UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the Fiscal Year Ended December 31, 2019
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the Transition Period From to
Commission File Number 1-15525
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||
(Address of Principal Executive Offices) | (Zip Code) | |||
(949 ) 250-2500
Registrant's telephone number, including area code
Securities registered pursuant to Section 12(b) of the Act: | ||
Title of each class | Trading Symbols(s) | Name of each exchange on which registered: |
Securities registered pursuant to Section 12(g) of the Act: None | ||
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined by Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ý No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
☒ | Accelerated filer | ☐ | Non-accelerated filer | ☐ | Smaller reporting company | Emerging growth company | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ý
The aggregate market value of the registrant's common stock held by non-affiliates as of June 28, 2019 (the last trading day of the registrant's most recently completed second quarter): $34,109,197,847 based on the closing price of the registrant's common stock on the New York Stock Exchange. This calculation does not reflect a determination that persons are affiliates for any other purpose.
The number of shares outstanding of the registrant's common stock, $1.00 par value, as of January 31, 2020, was 209,122,578 .
Documents Incorporated by Reference
EDWARDS LIFESCIENCES CORPORATION
Form 10-K Annual Report—2019
Table of Contents
PART I
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. We intend the forward-looking statements contained in this report to be covered by the safe harbor provisions of such Acts. All statements other than statements of historical fact in this report or referred to or incorporated by reference into this report are "forward-looking statements" for purposes of these sections. These statements include, among other things, any predictions of earnings, revenues, expenses or other financial items, plans or expectations with respect to development activities, clinical trials or regulatory approvals, any statements of plans, strategies and objectives of management for future operations, any statements concerning our future operations, financial conditions and prospects, and any statements of assumptions underlying any of the foregoing. These statements can sometimes be identified by the use of the forward-looking words such as "may," "believe," "will," "expect," "project," "estimate," "should," "anticipate," "plan," "goal," "continue," "seek," "pro forma," "forecast," "intend," "guidance," "optimistic," "aspire," "confident," other forms of these words or similar words or expressions or the negative thereof. Statements of past performance, efforts, or results about which inferences or assumptions may be made can also be forward-looking statements and are not indicative of future performance or results; these statements can be identified by the use of words such as "preliminary," "initial," diligence," "industry-leading," "compliant," "indications," or "early feedback" or other forms of these words or similar words or expressions or the negative thereof. Investors are cautioned not to unduly rely on such forward-looking statements. These forward-looking statements are subject to substantial risks and uncertainties that could cause our results or future business, financial condition, results of operations or performance to differ materially from our historical results or experiences or those expressed or implied in any forward-looking statements contained in this report. See "Risk Factors" in Part I, Item 1A below for a discussion of these risks, as such risks may be amended, supplemented or superseded from time to time by our subsequent reports on Forms 10-Q and 8-K. These forward-looking statements speak only as of the date on which they are made and we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of the statement. If we do update or correct one or more of these statements, investors and others should not conclude that we will make additional updates or corrections.
Unless otherwise indicated or otherwise required by the context, the terms "we," "our," "it," "its," "Company," "Edwards," and "Edwards Lifesciences" refer to Edwards Lifesciences Corporation and its subsidiaries.
1
Item 1. Business
Overview
Edwards Lifesciences Corporation is the global leader in patient-focused medical innovations for structural heart disease, as well as critical care and surgical monitoring. Driven by a passion to help patients, we partner with the world’s leading clinicians and researchers and invest in research and development to transform care for those impacted by structural heart disease or who require hemodynamic monitoring during surgery or in intensive care. Edwards Lifesciences has been a leader in these areas for over six decades. Since our founder, Lowell Edwards, first dreamed of using engineering to address diseases of the human heart, we have steadily built a company on the premise of imagining, building, and realizing a better future for patients.
A pioneer in the development of heart valve therapies, we are the world's leading manufacturer of heart valve systems and repair products used to replace or repair a patient's diseased or defective heart valve. Our innovative work in heart valves encompasses both surgical and transcatheter therapies for heart valve replacement and repair. In addition, our robust pipeline of future technologies is focused on the less invasive repair or replacement of the mitral and tricuspid valves of the heart, which are more complex and more challenging to treat than the aortic valve that is currently the focus of many of our commercially approved valve technologies. We are also a global leader in hemodynamic and noninvasive brain and tissue oxygenation monitoring systems used to measure a patient's cardiovascular function in the hospital setting.
Cardiovascular disease is the number-one cause of death in the world, and is the top disease in terms of health care spending in nearly every country. Cardiovascular disease is progressive in that it tends to worsen over time and often affects the structure of an individual's heart.
Patients undergoing treatment for cardiovascular disease can be treated with a number of our medical technologies, which are designed to address individual patient needs with respect to disease process, comorbidities, and health status. For example, an individual with a heart valve disorder may have a faulty valve that is affecting the function of his or her heart or blood flow throughout his or her body. A clinician may elect to remove the valve and replace it with one of our bioprosthetic surgical tissue heart valves or surgically re-shape and repair the faulty valve with an Edwards Lifesciences annuloplasty ring. Alternatively, a clinician may implant an Edwards Lifesciences transcatheter valve or repair system via a catheter-based approach that does not require traditional open-heart surgery and can be done while the heart continues to beat. Patients in the hospital setting, including high-risk patients in the operating room or intensive care unit, are candidates for having their cardiac function or fluid levels monitored by our Critical Care products through multiple monitoring options, including noninvasive and minimally invasive technologies. These technologies enable proactive clinical decisions while also providing the opportunity for improving diagnoses and developing individualized therapeutic management plans for patients.
Corporate Background
Edwards Lifesciences Corporation was incorporated in Delaware on September 10, 1999.
Our principal executive offices are located at One Edwards Way, Irvine, California 92614. The telephone number at that address is (949) 250-2500. We make available, free of charge on our website located at www.edwards.com, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports, as soon as reasonably practicable after filing such reports with the Securities and Exchange Commission ("SEC"). The contents of our website are not incorporated by reference into this report.
Edwards Lifesciences' Product and Technology Offerings
The following discussion summarizes the main areas of products and technologies we offer to treat advanced cardiovascular disease. Our products and technologies are categorized into four main areas: Transcatheter Aortic Valve Replacement, Transcatheter Mitral and Tricuspid Therapies, Surgical Structural Heart, and Critical Care. For more information on net sales from these four main areas, see "Net Sales by Product Group" in Part II, Item 7 "Management's Discussion and Analysis of Financial Condition and Results of Operations."
Transcatheter Aortic Valve Replacement
We are the global leader in transcatheter heart valve replacement technologies designed for the minimally invasive replacement of heart valves. The Edwards SAPIEN family of valves, including Edwards SAPIEN XT, the Edwards SAPIEN 3, and the Edwards SAPIEN 3 Ultra transcatheter aortic heart valves, and their respective delivery systems, are used to treat heart
2
valve disease using catheter-based approaches for patients who have severe symptomatic aortic stenosis and certain patients with congenital heart disease. Delivered while the heart is beating, these valves can enable patients to experience a better quality of life sooner than patients receiving traditional surgical therapies. We began offering our transcatheter heart valves to patients commercially in Europe in 2007, in the United States in 2011, and in Japan in 2013. Supported by extensive customer training and service, and a growing body of compelling clinical evidence, our SAPIEN family of transcatheter aortic heart valves are the most widely prescribed transcatheter heart valves in the world.
Sales of our transcatheter aortic valve replacement products represented 63%, 61%, and 59% of our net sales in 2019, 2018, and 2017, respectively.
Transcatheter Mitral and Tricuspid Therapies
We are making significant investments in the development of transcatheter heart valve repair and replacement technologies designed to treat mitral and tricuspid valve diseases. While many of these technologies are in early development and clinical phases, the PASCAL transcatheter valve repair system and the Cardioband systems for mitral and tricuspid valve repair are commercially available in Europe. The PASCAL system provides a differentiated, minimally-invasive therapy to address the needs of patients with mitral regurgitation through leaflet approximation, while the Cardioband system enables clinicians to restore a patient’s mitral or tricuspid valve to a more functional state by reducing the annulus and lowering regurgitation.
Surgical Structural Heart
The core of our surgical tissue heart valve product line is the Carpentier-Edwards PERIMOUNT pericardial valve platform, including the line of PERIMOUNT Magna Ease pericardial valves for aortic and mitral surgical valve replacement. With more long-term clinical publications on durability and performance than any other surgical valve, PERIMOUNT valves are the most widely implanted surgical tissue heart valves in the world. Our latest innovation, the INSPIRIS RESILIA aortic valve, is built on our PERIMOUNT platform and offers RESILIA tissue and VFit technology. INSPIRIS is now a leading aortic valve in the U.S. and in Japan. In addition to our replacement valves, we are the worldwide leader in surgical heart valve repair therapies, which include annuloplasty rings. We are also a global leader in cardiac cannula devices and offer a variety of procedure-enabling innovations that advance minimally invasive surgery. At the end of 2019, we received European regulatory approval for the HARPOON Beating Heart Mitral Valve Repair System, which can help transform care for many patients with degenerative mitral regurgitation.
Sales of our surgical tissue heart valve products represented 17%, 18%, and 21% of our net sales in 2019, 2018, and 2017, respectively.
Critical Care
We are a world leader in hemodynamic monitoring systems used to measure a patient's heart function and fluid status in surgical and intensive care settings. Hemodynamic monitoring plays an important role in enhancing surgical recovery. Edwards’ complete hemodynamic portfolio helps clinicians make proactive clinical decisions that can improve patient outcomes, and includes the minimally invasive FloTrac system, the noninvasive ClearSight system, and recently added ForeSight, the noninvasive tissue oximetry system. We also support clinical needs with our well-established Swan-Ganz line of pulmonary artery catheters, arterial pressure monitoring products, and Edwards Oximetry Central Venous Catheters. In conjunction with our sensors, our HemoSphere monitoring platforms display valuable physiological information in an easy to understand and actionable manner. Amplifying our sensor and monitoring platform portfolio is the addition of our first predictive algorithm, Acumen Hypotension Prediction Index, which alerts clinicians in advance of a patient developing low blood pressure.
Sales of our core hemodynamic products represented 9%, 10%, and 10% of our net sales in 2019, 2018, and 2017, respectively.
Competition
The medical technology industry is highly competitive. We compete with many companies, including divisions of companies much larger than us and smaller companies that compete in specific product lines or certain geographies. Furthermore, new product development and technological change characterize the areas in which we compete. Our present or future products could be rendered obsolete or uneconomical as a result of technological advances by one or more of our present or future competitors or by other therapies, including drug therapies. We must continue to develop and commercialize new products and technologies to remain competitive in the cardiovascular medical technology industry. We believe that we
3
compete primarily on the basis of clinical superiority supported by extensive data, and innovative features that enhance patient benefit, product performance, and reliability. Customer and clinical support, and data that demonstrate both improvement in a patient's quality of life and a product's cost-effectiveness, are additional aspects of competition.
The cardiovascular segment of the medical technology industry is dynamic and subject to significant change due to cost-of-care considerations, regulatory reform, industry and customer consolidation, and evolving patient needs. The ability to provide products and technologies that demonstrate value and improve clinical outcomes is becoming increasingly important for medical technology manufacturers.
We believe that we are a leading global competitor in each of our product lines. In Transcatheter Aortic Valve Replacement, our primary competitors include Medtronic PLC and Boston Scientific Corporation. In Transcatheter Mitral and Tricuspid Therapies, our primary competitor is Abbott Laboratories, and there are a considerable number of large and small companies with development efforts in these fields. In Surgical Structural Heart, our primary competitors include Medtronic PLC, Abbott Laboratories, LivaNova, and CryoLife. In Critical Care, we compete primarily with a variety of companies in specific product lines including ICU Medical, Inc., PULSION Medical Systems SE, a subsidiary of Getinge AB, Cheetah Medical, Inc., a subsidiary of Baxter International, and LiDCO Group PLC.
Sales and Marketing
Our portfolio includes some of the most recognizable cardiovascular device product brands in treating structural heart disease today. We have a number of product lines that require sales and marketing strategies tailored to deliver high-quality, cost-effective products and technologies to customers worldwide. Because of the diverse global needs of the population that we serve, our distribution system consists of several direct sales forces as well as independent distributors. We are not dependent on any single customer and no single customer accounted for 10% or more of our net sales in 2019.
To help provide awareness of our products and technologies, we conduct educational symposia and best practices training for our physician, hospital executive, service line leadership, nursing, and clinical-based customers. We rely extensively on our sales and field clinical specialist personnel who work closely with our customers in hospitals. Field clinical specialists routinely attend procedures where Edwards' products are being used in order to provide guidance on the use of our devices, thereby enabling physicians and staff to reach expert proficiency and deliver positive patient outcomes. Our customers include physicians, nurses, and other clinical personnel, but can also include decision makers such as service line leaders, material managers, biomedical staff, hospital administrators and executives, purchasing managers, and ministries of health. Also, for certain of our product lines and where appropriate, our corporate sales team actively pursues approval of Edwards Lifesciences as a qualified supplier for hospital group purchasing organizations ("GPOs") that negotiate contracts with suppliers of medical products. Additionally, we have contracts with a number of United States and European national and regional buying groups, including healthcare systems and Integrated Delivery Networks. Where we choose to market our products is also influenced by the existence of, or potential for, adequate reimbursement to hospitals and other providers by national healthcare systems.
United States. In the United States, we sell substantially all of our products through our direct sales forces. In 2019, 58% of our net sales were derived from sales to customers in the United States.
International. In 2019, 42% of our net sales were derived internationally through our direct sales forces and independent distributors. Of the total international sales, 52% were in Europe, 24% were in Japan, and 24% were in Rest of World. We sell our products in approximately 100 countries, and our major international markets include Canada, China, France, Germany, Italy, Japan, and the United Kingdom. A majority of the sales and marketing approach outside the United States is direct sales, although it varies depending on each country's size and state of development.
Raw Materials and Manufacturing
We operate manufacturing facilities in various geographies around the world. We manufacture our Transcatheter Aortic Valve Replacement, Transcatheter Mitral and Tricuspid technologies, and Structural Surgical Heart products primarily in the United States (California and Utah), Singapore, Costa Rica, and Ireland. We manufacture our Critical Care products primarily in our facilities located in Puerto Rico and the Dominican Republic.
We use a diverse and broad range of raw and organic materials in the design, development, and manufacture of our products. We manufacture our non-implantable products from fabricated raw materials including resins, chemicals, electronics, and metals. Most of our replacement heart valves are manufactured from natural tissues harvested from animal tissue, as well as fabricated materials. We purchase certain materials and components used in manufacturing our products from external
4
suppliers. In addition, we purchase certain supplies from single sources for reasons of sole source availability or constraints resulting from regulatory requirements.
We work with our suppliers to mitigate risk and seek continuity of supply while maintaining quality and reliability. Alternative supplier options are generally considered, identified, and approved for materials deemed critical to our products, although we do not typically pursue immediate regulatory qualification of alternative sources due to the strength of our existing supplier relationships and the time and expense associated with the regulatory validation process.
We comply with all current global guidelines regarding risks for products incorporating animal tissue intended to be implanted in humans. We follow rigorous sourcing and manufacturing procedures intended to safeguard humans from potential risks associated with diseases such as bovine spongiform encephalopathy ("BSE"). We obtain bovine tissue used in our pericardial tissue valve products only from sources within the United States and Australia, where strong control measures and surveillance programs exist. In addition, bovine tissue used in our pericardial tissue valve products is from tissue types considered by global health and regulatory organizations to have shown no risk of infectibility. Our manufacturing and sterilization processes are designed to render tissue biologically safe from all known infectious agents and viruses.
Quality Assurance
We are committed to providing to our patients quality products and have implemented modern quality systems and concepts throughout the organization. The quality system starts with the initial design concept, risk management, and product specification, and continues through the design of the product, packaging and labeling, and the manufacturing, sales, support, and servicing of the product. The quality system is intended to design quality into the products and utilizes continuous improvement concepts, including Lean/Six Sigma principles, throughout the product lifecycle.
Our operations are frequently inspected by the many regulators that oversee medical device manufacturing, including the United States Food and Drug Administration ("FDA"), our European Notified Bodies, and other regulatory entities. The medical technology industry is highly regulated and our facilities and operations are designed to comply with all applicable quality systems standards, including the International Organization for Standardization ("ISO") 13485. These standards require, among other items, quality system controls that are applied to product design, component material, suppliers, and manufacturing operations. These regulatory approvals and ISO certifications can be obtained only after a successful audit of a company's quality system has been conducted by regulatory or independent outside auditors. Periodic reexamination by an independent outside auditor is required to maintain these certifications.
Environmental, Health, and Safety
We are committed to providing a safe and healthy workplace and complying with all relevant regulations and medical technology industry standards. Through our corporate and site level Environmental, Health, and Safety functions, we establish and monitor programs to reduce pollution, prevent injuries, and maintain compliance with applicable regulations. In order to measure performance, we monitor and report on a number of metrics, including regulated and non-regulated waste disposal, energy usage, water consumption, air toxic emissions, and injuries from our production activities. Each of our manufacturing sites is evaluated regularly with respect to a broad range of Environmental, Health, and Safety criteria.
Research and Development
In 2019, we made significant investments in research and development as we worked to develop therapies that we believe have the potential to change the practice of medicine. Research and development spending increased 21% year over year to 17% of 2019 sales. This increase was primarily the result of significant investments in our transcatheter structural heart programs, including an increase in clinical research for our mitral and tricuspid therapies business. We are engaged in ongoing research and development to deliver clinically advanced new products, to enhance the effectiveness, ease of use, safety, and reliability of our current leading products, and to expand the applications of our products as appropriate. We focus on opportunities within specific areas of structural heart disease and critical care monitoring.
A considerable portion of our research and development investment includes clinical trials and the collection of evidence that provide data for use in regulatory submissions, and required post-market approval studies involving applications of our products. Our investment in clinical studies also includes outcomes and cost-effectiveness data for payers, clinicians, and healthcare systems.
5
In Transcatheter Aortic Valve Replacement, we are developing new products to further improve and streamline transcatheter aortic heart valve replacement procedures, and developing pulmonic platforms to expand therapies for congenital heart disease patients.
In Transcatheter Mitral and Tricuspid Therapies, we are making significant investments in innovation and clinical evidence to develop technologies designed to treat mitral and tricuspid valve diseases and other structural heart conditions. In addition to our internally developed programs, we have made investments in several companies that are independently developing minimally-invasive technologies to treat structural heart diseases.
Our Surgical Structural Heart development programs include innovative platforms for patients who are best treated surgically, specifically active patients and patients with more complex combined procedures.
In our Critical Care product line, we are pursuing the development of a variety of decision support solutions for our clinicians. This includes next-generation noninvasive and minimally-invasive hemodynamic monitoring systems, and a next-generation monitor platform. We are also developing a decision support software suite with advanced algorithms for proactive hemodynamic management, including a semi-closed loop system for standardized management of patient fluid levels.
Our research and development activities are conducted primarily in facilities located in the United States and Israel. Our experienced research and development staff are focused on product design and development, quality, clinical research, and regulatory compliance. To pursue primary research efforts, we have developed alliances with several leading research institutions and universities, and also work with leading clinicians around the world in conducting scientific studies on our existing and developing products.
Proprietary Technology
Patents, trademarks, and other proprietary rights are important to the success of our business. We also rely upon trade secrets, know-how, continuing innovations, and licensing opportunities to develop and maintain our competitive position.
We own more than 4,700 issued United States patents, pending United States patent applications, issued foreign patents, and pending foreign patent applications. We also have licensed various United States and foreign patents and patent applications that relate to aspects of the technology incorporated in certain of our products, including our heart valves and annuloplasty rings. We also own or have rights in United States and foreign patents and patent applications in the field of transcatheter heart valve repair and replacement. In addition, we own or have rights in United States and foreign patents and patent applications that cover catheters, systems and methods for hemodynamic monitoring, and vascular access products, among others.
We are a party to several license agreements with unrelated third parties pursuant to which we have obtained, for varying terms, the exclusive or non-exclusive rights to certain patents held by such third parties in consideration for cross-licensing rights and/or royalty payments. We have also licensed certain patent rights to others.
We monitor the products of our competitors for possible infringement of our owned and licensed patents. Litigation has been necessary to enforce certain patent rights held by us, and we plan to continue to defend and prosecute our rights with respect to such patents.
We own certain United States registered trademarks used in our business. Many of our trademarks have also been registered for use in certain foreign countries where registration is available and where we have determined it is commercially advantageous to do so.
Government Regulation and Other Matters
Our products and facilities are subject to regulation by numerous government agencies, including the FDA, European Community Notified Bodies, and the Japanese Pharmaceuticals and Medical Devices Agency, to confirm compliance with the various laws and regulations governing the development, testing, manufacturing, labeling, marketing, and distribution of our products. We are also governed by federal, state, local, and international laws of general applicability, such as those regulating employee health and safety, and the protection of the environment. Overall, the amount and scope of domestic and foreign laws and regulations applicable to our business has increased over time.
United States Regulation. In the United States, the FDA has responsibility for regulating medical devices. The FDA regulates design, development, testing, clinical studies, manufacturing, labeling, promotion, and record keeping for medical
6
devices, and reporting of adverse events, recalls, or other field actions by manufacturers and users to identify potential problems with marketed medical devices. Many of the devices that we develop and market are in a category for which the FDA has implemented stringent clinical investigation and pre-market clearance or approval requirements. The process of obtaining FDA clearance or approval to market a product is resource intensive, lengthy, and costly. FDA review may involve substantial delays that adversely affect the marketing and sale of our products. A number of our products are pending regulatory clearance or approval to begin commercial sales in various markets. Ultimately, the FDA may not authorize the commercial release of a medical device if it determines the device is not safe and effective or does not meet other standards for clearance. Additionally, even if a product is cleared or approved, the FDA may impose restrictions or require testing and surveillance programs to monitor the effects of these products once commercialized.
The FDA has the authority to halt the distribution of certain medical devices, detain or seize adulterated or misbranded medical devices, order the repair, replacement, or refund of the costs of such devices, or preclude the importation of devices that are or appear violative. The FDA also conducts inspections to determine compliance with the quality system regulations concerning the manufacturing and design of devices and current medical device reporting regulations, recall regulations, clinical testing regulations, and other requirements. The FDA may withdraw product clearances or approvals due to failure to comply with regulatory standards, or the occurrence of unforeseen problems following initial approval, and require notification of health professionals and others with regard to medical devices that present unreasonable risks of substantial harm to the public health. Additionally, the failure to comply with FDA or comparable regulatory standards or the discovery of previously unknown product problems could result in fines, delays, or suspensions of regulatory clearances or approvals, seizures, injunctions, recalls, refunds, civil money penalties, or criminal prosecution. Our compliance with applicable regulatory requirements is subject to continual review. Moreover, the FDA and several other United States agencies administer controls over the export of medical devices from the United States and the import of devices into the United States, which could also subject us to sanctions for noncompliance.
We are also subject to additional laws and regulations that govern our business operations, products, and technologies, including:
• | federal, state, and foreign anti-kickback laws and regulations, which generally prohibit payments to physicians or other purchasers of medical products as an inducement to purchase a product; |
• | the Stark law, which prohibits physicians from referring Medicare or Medicaid patients to a provider that bills these programs for the provision of certain designated health services if the physician (or a member of the physician's immediate family) has a financial relationship with that provider; |
• | federal and state laws and regulations that protect the confidentiality of certain patient health information, including patient records, and restrict the use and disclosure of such information, in particular, the Health Insurance Portability and Accountability Act of 1996; |
• | the Physician Payments Sunshine Act, which requires public disclosure of the financial relationships of United States physicians and teaching hospitals with applicable manufacturers, including medical device, pharmaceutical, and biologics companies; |
• | the False Claims Act, which prohibits the submission of false or otherwise improper claims for payment to a federally funded health care program, and health care fraud statutes that prohibit false statements and improper claims to any third-party payor; and |
• | the United States Foreign Corrupt Practices Act, which can be used to prosecute companies in the United States for arrangements with foreign government officials or other parties outside the United States. |
Failure to comply with these laws and regulations could result in criminal liability, significant fines or penalties, negative publicity, and substantial costs and expenses associated with investigation and enforcement activities. To assist in our compliance efforts, we adhere to many codes of ethics and conduct regarding our sales and marketing activities in the United States and other countries in which we operate. In addition, we have in place a dedicated team to improve our internal business compliance programs and policies.
International Regulation. Internationally, the regulation of medical devices is complex. In Europe, our products are subject to extensive regulatory requirements. The regulatory regime in the European Union for medical devices became mandatory in June 1998. It requires that medical devices may only be placed on the market if they do not compromise safety and health when properly installed, maintained, and used in accordance with their intended purpose. National laws conforming
7
to the European Union's legislation regulate our products under the medical devices regulatory system. Although the more variable national requirements under which medical devices were formerly regulated have been substantially replaced by the European Union Medical Devices Directive, individual nations can still impose unique requirements that may require supplemental submissions. The European Union medical device laws require manufacturers to declare that their products conform to the essential regulatory requirements after which the products may be placed on the market bearing the CE Mark. Manufacturers' quality systems for products in all but the lowest risk classification are also subject to certification and audit by an independent notified body. In Europe, particular emphasis is being placed on more sophisticated and faster procedures for the reporting of adverse events to the competent authorities.
In May 2017, the European Union (the "EU") implemented a new regulatory scheme for medical devices under the Medical Device Regulation ("MDR"). The MDR becomes fully effective in 2020 and will bring significant new requirements for many medical devices, including enhanced requirements for clinical evidence and documentation, increased focus on device identification and traceability, new definitions and registration of economic operators throughout the distribution chain, and additional postmarket surveillance and vigilance. Compliance with the MDR will require re-certification of many of our products to the enhanced standards, and will result in substantial additional expense. In addition, in the EU, we import some of our devices through our offices in Switzerland. Switzerland is not a member state of the EU, but is linked to the EU through bilateral treaties; therefore, the free movement of goods, including medical devices, between the EU and Switzerland after implementation of the MDR requires a revised Mutual Recognition Agreement ("MRA"), which continues to be under negotiation for the MDR. If an MRA covering the MDR is not put in place, then non-EU manufacturers may be required to make significant changes, including replacement of Swiss economic operators with operators based in EU Member States, and changes will need to be made to our device labeling and/or packaging to satisfy MDR requirements. If these measures are unable to be taken, it may no longer be possible to place such devices on the EU market.
In Japan, pre-market approval and clinical studies are required as is governmental pricing approval for medical devices. Clinical studies are subject to a stringent "Good Clinical Practices" standard. Approval time frames from the Japanese Ministry of Health, Labour and Welfare vary from simple notifications to review periods of one or more years, depending on the complexity and risk level of the device. In addition, importation of medical devices into Japan is subject to the "Good Import Practices" regulations. As with any highly regulated market, significant changes in the regulatory environment could adversely affect future sales.
In many of the other foreign countries in which we market our products, we may be subject to regulations affecting, among other things:
• | product standards and specifications; |
• | packaging requirements; |
• | labeling requirements; |
• | product collection and disposal requirements; |
• | quality system requirements; |
• | import restrictions; |
• | tariffs; |
• | duties; and |
• | tax requirements. |
Many of the regulations applicable to our devices and products in these countries are similar to those of the FDA. In some regions, the level of government regulation of medical devices is increasing, which can lengthen time to market and increase registration and approval costs. In many countries, the national health or social security organizations require our products to be qualified before they can be marketed and considered eligible for reimbursement.
Health Care Initiatives. Government and private sector initiatives to limit the growth of health care costs, including price regulation and competitive pricing, coverage and payment policies, comparative effectiveness reviews, technology assessments, increasing evidentiary demands, and managed-care arrangements, are continuing in many countries where we do
8
business, including the United States, Europe, and Japan. As a result of these changes, the marketplace has placed increased emphasis on the delivery of more cost-effective medical therapies. For example, government programs, private health care insurance, and managed-care plans have attempted to control costs by restricting coverage and limiting the level of reimbursement for procedures or treatments, and some third-party payors require their pre-approval before new or innovative devices or therapies are utilized by patients. These various initiatives have created increased price sensitivity over medical products generally and may impact demand for our products and technologies.
The delivery of our products is subject to regulation by the Department of Health and Human Services ("HHS") in the United States and comparable state and foreign agencies responsible for reimbursement and regulation of health care items and services. Foreign governments also impose regulations in connection with their health care reimbursement programs and the delivery of health care items and services. Reimbursement schedules regulate the amount the United States government will reimburse hospitals and doctors for the inpatient care of persons covered by Medicare. HHS' Centers for Medicare & Medicaid Services ("CMS") may also review whether and/or under what circumstances a procedure or technology is reimbursable for Medicare beneficiaries. Changes in current coverage and reimbursement levels could have an adverse effect on market demand and our pricing flexibility. The CMS National Coverage Determination for Transcatheter Aortic Valve Replacement was issued in June 2019. The modernized requirements and more streamlined patient evaluation process are meaningful enhancements that may help ensure equitable access for more patients suffering from severe aortic stenosis.
Health care cost containment efforts have also prompted domestic hospitals and other customers of medical device manufacturers to consolidate into larger purchasing groups to enhance purchasing power. The medical technology industry has also experienced some consolidation, partly in order to offer a broader range of products to large purchasers. As a result, transactions with customers are larger, more complex, and tend to involve more long-term contracts than in the past. These larger customers, due to their enhanced purchasing power, may attempt to increase the pressure on product pricing.
Health Care Legislation. In 2010, significant reforms to the health care system were adopted as law in the United States as part of the Affordable Care Act. The law included provisions that, among other things, created programs to encourage a shift to value-based care, required all individuals to have health insurance (with limited exceptions), and imposed increased taxes. The law required the medical technology industry to pay a 2.3% excise tax on United States sales of most medical devices. The excise tax, which increased our operating expenses, was suspended for calendar years 2016 through 2019. In December 2019, this legislation was repealed.
These laws or any future legislation, including deficit reduction legislation, could impact medical procedure volumes, reimbursement for our products, and demand for our products or the prices at which we sell our products.
Seasonality
Our quarterly net sales are influenced by many factors, including new product introductions, acquisitions, regulatory approvals, patient and physician holiday schedules, and other factors. Net sales in the third quarter are typically lower than other quarters of the year due to the seasonality of the United States and European markets, where summer vacation schedules normally result in fewer medical procedures.
Employees
As of December 31, 2019, we had approximately 13,900 employees worldwide, the majority of whom were located in the United States, Singapore, the Dominican Republic, and Puerto Rico. We emphasize competitive compensation, benefits, equity participation, and a positive and attractive work environment in our efforts to attract and retain qualified personnel, and employ a rigorous talent management system. None of our North American employees are represented by a labor union. In various countries outside of North America, we interact with trade unions and work councils that represent a limited number of employees.
9
Item 1A. Risk Factors
Our business and assets are subject to varying degrees of risk and uncertainty. An investor should carefully consider the risks described below, as well as other information contained in this Annual Report on Form 10-K and in our other filings with the SEC. Additional risks not presently known to us or that we currently deem immaterial may also adversely affect our business. If any of these events or circumstances occurs, our business, financial condition, results of operations, or prospects could be materially harmed. In that case, the value of our securities could decline and an investor could lose part or all of his or her investment. In addition, forward-looking statements within the meaning of the federal securities laws that are contained in this Annual Report on Form 10-K or in our other filings or statements may be subject to the risks described below as well as other risks and uncertainties. Please read the cautionary notice regarding forward-looking statements in Part I above.
Business and Operating Risks
If we do not introduce new and differentiated products in a timely manner, our products may become more susceptible to competition or technologically obsolete and our operating results may suffer.
The cardiovascular products industry is characterized by technological changes, frequent new product introductions, and evolving industry standards. Without the timely introduction of new and differentiated products, our products could become more susceptible to competition or technologically obsolete and our revenue and operating results would suffer. Even if we are able to develop new or differentiated products, our ability to market them could be limited by the need for regulatory clearance, restrictions imposed on approved indications, entrenched patterns of clinical practice, barriers in the patients' path to treatment, uncertainty over third-party reimbursement, or other factors.
We devote significant financial and other resources to our research and development activities; however, the research and development process is prolonged and entails considerable uncertainty. Accordingly, products we are currently developing may not complete the development process or obtain the regulatory or other approvals required to market such products in a timely manner or at all.
In addition, even if we are able to successfully develop new or differentiated products, they may not produce revenue in excess of the costs of development, and they may be rendered obsolete or less competitive by changing customer preferences or the introduction by our competitors of products with newer technologies or features or other factors.
We may experience supply interruptions that could harm our ability to manufacture products.
We use a broad range of raw and organic materials and other items from third party vendors in the design, manufacture and sterilization of our products. Our Transcatheter Aortic Valve Replacement, Transcatheter Mitral and Tricuspid Therapies, and Surgical Structural Heart products are manufactured from treated natural animal tissue and man-made materials. Our non-implantable products are manufactured from man-made raw materials including resins, chemicals, electronics, and metals. We purchase certain of the materials and components used in the manufacture of our products from external suppliers, and we purchase certain supplies from single sources for reasons of quality assurance, cost-effectiveness, availability, constraints resulting from regulatory requirements and other reasons. We also contract with third parties for important services related to infrastructure and information technology. General economic conditions could adversely affect the financial viability of our suppliers, resulting in their inability to provide materials and components used in the manufacture of our products. While we work closely with suppliers to monitor their financial viability, assure continuity of supply, and maintain high quality and reliability, these efforts may not be successful. In addition, due to the rigorous regulations and requirements of the FDA and foreign regulatory authorities regarding the manufacture of our products (including the need for approval of any change in supply arrangements), we may have difficulty establishing additional or replacement sources on a timely basis or at all if the need arises. Certain suppliers may also elect to no longer service medical technology companies due to the burdens of applicable quality requirements and regulation. Although alternative supplier options are often considered and identified, we typically do not pursue regulatory qualification of alternative sources due to the strength of our existing supplier relationships and the time and expense associated with the regulatory validation process. A change in suppliers could require significant effort or investment in circumstances where the items supplied are integral to product performance or incorporate unique technology, and the loss of any existing supply contract could have a material adverse effect on us.
Regulatory agencies in the United States or other international geographies from time to time have limited or banned the use of certain materials used in the manufacture of our products. In these circumstances, transition periods typically provide time to arrange for alternative materials. If we are unable to identify alternative materials or suppliers and secure approval for their use in a timely manner, our business could be harmed.
10
In addition, for our suppliers located outside the United States, trade or regulatory embargoes imposed by foreign countries or the United States could result in delays or shortages that could harm our business.
The manufacture of many of our products is highly complex and subject to strict quality controls. If we or one of our suppliers or logistics partners encounters manufacturing, logistics, or quality problems, our business could suffer.
The manufacture and sterilization of many of our products is highly complex due in part to rigorous regulatory requirements. In addition, quality is extremely important due to the serious and costly consequences of a product failure. Problems can arise for a number of reasons, including disruption of facility utilities, equipment malfunction, failure to follow protocols and procedures, raw material problems, software problems, or human error. Disruptions can occur at any time, including during implementation of new equipment and systems to replace aging equipment, as well as during production line transfers and expansions. As we expand into new markets, we may face unanticipated surges in demand which could strain our production capacity and lead to other types of disruption. Also, as we expand our manufacturing footprint, significant delays in construction and process validation could impact our production capacity. Further, scaling a new product for commercial production can sometimes be delayed. If these problems arise or if we otherwise fail to meet our internal quality standards or those of the FDA or other applicable regulatory body, which include detailed record-keeping requirements, our reputation could be damaged, we could become subject to a safety alert or a recall, we could incur product liability and other costs, product approvals could be delayed, and our business could otherwise be adversely affected.
In addition, our manufacturing and warehousing facilities, as well as those of our suppliers and logistics partners, could be materially damaged by earthquakes, hurricanes, volcanoes, fires, and other natural disasters or catastrophic circumstances. While we believe that our exposure to significant losses from a catastrophic disaster could be partially mitigated by our ability to manufacture, store, and distribute some of our products at other facilities, the losses could have a material adverse effect on our business for an indeterminate period of time before this transition is complete and operates without significant disruption.
We may be required, from time to time, to recognize charges in connection with the write-down of our assets or dispositions of business operations or for other reasons.
We manage a portfolio of research and development products. From time to time, we identify operations and products that are underperforming or not a fit with our longer term business strategy. We may seek to dispose of these underperforming operations or products. We may also seek to dispose of other operations or products for strategic or other business reasons. If we cannot dispose of an operation or product on acceptable terms, we may voluntarily cease operations related to that product. Any of these events could result in charges, which could be substantial and which could adversely affect our results of operations.
We may not successfully identify and complete acquisitions or strategic alliances on favorable terms or achieve anticipated synergies relating to any acquisitions or alliances, and such acquisitions could result in unforeseen operating difficulties and expenditures, require significant management resources, and require significant charges or write-downs.
We regularly explore potential acquisitions of complementary businesses, technologies, services, or products, as well as potential strategic alliances. We may be unable to find suitable acquisition candidates or appropriate partners with which to form alliances. Even if we identify appropriate acquisition or alliance candidates, we may be unable to complete the acquisitions or alliances on favorable terms, if at all. In addition, the process of integrating an acquired business, technology, service, or product into our existing operations could result in unforeseen difficulties and expenditures. Integration of an acquired company often requires significant expenditures as well as significant management resources that otherwise would be available for ongoing development of our other businesses. Moreover, we may not realize the anticipated financial or other benefits of an acquisition or alliance.
We may be required to take charges or write-downs in connection with acquisitions. In particular, acquisitions of businesses engaged in the development of new products may give rise to developed technology and/or in-process research and development assets. To the extent that the value of these assets declines, we may be required to write down the value of the assets. Also, in connection with certain asset acquisitions, we may be required to take an immediate charge related to acquired in-process research and development assets. Either of these situations could result in substantial charges, which could adversely affect our results of operations.
Acquisitions could also involve the issuance of equity securities, the incurrence of debt, contingent liabilities, or amortization of expenses related to other intangible assets, any of which could adversely impact our financial condition or results of operations. In addition, equity or debt financing required for such acquisitions may not be available.
11
We face intense competition, and if we do not compete effectively, our business will be harmed.
The cardiovascular medical technology industry is highly competitive. We compete with many companies, some of which are larger, with better brand or name recognition, and broader product offerings. Our customers consider many factors when selecting a product, including product reliability, breadth of product line, clinical outcomes, product availability, price, availability and rate of reimbursement, and services provided by the manufacturer. In addition, our ability to compete will depend in large part on our ability to develop and acquire new or differentiated products and technologies, anticipate technology advances, and keep pace with other developers of cardiovascular therapies, including drug therapies, and technologies. Our sales, technical, and other key personnel play an integral role in the development, marketing, and selling of new and existing products. If we are unable to recruit, hire, develop, and retain a talented, competitive workforce, our ability to compete may be adversely affected. Our competitive position can also be adversely affected by product problems, physician advisories, and safety alerts, reflecting the importance of quality in the medical technology industry. Our position can shift as a result of any of these factors. In addition, given the trend toward value-based healthcare, if we are not able to continue to demonstrate the full value of our differentiated products to healthcare providers and payors, our competitive position could be adversely affected. See "Competition" under "Business" included herein.
Unsuccessful clinical trials or procedures relating to products under development could have a material adverse effect on our prospects.
The regulatory approval process for new products and new indications for existing products requires extensive clinical trials and procedures, including early clinical feasibility and regulatory studies. Unfavorable or inconsistent clinical data from current or future clinical trials or procedures conducted by us, our competitors, or third parties, or perceptions regarding this clinical data, could adversely affect our ability to obtain necessary approvals and the market's view of our future prospects. Such clinical trials and procedures are inherently uncertain and there can be no assurance that these trials or procedures will be enrolled or completed in a timely or cost-effective manner or result in a commercially viable product or expanded indication; failure to do so could have a material adverse effect on our prospects. Clinical trials or procedures may experience significant setbacks even after earlier trials have shown promising results. Further, preliminary results from clinical trials or procedures may be contradicted by subsequent clinical analysis. In addition, results from our clinical trials or procedures may not be supported by actual long-term studies or clinical experience. If preliminary clinical results are later contradicted, or if initial results cannot be supported by actual long-term studies or clinical experience, our business could be adversely affected. Clinical trials or procedures may be delayed, suspended, or terminated by us, the FDA, or other regulatory authorities at any time if it is believed that the trial participants face unacceptable health risks or any other reasons.
The success of many of our products depends upon strong relationships with certain key physicians.
The development, marketing, and sale of many of our products requires us to maintain working relationships with physicians upon whom we rely to provide considerable knowledge and experience. These physicians may assist us as researchers, marketing consultants, product trainers and consultants, inventors, and as public speakers. If new laws, regulations, or other developments limit our ability to maintain strong relationships with these professionals or to continue to receive their advice and input, the development and marketing of our products could suffer, which could have a material adverse effect on our business, financial condition, and results of operations.
Failure to protect our information technology infrastructure against cyber-based attacks, network security breaches, service interruptions or data corruption could materially disrupt our operations and adversely affect our business and operating results.
The operation of our business depends on our information technology systems. We rely on our information technology systems to, among other things, effectively manage sales and marketing data, accounting and financial functions, inventory management, product development tasks, clinical data, customer service and technical support functions. Our information technology systems are vulnerable to damage or interruption from earthquakes, fires, floods and other natural disasters, terrorist attacks, power losses, computer system or data network failures, security breaches, data corruption, and cyber-based attacks. Cyber-based attacks can include computer viruses, computer denial-of-service attacks, phishing attacks, worms, and other malicious software programs or other attacks, covert introduction of malware to computers and networks, impersonation of authorized users, and efforts to discover and exploit any design flaws, bugs, security vulnerabilities, or security weaknesses, as well as intentional or unintentional acts by employees or other insiders with access privileges, intentional acts of vandalism by third parties and sabotage. In addition, federal, state, and international laws and regulations, such as the General Data Protection Regulation adopted by the European Union and the California Consumer Privacy Act, can expose us to enforcement actions and investigations by regulatory authorities, and potentially result in regulatory penalties and significant legal liability, if our information technology security efforts fail. In addition, a variety of our software systems are cloud‑based data management applications, hosted by third‑party service providers whose security and information technology systems are subject to similar risks.
12
The failure of either our or our service providers’ information technology could disrupt our entire operation or result in decreased sales, result in liability claims or regulatory penalties, or lead to increased overhead costs, product shortages, loss or misuse of proprietary or confidential information, intellectual property, or sensitive or personal information, all of which could have a material adverse effect on our reputation, business, financial condition, and operating results.
Market and Other External Risks
General economic and political conditions could have a material adverse effect on our business.
External factors can affect our profitability and financial condition. Such external factors include general domestic and global economic conditions, such as interest rates, tax law including tax rate changes, and factors affecting global economic stability, and the political environment regarding health care in general. We cannot predict to what extent the global economic conditions may negatively impact our business. For example, negative conditions in the credit and capital markets could impair our ability to access the financial markets for working capital or other funds and could negatively impact our ability to borrow. An increase in interest rates could result in an increase in our borrowing costs and could otherwise restrict our ability to access the capital markets. Such conditions could result in decreased liquidity and impairments in the carrying value of our investments, and could adversely affect our results of operations and financial condition. These and other conditions could also adversely affect our customers and may impact their ability or decision to purchase our products or make payments on a timely basis.
Various laws, including the Affordable Care Act, the Medicare Access and CHIP Reauthorization Act of 2015, and the 21st Century Cures Act, or any future legislation, including deficit reduction legislation, could impact medical procedure volumes, reimbursement for our products, and demand for our products or the prices at which we sell our products. For more information about these laws as they relate to our business, see the section entitled “Health Care Legislation” in Part I, Item 1, “Business.”
We operate globally and changes in tax laws could adversely affect our results.
We are subject to income taxes in the United States as well as other jurisdictions. Our effective tax rate could fluctuate due to changes in the mix of earnings and losses in countries with differing statutory tax rates. Our tax expense could be impacted by changes in excess tax benefits of stock-based compensation, federal and state tax credits, non-deductible expenses, changes in the valuation of deferred tax assets and liabilities and our ability to utilize them, the applicability and creditability of withholding taxes, and effects from acquisitions.
Our tax provision could be impacted by changes in accounting principles and tax legislation. Corporate tax reform, base-erosion efforts, and tax transparency continue to be high priorities in many tax jurisdictions where we have business operations. In addition, many countries are beginning to align their international tax rules with the Organisation for Economic Co-operation and Development’s Base Erosion and Profit Shifting recommendations and action plan that aim to standardize and modernize global corporate tax policy, including changes to cross-border tax, transfer pricing documentation rules, and nexus-based tax incentive practices. These changing tax laws could have a material adverse effect on our business.
We are subject to ongoing tax audits in the various jurisdictions in which we operate. Tax authorities may disagree with certain positions we have taken and assess additional taxes. We regularly assess the likely outcomes of these audits in order to determine the appropriateness of our tax provision. However, there can be no assurance that we will accurately predict the outcomes of these audits. We have recorded reserves for potential payments of tax to various tax authorities related to uncertain tax positions. However, the calculation of such tax liabilities involves the application of complex tax laws and regulations in many jurisdictions. If our estimate of tax liabilities proves to be less than the amount for which we are ultimately liable, we would incur additional charges, and such charges could have a material adverse effect on our business, financial condition, results of operations, and cash flows.
If the tax incentives or tax holiday arrangements we have negotiated change or cease to be in effect or applicable, our income taxes could increase significantly.
We benefit from tax incentives extended to our foreign subsidiaries to encourage investment or employment. Several jurisdictions have granted us tax incentives which require renewal at various times in the future. The incentives are conditioned on achieving various thresholds of investments and employment, or specific types of income. Our taxes could increase if the incentives are not renewed upon expiration. If we cannot or do not wish to satisfy all or parts of the tax incentive conditions, we
13
may lose the related tax incentive and could be required to refund tax incentives previously realized. As a result, our effective tax rate could be higher than it would have been had we maintained the benefits of the tax incentives.
Our business is subject to economic, political, and other risks associated with international sales and operations.
Because we sell our products in a number of countries, our business is subject to the risks of doing business internationally, including risks associated with anti-corruption and anti-bribery laws. Our net sales originating outside the United States, as a percentage of total net sales, were 42% in 2019. We anticipate that sales from international operations will continue to represent a substantial portion of our total sales. In addition, many of our manufacturing facilities and suppliers are located outside of the United States. Accordingly, our future results could be harmed by a variety of factors, including:
• | changes in local medical reimbursement policies and programs; |
• | changes in foreign regulatory requirements; |
• | changes in a specific country's or region's political or economic conditions, including changing circumstances in emerging regions, that may reduce the number of procedures that use our products; |
• | trade protection measures, quotas, embargoes, import or export licensing requirements, and duties, tariffs, or surcharges; |
• | potentially negative impact of tax laws, including transfer pricing liabilities and tax costs associated with the repatriation of cash; |
• | difficulty in staffing and managing global operations; |
• | currency exchange rate fluctuations; |
• | cultural or other local factors affecting financial terms with customers; |
• | local economic and financial conditions, including sovereign defaults and decline in sovereign credit ratings, affecting the collectability of receivables, including receivables from sovereign entities; |
• | an outbreak of any life-threatening communicable disease; |
• | economic and political instability and local economic and political conditions; |
• | differing labor regulations; and |
• | differing protection of intellectual property. |
In addition, a Mutual Recognition Agreement still under negotiation for the Medical Device Regulation can result in a lack of free movement of medical devices between the European Union and Switzerland, can impact our access in the European Union and can, ultimately, have a material effect on our business, financial condition, and results of operations. See "Government Regulation and Other Matters" under Item 1 "Business."
Substantially all of our sales outside of the United States are denominated in local currencies, principally in Europe (and primarily denominated in the Euro) and in Japan. The United States dollar value of our international sales varies with currency exchange rate fluctuations. Decreases in the value of the United States dollar to the Euro or the Japanese yen, as well as other currencies, have the effect of increasing our reported revenues even when the volume of international sales has remained constant. Increases in the value of the United States dollar relative to the Euro or the Japanese yen, as well as other currencies, have the opposite effect. Significant increases or decreases in the value of the United States dollar could have a material effect on our revenues, cost of sales, and results of operations. We have a hedging program for certain currencies that attempts to manage currency exchange rate risks to an acceptable level based on management's judgment of the appropriate trade-off between risk, opportunity, and cost; however, this hedging program does not completely eliminate the effects of currency exchange rate fluctuations.
The United States Foreign Corrupt Practices Act, the United Kingdom Bribery Act, and similar laws in other jurisdictions contain prohibitions against bribery and other illegal payments, and make it an offense to fail to have procedures in place that
14
prevent such payments. Recent years have seen an increasing number of investigations and other enforcement activities under these laws. Although we have compliance programs in place with respect to these laws, which may be used as a defense to prove we had adequate procedures, no assurance can be given that a violation will not be found, and if found, the resulting penalties could adversely affect us and our business.
The stock market can be volatile and fluctuations in our quarterly sales and operating results as well as other factors could cause our financial guidance to vary from actual results and our stock price to decline.
From time to time, the stock market experiences extreme price and volume fluctuations. This volatility can have a significant effect on the market prices of securities for reasons unrelated to underlying performance. These broad market fluctuations may materially adversely affect our stock price, regardless of our operating results. In addition, the market price of our common stock could fluctuate substantially in response to any of the other risk factors set out above and below, as well as a number of other factors, including the performance of comparable companies or the medical technology industry, or changes in financial estimates and recommendations of securities analysts.
Our sales and operating results may vary significantly from quarter to quarter. A high proportion of our costs are fixed, due in part to significant selling, research and development, and manufacturing costs. Thus, small declines in revenue could disproportionately affect our operating results in a quarter, and the price of our common stock could fall. Other factors that could affect our quarterly sales and operating results include:
• | announcements of innovations, new products, strategic developments, or business combinations by us or our competitors; |
• | demand for and clinical acceptance of products; |
• | the timing and execution of customer contracts, particularly large contracts that would materially affect our operating results in a given quarter; |
• | the timing and effectiveness of the introduction of new products; |
• | the timing of marketing, training, and other expenses related to the introduction of new products; |
• | the timing and substance of regulatory approvals; |
• | changes in foreign currency exchange rates; |
• | delays or problems in introducing new products, such as slower than anticipated adoption of transcatheter heart valves; |
• | changes in our pricing policies or the pricing policies of our competitors; |
• | governmental reimbursement rates, including the timing of approvals of governmental reimbursement rates or changes in reimbursement rates for our products; |
• | increased expenses, whether related to sales and marketing, raw materials or supplies, product development, or administration; |
• | changes in the level of economic activity in the United States or other regions in which we do business; |
• | changes to accounting standards; |
• | costs related to acquisitions of technologies or businesses; and |
• | our ability to expand our operations and the amount and timing of expansion-related expenditures. |
The quarterly and full-year financial guidance we provide to investors and analysts with insight to our view of our future performance is based on assumptions about our sales and operating results. Due to the nature of our business and the numerous factors that can impact our sales and operating performance, including those described above, our financial guidance may vary
15
from actual results. If we fail to meet any financial guidance that we provide, or if we find it necessary to revise such guidance during the year, the price of our common stock could decline.
Continued consolidation in the health care industry could have an adverse effect on our sales and results of operations.
The health care industry has been consolidating, and organizations such as GPOs, independent delivery networks, and large single accounts, such as the United States Veterans Administration, continue to consolidate purchasing decisions for many of our health care provider customers. As a result, transactions with customers are larger and more complex, and tend to involve more long-term contracts. The purchasing power of these larger customers has increased, and may continue to increase, causing downward pressure on product pricing. If we are not one of the providers selected by one of these organizations, we may be precluded from making sales to its members or participants. Even if we are one of the selected providers, we may be at a disadvantage relative to other selected providers that are able to offer volume discounts based on purchases of a broader range of medical equipment and supplies. Further, we may be required to commit to pricing that has a material adverse effect on our revenues, profit margins, business, financial condition, and results of operations. We expect that market demand, governmental regulation, third-party reimbursement policies, and societal pressures will continue to change the worldwide health care industry, resulting in further business consolidations and alliances, which may exert further downward pressure on the prices of our products and could adversely impact our business, financial condition, and results of operations.
If government and other third-party payors decline to reimburse our customers for our products or impose other cost containment measures to reduce reimbursement levels, our ability to profitably sell our products will be harmed.
We sell our products and technologies to hospitals and other health care providers, all of which receive reimbursement for the health care services provided to patients from third-party payors, such as government programs (both domestic and international), private insurance plans, and managed care programs. The ability of customers to obtain appropriate reimbursement for their products from private and governmental third-party payors is critical to the success of medical technology companies. The availability of reimbursement affects which products customers purchase and the prices they are willing to pay. Reimbursement varies from country to country and can significantly impact acceptance of new products.
Government and other third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for medical products and services. There can be no assurance that levels of reimbursement, if any, will not be decreased in the future, or that future legislation, regulation, or reimbursement policies of third-party payors will not otherwise adversely affect the demand for and price levels of our products. The introduction of cost containment incentives, combined with closer scrutiny of health care expenditures by both private health insurers and employers, has resulted in increased discounts and contractual adjustments to hospital charges for services performed. Hospitals or physicians may respond to such cost-containment pressures by substituting lower cost products or other therapies.
Initiatives to limit the growth of health care costs, including price regulation, are underway in countries around the world. In many countries, customers are reimbursed for our products under a government operated insurance system. Under such a system, the government periodically reviews reimbursement levels and may limit patient access. If a government were to decide to reduce reimbursement levels, our product pricing could be adversely affected.
Third-party payors may deny reimbursement if they determine that a device used in a procedure was not used in accordance with cost-effective treatment methods as determined by such third-party payors or was used for an unapproved indication. Third-party payors may also deny reimbursement for experimental procedures and devices. We believe that many of our existing products are cost-effective, even though the one-time cost may be significant, because they are intended to improve quality of life and reduce overall health care costs over a long period of time. We cannot be certain that these third-party payors will recognize these cost savings and quality of life benefits instead of merely focusing on the lower initial costs associated with competing therapies. If our products are not considered cost-effective by third-party payors, our customers may not be reimbursed for them, resulting in lower sales of our products.
Legal, Compliance, and Regulatory Risks
We may incur losses from product liability or other claims that could adversely affect our operating results.
Our business exposes us to potential product liability risks that are inherent in the design, manufacture, and marketing of medical technologies. Our products are often used in surgical and intensive care settings with seriously ill patients. In addition, many of the devices we manufacture and sell are designed to be implanted in the human body for long periods of time. Component failures, manufacturing and assembly flaws, design defects, software defects, medical procedure errors, or inadequate disclosure of product-related risks or product-related information could result in an unsafe condition or injury to, or
16
death of, patients. Such problems could result in product liability, medical malpractice or other lawsuits and claims, safety alerts, or product recalls in the future, which, regardless of their ultimate outcome, could have a material adverse effect on our business, reputation, and ability to attract and retain customers. Product liability claims may be brought from time to time either by individuals or by groups seeking to represent a class. We may incur charges related to such matters in excess of any established reserves and such charges, including the establishment of any such reserves, could have a material adverse impact on our net income and net cash flows.
Our inability to protect our intellectual property or failure to maintain the confidentiality and integrity of data or other sensitive company information, by cyber-attack or other event, could have a material adverse effect on our business.
Our success and competitive position are dependent in part upon our proprietary intellectual property. We rely on a combination of patents and trade secrets to protect our proprietary intellectual property, and we expect to continue to do so. Although we seek to protect our proprietary rights through a variety of means, we cannot guarantee that the protective steps we have taken are adequate to protect these rights. Patents issued to or licensed by us in the past or in the future may be challenged and held invalid. In addition, as our patents expire, we may be unsuccessful in extending their protection through patent term extensions. The expiration of, or the failure to maintain or extend our patents, could have a material adverse effect on us.
We also rely on confidentiality agreements with certain employees, consultants, and other third parties to protect, in part, trade secrets and other proprietary information. These agreements could be breached, and we may not have adequate remedies for such a breach. In addition, others could independently develop substantially equivalent proprietary information or gain access to our trade secrets or proprietary information.
Our intellectual property, other proprietary technology, and other sensitive company information is dependent on sophisticated information technology systems and is potentially vulnerable to cyber-attacks, loss, damage, destruction from system malfunction, computer viruses, loss of data privacy, or misappropriation or misuse of it by those with permitted access, and other events. While we have invested to protect our intellectual property and other information, and continue to upgrade and enhance our systems to keep pace with continuing changes in information processing technology, there can be no assurance that our precautionary measures will prevent breakdowns, breaches, cyber-attacks, or other events. Such events could have a material adverse effect on our reputation, financial condition, or results of operations.
We spend significant resources to enforce our intellectual property rights, sometimes resulting in litigation. Intellectual property litigation is complex and can be expensive and time-consuming. However, our efforts in this regard may not be successful. We may not be able to detect infringement. In addition, competitors may design around our technology or develop competing technologies. Patent litigation can result in substantial cost and diversion of effort. Intellectual property protection may also be unavailable or limited in some foreign countries, enabling our competitors to capture increased market position. The invalidation of key intellectual property rights or an unsuccessful outcome in lawsuits filed to protect our intellectual property could have a material adverse effect on our financial condition, results of operations, or prospects.
Third parties may claim we are infringing their intellectual property, and we could suffer significant litigation or licensing expenses or be prevented from selling products.
During recent years, we and our competitors have been involved in substantial litigation regarding patent and other intellectual property rights in the medical technology industry. From time to time, we have been and may in the future be forced to defend against claims and legal actions alleging infringement of the intellectual property rights of others, and such intellectual property litigation is typically costly and time-consuming. Adverse determinations in any such litigation could result in significant liabilities to third parties or injunctions that bar the sale of our products, or could require us to seek licenses from third parties and, if such licenses are not available on commercially reasonable terms, prevent us from manufacturing, selling, or using certain products, any one of which could have a material adverse effect on us. In addition, some licenses may be non-exclusive, which could provide our competitors access to the same technologies.
Third parties could also obtain patents that may require us to either redesign products or, if possible, negotiate licenses from such third parties. Such licenses may materially increase our expenses. If we are unable to redesign products or obtain a license, we might have to exit a particular product offering.
17
We and our customers are subject to rigorous governmental regulations and we may incur significant expenses to comply with these regulations and develop products that are compatible with these regulations. In addition, failure to comply with these regulations could subject us to substantial sanctions which could adversely affect our business, results of operations, and financial condition.
The medical technologies we manufacture and market are subject to rigorous regulation by the FDA and numerous other federal, state, and foreign governmental authorities, including regulations that cover the composition, labeling, testing, clinical study, design, sourcing, manufacturing, packaging, marketing, advertising, promotion, and distribution of our products.
We are required to register with the FDA as a device manufacturer. As a result, we are subject to periodic inspection by the FDA for compliance with the FDA's Quality System Regulation ("QSR") requirements, which require manufacturers of medical devices to adhere to certain regulations, including testing, design, quality control, and documentation procedures. The FDA may also inspect our compliance with requirements related to adverse event reporting, recalls or corrections (field actions), the conduct of clinical studies, and other requirements. In the European Union, we are required to maintain certain CE Mark and ISO certifications in order to sell our products, and are subject to periodic inspections by notified bodies to obtain and maintain these certifications. If we or our suppliers fail to adhere to QSR, CE Mark, ISO, or similar requirements, this could delay or interrupt product production or sales and/or lead to fines, difficulties in obtaining regulatory clearances, recalls, or other consequences, which in turn could have a material adverse effect on our financial condition and results of operations or prospects.
Medical devices must receive FDA clearance or approval before they can be commercially marketed in the United States. In addition, the FDA may require testing and surveillance programs to monitor the effects of approved products that have been commercialized, and can prevent or limit further marketing of a product based upon the results of post-marketing programs. Further, the federal Medical Device Reporting regulations require us to provide information to the FDA whenever there is evidence that reasonably suggests that a device may have caused or contributed to a death or serious injury or, if a malfunction were to occur, would be likely to cause or contribute to a death or serious injury. Federal regulations also require us to report certain recalls or corrective actions to the FDA. Furthermore, most major markets for medical devices outside the United States require clearance, approval, or compliance with certain standards before a product can be commercially marketed. The process of obtaining regulatory clearances or approvals to market a medical device, particularly from the FDA and certain foreign governmental authorities, can be costly and time-consuming, and clearances or approvals may not be granted for products or product improvements on a timely basis, if at all. Delays in receipt of, or failure to obtain, clearances or approvals for products or product improvements could result in delayed realization of product revenues or in substantial additional costs, which could have a material adverse effect on our business or results of operations or prospects. At any time after approval of a product for commercial sale, the FDA may conduct periodic inspections to determine compliance with QSR requirements, and/or current Medical Device Reporting regulations, or other regulatory requirements. Noncompliance with applicable requirements may subject us or responsible individuals to sanctions including civil money penalties, product seizure, injunction, or criminal prosecution. In addition, the FDA may withhold or delay pre-market approval of our products until the noncompliance is resolved. Product approvals by the FDA can also be withdrawn due to failure to comply with regulatory standards or the occurrence of unforeseen problems following initial approval.
The United States Physician Payment Sunshine Act, and similar laws in other jurisdictions, also impose reporting and disclosure requirements on device, pharmaceutical, and biologics companies for certain financial relationships with United States health care providers and teaching hospitals. Failure to submit required information or submitting incorrect information may result in significant civil monetary penalties.
We are also subject to various United States and international laws pertaining to health care pricing, anti-corruption, and fraud and abuse, including prohibitions on kickbacks and the submission of false claims laws and restrictions on relationships with physicians and other referral sources. These laws are broad in scope and are subject to evolving interpretation, which could require us to incur substantial costs to monitor compliance or to alter our practices if we are found not to be in compliance. Violations of these laws may be punishable by criminal or civil sanctions against us and our officers and employees, including substantial fines, imprisonment, and exclusion from participation in governmental health care programs.
Despite our implementation of compliance processes, we may be subject, from time to time, to inspections, investigations, and other enforcement actions by governmental authorities. If we are found not to be in compliance with applicable laws or regulations, the applicable governmental authority can impose fines, delay, suspend, or revoke regulatory clearances or approvals, institute proceedings to detain or seize our products, issue a recall, impose marketing or operating restrictions, enjoin future violations and assess civil penalties against us or our officers or employees, and institute criminal prosecution. Moreover, governmental authorities can ban or request the recall, repair, replacement, or refund of the cost of any device or product we manufacture or distribute. Any of the foregoing actions could result in decreased sales including as a
18
result of negative publicity and product liability claims, and could have a material adverse effect on our financial condition, results of operations, and prospects. In addition to the sanctions for noncompliance described above, commencement of an enforcement proceeding, inspection, or investigation could divert substantial management attention from the operation of our business and have an adverse effect on our business, results of operations, and financial condition.
Our industry is experiencing greater scrutiny and regulation by governmental authorities, which may lead to greater governmental regulation and scrutiny in the future.
In recent years, the medical technology industry has been subject to increased regulatory scrutiny, including by the FDA, numerous other federal, state, and foreign governmental authorities, as well as members of Congress. This has included increased regulation, enforcement, inspections, and governmental investigations of the medical technology industry and disclosure of financial relationships with health care professionals. We anticipate that the government will continue to scrutinize our industry closely, and that additional regulation by governmental authorities, both foreign and domestic, may increase compliance costs, exposure to litigation, and other adverse effects to our operations.
We are subject to risks arising from concerns and/or regulatory actions relating to animal borne illnesses, including “mad cow disease.”
Certain of our products, including pericardial tissue valves, are manufactured using bovine tissue. Concerns relating to the potential transmission of animal borne illnesses, including BSE, commonly known as "mad cow disease," from cows to humans may result in reduced acceptance of products containing bovine materials. Certain medical device regulatory agencies have considered whether to continue to permit the sale of medical devices that incorporate bovine material. We obtain bovine tissue only from closely controlled sources within the United States and Australia. The bovine tissue used in our pericardial tissue valves is from tissue types considered by global health and regulatory organizations to have shown no risk of infectibility for the suspected BSE infectious agent. We have not experienced any significant adverse impact on our sales as a result of concerns regarding BSE, but no assurance can be given that such an impact may not occur in the future.
Use of our products in unapproved circumstances could expose us to liabilities.
The marketing approval from the FDA and other regulators of certain of our products are, or are expected to be, limited to specific indications. We are prohibited from marketing or promoting any unapproved use of our products. Physicians, however, can use these products in ways or circumstances other than those strictly within the scope of the regulatory approval. Although the product training we provide to physicians and other health care professionals is limited to approved uses or for clinical trials, no assurance can be given that claims might not be asserted against us if our products are used in ways or for procedures that are not approved.
Our operations are subject to environmental, health, and safety regulations that could result in substantial costs.
Our operations are subject to environmental, health, and safety laws, and regulations concerning, among other things, the generation, handling, transportation, and disposal of hazardous substances or wastes, the cleanup of hazardous substance releases, and emissions or discharges into the air or water. We have incurred and may incur in the future expenditures in connection with environmental, health and safety laws, and regulations. New laws and regulations, violations of these laws or regulations, stricter enforcement of existing requirements, or the discovery of previously unknown contamination could require us to incur costs or could become the basis for new or increased liabilities that could be material.
Item 1B. Unresolved Staff Comments
None.
19
Item 2. Properties
The locations and uses of our major properties are as follows:
North America | ||||
Irvine, California | (1 | ) | Corporate Headquarters, Research and Development, Regulatory and Clinical Affairs, Manufacturing, Marketing, Administration | |
Draper, Utah | (1 | ) | Manufacturing, Administration | |
Haina, Dominican Republic | (2 | ) | Manufacturing | |
Añasco, Puerto Rico | (2 | ) | Manufacturing | |
Central America | ||||
Cartago, Costa Rica | (1),(2) | Manufacturing | ||
Europe | ||||
Nyon, Switzerland | (1 | ) | Administration, Marketing | |
Prague, Czech Republic | (2 | ) | Administration | |
Shannon, Limerick, Ireland | (1),(2) | Manufacturing (under construction) | ||
Asia | ||||
Tokyo, Japan | (2 | ) | Administration, Marketing, Distribution | |
Shanghai, China | (2 | ) | Administration, Marketing | |
Singapore | (1),(2) | Manufacturing, Distribution, Administration | ||
_______________________________________________________________________________
(1) | Owned property. |
(2) | Leased property. |
The Dominican Republic lease expires in 2022; the Puerto Rico property has two leases that expire in 2023; the Costa Rica lease expires in 2021; the Prague, Czech Republic lease expires in 2026; the Shannon, Ireland lease expires in 2024; the Tokyo, Japan lease expires in 2021; the Shanghai, China lease expires in 2021; and Singapore has one land lease that expires in 2036 and one that expires in 2041. We believe our properties have been well maintained, are in good operating condition, and are adequate for current needs.
Item 3. Legal Proceedings
For a description of our material pending legal proceedings, please see Note 18 to the "Consolidated Financial Statements" of this Annual Report on Form 10-K, which is incorporated by reference.
Item 4. Mine Safety Disclosures
Not applicable.
20
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is traded on the New York Stock Exchange (the "NYSE") under the symbol "EW."
Number of Stockholders
On January 31, 2020, there were 9,357 stockholders of record of our common stock.
Dividends
We have never paid any cash dividends on our capital stock and have no current plans to pay any cash dividends. Our current policy is to retain any future earnings for use in our business.
Issuer Purchases of Equity Securities
On November 15, 2017, the Board of Directors approved a stock repurchase program authorizing us to purchase on the open market, including pursuant to a Rule 10b5-1 plan, or in privately negotiated transactions, up to $1.0 billion of our common stock. On May 8, 2019, the Board of Directors approved a new stock repurchase program providing for an additional $1.0 billion of repurchases of our common stock. The repurchase programs do not have an expiration date. We did not purchase any of our common stock during the fourth quarter of 2019 and, as of December 31, 2019, we had remaining authority to purchase $1.2 billion of common stock.
21
Performance Graph
The following graph compares the performance of our common stock with that of the S&P 500 Index and the S&P 500 Health Care Equipment Index. The cumulative total return listed below assumes an initial investment of $100 at the market close on December 31, 2014 and reinvestment of dividends. Stockholder returns over the indicated period should not be considered indicative of future stockholder returns.
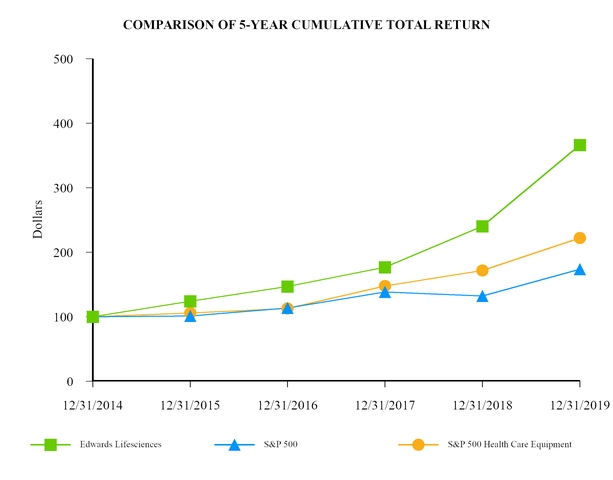
Total Cumulative Return | |||||||||||||||||||
2015 | 2016 | 2017 | 2018 | 2019 | |||||||||||||||
Edwards Lifesciences | $ | 124.01 | $ | 147.12 | $ | 176.97 | $ | 240.49 | $ | 366.29 | |||||||||
S&P 500 | 101.38 | 113.51 | 138.29 | 132.23 | 173.86 | ||||||||||||||
S&P 500 Health Care Equipment | 105.97 | 112.85 | 147.71 | 171.70 | 222.04 | ||||||||||||||
22
Item 6. Selected Financial Data
As of or for the Years Ended December 31, | ||||||||||||||||||||
2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||||
(in millions, except per share data) | ||||||||||||||||||||
OPERATING RESULTS | Net sales | $ | 4,348.0 | $ | 3,722.8 | $ | 3,435.3 | $ | 2,963.7 | $ | 2,493.7 | |||||||||
Gross profit | 3,233.6 | 2,783.4 | 2,560.0 | 2,166.3 | 1,876.5 | |||||||||||||||
Operating income (a) | 1,146.8 | 748.2 | 1,089.4 | 751.2 | 636.1 | |||||||||||||||
Net income (a) | 1,046.9 | 722.2 | 583.6 | 569.5 | 494.9 | |||||||||||||||
COMMON STOCK INFORMATION | Net income per common share (a): | |||||||||||||||||||
Basic | $ | 5.03 | $ | 3.45 | $ | 2.77 | $ | 2.67 | $ | 2.30 | ||||||||||
Diluted | 4.93 | 3.38 | 2.70 | 2.61 | 2.25 | |||||||||||||||
Cash dividends declared per common share | — | — | — | — | — | |||||||||||||||
BALANCE SHEET DATA | Total assets | $ | 6,488.1 | $ | 5,323.7 | $ | 5,666.4 | $ | 4,518.5 | $ | 4,056.3 | |||||||||
Long-term debt (b) | 594.4 | 593.8 | 438.4 | 822.3 | 596.9 | |||||||||||||||
_______________________________________________________________________________
(a) | The above results for 2019 include special charges of $64.6 million. The above results for 2018 include special charges of $109.1 million (primarily the impairment of intangible assets) and a $180.0 million ($137.5 million, net of tax) charge in 2018 related to a litigation settlement. The above results for 2017 include a $112.5 million ($70.3 million, net of tax) gain for a litigation payment received in 2017 and a $262.0 million tax expense related to the implementation of U.S. tax law changes. See Part II, Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" and Note 3, Note 4 and Note 17 to the "Consolidated Financial Statements" for additional information. |
(b) | In October 2013, we issued $600.0 million of 2.875% fixed-rate unsecured senior notes due October 15, 2018 (the "2013 Notes"). At December 31, 2017, the 2013 Notes were classified as short-term obligations as these obligations were due within one year. These 2013 Notes were paid in October 2018. In June 2018, we issued $600.0 million of 4.3% fixed-rate unsecured senior notes due June 15, 2028, which were classified as long-term obligations as of December 31, 2019 and 2018. Amounts outstanding under our Five-Year Credit Agreement ("Credit Agreement") have been classified as long-term obligations in accordance with the terms of the Credit Agreement. |
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis presents the factors that had a material effect on our results of operations during the two years ended December 31, 2019. Also discussed is our financial position as of December 31, 2019. You should read this discussion in conjunction with the historical consolidated financial statements and related notes included elsewhere in this Form 10-K. For a discussion related to the results of operations for 2018 compared to 2017, refer to Part II, Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our 2018 Annual Report on Form 10–K filed with the Securities and Exchange Commission on February 15, 2019.
Overview
We are the global leader in patient-focused medical innovations for structural heart disease, as well as critical care and surgical monitoring. Driven by a passion to help patients, we partner with the world's leading clinicians and researchers and invest in research and development to transform care for those impacted by structural heart disease or who require hemodynamic monitoring during surgery or in intensive care. We conduct operations worldwide and are managed in the following geographical regions: United States, Europe, Japan, and Rest of World. Our products are categorized into the following main areas: Transcatheter Aortic Valve Replacement ("TAVR"), Transcatheter Mitral and Tricuspid Therapies ("TMTT"), Surgical Structural Heart ("Surgical"), and Critical Care. Prior to 2019, TMTT and TAVR had been reported together. Therefore, prior periods have been presented to conform with the updated product categories.
23
Financial Highlights
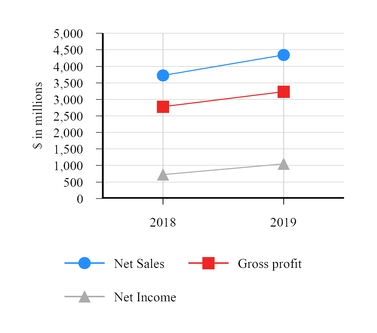
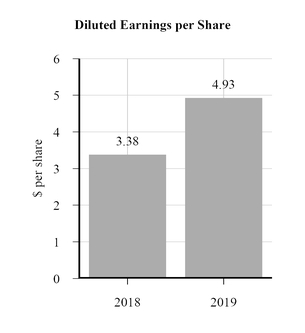
Our sales growth was driven by our TAVR products, primarily the Edwards SAPIEN 3 transcatheter heart valve and the Edwards SAPIEN 3 Ultra System. Our 2018 Surgical sales in the United States were reduced by a $82.5 million sales return reserve related to our conversion to a consignment inventory model.
Our gross profit increase was driven by our sales performance noted above and was positively impacted by an improved product mix, led by TAVR products. Partially offsetting this increase was a charge of $73.1 million recorded in 2019, primarily comprised of the write off of inventory related to strategic decisions regarding our TAVR portfolio, including the decision to discontinue our CENTERA program.
The increase in our net income and diluted earnings per share in 2019 was primarily driven by the aforementioned sales growth, partially offset by a 2018 tax benefit and the charge in 2019 related to strategic decisions regarding our TAVR portfolio.
Healthcare Environment, Opportunities, and Challenges
The medical technology industry is highly competitive and continues to evolve. Our success is measured both by the development of innovative products and the value we bring to our stakeholders. We are committed to developing new technologies and providing innovative patient care, and we are committed to defending our intellectual property in support of those developments. In 2019, we invested 17.3% of our net sales in research and development. The following is a summary of important developments during 2019:
• | we reached an agreement with Boston Scientific Corporation ("Boston Scientific") in January 2019 to settle all outstanding patent disputes for a one-time payment to Boston Scientific of $180.0 million; |
• | we completed the acquisition of CAS Medical Systems, Inc. ("CASMED"). CASMED is a medical technology company dedicated to noninvasive monitoring of tissue oxygenation in the brain; |
• | we received CE Mark for the Edwards PASCAL transcatheter valve repair system; |
• | we received FDA approval to expand use of the Edwards SAPIEN and SAPIEN 3 Ultra transcatheter heart valve systems to the treatment of severe, symptomatic aortic stenosis patients who are determined to be at low risk of open-heart surgery; |
• | we received CE Mark to expand use of the Edwards SAPIEN 3 transcatheter heart valve for the treatment of patients diagnosed with aortic stenosis who are at low risk for open-heart surgery; and |
24
• | we received FDA approval for an Early Feasibility Study to evaluate the safety and function of the Edwards EVOQUE tricuspid valve replacement system. |
We are dedicated to generating robust clinical, economic, and quality of life evidence increasingly expected by patients, clinicians, and payors in the current healthcare environment, with the goal of encouraging the adoption of innovative new medical therapies that demonstrate superior outcomes.
Results of Operations
Net Sales by Major Regions
(dollars in millions)
Year Ended December 31, | Change | |||||||||||||
2019 | 2018 | $ | % | |||||||||||
United States | $ | 2,532.7 | $ | 2,055.3 | $ | 477.4 | 23.2 | % | ||||||
Europe | 941.2 | 885.1 | 56.1 | 6.4 | % | |||||||||
Japan | 444.7 | 396.8 | 47.9 | 12.1 | % | |||||||||
Rest of World | 429.4 | 385.6 | 43.8 | 11.3 | % | |||||||||
International | 1,815.3 | 1,667.5 | 147.8 | 8.9 | % | |||||||||
Total net sales | $ | 4,348.0 | $ | 3,722.8 | $ | 625.2 | 16.8 | % | ||||||
International net sales include the impact of foreign currency exchange rate fluctuations. The impact of foreign currency exchange rate fluctuations on net sales is not necessarily indicative of the impact on net income due to the corresponding effect of foreign currency exchange rate fluctuations on international manufacturing and operating costs, and our hedging activities. For more information, see "Quantitative and Qualitative Disclosures About Market Risk."
Net Sales by Product Group
(dollars in millions)
Year Ended December 31, | Change | |||||||||||||
2019 | 2018 | $ | % | |||||||||||
Transcatheter Aortic Valve Replacement | $ | 2,737.9 | $ | 2,283.8 | $ | 454.1 | 19.9 | % | ||||||
Transcatheter Mitral and Tricuspid Therapies | 28.2 | 2.9 | 25.3 | NM | ||||||||||
Surgical Heart Valve Therapy | 841.7 | 761.6 | 80.1 | 10.5 | % | |||||||||
Critical Care | 740.2 | 674.5 | 65.7 | 9.7 | % | |||||||||
Total net sales | $ | 4,348.0 | $ | 3,722.8 | $ | 625.2 | 16.8 | % | ||||||
NM - Not meaningful
25
Transcatheter Aortic Valve Replacement
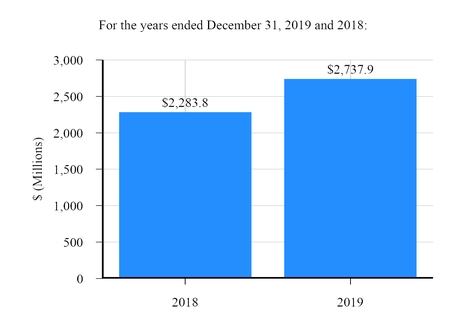
The increase in net sales of TAVR products was due primarily to:
• | higher sales of the Edwards SAPIEN 3 valve, particularly in the United States, driven by strong therapy adoption; and |
• | higher sales of the Edwards SAPIEN 3 Ultra System following its regulatory approval in Europe (November 2018) and the United States (December 2018); |
partially offset by:
• | foreign currency exchange rate fluctuations, which decreased net sales by $32.7 million, due primarily to the weakening of the Euro against the United States dollar. |
The March 2019 results of the PARTNER 3 Trial demonstrated superiority of SAPIEN 3 TAVR over surgery in the low risk patient population. In August 2019, we received FDA approval to expand use of the Edwards SAPIEN 3 and SAPIEN 3 Ultra transcatheter heart valve systems to the treatment of severe, symptomatic aortic stenosis patients who are determined to be at low risk of open-heart surgery. Given the approval for patients at low surgical risk and the continued excellence and versatility of our balloon expandable platform, we decided to discontinue the CENTERA program. While the CENTERA valve has demonstrated excellent clinical outcomes and is performing well for patients, the time and resources required to optimize deliverability and expand the indications to match the SAPIEN 3 valve are significant. In November 2019, we received CE Mark to expand use of the Edwards SAPIEN 3 transcatheter heart valve for the treatment of patients diagnosed with aortic stenosis who are at low risk for open-heart surgery.
26
Transcatheter Mitral and Tricuspid Therapies
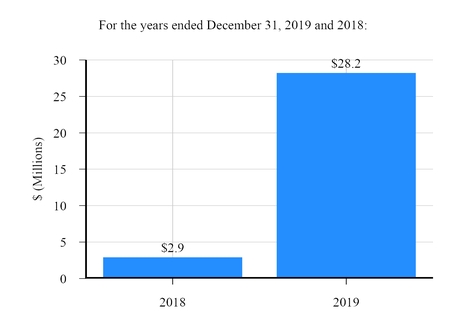
The increase in net sales of TMTT products was due primarily to sales of the Edwards PASCAL transcatheter valve repair system in Europe, which received CE Mark in February 2019.
In mitral repair, we continue to enroll our CLASP IID U.S. pivotal trial to study PASCAL in primary, or degenerative, mitral valve disease. We also have initiated enrollment in our CLASP IIF pivotal trial for patients with secondary, or functional, mitral valve disease. In September 2019, we received FDA approval for our CLASP IITR pivotal trial to study PASCAL in patients with symptomatic severe tricuspid regurgitation. In the fourth quarter of 2019, we received FDA approval for an Early Feasibility Study to evaluate the safety and function of the Edwards EVOQUE tricuspid valve replacement system.
Surgical Structural Heart
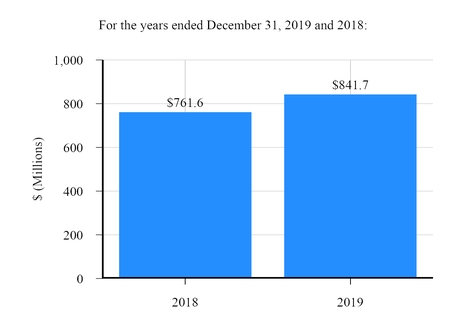
The increase in net sales of Surgical products was due primarily to:
• | sales return reserves in 2018 of $82.5 million in the United States related to our conversion to a consignment inventory model; and |
27
• | increased sales of aortic tissue valves in Japan, Europe and the United States, primarily the INSPIRIS RESILIA aortic valve; |
partially offset by:
• | foreign currency exchange rate fluctuations, which decreased net sales by $14.5 million, due primarily to the weakening of the Euro against the United States dollar. |
At the end of 2019, we received European regulatory approval for HARPOON Beating Heart Mitral Valve Repair System, and are in the process of beginning our commercial launch. HARPOON offers the potential for earlier treatment of degenerative mitral valve disease, with faster recovery and more consistent outcomes for surgical patients.
Critical Care

2019 Compared with 2018
The increase in net sales of Critical Care products was driven by our HemoSphere advanced monitoring platform, primarily in the United States, partially offset by foreign currency exchange rate fluctuations, which decreased net sales by $9.1 million, due primarily to the weakening of the Euro and various other currencies against the United States dollar.
On April 18, 2019, we completed the acquisition of CASMED, a medical technology company dedicated to noninvasive monitoring of tissue oxygenation in the brain. Our Critical Care sales for 2019 included $16.8 million related to CASMED.
We received FDA clearance in the third quarter of 2019 to use FORE-SIGHT, our cerebral oximetry technology, on our Hemosphere platform, and have initiated the commercial launch.
28
Gross Profit
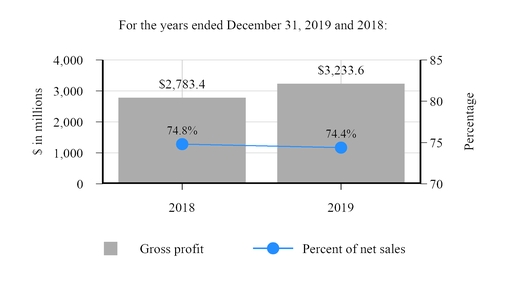
The decrease in gross profit as a percentage of net sales in 2019 compared to 2018 was driven by (1) a charge of $73.1 million related to strategic decisions regarding our transcatheter aortic valve portfolio, including the decision to discontinue our CENTERA program (for further information see the "Financial Highlights" section above), (2) the impact of multiple investments in our operations, including an increase in costs to improve our manufacturing processes and (3) spending in support of the new European device regulations. This decrease was partially offset by a 1.5 percentage point increase due to the impact of foreign currency exchange rate fluctuations, including the settlement of foreign currency hedging contracts.
Selling, General, and Administrative ("SG&A") Expenses
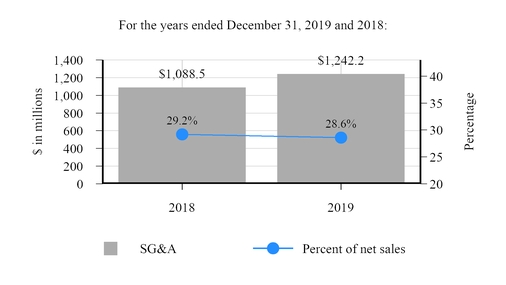
The increase in SG&A expenses in 2019 compared to 2018 was due primarily to higher transcatheter structural heart field personnel-related costs, primarily in the United States and Europe. These increases were partially offset by the impact of foreign currency, which decreased expenses by $16.7 million primarily due to the weakening of the Euro against the United States dollar. The decrease in SG&A expenses as a percentage of net sales was primarily due to leverage from our higher sales performance.
29
Research and Development ("R&D") Expenses

The increase in R&D expenses in 2019 compared to 2018 was due primarily to investments in our transcatheter structural heart programs, including generating clinical evidence.
Intellectual Property Litigation Expenses (Income), net
We incurred intellectual property litigation expenses, including settlements and external legal costs, of $33.4 million and $214.0 million during 2019 and 2018, respectively. In January 2019, we reached an agreement with Boston Scientific to settle all outstanding patent disputes for a one-time payment to Boston Scientific of $180.0 million, which was included as an expense in 2018. The settlement covers alleged past damages and no further royalties will be owed by either party.
Change in Fair Value of Contingent Consideration Liabilities, net
The change in fair value of contingent consideration liabilities resulted in income of $6.1 million and $5.7 million for the years ended December 31, 2019 and 2018, respectively. The income was due primarily to longer product development timelines, which reduced the probability of milestone achievements. The income was net of expenses associated with changes in the fair value of the liabilities associated with adjustments to discount rates, accretion of interest due to the passage of time, and the 2018 achievement by Valtech Cardio Ltd. of a regulatory milestone. For further information, see Note 11 to the "Consolidated Financial Statements."
Special Charges, net
For information on special charges, see Note 4 to the "Consolidated Financial Statements."
Interest Expense
Interest expense was $20.7 million and $29.9 million in 2019 and 2018, respectively. The decrease in interest expense resulted primarily from a lower average debt balance.
Interest Income
Interest income was $32.2 million and $32.0 million in 2019 and 2018, respectively. The increase in interest income resulted primarily from higher average interest rates, partially offset by a lower average investment balance.
30
Other (Income) Expense, net
(in millions)
Years Ended December 31, | |||||||
2019 | 2018 | ||||||
Foreign exchange gains, net | $ | (5.9 | ) | $ | (6.7 | ) | |
(Gain) loss on investments | (0.5 | ) | 1.7 | ||||
Non-service cost components of net periodic pension benefit cost (credit) | 0.2 | (0.1 | ) | ||||
Other | (2.0 | ) | 1.1 | ||||
Total other (income) expense, net | $ | (8.2 | ) | $ | (4.0 | ) | |
The net foreign exchange gains relate to the foreign currency fluctuations in our global trade and intercompany receivable and payable balances, offset by the gains and losses on derivative instruments intended as an economic hedge of those exposures.
The (gain) loss on investments represents our net share of gains and losses in investments accounted for under the equity method, and realized gains and losses on investments in equity securities.
The non-service cost components of net periodic pension benefit cost (credit) includes the costs of our defined benefit plans that are not attributed to services rendered by eligible employees during the year, such as interest costs, expected return on plan assets, and amortization of actuarial gains or losses. Certain costs associated with realignments, including settlements and curtailments, have been included as a component of "Special (Gains) Charges, net." For further information, see Notes 4 and 13 to the "Consolidated Financial Statements."
Provision for Income Taxes
Year Ended December 31, | Change | ||||||||||
2019 | 2018 | $ | % | ||||||||
Provision for income taxes | 119.6 | 39.2 | 80.4 | 205.1 | % | ||||||
Effective tax rate | 10.3 | % | 5.1 | % | |||||||
Our effective income tax rate in 2019 and 2018 was 10.3% and 5.1%, respectively. Our effective tax rate for 2019 increased in comparison to 2018 primarily because of the increase in the U.S. tax on global intangible low-taxed income that became effective with the Tax Cuts and Jobs Act of 2017 (the "2017 Act"), and the tax benefit in 2018 from audit settlements.
In 2019, the difference between our 10.3% effective tax rate and the Federal statutory rate of 21% was primarily due to the foreign tax rate differential on foreign operations, the recognition of excess tax benefits on stock-based compensation, and Federal and California research and development credits.
As of December 31, 2019, gross uncertain tax positions were $203.1 million. We estimate that these liabilities would be reduced by $50.1 million from offsetting tax benefits associated with the correlative effects of potential transfer pricing adjustments, state income taxes, and timing adjustments. The net amount of $153.0 million, if not required, would favorably affect our effective tax rate.
We strive to resolve open matters with each tax authority at the examination level and could reach agreement with a tax authority at any time. While we have accrued for matters we believe are more likely than not to require settlement, the final outcome with a tax authority may result in a tax liability that is more or less than that reflected in the consolidated financial statements. Furthermore, we may later decide to challenge any assessments, if made, and may exercise our right to appeal. The uncertain tax positions are reviewed quarterly and adjusted as events occur that affect potential liabilities for additional taxes, such as lapsing of applicable statutes of limitations, proposed assessments by tax authorities, negotiations between tax authorities, identification of new issues, and issuance of new legislation, regulations, or case law. We believe that adequate amounts of tax and related penalty and interest have been provided in income tax expense for any adjustments that may result from our uncertain tax positions.
At December 31, 2019, all material state, local, and foreign income tax matters have been concluded for years through 2008. During 2018, we signed agreements with the Internal Revenue Service ("IRS") to settle tax years 2009 through 2014,
31
including transfer pricing matters and the tax treatment of a portion of a litigation settlement payment received in 2014. The IRS began its examination of the 2015 and 2016 tax years during the fourth quarter of 2018 and its examination of the 2017 tax year during the first quarter of 2019.
During 2018, we executed an Advance Pricing Agreement ("APA") between the United States and Switzerland governments for tax years 2009 through 2020 covering various transfer pricing matters and we have updated our transfer pricing policies accordingly. Certain intercompany transactions covering tax years 2015 through 2019 were not resolved and those related tax positions remain uncertain. These transfer pricing matters may be significant to our consolidated financial statements. Based upon the information currently available and numerous possible outcomes, we cannot reasonably estimate what, if any, changes in our existing uncertain tax positions may occur in the next 12 months and, therefore, have recorded the gross uncertain tax positions as a long-term liability.
In addition, we executed other APAs as follows: during 2017, an APA between the United States and Japan covering tax years 2015 through 2019; and during 2018, APAs between Japan and Singapore and between Switzerland and Japan covering tax years 2015 through 2019. We are evaluating filing to renew some or all of these APAs for the years 2020 and forward. The execution of some or all of these APAs depends on a number of variables outside of our control.
We have received tax incentives in certain non-U.S. tax jurisdictions, the primary benefit for which will expire in 2029. The tax reductions as compared to the local statutory rates were $157.6 million ($0.75 per diluted share) and $144.9 million ($0.70 per diluted share) for the years ended December 31, 2019 and 2018, respectively.
Liquidity and Capital Resources
Our sources of cash liquidity include cash and cash equivalents, short-term investments, amounts available under credit facilities, and cash from operations. We believe that these sources are sufficient to fund the current requirements of working capital, capital expenditures, and other financial commitments for the next twelve months from the financial statement issuance date. However, we periodically consider various financing alternatives and may, from time to time, seek to take advantage of favorable interest rate environments or other market conditions.
The 2017 Act, which was signed into law on December 22, 2017, included extensive changes to the international tax regime. The 2017 Act required a deemed repatriation of post-1986 undistributed foreign earnings and profits. The deemed repatriation resulted in a $263.9 million tax obligation as of December 31, 2019. The one-time transition tax liability, as adjusted, is payable in six remaining annual installments, as outlined in the contractual obligations table below. See Note 17 to the "Consolidated Financial Statements" for additional information about the one-time transition tax.
As of December 31, 2019, cash and cash equivalents and short-term investments held in the United States and outside the United States were $909.8 million and $607.1 million, respectively. During 2019, we repatriated cash and notes receivable of $1.2 billion. We assert that $1.1 billion of our foreign earnings continue to be permanently reinvested and our intent is to repatriate $140.7 million of our foreign earnings as of December 31, 2019.
On April 18, 2019, we acquired CASMED for an aggregate cash purchase price of $2.45 per share of common stock, or
$100.8 million. For more information, see Note 8 to the "Consolidated Financial Statements."
Certain of our business acquisitions involve contingent consideration arrangements. Payment of additional consideration in the future may be required, contingent upon the acquired company reaching certain performance milestones, such as attaining specified revenue levels, or obtaining regulatory approvals. For further information, see Note 8 to the "Consolidated Financial Statements."
We have a Five-Year Credit Agreement ("the Credit Agreement") which matures on April 28, 2023. The Credit Agreement provides up to an aggregate of $750.0 million in borrowings in multiple currencies. Subject to certain terms and conditions, we may increase the amount available under the Credit Agreement by up to an additional $250.0 million in the aggregate. As of December 31, 2019, there were no borrowings outstanding under the Credit Agreement. The Credit Agreement is unsecured and contains various financial and other covenants, including a maximum leverage ratio, as defined in the Credit Agreement. The Company was in compliance with all covenants at December 31, 2019.
In June 2018, we issued $600.0 million of 4.3% fixed-rate unsecured senior notes (the "2018 Notes") due June 15, 2028. As of December 31, 2019, the total carrying value of our 2018 Notes was $594.4 million. For further information on our debt, see Note 10 to the "Consolidated Financial Statements."
32
We reached an agreement with Boston Scientific to settle all outstanding patent disputes for a one-time payment to Boston Scientific of $180.0 million, which was paid in January 2019.
From time to time, we repurchase shares of our common stock under share repurchase programs authorized by the Board of Directors. We consider several factors in determining when to execute share repurchases, including, among other things, expected dilution from stock plans, cash capacity, and the market price of our common stock. During 2019, under the Board authorized repurchase programs, we repurchased a total of 1.4 million shares at an aggregate cost of $255.0 million, and as of December 31, 2019, we had remaining authority to purchase $1.2 billion of our common stock. For further information, see Note 14 to the "Consolidated Financial Statements."
Consolidated Cash Flows - For the twelve months ended December 31, 2019 and 2018
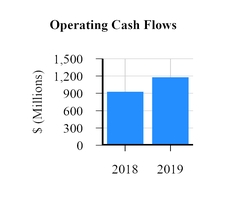
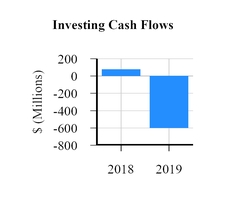
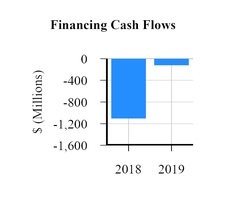
Net cash flows provided by operating activities of $1.2 billion for 2019 increased $252.6 million from 2018 due primarily to (1) improved operating performance in 2019, (2) higher tax payments in 2018 related to an audit settlement, and (3) a higher bonus payout in 2018 associated with 2017 performance, partially offset by (1) a payment of $180.0 million in 2019 for a litigation settlement and (2) higher working capital needs in 2019.
Net cash used in investing activities of $595.8 million in 2019 consisted primarily of (1) capital expenditures of $254.4 million, (2) net purchases of investments of $158.2 million, (3) a $100.2 million net cash payment associated with the acquisition of CASMED, (4) a $35.0 million payment for an option to acquire a company, and (5) a $24.0 million payment to acquire certain early-stage transcatheter intellectual property and associated clinical and regulatory experience.
Net cash provided by investing activities of $76.7 million in 2018 consisted primarily of net proceeds from investments of $342.0 million, partially offset by capital expenditures of $238.7 million.
We currently anticipate making capital expenditures of approximately $400 million in 2020 as we continue to invest in our operations.
Net cash used in financing activities of $115.6 million in 2019 consisted primarily of purchases of treasury stock of $263.3 million, partially offset by proceeds from stock plans of $160.5 million.
Net cash used in financing activities of $1.1 billion in 2018 consisted primarily of purchases of treasury stock of $795.5 million and net debt repayments of $437.3 million, partially offset by proceeds from stock plans of $147.0 million.
Consolidated Cash Flows - For the twelve months ended December 31, 2018 and 2017
For a discussion related to our consolidated cash flows for 2018 compared to 2017, refer to Part II, Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our 2018 Annual Report on Form 10–K filed with the Securities and Exchange Commission on February 15, 2019.
33
Contractual Obligations
A summary of all of our contractual obligations and commercial commitments as of December 31, 2019 is as follows (in millions):
Payments Due by Period | |||||||||||||||||||
Contractual Obligations | Total | Less Than 1 Year | 1-3 Years | 4-5 Years | After 5 Years | ||||||||||||||
Debt | $ | 600.0 | $ | — | $ | — | $ | — | $ | 600.0 | |||||||||
Operating leases | 91.8 | 27.4 | 34.2 | 15.7 | 14.5 | ||||||||||||||
Interest on debt | 164.3 | 19.9 | 39.8 | 38.5 | 66.1 | ||||||||||||||
Transition tax on unremitted foreign earnings and profits (a) | 263.9 | 25.1 | 50.3 | 110.0 | 78.5 | ||||||||||||||
Pension obligations (b) | 1.9 | 1.9 | — | — | — | ||||||||||||||
Purchase and other commitments (c) | 43.6 | 28.0 | 12.1 | 1.3 | 2.2 | ||||||||||||||
Total contractual cash obligations (d), (e) | $ | 1,165.5 | $ | 102.3 | $ | 136.4 | $ | 165.5 | $ | 761.3 | |||||||||
_______________________________________________________________________________
(a) | As of December 31, 2019, we had recorded $263.9 million of income tax liabilities related to the one-time transition tax that resulted from the enactment of the 2017 Act. The transition tax is due in eight annual installments, with the first annual installment paid in 2018 and the second annual installment paid in 2019. The remaining installment amounts will be equal to 8% of the total liability, payable in fiscal years 2020 through 2022, 15% in fiscal year 2023, 20% in fiscal year 2024, and 25% in fiscal year 2025. See Note 17 to the "Consolidated Financial Statements" for additional information about the one-time transition tax. |
(b) | The amount included in "Less Than 1 Year" reflects anticipated contributions to our various pension plans. Anticipated contributions beyond one year are not determinable. The total accrued benefit liability for our pension plans recognized as of December 31, 2019 was $42.0 million. This amount is impacted by, among other items, pension expense funding levels, changes in plan demographics and assumptions, and investment returns on plan assets. Therefore, we are unable to make a reasonably reliable estimate of the amount and period in which the liability might be paid, and did not include this amount in the contractual obligations table. See Note 13 to the "Consolidated Financial Statements" for further information. |
(c) | For certain purchase and other commitments, such as commitments to fund equity method or other investments, the timing of the payment may not be certain. In these cases, the maturity dates in the table reflect our best estimates. |
(d) | As of December 31, 2019, the gross liability for uncertain tax positions, including interest, was $215.9 million and relates primarily to transfer pricing matters. During 2018, we executed an APA between the United States and Switzerland governments for tax years 2009 through 2020 covering various transfer pricing matters and we have updated our transfer pricing policies accordingly. Certain intercompany transactions covering tax years 2015 through 2019 were not resolved and those related tax positions remain uncertain. These transfer pricing matters may be significant to our consolidated financial statements, and the final outcome of the negotiations is uncertain. Management believes that adequate amounts of tax and related penalty and interest have been provided in income tax expense for any adjustments that may result for our uncertain tax positions. We are unable to make a reasonably reliable estimate of the amount and period in which the liability might be paid, and did not include this amount in the contractual obligations table. |
(e) | We acquire assets still in development, enter into research and development arrangements, acquire businesses, and sponsor certain clinical trials that often require milestone, royalty, or other future payments to third-parties, contingent upon the occurrence of certain future events. In situations where we have no ability to influence the achievement of the milestone or otherwise avoid the payment, we have included those payments in the table above. However, we have excluded from the table contingent milestone payments and other contingent liabilities for which we cannot reasonably predict future payments or for which we can avoid making payment by unilaterally deciding to stop development of a product or cease progress of a clinical trial. We estimate that these contingent payments could be up to $565.0 million if all milestones or other contingent obligations are met. This amount includes certain milestone-based contingent obligations that may be paid through a combination of cash and issuance of common stock. |
Critical Accounting Policies and Estimates
Our results of operations and financial position are determined based upon the application of our accounting policies, as discussed in the notes to the "Consolidated Financial Statements." Certain of our accounting policies represent a selection
34
among acceptable alternatives under GAAP. In evaluating our transactions, management assesses all relevant GAAP and chooses the accounting policy that most accurately reflects the nature of the transactions.
The application of accounting policies requires the use of judgment and estimates. These matters that are subject to judgments and estimation are inherently uncertain, and different amounts could be reported using different assumptions and estimates. Management uses its best estimates and judgments in determining the appropriate amount to reflect in the consolidated financial statements, using historical experience and all available information. We also use outside experts where appropriate. We apply estimation methodologies consistently from year to year.
We believe the following are the critical accounting policies which could have the most significant effect on our reported results and require subjective or complex judgments by management.
Revenue Recognition
When we recognize revenue from the sale of our products, the amount of consideration we ultimately receive varies depending upon the return terms, sales rebates, discounts, and other incentives that we may offer, which are accounted for as variable consideration when estimating the amount of revenue to recognize. The estimate of variable consideration requires significant judgment. We include estimated amounts in the transaction price to the extent it is probable that a significant reversal of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is resolved. The estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely upon an assessment of historical payment experience, historical relationship to revenues, estimated customer inventory levels, and current contract sales terms with direct and indirect customers. Product returns are typically not significant because returns are generally not allowed unless the product is damaged at time of receipt. If the historical data and inventory estimates used to calculate the variable consideration do not approximate future activity, our financial position, results of operations, and cash flows could be impacted.
In addition, in limited circumstances, we may allow customers to return previously purchased products, such as for next-generation product offerings. For these transactions, we defer recognition of revenue on the sale of the earlier generation product based upon an estimate of the amount of product to be returned when the next-generation products are shipped to the customer. Uncertain timing of next-generation product approvals, variability in product launch strategies, product recalls, and variation in product utilization all affect the estimates related to sales returns and could cause actual returns to differ from these estimates.
Our sales adjustment related to distributor rebates given to our United States distributors represents the difference between our sales price to the distributor and the negotiated price to be paid by the end-customer. We validate the distributor rebate accrual quarterly through either a review of the inventory reports obtained from our distributors or an estimate of the distributor's inventory. This distributor inventory information is used to verify the estimated liability for future distributor rebate claims based on historical rebates and contract rates. We periodically monitor current pricing trends and distributor inventory levels to ensure the credit for future distributor rebates is fairly stated.
Excess and Obsolete Inventory
The valuation of our inventory requires us to estimate excess, obsolete, and expired inventory. We base our provisions for excess, obsolete, and expired inventory on our estimates of forecasted net sales. A significant change in the timing or level of demand for our products as compared to forecasted amounts may result in recording additional allowances for excess, obsolete, and expired inventory in the future. In addition, our industry is characterized by rapid product development and frequent new product introductions. Uncertain timing of next-generation product approvals, variability in product launch strategies, product recalls, increasing levels of consigned inventory, and variation in product utilization all affect our estimates related to excess, obsolete, and expired inventory.
Intangible Assets and Long-lived Assets
We acquire intangible assets in connection with business combinations and asset purchases. The acquired intangible assets are recorded at fair value, which is determined based on a discounted cash flow analysis. The determination of fair value requires significant estimates, including, but not limited to, the amount and timing of projected future cash flows, the discount rate used to discount those cash flows, the assessment of the asset's life cycle, including the timing and expected costs to complete in-process projects, and the consideration of legal, technical, regulatory, economic, and competitive risks.
35
In-process research and development assets acquired in business combinations is reviewed for impairment annually, or whenever an event occurs or circumstances change that would indicate the carrying amount may be impaired. Additionally, management reviews the carrying amounts of other intangible and long-lived assets whenever events or circumstances indicate that the carrying amounts of an asset may not be recoverable. The impairment reviews require significant estimates about fair value, including estimation of future cash flows, selection of an appropriate discount rate, and estimates of long-term growth rates.
Contingent Consideration
We record contingent consideration resulting from a business combination at its fair value on the acquisition date. We determine the fair value of the contingent consideration based primarily on the following factors:
• | discount rates used to present value the projected cash flows; |
• | the probability of success of clinical events and regulatory approvals, and/or meeting commercial milestones; |
• | projected payment dates; and |
• | volatility of future revenue. |
On a quarterly basis, we revalue these obligations and record changes in their fair value as an adjustment to earnings. Changes to contingent consideration obligations can result from adjustments to discount rates, accretion of the discount rates due to the passage of time, changes in our estimates of the likelihood or timing of achieving development or commercial milestones, changes in the probability of certain clinical events, or changes in the assumed probability associated with regulatory approval.
The assumptions related to determining the value of contingent consideration include a significant amount of judgment, and any changes in the underlying estimates could have a material impact on the amount of contingent consideration expense recorded in any given period.
Income Taxes
The determination of our provision for income taxes requires significant judgment, the use of estimates, and the interpretation and application of complex tax laws. Realization of certain deferred tax assets, primarily tax credits, net operating loss and other carryforwards, is dependent upon generating sufficient taxable income in the appropriate jurisdiction prior to the expiration of the carryforward periods. Failure to achieve forecasted taxable income in the applicable taxing jurisdictions could affect the ultimate realization of deferred tax assets and could result in an increase in our effective tax rate on future earnings.
We have made an accounting policy election to recognize the U.S. tax effects of global intangible low-taxed income as a component of income tax expense in the period the tax arises.
We are subject to income taxes in the United States and numerous foreign jurisdictions. Our income tax returns are periodically audited by domestic and foreign tax authorities. These audits include questions regarding our tax filing positions, including the timing and amount of deductions and the allocation of income amongst various tax jurisdictions. We evaluate our tax positions and establish liabilities in accordance with the applicable accounting guidance on uncertainty in income taxes. Significant judgment is required in evaluating our uncertain tax positions, including estimating the ultimate resolution to intercompany pricing controversies between countries when there are numerous possible outcomes. We review these tax uncertainties quarterly and adjust the liability as events occur that affect potential liabilities for additional taxes, such as the progress of tax audits, lapsing of applicable statutes of limitations, negotiations between tax authorities, identification of new issues, and issuance of new legislation, regulations, or case law.
For additional details on our income taxes, see Note 2 and Note 17 to the "Consolidated Financial Statements."
Stock-based Compensation
We measure and recognize compensation expense for all stock-based awards based on estimated fair values. Stock-based awards consist of stock options, service-based restricted stock units, market-based restricted stock units, performance-based restricted stock units, and employee stock purchase subscriptions. The fair value of each option award and employee stock
36
purchase subscription is estimated on the date of grant using the Black-Scholes option valuation model. The fair value of market-based restricted stock units is determined using a Monte Carlo simulation model, which uses multiple input variables to determine the probability of satisfying the market condition requirements. The Black-Scholes and Monte Carlo models require various highly judgmental assumptions, including stock price volatility, risk-free interest rate, and expected option term. For performance-based restricted stock units, expense is recognized if and when we conclude that it is probable that the performance condition will be achieved, which requires judgment. Stock-based compensation expense is recorded net of estimated forfeitures. Judgment is required in estimating the stock awards that will ultimately be forfeited. If actual results differ significantly from these estimates, stock-based compensation expense and our results of operations would be impacted.
New Accounting Standards
Information regarding new accounting standards is included in Note 2 to the "Consolidated Financial Statements."
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Our business and financial results are affected by fluctuations in world financial markets, including changes in currency exchange rates and interest rates. We manage these risks through a combination of normal operating and financing activities and derivative financial instruments. We do not use derivative financial instruments for trading or speculative purposes.
Interest Rate Risk
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio and our long-term debt. Our investment strategy is focused on preserving capital and supporting our liquidity requirements, while earning a reasonable market return. We invest in a variety of fixed-rate debt securities, primarily time deposits, commercial paper, U.S. and foreign government and agency securities, asset-backed securities, corporate debt securities, and municipal debt securities. The market value of our investments may decline if current market interest rates rise. As of December 31, 2019, we had $894.0 million of investments in fixed-rate debt securities which had an average remaining term to maturity of approximately 1.0 years. Taking into consideration the average maturity of our fixed-rate debt securities, a hypothetical 0.5% to 1.0% absolute increase in interest rates at December 31, 2019 would have resulted in a $4.6 million to $9.2 million decrease in the fair value of these investments. Such a decrease would only result in a realized loss if we choose or are forced to sell the investments before the scheduled maturity, which we currently do not anticipate.
For more information related to investments, see Note 7 to the "Consolidated Financial Statements."
We are also exposed to interest rate risk on our debt obligations. As of December 31, 2019, we had $600.0 million of Notes outstanding that carry a fixed rate, and also had available a $750.0 million Credit Agreement that carries a variable interest rate based on the London interbank offered rate ("LIBOR"). As of December 31, 2019, there were no borrowings outstanding under the Credit Agreement. Based on our December 31, 2019 variable debt levels, a hypothetical 1.0% absolute increase in our floating market interest rates would not have impacted our interest expense since we had no variable debt outstanding during the year. As of December 31, 2019, a hypothetical 1.0% absolute increase in market interest rates would decrease the fair value of the fixed-rate debt by approximately $44.7 million. This hypothetical change in interest rates would not impact the interest expense on the fixed-rate debt.
For more information related to outstanding debt obligations, see Note 10 to the "Consolidated Financial Statements."
Currency Risk
We are exposed to foreign currency risks that arise from normal business operations. These risks include the translation of local currency balances and results of our non-United States subsidiaries into United States dollars, currency gains and losses related to intercompany and third-party transactions denominated in currencies other than a location's functional currency, and currency gains and losses associated with intercompany loans. Our principal currency exposures relate to the Euro and the Japanese yen. Our objective is to minimize the volatility of our exposure to these risks through a combination of normal operating and financing activities and the use of derivative financial instruments in the form of foreign currency forward exchange contracts and cross currency swap contracts. The total notional amount of our derivative financial instruments entered into for foreign currency management purposes at December 31, 2019 was $1.6 billion. A hypothetical 10% increase/decrease in the value of the United States dollar against all hedged currencies would increase/decrease the fair value of these derivative contracts by $117.0 million. Any gains or losses on the fair value of derivative contracts would generally be offset by gains and losses on the underlying transactions, so the net impact would not be significant to our financial condition or results of operations.
37
For more information related to outstanding foreign exchange contracts, see Note 2 and Note 12 to the "Consolidated Financial Statements."
Credit Risk
Derivative financial instruments involve credit risk in the event the financial institution counterparty should default. It is our policy to execute such instruments with major financial institutions that we believe to be creditworthy. At December 31, 2019, all derivative financial instruments were with bank counterparties assigned investment grade ratings by national rating agencies. We further diversify our derivative financial instruments among counterparties to minimize exposure to any one of these entities. We have not experienced a counterparty default and do not anticipate any non-performance by our current derivative counterparties.
Concentrations of Risk
We invest excess cash in a variety of fixed-rate debt securities, and diversify the investments between financial institutions. Our investment policy limits the amount of credit exposure to any one issuer.
In the normal course of business, we provide credit to customers in the health care industry, perform credit evaluations of these customers, and maintain allowances for potential credit losses, which have historically been adequate compared to actual losses. In 2019, we had no customers that represented 10% or more of our total net sales or accounts receivable, net.
Investment Risk
We are exposed to investment risks related to changes in the underlying financial condition and credit capacity of certain of our investments. As of December 31, 2019, we had $894.0 million of investments in fixed-rate debt securities of various companies, of which $556.2 million were long-term. In addition, we had $29.3 million of investments in equity instruments of public and private companies. Should these companies experience a decline in financial condition or credit capacity, or fail to meet certain development milestones, a decline in the investments' values may occur, resulting in unrealized or realized losses.
38
Item 8. Financial Statements and Supplementary Data
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2019
Financial Statements: | |
For the Years Ended December 31, 2019, 2018, and 2017: | |
Other schedules are not applicable and have not been submitted. | |
39
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Edwards Lifesciences Corporation
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Edwards Lifesciences Corporation and its subsidiaries (the “Company”) as of December 31, 2019 and 2018, and the related consolidated statements of operations, of comprehensive income, of stockholders’ equity and of cash flows for each of the three years in the period ended December 31, 2019, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Change in Accounting Principle
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for leases in 2019.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the
40
company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Uncertain Tax Positions Related to Intercompany Transfer Pricing
As described in Notes 2 and 17 to the consolidated financial statements, the Company had a gross uncertain tax position liability balance of $203.1 million as of December 31, 2019, primarily related to transfer pricing. The Company is subject to income taxes in the United States and numerous foreign jurisdictions. As disclosed by management, the Company’s income tax returns in these jurisdictions are periodically audited by domestic and foreign tax authorities. These audits include questions regarding the Company’s tax filing positions, including the timing and amount of deductions and the allocation of income amongst various tax jurisdictions. Significant judgment is required by management in evaluating uncertain tax positions, including estimating the ultimate resolution to intercompany pricing controversies between countries when there are numerous possible outcomes.
The principal considerations for our determination that performing procedures relating to uncertain tax positions related to intercompany transfer pricing is a critical audit matter are there was significant judgment by management when determining uncertain tax positions related to intercompany transfer pricing, including a high degree of estimation uncertainty in evaluating whether certain tax filing positions taken by management will be upheld by the related local tax authority. This in turn led to a high degree of auditor judgment, effort, and subjectivity in performing procedures to evaluate the timely identification and accurate measurement of uncertain tax positions related to intercompany transfer pricing. Also, the evaluation of audit evidence available to support the tax liabilities for uncertain tax positions related to intercompany transfer pricing is complex and required significant auditor judgment as the nature of the evidence is highly subjective and the audit effort involved the use of professionals with specialized skill and knowledge to assist in evaluating the audit evidence obtained.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to identification and recognition of the liability for uncertain tax positions related to intercompany transfer pricing, and controls addressing completeness of the uncertain tax positions, as well as controls over measurement of the liability. These procedures also included, among others, (i) testing the information used in the calculation of the liability for uncertain tax positions, including intercompany agreements, international, federal, and state filing positions, and the related final tax returns; (ii) testing the calculation of the liability for uncertain tax positions related to intercompany transfer pricing, by jurisdiction, including management’s assessment of the technical merits of tax positions and estimates of the amount of tax benefit expected to be sustained; (iii) testing the completeness of management’s assessment of both the identification of uncertain tax positions related to intercompany transfer pricing and possible outcomes of each uncertain tax position; and (iv) evaluating the status and results of income tax audits with the relevant tax authorities. Professionals with specialized skill and knowledge were used to assist in the evaluation of the completeness and measurement of the Company’s uncertain tax positions related to intercompany transfer pricing, including evaluating the reasonableness of management’s assessment of whether tax positions are more-likely-than-not of being sustained and the amount of potential benefit to be realized, the application of relevant tax laws, and estimated interest and penalties.
Fair Value of Contingent Consideration Liabilities
As described in Note 11 to the consolidated financial statements, certain of the Company’s acquisitions involve contingent consideration arrangements. As of December 31, 2019, the Company had a contingent consideration liability of $172.5 million. As disclosed by management, payment of additional consideration is contingent upon the acquired company reaching certain performance milestones, such as attaining specified revenue levels or obtaining regulatory approvals. These contingent
41
consideration liabilities are measured by management at estimated fair value using either a probability weighted discounted cash flow analysis or a Monte Carlo simulation model, both of which consider significant unobservable inputs. These inputs include (1) the discount rate used to present value the projected cash flows, (2) the probability of milestone achievement, (3) the projected payment dates, and (4) the volatility of future revenue.
The principal considerations for our determination that performing procedures relating to the fair value of contingent consideration liabilities is a critical audit matter are there was significant judgment by management when estimating the fair value of these contingent obligations, including a high degree of estimation uncertainty in evaluating the discount rate, the probability of milestone achievement, the projected payment dates, and the volatility of future revenue. This in turn led to a high degree of auditor judgment, effort, and subjectivity in performing procedures to evaluate the fair value of contingent consideration liabilities. Also, the evaluation of audit evidence available to support the fair value of the contingent consideration liabilities is complex and required significant auditor judgment as the nature of the evidence is highly subjective and the audit effort involved the use of professionals with specialized skill and knowledge to assist in evaluating the audit evidence obtained.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s process for estimating the fair value of contingent consideration liabilities, including controls over the determination of the significant unobservable inputs selected by management. These procedures also included, among others, (i) testing management’s process for estimating the fair value of contingent consideration liabilities and (ii) testing management’s probability weighted discounted cash flow analysis or Monte Carlo simulation used to estimate the fair value of the contingent consideration liabilities. Testing management’s process included evaluating the appropriateness of the valuation methods used and the reasonableness of the significant assumptions, including the discount rate, the probability of milestone achievement, the projected payment dates, and the volatility of future revenue. Evaluating the reasonableness of the probability of milestone achievement and projected payment date of each milestone involved consideration of information obtained from the Company’s product engineers, clinical trial data, and third-party industry data. The discount rate was evaluated by considering the cost of capital of comparable businesses and other industry factors. Professionals with specialized skill and knowledge were used to assist in the evaluation of certain significant assumptions, including the discount rate and volatility of future revenue.
/s/ PricewaterhouseCoopers LLP
Irvine, California
February 14, 2020
We have served as the Company’s auditor since 1999.
42
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED BALANCE SHEETS
(in millions, except par value)
December 31, | |||||||
2019 | 2018 | ||||||
ASSETS | |||||||
Current assets | |||||||
Cash and cash equivalents | $ | $ | |||||
Short-term investments (Note 7) | |||||||
Accounts receivable, net (Note 5) | |||||||
Other receivables | |||||||
Inventories (Note 5) | |||||||
Prepaid expenses | |||||||
Other current assets | |||||||
Total current assets | |||||||
Long-term investments (Note 7) | |||||||
Property, plant, and equipment, net (Note 5) | |||||||
Operating lease right-of-use assets (Note 6) | — | ||||||
Goodwill (Note 9) | |||||||
Other intangible assets, net (Note 9) | |||||||
Deferred income taxes | |||||||
Other assets | |||||||
Total assets | $ | $ | |||||
LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||
Current liabilities | |||||||
Accounts payable | $ | $ | |||||
Accrued and other liabilities (Note 5) | |||||||
Operating lease liabilities (Note 6) | — | ||||||
Total current liabilities | |||||||
Long-term debt (Note 10) | |||||||
Contingent consideration liabilities (Notes 8 and 11) | |||||||
Taxes payable (Note 17) | |||||||
Operating lease liabilities (Note 6) | — | ||||||
Uncertain tax positions (Note 17) | |||||||
Other long-term liabilities | |||||||
Commitments and contingencies (Notes 6, 10 and 18) | |||||||
Stockholders' equity (Note 14) | |||||||
Preferred stock, $.01 par value, authorized 50.0 shares, no shares outstanding | |||||||
Common stock, $1.00 par value, 350.0 shares authorized, 218.1 and 215.2 shares issued, and 209.1 and 207.7 shares outstanding, respectively | |||||||
Additional paid-in capital | |||||||
Retained earnings | |||||||
Accumulated other comprehensive loss | ( | ) | ( | ) | |||
Treasury stock, at cost, 9.0 and 7.5 shares, respectively | ( | ) | ( | ) | |||
Total stockholders' equity | |||||||
Total liabilities and stockholders' equity | $ | $ | |||||
The accompanying notes are an integral part of these consolidated financial statements.
43
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(in millions, except per share information)
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Net sales | $ | $ | $ | ||||||||
Cost of sales | |||||||||||
Gross profit | |||||||||||
Selling, general, and administrative expenses | |||||||||||
Research and development expenses | |||||||||||
Intellectual property litigation expenses (income), net (Note 3) | ( | ) | |||||||||
Change in fair value of contingent consideration liabilities | ( | ) | ( | ) | ( | ) | |||||
Special charges, net (Note 4) | |||||||||||
Other operating expenses | |||||||||||
Operating income | |||||||||||
Interest expense | |||||||||||
Interest income | ( | ) | ( | ) | ( | ) | |||||
Special (gains) charges, net (Note 4) | ( | ) | |||||||||
Other (income) expense, net (Note 16) | ( | ) | ( | ) | |||||||
Income before provision for income taxes | |||||||||||
Provision for income taxes (Note 17) | |||||||||||
Net income | $ | $ | $ | ||||||||
Share information (Note 2): | |||||||||||
Earnings per share: | |||||||||||
Basic | $ | $ | $ | ||||||||
Diluted | $ | $ | $ | ||||||||
Weighted-average number of common shares outstanding: | |||||||||||
Basic | |||||||||||
Diluted | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
44
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(in millions)
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Net income | $ | $ | $ | ||||||||
Other comprehensive (loss) income, net of tax (Note 15): | |||||||||||
Foreign currency translation adjustments | ( | ) | ( | ) | |||||||
Unrealized (loss) gain on cash flow hedges | ( | ) | ( | ) | |||||||
Defined benefit pension plans | ( | ) | |||||||||
Unrealized gain (loss) on available-for-sale investments | ( | ) | ( | ) | |||||||
Reclassification of net realized investment loss to earnings | |||||||||||
Other comprehensive (loss) income, net of tax | ( | ) | |||||||||
Comprehensive income | $ | $ | $ | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
45
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in millions)
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Cash flows from operating activities | |||||||||||
Net income | $ | $ | $ | ||||||||
Adjustments to reconcile net income to cash provided by operating activities: | |||||||||||
Depreciation and amortization | |||||||||||
Non-cash operating lease cost | — | — | |||||||||
Stock-based compensation (Notes 2 and 14) | |||||||||||
Inventory write off | |||||||||||
Impairment charges (Note 4) | |||||||||||
Change in fair value of contingent consideration liabilities, net (Note 11) | ( | ) | ( | ) | ( | ) | |||||
Deferred income taxes | ( | ) | |||||||||
Purchased in-process research and development | |||||||||||
Other | ( | ) | ( | ) | |||||||
Changes in operating assets and liabilities: | |||||||||||
Accounts and other receivables, net | ( | ) | ( | ) | ( | ) | |||||
Inventories | ( | ) | ( | ) | ( | ) | |||||
Accounts payable and accrued liabilities | ( | ) | |||||||||
Income taxes | ( | ) | |||||||||
Prepaid expenses and other current assets | ( | ) | ( | ) | |||||||
Other | |||||||||||
Net cash provided by operating activities | |||||||||||
Cash flows from investing activities | |||||||||||
Capital expenditures | ( | ) | ( | ) | ( | ) | |||||
Deposit of cash in escrow | ( | ) | |||||||||
Purchases of held-to-maturity investments (Note 7) | ( | ) | ( | ) | ( | ) | |||||
Proceeds from sales and maturities of held-to-maturity investments (Note 7) | |||||||||||
Purchases of available-for-sale investments (Note 7) | ( | ) | ( | ) | ( | ) | |||||
Proceeds from sales and maturities of available-for-sale investments (Note 7) | |||||||||||
Acquisitions (Notes 8 and 9) | ( | ) | ( | ) | |||||||
Payment for acquisition option | ( | ) | |||||||||
Investments in intangible assets and in-process research and development | ( | ) | ( | ) | ( | ) | |||||
Other | ( | ) | ( | ) | ( | ) | |||||
Net cash (used in) provided by investing activities | ( | ) | ( | ) | |||||||
Cash flows from financing activities | |||||||||||
Proceeds from issuance of debt | |||||||||||
Payments on debt and finance lease obligations | ( | ) | ( | ) | ( | ) | |||||
Purchases of treasury stock | ( | ) | ( | ) | ( | ) | |||||
Proceeds from stock plans | |||||||||||
Payment of contingent consideration | ( | ) | |||||||||
Other | ( | ) | ( | ) | |||||||
Net cash used in financing activities | ( | ) | ( | ) | ( | ) | |||||
Effect of currency exchange rate changes on cash and cash equivalents | ( | ) | ( | ) | |||||||
Net increase (decrease) in cash and cash equivalents | ( | ) | ( | ) | |||||||
Cash and cash equivalents at beginning of year | |||||||||||
Cash and cash equivalents at end of year | $ | $ | $ | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
46
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
(in millions)
Common Stock | Treasury Stock | ||||||||||||||||||||||||||||
Shares | Par Value | Shares | Amount | Additional Paid-in Capital | Retained Earnings | Accumulated Other Comprehensive Loss | Total Stockholders' Equity | ||||||||||||||||||||||
BALANCE AT DECEMBER 31, 2016 | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | |||||||||||||||||||
Impact to retained earnings from adoption of ASU 2016-09 | |||||||||||||||||||||||||||||
BALANCE AT JANUARY 1, 2017 | ( | ) | ( | ) | |||||||||||||||||||||||||
Net income | |||||||||||||||||||||||||||||
Other comprehensive income, net of tax | |||||||||||||||||||||||||||||
Common stock issued under equity plans | |||||||||||||||||||||||||||||
Stock-based compensation expense | |||||||||||||||||||||||||||||
Shares issued to acquire business | ( | ) | |||||||||||||||||||||||||||
Purchases of treasury stock | ( | ) | ( | ) | |||||||||||||||||||||||||
Retirement of treasury stock | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||
BALANCE AT DECEMBER 31, 2017 | ( | ) | ( | ) | |||||||||||||||||||||||||
Impact to retained earnings from adoption of ASU 2016-16 and ASU 2018-02 | ( | ) | |||||||||||||||||||||||||||
BALANCE AT JANUARY 1, 2018 | ( | ) | ( | ) | |||||||||||||||||||||||||
Net income | |||||||||||||||||||||||||||||
Other comprehensive income, net of tax | |||||||||||||||||||||||||||||
Common stock issued under equity plans | |||||||||||||||||||||||||||||
Stock-based compensation expense | |||||||||||||||||||||||||||||
Shares issued in payment for contingent consideration liabilities | ( | ) | |||||||||||||||||||||||||||
Purchases of treasury stock | ( | ) | ( | ) | |||||||||||||||||||||||||
BALANCE AT DECEMBER 31, 2018 | ( | ) | ( | ) | |||||||||||||||||||||||||
Net income | |||||||||||||||||||||||||||||
Other comprehensive loss, net of tax | ( | ) | ( | ) | |||||||||||||||||||||||||
Common stock issued under equity plans | |||||||||||||||||||||||||||||
Stock-based compensation expense | |||||||||||||||||||||||||||||
Purchases of treasury stock | ( | ) | ( | ) | |||||||||||||||||||||||||
BALANCE AT DECEMBER 31, 2019 | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | |||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
47
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS
Edwards Lifesciences Corporation ("Edwards Lifesciences" or the "Company") conducts operations worldwide and is managed in the following geographical regions: United States, Europe, Japan, and Rest of World. Edwards Lifesciences is focused on technologies that treat structural heart disease and critically ill patients. The products and technologies provided by Edwards Lifesciences are categorized into the following main areas: Transcatheter Aortic Valve Replacement, Transcatheter Mitral and Tricuspid Therapies, Surgical Structural Heart, and Critical Care.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Edwards Lifesciences and its majority-owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation. The Company reviews its investments in other entities to determine whether the Company is the primary beneficiary of a variable interest entity ("VIE"). The Company would be the primary beneficiary of the VIE, and would be required to consolidate the VIE, if it has the power to direct the significant activities of the entity and the obligation to absorb losses or receive benefits from the entity that may be significant to the VIE. Based on the Company's analysis, it determined it is not the primary beneficiary of any VIEs; however, future events may require VIEs to be consolidated if the Company becomes the primary beneficiary.
Use of Estimates
The consolidated financial statements of Edwards Lifesciences have been prepared in accordance with generally accepted accounting principles in the United States of America ("GAAP") which have been applied consistently in all material respects. The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements. Actual results could differ from those estimates.
Foreign Currency Translation
When the local currency of the Company's foreign entities is the functional currency, all assets and liabilities are translated into United States dollars at the rate of exchange in effect at the balance sheet date. Income and expense items are translated at the weighted-average exchange rate prevailing during the period. The effects of foreign currency translation adjustments for these entities are deferred and reported in stockholders' equity as a component of "Accumulated Other Comprehensive Loss." The effects of foreign currency transactions denominated in a currency other than an entity's functional currency are included in "Other (Income) Expense, net."
Revenue Recognition
Revenue is recognized when control of the promised goods or services is transferred to the customer in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those products or services.
The Company generates nearly all of its revenue from direct product sales and sales of products under consignment arrangements. Revenue from direct product sales is recognized at a point in time when the performance obligation is satisfied upon delivery of the product. Revenue from sales of consigned inventory is recognized at a point in time when the performance obligation is satisfied once the product has been implanted or used by the customer. The Company periodically reviews consignment inventories to confirm the accuracy of customer reporting. The Company also generates a small portion of its revenue from service contracts, and recognizes revenue from service contracts ratably over the term of the contracts. Sales taxes and other similar taxes that the Company collects concurrent with revenue-producing activities are excluded from revenue. The Company does not typically have any significant unusual payment terms beyond 90 days in its contracts with customers. In addition, the Company receives royalty payments for the licensing of certain intellectual property and recognizes the royalty when the subsequent sale of product using the intellectual property occurs.
The amount of consideration the Company ultimately receives varies depending upon the return terms, sales rebates, discounts, and other incentives that the Company may offer, which are accounted for as variable consideration when estimating the amount of revenue to recognize. The Company includes estimated amounts in the transaction price to the extent it is
48
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
probable that a significant reversal of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is resolved. The estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely upon an assessment of historical payment experience, historical relationship to revenues, estimated customer inventory levels, and current contract sales terms with direct and indirect customers.
The Company's sales adjustment related to distributor rebates given to the Company's United States distributors represents the difference between the Company's sales price to the distributor and the negotiated price to be paid by the end-customer. This distributor rebate is recorded as a reduction to sales and a reduction to the distributor's accounts receivable at the time of sale to a distributor. The Company periodically monitors current pricing trends and distributor inventory levels to ensure the credit for future distributor rebates is fairly stated.
The Company also offers volume rebates to certain group purchasing organizations ("GPOs") and customers based upon target sales levels. Volume rebates offered to GPOs are recorded as a reduction to sales and an obligation to the GPOs, as the Company expects to pay in cash. Volume rebates offered to customers are recorded as a reduction to sales and either accounts receivable if the Company expects a net payment from the customer, or as an obligation to the customer if the Company expects to pay in cash. The provision for volume rebates is estimated based on customers' contracted rebate programs, projected sales levels, and historical experience of rebates paid. The Company periodically monitors its customer rebate programs to ensure that the allowance and liability for accrued rebates is fairly stated.
Product returns are typically not significant because returns are generally not allowed unless the product is damaged at time of receipt. In limited circumstances, the Company may allow customers to return previously purchased products, such as for next-generation product offerings. For these transactions, the Company defers recognition of revenue on the sale of the earlier generation product based upon an estimate of the amount of product to be returned when the next-generation products are shipped to the customer.
A limited number of the Company’s contracts with customers contain multiple performance obligations. For these contracts, the transaction price is allocated to each performance obligation based on its relative standalone selling price charged to other customers.
The Company applies the optional exemption of not disclosing the amount of the transaction price allocated to unsatisfied performance obligations for contracts with an original expected duration of one year or less.
Shipping and Handling Costs
Cash Equivalents
The Company considers highly liquid investments with original maturities of three months or less to be cash equivalents. These investments are valued at cost, which approximates fair value.
49
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Investments
The Company invests its excess cash in fixed-rate debt securities, including time deposits, commercial paper, U.S. government and agency securities, asset-backed securities, corporate debt securities, and municipal debt securities. Investments with maturities of one year or less are classified as short-term, and investments with maturities greater than one year are classified as long-term. Investments that the Company has the ability and intent to hold until maturity are classified as held-to-maturity and carried at amortized cost. Investments in debt securities that are classified as available-for-sale are carried at fair value with unrealized gains and losses included in "Accumulated Other Comprehensive Loss." The Company determines the appropriate classification of its investments in fixed-rate debt securities at the time of purchase and reevaluates such designation at each balance sheet date.
The Company also has long-term equity investments in companies that are in various stages of development. These investments are reported at fair value or under the equity method of accounting, as appropriate. Equity investments that do not have readily determinable fair values are recorded at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or similar investment of the same issuer. The Company accounts for investments in limited partnerships and limited liability corporations, whereby the Company owns a minimum of 5 % of the investee's outstanding voting stock, under the equity method of accounting. These investments are recorded at the amount of the Company's investment and adjusted each period for the Company's share of the investee's income or loss, and dividends paid.
Realized gains and losses on investments that are sold are determined using the specific identification method, or the first-in, first-out method, depending on the investment type, and recorded to "Other (Income) Expense, net." Income relating to investments in fixed-rate debt securities is recorded to "Interest Income."
The Company periodically reviews its investments for impairment. When the fair value of an investment declines below cost, management uses the following criteria to determine if such a decline should be considered other-than-temporary and result in a recognized loss:
• | the duration and extent to which the market value has been less than cost; |
• | the financial condition and near term prospects of the investee/issuer; |
• | the reasons for the decline in market value; |
• | the Company's ability and intent to hold the investment for a period of time sufficient to allow for any anticipated recovery in market value; and |
• | the investee's performance against product development milestones. |
Allowance for Doubtful Accounts
Inventories
Inventories are stated at the lower of cost (first-in, first-out method) or market value. Market value for raw materials is based on replacement costs, and for other inventory classifications is based on net realizable value.
50
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
A write-down for excess or slow moving inventory is recorded for inventory which is obsolete, nearing its expiration date (generally triggered at six months prior to expiration), is damaged, or slow moving (generally defined as quantities in excess of a two-year supply). The allowance for excess and slow moving inventory was $42.6 million and $30.3 million at December 31, 2019 and 2018, respectively.
At December 31, 2019 and 2018, $128.7 million and $106.5 million, respectively, of the Company's finished goods inventories were held on consignment.
Property, Plant, and Equipment
Property, plant, and equipment are recorded at cost. Depreciation is principally calculated for financial reporting purposes on the straight-line method over the estimated useful lives of the related assets, which range from 10 to 40 years for buildings and improvements, from 3 to 15 years for machinery and equipment, and from 3 to 5 years for software. Leasehold improvements are amortized over the life of the related facility leases or the asset, whichever is shorter. Straight-line and accelerated methods of depreciation are used for income tax purposes. Construction in progress is not depreciated until the asset is ready for its intended use and placed into service.
Depreciation expense for property, plant, and equipment was $84.7 million, $74.9 million, and $74.1 million for the years ended December 31, 2019, 2018, and 2017, respectively.
Impairment of Goodwill and Long-lived Assets
Goodwill is reviewed for impairment annually in the fourth quarter of each fiscal year, or whenever an event occurs or circumstances change that would indicate that the carrying amount may be impaired. Goodwill is tested for impairment at the reporting unit level by first performing a qualitative assessment to determine whether it is more likely than not that the fair value of the reporting unit is less than its carrying value. If the reporting unit does not pass the qualitative assessment, then the Company performs a quantitative impairment test. The Company determined, after performing a qualitative review of each reporting unit, that it is more likely than not that the fair value of each of its reporting units substantially exceeds the respective carrying amounts. Accordingly, in 2019, 2018, and 2017, the Company did not record any impairment loss.
Indefinite-lived intangible assets relate to in-process research and development acquired in business combinations. The estimated fair values of in-process research and development projects acquired in a business combination which have not reached technological feasibility are capitalized and accounted for as indefinite-lived intangible assets subject to impairment testing until completion or abandonment of the projects. Upon successful completion of the project, the capitalized amount is amortized over its estimated useful life. If the project is abandoned, all remaining capitalized amounts are written off immediately. Indefinite-lived intangible assets are reviewed for impairment annually, or whenever an event occurs or circumstances change that would indicate the carrying amount may be impaired. An impairment loss is recognized when the asset's carrying value exceeds its fair value. In-process research and development projects acquired in an asset acquisition are expensed unless the project has an alternative future use.
Management reviews the carrying amounts of other finite-lived intangible assets and long-lived tangible assets whenever events or circumstances indicate that the carrying amounts of an asset may not be recoverable. Impairment indicators include, among other conditions, cash flow deficits, historic or anticipated declines in revenue or operating profit, and adverse legal or regulatory developments. If it is determined that such indicators are present and the review indicates that the assets will not be fully recoverable, based on undiscounted estimated cash flows over the remaining amortization periods, their carrying values are reduced to estimated fair market value. Estimated fair market value is determined primarily using the anticipated cash flows discounted at a rate commensurate with the risk involved. For the purposes of identifying and measuring impairment, long-lived
51
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
assets are grouped with other assets and liabilities at the lowest level for which identifiable cash flows are largely independent of the cash flows of other assets and liabilities.
In 2019, the Company recorded a $40.6 million charge related to the impairment of certain in-process research and development assets. In 2018, the Company recorded a $116.2 million charge related to the impairment of certain developed technology and in-process research and development assets. See Note 4 for further information. In 2017, the Company did not record any impairment loss related to its in-process research and development assets.
The Company is subject to income taxes in the United States and numerous foreign jurisdictions. Significant judgment is required in evaluating the Company's uncertain tax positions and determining its provision for income taxes. The Company recognizes the financial statement benefit of a tax position only after determining that a position would more likely than not be sustained based upon its technical merit if challenged by the relevant taxing authority and taken by management to the court of last resort. For tax positions meeting the more-likely-than-not threshold, the amount recognized in the consolidated financial statements is the largest benefit that has a greater than 50% likelihood of being realized upon settlement with the relevant tax authority. The Company recognizes interest and penalties related to income tax matters in income tax expense. The Company has made an accounting policy election to recognize the U.S. tax effects of global intangible low-taxed income as a component of income tax expense in the period the tax arises.
Deferred tax assets and liabilities are recognized for the expected future tax consequences of events that have been recognized in the Company's financial statements or tax returns. The Company evaluates quarterly the realizability of its deferred tax assets by assessing its valuation allowance and adjusting the amount, if necessary. The factors used to assess the likelihood of realization are both historical experience and the Company's forecast of future taxable income and available tax planning strategies that could be implemented to realize the net deferred tax assets. Failure to achieve forecasted taxable income in the applicable taxing jurisdictions could affect the ultimate realization of deferred tax assets and could result in an increase in the Company's effective tax rate on future earnings.
Research and Development Costs
Research and development costs are charged to expense when incurred.
Earnings per Share
Basic earnings per share is computed by dividing net income by the weighted-average common shares outstanding during a period. Diluted earnings per share is computed based on the weighted-average common shares outstanding plus the effect of dilutive potential common shares outstanding during the period calculated using the treasury stock method. Dilutive potential common shares include employee equity share options, nonvested shares, and similar equity instruments granted by the Company. Potential common share equivalents have been excluded where their inclusion would be anti-dilutive.
52
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The table below presents the computation of basic and diluted earnings per share (in millions, except for per share information):
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Basic: | |||||||||||
Net income | $ | $ | $ | ||||||||
Weighted-average shares outstanding | |||||||||||
Basic earnings per share | $ | $ | $ | ||||||||
Diluted: | |||||||||||
Net income | $ | $ | $ | ||||||||
Weighted-average shares outstanding | |||||||||||
Dilutive effect of stock plans | |||||||||||
Dilutive weighted-average shares outstanding | |||||||||||
Diluted earnings per share | $ | $ | $ | ||||||||
Stock options, restricted stock units, and market-based restricted stock units to purchase approximately 0.5 million, 1.1 million, and 1.9 million shares for the years ended December 31, 2019, 2018, and 2017, respectively, were outstanding, but were not included in the computation of diluted earnings per share because the effect would have been anti-dilutive.
Stock-based Compensation
The Company measures and recognizes compensation expense for all stock-based awards based on estimated fair values. Stock-based awards consist of stock options, restricted stock units (service-based, market-based, and performance-based), and employee stock purchase subscriptions. Stock-based compensation expense is measured at the grant date based on the fair value of the award and is recognized as expense over the requisite service period (vesting period) on a straight-line basis. For performance-based restricted stock units, the Company recognizes stock-based compensation expense if and when the Company concludes that it is probable that the performance condition will be achieved, net of estimated forfeitures. The Company reassesses the probability of vesting at each quarter end and adjusts the stock-based compensation expense based on its probability assessment. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Upon exercise of stock options or vesting of restricted stock units, the Company issues common stock.
Total stock-based compensation expense was as follows (in millions):
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Cost of sales | $ | $ | $ | ||||||||
Selling, general, and administrative expenses | |||||||||||
Research and development expenses | |||||||||||
Total stock-based compensation expense | $ | $ | $ | ||||||||
Upon a participant's retirement, all unvested stock options and performance-based restricted stock units are immediately forfeited. In addition, upon retirement, a participant will immediately vest in 25 % of service-based restricted stock units for each full year of employment with the Company measured from the grant date. All remaining unvested service-based restricted stock units are immediately forfeited. For market-based restricted stock units, upon retirement and in certain other specified cases, a participant will receive a pro-rated portion of the shares that would ultimately be issued based on attainment of the performance goals as determined on the vesting date. The pro-rated portion is based on the participant's whole months of service with the Company during the performance period prior to the date of termination.
53
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Derivatives
The Company uses derivative financial instruments to manage interest rate and foreign currency risks. It is the Company's policy not to enter into derivative financial instruments for speculative purposes.
Derivative financial instruments involve credit risk in the event the counterparty should default. It is the Company's policy to execute such instruments with global financial institutions that the Company believes to be creditworthy. The Company diversifies its derivative financial instruments among counterparties to minimize exposure to any one of these entities. The Company also uses International Swap Dealers Association master-netting agreements. The master-netting agreements provide for the net settlement of all contracts through a single payment in a single currency in the event of default, as defined by the agreements.
The Company uses foreign currency forward exchange contracts, cross currency swap contracts, and foreign currency denominated debt to manage its exposure to changes in currency exchange rates from (1) future cash flows associated with intercompany transactions and certain local currency expenses expected to occur within the next 13 months (designated as cash flow hedges), (2) its net investment in certain foreign subsidiaries (designated as net investment hedges) and (3) foreign currency denominated assets or liabilities (designated as fair value hedges). The Company also uses foreign currency forward exchange contracts that are not designated as hedging instruments to offset the transaction gains and losses associated with certain assets and liabilities denominated in currencies other than their functional currencies resulting principally from intercompany and local currency transactions.
The Company at times has used interest rate swaps to convert a portion of its fixed-rate debt into variable-rate debt. These interest rate swaps were designated as fair value hedges and met the shortcut method requirements under the accounting standards for derivatives and hedging. Accordingly, changes in the fair values of the interest rate swaps were considered to exactly offset changes in the fair value of the underlying long-term debt.
All derivative financial instruments are recognized at fair value in the consolidated balance sheets. For each derivative instrument that is designated as a fair value hedge, the gain or loss on the derivative included in the assessment of hedge effectiveness is recognized immediately to earnings, and offsets the loss or gain on the underlying hedged item. The Company reports in "Accumulated Other Comprehensive Loss" the gain or loss on derivative financial instruments that are designated, and that qualify, as cash flow hedges. The Company reclassifies these gains and losses into earnings in the same line item and in the same period in which the underlying hedged transactions affect earnings. Changes in the fair value of net investment hedges are reported in "Accumulated Other Comprehensive Loss" as a part of the cumulative translation adjustment and would be reclassified into earnings if the underlying net investment is sold or substantially liquidated. The portion of the change in fair value related to components excluded from the hedge effectiveness assessment are amortized into earnings over the life of the derivative. The gains and losses on derivative financial instruments for which the Company does not elect hedge accounting treatment are recognized in the consolidated statements of operations in each period based upon the change in the fair value of the derivative financial instrument. Cash flows from net investment hedges are reported as investing activities in the consolidated statements of cash flows, and cash flows from all other derivative financial instruments are reported as operating activities.
In February 2016, the Financial Accounting Standards Board ("FASB") issued an amendment to the guidance on leases. The amendment improves transparency and comparability among companies by recognizing lease assets and lease liabilities on the balance sheet and by disclosing key information about leasing arrangements. The guidance was effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach was required upon adoption. Reporting entities could elect to adjust comparative periods and record the cumulative effect adjustment at the beginning of the earliest comparative period, or to not adjust comparative periods and record the cumulative effect adjustment at the effective date.
The Company adopted the new guidance as of the effective date of January 1, 2019 with no adjustments to the comparative period presented in the financial statements. In addition, the Company elected the package of practical expedients permitted under the transition guidance to not reassess (1) whether any expired or existing contracts are, or contain, leases, (2)
54
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
the lease classification for expired or existing leases, and (3) initial direct costs for existing leases. The Company implemented internal controls and system solutions to enable the preparation and disclosure of financial information about its leasing arrangements upon adoption.
The adoption of the guidance resulted in the recognition of right-of-use assets and additional lease liabilities for operating leases of $58.7 million as of January 1, 2019. The guidance did not have an impact on the Company's consolidated statements of operations. See Note 6 for disclosures related to the Company's leases.
New Accounting Standards Not Yet Adopted
In January 2020, the FASB issued an amendment clarifying the interaction between accounting standards related to equity securities, equity method investments, and certain derivatives. The guidance is effective for fiscal years beginning after December 15, 2020. The Company does not expect the adoption of this guidance will have a material impact on its consolidated financial statements.
In December 2019, the FASB issued an amendment to the guidance on income taxes which is intended to simplify the accounting for income taxes. The amendment eliminates certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period, and the recognition of deferred tax liabilities for outside basis differences. The amendment also clarifies existing guidance related to the recognition of franchise tax, the evaluation of a step up in the tax basis of goodwill, and the effects of enacted changes in tax laws or rates in the effective tax rate computation, among other clarifications. The guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020. The Company is currently evaluating the impact the guidance will have on its consolidated financial statements.
In August 2018, the FASB issued an amendment to the accounting guidance on cloud computing service arrangements. The guidance aligns the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. The guidance also requires an entity to expense the capitalized implementation costs of a hosting arrangement that is a service contract over the term of the hosting arrangement. The guidance is effective for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years. The Company does not expect the adoption of this guidance will have a material impact on its consolidated financial statements.
In August 2018, the FASB issued an amendment to the accounting guidance on retirement benefits. The guidance modifies the disclosure requirements for employers that sponsor defined benefit pension or other postretirement plans. The guidance is effective for fiscal years ending after December 15, 2020 and must be applied retrospectively to all periods presented. The Company does not expect the adoption of this guidance will have a material impact on its consolidated financial statements.
In August 2018, the FASB issued an amendment to the accounting guidance on fair value measurements. The guidance modifies the disclosure requirements on fair value measurements, including the removal of disclosures of the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy, the policy for timing of transfers between levels, and the valuation processes for Level 3 fair value measurements. The guidance also adds certain disclosure requirements related to Level 3 fair value measurements. The guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. The Company does not expect the adoption of this guidance will have a material impact on its consolidated financial statements.
In June 2016, the FASB issued an amendment to the guidance on the measurement of credit losses on financial instruments. The amendment updates the guidance for measuring and recording credit losses on financial assets measured at amortized cost by replacing the “incurred loss” model with an “expected loss” model. Accordingly, these financial assets will be presented at the net amount expected to be collected. The amendment also requires that credit losses related to available-for-sale debt securities be recorded as an allowance through net income rather than reducing the carrying amount under the current, other-than-temporary-impairment model. The guidance is effective for fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. Early adoption is permitted for annual periods after December 15, 2018.
55
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
3. INTELLECTUAL PROPERTY LITIGATION EXPENSES (INCOME), NET
The Company incurred intellectual property litigation expenses, including settlements and external legal costs, of $33.4 million, $214.0 million and $39.2 million during 2019, 2018 and 2017, respectively. In January 2019, the Company reached an agreement with Boston Scientific Corporation ("Boston Scientific") to settle all outstanding patent disputes for a one-time payment to Boston Scientific of $180.0 million, which was included as an expense in 2018. The settlement covers alleged past damages and no further royalties will be owed by either party.
In November 2017, the Company recorded a $112.5 million litigation gain related to the theft of trade secrets.
4. SPECIAL CHARGES
Impairment of Long-lived Assets
In December 2019, the Company recorded a charge of $40.6 million related to the impairment of certain in-process research and development assets, and in December 2018, the Company recorded a charge of $116.2 million related to the impairment of certain developed technology and in-process research and development assets. These assets were acquired as part of the acquisition of Valtech Cardio Ltd. ("Valtech"). The Company measured the amount of the impairments by calculating the amount by which the carrying values exceeded the estimated fair values, which were based on projected discounted future net cash flows. Based on market and clinical trial developments at the time of the impairments, the Company re-evaluated the clinical development plans for the technologies acquired from Valtech, which resulted in a reduction to the projected near-term discounted future net cash flows related to the acquired mitral technology for the 2018 charge, and related to the acquired mitral and tricuspid technology for the 2019 charge. The impairments were recorded to the Company’s Rest of World segment.
In June 2017, the Company recorded a $31.2 million charge related to the impairment of one of its cost method investments and an associated long-term asset related to the Company's option to acquire this investee. The Company concluded that the impairment of these assets was other-than-temporary based upon a recent review of the investee's clinical data and trial results, which did not support continuation of the product development effort, and the financial condition and near-term prospects of the investee.
Acquisition of Intellectual Property
In March 2019, the Company recorded a $24.0 million charge related to the acquisition of early-stage transcatheter intellectual property and associated clinical and regulatory experience.
Charitable Foundation Contribution
In December 2017, the Company contributed $25.0 million to the Edwards Lifesciences Foundation, a related-party not-for-profit organization whose mission is to support health- and community-focused charitable organizations. The contribution was irrevocable and was recorded as an expense at the time of payment.
Gain on Step Acquisition
In December 2017, the Company acquired Harpoon Medical, Inc. As a result of the acquisition, the Company remeasured at fair value its previously held ownership in Harpoon Medical, Inc. and recognized a gain of $6.5 million. See Note 8 for further information.
56
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. SPECIAL CHARGES (Continued)
Realignment Expenses
In March 2018, the Company recorded a $7.1 million gain related to the curtailment of its defined benefit plan in Switzerland resulting from the closure of its manufacturing plant.
In September 2017, the Company recorded a $10.2 million charge related primarily to severance expenses (impacting 232 employees) and other costs associated with the planned closure of its manufacturing plant in Switzerland. As of December 31, 2019, payments related to the realignment were complete.
57
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. OTHER CONSOLIDATED FINANCIAL STATEMENT DETAILS
Composition of Certain Financial Statement Captions
Components of selected captions in the consolidated balance sheets are as follows:
As of December 31, | |||||||
2019 | 2018 | ||||||
(in millions) | |||||||
Accounts receivable, net | |||||||
Trade accounts receivable | $ | $ | |||||
Allowance for doubtful accounts | ( | ) | ( | ) | |||
$ | $ | ||||||
Inventories | |||||||
Raw materials | $ | $ | |||||
Work in process | |||||||
Finished products | |||||||
$ | $ | ||||||
Property, plant, and equipment, net | |||||||
Land | $ | $ | |||||
Buildings and leasehold improvements | |||||||
Machinery and equipment | |||||||
Equipment with customers | |||||||
Software | |||||||
Construction in progress | |||||||
Accumulated depreciation | ( | ) | ( | ) | |||
$ | $ | ||||||
Accrued and other liabilities | |||||||
Employee compensation and withholdings | $ | $ | |||||
Litigation and insurance reserves (Note 18) | |||||||
Taxes payable | |||||||
Accrued rebates | |||||||
Property, payroll, and other taxes | |||||||
Research and development accruals | |||||||
Fair value of derivatives | |||||||
Accrued marketing expenses | |||||||
Accrued professional services | |||||||
Accrued realignment reserves | |||||||
Accrued relocation costs | |||||||
Other accrued liabilities | |||||||
$ | $ | ||||||
In 2019, the Company recorded a $73.1 million charge to "Cost of Sales," primarily comprised of the write off of inventory related to strategic decisions regarding its transcatheter aortic valve portfolio, including the decision to discontinue its CENTERA program.
58
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. OTHER CONSOLIDATED FINANCIAL STATEMENT DETAILS (Continued)
Supplemental Cash Flow Information
(in millions)
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Cash paid during the year for: | |||||||||||
Interest | $ | $ | $ | ||||||||
Income taxes | $ | $ | $ | ||||||||
Amounts included in the measurement of lease liabilities: | |||||||||||
Operating cash flows from operating leases | $ | $ | — | $ | — | ||||||
Non-cash investing and financing transactions: | |||||||||||
Fair value of shares issued in payment for contingent consideration liabilities (Note 11) | $ | $ | $ | ||||||||
Fair value of shares issued in connection with business combinations (Note 8) | $ | $ | $ | ||||||||
Right-of-use assets obtained in exchange for new lease liabilities | $ | $ | — | $ | — | ||||||
Capital expenditures accruals | $ | $ | $ | ||||||||
Retirement of treasury stock | $ | $ | $ | ||||||||
6. LEASES
The Company determines whether a contract is, or contains, a lease at inception. Right-of-use assets represent the Company’s right to use an underlying asset during the lease term, and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Right-of-use assets and lease liabilities are recognized at lease commencement based upon the estimated present value of unpaid lease payments over the lease term. The Company uses its incremental borrowing rate based on the information available at lease commencement in determining the present value of unpaid lease payments. The Company's incremental borrowing rate is determined based on the estimated rate of interest for collateralized borrowing over a similar term as the associated lease. Right-of-use assets also include any lease payments made at or before lease commencement and any initial direct costs incurred, and exclude any lease incentives received.
The Company determines the lease term as the noncancellable period of the lease, and may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option. Leases with a term of 12 months or less are not recognized on the balance sheet. Certain of the Company’s leases include variable lease payments that are based on costs incurred or actual usage, or adjusted periodically based on an index or a rate. The Company’s leases do not contain any residual value guarantees.
The Company accounts for the lease and non-lease components as a single lease component for all of its leases except vehicle leases, for which the lease and non-lease components are accounted for separately.
Operating leases are included in “Operating Lease Right-of-Use Assets” and “Operating Lease Liabilities” on the Company’s consolidated condensed balance sheets.
The Company leases certain office space, manufacturing facilities, land, apartments, warehouses, vehicles, and equipment with remaining lease terms ranging from less than 1 year to 21 years, some of which include options to extend or terminate the leases.
Operating lease costs for the year ended December 31, 2019 were $27.9 million. Short-term and variable lease costs were not material for the year ended December 31, 2019.
59
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. LEASES (Continued)
Supplemental balance sheet information related to operating leases was as follows (in millions, except lease term and discount rate):
December 31, 2019 | |||
Operating lease right-of-use assets | $ | ||
Operating lease liabilities, current portion | $ | ||
Operating lease liabilities, long-term portion | |||
Total operating lease liabilities | $ | ||
Maturities of operating lease liabilities at December 31, 2019 were as follows (in millions):
2020 | $ | ||
2021 | |||
2022 | |||
2023 | |||
2024 | |||
Thereafter | |||
Total lease payments | |||
Less: imputed interest | ( | ) | |
Total lease liabilities | $ | ||
Weighted-average remaining lease term (in years) | |||
Weighted-average discount rate | % | ||
As of December 31, 2019, the Company had additional operating lease commitments of $1.6 million for office space that have not yet commenced. These leases will commence during 2020 with lease terms of 6 months to 7 years.
Disclosures related to periods prior to adopting the new lease guidance
Certain facilities and equipment are leased under operating leases expiring at various dates. Most of the operating leases contain renewal options. Total expense for all operating leases was $27.0 million and $27.3 million for the years 2018 and 2017, respectively.
Future minimum lease payments (including interest) under non-cancelable operating leases at December 31, 2018 were as follows (in millions):
Operating Leases | |||
2019 | $ | ||
2020 | |||
2021 | |||
2022 | |||
2023 | |||
Thereafter | |||
Total obligations and commitments | $ | ||
60
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. INVESTMENTS
Debt Securities
Investments in debt securities at the end of each period were as follows (in millions):
December 31, 2019 | December 31, 2018 | ||||||||||||||||||||||||||||||
Held-to-maturity | Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | |||||||||||||||||||||||
Bank time deposits | $ | $ | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||
Available-for-sale | |||||||||||||||||||||||||||||||
Bank time deposits | $ | $ | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||
Commercial paper | |||||||||||||||||||||||||||||||
U.S. government and agency securities | ( | ) | |||||||||||||||||||||||||||||
Foreign government bonds | |||||||||||||||||||||||||||||||
Asset-backed securities | ( | ) | ( | ) | |||||||||||||||||||||||||||
Corporate debt securities | ( | ) | ( | ) | |||||||||||||||||||||||||||
Municipal securities | |||||||||||||||||||||||||||||||
$ | $ | $ | ( | ) | $ | $ | $ | $ | ( | ) | $ | ||||||||||||||||||||
The cost and fair value of investments in debt securities, by contractual maturity, as of December 31, 2019 were as follows:
Held-to-Maturity | Available-for-Sale | ||||||||||||||
Cost | Fair Value | Cost | Fair Value | ||||||||||||
(in millions) | |||||||||||||||
Due in 1 year or less | $ | $ | $ | $ | |||||||||||
Due after 1 year through 5 years | |||||||||||||||
Instruments not due at a single maturity date | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
Actual maturities may differ from the contractual maturities due to call or prepayment rights.
The following tables present gross unrealized losses and fair values for those investments that were in an unrealized loss position as of December 31, 2019 and 2018, aggregated by investment category and the length of time that individual securities have been in a continuous loss position (in millions):
December 31, 2019 | |||||||||||||||||||||||
Less than 12 Months | 12 Months or Greater | Total | |||||||||||||||||||||
Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | ||||||||||||||||||
Asset-backed securities | ( | ) | ( | ) | |||||||||||||||||||
Corporate debt securities | ( | ) | ( | ) | |||||||||||||||||||
$ | $ | ( | ) | $ | $ | $ | $ | ( | ) | ||||||||||||||
61
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. INVESTMENTS (Continued)
December 31, 2018 | |||||||||||||||||||||||
Less than 12 Months | 12 Months or Greater | Total | |||||||||||||||||||||
Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | ||||||||||||||||||
U.S. government and agency securities | $ | $ | ( | ) | $ | $ | ( | ) | $ | $ | ( | ) | |||||||||||
Asset-backed securities | ( | ) | ( | ) | |||||||||||||||||||
Corporate debt securities | ( | ) | ( | ) | ( | ) | |||||||||||||||||
$ | $ | ( | ) | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||||||
Investments in Unconsolidated Affiliates
The Company has a number of equity investments in privately and publicly held companies. Investments in these unconsolidated affiliates are recorded in " Long-term Investments" on the consolidated balance sheets, and are as follows:
December 31, | |||||||
2019 | 2018 | ||||||
(in millions) | |||||||
Equity method investments | |||||||
Cost | $ | $ | |||||
Equity in losses | ( | ) | ( | ) | |||
Carrying value of equity method investments | |||||||
Equity securities | |||||||
Carrying value of non-marketable equity securities | |||||||
Total investments in unconsolidated affiliates | $ | $ | |||||
Non-marketable equity securities consist of investments in privately held companies without readily determinable fair values, and are reported at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or similar investment of the same issuer. The Company recorded an upward adjustment of $0.3 million during 2019 based on observable price changes. As of December 31, 2019, the Company had recorded accumulated upward adjustments of $2.0 million based on observable price changes, and accumulated downward adjustments of $1.9 million due to impairment and observable price changes.
During 2019, 2018, and 2017, the gross realized gains or losses from sales of available-for-sale investments were not material.
8. ACQUISITIONS
CAS Medical Systems, Inc.
On February 11, 2019, the Company entered into an agreement and plan of merger to acquire all the outstanding shares of CAS Medical Systems, Inc. ("CASMED") for an aggregate cash purchase price of $2.45 per share of common stock, or an equity value of approximately $100 million. The transaction closed on April 18, 2019, and the cash purchase price was $100.8 million. Acquisition-related costs of $2.0 million were recorded in “Selling, General, and Administrative Expenses” during the year ended December 31, 2019.
62
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. ACQUISITIONS (Continued)
CASMED is a medical technology company dedicated to noninvasive monitoring of tissue oxygenation in the brain. The Company plans to integrate the acquired technology platform into its hemodynamic monitoring platform. The acquisition was accounted for as a business combination. Tangible and intangible assets acquired were recorded based on their estimated fair values at the acquisition date. The excess of the purchase price over the fair value of net assets acquired was recorded to goodwill. The following table summarizes the fair values of the assets acquired and liabilities assumed (in millions):
Current assets | $ | ||||
Property and equipment, net | |||||
Goodwill | |||||
Developed technology | |||||
Customer relationships | |||||
Deferred tax assets | |||||
Liabilities assumed | ( | ) | |||
Total purchase price | |||||
Less: cash acquired | ( | ) | |||
Total purchase price, net of cash acquired | $ | ||||
Goodwill includes expected synergies and other benefits the Company believes will result from the acquisition. Goodwill was assigned to the Company’s United States segment and is not deductible for tax purposes. Developed technology assets are being amortized over a weighted-average useful life of 14 years. Customer relationships assets are being amortized over a weighted-average useful life of 10 years.
The results of operations for CASMED have been included in the accompanying consolidated financial statements from the date of acquisition. Pro forma results have not been presented as the results of CASMED are not material in relation to the consolidated financial statements of Edwards Lifesciences.
Harpoon Medical, Inc.
On December 1, 2017, the Company acquired all the outstanding shares of Harpoon Medical, Inc. for an aggregate cash purchase price of $119.5 million, which includes $16.0 million paid previously for a cost method investment and an exclusive option to acquire Harpoon Medical, Inc., and is net of $8.0 million received from the sale of the Company's previous ownership interest. The Company remeasured its previously held ownership in Harpoon Medical, Inc., which had a carrying value at the date of acquisition of $1.5 million and represented approximately 6 % of the fully-diluted outstanding shares of Harpoon Medical, Inc., and recognized a gain of $6.5 million in "Special (Gains) Charges, net." In addition, the Company agreed to pay up to an additional $150.0 million in pre-specified milestone-driven payments over the next 10 years. The Company recognized in "Contingent Consideration Liabilities" a $59.7 million liability for the estimated fair value of the contingent milestone payments. The fair value of the contingent milestone payments are remeasured each quarter, with changes in the fair value recognized within operating expenses on the consolidated statements of operations. For further information on the fair value of the contingent milestone payments, see Note 11.
In connection with the acquisition, the Company placed $10.0 million of the purchase price into escrow to satisfy any claims for indemnification made in accordance with the merger agreement. Funds remaining 12 months after the acquisition date were disbursed to Harpoon Medical, Inc.'s former shareholders. Acquisition-related costs of $0.4 million were recorded in “Selling, General, and Administrative Expenses” during the year ended December 31, 2017.
63
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. ACQUISITIONS (Continued)
Harpoon Medical, Inc. is a medical technology company pioneering beating-heart repair for degenerative mitral regurgitation. The Company plans to add this technology to its portfolio of mitral and tricuspid repair products. The acquisition was accounted for as a business combination. Tangible and intangible assets acquired were recorded based on their estimated fair values at the acquisition date. The excess of the purchase price over the fair value of net assets acquired was recorded to goodwill. The following table summarizes the fair values of the assets acquired and liabilities assumed (in millions):
Current assets | $ | ||||
Property and equipment, net | |||||
Goodwill | |||||
In-process research and development assets | |||||
Other assets | |||||
Current liabilities assumed | ( | ) | |||
Deferred income taxes | ( | ) | |||
Total purchase price | |||||
Less: cash acquired | ( | ) | |||
Total purchase price, net of cash acquired | $ | ||||
Goodwill includes expected synergies and other benefits the Company believes will result from the acquisition. Goodwill was assigned to the Company’s United States segment and is not deductible for tax purposes. In-process research and development has been capitalized at fair value as an intangible asset with an indefinite life and will be assessed for impairment in subsequent periods. The fair value of the in-process research and development assets was determined using the income approach. This approach determines fair value based on cash flow projections which are discounted to present value using a risk-adjusted rate of return. The discount rates used to determine the fair value of the in-process research and development assets ranged from 18.0 % to 19.0 %. Completion of successful design developments, bench testing, pre-clinical studies and human clinical studies are required prior to selling any product. The risks and uncertainties associated with completing development within a reasonable period of time include those related to the design, development, and manufacturability of the product, the success of pre-clinical and clinical studies, and the timing of regulatory approvals. The valuation assumed $41.4 million of additional research and development expenditures would be incurred prior to the date of product introduction. In the valuation, net cash inflows were modeled to commence in Europe in 2018, and in the United States and Japan in 2022. The Company does not currently anticipate significant changes to forecasted research and development expenditures, and net cash inflows are now expected to commence in Europe in 2020. Upon completion of development, the underlying in-process research and development asset will be amortized over its estimated useful life.
The results of operations for Harpoon Medical, Inc. have been included in the accompanying consolidated financial statements from the date of acquisition. Pro forma results have not been presented as the results of Harpoon Medical, Inc. are not material in relation to the consolidated financial statements of Edwards Lifesciences.
Valtech Cardio Ltd.
On November 26, 2016, the Company entered into an agreement and plan of merger to acquire Valtech Cardio Ltd. ("Valtech") for approximately $340.0 million, subject to certain adjustments, with the potential for up to an additional $350.0 million in pre-specified milestone-driven payments over the next 10 years. The transaction closed on January 23, 2017, and the consideration paid included the issuance of approximately 2.8 million shares of the Company's common stock (fair value of $266.5 million) and cash of $86.2 million. The Company recognized in "Contingent Consideration Liabilities" a $162.9 million liability for the estimated fair value of the contingent milestone payments. For further information on the fair value of the contingent milestone payments, see Note 11.
64
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. ACQUISITIONS (Continued)
Prior to the close of the transaction, Valtech spun off its early-stage transseptal mitral valve replacement technology program. Concurrent with the closing, the Company entered into an agreement for an exclusive option to acquire that program and its associated intellectual property for approximately $200.0 million, subject to certain adjustments, plus an additional $50.0 million if a certain European regulatory approval is obtained within 10 years of the acquisition closing date. The option expired in January 2020.
In-process research and development assets acquired as part of this transaction were capitalized at fair value, which was determined using the income approach. This approach determines fair value based on cash flow projections which are discounted to present value using a risk-adjusted rate of return. The valuation assumed $87.3 million of additional research and development expenditures would be incurred prior to the date of product introduction and that net cash inflows would commence in 2019. In December 2018, the Company recorded a $116.2 million impairment charge related to Valtech's intangible assets, and in December 2019, the Company recorded a $40.6 million impairment charge to write off the remaining in-process research and development assets. For further information, see Note 4.
CardiAQ Valve Technologies, Inc.
On July 3, 2015, the Company entered into an agreement and plan of merger to acquire CardiAQ Valve Technologies, Inc. ("CardiAQ") for an aggregate cash purchase price of $350.0 million, subject to certain adjustments. The transaction closed on August 26, 2015, and the cash purchase price after the adjustments was $348.0 million. In addition, the Company agreed to pay an additional $50.0 million if a certain European regulatory approval is obtained within 48 months of the acquisition closing date. The Company recognized in "Contingent Consideration Liabilities" a $30.3 million liability for the estimated fair value of this contingent milestone payment. The Company does not expect this milestone to be achieved and reversed the liability in 2018. For further information on the fair value of the contingent milestone payment, see Note 11.
9. GOODWILL AND OTHER INTANGIBLE ASSETS
Goodwill and in-process research and development assets resulting from purchase business combinations are not subject to amortization. Other acquired intangible assets with finite lives are amortized over their expected useful lives on a straight-line basis, or if reliably determinable, based on the pattern in which the economic benefit of the asset is expected to be used. The Company expenses costs incurred to renew or extend the term of acquired intangible assets.
In April 2019, the Company acquired CASMED. This transaction resulted in an increase to goodwill of $64.4 million and developed technology of $35.9 million. For further information, see Note 8.
65
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. GOODWILL AND OTHER INTANGIBLE ASSETS (Continued)
The changes in the carrying amount of goodwill, by segment, during the years ended December 31, 2019 and 2018 were as follows:
United States | Europe | Rest of World | Total | ||||||||||||
(in millions) | |||||||||||||||
Goodwill at December 31, 2017 | $ | $ | $ | $ | |||||||||||
Currency translation adjustment | ( | ) | ( | ) | ( | ) | |||||||||
Goodwill at December 31, 2018 | |||||||||||||||
Goodwill acquired during the year | |||||||||||||||
Currency translation adjustment | ( | ) | ( | ) | ( | ) | |||||||||
Goodwill at December 31, 2019 | $ | $ | $ | $ | |||||||||||
Other intangible assets consist of the following (in millions):
December 31, | |||||||||||||||||||||||||
Weighted-Average Useful Life (in years) | 2019 | 2018 | |||||||||||||||||||||||
Cost | Accumulated Amortization | Net Carrying Value | Cost | Accumulated Amortization | Net Carrying Value | ||||||||||||||||||||
Finite-lived intangible assets | |||||||||||||||||||||||||
Patents | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | |||||||||||||||
Developed technology (Note 4) | ( | ) | ( | ) | |||||||||||||||||||||
Other | ( | ) | |||||||||||||||||||||||
( | ) | ( | ) | ||||||||||||||||||||||
Indefinite-lived intangible assets | |||||||||||||||||||||||||
In-process research and development (Note 4) | — | — | |||||||||||||||||||||||
$ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||||||||
Amortization expense related to other intangible assets for the years ended December 31, 2019, 2018, and 2017 was $4.6 million, $2.5 million, and $7.8 million, respectively. Estimated amortization expense for each of the years ending December 31 is as follows (in millions):
2020 | $ | ||
2021 | |||
2022 | |||
2023 | |||
2024 | |||
66
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. DEBT AND CREDIT FACILITIES
In June 2018, the Company issued $600.0 million of fixed-rate unsecured senior notes (the "Notes") due June 15, 2028. Interest is payable semi-annually in arrears, with payments due in June and December of each year. The Company may redeem the Notes, in whole or in part, at any time and from time to time at specified redemption prices. In addition, upon the occurrence of certain change of control triggering events, the Company may be required to repurchase all or a portion of the Notes at a price equal to 101 % of their principal amount, plus accrued and unpaid interest. The Notes also include covenants that limit the Company's ability to incur secured indebtedness, enter into sale and leaseback transactions, and consolidate, merge, or transfer all or substantially all of its assets.
The following is a summary of the Notes as of December 31, 2019 and 2018:
December 31, | |||||||||||||
2019 | 2018 | ||||||||||||
Amount | Effective Interest Rate | Amount | Effective Interest Rate | ||||||||||
(in millions) | (in millions) | ||||||||||||
Fixed-rate 4.300% 2018 Notes | $ | % | $ | % | |||||||||
Unamortized discount | ( | ) | ( | ) | |||||||||
Unamortized debt issuance costs | ( | ) | ( | ) | |||||||||
Total carrying amount | $ | $ | |||||||||||
As of December 31, 2019 and 2018, the fair value of the Notes was $667.6 million and $607.0 million, respectively, based on observable market prices in less active markets and categorized as Level 2 (Note 11). The debt issuance costs, as well as the discount, are being amortized to interest expense over the term of the notes.
The Company has a Five-Year Credit Agreement ("the Credit Agreement") which matures on April 28, 2023. The Credit Agreement provides up to an aggregate of $750.0 million in borrowings in multiple currencies. The Company may increase the amount available under the Credit Agreement, subject to agreement of the lenders, by up to an additional $250.0 million in the aggregate. Borrowings generally bear interest at the London interbank offered rate ("LIBOR") plus a spread ranging from 0.9 % to 1.3 %, depending on the leverage ratio, as defined in the Credit Agreement. The Company also pays a facility fee ranging from 0.1 % to 0.2 %, depending on the leverage ratio, on the entire credit commitment available, whether drawn or not. The facility fee is expensed as incurred. During 2019, the spread over LIBOR was 0.9 % and the facility fee was 0.1 %. Issuance costs of $2.4 million are being amortized to interest expense over the term of the Credit Agreement. As of December 31, 2019 and 2018, there were no borrowings outstanding under the Credit Agreement. All amounts outstanding under the Credit Agreement have been classified as long-term obligations in accordance with the terms of the Credit Agreement. The Credit Agreement is unsecured and contains various financial and other covenants, including a maximum leverage ratio, as defined in the Credit Agreement. The Company was in compliance with all covenants at December 31, 2019.
The weighted-average interest rate under all debt obligations was 3.4 % and 3.4 % at December 31, 2019 and 2018, respectively.
67
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. FAIR VALUE MEASUREMENTS
The consolidated financial statements include financial instruments for which the fair market value of such instruments may differ from amounts reflected on a historical cost basis. Financial instruments of the Company consist of cash deposits, accounts and other receivables, investments, accounts payable, certain accrued liabilities, and borrowings under a revolving credit agreement. The carrying value of these financial instruments generally approximates fair value due to their short-term nature. Financial instruments also include notes payable. See Note 10 for further information on the fair value of the notes payable.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. The Company prioritizes the inputs used to determine fair values in one of the following three categories:
Level 1—Quoted market prices in active markets for identical assets or liabilities.
Level 2—Inputs, other than quoted prices in active markets, that are observable, either directly or indirectly.
Level 3—Unobservable inputs that are not corroborated by market data.
In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, the level in the fair value hierarchy within which the fair value measurement in its entirety falls has been determined based on the lowest level input that is significant to the fair value measurement in its entirety.
68
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. FAIR VALUE MEASUREMENTS (Continued)
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following table summarizes the Company's financial instruments which are measured at fair value on a recurring basis as of December 31, 2019 and 2018 (in millions):
December 31, 2019 | Level 1 | Level 2 | Level 3 | Total | |||||||||||
Assets | |||||||||||||||
Cash equivalents | $ | $ | $ | $ | |||||||||||
Available-for-sale investments: | |||||||||||||||
Bank time deposits | |||||||||||||||
Corporate debt securities | |||||||||||||||
Asset-backed securities | |||||||||||||||
U.S. government and agency securities | |||||||||||||||
Foreign government bonds | |||||||||||||||
Commercial paper | |||||||||||||||
Investments held for deferred compensation plans | |||||||||||||||
Derivatives | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
Liabilities | |||||||||||||||
Derivatives | $ | $ | $ | $ | |||||||||||
Deferred compensation plans | |||||||||||||||
Contingent consideration liabilities | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
December 31, 2018 | |||||||||||||||
Assets | |||||||||||||||
Cash equivalents | $ | $ | $ | $ | |||||||||||
Available-for-sale investments: | |||||||||||||||
Corporate debt securities | |||||||||||||||
Asset-backed securities | |||||||||||||||
U.S. government and agency securities | |||||||||||||||
Foreign government bonds | |||||||||||||||
Commercial paper | |||||||||||||||
Municipal securities | |||||||||||||||
Investments held for deferred compensation plans | |||||||||||||||
Derivatives | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
Liabilities | |||||||||||||||
Derivatives | $ | $ | $ | $ | |||||||||||
Deferred compensation plans | |||||||||||||||
Contingent consideration liabilities | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
69
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. FAIR VALUE MEASUREMENTS (Continued)
The following table summarizes the changes in fair value of the contingent consideration obligation for the year ended December 31, 2019 (in millions):
December 31, | |||||||
2019 | 2018 | ||||||
Fair value, beginning of year | $ | $ | |||||
Payments (cash and issued shares) | ( | ) | |||||
Changes in fair value | ( | ) | ( | ) | |||
Fair value, end of year | $ | $ | |||||
During 2019 and 2018, the contingent consideration liability related to certain of the Company's previous business acquisitions was reduced by $24.1 million and $26.8 million, respectively, due to delays in product development, which reduced the probability of milestone achievement. These reductions were partially offset by changes in the fair value of the liabilities associated primarily with adjustments to discount rates and accretion of interest due to the passage of time.
Cash Equivalents and Available-for-sale Investments
The Company estimates the fair values of its money market funds based on quoted prices in active markets for identical assets. The Company estimates the fair values of its time deposits, commercial paper, U.S. and foreign government and agency securities, municipal securities, asset-backed securities, and corporate debt securities by taking into consideration valuations obtained from third-party pricing services. The pricing services use industry standard valuation models, including both income and market-based approaches, for which all significant inputs are observable, either directly or indirectly, to estimate fair value. These inputs include reported trades and broker-dealer quotes on the same or similar securities, benchmark yields, credit spreads, prepayment and default projections based on historical data, and other observable inputs. The Company independently reviews and validates the pricing received from the third-party pricing service by comparing the prices to prices reported by a secondary pricing source. The Company’s validation procedures have not resulted in an adjustment to the pricing received from the pricing service.
Deferred Compensation Plans
The Company holds investments in trading securities related to its deferred compensation plans. The investments are in a variety of stock, bond, and money market mutual funds. The fair values of these investments and the corresponding liabilities are based on quoted market prices.
Derivative Instruments
The Company uses derivative financial instruments in the form of foreign currency forward exchange contracts and cross currency swap contracts to manage foreign currency exposures. All derivatives contracts are recognized on the balance sheet at their fair value. The fair value of foreign currency derivative financial instruments and the cross currency swap contracts was estimated based on quoted market foreign exchange rates, cross currency swap basis rates, and market discount rates. Judgment was employed in interpreting market data to develop estimates of fair value; accordingly, the estimates presented herein are not necessarily indicative of the amounts that the Company could realize in a current market exchange. The use of different market assumptions or valuation methodologies could have a material effect on the estimated fair value amounts.
Contingent Consideration Liabilities
Certain of the Company's acquisitions involve contingent consideration arrangements. Payment of additional consideration is contingent upon the acquired company reaching certain performance milestones, such as attaining specified revenue levels or obtaining regulatory approvals. These contingent consideration liabilities are measured at estimated fair value using either a probability weighted discounted cash flow analysis or a Monte Carlo simulation model, both of which consider significant unobservable inputs. These inputs include (1) the discount rate used to present value the projected cash flows (ranging from 1.5 % to 10.6 %), (2) the probability of milestone achievement (ranging from 2.0 % to 98.5 %), (3) the projected
70
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. FAIR VALUE MEASUREMENTS (Continued)
payment dates (ranging from 2022 to 2026), and (4) the volatility of future revenue (45.0 %). The use of different assumptions could have a material effect on the estimated fair value amounts.
12. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
The Company uses derivative financial instruments to manage its currency exchange rate risk and its interest rate risk as summarized below. Notional amounts are stated in United States dollar equivalents at spot exchange rates at the respective dates. The Company does not enter into these arrangements for trading or speculation purposes.
Notional Amount | |||||||
December 31, 2019 | December 31, 2018 | ||||||
(in millions) | |||||||
Foreign currency forward exchange contracts | $ | $ | |||||
Cross currency swap contracts | |||||||
The following table presents the location and fair value amounts of derivative instruments reported in the consolidated balance sheets (in millions):
Fair Value | |||||||||
Balance Sheet Location | December 31, 2019 | December 31, 2018 | |||||||
Derivatives designated as hedging instruments | |||||||||
Assets | |||||||||
Foreign currency contracts | Other current assets | $ | $ | ||||||
Foreign currency contracts | Other assets | $ | |||||||
Cross currency swap contracts | Other assets | $ | $ | ||||||
Liabilities | |||||||||
Foreign currency contracts | Accrued and other liabilities | $ | $ | ||||||
Foreign currency contracts | Other long-term liabilities | $ | $ | ||||||
71
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES (Continued)
The following table presents the effect of master-netting agreements and rights of offset on the consolidated balance sheets (in millions):
Gross Amounts Not Offset in the Consolidated Balance Sheet | |||||||||||||||||||||||
Gross Amounts Offset in the Consolidated Balance Sheet | Net Amounts Presented in the Consolidated Balance Sheet | ||||||||||||||||||||||
December 31, 2019 | Gross Amounts | Financial Instruments | Cash Collateral Received | Net Amount | |||||||||||||||||||
Derivative Assets | |||||||||||||||||||||||
Foreign currency contracts | $ | $ | $ | $ | ( | ) | $ | $ | |||||||||||||||
Cross currency swap contracts | $ | $ | $ | $ | $ | $ | |||||||||||||||||
Derivative Liabilities | |||||||||||||||||||||||
Foreign currency contracts | $ | $ | $ | $ | ( | ) | $ | $ | |||||||||||||||
December 31, 2018 | |||||||||||||||||||||||
Derivative Assets | |||||||||||||||||||||||
Foreign currency contracts | $ | $ | $ | $ | ( | ) | $ | $ | |||||||||||||||
Cross currency swap contracts | $ | $ | $ | $ | $ | $ | |||||||||||||||||
Derivative Liabilities | |||||||||||||||||||||||
Foreign currency contracts | $ | $ | $ | $ | ( | ) | $ | $ | |||||||||||||||
The following tables present the effect of derivative and non-derivative hedging instruments on the consolidated statements of operations and consolidated statements of comprehensive income:
Amount of Gain or (Loss) Recognized in OCI on Derivative (Effective Portion) | Location of Gain or (Loss) Reclassified from Accumulated OCI into Income | Amount of Gain or (Loss) Reclassified from Accumulated OCI into Income | ||||||||||||||||
2019 | 2018 | 2019 | 2018 | |||||||||||||||
(in millions) | (in millions) | |||||||||||||||||
Cash flow hedges | ||||||||||||||||||
Foreign currency contracts | $ | $ | Cost of sales | $ | $ | ( | ) | |||||||||||
Selling, general, and administrative expenses | $ | $ | ( | ) | ||||||||||||||
Amount of Gain or (Loss) Recognized in OCI on Derivative (Effective Portion) | Location of Gain or (Loss) Reclassified from Accumulated OCI into Income | Amount of Gain or (Loss) Recognized in Income on Derivative (Amount Excluded from Effectiveness Testing) | ||||||||||||||||
2019 | 2018 | 2019 | 2018 | |||||||||||||||
(in millions) | (in millions) | |||||||||||||||||
Net investment hedges | ||||||||||||||||||
Cross currency swap contracts | $ | $ | Interest expense | $ | $ | |||||||||||||
Foreign currency denominated debt | $ | $ | ||||||||||||||||
In June 2018, the Company repaid and dedesignated its €370.0 million of outstanding long-term debt which had been previously designated as a net investment hedge, and concurrently entered into cross currency swap contracts, which were designated as a net investment hedge. The cross currency swaps have an expiration date of June 15, 2028. At maturity of the cross currency swap contracts, the Company will deliver the notional amount of €257.2 million and will receive $300.0 million from the counterparties. The Company will receive semi-annual interest payments from the counterparties based on a fixed interest rate until maturity of the agreements.
72
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES (Continued)
Amount of Gain or (Loss) Recognized in Income on Derivative (a) | |||||||||||||
Location of Gain or (Loss) Recognized in Income on Derivative | |||||||||||||
2019 | 2018 | 2017 | |||||||||||
(in millions) | |||||||||||||
Fair value hedges | |||||||||||||
Foreign currency contracts | Other (income) expense, net | $ | $ | $ | |||||||||
Interest rate swap agreements | Interest expense | $ | $ | $ | ( | ) | |||||||
___________________________________________________________
(a) | The gains and losses on the interest rate swap agreements were fully offset by the changes in the fair value of the fixed-rate debt being hedged. In December 2017, the interest rate swap was settled at a loss of $ |
Amount of Gain or (Loss) Recognized in Income on Derivative | |||||||||||||
Location of Gain or (Loss) Recognized in Income on Derivative | |||||||||||||
2019 | 2018 | 2017 | |||||||||||
(in millions) | |||||||||||||
Derivatives not designated as hedging instruments | |||||||||||||
Foreign currency contracts | Other (income) expense, net | $ | $ | $ | ( | ) | |||||||
The following table presents the effect of cash flow hedge accounting on the consolidated statements of operations:
Location and Amount of Gain or (Loss) Recognized in Income on Fair Value and Cash Flow Hedging Relationships | |||||||||||
Twelve Months Ended December 31, 2019 | |||||||||||
Cost of sales | Selling, general, and administrative expenses | Other (Income) Expense, net | |||||||||
Total amounts of income and expense line items shown in the consolidated statements of operations in which the effects of fair value or cash flow hedges are recorded | $ | ( | ) | $ | ( | ) | $ | ||||
The effects of fair value and cash flow hedging: | |||||||||||
Gain (loss) on fair value hedging relationships: | |||||||||||
Foreign currency contracts: | |||||||||||
Hedged items | |||||||||||
Derivatives designated as hedging instruments | ( | ) | |||||||||
Amount excluded from effectiveness testing recognized in earnings based on an amortization approach | |||||||||||
Gain (loss) on cash flow hedging relationships: | |||||||||||
Foreign currency contracts: | |||||||||||
Amount of gain (loss) reclassified from accumulated OCI into income | $ | $ | $ | ||||||||
73
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES (Continued)
Location and Amount of Gain or (Loss) Recognized in Income on Fair Value and Cash Flow Hedging Relationships | |||||||||||
Twelve Months Ended December 31, 2018 | |||||||||||
Cost of sales | Selling, general, and administrative expenses | Other (Income) Expense, net | |||||||||
Total amounts of income and expense line items shown in the consolidated statements of operations in which the effects of fair value or cash flow hedges are recorded | $ | ( | ) | $ | ( | ) | $ | ||||
The effects of fair value and cash flow hedging: | |||||||||||
Gain (loss) on fair value hedging relationships: | |||||||||||
Foreign currency contracts: | |||||||||||
Hedged items | |||||||||||
Derivatives designated as hedging instruments | |||||||||||
Amount excluded from effectiveness testing recognized in earnings based on an amortization approach | |||||||||||
Gain (loss) on cash flow hedging relationships: | |||||||||||
Foreign currency contracts: | |||||||||||
Amount of gain (loss) reclassified from accumulated OCI into income | $ | ( | ) | $ | ( | ) | $ | ||||
The Company expects that during 2020 it will reclassify to earnings a $6.5 million gain currently recorded in "Accumulated Other Comprehensive Loss." For the years ended December 31, 2019, 2018, and 2017, the Company did not record any gains or losses due to hedge ineffectiveness.
74
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. EMPLOYEE BENEFIT PLANS
Defined Benefit Plans
Edwards Lifesciences maintains defined benefit pension plans in Japan and certain European countries. In 2018, the Company curtailed its defined benefit plan in Horw, Switzerland (see Note 4) and at the end of 2017, redesigned one of its defined benefit plans in Nyon, Switzerland into a defined contribution plan upon changes in local legislation.
Years Ended December 31, | |||||||
2019 | 2018 | ||||||
(in millions) | |||||||
Change in projected benefit obligation: | |||||||
Beginning of year | $ | $ | |||||
Service cost | |||||||
Interest cost | |||||||
Participant contributions | |||||||
Actuarial loss | |||||||
Benefits paid | ( | ) | ( | ) | |||
Plan amendment | ( | ) | ( | ) | |||
Settlements and curtailment gain | ( | ) | |||||
Currency exchange rate changes and other | ( | ) | |||||
End of year | $ | $ | |||||
Change in fair value of plan assets: | |||||||
Beginning of year | $ | $ | |||||
Actual return on plan assets | ( | ) | |||||
Employer contributions | |||||||
Participant contributions | |||||||
Settlements | ( | ) | |||||
Benefits paid | ( | ) | ( | ) | |||
Currency exchange rate changes and other | ( | ) | |||||
End of year | $ | $ | |||||
Funded Status | |||||||
Projected benefit obligation | $ | ( | ) | $ | ( | ) | |
Plan assets at fair value | |||||||
Underfunded status | $ | ( | ) | $ | ( | ) | |
Net amounts recognized on the consolidated balance sheet: | |||||||
Other long-term liabilities | $ | $ | |||||
Accumulated other comprehensive loss, net of tax: | |||||||
Net actuarial loss | $ | ( | ) | $ | ( | ) | |
Net prior service cost | |||||||
Deferred income tax benefit | |||||||
Total | $ | ( | ) | $ | ( | ) | |
75
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. EMPLOYEE BENEFIT PLANS (Continued)
The accumulated benefit obligation ("ABO") for all defined benefit pension plans was $101.1 million and $93.5 million as of December 31, 2019 and 2018, respectively. The projected benefit obligation and ABO were in excess of plan assets for all pension plans as of December 31, 2019 and 2018.
The components of net periodic pension benefit cost (credit) are as follows (in millions):
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Service cost, net | $ | $ | $ | ||||||||
Interest cost | |||||||||||
Expected return on plan assets | ( | ) | ( | ) | ( | ) | |||||
Settlements and curtailment gain | ( | ) | ( | ) | |||||||
Special termination benefits | |||||||||||
Amortization of actuarial loss | |||||||||||
Amortization of prior service (credit) cost | ( | ) | ( | ) | |||||||
Net periodic pension benefit cost (credit) | $ | $ | ( | ) | $ | ||||||
The net actuarial loss and prior service credit that will be amortized from "Accumulated Other Comprehensive Loss" into net periodic pension benefit cost in 2020 are expected to be $1.5 million and $(0.7 ) million, respectively.
Expected long-term returns for each of the plans' strategic asset classes were developed through consultation with investment advisors. Several factors were considered, including survey of investment managers' expectations, current market data, minimum guaranteed returns in certain insurance contracts, and historical market returns over long periods. Using policy target allocation percentages and the asset class expected returns, a weighted-average expected return was calculated.
To select the discount rates for the defined benefit pension plans, the Company uses a modeling process that involves matching the expected duration of its benefit plans to a yield curve constructed from a portfolio of AA-rated fixed-income debt instruments, or their equivalent. For each country, the Company uses the implied yield of this hypothetical portfolio at the appropriate duration as a discount rate benchmark.
The weighted-average assumptions used to determine the benefit obligations are as follows:
December 31, | |||||
2019 | 2018 | ||||
Discount rate | % | % | |||
Rate of compensation increase | % | % | |||
Social securities increase | % | % | |||
Pension increase | % | % | |||
76
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. EMPLOYEE BENEFIT PLANS (Continued)
The weighted-average assumptions used to determine the net periodic pension benefit cost are as follows:
Years ended December 31, | ||||||||
2019 | 2018 | 2017 | ||||||
Discount rate | % | % | % | |||||
Expected return on plan assets | % | % | % | |||||
Rate of compensation increase | % | % | % | |||||
Social securities increase | % | % | % | |||||
Pension increase | % | % | % | |||||
Plan Assets
The Company's investment strategy for plan assets is to seek a competitive rate of return relative to an appropriate level of risk and to earn performance rates of return in accordance with the benchmarks adopted for each asset class. Risk management practices include diversification across asset classes and investment styles, and periodic rebalancing toward asset allocation targets.
The Administrative and Investment Committee decides on the defined benefit plan provider in each location and that provider decides the target allocation for the Company's defined benefit plan at that location. The target asset allocation selected reflects a risk/return profile the Company feels is appropriate relative to the plans' liability structure and return goals. In certain plans, asset allocations may be governed by local requirements. Target weighted-average asset allocations at December 31, 2019, by asset category, are as follows:
Equity securities | % | |
Debt securities | % | |
Real estate | % | |
Other | % | |
Total | % | |
The fair values of the Company's defined benefit plan assets at December 31, 2019 and 2018, by asset category, are as follows (in millions):
December 31, 2019 | Level 1 | Level 2 | Level 3 | Total | |||||||||||
Asset Category | |||||||||||||||
Cash | $ | $ | $ | $ | |||||||||||
Equity securities: | |||||||||||||||
United States equities | |||||||||||||||
International equities | |||||||||||||||
Debt securities: | |||||||||||||||
United States government bonds | |||||||||||||||
International government bonds | |||||||||||||||
Real estate | |||||||||||||||
Mortgages | |||||||||||||||
Insurance contracts | |||||||||||||||
Total plan assets measured at fair value | $ | $ | $ | ||||||||||||
Alternative investments measured at net asset value (a) | |||||||||||||||
Total plan assets | $ | ||||||||||||||
77
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. EMPLOYEE BENEFIT PLANS (Continued)
December 31, 2018 | Level 1 | Level 2 | Level 3 | Total | |||||||||||
Asset Category | |||||||||||||||
Cash | $ | $ | $ | $ | |||||||||||
Equity securities: | |||||||||||||||
United States equities | |||||||||||||||
International equities | |||||||||||||||
Debt securities: | |||||||||||||||
United States government bonds | |||||||||||||||
International government bonds | |||||||||||||||
Real estate | |||||||||||||||
Mortgages | |||||||||||||||
Insurance contracts | |||||||||||||||
Total plan assets | $ | $ | $ | $ | |||||||||||
Alternative investments measured at net asset value (a) | |||||||||||||||
Total plan assets | $ | ||||||||||||||
_______________________________________
(a) | Certain investments that were measured at net asset value per share have not been classified in the fair value hierarchy. The fair value amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the total plan assets. |
The following table summarizes the changes in fair value of the Company's defined benefit plan assets that have been classified as Level 3 for the years ended December 31, 2019 and 2018 (in millions):
Insurance Contracts | |||
Balance at December 31, 2017 | $ | ||
Actual return on plan assets: | |||
Relating to assets still held at December 31, 2018 | ( | ) | |
Currency exchange rate impact | ( | ) | |
Balance at December 31, 2018 | |||
Purchases, sales and settlements | ( | ) | |
Balance at December 31, 2019 | $ | ||
Equity and debt securities are valued at fair value based on quoted market prices reported on the active markets on which the individual securities are traded. Real estate investments are valued by discounting to present value the cash flows expected to be generated by the specific properties. Investments in mortgages are valued at cost, which is deemed to approximate its fair value. The insurance contracts are valued at the cash surrender value of the contracts, which is deemed to approximate its fair value. Alternative investments include hedge funds, private equity funds and other miscellaneous investments, and are valued using the net asset value provided by the fund administrator as a practical expedient. The net asset value is based on the fair value of the underlying assets owned by the fund divided by the number of shares outstanding.
78
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. EMPLOYEE BENEFIT PLANS (Continued)
The following benefit payments, which reflect expected future service, as appropriate, at December 31, 2019, are expected to be paid (in millions):
2020 | $ | ||
2021 | |||
2022 | |||
2023 | |||
2024 | |||
2024-2026 | |||
As of December 31, 2019, expected employer contributions for 2020 are $1.9 million.
Defined Contribution Plans
The Company's employees in the United States and Puerto Rico are eligible to participate in a qualified defined contribution plan. In the United States, participants may contribute up to 25 % of their eligible compensation (subject to tax code limitation) to the plan. Edwards Lifesciences matches the first 4 % of the participant's annual eligible compensation contributed to the plan on a dollar-for-dollar basis. Edwards Lifesciences matches the next 2 % of the participant's annual eligible compensation to the plan on a 50 % basis. In Puerto Rico, participants may contribute up to 25 % of their annual compensation (subject to tax code limitation) to the plan. Edwards Lifesciences matches the first 4 % of participant's annual eligible compensation contributed to the plan on a 50 % basis. The Company also provides a 2 % profit sharing contribution calculated on eligible earnings for each employee. Matching contributions relating to Edwards Lifesciences employees were $31.4 million, $26.6 million, and $19.9 million in 2019, 2018, and 2017, respectively.
The Company also has nonqualified deferred compensation plans for a select group of employees. The plans provide eligible participants the opportunity to defer eligible compensation to future dates specified by the participant with a return based on investment alternatives selected by the participant. The amount accrued under these nonqualified plans was $88.7 million and $68.5 million at December 31, 2019 and 2018, respectively.
14. COMMON STOCK
Treasury Stock
In November 2017, the Board of Directors approved a stock repurchase program authorizing the Company to purchase up to $1.0 billion of the Company's common stock. In May 2019, the Board of Directors approved a new stock repurchase program authorizing the Company to purchase up to an additional $1.0 billion of the Company's common stock. The repurchase programs do not have an expiration date. Stock repurchased under the programs may be used to offset obligations under the Company's employee stock-based benefit programs and stock-based business acquisitions, and will reduce the total shares outstanding.
During 2019, 2018, and 2017, the Company repurchased 1.5 million, 5.5 million, and 7.7 million shares, respectively, at an aggregate cost of $263.3 million, $795.5 million, and $763.3 million, respectively, including shares purchased under the accelerated share repurchase ("ASR") agreements described below and shares acquired to satisfy tax withholding obligations in connection with the vesting of restricted stock units issued to employees. The timing and size of any future stock repurchases are subject to a variety of factors, including expected dilution from stock plans, cash capacity, and the market price of the Company's common stock.
Accelerated Share Repurchase
During 2019, 2018, and 2017, the Company entered into ASR agreements providing for the repurchase of the Company's common stock based on the volume-weighted average price ("VWAP") of the Company's common stock during the term of the
79
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
14. COMMON STOCK (Continued)
agreements, less a discount. The following table summarizes the terms of the ASR agreements (dollars and shares in millions, except per share data):
Initial Delivery | Final Settlement | ||||||||||||||||||||||
Agreement Date | Amount Paid | Shares Received | Price per Share (a) | Value of Shares as % of Contract Value | Settlement Date | Total Shares Received | Average Price per Share (a) | ||||||||||||||||
November 2017 | $ | $ | % | December 2017 | $ | ||||||||||||||||||
April 2018 | $ | $ | % | July 2018 | $ | ||||||||||||||||||
October 2018 | $ | $ | % | November 2018 | $ | ||||||||||||||||||
May 2019 | $ | $ | % | May 2019 | $ | ||||||||||||||||||
May 2019 | $ | $ | % | June 2019 | $ | ||||||||||||||||||
The ASR agreements were accounted for as two separate transactions: (1) the value of the initial delivery of shares was recorded as shares of common stock acquired in a treasury stock transaction on the acquisition date and (2) the remaining amount of the purchase price paid was recorded as a forward contract indexed to the Company's own common stock and was recorded in "Additional Paid-in Capital" on the consolidated balance sheets. The initial delivery of shares resulted in an immediate reduction of the outstanding shares used to calculate the weighted-average common shares outstanding for basic and diluted earnings per share. The Company determined that the forward contract indexed to the Company's common stock met all the applicable criteria for equity classification and, therefore, was not accounted for as a derivative instrument.
Employee and Director Stock Plans
The Edwards Lifesciences Corporation Long-term Stock Incentive Compensation Program (the "Program") provides for the grant of incentive and non-qualified stock options, restricted stock, and restricted stock units for eligible employees and contractors of the Company. Under the Program, these grants are awarded at a price equal to the fair market value at the date of grant based upon the closing price on that date. Options to purchase shares of the Company's common stock granted under the Program generally vest over predetermined periods of between three to four years and expire seven years after the date of grant. Service-based restricted stock units of the Company's common stock granted under the Program generally vest over predetermined periods ranging from three to four years after the date of grant. Market-based restricted stock units of the Company's common stock granted under the Program vest over three years based on a combination of certain service and market conditions. The actual number of shares issued will be determined based on the Company's total stockholder return relative to a selected industry peer group. Performance-based restricted stock units vest based on a combination of certain service conditions and upon achievement of specified milestones. Under the Program, the number of shares of common stock available for issuance under the Program was 109.2 million shares. No more than 11.2 million shares reserved for issuance may be granted in the form of restricted stock or restricted stock units.
The Company also maintains the Nonemployee Directors Stock Incentive Compensation Program (the "Nonemployee Directors Program"). Under the Nonemployee Directors Program, annually each nonemployee director may receive up to 40,000 stock options or 16,000 restricted stock units of the Company's common stock, or a combination thereof, provided that in no event may the total value of the combined annual award exceed $0.2 million. These grants generally vest over one year from the date of grant. Under the Nonemployee Directors Program, an aggregate of 2.8 million shares of the Company's common stock has been authorized for issuance.
The Company has an employee stock purchase plan for United States employees and a plan for international employees (collectively "ESPP"). Under the ESPP, eligible employees may purchase shares of the Company's common stock at 85 % of the lower of the fair market value of Edwards Lifesciences common stock on the effective date of subscription or the date of purchase. Under the ESPP, employees can authorize the Company to withhold up to 12 % of their compensation for common stock purchases, subject to certain limitations. The ESPP is available to all active employees of the Company paid from the United States payroll and to eligible employees of the Company outside the United States, to the extent permitted by local law. The ESPP for United States employees is qualified under Section 423 of the Internal Revenue Code. The number of shares of common stock authorized for issuance under the ESPP was 15.3 million shares.
80
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
14. COMMON STOCK (Continued)
The fair value of each option award and employee stock purchase subscription is estimated on the date of grant using the Black-Scholes option valuation model that uses the assumptions noted in the following tables. The risk-free interest rate is estimated using the U.S. Treasury yield curve and is based on the expected term of the award. Expected volatility is estimated based on a blend of the weighted-average of the historical volatility of Edwards Lifesciences' stock and the implied volatility from traded options on Edwards Lifesciences' stock. The expected term of awards granted is estimated from the vesting period of the award, as well as historical exercise behavior, and represents the period of time that awards granted are expected to be outstanding. The Company uses historical data to estimate forfeitures and has estimated an annual forfeiture rate of 6.4 %.
The Black-Scholes option pricing model was used with the following weighted-average assumptions for options granted during the following periods:
Option Awards
2019 | 2018 | 2017 | |||||||||
Average risk-free interest rate | % | % | % | ||||||||
Expected dividend yield | |||||||||||
Expected volatility | % | % | % | ||||||||
Expected life (years) | |||||||||||
Fair value, per share | $ | $ | $ | ||||||||
The Black-Scholes option pricing model was used with the following weighted-average assumptions for ESPP subscriptions granted during the following periods:
ESPP
2019 | 2018 | 2017 | |||||||||
Average risk-free interest rate | % | % | % | ||||||||
Expected dividend yield | |||||||||||
Expected volatility | % | % | % | ||||||||
Expected life (years) | |||||||||||
Fair value, per share | $ | $ | $ | ||||||||
The fair value of market-based restricted stock units was determined using a Monte Carlo simulation model, which uses multiple input variables to determine the probability of satisfying the market condition requirements. The weighted-average assumptions used to determine the fair value of the market-based restricted stock units during the years ended December 31, 2019, 2018, and 2017 included a risk-free interest rate of 2.2 %, 2.7 %, and 1.7 %, respectively, and an expected volatility rate of 29.4 %, 29.7 %, and 30.2 %, respectively.
81
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
14. COMMON STOCK (Continued)
Stock option activity during the year ended December 31, 2019 under the Program and the Nonemployee Directors Program was as follows (in millions, except years and per-share amounts):
Shares | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||
Outstanding as of December 31, 2018 | $ | |||||||||||
Options granted | ||||||||||||
Options exercised | ( | ) | ||||||||||
Outstanding as of December 31, 2019 | $ | |||||||||||
Exercisable as of December 31, 2019 | ||||||||||||
Vested and expected to vest as of December 31, 2019 | ||||||||||||
The following table summarizes nonvested restricted stock unit activity during the year ended December 31, 2019 under the Program and the Nonemployee Directors Program (in millions, except per-share amounts):
Shares | Weighted- Average Grant-Date Fair Value | |||||
Nonvested as of December 31, 2018 | $ | |||||
Granted (a) | ||||||
Vested | ( | ) | ||||
Forfeited (b) | ( | ) | ||||
Nonvested as of December 31, 2019 | ||||||
_______________________________________________________________________________
(a) | The shares granted includes |
(b) | The shares forfeited includes |
The intrinsic value of stock options exercised and restricted stock units vested during the years ended December 31, 2019, 2018, and 2017 were $382.1 million, $281.1 million, and $205.2 million, respectively. The intrinsic value of stock options is calculated as the amount by which the market price of the Company's common stock exceeds the exercise price of the option. During the years ended December 31, 2019, 2018, and 2017, the Company received cash from exercises of stock options of $110.4 million, $103.7 million, and $77.6 million, respectively, and tax benefits from exercises of stock options and vesting of restricted stock units of $85.1 million, $62.5 million, and $66.9 million, respectively. The total grant-date fair value of stock options vested during the years ended December 31, 2019, 2018, and 2017 were $31.2 million, $29.0 million, and $26.3 million, respectively.
As of December 31, 2019, the total remaining unrecognized compensation expense related to nonvested stock options, restricted stock units, and employee stock purchase subscriptions amounted to $126.3 million, which will be amortized over the weighted-average remaining requisite service period of 30 months.
82
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
15. ACCUMULATED OTHER COMPREHENSIVE LOSS
Presented below is a summary of activity for each component of "Accumulated Other Comprehensive Loss" for the years ended December 31, 2019, 2018, and 2017.
Foreign Currency Translation Adjustments | Unrealized Gain (Loss) on Hedges | Unrealized Gain (Loss) on Available-for-sale Investments | Unrealized Pension Costs (a) | Total Accumulated Other Comprehensive Loss | |||||||||||||||
(in millions) | |||||||||||||||||||
December 31, 2016 | $ | ( | ) | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||
Other comprehensive income (loss) before reclassifications | ( | ) | ( | ) | |||||||||||||||
Amounts reclassified from accumulated other comprehensive loss | ( | ) | ( | ) | ( | ) | |||||||||||||
Deferred income tax benefit (expense) | ( | ) | |||||||||||||||||
December 31, 2017 | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||
Impact from adoption of ASU 2016-16 and ASU 2018-02 | ( | ) | ( | ) | ( | ) | |||||||||||||
January 1, 2018 | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||
Other comprehensive (loss) income before reclassifications | ( | ) | ( | ) | |||||||||||||||
Amounts reclassified from accumulated other comprehensive loss | ( | ) | |||||||||||||||||
Deferred income tax expense | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||
December 31, 2018 | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||
Other comprehensive (loss) income before reclassifications | ( | ) | ( | ) | |||||||||||||||
Amounts reclassified from accumulated other comprehensive loss | ( | ) | ( | ) | ( | ) | |||||||||||||
Deferred income tax (expense) benefit | ( | ) | ( | ) | |||||||||||||||
December 31, 2019 | $ | ( | ) | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||
_______________________________________________________________________________
83
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
15. ACCUMULATED OTHER COMPREHENSIVE LOSS (Continued)
(a) | For the years ended December 31, 2019, 2018, and 2017, the change in unrealized pension costs consisted of the following (in millions): |
Pre-Tax Amount | Tax (Expense) Benefit | Net of Tax Amount | |||||||||
2019 | |||||||||||
Prior service credit arising during period | $ | $ | ( | ) | $ | ||||||
Amortization of prior service credit | ( | ) | ( | ) | |||||||
Net prior service credit arising during period | ( | ) | |||||||||
Net actuarial loss arising during period | ( | ) | ( | ) | |||||||
Unrealized pension costs, net | $ | ( | ) | $ | $ | ( | ) | ||||
2018 | |||||||||||
Prior service credit arising during period | $ | $ | ( | ) | $ | ||||||
Amortization of prior service credit | ( | ) | ( | ) | |||||||
Net prior service credit arising during period | ( | ) | |||||||||
Net actuarial loss arising during period | ( | ) | ( | ) | |||||||
Unrealized pension credits, net | $ | $ | ( | ) | $ | ||||||
2017 | |||||||||||
Prior service credit arising during period | $ | $ | ( | ) | $ | ||||||
Amortization of prior service cost | |||||||||||
Net prior service credit arising during period | ( | ) | |||||||||
Net actuarial gain arising during period | ( | ) | |||||||||
Unrealized pension credits, net | $ | $ | ( | ) | $ | ||||||
84
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
15. ACCUMULATED OTHER COMPREHENSIVE LOSS (Continued)
The following table provides information about amounts reclassified from "Accumulated Other Comprehensive Loss" (in millions):
Years Ended December 31, | |||||||||
Details about Accumulated Other Comprehensive Loss Components | 2019 | 2018 | Affected Line on Consolidated Statements of Operations | ||||||
Foreign currency translation adjustments | $ | $ | Other (income) expense, net | ||||||
( | ) | Provision for income taxes | |||||||
$ | $ | Net of tax | |||||||
Gain (loss) on hedges | $ | $ | ( | ) | Cost of sales | ||||
( | ) | Selling, general, and administrative expenses | |||||||
Other (income) expense, net | |||||||||
( | ) | Total before tax | |||||||
( | ) | Provision for income taxes | |||||||
$ | $ | ( | ) | Net of tax | |||||
Gain (loss) on available-for-sale investments | $ | ( | ) | $ | ( | ) | Other (income) expense, net | ||
( | ) | Provision for income taxes | |||||||
$ | ( | ) | $ | ( | ) | Net of tax | |||
Amortization of pension adjustments | $ | $ | Special (gains) charges, net | ||||||
( | ) | ( | ) | Other (income) expense, net | |||||
( | ) | Total before tax | |||||||
( | ) | Provision for income taxes | |||||||
$ | ( | ) | $ | Net of tax | |||||
16. OTHER (INCOME) EXPENSE, NET
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in millions) | |||||||||||
Foreign exchange (gains) losses, net | $ | ( | ) | $ | ( | ) | $ | ||||
(Gain) loss on investments | ( | ) | |||||||||
Non-service cost components of net periodic pension benefit cost (credit) | ( | ) | ( | ) | |||||||
Other | ( | ) | ( | ) | |||||||
Total other (income) expense, net | $ | ( | ) | $ | ( | ) | $ | ||||
85
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
17. INCOME TAXES
The Company's income before provision for income taxes was generated from United States and international operations as follows (in millions):
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
United States | $ | $ | $ | ||||||||
International, including Puerto Rico | |||||||||||
$ | $ | $ | |||||||||
The provision for income taxes consists of the following (in millions):
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Current | |||||||||||
United States: | |||||||||||
Federal | $ | $ | $ | ||||||||
State and local | |||||||||||
International, including Puerto Rico | |||||||||||
Current income tax expense | $ | $ | $ | ||||||||
Deferred | |||||||||||
United States: | |||||||||||
Federal | $ | $ | ( | ) | $ | ||||||
State and local | ( | ) | ( | ) | ( | ) | |||||
International, including Puerto Rico | ( | ) | |||||||||
Deferred income tax expense (benefit) | ( | ) | |||||||||
Total income tax provision | $ | $ | $ | ||||||||
86
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
17. INCOME TAXES (Continued)
The components of deferred tax assets and liabilities are as follows (in millions):
December 31, | |||||||
2019 | 2018 | ||||||
Deferred tax assets | |||||||
Compensation and benefits | $ | $ | |||||
Benefits from uncertain tax positions | |||||||
Net tax credit carryforwards | |||||||
Net operating loss carryforwards | |||||||
Accrued liabilities | |||||||
Inventories | |||||||
State income taxes | |||||||
Investments | |||||||
Lease liability obligations | — | ||||||
Other | |||||||
Total deferred tax assets | |||||||
Deferred tax liabilities | |||||||
Property, plant, and equipment | ( | ) | ( | ) | |||
Cash flow and net investment hedges | ( | ) | ( | ) | |||
Deferred tax on foreign earnings | ( | ) | ( | ) | |||
Right-of-use assets | ( | ) | — | ||||
Inventories | ( | ) | |||||
Other intangible assets | ( | ) | ( | ) | |||
Other | ( | ) | ( | ) | |||
Total deferred tax liabilities | ( | ) | ( | ) | |||
Valuation allowance | ( | ) | ( | ) | |||
Net deferred tax assets | $ | $ | |||||
During 2019, net deferred tax assets decreased $8.7 million, including items that were recorded to stockholders' equity and which did not impact the Company's income tax provision.
The valuation allowance of $65.8 million as of December 31, 2019 reduces certain deferred tax assets to amounts that are more likely than not to be realized. This allowance primarily relates to the net operating loss carryforwards of certain non-United States subsidiaries and certain non-United States credit carryforwards.
87
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
17. INCOME TAXES (Continued)
Net operating loss and capital loss carryforwards and the related carryforward periods at December 31, 2019 are summarized as follows (in millions):
Carryforward Amount | Tax Benefit Amount | Valuation Allowance | Net Tax Benefit | Carryforward Period Ends | |||||||||||||
United States federal net operating losses | $ | $ | $ | $ | 2030-2037 | ||||||||||||
United States federal net operating losses | Indefinite | ||||||||||||||||
United States state net operating losses | ( | ) | 2026-2039 | ||||||||||||||
United States state net operating losses | Indefinite | ||||||||||||||||
Non-United States net operating losses | ( | ) | 2020-2027 | ||||||||||||||
Non-United States net operating losses | ( | ) | Indefinite | ||||||||||||||
United States capital losses | ( | ) | 2022 | ||||||||||||||
Total | $ | $ | $ | ( | ) | $ | |||||||||||
Certain tax attributes are subject to an annual limitation as a result of the acquisitions of Harpoon Medical, Inc. and CAS Medical Systems, Inc. (see Note 8), which constitute a change of ownership as defined under Internal Revenue Code Section 382.
The gross tax credit carryforwards and the related carryforward periods at December 31, 2019 are summarized as follows (in millions):
Carryforward Amount | Valuation Allowance | Net Tax Benefit | Carryforward Period Ends | ||||||||||
California research expenditure tax credits | $ | $ | $ | Indefinite | |||||||||
Federal research expenditure tax credits | 2026-2039 | ||||||||||||
Puerto Rico purchases credit | ( | ) | Indefinite | ||||||||||
Total | $ | $ | ( | ) | $ | ||||||||
The Company has $124.4 million of California research expenditure tax credits it expects to use in future periods. The credits may be carried forward indefinitely. Based upon anticipated future taxable income, the Company expects that it is more likely than not that all California research expenditure tax credits will be utilized, although the utilization of the full benefit is expected to occur over a number of years and into the distant future. Accordingly, no valuation allowance has been provided. The Company has $21.8 million of Puerto Rico purchases credit. Throughout its history and into the future, the Puerto Rico operations generate or are expected to generate credits each year in excess of its ability to utilize credits in those years. As a result, even though the credits have an indefinite life, the Company continues to record a valuation allowance on the credit carryforwards.
On December 22, 2017, Public Law 115-97, commonly referred to as the Tax Cuts and Jobs Act (the "2017 Act"), was signed into law. The 2017 Act (1) reduced the U.S. federal corporate tax rate from 35 percent to 21 percent for tax years beginning after December 31, 2017, (2) required companies to pay a one-time mandatory deemed repatriation tax on the cumulative earnings of certain foreign subsidiaries that were previously tax deferred, and (3) created new taxes on certain foreign earnings in future years.
On December 22, 2017, Staff Accounting Bulletin No. 118 ("SAB 118") was issued to address the application of generally accepted accounting principles in the United States of America in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the 2017 Act. In accordance with SAB 118, as of December 31, 2017, the Company had estimated provisional amounts for (1) $3.3 million of tax benefits in connection with the remeasurement of certain tax assets and liabilities, (2) $297.4 million of net tax expense (discussed below) in connection with the one-time mandatory deemed repatriation tax on cumulative earnings of certain foreign subsidiaries, and (3) $32.3 million of tax benefits associated with a tax reform related restructuring. In accordance with SAB 118, during 2018 the Company adjusted the provisional amounts as described below.
88
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
17. INCOME TAXES (Continued)
As a result of Internal Revenue Service ("IRS") guidance issued subsequent to the 2017 Act, the $32.3 million of tax benefits associated with the tax reform related restructuring mentioned above were reversed in 2018. In addition, during 2018, the Company recorded a $12.8 million reduction in the repatriation tax and an additional benefit of $3.7 million in connection with the remeasurement of deferred tax assets. In accordance with SAB 118, the Company completed its accounting for the 2017 Act during the fourth quarter of 2018. In addition, the Company elected to pay the repatriation tax in installments over eight years.
The Company asserts that $1.1 billion of its foreign earnings continue to be indefinitely reinvested and it intends to repatriate $140.7 million of its foreign earnings as of December 31, 2019. The estimated net tax liability (after credits) on the indefinitely reinvested earnings if repatriated is $13.6 million.
The Company has received tax incentives in certain non-U.S. tax jurisdictions, the primary benefit for which will expire in 2029. The tax reductions as compared to the local statutory rates were $157.6 million ($0.75 per diluted share), $144.9 million ($0.70 per diluted share), and $81.0 million ($0.39 per diluted share) for the years ended December 31, 2019, 2018, and 2017, respectively.
A reconciliation of the United States federal statutory income tax rate to the Company's effective income tax rate is as follows (in millions):
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Income tax expense at U.S. federal statutory rate | $ | $ | $ | ||||||||
Foreign income taxed at different rates | ( | ) | ( | ) | ( | ) | |||||
State and local taxes, net of federal tax benefit | |||||||||||
Tax credits, federal and state | ( | ) | ( | ) | ( | ) | |||||
Build (release) of reserve for prior years' uncertain tax positions | ( | ) | ( | ) | |||||||
U.S. tax on foreign earnings, net of credits | ( | ) | ( | ) | ( | ) | |||||
Tax on global intangible low-taxed income | |||||||||||
Foreign-derived intangible income deduction | ( | ) | ( | ) | |||||||
Deductible employee share-based compensation | ( | ) | ( | ) | ( | ) | |||||
Nondeductible employee share-based compensation | |||||||||||
Impact related to 2017 U.S. Tax Reform | |||||||||||
Other | ( | ) | |||||||||
Income tax provision | $ | $ | $ | ||||||||
The Company's effective tax rate for 2019 increased in comparison to 2018 primarily because of the increase in the U.S. tax on global intangible low-taxed income and the tax benefit in 2018 from audit settlements.
Uncertain Tax Positions
As of December 31, 2019 and 2018, the gross uncertain tax positions were $203.1 million and $150.7 million, respectively. The Company estimates that these liabilities would be reduced by $50.1 million and $42.7 million, respectively, from offsetting tax benefits associated with the correlative effects of potential transfer pricing adjustments, state income taxes, and timing adjustments. The net amounts of $153.0 million and $108.0 million, respectively, if not required, would favorably affect the Company's effective tax rate.
89
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
17. INCOME TAXES (Continued)
A reconciliation of the beginning and ending amount of uncertain tax positions, excluding interest, penalties, and foreign exchange, is as follows (in millions):
December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Uncertain gross tax positions, January 1 | $ | $ | $ | ||||||||
Current year tax positions | |||||||||||
Increase in prior year tax positions | |||||||||||
Decrease in prior year tax positions | ( | ) | ( | ) | ( | ) | |||||
Settlements | ( | ) | ( | ) | |||||||
Lapse of statutes of limitations | ( | ) | ( | ) | |||||||
Uncertain gross tax positions, December 31 | $ | $ | $ | ||||||||
The table above summarizes the gross amounts of uncertain tax positions without regard to reduction in tax liabilities or additions to deferred tax assets and liabilities if such uncertain tax positions were settled.
The Company recognizes interest and penalties, if any, related to uncertain tax positions in the provision for income taxes. As of December 31, 2019, the Company had accrued $9.3 million (net of $3.5 million tax benefit) of interest related to uncertain tax positions, and as of December 31, 2018, the Company had accrued $4.6 million (net of $1.9 million tax benefit) of interest related to uncertain tax positions. During 2019, 2018, and 2017, the Company recognized interest expense (benefit), net of tax benefit, of $4.7 million, $(2.8 ) million, and $(7.3 ) million, respectively, in "Provision for Income Taxes" on the consolidated statements of operations.
The Company strives to resolve open matters with each tax authority at the examination level and could reach agreement with a tax authority at any time. While the Company has accrued for matters it believes are more likely than not to require settlement, the final outcome with a tax authority may result in a tax liability that is more or less than that reflected in the consolidated financial statements. Furthermore, the Company may later decide to challenge any assessments, if made, and may exercise its right to appeal. The uncertain tax positions are reviewed quarterly and adjusted as events occur that affect potential liabilities for additional taxes, such as lapsing of applicable statutes of limitations, proposed assessments by tax authorities, negotiations between tax authorities, identification of new issues, and issuance of new legislation, regulations, or case law. Management believes that adequate amounts of tax and related penalty and interest have been provided in income tax expense for any adjustments that may result from these uncertain tax positions.
At December 31, 2019, all material state, local, and foreign income tax matters have been concluded for years through 2008. During 2018, the Company signed agreements with the IRS to settle tax years 2009 through 2014, including transfer pricing matters and the tax treatment of a portion of a litigation settlement payment received in 2014. The IRS began its examination of the 2015 and 2016 tax years during the fourth quarter of 2018 and its examination of the 2017 tax year during the first quarter of 2019.
During 2018, the Company executed an Advance Pricing Agreement ("APA") between the United States and Switzerland governments for tax years 2009 through 2020 covering various transfer pricing matters . Certain intercompany transactions covering tax years 2015 through 2019 were not resolved and those related tax positions remain uncertain. These transfer pricing matters may be significant to the Company's consolidated financial statements. Based upon the information currently available and numerous possible outcomes, the Company cannot reasonably estimate what, if any, changes in its existing uncertain tax positions may occur in the next 12 months and, therefore, has recorded the gross uncertain tax positions as a long-term liability.
In addition, the Company executed other APAs as follows: during 2017, an APA between the United States and Japan covering tax years 2015 through 2019; and during 2018, APAs between Japan and Singapore and between Switzerland and Japan covering tax years 2015 through 2019. The Company is evaluating filing to renew some or all of these APAs for the years 2020 and forward. The execution of some or all of these APAs depends on a number of variables outside of the Company's control.
90
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
18. LEGAL PROCEEDINGS
On January 28, 2019, Abbott Cardiovascular Systems, Inc. and Evalve, Inc., both subsidiaries of Abbott Laboratories (collectively "Abbott") filed a lawsuit against Edwards Lifesciences Corporation and Edwards Lifesciences, LLC, (“Edwards”) in the Federal District Court in the District of Delaware alleging that the Edwards PASCAL heart valve repair system infringes certain claims of Abbott’s U.S. Patent Nos. 7,288,097, 6,752,813, 7,563,267, 7,736,388, and 8,057,493, seeking unspecified monetary damages and preliminary and permanent injunctive relief. Thereafter, Abbott sought a preliminary injunction and a
temporary restraining order and the court denied these requests. Trial is scheduled for May 6, 2020.
On January 28, 2019, Abbott and its Abbott Medical UK Limited subsidiary (inclusively and collectively also "Abbott”) filed a lawsuit in the United Kingdom in the High Court of Justice, Chancery Division, Patents Court, against Edwards Lifesciences Limited, alleging that the Edwards PASCAL heart valve repair system infringes certain claims of Abbott’s UK national patents arising from EP 1 624 810 B1 (the " ‘810 patent") and EP 1 408 850 B1 (the “ ‘850 patent”). Abbott requested a preliminary injunction and the High Court denied this request. Trial proceedings began on December 9, 2019, and a decision is expected in early 2020.
On January 28, 2019, Abbott Medical GmbH (inclusively and collectively also "Abbott”) filed a lawsuit in the District Court in Düsseldorf, Germany against Edwards Lifesciences Corporation and its German subsidiary, Edwards Lifesciences Services GmbH, alleging that the Edwards PASCAL heart valve repair system infringes certain claims of Abbott’s German national patents arising from the ‘810 and ‘850 European patents. The District Court has scheduled trial for March 19, 2020 for the '810 patent and for July 14, 2020 for the '850 patent. On March 15, 2019, Edwards filed a lawsuit in the German Patent Court in Munich, Germany, alleging that Abbott’s ‘850 patent is invalid. The Abbott ‘810 patent is subject to opposition proceedings at the European Patent Office, where a first instance opposition panel determined that the '810 patent was valid with amendment. Edwards will appeal.
On January 28, 2019, Abbott and Abbott Medical AG (inclusively and collectively also "Abbott”) filed a lawsuit in the Federal Patent Court in St. Gallen, Switzerland against Edwards Lifesciences AG, Edwards Lifesciences Technology Sàrl, Edwards Lifesciences IPRM AG, and Mitral Valve Technologies Sàrl, alleging that the Edwards PASCAL heart valve repair system infringes Abbott’s Swiss national patents arising from the same European patents. The court subsequently granted Abbott's request for a preliminary injunction on the '850 patent and Edwards has appealed.
On January 28, 2019, Abbott Cardiovascular System Inc., Abbott Medical Italia S.p.A and Evalve Inc. (inclusively and collectively also "Abbott”) filed a lawsuit in the Civil Court of Milan, Italy against Edwards Lifesciences Corporation, Edwards Lifesciences LLC, and Edwards Lifesciences Italia SpA, alleging that the Edwards PASCAL heart valve repair system infringes Abbott’s Italian national patent arising from its ‘850 European patent. The lawsuit seeks a preliminary injunction. The Company intends to defend itself vigorously in all of the above matters.
On February 22, 2019, Edwards Lifesciences Corporation and Edwards Lifesciences, LLC filed a lawsuit against Abbott Cardiovascular Systems, Inc. in the Federal District Court in the Central District of California alleging that Abbott's MITRACLIP device infringes Edwards’ U.S. Patent Nos. 6,719,767, 7,011,669, and 8,062,313 related to heart implant technology and seeking unspecified monetary damages. A trial date has not yet been scheduled.
Because the ultimate outcome of the above matters involve judgments, estimates, and inherent uncertainties, charges related to such matters could have a material adverse impact on the Company's consolidated financial results. The Company records accruals for loss contingencies to the extent it is probable that a liability has been incurred and the amount of the related loss can be reasonably estimated. Where a loss is reasonably possible (including potential losses in excess of the amounts accrued by the Company), the Company will disclose a reasonable estimate of the amount of loss or range of possible losses with respect to the loss contingency (including potential losses in excess of the amounts accrued by the Company), unless the Company is not able to reasonably estimate the amount or range of reasonably possible losses. With respect to the matters above, the Company is not able to estimate the amount or range of any loss for these matters.
In addition, the Company is or may be a party to, or may otherwise be responsible for, pending or threatened lawsuits including those related to products and services currently or formerly manufactured or performed, as applicable, by the Company, workplace and employment matters or governmental investigations (the "Other Lawsuits"). The Other Lawsuits raise
91
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
18. LEGAL PROCEEDINGS (Continued)
difficult and complex factual and legal issues and are subject to many uncertainties, including, but not limited to, the facts and circumstances of each particular case or claim, the jurisdiction in which each suit is brought, and differences in applicable law. Management does not believe that any loss relating to the Other Lawsuits would have a material adverse effect on the Company's overall financial condition, results of operations or cash flows. However, the resolution of one or more of the Other Lawsuits in any reporting period, could have a material adverse impact on the Company's financial results for that period. The Company is not able to estimate the amount or range of any loss for legal contingencies related to the Other Lawsuits for which there is no reserve or additional loss for matters already reserved.
The Company is subject to various environmental laws and regulations both within and outside of the United States. The Company's operations, like those of other medical device companies, involve the use of substances regulated under environmental laws, primarily in manufacturing and sterilization processes. While it is difficult to quantify the potential impact of continuing compliance with environmental protection laws, management believes that such compliance will not have a material impact on the Company's financial results.
19. SEGMENT INFORMATION
The Company conducts operations worldwide and is managed in the following geographical regions: United States, Europe, Japan, and Rest of World. All regions sell products that are used to treat advanced cardiovascular disease.
The Company's geographic segments are reported based on the financial information provided to the Chief Operating Decision Maker (the Chief Executive Officer). The Company evaluates the performance of its geographic segments based on net sales and operating income. The accounting policies of the segments are substantially the same as those described in Note 2. Segment net sales and segment operating income are based on internally derived standard foreign exchange rates, which may differ from year to year, and do not include inter-segment profits. Because of the interdependence of the reportable segments, the operating profit as presented may not be representative of the geographical distribution that would occur if the segments were not interdependent. Net sales by geographic area are based on the location of the customer.
Certain items are maintained at the corporate level and are not allocated to the segments. The non-allocated items include net interest expense, global marketing expenses, corporate research and development expenses, manufacturing variances, corporate headquarters costs, special gains and charges, stock-based compensation, foreign currency hedging activities, certain litigation costs, changes in the fair value of contingent consideration liabilities, and most of the Company's amortization expense. Although most of the Company's depreciation expense is included in segment operating income, due to the Company's methodology for cost build-up, it is impractical to determine the amount of depreciation expense included in each segment, and, therefore, a portion is maintained at the corporate level. The Company neither discretely allocates assets to its operating segments, nor evaluates the operating segments using discrete asset information.
92
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
19. SEGMENT INFORMATION (Continued)
The table below presents information about Edwards Lifesciences' reportable segments (in millions):
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Segment Net Sales | |||||||||||
United States | $ | $ | $ | ||||||||
Europe | |||||||||||
Japan | |||||||||||
Rest of World | |||||||||||
Total segment net sales | $ | $ | $ | ||||||||
Segment Operating Income | |||||||||||
United States | $ | $ | $ | ||||||||
Europe | |||||||||||
Japan | |||||||||||
Rest of World | |||||||||||
Total segment operating income | $ | $ | $ | ||||||||
The table below presents reconciliations of segment net sales to consolidated net sales and segment operating income to consolidated income before provision for income taxes ("pre-tax income") (in millions):
Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
Net Sales Reconciliation | |||||||||||
Segment net sales | $ | $ | $ | ||||||||
Foreign currency | |||||||||||
Consolidated net sales | $ | $ | $ | ||||||||
Pre-tax Income Reconciliation | |||||||||||
Segment operating income | $ | $ | $ | ||||||||
Unallocated amounts: | |||||||||||
Corporate items | ( | ) | ( | ) | ( | ) | |||||
Special charges, net | ( | ) | ( | ) | ( | ) | |||||
Intellectual property litigation (expenses) income, net | ( | ) | ( | ) | |||||||
Change in fair value of contingent consideration liabilities, net | |||||||||||
Foreign currency | |||||||||||
Consolidated operating income | |||||||||||
Non-operating income (expense) | ( | ) | |||||||||
Consolidated pre-tax income | $ | $ | $ | ||||||||
93
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
19. SEGMENT INFORMATION (Continued)
Enterprise-Wide Information
Enterprise-wide information is based on actual foreign exchange rates used in the Company's consolidated financial statements.
As of or for the Years Ended December 31, | |||||||||||
2019 | 2018 | 2017 | |||||||||
(in millions) | |||||||||||
Net Sales by Geographic Area | |||||||||||
United States | $ | $ | $ | ||||||||
Europe | |||||||||||
Japan | |||||||||||
Rest of World | |||||||||||
$ | $ | $ | |||||||||
Net Sales by Major Product Area | |||||||||||
Transcatheter Aortic Valve Replacement | $ | $ | $ | ||||||||
Transcatheter Mitral and Tricuspid Therapies | |||||||||||
Surgical Structural Heart | |||||||||||
Critical Care | |||||||||||
$ | $ | $ | |||||||||
Long-lived Tangible Assets by Geographic Area | |||||||||||
United States | $ | $ | $ | ||||||||
Europe | |||||||||||
Japan | |||||||||||
Rest of World | |||||||||||
$ | $ | $ | |||||||||
94
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
20. QUARTERLY FINANCIAL RESULTS AND MARKET FOR THE COMPANY'S STOCK (UNAUDITED)
Years Ended December 31, | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | Total Year | ||||||||||||||
(in millions, except per share data) | |||||||||||||||||||
2019 | |||||||||||||||||||
Net sales | $ | $ | $ | $ | $ | ||||||||||||||
Gross profit | |||||||||||||||||||
Net income (a) | |||||||||||||||||||
Earnings per common share (a): | |||||||||||||||||||
Basic | |||||||||||||||||||
Diluted | |||||||||||||||||||
Market price: | |||||||||||||||||||
High | $ | $ | $ | $ | $ | ||||||||||||||
Low | |||||||||||||||||||
2018 | |||||||||||||||||||
Net sales | $ | $ | $ | $ | $ | ||||||||||||||
Gross profit | |||||||||||||||||||
Net income (b) | |||||||||||||||||||
Earnings per common share (b): | |||||||||||||||||||
Basic | |||||||||||||||||||
Diluted | |||||||||||||||||||
Market price: | |||||||||||||||||||
High | $ | $ | $ | $ | $ | ||||||||||||||
Low | |||||||||||||||||||
_______________________________________________________________________________
(a) | The first quarter of 2019 includes a $ |
(b) |
95
EDWARDS LIFESCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
21. VALUATION AND QUALIFYING ACCOUNTS
Additions | |||||||||||||||||||
Balance at Beginning of Period | Charged to Costs and Expenses | Charged to Other Accounts | Deductions From Reserves | Balance at End of Period | |||||||||||||||
(in millions) | |||||||||||||||||||
Year ended December 31, 2019 | |||||||||||||||||||
Allowance for doubtful accounts (a) | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
Tax valuation allowance (b) | |||||||||||||||||||
Year ended December 31, 2018 | |||||||||||||||||||
Allowance for doubtful accounts (a) | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
Tax valuation allowance (b) | ( | ) | ( | ) | |||||||||||||||
Year ended December 31, 2017 | |||||||||||||||||||
Allowance for doubtful accounts (a) | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
Tax valuation allowance (b) | ( | ) | |||||||||||||||||
_______________________________________________________________________________
(a) | The deductions related to allowances for doubtful accounts represent accounts receivable which are written off. |
(b) | The tax valuation allowances are provided for other-than-temporary impairments and unrealized losses related to certain investments that may not be recognized due to the uncertainty of the ready marketability of certain impaired investments, and net operating loss and credit carryforwards that may not be recognized due to insufficient taxable income. |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures. The Company's management, including the Chief Executive Officer and Chief Financial Officer, performed an evaluation of the effectiveness of the design and operation of the Company's disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) as of December 31, 2019.
Based on their evaluation, the Chief Executive Officer and Chief Financial Officer have concluded as of December 31, 2019 that the Company's disclosure controls and procedures are designed at a reasonable assurance level and are effective in providing reasonable assurance that the information required to be disclosed by the Company in the reports it files or submits under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized, and reported within the time periods specified in the SEC's rules and forms, and that such information is accumulated and communicated to the Company's management, including the Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Management's Report on Internal Control Over Financial Reporting. The Company's management, including the Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. Under the supervision and with the participation of the Company's management, including the Chief Executive Officer and Chief Financial Officer, the Company conducted an evaluation of the effectiveness of its internal control over financial reporting based on the framework in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on that evaluation, the Company's management concluded that its internal control over financial reporting was effective as of December 31, 2019. The effectiveness of the
96
Company's internal control over financial reporting as of December 31, 2019 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Changes in Internal Control Over Financial Reporting. There have been no changes in the Company's internal control over financial reporting that occurred during the Company's fourth fiscal quarter of 2019 that have materially affected, or are reasonably likely to materially affect, the Company's internal control over financial reporting.
Item 9B. Other Information
None.
97
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Certain information required by this Item will be set forth under the headings "Corporate Governance Policies and Practices," "Executive Compensation and Other Information—Executive Officers," and "Other Matters and Business—Additional Information" and "—Delinquent Section 16(a) Reports" in the definitive proxy materials to be filed in connection with the Company's 2020 Annual Meeting of Stockholders (the "Proxy Statement") (which Proxy Statement will be filed with the SEC within 120 days of December 31, 2019). The information required by this Item to be contained in the Proxy Statement is incorporated herein by reference. The Company has adopted a code of ethics that applies to all directors and employees, including the Company's principal executive officer, principal financial officer and controller or persons performing similar functions. The code of ethics (business practice standards) is posted on the Company's website, which is found at www.edwards.com under "Investors-Corporate Governance-Corporate Responsibility-Global Integrity Program." To the extent required by applicable rules of the SEC and the New York Stock Exchange, the Company intends to disclose on its website any amendments to, or waivers from, any provision of its code of ethics that apply to the Company's directors and executive officers, including the principal executive officer, principal financial officer or controller or persons performing similar functions.
Item 11. Executive Compensation
The information contained under the heading "Executive Compensation and Other Information" in the Proxy Statement is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information contained under the headings "Security Ownership of Certain Beneficial Owners and Management" and "Equity Compensation Plan Information" in the Proxy Statement is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information contained under the heading "Other Matters and Business—Related Persons Transactions" and under the heading "Corporate Governance Policies and Practices—Director Independence" in the Proxy Statement is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services
The information contained under the heading "Audit Matters—Fees Paid to Principal Accountants" in the Proxy Statement is incorporated herein by reference.
98
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a) The following documents are filed as part of this report:
1. Consolidated Financial Statements. See “Index to Consolidated Financial Statements” in Part II, Item 8 herein.
2. Financial Statement Schedules. Other schedules are not applicable and have not been included herein.
3. Exhibits.
Exhibit No. | Exhibit No. | |
3.1 | ||
3.2 | ||
4.1 | ||
4.2 | ||
4.3 | ||
4.4 | ||
4.5 | ||
4.6 | ||
10.1 | ||
#10.2 | ||
*10.3 | ||
*10.4 | ||
*10.5 | ||
*10.6 | ||
*10.7 | ||
*10.8 | ||
*10.9 | ||
99
Exhibit No. | Exhibit No. | |
*10.10 | ||
*10.11 | ||
*10.12 | ||
*10.13 | ||
*10.14 | ||
*10.15 | ||
*10.16 | ||
*10.17 | ||
*10.18 | ||
*10.19 | ||
*10.20 | ||
*10.21 | ||
*10.22 | ||
*10.23 | ||
*10.24 | ||
*10.25 | ||
*10.26 | ||
*10.27 | ||
*10.28 | ||
*10.29 | ||
100
Exhibit No. | Exhibit No. | |
*10.30 | ||
*10.31 | ||
*10.32 | ||
*10.33 | ||
*10.34 | ||
*10.35 | ||
*10.36 | ||
*10.37 | ||
*10.38 | ||
*10.39 | ||
*10.40 | ||
*10.41 | ||
*10.42 | ||
21.1 | ||
23 | ||
31.1 | ||
31.2 | ||
+32 | ||
101.INS | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | |
101.SCH | XBRL Taxonomy Extension Schema Document | |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | |
104 | Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) | |
______________________________________________________________________________
# Pursuant to a request for confidential treatment, confidential portions of this exhibit have been redacted and have been filed separately with the Securities and Exchange Commission
* Represents management contract or compensatory plan
+ Furnished herewith
101
Item 16. Form 10-K Summary
None.
102
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
EDWARDS LIFESCIENCES CORPORATION | |||
February 14, 2020 | By: | /s/ MICHAEL A. MUSSALLEM | |
Michael A. Mussallem Chairman of the Board and Chief Executive Officer | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title | Date | |
/s/ MICHAEL A. MUSSALLEM | Chairman of the Board and Chief Executive Officer | February 14, 2020 | |
Michael A. Mussallem | (Principal Executive Officer) | ||
/s/ SCOTT B. ULLEM | Corporate Vice President, Chief Financial Officer | February 14, 2020 | |
Scott B. Ullem | (Principal Financial Officer) | ||
/s/ ROBERT W.A. SELLERS | Vice President, Corporate Controller | February 14, 2020 | |
Robert W.A. Sellers | (Principal Accounting Officer) | ||
/s/ KIERAN T. GALLAHUE | Director | February 14, 2020 | |
Kieran T. Gallahue | |||
/s/ LESLIE S. HEISZ | Director | February 14, 2020 | |
Leslie S. Heisz | |||
/s/ WILLIAM J. LINK, PH.D. | Director | February 14, 2020 | |
William J. Link, Ph.D. | |||
/s/ STEVEN R. LORANGER | Director | February 14, 2020 | |
Steven R. Loranger | |||
/s/ MARTHA H. MARSH | Director | February 14, 2020 | |
Martha H. Marsh | |||
/s/ WESLEY W. VON SCHACK | Director | February 14, 2020 | |
Wesley W. von Schack | |||
/s/ NICHOLAS J. VALERIANI | Director | February 14, 2020 | |
Nicholas J. Valeriani | |||
103