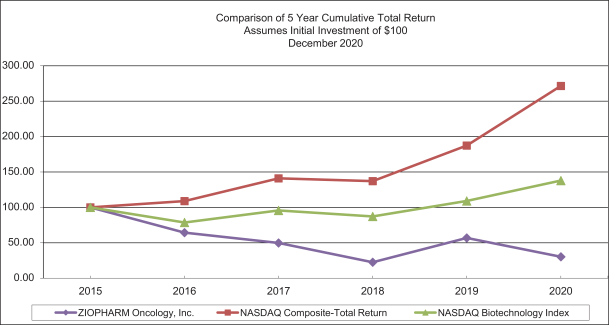
Item 6. Selected Financial Data
We have elected to comply with Item 301 of Regulation S-K, as amended February 10, 2021 and are omitting this disclosure in reliance thereon.
Item 7. Management Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes appearing elsewhere in this Annual Report on
Form 10-K. In
addition to historical financial information, the following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those contained in or implied by any forward-looking statements. Factors that could cause or contribute to these differences include those under “Risk Factors” included in Part I, Item 1A and under “Special Note Regarding Forward-Looking Statements” or in other parts of this Annual Report on Form 10-K.
Overview
We are a clinical-stage biopharmaceutical company focused on discovering, developing and commercializing next generation immunooncology platforms that leverage cell- and gene-based therapies to treat patients with cancer. We are developing platform technologies that utilize the immune system by employing innovative cell engineering and novel, controlled gene expression technologies designed to deliver safe and effective, cell and gene therapies for the treatment of multiple cancer types. Our major platform and priority is referred to as Sleeping Beauty and is based on the non-viral genetic engineering of immune cells using a transposon/transposase system that is intended to stably engineer T cells outside of the body for subsequent infusion. Our second platform is referred to as Controlled IL-12 and is designed to stimulate expression of interleukin 12, or
IL-12,
a master regulator of the immune system, in a controlled manner to focus the patient’s immune system to more effectively attack cancer cells. Using our Sleeping Beauty platform, we are developing T cell receptor, or TCR, T cell therapies to target neoantigens in solid tumors using two approaches, which we refer to as our “Library TCR-T Approach” and “our Personalized TCR-T Approach.” The Library TCR-T Approach uses third party (allogeneic) TCRs that have
74