UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM
(Mark One)
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
OR |
|
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
|
OR |
|
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
OR |
|
|
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
Date of event requiring this shell company report
For the transition period from to
Commission file number:
(Exact name of Registrant as specified in its charter) |
|
N/A |
(Translation of Registrant’s name into English) |
|
Antigua, West Indies |
(Jurisdiction of incorporation or organization) |
|
(Address of principal executive offices) |
|
Chief Financial Officer Tel: Fax: E-mail: |
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person) |
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None |
(Title of Class) |
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None |
(Title of Class) |
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report
|
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
☐ Yes ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
☐ Yes ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
☒
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or such shorter period that the registrant was required to submit such files).
☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ |
Non-accelerated filer ☐ |
|
|
|
Emerging growth company |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
|
International Financial Reporting Standards as issued by the International Accounting Standards Board ☐ |
|
Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐ Yes ☒ No
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court.
☐ Yes
CONTENTS
1 |
|||
|
|
|
|
1 |
|||
|
|
||
2 |
|||
|
|
|
|
|
2 |
||
|
2 |
||
|
2 |
||
|
32 |
||
|
49 |
||
|
49 |
||
|
60 |
||
|
70 |
||
|
71 |
||
|
76 |
||
|
76 |
||
|
89 |
||
|
90 |
||
|
|
|
|
91 |
|||
|
|
|
|
|
91 |
||
|
Material Modifications to the Rights of Security Holders and Use of Proceeds |
91 |
|
|
92 |
||
|
93 |
||
|
93 |
||
|
93 |
||
|
93 |
||
|
Purchases of Equity Securities by the Issuer and Affiliated Purchasers |
93 |
|
|
93 |
||
|
93 |
||
|
94 |
||
|
Disclosure Regarding Foreign Jurisdictions That Prevent Inspections |
94 |
|
94 |
|||
|
|
||
95 |
|||
|
|
|
|
|
95 |
||
|
95 |
||
|
95 |
||
INTRODUCTION
In this annual report on Form 20-F, unless otherwise indicated or unless the context otherwise requires,
· “Sinovac,” “Sinovac Biotech,” “Company,” “we,” “us,” “our company,” and “our” refer to Sinovac Biotech Ltd., its predecessor entities and its consolidated subsidiaries
· “Sinovac Antigua” refers to Sinovac Biotech Ltd.;
· “China,” “Chinese” or the “PRC” refers to the People’s Republic of China, excluding, for the purposes of this annual report on Form 20-F only, Taiwan and the special administrative regions of Hong Kong and Macau;
· “RMB” or “renminbi” refers to the legal currency of China; and “$” or “U.S. dollars” refers to the legal currency of the United States;
· “shares” or “common shares” refers to our common shares, par value $0.001 per share; and
· “U.S. GAAP” refers to generally accepted accounting principles in the United States.
Discrepancies in any table between the amounts identified as total amounts and the sum of the amounts listed therein are due to rounding.
This annual report contains translations of certain renminbi amounts into U.S. dollars at specified rates solely for the convenience of readers. Unless otherwise stated, all translations from renminbi to U.S. dollars were made at a rate of RMB6.8972 to $1.00, the exchange rate in effect as set forth in the H.10 statistical release of The Board of Governors of the Federal Reserve System, or the Federal Reserve Exchange Rate, in effect on December 30, 2022. We make no representation that the renminbi or U.S. dollar amounts referred to in this annual report could have been or could be converted into U.S. dollars or renminbi, as the case may be, at any particular rate or at all. On April 21, 2023, the Federal Reserve Exchange Rate was RMB6.8920 to $1.00.
FORWARD-LOOKING INFORMATION
This annual report contains forward-looking statements that reflect our current expectations and views of future events. These forward-looking statements are made under the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. Known and unknown risks, uncertainties and other factors, including those included in “Item 3. Key Information—D. Risk Factors,” may cause our actual results, performance, or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify some of these forward-looking statements by words or phrases such as “may,” “might,” “will,” “would,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,” “potential,” “continue,” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events that we believe may affect our financial condition, results of operations, business strategy, and financial needs. These forward-looking statements include statements relating to:
These forward-looking statements involve various risks and uncertainties. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. Our actual results could be materially different from our expectations. Important risks and factors that could cause our actual results to be materially different from our expectations are generally set forth in “Item 3. Key Information—D. Risk Factors,” “Item 4. Information on the Company—B. Business Overview,” “Item 5. Operating and Financial Review and Prospects,” and other sections in this annual report. You should read thoroughly this annual report and the documents that we refer to in this annual report with the understanding that our actual future results may be materially different from and worse than what we expect. We qualify all of our forward-looking statements by these cautionary statements.
We operate in an evolving environment. New risk factors and uncertainties emerge from time to time and it is not possible for our management to predict all risk factors and uncertainties, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. The forward-looking statements made in this annual report relate only to events or information as of the date on which the statements are made in this annual report. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
1
PART I
ITEM 1. Identity of Directors, Senior Management and Advisers
Not applicable.
ITEM 2. Offer Statistics and Expected Timetable
Not applicable.
ITEM 3. Key Information
Permissions Required from the PRC Authorities for Our Operations
We conduct our business primarily through our subsidiaries in China. Our operations in China are governed by PRC laws and regulations. We face various legal and operational risks and uncertainties associated with having a portion of our operations in China and the complex and evolving PRC laws and regulations. For example, we face risks associated with regulatory approvals on offerings conducted overseas and foreign investment in China-based issuers, anti-monopoly regulatory actions, and oversight on cybersecurity and data privacy, which may negatively impact our ability to conduct certain businesses or access foreign investments. These risks could result in a material adverse change in our operations and the value of our shares, significantly limit or completely hinder our ability to offer or continue to offer securities to investors, or cause the value of such securities to significantly decline or become worthless. For more detailed information, see “—D. Risk Factors—Risks Relating to Doing Business in China—Future changes in laws, regulations or enforcement policies in China and the PRC government’s oversight and discretion over our operations could adversely affect our business.”
The Holding Foreign Companies Accountable Act
The Holding Foreign Companies Accountable Act (“HFCAA”), as amended, was initially enacted on December 18, 2020. In accordance with the HFCAA, trading in our shares on a national securities exchange or in the over the counter trading market in the United States may be prohibited if the Public Company Accounting Oversight Board (United States) (“PCAOB”) determines that it cannot inspect or fully investigate our auditor for two consecutive years beginning in 2021, and, as a result, the United States Securities and Exchange Commission (“SEC”) may determine to delist our shares. On September 22, 2021, the PCAOB adopted a final rule implementing the HFCAA, which provides a framework for the PCAOB to use when determining, as contemplated under the HFCAA, whether the PCAOB is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction. On December 16, 2021, the PCAOB issued a report on its determinations that the PCAOB was unable to inspect or investigate completely PCAOB-registered public accounting firms headquartered in mainland China and Hong Kong, because of positions taken by PRC authorities in these jurisdictions. The PCAOB included in its report a list of registered public accounting firms headquartered in mainland China and Hong Kong that the PCAOB was unable to inspect or investigate completely, including our auditor.
Our auditor, Grant Thornton Zhitong Certified Public Accountants LLP (“Grant Thornton”), is an independent registered public accounting firm that issues the audit reports included elsewhere in this annual report. Our auditor was subject to the determinations made by the PCAOB, on December 16, 2021, and as a result, the PCAOB was not able to fully inspect our auditor. On May 4, 2022, we were identified by the SEC under the HFCAA. On December 15, 2022, the PCAOB issued a report that vacated its December 16, 2021 determination and removed mainland China and Hong Kong from the list of jurisdictions where it was unable to inspect or investigate completely registered public accounting firms. For this reason, we do not expect to be identified as a Commission-Identified Issuer under the HFCAA after we file this annual report on Form 20-F. Each year, the PCAOB will determine whether it can inspect and investigate completely accounting firms in mainland China and Hong Kong, among other jurisdictions. If PCAOB determines in the future that it no longer has full access to inspect and investigate completely accounting firms in mainland China and Hong Kong and we continue to use an accounting firm headquartered in one of these jurisdictions to issue an audit report on our financial statements filed with the SEC, we would be identified as a Commission-Identified Issuer following the filing of the annual report on Form 20-F for the relevant fiscal year. There can be no assurance that we would not be identified as a Commission-Identified Issuer for any future fiscal year, and if we were so identified for two consecutive years, we would become subject to the prohibition on trading under the HFCAA. See “—D. Risk Factors—Risks Related to Doing Business in China—Our shares will be prohibited from trading in the United States under the HFCAA in 2023 if the PCAOB is unable to inspect or fully investigate auditors located in China. The delisting of our shares, or the threat of their being delisted, may materially and adversely affect the value of your investment.”
Cash and Asset Flows Through Our Organization
Sinovac Antigua is a holding company, and we rely in part on dividends paid by our subsidiaries for our cash needs, including our operating expenses and additional investment opportunities. The payment of dividends from subsidiaries in China is subject to limitations. Regulations in the PRC currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and
2
regulations in China. Our subsidiary is also required to set aside at least a portion of its after-tax profit based on PRC accounting standards each year to fund the statutory surplus reserves.
The reserves can be used to recoup previous years’ losses, if any, and, subject to the approval of the relevant PRC government authority, may be converted into share capital in proportion to their existing shareholdings, or by increasing the par value of the shares currently held by them. Such reserves, however, are not distributable as cash dividends. In addition, at discretion of their board of directors, our subsidiaries may allocate a portion of their after-tax profits based on PRC accounting standards to the employee welfare and bonus funds, which shall be utilized for collective staff benefits. In addition, if our PRC subsidiaries incur debt on its own behalf in the future, the instruments governing the debt may restrict the ability of one or more of our PRC subsidiaries, as the case may be, to pay dividends or make other distributions to us.
The ability of our subsidiary to convert renminbi into U.S. dollars and make payments to us is subject to PRC foreign exchange regulations. Under these regulations, the renminbi is convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions. Conversion of renminbi for capital account items, such as direct investment, loan, security investment and repatriation of investment, however, is still subject to the approval of SAFE., see “—Risk Factors—Risks Relating to Doing Business in China—We rely on dividends paid by our PRC subsidiaries for our cash needs. If they are unable to pay us sufficient dividends due to statutory or contractual restrictions on their abilities to distribute dividends to us, our various cash needs may not be met.” and “Item 10. Additional Information — D. Exchange Controls.”
Under PRC laws, Sinovac Antigua may fund our PRC subsidiaries only through capital contributions or loans, subject to satisfaction of applicable government registration and approval requirements. In 2020, 2021 and 2022, no assets other than cash were transferred through our organization.
A. Reserved
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Risk Factors Summary
The following summarizes some, but not all, of the risks provided below. Please carefully consider all of the information discussed in this Item D. “Risk Factors” in this annual report for a more thorough description of these and other risks.
Risks Related to Our Company
3
Risks Related to Government Regulation
Risks Related to Our Intellectual Property
Risks Related to Doing Business in China
Risks Related to Our Company
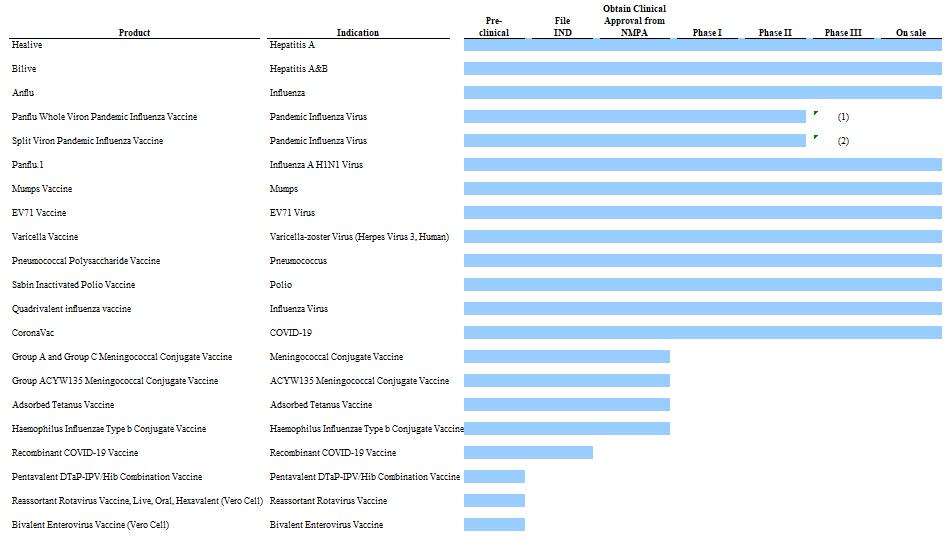
Our business performance relies on our ability to react to infectious disease threats and to continually introduce new vaccine products into the commercial market. Our failure to effectively develop and commercialize new products could materially and adversely affect our business, financial condition, results of operations and prospects.
The biopharmaceutical market in general and the vaccine product market in particular are developing rapidly as a result of ongoing infectious disease threats and new trends in the related research and technology developments. Consequently, our success depends on our ability to react to threats of disease and technology development trends and to identify, develop and commercialize in a timely and cost-effective manner effective vaccine products that meet evolving market needs.
Whether we are successful in developing and commercializing new products is determined by, among other things, our ability to:
4
Although we are profitable in 2020, 2021 and 2022, we incurred a loss in past years, and may incur losses again in the future.
Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We recorded a profit in 2020, 2021 and 2022. However, we incurred a loss in the past years, caused primarily by research and development expenses. None of the research and development expenses incurred were capitalized in our financial statements. We intend to continue to invest in research and development to sustain our long-term growth. We expect our research and development expenses to fluctuate depending on the progress we make on each project, with relatively more spending on clinical studies than preclinical studies. We expect that our spending on research and development will have a negative impact on our future net earnings. As a result, we may incur losses in the future, which will have an adverse impact on our working capital, total assets, shareholders’ equity and cash flow.
As required by the PRC laws, we sell vaccines in China through CDCs) which are PRC government agencies. This exposes us to risks relating to doing business with the government.
As required by the PRC laws, we sell our vaccines to CDCs, which exposes us to various risks relating to doing business with the government. For example, demand and ability to pay for our products may be affected by government budgetary cycles, shifting availability of public funds and changes in policy. Funding reductions, delays in payment or unilateral demands for changes to the terms of our contracts by our government customers could adversely impact our results of operations and financial condition, exacerbate the existing seasonality of our revenues and make it difficult for us to allocate resources or anticipate demand for our products. More importantly, we have little or no control over government procurement decisions, and government agencies that contract to purchase our products may reduce or cancel orders, or demand price adjustments or other changes to their contracts with us without our consent. Changes in the personnel of the PRC government agencies that purchase our products may result in changes or delays to or cancellations of purchase commitments due to, among others, differing policy and budgetary agendas of the personnel involved. Similar changes could occur if CDC or other relevant government agencies were to be consolidated with another ministry. In addition, if our vaccines are to be sold in other countries or regions other than China, regulatory approvals from the relevant governmental authorities of the target markets are to be obtained. Any of the above mentioned actions taken by government agencies could have a material adverse effect on our results of operations and expected earnings, or result in our failure to meet, or having to adjust downwards, our sales and gross margin guidance or estimates, which could adversely affect our share price and result in substantial losses. In addition, many of the remedies that are available to us when dealing with private parties, such as making claims for breach of contract or taking other legal actions, may not be available or practicable in our dealings with government agencies.
We currently have limited revenue sources. A reduction in revenues from sales of COVID-19 vaccine will cause our revenues to decline significantly and could materially harm our business.
We generate all of our revenues from sales of our vaccine products. We derived a substantial percentage of our revenues from COVID-19 vaccine, CoronaVac, in 2021 and 2022. We face risks and uncertainties related to production and sales of CoronaVac, including (i) the demand of COVID-19 vaccines throughout the world may be reduced or no longer exist in the future as more and more people have been vaccinated; (ii) the possibility that COVID-19 epidemic may diminish in severity or prevalence, or disappear entirely; and (iii) other companies may produce superior or competitive products, any of which will adversely impact the sales of CoronaVac. As a result of the relative lack of product diversification, an investment in our company will be riskier than investments in companies that offer a wide variety of products or services.
We expect our key products, which will likely shift over time, to account for a significant portion of our net revenues for the foreseeable future. As a result, continued market acceptance and popularity of these products are critical to our success and a reduction in demand due to, among other factors, the introduction of competing products by our competitors, the entry of new competitors, or end-users’ dissatisfaction with the quality of our products, could materially and adversely affect our financial condition and results of operations.
We could be subject to costly and time-consuming product liability actions and, because our insurance coverage is limited, our exposure to such claims could cause significant financial burden.
Our business exposes us to potential product liability risks that are inherent in the testing, manufacturing and marketing of biopharmaceutical products. We manufacture vaccines that are injected into healthy people to protect against infectious illnesses. If our products do not function as anticipated, whether as a result of flaws in our design, unanticipated health consequences or side effects, misuse or mishandling by third parties, or faulty or contaminated supplies, they could harm the vaccines and, as a result, subject us to product liability lawsuits. Claims against us also
5
could be based on failure to immunize as anticipated. Any product liability claim brought against us, with or without merit, could have a material adverse effect on us. Meritless and unsuccessful product liability claims can be time-consuming and expensive to defend and could result in the diversion of management’s attention from managing our core business or result in associated negative publicity.
Successful assertion of product liability claims against us could require us to pay significant monetary damages. Although we currently carry worldwide product liability insurance for Healive, Bilive, Anflu, Panflu and Inlive, we cannot assure that such coverage will be sufficient to cover any liabilities resulting from successful product liability claims. In such a case, we may be required to make substantial payments to cover any losses, damages or liabilities arising from product liability claims. For any amounts covered by insurance, foreign exchange or other regulatory restrictions may prevent the use of insurance proceeds to meet the liabilities.
In addition, while we have procured liability insurance for the clinical trials which we are currently carrying out outside mainland China, we did not procure the liability insurance for each of our clinical trials which we have completed in mainland China and we do not have or plan to procure clinical trial liability insurance for our clinical trials in mainland China in the future to mitigate any unsuccessful clinical trial expenses or product liability claims arising therefrom for all our vaccine products. Any of these factors could have a material adverse effect on our business, financial condition and results of operations.
We face risks related to health epidemics and other widespread outbreaks of contagious disease, which could disrupt our operations and impact our operating results.
Significant outbreaks of contagious diseases, and other adverse public health developments, could have a material impact on our business operations and operating results. In December 2019, a strain of novel coronavirus, COVID-19, causing respiratory illness emerged in the city of Wuhan in the Hubei province of China and has subsequently spread throughout the world. The outbreak of COVID-19 was recognized as a pandemic by the World Health Organization (“WHO”) on March 11, 2020. In response to the outbreak, governmental authorities of countries all over the world imposed lockdowns and other restrictions to contain the virus, and various businesses suspended or reduced operations. The COVID-19 pandemic has resulted in significant disruptions in the global economy. The PRC government took certain emergency measures to combat the spread of the virus, including implementation of travel bans and closure of factories and businesses throughout the whole country, including Beijing where our research and development functions and main production lines are located. Since January 1, 2022, certain areas in China have suffered from outbreaks of COVID-19 variants including Delta and Omicron virus variants. In response, local governments in the affected areas imposed various restrictions on business and social activities, including city lockdowns, restrictions on travel and other emergency quarantines.
As COVID-19 pandemic continues to evolve and there is great uncertainty as to the future progress of the virus, we cannot anticipate with any certainty the length or severity of the effects of COVID-19 pandemic. We believe the ultimate impact of the COVID-19 pandemic on our business, financial condition and results of operations will be affected by the speed and extent of the continued spread of the coronavirus globally, the emergence of additional virus variants, the duration of the pandemic, new information regarding the severity and incidence of the COVID-19 virus, the safety, efficacy and availability of vaccines and treatments for COVID-19 and the rate at which the population becomes vaccinated against COVID-19. We continue to monitor the spread of COVID-19 in China and globally and have put in place and will continue to put in place measures as appropriate and necessary for our business. Any prolonged lockdowns or deviations from normal daily operations could negatively impact our business.
We could face risks and uncertainties related to our efforts to develop a vaccine to help prevent COVID-19 and potential treatments for COVID-19, as well as challenges related to their manufacturing, supply and distribution.
We face uncertainties related to our efforts to develop a vaccine to prevent the COVID-19, including uncertainties and risks that our existing and future vaccines may not be successful, commercially viable or receive final approval from regulatory authorities. The pre-clinical, clinical data or safety data and further analysis of the existing pre-clinical, clinical or safety of our existing COVID-19 vaccine or future vaccines or treatments may be unfavorable, or we may not be able to produce comparable clinical or other results, including but not limited to the rate of vaccine effectiveness and safety and tolerability profile observed to date or in larger, more diverse populations upon commercialization. Our COVID-19 vaccine, CoronaVac, may not be able to prevent COVID-19 caused by emerging virus variants. The widespread use of the vaccine may lead to new information about efficacy, safety or other developments, including the risk of additional adverse reactions or side effects and regulatory authorities may not be satisfied with the results from any future pre-clinical and clinical studies and may not approve our existing or future vaccines or treatments, or may withdraw or terminate such approvals granted previously to us. Disruptions in the relationships between us and our collaboration partners, research and development institutes, clinical trial sites, countries where the trials are conducted or third-party suppliers, availability of raw materials to manufacture any such products, our ability to scale up or maintain the manufacturing capacity on a timely basis or have access to logistics or supply channels commensurate within global demand for any potential approved vaccine or product candidate, could delay the commercialization of our existing COVID-19 vaccine or any future vaccines or products or otherwise have a significant impact on our business, financial condition and results of operations. We cannot guarantee you that we can produce superior or more competitive products than our competitors, or whether the demand for our COVID-19 vaccine may still exist. Any of these factors could have a material adverse effect on our business, financial condition and results of operations.
Our financial prospects depend on the success of our clinical-stage and pre-clinical stage product pipeline.
6
We have invested significant time and resources on the development of our existing vaccine candidates, and we expect to continue to incur substantial and increasing expenditures for the development and commercialization of our vaccine candidates. Our ability to achieve revenue and profitability is dependent on our ability to complete the clinical development of our vaccine candidates, obtain necessary regulatory approvals, and have our vaccines manufactured and successfully marketed. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of pre-clinical studies and early clinical trials of our vaccine candidates may not be predictive of the results of later-stage clinical trials, and initial or interim results of a trial may not be predictive of the final results. If our vaccine candidates fail to achieve their expected success in a timely manner or at all, we could experience significant delays in our ability to obtain approval for and/or to successfully commercialize our vaccine candidates. We would have expended a significant amount of capital to progress the relevant vaccine candidates to that stage, and would not realize any revenue on such vaccine candidate if it then ultimately failed to receive regulatory approval due to poor clinical trial results. It would materially harm our business and we may not be able to generate sufficient revenues and cash flows to continue our operations.
We have devoted significant resources to research and develop various vaccines to address the pandemic threat of infectious diseases, including COVID-19, SARS, avian flu and swine flu, and will continue to devote resources to the development of vaccines to address any new needs.
However, the threat of a pandemic outbreak may subside before we realize any return on our investment in our research and development. For example, although we believed we were the first company to complete a phase I clinical trial of an inactivated SARS vaccine in December 2004, we did not proceed with the phase II and phase III trials as the SARS epidemic subsequently subsided. Other organizations may obtain licenses for their own pandemic vaccines, or government health organizations may acquire adequate stockpiles of pandemic vaccine or adopt other technologies or strategies to prevent or limit outbreaks before our pandemic vaccines achieve significant sales. We may not achieve a return on our investment before the threat of a pandemic outbreak subsides or a competing product is adopted. We have completed phase III trials of COVID-19 vaccine in Brazil, Turkey, Indonesia and Chile and have received a conditional marketing authorization for CoronaVac from China’s National Medical Products Administration (“NMPA”). CoronaVac has been granted emergency use approval under the WHO’s Emergency Use Listing (EUL) procedure. We cannot assure comparable clinical or other results, including the rate of vaccine effectiveness and safety and tolerability profile or in larger, more diverse populations upon commercialization. Major international and Chinese vaccine companies, universities and other research institutions are also pursuing the development of the COVID 19- vaccines. They may succeed in developing COVID-19 vaccine and obtaining regulatory approvals before us or gain better acceptance for the same target markets as ours, which will undermine our competitive position.
Moreover, because we have limited financial and managerial resources, we focus our product pipeline on research programs and vaccine candidates that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other vaccine candidates that later prove to have greater commercial potential.
Failure to achieve and maintain effective internal controls could have a material adverse effect on our business, results of operations and the trading price of our common shares.
We are subject to the reporting obligations under U.S. securities laws. Section 404 of the Sarbanes-Oxley Act of 2002 and related rules require public companies to include a report of management on their internal control over financial reporting in their annual reports. This report must contain an assessment by management of the effectiveness of a public company’s internal control over financial reporting. In addition, an independent registered public accounting firm for a public company must attest to and report on the effectiveness of our internal control over financial reporting.
Our management has concluded that our internal control over financial reporting was effective as of December 31, 2022. See “Item 15. Controls and Procedures.” Our independent registered public accounting firm has issued an attestation report on our internal control over financial reporting, which concludes that our internal control over financial reporting was effective in all material aspects as of December 31, 2022. However, we cannot assure that any material weakness or deficiency in our internal control over financial reporting will not be identified in the future. We may not always be able to maintain an effective internal control over financial reporting. If we fail to maintain effective internal control over financial reporting in the future, we and our independent registered public accounting firm may not be able to conclude that we have effective internal control over financial reporting at a reasonable assurance level. This could in turn result in the loss of investor confidence in the reliability of our financial statements and negatively impact the trading price of our common shares, inhibiting our ability to raise sufficient capital on favorable terms. Furthermore, we have incurred and anticipate that we will continue to incur considerable costs and use significant management time and other resources in an effort to comply with Section 404 and other requirements of the Sarbanes-Oxley Act.
If we are unable to successfully compete in the highly competitive biopharmaceutical industry, our business could be harmed.
We operate in a highly competitive environment and we expect the competition to increase in the future. Our competitors include large pharmaceutical and biotechnology companies, both domestic and international. Many of these competitors have greater resources than we do. New competitors may also enter into the markets in which we compete. Accordingly, even if we are successful in launching a product, we may not be able to outperform a competing product for any number of reasons, including the possibility that the competitor may:
7
The technologies applied by our competitors and us are rapidly evolving and new developments frequently result in price competition and product obsolescence. In addition, we may be impacted by competition from generic forms of our products, substitute products or imports of products from lower-priced markets. For a detailed description of our competitors, please see “Item 4. Information on the Company — B. Business Overview — Competition.”
We may not be able to maintain market share in China with our commercialized vaccines, which could adversely affect our ability to increase our revenues.
According to the NMPA, there are approximately 50 vaccine manufacturers in China. Many of our commercialized vaccine products are also marketed by other vaccine companies in China, of which we believe approximately 15 are our direct competitors for our non-covid vaccines. We are market leaders for certain products in China. We also compete internationally against multi-national corporations for market shares of certain vaccine products. Our revenue could be adversely impacted if we are not able to maintain our supplied quantity and market share.
We may not be able to maintain market share in the government-funded hepatitis A vaccine market, or other government-funded vaccine markets, which could adversely affect our revenues, and if we do maintain or expand market share in these markets, we may need to sell our vaccines at a lower price, which could adversely affect our gross margin.
Hepatitis A vaccines have been included in the Expanded Program of Immunization (“EPI”) in China since 2007. The PRC government purchases hepatitis A vaccines for each 18-month-old child. Although the hepatitis A vaccines have been included in the EPI, most provincial and municipal governments are not able to afford the two shots of inactivated hepatitis A vaccines due to insufficient financial support, which constrains the purchase of inactivated hepatitis A vaccines in government-funded markets. Most provincial and municipal governments prefer to purchase lower-priced live attenuated hepatitis A vaccines; however, a few affluent provincial and municipal governments, such as Beijing, Tianjin, Shanghai and Jiangsu province, have started to purchase inactivated hepatitis A vaccines. We are supplying vaccines in these government-funded markets at a lower price than we do in the private market, which could adversely affect our gross margin. Our revenue could also be adversely impacted if we are not able to maintain our market share of the government-funded markets in these cities and provinces. As we are making efforts to breakthrough into additional provincial and municipal public markets, we may be forced to lower our prices to win tenders, which will adversely affect our gross margin.
Since 2007, we have been selected as one of the seasonal flu vaccine suppliers by Beijing CDC and by Zhejiang CDC, respectively. In 2022, we supplied flu vaccine to Tibet Autonomous Region CDC. However, we cannot assure that we will continue to obtain orders in the future and maintain these market share. If the supply volume decreases, it would negatively impact our sales revenue in the future.
Since 2008, we have received three stockpiling orders for our H5N1 vaccine from China’s central government every two years in an amount of three million doses per order, and four stockpiling orders from Beijing government in an amount of 20,000 doses per order. The latest batch of stockpiled H5N1 vaccines for the central government expired in the first half of 2016 and we recognized the revenue upon the government inspection. The most recent batch ordered by Beijing government expired in 2020. We cannot assure that we will receive additional stockpiling orders from governments in the future.
If CDCs, hospitals, CDC doctors and end users do not accept our products, we may be unable to generate significant revenue.
Even if we have obtained regulatory approvals for commercialization of our vaccines in China or in other countries or regions, they still may not gain market acceptance among CDCs, regulatory agencies, CDC doctors, end users, patients and the medical community, which would limit our ability to generate revenue and adversely affect our results of operations. CDCs, regulatory agencies and CDC doctors may not recommend products developed by us or our collaborators until clinical data or other factors demonstrate superior or comparable safety and efficacy of our products as compared to other available products. Even if the clinical safety and efficacy of our products are established, CDCs, regulatory agencies and CDC doctors may elect not to recommend these products for a variety of reasons. There are other vaccines and prevention options for the conditions that many of our products and product candidates target, such as EV71, hepatitis A, influenza, varicella, etc.. In order to
8
successfully launch a product, we must educate CDC doctors and end users about the relative benefits of our products. If our products are not perceived as easy and convenient to use, perceived to present a greater risk of side effects or are not perceived to be as effective as other available vaccines, CDCs, CDC doctors , parents and end users might not adopt our products. A failure of our products to gain commercial acceptance would have a material adverse effect on our business, financial condition and results of operations.
Our business could be negatively affected as a result of actions of shareholders or others.
On March 5, 2018, we announced the re-election of the members of our board of directors—Mr. Weidong Yin, Mr. Yuk Lam Lo, Mr. Simon Anderson, Mr. Kenneth Lee, and Mr. Meng Mei—at our annual general meeting of shareholders held on February 6, 2018 (the “2017 AGM”). We also announced that we had determined, after consultation with our Antigua legal counsel, that an alternative, pre-printed ballot not made available to all our shareholders and purportedly submitted at the 2017 AGM by certain of our shareholders, including 1Globe Capital LLC (“1Globe”), The Chiang Li Family, OrbiMed Advisors LLC and OrbiMed Capital LLC (together “OrbiMed”), and certain additional shareholders (collectively, the “Shareholder Group”) was invalid. We refer to this ballot as the “Non-Public Submission.” On March 13, 2018, 1Globe filed a complaint against Sinovac Antigua in the Eastern Caribbean Supreme Court in the High Court of Justice, Antigua and Barbuda (the “Antigua Court”). The complaint sought a declaration that the five persons purportedly proposed by the Shareholder Group on the Non-Public Submission at the 2017 AGM were elected as directors of Sinovac Antigua at that meeting, an order that those directors be installed as Sinovac Antigua’s board of directors, and a declaration that any actions taken on behalf of Sinovac Antigua at the direction of the board of directors since the 2017 AGM are null and void. Following a trial in early December 2018, the Antigua Court issued a judgment on December 19, 2018 (the “Antigua Judgment”) that dismissed 1Globe’s claim and declared that Sinovac Antigua’s shareholder rights agreement (the “Rights Agreement”) was validly adopted as a matter of Antigua law. 1Globe filed notice to appeal the Antigua Court’s judgment on January 29, 2019. 1Globe’s appeal of the Antigua Court’s Judgment was heard on September 18, 2019. On December 9, 2021, the Eastern Caribbean Supreme Court, Court of Appeal (the “Court of Appeal”), handed down its judgment, dismissing all grounds of appeal and upholding the Antigua Judgment, including confirming that Sinovac Antigua’s Rights Agreement was consistent with its Articles of Incorporation and By-laws, and Antiguan business law. 1Globe applied for leave to appeal to the Judicial Committee of the Privy Council (the “Privy Council”), and the hearing of the application was held on February 24, 2022, in which the Court of Appeal granted 1Globe leave to appeal to the Privy Council on certain grounds, although not including the challenge to the validity of the Rights Agreement. On April 19, 2022, 1Globe renewed its application directly to the Privy Council for leave to appeal on its ground of appeal concerning the validity of the Rights Agreement. On July 13, 2022, 1Globe filed its Notice of Appeal on those grounds on which the Court of Appeal had granted 1Globe leave to appeal. On September 16, 2022, 1Globe filed an application to the Privy Council seeking permission to amend its existing application for permission to appeal and its existing Notice of Appeal, and to seek permission to appeal on another ground rejected by the Court of Appeal concerning the exercise of the Antigua Court’s discretion. Sinovac responded on October 21, 2022. On February 15, 2023, the Privy Council made a procedural decision to allow amendment of its existing application for permission to appeal, and decided to deal with procedural and substantive issues together at the Final Hearing. 1Globe has not yet taken steps to list a substantive hearing before the Privy Council. The appeal outcome is therefore pending.
On October 8, 2018, we became aware that unauthorized documents in respect of Sinovac Biotech (Hong Kong) Limited. (“Sinovac Hong Kong”) had been filed with the Hong Kong Companies Registry to change the directors of Sinovac Hong Kong from Mr. Weidong Yin and Ms. Nan Wang to Mr. Jianzeng Cao and Mr. Pengfei Li. Mr. Yin and Ms. Wang commenced legal proceedings before the High Court of the Hong Kong Special Administrative Region (“Hong Kong High Court”) (“HCMP 1731/2018”). In a hearing before the Hong Kong High Court on October 19, 2018, the judge granted an interlocutory injunction restraining Mr. Li and Mr. Cao from purporting to act or holding themselves out as directors of Sinovac Hong Kong or its subsidiaries, purporting to take any actions as directors of Sinovac Antigua or its subsidiaries, and relying on or using the forged documents in any way whatsoever. On November 28, 2018 at a further hearing in the Hong Kong High Court, the Hong Kong High Court made orders (“November 28 Order”) and held that it is beyond dispute that the documents in respect of Sinovac Hong Kong had been forged and unlawfully filed with the Hong Kong Companies Registry, based on the evidence filed by Mr. Yin and Ms. Wang as Plaintiff, and Mr. Cao and Mr. Li as the Defendants. The Hong Kong High Court therefore declared that Mr. Yin and Ms. Wang were and still are the lawful directors of Sinovac Hong Kong (“Lawful Directors”), and Mr. Li and Mr. Cao were not and are not the lawful directors of Sinovac Hong Kong. The Hong Kong High Court also granted a permanent injunction restraining Mr. Li and Mr. Cao from purporting to act or holding themselves out as directors of Sinovac Hong Kong or its subsidiaries (including but not limited to Sinovac Biotech Co., Ltd. (“Sinovac Beijing”), purporting to take any actions as directors of Sinovac Hong Kong or its subsidiaries, and relying on or using the forged documents in any way whatsoever. Furthermore, the Hong Kong High Court also ordered the Companies Registry to remove the forged documents in respect of Sinovac Hong Kong that had been unlawfully filed. The November 28 Order is effective and enforceable. The Companies Registry has removed the forged documents following the November 28 Order. On November 28, 2018, Mr. Cao and Mr. Li filed a Notice of Appeal with the Hong Kong Court of Appeal, indicating their intention to appeal the orders made by the Hong Kong High Court. The appeal does not operate as a stay on the November 28 Order except to the extent that the Court below, or the Court of Appeal otherwise directs: O.59, r. 13 (1)(a) of the Rules of the High Court. As of the date of this annual report, neither the Court of First Instance nor the Court of Appeal directed that the execution of the November 28 Order should be stayed. So far, Mr. Cao and Mr. Li have taken no further steps in respect of the appeal after the Notice of Appeal was filed on November 28, 2018. No hearing date has yet been fixed to hear the appeal.
On October 8, 2018, we also became aware that unauthorized documents in respect of Sinovac Beijing had been filed with the Industry and Commerce Bureau of Haidian District of Beijing (“Haidian AIC”) to change the directors of Sinovac Beijing from Mr. Yin, Ms. Wang and Mr. Dawei Mao to Mr. Cao, Mr. Li and Ms. Xiaomin Yang. Mr. Yin and Ms. Wang filed objection to such unlawful change to the Haidian AIC. On March 19, 2020, Haidian AIC issued an official decision (“AIC Decision”) declaring that (i) the unauthorized documents filed are forged and fake documents; (ii) the filing of change of directors with the forged documents is null and void; (iii) the unlawful filing to change the directors
9
will be removed and the registration of Mr. Yin, Ms. Wang and Mr. Mao as directors of Sinovac Beijing will be restored. The parties of material interest concerned in the AIC Decision may raise objection or file a lawsuit within 60 days. No one has filed the objection or lawsuit against the AIC Decision within 60 days thereof.
On May 31, 2019, Heng Ren Investments LP (“Heng Ren”) filed suit against Sinovac and Mr. Yin for alleged breach of fiduciary duties and wrongful equity dilution, in Massachusetts state court. Sinovac moved the matter from state court to the United States District Court for the District of Massachusetts. Subsequently, on April 29, 2021, Heng Ren filed an amended complaint which alleged that Mr. Yin breached fiduciary duties owed to minority shareholders, that Sinovac aided and abetted breaches of fiduciary duties, and that both Sinovac and Mr. Yin engaged in wrongful equity dilution. Heng Ren requested damages, attorneys’ fees, and prejudgment interest. On September 14, 2020, Sinovac filed a motion to dismiss Heng Ren’s claims. In July 2021, Sinovac moved to dismiss Heng Ren’s amended complaint in the federal court in Massachusetts. On March 4, 2022, the court granted the motion as to the breach of fiduciary duty claims and denied the motion as to the wrongful equity dilution claim, and denied reconsideration of its decision on the motion. Sinovac has answered the complaint. On February 15, 2023, the court stayed discovery in the Heng Ren matter pending the resolution of an outstanding motion to dismiss filed in a purported shareholder's matter by us.
On December 5, 2022, a purported shareholder filed a putative class action complaint in United States District Court for the District of Massachusetts, asserting a claim under Section 204 of the Antigua and Barbuda International Business Corporations Act related to the PIPE transaction, alleging that all shareholders were harmed in an identical manner to one another by the PIPE transaction because the shares that were issued in the PIPE transaction allegedly undervalued Sinovac and all shareholders were purportedly wrongfully diluted as a result. The purported shareholder is represented by the same attorney who represents Heng Ren, and requests damages, attorneys’ fees, and prejudgment interest. On January 18, 2023, we filed a motion to dismiss. The motion was fully briefed as of March 9, 2023, and is currently pending before the court.
We cannot predict the outcome of our ongoing litigation, including whether we will prevail. We also cannot predict how the litigation may affect our share price, which could be volatile during the pendency of each suit and following its conclusion. Preparing for the litigation, or any related litigation or related matters, has caused us to incur significant costs and we expect these costs to continue until the litigation concludes. In addition, preparing for litigation is time-consuming and may disrupt our operations and divert the attention of management and our employees from executing our strategic plan. In addition, the uncertainties as to the composition of the board of directors of Sinovac Antigua may materially and adversely affect business in unpredictable ways, which, in turn, could cause our revenue, earnings and operating cash flows to be materially and adversely affected.
The ongoing litigation regarding the Rights Agreement could have a material adverse effect on the results of our operations and our financial condition.
On March 5, 2018, we filed a lawsuit in the Court of Chancery of the State of Delaware seeking a determination whether the Shareholder Group had triggered the Rights Agreement, by forming a group holding approximately 45% of outstanding shares, in excess of the Right Agreement’s threshold of 15%, and acting in concert prior to the 2017 AGM. The Rights Agreement is intended to promote the fair and equal treatment of all Sinovac shareholders and ensure that no person or group can gain control of Sinovac through undisclosed voting arrangements, open market accumulation or other tactics potentially disadvantaging the interest of all our shareholders.
On April 12, 2018, 1Globe filed an amended answer to our complaint, counterclaims, and a third-party complaint against Mr. Weidong Yin alleging, among other allegations, that the Rights Agreement is not valid, that Mr. Yin and the Buyer Consortium (comprising Mr. Yin, SAIF partners IV L.P., or SAIF, C-Bridge Healthcare Fund II, L.P., Advantech Capital L.P., Vivo Capital Fund VIII, L.P. and Vivo Capital Surplus Fund VIII, L.P.) had previously triggered the Rights Agreement, and that 1Globe did not trigger the Rights Agreement. The Chiang Li Family and OrbiMed filed similar responses. We, and our board of directors, believe that the actions taken by our board of directors were appropriate under the circumstances and in the interests of our company and all our shareholders. We also believe that the allegations in the counterclaim and third-party complaint are without merit. 1Globe asks for various measures of equitable relief and also includes a claim for its costs, including attorneys’ fees. On March 6, 2019, the Delaware Court entered a status quo order preventing us from distributing Exchange Shares (defined below) to any shareholders or otherwise take any action pursuant to the Rights Agreement until the conclusion of the Delaware litigation or Court order. The case is stayed pending resolution of parallel litigation in Antigua, which we anticipate will resume following the conclusion of the Antigua litigation.
Following a trial on the validity of the Sinovac Antigua’s Rights Agreement, on December 19, 2018, the Antigua Court held that Sinovac Antigua’s Rights Agreement is valid under Antigua law, and found that “there was a secret plan to take control of the Company” at the 2017 AGM by the Shareholder Group. On February 18, 2019, after reviewing the Court’s judgment and considering all additional facts known to the Board, the Board determined that the Shareholder Group, together with their affiliates and associates (collectively, the “Collaborating Shareholders”) became Acquiring Persons on or prior to the 2017 AGM and that their conduct resulted in a “Trigger Event” under Sinovac Antigua’s Rights Agreement. Pursuant to the Rights Agreement, our board of directors elected to exchange (the “Exchange”) each valid and outstanding preferred share purchase right held by Sinovac Antigua’s shareholders (not including the Collaborating Shareholders) for a combination of 0.655 of Sinovac Antigua’s common shares and 0.345 of Sinovac Antigua’s newly created Series B Convertible preferred shares (the “Series B Preferred Shares” and, together, each an “Exchange Share”). On February 22, 2019, the Exchange Shares were issued into the Shareholder 2019 Rights Exchange Trust in the name of Wilmington Trust, National Association, which holds the Exchange Shares for the benefit of Sinovac Antigua’s shareholders (not including the Collaborating Shareholders). 1Globe filed notice to appeal the Antigua Court’s judgment on January 29, 2019. On April 4, 2019, the Eastern Caribbean Supreme Court, Court of Appeal issued an order that restrains Sinovac Antigua from taking further action under its Rights Agreement, including the distribution of the previously issued Exchange Shares, until the
10
conclusion of such appeal. 1Globe’s appeal of the Antigua Court’s Judgment was heard on September 18, 2019. On December 9, 2021, the Court of Appeal handed down its judgment, dismissing all grounds of appeal and upholding the Antigua Judgment. The Court of Appeal also confirmed that Sinovac Antigua’s Rights Agreement was consistent with its Articles of Incorporation and By-laws, and Antiguan business law. 1Globe applied for leave to appeal to the Privy Council, and the hearing of the application was held on February 24, 2022, in which the Court of Appeal refused 1Globe’s application to take the issue of the Rights Agreement to the Privy Council. In January 2022, the Court of Appeal extended the order initially made on April 4, 2019, that restrains Sinovac Antigua from taking further action under its Rights Agreement, including the distribution of the previously issued Exchange Shares, until the conclusion of any appeal to the Privy Council. 1Globe applied for leave to appeal to the Judicial Committee of the Privy Council, and the hearing of the application was held on February 24, 2022, in which the Court of Appeal granted 1Globe leave to appeal to the Privy Council on certain grounds, although not including the challenge to the validity of the Rights Agreement. On April 19, 2022, 1Globe renewed its application directly to the Privy Council for leave to appeal on its ground of appeal concerning the validity of the Rights Agreement. On July 13, 2022, 1Globe filed its Notice of Appeal on those grounds on which the Court of Appeal had granted 1Globe leave to appeal. On September 16, 2022, 1Globe filed an application to the Privy Council seeking permission to amend its existing application for permission to appeal, and its existing Notice of Appeal, and to seek permission to appeal on another ground rejected by the Court of Appeal concerning the exercise of the Antigua Court’s discretion. Sinovac responded on October 21, 2022. On February 15, 2023, the Privy Council made a procedural decision to allow amendment of its existing application for permission to appeal, and decided to deal with procedural and substantive issues together at the Final Hearing. 1Globe has not yet taken steps to list a substantive hearing before the Privy Council. The appeal outcome is therefore pending.
We cannot predict the outcome of the litigation. Preparing for this litigation, or any related litigation or related matters, has caused us to incur significant costs and we expect these costs to continue until the litigation concludes. In addition, preparing for this litigation is time-consuming and may disrupt our operations and divert the attention of management and our employees from executing our strategic plan.
Our ongoing litigation against 1Globe and The Chiang Li Family claiming violations of U.S. federal securities laws could have a material adverse effect on the results of our operations and our financial condition.
On March 5, 2018, Sinovac Antigua filed a lawsuit in the United States District Court for Massachusetts alleging violations of Section 13(d) and Section 13(g) of the Securities Exchange Act of 1934 (the “Exchange Act”) by 1Globe and The Chiang Li Family. The lawsuit alleges, among other things, that the defendant shareholders failed to make required disclosures on Schedule 13D regarding their intentions to attempt to replace Sinovac Antigua’s board of directors. 1Globe counterclaimed to allege violations of securities laws; specifically, abuse of process, negligent misrepresentation, and fraudulent misrepresentation by Sinovac Antigua.
The litigation is currently stayed pending resolution of the parallel litigation in Antigua, and we cannot predict when or how the litigation will be resolved. There can be no assurance that we will prevail in this litigation. Preparing for this litigation, or any related litigation or related matters may result in significant costs to our company or otherwise adversely affect our business.
Disruptive actions taken by the minority shareholder of Sinovac Beijing caused suspension of production, destruction of products and disruption of our website, which may materially and adversely affect our business, financial condition and results of operations.
Sinovac Beijing, our principal operating subsidiary, is a Sino-foreign equity joint venture in which we own a 73.09% interest and Sinobioway Bio-medicine Co., Ltd., formerly named Xiamen Bioway Group Co., Ltd (“Sinobioway Medicine”), owns a 26.91% interest. Certain events suggest that Sinobioway Medicine’s interests are not aligned with our interests. We cannot assure that Sinobioway Medicine will be cooperative with us in handling matters related to the operations of Sinovac Beijing.
As the minority shareholder of Sinovac Beijing, according to Sinovac Beijing’s articles of association, Sinobioway Medicine has the right to assign a director to the five-director board of Sinovac Beijing, and the director assigned by Sinobioway Medicine is the legal representative of Sinovac Beijing. Accordingly, the representative of Sinobioway Medicine has the ability to take actions that bind Sinovac Beijing or to block any action that requires unanimous board approval. In addition, if we wish to transfer our equity interest in Sinovac Beijing, in whole or in part, to a third party, Sinobioway Medicine has a right of first refusal to purchase our interest in accordance with relevant PRC regulations.
Sinobioway Medicine, the minority shareholder of Sinovac Beijing, has additional rights under the joint venture contract and articles of association of Sinovac Beijing. The joint venture contract and articles of association require the consent of each of Sinovac Beijing’s shareholders and/or unanimous board approval on matters such as a major change in the business line of the company, expansion or amendment of the business scope of the company, transfer of the registered capital by a shareholder, creation of a mortgage or pledge upon the company’s assets, a change in the organizational form of the company and designation or removal of the general manager.
In February 2018, Mr. Aihua Pan, the representative of Sinobioway Medicine, sent letters without the approval of the full board of Sinovac Beijing, to Mr. Yin, Ms. Wang, and other senior managers of Sinovac Beijing purporting to terminate their employment. The board of directors of Sinovac Beijing subsequently determined, with the advice of PRC legal counsel, that this action did not conform with the joint venture contract and articles of association and was unlawful. On March 5, 2018, Sinovac Biotech announced actions taken to enhance the corporate governance and management of Sinovac Beijing, including the appointment of Mr. Dawei Mao, Chairman of Zhongke Biopharmaceutical Co., Ltd., as a director of Sinovac Beijing. He replaced Ms. Xiaomin Yang, then President of Sinobioway Group Co., Ltd. In addition, in March 2018, Mr. Yin, Ms. Wang, and other senior managers of Sinovac Beijing signed new employment agreements with Sinovac Biotech Ltd. and Sinovac Beijing.
11
On April 17, 2018, Mr. Aihua Pan and dozens of unidentified individuals forcibly entered Sinovac Beijing’s corporate offices and limited the physical movements of employees in Sinovac Beijing’s general manager’s office and finance department in an attempt to wrongfully take control of Sinovac Beijing’s official seal, legal documents, accounting seal, financial documents and financial information systems. In addition, these individuals disrupted Sinovac Beijing’s hepatitis A vaccine production and seasonal flu vaccine production by cutting power, seriously impacting Sinovac Beijing’s production and manufacturing processes and possibly damaging product quality. Due to these disruptive actions, Sinovac Beijing was forced to destroy the affected products. To maintain product safety, Sinovac Beijing temporarily suspended production at the impacted facility, though production has resumed at this facility months later. Sinovac Beijing was also forced to destroy the bacterial seeds intended for use in the production of its 23-valent pneumococcal polysaccharide vaccine (“PPV”) and to suspend all preparations for and ultimately postpone the inspection by NMPA, formerly known as the PRC State Food and Drug Administration, of the manufacturing site necessary for 23-valent PPV production approval.
On September 17, 2020, the Fourth Intermediate People’s Court of Beijing (“Beijing Fourth Court”) issued a judgment holding Sinobioway Medicine and Mr. Aihua Pan liable for torts and breaches of shareholders fiduciary duty under the PRC Company Law and liable for Sinovac Beijing’s losses of RMB15.4 million caused by their disruptive actions. Sinovac Beijing, Sinobioway Medicine and Mr. Aihua Pan filed notice to appeal to the Higher People’s Court of Beijing Municipality. The Higher People’s Court of Beijing Municipality held a hearing in September 2021. On October 31, 2022, the Higher People’s Court of Beijing Municipality issued a judgment in favor of Sinovac Hong Kong, upholding the judgment issued by the Beijing Fourth Court and ruling that Shandong Sinobioway Biomedicine, as the sole shareholder of Sinobioway Medicine, is jointly and severally liable for all the relevant obligations of Sinobioway Medicine to Sinovac Hong Kong.
On November 15, 2021, Sinobioway Medicine filed a complaint against Sinovac Beijing and Sinovac Hong Kong in Beijing Fourth Court. The complaint sought to dissolve and liquidate Sinovac Beijing with the argument that the board of directors of Sinovac Beijing has been unable to function for the benefit of the company and the two shareholders of Sinovac Beijing have gotten into a deadlock. In December 2022, Sinobioway Medicine filed a request to the Beijing Fourth Court to voluntarily withdraw the case. The Beijing Fourth Court supported such voluntary withdrawal and made an official ruling to dismiss the case on January 20, 2023.
In November 2021, Sinobioway Medicine filed a complaint against Sinovac Life Sciences Co., Ltd. (“Sinovac LS”, formerly known as Sinovac Research and Development Co., Ltd.), Sinovac Hong Kong, Mr. Weidong Yin and Keding Investment (Hong Kong) Limited with Beijing Fourth Court, claiming that Sinovac LS has infringed the legitimate rights of Sinovac Beijing when doing the research and development of CoronaVac. Sinobioway Medicine listed Sinovac Beijing as a third party in the case. On March 13, 2023, Beijing Fourth Court notified us by telephone that Sinobioway Medicine had just filed a request to the Beijing Fourth Court to voluntarily withdraw the case. The Beijing Fourth Court supported such voluntary withdrawal and is in the process of making an official ruling to dismiss the case.
On February 13, 2023, Shandong Sinobioway Biomedicine filed a complaint against Sinobioway Medicine and Sinovac Beijing with the People’s Court of Zhangdian District, Zibo Municipality (“Zhangdian District Court”), requesting the court to rule and confirm that Shandong Sinobioway Biomedicine, instead of Sinobioway Medicine, should be the shareholder of Sinovac Beijing despite that Sinobioway Medicine has been and is currently registered as the shareholder of Sinovac Beijing with the company registrar. Shandong Sinobioway Biomedicine also requested Zhangdian District Court to rule and order Sinovac Beijing to correct the registration of Shandong Sinobioway Biomedicine as the shareholder of Sinovac Beijing, Mr. Jialin Yue as the director, Chairman and Legal Representative of Sinovac Beijing and Mr. Weining Luan as the supervisor of Sinovac Beijing with the company registrar. In response to this newly filed case, on February 24, 2023, Sinovac Beijing filed an objection to the jurisdiction of Zhangdian District Court to hear this case. On March 12, 2023, Zhangdian District Court ruled that it has the jurisdiction to hear this case. On March 24, 2023, Sinovac Beijing filed an appeal to the Intermediate People’s Court of Zibo Municipality (“Zibo Intermediate Court”), in objection to the ruling made by Zhangdian District Court regarding its jurisdiction to hear the case. As of the date of this annual report, the case is pending with the final ruling to be made by Zibo Intermediate Court on the jurisdiction of Zhangdian District Court.
These and other actions taken by Sinobioway Medicine and its representatives may materially and adversely affect our business, financial condition and results of operations. We also cannot assure you that Sinobioway Medicine and its representatives will cease from interfering with our business.
We do not currently intend to hold an annual general meeting of shareholders until after the final determination of the litigation concerning the Rights Agreement, which will delay the ability of our shareholders to vote in an election of our directors.
With the ongoing litigations concerning the Exchange and the Rights Agreement, we have not been able to hold an annual meeting of shareholders since February 2018, and will not be able to hold an annual meeting of shareholders before the final determination of such litigations. Therefore, our shareholders will not have the opportunity to vote in an election of our directors for an indeterminate amount of time. If our shareholders want us to hold an annual meeting prior to the final determination of these ongoing litigations, they may attempt to force us to hold one under Antigua law.
The interests of the minority shareholder of Sinovac Beijing, Sinovac LS and Sinovac Dalian may diverge from our own, which may adversely affect our ability to manage these subsidiaries.
We are the majority shareholder of and have equity interests in Sinovac Beijing, Sinovac LS and Sinovac (Dalian) Vaccine Technology Co., Ltd. (“Sinovac Dalian”). If our interests diverge from those of our minority shareholders, they may exercise their rights under the relevant articles of association, shareholder’s agreement or joint venture contracts of each of such subsidiaries and the relevant PRC laws to protect their own
12
interests, which may substantially differ from ours. As a result, our ability to manage these subsidiaries as well as their own subsidiaries and affiliates may be adversely affected, which in turn may materially and adversely affect our business, financial condition and results of operations.
Recent disruptive actions taken by Sinobioway Medicine, a minority shareholder of Sinovac Beijing, has shown that its interests are not aligned with ours. We cannot assure that Sinobioway Medicine will be cooperative in handling matters related to the operations of Sinovac Beijing in the future.
As of the date of this annual report, Dalian Jin Gang Group, a minority shareholder of Sinovac Dalian, has been cooperating with us with respect to the business of Sinovac Dalian, and the minority shareholders of Sinovac LS have been aligned with us with respect to the business of Sinovac LS. We cannot assure, however, that these minority shareholders will continue to act in a cooperative manner in the future.
Our growth may be adversely affected if market demand for our vaccine products and product candidates does not meet our expectations. We may encounter problems of inadequate supply or oversupply, which would materially and adversely affect our financial condition and results of operations and would also damage our reputation and brand.
The production of vaccine products is a lengthy and complex process. As a result, our inability to match our production to market demand may result in a failure to meet market demand, which could materially and adversely affect our financial condition and results of operations and could also damage our reputation and corporate brand. For example, many patients receive their seasonal flu vaccinations in the three-month period from September to November in anticipation of an upcoming flu season and we expect this period to be one of the most significant sales periods for this product each year. In anticipation of the flu season, we intend to build up inventory of our influenza vaccine product in line with what we believe will be the anticipated demand for the product. If actual demand does not meet our expectations, we may be required to write off significant inventory and may otherwise experience adverse consequences in our financial condition. If we overestimate demand, we may purchase more raw materials than required. If we underestimate demand, our third-party suppliers may have inadequate raw material inventories, which could interrupt our manufacturing, delay shipments and result in lost sales.
If we are unable to enroll sufficient subjects and identify clinical investigators for our clinical trials, our development programs could be delayed or terminated.
The rate of completion of our clinical trials significantly depends on the rate of enrollment of volunteers. Patients’ enrollment is a function of many factors, including:
We may have difficulty in obtaining sufficient volunteer subjects’ enrollment or finding qualified investigators to conduct the clinical trials as planned and we may need to expend substantial funds to obtain access to resources or delay or modify our plans significantly. These considerations may lead us to consider the termination of development of a product for a particular indication.
A setback in any of our clinical trials could adversely affect our share price.
Clinical trials are an important part of vaccine research before any vaccine is approved for commercial use in humans. Setbacks in any phase of the clinical trials of our product candidates could have a material adverse effect on our business and prospects and financial results and would likely cause a decline in the price of our common shares. We may not achieve our projected development goals in the time frames we announce and expect. If we fail to achieve one or more milestones as contemplated, the market price of our common shares could decline.
We set goals for, and make public statements regarding, our anticipated timing of the accomplishment of objectives material to our success, such as the commencement and completion of clinical trials and other milestones. The actual timing of these events can vary significantly due to factors such as delays or failures in our clinical trials, the uncertainties inherent in the regulatory approval process and delays in achieving manufacturing or marketing arrangements sufficient to commercialize our products. We may not complete our clinical trials or make regulatory
13
submissions or receive regulatory approvals as planned. Also, we may not be able to adhere to our anticipated schedule for the launch of any of our products. If we fail to achieve one or more milestones as contemplated, the market price of our shares could decline.
We rely on third parties to conduct clinical trials, who may not perform their duties satisfactorily.
After we obtain approval to conduct clinical trials for our product candidates, we rely on qualified research organizations, medical institutions and clinical investigators to enroll qualified patients and conduct clinical trials. Our reliance on these third parties for clinical development activities reduces our control over the clinical trial process. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not fulfill their contractual obligations, including failing to meet expected deadlines, we may not succeed or may experience delays in our efforts to obtain regulatory approvals and commercialize our vaccine candidates.
If any of our third-party suppliers or manufacturers cannot adequately meet our needs, our business could be harmed.
While we use raw materials and other key material supplies that are generally available from multiple commercial sources, certain raw materials may be in short supply or difficult for suppliers to produce in accordance with our specifications. Certain raw materials are from single-sourced suppliers, while we are expanding our supplier portfolio for supply alternatives, however, any efforts to substitute key materials from an alternate source may be delayed as they may require regulatory approval. Moreover, regulatory approvals to market our products may be conditioned upon obtaining certain materials from specified sources. The COVID-19 pandemic has also created a sudden surge in material used in COVID-19 vaccine and medical supplies. If third-party suppliers were to cease production or otherwise fail to supply us with quality raw materials, and if we were unable to contract on acceptable terms for these materials with alternative suppliers, our ability to deliver our products to the market would be adversely affected.
In addition, if we fail to secure supply sources for some of the raw materials we use, our business could be harmed. For example, we sourced hepatitis B antigens entirely from Beijing Institute of Biological Products Co., Ltd. (“Beijing Biological”) for Bilive production. Although we are developing our own hepatitis B vaccine, before it is approved to be commercialized, we have to rely on the supplier to receive hepatitis B antigen. We and Beijing Biological agreed to enter into annual hepatitis B antigens supply agreements after our previous ten-year exclusive supply framework agreement expired in October 2012. Beijing Biological supplied hepatitis B antigens to us from July 2013 to June 2015 based on the annual supply agreement. Thereafter, Beijing Biological ceased its hepatitis B antigens production due to facilities renovation. We are working closely with Beijing Biological to resume production of Bilive.
Our business is highly seasonal. This seasonality will contribute to our operating results fluctuating considerably throughout the year.
The seasonality in our business is expected to result in significant quarterly fluctuations in our ongoing operating results. For example, the influenza season generally runs from November through March of the next year and the largest percentage of influenza vaccinations is administered between September and November of each year. As a result, we expect to realize most of our annual revenues from influenza vaccines during this period.
We rely on a limited number of facilities for the manufacturing of our products in accordance with relevant regulatory requirements. Any disruption to our existing manufacturing facilities or in the development of new facilities could reduce or restrict our sales and harm our reputation.
According to the China GMP guidelines, each vaccine product can only be produced in a dedicated production facility. In Beijing, we conduct the primary production of each vaccine in dedicated production plants at our Shangdi site, Changping site and Daxing site, and secondary filling and packaging at our Changping and Daxing site. In Dalian, we manufacture mumps and varicella vaccine at one facility. Though we have been constructing additional manufacturing facilities for our products, we do not maintain back-up primary production facilities for our currently available products, so we are dependent on our existing facilities for the continued operation of our business.
As described more fully above, a representative of Sinobioway Medicine, who was the Chairman of the board of directors of Sinovac Beijing, and dozens of unidentified individuals forcibly entered Sinovac Beijing’s corporate offices and disrupted Sinovac Beijing’s hepatitis A vaccine production and seasonal flu vaccine production by cutting power to our Shangdi site, seriously impacting Sinovac Beijing’s production and manufacturing processes and possibly damaging product quality. Due to the actions of the representative of Sinobioway Medicine, Sinovac Beijing was forced to destroy the affected products. To maintain product safety, Sinovac Beijing temporarily decided to stop production at the impacted facility, though production subsequently resumed.
Natural disasters or other unanticipated catastrophic events, including power interruptions, water shortages, storms, fires, earthquakes and terrorist attacks, could significantly impair our ability to manufacture products and operate business and could also delay our research and development activities. Our facilities and certain equipment located in these facilities would be difficult to replace and could require substantial replacement lead-time. Catastrophic events may also destroy any inventory located in our facilities.
14
We do not maintain any business interruption insurance to cover lost income as a result of any such events. The occurrence of such events could materially and adversely affect our business. We may keep building additional manufacturing facilities in the future. There can be no assurance, however, that we will be able to expand our manufacturing capabilities to or realize the anticipated benefits of our new facilities. Any of these factors could reduce or restrict our sales, harm our reputation and have a material adverse effect on our business, financial condition, results of operations and prospects.
We may need additional capital to upgrade or expand our production capabilities, to continue development of our product pipeline and to market existing and future products on a large scale. We cannot guarantee that we will find adequate sources of capital in the future.
In the future, we may need to raise additional funds to finance equipment expenditures, to acquire intellectual property, to further expand the production facility for our pipeline products, to continue the development and commercialization of our product candidates and to fund other corporate purposes. As of December 31, 2022, we had approximately $4,286 million in cash and cash equivalents and restricted cash. We may undertake significant future financings in order to:
In the past, we funded most of our research and development and other expenditures through government grants, working capital, bank loans and proceeds from private placements and public offerings of our common shares. We may raise additional funds in the future because our current operating and capital resources may be insufficient to meet future requirements.
Sinovac Antigua is authorized to issue 100,000,000 common shares, 99,502,243 of which are issued and outstanding. To increase the number of authorized common shares, we must amend Sinovac Antigua’s Articles of Incorporation and By-laws, which requires (i) the majority of common shares be present for a quorum, and (ii) affirmative vote of two thirds of common shares (excluding Series B Preferred Shares) present and voting at the general meeting. We cannot assure that Sinovac Antigua will be able to collect sufficient affirmative votes to amend its Articles of Incorporation and By-laws. If we fail to increase the number of authorized commons shares of Sinovac Antigua, we will lack common shares for future issuance of equity securities.
If we raise additional funds by issuing equity securities, it will result in further dilution to our existing shareholders because the shares may be sold when the market price is low and shares issued in equity financing transactions will normally be sold at a discount to the current market price. Any additional equity securities issued also may provide for rights, preferences or privileges senior or otherwise preferential to those of holders of our existing common shares. Unforeseen problems including materially negative developments relating to, among other things, disease developments, product sales, new product rollouts, clinical trials, research and development programs, our strategic relationships, our intellectual property, litigation, regulatory changes in our industry, the Chinese market generally or general economic conditions, could interfere with our ability to raise additional funds or materially and adversely affect the terms upon which such funding is available.
If we raise additional funds by issuing debt securities, these debt securities would have rights, preferences and privileges senior to those of holders of our common shares, and the terms of the debt securities issued could impose significant restrictions on our operations. If we raise additional funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to certain of our technologies, marketing territories, product candidates or products that we would otherwise seek to develop or commercialize ourselves, or be required to grant licenses on terms that are not favorable to us. In the past, we have received different types of grants from the PRC government to finance the research and development and facility investment of our vaccine products. We may not receive additional grants in the future.
As described above, the actions of the Shareholder Group leading up to and at the 2017 AGM resulted in uncertainties as to the future direction of our company and the composition of our board of directors. As a result of these uncertainties, we do not know whether additional financing will be available to us on commercially acceptable terms when needed. If adequate funds are not available or are not available on commercially acceptable terms, we may be unable to continue developing our products. In any such event, our ability to bring a product to market and earn revenues could be delayed and competitors could develop products sooner than we do. As a result, our business, financial condition and results of operations could be materially and adversely affected.
15
We issued approximately 27.8 million common shares and 14.6 million Series B Preferred Shares in connection with the Exchange, and could issue additional common shares or Series B Preferred Shares, or one or more additional series of preferred shares with the effect of diluting existing shareholders and impairing their voting and other rights
Our articles of incorporation authorize the issuance of up to 100,000,000 common shares and 50,000,000 preferred shares with designations, rights, privileges, restrictions and conditions as may be determined from time to time by our board of directors. On February 22, 2019, in connection with the Exchange, we issued approximately 27.8 million common shares and 14.6 million Series B Preferred Shares for the benefit of the holders of valid and outstanding Rights as of that date. This issuance had the effect of significantly diluting the holdings of the shareholders that are not entitled to participate in the Exchange.
The Series B Preferred Shares share equally in all dividends and distributions made on our common shares and vote together with the common shares on all matters brought before the shareholders, in each case on an as-converted basis and subject to applicable law. The Series B Preferred Shares are convertible into common shares at our option, or automatically upon a successful shareholder vote to increase the authorized number of Sinovac Antigua’s common shares. Until the Series B Preferred Shares are converted into common shares (or until the Series B Preferred Shares are listed on a nationally recognized securities exchange), they will earn a preferred dividend equal to $0.41 per annum, payable quarterly in arrears. As a result of the ongoing litigation described elsewhere, there can be no assurance that this preferred share dividend will be paid in a timely manner, if at all.
Our board is empowered, without shareholder approval, to issue one or more additional series of preferred shares with dividend, liquidation, conversion, voting or other rights which could dilute the interest of, or impair the voting power of, our common shareholders. The issuance of such additional series of preferred shares, or the issuance of additional common shares, could be used as a method of discouraging, delaying or preventing a change in control.
The PIPE Investors (as defined below) may exercise influence over us, including through their ability to influence matters requiring the approval of holders of our Common Shares or Series A Preferred Shares.
On July 2, 2018, we completed a private placement of our common shares (the “PIPE”) with private investors Vivo Capital and Advantech Capital (the “PIPE Investors”), whereby we received gross proceeds of $86.73 million. The proceeds of this offering will be used to increase our capabilities in research relating to quality control and to build additional production facilities to support the development and commercialization of sabin inactivated polio vaccine (“sIPV”) -based combination vaccine and other new vaccine projects. These investments have not yet been made due in part to the disruptive actions of certain of our shareholders and the related litigation, which remains ongoing.
The shares owned by the PIPE Investors currently represent approximately 20.68% of the voting rights in respect of our share capital (after taking into account the shares issued in the Exchange under the Rights Agreement). Further, the PIPE Investors are entitled to appoint a designee and observer to Sinovac Antigua’s board of directors. Accordingly, the PIPE Investors may have the ability to influence the direction of Sinovac Antigua or the outcome of most matters submitted for the vote of our shareholders. In any of these matters, the interests of the PIPE Investors may differ from or conflict with the interests of our other shareholders.
In connection with the PIPE, Sinovac Antigua entered into a shareholders agreement with the PIPE Investors, pursuant to which the PIPE Investors agreed to vote their shares affirmatively in favor of all of the director designees nominated to serve on Sinovac Antigua’s board of directors, and the PIPE Investors agreed to transfer restrictions with respect to their shares and a standstill provision, which, among other things, bars each PIPE Investor and its affiliates from acquiring in excess of 10% of the share capital of Sinovac Antigua.
In addition, the PIPE Investors are in the business of making investments in companies and may, from time to time, acquire interests in businesses that directly or indirectly compete with our business, as well as businesses that are significant existing or potential customers.
If we are unable to attract, train, retain and motivate our third-party marketing agents in mainland China, sales of our products may be materially and adversely affected.
In mainland China, we rely on our third-party marketing agents, who are dispersed across the country, to market our products to CDCs and other healthcare institutions. We believe that our future success in the Chinese market will depend on the dedication, efforts and performance of our third-party marketing agents. There are only limited numbers of competent and qualified marketing agents in the China vaccine industry. Our competitors may provide commissions or other economic incentives to third-party marketing agents significantly above the market standard, which may cause such agents to cease marketing our products. If we are unable to attract, train, retain and motivate our marketing agents, sales of our products in the Chinese market may be materially and adversely affected.
Anti-corruption measures taken by the PRC government to correct corruptive practices in the vaccine industry could adversely affect our sales and reputation.
16
The PRC government has taken anti-corruption measures to correct corrupt practices. In the vaccine industry, such practices include, among others, acceptance of kickbacks, bribery or other illegal gains or benefits by the officials of CDCs in connection with recommendation of a certain vaccine. We do not control the business activities of our third-party marketing agents, who might engage in corrupt practices to promote our products, which may be unknown to us. While we maintain strict anti-corruption policies applicable to our internal sales force and third-party marketing agents, these policies may not be completely effective. If any individual of our sales staff or any of our third-party marketing agents engages in corrupt practices and the PRC government takes enforcement action, our own practices and the market agents’ practices may be checked or investigated. If this occurs, our sales and reputation may be materially and adversely affected.
Some of the predecessor shareholders of Sinovac Beijing were enterprises owning state-owned assets (“EOSAs”). Their failures to comply with PRC legal requirements in asset or share transfers could, under certain circumstances, result in such transfers being invalidated by government authorities. If this occurs, we could lose our ownership of intellectual property rights that are vital to our business as well as our equity ownership in Sinovac Beijing.
Sinovac Beijing is currently owned 73.09% by us and 26.91% by Sinobioway Medicine. The technologies related to our hepatitis A vaccine, hepatitis A and B vaccine and influenza vaccine that are vital to our business were directly or indirectly transferred to us by Tangshan Yian Biological Engineering Co., Ltd. (“Tangshan Yian”). Some of the predecessor shareholders of Sinovac Beijing, including Shenzhen Kexing Biological Engineering Ltd. (“Shenzhen Kexing”), Sinobioway Medicine, Tangshan Medicine Biotech Co., Ltd., Tangshan Yikang Biotech Co., Ltd. and Tangshan Yian, were EOSAs. Under applicable PRC laws, when EOSAs sell, transfer or assign assets or equity investments in their possession or under their control to third parties, they are required to obtain an independent appraisal of the transferred assets or shares and file such appraisal with or obtain approval of such appraisal from PRC government authorities. Since 2004, EOSAs have also been required to make such assets or equity transfers at government-designated marketplaces. Certain of our acquisitions of intellectual property rights and equity interests were subject to these requirements.
Tangshan Yian failed to file with the government authorities the appraisal of the hepatitis A vaccine technology that it transferred to Sinovac Beijing in 2001 as its capital contribution to Sinovac Beijing. Under PRC laws, Tangshan Yian also failed to:
These failures subject us to the risk of losing ownership or control of these vaccine technologies.
In addition, before we acquired our 73.09% equity interest in Sinovac Beijing, it had undergone multiple changes in its shareholders and the amounts held by its shareholders. Some of the EOSA shareholders of Sinovac Beijing have sold, transferred or assigned their respective equity interests in Sinovac Beijing without fully complying with laws to appraise the equity interests, to file such appraisals with or obtain regulatory approval of such appraisals from PRC government authorities or to make equity interest transfers at the government-designated marketplaces as required for transactions completed after 2004. Similar to the asset transfers, such failures subject us to the risk of losing the ownership or control of our equity interest in Sinovac Beijing.
PRC government authorities may take court actions to invalidate the transfers of the assets or equity investments discussed above for non-compliance with applicable appraisal, filing, approval and designated marketplace requirements. The government authorities could take such legal actions and such legal actions, if commenced, could be successful. If these transfers are invalidated, we would lose title to these assets and investments. Because we depend on these technologies and because Sinovac Beijing constitutes core part of our operations, our loss of these technologies or equity interest in Sinovac Beijing would materially and adversely affect our operations and financial condition.
The Rights Agreement and certain provisions of our By-laws may discourage a change of control.
In March 2016, we adopted the Rights Agreement that provides for the issuance of one right (a “Right”) for each of our outstanding common shares. We amended and restated the Rights Agreement in February 2019 that provides for the issuance of one Right for each of our outstanding common shares and Series B Preferred Shares. In February 2020, we further amended the amended and restated Rights Agreement to extend its term until February 2021. The Rights are designed to assure that all of our shareholders receive fair and equal treatment in the event of any proposed takeover and to guard against partial tender offers, open market accumulations, undisclosed voting arrangements and other abusive or coercive tactics to gain control of our company or our board of directors without paying all shareholders a control premium. The Rights will cause substantial dilution to a person or group that acquires 15% or more of the aggregate total of common shares and Series B Preferred Shares on terms not approved by our board of directors.
On December 9, 2021, the Court of Appeal handed down its judgment, dismissing all grounds of appeal and upholding the Antigua Judgment. The Court of Appeal also confirmed that Sinovac Antigua’s Rights Agreement was consistent with its Articles of Incorporation and By-laws, and Antiguan business law. 1Globe applied for leave to appeal to the Privy Council, and the hearing of the application was held on February 24, 2022, at which the Court of Appeal refused 1Globe’s application to take the issue of the validity of the Rights Agreement to the Privy Council,
17
but granted leave to appeal on certain other grounds. As described above, on April 21, 2022, 1Globe renewed its application to further appeal the judgment of the Court of Appeal that the Rights Agreement is valid, directly to the Privy Council. If 1Globe is successful in its appeal of this element of the judgment, our shareholders will not benefit from the protections of the Rights Agreement and our company may be subject to abusive or coercive tactics by certain shareholders to gain control of our company or our board of directors without paying all shareholders a control premium. On April 4, 2019, the Court of Appeal issued an order restraining our company from taking further action under the Rights Agreement, including the distribution of the previously issued Exchange Shares, until the conclusion of the appeal of the judgment. The parties have agreed a continuation of this interim injunction that restrains our company from taking further action under the Rights Agreement until the appeal process at the Privy Council is complete.
The Delaware litigation is stayed pending the resolution of the litigation in Antigua. The Delaware Court’s status quo order prevents us from distributing Exchange Shares to any shareholders or otherwise taking any action pursuant to the Rights Agreement until the conclusion of the Delaware litigation or Court order, which we anticipate will resume following the conclusion of the Antigua litigation.
On February 21, 2021, 2022 and 2023, we entered into the second, third and fourth amendments to the Rights Agreement, respectively, to extend the expiration date of the rights contained therein from February 22, 2021 to February 22, 2023 and further to February 22, 2024.
Some provisions of our By-laws may discourage, delay or prevent a change in control of our company or management that shareholders may consider favorable, including provisions that authorize our board of directors to issue preferred shares in one or more series and to designate the price, rights, preferences, privileges and restrictions of such preferred shares without any further vote or action by our shareholders.
These provisions could make it more difficult for a third party to acquire us, even if the third party’s offer may be considered beneficial by many shareholders. As a result, shareholders may be limited in their ability to obtain a premium for their shares.
We depend on our key personnel, the loss of whom would adversely affect our operations. If we fail to attract and retain the talent required for our business, our business will be materially harmed.
We had 3,558 full-time employees as of December 31, 2022 and we depend to a great extent on principal members of our management and scientific teams. If we lose the services of any key personnel, in particular Mr. Weidong Yin, the loss could significantly impede the key decision making on strategic choices and operational issues, which in turn will harm our business achievement. We do not have any key man life insurance policies. We have entered into employment agreements with our executive officers, under which they have agreed to restrictive covenants relating to non-competition and non-solicitation. These employment agreements do not, however, guarantee that we will be able to retain the services of all our executive officers in the future.
As described above, a representative of Sinobioway Medicine, who was the Chairman of the board of directors of Sinovac Beijing, sent letters without the approval of the full board of Sinovac Beijing, to Mr. Yin, Ms. Nan Wang, and other senior managers of Sinovac Beijing purporting to terminate their employment. The board of directors of Sinovac Beijing subsequently determined, with the advice of PRC legal counsel, that this action did not conform with the joint venture contract and the articles of association of Sinovac Beijing and was unlawful. As also described above, the representative of Sinobioway Medicine and dozens of unidentified individuals forcibly entered Sinovac Beijing’s corporate offices and limited the physical movements of employees in Sinovac Beijing’s general manager’s office and finance department in an attempt to wrongfully take control of Sinovac Beijing’s official seal, legal documents, accounting seal, financial documents and financial information systems. As a result of these actions, our ability to attract and retain the talent required for our business may be materially harmed.
In addition, recruiting and retaining additional qualified scientific, technical and managerial personnel and research partners will be critical to our success. Competition among biopharmaceutical and biotechnology companies for qualified employees in China is intense and turnover rates are high. There is a shortage of employees in China with expertise in our areas of research and clinical and regulatory affairs, and this shortage is likely to continue. In addition, we have a limited number of shares available for issuance under our share incentive award plan, which may affect our ability to retain and motivate our employees. We may not be able to retain existing personnel or attract and retain qualified staff in the future. If we fail to hire and retain personnel in key positions, we may be unable to develop or commercialize our product candidates in a timely manner.
We may encounter difficulties in managing our growth, which could adversely affect our results of operations.
We have experienced rapid and substantial growth and, if such growth continues, will place a strain on our administrative and operational infrastructure. We also plan to introduce new products to market that, if successful, could place a strain on our administrative and operational infrastructure. If we are unable to manage this growth effectively, our business, results of operations or financial condition may be materially and adversely affected. Our ability to manage our operations and growth effectively requires us to continue to improve our operational, financial and management controls, reporting systems and procedures and hiring programs. We may not be able to successfully implement these required improvements.
International expansion may be costly, time-consuming and difficult. If we do not successfully expand internationally, our growth strategy and prospects would be materially and adversely affected.
18
We have entered into certain selected international markets and intend to continue to expand the sales of our products into new international markets. In expanding our business internationally, we have entered, and intend to continue to enter, markets in which we have limited or no experience and in which our brand may be less recognized. To promote our brand and generate demand for our products to attract distributors in international markets, we expect to spend significantly more on marketing and promotion than we do in our existing domestic markets when appropriate. We may be unable to attract a sufficient number of distributors, and our selected distributors may not be suitable for selling our products.
In new markets, we may fail to anticipate competitive conditions that are different from those in our existing markets. These competitive conditions may make it difficult or impossible for us to effectively operate in these markets. If our expansion efforts in existing and new internal markets are unsuccessful, our growth strategy and prospects would be materially and adversely affected.
We are exposed to other risks associated with international operations, including:
We may undertake acquisitions which may have a material adverse effect on our ability to manage our business and may end up being unsuccessful.
Our growth strategy may involve the acquisition of new production lines, technologies, businesses, products or services or the creation of strategic alliances in areas in which we do not currently operate. These acquisitions and strategic alliances could require that our management develop expertise in new areas or new geographies, manage new business relationships and attract new types of customers. Furthermore, acquisitions may require significant attention from our management, and the diversion of our management’s attention and resources could have a material adverse effect on our ability to manage our business. We may experience difficulties integrating acquisitions into our existing business and operations. Future acquisitions may also expose us to potential risks, including risks associated with:
We may be unable to ensure compliance with United States economic sanctions laws, especially when we sell our products to distributors over which we have limited control.
19
The U.S. Department of the Treasury’s Office of Foreign Assets Control administers certain laws and regulations that impose penalties upon U.S. persons and, in some instances, foreign entities owned or controlled by U.S. persons, for conducting activities or transacting business with certain countries, governments, entities or individuals subject to U.S. economic sanctions (“U.S. Economic Sanctions Laws”). We will not use any proceeds, directly or indirectly, from sales of our common shares, to fund any activities or business with any country, government, entity or individual with respect to which U.S. persons or, as appropriate, foreign entities owned or controlled by U.S. persons, are prohibited by U.S. Economic Sanctions Laws from conducting such activities or transacting such business.
However, we sell our products in international markets through independent non-U.S. distributors which are responsible for interacting with the end-users of our products. We may not be able to ensure that such non-U.S. distributors fully comply with all applicable U.S. Economic Sanctions Laws. As a result of the foregoing, actions could be taken against us that could materially and adversely affect our reputation and have a material adverse effect on our business, financial condition, results of operations and prospects.
We may be classified as a passive foreign investment company, which could result in adverse U.S. federal income tax consequences to U.S. Holders of our common shares.
Based on our estimates of the fair market value of our assets (subject to the discussion below) as well as the composition of our income and assets, we do not believe we were a “passive foreign investment company” (“PFIC”), for U.S. federal income tax purposes for our taxable year ended December 31, 2022. However, the application of the PFIC rules is subject to uncertainty in several respects, and we cannot assure that we will not be a PFIC for any taxable year. In general, a non-U.S. corporation will be a PFIC for any taxable year if either (i) at least 75% of its gross income for such year is passive income or (ii) at least 50% of the value of its assets (generally based on a quarterly average) during such year is attributable to assets that produce passive income or are held for the production of passive income. We must make a separate determination after the close of each taxable year as to whether we were a PFIC for that year. In particular, under normal circumstances, the value of our assets for purposes of the PFIC test for a particular taxable year would generally be determined by reference to the market price of our common shares at the end of each quarter during such taxable year, and fluctuations in such market price (or changes in the composition of our income or assets) could cause us to become a PFIC for any subsequent year. However, as a result of the suspension of trading in our shares, we are unable to reference the actual market prices of our common shares in determining our PFIC status. As a result, we have based our determination of the fair market value of our assets for purposes of the PFIC determination on our estimated enterprise value, which we estimated by reference to our earnings per share and number of outstanding shares, and a comparison of such earnings per share to the earnings per share of certain other companies in industries similar to ours that have shares listed on a U.S. stock exchange. We cannot provide any assurances that the actual value of our shares is not materially different on the applicable measurement dates from such estimated value or as to whether the U.S. Internal Revenue Service will respect our approach. This uncertainty will continue so long as trading in our shares remains suspended. In addition, the composition of our income and assets will be affected by how, and how quickly, we use the cash we generate from our operations or raise in any offering. If we are a PFIC for any year during which a U.S. Holder (as defined in “Item 10. Additional Information — E. Taxation — United States Federal Income Taxation”) holds our common shares, additional reporting requirements and certain adverse U.S. federal income tax consequences could apply to such U.S. Holder. Please see “Item 10. Additional Information — E. Taxation — United States Federal Income Taxation — Passive Foreign Investment Company.”
If we were deemed to be an investment company under the U.S. Investment Company Act of 1940, as amended (the “1940 Act”), applicable restrictions could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business, financial condition and results of operations.
Under Sections 3(a)(1)(A) and (C) of the 1940 Act, a company generally will be deemed to be an “investment company” for purposes of the 1940 Act if (1) it is, or holds itself out as being, engaged primarily, or proposes to engage primarily, in the business of investing, reinvesting or trading in securities or (2) it engages, or proposes to engage, in the business of investing, reinvesting, owning, holding or trading in securities and it owns or proposes to acquire investment securities having a value exceeding 40% of the value of its total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis. We intend to conduct our operations so that we will not be deemed an investment company. However, if we were to be deemed an investment company, restrictions imposed by the 1940 Act, including limitations on our capital structure and our ability to transact with affiliates, could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business, financial condition and results of operations.
Negative publicity regarding vaccinations in China may lead to lower demand for vaccination, which could in turn negatively affect our business, financial condition and results of operations.
In December 2013, it was reported that several infants died shortly after receiving inoculations of hepatitis B vaccine produced by a domestic company in China. NMPA and National Health and Family Planning Commission have determined that the inoculated hepatitis B vaccines comply with the applicable regulatory standards. In March 2016, media reported on improperly stored vaccines illegally sold in Shandong province and all across China. The illegal distribution started in 2010 and two suspects were detained by police in 2015. Although experts from the WHO have confidence in China’s vaccine industry and publicly clarified their position several times since news of this scandal broke, public concerns remain. In July 2018, Changchun Changsheng Life Science Co., Ltd. was found by the government to have falsified production records.
20
Although the government has determined to levy a $1.3 billion fine on the company, such negative publicity has led to lower demand for vaccination in China in 2018, which has in turn negatively affected the whole vaccine industry.
As a foreign private issuer, we are subject to different U.S. securities laws and NASDAQ listing rules than domestic U.S. issuers.
As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, as an Antigua and Barbuda company listed on the NASDAQ Global Select Market, we are subject to NASDAQ’s corporate governance requirements. However, NASDAQ listing rules permit a foreign private issuer like us to elect to follow home country corporate governance practices in lieu of certain NASDAQ corporate governance standards, subject to certain conditions. Certain corporate governance practices in Antigua and Barbuda, which is our home country, may differ significantly from the NASDAQ standards. As a result of our status as a foreign private issuer, you may not be afforded the same information or protections that would be made available to you were you investing in a domestic U.S. issuer.
Trading of our common shares on NASDAQ has been halted since February 22, 2019.
In connection with the Exchange and the issuance of the Exchange Shares into the Shareholder 2019 Rights Exchange Trust, NASDAQ implemented a halt in trading in Sinovac Antigua’s common shares in order to facilitate the orderly distribution of the Exchange Shares. In light of the ongoing litigation concerning the Rights Agreement, there can be no assurance when or if this halt will be lifted. NASDAQ has continued listing standards that we must maintain on an ongoing basis in order to continue the listing of our common shares. If NASDAQ determines that we fail to meet these continued listing requirements, our common shares may be subject to delisting.
If our common shares are delisted and we are not able to list our common shares on another national securities exchange, we expect our securities would be quoted on an over-the-counter market. If this were to occur, our shareholders could face significant material adverse consequences, including limited availability of market quotations for our securities and reduced liquidity for the trading of our securities. In addition, we could experience a decreased ability to issue additional securities and obtain additional financing in the future.
Risks Related to Government Regulation
We may not be able to comply with applicable GMP standards and other regulatory requirements, which could have a material adverse effect on our business, financial condition and results of operations.
We are required to comply with applicable GMP regulations, which include, among other things, requirements relating to personnel, premises and equipment, raw material and products, qualification and validation, document management, production management, quality control and assurance and product distribution and recall. Manufacturing facilities must be approved by governmental authorities before they can be used to commercially manufacture our products and are subject to inspection by regulatory agencies. We had been required to comply with the new GMP standards implemented by NMPA since March 1, 2011 and all vaccine manufacturers were required to meet the new GMP standards and obtain certifications for their manufacturing facilities by December 31, 2013. Any manufacturer that failed to meet the deadline were forced to suspend production.
We have obtained the new GMP certificates for all of our commercial production facilities. However, we cannot assure that we will be able to continue to meet the applicable GMP standards and other regulatory requirements in the future.
In addition, in light of the incident where vaccines were illegally sold and distributed in Shandong province and other provinces around China in 2016, the government has changed policies and regulations related to the vaccine sales and distribution in China. Before the policy was issued, human vaccine sales were halted in China for months. The vaccine purchase and delivery were resumed in second half of 2016. We are not able to estimate whether there will be any other change of policies and regulations on our business in the future, which will negatively impact on business in the future.
The 2020 Chinese Pharmacopoeia came into effect on December 30, 2020. We have made a thorough assessment on the 2020 Chinese Pharmacopeia and updated our operation procedures according to the new regulatory requirements to ensure full compliance.
If we fail to comply with applicable regulatory requirements at any stage during the regulatory process, including following any product approval, we may be subject to sanctions, including:
21
We can only sell products that have received regulatory approvals. Many factors affect our ability to obtain such approvals.
Pre-clinical and clinical trials of our products, and the manufacturing and marketing of our products, are subject to extensive, costly and rigorous regulation by governmental authorities in the PRC and in other countries. Even if we complete pre-clinical and clinical trials successfully, we may not be able to obtain applicable regulatory approvals. We cannot market any product candidate until we have both completed our clinical trials and obtained the necessary regulatory approvals for that product candidate.
Conducting clinical trials and obtaining regulatory approvals are uncertain, time-consuming and expensive processes. The process of obtaining required regulatory approvals from NMPA and other regulatory authorities often takes many years and can vary significantly based on the type, complexity and novelty of the product candidates. For example, it took us approximately ten years to develop and obtain regulatory approval to commercialize Healive, and it took us five and a half years and four and a half years to develop and obtain regulatory approvals to commercialize Bilive and Anflu, respectively. EV71 vaccine took us eight years from 2008 to 2016 to develop and obtain regulatory approvals. Delays in obtaining NMPA approvals of our products could result in substantial additional costs and adversely affect our ability to compete with other companies. Even if regulatory approval is ultimately granted, we may not maintain the approval and the approval may be withdrawn. Any approval received may also restrict the intended use and marketing of the product we want to commercialize.
There can be no assurance that all of the clinical trials pertaining to our vaccines in development will be completed within the timeframes currently anticipated by us. We could encounter difficulties in enrolling patients for clinical trials or encounter setbacks while conducting clinical trials that result in delays or cancellation. Data obtained from pre-clinical and clinical studies are subject to varying interpretations that could delay, limit or prevent regulatory approvals, and failure to observe regulatory requirements or inadequate manufacturing processes are examples of other problems that could prevent approvals. In addition, we may encounter delays or rejections in the event of additional regulation from future legislation, administrative action or changes in the NMPA policy or if unforeseen health risks become an issue with the participants of clinical trials.
Clinical trials may fail at any stage. Results of early trials frequently do not predict results of later trials, and acceptable results in early trials may not be repeated. For these reasons, we do not know whether regulatory authorities will grant approval for any of our product candidates in the future. In addition, production permits for our products are valid for five years and we need to apply for renewal six months prior to their expiration. The process to approve our renewal applications could be lengthy and there is no assurance that we will be granted renewal in a timely manner or at all.
Outside the PRC, our ability to market some of our potential products is contingent upon receiving marketing authorizations from the appropriate foreign regulatory authorities. For example, our hepatitis A vaccine, Healive, can be supplied to certain international organizations and is eligible to participate into the tender process in some countries as it has passed the WHO prequalification assessment (“WHO PQ”). However, there are still countries that require additional marketing authorization to sell in such countries despite the WHO PQ status. These foreign regulatory approval processes include risks similar to those associated with the NMPA approval process as described above and may include additional risks.
Because the medical conditions that our vaccines are intended to prevent represent significant public health threats, we are at risk of governmental actions detrimental to our business, such as product seizure, compulsory licensing and additional regulations.
In response to a pandemic or the perceived risk of a pandemic, governments in the PRC and other countries may take actions to protect their citizens that could affect our ability to control the production and export of pandemic vaccines or otherwise impose burdensome regulations on our business. For example, an outbreak of influenza and the COVID-19 pandemic could subject our manufacturing facilities to be mandated by the PRC government. The PRC government might grant compulsory licenses to allow our competitors to manufacture products that are protected by our patents or use our technology, using funds received from government agencies.
We deal with hazardous materials that may cause injury to others. These materials are regulated by environmental laws that may impose significant costs and restrictions on our business.
Our research and development programs and manufacturing operations involve the controlled use of potentially harmful biological materials and other hazardous materials. We cannot eliminate the risk of accidental contamination or injury to our employees or others from the use, manufacture, storage, handling or disposal of hazardous materials and certain waste products. In the event of contamination or injury, we could be held liable for any resulting damages, and the liability could exceed our resources or applicable insurance coverage we may have.
We are also subject to PRC laws and regulations governing the construction and operation of production facilities that may have an impact on the environment and the use, manufacture, storage, handling or disposal of hazardous materials and waste products, such as the PRC Environmental Impact Assessment Law, the PRC Prevention and Control of Water Pollution Law and the PRC Environmental Protection Law, as well as waste-disposal standards set by relevant governmental agencies. It is likely that China will continue to adopt stricter pollution controls as the country is experiencing increasingly serious environmental pollution. Although our facilities have passed previous environmental examination conducted by the Beijing Municipal Environment Protection Bureau, we cannot assure that we will continue to pass similar environmental examinations on any future production facilities that we may construct.
22
We have already obtained the approval of the environmental impact assessment report from relevant regulatory authorities for our relevant construction plan of our facilities, however, we cannot assure that we will continue to obtain the approval on environmental impact assessment report for any future production facilities that we may construct. According to the PRC Environmental Impact Assessment Law, after the approval of previous environmental impact assessment report, if there is any material change in the nature, scale, location, production technology used and measures adopted to prevent damages to ecology, new environmental impact assessment reports need to be filed for approval. Moreover, we do not currently have a pollution and remediation insurance policy to mitigate any risk related to environmental pollution or violation of environmental law.
Failure to commence development of land which we have been granted right to use within the required timeframe may cause us to lose our land use rights.
Sinovac Dalian has land use rights to two parcels of land, with an aggregate area of 95,686 square meters (approximately 1,030,000 square feet) located in the Economic and Technical Development Zone of Dalian, Liaoning province by the local government. According to the relevant PRC regulations, a parcel of land may be treated as idle land if development of the land has not been commenced within one year after the commencement date stipulated in the land use rights grant contract or the issuance date of the construction land approval certificate. Land users can extend the deadline for commencing the construction work for one year.
All of our current facilities of Sinovac Dalian are located at one of the two parcels of the land with an aggregated area of 55,606 square meters (598,582 square feet). However, as of the date of this annual report, we have not commenced development of the other parcel of the land with 40,080 square meters (431,418 square feet) which Sinovac Dalian was granted the right to use. The PRC government may treat the land as idle land, in which case we may be required to pay idle land fees or penalties, change the intended use of the land, find another parcel of land, or even be required to forfeit the land to PRC government, any of which would adversely affect our financial condition.
Negative publicity regarding China-based companies listed in the United States may affect the trading price of our common shares and result in increased regulatory scrutiny of our business.
In the past, litigation and negative publicity surrounding companies with operations in China listed in the United States have resulted in declining stock prices for such companies. Various equity research organizations have published reports on China-based companies after examining their corporate governance practices, related party transactions, sales practices and financial statements that have led to special investigations and stock suspensions on national exchanges. Any similar scrutiny of us, regardless of merit, could result in a diversion of our management’s attention from managing our core business, negative publicity, potential costs to defend ourselves against rumors, volatility and loss in the trading price of our common shares and increased directors’ and officers’ insurance premiums, any of which could materially and adversely affect our business, financial condition and results of operations.
Uncertainties exist with respect to how the PRC Vaccine Administration Law may impact our current operations.
The PRC Vaccine Administration Law became effective on December 1, 2019. It is China’s first legislation dedicated to the regulation of vaccine industry. According to the law, the supervision of vaccines will cover the whole lifecycle from vaccine development, production and distribution to vaccination. Specialized inspection teams of pharmaceutical professionals will be established at the central and provincial levels to conduct the supervision work. An electronic information system will also be set up to make all information on vaccines trackable during vaccine production, distribution and vaccination. The vaccine tracking system requires vaccination data, including vaccine's information, expiry date and use date, the medical workers who issue the vaccines and their recipients, should be recorded and retained for at least five years after its expiry. The law imposes tough punishments on wrongdoers, stipulating that people whose violations constitute a crime shall bear heavier criminal responsibility. The move under the PRC Vaccine Administration Law could be a milestone in vaccine safety, while bringing back market confidence in the regulatory system. The new law is believed to be able to enable the regulators to close loopholes and rein in risks in vaccine management and boost the confidence of the public in vaccine products manufactured in China. Since the Vaccine Administration Law was recently promulgated, no detailed implementing rules have been promulgated so far, and it is unclear how this regulation will be interpreted, amended and implemented by the relevant PRC government authorities. In addition, PRC judicial and administrative authorities have significant discretion in interpreting and implementing statutory and contractual terms. We cannot predict how the new law will affect our business operations or future strategy.
Risks related to Our Intellectual Property
If we are unable to protect our technologies from competitors with patents or other forms of intellectual property protection, our business may be harmed.
Our success depends, in part, on our ability to protect our proprietary technologies. We try to protect the technology that we consider important to our business by filing patent applications and relying on trade secret and pharmaceutical regulatory protection, including our existing and potential vaccines.
23
We have a total of 94 issued patents and a number of pending patent applications relating to our vaccines in China. The process of seeking patent protection in China can be lengthy and expensive and we cannot assure you that our pending patent applications, or any patent applications we may make in the future with respect to other products, will result in issued patents, or that any patents issued in the future will be able to provide us with meaningful protection or commercial advantage. Our patent applications might be challenged, invalidated or circumvented.
In addition to patents, we rely on trade secrets and proprietary know-how to protect our intellectual property. We have entered into confidentiality agreements (which include, in the case of employees, non-competition provisions) with many of our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of our employees, the agreements provide that all of the technology which is conceived by the individual during the course of employment is our exclusive property. These agreements may not provide meaningful protection or adequate remedies in the event of unauthorized use or disclosure of our proprietary information. In addition, third parties could possibly independently develop information and techniques substantially similar to ours or otherwise gain access to our trade secrets.
Our current or potential competitors, many of whom have substantial resources and have made substantial investments in competing technologies, could develop products that compete directly with our products despite our intellectual property rights.
Intellectual property rights and confidentiality protections in China may not be as effective as in the United States or other developed countries. Policing unauthorized use of proprietary technology is difficult and expensive, and we might need to resort to litigation to enforce or defend patents issued to us or to determine the enforceability, scope and validity of our proprietary rights or those of others. The experience and capabilities of PRC courts in handling intellectual property litigation varies, and outcomes are unpredictable. Further, such litigation may require significant expenditures of cash and management efforts and could harm our business, financial condition and results of operations. An adverse determination in any such litigation could materially impair our intellectual property rights and may harm our business, prospects and reputation.
We may be exposed to infringement or misappropriation claims by third parties which, if determined adversely to us, could cause substantial liabilities to us, or we may be unable to sell some of our products. Please see “Item 4. Information on the Company — B. Business Overview — Intellectual Property and Proprietary Technology.”
Third parties may bring intellectual property infringement claims against us in the future.
Our commercial success depends significantly on our ability to operate without infringing the patents and other proprietary rights of third parties. Even after reasonable investigation, we may not know with certainty whether we have infringed upon a third party’s patent due to the complexity of patent claims, the inadequacy of patent clearance search procedures in the PRC and the fact that a third party may have filed a patent application without our knowledge while that product was under development by us.
Patent applications are maintained in secrecy until their publication 18 months after the filing date. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. China, similar to many other countries, adopts the first-to-file system under which the first party to file a patent application (instead of the first to invent the subject invention) may be awarded a patent. There may also be technologies licensed to us or acquired by us that are subject to infringement, misappropriation or other claims by others which could damage our ability to rely on such technologies.
If a third-party claims that we infringe upon its proprietary rights, any of the following may occur:
If any of these events occurs, our business will suffer and the market price of our common shares could decline.
The success of our business may depend on licensing vaccine components from, and entering into collaboration arrangements with, third parties. We cannot be certain that our licensing or collaboration efforts will succeed or that we will realize any revenue from them.
The success of our business strategy depends, in part, on our ability to enter into licensing and collaboration arrangements and to effectively manage the resulting relationships. Our ability to enter into agreements with commercial partners depends in part on our ability to convince them
24
of the value of our technology and know-how. This may require substantial time and effort. While we anticipate expending substantial funds and management effort, we cannot assure that strategic relationships will result or that we will be able to negotiate additional strategic agreements in the future on acceptable terms, if at all.
We may incur significant financial commitments to collaborators in connection with potential licenses and sponsored research agreements. In addition, we may not be able to control the areas of responsibility undertaken by our strategic partners and may be adversely affected should these partners prove to be unable to carry a product candidate forward to full commercialization or should they lose interest in dedicating the necessary resources toward developing any such product quickly.
Third parties may terminate our licensing and other strategic arrangements if we do not perform as required under these arrangements. Generally, we expect that agreements for rights to develop technologies will require us to exercise diligence in bringing product candidates to market and may require us to make milestone and royalty payments that, in some instances, could be substantial. Our failure to exercise the required diligence or make any required milestone or royalty payments could result in the termination of the relevant license agreement, which could have a material adverse effect on us and our operations. In addition, these third parties breach or terminate their agreements with us or otherwise fail to conduct their activities in connection with our relationships in a timely manner. If we or our partners terminate or breach any of our licenses or relationships, we may:
Licensing arrangements and strategic relationships in our industry can be complex, particularly with respect to intellectual property rights. Disputes may arise in the future regarding ownership rights to technology developed by or with other parties. These and other possible disagreements between us and third parties with respect to our licenses or our strategic relationships could lead to delays in the research, development, manufacture and commercialization of our product candidates. These disputes could also result in litigation or arbitration, both of which are time-consuming and expensive. Moreover, these third parties may pursue alternative technologies or product candidates either on their own or in strategic relationships with others in direct competition with us.
Any cessation or suspension of our collaborations with scientific advisors and academic institutions may increase our costs in research and development, lengthen our new vaccines development process and lower our efficiency in new products development.
We work with scientific advisors and academic collaborators who assist us in some of our research and development efforts. Some of our pre-clinical and research programs rely heavily on such collaborators and we generally benefit considerably from the resources, technology and experience these collaborations can provide. These scientists are not, however, our employees and may have other commitments that limit their availability to us. If a conflict of interest arises between their work for us and their work for another entity, we may lose the services of these scientists and institutions. Any cessation or suspension of our collaborations with scientific advisors and academic institutions may increase our research and development costs, lengthen our new vaccine development process and lower our efficiency in new products development. In addition, although our scientific advisors and academic collaborators generally sign agreements not to disclose our confidential information, valuable proprietary knowledge may become publicly known which would compromise our competitive advantage.
We may lose the right to use “科兴” (Kexing) on our vaccine products and/or as part of our trade name.
Since 2001, Sinovac Beijing has been using “科兴” (Kexing) as part of its Chinese trade name. Sinovac Dalian began to use “科兴” (Kexing) as part of its Chinese trade name in 2010. Shenzhen Kexing (an unrelated party) successfully registered “科兴” trademark in China for Class 5 (Pharmaceuticals) under the International Classification of Goods and Services in 2001. To protect our interest in using “科兴” in our trade names, we applied to register “科兴” in China for Class 42 (Scientific & Technological Services & Research) in 2006 and the PRC Trademark Office of the State Administration for Industry and Commerce approved our application in 2010.
As of the date of this annual report, the “科兴” trademark registered and owned by Shenzhen Kexing has not been identified as “Well-known Trademark” by the relevant PRC authorities. If the “科兴” trademark owned by Shenzhen Kexing is ever officially identified as a “Well-Known Trademark” in the future, however, we may be subject to trademark infringement claim for the use of “科兴” in our trade names. It is possible that we might lose our ability to use the “科兴” trademark in our trade names due to a successful trademark infringement claim, which may adversely affect our ability to maintain and protect our brands, cause us to incur litigation costs and divert resources and management attention.
Risks Related to Doing Business in China
25
Overall economic conditions of China, which could reduce the demand for our products and materially and adversely affect our competitive position.
We conduct a significant part of our operations in China, and generated approximately 74.8% of our sales in China in 2022. Accordingly, our business, financial condition, results of operations and prospects are affected significantly by economic, political and legal developments in China. The Chinese economy differs from the economies of most developed countries in many respects, including:
While the Chinese economy has experienced significant growth in the past 40 years, growth has been uneven, both geographically and among various sectors of the economy. The PRC government has implemented measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall Chinese economy, but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us.
The Chinese economy has been transitioning from a planned economy to a more market-oriented economy. Although in the recent decade the PRC government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, the Chinese government still owns a substantial portion of the productive assets in China. The PRC government also exercises significant control over Chinese economic growth by allocating resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies.
The political relationship among foreign countries and China is subject to sudden fluctuations and periodic tensions. Changes in political conditions in China and changes in the state of foreign relations are difficult to predict and could adversely affect our product export and international collaborations. This could lead to a decline in our profitability in the future.
Any adverse change in the economic conditions or government policies in China, including the economic slowdown in 2020 and 2022 due to the COVID-19 pandemic, could have a material adverse effect on overall economic growth and the level of healthcare investments and expenditures in China, which in turn could lead to a reduction in demand for our products and consequently have a material adverse effect on our businesses.
Future changes in laws, regulations or enforcement policies in China and the PRC government’s oversight and discretion over our operations could adversely affect our business.
Laws, regulations and enforcement policies in China, including those regulating our business, are evolving and subject to future change. In particular, the PRC government authorities may continue to promulgate new laws, regulations, rules and guidelines governing companies with respect to a wide range of issues, such as competition and antitrust, privacy and data protection, intellectual property, and other matters, which may result in additional obligations imposed on us. Future changes in laws, regulations or administrative interpretations, or stricter enforcement policies by the PRC government, could impose more stringent requirements on us, including fines or other penalties. Changes in applicable laws and regulations may also increase our operating costs. Compliance with such requirements could impose substantial additional costs or otherwise have a material adverse effect on our business, financial condition and results of operations. These changes may relax some requirements, which could be beneficial to our competitors or could lower market entry barriers and increase competition. Further, regulatory agencies in China may, sometimes abruptly, change their enforcement practices.
Prior enforcement activity, or lack of enforcement activity, is not necessarily predictive of future actions. Any enforcement actions against us could have a material adverse effect on us and the market price of our common shares. In addition, any litigation or governmental investigation or enforcement proceedings in China may be protracted and may result in substantial costs and diversion of resources and management attention, negative publicity, damage to our reputation and decline in the price of our common shares.
In addition, the PRC government has significant oversight and discretion over the conduct of our business, and may intervene or influence our operations as the government deems appropriate to advance regulatory and societal goals and policy positions. The PRC government published new policies that significantly affected certain industries.
26
The PRC government has indicated an intent to exert more oversight over offerings that are conducted overseas and/or foreign investment in China-based issuers. For instance, in July 2021, the relevant PRC governments promulgated the Opinions on Strictly Cracking Down on Illegal Securities Activities, among which, it is mentioned that the administration and supervision of overseas-listed China-based companies will be strengthened, and the special provisions of the State Council of the PRC on overseas issuance and listing of shares by such companies will be revised, clarifying the responsibilities of domestic industry regulators and regulatory authorities.
On December 24, 2021, the China Securities Regulatory Commission (“CSRC”) issued to solicit comments the Administrative Measures for the Filing of Overseas Securities Offering and Listing by Domestic Companies (Draft for comments), or the Draft Filing Measures, which, among others, set forth the standards in determination of an indirect overseas listing by a domestic company, the responsible filing persons, and the procedures for the filing. The period for which the CSRC solicits comments on the Draft Filing Measures ended on January 23, 2022.
On February 17, 2023, the CSRC promulgated the Provisional Measures on the Administration of Overseas Securities Offering and Listing by Domestic Companies (“Provisional Measures”), which has taken effect and been put into implementation since March 31, 2023. These Provisional Measures require that, among other things, domestic companies that seek to offer or list securities overseas, both directly and indirectly, should fulfill the filing procedure and report relevant information with the CSRC. Indirect offering or listing refers to offering or listing that is to be made and completed under the name of an overseas entity by a Chinese entity which has its main business activities conducted within China, on the strength of the overseas entity’s equity interest or similar interest in the Chinese entity, the assets of the Chinese entity, and gains or other similar interest from the Chinese entity. The Chinese entity that seek to offer or list securities overseas are required to stipulate its articles of association, set up effective internal control system and effectively regulate the corporate governance and accounting activities pursuant to the PRC Company Law and the PRC Accounting Law. In the meantime, under these Provisional Measures, the Chinese entity that seek to offer or list securities overseas must take the obligations and responsibilities to comply with and implement the state security regulations and take necessary security and safety measures and shall not divulge or disclose state secrets and secrets of all level government agencies. The overseas issuers shall appoint one of its entities which is located and carries out business activities in China as its responsible entity in China to make the filings and reporting under these Provisional Measures. Further, after the initial public offering, the relevant Chinese entity shall make the filing with CSRC within three business days of the completion of any issuance of new securities overseas. If a Chinese entity violates these Provisional Measures, such Chinese entity and its controlling shareholders, actual controllers, directors, supervisors, and senior executives may be subject to administrative penalties such as warnings and fines. Failure to comply with the filing requirements may result in an order of rectification, a warning and fines up to RMB10 million to the non-compliant domestic companies, and the directly responsible persons of the companies will be warned and fined between RMB500,000 and RMB5 million. Furthermore, if the controlling shareholder and the actual controller of the non-compliant companies organizes or instigates the breach, they will be fined between RMB1 million and RMB10 million. In addition to above filing requirements, the Filings Rules also requires an issuer to report to the CSRC within three business days after occurrence of any the following events: (i) its change of control; (ii) its being subject to investigation or sanctions by any overseas securities regulators or overseas authorities; (iii) its change of listing status or listing segment; (iv) voluntary or mandatory delisting; and (v) material change of its principal business operations to the extent that it ceases to be subject to the filing requirements of the Provisional Measures.
We cannot rule out the possibility that the Chinese government will in the future release regulations or policies that directly or indirectly affect our industry or require us to seek additional permission to continue our operations, which could result in a material adverse change in our operation and the value of our shares.
Furthermore, the PRC government authorities are continuously strengthening the oversight and law enforcement in recent years, such as enhancing joint supervision of relevant governmental departments, systemically promulgating and implementing new rules, policies, guidelines and interpretations, and taking other comprehensive actions, which may affect our business model, monetization methods, daily operation, acquisition, investment and business development. Therefore, investors of our company and our business face potential uncertainty from actions taken by the PRC government affecting our business.
Complying with evolving laws and regulations regarding cybersecurity, information security, privacy and data protection and other related laws and requirements may be expensive and may force us to make adverse changes to our business. Many of these laws and regulations are subject to change and uncertain interpretation, and any failure or perceived failure to comply with these laws and regulations could result in negative publicity, legal proceedings, suspension or disruption of operations, increased cost of operations, or otherwise harm our business.
Laws and regulations governing cybersecurity, information security, privacy and data protection, the use of the Internet as a commercial medium, the use of data in artificial intelligence and machine learning, and data sovereignty requirements are rapidly evolving, extensive, complex, and include inconsistencies and uncertainties. We and our partners may routinely receive, collect, generate, store, process, transmit and maintain medical data, trail records and other personal details of the subjects enrolled in our clinical trials, along with other personal or sensitive information.
On June 10, 2021, the Standing Committee of the National People’s Congress of China promulgated the PRC Data Security Law, which became effective in September 2021. The PRC Data Security Law provides for data security and privacy obligations on entities and individuals carrying
27
out data processing activities, introduces a data classification and hierarchical protection system based on the importance of data in economic and social development, as well as the degree of harm it will cause to national security, public interests, or legitimate rights and interests of individuals or organizations when such data is tampered with, destroyed, leaked, or illegally acquired or used, provides for a national security review procedure for those data activities which may affect national security and imposes export restrictions on certain data and information.
On November 14, 2021, the Cyberspace Administration of China (the “CAC”) commenced to publicly solicit comments on the Regulations on the Administration of Cyber Data Security (Draft for Comments) (the “Draft Data Security Regulations”). According to the Draft Data Security Regulations, data processors shall, in accordance with relevant state provisions, apply for cybersecurity review when carrying out the following activities: (i) the merger, reorganization or separation of Internet platform operators that have acquired a large number of data resources related to national security, economic development or public interests, which affect or may affect national security; (ii) data processors that handle personal information of more than one million people contemplating to list its securities on a foreign stock exchange;(iii) data processors contemplating to list its securities on a stock exchange in Hong Kong, which affects or may affect national security; and (iv) other data processing activities that affect or may affect national security. According to the PRC National Security Law, national security refers to a status in which the regime, sovereignty, unity, territorial integrity, welfare of the people, sustainable economic and social development, and other vital interests of the state are relatively not in danger and not threatened internally or externally and the ability to maintain a sustained security status. However, the criteria for determining “affect(s) or may affect national security” as stipulated in the Draft Data Security Regulations, remain uncertain, and are still subject to further clarification by the CAC.
On December 28, 2021, the CAC, the National Development and Reform Commission (“NDRC”), the Ministry of Industry and Information Technology of the People’s Republic of China (“MIIT”) and several other administrations jointly promulgated the Cybersecurity Review Measures (the “Review Measures”), which became effective on February 15, 2022. According to the Review Measures, (i) if a critical information infrastructure operator purchases network products and services or an online platform operator conducts data processing, either of which affects or may affect national security, a cybersecurity review shall be carried out according to the Review Measures; (ii) an issuer who is a network platform operator holding personal information of more than one million shall file for a cybersecurity review with respect to its proposed listing on a foreign stock exchange; and (iii) the relevant PRC governmental authorities may initiate cybersecurity review if such governmental authorities determine that the issuer’s network products or services, or data processing activities affect or may affect national security.
These and other similar legal and regulatory developments could affect how we design our IT systems, how we operate our business, how our partners process and share data, how we process and use data, and how we transfer personal data from one jurisdiction to another. We may incur substantial costs to comply with such laws and regulations and to establish and maintain internal compliance policies.
We rely on dividends paid by our PRC subsidiaries for our cash needs. If they are unable to pay us sufficient dividends due to statutory or contractual restrictions on their abilities to distribute dividends to us, our various cash needs may not be met.
We are a holding company, and we rely on the dividends paid by our PRC subsidiaries, including majority-owned subsidiaries Sinovac Beijing, Sinovac Dalian and Sinovac LS and our wholly owned subsidiary Sinovac Biomed Co., Ltd. (“Sinovac Biomed”) for our cash needs, including the funds necessary to pay any dividends and other cash distributions to our shareholders, service any debt we may incur and pay our operating expenses. The payment of dividends in the PRC is subject to limitations. Regulations in the PRC currently permit payment of dividends by our PRC subsidiaries only out of accumulated profits as determined in accordance with accounting standards and regulations in China. For instance, in accordance with the regulations in China, Sinovac Beijing, Sinovac Dalian, Sinovac LS and Sinovac Biomed are required to set aside at least 10% of their after-tax profits each year to contribute to its reserve fund until the accumulated balance of such reserve fund reaches 50% of the registered capital of each company.
Sinovac Beijing, Sinovac Dalian, Sinovac LS and Sinovac Biomed are also required to set aside, at the discretion of their respective board of directors, a portion of their annual income after taxes to their employee welfare and bonus funds. These funds reduce the ability of the subsidiaries to pay dividends in cash.
In addition, if Sinovac Beijing, Sinovac Dalian, Sinovac LS or Sinovac Biomed incurs debt on their own in the future, the instruments governing the debt may restrict their ability to pay dividends or make other distributions to us before the debt is fully paid.
Restrictions on currency exchange may limit our ability to receive and use our revenues effectively.
We receive over 74.8% of our revenues in renminbi, which currently is not a freely convertible currency. A portion of our revenues may be converted into other currencies to meet our foreign currency obligations, including, among others, payment of dividends declared by our subsidiaries. Under China’s existing foreign exchange regulations, Sinovac Beijing, Sinovac LS, Sinovac Dalian and Sinovac Biomed are able to pay dividends in foreign currencies without prior approval from the State Administration of Foreign Exchange (“SAFE”) by complying with certain procedural requirements. However, the PRC government could take future measures to restrict access to foreign currencies for current account transactions.
28
Our PRC subsidiaries’ ability to obtain foreign exchange is subject to significant foreign exchange controls and, in the case of amounts under the capital account, requires the approval of and/or registration with PRC government authorities, including SAFE. In particular, if we finance our PRC subsidiaries by means of foreign currency from us or other foreign lenders, the foreign borrowed amount is not allowed to exceed the difference between the amount of total investment and the amount of the registered capital as approved by the Ministry of Commerce (“MOFCOM”) and registered with SAFE. Such loans must also be registered with SAFE as foreign debts. If we finance our PRC subsidiaries by means of additional capital contributions from offshore, the amount of these capital contributions must first be approved by the relevant government approval authority. These limitations could affect the ability of our PRC subsidiaries to obtain foreign exchange through debt or equity financing.
Fluctuation in the value of the renminbi may have a material adverse effect on your investment.
The value of the renminbi against the U.S. dollar, Euro and other currencies is affected by, among other things, changes in China’s political and economic conditions and China’s foreign exchange policies. The PRC government allows the renminbi to fluctuate within a narrow and managed band against a basket of certain foreign currencies.
Since June 2010, the Renminbi has fluctuated against the U.S. dollar. Since October 1, 2016, the RMB has joined the International Monetary Fund’s basket of currencies that make up the Special Drawing Right, along with the U.S. dollar, the Euro, the Japanese yen and the British pound. Since the fourth quarter of 2016, the RMB depreciated significantly in the backdrop of a surging U.S. dollar and persistent capital outflows of China. Towards the end of 2020 and in 2021, the RMB saw this past depreciation trend reversed and the RMB gained more than 2% against the U.S. dollar. However, in 2022, the RMB lost over 8% in value against the U.S. dollar. With the development of the foreign exchange market and progress towards interest rate liberalization and renminbi internationalization, the PRC government may announce further changes to the exchange rate system and the RMB could appreciate or depreciate significantly in value against the U.S. dollar.
It is difficult to predict how long such depreciation of the RMB against the U.S. dollar may last and when and how the relationship between the renminbi and the U.S. dollar may change again. The PRC government indicated that it would make the foreign exchange rate of the renminbi more flexible and widen the trading band of renminbi, which increases the possibility of sharp fluctuations in renminbi’s value in the future as well as the unpredictability associated with renminbi’s exchange rate. There remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in further and more significant fluctuations of the renminbi against foreign currencies.
As the majority of our costs and expenses are denominated in renminbi, a resumption of the appreciation of the renminbi against the U.S. dollar would further increase our costs in U.S. dollar terms. In addition, as our operating subsidiaries in China receive revenues in renminbi, any significant depreciation of the renminbi against the U.S. dollar may have a material adverse effect on our revenues in U.S. dollar terms and financial condition, and the value of, and any dividends payable on, our common shares. For example, to the extent that we need to convert U.S. dollars into renminbi for our operations, appreciation of the renminbi against the U.S. dollar would have an adverse effect on the renminbi amount we receive from the conversion. Conversely, if we decide to convert our renminbi into U.S. dollars for the purpose of making payments for dividends on our common shares or for other business purposes, appreciation of the U.S. dollar against the renminbi would have a negative effect on the U.S. dollar amount available to us.
Our business benefits from certain government tax incentives. Expiration, reduction or elimination of these incentives will increase our tax expenses and in turn decrease our net income.
Pursuant to the PRC Enterprise Income Tax Law (the “EIT Law”) and its implementation rules, both domestic companies and the foreign invested enterprises (the “FIEs”) are subject to a unified income tax rate of 25%. Preferential tax treatments are expected to be granted to high and new technology enterprises that conduct business in encouraged sectors, whether FIEs or domestic companies.
Sinovac Beijing reconfirmed its “High and New Technology Enterprises” (“HNTE”), status and obtained the corresponding certificate in 2020 for a period of three years. As a result, subject to satisfaction of applicable criteria as confirmed by the competent authorities, Sinovac Beijing is entitled to a reduced enterprise income tax (“EIT”) rate of 15% from 2020 to 2022. Sinovac Dalian reconfirmed its HNTE status in 2020 for another three-year period, which is from 2020 to 2022. Sinovac LS, being confirmed as a HNTE in 2020 for a period of three years, is subject to the preferential EIT of 15% from 2020 to 2022. The PRC government could eliminate any of these preferential tax treatments before their scheduled expiration. Expiration, reduction or elimination of such tax incentives will increase our tax expenses and in turn decrease our net income. Sinovac Beijing, Sinovac Dalian and Sinovac LS will re-apply for HNTE in 2023.
Under the EIT Law, dividends payable by us and gains on the disposition of our shares may be subject to PRC taxation.
If we were considered a PRC resident enterprise under the EIT Law, our shareholders who are deemed non-resident enterprises may be subject to the EIT at the rate of 10% upon the dividends payable by us or upon any gains realized from the transfer of our shares, if such income is deemed derived from China, provided that (i) such foreign enterprise investor has no establishment or premises in China or (ii) it has an establishment or premises in China but its income derived from China has no real connection with such establishment or premises. If we were required under the EIT Law to withhold PRC income tax on our dividends payable to our non-PRC enterprise shareholders, or if any gains
29
realized from the transfer of our shares by our non-PRC enterprise shareholders were subject to the EIT, such shareholders’ investment in our shares would be materially and adversely affected.
PRC regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or our PRC subsidiaries to liability or penalties, limit our ability to inject capital into our PRC subsidiaries or limit our PRC subsidiaries’ ability to increase their registered capital or distribute profits.
SAFE promulgated the Circular on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Roundtrip Investment through Special Purpose Vehicles (“SAFE Circular 37”) on July 4, 2014, which replaced the former circular commonly known as “SAFE Circular 75” promulgated by SAFE on October 21, 2005. SAFE Circular 37 requires PRC residents to register with the local branches of SAFE in connection with their direct establishment or indirect control of an offshore entity, for the purpose of overseas investment and financing, with such PRC residents’ legally owned assets or equity interests in domestic enterprises or offshore assets or interests, referred to in SAFE Circular 37 as a “special purpose vehicle.”
SAFE Circular 37 further requires amendment to the registration in the event of any significant changes with respect to the special purpose vehicle, such as increase or decrease of capital contributed by PRC individuals, share transfer or exchange, merger, division, or other material events. In the event that a PRC shareholder holding interests in a special purpose vehicle fails to fulfill the required SAFE registration, the PRC subsidiaries of that special purpose vehicle may be prohibited from making profit distributions to the offshore parent and from carrying out subsequent cross-border foreign exchange activities, and the special purpose vehicle may be restricted in its ability to contribute additional capital into its PRC subsidiary.
Failure to comply with the various SAFE registration requirements described above could result in liability under PRC law for evasion of foreign exchange controls. According to the Notice on Further Simplifying and Improving Policies for the Foreign Exchange Administration of Direct Investment released on February 13, 2015 by SAFE, local banks will examine and handle foreign exchange registration for overseas direct investment, including the initial foreign exchange registration and amendment registration, under SAFE Circular 37 from June 1, 2015.
Mr. Weidong Yin has made the required SAFE registration with respect to his investments in our company. However, we may not be aware of the identities of all of our beneficial owners who are PRC residents. We do not control our beneficial owners and cannot assure you that all of our PRC-resident beneficial owners will comply with SAFE Circular 37 and subsequent implementation rules. The failure of our beneficial owners who are PRC residents to register or amend their foreign exchange registrations in a timely manner pursuant to SAFE Circular 37 and subsequent implementation rules, or the failure of future beneficial owners of our company who are PRC residents to comply with the registration procedures set forth in SAFE Circular 37 and subsequent implementation rules, may subject such beneficial owners or our PRC subsidiaries to fines and legal sanctions.
Furthermore, since it is unclear how any future regulation concerning offshore or cross-border transactions will be implemented by the relevant PRC government authorities, we cannot predict how these regulations will affect our business operations or future strategy. Failure to register or comply with relevant requirements may also limit our ability to contribute additional capital to our PRC subsidiaries and limit our PRC subsidiaries’ ability to distribute dividends to our company. These risks may have a material adverse effect on our business, financial condition and results of operations.
Any failure to comply with PRC regulations regarding our employee equity incentive plans may subject the PRC plan participants or us to fines and other legal or administrative sanctions.
Pursuant to SAFE Circular 37, PRC residents who participate in share incentive plans in overseas non-publicly listed companies due to their position as director, senior management or employees of the PRC subsidiaries of the overseas companies may submit applications to SAFE or its local branches for the foreign exchange registration with respect to offshore special purpose companies. Our directors, executive officers and other employees who are PRC residents and who have been granted options and restricted shares were able to follow SAFE Circular 37 to apply for the foreign exchange registration before our company became an overseas listed company.
Since our company has become an overseas listed company, we and our directors, executive officers and other employees who are PRC residents and who have been granted options are subject to the Notice on Issues Concerning the Foreign Exchange Administration for Domestic Individuals Participating in Stock Incentive Plan of Overseas Publicly Listed Company, issued by SAFE in February 2012, according to which, employees, directors, supervisors and other management members participating in any stock incentive plan of an overseas publicly listed company who are PRC residents are required to register with SAFE through a domestic qualified agent, which could be a PRC subsidiary of such overseas listed company, and complete certain other procedures.
Failure to complete SAFE registrations may subject them to fines and legal sanctions and may also limit the ability to make payments under our equity incentive plans or receive dividends or sales proceeds related thereto, or our ability to contribute additional capital into our subsidiaries in China and limit such subsidiaries’ ability to distribute dividends to us. We also face regulatory uncertainties that could restrict our ability to adopt additional equity incentive plans for our directors and employees under PRC law.
30
In addition, the State Administration for Taxation has issued circulars concerning employee share options or restricted shares. Under these circulars, employees working in the PRC who exercise share options, or whose restricted shares or restricted share units, or RSUs, vest, will be subject to PRC individual income tax. The PRC subsidiaries of an overseas listed company have obligations to file documents related to employee share options or restricted shares with relevant tax authorities and to withhold individual income taxes of those employees related to their share options, restricted shares or RSUs. If the employees fail to pay, or the PRC subsidiaries fail to withhold, their income taxes according to relevant laws, rules and regulations, the PRC subsidiaries may face sanctions imposed by the tax authorities or other PRC government authorities.
PRC regulation of loans and direct investment by offshore holding companies to PRC entities may delay or prevent us from making loans or additional capital contributions to our PRC operating subsidiaries and affiliated entities.
In funding our PRC subsidiaries, we must comply with PRC legal requirements relating to foreign debt registration and to PRC foreign-investment companies’ “registered capital” and “total investment” ratio. “Registered capital” refers to the capital contributed to or paid into a PRC foreign-investment company in cash or in kind, and “total investment” refers to the estimated amount of the total capital as required to enable and support the full-scale operation of a PRC foreign-investment company when the company is initially established. The amounts of a PRC foreign-investment company’s registered capital and total investment are set forth in the company’s articles of association and joint venture contract (in the case of a Sino-foreign joint venture) and approved by the competent government authority in advance. The balance between the required “total investment” and the “registered capital” can be satisfied by borrowings or loans obtained by the company. In other words, such loans cannot exceed the difference between such company’s registered capital and total investment.
Loans by us or Sinovac Hong Kong to Sinovac Beijing, Sinovac LS, Sinovac Dalian or Sinovac Biomed cannot exceed the difference between such company’s registered capital and total investment. The total investment and registered capital can be adjusted after the establishment of a foreign-investment companies with the approvals of all the shareholders or unanimous approvals of the board of directors. In the case of Sinovac Beijing, Sinovac Dalian or Sinovac LS, the approval from its respective minority shareholders is required to increase the amount of total investment. Further, all the loans from the overseas lenders must be registered with SAFE as foreign debts.
We may also decide to finance our PRC subsidiaries by making additional capital contributions. These additional contributions must be approved by the government approval authority and, in the case of Sinovac Beijing or Sinovac Dalian and Sinovac LS, the approval from its respective minority shareholders. We cannot assure you that we will be able to obtain these government registrations or approvals, or the approval of the minority shareholders on a timely basis, if at all, with respect to future loans or additional capital contributions by us to our subsidiaries. If we fail to obtain such registrations or approvals, our ability to capitalize our PRC operations would be negatively affected, which could adversely and materially affect the liquidity of our subsidiaries and our ability to expand the business.
Because we are incorporated under Antigua and Barbuda law, substantially all of our operations, property and assets are located in China and all of our major shareholders, directors and officers and substantially all of their assets are located outside of the United States, you may be unable to protect your shareholder rights under U.S. law in a court in the United States.
We are incorporated in Antigua and Barbuda. Our corporate affairs are governed by our Articles of Incorporation and By-laws and by the International Business Corporations Act and common law of Antigua and Barbuda. The rights of shareholders to take legal action against our directors, officers and us, actions by minority shareholders and the fiduciary responsibilities of our directors to us are to a large extent governed by the International Business Corporations Act and common law of Antigua and Barbuda. The International Business Corporations Act was modelled on Canadian company law and the common law of Antigua and Barbuda is derived from comparatively limited judicial precedent in Antigua and Barbuda, as well as from English common law, which has persuasive, but not binding, authority on a court in Antigua and Barbuda.
The rights of our shareholders and the fiduciary responsibilities of our directors under Antigua and Barbuda law are not as clearly established as they would be under statutes or judicial precedents in the United States. Among other things, Antigua and Barbuda has a less developed body of securities laws as compared to the United States, and provides significantly less protection to investors. Further, Antigua and Barbuda’s body of securities law, and the experience of its courts in addressing corporate and securities law issues of a type often experienced by public companies, is likely less developed than that of some of the other jurisdictions where publicly traded China-based companies are incorporated, such as the Cayman Islands.
It may be difficult or impossible for you to bring an action against us or our directors or officers in Antigua and Barbuda courts or to enforce or protect your rights under U.S. securities laws or otherwise. Even if you are successful in bringing an action of this kind, you may be unable to enforce a judgment against our assets or the assets of our directors and officers under the laws of Antigua and Barbuda.
There is doubt as to whether Antigua and Barbuda courts would enforce judgments of United States courts obtained in actions against us or our directors or officers that are predicated upon the civil liability provisions of the Securities Act, or in original actions brought against us or such persons predicated upon the Securities Act. There is no treaty in effect between the United States and Antigua and Barbuda providing for such enforcement, and there are grounds upon which Antigua and Barbuda courts may not enforce judgments of United States courts. In addition, Antigua and Barbuda corporations may not have standing to initiate a shareholder derivative action before the federal courts of the United States.
PRC courts may recognize and enforce foreign judgments in accordance with the PRC Civil Procedures Law based either on treaties between the PRC and the country where the judgment is made or on reciprocity between jurisdictions. If there are no treaties or reciprocity arrangements
31
between the PRC and a foreign jurisdiction where a judgment is rendered, matters relating to the recognition and enforcement of the foreign judgment in the PRC may be resolved through diplomatic channels. The PRC does not have any treaties or other arrangements with the United States or Antigua and Barbuda that provide for the reciprocal recognition and enforcement of foreign judgments. As a result, it is generally difficult to enforce in the PRC a judgment rendered by a U.S. or Antigua and Barbuda court.
As a result of all of the above, as well as the fact that substantially all of our property, assets and operations are located in China and all of our major shareholders, directors and officers and substantially all of their assets are located outside of the United States, you may be unable to protect your shareholder interests through actions against us or our officers, directors or major shareholders.
The Public Company Accounting Oversight Board (“PCAOB”) had historically been unable to inspect our auditor in relation to their audit work performed for our financial statements and the inability of the PCAOB to conduct inspections over our auditor deprives our investors with the benefits of such inspections.
Our auditor, the independent registered public accounting firms that issues the audit report included elsewhere in this annual report, as an auditor of companies that are traded publicly in the United States and a firm registered with the PCAOB, is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess its compliance with applicable professional standards. Since our auditor is located in China, a jurisdiction where the PCAOB was historically unable to conduct inspections and investigations completely before 2022, our auditor had historically been unable to be inspected by the PCAOB. As a result, we and investors in our shares are deprived of the benefits of such PCAOB inspections. The inability of the PCAOB to conduct inspections of auditors in China makes it more difficult to evaluate the effectiveness of our independent registered public accounting firm’s audit procedures or quality control procedures as compared to auditors outside of China that are subject to PCAOB inspections, which could cause investors and potential investors in our shares to lose confidence in the audit, reported financial information, and the quality of our financial statements.
Our shares will be prohibited from trading in the United States under the HFCAA in the future if the PCAOB is unable to inspect or fully investigate auditors located in China. The delisting of our shares, or the threat of their being delisted, may materially and adversely affect the value of your investment.
The HFCAA, which was signed into U.S. law on December 18, 2020, states that if the SEC determines that we have filed audit reports issued by a registered public accounting firm that has not been subject to inspection for the PCAOB for two consecutive years beginning in 2021, the SEC shall prohibit our shares from being traded on a national securities exchange or in the over-the-counter trading market in the United States. On December 16, 2021, the PCAOB issued a report to notify the SEC of its determination that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in mainland China and Hong Kong. The PCAOB identified our auditor as one of the registered public accounting firms that the PCAOB is unable to inspect or investigate completely. On May 4, 2022, we were identified by the SEC under the HFCAA. On December 15, 2022, the PCAOB issued a report that vacated its December 16, 2021 determination and removed mainland China and Hong Kong from the list of jurisdictions where it was unable to inspect or investigate completely registered public accounting firms. For this reason, we do not expect to be identified as a Commission-Identified Issuer under the HFCAA after we file this annual report on Form 20-F. Each year, the PCAOB will determine whether it can inspect and investigate completely audit firms in mainland China and Hong Kong, among other jurisdictions. If PCAOB determines in the future that it no longer has full access to inspect and investigate completely accounting firms in mainland China and Hong Kong and we continue to use an accounting firm headquartered in one of these jurisdictions to issue an audit report on our financial statements filed with the SEC, we would be identified as a Commission-Identified Issuer following the filing of the annual report on Form 20-F for the relevant fiscal year. There can be no assurance that we would not be identified as a Commission-Identified Issuer for any future fiscal year, and if we were so identified for two consecutive years, we would become subject to the prohibition on trading under the HFCAA.
If our shares are prohibited from trading in the United States, there is no certainty that we will be able to list on a non-U.S. exchange or that a market for our shares will develop outside of the United States. Such a prohibition would substantially impair your ability to sell or purchase our shares when you wish to do so, and the risk and uncertainty associated with delisting would have a negative impact on the price of our shares. Also, such a prohibition would significantly affect our ability to raise capital on terms acceptable to us, or at all, which would materially and adversely affect our business, financial condition, and prospects.
On February 24, 2023, the CSRC and other PRC governmental authorities jointly issued the Provisions on Strengthening Confidentiality and Archives Administration of Overseas Securities Offering and Listing by Domestic Companies (the “Confidentiality Provisions”), which came into effect on March 31, 2023. The Confidentiality Provisions outline obligations of issuers listed in overseas markets with operations in China when they provide information involving state secrets or sensitive information to their securities service providers (e.g., auditors) and overseas regulators. In addition, under the Confidentiality Provisions, such issuers are also required to obtain approval from the CSRC and other PRC authorities before accepting any investigation or inspection by overseas regulators. As the Confidentiality Provisions are recently promulgated and in effect, there are uncertainties with respect to their interpretation and implementation.
ITEM 4. Information on the Company
32
A. History and Development of the Company
Our legal and commercial name is Sinovac Biotech Ltd. Our principal executive offices are located at No. 39 Shangdi Xi Road, Haidian District, Beijing 100085, PRC. Our telephone number at this address is +86-10-5693-1800. Our registered address is located at the office of APN Corporate and Management Services Limited, Unit #4 Bryson’s Complex, Friars Hill Road, St. John’s, Antigua. Our agent for service of process in the United States is Cogency Global Inc., located at 122 East 42nd Street, 18th Floor, New York, NY 10168.
We are a holding company and conduct our business through our 73.09% majority-owned subsidiary Sinovac Beijing, our 59.24% majority-owned subsidiary Sinovac LS, our 68% majority-owned subsidiary Sinovac Dalian, and our wholly owned subsidiaries Sinovac Biomed, Sinovac Hong Kong and Sinovac Biotech (Singapore) Pte. Ltd. (“Sinovac Singapore”). Sinovac Beijing was incorporated on April 28, 2001, Sinovac LS was incorporated on May 7, 2009, Sinovac Dalian was established on January 19, 2010, Sinovac Biomed was incorporated on April 16, 2015, Sinovac Hong Kong was incorporated on October 21, 2008 and Sinovac Singapore was incorporated on August 6, 2020.
We were incorporated in Antigua and Barbuda on March 1, 1999 as an Antiguan company with limited liability under the laws of Antigua and Barbuda pursuant to the International Business Corporations Act. Before we adopted our current name on October 21, 2003, we were called Net-Force System Inc. and were primarily engaged in the online gaming business. In September 2003, we issued ten million new shares to Lily Wang, one of our then principal shareholders to acquire a 51% equity interest in Sinovac Beijing. Ms. Wang had contracted to purchase these shares from certain of Sinovac Beijing’s then shareholders for cash immediately before the above 51% share transfer. However, this 51% equity interest in Sinovac Beijing was transferred to us directly from those shareholders and was recorded under applicable PRC law transfer documents as a cash transaction. Lily Wang was responsible for paying the cash to those shareholders. The transfer of the Sinovac Beijing equity interest to us was registered and approved by PRC government authorities in August 2004. In September 2004, we acquired an additional 20.6% equity interest in Sinovac Beijing for approximately $3.3 million in cash. In October 2011, we further acquired an additional 1.53% equity interest in Sinovac Beijing by contributing the dividends declared to Sinovac Hong Kong but unpaid in amount of RMB18.6 million. We currently own 73.09% of the equity interests in Sinovac Beijing and Sinobioway Medicine owns a 26.91% interest.
In January 2004, we entered into a share purchase agreement with Heping Wang and issued him 3.5 million of our common shares and a promissory note in the amount of $2.2 million to acquire from him a 100% equity interest in Tangshan Yian. Mr. Wang had contracted to purchase these shares from Tangshan Yian’s then two shareholders immediately before the above 100% share transfer. However, this 100% equity interest in Tangshan Yian was transferred to us directly from those shareholders and was recorded under applicable PRC law transfer documents as a cash transaction. Heping Wang was responsible for paying the cash to the two shareholders. The transfer of the Tangshan Yian equity interest by Mr. Wang to us was registered and approved by PRC government authorities in November 2004.
In the first quarter of 2008, we issued and sold an aggregate of 2.5 million common shares at $3.90 per share to Sansar Capital Management. We received approximately $9.75 million in gross proceeds from this private placement of our common shares.
In October 2008, we established Sinovac Hong Kong, a wholly owned subsidiary focused primarily on registering and distributing current and newly-developed vaccine products in Hong Kong and exporting our products abroad. In addition, Sinovac Hong Kong seeks research and development collaboration opportunities with third parties in Hong Kong.
In May 2009, Sinovac LS was incorporated with a registered capital of $5 million. In June 2016, our board of directors approved an additional capital contribution of $4.6 million, which has been fully provided.
In November 2009, we entered into a joint venture agreement with Dalian Jin Gang Group to establish Sinovac Dalian. In January 2010, we established Sinovac Dalian which focuses on the research, development, manufacturing and commercialization of live attenuated vaccines, such as varicella and mumps vaccines for human use. Pursuant to the joint venture agreement, we made an initial cash contribution of RMB60.0 million in exchange for a 30% equity interest in Sinovac Dalian and Dalian Jin Gang Group made an asset contribution of RMB 140.0 million, including manufacturing facilities, production lines and land use rights, in exchange for the remaining 70% interest in Sinovac Dalian.
In December 2010, we purchased an additional 25% equity interest in Sinovac Dalian from Dalian Jin Gang Group for consideration of RMB50.0 million. In 2014, the board of directors passed a resolution to increase our capital contribution to Sinovac Dalian in the amount of RMB80.0 million, which aimed to increase Sinovac’s equity ownership from 55% to 67.86%. RMB50.0 million was initially provided through foreign debt with the expectation of a debt to equity swap of the total amount after the remaining RMB30.0 million is provided to Sinovac Dalian. In 2016, an additional RMB30.0 million was made to Sinovac Dalian through foreign debt and subsequently the debt to equity swap for a total of RMB80.0 million was completed. In October 2016, our equity ownership in Sinovac Dalian increased to 67.86%.
In February 2010, we closed a public offering of our common shares. We issued and sold 11.5 million common shares at $5.75 per share. We received net proceeds of approximately $61.8 million, after deducting underwriting discounts and commissions and offering expenses payable.
In 2013, we increased the capital investment to Tangshan Yian with the total amount of $4 million, which we lent to Tangshan Yian in 2010. In the same year, we lent Tangshan Yian $1 million to be used for sales and marketing spending and other corporate purposes and operational activities. In December 2015, we entered into an equity interest transfer agreement with Beijing Kuai Le Xing Biotech Co., Ltd. to transfer our
33
100% equity interest in Tangshan Yian to Beijing Kuai Le Xing Biotech Co., Ltd. for consideration of RMB13.0 million. The disposal of Tangshan Yian was completed in February 2016.
In April 2015, we established Sinovac Biomed, which is 100% owned by Sinovac Hong Kong. Sinovac Biomed focuses on the distribution of vaccine products as well as providing consulting services in the vaccination industry.
In March 2016, we adopted the Rights Agreement. Pursuant to the Rights Agreement, subject to limited exceptions, upon (i) a person or group obtaining ownership of 15% or more of our common shares or (ii) the commencement or announcement of an intention to make a tender offer or exchange offer, the consummation of which would result in the beneficial ownership by a person or group of 15% or more of our common shares, in each case, without the approval of our board of directors, each Right will entitle the holders, other than the Acquiring Person, to buy, at an exercise price of $30.00, one one-thousandth of a share of our newly created series A junior participating preferred shares (the “Series A Preferred Shares”). Holders are entitled to receive, in lieu of each one one-thousandths of a Series A Preferred Share, common shares having a market value at that time of twice the Right’s exercise price. Our board of directors is entitled to redeem the Rights at $0.001 per Right at any time before the Rights are exercisable. We refer to the person who acquired 15% or more of the outstanding common shares of Sinovac Antigua as the “Acquiring Person.” As described above, on March 5, 2018, Sinovac Antigua filed a lawsuit in the Court of Chancery of the State of Delaware seeking a determination whether the Shareholder Group had triggered the Rights Agreement by forming a group holding approximately 45% of Sinovac Antigua’s outstanding shares, in excess of the plan’s threshold of 15%, and acting in concert prior to the 2017 AGM.
On February 18, 2019, after reviewing the judgment of the Antigua Court of December 19, 2018 and considering all additional facts known to the board of directors, our board of directors determined that the Collaborating Shareholders became Acquiring Persons as defined under the Rights Agreement, and that their conduct resulted in a Trigger Event under the Rights Agreement. As a result, the Rights held by the Collaborating Shareholders were deemed void.
Pursuant to the Rights Agreement, the board of directors elected to exchange each valid and outstanding Right held by Sinovac Antigua’s shareholders (not including the Collaborating Shareholders) for an Exchange Share. The total Exchange Shares to be received by any holder will be rounded up to the nearest whole common share and rounded down to the nearest whole Series B preferred share. On February 22, 2019, in order to facilitate the Exchange, approximately 27.8 million Common Shares and approximately 14.6 million Series B Preferred Shares were issued into a trust for the benefit of the holders of the valid and outstanding Rights (not including the Collaborating Shareholders). As of the close of trading in the United States on February 22, 2019, the Rights converted into the right to receive the Exchange Shares and will no longer trade with the common shares, and will not otherwise trade on any securities market.
In February 2019, we amended and restated the Rights Agreement. Pursuant to the amended and restated Rights Agreement, subject to limited exceptions, upon (i) a person or group obtaining ownership of 15% or more of the aggregate total of our common shares and Series B Preferred Shares then issued and outstanding or (ii) the commencement or announcement of an intention to make a tender offer or exchange offer, the consummation of which would result in the beneficial ownership by a person or group of 15% or more of the aggregate total of our common shares and Series B Preferred Shares then issued and outstanding, in each case, without the approval of our board of directors, each Right will entitle the holders, other than the acquiring person, to buy, at an exercise price of $20.00, one one-thousandth of a share of our newly created series C junior participating preferred shares (the “Series C Preferred Shares”). Holders are entitled to receive, in lieu of each one one-thousandths of a Series C Preferred Share, common shares and/or Series B Preferred Shares having a market value at that time of twice the Right’s exercise price. Our board of directors is entitled to redeem the Rights at $0.001 per Right at any time before the Rights are exercisable. We refer to the person who acquired 15% or more of the outstanding common shares or Series B Preferred Shares of Sinovac Antigua as the “acquiring person.” In February 2021, 2022 and 2023, we further amended the amended and restated Rights Agreement to extend its term until February 2024.
On March 6, 2019, the Delaware Chancery Court entered a status quo order providing that Sinovac Antigua not distribute any of the Exchange Shares from the trust until the final disposition of the pending Delaware litigation or further order of the Court. On April 4, 2019, the Eastern Caribbean Supreme Court, Court of Appeal issued an order restraining Sinovac Antigua from taking further action under the Rights Agreement, including the distribution of the previously issued Exchange Shares to the holders of valid Rights, until the conclusion of 1Globe Capital, LLC’s appeal of the December 19, 2018 Judgment of the Antigua Court, and this order was extended in January 2022 until the conclusion of the appeal process to the Privy Council. On April 8, 2019, the Delaware Chancery Court stayed the Delaware litigation pending the outcome of 1Globe’s appeal of the Antigua Judgment. 1Globe’s appeal of the Antigua Court’s Judgment was heard by the Court of Appeal on September 18, 2019. On December 9, 2021, the Court of Appeal handed down its judgment, dismissing all grounds of appeal and upholding the Antigua Judgment. On February 24, 2022, 1Globe obtained permission from the Court of Appeal for leave to appeal certain appeal grounds to the Privy Council, although not including the challenge to the validity of the Rights Agreement.. On April 19, 2022, 1Globe renewed its application directly to the Privy Council for leave to appeal on its ground of appeal concerning the validity of the Rights Agreement. On July 13, 2022, 1Globe filed its Notice of Appeal on those grounds on which the Court of Appeal had granted 1Globe leave to appeal. On September 16, 2022, 1Globe filed an application to the Privy Council seeking permission to amend its existing application for permission to appeal and its existing Notice of Appeal, and to seek permission to appeal on another ground rejected by the Court of Appeal concerning the exercise of the Antigua Court’s discretion. Sinovac responded on October 21, 2022. On February 15, 2023, the Privy Council made a procedural decision to allow amendment of its existing application for permission to appeal, and decided to deal with procedural and substantive issues together at the Final Hearing. 1Globe has not yet
34
taken steps to list a substantive hearing before the Privy Council. The appeal outcome is therefore pending. See “Legal and Administrative Proceedings” for additional information.
In May 2020, Prime Success and Vivo Capital invested $15 million in our then wholly owned subsidiary, Sinovac LS, to further the development of CoronaVac. The two investors each loaned $7.5 million in the form of a convertible loan that bore interest, or, at the investor’s election, converted into 7.5% of the total equity interest of Sinovac LS. Later each of Prime Success and Vivo Capital exercised its right to convert its convertible loan into 7.5% of the total equity interests of Sinovac LS. After the investment made by Sino Biopharmaceutical Limited as described below, Prime Success and Vivo Capital each holds approximately 6.3% stake in Sinovac LS.
On August 6, 2020, we established Sinovac Singapore, a wholly owned subsidiary focuses primarily on registering and distributing vaccine products in Singapore and exporting our products abroad. In addition, Sinovac Singapore seeks research and development collaboration opportunities with third parties in Asia.
In November 2020, we increased our equity ownership of Sinovac Dalian from 67.86% to 68%, by converting RMB46.6 million debt into equity.
In December 2020, Sino Biopharmaceutical Limited, an innovative research and development driven pharmaceutical conglomerate in China, through its affiliates, invested approximately $500 million in exchange for approximately 15% equity interest in Sinovac LS in funding for further development, capacity expansion and manufacturing of the CoronaVac. After this investment, our equity ownership of Sinovac LS decreased to 59.24%.
In 2022, we set up subsidiaries in Thailand, the Philippines, Mexico, Peru, Colombia, Ecuador, Bangladesh, Indonesia, Pakistan as part of our globalization strategy.
For additional information regarding our principal capital expenditures, see “— D. Property, Plants and Equipment” and “Item 5. Operating and Financial Review and Prospects —B. Liquidity and Capital Resources — Capital Expenditures.”
The SEC maintains an Internet site that contains our reports, proxy and information statements, and other information that we filed electronically with the SEC at http:// www.sec.gov.
Investor inquiries should be directed to us at the address and telephone number of our principal executive offices set forth above. Our website is http://www.sinovac.com. The information contained on our website does not form part of this annual report.
B. Business Overview
We are a fully integrated China-based biopharmaceutical company that focuses on research, development, manufacturing and commercialization of vaccines that protect against human infectious diseases including, without limitation, hepatitis A, hepatitis B, hand foot and mouth disease (“HFMD”) caused by EV71, seasonal influenza, H5N1 and H1N1 pandemic influenza, coronavirus, pneumococcus, poliomyelitis, varicella and mumps.
In 2002, we launched our first product, Healive, which was the first inactivated hepatitis A vaccine developed, produced and marketed by a China-based manufacturer. In 2005, we received regulatory approvals for the production of Bilive in China, a combined hepatitis A and B vaccine, and Anflu, a split viron influenza vaccine. In April 2008, we received the regulatory approval for the production in China of our whole viron H5N1 pandemic influenza (avian flu) vaccine, which is the only vaccine approved for sale to the Chinese national vaccine stockpiling program.
In September 2009, we were granted a production license for Panflu.1, which was the first approved vaccine in the world against the influenza A H1N1 virus (swine flu). In December 2011, we obtained the production license from NMPA for its mumps vaccine product and launched the mumps vaccine in late 2012. In December 2015, NMPA issued the new drug certificate and production license for Inlive, our EV71 vaccine. In January 2016, NMPA issued the GMP certificate and Inlive, our EV71 vaccine, was commercially launched in China in June 2016.
In December 2019, the NMPA approved and issued a product license for our varicella vaccine. In June and December 2020, we obtained production license for our quadrivalent influenza vaccine and pneumococcal polysaccharide vaccine from the NMPA, respectively.
We initiated the development of CoronaVac, an inactivated vaccine against COVID-19, in January 2020. On February 5, 2021, NMPA granted a conditional marketing authorization for CoronaVac to be used in individuals aged 18 and above, and we obtained the approval of its emergency use for children aged 3-17 years in June 2021. In June 2021, CoronaVac was approved for the WHO’s EUL procedure. As of the date of this annual report, CoronaVac have been authorized in more than 60 countries and regions under conditional marketing authorizations or emergency use. We continue to actively seek regulatory approval of CoronaVac in other countries and regions around the world in an effort to maximize global accessibility and affordability of the COVID-19 vaccine. In July 2021, we entered into an advance purchase agreement with the Global Alliance for Vaccines and Immunization (“Gavi Alliance”) to provide up to 380 million doses of CoronaVac for global distribution. CoronaVac has become the only inactivated COVID-19 vaccine in the world approved for use for children as young as six months old, after the Secretary of
35
Health of Hong Kong approved the lowering of the minimum age for receiving CoronaVac from three years to six months old for “off-label use” in early August 2022. CoronaVac was approved for pediatric use in over ten countries.
In July 2021, we were granted a production license for our sabin inactivated polio vaccine (sIPV). In June 2022, our sIPV vaccine was prequalified by the WHO, and our sIPV vaccine is now available for United Nations agencies to purchase to support the global polio eradication strategy.
In November 2022, our live attenuated varicella vaccine was prequalified by the WHO, marking the first WHO prequalified Chinese varicella vaccine.
Our Products
We specialize in the research, development, manufacturing and commercialization of vaccines for infectious diseases with significant unmet medical need. Set forth below is a chart that outlines our current marketed products and those that we have developed or are developing.

36
37
38
Our pipeline consists of vaccine candidates in the clinical and pre-clinical development phases in China, as follows:
39
Research and Development
We have established a leadership position in the research and development of vaccines in China. Since our inception, we have successfully developed and marketed Healive, Bilive, Anflu, Panflu, Panflu.1, mumps vaccine, Inlive, varicella vaccine, QIV, PPV, sIPV and CoronaVac. Please see “— Our Products.” We believe our R&D capabilities provide us with a key competitive advantage. We intend to focus our research and development efforts on developing vaccines for infectious diseases with significant unmet medical needs, as well as the vaccine products with extensive market demand in China and other countries. We have undertaken nearly 60 national, provincial and ministerial science and technology R&D projects, received two national level technology awards, and published more than 140 SCI papers, many of which were published in top academic journals including New England Journal of Medicine, The Lancet, Science and Nature, etc. Furthermore, we also lead the Chinese branch of the BRICS Vaccine Research and Development Center. So far we have obtained 94 patents for our core technologies in China.
In 2008, we restructured our R&D team in Beijing to better utilize our scientific and personnel resources. In 2009, we built an R&D center of approximately 13,300 square feet in the campus of our Beijing headquarters to meet our R&D demand. In 2011, we built a lab of 6,778 square feet, which is focused on maintaining quality control of our pipeline products. In 2021, we moved pipeline product development to our new site in Daxing District of Beijing, where we also produce CoronaVac. Currently, we have a research and development team of over 500 people and have established an academic workstation, as well as a national-level post-doctoral scientific research workstation.
In order to achieve our R&D goal, part of our R&D strategy is to focus on in-house development and to establish collaborations with domestic and international partners on technology and key material licensing, including but not limit to strains and cell lines. We have entered into collaborations with a group of leading universities, colleges and research institutes that have strong vaccine research capabilities and proven track records in China. In most cases, we will own the commercial rights to the products that result from our existing R&D strategic collaborations.
The investment in R&D is one of our strategies, which, we believe, will ensure our future growth. Our research and development expenses were $422.1 million, $155.0 million and $48.8 million in 2022, 2021 and 2020, respectively. We have obtained financial support from the PRC government to conduct preclinical and clinical research of vaccines for government-sponsored programs.
Sales and Marketing
Our sales strategy is to increase our market share and enhance our competitive advantage in the private vaccine sales market in China while building on this strength to encourage government to expand market size in the government-paid market. We also intend to establish our presence, increase our sales to international markets and enhance awareness of our products outside China.
In 2018, our sales model was totally transformed to a collaborative model between our sales team and third-party marketing agents. We have formed a marketing management team, strengthened the compliance management to third-party marketing agents, and expanded market coverage, improved our competitive position in the market, and improved the quality of customer services through professional and academic promotion activities. As of December 31, 2022, our internal sales and marketing team covered 2,394 district CDC customers in 31 provinces and municipal cities in China. Our sales and marketing team is mainly responsible for developing marketing strategy for each product, medical education of our products, maintenance of customer relationship at or above the provincial level, bidding access at the provincial level, development of the public market, as well as product sales related services and the support and management of third-party marketing promotion agents. We cooperate with 48 third-party marketing promotion agents, engaging approximately 1,500 marketing and promotional staff. The team of the third-party agents carries out business with district CDC customers and point of vaccination (POVs) with our support in all aspects. In addition, we have taken the lead in placing commercial insurance compensation mechanism for adverse events response to vaccination nationwide in the private vaccine market and certain government sponsored programs to provide more professional services for CDC customers and end-users. We believe these efforts contributed to our reputation for quality and brand awareness in the Chinese vaccine market.
In 2022, 2021 and 2020, our sales in China contributed 74.8%, 56.3% and 71.6%, respectively, of our total sales. As of December 31, 2022, we had already exported our vaccine products to 53 countries. In order to speed up the business globalization, as well as strengthening our reputation for quality, we obtained WHO prequalifications for hepatitis A vaccine, Healive, in December 2017, sIPV vaccine in June 2022 and for live attenuated varicella vaccine in November 2022. In June 2021, CoronaVac was approved for the WHO’s EUL procedure.
40
We will continue to explore the globalization of our product portfolio and develop products targeting potential international markets where we believe we can be successful.
Seasonality
Our business is highly seasonal. For example, the influenza season generally runs from November through March of the next year, and the largest percentage of influenza vaccinations is administered between September and November of each year. As a result, we expect to realize most of our annual revenues from seasonal flu vaccines from August to October. We expect this seasonality in our business to contribute to significant quarterly fluctuations in our operating results. In the first quarter, our strong winter-season sales are usually offset by the slow-down of business during the Chinese New Year holiday season that effectively lasts more than half a month. During this holiday season, many businesses in China, including CDCs and most departments in hospitals, are either closed or substantially reduce the level of their activities. Please see “Item 3. Key Information — D. Risk Factors — Risks Related to Our Company — Our business is highly seasonal. This seasonality will contribute to our operating results fluctuating considerably throughout the year.”
Suppliers
We obtain the raw materials from local and overseas suppliers. We generally maintain at least two suppliers for each key raw material, with the exception of hepatitis B antigens we have used for Bilive production. We have sourced hepatitis B antigens entirely from Beijing Biological. Please see “Item 3. Key Information — D. Risk Factors — Risks Related to Our Company — If any of our third-party suppliers or manufacturers cannot adequately meet our needs, our business could be harmed.” Raw materials generally are in good supply and the prices we pay for them have remained stable. We target to maintain our gross margin in the event of rising raw materials costs by improving our production processes and technical methods.
Manufacturing, Safety and Quality Assurance
We have four manufacturing bases located in the Haidian, Changping and Daxing districts of Beijing and Dalian in Liaoning province.
We have three upstream production facilities in Haidian District, Beijing for commercialized products. Our Healive has an annual capacity of 20 million doses. Our influenza production line has an annual capacity of 15 million doses, which can also be used to produce 20 million doses of Panflu or Panflu.1 annually. Our PPV has an annual capacity of 15 million doses.
We received GMP certificates for our Healive, Bilive and influenza production facilities initially in March 2002, June 2005 and October 2005, respectively, and renewed their GMP certificates for another five years in 2018. The upstream production plants for our hepatitis vaccines and influenza vaccines in Haidian District passed the new GMP certification and obtained the new GMP certificate on April 17, 2013, which was renewed on April 13, 2018 for five years. Our hepatitis A vaccine production lines in both Shangdi site and Changping site passed GMP inspection by WHO for prequalification purpose in December 2017. Our upstream production line for PPV, with an annual production capacity of 15 million doses, was built in Shangdi site in 2014, which passed the GMP inspection by the NMPA in June 2020 and production license was granted on December 2, 2020.
Our production site in Changping District, Beijing primarily consists of a filling and packaging facilities that complies with the new PRC GMP standards, as well as the EV71 production facility and the sIPV production facility. The EV71 vaccine production line has a designed annual capacity of 20 million doses and was granted the GMP certificate in January 2016. Our GMP compliant sIPV vaccine production line was built in 2017 with a designed annual production capacity of 40 million doses. Our sIPV vaccine production lines in Changping site passed GMP inspection by WHO for prequalification purpose in June 2022.
We have three production sites in Daxing District, Beijing for CoronaVac which can also be used for other products in the future. The sites are in compliance with the new PRC GMP standards.
Our production site in Sinovac Dalian focuses on the research, development, manufacturing and commercialization of live-attenuated vaccines, such as varicella, mumps and combination vaccines containing measles, mumps, rubella, and/or varicella. Sinovac Dalian received its GMP certificate from NMPA for its mumps vaccine in September 2012 and launched mumps vaccine, its first commercial product, in late 2012. The renewed GMP certificate issued by Food and Drug Administration of Liaoning Province was obtained on February 13, 2018. Our varicella vaccine production line was inspected by the Medical Products Administration of Liaoning Province and a production license was granted in December 2019.
Each of our vaccine producing subsidiaries has its own quality assurance department. The quality assurance department of each subsidiary plays a role to supervise the R&D, manufacturing, procurement, quality control, sales and marketing, logistics and plant construction of each subsidiary under the guidance of the applicable regulations and guidelines. Regular training or seminars are organized among quality assurance departments of subsidiaries to share and exchange knowledge and experiences.
41
We have built a pharmacovigilance system, which includes organization structure, documentation, working procedures and SOPs. The organization structure indicates staff organization and their relevant duties and responsibilities. According to the requirements of the regulatory authorities, we regularly report the severe Adverse Event Following Immunization (“AEFI”) in time. We summarize and analyze safety information coming from post-marketing surveillance, phase IV clinical trials, safety studies and literatures, and to submit the Periodic Safety Update Reports to the regulatory authorities regularly. Meanwhile, we are also required to assist the regulatory authorities to investigate on the AEFIs and provide related information as required.
With respect to compliance with environmental laws, we have also obtained the approval of the environmental impact assessment report from the Beijing Municipal Environment Protection Bureau for the construction plan of our facilities in Changping District, Beijing in 2011. We produce Bilive vaccine at our production facility for hepatitis A vaccine and produce Panflu and Panflu.1 vaccines at our production facility for seasonal influenza vaccine. According to the PRC Environmental Impact Assessment Law, after the approval of previous environmental impact assessment reports, if there is any material change in the nature, scale, location, production technology used and measures adopted to prevent damages to ecology, new environmental impact assessment reports need to be filed for approval. We also added a sIPV production facility to the Changping construction plan in 2016. The relevant environmental impact assessment report was submitted to the relevant government authorities and passed the government evaluation. In addition, we have also obtained approval for the environmental impact assessment report for PPV production facility at our Shangdi site in 2014. In 2020, we obtained the approval for the environmental impact assessment report for the CoronaVac production facility.
Collaborations
In March 2009, we entered into a technology transfer agreement (with an amendment agreement entered into on December 14, 2011) with Tianjin CanSino Biotechnology Inc. (“Tianjin Cansino”). According to the agreement, Tianjin Cansino will transfer the technology related to pneumococcal vaccine to us and jointly develop the technology with us. The collaboration term under the technology transfer agreement is from March 12, 2009 to eight years after the first sale of the vaccine developed under the technology transfer agreement in the Chinese market.
Under the terms of the technology transfer agreement, we will make milestone payments of up to $3 million and royalty payments ranging from 6% to 10% of net sales in China. Both parties will work together to develop international markets for the products. On November 17, 2009 and December 14, 2011, two amendment agreements were signed for the payment of $0.3 million for the transfer of an additional six serotypes and related technology. As of December 31, 2022, we made total milestone payments of $1.2 million ($1.0 million under the agreement dated as of March 12, 2009 and $0.2 million under the amendment agreement dated as of December 14, 2011). The remaining milestone payments will be paid when we achieve each specific milestone, which includes obtaining clinical trials approval, completing clinical trials and achievement of desired results, and achievement of commercial sales.
In January 2015, we entered into the third amendment to the technology transfer agreement dated March 12, 2009, as amended on November 17, 2009 and December 24, 2011, respectively. By entering into this third amendment, the technology transfer agreement was amended to be a licensing agreement. The remaining milestone and royalty payments under the technology transfer agreement have been reduced. Both we and Tianjin Cansino are free to develop pneumococcal vaccines or to collaborate with other companies for the same purpose. We did not make any payment in this regard for the years ended December 31, 2022, 2021 and 2020.
In August 2009, we entered into a patent license agreement with the National Institutes of Health (“NIH”), an agency of the United States Public Health Services within the Department of Health and Human Services. NIH has granted us a non-exclusive license to import and use certain Rotavirus Strains and Monoclonal Antibodies (“Biological Materials”) to develop an oral rotavirus vaccine and produce the vaccine in commercial sales and launch into market. NIH has also granted us the right to use certain documentation associated with the Biological Materials for this research and development project. The term of the license under the patent license agreement, as amended in 2022, is from August 18, 2009 to the later of (a) the expiration of all royalty obligations under the licensed rights where such rights exist and (b) twelve years after the first commercial sale by us, unless the agreement is terminated earlier per the provisions included therein.
We agreed to pay NIH a license royalty of $80,000 upon execution of the agreement and a non-refundable minimum annual royalty of $7,500, and royalty payments on net sales ranging from 1.5% to 4% depending on the sales territory and the customers. For each country in the licensed territory under the patent license agreement, we also agreed to pay NIH benchmark royalties in the total amount of $0.3 million upon achieving the benchmarks as specified in the patent license agreement, including completion of clinical trials, obtaining regulatory approval for marketing, and achievement of commercial sales. We recorded a license royalty of nil, nil and $1,000 for the year ended December 31, 2022, 2021 and 2020, respectively, as research and development expenses.
In August 2011, we licensed from Medimmune, LLC, a US based pharmaceutical company, certain non-exclusive rights to use patented reverse genetics technology pertaining to H5N1 influenza virus strain production for vaccines. We agreed to pay an upfront license fee and milestone payments of up to an aggregate of $9.9 million based upon achievement of cumulative net sales of licensed products in China (including Hong Kong and Macau), as well as royalty payments in single digit of net sales of the licensed products in China (including Hong Kong and Macau).
42
License fee and royalties of $3.4 million accrued at the end of 2011 were paid in 2012. We did not accrue any royalty payment in 2022, 2021 and 2020.
In April 2014, we entered into a non-exclusive license agreement with INTRAVACC, a governmental institute working under the Dutch Ministry of Public Health, Welfare and Sports, to develop and commercialize sIPV for distribution in China and other countries. The agreement has a term of 50 years.
We agreed to pay INTRAVACC a license fee of up to $2.4 million (€1.5 million) net of PRC withholding tax, including an entrance fee and milestone payments upon achievement of specific milestones. We also agreed to pay royalty payments in a single digit percentage of net sales generated worldwide from the product or products developed under the license agreement. We recorded a payment of $67,796 (€60,000) for the year ended December 31, 2022, as cost of goods sold. We recorded a payment of $72,345 (€60,000) for the year ended December 31, 2021, as cost of goods sold. We recorded a payment of $35,000 (€30,000) for the year ended December 31, 2020, as research and development expense.
In September 2015, Sinovac Dalian entered into a technology transfer and supply agreement with GlaxoSmithKline Biologicals SA (“GSK”), to use GSK’s measles seeds to develop combination vaccines containing measles for the China market. Under this agreement, GSK agreed to transfer its measles seeds, and provide reasonable assistance and relevant technical materials to Sinovac Dalian for developing and producing combination vaccines containing measles. We did not make any payment for purchasing measles seeds to GSK for the years ended December 31, 2022, 2021 and 2020.
Competition
The pharmaceutical, biopharmaceutical and biotechnology industries both within China and globally are intensely competitive and are characterized by rapid and significant technological progress, and our operating environment is increasingly competitive. In 2010, the NMPA increased the quality standard of some vaccine products by issuing a new version of Pharmacopeia. As a result, some vaccine products manufactured by multinational companies could no longer be sold in China. According to the NMPA, there are approximately 50 vaccine companies in China, of which we believe approximately 15 are our direct competitors for our non-COVID-19 vaccines.
Even with the advent of private medical and healthcare insurance programs in China and the government vaccine purchase program’s expanded vaccine list, most Chinese citizens must pay for vaccines by their own because these insurance programs do not typically cover vaccines and the government vaccine purchase program covers only infants and young children. We believe the consumer market for conventional products is health conscious yet price sensitive and accordingly would favor our products over both the cheaper vaccines with lower quality provided by local manufacturers and the more expensive vaccines with comparable quality manufactured by international competitors. Our competitors, both domestic and international, include large integrated multinational pharmaceutical, domestic state-owned entities and domestic private companies that currently engage in, have engaged in or may engage in, efforts related to the discovery and development of new biopharmaceuticals and vaccines. Many of these entities have substantially greater research and development capabilities and financial, scientific, manufacturing, marketing and sales resources than we do. They are also more experienced in research and development, clinical trials, regulatory matters, manufacturing, marketing and sales.
Multiple vaccine products have been approved for sales worldwide. Many of these vaccine products are marketed by our major competitors in particular for hepatitis A, influenza, COVID-19 vaccine, varicella and sIPV. Specifically, with respect to the inactivated hepatitis A vaccine, we consider Merck Sharp & Dohme Corp.(“MSD”) as key competitors in China, and GlaxoSmithKline Biologicals (“GSK”)and MSD for the markets outside China. The live attenuated hepatitis A vaccine manufacturers include Institute of Medical Biology - Chinese Academy of Medical Sciences (IMBCAM), Pukang Biological Co., Ltd., and Changchun Institute of Biological Products. With respect to the hepatitis A and B vaccines, we are the only company with this product in China. With respect to the influenza vaccines, we consider Hualan Biological Engineering Inc. and Changchun Institute of Biological Products as key competitors in China, and Sanofi Pasteur S.A. as our major competitor for the markets outside China. With respect to the EV71 vaccines, we consider IMBCAM and China National Biotec Group Co., Ltd. as our key competitors in China, as well as GSK, MSD, GC Biopharm and SK Biosicence Worldwide. With respect to the COVID-19 vaccine, we consider China National Biotec Group, Pfizer, Moderna and AstraZeneca as our key competitors. With respect to the varicella vaccine, we consider Changchun BCHT Biotechnology Co., Ltd. and Changchun Keygen Biological Products Co., Ltd. as key competitors in China. With respect to the 23-valent pneumococcal polysaccharide vaccine, we consider Chengdu Institute of Biological Products, Walvax Biotechnology Co., Ltd. and Shenzhen Kangtai Biological Products Co., Ltd. as key competitors in China. For inactivate polio vaccine we consider IMBCAM and Sinopharm as our key competitor in China.
We believe we enjoy a number of advantages over the PRC domestic competitors and multinational competitors in China. Generally, we believe that the principal competitive advantage in the markets for our products and product candidates include:
43
Intellectual Property and Proprietary Technology
Protection of our intellectual property and proprietary technology is important to our business. We rely primarily on a combination of trademark, patent and trade secret protection laws in China and other jurisdictions, as well as employee and third-party confidentiality agreements to safeguard our intellectual property, know-how and brand. Our ability to protect and use our intellectual property rights in the development and commercialization of our technologies and products, operate without infringing the proprietary rights of others and prevent others from infringing our proprietary rights is crucial to our long term success. We will be able to protect our products and technologies from unauthorized use by third parties only to the extent that they are covered by valid and enforceable patents, trademarks or copyrights, or are effectively maintained as trade secrets, know-how or other proprietary information.
We have a total of 94 issued patents and a number of pending patent applications related to our vaccines in China.
With respect to, among other things, proprietary know-how that is not patentable and processes for which patents are difficult to enforce, we rely on trade secret protection and confidentiality agreements to safeguard our interests. We believe that many elements of our vaccine products, clinical trial data and manufacturing processes involve proprietary know-how, technology or data that are not covered by patents or patent applications. We have taken appropriate security measures to protect such assets. We have entered into confidentiality agreements (which include, in the case of employees, non-competition provisions) with all our employees and many of our consultants, outside scientific collaborators, sponsored researchers and other advisors. These agreements provide that all confidential information developed or made known to the individual or organization or company during the course of its relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances permitted by such agreements. In the case of our employees, the agreements provide that all of the technology conceived by the individual during the course of employment is our exclusive property and require our employees to assign to us all of their inventions, designs and technologies they develop once the technology is conceived and cooperate with us to secure patent protection for these inventions if we wish to pursue such protection.
We maintain 44 trademark registrations in China and countries and regions where we conduct business, including (i) “Sinovac”, (ii) Sinovac’s Chinese name and its logo, (iii) “Healive”, its Chinese name and its logo, (iv) “Bilive” and its Chinese name, (v) “Anflu” and its Chinese name, (vi) “Panflu”, its Chinese name and its logo, (vii) “PANFLU.1” and its Chinese name, (viii) “Inlive” and its Chinese name, (ix) “EV71Vac” , (x) “EntV71” and its Chinese name, and (xi) “CoronaVac” and its Chinese name.
As our brand names “Sinovac” and “科兴” are becoming more recognized in the vaccine market, we are working to maintain, increase and enforce our rights in the trademark portfolio. Since 2001, Sinovac Beijing has been using “科兴” (Kexing) as part of its Chinese trade name. Sinovac Dalian began to use “科兴” (Kexing) as part of its Chinese trade name in 2010. Shenzhen Kexing successfully registered “科兴” trademark in China for Class 5 (Pharmaceuticals) under the International Classification of Goods and Services in 2001. To protect our interest in using “科兴” in our trade names, we applied to register “科兴” in China for Class 42 (Scientific & Technological Services & Research) in 2006 and the PRC Trademark Office of the State Administration for Industry and Commerce approved our application in 2010. As of the date of this annual report, the “科兴” trademark registered and owned by Shenzhen Kexing has not been identified as “Well-known Trademark” by the relevant PRC authorities. If the “科兴” trademark owned by Shenzhen Kexing is ever officially identified as a “Well-Known Trademark” in the future, however, we may be subject to trademark infringement claim for the use of “科兴” in our trade names.
We have registered our own domain names, including www.sinovac.com.cn and www.sinovac.com, with the China Internet Network Information Center.
Insurance
We maintain property insurance coverage with an annual aggregate insured amount of approximately RMB6,449 million ($935 million) in 2022 to cover our property and facilities from claims arising from fire, earthquake, flood and a wide range of other natural disasters. We are carrying worldwide product liability insurance for Healive, Bilive, Anflu, Panflu and Inlive (excluding the United States and Europe) from April 2022 to April 2023. We also carry product liability insurance for all of our products in China. We maintain liability insurance to cover liability claims that may arise from the incidents relating to certain of the clinical trials of our vaccine products. Our insurance coverage may not be sufficient to cover any claim for product liability or damage to our fixed assets. We do not maintain any business interruption insurance. We are negotiating with the insurance providers for a renewal of our product liabilities insurance policies. See “Item 3. Key Information — D. Risk Factors — Risks Related to Our Company — We could be subject to costly and time-consuming product liability actions and, because our insurance coverage is limited, our exposure to such claims could cause significant financial burden.”
44
Regulatory Framework of the Pharmaceutical Industry in the PRC
The testing, approval, manufacturing, labeling, advertising and marketing, delivery, post-approval safety reporting, and export of our vaccine products or product candidates are extensively regulated by governmental authorities in the PRC and other countries.
In the PRC, the NMPA regulates and supervises vaccine products under the Pharmaceutical Administration Law, the Implementing Regulations on Pharmaceutical Administration Law, the Vaccine Administration Law, the Administration of Registration of Pharmaceuticals Procedures, and other relevant rules and regulations which are applicable to manufacturers in general. Every step of our vaccine production is subject to the requirements on the manufacture and sale of pharmaceutical products as provided by these laws and regulations, including but not limited to, the standards of clinical trial, approval and transfer of new medicine registrations, applicable industry standards of manufacturing, distribution, packaging, advertising and pricing.
Pre-clinical Studies. Pre-clinical studies include in-vitro laboratory evaluation of the product candidate, as well as in-vivo animal studies to assess the potential safety and efficacy of the product candidate. Non-clinical studies must be conducted in compliance with Good Laboratory Practice for Non-clinical Studies of Pharmaceuticals. With respect to vaccines, the pre-clinical studies should also comply with Technical Guidance for Pre-clinical Studies on Preventive Vaccines. We must submit a file package for investigational new drug (“IND”) application to the Centers for Drug Evaluation. The applicant shall be provided with a decision on whether a consent is granted to conduct clinical study. If no decision is provided within 60 days, it’s regarded as permission granted. We cannot assure that submission of an IND will result in the Centers for Drug Evaluation allowing clinical trials to begin, after these trials commence, issues could arise that result in the suspension or termination of such clinical trials.
Communication Meeting. In order to improve review process of regulatory approval, the NMPA has set up a communication channel between the applicant and reviewing agencies. Applicant can discuss material safety issues during the human clinical study or significant technical issues arise during the development process with regulatory agencies. This kind of meetings can also be held at critical stages in the entire process of drug development, including before IND application, before phase III human clinical studies, or before NDA.
Clinical trials. Clinical trials involve the administration of the product candidate to healthy volunteers or patients under the supervision of principal investigators, who are generally physicians or an independent third party not employed by us or under our control. Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. In phase I, the initial introduction of the drug into human subjects, the drug is usually tested for safety (adverse effects), dosage tolerance, and pharmacologic action. phase II usually involves studies in a limited patient population to evaluate preliminarily the efficacy of the drug for specific, targeted conditions and to determine dosage tolerance and appropriate dosage and to identify possible adverse effects and safety risks. Phase III trials generally further evaluate clinical efficacy and test further for safety within an expanded patient population. Clinical trials have to be conducted in compliance with the Good Clinical Trial Practice of Pharmaceuticals.
With respect to vaccines, we also have to comply with the NMPA’s Requirements on Application for Clinical Trial of New Preventive Biological Products. The sample vaccine products must be tested by the National Institutes for Food and Drug Control (the “NIFDC”) before they may be used in the clinical trials. We or the NMPA may suspend clinical trials at any time on various grounds, including a finding that subjects are being exposed to an unacceptable health risk.
After three phases of clinical trials, we apply for New Drug Application (“NDA”). We submit to the Centers for Drug Evaluation the NDA file package, which includes a clinical trial research report, pharmaceutical research data, and records of manufacturing and testing of three batches of products, to apply for the marketing authorization. For vaccines, we have to comply with the NMPA’s Guidelines for Clinical Trial Report on Vaccines.
Marketing Authorization. The applicant can submit an application for a marketing authorization with submission of relevant research materials after completing the research on pharmacology, pharmacological toxicology and clinical trials to support the registration of drugs on the market, establishing quality standards, completing the verification of commercial-scale production processes, and preparing to accept the verification and inspection of drug registration. If the application dossiers pass the formal examination, they will be accepted. The Center for Drug Evaluation would organize pharmaceutical, medical and other technicians to evaluate the accepted application of drug marketing authorization according to the requirements. The Center for Drug Evaluation would then initiate the verification and inspection action based on the risks identified during the evaluation and the relevant technical institutes would conduct the verification and inspection within the time limit. NMPA will verify the authenticity of submitted document, reliability of submitted data and conduct site inspection on research lab and production site, as well as other inspections as NMPA thinks necessary. For vaccine products, the on-site inspection on production site and the inspection of the quality management on the production of vaccine products shall be conducted. Vaccine products shall be tested before marketing authorizations are issued. The testing includes confirmation on the quality stand and sample testing. Subsequently the Center for Drug Evaluation shall conduct a comprehensive review of the safety, effectiveness and quality controllability of drugs on the basis of the drug registration declaration information, verification results and inspection results, etc., and, if the conclusions of the comprehensive review are adopted, the drug marketing authorization will be approved and a drug registration certificate will be sent.
The marketing authorization is valid for a term of five years and must be renewed before its expiration. During the renewal process, our production facilities will be re-evaluated by the appropriate governmental authorities and must comply with effective standards and regulations.
45
During or after a public health emergency, the NMPA may decide, in accordance with the law, to apply special approval for the prevention and treatment of medicines necessary for emergency response to public health emergencies. For applications for the registration of drugs subject to special approval, the NMPA shall, organize and carry out the processing, review, verification and inspection of drug registration in a simultaneous manner, following the principles of centralized coordination, early intervention, and rapid, efficient and scientific examination and approval. The circumstances, procedures, time limits and requirements of special approval shall be implemented in accordance with the provisions of the special approval procedure for medicines.
We may also be required to conduct clinical trials prior to commencing the manufacturing of pharmaceutical products for which there are published state pharmaceutical standards.
Batch Approval. Our vaccine products cannot be distributed in the market before receiving batch approval. After we obtain the production permit, we will start commercial production, after which we need to apply for batch release approval by the NIFDC for the commercial lots. For each batch of products, we will provide samples taken from cold rooms by inspectors, together with manufacturing records, self-testing records and other quality control documents. The NIFDC will review the documents and test the samples and issue a batch approval within approximately two months if our manufacture procedures and the quality of our products meet the NMPA standards. With the batch approval, we may distribute the approved batch of vaccines to the market.
Regulatory Framework of the Vaccine Administration in the PRC
On December 1, 2019, the PRC Vaccine Administration Law, China’s first legislation dedicated to vaccine management, became effective.
The new law is expected to enable the regulators to close loopholes and rein in risks in vaccine management. In addition, the strategic position of the vaccine industry to the whole country and its welfare nature concerning the general public have been clearly recognized in the new law. The Chinese government will support the fundamental scientific research and commercialization research of vaccine products to encourage the development of innovation technologies and new vaccine products. The research and development, production and stockpiling of vaccine products preventing serious diseases will be part of the state strategy. The new law also makes it very clear that the PRC government will encourage the further consolidation of the vaccine industry so that manufacturers with large scale production capacities of more quality products, using more advanced technologies, could emerge. All of these new regulatory regimes to be established under the new law may largely boost the confidence of the public in vaccine products manufactured in China.
The new PRC Vaccine Administration Law implements more stringent supervision of the entire process of vaccine development, production, delivery, and inoculation. The legislation mandates both government oversight and the duty of manufacturers to report compliance in all substantial aspects of the whole lifecycle of vaccine products. The sanctions and penalties for the illegal activities have been significantly increased. For instance, the sanctions for production or selling of fake or substandard vaccines extend to include confiscating of all illegal gains obtained from and the materials, equipment and other facilities and resources used for production or selling of fake or substandard vaccines, suspension of business for corrections, revoking of drug registration certificate or production license. The fines can be as high as 15 times to 50 times of the market value of the fake vaccines or 10 times to 30 times of the market value of the substandard vaccines. In the case of serious circumstances, the legal representative, the person in charge or the key personnel who are directly responsible for production or selling of fake or substandard vaccines and the other persons responsible are also subject to sanctions of confiscating their income during the production period of the fake or substandard vaccines, a fine of one time to ten times of the said income. Such persons will be permanently banned from engaging in drug production activities and will be subject to five to 15 days of detention.
Classification of Vaccines
Vaccines refer to preventive biological products for human vaccination so as to prevent and control the occurrence and prevalence of diseases, including the Expanded Program of Immunization (EPI) and vaccines not covered by the immunization program (the “Private-Pay Market”).
Mandated Manufacturing
Market authorization holder of vaccines refers to the enterprise who obtains both a vaccine registration certificate and a drug manufacturing license. Market authorization holders of vaccines must have adequate vaccines manufacturing capacity. Where the mandated manufacturing is necessary due to inadequate vaccines manufacturing capacity, the market authorization holder of vaccines must obtain an approval of the NMPA for such mandated manufacturing.
46
Keeping of Sales Records
Market authorization holders of vaccines must keep accurate and complete sales records and keep the same for reference for at least five years after the shelf life of the relevant vaccines.
Compulsory Vaccines Liability Insurance
The PRC government will implement the rules for compulsory vaccines liability insurance. The market authorization holders of vaccines must underwrite the compulsory vaccine liability insurance. Specific implementing measures for the compulsory vaccine liability insurance system will be formulated by the medical products administration under the State Council in collaboration with the health administration and insurance regulatory authority under the State Council. In case of failure of complying with such obligation, the market authorization holder of vaccines will be imposed a fine up to RMB2 million.
Post-Market Management of Vaccines
(a) Post-market investigation
The market authorization holders of vaccines must establish the whole-lifecycle quality management system of vaccines, and carry out post-market investigation to further confirm the safety, efficacy and quality controllability of the vaccines supplied to the market. In case of failure of complying with such obligation, the market authorization holder of vaccines will be imposed a fine up to RMB2 million.
(b) Quality retrospection analysis and risk reporting
The market authorization holders of vaccines must set up a vaccines quality retrospection analysis and risk reporting system, and faithfully report relevant information on vaccine manufacturing, distribution, post-market investigation and risk management to NMPA on a yearly basis. In case of failure of complying with such obligation, the market authorization holder of vaccines will be imposed a fine up to RMB2 million.
(c) Post-market evaluation
The NMPA has the right to request a market authorization holder of vaccines to conduct post-market evaluation or directly organize post-market evaluation. The NMPA will cancel the drug registration certificate for vaccines with serious adverse event to vaccination or endangering human health due to other causes.
Information Disclosure
The market authorization holders of vaccines must establish an information disclosure system and promptly disclose vaccine product information, package insert and labels, situations concerning the implementation of quality control, lot release, recall, inspection and punishment imposed and compulsory vaccine liability insurance effected, etc. on its website as required. In case of failure of complying with such obligation the market authorization holder of vaccines will be imposed a fine up to RMB2 million.
Following the promulgation of the PRC Vaccine Administration Law in June 2019, the MIIT announced that the thresholds to entry in the Chinese vaccine industry will be raised and they will more strictly control the number of new vaccine manufacturers to be established.
C. Organizational Structure
The chart below summarizes our corporate structure and identifies our significant subsidiaries, as that term is defined under Section 1-02 of Regulation S-X under the U.S. Securities Act, and subsidiaries representative of our major business, as of the date of this report.
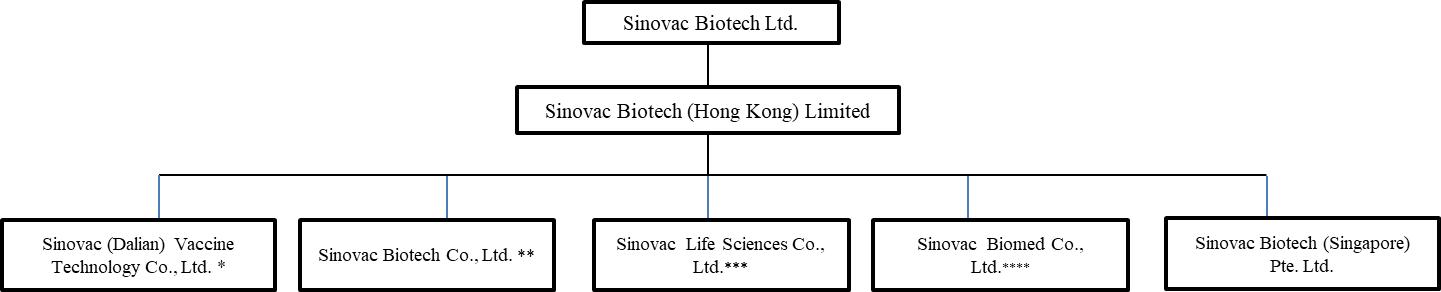
47
As of December 31, 2022, we held 100% equity interest in Sinovac Biotech (Hong Kong) Limited, our subsidiary incorporated in Hong Kong, Sinovac Biotech (Singapore) Pte. Ltd., our subsidiary established in Singapore, and Sinovac Biomed Co., Ltd., our subsidiary established in the PRC; we hold 73.09% of equity interests in Sinovac Biotech Co., Ltd., our subsidiary established in the PRC, 59.24% of the equity interests in Sinovac Life Sciences Co., Ltd. (formerly known as Sinovac Research & Development Co., Ltd.), our subsidiary established in the PRC, and 68% of the equity interests in Sinovac (Dalian) Vaccine Technology Co., Ltd., our subsidiary established in the PRC.
* Dalian Jin Gang Group Co., Ltd. owns the remaining 32% equity interest in Sinovac (Dalian) Vaccine Technology Co., Ltd.
** Sinobioway Bio-medicine Co., Ltd., formerly named Xiamen Bioway Group Co., Ltd, owns the remaining 26.91% equity interest in Sinovac Biotech Co., Ltd.
*** Affiliates of Sino Biopharmaceutical Limited, Keding Investment (Hong Kong) Limited, Vivo Capital Fund IX and Prime Success, L.P. owns 15.38%, 12.69%, 6.345% and 6.345%, respectively, of the remaining equity interest in Sinovac LS, former name of which was Sinovac
Research & Development Co., Ltd.
**** The former name is Sinovac Zhong Yi Bio-pharmaceutical Co., Ltd.
D. Property, Plants and Equipment
We are headquartered in the Peking University Biological Industry Park in Haidian District in Beijing, with the total construction area of 48,980 square feet (4,550 square meters), of which approximately 16,700 square feet (1,551 square meters) are used as office space and approximately 32,300 square feet (2,999 square meters) are used for the production plant for Healive. We own the facilities on this construction site.
In August 2004, we signed two 20-year leases with SinoBioway Biotech Group Co. Ltd. (“SinoBioway”), pursuant to which we leased two buildings of approximately 35,800 square feet (3,330 square meters) and 13,300 square feet (1,236 square meters), respectively, located at the Peking University Biological Park in Beijing. We house our Anflu manufacturing and R&D center in these two buildings. One of the lease agreements was amended on August 12, 2010 to reflect an increase in the lease rental. In June 2007, we signed another 20-year lease with SinoBioway, in order to expand Sinovac Beijing’s production facilities in our Shangdi site, pursuant to which we lease one building of approximately 37,000 square feet (3,437 square meters), located at Peking University Biological Park. Part of our administrative offices and filling facilities are located in this building until 2013. The filling facilities have been moved to Changping site since 2013, and the original filling facilities space is set up as the commercial production facility for our pneumococcal vaccines.
In September 2010, we entered into an agreement with SinoBioway, under which we lease a space of 6,780 square feet. The lease term is five years and we use it for our research and development function. On April 8, 2013, we entered into three supplemental agreements with SinoBioway, under which the expiration date of each of the four operating lease agreements was extended to April 7, 2033.
All these offices and production facilities in the Peking University Biological Industry Park are known as our Shangdi site. We have three production lines located at the Shangdi site. The production line to manufacture hepatitis vaccines 20 million doses annually. The production line to manufacture influenza vaccines, Anflu, QIV, Panflu and Panflu.1, interchangeably has an annual production capacity of approximately 15 million doses of Anflu. We have built a PPV production line at the Shangdi site with designed annual capacity of 15 million doses per year.
In February 2010, we acquired a right to use approximately 312,380 square feet (29,021 square meters) of land located in Changping District, Beijing (“Changping Site”) with five buildings with a total built-out area of 347,900 square feet (32,322 square meters) for a total consideration of approximately RMB123.6 million. We have made all required payments by December 31, 2012. We built a new filling and packaging line, EV71 production facilities and a storage warehouse at the Changping site. In May 2013, the new filling and packaging facility at the Changping site was granted the GMP certificate, following which, we moved all the filling and packaging activities to the Changping site. The five-year GMP renewal at the Changping site for the filling and package line was successfully completed on April 13, 2018. The new storage warehouse was put into operation in December 2010. The EV71 vaccine production line at the Changping site has a designed annual capacity of 20 million doses and was granted the new GMP certificate in January 2016. In July 2016, we started to build our sIPV plant for bulk production at the Changping site with capacity approximately 40 million doses and we obtained the approval of sIPV from the NMPA in 2021. In 2021, we expanded the filling and packaging facilities to add additional filling and packaging lines and moved the storage to leased warehouse.
In November 2009, we entered into an agreement with Dalian Jin Gang Group to establish Sinovac Dalian. In January 2010, we established Sinovac Dalian which focuses on the research, development, manufacturing and commercialization of live-attenuated vaccines, such as varicella, mumps and rubella vaccines for human use. Sinovac Dalian has nine existing buildings with a total construction area of 581,200 square feet (54,000 square meters), located at DD Port, Economic and Technical Development Zone, Dalian City, Liaoning province. Sinovac Dalian received its GMP certificate (2010 version) from the NMPA for its mumps vaccine in September 2012 for five years. The renewed GMP certificate issued by Food and Drug Administration of Liaoning Province was obtained on February 13, 2018, which will remain valid until February 12, 2023. The construction of a varicella vaccine production plant was completed in 2019. The production permit was granted in December 2019 after the Medical Products Administration of Liaoning Province’s inspection of our varicella vaccine production line. The annual capacity of the varicella production line is 5 million doses.
In 2009, we established Sinovac LS focusing on R&D. In 2020, Sinovac LS obtained a drug production license. Currently, Sinovac LS is mainly committed to the research and development, production and sales of COVID-19 vaccine, CoronaVac, as well as the research and development of other new products. Sinovac LS is located in the biomedical industry base in Daxing District, Beijing, which has five production bases through
48
direct ownership or lease. Our CoronaVac production sites are in compliance with the new PRC GMP standards. Sinovac LS had a rapid growth in 2020 and 2021, and in order to host future pipeline product manufacturing facilities, Sinovac LS also acquired an additional campus in the same area. As of the date of this annual report, Sinovac LS has a total built-out area of 511,000 square meters and has a total construction area of 376,000 square meters in the Daxing District.
In September 2022, we initiated the construction of a new industrialization base in Zhongguancun Life Science Park in Changping District of Beijing, which is designed to be a base for the industrialization of our high-tech achievements. It is planned to consist of seven main buildings, with a total construction area of about 1,324,000 square feet (123,000 square meters), and the total investment amount is expected to exceed RMB2 billion. It is expected that the construction of the main structure will be completed by the end of 2023 and the buildings will be put into use in 2024. After completion, the base will serve as the generic technology platform, service platform and manufacturing base for frontier fields in biomedicine in China to accelerate the transformation of biomedical research achievements.
In July 2022, we commenced the construction of the Vaccine Quality Research Center in our Changping site, with a construction area of 280,000 square feet (26,000 square meters). The Vaccine Quality Research Center is expected to be put into use in the second half of 2023. It is mainly used for the research on storage and quality control of vaccines during their whole manufacturing process from active pharmaceutical ingredient to finished products, providing support for improving vaccine quality standards and quality control abilities and housing the industrialization of different varieties of vaccines.
ITEM 4A. Unresolved Staff Comments
Not applicable.
ITEM 5. Operating and Financial Review and Prospects
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our consolidated financial statements and the related notes included elsewhere in this annual report on Form 20-F. This discussion may contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Item 3. Key Information — D. Risk Factors” or in other parts of this annual report on Form 20-F.
49
Overview
We are a fully integrated, China-based biopharmaceutical company that focuses on the research, development, manufacturing and commercialization of vaccines against infectious diseases. We have successfully developed a portfolio of products, consisting of vaccines against hepatitis A, hepatitis B, EV71, influenza viruses, mumps, varicella, pneumococcal, COVID-19 and poliomyelitis. The following table sets forth certain information on our commercialized products.
|
|
|
Products |
|
Date of Approval |
Healive |
|
May 2002 |
Bilive |
|
June 2005 |
Anflu |
|
October 2005 |
Inlive |
|
January 2016 |
Panflu(1) |
|
April 2008 |
Panflu.1(1) |
|
September 2009 |
Mumps |
|
September 2012 |
Varicella |
|
December 2019 |
Quadrivalent influenza |
|
June 2020 |
Pneumococcal polysaccharide |
|
December 2020 |
CoronaVac |
|
February 2021 |
sIPV |
|
June 2021 |
Our Proprietary Rights
Healive was co-developed by Tangshan Yian and the NIFDC. In April 2001, Tangshan Yian contributed its proprietary rights to Healive to Sinovac Beijing as its capital contribution. In 2002, the NIFDC, Tangshan Yian and Sinovac Beijing agreed that Sinovac Beijing owned the right to market and sell Healive, and that Sinovac Beijing was required to pay the NIFDC approximately $1 million for the Healive technology consulting fee that Tangshan had not paid by that time. We obtained Healive’s new drug certificate from the NMPA in December 1999, the production license in May 2002, and final PRC regulatory approval for production of Healive in May 2002. Production of Healive commenced in July 2002.
Bilive was initially developed by Tangshan Yian. In March 2002, Tangshan Yian and Beijing Keding entered into an agreement under which Tangshan Yian transferred to Beijing Keding its proprietary rights to Bilive at no cost. In August 2002, Sinovac Beijing acquired the proprietary rights to Bilive from Beijing Keding in consideration of a 10.7% equity interest in Sinovac Beijing and a cash payment of $18,000. Beijing Keding is owned by Mr. Weidong Yin and three other senior officers of Sinovac Beijing. We received the production license for Bilive from the NMPA in January 2005. In June 2005, we obtained the final PRC regulatory approval for production of Bilive. The cost of the proprietary rights to Bilive was expensed as purchased in-process research and development. Production of Bilive commenced in June 2005.
In March 2003, Sinovac Beijing acquired the proprietary rights to Anflu from Tangshan Yian at the vendor’s cost. In November 2004, we completed the acquisition of 100% of the shares of Tangshan Yian. We received final PRC regulatory approval for the production of Anflu in October 2005. The cost of the proprietary rights to Anflu was expensed as purchased in-process research and development.
Sinovac Beijing started to research and develop the H5N1 vaccine in 2004. In 2004, Sinovac Beijing entered into an agreement with the National Institute for Biological Standards and Controls (“NIBSC”), an England based laboratory under the WHO, on transferring the H5N1 virus strain. According to the agreement, Sinovac Beijing as the recipient would receive the materials and information from NIBSC. The agreement indicated that Sinovac Beijing can only use received materials and information for academic in-house research purposes and Sinovac Beijing shall negotiate with the owner of reverse genetics technology pertaining to virus strain for any commercial purpose. In April 2008, Sinovac Beijing received a production license for H5N1 from the PRC government and started to produce H5N1 vaccines for the government-stockpiling program in June 2008.
In 2011, we licensed from MedImmune certain rights to use patented reverse genetics technology pertaining to virus strain production for H5N1 influenza vaccine. We have agreed to pay an upfront license fee, milestone payments up to an aggregate of $9.9 million based upon the achievement of cumulative net sales of licensed products in China (including Hong Kong and Macau), as well as royalty payments in single digit of net sales of the licensed products in China (including Hong Kong and Macau). On August 15, 2012, we entered into amended agreements with MedImmune to, among other things, extend the effectiveness of each agreement to reflect revised termination dates between December 2015 and
50
May 2021. License fee and royalties of $3.4 million accrued at the end of 2011 was paid in 2012. We accrued a royalty of $9,000 at the end of 2018, which was paid in 2019. No royalties were incurred for the years ended December 31, 2022, 2021 and 2020.
No amortization expenses were recorded in 2022, 2021 and 2020 for proprietary rights as they were fully amortized.
Research and Development Programs
The research and development strategy is developed by management and reviewed and approved by the board of directors of our company. Utilizing the resources and platform of each subsidiary, the R&D team of each subsidiary selects a R&D project and develops a feasibility analysis for review and approval by the board of directors. Once the project is approved, the R&D progress as well as the spending of each project will be tracked. Each year all the ongoing R&D projects will be reviewed along with the budgeting for the following year.
We also use our research and development resources, including employees and our technology, across multiple product development programs.
The process of developing, obtaining and maintaining regulatory approvals for new products is lengthy, expensive and uncertain. While the development may take years to complete, the market environment may change from the time when the project is selected, which will have an impact on the expected return of the investment. We anticipate that we will frequently monitor the progress of each key project and determine which of our early-stage product candidates is best suited for further development, as well as how much funding to direct to each program, on an on-going basis in response to the scientific and clinical success and commercial potential of each product candidate.
Our pipeline consists of vaccine candidates with 5 types of vaccine technology in the clinical and pre-clinical development phases in China, as follows:
Group A and Group C Meningococcal Conjugate Vaccine, Freeze-dried.Neisseria meningitidis causes meningococcal disease, which are often severe, can be deadly, and include infections of the lining of the brain and spinal cord (meningitis) and bloodstream. Group A and Group C Meningococcal Conjugate Vaccine is a freeze-dried formulation containing Neisseria meningitidis serogroups A and C polysaccharides conjugated to a CRM197 protein carrier. The vaccine can effectively prevent meningococcal disease, especially for younger children (down to 2 months old) and teens. Our Group A and Group C Meningococcal Conjugate Vaccine was approved for clinical studies by NMPA in December 2022.
Group ACYW135 Meningococcal Conjugate Vaccine. This vaccine is a quadrivalent meningococcal vaccine containing polysaccharides from serogroups A, C, W, and Y conjugated to a CRM197 carrier protein, which protects against four types of meningococcal bacteria (types A, C, W, and Y). The vaccine effectively prevents younger children (down to 2 months old) and teens from meningococcal disease. Our company ACYW135 Meningococcal Conjugate Vaccine was approved for clinical studies by NMPA in April 2023.
Adsorbed Tetanus Vaccine. Tetanus is a specific infection in which Clostridium tetanus invades the body through skin or mucosal wounds, grows and multiplies in a hypoxic environment, and produces toxins that cause muscle spasms. Adsorption Tetanus Vaccine is mainly used for people with a high chance of trauma, also for the prevention of maternal and neonatal tetanus in pregnant women. Our Adsorbed Tetanus Vaccine was approved for clinical studies by the NMPA in June 2021.
Haemophilus Influenzae Type b Conjugate Vaccine. Haemophilus Influenzae Type b can cause a variety of sicknesses in humans, the most common and severe being meningitis, followed by pneumonia. Haemophilus Influenzae Type b Conjugate vaccine induces humoral immune responses to prevent aggressive infections (including meningitis, pneumonia, sepsis, cellulitis, epiglottitis, etc.) caused by Haemophilus Influenzae Type b. Our Haemophilus Influenzae Type b Conjugate Vaccine was approved for clinical study by the NMPA in August 2021.
Recombinant COVID-19 Vaccine (CHO Cell, Omicron Strain). Coronavirus Disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 is a highly contagious virus that causes the pandemic in the past three years. The severity of COVID-19 symptoms can range from very mild to severe, though mostly mild, some (especially the elderly) may experience worsened symptoms, such as worsened shortness of breath and pneumonia. COVID-19 vaccines are crucial tools to protect against severe disease and death. The Recombinant COVID-19 Vaccine stimulates the body to produce immunity against SARS-CoV-2 Omicron variant infection. We have submitted the Investigational New Drug Application (IND) to NMPA for Recombinant COVID-19 Vaccine (CHO Cell, Omicron Strain) in December 2022.
Reassortant Rotavirus Vaccine, Live, Oral, Hexavalent (Vero Cell). Rotavirus is a highly contagious virus that causes severe watery diarrhea, vomiting, fever, and abdominal pain, which spreads easily among infants and young children. The Reassortant Rotavirus Vaccine is an orally administered vaccine used for prevention of rotavirus gastroenteritis in infants caused by rotavirus infection of six serotypes. We have submitted the written request to NMPA for a Pre-IND meeting for Reassortant Rotavirus Vaccine, Live, Oral, Hexavalent (Vero Cell) in March 2023.
Bivalent Enterovirus Vaccine (Vero Cell), Inactivated. Hand, foot, and mouth disease (HFMD) is a contagious viral infection caused by Enterovirus or Coxsackievirus, with symptoms of fever, malaise, skin rash, sore throat, and small blisters that ulcerate, and few with severe
51
symptoms of viral encephalitis, brainstem encephalitis, pulmonary edema, acute flaccid paralysis, and even death. The Bivalent Enterovirus Vaccine can prevent infants and younger children from affecting EV-A71 and CV-A16, the two main pathogens associated with hand, foot, and mouth disease. We have submitted the written request to NMPA for a Pre-IND meeting for Bivalent Enterovirus Vaccine (Vero Cell), Inactivated in July 2021.
Pentavalent DTaP-IPV/Hib Combination Vaccine. Based on the above two developing vaccines—Adsorbed Tetanus Vaccine and Haemophilus Influenzae Type b Conjugate Vaccine, and our listed product Poliomyelitis Vaccine (Vero Cell), Inactivated, Sabin Strains, we are in the preclinical development process of the Diphtheria, Tetanus, Pertussis (acellular, component), Poliomyelitis (Sabin, inactivated) Vaccine (adsorbed) and Haemophilus Influenzae Type b Conjugate Vaccine (DTaP-IPV/Hib).
Other vaccines. Currently we are developing different types of vaccines to prevent more than 20 life-threatening diseases, which are under preclinical studies or have completed preclinical studies.
Government Grants
Deferred government grants represent funding received from the government for research and development, or investment in building or improving production facilities. The amount of deferred government grants as of year-end is net of research and development expenditures or depreciation incurred or those recognized as government grants income. We received government grants that were deferred in the amount of RMB48.2 million ($7.0 million), RMB17.0 million and RMB92.4 million in 2022, 2021 and 2020, respectively. In addition, we received RMB51.4 million ($7.6 million), RMB10.9 million and RMB21.7 million in other government grants and subsidies that were recognized in the statements of comprehensive income (loss) in 2022, 2021 and 2020, respectively.
Deferred government grants included the following:
Government grants for property, plant and equipment
We have three deferred government grants related to property, plant and equipment. We have fulfilled the conditions attached to two grants and expect to fulfill another one in 2023. RMB2.43 million ($0.35 million) will be amortized in 2023 which was included in the current portion of deferred government grant and RMB13.64 million ($1.98 million) will be amortized after 2023 which was included in the non-current portion of deferred government grants. RMB3.08 million ($0.5 million) was recorded as a reduction to depreciation expense for the year ended December 31, 2022, as compared to $0.6 million and $0.4 million for the years ended December 31, 2021 and 2020, respectively, and nil was recorded as government grant recognized in income for the year ended December 31, 2022, as compared to $79,000 and $80,000 for the years ended December 31, 2021 and 2020.
Government grants for research and development
We have twelve deferred government grants related to various research and development projects. We expect to fulfil the conditions attached to eight grants in 2023 and recorded RMB101.9 million ($14.9 million) as the current portion of deferred government grants, while the remaining four grant’s condition is expected to be fulfilled after 2023 and RMB17.2 million ($2.5 million) was recorded in the non-current portion of deferred government grants.
52
RESULTS OF OPERATIONS
|
|
Year ended December 31, |
|
|||||||||
Consolidated statements of comprehensive income (loss) data |
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
|
(in thousands except share and per share data) |
|
|||||||||
Sales |
|
$ |
1,492,761 |
|
|
$ |
19,374,904 |
|
|
$ |
510,624 |
|
Cost of sales(1) |
|
|
684,456 |
|
|
|
1,072,221 |
|
|
|
67,180 |
|
Gross profit |
|
|
808,305 |
|
|
|
18,302,683 |
|
|
|
443,444 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|||
Selling, general and administrative expenses(1) |
|
|
823,543 |
|
|
|
591,167 |
|
|
|
176,534 |
|
Provision for doubtful accounts |
|
|
4,268 |
|
|
|
2,967 |
|
|
|
2,640 |
|
Research and development expenses(1) |
|
|
442,108 |
|
|
|
155,040 |
|
|
|
48,760 |
|
Loss on disposal of property, plant and equipment |
|
|
5,213 |
|
|
|
977 |
|
|
|
163 |
|
Government grants recognized in income |
|
|
(760 |
) |
|
|
(725 |
) |
|
|
(297 |
) |
Total operating expenses |
|
|
1,274,372 |
|
|
|
749,426 |
|
|
|
227,800 |
|
Operating (loss) income |
|
|
(466,067 |
) |
|
|
17,553,257 |
|
|
|
215,644 |
|
Interest and financing expenses |
|
|
(1,264 |
) |
|
|
(2,836 |
) |
|
|
(1,453 |
) |
Interest income |
|
|
190,818 |
|
|
|
102,568 |
|
|
|
1,930 |
|
Other income (expense), net |
|
|
301,751 |
|
|
|
(89,948 |
) |
|
|
496 |
|
Income before income taxes |
|
|
25,238 |
|
|
|
17,563,041 |
|
|
|
216,617 |
|
Income tax recovery (expense) |
|
|
62,893 |
|
|
|
(3,104,130 |
) |
|
|
(31,438 |
) |
Net income |
|
|
88,131 |
|
|
|
14,458,911 |
|
|
|
185,179 |
|
Less: (income) loss attributable to non-controlling interests |
|
|
25,735 |
|
|
|
(5,991,431 |
) |
|
|
(74,810 |
) |
Net income attributable to the shareholders of Sinovac |
|
|
113,866 |
|
|
|
8,467,480 |
|
|
|
110,369 |
|
Preferred stock dividends |
|
|
(5,982 |
) |
|
|
(5,982 |
) |
|
|
(6,015 |
) |
Net income attributable to common shareholders of Sinovac |
|
|
107,884 |
|
|
|
8,461,498 |
|
|
|
104,354 |
|
Comprehensive (loss) / income |
|
|
(770,914 |
) |
|
|
14,652,009 |
|
|
|
217,507 |
|
Less: comprehensive (income) loss attributable to non-controlling interests |
|
|
370,882 |
|
|
|
(6,073,832 |
) |
|
|
(82,892 |
) |
Comprehensive income (loss) attributable to shareholders of Sinovac |
|
$ |
(400,032 |
) |
|
$ |
8,578,177 |
|
|
$ |
134,615 |
|
Sales
Revenues from sales represent: (1) the invoiced value of goods, net of value added taxes, and sales returns. See “Item 5. Operating and Financial Review and Prospects — A. Operating Results — Taxes and incentives.” We recognize revenues when control of promised goods is transferred to our customers in an amount of consideration of which we expect to be entitled to in exchange for the goods, and we can reasonably estimates return provision for the goods; and (2) the value of goods produced for government stockpiling program. We recognize revenues from the sales of products to the government stockpiling program when cash has been received and the products have expired and passed government inspection or are delivered per government instruction.
Our revenues, growth and results of operations depend on several factors, including the level of acceptance of our products among doctors, hospitals and patients, and our ability to maintain or increase prices for our products at levels that provide favorable margins. The level of acceptance among doctors, hospitals and patients is influenced by the performance, promotion and academic research, and pricing of our products.
We market and sell our vaccine products primarily through provincial and municipal CDCs. We enter into sales agreements with CDCs each time a CDC places a purchase order. Pursuant to these sales agreements, CDCs typically agree not to re-sell our products to regions outside the territory the pertinent CDC covers administratively. For vaccines included in the government sponsored expended immunization program, we actively participate in the tender and bidding organized by various provincial level CDCs. We enter into sales agreements with CDCs when we win a bid.
53
Pricing
In the private market, we set our price based on our production cost, the price of competitive products and acceptance level of CDCs and patients. We also adjust our product price according to changes in the external environment to balance sales volume and gross profit, and ultimately to maximize sales profit margins.
In the public market, the government purchases vaccines for EPI market typically by issuing government tenders. During the evaluation process, price is a key factor which impacts the result of the tender. Therefore, we need to price our products competitively to win the tenders. We believe that our emphasis on product quality is an advantage and increases our competitiveness.
Cost of sales
Our cost of sales primarily consists of material, direct labor and production overheads. Depreciation of property, plant and equipment attributable to manufacturing activities and license amortization are capitalized as part of inventory, and expensed as cost of sales when product is sold. Cost of goods sold in 2022, 2021 and 2020 amounted to $684.5 million, $1,072.2 million and $67.2 million, respectively, of which idle capacity amounted to $97.5 million, $8.6 million and $1.7 million, respectively. We produce our products and conduct the final product packaging in-house.
Our production capacities have not been fully utilized. If we successfully commercialized new products and increase sales of existing products, we expect the unit production cost to decrease.
Selling, general and administrative expense
Selling and marketing expenses consist primarily of salaries and related expenses for personnel engaged in sales, marketing and customer support functions and costs associated with marketing activities and shipping. Selling expense decreased from $376.3 million in 2021 to $262.9 million due to decreased sales in 2022 , which accounted for 18% of total sales revenue of 2022.
General and administrative expense consists primarily of compensation for employees in executive and operational functions, including finance and accounting, business development and human resources. Other significant costs include facilities costs, professional fees for accounting and legal services.
Research and development expenses
Our research and development expenses consist primarily of:
We expense both internal and external research and development costs as incurred, other than capital expenditures that have alternative future uses, such as the build-out of our plant, or license fees and milestone payments made to third parties after regulatory approval is received. We expect our research and development costs will continue to be substantial and that they will increase as we advance our current portfolio of product candidates through clinical trials and move other product candidates into pre-clinical and clinical trials.
Taxes and incentives
Our PRC subsidiaries are subject to income taxes in China on their taxable income calculated at a tax rate in accordance with the relevant income tax laws and regulations. In general, the PRC tax authorities have up to five years to conduct examinations of the tax filings of the Company’s PRC subsidiaries. Accordingly, income tax returns filed by our PRC subsidiaries for tax years beginning in 2017 remain open to examination by tax authorities.
Effective from January 1, 2008, the PRC’s statutory income tax rate is 25%. An enterprise may benefit from a preferential tax rate of 15% under the EIT Law if it qualifies as a “High and New Technology Enterprise”, or HNTE. Sinovac Beijing and Sinovac Dalian, have been reconfirmed as HNTE in 2020 for a period of three years, and Sinovac LS, has been confirmed as a HNTE in 2020 for a period of three years, and are subject
54
to a preferential income tax rate of 15% from 2020 to 2022. Our other PRC subsidiaries are subject to income tax at the statutory rate of 25%. We determine deferred taxes for each tax-paying entity in each tax jurisdiction. The potential tax benefits arising from the losses incurred by the subsidiaries have been recorded in our financial statements.
We evaluate our valuation allowances requirements at each reporting period by reviewing all available evidence, both positive and negative, and considering whether, based on the weight of that evidence, a valuation allowance is needed. When a change in circumstances causes a change in management’s judgment about the ability to realize deferred tax assets, the impact of the change on the valuation allowance is generally reflected in income from operations. The future realization of the tax benefit of an existing deductible temporary difference ultimately depends on the existence of sufficient taxable income of the appropriate character within the carry forward period available under applicable tax law.
Tax losses of our PRC subsidiaries in the amount of RMB467.9 million ($67.8 million) as of December 31, 2022 will expire from 2023 to 2027, if not utilized.
Year Ended December 31, 2022 Compared to Year Ended December 31, 2021
Sales. Total sales in 2022 decreased to $1.5 billion from $19.4 billion in 2021. The decrease was mainly due to the decreased sales of COVID-19 vaccine.
The table below sets forth a breakdown of our sales by market type:
|
|
Year ended December 31, |
|
|||||
Sales |
|
2022 |
|
|
2021 |
|
||
|
|
(in thousands) |
|
|||||
EPI |
|
$ |
732,578 |
|
|
$ |
10,552,059 |
|
Private Pay |
|
|
384,192 |
|
|
|
349,083 |
|
Export |
|
|
375,991 |
|
|
|
8,473,762 |
|
Total sales |
|
$ |
1,492,761 |
|
|
$ |
19,374,904 |
|
Gross Profit. Gross profit in 2022 decreased to $0.8 billion from $18.3 billion in 2021. Gross margin percentage decreased to 57.8% in 2022 from 94.5% in 2021, primarily due to the decrease of average selling price of COVID-19 vaccines.
Selling, General and Administrative Expenses. Selling, general and administrative expenses in 2022 increased to $823.5 million from $591.2 million in 2021. The increase was mainly due to the establishment of a long-term employee incentive plan in 2022 (the "Employee Incentive Plan") following our successful COVID-19 campaign offset by lower selling expense due to reduced sales.
We recorded total share-based compensation of nil in 2022, compared to $7.7 million in 2021. As of December 31, 2022 and 2021, we had no unrecognized compensation costs.
Research and Development Expenses. Research and development expenses in 2022, primarily represented expenditures on the advancement of pipeline vaccines, increased to $442.1 million in 2022 from $155.0 million in 2021.
Interest and Financing Expenses. Interest and financing expense decreased to $1.3 million in 2022 from $2.8 million in 2021.
Income Tax Recovery (Expenses). Income tax recovery was $62.9 million in 2022, compared to $3.1 billion in income tax expense in 2021.
Net Income. Net income was $88.1 million in 2022, compared to $14.5 billion in 2021. Net income attributable to shareholders of Sinovac was $113.9 million in 2022, compared to $8.5 billion in 2021. Net income attributable to common shareholders of Sinovac was $107.9 million in 2022, compared to $8.5 billion in 2021.
Year Ended December 31, 2021 Compared to Year Ended December 31, 2020
Sales. Total sales in 2021 increased to $19.4 billion from $510.6 million in 2020. The growth was mainly contributed by sales of COVID-19 vaccines.
55
The table below sets forth a breakdown of our sales by market type:
|
|
Year ended December 31, |
|
|||||
Sales |
|
2021 |
|
|
2020 |
|
||
|
|
(in thousands) |
|
|||||
EPI |
|
$ |
10,552,059 |
|
|
$ |
96,799 |
|
Private Pay |
|
|
349,083 |
|
|
|
268,821 |
|
Export |
|
|
8,473,762 |
|
|
|
145,004 |
|
Total sales |
|
$ |
19,374,904 |
|
|
$ |
510,624 |
|
Gross Profit. Gross profit in 2021 increased to $18.3 billion from $443.4 million in 2020. Gross margin percentage increased to 94.5% in 2021 from 86.8% in 2020, primarily due to economies of scales from significant increase in production volume.
Selling, General and Administrative Expenses. Selling, general and administrative expenses in 2021 increased to $591.2 million from $176.5 million in 2020. The increase was mainly due to increased resources dedicated to revenue growth and operation expansion.
We recorded total share-based compensation of $7.7 million in 2021, compared to $10.2 million in 2020. As of December 31, 2021 and 2020, we had unrecognized compensation costs of $nil and $6.6 million, respectively.
Research and Development Expenses. Research and development expenses in 2021, primarily represented expenditures on the advancement of pipeline vaccines, increased to $155.0 million in 2021 from $48.8 million in 2020.
Interest and Financing Expenses. Interest and financing expense increased to $2.8 million in 2021 from $1.5 million in 2020.
Income Tax Expenses. Income tax expense was $3.1 billion in 2021, compared to an income tax expense of $31.4 million in 2020.
Net Income. Net income was $14.5 billion in 2021, compared to $185.2 million in 2020. Net income attributable to shareholders of Sinovac was $8.5 billion in 2021, compared to $110.4 million in 2020. Net income attributable to common shareholders of Sinovac was $8.5 billion in 2021, compared to $104.4 million in 2020.
To date, we have financed our operating and investing activities through cash flows from operations, bank borrowings and cash provided by financing activities. As of December 31, 2022, 2021 and 2020, our cash and cash equivalents and restricted cash were $4.3 billion, $11.6 billion and $1.1 billion, respectively.
Based on our current business plan, we believe that our existing capital resources is sufficient to meet our cash requirements to fund planned operations and other commitments for at least the next 12 months. However, we may decide to enhance our liquidity position or increase our cash reserve for future operations and investments through additional financing. For more information, see “Item 3. Key Information—D. Risk Factors—Risks Relating to Our Company—We may need additional capital to upgrade or expand our production capabilities, to continue development of our product pipeline and to market existing and future products on a large scale. We cannot guarantee that we will find adequate sources of capital in the future.”
Cash Flows and Working Capital
The following table sets forth a summary of our net cash flows for the periods indicated:
|
|
Year ended December 31, |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
|
(in thousands) |
|
|||||||||
Net cash (used in)/provided by operating activities |
|
$ |
(770,745 |
) |
|
$ |
15,352,541 |
|
|
$ |
479,309 |
|
Net cash used in investing activities |
|
|
(5,760,470 |
) |
|
|
(3,023,574 |
) |
|
|
(204,756 |
) |
Net cash (used in)/provided by financing activities |
|
|
(241,399 |
) |
|
|
(1,896,680 |
) |
|
|
592,566 |
|
Effect of exchange rate changes on cash and cash equivalents and restricted cash |
|
|
(560,769 |
) |
|
|
137,269 |
|
|
|
27,207 |
|
(Decrease)/increase in cash and cash equivalents and restricted cash |
|
|
(7,333,383 |
) |
|
|
10,569,556 |
|
|
|
894,326 |
|
Cash and cash equivalents and restricted cash at beginning of period |
|
|
11,619,760 |
|
|
|
1,050,204 |
|
|
|
155,878 |
|
Cash and cash equivalents and restricted cash at end of period |
|
$ |
4,286,377 |
|
|
$ |
11,619,760 |
|
|
$ |
1,050,204 |
|
56
Operating Activities
Net cash used in operating activities was $770.7 million in 2022, compared to net cash provided by operating activities of $15.4 billion in 2021. Net cash used in our operating activities in 2022 resulted primarily from our research and development expenses and the Employee Incentive Plan.
Net cash provided by operating activities was $15.4 billion in 2021, compared to net cash provided by operating activities of $479.3 million in 2020. Net cash provided by our operating activities in 2021 resulted primarily from our net income of $14.5 billion.
Investing Activities
Net cash used in investing activities was $5.8 billion in 2022, compared to $3.0 billion in 2021. We invested primarily in short-term and long-term investments and property, plant and equipment in 2022.
Net cash used in investing activities was $3.0 billion in 2021, compared to net cash used in investing activities of $204.8 million in 2020. We invested primarily in short-term investments and property, plant and equipment in 2021.
Financing Activities
Net cash used in financing activities was $241.4 million in 2022, compared to $1.9 billion in 2021. In 2022, we paid dividend of $263.2 million.
Net cash used in financing activities was $1.9 billion in 2021, compared to net cash provided by financing activities of $592.6 million in 2020. In 2021, we received loan proceeds of $13.5 million, made dividend payment of $1.9 billion and loan repayments of $33.4 million.
Accounts Receivable
Our total accounts receivable, including other receivables, decreased from $952.4 million as of December 31, 2021 to $537.1 million as of December 31, 2022 due to lower sales. Our average accounts receivable turnover time in 2022 was 174 days, compared to 12 days in 2021.
Our maximum exposure to credit risk at the balance sheet dates relating to accounts receivables is summarized as follows:
|
|
Year ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
|
|
(in thousands) |
|
|||||
Aging within one year, net of allowance for doubtful accounts |
|
$ |
424,060 |
|
|
$ |
902,995 |
|
Aging greater than one year, net of allowance for doubtful accounts |
|
|
72,954 |
|
|
|
20,803 |
|
Total trade receivable |
|
$ |
497,014 |
|
|
$ |
923,798 |
|
57
Borrowings
As of December 31, 2022, we had $0.3 million in short-term bank loans, offset by $4.3 billion in cash and cash equivalents, resulting in a liquid assets balance of $4.3 billion, compared with $11.6 billion at the end of December 31, 2021. The following tables summarize our short-term and long-term bank borrowings as of December 31, 2022:
Type |
|
Amount |
|
Annual |
|
Interest |
|
Maturity Date |
|
Purpose |
Bank loan from China Everbright Bank |
|
RMB81.4 million |
|
5.88% |
|
Quarterly |
|
November 16, 2028 |
|
Purchase of property, plant and equipment |
On November 17, 2020, Sinovac Dalian entered into a maximum credit facility of $30.7 million (RMB200 million) with China Everbright Bank to finance Sinovac Dalian’s purchase of property plant and equipment, with a term from November 17, 2020 to November 16, 2028. The loan bears annual interest rate at 123 basis point above the prime rate of a five-year term loan published by the People’s Bank of China, at 5.88%. Interest is payable quarterly and principal installment repayments begin in 2023 and shall be fully paid by November 16, 2028. As of December 31 2022, $0.3 million was recorded in bank loans due within one year and $11.5 million was recorded in long-term bank loans. Certain machinery and equipment of Sinovac Dalian with a net book value of $33.6 million (RMB232.0 million) were pledged as collateral.
Our weighted average effective interest rate on outstanding borrowings was 5.88%, 5.64% and 4.84% for the years ended December 31, 2022, 2021 and 2020, respectively. We have not historically used, and do not expect to use in the future, any derivative financial instruments to manage our exposure to interest risk.
Treasury Policy
We have established a treasury policy to better utilize our financial resources and manage our cash that we generate from our operations. Under this policy, when our internal cash flow and liquidity forecast indicates that we have sufficient capital resources for our operating activities and our capital expenditure, we make liquid investments with a portion of our excess cash to achieve a better return on our assets than generating interest on bank deposits. Our cash and cash equivalents consists of cash on hand and investments in interest bearing demand deposits, time deposits, and other highly liquid investments which have original maturities of three months or less when purchased. Short-term and long-term investments primarily consists of deposits in commercial banks and wealth management products issued by commercial banks and other financial institutions.
Restrictions on Cash Dividends
We are a holding company, and we rely in part on dividends paid by our subsidiaries for our cash needs, including our operating expenses and additional investment opportunities. The payment of dividends from subsidiaries in China is subject to limitations. Regulations in the PRC currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China. Our subsidiary is also required to set aside at least a portion of its after-tax profit based on PRC accounting standards each year to fund the statutory surplus reserves.
The reserves can be used to recoup previous years’ losses, if any, and, subject to the approval of the relevant PRC government authority, may be converted into share capital in proportion to their existing shareholdings, or by increasing the par value of the shares currently held by them. Such reserves, however, are not distributable as cash dividends. In addition, at discretion of their board of directors, our subsidiaries may allocate a portion of their after-tax profits based on PRC accounting standards to the employee welfare and bonus funds, which shall be utilized for collective staff benefits. In addition, if our PRC subsidiaries incurs debt on its own behalf in the future, the instruments governing the debt may restrict the ability of one or more of our PRC subsidiaries, as the case may be, to pay dividends or make other distributions to us.
The ability of our subsidiary to convert renminbi into U.S. dollars and make payments to us is subject to PRC foreign exchange regulations. Under these regulations, the renminbi is convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions. Conversion of renminbi for capital account items, such as direct investment, loan, security investment and repatriation of investment, however, is still subject to the approval of SAFE. See “Item 10. Additional Information — D. Exchange Controls.”
Material Cash Requirements
Other than the ordinary cash requirements for our operations, our material cash requirements as of December 31, 2022 and any subsequent interim period primarily include our short-term and long-term bank borrowings, capital expenditures and cash requirements for potential investments.
We intend to fund our existing and future material cash requirements primarily with anticipated cash flows from operations, our existing cash balance and other financing alternatives. We will continue to make cash commitments, including capital expenditures, to support the growth of our business.
58
Capital Expenditures
We made capital expenditures of $439.1 million, $751.0 million and $127.7 million in 2022, 2021 and 2020, respectively. As of December 31, 2022, our commitments related to capital expenditures of approximately $34.6 million were primarily for the construction of vaccine production facilities for pipeline products. We will finance such commitments through cash generated from operations.
Off-Balance Sheet Commitments and Arrangements
We have not entered into any financial guarantees or other commitments or obligations to guarantee the payment obligations of any unconsolidated third parties. In addition, we have not entered into any derivative contracts that are indexed to our shares and classified as shareholders’ equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. Moreover, we do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or product development services with us.
C. Research and Development, Patents and Licenses, Etc.
See discussions under “Item 5. Operating and Financial Review and Prospects — A. Operating Results — Research and Development Programs.”
D. Trend Information
The COVID-19 pandemic has created a sudden surge in material used in COVID-19 vaccines. Because of the significant uncertainties surrounding the COVID-19 pandemic, the demand for our COVID-19 vaccine and related financial impact cannot be reasonably estimated at this time. See “Item 3. Key Information- D. Risk Factors” of this annual report.
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the period from January 1, 2022 to December 31, 2022 that are reasonably likely to have a material adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
E. Critical Accounting Estimates
Our consolidated financial information has been prepared in accordance with U.S. GAAP, which requires us to make judgments, estimates and assumptions that affect (1) the reported amounts of our assets and liabilities, (2) the disclosure of our contingent assets and liabilities at the end of each fiscal period and (3) the reported amounts of revenues and expenses during each fiscal period. We continually evaluate these estimates based on our own historical experience, knowledge and assessment of current business and other conditions, our expectations regarding the future based on available information and reasonable assumptions, which together form our basis for making judgments about matters that are not readily apparent from other sources. Since the use of estimates is an integral component of the financial reporting process, our actual results could differ from those estimates. Some of our accounting policies require a higher degree of judgment than others in their application.
When reviewing our financial statements, you should consider (1) our selection of critical accounting policies, (2) the judgment and other uncertainties affecting the application of those policies and (3) the sensitivity of reported results to changes in conditions and assumptions. We believe the following accounting policies involve the most significant judgment and estimates used in the preparation of our financial statements.
Revenue from Contracts with Customers
Revenue is recognized at a point in time when performance obligation is satisfied where control of promised goods is transferred to our customers in an amount of consideration of which we expect to be entitled to in exchange for the goods, and we can reasonably estimate return provisions for the goods.
Product return provisions are estimated based on historical return and exchange data as well as inventory levels and remaining shelf lives of the products in distribution channels.
As of December 31, 2022, sales return provision for our vaccine products was $17.7 million, compared to $33.7 million as of December 31, 2021. Sales return provision as a percentage of net sales was 1.2% and 0.2% in 2022 and 2021, respectively.
For the year ended December 31, 2022, we did not have any significant incremental costs of obtaining contracts with customers or costs incurred in fulfilling contracts with customers within the scope of ASC Topic 606, that shall be recognized as an asset and amortized to expenses in a pattern that matches the timing of the revenue recognition of the related contract.
We do not have contract assets since revenue is recognized as control of goods is transferred. Contract liabilities consist of advance payments from customers. Contract liabilities are reported in a net position on a customer-by-customer basis at the end of each reporting period. All contract liabilities are included in deferred revenue on the consolidated balance sheets.
For the year ended December 31, 2022, we recognized sales of $54.5 million related to contract liabilities as of January 1, 2022.
59
Allowance for Doubtful Accounts
We extend unsecured credit to our customers in the ordinary course of business and actively pursuing past due accounts. An allowance for doubtful accounts is established and recorded based on management’s assessment of the credit history with the customer and current relationships with them.
We also maintain an allowance for doubtful accounts for estimated losses based on our assessment of the collectability of specific customer accounts and the aging of the accounts receivable. We analyze accounts receivable and historical bad debts, customer concentrations, customer solvency, current economic and geographic trends, and changes in customer payment terms and practices when evaluating the adequacy of our current and future allowance. In circumstances where we are aware of a specific customer’s inability to meet its financial obligations to us, a specific allowance for bad debt is estimated and recorded, which reduces the recognized receivable to the estimated amount we believe will ultimately be collected. We monitor and analyze the accuracy of the allowance for doubtful accounts estimate by reviewing past collectability and adjust it for future expectations to determine the adequacy of our current and future allowance. Our reserve levels have generally been sufficient to cover credit losses. Our allowance for doubtful accounts as of December 31, 2022 was $12.5 million, compared to $9.1 million as of December 31, 2021. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required. Bad debt provision was $4.3 million for the year ended December 31, 2022, compared to $3.0 million for the year ended December 31, 2021.
Inventory Provision
We write off all the unsold seasonal influenza vaccines before the end of the flu season at the end of the fiscal year, except for those distributed after the end of the fiscal year. In addition, we estimate an inventory provision for existing Healive, Bilive, Inlive, Mumps, Varicella, CoronaVac, PPV and sIPV products in inventory after considering the sales forecasts, the conditions of the raw material inventory, as well as the expiration dates of these products. The inventory provision in 2022, 2021 and 2020 was $140.0 million, $70.1 million and $5.8 million, respectively.
Impairment of Long-Lived Assets
Long-lived assets, including property, plant and equipment and intangible assets subject to amortization, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of an asset group may not be recoverable from the future undiscounted net cash flows expected to be generated by the asset group. An asset group is identified as assets at the lowest level for which identifiable cash flows are largely independent of the cash flows of other assets.
If the asset group is not fully recoverable, an impairment loss would be recognized for the difference between the carrying value of the asset group and its estimated fair value, based on the discounted net future cash flows or other appropriate methods, such as comparable market values. We use estimates and judgments in the impairment tests and the timing and amount of impairment charges could be materially different if different estimates or judgments are utilized. We did not record any impairment charges on long-lived assets in 2022, 2021 and 2020.
Income Tax Valuation Allowance
In 2022, we recorded $71.1 million of deferred income tax assets based on the difference in timing of certain deductions for income tax and accounting purposes. We evaluate our valuation allowance requirements at each reporting period by reviewing all available evidence, both positive and negative, and considering whether, based on the weight of that evidence, a valuation allowance is needed. When a change in circumstances causes a change in management’s judgment about the reliability of deferred tax assets, the impact of the change on the valuation allowance is generally reflected in income from operations. The future realization of the tax benefit of an existing deductible temporary difference ultimately depends on the existence of sufficient taxable income of the appropriate character within the carry forward period available under applicable tax law.
Recently Issued Accounting Standards
In June 2022, the FASB issued ASU 2022-03, Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions, which clarifies that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments also clarify that an entity cannot, as a separate unit of account, recognize and measure a contractual sale restriction. This guidance also requires certain disclosures for equity securities subject to contractual sale restrictions. The new guidance is required to be applied prospectively with any adjustments from the adoption of the amendments recognized in earnings and disclosed on the date of adoption.
This guidance is effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. Early adoption is permitted. We do not expect that the adoption of this guidance to have a material impact on our consolidated financial statements.
ITEM 6. Directors, Senior Management and Employees
60
A. Directors and Senior Management
The following table sets forth information regarding our directors and executive officers as of the date of this annual report:
Directors and Executive Officers |
|
Age |
|
Position/Title |
Weidong Yin |
|
59 |
|
Chairman, President, Chief Executive Officer |
Simon Anderson(1) (2) (3) (4) |
|
62 |
|
Independent Director |
Yuk Lam Lo(1) (2) (3) |
|
73 |
|
Independent Director |
Kenneth Lee(2) (3) |
|
55 |
|
Independent Director |
Meng Mei(1) (2) (3) |
|
68 |
|
Independent Director |
Shan Fu(3) (4) |
|
56 |
|
Independent Director |
Nan Wang |
|
56 |
|
Chief Financial Officer, Vice President |
Qiang Gao |
|
46 |
|
Chief Operating Officer, Vice President |
Guang (Helen) Yang |
|
43 |
|
Chief Business Officer |
Jing Li |
|
49 |
|
Vice President, Quality and Production |
Mr. Weidong Yin has served as our chairman, president, chief executive officer and secretary since September 2003. He previously worked as a medical doctor in infectious disease at the China Center for Disease Control and Prevention, Tangshan City, Hebei province. Mr. Yin has been dedicated to hepatitis research for over 20 years and was instrumental in the development of Healive. In addition, Mr. Yin has been appointed as the principal investigator by the Chinese Ministry of Science and Technology for many key governmental R&D programs such as Inactivated Hepatitis A Vaccine R&D, Inactivated SARS Vaccine R&D and New Human Influenza Vaccine (H5N1) R&D. He is also the president of Zhongguancun Listed Companies Association. He obtained his MBA from the National University of Singapore.
Mr. Simon Anderson has served as an independent director of our company since July 2004. He is the chairman of our audit committee and a member of our compensation committee and corporate governance and nominating committee. Mr. Anderson advises companies listed on North American stock exchanges and private businesses in the areas of regulatory compliance, exchange listings and financial operations. He is a member of the Chartered Professional Accountants of British Columbia, having qualified as a Chartered Accountant in 1986. Mr. Anderson serves as a director of IBC Advanced Alloys Corp., which manufactures and processes alloys at its U.S. plants.
Mr. Yuk Lam Lo has served as an independent director of our company since March 2006. Mr. Lo is the chairman of our corporate governance and nominating committee and a member of our audit committee and compensation committee. Currently Mr. Lo is serving as the Founding President of HK Bio-Med Innotech Association. He is also the Honorary Founding Chairman of Hong Kong Biotechnology Organization. In the educational area, Mr. Lo has been elected an Honorary Fellow of the Hong Kong University of Science and Technology. He is also the Honorary Professor of several universities in China. Mr. Lo was heavily involved in several committees of the HKSAR Government. He had been served as the Chairman of the Advisory Council for Food Safety of the Food and Health Bureau HKSAR, Director of the Hong Kong Applied R&D Fund Co. Ltd., Chairman of the Biotechnology Committee of the Hong Kong Industry & Technology Development Council, and Chairman of Biotechnology Projects Vetting Committee of the Innovation and Technology Fund, HKSAR. In mainland China, Mr. Lo is a member of Chinese People’s Political Consultative Conference in Jilin Province. He was also a consultant of the Centre for Disease Control and Prevention of China. In recognition of his leadership in the community and dedication to his field, Mr. Lo has received many awards, such as the “Pericles International Prize” in 2019. He is the second Asian and the first person from Hong Kong to be awarded the Prize since it was founded in 1986. In 2020, Mr. Lo was awarded the Bronze Bauhinia Star from the HKSAR government for his outstanding services over the past decades. In the business sector, Mr. Lo is the Chairman of GT Healthcare Capital Partners, and Partner & Investment Committee Member of Hongsen Investment Management Limited. As at the date of this annual report, Mr. Lo holds directorships in the following listed companies: He is an Independent Non-executive Director of Luye Pharma Group Limited (2186.HK) and an Independent Non-executive Director of Zhaoke Ophthalmology Limited (06622.HK).
Mr. Kenneth Lee is an independent director of Sinovac. He has served on our board of directors since May 2011. In July 2012, the board appointed him as a member of our compensation committee and corporate governance and nominating committee. He has more than 20 years of experience across private equity investments, corporate finance, and business development in China. Mr. Lee was a partner at SAIF Partners. Mr. Lee graduated from Amherst College.
Mr. Meng Mei has served as an independent director of our company since March 2012. Mr. Mei is the chairman of our compensation committee, and member of our audit committee and corporate governance and nominating committee. Mr. Mei founded TusPark, a science park established by Tsinghua University in 1994, to incubate high growth companies. He has been the director of TusPark’s development center since its inception. Mr. Mei is also the Chairman of TusHoldings Co., Ltd., which is engaged in the development, construction, and management of TusPark and is
61
providing services to enterprises based in TusPark. TusHoldings Co., Ltd. is also involved in venture capital investments in China. Mr. Mei sits on the judging expert panel of China’s National Science & Technology Award. He has developed courses on entrepreneurship and new venture formation as a Tsinghua University professor and an entrepreneur. Mr. Mei holds a bachelor’s degree in automation from Tsinghua University, PRC.
Mr. Shan Fu has served as an independent director since July 2018, when he was appointed as a director by the PIPE Investors in connection with the PIPE transaction described above. Mr. Fu is the chairman of our investment committee and a member of our compensation committee. Mr. Fu is a Managing Partner at Vivo Capital. Vivo Capital is a healthcare focused investment firm formed in 1996 with almost $7 billion under management. Prior to joining Vivo in 2013, Mr. Fu was Senior Managing Director in the Private Equity group and the Chief Representative of Blackstone’s Beijing Office. Additionally, Mr. Fu’s qualifications include experience in the Department of Foreign Investment in China’s National Development and Reform Commission, the State Economic and Trade Commission, the Office of Economic and Trade in State Council, and the Office of Production in State Council. Mr. Fu is currently a director on the boards of 11 biotech companies.
Ms. Nan Wang has served as our chief financial officer since June 2013. Ms. Wang served as the vice president of Sinovac Beijing from 2001 and the board director since 2009. Ms. Wang oversaw business development, investment and clinical research. Ms. Wang also served as the first general manager of Sinovac Dalian since establishment of this company. During her 20 years of service, Ms. Wang was responsible for our business development, investment and clinical research. She has been actively promoted our foreign cooperation, leading domestic and international cooperation negotiations on a number of projects including equity, technology and market, and successfully achieving a number of foreign cooperation. Ms. Wang has led clinical research on many important projects including SARS vaccine (phase I), inactivated H5N1 influenza (avian flu) vaccine, influenza, H1N1 influenza vaccine and EV71 vaccine (Vero Cell), inactivated, and has actively promoted the listing of new products. Ms. Wang received her bachelor’s degree in biology from Peking University and her master’s degree from University of International Business and Economics, PRC. Ms. Wang also received a diploma in financial management from Beijing College for Entrepreneurs, PRC in 2003.
Mr. Qiang Gao has served as our chief operating officer since April 2020. Mr. Gao joined Sinovac Beijing in 2002 and has served as quality control manager, quality assurance manager, R&D manager and R&D director at Sinovac Beijing in the past years, the general manager of Sinovac LS since 2010, and our vice president since April 2016. Mr. Gao has participated in the development of several vaccine varieties, including influenza vaccine, SARS vaccine, inactivated H5N1 influenza (avian flu) vaccine, EV71 vaccine, COVID-19 vaccine, ongoing sIPV vaccine and declared 23-valent pneumonia vaccine. Under his leadership, we successfully passed the WHO assessment and were selected to be eligible to import inactivated polio vaccine technology from the Netherlands and participate in the global polio eradication project. This project makes China become one of only six developing countries eligible for the technology transfer. Mr. Gao is currently a member of the Beijing Virus Society, Master of Engineering Supervisor of Institute of Microbiology (Chinese Academy of Sciences), and a subject review expert of the Beijing Municipal Science and Technology Commission. Mr. Gao received a master’s degree and a bachelor’s degree in microbiology from the University of Agriculture, PRC.
Ms. Guang (Helen) Yang has served as our Chief Business Officer since April 2021. Ms. Yang is a key member of our executive team and is responsible for our worldwide commercial strategic development and operation. Ms. Yang joined Sinovac Beijing in 2003 and has nearly 20 years of experience in human vaccine industry. Ms. Yang is currently in charge of our global sales and marketing management, focusing on global marketing commercial strategy layout, development, transformation, building and upgrading our marketing commercial network and system. She is committed to continuously ramping up our marketing business and commercial capabilities, promoting and strengthening our global brand influence. Under her leadership, our products and services have benefited more than 60 countries and regions around the world during the COVID-19 period. In the domestic market, Ms. Yang leads our team to enhance the market positioning capabilities and brand influence recognition in China's vaccine industry. Ms. Yang obtained her MBA with outstanding graduate results from Peking University & Vlerick Leuven Gent Management School, Belgium in 2011. She received her master’s degree of science (Hons) in ISMA International Securities Investment and Banking from the University of Reading, United Kingdom in 2003 and her bachelor’s degree in economics (Hons) from China Agricultural University and University of Colorado Denver International School in 2002.
Ms. Jing Li has served as our vice president since April 2016. Ms. Li was named as quality person of Sinovac Beijing in March 2015. Since she joined Sinovac Beijing in 2003, she has worked in different roles in production and quality function, including quality assurance vice manager, department manager of hepatitis A vaccine production and director of vaccine production at Sinovac Beijing. Ms. Li has successively organized and completed the production and site inspection of EV71 vaccine, the commercial production and application of 23-valent pneumococcal polysaccharide vaccine. As the project leader, she organized and led the effort to pass WHO pre-certification assessment of hepatitis A vaccine, which significantly promoted the export sales of hepatitis A vaccine. Ms. Li received a master’s degree in physiology from the University of Agriculture, PRC.
No family relationship exists among any of our directors or members of our executive officers named above and no arrangement or understanding exists between any of our major shareholders, customers, suppliers or others, pursuant to which any person referred to above was selected as a director or executive officers.
62
B. Compensation
For 2022, we paid approximately $244.8 million and accrued approximately $150.5 million in compensations to our directors and executive officers. We have not set aside or accrued any amount of cash to provide pension, retirement or other similar benefits to our officers and directors. Our PRC subsidiaries and consolidated affiliated entities as well as their subsidiaries are required by law to make contributions equal to certain percentages of each employee’s salary for his or her retirement benefits, medical insurance benefits, housing funds, unemployment and other statutory benefits.
INDEMNIFICATION AGREEMENTS
We have entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we may agree to indemnify our directors and executive officers against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being a director or officer of our company.
EMPLOYMENT AGREEMENTS; NON-DISCLOSURE, NON-COMPETITION AND PROPRIETARY INFORMATION AGREEMENT
We have entered into employment agreements with each of our executive officers. Under these agreements, each of our executive officers is employed for a specified time period. We may terminate the employment of any officers for cause, at any time, without notice or remuneration, for certain acts of such officer, such as conviction of or plea of guilty to a felony or to an act of fraud, misappropriation or embezzlement, gross negligence or dishonest acts to our detriment, gross misconduct or a failure to perform agreed duties, death or disability (physical or mental impairment). We may also terminate his or her employment without cause, at any time, upon a one month’s written notice. Our officers may terminate their employment, at any time, with a one-month prior written notice to our company for good reason, including material diminution in their authority, duties, responsibilities or cash compensation as detailed in their employment agreements, or in event of any action or inaction that constitutes a material breach by our company under the employment agreement, in the manner set forth in their employment agreements. Upon termination of his or her employment with us by our company without cause or by him or her for good reason, such executive officer is entitled to receive severance benefits including cash payment equal to the amount set forth in his or her employment agreement. In addition, all the share options and restricted share award granted to him or her under our stock/share incentive plans will become fully vested on the employment termination date and such share options will remain exercisable for eighteen months following the employment termination date. In addition, each of our executive officer has entered into a non-disclosure, non-competition and proprietary information agreement and agreed to be bound by non-competition and non-solicitation restrictions during the term of his or her employment and typically for one year and four years, respectively, following the last date of employment.
The bonus plan of the executive officers is made based on our annual performance in different functions and the respective key result areas of these functional teams. Each executive officer’s bonus is determined based on the key corporate development objectives and key performance index set by the compensation committee and approved by the board at the beginning of the year. The bonus plan is approved by the board.
Our shareholders have authorized the board of directors to administer two share incentive plans which in aggregate provide for the issuance of up to 9,000,000 shares of common stock, including 5,000,000 shares reserved under the 2003 Stock Option Plan and 4,000,000 shares reserved under 2012 Share Incentive Plan. As of December 31, 2022, an aggregate of 42,800 shares, consisting of 42,800 shares under the 2003 Stock Option Plan and no shares under the 2012 Share Incentive Plans, are still available for any future grant of incentive awards under the two share incentive plans. The following tables summarize, as of December 31, 2022, the outstanding options that we granted to several of our directors, executive officers, principal shareholders and to other individuals as a group, all of which were made under our 2012 Share Incentive Plan.
Name |
|
Number of |
|
|
Exercise |
|
|
Grant Date |
|
Expiration Date |
||
Weidong Yin |
|
|
— |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Simon Anderson |
|
|
40,000 |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Yuk Lam Lo |
|
|
40,000 |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Meng Mei |
|
|
10,000 |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Kenneth Lee |
|
|
40,000 |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Nan Wang |
|
|
— |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Qiang Gao |
|
|
— |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Guang Yang |
|
|
— |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Jing Li |
|
|
— |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Others as a group |
|
|
42,500 |
|
|
|
4.98 |
|
|
May 1, 2015 |
|
April 30, 2023 |
Subtotal |
|
|
172,500 |
|
|
|
|
|
|
|
|
|
63
2003 STOCK OPTION PLAN
Our board of directors adopted the 2003 Stock Option Plan (the “2003 Plan”) on November 1, 2003. The purpose of the plan is to attract and retain the best available personnel for positions of substantial responsibility, provide additional incentive to employees, directors and consultants and promote the success of our business. Our board of directors believes that our company’s long-term success depends on our ability to attract and retain superior individuals who, by virtue of their ability, experience and qualifications, make important contributions to our business.
Set forth below is a summary of the principal terms of the 2003 Plan.
2012 SHARE INCENTIVE PLAN
In August 2012, our shareholders adopted a 2012 Share Incentive Plan, or the 2012 Plan. The maximum aggregate number of common shares which may be issued pursuant to all awards under the 2012 Plan is 4,000,000 shares. As of December 31, 2022, 3,073,700 common shares were issued under the 2012 Plan. The following paragraphs describe the principal terms of the 2012 Plan.
64
For common share awards granted under the 2012 Plan, namely (1) restricted shares, (2) restricted share units, (3) dividend equivalents, (4) deferred shares, and (5) share payments, the consideration shall not be less than the par value of the shares purchased. The terms of the share awards are set by the plan administrator in its sole discretion.
The exercise price of share appreciation right under the 2012 Plan is determined by the plan administrator and set forth in the award agreement which may be a fixed or variable price related to the fair market value of the shares. The term of the share appreciation right will not exceed ten years.
The approval of shareholders is required for downward adjustment of the exercise prices of options or share appreciation rights. A downward adjustment of the exercise prices of options or share appreciation rights means (i) lowering the exercise price of outstanding options or share appreciation rights, or (ii) cancelling outstanding options or share appreciation rights in exchange for cash, other awards, or options or share appreciation rights with an exercise price that is less than the exercise price of the original options or share appreciation rights.
C. Board Practices
Board of Directors
Our Articles of Incorporation prescribe that we should have a minimum of one and a maximum of 15 directors. Currently, our board of directors comprises six board members, five of whom are independent. A director is not required to hold any shares in the company by way of qualification. A director may vote with respect to any contract, proposed contract or arrangement in which he is materially interested provided that such director must disclose his interest in the contract or arrangement. There is no age limit requirement for directors. Under Antigua law, our directors have a duty of loyalty to act honestly, in good faith and with a view to our best interests. Our directors also have a duty to exercise the skill they actually possess and such care and diligence that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our Articles of Incorporation and By-laws, as amended and re-stated from time to time. A shareholder has the right to seek damages if a duty owed by our directors is breached.
The functions and powers of our board of directors include, among others:
65
As described above, on March 5, 2018, we announced the re-election of the members of our board of directors—Mr. Weidong Yin, Mr. Yuk Lam Lo, Mr. Simon Anderson, Mr. Kenneth Lee, and Mr. Meng Mei—at the 2017 AGM. We also announced that we had determined, after consultation with our Antigua legal counsel, that an alternative, pre-printed ballot not made available to all our shareholders and purportedly submitted at our 2017 AGM by the Shareholder Group was invalid. On March 13, 2018, 1Globe filed a complaint against our company in the Antigua Court to dispute the results of the election. See “Item 8. Financial Information — A. Consolidated Statements and Other Financial Information — Legal and Administrative Proceedings” for additional information. In July 2018, Mr. Shan Fu was appointed to our board of directors in connection with the PIPE transaction.
Board Diversity
The table below provides certain information regarding the diversity of our board of directors as of the date of this annual report.
Board Diversity Matrix (As of April 28, 2023)
|
||||
Country of Principal Executive Offices: |
PRC |
|||
Foreign Private Issuer |
Yes |
|||
Disclosure Prohibited under Home Country Law |
No |
|||
Total Number of Directors |
6 |
|||
Part I: Gender Identity |
|
|||
|
Female |
Male |
Non-binary |
Did Not Disclose Gender |
Directors |
0 |
6 |
0 |
0 |
Part II: Demographic Background |
|
|||
Underrepresented Individual in Home Country Jurisdiction |
1 |
|||
LGBTQ |
0 |
|||
Did Not Disclose Demographic Background |
0 |
|||
Directors who are Jewish People |
0 |
|||
Directors with Disabilities |
0 |
|||
We currently have one member of our board of directors who is Diverse within the meaning of Nasdaq Rule 5605(f)(2)(B) and three women among the four senior executive officers. We believe that drawing from a broad range and variety of perspectives is beneficial to our success and helps us achieve our objectives in terms of efficiency for the benefit of our shareholders. Therefore, we are committed to increasing the range and variety of perspectives of our directors and senior management over time and, as such, supports initiatives aimed at identifying candidates with a broad range and variety of skills, qualifications, capabilities, talents, insights and professional and life experiences. We have not adopted a written diversity policy or target quota relating to the identification and nomination of Diverse directors and members of senior management. Diversity encompasses gender, age, experience, education, ethnicity, religious and cultural backgrounds as well as other dimensions such as lifestyle choices and family responsibilities (the “Designated Groups”). We focus on factors such as skills, qualifications, capabilities, insights, talents, personal attributes and professional and life experiences in the director and senior management identification and selection process. Recommendations for election and appointment are made on merit, in light of the skills, experience, independence and knowledge that we require to be most effective with regards to our current and future plans and objectives, as well as anticipated regulatory and market developments. We must retain the flexibility to add qualified directors and senior management. While consideration of the number of individuals from Designated Groups who are members of the Board and members of senior management will continue to be a component of the selection process, the necessity of obtaining the right synergy and balance among directors and senior management so as to optimize the Company's ability to meet the challenges it faces is paramount.
Our commitment to diversity is further reflected in the equal opportunities policy we adopted, which recognizes our commitment to equality of opportunity and to the recruitment, retention, development and promotion of qualified female candidates among our workforce, including at the highest level.
66
Terms of Directors and Executive Officers
Our officers are elected by and serve at the discretion of the board of directors. Our directors are not subject to a term of office and hold office until a successor is elected at the next annual shareholders’ meeting. A director will be removed from office automatically if, among other things, the director (i) becomes bankrupt or makes any arrangement or composition with his creditors, or (ii) dies or is found by our company to be or becomes of unsound mind. None of our directors has a service contract with us or any of our subsidiaries providing for benefits upon termination of employment.
Committees of the Board of Directors
Our board of directors has established an audit committee, a compensation committee, a corporate governance and nominating committee and an investment committee.
Audit Committee
Our audit committee consists of Messrs. Simon Anderson, Yuk Lam Lo and Meng Mei, and is chaired by Simon Anderson, an audit committee financial expert, all of whom satisfy the “independence” requirements of Rule 5605 of the NASDAQ Listing Rules and Rule 10A-3 under the Securities Exchange Act of 1934. The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
In 2022, our audit committee held meetings or passed resolutions by unanimous written consent three times.
Compensation Committee
Our compensation committee consists of Messrs. Meng Mei, Simon Anderson, Yuk Lam Lo, Kenneth Lee and Shan Fu, and is chaired by Mr. Meng Mei, all of whom satisfy the “independence” requirements of Rule 5605 of the NASDAQ Listing Rules and Rule 10C-1 under the Securities Exchange Act of 1934. Our compensation committee assists the board in reviewing and approving the compensation structure of our directors and executive officers, including all forms of compensation to be provided to our directors and executive officers. Members of the compensation committee are not prohibited from direct involvement in determining their own compensation. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
67
In 2022, our compensation committee held meetings or passed resolutions by unanimous written consent twice.
Corporate Governance and Nominating Committee
Our corporate governance and nominating committee consist of Messrs. Yuk Lam Lo, Simon Anderson, Kenneth Lee and Meng Mei, and is chaired by Mr. Yuk Lam Lo, all of whom satisfy the “independence” requirements of Rule 5605 of the NASDAQ Listing Rules. The corporate governance and nominating committee assists the board of directors in identifying individuals qualified to become our directors and in determining the composition of the board and its committees. The corporate governance and nominating committee is responsible for, among other things:
In 2022, our corporate governance and nominating committee held meetings or passed resolutions by unanimous written consent once.
Investment Committee
Our investment committee consist of Messrs. Shan Fu and Simon Anderson, and is chaired by Mr. Shan Fu, all of whom satisfy the “independence” requirements of Rule 5605 of the NASDAQ Listing Rules. The investment committee assists the board of directors in fulfilling their responsibilities in relation to investment strategies, management and practices of the us and oversee the our investment transactions, management, policies and guidelines. The investment committee is responsible for, among other things:
· review, evaluate and recommend to the board of directors to approve investment projects within the prescribe scope of investment by the charter;
· establish and periodically review the our investment policies and guidelines;
· oversee and periodically review the performance of our investments, including the impact on such performance of our investment policies and guidelines;
· periodically review the structure, approach and effectiveness of our investment function, including the performance of, and allocation of responsibilities between, our personnel and third-party advisors
· select our money managers and investment advisors, monitor their performance and, when appropriate, terminate their engagement; and
· monitor on an ongoing basis the performance of our investment advisors and retain and terminate such advisors as it deems appropriate
In 2022, our investment committee held no meetings or passed resolutions by unanimous written consent.
Interested Transactions
A director may vote in respect of any contract or transaction in which he or she is interested, provided that the nature of the interest of any directors in such contract or transaction is disclosed by him or her at or prior to its consideration and any vote in that matter.
Remuneration and Borrowing
The directors may determine remuneration to be paid to the directors. The compensation committee assists the directors in reviewing and approving the compensation structure for the directors. The directors may exercise all our powers to borrow money and to mortgage or charge its undertaking, property and uncalled capital, and to issue debentures or other securities whether outright or as security for any debt obligations of our company or of any third party.
D. Employees
68
As of December 31, 2022, 2021 and 2020, we had 3,558, 4,281 and 1,959 full-time employees, respectively. Of our workforce as of December 31, 2022, about 622 employees are primarily engaged in research and development, 179 employees are engaged in sales and marketing, 2,418 employees in production related and 339 employees in administration. As of December 31, 2022, we have a total of 717 temporary employees. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We consider our relationship with our employees to be good.
E. Share Ownership
The following table sets forth information with respect to the beneficial ownership of our common shares, as of March 31, 2023, by:
The calculations in the table below are based on 71,724,902 common shares outstanding as of March 31, 2023 before taking into account the issuance of the Exchange Shares in the Exchange and 114,133,056 shares, including 99,502,243 common shares and 14,630,813 Series B Preferred Shares, after taking into account the issuance of the Exchange Shares in the Exchange. Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days, including through the exercise of any option, warrant or other right or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person. Additionally, for purposes of this item, share counts and calculations in the table below do not reflect the issuance of the Exchange Shares into the Shareholder 2019 Rights Exchange Trust in connection with the Exchange.
|
|
Before issuance of the Exchange Shares |
|
|
After issuance of the Exchange Shares |
|
||||||||||
|
|
Number |
|
|
% |
|
|
Number |
|
|
% |
|
||||
Directors and Executive Officers: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Weidong Yin |
|
|
6,359,500 |
|
|
|
8.87 |
|
|
|
12,569,000 |
|
|
|
11.01 |
|
Simon Anderson |
|
* |
|
|
* |
|
|
* |
|
|
* |
|
||||
Yuk Lam Lo |
|
* |
|
|
* |
|
|
* |
|
|
* |
|
||||
Meng Mei |
|
* |
|
|
* |
|
|
* |
|
|
* |
|
||||
Kenneth Lee |
|
* |
|
|
* |
|
|
* |
|
|
* |
|
||||
Shan Fu |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
- |
|
Nan Wang |
|
* |
|
|
* |
|
|
* |
|
|
* |
|
||||
Guang (Helen) Yang |
|
* |
|
|
* |
|
|
* |
|
|
* |
|
||||
Qiang Gao |
|
* |
|
|
* |
|
|
* |
|
|
* |
|
||||
Jing Li |
|
* |
|
|
* |
|
|
* |
|
|
* |
|
||||
All directors and executive officers as a group |
|
|
7,536,847 |
|
|
|
10.51 |
|
|
|
14,599,714 |
|
|
|
12.79 |
|
Principal Shareholders |
|
|
|
|
|
|
|
|
|
|
|
|
||||
SAIF Partners IV(1) |
|
|
10,780,820 |
|
|
|
15.03 |
|
|
|
21,561,640 |
|
|
|
18.89 |
|
Prime Success, L.P.(2) |
|
|
5,900,000 |
|
|
|
8.23 |
|
|
|
11,800,000 |
|
|
|
10.34 |
|
Vivo Capital(3) |
|
|
5,900,000 |
|
|
|
8.23 |
|
|
|
11,800,000 |
|
|
|
10.34 |
|
CDH Utopia Limited(4) |
|
|
6,000,000 |
|
|
|
8.37 |
|
|
|
6,000,000 |
|
|
|
5.26 |
|
1Globe Capital LLC(5) |
|
|
3,353,092 |
|
|
|
4.67 |
|
|
|
3,353,092 |
|
|
|
2.94 |
|
Total share outstanding |
|
|
71,724,902 |
|
|
|
100.00 |
|
|
|
114,133,056 |
|
|
|
100.00 |
|
* Less than 1% of our common shares.
(1) According to the Amendment No. 8 to Schedule 13D filed with the SEC on March 15, 2023 by SAIF Partners IV L.P., SAIF IV GP, L.P. and SAIF IV GP Capital Ltd.
(2) According to the Schedule 13G filed with the SEC on July 10, 2018 by Prime Success, L.P., Green Vision Partners Limited and Advantech Capital Partners Ltd.
(3) According to the Amendment No. 2 to Schedule 13D filed with the SEC on August 27, 2018 by Vivo Capital, LLC, Vivo Capital VIII, LLC and Vivo Capital IX, LLC.
(4) According to the Schedule 13D filed with the SEC on December 22, 2020 by CDH Utopia Limited, CDH Fund VI, L.P., CDH VI Holdings Company Limited and CDH Griffin Holdings Company Limited.
69
(5) According to the Schedule 13D filed with the SEC on July 7, 2017, the Amendment No. 1 to the Schedule 13D filed with the SEC on March 23, 2018 and the Amendment No. 2 filed with the SEC on March 19, 2019 and the Amendment No. 3 filed with the SEC on December 21, 2020 by 1Globe Capital LLC.
None of our existing shareholders has different voting rights from other shareholders. Holders of our Series B Preferred Shares vote together with the common shares on an as-converted basis on all matters presented to the shareholders for a vote, subject to applicable law. Except for the complaint against filed by 1Globe against Sinovac Antigua in the Antigua Court, as disclosed in “Item 8. Financial Information — A. Consolidated Statements and Other Financial Information — Legal and Administrative Proceedings” or elsewhere in this annual report, we are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
As of December 31, 2018, 71,139,402 of common shares were issued and outstanding. On February 22, 2019, 27,777,341 of Sinovac Antigua’s common shares and 14,630,813 of Sinovac Antigua’s Series B preferred shares were issued into the Shareholder 2019 Rights Exchange Trust in connection with the Exchange. As described below under “Item 8. Financial Information — A. Consolidated Statements and Other Financial Information — Legal and Administrative Proceedings”, courts in Antigua and Delaware have enjoined the Company from issuing Exchange Shares from the Trust until final resolution of such matters. Taking into account issuance of the Exchange Shares, immediately following such issuance, 98,918,243 of common shares and 14,630,813 of Series B Preferred Shares were issued and outstanding. As of December 31, 2022, 99,502,243 of common shares and 14,630,813 of Series B Preferred Shares were issued and outstanding. Approximately 89% of the aggregate total of common shares and Series B Preferred Shares issued and outstanding were held by the record shareholders in the United States.
To our knowledge, except as disclosed elsewhere in this annual report, we are not directly or indirectly owned or controlled by another corporation, any foreign government or any other natural or legal person, severally or jointly.
For the options granted to our directors, officers and employees, please refer to “— B. Compensation.”
F. Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation.
Not Applicable.
A. Major Shareholders
Please refer to “Item 6. Directors, Senior Management and Employees — E. Share Ownership.”
B. Related Party Transactions
Transaction with Yuk Lam Lo
Sinovac Hong Kong is using part of the office of Mr. Yuk Lam Lo, one of our independent directors, as its office. We do not pay any rent to Mr. Lo and only pay our share of the utilities and property management fees, which totaled $3,155, $4,877 and $3,998 in 2022, 2021 and 2020, respectively.
Loan from a non-controlling shareholder
We have a loan due to Dalian Jin Gang Group, the non-controlling shareholder of Sinovac Dalian, with a total amount of $4.4 million (RMB30 million) borrowed in August 2020 and repayable on August 9, 2023. This loan is unsecured, bearing interest at 6.5% per year and payable monthly.
Transactions with other related parties
We entered into two operating lease agreements with SinoBioway, the non-controlling shareholder of Sinovac Beijing, with respect to Sinovac Beijing’s production plant and laboratory in Beijing, China with annual lease payments totaling RMB1.4 million. The leases commenced on August 12, 2004 and have a term of 20 years. One of the lease agreements was amended on August 12, 2010 with the rent increasing from RMB0.5 million to RMB1.4 million per year.
In June 2007, we entered into another operating lease agreement with SinoBioway, with respect to the expansion of Sinovac Beijing’s production plant in Beijing, China, for an annual lease payment of RMB2.0 million. The lease commenced in June 2007 and has a term of 20 years.
70
In September 2010, we entered into another operating lease agreement with SinoBioway with respect to expansion of Sinovac LS’ business in research and development activities for an annual lease payment of RMB1.0 million. The lease commenced on September 30, 2010 and has an initial term of five years.
On April 8, 2013, we entered into four supplemental agreements with SinoBioway, under which the expiration date of all operating lease agreements was extended to April 7, 2033.
In 2019, we entered into an operating lease agreement with Dalian Jin Gang Group, the non-controlling shareholder of Sinovac Dalian, to rent refrigeration storage with the space of 2,000 sq.m. with an annual rent amounted RMB0.3 million. The lease commenced on January 1, 2019 and had a term of five years. On June 30, 2019, the lease agreement was amended. The term of the lease was changed to from July 1, 2019 to December 31, 2024, and the annual rent was changed to RMB0.2 million as the space of the leased refrigeration storage was reduced to 1,000 sq.m. In 2019, we also entered into a management service agreement with Dalian Jin Gang Group, pursuant to which it provided us with management service related to the operating lease agreement with an annual management service fee of RMB100,000. The management service agreement was amended on June 30, 2019, and the annual management service fee was changed to RMB44,000.
As of December 31, 2022, $6.4 million in right-of use asset and $6.0 million in current and non-current lease liability are related to the lease with SinoBioway and Jin Gang.
In 2022, we invested in $88.4 million to certain Vivo Capitals funds, where Mr. Shan Fu is the Managing Partner at Vivo Capital.
Share Options
See “Item 6. Directors, Senior Management and Employees — B. Compensation — 2003 Stock Option Plan” and “Item 6. Directors, Senior Management and Employees — B. Compensation — 2012 Share Incentive Plan.”
Trust for Incentive
See “Item 6. Directors, Senior Management and Employees — B. Compensation — Trust for Incentive”.
Indemnification Agreements
See “Item 6. Directors, Senior Management and Employees — B. Compensation — Indemnification Agreements.”
Employment Agreements; Non-Disclosure, Non-Competition and Proprietary Information Agreement
See “Item 6. Directors, Senior Management and Employees — B. Compensation — Employment Agreements; Non-Disclosure, Non-Competition and Proprietary Information Agreement.”
C. Interests of Experts and Counsel
Not applicable.
ITEM 8. Financial Information
A. Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report.
Legal and Administrative Proceedings
We may be subject to legal proceedings, investigations and claims relating to the conduct of our business from time to time.
US Litigation
Delaware Chancery Court Action
On March 5, 2018, we filed a lawsuit in the Court of Chancery of the State of Delaware seeking a determination whether 1Globe, The Chiang Li Family, OrbiMed and other shareholders of Sinovac Biotech Ltd. had triggered the Rights Agreement by forming a group holding approximately 45% of outstanding shares of Sinovac Biotech Ltd., in excess of the plan’s threshold of 15%, and acting in concert prior to the 2017 AGM. The Rights Agreement is intended to promote the fair and equal treatment of all Sinovac shareholders and ensure that no person or group can gain control of Sinovac through undisclosed voting arrangements, open market accumulation or other tactics potentially disadvantaging the interest of all shareholders.
71
On April 12, 2018, 1Globe filed an amended answer to Sinovac Antigua’s complaint, counterclaims, and a third-party complaint against Mr. Weidong Yin alleging, among other allegations, that the Rights Agreement is not valid, that Mr. Weidong Yin and the Buyer Consortium had previously triggered the Rights Agreement, and that 1Globe did not trigger the Rights Agreement. Sinovac Antigua and its board of directors believes that the actions taken by the board of directors were appropriate under the circumstances and that the allegations of the counterclaims and third-party complaint are without merit. 1Globe asks for various measures of equitable relief and also includes a claim for its costs, including attorneys’ fees.
On July 31, 2018, following Sinovac Antigua motions for partial summary judgment and an expedited trial date, the Delaware Chancery Court effectively stayed the action pending receipt of a post-trial decision from the Antigua Court in the matter captioned 1Globe Capital, LLC and Sinovac Biotech Ltd., Claim No. ANUHCV 2018/0120. On December 19, 2018, the Antigua Court issued a judgment affirming the validity of Sinovac Antigua’s Rights Agreement under Antigua law, and finding that “there was a secret plan to take control” of Sinovac Antigua at the 2017 AGM.
Based upon the Antigua Court’s judgment and other facts known to the board of directors, our board of directors determined that the Collaborating Shareholders became Acquiring Persons on or prior to the 2017 AGM and their conduct resulted in a Trigger Event under Sinovac Antigua’s Rights Agreement. As a result of becoming Acquiring Persons, the approximately 28.7 million Rights held by the Collaborating Shareholders automatically became void under the terms of the Rights Agreement. Pursuant to the Rights Agreement, our board of directors elected to exchange the approximately 42.4 million valid and outstanding Rights held by Sinovac Antigua’s shareholders (not including the Collaborating Shareholders) for a combination of approximately 27.8 million common shares and approximately 14.6 million Series B preferred shares, all of which Sinovac Antigua issued into a trust on February 22, 2019 for the benefit of the holders of the valid and outstanding Rights. See “History and Development of the Company” for additional information.
On March 6, 2019, the Delaware Chancery Court entered a status quo order providing that Sinovac Antigua not distribute any of the Exchange Shares to rights holders until the final disposition of the pending Delaware litigation or further order of the Court. On April 8, 2019, the Delaware Chancery Court stayed the Delaware litigation pending the final outcome of 1Globe’s appeal of the Antigua Judgment. The Antigua litigation is ongoing, see “– Antigua Litigation” below, and therefore the Delaware Chancery Court action remains stayed.
Massachusetts District Court Actions
On March 5, 2018, Sinovac Antigua also filed a lawsuit in the United States District Court for Massachusetts alleging violations of Section 13(d) of the Securities Exchange Act of 1934 by 1Globe and The Chiang Li Family. The lawsuit alleges, among other things, that the defendant shareholders failed to make required disclosures on Schedule 13D regarding their intentions to attempt to replace Sinovac Antigua’s board of directors.
On April 9, 2018, we received a document request from the SEC requesting all of our documents concerning 1Globe, the Chiang Li Family, OrbiMed, certain other shareholders, and their affiliates. We have been cooperating with the SEC. We understand the SEC is investigating whether 1Globe, and possibly other shareholders, violated the U.S. securities laws. We do not have any information to suggest the SEC is investigating the actions of Sinovac Antigua or its officers and directors.
On May 21, 2018, 1Globe answered and filed counterclaims against Sinovac Antigua and certain of its executives, alleging violations of Section 10(b) of the Exchange Act and various state law claims. In response to Sinovac Antigua motion to dismiss 1Globe’s counterclaims, on August 1, 2018, 1Globe filed amended counterclaims against Sinovac Antigua and certain of its executives, alleging violations of Section 10(b) of the Exchange Act and Rule 10b-5, as well as state law claims of abuse of process, fraudulent misrepresentation, negligent misrepresentation, and aiding and abetting such violations, primarily arising out of allegedly false and/or misleading statements made by us regarding our business, operational, and financial results.
On August 17, 2018, the Massachusetts Court granted a consent motion to extend the deadline for Sinovac Antigua’s response to 1Globe’s counterclaims (and for any subsequent opposition by 1Globe) until after the Antigua Court issued a ruling in the matter captioned 1Globe Capital, LLC and Sinovac Biotech Ltd., Claim No. ANUHCV 2018/0120. On December 19, 2018, the Antigua Court issued a judgment, which 1Globe appealed on January 29, 2019. Per the Massachusetts Court’s order, the parties have filed periodic status reports regarding the pending court proceedings in Antigua. No date for Sinovac Antigua’s response to 1Globe’s counterclaims has been set. We are vigorously pursuing this lawsuit; however, we cannot predict whether an ultimate outcome will be favorable or unfavorable, nor estimate the amount or range of potential loss (if any) at this time.
Also on August 1, 2018, 1Globe filed a motion for preliminary injunction seeking to enjoin Sinovac Antigua from, inter alia, altering its capital structure. On October 15, 2018, the Massachusetts Court denied 1Globe’s motion. On November 14, 2018, 1Globe filed an appeal of the denial of its motion for preliminary injunction to the United States Court of Appeals for the First Circuit. On January 10, 2019, 1Globe filed a motion to hold its appeal in abeyance pending the outcome of its separate appeal of the Antigua Court’s judgment, which Sinovac Antigua opposed. In October 2019, 1Globe voluntarily dismissed the appeal.
72
Separately, Heng Ren Investments LP (“Heng Ren”) filed suit against Sinovac Antigua and Weidong Yin for alleged breach of fiduciary duties and wrongful equity dilution on May 31, 2019, in Massachusetts state court. Sinovac Antigua moved the matter from state court to the United States District Court for the District of Massachusetts. Subsequently, on April 29, 2021, Heng Ren filed an amended complaint which alleged that Mr. Yin breached fiduciary duties owed to minority shareholders, that Sinovac Antigua aided and abetted breaches of fiduciary duties, and that both Sinovac Antigua and Mr. Yin engaged in wrongful equity dilution. Heng Ren requested damages, attorneys’ fees, and prejudgment interest. On September 14, 2020, Sinovac Antigua filed a motion to dismiss Heng Ren’s claims. In July 2021, Sinovac moved to dismiss Heng Ren’s amended complaint in the federal court in Massachusetts. On March 4, 2022, the court granted the motion as to the breach of fiduciary duty claims and denied the motion as to the wrongful equity dilution claim, and denied reconsideration of its decision on the motion. Sinovac has answered the complaint. On February 15, 2023, the court stayed discovery in the Heng Ren matter pending the resolution of an outstanding motion to dismiss filed in a purported shareholder's matter.
On December 5, 2022, a purported shareholder filed a putative class action complaint in United States District Court for the District of Massachusetts, asserting a claim under Section 204 of the Antigua and Barbuda International Business Corporations Act related to the PIPE transaction, alleging that all shareholders were harmed in an identical manner to one another by the PIPE transaction because the shares that were issued in the PIPE transaction allegedly undervalued Sinovac and all shareholders were purportedly wrongfully diluted as a result. The purported shareholder is represented by the same attorney who represents Heng Ren, and requests damages, attorneys’ fees, and prejudgment interest. On January 18, 2023, Sinovac filed a motion to dismiss. The motion was fully briefed as of March 9, 2023, and is currently pending before the court.
On January 19, 2023, Sinovac filed a motion to stay the Heng Ren action pending the resolution of the putative class action. On February 16, 2023, Massachusetts federal court stayed the Heng Ren action.
Antigua Litigation
On March 13, 2018, 1Globe filed a complaint against Sinovac Antigua in the Antigua Court. The complaint seeks a declaration that the five persons purportedly proposed on the Non-Public Submission at the 2017 AGM were elected as directors of Sinovac Antigua at that meeting, an order of the Antigua Court that those directors be installed as Sinovac Antigua’s board of directors, and a declaration that any actions taken on behalf of Sinovac Antigua at the direction of the board of directors since the 2017 AGM are null and void. On April 10, 2018, 1Globe filed a notice of application in the Antigua Court seeking an order declaring the result of the disputed election, an urgent order restraining Sinovac Antigua’s board of directors from acting, pending determination of the dispute, including acting to initiate or continue litigation against the Shareholder Group, and other related relief. We attended the first hearing on May 9, 2018. In July 2018, the Antigua court heard an application by 1Globe for interim injunctive relief preventing Sinovac Antigua from exercising its rights under the Rights Agreement. This application was unsuccessful, but the judge set an expedited timetable to trial. The trial of the matter took place from December 3 to 5, 2018. On December 19, 2018, the judge handed down his judgment, finding in Sinovac Antigua’s favor in full, dismissing 1Globe’s claim and declaring that the Rights Agreement was validly adopted as a matter of Antigua law. On January 29, 2019, 1Globe filed a Notice of Appeal. On March 4, 2019, 1Globe filed an application for urgent interim relief, seeking an injunction to prevent Sinovac Antigua from continuing to implement its Rights Agreement until the resolution of the appeal. This urgent interim relief application was heard on April 4, 2019, at which the Court of Appeal made an order restraining Sinovac Antigua in similar terms to the Delaware Court order of March 6, 2019, together with restraint from operating the Rights Agreement in any way that affects 1Globe’s rights or shareholding until determination of the appeal. 1Globe’s appeal of the Antigua Court’s Judgment was heard on September 18, 2019. On December 9, 2021, the Court of Appeal handed down its judgment, dismissing all grounds of appeal and upholding the Antigua Judgment. The Court of Appeal also confirmed that Sinovac Antigua’s Rights Agreement was consistent with its Articles of Incorporation and By-laws, and Antiguan business law. In January 2022, the Court of Appeal extended the order initially made on April 4, 2019, that restrains Sinovac Antigua from taking further action under its Rights Agreement, including the distribution of the previously issued Exchange Shares, until the conclusion of any appeal to the Privy Council. 1Globe applied for leave to appeal to the Privy Council, and the hearing of that application was held on February 24, 2022, in which the Court of Appeal granted 1Globe leave to appeal certain grounds to the Privy Council. On April 19, 2022, 1Globe renewed its application directly to the Privy Council for leave to appeal on its ground of appeal concerning the validity of the Rights Agreement. On July 13, 2022, 1Globe filed its Notice of Appeal on those grounds on which the Court of Appeal had granted 1Globe leave to appeal. On September 16, 2022, 1Globe filed an application to the Privy Council seeking permission to amend its existing application for permission to appeal and its existing Notice of Appeal, and to seek permission to appeal on another ground rejected by the Court of Appeal concerning the exercise of the Antigua Court’s discretion. Sinovac responded on October 21, 2022. On February 15, 2023, the Privy Council made a procedural decision to allow amendment of its existing application for permission to appeal, and decided to deal with procedural and substantive issues together at the Final Hearing. 1Globe has not yet taken steps to list a substantive hearing before the Privy Council. The appeal outcome is therefore pending.
As such, the final appeal is ongoing as of the date of this annual report. We cannot predict or estimate an outcome or economic burden for this case at this time.
Hong Kong Litigation
On October 8, 2018, Sinovac became aware that unauthorized documents in respect of Sinovac Hong Kong had been unlawfully filed with the Hong Kong Companies Registry to change the directors of Sinovac Hong Kong from Mr. Weidong Yin and Ms. Nan Wang to Mr. Jianzeng Cao and Mr. Pengfei Li. On October 15, 2018, Mr. Yin and Ms. Nan Wang commenced proceedings HCMP 1731/2018 before the Hong Kong High Court.
73
In a hearing before the Hong Kong High Court on October 19 2018, the Lawful Directors asked the court to grant an urgent interim injunction order to restrain Mr. Li and Mr. Cao from taking further unlawful actions against Sinovac HK and its subsidiaries. At the hearing, the judge granted an interlocutory injunction in the same terms sought by the Lawful Directors restraining Mr. Pengfei Li and Mr. Jianzeng Cao from purporting to act or holding themselves out as directors of Sinovac Hong Kong or its subsidiaries, purporting to take any actions as directors of Sinovac Hong Kong or its subsidiaries, and relying on or using the forged documents in any way whatsoever.
On November 28, 2018 at a further hearing in the Hong Kong High Court, the Hong Kong High Court made the November 28 Order and held that it is beyond dispute that the documents in respect of Sinovac Hong Kong had been forged and unlawfully filed with the Hong Kong Companies Registry, based on the evidence filed by Mr. Cao, Mr. Li and the Lawful Directors. The Hong Kong High Court therefore declared that Mr. Yin and Ms. Wang were and still are the lawful directors of Sinovac Hong Kong, and Mr. Li and Mr. Cao were not and are not the lawful directors of Sinovac Hong Kong. The Hong Kong High Court also granted a permanent injunction restraining Mr. Li and Mr. Cao from purporting to act or holding themselves out as directors of Sinovac Hong Kong or its subsidiaries (including but not limited to Sinovac Beijing), purporting to take any actions as directors of Sinovac Hong Kong or its subsidiaries, and relying on or using the forged documents in any way whatsoever. Furthermore, the Hong Kong High Court also ordered the Companies Registry to remove the forged documents in respect of Sinovac Hong Kong that had been unlawfully filed.
On November 28, 2018, Mr. Cao and Mr. Li filed a Notice of Appeal with the Hong Kong Court of Appeal, indicating their intention to appeal the orders made by the Hong Kong High Court. No hearing date has yet been fixed to hear the appeal. Mr. Yin and Ms. Wang intends to vigorously contest the appeal filed by Mr. Cao and Mr. Li. Pending the determination of the appeal, the November 28 Order remains effective and enforceable. Pursuant to the November 28 Order, the Hong Kong Companies Registry has removed the purported Sinovac Hong Kong documents from the Companies Register and updated Sinovac Hong Kong’s register of director such that the directors on record are Mr. Yin, Ms. Wang and Mr. Yuk Lam Lo.
As of the date of this annual report, neither the Court of First Instance nor the Court of Appeal directed that the execution of the November 28 Order should be stayed. So far, Mr. Cao and Mr. Li have taken no further steps in respect of the appeal after the Notice of Appeal was filed on November 28, 2018.
PRC Litigation
On May 16, 2018, Sinovac Hong Kong filed a complaint against Sinobioway Medicine, Mr. Aihua Pan, and Shandong Sinobioway Biomedicine Co., Ltd. in the Beijing Fourth Court. The complaint sought to hold the defendants jointly and severally liable for the torts they committed during an attempt of the defendants to take physical control of our facility in Shangdi site in Beijing on April 17, 2018. Later, Sinovac Hong Kong made an application to the court to add Sinovac Beijing as a third party to participate in the proceedings. The court has granted an order, permitting Sinovac Beijing to participate in the proceedings as a third party. At the hearing held on July 2, 2019, Sinovac Hong Kong, the defendants and Sinovac Beijing cross-examined the evidences submitted by each party. Based on the result of the cross-examination, the court declared that an independent evaluation firm shall be engaged by both the defendants and Sinovac Hong Kong to evaluate the losses and damages sustained by Sinovac Beijing as the result of the actions taken by the defendants on April 17, 2019. An independent evaluation firm was selected by the court and the evaluation was conducted accordingly. On September 17, 2020, the Fourth Intermediate People’s Court of Beijing issued a judgment holding Sinobioway Medicine and Mr. Aihua Pan liable for torts and breaches of shareholders fiduciary duty under the PRC Company Law and liable for Sinovac Beijing’s losses of RMB15.4 million caused by their disruptive actions. Sinovac Beijing, Sinobioway Medicine and Mr. Aihua Pan filed notice to appeal to the Higher People’s Court of Beijing Municipality. The Higher People’s Court of Beijing Municipality held a hearing in September 2021. On October 31, 2022, the Higher People’s Court of Beijing Municipality issued a judgment in favor of Sinovac Hong Kong, upholding the judgment issued by the Beijing Fourth Court and ruling that Shandong Sinobioway Biomedicine, as the sole shareholder of Sinobioway Medicine, is jointly and severally liable for all the relevant obligations of Sinobioway Medicine to Sinovac Hong Kong.
On September 13, 2018, Sinovac Beijing filed a complaint against Mr. Aihua Pan in the Haidian District Court of Beijing (the “Haidian Court”). The complaint sought to request Mr. Pan return a business license of Sinovac Beijing which was reissued by the Haidian Branch of Beijing Administration for Industry and Commerce on May 10, 2018 based on the false reporting made by Mr. Pan and the seals of Sinovac Beijing which are forged by Mr. Pan. Sinovac Beijing filed a preservation application to the court. The court supported Sinovac Beijing’s preservation application and prohibited Mr. Pan from using or authorizing others to use the above-mentioned license and seals during the case hearing. The court held a preliminary and brief hearing on November 18, 2019. At the hearing, the court has decided and declared to suspend the proceedings until the final verdict of the September 5 Board Resolution Case (as described below) is given by the Beijing Fourth Court. On November 30, 2021, Sinovac settled the litigation with Mr. Aihua Pan. With the settlement, Mr. Pan has returned the business license and the seals of Sinovac Beijing to Sinovac Beijing, and Sinovac Beijing has withdrawn its claims with the Haidian Court.
On October 8, 2018, the Company also became aware that unauthorized documents in respect of Sinovac Beijing had been filed with the Industry and Commerce Bureau of Haidian District of Beijing (“Haidian AIC”) to change the directors of Sinovac Beijing from Mr. Weidong Yin, Ms. Nan Wang and Mr. Dawei Mao to Mr. Jianzeng Cao, Mr. Pengfei Li and Ms. Xiaomin Yang. Mr. Yin and Ms. Wang filed an objection to such unlawful change with the Haidian AIC. On March 19, 2020, Haidian AIC issued an official decision (“AIC Decision”) declaring that (i) the unauthorized documents are forged and fake documents; (ii) the filing of change of directors made based on the forged documents is null and
74
void; (iii) the unlawful filing to change the said directors will be removed and (iv) the registration of directors of Sinovac Beijing will be restored. The parties of material interest in the AIC Decision may raise objection or file a lawsuit within 60 days. No one had filed the objection or lawsuit against the AIC Decision within 60 days thereof.
On December 24, 2018, Sinobioway Medicine filed a complaint against Sinovac Beijing in the Haidian Court. The complaint sought a declaration that all the board resolutions dated September 5, 2018, including the composition of the board, the appointment of the senior managers and the management of the corporate seals, are invalid (“September 5 Board Resolution Case”). Sinovac Hong Kong has filed an application for adding itself as the third party in this lawsuit. The court decided to accept its application. As a result of Sinovac Hong Kong, which is deemed as a foreign entity under the PRC Civil Procedural Law, participating in the litigation, the Haidian Court does not have the jurisdiction over the case and has transferred the case to the Beijing Fourth Court. In January 2020, the Beijing Fourth Court requested all the participants in the litigation to submit evidence. Both Sinovac Beijing and Sinovac Hong Kong submitted all the valid evidence to the court in February and March 2020. Then, Sinobioway Medicine filed a request to the Beijing Fourth Court to voluntarily withdrew the case on November 2, 2020. The Beijing Fourth Court supported such voluntary withdrawal and made a ruling to dismiss the case on November 6, 2020.
On July 25, 2019, Sinobioway Medicine filed a complaint against Sinovac Beijing in the Haidian Court. The complaint sought to request Sinovac Beijing to provide (i) all the corporate documents of Sinovac Beijing, including the Articles of Association and board resolutions (ii) all the books and the related accounting vouchers and records of Sinovac Beijing, created from the date of January 1, 2017, and (iii) the monthly financial reports of Sinovac Beijing for the lawyers and auditors of Sinobioway Medicine to review and/or copy. The complaint also sought to request Sinovac Beijing to agree the auditors of Sinobioway to audit its annual and quarterly financial reports. On October 28, 2019, one judge of the Haidian Court held a preliminary and brief hearing and declared that the simplified procedures shall not apply to this case, which shall be heard by a panel of three judges. On August 25, 2020, the Haidian Court issued a judgment only supporting Sinobioway Medicine’s request for inspecting and copying the Articles of Association and board resolutions of Sinovac Beijing and inspecting books and related accounting vouchers and records of Sinovac Beijing, and dismissing the rest of Sinobioway Medicine’s claims. Sinobioway Medicine filed a notice to appeal to Beijing First Intermediate Court on September 7, 2020. Neither party provided any new evidence nor questioned the procedures applied by Haidian Court. On March 15, 2021, after a hearing, the Beijing First Intermediate Court made a ruling after a hearing, rejecting the appeal made by Sinobioway Medicine and endorsing the judgment made by Haidian Court. The lawsuit was closed.
On November 15, 2021, Sinobioway Medicine filed a complaint against Sinovac Beijing and Sinovac Hong Kong in Beijing Fourth Court. The complaint sought to dissolve and liquidate Sinovac Beijing with the argument that the board of directors of Sinovac Beijing has been unable to function for the benefit of the company and the two shareholders of Sinovac Beijing have gotten into a deadlock. In December 2022, Sinobioway Medicine filed a request to the Beijing Fourth Court to voluntarily withdraw the case. The Beijing Fourth Court supported such voluntary withdrawal and made an official ruling to dismiss the case on January 20, 2023.
In November 2021, Sinobioway Medicine filed a complaint against Sinovac LS, Sinovac Hong Kong, Mr. Weidong Yin and Keding Investment (Hong Kong) Limited with the Beijing Fourth Court, claiming that Sinovac LS has infringed the legitimate rights of Sinovac Beijing when doing the research and development of CoronaVac. Sinobioway Medicine listed Sinovac Beijing as a third party in the case. On March 13, 2023, Beijing Fourth Court notified us by telephone that Sinobioway Medicine had just filed a request to the Beijing Fourth Court to voluntarily withdraw the case. The Beijing Fourth Court supported such voluntary withdrawal and is in the process of making an official ruling to dismiss the case.
On February 13, 2023, Shandong Sinobioway Biomedicine filed a complaint against Sinobioway Medicine and Sinovac Beijing with Zhangdian District Court, requesting the court to rule and confirm that Shandong Sinobioway Biomedicine, instead of Sinobioway Medicine, should be the shareholder of Sinovac Beijing despite that Sinobioway Medicine has been and is currently registered as the shareholder of Sinovac Beijing with the company registrar. Shandong Sinobioway Biomedicine also requested Zhangdian District Court to rule and order Sinovac Beijing to correct the registration of Shandong Sinobioway Biomedicine as the shareholder of Sinovac Beijing, Mr. Jialin Yue as the director, Chairman and Legal Representative of Sinovac Beijing and Mr. Weining Luan as the supervisor of Sinovac Beijing with the company registrar. In response to this newly filed case, on February 24, 2023, Sinovac Beijing filed an objection to the jurisdiction of Zhangdian District Court to hear this case. On March 12, 2023, Zhangdian District Court ruled that it has the jurisdiction to hear this case. On March 24, 2023, Sinovac Beijing filed an appeal to Zibo Intermediate Court, in objection to the ruling made by Zhangdian District Court regarding its jurisdiction to hear the case. As of the date of this annual report, the case is pending with the final ruling to be made by Zibo Intermediate Court on the jurisdiction of Zhangdian District Court.
Dividend Policy
We have never declared or paid any dividends, nor do we have any present plan to pay any cash dividends on our common shares in the foreseeable future. We currently intend to retain most, if not all, of our available funds and any future earnings to operate and expand our business.
Our board of directors has complete discretion on whether to pay dividends. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant. Cash dividends on our common shares, if any, will be paid in U.S. dollars.
75
We are a holding company, and we rely on the dividends paid by our subsidiaries for our cash needs, including the funds necessary to pay any dividends and other cash distributions to our shareholders, service any debt we may incur and pay our operating expenses. The payment of dividends in China is subject to limitations. Regulations in the PRC currently permit payment of dividends by our PRC subsidiaries only out of accumulated profits as determined in accordance with accounting standards and regulations in China. In accordance with the regulations in China, our PRC subsidiaries are required to set aside at least 10% of their after-tax profits each year to contribute to its reserve fund until the accumulated balance of such reserve fund reaches 50% of the registered capital of each company. Our PRC subsidiaries are required to set aside, at the discretion of their respective board of directors, a portion of its after-tax profits to their employee welfare and bonus funds.
Furthermore, pursuant to the double tax arrangement between Hong Kong and PRC, dividends paid by a foreign-invested enterprise in China to its direct holding company in Hong Kong will be subject to withholding tax at a rate of 5% (if the foreign investor owns directly at least 25% of the shares of the foreign-invested enterprise for a period greater than 12 months and meets the relevant requirements pursuant to the tax arrangement between Hong Kong and PRC), or otherwise at 10%. Prior to May 2012, whether the favorable rate will be applicable to dividends received by Sinovac Hong Kong from our PRC subsidiaries is subject to the approval of the PRC tax authorities. The PRC tax authorities have discretion to assess whether a recipient of the PRC-sourced income is only an agent or a conduit, or lacks the requisite amount of business substance, in which case the application of the tax arrangement may be denied. This withholding tax imposed on dividends paid to us by our PRC subsidiaries would reduce our net income attributable to the shareholders. In May 2012, Sinovac Hong Kong was granted by the local tax bureau the preferential dividend withholding tax rate of 5% on dividends declared by Sinovac Beijing for three years from 2012 to 2014. The preferential dividend withholding tax rate expired in 2014. Subsequent to May 2012, the preferential dividend withholding tax rate no longer needed to be approved by the PRC tax authorities, instead companies can apply the 5% rate if a self-assessment determined the recipient of the PRC-sourced income qualify for the preferential rate. However, such self-assessment could be overturned upon an inspection by the PRC tax authorities. We have assessed Sinovac Hong Kong meets the relevant requirements pursuant to the tax arrangement between Hong Kong and PRC, and determined the preferential dividend withholding tax rate of 5% is applicable to Sinovac Hong Kong.
B. Significant Changes
Except with respect to the Exchange and the related issuance of common shares and Series B Preferred Shares pursuant to Sinovac Antigua’s Rights Agreement, as well as the related ongoing litigation, in each case disclosed elsewhere in this annual report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
ITEM 9. The Offer and Listing
A. Offer and Listing Details
See “—C. Markets.”
B. Plan of Distribution
Not applicable.
C. Markets
Our common shares have been listed on the NASDAQ Global Select Market since January 3, 2011 under the symbol “SVA.” In connection with the Exchange and the issuance of the Exchange Shares into the Shareholder 2019 Rights Exchange Trust, trading of our common shares on Nasdaq has been halted since February 22, 2019.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
ITEM 10. Additional Information
A. Share Capital
76
Not applicable.
B. Memorandum and Articles of Association
We are an Antiguan company (Company No. 11949) with limited liability and our affairs are governed by our Articles of Incorporation, By-laws and the International Business Corporations Act. The following are summaries of material provisions of our Articles of Incorporation, By-laws and the International Business Corporations Act.
General
All of our outstanding common shares are fully paid and non-assessable. The common shares are issued in registered form. Holders of common shares are entitled to receive share certificates. Our shareholders who are non-residents of Antigua may freely hold and vote their common shares.
Corporate Purpose
The objects for which the Company is established are set forth in the Company’s Articles of Incorporation, as follows:
Sinovac Antigua shall not engage in International Banking, Trust, Insurance, Betting and Bookmaking or any other activity which requires a License under the International Business Corporations Act.
Sinovac Antigua shall be primarily engaged in research, development and commercialization of human vaccines for infectious diseases.
Dividends: Rights to Share Profits
The holders of our common shares are entitled to such dividends as may be declared by our board of directors subject to the International Business Corporations Act. For example, under the International Business Corporations Act, a company shall not declare or pay a dividend if this would result in the company’s inability to pay its liabilities as they become due or the realizable value of the company’s assets less than the aggregate of its liabilities and stated capital of all class. In addition, a company shall not pay a dividend out of unrealized profits.
Voting Rights
Each common share is entitled to one vote on all matters upon which the common shares are entitled to vote.
A quorum required for a meeting of shareholders consists of shareholders who hold at least a majority of our shares at the meeting present in person or by proxy. Shareholders’ meetings are held annually and may be convened by our board of directors on its own initiative or upon a request to the directors by shareholders holding in aggregate at least five percent of our issued share capital. Advance notice of at least 21 days is required for the convening of our annual general meeting and other shareholders meetings.
Unless the International Business Corporations Act otherwise requires, resolutions to be passed by the shareholders require a simple majority vote. Important matters such as changes to our By-laws require a resolution passed by a vote of shareholders holding a majority of all the outstanding and issued shares.
Transfer of Common Shares
Our shareholders may transfer common shares by endorsing the relevant share certificates, completing a share transfer form or by other proper evidence of succession, assignment or authority to transfer.
77
Liquidation
On a return of capital on winding up or otherwise (other than on conversion, redemption or purchase of common shares), assets available for distribution among the holders of common shares shall be distributed among the holders of the common shares on a pro rata basis. If our assets available for distribution are insufficient to repay all of the paid-up capital, the assets will be distributed so that the losses are borne by our shareholders proportionately.
Reserve Fund
Subject to the provisions of the International Business Corporations Act, as amended, we may by special resolution reduce any capital redemption reserve fund or any share premium account.
Redemption, Repurchase and Surrender of Shares
Subject to the provisions of the International Business Corporations Act, as amended, we may by special resolution reduce our share capital, any capital redemption on reserve fund or any share premium account. However, in accordance with the International Business Corporations Act, we must not make any payment to purchase or redeem any redeemable issued by it if there are reasonable grounds for us believing that
Calls on Shares and Forfeiture of Shares
There are no provisions in our Articles of Incorporation and By-laws, as amended, governing the calls on shares and forfeiture of shares.
Limitations on the Rights to Own Shares
There are no provisions in our Articles of Incorporation and By-laws, as amended, governing the limitations on the rights to own shares in the Corporation.
Ownership Threshold
There are no provisions in our Articles of Incorporation and By-laws, as amended, governing the ownership threshold above which shareholder ownership must be disclosed. Shareholders will, however, be required to disclose shareholder ownership in accordance with applicable laws and regulations.
Inspection of Books and Records
Holders of our common shares will have no general right under Antigua law to inspect or obtain copies of our list of shareholders or our corporate records. They may, however, access such corporate information as is publicly available in the Companies Registry in St. John’s, Antigua. We will also provide our shareholders with annual audited consolidated financial statements.
Changes in Capital
We may from time to time by a resolution passed by a majority of the shares entitled to vote:
78
We may by special resolution reduce our share capital and any capital redemption reserve in any manner authorized by law.
Director’s Powers and Qualification
Pursuant to the International Business Corporation Act, a director or officer of the corporation, (a) who is a party to a material contract or proposed material contract with the corporation; or (b) who is a director or an officer of any body, or has material interest in any body, that is a party to a material contract or proposed material contract with the corporation, must disclose in writing to the corporation or request to have entered in the minutes of meetings of directors the nature and extent of his interest. The disclosure must be made, in the case of a director of a corporation, (a) at the meeting at which a proposed contract is first considered; (b) if the director was not then interested in the proposed contract, at the first meeting after he becomes so interested; (c) if the director becomes interested after a contract is made at the first meeting after he becomes so interested; or (d) if a person who is interested in a contract later becomes a director of the corporation, at the first meeting after he becomes a director. A director of the corporation may vote on any resolution to approve a contract that he has an interest in, if the contract (a) is an arrangement by way of a security for money loaned to or obligation undertaken by him for the benefit of the corporation or an affiliate of the corporation; (b) is a contract that relates primarily to his remuneration as a director, officer, employee or agent of the corporation or affiliate of the corporation; (c) is a contract for indemnity or insurance under section 99 to 101 of the International Business Corporation Act; (d) is a contract with an affiliate of the corporation; or (e) is a contract other than one referred to in (a) to (d) above. But, in the case of a contract described in paragraph (e), no resolution is valid unless it is approved by not less than two-thirds of the votes of the shareholders of corporation to whom notice of the nature and extent of the director’s interest in the contract is declared and disclosed in reasonable details. A general notice to the directors of the corporation by a director or an officer of the corporation declaring that he is a director or officer of or has a material interest in another body and is to be regarded as interested in any contract with that body is a sufficient declaration of interest in relation to any such contract.
There are no provisions in our Articles of Incorporation and By-laws, as amended, governing the directors’ powers to vote compensation to themselves or any members of their body.
Pursuant to the International Business Corporation Act, unless the articles or by-laws, or any unanimous shareholder agreement relating to, the corporation otherwise provide, the articles of a corporation are presume to provide that the directors of the corporation may, without authorization of the shareholders, (i) borrow money upon the credit of the corporation; (ii) issue, re-issue, sell or pledge debenture of the corporation; (iii) give guarantee on behalf of the corporation to secure performance of an obligation of any person; and (iv) mortgage, charge, pledge, or otherwise create to secure any obligation of the corporation a security interest in all or any property of the corporation that is owned or subsequently acquired by the corporation. “Security interest” means any interest in or charge upon any property of a corporation, by way of mortgage, bond, lien, pledge or other mean, that is created or taken to secure the payment of an obligation of the corporation. Notwithstanding, when circumstances prejudicial to the corporation exist, the corporation shall not directly or directly, give financial assistance by means of a loan, guarantee or otherwise to a shareholder, director, officer or employee of the corporation or affiliated corporation; or to any person for the purpose of or in connection with a purchase of a share issued or to be issued by the corporation or a corporation with which it is affiliated. Unless the articles or by-laws, or any unanimous shareholder agreement relating to, the corporation otherwise provide, the directors of the corporation may by resolution delegate the powers mentioned above to a director, a committee of directors or an officer of the corporation.
There are no provisions in our Articles of Incorporation and By-laws, as amended, governing the directors’ powers as it relates to retirement or non-retirement of directors under the age limit requirement.
There are no provisions in our Articles of Incorporation and By-laws, as amended, that make provisions for number of shares required for director’s qualification.
General Meetings of Shareholders
We must hold an annual shareholders’ meeting every year. The meeting must take place within Antigua and Barbuda at a place and time prescribed by our board of directors. As it relates to a special shareholders’ meeting, the board of directors may, whenever it thinks fit, convene a special shareholders’ meeting. Our board of directors shall also on the requisition of the holders of not less than one-twentieth of our issued share capital proceed to convene a special shareholders’ meeting. No business shall be transacted at any shareholders’ meeting unless a quorum of shareholders is present at the time when the meeting proceeds to business. Shareholders present in person or by proxy representing a majority of our shares shall constitute a quorum. All meetings shall be chaired by a director appointed by our board of directors to act as the chairman. Minutes of the proceedings of every annual shareholders’ meeting shall be kept, and shall be signed by the chairman of the same meeting, or by the chairman of the next succeeding meeting, and the same, when so signed, shall be conclusive evidence of all such proceedings and of the proper election of the chairman.
Subject to any rights or restrictions for the time being attached to any class or classes of shares, every shareholder shall have one vote for each share of which he is the holder. All elections for director shall be decided by majority vote; all other questions shall be decided by majority vote except as otherwise required by the International Business Corporations Act, as amended. Unless otherwise provided by law, any action required to be taken at a meeting of the shareholders, or any other action which may be taken at a meeting of the shareholders, may be taken without a meeting if a consent in writing, setting forth the action so taken, shall be signed by all of the shareholders entitled to vote with respect to the subject matter thereof. Votes may be given either personally or by proxy. The instrument appointing a proxy shall be in writing under the hand
79
of the appointer of his attorney duly authorized in writing, or if the appointer is a corporation, either under seal or under the hand of an officer or attorney duly authorized. A proxy need not be our shareholder.
Written or printed notice stating the place, day and hour of the meeting and, in case of a special meeting, the purpose or purpose for which the meeting is called, shall be delivered not less than 21 days before the date of the meeting, either personally by mail or facsimile, to each shareholder on record entitled to vote at such meeting. If mailed such notice is deemed to be delivered when deposited in the mail, addressed to the shareholder at his address as it appears on our share transfer books, with postage thereon prepaid.
Series B Preferred Shares
Ranking. The Series B Preferred Shares rank senior to Sinovac Antigua’s common shares, Series A Junior Participating Preferred Shares, par value $0.001 per share, and Series C Junior Participating Preferred Shares, par value $0.001 per share, and junior to all series or any other class of Sinovac Antigua’s Preferred Shares, except to the extent that any such other series or class specifically provides that it will rank on a parity with or junior to the Series B Preferred Shares.
Dividends. Holders of Series B Preferred Shares are entitled to receive (i) the same aggregate amount per share (on an as-converted basis) of all dividends (cash or in-kind) declared on the common shares and (ii) cumulative preferential dividends, payable quarterly in arrears, at an annual rate of $0.41 per annum in cash until the earlier of (a) the conversion of the Series B Preferred Shares into the common shares or (b) the listing of the Series B Preferred Shares on a nationally recognized securities exchange.
Voting Rights. Holders of Series B Preferred Shares are entitled to vote with the holders of common shares, voting together as a single class, on all matters submitted for a vote of the shareholders of Sinovac Antigua, subject to applicable law. Each Series B Preferred Share entitles the holder to a number of votes equal to the number of common shares issuable upon the conversion of such Series B Preferred Share to which such share is entitled as of the applicable record date.
Conversion. Either (i) at our option or (ii) within 90 days of approval by the shareholders of Sinovac Antigua of an increase in the number of Sinovac Antigua’s authorized but unissued common shares to such number as would be sufficient to effect the conversion of all or any portion of the outstanding Series B Preferred Shares (a “Common Share Increase”), all or such portion of the Series B Preferred Shares will be convertible into common shares on a one-for-one basis, subject to customary anti-dilution adjustments.
Listing. In the event the shareholders of Sinovac Antigua do not vote to approve a Common Share Increase at the next annual general meeting following the initial issuance of any Series B Preferred Shares, Sinovac Antigua will use its best efforts to list the Series B Preferred Shares for trading on a nationally recognized securities exchange within 180 days of such annual general meeting.
Consolidation, Merger, etc. In case Sinovac Antigua shall enter into any consolidation, amalgamation, merger, combination or other transaction in which the common shares are exchanged for or changed into other shares or securities, cash and/or any other property, then in any such case each Series B Preferred Share shall at the same time be similarly exchanged or changed into an amount per share (on an as-converted basis) equal to the aggregate amount of shares, securities, cash and/or any other property (payable in kind), as the case may be, into which or for which each common share is changed or exchanged.
Liquidation. Upon any liquidation, dissolution or winding up of Sinovac Antigua, voluntary or otherwise, the holders of Series B Preferred Shares shall be entitled to receive a preferential payment of $0.01 per share, plus an aggregate amount per share (on an as-converted basis) equal to the aggregate amount to be distributed per share to holders of common shares.
Differences in Corporate Law
The International Business Corporations Act is modeled after Canadian corporate law and differs from laws applicable to United States corporations and their shareholders. Set forth below is a summary of the significant differences between the provisions of the International Business Corporations Act applicable to us and the laws applicable to companies incorporated in the State of Delaware and their stockholders.
Mergers and Similar Arrangements
Antigua and Barbuda law does not provide for mergers as that expression is understood under United States corporate law. However, there are statutory provisions for amalgamation that facilitate the consolidation of companies, provided that the arrangement is approved by a majority number of each class of shareholders and creditors with whom the arrangement is to be made, and who must in addition represent two-thirds in value of each such class of shareholders or creditors, as the case may be, that are present and voting either in person or by proxy at a meeting, or meetings, convened for that purpose. The convening of the meetings and subsequently the arrangement may be, but is not required to be, sanctioned by the High Court of Antigua and Barbuda. While a dissenting shareholder has the right to express to the court his view that the transaction ought not to be approved, the court can be expected to approve the arrangement if it determines that:
80
When a take-over offer is made and accepted (within four months) by holders of 90% of the shares affected, the offeror may, within a two-month period, require the holders of the remaining shares to transfer such shares on the terms of the offer. An objection can be made to the High Court of Antigua and Barbuda but this is unlikely to succeed unless there is evidence of fraud, bad faith or collusion.
If the arrangement and reconstruction is thus approved, the dissenting shareholder would have no rights comparable to appraisal rights, which would otherwise ordinarily be available to dissenting shareholders of United States corporations, providing rights to receive payment in cash for the judicially determined value of the shares.
Shareholders’ Suits
We are not aware of any reported class action or derivative action having been brought in a court in Antigua and Barbuda. In principle, the company itself will normally be the proper claimant in actions against directors, and derivative actions may not generally be brought by a minority shareholder. However, Canadian authorities provide exceptions to the foregoing principle, including when:
Directors’ Fiduciary Duties
Under Delaware corporate law, a director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally.
In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, a director must prove the procedural fairness of the transaction, and that the transaction was of fair value to the corporation. As a matter of Antigua and Barbuda law, a director of an Antigua and Barbuda company is in the position of a fiduciary with respect to the company and therefore it is considered that he owes the following duties to the company — a duty to act bona fide in the best interests of the company, a duty not to make a profit out of his position as director (unless the company permits him to do so) and a duty not to put himself in a position where the interests of the company conflict with his personal interest or his duty to a third-party.
A director of an Antigua and Barbuda company owes to the company a duty to act with skill and care. It was previously considered that a director need not exhibit in the performance of his duties a greater degree of skill than may reasonably be expected from a person of his knowledge and experience. However, Canadian and Commonwealth courts have moved towards an objective standard with regard to the required skill and care and these authorities are likely to be followed in Antigua and Barbuda.
Shareholder Action by Written Consent
Under the Delaware General Corporation Law, a corporation may eliminate the right of shareholders to act by written consent by amendment to its certificate of incorporation. Antigua and Barbuda law and our By-laws provide that shareholders may approve corporate matters by way of a unanimous written resolution signed by or on behalf of each shareholder who would have been entitled to vote on such matter at a general meeting without a meeting being held.
Shareholder Proposals
Under the Delaware General Corporation Law, a shareholder has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions in the governing documents. A special meeting may be called by the board of directors or any other person authorized to do so in the governing documents, but shareholders may be precluded from calling special meetings. Antigua and Barbuda law and our By-laws allow our shareholders holding not less than five per cent of the paid up voting share capital of the company to requisition a shareholder’s meeting. We are obligated under our By-laws and the International Business Corporations Act to call shareholders’ annual general
81
meetings. See “Risk Factors — We do not currently intend to hold an annual general meeting of shareholders until after the final determination of the litigation concerning the Rights Agreement, which will delay the ability of our shareholders to vote in an election of our directors.”
Cumulative Voting
Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides for it. Cumulative voting potentially facilitates the representation of minority shareholders on a board of directors since it permits the minority shareholder to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s voting power with respect to electing such director. As permitted under Antigua and Barbuda law, our By-laws will not provide for cumulative voting. As a result, our shareholders are not afforded any less protections or rights on this issue than shareholders of a Delaware corporation.
Removal of Directors
Under the Delaware General Corporation Law, a director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. Under our By-laws, directors can be removed by a majority vote of the shareholders.
Transactions with Interested Shareholders
The Delaware General Corporation Law contains a business combination statute applicable to Delaware public corporations whereby, unless the corporation has specifically elected not to be governed by such statute by amendment to its certificate of incorporation, it is prohibited from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or a group who or which owns or owned 15% or more of the target’s outstanding voting stock within the past three years. This has the effect of limiting the ability of a potential acquirer to make a two-tiered bid for the target in which all shareholders would not be treated equally. The statute does not apply if, among other things, prior to the date on which such shareholder becomes an interested shareholder, the board of directors approves either the business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages any potential acquirer of a Delaware public corporation to negotiate the terms of any acquisition transaction with the target’s board of directors.
Antigua and Barbuda law has no comparable statute. As a result, we cannot avail ourselves of the types of protections afforded by the Delaware business combination statute. However, although Antigua and Barbuda law does not regulate transactions between a company and its significant shareholders, it does provide that such transactions must be entered into bona fide in the best interests of the company and not with the effect of constituting a fraud on the minority shareholders.
Dissolution; Winding Up
Under the Delaware General Corporation Law, unless the board of directors approves the proposal to dissolve, dissolution must be approved by shareholders holding 100% of the total voting power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority voting requirement in connection with dissolutions initiated by the board. Under the International Business Corporations Act, our company may be dissolved, liquidated or wound up only by the vote of holders of two-thirds of our shares voting at a meeting or the unanimous written resolution of all shareholders.
Variation of Rights of Shares
Under the Delaware General Corporation Law, a corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise. Under Antigua and Barbuda law and our By-laws, if our share capital is divided into more than one class of shares, we may vary the rights attached to any class only with the vote at a class meeting of holders of two-thirds of the shares of such class or unanimous written resolution.
Amendment of Governing Documents
Under the Delaware General Corporation Law, a corporation’s governing documents may be amended with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. As permitted by Antigua and Barbuda law, our By-laws may only be amended with the vote of holders representing a majority of all our shares voting issued and outstanding or the unanimous written resolution of all shareholders. By-laws can be amended by a vote or unanimous written resolution of the directors.
Indemnification of Directors and Executive Officers and Limitation of Liability
Antigua and Barbuda law does not limit the extent to which a company’s by-laws may provide for indemnification of officers and directors, except to the extent any such provision may be held by the Antigua and Barbuda courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Our By-laws permit indemnification of officers and directors for
82
losses, damages, costs and expenses incurred in their capacities as such unless such losses or damages arise from negligence or illegal action of such directors or officers. This standard of conduct is generally the same as permitted under the Delaware General Corporation Law to a Delaware corporation. In addition, we have entered into indemnification agreements with our directors and senior executive officers that provide such persons with additional indemnification beyond that provided in our By-laws.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable as a matter of United States law.
Anti-takeover Provisions in the By-laws
Some provisions of our By-laws may discourage, delay or prevent a change in control of our company or management that shareholders may consider favorable, including provisions that authorize our board of directors to issue preferred shares in one or more series and to designate the price, rights, preferences, privileges and restrictions of such preferred shares without any further vote or action by our shareholders.
However, under Antigua and Barbuda law, our directors may only exercise the rights and powers granted to them under our By-laws for what they believe in good faith to be in the best interests of our company.
Rights of Non-resident or Foreign Shareholders
There are no limitations imposed by our By-laws on the rights of non-resident or foreign shareholders to hold or exercise voting rights on our shares. In addition, there are no provisions in our By-laws governing the ownership threshold above which shareholder ownership must be disclosed.
Rights Agreement
In March 2016, we adopted the Rights Agreement that provides for the issuance of one Right for each of our outstanding common shares. In February 2019, we amended and restated the Rights Agreement that provides for the issuance of one Right for each of our outstanding common shares or Series B Preferred Shares. In February 2020, 2021 and 2022, we further amended the amended and restated Rights Agreement to extend its term until February 2023. The Rights are designed to assure that all of our shareholders receive fair and equal treatment in the event of any proposed takeover and to guard against partial tender offers, open market accumulations, undisclosed voting arrangements and other abusive or coercive tactics to gain control of our company or our board of directors without paying all shareholders a control premium. The Rights will cause substantial dilution to a person or group that acquires 15% or more of the aggregate total of our common shares and Series B Preferred Shares on terms not approved by our board of directors.
Rights agreements are allowable under Delaware law. Additionally, as discussed above, on December 19, 2018, the Antigua Court held that Sinovac Antigua’s Rights Agreement is valid under Antigua law. 1Globe filed notice to appeal the Antigua Court’s judgment on January 29, 2019. 1Globe’s appeal of the Antigua Court’s Judgment was heard on September 18, 2019, and the appeal decision was announced by the Court of Appeal on December 9, 2021, upholding the Antigua Judgment in each point. On April 19, 2022, 1Globe applied to the Privy Council for permission to appeal the determination in the Antigua Judgment that the Rights Agreement is valid under Antigua law. 1Globe has not yet taken steps to list a substantive hearing before the Privy Council. The appeal outcome is therefore pending.
C. Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company” or elsewhere in this annual report on Form 20-F.
D. Exchange Controls
Foreign Currency Exchange
Under the Foreign Exchange Administration Rules, Renminbi is convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions. As for capital account items, such as direct investments, loans, security investments and the repatriation of investment returns, however, the conversion of foreign currency is still subject to the approval of, or registration with, SAFE or its competent local branches. SAFE approval is not necessary for the conversion of Renminbi for foreign currency payments for current account items except as otherwise explicitly provided by laws and regulations. Under the Administration Rules of the Settlement, Sale and Payment of Foreign Exchange, enterprises may only buy, sell or remit foreign currencies at banks that are authorized to conduct foreign exchange business after the enterprise provides valid commercial documents and relevant supporting documents and, in the case of certain capital account transactions, after obtaining approval from SAFE or its competent local branches. If we provide loans to any of our PRC subsidiaries, the total amount of such loans may not exceed the difference between its total investment as approved by the foreign investment authorities and its registered capital at the time of the provision of such loans. Such loans need to be registered with the SAFE, which usually takes no more than 20 working days to complete. The cost of completing such registration is minimal. Capital investments by enterprises outside of the PRC are subject to further limitations, which include approvals by MOFCOM, SAFE and NDRC, or their respective competent local branches.
83
On August 29, 2008, SAFE issued the Circular on the Relevant Operating Issues Concerning the Improvement of the Administration of the Payment and Settlement of Foreign Currency Capital of Foreign-Invested Enterprises, or SAFE Circular 142. Pursuant to SAFE Circular 142, Renminbi capital obtained from settlement of the foreign currency capital of a foreign-invested enterprise must be used within the business scope as approved by the applicable government authority and unless otherwise specifically provided by law, such Renminbi capital cannot be used for domestic equity investments. In addition, SAFE strengthened its oversight of the flow and use of the Renminbi capital converted from foreign currency registered capital of a foreign-invested company. As a result, the use of such Renminbi capital may not be altered without the SAFE’s approval, and such Renminbi capital may not be used to repay Renminbi loans if the relevant loan proceeds have not been used. As to the latest development, on March 30, 2015, SAFE issued the Circular on the Management Concerning the Reform of the Payment and Settlement of Foreign Currency Capital of Foreign-Invested Enterprises, or SAFE Circular 19, which became effective on June 1, 2015 and replaced SAFE Circular 142. Pursuant to SAFE Circular 19, up to 100% of foreign currency capital of foreign-invested enterprise may be converted into RMB capital according to the actual operation of the enterprise within the business scope at its will and the RMB capital converted from foreign currency registered capital of a foreign-invested enterprise may be used for equity investments within the PRC. However, under SAFE Circular 19, RMB capital converted from foreign currency registered capital of a foreign-invested company still may not in any case be used to advance the RMB entrusted loan or repay RMB loans if the proceeds of such loans have not been used.
On November 19, 2012, SAFE promulgated the Circular of Further Improving and Adjusting Foreign Exchange Administration Policies on Foreign Direct Investment, or SAFE Circular 59, which became effective on December 17, 2012. SAFE Circular 59 substantially amends and simplifies the current foreign exchange procedure. The major developments under SAFE Circular 59 are that the opening of various special purpose foreign exchange accounts, such as pre-establishment expenses accounts, foreign exchange capital accounts and guarantee accounts, no longer requires the approval of SAFE. Furthermore, multiple capital accounts for the same entity may be opened in different provinces, which was not possible before the issuance of SAFE Circular 59. The reinvestment of lawful incomes, such as profit and proceeds of equity transfer, capital reduction, liquidation and early repatriation of investment, by foreign investors in the PRC and the purchase and remittance of foreign exchange as a result of capital reduction, liquidation, early repatriation or share transfer in a foreign-invested enterprise no longer requires SAFE approval.
On May 10, 2013, SAFE promulgated the Circular on Printing and Distributing the Provisions on Foreign Exchange Administration over Domestic Direct Investment by Foreign Investors and the Supporting Documents, which specifies that the administration by SAFE or its local branches over direct investment by foreign investors in the PRC shall be conducted by way of registration. Institutions and individuals shall register with SAFE and/or its branches for their direct investment in the PRC. Banks shall process foreign exchange business relating to the direct investment in the PRC based on the registration information provided by SAFE and its branches.
On February 13, 2015, SAFE issued the Circular on Further Simplifying and Improving the Foreign Exchange Administration Policies on Direct Investments, or SAFE Circular 13, pursuant to which the administrative examination and approval procedures with SAFE or its local branches relating to the foreign exchange registration approval for domestic direct investments as well as overseas direct investments have been cancelled, and qualified banks are delegated the power to directly conduct such foreign exchange registrations under the supervision of SAFE or its local branches. SAFE Circular 13 took effect on June 1, 2015.
On April 26, 2016, SAFE issued the Circular of the State Administration of Foreign Exchange on Further Promoting Trade and Investment Facility and Improving the Examination and Verification of the Authenticity, pursuant to which when handling the remittance of profits exceeding the equivalent of US$50,000 abroad for a domestic institution, a bank shall examine, according to the principle of transaction authenticity, the profit distribution resolution of the board of directors (or the profit distribution resolution of all partners) that is related to this remittance of profits abroad, the original of its tax record-filing form and the financial statements in proof of the profits involved in this remittance.
On June 9, 2016, SAFE issued the Circular of the State Administration of Foreign Exchange on Reforming and Regulating Policies on the Control over Foreign Exchange Settlement of Capital Accounts, to promote nationwide the reform of control approaches to foreign exchange settlement of foreign debts of enterprises and in the meantime to unify and regulate control over discretionary settlement and payment of foreign exchange receipts under capital accounts. Pursuant to this circular, domestic enterprises (including foreign-invested enterprises) may go through foreign exchange settlement formalities for their foreign debts at their discretion. In addition, domestic institutions may, at their discretion, settle up to 100% of foreign exchange receipts under capital accounts for the time being.
On October 23, 2019, SAFE issued the Circular Regarding Further Promotion of the Facilitation of Cross-Border Trade and Investment, or SAFE Circular 28, pursuant to which all foreign-invested enterprises can make domestic equity investments with their capital funds in accordance with the law.
E. Taxation
Antigua and Barbuda Taxation
We and our securities holders, other than those resident in Antigua and Barbuda, are exempt from Antigua and Barbuda income, corporation or profits tax, withholding tax, capital gains tax, capital transfer tax, estate duty or inheritance tax. We are not subject to stamp or other similar duty on the issuance, transfer or redemption of our common shares. Under Section 276 of the International Business Corporations Act of Antigua and Barbuda, the tax exemption we and our securities holders currently enjoy will continue in effect for a period of 50 years from our date of incorporation, which is March 1, 1999. No reciprocal income tax treaty affecting us exists between Antigua and Barbuda and the United States.
United States Federal Income Taxation
84
The following discussion describes the material U.S. federal income tax consequences to U.S. Holders (as defined below) under current law of an investment in our common shares. The effects of any applicable state or local laws and other U.S. federal tax laws such as estate and gift tax laws, and the impact of the alternative minimum tax and the Medicare contribution tax on net investment income, are not discussed. This discussion applies only to U.S. Holders that hold our common shares as capital assets (generally, property held for investment) and have the U.S. dollar as their functional currency. This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), U.S. Treasury regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the U.S. Internal Revenue Service, in each case, in effect or, in some cases, proposed as of the date of this annual report. All of the foregoing authorities are subject to differing interpretations or change, which change could apply retroactively and could affect the tax consequences described below. We have not sought and will not seek any rulings from the U.S. Internal Revenue Service regarding the matters discussed below. There can be no assurance the U.S. Internal Revenue Service or a court will not take a contrary position to that discussed below regarding the U.S. federal tax consequences of the purchase, ownership, and disposition of our common shares. The following discussion does not address all U.S. federal income tax consequences relevant to a U.S. Holder’s particular circumstances or to holders subject to particular rules, including:
INVESTORS ARE URGED TO CONSULT THEIR TAX ADVISORS REGARDING THE APPLICATION OF THE U.S. FEDERAL INCOME TAX RULES TO THEIR PARTICULAR CIRCUMSTANCES AS WELL AS THE ESTATE AND GIFT, STATE, LOCAL AND NON-U.S. TAX CONSEQUENCES TO THEM OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON SHARES.
For purposes of this discussion, a “U.S. Holder” means a beneficial owner of our common shares that is, for U.S. federal income tax purposes:
If a partnership (or other entity taxable as a partnership for U.S. federal income tax purposes) is a beneficial owner of our common shares, the tax treatment of a partner in the partnership generally will depend upon the status of the partner and the activities of the partnership. If you are a partner in such partnership, you should consult your tax advisor.
U.S. Treasury regulations (the “Foreign Tax Credit Regulations”) may in some circumstances prohibit you from claiming a foreign tax credit with respect to certain non-U.S. taxes that are not creditable under applicable income tax treaties. Accordingly, a U.S. Holder that is not eligible
85
for the benefits of the income tax treaty between the United States and the PRC (the “Treaty”) should consult its tax advisor regarding the creditability or deductibility of any PRC taxes imposed on dividends on, or dispositions of, the common shares. Accordingly, the discussions below with respect to foreign tax credits does not apply to U.S. Holders in this situation.
Taxation of Dividends and Other Distributions on Our Common Shares
Subject to the passive foreign investment company (“PFIC”) rules discussed below, the gross amount of any distributions we make to you with respect to our common shares generally will be includible in your gross income in the year received as dividend income to the extent the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent the amount of the distribution exceeds our current and accumulated earnings and profits, such excess amount will be treated first as a tax-free return of your tax basis in your common shares, and then, to the extent such excess amount exceeds your tax basis, as capital gain. We currently do not, and we do not intend to, calculate our earnings and profits under U.S. federal income tax principles. Therefore, a U.S. Holder should expect that a distribution will generally be reported as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above. Any dividends we pay will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from U.S. corporations.
The amount of any dividend paid in foreign currency will be the U.S. dollar amount calculated by reference to the exchange rate in effect on the date of receipt, regardless of whether the payment is in fact converted into U.S. dollars. If the dividend is converted into U.S. dollars on the date of receipt, a U.S. Holder should not be required to recognize foreign currency gain or loss in respect of the dividend income. A U.S. Holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of receipt.
With respect to certain non-corporate U.S. Holders, including individual U.S. Holders, dividends may constitute “qualified dividend income” eligible to be taxed at the preferential rate applicable to capital gains, provided that (1) our common shares are readily tradable on an established securities market in the United States, or we are eligible for the benefits of a qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are neither a PFIC nor treated as such with respect to you (as discussed below) for the taxable year in which the dividend is paid or the preceding taxable year and (3) certain holding period requirements are met. Under U.S. Internal Revenue Service authority, common shares are considered for the purpose of clause (1) above to be readily tradable on an established securities market in the United States if they are listed on the NASDAQ Global Select Market, as are our common shares. There can be no assurance our common shares will continue to be readily tradable on an established securities market in the future. Consequently, there can be no assurance dividends paid on our common shares will continue to qualify for the reduced tax rates. If we are treated as a “resident enterprise” for PRC tax purposes under the EIT Law (see “Item 10. Additional Information — E. Taxation — PRC Taxation”), we may be eligible for the benefits of the Treaty. You should consult your tax advisors regarding the availability of the lower capital gains rate applicable to qualified dividend income for dividends paid with respect to our common shares.
Dividends generally will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the U.S. foreign tax credit limitation generally will be limited to the gross amount of the dividend, multiplied by the reduced tax rate applicable to qualified dividend income and divided by the highest tax rate that would be applicable to dividends if not for the reduced tax rate applicable to qualified dividend income. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by us with respect to our common shares generally will constitute “passive category income.”
If PRC withholding taxes apply to dividends paid to you with respect to the common shares (see “Item 10. Additional Information — E. Taxation — PRC Taxation”), subject to certain conditions and limitations, such PRC withholding taxes may be treated as foreign taxes eligible for credit against your U.S. federal income tax liability. The rules relating to the determination of the foreign tax credit are complex, and you should consult your tax advisors regarding the availability of a foreign tax credit in your particular circumstances.
Taxation of Disposition of Our Common Shares
Subject to the PFIC rules discussed below, you will recognize taxable gain or loss on any sale, exchange or other taxable disposition of a common share equal to the difference between the amount realized for the common share and your tax basis in the common share. The gain or loss generally will be capital gain or loss. If you are a non-corporate U.S. Holder, including an individual U.S. Holder, who has held the common share for more than one year, you will be eligible for reduced tax rates. The deductibility of capital losses is subject to limitations.
Any gain or loss you recognize on a disposition of our common shares generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes. However, if we are treated as a PRC resident enterprise for PRC tax purposes and PRC tax is imposed on any gain from the disposition of the common shares, a U.S. Holder that is eligible for the benefits of the Treaty (see “Item 10. Additional Information — E. Taxation — PRC Taxation”) may elect to treat the gain as PRC source income under the Treaty. The Foreign Tax Credit Regulations generally preclude you from claiming a foreign tax credit with respect to PRC income taxes on gains from dispositions of common shares if you do not elect to apply the benefits of the Treaty. However, in that case it is possible that any PRC taxes on disposition gain may either be deductible or reduce the amount realized on the disposition. You should consult your tax advisors regarding the proper treatment of gain or loss in your particular circumstances.
Passive Foreign Investment Company
86
Based on our estimates of the fair market value of our assets, and the composition of our income and assets, we do not believe we were a PFIC for U.S. federal income tax purposes for our taxable year ended December 31, 2022. However, the application of the PFIC rules is subject to uncertainty in several respects, and we cannot assure that we will not be a PFIC for any taxable year.
In general, a non-U.S. corporation will be a PFIC for any taxable year if either:
For this purpose, passive income generally includes, among other things, dividends, interest, royalties, rents, annuities, and net gains from certain commodity and foreign currency transactions, subject to certain exceptions. Passive income generally does not include rents and royalties derived from the active conduct of a trade or business (other than from a related person). We will be treated as owning our proportionate share of the assets and receiving our proportionate share of the income of any other corporation in which we own, directly or indirectly, at least 25% (by value) of the stock.
We must make a separate determination after the close of each taxable year as to whether we were a PFIC for that year. In particular, under normal circumstances, the value of our assets for purposes of the PFIC test for a particular taxable year would generally be determined by reference to the market price of our common shares at the end of each quarter during such taxable year, and fluctuations in such market price (or changes in the composition of our income or assets) could cause us to become a PFIC for any subsequent year. However, as a result of the suspension of trading in our shares, we are unable to reference the actual market prices of our common shares in determining our PFIC status. As a result, we have based our determination of the fair market value of our assets for purposes of the PFIC determination on our estimated enterprise value, which we estimated by reference to our earnings per share and number of outstanding shares, and a comparison of such earnings per share to the earnings per share of certain other companies in industries similar to ours and that have shares listed on a U.S. stock exchange. We cannot provide any assurances that the actual value of our shares is not materially different on the applicable measurement dates from such estimated value or as to whether the U.S. Internal Revenue Service will respect our approach. This uncertainty will continue so long as trading in our shares remains suspended. In addition, the composition of our income and assets will be affected by how, and how quickly, we use any cash we generate from our operations or raise in any offering. If we are a PFIC for any taxable year during which you hold our common shares, we generally will continue to be treated as a PFIC with respect to you for that year and for all succeeding years during which you hold our common shares, regardless of whether we continue to meet the income or asset tests described above, unless we cease to be a PFIC and you make a “deemed sale” election with respect to our common shares. If such election is made, you will be deemed to have sold common shares you hold at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain from such deemed sale would be subject to the rules described in the following two paragraphs. After the deemed sale election, your common shares with respect to which such election was made will not be treated as shares in a PFIC unless we subsequently become a PFIC. You are urged to consult your tax advisor about this election.
For each taxable year we are treated as a PFIC with respect to you, you will be subject to additional reporting requirements as well as special tax rules with respect to any “excess distribution” you receive and any gain you realize from a sale or other disposition (including a pledge) of the common shares, unless (i) you make a “mark-to-market” election as discussed below or (ii) we have ceased to be a PFIC and you have previously made the deemed sale election described above. Distributions you receive in a taxable year that are greater than 125% of the average annual distributions you received during the shorter of the three preceding taxable years or your holding period for the common shares before the current taxable year will be treated as excess distributions. Under these special tax rules:
Gains (but not losses) from a sale or other disposition of the common shares are not taxed at reduced tax rates, even if you hold the common shares as capital assets.
If we are treated as a PFIC with respect to you for any taxable year, to the extent any of our subsidiaries are also PFICs or we make direct or indirect equity investments in other entities that are PFICs, you will be deemed to own shares in such lower-tier PFICs directly or indirectly owned by us in the proportion that the value of the common shares you own bears to the value of all of our common shares, and you may be subject to the rules described in the preceding two paragraphs with respect to the shares of such lower-tier PFICs that you would be deemed to own. You should consult your tax advisors regarding the application of the PFIC rules to any of our subsidiaries.
87
A U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market election for such stock to elect out of the PFIC rules described above regarding excess distributions and recognized gains. If you make a mark-to-market election for the common shares, you will include in income for each taxable year that we are a PFIC an amount equal to the excess, if any, of the fair market value of the common shares as of the close of your taxable year over your adjusted basis in such common shares. You will be allowed a deduction for the excess, if any, of the adjusted basis of the common shares over their fair market value as of the close of the taxable year. However, deductions will be allowable only to the extent of any net mark-to-market gains on the common shares included in your income for prior taxable years. Amounts included in your income under a mark-to-market election, as well as gain from the actual sale or other disposition of the common shares will be treated as ordinary income. Ordinary loss treatment will apply to the deductible portion of any mark-to-market loss on the common shares, as well as to any loss from the actual sale or other disposition of the common shares, to the extent that the amount of such loss does not exceed the net mark-to-market gains previously included for such common shares. Your basis in the common shares will be adjusted to reflect any such income or loss amounts. If you make a valid mark-to-market election, any distributions we make would generally be subject to the tax rules discussed above under “— Taxation of Dividends and Other Distributions on Our Common Shares,” and the lower capital gains rate applicable to qualified dividend income would not apply.
The mark-to-market election is available only for “marketable stock,” which generally is defined as stock that is traded in greater than de minimis quantities on at least 15 days during each calendar quarter (“regularly traded”) on a “qualified exchange or other market,” as defined in applicable U.S. Treasury regulations. Any trades that have as their principal purpose satisfying this requirement will be disregarded. Our common shares are listed on the NASDAQ Global Select Market, which is a qualified exchange or other market for these purposes. Consequently, if the common shares remain listed on the NASDAQ Global Select Market and are regularly traded (which, presently, they may not be, due to the current suspension of trading on our shares), and you are a holder of common shares, we expect the mark-to-market election would be available to you if we are or become a PFIC. There can be no assurance the common shares are or will be “regularly traded” for purposes of the mark-to-market election. Once made, the election cannot be revoked without the consent of the U.S. Internal Revenue Service unless the common shares cease to be marketable stock. Because a mark-to-market election cannot be made for equity interests in any lower-tier PFICs that we own, a U.S. Holder may continue to be subject to the PFIC rules described above regarding excess distributions and recognized gains with respect to its indirect interest in any investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes. You should consult your tax advisors as to the availability and desirability of a mark-to-market election, as well as the impact of such election on interests in any lower-tier PFICs.
Alternatively, a U.S. Holder of stock in a PFIC may make a “qualified electing fund” election with respect to such corporation to elect out of the PFIC rules described above regarding excess distributions and recognized gains. A U.S. Holder that makes a qualified electing fund election with respect to a PFIC will generally include in income such holder’s pro rata share of the corporation’s income on a current basis. However, you may make a qualified electing fund election with respect to your common shares only if we furnish you annually with certain tax information, and we currently do not intend to prepare or provide such information.
Each U.S. shareholder of a PFIC is required to file an annual report containing such information as the U.S. Treasury requires. If we are or become a PFIC, you should consult your tax advisors regarding any reporting requirements that may apply to you.
You are urged to consult your tax advisors regarding the application of the PFIC rules to your investment in our common shares.
Information Reporting and Backup Withholding
Dividend payments with respect to our common shares and proceeds from the sale, exchange or redemption of our common shares may be subject to information reporting to the U.S. Internal Revenue Service and possible U.S. backup withholding at a current rate of 24%. Backup withholding will not apply, however, to a U.S. Holder that furnishes a correct taxpayer identification number and makes any other required certification on U.S. Internal Revenue Service Form W-9 or that is otherwise exempt from backup withholding. U.S. Holders that are required to establish their exempt status generally must provide such certification on U.S. Internal Revenue Service Form W-9. We do not assume responsibility for backup withholding.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the U.S. Internal Revenue Service and furnishing any required information in a timely manner. You should consult your tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
Additional Reporting Requirements
Certain U.S. Holders who are individuals (and certain entities) are required to report information relating to an interest in our common shares, subject to certain exceptions (including an exception for common shares held in accounts maintained by certain financial institutions). U.S. Holders should consult their tax advisors regarding the effect, if any, of these rules on their ownership and disposition of our common shares.
PRC Taxation
Under the EIT Law, enterprises established under the laws of non-PRC jurisdictions but whose “de facto management body” is located in China are considered “resident enterprises” for PRC tax purposes. Under the implementation regulations issued by the State Council relating to the EIT
88
Law, “de facto management bodies” are defined as the bodies that have material and overall management control over the business, personnel, accounts and properties of an enterprise. In 2009, the State Administration of Taxation issued a circular, known as Circular 82, which provides certain specific criteria for determining whether the “de facto management body” of a PRC-controlled offshore incorporated enterprise is located in China. Although this circular only applies to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled by PRC individuals or foreigners, the criteria set forth in the circular may reflect the State Administration of Taxation’s general position on how the “de facto management body” text should be applied in determining the tax resident status of all offshore enterprises. According to Circular 82, an offshore incorporated enterprise controlled by a PRC enterprise or a PRC enterprise group will be regarded as a PRC tax resident by virtue of having its “de facto management body” in China only if all of the following conditions are met: (i) the primary location of the day-to-day operational management is in the PRC; (ii) decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in the PRC; (iii) the enterprise’s primary assets, accounting books and records, company seals, and board and shareholders minutes, are located or maintained in the PRC; and (iv) at least 50% of voting board members or senior executives habitually reside in the PRC. Substantially all of our management are currently based in China, and may remain in China in the future. If we were treated as a “resident enterprise” for PRC tax purposes, we would be subject to PRC income tax on our worldwide income at a uniform tax rate of 25%. Dividends received by us from our PRC subsidiaries may be exempt from PRC withholding tax.
Under the EIT Law and its implementation regulations, dividends paid to a non-PRC investor are generally subject to a 10% PRC withholding tax, if such dividends are derived from sources within China and the non-PRC investor is considered to be a non-resident enterprise without any establishment or place of business within China or if the dividends paid have no connection with the non-PRC investor’s establishment or place of business within China, unless such tax is eliminated or reduced under an applicable tax treaty. Similarly, any gain realized on the transfer of common shares by such investor is also subject to a 10% PRC withholding tax if such gain is regarded as income derived from sources within China, unless such tax is eliminated or reduced under an applicable tax treaty.
If we were considered a PRC “resident enterprise,” it is possible that the dividends we pay with respect to our common shares, or the gain you may realize from the transfer of our common shares, would be treated as income derived from sources within China and be subject to income tax at 10%.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to the periodic reporting and other informational requirements of the Exchange Act. Under the Exchange Act, we are required to file reports and other information with the SEC. Specifically, we are required to file annually a Form 20-F within four months after the end of each fiscal year. You can access the reports that we file with the SEC at the SEC’s web site at www.sec.gov, which contains reports, proxy and information statements, and other information regarding registrants that make electronic filings with the SEC using its EDGAR system. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
We will furnish the transfer agent of our common shares, with our annual reports, which will include a review of operations and annual audited consolidated financial statements prepared in conformity with U.S. GAAP, and all notices of shareholders’ meetings and other reports and communications that are made generally available to our shareholders. The transfer agent will make such notices, reports and communications available to holders of our common shares and, upon our request, will mail to all record holders of our common shares the information contained in any notice of a shareholders’ meeting received by the transfer agent from us.
In accordance with the NASDAQ Rules, we will post this annual report on Form 20-F on our website www.sinovac.com. In addition, we will provide hardcopies of our annual report free of charge to shareholders upon request.
I. Subsidiary Information
For a listing of our subsidiaries, see “Item 4. Information on the Company — C. Organizational Structure.”
J. Annual Report to Security Holders
Not applicable.
ITEM 11. Quantitative and Qualitative Disclosures about Market Risk
89
Foreign Exchange Risk
Majority of our revenues and most of our costs and our expenses are denominated in renminbi. Our exposure to foreign exchange risk primarily relates to cash and cash equivalents denominated in U.S. dollars from international sales. Furthermore, the renminbi prices of some of the materials and supplies for reagent kits that are imported from companies in the United States, Sweden and United Kingdom may be affected by fluctuations in the value of renminbi against the currencies of those countries. We also incur professional, investor relations, director compensation and miscellaneous fees related to our operations as a public company that are denominated in U.S. dollars.
The conversion of Renminbi into foreign currencies, including U.S. dollars, is based on rates set by the People’s Bank of China. The Renminbi has fluctuated against the U.S. dollar, at times significantly and unpredictably. The value of Renminbi against the U.S. dollar and other currencies is affected by changes in China’s political and economic conditions and by China's foreign exchange policies, among other things. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between Renminbi and the U.S. dollar in the future. The PRC government has indicated that it will make effort to widen the trading band of the renminbi exchange rate, which increases the possibility of sharp fluctuations in renminbi’s value in the future as well as the unpredictability associated with renminbi’s exchange rate. By way of example, assuming we had converted a U.S. dollar denominated cash balance of $1.0 million as of December 31, 2022 into renminbi at the noon buying rate of $1.00 for RMB6.8972 as of December 30, 2022, such a cash balance would have been RMB6.89 million. Assuming a 1% appreciation/depreciation of the renminbi against the U.S. dollar, such a cash balance would have decreased/increased by RMB68,972 as of December 31, 2022.
Our financial statements are expressed in U.S. dollars but our subsidiaries’ functional currency is renminbi. The value of our shares will be affected by the foreign exchange rate between U.S. dollars and renminbi. To the extent we hold assets denominated in U.S. dollars, any appreciation of the renminbi against the U.S. dollar could result in a change to our statements of comprehensive income and a reduction in the value of our U.S. dollar denominated assets. On the other hand, a decline in the value of renminbi against the U.S. dollar could reduce the U.S. dollar equivalent amounts of our financial results, the value of your investment in our company and the dividends we may pay in the future, if any, all of which may have a material adverse effect on the prices of our shares.
Interest Rate Risk
Our exposure to interest rate risk relates primarily to the interest expense associated with our short-term and/or long-term bank borrowings as well as interest income provided by excess cash invested in demand deposits, term deposits, and other investments placed with financial institutions. Such borrowing and interest-earning instruments carry a degree of interest rate risk. We have not historically used, and do not expect to use in the future, any derivative financial instruments to manage our exposure to interest risk. We have not been exposed nor do we anticipate being exposed to material risks due to changes in interest rates. The weighted effective interest rate on our outstanding bank loans was 5.88%, 5.64% and 4.84%for the years ended December 31, 2022, 2021 and 2020. A hypothetical increase or decrease in interest rates of 1% would increase or decrease our annual interest and financing expenses by $0.1 million based on our outstanding indebtedness as of December 31, 2022.
Concentration of Credit Risks
Financial instruments that potentially subject us to concentration of credit risks consist primarily of cash and cash equivalents, restricted cash, short-term investment and accounts receivable, the balances of which are stated on the consolidated balance sheets which represent our maximum exposure. We place our cash and cash equivalents, restricted cash, and short-term investment in good credit quality financial institutions in Hong Kong and China. Concentration of credit risks with respect to accounts receivables is linked to the concentration of revenue. Our customers are mainly various government agencies. For the year ended December 31, 2020, our largest customer accounted for 11% of our total revenue, and no single customer accounted for more than 10% of the total sales for the year ended December 31, 2021 and 2022. To manage credit risk, we perform ongoing credit evaluations of customers’ financial condition.
PRC state-owned banks, such as Bank of China, are subject to a series of risk control regulatory standards, and PRC bank regulatory authorities are empowered to take over the operation and management when any of those banks faces a material credit crisis. We do not foresee substantial credit risk with respect to cash and cash equivalents, restricted cash and short-term investments held at the PRC state-owned banks. Meanwhile, China does not have an official deposit insurance program, nor does it have an agency similar to what was the Federal Deposit Insurance Corporation (FDIC) in the U.S. In the event of bankruptcy of one of the financial institutions in which we have deposits or investments, it may be unlikely to claim its deposits or investments back in full. We have selected reputable international financial institutions with high rating rates to place its foreign currencies. We regularly monitor the rating of the international financial institutions to avoid any potential defaults. There has been no recent history of default in relation to these financial institutions.
ITEM 12. Description of Securities other than Equity Securities
|
90
A. Debt Securities.
Not applicable.
B. Warrants and Rights.
With respect to the preferred share purchase right, see Form 8-A and Amendment No. 1, Amendment No. 2, Amendment No. 3 and Amendment No. 4 thereto (file no. 001-32371) which we filed with the Securities and Exchange Commission on February 22, 2019, February 21, 2020, February 22, 2021, February 22, 2022 and February 22, 2023, respectively.
Not applicable.
Not applicable.
PART II
ITEM 13. Defaults, Dividend Arrearages and Delinquencies
None.
ITEM 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
A. — D. Material Modifications to the Rights of Security Holders
In March 2016, we adopted the Rights Agreement. In February 2019, we amended and restated the Rights Agreement. Pursuant to the amended and restated Rights Agreement, subject to limited exceptions, upon (i) a person or group obtaining ownership of 15% or more of aggregate total of our common shares and Series B Preferred Shares (on an as converted basis) then issued and outstanding or (ii) the commencement or announcement of an intention to make a tender offer or exchange offer, the consummation of which would result in the beneficial ownership by a person or group of 15% or more of our common shares and Series B Preferred Shares (on an as converted basis) then issued and outstanding, in each case, without the approval of our board of directors, each Right will entitle the holders, other than the acquiring person, to buy, at an exercise price of $20.00, one one-thousandth of a Series C Preferred Share. Holders are entitled to receive, in lieu of each one one-thousandths of a Series C Preferred Share, common shares or Series B Preferred Shares having a market value at that time of twice the Right’s exercise price. Our board of directors is entitled to redeem the Rights at $0.001 per Right at any time before the Rights are exercisable. We refer to the person who acquired 15% or more of the outstanding common shares or Series B Preferred Shares of Sinovac Antigua as the “acquiring person.” In February 2020, 2021, 2022 and 2023, we further amended the amended and restated Rights Agreement to extend its term until February 2024.
On February 18, 2019, after reviewing the judgment of the Antigua Court of December 19, 2018 and considering all additional facts known to the board of directors, our board of directors determined that the Collaborating Shareholders became Acquiring Persons as defined under Sinovac Antigua’s Rights Agreement, and that their conduct resulted in a Trigger Event under the Rights Agreement. As a result, the Rights held by the Collaborating Shareholders were deemed void. Pursuant to the Rights Agreement, the board of directors elected to exchange each valid and outstanding Right held by Sinovac Antigua’s shareholders (not including the Collaborating Shareholders) for an Exchange Share. On March 6, 2019, the Delaware Chancery Court entered a status quo order providing that Sinovac Antigua not distribute any of the Exchange Shares from the trust until the final disposition of the pending Delaware litigation or further order of the Court. On April 4, 2019, the Eastern Caribbean Supreme Court, Court of Appeal issued an order restraining Sinovac Antigua from taking further action under its Rights Agreement, including the distribution of the previously issued Exchange Shares to the holders of valid Rights, until the conclusion of 1Globe Capital, LLC’s appeal of the December 19, 2018 Judgment of the Antigua Court. In January 2022, the Court of Appeal extended the order until the conclusion of any appeal to the Privy Council. On April 8, 2019, the Delaware Chancery Court stayed the Delaware litigation pending the outcome of 1Globe’s appeal of the Antigua Judgment. 1Globe’s appeal of the Antigua Court’s Judgment was heard on September 18, 2019. On December 9, 2021, the Court of Appeal handed down its judgment, dismissing all grounds of appeal and upholding the Antigua Judgment. The Court of Appeal also confirmed that Sinovac Antigua’s Rights Agreement was consistent with its Articles of Incorporation and By-laws, and Antiguan business law. 1Globe applied for leave to appeal to the Privy Council, and the hearing of the application was held on February 24, 2022, at which the Court of Appeal refused 1Globe’s application to take the issue of the validity of the Rights Agreement to the Privy Council, but granted leave to appeal on certain other grounds. On April 19, 2022, 1Globe renewed its application directly to the Privy Council for leave to appeal on its ground of appeal concerning the validity of the Rights Agreement. 1Globe has not yet taken steps to list a substantive hearing before the Privy Council. The appeal outcome is therefore pending.
The Delaware litigation is stayed pending the resolution of the litigation in Antigua. The Delaware Court’s status quo order prevents us from distributing Exchange Shares to any shareholders or otherwise taking any action pursuant to the Rights Agreement until the conclusion of the Delaware litigation or Court order, which we anticipate will resume following the conclusion of the Antigua litigation.
91
On February 22, 2019, in connection with the Exchange, we issued approximately 27.8 million common shares and 14.6 million Series B Preferred Shares for the benefit of the holders of valid and outstanding Rights as of that date. This issuance had the effect of significantly diluting the holdings of the shareholders that are not entitled to participate in the Exchange. The Series B Preferred Shares share equally in all dividends and distributions made on our common shares and vote together with the common shares on all matters brought before the shareholders, in each case on an as-converted basis and subject to applicable law. The Series B Preferred Shares are convertible into common shares at our option, or automatically upon a successful shareholder vote to increase the authorized number of common shares of Sinovac Antigua. Until the Series B Preferred Shares are converted into common shares (or until the Series B Preferred Shares are listed on a nationally recognized securities exchange), they will earn a preferred dividend equal to $0.41 per annum, payable quarterly in arrears.
E. Use of Proceeds
Not applicable.
ITEM 15. Controls and Procedures
Disclosure Controls and Procedures
In connection with the preparation of this annual report on Form 20-F, we carried out an evaluation of the effectiveness of our disclosure controls and procedures, which is defined in Rules 13a-15(e) of the Exchange Act, as of the period covered by this annual report.
Based on this evaluation, our chief executive officer and chief financial officer concluded that, as of December 31, 2022, our disclosure controls and procedures were effective in ensuring that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act was recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, which is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation and fair presentation of the consolidated financial statements for external purposes in accordance with accounting principles generally accepted in the United States and includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of a company’s assets, (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that a company’s receipts and expenditures are made only in accordance with authorization of a company’s management and directors, and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of a company’s assets that could have a material effect on the consolidated financial statements.
Our management conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2022. In making this assessment, we used the criteria established within the Internal Control —Integrated Framework (2013 Framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission. This evaluation included a review of the documentation of controls, an evaluation of the design effectiveness of controls, the testing of the operating effectiveness of controls and a conclusion on this evaluation. All internal control systems, no matter how well designed, have inherent limitations. Even those systems determined to be effective may not prevent or detect misstatements and can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement to our annual or interim financial statements will not be prevented or detected on a timely basis.
Based on our evaluation, our management has concluded that our internal control over financial reporting was effective as of December 31, 2022.
Grant Thornton, an independent registered public accounting firm that audited our financial statements included in this annual report, has issued an attestation report on the effectiveness of our internal control over financial reporting as of December 31, 2022.
Attestation Report of the Registered Public Accounting Firm
The attestation report issued by Grant Thornton, our independent registered public accounting firm, on the effectiveness of internal control over financial reporting can be found on page F-5 of this annual report.
Changes in Internal Control over Financial Reporting
92
As required by Rule 13a-15(d), under the Exchange Act, our management, including our chief executive officer and chief financial officer, has conducted an evaluation of our internal control over financial reporting to determine whether any changes occurred during the period covered since last report have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. Based on this evaluation, it has been determined that there has been no change during the period covered by this annual report.
ITEM 16A. Audit Committee Financial Expert
Our board of directors has determined that we have at least one audit committee financial expert serving on our audit committee. Our audit committee financial expert is Mr. Simon Anderson. Each member of our audit committee, including Mr. Anderson, satisfies the “independence” requirements of the NASDAQ Marketplace rule and Rule 10A-3 under the Exchange Act.
ITEM 16B. Code of Ethics
Our board of directors has adopted a code of business conduct and ethics that applies to our directors, officers, employees and agents, including certain provisions that specifically apply to our chief executive officer, chief financial officer, vice presidents and any other persons who perform similar functions for us. We have filed our code of business conduct and ethics as an exhibit our annual report on Form 20-F (file no. 001-32371) filed with the SEC on July 14, 2006, and posted the code on our website at www.sinovac.com. We hereby undertake to provide to any person without charge, a copy of our code of business conduct and ethics within ten working days after we receive such person’s written request.
ITEM 16C. Principal Accountant Fees and Services
Grant Thornton audited our financial statements for the year ended December 31, 2022 and 2021. The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered by Grant Thornton, for the periods indicated below.
|
|
2022 |
|
|
2021 |
|
||
Audit fees(1) |
|
$0.8 million |
|
|
$0.8 million |
|
||
Audited-related fees(2) |
|
|
— |
|
|
|
— |
|
Tax fees(3) |
|
|
— |
|
|
|
— |
|
All other fees(4) |
|
|
— |
|
|
|
— |
|
Before our independent auditors are engaged to render any services, the terms and fees of the engagement are reviewed by the audit committee before our audit committee grants approval. All services as described above have been approved by our audit committee.
ITEM 16D. Exemptions from the Listing Standards for Audit Committees
None.
ITEM 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
ITEM 16F. Change in Registrant’s Certifying Accountant
None.
ITEM 16G. Corporate Governance
NASDAQ Stock Market Rule 5620 requires each issuer to hold an annual meeting of shareholders no later than one year after the end of the issuer’s fiscal year-end. However, NASDAQ Stock Market Rule 5615(a)(3) permits foreign private issuers like us to follow “home country practice” in certain corporate governance matters. We did not have an annual meeting of shareholders in 2022, 2021 and 2020. Dentons, our
93
Antigua and Barbuda counsel, has provided a letter to the NASDAQ Global Select Market certifying that our current practice relating to the annual meeting of shareholders will not breach our Articles of Incorporation and By-laws nor any applicable law in Antigua and Barbuda.
Other than the annual meeting practice described above, there are no significant differences between our corporate governance practices and those followed by U.S. domestic companies under NASDAQ Stock Market Rules.
ITEM 16H. Mine Safety Disclosure
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
In May 2022, the SEC conclusively listed Sinovac Biotech Ltd. as a Commission-Identified Issuer under the HFCAA following the filing of our annual report on Form 20-F for the fiscal year ended December 31, 2021. Our auditor who issued the audit report for us for the fiscal year ended December 31, 2021 is a registered public accounting firm headquartered in mainland China, a jurisdiction where the PCAOB determined that it was unable to inspect or investigate registered public accounting firms headquartered there until December 2022.
On December 15, 2022, the PCAOB issued a report that vacated its December 16, 2021 determination and removed mainland China and Hong Kong from the list of jurisdictions where it is unable to inspect or investigate completely registered public accounting firms. For this reason, we do not expect to be identified as a Commission-Identified Issuer under the HFCAA after we file this annual report on Form 20-F.
As of the date of this annual report, to our knowledge, (i) no Antigua or PRC governmental entities owns any shares of Sinovac Antigua or its subsidiaries, (ii) the Antigua or PRC governmental entities do not have a controlling financial interest in Sinovac Antigua or its subsidiaries, (iii) none of the members of the board of directors of Sinovac Antigua or its subsidiaries is an official of the Communist Party of China, and (iv) none of the currently effective memorandum and articles of association (or equivalent organizing document) of Sinovac Antigua or its subsidiaries contains any charter of the Communist Party of China.
ITEM 16J. INSIDER TRADING POLICIES
Our board of directors has adopted insider trading policies and procedures governing the purchase, sale, and other dispositions of the our securities by directors, senior management, and employees that are reasonably designed to promote compliance with applicable insider trading laws, rules and regulations, and any listing standards applicable to us.
94
PART III
ITEM 17. Financial Statements
We have elected to provide financial statements pursuant to Item 18.
ITEM 18. Financial Statements
The consolidated financial statements of our company are included at the end of this annual report.
ITEM 19. EXHIBITS
Exhibit Number |
|
Description of Document |
|
|
|
1.1 |
|
|
1.2 |
|
|
1.3 |
|
|
1.4 |
|
|
2.1 |
|
|
2.2 |
|
|
2.3 |
|
|
4.1 |
|
|
4.2 |
|
|
4.3 |
|
|
4.4 |
|
|
4.5 |
|
|
4.6 |
|
|
4.7 |
|
|
4.8 |
|
|
4.9 |
|
|
4.10 |
|
|
4.11 |
|
95
4.12 |
|
|
4.13 |
|
|
4.14 |
|
|
4.15 |
|
|
4.16 |
|
|
4.17 |
|
|
4.18 |
|
|
4.19 |
|
|
4.20 |
|
|
4.21 |
|
|
4.22 |
|
|
4.23 |
|
|
4.24 |
|
|
8.1* |
|
|
11.1 |
|
|
12.1* |
|
CEO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
12.2* |
|
CFO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
13.1** |
|
CEO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
13.2** |
|
CFO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
15.1* |
|
Consent of Marcum Bernstein & Pinchuk LLP(Formerly Marcum Bernstein & Pinchuk LLP) |
15.2* |
|
Consent of Grant Thornton Zhitong Certified Public Accountants LLP |
101.INS* |
|
Inline XBRL Instance Document—this instance document does not appear in the Interactive Data File because its XBRL tags embedded within the Inline XBRL document |
101.SCH* |
|
Inline XBRL Taxonomy Extension Schema Document |
101.CAL* |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF* |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB* |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
101.PRE* |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* |
|
Filed with this annual report on Form 20-F |
** |
|
Furnished with this annual report on Form 20-F |
96
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
Sinovac Biotech Ltd. |
|
|
|
|
|
By: |
/s/ Weidong Yin |
|
|
Name: Weidong Yin |
|
|
Title: Chairman and Chief Executive Officer |
|
|
|
Date: April 28, 2023 |
|
|
97
SINOVAC BIOTECH LTD.
Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
December 31, 2022 and 2021
F-1
Index
F-2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of Sinovac Biotech Ltd.
Opinion on the financial statements
We have audited the accompanying consolidated balance sheets of Sinovac Biotech Ltd. and subsidiaries (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of comprehensive income (loss), changes in shareholders’ equity, and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of December 31, 2022, based on criteria established in the 2013 Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”), and our report dated expressed an unqualified opinion.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical audit matter
The critical audit matter communicated below is a matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Ongoing litigation
Description of the Matter
As described further in Note 18(b) and Note 22 to the financial statements, there is a series of ongoing litigation between the Company and 1Globe, and certain other minority shareholders. The Company’s board of directors determined that those shareholders became “Acquiring Person” and their conduct resulted in a “Trigger Event” under the Company’s Rights Agreement. As a result, 27,777,341 new common shares and 14,630,813 preferred shares of the Company were issued into a trust for the benefit of the holders of the valid and outstanding rights. 1Globe filed an amended answer to the Company’s compliant, counterclaims, and a third-party complaint alleging, among other allegations, that the Rights Agreement is not valid, and that 1Globe did not trigger the Rights Agreement. On December 9, 2021, the Court of Appeal confirmed that the Company’s Rights Agreement was consistent with its Articles of Incorporation and By-laws, and Antiguan business law. 1Globe applied for leave to appeal to the Privy Council, and the hearing of that application was held on February 24, 2022, in which the Court of Appeal granted 1Globe leave to appeal certain grounds to the Privy Council. On April 19, 2022, 1Globe renewed its application directly to the Privy Council for leave to appeal on its ground of appeal concerning the validity of the Rights Agreement. 1Globe has not yet taken steps to list a substantive hearing before the Privy Council. On July 13, 2022, 1Globe filed its Notice of Appeal on those grounds on which the Court of Appeal had granted 1Globe leave to appeal. On September 16, 2022, 1Globe filed an application to the Privy Council seeking permission to amend its existing application for permission to appeal and its existing Notice of Appeal, and to seek permission to appeal on another ground rejected by the Court of Appeal concerning the exercise of the Antigua Court’s discretion. The Company responded on October 21, 2022. On February 15, 2023, the
F-3
Privy Council made a procedural decision to allow amendment of its existing application for permission to appeal, and decided to deal with procedural and substantive issues together at the Final Hearing. 1Globe has not yet taken steps to list a substantive hearing before the Privy Council. The appeal outcome is therefore pending. The final appeal is ongoing as of the date of this annual report. The Company cannot predict or estimate an outcome or economic burden for this litigation at this time.
The principal considerations for our determination that this litigation is a critical audit matter relates to material disclosure of contingencies and calculation of earnings per share, and involved subjective and complex auditor judgement.
How we Addressed the Matter in Our Audit
Our audit procedures related to the CAM included the following, amongst others:
/s/
We have served as the Company’s auditor since 2021.
April 28, 2023
F-4
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of Sinovac Biotech Ltd.
Opinion on internal control over financial reporting
We have audited the internal control over financial reporting of Sinovac Biotech Ltd. and subsidiaries (the “Company”) as of December 31, 2022, based on criteria established in the 2013 Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2022, based on criteria established in the 2013 Internal Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated financial statements of the Company as of and for the year ended December 31, 2022, and our report dated April 28, 2023 expressed an unqualified opinion on those financial statements.
Basis for opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and limitations of internal control over financial reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Grant Thornton Zhitong Certified Public Accountants LLP
Beijing, China
April 28, 2023
F-5
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of Sinovac Biotech Ltd.
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of comprehensive income, shareholders’ equity and cash flows of Sinovac Biotech Ltd. (the “Company”) for the year ended December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the results of its operations and its cash flows of the Company for the year ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Marcum Asia CPAs LLP
We have served as the Company’s auditor from 2019 to 2021.
April 22, 2021
F-6
SINOVAC BIOTECH LTD.
Consolidated Balance Sheets
As of December 31, 2022 and 2021
(Expressed in thousands of U.S. dollars, except for number of shares and per share data)
|
|
December 31, |
|
|
December 31, |
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Restricted cash (note 3) |
|
|
|
|
|
|
||
Short-term investments (note 4) |
|
|
|
|
|
|
||
Accounts receivable – net (note 6) |
|
|
|
|
|
|
||
Inventories (note 7) |
|
|
|
|
|
|
||
Prepaid expenses and deposits |
|
|
|
|
|
|
||
Income tax receivable |
|
|
|
|
|
— |
|
|
Total current assets |
|
|
|
|
|
|
||
Property, plant and equipment – net (note 8) |
|
|
|
|
|
|
||
Prepaid land lease payments (note 9) |
|
|
|
|
|
|
||
Intangible assets - net (note 10) |
|
|
|
|
|
|
||
Long-term prepaid expenses (note 13(b)) |
|
|
|
|
|
|
||
Long-term investments (note 4) |
|
|
|
|
|
|
||
Prepayments for acquisition of equipment |
|
|
|
|
|
|
||
Deferred tax assets (note 15) |
|
|
|
|
|
|
||
Right-of-use assets (notes 11 and 13(b)) |
|
|
|
|
|
|
||
Other non-current assets |
|
|
|
|
|
— |
|
|
Total assets |
|
$ |
|
|
$ |
|
||
LIABILITIES AND EQUITY |
|
|
|
|
|
|
||
Current liabilities |
|
|
|
|
|
|
||
Short-term bank loans and current portion of long-term bank loans (note 12) |
|
$ |
|
|
$ |
|
||
Loan from a non-controlling shareholder (note 13 (a)) |
|
|
|
|
|
|
||
Accounts payable and accrued liabilities (note 14) |
|
|
|
|
|
|
||
Income tax payable |
|
|
— |
|
|
|
|
|
Deferred revenue (note 16) |
|
|
|
|
|
|
||
Deferred government grants (note 17) |
|
|
|
|
|
|
||
Dividend payable (note 19) |
|
|
|
|
|
|
||
Lease liability (notes 11 and 13(b)) |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
Deferred government grants (note 17) |
|
|
|
|
|
|
||
Long-term bank loans (note 12) |
|
|
|
|
|
|
||
Deferred tax liability |
|
|
|
|
|
|
||
Loan from a non-controlling shareholder (note 13 (a)) |
|
|
— |
|
|
|
|
|
Lease liability (notes 11 and 13(b)) |
|
|
|
|
|
|
||
Other non-current liabilities (note 15) |
|
|
|
|
|
|
||
Total long-term liabilities |
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
||
and contingencies (notes 18 and 24) |
|
|
|
|
|
|
||
EQUITY |
|
|
|
|
|
|
||
Preferred stock (note 19) |
|
|
|
|
|
|
||
Authorized |
|
|
|
|
|
|
||
Issued and outstanding: |
|
|
|
|
|
|
||
Common stock (note 19) |
|
|
|
|
|
|
||
Authorized: |
|
|
|
|
|
|
||
Issued and outstanding: |
|
|
|
|
|
|
||
Additional paid-in capital |
|
|
|
|
|
|
||
Subscriptions receivable |
|
|
— |
|
|
|
( |
) |
Accumulated other comprehensive income (loss) |
|
|
( |
) |
|
|
|
|
Statutory surplus reserves (note 21) |
|
|
|
|
|
|
||
Accumulated earnings |
|
|
|
|
|
|
||
Total shareholders' equity |
|
|
|
|
|
|
||
Non-controlling interests |
|
|
|
|
|
|
||
Total equity |
|
|
|
|
|
|
||
Total liabilities and equity |
|
$ |
|
|
$ |
|
||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
SINOVAC BIOTECH LTD.
Consolidated Statements of Comprehensive Income (Loss)
For the years ended December 31, 2022, 2021 and 2020
(Expressed in thousands of U.S. Dollars, except for number of shares and per share data)
|
|
For the year ended December 31 |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Sales (note 23) |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Cost of sales |
|
|
|
|
|
|
|
|
|
|||
Gross profit |
|
|
|
|
|
|
|
|
|
|||
Selling, general and administrative expenses (including rent expenses incurred |
|
|
|
|
|
|
|
|
|
|||
Provision for doubtful accounts |
|
|
|
|
|
|
|
|
|
|||
Research and development expenses |
|
|
|
|
|
|
|
|
|
|||
Loss on disposal of property, plant and equipment (note 8) |
|
|
|
|
|
|
|
|
|
|||
Government grants recognized in income |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Total operating expenses |
|
|
|
|
|
|
|
|
|
|||
Operating (loss) income |
|
|
( |
) |
|
|
|
|
|
|
||
Interest and financing expenses – (including interest expenses incurred |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Interest income |
|
|
|
|
|
|
|
|
|
|||
Other income (expense), net |
|
|
|
|
|
( |
) |
|
|
|
||
Income before income taxes |
|
|
|
|
|
|
|
|
|
|||
Income tax recovery (expense) (note 15) |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Net income |
|
|
|
|
|
|
|
|
|
|||
Less: (Income) loss attributable to non-controlling interests |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Net income attributable to shareholders of Sinovac |
|
|
|
|
|
|
|
|
|
|||
Preferred stock dividends |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net income attributable to common shareholders of Sinovac |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Net income |
|
|
|
|
|
|
|
|
|
|||
Other comprehensive income, net of tax of nil |
|
|
|
|
|
|
|
|
|
|||
Foreign currency translation adjustments |
|
|
( |
) |
|
|
|
|
|
|
||
Comprehensive (loss) / income |
|
|
( |
) |
|
|
|
|
|
|
||
Less: comprehensive (income) loss attributable to non-controlling interests |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Comprehensive income (loss) attributable to shareholders of Sinovac |
|
|
( |
) |
|
|
|
|
|
|
||
Earnings per share (note 22) |
|
|
|
|
|
|
|
|
|
|||
Basic net income per share |
|
|
|
|
|
|
|
|
|
|||
Diluted net income per share |
|
|
|
|
|
|
|
|
|
|||
Weighted average number of shares of common stock outstanding |
|
|
|
|
|
|
|
|
|
|||
– Basic |
|
|
|
|
|
|
|
|
|
|||
– Diluted |
|
|
|
|
|
|
|
|
|
|||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
SINOVAC BIOTECH LTD.
For the years ended December 31, 2022, 2021 and 2020
(Expressed in thousands of U.S. dollars, expect number of shares data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
Common stock |
|
|
Preferred stock |
|
|
Additional |
|
|
Subscriptions |
|
|
currency |
|
|
Statutory |
|
|
Accumulated |
|
|
Total |
|
|
Non- |
|
|
Total |
|
||||||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
capital |
|
|
receivable |
|
|
adjustment) |
|
|
reserves |
|
|
earnings |
|
|
equity |
|
|
interests |
|
|
equity |
|
||||||||||||
Balance, December 31, 2019 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||||||||||
Share-based compensation (note 20) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
Exercise of stock options (note 20) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
Subscriptions receivable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
||||||||
Cancellation of outstanding shares (note 20) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Equity transactions of subsidiaries |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Dividend accrued (note 19) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
( |
) |
|||||||||
Other comprehensive Income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Other comprehensive income attributable to non-controlling interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Other comprehensive income attributable to shareholders of Sinovac |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
Net income for the year |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
-Net income attributable to non-controlling interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Net income attributable to shareholders of Sinovac |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Transfer to statutory surplus reserves (note 21) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||||||||||
Balance, December 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-9
SINOVAC BIOTECH LTD.
Consolidated Statements of Shareholders’ Equity
For the years ended December 31, 2022, 2021 and 2020
(Expressed in thousands of U.S. dollars, expect number of shares data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
Common stock |
|
|
Preferred stock |
|
|
Additional |
|
|
Subscriptions |
|
|
currency |
|
|
Statutory |
|
|
Accumulated |
|
|
Total |
|
|
Non- |
|
|
Total |
|
||||||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
capital |
|
|
receivable |
|
|
adjustment) |
|
|
reserves |
|
|
earnings |
|
|
equity |
|
|
interests |
|
|
equity |
|
||||||||||||
Balance, December 31, 2020 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
$ |
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|||||||||||
Share-based compensation (note 20) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
Exercise of stock options (note 20) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
Dividend accrued and paid (note 19) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
||||||||
Other comprehensive income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|||||||||||
- Other comprehensive income attributable to non-controlling interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Other comprehensive income attributable to shareholders of Sinovac |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
Net income for the year |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Net income attributable to non-controlling interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Net income attributable to shareholders of Sinovac |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Transfer to statutory surplus reserves (note 21) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||||||||||
Balance, December 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-10
SINOVAC BIOTECH LTD.
Consolidated Statements of Shareholders’ Equity
For the years ended December 31, 2022, 2021 and 2020
(Expressed in thousands of U.S. dollars, expect number of shares data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
Common stock |
|
|
Preferred stock |
|
|
Additional |
|
|
Subscriptions |
|
|
currency |
|
|
Statutory |
|
|
Accumulated |
|
|
Total |
|
|
Non- |
|
|
Total |
|
||||||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
capital |
|
|
receivable |
|
|
adjustment) |
|
|
reserves |
|
|
earnings |
|
|
equity |
|
|
interests |
|
|
equity |
|
||||||||||||
Balance, December 31, 2021 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||||||||||
Subscriptions receivable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Dividend accrued and paid (note 19) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
||||||||
Other comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Other comprehensive loss attributable to non-controlling interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
||||||||||
- Other comprehensive loss attributable to shareholders of Sinovac |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|||||||||
Net income for the year |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
-Net loss attributable to non-controlling interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
||||||||||
- Net income attributable to shareholders of Sinovac |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
- Transfer to statutory surplus reserves (note 21) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||||||||||
Balance, December 31, 2022 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-11
SINOVAC BIOTECH LTD.
Consolidated Statements of Cash Flows
For the years ended December 31, 2022, 2021 and 2020
(Expressed in thousands of U.S. dollars)
|
|
For the year ended December 31 |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Operating activities |
|
|
|
|
|
|
|
|
|
|||
Net Income |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Adjustments to reconcile net income to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
|||
- Deferred income taxes (note 15) |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
- Share-based compensation (note 20) |
|
|
|
|
|
|
|
|
|
|||
- Inventory provision (note 7) |
|
|
|
|
|
|
|
|
|
|||
- Provision for doubtful accounts |
|
|
|
|
|
|
|
|
|
|||
- Loss on disposal of property, plant and equipment (note 8) |
|
|
|
|
|
|
|
|
|
|||
- Depreciation of property, plant and equipment (note 8) |
|
|
|
|
|
|
|
|
|
|||
- Amortization of prepaid land lease payments (note 9) |
|
|
|
|
|
|
|
|
|
|||
- Amortization of intangible assets (note 10) |
|
|
|
|
|
|
|
|
|
|||
- Government grants recognized in income |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Changes in: |
|
|
|
|
|
|
|
|
|
|||
- Accounts receivable |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
- Inventories |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
- Income tax (recoverable) / payable |
|
|
( |
) |
|
|
|
|
|
|
||
- Prepaid expenses and deposits |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
- Deferred revenue |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
- Accounts payable and accrued liabilities |
|
|
( |
) |
|
|
|
|
|
|
||
- Other non-current liabilities |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net cash (used in) provided by operating activities |
|
|
( |
) |
|
|
|
|
|
|
||
Financing activities |
|
|
|
|
|
|
|
|
|
|||
- Proceeds from bank loans |
|
|
|
|
|
|
|
|
|
|||
- Repayments of bank loans |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
- Proceeds from issuance of common stock, net of share issuance costs |
|
|
|
|
|
|
|
|
|
|||
- Dividend paid |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
- Proceeds from subsidiary's financing |
|
|
|
|
|
|
|
|
|
|||
- Government grants received (note 17) |
|
|
|
|
|
|
|
|
|
|||
- Loan from a non-controlling shareholder (note 13(a)) |
|
|
|
|
|
|
|
|
|
|||
- Repayments of loan from a non-controlling shareholder (note 13(a)) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net cash provided by (used in) financing activities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Investing activities |
|
|
|
|
|
|
|
|
|
|||
- Proceeds from redemption of investments |
|
|
|
|
|
|
|
|
|
|||
- Proceeds from dissolution of subsidiary |
|
|
|
|
|
|
|
|
|
|||
- Proceeds from disposal of equipment |
|
|
|
|
|
|
|
|
|
|||
- Acquisition of intangible assets |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
- Prepaid land lease payments |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
- Purchase of investments |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
- Purchase of property, plant and equipment |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
- Purchase of equity investments |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Net cash used in investing activities |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Effect of exchange rate changes on cash and cash equivalents and restricted cash |
|
|
( |
) |
|
|
|
|
|
|
||
(Decrease) increase in cash and cash equivalents and restricted cash |
|
|
( |
) |
|
|
|
|
|
|
||
Cash and cash equivalents and restricted cash, beginning of year |
|
|
|
|
|
|
|
|
|
|||
Cash and cash equivalents and restricted cash, end of year |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
|||
Cash paid for interest |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Cash paid for income taxes |
|
$ |
|
|
$ |
|
|
$ |
|
|||
The accompanying notes are an integral part of these consolidated financial statements
F-12
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
1. Basis of Presentation
These consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”). They include the accounts of Sinovac Biotech Ltd., which is incorporated under the laws of Antigua and Barbuda, and its wholly owned or controlled subsidiaries (collectively, the “Company”).
|
Name |
|
Date of |
|
Place of |
|
Percentage of |
|
|
Percentage of |
|
|
Principal activities |
||
|
Sinovac Biotech |
|
October 2008 |
|
Hong Kong |
|
|
% |
|
|
% |
|
International sales |
||
|
Sinovac Biotech Co., |
|
April 2001 |
|
People’s |
|
|
% |
|
|
% |
|
Research and |
||
|
Sinovac Life Sciences |
|
May 2009 |
|
PRC |
|
|
% |
|
|
% |
|
Research and |
||
|
Sinovac (Dalian) Vaccine |
|
January 2010 |
|
PRC |
|
|
% |
|
|
% |
|
Research and |
||
|
Sinovac Biomed Co., Ltd. ("Sinovac Biomed") |
|
April 2015 |
|
PRC |
|
|
% |
|
|
% |
|
Distribution of |
||
|
Sinovac Biotech (Singapore) |
|
August 2020 |
|
Singapore |
|
|
% |
|
|
% |
|
International sales |
||
F-13
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
2. Significant Accounting Policies
(a) Use of Estimates
In preparation of the Company’s consolidated financial statements, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting periods. Significant estimates made by management include provision for product returns, allowance for doubtful accounts, inventory provisions, impairment of long-lived assets, fair value of options granted and related forfeiture rates, and realizability of deferred tax assets. On an ongoing basis, management reviews its estimates to ensure that these estimates appropriately reflect changes in the Company’s business and new information as it becomes available. If historical experience and other factors used by management to make these estimates do not reasonably reflect future activity, the Company’s consolidated financial statements could be materially impacted.
(b) Cash and Cash Equivalents
Cash equivalents consist of highly liquid investments that are readily convertible to cash generally with maturities of three months or less when purchased.
(c) Restricted Cash
Restricted cash is cash held as collateral for transactions the Company has entered into.
The ending balance of cash and cash equivalents and restricted cash presented in the consolidated statements of cash flows in 2022 is $
(d) Investments
Short-term investments
All highly liquid investments with original maturities between three months and one year are classified as short-term investments. Investments that are expected to be realized in cash during the next twelve months are also included in short-term investments.
The Company accounts for short-term debt investments in accordance with ASC Topic 320, Investments—Debt Securities (“ASC 320”). The Company classifies the short-term investments in debt as “held-to-maturity,” “trading” or “available-for-sale,” whose classification determines the respective accounting methods stipulated by ASC 320. Dividend and interest income, including amortization of the premium and discount arising at acquisition, for all categories of investments in securities are included in earnings. Any realized gains or losses on the sale of the short-term investments are determined on a specific identification method, and such gains and losses are reflected in earnings during the period in which gains or losses are realized.
Securities that the Company has the positive intent and ability to hold to maturity are classified as held-to-maturity securities and stated at amortized cost less allowance for credit losses.
Securities that are bought and held principally for the purpose of selling them in the near term are classified as trading securities. Unrealized holding gains and losses for trading securities are included in earnings.
Debt investments not classified as trading or as held-to-maturity are classified as available-for-sale debt securities, which are reported at fair value, with unrealized gains and losses recorded in “Accumulated other comprehensive income (loss)” on the consolidated balance sheets.
The allowance for credit losses of the held-to-maturity debt securities reflects the Company’s estimated expected losses over the contractual lives of the held-to-maturity debt securities and is charged to “Other income, net” in the consolidated statements of comprehensive income. Estimated allowances for credit losses are determined by considering reasonable and supportable forecasts of future economic conditions in addition to information about past events and current conditions. As of December 31, 2022 and 2021, the allowance for credit losses provided for the held-to-maturity debt securities held by the Company was nil.
Long-term investments
F-14
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
The Company’s long-term investments consist of equity method investments and held-to-maturity debt investments with original maturities greater than one year.
Investments in entities in which the Company can exercise significant influence but does not own a majority equity interest or control are accounted for using the equity method of accounting in accordance with ASC Topic 323, Investments-Equity Method and Joint Ventures (“ASC 323”). Under the equity method, the Company initially records its investment at cost and the difference between the cost of the equity investee and the amount of the underlying equity in the net assets of the equity investee is accounted for as if the investee were a consolidated subsidiary. The Company subsequently adjusts the carrying amount of its investment to recognize the Company’s proportionate share of each equity investee’s net income or loss into earnings. The Company will discontinue applying the equity method if an investment (plus additional financial support provided to the investee, if any) has been reduced to zero. The Company evaluates its equity method investments for impairment at each reporting date, or more frequently if events or changes in circumstances indicate that the carrying amount of the investment might not be recoverable. An impairment loss on the equity method investments is recognized in earnings when the decline in value is determined to be other-than-temporary.
(e) Accounts Receivable
The Company adopted Accounting Standards Update (ASU) 2016-13, “Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments” (“ASU 2016-13”) which requires the measurement and recognition of expected credit losses for financial assets held at amortized costs. ASU 2016-13 replaces the existing incurred loss impairment model with an expected loss methodology, which results in more timely recognition of credit losses. The Company estimates an allowance for doubtful accounts based on historical experience, the age of the accounts receivable balances, credit quality of the Company’s customers, current and forecasted future economic conditions and other factors that may affect its customers’ ability to pay.
(f) Inventories
Inventories are stated at the lower of cost or net realizable value. The cost of work in progress and finished goods is determined on a weighted-average cost basis and includes direct material, direct labor and overhead costs. Net realizable value represents the anticipated selling price, net of distribution cost, less estimated costs to completion for work in progress.
(g) Property, Plant and Equipment
Property, plant and equipment are recorded at cost. Significant additions and improvements are capitalized, while repairs and maintenance are charged to expenses as incurred. Equipment purchased for specific research and development projects with no alternative use are expensed. Assets under construction are not depreciated until construction is completed and the assets are ready for their intended use. Gains and losses from the disposal of property, plant and equipment are recorded in gain or loss on disposal and impairment of property, plant and equipment included in the consolidated statements of comprehensive income (loss).
Depreciation of property, plant and equipment is computed using the straight-line method based on the estimated useful lives of the assets as follows:
Plant and buildings |
|
Machinery and equipment |
|
Motor vehicles |
|
Office equipment and furniture |
|
Leasehold improvements |
(h) Prepaid Land Lease Payments
Prepaid land lease payments represent amounts paid for the rights to use land in the PRC and is recorded at purchased cost less accumulated amortization. Amortization is provided on a straight-line basis over the term of the lease agreement, which ranges from
(i) Intangible Assets
The Company capitalizes the patent payment and the purchased cost of vaccines if the vaccine has received a new drug certificate from the National Medical Products Administration (“NMPA) of China. If the vaccine has not received a new drug certificate, the purchase cost is expensed as in-process research and development.
F-15
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
Licenses in relation to the production and sales of pharmaceutical products are amortized on a straight-line basis over their respective useful lives. Costs incurred to renew or extend the term of licenses are capitalized and amortized over the license’s useful life on a straight-line basis.
The costs of acquiring and developing computer software and cloud computing websites for internal use are capitalized as intangible assets. Computer software and cloud computing related intangible assets are amortized over
(j) Impairment of Long-Lived Assets
Long-lived assets including property, plant and equipment and intangible assets subject to amortization are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of an asset group may not be recoverable from the future undiscounted net cash flows expected to be generated by the asset group. An asset group is identified as assets at the lowest level for which identifiable cash flows are largely independent of the cash flows of other assets. If the asset group is not fully recoverable, an impairment loss would be recognized for the difference between the carrying value of the asset group and its estimated fair value, based on the discounted net future cash flows or other appropriate methods, such as comparable market values. The Company uses estimates and judgments in its impairment tests and if different estimates or judgment had been utilized, the timing or the amount of any impairment charges could be materially different.
(k) Income Taxes
The Company follows the liability method of accounting for income taxes. Under this method, deferred tax liabilities and assets are determined based on the temporary differences between the carrying values and tax bases of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. A valuation allowance is provided if, based on the weight of available evidence, it is more-likely-than-not that some portion, or all, of the deferred tax assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates and laws.
The tax benefit from an uncertain tax position is recognized only if it is more likely than not that the tax position will be sustained upon examination by the appropriate taxing authority, based on the technical merits of the position. The tax benefits recognized from such a position are measured based on the amount that is greater than 50% likely of being realized upon settlement. The Company recognizes a change in available facts after the reporting date but before issuance of the financial statements in the period when the change in facts occur, even if that new information provides a better estimate of the ultimate outcome of an uncertainty. Liabilities associated with uncertain tax positions are classified as long−term unless expected to be settled within one year. Interest and penalties related to uncertain tax positions, if any, are recorded in the provision for income taxes and classified with the related liability on the consolidated balance sheets.
(l) Value-added Taxes
Value-added taxes (“VAT”) collected from customers relating to product sales and payable to governmental authorities are presented on a net basis. VAT collected from customers is excluded from revenue.
F-16
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
(m) Revenue from Contracts with Customers
Revenue is recognized at a point in time when performance obligation is satisfied where control of promised goods is transferred to the Company’s customers in an amount of consideration of which the Company expect to be entitled to in exchange for the goods, and the Company can reasonably estimates return provisions for the goods.
Product return provisions are estimated based on historical return and exchange data as well as inventory levels and remaining shelf lives of products in distribution channels.
As of December 31, 2022, sales return provision for the Company’s vaccine products was $
Deferred revenue is generally related to government stockpiling programs and advances received from customers. For government stockpiling programs, the Company generally obtains purchase authorizations from the government for specified amount of products at a specified price and no rights of return are provided. Revenue is recognized when the government takes delivery of the products. If the products expire prior to delivery, these expired products are recognized as revenue once cash is received and have passed government inspection.
For the year ended December 31, 2022, the Company did not have any significant incremental costs of obtaining contracts with customers or costs incurred in fulfilling contracts with customers that shall be recognized as an asset and amortized to expenses in a pattern that matches the timing of the revenue recognition of the related contract.
The Company does not have contract assets since revenue is recognized as control of goods is transferred. Contract liabilities consist of advance payments from customers. Contract liabilities are reported in a net position on a customer-by-customer basis at the end of each reporting period. All contract liabilities are included in deferred revenue on the consolidated balance sheets. For the year ended December 31, 2022, the Company recognized sales of $
(n) Shipping and Handling
(o) Advertising Expenses
Advertising costs are expensed as incurred and included in selling, general and administrative expenses. Advertising costs were $
(p) Research and Development
Research and development ("R&D") costs are expensed as incurred and are disclosed as a separate line item in the Company’s consolidated statements of comprehensive income (loss). R&D costs consist primarily of the remuneration of R&D staff, depreciation, material, clinical trial costs as well as amortization of acquired technology and know-how used in R&D with alternative future uses. R&D costs also include costs associated with collaborative R&D and in-licensing arrangements, including upfront fees paid to collaboration partners in connection with technologies which have not reached technological feasibility and do not have an alternative future use. Reimbursement of R&D costs for arrangements with collaboration partners is recognized when the obligations are incurred.
Under certain R&D arrangements with third parties, the Company may be required to make payments that are contingent on the achievement of specific development, regulatory and/or commercial milestones. Before a product receives regulatory approval, license fees and milestone payments made to third parties are expensed as incurred. License fees and milestone payments made to third parties after regulatory approval is received are capitalized and amortized over the remaining life of the agreement with third parties.
F-17
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
(q) Government Grants
Government grants received from the PRC government by the PRC operating subsidiaries of the Company are recognized when there is reasonable assurance that the amount is receivable and all the conditions specified in the grant have been met. Government grants for R&D are recognized as a reduction to R&D expenses when the expenses are incurred in the same period as when the conditions attached to the grants are met, or recognized as government grants in income in the period when the conditions are met after the expenses are incurred. Government grants for property, plant and equipment are deferred and recognized as a reduction to the related depreciation and amortization expenses in the same manner as the property, plant and equipment are depreciated. Interest subsidies are recorded as a reduction to interest and financing expenses in the consolidated statements of comprehensive income (loss), or recorded as a reduction to interest capitalized if the subsidies granted are related to a specific borrowing associated with building a qualifying asset. For government loans received at below market interest rate, the difference between the face value of the loan and fair value using the effective interest rate method is recorded as deferred government grants.
(r) Retirement and Other Post-retirement Benefits
Full-time employees of the Company in the PRC participate in a government mandated defined contribution plan pursuant to which certain pension benefits, medical care, unemployment insurance, employee housing fund and other welfare benefits are provided to employees. PRC labor regulations require that the Company make contributions to the government for these benefits based on certain percentages of the employees’ salaries. The Company has no legal obligation for the benefits beyond the contributions. Total amounts for employee retirement and other post-retirement benefits incurred was $
(s) Foreign Currency Translation and Transactions
The Company maintains accounting records in functional currencies as follows: U.S. dollars (“$”) for Sinovac Biotech. Ltd., Sinovac Hong Kong and Sinovac Singapore, and Renminbi Yuan (“RMB”) for the PRC subsidiaries. The Company uses the US$ as its reporting currency.
At the transaction date, each asset, liability, revenue and expense is re-measured into the functional currency by the use of the exchange rate in effect at that date. At each period end, foreign currency monetary assets, and liabilities are re-measured into the functional currency by using the exchange rate in effect at the balance sheet date. The Company recognized foreign exchange gain of $
Assets and liabilities of subsidiaries with functional currencies other than US$ are translated into US$ at the exchange rates in effect at the balance sheet date. Revenue and expenses are translated at average exchange rates. Gains and losses from such translations are recorded in accumulated other comprehensive income, a component of shareholders’ equity.
(t) Share-based Compensation
Compensation expense for costs related to all share-based payments, including grants of stock options, is recognized through a fair-value based method. The Company uses the Black-Scholes option-pricing model to determine the grant date fair value for stock options. The Company uses the grant date stock price to determine the grant date fair value of restricted shares. The Company has elected to recognize share-based compensation costs using the straight-line method over the requisite service period with a graded vesting schedule, provided that the amount of compensation costs recognized at any date is at least equal to the portion of the grant date value of the awards that are vested at that date. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from initial estimates. Share based compensation costs are recorded net of estimated forfeitures such that expense is recorded only for those awards that are expected to vest.
F-18
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
(u) Comprehensive Income
The Company’s comprehensive income consists of net income and foreign currency translation adjustments.
(v) Earnings Per Share
Earnings per share is calculated in accordance with Accounting Standards Codification (“ASC”) 260 Earnings per Share. Basic earnings per share is computed by dividing the net income attributable to shareholders of Sinovac by the weighted average number of common shares outstanding during the year. Diluted earnings per share is computed in accordance with the treasury stock method and based on the weighted average number of common shares and dilutive common share equivalents. Dilutive common share equivalents are excluded from the computation of diluted earnings per share if their effects would be anti-dilutive.
(w) Leases
The Company determines if an arrangement is a lease or contains a lease at lease inception. For operating leases, the Company recognizes a right-of-use asset and a lease liability based on the present value of the lease payments over the lease term on the consolidated balance sheets at commencement date. As most of the Company’s leases do not provide an implicit rate, the Company estimates its incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The incremental borrowing rate is estimated to approximate the interest rate on a collateralized basis with similar terms and payments, and in economic environments where the leased asset is located.
(x) Fair Value Measurements
Assets and liabilities subject to fair value measurements are required to be disclosed within a specified fair value hierarchy. The fair value hierarchy ranks the quality and reliability of inputs, or assumptions, used in the determination of fair value and requires assets and liabilities carried at fair value to be classified and disclosed in one of the following categories based on the lowest level input used that is significant to a particular fair value measurement:
As of December 31, 2022, the Company recorded $
The carrying values of cash equivalents, restricted cash, accounts receivable, accounts payable and accrued liabilities and short-term bank loans and the current portion of long-term debt approximate their fair value because of their short-term nature. Fair value of held-to-maturity debt investments as disclosed are determined based on level 2 inputs based on the discounted cash flow model using the discount curve of market interest rates and their fair values approximate the carrying values. Fair value of the long-term bank loans is determined based on level 2 inputs, and the carrying amounts of long-term bank loans approximate fair value as the related interest rates approximate rates currently offered by financial institutions for similar debt instruments.
For equity securities accounted for under the measurement alternative, when there are observable price changes in orderly transactions for identical or similar investments of the same issuer, the investments are re-measured to fair value. The Company also measures property, plant and equipment at fair value on a non-recurring basis only if an impairment charge were to be recognized. There were no non-recurring fair value measurements for the years ended December 31, 2022 and 2021.
F-19
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
(y) Concentration of Risks
Exchange Rate Risks
The Company operates in China, which may give rise to significant foreign currency risks from fluctuations and the degree of volatility of foreign exchange rates between the U.S. dollars and the RMB. In 2022, foreign exchange gain is $
Currency Convertibility Risks
Substantially all of the Company’s operating activities are transacted in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the People’s Bank of China or other regulatory institutions requires submitting a payment application form together with other information such as suppliers’ invoices, shipping documents and signed contracts.
Concentration of Credit Risks
Financial instruments that potentially subject the Company to concentration of credit risks consist primarily of cash and cash equivalents, restricted cash, short-term investments and accounts receivable, the balances of which are stated on the consolidated balance sheets which represent the Company’s maximum exposure. The Company places its cash and cash equivalents, restricted cash, and short-term investments in reputable financial institutions in Hong Kong and China. Concentration of credit risks with respect to accounts receivables is linked to the concentration of revenue. The Company’s customers are mainly various government agencies in China. For the year ended December 31, 2020, one of the Company’s customers accounted for 11% of the Company’s total revenue, and no single customer of the Company accounted for more than 10% of the total sales for the years ended December 31, 2022 and 2021. To manage credit risk, the Company performs ongoing credit evaluations of customers’ financial condition.
Interest Rate Risks
The Company is subject to interest rate risk. Other than loans from a non-controlling shareholder of $
(z) Recently Issued Accounting Standards
In June 2022, the FASB issued ASU 2022-03, Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions, which clarifies that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments also clarify that an entity cannot, as a separate unit of account, recognize and measure a contractual sale restriction. This guidance also requires certain disclosures for equity securities subject to contractual sale restrictions. The new guidance is required to be applied prospectively with any adjustments from the adoption of the amendments recognized in earnings and disclosed on the date of adoption.
This guidance is effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. Early adoption is permitted. The Company does not expect that the adoption of this guidance to have a material impact on its consolidated financial statements.
F-20
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
3. Restricted Cash
As of December 31, 2022, the balance of $
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Restricted Cash |
|
$ |
|
|
$ |
|
||
|
Total Restricted Cash |
|
$ |
|
|
$ |
|
||
4. Investments
Short-term investments
As of December 31, 2022 and 2021, the Company’s short-term investments were comprised of only debt securities. Short-term held-to-maturity investments were mainly deposits in commercial banks and wealth management products issued by commercial banks and other financial institutions for which the Company has the positive intent and ability to hold those securities to maturity, which are more than three months but less than one year. The carrying value of short-term investments as of December 31, 2022 and 2021 approximate their fair value.
During the years ended December 31, 2022, 2021 and 2020, the Company recorded interest income from its short-term investments of $
Short-term investments classification as of December 31, 2022 and 2021 were shown as below:
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Held-to-maturity debt investments |
|
$ |
|
|
|
|
||
|
|
|
|
|
|
|
|
||
Long-term investments
The following table sets forth a breakdown of the categories of long-term investments held by the Company as of the dates indicated:
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Long-term held-to-maturity debt investments |
|
$ |
|
|
$ |
|
||
|
Equity method investments and private equity |
|
|
|
|
|
|
||
|
Total long-term investments |
|
$ |
|
|
$ |
|
||
Long-term held-to-maturity debt investments
Long-term held-to-maturity debt investment represents a deposit in a financial institution with a contractual maturity due over one year for which the Company has the positive intent and ability to hold those securities to maturity.
During the years ended December 31, 2022, 2021 and 2020, the Company recorded interest income from its long-term held-to-maturity investment of $
Equity method investments
F-21
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
Equity investment in Keyvac Biyolojik Ürünler Sanayi ve Ticaret Anonim Şirketi
In 2021, Sinovac LS through one of its wholly owned subsidiaries Sinovac Life Sciences (Hainan) Co., Ltd. and a business partner in the Republic of Turkey formed a joint venture Keyvac Biyolojik Ürünler Sanayi ve Ticaret Anonim Şirketi (“Keyvac”) in the Republic of Turkey, with the focus on manufacturing and commercialization of vaccines. The Company owns about
The Company invests in equity securities of certain companies whose securities are not publicly traded and fair value is not readily determinable and where the Company has concluded it does not have significant influence based on its ownership percentage and other factors. These investments are recorded at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. In 2022, the Company, through its wholly owned subsidiary, Sinovac Hong Kong, and Sinovac Biomed, invested in companies that operate in the biotech supply chain industry.
In 2022, the Company, through its subsidiaries, invested in certain of Vivo Capital as limited partner and accounts for the investments under the equity method.
5. Trust for Incentive
In June 2022, the Company approved a long term incentive plan in the aggregate amount of $
The Company has the direct ability to control the Trust as the Trustee is required to act in accordance with the Trust deed and because the Company has the power to direct the activities of the Trust, the Trust is consolidated into the Company's consolidated financial statements. The assets held in the Trust that are being used as investments to satisfy the Company’s obligations under the Incentive Plan are recorded in short-term investments in the amount of $
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Trade receivables |
|
$ |
|
|
$ |
|
||
|
Allowance for doubtful accounts |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
||
|
Other receivables |
|
|
|
|
|
|
||
|
Total accounts receivable |
|
$ |
|
|
$ |
|
||
The allowance for doubtful accounts reflects the Company's best estimate of probable losses inherent in the accounts receivable balance.
The Company estimates the allowance based on historical experience, the age of the accounts receivable balances, credit quality of the Company’s customers, current and forecasted future economic conditions, and other factors that may affect customers’ ability to pay.
The Company’s maximum exposure to credit risk at the balance sheets date relating to trade receivables is summarized as follows:
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Aging within one year, net of allowance for doubtful accounts |
|
$ |
|
|
$ |
|
||
|
Aging greater than one year, net of allowance for doubtful accounts |
|
|
|
|
|
|
||
|
Total trade receivables |
|
$ |
|
|
$ |
|
||
F-22
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
7. Inventories
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Raw materials |
|
$ |
|
|
$ |
|
||
|
Work in progress |
|
|
|
|
|
|
||
|
Finished goods |
|
|
|
|
|
|
||
|
Total inventories |
|
$ |
|
|
$ |
|
||
For the year ended December 31, 2022, the Company charged $
For the year ended December 31, 2022, cost of sales includes $
8. Property, Plant and Equipment - net
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Cost |
|
|
|
|
|
|
||
|
Construction in progress |
|
$ |
|
|
$ |
|
||
|
Plant and buildings |
|
|
|
|
|
|
||
|
Machinery and equipment |
|
|
|
|
|
|
||
|
Motor vehicles |
|
|
|
|
|
|
||
|
Office equipment and furniture |
|
|
|
|
|
|
||
|
Leasehold improvements |
|
|
|
|
|
|
||
|
Total cost |
|
$ |
|
|
$ |
|
||
|
Less: Accumulated depreciation |
|
|
|
|
|
|
||
|
Plant and buildings |
|
$ |
|
|
$ |
|
||
|
Machinery and equipment |
|
|
|
|
|
|
||
|
Motor vehicles |
|
|
|
|
|
|
||
|
Office equipment and furniture |
|
|
|
|
|
|
||
|
Leasehold improvements |
|
|
|
|
|
|
||
|
Total accumulated depreciation |
|
$ |
|
|
$ |
|
||
|
Property, plant and equipment, net |
|
$ |
|
|
$ |
|
||
Buildings and machinery and equipment of Sinovac Dalian with a net book value of $
Net depreciation expense for the year ended December 31, 2022 was $
Loss on disposal of equipment for the year ended December 31, 2022 was $
9. Prepaid Land Lease Payments
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Prepaid land lease payments |
|
$ |
|
|
$ |
|
||
|
Less: accumulated amortization |
|
|
( |
) |
|
|
( |
) |
|
Net carrying value |
|
$ |
|
|
$ |
|
||
Amortization expense for prepaid land lease payments for the year ended December 31, 2022 was $
F-23
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
10.
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Computer software |
|
$ |
|
|
$ |
|
||
|
Less: accumulated amortization |
|
|
|
|
|
|
||
|
Net carrying value |
|
$ |
|
|
$ |
|
||
Amortization expense for intangible assets for the year ended December 31, 2022 was $
11. Leases
The Company’s operating leases mainly related to plants and buildings, some of which include options to extend leases that have not been included in the calculation of the Company’s lease liabilities and right-of-use assets. The Company recognizes rent on a straight-line basis over the expected term of the lease, which includes rent holidays and scheduled rent increases. For leases with terms greater than 12 months, the Company records the related asset and lease liability at the present value of lease payments over the lease term.
As of December 31, 2022, there were no finance leases entered into by the Company.
As of December 31, 2022, the weighted average remaining lease term was
|
|
|
For the year |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Cash payments for operating leases |
|
$ |
|
|
$ |
|
||
|
Right-of-use assets obtained in exchange for operating lease liabilities |
|
|
— |
|
|
|
|
|
Future lease payments under operating leases as of December 31, 2022 were as follows:
|
2023 |
|
$ |
|
|
|
2024 |
|
|
|
|
|
2025 |
|
|
|
|
|
2026 |
|
|
|
|
|
2027 |
|
|
|
|
|
Thereafter |
|
|
|
|
|
Total future lease payments |
|
|
|
|
|
Less: Imputed interest |
|
|
|
|
|
Total lease liability balance |
|
$ |
|
Minimum future rental payments under short-term leases for the year ending December 31, 2022 was $
As of December 31, 2022, additional operating leases that have not yet commenced were immaterial.
F-24
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
12. Bank Loans
Summarized below are bank loans as of December 31, 2022 and 2021:
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Bank of China (a) |
|
$ |
— |
|
|
$ |
|
|
|
China Merchants Bank (b) |
|
|
— |
|
|
|
|
|
|
China Everbright Bank (c) |
|
|
|
|
|
|
||
|
Bank loans due within one year |
|
|
|
|
|
|
||
|
China Merchants Bank (b) |
|
|
— |
|
|
|
|
|
|
China Everbright Bank (c) |
|
|
|
|
|
|
||
|
Long-term bank loans |
|
|
|
|
|
|
||
|
Total bank loans |
|
$ |
|
|
$ |
|
||
Aggregate maturities of loans for each of the next 5 years following December 31, 2022 are as follows:
|
Within 1 year |
|
$ |
|
|
|
In 2024 |
|
|
|
|
|
In 2025 |
|
|
|
|
|
In 2026 |
|
|
|
|
|
After 2026 |
|
|
|
|
|
Total |
|
$ |
|
F-25
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
The weighted average interest rate for all short-term and long-term bank loans was
13. Related Party Transactions and Balances
(a) Loan from a non-controlling shareholder
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Loan - current |
|
$ |
|
|
$ |
|
||
|
Loan - non - current |
|
|
— |
|
|
|
|
|
|
|
|
$ |
|
|
$ |
|
||
The Company has loan due to Dalian Jin Gang Group, the non-controlling shareholder of Sinovac Dalian, with a total amount of $
(b) The Company entered into the following transactions in the normal course of operations with related parties:
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Rent expenses to SinoBioway Biotech Group Co. Ltd. (“SinoBioway”). |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
Rent expenses to Dalian Jin Gang Group (“Jin Gang”). |
|
|
|
|
|
|
|
|
|
|||
|
Investment income from VIVO Capital's funds |
|
|
|
|
— |
|
|
— |
|
|||
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
In 2004, the Company entered into
In June 2007, the Company entered into another operating lease agreement with SinoBioway, with respect to the expansion of Sinovac Beijing’s production plant in Beijing, China, for an annual lease payment of $
In September 2010, the Company entered into another operating lease agreement with SinoBioway with respect to expansion of Sinovac R&D’s business in research and development activities for an annual lease payment of $
On April 8, 2013, the Company entered into
In 2019, the Company entered into an operating lease agreement with Jin Gang, the non-controlling shareholder of Sinovac Dalian, to rent refrigeration storage with the space of 2,000 sq.m. with an annual rent amounted $
F-26
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
As of December 31, 2022, $
In 2022, the Company invested $
14. Accounts Payable and Accrued Liabilities
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Trade payables |
|
$ |
|
|
$ |
|
||
|
Machinery and equipment payables |
|
$ |
|
|
|
|
||
|
Accrued expenses |
|
$ |
|
|
|
|
||
|
Value added tax payable |
|
$ |
|
|
|
|
||
|
Withholding tax payable |
|
$ |
|
|
|
|
||
|
Other tax payable |
|
$ |
|
|
|
|
||
|
Bonus and benefit payables |
|
$ |
|
|
|
|
||
|
Other payables |
|
$ |
|
|
|
|
||
|
Total accounts payable and accrued liabilities |
|
$ |
|
|
$ |
|
||
15. Income Taxes
Antigua and Barbuda
Under the current laws of Antigua and Barbuda, the Company is not subject to tax on income or capital gains. Additionally, upon payments of dividends by the Company to its shareholders, no Antigua and Barbuda withholding tax will be imposed.
Hong Kong
Under Hong Kong tax laws, Sinovac Hong Kong is subject to Hong Kong Profits Tax rate at
Singapore
Under Singapore tax laws, Sinovac Singapore is subject to Singapore Income Tax rate at
China
Effective from January 1, 2008, the PRC’s statutory income tax rate is
The Company’s income before income tax consists of:
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Non-PRC |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
PRC |
|
|
( |
) |
|
|
|
|
|
|
||
|
Total |
|
$ |
|
|
$ |
|
|
$ |
|
|||
F-27
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
The Company’s income taxes consists of:
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Current income tax expense |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
Deferred tax recovery (expense) |
|
|
|
|
|
( |
) |
|
|
|
||
|
Total income tax recovery (expense) |
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
The following is a reconciliation of the Company’s total income tax expenses to the amount computed by applying the PRC statutory income tax rate of
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Income before income taxes |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
Income tax expense at the PRC statutory rate |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
International tax rate differential |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
Super deduction for research and development expenses |
|
|
|
|
|
|
|
|
|
|||
|
Non-deductible expenses |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Effect of preferential tax rate |
|
|
( |
) |
|
|
|
|
|
|
||
|
Change in valuation allowance |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
Effect of PRC withholding tax |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
Other adjustments |
|
|
|
|
|
|
|
|
( |
) |
||
|
Income tax recovery (expense) |
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
The tax effects of temporary differences that give rise to the Company’s deferred tax assets are as follows:
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Inventories |
|
|
|
|
|
|
||
|
Accrued expenses |
|
|
|
|
|
|
||
|
Deferred government grants |
|
|
|
|
|
|
||
|
Fixed assets |
|
|
( |
) |
|
|
( |
) |
|
Tax losses carried forward |
|
|
|
|
|
|
||
|
Less: valuation allowance |
|
|
( |
) |
|
|
( |
) |
|
Deferred tax assets |
|
$ |
|
|
$ |
|
||
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which the temporary differences become deductible or utilized. The Company considers projected future taxable income and tax planning strategies in making this assessment. Based upon an assessment of the level of historical taxable income and projections for future taxable income over the periods in which the deferred tax assets are deductible or can be utilized.
The Company evaluates its valuation allowance requirements at end of each reporting period by reviewing all available evidence, both positive and negative, and considering whether, based on the weight of that evidence, a valuation allowance is needed. When circumstances cause a change in management’s judgment about the realizability of deferred tax assets, the impact of the change on the valuation allowance is generally reflected in income from operations. The future realization of the tax benefit of an existing deductible temporary difference ultimately depends on the existence of sufficient taxable income of the appropriate character within the carry forward period available under applicable tax law. The Company’s valuation allowance increased by $
Tax loss carry-forwards of the Company’s PRC subsidiaries in the amount of $
As of December 31, 2022, deferred tax liabilities of $
F-28
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
companies to their overseas parents in respect of earnings derived from January 1, 2008 onwards are subject to PRC dividend withholding tax at
As of December 31, 2022, the Company has not recognized any deferred tax liability on Sinovac Beijing’s undistributed earnings of approximately $
The changes in unrecognized tax benefits are as follows:
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Balance on January 1 |
|
|
|
|
|
|
|
|
|
|||
|
Additions for tax positions of the current year |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Additions for tax positions of the prior years |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Settlement with the taxing authority |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Lapse of statute of limitations |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Balance on December 31 |
|
$ |
|
|
$ |
|
|
$ |
|
|||
The Company recognizes interest and penalties, if any, related to unrecognized tax benefits, and such interest and penalties are reversed when statute of limitations lapse. For the year ended December 31, 2022, the Company reversed $
As of December 31, 2022, the Company had unrecognized tax benefits of approximately $
16. Deferred Revenue
Current deferred revenue included $
17. Deferred Government Grants
Deferred government grants represent funding received from the government for research and development (“R&D”) or investment in constructing or improving production facilities. The amount of deferred government grants as of December 31, 2022 is net of R&D expenditures, deduction of depreciation expenses, and the amount recognized as government grant income. The Company received $
F-29
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
Summarized below are deferred government grants as of December 31, 2022 and 2021:
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Government grants for property, plant and equipment (a) |
|
$ |
|
|
$ |
|
||
|
Government grants for research and development (b) |
|
|
|
|
|
|
||
|
Current deferred government grants |
|
|
|
|
|
|
||
|
Government grants for property, plant and equipment (a) |
|
|
|
|
|
|
||
|
Government grants for research and development (b) |
|
|
|
|
|
|
||
|
Non-current deferred government grants |
|
|
|
|
|
|
||
|
Total deferred government grants |
|
|
|
|
|
|
||
(a)
(b)
F-30
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
18. Commitments and Contingencies
(a) Other Commitments
In addition to commitments disclosed in note 24, commitments related to R&D expenditures are $
Commitments related to capital expenditures for the Company are approximately $
(b) Litigation Matters
US Litigation
Delaware Chancery Court Action
On April 12, 2018, 1Globe filed an amended answer to the Company’s complaint, counterclaims, and a third-party complaint against Mr. Weidong Yin alleging, among other allegations, that the Rights Agreement is not valid, that Mr. Yin and the Buyer Consortium (comprising Mr. Weidong Yin, the chairman, president and chief executive officer of Sinovac Biotech Ltd., SAIF partners IV L.P., or SAIF, C-Bridge Healthcare Fund II, L.P., Advantech Capital L.P., Vivo Capital Fund VIII, L.P. and Vivo Capital Surplus Fund VIII, L.P.) had previously triggered the Rights Agreement, and that 1Globe did not trigger the Rights Agreement. The Company and its board of directors believes that the actions taken by the board of directors were appropriate under the circumstances and that the allegations of the counterclaims and third-party complaint are without merit. 1Globe asks for various measures of equitable relief and also includes a claim for its costs, including attorneys’ fees.
On July 31, 2018, following the Company motions for partial summary judgment and an expedited trial date, the Delaware Chancery Court effectively stayed the action pending receipt of a post-trial decision from the Antigua Court in the matter captioned 1Globe Capital, LLC and Sinovac Biotech Ltd., Claim No. ANUHCV 2018/0120. On December 19, 2018, the Antigua Court issued a judgment (the “Antigua Court’s Judgment”) affirming the validity of Sinovac Antigua’s Rights Agreement under Antigua law, and finding that “there was a secret plan to take control” of the Company at the 2017 AGM.
Based upon the Antigua Court’s judgment and other facts known to the board of directors, the Company’s board of directors determined that certain of the Company’s shareholders, including 1Globe Capital LLC (“1Globe”), The Chiang Li Family, OrbiMed Advisors LLC and OrbiMed Capital LLC (together “OrbiMed”), and certain additional shareholders (collectively, the “Shareholder Group”), together with their affiliates and associates (collectively, the “Collaborating Shareholders”) became Acquiring Persons as defined under the Rights Agreement, on or prior to the 2017 AGM and their conduct resulted in a “Trigger Event” under the Rights Agreement. As a result of becoming Acquiring Persons, the approximately
On March 6, 2019, the Delaware Chancery Court entered a status quo order providing that the Company not distribute any of the Exchange Shares to rights holders until the final disposition of the pending Delaware litigation or further order of the Court. On April 8, 2019, the Delaware Chancery Court stayed the Delaware litigation pending the final outcome of 1Globe’s appeal of the Antigua Judgment. The Antigua litigation is ongoing.
F-31
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
Massachusetts District Court Actions
On March 5, 2018, the Company also filed a lawsuit in the United States District Court for Massachusetts alleging violations of Section 13(d) of the Securities Exchange Act of 1934 by 1Globe and The Chiang Li Family. The lawsuit alleges, among other things, that the defendant shareholders failed to make required disclosures on Schedule 13D regarding their intentions to attempt to replace the Company's board of directors.
On April 9, 2018, the Company received a document request from SEC requesting all of the Company’s documents concerning 1Globe, the Chiang Li Family, OrbiMed, certain other shareholders, and their affiliates. The Company has been cooperating with the SEC. The Company understands the SEC is investigating whether 1Globe, and possibly other shareholders, violated the U.S. securities laws. The Company does not have any information to suggest the SEC is investigating the actions of the Company or its officers and directors.
On May 21, 2018, 1Globe answered and filed counterclaims against the Company and certain of its executives, alleging violations of Section 10(b) of the Exchange Act and various state law claims. In response to the Company’s motion to dismiss 1Globe’s counterclaims, on August 1, 2018, 1Globe filed amended counterclaims against the Company and certain of its executives, alleging violations of Section 10(b) of the Exchange Act and Rule 10b-5, as well as state law claims of abuse of process, fraudulent misrepresentation, negligent misrepresentation, and aiding and abetting such violations, primarily arising out of allegedly false and/or misleading statements made by the Company regarding its business, operational, and financial results.
On August 17, 2018, the Massachusetts Court granted a consent motion to extend the deadline for the Company’s response to 1Globe’s counterclaims (and for any subsequent opposition by 1Globe) until after the Antigua Court issued a ruling in the matter captioned 1Globe Capital, LLC and Sinovac Biotech Ltd., Claim No. ANUHCV 2018/0120. On December 19, 2018, the Antigua Court issued a judgment, which 1Globe appealed on January 29, 2019. Per the Massachusetts Court’s order, the parties have filed periodic status reports regarding the pending court proceedings in Antigua. No date for the Company’s response to 1Globe’s counterclaims has been set. The Company is vigorously pursuing this lawsuit; however, the Company cannot predict whether an ultimate outcome will be favorable or unfavorable, nor estimate the amount or range of potential loss (if any) at this time.
Also on August 1, 2018, 1Globe filed a motion for preliminary injunction seeking to enjoin the Company from, inter alia, altering the capital structure of the Company. On October 15, 2018, the Massachusetts Court denied 1Globe’s motion. On November 14, 2018, 1Globe filed an appeal of the denial of its motion for preliminary injunction to the United States Court of Appeals for the First Circuit. On January 10, 2019, 1Globe filed a motion to hold its appeal in abeyance pending the outcome of its separate appeal of the Antigua Court’s judgment, which the Company opposed. In October 2019, 1Globe voluntarily dismissed the appeal.
Separately, Heng Ren Investments LP (“Heng Ren”) filed suit against the Company and Mr. Weidong Yin for alleged breach of fiduciary duties and wrongful equity dilution on May 31, 2019, in Massachusetts state court. The Company removed the matter from state court to the United States District Court for the District of Massachusetts. Heng Ren alleged that Mr. Yin breached fiduciary duties owed to minority shareholders, that the Company aided and abetted breaches of fiduciary duties, and that both the Company and Mr. Yin engaged in wrongful equity dilution. Heng Ren requested damages, attorneys’ fees, and prejudgment interest. On September 14, 2020, the Company filed a motion to dismiss Heng Ren’s claims. In July 2021, the Company moved to dismiss Heng Ren’s amended complaint in the federal court in Massachusetts. On March 4, 2022, the court granted the motion as to the breach of fiduciary duty claims and denied the motion as to the wrongful equity dilution claim. The Company is presently appealing the denial to the United States Court of Appeals for the First Circuit and has answered the complaint. On February 15, 2023, the court stayed discovery in the Heng Ren matter pending the resolution of an outstanding motion to dismiss filed in a purported shareholder's matter by us.
On December 5, 2022, a purported shareholder filed a putative class action complaint in Massachusetts federal court, asserting a claim under Section 204 of the Antigua and Barbuda International Business Corporations Act related to the PIPE transaction, alleging that all shareholders were harmed in an identical manner to one another by the PIPE transaction because the shares that were issued in the PIPE transaction allegedly undervalued Sinovac and all shareholders were purportedly wrongfully diluted as a result. The purported shareholder is represented by the same attorney who represents Heng Ren, and requests damages, attorneys’ fees, and prejudgment interest. On January 18, 2023, Sinovac filed a motion to dismiss. On February 15, 2023, Mr. Lerner opposed the motion to dismiss. On March 9, 2023, Sinovac filed a reply in support of its motion to dismiss. The motion was fully briefed as of March 9, 2023, and is currently pending before the court.
On January 19, 2023, Sinovac filed a motion to stay the Heng Ren action pending the resolution of the putative class action. On February 16, 2023, Massachusetts federal court stayed the Heng Ren action.
Antigua Litigation
F-32
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
On March 13, 2018, 1Globe filed a complaint against Sinovac Antigua in the Antigua Court. The complaint seeks a declaration that the five persons purportedly proposed on the Non-Public Submission at the 2017 AGM were elected as directors of Sinovac Antigua at that meeting, an order of the Antigua Court that those directors be installed as Sinovac Antigua’s board of directors, and a declaration that any actions taken on behalf of Sinovac Antigua at the direction of the board of directors since the 2017 AGM are null and void. On April 10, 2018, 1Globe filed a notice of application in the Antigua Court seeking an order declaring the result of the disputed election, an urgent order restraining Sinovac Antigua’s board of directors from acting, pending determination of the dispute, including acting to initiate or continue litigation against the Shareholder Group, and other related relief. We attended the first hearing on May 9, 2018. In July 2018, the Antigua court heard an application by 1Globe for interim injunctive relief preventing Sinovac Antigua from exercising its rights under the Rights Agreement. This application was unsuccessful, but the judge set an expedited timetable to trial. The trial of the matter took place from December 3 to 5, 2018. On December 19, 2018, the judge handed down his judgment, finding in Sinovac Antigua’s favor in full, dismissing 1Globe’s claim and declaring that the Rights Agreement was validly adopted as a matter of Antigua law. On January 29, 2019, 1Globe filed a Notice of Appeal. On March 4, 2019, 1Globe filed an application for urgent interim relief, seeking an injunction to prevent Sinovac Antigua from continuing to implement its Rights Agreement until the resolution of the appeal. This urgent interim relief application was heard on April 4, 2019, at which the Court of Appeal made an order restraining Sinovac Antigua in similar terms to the Delaware Court order of March 6, 2019, together with restraint from operating the Rights Agreement in any way that affects 1Globe’s rights or shareholding until determination of the appeal. 1Globe’s appeal of the Antigua Court’s Judgment was heard on September 18, 2019. On December 9, 2021, the Court of Appeal handed down its judgment, dismissing all grounds of appeal and upholding the Antigua Judgment. The Court of Appeal also confirmed that Sinovac Antigua’s Rights Agreement was consistent with its Articles of Incorporation and By-laws, and Antiguan business law. In January 2022, the Court of Appeal extended the order initially made on April 4, 2019, that restrains Sinovac Antigua from taking further action under its Rights Agreement, including the distribution of the previously issued Exchange Shares, until the conclusion of any appeal to the Privy Council. 1Globe applied for leave to appeal to the Privy Council, and the hearing of that application was held on February 24, 2022, in which the Court of Appeal granted 1Globe leave to appeal certain grounds to the Privy Council. On April 19, 2022, 1Globe renewed its application directly to the Privy Council for leave to appeal on its ground of appeal concerning the validity of the Rights Agreement. On July 13, 2022, 1Globe filed its Notice of Appeal on those grounds on which the Court of Appeal had granted 1Globe leave to appeal. On September 16, 2022, 1Globe filed an application to the Privy Council seeking permission to amend its existing application for permission to appeal and its existing Notice of Appeal, and to seek permission to appeal on another ground rejected by the Court of Appeal concerning the exercise of the Antigua Court’s discretion. Sinovac responded on October 21, 2022. On February 15, 2023, the Privy Council made a procedural decision to allow amendment of its existing application for permission to appeal, and decided to deal with procedural and substantive issues together at the Final Hearing. 1Globe has not yet taken steps to list a substantive hearing before the Privy Council. The appeal outcome is therefore pending.
As such, the final appeal is ongoing as of the date of this annual report. We cannot predict or estimate an outcome or economic burden for this case at this time.
19. Preferred and Common Stock
Share Capital
On February 22, 2019, pursuant to the Rights Agreement, the Company’s board of directors elected to exchange the approximately
Each share of common stock is entitled to
F-33
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
In 2020, the Company issued
In 2021, the Company issued
In 2022, there was
20. Stock Options
(a) Stock Option Plan
The board of directors approved a stock option plan (the “2003 Plan”) effective on November 1, 2003, pursuant to which directors, officers, employees and consultants of the Company are eligible to receive grants of options for the Company’s common stock. The 2003 Plan expires on November 1, 2023. Up to
On August 22, 2012, the board of directors approved a new stock option plan (the “2012 Plan”), which allowed the Company to issue up to
On May 1, 2015, the Company granted
On March 7, 2018, the Company granted
On September 16, 2020, the board of directors approved an employee share ownership plan (the “2020 ESOP”), where options were granted to officers and employees of the Company the right to purchase up to a
(b) Valuation Assumptions
The Company used the Black-Scholes option-pricing model in determining the fair value of stock options issued under the 2020 ESOP, and valuation assumptions include expected volatility of
F-34
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
(c) Share-based Payment Award Activity
A summary of the Company’s stock options activity for the 2003 and 2012 Plan is presented below:
|
|
|
Number |
|
|
Weighted |
|
|
Aggregate |
|
|||
|
Outstanding as of January 1, 2022 |
|
|
|
|
$ |
|
|
$ |
|
|||
|
Granted |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Forfeited / Expired |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Outstanding as of December 31, 2022 |
|
|
|
|
$ |
|
|
$ |
|
|||
|
Vested and expected to vest at December 31, 2022 |
|
|
|
|
$ |
|
|
|
|
|||
|
Exercisable as of December 31, 2022 |
|
|
|
|
$ |
|
|
$ |
|
|||
As of December 31, 2022
|
|
Exercise |
|
|
Number of |
|
|
Remaining |
|
|
Average |
|
|
Number |
|
|
Remaining |
|
|
Average |
|
|||||||
|
|
$ |
4.98 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
|
||||||
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
The grant date fair value of options issued under the 2020 ESOP is $
Share-based compensation expense, included in cost of sales, selling, general and administrative expenses and R&D expenses is charged to operations over the vesting period of the options using the straight-line amortization method. The share-based compensation expense was $nil in 2022 (2021 - $
The aggregate intrinsic value of the Company’s stock options is calculated as the difference between the exercise price of the options and the quoted price of the common shares that were in the money. The aggregate intrinsic value of the Company’s stock options exercised under the 2003 Plan and the 2012 Plan was $nil for year ended December 31, 2022, determined as of the date of option exercise (2021 - $
The estimated fair value of stock options vested during the year ended December 31, 2022 was nil (2021 - $nil, 2020 – $nil).
21. Statutory Surplus Reserves
Pursuant to Chinese company law applicable to foreign investment companies, the Company’s PRC subsidiaries are required to maintain statutory surplus reserves. The statutory surplus reserves are to be appropriated from net income after taxes, and should be at least
Sinovac Biomed has not accumulated any profit since inception. No appropriation to the statutory surplus reserves and staff welfare and bonus were made.
Dividends declared by the Company’s PRC subsidiaries are based on the distributable profits as reported in their statutory financial statements reported in accordance with PRC GAAP, which differ from the results of operations reflected in the consolidated financial statements prepared in accordance with US GAAP. The Company’s ability to pay dividends is primarily dependent on the Company
F-35
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
receiving distributions of funds from its PRC subsidiaries. As of December 31, 2022, the Company has $nil dividend payable to common shareholders (December 31, 2021 - $nil), and has $
Under PRC laws and regulations, statutory surplus reserves are restricted to set-off against losses, expansion of production and operation and increasing registered capital of the respective company, and are not distributable other than upon liquidation. Staff welfare and bonus funds are restricted to expenditures for the collective welfare of employees. The reserves are not allowed to be transferred to the Company in terms of cash dividends, loans or advances, nor are they allowed for distribution except under liquidation. Amounts restricted include the PRC subsidiaries’ paid-in capital, additional paid-in capital and statutory surplus reserves of the Company’s PRC subsidiaries totaling $
22. Earnings per Share
The following table sets forth the computation of basic and diluted income attributable to common shareholders of Sinovac per share (in thousands, except for number of shares and per share data):
|
|
|
For the year ended December 31 |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Numerator |
|
|
|
|
|
|
|
|
|
|||
|
Net income |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
Less: Income (loss) attributable to non-controlling interests |
|
|
( |
) |
|
|
|
|
|
|
||
|
Income attributable to shareholders of Sinovac |
|
|
|
|
|
|
|
|
|
|||
|
Less: Preferred stock dividends |
|
|
|
|
|
|
|
|
|
|||
|
Net income attributable to shareholders of Sinovac |
|
|
|
|
|
|
|
|
|
|||
|
Net income attributable to shareholders of Sinovac for computing diluted net income per share |
|
|
|
|
|
|
|
|
|
|||
|
Denominator |
|
|
|
|
|
|
|
|
|
|||
|
Basic weighted average number of common shares outstanding |
|
|
|
|
|
|
|
|
|
|||
|
Dilutive effect of stock options and preferred shares |
|
|
|
|
|
|
|
|
|
|||
|
Diluted weighted average number of common shares outstanding |
|
|
|
|
|
|
|
|
|
|||
|
Earnings per share |
|
|
|
|
|
|
|
|
|
|||
|
Basic net income per share |
|
|
|
|
|
|
|
|
|
|||
|
Diluted net income per share |
|
|
|
|
|
|
|
|
|
|||
As the Company announced on February 22, 2019, the Company’s Board of Directors determined that certain shareholders became Acquiring Persons, and a Trigger Event occurred under the Rights Agreement. As a result,
F-36
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
23. Segment Information
The Company operates exclusively in the biotechnology sector. The Company’s business is considered as operating in
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
Assets |
|
|
|
|
|
|
||
|
Mainland China |
|
$ |
|
|
$ |
|
||
|
Outside Mainland China |
|
|
|
|
|
|
||
|
Total Assets |
|
$ |
|
|
$ |
|
||
The Company’s revenues by market type are as follows:
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Sales |
|
|
|
|
|
|
|
|
|
|||
|
EPI |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
Private Pay |
|
|
|
|
|
|
|
|
|
|||
|
Export |
|
|
|
|
|
|
|
|
|
|||
|
Total Sales |
|
$ |
|
|
$ |
|
|
$ |
|
|||
The Company’s revenues are attributed to geographic locations as follows:
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Sales |
|
|
|
|
|
|
|
|
|
|||
|
Mainland China |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
Outside Mainland China |
|
|
|
|
|
|
|
|
|
|||
|
Total Sales |
|
$ |
|
|
$ |
|
|
$ |
|
|||
F-37
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
24. Collaboration Agreements
Under the terms of the technology transfer agreement, the Company will make milestone payments of up to $
In January 2015, the Company entered into the third amendment to the technology transfer agreement dated March 12, 2009 and the two amendment agreements dated November 17, 2009 and December 24, 2011, respectively. By entering into this third amendment, the technology transfer agreement was amended to be a licensing agreement. The remaining milestone and royalty payments under the technology transfer agreement have been reduced. Both the Company and Tianjin Cansino are free to develop pneumococcal vaccines or to collaborate with other companies for the same purpose. The Company did not make any payment or recorded any research and development expenses for the years ended December 31, 2022, 2021 and 2020, respectively.
The Company has agreed to pay NIH a license issue royalty of $
The Company has agreed to pay INTRAVACC up to $
F-38
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
December 31, 2021 as cost of goods sold, and recorded a milestone fee of $
25. Subsequent Events
Aside from those disclosed in note 18 to the financial statements, no other reportable events or transactions take place after the balance sheet date.
26. Condensed Financial Information of the Parent Company
Balance Sheets
|
|
|
December 31, |
|
|||||
|
|
|
2022 |
|
|
2021 |
|
||
|
ASSETS |
|
|
|
|
|
|
||
|
Current assets |
|
|
|
|
|
|
||
|
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
|
Prepaid expenses and other receivables |
|
|
|
|
|
|
||
|
Amount due from subsidiaries |
|
|
|
|
|
|
||
|
Dividends receivable |
|
|
|
|
|
|
||
|
Total current assets |
|
|
|
|
|
|
||
|
Investment in subsidiaries |
|
|
|
|
|
|
||
|
Total assets |
|
$ |
|
|
$ |
|
||
|
LIABILITIES AND EQUITY |
|
|
|
|
|
|
||
|
Current liabilities |
|
|
|
|
|
|
||
|
Accrued expenses and other payables |
|
$ |
|
|
$ |
|
||
|
Amount due to subsidiaries |
|
|
|
|
|
|
||
|
Dividend payable |
|
|
|
|
|
|
||
|
Total current liabilities |
|
|
|
|
|
|
||
|
Total liabilities |
|
$ |
|
|
$ |
|
||
|
EQUITY |
|
|
|
|
|
|
||
|
Preferred stock |
|
|
|
|
|
|
||
|
Authorized |
|
|
|
|
|
|
||
|
Issued and outstanding: |
|
|
|
|
|
|
||
|
Common stock |
|
|
|
|
|
|
||
|
Authorized: |
|
|
|
|
|
|
||
|
Issued and outstanding: |
|
|
|
|
|
|
||
|
Additional paid-in capital |
|
|
|
|
|
|
||
|
Subscriptions receivable |
|
|
|
|
|
( |
) |
|
|
Accumulated other comprehensive income (loss) |
|
|
( |
) |
|
|
|
|
|
Retained earnings |
|
|
|
|
|
|
||
|
Total shareholders' equity |
|
|
|
|
|
|
||
|
Total liabilities and equity |
|
$ |
|
|
$ |
|
||
Statements of Comprehensive Income (Loss)
F-39
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
|
|
|
For the year ended December 31 |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Selling, general and administrative expenses |
|
|
|
|
|
|
|
|
|
|||
|
Total operating expenses |
|
|
|
|
|
|
|
|
|
|||
|
Loss from operations |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Other income (expenses) |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
Interest income |
|
|
|
|
|
|
|
|
|
|||
|
Equity earnings of subsidiaries, net of tax |
|
|
|
|
|
|
|
|
|
|||
|
Net income |
|
|
|
|
|
|
|
|
|
|||
|
Preferred stock dividends |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Net income attributable to common shareholders |
|
|
|
|
|
|
|
|
|
|||
|
Net income |
|
|
|
|
|
|
|
|
|
|||
|
Foreign currency translation adjustments |
|
|
( |
) |
|
|
|
|
|
|
||
|
Total comprehensive (loss) / income |
|
$ |
( |
) |
|
$ |
|
|
$ |
|
||
Statements of Cash Flows
|
|
|
For the year ended December 31 |
|
|||||||||
|
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
|
Operating activities |
|
|
|
|
|
|
|
|
|
|||
|
Net income |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
Adjustments to reconcile net loss to net cash provided by (used in) |
|
|
|
|
|
|
|
|
|
|||
|
- Share-based compensation |
|
|
|
|
|
|
|
|
|
|||
|
- Equity in earnings of subsidiaries |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Changes in: |
|
|
|
|
|
|
|
|
|
|||
|
- Amount due from subsidiaries |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
- Prepaid expenses and other receivables |
|
|
|
|
|
|
|
|
( |
) |
||
|
- Amount due to subsidiaries |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
- Accrued expenses and other payables |
|
|
( |
) |
|
|
|
|
|
|
||
|
Net cash (used in) provided by operating activities |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
Financing activities |
|
|
|
|
|
|
|
|
|
|||
|
- Proceeds from issuance of common stock, net of share issuance costs |
|
|
|
|
|
|
|
|
|
|||
|
Net cash provided by financing activities |
|
|
|
|
|
|
|
|
|
|||
|
(Decrease) increase in cash and cash equivalents |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
Cash and cash equivalents, beginning of year |
|
|
|
|
|
|
|
|
|
|||
|
Cash and cash equivalents, end of year |
|
$ |
|
|
$ |
|
|
$ |
|
|||
(a) Basis of presentation
The condensed financial information has been prepared using the same accounting policies as set out in the accompanying consolidated financial statements except that the Company used the equity method to account for investment in its subsidiaries.
The Company records its investment in its subsidiaries under the equity method of accounting. Such investment is presented on the balance sheets as “Investment in subsidiaries” and share of their income (loss) as “Equity earnings (losses) of subsidiaries” in the statements of comprehensive income (loss).
F-40
SINOVAC BIOTECH LTD.
Notes to Consolidated Financial Statements
(Expressed in thousands of U.S. dollars, unless otherwise stated)
Each of the Company’s PRC subsidiaries has restrictions on its ability to pay dividends to the Company under PRC laws and regulations (Note 21). The subsidiaries did not pay any dividends to the Company for the years presented.
Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted by reference to the consolidated financial statements.
(b) Commitments
The Company does not have any significant commitments or long-term obligations as of any of the periods presented, except for those disclosed in the consolidated financial statements (notes 18 and 24).
F-41