UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________________________________________________
FORM 10-K
_________________________________________________________________
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the fiscal year ended December 31 , 2021
or
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the transition period from to
COMMISSION FILE NUMBER 000-31161
_________________________________________________________________
(Exact name of registrant as specified in its charter)
_________________________________________________________________
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||||||
| (Address of principal executive offices) | (Zip Code) | |||||||
(Registrant’s telephone number, including area code)
Securities registered pursuant to 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
Securities registered pursuant to 12(g) of the Act: None
_________________________________________________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| ☒ | Accelerated filer | ☐ | ||||||||||||
| Non-accelerated filer | ☐ | Smaller reporting company | ||||||||||||
| Emerging growth company | ||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $4.2 billion as of June 30, 2021, based on the last sale price of the registrant’s common stock as reported on the Nasdaq Global Select Market on such date. For purposes of this calculation, shares of the registrant’s common stock held by directors and executive officers have been excluded. This number is provided only for purposes of this Annual Report on Form 10-K and does not represent an admission that any particular person or entity is an affiliate of the registrant.
As of February 17, 2022, there were 61,659,385 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
ARENA PHARMACEUTICALS, INC.
FORM 10-K – ANNUAL REPORT
For the Fiscal Year Ended December 31, 2021
Table of Contents
| Page | ||||||||
i
INFORMATION RELATING TO FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, or Annual Report, includes forward-looking statements, which involve a number of risks and uncertainties. These forward-looking statements can generally be identified as such because the context of the statement will include words such as “may,” “could,” “will,” “intend,” “plan,” “believe,” “anticipate,” “expect,” “estimate,” “predict,” “potential,” “prospect,” “continue,” “likely,” “opportunity,” “focused on,” “evaluating for,” “in development for,” the negative of these words or other similar words. Similarly, statements that describe our future plans, strategies, intentions, expectations, objectives, goals or prospects and other statements that are not historical facts are also forward-looking statements. Discussions containing these forward-looking statements may be found, among other places, in “Business” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Annual Report. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. Readers of this Annual Report are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the time this Annual Report was filed with the Securities and Exchange Commission, or SEC. These forward-looking statements are based largely on our expectations and projections about future events and future trends affecting our business and are subject to risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. These risks and uncertainties include, without limitation, those discussed in “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Annual Report. In addition, past financial or operating performance is not necessarily a reliable indicator of future performance, and you should not use our historical performance to anticipate results or future period trends. We can give no assurances that any of the events anticipated by the forward-looking statements will occur or, if any of them do, what impact they will have on our results of operations and financial condition. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and you are cautioned not to unduly rely upon these statements. Except as required by law, we undertake no obligation to update publicly or revise our forward-looking statements to reflect events or circumstances that arise after the filing of this Annual Report or documents incorporated by reference herein that include forward-looking statements.
TRADEMARKS AND CERTAIN TERMS
In this Annual Report, “Arena Pharmaceuticals,” “Arena,” “Company,” “we,” “us” and “our” refer to Arena Pharmaceuticals, Inc., and our wholly owned subsidiaries on a consolidated basis, unless the context otherwise provides. “APD” is an abbreviation for Arena Pharmaceuticals Development.
Arena Pharmaceuticals ® and Arena ® are registered service marks of Arena. Any other brand names or trademarks appearing in this Annual Report are the property of their respective holders.
RISK FACTOR SUMMARY
Below is a summary of the material factors that make an investment in our stock speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below under the heading “Risk Factors” in Item 1A of Part I of this Annual Report and should be carefully considered, together with other information in this Annual Report and our other filings with the Securities and Exchange Commission, or SEC, before making investment decisions regarding our stock.
•Failure to complete, or delays in completing, the pending transaction with Pfizer Inc. announced on December 13, 2021 could materially and adversely affect our results of operations and our stock price.
•Drug development programs are expensive, time consuming, uncertain, and susceptible to change, interruption, delay, or termination.
•We will need to obtain additional funds or enter into collaboration agreements to execute on our corporate strategy, and we may not be able to do so at all or on terms you view as favorable; your ownership may be substantially diluted if we do obtain additional funds; you may not agree with the manner in which we allocate our available resources; and we may not be profitable.
•Our business may be negatively impacted based on the clinical trials and preclinical studies of, and decisions affecting, one or more of our drug candidates.
1
•The development, approval, or commercialization of any of our drug candidates could be negatively affected by circumstances related to other drug candidates or approved products.
•Topline data may not accurately reflect the complete results of a particular study or trial.
•The results of preclinical studies and completed clinical trials are not necessarily predictive of future results, and our current drug candidates or any approved drugs may not be further developed or have favorable results in later studies or trials.
•Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
•Drug discovery and development is intensely competitive in the therapeutic areas on which we focus. If the number of our competitors increase or they develop treatments that are approved faster, marketed better, less expensive or demonstrated to be more effective or safer than our drugs or drug candidates, our commercial opportunities could be reduced or eliminated.
•Our ability to generate revenues from any of our drugs that receive regulatory approval will be subject to a variety of risks, many of which are out of our control. For example, even if approved, our drugs may not be commercially successful for various reasons, including if they are not widely covered and adequately reimbursed by third-party payers.
•Our efforts will be seriously jeopardized if we are unable to attract and retain key and other employees or manage the growth of our organization.
•We rely on other companies to carry out certain key activities, including conducting clinical trials and preclinical studies and manufacturing all our drugs and drug candidates.
•Our business, including our preclinical and clinical programs, may be significantly and adversely affected by the COVID-19 pandemic.
•Our success is dependent on intellectual property rights held by us and third parties and our interest in these rights is complex and uncertain. We cannot protect these rights throughout the world.
2
PART I
Item 1. Business.
Overview
We are a biopharmaceutical company focused on delivering novel, transformational medicines with optimized pharmacology and pharmacokinetics to patients globally. Our internally developed pipeline includes multiple potentially first- or best-in-class assets with broad clinical utility.
Our most advanced investigational clinical programs include:
•Etrasimod, which we are evaluating in a Phase 3 program for ulcerative colitis, or UC, a Phase 2b/3 program for Crohn’s disease, or CD, a Phase 2 program in alopecia areata, or AA, and a Phase 2b program for eosinophilic esophagitis, or EoE. We also plan to evaluate etrasimod in a Phase 3 program in atopic dermatitis, or AD.
•APD418, for acute heart failure, or AHF, which we are evaluating in a Phase 2 trial.
•Temanogrel, a second compound in our cardiovascular therapeutic area, which we are evaluating in a Phase 2 trial for microvascular obstruction, or MVO, and a Phase 2 trial for Raynaud’s phenomenon secondary to systemic sclerosis, or SSc-RP.
•Olorinab, which we were evaluating for a broad range of visceral pain conditions associated with gastrointestinal diseases. We are evaluating possible strategic options for olorinab.
We continue to leverage our two decades of world-class G-protein-coupled receptor, or GPCR, target discovery research to develop breakthrough drugs and ultimately deliver these to patients with unmet needs. Our long-term pipeline prospects include a collaboration with Beacon Discovery, Inc., or Beacon, across a broad range of immune-mediated inflammatory targets and compounds.
We have license agreements or collaborations with various companies, including:
•United Therapeutics (ralinepag in a Phase 3 program for pulmonary arterial hypertension),
•Everest Medicines Limited (etrasimod in a Phase 3 program for UC in Greater China and select countries in Asia),
•Beacon Discovery (early research platform for GPCR targets),
•Boehringer Ingelheim International GmbH (undisclosed orphan GPCR program for central nervous system – preclinical), and
•Aristea Therapeutics (RIST4721 in a Phase 2 program for the treatment of palmoplantar pustulosis and other neutrophil-mediated diseases).
In response to the COVID-19 pandemic, substantially all of our workforce began working from home in March 2020, either all or substantially all of the time, and continues to do so as of the date of this filing. While many jurisdictions have lifted COVID-19-related restrictions, the continued spread of the coronavirus and its variants continues to cause a risk of potential delays in site initiation and participant enrollment and screening rates in certain of our clinical development programs. The potential impact, if any, that these site-level delays could have on our development program timelines remains uncertain, depending on a variety of factors including vaccination rates, which vary greatly from region to region, and the emergence and spread of new variants of the coronavirus. Our future research and development expenses and selling, general and administrative expenses may vary significantly if we experience an increased impact from COVID-19 on the costs and timing associated with the conduct of our clinical trials and other related business activities. For further information, refer to “Part I - Item 1A - Risk Factors” of this Annual Report.
On December 12, 2021, Arena entered into an Agreement and Plan of Merger with Pfizer Inc., a Delaware corporation, and Antioch Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of Pfizer Inc., providing for, among other things, the merger of Merger Sub with and into Arena, with Arena surviving the Merger. For further
3
information, refer to “Part II - Item 7 - Management's Discussion and Analysis of Financial Condition and Results of Operations” of this Annual Report.
Our Strategy
The primary elements of our strategy are:
•Develop etrasimod – a modulator of the sphingosine 1-phosphate, or S1P, receptor intended for the treatment of a broad range of immune-mediated inflammatory diseases including gastrointestinal and dermatologic diseases;
•Evaluate possible strategic options for olorinab – an agonist of the cannabinoid receptor 2, or CB2, intended for the treatment of a range of visceral pain associated with gastrointestinal conditions;
•Develop APD418 – a β3-AdrR antagonist and cardiac myotrope intended for AHF;
•Develop temanogrel – a peripherally acting and selective 5HT2a inverse agonist intended for MVO and SSc-RP;
•Progress a broad early-stage pipeline build with Beacon; consider other external opportunities with potential for transformational innovation in our disease areas of interest;
•Develop our pipeline by efficiently managing our cash and development timelines, which may include entering strategic agreements for certain clinical and preclinical programs;
•Explore existing compounds in new indications and progress additional pipeline programs over time in select therapeutic areas; and
•Prudently build a vibrant, sustainable, high-performing organization.
Pipeline of Development Programs
Below is a summary of our internally developed, proprietary portfolio:

Etrasimod Program
Etrasimod is a next-generation, oral, highly selective sphingosine 1-phosphate, or S1P, receptor modulator, discovered by Arena, designed to provide systemic and local cell modulation by selectively targeting S1P receptor subtypes 1, 4 and 5. S1P receptors have been demonstrated to be involved in the modulation of several biological responses, including lymphocyte trafficking from lymph nodes to the peripheral blood. By isolating subpopulations of lymphocytes in lymph nodes, fewer immune cells are available in the circulating blood to effect tissue damage. Etrasimod has therapeutic
4
potential in immune-mediated inflammatory disease areas including gastroenterology and dermatology. We are currently evaluating etrasimod in ulcerative colitis, Crohn’s disease, eosinophilic esophagitis, atopic dermatitis, and alopecia areata.
Gastrointestinal Diseases
Inflammatory bowel diseases
Inflammatory bowel diseases, or IBD, like UC and CD are chronic life-long immune-mediated inflammatory conditions of the gastrointestinal tract that affect approximately 1.7 million patients in the United States, or US, alone. The prevalence of UC and CD in the US are currently estimated at approximately 0.9 million and 0.8 million patients, respectively. The prevalence of IBD in France, Germany, Italy and Spain collectively, or EU4, and the United Kingdom, is estimated at 1.3 million with approximately 0.7 million patients with UC and 0.6 million patients with CD. Both conditions represent a significant burden to patients, including hospitalization, surgery, and a longer-term risk of colon cancer, as well as impaired quality of life, economic productivity and social functioning. Additionally, Japan has an estimated prevalence of approximately 0.2 million IBD patients. In aggregate, there are approximately 3.2 million patients across the US, EU4, the United Kingdom, and Japan that are currently living with IBD.
UC is characterized by contiguous mucosal inflammation limited to the colon which involves the rectum in approximately 95% of cases and may extend to involve parts or all of the large intestine. In contrast, CD is characterized by full thickness inflammation that can occur anywhere in the gastrointestinal, or GI, tract but most typically involves the terminal ileum and colon; and causes fistulation and scarring. Symptoms for UC and CD can vary, depending on the location and severity of inflammation, but some of the most common are diarrhea, abdominal cramps, and rectal bleeding.
An important goal of therapy for IBD is durable remission while improving the patient’s quality of life. Although a number of therapies are approved for the treatment of IBD, they are often associated with an inability to induce or maintain remission, serious side effects, and complicated administration regimens. There is therefore an unmet medical need for novel oral agents with an enhanced risk-benefit profile and more convenient administration for the treatment of moderately to severely active IBD.
Eosinophilic Esophagitis
Eosinophilic esophagitis, or EoE, is a chronic, relapsing and remitting, immune mediated disease of the esophagus driven by an allergic Th2 T cell inflammatory profile. Histologically, EoE is characterized by the accumulation of eosinophils in the lining of the esophagus, a tissue that under normal conditions lacks these cells. In addition to eosinophils, CD4 and CD8 T cells, dendritic cells and mast cells increase in the tissue. The presence of these unwelcome inflammatory cells has been shown to have a direct effect on immune function and tissue damage. Both pediatric and adult populations can develop EoE, however, the disorder is most common in individuals between the ages of 20 and 40 years old. EoE also exhibits a strong hereditability pattern and predominance for males and for Caucasians, and principally in socioeconomically developed countries. EoE affects approximately 135,000 patients of all ages in the US and an estimated 132,000 patients in the EU4 and United Kingdom. There are no approved therapies in the US for the treatment of EoE. We believe that etrasimod may represent a significant opportunity to provide an effective treatment to EoE due to its potential to modulate the trafficking of multiple immune subsets.
Dermatologic Conditions
Atopic Dermatitis
Atopic dermatitis, or AD, is a chronic, inflammatory skin disorder characterized by dry skin, pruritus, and relapsing lesions. AD has a severe impact on quality of life, including potential occupational, social, and psychological impairments. The adult diagnosed prevalence is approximately 8.5 million patients in the US, and 6.5 million patients in the EU4 and United Kingdom. According to a recent survey with dermatologists in the US, 55% of dermatologists reported a high unmet need in atopic dermatitis.
Two new therapies have been marketed in the US since 2016, however these treatments have less desirable administration routes and are not effective in all patients. Therefore, we believe a significant unmet need remains for differentiated, safe, oral agents that are effective and have a favorable side effect profile. AD pathology is driven by a combination of impaired skin epithelial barriers, altered microbiota, and aberrant inflammation driven by activated immune cells, including skin-infiltrating T cells and dendritic cells, or DCs. Etrasimod may have the potential to reduce DC migration/activation (S1P receptor subtypes 1 and 4 mediated) and T cell infiltration (S1P receptor subtype 1 mediated) in
5
the skin. These effects could reduce the T cell-mediated inflammation in the skin that underlies atopic dermatitis pathogenesis.
Alopecia Areata
Alopecia areata is a T-cell-mediated autoimmune skin disorder with unmet medical need that causes non-scarring patchy hair loss, most often on the scalp. The prevalence is approximately 2.6 million patients in the US and 2.1 million in the EU4 and United Kingdom.
The disease may be limited to 1 or more discrete, round or oval patches of hair loss the size of a coin on the scalp, or it may progress to full hair loss of the scalp (alopecia totalis) or the entire body (alopecia universalis). The course of disease is unpredictable, with spontaneous regrowth of hair occurring in 80% of patients within the first year, and sudden relapse at any given time. Patients with extensive disease (at least 50% total scalp hair loss) rarely have spontaneous hair regrowth. Patients with persistent moderate-to-severe AA also often suffer tremendous emotional and psychosocial distress and reduced quality of life as a result of their hair loss. Thus, psychosocial support and therapy is an important part of disease management, as this often-disfiguring disease can be psychosocially burdensome. The estimated lifetime risk of AA is 2% among the general population and represents the second most common form of human hair loss, second only to androgenetic alopecia. The current standard of care is injected corticosteroids which have limitations in terms of efficacy. Etrasimod may have the potential to reduce circulating CD4+ and CD8+ lymphocytes available to infiltrate the hair follicle which may decrease inflammation and restore hair growth.
Etrasimod Development
Inflammatory Bowel Disease
We are currently in a Phase 3 program in UC and a Phase 2b/3 program in CD.
ELEVATE UC constitutes our Phase 3 global registrational program to assess the safety and efficacy of once-daily etrasimod 2 mg in participants with moderately to severely active UC. In January 2021, the pivotal, 52-week ELEVATE UC 52 trial completed enrollment. In August 2021, the pivotal, 12-week ELEVATE UC 12 trial reached full enrollment. We expect topline data from both ELEVATE UC 12 and ELEVATE UC 52 in the first quarter of 2022.
In April 2021, we enrolled the first patient in a Phase 2 trial evaluating etrasimod for the potential treatment of moderately active UC, called GLADIATOR UC. This trial is a multicenter, randomized, placebo-controlled 52-week trial to assess the safety and efficacy of once-daily etrasimod 2 mg in participants with moderately active UC. This trial is intended to evaluate etrasimod in a less severe patient population than ELEVATE UC, potentially broadening the patient population and demonstrating the potential use of a once-a-day oral therapy earlier in the treatment paradigm.
In August 2021, we increased the target enrollment for our Phase 2/3 CULTIVATE Study A for etrasimod in CD from 50 to 70 participants and reached target enrollment in November 2021. We expect dose-ranging data to read out in the second quarter of 2022. Study A is a Phase 2, multicenter, randomized 14-week trial, intended to provide supportive data for dose selection evaluating 2 mg and 3 mg etrasimod in subjects with moderately to severely active CD. The Phase 2/3 program also consists of a Phase 2b dose-ranging multicenter, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of once-daily etrasimod in participants with moderately to severely active CD. The Phase 3 portion of the program will include two induction trials, with re-randomization of clinical responders into a single maintenance trial. The program is intended to provide an operationally seamless transition from Phase 2 to Phase 3. We have withdrawn previously announced overall program guidance for CULTIVATE based on the expected COVID-19 impact on trial execution, including site activation and participant enrollment.
In 2019, we announced positive results from a 34-week open-label extension, or OLE, of the Phase 2 OASIS trial of etrasimod for the treatment of UC. The trial enrolled 118 participants (84% of OASIS study completers), of which 22 completers also received 2 mg in OASIS, for a total of 46 weeks of treatment with etrasimod. Overall, etrasimod demonstrated durable, long-term clinical remission and was generally well tolerated in this trial. Adverse events in the OLE study were generally mild to moderate in severity and no new safety findings were noted. Impact on heart rate and atrioventricular, or AV, conduction was minimal throughout the study with no discontinuations from study related to bradycardia or AV block.
In 2018, we announced topline results from OASIS, a dose finding 12-week randomized, double-blind, placebo-controlled multinational Phase 2 clinical trial of etrasimod in moderate to severe UC. The aim of the trial was to
6
investigate dose response and compare the active arm(s) to placebo. The trial evaluated the effects of etrasimod at 1 mg and 2 mg versus placebo on multiple efficacy measures including a three-component partial Mayo Clinic Score, clinical remission, clinical response, and endoscopic improvement in 156 participants. Etrasimod demonstrated a clear dose response and statistically significant improvements versus placebo in the primary, all secondary, and clinical remission endpoints at the 2 mg dose. There were fewer participants with serious adverse events, or SAEs, compared to placebo (0% in 2 mg, 5.8% in 1 mg and 11.1% in placebo). Impact on heart rate and AV conduction was low throughout the study with no discontinuations from study related to bradycardia or AV block. There were no increases in liver function tests compared to placebo and no reports of macular edema or pulmonary function test abnormalities. In this trial, etrasimod was well tolerated.
Eosinophilic Esophagitis
We are recruiting for a Phase 2b program for EoE. In February 2021, we dosed the first participant in the Phase 2b VOYAGE trial for etrasimod in EoE. Our Phase 2b VOYAGE trial of etrasimod is a randomized, double-blind, placebo-controlled trial, with a primary efficacy measurement at week 16 and a secondary efficacy analysis at week 24, to assess the safety and efficacy of 1 mg and 2 mg etrasimod in approximately 100 adult participants with EoE. In June 2021, the FDA granted Orphan Drug Designation status to etrasimod for the treatment of EoE.
Atopic Dermatitis
We are currently in planning for a Phase 3 program for AD and have ongoing discussions with regulatory authorities on trial design.
In July 2021, we evaluated an updated open-label extension data set from the Phase 2 ADVISE trial for 2 mg etrasimod in atopic dermatitis that demonstrated meaningful effects at week 16 of the OLE period on validated Investigator Global Assessment, or vIGA, at 47%, Eczema Area and Severity Index, or EASI-75, at 72%, and Peak Pruritis Numeric Rating Scale, or PP-NRS, at 61% with consistent safety profile out to one year.
In 2020, we announced topline results from ADVISE, a multicenter, randomized, double-blind, placebo-controlled Phase 2b trial evaluating safety and efficacy of once-daily etrasimod in approximately 140 participants with moderate to severe AD. The primary endpoint measured was percent change in EASI from baseline to week 12, followed by a 4-week follow-up observation period. Secondary endpoints included the proportion of participants achieving EASI-75, proportion of participants with a vIGA 0 to 1, and percent change in peak pruritis. In the primary analysis, nearly one-third of participants in the 2 mg etrasimod group achieved clear or almost clear skin, as defined by the vIGA, which is the FDA endpoint for Phase 3 registration. The vIGA improvement was statistically significant as compared to placebo at 12 weeks. Across EASI-75 and peak change in pruritis, etrasimod 2 mg demonstrated early and statistically significant effect at week 4. Etrasimod did not meet the Phase 2b primary endpoint of EASI change from baseline at week 12 as compared to placebo. Between weeks 4-8, the trial was impacted by unwarranted dose interruption in 19% (n=9) of the etrasimod 2 mg group. Adjusting for this dose interruption, a post-hoc Completer Analysis, which included only those participants receiving full therapeutic exposure of etrasimod 2 mg, showed statistically significant effect on the EASI score compared to placebo (weeks 4 and 12), EASI-75 at week 4, vIGA at week 12 and pruritis through week 8. The Completer Analysis also showed no plateau of effect over the 12-week trial. Overall, the safety profile was consistent with previous trials of etrasimod including low first-dose heart rate effect with no titration, and no serious adverse events across the groups. Additionally, none of the other adverse events commonly associated with first-generation S1P receptor modulators were seen in this trial.
Alopecia Areata
We are currently recruiting a Phase 2 program in Alopecia Areata.
In July 2021, the Phase 2 trial for etrasimod in AA was amended to add a 3 mg cohort and expand patient population subtypes. The Phase 2 randomized, placebo-controlled trial will assess the safety and efficacy of once-daily etrasimod 2 mg and 3 mg in 78 participants with moderate-to-severe AA. We expect to announce topline data for this trial in the second half of 2022.
Etrasimod Controlled-Release Formulation
In April 2020, we announced data from a Phase 1 clinical trial evaluating a controlled-release delivery profile for etrasimod.
7
The controlled-release profiles were designed to retain etrasimod's rapid onset of action while further improving its potentially best-in-class non-titrated, low intrinsic first-dose heart rate effect. A controlled-release formulation has the potential to deliver rapid life-cycle management and extend etrasimod's intellectual property portfolio.
Based on the results, we have initiated a product development program.
Prior Development
In January 2015, we announced top-line results from a Phase 1b multiple-ascending dose clinical trial for etrasimod. In this trial, etrasimod demonstrated a dose-dependent effect on lymphocyte count lowering in blood, with mean decreases from baseline of up to 69%. Lymphocyte counts, on average, recovered to baseline within one week of conclusion of dosing. There was a modest impact on heart rate, but none of the changes were classified by the investigator as clinically significant. There were also no findings with respect to pulmonary function or liver enzyme tests that were classified by the investigator as clinically significant. The most common treatment-emergent adverse events were mild or moderate contact dermatitis, headache, constipation and diarrhea, with none being clearly drug related. There were no discontinuations for adverse events, and no serious adverse events were observed.
The randomized, double-blind, placebo-controlled Phase 1b clinical trial evaluated the safety, tolerability, pharmacodynamics and pharmacokinetics of multiple-ascending doses of etrasimod. In five different dosing cohorts, 50 healthy volunteers received etrasimod and 10 healthy volunteers received placebo for 21 days.
Prior to commencing the Phase 1b multiple-ascending dose clinical trial for etrasimod, we completed a Phase 1 single-ascending dose clinical trial of the compound. This randomized, double-blind and placebo-controlled trial evaluated the safety, tolerability and pharmacokinetics of single-ascending doses of etrasimod in 40 healthy adult volunteers. In the trial, etrasimod demonstrated favorable pharmacokinetic and pharmacodynamic effects, a dose-responsive reduction in blood lymphocyte count and a slowing of heart rate that appears comparable to other S1P receptor modulators. The terminal half-life was approximately 35 hours.
Etrasimod Intellectual Property
As of February 4, 2022, we owned issued patents that cover compositions of matter for etrasimod and related compounds, and methods of treatment utilizing etrasimod and related compounds, in 62 jurisdictions, including the United States, China, Japan, Germany, France, Italy, the United Kingdom, Brazil, Spain, Canada, India, Russia, South Korea and Australia. The earliest priority date for the patents on etrasimod is 2008. The terms of these patents are capable of continuing into 2029 in most jurisdictions without taking into account any patent term adjustment or extension regimes of any country or any additional term of exclusivity we might obtain by virtue of the later filed patent applications.
APD418 Program
APD418 is a potential first-in-class β3-adrenergic receptor (AdrR) antagonist and cardiac myotrope designed to regulate myofilament calcium sensitivity in order to improve contractility without inducing the serious adverse events associated with currently available inotropes. APD418 is in early-stage clinical development for patients with AHF.
AHF is broadly defined as a rapid onset of new or worsening signs and symptoms of HF. It is a potentially life-threatening condition, requiring hospitalization, and emergency treatment is aimed predominantly at managing fluid overload and achieving hemodynamic stability. In most cases, hospitalization for AHF results when the neurohormonal compensatory mechanisms, which act to maintain hemodynamic stability in chronic heart failure, are inadequate.
Management of AHF is focused on treating clinical conditions and precipitating factors, immediate stabilization, and symptom relief. Most patients presenting to the emergency department with AHF are congested and have normal to high blood pressures. Diuretics are first line therapy to treat volume overload including congestion; vasodilators are also used as first line therapy for symptom relief in normotensive and hypertensive patients who fail to respond adequately to diuretics. Unfortunately, neither diuretics nor vasodilators have been associated with improved survival.
For patients presenting as hypotensive, who are at the highest risk of mortality, and patients who fail to adequately respond to initial therapy, treatment options are particularly limited and may require the use of inotropic agents. These agents are associated with significant risk of unwanted hemodynamic effects, arrhythmias, and pathway dependent cardiotoxicity, leading to adverse outcomes and increased mortality.
8
Arena is developing APD418, an internally discovered and developed investigational drug candidate, to address the significant unmet need in AHF.
APD418 Development
In July 2021, we announced that a Phase 2 trial for APD418 was initiated. The randomized, double-blind, placebo-controlled trial is intended to evaluate the safety, pharmacokinetics, and effect on cardiac function of intravenous APD418 in approximately 80 adult participants with heart failure with reduced ejection fraction.
In November 2020, we announced the completion of a first-in-human Phase 1 safety and tolerability trial of APD418. The trial found APD418 was generally well tolerated.
The FDA has granted us Fast Track designation for development of APD418 in AHF.
APD418 Intellectual Property
As of February 4, 2022, we owned issued patents covering compositions of matter for APD418 and related compounds, and methods of treatment utilizing APD418 and related compounds, in the United States, Japan, Australia, and three other jurisdictions, and we had applications pending in 37 other jurisdictions, of which the ones with the largest pharmaceutical markets were Europe and China. The earliest priority date for the patents on APD418 is 2016. The terms of these patents are capable of continuing into 2037 without taking into account any patent term extension.
Temanogrel Program
Temanogrel is a peripherally acting and selective 5HT2a inverse agonist designed to inhibit serotonin (5-HT)-mediated amplification of platelet aggregation and vasoconstriction.
Microvascular Obstruction
A significant proportion of patients undergoing percutaneous coronary intervention, or PCI, for treatment of acute coronary syndrome, or ACS, fail to achieve full myocardial or microcirculatory reperfusion despite resolution of the epicardial coronary occlusion. This condition is referred to as MVO and has been reported in approximately 40% to 60% patients undergoing PCI for ST-elevation myocardial infraction, or STEMI, and approximately 30% patients undergoing PCI for non-ST-elevation myocardial infarction, or NSTEMI, with the greatest incidence in high-risk NSTEMI patients. Importantly, the presence of MVO post-PCI has been associated with worse clinical outcomes, including mortality and hospitalization for heart failure, related to suboptimal cardiac function and recovery in the days and months following the PCI procedure.
During PCI, platelets become activated following rupture of the atherosclerotic plaque, which can be either procedure-induced or occur spontaneously. Upon activation, platelets release 5-HT in substantial quantities. Serotonin acts on 5-HT2A receptors on platelets and smooth muscle cells, resulting in amplification of platelet aggregation and vasoconstriction, both of which may detrimentally affect the microcirculation. We believe blocking these 5-HT-mediated effects with temanogrel during and following PCI for STEMI and NSTEMI may prevent or minimize the development of MVO and facilitate cardiac functional recovery, resulting in improved clinical outcomes.
Raynaud’s Phenomenon Secondary to Systemic Sclerosis
Raynaud’s phenomenon in SSc is associated with more severe disease manifestation owing to a complex underlying pathophysiology, including vasculopathy and endothelial dysfunction, which detrimentally affect the microcirculation. Intravascular factors, including platelet activation and 5-HT release, likely also contribute to Raynaud’s phenomenon in SSc.
Raynaud’s phenomenon in SSc is commonly triggered by cold temperature, which is known to enhance activation of platelets. In addition, platelets from SSc patients have an increased aggregation response to collagen or 5-HT compared to platelets from healthy individuals, suggesting hypersensitivity of platelets may contribute to the elevated levels of 5-HT observed in patients with SSc-RP. It has been reported that 5-HT induces arterial vasoconstriction and reduces blood flow in human hands to a similar extent as observed with exposure to cold, and that 5-HT2A blockade attenuates these 5-HT-induced effects. In addition, enhanced vasoconstriction response to 5-HT has been observed in SSc patients compared to healthy individuals.
9
Importantly, the relevance of 5-HT and the 5-HT2A pathway in SSc-RP has been evaluated in clinical studies using ketanserin and sarpogrelate, which indicated that 5-HT2A antagonism may provide beneficial effects on both digital blood flow and improvement of Raynaud’s symptoms.
Temanogrel Development
Microvascular Obstruction
We are recruiting for a Phase 2b program for MVO.
In June 2021, the first participant was randomized in the Phase 2 trial for temanogrel in MVO. The randomized, double-blind, placebo-controlled trial is intended to evaluate the safety and effect on MVO of intravenous temanogrel in approximately 99 adult participants undergoing percutaneous coronary intervention.
The FDA has granted us Fast Track designation for development of temanogrel for MVO.
Raynaud’s Phenomenon Secondary to Systemic Sclerosis
We are recruiting for a Phase 2b program for SSc-RP.
In November 2021, the first participant was randomized in the Phase 2 trial for temanogrel in SSc-RP. The randomized, double-blind, placebo-controlled, crossover trial is intended to evaluate the safety and effect of oral temanogrel on digital blood flow in approximately 48 participants with SSc-RP.
Prior Development
We have completed four Phase 1 studies with the oral formulation and one Phase 1 study with the IV formulation of temanogrel.
Temanogrel Intellectual Property
As of February 4, 2022, we owned issued patents that cover compositions of matter for temanogrel and related compounds, and methods of treatment utilizing temanogrel and related compounds, in 9 jurisdictions, including the United States, Japan, Germany, France, Italy, the United Kingdom, and Spain. The earliest priority date for the patents on temanogrel is 2004. The terms of these patents are capable of continuing into 2025 in most jurisdictions without taking into account any patent term adjustment or extension regimes of any country or any additional term of exclusivity we might obtain by virtue of the later filed patent applications.
Olorinab Program
Olorinab, a potentially first-in-class, orally available, potent, peripherally acting, highly selective, full agonist of the CB2 receptor, is an internally discovered investigational drug candidate for the treatment of visceral pain.
The CB2 receptor is expressed in the GI nervous system, and in many tissues and organs of the abdomen. CB2 receptors are found on immune cells but also on microglia, terminal neurons, dorsal root ganglia, and on visceral sensory neurons. We believe selectively targeting the CB2 receptor may provide therapeutic benefit for visceral pain without the potential for dependence, abuse, and GI and cardiovascular side effects associated with opiates or nonsteroidal anti-inflammatory drugs, or NSAIDs, which are among the most common pain relievers. In addition to analgesic effects, olorinab may have anti-inflammatory properties.
Olorinab is designed to be a peripherally acting and selective CB2 receptor agonist and is intended to provide pain relief without the unwanted side effects associated with CB1 receptor activation.
Olorinab Development
In March 2021, we announced topline results from the CAPTIVATE trial for treatment of abdominal pain associated with irritable bowel syndrome and showed that although olorinab was well tolerated it did not meet the primary efficacy endpoint of statistically significant improvement in the overall AAPS from baseline to week 12. CAPTIVATE was a Phase 2, 4-arm multi-center, randomized, double-blind, placebo-controlled, 12-week trial to assess the safety and efficacy of olorinab administered three times daily in 273 participants. In CAPTIVATE participants were randomized to placebo, 10 mg, 25 mg or 50 mg. A pre-specified analysis assessed participants with baseline AAPS ≥ 6.5 (median), representing those
10
with moderate to severe pain. This subgroup accounted for 50% of the overall study population. Within this subgroup, the 50 mg treatment group showed a clinically meaningful and statistically significant (p=0.01) reduction in AAPS of 1.64 points compared to placebo and 3.93 points from baseline at week 12. Olorinab was generally safe and well tolerated in the study, consistent with the safety profile of previous trials. Discontinuation rates and adverse events were similar to placebo, notably with no worsening of bowel habits and no treatment interruptions. There were no serious adverse events observed in the study. We are evaluating possible strategic options for olorinab.
Prior Development
In 2018, we announced positive topline results from our Phase 2a trial of olorinab in development for the treatment of pain associated with CD. This exploratory study was an open‑label investigation to evaluate safety and tolerability of olorinab in this patient population and to gain initial insights into its efficacy via a pain visual analog scale, or VAS. Fourteen participants were enrolled into two cohorts at 25 mg and 100 mg administered three times daily for up to eight weeks. Reductions in pain were seen within the first week of treatment and statistically significant improvement from baseline in Average Abdominal Pain Score, or AAPS, at weeks four and eight. In this trial, olorinab appeared generally well tolerated with no clinically significant changes in heart rate or blood pressure, no psychotropic effects, and no discontinuations due to adverse events.
In April 2016, we announced favorable results from a Phase 1b multiple-ascending dose clinical trial of olorinab. This randomized, double-blind, placebo-controlled Phase 1b clinical trial enrolled 36 healthy adults to evaluate the safety, tolerability and pharmacokinetics of multiple-ascending doses of olorinab. Cohorts of 12 subjects (nine active, three placebo) were administered doses of 50 mg, 100 mg, or 200 mg of olorinab or placebo three times daily for 10 days and, in connection with the pharmacokinetic evaluation, one time on the 11th day. The most common adverse events were headache and nausea. All adverse events were classified as mild, and there were no serious adverse events reported. There was one discontinuation in the high-dose group due to an adverse event of mild thirst and somnolence. Reductions in blood pressure and heart rate were observed, but none were symptomatic or resulted in an adverse event. Drug levels at all doses tested in the trial, including the lowest dose, were well above those believed to be needed to stimulate the CB2 receptor.
In April 2015, we announced favorable top-line results from a Phase 1 single-ascending dose clinical trial of olorinab. The randomized, double-blind and placebo-controlled trial enrolled 56 healthy adults to evaluate the safety, tolerability and pharmacokinetics of single-ascending doses of olorinab. Dose-responsive exposure was observed over the explored dose range of 10-400 mg with good tolerability at all doses administered.
Olorinab Intellectual Property
As of February 4, 2022, we owned issued patents covering compositions of matter for olorinab and related compounds, and methods of treatment utilizing olorinab and related compounds, in 23 jurisdictions, including the United States, China, Japan, Canada, India, Russia, South Korea and Australia, and we had applications pending in 8 other jurisdictions, of which the ones with the largest pharmaceutical markets were Europe, Venezuela, and Brazil. The earliest priority date for the patents on olorinab is 2009. The terms of these patents are capable of continuing into 2030 in most jurisdictions without taking into account any patent term adjustment or extension regimes of any country or any additional term of exclusivity we might obtain by virtue of the later filed patent applications.
Additional Internal Preclinical and Clinical Programs
In January 2020, we entered an agreement with Beacon Discovery for the development of multiple novel, early stage, oral autoimmune programs. The multiple G-protein-coupled receptor, or GPCR, targets in this collaboration with Beacon Discovery and their associated chemistry represent the next generation of oral compounds which, if successfully developed and approved, may transform the way autoimmune diseases are approached and treated. This collaboration includes both novel and validated targets and compounds.
We have additional assets, including other 5-HT2A modulators, which are either in or being evaluated for future clinical and preclinical development. We are also evaluating additional delivery forms of the products in our pipeline to extend clinical utility or improve the product profile.
11
Collaborations and License Agreements
We have strategic collaborations and licenses with pharmaceutical companies, including United Therapeutics, Everest Medicines Limited, or Everest, Boehringer Ingelheim International GmbH, or Boehringer Ingelheim, Beacon, Eisai, and Aristea Therapeutics, Inc., or Aristea.
Aristea Collaboration and Option Agreement
In July 2021, we entered into a strategic collaboration and option agreement to advance the clinical development of RIST4721, an oral CXCR2 antagonist being developed by Aristea for the treatment of palmoplantar pustulosis (“PPP”) and other neutrophil-mediated diseases.
Under the terms of the agreement, we paid $60.0 million upfront to Aristea and invested $10.0 million in Aristea’s Series B preferred stock, both of which were paid in July 2021. In return, Aristea granted us an exclusive option to acquire Aristea, including rights to all CXCR2 programs, upon completion of the Phase 2b study of RIST4721 in PPP. The agreement also provided a framework during the option period for the companies to jointly explore the development of additional neutrophil-mediated diseases, including hidradenitis suppurativa and inflammatory bowel disease.
United Therapeutics License Agreement
In November 2018, we entered into a collaboration and license agreement with United Therapeutics. Under the United Therapeutics Agreement, we granted United Therapeutics an exclusive, worldwide, royalty-bearing license to develop, manufacture and commercialize ralinepag. This transaction was completed in January 2019. At the closing of the transaction, we transferred to United Therapeutics certain other assets relating to ralinepag, including, among others, related domain names and trademarks, permits, certain contracts, inventory, regulatory documentation, IND, and non-clinical, pre-clinical and clinical trial data. United Therapeutics has agreed to assume certain limited liabilities, including, among others, all post-closing obligations under assumed contracts and the IND. United Therapeutics is responsible for all development, manufacture and commercialization of the licensed products globally.
Upon the closing of this transaction, in January 2019, we received an upfront payment of $800.0 million. We are eligible to receive a payment of $150.0 million upon first marketing approval of ralinepag in a major non-US market, and a payment of $250.0 million upon US marketing approval of an inhaled formulation of ralinepag. In addition, we are entitled to receive low double-digit, tiered royalties on net sales of ralinepag products, subject to certain adjustments for third-party license payments.
The United Therapeutics Agreement contains various representations and warranties of Arena and United Therapeutics, and various covenants of the parties, including covenants to cooperate in seeking regulatory approvals, as well as our agreement not to compete, during the period in which royalties are payable (or during the five-year period following the closing if we are subject to a change of control transaction) in the development of a prostacyclin to treat pulmonary hypertension.
Ralinepag Program
Ralinepag is in a Phase 3 program for pulmonary arterial hypertension, or PAH. Ralinepag is a next-generation potent, highly selective oral IP receptor agonist intended for the treatment of PAH. Ralinepag was designed by us to deliver intravenous prostacyclin-like potency and pharmacokinetics in an oral tablet. In non-clinical experiments, ralinepag demonstrated potentially best-in-class activation of the IP receptor resulting in vasodilation, inhibition of smooth muscle cell proliferation and inhibition of platelet aggregation. Additionally, early-stage studies of ralinepag pharmacokinetics in humans revealed an approximately 24-hour half-life and a low peak-to-trough ratio supporting therapeutic blood levels with once daily dosing.
Ralinepag was granted orphan drug status for the treatment of PAH by the US Food and Drug Administration, or FDA, in September 2014, and by the European Medicines Agency in January 2019.
PAH is a progressive, life-threatening disorder characterized by increased pressure in the pulmonary arteries that carry blood from the heart to the lungs. PAH occurs when the pulmonary arteries thicken or grow rigid. This makes blood flow more difficult. The heart must work harder to push blood through the arteries, and the arteries are unable to carry adequate blood to the lungs. The increased pressure strains the heart, which can limit physical activity, result in heart failure and reduce life expectancy. PAH will continue to worsen over time, even with proper treatment. Based on data from the Registry to EValuate Early And Long-term PAH disease management, or REVEAL, of patients in the US, there is an estimated five-year survival rate of 57% from diagnosis.
12
PAH involves several interrelated mechanisms, with prostacyclin and thromboxane A2 playing a major role in maintaining pulmonary vascular tone through their balanced activity. Prostacyclin, released by endothelial cells, promotes vasodilation and inhibits platelet aggregation. Prostacyclin also has antiproliferative effects on vascular smooth muscle. Despite treatment guidelines, targeting the prostacyclin pathway has been primarily reserved for patients with advanced disease due to limitations of currently available options including parenteral prostacyclins which are the only PAH treatment that have demonstrated a mortality benefit.
Everest Collaboration
In December 2017, we entered into a Collaboration and License Agreement, or the Everest Agreement, with Everest regarding the development and commercialization of ralinepag and etrasimod in China, Taiwan, Hong Kong, Macau and South Korea, or the Everest Territories. In January 2019, we and Everest amended the Everest Agreement by entering into two separate agreements, one for each of ralinepag and etrasimod, with the terms for each program that are substantially the same as in the original Everest Agreement. Under the United Therapeutics Agreement, we assigned the separate Everest Agreement related to ralinepag to United Therapeutics.
Under the separate Everest Agreement related to etrasimod, we granted Everest an exclusive, royalty-bearing license to develop, manufacture and commercialize etrasimod (in oral formulations only), in the Everest Territories.
Everest is responsible for all development, manufacture and commercialization of the licensed products in the Everest Territories, and may participate in the portion of our global clinical trials that is conducted in the Everest Territories.
We are eligible to receive development, regulatory and commercial milestone payments from Everest, as well as tiered royalties on net sales ranging from the high single digits to low double digits. Following an initial royalty term, we are eligible to receive a lower trademark royalty if Everest continues to use our licensed product-related trademarks.
In the fourth quarter of 2018, the National Medical Products Administration of China, formerly known as the China Food and Drug Administration, or CFDA, accepted the initial clinical trial applications for an oral formulation of ralinepag and for etrasimod. Subsequently, in the fourth quarter of 2019, Everest announced that the first subject has been dosed in a Phase 3 trial evaluating etrasimod in development for the treatment of UC in Greater China and South Korea. This Phase 3 trial is ongoing.
Boehringer Ingelheim Collaboration
In 2015, we entered into an exclusive agreement with Boehringer Ingelheim, to conduct joint research to identify drug candidates targeting a GPCR that belongs to a group of orphan central nervous system, or CNS, receptors. An “orphan receptor” is structurally related to a family of proteins that are known to act as functional cell-surface receptors but whose ligand has not yet been identified. A lead molecule is currently in preclinical development.
In the past, we contracted with Beacon to perform our research obligations under the Boehringer Ingelheim collaboration. In exchange, we agreed to share limited near-term milestones with Beacon as well as the full-time equivalent funding paid to us by Boehringer Ingelheim. We have retained the longer-term success milestones and all royalties.
Beacon Discovery Agreements
In the first quarter of 2021, we received a $1.1 million payment as a result of the merger (“Merger”) between Eurofins Beacon Discovery Holdings, Inc. (“Eurofins”) and Beacon. This payment satisfied Beacon’s obligation to pay us a percentage of the consideration for such sale transaction in the event that Beacon was sold as outlined in the 2016 License and Collaboration Agreement. We are eligible to receive future contingent consideration payments based on certain performance metrics achieved by Beacon over a four-year performance period through the first quarter of 2025 up to an aggregate of $2.0 million.
Following the Merger, we entered into a Consent and Release Agreement that terminated the Company’s rights of negotiation and rights of first refusal to potentially obtain licenses to certain compounds discovered and developed by Beacon. In addition, the Consent and Release Agreement terminated our right, under the 2016 License and Collaboration Agreement, to receive any revenue received by Beacon including upfront payments, milestone payments and royalties. The 2020 Collaboration and License Agreement with Beacon remains in effect and was not impacted by the Merger.
In January 2020, we entered into a multi-year strategic Collaboration and License Agreement with Beacon, aimed at building novel medicines across a range of GPCR targets believed to play a role in immune and inflammatory
13
diseases. Under the terms of this agreement, Beacon is responsible for early drug discovery activities and Arena will be responsible for any potential future development and, ultimately, commercialization activities. We are required to pay to Beacon research initiation fees, make quarterly research funding payments for the duration of Beacon’s research activities as well as research, development and regulatory milestone payments. We are also obligated to pay Beacon tiered royalties on net sales of low single digits levels.
Beginning in September 2016, we entered into a series of agreements with Beacon.
In 2016, we entered into a License and Collaboration Agreement with Beacon, pursuant to which we granted Beacon a non-exclusive, non-assignable and non-sublicensable license to certain database information relating to compounds, receptors and pharmacology, and transferred certain equipment to Beacon. Beacon will seek to engage global partners to facilitate discovery and development. Beacon has agreed to assign to us any intellectual property relating to our existing research and development programs developed in the course of performing research for us and grant us a non-exclusive license to any intellectual property developed outside the course of performing work for us that is reasonably necessary or useful for developing or commercializing the products under our research and development programs. We are also entitled to rights of negotiation and rights of first refusal to potentially obtain licenses to certain compounds discovered and developed by Beacon. In addition, we are entitled to receive (i) a percentage of any revenue received by Beacon on or after the second anniversary of the effective date of the agreement from any third party pursuant to a third-party license, including upfront payments, milestone payments and royalties; (ii) single-digit royalties on the aggregate net sales of any related products sold by Beacon and its affiliates; and (iii) in the event that Beacon is sold, a percentage of the consideration for such sale transaction.
In 2016, we also entered into a Master Services Agreement with Beacon, pursuant to which Beacon performs certain other research services for us relating to our proprietary pipeline.
Eisai Agreement
In December 2016, we entered into a Transaction Agreement with Eisai regarding lorcaserin. Pursuant to the Transaction Agreement, we granted Eisai an exclusive, royalty-bearing license, or transferred intellectual property, to develop, manufacture and commercialize lorcaserin in all countries and territories of the world. Under the Transaction Agreement we are entitled to receive tiered royalty payments starting at 9.5% on net sales of lorcaserin.
Lorcaserin was approved for marketing in the US, and in certain other territories, for the indication of weight management. On February 13, 2020, the FDA issued a drug safety communication announcing that it requested Eisai voluntarily withdraw lorcaserin from the US market based on the FDA’s analysis of data from the Cardiovascular and Metabolic Effects of Lorcaserin in Overweight and Obese Patients – Thrombolysis in Myocardial Infarction 61 (CAMELLIA-TIMI 61) study, and that Eisai has submitted a request to voluntarily withdraw lorcaserin from the US market. On September 30, 2020, Eisai announced that, after consulting with the FDA, it is continuing the lorcaserin expanded access program and has initiated a Phase 3 clinical study of lorcaserin in patients with Dravet syndrome, a severe type of epilepsy characterized by prolonged seizures that begin in the first year of life.
In October 2020, we entered into a Royalty Purchase Agreement with Longboard pursuant to which Longboard purchased from us the right to receive all milestone payments, royalties, interest and other payments relating to net sales of lorcaserin owed or otherwise payable by Eisai.
We believe that Eisai is solely responsible for any expenses and losses associated with product liability claims, except we and Eisai share 50% of losses for any alleged defective manufacturing of lorcaserin that was manufactured by us prior to entering into the Transaction Agreement.
Intellectual Property
Our success depends in large part on our ability to protect our compounds and information, and to operate without infringing the proprietary rights of third parties. We rely on a combination of patent, trade secret, copyright, and trademark laws, as well as confidentiality, licensing and other agreements, to establish and protect our proprietary rights. We seek patent protection for our key inventions, including drug candidates we identify, routes for chemical synthesis, pharmaceutical formulations and methods of treatment.
There is no assurance that any of our patent applications will issue, or that any of the patents will be enforceable or will cover a drug or other commercially significant product or method. In addition, we regularly review our
14
patent portfolio to identify patents and patent applications for potential abandonment that we deem to have relatively low value to our ongoing business operations. There is also no assurance that we will correctly identify which of our patents and patent applications should be maintained and which should be abandoned. The term of most of our current patents commenced, and most of our future patents, if any, will commence, on the date of issuance and terminate 20 years from the earliest effective filing date of the patent application. Because any marketing and regulatory approval for a drug often occurs several years after the related patent application is filed, the resulting exclusivity afforded by any patent on our drug candidates will likely be substantially less than 20 years.
In the United States, patent term adjustment is available for certain delays in patent office proceedings. In addition, under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, the term of a patent that covers an FDA-approved drug may be eligible for patent term extension, or PTE. PTE permits patent term restoration of a US patent as compensation for the patent term lost during product development and the FDA regulatory review process. The Hatch-Waxman Act permits a PTE of up to five years beyond the expiration of the patent. This period is generally one-half the time between the effective date of an Investigational New Drug, or IND (falling after issuance of the patent), and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application, provided the sponsor acted with diligence. A PTE cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. The application for PTE is subject to approval by the US Patent and Trademark Office in conjunction with the FDA.
Outside of the United States, similar provisions may be available in the European Union, Japan, South Korea and some other jurisdictions to extend the term of a patent that covers an approved drug. The length of any such extension may vary by country. Our European patents may be eligible for supplemental protection certificates of up to five years in one or more countries.
Due to the specific requirements for obtaining these extensions, there is no assurance that our patents will be afforded extensions even if we encounter significant delays in patent office proceedings or marketing and regulatory approval.
In addition to patent protection, we rely on trade secrets, proprietary know-how and continuing technological advances to develop and maintain our competitive position. To maintain the confidentiality of our trade secrets and proprietary information, all of our employees are required to enter into and adhere to an employee confidentiality and invention assignment agreement, and invention disclosure procedures as a condition of employment. Additionally, our employee confidentiality and invention assignment agreements require that our employees not bring to us, or use without proper authorization, any third-party proprietary technology. We also generally require our consultants and collaborators that have access to proprietary property and information to execute confidentiality and invention rights agreements in our favor. While such arrangements are intended to enable us to better control the use and disclosure of our proprietary property and provide for our ownership of proprietary technology developed on our behalf, they may not provide us with meaningful protection for such property and technology in the event of unauthorized use or disclosure.
Competition
The biotechnology and pharmaceutical industries are highly competitive and are subject to rapid and significant change. We face significant competition from many organizations with drugs or drug candidates that do or may compete with drug candidates we are developing. We may not be able to compete successfully against these organizations, which include many large, well-financed and experienced pharmaceutical and biotechnology companies, as well as academic and research institutions and government agencies.
Developments by others may render our drug candidates obsolete or noncompetitive, and we or our collaborators may not be successful in developing either first- or best-in-class drugs.
Etrasimod is currently in development for the treatment of IBD, including in a Phase 3 program in UC and in a Phase 2b/3 program in CD. If approved in IBD, etrasimod would compete with generic drugs, branded oral agents, biologics and biosimilars. There are multiple FDA-approved products for treatment of moderate-to-severe IBD. Many of these products are biologics and include but are not limited to adalimumab by AbbVie, vedolizumab by an affiliate of Takeda Pharmaceuticals, and infliximab and ustekinumab by Janssen Biotech. Additionally, there are approved biosimilar agents to infliximab. There are also approved oral agents, including tofacitinib by Pfizer, as well as multiple oral agents in development. Ozanimod, by Bristol Myers Squibb, or BMS, is an S1P receptor modulator that is approved for use in multiple sclerosis and ulcerative colitis. There are also JAK inhibitors in development, including but not limited to filgotinib by Gilead Sciences and Galapagos, and upadacitinib by AbbVie. In addition oral generic agents such as
15
mesalamine and methotrexate are used in the treatment of IBD. There are multiple IL-23 biologics in development for IBD, including Tremfya (guselkumab) by Jannsen Biotech, Skyrizi (risankizumab-rzaa) by AbbVie and mirikizumab by Eli Lilly. There are also oral TYK2 inhibitors in development for IBD, including deucravacitinib by Bristol Myers Squibb.
Etrasimod has completed a Phase 2 study in AD and we intend to advance into a Phase 3 program for etrasimod in AD. If approved for that indication, etrasimod would compete with generic topical agents, branded topical agents, oral agents, and biologics. Currently AD patients are treated with generic topical steroids, generic topical calcineurin inhibitors, oral generic agents including cyclosporine and methotrexate, and a branded biologic agent, dupilumab, from Regeneron and Sanofi, which was approved in 2017. There are a number of topical treatments, oral agents, and biologic agents in development for AD. Topical agents may have different indications and limitations of use. Three oral agents have completed Phase 3 trials in AD and include baricitinib by Eli Lilly, abrocitinb by Pfizer, and upadacitinib by AbbVie. Baricitinib, abrocitinib, and upadacitinib are approved in the EU and Japan for moderate to severe atopic dermatitis. Physicians may also use agents such as tofacitinib off-label in the treatment of AD. There are also biologic agents in development for AD including but not limited to IL-13 inhibitors tralokinumab by Leo Pharma and lebrikizumab by Eli Lilly. In September 2021, the FDA approved topical JAK Opzelura (ruxolitinib) for the short term treatment of moderate to severe atopic dermatitis. In January 2022, the FDA approved tralokinumab for the treatment of moderate to severe atopic dermatitis in adult patients. In January 2022, the FDA approved Rinvoq (upadacitinib) for the treatment of moderate to severe atopic dermatitis in patients 12 years of age and older and also approved Cibinqo (abrocitinib) for the treatment of moderate to severe atopic dermatitis in adult patients. This list does not include all topical, oral, or biologic agents in development.
Etrasimod is currently in Phase 2 programs for the treatment of EoE and AA. If approved for EoE, etrasimod would compete with generic oral steroids, branded oral steroids, generic and branded proton pump inhibitors, and biologics. If approved for AA, etrasimod would compete with generic topical agents, branded topical agents, oral agents, and biologics.
APD418 is in development for AHF. There are currently no approved products to improve outcomes in AHF patients. A number of agents are used off-label, including, inotropic agents, loop diuretics, nitrate vasodilators, PDE3 inhibitors, and calcium sensitizers. Other agents are in development—for example, BMS is developing cimlanod (BMS-986231) in a Phase 2 study and Lee’s Pharma is developing istaroxime, a positive inotropic agent.
Temanogrel is in development for prevention of MVO in the setting of PCI. A number of drugs are used off-label to manage these patients, including direct thrombin inhibitors, glycoprotein IIb/IIIa inhibitors, and calcium channel blockers. There is one agent in Phase 2 development, revacept, by AdvanceCor, with a mechanism of action of platelet membrane glycoprotein VI inhibition.
Many of our existing and potential competitors have substantially greater drug development capabilities and financial, scientific and marketing resources than we do. Additional consolidation in the pharmaceutical industry may result in even more resources being concentrated with our competitors. As a result, our competitors may be able to devote greater resources than we can to the research, development, marketing and promotion of therapeutic products or drug discovery techniques, or to adapt more readily to technological advances than we can. Accordingly, our competitors may succeed in obtaining patent protection, receiving regulatory approval or commercializing drugs before we do.
We may rely on collaborators for support of development programs and for the manufacturing and marketing of drug candidates. Such collaborators may be conducting multiple drug development efforts within the same disease areas that are the subject of their agreements with us, which may negatively impact the development of drugs that are subject to our agreements. In addition, we face and will continue to face intense competition from other companies for such collaboration arrangements, and technological and other developments by others may make it more difficult for us to establish such relationships.
Government Regulation
We and our collaborators are subject to significant governmental regulation. The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the preclinical and clinical development, pre-market approval, manufacture, import, export, marketing and distribution of pharmaceutical products. These agencies and other regulatory agencies regulate research and development activities and the testing, approval, manufacture, quality control, safety, effectiveness, labeling, storage, tracking, recordkeeping, advertising, pricing and promotion of drug candidates and commercialized drugs. Failure to comply with applicable FDA or other regulatory requirements may result in inspectional notices of violation, warning letters, civil or criminal penalties,
16
suspension or delays in clinical development, recall or seizure of products, partial or total suspension of production, withdrawal of a product from the market or other negative consequences.
In the United States
In the United States, the FDA regulates drug products under the Federal Food, Drug, and Cosmetic Act, or FD&C Act, and its implementing regulations. The process required by the FDA before drug candidates may be marketed in the United States generally involves the following:
•completion of extensive preclinical laboratory tests and preclinical animal studies, many of which are required to be performed in accordance with the FDA’s Good Laboratory Practice, or GLP, regulations;
•submission to the FDA of an IND, which must become effective before human clinical trials may begin and be updated annually;
•performance of adequate and well-controlled human clinical trials, performed in accordance with the FDA’s Good Clinical Practice, or GCP, regulations, to establish the safety and efficacy of the drug candidate for each proposed indication;
•submission to the FDA of a New Drug Application, or NDA, after completion of adequate and well-controlled human clinical trials, generally accompanied by payment of a substantial user fee to the FDA;
•a determination by the FDA within 60 days of its receipt of the NDA to file the NDA for review;
•satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities at which the active pharmaceutical ingredient and finished drug product are produced and tested to assess compliance with Current Good Manufacturing Practices, or cGMP, regulations;
•FDA review and approval of the NDA prior to any commercial marketing or sale of the drug in the United States; and
•Prior to commercialization, centrally acting drugs may be subject to review and potential scheduling by the DEA.
The development and approval process requires substantial expertise, time, effort and financial resources, and we cannot be certain that any approvals for our drug candidates will be granted on a timely basis, if at all.
The results of preclinical tests (which include laboratory evaluation as well as GLP studies to evaluate toxicity in animals) for a particular drug candidate, together with related manufacturing information and analytical data, are submitted as part of an IND to the FDA. The initial IND becomes effective 30 days after receipt by the FDA, following its initial safety review. During the 30-day time period the FDA may require additional information. The FDA may institute a clinical hold at the 30-day time period if any questions are not fully addressed or because of other concerns about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. The FDA may place an IND on partial or full clinical hold at any time during a product candidate’s development. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development. Further, an independent institutional review board, or IRB, for each medical center proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that center and it must monitor the study until completed. The FDA, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive Good Clinical Practice, or GCP, regulations and regulations for informed consent and privacy of individually identifiable information.
Clinical trials. For purposes of NDA submission and approval, clinical trials are typically conducted in the following sequential phases, which may overlap:
•Phase 1 clinical trials. Studies are initially conducted in a limited population to test the drug candidate for safety, dose tolerance, absorption, metabolism, distribution and excretion, typically in healthy volunteers, but in some cases in patients.
•Phase 2 clinical trials. Studies are generally conducted in a limited patient population to identify possible adverse effects and safety risks, explore the initial efficacy of the product for specific
17
targeted indications and to determine dose range or pharmacodynamics. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
•Phase 3 clinical trials. These are commonly referred to as pivotal studies or adequate and well-controlled studies. When Phase 2 evaluations demonstrate that a dose range of the product is effective and has an acceptable safety profile, Phase 3 clinical trials are undertaken in large patient populations to further evaluate dosage, provide substantial evidence of clinical efficacy and further test for safety in an expanded and diverse patient population at multiple, geographically dispersed clinical trial centers.
•Phase 4 clinical trials. The FDA may approve an NDA for a drug candidate but require that the sponsor conduct additional clinical trials to further assess the drug after NDA approval under a post-approval commitment. In addition, a sponsor may decide to conduct additional clinical trials after the FDA has approved an NDA. Post-approval trials are typically referred to as Phase 4 clinical trials.
New drug applications. The results of drug development, preclinical studies and clinical trials are submitted to the FDA as part of an NDA. NDAs also must contain extensive chemistry, manufacturing and control, or CMC, information. An NDA is usually accompanied by a significant user fee. The FDA reviews all NDAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing, which occurs, if at all, 60 days after submission by the NDA sponsor. Once the submission has been accepted for filing, the FDA’s goal is to review applications within 10 months from its acceptance of the filing or, if the application relates to an unmet medical need in a serious or life-threatening indication, six months from its acceptance of the filing. The review process can be significantly extended by FDA requests for additional information or clarification. The FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee. The FDA may deny approval of an NDA by issuing a Complete Response Letter, or CRL, if the applicable regulatory criteria are not satisfied. A CRL may require additional clinical data and/or an additional pivotal Phase 3 clinical trial(s), and/or other significant, expensive and time-consuming requirements related to clinical trials, preclinical studies or manufacturing. Data are not always conclusive, and the FDA may interpret data differently than we or our collaborators interpret data. Approval may occur with Risk Evaluation and Mitigation Strategies, or REMS, that may limit the labeling, distribution or promotion of a drug product. Once issued, the FDA may withdraw product approval if ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require testing, including Phase 4 clinical trials, and surveillance programs to monitor the safety effects of approved products which have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these postmarketing programs or other information.
Expedited Development and Review Programs. The FDA has four programs that are intended to facilitate and expedite development and review of new drugs to address unmet medical need in the treatment of a serious or life-threatening condition: fast track designation, breakthrough therapy designation, accelerated approval, and priority review designation. These programs do not change the standards for approval but may expedite the development or approval process.
New investigational drugs may qualify for fast track designation if they are intended to treat a serious condition and nonclinical or clinical data demonstrate the potential to address unmet medical need. Fast track designation applies to the drug (either alone or in combination with other drugs) and the specific indication for which it is being studied. A fast track product is afforded opportunities for frequent interactions with the FDA to support efficient development and may be eligible for priority review if supported by clinical data at the time of NDA submission. After preliminary evaluation of clinical data and a determination that a fast track product may be effective, the FDA may consider reviewing portions of an NDA before the complete application is submitted (“rolling review”), if the sponsor gains FDA agreement on the proposed schedule for submission of the various sections of the NDA and the sponsor pays any required user fees upon submission of the first section of the NDA.
An NDA for a drug that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness compared to marketed products may qualify for priority review designation. A priority review designation means that the FDA’s goal is to take action on the NDA within six months of the filing date as compared to ten months under its standard review.
In addition, a product may qualify for an accelerated approval pathway if it meets three criteria: treats a serious condition; generally provides a meaningful advantage over available therapies; demonstrates an effect on a surrogate
18
endpoint that is reasonably likely to predict clinical benefit or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality that is reasonably likely to predict an effect on irreversible morbidity or other clinical benefit. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform adequate and well controlled post marketing clinical trials to verify the predicted clinical benefit. Products receiving accelerated approval may be subject to expedited withdrawal procedures if the sponsor fails to conduct the required post-market studies or if such studies fail to verify the predicted clinical benefit. In addition, the FDA currently requires, as a condition for accelerated approval, pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product.
The Food and Drug Administration Safety and Innovation Act established a category of drugs and biologics referred to as “breakthrough therapies”, and a drug candidate may qualify for breakthrough therapy designation if it is intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement on a clinically significant endpoint(s) over available therapies. Breakthrough therapy designation affords all of the fast track designation features, as well as more intensive FDA interaction and guidance. Breakthrough therapy designation is distinct from both the accelerated approval pathway and priority review designation, which can also be granted to the same drug if relevant criteria are met.
Other US regulatory requirements. Products manufactured or distributed pursuant to FDA approvals are subject to continuing regulation by the FDA, including recordkeeping, annual product quality review and reporting requirements. Adverse event experience with the product must be reported to the FDA in a timely fashion and pharmacovigilance programs to proactively look for these adverse events are mandated by the FDA. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies and are subject to periodic inspections (which may be unannounced) by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMP regulations, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Following such inspections, the FDA may issue notices on Form FDA 483 and warning letters that could cause us to modify certain activities. A Form FDA 483 notice, if issued at the conclusion of an FDA inspection or after the appropriate FDA office review of the Establishment Inspection Report prepared by the investigator, can list conditions the FDA believes may have violated cGMP or other FDA regulations. FDA guidelines specify that a warning letter be issued for violations of “regulatory significance,” also known as Official Action Indicated, or OAI. Failure to adequately and promptly correct the observation(s) can result in regulatory action. In addition to Form FDA 483 notices and warning letters, failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as suspension of manufacturing, recall of product, seizure of product, injunctive action or possible civil or criminal penalties.
The FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for healthcare professional marketing activities and materials, direct-to-consumer advertising, dissemination of off-label information, industry-sponsored scientific and educational activities and promotional activities involving the Internet. Drugs may be marketed only for their approved indications and in accordance with the provisions of the confines of the pivotal studies and the approved label. Further, we may be required to develop additional data or conduct additional preclinical studies and clinical trials, and we may be required to submit and obtain FDA approval of a new or supplemental NDA for changes to, among other things, the indications, labeling, or manufacturing processes or facilities of a drug. Failure to comply with these requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, corrective advertising, suspension of manufacturing, seizure of product, injunctive action or potential civil and criminal penalties.
Physicians may prescribe legally available drugs for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA, if in their professional medical judgment, the physicians deem such use to be appropriate. Such off-label uses are common across certain medical specialties. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’ communications regarding off-label use.
To distribute products commercially, we or our collaborators, as applicable, must comply with state laws that require the registration of manufacturers and wholesale distributors of pharmaceutical products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution.
19
Drug Enforcement Administration regulation. The DEA regulates drugs that are controlled substances. Controlled substances are those drugs that appear on one of the five schedules promulgated and administered by the DEA under the Controlled Substances Act, or CSA. The CSA governs, among other things, the inventory, distribution, recordkeeping, handling, security and disposal of controlled substances. Any drug that acts on the central nervous system has the potential to become a controlled substance based on an evaluation of its abuse potential, and scheduling by the DEA is a separate process that may delay the commercial launch of a drug even after FDA approval of the NDA. Companies with a scheduled drug are subject to periodic and ongoing inspections by the DEA and similar state drug enforcement authorities to assess ongoing compliance with the DEA’s regulations. Any failure to comply with these regulations could lead to a variety of sanctions, including the revocation or a denial of renewal of any DEA registration, injunctions, or civil or criminal penalties.
Hatch-Waxman Exclusivity. Market exclusivity provisions of the Hatch-Waxman Act can delay the submission or approval of applications seeking to rely upon the FDA’s findings of safety and effectiveness for a previously approved NDA. A new chemical entity, or NCE, subject to an NDA is entitled to a five-year period of non-patent marketing exclusivity in the United States. A drug is an NCE if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, such an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement of patents listed with the FDA by the NDA holder. The Hatch-Waxman Act also provides three years of marketing exclusivity for an NDA, or supplement to an existing NDA, if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active ingredient. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Orphan drug designation and exclusivity. Under the Orphan Drug Act, the FDA may designate a drug product as an “orphan drug” if it is intended to treat a rare disease or condition (generally meaning that it affects fewer than 200,000 individuals in the United States, or more in cases in which there is no reasonable expectation that the cost of developing and making a drug product available in the United States for treatment of the disease or condition will be recovered from sales of the product). A company must request orphan product designation before submitting an NDA. If the request is granted, the FDA will disclose the identity of the therapeutic agent and its potential use. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product with orphan status receives the first FDA approval for the disease or condition for which it has such designation or for a select indication or use within the rare disease or condition for which it was designated, the product generally will receive orphan product exclusivity. Orphan product exclusivity means that the FDA may not approve any other applications for the same product for the same indication for seven years, except in certain limited circumstances. Competitors may receive approval of different products for the indication for which the orphan product has exclusivity and may obtain approval for the same product but for a different indication or the same product for the same indication if demonstrated to be clinically superior. If a drug or drug product designated as an orphan product ultimately receives marketing approval for an indication broader than what was designated in its orphan product application, it may not be entitled to exclusivity.
Pediatric exclusivity. The FDA may issue Written Requests for pediatric studies prior to approval of a new drug application, if the FDA has determined that information related to the use of the drug in the pediatric population may produce health benefits. As an incentive to industry to conduct such studies requested by the FDA, a 6-month period of additional exclusivity may be granted as an add-on to existing marketing exclusivity periods and patent terms.
Outside of the United States
In addition to regulations in the United States, we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical studies and any commercial sales and distribution of products. Whether or not we obtain FDA approval for a product candidate, we must obtain the requisite approvals from regulatory authorities in foreign jurisdictions prior to the commencement of clinical studies or marketing and sale of the product in those jurisdictions.
20
Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In the EU, for example, a Clinical Trial Application, or CTA, must be submitted to the national health authority and an independent ethics committee in each country in which we intend to conduct clinical trials, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed in that country. Under the new Regulation on Clinical Trials, which took effect in January 2022, there will be a centralized application procedure in respect of clinical trials to be conducted in the EU where one national authority will take the lead in reviewing the application and the other national authorities have more limited involvement. Any substantial changes to the trial protocol or other information submitted with the clinical trial applications must be notified to or approved by the relevant competent authorities and ethics committees.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of an investigational drug or biological product in the EU, we must submit a marketing authorization application either under the so-called centralized or national authorization procedures.
Centralized procedure. The centralized procedure provides for the grant of a single marketing authorization, which is issued by the European Commission based on the opinion of the Committee for Medicinal Products for Human Use, or the CHMP, of the European Medicines Agency, or EMA, and that is valid in all EU member states, as well as Iceland, Liechtenstein and Norway (together, the “EEA”). The Centralized Procedure is mandatory for certain types of products, such as biotechnology medicinal products, orphan medicinal products, and medicines that contain a new active substance indicated for the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU. Under the Centralized Procedure the maximum timeframe for the evaluation of an MAA is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases when the authorization of a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. Under the accelerated procedure the standard 210-day review period is reduced to 150 days.
National authorization procedures. There are also two other possible routes to authorize medicinal products in several EU Member States, which are available for investigational medicinal products that fall outside the scope of the centralized procedure:
•Decentralized procedure. Using the decentralized procedure, an applicant may apply for simultaneous authorizations in more than one EU country of medicinal products that have not yet been authorized in any EU Member State and that do not fall within the mandatory scope of the centralized procedure.
•Mutual recognition procedure. In the mutual recognition procedure, a medicine is first authorized in one EU Member State, in accordance with the national procedures of that country. Following this, further marketing authorizations can be sought from other EU Member States in a procedure whereby the countries concerned agree to recognize the validity of the original, national marketing authorization.
In the EEA, upon receiving marketing authorization, new chemical entities generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the EU from referencing the innovator’s data to assess a generic application. During the additional two-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic product can be marketed until the expiration of the market exclusivity. However, there is no guarantee that a product will be considered by the EU’s regulatory authorities to be a new chemical entity and qualify for data exclusivity.
The EMA grants orphan drug designation to promote the development of products that may offer therapeutic benefits for life-threatening or chronically debilitating conditions affecting not more than five in 10,000 people in the EU. In addition, orphan drug designation can be granted if the drug is intended for a life threatening, seriously debilitating or serious and chronic condition in the EU and without incentives it is unlikely that sales of the drug in the EU would be
21
sufficient to justify developing the drug. Orphan drug designation is only available if there is no other satisfactory method approved in the EU of diagnosing, preventing or treating the condition, or if such a method exists, the proposed orphan drug will be of significant benefit to patients. Orphan drug designation provides opportunities for free protocol assistance, fee reductions for access to the centralized regulatory procedures and ten years of market exclusivity following drug approval, which can be extended to 12 years if trials are conducted in accordance with an agreed-upon pediatric investigational plan. The exclusivity period may be reduced to six years if the designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity.
Great Britain, or GB, is no longer covered by the EEA’s procedures outlined above (Northern Ireland will be covered by the centralized authorization procedure and can be covered under the decentralized or mutual recognition procedures). A separate marketing authorization will be required to market drugs in GB. However, for two years from 1 January 2021, the MHRA may adopt decisions taken by the European Commission on the approval of new marketing authorizations through the centralized procedure, and the MHRA will have regard to marketing authorizations approved in a country in the EEA (although in both cases a marketing authorization will only be granted if any GB-specific requirements are met). Various national procedures are now available to place a drug on the market in the UK, GB, or Northern Ireland, with the main national procedure having a maximum timeframe of 150 days (excluding time taken to provide any further information or data required). The data exclusivity periods in the UK are currently in line with those in the EU, but the Trade and Cooperation Agreement agreed between the UK and EU provides that the periods for both data and market exclusivity are to be determined by domestic law, and so there could be divergence in the future.
Orphan designation in GB following Brexit is largely aligned with the position in the EU but is based on the prevalence of the condition in GB. It is therefore possible that conditions that are currently designated as orphan conditions in GB will no longer be and that conditions that are not currently designated as orphan conditions in the EU will be designated as such in GB.
Prescription drug reimbursement and healthcare reform. In the United States and markets in other countries, sales of prescription drug products depend in part on the availability of reimbursement from third-party payers. Third-party payers include government health administrative authorities, managed care organizations, private health insurers and other organizations. The process for determining whether a payer will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payer will pay for the drug product. Third-party payers may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drug products for a particular indication. Third-party payers are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. We may need to conduct expensive pharmacoeconomic studies to demonstrate the cost-effectiveness of our products. A payer’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. Patients who are prescribed medications for the treatment of their conditions, and their prescribing physicians, generally rely on third-party payers to reimburse all or part of the costs associated with their prescription drugs. Patients are less likely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Therefore, coverage and adequate reimbursement are important to new product acceptance.
If a drug is reimbursed by Medicare or Medicaid, pricing and rebate programs must comply with, as applicable, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 as well as the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and the Veterans Health Care Act of 1992, or VHCA, each as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Under the VHCA, drug companies are required to offer certain drugs at a reduced price to a number of federal agencies including US Department of Veterans Affairs and US Department of Defense, the Public Health Service and certain private Public Health Service designated entities in order to participate in other federal funding programs including Medicare and Medicaid. Participation under the VHCA requires submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as entry into government procurement contracts governed by the Federal Acquisition Regulations.
The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of drugs have been a focus in this effort, which has resulted in several recent congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. In addition, emphasis on managed care in the United States has increased and
22
we expect will continue to increase the pressure on drug pricing. Coverage policies, third-party reimbursement rates and drug pricing regulation may change at any time. Under the Biden administration the Build Back Better plan has attempted to address several of these issues but has been met with resistance in the Senate. According to a report from the Kaiser Family Foundation, the key elements included in this framework are 1) Allowing the federal government to negotiate prices for some high-cost drugs covered under Medicare Part B and Part D, 2) Requiring inflation rebates to limit annual increase in drug prices in Medicare and private insurers, 3) Capping out of pocket spending for Medicare Part D enrollees, 4) Limiting cost sharing requirements for insulin for people with Medicare and private insurance, 5) Eliminating cost-sharing for adult vaccines covered under Part D, 6) Repealing the Trump Administration’s proposed drug rebate rule that had already been delayed. Although the bill received support from the House of Representatives, it may lack the support needed to pass in the Senate. The administration is considering breaking up the bill in order to gain Senate support. Additional legislation outside of the Build Back Better plan is being considered to reduce pharmacy fees paid to middlemen payers with the goal of reducing patient out-of-pocket and domestic and international reference pricing will continue to be evaluated, but as with other legislation may face resistance within the Senate. This further highlights potential difficulties to gain unanimous support for pharmaceutical pricing legislation regardless of the administration in charge.
In addition, there has been heightened scrutiny in the United States and other countries of pharmaceutical pricing practices in light of the rising cost of prescription drugs and biologics. In the United States, such scrutiny has resulted in congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. At the federal level, the Trump administration used several means to propose or implement drug pricing reform, including through federal budget proposals, executive orders and policy initiatives. For example, on July 24, 2020 and September 13, 2020, President Trump announced several executive orders related to prescription drug pricing that attempted to implement several of the administration’s proposals. As a result, the FDA concurrently released a final rule and guidance in September 2020 providing pathways for states to build and submit importation plans for drugs from Canada. Further, on November 20, 2020, the U.S. Department of Health and Human Services, or HHS, finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The implementation of the rule has been delayed by the Biden administration from January 1, 2022 to January 1, 2023 due to ongoing litigation. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers, the implementation of which have also been delayed by the Biden administration until January 1, 2023. On November 20, 2020, the Centers for Medicare and Medicaid Services, or CMS, issued an interim final rule implementing President Trump’s Most Favored Nation executive order, which would tie Medicare Part B payments for certain physician-administered drugs to the lowest price paid in other economically advanced countries. As a result of litigation challenging the Most Favored Nation model, on December 27, 2021, CMS published a final rule that rescinded the Most Favored Nation Model interim final rule. In July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to Biden’s executive order, on September 9, 2021, HHS released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue to advance these principles. In addition, Congress is considering drug pricing as part of other reform initiatives. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. It is possible that additional governmental action is taken in response to the COVID-19 pandemic. For example, the Coronavirus Aid, Relief, and Economic Security Act, or the CARES Act, and other COVID-19 relief legislation suspended the 2% Medicare rate reduction sequester from May 1, 2020 through March 31, 2022. Under current legislation the actual reduction in Medicare payments will vary from 1% in 2022 to up to 3% in the final fiscal year of this sequester. Additionally, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. Congress is considering additional health reform measures.
In countries outside the United States, pricing of pharmaceutical products may be subject to governmental control. Evaluation criteria used by many government agencies for the purposes of pricing and reimbursement typically focus on a product’s degree of innovation and its ability to meet a clinical need unfulfilled by currently available therapies. Some countries operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost-effectiveness of a particular drug candidate to currently available
23
therapies. Other countries allow companies to fix their own prices for medicines but monitor and control company profits. In addition, in some countries, cross-border imports from low-priced markets exert a commercial pressure on pricing within a country. There can be no assurance that any country that has price controls or reimbursement limitations for drug products will allow favorable reimbursement and pricing arrangements for any of our products.
Healthcare fraud and abuse. Pharmaceutical companies are subject to various federal and state laws pertaining to healthcare fraud and abuse, including, but not limited to, anti-kickback and false claims laws.
The Federal Anti-Kickback Statute makes it illegal for any person or entity, including a prescription drug manufacturer, or a party acting on its behalf, to knowingly and willfully solicit, offer, receive or provide any remuneration, directly or indirectly, in exchange for, or to induce, the referral of business, including the purchase, order, lease of any good, facility, service or item, including the prescription of a particular drug, for which payment may be made under federal healthcare programs such as Medicare and Medicaid. Some of the state prohibitions are broader in scope and apply to referral of patients for healthcare services reimbursed by any source, not only the Medicare and Medicaid programs.
In the course of practicing medicine, physicians may legally prescribe FDA-approved drugs for an indication that has not been approved by the FDA and which, therefore, is not described in the product’s approved labeling, so-called “off-label use” or “the practice of medicine,” if deemed appropriate in the physicians’ professional medical judgment. The FDA does not ordinarily regulate the behavior of physicians in their choice of treatments. The FDA and other government agencies do, however, restrict communications on the subject of off-label use by a manufacturer or those acting on behalf of a manufacturer. Companies may not promote FDA-approved drugs for off-label uses. The FDA and other governmental agencies do permit a manufacturer (and those acting on its behalf) to engage in some limited, non-misleading, non-promotional exchanges of scientific information regarding unapproved indications.
There are numerous federal false claims laws and civil monetary penalty laws, including the Federal Civil False Claims Act and Civil Monetary Penalties Law, that forbid, among other things, anyone from knowingly presenting, or causing to be presented for payment to third-party payers (including Medicare and Medicaid) claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed or claims for medically unnecessary items or services. Suits filed under the Federal Civil False Claims Act can be brought by any individual on behalf of the government, known as “qui tam” actions, and such individuals, commonly known as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The filing of qui tam actions has caused a number of pharmaceutical, medical device, and other healthcare companies to have to defend a Federal Civil False Claims Act action.
The Federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created federal criminal statutes that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payers, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. Additionally, the Civil Monetary Penalties Law imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal healthcare program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
In addition, the federal transparency requirements under the Physician Payments Sunshine Act require applicable manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to track and annually report to the Centers for Medicare & Medicaid Services, or CMS, payments and other transfers of value provided to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), other healthcare professionals (such as physician assistants and nurse practitioners) and teaching hospitals, and information regarding certain ownership and investment interests held by physicians or their immediate family members.
We may also be subject to state equivalents of these fraud and abuse laws.
Violations of fraud and abuse laws may be punishable by significant criminal, civil and/or administrative sanctions, including individual imprisonment, disgorgement, criminal fines and civil monetary penalties, possible exclusion from federal healthcare programs (including Medicare and Medicaid), and integrity oversight and reporting obligations to resolve allegations of non-compliance with these laws. In addition, under certain healthcare fraud and abuse laws, there is
24
an ability for private individuals to bring similar actions. Additionally, many states have analogous fraud and abuse laws, some of which may be broader in scope. Further, there are an increasing number of state laws that require pharmaceutical companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities, or register their sales representatives, as well as prohibiting certain other sales and marketing practices. The federal transparency requirements under the ACA require certain manufacturers of drugs, devices, biologics and medical supplies to annually report to the Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals and physician ownership and investment interests. Additionally, recent federal legislation imposes additional obligations on certain pharmaceutical manufacturers, among others, regarding drug product tracking and tracing.
Our activities are also potentially subject to federal and state consumer protection and unfair competition laws. We are also subject to the US Foreign Corrupt Practices Act, or the FCPA, which prohibits companies and individuals from engaging in specified activities to obtain or retain business or to influence a person working in an official capacity. Under the FCPA, it is illegal to pay, offer to pay, or authorize the payment of anything of value to any foreign government official, governmental staff members, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity.
Healthcare and other privacy and security laws. We are subject to numerous laws and regulations regarding the privacy, protection, and security of health information and other personal information. These laws and regulations impose obligations and restrictions on us with respect to the collection, storage, use, disclosure, transfer and security of personal information.
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, imposes obligations with respect to safeguarding the privacy, security and transmission of individually identifiable health information on covered entities and their business associations and covered subcontractors that perform services for them involving individually identifiable health information. We may be subject to, or our collaborators’ marketing activities may be limited by, HIPAA and its implementing regulations. In addition, many state laws apply to the use and disclosure of health and other personal information.
For example, as of January 1, 2020, we are subject to the California Consumer Privacy Act, or CCPA. The CCPA has created new individual privacy rights for California residents and places increased privacy and security obligations on entities handling the personal information of such residents. Among other obligations, the CCPA requires covered businesses to provide new disclosures to California residents and to respond to requests to access and/or delete personal information. The CCPA also allows for a private right of action for data breaches.
The European General Data Protection Regulation, or GDPR, imposes many requirements and restrictions that impact our collection, transfer, use and retention of personal data of clinical trial subjects and other individuals in the European Union. For example, the GDPR dictates mandatory contractual terms for service providers that process personal data for us and restricts our ability to transfer data from the European Economic Area to our offices and service providers in the United States and other countries. Penalties for non-compliance with the GDPR are steep, with potential fines of up to 4% of our global revenue.
Because we have operations in Switzerland, we also are required to comply with the data privacy and security laws and regulations of that country. As we conduct clinical trials and other activities that involve the collection of personal data from individuals in other countries and regions, we may be subject to additional data privacy and security laws and regulations.
Across the United States and globally, laws and regulations governing data privacy and security continue to develop and evolve. The data privacy and security laws and regulations to which we are subject will likely impact (possibly significantly) our business activities. Many of the laws and regulations that apply to us contain ambiguous provisions or impose requirements that differ from country to country, creating uncertainty. Compliance with the enhanced obligations imposed by such laws and regulations may require us to revise our business practices, allocate more resources to privacy and security, and implement new technologies. Such efforts may result in significant costs to our business.
Failure to comply with data privacy and security laws and regulations could result in regulatory penalties and significant legal liability and could have a material adverse impact on our financial results.
25
Manufacturing, Revenues from External Customers, and Sources and Availability of Materials
We recognized revenues of $0.1 million for the year ended December 31, 2021. Our revenues of $0.3 million for the year ended December 31, 2020 primarily included royalty revenue from Eisai. Our revenues of $806.4 million for the year ended December 31, 2019 included an $800.0 million upfront payment from United Therapeutics, $5.0 million from Everest, and $1.7 million from Boehringer Ingelheim. This information excludes revenue activity reported within discontinued operations. We do not currently engage in manufacturing activities and we are not dependent on availability of materials for our core business operations.
Compliance with Environmental Regulations
Our business involves the controlled use of hazardous materials, chemicals, biological materials and various radioactive compounds. In the United States, we are subject to regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the US Environmental Protection Agency, the California Environmental Protection Agency, the Toxic Substances Control Act, the Resource Conservation and Recovery Act, the CSA and other federal, state or local regulations.
We may be subject to further such regulations in the future. Although we believe that our operations comply in all material respects with the applicable environmental laws and regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. In the event of such an accident, we could be held liable for any damages that result, and the extent of that liability could exceed our resources. Our compliance with these laws and regulations has not had, and is not expected to have, a material effect upon our capital expenditures, results of operations or competitive position.
Human Capital
Arena’s leadership and employees are guided by our purpose – to deliver our important medicines and a simple statement that defines how we approach everything we do: Care More. Act Differently.
As of December 31, 2021, we employed 448 full and part-time employees located primarily in our three “hubs” – San Diego, CA, Boston, MA and Zug, Switzerland, as well as home-based employees across the US and Europe. In addition to our employees, we engage consultants, independent contractors, and temporary employees to provide flexibility in support of our business execution and objectives.
We know our success is anchored in our ability to recruit and retain talented, highly skilled team members. We operate in a very competitive employment landscape and recognize that what differentiates Arena from others is more than fair, equitable and competitive salaries, broad-based equity ownership, high-quality health and well-being programs, significant investments in employee development and safe, healthy and conscious workspaces – these are price of entry. We think what differentiates Arena is that we are guided by both our purpose and through the lens of our values – Put People First, Be Curious, Think Disruptive and Be Daring –- we actively strive to inspire and cultivate a vibrant, thriving and resilient culture that engenders a sense of purpose, community, connection and gratitude. We also encourage a workplace that is diverse and inclusive at all levels and we continue to strengthen our diversity and inclusion initiatives through our IDE@A (Inclusion, Diversity and Equity at Arena) employee advocacy committee that promotes sharing, learning and engagements in diversity, equity, and inclusion topics and activities.
Our Human Capital practices and commitments are also incorporated into our annual corporate goals and corporate performance metrics. In 2021, we have also added a specific corporate objective related to diversity and inclusion and our Environmental, Social and Governance (ESG) initiatives.
For additional perspective on our human capital practices and resources, please also see our Corporate ESG Report, proxy statement and corporate website.
Available Information
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, or the Exchange Act, are available free of charge on our website (www.arenapharm.com) as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC.
26
Item 1A. Risk Factors.
RISK FACTORS
Investment in our stock involves a high degree of risk. You should consider carefully the risks described below, together with other information in this Annual Report on Form 10-K and our other filings with the SEC, before making investment decisions regarding our stock. If any of the following events actually occur, our business, operating results, prospects or financial condition could be materially and adversely affected. This could cause the trading price of our common stock to decline and you may lose all or part of your investment. Moreover, the risks described below are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also affect our business, operating results, prospects or financial condition.
Risks Relating to Our Pending Transaction with Pfizer
Failure to complete, or delays in completing, the pending transaction with Pfizer announced on December 13, 2021 could materially and adversely affect our results of operations and our stock price.
On December 12, 2021, we entered into an Agreement and Plan of Merger, or the Merger Agreement, with Pfizer Inc. and Antioch Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of Pfizer, or Merger Sub, pursuant to which, if all of the conditions to closing are satisfied or waived, Merger Sub will be merged with and into our company with our company (the "Merger") surviving as a wholly owned subsidiary of Pfizer. Consummation of the transaction is subject to certain closing conditions, some of which are not within our control, and may prevent, delay, or otherwise materially adversely affect the completion of the transaction. We cannot predict with certainty whether and when any of the required closing conditions will be satisfied or if additional uncertainties may arise and cannot assure that we will be able to successfully consummate the pending transaction as currently contemplated under the Merger Agreement or at all. Risks related to the failure of the pending transaction to be consummated include, but are not limited to, the following:
•the trading price of our stock may decline to the extent that the current market price for our stock reflects a market assumption that the Merger will be completed;
•under some circumstances, we may be required to pay a termination fee to Pfizer of $235 million;
•potential adverse effects on our relationships with current collaboration partners, suppliers and other business partners, or those with which we are seeking to establish business relationships, due to uncertainties about the Merger;
•we will remain liable for significant transaction costs, including legal, financial advisory, accounting, and other costs relating to the transaction regardless of whether the Merger is consummated;
•the attention of our management may have been diverted to the Merger; and
•we could be subject to litigation related to any failure to complete the Merger.
Additionally, the performance of our employees may be negatively affected during the pendency of the transaction as employees may experience uncertainty about their future roles following completion of the Merger, and likewise it may be difficult to attract new employees during the pendency of the transaction.
Further, under the Merger Agreement, we are generally required to conduct in all material respects our business in the ordinary course, consistent with past practice and are restricted from taking certain specified actions absent Pfizer’s prior written consent, including pursuing opportunistic financing through the sale of our equity or from sources on terms that we find favorable, which restrictions could adversely affect our ability to conduct our business as we otherwise would have done if we were not subject to these restrictions.
The occurrence of any of these events individually or in combination could materially and adversely affect our business, results of operations, financial condition, and our stock price.
27
Lawsuits have been filed against us and the members of our board of directors arising out of the pending transaction, and additional such lawsuits may be filed in the future, which may delay or prevent the pending transaction.
Stockholder complaints and other complaints have been filed against us and our board of directors in connection with the transactions contemplated by the Merger Agreement, and additional such lawsuits may be filed in the future. The outcome of litigation is uncertain, and we may not be successful in defending against any such claims. These lawsuits could delay or prevent the transaction, divert the attention of our management and employees from our day-to-day business, result in substantial costs to us, and otherwise adversely affect our business, results of operations, and financial condition.
The ability to complete the transaction is subject to the receipt of consents and approvals from government entities, which may impose conditions that could have an adverse effect or could cause either party to abandon the transaction.
Completion of the transaction is conditioned upon, among other things, the expiration or termination of the required waiting periods under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, or the HSR Act, and the receipt of certain other regulatory approvals. In deciding whether to grant antitrust approvals, the Federal Trade Commission, or the FTC, and other regulatory agencies will consider the effect of the transaction on competition. The FTC, or other regulatory agencies, may condition their approval of the transaction on Pfizer’s or our agreement to various requirements, limitations, or costs, or require divestitures or place restrictions on the conduct of Pfizer’s business following the transaction. In addition, these requirements, limitations, costs, divestitures, or restrictions may result in the delay or abandonment of the transaction.
The Merger Agreement with Pfizer limits our ability to pursue alternative transactions which could deter a third party from proposing an alternative transaction.
The Merger Agreement contains provisions that, subject to certain exceptions, limit our ability to initiate, solicit, knowingly encourage or facilitate the making of any offer or proposal which constitutes or is reasonably likely to lead to an acquisition proposal of Arena. It is possible that these or other provisions in the Merger Agreement might discourage a potential competing acquirer that might have an interest in acquiring all or a significant part of our outstanding common stock from considering or proposing an acquisition or that the price at which Pfizer has proposed to acquire the Company might result in a potential competing acquirer proposing to pay a lower per share price to acquire our common stock than it might otherwise have proposed to pay.
Risks Relating to Our Business
Drug development programs are expensive, time consuming, uncertain and susceptible to change, interruption, delay or termination.
Drug development programs are very expensive, time consuming and difficult to design and implement. Our drug candidates are in various stages of clinical and preclinical development and are prone to the risks of failure inherent in research and development. Clinical trials and preclinical studies are needed to demonstrate that drug candidates are safe and effective to the satisfaction of the FDA, and similar non-US regulatory authorities, and the FDA or other regulatory authority may require us to, or we or others may decide to, conduct additional research and development even after a drug is approved. The commencement or completion of our clinical trials or preclinical studies could be substantially delayed or prevented by several factors, including the following:
•limited number of, and competition for, suitable participants required for enrollment in our clinical trials or animals to conduct our preclinical studies;
•limited number of, and competition for, suitable sites to conduct our clinical trials or preclinical studies;
•delay or failure to obtain a meeting, approval or agreement from the applicable regulatory authority to commence a clinical trial or approve a study protocol or change;
28
•supply chain issues, such as delay or failure to obtain supplies of drug candidates, drugs or other materials, with appropriate packaging and labeling, sufficient for the trial or study;
•delay or failure to reach agreement on acceptable agreement terms or protocols;
•delay or other disruption related to the COVID-19 pandemic; and
•delay or failure to obtain institutional review board, or IRB, approval to conduct a clinical trial at a prospective site.
For example, recruitment for the indications in our ongoing and planned clinical studies is competitive and challenging, and it is difficult to predict when such trials will be fully enrolled or when data will be available, if at all.
In addition, the FDA, other regulatory authorities, collaborators, or we may suspend, delay or terminate our development programs at any time for various reasons, including those listed above affecting the commencement or completion of trials and the following:
•side effects experienced by study participants or other safety issues;
•lack of effectiveness of any drug candidate during clinical trials;
•slower than expected rates of patient recruitment and enrollment or lower than expected patient retention rates;
•difficulty in maintaining contact with participants during or after treatment, which may result in incomplete data;
•inability or unwillingness of medical investigators to follow our clinical protocols;
•inadequacy of or changes in the manufacturing process or compound formulation;
•delays in obtaining regulatory approvals to commence a study, or “clinical holds,” or delays requiring suspension or termination of a study by a regulatory authority, such as the FDA, after a study is commenced;
•changes in applicable regulatory policies and regulations;
•delays in identifying and reaching agreement on acceptable terms with prospective clinical trial sites;
•uncertainty regarding proper dosing;
•unfavorable results from clinical trials or preclinical studies, including those conducted by us, our partners or our licensees;
•failure of our clinical research organizations to comply with all regulatory and contractual requirements or otherwise perform their services in a timely or acceptable manner;
•scheduling conflicts with participating clinicians and clinical institutions;
•failure to design appropriate clinical trial protocols;
•insufficient data to support regulatory approval;
•failure of participating clinicians and clinical institutions to comply with all legal, regulatory and contractual requirements or otherwise perform in a timely or acceptable manner;
•lack of sufficient funding to continue clinical trials or preclinical studies; or
•changes in business priorities or perceptions of the value of the program.
There is typically a high rate of attrition from the failure of drug candidates proceeding through clinical trials, and many companies have experienced significant setbacks in advanced development programs even after promising results were observed in earlier studies or trials. We have experienced setbacks in our internal and partnered development programs and expect to experience additional setbacks from time to time in the future. In addition, even if the earlier-stage results of our development programs are favorable, these programs may take significantly longer than expected to complete or may not be completed at all. If we or our collaborators abandon or are delayed in our development efforts related to any drug or drug candidate, we may not be able to generate sufficient revenues to continue our operations at the current or planned level or be profitable, our reputation in the industry and in the investment community would likely be significantly
29
damaged, additional funding may not be available to us or may not be available on terms we or others believe are favorable, and our stock price may decrease significantly.
We may not be successful in initiating, enrolling participants in, or completing our studies or trials or advancing our programs on our projected timetable, if at all. Any failure to initiate or delays in our studies, trials or development programs, or unfavorable results or decisions or negative perceptions regarding any of our programs, could cause our stock price to decline significantly. This is particularly the case with respect to our clinical programs.
We will need to obtain additional funds or enter into collaboration agreements to execute on our corporate strategy, and we may not be able to do so at all or on terms you view as favorable; your ownership may be substantially diluted if we do obtain additional funds; you may not agree with the manner in which we allocate our available resources; and we may not be profitable.
It takes many years and potentially hundreds of millions of dollars to successfully develop a compound into a marketed drug. We have accumulated a large deficit that has primarily resulted from the significant expenditures we have made in research and development since our inception. We expect that our losses and operating expenses will continue to be substantial.
All of our internal programs are in the development stage, and we may not have adequate funds to develop all of our compounds into marketed drugs.
We may seek to obtain additional funding through the capital markets or other financing sources. Additional funding may not be available to us or may not be available on terms we or others believe are favorable, including due to negative impacts on the stock market and investor sentiment resulting from the COVID-19 pandemic. Our ability to obtain additional funding may depend on many factors, including those outside our control. Should we obtain additional funding, your ownership interest may be diluted or otherwise negatively impacted.
We have entered into, and may in the future seek to enter into, collaboration or other agreements with other entities to continue to develop and, if successful, commercialize one or more of our drug candidates. We may not be able to enter into any such agreements on terms that we or third parties, including investors or analysts, view as favorable, if at all. Our ability to enter into any such agreement for any of our programs or drug candidates depends on many factors, potentially including the outcomes of additional testing (including clinical trial results) or regulatory applications for marketing approval, and we do not control these outcomes.
We may allocate our resources in ways that do not improve our results of operations or enhance the value of our assets, and our stockholders and others may also not agree with the manner in which we choose to allocate our resources or obtain additional funding. We may also eliminate, scale back, or delay some or all of our research and development programs, and any such reductions or failure to apply our resources effectively or to obtain additional funding could narrow, slow, or otherwise adversely impact the development and commercialization of one or more of our drug candidates, which could reduce our opportunities for success and have a material adverse effect on our business, our prospects, and the market price of our common stock.
In addition, we cannot assure you that we will be profitable or, if we are profitable for any particular time period, that we will be profitable in the future.
Our business may be negatively impacted based on the clinical trials and preclinical studies of, and decisions affecting, one or more of our drug candidates.
The results and timing of clinical trials and preclinical studies obtained by us or our collaborators or licensees, as well as related decisions by us, collaborators, licensees, and regulators, can affect our stock price. Results of clinical trials and preclinical studies are uncertain and subject to different interpretations by regulatory agencies, us, or others. The design of these trials and studies (which may change significantly and be more expensive than anticipated depending on results and regulatory decisions), as well as related analyses of such results, including adverse effects, may not be viewed favorably by us or third parties, including investors, analysts, current or potential collaborators, the academic and medical communities, and regulators, which could adversely impact the development and opportunities for regulatory approval of drug candidates and commercialization (and even result in withdrawal from the market) of approved drugs. The same may be true of decisions regarding the focus and prioritization of our research and development efforts. Stock prices of
30
companies in our industry have declined significantly when such results and decisions were unfavorable or perceived negatively or when a drug candidate or product did not otherwise meet expectations.
The development, approval or commercialization of any of our drug candidates could be negatively affected by circumstances related to other drug candidates or approved products.
Information on our drug candidates in clinical development is preliminary and incomplete, and for such drug candidates, particularly in the earlier stages of development, information on approved products in the same or related drug classes may indicate potential risks related to the development of our drug candidates. In particular, safety issues affecting other drugs or drug candidates may result in increased regulatory scrutiny of the safety of our drugs or drug candidates, may raise potential adverse publicity, and may affect product sales or result in litigation.
For example, etrasimod is an orally available modulator of the S1P receptors. Other orally available modulators of the S1P receptors, such as GILENYA, have been associated with risks such as adverse cardiovascular effects, including lowering of the heart rate and heart blocks, infection, macular edema, respiratory effects, fetal risk, a rare brain infection, and elevations in liver enzymes. These adverse reactions and risks may be associated with S1P receptor modulation and could be found to be associated with the use of etrasimod. Such adverse reactions and risks, either actual or perceived, could negatively impact the development, approval, or commercialization of etrasimod, or our ability to enter into a collaboration on acceptable terms.
Topline data may not accurately reflect the complete results of a particular study or trial.
We may publicly disclose topline or interim data from time to time, which are based on preliminary analyses of then-available efficacy and safety data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial.
We also make assumptions, estimations, calculations, and conclusions as part of our analyses of data, and others, including regulatory agencies, may not accept or agree with our assumptions, estimations, calculations, conclusions, or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular drug candidate or drug, and our company in general. In addition, the information we may publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you, regulators, or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure, and any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities, or otherwise regarding a particular drug, drug candidate or our business.
Our hypothesis that selectively targeting receptors can lead to more efficacious or safer drugs may not be correct.
In general, we have designed and optimized the drug candidates that we or our collaborators and licensees are developing (including etrasimod, olorinab, APD418, temanogrel and ralinepag) to selectively target certain receptors found on cells in humans. Our hypothesis is that selectivity may allow our drug candidates to address diseases more efficaciously or without some of the negative effects associated with less selective drugs. In certain cases, we believe early research and, if available, early clinical testing, provides preliminary support for our hypothesis. However, our hypothesis may not be correct, early research and early phase clinical testing may not be predictive of efficacy or safety in later trials, and our drug candidates may not be approved or, if approved, have the desired efficacy or safety profile. For example, our Phase 2b trial of olorinab for IBS failed to meet its primary endpoint.
It is generally our strategy to develop drug candidates that we believe will be first-in-class, best-in-class, or similar descriptions, or otherwise have broad clinical utility, optimized pharmacology, or optimized pharmacokinetics. Some or all of our drug candidates may not achieve these goals. For example, failure to complete enrollment in clinical trials on schedule or at all could prevent a drug candidate from being first-in-class. Similarly, comparing data from different trials, or making predictions based on preclinical data, may not allow us to correctly determine whether our drug candidates are superior to competitive drugs or drug candidates in the same way that comparisons can be made from conducting trials in which our and a competitive drug is tested “head to head” in the same trial. The failure of our drugs or drug candidates to be first-in-class, best-in-class, or similar descriptions, or have broad clinical utility, optimized pharmacology, or optimized pharmacokinetics, or a lack of “head to head” data, could adversely affect development, regulatory approval, third-party payer support, or market adoption, which could have a material adverse impact on our business.
31
The results of preclinical studies and completed clinical trials are not necessarily predictive of future results, and our current drug candidates or any approved drugs may not be further developed or have favorable results in later studies or trials.
Preclinical studies and Phase 1 and Phase 2 clinical trials are not primarily designed to test the efficacy of a drug candidate, but rather to establish potential mechanisms of action, test safety, study pharmacokinetics and pharmacodynamics, and understand the drug candidate’s side effects at various doses, schedules, or routes of administration. Favorable results in early studies or trials may not be confirmed in later studies or trials, including preclinical studies that continue or that are initiated after earlier clinical trials and large-scale clinical trials, and our drug candidates or drugs in subsequent trials or studies may fail to show desired safety and efficacy despite having progressed through earlier-stage trials. For example, we have announced positive topline Phase 2 results for etrasimod in participants with ulcerative colitis, but these results may not be confirmed in any subsequent Phase 3 study. By way of another example, the impact of etrasimod on heart rate that was observed in completed clinical trials may be observed in subsequent trials, and it could be viewed negatively by the FDA or other regulatory agencies.
Unfavorable results from clinical trials or preclinical studies could result in delays, modifications, or abandonment of ongoing or future clinical trials, or abandonment of a program. Clinical and preclinical results are frequently susceptible to varying interpretations that may delay, limit, or prevent regulatory approvals or commercialization. Negative or inconclusive results or adverse medical events during such trials or studies could cause a clinical trial to be delayed, repeated, or terminated; a program to be abandoned; or negatively impact a related marketed drug, which could have a material adverse effect on our business, financial condition, and results of operations.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay, or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, EMA, or other comparable foreign authorities. Results of our trials could reveal a high and unacceptable severity and prevalence of these or other side effects. In such an event, our trials could be suspended or terminated, and the FDA, EMA, or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. The drug-related side effects could affect patient recruitment or the ability of enrolled participants to complete the trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition, and prospects significantly.
Additionally, if one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including:
•regulatory authorities may withdraw approvals of such product;
•regulatory authorities may require additional warnings on the label;
•we may be required to create a medication guide outlining the risks of such side effects for distribution to patients;
•we could be sued and held liable for harm caused to patients; and
•our reputation may suffer.
For example, in February 2020 the FDA issued a drug safety communication announcing that it requested Eisai voluntarily withdraw lorcaserin (previously marketed in the United States as BELVIQ and BELVIQ XR) from the US market based on the FDA’s analysis of data from a study completed by Eisai and a change in the FDA’s risk-benefit assessment of BELVIQ. Eisai agreed to voluntarily withdraw lorcaserin products from the US market, as requested by the FDA, and from foreign markets. Following these events, lawsuits relating to lorcaserin against us and others have been filed in the United States and abroad.
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results of operations and prospects.
32
Drug discovery and development is intensely competitive in the therapeutic areas on which we focus. If the number of our competitors increase or they develop treatments that are approved faster, marketed better, less expensive, or demonstrated to be more effective or safer than our drugs or drug candidates, our commercial opportunities could be reduced or eliminated.
Many of the drugs we or our collaborators are attempting or may attempt to discover and develop may compete with existing therapies in the United States and other territories. In addition, many companies are pursuing the development of new drugs that target the same diseases and conditions that we target. For example, with regard to etrasimod, there are other drugs that have a similar mechanism of action that entered Phase 3 clinical development before etrasimod for the same indications that we are pursuing, such as ulcerative colitis.
Our competitors, particularly large pharmaceutical companies, may have substantially greater research, development, marketing, and sales capabilities and greater financial, scientific, and human resources than we do. Companies that complete clinical trials, obtain required regulatory agency approvals, and commence commercial sale of their drugs before we do for the same indication may achieve a significant competitive advantage, including certain patent and marketing exclusivity rights. In addition, our competitors’ drugs may have fewer side effects, more desirable characteristics (such as efficacy, route of administration, or frequency of dosing), or be viewed more favorably by patients, healthcare providers, healthcare payers, the medical community, the media, or others than our drug candidates or drugs, if any, for the same indication. Our competitors may also market generic or other drugs that compete with our drugs at a lower price than our drugs, which may negatively impact our drug sales, if any. Any results from our research and development efforts, or from our joint efforts with our existing or any future collaborators, may not compete successfully with existing or newly discovered products or therapies.
Our revenues in the future will be substantially dependent on the success of our or our collaborators’ and licensees’ marketing of drugs we have discovered or developed. To the extent such drugs are not commercially successful, our business, financial condition, and results of operations may be materially adversely affected, and the price of our common stock may decline.
We believe our revenues will be substantially dependent on the success of the drugs we or our collaborators and licensees successfully develop. We do not know whether or when such drug candidates will be approved by regulatory authorities for sale or commercialized. Even if approved and commercialization begins, we do not know if such commercialization will be successful or otherwise meet our, your, analysts’, or others’ expectations, and the market price of our common stock could decline significantly. For example, sales of lorcaserin to date have been less than we and others initially anticipated, and, in February 2020, Eisai (as well as its distributor in South Korea) determined to withdraw lorcaserin (previously marketed in the United States as BELVIQ and BELVIQ XR) from the market based on concerns raised by the FDA.
We cannot guarantee future product sales or achievement of milestones under our collaborations and license agreements. For example, our license agreement with United Therapeutics for ralinepag does not contain a covenant obligating United Therapeutics to use any particular efforts to develop or commercialize any product, and we may never receive any milestone or royalty payments under this license agreement. In addition, our collaboration and license agreements may be terminated in certain circumstances, which may result in us not receiving additional milestone or other payments under the terminated agreement.
The degree of market acceptance and commercial success of a drug will depend on a number of factors, including the following, as well as risks identified in other risk factors:
•the number of patients treated with the drug and their results;
•market acceptance and use of the drug, which may depend on the public’s awareness and view of the drug, economic changes, national and world events, potentially seasonal and other fluctuations in demand, the timing and impact of current or new competition, and the drug’s perceived advantages or disadvantages over alternative treatments (including relative convenience, ease of administration, and prevalence and severity of any adverse events, including any unexpected adverse events);
•the actual and perceived safety and efficacy of the drug on both a short- and long-term basis among actual or potential patients, healthcare providers and others in the medical community, regulatory agencies, and insurers and other payers, including related decisions by any such entity or individual;
•incidence and severity of any side effects, including as a result of off-label use or in combination with one or more drugs;
33
•new data relating to the drug, including as a result of additional studies, trials, or analyses of the drug or related drugs or drug candidates, whether conducted by us or by others;
•physicians’ awareness of the drug, and the willingness of physicians to prescribe and of patients to use the drug;
•the claims, limitations, warnings, and other information in the drug’s current or future labeling;
•any current or future scheduling designation for the drug by the US Drug Enforcement Administration, or DEA, or any comparable foreign authorities;
•our or our collaborators’ maintenance of an effective sales force, marketing team, strategy, program, medical affairs group, and related functions, as well as our or our collaborators’ sales, marketing, and other representatives accurately describing the drug consistent with its approved labeling;
•the price and perceived cost-effectiveness of the drug, including as compared to possible alternatives;
•the ability of patients and physicians and other providers to obtain and maintain coverage and adequate reimbursement, if any, by third-party payers, including government payers;
•the ability and desire of group purchasing organizations, or GPOs, including distributors and other network providers, to sell the drug to their constituencies;
•introduction of counterfeit or unauthorized versions of the drug;
•to the extent the drug is approved and marketed in a jurisdiction with a significantly lower price than in another jurisdiction, the impact of the lower pricing in the higher-priced territory, including on the pricing of reimbursement, if available, and by the diversion of lower-priced of the drug into the higher-priced territory; and
•the availability of adequate commercial manufacturing and supply chain for the drug.
Our drugs may not be commercially successful if not widely covered and adequately reimbursed by third-party payers, and we may depend on others to obtain and maintain third-party payer access; inadequate third-party coverage and reimbursement could make entering into agreements with pharmaceutical companies to collaborate or commercialize our drugs more difficult and diminish our revenues.
Our and our collaborators’ and licensee’s ability to successfully commercialize any of our drugs that have been or may be approved will depend, in part, on government regulation and the availability of coverage and adequate reimbursement from third-party payers, including private health insurers and government payers, such as the Medicaid and Medicare programs, increases in government-run, single-payer health insurance plans, and compulsory licenses of drugs. We expect government and third-party payers will continue their efforts to contain healthcare costs by limiting coverage and reimbursement levels for new drugs. In addition, many countries outside of the US have nationalized healthcare systems in which the government pays for all such products and services and must approve product pricing, and some US politicians advocate for implementation of a comparable system in the United States. A government or third-party payer decision not to approve pricing, or provide adequate coverage and reimbursements, for our drugs, if any, could limit market acceptance of and demand for our drugs.
It is increasingly difficult to obtain coverage and adequate reimbursement levels from third-party payers, and significant uncertainty exists as to the coverage and reimbursement of newly approved prescription drug products. We or our collaborators also face competition in negotiating for coverage from pharmaceutical companies and others with competitive drugs or other treatment, and these competitors may have significantly more negotiating leverage or success with respect to individual payers than we or our collaborators may have.
Federal and state healthcare reform measures that have been or may be implemented in the future may result in more rigorous coverage criteria, more limited coverage and downward pressure on the price that we may receive for any approved product, which could seriously decrease our future revenues. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the ACA, which was enacted in 2010, is one such healthcare reform measure that has made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. In the years since its enactment, there have been significant developments in, and legislative, executive, and judicial activity around, attempts to repeal, replace, or modify the ACA. While Congress has not passed comprehensive repeal legislation, several bills affecting the implementation of certain taxes under the ACA have
34
been signed into law. Legislation enacted in 2017, informally titled the Tax Cuts and Jobs Act of 2017, or TCJA, includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Additionally, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the ACA-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminated the health insurer tax. Further, the Bipartisan Budget Act of 2018, or the BBA, among other things, amended the ACA, effective January 1, 2019, to increase from 50 percent to 70 percent the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole”. On June 17, 2021 the US Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Thus, the ACA will remain in effect in its current form. Further, prior to the US Supreme Court ruling, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace, which began on February 15, 2021 and remained open through August 15, 2021. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges and the healthcare reform measures of the Biden administration will impact the ACA and our business and operations.
In addition, there has been heightened scrutiny in the United States and other countries of pharmaceutical pricing practices in light of the rising cost of prescription drugs and biologics. In the United States, such scrutiny has resulted in congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. At the federal level, the Trump administration used several means to propose or implement drug pricing reform, including through federal budget proposals, executive orders and policy initiatives. For example, on July 24, 2020 and September 13, 2020, President Trump announced several executive orders related to prescription drug pricing that attempted to implement several of the administration’s proposals. As a result, the FDA concurrently released a final rule and guidance in September 2020, providing pathways for states to build and submit importation plans for drugs from Canada. Further, on November 20, 2020, the US Department of Health and Human Services, or HHS, finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The implementation of the rule has been delayed by the Biden administration from January 1, 2022 to January 1, 2023 due to ongoing litigation. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers, the implementation of which have also been delayed by the Biden administration until January 1, 2023. On November 20, 2020, the Centers for Medicare and Medicaid Services, or CMS, issued an interim final rule implementing President Trump’s Most Favored Nation executive order, which would tie Medicare Part B payments for certain physician-administered drugs to the lowest price paid in other economically advanced countries. As a result of litigation challenging the Most Favored Nation model, on December 27, 2021, CMS published a final rule that rescinded the Most Favored Nation Model interim final rule. In July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to Biden’s executive order, on September 9, 2021, HHS released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue to advance these principles. In addition, Congress is considering drug pricing as part of other reform initiatives. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. It is possible that additional governmental action is taken in response to the COVID-19 pandemic. For example, the CARES Act and other COVID-19 relief legislation suspended the 2% Medicare rate reduction sequester from May 1, 2020 through March 31, 2022. Under current legislation the actual reduction in Medicare payments will vary from 1% in 2022 to up to 3% in the final fiscal year of this sequester. Additionally, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. Congress is considering additional health reform measures.
Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payers. The implementation of additional cost containment measures or other
35
healthcare reforms may also limit our commercial opportunities by reducing the amount a potential collaborator or licensee is willing to pay to license our programs or drug candidates in the future, which may prevent us from being able to establish and maintain collaborations and license agreements, generate revenue, attain profitability, or commercialize our products.
In the European Union, similar political, economic and regulatory developments may affect our ability to profitably commercialize our product candidates, if approved. In addition to continuing pressure on prices and cost containment measures, legislative developments at the European Union or member state level may result in significant additional requirements or obstacles that may increase our operating costs. The delivery of healthcare in the European Union, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than European Union, law and policy. National governments and health service providers have different priorities and approaches to the delivery of healthcare and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most European Union member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing European Union and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize our product candidates, if approved.
In markets outside of the United States and the European Union, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies.
Certain Governments outside of the United States impose strict price controls, which may adversely affect our revenues, if any.
In some countries, particularly the countries of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed, possibly materially.
Forecasting potential sales for drugs will be difficult, and if our projections are inaccurate, our business and stock price may be adversely affected.
Our business planning requires us to forecast or make assumptions regarding demand and revenues for our drugs if they are approved despite numerous uncertainties. These uncertainties may be increased if we rely on our collaborators to conduct commercial activities and provide us with accurate and timely information. Actual results may deviate materially from projected results for various reasons, including the following, as well as risks identified in other risk factors:
•the rate of adoption in the particular market, including fluctuations in demand for various reasons, such as fluctuations related to economic changes, national and world events, holidays, and seasonal changes;
•pricing (including discounting or other promotions), reimbursement, product returns or recalls, competition, labeling, DEA scheduling, adverse events, and other items that impact commercialization;
•lack of patient and physician familiarity with the drug;
•lack of patient use and physician prescribing history;
•lack of commercialization experience with the drug;
•actual sales to patients may significantly differ from expectations based on sales to wholesalers;
•uncertainty relating to when the drug may become commercially available to patients and rate of adoption in other territories; and
•other changes in regulatory or commercial conditions.
36
Revenues from drug sales may be based in part on estimates, judgment, and accounting policies, and incorrect estimates or regulators’ or others’ disagreement regarding such estimates or accounting policies may result in changes to guidance, projections, or previously reported results. Expected and actual product sales and quarterly and other results may greatly fluctuate, and such fluctuations can adversely affect the market price of our common stock, perceptions of our ability to forecast demand and revenues, and our ability to maintain and fund our operations.
Our efforts will be seriously jeopardized if we are unable to attract and retain key and other employees.
Our success depends on the continued contributions of our principal management, development, and scientific personnel and the ability to hire and retain key and other personnel. We face competition for such personnel, and we believe that risks and uncertainties related to our business may impact our ability to hire and retain key and other personnel. If we do not recruit and retain effective management and other key employees, particularly our executive officers, our operations, our ability to generate or raise additional capital, and our business in general, may be adversely impacted. For example, to execute our clinical programs, our strategy is to maintain a sufficient and robust program management function with clinical expertise. We are in the process of modifying and building this function, and we may not be able to establish the function we believe necessary to support our clinical goals and meet our corporate objectives.
We are expanding our organization and may experience difficulties in managing this growth, which could disrupt our operations.
Although we reduced our recruiting and hiring activities in light of the ongoing COVID-19 pandemic, as part of our long-term business plan, we are seeking to expand our employee base to increase our managerial, scientific, operational, manufacturing supply, commercial, financial and other resources and to hire more consultants and contractors, including in and outside of our office locations to scale Arena for growth, provide visibility to existing and planned projects, and improve program prioritization across the organization. Future growth will impose significant additional responsibilities on our management, including the need to identify, recruit, maintain, motivate and integrate additional employees, consultants and contractors. Also, our management may need to divert attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees, and reduced productivity among remaining employees. Our future growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. Moreover, if our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenues could be reduced, and we may not be able to implement our business strategy. Our future financial performance and our ability to develop and then commercialize any approved products and compete effectively will depend, in part, on our ability to effectively manage any future growth.
Data generated or analyzed with respect to product use in the market or required postmarketing or other studies or trials may result in decreased demand, lower sales, product recall, regulatory action, or litigation.
An NDA holder (or the equivalent outside the United States) is responsible for assessing and monitoring the safety of a drug that has been approved for marketing, including reviewing reports of adverse safety events. In addition, NDA holders often conduct additional studies or trials or analyze new or previous data related to an approved drug, including with respect to required postmarketing studies and in connection with seeking additional regulatory approvals in new territories.
Any new data generated, including from adverse event reports or required postmarketing, registration, or other studies or trials, may result in label changes, adversely affect sales or development, result in withdrawal of the drug from the market, or result in litigation. In addition, analyses of previous data can have similar risks. Regulatory agencies may consider the new data or analyses in reviewing marketing applications for drug candidates in their territories or impose post-approval requirements that require significant additional expenditures. For example, in February 2020, the FDA requested that Eisai withdraw lorcaserin (previously marketed in the United States as BELVIQ and BELVIQ XR) from the US market based on the FDA’s analysis of data from a study completed by Eisai and a change in the FDA’s risk-benefit assessment of BELVIQ, and regulators in other countries have taken similar steps. Eisai agreed to voluntarily withdraw lorcaserin products from the US market, as requested by the FDA, and from foreign markets. Following these events, lawsuits relating to lorcaserin against us and others have been filed in the United States and abroad. While these lawsuits remain in preliminary stages and we are actively disputing the allegations contained therein, to the extent they proceed, and
37
the claims they allege are found to have merit and an adverse judgment ensues, the lawsuits may have a material adverse effect on our business or financial condition.
The discovery of significant problems with a product or class of products similar to any approved drug could have an adverse effect on our or our collaborator’s or licensee’s commercialization.
If we license or otherwise partner our drugs, our failure to maintain such agreements or poor performance or results under such agreements could negatively impact our business.
Our collaborators and licensees may have primary responsibility for the regulatory approval, marketing and distribution, and, in certain circumstances, development, of our drug candidate(s) in the territory or territories under the applicable collaboration. We may have limited or no control over our collaborator’s decisions, including the amount and timing of resources that any of these collaborators will dedicate to such activities. This is the case for our ralinepag exclusive license agreement with United Therapeutics and our lorcaserin Transaction Agreement with Eisai.
When we enter collaboration and license agreements, we are subject to a number of other risks, including:
•our collaborators and licensees may not comply with applicable laws or regulatory guidelines, which could adversely impact the development or commercialization of the drug or drug candidate;
•there could be disagreements regarding the agreements or the study or development that delay or terminate the commercialization, research, study, or development, delay or eliminate potential payments under the agreements or increase our costs under or outside of the agreements;
•our collaborators and licensees may not effectively allocate adequate resources, may have limited experience in a particular territory, or may generate unfavorable data or results; and
•our collaborators and licensees may not perform as expected, including with regard to making any required payments, and the agreements may not provide adequate protection or may not be effectively enforced.
We or our collaborators or licensees might terminate our agreements in certain circumstances or amend the terms of our agreement, and investors and analysts may not view any termination or amendment as favorable.
We rely on other companies, including third-party manufacturers and sole-source suppliers, to manufacture all our drugs and drug candidates, and we or such other companies may encounter failures or difficulties or not receive or provide adequate supply, which could adversely affect development or commercialization.
We do not own or operate manufacturing facilities that can produce active pharmaceutical ingredient, or API, intermediates and other material required to make our drug candidates. Instead, we rely on other companies to supply API, intermediates and other materials. Certain of these materials are available from only one or a small number of suppliers, and using a new supplier, if available, could result in substantial delay and greater cost. Our and our manufacturers’ dependence on single or limited sources of materials may adversely affect our ability to develop and deliver drug products on a timely and competitive basis, or at all.
Any performance failure on the part of us or a third-party manufacturer could result in a product recall or seizure or a delay or other adverse effect on sales of an approved product or the clinical development or regulatory approval of one or more of our other drug candidates. We or third-party manufacturers may encounter difficulties involving production yields, regulatory compliance, lot release, quality control, and quality assurance, as well as shortages of qualified personnel.
The ability to adequately and timely manufacture and supply drug product is dependent on the uninterrupted and efficient operation of the manufacturing facilities, which is impacted by many manufacturing variables, including:
•availability or contamination of raw materials and components used in the manufacturing process, particularly those for which we have no other source or supplier;
•ability to accommodate changes in dosage or formulation;
•capacity of our facilities or those of our contract manufacturers;
38
•having the ability to adjust to changes in actual or anticipated use of the facility, including with respect to having sufficient capacity and a sufficient number of qualified personnel;
•facility contamination by microorganisms or viruses or cross contamination;
•compliance with regulatory requirements, including inspectional notices of violation and warning letters;
•maintenance and renewal of any required licenses or certifications;
•changes in actual or forecasted demand;
•timing and number of production runs;
•production success rates and bulk drug yields; and
•timing and outcome of product quality testing.
In addition, we or our third-party manufacturers may encounter delays and problems in manufacturing our drug candidates or drugs for a variety of reasons, including accidents during operation, failure of equipment, delays in receiving materials, natural or other disasters, health epidemics (including COVID-19), political or governmental unrest or changes, social unrest, intentional misconduct, or other factors inherent in operating complex manufacturing facilities. Commercially available starting materials, reagents and excipients may be or become scarce or more expensive to procure, and we may not be able to obtain favorable terms in agreements with subcontractors. We or our third-party manufacturers may not be able to operate our respective manufacturing facilities in a cost-effective manner or in a time frame that is consistent with our expected future manufacturing needs. If we or our third-party manufacturers cease or interrupt production or if our third-party manufacturers and other service providers fail to supply materials, products, or services to us for any reason, such interruption could delay progress on our programs, or interrupt the commercial supply of drug products, with the potential for additional costs and lost revenues. If this were to occur, we may also need to seek alternative means to fulfill our manufacturing needs.
We may not be able to enter into or maintain agreements with manufacturers whose facilities and procedures comply with applicable law. Manufacturers are subject to ongoing periodic inspection (which may be unannounced) by the FDA, the DEA, corresponding state and foreign authorities and other regulatory authorities to ensure strict compliance with Current Good Manufacturing Practices, or cGMPs, regulations, and other applicable government regulations and corresponding foreign standards. We do not have control over a third-party manufacturer’s compliance with these regulations and standards. If we or one of our manufacturers or other company in the supply chain fail to maintain compliance or otherwise experience setbacks, we or they could be subject to civil or criminal penalties, the production of one or more of our drug candidates or any approved products could be interrupted or suspended, or our product could be recalled or withdrawn, resulting in delays, additional costs, and potentially lost revenues.
Our drug candidates are subject to extensive regulation, and we may not receive required regulatory approvals, or timely approvals, for any of our drug candidates.
Preclinical and clinical development, manufacturing, labeling, packaging, storage, recordkeeping, advertising, promotion, export, marketing and distribution, and other activities relating to developing and manufacturing drugs are subject to extensive regulation by the FDA, EMA, and other regulatory agencies. We and others we contract with are subject to periodic inspections (which may be unannounced) by the FDA, DEA, EMA, and other regulatory agencies. Failure to comply with applicable regulatory requirements may, either before or after product approval, subject us to administrative or judicially imposed sanctions that may negatively impact research and development or commercialization, or otherwise negatively impact our business. Regulatory agencies have in the past inspected certain aspects of our business, and we were provided with observations of objectionable conditions or practices with respect to our business. There is no assurance that regulatory agencies will not provide us with observations in future inspections or that we satisfactorily addressed observations provided to us in past inspections.
Regulatory approval of a drug candidate is not guaranteed, and our business and reputation may be harmed by any failure or significant delay in receiving regulatory approval. The number and types of preclinical studies and clinical trials that will be required for FDA approval varies depending on the drug candidate, the disease or condition that the drug candidate is designed to target and the regulations applicable to any particular drug candidate. Despite the time and expense exerted in preclinical and clinical studies, failure can occur at any stage, and we could encounter problems that cause us to abandon clinical trials or to repeat or perform additional preclinical studies and clinical trials.
39
We cannot predict when or whether, or assure you that, our collaborators’ or our past or any future regulatory submissions or responses will be sufficient to the applicable regulatory authority or others, that the applicable regulatory authority or others will consider data or our analyses, interpretations or procedures related to any of our drug candidates as sufficient or persuasive, or that any regulatory authority will ever approve any of our drug candidates in the future.
To market any drugs outside of the United States, we and our current or future collaborators must comply with numerous and varying regulatory requirements of other countries. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks associated with FDA approval as well as additional risks, some of which may be unanticipated. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We or these third parties may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA or any other regulatory authority does not assure or predict with any certainty that any other regulatory authority will approve the drug. The failure to obtain approval in one jurisdiction may negatively impact our ability to obtain approval elsewhere. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any jurisdiction, which could materially impair our ability to generate revenue. In addition, existing regulatory policies and laws may change. We cannot predict the likelihood, nature, or extent of new government regulation, either in the United States or in other countries, or the impact on our drug candidates or drugs. For example, new FDA regulation could delay or prevent marketing approvals, increase the cost of research and development, and result in narrower product labeling and expensive post-marketing requirements.
Fast Track, Breakthrough Therapy, Accelerated Approval, Priority Review, Orphan Drug, or similar designations by the FDA or other applicable regulatory agencies may not lead to a faster development or review process or other intended benefits of such designation.
The FDA may grant Fast Track, Breakthrough Therapy, Accelerated Approval, Priority Review, or other designations to product candidates that meet applicable guidelines in order to speed the availability of certain drugs. Orphan Drug Designation status provides certain financial incentives, including market exclusivity, to drugs intended to treat a rare disease or condition. Other applicable regulatory agencies may grant similar designations. These designations may apply only to the combination of a product candidate and a specific indication or patient population. Product candidates that receive these designations may not actually receive faster clinical development or regulatory review or approval any sooner than other product candidates that do not have such designation, or at all. Furthermore, a product’s receipt of such a designation does not increase the likelihood that the product candidate will receive marketing approval. The FDA or other regulatory agency may also withdraw a designation if it determines that the product candidate no longer meets the relevant criteria.
For example, the FDA has granted Fast Track designation for APD418 for treatment of decompensated heart failure, or DHF, which we refer to as acute heart failure, or AHF, in patients who have heart failure with reduced ejection fraction and for temanogrel for improvement of cardiovascular outcomes and myocardial recovery by the prevention and treatment of microvascular obstruction in patients undergoing percutaneous coronary intervention. Despite receiving these Fast Track designations, such designations may be withdrawn in the future, and in any event APD418 and temanogrel may not actually receive faster clinical development or regulatory review or approval any sooner than other product candidates that do not have such designation, or at all.
Similarly, the FDA has granted Orphan Drug Designation status to etrasimod for the treatment of EoE. Though etrasimod has received Orphan Drug Designation from the FDA, there may be limitations to the exclusivity afforded by such designation, including if the FDA concludes that a later drug is safer, more effective or makes a major contribution to patient care. Further, the granting of Orphan Drug Designation does not alter the standard regulatory requirements and process for obtaining marketing approval.
Our activities and drugs will still be subject to extensive postmarketing regulation if approved.
Following regulatory approval of any of our drug candidates, we and our collaborators will be subject to ongoing obligations and continued regulatory review from the FDA, EMA, and other applicable regulatory agencies, such as continued adverse event reporting requirements. There may also be additional postmarketing obligations imposed by the
40
FDA, EMA, or other regulatory agencies. These obligations may result in significant expense and limit the ability to commercialize such drugs.
The FDA, EMA, or other regulatory agencies may also require that the sponsor of the NDA or foreign equivalent, as applicable, conduct additional clinical trials to further assess approved drugs after approval under a post-approval commitment. Such additional studies may be costly and may impact the commercialization of the drug. Unfavorable trial results from postmarketing studies could negatively impact market acceptance of the drug, limit the revenues we generate from sales, result in the drug’s withdrawal from the market, negatively impact the potential approval of the drug in other territories, and result in litigation.
The FDA, EMA, or other regulatory agencies may also impose significant restrictions on the indicated uses for which a drug may be marketed. Additionally, the FDA may require a Risk Evaluation and Mitigation Strategies, or REMS, program, including in connection with a drug’s approval, to help ensure that the benefits of the drug outweigh its risks. A REMS may be required to include various elements, such as a medication guide or patient package insert, a communication plan to educate healthcare providers of the drug’s risks, limitations on who may prescribe or dispense the drug, requirements that patients enroll in a registry or undergo certain health evaluations or other measures that the FDA deems necessary to ensure the safe use of the drug.
With regard to any drug that receives regulatory approval, the labeling, packaging, adverse event reporting, storage, advertising, and promotion for the drug will be subject to extensive regulatory requirements. We and the manufacturers of our products are also required to comply with cGMP regulations, which include requirements relating to quality control and quality assurance, as well as the corresponding maintenance of records and documentation. Further, regulatory agencies must approve these manufacturing facilities before they can be used to manufacture our products, and these facilities are subject to ongoing regulatory inspections. In addition, regulatory agencies subject a drug, its manufacturer and the manufacturer’s facilities to continual review and inspections. The subsequent discovery of previously unknown problems with a drug, including adverse events of unanticipated severity or frequency, or problems with the facility where the drug is manufactured, may result in restrictions on the marketing of that drug, up to and including withdrawal of the drug from the market. In the United States, the DEA and comparable state-level agencies also heavily regulate the manufacturing, holding, processing, security, recordkeeping, and distribution of drugs that are considered controlled substances, and the DEA periodically inspects facilities for compliance with its rules and regulations.
Our ability to generate revenues from any of our drugs that receive regulatory approval will be subject to a variety of risks, many of which are out of our control.
Despite having been approved for marketing by a regulatory agency, a drug may not gain market acceptance among patients, healthcare providers, healthcare payers or the medical community. We believe that the degree of market acceptance and our ability to generate revenues from such products will depend on a number of factors, including:
•timing of market introduction of our drugs and competitive drugs and alternative treatments;
•physician and patient awareness of our drugs;
•actual and perceived efficacy and safety of our drugs;
•incidence and severity of any side effects;
•potential or perceived advantages or disadvantages as compared to alternative treatments;
•effectiveness of sales, marketing and distribution support;
•price of our future products, both in absolute terms and relative to alternative treatments;
•the general marketplace for the particular drug;
•the effect of current and future healthcare laws on our drug candidates;
•availability of coverage and adequate reimbursement from government and other third-party payers; and
•product labeling or product insert requirements of the FDA or other regulatory authorities.
If our approved drugs fail to achieve market acceptance, we may not be able to generate significant revenues to be profitable.
41
Collaboration and license agreement relationships may lead to disputes, divert management’s attention, expose us to liability, and delay drug development and commercialization, and we may not realize the full commercial potential of our drug candidates or drugs.
We may have conflicts with our prospective, current, or past collaborators or licensees, such as conflicts concerning rights and obligations under our agreements (including, for example, relating to indemnification for product liability claims and losses), the interpretation of preclinical or clinical data, the achievement of milestone or other payments, the ownership of intellectual property, or research and development, regulatory, commercialization, litigation, or other strategy. Collaborators or licensees may stop supporting our drug candidates or drugs, including if they no longer view the program as in their best financial or other interests or they develop or obtain rights to competing drug candidates or drugs. In addition, collaborators or licensees may fail to effectively develop, obtain approval for, or commercialize our drugs, which may result in us not realizing their full commercial potential. If any conflicts arise with any of our current, past, or prospective collaborators or licensees, the other party may act in a manner that is adverse to our interests. Any such disagreement could result in one or more of the following, each of which could delay, or lead to termination of, development or commercialization of our drug candidates or drugs, and in turn prevent us from generating revenues or cause us to incur liabilities:
•unwillingness or inability on the part of a collaborator or licensee to pay for studies or other research, milestones, royalties or other payments that we believe are due to us under a collaboration;
•uncertainty regarding ownership of intellectual property rights arising from our collaboration or license agreement activities, which could prevent us from entering into additional collaborations;
•unwillingness on the part of a collaborator or licensee to keep us informed regarding the progress of its development, regulatory, commercialization, pharmacovigilance, or other activities or to permit public disclosure of the results of those activities;
•slowing or cessation of a collaborator’s or licensee’s research, development, regulatory, or commercialization efforts with respect to our drug candidates or drugs; or
•litigation or arbitration with our collaborator or licensee, or with third parties (including relating to product liability, intellectual property, or other subject matters).
Setbacks, consolidation, and regulation in the pharmaceutical and biotechnology industries could make entering into agreements with pharmaceutical companies, including agreements to collaborate or commercialize our drugs more difficult.
Setbacks in the pharmaceutical and biotechnology industries, such as those caused by safety concerns relating to drugs or drug candidates, as well as competition from generic drugs, litigation and industry consolidation, may have an adverse effect on us, including by making it more difficult to enter into agreements with pharmaceutical companies to collaborate or commercialize our drugs, which in turn could diminish our revenues. For example, the FDA may be more cautious in approving our drug candidates based on safety concerns relating to these or other drugs or drug candidates, or pharmaceutical companies may be less willing to enter into new collaborations or continue existing collaborations if they are integrating a new operation as a result of a merger or acquisition or if their therapeutic areas of focus change following a merger.
Additionally, enhanced regulatory scrutiny of transactions in the healthcare and pharmaceutical industries could also limit our ability to enter into agreements with other pharmaceutical companies on favorable terms or at all. For example, on July 9, 2021, President Biden issued an executive order outlining 72 initiatives by multiple federal agencies, including the FDA and Federal Trade Commission, to reduce "excessive" corporate consolidation and increase competition across the US economy, including in the pharmaceutical industry. Potential actions taken by such agencies in response to the executive order may also make it more difficult for us to enter into agreements with other pharmaceutical companies, on terms we or you view as favorable, or at all.
We and our collaborators rely on third parties to conduct clinical trials and preclinical studies. If those parties do not comply with regulatory and contractual requirements, successfully carry out their contractual obligations, or meet expected deadlines, our drug candidates may not advance in a timely manner or at all.
In the course of our discovery, preclinical testing, and clinical trials, we and our collaborators rely on third parties, including investigators, clinical research organizations, manufacturers, and laboratories, to perform critical services. For example, we rely on third parties to conduct our clinical trials and many of our preclinical studies. Clinical research organizations are responsible for many aspects of the trials, including finding and enrolling participants for testing
42
and administering the trials. Although we rely on these third parties to conduct our clinical trials, we are responsible for ensuring that each of our clinical trials is conducted in accordance with its investigational plan and protocol. Moreover, the FDA and foreign regulatory authorities require us to comply with regulations and standards, commonly referred to as Good Clinical Practices, or GCPs, for conducting, monitoring, recording, and reporting the results of clinical trials to ensure that the data and results are scientifically credible and accurate and that the trial participants are adequately informed of the potential risks of participating in clinical trials. Our reliance on third parties does not relieve us of these responsibilities and requirements. These third parties may not be available when we need them or, if they are available, may not comply with all legal, regulatory, and contractual requirements or may not otherwise perform their services in a timely or acceptable manner, and we may need to enter into new arrangements with alternative third parties, and our preclinical studies or clinical trials may be extended, delayed, or terminated. These independent third parties may also have relationships with other commercial entities, some of which may compete with us. If these third parties cannot adequately and timely fulfill their obligations to us for any reason, including due to disruptions caused by the COVID-19 pandemic, or if such third parties otherwise fail to meet deadlines, our development programs and/or regulatory reviews for marketing approvals may be extended, delayed, or terminated. In addition, if such third parties fail to perform their obligations in compliance with legal and regulatory requirements and our protocols, our preclinical studies or clinical trials may not meet regulatory requirements or may need to be repeated. As a result of our dependence on third parties, we may face delays or failures outside of our direct control. These risks also apply to the development activities of collaborators, and we do not control their research and development, clinical trial, or regulatory activities.
We may participate in new strategic transactions that could impact our liquidity, increase our expenses, present significant distractions to our management, and be viewed as unfavorable.
From time to time we consider strategic transactions, such as out-licensing or in-licensing of compounds or technologies, acquisitions of companies, asset purchases, and spin-offs. Additional potential transactions we may consider include a variety of different business arrangements, such as strategic collaborations, joint ventures, restructurings, divestitures, business combinations, and investments. In addition, another entity may pursue us as an acquisition target. Any such transaction may be viewed as unfavorable by our stockholders or others and may require us to incur non-recurring or other charges, may create potential liabilities, may increase our near- and long-term expenditures, and may pose significant integration challenges, require additional expertise, or disrupt our management or business, any of which could harm our operations and financial results.
When we evaluate significant proposed transactions we conduct business, legal, and financial due diligence with the goal of identifying and evaluating material risks involved in the transaction. Despite our efforts, we ultimately may be unsuccessful in ascertaining or evaluating all such risks and, as a result, might not realize the intended advantages of the transaction. If we fail to realize the expected benefits from any transaction we may consummate, whether as a result of unidentified risks, integration difficulties, regulatory setbacks, or other events, our business, results of operations, and financial condition could be adversely affected.
We may incur substantial liabilities for any product liability claims or otherwise as a drug product developer.
We develop, test, manufacture, and expect to commercialize drugs for use by humans. We face an inherent risk of product liability exposure related to the testing of our drug candidates in clinical trials, and a risk with the commercialization of lorcaserin (previously marketed in the United States as BELVIQ and BELVIQ XR) as well as any other drug that may be approved for marketing.
Whether or not we are ultimately successful in any product liability or related litigation, such litigation would consume substantial amounts of our financial and managerial resources and might result in adverse publicity, all of which would impair our business. In addition, damages awarded in a product liability action could be substantial and could have a negative impact on our financial condition.
An individual may bring a liability claim against us if one of our drugs or drug candidates causes, or merely appears to have caused, an injury. Regardless of merit or eventual outcome, liability claims may result in:
•decreased demand for our drug;
•injury to our reputation;
•increased difficulty to attract, or withdrawal of, clinical trial participants;
•costs of related litigation;
43
•substantial monetary awards to clinical trial participants, patients, or other claimants;
•loss of revenues; and
•the inability to commercialize our drugs or drug candidates.
We have limited product liability insurance that covers our clinical trials and products as well as indemnification protection in certain of our collaboration or license agreements. Our insurance costs continue to rise, and we may not be able to maintain or obtain insurance coverage at a reasonable cost, we may not have insurance coverage that will be adequate to satisfy any liability that may arise, and our collaborators or licensees may not indemnify us, each of which could have an adverse effect on our results of operations and financial condition.
For example, in December 2016 we granted Eisai an exclusive, royalty-bearing license, or transferred intellectual property, to develop, manufacture, and commercialize lorcaserin (previously marketed in the United States as BELVIQ and BELVIQ XR) in all countries and territories of the world. Our former subsidiary Arena Pharmaceuticals GmbH, or Arena GmbH, manufactured BELVIQ and other products for commercialization or clinical trials up until the sale of that manufacturing business to Siegfried effective March 31, 2018. Under our agreements with Eisai, we and Eisai will each bear 50% of losses arising from any alleged defective manufacturing of BELVIQ by Arena GmbH prior to the date of the sale to Siegfried, and Eisai will be solely responsible for any expenses and losses associated with other product liability claims. In February 2020, the FDA requested that Eisai withdraw lorcaserin products from the US market based on the FDA’s analysis of data from a study completed by Eisai and a change in the FDA’s risk-benefit assessment of lorcaserin, and regulators in other countries have taken similar steps. Eisai agreed to voluntarily withdraw lorcaserin products from the US market, as requested by the FDA, and from foreign markets. Following these events, lawsuits relating to lorcaserin against us and others have been filed in the United States and abroad. Any damages awarded in connection with lorcaserin litigation could be substantial and have a negative impact on our financial condition. Eisai or others could also take the position that some or all claims fall outside their indemnification obligations, or they could be unable to indemnify us, in connection with litigation or other claims, and seeking to enforce rights to indemnification could require significant financial resources and management attention. Even if we are successful in defending against all claims or are fully indemnified or insured, such claims could still consume significant financial resources, divert attention away from our day-to-day activities, and result in adverse publicity, all of which could have a negative impact on our financial condition and our business.
We have significant contractual obligations that may adversely affect our cash flow, cash position, and stock price.
We have long-term leases on real properties and other contractual obligations, and limited revenues. If we are unable to generate cash from operations in the future sufficient to meet our financial obligations we will need to obtain additional funds from other sources, and we may not be able to do so at all or on terms favorable to our stockholders or us.
Also, if we do not have sufficient cash in the future and are unable to generate cash from operations or obtain additional funds from other sources sufficient to meet our contractual obligations, we may have to delay or curtail some or all of our development and commercialization programs, sell or license some or all of our assets on terms that you or others may view as unfavorable, or default on obligations under our agreements.
We may be subject, directly or indirectly, to federal, state, and foreign healthcare laws and regulations, including but not limited to fraud and abuse and false claims laws as well as data privacy and data protection. If we are unable to comply, or have not fully complied, with such laws or regulations, we could face substantial penalties and prosecution.
In the United States, drug manufacturers and marketers are subject to various state and federal fraud and abuse laws, including, without limitation, the Federal Anti-Kickback Statute and Federal False Claims Act. There are similar laws in other countries. These laws may impact, among other things, the research, manufacturing, sales, marketing, and education programs for our drugs.
The Federal Anti-Kickback Statute prohibits persons and entities from knowingly and willingly soliciting, offering, receiving, or providing any remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the purchase, lease, order, or furnishing or arranging for, a good, item, facility, or service, for which payment may be made, in whole or in part, under a federal healthcare program such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been
44
violated. The Federal Anti-Kickback Statute is broad and, despite a series of narrow statutory exceptions and regulatory safe harbors, prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Moreover, the ACA, among other things, amended the intent requirement of the Federal Anti-Kickback Statute and certain criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of these statutes or specific intent to violate them. The ACA also provides that the government may assert that a claim including items or services resulting from a violation of the Federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the Federal Civil False Claims Act. Many states have also adopted laws similar to the Federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The Federal Civil False Claims Act prohibits, among other things, persons or entities from knowingly presenting, or causing to be presented, a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Suits filed under the Federal Civil False Claims Act can be brought by any individual on behalf of the government, known as “qui tam” actions, and such individuals, commonly known as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The filing of qui tam actions has caused a number of pharmaceutical, medical device, and other healthcare companies to have to defend a Federal Civil False Claims Act action. When an entity is determined to have violated the Federal Civil False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim, in addition to other penalties that may apply. Various states have also enacted laws modeled after the Federal Civil False Claims Act, some of which are broader in scope and may apply regardless of payer.
The Federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created federal criminal statutes that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payers, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. Additionally, the Civil Monetary Penalties Law imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal healthcare program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
The Federal Physician Payments Sunshine Act, created under the ACA, and its implementing regulations require certain manufacturers of drugs, devices, biologicals, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program (with certain exceptions) to report annually to CMS information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists, and chiropractors), other healthcare professionals (such as physicians assistants and nurse practitioners) and teaching hospitals, as well as information regarding ownership and investment interests held by physicians and their immediate family members.
We may be subject to healthcare data privacy and security regulation by foreign, federal, state and local governments in the jurisdictions in which we conduct our business. In the US, at the federal level, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their respective implementing regulations, impose specified requirements relating to the privacy, security, and transmission of individually identifiable health information on certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, as well as their business associates and their covered subcontractors that perform services involving the use or disclosure of individually identifiable health information. Further, we may also be subject to US state health information privacy and data breach notification laws which govern the collection, use, disclosure, and protection of health-related and other personal information, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus requiring additional compliance efforts.
Additionally, the Drug Supply Chain Security Act imposes obligations on manufacturers of pharmaceutical products, among others, related to product tracking and tracing. Among the requirements, manufacturers will be required to provide certain information regarding the drug product to individuals and entities to which product ownership is transferred, label drug product with a product identifier, and keep certain records regarding the drug product. The transfer of information to subsequent product owners by manufacturers will eventually be required to be done electronically. Manufacturers will also be required to verify that purchasers of the manufacturers’ products are appropriately licensed. Further, manufacturers will have drug product investigation, quarantine, disposition, and notification responsibilities related to counterfeit, diverted, stolen, and intentionally adulterated products, as well as products that are the subject of
45
fraudulent transactions or which are otherwise unfit for distribution such that they would be reasonably likely to result in serious health consequences or death.
Outside the United States, interactions between pharmaceutical companies and physicians are also governed by strict laws, such as national anti-bribery and kickback laws of European countries, regulations, industry self-regulation codes of conduct, and physicians’ codes of professional conduct. Payments made to physicians in certain European Union Member States must be publicly disclosed. Moreover, agreements with physicians often must be the subject of prior notification and approval by the physician’s employer, his or her competent professional organization, and/or the regulatory authorities of the individual European Union Member States. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines, or imprisonment. Risks associated with these laws may increase as new laws and regulations are adopted and as enforcement agencies adopt or increase enforcement efforts.
We are unable to predict whether we could be subject to actions under any of these fraud and abuse or other laws, or the impact of such actions. If we are found to be in violation of any of the laws described above and other applicable federal, state and international laws, we may be subject to penalties, including significant civil, criminal and/or administrative penalties, damages, fines, individual imprisonment, disgorgement, possible exclusion from government healthcare reimbursement programs, integrity oversight and reporting obligations to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, all of which could have a material adverse effect on our business and results of operations.
We may be subject to evolving laws, regulations, rules, contracts and other obligations regarding data privacy and protection, and our actual or perceived failure to comply with such obligations could harm our reputation, subject us to government enforcement actions or private litigation (that could carry potentially significant fines or penalties for non-compliance), disrupt our clinical trials or commercialization of products, or other adverse effects on our business or prospects.
Across the United States and globally, laws and regulations governing data privacy and security continue to develop and evolve. We process personal data and other sensitive and confidential information, including trade secrets, intellectual property, and information we collect about patients in connection with clinical trials, which subjects us to various obligations relating to data privacy and information security, such as data privacy and information security laws, regulations, rules, contracts and other obligations, which may significantly affect our business activities. Many of the obligations that apply to us contain ambiguous provisions or impose requirements that differ from country to country, creating uncertainty. Compliance with the enhanced obligations imposed by such obligations may require us to revise our business practices, allocate more resources to privacy and information security, and implement new technologies. Such efforts may result in significant costs to our business. Noncompliance or perceived noncompliance could result in proceedings against us by governmental and regulatory entities, collaborators, data subjects or others, fines, or other consequences.
The legislative and regulatory framework relating to the collection, use, retention, safeguarding, disclosure, sharing, transfer, security and other processing (or, collectively, Process or Processing) of personal data worldwide is rapidly evolving and is likely to remain unsettled for the foreseeable future. The regulatory frameworks for privacy issues may result in ever-increasing regulatory and public scrutiny and escalating levels of enforcement and sanctions, including monetary penalties and personal data processing penalties (including an inability to process personal data).
We are, or may become, subject to foreign privacy laws and regulations. For example, the European Economic Area (EEA), Switzerland, United Kingdom (UK) and certain other foreign territories have restrictions on the Processing of certain personal data, including providing that transfers of personal data outside of their territories may only take place if the country to which the personal data is transferred ensures an “adequate” level of privacy protection. One of the primary safeguards that allowed US companies to import personal data from Europe had been certification to the EU-US Privacy Shield and Swiss US Privacy Shield frameworks administered by the US Department of Commerce. However, the Court of Justice of the European Union (EU), or CJEU, invalidated the EU US Privacy Shield, in a case known as “Schrems II.” Following this decision, the UK government has similarly invalidated the EU-US Privacy Shield as a mechanism for lawful personal data transfers from the UK to the United States under the UK General Data Protection Regulation (UK GDPR); and the Swiss Federal Data Protection and Information Commissioner announced that the Swiss-US Privacy Shield does not provide adequate safeguards for the purposes of personal data transfers from Switzerland to the United States. The CJEU’s decision in Schrems II also raised questions about whether one of the primary alternatives to the EU-US Privacy Shield, namely, the European Commission’s Standard Contractual Clauses, can lawfully be used for personal data transfers
46
from Europe to the United States or other third countries that are not the subject of an adequacy decision of the European Commission. At present, there are few, if any, viable alternatives to the Standard Contractual Clauses. As such, if we are unable to implement a valid mechanism for personal data transfers from Europe, we will face increased exposure to regulatory actions, substantial fines and injunctions against Processing personal data from Europe. Inability to export personal data may also: restrict our activities outside Europe; limit our ability to collaborate with partners as well as other service providers, contractors and other companies outside of Europe; and/or require us to increase our Processing capabilities within Europe at significant expense or otherwise cause us to change the geographical location or segregation of our relevant systems and operations – any or all of which could adversely affect our operations or financial results. Additionally, other countries outside of Europe have enacted or are considering enacting similar cross-border data transfer restrictions and laws requiring local data residency, which could increase the cost and complexity of delivering our services and operating our business. The type of challenges we face in Europe may also arise in other jurisdictions that adopt similar frameworks.
Collectively, European data protection laws (including the General Data Protection Regulation, or GDPR) are wide-ranging in scope (including extra-territoriality measures intended to bring non-EU companies under the regulation) and impose numerous, significant and complex compliance burdens in relation to the Processing of personal data. In particular, the Processing of “special category personal data” (such as personal data related to health and genetic information even if key-coded), which will be relevant to our operations in the context of our conduct of clinical trials, is subject to heightened compliance burdens under European data protection laws and is a topic of active interest among relevant regulators. Fines for non-compliance with the GDPR are steep, with potential fines of up to 20 million Euros or 4% of our global revenue, whichever is greater. In addition, the GDPR authorizes civil litigation claims and penalties for non-compliance.
In addition, the GDPR provides that EEA member states may introduce specific requirements related to the Processing of special categories of personal data, and the UK Data Protection Act of 2018 complements the UK GDPR in this regard, which may lead to greater divergence on the law that applies to the Processing of such personal data across the EEA and/or UK and increase our costs and overall compliance risk. Such country-specific regulations could also limit our ability to Process relevant personal data in the context of our EEA and/or UK operations ultimately having an adverse impact on our business and financial condition.
Further, the UK’s decision to leave the EU, often referred to as Brexit, and ongoing developments in the UK have created some uncertainty regarding data protection regulation in the UK. Following December 31, 2020, and the expiration of transitional arrangements between the UK and EU, the data protection obligations of the GDPR continue to apply to UK-related Processing of personal data in substantially unvaried form under the so-called ‘UK GDPR’ (i.e., the GDPR as it continues to form part of UK law by virtue of section 3 of the EU (Withdrawal) Act 2018, as amended). However, going forward, there is increasing risk for divergence in application, interpretation and enforcement of the data protection laws as between the UK and EEA. Furthermore, the relationship between the UK and the EEA in relation to certain aspects of data protection law, including cross-border data transfers, remains unsettled.
Additionally, we are, or may become, subject to US privacy laws. For example, the California Consumer Privacy Act, or CCPA, went into effect on January 1, 2020 and establishes a privacy framework for covered businesses, including a broad definition of personal data and data privacy rights. The CCPA created new individual privacy rights for California residents and places increased privacy and security obligations on covered businesses processing personal data. The CCPA requires covered businesses to provide new disclosures to California residents and provide such individuals with new ways to opt-out of certain sales of personal data. The CCPA also provides a private cause of action and statutory damages for violations, including for data breaches. Although the CCPA exempts certain data regarding clinical trials, the CCPA, to the extent applicable to our business and operations, may impact our business activities by increasing our compliance costs and potential liability with respect to personal information that we maintain about California residents. It is anticipated that the CCPA will be expanded on January 1, 2023, when the California Privacy Rights Act of 2020 (“CPRA”) becomes operative. The CPRA will, among other things, give California residents the ability to limit use of certain sensitive personal data, further restrict the use of cross-contextual advertising, establish restrictions on the retention of personal data, expand the types of data breaches subject to the CCPA’s private right of action, provide for increased penalties for CPRA violations concerning California residents under the age of 16, and establish a new California Privacy Protection Agency to implement and enforce the law. Other US states have also enacted or proposed data privacy laws, and new laws may be enacted in the future. Collectively, these laws exemplify the vulnerability of our business to the evolving regulatory environment related to personal data.
47
Also, we publish privacy policies and other documentation regarding our Processing of personal information. Although we endeavor to comply with our published policies and other documentation, we may at times fail to do so or may be perceived to have failed to do so. Moreover, despite our efforts, we may not be successful in achieving compliance if our employees or third-party vendors fail to comply with our published policies and documentation. Such failures can subject us to potential foreign, local, state and federal action if such documentation is found to be deceptive, unfair, or misrepresentative of our actual practices. Moreover, subjects about whom we or our partners obtain information, as well as the providers who share this information with us, may contractually limit our ability to use and disclose the information. Claims that we have violated individuals’ privacy rights or failed to comply with data protection laws or applicable privacy notices or other obligations, even if we were found not liable, could be expensive and time-consuming to defend and could result in adverse effects, including but not limited to publicity that could harm our business.
We may not be able to effectively integrate, manage or maintain our international operations, and such difficulty could adversely affect our business operations, financial condition, results of operations and stock price.
We have personnel in Switzerland, and we engage in clinical trials and other activities in many territories outside of the United States. There are significant risks associated with foreign operations, including but not limited to:
•compliance with various local laws and regulations, which may conflict or change, such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements, drug pricing and reimbursement, and other governmental approvals, permits, and licenses;
•complexities and difficulties in obtaining protection for and enforcing our intellectual property rights;
•difficulties in staffing and managing foreign operation, such as the integration of our corporate culture with local customs and cultures;
•the distraction to our management;
•foreign currency exchange rates and the impact of shifts in the United States and local economies on those rates;
•certain expenses including, among others, expenses for travel, translation, and insurance; and
•integration of our policies and procedures, including disclosure controls and procedures and internal control over financial reporting, with our international operations.
Any of these risks could adversely affect our business.
We and third parties we contract with use hazardous materials in our operations.
Our activities involve the use of materials that could be hazardous to human health and safety or the environment. We cannot completely eliminate the risks associated with their use, storage, or disposal, which could cause:
•interruption of our development or manufacturing efforts;
•injury to our employees and others;
•environmental damage resulting in costly cleanup; and
•liabilities under domestic or foreign laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste products.
In such an event, we may be held liable for any resulting damages, and any such liability could exceed our resources. Although we carry insurance in amounts and type that we consider commercially reasonable, we cannot be certain that the coverage or coverage limits of our insurance policies will be adequate, and we do not have insurance coverage for losses relating to an interruption of our research and development efforts caused by contamination.
Our business, including our preclinical and clinical programs, may be significantly and adversely affected by the COVID-19 pandemic.
Coronavirus disease 2019, or COVID-19, has spread globally, including in the United States and Switzerland, where we have operations, and in many other countries where we are conducting or plan to conduct clinical trials or have manufacturing activities conducted. The COVID-19 pandemic poses the risk that we or our clinical trial participants,
48
employees, contractors, collaborators and vendors may be prevented from conducting certain clinical trials or other business activities for an indefinite period of time, including due to “stay-at-home” orders or shutdowns that may be requested or mandated by governmental authorities. Beginning the week of March 16, 2020, substantially all of our workforce began working from home either all or substantially all of the time, and they continue to do so as of the date of this filing. Due to the COVID-19 pandemic and our remote workforce, there exists an increased risk to our information technology assets and data. The pandemic, stay-at-home orders, and our work-from-home policies may negatively impact productivity, disrupt our business, and delay our development programs, all of which may delay our regulatory and commercialization timelines. The magnitude of these impacts will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course, which will in turn depend on a variety of factors including vaccination rates, which vary greatly from region to region, and the emergence and spread of new variants of the coronavirus.
The COVID-19 pandemic has impacted and may continue to impact our clinical programs, including our etrasimod, olorinab, and APD418 programs. For example, some clinical site activations and participant enrollment and screening rates slowed for certain periods in certain regions. These timing changes, however, have been highly variable and their aggregate impact remains uncertain. As a result, it is not possible at this time to estimate the total impact COVID-19 will have on our clinical programs. If the COVID-19 pandemic continues in the United States and around the world, we may experience, or continue to experience, disruptions that could severely impact our preclinical studies and clinical trials, including:
•delays in receiving approval from local regulatory authorities to initiate our planned clinical trials;
•delays or difficulties in enrolling or retaining participants in our clinical trials;
•delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff;
•delays in clinical sites receiving the supplies and materials needed to conduct our clinical trials, including interruption in global shipping that may affect the transport of clinical trial materials;
•changes in local regulations as part of a response to the COVID-19 pandemic which may require us to change the ways in which our clinical trials are conducted, which may result in unexpected costs, or to discontinue the clinical trials altogether;
•diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials;
•interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others, or interruption of clinical trial participant visits and study procedures, the occurrence of which could affect the integrity of clinical trial data;
•risk that participants enrolled in our clinical trials will acquire COVID-19 while the clinical trial is ongoing, which could impact the results of the clinical trial, including by increasing the number of observed adverse events;
•interruptions in preclinical studies due to restricted or limited operations at our research and development facilities;
•delays in necessary interactions with local regulators, ethics committees, and other important agencies and contractors due to limitations in employee resources or forced furlough of employees;
•limitations in employee resources that would otherwise be focused on the conduct of our clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people;
•disrupting our talent attraction and retention strategy;
•refusal of the FDA or other regulatory authorities to accept data from clinical trials in affected geographies; and
•interruption or delays to our sourced discovery and clinical activities.
The COVID-19 pandemic continues to rapidly evolve. The extent to which the outbreak impacts our business, preclinical studies, and clinical programs, as well as our industry and conditions in the greater workforce, will depend on
49
future developments, which are highly uncertain and cannot be predicted with confidence, such as the ultimate geographic spread of the disease, the emergence and spread of novel variants of the coronavirus, the duration of the pandemic, potential stay-at-home orders, travel restrictions, and “social distancing” in the United States, Switzerland and other countries, business closures or business disruptions, and the effectiveness of actions taken in the United States and other countries to contain and treat the disease and vaccinate their populations.
If our information technology or data is compromised by catastrophic events, security breaches, including any cybersecurity incidents, or otherwise, our business and operations might be disrupted or adversely affected.
Our US operations are primarily located in a business park in San Diego, California and an office in Boston, Massachusetts, and our principal executive offices are located in Park City, Utah. We also have certain operations in Zug, Switzerland. We depend on our facilities and on collaborators, licensees, contractors, and vendors for the continued operation of our business, some of whom are located in Europe and Asia. As a result, natural disasters or other catastrophic events in various parts of the world, including interruptions in the supply of natural resources, political and governmental changes, disruption in transportation networks or delivery services, severe weather conditions, wildfires and other fires, explosions, actions of animal rights activists, terrorist attacks, earthquakes, wars, and public health issues (including the COVID-19 pandemic) could disrupt our operations or those of our collaborators, contractors, and vendors or contribute to unfavorable economic or other conditions that could adversely impact us.
In addition, we depend on the efficient and uninterrupted operation of information technology (IT) and communications systems, which we use for, among other things, processing sensitive company data, including our financial data, intellectual property, personal information, and other proprietary business information. We may use third-party service providers to help us operate our business and process company data. We may also share such company data with our partners and other third parties in conjunction with our business. Relevant laws, regulations, industry standards, contractual obligations, and published statements, may require us to implement specific security measures or use industry-standard or reasonable measures to protect against security breaches. If we, our service providers, partners or other relevant third parties have experienced, or in the future experience, any security incident(s) that result in, any data loss, deletion, or destruction; unauthorized acquisition, disclosure or access; or other compromise related to the security, confidentiality, or integrity of our (or their) information technology systems or data, it may materially and adversely affect our business such as by requiring the diversion of funds to address the security incident(s); causing interruptions, delays or outages in our operations and development programs; subjecting us to regulatory investigations and enforcement actions; imposing significant regulatory fines; and exposing us to private litigation. We may be required to expend significant resources, fundamentally change our business activities and practices, or modify our operations, including our clinical trial activities, or information technology in an effort to protect against security incidents and to mitigate, detect, and remediate actual and potential vulnerabilities.
While certain of our operations have business continuity and disaster recovery plans and other security measures intended to prevent and minimize the impact of IT-related interruptions, our IT infrastructure and the IT infrastructure of our current and any future collaborators, contractors, and vendors are vulnerable to damage from a variety of evolving threats, included but not limited to cyberattacks, computer viruses, software bugs, unauthorized access, user error or malfeasance, data corruption, server malfunctions, software or hardware failures, electrical failures and natural disasters or other catastrophic events. Ransomware and supply chain attacks have also become increasingly prevalent and severe. The aforementioned threats may come from a wide variety of actors, including traditional hackers, employees, sophisticated nation-states, and state-sponsored actors. Our security measures, or those maintained on our behalf, may fail in the future, and if such measures are compromised now, or in the future, or the security, confidentiality, integrity or availability of, our IT systems or data is compromised or fails, we could experience material adverse impacts. We could experience failures in our information systems, which could result in an interruption of our normal business operations and require substantial expenditure of financial and administrative resources to remedy. System failures, accidents, or security breaches can cause interruptions in our operations and can result in a material disruption of our research and development programs and other business operations. The loss of data from completed or future studies or clinical trials could result in delays in our research, development, or regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Similarly, we and our licensees rely on third parties to conduct studies and clinical trials of our drug candidates and manufacture our drug candidates, and similar events relating to these third parties’ information computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liabilities and the development of any of our other drug candidates and the commercialization of drugs could be delayed or otherwise adversely affected. Actual or perceived security incidents, and concerns regarding data privacy, security or processing may cause our actual or perspective customers, collaborators, partners and/or clinical
50
trial participants to stop participating in our trials, using our products or working with us. This discontinuance of relationships with third parties, or failure to meet the expectations of such third parties, could result in material harm to our operations, financial performance or reputation and affect our ability to grow and operate our business.
If a security incident affects our or third parties’ systems upon which we rely, corrupts our data or results in the unauthorized disclosure or release of personal information, our reputation could be materially damaged or our operations could be disrupted. In addition, such a breach may require notification to governmental agencies, supervisory bodies, credit reporting agencies, the media, individuals or others pursuant to various federal, state and foreign data protection, privacy and security laws, regulations, guidelines, contracts and published statements, if applicable. Such disclosures are costly, and the disclosure or the failure to comply with such requirements could lead to material adverse effect on our reputation, business, or financial condition.
Even though we believe we carry commercially reasonable business interruption and liability insurance, and our contractors may carry liability insurance that protect us in certain events, we might suffer losses as a result of business interruptions or security incidents that exceed the coverage available under our and our contractors’ insurance policies or for which we or our contractors do not have coverage. For example, we are not insured against a terrorist attack. Moreover, any limitations in liability in our contracts that might protect us from liabilities or damages if we fail to comply with applicable obligations related to information security may be inadequate. Any natural disaster or other catastrophic event could have a significant negative impact on our operations and financial results. Moreover, any such event could delay our research and development programs and adversely affect, which may include stopping, our commercial production.
We and our employees and directors may be named as defendants in litigation that could result in substantial costs and divert management’s attention.
Securities class action litigation may be brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because companies in the pharmaceuticals industry often experience significant stock price volatility. For example, beginning in 2010, a number of lawsuits were filed against us and certain of our employees and directors alleging we and the other defendants violated federal securities laws by making materially false and misleading statements regarding our lorcaserin trials, thereby artificially inflating the price of our common stock. These lawsuits were settled in 2018.
While we carry liability insurance, any losses we incur in connection with any current or future lawsuits may not be covered by insurance in an amount sufficient to cover our losses or at all, and our assets may be insufficient to cover any amounts that exceed our insurance coverage. We may have to pay damage awards or otherwise may enter into settlement arrangements in connection with any future claims. A settlement of any of future lawsuit against us could also involve the issuance of common stock or other equity, which may dilute your ownership interest. Any payments or settlement arrangements could have material adverse effects on our business, operating results, financial condition, or your ownership interest. Even if the plaintiffs’ claims are not successful, any future lawsuit against us and/or our directors or executive officers could result in substantial costs and significantly and adversely impact our reputation and divert our management’s attention and resources, which could have a material adverse effect on our business, operating results or financial condition. In addition, any such lawsuits may make it more difficult to finance our operations, obtain certain types of insurance (including directors’ and officers’ liability insurance), and attract and retain qualified executive officers, other employees, and directors.
Negative US and global economic conditions may pose challenges to our business strategy, which relies on funding from collaborators or the financial markets, and may create other financial risks for us.
While significant uncertainty remains as to the aggregate impact of the COVID-19 pandemic on our operations and liquidity, and on the global economy as a whole, the COVID-19 pandemic has already had a significant adverse impact on domestic and global economies, as well as global financial markets. Negative conditions in the US or global economy, including financial markets, may adversely affect our business and the business of our current and prospective collaborators, distributors, and licensees, which we sometimes refer to generally as our collaborators, and others with which we do or may conduct business. The duration and severity of these conditions is uncertain. If negative economic conditions persist or worsen, we may be unable to secure funding to sustain our operations or to find suitable collaborators to advance our internal programs, even if we achieve positive results from our research and development or business development efforts. Such negative conditions could also impact commercialization of any drugs we and our collaborators and licensees develop, as well as our financial condition. From time to time we may maintain a portfolio of investments in marketable debt securities, which are recorded at fair value. Although we have established investment guidelines relative to
51
diversification and maturity with the objectives of maintaining safety of principal and liquidity, we rely on credit rating agencies to help evaluate the riskiness of investments, and such agencies may not accurately predict such risk. In addition, such agencies may reduce the credit quality of our individual holdings, which could adversely affect their value. Lower credit quality and other market events, such as changes in interest rates and deterioration in credit markets, may have an adverse effect on the fair value of our investment holdings and cash position.
Currency fluctuations may negatively affect our financial condition.
We primarily spend and generate cash in US dollars and present our consolidated financial statements in US dollars. However, a portion of our expected and potential payments and receipts, including relating to our Swiss operations and under certain of our agreements, are in foreign currencies. A fluctuation of the exchange rates of foreign currencies versus the US dollar may, thus, adversely affect our financial results, including cash balances, expenses and revenues. We may in the future enter into hedging transactions to try to reduce our foreign currency exposure, but there is no assurance that such transactions will occur or be successful.
Our ability to use our net operating losses and certain other tax attributes to offset future taxable income or taxes may be limited.
As of December 31, 2021, we had federal and state net operating loss carryforwards of $1,437.6 million and $951.2 million, respectively. Portions of our federal net operating loss carryforwards, if not utilized, will begin to expire in 2028, and our state net operating loss carryforwards will begin to expire in 2028. Such net operating loss carryforwards could expire unused and be unavailable to offset future income tax liabilities. Under current law, federal net operating losses incurred in tax years beginning after December 31, 2017 may be carried forward indefinitely, but the deductibility of such federal net operating losses in tax years beginning after December 31, 2020 is limited to 80% of taxable income. It is uncertain if and to what extent various states will conform to federal tax laws. In addition, under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the IRC, and corresponding provisions of state law, if a corporation undergoes an “ownership change,” which generally is defined as a greater than 50% change, by value, in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income or taxes may be limited. We have experienced ownership changes in the past and we may experience additional ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. If an ownership change occurs and our ability to use our net operating loss carryforwards is materially limited, it would harm our future operating results by effectively increasing our future tax obligations. Similar provisions of state law also may apply to limit the use of our state net operating loss carryforwards. In addition, at the state level, there may be periods during which the use of net operating losses is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed. For example, California passed legislation imposing limits on the usability of California state net operating losses and certain tax credits in tax years beginning after 2019 and before 2023.
Risks Relating to Our Intellectual Property
Our success is dependent on intellectual property rights held by us and third parties and our interest in these rights is complex and uncertain.
Our success will depend on our own and on current or future collaborators’ abilities to obtain, maintain, and defend patents. In particular, the patents directed to our drug candidates and drugs are important to developing and commercializing drugs and to our revenue. We have numerous US and foreign patents issued and patent applications pending for our technologies. There is no assurance that any of our patent applications will issue, or that any of the patents will be enforceable or will cover a drug or other commercially significant technology or method, or that the patents will be held to be valid for their expected terms.
The procedures for obtaining a patent are complex. These procedures require an analysis of the scientific technology related to the invention and many sophisticated legal issues. Obtaining patent rights outside the United States often requires the translation of highly technical documents and an improper translation may jeopardize our patent protection. Ensuring adequate quality of translators and foreign patent attorneys is often very challenging. Consequently, the process for having our pending patent applications issue as patents will be difficult, complex, time consuming, and expensive. Our patent position is very uncertain, and we do not know when, or if, we will obtain additional patents, or if the scope of the patents obtained will be sufficient to protect our drugs or be considered sufficient by parties reviewing our patent positions pursuant to a potential marketing, licensing, or financing transaction.
52
In addition, other entities may challenge the validity or enforceability of our patents in litigation or administrative proceedings. We cannot make assurances as to how much protection, if any, our patents will provide if we attempt to enforce them or if they are challenged. It is possible that a competitor or a generic pharmaceutical provider may successfully challenge our patents and those challenges may result in reduction or elimination of our patent coverage.
We also rely on confidentiality agreements and trade secrets to protect our technologies. However, such information is difficult to protect. We require our employees to contractually agree not to improperly use our confidential information or disclose it to others, but we may be unable to determine if our employees have conformed or will conform to their legal obligations under these agreements. We also enter into confidentiality agreements with prospective collaborators, collaborators, service providers, and consultants, but we may not be able to adequately protect our trade secrets or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of this information. Many of our employees and consultants were, and many of them may currently be, parties to confidentiality agreements with other pharmaceutical and biotechnology companies, and the use of our technologies could violate these agreements. In addition, third parties may independently discover our trade secrets or other proprietary information.
Some of our research and development collaborators and scientific consultants have rights to publish data and information to which we have rights. We generally seek to prevent our collaborators and consultants from disclosing scientific discoveries before we have the opportunity to file patent applications on such discoveries. In some of our collaborations we do not control our collaborators’ ability to disclose their own discoveries under the collaboration, and in some of our academic relationships we are limited to relatively short periods to review a proposed publication and file a patent application. If we cannot maintain confidentiality in connection with our collaborations and relationships, our ability to receive patent protection or protect our proprietary information will be impaired.
We believe that the United States is by far the largest single market for pharmaceuticals in the world. Because of the critical nature of patent rights to our industry, changes in US patent laws could have a profound effect on our future profits, if any. It is unknown which, if any, patent laws will change, how changes to patent laws would ultimately be enforced by the courts, and how they would impact our business.
A dispute regarding the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be costly and result in delays or termination of our future research, development, manufacturing, and sales activities.
Our commercial success depends upon our ability to develop and manufacture our drugs and drug candidates, market and sell drugs, and conduct our research and development activities without infringing or misappropriating the proprietary rights of others. There are many issued patents and pending patent applications owned by others relating to research and development programs that could be determined to be similar, identical, or superior to ours or our licensors or collaborators. We may be exposed to future litigation by others based on claims that our drugs, drug candidates, technologies, or activities infringe the intellectual property rights of others. Numerous issued patents and pending patent applications owned by others exist in the areas of our research and development, including some that purport to allow the patent holder to control the use of all drugs that modulate a particular drug target regardless of whether the infringing drug bears any structural resemblance to a chemical compound known to the patent holder at the time of patent filing. Numerous issued patents and pending patent applications owned by others also exist in the therapeutic areas in which we are developing drugs. There are also numerous issued patents and pending patent applications owned by others that are directed to chemical compounds or synthetic processes that may be necessary or useful to our research, development, manufacturing, or commercialization activities. These could materially affect our ability to develop our drug candidates or manufacture, import, or sell drugs, and our activities, or those of our licensors or collaborators, could be determined to infringe these patents. Because patent applications can take many years to issue, there may be currently pending applications, unknown to us, that may later result in issued patents that our drugs, drug candidates, or technologies may infringe. There also may be existing patents owned by others, of which we are not aware, that our drug candidates or technologies may infringe. Further, there may be issued patents or pending patent applications owned by others in fields relevant to our business, of which we are or may become aware, that we believe (i) are invalid, unenforceable, or we do not infringe; (ii) relate to immaterial portions of our overall research and development, manufacturing, and commercialization efforts; or (iii) in the case of pending patent applications, the resulting patent would not be granted or, if granted, would not likely be enforced in a manner that would materially impact such efforts. We cannot assure you that others holding any of these patents or patent applications will not assert infringement claims against us and seek damages or enjoinment of our activities. We also cannot assure you that, in the event of litigation, we will be able to successfully assert non-infringement, unenforceability, invalidity, or immateriality, or that any infringement claims will be resolved in our favor.
53
In addition, others may infringe or misappropriate our proprietary rights. We may have to institute costly legal action to protect our intellectual property rights, or we may not be able to afford the costs of enforcing or defending our intellectual property rights.
There could be significant litigation and other administrative proceedings in our industry that affect us regarding patent and other intellectual property rights. Any legal action or administrative action against us, or our collaborators, claiming damages or seeking to enjoin commercial activities relating to our research and development, manufacturing, and commercialization activities could:
•require us, or our collaborators, to obtain a license which may not be available on commercially reasonable terms, if at all;
•prevent us from importing, making, using, selling, or offering to sell the subject matter claimed in patents held by others and subject us to potential liability for damages;
•consume a substantial portion of our managerial, scientific, and financial resources; or
•be costly, regardless of the outcome.
Furthermore, because of the substantial amount of pre-trial document and witness discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised. In addition, during the course of intellectual property litigation, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the trading price of our common stock.
We are aware of third-party patents, as well as third-party patent applications, that could adversely affect the potential commercialization of etrasimod. For example, we are aware of third-party patents, as well as a third-party patent application, with broad claims to administering an S1P modulator by starting with a lower dose and then increasing to a higher, standard daily dose. We are also aware of third-party litigation in which one such patent has been asserted. While we do not believe that any claims of such patents that would cover the potential commercialization of etrasimod are valid and enforceable, we may be incorrect in this belief.
We have been contacted from time to time by third parties regarding their intellectual property rights, sometimes asserting that we may need a license to use their technologies. If we fail to obtain any required licenses or make any necessary changes to our technologies, we may become involved in expensive and time-consuming litigation or we may be unable to develop or commercialize some or all of our drugs or drug candidates.
We cannot predict the outcome of any litigation matter. For example, our existing patents could be invalidated, found unenforceable or found not to cover a generic form of our drugs.
We cannot protect our intellectual property rights throughout the world.
Filing, prosecuting, defending, and enforcing patents on all of our drug candidates throughout the world would be prohibitively expensive. The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties (for example, the patent owner has failed to “work” the invention in that country or the third party has patented improvements). In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. Compulsory licensing of life-saving drugs is also becoming increasingly popular in developing countries either through direct legislation or international initiatives. Such compulsory licenses could be extended to include some of our drug candidates, which could limit our potential revenue opportunities. Moreover, the legal systems of certain countries, particularly certain developing countries, do not favor the aggressive enforcement of patents and other intellectual property protection, particularly those relating to pharmaceuticals, which makes it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
54
Risks Relating to Our Securities
Our stock price will likely be volatile, and your investment in our stock could decline in value.
Our stock price has fluctuated historically. From January 1, 2021, to February 17, 2022, the market price of our stock was as low as $45.50 per share and as high as $94.50 per share. The stock market, particularly in recent years, has experienced significant volatility particularly with respect to pharmaceutical and biotechnology stocks, and this trend may continue. The ongoing COVID-19 pandemic, for example, has previously negatively affected the stock market and investor sentiment and has resulted in significant volatility, and the COVID-19 pandemic may have these effects in the future.
Very few drug candidates being tested will ultimately receive regulatory approval, and companies in our industry sometimes experience significant volatility in their stock price. Our stock price may fluctuate significantly depending on a variety of factors, including:
•results or decisions affecting the development or commercialization of any of our drug candidates or drugs, including the results of studies, trials and other analyses;
•the success, failure or setbacks of our or a perceived competitor’s drugs or drug candidates;
•the timing of the development of our drug candidates;
•discussions or recommendations affecting our drugs or drug candidates by the FDA or other reviewers of preclinical or clinical data or other information related to our drug candidates or drugs;
•regulatory actions or decisions or legislation affecting drugs or drug candidates, including ours and those of our competitors;
•the commercial availability and success or failure of any of our drug candidates;
•the development and implementation of our continuing development and research plans;
•the entrance into, or failure to enter into, a new collaboration or the modification or termination of an existing collaboration or other material transaction;
•the timing and receipt by us of milestone and other payments or failing to achieve and receive the same;
•fluctuation in prescriptions, sales, or financial results (including with respect to revenue recognition, expenses, and other operating results) or inaccurate sales or cash forecasting;
•accounting restatements and changes;
•supply chain or manufacturing issues;
•changes in our research and development budget or the research and development budgets of our existing or potential collaborators;
•the introduction, development or withdrawal of drug candidates or drugs by others that target the same diseases and conditions that we or our collaborators target or the introduction of new drug discovery techniques;
•expenses related to, and the results of, litigation, other disputes and other proceedings;
•financing strategy or decisions;
•the allocation of our resources;
•our ability, or the perception by investors of our ability, to continue to meet all applicable requirements for continued listing of our common stock on The Nasdaq Stock Market, and the possible delisting of our common stock if we are unable to do so;
•developments in intellectual property rights or related announcements;
•disruptions caused by man-made or natural disasters or public health pandemics or epidemics or other business interruptions, including, for example, the COVID-19 pandemic; and
•capital market and other macroeconomic conditions.
We are not able to control many of these factors. If our financial or scientific results in a particular period do not meet stockholders’ or analysts’ expectations, our stock price may decline, and such decline could be significant.
55
Any future equity or debt issuances or other financing transactions may have dilutive or adverse effects on our existing stockholders.
We have been opportunistic in our efforts to obtain cash, and we expect we will evaluate various funding alternatives from time to time. We may issue additional shares of common stock or convertible securities that could dilute your ownership in our company and may include terms that give new investors rights that are superior to yours. We have effective registration statements to sell shares of our common stock and certain other securities, and we may elect to sell shares pursuant to such registration from time to time. In February 2020, we entered into a sales agreement with Credit Suisse Securities (USA) LLC, SVB Leerink LLC and Cantor Fitzgerald & Co., pursuant to which we may sell and issue shares of our common stock having an aggregate offering price of up to $250.0 million from time to time in transactions that are deemed to be “at-the-market offering” as defined in Rule 415(a)(4) under the Securities Act. As of February 17, 2022 we have sold 1.2 million shares for aggregate gross proceeds of $100.6 million under the sales agreement.
Moreover, any issuances by us of equity securities may be at or below the prevailing market price of our common stock and in any event may have a dilutive impact on your ownership interest, which could cause the market price of our common stock to decline. In addition, we may also raise additional funds through the incurrence of debt or other financing transaction, and the investors may have rights superior to your rights in the event we are not successful and are forced to seek the protection of bankruptcy laws or the transaction may otherwise adversely affect our business prospects and existing stockholders.
There are a substantial number of shares of our common stock that may become eligible for future sale in the public market, and the sale of our common stock could cause the market price of our common stock to fall.
As of February 17, 2022, there were (i) options to purchase 7,864,700 shares of our common stock outstanding under our equity incentive plans at a weighted-average exercise price of $47.40 per share, (ii) 1,504,563 restricted stock unit awards outstanding under our equity incentive plans, (iii) performance restricted stock units outstanding under our equity incentive plans under which up to 365,744 shares of common stock may be issuable upon achievement of all specified performance goals, (iv) 2,117,674 additional shares of common stock remaining issuable under our Amended and Restated 2020 Long-Term Incentive Plan, and (v) 883,364 shares issuable under our 2019 Employee Stock Purchase Plan.
Once issued, the shares described above will be available for immediate resale in the public market. The market price of our common stock could decline as a result of such resales due to the increased number of shares available for sale in the market. As of February 17, 2022, there were 61,659,385 shares of our common stock outstanding.
Our executive officers and directors, and other holders of our common stock and other securities, may take actions that are contrary to your interests, including selling their stock.
Sales of our stock by our executive officers and directors, or the perception that such sales may occur, could adversely affect the market price of our stock. Our executive officers and directors may sell stock in the future, either as part, or outside, of trading plans under Rule 10b5-1 of the SEC.
A small number of stockholders may hold or acquire a significant amount of our outstanding stock. From time to time, there is a large short interest in our stock. These holders of such stock or positions may seek control of us, may support transactions that we or you do not believe are favorable, and may have interests that are different from yours. In addition, sales of a large number of shares of our stock by these large stockholders or other stockholders within a short period of time could adversely affect our stock price.
We may also be involved in disagreements with the holders of our stock, warrants, or other securities in the future. Such disagreements may lead to proxy contests or litigation, which may be expensive and consume management’s time, involve settlements, the terms of which may not be favorable to us, or result in other negative consequences to our business.
Certain of our agreements, provisions in our charter documents, possible future agreements and Delaware law could delay or prevent a change in management or a takeover attempt that you may consider to be in your best interests.
There is a standstill provision in our transaction agreement with Eisai, and we may enter into agreements with others that contain similar provisions. In addition, we may in the future adopt a stockholders’ rights agreement, which would cause substantial dilution to any person who attempts to acquire us in a manner or on terms not approved by our
56
board of directors. These provisions or agreements, as well as other provisions in our certificate of incorporation and bylaws and under Delaware law, could delay or prevent the removal of directors and management and could make more difficult a merger, tender offer, or proxy contest involving us that you may consider to be in your best interests. For example, our charter provisions:
•allow our board of directors to issue preferred stock without stockholder approval;
•limit who can call a special meeting of stockholders;
•eliminate stockholder action by written consent; and
•establish advance notice requirements for nomination for election to the board of directors or for proposing matters to be acted upon at stockholders’ meetings.
Our bylaws provide that the Court of Chancery of the State of Delaware and the federal district courts of the United States of America are the exclusive forums for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our bylaws provide that the Court of Chancery of the State of Delaware will be the exclusive forum for (a) any derivative action or proceeding brought on behalf of us, (b) any action asserting a claim of breach of a fiduciary duty owed by any of our directors or any of our officers or other employees to us or our stockholders, (c) any action asserting a claim against us or any of our directors or any of our officers or other employees arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our bylaws, or (d) any action asserting a claim against us or any of our directors or any of our officers or other employee governed by the internal affairs doctrine. This provision does not apply to suits brought to enforce a duty or liability created by the Securities Act or the Exchange Act. Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly, both state and federal courts have jurisdiction to entertain such claims. To prevent having to litigate claims in multiple jurisdictions and the threat of inconsistent or contrary rulings by different courts, among other considerations, our bylaws further provides that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions. In such instance, we would expect to vigorously assert the validity and enforceability of the exclusive forum provisions of our bylaws. This may require significant additional costs associated with resolving such action in other jurisdictions and there can be no assurance that the provisions will be enforced by a court in those other jurisdictions.
These exclusive forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage lawsuits against us and our directors, officers and other employees. If a court were to find either exclusive forum provision in our bylaws to be inapplicable or unenforceable in an action, we may incur further significant additional costs associated with resolving the dispute in other jurisdictions, all of which could seriously harm our business.
General Risk Factors
Laws, rules, and regulations, including relating to public companies, may be costly and impact our ability to attract and retain directors and executive officers.
Laws and regulations affecting public companies, including rules adopted by the SEC and by Nasdaq, judicial rulings, and other laws and regulations, including, for example, of state, federal, and foreign governments, may result in increased costs to us, particularly as we continue to develop the required capabilities in the United States and abroad to develop and commercialize our product candidates. These laws, rules, and regulations could make it more difficult or costly for us to obtain certain types of insurance, including directors’ and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. These laws, rules, and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, on our board committees, or as executive officers. We cannot estimate accurately the amount or timing of additional costs we may incur to respond to these laws, rules, and regulations.
57
Changes in funding for the FDA, the SEC, and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner, or otherwise prevent those agencies from performing normal functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the US government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC, and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns could impact our ability to access the public markets and obtain capital necessary to properly capitalize and continue our operations.
The withdrawal of the United Kingdom from the European Union, commonly referred to as “Brexit,” may adversely impact our ability to obtain regulatory approvals of our product candidates in the European Union, result in restrictions or imposition of taxes and duties for importing our product candidates into the European Union, and may require us to incur additional expenses in order to develop, manufacture and commercialize our product candidates in the European Union.
Following the result of a referendum in 2016, the United Kingdom left the European Union on January 31, 2020, commonly referred to as “Brexit.” Pursuant to the formal withdrawal arrangements agreed between the United Kingdom and the European Union, the United Kingdom was subject to a transition period that ended December 31, 2020, or the Transition Period, during which EU rules continued to apply. A trade and cooperation agreement, or the Trade and Cooperation Agreement, that outlines the future trading relationship between the United Kingdom and the European Union was agreed in December 2020.
Since a significant proportion of the regulatory framework in the United Kingdom applicable to our business and our product candidates is derived from EU directives and regulations, Brexit has had, and may continue to have, a material impact on the regulatory regime with respect to the development, manufacture, importation, approval and commercialization of our product candidates in the United Kingdom or the European Union. For example, Great Britain is no longer covered by the centralized procedures for obtaining EU-wide marketing authorization from the EMA and, and a separate marketing authorization will be required to marker our product candidates in Great Britain. It is currently unclear whether the Medicines & Healthcare products Regulatory Agency, or MHRA, in the U.K. is sufficiently prepared to handle the increased volume of marketing authorization applications that it is likely to receive.
While the Trade and Cooperation Agreement provides for the tariff-free trade of medicinal products between the UK and the EU there may be additional non-tariff costs to such trade which did not exist prior to the end of the Transition Period. Further, should the UK diverge from the EU from a regulatory perspective in relation to medicinal products, tariffs could be put into place in the future. We could therefore, both now and in the future, face significant additional expenses (when compared to the position prior to the end of the Transition Period) to operate our business, which could significantly and materially harm or delay our ability to generate revenues or achieve profitability of our business. Any further changes in international trade, tariff and import/export regulations as a result of Brexit or otherwise may impose unexpected duty costs or other non-tariff barriers on us. These developments, or the perception that any of them could occur, may significantly reduce global trade and, in particular, trade between the impacted nations and the UK It is also possible that Brexit may negatively affect our ability to attract and retain employees, particularly those from the EU. These developments, or the perception that any of them could occur, may significantly reduce global trade and, in particular, trade between the impacted nations and the United Kingdom.
58
Our employees, clinical trial investigators, CROs, CMOs, consultants, vendors, and collaborators may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, clinical trial investigators, CROs, CMOs, consultants, vendors, and collaborators. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates:
•FDA regulations or those of comparable foreign regulatory authorities, including those laws that require the reporting of true, complete and accurate information;
•manufacturing standards;
•federal and state health and data privacy, security, fraud and abuse, government price reporting, transparency reporting requirements, and other healthcare laws and regulations in the United States and abroad;
•sexual harassment and other workplace misconduct; or
•laws that require the true, complete, and accurate reporting of financial information or data.
Such misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation.
We have adopted a Code of Business Conduct and Ethics and other policies and procedures, but it is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal, and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from government funded healthcare programs, such as Medicare, Medicaid, and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional integrity reporting and oversight obligations, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Our disclosure controls and procedures and our internal control over financial reporting may not prevent potential errors and fraud.
Our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all potential errors and fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. There are inherent limitations in all control systems, and no system of controls can provide absolute assurance that all control issues and instances of fraud, if any, or misstatements due to error, if any, within the company have been detected. While we believe that our disclosure controls and procedures and internal control over financial reporting are and have been effective at the reasonable assurance level, we intend to continue to examine and refine our disclosure controls and procedures and internal control over financial reporting and to monitor ongoing developments in these areas.
Current and future tax laws and regulations could adversely affect our business and financial condition.
New income, sales, use or other tax laws, statutes, rules, regulations, or ordinances could be enacted at any time, which could affect the tax treatment of our domestic and foreign earnings. Any new taxes could adversely affect our domestic and international business operations, and our business and financial performance. Further, existing tax laws, statutes, rules, regulations, or ordinances could be interpreted, changed, modified, or applied adversely to us. For example, the TCJA, together with the CARES Act, significantly revised the IRC. The Biden administration and Congress have proposed various U.S. federal tax law changes, which if enacted could have a material impact on our business, cash flows, financial condition, or results of operations. In addition, it is uncertain if and to what extent various states will conform to the TCJA, the CARES Act, or any newly enacted US federal tax legislation. Changes in corporate tax rates, the realization of net deferred tax assets relating to our operations, the taxation of foreign earnings, and the deductibility of expenses under the TCJA or future reform legislation could have a material impact on the value of our deferred tax assets, could result in significant one-time charges, and could increase our future US tax expense.
59
We have an international corporate structure and intercompany arrangements that include licensing of worldwide intellectual property rights related to certain of our drug candidates to one or more wholly owned subsidiaries in order to, among other things, build a platform for long-term operational and financial efficiencies. One such efficiency is the potential reduction of our worldwide effective tax rate on certain potential future revenues. The application of the tax laws of the jurisdictions in which we operate our international business activities is subject to interpretation and depends on our ability to operate our business in a manner consistent with our corporate structure and intercompany arrangements. Future changes in US and non-US tax laws, including implementation of international tax reform relating to the tax treatment of multinational corporations, if enacted, may reduce or eliminate any potential financial efficiencies that we hoped to achieve by establishing this operational structure. Additionally, taxing authorities, such as the US Internal Revenue Service, may audit and otherwise challenge these types of arrangements, and have done so with other companies in the pharmaceutical industry. If any such changes in tax law are enacted, or our international corporate structure and intercompany arrangements are otherwise challenged, our business could be materially adversely impacted.
Changes or modifications in financial accounting standards, including those related to revenue recognition, may harm our results of operations.
From time to time the Financial Accounting Standards Board, or FASB, either alone or jointly with other organizations, promulgates new accounting principles that could have an adverse impact on our financial position, results of operations, or reported cash flows. Any difficulties in adopting or implementing any new accounting standard, or updating or modifying our internal controls as needed on a timely basis, could result in our failure to meet our financial reporting obligations, which could result in regulatory discipline and harm investors’ confidence in us. In addition, if we were to change our critical accounting estimates, including those related to the recognition of revenue, our operating results could be significantly affected.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
We were incorporated in the state of Delaware in April 1997 and our principal executive offices are in Park City, Utah. We also have operations in San Diego, California; Boston, Massachusetts and Zug, Switzerland.
We lease real property to support our business, including research and development, sales, marketing and administration. We believe our facilities are suitable and adequate for our current and near-term needs, and that we will be able to locate additional facilities as needed.
Item 3. Legal Proceedings.
Between January 4, 2022, and January 21, 2022, nine (9) civil actions were filed challenging the adequacy of certain public disclosures made by the Company concerning the Company’s pending transaction with Pfizer. On January 4, 2022, Elaine Wang, a purported stockholder of the Company, commenced an action in the United States District Court for the Southern District of New York, captioned Elaine Wang v. Arena Pharmaceuticals, Inc., et al., Case No. 1:22-cv-00047, against the Company and current members of its board of directors (the “Wang Complaint”). On January 6, 2022, Alex Ciccotelli, a purported stockholder of the Company, commenced an action in the United States District Court for the Southern District of New York, captioned Alex Ciccotelli v. Arena Pharmaceuticals, Inc., et al., Case No. 1:22-cv-00144, against the Company and current members of its board of directors (the “Ciccotelli Complaint”). On January 11, 2022, Darrell Nasalroad, a purported stockholder of the Company, commenced an action in the United States District Court for the Eastern District of New York, captioned Darrell Nasalroad v. Arena Pharmaceuticals, Inc., et al., Case No. 1:22-cv-00167, against the Company and current members of its board of directors (the “Nasalroad Complaint”). On January 12, 2022, Katherine Finger, a purported stockholder of the Company, commenced an action in the United States District Court for the Southern District of California, captioned Katherine Finger v. Arena Pharmaceuticals, Inc., et al., Case No. 3:22-cv-00039-LL-AHG, against the Company and current members of its board of directors (the “Finger Complaint”). On January 13, 2022, Michael Kent, a purported stockholder of the Company, commenced an action in the United States District Court for the District of Delaware, captioned Michael Kent v. Arena Pharmaceuticals, Inc., et al., Case No. 1:22-cv-00052-UNA, against the Company and current members of its board of directors (the “Kent Complaint”). On January 14, 2022, Jeffrey D. Justice, II, a purported stockholder of the Company, commenced an action in the United States District Court for the Eastern District of Pennsylvania, captioned Jeffrey D. Justice, II v. Arena Pharmaceuticals, Inc., et al., Case
60
No. 2:22-cv-00178, against the Company and current members of its board of directors (the “Justice Complaint”). On January 17, 2022, David Kaufmann, a purported stockholder of the Company, commenced an action in the United States District Court for the Southern District of New York, captioned David Kaufmann v. Arena Pharmaceuticals, Inc., et al., Case No. 1:22-cv-00405, against the Company and current members of its board of directors (the “Kaufmann Complaint”). On January 21, 2022, Deatra Harrington, a purported stockholder of the Company, commenced an action in the United States District Court for the Southern District of New York, captioned Deatra Harrington v. Arena Pharmaceuticals, Inc., et al., Case No. 1:22-cv-00551, against the Company and its board of directors (the “Harrington Complaint,” collectively with the Wang Complaint, the Ciccotelli Complaint, the Nasalroad Complaint, the Finger Complaint, the Kent Complaint, the Justice Complaint, and the Kaufmann Complaint, the “Complaints”). The Complaints asserted claims under Sections 14(a) and 20(a) of the Securities Exchange Act of 1934 (“Exchange Act”) and sought, among other things, an injunction preventing consummation of the pending transaction with Pfizer, rescission of the pending transaction or rescissory damages in the event it is consummated, declaratory relief that defendants violated the Exchange Act and requiring defendants to issue a proxy statement that includes the allegedly omitted information, an accounting by the defendants for all damages caused to the plaintiffs, and the award of attorneys’ fees and expenses. On January 5, 2022, Herbert Silverberg, a purported stockholder of the Company, commenced a putative class action in the Court of Chancery of the State of Delaware, captioned Herbert Silverberg v. Amit D. Munshi, et al., C.A. No. 2022-0018-PAF, against the Company and current members of its board of directors, and contemporaneously filed motions to expedite discovery and for preliminary injunction (collectively, the “Silverberg Action”). The Silverberg Action asserted a claim for breach of fiduciary duty and sought, among other things, an injunction preventing consummation of the pending transaction with Pfizer, rescission of the pending transaction or damages in the event it is consummated, and the award of attorneys’ fees and expenses. The motion to expedite discovery in the Silverberg Action was denied on January 10, 2022 and, on February 3, 2022, the Silverberg Action was voluntarily dismissed. Defendants believe the claims that were asserted in the Complaints and Silverberg Action were without merit and deny any wrongdoing in connection with the pending transaction with Pfizer or the disclosures related thereto.
As of February 23, 2022, all of the Complaints had been voluntarily dismissed.
Item 4. Mine Safety Disclosures.
Not applicable.
61
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market information
Our common stock is listed on the Nasdaq Global Select Market under the symbol “ARNA.”
Holders
As of February 17, 2022, there were approximately 69 stockholders of record of our common stock, one of which is Cede & Co., a nominee for Depository Trust Company, or DTC. Shares of common stock that are held by financial institutions as nominees for beneficial owners are deposited into participant accounts at DTC and are considered to be held of record by Cede & Co. as one stockholder.
Dividends
We have never paid cash dividends on our capital stock. We anticipate that we will retain earnings, if any, to support operations and finance the growth and development of our business and, therefore, do not expect to pay cash dividends in the foreseeable future.
62
Performance graph
The graph below compares the cumulative five-year total return on our common stock from December 31, 2016, through December 31, 2021, to the cumulative total return over such period for (i) the Nasdaq Composite Index and (ii) the Nasdaq Biotechnology Index. The graph assumes the investment of $100 on December 31, 2016, with the reinvestment of dividends, although dividends have not been declared on our common stock, and is calculated according to the Securities and Exchange Commission’s methodology. We caution that the stock price performance shown in the graph may not be indicative of future stock price performance. The graph, including each of the graph lines, was provided by Research Data Group, Inc.
This information, including the graph below, is not deemed to be “soliciting material” or to be “filed” with the Securities and Exchange Commission, or subject to the Securities and Exchange Commission’s proxy rules, other than as provided in such rules, or to the liabilities of Section 18 of the Securities Exchange Act of 1934, and shall not be deemed incorporated by reference into any prior or subsequent filing by us under the Securities Act of 1933 or the Securities Exchange Act of 1934, except to the extent that we specifically incorporate it by reference into any such filing.
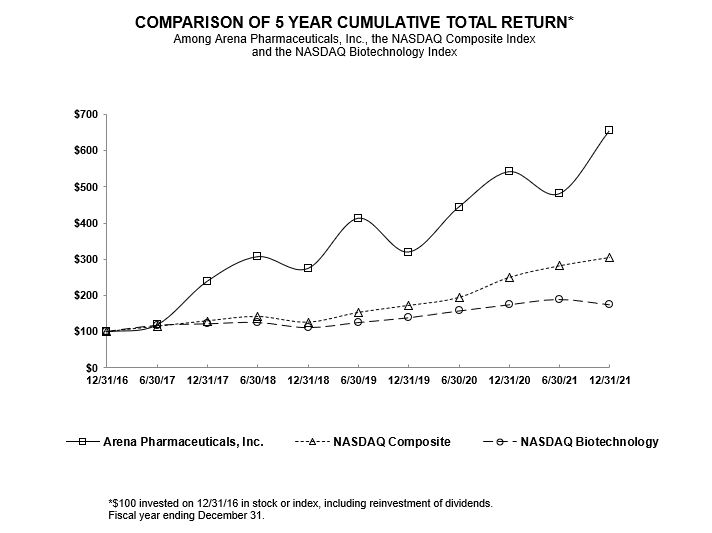
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis in conjunction with “Item 8. Financial Statements and Supplementary Data” included below in this Annual Report on Form 10-K, or Annual Report. Operating results are not necessarily indicative of results that may occur in future periods.
This discussion and analysis contains forward-looking statements that involve a number of risks, uncertainties and assumptions. Actual events or results may differ materially from our expectations. Important factors that could cause
63
actual results to differ materially from those stated or implied by our forward-looking statements include, but are not limited to, those set forth in “Item 1A. Risk Factors” in this Annual Report. All forward-looking statements included in this Annual Report are based on information available to us as of the time we file this Annual Report and, except as required by law, we undertake no obligation to update publicly or revise any forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain.
Pending Transaction with Pfizer
On December 12, 2021, Arena entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Pfizer Inc., a Delaware corporation (“Parent”), and Antioch Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of Parent (“Merger Sub”), providing for, among other things, the merger of Merger Sub with and into Arena (the “Merger”), with Arena surviving the Merger.
At the effective time of the Merger (the “Effective Time”), each:
(i) share of common stock of the Company, par value $0.0001 per share (each, a “Share”), issued and outstanding immediately prior to the Effective Time (other than (A) Shares owned by the Company as treasury stock, (B) Shares owned by Parent or Merger Sub and (C) any dissenting shares) will no longer be outstanding and will automatically be cancelled, retired and converted into the right to receive an amount in cash equal to $100.00, without interest thereon (the “Merger Consideration”);
(ii) option to purchase Shares (each, a “Company Option”) granted by the Company under the Company’s 2021 Long-Term Incentive Plan or prior stock plans (collectively, the “Company Stock Plans”) that is outstanding as of immediately prior to the Effective Time, whether or not then vested, will be cancelled and immediately cease to be outstanding and converted into the right to receive an amount in cash equal to the product of (1) the excess, if any, of the Merger Consideration over the per-share exercise price of such Company Option, multiplied by (2) the number of Shares then subject to such Company Option;
(iii) Company restricted stock unit, except as described in “(iv)” below, subject to vesting conditions based solely on continued employment or service to the Company or any of its subsidiaries granted by the Company under a Company Stock Plan that is unvested and outstanding as of immediately prior to the Effective Time will be cancelled and immediately cease to be outstanding and converted into the right to receive an amount in cash equal to the Merger Consideration;
(iv) Company restricted stock unit that is granted after December 12, 2021 (each, a “2022 Company RSU”) that is unvested and outstanding as of immediately prior to the Effective Time will be substituted automatically with a Parent restricted stock unit with respect to that number of shares of Parent common stock (each, an “Adjusted RSU”) that is equal to the product of (1) the total number of Shares subject to the 2022 Company RSU immediately prior to the Effective Time multiplied by (2)(a) the Merger Consideration divided by (b) the average of the volume-weighted average sales price per share of common stock of Parent on the New York Stock Exchange for the consecutive period of 15 trading days ending on (and including) the trading day that is four trading days prior to the Effective Time, with any fractional shares rounded to the nearest whole share. Each Adjusted RSU will otherwise be subject to the same terms and conditions applicable to such 2022 Company RSU immediately prior to the Effective Time (including vesting terms, and subject to accelerated vesting in connection with certain qualifying terminations of employment following the Effective Time); and
(v) Company restricted stock unit granted by the Company under a Company Stock Plan that is subject to performance-based vesting conditions (each, a “Company PRSU”) that is unvested and outstanding as of immediately prior to the Effective Time will be cancelled and immediately cease to be outstanding and converted into the right to receive an amount in cash equal to the Merger Consideration (with all the performance-based vesting conditions associated with such Company PRSU being deemed achieved at the greater of actual completed performance at the Effective Time or at target for any Company PRSU).
Consummation of the Merger is subject to certain conditions, including, but not limited to, the: (i) Company’s receipt of the approval of the Company’s stockholders representing a majority of the outstanding Shares (the “Company Requisite Vote”), which was obtained on February 2, 2022; (ii) expiration or termination of any waiting periods applicable to the consummation of the Merger under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (“HSR Act”) and the receipt of certain additional clearances or approvals of certain other governmental bodies applicable to the Merger; and (iii)
64
the absence of any law or order prohibiting or making illegal the consummation of the Merger. The Merger is targeted to close in the first half of 2022.
OVERVIEW AND RECENT DEVELOPMENTS
We are a biopharmaceutical company focused on delivering novel, transformational medicines with optimized pharmacology and pharmacokinetics to patients globally. Our internally developed pipeline includes multiple potentially first- or best-in-class assets with broad clinical utility.
Our most advanced investigational clinical programs include:
•Etrasimod, which we are evaluating in a Phase 3 program for ulcerative colitis, or UC, a Phase 2b/3 program for Crohn’s disease, or CD, a Phase 2 program in alopecia areata, or AA, and a Phase 2b program for eosinophilic esophagitis, or EoE. We also plan to evaluate etrasimod in a Phase 3 program in atopic dermatitis, or AD.
•APD418, for acute heart failure, or AHF, which we are evaluating in a Phase 2 trial.
•Temanogrel, a second compound in our cardiovascular therapeutic area, which we have advanced into a Phase 2 proof of mechanism study in microvascular obstruction, or MVO and initiated a Phase 2 trial in Raynaud’s phenomenon secondary to systemic sclerosis, or SSc-RP.
•Olorinab, which we were evaluating for a broad range of visceral pain conditions associated with gastrointestinal diseases. We are evaluating possible strategic options for olorinab.
We continue to leverage our two decades of world-class G-protein-coupled receptor, or GPCR, target discovery research to develop breakthrough drugs and ultimately deliver these to patients with large unmet needs. Our long-term pipeline prospects include an enhanced collaboration with Beacon Discovery across a broad range of immune-mediated inflammatory targets and compounds.
We have license agreements or collaborations with various companies, including:
•United Therapeutics (ralinepag in a Phase 3 program for pulmonary arterial hypertension),
•Everest Medicines Limited (etrasimod in a Phase 3 program for UC in Greater China and select countries in Asia),
•Beacon Discovery (early research platform for GPCR targets),
•Boehringer Ingelheim International GmbH (undisclosed orphan GPCR program for central nervous system – preclinical), and
•Aristea Therapeutics (RIST4721 in a Phase 2 program for the treatment of palmoplantar pustulosis and other neutrophil-mediated diseases).
To limit the spread of COVID-19, governments have taken various actions including the issuance of stay-at-home orders and social distancing guidelines, causing some businesses to suspend operations and a reduction in demand for many products from direct or ultimate customers. Accordingly, many businesses have adjusted, reduced or suspended operating activities. The impact of this pandemic has been and will likely continue to be extensive in many aspects of society, which has resulted in and will likely continue to result in significant disruptions to businesses and capital markets around the world. The extent to which COVID-19 impacts us will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the coronavirus and the actions to contain the coronavirus or treat its impact, among others. Beginning the week of March 16, 2020, substantially all of our workforce began working from home, either all or substantially all of the time. In addition, we have experienced delays in site initiation and participant enrollment and screening rates in certain of our clinical development programs as a result of the COVID-19 pandemic. The potential impact, if any, that these site-level delays could have on our development program timelines remains uncertain. The effects of the stay-at-home orders and our work-from-home policies may negatively impact productivity, disrupt our business and delay our development programs, and may delay our regulatory and commercialization timelines, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. Our future research and development expenses and selling, general and administrative expenses may vary significantly based on developments related to the coronavirus outbreak and impact of it and COVID-19 on the costs and timing associated with the conduct of our clinical trials and other related business activities.
65
Program development update.
Gastroenterology
ELEVATE UC constitutes our Phase 3 global registrational program to assess the safety and efficacy of once-daily etrasimod 2 mg in participants with moderately to severely active UC. In January 2021, the pivotal, 52-week ELEVATE UC 52 trial completed enrollment. In August 2021, the pivotal, 12-week ELEVATE UC 12 trial reached full enrollment. We expect topline data from both ELEVATE UC 12 and ELEVATE UC 52 in the first quarter of 2022.
In November 2021, we reached target enrollment of 70 participants in Study A of the Phase 2/3 CULTIVATE trial evaluating the safety and efficacy of 2 mg and 3 mg etrasimod, a highly selective, once-daily, oral sphingosine 1-phosphate (S1P) receptor modulator, in participants with moderate to severe Crohn's disease (CD).
In February 2021, we dosed the first participant in our Phase 2b VOYAGE trial of etrasimod in EoE. VOYAGE is a Phase 2b randomized, double-blind, placebo-controlled trial, with a primary efficacy measurement at week 16 and a secondary efficacy analysis at week 24, to assess the safety and efficacy of 1 mg and 2 mg etrasimod in participants with EoE. In June 2021, the FDA granted Orphan Drug Designation status to etrasimod for the treatment of eosinophilic esophagitis.
Dermatology
In July 2021, we evaluated an updated open-label extension (“OLE”) data set from the Phase 2 ADVISE trial for 2 mg etrasimod in atopic dermatitis which demonstrated meaningful effects at week 16 of the OLE period on validated Investigator Global Assessment (“vIGA”) at 47%, Eczema Area and Severity Index (“EASI-75”) at 72%, and Peak Pruritis Numeric Rating Scale (“PP-NRS”) at 61% with consistent safety profile out to one year.
In July 2021, the Phase 2 trial for etrasimod in alopecia areata was amended to add a 3 mg cohort and expand patient population subtypes. We expect to announce topline data for this trial in the second half of 2022.
Cardiovascular
In July 2021, a Phase 2 trial for APD418 was initiated. The FDA has granted us Fast Track designation for development of APD418 in AHF.
In June 2021, the first participant was randomized in the Phase 2 trial for temanogrel in microvascular obstruction.
In November 2021, the first participant was randomized in the Phase 2 trial for temanogrel in Raynaud’s phenomenon secondary to systemic sclerosis.
Collaborations and license agreements update.
In July 2021, the Company entered into a strategic collaboration and option agreement to advance the clinical development of RIST4721, an oral CXCR2 antagonist being developed by Aristea Therapeutics, Inc. (“Aristea”) for the treatment of palmoplantar pustulosis (“PPP”) and other neutrophil-mediated diseases. Refer to Note 8. Collaborations and License Agreements of the consolidated financial statements for further detail.
In 2020, we entered into a multi-year strategic collaboration and license agreement (“2020 Collaboration and License Agreement”) with Beacon, aimed at building novel medicines across a range of GPCR targets believed to play a role in immune and inflammatory diseases. Under the terms of this agreement, Beacon is responsible for early drug discovery activities and we are responsible for any potential future development and, ultimately, commercialization activities. We are required to pay Beacon research initiation fees, make quarterly research funding payments for the duration of Beacon’s research activities as well as research, development and regulatory milestone payments. We are also obligated to pay Beacon tiered royalties on net sales of low single digits levels. In the first quarter of 2021, we received a $1.1 million payment as a result of the merger between Eurofins Beacon Discovery Holdings, Inc. (“Eurofins”) and Beacon. This payment satisfied Beacon’s obligation to pay us a percentage of the consideration for such sale transaction in the event that Beacon was sold as outlined in the 2016 License and Collaboration Agreement. We are eligible to receive future contingent consideration payments based on certain performance metrics achieved by Beacon over a four-year performance period through the first quarter of 2025 up to an aggregate of $2.0 million. Following the merger, we entered
66
into a Consent and Release Agreement that terminated our rights of negotiation and rights of first refusal to potentially obtain licenses to certain compounds discovered and developed by Beacon. In addition, the Consent and Release Agreement terminated the Company’s right, under the 2016 License and Collaboration Agreement, to receive any revenue received by Beacon including upfront payments, milestone payments and royalties. The 2020 Collaboration and License Agreement with Beacon remains in effect and was not impacted by the merger.
Other corporate events.
In July 2021, we announced the appointment of Douglas J. Manion, M.D., F.R.C.P. (C), as Executive Vice President of Research & Development. In June 2021, we announced the appointment of Steven J. Schoch as an independent director and as chair of the Audit Committee.
See the above “Business” section for a more complete discussion of our business.
RESULTS OF OPERATIONS
We are providing the following summary of our revenues, research and development expenses and selling, general and administrative expenses to supplement the more detailed discussion below. The dollar values in the following tables are in millions.
For our discussion of the year ended December 31, 2020, compared to the year ended December 31, 2019, please read Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations located in our Annual Report on Form 10-K for the year ended December 31, 2020, as filed with the SEC on February 23, 2021.
YEAR ENDED DECEMBER 31, 2021, COMPARED TO YEAR ENDED DECEMBER 31, 2020
Revenues
We recognized revenues of $0.1 million for the year ended December 31, 2021, compared to revenues of $0.3 million for the year ended December 31, 2020. Absent any new collaborations, we expect our 2022 revenues will primarily consist of potential milestone payments from our existing collaborations and license agreements.
Revenues from milestones and royalties are difficult to predict, and our overall revenues will likely continue to vary from quarter to quarter and year to year. In the short term, we expect the amount of revenue we earn to fluctuate.
Research and development expenses
Research and development expenses, which account for the majority of our expenses, consist primarily of clinical trial costs (including payments to contract research organizations, or CROs), salaries and other personnel costs, preclinical study fees, manufacturing costs for non-commercial products, research supply costs and facility and equipment costs. We expense research and development costs as they are incurred when these expenditures have no alternative future uses. We generally do not track our earlier-stage, internal research and development expenses by project; rather, we track such expenses by the type of cost incurred.
| Years ended December 31, | % change from 2020 to 2021 | |||||||||||||||||||
| Type of expense | 2021 | 2020 | ||||||||||||||||||
| External clinical and preclinical study fees | $ | 296.0 | $ | 220.4 | 34.3 | % | ||||||||||||||
Salary and other personnel costs (excluding non-cash share-based compensation) | 75.7 | 64.7 | 17.0 | % | ||||||||||||||||
| Non-cash share-based compensation | 33.5 | 26.0 | 28.8 | % | ||||||||||||||||
| Facility and other costs | 14.3 | 12.6 | 13.5 | % | ||||||||||||||||
| Total research and development expenses | $ | 419.5 | $ | 323.7 | 29.6 | % | ||||||||||||||
Research and development expenses increased by $95.8 million to $419.5 million for the year ended December 31, 2021, from $323.7 million for the year ended December 31, 2020. The increase in external clinical and preclinical study fees was primarily due to the progression of the etrasimod UC and CD programs as well as the temanogrel and APD418 programs, partially offset by a decrease in olorinab program expenses. The increase in salary and other personnel costs and
67
non-cash share-based compensation was primarily due to an increase in the number of research and development employees and compensation expense related to RSUs and PRSUs.
Of the $296.0 million external clinical and preclinical study fees noted in the table above for the year ended December 31, 2021, $250.9 million relates to etrasimod. Of the $220.4 million external clinical and preclinical study fees noted in the table above for the year ended December 31, 2020, $170.7 million relates to etrasimod.
Cumulatively from our inception through December 31, 2021, we have recognized (i) external clinical and preclinical study fees of $307.8 million for lorcaserin, $616.0 million for etrasimod, $64.2 million for ralinepag, $43.8 million for nelotanserin, $67.1 million for olorinab and $27.8 million for temanogrel and (ii) $53.2 million for non-commercial manufacturing and other development costs for lorcaserin and, to a lesser extent, nelotanserin.
We expect to incur substantial research and development expenses in 2022 and for the aggregate amount in 2022 to be greater than the amount incurred in 2021. We expect our research and development costs to be higher primarily due to a higher number of clinical studies and associated external clinical trial costs and increasing headcount in connection with advancing our pipeline. Our actual expenses may be higher or lower than anticipated due to various factors, including our progress and results. For example, patient enrollment in our Phase 3 clinical program for etrasimod is expected to be competitive and challenging, and could take longer than originally projected, which may result in our related external expenses being lower in 2022 than anticipated (but which might increase the overall costs for completing this multi-year program).
Expenditures on current and future clinical development programs are expected to be substantial and subject to many uncertainties, which include having adequate funding and developing our drug candidates independently or with collaborators. As a result of such uncertainties, we cannot predict with any significant degree of certainty the duration and completion costs of our research and development projects or whether, when and to what extent we will generate revenues from the commercialization and sale of any of our drug candidates. The duration and cost of clinical trials may vary significantly over the life of a project as a result of unanticipated events arising during clinical development and a variety of factors, including:
•the nature and number of trials and studies in a clinical program;
•the potential therapeutic indication;
•the number of patients who participate in the trials;
•the number and location of sites included in the trials;
•the rates of patient recruitment, enrollment and withdrawal;
•the duration of patient treatment and follow-up;
•the costs of manufacturing drug candidates; and
•the costs, requirements, timing of, and the ability to secure and maintain regulatory approvals.
Acquired in-process research and development
In July 2021, we entered into a strategic collaboration and option agreement with Aristea to advance the clinical development of RIST4721, an oral CXCR2 antagonist. We made a $60.0 million upfront payment and $10.0 million investment in Aristea’s preferred stock, both of which were expensed as acquired in-process research and development expenses in the consolidated statement of operations during the year ended December 31, 2021.
Selling, general and administrative expenses
68
| Years ended December 31, | % change from 2020 to 2021 | |||||||||||||||||||
| Type of expense | 2021 | 2020 | ||||||||||||||||||
Salary and other personnel costs (excluding non-cash share-based compensation) | $ | 41.5 | $ | 31.8 | 30.5 | % | ||||||||||||||
| Non-cash share-based compensation | 36.9 | 33.9 | 8.8 | % | ||||||||||||||||
| Legal, accounting and other professional fees | 31.8 | 23.8 | 33.6 | % | ||||||||||||||||
| Facility and other costs | 16.0 | 13.7 | 16.8 | % | ||||||||||||||||
| Total selling, general and administrative expenses | $ | 126.2 | $ | 103.2 | 22.3 | % | ||||||||||||||
Selling, general and administrative expenses increased by $23.0 million to $126.2 million for the year ended December 31, 2021, from $103.2 million for the year ended December 31, 2020. The increase in salary and other personnel costs and non-cash share-based compensation was primarily due to an increase in the number of selling, general and administrative employees and compensation expense related to RSUs and PRSUs. The increase in legal, accounting and other professional fees was primarily related to legal expenses associated with the ongoing BELVIQ litigation as well as marketing and commercialization costs associated with early commercialization preparedness. The increase in facility and other costs was primarily due to increased software license subscriptions and computer equipment purchases associated with higher headcount and new system implementations.
Transaction costs
In December 2021, we entered into a definitive agreement under which Pfizer, Inc. will acquire all of Arena's outstanding shares. In connection with this transaction, we incurred transaction fees of approximately $7.3 million. In addition to the transaction fees associated with the Pfizer transaction, we also incurred approximately $1.3 million of fees related to the Aristea transaction. These expenses are presented as transaction costs in the accompanying consolidated statements of operations.
Interest and other income, net. Interest and other income, net, was $7.9 million for the year ended December 31, 2021, compared to $21.9 million for the year ended December 31, 2020. This change was primarily due to a decline of $9.6 million in interest income from our available-for-sale investments and an increase of $5.9 million from equity in losses from our equity method investment in Longboard. During the year ended December 31, 2021, we recorded a gain from a change in ownership percentage of our equity method investment in Longboard of approximately $13.9 million and a $1.1 million gain from the Beacon Discovery merger with Eurofins. During the year ended December 31, 2020, we recorded a gain of $13.0 million from deconsolidating our investment in Arena Neurosciences, Inc. (now Longboard).
Income tax expense. We did not record a benefit for income taxes for the years ended December 31, 2021 or 2020 because we have a full valuation allowance against our deferred tax assets.
LIQUIDITY AND CAPITAL RESOURCES
On December 12, 2021, Arena entered into an Agreement and Plan of Merger with Pfizer Inc. We have agreed to various covenants and agreements, including, among others, agreements to conduct our business in the ordinary course during the period between the execution of the Plan of Merger and the effective time of the Merger. Outside of certain
69
limited exceptions, we may not take, authorize, commit, resolve, or agree to do certain actions without Pfizer’s consent, including:
•acquiring businesses and disposing of significant assets;
•incurring expenditures above specified thresholds;
•issuing equity;
•issuing debt facilities; and
•repurchasing shares of our outstanding common stock.
We do not believe these restrictions will prevent us from meeting our ongoing costs of operations, working capital needs, or capital expenditure requirements.
We have accumulated a large deficit since inception that has primarily resulted from the significant research and development expenditures we have made in seeking to identify and develop compounds that could become marketed drugs. We expect to continue to incur substantial losses for at least the short term.
To date, we have obtained cash and funded our operations primarily through the sale of common and preferred stock, the issuance of debt and related financial instruments, payments from collaborators and customers and sale leaseback transactions. From our inception through December 31, 2021, we have generated $4.0 billion in cash from these sources, of which approximately $2.5 billion was through sales of equity, $1.4 billion was through payments from collaborators and customers, $96.9 million was through the issuance of debt and related financial instruments and $77.1 million was from sale and leaseback transactions.
We believe our cash resources are sufficient to allow us to continue operations for at least the next 12 months from the date this Annual Report is filed with the SEC. There is no guarantee that adequate funds will be available when needed from additional debt or equity financing, development and commercialization partnerships or from other sources, or on terms acceptable to us. If our efforts to obtain sufficient additional funds are not successful, we would be required to delay, scale back, or eliminate some or all of our research or development, manufacturing operations, administrative operations, and clinical or regulatory activities, which could negatively affect our ability to achieve certain corporate goals.
Short term liquidity
The following discussion of our short-term liquidity assumes that the Merger with Pfizer is not consummated and we continue to operate as an independent entity. During the pendency of the Merger we are restricted from pursuing financing opportunities through the manners listed in items (ii)-(iv) in the below paragraph.
At December 31, 2021, we had $0.7 billion in cash and cash equivalents and available-for-sale investments. Our potential sources of liquidity in the short term include (i) milestone and other payments from collaborators, (ii) entering into new collaboration, licensing or commercial agreements for one or more of our drug candidates or programs, (iii) the lease of our facilities or sale of other assets and (iv) sale of equity, issuance of debt or other transactions.
Long term liquidity
The following discussion of our long-term liquidity assumes that the Merger with Pfizer is not consummated and we continue to operate as an independent entity.
It will require substantial cash to achieve our objectives of discovering, developing and commercializing drugs, and this process typically takes many years and potentially several hundreds of millions of dollars for an individual drug. We may not have adequate available cash, or assets that could be readily turned into cash, to meet these objectives in the long term. We will need to obtain significant funds under our existing collaborations, under new collaboration, licensing or other commercial agreements for one or more of our drug candidates and programs or patent portfolios, or from other potential sources of liquidity, which may include the sale of equity, issuance of debt or other transactions.
In addition to potential payments from our current collaborators, as well as funds from public and private financial markets, potential sources of liquidity in the long term include (i) upfront, milestone, royalty and other payments from any future collaborators or licensees and (ii) revenues from sales of any drugs we obtain regulatory approval to commercialize on our own. The length of time that our current cash and cash equivalents and any available borrowings will sustain our operations is based on, among other things, the rate of adoption and commercial success of any drugs we or our
70
collaborators obtain regulatory approval to market, regulatory decisions affecting our and our collaborator’s drug candidates, prioritization decisions regarding funding for our programs, progress in our clinical and earlier-stage programs, the time and costs related to current and future clinical trials and nonclinical studies, our research, development, manufacturing and commercialization costs (including personnel costs), our progress in any programs under collaborations, costs associated with intellectual property, our capital expenditures, and costs associated with securing any in-licensing opportunities. Any significant shortfall in funding may result in us reducing our development and/or research activities, which, in turn, would affect our development pipeline and ability to obtain cash in the future.
We evaluate from time to time potential acquisitions, in-licensing and other opportunities. Any such transaction may impact our liquidity as well as affect our expenses if, for example, our operating expenses increase as a result of such acquisition or license or we use our cash to finance the acquisition or license.
Sources and uses of our cash
Net cash used in operating activities was $452.0 million in the year ended December 31, 2021 compared to $353.1 million in the year ended December 31, 2020. This change was primarily the result of an increase of $44.4 million in payments made for external clinical study fees, and an increase in cash expenditures of approximately $36.0 million for personnel costs resulting primarily from an increase in the number of employees.
Net cash provided by investing activities was $321.4 million in the year ended December 31, 2021 compared to net cash used in investing activities of $21.4 million in the year ended December 31, 2020. This change was primarily due to a decrease of $421.4 million in purchases of available-for-sale investments, partially offset by a $70.0 million payment to Aristea in July 2021 for acquired in-process research and development.
Net cash of $135.4 million was provided by financing activities in the year ended December 31, 2021, primarily as a result of proceeds from the issuance of common stock under the ATM facility. Net cash of $350.6 million was provided by financing activities in the year ended December 31, 2020, primarily as a result of proceeds from the issuance of common stock in a public offering.
Contractual Obligations
Our financing obligations relate to sale and leaseback transactions for certain of our properties. We have applied the financing method to these sale and leaseback transactions, which requires that the book value of the properties and related accumulated depreciation remain on our balance sheet with no sale recognized. The sales price of the properties is recorded as a financing obligation and a portion of each lease payment is recorded as interest expense. At December 31, 2021, we expect our interest expense over the remaining term of these leases to total $12.6 million. Our other properties are under operating leases.
CRITICAL ACCOUNTING POLICIES AND MANAGEMENT ESTIMATES
The SEC defines critical accounting policies as those that are, in management’s view, important to the portrayal of our financial condition and results of operations and demanding of management’s judgment. Our discussion and analysis of financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with the US generally accepted accounting principles, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosures. We base our estimates on historical experience and on various assumptions that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ significantly from those estimates.
Our significant accounting policies are more fully described in Note 1 of the consolidated financial statements included in this Annual Report. As disclosed in Note 1, the preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions about future events that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ significantly from those estimates. We believe that the following discussion addresses our most critical accounting policies, which are those that are most important to the portrayal of our financial condition and results of operations and require management’s most difficult, subjective and complex judgments.
71
Revenue recognition. Our revenues to date have been generated primarily through collaboration or license agreements. Our collaboration and license agreements frequently contain multiple types of promised goods or services including (i) intellectual property licenses, (ii) product research, development and regulatory services and (iii) product manufacturing. Consideration we receive under these arrangements may include upfront payments, research and development funding, cost reimbursements, milestone payments, payments for product sales and royalty payments.
We recognize revenue when a customer obtains control of promised goods or services. The amount of revenue recognized reflects the consideration that we expect to be entitled to receive in exchange for these services and excludes sales incentives and amounts collected on behalf of third parties. We analyze the nature of these performance obligations in the context of individual collaboration and license agreements in order to assess the distinct performance obligations. We apply the following five steps to recognize revenue:
i)Identify the contract with a customer. We consider the terms and conditions of our collaboration and license agreements to identify contracts within the scope of ASC 606. We consider that we have a contract with a customer when the contract is approved, we can identify each party's rights regarding the goods and services to be transferred, we can identify the payment terms for the goods and services, we have determined the customer has the ability and intent to pay and the contract has commercial substance. We use judgment in determining the customer's ability and intent to pay, which is based upon factors including the customer's historical payment experience or, for new customers, credit and financial information pertaining to the customers.
ii)Identify the performance obligations in the contract. Performance obligations in our collaboration and license agreements are identified based on the goods and services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the service either on its own or together with other resources that are readily available from third parties or from us, and are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from other promises in the contract. Our performance obligations generally consist of intellectual property licenses, research, development and/or regulatory services and manufacturing and supply commitments. Determining whether a promised goods or service is a separate performance obligation requires the use of significant judgment. A change in such judgment could result in a significant change in the period in which revenue is recognized.
Most of our collaboration and license agreements with customers contain multiple promised goods or services. Based on the characteristics of the promised goods and services we analyze whether they are separate or combined performance obligations. The transaction price is allocated to the separate performance obligations on a relative standalone selling price basis. We determine standalone selling price based on our overall pricing and discounting objectives, taking into consideration the type of services, estimates of hourly market rates, and stage of the research, development or clinical trials.
iii)Determine the transaction price. We determine the transaction price based on the consideration to which we expect to be entitled in exchange for transferring goods and services to the customer. In determining the transaction price, any variable consideration would be considered, to the extent applicable, if, in our judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. In accordance with the royalty exception under ASC 606 for licenses of intellectual property, the transaction price excludes future royalty payments to be received from our customers. None of our collaboration and license agreements contain consideration payable to our customer or a significant financing component. The process for determining the transaction price involves significant judgment and includes consideration of multiple factors such as estimated revenues, market size, and development risk, among other factors contemplated in negotiating the arrangement with the customer.
Our contracts with customers primarily include two types of variable consideration: (i) development and regulatory milestone payments, which are due to us upon achievement of specific development and regulatory milestones and (ii) one-time sales-based payments and sales-based royalties associated with sold or licensed intellectual property.
Due to uncertainty associated with achievement of the development and regulatory milestones, the related milestone payments are excluded from the contract consideration and the corresponding revenue is not recognized until we conclude it is probable that reversal of such milestone revenue will not occur.
Product sales-based royalties under licensed intellectual property and one-time payments are accounted for under the royalty exception. We recognize revenue for sales-based royalties under licensed intellectual property and one-time payments at the later of when the sales occur or the performance obligation is satisfied or partially satisfied.
72
iv)Allocate the transaction price to performance obligations in the contract. If the contract contains a single performance obligation, the entire transaction price is allocated to that performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation based on a relative standalone selling price.
v)Recognize revenue when or as we satisfy a performance obligation. Revenue is recognized at the time the related performance obligation is satisfied by transferring the promised goods or services to a customer. We recognize revenue when we transfer control of the goods or services to our customers for an amount that reflects the consideration that we expect to receive in exchange for those services.
Performance Obligations.
The following is a description of principal goods and services from which we generate revenue.
Intellectual property licenses
We generate revenue from licensing our intellectual property including know-how and development and commercialization rights. These licenses provide customers with a term-based license to further research, develop and commercialize our internally-discovered drug candidates. The consideration we receive in the form of nonrefundable upfront consideration related to the functional intellectual property licenses is recognized when we transfer such license to the customer unless the license is combined with other goods or services into one performance obligation, in which case the revenue is recognized over a period of time based on our estimated pattern in which we satisfy the combined performance obligation. Our licensing agreements are generally cancellable.
Customers have the right to terminate their contracts upon notice. We have the right to terminate the contracts generally only if the customer is in breach of the contract and fails to remedy the breach in accordance with the contractual terms.
Intellectual property sales
We generate royalty revenue from sales of our intellectual property. We estimate the future royalty payments and recognize revenue with a corresponding contract asset at a point in time when we transfer the intellectual property to the customer. We periodically reassess our estimate of the future royalty payments and recognize any estimate adjustments as revenue in the current period.
Research, development and regulatory services
We generate revenue from research, development and regulatory services we provide to our customers in connection with the licensed intellectual property. The services we provide to our customers primarily include scientific research activities, preparation for and management of clinical trials, and assistance during the regulatory approval application process. Revenue associated with these services is recognized based on our estimate of total consideration to be received for such services and the pattern in which we perform the services. The pattern of performance is generally determined to be the amount of incurred expenses reimbursed by the customer as a percentage of total expected reimbursable expenses associated with the contract.
Clinical trial expenses. We accrue clinical trial expenses based on work performed. In determining the amount to accrue, we rely on estimates of total costs incurred based on enrollment, the completion of trials and other events. We follow this method because we believe reasonably dependable estimates of the costs applicable to various stages of a clinical trial can be made. However, the actual costs and timing of clinical trials are uncertain, subject to risks and may change depending on a number of factors. Differences between the actual clinical trial costs and the estimated clinical trial costs that we have accrued in any prior period are recognized in the subsequent period in which the actual costs become known. Historically, these differences have not been material; however, material differences could occur in the future.
Share-based compensation. Our share-based awards are measured at fair value and recognized over the requisite service or performance period. We estimate the fair value of each stock option on the date of grant using the Black-Scholes option pricing model which requires the input of subjective assumptions, including price volatility of the underlying stock, risk-free interest rate, dividend yield, and expected life of the option. Expected volatility is computed using historical volatility for a period equal to the expected term. The expected term of options is determined based on historical experience of similar awards, giving consideration to the contractual terms of the share-based awards, vesting schedules and post-vesting terminations. The risk-free interest rates are based on the US Treasury yield curve, with a
73
remaining term approximately equal to the expected term used in the option pricing model. We account for forfeitures in the period they occur. The fair value of each restricted stock unit award is determined based on the market price of the underlying common stock on the date of the grant. We estimate the fair value of restricted stock unit awards that include market-based performance conditions on the date of grant using a Monte Carlo simulation model, based on the market price of the underlying common stock, expected performance measurement period, expected stock price volatility and expected risk-free interest rate.
Income taxes. Significant judgment is required by management to determine our provision for income taxes, our deferred tax assets and liabilities, and the valuation allowance to record against our net deferred tax assets, which are based on complex and evolving tax regulations throughout the world. Our tax calculation is impacted by tax rates in the jurisdictions in which we are subject to tax and the relative amount of income earned in each jurisdiction. Our deferred tax assets and liabilities are determined using the enacted tax rates expected to be in effect for the years in which those tax assets are expected to be realized.
The effect of an uncertain income tax position is recognized at the largest amount that is “more-likely-than-not” to be sustained under audit by the taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained.
The realization of our deferred tax assets is dependent upon our ability to generate sufficient future taxable income. We establish a valuation allowance when it is more-likely-than-not that the future realization of all or some of the deferred tax assets will not be achieved. The evaluation of the need for a valuation allowance is performed on a jurisdiction-by-jurisdiction basis, and includes a review of all available evidence, both positive and negative.
Impairment of investments. At each reporting date, we perform an evaluation of impairment of our available-for-sale investments to determine if any unrealized losses are the result of credit losses. Impairment is assessed at the individual security level. Factors considered in determining whether a loss resulted from a credit loss or other factors include the Company’s intent and ability to hold the investment until the recovery of its amortized cost basis, the extent to which the fair value is less than the amortized cost basis, the financial condition of the issuer, any historical failure of the issuer to make scheduled interest or principal payments, any changes to the rating of the security by a rating agency, any adverse legal or regulatory events affecting the issuer or issuer’s industry, and any significant deterioration in economic conditions. If a decline in the fair value below the amortized cost basis of available-for-sale securities is determined to be due to credit-related factors, we will record the losses in earnings through an allowance account. Unrealized gains and losses that are not credit-related are included in accumulated other comprehensive income.
The above listing is not intended to be a comprehensive list of all of our accounting policies. In many cases, the accounting treatment of a particular transaction is specifically dictated by GAAP. See our audited consolidated financial statements and notes thereto included elsewhere in this Annual Report, which contain additional accounting policies and other disclosures required by GAAP.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Interest Rate Risk
We invest our excess cash in investment-grade, interest-bearing securities. The primary objective of our investment activities is to preserve principal and liquidity. To achieve this objective, we invest in money market funds, US Treasury notes, and high-quality marketable debt instruments of corporations and government-sponsored enterprises with contractual maturity dates of generally less than two years. All investment securities have a credit rating of at least A or better, as determined by Moody’s Investors Service, Standard & Poor’s or Fitch Ratings. We have the ability to hold our investments until maturity, and therefore we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a change in market interest rates on our investments.
Foreign Currency Exchange Risk
We have a wholly owned subsidiary in Switzerland, which exposes us to foreign currency exchange risk. The functional currency of our subsidiary in Switzerland is the Swiss franc. Accordingly, all assets and liabilities of our Swiss subsidiary are translated to US dollars based on the applicable exchange rate on the balance sheet date. Revenue and expense components are translated to US dollars at weighted-average exchange rates in effect during the period. Gains and losses resulting from foreign currency translation are reported as a separate component of accumulated other comprehensive income in the equity section of our consolidated balance sheets.
74
Foreign currency transaction gains and losses recorded in continuing operations are insignificant. If a 10% change in the US dollar-to-Swiss franc exchange rate were to have occurred on December 31, 2021, this change would not have had a material effect on the financial results of our continuing operations.
We have not hedged exposures denominated in foreign currencies but may do so in the future.
75
Item 8. Financial Statements and Supplementary Data.
ARENA PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | |||||
76
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Arena Pharmaceuticals, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Arena Pharmaceuticals, Inc. and subsidiaries (the Company) as of December 31, 2021 and 2020, the related consolidated statements of operations and comprehensive (loss) income, equity, and cash flows for each of the years in the three-year period ended December 31, 2021, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2021, in conformity with US generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 23, 2022 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the US federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Evaluation of the accrued clinical and preclinical study fees
As discussed in Note 1 to the consolidated financial statements, the Company accrues clinical trial costs and preclinical study fees based on the work performed. The Company has entered into various contracts with contract research organizations (CROs) to perform research and development activities. When billing terms under these contracts do not coincide with the timing of the work performed, the Company is required to make estimates of outstanding obligations as of year-end to those CROs. These estimates are based on a number of factors, including the Company’s knowledge of patient enrollment, the status of each of the clinical trials, invoicing to date, and the provisions in the contracts. The accrued clinical and preclinical study fees were $29.1 million as of December 31, 2021. The clinical and preclinical study fees included within research and development expenses were $296.0 million for the year ended December 31, 2021.
77
We identified the evaluation of accrued clinical and preclinical study fees as a critical audit matter. Challenging auditor judgment was involved in evaluating the status of each of the clinical trials.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls over the Company’s process to estimate the outstanding obligations for the work performed by the CROs over clinical trials, including controls over the status of the clinical trials. We assessed the amount billed to date for a selection of contracts with CROs by confirming the costs incurred with the respective CROs and the underlying invoices. We further evaluated the status of the selected clinical trials by inquiring with individuals of the Company responsible for monitoring and tracking the status of clinical trials, reviewing the provisions of the contracts, and inspecting government clinical trial databases. We also examined certain invoices received after December 31, 2021 and evaluated whether services received prior to December 31, 2021 were included in the accrued clinical and preclinical study fee balance as of December 31, 2021.
/s/ | |||||
| We have served as the Company’s auditor since 2010. | |||||
| February 23, 2022 | |||||
78
ARENA PHARMACEUTICALS, INC.
Consolidated Balance Sheets
(In thousands, except share and per share data)
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Assets | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Short-term investments, available-for-sale | |||||||||||
| Prepaid expenses and other current assets | |||||||||||
| Total current assets | |||||||||||
| Investments, available-for-sale | |||||||||||
| Land, property and equipment, net | |||||||||||
| Other non-current assets | |||||||||||
| Total assets | $ | $ | |||||||||
| Liabilities and Stockholders' Equity | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable and other accrued liabilities | $ | $ | |||||||||
| Accrued clinical and preclinical study fees | |||||||||||
| Current portion of lease financing obligations | |||||||||||
| Total current liabilities | |||||||||||
| Lease financing obligations, less current portion | |||||||||||
| Other long-term liabilities | |||||||||||
| Commitments and contingencies | |||||||||||
| Stockholders' equity: | |||||||||||
Preferred stock, $ | |||||||||||
Common stock, $ | |||||||||||
| Additional paid-in capital | |||||||||||
| Accumulated other comprehensive (loss) income | ( | ||||||||||
| Accumulated deficit | ( | ( | |||||||||
| Total stockholders' equity | |||||||||||
| Total liabilities and equity | $ | $ | |||||||||
See accompanying notes to consolidated financial statements.
79
ARENA PHARMACEUTICALS, INC.
Consolidated Statements of Operations and Comprehensive (Loss) Income
(In thousands, except per share data)
Years ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Revenues | |||||||||||||||||
| Collaboration and other revenue | $ | $ | $ | ||||||||||||||
| United Therapeutics revenue | |||||||||||||||||
| Royalty revenue | ( | ||||||||||||||||
| Total revenues | |||||||||||||||||
| Operating costs and expenses | |||||||||||||||||
| Research and development | |||||||||||||||||
| Acquired in-process research and development | |||||||||||||||||
| Selling, general and administrative | |||||||||||||||||
| Transaction costs | |||||||||||||||||
| Total operating costs and expenses | |||||||||||||||||
| (Loss) income from operations | ( | ( | |||||||||||||||
| Interest and other income (expense) | |||||||||||||||||
| Interest income | |||||||||||||||||
| Interest expense | ( | ( | ( | ||||||||||||||
| Other (expense) income, net | ( | ||||||||||||||||
| Gain from Longboard equity method investment | |||||||||||||||||
| Total interest and other income, net | |||||||||||||||||
| (Loss) income before income taxes | ( | ( | |||||||||||||||
| Income tax provision | ( | ||||||||||||||||
| Net (loss) income | $ | ( | $ | ( | $ | ||||||||||||
| Net (loss) income per share, basic: | $ | ( | $ | ( | $ | ||||||||||||
| Net (loss) income per share, diluted: | $ | ( | $ | ( | $ | ||||||||||||
| Shares used in calculating net (loss) income per share, basic | |||||||||||||||||
| Shares used in calculating net (loss) income per share, diluted | |||||||||||||||||
| Comprehensive (Loss) Income: | |||||||||||||||||
| Net (loss) income | $ | ( | $ | ( | $ | ||||||||||||
| Foreign currency translation adjustment | ( | ||||||||||||||||
| Unrealized (loss) gain on available-for-sale investments | ( | ( | |||||||||||||||
| Comprehensive (loss) income | $ | ( | $ | ( | $ | ||||||||||||
See accompanying notes to consolidated financial statements.
80
ARENA PHARMACEUTICALS, INC.
Consolidated Statements of Equity
(In thousands, except share data)
| Common Stock | Additional Paid-In Capital | Accumulated Other Comprehensive Income (Loss) | Accumulated Deficit | Total Equity | |||||||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||||||||
Balance at December 31, 2018 | $ | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||||
| Shares issued from stock plans, net of payroll taxes paid | — | — | — | ||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | — | — | |||||||||||||||||||||||||||||||
| Unrealized gain on available-for-sale investments | — | — | — | — | |||||||||||||||||||||||||||||||
| Translation loss | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Net income | — | — | — | — | |||||||||||||||||||||||||||||||
Balance at December 31, 2019 | ( | ||||||||||||||||||||||||||||||||||
| Issuance of common stock to underwriters, net | — | — | |||||||||||||||||||||||||||||||||
| Shares issued from stock plans, net of payroll taxes paid | — | — | — | ||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | — | — | |||||||||||||||||||||||||||||||
| Unrealized loss on available-for-sale investments | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Translation gain | — | — | — | — | |||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
Balance at December 31, 2020 | ( | ||||||||||||||||||||||||||||||||||
| Shares issued from stock plans, net of payroll taxes paid | — | — | — | ||||||||||||||||||||||||||||||||
| Issuance of common stock under the ATM facility, net | — | — | — | ||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | — | — | |||||||||||||||||||||||||||||||
| Unrealized loss on available-for-sale investments | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Translation gain | — | — | — | — | |||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
Balance at December 31, 2021 | $ | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||||
See accompanying notes to consolidated financial statements.
81
ARENA PHARMACEUTICALS, INC.
Consolidated Statements of Cash Flows
(In thousands)
Years ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Operating activities: | |||||||||||||||||
| Net (loss) income | $ | ( | $ | ( | $ | ||||||||||||
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | |||||||||||||||||
| Acquired in-process research and development | |||||||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Deferred income taxes | |||||||||||||||||
| Share-based compensation | |||||||||||||||||
| Gain from Longboard equity method investment | ( | ( | |||||||||||||||
Amortization of original issue discounts, net of premiums, on available- for-sale investments | ( | ||||||||||||||||
| Other operating activities, net | |||||||||||||||||
| Changes in operating assets and liabilities: | |||||||||||||||||
| Accounts receivable | |||||||||||||||||
| Prepaid expenses and other assets | ( | ( | |||||||||||||||
| Accounts payable, accrued clinical and other current liabilities | |||||||||||||||||
| Net cash (used in) provided by operating activities - continuing operations | ( | ( | |||||||||||||||
| Investing activities: | |||||||||||||||||
| Cash paid for acquired in-process research and development | ( | ||||||||||||||||
| Purchases of available-for-sale investments | ( | ( | ( | ||||||||||||||
| Proceeds from sale and maturity of available-for-sale investments | |||||||||||||||||
| Purchases of property and equipment | ( | ( | ( | ||||||||||||||
| Other non-current assets | ( | ||||||||||||||||
| Net cash provided by (used in) investing activities - continuing operations | ( | ( | |||||||||||||||
Net cash provided by investing activities - discontinued operations | |||||||||||||||||
| Net cash provided by (used in) investing activities | ( | ( | |||||||||||||||
| Financing activities: | |||||||||||||||||
| Principal payments on lease financing obligations | ( | ( | ( | ||||||||||||||
| Proceeds from issuance of common stock under ATM facility, net | |||||||||||||||||
| Proceeds from issuance of common stock in public offering, net | |||||||||||||||||
| Proceeds from issuance of common stock from stock plans, net | |||||||||||||||||
| Net cash provided by financing activities | |||||||||||||||||
| Effect of exchange rate changes on cash | ( | ||||||||||||||||
| Net increase in cash, cash equivalents and restricted cash | ( | ||||||||||||||||
| Cash, cash equivalents and restricted cash at beginning of year | |||||||||||||||||
| Cash, cash equivalents and restricted cash at end of year | $ | $ | $ | ||||||||||||||
| Supplemental disclosure of cash flow information: | |||||||||||||||||
| Interest paid | $ | $ | $ | ||||||||||||||
See accompanying notes to consolidated financial statements.
82
ARENA PHARMACEUTICALS, INC.
Notes to Consolidated Financial Statements
1. The Company and Summary of Significant Accounting Policies
The Company
Arena Pharmaceuticals, Inc., or Arena, was incorporated on April 14, 1997, and commenced operations in July 1997. The Company is a biopharmaceutical company focused on delivering novel, transformational medicines with optimized pharmacology and pharmacokinetics to patients globally. The Company’s internally developed pipeline includes multiple potentially first- or best-in-class assets with broad clinical utility.
The Company’s most advanced investigational clinical programs include: etrasimod (APD334), being evaluated in a Phase 3 program for ulcerative colitis, or UC, a Phase 2b/3 program for Crohn’s disease, or CD, a Phase 2 program in alopecia areata, or AA, and a Phase 2b program for eosinophilic esophagitis, or EoE. The Company also plans to evaluate etrasimod in a Phase 3 program in atopic dermatitis, or AD. In addition, the Company is evaluating APD418, which is being developed for the treatment of acute heart failure, or AHF, in a Phase 2 trial. A second compound in its cardiovascular therapeutic area, temanogrel, is being evaluated in a Phase 2 trial in microvascular obstruction, or MVO, and a Phase 2 trial in Raynaud’s phenomenon secondary to systemic sclerosis, or SSc-RP. The Company is also evaluating possible strategic options for olorinab, a potential treatment for a broad range of visceral pain conditions associated with gastrointestinal diseases.
The Company operates in one business segment. The Company’s principal executive offices are located in Park City, Utah, and its primary clinical operations are conducted in San Diego, California and Boston, Massachusetts; and in Zug, Switzerland by Arena Pharmaceuticals Development GmbH, or APD GmbH, the Company’s wholly-owned subsidiary.
Pending Transaction with Pfizer
On December 12, 2021, Arena entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Pfizer Inc., a Delaware corporation (“Parent”), and Antioch Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of Parent (“Merger Sub”), providing for, among other things, the merger of Merger Sub with and into Arena (the “Merger”), with Arena surviving the Merger.
At the effective time of the Merger (the “Effective Time”), each:
(i) share of common stock of the Company, par value $0.0001 per share (each, a “Share”), issued and outstanding immediately prior to the Effective Time (other than (A) Shares owned by the Company as treasury stock, (B) Shares owned by Parent or Merger Sub and (C) any dissenting shares) will no longer be outstanding and will automatically be cancelled, retired and converted into the right to receive an amount in cash equal to $100.00 , without interest thereon (the “Merger Consideration”);
(ii) option to purchase Shares (each, a “Company Option”) granted by the Company under the Company’s 2021 Long-Term Incentive Plan or prior stock plans (collectively, the “Company Stock Plans”) that is outstanding as of immediately prior to the Effective Time, whether or not then vested, will be cancelled and immediately cease to be outstanding and converted into the right to receive an amount in cash equal to the product of (1) the excess, if any, of the Merger Consideration over the per-share exercise price of such Company Option, multiplied by (2) the number of Shares then subject to such Company Option;
(iii) Company restricted stock unit, except as described in “(iv)” below, subject to vesting conditions based solely on continued employment or service to the Company or any of its subsidiaries granted by the Company under a Company Stock Plan that is unvested and outstanding as of immediately prior to the Effective Time will be cancelled and immediately cease to be outstanding and converted into the right to receive an amount in cash equal to the Merger Consideration;
(iv) Company restricted stock unit that is granted after December 12, 2021 (each, a “2022 Company RSU”) that is unvested and outstanding as of immediately prior to the Effective Time will be substituted automatically with a Parent restricted stock unit with respect to that number of shares of Parent common stock (each, an “Adjusted RSU”) that is equal to the product of (1) the total number of Shares subject to the 2022 Company RSU immediately prior to the Effective Time multiplied by (2)(a) the Merger Consideration divided by (b) the average of the volume-weighted average sales price per share of common stock of Parent on the New York Stock Exchange for the consecutive period of 15 trading days ending on
83
(and including) the trading day that is four trading days prior to the Effective Time, with any fractional shares rounded to the nearest whole share. Each Adjusted RSU will otherwise be subject to the same terms and conditions applicable to such 2022 Company RSU immediately prior to the Effective Time (including vesting terms, and subject to accelerated vesting in connection with certain qualifying terminations of employment following the Effective Time); and
(v) Company restricted stock unit granted by the Company under a Company Stock Plan that is subject to performance-based vesting conditions (each, a “Company PRSU”) that is unvested and outstanding as of immediately prior to the Effective Time will be cancelled and immediately cease to be outstanding and converted into the right to receive an amount in cash equal to the Merger Consideration (with all the performance-based vesting conditions associated with such Company PRSU being deemed achieved at the greater of actual completed performance at the Effective Time or at target for any Company PRSU).
Consummation of the Merger is subject to certain conditions, including, but not limited to, the: (i) Company’s receipt of the approval of the Company’s stockholders representing a majority of the outstanding Shares (the “Company Requisite Vote”), which was obtained on February 2, 2022; (ii) expiration or termination of any waiting periods applicable to the consummation of the Merger under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (“HSR Act”) and the receipt of certain additional clearances or approvals of certain other governmental bodies applicable to the Merger; and (iii) the absence of any law or order prohibiting or making illegal the consummation of the Merger. The Merger is targeted to close in the first half of 2022.
Other than transaction expenses associated with the Merger Agreement of $7.3 million recorded in Transaction costs in the accompanying consolidated statements of operations for the year ended December 31, 2021, the terms of the Merger Agreement did not have a material impact on the Company’s consolidated financial statements.
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with US generally accepted accounting principles, or GAAP, and reflect all of the Company’s activities, including those of its wholly owned subsidiaries. All material intercompany accounts and transactions have been eliminated in consolidation. Certain prior period amounts in the consolidated financial statements have been reclassified to conform to the current period presentation.
Liquidity
As of December 31, 2021, the Company had cash, cash equivalents and available-for-sale investments of approximately $0.7 billion. The Company believes its cash, cash equivalents and available-for-sale investments will be sufficient to fund its operations for at least the next 12 months from the date these consolidated financial statements are issued.
The Company will require substantial cash to achieve its objectives of discovering, developing and commercializing drugs, as this process typically takes many years and potentially hundreds of millions of dollars for an individual drug. The Company may not have adequate available cash, or assets that could be readily turned into cash, to meet these objectives in the long term. The Company will need to obtain significant funds under its existing collaborations and license agreements, under new collaboration, licensing or other commercial agreements for one or more of its drug candidates and programs or patent portfolios, or from other potential sources of liquidity, which may include the sale of equity, issuance of debt or other transactions.
84
Recent Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies and adopted by the Company as of the specified effective date. Unless otherwise discussed below, the Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on its consolidated financial statements upon adoption.
The following table provides a brief description of recently issued or adopted accounting standards:
| Standard | Description | Effective Date | Effect on the Financial Statements or Other Significant Matters | |||||||||||||||||
ASU 2016-13, Measurement of Credit Losses (Topic 326): Financial Statements – Credit Losses | ASU 2016-13 revised the measurement of credit losses for financial assets measured at amortized cost from an incurred loss to an expected loss methodology. | January 1, 2020 | The Company adopted ASU 2016-13 on January 1, 2020 which did not have a material impact on its consolidated financial statements. | |||||||||||||||||
ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes | ASU 2019-12 modifies ASC 740, Income Taxes to simplify the accounting for income taxes in various areas. | January 1, 2021 | The Company adopted ASU 2019-12 on January 1, 2021 which did not have a material impact on its consolidated financial statements. | |||||||||||||||||
ASU 2020-01, Investments—Equity Securities (Topic 321), Investments—Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815) | ASU 2020-01 clarifies the interactions between Topic 321 (equity securities), Topic 323 (equity method and joint ventures) and Topic 815 (derivatives and hedge accounting). The ASU addresses the accounting for the transition into and out of the equity method and measuring certain purchased options and forward contracts to acquire investments. | January 1, 2021 | The Company adopted ASU 2020-01 on January 1, 2021 which did not have a material impact on its consolidated financial statements. | |||||||||||||||||
ASU 2020-08, Codification Improvements to Subtopic 310-20, Receivables—Nonrefundable Fees and Other Cost | ASU 2020-08 clarifies an entity should, for each reporting period, reevaluate the amortization period for a premium paid on an individual callable debt security that has multiple call dates. | January 1, 2021 | The Company adopted ASU 2020-08 on January 1, 2021 which did not have a material impact on its consolidated financial statements. | |||||||||||||||||
Use of Estimates
The preparation of financial statements in conformity with GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts (including assets, liabilities, revenues and expenses) and related disclosures. The amounts reported could differ under different estimates and assumptions.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash and highly liquid investments with remaining maturities of three months or less when purchased. The following table provides a reconciliation of the components of cash, cash equivalents and restricted cash reported in the consolidated balance sheets to the total of the amount presented in the consolidated statements of cash flows, in thousands:
85
| December 31, 2021 | December 31, 2020 | ||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Restricted cash included in other non-current assets | |||||||||||
Total cash, cash equivalents and restricted cash presented in the consolidated statements of cash flows | $ | $ | |||||||||
The restricted cash relates to the Company’s property leases. The restriction will lapse when the related leases expire.
Available-for-Sale Investments
The Company defines investments as income-yielding securities that can be readily converted to cash and classifies such investments as available-for-sale. The Company carries these securities at fair value and reports unrealized gains and losses as a separate component of accumulated other comprehensive income or loss. The cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion is included in interest income. Realized gains and losses and declines in securities are included in other income or expense. The cost of securities sold is based on the specific identification method. Interest and dividends on available-for-sale securities are included in interest income.
Concentrations of Risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist primarily of cash, cash equivalents and available-for-sale investments. The Company limits its exposure to credit loss by holding cash primarily in US dollars or placing its cash and investments in US government, agency or government-sponsored enterprise obligations and in corporate debt instruments that are rated investment grade, in accordance with an investment policy approved by its Board of Directors.
The Company’s customers are typically other biopharmaceutical companies to which it licenses its intellectual property, or sells research and development services or other services under license or collaboration agreements. For the year ended December 31, 2021 and 2020, the Company recognized an immaterial amount of revenue. For the year ended December 31, 2019, more than 99 % of the Company’s annual revenue was from United Therapeutics.
The Company monitors its customers’ financial credit worthiness in order to assess and respond to any changes in their credit profile. During the years ended December 31, 2021, 2020, and 2019, the Company did not record any write-offs or reserves against accounts receivable.
Equity Method Investment
Investments and ownership interests are accounted for under equity method accounting if the Company has the ability to exercise significant influence but does not have a controlling financial interest. The Company records its interest in the net earnings of its equity method investees within other income or loss in the consolidated statements of operations. The Company records its interest in the net earnings of its equity method investments based on the most recently available financial statements of the investees.
The carrying amount of the investment in equity interests is adjusted to reflect the Company's interest in net earnings, dividends received and impairments. The Company reviews for impairment whenever factors indicate that the carrying amount of the investment might not be recoverable. In such a case, the decrease in value is recognized in the period the impairment occurs in the consolidated statements of operations.
Property and Equipment
Property and equipment are stated at cost and depreciated over the estimated useful lives of the assets (generally) 3 to 15 years using the straight-line method. Buildings are stated at cost and depreciated over an estimated useful life of approximately 20 years using the straight-line method. Leasehold improvements are stated at cost and amortized over the shorter of the estimated useful lives of the assets or the lease term using the straight-line method. Capital improvements are stated at cost and amortized over the estimated useful lives of the underlying assets using the straight-line method.
86
Long-lived Assets
If indicators of impairment exist, the Company assesses the recoverability of the affected long-lived assets by determining whether the carrying value of such assets can be recovered through undiscounted cash flows. If impairment is indicated, the Company measures the impairment loss by comparing the fair value to the carrying value of the asset.
Foreign Currency
The functional currency of the Company’s wholly owned subsidiary in Switzerland, APD GmbH, is the Swiss franc. Accordingly, all assets and liabilities of this subsidiary are translated to US dollars based on the applicable exchange rate on the balance sheet date. Revenue and expense components are translated to US dollars at weighted-average exchange rates in effect during the period. Gains and losses resulting from foreign currency translation are reported as a separate component of accumulated other comprehensive income or loss in the equity section of the Company’s consolidated balance sheets. Foreign currency transaction gains and losses are primarily the result of remeasuring US dollar-denominated receivables and payables of the Company’s foreign subsidiaries.
Share-based Compensation
The Company’s share-based awards are measured at fair value and recognized over the requisite service or performance period. The fair value of each stock option is estimated on the date of grant using the Black-Scholes option pricing model, based on the market price of the underlying common stock, expected term, expected stock price volatility and expected risk-free interest rate. Expected volatility is computed using historical volatility for a period equal to the expected term. The expected term of options is determined based on historical experience of similar awards, giving consideration to the contractual terms of the share-based awards, vesting schedules and post-vesting terminations. The risk-free interest rates are based on the US Treasury yield curve, with a remaining term approximately equal to the expected term used in the option pricing model. The Company accounts for forfeitures in the period they occur. The fair value of each restricted stock unit award is estimated based on the market price of the underlying common stock on the date of the grant. The fair value of restricted stock unit awards that include market-based performance conditions is estimated on the date of grant using a Monte Carlo simulation model, based on the market price of the underlying common stock, expected performance measurement period, expected stock price volatility and expected risk-free interest rate.
Revenue Recognition
The Company’s revenues to date have been generated primarily through collaboration and license agreements. The Company’s collaboration and license agreements frequently contain multiple types of promised goods or services including (i) intellectual property licenses, (ii) product research, development and regulatory services and (iii) product manufacturing. Consideration received under these arrangements may include upfront payments, research and development funding, cost reimbursements, milestone payments, payments for product sales and royalty payments.
Under ASC 606, revenue is recognized when a customer obtains control of promised goods or services. The amount of revenue recognized reflects the consideration that the Company expects to be entitled to receive in exchange for these services and excludes sales incentives and amounts collected on behalf of third parties. The Company analyzes the nature of these performance obligations in the context of individual collaboration and license agreements in order to assess the distinct performance obligations. The Company applies the following five steps to recognize revenue:
i)Identify the contract with a customer. The Company considers the terms and conditions of its collaboration and license agreements to identify contracts within the scope of ASC 606. The Company considers that it has a contract with a customer when the contract is approved, it can identify each party's rights regarding the goods and services to be transferred, it can identify the payment terms for the goods and services, it has determined the customer has the ability and intent to pay and the contract has commercial substance. The Company uses judgment in determining the customer's ability and intent to pay, which is based upon factors including the customer's historical payment experience or, for new customers, credit and financial information pertaining to the customers.
ii)Identify the performance obligations in the contract. Performance obligations in the Company’s collaboration and license agreements are identified based on the goods and services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the service either on its own or together with other resources that are readily available from third parties or from the Company, and are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from other promises in the contract. The Company’s performance obligations generally consist of intellectual property licenses, research, development and/or regulatory services and manufacturing and supply commitments.
87
iii)Determine the transaction price. The Company determines the transaction price based on the consideration to which it expects to be entitled in exchange for transferring goods and services to the customer. In determining the transaction price, any variable consideration would be considered, to the extent applicable, if, in its judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. In accordance with the royalty exception under ASC 606 for licenses of intellectual property, the transaction price excludes future royalty payments to be received from the Company’s customers. None of the Company’s collaboration and license agreements contain consideration payable to its customer or a significant financing component.
iv)Allocate the transaction price to performance obligations in the contract. If the contract contains a single performance obligation, the entire transaction price is allocated to that performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation based on a relative standalone selling price.
v)Recognize revenue when or as performance obligation are satisfied. Revenue is recognized at the time the related performance obligation is satisfied by transferring the promised goods or services to a customer. The Company recognizes revenue when it transfers control of the goods or services to its customers for an amount that reflects the consideration that it expects to receive in exchange for those services.
Performance Obligations
The following is a description of principal goods and services from which the Company generates revenue.
Intellectual property licenses
The Company generates revenue from licensing its intellectual property including know-how and development and commercialization rights. These licenses provide customers with a term-based license to further research, develop and commercialize its internally discovered drug candidates. The consideration the Company receives in the form of nonrefundable upfront consideration related to the functional intellectual property licenses is recognized when it transfers such license to the customer unless the license is combined with other goods or services into one performance obligation, in which case the revenue is recognized over a period of time based on the Company’s estimated pattern in which it satisfies the combined performance obligation. The Company’s licensing agreements are generally cancellable. Customers have the right to terminate their contracts upon notice. The Company has the right to terminate the contracts generally only if the customer is in breach of the contract and fails to remedy the breach in accordance with the contractual terms.
Intellectual property sales
The Company generates royalty revenue from sales of its intellectual property. The Company estimates the future royalty payments and recognizes revenue with a corresponding contract asset at a point in time when it transfers the intellectual property to the customer. The Company periodically reassesses its estimate of the future royalty payments and recognizes any estimate adjustments as revenue in the current period.
Research, development and regulatory services
The Company generates revenue from research, development and regulatory services it provides to its customers in connection with the licensed intellectual property. The services the Company provides to its customers primarily includes scientific research activities, preparation for and management of clinical trials, and assistance during the regulatory approval application process. Revenue associated with these services is recognized based on its estimate of total consideration to be received for such services and the pattern in which it performs the services. The pattern of performance is generally determined to be the amount of incurred expenses reimbursed by the customer as a percentage of total expected reimbursable expenses associated with the contract.
Contracts with Multiple Performance Obligations
Most of the Company’s collaboration and license agreements with customers contain multiple promised goods or services. Based on the characteristics of the promised goods and services the Company analyzes whether they are separate or combined performance obligations. The transaction price is allocated to the separate performance obligations on a relative standalone selling price basis. The Company determines standalone selling price based on its overall pricing and discounting objectives, taking into consideration the type of services, estimates of hourly market rates, and stage of the research, development or clinical trials.
88
Variable Consideration
The Company’s contracts with customers primarily include two types of variable consideration: (i) development and regulatory milestone payments, which are due to the Company upon achievement of specific development and regulatory milestones and (ii) one-time sales-based payments and sales-based royalties associated with sold or licensed intellectual property.
Due to uncertainty associated with achievement of the development and regulatory milestones, the related milestone payments are excluded from the contract consideration and the corresponding revenue is not recognized until the Company concludes it is probable that reversal of such milestone revenue will not occur.
Product sales-based royalties under licensed intellectual property and one-time payments are accounted for under the royalty exception. The Company recognizes revenue for sales-based royalties under licensed intellectual property and one-time payments at the later of when the sales occur or the performance obligation is satisfied or partially satisfied.
Disaggregation of Revenue
The Company operates in one reportable business segment. The Company provides goods and services to its customers in collaboration and license agreements pursuant to various geographical markets.
Cost to Obtain and Fulfill a Contract
The Company generally does not incur costs to obtain new contracts. Costs to fulfill contracts are expensed as incurred.
Remaining Performance Obligations
The Company continues to be eligible to receive consideration in the form of milestones and royalties.
Under the royalty exception in ASC 606 for licensed intellectual property, the Company does not recognize any revenue for the variable amounts related to sales-based royalties and milestones until the later of when the sales occur or the performance obligation is satisfied or partially satisfied. Accordingly, the revenue related to future sales-based royalties and milestones are excluded from the estimated revenue expected to be recognized in the future related to performance obligations that are unsatisfied.
Research and Development Expenses
Research and development expenses, which consist primarily of salaries and other personnel costs, clinical trial costs and preclinical study fees, manufacturing costs for non-commercial products, and the development of earlier-stage programs and technologies, are expensed as incurred when these expenditures have no alternative future use.
The Company accrues clinical trial expenses based on work performed. In determining the amount to accrue, the Company relies on estimates of total costs incurred based on enrollment, the completion of trials and other events. The Company follows this method because it believes reasonably dependable estimates of the costs applicable to various stages of a clinical trial can be made. However, the actual costs and timing of clinical trials are uncertain, subject to risks and may change depending on a number of factors. Differences between the actual clinical trial costs and the estimated clinical trial costs that the Company has accrued in any prior period are recognized in the subsequent period in which the actual costs become known. Historically, these differences have not been material; however, material differences could occur in the future. Payments made to reimburse collaborators for the Company’s share of their research and development activities are recorded as research and development expenses, and are recognized as the work is performed.
Comprehensive Income (Loss)
Comprehensive income or loss is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. The Company reports components of comprehensive income or loss in the period in which they are recognized. For the years ended December 31, 2021, 2020 and 2019, comprehensive income (loss) consisted of net income (loss), foreign currency translation gains and losses, and unrealized gains and losses related to available-for-sale investments.
89
(Loss) Income Per Share
The Company calculates basic and diluted loss per share using the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share is computed by dividing the net loss by the weighted average number of common shares and common stock equivalents outstanding for the period determined using the treasury stock method. For purposes of this calculation, stock options, employee stock purchase plan rights, restricted stock units, and performance-based restricted stock units are considered to be common stock equivalents but are not included in the calculations of diluted net loss per share for periods of losses as their effect would be anti-dilutive.
Since the Company reported a net loss for the years ended December 31, 2021, and 2020, in addition to excluding potentially dilutive out-of-the money securities, the Company excluded from its calculation of loss per share all potentially dilutive (i) in-the-money stock options, (ii) RSUs, (iii) PRSUs and (iv) shares to be purchased under the employee stock purchase plan. The diluted net loss per share is the same as basic net loss per share for periods a net loss was reported.
Diluted weighted average shares excluded potential common shares related to outstanding stock options, non-vested restricted stock units and shares to be purchased under the employee stock purchase plan totaling 4.2 million, 5.2 million, and 4.0 million for the years 2021, 2020, and 2019, respectively, as they were anti-dilutive.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. The Company’s deferred tax assets and liabilities are determined using the enacted tax rates expected to be in effect for the years in which those tax assets are expected to be realized.
The realization of the Company’s deferred tax assets is dependent upon its ability to generate sufficient future taxable income. The Company establishes a valuation allowance when it is more-likely-than-not the future realization of all or some of the deferred tax assets will not be achieved. The evaluation of the need for a valuation allowance is performed on a jurisdiction-by-jurisdiction basis, and includes a review of all available evidence, both positive and negative.
The impact of an uncertain income tax position is recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained.
2. Fair Value Disclosures
The Company’s investments include cash equivalents and available-for-sale investment securities consisting of money market funds, US treasury notes, and high quality, marketable debt instruments of corporations and government sponsored enterprises in accordance with the Company’s investment policy. The Company’s investment policy defines allowable investment securities and establishes guidelines relating to credit quality, diversification, and maturities of its investments to preserve principal and maintain liquidity.
The Company measures its financial assets and liabilities at fair value, which is defined as the exit price, or the amount that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
The Company uses the following three-level valuation hierarchy that maximizes the use of observable inputs and minimizes the use of unobservable inputs to value its financial assets and liabilities:
| Level 1 | –Observable inputs such as unadjusted quoted prices in active markets for identical instruments. | ||||
| Level 2 | –Quoted prices for similar instruments in active markets or inputs that are observable for the asset or liability, either directly or indirectly. | ||||
| Level 3 | –Significant unobservable inputs based on the Company’s assumptions. | ||||
90
The following tables present the Company’s valuation hierarchy for its financial assets that are measured at fair value on a recurring basis, in thousands:
Fair Value Measurements as of December 31, 2021 | |||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||
Money market funds(1) | $ | $ | $ | $ | |||||||||||||||||||
US government and government agency notes(2) | |||||||||||||||||||||||
Corporate debt securities(2) | |||||||||||||||||||||||
Commercial paper(2) | |||||||||||||||||||||||
| $ | $ | $ | $ | ||||||||||||||||||||
Fair Value Measurements as of December 31, 2020 | |||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||
Money market funds(1) | $ | $ | $ | $ | |||||||||||||||||||
US government and government agency notes(2) | |||||||||||||||||||||||
Corporate debt securities(2) | |||||||||||||||||||||||
Commercial paper(3) | |||||||||||||||||||||||
| $ | $ | $ | $ | ||||||||||||||||||||
______________________
(1)Included in cash and cash equivalents in the accompanying consolidated balance sheets.
(2)Included in available-for-sale investments in the accompanying consolidated balance sheets.
(3)Included in either cash and cash equivalents or available-for-sale investments in the accompanying consolidated balance sheets.
The Company obtains the fair value of its Level 2 financial instruments from third-party pricing services. The pricing services utilize industry standard valuation models whereby all significant inputs, including benchmark yields, reported trades, broker/dealer quotes, issuer spreads, bids, offers, or other market-related data, are observable. The Company validates the prices provided by the third-party pricing services by reviewing their pricing methods and matrices and obtaining market values from other pricing sources. The Company did not adjust or override any fair value measurements provided by these pricing services as of December 31, 2021 and 2020, respectively. The Company has not transferred any investment securities between the classification levels.
91
3. Investments, Available-for-Sale
Investments, available-for-sale, consisted of the following, in thousands:
December 31, 2021 | Maturity in years | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | |||||||||||||||||||||||||||
US government and government agency notes | Less than 1 | $ | $ | $ | ( | $ | ||||||||||||||||||||||||||
| Corporate debt securities | Less than 1 | ( | ||||||||||||||||||||||||||||||
| Commercial paper | Less than 1 | ( | ||||||||||||||||||||||||||||||
| Short-term investments, available-for-sale | $ | $ | $ | ( | $ | |||||||||||||||||||||||||||
US government and government agency notes | 1 - 5 | $ | $ | $ | ( | $ | ||||||||||||||||||||||||||
| Corporate debt securities | 1 - 5 | ( | ||||||||||||||||||||||||||||||
| Investments, available-for-sale | $ | $ | $ | ( | $ | |||||||||||||||||||||||||||
December 31, 2020 | Maturity in years | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | |||||||||||||||||||||||||||
US government and government agency notes | Less than 1 | $ | $ | $ | ( | $ | ||||||||||||||||||||||||||
| Corporate debt securities | Less than 1 | ( | ||||||||||||||||||||||||||||||
| Commercial paper | Less than 1 | ( | ||||||||||||||||||||||||||||||
| Short-term investments, available-for-sale | $ | $ | $ | ( | $ | |||||||||||||||||||||||||||
4. Balance Sheet Details
Land, property and equipment, net consisted of the following, in thousands:
December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Land | $ | $ | |||||||||
| Building and capital improvements | |||||||||||
| Leasehold improvements | |||||||||||
| Machinery and equipment | |||||||||||
| Computers and software | |||||||||||
| Furniture and office equipment | |||||||||||
| Less accumulated depreciation and amortization | ( | ( | |||||||||
| Land, property and equipment, net | $ | $ | |||||||||
As of December 31, 2021, the majority of the Company’s long-lived assets were located in the United States.
92
Accounts payable and other accrued liabilities consisted of the following, in thousands:
December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued compensation | |||||||||||
| Other accrued liabilities | |||||||||||
| Total accounts payable and other accrued liabilities | $ | $ | |||||||||
5. Leases
San Diego, California
The Company has three properties in San Diego, California, under sale and leaseback agreements. The terms of these leases contain a purchase option and stipulate annual increases in monthly lease payments of 2.5 4.1 million, $4.5 million, and $4.8 million respectively, related to these leases. The Company expects interest expense related to its facilities to total $12.6 million from December 31, 2021, through the remaining terms of the leases in fiscal year 2027. At December 31, 2021, the total financing obligation associated with these sale and leaseback agreements was $41.2 million. The aggregate residual value of the facilities at the end of the lease terms is $5.0 million.
The Company leases an additional property in San Diego, California under an operating lease, which expires in May 2027 , contains a purchase option and stipulates annual increases in monthly lease payments of 2.5 6.3 million based on the present value of the remaining minimum lease payments under the terms of its existing operating lease with a corresponding right-of-use asset of $5.9 million. As this lease did not provide an implicit rate, the Company used its estimated incremental borrowing rate based on the information available at the effective date of adoption in determining the present value of the remaining minimum lease payments. The weighted-average discount rate used was 7.25 %.
Boston, Massachusetts
In the third quarter of 2019, the Company entered into a lease agreement for approximately 12,755 square feet of office space in Boston, Massachusetts with a lease inception date of September 1, 2019 . This lease is classified as an operating lease and expires in December 2026. The lease stipulates annual increases in monthly lease payments of 2.0 %. At the lease inception date, the Company recorded an operating lease liability of $5.2 million based on the present value of the remaining minimum lease payments under the terms of this lease with a corresponding right-of-use asset of $5.2 million. As this lease did not provide an implicit rate, the Company used its estimated incremental borrowing rate based on the information available at the effective date of adoption in determining the present value of remaining minimum lease payments. The weighted-average discount rate used was 7.25 %.
Zug, Switzerland
In the second quarter of 2019, the Company entered into a lease in Zug, Switzerland, for approximately 10,500 square feet of office space with a lease inception date of June 1, 2019 . This lease expires in May 2024 . At the lease inception, the Company recorded an operating lease liability of $1.4 million based on the present value of the remaining minimum lease payments under the terms of this operating lease with a corresponding right-of-use asset of $1.5 million. As this lease did not provide an implicit rate, the Company used its estimated incremental borrowing rate based on the information available as of the lease inception in determining the present value of remaining minimum lease payments. The weighted-average discount rate used was 7.25 %. In the third quarter of 2019, the Company entered into an addendum to this lease for approximately 4,050 square feet of additional office space in the same location with the same landlord and a lease inception date of January 1, 2020 .
As of December 31, 2021, the balance of the right-of-use assets associated with the leases described above was $9.1 million and is included in in the accompanying consolidated balance sheets. As of December 31, 2021, the current portion of the corresponding lease liabilities of $1.8 million is
93
and other accrued liabilities and the non-current portion of the lease liabilities of $8.3 million is included in in the accompanying consolidated balance sheets. The operating lease costs and cash paid for the amounts included in the measurement of lease liabilities are classified as operating activities in the accompanying consolidated statements of cash flows. The Company recognizes rent expense on a straight-line basis over the term of each lease. Rent expense of $2.4 million, $2.4 million and $1.9 million was recognized for the years ended December 31, 2021, 2020, and 2019, respectively. The weighted-average remaining lease term for all operating leases as of December 31, 2021 was 4.8 years.
At December 31, 2021, the future lease payments under the Company’s existing financing and non-cancellable operating leases were as follows, in thousands:
Year ending December 31, | Financing Obligations | Operating Leases | ||||||||||||
| 2022 | $ | $ | ||||||||||||
| 2023 | ||||||||||||||
| 2024 | ||||||||||||||
| 2025 | ||||||||||||||
| 2026 | ||||||||||||||
| Thereafter | ||||||||||||||
| Total minimum lease payments | $ | |||||||||||||
| Less amounts representing interest | ( | |||||||||||||
| Add amounts representing residual value | ||||||||||||||
| Lease financing obligations | ||||||||||||||
| Less current portion | ( | |||||||||||||
| $ | ||||||||||||||
The Company has other leases primarily for office space that it enters into from time to time. These leases have terms of 12 months or less from lease commencement date and are considered short-term leases and not recorded on the consolidated balance sheets; however, the lease expenditures recognized are captured and reported as incurred.
Subleases
In 2016 and 2018, the Company entered into agreements to sublease several of its California properties. All the Company’s subleases expire in May 2027 3.0
The Company recognizes rent income on a straight-line basis over the term of the subleases.
Expected minimum rental payments to be received under the sublease are as follows, in thousands:
Year ending December 31, | ||||||||
| 2022 | $ | |||||||
| 2023 | ||||||||
| 2024 | ||||||||
| 2025 | ||||||||
| 2026 | ||||||||
| Thereafter | ||||||||
| Total | $ | |||||||
6. Equity Method Investment
94
developing novel central nervous system, or CNS, targeted assets discovered by the Company’s G-protein-coupled receptor, or GPCR, research engine. Longboard was previously a wholly owned subsidiary of Arena. As of the completion of Longboard’s Series A financing in October 2020, the Company’s ownership in Longboard comprised approximately 33.4 % of the outstanding shares of capital stock of Longboard. The Company has licensed certain development and worldwide commercialization rights to Longboard and is entitled to receive royalties on potential sales of LP352, LP143 and LP659, in the future. In October 2020, the Company also entered into a separate services agreement with Longboard, pursuant to which it agreed to perform certain research and development services, general and administrative services, management services and other mutually agreed services for Longboard and receive service fees. The Company’s investment is accounted for as an equity method investment, and the investee, Longboard, is considered a related party.
In March 2021, Longboard completed an initial public offering (“IPO”) and the Company’s ownership was diluted to 23.5 %. The Company recorded a gain of approximately $13.9 million during the three months ended March 31, 2021 as a result of the offering to account for the related ownership dilution of its equity method investment. The gain was determined based upon the Company’s proportionate share of the increase in the net assets of Longboard from the offering.
The carrying value and ownership percentage of the Company’s equity method investment is as follows, in thousands, except ownership percentages:
| December 31, 2021 | December 31, 2020 | |||||||||||||||||||||||||||||||
| Balance Sheet Location | Carrying Value | Ownership % | Carrying Value | Ownership % | ||||||||||||||||||||||||||||
| Longboard | Other non-current assets | $ | % | $ | % | |||||||||||||||||||||||||||
Equity method investment activity included in the Company’s consolidated statements of operations is as follows, in thousands:
| Income Statement Location | Year Ended December 31, 2021 | Year Ended December 31, 2020 | ||||||||||||||||||
| Equity in losses from Longboard | Other (expense) income, net | $ | ( | $ | ( | |||||||||||||||
| Gain from Longboard IPO | Gain from Longboard equity method investment | |||||||||||||||||||
| Gain from deconsolidation | Gain from Longboard equity method investment | |||||||||||||||||||
Accounts receivable due from Longboard related to the service agreement was approximately $0.3 million as of December 31, 2021 and is classified in “Prepaid expenses and other current assets” in the consolidated balance sheets.
7. Stockholders’ Equity
In February 2020, the Company entered into a sales agreement with Credit Suisse Securities (USA) LLC, SVB Leerink LLC and Cantor Fitzgerald & Co., pursuant to which it may sell and issue shares of its common stock having an aggregate offering price of up to $250.0 million from time to time in transactions that are deemed to be “at-the-market offering” as defined in Rule 415(a)(4) under the Securities Act of 1933, as amended, or Securities Act.
During the first quarter of 2021, the Company sold 1.2 million shares of common stock under the sales agreement at a weighted average price of $81.06 per share and realized gross proceeds of $100.6 million.
In June 2020, the Company completed the sale of an aggregate of 6,325,000 shares of common stock in an underwritten public offering. Net proceeds from the offering were approximately $301.8 million after deducting underwriting discounts and commissions and offering expenses payable by the Company.
Equity Compensation Plans
In June 2021, the Company’s stockholders approved the 2021 Long-Term Incentive Plan, or 2021 LTIP. Upon such approval, the Company’s Amended and Restated 2020 Long-Term Incentive Plan, or 2020 LTIP, was terminated. Notwithstanding such termination or the previous termination of its 2017 Long-Term Incentive Plan, 2013 Long-Term Incentive Plan, 2012 Long-Term Incentive Plan, 2009 Long-Term Incentive Plan, and 2006 Long-Term Incentive Plan, as amended, or, together with the 2020 LTIP, the Prior Plans, all outstanding awards under the Prior Plans continue to be governed under the terms of the Prior Plans. Since the Company’s stockholders approved the 2021 LTIP, the Compensation Committee of the Company’s Board of Directors has from time to time amended the 2021 LTIP to incorporate into the 2021 LTIP inducement equity awards granted to new employees of the Company or its subsidiary APD GmbH. The number of shares of common stock authorized for issuance under the 2021 LTIP may be increased by the
95
number of shares subject to any stock awards under the Prior Plans that are forfeited, expire or otherwise terminate without the issuance of such shares and would otherwise be returned to the share reserve under the Prior Plans but for their termination and as otherwise provided in the 2021 LTIP.
The aggregate number of shares of the Company’s common stock that initially could be issued pursuant to stock awards granted under the 2021 LTIP is 1,466,561 shares. Shares issued after the effective date of the 2021 LTIP pursuant to awards granted under the 2021 LTIP or any of the Company’s Prior Plans reduce the number of shares available for issuance under the 2021 LTIP by 1 share for every share issued.
Shares under the 2021 LTIP may be granted as incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards and performance awards. Performance awards may be based on the achievement of operational, financial, research and development, collaboration and license arrangements and other performance metrics provided under the 2021 LTIP, such as total stockholder return, revenue, research, development and regulatory achievements and strategic and operational initiatives.
A total of 12,862,657 shares of the Company’s common stock were reserved for future issuance at December 31, 2021, pursuant to the 2021 LTIP and the Company’s Prior Plans, or collectively, its Equity Compensation Plans.
Share-Based Compensation Expense
The Company recognized share-based compensation expense by function as follows, in thousands:
| Years Ended December 31, | ||||||||||||||||||||
| 2021 | 2020 | 2019 | ||||||||||||||||||
| Selling, general and administrative | $ | $ | $ | |||||||||||||||||
| Research and development | ||||||||||||||||||||
| Total share-based compensation expense | $ | $ | $ | |||||||||||||||||
The Company recognized share-based compensation expense by grant type as follows, in thousands:
| Years Ended December 31, | ||||||||||||||||||||
| 2021 | 2020 | 2019 | ||||||||||||||||||
| Stock options | $ | $ | $ | |||||||||||||||||
| Performance-based restricted stock units | ||||||||||||||||||||
| Restricted stock units | ||||||||||||||||||||
| Employee stock purchase plan | ||||||||||||||||||||
| Total share-based compensation expense | $ | $ | $ | |||||||||||||||||
Stock Options
The fair value of each option issued to employees was estimated on the date of grant using the Black-Scholes option pricing model with the following weighted-average assumptions:
| Years Ended December 31, | ||||||||||||||||||||
| 2021 | 2020 | 2019 | ||||||||||||||||||
| Expected volatility | % | % | % | |||||||||||||||||
| Expected term (in years) | ||||||||||||||||||||
| Risk-free interest rate | % | % | % | |||||||||||||||||
| Expected dividend yield | % | % | % | |||||||||||||||||
96
The following table summarizes the stock option activity under the Company’s stock option plans during the year ended December 31, 2021 (in thousands, except per share amounts and years):
| Options | Weighted- Average Exercise Price | Weighted- Average Contractual Life (in years) | Intrinsic Value(1) | ||||||||||||||||||||
| Outstanding at January 1, 2021 | $ | ||||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | ( | ||||||||||||||||||||||
| Forfeited/cancelled/expired | ( | ||||||||||||||||||||||
| Outstanding at December 31, 2021 | $ | $ | |||||||||||||||||||||
| Exercisable at December 31, 2021 | $ | $ | |||||||||||||||||||||
(1)The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying options and the estimated fair value of the common stock for the options that were in the money at December 31, 2021.
The aggregate intrinsic value of options exercised during the year ended December 31, 2021 was $55.6 million.
As of December 31, 2021, there was approximately $81.1 million of unrecognized compensation expense related to unvested stock options that is expected to be recognized over a weighted-average period of 2.5 years.
Restricted Stock Units
The recipient of a restricted stock award has all rights of a stockholder at the date of grant, subject to certain restrictions on transferability and a risk of forfeiture. Restricted stock unit awards generally vest over or four years from the date of grant. Neither the exercise price of an option nor the grant price of a stock appreciation right may be less than 100 % of the fair market value of the common stock on the date such equity award is granted, except in specified situations. The 2021 LTIP prohibits option and stock appreciation right repricings (other than to reflect stock splits, spin-offs or certain other corporate events) without stockholder approval.
Restricted stock awards, restricted stock unit awards and performance awards are share awards that, upon vesting, will deliver to the holder shares of the Company’s common stock. The following table summarizes the Company’s RSU activity during the year ended December 31, 2021, in thousands (except grant date fair value data):
| Number of Shares | Weighted- Average Grant Date Fair Value | ||||||||||
Non-vested at December 31, 2020 | $ | ||||||||||
| Granted | |||||||||||
| Released | ( | ||||||||||
| Forfeited/cancelled | ( | ||||||||||
Non-vested at December 31, 2021 | $ | ||||||||||
As of December 31, 2021, there was approximately $41.5 million of unrecognized compensation expense related to unvested RSUs that is expected to be recognized over a remaining weighted-average period of 3.1 years.
Performance-Based Restricted Stock Units
In 2021, a total of 216,772 target Performance-Based Restricted Stock Units, or PRSUs, were granted to employees in a company-wide grant. The PRSUs vest upon the closing price of the Company’s common stock, or the Closing Price, reaching certain price thresholds during the three-year performance period ending February 2024, or the Performance Period, and the participant’s subsequent satisfaction of a continuing service requirement of generally 90 calendar days. If, on five consecutive trading days or ten non-consecutive trading days during the Performance Period, the Closing Price equals or exceeds $120.00 , $130.00 or $145.00 , and the participant thereafter satisfies the continuing service requirement, then the PRSUs are deemed vested at 50 %, 100 % or 200 %, respectively, of the participant’s respective target
97
PRSU amount. The shares may be issued following achievement of each price threshold, and the maximum number of common shares that may be issued pursuant to each PRSU grant equals 200 % of the target number of PRSUs granted. As these awards contain a market condition, the Company used a Monte Carlo simulation model to estimate the grant-date fair value, which totaled $22.0 million. The grant-date fair value is recognized as compensation expense over the requisite service period of approximately 1.2 years which was derived from the Monte Carlo simulation; no compensation expense is recognized for service not provided upon separation from the Company. There is no adjustment of compensation expense recognized for service performed regardless of the number of PRSUs, if any, that ultimately vest.
Performance awards are share awards that, upon vesting, will deliver to the holder shares of the Company’s common stock. The following table summarizes the Company’s PRSU activity during the year ended December 31, 2021, in thousands (except grant date fair value data):
| Number of Shares | Weighted- Average Grant Date Fair Value | ||||||||||
| Non-vested at January 1, 2021 | $ | ||||||||||
Granted(1) | |||||||||||
| Released | ( | ||||||||||
| Forfeited/cancelled | ( | ||||||||||
| Non-vested at December 31, 2021 | $ | ||||||||||
(1)Pursuant to the terms of the awards granted, the actual number of awards earned could range between 0 % and 200 % of the above number of awards granted. The amount disclosed represents PRSU grants at maximum payout.
As of December 31, 2021, there was approximately $5.8 million of unrecognized compensation expense related to unvested PRSUs.
Employee Stock Purchase Plan
In June 2019, the Company’s stockholders approved the 2019 Employee Stock Purchase Plan, or 2019 ESPP. Under the 2019 ESPP, substantially all employees can elect to have up to 15 % of their annual compensation withheld to purchase up to 2,000 shares of common stock per purchase period, subject to certain limitations. The shares of common stock can be purchased over an offering period with a maximum duration of 12 months and at a price of not less than 85 % of the lesser of the fair market value of the common stock on (i) the first trading day of the applicable offering period or (ii) the last trading day of the applicable six-month purchase period. Under applicable accounting guidance, the 2019 ESPP is considered a compensatory plan.
During the year ended December 31, 2021 and 2020, a total of 52,180 and 64,456 shares, respectively, were purchased by the Company’s employees under the 2019 ESPP. There were no ESPP purchases in 2019.
8. Collaborations and License Agreements
98
In the following table, revenue is disaggregated by major customers and timing of revenue recognition, in thousands:
| Year Ended December 31, | |||||||||||
| Customers | 2021 | 2020 | |||||||||
| Eisai | $ | $ | |||||||||
| Other | |||||||||||
| Total | $ | $ | |||||||||
| Year Ended December 31, | |||||||||||
| Timing of revenue recognition | 2021 | 2020 | |||||||||
| Revenue recognized over time | $ | $ | |||||||||
| Revenue recognized at a point in time | |||||||||||
| Total | $ | $ | |||||||||
Aristea Therapeutics, Inc.
In July 2021, the Company entered into a strategic collaboration and option agreement to advance the clinical development of RIST4721, an oral CXCR2 antagonist being developed by Aristea Therapeutics, Inc. (“Aristea”) for the treatment of palmoplantar pustulosis (“PPP”) and other neutrophil-mediated diseases.
Under the terms of the agreement, the Company paid $60.0 million upfront to Aristea and invested $10.0 million in Aristea’s Series B preferred stock, both of which were paid in July 2021. In return, Aristea granted the Company an exclusive option to acquire Aristea, including rights to all CXCR2 programs, upon completion of the Phase 2b study of RIST4721 in PPP. The agreement also provided a framework during the option period for the companies to jointly explore the development of additional neutrophil-mediated diseases, including hidradenitis suppurativa and inflammatory bowel disease. The Company accounted for the transaction as an asset acquisition and expensed the total $70.0 million payment to Aristea as acquired in-process research and development in the consolidated statement of operations during the year ended December 31, 2021, because: i) the Company is not the primary beneficiary and does not have a controlling financial interest in Aristea, ii) Aristea does not meet the definition of a business from an accounting perspective, and iii) the asset has no alternative future use.
United Therapeutics Corporation
The Company received an upfront payment of $800.0 million under the agreement in the first quarter of 2019. The Company is also eligible to receive up to an aggregate of $400.0 million in regulatory milestone payments related to ralinepag, consisting of a payment of $150.0 million upon first marketing approval of an oral formulation of ralinepag in a major non-US market, and a payment of $250.0 million upon US marketing approval of an inhaled formulation of ralinepag to treat pulmonary arterial hypertension, as well as low double-digit, tiered royalties on net sales of ralinepag products, subject to certain adjustments for third party license payments.
The promised goods and services under this agreement are accounted for as a single performance obligation consisting of a research, development and commercialization license. The Company’s performance obligation under this agreement was satisfied upon the closing of the transaction in January 2019, and accordingly, the estimated total transaction price of the agreement of $800.0 million was recognized as revenue at the commencement of this agreement in 2020. The future potential milestone payments were excluded from the estimated total transaction price as they are considered constrained. Under the royalty exception in ASC 606 for licensed intellectual property, the Company does not include any variable amounts related to sales-based royalties in the transaction price until the later of when the sales occur or the performance obligation is satisfied or partially satisfied.
99
Everest
In December 2018, the Company and Everest entered into an exclusive agreement, or the Everest Agreement, to conduct joint development for the ralinepag and etrasimod programs. Under the Everest Agreement, the Company granted Everest an exclusive, royalty-bearing license to develop and commercialize ralinepag (in any formulation) and etrasimod (in oral formulations), in mainland China, Taiwan, Hong Kong, Macau and South Korea, or collectively, the Territories. Everest is generally responsible for development and commercialization of the licensed products in the Territories and may participate in the portion of the Company’s global clinical trials that is conducted in the Territories. In January 2019, the Company and Everest amended the Everest Agreement by entering into two separate agreements, one for each development program with the terms identical to the original Everest Agreement. Under the agreement with United Therapeutics described above, the Company assigned all its rights and obligations with respect to the ralinepag program under the Everest Agreement, to United Therapeutics.
The Company is also eligible to receive up to an aggregate of $110.0 million in success milestones in case of full commercial success of etrasimod products. The Company is also eligible to receive tiered royalties on net sales of etrasimod products in the Territories.
The promised goods and services under the Everest Agreement are accounted for as a single performance obligation consisting of a development and commercialization license. As of December 31, 2021, all remaining future potential milestone payments were excluded from the estimated total transaction price as they are considered constrained.
For the year ended December 31, 2019, the Company recognized revenues of $5.0 million from the Everest Agreement. The Company did not
Eisai
In December 2016, the Company amended and restated the terms of the marketing and supply agreement for lorcaserin with Eisai by entering into a Transaction Agreement and a Supply Agreement (collectively, the Eisai Agreement). Under the Transaction Agreement, Eisai acquired an exclusive royalty-bearing license or transfer of intellectual property to global commercialization and manufacturing rights to lorcaserin, including in the territories retained by the Company under the prior agreement, with control over global development and commercialization decisions. Eisai is responsible for all lorcaserin development expenses in the future. The Company also assigned to Eisai its rights under the commercial lorcaserin distribution agreements with Ildong Pharmaceutical Co., Ltd., or Ildong, for South Korea; CY Biotech Company Limited, or CYB, for Taiwan; and Teva Pharmaceuticals Ltd.’s Israeli subsidiary, Abic Marketing Limited, or Teva, for Israel.
Until March 31, 2018, when the Company sold the Manufacturing Operations, including the assignment of the Supply Agreement, to Siegfried, it manufactured lorcaserin at its manufacturing facility in Zofingen, Switzerland.
Lorcaserin was approved for marketing in the US, and in certain other territories, for the indication of weight management. On February 13, 2020, the FDA issued a drug safety communication announcing that it requested Eisai voluntarily withdraw lorcaserin from the US market based on the FDA’s analysis of data from the Cardiovascular and Metabolic Effects of Lorcaserin in Overweight and Obese Patients – Thrombolysis in Myocardial Infarction 61 (CAMELLIA-TIMI 61) study, and that Eisai has submitted a request to voluntarily withdraw lorcaserin from the US market. On September 30, 2020, Eisai announced that, after consulting with the FDA, it is continuing the lorcaserin expanded access program and has initiated a Phase 3 clinical study of lorcaserin in patients with Dravet syndrome, a severe type of epilepsy characterized by prolonged seizures that begin in the first year of life.
In October 2020, the Company entered into a Royalty Purchase Agreement with Longboard pursuant to which Longboard purchased from the Company the right to receive all milestone payments, royalties, interest and other payments relating to net sales of lorcaserin owed or otherwise payable by Eisai.
The Company believes that Eisai is solely responsible for any expenses and losses associated with product liability claims, except the Company and Eisai share 50 % of losses for any alleged defective manufacturing of lorcaserin that was manufactured by us prior to entering into the Transaction Agreement.
100
For the years ended December 31, 2020 and 2019, the Company recorded royalty revenues of $0.3 million and $(1.1 ) million, respectively related to the Transaction Agreement.
Boehringer Ingelheim International GmbH
In December 2015, the Company and Boehringer Ingelheim entered into a collaboration and license agreement, or Boehringer Ingelheim Agreement, under which the Company and Boehringer Ingelheim conduct joint research to identify drug candidates targeting an undisclosed G-protein-coupled receptor, or GPCR, that belongs to the group of orphan central nervous system receptors. Under the Boehringer Ingelheim Agreement, the Company granted Boehringer Ingelheim exclusive rights to its internally discovered, novel compounds and intellectual property for an orphan CNS receptor. The agreement grants Boehringer Ingelheim exclusive worldwide rights to develop, manufacture and commercialize products resulting from the collaboration.
In December 2018 and October 2019, the Company earned a milestone payment of $3.5 million and $1.5 million, respectively, upon Boehringer Ingelheim’s initiation of preclinical development of a first and an additional compound.
The Company is also eligible to receive up to an aggregate of $246.0 million (of which the first $7.0 million is also payable to Beacon) in additional success milestone payments in case of full commercial success of multiple drug products.
The promised goods and services under the Boehringer Ingelheim Agreement are accounted for as a single combined performance obligation consisting of a research license, a development and commercialization license and research services. The Company’s research services performance obligation under the original term of the Boehringer Ingelheim Agreement was completely satisfied as of January 2018, and accordingly the estimated total transaction price of the Boehringer Ingelheim Agreement under the original contractual term was fully recognized as revenue over the period from January 2016 through January 2018. The Company recognizes revenue for the combined performance obligation based on the amount of incurred development expenses reimbursed by the customer as a percentage of total expected reimbursable expenses associated with the contract. As of December 31, 2021, all future potential milestone payments were excluded from the estimated total transaction price as they are considered constrained.
For the years ended December 31, 2019, the Company recognized revenue of $1.7 million from the Boehringer Ingelheim Agreement. The Company did no
Beacon Discovery, Inc.
In September 2016, the Company entered into a series of agreements with Beacon, a privately held drug discovery incubator which focuses on identifying and advancing molecules targeting GPCRs. Beacon was founded in 2016 by several of the Company’s former employees.
The Company entered into an agreement, or License and Collaboration Agreement, with Beacon, pursuant to which the Company transferred certain equipment to Beacon and granted Beacon a non-exclusive, non-assignable and non-sublicensable license to certain database information relating to compounds, receptors and pharmacology, and transferred certain equipment to Beacon. Beacon will seek to engage global partners to facilitate discovery and development. Beacon has agreed to assign to the Company any intellectual property relating to its existing research and development programs developed in the course of performing research for the Company and grant the Company a non-exclusive license to any intellectual property developed outside the course of performing work for it that is reasonably necessary or useful for developing or commercializing the products under the Company’s research and development programs. The Company is also entitled to rights of negotiation and rights of first refusal to potentially obtain licenses to compounds discovered and developed by Beacon. In addition, the Company is entitled to receive (i) a percentage of any revenue received by Beacon on or after the second anniversary of the effective date of the agreement from any third party pursuant to a third-party license, including upfront payments, milestone payments and royalties; (ii) single-digit royalties on the aggregate net sales of any related products sold by Beacon and its affiliates; and (iii) in the event that Beacon is sold, a percentage of the consideration for such sale transaction.
The Company entered a services agreement with Beacon, or Master Services Agreement, pursuant to which Beacon performs certain research services for it.
101
The Company also entered into a separate services agreement with Beacon, or Beacon Services Agreement, pursuant to which Beacon performed its research obligations under the Company’s agreement with Boehringer Ingelheim. In consideration for performing these research obligations, Beacon is entitled to receive the applicable FTE payments that are paid to the Company by Boehringer Ingelheim for the research services and certain milestone payments.
The Company also entered into a sublease agreement, or Sublease, with Beacon, pursuant to which it subleased approximately 30,000 square feet of laboratory, office and meeting room space to Beacon until May 2027.
In 2020, the Company entered into a new multi-year strategic Collaboration and License Agreement with Beacon, aimed at building novel medicines across a range of GPCR targets believed to play a role in immune and inflammatory diseases. Under the terms of this agreement Beacon is responsible for early drug discovery activities and the Company will be responsible for any potential future development and, ultimately, commercialization activities. The Company is required to pay to Beacon research initiation fees, make quarterly research funding payments for the duration of Beacon’s research activities as well as research, development and regulatory milestone payments depending on the future research and development progress. The Company is also obligated to pay Beacon tiered royalties on net sales of low single digits levels.
In the first quarter of 2021, the Company received a $1.1 million payment as a result of the merger (“Merger”) between Eurofins Beacon Discovery Holdings, Inc. (“Eurofins”) and Beacon. This payment satisfied Beacon’s obligation to pay the Company a percentage of the consideration for such sale transaction in the event that Beacon was sold as outlined in the 2016 License and Collaboration Agreement. The Company is eligible to receive future contingent consideration payments based on certain performance metrics achieved by Beacon over a four-year performance period through the first quarter of 2025 up to an aggregate of $2.0 million.
Following the Merger, the Company entered into a Consent and Release Agreement that terminated the Company’s rights of negotiation and rights of first refusal to potentially obtain licenses to certain compounds discovered and developed by Beacon. In addition, the Consent and Release Agreement terminated the Company’s right, under the 2016 License and Collaboration Agreement, to receive any revenue received by Beacon including upfront payments, milestone payments and royalties. The 2020 Collaboration and License Agreement with Beacon remains in effect and was not impacted by the Merger.
Outpost Medicine LLC
In April 2018, the Company and Outpost Medicine entered into a license agreement, or Outpost Agreement, under which Outpost Medicine has an exclusive right to advance LP352 for the potential treatment of genitourinary disorders.
For the year ended December 31, 2019, the Company recognized revenues of $0.5 million from the Outpost Agreement. During the year ended December 31, 2020, the Outpost Agreement was terminated.
Axovant Sciences GmbH
In 2015, the Company entered into a development, marketing and supply agreement with Roivant Sciences Ltd., which subsequently assigned the exclusive rights to develop and commercialize nelotanserin to its subsidiary, Axovant. Under this agreement, Axovant had exclusive worldwide rights to develop and commercialize nelotanserin, subject to regulatory approval. The Company also provided certain services and manufactured and sold nelotanserin to Axovant. The Company refers to this agreement as the Axovant Agreement.
In the fourth quarter of 2019, the Axovant Agreement was terminated.
102
9. Income Taxes
The following table summarizes the Company’s (loss) income before income taxes by region for the years presented, in thousands:
Years ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| United States | $ | ( | $ | ( | $ | ||||||||||||
| Foreign | ( | ( | |||||||||||||||
| Total (loss) income before income taxes | $ | ( | $ | ( | $ | ||||||||||||
The Company did not
The Company’s effective income tax rate differs from the statutory federal rate of 21
Years ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| (Benefit) Provision for income taxes at statutory federal rate | $ | ( | $ | ( | $ | ||||||||||||
| Change in federal and foreign valuation allowance | |||||||||||||||||
| Deferred adjustment related to foreign net operating losses | |||||||||||||||||
| Capital loss on foreign subsidiary liquidation | ( | ||||||||||||||||
| State Tax, net of federal benefit | ( | ( | |||||||||||||||
| Share-based compensation expense | ( | ( | |||||||||||||||
| Foreign losses at lower effective rates | |||||||||||||||||
| Research and development and Orphan Drug credits | ( | ( | ( | ||||||||||||||
| Permanent differences and other | |||||||||||||||||
| Provision for income taxes | $ | $ | $ | ||||||||||||||
The components of the Company’s net deferred tax assets are as follows, in thousands:
103
December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Deferred tax assets: | |||||||||||
| Federal and California NOL carryforwards | $ | $ | |||||||||
| Federal and California research and development credit carryforwards | |||||||||||
| Foreign NOL carryforwards | |||||||||||
| Share-based compensation expense | |||||||||||
| Depreciation | |||||||||||
| Lease liability | |||||||||||
| Other, net | |||||||||||
| Total deferred tax assets | |||||||||||
| Deferred tax liabilities: | |||||||||||
| Right-of-use assets | ( | ( | |||||||||
| Equity investment | ( | ( | |||||||||
| Total deferred tax liabilities | ( | ( | |||||||||
| Net deferred tax assets | |||||||||||
| Valuation allowance | ( | ( | |||||||||
| Net deferred tax assets | $ | — | $ | — | |||||||
A valuation allowance is recorded against all of the Company’s deferred tax assets, as realization of any of these assets does not meet the more-likely-than-not criteria for recognition. The realization of the Company’s deferred tax assets is dependent upon future taxable income. The Company’s ability to generate taxable income is analyzed regularly on a jurisdiction-by-jurisdiction basis. At such time as it is more-likely-than-not that the Company will generate taxable income in a jurisdiction, it will further reduce or remove the valuation allowance. The valuation allowance increased by $173.1 million from December 31, 2020, to December 31, 2021.
At December 31, 2021, the Company had federal NOL carryforwards of $1,437.6 million that will begin to expire in 2028 unless previously utilized. At the same date, the Company had California NOL carryforwards of $951.2 million, which begin expiring in 2028 1,047.2 million of net operating losses with no expiration. Federal net operating losses generated after December 31, 2017 are also subject to an 80 % limitation if utilized after 2020.
At December 31, 2021, the Company had federal and California research and development tax credit carryforwards, net of reserves, of $58.9 million and $29.5 million, respectively. At December 31, 2021, the Company had a Federal Orphan Drug Credit carryforward, net of reserves, of $15.0 million. Federal credit carryforwards will begin to expire after 2026 unless previously utilized. The California research and development credit carries forward indefinitely.
Additionally, utilization of net operating loss and R&D tax credit carryforwards to offset future taxable income may be subject to an annual limitation, pursuant to Sections 382 and 383 of the IRC. The Company has completed an analysis under Sections 382 and 383 of the IRC through 2021 and no ownership changes limiting its utilization of tax attribute carryforwards has occurred since 2010. Future ownership changes may occur that limit the Company's ability to utilize its tax attribute carryforwards.
In accordance with authoritative guidance, the impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained.
The following table reconciles the beginning and ending amount of unrecognized tax benefits for the years presented, in thousands:
104
Years ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Gross unrecognized tax benefits at the beginning of the year | $ | $ | $ | ||||||||||||||
| Additions from tax positions taken in the current year | |||||||||||||||||
| Additions from tax positions taken in prior years | |||||||||||||||||
| Reductions from tax positions taken in prior years | ( | ||||||||||||||||
| Gross unrecognized tax benefits at end of the year | $ | $ | $ | ||||||||||||||
Of the Company’s total unrecognized tax benefits at December 31, 2021, $12.8 million will impact its effective tax rate in the event the valuation allowance is removed. The Company does not anticipate that there will be a substantial change in unrecognized tax benefits within the next 12 months.
The Company’s practice is to recognize interest and/or penalties related to income tax matters in income tax expense. Because the Company has incurred net losses since its inception, it did not not
The Company is subject to income taxation in the United States at the Federal and state levels. Tax years beginning in 2003 are subject to examination by US and California tax authorities due to the carryforward of unutilized NOLs and tax credits. The Company is also subject to foreign income taxes in the countries in which it operates. To the Company’s knowledge, it is not currently under examination by any taxing authorities.
10. Legal Proceedings
105
11. Subsequent Event
Item 9 Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our periodic and current reports that we file with the SEC is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
As of December 31, 2021, we conducted an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of the end of the period covered by this Annual Report on Form 10-K.
Management’s Report on Internal Control Over Financial Reporting
Our management is also responsible for establishing and maintaining for us adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the Internal Control—Integrated Framework (2013 framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under this framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2021.
The registered public accounting firm that audited our financial statements as of and for the year ended December 31, 2021, included in this Annual Report on Form 10-K, has issued an attestation report on our internal control over financial reporting, and such report is included below.
Changes in Internal Control Over Financial Reporting
An evaluation was also performed under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of any changes in our internal control over financial
106
reporting that occurred during our last fiscal quarter and that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. That evaluation did not identify any change in our internal control over financial reporting during the fourth quarter of the year ended December 31, 2021 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
107
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Arena Pharmaceuticals, Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited Arena Pharmaceuticals, Inc. and subsidiaries' (the Company) internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2021 and 2020, the related consolidated statements of operations and comprehensive (loss) income, equity, and cash flows for each of the years in the three-year period ended December 31, 2021, and the related notes (collectively, the consolidated financial statements), and our report dated February 23, 2022 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the US federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| /s/ KPMG LLP | |||||
| San Diego, California | |||||
February 23, 2022 | |||||
108
Item 9B. Other Information.
Not applicable.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
109
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
We have adopted a Code of Business Conduct and Ethics that applies to our directors and employees (including our principal executive officer, principal financial officer, principal accounting officer and controller), and have posted the text of the policy on our website (www.arenapharm.com) in connection with “Investor” materials. In addition, we intend to promptly disclose on our website in the future (i) the date and nature of any amendment (other than technical, administrative or other non-substantive amendments) to the policy that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and relates to any element of the code of ethics definition enumerated in Item 406(b) of Regulation S-K, and (ii) the nature of any waiver, including an implicit waiver, from a provision of the policy that is granted to one of these specified individuals that relates to one or more of the elements of the code of ethics definition enumerated in Item 406(b) of Regulation S-K, the name of such person who is granted the waiver and the date of the waiver.
The other information required by this item will be included under the captions “Election of Directors,” “Compensation and Other Information Concerning Executive Officers, Directors and Certain Stockholders” and “Delinquent Section 16(a) Reports” in our definitive proxy statement for the annual meeting of stockholders to be held in June 2022 to be filed with the SEC on or before May 2, 2022, or the Proxy Statement, and is incorporated herein by reference.
Item 11. Executive Compensation.
The information required by this item will be included under the captions “Compensation and Other Information Concerning Executive Officers, Directors and Certain Stockholders” and “Compensation Committee Interlocks and Insider Participation” in the Proxy Statement and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item will be included under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Securities Authorized for Issuance Under Equity Compensation Plans” in the Proxy Statement and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item will be included under the captions “Certain Relationships and Related Transactions” and “Election of Directors” in the Proxy Statement and is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services.
Our independent registered public accounting firm is KPMG LLP, San Diego, CA, Auditor Firm ID: 185 .
The information required by this item will be included under the captions “Independent Auditors’ Fees” and “Pre-approval Policies and Procedures” in the Proxy Statement and is incorporated herein by reference.
110
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a)1. FINANCIAL STATEMENTS
Reference is made to the Index to Financial Statements under Item 8, Part II hereof.
2. FINANCIAL STATEMENT SCHEDULES
The financial statement schedules have been omitted either because they are not required or because the information has been included in the consolidated financial statements or the notes thereto included in this annual report.
3. EXHIBITS
Exhibit No. | Exhibit Description | |||||||
| 2.1* | ||||||||
| 2.2+* | ||||||||
| 2.3* | ||||||||
| 3.1 | ||||||||
| 3.2 | ||||||||
| 3.3 | ||||||||
| 3.4 | ||||||||
| 3.5 | ||||||||
111
Exhibit No. | Exhibit Description | |||||||
| 3.6 | ||||||||
| 3.7 | ||||||||
| 4.1 | ||||||||
| 4.2 | ||||||||
| 4.3 | ||||||||
| 10.1 | ||||||||
| 10.2 | ||||||||
| 10.3 | ||||||||
| 10.4 | ||||||||
| 10.5 | ||||||||
| 10.6+ | ||||||||
| 10.7 | ||||||||
112
Exhibit No. | Exhibit Description | |||||||
| 10.8 | ||||||||
| 10.9+ | ||||||||
| 10.10 | ||||||||
| 10.11 | ||||||||
| 10.12** | ||||||||
| 10.13** | ||||||||
| 10.14** | ||||||||
| 10.15** | ||||||||
| 10.16** | ||||||||
| 10.17** | ||||||||
| 10.18** | ||||||||
| 10.19** | ||||||||
113
Exhibit No. | Exhibit Description | |||||||
| 10.20** | ||||||||
| 10.21** | ||||||||
| 10.22** | ||||||||
| 10.23** | ||||||||
| 10.24** | ||||||||
| 10.25** | ||||||||
| 10.26** | ||||||||
| 10.27** | ||||||||
| 10.28** | ||||||||
| 10.29** | ||||||||
| 10.30** | ||||||||
114
Exhibit No. | Exhibit Description | |||||||
| 10.31** | ||||||||
| 10.32** | ||||||||
| 10.33** | ||||||||
| 10.34** | ||||||||
| 10.35** | ||||||||
| 10.36** | ||||||||
| 10.37** | ||||||||
| 10.38** | ||||||||
| 10.39** | ||||||||
| 10.40** | ||||||||
| 10.41** | ||||||||
115
116
117
Exhibit No. | Exhibit Description | |||||||
| 32.1 | ||||||||
| 101.INS | Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | |||||||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document | |||||||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | |||||||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | |||||||
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | |||||||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | |||||||
| 104 | Cover Page Interactive Data File (formatted as inline XBRL with applicable taxonomy extension information contained in Exhibit 101.INS) | |||||||
+ Confidential treatment has been requested or granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
* Exhibits and schedules to this agreement have been omitted pursuant to the rules of the Securities and Exchange Commission. We will submit copies of such exhibits and schedules to the Securities and Exchange Commission upon request.
** Management contract or compensatory plan or arrangement.
(b)EXHIBITS
See Item 15(a)(3) above.
(c)FINANCIAL STATEMENT SCHEDULES
See Item 15(a)(2) above.
Item 16. Form 10-K Summary.
None.
118
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ARENA PHARMACEUTICALS, INC. | ||||||||
Date: February 23, 2022 | By: | / S / AMIT D. MUNSHI | ||||||
Amit D. Munshi President and Chief Executive Officer (principal executive office) | ||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signatures | Title | Date | ||||||||||||||||||
| By: | / S / AMIT D. MUNSHI | President and Chief Executive Officer and Director (principal executive officer) | February 23, 2022 | |||||||||||||||||
| Amit D. Munshi | ||||||||||||||||||||
| By: | / S / LAURIE STELZER | Executive Vice President and Chief Financial Officer (principal financial and accounting officer) | February 23, 2022 | |||||||||||||||||
| Laurie Stelzer | ||||||||||||||||||||
| By: | / S / GARRY A. NEIL | Chair of the Board | February 23, 2022 | |||||||||||||||||
| Garry A. Neil, M.D. | ||||||||||||||||||||
| By: | / S / JAYSON DALLAS | Director | February 23, 2022 | |||||||||||||||||
| Jayson Dallas, M.D. | ||||||||||||||||||||
| By: | / S / OLIVER FETZER | Director | February 23, 2022 | |||||||||||||||||
| Oliver Fetzer, Ph.D. | ||||||||||||||||||||
| By: | / S / KIERAN T. GALLAHUE | Director | February 23, 2022 | |||||||||||||||||
| Kieran T. Gallahue | ||||||||||||||||||||
| By: | / S / JENNIFER JARRETT | Director | February 23, 2022 | |||||||||||||||||
| Jennifer Jarrett | ||||||||||||||||||||
| By: | / S / KATHARINE KNOBIL | Director | February 23, 2022 | |||||||||||||||||
| Katharine Knobil, M.D. | ||||||||||||||||||||
| By: | / S / TINA S. NOVA | Director | February 23, 2022 | |||||||||||||||||
| Tina S. Nova, Ph.D. | ||||||||||||||||||||
| By: | / S / NAWAL OUZREN | Director | February 23, 2022 | |||||||||||||||||
| Nawal Ouzren | ||||||||||||||||||||
| By: | / S / STEVEN J. SCHOCH | Director | February 23, 2022 | |||||||||||||||||
| Steven J. Schoch | ||||||||||||||||||||
119