant and equipment and financial assets.
- (3)
- Other exceptional items: Cost of goods sold and Other expense include net restructuring charges; Other income includes the reversal of restructuring charges; Other expense also includes an expense of $18 million related to the advancement of the timing of recording the US Healthcare Fee liability as a result of final regulations.
2013
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Other exceptional items(3) |
Core results |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|
$ m$ m |
|||||||||||
Gross profit |
3,995 | 407 | 20 | 2 | 4,424 | |||||||||||
| | | | | | | | | | | | | | | | | |
Operating income |
1,028 | 409 | 17 | 87 | 1,541 | |||||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
||||||||||||||||
Cost of goods sold |
(5,476 | ) | 407 | 20 | 2 | (5,047 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
||||||||||||||||
Research & Development |
(787 | ) | 2 | (785 | ) | |||||||||||
Other income |
106 | (6 | ) | 100 | ||||||||||||
Other expense |
(240 | ) | 3 | 85 | (152 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms.
- (2)
- Impairments:
Cost of goods sold includes impairment charges related to intangible assets; Other income includes a reversal of impairment
charges related to property, plant and equipment; Other expense includes impairment charges related to property, plant and equipment.
- (3)
- Other exceptional items: Cost of goods sold includes net restructuring charges related to the Group-wide rationalization of manufacturing sites; Other expense includes provisions for legal matters.
171
2015, 2014 AND 2013 RECONCILIATION OF IFRS RESULTS TO CORE RESULTS—CORPORATE
2015
|
IFRS results |
Impairments(1) | Acquisition or divestment related items, including restructuring and integration charges(2) |
Other exceptional items(3) |
Core results |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
|
$ m |
$ m |
$ m$ m |
|||||||||||
Gross profit |
276 | 276 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Operating loss |
(419 | ) | 112 | (10 | ) | (35 | ) | (352 | ) | |||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Loss |
||||||||||||||||
General & Administration |
(648 | ) | 54 | (594 | ) | |||||||||||
Other income |
737 | (260 | ) | (127 | ) | 350 | ||||||||||
Other expense |
(784 | ) | 112 | 250 | 38 | (384 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Impairments:
Other expense includes impairment charges related to property, plant and equipment and financial assets.
- (2)
- Acquisition
or divestment related items, including restructuring and integration charges: Other income and Other expense include items
related to the portfolio transformation.
- (3)
- Other exceptional items: General & Administration and Other expense include expenses related to setup costs for Novartis Business Services; Other income includes a gain from a Swiss pension plan amendment, a reversal of a provision and items related to portfolio transformation; Other expense also includes a credit for a legal settlement charged to the divisions.
2014
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Other exceptional items(3) |
Core results |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|
$ m$ m |
|||||||||||
Gross profit |
670 | (302 | ) | 368 | ||||||||||||
| | | | | | | | | | | | | | | | | |
Operating loss |
(67 | ) | 3 | 114 | (473 | ) | (423 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
||||||||||||||||
Other revenues |
540 | (302 | ) | 238 | ||||||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Loss |
||||||||||||||||
General & Administration |
(618 | ) | 18 | (600 | ) | |||||||||||
Other income |
481 | (307 | ) | 174 | ||||||||||||
Other expense |
(600 | ) | 3 | 114 | 118 | (365 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Other expense includes amortization of intangible assets.
- (2)
- Impairments:
Other expense includes impairment charges related to property, plant and equipment and financial assets.
- (3)
- Other exceptional items: Other revenues includes an amount for a commercial settlement; General & Administration includes expenses related to setup costs for Novartis Business Services; Other income includes an insurance recovery transferred to Pharmaceuticals net of a deferred amount and gains in the Novartis Venture Fund; Other expense includes charges for transforming IT and finance processes, as well as a provision for a legal settlement.
172
2013
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Acquisition or divestment related items, restructuring and integration charges(3) |
Other exceptional items(4) |
Core results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Gross profit |
211 | 211 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating loss |
(653 | ) | 4 | 58 | 1 | 39 | (551 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Loss |
|||||||||||||||||||
Other income |
321 | (75 | ) | 246 | |||||||||||||||
Other expense |
(596 | ) | 4 | 58 | 1 | 114 | (419 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Other expense includes amortization of intangible assets
- (2)
- Impairments:
Other expense includes impairment charges related to property, plant and equipment and to a financial asset.
- (3)
- Acquisition
or divestment related items, restructuring and integration charges: Other expense reflects Alcon integration costs.
- (4)
- Other exceptional items: Other income includes a reversal of a provision; Other expense includes charges for transforming IT and finance processes.
2015, 2014 AND 2013 RECONCILIATION OF IFRS RESULTS TO CORE RESULTS—DISCONTINUED OPERATIONS
| |
|
|
|
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Acquisition or divestment related items, including restructuring and integration charges(2) |
|
|
|||||||||||
| |
IFRS results |
|
Other exceptional items(3) |
Core results |
||||||||||||
2015
|
Impairments(1) | |||||||||||||||
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Gross profit |
267 | 6 | 273 | |||||||||||||
| | | | | | | | | | | | | | | | | |
Operating income/loss |
12,477 | (83 | ) | (12,627 | ) | 8 | (225 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
Income/loss before taxes |
12,479 | (83 | ) | (12,627 | ) | 8 | (223 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
Taxes(4) |
(1,713 | ) | (33 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | |
Net income/loss |
10,766 | (256 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
EPS ($)(5) |
4.48 | (0.11 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
||||||||||||||||
Cost of goods sold |
(376 | ) | 6 | (370 | ) | |||||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Loss |
||||||||||||||||
Other income |
13,420 | (13,310 | ) | (1 | ) | 109 | ||||||||||
Other expense |
(727 | ) | (83 | ) | 683 | 3 | (124 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Impairments: Other expense includes the partial reversal of the influenza Vaccines business impairment charge recorded in 2014.
173
- (2)
- Acquisition
or divestment related items, including restructuring and integration charges: Other income includes gains from the divestment of
Animal Health ($4.6 billion) and from the transactions with GSK ($2.8 billion for the non-influenza Vaccines business and $5.9 billion resulting from the contribution of the
former Novartis OTC division into the GSK consumer healthcare joint venture in exchange for 36.5% interest in this newly created entity); Other expense includes additional transaction related expenses
of $0.6 billion and other portfolio transformation related costs.
- (3)
- Other
exceptional items: Cost of goods sold, Other income and Other expense include restructuring charges.
- (4)
- Taxes
on the adjustments between IFRS and core results take into account, for each individual item included in the adjustment, the tax rate
that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. There is usually a tax impact on exceptional items although this is not
always the case for items arising from legal settlements in certain jurisdictions. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total
adjustments of $12.7 billion to arrive at the core results before tax amounts to $1.7 billion. The average tax rate on the adjustments is 13.2%.
- (5)
- Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.
2014
|
IFRS results |
Amortization of intangible assets(1) |
Impairments(2) | Acquisition or divestment related items, including restructuring and integration charges(3) |
Other exceptional items(4) |
Core results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Gross profit |
2,886 | 65 | 302 | 19 | 3,272 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Operating loss/income |
(353 | ) | 73 | 1,141 | (680 | ) | (38 | ) | 143 | ||||||||||
| | | | | | | | | | | | | | | | | | | | |
Loss/income before taxes |
(351 | ) | 73 | 1,141 | (680 | ) | (38 | ) | 145 | ||||||||||
| | | | | | | | | | | | | | | | | | | | |
Taxes(5) |
(96 | ) | (43 | ) | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net loss/income |
(447 | ) | 102 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
EPS ($)(6) |
(0.18 | ) | 0.04 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
|||||||||||||||||||
Cost of goods sold |
(3,073 | ) | 65 | 302 | 19 | (2,687 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Income |
|||||||||||||||||||
Research & Development |
(857 | ) | 8 | (849 | ) | ||||||||||||||
Other income |
1,007 | (1 | ) | (876 | ) | (89 | ) | 41 | |||||||||||
Other expense |
(1,146 | ) | 840 | 196 | 32 | (78 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets up to the portfolio transformation annoucement date; Research & Development includes the recurring amortization of acquired rights for technology platforms
up to the portfolio transformation annoucement date.
- (2)
- Impairments:
Cost of goods sold and Other expense include the $1.1 billion impairment charge as a result of the proposed sale of the
influenza vaccines business; Other income includes a reduction of an impairment charge for property, plant and equipment; Other expense relates to an additional impairment charge in Corporate, for an
in-process project which is pending divestment as a result of the proposed portfolio transformation transactions.
- (3)
- Acquisition
or divestment related items, including restructuring and integration charges: Other income includes the gain on the disposal of
the blood transfusion diagnostics unit on January 9, 2014; Other expense includes professional service fees related to the portfolio transformation divestment activities.
- (4)
- Other exceptional items: Cost of goods sold and Other expense include restructuring charges related to the Group-wide rationalization of manufacturing sites; Other income includes the gain on the sale of a divested product, which was sold as a result of the proposed portfolio transformation transaction, the reversal of restructuring charges related to the Group-wide rationalization of manufacturing sites, the partial reversal of a legal expense provision, and
174
the impact from a post-retirement medical plan amendment; Other expense also includes the write-off of a receivable as a result of the proposed portfolio transformation transactions.
- (5)
- Taxes
on the adjustments between IFRS and core results take into account, for each individual item included in the adjustment, the tax rate
that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. There is usually a tax impact on exceptional items although this is not
always the case for items arising from legal settlements in certain jurisdictions. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total
adjustments of $496 million to arrive at the core results before tax amounts to $53 million. The average tax rate on the adjustments is 10.7%.
- (6)
- Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.
2013
|
IFRS results | Amortization of intangible assets(1) |
Impairments(2) | Other exceptional items(3) |
Core results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Gross profit |
3,086 | 250 | 8 | 21 | 3,365 | |||||||||||
| | | | | | | | | | | | | | | | | |
Operating loss/income |
(73 | ) | 275 | 49 | 27 | 278 | ||||||||||
| | | | | | | | | | | | | | | | | |
Loss/income before taxes |
(72 | ) | 275 | 49 | 27 | 279 | ||||||||||
| | | | | | | | | | | | | | | | | |
Taxes(4) |
55 | (41 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | |
Net loss/income |
(17 | ) | 238 | |||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
EPS ($)(5) |
0 | 0.10 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Gross Profit |
||||||||||||||||
Cost of goods sold |
(3,322 | ) | 250 | 9 | 21 | (3,042 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
The following are adjustments to arrive at Core Operating Loss |
||||||||||||||||
Research & Development |
(781 | ) | 24 | (757 | ) | |||||||||||
Other income |
174 | (1 | ) | (1 | ) | 172 | ||||||||||
Other expense |
(184 | ) | 1 | 41 | 7 | (135 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Amortization
of intangible assets: Cost of goods sold includes recurring amortization of acquired rights to in-market products and other
production-related intangible assets; Research & Development includes the recurring amortization of acquired rights for technology platforms.
- (2)
- Impairments:
Cost of goods sold includes impairment charges related to intangible assets; Other income includes a reduction of an impairment
charge; Other expense includes impairment charges for financial assets and property, plant and equipment and impairments related to the Group-wide rationalization of manufacturing sites.
- (3)
- Other
exceptional items: Cost of goods sold and Other expense include restructuring charges related to the Group-wide rationalization of
manufacturing sites; Other income includes reversal of charges related to the Group-wide rationalization of manufacturing sites.
- (4)
- Taxes
on the adjustments between IFRS and core results take into account, for each individual item included in the adjustment, the tax rate
that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. There is usually a tax impact on exceptional items although this is not
always the case for items arising from legal settlements in certain jurisdictions. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total
adjustments of $351 million to arrive at the core results before tax amounts to $96 million. The average tax rate on the adjustments is 27.4%.
- (5)
- Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.
175
2015 and 2014 Reconciliation of segment operating income to Core Results
| |
Pharmaceuticals | Alcon | Sandoz | Corporate | Total Group | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | |||||||||||||||||||||
| |
$ millions |
$ millions |
$ millions |
$ millions |
$ millions |
$ millions |
$ millions |
$ millions |
$ millions |
$ millions |
|||||||||||||||||||||
IFRS Operating income from continuing operations |
7,597 | 8,471 | 794 | 1,597 | 1,005 | 1,088 | (419 | ) | (67 | ) | 8,977 | 11,089 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization of intangible assets |
1,290 | 276 | 2,063 | 2,064 | 356 | 400 | 3 | 3,709 | 2,743 | ||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Impairments |
|||||||||||||||||||||||||||||||
Intangible assets |
19 | 231 | 120 | 7 | 27 | 39 | 166 | 277 | |||||||||||||||||||||||
Property, plant & equipment related to the Group-wide rationalization of manufacturing sites |
6 | 23 | 83 | 89 | 23 | ||||||||||||||||||||||||||
Other property, plant & equipment |
(45 | ) | (8 | ) | 1 | (1 | ) | 14 | 7 | 21 | 23 | (9 | ) | 21 | |||||||||||||||||
Financial assets |
32 | 20 | 1 | 91 | 91 | 123 | 112 | ||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total impairment charges |
12 | 266 | 121 | 6 | 124 | 47 | 112 | 114 | 369 | 433 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Acquisition or divestment related items |
|||||||||||||||||||||||||||||||
—Income |
(22 | ) | (1 | ) | (260 | ) | (283 | ) | |||||||||||||||||||||||
—Expense |
214 | 33 | 1 | 250 | 465 | 33 | |||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total acquisition or divestment related items, net |
192 | 33 | (10 | ) | 182 | 33 | |||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Other exceptional items |
|||||||||||||||||||||||||||||||
Exceptional divestment gains |
(626 | ) | (237 | ) | (54 | ) | (294 | ) | (680 | ) | (531 | ) | |||||||||||||||||||
Restructuring items |
|||||||||||||||||||||||||||||||
—Income |
(27 | ) | (56 | ) | (7 | ) | (24 | ) | (3 | ) | (5 | ) | (39 | ) | (83 | ) | |||||||||||||||
—Expense |
391 | 632 | 60 | 95 | 121 | 21 | 57 | 1 | 629 | 749 | |||||||||||||||||||||
Legal-related items |
|||||||||||||||||||||||||||||||
—Expense |
578 | 125 | 4 | 40 | (30 | ) | 30 | 592 | 155 | ||||||||||||||||||||||
Additional exceptional income |
(119 | ) | (158 | ) | (5 | ) | (29 | ) | (2 | ) | (68 | ) | (315 | ) | (194 | ) | (502 | ) | |||||||||||||
Additional exceptional expense |
132 | 162 | 33 | 102 | 15 | 18 | 65 | 105 | 245 | 387 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total other exceptional items |
329 | 468 | 85 | 144 | 174 | 36 | (35 | ) | (473 | ) | 553 | 175 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total adjustments |
1,823 | 1,043 | 2,269 | 2,214 | 654 | 483 | 67 | (356 | ) | 4,813 | 3,384 | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core operating income from continuing operations |
9,420 | 9,514 | 3,063 | 3,811 | 1,659 | 1,571 | (352 | ) | (423 | ) | 13,790 | 14,473 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
as % of net sales |
30.9 | % | 29.9 | % | 31.2 | % | 35.2 | % | 18.1 | % | 16.4 | % | 27.9 | % | 27.7 | % | |||||||||||||||
Income from associated companies |
812 | 2 | 4 | 264 | 1,102 | 266 | 1,918 | ||||||||||||||||||||||||
Core adjustments to income from associated companies, net of tax |
(812 | ) | 715 | (163 | ) | 715 | (975 | ) | |||||||||||||||||||||||
Interest expense |
(655 | ) | (704 | ) | |||||||||||||||||||||||||||
Other financial income and expense(1) |
(24 | ) | (31 | ) | |||||||||||||||||||||||||||
Taxes (adjusted for above items) |
(2,051 | ) | (2,028 | ) | |||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core net income from continuing operations |
12,041 | 12,653 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core net loss/income from discontinued operations(2) |
(256 | ) | 102 | ||||||||||||||||||||||||||||
Core net income |
11,785 | 12,755 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core net income attributable to shareholders |
11,774 | 12,685 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core basic EPS from continuing operations ($)(3) |
5.01 | 5.19 | |||||||||||||||||||||||||||||
Core basic EPS from discontinued operations ($)(3) |
(0.11 | ) | 0.04 | ||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total core basic EPS ($)(3) |
4.90 | 5.23 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Adjustments
for charges of $0.4 billion are related to Venezuela subsidiaries.
- (2)
- For
details on discontinued operations reconciliation from IFRS to core net income, please see "—Non-IFRS Measures as Defined by
Novartis".
- (3)
- Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.
176
2014 and 2013 Reconciliation of segment operating income to Core Results
| |
Pharmaceuticals | Alcon | Sandoz | Corporate | Total Group | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | |||||||||||||||||||||
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||||||||||
IFRS Operating income from continuing operations |
8,471 | 9,376 | 1,597 | 1,232 | 1,088 | 1,028 | (67 | ) | (653 | ) | 11,089 | 10,983 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization of intangible assets |
276 | 278 | 2,064 | 1,989 | 400 | 409 | 3 | 4 | 2,743 | 2,680 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Impairments |
|||||||||||||||||||||||||||||||
Intangible assets |
231 | 29 | 7 | 57 | 39 | 20 | 277 | 106 | |||||||||||||||||||||||
Property, plant & equipment related to the Group-wide rationalization of manufacturing sites |
23 | 1 | 23 | 1 | |||||||||||||||||||||||||||
Other property, plant & equipment |
(8 | ) | 28 | (1 | ) | 4 | 7 | (3 | ) | 23 | 17 | 21 | 46 | ||||||||||||||||||
Financial assets |
20 | 16 | 1 | 91 | 41 | 112 | 57 | ||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total impairment charges |
266 | 74 | 6 | 61 | 47 | 17 | 114 | 58 | 433 | 210 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Acquisition or divestment related items |
|||||||||||||||||||||||||||||||
—Expense |
33 | 330 | 1 | 33 | 331 | ||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total acquisition or divestment related items, net |
33 | 330 | 1 | 33 | 331 | ||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Other exceptional items |
|||||||||||||||||||||||||||||||
Exceptional divestment gains |
(237 | ) | (313 | ) | (294 | ) | (531 | ) | (313 | ) | |||||||||||||||||||||
Restructuring items |
|||||||||||||||||||||||||||||||
—Income |
(56 | ) | (40 | ) | (24 | ) | (3 | ) | (83 | ) | (40 | ) | |||||||||||||||||||
—Expense |
632 | 122 | 95 | 77 | 21 | 2 | 1 | 749 | 201 | ||||||||||||||||||||||
Legal-related items |
|||||||||||||||||||||||||||||||
—Expense |
125 | 33 | 85 | 30 | 155 | 118 | |||||||||||||||||||||||||
Additional exceptional income |
(158 | ) | (70 | ) | (29 | ) | (56 | ) | (4 | ) | (315 | ) | (75 | ) | (502 | ) | (205 | ) | |||||||||||||
Additional exceptional expense |
162 | 63 | 102 | 61 | 18 | 4 | 105 | 114 | 387 | 242 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total other exceptional items |
468 | (205 | ) | 144 | 82 | 36 | 87 | (473 | ) | 39 | 175 | 3 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total adjustments |
1,043 | 147 | 2,214 | 2,462 | 483 | 513 | (356 | ) | 102 | 3,384 | 3,224 | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core operating income from continuing operations |
9,514 | 9,523 | 3,811 | 3,694 | 1,571 | 1,541 | (423 | ) | (551 | ) | 14,473 | 14,207 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
as % of net sales |
29.9 | % | 29.6 | % | 35.2 | % | 35.2 | % | 16.4 | % | 16.8 | % | 27.7 | % | 27.4 | % | |||||||||||||||
Income from associated companies |
812 | 4 | 2 | 1,102 | 597 | 1,918 | 599 | ||||||||||||||||||||||||
Core adjustments to income from associated companies, net of tax |
(812 | ) | (163 | ) | 277 | (975 | ) | 277 | |||||||||||||||||||||||
Interest expense |
(704 | ) | (683 | ) | |||||||||||||||||||||||||||
Other financial income and expense(1) |
(31 | ) | (48 | ) | |||||||||||||||||||||||||||
Taxes (adjusted for above items) |
(2,028 | ) | (2,057 | ) | |||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core net income from continuing operations |
12,653 | 12,295 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core net income from discontinued operations(2) |
102 | 238 | |||||||||||||||||||||||||||||
Core net income |
12,755 | 12,533 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core net income attributable to shareholders |
12,685 | 12,416 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Core basic EPS from continuing operations ($)(3) |
5.19 | 4.99 | |||||||||||||||||||||||||||||
Core basic EPS from discontinued operations ($)(3) |
0.04 | 0.10 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total core basic EPS ($)(3) |
5.23 | 5.09 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- 2013
adjusted for $44 million of devaluation loss.
- (2)
- For
details on discontinued operations reconciliation from IFRS to core net income, please see "—Non-IFRS Measures as Defined by
Novartis".
- (3)
- Earnings per share (EPS) is calculated on the amount of net income attributable to shareholders of Novartis AG.
177
5.B Liquidity and Capital Resources
The following tables summarize the Group's cash flow and net debt.
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Cash flows from operating activities from continuing operations |
12,085 | 13,898 | 12,617 | |||||||
Cash flows used in investing activities from continuing operations |
(19,666 | ) | (8 | ) | (3,219 | ) | ||||
Cash flows from operating and investing activities from discontinued operations |
8,694 | 888 | 424 | |||||||
Cash flows used in financing activities |
(9,176 | ) | (8,147 | ) | (8,769 | ) | ||||
Currency translation effect on cash and cash equivalents |
(286 | ) | (295 | ) | 82 | |||||
| | | | | | | | | | | |
Net change in cash and cash equivalents |
(8,349 | ) | 6,336 | 1,135 | ||||||
Change in marketable securities, commodities, time deposits and derivative financial instruments |
(66 | ) | (1,696 | ) | (32 | ) | ||||
Change in current and non-current financial debts and derivative financial instruments |
(1,520 | ) | (2,393 | ) | 1,708 | |||||
| | | | | | | | | | | |
Change in net debt |
(9,935 | ) | 2,247 | 2,811 | ||||||
Net debt at January 1 |
(6,549 | ) | (8,796 | ) | (11,607 | ) | ||||
| | | | | | | | | | | |
Net debt at December 31 |
(16,484 | ) | (6,549 | ) | (8,796 | ) | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Financial year 2015
Cash flow from operating activities of continuing operations decreased to $12.1 billion from $13.9 billion in 2014.
The decrease was primarily due to the negative currency impact on operations. The prior year also included higher proceeds from commercial settlements.
The cash outflow for investing activities from continuing operations amounted to $19.7 billion in 2015. This was primarily due to the outflow of $16.5 billion for acquisitions of businesses, mainly the oncology business from GSK for $16.0 billion, the net outflow of $2.8 billion for the purchase of property, plant and equipment, intangible and other non-current assets and the net outflow of $0.3 billion from the change in marketable securities.
In 2014, cash flows used in investing activities from continuing operations was a small net outflow of $8 million. This was primarily due to net outflows of $0.3 billion from the acquisition of businesses, $3.0 billion mainly from purchase of property, plant and equipment, offset by $1.4 billion of proceeds from the sale of investments in associated companies, particularly LTS Lohmann Therapie-Systeme AG and Idenix Pharmaceuticals, Inc. and $1.9 billion proceeds from the net sale of other marketable securities, including maturing long-term deposits.
The cash flows used in financing activities amounted to $9.2 billion, compared to $8.1 billion in 2014. The 2015 amount includes a cash outflow of $6.6 billion for the dividend payment and $4.5 billion for treasury share transactions, net. The net inflow from the increase in current and non-current financial debt of $2.0 billion was mainly due to the issuance of three Swiss franc denominated bonds for a total amount of $1.5 billion in the first half of 2015, the issuance of two US dollar denominated bonds totaling $3.0 billion in the fourth quarter 2015 and the increase in commercial paper outstanding of $0.4 billion, partially offset by the repayment at maturity of a US dollar denominated bond of $2.0 billion and a Swiss franc denominated bond of $0.9 billion. In 2014, the cash outflows included $6.8 billion for the dividend
178
payment and $4.5 billion for treasury share transactions, net. These outflows were partially offset by increase in the current and non-current financial debt of $3.3 billion.
The net cash inflows from discontinued operations of $8.7 billion in 2015 were mainly driven by the net proceeds of $8.9 billion from the divestments in connection with the portfolio transformation transactions. In 2014, the net cash inflow of $0.9 billion consisted mainly of proceeds from the divestment of the blood transfusion diagnostics unit to Grifols S.A.
Financial year 2014
Cash flow from operating activities of continuing operations increased to $13.9 billion from $12.6 billion in 2013, an increase of $1.3 billion. This was primarily due to higher operating income adjusted for non-cash items, despite negative currency effects and increased hedging gains, partially offset by payments for legal settlements and restructuring.
In 2014, cash flow used in investing activities of continuing operations was a small net outflow of $8 million compared to an outflow of $3.2 billion in 2013. In 2014, there were proceeds from the sale of investments in associated companies included, in particular LTS Lohmann Therapie-Systeme AG and Idenix Pharmaceuticals, Inc. of $0.6 billion and $0.8 billion respectively and of $1.9 billion from the net sale of other marketable securities including maturing long-term deposits. These inflows were offset by outflows of $2.6 billion for property, plant and equipment and a net amount of $0.7 billion for acquisition of businesses mainly the acquisition of WaveTec ($0.4 billion) and other non-current assets, primarily intangible assets. The prior year outflow for investing activities of $3.2 billion was primarily related to investments in property, plant and equipment of $2.9 billion and a net outflow of $0.3 billion for the acquisition of businesses and other non-current assets, mainly intangible assets.
In 2014, cash inflows from investing activities of discontinued operations amounted to $ 0.9 billion, mainly on account of the net proceeds from the divestment of the blood transfusion diagnostics unit to Grifols S.A.
The cash flows used in financing activities amounted to $8.1 billion, compared to $8.8 billion, in 2013. The 2014 amount includes the dividend payment of $6.8 billion, net treasury share transactions of $4.5 billion and a net increase in financial debt of $3.3 billion, principally due to the issuance of four bonds totaling $5.5 billion reduced by the repayment at maturity of a bond of $2.0 billion. In 2013, the dividend payment amounted to $6.1 billion, net treasury share transactions were $1.2 billion and financial debt decreased by a net amount of $1.3 billion.
Financial year 2013
In 2013, cash flow from operating activities of continuing operations amounted to $12.6 billion compared to $13.8 billion in the prior year, mainly due to lower operating income and higher working capital requirements.
In 2013, cash flow used in investing activities of continuing operations was $3.2 billion compared to $ 5.4 billion in the prior year. It includes investments in property, plant and equipment, which amounted to $2.9 billion compared to $2.5 billion in the prior year. These expenditures represent 5.6% and 4.8% of net sales in 2013 and 2012, respectively. The prior year cash flow used in investing activities of continuing operations included higher net investments in marketable securities of $1.1 billion and $1.7 billion for the acquisition of businesses mainly for the acquisition of Fougera Pharmaceuticals, Inc.
In 2013 the cash flow used in investing activities of discontinued operations amounted to $0.1 billion compared to $0.3 billion in the prior year, mainly on account of net investments in property, plant and equipment.
In 2013, cash flow used in financing activities amounted to $8.8 billion compared to $6.7 billion in 2012. The 2013 amount included a dividend payment of $6.1 billion, compared to $6.0 billion in 2012.
179
There was a further $2.7 billion cash outflow in 2013, mainly related to net repayments of financial debts of $1.3 billion as well as a net outflow of $1.2 billion for treasury share purchases. This net outflow results from $2.9 billion spent on the acquisition of treasury shares and $1.7 billion of proceeds mainly from exercised options. In 2012, besides the dividend payment the cash flow used in financing activities mainly includes a net repayment of financial debts of $0.5 billion and a net cash outflow of $0.1 billion for treasury share transactions.
Net debt constitutes a non-IFRS financial measure, which means that it should not be interpreted as a measure determined under International Financial Reporting Standards (IFRS). Net debt is presented as additional information as it is a useful indicator of the Group's ability to meet financial commitments and to invest in new strategic opportunities, including strengthening its balance sheet.
Financial year 2015
Total financial debt, including derivatives, amounted to $21.9 billion at December 31, 2015 compared to $20.4 billion at December 31, 2014.
Non-current financial debt increased by $2.5 billion to $16.3 billion at December 31, 2015, from $13.8 billion at December 31, 2014. The increase was mainly due to the issuance of three Swiss franc denominated bonds for a total amount of $1.5 billion and the issuance of two US dollar denominated bonds for a total of $3.0 billion, partially offset by the reclassification to current financial debt of a euro denominated bond of $1.6 billion.
Current financial debt decreased by $1.0 billion to $5.6 billion at December 31, 2015, from $6.6 billion at December 31, 2014. The decrease was mainly due to repayment at maturity of a US dollar denominated bond of $2.0 billion and a Swiss franc denominated bond of $0.9 billion, partially offset by the reclassification from non-current financial debt of the $1.6 billion euro denominated bond mentioned above.
Overall current financial debt consists of the current portion of non-current debt of $1.7 billion and other short-term borrowings (including derivatives and commercial paper) of $3.9 billion. Group net debt increased to $16.5 billion at the end of 2015 compared to $6.5 billion at the end of 2014.
Novartis has two US commercial paper programs under which it can issue up to $9 billion in the aggregate of unsecured commercial paper notes. Novartis also has a Japanese commercial paper program under which it can issue up to JPY 150 billion (approximately $1.25 billion) of unsecured commercial paper notes. Commercial paper notes totaling $1.1 billion under these three programs were outstanding as per December 31, 2015. Novartis further has a committed credit facility of $6 billion, entered into on September 23, 2015. This credit facility is provided by a syndicate of banks and is intended to be used as a backstop for the US commercial paper programs. It matures in September 2020 and was undrawn as per December 31, 2015.
The long-term credit rating for the company continues to be double-A (Moody's Aa3; Standard & Poor's AA–; Fitch AA).
Financial year 2014
In 2014, the total financial debt, including derivatives, increased by $2.4 billion, and amounted to $20.4 billion compared to $18.0 billion in 2013.
Non-current financial debt amounted to $13.8 billion which is a net increase of $2.6 billion compared to 2013, mainly due to the issuance of four bonds and additional long-term debt totaling $5.5 billion. This is partly offset by $2.9 billion bond and loan reclassification to current financial debt for the portion which
180
is due within the next twelve months. Non-current financial debt consists of bonds of $13.2 billion and other non-current financial debt of $0.6 billion.
Current financial debt decreased by $0.2 billion from $6.8 billion at December 31, 2013 to $6.6 billion at December 31, 2014, mainly due to a decrease of commercial paper and other financial debt, including derivatives, totaling $0.6 billion. This was partially offset by the reclassification of non-current financial debt of $3.0 billion, combined with repayments in 2014 of non-current financial debts amounting to $2.6 billion, totaling to a net increase of $0.4 billion.
Overall current financial debt consists of commercial paper of $0.6 billion, the current portion of non-current debt of $3.0 billion and other short-term borrowings (including derivatives) of $3.0 billion.
Net debt decreased to $6.5 billion at the end of 2014 compared to $8.8 billion at the end of 2013.
An overview of our current financial debt and related interest rates is set forth below:
| |
December 31 | Average interest rate at year end |
Average balance during the year |
Average interest rate during the year |
Maximum balance during the year |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
% |
$ m |
% |
$ m |
|||||||||||
2015 |
||||||||||||||||
Interest-bearing accounts of associates payable on demand |
1,645 | 0.62 | 1,720 | 0.59 | 1,803 | |||||||||||
Other bank and financial debt |
1,185 | 5.98 | 1,280 | 5.54 | 2,785 | |||||||||||
Commercial paper |
1,085 | 0.62 | 3,545 | 0.19 | 5,686 | |||||||||||
Current portion of non-current financial debt |
1,659 | na | 1,916 | na | 3,044 | |||||||||||
Fair value of derivative financial instruments |
30 | na | 79 | na | 188 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total current financial debt |
5,604 | 8,540 | 13,506 | |||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
2014 |
||||||||||||||||
Interest-bearing accounts of associates payable on demand |
1,651 | 1.00 | 1,792 | 1.00 | 1,891 | |||||||||||
Other bank and financial debt |
1,272 | 5.32 | 1,537 | 4.40 | 2,074 | |||||||||||
Commercial paper |
648 | 0.26 | 1,260 | 0.13 | 3,076 | |||||||||||
Current portion of non-current financial debt |
2,989 | na | 2,565 | na | 3,500 | |||||||||||
Fair value of derivative financial instruments |
52 | na | 50 | na | 92 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total current financial debt |
6,612 | 7,204 | 10,633 | |||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
na = not applicable or available
Interest bearing accounts of associates payable on demand relate to employee deposits in CHF from the compensation of associates employed by Swiss entities (December 31, 2015 interest rate: 0.5%). Other bank and financial debt refer to usual lending and overdraft facilities.
181
The maturity schedule of our net debt is as follows:
December 31, 2015
|
Due within one month |
Due later than one month but less than three months |
Due later than three months but less than one year |
Due later than one year but less than five years |
Due after five years |
Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Current assets |
|||||||||||||||||||
Marketable securities and time deposits |
22 | 11 | 200 | 247 | 62 | 542 | |||||||||||||
Commodities |
86 | 86 | |||||||||||||||||
Derivative financial instruments and accrued interest |
40 | 67 | 38 | 145 | |||||||||||||||
Cash and cash equivalents |
4,674 | 4,674 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total current financial assets |
4,736 | 78 | 238 | 247 | 148 | 5,447 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Non-current liabilities |
|||||||||||||||||||
Financial debt |
(4,664 | ) | (11,663 | ) | (16,327 | ) | |||||||||||||
Financial debt—undiscounted |
(4,676 | ) | (11,797 | ) | (16,473 | ) | |||||||||||||
Total non-current financial debt |
(4,664 | ) | (11,663 | ) | (16,327 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Current liabilities |
|||||||||||||||||||
Financial debt |
(3,258 | ) | (289 | ) | (2,027 | ) | (5,574 | ) | |||||||||||
Financial debt—undiscounted |
(3,258 | ) | (289 | ) | (2,028 | ) | (5,575 | ) | |||||||||||
Derivative financial instruments |
(8 | ) | (20 | ) | (2 | ) | (30 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total current financial debt |
(3,266 | ) | (309 | ) | (2,029 | ) | (5,604 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net debt |
1,470 | (231 | ) | (1,791 | ) | (4,417 | ) | (11,515 | ) | (16,484 | ) | ||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
December 31, 2014
|
Due within one month |
Due later than one month but less than three months |
Due later than three months but less than one year |
Due later than one year but less than five years |
Due after five years |
Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Current assets |
|||||||||||||||||||
Marketable securities and time deposits |
21 | 68 | 37 | 181 | 76 | 383 | |||||||||||||
Commodities |
97 | 97 | |||||||||||||||||
Derivative financial instruments and accrued interest |
161 | 126 | 72 | 359 | |||||||||||||||
Cash and cash equivalents |
9,623 | 3,400 | 13,023 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total current financial assets |
9,902 | 3,594 | 109 | 181 | 76 | 13,862 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Non-current liabilities |
|||||||||||||||||||
Financial debt |
(5,423 | ) | (8,376 | ) | (13,799 | ) | |||||||||||||
Financial debt—undiscounted |
(5,434 | ) | (8,470 | ) | (13,904 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total non-current financial debt |
(5,423 | ) | (8,376 | ) | (13,799 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Current liabilities |
|||||||||||||||||||
Financial debt |
(2,678 | ) | (335 | ) | (3,547 | ) | (6,560 | ) | |||||||||||
Financial debt—undiscounted |
(2,678 | ) | (335 | ) | (3,549 | ) | (6,562 | ) | |||||||||||
Derivative financial instruments |
(18 | ) | (32 | ) | (2 | ) | (52 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total current financial debt |
(2,696 | ) | (367 | ) | (3,549 | ) | (6,612 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net debt |
7,206 | 3,227 | (3,440 | ) | (5,242 | ) | (8,300 | ) | (6,549 | ) | |||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
182
The following table provides a breakdown of liquidity and financial debt by currency as of December 31:
LIQUIDITY AND FINANCIAL DEBT BY CURRENCY
| |
Liquidity in % 2015(1) |
Liquidity in % 2014(1) |
Financial debt in % 2015(2) |
Financial debt in % 2014(2) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
$ |
50 | 80 | 64 | 59 | |||||||||
EUR |
16 | 1 | 14 | 17 | |||||||||
CHF |
13 | 10 | 14 | 13 | |||||||||
JPY |
1 | 5 | 8 | ||||||||||
Other |
20 | 9 | 3 | 3 | |||||||||
| | | | | | | | | | | | | | |
|
100 | 100 | 100 | 100 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- (1)
- Liquidity
includes cash and cash equivalents, marketable securities, commodities and time deposits.
- (2)
- Financial debt includes non-current and current financial debt.
EFFECTS OF CURRENCY FLUCTUATIONS
We transact our business in many currencies other than the US dollar, our reporting currency.
The following provides an overview of net sales and operating expenses for our continuing operations based on IFRS values for 2015, 2014 and 2013 for currencies most important to the Group:
| |
2015 | 2014 | 2013 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Currency
|
Net sales | Operating expenses |
Net sales | Operating expenses |
Net sales | Operating expenses |
|||||||||||||
| |
% |
% |
% |
% |
% |
% |
|||||||||||||
US dollar ($) |
40 | 42 | 36 | 39 | 36 | 40 | |||||||||||||
Euro (EUR) |
24 | 23 | 26 | 25 | 26 | 25 | |||||||||||||
Swiss franc (CHF) |
2 | 13 | 2 | 13 | 2 | 12 | |||||||||||||
Japanese yen (JPY) |
6 | 4 | 7 | 5 | 8 | 5 | |||||||||||||
Chinese yuan (CNY) |
4 | 3 | 3 | 3 | 3 | 3 | |||||||||||||
British pound (GBP) |
3 | 3 | 3 | 2 | 2 | 2 | |||||||||||||
Canadian dollar (CAD) |
3 | 1 | 3 | 1 | 3 | 1 | |||||||||||||
Brazilian real (BRL) |
2 | 2 | 2 | 2 | 2 | 2 | |||||||||||||
Australian dollar (AUD) |
2 | 1 | 2 | 1 | 2 | 1 | |||||||||||||
Russian ruble (RUB) |
1 | 1 | 2 | 1 | 2 | 1 | |||||||||||||
Other currencies |
13 | 7 | 14 | 8 | 14 | 8 | |||||||||||||
Operating expenses in the above table include Cost of goods sold, Marketing & Sales, Research & Development, General & Administration, Other income and Other expense.
We prepare our consolidated financial statements in US dollars. As a result, fluctuations in the exchange rates between the US dollar and other currencies can have a significant effect on both the Group's results of operations as well as on the reported value of our assets, liabilities and cash flows. This in turn may significantly affect reported earnings (both positively and negatively) and the comparability of period-to-period results of operations.
For purposes of our consolidated balance sheets, we translate assets and liabilities denominated in other currencies into US dollars at the prevailing market exchange rates as of the relevant balance sheet
183
date. For purposes of the Group's consolidated income and cash flow statements, revenue, expense and cash flow items in local currencies are translated into US dollars at average exchange rates prevailing during the relevant period. As a result, even if the amounts or values of these items remain unchanged in the respective local currency, changes in exchange rates have an impact on the amounts or values of these items in our consolidated financial statements.
Because our expenditures in Swiss francs are significantly higher than our revenues in Swiss francs, volatility in the value of the Swiss franc can have a significant impact on the reported value of our earnings, assets and liabilities, and the timing and extent of such volatility can be difficult to predict. In addition, there is a risk that certain countries could take steps which could significantly impact the value of their currencies.
There is also a risk that certain countries could devalue their currency. If this occurs, then it could impact the effective prices we would be able to charge for our products and also have an adverse impact on both our consolidated income statement and balance sheet. The Group is exposed to a potential adverse devaluation risk on its intercompany funding and total investment in certain subsidiaries operating in countries with exchange controls.
The most significant country in this respect is Venezuela, where the Group is exposed to potential devaluation losses in the income statement on its total intercompany balances with its subsidiaries in Venezuela, which at December 31, 2015 amounted to $0.3 billion. The Group also has an equivalent of approximately $0.2 billion of cash in local currency, which is only slowly being approved for remittance outside of the country and which is subject to loss of purchase power due to high inflation in the country.
Subsidiaries whose functional currencies have experienced a cumulative inflation rate of more than 100% over the past three years apply the rules of IAS 29 "Financial Reporting in Hyperinflationary Economies". Gains and losses incurred upon adjusting the carrying amounts of non-monetary assets and liabilities for inflation are recognized in the income statement. The subsidiaries in Venezuela restate non-monetary items in the balance sheet in line with the requirements of IAS 29. The corresponding monetary loss of $72 million is included in the 2015 financial results.
In 2014 and through October 2015, the exchange rate used by the Group for consolidation of the financial statements of its Venezuela subsidiaries was the official exchange rate for the Venezuela bolivar (VEF) of VEF 6.3/$, which is available for imports of specific goods and services of national priority, including medicines and medical supplies, as published by the Centro Nacional de Comercio Exterior (CENCOEX, formerly CADIVI).
In November 2015, a Venezuela subsidiary of the Group agreed with CENCOEX to settle a substantial part of our intercompany trade payables dated on or before December 31, 2014 in a transaction that required the Venezuela subsidiary to purchase a $ denominated bond at par value issued by Petróleos de Venezuela (PDVSA), with a coupon rate of 6% per annum maturing in 2024. In Venezuela there are differing official exchange rates against the $ and for the settlement of these intercompany trade payables, through the purchase of the $ bond, CENCOEX set the exchange rate at VEF 11.0/$. As a result, from November 2015 the Group changed its exchange rate used for the consolidation of the financial statements of its Venezuela subsidiaries. The use of the new exchange rate by the Venezuela subsidiaries resulted in a $211 million loss from the re-measurement of the intra-Group and third party liabilities.
As agreed with CENCOEX, the Venezuela subsidiary purchased the PDVSA bond on December 9, 2015. The bond was sold on December 11, 2015. The proceeds from the sale of this bond were $73 million resulting in a loss of $127 million.
We seek to manage currency exposure by engaging in hedging transactions where management deems appropriate, after taking into account the natural hedging afforded by our global business activity. For 2015, we entered into various contracts that change in value with movements in foreign exchange rates in
184
order to preserve the value of assets, commitments and expected transactions. We use forward contracts and foreign currency options to hedge. For more information on how these transactions affect our consolidated financial statements and on how foreign exchange rate exposure is managed, see "Item 18. Financial Statements—Notes 1, 5, 16 and 29".
In 2015, the US dollar significantly increased in value against most currencies. In particular, the average value of the euro, Japanese yen and emerging market currencies (especially the Brazilian real and Russian ruble) decreased in 2015 against the $ dollar. In January 2015, following an announcement by the Swiss National Bank that it was discontinuing its minimum exchange rate with the euro, the value of the Swiss franc increased versus the euro and the $.
The following table sets forth the foreign exchange rates of the US dollar ($) against key currencies used for foreign currency translation when preparing the Group's consolidated financial statements:
| |
Average for year |
|
|
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Year-end | |
||||||||||||||||
| |
Change in % |
Change in % |
|||||||||||||||||
$ per unit
|
2015 | 2014 | 2015 | 2014 | |||||||||||||||
AUD |
0.753 | 0.903 | (17 | ) | 0.731 | 0.819 | (11 | ) | |||||||||||
BRL |
0.305 | 0.426 | (28 | ) | 0.253 | 0.376 | (33 | ) | |||||||||||
CAD |
0.784 | 0.906 | (13 | ) | 0.721 | 0.861 | (16 | ) | |||||||||||
CHF |
1.040 | 1.094 | (5 | ) | 1.011 | 1.010 | 0 | ||||||||||||
CNY |
0.159 | 0.162 | (2 | ) | 0.154 | 0.161 | (4 | ) | |||||||||||
EUR |
1.110 | 1.329 | (16 | ) | 1.093 | 1.215 | (10 | ) | |||||||||||
GBP |
1.529 | 1.648 | (7 | ) | 1.483 | 1.556 | (5 | ) | |||||||||||
JPY (100) |
0.826 | 0.947 | (13 | ) | 0.831 | 0.836 | (1 | ) | |||||||||||
RUB (100) |
1.649 | 2.649 | (38 | ) | 1.362 | 1.722 | (21 | ) | |||||||||||
| |
Average for year |
|
|
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Year-end | |
||||||||||||||||
| |
Change in % |
Change in % |
|||||||||||||||||
$ per unit
|
2014 | 2013 | 2014 | 2013 | |||||||||||||||
AUD |
0.903 | 0.968 | (7 | ) | 0.819 | 0.892 | (8 | ) | |||||||||||
BRL |
0.426 | 0.465 | (8 | ) | 0.376 | 0.424 | (11 | ) | |||||||||||
CAD |
0.906 | 0.971 | (7 | ) | 0.861 | 0.939 | (8 | ) | |||||||||||
CHF |
1.094 | 1.079 | 1 | 1.010 | 1.124 | (10 | ) | ||||||||||||
CNY |
0.162 | 0.163 | (1 | ) | 0.161 | 0.165 | (2 | ) | |||||||||||
EUR |
1.329 | 1.328 | 0 | 1.215 | 1.378 | (12 | ) | ||||||||||||
GBP |
1.648 | 1.564 | 5 | 1.556 | 1.653 | (6 | ) | ||||||||||||
JPY (100) |
0.947 | 1.026 | (8 | ) | 0.836 | 0.952 | (12 | ) | |||||||||||
RUB (100) |
2.649 | 3.142 | (16 | ) | 1.722 | 3.044 | (43 | ) | |||||||||||
The following table provides a summary of the currency impact on key Group figures due to their conversion into $, the Group's reporting currency, of the financial data from entities reporting in non-US dollars. Constant currency (cc) calculations apply the exchange rates of the prior year to the current year financial data for entities reporting in non-US dollars.
185
CURRENCY IMPACT ON KEY FIGURES
| |
Change in constant currencies % 2015 |
Change in $ % 2015 |
Percentage point currency impact 2015 |
Change in constant currencies % 2014 |
Change in $ % 2014 |
Percentage point currency impact 2014 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net sales from continuing operations |
5 | (5 | ) | (10 | ) | 3 | 1 | (2 | ) | ||||||||||
Operating income from continuing operations |
(2 | ) | (19 | ) | (17 | ) | 7 | 1 | (6 | ) | |||||||||
Net income from continuing operations |
(18 | ) | (34 | ) | (16 | ) | 21 | 15 | (6 | ) | |||||||||
Core operating income from continuing operations |
10 | (5 | ) | (15 | ) | 7 | 2 | (5 | ) | ||||||||||
Core net income from continuing operations |
9 | (5 | ) | (14 | ) | 8 | 3 | (5 | ) | ||||||||||
For additional information on the effects of currency fluctuations, see "Item 18. Financial Statements—note 29".
Novartis defines free cash flow as cash flow from operating activities and cash flow associated with the purchase or sale of property, plant and equipment, intangible, other non-current and financial assets. Cash flows in connection with the acquisition or divestment of subsidiaries, associated companies and non-controlling interests in subsidiaries are not taken into account to determine free cash flow. The free
186
cash flow measure is a non-IFRS measure, see "—Non-IFRS Measures as Defined by Novartis" above. The following is a summary of the free cash flow:
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Operating income from continuing operations |
8,977 | 11,089 | 10,983 | |||||||
Reversal of non-cash items |
||||||||||
Depreciation, amortization and impairments |
5,575 | 4,751 | 4,462 | |||||||
Change in provisions and other non-current liabilities |
1,642 | 1,490 | 736 | |||||||
Other |
(96 | ) | 122 | 307 | ||||||
| | | | | | | | | | | |
Operating income adjusted for non-cash items |
16,098 | 17,452 | 16,488 | |||||||
Interest and other financial receipts |
1,180 | 1,067 | 539 | |||||||
Interest and other financial payments |
(669 | ) | (692 | ) | (631 | ) | ||||
Taxes paid |
(2,454 | ) | (2,179 | ) | (2,054 | ) | ||||
Payments out of provisions and other net cash movements in non-current liabilities |
(1,207 | ) | (1,125 | ) | (947 | ) | ||||
Change in inventory and trade receivables less trade payables |
(617 | ) | (731 | ) | (588 | ) | ||||
Change in other net current assets and other operating cash flow items |
(246 | ) | 106 | (190 | ) | |||||
| | | | | | | | | | | |
Cash flows from operating activities from continuing operations |
12,085 | 13,898 | 12,617 | |||||||
Purchase of property, plant & equipment |
(2,367 | ) | (2,624 | ) | (2,903 | ) | ||||
Purchase of intangible assets |
(1,138 | ) | (780 | ) | (475 | ) | ||||
Purchase of financial assets |
(264 | ) | (239 | ) | (152 | ) | ||||
Purchase of other non-current assets |
(82 | ) | (60 | ) | (38 | ) | ||||
Proceeds from sales of property, plant & equipment |
237 | 60 | 48 | |||||||
Proceeds from sales of intangible assets |
621 | 246 | 96 | |||||||
Proceeds from sales of financial assets |
166 | 431 | 313 | |||||||
Proceeds from sales of other non-current assets |
1 | 2 | 15 | |||||||
| | | | | | | | | | | |
Free cash flow from continuing operations |
9,259 | 10,934 | 9,521 | |||||||
Free cash flow from discontinued operations |
(230 | ) | (172 | ) | 424 | |||||
| | | | | | | | | | | |
Free cash flow |
9,029 | 10,762 | 9,945 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Financial year 2015
In 2015, free cash flow from continuing operations decreased by 15% to $9.3 billion compared to $10.9 billion in 2014. This decrease was primarily due to the negative currency impact on operations. The prior year also included higher proceeds from Novartis Venture Fund divestments and commercial settlements. Total free cash flow including the continuing and discontinued operations was $9.0 billion in 2015 compared to $10.8 billion in 2014.
Financial year 2014
The free cash flow from continuing operations increased by $1.4 billion to $10.9 billion. This was primarily due to higher cash flows from operating activities, which mainly benefited from higher operating income adjusted for non-cash items, despite negative currency effects and increased hedging gains, partially offset by higher investments in intangible assets.
187
In 2014, free cash flow of the total Group increased by $0.8 billion to $10.8 billion compared to $9.9 billion in 2013.
Financial year 2013
In 2013, free cash flow from continuing operations amounted to $9.5 billion which was 15% below the prior year. Aside from the significant currency impact, major reasons for the decline were increased trade receivables and higher capital investments in manufacturing and research facilities.
In 2013, the total Group free cash flow of $9.9 billion was 13% below the prior year.
The total Group free cash flow was primarily used for the dividend payments to shareholders of $6.1 billion as well as a $1.3 billion net repayment of financial debt and for treasury share purchases of net $1.2 billion.
This allocation reflected management's intention to optimize shareholder returns whilst at the same time reinvesting surplus funds in the business to promote future growth.
CONDENSED CONSOLIDATED BALANCE SHEETS
| |
Dec 31, 2015 | Dec 31, 2014 | Change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Assets |
||||||||||
Property, plant & equipment |
15,982 | 15,983 | (1 | ) | ||||||
Goodwill |
31,174 | 29,311 | 1,863 | |||||||
Intangible assets other than goodwill |
34,217 | 23,832 | 10,385 | |||||||
Financial and other non-current assets |
27,338 | 18,700 | 8,638 | |||||||
| | | | | | | | | | | |
Total non-current assets |
108,711 | 87,826 | 20,885 | |||||||
| | | | | | | | | | | |
Inventories |
6,226 | 6,093 | 133 | |||||||
Trade receivables |
8,180 | 8,275 | (95 | ) | ||||||
Other current assets |
2,992 | 2,530 | 462 | |||||||
Cash, marketable securities, commodities, time deposits and derivative financial instruments |
5,447 | 13,862 | (8,415 | ) | ||||||
Assets related to discontinued operations(1) |
0 | 6,801 | (6,801 | ) | ||||||
| | | | | | | | | | | |
Total current assets |
22,845 | 37,561 | (14,716 | ) | ||||||
| | | | | | | | | | | |
Total assets |
131,556 | 125,387 | 6,169 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Equity and liabilities |
||||||||||
Total equity |
77,122 | 70,844 | 6,278 | |||||||
| | | | | | | | | | | |
Financial debts |
16,327 | 13,799 | 2,528 | |||||||
Other non-current liabilities |
14,399 | 13,771 | 628 | |||||||
| | | | | | | | | | | |
Total non-current liabilities |
30,726 | 27,570 | 3,156 | |||||||
| | | | | | | | | | | |
Trade payables |
5,668 | 5,419 | 249 | |||||||
Financial debts and derivatives |
5,604 | 6,612 | (1,008 | ) | ||||||
Other current liabilities |
12,436 | 12,524 | (88 | ) | ||||||
Liabilities related to discontinued operations(1) |
0 | 2,418 | (2,418 | ) | ||||||
| | | | | | | | | | | |
Total current liabilities |
23,708 | 26,973 | (3,265 | ) | ||||||
| | | | | | | | | | | |
Total liabilities |
54,434 | 54,543 | (109 | ) | ||||||
| | | | | | | | | | | |
Total equity and liabilities |
131,556 | 125,387 | 6,169 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- For details of discontinued operations in the consolidated balance sheet, refer to "Item 18. Financial Statements—Note 30".
188
Total non-current assets of $108.7 billion at December 31, 2015 increased by $20.9 billion compared to December 31, 2014. Intangible assets other than goodwill increased by $10.4 billion to $34.2 billion, mainly on account of the new oncology assets acquired from GSK, which added product rights amounting to $13.0 billion to the intangible assets of the Group. This increase was partially offset by the amortization of intangible assets of $3.8 billion. Goodwill increased by $1.9 billion to $31.2 billion, mainly on account of the goodwill of $2.4 billion recorded on the new oncology assets, partially offset by currency translation adjustments of $0.6 billion.
Financial and other non-current assets increased by $8.6 billion to $27.3 billion, mainly on account of the 36.5% investment in the GSK consumer healthcare joint venture of $7.6 billion, while investments in property, plant and equipment were in line with the prior year.
Total current assets decreased by $14.7 billion to $22.8 billion at December 31, 2015, as cash and cash equivalents decreased by $8.4 billion to $5.4 billion, mainly on account of the net cash outflows from the portfolio transformation transactions as well as the dividend payment. The assets related to discontinued operations and held for sale reduced by $6.8 billion as a result of the closing of the portfolio transformation transactions in 2015. Trade receivables, inventories and other current assets were in line with the prior year.
Based on our current incurred loss provisioning approach, we consider that our doubtful debt provisions are adequate. However, we intend to continue to monitor the level of trade receivables in Greece, Italy, Portugal and Spain (the "GIPS countries"). Should there be a substantial deterioration in our economic exposure with respect to those countries, we may increase our level of provisions by moving to an expected loss provisioning approach or may change the terms of trade on which we operate.
The following table provides an overview of our aging analysis of our trade receivables as of December 31, 2015 and 2014:
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Not overdue |
7,318 | 7,406 | |||||
Past due for not more than one month |
265 | 334 | |||||
Past due for more than one month but less than three months |
255 | 275 | |||||
Past due for more than three months but less than six months |
193 | 174 | |||||
Past due for more than six months but less than one year |
156 | 102 | |||||
Past due for more than one year |
135 | 140 | |||||
Provisions for doubtful trade receivables |
(142 | ) | (156 | ) | |||
| | | | | | | | |
Total trade receivables, net |
8,180 | 8,275 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
With regard to the GIPS countries, the majority of the outstanding trade receivables from these countries are due directly from local governments or from government-funded entities. The gross trade receivables from GIPS countries at December 31, 2015 amount to $920 million (2014: $915 million), of which $58 million are past due for more than one year (2014: $69 million) and for which provisions of $37 million have been recorded (2014: $48 million). At December 31, 2015 amounts past due for more than one year are not significant in any of the GIPS countries on a standalone basis.
There is also a risk that certain countries could devalue their currency. The most significant exposure for Novartis in this respect is in Venezuela, which is described in more detail, see "—Effects of currency fluctuations" above.
Trade payables, other current and non-current liabilities of $32.5 billion increased by $0.8 billion compared to $31.7 billion at December 31, 2014. This change was due to an increase in other non-current liabilities of $0.6 billion and an increase in trade payables of $0.2 billion. The liabilities related to
189
discontinued operations and held for sale reduced by $2.4 billion as a result of the closing of the portfolio transformation transactions in 2015.
Included in other current liabilities are $1.7 billion relating to outstanding taxes. While there is some uncertainty about the final taxes to be assessed in our major countries, we consider this uncertainty to be limited since our tax assessments are generally relatively current. In our key countries Switzerland and the US, assessments have been agreed by the tax authorities up to 2010 in Switzerland and in the US up to 2009, with the exception of one open US position in 2007.
The Group's equity increased by $6.3 billion to $77.1 billion at December 31, 2015, compared to $70.8 billion at December 31, 2014. The increase was on account of our net income of $17.8 billion, share-based compensation of $0.8 billion and the settlement of the obligation under the share repurchase agreement of $0.7 billion. The increase was partially offset by the $6.6 billion dividend payment, net purchases of treasury shares of $4.5 billion, unfavorable currency translation differences of $1.7 billion and net actuarial losses from defined benefit plans of $0.1 billion.
The Group's liquidity amounted to $5.4 billion at December 31, 2015, compared to $13.9 billion at December 31, 2014, and net debt increased over the same period by $10.0 billion to $16.5 billion. The debt/equity ratio decreased to 0.28:1 at December 31, 2015 compared to 0.29:1 at December 31, 2014.
SUMMARY OF EQUITY MOVEMENTS ATTRIBUTABLE TO NOVARTIS AG SHAREHOLDERS
| |
Number of outstanding shares (in millions) |
Issued share capital and reserves attributable to Novartis AG shareholders |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | Change | 2015 | 2014 | Change | |||||||||||||
| |
|
|
|
$ m |
$ m |
$ m |
|||||||||||||
Balance at beginning of year |
2,398.6 | 2,426.1 | (27.5 | ) | 70,766 | 74,343 | (3,577 | ) | |||||||||||
Shares acquired to be held in Group Treasury |
(9.6 | ) | (46.8 | ) | 37.2 | (897 | ) | (4,057 | ) | 3,160 | |||||||||
Shares acquired to be canceled |
(49.9 | ) | (27.0 | ) | (22.9 | ) | (4,805 | ) | (2,396 | ) | (2,409 | ) | |||||||
Other share purchases |
(4.1 | ) | (5.4 | ) | 1.3 | (417 | ) | (473 | ) | 56 | |||||||||
Increase in equity from exercise of options and employee transactions |
27.0 | 41.4 | (14.4 | ) | 1,592 | 2,400 | (808 | ) | |||||||||||
Equity-based compensation |
11.9 | 10.3 | 1.6 | 815 | 1,143 | (328 | ) | ||||||||||||
Decrease/(Increase) of treasury share repurchase obligation under a share buy-back trading plan |
658 | (658 | ) | 1,316 | |||||||||||||||
Dividends |
(6,643 | ) | (6,810 | ) | 167 | ||||||||||||||
Net income of the year attributable to shareholders of Novartis AG |
17,783 | 10,210 | 7,573 | ||||||||||||||||
Other comprehensive income attributable to shareholders of Novartis AG |
(1,806 | ) | (2,936 | ) | 1,130 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balance at end of year |
2,373.9 | 2,398.6 | (24.7 | ) | 77,046 | 70,766 | 6,280 | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
During 2015, 38.9 million treasury shares were delivered as a result of options being exercised and physical share deliveries related to equity-based participation plans (2014: 51.7 million shares). 9.6 million shares were repurchased on the SIX Swiss Exchange first trading line (2014: 46.8 million), 4.1 million shares were acquired from employees which were previously granted to them under the respective programs (2014: 5.4 million). In addition, Novartis repurchased 49.9 million shares on the SIX Swiss Exchange second trading line under the $5 billion share buyback announced in 2013, which was completed
190
in November 2015, and also to offset the dilutive impact from equity-based participation plans (2014: 27.0 million). With these transactions, the total number of shares outstanding was reduced by 24.7 million in 2015 (2014: reduction of 27.5 million shares) and the sixth share buyback program, which was approved by the shareholders at the AGM 2008 has been completed.
Treasury shares
At December 31, 2015, our holding of treasury shares amounted to 303.1 million shares or 11% of the total number of issued shares. Approximately 137 million treasury shares are held in entities that limit their availability for use.
At December 31, 2014, our holding of treasury shares amounted to 307.6 million shares or 11% of the total number of issued shares. Approximately 153 million treasury shares are held in entities that limit their availability for use.
At December 31, 2013, our holding of treasury shares amounted to 280.1 million shares or 10% of the total number of issued shares. Approximately 149 million treasury shares are held in entities that limit their availability for use.
Bonds
In February 2015, three Swiss franc bonds totaling CHF 1.375 billion were completed; a 10-year bond of CHF 0.5 billion with a coupon of 0.25%, a 14-year bond of CHF 0.550 billion with a coupon of 0.625% and a 20-year bond of CHF 0.325 billion with a coupon of 1.050%.
In November 2015, two US Dollar bonds totaling $3.0 billion were issued: a 10-year bond of $1.75 billion with a coupon of 3.0% and a 30-year bond of $1.25 billion with a coupon of 4.0%.
In 2015, a 2.9% US Dollar bond of $2.0 billion and a 3.625% CHF bond of 0.8 billion were repaid.
In February 2014, a $4.0 billion bond offering was completed in the United States consisting of two tranches; one 10 year bond of $2.15 billion with a coupon of 3.4% and one 30 year bond of $1.85 billion with a coupon of 4.4%. Further, a 4.125% US Dollar bond of $2 billion was repaid at maturity.
In October 2014, a EUR 1.2 billion bond offering was completed consisting of two tranches; one 7 year bond of EUR 0.6 billion with a coupon of 0.75% and one 12 year bond of EUR 0.6 billion with a coupon of 1.625%.
In April 2013, a 1.9% US Dollar bond of $2.0 billion was repaid.
Liquidity/Short-term Funding
We continuously track our liquidity position and asset/liability profile. This involves modeling expected cash flows based on both historical experiences and contractual expectations to project our liquidity requirements. We seek to preserve prudent liquidity and funding capabilities.
We are not aware of significant demands to change our level of liquidity needed to support our normal business activities. We make use of various borrowing facilities provided by several financial institutions. We also successfully issued various bonds in 2009, 2010, 2012, 2014 and 2015 and raised funds through our commercial paper programs. In addition, reverse repurchasing agreements are contracted and Novartis has entered into credit support agreements with various banks for derivative transactions. We have no commitments from repurchase or securities lending transactions at the end of 2015. For details of the maturity profile of debt, currency and interest rate structure, see "Item 18. Financial Statements—Note 29".
191
5.C Research & Development, Patents and Licenses
Our R&D spending for continuing operations totaled $8.9 billion, $9.1 billion and $9.1 billion ($8.7 billion, $8.7 billion and $8.9 billion excluding impairments and amortization charges) for the years 2015, 2014 and 2013, respectively. Each of our divisions has its own R&D and patents policies. Our divisions have numerous products in various stages of development. For further information on these policies and these products in development, see "Item 4. Information on the Company—4.B Business Overview."
As described in the "Risk Factors" section and elsewhere in this Form 20-F, our drug development efforts are subject to the risks and uncertainties inherent in any new drug development program. Due to the risks and uncertainties involved in progressing through pre-clinical development and clinical trials, and the time and cost involved in obtaining regulatory approvals, among other factors, we cannot reasonably estimate the timing, completion dates, and costs, or range of costs, of our drug development program, or of the development of any particular development compound, see "Item 3. Key Information—3.D Risk Factors." In addition, for a description of the research and development process for the development of new drugs and our other products, and the regulatory process for their approval, see "Item 4. Information on the Company—4.B Business Overview."
Please see "—5.A Operating Results—Factors Affecting Results of Operations" and "Item 4, Information on the Company—4.B Business Overview" for trend information.
5.E Off-Balance Sheet Arrangements
We have no unconsolidated special purpose financing or partnership entities or other off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources, that is material to investors, see also "Item 18. Financial Statements—Note 28" and matters described in "Item 5.F Aggregate Contractual Obligations".
192
5.F Aggregate Contractual Obligations
The following table summarizes the Group's contractual obligations and other commercial commitments at December 31, 2015, as well as the effect these obligations and commitments are expected to have on the Group's liquidity and cash flow in future periods:
| |
Payments due by period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total | Less than 1 year | 2–3 years | 4–5 years | After 5 years | |||||||||||
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Non-current financial debt, including current portion |
17,986 | 1,659 | 505 | 5,460 | 10,362 | |||||||||||
Operating leases |
2,996 | 273 | 335 | 207 | 2,181 | |||||||||||
Unfunded pensions and other post-employment benefit plans |
2,165 | 113 | 234 | 251 | 1,567 | |||||||||||
Research & Development |
||||||||||||||||
Unconditional commitments |
650 | 88 | 147 | 265 | 150 | |||||||||||
Potential milestone commitments |
2,405 | 601 | 781 | 626 | 397 | |||||||||||
Purchase commitments |
||||||||||||||||
Property, plant & equipment |
359 | 304 | 55 | |||||||||||||
| | | | | | | | | | | | | | | | | |
Total contractual cash obligations |
26,561 | 3,038 | 2,057 | 6,809 | 14,657 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
We expect to fund the R&D and purchase commitments with internally generated resources.
For other contingencies, see "Item 4. Information on the Company—4.D Property, Plants and Equipment—Environmental Matters", "Item 8. Financial Information—8.A Consolidated Statements and Other Financial Information" and "Item 18. Financial Statements—Note 20 and 28".
Item 6. Directors, Senior Management and Employees
6.A Directors and Senior Management
Joerg Reinhardt, Ph.D.
Chairman of the Board of Directors
German, age 59
Function at Novartis AG Joerg Reinhardt, Ph.D., has been Chairman of the Board of Directors of Novartis since 2013. He is also Chairman of the Research & Development Committee and Chairman of the Board of Trustees of the Novartis Foundation.
Other activities Mr. Reinhardt previously was chairman of the board of management and the executive committee of Bayer HealthCare, Germany. Prior to that, he was Chief Operating Officer of Novartis from 2008 to 2010, and Head of the Vaccines and Diagnostics Division of Novartis from 2006 to 2008. He was also Chairman of the Board of the Genomics Institute of the Novartis Research Foundation in the United States from 2000 to 2010, a member of the supervisory board of MorphoSys AG in Germany from 2001 to 2004, and a member of the board of directors of Lonza Group AG in Switzerland from 2012 to 2013.
Professional background Mr. Reinhardt graduated with a Ph.D. in pharmaceutical sciences from Saarland University in Germany. He joined Sandoz Pharma Ltd. in 1982 and held various positions at Sandoz and later Novartis, including Head of Development.
193
Key knowledge/experience Leadership, global and industry experience—former chairman of global healthcare company; former Chief Operating Officer of Novartis and former Chairman of Novartis research institution; former board member of leading biotechnology company and of global supplier for pharmaceutical, healthcare and life sciences industries.
Enrico Vanni, Ph.D.
Vice Chairman of the Board of Directors
Swiss, age 64
Function at Novartis AG Enrico Vanni, Ph.D., has been a member of the Board of Directors since 2011. He qualifies as an independent Non-Executive Director. He is Vice Chairman of the Board of Directors and Chairman of the Compensation Committee. He is also a member of the Audit and Compliance Committee and the Research & Development Committee.
Other activities Since his retirement as director of McKinsey & Company in 2007, Mr. Vanni has been an independent consultant. He is a board member of several companies in industries from healthcare to private banking—including Advanced Oncotherapy PLC in England, and non-listed companies such as Lombard Odier SA, Banque Privée BCP (Suisse) SA, Eclosion2, and Denzler & Partners SA, all based in Switzerland.
Professional background Mr. Vanni holds an engineering degree in chemistry from the Federal Polytechnic School of Lausanne, Switzerland; a Ph.D. in chemistry from the University of Lausanne; and a Master of Business Administration from INSEAD in Fontainebleau, France. He began his career as a research engineer at the International Business Machines Corp. (IBM) in California, United States, and joined McKinsey in Zurich in 1980. He managed the Geneva office for McKinsey from 1988 to 2004, and consulted for companies in the pharmaceutical, consumer and finance sectors. He led McKinsey's European pharmaceutical practice and served as a member of the firm's partner review committee prior to his retirement in 2007. As an independent consultant, Mr. Vanni has continued to support leaders of pharmaceutical and biotechnology companies on core strategic challenges facing the healthcare industry.
Key knowledge/experience Global and industry experience—senior consultant of global pharmaceutical/biotechnology and consumer goods companies, and financial institutions. Science experience—research engineer at technology company and manager of projects in global pharmaceutical R&D. Leadership experience—office management of global consulting company and leadership of its European pharmaceutical practice.
Nancy C. Andrews, M.D., Ph.D.
Member of the Board of Directors
American, age 57
Function at Novartis AG Nancy C. Andrews, M.D., Ph.D., has been a member of the Board of Directors since February 27, 2015. She qualifies as an independent Non-Executive Director and is a member of the Research & Development Committee.
Other activities Dr. Andrews is dean of the Duke University School of Medicine and vice chancellor for academic affairs at Duke University in the United States. She is also a professor of pediatrics, pharmacology and cancer biology at Duke. Prior to joining Duke, she was director of the Harvard/MIT M.D.-Ph.D. Program, and dean of basic sciences and graduate studies as well as professor of pediatrics at Harvard Medical School in the US. From 1993 to 2006, Dr. Andrews was a biomedical research investigator at the Howard Hughes Medical Institute, also in the US. Her research expertise is in iron homeostasis and mouse models of human diseases.
Professional background Dr. Andrews received her Ph.D. in biology from the Massachusetts Institute of Technology in the US and her M.D. from Harvard Medical School. She completed her residency and
194
fellowship trainings in pediatrics and hematology/oncology at Boston Children's Hospital and the Dana-Farber Cancer Institute, both in the US, and served as an attending physician at Boston Children's Hospital. Dr. Andrews also served as president of the American Society for Clinical Investigation. Additionally, she was elected as a fellow of the American Association for the Advancement of Science and to membership in the US National Academy of Sciences, the National Academy of Medicine, and the American Academy of Arts and Sciences. She serves on the council of the National Academy of Medicine and on the board of directors of the American Academy of Arts and Sciences.
Key knowledge/experience Leadership and healthcare experience—dean of leading US university medical school; member of various medical, scientific and ethical institutions and commissions. Education and scientific experience—research scientist and professor at leading US universities.
Dimitri Azar, M.D.
Member of the Board of Directors
American, age 56
Function at Novartis AG Dimitri Azar, M.D., has been a member of the Board of Directors since 2012. He qualifies as an independent Non-Executive Director and is a member of the Audit and Compliance Committee and the Research & Development Committee.
Other activities Dr. Azar is dean of the College of Medicine and professor of ophthalmology, bioengineering and pharmacology at the University of Illinois at Chicago in the United States, where he formerly was head of the Department of Ophthalmology and Visual Sciences. He is a member of the American Ophthalmological Society and is on the boards of trustees of the Chicago Medical Society, the Chicago Ophthalmological Society, the Association for Research in Vision and Ophthalmology, and the Tear Film and Ocular Surface Society.
Professional background Dr. Azar began his career at the American University of Beirut Medical Center in Lebanon, and completed his fellowship and residency training at the Massachusetts Eye and Ear Infirmary at Harvard Medical School in the US. His research on matrix metalloproteinases in corneal wound healing and angiogenesis has been funded by the US National Institutes of Health since 1993. Dr. Azar practiced at the Wilmer Eye Institute at the Johns Hopkins Hospital School of Medicine in the US, and then returned to the Massachusetts Eye and Ear Infirmary as director of cornea and external disease. He became professor of ophthalmology with tenure at Harvard Medical School in 2003. Dr. Azar holds an Executive Master of Business Administration from the University of Chicago Booth School of Business in the US.
Key knowledge/experience Leadership, healthcare and education experience—dean and professor at leading US university medical school. Biomedical science experience—federally-funded clinician-scientist and research fellowship recipient.
Verena A. Briner, M.D.
Member of the Board of Directors
Swiss, age 64
Function at Novartis AG Verena A. Briner, M.D., has been a member of the Board of Directors since 2013. She qualifies as an independent Non-Executive Director and is a member of the Risk Committee.
Other activities Dr. Briner is professor of internal medicine at the University of Basel, and visiting professor at the University of Lucerne, both in Switzerland. She is chief medical officer and head of the Department of Medicine at the Lucerne Cantonal Hospital in Switzerland. Additionally, she is a member of several medical and ethical institutions and commissions, including the board of the Foundation for the Development of Internal Medicine in Europe, the senate of the Swiss Academy of Medical Sciences, and
195
the journal of the inter-cantonal convention on highly-specialized medicine (IVHSM), Switzerland. She is also a member and former president of the Swiss Society of Internal Medicine.
Professional background Dr. Briner graduated with an M.D. from the University of Basel in 1978, and has a specialized degree in internal medicine and nephrology from the Swiss Medical Association. She has received several prestigious scholarships and scientific grants, including the President's Grant of the Swiss Society of General Internal Medicine in 2011. Additionally, she is a fellow of the Royal College of Physicians, United Kingdom, and an honorary fellow of the American College of Physicians, the European Federation of Internal Medicine, the Polish Society of Internal Medicine, and the Swiss Society of General Internal Medicine.
Key knowledge/experience Leadership and healthcare experience—chief medical officer and department head at leading Swiss hospital; former president of Swiss medical society; member of various medical and ethical institutions and commissions. Education experience—professor and visiting professor at leading Swiss universities.
Srikant Datar, Ph.D.
Member of the Board of Directors
American, age 62
Function at Novartis AG Srikant Datar, Ph.D., has been a member of the Board of Directors since 2003. He qualifies as an independent Non-Executive Director. He is Chairman of the Audit and Compliance Committee, and a member of the Risk Committee and the Compensation Committee. The Board of Directors has appointed him as Audit Committee Financial Expert.
Other activities Mr. Datar is Arthur Lowes Dickinson Professor at the Graduate School of Business Administration at Harvard University in the United States. He is also a member of the boards of directors of ICF International Inc., Stryker Corp. and T-Mobile US, all in the US.
Professional background Mr. Datar graduated in 1973 with distinction in mathematics and economics from the University of Bombay in India. He is a chartered accountant, and holds two master's degrees and a doctorate from Stanford University in the US. Mr. Datar has worked as an accountant and planner in industry, and as a professor at Carnegie Mellon University, Stanford University and Harvard University, all in the US. His research interests are in the areas of cost management, measurement of productivity, new product development, innovation, time-based competition, incentives and performance evaluation. He is the author of many scientific publications and has received several academic awards and honors. Mr. Datar has also advised and worked with numerous companies in research, development and training.
Key knowledge/experience Leadership and education experience—former senior associate dean and current professor at leading US university. Global and industry experience board member of global professional services firm, leading global medical technology company, and major US telecommunications company.
Ann Fudge
Member of the Board of Directors
American, age 64
Function at Novartis AG Ann Fudge has been a member of the Board of Directors since 2008. She qualifies as an independent Non-Executive Director and is a member of the Risk Committee; the Compensation Committee; and the Governance, Nomination and Corporate Responsibilities Committee.
Other activities Ms. Fudge is vice chairman and senior independent director of Unilever NV, London and Rotterdam. She is a trustee of the New York-based Rockefeller Foundation and the Washington, D.C.-based Brookings Institution, and is chair of the US Programs Advisory Panel of the
196
Bill & Melinda Gates Foundation. Ms. Fudge is also a trustee of WGBH public media and serves on the board of the Council on Foreign Relations.
Professional background Ms. Fudge received her bachelor's degree from Simmons College in the United States and her Master of Business Administration from Harvard University Graduate School of Business, also in the US. She is former chairman and CEO of Young & Rubicam Brands, New York. Before that, she served as president of the Beverages, Desserts and Post Division of Kraft Foods Inc. in the US.
Key knowledge/experience Leadership and marketing experience—former chairman and CEO of global marketing communications company; former president of leading consumer products business unit. Global and industry experience—former board member of global technology company; board member of global consumer goods company.
Pierre Landolt, Ph.D.
Member of the Board of Directors
Swiss, age 68
Function at Novartis AG Pierre Landolt, Ph.D., has been a member of the Board of Directors since 1996. He qualifies as an independent Non-Executive Director and is Chairman of the Governance, Nomination and Corporate Responsibilities Committee.
Other activities Mr. Landolt is chairman of the Sandoz Family Foundation, overseeing its development in several investment fields. He is also chairman of the Swiss private bank Landolt & Cie SA. In Switzerland, he is chairman of Emasan AG and Vaucher Manufacture Fleurier SA, and vice chairman of Parmigiani Fleurier SA. Additionally, he is vice chairman of the Montreux Jazz Festival Foundation and a board member of Amazentis SA, Switzerland. In Brazil, Mr. Landolt is president of AxialPar Ltda. and Moco Agropecuaria Ltda., the Instituto Fazenda Tamanduá and the Instituto Estrela de Fomento ao Microcrédito.
Professional background Mr. Landolt graduated with a bachelor's degree in law from the University of Paris—Assas. From 1974 to 1976, he worked for Sandoz Brazil. In 1977, he acquired an agricultural estate in the semi-arid Northeast Region of Brazil, and within several years converted it into a model farm in organic and biodynamic production. Since 1997, Mr. Landolt has been associate and chairman of AxialPar Ltda., Brazil, an investment company focused on sustainable development. In 2000, he co-founded Eco-Carbone SAS, a company active in the design and development of carbon-sequestration processes. In 2007, he co-founded Amazentis SA, a startup company active in the convergence space of medication and nutrition. In 2011, Mr. Landolt received the title of Docteur des Sciences Économiques Honoris Causa from the University of Lausanne in Switzerland.
Key knowledge/experience Banking and industry experience in international and emerging markets—chairman of private bank; chairman and vice chairman of luxury goods companies; board member of agribusiness company. Leadership and global experience chairman of large family investment holding.
Andreas von Planta, Ph.D.
Member of the Board of Directors
Swiss, age 60
Function at Novartis AG Andreas von Planta, Ph.D., has been a member of the Board of Directors since 2006. He qualifies as an independent Non-Executive Director. He is Chairman of the Risk Committee and a member of the Audit and Compliance Committee and the Governance, Nomination and Corporate Responsibilities Committee.
Other activities Mr. von Planta is a board member of Helvetia Holding AG in Switzerland, and also serves on the boards of various Swiss subsidiaries of foreign companies and other non-listed Swiss
197
companies, including A.P. Moller Finance SA, HSBC Private Bank (Switzerland) SA, Socotab Frana SA, Raymond Weil SA and Générale-Beaulieu Holding SA. Additionally, he is chairman of the regulatory board of the SIX Swiss Exchange AG.
Professional background Mr. von Planta holds lic. iur. and Ph.D. degrees from the University of Basel in Switzerland, and an LL.M. from Columbia University School of Law in the United States. He passed his bar examinations in Basel in 1982. Since 1983, he has lived in Geneva and worked for the law firm Lenz & Staehelin, where he became a partner in 1988. His areas of specialization include corporate law, corporate governance, corporate finance, company reorganizations, and mergers and acquisitions.
Key knowledge/experience Leadership and global experience—board member of insurance company. Industry experience—partner at leading Swiss law firm.
Charles L. Sawyers, M.D.
Member of the Board of Directors
American, age 56
Function at Novartis AG Charles L. Sawyers, M.D., has been a member of the Board of Directors since 2013. He qualifies as an independent Non-Executive Director and is a member of the Research & Development Committee and the Governance, Nomination and Corporate Responsibilities Committee.
Other activities In the United States, Dr. Sawyers is chair of the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center, professor of medicine and of cell and developmental biology at the Weill Cornell Graduate School of Medical Sciences, and an investigator at the Howard Hughes Medical Institute. He serves on US President Barack Obama's National Cancer Advisory Board, and is former president of the American Association for Cancer Research and of the American Society for Clinical Investigation. He is also a member of the US National Academy of Sciences and Institute of Medicine.
Professional background Dr. Sawyers received his M.D. from the Johns Hopkins School of Medicine in the US, and worked at the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles in the US for nearly 18 years before joining Memorial Sloan Kettering in 2006. An internationally-acclaimed cancer researcher, he co-developed the Novartis cancer drug Gleevec/Glivec and has received numerous honors and awards, including the Lasker-DeBakey Clinical Medical Research Award in 2009. Dr. Sawyers is a member of the scientific advisory board of Agios Pharmaceuticals Inc. in the US.
Key knowledge/experience Leadership, healthcare and science experience—program chair at leading cancer treatment and research institution; member of US cancer advisory board; former president of scientific organization and of medical honor society. Education experience—professor at leading US university.
William T. Winters
Member of the Board of Directors
British/American, age 54
Function at Novartis AG William T. Winters has been a member of the Board of Directors since 2013. He qualifies as an independent Non-Executive Director and is a member of the Compensation Committee.
Other activities Mr. Winters is CEO and a board member of Standard Chartered, based in London. He previously ran Renshaw Bay, an alternative asset management firm, and was co-CEO of JPMorgan's investment bank from 2003 to 2010. Additionally, he was a commissioner on the UK Independent Commission on Banking in 2010 and 2011.
198
Professional background Mr. Winters received his bachelor's degree from Colgate University in the United States, and his Master of Business Administration from the Wharton School of the University of Pennsylvania, also in the US. He joined JPMorgan in 1983 and held management roles across several market areas and in corporate finance. Mr. Winters is a board member of Colgate University, and also serves on the boards of the International Rescue Committee, the Young Vic theater and the Print Room theater in the United Kingdom. He was awarded the title of Commander of the Order of the British Empire in 2013.
Key knowledge/experience Leadership and global experience CEO and executive director of leading international banking group; former chairman and CEO of alternative asset management firm; former co-CEO of investment banking at global financial services firm. Education experience—board member of leading US university.
Honorary Chairmen
Alex Krauer, Ph.D.
Daniel Vasella, M.D.
Corporate Secretary
Charlotte Pamer-Wieser, Ph.D.
Joseph Jimenez
Chief Executive Officer of Novartis
American, age 56
Joseph Jimenez has been Chief Executive Officer (CEO) of Novartis since 2010. Under his leadership, and driven by a commitment to R&D investment, Novartis has developed one of the largest pipelines of self-originated drugs in the industry. Mr. Jimenez has also transformed the company's portfolio to focus on leading businesses with innovation power and global scale in pharmaceuticals, eye care and generics.
Prior to serving as CEO of Novartis, Mr. Jimenez held the position of Division Head, Novartis Pharmaceuticals. He joined Novartis in 2007 as Division Head, Novartis Consumer Health.
Previously, Mr. Jimenez served as president and CEO of the North American and European businesses for the H.J. Heinz Company. Additionally, he served on the board of directors of Colgate-Palmolive Co. from 2009 to 2015, and of AstraZeneca PLC from 2002 to 2007.
Mr. Jimenez is a member of the board of directors of General Motors Co. He graduated in 1982 with a bachelor's degree from Stanford University and in 1984 with a Master of Business Administration from the University of California, Berkeley, both in the United States.
Steven Baert
Head of Human Resources of Novartis
Belgian, age 41
Steven Baert has been Head of Human Resources (HR) of Novartis since February 2014. He is a member of the Executive Committee of Novartis.
Mr. Baert joined Novartis in 2006 as Head of Human Resources Global Functions in Switzerland. He has held several senior HR roles, including Head of Human Resources for Emerging Growth Markets,
199
and Global Head, Human Resources, Oncology. Mr. Baert also served as Head of Human Resources, US and Canada, for Novartis Pharmaceuticals Corporation.
Prior to joining Novartis, Mr. Baert held HR positions at Bristol-Myers Squibb Co. and Unilever.
Mr. Baert represents Novartis on the board of GSK Consumer Healthcare. He holds a Master of Business Administration from the Vlerick Business School in Belgium and a Master in Law from the Katholieke Universiteit Leuven, also in Belgium. Additionally, he has a Bachelor in Law from the Katholieke Universiteit Brussels.
Felix R. Ehrat, Ph.D.
Group General Counsel of Novartis
Swiss, age 58
Felix R. Ehrat, Ph.D., has been Group General Counsel of Novartis since 2011. He is a member of the Executive Committee of Novartis.
Mr. Ehrat is a leading practitioner of corporate, banking, and mergers and acquisitions law, as well as an expert in corporate governance and arbitration. He started his career as an associate with Baer & Karrer Ltd. in Zurich in 1987, became partner in 1992, and advanced to senior partner (2003 to 2011) and executive chairman of the board (2007 to 2011) of the firm. Mr. Ehrat is chairman of Globalance Bank AG in Switzerland, and chairman of SwissHoldings (Federation of Industrial and Service Groups in Switzerland). He is a board member of Geberit AG and avenir suisse (a think tank for economic and social issues). Previously, he was, among other things, chairman and a board member of several listed and non-listed companies.
Mr. Ehrat was admitted to the Zurich bar in 1985 and received his doctorate of law from the University of Zurich in Switzerland in 1990. In 1986, he completed an LL.M. at McGeorge School of Law in the United States. Some of his past memberships include the International Bar Association, where he was co-chair of the Corporate and M&A Law Committee from 2007 to 2008, and Association Internationale des Jeunes Avocats, where he was president from 1998 to 1999.
David Epstein
Division Head, Novartis Pharmaceuticals
American, age 54
David Epstein has been Division Head of Novartis Pharmaceuticals since 2010. He is a member of the Executive Committee of Novartis.
Since taking this role, Mr. Epstein has set a course for Novartis Pharmaceuticals to develop into the world's best pharmaceutical business. He previously served as Head of Novartis Oncology, building the Oncology business from start-up to number two in the world through six new drug approvals and more than 10 indication expansions.
Before joining Novartis, Mr. Epstein was an associate in the strategy practice of the consulting firm Booz Allen Hamilton in the United States. He joined Sandoz, a Novartis predecessor company, in 1989 and held various leadership positions of increasing responsibility, including Chief Operating Officer of Novartis Pharmaceuticals Corporation in the US and Global Head of Novartis Specialty Medicines.
Mr. Epstein received a bachelor's degree in pharmacy, with honors, from the Ernest Mario School of Pharmacy at Rutgers, The State University of New Jersey, in the US in 1984. He received a Master of Business Administration in finance and marketing from New York's Columbia University Graduate School of Business, also in the US, in 1987.
200
Mark C. Fishman, M.D.
President of the Novartis Institutes for BioMedical Research
American, age 64
Mark C. Fishman, M.D., has been President of the Novartis Institutes for BioMedical Research (NIBR) since 2002. He is a member of the Executive Committee of Novartis.
Before joining Novartis in 2002, Dr. Fishman was chief of cardiology and director of the Cardiovascular Research Center at Massachusetts General Hospital, as well as professor of medicine at Harvard Medical School, both in the United States. He completed his internal medicine residency, chief residency and cardiology training at Massachusetts General Hospital.
Dr. Fishman graduated with a bachelor's degree from Yale College in the US in 1972, and with an M.D. from Harvard Medical School in 1976. He has been honored with many awards and distinguished lectureships, and serves on the council of the Institute of Medicine of the National Academies in the US. Additionally, he is a fellow of the American Academy of Arts and Sciences, also in the US.
Richard Francis
Division Head, Sandoz
British, age 47
Richard Francis has been Division Head of Sandoz since May 2014. He is a member of the Executive Committee of Novartis.
Mr. Francis joined Novartis from Biogen Idec, where he held global and country leadership positions during his 13-year career with the company. Most recently, he was senior vice president of the company's US commercial organization. From 1998 to 2001, he was at Sanofi in the United Kingdom, where he held various marketing roles across the company's urology, analgesics and cardiovascular products. He has also held sales and marketing positions at Lorex Synthelabo and Wyeth.
Mr. Francis holds a B.A. in economics from the Manchester Metropolitan University, England.
Jeff George
Division Head, Alcon
American, age 42
Jeff George has been Division Head of Alcon since May 2014. He is a member of the Executive Committee of Novartis.
For more than five years prior to joining Alcon, Mr. George led Sandoz, the generics division of Novartis and the world's second-largest generics company with more than 26,000 associates across 164 countries. Prior to Sandoz, he was Head of Emerging Markets for the Middle East, Africa, Southeast Asia and CIS for Novartis Pharmaceuticals.
Mr. George joined Novartis in 2007 as Head of Commercial Operations for Western and Eastern Europe for Novartis Vaccines. Before joining Novartis, he was senior director of strategic planning and business development at Gap Inc. in San Francisco, United States. Between 2001 and 2004, he worked at McKinsey & Company, also in San Francisco, as an engagement manager.
Mr. George received a Master of Business Administration from Harvard University in the US in 2001. He graduated in 1999 with a master's degree from the Johns Hopkins University's School of Advanced International Studies, also in the US, where he studied international economics and emerging markets political economy. In 1996, he received his bachelor's degree, magna cum laude, in international relations from Carleton College in the US.
201
Harry Kirsch
Chief Financial Officer of Novartis
German, age 50
Harry Kirsch has been Chief Financial Officer (CFO) of Novartis since 2013. He is a member of the Executive Committee of Novartis.
Mr. Kirsch joined Novartis in 2003 and, prior to his current position, served as CFO of the company's Pharmaceuticals Division. Under his leadership, the division's core operating income margin increased, in constant currencies, every quarter of 2011 and 2012 despite patent expirations. At Novartis, he also served as CFO of Pharma Europe, and as Head of Business Planning & Analysis and Financial Operations for the Pharmaceuticals Division. Mr. Kirsch joined Novartis from Procter & Gamble (P&G) in the United States, where he was CFO of P&G's global pharmaceutical business. Prior to that, he held finance positions in different categories of P&G's consumer goods business, technical operations, and Global Business Services organization.
Mr. Kirsch represents Novartis on the board of GSK Consumer Healthcare. He studied industrial engineering and economics at the University of Karlsruhe in Germany ("Diplom-Wirtschaftsingenieur").
André Wyss
Global Head, Novartis Business Services and Country President for Switzerland
Swiss, age 48
André Wyss has been Global Head of Novartis Business Services (NBS) since May 2014. In July 2014, he was also appointed Country President for Switzerland. He is a member of the Executive Committee of Novartis.
Mr. Wyss joined Novartis in 1984 as a chemistry apprentice. Before being appointed Head of NBS, he served as US Country Head and President of Novartis Pharmaceuticals Corporation. Prior to that, he was Head of the Pharmaceuticals Division Region Asia-Pacific, Middle East and African Countries (AMAC). Before leading AMAC, he served as Group Emerging Markets Head, and as Country President and Head of Pharmaceuticals, Greece.
Mr. Wyss received a graduate degree in economics from the School of Economics and Business Administration (HWV) in Switzerland in 1995. He is a member of the board of economiesuisse.
As Chairman of the Compensation Committee of the Board of Directors, I am pleased to share with you the 2015 Compensation Report of Novartis AG.
At Novartis, our mission is to discover new ways to improve and extend people's lives. We use science-based innovation to address some of society's most challenging healthcare issues, discovering and developing breakthrough treatments and finding new ways to deliver them to as many people as possible. Our company also wants to be an employer of choice and to provide superior returns to our shareholders. During the last two years, the Compensation Committee undertook significant work to:
- –
- Better align the executive compensation system with our long-term business strategy and shareholder interests
202
- –
- Strengthen the corporate governance framework
- –
- Implement all elements of the Minder Ordinance to Board and executive compensation
The Compensation Committee would like to acknowledge the strong shareholder support at the 2015 Annual General Meeting (AGM) for all of the remuneration-related resolutions, and express appreciation for the opportunity to engage many of our shareholders on compensation topics in 2015. The Compensation Committee would also like to thank Dr. Ulrich Lehner for his services on the Compensation Committee and welcome William Winters as a new member.
2015 company performance
In 2015, Novartis progressed in all of its key priorities. The company completed its portfolio transformation ahead of schedule, achieved major innovation milestones with Entresto, Cosentyx and biosimilars, captured cross-divisional synergies with the creation of the Novartis Business Services unit and continued to build a high-performing organization. Currencies had a very negative impact on our reported results in US dollars as the US dollar strengthened significantly vs. all major currencies in 2015. Operationally, in constant currencies, the company was marginally below its sales target but slightly above its net income and free cash flow targets. Pharmaceuticals and Sandoz delivered strong performances, while Alcon negatively impacted consolidated results. The company improved core margin despite the currency impact. Although, in US dollars, Novartis' TSR was –3.5% in 2015, TSR was +53.4% for the period 2013-2015, corresponding to the usual three-year cycle of our long-term plans.
2015 CEO compensation
For 2015, our CEO was awarded total compensation of CHF 11,596,560. This amount included an Annual Incentive of CHF 3,090,758 (representing 100% of target) based on a combination of his and our company's performance, as summarized above. Half of the Annual Incentive was delivered in cash, and the remaining half was delivered in restricted share units, which will have a three-year vesting period. His total compensation also included Long-Term Incentive grants with a target value of CHF 6,181,580, which will be subject to performance conditions for the 2015-2017 cycle.
Compensation systems
While the Compensation Committee continued to evaluate the effectiveness of our compensation program, 2015 was a year of stability and refinement of our existing compensation systems following major changes to the Swiss and international regulatory environment. During 2015, the Compensation Committee made only small changes to further align compensation to long-term business strategy and shareholder interests for all associates of Novartis. With effect from 2016, the new compensation system for Executive Committee members will be rolled out to all key executives. Our company has also embedded our Values and Behaviors in the talent framework and ensured that our rigorous performance management process is upheld at all levels of the organization. The new program has the full support of our Board of Directors. We believe that it provides a competitive advantage to Novartis in the marketplace for executive talent.
2016 AGM
The Compensation Committee is committed to continued engagement between shareholders and our company to fully understand diverse viewpoints and discuss the important connections between our company's compensation program, business strategy, and long-term financial and operating performance. As was the case last year and in line with our Articles of Incorporation, shareholders will be asked to approve the following:
- –
- Total maximum amount of Board compensation from the 2016 AGM to the 2017 AGM
203
- –
- Total maximum amount of Executive Committee compensation for the 2017 financial year
Shareholders will also be asked to endorse this Compensation Report in an advisory vote.
On behalf of Novartis and the Compensation Committee, I would like to thank you for your continued support and feedback, which I consider extremely valuable in driving improvements in our compensation systems and practices. I invite you to send your comments to me at the following email address: investor.relations@novartis.com.
Respectfully,
Enrico Vanni, Ph.D.
Chairman of the Compensation Committee
204
COMPENSATION REPORT AT A GLANCE
Executive Committee compensation
2015 Executive Committee Compensation System (see "—2015 Executive Committee Compensation System" below)
The following components are included:
| |
Fixed compensation and benefits |
Variable compensation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Annual base compensation |
Pension and other benefits |
Annual Incentive | Long-Term Performance Plan (LTPP) |
Long-Term Relative Performance Plan (LTRPP) |
|||||
Purpose |
Reflects associates' responsibilities, job characteristics, experience and skill sets | Establish a level of security for associates and their dependents tailored to local market practices and regulations | Rewards performance against key short-term targets and Values & Behaviors | Rewards long-term shareholder value creation and long-term innovation | Rewards relative total shareholder return | |||||
Performance period |
n/a | n/a | 1 year (2015) | 3 years (2015-2017) | 3 years (2015-2017) | |||||
Performance measures |
n/a | n/a | Based on a payout matrix made up of: —Individual balanced scorecard, including financial targets and individual objectives —Assessed Values and Behaviors |
Based on: —75% Novartis Cash Value Added —25% divisional long-term innovation milestones |
Based on Novartis relative total shareholder Return vs. versus our peer group of 12 healthcare companies(1) | |||||
Delivery (at the end of the performance period for variable compensation) |
Cash | Country specific | 50% cash 50% deferred equity(2) (3-year holding of restricted shares/ restricted share units) |
Equity (includes dividend equivalents) | Equity (includes dividend equivalents) | |||||
- (1)
- The
companies in our peer group consist of Abbott, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co.,
GlaxoSmithKline, Johnson & Johnson, Merck & Co., Pfizer, Roche and Sanofi.
- (2)
- Executive Committee members may elect to receive more of their Annual Incentive in shares instead of cash.
205
| |
Fixed compensation and benefits |
Variable compensation | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Annual base compensation |
Pension and other benefits |
Annual Incentive | Long-Term Performance Plan (LTPP) |
Long-Term Relative Performance Plan (LTRPP) |
Total variable compensation |
||||||
CEO variable opportunity as % of base salary |
n/a | n/a | Target: 150% (range 0-200% of target) | Target: 200% (range 0-200% of target) | Target: 100% (range 0-200% of target) | Target: 450% (range 0-200% of target) | ||||||
Executive Committee variable opportunity as % of base salary (excluding CEO) |
n/a |
n/a |
Target: 90%-120% (range 0-200% of target) |
Target: 140%-190% (range 0-200% of target) |
Target: 30%-90% (range 0-200% of target) |
Target: 260%-400% (range 0-200% of target) |
||||||
2015 Executive Committee Compensation (see "—2015 Executive Committee Compensation" below)
Amounts paid or granted during the 2015 financial year:
| |
Fixed compensation and benefits |
Variable compensation | |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Annual base compensation |
Pension and other benefits |
Annual Incentive | Long-Term Performance Plan (LTPP) |
Long-Term Relative Performance Plan (LTRPP) |
Total variable compensation |
|||||||||||||
(CHF) |
|||||||||||||||||||
Purpose |
|||||||||||||||||||
CEO compensation |
2,060,500 | 263,721 | 3,090,758 | 4,121,054 | (1) | 2,060,527 | (1) | 11,596,560 | |||||||||||
Executive Committee compensation (excluding CEO) |
7,429,769 | 5,071,392 | 11,230,142 | 11,973,697 | (1) | 4,652,661 | (1) | 40,357,661 | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total |
9,490,269 | 5,335,113 | (2) | 14,320,900 | 16,094,751 | 6,713,188 | 51,954,221 | (2) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
- (1)
- The
amounts shown in these columns represent the underlying share value of the grant date target value of the number of Performance Share
Units granted to each Executive Committee member for the performance cycle 2015-2017.
- (2)
- It includes an amount of CHF 58,757 for mandatory employer contributions paid by Novartis to governmental social security systems. This amount is out of total employer contributions of CHF 3,457,097, and provides a right to the maximum future insured government pension benefit for the Executive Committee member.
2016 Executive Committee Compensation System
Compensation opportunity
As for all associates, Executive Committee members may have received a merit increase, based on their 2015 performance, and/or an adjustment to benchmark.
206
Performance measures
Annual Incentive
No changes have been made to the performance measures under the Annual Incentive.
Long-Term Incentives
No changes have been made to the performance measures under either the Long-Term Performance Plan or the Long-Term Relative Performance Plan.
Board compensation
2015 Board Compensation System (see "—2015 Board Compensation System" below)
Delivery: 50% cash, 50% shares
| |
Annual fee | |||
|---|---|---|---|---|
(CHF) |
||||
Chairman of the Board |
3,800,000 | (1) | ||
Board membership |
300,000 | |||
Vice Chairman |
50,000 | |||
Chairman of Audit and Compliance Committee |
120,000 | |||
Chairman of the following committees: |
||||
—Compensation Committee |
||||
—Governance, Nomination and Corporate Responsibilities Committee |
||||
—Research & Development Committee(2) |
||||
—Risk Committee |
60,000 | |||
Membership of Audit and Compliance Committee |
60,000 | |||
Membership of the following committees: |
||||
—Compensation Committee |
||||
—Governance, Nomination and Corporate Responsibilities Committee |
||||
—Research & Development Committee |
||||
—Risk Committee |
30,000 | |||
- (1)
- The
Chairman also received company pension contributions until the 2015 AGM (when they ceased), and payment for loss of other entitlements
with his previous employer for a total value of EUR 2,665,051 staggered over the period from 2014 to 2016.
- (2)
- The Chairman receives no additional committee fees for chairing the Research & Development Committee.
2015 Board Compensation (see "—2015 Board Compensation" below)
Amounts earned during the 2015 financial year
| |
Cash | Equity | Other benefits(1) |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(CHF) |
|||||||||||||
Chairman Dr. Joerg Reinhardt |
1,900,000 | 1,900,000 | 29,197 | 3,829,197 | |||||||||
Other Board members |
1,601,417 | 2,331,917 | 17,145 | 3,950,479 | |||||||||
| | | | | | | | | | | | | | |
Total |
3,501,417 | 4,231,917 | 46,342 | 7,779,676 | (2) | ||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- (1)
- It includes an amount of CHF 21,502 for mandatory employer contributions paid by Novartis to Swiss governmental social security systems. This amount is out of total employer contributions of CHF 429,806, and provides a right to the maximum
207
future insured government pension benefit for the Board member. No occupational pension contributions have been provided to the Chairman from the 2015 AGM onwards.
- (2)
- Please see "—2015 Board Compensation—Reconciliation Between the Reported Board Compensation and the Amount Approved by Shareholders at the AGM" for a reconciliation between the amount reported in this table and the amount approved by shareholders at the 2015 AGM to be used to compensate Board members for the period from the 2015 AGM to the 2016 AGM. The amount paid is within the maximum amount approved by shareholders.
2016 Board Compensation System
The Board compensation system will remain unchanged in 2016.
Compensation governance
Governance and risk management (see "—Compensation Governance" below)
Decision-making authorities with regard to compensation, within the parameters set by the shareholders' meeting
Decision on
|
Authority | |
|---|---|---|
| Compensation of Chairman and other Board members | Board of Directors | |
| Compensation of CEO | Board of Directors | |
| Compensation of Executive Committee members | Compensation Committee |
- –
- Rigorous performance management process
- –
- Balanced mix of short-term and long-term variable compensation elements
- –
- Matrix approach to performance evaluation under the Annual Incentive, including an individual balanced scorecard and assessed Novartis
Values and Behaviors
- –
- Performance-vesting Long-Term Incentives only, with three-year overlapping cycles
- –
- All variable compensation is capped at 200% of target
- –
- Contractual notice period of 12 months
- –
- Post-contractual non-compete limited to a maximum of 12 months (annual base compensation and Annual Incentive of the prior year
only)
- –
- No severance payments or change-of-control clauses
- –
- Clawback principles apply to all elements of variable compensation
- –
- Share ownership requirements; no hedging or pledging of Novartis share ownership position by Board and Executive Committee members
Executive Committee compensation risk management principles
208
EXECUTIVE COMMITTEE COMPENSATION PHILOSOPHY AND PRINCIPLES
Novartis Compensation Philosophy
The compensation philosophy aims to ensure that the Executive Committee is rewarded according to its success in implementing the company strategy and to its contribution to company performance. The Executive Committee compensation system is designed in line with the following key elements:
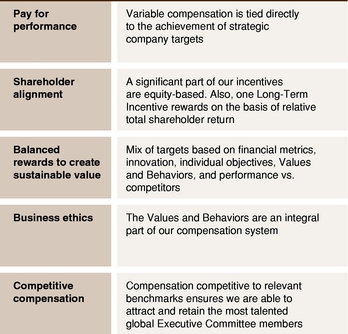
Alignment With Company Strategy
The Novartis strategy is to use science-based innovation to deliver better patient outcomes. We aim to lead in growing areas of healthcare. To align the compensation system with this strategy, the Board of Directors determines specific, measurable and time-bound performance metrics, including financial metrics such as sales, profit and cash flow, as well as non-financial metrics, which indicate the success of its implementation. The Board of Directors then sets short-term and long-term targets for each of these performance metrics and compensates the Executive Committee according to the extent to which the targets are achieved. In line with the company's focus on science-based innovation, the Board of Directors sets a number of specific targets for each division to fulfill within specific timeframes. In line with the company's aim to lead in growing areas of healthcare, Novartis has focused its portfolio to have three market-leading divisions in innovative pharmaceuticals, eye care and generics. Finally, to ensure that Novartis is a high-performing organization over the long term, the Board of Directors also sets targets in areas such as quality, talent, integrity and reputation, which are reinforced by the Novartis Values and Behaviors.
Executive Committee Compensation Benchmarking
To attract and retain key talent, it is important for us to offer competitive compensation opportunities. Executives meeting their objectives are generally awarded target compensation at a level comparable to the median level of similar roles within the benchmark companies (see "—Benchmark Companies" below). In the event of under- or over-performance, the actual compensation may be lower or higher than the benchmark median.
209
While benchmarking information regarding executive pay is considered by the Compensation Committee, any decisions on compensation are ultimately based on the specific business needs of Novartis and the performance of the individual.
The Compensation Committee reviews the compensation of the CEO and Executive Committee members annually in comparison to the relevant compensation levels of similar positions at peer companies. For this purpose, the Compensation Committee uses benchmark data from publicly available sources, as well as reputable market data providers. All data is reviewed and evaluated by the Compensation Committee's independent advisor, who also provides independent research and advice regarding the compensation of the CEO and other Executive Committee members.
For the CEO and Executive Committee members, the company benchmarks against global competitors in the healthcare industry with similar business models, size and needs for talent and skills. The Compensation Committee reviews the companies in our compensation peer group annually and considers adjustments over time in line with the evolution of the competitive environment in the healthcare industry.

Within this peer group, Novartis is among the largest in key dimensions including market capitalization, sales and operating income.
2015 EXECUTIVE COMMITTEE COMPENSATION SYSTEM
The 2015 Executive Committee compensation system consists of the following components:

Fixed Compensation And Benefits
Annual Base Compensation
The level of base compensation reflects each associate's key responsibilities, job characteristics, experience and skill sets. It is paid in cash, typically monthly.
Base compensation is reviewed annually, and any increase reflects merit based on performance, as well as market movements.
Pension and Other Benefits
The primary purpose of pension and insurance plans is to establish a level of security for associates and their dependents with respect to age, health, disability and death. The level and scope of pension and insurance benefits provided are country-specific, influenced by local market practices and regulations.
210
Company policy is to change from defined-benefit pension plans to defined-contribution pension plans. All major plans have now been aligned with this policy as far as reasonably practicable. See also "Item 18. Financial Statements—Note 25."
Novartis may provide other benefits in a specific country according to local market practices and regulations, such as a company car, and tax and financial planning services. Executive Committee members who have been transferred on an international assignment also receive benefits (such as tax equalization) in line with the company's international assignment policies.
Annual Incentive
For the Annual Incentive of the CEO and Executive Committee members, a target incentive is defined as a percentage of base compensation at the beginning of each performance year. The target incentive is 150% of base compensation for the CEO, and ranges from 90% to 120% for other Executive Committee members. It is paid half in cash and half in shares deferred for three years. The formula for the target Annual Incentive is outlined below:

Performance measures
The Annual Incentive is based on a payout matrix made up of two elements: a balanced scorecard and the Novartis Values and Behaviors, which are described in more detail below.
Balanced scorecard
The first element used to determine the payout of the Annual Incentive is a balanced scorecard within which Group or divisional financial targets are weighted 60% and individual objectives are weighted 40%. As reported last year, as of 2015, innovation was removed from the Group financial targets of the Annual Incentive and instead included in the Long-Term Performance Plan, as the Compensation Committee's view is that innovation achievements are more effectively measured on a multiyear basis. For more details on the target-setting and performance management process, please refer to "—Executive Committee Performance Management Process."
Group or divisional financial targets
Within the Group or divisional financial targets, each measure such as sales or net income is weighted individually. The CEO and function heads share the same Group financial targets (described further below). In place of the Group targets, division heads have divisional targets that include divisional sales, operating income, free cash flow as a percentage of sales, and market share of peers. The Board of Directors sets the Group and divisional financial targets at the start of each performance year in constant currencies, and evaluates achievement against these targets at the end of that year.
Individual objectives
Individual objectives differ for each Executive Committee member depending on his responsibilities, and may include additional financial and non-financial targets. Examples of additional financial targets are implementation of growth, productivity and development initiatives. Non-financial targets may include
211
leadership and people management, workforce diversity, quality, social initiatives such as access to medicines, and ethical business practices.
By way of illustration, the balanced scorecard measures used for the CEO in 2015 are set out in the following table:
2015 BALANCED SCORECARD MEASURES USED FOR THE CEO
Performance measures
|
Weight | Breakdown of performance measures | |||
|---|---|---|---|---|---|
Group financial targets |
60 | % | Group net sales Corporate net result Group net income Group free cash flow as % of sales |
||
CEO individual objectives |
40 | % | Additional financial targets (e.g., EPS) Innovation and growth Portfolio review Cross-divisional synergies High-performing organization |
||
Overall total |
100 | % | |||
| | | | | | |
| | | | | | |
| | | | | | |
Novartis Values and Behaviors
The second element used to determine the payout of the Annual Incentive ensures that the associate's performance is achieved in line with the highest standards of business conduct, as outlined in the Novartis Values and Behaviors. Novartis requires Executive Committee members to be action-oriented and full of energy to face challenging situations, to assign the highest priority to customer satisfaction, and to commit to honesty in every facet of behavior, demonstrating strong ethical and legal conduct. Novartis leaders are expected to live up to these behaviors on a daily basis, and to align and energize other associates to do the same. Novartis Values and Behaviors are an essential element in the annual assessment of Executive Committee members. For more details on the performance assessment process of the Novartis Values and Behaviors, please refer to "—Executive Committee Performance Management Process—Assessment of Values and Behaviors at Novartis."
Performance evaluation and payout determination
Following a thorough review of the two elements that compose the Annual Incentive—performance against the balanced scorecard objectives and an assessment against the Novartis Values and Behaviors—a rating from 1 to 3 is assigned to each.
The following payout matrix shows how the Annual Incentive performance factor is derived using a combination of performance against the balanced scorecard and demonstration of the Novartis Values and Behaviors. The Compensation Committee determines the final payout factor for Executive Committee members taking into account the ranges shown. Payouts are capped at 200% of target.
212
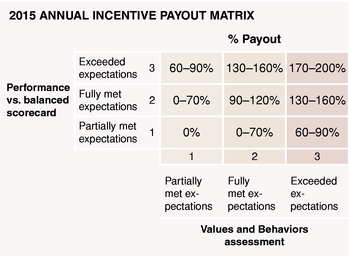
The payout matrix for the Annual Incentive equally recognizes performance against the objectives in the balanced scorecard, and the assessment against the Novartis Values and Behaviors.
Form and delivery of the award
The Annual Incentive is paid 50% in cash in March of the year following the performance period, and 50% in Novartis shares (or restricted share units, known as RSUs) that are deferred and restricted for three years. Each restricted share is entitled to voting rights and payment of dividends during the vesting period. Each RSU is equivalent in value to one Novartis share and is converted into one share at the vesting date. RSUs under this plan do not carry any dividend, dividend equivalent or voting rights. Following the vesting period, settlement is made in unrestricted Novartis shares or American Depositary Receipts (ADRs).
If a participant leaves Novartis due to voluntary resignation or misconduct, unvested shares (and RSUs) are forfeited. The Board of Directors and the Compensation Committee retain accountability for ensuring that rules are applied correctly, and for determining whether a different treatment should apply in exceptional circumstances. This is necessary to ensure that the treatment of any award in the event of cessation of employment is appropriate.
Executives may choose to receive some or all of the cash portion of their Annual Incentive in Novartis shares or ADRs (US only) that will not be subject to conditions. In the US, awards may also be delivered in cash under the US-deferred compensation plan.
Long-Term incentives
Novartis operates two Long-Term Incentives (the Long-Term Performance Plan and the Long-Term Relative Performance Plan) for the Executive Committee members, which function in an identical way except for the performance conditions applied.
213
Grant of Long-Term Incentives
At the beginning of every performance period, Executive Committee members are granted a target number of performance share units (PSUs) under each of the Long-Term Incentives according to the following formula:

Vesting of Long-Term Incentives
At the end of the three-year performance period, the Compensation Committee adjusts the number of PSUs realized based on actual performance against target.

The performance factor can range from 0% to 200% of target. Each realized PSU is converted into one Novartis share at the vesting date. PSUs do not carry voting rights, but do carry dividend equivalents that are reinvested in additional PSUs and paid at vesting to the extent that performance conditions have been met. In the US, awards may also be delivered in cash under the US-deferred compensation plan.
If a participant leaves Novartis due to voluntary resignation or termination by the company for misconduct, none of the awards vest. When a member is terminated by the company for reasons other than for performance or conduct, the award vests on a pro-rata basis for time spent with the company during the performance period. In such a case, the award will vest on the regular vesting date (no acceleration), will be subject to performance should an evaluation be possible, and will also be subject to other conditions such as observing the conditions of a non-compete agreement. Executives leaving Novartis due to approved retirement, including approved early retirement, death or disability, will receive full vesting of their award on the normal vesting date (acceleration will only apply in the case of death). The award will be subject to performance, should an evaluation be possible, and will also be subject to other conditions such as observing the conditions of a non-compete agreement. Further details can be found in "Item 18. Financial Statements—Note 26."
The Board of Directors and the Compensation Committee retain accountability for ensuring that rules are applied correctly, and for determining whether different treatment should apply in exceptional circumstances. This is necessary to ensure that the treatment of any award in the event of cessation of employment is appropriate.
Long-Term Performance Plan (LTPP)
This is the first of the two Long-Term Incentive plans.
Overview
The LTPP, as described below, was granted for the first time to the CEO and Executive Committee members in 2014. The target incentive is 200% of base compensation for the CEO, and ranges from 140%
214
to 190% for other Executive Committee members. Additional executives in key positions who have a significant impact on the long-term success of Novartis were invited to participate in the LTPP, as of 2015.
In the 2013 and earlier Compensation Reports, there was a different plan that was also called LTPP. In this Compensation Report (as in the 2014 Compensation Report), that plan has been renamed Old Long-Term Performance Plan (OLTPP), and is described under "—Performance Vesting of Old Long-Term Performance Plan (2013-2015)."
Performance measures
Awards under the LTPP are based on three-year performance objectives and split as follows:
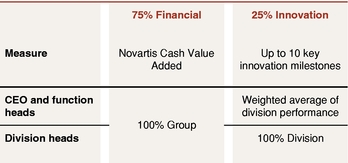
Financial measure (Novartis Cash Value Added): 75% of LTPP
The Novartis Cash Value Added (NCVA) is a metric that incentivizes both sales growth and margin improvement as well as asset efficiency. A summary of the calculation is below:

The NCVA targets are determined considering expected growth rates in sales, operating income and return from invested capital, under foreseen economic circumstances.
At the end of the performance cycle, the NCVA performance factor is calculated in constant currencies. The NCVA performance factor is based on a 1:3 payout curve, where a 1% deviation in realization versus target leads to a 3% change in payout (for example, a realization of 105% leads to a payout factor of 115%). If performance over the three-year vesting period falls below 67% of target, no payout is made for this portion of LTPP. If performance over the three-year vesting period is above 133% of target, payout for this portion of LTPP is capped at 200% of target.
The calculated performance realization is adjusted for unplanned major events during the cycle (e.g., significant merger and acquisition transactions).
215
Innovation measure: 25% of LTPP
Innovation is a key element of the Novartis strategy. Divisional innovation targets are set at the beginning of the performance cycle, comprised of up to 10 target milestones that represent the most important research and development project milestones for each division. These milestones are chosen because of the expected future impact to Novartis in terms of potential revenue, or due to their qualitative potential impact to science, medicine, and the treatment or care of patients.
A payout matrix has been established for this metric that allows a 0-150% payout for the achievement of target milestones. If all target milestones are achieved, a 150-200% payout may be awarded for extraordinary additional achievement. The CEO and function heads receive the weighted average of divisional innovation payouts.
The Research & Development Committee assists the Board of Directors and the Compensation Committee in setting the innovation targets and reviewing achievements at the end of the cycle.
Long-Term Relative Performance Plan (LTRPP)
This is the second of the two Long-Term Incentive plans.
Overview
The LTRPP was granted for the first time to the CEO and Executive Committee members in 2014. The target incentive is 100% of base compensation for the CEO, and ranges from 30% to 90% for other Executive Committee members.
Performance measure
The LTRPP is based on the achievement of long-term relative Group total shareholder return (TSR) versus the peer group of 12 companies in the healthcare industry over rolling three-year performance periods. TSR is calculated in US dollars as share price growth plus dividends over the three-year performance period. The calculation will be based on Bloomberg standard published TSR data, which is publicly available.
The peer group for the 2015-2017 performance cycle is the same as for benchmarking the compensation of Executive Committee members and is comprised of: Abbott, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., GlaxoSmithKline, Johnson & Johnson, Merck & Co., Pfizer, Roche and Sanofi.
At the end of the performance period, all companies are ranked in order of highest to lowest TSR, and the position in the peer group determines the payout range as follows:
PAYOUT MATRIX Position in peer group |
Payout range | |
|---|---|---|
Positions 1-3 |
160-200% | |
Positions 4-6 |
100-140% | |
Positions 7-10 |
20-80% | |
Positions 11-13 |
0% | |
| | | |
The Compensation Committee determines the payout within the ranges shown, and takes into consideration factors such as absolute TSR, overall economic conditions, currency fluctuations and other unforeseeable situations.
216
Target Disclosure
In line with our principle to allow shareholders to assess the relationship between company performance and pay, the financial, innovation and individual targets under the Annual Incentive plan and the LTPP will be disclosed in the Compensation Report with the achievements against such targets at the end of each performance cycle. Targets under the Annual Incentive plan and the LTPP are considered confidential at the time of setting. Communicating such targets before the end of the performance cycle would allow substantial insight into the company's forward-looking strategies and could therefore place the company at a competitive disadvantage.
2016 Executive Committee compensation system
The Compensation Committee has evaluated the Executive Committee compensation system and has decided that it will remain unchanged in 2016. The Compensation Committee believes that it is operating as intended, supports the company's strategy, and is aligned with market and best practice.
EXECUTIVE COMMITTEE PERFORMANCE MANAGEMENT PROCESS
To foster a high-performance culture, the company applies a uniform performance management process worldwide based on quantitative and qualitative criteria, including Novartis Values and Behaviors. Novartis associates, including the CEO and Executive Committee members, are subject to a three-step formal process:
![]()
At the beginning of the year, the CEO presents the Group and divisional financial and innovation targets of our variable compensation plans to both the Compensation Committee and the Board of Directors for approval. At the same time, the CEO discusses his individual objectives for the coming year with the Chairman of the Board of Directors.
The Board of Directors reviews and approves these objectives, which are incorporated into the Annual Incentive and Long-Term Incentive plans.
Annual Incentive
The Group financial and individual targets proposed by the CEO are challenged and approved by both the Compensation Committee and the Board of Directors. The targets set for the Annual Incentive support our ambition to be a leader in the healthcare industry.
Financial and innovation measure of LTPP
The NCVA target is based on the company's long-range strategic plan approved by the Board of Directors to deliver long-term sustainable growth and productivity as well as efficient use of its assets. The Compensation Committee believes that the NCVA target is ambitiously set to create long-term value for shareholders.
The innovation targets of the LTPP are largely aligned with the major development projects outlined in "Item. 4 Information on the Company—Item 4.B Business Overview—Pharmaceuticals—Selected Development Projects," "Item. 4 Information on the Company—Item 4.B Business Overview—Alcon—Selected Development Projects," and "Item. 4 Information on the Company—Item 4.B Business Overview—Sandoz—Biosimilars in Phase III Development and Registration." The targets are
217
recommended by the divisions and reviewed by the Research & Development Committee. The innovation targets are focused on challenging milestones of critical importance to the long-term success of the business, and should be best- or first-in-class development projects that can significantly advance treatment outcomes for patients worldwide.
Relative TSR: 100% of LTRPP
The payout matrix for the LTRPP can be found in "—2015 Executive Committee Compensation System—Variable Compensation—Long-Term Incentives—Long-Term Relative Performance Plan (LTRPP)." The Compensation Committee believes that the LTRPP payout matrix is aligned with the company's pay-for-performance principle, including a very significant reduction in the actual payout relative to target payout if the company's TSR is below the median of the peer group.
The Board of Directors periodically assesses Group business performance as well as progress of the CEO against his objectives and incentive plan targets. At the mid-year performance review, the performance of the CEO is reviewed by the Chairman of the Board of Directors.
For the year-end review, the CEO prepares and presents to the Chairman of the Board of Directors, and later to the full Board of Directors, the actual results against the previously agreed-upon objectives, taking into account the audited financial results as well as an assessment against the Novartis Values and Behaviors. At the year-end review, the Board of Directors discusses the performance of the CEO without him being present. It evaluates the extent to which targeted objectives have been achieved and, to the extent possible, compares these results with peer industry companies, taking into account general economic and financial criteria and industry developments. The Board of Directors later shares its assessment with the CEO.
CEO Compensation determination
At its January meeting, following a recommendation from the Compensation Committee, the Board of Directors decides on the CEO's variable compensation for the prior performance cycles and on the target compensation for the coming year. This meeting takes place without the CEO being present. The Board of Directors later shares its decisions with the CEO.
Performance management process for other Executive Committee members (excluding the CEO)
Executive Committee members propose the divisional financial and innovation targets for approval by the CEO and, subsequently, by the Board of Directors and Compensation Committee. In addition, each Executive Committee member agrees on individual objectives with the CEO, who also reviews members' performance at mid-year and year-end.
At year-end, following his evaluation, the CEO meets with the Chairman of the Board of Directors, who reviews the performance of Executive Committee members. Subsequently, the CEO presents and discusses at the Board of Directors meeting his recommended performance rating for each member.
Later, in the presence of the CEO and taking into consideration the recommendations of the Board of Directors, the Compensation Committee decides at its January meeting on the variable compensation of Executive Committee members for the prior year and on their target compensation for the coming year. The Compensation Committee informs the Board of Directors of its final decisions, and the CEO later shares these decisions with Executive Committee members.
218
Assessment of Values and Behaviors at Novartis
Values and Behaviors have been an integral part of the company's compensation system since its foundation. In 2015, to reinforce the culture of the company, Novartis rolled out new Values and Behaviors—which are innovation, quality, collaboration, performance, courage and integrity.
What we value
|
Observed behaviors | |
|---|---|---|
Innovation by experimenting and delivering solutions |
—Experiments and encourages others to do so | |
|
—Takes smart risks that benefit patients and customers | |
|
—Delivers new solutions with speed and simplicity | |
Quality by taking pride in doing ordinary things extraordinarily well |
—Is always looking for better ways to do things |
|
|
—Does not compromise on quality and safety, and strives for excellence | |
|
—Continuously works to improve own strengths and weaknesses | |
Collaboration by championing high-performing teams with diversity and inclusion |
—Champions working together in high-performing teams |
|
|
—Knows self and impact on others | |
|
—Welcomes diversity and inclusion of styles, ideas and perspectives | |
Performance by prioritizing and making things happen with urgency |
—Is passionate to achieve goals and goes the extra mile |
|
|
—Puts team results before own success and acknowledges contributions of others | |
|
—Prioritizes, decides and makes things happen with urgency | |
Courage by speaking up, giving and receiving feedback |
—Speaks up and challenges the norm |
|
|
—Acknowledges when things don't work and learns | |
|
—Gives and accepts constructive feedback | |
Integrity by advocating and applying high ethical standards every day |
— Operates with high ethical standards |
|
|
—Is humble and caring, and shows trust, respect and empathy | |
|
—Lives by the Code of Conduct even when facing resistance or difficulties |
These values are embedded in all aspects of employees' lives at Novartis, from recruitment and development to promotions, performance assessments through 360-degree evaluations and organizational employee surveys, as well as Annual Incentive awards to measure individual and organizational performance against our values. As part of the Annual Incentive award process, training programs and toolkits were established to evaluate behavior related to the six new values. They are one of the elements used to assess associates' performance.
219
During 2015, we further improved the framework for measuring individual performance against our values, ensuring that fair, objective assessments can be made in a uniform way across all levels of the organization. The assessment is part of a rigorous management process review in which observed Values and Behaviors are evaluated based on globally-defined principles. The assessment initially takes place during a discussion between associates and line managers, followed by a calibration and validation at multiple levels of the organization to allow for a fair, consistent, objective and transparent evaluation. During the calibration sessions, line managers share the proposed ratings of their direct reports with peers to ensure all apply a common framework, and they seek input and feedback on observed behaviors.
The Values and Behaviors assessment for the CEO and other Executive Committee members is calibrated by the Board of Directors.
2015 EXECUTIVE COMMITTEE COMPENSATION
The 2015 compensation of the CEO is outlined in detail within this section:
Base salary: The CEO's base salary remained CHF 2,060,500 for 2015.
Benefits: The CEO received pension benefits of CHF 175,289 and other benefits of CHF 88,432 during 2015.
Annual Incentive: The Annual Incentive performance is measured in constant currencies to reflect the operational performance that can be influenced. Overall, the company met most of its financial targets for the year set by the Board of Directors in constant currencies. Group results were negatively impacted by Alcon's performance and by the slow-down of emerging markets, offset by strong results from Pharmaceuticals and Sandoz. The Group was marginally behind its sales target, while Group net income was slightly ahead of target mainly due to strong cost management. Corporate net result was significantly ahead of target mainly due to lower corporate costs and taxes. Performance in Group free cash flow as a percentage of sales was slightly above target mainly due to higher cash flows from operating activities.
Currency movements had a significant negative impact on the reported results vs. target (in US dollars, sales: –5.2 billion, net income and free cash flow (FCF): –1.6 billion each) that were adjusted in the Annual Incentive calculation.
Group financial targets (60%) |
Performance metrics for continuing operations (weight) | Target(1) | Achievement vs. target(2) (in constant currencies) |
|||
|---|---|---|---|---|---|---|
| Group net sales (30%) | $55,289 m | Slightly below | ||||
| Corporate net result(3) (20%) | $–2,284 m | Significantly exceeded | ||||
| Group net income (30%) | $8,996 m | Slightly exceeded | ||||
| Group FCF as % of sales (20%) | 20.5% | Slightly exceeded | ||||
| Overall achievement for Group financial targets | Slightly above target | |||||
| Individual objectives (40%) |
Additional key financial targets for continuing operations Additional financial targets were not all met. Including adjustments, in constant currencies, core operating income, EPS and core EPS targets were met, while reported operating income was slightly missed. Emerging Market growth and Divisional share of peers (Pharmaceuticals, Alcon and Sandoz) were below target (for the latter mainly due to currency impact). |
Slightly below | ||||
| Continued | ||||||
220
| Innovation and growth | Exceeded | |||||
| 2015 was another excellent year for innovation and growth. The company successfully achieved 20 major approvals and 14 major submissions. Novartis had the highest number of FDA approvals(4) in the industry (4 out of 45 novel drugs). Major innovation milestones were achieved in 2015 with Entresto (approved in the EU), Cosentyx (approved for AS and PsA in EU) and submission of biosimilars etanercept and pegfilgrastim. Zarxio was the first biosimilar approved under the BPCIA pathway. Sandoz also received US approval of Glatopa. The NIBR unit launched a new immuno-oncology research team that delivered significant progress in building a portfolio with several candidates already in clinical trials and more expected to enter the clinic by the end of 2016. | ||||||
| Individual objectives (40%) |
|
|||||
|---|---|---|---|---|---|---|
| Individual objectives (40%) |
Portfolio review With the announcement on March 2, 2015 of the completion of the transactions with GSK, and the announcement on July 31, 2015 of the divestment of the Vaccines influenza business to CSL, Novartis successfully completed its portfolio review ahead of schedule (target for completion: H2 2015). A total of 17,000 associates transferred from Novartis to GSK and CSL. The completion of the portfolio review has improved Novartis' competitive position resulting in a more focused company with leading positions in innovative pharmaceuticals, generics and eye care. |
Slightly exceeded | ||||
|
Cross-divisional synergies Novartis Business Services, our shared services organization, continued to execute on its priorities and the transformation of the organization is developing as scheduled. The company generated approximately $3,216 million in total productivity gains (target: $2,746 million) by leveraging our scale. In 2015 we announced plans to close or divest 6 sites. All of these actions increased the productivity of the company. |
Exceeded | |||||
| High-performing organization (e.g., quality, talent) | At target | |||||
| Across the Novartis network, for the full year, there were 192 inspections, including 31 conducted by the FDA. 189 of the 192 inspections in the full year were good or satisfactory. The outcomes of three inspections are still pending. In addition, the company continued to roll out the process of upgrading its compliance and integrity processes as well as Novartis Values and Behaviors. A new talent management strategy was established and some progress was made on the talent pipeline and talent management initiatives. The company was disappointed with certain compliance and reputational challenges. | ||||||
| Overall achievement for individual objectives | At target | |||||
- (1)
- The
target was set using July 2014 forward currency exchange rates
- (2)
- Adjusted
for significant currency movements (in US dollars, sales: –5.2 billion, net income and FCF:
–1.6 billion each) and other adjustments including the changes in income from associated companies
- (3)
- Includes
corporate cost, income from associated companies, net financial income and income taxes
- (4)
- Source: FDA's Center for Drug Evaluation and Research's (CDER's) 2015 annual report
221
Following a thorough performance evaluation, including assessed Values and Behaviors (see "—Executive Committee Performance Management Process—Assessment of Values and Behaviors at Novartis" for further details of the performance management process and assessment of Values and Behaviors), the Compensation Committee determined that the CEO's Annual Incentive performance factor would be 100%. The value of his Annual Incentive award was determined as follows:
| |
Annual base salary CHF thousands |
x |
Target incentive % |
x |
Performance factor % |
= |
Final award CHF thousands |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Annual Incentive |
2,061 | x | 150 | % | x | 100 | % | = | 3,091 | (1) | |||||||||
- (1)
- 50% of the Annual Incentive was paid in cash and 50% was paid as 19,390 RSUs, which have a three-year vesting period.
The table below shows how the 2015 Long-Term Incentive grants of the CEO were determined. These grants were awarded under the LTPP and LTRPP, and will vest to the extent that performance conditions have been met for the 2015-2017 cycle. An overview of these plans is outlined in "—2015 Executive Committee Compensation System—Variable Compensation—Long-Term Incentives."
CEO LONG-TERM INCENTIVE GRANTS CYCLE 2015-2017
| |
Annual base salary CHF thousands |
x |
Target incentive % |
= |
Grant value CHF thousands |
Target number of PSUs(1) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
LTPP |
2,061 | x | 200 | % | = | 4,122 | 48,626 | ||||||||||
LTRPP |
2,061 | x | 100 | % | = | 2,061 | 24,313 | ||||||||||
- (1)
- Achievement will be reported in the 2017 Compensation Report. The grant value has been converted into a target number of PSUs based on a price of CHF 84.75 per Novartis share.
222
In January 2015, at target, the CEO's compensation was made up of 18% annual base compensation, 2% pension and other benefits, 27% Annual Incentive and 53% Long-Term Incentive. The Long-Term Incentive was split according to a ratio of 2:1 LTPP to LTRPP.
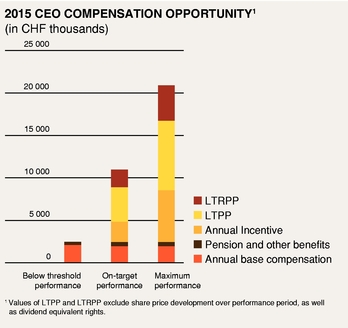
Executive Committee Compensation Tables (Audited)
Compensation of Executive Committee members for 2015
The following table discloses the compensation paid or granted to the CEO and other Executive Committee members for performance in 2015.
Alignment of reporting and performance
The compensation table synchronizes the reporting of Annual Incentive compensation with the performance in the given year (i.e., all amounts awarded for performance in 2015 are disclosed in full). This includes the restricted shares and RSUs granted under the Annual Incentive, which will vest three years following the grant based on plan rules. For awards granted under the LTPP and LTRPP, the target values (based on 100% achievement) at the time of grant are shown.
The performance and vesting value of the LTPP and LTRPP for the performance cycle 2015-2017 will be reported in the 2017 Compensation Report. The achievement against target and the vesting value of the OLTPP for the performance cycle 2013-2015 are shown in a separate table under "—Performance Vesting of Old Long-Term Performance Plan (2013-2015)."
Valuation principles
For the purpose of the tables contained within this Compensation Report, and to allow a comparison with other companies, Novartis shares and ADRs are disclosed at their market value on the date of grant. Market value is the quoted closing share price at that date. Restricted shares and RSUs are disclosed at the underlying value of Novartis shares and ADRs. PSUs are also valued for the purpose of this Compensation Report at the underlying value of the Novartis shares and ADRs at the grant date, and are disclosed at target value, assuming that they will vest at 100% achievement.
223
EXECUTIVE COMMITTEE MEMBER COMPENSATION FOR FINANCIAL YEAR 2015(1)
| |
|
Fixed compensation and pension benefits |
Variable compensation | |
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Base compensation |
|
|
|
LTPP 2015-2017 cycle |
LTRPP 2015-2017 cycle |
|
|
|||||||||||||||||||
| |
|
Pension benefits |
2015 Annual Incentive | |
Total compensation |
|||||||||||||||||||||||
| |
|
Other | ||||||||||||||||||||||||||
| |
|
Cash (amount) |
Cash (amount) | Equity (value at grant date)(3) |
PSUs (target value at grant date)(4) |
PSUs (target value at grant date)(4) |
||||||||||||||||||||||
| |
Currency | Amount(2) | Amount(5) | Amount(6) | ||||||||||||||||||||||||
| Joseph Jimenez (CEO) | CHF | 2,060,500 | 175,289 | 1,545,375 | 1,545,383 | 4,121,054 | 2,060,527 | 88,432 | 11,596,560 | |||||||||||||||||||
| Steven Baert | CHF | 653,333 | 158,099 | 543,900 | 543,953 | 960,048 | 256,030 | 94,716 | 3,210,079 | |||||||||||||||||||
| Felix R. Ehrat | CHF | 892,500 | 153,054 | 648,875 | 648,917 | 1,521,517 | 447,565 | 12,669 | 4,325,097 | |||||||||||||||||||
| David Epstein | $ | 1,400,000 | 362,819 | 1,428,000 | 1,428,054 | 2,520,001 | 1,260,050 | 569,737 | 8,968,661 | |||||||||||||||||||
| Mark C. Fishman(7) | $ | 990,000 | 248,910 | 861,300 | 861,323 | 1,881,089 | 891,021 | 129,825 | 5,863,468 | |||||||||||||||||||
| Richard Francis | CHF | 716,667 | 193,635 | 599,400 | 599,424 | 1,080,054 | 360,018 | 954,170 | 4,503,368 | |||||||||||||||||||
| Jeff George | $ | 956,539 | 200,946 | 158,400 | 158,404 | 1,536,056 | 576,009 | 1,260,286 | 4,846,640 | |||||||||||||||||||
| Harry Kirsch | CHF | 950,000 | 160,431 | 757,625 | 757,628 | 1,480,074 | 647,575 | 51,476 | 4,804,809 | |||||||||||||||||||
| Brian McNamara (until March 1, 2015)(8) | $ | 131,154 | 69,008 | 115,100 | 0 | 58,361 | 11,751 | 40,670 | 426,044 | |||||||||||||||||||
| Andrin Oswald (until March 1, 2015)(8) | CHF | 138,333 | 27,634 | 136,500 | 0 | 64,580 | 13,899 | 283,236 | 664,182 | |||||||||||||||||||
| André Wyss | CHF | 735,000 | 127,237 | 0 | 1,176,053 | 1,102,513 | 294,083 | 83,688 | 3,518,574 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total(9) | CHF | 9,490,269 | 1,843,151 | 6,695,906 | 7,624,994 | 16,094,751 | 6,713,188 | 3,491,962 | 51,954,221 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See following table for 2014 compensation figures
- (1)
- Does
not include reimbursement for travel and other necessary business expenses incurred by Executive Committee members in the performance of
their services, as these amounts are not considered compensation
- (2)
- Includes
service costs of pension and post-retirement healthcare benefits accumulated in 2015, in accordance with IAS19. It also includes an
amount of CHF 58,757 for mandatory employer contributions paid by Novartis to governmental social security systems. This amount is out of total employer contributions of CHF 3,457,097,
and provides a right to the maximum future insured government pension benefit for the Executive Committee member.
- (3)
- The
portion(s) of the Annual Incentive delivered in shares is rounded up to the nearest share based on the closing share price on the grant
date (January 20, 2016). The closing share price on this date was CHF 79.70 per Novartis share and $80.49 per ADR.
- (4)
- The
amounts shown in these columns represent the underlying share value of the target number of PSUs granted to each Executive Committee
member for the performance cycle 2015-2017 based on the closing share price on the grant date (January 21, 2015). The closing share price on this date was CHF 84.75 per Novartis share
and $98.75 per ADR.
- (5)
- Includes
any other perquisites, benefits in kind and international assignment benefits as per global mobility policy (e.g., housing,
international health insurance, children's school fees, tax equalization). Tax equalization benefits included for David Epstein, Richard Francis, Jeff George and Andrin Oswald are $305,867,
CHF 739,086, $1,153,361 and CHF 249,728, respectively.
- (6)
- All amounts are before deduction of employee's social security contribution and income tax due by the Executive Committee member
224
Continued from page 223
- (7)
- Mark C. Fishman, President NIBR and Executive Committee member, will step down from the Executive Committee on
February 29, 2016 and retire from Novartis. He will receive further contractual compensation that includes the base salary, pension and other benefits (pro-rata until February 29, 2016)
and the vesting of his incentive awards in accordance with the terms of the Novartis plan rules. As of March 1, 2016, Mark C. Fishman will provide certain consulting services to Novartis
for which he will be compensated for a period of up to two years until February 28, 2018. The fees for these services are capped at $250,000 p.a. and are in line with those paid to other
scientists who provide consultancy services to the NIBR organization.
- (8)
- Brian McNamara (Division Head, Novartis OTC) and Andrin Oswald (Division Head, Novartis Vaccines) transitioned to the
GlaxoSmithKline (GSK) group on March 2, 2015 following the completion of the Novartis OTC and Vaccines transactions with GSK. The information disclosed under columns "LTPP" and "LTRPP" in the
table above reflects their pro-rata compensation at target. Following their transition to GSK, and in accordance with the applicable plan rules, the LTPP and LTRPP awards for cycle 2015-2017 (as well
as for those granted for cycle 2014-2016) will be eligible to vest on the normal vesting date and on a pro-rata basis based on the number of months worked with Novartis during the performance period.
The vesting of these awards is subject to performance conditions assessed at the end of the cycle.
- (9)
- Amounts in US dollars for David Epstein, Mark C. Fishman, Jeff George and Brian McNamara were converted at a rate of CHF 1.00 = $1.040, which is the same average exchange rate used in the Group's consolidated financial statements.
225
EXECUTIVE COMMITTEE MEMBERS COMPENSATION FOR FINANCIAL YEAR 2014(1)
| |
|
Fixed compensation and pension benefits |
Variable compensation | |
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Base compensation | |
|
|
LTPP 2014-2016 cycle |
LTRPP 2014-2016 cycle |
|
|
|||||||||||||||||||
| |
|
Pension benefits | 2014 Annual Incentive | |
Total compensation |
|||||||||||||||||||||||
| |
|
Other | ||||||||||||||||||||||||||
| |
|
Cash (amount) |
Cash (amount) | Equity (value at grant date)(3) |
PSUs (target value at grant date)(4) |
PSUs (target value at grant date)(4) |
||||||||||||||||||||||
| |
Currency | Amount(2) | Amount(5) | Amount(6) | ||||||||||||||||||||||||
Joseph Jimenez (CEO) |
CHF | 2,060,500 | 165,584 | 2,009,000 | 2,009,084 | 4,121,003 | 2,060,501 | 222,818 | 12,648,490 | |||||||||||||||||||
Steven Baert (from February 26, 2014) |
CHF | 482,426 | 68,963 | 309,212 | 309,253 | 709,328 | 136,438 | 103,147 | 2,118,767 | |||||||||||||||||||
Juergen Brokatzky-Geiger (until February 25, 2014)(7) |
CHF | 110,650 | 22,454 | 0 | 0 | 0 | 0 | 3,245,256 | 3,378,360 | |||||||||||||||||||
Kevin Buehler (until April 30, 2014)(8) |
$ | 382,691 | 82,991 | 230,400 | 230,384 | 729,614 | 345,620 | 4,139,920 | 6,141,620 | |||||||||||||||||||
Felix R. Ehrat |
CHF | 875,000 | 154,299 | 0 | 1,408,037 | 1,496,019 | 440,066 | 8,928 | 4,382,349 | |||||||||||||||||||
David Epstein |
$ | 1,400,000 | 343,460 | 1,260,000 | 1,260,050 | 2,520,002 | 1,260,001 | 277,804 | 8,321,317 | |||||||||||||||||||
Mark C. Fishman |
$ | 990,000 | 294,572 | 1,009,800 | 1,009,818 | 1,881,034 | 891,033 | 78,369 | 6,154,626 | |||||||||||||||||||
Richard Francis (from May 1, 2014)(9) |
CHF | 466,667 | 114,435 | 211,450 | 211,451 | 871,135 | 186,735 | 3,364,623 | 5,426,496 | |||||||||||||||||||
Jeff George |
$ | 924,520 | 127,826 | 654,341 | 654,416 | 1,470,358 | 275,692 | 1,084,850 | 5,192,003 | |||||||||||||||||||
George Gunn(10) |
CHF | 865,000 | 116,542 | 622,800 | 622,828 | 1,384,066 | 346,035 | 0 | 3,957,271 | |||||||||||||||||||
Harry Kirsch |
CHF | 829,167 | 148,526 | 888,250 | 888,265 | 1,360,024 | 425,021 | 31,980 | 4,571,233 | |||||||||||||||||||
Brian McNamara |
$ | 673,077 | 76,484 | 578,000 | 578,083 | 1,020,055 | 204,076 | 77,717 | 3,207,492 | |||||||||||||||||||
Andrin Oswald |
CHF | 827,500 | 125,406 | 539,500 | 539,519 | 1,162,005 | 249,054 | 233,675 | 3,676,659 | |||||||||||||||||||
André Wyss (from May 1, 2014) |
CHF | 466,667 | 59,703 | 0 | 736,223 | 935,003 | 249,349 | 58,045 | 2,504,990 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total(11) |
CHF | 10,978,356 | 1,821,737 | 7,992,041 | 10,136,681 | 19,004,820 | 6,813,877 | 12,440,922 | 69,188,434 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
As published in the 2014 Compensation Report
- (1)
- Does
not include reimbursement for travel and other necessary business expenses incurred in the performance of their services, as these
amounts are not considered compensation. In general, for those who have left the Executive Committee in the course of 2014, the information under the columns "Base compensation", "Pension benefits",
"Annual Incentive", "LTPP" and "LTRPP" in the table above reflects their pro-rata compensation over 2014 for the period they were a member of the Executive Committee. The information under the column
"Other" includes inter alia their pro-rata compensation from the date they stepped down from the Executive Committee to December 31, 2014. For those who have joined the Executive Committee in
the course of 2014, the information under the columns "Base compensation", "Pension benefits" and "Annual Incentive" includes their pro-rata compensation from the date they joined the Executive
Committee to December 31, 2014. The information under the "LTPP" and "LTRPP" in the table above reflects their pro-rata compensation at target from the date they joined the Executive Committee
to December 31, 2016.
- (2)
- Includes
service costs of pension and post-retirement healthcare benefits accumulated in 2014, in accordance with IAS19. In addition, in
compliance with the Minder Ordinance, it includes an amount of mandatory employer social security contributions of CHF 76,534. This amount provides a right to the maximum future insured
government benefit for the members. This is out of a mandatory total of CHF 2,980,528 paid by Novartis to both Swiss and US governmental social security systems.
- (3)
- The
portion(s) of the Annual Incentive delivered in shares is rounded up to the nearest share based on the closing share price on the grant
date (January 21, 2015).
- (4)
- The amounts shown in these columns represent the underlying share value of the target number of PSUs granted to each Executive Committee member for the performance cycle 2014-2016 based on the closing share price on January 22, 2014. The closing share price on this date was CHF 73.75 per Novartis share and $80.79 per ADR.
226
|
Continued from page 225
- (5)
- Includes any other perquisites, benefits in kind, international assignment benefits as per global mobility policy
(e.g. housing, international health insurance, children's school fees, tax equalization) and other compensation. Does not include relocation costs paid in 2014
- (6)
- All amounts are before deduction of employee's social security contribution and income tax due by the Executive Committee
member
- (7)
- Juergen Brokatzky-Geiger stepped down from the Executive Committee on February 25, 2014, and as of February 26,
2014, he has been appointed as Global Head of Corporate Social Responsibility. He remained under the old Executive Committee incentive compensation system. As a result, his variable compensation has
been reported in full under the column "Other".
- (8)
- Kevin Buehler stepped down from the Executive Committee on April 30, 2014. In accordance with the contractual
12 month notice period of his employment agreement, he will retire from the company on April 30, 2015. He will receive further contractual compensation that includes the base salary,
pension and other benefits (pro-rata until April 30, 2015) and the vesting of his incentive awards in accordance with the terms of the Novartis plan rules. His compensation does not include an
annual pension in payment ($507,017) following the acquisition of Alcon in 2011.
- (9)
- Richard Francis will receive compensation in the form of 41,500 RSUs for lost entitlements at his former employer with a total
value at grant of CHF 3.2 million. The vesting of the RSUs will be staggered based on the vesting period at his former employer, and extend over the period from 2015-2017, provided that
he remains employed with Novartis at the respective due dates. 21,500, 13,500 and 6,500 RSUs will respectively vest on February 1, 2015, 2016 and 2017.
- (10)
- Following the completion on January 1, 2015 of the transaction with Eli Lilly, George Gunn (Division Head, Novartis
Animal Health), stepped down from the Executive Committee. He will provide assistance with regard to the post-closing divestment of Animal Health until he will reach his contractual retirement age in
July 2015. George Gunn will receive further contractual compensation that includes the base salary, pension and other benefits (pro-rata until July 31, 2015) and the vesting of his incentive
awards in accordance with the terms of the Novartis plan rules.
- (11)
- Amounts in US dollars for Kevin Buehler, David Epstein, Mark C. Fishman, Jeff George and Brian McNamara were converted at a rate of CHF 1.00 = $1.094, which is the same average exchange rate used in the Group's consolidated financial statements. At the time of his appointment as Head of Alcon, Mr. George's Swiss employment agreement was replaced with a US employment agreement in US dollars.
227
EXECUTIVE COMMITTEE MEMBERS—EQUITY AWARDS FOR FINANCIAL YEAR 2015 (Number of equity instruments)(1)
| |
Variable compensation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 Annual Incentive |
LTPP 2015-2017 cycle |
LTRPP 2015-2017 cycle |
|||||||
| |
Equity (number)(2) |
PSUs (target number)(3) |
PSUs (target number)(3) |
|||||||
Joseph Jimenez |
19,390 | 48,626 | 24,313 | |||||||
Steven Baert |
6,825 | 11,328 | 3,021 | |||||||
Felix R. Ehrat |
8,142 | 17,953 | 5,281 | |||||||
David Epstein |
17,742 | 25,519 | 12,760 | |||||||
Mark C. Fishman |
10,701 | 19,049 | 9,023 | |||||||
Richard Francis |
7,521 | 12,744 | 4,248 | |||||||
Jeff George |
1,968 | 15,555 | 5,833 | |||||||
Harry Kirsch |
9,506 | 17,464 | 7,641 | |||||||
Brian McNamara (until March 1, 2015)(4) |
0 | 591 | 119 | |||||||
Andrin Oswald (until March 1, 2015)(4) |
0 | 762 | 164 | |||||||
André Wyss |
14,756 | 13,009 | 3,470 | |||||||
| | | | | | | | | | | |
Total |
96,551 | 182,600 | 75,873 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See table below for 2014 compensation figures
- (1)
- The
value of the awards included in this table are reported in the table "EXECUTIVE COMMITTEE MEMBER COMPENSATION FOR FINANCIAL YEAR 2015"
under "—2015 Executive Committee Compensation—Executive Committee Compensation Tables (Audited)."
- (2)
- Vested
shares, restricted shares and/or RSUs granted under the Annual Incentive for performance year 2015
- (3)
- Target
number of PSUs granted under the LTPP and LTRPP as applicable for the 2015-2017 performance cycle
- (4)
- Target number of PSUs granted under the LTPP and LTRPP are reported on a pro-rata basis. See footnote 8 of the table "EXECUTIVE COMMITTEE MEMBER COMPENSATION FOR FINANCIAL YEAR 2015" under "—2015 Executive Committee Compensation—Executive Committee Compensation Tables (Audited)."
228
EXECUTIVE COMMITTEE MEMBERS—EQUITY AWARDS FOR PERFORMANCE YEAR 2014 (Number of equity instruments)(1)
| |
Variable compensation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 Annual Incentive |
LTPP 2014-2016 cycle |
LTRPP 2014-2016 cycle |
|
|||||||||
| |
Other Equity/ Target PSUs (number) |
||||||||||||
| |
Equity (number)(2) |
Target PSUs (number)(3) |
Target PSUs (number)(3) |
||||||||||
Joseph Jimenez |
23,706 | 55,878 | 27,939 | 0 | |||||||||
Steven Baert (from February 26, 2014) |
3,649 | 9,618 | 1,850 | 0 | |||||||||
Juergen Brokatzky-Geiger (until February 25, 2014) |
0 | 0 | 0 | 30,953 | (4) | ||||||||
Kevin Buehler (until April 30, 2014) |
2,333 | 9,031 | 4,278 | 31,936 | |||||||||
Felix R. Ehrat |
16,614 | 20,285 | 5,967 | 0 | |||||||||
David Epstein |
12,760 | 31,192 | 15,596 | 0 | |||||||||
Mark C. Fishman |
10,226 | 23,283 | 11,029 | 0 | |||||||||
Richard Francis (from May 1, 2014) |
2,495 | 11,812 | 2,532 | 41,500 | (5) | ||||||||
Jeff George |
6,627 | 18,224 | 3,417 | 0 | |||||||||
George Gunn |
7,349 | 18,767 | 4,692 | 0 | |||||||||
Harry Kirsch |
10,481 | 18,441 | 5,763 | 0 | |||||||||
Brian McNamara |
5,854 | 12,626 | 2,526 | 0 | |||||||||
Andrin Oswald |
6,366 | 15,756 | 3,377 | 0 | |||||||||
André Wyss (from May 1, 2014) |
8,687 | 12,678 | 3,381 | 0 | |||||||||
| | | | | | | | | | | | | | |
Total |
117,147 | 257,591 | 92,347 | 104,389 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
As published in the 2014 Compensation Report
- (1)
- See
also corresponding footnote 1 of the table "EXECUTIVE COMMITTEE MEMBER COMPENSATION FOR FINANCIAL YEAR 2014" with regard to the Executive
Committee members who left or joined the Committee in the course of 2014.
- (2)
- Vested
shares, restricted shares and/or RSUs granted under the Annual Incentive for performance year 2014
- (3)
- Target
number of PSUs granted under the LTPP and LTRPP as applicable for the 2014–2016 performance cycle
- (4)
- Juergen
Brokatzky-Geiger remained under the old Executive Committee compensation system. The information under the column "Other" includes
the following equity awards: 12,638 restricted shares granted under the Novartis Equity Plan Select, 6,342 investment shares and 3,171 matching shares under the Employee Share Ownership Plan, and
8,802 target PSUs under the OLTPP for the 2014-2016 performance cycle.
- (5)
- This amount reflects the total number of RSUs granted to Richard Francis in 2014 as compensation for lost entitlements at his former employer on joining Novartis.
229
EXECUTIVE COMMITTEE MEMBER COMPENSATION BASE AND VARIABLE COMPENSATION MIX FOR FINANCIAL YEAR 2015(1)
| |
Base salary | Variable compensation(2) |
|||||
|---|---|---|---|---|---|---|---|
Joseph Jimenez |
18.2 | % | 81.8 | % | |||
Steven Baert |
22.1 | % | 77.9 | % | |||
Felix R. Ehrat |
21.5 | % | 78.5 | % | |||
David Epstein |
17.4 | % | 82.6 | % | |||
Mark C. Fishman |
18.1 | % | 81.9 | % | |||
Richard Francis |
21.4 | % | 78.6 | % | |||
Jeff George |
28.3 | % | 71.7 | % | |||
Harry Kirsch |
20.7 | % | 79.3 | % | |||
André Wyss |
22.2 | % | 77.8 | % | |||
| | | | | | | | |
Total |
20.1 | % | 79.9 | % | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- (1)
- Excludes
pension and other benefits, as well as Brian McNamara and Andrin Oswald, who stepped down from the Executive Committee on
March 1, 2015 as a result of the GlaxoSmithKline transaction.
- (2)
- See the table "EXECUTIVE COMMITTEE MEMBER COMPENSATION FOR FINANCIAL YEAR 2015" under "—2015 Executive Committee Compensation—Executive Committee Compensation Tables (Audited)" with regard to the disclosure principles of variable compensation.
Loans to Executive Committee members
No loans were granted to current or former Executive Committee members or to "persons closely linked" to them in 2015. No such loans were outstanding as of December 31, 2015.
Other payments to Executive Committee members
During 2015, no other payments (or waivers of claims) were made to Executive Committee members or to "persons closely linked" to them.
Payments to former Executive Committee members
During 2015, under the former Executive Committee members' contracts and in line with the company's plan rules and policies, payments were made to Kevin Buehler, the former Division Head of Alcon, and George Gunn, the former Division Head of Animal Health, who retired from the company on May 1, 2015 and on August 1, 2015, respectively. In 2015, an amount of $1,127,324 and CHF 1,214,583 was paid to Mr. Buehler and Mr. Gunn, respectively. These amounts exclude the value of the vested OLTPP awards for cycle 2013-2015 of Mr. Buehler and Mr. Gunn, who received, in accordance with the plan rules, $1,763,889 and CHF 1,527,285 (value of the shares delivered at vesting), respectively. In addition, in line with their contracts and the company's policies, a total amount of CHF 24,116 was paid by the company for tax and financial services provided to two other former Executive Committee members. With the exception of the above amounts, during 2015, no other payments (or waivers of claims) were made to former Executive Committee members or to "persons closely linked."
James E. Bradner, future President of NIBR and Executive Committee member
As announced on September 24, 2015, James E. Bradner will succeed Mark Fishman as President of the Novartis Institutes for BioMedical Research (NIBR) and become an Executive Committee member with effect from March 1, 2016. Prior to joining Novartis, Dr. Bradner served as a board member and
230
advisor to many scientific companies he founded, and as a supervisory board member of another company. In reaching the terms of the offer for Dr. Bradner, the Board of Directors recognized the need to make up for compensation that Dr. Bradner would be forfeiting on joining Novartis. In extending our offer to Dr. Bradner, the following compensation for lost entitlements was agreed to attract him to Novartis:
- –
- In January 2016, as compensation for lost entitlements at one of his scientific companies on joining Novartis, Dr. Bradner has
been paid an amount of $844,250 for the 275,000 shares that he forfeited. The fair market value of the forfeited shares was determined by an independent valuation expert.
- –
- In January 2016, Dr. Bradner received compensation in the form of 3,607 RSUs for lost entitlements in connection with his supervisory board mandate with a total value at grant of $309,300. The vesting of the RSUs will be staggered based on the original vesting period of the forfeited entitlements, provided that he remains employed with Novartis at the respective due dates.
Please also see the additional related disclosure made in "Item 18. Financial Statements—Note 27." These disclosures are made on a voluntary basis and will be further communicated in next year's annual report on Form 20-F.
Award and delivery of equity to Novartis associates
During 2015, 12.4 million unvested restricted shares (or ADRs), RSUs and target PSUs were granted and 14.4 million Novartis shares (or ADRs) were delivered to Novartis associates under various equity-based participation plans. Current unvested equity instruments (restricted shares, RSUs and target PSUs) as well as outstanding equity options held by associates represent 2.4% of shares issued of Novartis. Novartis delivers treasury shares to associates to fulfill these obligations and aims to offset the dilutive impact from its equity-based participation plans.
Share Ownership Requirements for Executive Committee members
Executive Committee members are required to own at least a minimum multiple of their annual base compensation in Novartis shares or share options within three years of hire or promotion, as set out in the table below.
CEO |
5 × base compensation | |
Executive Committee members |
3 × base compensation | |
| | | |
In the event of a substantial rise or drop in the share price, the Board of Directors may, at its discretion, amend that time period accordingly.
The determination of equity amounts against the share ownership requirements is defined to include vested and unvested Novartis shares or ADRs, as well as RSUs acquired under the compensation plans, but excluding unvested matching shares granted under the Leveraged Share Savings Plan (LSSP) and the Employee Share Ownership Plan (ESOP), and unvested PSUs from LTPP and LTRPP. The determination includes other shares as well as vested options of Novartis shares or ADRs that are owned directly or indirectly by "persons closely linked" to them. The Compensation Committee reviews compliance with the share ownership guideline on an annual basis.
As of December 31, 2015, all members who have served at least three years on the Executive Committee have met or exceeded their personal Novartis share ownership requirements.
231
As of January 1, 2016, to better align with prevalent market practice and the change to our compensation system, Executive Committee members will be required to meet their share ownership requirement within five years of hire/promotion.
Shares, ADRs, equity rights and share options owned by Executive Committee members
The following tables show the total number of shares, ADRs, other equity rights and share options owned by Executive Committee members and "persons closely linked" to them as of December 31, 2015.
As of December 31, 2015, no Executive Committee members together with "persons closely linked" to them owned 1% or more of the outstanding shares (or ADRs) of Novartis, either directly or through share options.
The market value of share options (previously granted) is calculated using an option pricing valuation model as at the grant date.
SHARES, ADRs AND OTHER EQUITY RIGHTS OWNED BY EXECUTIVE COMMITTEE MEMBERS(1)
| |
Vested shares and ADRs |
Unvested shares and other equity rights(2) |
Total at December 31, 2015 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Joseph Jimenez |
284,405 | 322,200 | 606,605 | |||||||
Steven Baert |
1,700 | 44,977 | 46,677 | |||||||
Felix R. Ehrat |
92,435 | 107,870 | 200,305 | |||||||
David Epstein |
70,371 | 230,535 | (3) | 300,906 | ||||||
Mark C. Fishman |
52,242 | 276,622 | (3) | 328,864 | ||||||
Richard Francis |
14,357 | 37,722 | 52,079 | |||||||
Jeff George |
119,247 | 99,373 | 218,620 | |||||||
Harry Kirsch |
46,579 | 100,359 | 146,938 | |||||||
André Wyss |
44,660 | 79,917 | 124,577 | |||||||
| | | | | | | | | | | |
Total(4) |
725,996 | 1,299,575 | 2,025,571 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- Includes
holdings of "persons closely linked" to Executive Committee members (see definition under "—2015 Executive Committee
Compensation—Executive Committee Compensation Tables (Audited)—Persons Closely Linked," below)
- (2)
- Includes
restricted shares, RSUs and target number of PSUs. Matching shares under the ESOP, LSSP, and target number of PSUs are disclosed
pro-rata to December 31, unless the award qualified for full vesting under the relevant plan rules. Awards under all other incentive plans are disclosed in full.
- (3)
- Includes
both deferred and unvested cash-settled equity awards and holdings of Novartis shares in US-defined contribution plans.
- (4)
- As a result of the GlaxoSmithKline transaction, Brian McNamara and Andrin Oswald stepped down from the Executive Committee on March 1, 2015. Brian McNamara owned 52,251 vested shares and 15,200 unvested shares and other equity rights at March 1, 2015. Andrin Oswald owned 122,892 vested shares and 41,547 unvested shares and other equity rights at March 1, 2015.
232
SHARE OPTIONS OWNED BY EXECUTIVE COMMITTEE MEMBERS(1)
| |
Number of share options(2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | Other | Total at December 31, 2015 |
|||||||
Jeff George |
141,396 | 0 | 141,396 | |||||||
André Wyss |
0 | 378,390 | 378,390 | |||||||
| | | | | | | | | | | |
Total(3) |
141,396 | 378,390 | 519,786 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- The
last share option grants under the Novartis Equity Plan Select were made in January 2013.
- (2)
- Share
options disclosed for a specific year were granted in that year under the Novartis Equity Plan Select. The column "Other" refers to
share options granted in 2008 or earlier, to share options granted to these executives while they were not Executive Committee members, and to share options bought on the market by the Executive
Committee members or "persons closely linked" to them (see definition under "—2015 Executive Committee Compensation—Executive Committee Compensation Tables
(Audited)—Persons Closely Linked," below).
- (3)
- No other current Executive Committee members owned share options at December 31, 2015. As a result of the GlaxoSmithKline transaction, Brian McNamara and Andrin Oswald stepped down from the Executive Committee on March 1, 2015. At March 1, 2015, Brian McNamara and Andrin Oswald did not own any share options.
Persons closely linked
"Persons closely linked" are (I) their spouse, (II) their children below age 18, (III) any legal entities that they own or otherwise control, and (IV) any legal or natural person who is acting as their fiduciary.
PERFORMANCE VESTING OF OLD LONG-TERM PERFORMANCE PLAN (2013-2015)
Overview
As of 2014, grants are no longer made under this plan to Executive Committee members, but performance for the last cycle of the OLTPP is reported in this Compensation Report. The performance for the first cycle of the LTPP and LTRPP (cycle 2014-2016) will be reported in the 2016 Compensation Report.
The OLTPP provided grants based on a target percentage of base compensation at the beginning of each plan cycle. It represented 175% of base salary for the CEO.
Form of award at grant
At the beginning of the performance period, participants were granted a target number of PSUs according to the following formula:

233
Performance measure
The rewards were based on rolling three-year Group performance objectives focused on the Novartis Economic Value Added (NVA) measured annually. NVA takes into account Group operating income adjusted for interest, taxes and cost of capital charge. The formula is included under "Item 5. Operating and Financial Review and Prospects—Item 5.A Operating Results—Non-IFRS Measures as Defined by Novartis—Novartis Economic Value Added."
The NVA performance factor was based on a 1:5 payout curve, where a 1% deviation in realization versus target led to a 5% change in payout (for example, a performance ratio of 105% would have led to a performance factor of 125%). If performance over the three-year vesting period would have fallen below 80% of target, no shares would have vested. The performance factor was capped at 200% of target, corresponding to an achievement of 20% above target.
Delivery at vesting
At the end of the three-year performance period, the target number of PSUs was multiplied by the performance factor approved by the Compensation Committee. PSUs were converted into Novartis shares and immediately vested. In the US, awards may also have been delivered in cash under the US-deferred compensation plan.
Outcome of the Performance Cycle 2013-2015
Over the three-year performance period, 2013 to 2015, Novartis performed 3.5% ahead of the $7.4 billion NVA target, corresponding to a payout of 118% following the application of the 1:5 payout curve. This achievement was mainly driven by operating income performance and productivity initiatives. In arriving at the NVA performance score, the Compensation Committee excluded, as major items, the favorable impact from the delayed entry of generic competition for Diovan monotherapy in the US, income generated from the sale of the Idenix Pharmaceuticals Inc. and LTS Lohmann Therapie-Systeme AG stakes, the negative impact from executing the Group portfolio transformation (including an exceptional pre-tax impairment charge of $1.1 billion related to the divestment of the Vaccines influenza business). Over the entire three-year cycle, currency movements had a significant negative impact (more than $2.1 billion) in NVA well above the impact on the previous cycle of the OLTPP. Considering the total shareholder return of the three years (in US dollars, +53.4%), the Compensation Committee decided to exclude, on a discretionary basis, a portion of this currency impact.
234
The table below shows the vesting of the OLTPP 2013-2015 cycle for the CEO and other Executive Committee members.
PAYOUT SCHEDULE FOR OLTPP 2013-2015 PERFORMANCE CYCLE(1)
| |
Currency | PSUs (target value at grant date) |
PSUs (target number) |
Performance factor payout for OLTPP 2013-2015 cycle |
Shares delivered at vesting (number) |
Shares delivered at vesting (value at vesting price) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Joseph Jimenez |
CHF | 3,605,933 | 58,443 | 118 | % | 68,963 | 5,496,351 | |||||||||||
Other 8 members of the Executive Committee(2) |
CHF | 5,363,227 | 86,864 | 118 | % | 102,500 | 8,214,409 | |||||||||||
| | | | | | | | | | | | | | | | | | | |
Total |
CHF | 8,969,160 | 145,307 | 118 | % | 171,463 | 13,710,760 | |||||||||||
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
See below for 2014 compensation figures
- (1)
- For
those who have left or joined the ECN in the course of the 2013-2015 performance period, the information disclosed under this table
reflects the pro-rata LTPP 2013-2015 payout attributable to the period they were a member of the Executive Committee.
- (2)
- This table excludes the awards which were originally granted to Brian McNamara (10,780 target PSUs) and Andrin Oswald (13,688 target PSUs) for OLTPP 2013-2015 performance cycle. As a result of the GlaxoSmithKline transaction, and in accordance with the OLTPP plan rules, these awards were forfeited.
For the Executive Committee members, including the CEO, the impact of the share price appreciation over the vesting period on the total value realized at vesting was CHF 3.1 million. For the CEO, the impact of the share price appreciation was CHF 1.4 million. This represents 25% of the overall vesting value.
For comparative purposes, the table below shows the vesting of the OLTPP 2012-2014 cycle for the CEO and other Executive Committee members, as published in the 2014 Compensation Report.
PAYOUT SCHEDULE FOR OLTPP 2012-2014 PERFORMANCE CYCLE(1)
| |
Currency | PSUs (target value at grant date) |
PSUs (target number) |
Performance factor payout for OLTPP 2012-2014 cycle |
Shares delivered at vesting (number) |
Shares delivered at vesting (value at vesting price) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Joseph Jimenez |
CHF | 3,605,926 | 66,530 | 168 | % | 111,771 | 9,472,592 | |||||||||||
Other 13 members of the Executive Committee |
CHF | 7,783,335 | 142,747 | 168 | % | 239,822 | 20,539,978 | |||||||||||
| | | | | | | | | | | | | | | | | | | |
Total |
CHF | 11,389,261 | 209,277 | 168 | % | 351,593 | 30,012,570 | |||||||||||
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
As published in the 2014 Compensation Report
- (1)
- For those who left or joined the Executive Committee in the course of the 2012-2014 performance period, the information disclosed under this table reflects the pro-rata LTPP 2012-2014 payout attributable to the period they were a member of the Executive Committee.
For the Executive Committee members, including the CEO, the impact of the share price appreciation over the vesting period of the OLTPP 2012-2014 cycle on the total value realized at vesting was CHF 10.9 million. For the CEO, the impact of the share price appreciation was CHF 3.4 million. This represents 36% of the overall vesting value.
235
2015 BOARD COMPENSATION SYSTEM
Board Compensation Philosophy and Benchmarking
The Board of Directors sets compensation for its members at a level that allows for the attraction and retention of high-caliber individuals with global experience, including a mix of Swiss and international members. Board members do not receive variable compensation, underscoring their focus on corporate strategy, supervision and governance.
The Board of Directors sets the level of compensation for its Chairman and the other members to be in line with relevant benchmark companies, which include other large Swiss-headquartered multinational companies, ABB, Credit Suisse, Holcim, Nestlé, Roche, Syngenta and UBS. This peer group has been chosen for Board compensation due to the comparability of Swiss legal requirements, including broad personal and individual liabilities under Swiss law (and new criminal liability under the Swiss rules regarding compensation of Board and Executive Committee members related to the Ordinance Against Excessive Compensation in Stock Exchange Listed Companies) and under US law (due to the company's secondary listing on the New York Stock Exchange).
The Board of Directors reviews the compensation of its members, including the Chairman, each year based on a proposal by the Compensation Committee and advice from its independent advisor, including relevant benchmarking information.
Compensation of the Chairman of the Board of Directors
As Chairman, Dr. Joerg Reinhardt receives total annual compensation valued at CHF 3.8 million. The total compensation is comprised equally of cash and shares, as follows:
- –
- Cash compensation: CHF 1.9 million per year
- –
- Share compensation: annual value equal to CHF 1.9 million of unrestricted Novartis shares
From the 2015 Annual General Meeting (AGM), Dr. Reinhardt voluntarily waived the company contribution for pension and insurance benefits. Until this date, the company made employer contributions regarding the Chairman's participation in the Novartis Swiss standard pension and life insurance benefit plans. These contributions amounted to CHF 24,840.
Dr. Reinhardt also receives compensation for lost entitlements at his former employer, with a total value of EUR 2.6 million, as reported in the 2014 and 2013 Compensation Reports. Payments are staggered based on the vesting period at his former employer, and extend over the period from 2014-2016, provided that he remains in office as Chairman at the respective due dates. On January 31, 2015, he received EUR 871,251 in cash(1).
For 2015, the Chairman voluntarily waived the increase in compensation to which he is entitled, which is an amount not lower than the average annual compensation increase awarded to associates based in Switzerland (1.5% for 2015). For the year 2016, the Chairman will also voluntarily waive this increase.
Compensation of the other Board members
The annual fee rates for Board membership and additional functions are included in the table below. These were approved by the Board of Directors with effect from the 2014 AGM and align our aggregate Board compensation to the current levels of other large Swiss companies.
- (1)
- On January 31, 2016 he will receive the third and final installment of EUR 1,045,800.
236
2015 BOARD MEMBER ANNUAL FEE RATES
| |
Annual fee (CHF) | |||
|---|---|---|---|---|
Chairman of the Board |
3,800,000 | (1) | ||
Board membership |
300,000 | |||
Vice Chairman |
50,000 | |||
Chair of Audit and Compliance Committee |
120,000 | |||
Chair of the following committees: |
||||
—Compensation Committee |
||||
—Governance, Nomination and Corporate Responsibilities Committee |
||||
—Research & Development Committee(2) |
||||
—Risk Committee |
60,000 | |||
Membership of Audit and Compliance Committee |
60,000 | |||
Membership of the following committees: |
||||
—Compensation Committee |
||||
—Governance, Nomination and Corporate Responsibilities Committee |
||||
—Research & Development Committee |
||||
—Risk Committee |
30,000 | |||
- (1)
- The
Chairman also received company pension contributions until the 2015 AGM (when they ceased), and payment for loss of other entitlements at
his previous employer for total EUR 2,665,051 staggered over 2014 to 2016.
- (2)
- The Chairman receives no additional committee fees for chairing the Research & Development Committee.
In addition, the following policies apply regarding their compensation:
- –
- 50% of compensation is delivered in cash, paid on a quarterly basis in arrears.
- –
- 50% of compensation is delivered in shares in two installments: one six months after the AGM and one 12 months after the AGM.
- –
- Board members bear the full cost of their employee social security contributions, if any, and do not receive share options or pension benefits.
The Board compensation system will remain unchanged in 2016.
237
2015 BOARD COMPENSATION
Board Member Compensation Table (Audited)
The following table discloses the 2015 Board member compensation. Board compensation is reported as the amount earned in the financial year.
BOARD MEMBER COMPENSATION EARNED FOR FINANCIAL YEAR 2015(1)
| |
Board membership |
Vice Chairman |
Audit and Compliance Committee |
Compensation Committee |
Governance, Nomination and Corporate Responsibilities Committee |
Research & Development Committee |
Risk Committee |
Cash (CHF) (A) |
Shares (CHF) (B) |
Shares (number)(2) |
Other (CHF) (C)(3) |
Total (CHF) (A)+(B)+(C)(4) |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Joerg Reinhardt(5) |
Chair | Chair | 1,900,000 | 1,900,000 | 19,397 | 29,197 | 3,829,197 | ||||||||||||||||||||||||||||||
Ulrich Lehner (until February 26, 2015) |
· | · | · | · | · | 39,167 | 39,167 | 1,242 | 582 | 78,916 | |||||||||||||||||||||||||||
Enrico Vanni |
· | · | · | Chair | · | 250,000 | 250,000 | 2,552 | 4,357 | 504,357 | |||||||||||||||||||||||||||
Nancy Andrews (from February 27, 2015) |
· | · | 137,500 | 137,500 | 812 | — | 275,000 | ||||||||||||||||||||||||||||||
Dimitri Azar |
· | · | · | 172,250 | 217,750 | 2,712 | — | 390,000 | |||||||||||||||||||||||||||||
Verena A. Briner |
· | · | 165,000 | 165,000 | 1,684 | 4,357 | 334,357 | ||||||||||||||||||||||||||||||
Srikant Datar |
· | Chair | · | · | 240,000 | 240,000 | 2,450 | — | 480,000 | ||||||||||||||||||||||||||||
Ann Fudge |
· | · | · | · | 195,000 | 195,000 | 1,990 | — | 390,000 | ||||||||||||||||||||||||||||
Pierre Landolt(6) |
· | Chair | — | 360,000 | 3,674 | 3,492 | 363,492 | ||||||||||||||||||||||||||||||
Charles L. Sawyers |
· | · | (7) | · | 177,500 | 177,500 | 1,757 | — | 355,000 | ||||||||||||||||||||||||||||
Andreas von Planta |
· | · | · | Chair | 225,000 | 225,000 | 2,296 | 4,357 | 454,357 | ||||||||||||||||||||||||||||
William T. Winters |
· | · | (7) | — | 325,000 | 3,210 | — | 325,000 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total |
3,501,417 | 4,231,917 | 43,776 | 46,342 | 7,779,676 | ||||||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See table below for 2014 compensation figures
- (1)
- Does
not include reimbursement for travel and other necessary business expenses incurred by Board members in the performance of their
services, as these are not considered compensation
- (2)
- Represents
the gross number of shares delivered to each Board member in 2015. The number of shares reported in this column represents:
(i) the second and final equity installment delivered in February 2015 for the services from the 2014 AGM to the 2015 AGM, and (ii) the first of two equity installment delivered in
August 2015 for the services from the 2015 AGM to the 2016 AGM. The second and final equity installment for the services from the 2015 AGM to the 2016 AGM will take place in February 2016.
- (3)
- It
includes an amount of CHF 21,502 for mandatory employer contributions paid by Novartis to Swiss governmental social security
systems. This amount is out of total employer contributions of CHF 429,806, and provides a right to the maximum future insured government pension benefit for the Board member.
- (4)
- All
amounts are before deduction of employee's social security contribution and income tax due by the Board member
- (5)
- Does
not include EUR 871,251 paid to Joerg Reinhardt on January 31, 2015 for lost entitlements at his former employer. This amount is
the second of three installments comprising to a total amount of EUR 2,665,051, which compensates him for lost entitlements with his previous employer due to him on joining Novartis. The third and
last installment of EUR 1,045,800 will be delivered on January 31, 2016, provided that he remains in office as our Chairman at the due dates. The lost entitlements of EUR 2,665,051 of Joerg
Reinhardt were included in full in the table entitled "BOARD MEMBER COMPENSATION IN 2013" under "Item. 6 Directors, Senior Management and Employees—Item 6.B
Compensation—2013 Comparative Information—2013 Board Compensation" of our 2014 Annual Report on Form 20-F as filed with the SEC on January 27, 2015, based on our
disclosure policy to report compensation for lost entitlements in full in the year the member of the Board or Executive Committee joined Novartis.
- (6)
- According
to Pierre Landolt, the Sandoz Family Foundation is the economic beneficiary of the compensation.
- (7)
- From February 27, 2015
238
BOARD MEMBER COMPENSATION EARNED FOR FINANCIAL YEAR 2014(1)
| |
Board membership |
Vice Chairman |
Audit and Compliance Committee |
Compensation Committee |
Governance, Nomination and Corporate Responsibilities Committee |
Research & Development Committee(2) |
Risk Committee |
Chairman's Committee(2) |
Delegated Board membership |
Cash (CHF) (A) |
Shares (CHF) (B) |
Shares (number)(3) |
Other (CHF) (C)(4) |
Total (CHF) (A)+(B)+(C)(5) |
|||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Joerg Reinhardt(6) |
Chair | Chair | Chair | 2,058,334 | 1,741,666 | 12,180 | 157,844 | (7) | 3,957,844 | ||||||||||||||||||||||||||||||||||
Ulrich Lehner |
· | · | · | · | · | · | (8) | · | 262,500 | 262,500 | 1,527 | 37,851 | (9) | 562,851 | |||||||||||||||||||||||||||||
Enrico Vanni |
· | · | · | Chair | · | · | 267,500 | 267,500 | 1,625 | 11,173 | (9) | 546,173 | |||||||||||||||||||||||||||||||
Dimitri Azar |
· | · | · | 86,250 | 313,750 | 2,154 | — | 400,000 | |||||||||||||||||||||||||||||||||||
Verena A. Briner |
· | · | (10) | 166,667 | 166,667 | 1,073 | 7,468 | (9) | 340,802 | ||||||||||||||||||||||||||||||||||
William Brody (until February 25, 2014) |
· | · | · | (11) | 43,750 | 43,750 | — | 83,333 | (12) | 170,833 | |||||||||||||||||||||||||||||||||
Srikant Datar |
· | Chair | · | · | · | 260,000 | 260,000 | 1,560 | — | 520,000 | |||||||||||||||||||||||||||||||||
Ann Fudge |
· | · | · | · | 204,167 | 204,167 | 1,268 | — | 408,334 | ||||||||||||||||||||||||||||||||||
Pierre Landolt(13) |
· | Chair | — | 368,333 | 2,340 | 7,031 | (9) | 375,364 | |||||||||||||||||||||||||||||||||||
Charles L. Sawyers |
· | · | 166,667 | 166,667 | 1,073 | — | 333,334 | ||||||||||||||||||||||||||||||||||||
Andreas von Planta |
· | · | · | Chair | 234,167 | 234,167 | 1,462 | 9,175 | (9) | 477,509 | |||||||||||||||||||||||||||||||||
Wendelin Wiedeking (until February 25, 2014) |
· | · | · | — | 75,000 | — | 4,482 | (9) | 79,482 | ||||||||||||||||||||||||||||||||||
William T. Winters |
· | 29,167 | 279,167 | 1,950 | — | 308,334 | |||||||||||||||||||||||||||||||||||||
Rolf M. Zinkernagel (until February 25, 2014) |
· | · | · | (14) | 54,167 | 54,167 | — | 175,870 | (9),(15) | 284,204 | |||||||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total |
3,833,336 | 4,437,501 | 28,212 | 494,227 | 8,765,064 | ||||||||||||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
As published in the 2014 Compensation Report
- (1)
- Does
not include reimbursement for travel and other necessary business expenses incurred in the performance of their services, as these are
not considered compensation.
- (2)
- As
of February 26, 2014, the Research & Development Committee has been introduced and the Chairman's Committee disbanded.
- (3)
- Represents
the gross number of shares delivered to each Board member in 2014 in respect of the first of two equity installments for the
services from the 2014 AGM to the 2015 AGM. The second equity installment will take place in February 2015. This number does not include the number of shares for the compensation for services for the
period from January 1, 2014 to the 2014 AGM.
- (4)
- In
compliance with the Minder Ordinance, it includes an amount of mandatory employer social security contributions of CHF 27,771. This
amount provides a right to the maximum future insured government benefit for the members. This is out of a mandatory total of CHF 359,890 paid by Novartis to both Swiss governmental social
security systems.
- (5)
- All amounts are before deduction of employee's social security contribution and income tax due by the Board member.
239
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Continued from page 238
- (6)
- Does
not include EUR 748,000 paid to Joerg Reinhardt on January 31, 2014 for lost entitlements at his former employer. This amount is
the first of three installments comprising to a total amount of EUR 2,665,051, which compensates him for lost entitlements with his previous employer due to him on joining Novartis. The second and
third installment are staggered based on the vesting period at his former employer, and extend over the period from 2015-2016, provided that he remains in office as our Chairman at the respective due
dates. On January 31, 2015 and 2016, he will respectively receive EUR 871,251 and EUR 1,045,800. The lost entitlements of EUR 2,665,051 of Joerg Reinhardt are included in full in the table
entitled "BOARD MEMBER COMPENSATION IN 2013" under "Item. 6 Directors, Senior Management and Employees—Item 6.B Compensation—2013 Comparative
Information—2013 Board Compensation" of our 2014 Annual Report on Form 20-F as filed with the SEC on January 27, 2015, based on our disclosure policy to report compensation
for lost entitlements in full in the year the member of the Board or ECN joined Novartis.
- (7)
- Includes
social security costs due by the individual and paid by the company until January 31, 2014, and service costs of pension and
post-retirement healthcare benefits accumulated in 2014 in accordance with IAS19
- (8)
- Until
February 25, 2014
- (9)
- Includes
social security costs due by the individual and paid by the company until February 25, 2014. As of February 26, 2014,
all Board members bear the full cost of their employee social security.
- (10)
- As
of February 26, 2014
- (11)
- The
Board of Directors has delegated William Brody to the Board of Directors of the Genomics Institute of the Novartis Research Foundation
(GNF) for the period from the 2014 AGM to the 2016 AGM.
- (12)
- Includes
his pro-rata compensation for the delegated Board membership of GNF from February 26, 2014 to December 31, 2014
- (13)
- According
to Pierre Landolt, the Sandoz Family Foundation is the economic beneficiary of the compensation.
- (14)
- The
Board of Directors has delegated Rolf M. Zinkernagel to the Scientific Advisory Board of the Novartis Institute for Tropical Diseases
(NITD) and to the Board of Directors of the Genomics Institute of the Novartis Research Foundation (GNF) for the period from the 2014 AGM to the 2016 AGM.
- (15)
- Includes his pro-rata compensation for the delegated Board memberships of NITD and GNF from February 26, 2014 to December 31, 2014
240
RECONCILIATION BETWEEN THE REPORTED BOARD COMPENSATION AND THE AMOUNT APPROVED BY SHAREHOLDERS AT THE AGM
(CHF)
|
Compensation earned during the financial year (A)(1) 2015 |
Compensation earned for the period from January 1 to AGM (2 months) of the financial year (B) January 1, 2015 to 2015 AGM |
Compensation to be earned for the period from January 1 to the AGM (2 months) in the year following the financial year (C) January 1, 2016 to 2016 AGM(2) |
Total compensation earned from AGM to AGM (A)–(B)+(C) 2015 AGM to 2016 AGM |
Amount approved/ endorsed by shareholders at the respective AGM 2015 AGM |
Amount within the amount approved/ endorsed by shareholders at the AGM 2015 AGM |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Joerg Reinhardt |
3,829,197 | (658,174 | ) | 633,334 | 3,804,357 | 3,805,000 | Yes | ||||||||||||
Other Board members |
3,950,479 | (667,250 | ) | 653,334 | 3,936,563 | 3,940,000 | Yes | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total |
7,779,676 | (1,325,424 | ) | 1,286,668 | 7,740,920 | 7,745,000 | Yes | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| |
2014 | January 1, 2014 to 2014 AGM(3) |
January 1, 2015 to 2015 AGM |
2014 AGM to 2015 AGM |
2014 AGM | 2014 AGM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Joerg Reinhardt |
3,957,844 | (670,497 | ) | 658,174 | 3,945,521 | 3,962,000 | Yes | ||||||||||||
Other Board members |
4,807,220 | (1,446,909 | )(4) | 667,250 | 4,027,561 | 4,060,000 | Yes | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total |
8,765,064 | (2,117,406 | ) | 1,325,424 | 7,973,082 | 8,022,000 | Yes | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
- (1)
- See
above for 2015 and 2014 Board member compensation.
- (2)
- To
be confirmed and reported in the 2016 Compensation Report.
- (3)
- Includes
an amount of CHF 27,771 for mandatory employer social security contributions paid by Novartis to Swiss governmental social
security systems. This amount is out of total employer contributions of CHF 359,890, and provides a right to the maximum future insured government pension benefit for the Board member.
- (4)
- Delegated Board membership fees earned after the 2014 AGM by William Brody and Rolf M. Zinkernagel are included in this amount.
241
Loans to Board members
No loans were granted to current or former members of the Board of Directors or to "persons closely linked" to them during 2015. No such loans were outstanding as of December 31, 2015.
Other payments to Board members
During 2015, no payments (or waivers of claims) other than those set out in the table entitled "Board Member Compensation Earned for Financial Year 2015" (including its footnotes) under "—2015 Board Compensation—Board Member Compensation Table (Audited)" were made to current members of the Board of Directors or to "persons closely linked" to them.
Share ownership requirements for Board members
The Chairman is required to own a minimum of 30,000 shares, and other members of the Board of Directors are required to own at least 4,000 Novartis shares within three years after joining the Board of Directors, to ensure alignment of their interests with shareholders. Board members are prohibited from hedging or pledging their ownership positions in Novartis shares that are part of their guideline share ownership requirement, and are required to hold these shares for 12 months after retiring from the Board of Directors. As of December 31, 2015, all members of the Board of Directors who have served at least three years on the Board of Directors have complied with the share ownership guidelines.
Shares, ADRs and share options owned by Board members
The total number of vested Novartis shares and ADRs owned by members of the Board of Directors and "persons closely linked" to them as of December 31, 2015 is shown in the table below.
As of December 31, 2015, no members of the Board of Directors together with "persons closely linked" to them owned 1% or more of the outstanding shares (or ADRs) of Novartis. As of the same date, no members of the Board of Directors held any share options.
SHARES AND ADRs OWNED BY BOARD MEMBERS(1)
| |
Number of shares(2) |
|||
|---|---|---|---|---|
| |
At December 31, 2015 |
|||
Joerg Reinhardt |
480,404 | |||
Enrico Vanni |
15,566 | |||
Nancy Andrews |
609 | |||
Dimitri Azar |
9,292 | |||
Verena A. Briner |
6,429 | |||
Srikant Datar |
32,629 | |||
Ann Fudge |
15,605 | |||
Pierre Landolt(3) |
54,866 | |||
Charles L. Sawyers |
4,252 | |||
Andreas von Planta |
124,868 | |||
William T. Winters |
5,998 | |||
| | | | | |
Total(4) |
750,518 | |||
| | | | | |
| | | | | |
| | | | | |
- (1)
- Includes holdings of "persons closely linked" to Board members (see definition under "—2015 Executive Committee Compensation—Executive Committee Compensation Tables (Audited)—Persons Closely Linked")
242
- (2)
- Each
share provides entitlement to one vote.
- (3)
- According
to Pierre Landolt, the Sandoz Family Foundation is the economic beneficiary of the shares.
- (4)
- Ulrich Lehner stepped down from the Board of Directors on February 26, 2015. At February 26, 2015, Ulrich Lehner owned 37,263 shares.
Payments to former Board members
During 2015, no payments (or waivers of claims) were made to former Board members or to "persons closely linked" to them, except for the following amounts:
- –
- Prof. Dr. William R. Brody and Prof. Dr. Rolf M. Zinkernagel, who stepped down from the Board of Directors at the 2014
AGM, received delegated Board membership fees for their work on the Boards of the Novartis Institute for Tropical Diseases (Prof. Dr. Zinkernagel) and the Genomics Institute of the Novartis
Research Foundation (Prof. Dr. Brody and Prof. Dr. Zinkernagel). During 2015, an amount of CHF 100,000 and CHF 200,000 was paid to Prof. Dr. Brody and Prof.
Dr. Zinkernagel, respectively, for their work on these Boards. Their mandate on the Board of the Genomics Institute of the Novartis Research Foundation ended as of November 19, 2015. The
company is appreciative of their many years of service on this Board.
- –
- The payments reported in "Item 18. Financial Statements—Note 27."
Item 18. Financial Statements—Note 27
The total expense for the year for the compensation awarded to Board and Executive Committee members using IFRS measurement rules is presented in the Financial Report in "Item 18. Financial Statements—Note 27."
The Swiss Code of Obligations and the Corporate Governance Guidelines of the SIX Swiss Exchange require listed companies to disclose certain information about the compensation of Board and Executive Committee members, their equity participation in the Group, and loans made to them. This Annual Report fulfills that requirement. In addition, the Annual Report is in line with the principles of the Swiss Code of Best Practice for Corporate Governance of the Swiss Business Federation (economiesuisse).
Compensation decision-making authorities
Authority for decisions related to compensation is governed by the Articles of Incorporation, the Board Regulations and the Compensation Committee Charter, which are all published on the company website: www.novartis.com/corporate-governance.
The Compensation Committee serves as the supervisory and governing body for compensation policies and plans within Novartis, and has overall responsibility for determining, reviewing and proposing compensation policies and plans for approval by the Board of Directors in line with the Compensation
243
Committee Charter. A summary of discussions and conclusions of each committee meeting is delivered to the full Board of Directors. A summary of the compensation decision-making authorities is set out below:
COMPENSATION AUTHORIZATION LEVELS WITHIN THE PARAMETERS SET BY THE SHAREHOLDERS' MEETING
Decision on
|
Authority | |
|---|---|---|
Compensation of Chairman and other Board members |
Board of Directors | |
Compensation of CEO |
Board of Directors |
|
Compensation of Executive Committee members |
Compensation Committee |
The Compensation Committee is composed exclusively of members of the Board of Directors who meet the independence criteria set forth in the Board Regulations. From the 2015 AGM, the Compensation Committee had the following four members: Ann Fudge, Enrico Vanni, Srikant Datar and William Winters. Enrico Vanni has served as Chair since 2012. Ulrich Lehner did not stand for re-election to the Board of Directors at the 2015 AGM.
Role of the Compensation Committee's independent advisor
The Compensation Committee retained Frederic W. Cook & Co. Inc. as its independent external compensation advisor for 2015. The advisor was hired directly by the Compensation Committee in 2011, and the Compensation Committee has been fully satisfied with the performance and independence of the advisor since its engagement. Frederic W. Cook & Co. Inc. is independent of management and does not perform any other consulting work for Novartis. In determining whether or not to renew the engagement with the advisor, the Compensation Committee evaluates, at least annually, the quality of the consulting service, the independence of the advisor, and the benefits of rotating advisors.
Compensation Committee meetings held in 2015
In 2015, the Compensation Committee held five formal meetings. The Compensation Committee conducted a performance self-evaluation in 2015 and a review of its charter, as it does every year.
Compensation governance and risk management
The Compensation Committee, with support from its independent advisor, reviews market trends in compensation and changes in corporate governance rules. It also reviews, together with the Risk Committee, the Novartis compensation systems to ensure that it does not encourage inappropriate or excessive risk taking and instead encourages behaviors that support sustainable value creation.
244
A summary of the risk management principles is outlined below:

Executive Committee employment contracts provide for a notice period of up to 12 months and contain no change-of-control clauses or severance provisions (e.g., agreements concerning special notice periods, longer-term contracts, "golden parachutes," waiver of lock-up periods for equities and bonds, shorter vesting periods, and additional contributions to occupational pension schemes).
Malus and clawback
Any incentive compensation paid to Executive Committee members is subject to "malus" and "clawback" rules. This means that the Board of Directors for the CEO, or the Compensation Committee for other Executive Committee members, may decide, subject to applicable law, not to pay any unpaid or unvested incentive compensation (malus), or seek to recover incentive compensation that has been paid in the past (clawback), where the payout has been proven to conflict with internal management standards including company policies and accounting policies or a violation of law. This principle applies to both the Annual Incentive and to the Long-Term Incentives.
In 2015, we took steps to further strengthen our corporate governance, reinforce the role of our Board in innovation, increase the diversity of our Board, and embed strong values in our company's culture.
Our mandate
Our Board is accountable for striving to create sustainable value as described in article 2 of Novartis AG's Articles of Incorporation. We achieve this by setting a clear strategy for Novartis and through effective governance focused on target setting, risk management, and performance optimization to provide accountability and control.
This requires an effective Board with the right composition, structure and processes, and with a clear understanding of its role. Our Board meets these requirements.
245
Our Board includes members with diverse educations, experiences, nationalities and interpersonal skills. This diversity was further strengthened when Nancy C. Andrews joined our Board in 2015. It will be further strengthened if Elizabeth Doherty and Ton Buechner are elected as new Board members at the forthcoming Annual General Meeting. For more information on these two Board member candidates, please consult our Notice of Annual General Meeting, dated January 27, 2016.
We emphasize training, performance evaluation, and ongoing improvement of our Board and its members, as well as succession planning. To get an outside view on where we could improve further, in 2014 we initiated a performance and effectiveness evaluation by an independent expert. In 2015, we conducted this performance evaluation in-house. As a result of these evaluations, our Board launched a search for the above-mentioned two new Board members to strengthen the general management and finance expertise of our Board, and decided to further deepen the business understanding of our Board members by broadening their continuing education program.
All Board members are independent, as defined by our rules and, with the exception of two of our Board members, those of key investors and proxy advisors. We have established processes to ensure our Board functions effectively. They promote efficient and balanced decision-making and seamless information transfer, enabling our Board to effectively fulfill its duties.
Our Board is primarily responsible for setting the strategic direction of Novartis and for appointing our CEO and the other Executive Committee members. We assert independent judgment and work closely with our Executive Committee, making sure our strategy is properly implemented and our ethical standards are applied.
Important Board decisions
One of the most important tasks of our Board is to set the strategic direction of Novartis, re-evaluate it each year, and make necessary changes in line with our mandate to create sustainable value. Active portfolio management is part of this role.
To fulfill this task, our Board holds a dedicated two-day strategy meeting each August. In 2015, we completed our portfolio transformation, approved by our Board in 2014, to focus on our core businesses—Pharmaceuticals, Alcon and Sandoz—and to bring our Over-the-Counter business into a joint venture, with Novartis holding a significant minority stake. Our strategy for these businesses has not changed. It is to use science-based innovation to deliver better outcomes for patients. We aim to lead in growing areas of healthcare.
The new Research & Development Committee of the Board, created to oversee our research and development strategy and to strengthen the Board's role in innovation, met four times in 2015 to evaluate various aspects of the effectiveness and competitiveness of our research and development organization.
Novartis also implemented a Board decision to create a centralized services group, Novartis Business Services, to facilitate collaboration across our divisions, and drive efficiency and productivity gains.
Finally, in 2015 we endorsed a proposal from our Executive Committee to introduce a revised set of six values to guide our employees' behavior at work. They include integrity and collaboration, and I believe they are important to the long-term success of Novartis.
Role of the Chairman
As independent, non-executive Chairman, I provide direction to our Board and make sure we effectively collaborate with our CEO and Executive Committee.
I ensure that our Board and its committees work effectively, setting the agenda, style and tone of Board discussions; promoting constructive debate and effective decision-making; and ensuring that our performance is regularly evaluated and that our members are properly trained.
246
In addition, I support and mentor our CEO, but do not interfere with the operational management of Novartis. I also promote effective communication with shareholders, so that we understand your views. In this task, I am supported by our Vice Chairman, Enrico Vanni.
Strengthened governance framework
As of last year, we introduced annual elections of the Chairman of the Board, of all Board members, and of Compensation Committee members, and we instituted the option for our shareholders to provide their voting instructions to the Independent Proxy electronically. Moreover, we introduced yearly binding shareholder votes on the aggregate compensation of our Board and Executive Committee, as well as a yearly non-binding shareholder vote on the Compensation Report.
Importance of shareholder engagement
Shareholder engagement is critical to our company's long-term success. Our Board of Directors is dedicated to enhancing interactions with our shareholders. We conduct interactions in an atmosphere of trust and respect that promotes a collaborative dialogue between Novartis and our shareholders—with views and positions expressed openly to enhance mutual understanding. As part of these efforts, our governance specialists meet regularly with their peers from shareholder groups. I have also personally met with many of our shareholders and intend to continue this dialogue.
Joerg Reinhardt
Chairman of the Board of Directors
SUMMARY OF OUR CORPORATE GOVERNANCE APPROACH
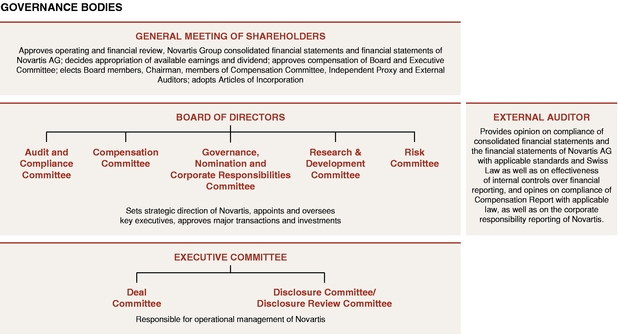
Independent, non-executive Chairman and separate CEO
247
Structure
All Board members are non-executive and independent as defined by our rules. The Board has assigned responsibilities to five committees:
- –
- Audit and Compliance Committee
- –
- Compensation Committee
- –
- Governance, Nomination and Corporate Responsibilities Committee
- –
- Research & Development Committee
- –
- Risk Committee
Composition
Board members have diverse educations, experience, nationalities and interpersonal skills. Their biographies (see "—Item 6.A Directors and Senior Management") describe their specific qualifications.
Processes
The Board's processes significantly influence its effectiveness. The Board has implemented best practices for all such processes. Important elements include Board meeting agendas (to address all important topics), information submitted to the Board (to ensure the Board receives sufficient information from management to perform its supervisory duty and to make decisions that are reserved for it), and boardroom behavior (to promote an efficient and balanced decision-making process).
Board and Executive Committee Compensation
Information on Board and Executive Committee compensation is outlined in our Compensation Report. See "—Item 6.B Compensation".
Full Implementation of Minder Ordinance
In 2015, all elements of the rules implementing the Minder Initiative were fully introduced with the amendment of the Articles of Incorporation of Novartis AG. Key Articles of Incorporation content is presented in this Corporate Governance Report, including information on the maximum number of Board mandates of Board and Executive Committee members, and on the "say-on-pay" votes at the Annual General Meeting of Shareholders (AGM).
OUR SHARES AND OUR SHAREHOLDERS
Share Capital of Novartis AG
As of December 31, 2015, the share capital of Novartis AG is CHF 1,338,496,500 fully paid-in and divided into 2,676,993,000 registered shares, each with a nominal value of CHF 0.50. Novartis AG has neither authorized nor conditional capital. There are no preferential voting shares; all shares have equal voting rights. No participation certificates, non-voting equity securities (Genussscheine), or profit-sharing certificates have been issued.
Novartis shares are listed on the SIX Swiss Exchange (ISIN CH0012005267, symbol: NOVN), as well as on the New York Stock Exchange (NYSE) in the form of American depositary receipts (ADRs) representing Novartis American depositary shares (ADSs) (ISIN US66987V1098, symbol: NVS).
248
The holder of an ADR has the rights enumerated in the deposit agreement (such as the right to give voting instructions and to receive a dividend). The ADS depositary of Novartis AG—JPMorgan Chase Bank, New York—holding the Novartis shares underlying the ADRs is registered as a shareholder in the Novartis Share Register. An ADR is not a Novartis share and an ADR holder is not a Novartis AG shareholder. ADR holders exercise their voting rights by instructing the depositary to exercise their voting rights. Each ADR represents one Novartis share.
Changes in Share Capital
During the last three years, the following changes were made to the share capital of Novartis AG:
In 2013 and 2014, the share capital of Novartis AG did not change. In 2015, Novartis AG reduced its share capital by CHF 14.6 million (from CHF 1,353,096,500 to CHF 1,338,496,500) by canceling 29.2 million Novartis shares repurchased on the second trading line during 2013 and 2014.
| Capital Changes |
|
|
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of shares | |
|||||||||||
Year
|
As of Jan 1 | Changes in shares |
As of Dec 31 | Changes in CHF |
|||||||||
2013 |
2,706,193,000 | 2,706,193,000 | |||||||||||
2014 |
2,706,193,000 | 2,706,193,000 | |||||||||||
2015 |
2,706,193,000 | (29,200,000 | ) | 2,676,993,000 | (14,600,000 | ) | |||||||
Convertible or Exchangeable Securities
Novartis AG has not issued convertible or exchangeable bonds, warrants, options or other securities granting rights to Novartis shares, other than options (and similar instruments such as stock appreciation rights) granted under or in connection with equity-based participation plans of associates. Novartis AG does not grant any new stock options under these plans.
Share Repurchase Programs
At the AGM in February 2008, shareholders approved the sixth share repurchase program authorizing the Board to repurchase Novartis shares up to a maximum of CHF 10 billion via a second trading line on the SIX Swiss Exchange. In 2008, a total of 6 million Novartis shares were repurchased at an average price of CHF 49.42 per Novartis share and canceled in 2009. In April 2008, the share repurchases were suspended in favor of debt repayment. In December 2010, the Board announced the reactivation of the share repurchases. In 2011, 39,430,000 Novartis shares were repurchased at an average price of CHF 52.81 per Novartis share and canceled in 2012. In 2012, no Novartis shares were repurchased. In 2013, 2,160,000 Novartis shares were repurchased at an average price of CHF 70.58 per Novartis share. In 2014, 27,040,000 Novartis shares were repurchased at an average price of CHF 81.18 per Novartis share. In 2015, 29,200,000 Novartis shares bought in 2013 and 2014 were canceled, and 49,878,180 Novartis shares were repurchased at an average price of CHF 93.24 per Novartis share. With those repurchases, the sixth share repurchase program has been completed.
Share Developments
- –
- Swiss-listed Novartis shares decrease 6% to CHF 86.80
- –
- ADRs decrease 7% to $86.04
Novartis shares finished at CHF 86.80, a decrease of 6% from the 2014 year-end closing price of CHF 92.35. Novartis ADRs decreased in 2015 by 7% to $86.04 from $92.66. The Swiss Market Index
249
(SMI) in comparison decreased by 1.8% in 2015, whereas the world pharmaceutical index (MSCI) grew by 2.6% during the year. Total shareholder return in 2015 was –3.4% in CHF and –3.5% in US dollars. Over a longer-term period, Novartis AG has consistently delivered a solid performance, providing a 9.9% compounded annual total shareholder return between January 1, 1996 and December 31, 2015, exceeding the 8.9% compounded returns of its large pharmaceutical peers (see "—Item 6.B Compensation—Benchmark Companies") or the returns of 9.2% of the MSCI.
The market capitalization of Novartis AG based on the number of Novartis shares outstanding (excluding Novartis treasury shares) amounted to $208 billion as of December 31, 2015, compared to $224 billion as of December 31, 2014.
Continuously rising dividend since 1996
The Board proposes a 4% increase in the dividend payment for 2015 to CHF 2.70 per Novartis share (2014: CHF 2.60) for approval at the AGM on February 23, 2016. This represents the 19th consecutive increase in the dividend paid per share since the creation of Novartis AG in December 1996. If the 2015 dividend proposal is approved by shareholders, dividends to be paid out will total approximately $6.6 billion (2014: $6.6 billion). This would result in an expected payout ratio of 93% of net income from continuing operations (2014: 62%) and 37% of net income attributable to shareholders of Novartis AG (2014: 65%). Based on the 2015 year-end share price of CHF 86.80, the dividend yield will be 3.1% (2014: 2.8%). The dividend payment date has been set for February 29, 2016.
Novartis AG offers a Direct Share Purchase Plan to investors residing in Switzerland. It provides an easy and inexpensive way for investors to directly purchase registered Novartis shares and for them to be held at no cost in a deposit account with SIX SAG AG. Due to legal restrictions, investors residing outside Switzerland may not participate in the plan. At the end of 2015, 7,814 shareholders were enrolled in this plan.
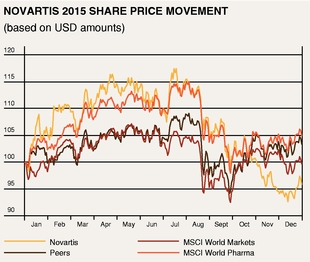
250
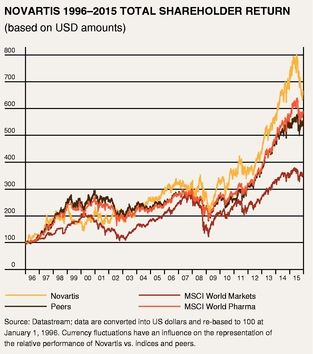
| Key Novartis Share Data |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Issued shares |
2,676,993,000 | 2,706,193,000 | 2,706,193,000 | |||||||
Treasury shares(1) |
303,098,183 | 307,566,743 | 280,108,692 | |||||||
Outstanding shares at December 31 |
2,373,894,817 | 2,398,626,257 | 2,426,084,308 | |||||||
Weighted average number of shares outstanding |
2,402,806,352 | 2,425,782,324 | 2,440,849,805 | |||||||
- (1)
- Approximately 137 million treasury shares (2014: 153 million; 2013: 149 million) are held in entities that restrict their availability for use.
| Per-share information(1) |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Basic earnings per share (US dollars) from continuing operations |
2.92 | 4.39 | 3.76 | |||||||
Basic earnings per share (US dollars) from discontinued operations |
4.48 | (0.18 | ) | 0.00 | ||||||
Total basic earnings per share (US dollars) |
7.40 | 4.21 | 3.76 | |||||||
Diluted earnings per share (US dollars) from continuing operations |
2.88 | 4.31 | 3.70 | |||||||
Diluted earnings per share (US dollars) from discontinued operations |
4.41 | (0.18 | ) | 0.00 | ||||||
Total diluted earnings per share |
7.29 | 4.13 | 3.70 | |||||||
Operating cash flow (US dollars) from continuing operations |
5.03 | 5.73 | 5.17 | |||||||
Year-end equity for Novartis AG shareholders (US dollars) |
32.46 | 29.50 | 30.64 | |||||||
Dividend (CHF)(2) |
2.70 | 2.60 | 2.45 | |||||||
- (1)
- Calculated
on the weighted average number of shares outstanding, except year-end equity.
- (2)
- 2015: Proposal to shareholders for approval at the Annual General Meeting on February 23, 2016.
251
| Key ratios—December 31 |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Price/earnings ratio(1) |
11.9 | 22.2 | 21.3 | |||||||
Price/earnings ratio from continuing operations(1) |
30.1 | 21.3 | 21.3 | |||||||
Enterprise value/EBITDA from continuing operations |
16 | 15 | 13 | |||||||
Dividend yield (%)(1) |
3.1 | 2.8 | 3.4 | |||||||
- (1)
- Based on the Novartis share price at December 31 of each year.
| Key data on ADRs issued in the US |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Year-end ADR price ($) |
86.04 | 92.66 | 80.38 | |||||||
High(1) |
106.12 | 96.65 | 80.39 | |||||||
Low(1) |
83.96 | 78.20 | 63.70 | |||||||
Number of ADRs outstanding(2) |
299,578,398 | 307,623,364 | 317,193,803 | |||||||
- (1)
- Based
on the daily closing prices.
- (2)
- The depositary, JPMorgan Chase Bank, holds one Novartis AG share for every ADR issued.
| Share price (CHF) |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Year-end share price |
86.80 | 92.35 | 71.20 | |||||||
High(1) |
102.30 | 93.80 | 73.65 | |||||||
Low(1) |
82.20 | 70.65 | 58.70 | |||||||
Year-end market capitalization ($ billions)(2) |
208.3 | 223.7 | 194.2 | |||||||
Year-end market capitalization (CHF billions)(2) |
206.1 | 221.5 | 172.7 | |||||||
- (1)
- Based
on the daily closing prices.
- (2)
- Market capitalization is calculated based on the number of shares outstanding (excluding treasury shares).
Significant Shareholders
According to the Novartis Share Register, as of December 31, 2015, the following registered shareholders (including nominees and the ADS depositary) held more than 2% of the total share capital of Novartis AG with the right to vote these shares:1
- –
- Shareholders: Novartis Foundation for Employee Participation, with its registered office in Basel, holding 2.6%; and Emasan AG, with
its registered office in Basel, holding 3.3%
- –
- Nominees: Chase Nominees Ltd., London,2 holding 8.8%; Nortrust Nominees, London, holding 3.2%; and The Bank of
New York Mellon, New York, holding 4.6% through its nominees, Mellon Bank, Everett, holding 1.7% and The Bank of New York Mellon, Brussels, holding 2.9%
- –
- ADS depositary: JPMorgan Chase Bank, New York, holding 11.2%
According to disclosure notifications filed with Novartis AG and the SIX Swiss Exchange, each of the following shareholders held between 3% and 5% of the share capital of Novartis AG as of December 31, 2015:
- –
- Capital Group Companies Inc., Los Angeles
- –
- BlackRock Inc., New York
- 1
- Excluding
6.2% of the share capital held as treasury shares by Novartis AG and its entities that restrict their availability for use
- 2
- Previously reported as JPMorgan Chase Bank, New York, but changed to its affiliate Chase Nominees Ltd., London, which is entered as nominee in the Novartis Share Register
252
Disclosure notifications pertaining to shareholdings in Novartis AG that were filed with Novartis AG and the SIX Swiss Exchange are published on the latter's electronic publication platform, and can be accessed via:
www.six-exchange-regulation.com/de/home/publications/significant-shareholders.html.
Cross Shareholdings
Novartis AG has no cross shareholdings in excess of 5% of capital or voting rights with any other company.
Distribution of Novartis Shares
The information in the following tables relates only to registered shareholders and does not include holders of unregistered shares. Also, the information provided in the tables below cannot be assumed to represent the entire Novartis AG investor base because nominees and JPMorgan Chase Bank, as ADS depositary, are registered as shareholders for a large number of beneficial owners.
As of December 31, 2015, Novartis AG had approximately 161,000 registered shareholders.
Number of Shares Held As of December 31, 2015 |
Number of registered shareholders |
% of registered share capital |
|||||
|---|---|---|---|---|---|---|---|
| 1–100 | 24,096 | 0.06 | |||||
| 101–1,000 | 96,203 | 1.53 | |||||
| 1,001–10,000 | 36,616 | 3.83 | |||||
| 10,001–100,000 | 3,387 | 3.32 | |||||
| 100,001–1,000,000 | 470 | 5.16 | |||||
| 1,000,001–5,000,000 | 73 | 5.79 | |||||
| 5,000,001 or more(1) | 34 | 50.79 | |||||
| | | | | | | | |
| Total registered shareholders/shares | 160,879 | 70.48 | |||||
| Unregistered shares | 29.52 | ||||||
| | | | | | | | |
| Total | 100.00 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- (1)
- Including significant registered shareholders as listed above
Registered Shareholders by Type As of December 31, 2015 |
Shareholders in % |
Shares in % |
|||||
|---|---|---|---|---|---|---|---|
Individual shareholders |
96.14 | 11.76 | |||||
Legal entities |
3.79 | 39.65 | |||||
Nominees, fiduciaries and ADS depositary |
0.07 | 48.59 | |||||
| | | | | | | | |
Total |
100.00 | 100.00 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
253
Registered Shareholders by Country As of December 31, 2015 |
Shareholders in % |
Shares in % |
|||||
|---|---|---|---|---|---|---|---|
France |
2.49 | 0.92 | |||||
Germany |
5.21 | 1.91 | |||||
Luxembourg |
0.03 | 1.08 | |||||
Switzerland(1) |
88.60 | 40.93 | |||||
United Kingdom |
0.50 | 23.77 | |||||
United States |
0.30 | 27.53 | |||||
Other countries |
2.87 | 3.86 | |||||
| | | | | | | | |
Total |
100.00 | 100.00 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Registered shares held by nominees are shown in the country where the company/affiliate entered in the Novartis Share Register as shareholder has its registered seat
- (1)
- Excluding 6.2% of the share capital held as treasury shares by Novartis AG and its entities that restrict their availability for use
Shareholder Rights
Shareholders have the right to receive dividends, to vote and to execute such other rights as granted under Swiss law and the Articles of Incorporation.
Each Novartis share registered with the right to vote entitles the holder to one vote at General Meetings. Novartis shares can only be voted if they are registered with voting rights with the Novartis Share Register by the third business day before the General Meeting (for shareholder registration and voting restrictions, see "—Shareholder Registration").
ADR holders may vote by instructing JPMorgan Chase Bank, the ADS depositary, to exercise the voting rights attached to the registered shares underlying the ADRs. JPMorgan Chase Bank exercises the voting rights for registered shares underlying ADRs for which no voting instructions have been given by providing a discretionary proxy to an uninstructed independent designee. Such designee has to be a Novartis AG shareholder.
The following powers are vested exclusively in the General Meeting:
- –
- Adoption and amendment of the Articles of Incorporation
- –
- Election and removal of the Chairman of the Board, Board and Compensation Committee members, the Independent Proxy and external
auditors
- –
- Approval of the management report (if required) and of the consolidated financial statements
- –
- Approval of the financial statements of Novartis AG and decision on the appropriation of available earnings shown on the balance
sheet, including with regard to dividends
- –
- Approval of the maximum aggregate amounts of compensation of the Board (for the period from an AGM until the next AGM) and of the
Executive Committee (for the financial year following the AGM)
- –
- Grant of discharge to Board and Executive Committee members
- –
- Decision of other matters that are reserved by law or by the Articles of Incorporation to the General Meeting of Shareholders
254
Resolutions and elections at General Meetings
The General Meeting passes resolutions and elections with the absolute majority of the votes represented at the meeting. However, under the Articles of Incorporation (www.novartis.com/corporate-governance), the approval of two-thirds of the votes represented at the meeting is required for:
- –
- An alteration of the purpose of Novartis AG
- –
- The creation of shares with increased voting powers
- –
- An implementation of restrictions on the transfer of registered shares and the removal of such restrictions
- –
- An authorized or conditional increase of the share capital
- –
- An increase of the share capital out of equity, by contribution in kind, for the purpose of an acquisition of property or the grant of
special rights
- –
- A restriction or suspension of rights or options to subscribe
- –
- A change of location of the registered office of Novartis AG
- –
- The dissolution of Novartis AG
In addition, the law provides for a qualified majority for other resolutions, such as a merger or spin-off.
Shareholders representing at least 10% of the share capital may request that an extraordinary General Meeting of Shareholders be convened. Shareholders representing Novartis shares with an aggregate nominal value of at least CHF 1 million may request that an item be included in a General Meeting agenda. Such requests must be made in writing at least 45 days before the meeting, specify the agenda item to be included, and contain the proposal on which the shareholder requests a vote.
Shareholders can vote their Novartis shares by themselves or appoint another shareholder or the Independent Proxy to vote on their behalf. All shareholders (who are not yet registered on the Sherpany Platform; see "—Shareholder Registration" below) receive a General Meeting invitation letter with a proxy appointment form for the appointment of the Independent Proxy. On this form shareholders can instruct the Independent Proxy to vote on alternative or additional motions related to the agenda items either (i) according to the motions of the Board for such alternative or additional motions, or (ii) against such alternative or additional motions, or (iii) to abstain from voting.
Novartis AG offers shareholders the opportunity to use an online platform (the Sherpany Platform) to receive notices of future General Meetings exclusively by email and to electronically give their instructions to the Independent Proxy, grant powers of attorney to other shareholders, and order their admission cards online. The General Meeting registration form enables shareholders who are not yet registered on the Sherpany Platform to order detailed documents related to opening a Sherpany account. They may also do so by contacting the Novartis Share Register. Shareholders can deactivate their online account at any time and again receive invitations in paper form.
Other rights associated with a registered Novartis share may only be exercised by the shareholder, its legal representative, another shareholder with the right to vote, or the Independent Proxy, or a usufructuary (a person not the owner of the share who is entitled to exercise the shareholder rights) or nominee who is registered in the Novartis Share Register.
255
Shareholder Registration
Only shareholders, usufructuaries or nominees registered in the Novartis Share Register with voting rights may exercise their voting rights. To be registered with voting rights, a shareholder must declare that he or she acquired the shares in his or her own name and for his or her own account. According to the Articles of Incorporation, the Board may register nominees with the right to vote. For restrictions on the registration of nominees, please see below.
The Articles of Incorporation provide that no shareholder shall be registered with the right to vote for more than 2% of the registered share capital. The Board may, upon request, grant an exemption from this restriction. Considerations include whether the shareholder supports the Novartis goal of creating sustainable value and has a long-term investment horizon. In 2015, no exemptions were requested. Exemptions are in force for the registered significant shareholders listed under "—Our Shareholders—Significant Shareholders," and for Norges Bank (Central Bank of Norway), Oslo, which as of December 31, 2015, held less than 2% of the share capital of Novartis AG.
The same registration and voting restrictions indirectly apply to holders of ADRs.
Given that shareholder representation at General Meetings traditionally has been rather low in Switzerland, Novartis AG considers registration restrictions necessary to prevent a minority shareholder from dominating a General Meeting.
The Articles of Incorporation provide that no nominee shall be registered with the right to vote for more than 0.5% of the registered share capital. The Board may, upon request, grant an exemption from this restriction if the nominee discloses the names, addresses and number of shares of the persons for whose account it holds 0.5% or more of the registered share capital. Exemptions are in force for the nominees listed under "—Our Shareholders—Significant Shareholders," and for the nominee Citi Bank, London, which in 2015 requested an exemption, but as of December 31, 2015 was not registered in the Novartis Share Register.
The same restrictions indirectly apply to holders of ADRs.
Registration restrictions in the Articles of Incorporation may only be removed through a resolution of the General Meeting of Shareholders, with approval of at least two-thirds of the votes represented at the meeting.
Shareholders, ADR holders or nominees who are linked to each other or who act in concert to circumvent registration restrictions are treated as one person or nominee for the purposes of the restrictions on registration.
No Restrictions on Trading Of Shares
No restrictions are imposed on the transferability of Novartis shares. The registration of shareholders in the Novartis Share Register or in the ADR register kept by JPMorgan Chase Bank does not affect the tradability of Novartis shares or ADRs. Registered Novartis shareholders or ADR holders may, therefore, purchase or sell their Novartis shares or ADRs at any time, including before a General Meeting regardless of the record date. The record date serves only to determine the right to vote at a General Meeting.
Change-of-Control Provisions
According to the Swiss Stock Exchange Act (as per January 1, 2016, according to the Swiss Federal Act on Financial Infrastructures), anyone who—directly, indirectly or acting in concert with third parties—acquires equity securities exceeding 331/3% of the voting rights of a company (whether or not such rights are exercisable) is required to make an offer to acquire all listed equity securities of that
256
company. A company may raise this threshold to 49% of the voting rights ("opting up") or may, under certain circumstances, waive the threshold ("opting out"). Novartis AG has not adopted any such measures.
In accordance with good corporate governance and the rules implementing the Minder Initiative, there are no change-of-control clauses and "golden parachute" agreements benefiting Board members, Executive Committee members, or other members of senior management. Furthermore, employment contracts with Executive Committee members do not contain notice periods or contract periods exceeding 12 months, or commissions for the acquisition or transfer of enterprises or severance payments.
General Compensation Provisions
Non-executive members of the Board of Directors
Compensation of non-executive members of the Board includes fixed compensation elements only. In particular, non-executive members of the Board of Directors shall receive no company contributions to any pension plan, no performance-related elements, and no financial instruments (e.g., options).
Members of the Executive Committee
The members of the Executive Committee receive fixed and variable performance-related compensation award. Fixed compensation comprises of the base salary and may include other elements and benefits such as contributions to pension plans. Variable compensation may be structured into short-term and long-term compensation elements. Short-term variable compensation elements shall be governed by performance metrics that take into account the performance of Novartis and/or parts thereof, and/or individual targets. Achievements are generally measured based on the one-year period to which the short-term compensation relates. The long-term compensation plans are based on performance metrics that take into account strategic objectives of Novartis (such as financial, innovation, shareholder return and/or other metrics). Achievements are generally measured based on a period of not less than three years.
If the maximum aggregate amount of compensation already approved by the General Meeting is not sufficient to cover the compensation of newly appointed or promoted Executive Committee members, Novartis may pay out compensation, in a total amount up to 40% of the total maximum aggregate amount last approved for the Executive Committee per compensation period, to newly appointed or promoted Executive Committee members.
For detailed information on the compensation of the Board and Executive Committee, see "—Item 6.B Compensation".
257

Board members, the Chairman, and Compensation Committee members are elected annually and individually by shareholders at the General Meeting. Board members whose term of office has expired are immediately eligible for re-election.
The average tenure of Board members is six years. A Board member must retire after reaching age 70. Under special circumstances, shareholders may grant an exemption from this rule and re-elect a Board member for additional terms of office. There is no mandatory term limit for Board members, so as not to lose the value of the insight and knowledge of the company's operations and practices that long-serving Board members have developed.
Name
|
Nationality | Year of birth |
First election at AGM |
Last election at AGM |
End of current term |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Joerg Reinhardt, Ph.D. |
D | 1956 | 2013 | 2015 | 2016 | ||||||||||
Enrico Vanni, Ph.D. |
CH | 1951 | 2011 | 2015 | 2016 | ||||||||||
Nancy C. Andrews, M.D., Ph.D |
US | 1958 | 2015 | 2015 | 2016 | ||||||||||
Dimitri Azar, M.D. |
US | 1959 | 2012 | 2015 | 2016 | ||||||||||
Verena A. Briner, M.D. |
CH | 1951 | 2013 | 2015 | 2016 | ||||||||||
Srikant Datar, Ph.D. |
US | 1953 | 2003 | 2015 | 2016 | ||||||||||
Ann Fudge |
US | 1951 | 2008 | 2015 | 2016 | ||||||||||
Pierre Landolt, Ph.D. |
CH | 1947 | 1996 | 2015 | 2016 | ||||||||||
Andreas von Planta, Ph.D. |
CH | 1955 | 2006 | 2015 | 2016 | ||||||||||
Charles L. Sawyers, M.D. |
US | 1959 | 2013 | 2015 | 2016 | ||||||||||
William T. Winters |
UK/US | 1961 | 2013 | 2015 | 2016 | ||||||||||
Board Composition
The composition of the Board must align with our status as a listed company, business portfolio, geographic reach and culture. The Board must be diverse in all aspects. Knowledge and experience in the
258
following fields must be represented on the Board: leadership and management; healthcare, life sciences and medicine; research and development; engineering and technology; marketing; banking, finance and accounting; human resources; legal and public affairs; and risk management.
Individual Board Member Profile
Board members should have the following personal qualities:
- –
- Interact with other Board members to build an effective and complementary Board
- –
- Establish trusting relationships
- –
- Apply independence of thought
- –
- Be challenging but supportive in the boardroom
- –
- Influence without creating conflict by applying a constructive, non-confrontational style
- –
- Listen well and offer advice based on sound judgment
- –
- Be able and willing to commit adequate time to Board and committee responsibilities
- –
- Be open to personal feedback and seek to be responsive
- –
- Do not have existing board memberships or hold other positions that could lead to a permanent conflict of interest
- –
- Understand and respect the boundaries of their role, leaving the operational management of the company to the CEO and his Executive Committee
Board members' biographies (see "—Item 6.A Directors and Senior Management") highlight the specific qualifications that led the Board to conclude they are qualified to serve on the Board, which is diverse in terms of background, credentials, interests and skills.
The diversity of a board of directors is critical to its effectiveness. Thus, when the Governance, Nomination and Corporate Responsibilities Committee of Novartis identifies new Board member candidates to be proposed to shareholders for election, the maintenance and improvement of the Board's diversity is an important criterion. The Board's aspiration is to have a diverse Board in all aspects. This includes nationality, gender, background and experience, age, tenure, viewpoints, interests, and technical and interpersonal skills.
259
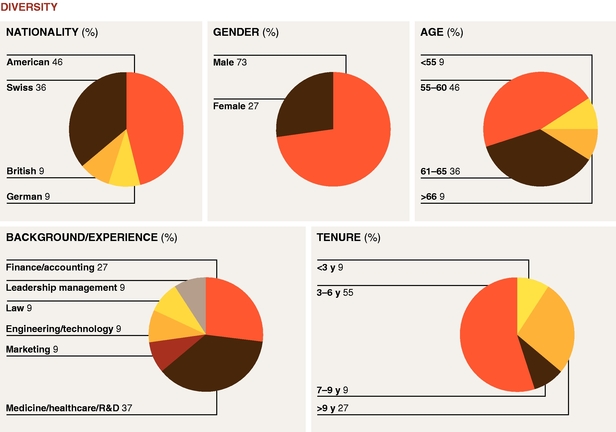
Role of the Board and its Committees
The Board is responsible for the overall direction and supervision of management and holds the ultimate decision-making authority for Novartis AG, except for those decisions reserved for shareholders.
The Board has delegated certain responsibilities to five committees, as set out below. Responsibilities described with the terms "overseeing" or "reviewing" are subject to final Board approval. The committees enable the Board to work in an efficient and effective manner, ensuring a thorough review and discussion of issues, while giving the Board more time for deliberation and decision-making. Moreover, committees
260
ensure that only Board members who are independent oversee audit and compliance, governance and compensation—as only independent Board members are delegated in the respective committees.
Responsibilities
|
Members | Number of meetings held in 2015/approximate average duration (hrs) of each meeting attendance |
Link | |||
|---|---|---|---|---|---|---|
Board of Directors |
10/6:00 | |||||
The primary responsibilities of the Board of Directors include: |
Joerg Reinhardt(1) Enrico Vanni Nancy C. Andrews(3) Dimitri Azar Verena A. Briner Srikant Datar Ann Fudge Pierre Landolt Andreas von Planta Charles L. Sawyers William T. Winters |
10 10 8 10 10 10 10 10 10 10 10 |
Articles of Incorporation of Novartis AG Regulations of the Board of Directors, its Committees and the Executive Committee of Novartis AG (Board Regulations) www.novartis.com/ corporate-governance |
|||
Audit and Compliance Committee |
7/2:30 |
|||||
The primary responsibilities of this committee include: |
Srikant Datar(1),(2) Dimitri Azar Enrico Vanni Andreas von Planta |
7 7 7 7 |
Charter of the Audit and Compliance Committee www.novartis.com/ corporate-governance |
|||
Compensation Committee |
5/2:30 |
|||||
The primary responsibilities of this committee include: |
Enrico Vanni(1) Srikant Datar Ann Fudge William T. Winters(4) |
5 5 5 4 |
Charter of the Compensation Committee www.novartis.com/ corporate-governance |
- (1)
- Chairman
- (2)
- Audit
Committee Financial Expert as defined by the US Securities and Exchange Commission
- (3)
- as
of AGM February 2015
- (4)
- as of April meeting
261
Responsibilities
|
Membership comprises |
Number of meetings held in 2015/approximate average duration (hrs) of each meeting attendance |
Link | |||
|---|---|---|---|---|---|---|
Governance, Nomination and Corporate Responsibilities Committee |
3/2:00 | |||||
The primary responsibilities of this committee include: |
Pierre Landolt(1) Ann Fudge Charles L. Sawyers Andreas von Planta |
3 2 3 3 |
Charter of the Governance, Nomination and Corporate Responsibilities Committee www.novartis.com/corporate-governance |
262
Responsibilities
|
Membership comprises |
Number of meetings held in 2015/approximate average duration (hrs) of each meeting attendance |
Link | |||
|---|---|---|---|---|---|---|
Research & Development Committee |
4/8:00 |
|||||
The primary responsibilities of this committee include: |
Joerg Reinhardt(1) Nancy C. Andrews(2) Dimitri Azar Charles L. Sawyers Enrico Vanni |
4 3 4 4 3 |
Charter of the Research & Development Committee www.novartis.com/corporate-governance |
|||
Risk Committee |
4/2:00 |
|||||
The primary responsibilities of this committee include: |
Andreas von Planta(1) Verena A. Briner Srikant Datar Ann Fudge |
4 4 4 4 |
Charter of the Risk Committee www.novartis.com/corporate-governance |
- (1)
- Chairman
- (2)
- as of AGM February 2015
263
The Board takes decisions as a whole, supported by its five committees. Each committee has a written charter outlining its duties and responsibilities, and is led by a Board-elected chairman.
The Board and its committees meet regularly throughout the year. The chairmen set their meeting agendas. Any Board member may request a Board or committee meeting, and the inclusion of an agenda item. Before meetings, Board members receive materials to help them prepare the discussions and decision-making.
Joerg Reinhardt has been independent, non-executive Chairman since August 1, 2013. He has both industry and Novartis experience, and meets the company's independence criteria. As independent Chairman, he can lead the Board to represent the interests of all stakeholders, being accountable to them and creating sustainable value through effective governance. The independent chairmanship also ensures an appropriate balance of power between the Board and Executive Committee.
In this role, Joerg Reinhardt:
- –
- Provides leadership to the Board
- –
- Supports and mentors the CEO
- –
- Supported by the Governance, Nomination and Corporate Responsibilities Committee, ensures effective succession plans for the Board and
the Executive Committee
- –
- Ensures that the Board and its committees work effectively
- –
- Sets the agenda, style and tone of Board discussions, promoting constructive dialogue and effective decision-making
- –
- Supported by the Governance, Nomination and Corporate Responsibilities Committee, ensures that all Board committees are properly
established, composed and operated
- –
- Ensures that the Board's performance is annually evaluated
- –
- Ensures introduction programs for new Board members and continuing education as well as specialization for all Board members
- –
- Ensures effective communication with the company's shareholders
- –
- Promotes effective relationships and communication between Board and Executive Committee members
Enrico Vanni has been independent, non-executive Vice Chairman since February 22, 2013.
In this role, he:
- –
- Leads the Board in case and as long as the Chairman is incapacitated
- –
- Chairs the sessions of independent Board members and leads independent Board members if and as long as the Chairman is not independent
The Board has meetings with Executive Committee members as well as private meetings without them.
264
In 2015, there were 10 Board meetings. Because all Board members are independent, no separate meetings of the independent Board members were held in 2015.
Key activities of our Board and committees in 2015
The Board meeting agendas in 2015 included the following standard topics: strategy; Group targets; personal objectives of the CEO; mergers and acquisitions, and business development and licensing review; financial and business reviews; major projects; investments and transactions; the Annual Report; and the General Meeting agenda. Topics addressed during private meetings included Board self-evaluation and performance assessment of senior management, as well as succession planning.
In addition, in 2015 our Board and its committees focused on a number of special topics, including:
Board of Directors:
Our biosimilars development pipeline, the pricing and competitive environment in pharmaceuticals, the rollout of our new Values and Behaviors, a review of our brand identity, the proposal to revise our Articles of Incorporation and Board regulations to implement the "Minder Legislation," the analysis of the AGM 2015 and investor feedback from our corporate governance roadshow, the issue of new bonds, and the renewal of existing credit facilities.
Governance, Nomination and Corporate Responsibilities Committee:
Investor feedback from our corporate governance roadshow and how to address it; the search profile for and discussion of potential new Board members to strengthen the general management and financial expertise background of our Board; a review of our corporate responsibility activities, including the proposal to introduce an "Access Brand" (a first-of-its-kind portfolio of products aimed at increasing access to medicines in low- and low-middle-income countries); and reviewing the activities of the Novartis Foundation (a philanthropic organization pioneering innovative healthcare models that have a transformational impact on the health of the poorest populations).
Compensation Committee:
The metrics that underpin the Annual Incentive and the performance-based Long-Term Incentive plans; the constituents of the Novartis healthcare peer group used for benchmarking and variable compensation purposes; the rollout of the compensation system of Executive Committee members to the broader Novartis executive group, as well as approving the Long-Term Incentive plans for the rest of the Novartis employee population; investor feedback from the corporate governance roadshow; and expense policies.
Audit and Compliance Committee:
The accounting of the portfolio transformation, the Novartis IT security organization and challenges, the roles of the Audit and Compliance Committee and the Risk Committee to avoid potential gaps or overlaps, working toward integrated assurance, specific accounting and compliance topics, compensation disclosures, the revision of the Internal Audit Charter, and the definition of growth products.
Risk Committee:
Key business risks at Alcon; pharmacovigilance and quality preparedness; benchmarking the enterprise risk management organization and processes; risks related to pricing, data privacy, IT security, and data integrity in manufacturing and development; and risks and opportunities related to the Step Change program (a program evolving our approach to business practices and customer relationships to strengthen our focus on performance with integrity).
265
Research & Development Committee:
The Novartis portfolio of R&D projects in the following areas: respiratory diseases; infectious diseases; autoimmune, transplantation and immunological diseases; cardiovascular and metabolic diseases; immuno-oncology; and musculoskeletal diseases. The committee also supported the setting and evaluation of innovation-related long-term performance metrics.
Dr. Alex Krauer and Dr. Daniel Vasella have been appointed Honorary Chairmen in recognition of their significant achievements on behalf of Novartis. They are not provided with Board documents and do not attend Board meetings.
The independence of Board members is a key corporate governance issue. An independent Board member is one who is independent of management and has no business or relationship that could materially interfere with the exercise of objective, unfettered and independent judgment. Only with a majority of Board members being independent can the Board fulfill its obligation to represent the interests of shareholders, being accountable to them and creating sustainable value through an effective governance of Novartis. Accordingly, Novartis established independence criteria based on international best-practice standards and outlined on the Novartis website:
www.novartis.com/investors/governance-documents.shtml.
- –
- The majority of Board members and any member of the Audit and Compliance Committee; the Compensation Committee; and the Governance,
Nomination and Corporate Responsibilities Committee must meet the company's independence criteria. These include, inter alia, (i) a Board member not having received direct compensation of more
than $120,000 per year from Novartis, except for dividends or Board compensation, within the last three years, (ii) a Board member not having been an employee of Novartis within the last three
years, (iii) a family member not having been an executive officer of Novartis within the last three years, (iv) a Board member or family member not being employed by the external auditor
of Novartis, (v) a Board member or family member not being a board member, employee or 10% shareholder of an enterprise that has made payments to, or received payments from, Novartis, in excess
of the greater of $1 million or 2% of that enterprise's gross revenues. For members of the Audit and Compliance Committee and the Compensation Committee, even stricter rules apply.
- –
- In addition, Board members are bound by the Novartis Conflict of Interest Policy, which prevents a Board member's potential personal
interests from influencing the decision-making of the Board.
- –
- The Governance, Nomination and Corporate Responsibilities Committee annually submits to the Board a proposal concerning the
determination of the independence of each Board member. For this assessment, the committee considers all relevant facts and circumstances of which it is aware—not only the explicit formal
independence criteria. This includes an assessment of whether a Board member is truly independent, in character and judgment, from any member of the senior management and from any of his/her current
or former colleagues.
- –
- In its meeting on December 17, 2015, the Board determined that all of its members are independent.
Relationship of Non-Executive Board Members with Novartis
No Board member is or was a member of the management of Novartis AG or of any other Novartis Group company in the last three financial years up to December 31, 2015. There are no significant
266
business relationships of any Board member with Novartis AG or with any other Novartis Group company.
Mandates Outside the Novartis Group
No Board member may hold more than 10 additional mandates in other companies, of which no more than four shall be in other listed companies. Chairmanships of the boards of directors of other listed companies count as two mandates. Each of these mandates is subject to Board approval.
The following mandates are not subject to these limitations:
- a)
- Mandates
in companies that are controlled by Novartis AG
- b)
- Mandates
that a Board member holds at the request of Novartis AG or companies controlled by it. No Board member shall hold more than five such mandates.
- c)
- Mandates in associations, charitable organizations, foundations, trusts and employee welfare foundations. No Board member may hold more than 10 such mandates.
"Mandates" means those in the supreme governing body of a legal entity that is required to be registered in the commercial register or a comparable foreign register. Mandates in different legal entities that are under joint control are deemed one mandate.
The Board may issue regulations that determine additional restrictions, taking into account the position of the respective member.
No loans or credits shall be granted to members of the Board.
Board Performance and Effectiveness Evaluation
Process
The Board conducts an annual review to evaluate its performance and that of individual committees and members. As part of this process, each Board member completes a questionnaire on the performance and effectiveness of the Board and his/her committees, which lays the groundwork for a qualitative review led by the Chairman. The Chairman has discussions with each Board member, and then with the entire Board. Further, the committee evaluations are discussed by the respective committee and the results are debriefed to the Board. Any suggestion for improvement is recorded and actions are agreed upon.
Periodically, this process is conducted by an independent consultant. In 2014, an independent performance and effectiveness evaluation of the Board and its committees, including an individual Board member assessment, was conducted by the independent expert company Russell Reynolds Associates. In 2015, the performance evaluation was conducted internally.
Content and Results
The performance review examined the performance and effectiveness, and strengths and weaknesses, of individual Board members and of the full Board and each Board committee.
This review covered topics including Board composition; purpose, scope and responsibilities; processes and governance of the Board and its committees; meetings and pre-reading material; team effectiveness; and leadership and culture.
The review also evaluated the ability and willingness of each Board member to commit adequate time and effort to his/her responsibilities as provided for in the charter of the Governance, Nomination and Corporate Responsibilities Committee.
267
The results were discussed at the January 2016 meeting of the Board. It was concluded that the Board and its committees operate effectively.
Information and Control Systems of the Board vis-a-vis Management
Information on Management
The Board ensures that it receives sufficient information from the Executive Committee to perform its supervisory duty and to make decisions that are reserved for it. The Board obtains this information through several means:
- –
- The CEO informs the Board regularly about current developments
- –
- Executive Committee meeting minutes are made available to the Board
- –
- Meetings or teleconferences are held as required between Board members and the CEO
- –
- The Board regularly meets with all Executive Committee members
- –
- The Board receives detailed, quarterly updates from each Division Head
- –
- By invitation, other members of management attend Board meetings to report on areas of the business for which they are responsible
- –
- Board members are entitled to request information from Executive Committee members or any other Novartis associate, and they may visit any Novartis site
Board Committees
Board committees regularly meet with management and, at times, outside consultants to review the business, better understand applicable laws and policies affecting the Group, and support the Board and management in meeting the requirements and expectations of stakeholders and shareholders.
In particular, the Chief Financial Officer (CFO), the Group General Counsel, and representatives of the external auditors are invited to Audit and Compliance Committee meetings. Additionally, the heads of Internal Audit, Financial Reporting & Accounting, Compliance and Quality, as well as the Head of the Global Business Practices Office report on a regular basis to the Audit and Compliance Committee. This committee reviews financial reporting processes on behalf of the Board. For each quarterly and annual release of financial information, the Disclosure Review Committee is responsible for ensuring the accuracy and completeness of disclosures. The Disclosure Review Committee, which is a management committee, is chaired by the CFO and includes the CEO; the Group General Counsel; the heads of the divisions, Novartis Business Services (NBS) and the Novartis Institutes for BioMedical Research (NIBR); the heads of finance of the divisions, NBS and NIBR; and the heads of the following corporate functions: Treasury, Tax, Financial Reporting & Accounting, Internal Audit and Investor Relations. The Audit and Compliance Committee reviews decisions made by the Disclosure Review Committee before the quarterly and annual releases are published.
The Risk Committee oversees the risk management system and processes, and also reviews the risk portfolio of the Group to ensure appropriate and professional risk management. For this purpose, the Group Risk Office and the risk owners of the divisions report on a regular basis to the Risk Committee. The Group General Counsel, the Head of Group Risk, the Head of Internal Audit, and other senior executives are invited to these meetings on a regular basis.
268
Novartis Management Information System
Novartis produces comprehensive, consolidated (unaudited) financial statements on a monthly basis for the total Group and its divisions. These are typically available within 10 days of the end of the month and include the following:
- –
- Consolidated income statement of the month, quarter-to-date and year-to-date in accordance with International Financial Reporting
Standards (IFRS), as well as adjustments to arrive at core results as defined by Novartis. The IFRS and core figures are compared to the prior-year period and targets in both US dollars and on a
constant currency basis.
- –
- Consolidated balance sheet as of the month end in accordance with IFRS in US dollars
- –
- Consolidated cash flow on a monthly, quarter-to-date and year-to-date basis in accordance with IFRS in US dollars
- –
- Supplementary data on a monthly, quarterly and year-to-date basis such as free cash flow, gross and net debt, headcount, personnel costs, working capital, and earnings per share on a US dollar basis where applicable
Constant currencies, core results, free cash flow, net debt and related target figures are non-IFRS measures. An explanation of non-IFRS measures can be found in "Item 5. Operating and Financial Review and Prospects—5.A Operating Results—Non-IFRS Measures as Defined by Novartis."
The above information is made available to Board members on a monthly basis. An analysis of key deviations from the prior year or target is also provided.
The Board also receives twice a year an outlook of the full-year results in accordance with IFRS and core, along with related commentary prior to the release of the quarterly results.
On an annual basis, in the fourth quarter of the year, the Board receives and approves the operating and financial targets for the following year.
In the middle of the year, the Board also reviews and approves the strategic plan for the next five years, which includes a projected consolidated income statement in US dollars prepared in accordance with IFRS and core (as defined by Novartis).
The Board does not have direct access to the company's financial and management reporting systems but can, at any time, request more detailed financial information on any aspect that is presented to it.
Internal Audit
The Internal Audit function carries out operational and system audits in accordance with an audit plan approved by the Audit and Compliance Committee. This function helps organizational units accomplish objectives by providing an independent approach to the evaluation, improvement and effectiveness of their internal control framework. It prepares reports on the audits it has performed, and reports actual or suspected irregularities to the Audit and Compliance Committee and the CEO. The Audit and Compliance Committee regularly reviews the Internal Audit scope, audit plans and results.
Risk Management
The Group Risk Office is overseen by the Board's independent Risk Committee. The Compensation Committee works closely with the Risk Committee to ensure that the compensation system does not lead to excessive risk-taking by management (for details, see "—Item 6.B Compensation").
Organizational and process measures have been established to identify and mitigate risks at an early stage. Organizationally, the individual divisions and functions are responsible for risk and risk mitigation, with specialized corporate functions—such as Group Finance, Group Quality Assurance, Corporate
269
Health, Safety and Environment, Business Continuity Management and Integrity & Compliance, and the Business Practices Office—providing support and controlling the effectiveness of risk management by the divisions and functions in these respective areas.
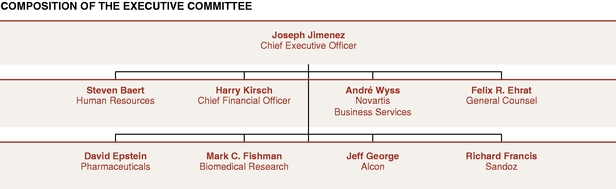
Executive Committee Composition
The Executive Committee is headed by the CEO. Its members are appointed by the Board.
There are no contracts between Novartis and third parties whereby Novartis would delegate any business management tasks to such third parties.
Executive Committee Role and Functioning
The Board has delegated to the Executive Committee overall responsibility for and oversight of the operational management of Novartis. This includes:
- –
- Developing policies and strategic plans for Board approval, and implementing those approved
- –
- Submitting to the Board and its committees proposed changes in management positions of material significance, investments, financial
measures, acquisitions or divestments, contracts of material significance, and targets—and implementing those approved
- –
- Preparing and submitting quarterly and annual reports to the Board and its committees
- –
- Informing the Board of all matters of fundamental significance to the businesses
- –
- Recruiting, appointing and promoting senior management
- –
- Ensuring the efficient operation of the Group and achievement of optimal results
- –
- Promoting an active internal and external communications policy
- –
- Dealing with any other matters delegated by the Board
The Executive Committee is supported by two sub-committees: The Deal Committee (members are the CEO, CFO, Division Head Pharmaceuticals, Group General Counsel, and Head of Biomedical Research) reviews important acquisitions and divestments of companies and businesses, and business development deals, and makes recommendations to the Executive Committee. The Disclosure Committee (members are the CEO, CFO, and Group General Counsel) determines whether an event constitutes information that is material to the Group, determines the appropriate disclosure and update of such information, and reviews media releases concerning such information.
270
In addition to other Board-assigned duties, the CEO leads the Executive Committee, building and maintaining an effective executive team. With the support of the Executive Committee, the CEO:
- –
- Is responsible for the operational management of Novartis
- –
- Develops strategy proposals to be recommended to the Board and ensures that approved strategies are implemented
- –
- Plans human resourcing to ensure that Novartis has the capabilities and means to achieve its plans, and that robust management
succession and management development plans are in place and presented to the Board
- –
- Develops an organizational structure, and establishes processes and systems to ensure the efficient organization of resources
- –
- Ensures that financial results, business strategies and, when appropriate, targets and milestones are communicated to the investment
community—and generally develops and promotes effective communication with shareholders and other stakeholders
- –
- Ensures that business performance is consistent with business principles, as well as legal and ethical standards
- –
- Develops processes and structures to ensure that capital investment proposals are reviewed thoroughly, that associated risks are
identified, and that appropriate steps are taken to manage these risks
- –
- Develops and maintains an effective framework of internal controls over risk in relation to all business activities of the company
- –
- Ensures that the flow of information to the Board is accurate, timely and clear
Mandates Outside the Novartis Group
No Executive Committee member may hold more than six additional mandates in other companies, of which no more than two additional mandates shall be in other listed companies. Each of these mandates is subject to Board approval. Executive Committee members are not allowed to hold chairmanships of the boards of directors of other listed companies.
The following mandates are not subject to these limitations:
- a)
- Mandates
in companies that are controlled by Novartis AG
- b)
- Mandates
that an Executive Committee member holds at the request of Novartis AG or companies controlled by it. No Executive Committee member shall hold more
than five such mandates.
- c)
- Mandates in associations, charitable organizations, foundations, trusts and employee welfare foundations. No Executive Committee member may hold more than 10 such mandates.
"Mandates" means those in the supreme governing body of a legal entity that is required to be registered in the commercial register or a comparable foreign register. Mandates in different legal entities that are under joint control are deemed one mandate.
The Board may issue regulations that determine additional restrictions, taking into account the position of the respective member.
271
No loans or credits shall be granted to members of the Executive Committee.
OUR INDEPENDENT EXTERNAL AUDITORS
Duration of the Mandate and Terms of Office of the Auditors
Based on a recommendation by the Audit and Compliance Committee, the Board nominates an independent auditor for election at the AGM. PricewaterhouseCoopers (PwC) assumed its existing auditing mandate for Novartis in 1996. Bruno Rossi, auditor in charge, began serving in his role in 2013, and Stephen Johnson, global relationship partner, began serving in his role in 2014. The Audit and Compliance Committee ensures that these partners are rotated at least every five years.
Information to the Board and the Audit and Compliance Committee
PwC is responsible for providing an opinion on whether the Group-consolidated financial statements comply with IFRS and Swiss law, and whether the separate parent company financial statements of Novartis AG comply with Swiss law. Additionally, PwC is responsible for opining on the effectiveness of internal control over financial reporting, on the Compensation Report as well as on the corporate responsibility reporting of Novartis.
The Audit and Compliance Committee, acting on behalf of the Board, is responsible for overseeing the activities of PwC. In 2015, this committee held seven meetings. PwC was invited to six of these meetings to attend during the discussion of agenda items that dealt with accounting, financial reporting or auditing matters, and any other matters relevant to its audit.
On an annual basis, PwC provides the Audit and Compliance Committee with written disclosures required by the US Public Company Accounting Oversight Board (PCAOB), and the committee and PwC discuss PwC's independence from Novartis and its management.
The Audit and Compliance Committee recommended to the Board to approve the audited Group-consolidated financial statements and the separate parent company financial statements of Novartis AG for the year ended December 31, 2015. The Board proposed the acceptance of these financial statements for approval by the AGM.
The Audit and Compliance Committee regularly evaluates the performance of PwC and once a year determines whether PwC should be proposed to the AGM for election. Also once a year, the auditor in charge and the global relationship partner report to the Board on PwC's activities during the current year and on the audit plan for the coming year. They also answer any questions or concerns Board members have about the performance of PwC, or about the work it has conducted or is planning to conduct.
To assess the performance of PwC, the Audit and Compliance Committee holds private meetings with the CFO and the Global Head of Internal Audit and, if necessary, obtains an independent external assessment. Criteria applied for the performance assessment of PwC include an evaluation of its technical and operational competence; its independence and objectivity; the sufficiency of the resources it has employed; its focus on areas of significant risk to Novartis; its willingness to probe and challenge; its ability to provide effective, practical recommendations; and the openness and effectiveness of its communications and coordination with the Audit and Compliance Committee, the Internal Audit function, and management.
Approval of Audit and Non-Audit Services
The Audit and Compliance Committee approves a budget for audit services whether recurring or non-recurring in nature, as well as audit-related services not related to internal controls over financial reporting. PwC reports quarterly to the Audit and Compliance Committee regarding the extent of services
272
provided in accordance with the applicable pre-approval and the fees for services performed to date. The Audit and Compliance Committee individually approves all audit-related services relating to internal controls over financial reporting, tax services and other services prior to the start of work.
PwC charged the following fees for professional services rendered for the 12-month periods ended December 31, 2015 and December 31, 2014:
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Audit Services |
25.9 | 29.7 | |||||
Audit-Related Services |
1.7 | 2.0 | |||||
Tax Services |
0.0 | 0.2 | |||||
Other Services |
0.1 | 0.1 | |||||
| | | | | | | | |
Total |
27.7 | 32.0 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Audit services include work performed to issue opinions on Group-consolidated financial statements and parent company financial statements of Novartis AG, to issue opinions relating to the effectiveness of the Group's internal control over financial reporting, and to issue reports on local statutory financial statements. Also included are audit services that generally can only be provided by the statutory auditor, such as the audit of the Compensation Report, audits of non-recurring transactions, audits of the adoption of new accounting policies, audits of information systems and the related control environment, reviews of quarterly financial results, as well as procedures required to issue consents and comfort letters.
Audit-related services include other assurance services provided by the independent auditor but not restricted to those that can only be provided by the statutory auditor. They include services such as audits of pension and other employee benefit plans, contract audits of third-party arrangements, corporate responsibility assurance, compliance with corporate integrity agreements, and other audit-related services.
Tax services represent tax compliance, assistance with historical tax matters and other tax-related services.
Other services include training in the finance area, benchmarking studies, and license fees for use of accounting and other reporting guidance databases.
OUR CORPORATE GOVERNANCE FRAMEWORK
Novartis AG is subject to the laws of Switzerland, in particular Swiss company and securities laws, and to the securities laws of the US as applicable to foreign private issuers of securities.
In addition, Novartis AG is subject to the rules of the SIX Swiss Exchange, including the Directive on Information Relating to Corporate Governance.
Novartis AG is also subject to the rules of NYSE as applicable to foreign private issuers of securities. NYSE requires Novartis AG to describe any material ways in which its corporate governance differs from that of domestic US companies listed on the exchange. These differences are:
- –
- Novartis AG shareholders do not receive written reports directly from Board committees.
- –
- External auditors are appointed by shareholders at the AGM, as opposed to being appointed by the Audit and Compliance Committee.
273
- –
- While shareholders cannot vote on all equity compensation plans, they are entitled to hold separate, yearly binding shareholder votes
on Board and Executive Committee compensation.
- –
- The Board has set up a separate Risk Committee that is responsible for business risk oversight, as opposed to delegating this
responsibility to the Audit and Compliance Committee.
- –
- The full Board is responsible for overseeing the performance evaluation of the Board and Executive Committee.
- –
- The full Board is responsible for setting objectives relevant to the CEO's compensation and for evaluating his performance.
Swiss Code of Best Practice for Corporate Governance
Novartis applies the Swiss Code of Best Practice for Corporate Governance.
Novartis Corporate Governance Standards
Novartis has incorporated the corporate governance standards described above into the Articles of Incorporation and the Regulations of the Board of Directors, its Committees and the Executive Committee of Novartis AG (www.novartis.com/corporate-governance).
The Governance, Nomination and Corporate Responsibilities Committee regularly reviews these standards and principles, taking into account best practices, and recommends improvements to the corporate governance framework for consideration by the full Board.
Additional corporate governance information can be found on the Novartis website: www.novartis.com/corporate-governance.
Printed copies of the Novartis Articles of Incorporation, Regulations of the Board, and Charters of Board Committees can be obtained by writing to: Novartis AG, Attn: Corporate Secretary, Lichtstrasse 35, CH-4056 Basel, Switzerland.
Novartis AG and Group Companies
Under Swiss company law, Novartis AG is organized as a corporation that has issued shares of common stock to investors. The registered office of Novartis AG is Lichtstrasse 35, CH-4056 Basel, Switzerland.
Business operations are conducted through Novartis Group companies. Novartis AG, a holding company, owns or controls directly or indirectly all entities worldwide belonging to the Novartis Group. Except as described below, the shares of these companies are not publicly traded. The principal Novartis subsidiaries and associated companies are listed in "Item 18. Financial Statements—Note 32."
Divisions
The businesses of Novartis are divided on a worldwide basis into three operating divisions: Pharmaceuticals, Alcon (eye care), and Sandoz (generics). In addition, there are NBS (shared services organization, delivering services to the divisions), NIBR (the company's global pharmaceutical research organization), and Group Corporate activities. In 2015, Animal Health and Vaccines were divested, and OTC was brought into a joint venture with GlaxoSmithKline's (GSK) business in this area—with Novartis holding a 36.5% minority stake in this joint venture.
274
Majority Holdings in Publicly-traded Group Companies
The Novartis Group owns 75% of Novartis India Limited, with its registered office in Mumbai, India, and listed on the Bombay Stock Exchange (ISIN INE234A01025, symbol: HCBA). The total market value of the 25% free float of Novartis India Limited was $97.6 million at December 31, 2015, using the quoted market share price at year end. Applying this share price to all the shares of the company, the market capitalization of the whole company was $390.5 million and that of the shares owned by Novartis was $292.9 million.
Significant Minority Shareholding owned by the Novartis Group
The Novartis Group owns 33.3% of the bearer shares of Roche Holding AG, with its registered office in Basel, Switzerland, and listed on the SIX Swiss Exchange (ISIN CH0012032113, symbol: RO). The market value of the Group's interest in Roche Holding AG, as of December 31, 2015, was $14.9 billion. The total market value of Roche Holding AG was $241.08 billion. Novartis does not exercise control over Roche Holding AG, which is independently governed, managed and operated.
The Novartis Group owns a 36.5% share of a joint venture created by GSK and Novartis, which combined the Novartis OTC and the GSK Consumer Healthcare businesses. Novartis holds four of the 11 seats of the joint venture's board. Furthermore, Novartis has certain minority rights and exit rights, including a put option that is exercisable as of March 2, 2018.
Novartis makes political contributions to support the political dialogue on public policy issues of relevance to Novartis, such as healthcare innovation and access to medicines.
Political contributions made by Novartis are not intended to give rise to any obligations of the party receiving it. Moreover, rules and procedures are in place to make sure that political contributions are never made with the expectation of a direct or immediate return for Novartis, and that they fully comply with applicable laws, regulations and industry codes.
Novartis only makes political contributions in countries where such contributions by corporations are legal and where political contributions from corporations are considered to reflect "good corporate citizenship." Moreover, Novartis only makes modest political contributions so as to not create any dependency from the political parties receiving these contributions.
In 2015, Novartis made political contributions totaling approximately $1.13 million, thereof approximately $680,000 in Switzerland, $235,000 in the US, $150,000 in Japan, $45,000 in Australia, $11,000 in Canada, and $8,000 in the UK. In addition, in the US, a political action committee established by Novartis used funds received from Novartis employees (but not from the company) to make political contributions totaling approximately $280,000.
In Switzerland, Novartis supports political parties that have a political agenda and hold positions that support the strategic interests of Novartis, its shareholders and other stakeholders. Swiss political parties are completely privately financed and the contributions of companies are a crucial part thereof. This private financing of parties is a deeply-rooted trait of the Swiss political culture, and contributing to that system is an important element of being a good corporate citizen.
The CEO, with the CFO and Investor Relations team, supported by the Chairman, is responsible for ensuring effective communication with shareholders to keep them informed of the company's strategy, business operations and governance. Through communication, the Board also learns about and addresses shareholders' expectations and concerns.
275
Novartis communicates with its shareholders through the AGM, meetings with groups of shareholders and individual shareholders, and written and electronic communications.
At the AGM, the Chairman, CEO and other Executive Committee members, and representatives of the external auditors are present and can answer shareholders' questions. Other meetings with shareholders may be attended by the Chairman, CEO, CFO, Executive Committee members, and other members of senior management.
Topics discussed, in full respect of applicable laws, with shareholders may include strategy, business performance and corporate governance.
Information for Our Stakeholders
Introduction
Novartis is committed to open and transparent communication with shareholders, financial analysts, customers, suppliers and other stakeholders. Novartis aims to disseminate material developments in its businesses in a broad and timely manner that complies with the rules of the SIX Swiss Exchange and NYSE.
Communications
Novartis publishes an Annual Report that provides information on the Group's results and operations. In addition, Novartis prepares an annual report on Form 20-F that is filed with the US Securities and Exchange Commission (SEC). Novartis discloses quarterly financial results in accordance with IFRS, and issues press releases from time to time regarding business developments.
Novartis furnishes press releases relating to financial results and material events to the SEC via Form 6-K. An archive containing recent Annual Reports, annual reports on Form 20-F, and quarterly results releases—as well as related materials such as slide presentations and conference call webcasts—is on the Novartis website at www.novartis.com/investors.
Novartis also publishes a consolidated Corporate Responsibility Performance Report, which details progress and demonstrates the company's commitment to be a leader in corporate responsibility. This report reflects the best-in-class reporting standard, the Global Reporting Initiative's (GRI) G4 guidelines, and fulfills the company's reporting requirement as a signatory of the UN Global Compact.
Information contained in reports and releases issued by Novartis is only correct and accurate at the time of release. Novartis does not update past releases to reflect subsequent events, and advises against relying on them for current information.
Investor Relations Program
An Investor Relations team manages the Group's interaction with the international financial community. Several events are held each year to provide institutional investors and analysts with various opportunities to learn more about Novartis.
Investor Relations is based at the Group's headquarters in Basel. Part of the team is located in the US to coordinate interaction with US investors. Information is available on the Novartis website: www.novartis.com/investors. Investors are also welcome to subscribe to a free email service on this site.
276
| Topic |
Information |
|
|---|---|---|
|
|
||
| Share Capital | Articles of Incorporation of Novartis AG www.novartis.com/corporate-governance Novartis key share data www.novartis.com/key-share-data |
|
| | | |
| Shareholder Rights | Articles of Incorporation of Novartis AG www.novartis.com/corporate-governance Investor Relations information www.novartis.com/investors |
|
| | | |
| Board Regulations | Board Regulations www.novartis.com/corporate-governance |
|
| | | |
| Executive Committee | Executive Committee www.novartis.com/executive-committee |
|
| | | |
| Novartis Code for Senior Financial Officers | Novartis Code of Ethical Conduct for CEO and Senior Financial Officers www.novartis.com/corporate-governance |
|
| | | |
| Additional Information | Novartis Investor Relations www.novartis.com/investors |
|
| | | |
The table below sets forth the breakdown of the total year-end number of our full time equivalent employees by main category of activity and geographic area for the past three years.
For the year ended December 31, 2015 (full time equivalents) |
Research & Development |
Production & Supply |
Marketing & Sales |
General & Administration |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
USA |
7,684 | 6,735 | 6,027 | 2,236 | 22,682 | |||||||||||
Canada and Latin America |
469 | 1,470 | 4,756 | 1,313 | 8,008 | |||||||||||
Europe |
10,014 | 19,767 | 18,278 | 7,383 | 55,442 | |||||||||||
Asia/Africa/Australasia |
3,413 | 6,819 | 18,611 | 3,725 | 32,568 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
21,580 | 34,791 | 47,672 | 14,657 | 118,700 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
For the year ended December 31, 2014 (full time equivalents) |
Research & Development |
Production & Supply |
Marketing & Sales |
General & Administration |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
USA |
8,147 | 8,283 | 6,529 | 2,341 | 25,300 | |||||||||||
Canada and Latin America |
515 | 2,435 | 5,309 | 1,327 | 9,586 | |||||||||||
Europe |
11,052 | 23,997 | 20,884 | 7,134 | 63,067 | |||||||||||
Asia/Africa/Australasia |
3,693 | 7,739 | 21,454 | 2,574 | 35,460 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
23,407 | 42,454 | 54,176 | 13,376 | 133,413 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Thereof Continuing Operations |
21,181 | 36,106 | 48,638 | 11,884 | 117,809 | |||||||||||
Thereof Discontinued Operations |
2,226 | 6,348 | 5,538 | 1,492 | 15,604 | |||||||||||
277
For the year ended December 31, 2013 (full time equivalents) |
Research & Development |
Production & Supply |
Marketing & Sales |
General & Administration |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
USA |
8,255 | 8,600 | 7,253 | 2,963 | 27,071 | |||||||||||
Canada and Latin America |
570 | 2,943 | 5,611 | 1,325 | 10,449 | |||||||||||
Europe |
11,438 | 23,449 | 20,719 | 7,009 | 62,615 | |||||||||||
Asia/Africa/Australasia |
3,674 | 7,331 | 21,986 | 2,570 | 35,561 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
23,937 | 42,323 | 55,569 | 13,867 | 135,696 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Thereof Continuing Operations |
21,658 | 35,847 | 49,643 | 12,214 | 119,362 | |||||||||||
Thereof Discontinued Operations |
2,279 | 6,476 | 5,926 | 1,653 | 16,334 | |||||||||||
As of December 31, 2015, the number of our full time equivalent employees decreased by approximately 15,000 compared to December 31, 2014, mainly due to the completion in 2015 of a series of transactions intended to transform our portfolio of businesses. For more information on these transactions see "Item 18. Financial Statements—Note 2."
A significant number of our associates are represented by unions or works councils. We have not experienced any material work stoppages in recent years, and we consider our employee relations to be good.
The aggregate amount of our shares owned by our non-executive Directors and the members of our Executive Committee in 2015 (including persons closely linked to them) as of December 31, 2015 was 1,688,920 shares. This excludes certain unvested shares and other equity rights (such as Restricted Stock Units and Phantom Shares) because such unvested shares and equity rights do not represent shares held by these persons as of December 31, 2015.
278
The aggregate amount of Novartis share and ADR options, including other information regarding the options, held by our non-executive Directors and the members of our Executive Committee in 2015, as of December 31, 2015 is set forth below:
Title of Options
|
Amount of shares called for by the options |
Exercise Price(1) |
Purchase Price (if any) |
Expiration Date | Total number of options held |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Novas16 Options |
1 | 71.30 | 0 | February 5, 2016 | 0 | |||||||||||
Novas17 Options |
1 | 72.85 | 0 | February 3, 2017 | 0 | |||||||||||
Novas18 Options |
1 | 64.05 | 0 | January 10, 2018 | 0 | |||||||||||
Novas19 Options |
1 | 53.65 | 0 | January 18, 2019 | 0 | |||||||||||
Novas20 Options |
1 | 55.85 | 0 | January 19, 2020 | 0 | |||||||||||
Novas21 Options |
1 | 54.70 | 0 | January 19, 2021 | 141,396 | |||||||||||
Novas22 Options |
1 | 54.20 | 0 | January 19, 2022 | 0 | |||||||||||
Novas23 Options |
1 | 61.70 | 0 | January 17, 2023 | 378,390 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total Novartis Share Options |
519,786 | |||||||||||||||
Novartis ADR Options Cycle X |
1 |
$ |
54.70 |
0 |
February 5, 2016 |
0 |
||||||||||
Novartis ADR Options Cycle XI |
1 | $ | 58.38 | 0 | February 3, 2017 | 0 | ||||||||||
Novartis ADR Options Cycle XII |
1 | $ | 57.96 | 0 | January 10, 2018 | 0 | ||||||||||
Novartis ADR Options Cycle XIII |
1 | $ | 46.42 | 0 | January 18, 2019 | 0 | ||||||||||
Novartis ADR Options Cycle XIV |
1 | $ | 53.70 | 0 | January 19, 2020 | 0 | ||||||||||
Novartis ADR Options Cycle XV |
1 | $ | 57.07 | 0 | January 19, 2021 | 0 | ||||||||||
Novartis ADR Options Cycle XVI |
1 | $ | 58.33 | 0 | January 19, 2022 | 0 | ||||||||||
Novartis ADR Options Cycle XVI |
1 | $ | 66.07 | 0 | January 17, 2023 | 0 | ||||||||||
| | | | | | | | | | | | | | | | | |
Total Novartis ADR Options |
0 | |||||||||||||||
- (1)
- Exercise price indicated is per share, and denominated in Swiss francs for share options and US dollars for ADR options.
Information above for any non-executive Directors and members of our Executive Committee who stepped down during 2015 is reported as of the date of their resignation.
Since 2014, we no longer grant any new share or ADR options to our non-executive Directors, the members of our Executive Committee and our associates under our equity-based participation plans. For more information on the Novartis shares, share options and other equity-based instruments owned by individual members of our Executive Committee and by our current non-executive Directors, see "—Item 6.B Compensation—Shares, ADRs, equity rights and share options owned by Executive Committee Members." and "—Item 6.B Compensation—Shares, ADRs and share options owned by Board members." For more information on our equity-based participation plans, see "Item 18. Financial Statements—Note 26."
Item 7. Major Shareholders and Related Party Transactions
Novartis shares are widely held. As of December 31, 2015, Novartis had approximately 161,000 shareholders listed in its share register, representing approximately 70.5% of issued shares. Based on the Novartis AG share register and excluding treasury shares, approximately 40.9% of the shares registered by name were held in Switzerland and approximately 27.5% were held in the US. Approximately 11.8% of the shares registered in the share register were held by individual investors, while approximately 88.2% were held by legal entities, nominees, fiduciaries and the ADS depositary.
279
Based on our share register, we believe that we are not directly or indirectly owned or controlled by another corporation or government, or by any other natural or legal persons. There are no arrangements that may result in a change of control.
2015
According to the share register, on December 31, 2015, no person or entity was registered as the owner of more than 5% of our shares. As of that date, excluding 6.2% of our share capital held as treasury shares by Novartis AG and its entities that restrict their availability for use, the following registered shareholders (including nominees and the ADS depositary) held more than 2% of the total share capital of Novartis with the right to vote these shares:
- •
- Shareholders: Novartis Foundation for Employee Participation, with its registered office in Basel, Switzerland, holding 2.6%; and
Emasan AG, with its registered office in Basel, Switzerland, holding 3.3%;
- •
- Nominees: Chase Nominees Ltd., London, England (holding 8.8%) (Previously reported as JPMorgan Chase Bank, New York, NY but
changed to its affiliate Chase Nominees Ltd., London, England, which is entered as nominee in our share register.); Nortrust Nominees, London, England (holding 3.2%); and The Bank of New York
Mellon, New York, NY (holding 4.6%) through its nominees, Mellon Bank, Everett, MA (holding 1.7%) and The Bank of New York Mellon, Brussels, Belgium (2.9%); and
- •
- ADS depositary: JPMorgan Chase Bank, New York, NY (holding 11.2%).
According to disclosure notifications filed with Novartis AG and the SIX Swiss Exchange, each of the following shareholders held between 3% and 5% of the share capital of Novartis AG as of December 31, 2015:
- •
- Capital Group Companies, Inc., Los Angeles, CA; and
- •
- BlackRock, Inc., New York, NY
As of December 31, 2015, no other shareholder was registered as owner of more than 2% of the registered share capital. Novartis has not entered into any agreement with any shareholder regarding the voting or holding of Novartis shares.
2014
According to the share register, on December 31, 2014, no person or entity was registered as the owner of more than 5% of our shares. As of that date, excluding 5.7% of our share capital held by Novartis AG, together with Novartis affiliates (excluding foundations), as treasury shares, the following registered shareholders (including nominees and the ADS depositary) held more than 2% of the total share capital of Novartis with the right to vote these shares:
- •
- Shareholders: Novartis Foundation for Employee Participation, with its registered office in Basel, Switzerland, holding 3.2%; and
Emasan AG, with its registered office in Basel, Switzerland, holding 3.3%;
- •
- Nominees: JPMorgan Chase Bank, New York, NY (holding 9.1%); Nortrust Nominees, London, England (holding 3.2%); and The Bank of New
York Mellon, New York, NY (holding 4.6%) through its nominees, Mellon Bank, Everett, MA (holding 2.6%) and The Bank of New York Mellon, Brussels, Belgium (2.0%); and
- •
- ADS depositary: JPMorgan Chase Bank, New York, NY (holding 11.4%).
280
According to disclosure notifications filed with Novartis AG and the SIX Swiss Exchange, each of the following shareholders held between 3% and 5% of the share capital of Novartis AG as of December 31, 2014:
- •
- Capital Group Companies, Inc., Los Angeles, CA; and
- •
- BlackRock, Inc., New York, NY
As of December 31, 2014, no other shareholder was registered as owner of more than 2% of the registered share capital. Novartis has not entered into any agreement with any shareholder regarding the voting or holding of Novartis shares.
2013
According to the share register, on December 31, 2013, no person or entity was registered as the owner of more than 5% of our shares. As of that date, excluding 4.9% of our share capital held by Novartis AG, together with Novartis affiliates (excluding foundations), as treasury shares, the following registered shareholders (including nominees and the ADS depositary) held more than 2% of the total share capital of Novartis with the right to vote these shares:
- •
- Shareholders: Novartis Foundation for Employee Participation, with its registered office in Basel, Switzerland, holding 3.0%; and
Emasan AG, with its registered office in Basel, Switzerland, holding 3.3%;
- •
- Nominees: JPMorgan Chase Bank, New York, NY (holding 11.1%); Nortrust Nominees, London, England (holding 3.2%); and The Bank of New
York Mellon, New York, NY (holding 4.6%) through its nominees, Mellon Bank, Everett, MA (holding 2.8%) and The Bank of New York Mellon, Brussels, Belgium (1.8%); and
- •
- ADS depositary: JPMorgan Chase Bank, New York, NY (holding 11.7%).
According to a disclosure notification filed with Novartis AG, Norges Bank (Central Bank of Norway), Oslo, Norway, held 2.03% of the share capital of Novartis AG as of December 31, 2013.
According to disclosure notifications filed with Novartis AG and the SIX Swiss Exchange, each of the following shareholders held between 3% and 5% of the share capital of Novartis AG as of December 31, 2013:
- •
- Capital Group Companies, Inc., Los Angeles, CA; and
- •
- BlackRock, Inc., New York, NY
As of December 31, 2013, no other shareholder was registered as owner of more than 2% of the registered share capital. Novartis has not entered into any agreement with any shareholder regarding the voting or holding of Novartis shares.
7.B Related Party Transactions
See "Item 18. Financial Statements—Note 27".
7.C Interests of Experts and Counsel
Not applicable.
281
8.A Consolidated Statements and Other Financial Information
See "Item 18. Financial Statements."
Dividend policy
Subject to the dividend policy described below, our Board of Directors expects to recommend the payment of a dividend in respect of each financial year. If approved by our shareholders at the relevant annual Shareholders' Meeting, the dividends will be payable shortly following such approval. Any shareholder who purchases our shares before the ex-dividend date and holds the shares until that date shall be deemed to be entitled to receive the dividends approved at that meeting. Dividends are reflected in our financial statements in the year in which they are approved by our shareholders.
Our dividend policy is to pay a growing annual dividend. This policy is subject to our financial conditions and outlook at the time, the results of our operations and other factors.
The Board will propose a dividend of CHF 2.70 per share to the shareholders for approval at the Annual General Meeting to be held on February 23, 2016. Because we pay dividends in Swiss francs, exchange rate fluctuations will affect the US dollar amounts received by holders of ADRs. For a summary of dividends we paid in the past five years, see "Item 3. Key Information—3.A Selected Financial Data—Cash Dividends per Share." See also "Item 3. Key Information—3.D Risk Factors—The price of our ADRs and the US dollar value of any dividends may be negatively affected by fluctuations in the US dollar/Swiss franc exchange rate."
Disclosure pursuant to Section 219 of the Iran Threat Reduction & Syria Human Rights Act (ITRA)
At Novartis, it is our mission to discover, develop and successfully market innovative products to prevent and cure diseases, to ease suffering and to enhance the quality of life of all people, regardless of where they live. This mission includes the compliant sale of medicines and other healthcare products worldwide. To help us fulfill this mission, we have representative offices located in Iran.
As of October 18, 2010, a non-US affiliate within our Pharmaceuticals Division entered into a non-binding Memorandum of Understanding (MoU) with the Ministry of Health and Medical Education of the Islamic Republic of Iran. Pursuant to the MoU, the Iranian Ministry of Health acknowledges certain benefits that may apply to sales of certain Novartis Pharmaceuticals medicines by third-party distributors in Iran. These include fast-track registration, market exclusivity, end-user subsidies and exemptions from customs tariffs. Novartis receives no payments from the Iranian Ministry of Health under the MoU and the MoU creates no obligations on the part of either Novartis or the Iranian Ministry of Health.
In 2015, non-US affiliates relating to our Pharmaceuticals and Sandoz Divisions made payments to government entities in Iran related to exit fees and other transactions ordinarily incident to travel by doctors and other medical professionals resident in Iran to attend conferences or other events outside Iran.
From time to time, including in 2015, non-US affiliates relating to our Pharmaceuticals and Sandoz Divisions enter into agreements with hospitals, research institutes, medical associations and universities in Iran to provide grants, sponsor congresses, seminars and symposia, and with doctors and other healthcare professionals for consulting services, including participation in advisory boards and investigator services for observational (non-interventional) studies. Some of these hospitals and research institutes are owned or controlled by the government of Iran, and some of these doctors and healthcare professionals are employed by hospitals that may be public or government-owned.
282
Because our Pharmaceuticals and Sandoz Divisions have operations in Iran, including employees, they obtain services and have other dealings incidental to their activities in that country, including paying taxes and salaries, and obtaining office rentals, insurance, electricity, water and telecommunications services, office and similar supplies and customs-related services from Iranian companies that may be owned or controlled by the government of Iran.
Some beneficiaries of payments made by non-US affiliates relating to our Pharmaceuticals and Sandoz Divisions in the course of the operations described above maintain accounts at banks that are included on the list of Specially Designated Nationals (SDNs). Nonetheless, since such payments relate to lawful and authorized transactions, use of a blocked Iranian financial institution is permitted in accordance with applicable laws and given that such institution is identified on the SDN List with the tag [IRAN].
None.
Our shares are listed in Switzerland on the SIX Swiss Exchange (SIX).
American Depositary Shares (ADSs), each representing one share, have been available in the US through an American Depositary Receipts (ADR) program since December 1996. This program was established pursuant to a Deposit Agreement which we entered into with JPMorgan Chase Bank N.A. as Depositary (Deposit Agreement). Our ADRs have been listed on the NYSE since May 2000, and are traded under the symbol "NVS."
The table below sets forth, for the periods indicated, the high and low closing sales prices for our shares traded in Switzerland and for ADRs traded in US. The data below regarding our shares reflects price and volume information for trades completed by members of the SIX during the day as well as for inter- dealer trades completed off the SIX and certain inter-dealer trades completed during trading on the previous business day.
283
The following share data was taken from SIX; the ADR data was taken from Bloomberg:
| |
Shares | ADRs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
High CHF per share |
Low CHF per share |
High $ per ADR |
Low $ per ADR |
|||||||||
Annual information for the past five years |
|||||||||||||
2011 |
55.80 | 39.99 | 64.52 | 51.65 | |||||||||
2012 |
59.00 | 48.80 | 63.96 | 51.48 | |||||||||
2013 |
73.65 | 58.70 | 80.39 | 63.70 | |||||||||
2014 |
93.80 | 70.65 | 96.65 | 78.20 | |||||||||
2015 |
102.30 | 82.20 | 106.12 | 83.96 | |||||||||
Quarterly information for the past two years |
|||||||||||||
2015 |
|||||||||||||
First Quarter |
99.70 | 84.30 | 103.00 | 91.67 | |||||||||
Second Quarter |
101.40 | 92.00 | 105.50 | 98.34 | |||||||||
Third Quarter |
102.30 | 87.35 | 106.12 | 89.52 | |||||||||
Fourth Quarter |
91.70 | 82.20 | 95.03 | 83.96 | |||||||||
2014 |
|||||||||||||
First Quarter |
75.30 | 70.65 | 85.02 | 78.20 | |||||||||
Second Quarter |
81.40 | 72.90 | 90.98 | 82.51 | |||||||||
Third Quarter |
90.15 | 76.95 | 94.80 | 85.25 | |||||||||
Fourth Quarter |
93.80 | 80.00 | 96.65 | 85.02 | |||||||||
Monthly information for most recent six months |
|||||||||||||
August 2015 |
101.60 | 89.60 | 104.36 | 94.81 | |||||||||
September 2015 |
94.80 | 87.35 | 97.96 | 89.52 | |||||||||
October 2015 |
91.70 | 87.45 | 95.03 | 90.14 | |||||||||
November 2015 |
90.35 | 87.05 | 91.04 | 85.24 | |||||||||
December 2015 |
88.05 | 82.20 | 88.05 | 83.96 | |||||||||
January 2016 (through January 20) |
86.45 | 79.70 | 86.21 | 80.23 | |||||||||
Fluctuations in the exchange rate between the Swiss franc and the US dollar will affect any comparisons of Swiss share prices and US ADR prices.
The average daily volumes of shares traded on the SIX (ON/OFF exchange) for the years 2015, 2014 and 2013 were 5,870,894, 4,963,517, and 4,568,858, respectively. These numbers are based on total annual turnover statistics supplied by the SIX via the Swiss Market Feed, which supplies such data to subscribers and to other information providers. The average daily volumes of ADRs traded in the US for the years 2015, 2014 and 2013 were 1,787,735, 1,504,087, and 1,440,718, respectively.
The Depositary has informed us that as of January 20, 2016, there were 301,119,296 ADRs outstanding, each representing one Novartis share (approximately 11% of total Novartis shares issued). On January 20, 2016, the closing sales price per share on the SIX was CHF 79.70 and $80.49 per ADR on the NYSE.
Not applicable.
284
See "9.A Offer and Listing Details."
Not applicable.
Not applicable.
Not applicable.
Item 10. Additional Information
Not applicable.
10.B Memorandum and Articles of Association
The following is a summary of certain provisions of our Articles of Incorporation (Articles), our Regulations of the Board of Directors (Board Regulations) and of Swiss law, particularly, the Swiss Code of Obligations (Swiss CO). This is not a summary of all the significant provisions of the Articles, the Board Regulations or of Swiss law and does not purport to be complete. This description is qualified in its entirety by reference to the Articles and the Board Regulations, which are an exhibit to this Form 20-F, and to Swiss law.
At our 2015 Annual General Meeting held on February 27, 2015, our shareholders approved amendments to our Articles to align with the Swiss Ordinance against Excessive Compensation in Stock Exchange Listed Companies on Board and Executive Compensation (the "Ordinance"). Key aspects of these amendments included determining (i) the maximum number of allowable external mandates for members of our Board of Directors (Board) and Executive Committee, (ii) the principles concerning the tasks and responsibilities of our Compensation Committee, (iii) the details concerning the procedure for the new yearly binding separate shareholder votes on the aggregate compensation of our Board and Executive Committee, and (iv) the principles of our compensation policy.
Novartis AG is registered in the commercial register of the Canton of Basel-Stadt, Switzerland, under number CHE-103.867.266. Our business purpose, as stated in Article 2 of the Articles, is to hold interests in enterprises in the area of health care or nutrition. We may also hold interests in enterprises in the areas of biology, chemistry, physics, information technology or related areas. We may acquire, mortgage, liquidate or sell real estate and intellectual property rights in Switzerland or abroad. In pursuing our business purpose, we strive to create sustainable value.
285
(a) According to our Board Regulations, a member of our Board (Director) may not participate in deliberations or resolutions on matters which affect, or reasonably might affect, the Director's interests, or the interests of a person close to the Director. In addition, the Swiss CO sets forth that if, in connection with the conclusion of a contract, the Company is represented by the person with whom it is concluding the contract, such contract shall be in writing. Furthermore, the Swiss CO does require directors and members of senior management to safeguard the interests of the corporation and, in this connection, imposes a duty of care and a duty of loyalty on such persons. This rule is generally interpreted to mean that directors and members of senior management are disqualified from participating in decisions which affect them personally.
(b) A Board resolution requires the affirmative majority of the votes cast. As with any Board resolution, Directors may not vote on their own compensation unless at least a majority of the Directors are present. Such votes are subject to the approval of the aggregate amounts of compensation of the Directors and the members of the Executive Committee by a shareholders' resolution under the Ordinance.
(c) The Articles prohibit the granting of loans or credits to Directors.
(d) Directors who have turned seventy years of age at the date of the General Meeting of Shareholders may no longer be elected as members of the Board. The General Meeting of Shareholders may, under special circumstances, grant an exemption from this rule.
(e) Our Directors are not required to be shareholders under our Articles.
Because Novartis AG has only one class of registered shares, the following information applies to all shareholders.
(a) The Swiss CO requires that at least 5% of our annual profit be retained as general reserves, so long as these reserves amount to less than 20% of our registered share capital. The law and the Articles permit us to accrue additional reserves.
Under the Swiss CO, we may only pay dividends out of the balance sheet profit, out of reserves created for this purpose or out of free reserves. In any event, under the Swiss CO, while the Board may propose that a dividend be paid, we may only pay dividends upon shareholders' approval at a General Meeting of Shareholders. Our auditors must confirm that the dividend proposal of our Board conforms with the Swiss CO and the Articles. Our Board intends to propose a dividend once each year. See "Item 3. Key Information—3.A. Selected Financial Data—Cash Dividends per Share" and "Item 8. Financial Information—8.A. Consolidated Financial Statements and Other Financial Information—Dividend Policy."
Dividends are usually due and payable shortly after the shareholders have passed a resolution approving the payment. Dividends which have not been claimed within five years after the due date revert to us, and are allocated to our general reserves. For information about deduction of the withholding tax or other duties from dividend payments, see "Item 10. Additional Information—10.E Taxation."
(b) Each share is entitled to one vote at a General Meeting of Shareholders. Voting rights may only be exercised for shares registered with the right to vote on the Record Date. In order to do so, the shareholder must file a share registration form with us, setting forth the shareholder's name, address and citizenship (or, in the case of a legal entity, its registered office). If the shareholder has not timely filed the form, then the shareholder may not vote at, or participate in, General Meetings of Shareholders.
286
To vote its shares, the shareholder must also explicitly declare that it has acquired the shares in its own name and for its own account. If the shareholder refuses to make such a declaration, the shares may not be voted unless the Board recognizes such shareholder as a nominee.
The Articles provide that no shareholder shall be registered with the right to vote shares comprising more than 2% of the registered share capital. The Board may, upon request, grant an exemption from this restriction. Considerations include whether the shareholder supports our goal of creating sustainable value and has a long-term investment horizon. Furthermore, the Articles provide that no nominee shall be registered with the right to vote shares comprising more than 0.5% of the registered share capital. The Board may, upon request, grant an exemption from this restriction if the nominee discloses the names, addresses and number of shares of the persons for whose account it holds more than 0.5% of the registered share capital. The same restrictions indirectly apply to holders of ADRs. We have in the past granted exemptions from the 2% rule for shareholders and the 0.5% rule for nominees. Under the Articles, the Board may delegate the power to grant such exemptions. The Board has delegated this power to the Chairman of the Board.
For purposes of the 2% rule for shareholders and the 0.5% rule for nominees, groups of companies and groups of shareholders acting in concert are considered to be one shareholder. These rules also apply to shares acquired or subscribed by the exercise of subscription, option or conversion rights.
After hearing the registered shareholder or nominee, the Board may cancel, with retroactive effect as of the date of registration, the registration of the shareholders if the registration was effected based on false information.
Registration restrictions in the Articles may only be removed upon a resolution carrying a two-thirds majority of the votes represented at a General Meeting of Shareholders.
Shareholders' resolutions generally require the approval of a majority of the votes present at a General Meeting of Shareholders. As a result, abstentions have the effect of votes against the resolution. Shareholder resolutions requiring a vote by such "absolute majority of the votes" include among others (1) amendments to the Articles; (2) elections of Directors, the Chairman, the Compensation Committee members, the independent proxy and the statutory auditors; (3) approval of the management report and the financial statements; (4) setting the annual dividend; (5) approval of the aggregate amounts of compensation of the Directors and the members of the Executive Committee; (6) decisions to discharge Directors and management from liability for matters disclosed to the General Meeting of Shareholders; and (7) the ordering of an independent investigation into specific matters proposed to the General Meeting of Shareholders.
According to the Articles and Swiss law, the following types of shareholders' resolutions require the approval of a "supermajority" of at least two-thirds of the votes present at a General Meeting of Shareholders: (1) an alteration of our corporate purpose; (2) the creation of shares with increased voting powers; (3) an implementation of restrictions on the transfer of registered shares and the removal of such restrictions; (4) an authorized or conditional increase of the share capital; (5) an increase of the share capital by conversion of equity, by contribution in kind, or for the purpose of an acquisition of property or the grant of special rights; (6) a restriction or an elimination of shareholders' preemptive rights; (7) a change of our domicile; (8) our dissolution; or (9) any amendment to the Articles which would create or eliminate a supermajority requirement.
Our shareholders annually elect all of the members of the Board, as well as the Chairman of the Board, the members of the Compensation Committee and the independent proxy. Cumulative voting of shares is not permitted under Swiss law.
At General Meetings of Shareholders, shareholders can be represented by proxy. However, a proxy must either be the shareholder's legal representative, another shareholder with the right to vote, or the independent proxy. Votes are taken either by a show of hands or by electronic voting, unless the General
287
Meeting of Shareholders resolves to have a ballot or where a ballot is ordered by the chairman of the meeting.
American Depositary Shares (ADSs) each representing one Novartis share and evidenced by American Depositary Receipts (ADRs) are issued by our depositary JPMorgan Chase Bank, New York, and not by us. The ADR is vested with rights defined and enumerated in the Deposit Agreement (such as the rights to vote, to receive a dividend and to receive a share of Novartis in exchange for a certain number of ADRs). The enumeration of rights, including any limitations on those rights in the Deposit Agreement, is final. There are no other rights given to the ADR holders. Only the ADS depositary, holding our shares underlying the ADRs, is registered as shareholder in our share register. An ADR is not a Novartis share and an ADR holder is not a Novartis shareholder.
The Deposit Agreement between our depositary, the ADR holder and us has granted certain indirect rights to vote to the ADR holders. ADR holders may not attend Novartis General Meetings in person. ADR holders exercise their voting rights by instructing JPMorgan Chase Bank, our depositary, to exercise the voting rights attached to the registered shares underlying the ADRs. Each ADR represents one Novartis share. JPMorgan Chase Bank exercises the voting rights for registered shares underlying ADRs for which no voting instructions have been given by providing a discretionary proxy to an uninstructed independent designee pursuant to paragraph 13 of the form of ADR. Such designee has to be a shareholder of Novartis. The same voting restrictions apply to ADR holders as to those holding Novartis shares (i.e., the right to vote up to 2% of the Novartis registered share capital—unless otherwise granted an exemption by the Board—and disclosure requirement for nominees).
(c) Shareholders have the right to allocate the profit shown on our balance sheet by vote taken at the General Meeting of Shareholders, subject to the legal requirements described in "Item 10.B.3(a) Shareholder Rights".
(d) Under the Swiss CO, any surplus arising out of a liquidation of our Company (i.e., after the settlement of all claims of all creditors) would be distributed to the shareholders in proportion to the paid-in nominal value of their shares.
(e) The Swiss CO limits a corporation's ability to hold or repurchase its own shares. We and our subsidiaries may only repurchase shares if we have freely disposable equity, in the amount necessary for this purpose, available. The aggregate nominal value of all Novartis shares held by us and our subsidiaries may not exceed 10% of our registered share capital. However, it is accepted that a corporation may repurchase its own shares beyond the statutory limit of 10%, if the repurchased shares are clearly dedicated for cancellation and if the shareholders passed a respective resolution at a General Meeting of Shareholders. In addition, we are required to create a special reserve on our balance sheet in the amount of the purchase price of the acquired shares. Repurchased shares held by us or our subsidiaries do not carry any rights to vote at a General Meeting of Shareholders, but are entitled to the economic benefits generally connected with the shares. It should be noted that the definition of what constitutes subsidiaries, and therefore, treasury shares, for purposes of the above described reserves requirement and voting restrictions differs from the definition included in the consolidated financial statements. The definition in the consolidated financial statements requires consolidation for financial reporting purposes of special purpose entities, irrespective of their legal structure, in instances where we have the power to govern the financial and operating policies of the entity so as to obtain benefits from its activities.
Under the Swiss CO, we may not cancel treasury shares without the approval of a capital reduction by our shareholders.
(f) Not applicable.
(g) Since all of our issued and outstanding shares have been fully paid in, we can make no further capital calls on our shareholders.
(h) See Items "10.B.3(b) Shareholder Rights" and "10.B.7 Change in Control".
288
10.B.4 Changes To Shareholder Rights
Under the Swiss CO, we may not issue new shares without the prior approval of a capital increase by our shareholders. If a capital increase is approved, then our shareholders would generally have certain preemptive rights to obtain newly issued shares in an amount proportional to the nominal value of the shares they already hold. These preemptive rights could be modified in certain limited circumstances with the approval of a resolution adopted at a General Meeting of Shareholders by a supermajority of votes. In addition, we may not create shares with increased voting powers or place restrictions on the transfer of registered shares without the approval of a resolution adopted at a General Meeting of Shareholders by a supermajority of votes. In addition, see Item 10.B.3(b) with regard to the Board's ability to cancel the registration of shares under limited circumstances.
Under the Swiss CO and the Articles, we must hold an annual ordinary General Meeting of Shareholders within six months after the end of our financial year. General Meetings of Shareholders may be convened by the Board or, if necessary, by the statutory auditors. The Board is further required to convene an extraordinary General Meeting of Shareholders if so resolved by a General Meeting of Shareholders, or if so requested by shareholders holding an aggregate of at least 10% of the registered shares, specifying the items for the agenda and their proposals. Shareholders holding shares with a nominal value of at least CHF 1,000,000 (i.e., 2,000,000 Novartis shares) have the right to request that a specific proposal be put on the agenda and voted upon at the next General Meeting of Shareholders. A General Meeting of Shareholders is convened by publishing a notice in the official Swiss Commercial Gazette (Schweizerisches Handelsamtsblatt) at least 20 days prior to such meeting. Shareholders may also be informed by mail. There is no provision in the Swiss CO or our Articles requiring a quorum for the holding of a General Meeting of Shareholders. In addition, see "Item 10.B.3(b) Shareholder Rights" regarding conditions for exercising a shareholder's right to vote at a General Meeting of Shareholders.
There are no limitations under the Swiss CO or our Articles on the right of non-Swiss residents or nationals to own or vote shares other than the restrictions applicable to all shareholders. But see "Item 10.B.3(b) Shareholder Rights" regarding conditions for exercising an ADR holder's right to vote at a shareholder meeting.
The Articles and the Board Regulations contain no provision that would have an effect of delaying, deferring or preventing a change in control of Novartis and that would operate only with respect to a merger, acquisition or corporate restructuring involving us or any of our subsidiaries.
According to the Swiss Merger Act, shareholders may pass a resolution to merge with another corporation at any time. Such a resolution would require the consent of at least two-thirds of all votes present at the necessary General Meeting of Shareholders.
Under the Swiss Financial Market Infrastructure Act, shareholders and groups of shareholders acting in concert who acquire more than 331/3% of our shares would be under an obligation to make an offer to acquire all remaining Novartis shares.
289
10.B.8 Disclosure of Shareholdings
Under the Swiss Financial Market Infrastructure Act, holders of our voting shares acting alone or acting in concert with others are required to notify us and the SIX Swiss Exchange of the level of their holdings whenever such holdings reach or exceed, or in some cases, fall short of, certain thresholds—3%, 5%, 10%, 15%, 20%, 25%, 331/3%, 50% and 662/3%—of our registered share capital. Following receipt of such notification we are required to inform the public by publishing the information via the electronic publication platform operated by the competent Disclosure Office.
An additional disclosure obligation exists under the Swiss CO which requires us to disclose, once a year in the notes to the financial statements published in our annual report, the identity of all of our shareholders (or related groups of shareholders) who have been granted exemption entitling them to vote more than 2% of our registered share capital, as described in "Item 10.B.3(b) Shareholder Rights".
See the references to Swiss law throughout this "Item 10.B Memorandum and Articles of Association".
The requirements of the Articles regarding changes in capital are not more stringent than the requirements of Swiss law.
Transactions with GSK
On April 22, 2014 (and as amended and restated on May 29, 2014), we entered into an overarching framework agreement (the "Implementation Agreement") with GSK for the Consumer Healthcare Joint Venture, the Vaccines Sale and the Oncology Acquisition (each as defined below and, together with the Influenza Put Option (as defined below), the "Transactions"). The Consumer Healthcare Joint Venture, the Vaccines Sale and the Oncology Acquisition were completed on March 2, 2015.
Consumer Healthcare Joint Venture with GSK
On April 22, 2014 (and as amended and restated on May 29, 2014, and March 1, 2015), we entered into a Contribution Agreement with GSK under which GSK contributed its consumer healthcare business (the "GSK Consumer Healthcare Business") and we contributed our OTC Division, with certain limited exceptions which include the over-the-counter business of our Sandoz Division, into a newly-created joint venture which operates under the GSK Consumer Healthcare name (the "Consumer Healthcare Joint Venture"). In consideration for those contributions, GSK owns 63.5% of the issued share capital of the Consumer Healthcare Joint Venture and we own 36.5% of the issued share capital of the Consumer Healthcare Joint Venture.
The operation of the Consumer Healthcare Joint Venture is governed by a Shareholders' Agreement, under which GSK has the right to appoint seven directors to the board of the Consumer Healthcare Joint Venture and we have the right to appoint four directors to the board of the Consumer Healthcare Joint Venture. The Shareholders' Agreement also contains certain minority shareholder protections, including the right to exit the Consumer Healthcare Joint Venture via a put option exercisable in certain windows in the period from the third to the twentieth anniversary of the creation of the Consumer Healthcare Joint Venture. The Shareholders' Agreement became operative concurrently with the creation of the Consumer Healthcare Joint Venture on March 2, 2015.
290
Sale of Vaccines Business (Excluding our Influenza Vaccines Business) to GSK
On April 22, 2014 (and as amended and restated on May 29, 2014, as further amended on October 9, 2014, and as further amended and restated on March 1, 2015), we entered into a Sale and Purchase Agreement with GSK under which we sold our Vaccines Division (with certain limited exceptions, and except for our influenza vaccines business) to GSK (the "Vaccines Sale") for up to $7.1 billion, consisting of $5.25 billion upfront and up to $1.8 billion in milestones, of which we have received $450 million as of December 31, 2015, plus royalties. We completed the Vaccines Sale on March 2, 2015.
Oncology Acquisition from GSK
On April 22, 2014 (and as amended and restated on May 29, 2014, November 21, 2014, and March 1, 2015), we entered into a Sale and Purchase Agreement with GSK under which we acquired GSK oncology products and certain related assets (the "Oncology Acquisition"). GSK has also granted us a right of first negotiation over the co-development and commercialization of GSK's current and future oncology R&D pipeline, excluding oncology vaccines, for a period of twelve and one half years from closing. We completed the Oncology Acquisition on March 2, 2015. Novartis paid an aggregate cash consideration of $16 billion for the Oncology Acquisition. Up to $1.5 billion of the cash consideration is contingent on certain development milestones and is potentially refundable.
Influenza Vaccines Business Put Option with GSK
On April, 22 2014 (and as amended and restated on May 29, 2014), we entered into a Put Option Deed with GSK pursuant to which we had the right to unilaterally require GSK to acquire our Vaccines Division's influenza vaccines business for $250 million, or certain parts of the influenza vaccines business for a pro-rata amount (the "Influenza Put Option") if the divestment to CSL discussed below was not completed. The Influenza Put Option expired concurrently with the closing of the divestment of our influenza vaccines business to CSL on July 31, 2015.
Sale of Influenza Vaccines Business to CSL
On October 26, 2014 (and as amended and restated on July 31, 2015), we entered into a Share and Business Sale Agreement with CSL under which we divested our Vaccines Division's influenza vaccines business to CSL for $275 million. This transaction was completed effective July 31, 2015.
Sale of Animal Health Division to Lilly
On April 22, 2014 (and as amended on December 17, 2014), we entered into a Stock and Asset Purchase Agreement with Lilly. Under this agreement, Lilly agreed to purchase our Animal Health Division (with certain limited exceptions) for approximately $5.4 billion. This transaction was completed on January 1, 2015.
There are no Swiss governmental laws, decrees or regulations that restrict, in a manner material to Novartis, the export or import of capital, including any foreign exchange controls, or that generally affect the remittance of dividends or other payments to non-residents or non-citizens of Switzerland who hold Novartis' shares.
The taxation discussion set forth below is intended only as a descriptive summary and does not purport to be a complete analysis or listing of all potential tax effects relevant to the ownership or
291
disposition of our shares or ADRs. The statements of US and Swiss tax laws set forth below are based on the laws and regulations in force as of the date of this 20-F, including the current Convention Between the US and the Swiss Confederation for the Avoidance of Double Taxation with Respect to Taxes on Income, entered into force on December 19, 1997 (the "Treaty"), and the US Internal Revenue Code of 1986, as amended (the "Code"), Treasury regulations, rulings, judicial decisions and administrative pronouncements, and may be subject to any changes in US and Swiss law, and in any double taxation convention or treaty between the US and Switzerland occurring after that date, which changes may have retroactive effect.
Swiss Residents
Withholding Tax on Dividends and Distributions. Dividends which we pay and similar cash or in-kind distributions which we may make to a holder of shares or ADRs (including distributions of liquidation proceeds in excess of the nominal value, stock dividends and, under certain circumstances, proceeds from repurchases of shares by us in excess of the nominal value) are generally subject to a Swiss federal withholding tax (the "Withholding Tax") at a current rate of 35%. Under certain circumstances distributions out of capital contribution reserves made by shareholders after December 31, 1996 are exempt from Withholding Tax. We are required to withhold this Withholding Tax from the gross distribution and to pay the Withholding Tax to the Swiss Federal Tax Administration. The Withholding Tax is refundable in full to Swiss residents who are the beneficial owners of the taxable distribution at the time it is resolved and duly report the gross distribution received on their personal tax return or in their financial statements for tax purposes, as the case may be.
Income Tax on Dividends. A Swiss resident who receives dividends and similar distributions (including stock dividends and liquidation surplus) on shares or ADRs is required to include such amounts in the shareholder's personal income tax return. However, distributions out of qualified capital contribution reserves are not subject to income tax. A corporate shareholder may claim substantial relief from taxation of dividends and similar distributions received if the shares held represent a fair market value of at least CHF 1 million.
Capital Gains Tax upon Disposal of Shares. Under current Swiss tax law, the gain realized on shares held by a Swiss resident who holds shares or ADRs as part of his private property is generally not subject to any federal, cantonal or municipal income taxation on gains realized on the sale or other disposal of shares or ADRs. However, gains realized upon a repurchase of shares by us may be characterized as taxable dividend income if certain conditions are met. Book gains realized on shares or ADRs held by a Swiss corporate entity or by a Swiss resident individual as part of the shareholder's business property are, in general, included in the taxable income of such person. However, the Federal Law on the Direct Federal Tax of December 14, 1990 and several cantonal laws on direct cantonal taxes provide for exceptions for Swiss corporate entities holding more than 10% of our voting stock for more than one year.
Residents of Other Countries
Recipients of dividends and similar distributions on our shares who are neither residents of Switzerland for tax purposes nor holding shares as part of a business conducted through a permanent establishment situated in Switzerland ("Non-resident Holders") are not subject to Swiss income taxes in respect of such distributions. Moreover, gains realized by such recipients upon the disposal of shares are not subject to Swiss income taxes.
Non-resident Holders of shares are, however, subject to the Withholding Tax on dividends and similar distributions mentioned above and under certain circumstances to the Stamp Duty described below. Such Non-resident Holders may be entitled to a partial refund of the Withholding Tax if the country in which they reside has entered into a bilateral treaty for the avoidance of double taxation with Switzerland.
292
Non-resident Holders should be aware that the procedures for claiming treaty refunds (and the time frame required for obtaining a refund) may differ from country to country. Non-resident Holders should consult their own tax advisors regarding receipt, ownership, purchase, sale or other dispositions of shares or ADRs and the procedures for claiming a refund of the Withholding Tax.
As of January 1, 2016, Switzerland has entered into bilateral treaties for the avoidance of double taxation with respect to income taxes with the following countries, whereby a part of the above-mentioned Withholding Tax may be refunded (subject to the limitations set forth in such treaties):
| Albania Algeria Argentina Armenia Australia Austria Azerbaijan Bahrain Bangladesh Belarus Belgium Bulgaria Canada Chile China Colombia Croatia Cyprus Czech Republic Denmark Ecuador Egypt Estonia |
Finland France Germany Georgia Ghana Greece Hong Kong Hungary Iceland India Indonesia Iran Israel Italy Ivory Coast Republic of Ireland Jamaica Japan Kazakhstan Republic of Korea (South Korea) Kuwait Kyrgyzstan |
Latvia Lithuania Luxembourg Macedonia Malaysia Malta Mexico Moldova Mongolia Montenegro Morocco Netherlands New Zealand Norway Pakistan Peru Philippines Poland Portugal Quatar Romania Russia Serbia |
Singapore Slovak Republic Slovenia South Africa Spain Sri Lanka Sweden Taiwan Tajikistan Thailand Trinidad and Tobago Tunisia Turkey Turkmenistan Ukraine United Arab Emirates United Kingdom United States of America Uruguay Uzbekistan Venezuela Vietnam |
The tax treaty with Bahrain is not applicable to the healthcare industry. Tax treaty negotiations are under way, or have been conducted, with Bosnia and Herzegovina, Brazil, Costa Rica, Libya, Liechtenstein, North Korea, Oman, Saudi Arabia, Senegal, Syria, and Zimbabwe. Tax treaty negotiations between Switzerland and some of the countries listed in the immediately preceding sentence have been ongoing for an extended period of time, and we are not certain when or if such negotiations will be completed, and when or if the corresponding treaties will come into effect.
A Non-resident Holder of shares or ADRs will not be liable for any Swiss taxes other than the Withholding Tax described above and, if the transfer occurs through or with a Swiss bank or other Swiss securities dealer, the Stamp Duty described below. If, however, the shares or ADRs of Non-resident Holders can be attributed to a permanent establishment or a fixed place of business maintained by such person within Switzerland during the relevant tax year, the shares or ADRs may be subject to Swiss income taxes in respect of income and gains realized on the shares or ADRs and such person may qualify for a full refund of the Withholding Tax based on Swiss tax law.
Residents of the US. A Non-resident Holder who is a resident of the US for purposes of the Treaty is eligible for a reduced rate of tax on dividends equal to 15% of the dividend, provided that such holder (i) qualifies for benefits under the Treaty, (ii) holds, directly and indirectly, less than 10% of our voting stock, and (iii) does not conduct business through a permanent establishment or fixed base in Switzerland
293
to which the shares or ADRs are attributable. Such an eligible holder must apply for a refund of the amount of the Withholding Tax in excess of the 15% Treaty rate. A Non-resident Holder who is a resident of the US for purposes of the Treaty is eligible for a reduced rate of tax on dividends equal to 5% of the dividend, provided that such holder (i) is a company, (ii) qualifies for benefits under the Treaty, (iii) holds directly at least 10% of our voting stock, and (iv) does not conduct business through a permanent establishment or fixed place of business in Switzerland to which the shares or ADRs are attributable. Such an eligible holder must apply for a refund of the amount of the Withholding Tax in excess of the 5% Treaty rate. Claims for refunds must be filed on Swiss Tax Form 82 (82C for corporations; 82I for individuals; 82E for other entities), which may be obtained from any Swiss Consulate General in the US or from the Federal Tax Administration of Switzerland at the address below, together with an instruction form. Four copies of the form must be duly completed, signed before a notary public of the US, and sent to the Federal Tax Administration of Switzerland, Eigerstrasse 65, CH-3003 Berne, Switzerland. The form must be accompanied by suitable evidence of deduction of Swiss tax withheld at source, such as certificates of deduction, signed bank vouchers or credit slips. The form may be filed on or after July 1 or January 1 following the date the dividend was payable, but no later than December 31 of the third year following the calendar year in which the dividend became payable. For US resident holders of ADRs, JPMorgan Chase Bank, N.A., as Depositary, will comply with these Swiss procedures on behalf of the holders, and will remit the net amount to the holders.
Stamp Duty upon Transfer of Securities. The sale of shares, whether by Swiss residents or Non-resident Holders, may be subject to federal securities transfer Stamp Duty of 0.15%, calculated on the sale proceeds, if the sale occurs through or with a Swiss bank or other Swiss securities dealer, as defined in the Swiss Federal Stamp Duty Act. The Stamp Duty has to be paid by the securities dealer and may be charged to the parties in a taxable transaction who are not securities dealers. Stamp Duty may also be due if a sale of shares occurs with or through a non-Swiss bank or securities dealer, provided (i) such bank or dealer is a member of the SIX, and (ii) the sale takes place on the SIX. In addition to this Stamp Duty, the sale of shares by or through a member of the SIX may be subject to a minor stock exchange levy.
The following is a general discussion of the material US federal income tax consequences of the ownership and disposition of our shares or ADRs that may be relevant to you if you are a US Holder (as defined below). Because this discussion does not consider any specific circumstances of any particular holder of our shares or ADRs, persons who are subject to US taxation are strongly urged to consult their own tax advisers as to the overall US federal, state and local tax consequences, as well as to the overall Swiss and other foreign tax consequences, of the ownership and disposition of our shares or ADRs. In particular, additional or different rules may apply to US expatriates, banks and other financial institutions, regulated investment companies, traders in securities who elect to apply a mark-to-market method of accounting, dealers in securities or currencies, tax-exempt entities, insurance companies, broker-dealers, investors liable for alternative minimum tax, investors that hold shares or ADRs as part of a straddle, hedging or conversion transaction, holders whose functional currency is not the US dollar, partnerships or other pass through entities, persons who acquired our shares pursuant to the exercise of employee stock options or otherwise as compensation and persons who hold directly, indirectly or by attribution, 10% or more of the voting power of our outstanding shares. This discussion generally applies only to US Holders who hold the shares or ADRs as a capital asset (generally, for investment purposes), and whose functional currency is the US dollar. Investors are urged to consult their own tax advisors concerning whether they are eligible for benefits under the Treaty.
For purposes of this discussion, a "US Holder" is a beneficial owner of our shares or ADRs who is (i) an individual who is a citizen or resident of the US for US federal income tax purposes, (ii) a corporation (or other entity taxable as a corporation for US federal income tax purposes) created or organized in or under the laws of the US or a state thereof or the District of Columbia, (iii) an estate the income of which is subject to US federal income taxation regardless of its source, or (iv) a trust (i) subject
294
to the primary supervision of a US court and the control of one or more US persons or (ii) that has a valid election in place to be treated as a US person. If a partnership (or other entity treated as a partnership for US federal income tax purposes) holds shares or ADRs, the tax treatment of a partner generally will depend upon the status of the partner and the activities of the partnership. Partners in a partnership that holds shares or ADRs are urged to consult their own tax advisor regarding the specific tax consequences of the owning and disposing of such shares or ADRs by the partnership.
For US federal income tax purposes, a US Holder of ADRs generally will be treated as the beneficial owner of our shares represented by the ADRs. However, see the discussion below under "—Dividends" regarding certain statements made by the US Treasury concerning depositary arrangements.
This discussion assumes that each obligation in the Deposit Agreement and any related agreement will be performed in accordance with its terms.
Dividends. US Holders will be required to include in gross income, as an item of ordinary income, the full amount (including the amount of any Withholding Tax) of a dividend paid with respect to our shares or ADRs at the time that such dividend is received by the US Holder, in the case of shares, or by the Depository, in the case of ADRs. For this purpose, a "dividend" will include any distribution paid by us with respect to our shares or ADRs (other than certain pro rata distributions of our capital stock) paid out of our current or accumulated earnings and profits, as determined under US federal income tax principles. To the extent the amount of a distribution by us exceeds our current and accumulated earnings and profits, such excess will first be treated as a tax-free return of capital to the extent of a US Holder's tax basis in the shares or ADRs (with a corresponding reduction in such tax basis), and thereafter will be treated as capital gain, which will be long-term capital gain if the US Holder held our shares or ADRs for more than one year. Under the Code, dividend payments by us on the shares or ADRs are not eligible for the dividends received deduction generally allowed to corporate shareholders.
Dividend income in respect of our shares or ADRs will constitute income from sources outside the US for US foreign tax credit purposes. Subject to the limitations and conditions provided in the Code, US Holders generally may claim as a credit against their US federal income tax liability, any Withholding Tax withheld from a dividend. The rules governing the foreign tax credit are complex. Each US Holder is urged to consult its own tax advisor concerning whether, and to what extent, a foreign tax credit will be available with respect to dividends received from us. Alternatively, a US Holder may claim the Withholding Tax as a deduction for the taxable year within which the Withholding Tax is paid or accrued, provided a deduction is claimed for all of the foreign income taxes the US Holder pays or accrues in the particular year. A deduction does not reduce US tax on a dollar-for-dollar basis like a tax credit. The deduction, however, is not subject to the limitations applicable to foreign tax credits.
The US Treasury has expressed concern that parties to whom ADRs are released may be taking actions inconsistent with the claiming of foreign tax credits for US Holders of ADRs. Accordingly, the summary above of the creditability of the Withholding Tax could be affected by future actions that may be taken by the US Treasury.
In general, a US Holder will be required to determine the amount of any dividend paid in Swiss francs, including the amount of any Withholding Tax imposed thereon, by translating the Swiss francs into US dollars at the spot rate on the date the dividend is actually or constructively received by a US Holder, in the case of shares, or by the Depositary, in the case of ADRs, regardless of whether the Swiss francs are in fact converted into US dollars. If a US Holder converts the Swiss francs so received into US dollars on the date of receipt, the US Holder generally should not recognize foreign currency gain or loss on such conversion. If a US Holder does not convert the Swiss francs so received into US dollars on the date of receipt, the US Holder will have a tax basis in the Swiss francs equal to the US dollar value on such date. Any foreign currency gain or loss that a US Holder recognizes on a subsequent conversion or other disposition of the Swiss francs generally will be treated as US source ordinary income or loss.
295
For a non-corporate US Holder, the US dollar amount of any dividends paid to it prior to January 1, 2013 that constitute qualified dividend income generally will be taxable at a maximum rate of 15%. For tax years beginning after 2012, the top rate is 20% for taxpayers with incomes exceeding $413,200 ($464,850 for joint filing taxpayers) provided that the US Holder meets certain holding period and other requirements. In addition, the dividends could be subject to a 3.8% net investment income tax. This tax is applied against the lesser of the US Holder's net investment income or modified adjusted gross income over $200,000 ($250,000 for joint filing taxpayers). We currently believe that dividends paid with respect to our shares and ADRs will constitute qualified dividend income for US federal income tax purposes. However, the US Treasury and the US Internal Revenue Service ("IRS") have announced their intention to promulgate rules pursuant to which US Holders of shares and ADRs, among others, will be permitted to rely on certifications from issuers to establish that dividends are treated as qualified dividends. US Holders of shares or ADRs are urged to consult their own tax advisors regarding the availability to them of the reduced dividend rate in light of their own particular situation and the computations of their foreign tax credit limitation with respect to any qualified dividends paid to them, as applicable.
Sale or Other Taxable Disposition. Upon a sale or other taxable disposition of shares or ADRs, US Holders generally will recognize capital gain or loss in an amount equal to the difference between the US dollar value of the amount realized on the disposition and the US Holder's tax basis (determined in US dollars) in the shares or ADRs. This capital gain or loss generally will be US source gain or loss and will be treated as long-term capital gain or loss if the holding period in the shares or ADRs exceeds one year. In the case of certain US Holders (including individuals), any long term capital gain generally will be subject to US federal income tax at preferential rates, which rates are subject to a maximum of 20% for taxpayers with incomes exceeding $413,200 ($464,850 for joint filing taxpayers) for gains recognized after January 1, 2013. In addition, the gains could be subject to a 3.8% investment income tax. This tax is applied against the lesser of the US Holder's net investment income or modified adjusted gross income over $200,000 ($250,000 for joint filing taxpayers). The deductibility of capital losses is subject to significant limitations under the Code. Deposits or withdrawals of our shares by US Holders in exchanges for ADRs will not result in the realization of gain or loss for US federal income tax purposes.
US Information Reporting and Backup Withholding. Dividend payments with respect to shares or ADRs and proceeds from the sale, exchange or other disposition of shares or ADRs received in the United States or through US-related financial intermediaries, may be subject to information reporting to the IRS and possible US backup withholding. Certain exempt recipients (such as corporations) are not subject to these information reporting and backup withholding requirements. Backup withholding will not apply to a US Holder who furnishes a correct taxpayer identification number and makes any other required certification or who is otherwise exempt from backup withholding. Any US Holders required to establish their exempt status generally must provide a properly-executed IRS Form W-9 (Request for Taxpayer Identification Number and Certification). Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a US Holder's US federal income tax liability, and a US Holder may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
10.F Dividends and paying agents
Not applicable.
Not applicable.
296
Any statement in this Form 20-F about any of our contracts or other documents is not necessarily complete. If the contract or document is filed as an exhibit to the Form 20-F the contract or document is deemed to modify the description contained in this Form 20-F. You must review the exhibits themselves for a complete description of the contract or document.
You may review a copy of our filings with the SEC, as well as other information furnished to the SEC, including exhibits and schedules filed with it, at the SEC's public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information. In addition, the SEC maintains an Internet site at http://www.sec.gov that contains reports and other information regarding issuers that file electronically with the SEC. These SEC filings are also available to the public from commercial document retrieval services.
We are required to file or furnish reports and other information with the SEC under the Securities Exchange Act of 1934 and regulations under that act. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the form and content of proxy statements and our officers, directors and principal shareholders are exempt from the reporting and short swing profit recovery provisions contained in Section 16 of the Exchange Act.
Not applicable.
Item 11. Quantitative and Qualitative Disclosures about Market Risk
The major financial risks facing the Group are managed centrally by Group Treasury. We have a written Treasury Directive and have implemented a strict segregation of front office and back office controls. The Group does regular reconciliations of its positions with its counterparties. In addition the Treasury function is included in management's internal control assessment.
For information about the effects of currency fluctuations and how we manage currency risk, see "Item 5. Operating and Financial Review and Prospects—Item 5.B Liquidity and Capital Resources".
For further information, see "Item 18. Financial Statements—Note 29".
Item 12. Description of Securities Other than Equity Securities
Not applicable.
Not applicable.
Not applicable.
297
12.D American Depositary Shares
Fees Payable By ADR Holders
According to our Deposit Agreement with the ADS depositary, JPMorgan Chase Bank (JPMorgan), holders of our ADRs may have to pay to JPMorgan, either directly or indirectly, fees or charges up to the amounts set forth below:
Category
|
Depositary actions | Associated Fee | ||
|---|---|---|---|---|
| Depositing or substituting underlying shares | Acceptance of shares surrendered, and issuance of ADRs in exchange, including surrenders and issuances in respect of: | $5.00 for each 100 ADSs (or portion thereof) evidenced by the new ADRs delivered | ||
| —Share distributions | ||||
| —Stock split | ||||
| —Rights | ||||
| —Merger | ||||
| —Exchange of shares or any other transaction or event or other distribution affecting the ADSs or the deposited shares | ||||
Withdrawing underlying shares |
Acceptance of ADRs surrendered for withdrawal of deposited shares |
$5.00 for each 100 ADSs (or portion thereof) evidenced by the ADRs surrendered |
||
Selling or exercising rights |
Distribution or sale of shares, the fee being in an amount equal to the fee for the execution and delivery of ADRs which would have been charged as a result of the deposit of such shares |
$5.00 for each 100 ADSs (or portion thereof) |
||
Transferring, splitting or grouping receipts |
Transfers, combining or grouping of depositary receipts |
$1.50 per ADR |
||
Expenses of the depositary |
Expenses incurred on behalf of holders in connection with —compliance with foreign exchange control regulations or any law or regulation relating to foreign investment —the depositary's or its custodian's compliance with applicable law, rule or regulation. —stock transfer or other taxes and other governmental charges —cable, telex and facsimile transmission and delivery |
Expenses payable at the sole discretion of the Depositary by billing Holders or by deducting charges from one or more cash dividends or other cash distributions. |
||
| —expenses of the depositary in connection with the conversion of foreign currency into US dollars (which are paid out of such foreign currency) | ||||
| —any other charge payable by any of the depositary or its agents | ||||
Advance tax relief |
Tax relief/reclamation process for qualified holders. |
A depositary service charge of $0.0075 per ADS |
298
Fees Payable By The Depositary To The Issuer
Pursuant to an agreement effective as of May 11, 2012, JPMorgan, as depositary, has agreed to reimburse Novartis $1.0 million per quarter, a total of $4.0 million per contract year, for expenses incurred directly related to our ADR program (the "Program") which were incurred during the contract year, including Program-related legal fees, expenses related to investor relations in the US, US investor presentations, ADR-related financial advertising and public relations, reasonable accountants' fees in relation to our Form 20-F, maintenance and broker reimbursement expenses. Because our expenses related to these categories exceed $4.0 million (see, for example, the amount of our accountants' fees set forth at "Item 16C. Principal Accountant Fees and Services—Auditing and Additional Fees"), the $4.0 million cannot be deemed to have reimbursed us for any particular one or more of these expenses.
JPMorgan has further agreed not to seek reimbursement of up to $50,000 of out-of-pocket expenses incurred annually in providing such administrative services.
299
Item 13. Defaults, Dividend Arrearages and Delinquencies
None.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
None.
Item 15. Controls and Procedures
(a) Novartis AG's chief executive officer and chief financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Exchange Act Rule 13a-15(e)) as of the end of the period covered by this Form 20-F, have concluded that, as of such date, our disclosure controls and procedures were effective.
(b) Report of Novartis Management on Internal Control Over Financial Reporting: Novartis' Board of Directors and management of the Group are responsible for establishing and maintaining adequate internal control over financial reporting. The Group's internal control system was designed to provide reasonable assurance to the Group's management and Board of Directors regarding the reliability of financial reporting and the preparation and fair presentation of its published consolidated financial statements.
All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective may not prevent or detect misstatements and can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Group management assessed the effectiveness of the Group's internal control over financial reporting as of December 31, 2015. In making this assessment, it used the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on our assessment management concluded that, as of December 31, 2015, Group's internal control over financial reporting is effective based on those criteria.
PricewaterhouseCoopers AG, Switzerland (PwC), an independent registered public accounting firm, has issued an unqualified opinion on the effectiveness of the Group's internal control over financial reporting which is included under "Item 18. Financial Statements" on page F-2.
(c) See the report of PwC, an independent registered public accounting firm, included under "Item 18. Financial Statements" on page F-2.
(d) There were no changes to our internal control over financial reporting that occurred during the period covered by this Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 16A. Audit Committee Financial Expert
Our Audit and Compliance Committee has determined that Srikant Datar possesses specific accounting and financial management expertise and that he is an Audit Committee Financial Expert as defined by the US Securities and Exchange Commission (SEC). The Board of Directors has also determined that Srikant Datar is "independent" in accordance with the applicable requirements of Rule 10A-3 of the US Securities Exchange Act of 1934, and that other members of the Audit and
300
Compliance Committee have sufficient experience and ability in finance and compliance matters to enable them to adequately discharge their responsibilities.
In addition to our Code of Conduct, which is applicable to all of our associates, we have adopted a Code of Ethical Conduct that imposes additional obligations on our principal executive officer, principal financial officer, principal accounting officer, and persons performing similar functions. This document is accessible on our Internet website at
https://www.novartis.com/investors/company-overview/corporate-governance
Item 16C. Principal Accountant Fees and Services
Refer to "Item 6. Directors, Senior Management and Employees—Item 6.C Board Practices—Our Independent External Auditors."
Item 16D. Exemptions from the Listing Standards for Audit Committees
Not Applicable.
301
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
2015
|
Total Number of Shares Purchased (a)(1) |
Average Price Paid per Share in $ (b) |
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs (c)(2) |
Maximum Approximate Value of Shares that may yet be purchased under the Plans or Programs in CHF (d) |
Maximum Approximate Value of Shares that may yet be purchased under the Plans or Programs in $ (e) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
|
(CHF millions) |
($ millions)(3) |
|||||||||||
Jan. 1–31 |
3,937,701 | 96.43 | 2,160,000 | 5,076 | 5,468 | |||||||||||
Feb. 1–28 |
7,505,191 | 100.25 | 2,100,000 | 4,878 | 5,145 | |||||||||||
Mar. 1–31 |
2,969,714 | 98.99 | 2,340,000 | 4,650 | 4,792 | |||||||||||
Apr. 1–30 |
2,379,328 | 103.16 | 2,040,000 | 4,448 | 4,726 | |||||||||||
May 1–31 |
2,009,752 | 102.56 | 1,840,000 | 4,271 | 4,508 | |||||||||||
Jun. 1–30 |
2,353,429 | 101.67 | 2,270,000 | 4,056 | 4,348 | |||||||||||
Jul. 1–31 |
6,904,824 | 102.67 | 6,685,000 | 3,402 | 3,529 | |||||||||||
Aug. 1–31 |
6,479,905 | 101.30 | 6,305,000 | 2,784 | 2,894 | |||||||||||
Sep. 1–30 |
6,854,308 | 95.09 | 6,755,000 | 2,159 | 2,215 | |||||||||||
Oct. 1–31 |
6,902,592 | 92.38 | 6,830,000 | 1,548 | 1,568 | |||||||||||
Nov. 1–30 |
8,078,310 | 87.78 | 6,629,280 | 958 | 931 | |||||||||||
Dec. 1–31 |
7,212,944 | 85.89 | 3,923,900 | 623 | 630 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
63,587,998 | 95.94 | 49,878,180 | |||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- Column
(a) shows shares we purchased as part of our sixth share repurchase program plus the following types of share purchases outside
of our publicly announced repurchase program: (1) shares which we purchased on the open market; and (2) shares which we purchased from Swiss employees who had obtained the shares through
a Novartis Employee Ownership Plan. See "Item 18. Financial Statements—Note 26"
- (2)
- Column
(c) shows shares purchased as part of our sixth share repurchase program which was approved by the shareholders
February 26, 2008 for an amount of up to CHF 10.0 billion. See "Item 6. Directors, Senior Management and Employes—5.C Board Practices—Our Shares and
Our Shareholders—Share Repurchase Programs."
- (3)
- Column (e) shows the Swiss franc amount from column (d) converted into US dollars as of the month-end, using the Swiss franc/US dollar exchange rate at the applicable month-end.
Item 16F. Change in Registrant's Certifying Accountant
Not applicable.
Item 16G. Corporate Governance
Refer to "Item 6. Directors, Senior Management and Employees—Item 6.C Board Practices—Our Corporate Governance Framework."
Item 16H. Mine Safety Disclosure
Not applicable.
302
See "Item 18. Financial Statements."
The following financial statements are filed as part of this annual report on Form 20-F.
303
| 1.1 | Articles of Incorporation of Novartis AG, as amended February 27, 2015 (English translation). | ||
1.2 |
Regulations of the Board and Committee Charters of Novartis AG, as amended in relevant part January 1, 2014, March 1, 2015, and November 1, 2015. |
||
2.1 |
Amended and Restated Deposit Agreement, dated as of May 11, 2000 among Novartis AG, JPMorgan Chase Bank (fka Morgan Guaranty Trust Company of New York), as depositary, and all holders from time to time of ADRs issued thereunder (incorporated by reference to Exhibit (a)(1) to Post-Effective Amendment No. 1 to Novartis AG's registration statement on Form F-6 (File No. 333-11758) as filed with the SEC on September 8, 2000). |
||
2.2 |
Amendment No. 1 to the Amended and Restated Deposit Agreement (incorporated by reference to Exhibit (a)(2) to Post-Effective Amendment No. 1 to Novartis AG's registration statement on Form F-6 (File No. 333-11758) as filed with the SEC on September 8, 2000). |
||
2.3 |
Amendment No. 2 to the Amended and Restated Deposit Agreement (incorporated by reference to Exhibit (a)(3) to Novartis AG's registration statement on Form F-6 (File No. 333-13446) as filed with the SEC on May 7, 2001). |
||
2.4 |
Restricted Issuance Agreement dated as of January 11, 2002 among Novartis AG, J.P. Morgan Chase Bank, as depositary, and all holders from time to time of ADRs representing ADSs issued thereunder (incorporated by reference to Exhibit 4 to the Registration Statement on Form F-3, File No. 333-81862, as filed with the SEC on January 31, 2002). |
||
2.5 |
Letter Agreement dated December 14, 2007 between Novartis AG and JPMorgan Chase Bank, as depositary (incorporated by reference to Exhibit 2.4 to the Form 20-F for the year ended December 31, 2007 as filed with the SEC on January 28, 2008). |
||
2.6 |
Form of American Depositary Receipt (incorporated by reference to Exhibit (a)(7) to the Registration Statement on Form F-6, File No. 333-198623, as filed with the SEC on September 8, 2014). |
||
2.7 |
The total amount of long-term debt securities authorized under any instrument does not exceed 10% of the total assets of the Company and its subsidiaries on a consolidated basis. We hereby agree to furnish to the SEC, upon its request, a copy of any instrument defining the rights of holders of long-term debt of the Company or of its subsidiaries for which consolidated or unconsolidated financial statements are required to be filed. |
||
4.1 |
Implementation Agreement made on April 22, 2014, and amended and restated on May 29, 2014, between GlaxoSmithKline plc and Novartis AG. (Incorporated by reference to Exhibit 4.1 of the Form 20-F for the year ended December 31, 2014, as filed with the SEC on January 27, 2015.) |
||
4.2 |
Contribution Agreement relating to the Consumer Healthcare Joint Venture made on April 22, 2014, as amended and restated on May 29, 2014 and March 1, 2015, between Novartis AG, GlaxoSmithKline plc and GlaxoSmithKline Consumer Healthcare Holdings Limited (formerly known as Leo Constellation Limited). Confidential portions of this exhibit have been omitted pursuant to a request for confidential treatment and filed separately with the SEC. |
||
4.3 |
Share and Business Sale Agreement relating to the Vaccines Group made on April 22, 2014, as amended and restated on May 29, 2014, as further amended on October 9, 2014, and as further amended and restated on March 1, 2015, between Novartis AG and GlaxoSmithKline plc. Confidential portions of this exhibit have been omitted pursuant to a request for confidential treatment and filed separately with the SEC. |
304
| 4.4 | Sale and Purchase Agreement in relation to the Oncology Business made on April 22, 2014, as amended and restated on May 29, 2014, November 21, 2014 and March 1, 2015, between GlaxoSmithKline plc and Novartis AG. Confidential portions of this exhibit have been omitted pursuant to a request for confidential treatment and filed separately with the SEC. | ||
4.5 |
Put Option Deed relating to all or part of the Influenza Business of the Novartis Group made on April 22, 2014, and amended and restated on May 29, 2014, between Novartis AG and GlaxoSmithKline plc. Confidential portions of this exhibit have been omitted pursuant to a request for confidential treatment and filed separately with the SEC. (Incorporated by reference to Exhibit 4.5 of the Form 20-F for the year ended December 31, 2014, as filed with the SEC on January 27, 2015.) |
||
4.6 |
Stock and Asset Purchase Agreement made on April 22, 2014, as amended on December 17, 2014, between Novartis AG and Eli Lilly and Company. Confidential portions of this exhibit have been omitted pursuant to a request for confidential treatment and filed separately with the SEC. (Incorporated by reference to Exhibit 4.6 of the Form 20-F for the year ended December 31, 2014, as filed with the SEC on January 27, 2015.) |
||
4.7 |
Share and Business Sale Agreement relating to the Flu Group made on October 26, 2014, as amended and restated on July 31, 2015, between Novartis AG and CSL Limited. Confidential portions of this exhibit have been omitted pursuant to a request for confidential treatment and filed separately with the SEC. |
||
4.8 |
Shareholders' Agreement relating to GlaxoSmithKline Consumer Healthcare Holdings Limited made on March 2, 2015, between GlaxoSmithKline Consumer Healthcare Holdings Limited, GlaxoSmithKline plc, Setfirst Limited, Novartis AG, Novartis Holding AG and Novartis Finance Corporation. Confidential portions of this exhibit have been omitted pursuant to a request for confidential treatment and filed separately with the SEC. |
||
6.1 |
For earnings per share calculation, see "Item 18. Financial Statements—Note 7." |
||
8.1 |
For a list of all of our principal Group subsidiaries and associated companies, see "Item 18. Financial Statements—Note 32." |
||
12.1 |
Certification of Joseph Jimenez, Chief Executive Officer of Novartis AG, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
||
12.2 |
Certification of Harry Kirsch, Chief Financial Officer of Novartis AG, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
||
13.1 |
Certification of Joseph Jimenez, Chief Executive Officer of Novartis AG, pursuant to Section 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
||
13.2 |
Certification of Harry Kirsch, Chief Financial Officer of Novartis AG, pursuant to Section 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
||
15.1 |
Consent of Independent Registered Public Accounting Firm, PricewaterhouseCoopers AG, to the incorporation by reference of the audit report contained in this Form 20-F into Novartis AG's Registration Statements on Form S-8 filed on October 1, 2004 (File No. 333-119475), on Form S-8 filed on September 5, 2006 (File No. 333-137112), on Form S-8 filed on October 29, 2009 (File No. 333-162727), on Form S-8 filed on January 18, 2011 (File No. 333-171739), on Form S-8 filed on April 8, 2011 (File No. 333-173382), on Form S-8 filed on September 12, 2014 (File No. 333-198706), and on Form F-3 filed on September 18, 2015 (File No. 333-207004). |
305
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| NOVARTIS AG | ||||
By: |
/s/ HARRY KIRSCH Name: Harry Kirsch Title: Chief Financial Officer, Novartis Group |
|||
By: |
/s/ FELIX R. EHRAT Name: Felix R. Ehrat Title: General Counsel, Novartis Group |
|||
Date: January 27, 2016
306
NOVARTIS GROUP
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors of Novartis AG, Basel
In our opinion, the accompanying consolidated balance sheets and the related consolidated income statements, consolidated statements of comprehensive income, consolidated statements of changes in equity, consolidated cash flow statements and notes (pages F-4 through F-118 in this Form 20-F) present fairly, in all material respects, the financial position of Novartis AG and its consolidated subsidiaries (Group or Company) at December 31, 2015 and December 31, 2014, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2015 in conformity with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
The Novartis' Board of Directors and management of the Group are responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying "Report of Novartis Management on Internal Control Over Financial Reporting" appearing under Item 15(b). Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board of the United States of America. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk
F-2
that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| PricewaterhouseCoopers AG | ||
/s/ BRUNO ROSSI Bruno Rossi Audit expert Auditor in charge |
/s/ STEPHEN JOHNSON Stephen Johnson Global relationship partner |
|
Basel, January 26, 2016 |
F-3
NOVARTIS GROUP CONSOLIDATED FINANCIAL STATEMENTS
CONSOLIDATED INCOME STATEMENTS
(For the years ended December 31, 2015, 2014 and 2013)
| |
Note | 2015 | 2014 | 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
$ m |
$ m |
$ m |
|||||||||
Net sales to third parties from continuing operations |
3 | 49,414 | 52,180 | 51,869 | |||||||||
Sales to discontinued segments |
26 | 239 | 221 | ||||||||||
| | | | | | | | | | | | | | |
Net sales from continuing operations |
3 | 49,440 | 52,419 | 52,090 | |||||||||
Other revenues |
947 | 1,215 | 626 | ||||||||||
Cost of goods sold |
(17,404 | ) | (17,345 | ) | (16,579 | ) | |||||||
| | | | | | | | | | | | | | |
Gross profit from continuing operations |
32,983 | 36,289 | 36,137 | ||||||||||
Marketing & Sales |
(11,772 | ) | (12,377 | ) | (12,638 | ) | |||||||
Research & Development |
(8,935 | ) | (9,086 | ) | (9,071 | ) | |||||||
General & Administration |
(2,475 | ) | (2,616 | ) | (2,603 | ) | |||||||
Other income |
2,049 | 1,391 | 1,205 | ||||||||||
Other expense |
(2,873 | ) | (2,512 | ) | (2,047 | ) | |||||||
| | | | | | | | | | | | | | |
Operating income from continuing operations |
3 | 8,977 | 11,089 | 10,983 | |||||||||
Income from associated companies |
4 | 266 | 1,918 | 599 | |||||||||
Interest expense |
5 | (655 | ) | (704 | ) | (683 | ) | ||||||
Other financial income and expense |
5 | (454 | ) | (31 | ) | (92 | ) | ||||||
| | | | | | | | | | | | | | |
Income before taxes from continuing operations |
8,134 | 12,272 | 10,807 | ||||||||||
Taxes |
6 | (1,106 | ) | (1,545 | ) | (1,498 | ) | ||||||
| | | | | | | | | | | | | | |
Net income from continuing operations |
7,028 | 10,727 | 9,309 | ||||||||||
Net income/(loss) from discontinued operations |
30 | 10,766 | (447 | ) | (17 | ) | |||||||
| | | | | | | | | | | | | | |
Net income |
17,794 | 10,280 | 9,292 | ||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Attributable to: |
|||||||||||||
Shareholders of Novartis AG |
17,783 | 10,210 | 9,175 | ||||||||||
Non-controlling interests |
11 | 70 | 117 | ||||||||||
Basic earnings per share ($) from continuing operations |
2.92 |
4.39 |
3.76 |
||||||||||
Basic earnings per share ($) from discontinued operations |
4.48 | (0.18 | ) | 0.00 | |||||||||
| | | | | | | | | | | | | | |
Total basic earnings per share ($) |
7 | 7.40 | 4.21 | 3.76 | |||||||||
| | | | | | | | | | | | | | |
Diluted earnings per share ($) from continuing operations |
2.88 | 4.31 | 3.70 | ||||||||||
Diluted earnings per share ($) from discontinued operations |
4.41 | (0.18 | ) | 0.00 | |||||||||
| | | | | | | | | | | | | | |
Total diluted earnings per share ($) |
7 | 7.29 | 4.13 | 3.70 | |||||||||
| | | | | | | | | | | | | | |
The accompanying Notes form an integral part of the consolidated financial statements.
F-4
NOVARTIS GROUP CONSOLIDATED FINANCIAL STATEMENTS
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(For the years ended December 31, 2015, 2014 and
2013)
| |
Note | 2015 | 2014 | 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
$ m |
$ m |
$ m |
|||||||||
Net income |
17,794 | 10,280 | 9,292 | ||||||||||
Other comprehensive income to be eventually recycled into the consolidated income statement: |
|||||||||||||
Fair value adjustments on marketable securities, net of taxes |
8.1 | 28 | 89 | 132 | |||||||||
Fair value adjustments on deferred cash flow hedges, net of taxes |
8.1 | 20 | 21 | 41 | |||||||||
| | | | | | | | | | | | | | |
Total fair value adjustments on financial instruments, net of taxes |
8.1 | 48 | 110 | 173 | |||||||||
Novartis share of other items recorded in comprehensive income recognized by associated companies, net of taxes |
8.2 | (48 | ) | (5 | ) | 5 | |||||||
Currency translation effects |
8.3 | (1,662 | ) | (2,220 | ) | 676 | |||||||
| | | | | | | | | | | | | | |
Total of items to eventually recycle |
(1,662 | ) | (2,115 | ) | 854 | ||||||||
| | | | | | | | | | | | | | |
Other comprehensive income never to be recycled into the consolidated income statement: |
|||||||||||||
Actuarial (losses)/gains from defined benefit plans, net of taxes |
8.4 | (147 | ) | (822 | ) | 1,504 | |||||||
| | | | | | | | | | | | | | |
Total comprehensive income |
15,985 | 7,343 | 11,650 | ||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Attributable to: |
|||||||||||||
Shareholders of Novartis AG |
15,977 | 7,274 | 11,538 | ||||||||||
Continuing operations |
5,238 | 7,820 | 11,512 | ||||||||||
Discontinued operations |
10,739 | (546 | ) | 26 | |||||||||
Non-controlling interests |
8 | 69 | 112 | ||||||||||
The accompanying Notes form an integral part of the consolidated financial statements.
F-5
NOVARTIS GROUP CONSOLIDATED FINANCIAL STATEMENTS
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
(For the years ended December 31, 2015, 2014 and 2013)
| |
Note | Share capital |
Treasury shares |
Retained earnings |
Total value adjustments |
Issued share capital and reserves attributable to Novartis shareholders |
Non- controlling interests |
Total equity |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||||||
Total equity at January 1, 2013 |
1,001 | (92 | ) | 70,220 | (1,992 | ) | 69,137 | 126 | 69,263 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income |
9,175 | 9,175 | 117 | 9,292 | |||||||||||||||||||||
Other comprehensive income |
8 | 5 | 2,358 | 2,363 | (5 | ) | 2,358 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income |
9,180 | 2,358 | 11,538 | 112 | 11,650 | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Dividends |
9.1 | (6,100 | ) | (6,100 | ) | (6,100 | ) | ||||||||||||||||||
Purchase of treasury shares |
9.2 | (22 | ) | (2,968 | ) | (2,990 | ) | (2,990 | ) | ||||||||||||||||
Increase in equity from exercise of options and employee transactions |
9.5 | 19 | 1,672 | 1,691 | 1,691 | ||||||||||||||||||||
Equity-based compensation |
9.6 | 6 | 1,071 | 1,077 | 1,077 | ||||||||||||||||||||
Impact of change in ownership of consolidated entities |
9.8 | (10 | ) | (10 | ) | (10 | ) | ||||||||||||||||||
Changes in non-controlling interests |
9.7 | (109 | ) | (109 | ) | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total of other equity movements |
3 | (6,335 | ) | (6,332 | ) | (109 | ) | (6,441 | ) | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total equity at December 31, 2013 |
1,001 | (89 | ) | 73,065 | 366 | 74,343 | 129 | 74,472 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income |
10,210 | 10,210 | 70 | 10,280 | |||||||||||||||||||||
Other comprehensive income |
8 | (5 | ) | (2,931 | ) | (2,936 | ) | (1 | ) | (2,937 | ) | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income |
10,205 | (2,931 | ) | 7,274 | 69 | 7,343 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Dividends |
9.1 | (6,810 | ) | (6,810 | ) | (6,810 | ) | ||||||||||||||||||
Purchase of treasury shares |
9.2 | (43 | ) | (6,883 | ) | (6,926 | ) | (6,926 | ) | ||||||||||||||||
Increase of Treasury share repurchase obligation under a share buy-back trading plan |
9.4 | (658 | ) | (658 | ) | (658 | ) | ||||||||||||||||||
Increase in equity from exercise of options and employee transactions |
9.5 | 23 | 2,377 | 2,400 | 2,400 | ||||||||||||||||||||
Equity-based compensation |
9.6 | 6 | 1,137 | 1,143 | 1,143 | ||||||||||||||||||||
Changes in non-controlling interests |
9.7 | (120 | ) | (120 | ) | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total of other equity movements |
(14 | ) | (10,837 | ) | (10,851 | ) | (120 | ) | (10,971 | ) | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total equity at December 31, 2014 |
1,001 | (103 | ) | 72,433 | (2,565 | ) | 70,766 | 78 | 70,844 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income |
17,783 | 17,783 | 11 | 17,794 | |||||||||||||||||||||
Other comprehensive income |
8 | (48 | ) | (1,758 | ) | (1,806 | ) | (3 | ) | (1,809 | ) | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income |
17,735 | (1,758 | ) | 15,977 | 8 | 15,985 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Dividends |
9.1 | (6,643 | ) | (6,643 | ) | (6,643 | ) | ||||||||||||||||||
Purchase of treasury shares |
9.2 | (33 | ) | (6,086 | ) | (6,119 | ) | (6,119 | ) | ||||||||||||||||
Reduction of share capital |
9.3 | (10 | ) | 15 | (5 | ) | |||||||||||||||||||
Decrease of treasury share repurchase obligation under a share buy-back trading plan |
9.4 | 658 | 658 | 658 | |||||||||||||||||||||
Increase in equity from exercise of options and employee transactions |
9.5 | 14 | 1,578 | 1,592 | 1,592 | ||||||||||||||||||||
Equity-based compensation |
9.6 | 6 | 809 | 815 | 815 | ||||||||||||||||||||
Changes in non-controlling interests |
9.7 | (10 | ) | (10 | ) | ||||||||||||||||||||
Fair value adjustments related to divestments |
8 | (100 | ) | 100 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total of other equity movements |
(10 | ) | 2 | (9,789 | ) | 100 | (9,697 | ) | (10 | ) | (9,707 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total equity at December 31, 2015 |
991 | (101 | ) | 80,379 | (4,223 | ) | 77,046 | 76 | 77,122 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying Notes form an integral part of the consolidated financial statements.
F-6
NOVARTIS GROUP CONSOLIDATED FINANCIAL STATEMENTS
CONSOLIDATED BALANCE SHEETS
(At December 31, 2015 and 2014)
| |
Note | 2015 | 2014 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
$ m |
$ m |
|||||||
Assets |
||||||||||
Non-current assets |
||||||||||
Property, plant & equipment |
10 | 15,982 | 15,983 | |||||||
Goodwill |
11 | 31,174 | 29,311 | |||||||
Intangible assets other than goodwill |
11 | 34,217 | 23,832 | |||||||
Investments in associated companies |
4 | 15,314 | 8,432 | |||||||
Deferred tax assets |
12 | 8,957 | 7,994 | |||||||
Financial assets |
13 | 2,466 | 1,720 | |||||||
Other non-current assets |
13 | 601 | 554 | |||||||
| | | | | | | | | | | |
Total non-current assets related to continuing operations |
108,711 | 87,826 | ||||||||
| | | | | | | | | | | |
Current assets |
||||||||||
Inventories |
14 | 6,226 | 6,093 | |||||||
Trade receivables |
15 | 8,180 | 8,275 | |||||||
Marketable securities, commodities, time deposits and derivative financial instruments |
16 | 773 | 839 | |||||||
Cash and cash equivalents |
16 | 4,674 | 13,023 | |||||||
Other current assets |
17 | 2,992 | 2,530 | |||||||
| | | | | | | | | | | |
Total current assets related to continuing operations |
22,845 | 30,760 | ||||||||
Assets related to discontinued operations |
30 | 0 | 6,801 | |||||||
| | | | | | | | | | | |
Total current assets |
22,845 | 37,561 | ||||||||
| | | | | | | | | | | |
Total assets |
131,556 | 125,387 | ||||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Equity and liabilities |
||||||||||
Equity |
||||||||||
Share capital |
18 | 991 | 1,001 | |||||||
Treasury shares |
18 | (101 | ) | (103 | ) | |||||
Reserves |
76,156 | 69,868 | ||||||||
| | | | | | | | | | | |
Issued share capital and reserves attributable to Novartis AG shareholders |
77,046 | 70,766 | ||||||||
Non-controlling interests |
76 | 78 | ||||||||
| | | | | | | | | | | |
Total equity |
77,122 | 70,844 | ||||||||
| | | | | | | | | | | |
Liabilities |
||||||||||
Non-current liabilities |
||||||||||
Financial debts |
19 | 16,327 | 13,799 | |||||||
Deferred tax liabilities |
12 | 6,355 | 6,099 | |||||||
Provisions and other non-current liabilities |
20 | 8,044 | 7,672 | |||||||
| | | | | | | | | | | |
Total non-current liabilities related to continuing operations |
30,726 | 27,570 | ||||||||
| | | | | | | | | | | |
Current liabilities |
||||||||||
Trade payables |
5,668 | 5,419 | ||||||||
Financial debts and derivative financial instruments |
21 | 5,604 | 6,612 | |||||||
Current income tax liabilities |
1,717 | 2,076 | ||||||||
Provisions and other current liabilities |
22 | 10,719 | 10,448 | |||||||
| | | | | | | | | | | |
Total current liabilities related to continuing operations |
23,708 | 24,555 | ||||||||
Liabilities related to discontinued operations |
30 | 0 | 2,418 | |||||||
| | | | | | | | | | | |
Total current liabilities |
23,708 | 26,973 | ||||||||
| | | | | | | | | | | |
Total liabilities |
54,434 | 54,543 | ||||||||
| | | | | | | | | | | |
Total equity and liabilities |
131,556 | 125,387 | ||||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The accompanying Notes form an integral part of the consolidated financial statements.
F-7
NOVARTIS GROUP CONSOLIDATED FINANCIAL STATEMENTS
CONSOLIDATED CASH FLOW STATEMENTS
(For the years ended December 31, 2015, 2014 and 2013)
| |
Note | 2015 | 2014 | 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
$ m |
$ m |
$ m |
|||||||||
Net income from continuing operations |
7,028 | 10,727 | 9,309 | ||||||||||
Reversal of non-cash items |
23.1 | 9,070 | 6,725 | 7,179 | |||||||||
Dividends received from associated companies and others |
432 | 479 | 444 | ||||||||||
Interest received |
34 | 35 | 40 | ||||||||||
Interest paid |
(646 | ) | (668 | ) | (609 | ) | |||||||
Other financial receipts |
714 | 553 | 55 | ||||||||||
Other financial payments |
(23 | ) | (24 | ) | (22 | ) | |||||||
Taxes paid(1) |
(2,454 | ) | (2,179 | ) | (2,054 | ) | |||||||
| | | | | | | | | | | | | | |
Cash flows before working capital and provision changes from continuing operations |
14,155 | 15,648 | 14,342 | ||||||||||
Payments out of provisions and other net cash movements in non-current liabilities |
(1,207 | ) | (1,125 | ) | (947 | ) | |||||||
Change in net current assets and other operating cash flow items |
23.2 | (863 | ) | (625 | ) | (778 | ) | ||||||
| | | | | | | | | | | | | | |
Cash flows from operating activities from continuing operations |
12,085 | 13,898 | 12,617 | ||||||||||
Cash flows used in/from operating activities from discontinued operations(1) |
(188 | ) | (1 | ) | 557 | ||||||||
| | | | | | | | | | | | | | |
Total cash flows from operating activities |
11,897 | 13,897 | 13,174 | ||||||||||
| | | | | | | | | | | | | | |
Purchase of property, plant & equipment |
(2,367 | ) | (2,624 | ) | (2,903 | ) | |||||||
Proceeds from sales of property, plant & equipment |
237 | 60 | 48 | ||||||||||
Purchase of intangible assets |
(1,138 | ) | (780 | ) | (475 | ) | |||||||
Proceeds from sales of intangible assets |
621 | 246 | 96 | ||||||||||
Purchase of financial assets |
(264 | ) | (239 | ) | (152 | ) | |||||||
Proceeds from sales of financial assets |
166 | 431 | 313 | ||||||||||
Purchase of other non-current assets |
(82 | ) | (60 | ) | (38 | ) | |||||||
Proceeds from sales of other non-current assets |
1 | 2 | 15 | ||||||||||
Divestments/acquisitions of interests in associated companies |
1,370 | (52 | ) | ||||||||||
Acquisitions of businesses |
23.3 | (16,507 | ) | (331 | ) | (42 | ) | ||||||
Purchase of marketable securities and commodities |
(595 | ) | (169 | ) | (278 | ) | |||||||
Proceeds from sales of marketable securities and commodities |
262 | 2,086 | 249 | ||||||||||
| | | | | | | | | | | | | | |
Cash flows used in investing activities from continuing operations |
(19,666 | ) | (8 | ) | (3,219 | ) | |||||||
Cash flows from/used in investing activities from discontinued operations(1) |
23.4 | 8,882 | 889 | (133 | ) | ||||||||
| | | | | | | | | | | | | | |
Total cash flows used in/from investing activities |
(10,784 | ) | 881 | (3,352 | ) | ||||||||
| | | | | | | | | | | | | | |
Dividends paid to shareholders of Novartis AG |
(6,643 | ) | (6,810 | ) | (6,100 | ) | |||||||
Acquisition of treasury shares |
(6,071 | ) | (6,915 | ) | (2,930 | ) | |||||||
Proceeds from exercise options and other treasury share transactions |
1,581 | 2,400 | 1,693 | ||||||||||
Increase in non-current financial debts |
4,596 | 6,024 | 93 | ||||||||||
Repayment of non-current financial debts |
(3,086 | ) | (2,599 | ) | (2,022 | ) | |||||||
Change in current financial debts |
451 | (107 | ) | 596 | |||||||||
Impact of change in ownership of consolidated entities |
4 | ||||||||||||
Dividends paid to non-controlling interests and other financing cash flows |
(4 | ) | (140 | ) | (103 | ) | |||||||
| | | | | | | | | | | | | | |
Cash flows used in financing activities |
(9,176 | ) | (8,147 | ) | (8,769 | ) | |||||||
| | | | | | | | | | | | | | |
Net effect of currency translation on cash and cash equivalents |
(286 | ) | (295 | ) | 82 | ||||||||
| | | | | | | | | | | | | | |
Net change in cash and cash equivalents |
(8,349 | ) | 6,336 | 1,135 | |||||||||
Cash and cash equivalents at January 1 |
13,023 | 6,687 | 5,552 | ||||||||||
| | | | | | | | | | | | | | |
Cash and cash equivalents at December 31 |
4,674 | 13,023 | 6,687 | ||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- (1)
- In 2015, total tax payment amounted to $3.3 billion (2014: $2.6 billion) of which a refund of $94 million (2014: $7 million) was included in the cash flows used in operating activities of discontinued operations and a $965 million payment (2014: $459 million) in the cash flows from investing activities of discontinued operations.
The accompanying Notes form an integral part of the consolidated financial statements.
F-8
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS
1. Significant Accounting Policies
The Novartis Group (Novartis or Group) is a multinational group of companies specializing in the research, development, manufacturing and marketing of a broad range of healthcare products led by innovative pharmaceuticals and also including eye care products and cost saving generic pharmaceuticals. It is headquartered in Basel, Switzerland.
The consolidated financial statements of the Group are prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB). They are prepared in accordance with the historical cost convention except for items that are required to be accounted for at fair value.
The Group's financial year-end is December 31 which is also the annual closing date of the individual entities' financial statements incorporated into the Group's consolidated financial statements.
The preparation of financial statements requires management to make certain estimates and assumptions, either at the balance sheet date or during the year that affect the reported amounts of assets and liabilities, including any contingent amounts, as well as of revenues and expenses. Actual outcomes and results could differ from those estimates and assumptions.
Listed below are accounting policies of significance to Novartis or, in cases where IFRS provides alternatives, the option adopted by Novartis.
The consolidated financial statements include all entities, including structured entities, over which Novartis AG, Basel, Switzerland, directly or indirectly has control (generally as a result of owning more than 50% of the entity's voting interest). Consolidated entities are also referred to as "subsidiaries".
In cases where Novartis does not fully own a subsidiary it has elected to value any remaining outstanding non-controlling interest at the time of acquiring control of the subsidiary at its proportionate share of the fair value of the net identified assets.
The contribution of a business to an associate or joint venture is accounted for by applying the option under IFRS that permits the accounting for the retained interest of the business contributed at its net book value at the time of the contribution.
Investments in associated companies (generally defined as investments in entities in which Novartis holds between 20% and 50% of voting shares or over which it otherwise has significant influence) and joint ventures are accounted for using the equity method except for selected venture fund investments for which the Group has elected to apply the method of fair value through the consolidated income statement.
The consolidated financial statements of Novartis are presented in US dollars ($). The functional currency of subsidiaries is generally the local currency of the respective entity. The functional currency used for the reporting of certain Swiss and foreign finance entities is $ instead of their respective local currencies. This reflects the fact that the cash flows and transactions of these entities are primarily denominated in these currencies.
F-9
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
For subsidiaries not operating in hyperinflationary economies, the subsidiary's results, financial position and cash flows that do not have $ as their functional currency are translated into $ using the following exchange rates:
- –
- income, expense and cash flows using for each month the average exchange rate with the US dollar values for each month being
aggregated during the year.
- –
- balance sheets using year-end exchange rates.
- –
- resulting exchange rate differences are recognized in other comprehensive income.
The only hyperinflationary economy applicable to Novartis is Venezuela. The financial statements of the major subsidiaries in this country are first adjusted for the effect of inflation with any gain or loss on the net monetary position recorded in the related functional lines in the consolidated income statement and then translated into $.
Acquired assets are initially recognized on the balance sheet at cost if they meet the criteria for capitalization. If acquired as part of a business combination, the fair value of identified assets represents the cost for these assets. If separately acquired, the cost of the asset includes the purchase price and any directly attributable costs for bringing the asset into the condition to operate as intended. Expected costs for obligations to dismantle and remove property, plant and equipment when it is no longer used are included in their cost.
Property, plant and equipment are depreciated on a straight-line basis in the consolidated income statement over their estimated useful lives. Leasehold land is depreciated over the period of its lease whereas freehold land is not depreciated. The related depreciation expense is included in the costs of the functions using the asset.
Property, plant and equipment are assessed for impairment whenever there is an indication that the balance sheet carrying amount may not be recoverable using cash flow projections for the useful life.
The following table shows the respective useful lives for property, plant and equipment:
| |
Useful life | |||
|---|---|---|---|---|
Buildings |
20 to 40 years | |||
Machinery and other equipment |
||||
Machinery and equipment |
7 to 20 years | |||
Furniture and vehicles |
5 to 10 years | |||
Computer hardware |
3 to 7 years | |||
Government grants obtained for construction activities, including any related equipment, are deducted from the gross acquisition cost to arrive at the balance sheet carrying value of the related assets.
F-10
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
Goodwill and Intangible Assets
Goodwill
Goodwill arises in a business combination and is the excess of the consideration transferred to acquire a business over the underlying fair value of the net identified assets acquired. It is allocated to groups of cash generating units (CGUs) which are usually represented by the reported segments. Goodwill is tested for impairment annually at the CGU level and any impairment charges are recorded under "Other Expense" in the consolidated income statement.
Intangible Assets Available-for-Use
Novartis has the following classes of available-for-use intangible assets: Currently marketed products; Marketing know-how; Technologies; Other intangible assets (including computer software) and the Alcon brand name.
Currently marketed products represent the composite value of acquired intellectual property, patents, and distribution rights and product trade names.
Marketing know-how represents the value attributable to the expertise acquired for marketing and distributing Alcon surgical equipment.
Technologies represent identified and separable acquired know-how used in the research, development and production processes.
Significant investments in internally developed and acquired computer software are capitalized and included in the "Other" category and amortized once available for use.
The Alcon brand name is shown separately as it is the only Novartis intangible asset that is available for use with an indefinite useful life. Novartis considers that it is appropriate that the Alcon brand name has an indefinite life since Alcon has a history of strong revenue and cash flow performance, and Novartis has the intent and ability to support the brand with spending to maintain its value for the foreseeable future.
Except for the Alcon brand name, intangible assets available for use are amortized over their estimated useful lives on a straight-line basis and evaluated for potential impairment whenever facts and circumstances indicate that their carrying value may not be recoverable. The Alcon brand name is not amortized, but evaluated for potential impairment annually.
F-11
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
The following table shows the respective useful lives for available-for-use intangible assets and the location in the consolidated income statement in which the respective amortization and any potential impairment charge is recognized:
| |
Useful life | Income statement location for amortization and impairment charges |
||
|---|---|---|---|---|
Currently marketed products |
5 to 20 years | "Cost of goods sold" | ||
Marketing know-how |
25 years | "Cost of goods sold" | ||
Technologies |
10 to 30 years | "Cost of goods sold" or "Research and Development" | ||
Other (including computer software) |
3 to 5 years | In the respective functional expense | ||
Alcon brand name |
Not amortized, indefinite useful life | Not applicable |
Intangible Assets Not Yet Available-for-Use
Acquired research and development intangible assets, which are still under development and have accordingly not yet obtained marketing approval, are recognized as In-Process Research & Development (IPR&D). IPR&D assets are only capitalized if they are deemed to enhance the intellectual property of Novartis and include items such as initial upfront and milestone payments on licensed or acquired compounds.
IPR&D is not amortized, but evaluated for potential impairment on an annual basis or when facts and circumstances warrant. Any impairment charge is recorded in the consolidated income statement under "Research & Development". Once a project included in IPR&D has been successfully developed it is transferred to the "Currently marketed product" category.
Impairment of Goodwill and Intangible Assets
An asset is considered impaired when its balance sheet carrying amount exceeds its estimated recoverable amount, which is defined as the higher of its fair value less costs of disposal and its value in use. Usually, Novartis applies the fair value less costs of disposal method for its impairment assessment. In most cases no directly observable market inputs are available to measure the fair value less costs of disposal. Therefore, an estimate is derived indirectly and is based on net present value techniques utilizing post-tax cash flows and discount rates. In the limited cases where the value in use method would be applied, net present value techniques would be applied using pre-tax cash flows and discount rates.
Fair value less costs of disposal reflects estimates of assumptions that market participants would be expected to use when pricing the asset or CGU, and for this purpose management considers the range of economic conditions that are expected to exist over the remaining useful life of the asset.
The estimates used in calculating the net present values are highly sensitive and depend on assumptions specific to the nature of the Group's activities with regard to:
- –
- amount and timing of projected future cash flows;
- –
- outcome of R&D activities (compound efficacy, results of clinical trials, etc.);
- –
- amount and timing of projected costs to develop IPR&D into commercially viable products;
- –
- probability of obtaining regulatory approval;
- –
- long-term sales forecasts for periods of up to 25 years;
F-12
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
- –
- sales erosion rates after the end of patent or other intellectual property rights protection and timing of the entry of generic
competition;
- –
- selected tax rate;
- –
- behavior of competitors (launch of competing products, marketing initiatives, etc.); and
- –
- selected discount rate.
Generally, for intangible assets with a definite useful life Novartis uses cash flow projections for the whole useful life of these assets, and for goodwill and the Alcon brand name, Novartis utilizes cash flow projections for a five-year period based on management forecasts, with a terminal value based on cash flow projections usually in line with or lower than inflation rates for later periods. Probability-weighted scenarios are typically used.
Discount rates used are based on the Group's estimated weighted average cost of capital adjusted for specific country and currency risks associated with cash flow projections as an approximation of the weighted average cost of capital of a comparable market participant.
Due to the above factors, actual cash flows and values could vary significantly from forecasted future cash flows and related values derived using discounting techniques.
Impairment of Associated Companies Accounted For at Equity
Novartis considers investments in associated companies for impairment evaluation whenever there is a quoted share price indicating a fair value less than the per-share balance sheet carrying value for the investment. For unquoted investments in associated companies recent financial information is taken into account to assess whether an impairment evaluation is necessary.
If the recoverable amount of the investment is estimated to be lower than the balance sheet carrying amount an impairment charge is recognized for the difference in the consolidated income statement under "Income from associated companies".
Cash and Cash equivalents, Marketable Securities, Commodities, Derivative Financial Instruments and Non-Current Financial Assets
Cash and cash equivalents include highly liquid investments with original maturities of three months or less which are readily convertible to known amounts of cash. Bank overdrafts are usually presented within current financial debts on the consolidated balance sheet except in cases where a right of offset has been agreed with a bank which then allows for presentation on a net basis.
The Group defines "marketable securities" as those financial assets which are managed by the Group's Corporate Treasury and consist principally of quoted equity and quoted debt securities as well as fund investments which are principally traded in liquid markets. Certain marketable securities are managed independently of Corporate Treasury, and these are typically held for long-term strategic purposes and are therefore classified as non-current financial assets. They include equity securities and fund investments.
Marketable securities are initially recorded at fair value on their trade date which is different from the settlement date when the transaction is ultimately effected. Quoted securities are re-measured at each
F-13
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
reporting date to fair value based on current market prices. If the market for a financial asset is not active or no market is available, fair values are established using valuation techniques. Apart from discounted cash flow analysis and other pricing models, for the majority of investments in what is known as the "Level 3" hierarchy, the valuation is based on the acquisition cost as the best approximation of the fair value of the investee. This is adjusted for a higher or lower valuation in connection with a partial disposal, a new round of financing and for the investee's performance below or above expectations. The fair value of investments in "Level 3" is reviewed regularly for a possible diminution in value.
The Group has classified all its equity and quoted debt securities as well as fund investments as available-for-sale, as they are not acquired to generate profit from short-term fluctuations in price. Unrealized gains, except exchange gains related to quoted debt instruments, are recorded as a fair value adjustment in the consolidated statement of comprehensive income. They are recognized in the consolidated income statement when the financial asset is sold at which time the gain is transferred either to "Other financial income and expense" for the marketable securities managed by the Group's Corporate Treasury or to "Other income" in the consolidated income statement for all other equity securities and fund investments. Exchange gains related to quoted debt instruments are immediately recognized in the consolidated income statement under "Other financial income and expense".
A security is assessed for impairment when its market value at the balance sheet date is less than initial cost reduced by any previously recognized impairment. Impairments on equity securities, quoted debt securities and fund investments, and exchange rate losses on quoted debt securities in a foreign currency which are managed by the Group's Corporate Treasury are immediately recorded in "Other financial income and expense". Impairments are recorded for all other equity securities and other fund investments in "Other expense" in the consolidated income statement.
Commodities include gold bullion or coins which are valued at the lower of cost or fair value using current market prices. The changes in fair value below cost are immediately recorded in "Other financial income and expense".
Other non-current financial assets including loans are carried at either amortized cost, which reflects the time value of money, or cost adjusted for any accrued interest, less any allowances for uncollectable amounts. Impairments and exchange rate gains and losses on other non-current financial assets, including loans, as well as interest income using the effective interest rate method, are immediately recorded in "Other income" or "Other expense" in the consolidated income statement.
Derivative financial instruments are initially recognized in the balance sheet at fair value and are re-measured to their current fair value at the end of each subsequent reporting period. The valuation of a forward exchange rate contract is based on the discounted cash flow model, using interest curves and spot rates at the reporting date as observable inputs.
Options are valued based on a modified Black-Scholes model using volatility and exercise prices as major observable inputs.
The Group utilizes derivative financial instruments for the purpose of hedging to reduce the volatility in the Group's performance due to the exposure to various types of business risks. The Group, therefore, enters into certain derivative financial instruments which provide effective economic hedges. The risk reduction is obtained because the derivative's value or cash flows are expected, wholly or partly, to move inversely to the hedged item and, therefore, offset changes in the value or cash flows of the hedged item. The overall hedging strategy is aiming to mitigate the currency and interest exposure risk of positions
F-14
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
which are contractually agreed and to partially hedge the exposure risk of selected anticipated transactions. However, the Group generally does not hedge the translation risk related to its foreign investments.
Not all of the financial impact of derivative financial instruments can be matched with the financial impact of the economically hedged item. A prerequisite for obtaining this accounting-hedge relationship is extensive documentation on inception and proving on a regular basis that the economic hedge is effective for accounting purposes. Changes in the fair value of any derivative instruments that do not qualify for cash flow hedge accounting are recognized immediately in "Other financial income and expense" in the consolidated income statement.
Inventory is valued at acquisition or production cost determined on a first-in first-out basis. This value is used for the "Cost of goods sold" in the consolidated income statement. Unsalable inventory is fully written off in the consolidated income statement under "Cost of goods sold".
Trade receivables are initially recognized at their invoiced amounts including any related sales taxes less adjustments for estimated revenue deductions such as rebates, chargebacks and cash discounts.
Provisions for doubtful trade receivables are established once there is an indication that it is likely that a loss will be incurred. These provisions represent the difference between the trade receivable's carrying amount in the consolidated balance sheet and the estimated net collectible amount. Significant financial difficulties of a customer, such as probability of bankruptcy, financial reorganization, default or delinquency in payments are considered indicators that recovery of the trade receivable is doubtful. Charges for doubtful trade receivables are recognized in the consolidated income statement within "Marketing & Sales" expenses.
Legal and Environmental Liabilities
Novartis and its subsidiaries are subject to contingencies arising in the ordinary course of business such as patent litigation, environmental remediation liabilities and other product-related litigation, commercial litigation, and governmental investigations and proceedings. Provisions are made where a reliable estimate can be made of the probable outcome of legal or other disputes including related fees and expenses against the subsidiary. Novartis believes that its total provisions are adequate based upon currently available information, however, given the inherent difficulties in estimating liabilities in this area, Novartis may incur additional costs beyond the amounts provided. Management believes that such additional amounts, if any, would not be material to the Group's financial condition but could be material to the results of operations or cash flows in a given period.
In a business combination or divestment of a business, it is necessary to recognize contingent future payments to previous or from new owners representing contractually defined potential amounts as a liability or asset. Usually for Novartis, these are linked to milestone or royalty payments related to certain assets and are recognized as a financial liability or asset at their fair value which is then re-measured at
F-15
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
each subsequent reporting date. These estimations typically depend on factors such as technical milestones or market performance and are adjusted for the probability of their likelihood of payment and if material, appropriately discounted to reflect the impact of time. Changes in the fair value of contingent liabilities in subsequent periods are recognized in the consolidated income statement in "Cost of goods sold" for currently marketed products and in "Research & Development" for IPR&D. Changes in contingent assets are recognized in "Other income" or "Other expense". The effect of unwinding the discount over time is recognized in "Interest expense" in the consolidated income statement. Novartis does not recognize contingent consideration associated with asset purchases outside of a business combination that are conditional upon future events which are within its control until such time as there is an unconditional obligation. If the contingent consideration is outside the control of Novartis, a liability is recognized once it becomes probable that the contingent consideration will become due. In both cases, if appropriate, a corresponding asset is recorded.
Defined Benefit Pension Plans and Other Post-Employment Benefits
The liability in respect of defined benefit pension plans and other post-employment benefits is the defined benefit obligation calculated annually by independent actuaries using the projected unit credit method. The current service cost for such post-employment benefit plans is included in the personnel expenses of the various functions where the associates are employed, while the net interest on the net defined benefit liability or asset is recognized as "Other expense" or "Other income".
Treasury shares are initially recorded at fair value on their trade date which is different from the settlement date when the transaction is ultimately effected. Treasury shares are deducted from consolidated equity at their nominal value of CHF 0.50 per share. Differences between the nominal amount and the transaction price on purchases or sales of treasury shares with third parties, or the value of services received for the shares allocated to associates as part of share-based compensation arrangements, are recorded in "Retained earnings" in the consolidated statement of changes in equity.
Revenue
Revenue is recognized on the sale of Novartis Group products and services and recorded as "Net sales" in the consolidated income statement when there is persuasive evidence that a sales arrangement exists, title and risks and rewards for the products are transferred to the customer, the price is determinable and collectability is reasonably assured. When contracts contain customer acceptance provisions, sales are recognized upon the satisfaction of acceptance criteria. If products are stockpiled at the request of the customer, revenue is only recognized once the products have been inspected and accepted by the customer and there is no right of return or replenishment on product expiry.
Provisions for rebates and discounts granted to government agencies, wholesalers, retail pharmacies, managed healthcare organizations and other customers are recorded as a deduction from revenue at the time the related revenues are recorded or when the incentives are offered. They are calculated on the basis of historical experience and the specific terms in the individual agreements. Provisions for refunds granted to healthcare providers under innovative pay-for-performance agreements are recorded as revenue deduction at the time the related sales are recorded. They are calculated on the basis of historical
F-16
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
experience and clinical data available for the product as well as the specific terms in the individual agreements. In cases where historical experience and clinical data are not sufficient for a reliable estimation of the outcome, revenue recognition is deferred until such history is available.
Cash discounts are offered to customers to encourage prompt payment and are recorded as revenue deductions. Following a decrease in the price of a product, we generally grant customers a "shelf stock adjustment" for a customer's existing inventory for the involved product. Provisions for shelf stock adjustments, which are primarily relevant within the Sandoz Division, are determined at the time of the price decline or at the point of sale, if the impact of a price decline on the products sold can be reasonably estimated based on the customer's inventory levels of the relevant product. When there is historical experience of Novartis agreeing to customer returns and Novartis can reasonably estimate expected future returns, a provision is recorded for estimated sales returns. In doing so the estimated rate of return is applied, determined based on historical experience of customer returns and considering any other relevant factors. This is applied to the amounts invoiced also considering the amount of returned products to be destroyed versus products that can be placed back in inventory for resale. Where shipments are made on a re-sale or return basis, without sufficient historical experience for estimating sales returns, revenue is only recorded when there is evidence of consumption or when the right of return has expired.
Provisions for revenue deductions are adjusted to actual amounts as rebates, discounts and returns are processed. The provision represents estimates of the related obligations, requiring the use of judgment when estimating the effect of these sales deductions.
Revenue from Lease Arrangements
For surgical equipment, in addition to cash and instalment sales, revenue is recognized under finance and operating lease arrangements. An arrangement that is not in the legal form of a lease is accounted for as a lease if it is dependent on the use of a specific asset or assets and the arrangement conveys a right to use the asset. Arrangements in which Novartis transfers substantially all the risks and rewards incidental to ownership to the customer are treated as finance lease arrangements. Revenue from finance lease arrangements is recognized at amounts equal to the fair values of the equipment, which approximate the present values of the minimum lease payments under the arrangements. As interest rates embedded in lease arrangements are approximately market rates, revenue under finance lease arrangements is comparable to revenue for outright sales. Finance income for arrangements in excess of twelve months is deferred and subsequently recognized based on a pattern that approximates the use of the effective interest method and recorded in "Other income". Operating lease revenue for equipment rentals is recognized on a straight-line basis over the lease term.
Other Revenue
"Other revenue" includes royalty income and revenue from activities such as manufacturing services or other services rendered to the extent such revenue is not recorded under net sales.
Internal Research & Development (R&D) costs are fully charged to "Research & Development" in the consolidated income statement in the period in which they are incurred. The Group considers that regulatory and other uncertainties inherent in the development of new products preclude the capitalization of internal development expenses as an intangible asset until marketing approval from a
F-17
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
regulatory authority is obtained in a major market such as the United States, the European Union, Switzerland or Japan.
Payments made to third parties in compensation for subcontracted R&D, such as contract research and development organizations, that is deemed not to enhance the intellectual property of Novartis are expensed as internal R&D expenses in the period in which they are incurred. Such payments are only capitalized if they meet the criteria for recognition of an internally generated intangible asset, usually when marketing approval has been achieved from a regulatory authority in a major market.
Payments made to third parties in order to in-license or acquire intellectual property rights, compounds and products, including initial upfront and subsequent milestone payments, are capitalized as are payments for other assets, such as technologies to be used in R&D activities. If additional payments are made to the originator company to continue to perform R&D activities, an evaluation is made as to the nature of the payments. Such additional payments will be expensed if they are deemed to be compensation for subcontracted R&D services not resulting in an additional transfer of intellectual property rights to Novartis. By contrast, such additional payments will be capitalized if they are deemed to be compensation for the transfer to Novartis of additional intellectual property developed at the risk of the originator company. Subsequent internal R&D costs in relation to IPR&D and other assets are expensed since the technical feasibility of the internal R&D activity can only be demonstrated by the receipt of marketing approval for a related product from a regulatory authority in a major market.
Costs for post-approval studies performed to support the continued registration of a marketed product are recognized as marketing expenses. Costs for activities that are required by regulatory authorities as a condition for obtaining marketing approval are charged as development expenses as they are incurred, in cases where it is anticipated that the related product will be sold over a longer period than the activities required to be performed to obtain the marketing approval. As a result, all activities necessary as a condition to maintain a received approval, whether conditional or not, are expensed in the consolidated income statement.
IPR&D assets are transferred to "Currently marketed products" once the related project has been successfully developed and then are amortized straight-line in the consolidated income statement over their useful life. Other acquired technologies included in intangible assets are amortized straight-line in the consolidated income statement over their estimated useful lives.
Inventory produced ahead of regulatory approval is provisioned against and the charge is included in "Other expense" in the consolidated income statement as its ultimate use cannot be assured. If this inventory can be subsequently sold, the provision is released to "Other income" in the consolidated income statement either on approval by the appropriate regulatory authority or, exceptionally in Europe, on recommendation by the Committee for Medicinal Products for Human Use (CHMP) if approval is virtually certain.
Vested Novartis shares and ADRs which are granted as compensation are valued at their market value on the grant date and are immediately expensed in the consolidated income statement.
The fair values of unvested restricted shares, restricted share units (RSUs) and performance share units (PSUs) in Novartis shares and American Depositary Receipts (ADRs) and related options granted to associates as compensation are recognized as an expense over the related vesting period. The expense
F-18
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
recorded in the consolidated income statement is included in the personnel expenses of the various functions where the associates are employed.
Unvested restricted shares, restricted ADRs and RSUs and any related options are only conditional on the provision of services by the plan participant during the vesting period. As a result, restricted shares, restricted ADRs, RSUs and any related options are valued using their market value on the grant date. The value of these grants, after making adjustment for assumptions related to their forfeiture during the vesting period, are expensed on a straight-line basis over the respective vesting period.
PSUs require the plan participant to not only provide services during the vesting period but they are also subject to certain performance criteria being achieved during the vesting period. PSUs granted under plans defined as "Long-Term Performance Plans" are subject to performance criteria based on Novartis internal performance metrics. The expense is determined taking into account assumptions concerning performance during the period against targets and expected forfeitures due to plan participants not meeting their service conditions. These assumptions are periodically adjusted. Any change in estimates for past services are recorded immediately as an expense or income in the consolidated income statement and amounts for future periods are expensed over the remaining vesting period. As a result, at the end of the vesting period, the total charge during the whole vesting period represents the amount which will finally vest. The number of equity instruments that finally vest is determined at the vesting date.
In 2014, a Long-Term Relative Performance Plan (LTRPP) was introduced. PSUs granted under this plan are not only conditional on the provision of services by the plan participant during the vesting period but are also conditional on the Total Shareholder Return (TSR) performance of Novartis relative to a specific peer group of companies over the vesting period. These performance conditions are based on variables which can be observed in the market. IFRS requires that these observations are taken into account in determining the fair value of these PSUs at the date of grant. Novartis has determined the fair value of these PSUs at the date of grant using a "Monte Carlo" simulation model. The total fair value of this grant is expensed on a straight-line basis over the vesting period. Adjustments to the number of equity instruments granted are only made if a plan participant does not fulfill the service conditions.
If a plan participant leaves Novartis, for reasons other than retirement, disability or death, then unvested restricted shares, restricted ADRs, RSUs and related share options and PSUs are forfeited, unless determined otherwise by the provision of the plan rules or by the Compensation Committee, for example, in connection with a reorganization or divestment.
Measuring the fair values of PSUs granted under the LTRPP and share and ADR options granted under other plans, requires an estimation of the probability of uncertain future events and various other factors used in the valuation models. The Monte Carlo simulation used for determining the fair value of the PSUs related to the LTRPP requires as input parameters the probability of factors related to uncertain future events; the term of the award; grant price of underlying shares or ADRs; expected volatilities; expected correlation matrix of the underlying equity instruments with those of the peer group of companies and the risk free interest rate. The fair values of options on Novartis shares and ADRs are calculated using the trinomial valuation method and has as input parameters the expected dividend yield and expected price volatility. Expected volatilities are based on those implied from listed financial instruments on Novartis shares, and—to the extent that equivalent values are not available—a future extrapolation based on historical volatility.
F-19
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
Grants from governments or similar organizations are recognized at their fair value when there is a reasonable assurance that the grant will be received and the Group will comply with all attached conditions.
Government grants related to income are deferred and recognized in the consolidated income statement over the period necessary to match them with the related costs which they are intended to compensate.
The accounting policy for property, plant and equipment describes the treatment of any related grants.
Charges to increase restructuring provisions are included in "Other expense" in the consolidated income statements. Corresponding releases are recorded in "Other income" in the consolidated income statement.
Taxes on income are provided in the same periods as the revenues and expenses to which they relate and include any interest and penalties incurred during the period. Deferred taxes are determined using the comprehensive liability method and are calculated on the temporary differences that arise between the tax base of an asset or liability and its carrying value in the balance sheet prepared for consolidation purposes, except for those temporary differences related to investments in subsidiaries and associated companies, where the timing of their reversal can be controlled and it is probable that the difference will not reverse in the foreseeable future. Furthermore, withholding or other taxes on eventual distribution of a subsidiary's retained earnings are only taken into account when a dividend has been planned since generally the retained earnings are reinvested.
The estimated amounts for current and deferred tax assets or liabilities, including any amounts related to any uncertain tax positions, are based on currently known facts and circumstances. Tax returns are based on an interpretation of tax laws and regulations and reflect estimates based on these judgments and interpretations. The tax returns are subject to examination by the competent taxing authorities which may result in an assessment being made requiring payments of additional tax, interest or penalties. Inherent uncertainties exist in the estimates of the tax positions.
Non-Current Assets Held for Sale or Related to Discontinued Operations
Non-current assets are classified as assets held for sale or related to discontinued operations when their carrying amount is to be recovered principally through a sale transaction and a sale is considered highly probable. They are stated at the lower of carrying amount and fair value less costs to sell. Assets held for sale or included within a disposal group are not depreciated or amortized.
F-20
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Significant Accounting Policies (Continued)
Status of Adoption of Significant New or Amended IFRS Standards or Interpretations
The adoption of new or amended standards and interpretations which are effective for the financial year beginning on January 1, 2015 did not have a material impact on the Group's consolidated financial statements.
The following new IFRS standards will, based on a Novartis analysis, be of significance to the Group, but have not yet been early adopted:
- –
- IFRS 9 Financial Instruments will substantially change the classification and
measurement of financial instruments; will require impairments to be based on a forward-looking model; will change the approach to hedging financial exposures and related documentation and also the
recognition of certain fair value changes. The mandatory effective date for requirements issued as part of IFRS 9 is January 1, 2018 with early adoption permitted. The Group is currently
assessing the impact of IFRS 9.
- –
- IFRS 15 Revenue from contracts with customers amends revenue recognition
requirements and establishes principles for reporting information about the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The standard
replaces IAS 18 Revenue and IAS 11 Construction contracts and related interpretations. The
standard is effective for annual periods beginning on or after January 1, 2018 with earlier adoption permitted. The Group is currently assessing the impact of adopting IFRS 15.
- –
- IFRS 16 Leases substantially changes the financial statements as the majority of leases will become on-balance sheet liabilities with corresponding right of use assets on the balance sheet. The standard replaces IAS 17 Leases and is effective January 1, 2019. Early application is permitted for companies that also apply IFRS 15 Revenue from Contracts with Customers. The Group is currently assessing the impact of adopting IFRS 16.
There are no other IFRS standards or interpretations which are not yet effective which would be expected to have a material impact on the Group.
2. Significant Transactions
Significant Transactions in 2015
Portfolio Transformation Transactions
Transaction with Eli Lilly and Company
On January 1, 2015, Novartis closed its transaction with Eli Lilly and Company, USA (Lilly) announced in April 2014 to divest its Animal Health business for $5.4 billion in cash. This resulted in a pre-tax gain of $4.6 billion which is recorded in operating income from discontinued operations.
F-21
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant Transactions (Continued)
Transactions with GlaxoSmithKline plc
On March 2, 2015, Novartis closed its transactions with GlaxoSmithKline plc, Great Britain (GSK) announced in April 2014, with the following consequences:
Pharmaceuticals—Acquisition of GSK oncology products
Novartis acquired GSK's oncology products and certain related assets for an aggregate cash consideration of $16.0 billion. Up to $1.5 billion of this cash consideration at the acquisition date is contingent on certain development milestones. The fair value of this potentially refundable consideration is $0.1 billion. In addition, under the terms of the agreement, Novartis is granted a right of first negotiation over the co-development or commercialization of GSK's current and future oncology R&D pipeline, excluding oncology vaccines. The right of first negotiation is for a period of 12.5 years from the acquisition closing date. The purchase price allocation of the fair value of the consideration of $15.9 billion resulted in net identified assets of $13.5 billion and goodwill of $2.4 billion. Since the acquisition the business generated net sales of $1.8 billion. Management estimates net sales for the entire year 2015 would have amounted to $2.1 billion had the Oncology products been acquired at the beginning of the 2015 reporting period. The net results from operations on a reported basis since the acquisition date were not material.
Vaccines—Divestment
Novartis has divested its Vaccines business (excluding its Vaccines influenza business) to GSK for up to $7.1 billion plus royalties. The $7.1 billion consists of $5.25 billion paid at closing and up to $1.8 billion in future milestone payments. The fair value of the contingent future milestones and royalties is $1.0 billion, resulting in a fair value of consideration received of $6.25 billion. Included in this amount, is a $450 million milestone payment received in late March 2015. The sale of this business resulted in a pre-tax gain of $2.8 billion which is recorded in operating income from discontinued operations.
Novartis's Vaccines influenza business is excluded from the GSK Vaccines business acquisition. However, GSK has entered into a future option arrangement with Novartis in relation to the Vaccines influenza business, pursuant to which Novartis could have unilaterally required GSK to acquire the entire or certain parts of its Vaccines influenza business for consideration of up to $250 million (the Influenza Put Option) if the divestment to CSL Limited, Australia (CSL), discussed below, had not been completed. The option period was 18 months from the closing date of the GSK transaction, but terminated with the sale of the Vaccines influenza business to CSL on July 31, 2015. Novartis paid GSK a fee of $5 million in consideration for the grant of the Influenza Put Option.
Consumer Health—Combination of Novartis OTC with GSK consumer healthcare in a joint venture
Novartis and GSK have agreed to create a combined Consumer Healthcare business through a joint venture between Novartis OTC and GSK Consumer Healthcare. On March 2, 2015, a new entity was formed via contribution of businesses from both Novartis and GSK. Novartis has a 36.5% interest in the newly created entity. Novartis has valued the contribution of 63.5% of its OTC Division in exchange for 36.5% of the GSK Consumer Healthcare business at fair value. Based on the estimates of fair values exchanged, an investment in an associated company of $7.6 billion was recorded. The resulting pre-tax gain, net of transaction related costs, of $5.9 billion is recorded in operating income from discontinued operations.
F-22
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant Transactions (Continued)
Novartis has four of eleven seats on the joint venture entity's Board of Directors. Furthermore, Novartis has customary minority rights and also exit rights at a pre-defined, market based pricing mechanism.
The investment is accounted for using the equity method of accounting using estimated results for the last quarter of the year. Any differences between this estimate and actual results, when available, will be adjusted in the Group's 2016 consolidated financial statements.
Additional GSK related costs
The GSK transaction resulted in $0.6 billion of additional transaction-related costs that were expensed.
Transaction with CSL
On October 26, 2014, Novartis entered into an agreement with CSL to sell its Vaccines influenza business to CSL for $275 million. Entering into the separate divestment agreement with CSL resulted in the Vaccines influenza business being classified as a separate disposal group consisting of a group of cash generating units within the Vaccines Division, requiring the performance of a separate valuation of the Vaccines influenza business net assets. This triggered the recognition of an exceptional impairment charge in 2014 of $1.1 billion as the estimated net book value of the Vaccines influenza business net assets was above the $275 million consideration. The transaction with CSL was completed on July 31, 2015, resulting in a partial reversal of the impairment recorded in 2014 in the amount of $0.1 billion, which is included in operating income from discontinued operations.
Other Significant Transactions in 2015
Pharmaceuticals—Acquisition of Spinifex Pharmaceuticals, Inc.
On June 29, 2015 Novartis entered into an agreement to acquire Spinifex Pharmaceuticals, Inc. (Spinifex), a US and Australian-based, privately held development stage company, focused on developing a peripheral approach to treat neuropathic pain. The transaction closed on July 24, 2015, and the total purchase consideration was $312 million. The amount consisted of an initial cash payment of $196 million and the net present value of the contingent consideration of $116 million due to previous Spinifex shareholders, which they are eligible to receive upon achievement of specified development and commercialization milestones. The purchase price allocation resulted in net identifiable assets of $263 million and goodwill of $49 million. Results of operations since the date of acquisition were not material.
Pharmaceuticals—Acquisition of Admune Therapeutics LLC
On October 16, 2015, Novartis acquired Admune Therapeutics LLC (Admune), a US-based, privately held company, broadening Novartis' pipeline of cancer immunotherapies. The total purchase consideration amounted to $258 million. This amount consists of an initial cash payment of $140 million and the net present value of the contingent consideration of $118 million due to Admune's previous owners, which they are eligible to receive upon the achievement of specified development and commercialization milestones. The purchase price allocation resulted in net identifiable assets of
F-23
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant Transactions (Continued)
$258 million. No goodwill was recognized. Results of operations since the date of acquisition were not material.
Significant Transaction in 2014
Vaccines—Divestment of Blood Transfusion Diagnostic Unit
On January 9, 2014, Novartis completed the divestment of its blood transfusion diagnostics unit announced on November 11, 2013 to the Spanish company Grifols S.A., for $1.7 billion in cash. The pre-tax gain on this transaction is approximately $0.9 billion and was recorded in operating income from discontinued operations.
Pharmaceuticals—Acquisition of CoStim Pharmaceuticals Inc.
On February 17, 2014, Novartis acquired all of the outstanding shares of CoStim Pharmaceuticals Inc., a Cambridge, Massachusetts, US-based, privately held biotechnology company focused on harnessing the immune system to eliminate immune-blocking signals from cancer, for a total purchase consideration of $248 million (excluding cash acquired). This amount consists of an initial cash payment and the net present value of contingent consideration of $153 million due to previous CoStim shareholders, which they are eligible to receive upon the achievement of specified development and commercialization milestones. The purchase price allocation resulted in net identified assets of $152 million (excluding cash acquired) and goodwill of $96 million. Results of operations since the acquisition were not material.
Pharmaceuticals—Divestment of Idenix Pharmaceuticals Inc. (Idenix) Shareholding
On August 5, 2014, Merck & Co., USA completed a tender offer for Idenix. As a result, Novartis divested its 22% shareholding in Idenix and realized a gain of approximately $0.8 billion which was recorded in income from associated companies.
Alcon—Acquisition of WaveTec Vision Systems, Inc. (WaveTec)
On October 16, 2014, Alcon acquired all of the outstanding shares of WaveTec, a privately held company, for $350 million in cash. The purchase price allocation resulted in net identified assets of $180 million and goodwill of $170 million. Results of operations since the acquisition were not material.
Corporate—Divestment of LTS Lohmann Therapie-Systeme AG (LTS) Shareholding
On November 5, 2014, Novartis divested its 43% shareholding in LTS and realized a gain of approximately $0.4 billion which was recorded in income from associated companies.
Significant Transaction in 2013
There were no significant acquisition or divestment transactions during 2013.
3. Segmentation of Key Figures 2015, 2014 and 2013
The businesses of Novartis are divided operationally on a worldwide basis into three reporting segments. In addition, we separately report Corporate activities.
F-24
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Segmentation of Key Figures 2015, 2014 and 2013 (Continued)
Reporting segments are presented in a manner consistent with the internal reporting to the chief operating decision maker which is the Executive Committee of Novartis. The reporting segments are managed separately because they each research, develop, manufacture, distribute, and sell distinct products that require differing marketing strategies.
The Executive Committee of Novartis is responsible for allocating resources and assessing the performance of the reporting segments.
The reporting segments are as follows:
Pharmaceuticals researches, develops, manufactures, distributes and sells patented prescription medicines. The Pharmaceuticals Division is organized into global business franchises responsible for the commercialization of various products. These franchises are: Oncology, Neuroscience, Retina, Immunology and Dermatology, Respiratory, Cardio-Metabolic, Established Medicines and Cell and Gene Therapies.
Alcon researches, discovers, develops, manufactures, distributes and sells eye care products. The Alcon Division is the global leader in eye care with product offerings in surgical, ophthalmic pharmaceuticals and vision care. The Alcon Division is organized globally in three global business franchises as follows: In Surgical, Alcon develops, manufactures, distributes and sells ophthalmic surgical equipment, instruments, disposable products and intraocular lenses. In Ophthalmic Pharmaceuticals, Alcon discovers, develops, manufactures, distributes and sells medicines to treat chronic and acute diseases of the eye, as well as over-the-counter medicines for the eye. In Vision Care, Alcon develops, manufactures, distributes and sells contact lenses and lens care products.
Sandoz develops, manufactures, distributes and sells prescription medicines, as well as pharmaceutical active substances, which are not protected by valid and enforceable third-party patents. The Sandoz Division is organized globally in three franchises, Retail Generics, Anti-Infectives and Biopharmaceuticals & Oncology Injectables. In Retail Generics, Sandoz develops, manufactures and markets active ingredients and finished dosage forms of pharmaceuticals to third parties. Retail Generics includes the areas of dermatology, respiratory and ophthalmics, as well as cardiovascular, metabolism, central nervous system, pain, gastrointestinal, and hormonal therapies. Finished dosage form anti-infectives sold to third parties are also part of Retail Generics. In Anti-Infectives, Sandoz manufactures active pharmaceutical ingredients and intermediates—mainly antibiotics- for internal use by Retail Generics and for sale to third party customers. In Biopharmaceuticals, Sandoz develops, manufactures and markets protein- or other biotechnology-based products known as biosimilars and provides biotechnology manufacturing services to other companies. In Oncology Injectables, Sandoz develops, manufactures and markets cytotoxic products for the hospital market.
Income and expenses relating to Corporate include the costs of the Group headquarters and those of corporate coordination functions in major countries. In addition, Corporate includes other items of income and expense which are not attributable to specific segments such as certain expenses related to post-employment benefits, environmental remediation liabilities, charitable activities, donations and sponsorships. Usually, no allocation of Corporate items is made to the segments. As a result, Corporate assets and liabilities principally consist of net liquidity (cash and cash equivalents, marketable securities less financial debts), investments in associated companies and current and deferred taxes and non-segment specific environmental remediation and post-employment benefit liabilities.
F-25
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Segmentation of Key Figures 2015, 2014 and 2013 (Continued)
Our divisions are supported by the Novartis Institutes for BioMedical Research and by Novartis Business Services.
- –
- The Novartis Institutes for BioMedical Research (NIBR) was created in 2003, and is headquartered in Cambridge, Massachusetts. NIBR
conducts the Pharmaceuticals and Alcon divisions research activities.
- –
- Novartis Business Services (NBS) was created in July 2014 and started operations in January 2015 as a shared services organization providing business support services across the Group such as information technology, real estate and facility services, procurement, product lifecycle services, human resources and financial reporting and accounting operations.
Following the Portfolio Transformation transactions described in Note 2, Novartis has separated the Group's reported financial data for the current and prior year into "continuing" operations and "discontinued" operations:
Continuing operations comprise:
- –
- Pharmaceuticals: Innovative patent-protected prescription medicines
- –
- Alcon: Surgical, ophthalmic pharmaceutical and vision care products
- –
- Sandoz: Generic pharmaceuticals
- –
- Corporate activities
Discontinued operations comprise:
- –
- Vaccines: Preventive human vaccines and the blood transfusion diagnostics unit. Excluded are certain intellectual property rights and
related other revenues of the Vaccines Division which are now reported under Corporate activities.
- –
- Consumer Health: OTC (over-the-counter medicines) and Animal Health. These two divisions were managed separately. However, neither was
material enough to the Group to be disclosed separately as a reporting segment.
- –
- Corporate: certain transactional and other expenses related to the portfolio transformation.
The accounting policies mentioned in Note 1 are used in the reporting of segment results. Inter-segmental sales are made at amounts which are considered to approximate arm's length transactions. The Executive Committee of Novartis evaluates segmental performance and allocates resources among the segments based on a number of measures including net sales, operating income and net operating assets. Segment net operating assets consist primarily of property, plant and equipment, intangible assets, inventories and trade and other operating receivables less operating liabilities.
F-26
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Segmentation of Key Figures 2015, 2014 and 2013 (Continued)
SEGMENTATION—CONSOLIDATED INCOME STATEMENTS 2015 and 2014
| |
Pharmaceuticals | Alcon | Sandoz | Corporate (including eliminations) | Group | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
($ m)
|
2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | |||||||||||||||||||||
Net sales to third parties from continuing operations |
30,445 | 31,791 | 9,812 | 10,827 | 9,157 | 9,562 | 49,414 | 52,180 | |||||||||||||||||||||||
Sales to other segments |
137 | 262 | 45 | 49 | 128 | 286 | (284 | ) | (358 | ) | 26 | 239 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net sales from continuing operations |
30,582 | 32,053 | 9,857 | 10,876 | 9,285 | 9,848 | (284 | ) | (358 | ) | 49,440 | 52,419 | |||||||||||||||||||
Other revenues |
790 | 629 | 25 | 34 | 25 | 12 | 107 | 540 | 947 | 1,215 | |||||||||||||||||||||
Cost of goods sold |
(7,379 | ) | (6,889 | ) | (5,153 | ) | (5,193 | ) | (5,325 | ) | (5,751 | ) | 453 | 488 | (17,404 | ) | (17,345 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit from continuing operations |
23,993 | 25,793 | 4,729 | 5,717 | 3,985 | 4,109 | 276 | 670 | 32,983 | 36,289 | |||||||||||||||||||||
Marketing & Sales |
(7,789 | ) | (8,178 | ) | (2,398 | ) | (2,474 | ) | (1,585 | ) | (1,725 | ) | (11,772 | ) | (12,377 | ) | |||||||||||||||
Research & Development |
(7,232 | ) | (7,331 | ) | (926 | ) | (928 | ) | (777 | ) | (827 | ) | (8,935 | ) | (9,086 | ) | |||||||||||||||
General & Administration |
(937 | ) | (1,009 | ) | (544 | ) | (613 | ) | (346 | ) | (376 | ) | (648 | ) | (618 | ) | (2,475 | ) | (2,616 | ) | |||||||||||
Other income |
1,145 | 734 | 58 | 79 | 109 | 97 | 737 | 481 | 2,049 | 1,391 | |||||||||||||||||||||
Other expense |
(1,583 | ) | (1,538 | ) | (125 | ) | (184 | ) | (381 | ) | (190 | ) | (784 | ) | (600 | ) | (2,873 | ) | (2,512 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating income from continuing operations |
7,597 | 8,471 | 794 | 1,597 | 1,005 | 1,088 | (419 | ) | (67 | ) | 8,977 | 11,089 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income from associated companies |
812 | 2 | 4 | 264 | 1,102 | 266 | 1,918 | ||||||||||||||||||||||||
Interest expense |
(655 | ) | (704 | ) | |||||||||||||||||||||||||||
Other financial income and expense |
(454 | ) | (31 | ) | |||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income before taxes from continuing operations |
8,134 | 12,272 | |||||||||||||||||||||||||||||
Taxes |
(1,106 | ) | (1,545 | ) | |||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income from continuing operations |
7,028 | 10,727 | |||||||||||||||||||||||||||||
Net income/(loss) from discontinued operations |
10,766 | (447 | ) | ||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income |
17,794 | 10,280 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Attributable to: |
|||||||||||||||||||||||||||||||
Shareholders of Novartis AG |
17,783 | 10,210 | |||||||||||||||||||||||||||||
Non-controlling interests |
11 | 70 | |||||||||||||||||||||||||||||
Included in net income from continuing operations are: |
|||||||||||||||||||||||||||||||
Interest income |
33 | 33 | |||||||||||||||||||||||||||||
Depreciation of property, plant & equipment |
(796 | ) | (856 | ) | (280 | ) | (307 | ) | (277 | ) | (317 | ) | (117 | ) | (106 | ) | (1,470 | ) | (1,586 | ) | |||||||||||
Amortization of intangible assets |
(1,305 | ) | (287 | ) | (2,079 | ) | (2,080 | ) | (362 | ) | (403 | ) | (9 | ) | (5 | ) | (3,755 | ) | (2,775 | ) | |||||||||||
Impairment charges on property, plant & equipment, net |
39 | (15 | ) | (1 | ) | 1 | (97 | ) | (7 | ) | (21 | ) | (23 | ) | (80 | ) | (44 | ) | |||||||||||||
Impairment charges on intangible assets, net |
(19 | ) | (231 | ) | (120 | ) | (7 | ) | (27 | ) | (39 | ) | (166 | ) | (277 | ) | |||||||||||||||
Impairment charges and fair value gains on financial assets, net |
(32 | ) | (20 | ) | (1 | ) | (72 | ) | (48 | ) | (104 | ) | (69 | ) | |||||||||||||||||
Additions to restructuring provisions |
(206 | ) | (433 | ) | (51 | ) | (64 | ) | (93 | ) | (4 | ) | (49 | ) | (3 | ) | (399 | ) | (504 | ) | |||||||||||
Equity-based compensation of Novartis and Alcon equity plans |
(600 | ) | (685 | ) | (86 | ) | (92 | ) | (53 | ) | (51 | ) | (164 | ) | (179 | ) | (903 | ) | (1,007 | ) | |||||||||||
F-27
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Segmentation of Key Figures 2015, 2014 and 2013 (Continued)
SEGMENTATION—CONSOLIDATED INCOME STATEMENTS 2014 and 2013
| |
Pharmaceuticals | Alcon | Sandoz | Corporate (including eliminations) | Group | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In $ m)
|
2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | |||||||||||||||||||||
Net sales to third parties from continuing operations |
31,791 | 32,214 | 10,827 | 10,496 | 9,562 | 9,159 | 52,180 | 51,869 | |||||||||||||||||||||||
Sales to other segments |
262 | 202 | 49 | 50 | 286 | 294 | (358 | ) | (325 | ) | 239 | 221 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net sales from continuing operations |
32,053 | 32,416 | 10,876 | 10,546 | 9,848 | 9,453 | (358 | ) | (325 | ) | 52,419 | 52,090 | |||||||||||||||||||
Other revenues |
629 | 497 | 34 | 27 | 12 | 18 | 540 | 84 | 1,215 | 626 | |||||||||||||||||||||
Cost of goods sold |
(6,889 | ) | (6,655 | ) | (5,193 | ) | (4,900 | ) | (5,751 | ) | (5,476 | ) | 488 | 452 | (17,345 | ) | (16,579 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit from continuing operations |
25,793 | 26,258 | 5,717 | 5,673 | 4,109 | 3,995 | 670 | 211 | 36,289 | 36,137 | |||||||||||||||||||||
Marketing & Sales |
(8,178 | ) | (8,514 | ) | (2,474 | ) | (2,452 | ) | (1,725 | ) | (1,672 | ) | (12,377 | ) | (12,638 | ) | |||||||||||||||
Research & Development |
(7,331 | ) | (7,242 | ) | (928 | ) | (1,042 | ) | (827 | ) | (787 | ) | (9,086 | ) | (9,071 | ) | |||||||||||||||
General & Administration |
(1,009 | ) | (1,051 | ) | (613 | ) | (589 | ) | (376 | ) | (374 | ) | (618 | ) | (589 | ) | (2,616 | ) | (2,603 | ) | |||||||||||
Other income |
734 | 699 | 79 | 79 | 97 | 106 | 481 | 321 | 1,391 | 1,205 | |||||||||||||||||||||
Other expense |
(1,538 | ) | (774 | ) | (184 | ) | (437 | ) | (190 | ) | (240 | ) | (600 | ) | (596 | ) | (2,512 | ) | (2,047 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating income from continuing operations |
8,471 | 9,376 | 1,597 | 1,232 | 1,088 | 1,028 | (67 | ) | (653 | ) | 11,089 | 10,983 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income from associated companies |
812 | 4 | 2 | 1,102 | 597 | 1,918 | 599 | ||||||||||||||||||||||||
Interest expense |
(704 | ) | (683 | ) | |||||||||||||||||||||||||||
Other financial income and expense |
(31 | ) | (92 | ) | |||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income before taxes from continuing operations |
12,272 | 10,807 | |||||||||||||||||||||||||||||
Taxes |
(1,545 | ) | (1,498 | ) | |||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income from continuing operations |
10,727 | 9,309 | |||||||||||||||||||||||||||||
Net loss from discontinued operations |
(447 | ) | (17 | ) | |||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income |
10,280 | 9,292 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Attributable to: |
|||||||||||||||||||||||||||||||
Shareholders of Novartis AG |
10,210 | 9,175 | |||||||||||||||||||||||||||||
Non-controlling interests |
70 | 117 | |||||||||||||||||||||||||||||
Included in net income are: |
|||||||||||||||||||||||||||||||
Interest income |
33 | 34 | |||||||||||||||||||||||||||||
Depreciation of property, plant & equipment |
(856 | ) | (822 | ) | (307 | ) | (319 | ) | (317 | ) | (307 | ) | (106 | ) | (106 | ) | (1,586 | ) | (1,554 | ) | |||||||||||
Amortization of intangible assets |
(287 | ) | (284 | ) | (2,080 | ) | (1,999 | ) | (403 | ) | (411 | ) | (5 | ) | (4 | ) | (2,775 | ) | (2,698 | ) | |||||||||||
Impairment charges on property, plant & equipment, net |
(15 | ) | (29 | ) | 1 | (4 | ) | (7 | ) | 3 | (23 | ) | (17 | ) | (44 | ) | (47 | ) | |||||||||||||
Impairment charges on intangible assets, net |
(231 | ) | (29 | ) | (7 | ) | (57 | ) | (39 | ) | (20 | ) | (277 | ) | (106 | ) | |||||||||||||||
Impairment charges/fair value gains on financial assets |
(20 | ) | (16 | ) | (1 | ) | (48 | ) | (41 | ) | (69 | ) | (57 | ) | |||||||||||||||||
Additions to restructuring provisions |
(433 | ) | (88 | ) | (64 | ) | (71 | ) | (4 | ) | (3 | ) | (3 | ) | (1 | ) | (504 | ) | (163 | ) | |||||||||||
Equity-based compensation of Novartis and Alcon equity plans |
(685 | ) | (610 | ) | (92 | ) | (105 | ) | (51 | ) | (38 | ) | (179 | ) | (139 | ) | (1,007 | ) | (892 | ) | |||||||||||
F-28
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Segmentation of Key Figures 2015, 2014 and 2013 (Continued)
SEGMENTATION—CONSOLIDATED BALANCE SHEETS
| |
Pharmaceuticals | Alcon | Sandoz | Corporate (including eliminations) | Group | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
($ m)
|
2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | |||||||||||||||||||||
Assets related to continuing operations |
41,552 | 25,657 | 40,330 | 42,494 | 17,688 | 18,771 | 31,986 | 31,664 | 131,556 | 118,586 | |||||||||||||||||||||
Assets related to discontinued operations |
6,801 | ||||||||||||||||||||||||||||||
Total assets |
41,552 | 25,657 | 40,330 | 42,494 | 17,688 | 18,771 | 31,986 | 31,664 | 131,556 | 125,387 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Liabilities related to continuing operations |
(10,798 | ) | (10,532 | ) | (2,403 | ) | (2,709 | ) | (3,545 | ) | (3,449 | ) | (37,688 | ) | (35,435 | ) | (54,434 | ) | (52,125 | ) | |||||||||||
Liabilities related to discontinued operations |
(2,418 | ) | |||||||||||||||||||||||||||||
Total liabilities |
(10,798 | ) | (10,532 | ) | (2,403 | ) | (2,709 | ) | (3,545 | ) | (3,449 | ) | (37,688 | ) | (35,435 | ) | (54,434 | ) | (54,543 | ) | |||||||||||
Total equity |
77,122 | 70,844 | |||||||||||||||||||||||||||||
Net debt |
16,484 | 6,549 | |||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net operating assets |
30,754 | 15,125 | 37,927 | 39,785 | 14,143 | 15,322 | 93,606 | 77,393 | |||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Included in assets and liabilities related to continuing operations(1) are: |
|||||||||||||||||||||||||||||||
Total property, plant & equipment |
9,985 | 9,732 | 2,504 | 2,413 | 2,788 | 3,123 | 705 | 715 | 15,982 | 15,983 | |||||||||||||||||||||
Additions to property, plant & equipment(2) |
1,309 | 1,676 | 565 | 517 | 421 | 531 | 224 | 180 | 2,519 | 2,904 | |||||||||||||||||||||
Total goodwill and intangible assets |
21,345 | 6,096 | 33,604 | 35,642 | 10,410 | 11,378 | 32 | 27 | 65,391 | 53,143 | |||||||||||||||||||||
Additions to goodwill and intangible assets(2) |
994 | 493 | 110 | 192 | 44 | 110 | 11 | 4 | 1,159 | 799 | |||||||||||||||||||||
Total investment in associated companies |
8 | 11 | 15 | 16 | 15,291 | 8,405 | 15,314 | 8,432 | |||||||||||||||||||||||
Additions to investment in associated companies(2) |
5 | 9 | 57 | 44 | 62 | 53 | |||||||||||||||||||||||||
Cash and cash equivalents, marketable securities, commodities, time deposits and derivative financial instruments |
5,447 | 13,862 | 5,447 | 13,862 | |||||||||||||||||||||||||||
Financial debts and derivative financial instruments |
21,931 | 20,411 | 21,931 | 20,411 | |||||||||||||||||||||||||||
Current income tax and deferred tax liabilities |
8,072 | 8,175 | 8,072 | 8,175 | |||||||||||||||||||||||||||
- (1)
- Items
reflect the allocation to continuing operations as described on page F-26.
- (2)
- Excluding impact of business combinations.
F-29
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Segmentation of Key Figures 2015, 2014 and 2013 (Continued)
The following table shows countries that accounted for more than 5% of at least one of the respective Group totals and regional information for the years ended December 31, 2015, 2014 and 2013:
| |
Net sales(1) | Total of selected non-current assets(2) |
|||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
$ m
|
2015 | % | 2014 | % | 2013 | % | 2015 | % | 2014 | % | |||||||||||||||||||||
| Country |
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
Switzerland |
774 | 2 | 658 | 1 | 625 | 1 | 47,054 | 49 | 34,399 | 44 | |||||||||||||||||||||
United States |
18,079 | 37 | 17,337 | 33 | 17,257 | 33 | 28,677 | 30 | 28,329 | 37 | |||||||||||||||||||||
United Kingdom |
1,277 | 3 | 1,379 | 3 | 1,267 | 2 | 7,769 | 8 | 612 | 1 | |||||||||||||||||||||
Germany |
3,262 | 7 | 3,742 | 7 | 3,628 | 7 | 2,908 | 3 | 3,365 | 4 | |||||||||||||||||||||
France |
2,269 | 5 | 2,638 | 5 | 2,779 | 5 | 188 | 228 | |||||||||||||||||||||||
Japan |
3,163 | 6 | 3,781 | 7 | 4,412 | 9 | 142 | 141 | |||||||||||||||||||||||
Other |
20,590 | 40 | 22,645 | 44 | 21,901 | 43 | 9,949 | 10 | 10,484 | 14 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Group |
49,414 | 100 | 52,180 | 100 | 51,869 | 100 | 96,687 | 100 | 77,558 | 100 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Region |
|||||||||||||||||||||||||||||||
Europe |
16,472 | 33 | 18,690 | 36 | 18,421 | 36 | 63,681 | 66 | 45,040 | 58 | |||||||||||||||||||||
Americas |
22,414 | 45 | 22,218 | 43 | 21,984 | 42 | 30,375 | 31 | 30,074 | 39 | |||||||||||||||||||||
Asia/Africa/Australasia |
10,528 | 22 | 11,272 | 21 | 11,464 | 22 | 2,631 | 3 | 2,444 | 3 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Group |
49,414 | 100 | 52,180 | 100 | 51,869 | 100 | 96,687 | 100 | 77,558 | 100 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Net
sales from operations by location of third party customer.
- (2)
- Total of property, plant and equipment; goodwill; intangible assets; and investment in associated companies
The Group's largest, second and third largest customer accounts for approximately 14%, 11% and 5% of net sales, respectively (2014: 12%, 11% and 5%; 2013: 15%, 10% and 7% respectively). No other customer accounted for 5% or more of net sales, in either year.
The highest amounts of trade receivables outstanding were for these same three customers. They amounted to 13%, 9% and 6%, respectively, of the trade receivables at December 31, 2015 (2014: 13%, 9% and 5%).
F-30
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Segmentation of Key Figures 2015, 2014 and 2013 (Continued)
PHARMACEUTICALS BUSINESS FRANCHISE NET SALES
| |
2015 | 2014 | Change (2014 to 2015) |
2013 | Change (2013 to 2014) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ % |
$ m |
$ % |
|||||||||||
Oncology |
||||||||||||||||
Gleevec/Glivec |
4,658 | 4,746 | (2 | ) | 4,693 | 1 | ||||||||||
Tasigna |
1,632 | 1,529 | 7 | 1,266 | 21 | |||||||||||
| | | | | | | | | | | | | | | | | |
Subtotal Bcr-Abl franchise |
6,290 | 6,275 | 0 | 5,959 | 5 | |||||||||||
Sandostatin |
1,630 | 1,650 | (1 | ) | 1,589 | 4 | ||||||||||
Afinitor/Votubia |
1,607 | 1,575 | 2 | 1,309 | 20 | |||||||||||
Exjade |
917 | 926 | (1 | ) | 893 | 4 | ||||||||||
Votrient |
565 | 0 | nm | 0 | nm | |||||||||||
Tafinlar/Mekinist |
453 | 0 | nm | 0 | nm | |||||||||||
Jakavi |
410 | 279 | 47 | 163 | 71 | |||||||||||
Revolade/Promacta |
402 | 0 | nm | 0 | nm | |||||||||||
Femara |
304 | 380 | (20 | ) | 384 | (1 | ) | |||||||||
Zykadia |
79 | 31 | 155 | 0 | nm | |||||||||||
Other |
819 | 587 | 40 | 919 | (36 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
Total Oncology |
13,476 | 11,703 | 15 | 11,216 | 4 | |||||||||||
| | | | | | | | | | | | | | | | | |
Neuroscience |
||||||||||||||||
Gilenya |
2,776 | 2,477 | 12 | 1,934 | 28 | |||||||||||
Exelon/Exelon Patch |
728 | 1,009 | (28 | ) | 1,032 | (2 | ) | |||||||||
Comtan/Stalevo |
294 | 371 | (21 | ) | 401 | (7 | ) | |||||||||
Other |
141 | 243 | (42 | ) | 237 | 3 | ||||||||||
| | | | | | | | | | | | | | | | | |
Total Neuroscience |
3,939 | 4,100 | (4 | ) | 3,604 | 14 | ||||||||||
| | | | | | | | | | | | | | | | | |
Retina |
||||||||||||||||
Lucentis |
2,060 | 2,441 | (16 | ) | 2,383 | 2 | ||||||||||
Other |
50 | 63 | (21 | ) | 61 | 3 | ||||||||||
| | | | | | | | | | | | | | | | | |
Total Retina |
2,110 | 2,504 | (16 | ) | 2,444 | 2 | ||||||||||
| | | | | | | | | | | | | | | | | |
Immunology and Dermatology |
||||||||||||||||
Neoral/Sandimmun(e) |
570 | 684 | (17 | ) | 750 | (9 | ) | |||||||||
Myfortic |
441 | 543 | (19 | ) | 637 | (15 | ) | |||||||||
Zortress/Certican |
335 | 327 | 2 | 249 | 31 | |||||||||||
Cosentyx |
261 | 0 | nm | 0 | nm | |||||||||||
Ilaris |
236 | 199 | 19 | 119 | 67 | |||||||||||
Other |
160 | 173 | (8 | ) | 169 | 2 | ||||||||||
| | | | | | | | | | | | | | | | | |
Subtotal Immunology and Dermatology excluding Everolimus stent drug |
2,003 | 1,926 | 4 | 1,924 | 0 | |||||||||||
Everolimus stent drug |
134 | 205 | (35 | ) | 247 | (17 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
Total Immunology and Dermatology |
2,137 | 2,131 | 0 | 2,171 | (2 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
nm = not meaningful
F-31
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Segmentation of Key Figures 2015, 2014 and 2013 (Continued)
PHARMACEUTICALS BUSINESS FRANCHISE NET SALES (Continued)
| |
2015 | 2014 | Change (2014 to 2015) |
2013 | Change (2013 to 2014) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ % |
$ m |
$ % |
|||||||||||
Respiratory |
||||||||||||||||
Ultibro Breezhaler |
260 | 118 | 120 | 6 | nm | |||||||||||
Onbrez Breezhaler/Arcapta Neohaler |
166 | 220 | (25 | ) | 192 | 15 | ||||||||||
Seebri Breezhaler |
150 | 146 | 3 | 58 | 152 | |||||||||||
| | | | | | | | | | | | | | | | | |
Subtotal COPD(1) portfolio |
576 | 484 | 19 | 256 | 89 | |||||||||||
Xolair(2) |
755 | 777 | (3 | ) | 613 | 27 | ||||||||||
Other |
263 | 320 | (18 | ) | 428 | (25 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
Total Respiratory |
1,594 | 1,581 | 1 | 1,297 | 22 | |||||||||||
| | | | | | | | | | | | | | | | | |
Cardio-Metabolic |
||||||||||||||||
Galvus |
1,140 | 1,224 | (7 | ) | 1,200 | 2 | ||||||||||
Entresto |
21 | 0 | nm | 0 | nm | |||||||||||
Other |
0 | 8 | nm | 0 | nm | |||||||||||
| | | | | | | | | | | | | | | | | |
Total Cardio-Metabolic |
1,161 | 1,232 | (6 | ) | 1,200 | 3 | ||||||||||
| | | | | | | | | | | | | | | | | |
Established medicines |
||||||||||||||||
Diovan |
1,284 | 2,345 | (45 | ) | 3,524 | (33 | ) | |||||||||
Exforge |
1,047 | 1,396 | (25 | ) | 1,456 | (4 | ) | |||||||||
Voltaren |
558 | 632 | (12 | ) | 675 | (6 | ) | |||||||||
Ritalin/Focalin |
365 | 492 | (26 | ) | 594 | (17 | ) | |||||||||
Other |
2,774 | 3,675 | (25 | ) | 4,033 | (9 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
Total Established Medicines |
6,028 | 8,540 | (29 | ) | 10,282 | (17 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
Total Division net sales |
30,445 | 31,791 | (4 | ) | 32,214 | (1 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- Chronic
Obstructive Pulmonary Disease
- (2)
- Net sales reflect Xolair sales for all indications (e.g. including Xolair SAA and Xolair CSU, which are managed by the Immunology & Dermatology franchise).
nm = not meaningful
The product portfolio of other segments is widely spread in 2015, 2014 and 2013.
F-32
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Associated Companies
Novartis has significant investments in Roche Holding AG, Basel (Roche) and in GlaxoSmithKline Consumer Healthcare Holdings Ltd, Brentford, Middlesex, UK as well as certain other smaller investments which are accounted for as associated companies:
| |
Balance sheet value |
||||||
|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | |||||
| |
$ m |
$ m |
|||||
Roche Holding AG, Switzerland |
7,919 | 8,159 | |||||
GlaxoSmithKline Consumer Healthcare Holdings Ltd., UK |
7,194 | ||||||
Others |
201 | 273 | |||||
| | | | | | | | |
Total |
15,314 | 8,432 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| |
Net income statement effect |
Other comprehensive income effect |
Total comprehensive income effect |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | 2015 | 2014 | 2013 | 2015 | 2014 | 2013 | |||||||||||||||||||
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||||||||
Roche Holding AG, Switzerland |
343 | 599 | 604 | (149 | ) | (51 | ) | (37 | ) | 194 | 548 | 567 | ||||||||||||||||
GlaxoSmithKline Consumer Healthcare Holdings Ltd., UK |
(79 | ) | (4 | ) | (83 | ) | ||||||||||||||||||||||
Idenix Pharmaceuticals Inc., US |
812 | 812 | ||||||||||||||||||||||||||
LTS Lohmann Therapie-Systeme AG, Germany |
436 | 31 | (6 | ) | 436 | 25 | ||||||||||||||||||||||
Others |
2 | 71 | (36 | ) | 20 | 11 | 2 | 91 | (25 | ) | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Associated companies related to continuing operations |
266 | 1,918 | 599 | (153 | ) | (31 | ) | (32 | ) | 113 | 1,887 | 567 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The Group's holding in Roche voting shares was 33.3% at December 31, 2015, 2014 and 2013. This investment represents approximately 6.3% of Roche's total outstanding voting and non-voting equity instruments at December 31, 2015, 2014 and 2013.
Since full-year 2015 financial data for Roche are not available when Novartis produces its consolidated financial results, a survey of analyst estimates is used to estimate the Group's share of Roche's net income. Any differences between these estimates and actual results will be adjusted in the Group's 2016 consolidated financial statements when available.
F-33
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Associated Companies (Continued)
The following tables show summarized financial information of Roche, including current values of fair value adjustments made at the time of the acquisition of the shares, for the year ended December 31, 2014 and for the six months ended June 30, 2015 since full year 2015 data is not yet available:
| |
Current assets |
Non-current assets |
Current liabilities |
Non-current liabilities |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
CHF billions |
CHF billions |
CHF billions |
CHF billions |
|||||||||
December 31, 2014 |
31.1 | 63.5 | 23.1 | 31.0 | |||||||||
June 30, 2015 |
25.2 | 61.3 | 21.7 | 28.0 | |||||||||
| |
Revenue | Net income | Other comprehensive income |
Total comprehensive income |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
CHF billions |
CHF billions |
CHF billions |
CHF billions |
|||||||||
December 31, 2014 |
47.5 | 7.3 | (2.3 | ) | 5.0 | ||||||||
June 30, 2015 |
23.6 | 4.1 | (1.1 | ) | 3.0 | ||||||||
A purchase price allocation was performed on the basis of publicly available information at the time of acquisition of the investment. The December 31, 2015 balance sheet value allocation is as follows:
| |
$ m | |||
|---|---|---|---|---|
Novartis share of Roche's estimated net assets |
2,314 | |||
Novartis share of re-appraised intangible assets |
1,039 | |||
Implicit Novartis goodwill |
2,879 | |||
| | | | | |
Current value of share in net identifiable assets and goodwill |
6,232 | |||
Accumulated equity accounting adjustments and translation effects less dividends received |
1,687 | |||
| | | | | |
December 31, 2015 balance sheet value |
7,919 | |||
| | | | | |
| | | | | |
| | | | | |
The identified intangible assets principally relate to the value of currently marketed products and are amortized on a straight-line basis over their estimated average useful life of 20 years.
In 2015, dividends received from Roche in relation to the distribution of its 2014 net income amounted to $429 million (2014: $473 million in relation with the distribution of its 2013 net income).
F-34
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Associated Companies (Continued)
The consolidated income statement effects from applying Novartis accounting principles for this investment in 2015, 2014 and 2013 are as follows:
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Novartis share of Roche's estimated current-year consolidated net income |
650 | 813 | 817 | |||||||
Prior-year adjustment |
(157 | ) | (56 | ) | (59 | ) | ||||
Amortization of fair value adjustments relating to intangible assets, net of taxes of $41 million (2014: $45 million, 2013: $45 million) |
(150 | ) | (158 | ) | (154 | ) | ||||
| | | | | | | | | | | |
Net income effect |
343 | 599 | 604 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The publicly quoted market value of the Novartis interest in Roche (Reuters symbol: RO.S) at December 31, 2015, was $14.9 billion (2014: $14.4 billion).
GlaxoSmithKline Consumer Healthcare Holdings Ltd
On March 2, 2015, Novartis closed its transactions with GlaxoSmithKline plc, Great Britain (GSK) announced in April 2014. As part of these transactions, Novartis and GSK have agreed to create a combined consumer healthcare business through a joint venture between Novartis OTC and GSK Consumer Healthcare. On March 2, 2015, a new entity GlaxoSmithKline Consumer Healthcare Holdings Ltd (GSK Consumer Healthcare) was formed via contribution of businesses from both Novartis and GSK.
At December 31, 2015, Novartis has a 36.5% interest in GSK Consumer Healthcare and four of eleven seats on the joint venture entity's Board of Directors. Furthermore, Novartis has customary minority rights and also exit rights at a pre-defined, market based pricing mechanism.
Novartis has valued the contribution of 63.5% of its OTC Division in exchange for 36.5% of the GSK Consumer Healthcare business at fair value. Based on the estimates of values exchanged, an investment in associated company of $7.6 billion was recorded on March 2, 2015.
The December 31, 2015 balance sheet value allocation is as follows:
| |
$ m | |||
|---|---|---|---|---|
Novartis share of GSK Consumer Healthcare's estimated net assets |
957 | |||
Novartis share of re-appraised intangible assets |
4,273 | |||
Implicit Novartis goodwill |
1,941 | |||
| | | | | |
Current value of share in net identifiable assets and goodwill |
7,171 | |||
Accumulated equity accounting adjustments and translation effects |
23 | |||
| | | | | |
December 31, 2015 balance sheet value |
7,194 | |||
| | | | | |
| | | | | |
| | | | | |
The identified intangible assets principally relate to the value of the indefinite life GSK Consumer Healthcare intangible assets. The identified intangible assets with a definite life are amortized on a straight-line basis over their estimated average useful life of 20 years.
F-35
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Associated Companies (Continued)
The following tables show interim unaudited financial information of GSK Consumer Healthcare, including current values of fair value adjustments made at the time of acquisition, for the seven months ended September 30, 2015 since full year 2015 data is not yet available:
| |
Current assets |
Non-current assets |
Current liabilities |
Non-current liabilities |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
GBP billions |
GBP billions |
GBP billions |
GBP billions |
|||||||||
September 30, 2015 |
3.3 | 21.3 | 1.8 | 2.1 | |||||||||
| |
Revenue | Net income | Other comprehensive income |
Total comprehensive income |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
GBP millions |
GBP millions |
GBP millions |
GBP millions |
|||||||||
September 30, 2015 |
3,241 | 4 | (44 | ) | (40 | ) | |||||||
Since full-year 2015 financial data for GSK Consumer Healthcare are not available when Novartis produces its consolidated financial results, a projection of the latest internal management reporting is used to estimate the Group's share of GSK Consumer Healthcare's net result for the year. Any differences between this estimate and actual results will be adjusted in the Group's 2016 consolidated financial statements when available.
The consolidated income statement effects from applying Novartis accounting principles for this investment in 2015 are as follows:
| |
2015 | |||
|---|---|---|---|---|
| |
$ m |
|||
Novartis share of GSK Consumer Healthcare's estimated current-year consolidated net income |
(17 | ) | ||
Amortization of fair value adjustments relating to intangible assets and inventory, net of taxes of $18 million |
(62 | ) | ||
| | | | | |
Net income effect |
(79 | ) | ||
| | | | | |
| | | | | |
| | | | | |
Other Associated Companies
During 2014, the shareholdings of 22% in Idenix Pharmaceuticals Inc. and 43% in LTS Lohmann Therapie-Systeme AG were sold realizing pre-tax gains of $812 million and $421 million, respectively. Others include a gain of $64 million recorded on investments in associated companies held by the Novartis Venture Funds, which are accounted at fair value from January 1, 2014 onwards, consistent with other investments held by these Funds.
F-36
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Interest Expense and Other Financial Income and Expense
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Interest expense |
(669 | ) | (701 | ) | (664 | ) | ||||
Income/(expense) due to discounting long-term liabilities |
14 | (3 | ) | (19 | ) | |||||
| | | | | | | | | | | |
Total interest expense |
(655 | ) | (704 | ) | (683 | ) | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Other Financial Income and Expense
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Interest income |
33 | 33 | 34 | |||||||
Dividend income |
1 | 1 | 1 | |||||||
Net capital (losses)/gains on available-for-sale securities |
(8 | ) | (2 | ) | 28 | |||||
Net capital losses on cash and cash equivalents |
(1 | ) | ||||||||
Income on forward contracts and options |
1 | 1 | 2 | |||||||
Impairment of commodities and available-for-sale securities |
(132 | ) | (14 | ) | ||||||
Other financial expense |
(23 | ) | (25 | ) | (20 | ) | ||||
Monetary loss from hyperinflation accounting |
(72 | ) | (61 | ) | (32 | ) | ||||
Currency result, net |
(254 | ) | 22 | (90 | ) | |||||
| | | | | | | | | | | |
Total other financial income and expense |
(454 | ) | (31 | ) | (92 | ) | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
6. Taxes
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Switzerland |
5,765 | 5,245 | 5,435 | |||||||
Foreign |
2,369 | 7,027 | 5,372 | |||||||
| | | | | | | | | | | |
Income before taxes from continuing operations |
8,134 | 12,272 | 10,807 | |||||||
Income/(loss) before taxes from discontinued operations |
12,479 | (351 | ) | (72 | ) | |||||
| | | | | | | | | | | |
Total income before taxes |
20,613 | 11,921 | 10,735 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-37
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Taxes (Continued)
Current and Deferred Income Tax Expense
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Switzerland |
(317 | ) | (661 | ) | (524 | ) | ||||
Foreign |
(1,333 | ) | (1,952 | ) | (1,793 | ) | ||||
| | | | | | | | | | | |
Current income tax expense from continuing operations |
(1,650 | ) | (2,613 | ) | (2,317 | ) | ||||
| | | | | | | | | | | |
Switzerland |
(68 | ) | 309 | 160 | ||||||
Foreign |
612 | 759 | 659 | |||||||
| | | | | | | | | | | |
Deferred tax income from continuing operations |
544 | 1,068 | 819 | |||||||
| | | | | | | | | | | |
Income tax expense from continuing operations |
(1,106 | ) | (1,545 | ) | (1,498 | ) | ||||
Income tax (expense)/income from discontinued operations |
(1,713 | ) | (96 | ) | 55 | |||||
| | | | | | | | | | | |
Total income tax expense |
(2,819 | ) | (1,641 | ) | (1,443 | ) | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The main elements contributing to the difference between the Group's overall expected tax rate (which can change each year since it is calculated as the weighted average tax rate based on pre-tax income of each subsidiary) and the effective tax rate are:
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
% |
% |
% |
|||||||
Expected tax rate |
12.4 | 11.7 | 12.9 | |||||||
Effect of disallowed expenditures |
3.5 | 2.9 | 3.4 | |||||||
Effect of utilization of tax losses brought forward from prior periods |
(0.2 | ) | (0.3 | ) | (0.1 | ) | ||||
Effect of income taxed at reduced rates |
(0.3 | ) | (0.6 | ) | (0.1 | ) | ||||
Effect of tax credits and allowances |
(2.7 | ) | (1.8 | ) | (2.0 | ) | ||||
Effect of write-off of deferred tax assets |
0.1 | |||||||||
Effect of tax rate change on opening balance |
(0.5 | ) | (0.2 | ) | ||||||
Effect of tax benefits expiring in 2017 |
(0.4 | ) | (0.8 | ) | (0.7 | ) | ||||
Effect of non-deductible losses in Venezuela |
1.2 | |||||||||
Effect of write down and reversal of write-down of investments in subsidiaries |
(0.9 | ) | 0.9 | |||||||
Prior year and other items |
1.5 | 0.6 | 0.6 | |||||||
| | | | | | | | | | | |
Effective tax rate for continuing operations |
13.6 | 12.6 | 13.9 | |||||||
Effective tax rate for discontinued operations |
13.7 | (27.4 | ) | 76.4 | ||||||
| | | | | | | | | | | |
Effective tax rate |
13.7 | 13.8 | 13.4 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The utilization of tax-loss carry-forwards lowered the tax charge by $15 million in 2015 and by $34 million and $13 million in 2014 and 2013 respectively.
F-38
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Earnings per Share
Basic earnings per share (EPS) is calculated by dividing net income attributable to shareholders of Novartis AG by the weighted average number of shares outstanding in a reporting period. This calculation excludes the average number of issued shares purchased by the Group and held as treasury shares.
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Basic earnings per share |
||||||||||
Weighted average number of shares outstanding (in millions) |
2,403 | 2,426 | 2,441 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Net income/(loss) attributable to shareholders of Novartis AG ($ m) |
||||||||||
— Continuing operations |
7,025 | 10,654 | 9,189 | |||||||
— Discontinued operations |
10,758 | (444 | ) | (14 | ) | |||||
| | | | | | | | | | | |
— Total |
17,783 | 10,210 | 9,175 | |||||||
| | | | | | | | | | | |
Basic earnings per share ($) |
||||||||||
— Continuing operations |
2.92 | 4.39 | 3.76 | |||||||
— Discontinued operations |
4.48 | (0.18 | ) | 0.00 | ||||||
| | | | | | | | | | | |
— Total |
7.40 | 4.21 | 3.76 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
For diluted EPS, the weighted average number of shares outstanding is adjusted to assume the vesting of all restricted shares and the conversion of all potentially dilutive shares arising from options on Novartis shares that have been issued.
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Diluted earnings per share |
||||||||||
Weighted average number of shares outstanding (in millions) |
2,403 | 2,426 | 2,441 | |||||||
Adjustment for vesting of restricted shares and dilutive shares from options (in millions) |
35 | 44 | 38 | |||||||
| | | | | | | | | | | |
Weighted average number of shares for diluted earnings per share (in millions) |
2,438 | 2,470 | 2,479 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Net income/(loss) attributable to shareholders of Novartis AG ($ m) |
||||||||||
— Continuing operations |
7,025 | 10,654 | 9,189 | |||||||
— Discontinued operations |
10,758 | (444 | ) | (14 | ) | |||||
| | | | | | | | | | | |
— Total |
17,783 | 10,210 | 9,175 | |||||||
| | | | | | | | | | | |
Diluted earnings per share ($) |
||||||||||
— Continuing operations |
2.88 | 4.31 | 3.70 | |||||||
— Discontinued operations |
4.41 | (0.18 | ) | 0.00 | ||||||
| | | | | | | | | | | |
— Total |
7.29 | 4.13 | 3.70 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
No options were excluded from the calculation of diluted EPS in 2013, 2014 or 2015, as all options were dilutive in both years.
F-39
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Changes in Consolidated Statements of Comprehensive Income
The consolidated statements of comprehensive income include the Group's net income for the year as well as all other valuation adjustments recorded in the Group's consolidated balance sheet but which under IFRS are not recorded in the consolidated income statement. These include fair value adjustments to financial instruments, actuarial gains or losses on defined benefit pension and other post-employment plans and currency translation effects, net of tax.
The following table summarizes these value adjustments and currency translation effects attributable to Novartis shareholders:
| |
Fair value adjustments on marketable securities |
Fair value adjustments on deferred cash flow hedges |
Actuarial losses from defined benefit plans |
Cumulative currency translation effects |
Total value adjustments |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Fair value adjustments at January 1, 2013 |
212 | (100 | ) | (6,048 | ) | 3,944 | (1,992 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
Fair value adjustments on financial instruments |
132 | 41 | 173 | |||||||||||||
Net actuarial gains from defined benefit plans(1) |
1,504 | 1,504 | ||||||||||||||
Currency translation effects(2) |
681 | 681 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Total fair value adjustments in 2013 |
132 | 41 | 1,504 | 681 | 2,358 | |||||||||||
| | | | | | | | | | | | | | | | | |
Fair value adjustments at December 31, 2013 |
344 | (59 | ) | (4,544 | ) | 4,625 | 366 | |||||||||
| | | | | | | | | | | | | | | | | |
Fair value adjustments on financial instruments |
89 | 21 | 110 | |||||||||||||
Net actuarial losses from defined benefit plans(1) |
(822 | ) | (822 | ) | ||||||||||||
Currency translation effects(2) |
(2,219 | ) | (2,219 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | |
Total fair value adjustments in 2014 |
89 | 21 | (822 | ) | (2,219 | ) | (2,931 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
Fair value adjustments at December 31, 2014 |
433 | (38 | ) | (5,366 | ) | 2,406 | (2,565 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
Fair value adjustments on financial instruments |
28 | 20 | 48 | |||||||||||||
Net actuarial losses from defined benefit plans(1) |
(147 | ) | (147 | ) | ||||||||||||
Currency translation effects(2) |
(1,659 | ) | (1,659 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | |
Total value adjustments in 2015 |
28 | 20 | (147 | ) | (1,659 | ) | (1,758 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
Fair value adjustments related to divestments |
100 | 100 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Value adjustments at December 31, 2015 |
461 | (18 | ) | (5,413 | ) | 747 | (4,223 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- Net
actuarial gains of $10 million are attributable to discontinued operations up to the respective divestment dates (2014: net
actuarial losses of $65 million, 2013: net actuarial gains of $39 million).
- (2)
- $29 million currency translation losses are attributable to discontinued operations up to the respective divestment dates (2014: losses of $37 million, 2013: gains of $1 million).
F-40
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Changes in Consolidated Statements of Comprehensive Income (Continued)
8.1) The 2015, 2014 and 2013 changes in the fair value of financial instruments were as follows:
| |
Fair value adjustments on marketable securities |
Fair value adjustments on deferred cash flow hedges |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Fair value adjustments at January 1, 2015 |
433 | (38 | ) | 395 | ||||||
| | | | | | | | | | | |
Changes in fair value: |
||||||||||
— Available-for-sale marketable securities |
(130 | ) | (130 | ) | ||||||
— Available-for-sale financial investments |
80 | 80 | ||||||||
— Associated companies' movements in comprehensive income |
(8 | ) | (8 | ) | ||||||
Realized net gains transferred to the consolidated income statement: |
||||||||||
— Marketable securities sold |
(1 | ) | (1 | ) | ||||||
— Other financial assets sold |
(103 | ) | (103 | ) | ||||||
Amortized net losses on cash flow hedges transferred to the consolidated income statement |
21 | 21 | ||||||||
Impaired financial assets transferred to the consolidated income statement |
194 | 194 | ||||||||
Deferred tax on above items |
(4 | ) | (1 | ) | (5 | ) | ||||
| | | | | | | | | | | |
Fair value adjustments during the year |
28 | 20 | 48 | |||||||
| | | | | | | | | | | |
Fair value adjustments at December 31, 2015 |
461 | (18 | ) | 443 | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-41
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Changes in Consolidated Statements of Comprehensive Income (Continued)
| |
Fair value adjustments on marketable securities |
Fair value adjustments on deferred cash flow hedges |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Fair value adjustments at January 1, 2014 |
344 | (59 | ) | 285 | ||||||
| | | | | | | | | | | |
Changes in fair value: |
||||||||||
— Available-for-sale marketable securities |
(3 | ) | (3 | ) | ||||||
— Available-for-sale financial investments |
91 | 91 | ||||||||
— Associated companies' movements in comprehensive income |
5 | 5 | ||||||||
Realized net gains transferred to the consolidated income statement: |
||||||||||
— Marketable securities sold |
(4 | ) | (4 | ) | ||||||
— Other financial assets sold |
(81 | ) | (81 | ) | ||||||
Amortized net losses on cash flow hedges transferred to the consolidated income statement |
23 | 23 | ||||||||
Impaired financial assets transferred to the consolidated income statement |
87 | 87 | ||||||||
Deferred tax on above items |
(6 | ) | (2 | ) | (8 | ) | ||||
| | | | | | | | | | | |
Fair value adjustments during the year |
89 | 21 | 110 | |||||||
| | | | | | | | | | | |
Fair value adjustments at December 31, 2014 |
433 | (38 | ) | 395 | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-42
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Changes in Consolidated Statements of Comprehensive Income (Continued)
| |
Fair value adjustments on marketable securities |
Fair value adjustments on deferred cash flow hedges |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Fair value adjustments at January 1, 2013 |
212 | (100 | ) | 112 | ||||||
| | | | | | | | | | | |
Changes in fair value: |
||||||||||
— Available-for-sale marketable securities |
3 | 3 | ||||||||
— Available-for-sale financial investments |
204 | 204 | ||||||||
— Associated companies' movements in comprehensive income |
7 | 7 | ||||||||
Realized net gains transferred to the consolidated income statement: |
||||||||||
— Marketable securities sold |
(46 | ) | (46 | ) | ||||||
— Other financial assets sold |
(74 | ) | (74 | ) | ||||||
Amortized net losses on cash flow hedges transferred to the consolidated income statement |
44 | 44 | ||||||||
Impaired financial assets transferred to the consolidated income statement |
65 | 65 | ||||||||
Deferred tax on above items |
(27 | ) | (3 | ) | (30 | ) | ||||
| | | | | | | | | | | |
Fair value adjustments during the year |
132 | 41 | 173 | |||||||
| | | | | | | | | | | |
Fair value adjustments at December 31, 2013 |
344 | (59 | ) | 285 | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
8.2) The Group has investments in associated companies, principally Roche Holding AG and GlaxoSmithKline Consumer Healthcare Holdings Ltd. The Group's share in movements in these companies' other comprehensive income are recognized directly in the respective categories of the Novartis consolidated statement of comprehensive income, net of tax. The currency translation effects and fair value adjustments of associated companies are included in the corresponding Group amounts. All other movements in these companies' statements of comprehensive income are recognized directly in the consolidated statement of comprehensive income under "Novartis share of other items recorded in comprehensive income recognized by associated companies, net of taxes". These amounted to a loss of $48 million (2014: loss of $5 million, 2013: income of $5 million).
8.3) In 2015, cumulative currency translation losses of $10 million have been recycled through the income statement as a result of the divestments of subsidiaries (2014: nil, 2013: gain of $1 million as a result of the liquidation of a subsidiary).
Currency translation losses of associated companies of $97 million were recognized in 2015 (2014: loss of $31 million, 2013: loss of $43 million).
F-43
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Changes in Consolidated Statements of Comprehensive Income (Continued)
8.4) Remeasurements from defined benefit plans arise as follows:
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Defined benefit pension plans before tax |
(252 | ) | (999 | ) | 1,977 | |||||
Other post-employment benefit plans before tax |
168 | (235 | ) | 163 | ||||||
Taxation on above items |
(63 | ) | 412 | (636 | ) | |||||
| | | | | | | | | | | |
Total after tax |
(147 | ) | (822 | ) | 1,504 | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
8.5) The following table shows contributions of associated companies to other comprehensive income:
| |
Note | 2015 | 2014 | 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
$ m |
$ m |
$ m |
|||||||||
Fair value adjustments attributable to associated companies |
(8 | ) | 5 | 6 | |||||||||
Novartis share of other items recorded in comprehensive income recognized by associated companies, net of taxes. |
8.2 | (48 | ) | (5 | ) | 5 | |||||||
Currency translation adjustments |
(97 | ) | (31 | ) | (43 | ) | |||||||
| | | | | | | | | | | | | | |
Other comprehensive income attributable to associated companies |
4 | (153 | ) | (31 | ) | (32 | ) | ||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
9. Changes in Consolidated Equity
9.1) A dividend of CHF 2.60 per share was approved at the 2015 Annual General meeting for the year ended December 31, 2014, resulting in a total dividend payment of $6.6 billion in 2015. (2014: the CHF 2.45 per share dividend amounted to $6.8 billion, 2013: the CHF 2.30 per share dividend payment amounted to $6.1 billion). The amount available for distribution as a dividend to shareholders is based on the available distributable retained earnings of Novartis AG determined in accordance with the legal provisions of the Swiss Code of Obligations.
9.2) During 2015, 63.6 million shares were purchased for $6.1 billion (2014: 79.2 million shares for $6.9 billion, 2013: 40.3 million shares for $3.0 billion). These share purchases comprise of 9.6 million shares which were repurchased for $897 million on the SIX Swiss Exchange first trading line (2014: 46.8 million shares for $4.1 billion, 2013: 33.3 million shares for $2.5 billion), 4.1 million shares were acquired for $417 million from employees which were previously granted to them under the respective programs (2014: 5.4 million shares for $473 million, 2013: 4.8 million shares for $356 million), and in addition, Novartis repurchased 49.9 million shares for $4.8 billion on the SIX Swiss Exchange second trading line under the $5 billion share buy-back announced in November 2013, which was completed in November 2015, and also to offset the dilutive impact from equity-based participation plans (2014: 27.0 million shares for $2.4 billion, 2013: 2.2 million shares for $170 million).
9.3) In 2015, Novartis reduced its share capital by cancelling a total of 29.2 million shares which were repurchased during 2013 and 2014 on the SIX Swiss Exchange second trading line. In 2014 and 2013, no shares were cancelled.
F-44
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Changes in Consolidated Equity (Continued)
9.4) In 2014, Novartis has entered into an irrevocable, non-discretionary arrangement with a bank to repurchase Novartis own shares on the second trading line under its $5 billion share buy-back as well as to mitigate dilution from equity-based participation plans. The commitment under this arrangement amounted to $658 million as of December 31, 2014 (2013: nil), reflecting the expected purchases by the bank under such trading plan over a rolling 90 days period. This trading plan was fully executed and has expired. As a result, there is no contingent liability related to this plan as of December 2015.
9.5) 27.0 million shares were delivered as a result of options being exercised related to equity-based participation plans and delivery of treasury shares, which contributed $1.6 billion (2014: 41.4 million shares for $2.4 billion, 2013: 34.3 million shares for $1.7 billion). The average share price of the shares delivered was significantly below market price reflecting the strike price of the options exercised.
9.6) Equity-settled share-based compensation is expensed in the consolidated income statement in accordance with the vesting period of the share-based compensation plans. The value for the shares and options granted is credited to consolidated equity over the respective vesting period. In 2015, 11.9 million shares were transferred to associates as part of equity-settled compensation (2014: 10.3 million shares, 2013: 11.5 million shares). In addition, tax benefits arising from tax deductible amounts exceeding the expense recognized in the income statement are credited to equity.
9.7) Changes in non-controlling interests in subsidiaries resulted in a reduction in consolidated equity of $10 million (2014: reduction of $120 million, 2013: reduction of $109 million).
9.8) In 2013 additional interests in subsidiaries were acquired. The reduction in equity of $10 million represents the excess of the amount paid over the amount recognized for the acquired non-controlling interest. In 2015 and 2014 no such transaction took place.
F-45
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Property, Plant & Equipment Movements
2015
|
Land | Buildings | Construction in progress |
Machinery & other equipment |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Cost |
||||||||||||||||
January 1 |
744 | 11,312 | 3,985 | 15,387 | 31,428 | |||||||||||
Reclassifications(1) |
12 | 1,833 | (2,601 | ) | 756 | |||||||||||
Additions |
4 | 408 | 1,665 | 442 | 2,519 | |||||||||||
Disposals and derecognitions(2) |
(41 | ) | (332 | ) | (59 | ) | (704 | ) | (1,136 | ) | ||||||
Currency translation effects |
(31 | ) | (364 | ) | (180 | ) | (788 | ) | (1,363 | ) | ||||||
| | | | | | | | | | | | | | | | | |
December 31 |
688 | 12,857 | 2,810 | 15,093 | 31,448 | |||||||||||
| | | | | | | | | | | | | | | | | |
Accumulated depreciation |
||||||||||||||||
January 1 |
(30 | ) | (5,093 | ) | (37 | ) | (10,285 | ) | (15,445 | ) | ||||||
Depreciation charge |
(3 | ) | (462 | ) | (1,005 | ) | (1,470 | ) | ||||||||
Accumulated depreciation on disposals and derecognitions(2) |
2 | 246 | 32 | 594 | 874 | |||||||||||
Impairment charge |
(12 | ) | (37 | ) | (4 | ) | (82 | ) | (135 | ) | ||||||
Reversal of impairment charge |
9 | 46 | 55 | |||||||||||||
Currency translation effects |
3 | 149 | 2 | 501 | 655 | |||||||||||
| | | | | | | | | | | | | | | | | |
December 31 |
(40 | ) | (5,188 | ) | (7 | ) | (10,231 | ) | (15,466 | ) | ||||||
| | | | | | | | | | | | | | | | | |
Net book value at December 31 |
648 | 7,669 | 2,803 | 4,862 | 15,982 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Net book value of property, plant & equipment under finance lease contracts |
85 | |||||||||||||||
| | | | | | | | | | | | | | | | | |
Commitments for purchases of property, plant & equipment |
359 | |||||||||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Reclassifications
between various asset categories due to completion of plant and other equipment under construction.
- (2)
- Derecognition of assets that are no longer used and are not considered to have a significant disposal value or other alternative use.
Borrowing costs on new additions to property, plant and equipment eligible for capitalization have been capitalized and amounted to $21 million in 2015 (2014: $20 million). The capitalization rate used to determine the amount of borrowing costs eligible for capitalization is 25% (2014: 25%) and the interest rate used is 4% (2014: 4%).
F-46
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Property, Plant & Equipment Movements (Continued)
2014
|
Land | Buildings | Construction in progress |
Machinery & other equipment |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Cost |
||||||||||||||||
January 1 |
920 | 12,933 | 3,635 | 17,813 | 35,301 | |||||||||||
Cost of assets related to discontinued operations |
(115 | ) | (1,175 | ) | (445 | ) | (1,597 | ) | (3,332 | ) | ||||||
Reclassifications(1) |
455 | (1,291 | ) | 836 | ||||||||||||
Additions(2) |
5 | 113 | 2,397 | 389 | 2,904 | |||||||||||
Disposals and derecognitions(3) |
(8 | ) | (127 | ) | (15 | ) | (544 | ) | (694 | ) | ||||||
Currency translation effects |
(58 | ) | (887 | ) | (296 | ) | (1,510 | ) | (2,751 | ) | ||||||
| | | | | | | | | | | | | | | | | |
December 31 |
744 | 11,312 | 3,985 | 15,387 | 31,428 | |||||||||||
| | | | | | | | | | | | | | | | | |
Accumulated depreciation |
||||||||||||||||
January 1 |
(29 | ) | (5,560 | ) | (29 | ) | (11,486 | ) | (17,104 | ) | ||||||
Accumulated depreciation on assets related to discontinued operations |
1 | 377 | 4 | 827 | 1,209 | |||||||||||
Depreciation charge(4) |
(3 | ) | (450 | ) | (1,133 | ) | (1,586 | ) | ||||||||
Accumulated depreciation on disposals and derecognitions(3) |
1 | 91 | 464 | 556 | ||||||||||||
Impairment charge |
(1 | ) | (10 | ) | (37 | ) | (18 | ) | (66 | ) | ||||||
Reversal of impairment charge |
21 | 1 | 22 | |||||||||||||
Currency translation effects |
1 | 459 | 4 | 1,060 | 1,524 | |||||||||||
| | | | | | | | | | | | | | | | | |
December 31 |
(30 | ) | (5,093 | ) | (37 | ) | (10,285 | ) | (15,445 | ) | ||||||
| | | | | | | | | | | | | | | | | |
Net book value at December 31 |
714 | 6,219 | 3,948 | 5,102 | 15,983 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Net book value of property, plant & equipment under finance lease contracts |
1 | |||||||||||||||
| | | | | | | | | | | | | | | | | |
Commitments for purchases of property, plant & equipment |
826 | |||||||||||||||
| | | | | | | | | | | | | | | | | |
- (1)
- Reclassifications
between various asset categories due to completion of plant and other equipment under construction.
- (2)
- Additions
in discontinued operations, for the period from January 1, 2014 to the portfolio transformation announcement on
April 22, 2014, were $50 million.
- (3)
- Derecognition
of assets that are no longer used and are not considered to have a significant disposal value or other alternative use.
- (4)
- Depreciation charge in discontinued operations, for the period from January 1, 2014 to the portfolio transformation announcement on April 22, 2014, was $66 million.
F-47
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Goodwill and Intangible Assets Movements
2015
|
Goodwill | Acquired research & development |
Alcon brand name |
Technologies | Currently marketed products |
Marketing know-how |
Other intangible assets |
Total of intangible assets other than goodwill |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||||||
Cost |
|||||||||||||||||||||||||
January 1 |
29,737 | 2,843 | 2,980 | 6,658 | 20,916 | 5,960 | 1,251 | 40,608 | |||||||||||||||||
Impact of business combinations |
2,438 | 730 | 12,970 | 15 | 13,715 | ||||||||||||||||||||
Reclassifications(1) |
(36 | ) | 5 | 31 | |||||||||||||||||||||
Additions |
881 | 217 | 61 | 1,159 | |||||||||||||||||||||
Disposals and derecognitions(2) |
(294 | ) | (26 | ) | (4 | ) | (324 | ) | |||||||||||||||||
Currency translation effects |
(590 | ) | (5 | ) | (95 | ) | (697 | ) | (13 | ) | (810 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
December 31 |
31,585 | 4,119 | 2,980 | 6,563 | 33,385 | 5,960 | 1,341 | 54,348 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Accumulated amortization |
|||||||||||||||||||||||||
January 1 |
(426 | ) | (685 | ) | (2,539 | ) | (11,684 | ) | (954 | ) | (914 | ) | (16,776 | ) | |||||||||||
Amortization charge |
(580 | ) | (2,848 | ) | (238 | ) | (89 | ) | (3,755 | ) | |||||||||||||||
Accumulated amortization on disposals and derecognitions(2) |
68 | 241 | 4 | 313 | |||||||||||||||||||||
Impairment charge |
(33 | ) | (164 | ) | (9 | ) | (206 | ) | |||||||||||||||||
Reversal of impairment charge |
40 | 40 | |||||||||||||||||||||||
Currency translation effects |
15 | 49 | 194 | 10 | 253 | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
December 31 |
(411 | ) | (650 | ) | (3,070 | ) | (14,221 | ) | (1,192 | ) | (998 | ) | (20,131 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net book value at December 31 |
31,174 | 3,469 | 2,980 | 3,493 | 19,164 | 4,768 | 343 | 34,217 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Reclassifications
between various asset categories as a result of product launches of acquired In-Process Research & Development.
- (2)
- Derecognitions of assets that are no longer used or being developed and are not considered to have a significant disposal value or other alternative use.
F-48
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Goodwill and Intangible Assets Movements (Continued)
Segmentation of Goodwill and Intangible Assets
The net book values at December 31, 2015 of goodwill and intangible assets are allocated to the Group's reporting segments as summarized below.
| |
Goodwill | Acquired research & development |
Alcon brand name |
Technologies | Currently marketed products |
Marketing know-how |
Other intangible assets |
Total of intangible assets other than goodwill |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||||||
Pharmaceuticals |
5,530 | 2,511 | 13 | 13,151 | 140 | 15,815 | |||||||||||||||||||
Alcon |
17,947 | 461 | 2,980 | 2,850 | 4,435 | 4,768 | 163 | 15,657 | |||||||||||||||||
Sandoz |
7,690 | 490 | 630 | 1,578 | 22 | 2,720 | |||||||||||||||||||
Corporate |
7 | 7 | 18 | 25 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total |
31,174 | 3,469 | 2,980 | 3,493 | 19,164 | 4,768 | 343 | 34,217 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Potential impairment charge, if any, if discounted cash flows fell by 5% |
4 | ||||||||||||||||||||||||
Potential impairment charge, if any, if discounted cash flows fell by 10% |
9 | ||||||||||||||||||||||||
2014
|
Goodwill | Acquired research & development |
Alcon brand name |
Technologies | Currently marketed products |
Marketing know-how |
Other intangible assets |
Total of intangible assets other than goodwill |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||||||
Cost |
|||||||||||||||||||||||||
January 1 |
31,554 | 2,648 | 2,980 | 7,104 | 24,160 | 5,960 | 1,479 | 44,331 | |||||||||||||||||
Cost of assets related to discontinued operations |
(1,222 | ) | (25 | ) | (346 | ) | (2,833 | ) | (359 | ) | (3,563 | ) | |||||||||||||
Impact of business combinations |
131 | 248 | 234 | 482 | |||||||||||||||||||||
Reclassifications(1) |
(139 | ) | (125 | ) | 95 | 169 | |||||||||||||||||||
Additions(2) |
405 | 125 | 216 | 53 | 799 | ||||||||||||||||||||
Disposals and derecognitions(3) |
(159 | ) | (286 | ) | (18 | ) | (463 | ) | |||||||||||||||||
Currency translation effects |
(726 | ) | (135 | ) | (100 | ) | (670 | ) | (73 | ) | (978 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
December 31 |
29,737 | 2,843 | 2,980 | 6,658 | 20,916 | 5,960 | 1,251 | 40,608 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Accumulated amortization |
|||||||||||||||||||||||||
January 1 |
(528 | ) | (575 | ) | (2,168 | ) | (11,953 | ) | (715 | ) | (1,079 | ) | (16,490 | ) | |||||||||||
Accumulated amortization of assets related to discontinued operations |
61 | 13 | 167 | 1,369 | 213 | 1,762 | |||||||||||||||||||
Amortization charge(4) |
(587 | ) | (1,868 | ) | (239 | ) | (81 | ) | (2,775 | ) | |||||||||||||||
Accumulated amortization on disposals and derecognitions(3) |
159 | 283 | 17 | 459 | |||||||||||||||||||||
Impairment charge |
(271 | ) | (46 | ) | (30 | ) | (347 | ) | |||||||||||||||||
Reversal of impairment charge |
70 | 70 | |||||||||||||||||||||||
Currency translation effects |
41 | (11 | ) | 49 | 461 | 46 | 545 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
December 31 |
(426 | ) | (685 | ) | (2,539 | ) | (11,684 | ) | (954 | ) | (914 | ) | (16,776 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net book value at December 31 |
29,311 | 2,158 | 2,980 | 4,119 | 9,232 | 5,006 | 337 | 23,832 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Reclassifications
between various asset categories as a result of product launches of acquired In-Process Research & Development.
- (2)
- Additions
in discontinued operations, for the period from January 1, 2014 to the portfolio transformation announcement on
April 22, 2014, were $11 million.
- (3)
- Derecognitions
of assets that are no longer used or being developed and are not considered to have a significant disposal value or other
alternative use.
- (4)
- Amortization charge in discontinued operations, for the period from January 1, 2014 to the portfolio transformation announcement on April 22, 2014, was $77 million.
F-49
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Goodwill and Intangible Assets Movements (Continued)
The Pharmaceuticals, Alcon and Sandoz divisions' cash generating units, to which indefinite life intangibles and/or goodwill are allocated, each comprise a group of smaller cash generating units. The valuation method of the recoverable amount of the cash generating units, to which indefinite life intangibles and/or goodwill are allocated, is based on the fair value less costs of disposal. The following assumptions are used in the calculations:
| |
Pharmaceutical | Alcon | Sandoz | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
% |
% |
% |
|||||||
Cash flows growth rate assumptions after forecast period |
1 | 3 | 0 to 2 | |||||||
Discount rate (post-tax) |
6 | 6 | 6 | |||||||
In 2015, intangible asset impairment charges for continuing operations of $206 million were recognized, of which $120 million were recorded in the Alcon Division and $86 million in total in the Pharmaceuticals and Sandoz divisions.
In 2014, intangible asset impairment charges in continuing operations amounted to $347 million ($302 million in the Pharmaceuticals Division and $45 million in total in the Sandoz and Alcon divisions).
In 2015 the reversal of prior year impairment charges amounted to $40 million (2014: $70 million).
F-50
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Deferred Tax Assets and Liabilities
| |
Property, plant & equipment |
Intangible assets |
Pensions and other benefit obligations of associates |
Inventories | Tax loss carryforwards |
Other assets, provisions and accruals |
Valuation allowance |
Total | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||||||
Gross deferred tax assets at January 1, 2015 |
268 | 214 | 1,749 | 3,470 | 85 | 2,601 | (14 | ) | 8,373 | ||||||||||||||||
Gross deferred tax liabilities at January 1, 2015 |
(639 | ) | (4,242 | ) | (410 | ) | (578 | ) | (3 | ) | (606 | ) | (6,478 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net deferred tax balance at January 1, 2015 |
(371 | ) | (4,028 | ) | 1,339 | 2,892 | 82 | 1,995 | (14 | ) | 1,895 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
At January 1, 2015 |
(371 | ) | (4,028 | ) | 1,339 | 2,892 | 82 | 1,995 | (14 | ) | 1,895 | ||||||||||||||
Credited/(charged) to income |
(57 | ) | 296 | 83 | 376 | (22 | ) | (129 | ) | (3 | ) | 544 | |||||||||||||
Charged to equity |
(216 | ) | (216 | ) | |||||||||||||||||||||
(Charged)/credited to other comprehensive income |
(63 | ) | 29 | (34 | ) | ||||||||||||||||||||
Impact of business combinations |
390 | (13 | ) | 377 | |||||||||||||||||||||
Other movements |
5 | (9 | ) | (30 | ) | (12 | ) | (3 | ) | 73 | 12 | 36 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net deferred tax balance at December 31, 2015 |
(423 | ) | (3,351 | ) | 1,329 | 3,256 | 57 | 1,739 | (5 | ) | 2,602 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross deferred tax assets at December 31, 2015 |
216 | 611 | 1,730 | 3,821 | 62 | 2,871 | (5 | ) | 9,306 | ||||||||||||||||
Gross deferred tax liabilities at December 31, 2015 |
(639 | ) | (3,962 | ) | (401 | ) | (565 | ) | (5 | ) | (1,132 | ) | (6,704 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net deferred tax balance at December 31, 2015 |
(423 | ) | (3,351 | ) | 1,329 | 3,256 | 57 | 1,739 | (5 | ) | 2,602 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
After offsetting $349 million of deferred tax assets and liabilities within the same tax jurisdiction the balance amounts to: |
|||||||||||||||||||||||||
Deferred tax assets at December 31, 2015 |
8,957 | ||||||||||||||||||||||||
Deferred tax liabilities at December 31, 2015 |
(6,355 | ) | |||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net deferred tax balance at December 31, 2015 |
2,602 | ||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross deferred tax assets at January 1, 2014 |
159 | 270 | 1,515 | 3,026 | 142 | 2,651 | (22 | ) | 7,741 | ||||||||||||||||
Gross deferred tax liabilities at January 1, 2014 |
(886 | ) | (4,796 | ) | (448 | ) | (514 | ) | (4 | ) | (622 | ) | (7,270 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net deferred tax balance at January 1, 2014 |
(727 | ) | (4,526 | ) | 1,067 | 2,512 | 138 | 2,029 | (22 | ) | 471 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
At January 1, 2014 |
(727 | ) | (4,526 | ) | 1,067 | 2,512 | 138 | 2,029 | (22 | ) | 471 | ||||||||||||||
Net deferred tax balance related to discontinued operations |
39 | 92 | (73 | ) | (40 | ) | (19 | ) | (93 | ) | (94 | ) | |||||||||||||
Credited/(charged) to income |
256 | 525 | 17 | 395 | (60 | ) | (60 | ) | (5 | ) | 1,068 | ||||||||||||||
Credited to equity |
157 | 157 | |||||||||||||||||||||||
Credited/(charged) to other comprehensive income |
389 | (8 | ) | 381 | |||||||||||||||||||||
Impact of business combinations |
(159 | ) | 30 | (1 | ) | (130 | ) | ||||||||||||||||||
Other movements |
61 | 40 | (61 | ) | 25 | (7 | ) | (29 | ) | 13 | 42 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net deferred tax balance at December 31, 2014 |
(371 | ) | (4,028 | ) | 1,339 | 2,892 | 82 | 1,995 | (14 | ) | 1,895 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross deferred tax assets at December 31, 2014 |
268 | 214 | 1,749 | 3,470 | 85 | 2,601 | (14 | ) | 8,373 | ||||||||||||||||
Gross deferred tax liabilities at December 31, 2014 |
(639 | ) | (4,242 | ) | (410 | ) | (578 | ) | (3 | ) | (606 | ) | (6,478 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net deferred tax balance at December 31, 2014 |
(371 | ) | (4,028 | ) | 1,339 | 2,892 | 82 | 1,995 | (14 | ) | 1,895 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
After offsetting $379 million of deferred tax assets and liabilities within the same tax jurisdiction the balance amounts to: |
|||||||||||||||||||||||||
Deferred tax assets at December 31, 2014 |
7,994 | ||||||||||||||||||||||||
Deferred tax liabilities at December 31, 2014 |
(6,099 | ) | |||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net deferred tax balance at December 31, 2014 |
1,895 | ||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
F-51
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Deferred Tax Assets and Liabilities (Continued)
A reversal of valuation allowance could occur when circumstances make the realization of deferred taxes probable. This would result in a decrease in the Group's effective tax rate.
Deferred tax assets of $3.9 billion (2014: $3.6 billion) and deferred tax liabilities of $5.8 billion (2014: $5.6 billion) are expected to have an impact on current taxes payable after more than twelve months.
At December 31, 2015, unremitted earnings of $65 billion (2014: $55 billion) have been retained by consolidated entities for reinvestment. Therefore, no provision is made for income taxes that would be payable upon the distribution of these earnings. If these earnings were remitted, an income tax charge could result based on the tax statutes currently in effect.
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Temporary differences on which no deferred tax has been provided as they are permanent in nature related to: |
|||||||
—Investments in subsidiaries |
2,644 | 7,802 | |||||
—Goodwill from acquisitions |
(28,202 | ) | (28,567 | ) | |||
The gross value of tax-loss carry-forwards that have, or have not, been capitalized as deferred tax assets, with their expiry dates is as follows:
| |
Not capitalized | Capitalized | 2015 total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
One year |
22 | 39 | 61 | |||||||
Two years |
80 | 25 | 105 | |||||||
Three years |
37 | 6 | 43 | |||||||
Four years |
54 | 7 | 61 | |||||||
Five years |
222 | 222 | ||||||||
More than five years |
465 | 712 | 1,177 | |||||||
| | | | | | | | | | | |
Total |
880 | 789 | 1,669 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
In 2015, $13 million (2014: $14 million, 2013: $181 million) of tax-loss carry-forwards expired.
| |
Not capitalized | Capitalized | 2014 total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
One year |
12 | 3 | 15 | |||||||
Two years |
22 | 26 | 48 | |||||||
Three years |
14 | 14 | ||||||||
Four years |
13 | 5 | 18 | |||||||
Five years |
52 | 8 | 60 | |||||||
More than five years |
345 | 396 | 741 | |||||||
| | | | | | | | | | | |
Total |
458 | 438 | 896 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-52
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Deferred Tax Assets and Liabilities (Continued)
Deferred tax assets related to taxable losses of relevant Group entities are recognized to the extent it is considered probable that future taxable profits will be available against which such losses can be utilized in the foreseeable future.
13. Financial and Other Non-Current Assets
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Available-for-sale long-term financial investments |
1,263 | 1,008 | |||||
Long-term receivables from customers |
317 | 334 | |||||
Minimum lease payments from finance lease agreements |
216 | 199 | |||||
Contingent consideration receivables |
550 | ||||||
Long-term loans, advances and security deposits |
120 | 179 | |||||
| | | | | | | | |
Total financial assets |
2,466 | 1,720 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Deferred compensation plans |
409 | 381 | |||||
Prepaid post-employment benefit plans |
36 | 37 | |||||
Other non-current assets |
156 | 136 | |||||
| | | | | | | | |
Total other non-current assets |
601 | 554 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Minimum Finance Lease Payments
The following table shows the receivables of the gross investments in finance leases and the net present value of the minimum lease payments, as well as unearned finance income. The finance income is recorded in "Other income".
| |
2015 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
$ m
|
Total future payments |
Unearned interest income |
Present value |
Provision | Net book value |
|||||||||||
Not later than one year(1) |
89 | (6 | ) | 83 | (1 | ) | 82 | |||||||||
Between one and five years |
221 | (17 | ) | 204 | (10 | ) | 194 | |||||||||
Later than five years |
61 | (5 | ) | 56 | (34 | ) | 22 | |||||||||
| | | | | | | | | | | | | | | | | |
Total |
371 | (28 | ) | 343 | (45 | ) | 298 | |||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- The current portion of the minimum lease payments is recorded in trade receivables or other current assets (to the extent not yet invoiced).
F-53
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. Financial and Other Non-Current Assets (Continued)
| |
2014 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
$ m
|
Total future payments |
Unearned interest income |
Present value |
Provision | Net book value |
|||||||||||
Not later than one year(1) |
50 | (3 | ) | 47 | (1 | ) | 46 | |||||||||
Between one and five years |
149 | (8 | ) | 141 | (6 | ) | 135 | |||||||||
Later than five years |
69 | (5 | ) | 64 | 64 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
268 | (16 | ) | 252 | (7 | ) | 245 | |||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- The current portion of the minimum lease payments is recorded in trade receivables or other current assets (to the extent not yet invoiced).
14. Inventories
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Raw material, consumables |
658 | 756 | |||||
Finished products and work in progress |
5,568 | 5,337 | |||||
| | | | | | | | |
Total inventories |
6,226 | 6,093 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The amount of inventory recognized as an expense in "Cost of goods sold" in the consolidated income statements during 2015 amounted to $10.5 billion (2014: $11.6 billion, 2013: $13.3 billion). The group recognized inventory provisions amounting to $356 billion (2014: $1.1 billion, 2013: $1.4 billion) and reversed inventory provisions amounting to $148 million (2014: $379 million, 2013: $474 million).
The reversals mainly result from the release of products initially requiring additional quality control inspections and from the reassessment of inventory values manufactured prior to regulatory approval but for which approval was subsequently received.
15. Trade Receivables
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Total gross trade receivables |
8,322 | 8,431 | |||||
Provisions for doubtful trade receivables |
(142 | ) | (156 | ) | |||
| | | | | | | | |
Total trade receivables, net |
8,180 | 8,275 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
F-54
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
15. Trade Receivables (Continued)
The following table summarizes the movement in the provision for doubtful trade receivables:
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
January 1 |
(156 | ) | (195 | ) | (217 | ) | ||||
Provisions for doubtful trade receivables related to discontinued operations |
15 | 1 | ||||||||
Provisions for doubtful trade receivables charged to the consolidated income statement |
(68 | ) | (92 | ) | (98 | ) | ||||
Utilization or reversal of provisions for doubtful trade receivables |
71 | 101 | 120 | |||||||
Currency translation effects |
11 | 15 | (1 | ) | ||||||
| | | | | | | | | | | |
December 31 |
(142 | ) | (156 | ) | (195 | ) | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The following sets forth details of the age of trade receivables that are not overdue as specified in the payment terms and conditions established with Novartis customers as well as an analysis of overdue amounts and related provisions for doubtful trade receivables:
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Not overdue |
7,318 | 7,406 | |||||
Past due for not more than one month |
265 | 334 | |||||
Past due for more than one month but less than three months |
255 | 275 | |||||
Past due for more than three months but less than six months |
193 | 174 | |||||
Past due for more than six months but less than one year |
156 | 102 | |||||
Past due for more than one year |
135 | 140 | |||||
Provisions for doubtful trade receivables |
(142 | ) | (156 | ) | |||
| | | | | | | | |
Total trade receivables, net |
8,180 | 8,275 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Trade receivable balances include sales to drug wholesalers, retailers, private health systems, government agencies, managed care providers, pharmacy benefit managers and government-supported healthcare systems. Novartis continues to monitor sovereign debt issues and economic conditions in Greece, Italy, Portugal, Spain (GIPS) and other countries where the trade receivables are due directly from local governments or from government-funded entities, and evaluates trade receivables in these countries for potential collection risks. Deteriorating credit and economic conditions and other factors in these countries have resulted in, and may continue to result in an increase in the average length of time that it takes to collect these trade receivables and may require Novartis to re-evaluate the collectability of these trade receivables in future periods.
With regard to the GIPS countries, the majority of the outstanding trade receivables from these countries are due directly from local governments or from government-funded entities. The gross trade receivables from GIPS countries at December 31, 2015 amount to $920 million (2014: $915 million), of which $58 million are past due for more than one year (2014: $69 million) and for which provisions of $37 million have been recorded (2014: $48 million). At December 31, 2015 amounts past due for more than one year are not significant in any of the GIPS countries on a standalone basis.
F-55
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
15. Trade Receivables (Continued)
Trade receivables include amounts denominated in the following major currencies:
Currency
|
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
CHF |
124 | 184 | |||||
CNY |
244 | 238 | |||||
EUR |
1,536 | 1,562 | |||||
GBP |
187 | 184 | |||||
JPY |
740 | 951 | |||||
$ |
3,311 | 3,059 | |||||
Other |
2,038 | 2,097 | |||||
| | | | | | | | |
Total trade receivables, net |
8,180 | 8,275 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
16. Marketable Securities, Commodities, Time Deposits, Derivative Financial Instruments and Cash and Cash Equivalents
Marketable Securities, Commodities, Time Deposits and Derivative Financial Instruments |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Debt securities |
339 | 327 | |||||
Equity securities |
6 | 15 | |||||
Fund investments |
33 | 35 | |||||
| | | | | | | | |
Total available-for-sale marketable securities |
378 | 377 | |||||
Commodities |
86 | 97 | |||||
Time deposits with original maturity more than 90 days |
164 | 6 | |||||
Derivative financial instruments |
143 | 356 | |||||
Accrued interest on debt securities and time deposits |
2 | 3 | |||||
| | | | | | | | |
Total marketable securities, commodities, time deposits and derivative financial instruments |
773 | 839 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
At December 31, 2015 all debt securities are denominated in $ except for $22 million in EUR (2014: $25 million). In addition, at December 31, 2014 debt securities of $1 million are denominated in CHF.
Cash and Cash Equivalents
|
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Current accounts |
3,074 | 3,607 | |||||
Time deposits and short-term investments with original maturity less than 90 days |
1,600 | 9,416 | |||||
| | | | | | | | |
Total cash and cash equivalents |
4,674 | 13,023 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
F-56
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
17. Other Current Assets
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
VAT receivable |
609 | 509 | |||||
Withholding tax recoverable |
97 | 144 | |||||
Income tax receivables |
171 | 202 | |||||
Reimbursements from insurers |
87 | ||||||
Prepaid expenses |
|||||||
—Third parties |
617 | 547 | |||||
—Associated companies |
4 | 3 | |||||
Other receivables |
|||||||
—Third parties |
1,463 | 1,033 | |||||
—Associated companies |
31 | 5 | |||||
| | | | | | | | |
Total other current assets |
2,992 | 2,530 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
18. Details of Share Capital and Share Movements
The following table shows the movement in the share capital:
| |
Dec 31, 2013 | Movement in year |
Dec 31, 2014 | Movement in year |
Dec 31, 2015 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Share capital |
1,001 | 1,001 | (10 | ) | 991 | |||||||||||
Treasury shares |
(89 | ) | (14 | ) | (103 | ) | 2 | (101 | ) | |||||||
| | | | | | | | | | | | | | | | | |
Outstanding share capital |
912 | (14 | ) | 898 | (8 | ) | 890 | |||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
The following table shows the movement in the shares:
| |
Number of shares(1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Dec 31, 2013 | Movement in year |
Dec 31, 2014 | Movement in year |
Dec 31, 2015 | |||||||||||
Total Novartis shares |
2,706,193,000 | 2,706,193,000 | (29,200,000 | ) | 2,676,993,000 | |||||||||||
Total treasury shares |
(280,108,692 | ) | (27,458,051 | ) | (307,566,743 | ) | 4,468,560 | (303,098,183 | ) | |||||||
| | | | | | | | | | | | | | | | | |
Total outstanding shares |
2,426,084,308 | (27,458,051 | ) | 2,398,626,257 | (24,731,440 | ) | 2,373,894,817 | |||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- All shares are voting shares, which are registered, authorized, issued and fully paid.
In 2015, Novartis reduced its share capital by cancelling a total of 29.2 million shares which were repurchased during 2013 and 2014 on the SIX Swiss Exchange second trading line.
During 2015, 38.9 million treasury shares were delivered as a result of options being exercised and physical share deliveries related to equity-based participation plans (2014: 51.7 million shares). 9.6 million shares were repurchased on the SIX Swiss Exchange first trading line (2014: 46.8 million). 4.1 million
F-57
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
18. Details of Share Capital and Share Movements (Continued)
shares were acquired from employees which were previously granted to them under the respective programs (2014: 5.4 million). In addition, Novartis repurchased 49.9 million shares on the SIX Swiss Exchange second trading line under the $5 billion share buy-back announced in November 2013, which was completed in November 2015, and also to offset the dilutive impact from equity-based participation plans (2014: 27.0 million shares). With these transactions, the total number of shares outstanding was reduced by 24.7 million shares in 2015 (2014: reduction of 27.5 million shares) and the sixth share buy-back program which was approved by the shareholders at the AGM 2008 has been completed. The market maker has acquired 7 million written call options, originally issued as part of the share-based compensation for associates that have not yet been exercised. The weighted average exercise price of these options is $58.27 and they have contractual lives of 10 years.
F-58
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
19. Non-Current Financial Debt
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Straight bonds |
17,193 | 15,982 | |||||
Liabilities to banks and other financial institutions(1) |
706 | 803 | |||||
Finance lease obligations |
87 | 3 | |||||
| | | | | | | | |
Total, including current portion of non-current financial debt |
17,986 | 16,788 | |||||
Less current portion of non-current financial debt |
(1,659 | ) | (2,989 | ) | |||
| | | | | | | | |
Total non-current financial debts |
16,327 | 13,799 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Straight bonds |
|||||||
3.625% CHF 800 million bond 2008/2015 of Novartis AG, Basel, Switzerland, issued at 100.35% |
807 | ||||||
5.125% $3,000 million bond 2009/2019 of Novartis Securities Investment Ltd., Hamilton, Bermuda, issued at 99.822% |
2,993 | 2,991 | |||||
4.25% EUR 1,500 million bond 2009/2016 of Novartis Finance S.A., Luxembourg, Luxembourg, issued at 99.757% |
1,639 | 1,821 | |||||
2.9% $2,000 million bond 2010/2015 of Novartis Capital Corporation, New York, United States, issued at 99.522% |
1,999 | ||||||
4.4% $1,000 million bond 2010/2020 of Novartis Capital Corporation, New York, United States, issued at 99.237% |
994 | 993 | |||||
2.4% $1,500 million bond 2012/2022 of Novartis Capital Corporation, New York, United States, issued at 99.225% |
1,488 | 1,486 | |||||
3.7% $500 million bond 2012/2042 of Novartis Capital Corporation, New York, United States, issued at 98.325% |
488 | 488 | |||||
3.4% $2,150 million bond 2014/2024 of Novartis Capital Corporation, New York, United States, issued at 99.287% |
2,130 | 2,128 | |||||
4.4% $1,850 million bond 2014/2044 of Novartis Capital Corporation, New York, United States, issued at 99.196% |
1,823 | 1,823 | |||||
0.75% EUR 600 million bond 2014/2021 of Novartis Finance S.A., Luxembourg, Luxembourg, issued at 99.134% |
650 | 721 | |||||
1.625% EUR 600 million bond 2014/2026 of Novartis Finance S.A., Luxembourg, Luxembourg, issued at 99.697% |
652 | 725 | |||||
0.25% CHF 500 million bond 2015/2025 of Novartis AG, Basel, Switzerland, issued at 100.64% |
507 | ||||||
0.625% CHF 550 million bond 2015/2029 of Novartis AG, Basel, Switzerland, issued at 100.502% |
557 | ||||||
1.050% CHF 325 million bond 2015/2035 of Novartis AG, Basel, Switzerland, issued at 100.479% |
329 | ||||||
3.0% $1,750 million bond 2015/2025 of Novartis Capital Corporation, New York, United States, issued at 99.010% |
1,726 | ||||||
4.0% $1,250 million bond 2015/2045 of Novartis Capital Corporation, New York, United States, issued at 98.029% |
1,217 | ||||||
| | | | | | | | |
Total straight bonds |
17,193 | 15,982 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- (1)
- Average interest rate 0.7% (2014: 0.9%)
F-59
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
19. Non-Current Financial Debt (Continued)
The following tables provide a breakdown of total non-current financial debt, including current portion by maturity and currency:
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Breakdown by maturity |
|||||||
2015 |
2,989 | ||||||
2016 |
1,659 | 1,838 | |||||
2017 |
170 | 175 | |||||
2018 |
335 | 342 | |||||
2019 |
3,161 | 3,068 | |||||
2020 |
998 | 1,004 | |||||
After 2020 |
11,663 | 7,372 | |||||
| | | | | | | | |
Total |
17,986 | 16,788 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Breakdown by currency |
|||||||
$ |
12,946 | 11,912 | |||||
EUR |
2,981 | 3,329 | |||||
JPY |
665 | 669 | |||||
CHF |
1,393 | 807 | |||||
Others |
1 | 71 | |||||
| | | | | | | | |
Total |
17,986 | 16,788 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Fair value comparison
|
2015 Balance sheet |
2015 Fair values |
2014 Balance sheet |
2014 Fair values |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
|||||||||
Straight bonds |
17,193 | 17,770 | 15,982 | 17,013 | |||||||||
Others |
793 | 793 | 806 | 806 | |||||||||
| | | | | | | | | | | | | | |
Total |
17,986 | 18,563 | 16,788 | 17,819 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The fair values of straight bonds are determined by quoted market prices. Other financial debts are recorded at notional amounts which are a reasonable approximation of the fair values.
Collateralized non-current financial debt and pledged assets
|
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Total amount of collateralized non-current financial debts |
7 | 1 | |||||
Total net book value of property, plant & equipment pledged as collateral for non-current financial debts |
112 | 184 | |||||
F-60
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
19. Non-Current Financial Debt (Continued)
The Group's collateralized non-current financial debt consists of loan facilities at usual market conditions.
The percentage of fixed rate financial debt to total financial debt was 82% at December 31, 2015 and December 31, 2014.
Financial debts, including current financial debts, contain only general default covenants. The Group is in compliance with these covenants.
The average interest rate on total financial debt in 2015 was 2.9% (2014: 3.4%, 2013: 3.3%).
20. Provisions and Other Non-Current Liabilities
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Accrued liability for employee benefits: |
|||||||
Defined benefit pension plans |
3,952 | 3,839 | |||||
Other long-term employee benefits and deferred compensation |
507 | 518 | |||||
Other post-employment benefits |
960 | 1,054 | |||||
Environmental remediation provisions |
791 | 828 | |||||
Provisions for product liabilities, governmental investigations and other legal matters |
451 | 521 | |||||
Contingent consideration |
712 | 465 | |||||
Other non-current liabilities |
671 | 447 | |||||
| | | | | | | | |
Total |
8,044 | 7,672 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Environmental Remediation Provisions
The material components of the environmental remediation provisions consist of costs to sufficiently clean and refurbish contaminated sites to the extent necessary and to treat and where necessary continue surveillance at sites where the environmental remediation exposure is less significant. The provision recorded at December 31, 2015 totals $0.9 billion (2014: $0.9 billion) of which $80 million (2014: $95 million) is current.
A substantial portion of the environmental remediation provisions relate to the remediation of Basel regional landfills in the adjacent border areas in Switzerland, Germany and France. The provisions are re-assessed on a yearly basis and are adjusted as necessary.
In the United States, Novartis has been named under federal legislation (the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended) as a potentially responsible party (PRP) in respect of certain sites. Novartis actively participates in, or monitors, the clean-up activities at the sites in which it is a PRP. The provision takes into consideration the number of other PRPs at each site and the identity and financial position of such parties in light of the joint and several nature of the liability.
F-61
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. Provisions and Other Non-Current Liabilities (Continued)
The following table shows the movements in the environmental liability provisions during 2015, 2014 and 2013:
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
January 1 |
923 | 1,061 | 1,120 | |||||||
Cash payments |
(52 | ) | (33 | ) | (68 | ) | ||||
Releases |
(5 | ) | (6 | ) | (19 | ) | ||||
Additions |
6 | 2 | 2 | |||||||
Currency translation effects |
(1 | ) | (101 | ) | 26 | |||||
| | | | | | | | | | | |
December 31 |
871 | 923 | 1,061 | |||||||
Less current provision |
(80 | ) | (95 | ) | (100 | ) | ||||
| | | | | | | | | | | |
Non-current environmental remediation provisions at December 31 |
791 | 828 | 961 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The expected timing of the related cash outflows as of December 31, 2015 is currently projected as follows:
| |
Expected cash outflows |
|||
|---|---|---|---|---|
| |
$ m |
|||
Due within two years |
180 | |||
Due later than two years, but within five years |
118 | |||
Due later than five years but within ten years |
457 | |||
Due after ten years |
116 | |||
| | | | | |
Total environmental remediation liability provisions |
871 | |||
| | | | | |
| | | | | |
| | | | | |
Provisions for Product Liabilities, Governmental Investigations and Other Legal Matters
Novartis has established provisions for certain product liabilities, governmental investigations and other legal matters, including provisions for expected legal costs where a potential cash outflow is probable and Novartis can make a reliable estimate of the amount of the outflow. These provisions represent the Group's current best estimate of the total financial effect for the matters listed below and for other less significant matters. Potential cash outflows reflected in a provision might be fully or partially off-set by insurance in certain circumstances. Novartis has not established provisions for potential damage awards for certain additional legal claims against our subsidiaries if Novartis currently believes that a payment is either not probable or cannot be reliably estimated. In total, these not-provisioned-for matters include fewer than 500 individual product liability cases and certain other legal matters. Plaintiffs' alleged claims in these matters, which Novartis does not believe to be entirely remote but which do not fulfill the conditions for the establishment of provisions, currently aggregate to, according to Novartis' current best belief, approximately $1.2 billion. In addition, in some of these matters there are claims for punitive or multiple (treble) damages, civil penalties and disgorgement of profits that in Novartis' view are either wholly or partially unspecified or wholly or partially unquantifiable at present; the Group believes that
F-62
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. Provisions and Other Non-Current Liabilities (Continued)
information about these amounts claimed by plaintiffs generally is not meaningful for purposes of determining a reliable estimate of a loss that is probable or more than remote. A number of other legal matters are in such early stages or the issues presented are such that the Group has not made any provisions other than for legal fees since it cannot currently estimate either a potential outcome or the amount of any potential losses. For these reasons, among others, the Group generally is unable to make a reliable estimate of possible loss with respect to such cases. It is therefore not practicable to provide information about the potential financial impact of those cases. There might also be cases for which the Group was able to make a reliable estimate of the possible loss or the range of possible loss, but the Group believes that publication of such information on a case-by-case basis would seriously prejudice the Group's position in ongoing legal proceedings or in any related settlement discussions. Accordingly, in such cases, information has been disclosed with respect to the nature of the contingency, but no disclosure is provided as to an estimate of the possible loss or range of possible loss.
A number of Novartis companies are, and will likely continue to be, subject to various legal proceedings and investigations that arise from time to time, including proceedings regarding product liability, sales and marketing practices, commercial disputes, employment, and wrongful discharge, antitrust, securities, health and safety, environmental, tax, international trade, privacy, and intellectual property matters. As a result, the Group may become subject to substantial liabilities that may not be covered by insurance and could affect our business and reputation. While Novartis does not believe that any of these legal proceedings will have a material adverse effect on its financial position, litigation is inherently unpredictable and large judgments sometimes occur. As a consequence, Novartis may in the future incur judgments or enter into settlements of claims that could have a material adverse effect on its results of operations or cash flow.
Governments and regulatory authorities around the world have been stepping up their compliance and law enforcement activities in recent years in key areas, including marketing practices, pricing, corruption, trade restrictions, embargo legislation, insider trading, antitrust, cyber security and data privacy. Further, when one government or regulatory authority undertakes an investigation, it is not uncommon for other governments or regulators to undertake investigations regarding the same or similar matters. Responding to such investigations is costly and requires an increasing amount of management's time and attention. In addition, such investigations may affect our reputation, create a risk of potential exclusion from government reimbursement programs in the US and other countries, and may lead to (or arise from) litigation. These factors have contributed to decisions by Novartis and other companies in the healthcare industry, when deemed in their interest, to enter into settlement agreements with governmental authorities around the world prior to any formal decision by the authorities or a court. Those government settlements have involved and may continue to involve, in current government investigations and proceedings, large cash payments, sometimes in the hundreds of millions of dollars or more, including the potential repayment of amounts allegedly obtained improperly and other penalties, including treble damages. In addition, settlements of government healthcare fraud cases often require companies to enter into corporate integrity agreements, which are intended to regulate company behavior for a period of years. Our affiliate Novartis Pharmaceuticals Corporation is a party to such an agreement, which will expire in 2020. Also, matters underlying governmental investigations and settlements may be the subject of separate private litigation.
F-63
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. Provisions and Other Non-Current Liabilities (Continued)
The following is a summary of significant legal proceedings to which Novartis or its subsidiaries are a party or were a party and that concluded in 2015.
Investigations and related litigations
Southern District of New York (SDNY) marketing practices investigation and litigation
In April 2013, the US government filed a civil complaint in intervention to an individual qui tam action against Novartis Pharmaceuticals Corporation (NPC) in the United States District Court (USDC) for the SDNY involving several of NPC's cardiovascular medications. The suit is related to a previously disclosed 2011 investigation of the United States Attorney's Office (USAO) for the SDNY relating to marketing practices, including the remuneration of healthcare providers, in connection with three NPC products (Lotrel, Starlix and Valturna). The complaint, as subsequently amended, asserts federal False Claims Act and common law claims with respect to speaker programs and other promotional activities for certain NPC cardiovascular medications allegedly serving as mechanisms to provide kickbacks to healthcare professionals. It seeks unspecified damages, which according to the complaint are "substantial", including treble damages and maximum civil penalties per claim, as well as disgorgement of Novartis profits from the alleged unlawful conduct. In August 2013, New York State filed a civil complaint in intervention asserting similar claims. Neither government complaint in intervention adopted the individual relator's claims with respect to off-label promotion of Valturna, which were subsequently dismissed with prejudice by the court. The individual relator continues to litigate the kickback claims on behalf of other states and municipalities. NPC vigorously contests the SDNY, New York State and individual claims, both as to alleged liability and amount of damages and penalties.
SDNY / Western District of New York healthcare fraud investigation
In 2011, Alcon Laboratories, Inc. (ALI) received a subpoena from the United States Department of Health & Human Services relating to an investigation into allegations of healthcare fraud. The subpoena requests the production of documents relating to marketing practices, including the remuneration of healthcare providers, in connection with certain ALI products (Vigamox, Nevanac, Omnipred, Econopred; surgical equipment). ALI is cooperating with the investigation, which is civil in nature.
Northern District of Texas (NDTX) investigation
In 2012, Alcon was notified that the USAO for the NDTX is conducting an investigation relating to the export of Alcon products to various countries subject to United States trade sanctions, including Iran, allegedly in violation of applicable trade sanctions, and received a grand jury subpoena requesting the production of documents for a period beginning in 2005 relating to this investigation. Alcon is cooperating with the investigation.
SDNY Gilenya investigation
In 2013, NPC received a civil investigative demand from the USAO for the SDNY requesting the production of documents and information relating to marketing practices for Gilenya, including the remuneration of healthcare providers in connection therewith. NPC is cooperating with this civil investigation.
F-64
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. Provisions and Other Non-Current Liabilities (Continued)
New York state investigation
In November 2014, ALI received a civil subpoena from the New York state attorney general relating to an investigation into a unilateral pricing policy program. ALI is cooperating with this civil investigation.
Lucentis/Avastin® matters in Italy and France
In 2013, the Italian Competition Authority (ICA) opened an investigation to assess whether Novartis Farma S.p.A., Novartis AG (NAG), F. Hoffmann-La Roche AG, Genentech Inc. and Roche S.p.A. colluded to artificially preserve the market positions of Avastin® and Lucentis. In March 2014, the ICA imposed a fine equivalent to $125 million on NAG and Novartis Farma S.p.A. and a fine on F. Hoffmann-La Roche AG and Roche S.p.A. equivalent to $122 million. As required by Italian law, Novartis has paid the ICA fine, subject to the right to later claim recoupment. In February 2015, Novartis appealed at the council of state the decision of the Tribunale amministrativo regionale (TAR) del Lazio which had upheld the fines. The decision is pending. Novartis' appeal of a decision by the Italian Medicines Agency to include Avastin® in a list of drugs to be reimbursed off-label for age-related macular degeneration (AMD) was rejected by the TAR Lazio in January 2016. Novartis will appeal this decision. In the second quarter of 2014, the Italian Ministry of Health (MoH) indicated in a letter that it intended to seek a total equivalent of approximately $1.3 billion in damages from Novartis and Roche entities based on the above allegations, and in the first quarter of 2015 the Lombardia region sent a payment request equivalent to approximately $63 million. Novartis vigorously contests the MoH and Lombardia claims.
In France, Novartis' appeal is pending against an inspection in April 2014 by the French Competition Authority on the premises of Novartis Groupe France and Roche with respect to the French market for anti-vascular endothelial growth factor (VEGF) products indicated for the treatment of wet AMD. Also in France, Novartis is appealing a temporary recommendation of use and reimbursement of off-label Avastin® for neovascular AMD by hospital ophthalmologists, in force since September 2015, as well as the decree on which the recommendation is based. In both Italy and France, Novartis believes that allowing the widespread off-label use and reimbursement of Avastin®, despite the presence of available licensed alternatives, would result in a breach of applicable regulations.
Japan investigation
In December 2015, trial started against a former Novartis Pharma K.K. (NPKK) employee, and also NPKK under the dual liability concept in Japanese law, over allegations brought by the Tokyo District Public Prosecutor Office in two counts for alleged manipulation of data in sub-analysis publications of the Kyoto Heart Study regarding valsartan. The charges against NPKK are subject to a maximum total fine of JPY 4 million.
In February 2015, the Japanese Ministry of Health, Labor and Welfare (MHLW) issued a business suspension order for failure to report adverse events, which required NPKK to halt manufacturing and sales in Japan for the period from March 5 to 19, 2015. NPKK has implemented a corrective and preventive action plan in response to a business improvement order and instruction issued by the MHLW in the fourth quarter of 2015 regarding additional instances of delayed adverse events reporting.
F-65
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. Provisions and Other Non-Current Liabilities (Continued)
Internal travel agencies investigation
After reports of Chinese government investigations of competitors for alleged improper use of certain China-based travel agencies to reward healthcare providers, Novartis commenced an internal investigation in 2013 concerning its local affiliates' relationships with China-based travel agencies (and other vendors). Novartis is communicating with the US Securities and Exchange Commission (SEC) about this internal investigation.
Italy MF59 investigation
In May 2014, the public prosecutor of Siena initiated a criminal investigation with respect to allegations that the transfer price of the adjuvant MF59 was unlawfully marked up. The investigation concerns whether the Focetria and Fluad vaccines sold to the government were over-priced and whether the Italian Ministry of Health paid an inflated amount in a dispute settlement relating to the supply of Focetria during the 2009 pandemic.
Product liability matters
Reclast/Aclasta product liability litigation
NPC is a defendant in 21 US product liability actions involving Reclast and alleging atypical femur fracture injuries, most of which are in New Jersey state or federal court coordinated with claims against other bisphosphonate manufacturers. There are also three Canadian putative class actions brought against numerous bisphosphonate manufacturers including NPC, Novartis Pharmaceuticals Canada Inc. and Novartis International AG in Quebec, Alberta and Saskatchewan. All claims are being vigorously contested.
Metoclopramide product liability litigation
Sandoz is a defendant, along with numerous manufacturers of brand pharmaceuticals, in 395 product liability actions in the state courts in Pennsylvania and California claiming that the use of metoclopramide, the generic version of the brand name drug Reglan®, caused personal injuries including tardive dyskinesia. Sandoz denies the allegations and is vigorously contesting the claims.
Tekturna/Rasilez/Valturna product liability litigation
NPC and certain other Novartis affiliates are defendants in 12 individual lawsuits pending in the USDC for the District of New Jersey (DNJ), and one in Alberta, Canada, claiming that treatment with Tekturna, Rasilez and/or Valturna caused renal failure, kidney disease or stroke. The claims are being vigorously contested.
Arbitration
Equa arbitration
In 2013, Sanofi K.K. (Sanofi) commenced an arbitration against NPKK relating to the termination of a co-promotion agreement in Japan of Equa (Galvus), which is used to treat type 2 diabetes. Sanofi seeks an award equivalent to $356 million, at a minimum, together with a request for payment of additional
F-66
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. Provisions and Other Non-Current Liabilities (Continued)
interest and expenses as well as legal and other costs of the proceedings. NPKK is vigorously defending the action as well as prosecuting a counterclaim against Sanofi.
Other matters
Average Wholesale Price (AWP) litigation
Claims have been brought by various US state governmental entities against various pharmaceutical companies, including certain Sandoz entities and NPC, alleging that they fraudulently overstated the AWP that is or has been used by payors, including state Medicaid agencies, to calculate reimbursements to healthcare providers. NPC and Sandoz reached settlements in the first, third, and fourth quarters of 2015 of the Wisconsin and Utah claims against them for amounts that are not material to Novartis. Sandoz has filed a motion for reconsideration against a Mississippi Supreme Court decision which in the fourth quarter of 2015 upheld the $30 million Chancery Court verdict against it. NPC remains a defendant in an action brought by the state of Illinois and in a putative class action brought by private payors in New Jersey. The claims are being vigorously contested.
Qui tam actions
NPC is a defendant in a relator's qui tam action in the USDC for the Eastern District of Pennsylvania asserting federal and state False Claims Act claims relating to certain alleged marketing practices involving Elidel®. The federal government and several states declined to intervene in the relator's action. NPC is vigorously contesting the claims.
In 2006, 2010 and 2012, qui tam complaints were filed in the District of Massachusetts (D. Mass.) asserting various federal False Claims Act and state claims relating to certain alleged improper marketing practices involving Xolair against various Novartis, Genentech and Roche entities. In 2011, the US and various state governments declined to intervene in the relators' actions, and closed their investigations. In June 2014, the relator in the 2010 action voluntarily dismissed his complaint with prejudice; the US and various states subsequently consented to the dismissal. In the first quarter of 2015, the USDC for the D. Mass. dismissed with prejudice all claims in connection with alleged improper marketing practices asserted by the relators and dismissed without prejudice all claims asserted in the name of the federal and various state governments. The relators have appealed. Novartis continues to vigorously contest the claims.
Antitrust class actions
Since the third quarter of 2013, approximately sixteen putative class action complaints have been filed against manufacturers of the brand drug Solodyn® and its generic equivalents, including Sandoz Inc. The cases have been consolidated and transferred for pretrial purposes to a federal district court in Massachusetts. The plaintiffs purport to represent direct and indirect purchasers of Solodyn® branded products and assert violations of federal and state antitrust laws, including allegations in connection with separate settlements by Medicis with each of the other defendants, including Sandoz Inc., of patent litigation relating to generic Solodyn®. Sandoz is vigorously contesting the claims.
Since March 2015, more than 50 putative class action complaints have been filed in several courts across the US naming contact-lens manufacturers, including ALI, and alleging violations of federal antitrust law as well as state antitrust, consumer protection and unfair competition laws of various states in
F-67
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. Provisions and Other Non-Current Liabilities (Continued)
connection with the sale of contact lenses. The cases have been consolidated in the Middle District of Florida by the Judicial Panel on Multidistrict Litigation and the claims are being vigorously contested.
Since June 2015, NPC, Novartis Corporation (NC) and NAG have been sued in five putative class action complaints brought in federal district court in Massachusetts on behalf of proposed classes of all direct and indirect purchasers, including end-payors, of Gleevec. The complaints assert violations of federal antitrust law and various state laws, and seek to prevent Novartis from enforcing a previously reported 2014 agreement under which Sun Pharmaceuticals agreed not to launch a generic version of Gleevec, until February 1, 2016, as well as damages and other relief. The claims are being vigorously contested.
In October 2015, Sandoz and Momenta Pharmaceuticals were sued in a putative antitrust class action in federal court in Tennessee alleging that Momenta and Sandoz engaged in anticompetitive conduct with regard to sales of enoxaparin, and the same allegations were made by Amphastar in a lawsuit filed in federal court in California (Sandoz, Momenta Pharmaceuticals and Amphastar are currently engaged in litigation concerning certain enoxaparin patents in federal court in Massachusetts). The claims are being vigorously contested.
Oriel litigation
In October 2013, Shareholder Representative Services LLC filed a complaint in New York State Court against Sandoz Inc., two affiliates and two former officers of Sandoz AG asserting various common law and statutory contract, fraud and negligent misrepresentation claims arising out of the Sandoz Inc. purchase of Oriel Therapeutics, Inc. In March 2015, the court dismissed all claims except a breach of contract claim against Sandoz Inc. Sandoz Inc. continues to vigorously contest the claim.
Eye drop products consumer class actions
Since November 2012, six putative consumer fraud class action litigations were commenced against Alcon (and in four cases Sandoz) in federal courts in the Southern Districts of Illinois (S.D. Ill.) and Florida and the Districts of Missouri, Massachusetts and New Jersey. They claim that Alcon's, Sandoz's and many other manufacturers defendants' eye drop products were deceptively designed so that the drop dosage is more than necessary to be absorbed in the eye or there is too much solution in each bottle for the course of the treatment, leading to wastage and higher costs to patient consumers. Three cases remain pending in the S.D. Ill., D. Mass. and DNJ. Novartis is vigorously contesting the claims.
Employment action
In March 2015, ALI and NC were sued in an individual and collective action filed in the SDNY. The parties negotiated a class settlement and a settlement for the individual plaintiffs (excluding one plaintiff) for an amount that is not material to Novartis, which settlements and amended complaint were filed with the court for approval in December 2015. The claims assert inter alia gender discrimination, pay discrimination and retaliation at Alcon. The one remaining individual claim continues to be vigorously contested.
F-68
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. Provisions and Other Non-Current Liabilities (Continued)
Concluded legal matters
Western District of Kentucky (WDKY) investigation
In 2012, NPC received a subpoena from the USAO for the WDKY requesting the production of documents relating to marketing practices, including alleged remuneration of healthcare providers and off-label promotion, in connection with certain NPC products (including Tekturna, Valturna, Reclast, Exelon Patch and other products). In the third quarter of 2015, the USAO declined to intervene in the relators' complaint and has closed the investigation.
SDNY specialty pharmacies investigation and litigation
In April 2013, the US government filed a civil complaint in intervention to a qui tam action against NPC in the USDC for the SDNY. The complaint, as subsequently amended, asserted federal False Claims Act and state law claims related to alleged unlawful contractual discounts and rebates to specialty pharmacies in connection with Myfortic, and alleged unlawful contractual discounts, rebates and patient referrals to one specialty pharmacy in connection with Exjade. In January 2014, eleven states filed three complaints in intervention asserting similar claims related to Exjade; and the qui tam relator served on NPC an amended complaint also asserting similar claims with respect to Myfortic and Exjade, as well as claims involving Tasigna, Gleevec and TOBI that the federal and various state governments declined to pursue. In the second half of 2015, NPC reached a settlement with all plaintiffs, including the United States Department of Justice, 45 states (made up of the eleven intervening states, as well as all the other states which were either part of the relator's complaint, or which reimbursed prescriptions of Myfortic and Exjade during the relevant time period), the District of Columbia and the qui tam relator. This resolves all the above-described claims related to Myfortic, Exjade, Tasigna, Gleevec and TOBI. As part of the settlement, NPC agreed to pay $390 million plus additional legal expenses to plaintiffs, and agreed with the Office of Inspector General of the US Department of Health & Human Services on an amendment and extension of its current Corporate Integrity Agreement until 2020.
DNJ investigation
In late September 2014, ALI received a subpoena from the USAO for the DNJ relating to an investigation of Alcon sales practices. In the third quarter of 2015, the USAO declined to proceed, and no charges were brought or sanctions imposed. The relator dismissed the complaint voluntarily.
Italy Sandostatin investigation
In January 2014, the ICA opened an investigation to assess whether Novartis Farma S.p.A. and Italfarmaco S.p.A. colluded on the supply of octreotide acetate (Sandostatin LAR and Longastatina® LAR, respectively). In consideration of commitments to amend certain provisions of the co-marketing agreement with Italfarmaco, the ICA decided to close the investigation with no finding of an infringement and thus without a fine. The decision became final in October 2015.
Zometa/Aredia product liability litigation
NPC had been a defendant in more than 880 cases brought in US courts in which plaintiffs generally claimed to have experienced osteonecrosis of the jaw or atypical femur fracture after treatment with Zometa or Aredia, which are used to treat patients whose cancer has spread to the bones. Nearly all the
F-69
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. Provisions and Other Non-Current Liabilities (Continued)
cases have been resolved through voluntary dismissals, pre-trial motion practice, trial, or settlements, the payments of which were not material to Novartis. Three cases where NPC prevailed at the trial level remain on appeal, and one other case remains pending. The remaining claims are being vigorously contested, but they are not material to Novartis.
Solodyn® Federal Trade Commission (FTC) investigation
The conduct challenged in the above-described Solodyn® antitrust class actions has also been the subject of an FTC investigation. In the fourth quarter of 2015, the FTC closed the investigation with no finding of an infringement or a fine. This matter is therefore concluded.
Excedrin consumer class actions
Four putative class actions were brought in December 2013 and January 2014 against Novartis and its consumer health unit. They generally claim that it was a deceptive practice to sell Excedrin Migraine at a higher price than Excedrin Extra Strength when the two have the same active ingredients, even though the products have different labels and clearly disclose their active ingredients. In 2014, three of the four putative class actions were dismissed; the remaining one is not material to Novartis.
Summary of Product Liability, Governmental Investigations and Other Legal Matters Provision Movements:
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
January 1 |
849 | 924 | 998 | |||||||
Provisions related to discontinued operations |
(37 | ) | ||||||||
Cash payments |
(256 | ) | (454 | ) | (373 | ) | ||||
Releases of provisions |
(223 | ) | (135 | ) | (184 | ) | ||||
Additions to provisions |
832 | 549 | 499 | |||||||
Currency translation effects |
(8 | ) | 2 | (16 | ) | |||||
| | | | | | | | | | | |
December 31 |
1,194 | 849 | 924 | |||||||
Less current portion |
(743 | ) | (328 | ) | (461 | ) | ||||
| | | | | | | | | | | |
Non-current product liabilities, governmental investigations and other legal matters provisions at December 31 |
451 | 521 | 463 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Novartis believes that its total provisions for investigations, product liability, arbitration and other legal matters are adequate based upon currently available information. However, given the inherent difficulties in estimating liabilities, there can be no assurance that additional liabilities and costs will not be incurred beyond the amounts provided.
F-70
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
21. Current Financial Debt and Derivative Financial Instruments
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Interest-bearing accounts of associates payable on demand |
1,645 | 1,651 | |||||
Bank and other financial debt |
1,185 | 1,272 | |||||
Commercial paper |
1,085 | 648 | |||||
Current portion of non-current financial debt |
1,659 | 2,989 | |||||
Fair value of derivative financial instruments |
30 | 52 | |||||
| | | | | | | | |
Total current financial debt and derivative financial instruments |
5,604 | 6,612 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The consolidated balance sheet amounts of current financial debt, other than the current portion of non-current financial debt, approximate the estimated fair value due to the short-term nature of these instruments.
The weighted average interest rate on the bank and other current financial debt (including employee deposits from the compensation of associates employed by Swiss entities) was 2.7% in 2015 and 2.6% in 2014.
Details on commercial papers are provided in Note 29—Liquidity risk.
22. Provisions and Other Current Liabilities
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Taxes other than income taxes |
551 | 549 | |||||
Restructuring provisions |
260 | 333 | |||||
Accrued expenses for goods and services received but not invoiced |
1,124 | 1,076 | |||||
Accruals for royalties |
550 | 561 | |||||
Provisions for revenue deductions |
3,790 | 3,533 | |||||
Accruals for compensation and benefits including social security |
1,932 | 1,968 | |||||
Environmental remediation liabilities |
80 | 95 | |||||
Deferred income |
385 | 329 | |||||
Provision for product liabilities, governmental investigations and other legal matters |
743 | 328 | |||||
Accrued share-based payments |
209 | 248 | |||||
Contingent considerations |
78 | 291 | |||||
Commitment for repurchase of own shares (see Note 9) |
658 | ||||||
Other payables |
1,017 | 479 | |||||
| | | | | | | | |
Total provisions and other current liabilities |
10,719 | 10,448 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Provisions are based upon management's best estimate and adjusted for actual experience. Such adjustments to the historic estimates have not been material.
F-71
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
22. Provisions and Other Current Liabilities (Continued)
Provision for Deductions from Revenue
The following table shows the movement of the provision for deductions from revenue:
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
January 1 |
3,533 | 4,182 | 4,072 | |||||||
Provisions related to discontinued operations |
(234 | ) | ||||||||
Impact of business combinations |
3 | |||||||||
Additions |
15,603 | 14,119 | 13,095 | |||||||
Payments/utilizations |
(15,218 | ) | (13,907 | ) | (12,762 | ) | ||||
Changes in offset against gross trade receivables |
50 | (420 | ) | (224 | ) | |||||
Currency translation effects |
(181 | ) | (207 | ) | 1 | |||||
| | | | | | | | | | | |
December 31 |
3,790 | 3,533 | 4,182 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Restructuring Provision Movements
| |
$ m | |||
|---|---|---|---|---|
January 1, 2013 |
221 | |||
Additions |
175 | |||
Cash payments |
(134 | ) | ||
Releases |
(47 | ) | ||
Transfers |
(42 | ) | ||
Currency translation effects |
1 | |||
| | | | | |
December 31, 2013 |
174 | |||
Provisions related to discontinued operations |
(4 | ) | ||
Additions |
504 | |||
Cash payments |
(295 | ) | ||
Releases |
(52 | ) | ||
Currency translation effects |
6 | |||
| | | | | |
December 31, 2014 |
333 | |||
Additions |
399 | |||
Cash payments |
(435 | ) | ||
Releases |
(36 | ) | ||
Currency translation effects |
(1 | ) | ||
| | | | | |
December 31, 2015 |
260 | |||
| | | | | |
| | | | | |
| | | | | |
In 2015, additions to provisions of $399 million in continuing operations were to a large extent related to reorganizations in the Pharmaceuticals Division. Thereby two initiatives totaling $106 million were targeted at efficiency gains in the business franchises other than Oncology and Cell and Gene Therapies. The integration of the Oncology business acquired from GSK resulted in restructuring expenses of $78 million. Alcon extended its initiative to realize productivity opportunities ($45 million). Finally group
F-72
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
22. Provisions and Other Current Liabilities (Continued)
wide initiatives to simplify the organizational structure ($159 million), mainly related to the manufacturing footprint and support services as well as a NIBR initiative ($11 million) resulted in an increase of the provision.
In 2014, additions to provisions of $504 million in continuing operations were mainly related to reorganizations in the Pharmaceuticals Division. In Pharmaceuticals an initiative in Development totaling $72 million was targeted at establishing an organizational model for the development activities which allows for greater focus on high priority programs in specialty medicines, more flexibility to adapt to changes in the portfolio, and which strengthens operational excellence. Activities in the Pharmaceuticals Division were also subject to a restructuring program totaling $286 million which was targeted at increasing operational leverage. Alcon has established a $56 million initiative to realize productivity opportunities.
In 2013, additions to provisions of $175 million in the Group were mainly related to reorganizations of the Pharmaceuticals research and development activities and the integration of Alcon.
The releases to income in 2015 of $36 million in continuing operations, in 2014 of $52 million in continuing operations and $5 million in discontinued operations and in 2013 of $47 million for the entire Group, were mainly due to settlement of liabilities at lower amounts than originally anticipated.
| |
Third party costs(1) |
Termination costs |
Additions to provision |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Restructuring initiatives
|
2015 | 2014 | 2015 | 2014 | 2015 | 2014 | |||||||||||||
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Pharmaceuticals—Research & Development |
11 | 72 | 11 | 72 | |||||||||||||||
Pharmaceuticals—Business Franchises |
8 | 106 | 278 | 106 | 286 | ||||||||||||||
Pharmaceuticals—GSK Oncology Integration |
78 | 78 | |||||||||||||||||
Alcon initiative to increase operating leverage |
45 | 56 | 45 | 56 | |||||||||||||||
Various Group initiatives to simplify organizational structure—including manufacturing sites and support services |
31 | 1 | 128 | 89 | 159 | 90 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total |
31 | 9 | 368 | 495 | 399 | 504 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
- (1)
- Third party costs are mainly associated with lease and other obligations due to abandonment of certain facilities.
F-73
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
23. Details to the Consolidated Cash Flow Statements
23.1) Adjustments for Non-Cash Items from Continuing Operations
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Taxes |
1,106 | 1,545 | 1,498 | |||||||
Depreciation, amortization and impairments on: |
||||||||||
Property, plant & equipment |
1,550 | 1,630 | 1,601 | |||||||
Intangible assets |
3,921 | 3,052 | 2,804 | |||||||
Financial assets(1) |
104 | 69 | 57 | |||||||
Income from associated companies |
(266 | ) | (1,918 | ) | (599 | ) | ||||
Gains on disposal of property, plant & equipment, intangible, financial and other non-current assets, net |
(869 | ) | (622 | ) | (347 | ) | ||||
Equity-settled compensation expense |
773 | 744 | 654 | |||||||
Change in provisions and other non-current liabilities |
1,642 | 1,490 | 736 | |||||||
Net financial income |
1,109 | 735 | 775 | |||||||
| | | | | | | | | | | |
Total |
9,070 | 6,725 | 7,179 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- Including unrealized fair value gains
In 2015, the Group acquired property, plant and equipment of $85 million through finance lease contracts.
23.2) Cash Flows from Changes in Working Capital and Other Operating Items Included in Operating Cash Flow from Continuing Operations
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
(Increase) in inventories |
(482 | ) | (506 | ) | (454 | ) | ||||
(Increase) in trade receivables |
(513 | ) | (367 | ) | (548 | ) | ||||
Increase in trade payables |
378 | 142 | 414 | |||||||
Change in other net current assets and other operating cash flow items |
(246 | ) | 106 | (190 | ) | |||||
| | | | | | | | | | | |
Total |
(863 | ) | (625 | ) | (778 | ) | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-74
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
23. Details to the Consolidated Cash Flow Statements (Continued)
23.3) Cash Flow Arising from Acquisitions and Divestments of Businesses
The following is a summary of the cash flow impact of acquisitions and divestments. The most significant transactions are described in Note 2.
| |
2015 Acquisitions |
2015 Divestments |
2014 Acquisitions |
2014 Divestments |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
|||||||||
Property, plant & equipment |
1,000 | 145 | |||||||||||
Currently marketed products |
(12,970 | ) | 646 | (234 | ) | 91 | |||||||
(Acquired)/divested research & development |
(730 | ) | 13 | (248 | ) | ||||||||
Technologies |
113 | ||||||||||||
Other intangible assets |
(15 | ) | 86 | ||||||||||
Financial and other assets including deferred tax assets(1) |
(555 | ) | 40 | (53 | ) | 7 | |||||||
Inventories |
893 | (1 | ) | 87 | |||||||||
Trade receivables and other current assets |
(3 | ) | 529 | (3 | ) | 159 | |||||||
Cash and cash equivalents |
(25 | ) | 311 | (2 | ) | ||||||||
Current and non-current financial debts |
(601 | ) | |||||||||||
Trade payables and other liabilities including deferred tax liabilities |
212 | (841 | ) | 186 | (50 | ) | |||||||
| | | | | | | | | | | | | | |
Net identifiable assets (acquired) or divested |
(14,086 | ) | 2,189 | (355 | ) | 439 | |||||||
Currency translation effects |
98 | (3 | ) | ||||||||||
Acquired/(divested) liquidity |
25 | (479 | ) | 2 | |||||||||
| | | | | | | | | | | | | | |
Subtotal |
(14,061 | ) | 1,808 | (353 | ) | 436 | |||||||
Refinancing of intercompany financial debt, net |
578 | ||||||||||||
Goodwill(1) |
(2,438 | ) | 1,042 | (131 | ) | 267 | |||||||
Divestment gain |
7,401 | 876 | |||||||||||
Taxes paid and other portfolio transformation related cash flows |
(1,337 | ) | (566 | ) | |||||||||
Receivables and payables contingent consideration, net |
(8 | ) | (519 | ) | 153 | ||||||||
Prepaid/deferred portion of sales price(2) |
(49 | ) | 47 | ||||||||||
| | | | | | | | | | | | | | |
Net cash flow |
(16,507 | ) | 8,924 | (331 | ) | 1,060 | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Of which: |
|||||||||||||
Net cash flow from discontinued operations |
8,924 | 1,060 | |||||||||||
Net cash flow used in continuing operations |
(16,507 | ) | (331 | ) | |||||||||
- (1)
- 2014
Acquisitions include an adjustment regarding a previous acquisition to deferred tax assets of $21 million and goodwill of
$135 million.
- (2)
- Divestments include $49 million proceeds for the divestment of the Animal Health business received in 2014.
There were no significant acquisitions or divestments which had an impact on the cash flow statement in 2013, however $42 million were paid for contingent considerations regarding acquisitions from previous years.
Notes 2 and 24 provide further information regarding acquisitions and divestments of businesses. All acquisitions were for cash.
F-75
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
23. Details to the Consolidated Cash Flow Statements (Continued)
23.4) Cash Flow From Discontinued Operations
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Cash flows used in/from operating activities |
(188 | ) | (1 | ) | 557 | |||||
Purchase of property, plant & equipment |
(41 | ) | (223 | ) | (161 | ) | ||||
Proceeds from sales of property, plant & equipment |
1 | 4 | 12 | |||||||
Purchase of intangible assets |
(18 | ) | (32 | ) | ||||||
Proceeds from sales of intangible assets |
79 | 58 | ||||||||
Purchase of financial and other non-current assets, net |
(2 | ) | (13 | ) | (10 | ) | ||||
Divestments of businesses(1) |
8,924 | 1,060 | ||||||||
| | | | | | | | | | | |
Cash flows from/used in investing activities |
8,882 | 889 | (133 | ) | ||||||
| | | | | | | | | | | |
Total net cash flows from discontinued operations |
8,694 | 888 | 424 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- Includes proceeds of $10,925 million reduced by $2,001 million, for payments of taxes, transaction-related costs and purchase price adjustments.
24. Acquisitions of Businesses
Assets and Liabilities Arising from Acquisitions
Fair value
|
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Currently marketed products |
12,970 | 234 | |||||
Acquired research & development |
730 | 248 | |||||
Other intangible assets |
15 | ||||||
Deferred tax assets(1) |
555 | 53 | |||||
Inventories |
1 | ||||||
Trade receivables and other current assets |
3 | 3 | |||||
Cash and cash equivalents |
25 | 2 | |||||
Payables and other liabilities including deferred tax liabilities |
(212 | ) | (186 | ) | |||
| | | | | | | | |
Net identifiable assets acquired |
14,086 | 355 | |||||
Acquired liquidity |
(25 | ) | (2 | ) | |||
Goodwill(1) |
2,438 | 131 | |||||
| | | | | | | | |
Net assets recognized as a result of business combinations |
16,499 | 484 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- (1)
- 2014 includes an adjustment regarding a previous acquisition to deferred tax assets of $21 million and goodwill of $135 million.
Note 2 details significant acquisition of businesses, which in 2015, were the GSK Oncology products, Spinifex and Admune. The goodwill arising out of these acquisitions is attributable to buyer specific synergies, assembled workforce and to the accounting for deferred tax liabilities on the acquired assets. Goodwill of $2.4 billion is tax deductible. In 2014 the significant transactions related to CoStim Pharmaceuticals and WaveTec.
There were no significant acquisitions in 2013.
F-76
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
25. Post-Employment Benefits for Associates
In addition to the legally required social security schemes, the Group has numerous independent pension and other post-employment benefit plans. In most cases these plans are externally funded in entities which are legally separate from the Group. For certain Group companies, however, no independent plan assets exist for the pension and other post-employment benefit obligations of associates. In these cases the related unfunded liability is included in the balance sheet. The defined benefit obligations (DBO) of all major pension and other post-employment benefit plans are reappraised annually by independent actuaries. Plan assets are recognized at fair value. The major plans are based in Switzerland, United States, United Kingdom, Germany and Japan, which represent 95% of the Group's total DBO for pension plans. Details of the plans in the two most significant countries of Switzerland and the US are provided below.
Swiss-based pension plans represent the most significant portion of the Group's total DBO and plan assets. For the active insured members born on or after January 1, 1956, or having joined the plans after December 31, 2010 the benefits are partially linked to the contributions paid into the plan. Certain features of Swiss pension plans required by law preclude the plans being categorized as defined contribution plans. These factors include a minimum interest guarantee on retirement savings accounts, a pre-determined factor for converting the accumulated savings account balance into a pension and embedded death and disability benefits.
All benefits granted under Swiss pension plans are vested and Swiss legislation prescribes that the employer has to contribute a fixed percentage of an associate's pay to an external pension fund. Additional employer's contributions may be required whenever the plan's statutory funding ratio falls below a certain level. The associate also contributes to the plan. The pension plans are run by separate legal entities, each governed by a Board of Trustees which for the principal plans consists of representatives nominated by Novartis and by the active insured associates. The Boards of Trustees are responsible for the plan design and the asset investment strategy.
In June 2015 the Board of Trustees of the Novartis Swiss Pension Fund agreed to adjust the annuity conversion rate at retirement with effect from January 1, 2016. This amendment does not have an impact on existing members receiving benefits or on plan members, born before January 1, 1956. This amendment resulted in a net pre-tax curtailment gain of $110 million (CHF 103 million).
The US pension plans represent the second largest component of the Group's total DBO and plan assets. The principal plans (Qualified Plans) are funded whereas plans providing additional benefits for executives (Restoration Plans) are unfunded. Employer contributions are required for Qualified Plans whenever the statutory funding ratio falls below a certain level. Furthermore, associates in the US are covered under other post-employment benefit plans and post-retirement medical plans.
F-77
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
25. Post-Employment Benefits for Associates (Continued)
The following tables are a summary of the funded and unfunded defined benefit obligation for pension and other post-employment benefit plans of associates at December 31, 2015 and 2014:
| |
Pension plans | Other post-employment benefit plans |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2015 | 2014 | |||||||||
| |
$ m |
$ m |
$ m |
$ m |
|||||||||
Benefit obligation at January 1 |
24,178 | 24,801 | 1,253 | 1,069 | |||||||||
Benefit obligations related to discontinued operations |
(848 | ) | (21 | ) | |||||||||
Current service cost |
451 | 418 | 32 | 35 | |||||||||
Interest cost |
399 | 654 | 46 | 49 | |||||||||
Past service costs and settlements |
(138 | ) | 6 | (89 | ) | ||||||||
Administrative expenses |
23 | 21 | |||||||||||
Remeasurement (gains)/losses arising from changes in financial assumptions |
(16 | ) | 2,129 | (34 | ) | 164 | |||||||
Remeasurement (gains)/losses arising from changes in demographic assumptions |
(41 | ) | 229 | (30 | ) | 121 | |||||||
Experience related remeasurement losses/(gains) |
56 | (14 | ) | (110 | ) | (22 | ) | ||||||
Currency translation effects |
(358 | ) | (2,156 | ) | (14 | ) | (5 | ) | |||||
Benefit payments |
(1,406 | ) | (1,282 | ) | (50 | ) | (48 | ) | |||||
Contributions of associates |
223 | 210 | |||||||||||
Effect of acquisitions, divestments or transfers |
31 | 10 | 39 | ||||||||||
| | | | | | | | | | | | | | |
Benefit obligation at December 31 |
23,402 | 24,178 | 1,132 | 1,253 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Fair value of plan assets at January 1 |
20,434 | 21,481 | 199 | 209 | |||||||||
Plan assets related to discontinued operations |
(530 | ) | |||||||||||
Interest income |
300 | 550 | 6 | 10 | |||||||||
Return on plan assets excluding interest income |
(286 | ) | 1,442 | (6 | ) | 28 | |||||||
Currency translation effects |
(223 | ) | (1,917 | ) | |||||||||
Novartis Group contributions |
494 | 485 | 23 | ||||||||||
Contributions of associates |
223 | 210 | |||||||||||
Settlements |
(3 | ) | (9 | ) | |||||||||
Benefit payments |
(1,406 | ) | (1,282 | ) | (50 | ) | (48 | ) | |||||
Effect of acquisitions, divestments or transfers |
3 | 4 | |||||||||||
| | | | | | | | | | | | | | |
Fair value of plan assets at December 31 |
19,536 | 20,434 | 172 | 199 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Funded status |
(3,866 | ) | (3,744 | ) | (960 | ) | (1,054 | ) | |||||
Limitation on recognition of fund surplus at January 1 |
(58 | ) | (45 | ) | |||||||||
Change in limitation on recognition of fund surplus (incl. exchange rate differences) |
12 | (9 | ) | ||||||||||
Interest income on limitation of fund surplus |
(4 | ) | (4 | ) | |||||||||
| | | | | | | | | | | | | | |
Limitation on recognition of fund surplus at December 31 |
(50 | ) | (58 | ) | |||||||||
| | | | | | | | | | | | | | |
Net liability in the balance sheet at December 31 |
(3,916 | ) | (3,802 | ) | (960 | ) | (1,054 | ) | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
F-78
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
25. Post-Employment Benefits for Associates (Continued)
The reconciliation of the net liability from January 1 to December 31 is as follows:
| |
Pension plans | Other post- employment benefit plans |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2015 | 2014 | |||||||||
| |
$ m |
$ m |
$ m |
$ m |
|||||||||
Net liability at January 1 |
(3,802 | ) | (3,365 | ) | (1,054 | ) | (860 | ) | |||||
Less: Net liability related to discontinued operations |
318 | 21 | |||||||||||
Current service cost |
(451 | ) | (418 | ) | (32 | ) | (35 | ) | |||||
Net interest expense |
(103 | ) | (108 | ) | (40 | ) | (39 | ) | |||||
Administrative expenses |
(23 | ) | (21 | ) | |||||||||
Past service costs and settlements |
135 | (15 | ) | 89 | |||||||||
Remeasurements |
(285 | ) | (902 | ) | 168 | (235 | ) | ||||||
Currency translation effects |
135 | 239 | 14 | 5 | |||||||||
Novartis Group contributions |
494 | 485 | 23 | ||||||||||
Effect of acquisitions, divestments or transfers |
(28 | ) | (6 | ) | (39 | ) | |||||||
Change in limitation on recognition of fund surplus |
12 | (9 | ) | ||||||||||
| | | | | | | | | | | | | | |
Net liability at December 31 |
(3,916 | ) | (3,802 | ) | (960 | ) | (1,054 | ) | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Amounts recognized in the consolidated balance sheet |
|||||||||||||
Prepaid benefit cost |
36 | 37 | |||||||||||
Accrued benefit liability |
(3,952 | ) | (3,839 | ) | (960 | ) | (1,054 | ) | |||||
The following table shows a breakdown of the DBO for pension plans by geography and type of member and the breakdown of plan assets into the geographical locations in which they are held.
| |
2015 $ m |
2014 $ m |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Switzerland | US | Rest of the World |
Total | Switzerland | US | Rest of the World |
Total | |||||||||||||||||
Benefit obligation at December 31 |
15,453 | 3,783 | 4,166 | 23,402 | 15,578 | 4,092 | 4,508 | 24,178 | |||||||||||||||||
Thereof unfunded |
736 | 466 | 1,202 | 820 | 484 | 1,304 | |||||||||||||||||||
By type of member |
|||||||||||||||||||||||||
Active |
6,196 | 990 | 1,392 | 8,578 | 6,268 | 1,182 | 1,502 | 8,952 | |||||||||||||||||
Deferred pensioners |
909 | 1,489 | 2,398 | 947 | 1,499 | 2,446 | |||||||||||||||||||
Pensioners |
9,257 | 1,884 | 1,285 | 12,426 | 9,310 | 1,963 | 1,507 | 12,780 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Fair value of plan assets at December 31 |
14,347 | 2,358 | 2,831 | 19,536 | 14,869 | 2,521 | 3,044 | 20,434 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Funded Status |
(1,106 | ) | (1,425 | ) | (1,335 | ) | (3,866 | ) | (709 | ) | (1,571 | ) | (1,464 | ) | (3,744 | ) | |||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
F-79
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
25. Post-Employment Benefits for Associates (Continued)
The following table shows the principal weighted average actuarial assumptions used for calculating defined benefit plans and other post-employment benefits of associates:
| |
Pension plans | Other post-employment benefit plans |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | 2015 | 2014 | 2013 | ||||||
| |
% |
% |
% |
% |
% |
% |
||||||
Weighted average assumptions used to determine benefit obligations at December 31 |
||||||||||||
Discount rate |
1.8% | 1.8% | 2.9% | 4.4% | 3.8% | 4.7% | ||||||
Expected rate of pension increase |
0.4% | 0.4% | 1.1% | |||||||||
Expected rate of salary increase |
2.9% | 3.2% | 3.5% | |||||||||
Interest on savings account |
0.8% | 0.9% | 2.1% | |||||||||
Current average life expectancy for a 65-year-old male/female |
21/24 years | 21/24 years | 21/23 years | 21/23 years | 22/24 years | 19/21 years | ||||||
Changes in the above-mentioned actuarial assumptions can result in significant volatility in the accounting for the Group's pension plans in the consolidated financial statements. This can result in substantial changes in the Group's other comprehensive income, long-term liabilities and prepaid pension assets.
The DBO is significantly impacted by assumptions regarding the rate that is used to discount the actuarially determined post-employment benefit liability. This rate is based on yields of high quality corporate bonds in the country of the plan. Decreasing corporate bond yields decrease the discount rate, so that the DBO increases and the funded status decreases.
In Switzerland an increase in the DBO due to lower discount rates is slightly offset by lower future benefits expected to be paid on the associate's savings account where the assumption on interest accrued changes in line with the discount rate.
The impact of decreasing interest rates on a plan's assets is more difficult to predict. A significant part of the plan assets is invested in bonds. Bond values usually rise when interest rates decrease and may therefore partially compensate for the decrease in the funded status. Furthermore, pension assets also include significant holdings of equity instruments. Share prices tend to rise when interest rates decrease and therefore often counteract the negative impact of the rising defined benefit obligation on the funded status although correlation of interest rates with equities is not as strong as with bonds, especially in the short term.
The expected rate for pension increases significantly affects the DBO of most plans in Switzerland, Germany and the United Kingdom. Such pension increases also decrease the funded status although there is no strong correlation between the value of the plan assets and pension/inflation increases.
Assumptions regarding life expectancy significantly impact the DBO. An increase in longevity increases the DBO. There is no offsetting impact from the plan assets as no longevity bonds or swaps are held by the pension funds. Generational mortality tables are used where this data is available.
F-80
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
25. Post-Employment Benefits for Associates (Continued)
The following table shows the sensitivity of the defined benefit pension obligation to the principal actuarial assumptions for the major plans in Switzerland, United States, United Kingdom, Germany and Japan on an aggregated basis:
| |
Change in 2015 year end defined benefit pension obligation |
|||
|---|---|---|---|---|
| |
$ m |
|||
25 basis point increase in discount rate |
(736 | ) | ||
25 basis point decrease in discount rate |
781 | |||
1 year increase in life expectancy |
797 | |||
25 basis point increase in rate of pension increase |
491 | |||
25 basis point decrease in rate of pension increase |
(111 | ) | ||
25 basis point increase of interest on savings account |
61 | |||
25 basis point decrease of interest on savings account |
(60 | ) | ||
25 basis point increase in rate of salary increase |
69 | |||
25 basis point decrease in rate of salary increase |
(71 | ) | ||
The healthcare cost trend rate assumptions for other post-employment benefits are as follows:
Healthcare cost trend rate assumptions used
|
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Healthcare cost trend rate assumed for next year |
7.5% | 7.0% | 7.0% | |||||||
Rate to which the cost trend rate is assumed to decline |
5.0% | 5.0% | 5.0% | |||||||
Year that the rate reaches the ultimate trend rate |
2022 | 2021 | 2021 | |||||||
The following table shows the weighted average plan asset allocation of funded defined benefit pension plans at December 31, 2015 and 2014:
| |
Pension plans | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Long-term target |
2015 | 2014 | |||||||
| |
% |
% |
% |
|||||||
Equity securities |
15–40 | 34 | 35 | |||||||
Debt securities |
20–60 | 35 | 34 | |||||||
Real estate |
5–20 | 14 | 13 | |||||||
Alternative investments |
0–20 | 14 | 10 | |||||||
Cash and other investments |
0–15 | 3 | 8 | |||||||
| | | | | | | | | | | |
Total |
100 | 100 | ||||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Cash, as well as most of the equity and debt securities have a quoted market price in an active market. Real estate and alternative investments, which include hedge fund and private equity investments usually do not have a quoted market price.
F-81
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
25. Post-Employment Benefits for Associates (Continued)
The strategic allocation of assets of the different pension plans are determined with the objective of achieving an investment return which, together with the contributions paid by the Group and its associates, is sufficient to maintain reasonable control over the various funding risks of the plans. Based upon the market and economic environments, actual asset allocations may temporarily be permitted to deviate from policy targets. The asset allocation currently includes investments in shares of Novartis AG which totaled at December 31, 2015, 11 million shares with a market value of $1.0 billion (2014: 11 million shares with a market value of $1.0 billion). The weighted average duration of the defined benefit obligation is 14.1 years (2014: 14.3 years). The Group's ordinary contribution to the various pension plans are based on the rules of each plan. Additional contributions are made whenever this is required by statute or law; i.e. usually when statutory funding levels fall below pre-determined thresholds. The only significant plans that are foreseen to require additional funding are those in UK.
The expected future cash flows in respect of pension and other post-employment benefit plans at December 31, 2015 were as follows:
| |
Pension plans | Other post-employment benefit plans |
|||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
Novartis Group contributions |
|||||||
2016 (estimated) |
531 | 58 | |||||
Expected future benefit payments |
|||||||
2016 |
1,201 | 58 | |||||
2017 |
1,232 | 61 | |||||
2018 |
1,239 | 64 | |||||
2019 |
1,243 | 66 | |||||
2020 |
1,236 | 68 | |||||
2021-2025 |
6,113 | 361 | |||||
In many subsidiaries associates are covered by defined contribution plans. Contributions charged to the 2015 consolidated income statement for the defined contribution plans were $359 million (2014: $348 million, 2013: $332 million). The 2015 amount excludes $1 million (2014: $14 million, 2013: $19 million) related to discontinued operations.
26. Equity-Based Participation Plans for Associates
The expense related to all equity-based participation plans in the 2015 consolidated income statement was $968 million (2014: $1.1 billion, 2013 $987 million) resulting in total liabilities arising from equity-based payment transactions of $209 million (2014: $277 million of which $248 million were recognized in continuing operations, 2013: $255 million). Out of the total expense, an amount of $903 million (2014: $1.0 billion, 2013: $892 million) was recognized in continuing operations and $65 million (2014: $124 million, 2013: $95 million) was recognized in discontinued operations.
Equity-based participation plans can be separated into the following plans.
F-82
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
26. Equity-Based Participation Plans for Associates (Continued)
The Annual Incentive of the CEO and other key executives is paid 50% in cash in February or March of the year following the performance period, and 50% in Novartis restricted shares or Restricted Share Units (RSUs) that are deferred and restricted for three years. Each restricted share is entitled to voting rights and payment of dividends during the vesting period. Each RSU is equivalent in value to one Novartis share and is converted into one share at the vesting date. RSUs do not carry any dividend, dividend equivalent or voting rights. The executives may elect to also receive their cash incentive partially or fully in shares which will not be subject to vesting conditions. In 2015, 14 executives received 0.1 million restricted shares and RSUs.
A number of associates in certain countries and certain key executives worldwide are encouraged to invest their Annual Incentive, and in the United Kingdom also their salary, in a share savings plan. Under the share savings plan, participants may elect to receive their Annual Incentive fully or partially in Novartis shares in lieu of cash. As a reward for their participation in the share savings plan, at no additional cost to the participant, Novartis matches their investments in shares after a holding period of three or five years.
Novartis currently has three share savings plans:
- –
- Worldwide 37 key executives were invited to participate in the Leveraged Share Savings Plan (LSSP) based on their performance in 2014.
At the participant's election, the Annual Incentive is awarded partly or entirely in shares. The elected number of shares was delivered in 2015 and is subject to a holding period of five years. At the
end of the holding period, Novartis will match the invested shares at a ratio of 1-to-1 (i.e. one share awarded for each invested share). In the US both the LSSP award and the corresponding
match are cash settled.
- –
- In Switzerland, the Employee Share Ownership Plan (ESOP) was available to 12,796 associates in 2014. ESOP participants may choose to
receive their Annual Incentive (i) 100% in shares, (ii) 50% in shares and 50% in cash or (iii) 100% in cash. After expiration of a three-year holding period for Novartis shares
invested under the ESOP, each participant will receive one matching share for every two Novartis shares invested. A total of 5,945 associates chose to receive shares under the ESOP for their
performance in 2014 and the invested shares were delivered in 2015.
- –
- In the United Kingdom, 1,618 associates can invest up to 5% of their monthly salary in shares (up to a maximum of GBP 125) and also may be invited to invest all or part of their net Annual Incentive in shares. Two invested shares are matched with one share with a holding period of three years. During 2015, 1,433 participants elected to participate in this plan.
Following the introduction of the new compensation programs in 2014, the CEO and the other Executive Committee members are no longer eligible to participate in the share savings plans.
Associates may only participate in one of these plans in any given year.
During 2015, a total of 4.1 million shares (2014: 4.8 million shares) were delivered to associates in lieu of their annual incentive (in the UK, also their salary).
F-83
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
26. Equity-Based Participation Plans for Associates (Continued)
The Equity Plan "Select" is a global equity incentive plan under which eligible associates, including Executive Committee members up to performance year 2013, may annually be awarded a grant subject to a three year vesting period. For certain associates the grant is subject to the achievement of predetermined business and individual performance objectives typically set at the start of the calendar year prior to the date of grant. For these associates the Select award is capped at 200% of target. No awards are granted for performance ratings below a certain threshold.
The Equity Plan "Select" currently allows its participants in Switzerland to choose the form of their equity compensation in restricted shares or restricted share units (RSUs). In all other jurisdictions, RSUs are typically granted. Until 2013, participants could also choose to receive part or the entire grant in the form of tradable share options.
Tradable share options expire on their 10th anniversary from the grant date. Each tradable share option entitles the holder to purchase after vesting (and before the 10th anniversary from the grant date) one Novartis share at a stated exercise price that equals the closing market price of the underlying share at the grant date.
The terms and conditions of the Novartis Equity Plan "Select" outside North America are substantially equivalent to the Novartis Equity Plan "Select" for North America.
Novartis Equity Plan "Select" outside North America
Participants in this plan were granted in 2015 a total of 1.7 million restricted shares and RSUs at CHF 84.75 (2014: 2.1 million restricted shares and RSUs at CHF 73.75).
The following table shows the activity associated with the share options during the period. The weighted average prices in the table below are translated from Swiss Francs into $ at historical rates.
| |
2015 | 2014 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Options | Weighted average exercise price |
Options | Weighted average exercise price |
|||||||||
| |
(millions) |
($) |
(millions) |
($) |
|||||||||
Options outstanding at January 1 |
16.1 | 59.2 | 26.4 | 57.3 | |||||||||
Sold or exercised |
(4.1 | ) | 56.7 | (9.8 | ) | 54.0 | |||||||
Forfeited or expired |
(0.3 | ) | 66.0 | (0.5 | ) | 62.2 | |||||||
| | | | | | | | | | | | | | |
Outstanding at December 31 |
11.7 | 59.9 | 16.1 | 59.2 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Exercisable at December 31 |
7.4 | 56.4 | 7.0 | 55.0 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
All share options were granted at an exercise price which was equal to the closing market price of the Group's shares at the grant date. The weighted average exercise price during the period the options were sold or exercised in 2015 was $56.74. The weighted average share price at the dates of sale was $97.89.
F-84
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
26. Equity-Based Participation Plans for Associates (Continued)
The following table summarizes information about share options outstanding at December 31, 2015:
| |
Options outstanding | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Range of exercise prices ($)
|
Number outstanding |
Average remaining contractual life |
Weighted average exercise price |
|||||||
| |
(millions) |
(years) |
($) |
|||||||
45–49 |
0.8 | 3.0 | 46.8 | |||||||
50–54 |
1.6 | 3.1 | 54.4 | |||||||
55–59 |
4.6 | 4.1 | 57.8 | |||||||
65–70 |
4.7 | 7.0 | 66.0 | |||||||
| | | | | | | | | | | |
Total |
11.7 | 5.1 | 59.9 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Novartis Equity Plan "Select" for North America
Participants in this plan were granted a total of 3.9 million RSUs at $98.75 (2014: 5.1 million RSUs at $80.79).
The following table shows the activity associated with the American Depositary Receipts (ADR) options during the period:
| |
2015 | 2014 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
ADR options |
Weighted average exercise price |
ADR options |
Weighted average exercise price |
|||||||||
| |
(millions) |
($) |
(millions) |
($) |
|||||||||
Options outstanding at January 1 |
44.4 | 59.6 | 58.8 | 58.9 | |||||||||
Sold or exercised |
(11.8 | ) | 57.8 | (12.2 | ) | 55.5 | |||||||
Forfeited or expired |
(0.7 | ) | 63.3 | (2.2 | ) | 62.6 | |||||||
| | | | | | | | | | | | | | |
Outstanding at December 31 |
31.9 | 60.2 | 44.4 | 59.6 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Exercisable at December 31 |
19.2 | 56.3 | 16.3 | 54.7 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
All ADR options were granted at an exercise price which was equal to the closing market price of the ADRs at the grant date. The weighted average exercise price during the period the ADR options were sold or exercised in 2015 was $57.75. The weighted average ADR price at the dates of sale or exercise was $100.58.
F-85
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
26. Equity-Based Participation Plans for Associates (Continued)
The following table summarizes information about ADR options outstanding at December 31, 2015:
| |
ADR options outstanding | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Range of exercise prices ($)
|
Number outstanding |
Average remaining contractual life |
Weighted average exercise price |
|||||||
| |
(millions) |
(years) |
($) |
|||||||
45–49 |
2.4 | 3.0 | 46.4 | |||||||
50–54 |
3.1 | 3.5 | 53.8 | |||||||
55–59 |
12.7 | 5.0 | 58.0 | |||||||
65–69 |
13.7 | 7.0 | 66.1 | |||||||
| | | | | | | | | | | |
Total |
31.9 | 5.6 | 60.2 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
In 2014, a new LTPP was introduced for the CEO and other key executives designed to not only drive long-term shareholder value, but also innovation. From 2015 onwards, this LTPP was extended to all key executives who previously participated in the now discontinued Old LTPP (OLTPP).
The rewards of the LTPP are based on three year performance objectives focused on financial and innovation measures. The financial measure is Novartis Cash Value Added (NCVA). The weighting of this measure is 75%. The NCVA target is approved by the Board of Directors.
The innovation measure is based on a holistic approach under which divisional innovation targets are set at the beginning of the cycle, comprised of up to ten target milestones that represent the most important research and development project milestones for each division. At the end of the performance period, the Research & Development Committee assists the Board of Directors and the Compensation Committee in evaluating performance against the innovation targets at the end of the cycle. The weighting of this measure is 25%.
Until 2014 (2013 for the CEO and other key executives), the OLTPP was available. The rewards are based on rolling three year performance objectives focused on the Novartis Economic Value Added (NVA). The NVA is calculated based on Group operating income and income from associated companies adjusted for interest, taxes and cost of capital charge. The performance realization of a plan cycle is obtained right after the end of the third plan year by adding together the annual NVA realizations of all plan years of the plan cycle. The performance ratio for a plan cycle is obtained by dividing the performance realization for the plan cycle with the performance target for the plan cycle, expressing the result as a percentage. The OLTPP only allows a payout if the actual NVA exceeds predetermined target thresholds. The payout is capped at 200% of target.
Under the LTPP and OLTPP, participants are granted a target number of Performance Share Units (PSUs) at the beginning of every performance period, which are converted into Novartis shares after the performance period. PSUs do not carry voting rights, but do carry dividend equivalents that are reinvested in additional PSUs and paid at vesting to the extent that performance conditions have been met. PSUs granted under the OLTPPs do not carry any dividend, dividend equivalent or voting rights.
F-86
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
26. Equity-Based Participation Plans for Associates (Continued)
At the end of the three-year performance period, the Compensation Committee adjusts the target number of PSUs earned based on actual performance. PSUs are converted into unrestricted Novartis shares without an additional vesting period.
In 2015, 0.4 million LTPP PSUs (2014: 0.3 million LTPP PSUs) based on achieving 100% of target were granted to 164 key executives. No PSUs were granted in 2015 under the OLTPP (2014: 0.2 million OLTPP PSUs).
Long-Term Relative Performance Plan (LTRPP)
The Long-Term Relative Performance Plan, was introduced in 2014, and is an equity plan for the CEO and other key executives. The target incentive is 100% of base compensation for the CEO and ranges from 30% to 90% for other key executives. It is capped at 200% of target. LTRPP is based on the achievement of long-term Group Total Shareholder Return (TSR) versus our peer group of 12 companies in the healthcare industry over rolling three-year performance periods. TSR is calculated in $ as share price growth plus dividends over the three-year performance period. The calculation will be based on Bloomberg standard published TSR data, which is publicly available. The position in the peer group determines the payout range.
The fair value of the LTRPP award was determined to be CHF 48.58 and $56.60 as of the grant date. In 2015, a total of 0.1 million LTRPP PSUs (2014: 0.1 million LTRPP PSUs) based on achieving 100% of target were granted to 12 executives.
Selected associates, excluding the Executive Committee members, may exceptionally receive Special Share Awards of restricted shares or RSUs. These Special Share Awards provide an opportunity to reward outstanding achievements or exceptional performance and aim at retaining key contributors. They are based on a formal internal selection process, in which the individual performance of each candidate is thoroughly assessed at several management levels. Special Share Awards generally have a five-year vesting period. In exceptional circumstances, Special Share Awards may be rewarded to attract special expertise and new talents into the organization. These grants are consistent with market practice and Novartis' philosophy to attract, retain and motivate best-in-class talents around the world.
Worldwide 848 associates at different levels in the organization were awarded 0.8 million restricted shares and RSUs in 2015 (2014: 0.8 million restricted shares and RSUs).
In addition, in 2015, Board members received 32,087 unrestricted shares as part of their regular compensation.
F-87
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
26. Equity-Based Participation Plans for Associates (Continued)
Summary of non-vested share movements
The table below provides a summary of non-vested share movements (restricted shares, RSUs and PSUs) for all plans:
| |
2015 | 2014 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of shares in millions |
Fair value in $ m |
Number of shares in millions |
Fair value in $ m |
|||||||||
Non-vested shares at January 1 |
24.2 | 1,702.5 | 23.1 | 1,370.6 | |||||||||
Granted |
12.4 | 1,157.0 | 14.5 | 1,153.4 | |||||||||
Vested |
(14.4 | ) | (968.9 | ) | (11.5 | ) | (709.2 | ) | |||||
Forfeited |
(2.1 | ) | (139.6 | ) | (1.9 | ) | (112.3 | ) | |||||
| | | | | | | | | | | | | | |
Non-vested shares at December 31 |
20.1 | 1,751.0 | 24.2 | 1,702.5 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Alcon, Inc., Equity Plans Granted to Associates Prior to the Merger
At the completion of the merger of Alcon, Inc., into Novartis on April 8, 2011, all awards outstanding under the Alcon equity plans were converted into awards based upon Novartis shares with a conversion factor of 3.0727 as defined in the Merger Agreement. There were no grants in 2015 and 2014, although certain of the unvested awards under the Alcon equity plans continued to have expense in 2014.
Share options and share-settled appreciation rights
Share options entitle the recipient to purchase Novartis shares at the closing market price of the former Alcon, Inc., share on the day of grant divided by the conversion factor.
Share-settled appreciation rights (SSAR) entitle the participant to receive, in the form of Novartis shares, the difference between the values of the former Alcon, Inc., share at the date of grant, converted into Novartis shares using the conversion factor, and the Novartis share price at the date of exercise.
F-88
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
26. Equity-Based Participation Plans for Associates (Continued)
The following table shows the activity associated with the converted Novartis share options and SSARs during 2015 and 2014:
| |
Number of options |
Weighted average exercise price |
Number of SSARs |
Weighted average exercise price |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(millions) |
($) |
(millions) |
($) |
|||||||||
Outstanding at January 1, 2014 |
1.2 | 27.7 | 3.1 | 36.3 | |||||||||
Exercised |
(0.5 | ) | 24.4 | (0.7 | ) | 38.7 | |||||||
| | | | | | | | | | | | | | |
Outstanding at December 31, 2014 |
0.7 | 30.1 | 2.4 | 35.6 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Exercisable at December 31, 2014 |
0.7 | 30.1 | 2.4 | 35.6 | |||||||||
| | | | | | | | | | | | | | |
Outstanding at January 1, 2015 |
0.7 | 30.1 | 2.4 | 35.6 | |||||||||
Exercised |
(0.5 | ) | 27.4 | (0.6 | ) | 32.5 | |||||||
| | | | | | | | | | | | | | |
Outstanding at December 31, 2015 |
0.2 | 36.8 | 1.8 | 36.6 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Exercisable at December 31, 2015 |
0.2 | 36.8 | 1.8 | 36.6 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
27. Transactions with Related Parties
Novartis has two agreements with Genentech, Inc., USA, a subsidiary of Roche Holding AG which is indirectly included in the consolidated financial statements using equity accounting since Novartis holds 33.3% of the outstanding voting shares of Roche.
Lucentis
Novartis has licensed the exclusive rights to develop and market Lucentis outside the United States for indications related to diseases of the eye. As part of this agreement, Novartis paid Genentech/Roche an initial milestone and shared the cost for the subsequent development by making additional milestone payments upon the achievement of certain clinical development points and product approval. Novartis also pays royalties on the net sales of Lucentis products outside the United States. In 2015, Lucentis sales of $2.1 billion (2014: $2.4 billion, 2013: $2.4 billion) have been recognized by Novartis.
In November 2015, Genentech/Roche entered into an agreement with Novartis resulting from an opt-in right related to Novartis entering into a Licensing and Commercialization agreement with Ophthotech Corporation to commercialize pegpleranib (otherwise known as Fovista and OAP030) to treat wet age-related macular degeneration (AMD) and various presentations or combinations with pegpleranib outside of the United States. Pursuant to the agreement, Novartis and Genentech/Roche will share in some development costs related to pegpleranib and if development is successful, Novartis will pay royalties on the net sales of pegpleranib outside of the United States.
F-89
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
27. Transactions with Related Parties (Continued)
Xolair
In February 2004, Novartis Pharma AG, Genentech, Inc., and Tanox, Inc., finalized a three-party collaboration to govern the development and commercialization of certain anti-IgE antibodies including Xolair and TNX-901. Under this agreement, all three parties co-developed Xolair. On August 2, 2007, Genentech, Inc. completed the acquisition of Tanox, Inc. and has taken over its rights and obligations. Novartis and Genentech/Roche are co-promoting Xolair in the United States where Genentech/Roche records all sales. Novartis records sales outside of the United States.
Novartis markets Xolair and records all sales and related costs outside the United States as well as co-promotion costs in the United States. Genentech/Roche and Novartis share the resulting profits from sales in the United States, Europe and other countries, according to agreed profit-sharing percentages. In 2015, Novartis recognized total sales of Xolair of $755 million (2014: $777 million, 2013: $613 million) including sales to them for the United States market.
The net expense for royalties, cost sharing and profit sharing arising out of the Lucentis and Xolair agreements with Genentech/Roche totaled $309 million in 2015 (2014: $536 million, 2013: $570 million).
Furthermore, Novartis has several patent license, supply and distribution agreements with Roche.
Executive Officer and Non-Executive Director Compensation
During 2015, there were 11 Executive Committee members ("Executive Officers"), including those who stepped down during the year (14 members in 2014 also including those who stepped down, 12 members in 2013 also including those who stepped down).
The total compensation for members of the Executive Committee and the 12 Non-Executive Directors (14 in 2014, 15 in 2013) using the Group's accounting policies for equity-based compensation and pension benefits was as follows:
| |
Executive Officers | Non-Executive Directors |
Total | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | 2015 | 2014 | 2013 | 2015 | 2014 | 2013 | |||||||||||||||||||
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||||||||
Benefits other than equity-based amounts |
17.1 | 18.3 | 16.0 | 4.7 | 6.2 | 8.6 | 21.8 | 24.5 | 24.6 | |||||||||||||||||||
Post-employment benefits |
1.9 | 2.1 | 1.9 | 0.1 | 1.4 | 1.9 | 2.2 | 3.3 | ||||||||||||||||||||
Termination benefits |
4.0 | 4.0 | ||||||||||||||||||||||||||
Equity-based compensation |
52.9 | 81.7 | 46.5 | 4.4 | 4.9 | 5.7 | 57.3 | 86.6 | 52.2 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total |
71.9 | 102.1 | 68.4 | 9.1 | 11.2 | 15.7 | 81.0 | 113.3 | 84.1 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
During 2015, there was a decrease in the IFRS compensation expense for Executive Committee members compared to 2015, mainly due to the decrease in number of Executive Committee members.
F-90
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
27. Transactions with Related Parties (Continued)
During 2014, there was an increase in the IFRS expense, compared to 2013, for equity-based compensation for Executive Committee members principally due to the following factors:
- –
- In the year, certain Executive Committee members either retired or met the early retirement conditions resulting in an accelerated
expense under IFRS, in accordance with the relevant plan terms.
- –
- There was a net increase in the number of Executive Committee members; higher equity-based compensation expense for prior year grants not yet fully vested; and the expense relating to the loss of equity-based entitlements from a previous employer.
The annual incentive award, which is fully included in equity-based compensation even when paid out in cash, is granted in January in the year following the reporting period.
Transactions with Former Members of the Board of Directors
During 2015 and 2014, no payments (or waivers of claims) were made to former Board members or to "persons closely" linked to them, except for the following amounts:
Prof. Dr. William R. Brody and Prof. Dr. Rolf M. Zinkernagel, who stepped down from the Board of Directors at the 2014 AGM, received delegated Board membership fees for their work on the Boards of the Novartis Institute for Tropical Diseases (Prof. Dr. Zinkernagel) and the Genomics Institute of the Novartis Research Foundation (Prof. Dr. Brody and Prof. Dr. Zinkernagel). During 2015, an amount of CHF 100,000 and CHF 200,000 was paid to Prof. Dr. Brody and Prof. Dr. Zinkernagel, respectively, for their work on these Boards. Their mandate on the Board of the Genomics Institute of the Novartis Research Foundation ended as of November 19, 2015.
Dr. Alex Krauer, Honorary Chairman, is entitled to an amount of CHF 60,000 for annual periods from one AGM to the next. This amount was fixed in 1998 upon his departure from the Board in 1999, and has not been revised since that date. An amount of CHF 60,000 was paid to Dr. Krauer during 2015. Due to a change in the timing of payments, an amount of CHF 45,000 was paid to Dr. Krauer, during 2014.
In 2015, Dr. Daniel Vasella, Honorary Chairman, received the contractual minimum compensation of $250,000 (2014: $363,552) under an agreement which became effective on November 1, 2013 and will last until the end of 2016. Under this agreement, Dr. Vasella is compensated at a rate of $25,000 per day, with an annual guaranteed minimum fee of $250,000. This amount is in line with compensation practices at other large companies when retired Chairmen or CEOs were retained in consulting agreements after leaving the board of directors.
In 2014, Dr. Vasella acquired an asset from a consolidated entity at fair value and exercised an option to acquire, at a future date, real estate in Risch, Zug, Switzerland. The real estate transaction closed in 2015 and Dr. Vasella acquired the Group assets from a consolidated entity for an arm's length transaction price determined on the basis of two independent external assessments.
In 2013, Dr. Vasella received a total amount of CHF 5.1 million from the date of the AGM, when he stepped down as Chairman and Board member, to December 31, 2013.
F-91
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
27. Transactions with Related Parties (Continued)
Transactions with a Future Executive Officer
As announced on September 24, 2015, Dr. James E. Bradner will succeed Dr. Mark Fishman as President of the Novartis Institutes for BioMedical Research (NIBR) and member of the ECN with effect from March 1, 2016. In 2015, a subsidiary acquired Dr. Bradner's 10 million shares (7% interest) in a non-material entity for $10 million. The arm's length transaction price was determined based on the most recent round of financing of this entity.
The above disclosures related to Dr. Vasella and Dr. Bradner are made on a voluntary basis.
28. Commitments and Contingencies
The Group has entered into various fixed term operational leases, mainly for cars and real estate. As of December 31, 2015 the Group's commitments with respect to these leases, including estimated payment dates, were as follows:
| |
2015 | |||
|---|---|---|---|---|
| |
$ m |
|||
2016 |
273 | |||
2017 |
202 | |||
2018 |
133 | |||
2019 |
103 | |||
2020 |
104 | |||
Thereafter |
2,181 | |||
| | | | | |
Total |
2,996 | |||
| | | | | |
| | | | | |
| | | | | |
Expense of current year |
313 | |||
| | | | | |
| | | | | |
| | | | | |
Research & Development Commitments
The Group has entered into long-term research agreements with various institutions which provide for potential milestone payments and other payments by Novartis that may be capitalized. As of
F-92
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
28. Commitments and Contingencies (Continued)
December 31, 2015 the Group's commitments to make payments under those agreements, and their estimated timing, were as follows:
| |
Unconditional commitments |
Potential milestone payments |
Total 2015 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
2016 |
88 | 601 | 689 | |||||||
2017 |
61 | 343 | 404 | |||||||
2018 |
86 | 438 | 524 | |||||||
2019 |
65 | 152 | 217 | |||||||
2020 |
200 | 474 | 674 | |||||||
Thereafter |
150 | 397 | 547 | |||||||
| | | | | | | | | | | |
Total |
650 | 2,405 | 3,055 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The Novartis Group entered into various purchase commitments for services and materials as well as for equipment in the ordinary course of business. These commitments are generally entered into at current market prices and reflect normal business operations.
Group companies have to observe the laws, government orders and regulations of the country in which they operate.
The Group's potential environmental remediation liability is assessed based on a risk assessment and investigation of the various sites identified by the Group as at risk for environmental remediation exposure. The Group's future remediation expenses are affected by a number of uncertainties. These uncertainties include, but are not limited to, the method and extent of remediation, the percentage of material attributable to the Group at the remediation sites relative to that attributable to other parties, and the financial capabilities of the other potentially responsible parties.
A number of Group companies are currently involved in administrative proceedings, litigations and investigations arising out of the normal conduct of their business. These litigations include product liabilities, governmental investigations and other legal matters. While provisions have been made for probable losses, which management deems to be reasonable or appropriate, there are uncertainties connected with these estimates.
Note 20 contains a more extensive discussion of these matters.
A number of Group companies are involved in legal proceedings concerning intellectual property rights. The inherent unpredictability of such proceedings means that there can be no assurances as to their ultimate outcome. A negative result in any such proceeding could potentially adversely affect the ability of certain Novartis companies to sell their products or require the payment of substantial damages or royalties.
In the opinion of management, however, the outcome of these actions will not materially affect the Group's financial position but could be material to the results of operations or cash flow in a given period.
F-93
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures
| |
Note | 2015(1) | 2014(1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
$ m |
$ m |
|||||||
Cash and cash equivalents |
16 | 4,674 | 13,023 | |||||||
Financial assets—measured at fair value through other comprehensive income |
||||||||||
Available-for-sale marketable securities |
||||||||||
Debt securities |
16 | 339 | 327 | |||||||
Equity securities |
16 | 6 | 15 | |||||||
Fund investments |
16 | 33 | 35 | |||||||
| | | | | | | | | | | |
Total available-for-sale marketable securities |
378 | 377 | ||||||||
| | | | | | | | | | | |
Available-for-sale long-term financial investments |
||||||||||
Equity securities |
13 | 1,173 | 937 | |||||||
Fund investments |
13 | 90 | 71 | |||||||
Contingent consideration receivables |
13 | 550 | ||||||||
| | | | | | | | | | | |
Total available-for-sale long-term financial investments |
1,813 | 1,008 | ||||||||
| | | | | | | | | | | |
Total financial assets—measured at fair value through other comprehensive income |
2,191 | 1,385 | ||||||||
| | | | | | | | | | | |
Financial assets—measured at amortized costs |
||||||||||
Trade receivables and other current assets (excluding pre-payments) |
15/17 | 10,551 | 10,255 | |||||||
Accrued interest on debt securities and time deposits |
16 | 2 | 3 | |||||||
Time deposits with original maturity more than 90 days |
16 | 164 | 6 | |||||||
Long-term loans and receivables from customers and finance lease, advances, security deposits |
13 | 653 | 712 | |||||||
| | | | | | | | | | | |
Total financial assets—measured at amortized costs |
11,370 | 10,976 | ||||||||
| | | | | | | | | | | |
Financial assets—measured at fair value through the consolidated income statement |
||||||||||
Associated companies at fair value through profit and loss |
181 | 234 | ||||||||
Derivative financial instruments |
16 | 143 | 356 | |||||||
| | | | | | | | | | | |
Total financial assets—measured at fair value through the consolidated income statement |
324 | 590 | ||||||||
| | | | | | | | | | | |
Total financial assets |
18,559 | 25,974 | ||||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Financial liabilities—measured at amortized costs |
||||||||||
Current financial debt |
||||||||||
Interest bearing accounts of associates payable on demand |
21 | 1,645 | 1,651 | |||||||
Bank and other financial debt |
21 | 1,185 | 1,272 | |||||||
Commercial paper |
21 | 1,085 | 648 | |||||||
Current portion of non-current debt |
21 | 1,659 | 2,989 | |||||||
| | | | | | | | | | | |
Total current financial debt |
5,574 | 6,560 | ||||||||
| | | | | | | | | | | |
Non-current financial debt |
||||||||||
Straight bonds |
19 | 17,193 | 15,982 | |||||||
Liabilities to banks and other financial institutions |
19 | 706 | 803 | |||||||
Finance lease obligations |
19 | 87 | 3 | |||||||
Current portion of non-current debt |
19 | (1,659 | ) | (2,989 | ) | |||||
| | | | | | | | | | | |
Total non-current financial debt |
16,327 | 13,799 | ||||||||
| | | | | | | | | | | |
Trade payables and commitment for repurchase of own shares (see Note 22) |
5,668 | 6,077 | ||||||||
| | | | | | | | | | | |
Total financial liabilites—measured at amortized costs |
27,569 | 26,436 | ||||||||
| | | | | | | | | | | |
Financial liabilities—measured at fair value through the consolidated income statement |
||||||||||
Contingent consideration (see Note 20/22) and other financial liabilities |
1,105 | 756 | ||||||||
Derivative financial instruments |
21 | 30 | 52 | |||||||
| | | | | | | | | | | |
Total financial liabilities—measured at fair value through the consolidated income statement |
1,135 | 808 | ||||||||
| | | | | | | | | | | |
Total financial liabilities |
28,704 | 27,244 | ||||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- Except for straight bonds (see Note 19) the carrying amount is a reasonable approximation of fair value.
Derivative Financial Instruments
The following tables show the contract or underlying principal amounts and fair values of derivative financial instruments analyzed by type of contract at December 31, 2015 and 2014. Contract or underlying principal amounts indicate the volume of business outstanding at the consolidated balance sheet date and
F-94
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
do not represent amounts at risk. The fair values are determined by reference to market prices or standard pricing models that use observable market inputs at December 31, 2015 and 2014.
| |
Contract or underlying principal amount |
Positive fair values |
Negative fair values |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2015 | 2014 | 2015 | 2014 | |||||||||||||
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Currency related instruments |
|||||||||||||||||||
Forward foreign exchange rate contracts |
8,795 | 10,072 | 142 | 283 | (30 | ) | (52 | ) | |||||||||||
Over-the-Counter currency options |
459 | 1,715 | 1 | 73 | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total of currency related instruments |
9,254 | 11,787 | 143 | 356 | (30 | ) | (52 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total derivative financial instruments included in marketable securities and in current financial debts |
9,254 | 11,787 | 143 | 356 | (30 | ) | (52 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The following table shows by currency contract or underlying principal amount the derivative financial instruments at December 31, 2015 and 2014:
December 31, 2015
|
EUR | $ | JPY | Other | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Currency related instruments |
||||||||||||||||
Forward foreign exchange rate contracts |
2,828 | 4,713 | 42 | 1,212 | 8,795 | |||||||||||
Over-the-Counter currency options |
459 | 459 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Total of currency related instruments |
3,287 | 4,713 | 42 | 1,212 | 9,254 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total derivative financial instruments |
3,287 | 4,713 | 42 | 1,212 | 9,254 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
December 31, 2014
|
EUR | $ | JPY | Other | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Currency related instruments |
||||||||||||||||
Forward foreign exchange rate contracts |
3,681 | 3,159 | 38 | 3,194 | 10,072 | |||||||||||
Over-the-Counter currency options |
1,215 | 500 | 1,715 | |||||||||||||
| | | | | | | | | | | | | | | | | |
Total of currency related instruments |
4,896 | 3,659 | 38 | 3,194 | 11,787 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total derivative financial instruments |
4,896 | 3,659 | 38 | 3,194 | 11,787 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Derivative financial instruments effective for hedge accounting purposes
At the end of 2015 and 2014, there were no open hedging instruments for anticipated transactions.
F-95
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
As required by IFRS, financial assets and liabilities recorded at fair value in the consolidated financial statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. There are three hierarchical levels, based on an increasing amount of subjectivity associated with the inputs to derive fair valuation for these assets and liabilities, which are as follows:
The assets carried at Level 1 fair value are equity and debt securities listed in active markets.
The assets generally included in Level 2 fair value hierarchy are foreign exchange and interest rate derivatives and certain debt securities. Foreign exchange derivatives and interest rate derivatives are valued using corroborated market data. The liabilities generally included in this fair value hierarchy consist of foreign exchange and interest rate derivatives.
Level 3 inputs are unobservable for the asset or liability. The assets generally included in Level 3 fair value hierarchy are various investments in hedge funds and unquoted equity security investments. Contingent consideration carried at fair value is included in this category.
2015
|
Level 1 | Level 2 | Level 3 | Valued at amortized cost |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Financial assets |
||||||||||||||||
Debt securities |
316 | 23 | 339 | |||||||||||||
Equity securities |
6 | 6 | ||||||||||||||
Fund investments |
29 | 4 | 33 | |||||||||||||
| | | | | | | | | | | | | | | | | |
Total available-for-sale marketable securities |
351 | 23 | 4 | 378 | ||||||||||||
Time deposits with original maturity more than 90 days |
164 | 164 | ||||||||||||||
Derivative financial instruments |
143 | 143 | ||||||||||||||
Accrued interest on debt securities |
2 | 2 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Total marketable securities, time deposits and derivative financial instruments |
351 | 166 | 4 | 166 | 687 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Available-for-sale financial investments |
700 | 473 | 1,173 | |||||||||||||
Fund investments |
90 | 90 | ||||||||||||||
Contingent consideration receivables |
550 | 550 | ||||||||||||||
Long-term loans and receivables from customers and finance lease, advances, security deposits |
653 | 653 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Financial investments and long-term loans |
700 | 1,113 | 653 | 2,466 | ||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Associated companies at fair value through profit and loss |
181 | 181 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Financial liabilities |
||||||||||||||||
Contingent consideration payables |
(790 | ) | (790 | ) | ||||||||||||
Other financial liabilities |
(315 | ) | (315 | ) | ||||||||||||
Derivative financial instruments |
(30 | ) | (30 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | |
Total financial liabilities at fair value |
(30 | ) | (1,105 | ) | (1,135 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
F-96
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
2014
|
Level 1 | Level 2 | Level 3 | Valued at amortized cost |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Financial assets |
||||||||||||||||
Debt securities |
301 | 26 | 327 | |||||||||||||
Equity securities |
15 | 15 | ||||||||||||||
Fund investments |
29 | 6 | 35 | |||||||||||||
| | | | | | | | | | | | | | | | | |
Total available-for-sale marketable securities |
345 | 26 | 6 | 377 | ||||||||||||
Time deposits with original maturity more than 90 days |
6 | 6 | ||||||||||||||
Derivative financial instruments |
356 | 356 | ||||||||||||||
Accrued interest on debt securities |
3 | 3 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Total marketable securities, time deposits and derivative financial instruments |
345 | 382 | 6 | 9 | 742 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Available-for-sale financial investments |
605 | 332 | 937 | |||||||||||||
Fund investments |
71 | 71 | ||||||||||||||
Long-term loans and receivables from customers and finance lease, advances, security deposits |
712 | 712 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Financial investments and long-term loans |
605 | 403 | 712 | 1,720 | ||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Associated companies at fair value through profit and loss |
66 | 168 | 234 | |||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Financial liabilities |
||||||||||||||||
Contingent consideration payables |
(756 | ) | (756 | ) | ||||||||||||
Derivative financial instruments |
(52 | ) | (52 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | |
Total financial liabilities at fair value |
(52 | ) | (756 | ) | (808 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
The analysis above includes all financial instruments including those measured at amortized cost or at cost.
F-97
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
The change in carrying values associated with Level 3 financial instruments using significant unobservable inputs during the year ended December 31 are set forth below:
2015
|
Associated Companies at fair value through profit and loss |
Fund investments |
Available- for-sale financial investments |
Contingent Consideration Receivables and other current financial assets |
Contingent consideration payables and other financial liabilities |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
January 1 |
168 | 77 | 332 | 756 | ||||||||||||
Impact of business combinations |
75 | |||||||||||||||
Fair value gains and other adjustments, including from divestments recognized in the consolidated income statement |
9 | 7 | 41 | 1,000 | ||||||||||||
Fair value losses (including impairments and amortizations) and other adjustments recognized in the consolidated income statement |
(25 | ) | (1 | ) | (35 | ) | (75 | ) | 644 | |||||||
Gains recognized in the consolidated statement of comprehensive income |
17 | 22 | ||||||||||||||
Purchases |
62 | 24 | 142 | 255 | ||||||||||||
Cash receipts and payments |
(450 | ) | (550 | ) | ||||||||||||
Proceeds from sales |
(15 | ) | (56 | ) | ||||||||||||
At equity investments reclassified due to loss of significant influence |
18 | |||||||||||||||
Reclassification |
(33 | ) | (15 | ) | 9 | |||||||||||
Currency translation effects |
||||||||||||||||
| | | | | | | | | | | | | | | | | |
December 31 |
181 | 94 | 473 | 550 | 1,105 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Total of fair value gains and losses recognized in the consolidated income statement for assets and liabilities held at December 31, 2015 |
(16 | ) | 6 | 6 | 925 | 644 | ||||||||||
F-98
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
2014
|
Associated Companies at fair value through profit and loss |
Equity securities |
Fund investments |
Available- for-sale financial investments |
Contingent consideration payables |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
January 1 |
0 | 26 | 63 | 366 | 572 | |||||||||||
Fair value gains recognized in the consolidated income statement |
12 | 2 | 17 | 51 | ||||||||||||
Fair value losses (including impairments and amortizations) recognized in the consolidated income statement |
(24 | ) | (51 | ) | (20 | ) | ||||||||||
Gains recognized in the consolidated statement of comprehensive income |
3 | 3 | 7 | |||||||||||||
Purchases |
27 | 7 | 140 | 153 | ||||||||||||
Proceeds from sales |
(26 | ) | (9 | ) | (23 | ) | ||||||||||
Reclassification |
179 | (29 | ) | 16 | (114 | ) | ||||||||||
Currency translation effects |
(5 | ) | (10 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | |
December 31 |
168 | 0 | 77 | 332 | 756 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Total of fair value gains and losses recognized in the consolidated income statement for assets and liabilities held at December 31, 2014 |
(12 | ) | 2 | (34 | ) | 31 | ||||||||||
No significant transfers from one level to the other occurred during the reporting period. Realized gains and losses associated with Level 3 available-for-sale marketable securities are recorded in the consolidated income statement under "Other financial income and expense" and gains and losses associated with Level 3 available-for-sale financial investments are recorded in the consolidated income statement under "Other income" or "Other expense", respectively.
If the pricing parameters for the Level 3 input were to change for associated companies at fair value through profit and loss, equity securities, fund investments and for available-for-sale financial investments by 10% positively or negatively, respectively, this would change the amounts recorded in the consolidated statement of comprehensive income by $75 million.
For the determination of the fair value of a contingent consideration various unobservable inputs are used. A change in these inputs might result in a significantly higher or lower fair value measurement. The significance and usage of these inputs may vary amongst the existing contingent considerations due to differences in the triggering events for payments or in the nature of the asset the contingent consideration relates to. Amongst others, the inputs used are the probability of success, sales forecast and assumptions regarding the discount rate, timing and different scenarios of triggering events. The inputs are interrelated. If the most significant parameters for the Level 3 input were to change by 10% positively or negatively, or where the probability of success (POS) is the most significant input parameter 10% were to be added or deducted from the applied POS for contingent consideration payables and other financial liabilities and contingent consideration receivables and other current financial assets, this would change
F-99
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
the amounts recorded in the consolidated income statement by $201 million and $196 million, respectively.
Nature and Extent of Risks Arising From Financial Instruments
Market Risk
Novartis is exposed to market risk, primarily related to foreign currency exchange rates, interest rates and the market value of the investments of liquid funds. The Group actively monitors and seeks to reduce, where it deems it appropriate to do so, fluctuations in these exposures. It is the Group's policy and practice to enter into a variety of derivative financial instruments to manage the volatility of these exposures and to enhance the yield on the investment of liquid funds. It does not enter any financial transactions containing a risk that cannot be quantified at the time the transaction is concluded. In addition, it does not sell short assets it does not have, or does not know it will have, in the future. The Group only sells existing assets or enters into transactions and future transactions (in the case of anticipatory hedges) that it confidently expects it will have in the future, based on past experience. In the case of liquid funds, the Group writes call options on assets it has or it writes put options on positions it wants to acquire and has the liquidity to acquire. The Group expects that any loss in value for these instruments generally would be offset by increases in the value of the underlying transactions.
Foreign currency Exchange Rate Risk
The Group uses the $ as its reporting currency. As a result, the Group is exposed to foreign currency exchange movements, primarily in European, Japanese and emerging market currencies. Fluctuations in the exchange rates between the US dollar and other currencies can have a significant effect on both the Group's results of operations, including reported sales and earnings, as well as on the reported value of our assets, liabilities and cash flows. This in turn may significantly affect the comparability of period-to-period results of operations.
Because our expenditures in Swiss francs are significantly higher than our revenues in Swiss francs, volatility in the value of the Swiss franc can have a significant impact on the reported value of our earnings, assets and liabilities, and the timing and extent of such volatility can be difficult to predict. In addition, there is a risk that certain countries could take other steps which could significantly impact the value of their currencies.
The Group is exposed to a potential adverse devaluation risk on its intercompany funding and total investment in certain subsidiaries operating in countries with exchange controls. The most significant country in this respect is Venezuela, where the Group has an equivalent of approximately $0.2 billion of cash in local currency, which is only slowly being approved for remittance outside of the country. As a result, the Group is exposed to a potential devaluation loss in the income statement on its total intercompany balances with its subsidiaries in Venezuela, which at December 31, 2015 amounted to $0.3 billion.
In 2014 and through October 2015, the exchange rate used by the Group for consolidation of the financial statements of its Venezuela subsidiaries was the official exchange rate for the Venezuela bolivar (VEF) of VEF 6.3/$, which is available for imports of specific goods and services of national priority, including medicines and medical supplies, as published by the Centro Nacional de Comercio Exterior (CENCOEX, formerly CADIVI).
F-100
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
In November 2015, a Venezuela subsidiary of the Group agreed with CENCOEX to settle a substantial part of our intercompany trade payables dated on or before December 31, 2014 in a transaction that required the Venezuela subsidiary to purchase a $ denominated bond at par value issued by Petróleos de Venezuela (PDVSA), with a coupon rate of 6% per annum maturing in 2024. In Venezuela there are differing official exchange rates against the $ and for the settlement of these intercompany trade payables, through the purchase of the $ bond, CENCOEX set the exchange rate at VEF 11.0/$. As a result, from November 2015 the Group changed its exchange rate used for consolidation of the financial statements of its Venezuela subsidiaries. The use of the new exchange rate by the Venezuela subsidiaries resulted in a $211 million loss from the re-measurement of the intra-Group and third party liabilities.
Novartis seeks to manage currency exposure by engaging in hedging transactions where management deems appropriate. Novartis may enter into various contracts that reflect the changes in the value of foreign currency exchange rates to preserve the value of assets, commitments and anticipated transactions. Novartis also uses forward contracts and foreign currency option contracts to hedge.
Net investments in subsidiaries in foreign countries are long-term investments. Their fair value changes through movements of foreign currency exchange rates. The Group only hedges the net investments in foreign subsidiaries in exceptional cases.
Commodity Price Risk
The Group has only a very limited exposure to price risk related to anticipated purchases of certain commodities used as raw materials by the Group's businesses. A change in those prices may alter the gross margin of a specific business, but generally by not more than 10% of the margin and thus below the Group's risk management tolerance levels. Accordingly, the Group does not enter into significant commodity futures, forward and option contracts to manage fluctuations in prices of anticipated purchases.
Interest Rate Risk
The Group addresses its net exposure to interest rate risk mainly through the ratio of its fixed rate financial debt to variable rate financial debt contained in its total financial debt portfolio. To manage this mix, Novartis may enter into interest rate swap agreements, in which it exchanges periodic payments based on a notional amount and agreed upon fixed and variable interest rates.
Equity Risk
The Group may purchase equities as investments of its liquid funds. As a policy, it limits its holdings in an unrelated company to less than 5% of its liquid funds. Potential investments are thoroughly analyzed. Call options are written on equities that the Group owns, and put options are written on equities which the Group wants to buy and for which cash is available.
Credit Risk
Credit risks arise from the possibility that customers may not be able to settle their obligations as agreed. To manage this risk the Group periodically assesses the financial reliability of customers, taking
F-101
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
into account their financial position, past experience and other factors. Individual risk limits are set accordingly.
The Group's largest customer accounts for approximately 14% of net sales, and the second and third largest customers account for 11% and 5% of net sales, respectively (2014: 12%, 11% and 5% respectively). No other customer accounted for 5% or more of net sales, in either year.
The highest amounts of trade receivables outstanding were for these same three customers. They amounted to 13%, 9% and 6%, respectively, of the trade receivables at December 31, 2015. There is no other significant concentration of credit risk (2014: 13%, 9% and 5% respectively).
Counterparty Risk
Counterparty risk encompasses issuer risk on marketable securities and money market instruments, credit risk on cash, time deposits and derivatives as well as settlement risk for different instruments. Issuer risk is reduced by only buying securities which are at least A- rated. Counterparty credit risk and settlement risk are reduced by a policy of entering into transactions with counterparties (banks or financial institutions) that feature a strong credit rating. For short-term investments of less than six months of maturity, the counterparty must be at least A-1/P-1/F-1 rated. Exposure to these risks is closely monitored and kept within predetermined parameters. The limits are regularly assessed and determined based upon credit analysis including financial statement and capital adequacy ratio reviews. In addition, reverse repurchasing agreements are contracted and Novartis has entered into credit support agreements with various banks for derivative transactions.
The Group's cash and cash equivalents are held with major regulated financial institutions, the three largest ones hold approximately 21.8%, 9.6% and 8.6%, respectively (2014: 11.8%, 7.7% and 7.7%, respectively).
The Group does not expect any losses from non-performance by these counterparties and does not have any significant grouping of exposures to financial sector or country risk.
Liquidity Risk
Liquidity risk is defined as the risk that the Group could not be able to settle or meet its obligations on time or at a reasonable price. Group Treasury is responsible for liquidity, funding as well as settlement management. In addition, liquidity and funding risks, related processes and policies are overseen by management. Novartis manages its liquidity risk on a consolidated basis based on business needs, tax, capital or regulatory considerations, if applicable, through numerous sources of financing in order to maintain flexibility. Management monitors the Group's net debt or liquidity position through rolling forecasts on the basis of expected cash flows.
Novartis has two US commercial paper programs under which it can issue up to $9 billion in the aggregate of unsecured commercial paper notes. Novartis also has a Japanese commercial paper program under which it can issue up to JPY 150 billion (approximately $1.25 billion) of unsecured commercial paper notes. Commercial paper notes totaling $1.1 billion under these three programs were outstanding as per December 31, 2015. Novartis further has a committed credit facility of $6 billion, entered into on September 23, 2015. This credit facility is provided by a syndicate of banks and is intended to be used as a backstop for the US commercial paper programs. It matures in September 2020 and was undrawn as per December 31, 2015.
F-102
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
The following table sets forth how management monitors net debt or liquidity based on details of the remaining contractual maturities of current financial assets and liabilities excluding trade receivables and payables and contingent considerations at December 31, 2015 and 2014:
December 31, 2015
|
Due within one month |
Due later than one month but less than three months |
Due later than three months but less than one year |
Due later than one year but less than five years |
Due after five years |
Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Current assets |
|||||||||||||||||||
Marketable securities and time deposits |
22 | 11 | 200 | 247 | 62 | 542 | |||||||||||||
Commodities |
86 | 86 | |||||||||||||||||
Derivative financial instruments and accrued interest |
40 | 67 | 38 | 145 | |||||||||||||||
Cash and cash equivalents |
4,674 | 4,674 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total current financial assets |
4,736 | 78 | 238 | 247 | 148 | 5,447 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Non-current liabilities |
|||||||||||||||||||
Financial debt |
(4,664 | ) | (11,663 | ) | (16,327 | ) | |||||||||||||
Financial debt—undiscounted |
(4,676 | ) | (11,797 | ) | (16,473 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total non-current financial debt |
(4,664 | ) | (11,663 | ) | (16,327 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Current liabilities |
|||||||||||||||||||
Financial debt |
(3,258 | ) | (289 | ) | (2,027 | ) | (5,574 | ) | |||||||||||
Financial debt—undiscounted |
(3,258 | ) | (289 | ) | (2,028 | ) | (5,575 | ) | |||||||||||
Derivative financial instruments |
(8 | ) | (20 | ) | (2 | ) | (30 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total current financial debt |
(3,266 | ) | (309 | ) | (2,029 | ) | (5,604 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net debt |
1,470 |
(231 |
) |
(1,791 |
) |
(4,417 |
) |
(11,515 |
) |
(16,484 |
) |
||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
F-103
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
December 31, 2014
|
Due within one month |
Due later than one month but less than three months |
Due later than three months but less than one year |
Due later than one year but less than five years |
Due after five years |
Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||||
Current assets |
|||||||||||||||||||
Marketable securities and time deposits |
21 | 68 | 37 | 181 | 76 | 383 | |||||||||||||
Commodities |
97 | 97 | |||||||||||||||||
Derivative financial instruments and accrued interest |
161 | 126 | 72 | 359 | |||||||||||||||
Cash and cash equivalents |
9,623 | 3,400 | 13,023 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total current financial assets |
9,902 | 3,594 | 109 | 181 | 76 | 13,862 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Non-current liabilities |
|||||||||||||||||||
Financial debt |
(5,423 | ) | (8,376 | ) | (13,799 | ) | |||||||||||||
Financial debt—undiscounted |
(5,434 | ) | (8,470 | ) | (13,904 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total non-current financial debt |
(5,423 | ) | (8,376 | ) | (13,799 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Current liabilities |
|||||||||||||||||||
Financial debt |
(2,678 | ) | (335 | ) | (3,547 | ) | (6,560 | ) | |||||||||||
Financial debt—undiscounted |
(2,678 | ) | (335 | ) | (3,549 | ) | (6,562 | ) | |||||||||||
Derivative financial instruments |
(18 | ) | (32 | ) | (2 | ) | (52 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total current financial debt |
(2,696 | ) | (367 | ) | (3,549 | ) | (6,612 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net debt |
7,206 |
3,227 |
(3,440 |
) |
(5,242 |
) |
(8,300 |
) |
(6,549 |
) |
|||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The consolidated balance sheet amounts of financial liabilities included in the above analysis are not materially different to the contractual amounts due on maturity. The positive and negative fair values on derivative financial instruments represent the net contractual amounts to be exchanged at maturity.
F-104
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
The Group's contractual undiscounted potential cash flows from derivative financial instruments to be settled on a gross basis are as follows:
December 31, 2015
|
Due within one month |
Due later than one month but less than three months |
Due later than three months but less than one year |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
|||||||||
Derivative financial instruments and accrued interest on derivative financial instruments |
|||||||||||||
Potential outflows in various currencies—from financial derivative liabilities |
(1,418 | ) | (2,800 | ) | (1,602 | ) | (5,820 | ) | |||||
Potential inflows in various currencies—from financial derivative assets |
1,448 | 2,819 | 1,601 | 5,868 | |||||||||
December 31, 2014
|
Due within one month |
Due later than one month but less than three months |
Due later than three months but less than one year |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
|||||||||
Derivative financial instruments and accrued interest on derivative financial instruments |
|||||||||||||
Potential outflows in various currencies—from financial derivative liabilities |
(3,549 | ) | (3,695 | ) | (2,527 | ) | (9,771 | ) | |||||
Potential inflows in various currencies—from financial derivative assets |
3,688 | 3,780 | 2,646 | 10,114 | |||||||||
F-105
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
Other contractual liabilities which are not part of management's monitoring of the net debt or liquidity consist of the following items:
December 31, 2015
|
Due later than one month but less than three months |
Due later than three months but less than one year |
Due later than one year but less than five years |
Due after five years |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Contractual interest on non-current liabilities |
(104 | ) | (499 | ) | (1,878 | ) | (4,332 | ) | (6,813 | ) | ||||||
Trade payables |
(5,668 | ) | (5,668 | ) | ||||||||||||
December 31, 2014
|
Due later than one month but less than three months |
Due later than three months but less than one year |
Due later than one year but less than five years |
Due after five years |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
$ m |
$ m |
|||||||||||
Contractual interest on non-current liabilities |
(154 | ) | (436 | ) | (1,778 | ) | (3,087 | ) | (5,455 | ) | ||||||
Trade payables and commitment for repurchase of own shares (see Note 22) |
(6,077 | ) | (6,077 | ) | ||||||||||||
Novartis strives to maintain a strong credit rating. In managing its capital, Novartis focuses on maintaining a strong balance sheet. Moody's rated the Group as Aa3 for long-term maturities and P-1 for short-term maturities and Standard & Poor's had a rating of AA- for long-term and A-1+ for short-term maturities. Fitch had a long-term rating of AA and a short-term rating of F1+.
The debt/equity ratio decreased to 0.28:1 at December 31, 2015 compared to 0.29:1 at the beginning of the year.
The Group uses a value at risk (VAR) computation to estimate the potential ten-day loss in the fair value of its financial instruments.
F-106
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
A ten-day period is used because of an assumption that not all positions could be undone in one day given the size of the positions. Apart from contingent consideration, finance lease obligations, and long-term loans and receivables, advances and security deposits the VAR computation includes all financial assets and financial liabilities as set forth above in this Note. Trade payables and receivables are considered only to the extent they comprise a foreign currency exposure. In addition, commodities are included in the computation.
The VAR estimates are made assuming normal market conditions, using a 95% confidence interval. The Group uses a "Delta Normal" model to determine the observed inter-relationships between movements in interest rates, stock markets and various currencies. These inter-relationships are determined by observing interest rate, stock market movements and forward foreign currency rate movements over a sixty-day period for the calculation of VAR amounts.
The estimated potential ten-day loss in pre-tax income from the Group's foreign currency instruments, the estimated potential ten-day loss of its equity holdings, and the estimated potential ten-day loss in fair value of its interest rate sensitive instruments (primarily financial debt and investments of liquid funds under normal market conditions) as calculated in the VAR model are the following:
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
All financial instruments |
387 | 272 | |||||
Analyzed by components: |
|||||||
Instruments sensitive to foreign currency exchange rates |
224 | 272 | |||||
Instruments sensitive to equity market movements |
50 | 48 | |||||
Instruments sensitive to interest rates |
353 | 254 | |||||
The average, high, and low VAR amounts are as follows:
2015
|
Average | High | Low | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
All financial instruments |
337 | 387 | 237 | |||||||
Analyzed by components: |
||||||||||
Instruments sensitive to foreign currency exchange rates |
313 | 418 | 173 | |||||||
Instruments sensitive to equity market movements |
55 | 111 | 33 | |||||||
Instruments sensitive to interest rates |
294 | 380 | 251 | |||||||
2014
|
Average | High | Low | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
All financial instruments |
240 | 306 | 193 | |||||||
Analyzed by components: |
||||||||||
Instruments sensitive to foreign currency exchange rates |
154 | 272 | 83 | |||||||
Instruments sensitive to equity market movements |
32 | 48 | 18 | |||||||
Instruments sensitive to interest rates |
177 | 254 | 96 | |||||||
The VAR computation is a risk analysis tool designed to statistically estimate the maximum potential ten day loss from adverse movements in foreign currency exchange rates, equity prices and interest rates
F-107
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
29. Financial Instruments—Additional Disclosures (Continued)
under normal market conditions. The computation does not purport to represent actual losses in fair value on earnings to be incurred by the Group, nor does it consider the effect of favorable changes in market rates. The Group cannot predict actual future movements in such market rates and it does not claim that these VAR results are indicative of future movements in such market rates or to be representative of any actual impact that future changes in market rates may have on the Group's future results of operations or financial position.
In addition to these VAR analyses, the Group uses stress testing techniques that aim to reflect a worst case scenario on the marketable securities which are monitored by Group Treasury. For these calculations, the Group uses the six-month period with the worst performance observed over the past twenty years in each category. For 2015 and 2014, the worst case loss scenario was calculated as follows:
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
|||||
All financial instruments |
12 | 16 | |||||
Analyzed by components: |
|||||||
Instruments sensitive to foreign currency exchange rates |
1 | 1 | |||||
Instruments sensitive to equity market movements |
4 | 8 | |||||
Instruments sensitive to interest rates |
7 | 7 | |||||
In the Group's risk analysis, Novartis considered this worst case scenario acceptable as it could reduce income, but would not endanger the solvency or the investment grade credit standing of the Group.
F-108
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
30. Discontinued Operations
Discontinued Operations Consolidated Income Statement Segmentation
| |
Vaccines | Consumer Health(1) |
Corporate (including eliminations) |
Total discontinued operations |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ m) |
2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | |||||||||||||||||
Net sales to third parties of discontinued operations |
145 | 1,537 | 456 | 4,279 | 601 | 5,816 | |||||||||||||||||||
Sales to continuing segments |
18 | 65 | 1 | 13 | 19 | 78 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net sales of discontinued operations |
163 | 1,602 | 457 | 4,292 | 620 | 5,894 | |||||||||||||||||||
Other revenues |
18 | 32 | 5 | 33 | 23 | 65 | |||||||||||||||||||
Cost of goods sold |
(192 | ) | (1,336 | ) | (184 | ) | (1,737 | ) | (376 | ) | (3,073 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit of discontinued operations |
(11 | ) | 298 | 278 | 2,588 | 267 | 2,886 | ||||||||||||||||||
Marketing & Sales |
(57 | ) | (280 | ) | (187 | ) | (1,532 | ) | (244 | ) | (1,812 | ) | |||||||||||||
Research & Development |
(151 | ) | (545 | ) | (30 | ) | (312 | ) | (181 | ) | (857 | ) | |||||||||||||
General & Administration |
(26 | ) | (118 | ) | (32 | ) | (313 | ) | (58 | ) | (431 | ) | |||||||||||||
Other income |
2,870 | 905 | 10,558 | 99 | (8 | ) | 3 | 13,420 | 1,007 | ||||||||||||||||
Other expense |
(57 | ) | (812 | ) | (14 | ) | (60 | ) | (656 | ) | (274 | ) | (727 | ) | (1,146 | ) | |||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating income/(loss) of discontinued operations |
2,568 | (552 | ) | 10,573 | 470 | (664 | ) | (271 | ) | 12,477 | (353 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Income from associated companies |
2 | 2 | 2 | 2 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Income/(loss) before taxes of discontinued operations |
12,479 | (351 | ) | ||||||||||||||||||||||
Taxes |
(1,713 | ) | (96 | ) | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income/(loss) of discontinued operations |
10,766 | (447 | ) | ||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Consumer Health is the aggregation of the OTC and Animal Health divisions.
F-109
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
30. Discontinued Operations (Continued)
Discontinued Operations Consolidated Income Statement Segmentation (Continued)
| |
Vaccines(1) | Consumer Health(2) |
Transfers to continuing Corporate(3) |
Corporate (including eliminations) |
Total discontinued operations |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (In $ m) |
2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | |||||||||||||||||||||
Net sales to third parties of discontinued operations |
1,537 | 1,987 | 4,279 | 4,064 | 5,816 | 6,051 | |||||||||||||||||||||||||
Sales to continuing segments |
65 | 61 | 13 | 11 | 78 | 72 | |||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net sales of discontinued operations |
1,602 | 2,048 | 4,292 | 4,075 | 5,894 | 6,123 | |||||||||||||||||||||||||
Other revenues |
32 | 333 | 33 | 36 | (84 | ) | 65 | 285 | |||||||||||||||||||||||
Cost of goods sold |
(1,336 | ) | (1,578 | ) | (1,737 | ) | (1,751 | ) | 7 | (3,073 | ) | (3,322 | ) | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit of discontinued operations |
298 | 803 | 2,588 | 2,360 | (77 | ) | 2,886 | 3,086 | |||||||||||||||||||||||
Marketing & Sales |
(280 | ) | (334 | ) | (1,532 | ) | (1,577 | ) | (1,812 | ) | (1,911 | ) | |||||||||||||||||||
Research & Development |
(545 | ) | (476 | ) | (312 | ) | (305 | ) | (857 | ) | (781 | ) | |||||||||||||||||||
General & Administration |
(118 | ) | (140 | ) | (313 | ) | (316 | ) | (1 | ) | (431 | ) | (457 | ) | |||||||||||||||||
Other income |
905 | 70 | 99 | 79 | 3 | 25 | 1,007 | 174 | |||||||||||||||||||||||
Other expense |
(812 | ) | (88 | ) | (60 | ) | (63 | ) | 4 | (274 | ) | (37 | ) | (1,146 | ) | (184 | ) | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating (loss)/income of discontinued operations |
(552 | ) | (165 | ) | 470 | 178 | (73 | ) | (271 | ) | (13 | ) | (353 | ) | (73 | ) | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income from associated companies |
2 | 1 | 2 | 1 | |||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Loss before taxes of discontinued operations |
(351 | ) | (72 | ) | |||||||||||||||||||||||||||
Taxes |
(96 | ) | 55 | ||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss of discontinued operations |
(447 | ) | (17 | ) | |||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- As
previously published, including the blood transfusion diagnostics unit.
- (2)
- Consumer
Health is the aggregation of the OTC and Animal Health divisions.
- (3)
- Other revenue contains royalties and out-licensing revenues of Vaccines which are to be retained by Novartis.
F-110
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
30. Discontinued Operations (Continued)
The following are included in net income from discontinued operations:
| |
2015 | 2014 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
$ m |
$ m |
$ m |
|||||||
Depreciation of property, plant & equipment |
(66 | ) | (201 | ) | ||||||
Amortization of intangible assets |
(77 | ) | (278 | ) | ||||||
Impairment charges on property, plant & equipment, net |
83 | (736 | ) | (33 | ) | |||||
Impairment charges on intangible assets, net |
(405 | ) | (8 | ) | ||||||
Impairment charges on financial assets |
(8 | ) | ||||||||
Additions to restructuring provisions |
(1 | ) | (14 | ) | (12 | ) | ||||
Equity-based compensation of Novartis equity plans |
(65 | ) | (124 | ) | (95 | ) | ||||
Discontinued Operations Consolidated Balance Sheet
| |
2014 | |||
|---|---|---|---|---|
| |
$ m |
|||
Assets of disposal groups classified as discontinued operations |
||||
Property, plant and equipment |
1,411 | |||
Goodwill |
1,119 | |||
Intangible assets other than goodwill |
1,343 | |||
Investments in associated companies |
1 | |||
Deferred tax assets |
304 | |||
Other non-current assets |
47 | |||
Inventories |
1,155 | |||
Trade receivables |
1,085 | |||
Other current assets |
336 | |||
| | | | | |
Total |
6,801 | |||
| | | | | |
| | | | | |
| | | | | |
Liabilities of disposal groups classified as discontinued operations |
||||
Deferred tax liabilities |
209 | |||
Provisions and other non-current liabilities |
497 | |||
Trade payables |
612 | |||
Current income tax liabilities |
176 | |||
Provisions and other current liabilities |
924 | |||
| | | | | |
Total |
2,418 | |||
| | | | | |
| | | | | |
| | | | | |
31. Events Subsequent to the December 31, 2015 Consolidated Balance Sheet Date
Dividend proposal for 2015 and approval of the Group's 2015 consolidated financial statements
On January 26, 2016, the Novartis AG Board of Directors proposed the acceptance of the 2015 consolidated financial statements of the Novartis Group for approval by the Annual General Meeting on February 23, 2016. Furthermore, also on January 26, 2016, the Board proposed a dividend of CHF 2.70 per share to be approved at the Annual General Meeting on February 23, 2016. If approved, total dividend payments would amount to approximately $6.6 billion (2014: $6.6 billion) using the CHF/$ December 31, 2015 exchange rate.
F-111
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
32. Principal Group Subsidiaries and Associated Companies
The following table lists the principal subsidiaries controlled by Novartis and associated companies in which Novartis is deemed to have significant influence. The equity interest percentage shown in the table also represents the share in voting rights in those entities, except where explicitly noted.
As at December 31, 2015
|
Share/paid-in capital(1) |
Equity interest % |
Activities | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Algeria |
|||||||||||||||||
Société par actions SANDOZ, Algiers |
DZD | 650.0 m | 100 | ‹*› | \*/ | ||||||||||||
Argentina |
|
||||||||||||||||
Novartis Argentina S.A., Buenos Aires |
ARS | 246.3 m | 100 | ‹*› | ▲ | ||||||||||||
Alcon Laboratorios Argentina S.A., Buenos Aires |
ARS | 83.9 m | 100 | ‹*› | |||||||||||||
Sandoz S.A., Buenos Aires |
ARS | 88.0 m | 100 | ‹*› | |||||||||||||
Australia |
|
||||||||||||||||
Novartis Australia Pty Ltd., North Ryde, NSW |
AUD | 11.0 m | 100 | /*/ | |||||||||||||
Novartis Pharmaceuticals Australia Pty Ltd., North Ryde, NSW |
AUD | 3.8 m | 100 | ‹*› | ▲ | ||||||||||||
Alcon Laboratories (Australia) Pty Ltd., Frenchs Forest, NSW |
AUD | 2.6 m | 100 | ‹*› | |||||||||||||
Sandoz Pty Ltd., North Ryde, NSW |
AUD | 11.6 m | 100 | ‹*› | |||||||||||||
Austria |
|
||||||||||||||||
Novartis Austria GmbH, Vienna |
EUR | 1.0 m | 100 | /*/ | |||||||||||||
Novartis Pharma GmbH, Vienna |
EUR | 1.1 m | 100 | ‹*› | |||||||||||||
Alcon Ophthalmika GmbH, Vienna |
EUR | 36,336.4 | 100 | ‹*› | |||||||||||||
Sandoz GmbH, Kundl |
EUR | 32.7 m | 100 | /*/ | ‹*› | \*/ | ▲ | ||||||||||
EBEWE Pharma Ges.m.b.H Nfg., Unterach am Attersee |
EUR | 1.0 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Bangladesh |
|
||||||||||||||||
Novartis (Bangladesh) Limited, Gazipur |
BDT | 162.5 m | 60 | ‹*› | \*/ | ||||||||||||
Belgium |
|
||||||||||||||||
N.V. Novartis Pharma S.A., Vilvoorde |
EUR | 7.1 m | 100 | ‹*› | |||||||||||||
S.A. Alcon-Couvreur N.V., Puurs |
EUR | 360.6 m | 100 | ‹*› | \*/ | ||||||||||||
N.V. Alcon S.A., Vilvoorde |
EUR | 141,856 | 100 | ‹*› | |||||||||||||
N.V. Sandoz S.A., Vilvoorde |
EUR | 19.2 m | 100 | ‹*› | |||||||||||||
Bermuda |
|
||||||||||||||||
Triangle International Reinsurance Ltd., Hamilton |
CHF | 1.0 m | 100 | /*/ | |||||||||||||
Novartis Securities Investment Ltd., Hamilton |
CHF | 30,000 | 100 | /*/ | |||||||||||||
Novartis International Pharmaceutical Ltd., Hamilton |
CHF | 100,000 | 100 | /*/ | ‹*› | \*/ | ▲ | ||||||||||
Trinity River Insurance Co. Ltd., Hamilton |
$ | 370,000 | 100 | /*/ | |||||||||||||
Novartis Investment Limited, Hamilton |
$ | 30,000 | 100 | /*/ | |||||||||||||
Novartis Pharmaceutical Proprietary Ltd., Hamilton |
CHF | 100,000 | 100 | /*/ | ‹*› | \*/ | ▲ | ||||||||||
Brazil |
|
||||||||||||||||
Novartis Biociências S.A., São Paulo |
BRL | 265.0 m | 100 | ‹*› | \*/ | ||||||||||||
Sandoz do Brasil Indústria Farmacêutica Ltda., Cambé, PR |
BRL | 190.0 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Canada |
|
||||||||||||||||
Novartis Pharmaceuticals Canada Inc., Dorval/Quebec |
CAD | 0 | (2) | 100 | ‹*› | ▲ | |||||||||||
Alcon Canada Inc., Mississauga, Ontario |
CAD | 0 | (2) | 100 | ‹*› | ||||||||||||
CIBA Vision Canada Inc., Mississauga, Ontario |
CAD | 1 | 100 | \*/ | |||||||||||||
Sandoz Canada Inc., Boucherville, Quebec |
CAD | 76.8 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Chile |
|
||||||||||||||||
Novartis Chile S.A., Santiago de Chile |
CLP | 2.0 bn | 100 | ‹*› | |||||||||||||
Alcon Laboratorios Chile Limitada, Santiago de Chile |
CLP | 2.0 bn | 100 | ‹*› | |||||||||||||
F-112
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
32. Principal Group Subsidiaries and Associated Companies (Continued)
As at December 31, 2015
|
Share/paid-in capital(1) |
Equity interest % |
Activities | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
China |
|||||||||||||||||
Beijing Novartis Pharma Co., Ltd., Beijing |
$ | 30.0 m | 100 | ‹*› | \*/ | ||||||||||||
Novartis Pharmaceuticals (HK) Limited, Hong Kong |
HKD | 200 | 100 | ‹*› | |||||||||||||
China Novartis Institutes for BioMedical Research Co., Ltd., Shanghai |
$ | 260.0 m | 100 | ▲ | |||||||||||||
Suzhou Novartis Pharma Technology Co., Ltd., Changshu |
$ | 103.4 m | 100 | \*/ | |||||||||||||
Shanghai Novartis Trading Ltd., Shanghai |
$ | 3.1 m | 100 | ‹*› | \*/ | ||||||||||||
Alcon Hong Kong Limited, Hong Kong |
HKD | 77,000 | 100 | ‹*› | |||||||||||||
Alcon (China) Ophthalmic Product Co., Ltd., Beijing |
$ | 2.2 m | 100 | ‹*› | |||||||||||||
Sandoz (China) Pharmaceutical Co., Ltd., Zhongshan |
$ | 36.5 m | 100 | ‹*› | \*/ | ||||||||||||
Colombia |
|
||||||||||||||||
Novartis de Colombia S.A., Santafé de Bogotá |
COP | 7.9 bn | 100 | ‹*› | |||||||||||||
Laboratorios Alcon de Colombia S.A., Santafé de Bogotá |
COP | 20.9 m | 100 | ‹*› | |||||||||||||
Croatia |
|
||||||||||||||||
Sandoz d.o.o., Zagreb |
HRK | 25.6 m | 100 | ‹*› | |||||||||||||
Czech Republic |
|
||||||||||||||||
Novartis s.r.o., Prague |
CZK | 51.5 m | 100 | ‹*› | |||||||||||||
Sandoz s.r.o., Prague |
CZK | 44.7 m | 100 | ‹*› | |||||||||||||
Alcon Pharmaceuticals (Czech Republic) s.r.o., Prague |
CZK | 31.0 m | 100 | ‹*› | |||||||||||||
Denmark |
|
||||||||||||||||
Novartis Healthcare A/S, Copenhagen |
DKK | 14.0 m | 100 | ‹*› | |||||||||||||
Alcon Nordic A/S, Copenhagen |
DKK | 0.5 m | 100 | ‹*› | |||||||||||||
Sandoz A/S, Copenhagen |
DKK | 10.0 m | 100 | ‹*› | |||||||||||||
Ecuador |
|
||||||||||||||||
Novartis Ecuador S.A., Quito |
$ | 4.0 m | 100 | ‹*› | |||||||||||||
Egypt |
|
||||||||||||||||
Novartis Pharma S.A.E., Cairo |
EGP | 33.8 m | 99 | ‹*› | \*/ | ||||||||||||
Sandoz Egypt Pharma S.A.E., New Cairo |
EGP | 250,000 | 100 | ‹*› | |||||||||||||
Finland |
|
||||||||||||||||
Novartis Finland Oy, Espoo |
EUR | 459,000 | 100 | ‹*› | |||||||||||||
France |
|
||||||||||||||||
Novartis Groupe France S.A., Rueil-Malmaison |
EUR | 103.0 m | 100 | /*/ | |||||||||||||
Novartis Pharma S.A.S., Rueil-Malmaison |
EUR | 43.4 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Laboratoires Alcon S.A., Rueil-Malmaison |
EUR | 12.9 m | 100 | ‹*› | \*/ | ||||||||||||
Sandoz S.A.S., Levallois-Perret |
EUR | 5.4 m | 100 | ‹*› | ▲ | ||||||||||||
Germany |
|
||||||||||||||||
Novartis Deutschland GmbH, Wehr |
EUR | 155.5 m | 100 | /*/ | |||||||||||||
Novartis Pharma GmbH, Nuremberg |
EUR | 25.6 m | 100 | ‹*› | ▲ | ||||||||||||
Novartis Pharma Produktions GmbH, Wehr |
EUR | 2.0 m | 100 | \*/ | |||||||||||||
Alcon Pharma GmbH, Freiburg |
EUR | 512,000 | 100 | ‹*› | |||||||||||||
WaveLight GmbH, Erlangen |
EUR | 6.6 m | 100 | ‹*› | |||||||||||||
CIBA Vision GmbH, Grosswallstadt |
EUR | 15.4 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Sandoz International GmbH, Holzkirchen |
EUR | 100,000 | 100 | /*/ | |||||||||||||
Sandoz Industrial Products GmbH, Frankfurt a. M. |
EUR | 2.6 m | 100 | ‹*› | \*/ | ||||||||||||
1 A Pharma GmbH, Oberhaching |
EUR | 26,000 | 100 | ‹*› | |||||||||||||
Salutas Pharma GmbH, Barleben |
EUR | 42.1 m | 100 | ‹*› | \*/ | ||||||||||||
Hexal AG, Holzkirchen |
EUR | 93.7 m | 100 | /*/ | ‹*› | \*/ | ▲ | ||||||||||
Gibraltar |
|
||||||||||||||||
Novista Insurance Limited, Gibraltar |
CHF | 130.0 m | 100 | /*/ | |||||||||||||
F-113
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
32. Principal Group Subsidiaries and Associated Companies (Continued)
As at December 31, 2015
|
Share/paid-in capital(1) |
Equity interest % |
Activities | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Greece |
|||||||||||||||||
Novartis (Hellas) S.A.C.I., Metamorphosis/Athens |
EUR | 23.4 m | 100 | ‹*› | |||||||||||||
Alcon Laboratories Hellas Commercial & Industrial S.A., Maroussi/Athens |
EUR | 5.7 m | 100 | ‹*› | |||||||||||||
Hungary |
|
||||||||||||||||
Novartis Hungary Healthcare Limited Liability Company, Budapest |
HUF | 545.6 m | 100 | ‹*› | |||||||||||||
Sandoz Hungary Limited Liability Company, Budapest |
HUF | 883.0 m | 100 | ‹*› | |||||||||||||
India |
|
||||||||||||||||
Novartis India Limited, Mumbai |
INR | 159.8 m | 75 | ‹*› | |||||||||||||
Novartis Healthcare Private Limited, Mumbai |
INR | 60.0 m | 100 | ‹*› | ▲ | ||||||||||||
Alcon Laboratories (India) Private Limited, Bangalore |
INR | 1.1 bn | 100 | ‹*› | |||||||||||||
Sandoz Private Limited, Mumbai |
INR | 32.0 m | 100 | ‹*› | \*/ | ||||||||||||
Indonesia |
|
||||||||||||||||
PT Novartis Indonesia, Jakarta |
IDR | 7.7 bn | 100 | ‹*› | \*/ | ||||||||||||
PT CIBA Vision Batam, Batam |
IDR | 11.9 bn | 100 | \*/ | |||||||||||||
Ireland |
|
||||||||||||||||
Novartis Ireland Limited, Dublin |
EUR | 25,000 | 100 | ‹*› | |||||||||||||
Novartis Ringaskiddy Limited, Ringaskiddy, County Cork |
EUR | 2.0 m | 100 | \*/ | |||||||||||||
Alcon Laboratories Ireland Limited, Cork City |
EUR | 541,251 | 100 | \*/ | |||||||||||||
Israel |
|
||||||||||||||||
Novartis Israel Ltd., Petach Tikva |
ILS | 1,000 | 100 | ‹*› | ▲ | ||||||||||||
Italy |
|
||||||||||||||||
Novartis Farma S.p.A., Origgio |
EUR | 18.2 m | 100 | /*/ | ‹*› | \*/ | ▲ | ||||||||||
Alcon Italia S.p.A., Milan |
EUR | 3.7 m | 100 | ‹*› | |||||||||||||
Sandoz S.p.A., Origgio |
EUR | 1.7 m | 100 | ‹*› | |||||||||||||
Sandoz Industrial Products S.p.A., Rovereto |
EUR | 2.6 m | 100 | \*/ | |||||||||||||
Japan |
|
||||||||||||||||
Novartis Holding Japan K.K., Tokyo |
JPY | 10.0 m | 100 | /*/ | |||||||||||||
Novartis Pharma K.K., Tokyo |
JPY | 6.0 bn | 100 | ‹*› | ▲ | ||||||||||||
Alcon Japan Ltd., Tokyo |
JPY | 500.0 m | 100 | ‹*› | |||||||||||||
Sandoz K.K., Tokyo |
JPY | 100.0 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Luxembourg |
|
||||||||||||||||
Novartis Investments S.à r.l., Luxembourg-Ville |
$ | 100.0 m | 100 | /*/ | |||||||||||||
Novartis Finance S.A., Luxembourg-Ville |
$ | 100,000 | 100 | /*/ | |||||||||||||
Malaysia |
|
||||||||||||||||
Novartis Corporation (Malaysia) Sdn. Bhd., Kuala Lumpur |
MYR | 3.3 m | 100 | ‹*› | |||||||||||||
Alcon Laboratories (Malaysia) Sdn. Bhd., Petaling Jaya |
MYR | 1.0 m | 100 | ‹*› | |||||||||||||
CIBA Vision Johor Sdn. Bhd., Gelang Patah |
MYR | 5.0 m | 100 | \*/ | |||||||||||||
Mexico |
|
||||||||||||||||
Novartis Farmacéutica, S.A. de C.V., Mexico City |
MXN | 205.0 m | 100 | ‹*› | \*/ | ||||||||||||
Alcon Laboratorios, S.A. de C.V., Mexico City |
MXN | 5.9 m | 100 | ‹*› | \*/ | ||||||||||||
Sandoz, S.A. de C.V., Mexico City |
MXN | 468.2 m | 100 | ‹*› | \*/ | ||||||||||||
Morocco |
|
||||||||||||||||
Novartis Pharma Maroc SA, Casablanca |
MAD | 80.0 m | 100 | ‹*› | \*/ | ||||||||||||
F-114
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
32. Principal Group Subsidiaries and Associated Companies (Continued)
As at December 31, 2015
|
Share/paid-in capital(1) |
Equity interest % |
Activities | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Netherlands |
|||||||||||||||||
Novartis Netherlands B.V., Arnhem |
EUR | 1.4 m | 100 | /*/ | |||||||||||||
Novartis Pharma B.V., Arnhem |
EUR | 4.5 m | 100 | ‹*› | ▲ | ||||||||||||
Alcon Nederland B.V., Breda |
EUR | 18,151 | 100 | ‹*› | |||||||||||||
Sandoz B.V., Almere |
EUR | 907,560 | 100 | ‹*› | \*/ | ||||||||||||
New Zealand |
|
||||||||||||||||
Novartis New Zealand Ltd., Auckland |
NZD | 820,000 | 100 | ‹*› | |||||||||||||
Norway |
|
||||||||||||||||
Novartis Norge AS, Oslo |
NOK | 1.5 m | 100 | ‹*› | ▲ | ||||||||||||
Pakistan |
|
||||||||||||||||
Novartis Pharma (Pakistan) Limited, Karachi |
PKR | 3.9 bn | 100 | ‹*› | |||||||||||||
Panama |
|
||||||||||||||||
Novartis Pharma (Logistics), Inc., Ciudad de Panama |
$ | 10,000 | 100 | ‹*› | |||||||||||||
Alcon Centroamerica S.A., Ciudad de Panama |
PAB | 1,000 | 100 | ‹*› | |||||||||||||
Philippines |
|
||||||||||||||||
Novartis Healthcare Philippines, Inc., Makati/Manila |
PHP | 298.8 m | 100 | ‹*› | |||||||||||||
Sandoz Philippines Corporation, Manila |
PHP | 30.0 m | 100 | ‹*› | \*/ | ||||||||||||
Alcon Laboratories (Philippines), Inc., Manila |
PHP | 16.5 m | 100 | ‹*› | |||||||||||||
Poland |
|
||||||||||||||||
Novartis Poland Sp. z o.o., Warszawa |
PLN | 44.2 m | 100 | ‹*› | ▲ | ||||||||||||
Alcon Polska Sp. z o.o., Warszawa |
PLN | 750,000 | 100 | ‹*› | |||||||||||||
Sandoz Polska Sp. z o.o., Warszawa |
PLN | 25.6 m | 100 | ‹*› | |||||||||||||
Lek S.A., Strykow |
PLN | 11.4 m | 100 | ‹*› | \*/ | ||||||||||||
Portugal |
|
||||||||||||||||
Novartis Portugal SGPS Lda., Porto Salvo |
EUR | 500,000 | 100 | /*/ | |||||||||||||
Novartis Farma—Produtos Farmacêuticos S.A., Porto Salvo |
EUR | 2.4 m | 100 | ‹*› | |||||||||||||
Alcon Portugal-Produtos e Equipamentos Oftalmologicos Lda., Porto Salvo |
EUR | 4.5 m | 100 | ‹*› | |||||||||||||
Sandoz Farmacêutica Lda., Porto Salvo |
EUR | 499,900 | 100 | ‹*› | |||||||||||||
Puerto Rico |
|
||||||||||||||||
Alcon (Puerto Rico) Inc., Catano |
$ | 15.5 | 100 | ‹*› | |||||||||||||
Romania |
|
||||||||||||||||
Sandoz S.R.L., Targu-Mures |
RON | 105.2 m | 100 | ‹*› | \*/ | ||||||||||||
Novartis Pharma Services Romania S.R.L., Bucharest |
RON | 3.0 m | 100 | ‹*› | |||||||||||||
Alcon Romania S.R.L., Bucharest |
RON | 10.8 m | 100 | ‹*› | |||||||||||||
Russian Federation |
|
||||||||||||||||
Novartis Pharma LLC, Moscow |
RUB | 20.0 m | 100 | ‹*› | |||||||||||||
Alcon Farmacevtika LLC, Moscow |
RUB | 44.1 m | 100 | ‹*› | |||||||||||||
ZAO Sandoz, Moscow |
RUB | 57.4 m | 100 | ‹*› | |||||||||||||
Novartis Neva LLC, St. Petersburg |
RUB | 1.3 bn | 100 | \*/ | |||||||||||||
Saudi Arabia |
|
||||||||||||||||
Saudi Pharmaceutical Distribution Co. Ltd., Riyadh |
SAR | 26.8 m | 75 | ‹*› | |||||||||||||
Singapore |
|
||||||||||||||||
Novartis (Singapore) Pte Ltd., Singapore |
SGD | 100,000 | 100 | ‹*› | |||||||||||||
Novartis Singapore Pharmaceutical Manufacturing Pte Ltd., Singapore |
SGD | 45.0 m | 100 | \*/ | |||||||||||||
Novartis Asia Pacific Pharmaceuticals Pte Ltd., Singapore |
SGD | 39.0 m | 100 | ‹*› | \*/ | ||||||||||||
F-115
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
32. Principal Group Subsidiaries and Associated Companies (Continued)
As at December 31, 2015
|
Share/paid-in capital(1) |
Equity interest % |
Activities | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Novartis Institute for Tropical Diseases Pte Ltd., Singapore |
SGD | 2,004 | 100 | ▲ | |||||||||||||
Alcon Singapore Manufacturing Pte Ltd., Singapore |
SGD | 101,000 | 100 | \*/ | |||||||||||||
CIBA Vision Asian Manufacturing and Logistics Pte Ltd., Singapore |
SGD | 1.0 m | 100 | \*/ | |||||||||||||
Alcon Pte Ltd, Singapore |
SGD | 164,000 | 100 | ‹*› | |||||||||||||
Slovakia |
|
||||||||||||||||
Novartis Slovakia s.r.o., Bratislava |
EUR | 2.0 m | 100 | ‹*› | |||||||||||||
Slovenia |
|
||||||||||||||||
Lek Pharmaceuticals d.d., Ljubljana |
EUR | 48.4 m | 100 | /*/ | ‹*› | \*/ | ▲ | ||||||||||
Sandoz Pharmaceuticals d.d., Ljubljana |
EUR | 1.5 m | 100 | ‹*› | |||||||||||||
South Africa |
|
||||||||||||||||
Novartis South Africa (Pty) Ltd., Kempton Park |
ZAR | 86.3 m | 100 | ‹*› | |||||||||||||
Alcon Laboratories (South Africa) (Pty) Ltd., Bryanston, Gauteng |
ZAR | 201,820 | 100 | ‹*› | |||||||||||||
Sandoz South Africa (Pty) Ltd., Kempton Park |
ZAR | 3.0 m | 100 | ‹*› | ▲ | ||||||||||||
South Korea |
|
||||||||||||||||
Novartis Korea Ltd., Seoul |
KRW | 24.5 bn | 99 | ‹*› | |||||||||||||
Alcon Korea Ltd., Seoul |
KRW | 33.8 bn | 100 | ‹*› | |||||||||||||
Sandoz Korea Ltd., Seoul |
KRW | 17.8 bn | 100 | ‹*› | |||||||||||||
Spain |
|
||||||||||||||||
Novartis Farmacéutica, S.A., Barcelona |
EUR | 63.0 m | 100 | /*/ | ‹*› | \*/ | |||||||||||
Alcon Cusi S.A., El Masnou |
EUR | 11.6 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Sandoz Farmacéutica, S.A., Madrid |
EUR | 270,450 | 100 | ‹*› | |||||||||||||
Sandoz Industrial Products, S.A., Les Franqueses del Vallés/Barcelona |
EUR | 9.3 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Sweden |
|
||||||||||||||||
Novartis Sverige AB, Täby/Stockholm |
SEK | 5.0 m | 100 | ‹*› | |||||||||||||
Switzerland |
|
||||||||||||||||
Novartis International AG, Basel |
CHF | 10.0 m | 100 | /*/ | |||||||||||||
Novartis Holding AG, Basel |
CHF | 100.2 m | 100 | /*/ | |||||||||||||
Novartis Research Foundation, Basel |
CHF | 29.3 m | 100 | /*/ | |||||||||||||
Novartis Foundation for Management Development, Basel |
CHF | 100,000 | 100 | /*/ | |||||||||||||
Novartis Foundation for Employee Participation, Basel |
CHF | 100,000 | 100 | /*/ | |||||||||||||
Novartis Sanierungsstiftung, Basel |
CHF | 2.0 m | 100 | /*/ | |||||||||||||
Novartis Pharma AG, Basel |
CHF | 350.0 m | 100 | /*/ | ‹*› | \*/ | ▲ | ||||||||||
Novartis Pharma Services AG, Basel |
CHF | 20.0 m | 100 | ‹*› | |||||||||||||
Novartis Pharma Schweizerhalle AG, Schweizerhalle |
CHF | 18.9 m | 100 | \*/ | |||||||||||||
Novartis Pharma Stein AG, Stein |
CHF | 251,000 | 100 | \*/ | ▲ | ||||||||||||
Novartis Pharma Schweiz AG, Rotkreuz |
CHF | 5.0 m | 100 | ‹*› | ▲ | ||||||||||||
Alcon Switzerland SA, Rotkreuz |
CHF | 100,000 | 100 | ‹*› | |||||||||||||
Alcon Pharmaceuticals Ltd., Fribourg |
CHF | 200,000 | 100 | /*/ | ‹*› | \*/ | ▲ | ||||||||||
ESBATech, a Novartis Company GmbH, Schlieren |
CHF | 14.0 m | 100 | ▲ | |||||||||||||
Sandoz AG, Basel |
CHF | 5.0 m | 100 | /*/ | ‹*› | \*/ | ▲ | ||||||||||
Sandoz Pharmaceuticals AG, Risch |
CHF | 100,000 | 100 | ‹*› | |||||||||||||
Roche Holding AG, Basel |
CHF | 160.0 m | 33/6 | (3) | /*/ | ||||||||||||
Taiwan |
|
||||||||||||||||
Novartis (Taiwan) Co., Ltd., Taipei |
TWD | 170.0 m | 100 | ‹*› | |||||||||||||
F-116
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
32. Principal Group Subsidiaries and Associated Companies (Continued)
As at December 31, 2015
|
Share/paid-in capital(1) |
Equity interest % |
Activities | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Thailand |
|
||||||||||||||||
Novartis (Thailand) Limited, Bangkok |
THB | 302.0 m | 100 | ‹*› | |||||||||||||
Alcon Laboratories (Thailand) Ltd., Bangkok |
THB | 228.1 m | 100 | ‹*› | |||||||||||||
Turkey |
|||||||||||||||||
Novartis Saglik, Gida ve Tarim Ürünleri Sanayi ve Ticaret A.S., Istanbul |
TRY | 98.0 m | 100 | ‹*› | \*/ | ||||||||||||
Alcon Laboratuvarlari Ticaret A.S., Istanbul |
TRY | 25.2 m | 100 | ‹*› | |||||||||||||
Sandoz Ilaç Sanayi ve Ticaret A.S., Istanbul |
TRY | 165.2 m | 100 | ‹*› | \*/ | ||||||||||||
United Arab Emirates |
|
||||||||||||||||
Novartis Middle East FZE, Dubai |
AED | 7.0 m | 100 | ‹*› | |||||||||||||
United Kingdom |
|
||||||||||||||||
Novartis UK Limited, Frimley/Camberley |
GBP | 25.5 m | 100 | /*/ | |||||||||||||
Novartis Pharmaceuticals UK Limited, Frimley/Camberley |
GBP | 5.4 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Novartis Grimsby Limited, Frimley/Camberley |
GBP | 250.0 m | 100 | \*/ | |||||||||||||
Alcon Eye Care (UK) Limited, Frimley/Camberley |
GBP | 550,000 | 100 | ‹*› | |||||||||||||
Sandoz Limited, Frimley/Camberley |
GBP | 2.0 m | 100 | ‹*› | |||||||||||||
Glaxosmithkline Consumer Healthcare Holdings Limited, Brentford, Middlesex |
GBP | 100,000 | 36.5 | /*/ | |||||||||||||
United States of America |
|
||||||||||||||||
Novartis Corporation, East Hanover, NJ |
$ | 72.2 m | 100 | /*/ | |||||||||||||
Novartis Finance Corporation, New York, NY |
$ | 1,002 | 100 | /*/ | |||||||||||||
Novartis Capital Corporation, New York, NY |
$ | 1 | 100 | /*/ | |||||||||||||
Novartis Pharmaceuticals Corporation, East Hanover, NJ |
$ | 5.2 m | 100 | ‹*› | \*/ | ▲ | |||||||||||
Novartis Institutes for BioMedical Research, Inc., Cambridge, MA |
$ | 1 | 100 | ▲ | |||||||||||||
CoStim Pharmaceuticals, Inc., Cambridge, MA |
$ | 1 | 100 | ▲ | |||||||||||||
Novartis Institute for Functional Genomics, Inc., San Diego, CA |
$ | 21,000 | 100 | ▲ | |||||||||||||
Genoptix, Inc., Carlsbad, CA |
$ | 1 | 100 | ‹*› | ▲ | ||||||||||||
Alcon Laboratories, Inc., Fort Worth, TX |
$ | 1,000 | 100 | /*/ | ‹*› | \*/ | |||||||||||
Alcon Refractive Horizons, LLC, Fort Worth, TX |
$ | 10 | 100 | \*/ | |||||||||||||
Alcon Research, Ltd., Fort Worth, TX |
$ | 12.5 | 100 | \*/ | ▲ | ||||||||||||
Alcon LenSx, Inc., Alisio Viejo, CA |
$ | 100 | 100 | \*/ | |||||||||||||
WaveTec Vision Systems, Inc., Alisio Viejo, CA |
$ | 1 | 100 | ‹*› | \*/ | ▲ | |||||||||||
Sandoz Inc., Princeton, NJ |
$ | 25,000 | 100 | ‹*› | \*/ | ▲ | |||||||||||
Fougera Pharmaceuticals, Inc., Melville, NY |
$ | 1 | 100 | ‹*› | \*/ | ▲ | |||||||||||
Eon Labs, Inc., Princeton, NJ |
$ | 1 | 100 | ‹*› | \*/ | ||||||||||||
Novartis Vaccines and Diagnostics, Inc., Cambridge, MA |
$ | 3.0 | 100 | ‹*› | |||||||||||||
Novartis Services, Inc., East Hanover, NJ |
$ | 1 | 100 | /*/ | |||||||||||||
Venezuela |
|
||||||||||||||||
Novartis de Venezuela, S.A., Caracas |
VEF | 1.4 m | 100 | ‹*› | |||||||||||||
Alcon Pharmaceutical, C.A., Caracas |
VEF | 5.5 m | 100 | ‹*› | |||||||||||||
In addition, the Group is represented by subsidiaries and associated companies in the following countries: Bosnia/Herzegovina, Bulgaria, Dominican Republic, Guatemala, the Former Yugoslav Republic of Macedonia, Peru, Ukraine and Uruguay.
- (1)
- Share/paid-in
capital may not reflect the taxable share/paid-in capital amount and does not include any paid-in surplus.
- (2)
- Shares
without par value
- (3)
- Approximately 33% of voting shares; approximately 6% of total net income and equity attributable to Novartis
m = million; bn = billion
F-117
NOTES TO THE NOVARTIS GROUP
CONSOLIDATED FINANCIAL STATEMENTS (Continued)
32. Principal Group Subsidiaries and Associated Companies (Continued)
The following describe the various types of entities within the Group:
| /*/ | Holding/Finance: This entity is a holding company and/or performs finance functions for the Group. | |
| ‹*› | Sales: This entity performs sales and marketing activities for the Group. | |
| \*/ | Production: This entity performs manufacturing and/or production activities for the Group. | |
| ▲ | Research and Development: This entity performs research and development activities for the Group. |
F-118
