
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
| For the fiscal year ended: |
or
| For the transition period from | to |
| Commission file number: |
| (Exact name of registrant as specified in its charter) |
|
| ||
| (State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) |
|
| ||
| (Address of principal executive offices) | (Zip Code) |
| Registrant’s telephone number, including area code: |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| The |
Securities registered pursuant to Section 12(g) of the Act:
| (Title of class) |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act.
Yes o
Indicate by check mark if the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | o | Accelerated filer | o | |
| x | Smaller reporting company | |||
| Emerging growth company |
If an emerging growth company, indicate
by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o
The aggregate market value of the voting and non-voting common stock held by non-affiliates of the Registrant, based on the average of the bid and asked price of the common stock on the OTC Pink Open Market of $9.51 per share, was $
The number of shares of the registrant’s common stock outstanding, par value $0.001 per share, as of June 24, 2022, was .
ANNUAL REPORT ON FORM 10-K
FOR THE YEAR ENDED MARCH 31, 2022
TABLE OF CONTENTS
| 2 |
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K (this “Report”) contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that relate to future events or to our future operations or financial performance. Any forward-looking statement involves known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statement.
Words such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “forecast,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “targets,” “likely,” “will,” “would,” “could,” “should,” “continue,” “scheduled” and similar expressions or phrases, or the negative of those expressions or phrases, are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Although we believe that we have a reasonable basis for each forward-looking statement contained in this report, we caution you that these statements are based on our estimates or projections of the future that are subject to known and unknown risks and uncertainties and other important factors that may cause our actual results, level of activity, performance, experience or achievements to differ materially from those expressed or implied by any forward-looking statement. Actual results, level of activity, performance, experience or achievements may differ materially from those expressed or implied by any forward-looking statement as a result of various important factors, including our critical accounting policies and risks and uncertainties relating, to:
| · | our strategies, prospects, plans, expectations, forecasts or objectives; |
| · | our ability to achieve a marketable product (i.e., our insulin pump) and the costs and timing thereof; |
| · | acceptance of our product candidate by our target market and our ability to compete in such market; |
| · | our ability to raise additional financing when needed and the terms and timing thereof; |
| · | our ability to expand, protect and maintain our intellectual property rights; |
| · | our future operations, financial position, revenues, costs, expenses, uses of cash, capital requirements, our need for additional financing or the period for which our existing cash resources will be sufficient to meet our operating requirements; |
| · | our analysis of the target market for our insulin pump; |
| · | the impact of COVID-19 and other adverse public health developments on our operations and our industry: |
| · | our ability to obtain all regulatory approvals and clearances relating to our insulin pump including those of the United States Food and Drug Administration, or FDA; |
| · | regulatory developments in the United States and other countries; |
| · | the timing and costs of our obtaining all regulatory approvals and clearances identified immediately above; |
| · | our compliance with all applicable laws, rules and regulations, including those of the Securities and Exchange Commission, or SEC, and the FDA; |
| · | our ability to compete in the diabetes marketplace with larger and more substantial medical device companies; |
| · | general economic, business, political and social conditions; |
| · | our reliance on and our ability to retain (and if necessary, timely recruit and replace) our officers, directors and key employees and their ability to timely and competently perform at levels expected of them; |
| · | our ability to generate significant revenues and achieve profitability; |
| · | our ability to manage the growth of our business; |
| · | our commercialization, marketing and manufacturing capabilities and strategies; |
| · | our ability to expand, protect and maintain our intellectual property position; |
| · | the success of competing third-party products; |
| · | our ability to comply with regulatory requirements relating to our business, and the costs of compliance with those requirements, including those on data privacy and security; |
| · | the specific risk factors discussed under the heading “Risk Factors” set forth in this report; and |
| · | various other matters, many of which are beyond our control. |
| 3 |
PART I
ITEM 1. BUSINESS
Our fiscal year ends on March 31 of each calendar year. Each reference to a fiscal year in this Report, refers to the fiscal year ended March 31 of the calendar year indicated (for example, fiscal 2022 refers to the fiscal year ended March 31, 2022). Unless the context requires otherwise, references to “we,” “us,” “our,” and the “Company” refer to Modular Medical, Inc. and its consolidated subsidiary.
Overview
We are a development stage medical device company focused on the design, development, and commercialization of an innovative insulin pump using modernized technology to increase pump adoption in the diabetes marketplace. Through the creation of a novel two-part patch pump, our MODD1 product candidate, or MODD1, we seek to fundamentally alter the trade-offs between cost and complexity and access to the higher standards of care that presently available insulin pumps provide. By simplifying and streamlining the user experience from introduction, prescription, reimbursement, training and day-to-day use, we seek to expand the wearable insulin delivery device market beyond the highly motivated “super users” and expand the category into the mass market. The product candidate seeks to serve both the Type 1 and the rapidly growing especially in terms of device adoption, type 2 diabetes markets.
Differentiation
We believe that there are a number of shortcomings and issues with currently available insulin pumps that prevent a substantial number of people who require insulin on a daily basis from choosing an insulin pump to treat their diabetes. We believe, that by tailoring our insulin pump to address such factors, we can expand the scope and adoption rate of insulin pump usage. We believe that to achieve broader market acceptance, an insulin pump must be easier to learn to use, be less time consuming to operate, more intuitive to both patients and physicians, and meet the standards for coverage by insurance providers so that co-payments required from patients are affordable and the hurdles to insurance coverage are significantly reduced.
Among the more prominent issues are:
| · | Complexity: Many existing pumps are highly complex and require significant technical expertise to use effectively. We believe such pumps were designed for “super users,” who have high levels of motivation and technical competence. The complexity of pumps proves daunting to less technically inclined users. |
| · | Cumbersome: We believe that a majority of existing pumps are bulky and difficult to manage, in many cases requiring additional equipment to introduce a catheter to the patient’s body and up to 48 inches of tubing, which must be replaced frequently, to connect the catheter to a pump. This requires users to carry spare parts and other equipment adding to the difficulty of using the pump. |
| · | Costs: Costs associated with insulin pump therapy are high and can be prohibitive, especially for those on fixed or limited incomes. These costs vary by pump, but multi-thousand-dollar upfront payments, often with substantial co-payments in addition to possible daily co-payments on consumables, can easily place current pumps out of reach for patients. This makes insurance providers hesitant to pay for them, leading to limited or absent reimbursement/coverage and high hurdles for patients to gain access. |
| · | Outdated style: Consumer electronics devices have evolved in both form and function. Diabetes pumps have not experienced similar progress. We believe that consumers will be more receptive of products designed with the user experience in mind and that many have low tolerance for complex, difficult procedures for use and maintenance of products. |
| · | Pump mechanism limitations: Traditional pumps generally utilize a syringe and plunger mechanism to deliver insulin. We believe this design limits the ability to reduce the size of the pump, and also potentially exposes the user to the unintended delivery of the full volume of insulin within the pump, which can cause hypoglycemia or death. We believe that the fear of adverse health events due to technical malfunctions related to traditional pump mechanism limitations deters the adoption of insulin pump therapy. |
| 4 |
Our team has substantial knowledge of the diabetes industry and experience in developing, obtaining regulatory authorization for, and bringing insulin pumps to market. Based on this experience, we believe that our innovative insulin pump, using a new and proprietary method of pumping insulin, can address most or all of these shortcomings. It provides a state-of-the-art insulin pump capable of both basal (steady flow) and bolus (mealtime dosing) insulin disbursement. It also has been designed considering a natural migration path to multi-chamber/multi-liquid pumps, potentially offering an exciting array of new therapies to patients with diabetes and other conditions.
Our goal is to become the leader in expanding access to insulin pump technology to a wider portion of diabetes sufferers and provide not just care for the super users, but “diabetes care for the rest of us.” We believe there is a substantial opportunity to penetrate the type 2 MDI marketplace, whether through this new insulin pump or further simplification of pumps for the type 2 marketplace.
The MODD1 is a high-precision, first-line pump that we believe represents the best choice for new pump patients because it is easy to afford, easy to learn, easy to use, and has a revolutionary design and technology that enable precision with low-cost manufacture and high reproducibility.
Key features include:
| · | Two parts - one reusable, one disposable - snap together to form the working system; |
| · | One button interface, easy to learn and use; |
| · | 90-day reusable, 3-day disposable; |
| · | Removable at any time from an adhesive bracket; |
| · | No external controller required, no charging, no battery replacement; and |
| · | Slim profile, lighter weight. |
A proprietary survey of American healthcare payors representing 50 million covered lives (approximately one-third of U.S. covered lives) performed for us by industry leading survey firm ISA has demonstrated that payors are willing to grant equivalent or preferential coverage for a product with this feature set at launch in exchange for rebates of approximately 20%. These costs are built into all of our models.
Diabetes Classifications and Therapies
Diabetes is typically classified as either type 1 or type 2:
| · | Type 1 diabetes is an auto-immune condition characterized by the body’s nearly complete inability to produce insulin. It is frequently diagnosed during childhood or adolescence. Individuals with type 1 diabetes require daily insulin therapy to survive. |
| · | Type 2 diabetes represents over 90% of all individuals diagnosed with diabetes and is characterized by the body’s inability to either properly utilize insulin or produce sufficient insulin. Initially, many people with type 2 diabetes attempt to manage their condition with improvements in diet and exercise and/or the use of oral medications and/or injection of glucagon-like peptide-1 (GLP-1) drugs. However, as their diabetes advances, patients often progress to require insulin therapies such as once-daily long-acting insulin and ultimately to intensified mealtime rapid-acting insulin therapy. This represents an important portion of the diabetes market with an estimated 1.6 million type 2 individuals with diabetes intensively treated with insulin currently in the United States |
Glucose, the primary source of energy for cells, must be maintained at certain levels in the blood in order to permit optimal cell function and health. In people with diabetes, blood glucose levels are not well controlled and frequently become very high, a condition known as hyperglycemia, and very low, a condition called hypoglycemia. Hyperglycemia can lead to serious long-term complications, including blindness, kidney disease, nervous system disease, occlusive vascular diseases, lower-limb amputation, stroke, cardiovascular disease, and death. Hypoglycemia can lead to confusion or loss of consciousness, often requiring a visit to the emergency room or, in certain cases, result in seizures, coma, and/or death.
All people with type 1 diabetes, which is our primary market, require daily insulin. According to the Seagrove 2021 Diabetes Blue Book, approximately 18% of people with type 2 diabetes in the United States, or 4.7 million people, require insulin (basal alone represent 3.1 million and basal plus mealtime represent 1.6 million) to manage their diabetes. In this Report, we refer to people with type 1 diabetes and people with type 2 diabetes who require mealtime insulin as “insulin-requiring people with diabetes.”
| 5 |
Currently, there are two primary therapies available for insulin-requiring people with diabetes: multiple daily insulin injections directly into the body through syringes or insulin pens, referred to as Multiple Daily Injection, or MDI therapy, or the use of an insulin pump to deliver mealtime insulin boluses (single dose) to help with glucose absorption after carbohydrate consumption and a continuous subcutaneous insulin infusion, or CSII therapy, into the body. Generally, CSII therapy is considered to provide a number of advantages over MDI therapy, primarily an improvement in glycemic control, as measured by certain diabetes management tests such as hemoglobin A1c (HbA1c) measure and more recently Time in Range (TIR) where a continuous glucose measuring device is used to calculate this test. Among other medical benefits, it has been demonstrated that insulin pump use can decrease glucose variability, reduce the number of hypoglycemia, decrease the daily doses of insulin and reduce the fear of hypoglycemia.

Notwithstanding these advantages, the difficulty in use resulting from the complexity and cumbersome design of available insulin pumps as well as high and often prohibitive costs for both the patient and insurance provider has resulted not only in dissatisfaction among many existing pump users (fewer than half purchase a new pump after warranty expires per Seagrove Partners (estimate), but also has severely limited the adoption rate of insulin pumps by a large segment of the MDI diabetes population, who we refer to in this Report as “Almost Pumpers.”
We define Almost Pumpers as insulin-requiring people with diabetes who are aware of pumps and their potential benefits but, because of past experience, pump shortcomings, cost, complexity and time and learning required to adopt and utilize available insulin pumps, continue to receive their daily insulin through MDI therapy.
Our initial focus for our insulin pump is the almost pumper segment population located in the United States.
Our research, along with marketplace data, estimates that 32% of Americans with type 1 diabetes use insulin pump therapy and 28% of Americans with type 1 diabetes (44% of those who currently utilize MDI) can be classified as having an interest in pump adoption and meeting the American Diabetes Association guidelines of glucose control if their objections to the currently available suite of products can be overcome. They do not want to closely manage their glucose levels and incur the associated time and effort involved. They are the Almost Pumpers. We have developed what we believe to be the most technologically advanced delivery system overcome the objections and provided motivation for this market. We believe that there are four addressable hurdles to adoption:
| · | Usability: the device needs to be easy to learn and to operate; |
| · | Affordability: we will focus on overcoming copay and insurance hurdles rather than leaving the “insurance journey” to the clinician and patient; |
| · | Accessibility and Education: we will seek to engage patients to sample this new technology by supplying clinicians with free samples and simple training to allow people to see first-hand the typical barriers to adoption that have been overcome; and |
| · | Service and Support: where we will answer their questions and concerns during this diabetes experience. |
We believe this conversion process, engaging people to try and thereby receive the benefits of our technology will substantially increase adoption of insulin pumps among both those with type 1 diabetes and type 2 diabetes who remain reliant upon multiple daily injections. Diabetes is a disease that appears throughout the world. Therefore, we cannot segment the market by socioeconomics, education or level of care. We intend to create an insulin pump that appeals to all Almost Pumpers.
Market
The International Diabetes Federation, or IDF, estimates that, in 2019, approximately 460 million people were living with diabetes worldwide and, that by 2045, this number will increase to approximately 700 million people.
An estimated 34 million people in the United States live with diabetes. Within this group, type 1 diabetes accounts for approximately 1.8 million people (7% of total) with the remainder being type 2 diabetes. However, of the people with type 2 diabetes about 1.6 million of them require intensive insulin treatments to manage their diabetes. This represents a large and growing market with the effects of diabetes accounting for roughly 25% of all healthcare dollars spent annually in the United States.
| 6 |
According to the National Diabetes Health Care Provider Survey conducted by Seagrove Partners, approximately 25% of the 1.6 million highly insulin intensive type 2 diabetes have considered going “on pump.”
Insulin pumps have been shown to provide a higher level of care for insulin dependent people with diabetes and result in better glycemic control, fewer comorbidities, fewer trips to the emergency room, and higher overall quality of life. They also result in lower overall costs to the healthcare system, reducing typical expense per patient year from $27,195 to $16,992.
Despite these benefits, only 1 in 3 (33%) of the 1.8 million Americans with type 1 diabetes and very few of the 1.6 million type 2 diabetes intensively treated with insulin currently use an insulin pump, for a total of approximately 670,000 current users, with only a slow increase of insulin pump use. The remaining 68% of type 1 diabetes’ and virtually all of the type 2 diabetes’ rely on multiple daily injections (MDI) for glucose control. Decades of advances in technology advances have left these non-pumpers at a significant disadvantage from a control perspective versus their “pumping” counterparts.
We have identified a large segment of the market that we refer to as “Almost Pumpers.” Almost Pumpers are those insulin-requiring people with diabetes (type 1 diabetes and type 2 diabetes) who feel that they would adopt the pump if it were less expensive, less time consuming, less technically intimidating, and if there was no separate controller. They represent approximately 32% of the type 1 diabetes market correlating to a $1.9 billion growth opportunity.
Insulin pumps on the market today require a substantial amount of time to manage the therapy, have high out of pocket costs that place these technologies out of reach for a large part of the population, and are feature-heavy with complex systems that have hampered adoption and intimidated many users. The most commonly used insulin pumps today require extensive training and hours of daily management. The average pump user must go through 42 steps of setup and refill process every 72 hours to “stay on track.”
 The
current reluctance to adopt the insulin pump has had serious consequences on the healthcare system. In the United States, people
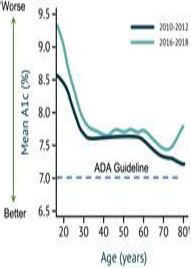
living with type 1 diabetes have struggled to attain glycemic targets. A 2019 analysis of the large type 1 diabetes Exchange clinical
registry found that only 21% of U.S. adults with type 1 diabetes achieved the ADA A1c goal (<7.0%). Further, according to a
study published in JAMA Internal Medicine, researchers found no significant improvements in diabetes care between 2005 and 2016,
with persistent gaps in care related to socioeconomic status.
The
current reluctance to adopt the insulin pump has had serious consequences on the healthcare system. In the United States, people
living with type 1 diabetes have struggled to attain glycemic targets. A 2019 analysis of the large type 1 diabetes Exchange clinical
registry found that only 21% of U.S. adults with type 1 diabetes achieved the ADA A1c goal (<7.0%). Further, according to a
study published in JAMA Internal Medicine, researchers found no significant improvements in diabetes care between 2005 and 2016,
with persistent gaps in care related to socioeconomic status.
The recent introduction and rapid adoption of Abbott Labs’ Freestyle Libre, or the Libre, has made continuous glucose monitoring, or CGM, easier and more affordable, expanding the product category, and doubling its size. Now for the first time, there is an easy, less painful (i.e., no more finger sticks) way for patients to have the data they need to understand more about their glucose levels and their insulin requirements. Access to such data has motivated patients to ask their diabetes clinician how they can achieve better glycemic control and made them more comfortable with using technology and wearables to treat their diabetes. Pumps offer a clear pathway to better control and better overall care. We believe that the insulin pump market is ready for a similar transition as that experienced in the CGM space. We believe our MODD1 pump represents a new and better offering to assist and induce a wide variety of patients to make the transition and bridge the void to superior control by becoming a “pumper.”
We believe the present pump marketplace is approximately a $1.9 billion market, comprising approximately 33% of type 1 diabetes pumpers and a small group of type 2 diabetes pumpers. Seagrove Partners estimates that 28% of type 1 diabetes patients and 25% of type 2 diabetes patients would adopt technology that was easier to use, access and pay for. We believe the total addressable market approximates $3 billion, assuming revenue of $4,128 per patient, per year. We expect to spend approximately 15% of our total revenue on discounts and free samples to encourage adoption of our pump product.
We are dedicated to helping all people with diabetes gain access to high quality care. We aim to help people with diabetes, especially Almost Pumpers and the historically underserved communities, gain access to insulin pump technology by making it affordable and easy to use.
| 7 |
Diabetes Care is at an Inflection Point
We believe that the insulin pump market stands at a crossroads as a confluence of events makes the timing for a new product introduction ideal.
2020 was a very difficult year in diabetes. Between COVID-19 and a loss of glycemic control during quarantines and isolation, deaths from diabetes rose by 17% in 2020 versus the prior year. This was sharpest among the young who saw deaths rise 29% in the 25 to 44 year-old demographic. This has created a pain point and a desire to find new and better solutions and has raised awareness among patients, caregivers, payors, and policy makers.
COVID-19 also encouraged (and required) trial and adoption of telehealth models and a great many people have found them to their liking with a high proportion of patients and of health care providers, or HCPs, that want to continue to use these technologies. We expect much of this shift and newfound comfort with distance care models to persist and believes that this can provide a patient acquisition and engagement model for insulin pumps and diabetes care, especially for pumps optimized for free trial and easy learning.
At the same time, reimbursement for patch pumps has been increasingly moving to a pharmacy benefits manager, or PBM, model, which simplifies reimbursement which will further aid in a “frictionless launch.” This represents a fundamental shift in the insulin pump market, making onboarding rapid and simplifying a previously complex and time-consuming “insurance journey.”
The CGM space (wearable devices that monitor blood glucose levels) has been experiencing explosive growth largely driven by the Libre. This product was a more affordable, easier to use version of the popular Dexcom CGM product. Not only is it now a larger (by revenues) product than Dexcom, but it accomplished this without seeming to slow Dexcom’s growth but rather by growing a new category with a new type of user.
These users are increasingly interested in adopting technology and wearables to manage their diabetes. We believe they are a natural market for a new type of pump if it can meet their needs and address their objections and that the conjunction of the above trends represents a unique opportunity in the insulin pump market’s history.
Diabetes technology companies understand that we are at a turning point with new markets. This can be seen with increased discussion around this topic during recent national diabetes conferences, as well as but also an increase in marketing promotion. For example, Dexcom aired a $5.5 million 30-second commercial during the 2021 Super Bowl.
All these recent changes support the high proportion of type 1 diabetes and type 2 diabetes intensively treated with insulin that are considered as Almost Pumpers, number that may grow in the next years and that may be more reachable with adequate marketing strategies.
Our Insulin Pump
Instead of building complex, bespoke, and difficult to manufacture and maintain pumping and control systems, we began with the technology and the user in mind. Using proprietary and patented methods of insulin measurement, we were able to eschew complex mechanisms and instead built a product candidate using only parts from high volume consumer electronics manufacturing lines, breaking the cost vs functionality curve that has existed in the insulin pump space and representing the first truly modern insulin pump design. This is a new kind of product for a new kind of patient.
The pre-production models of our low-cost insulin pump are now undergoing the testing required to submit to the FDA for clearance to market them in the United States. We continue to devote, substantial time and resources to better understand the needs and preferences of Almost Pumpers and the specific patent/provider/payor requirements to motivate change from MDI.
| 8 |
MODD1 has several distinguishing features:
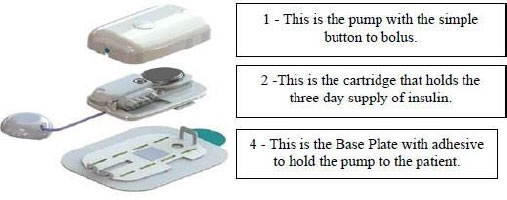
1 - The pump has a simple button to press to deliver insulin as the patient requires it. The electronic pump uses a simple motor and rotating cam to motivate the insulin into the patient along with a low power Bluetooth and near field communication chips to allow the patient to communicate with their smart phone, tablet, or other mobile computing platform, as appropriate.
2 - The pump snaps together with a three-day disposable cartridge that is patient filled with insulin for delivery. It includes the power source and a simple coin cell that allows it to run through the 80-hour life of the cartridge.
3 - There is a set (not shown) that contains a soft 6 millimeter cannula and an introducer for insertion into the skin and removal of the needle used to transfer insulin to the body.
4 - MODD1 comes with a variety of methods for the patient to wear the pump. Options include: a base plate with adhesive (shown) for attaching to the body that has features for holding the pump to the patient; overwraps to hold the product candidate to the patient; and a velcro strap with a base plate suitable for wrapping around the arm or leg of the patient.
The system will deliver a small continuous rate called a basal that will provide approximately 50% of the total daily dose required and the user will use the on-pump button to administer boluses, typically before and after meals.
The objective is to make the product candidate simple to acquire and take home, simple to learn and most importantly, simple to use to expand the pump market, drive adoption and ultimately better clinical outcomes.
Technological Advantages
The adoption of new ultra-high volume technologies will result in far easier manufacturing scale up as parts sourcing and assembly processes are far easier. The MODD1 was designed from the beginning for mass manufacturing processes and “lights out” or near lights out production assembly lines. This advantage is compounded by the high availability and already optimized cost reduction in its components. This has resulted in a cost of goods, estimated on the competitors’ announced margins and sales, 50% lower than our closest patch pump competitor.
The adoption of modern, miniaturized technologies has led to numerous other advantages as well. The MODD1 pump is smaller in overall volume than Insulet’s popular Omnipod product, or the Omnipod, and has a lower profile to the skin. Despite this, it holds a full 3mL (300 units) of insulin in line with full sized pumps such as Tandem and Medtronic, 50% more than the 2mL reservoir in the Omnipod. We believe that this volume advantage over other patch pumps will be significant as 24% of type 1 and over 50% of the rapidly growing type 2 market require more than 2mL of insulin every three days (the expected wear time of patch pumps).
In addition, our new and patented pumping modality will provide what we believe is the most even (and thus closest to the function of a healthy pancreas) delivery of basal insulin in the industry. Basal rate can be delivered almost continuously while other pumps are delivering micro-boluses every 5 minutes for the Omnipod and Tandem and Medtronic pumps. We plan to demonstrate the impact of our system on glycemic control in a future clinical study.
The technology allows the patient to simply add insulin and operate. The battery is included in each cartridge and the device is operated without a controller. Nothing needs charging. MODD1 has been made push button simple to appeal to a wider audience of users.
| 9 |
This new technology has also made the MODD1 lighter than existing offerings. Compared to the Omnipod, MODD1 weighs 20 grams (vs. 26 grams) empty and 23 grams (vs. 28 grams) fully filled (despite carrying 50% more insulin), a reduction of 23% and 18%, respectively. Also, unlike existing patch pumps, the MODD1 can be removed from the needle and taken off and replaced later if the user desires. This avoids loss of insulin in a pump due to accidental dislodging of the soft canula, an issue that users have expressed considerable dissatisfaction with on other patch pumps.
This technology is also uniquely suited to dual (or more) chamber pumps. We believe that such pumps will be integral to the realization of high time in range artificial pancreas solutions that require no human intervention, the next step forward from the cumbersome and awkward solutions today that require the user to announce meals, count and input carbs, and adjust delivery for exercise and sleep. The advantages of cost and miniaturization are multiplied in a multi-chamber setup and we expect to be able to reach price points, ease of use, and form factor unlike anything seen in the industry thus far. We believe that a prefilled, multi-hormone peel and stick patch pump able to function in a fully autonomous closed loop system with CGM’s represents the next generation of diabetes care. We believe that we have demonstrated our technology and are securing intellectual property protection on our approach.
We believe this technology, especially in dual chamber, will open up numerous applications outside of diabetes where medication compliance of complex therapy regimes is difficult addressing such spaces as weight loss, fertility, and simplifying complex delivery of multi-drug cocktails, especially those with diverse and challenging dosing schedules.
Our Solution
Our proposed pump is being designed and developed to address the aforementioned shortcomings of the existing pump market and to appeal to: (i) the substantial group of “Almost-Pumpers” who are currently interested in using an insulin pump, but have not done so because of the complexity, cost or cumbersome nature of existing products, and (ii) people who are using one of the currently available insulin pumps but are dissatisfied with such products. We believe that, owing to our new proprietary technology, our proposed insulin pump will be the simplest and least expensive product on the market and the easiest for providers to prescribe.
Our current pump prototype of our proposed pump has been built to test what we believe to be our novel approach to insulin pumps. By providing a pump that we believe will establish industry standards in terms of technology, simplicity to understand, ease of use and price, we believe our proposed pump will offer the vast majority of benefits afforded by more expensive and complex pumps but remain accessible to a substantially greater percentage of diabetes sufferers requiring daily insulin therapy.
We believe people generally will not use technology that intimidates them and physicians are hesitant to prescribe such technology. We believe mass market products, such as is intended for our proposed pump, must be “user friendly” and affordable. We believe this approach is fundamentally different from that applied to the existing pump market today where most pumps are continuously adding complex features and are “user friendly” to only the most technically astute.
Our current goal is to successfully design, develop and obtain all required regulatory approvals for our proposed insulin pump, and, thereafter, commercialize the finished product. Our long-term goal is to become a leading provider of insulin pump therapy by focusing on both consumer and clinical needs.
To achieve our above stated immediate and current goals, we intend to pursue the following business strategies:
| · | Use of innovative proprietary technology. |
Based upon the substantial experience of Paul DiPerna, our president, chief financial officer, treasurer and chairman of our board of directors, in engineering design and innovative technology in the medical device industry and, in particular, with insulin pumps, we have generated proprietary technology that has been incorporated into our proposed insulin pump. Generally, this technology is involved in the delivery of insulin to the user at the appropriate and necessary times. We believe this technology will greatly assist us in creating a simpler, user-friendly pump. We believe the proposed design, engineering and technology being incorporated into our proposed pump will make it substantially simpler and more affordable than those currently available. These features, together with the safety and reliability of our proposed pump, are designed to create the next generation of insulin pumps that will feature important and well-differentiated attributes compared to those currently available and make it available to consumers across mostly all socioeconomic groups in the United States and around the world.
| 10 |
| · | Keep costs low during our design and development process. |
To attempt to ensure that we have sufficient funds to design, develop, and obtain all required regulatory approvals for our proposed insulin pump without having to sacrifice quality and efficiency, we intend to maintain a tight budget and limit expenditures where possible. We believe this will be possible because of the extensive knowledge and experience of Mr. DiPerna, not only in the diabetes industry and more specifically in the insulin pump device market, but also his experience in designing and developing insulin pumps and other medical devices and his ability to manage a small, focused development team. We currently expect that various other expenses, such as product scale up, and sales and marketing costs, will not be incurred until such time as development work is completed and regulatory approvals obtained.
| · | Employ experienced engineers selected, supervised, and led by Mr. DiPerna, a highly experienced and respected engineer and executive in the insulin pump industry. |
To attempt to ensure our proposed insulin pump is “state of the art,” functional, and efficient, as well as to conserve funds, substantially all of our employees will initially be hand-picked engineers under the leadership of Mr. DiPerna. We believe that there is a strong pool of engineers with significant applicable experience and knowledge who we will be able to initially employ on a contract and/or outsource basis to help us design and develop our proposed insulin pump. We believe by hiring such persons on an out-source basis, we will save substantial resources and by having Mr. DiPerna lead and focus the team on technological and mechanical aspects of our proposed insulin pump, we believe our team will be well guided, focused, cost efficient, and able to efficiently design and develop our product candidate that we believe can eventually be a competitive and popular choice for people with insulin requiring diabetes.
Commercialization Strategy: Overcoming the Insurance Hurdles
Our goal is to establish MODD1 as the best option for new pump patients as we expand the market into the Almost Pumpers (Type 1 and Type 2) and the newly motivated CGM users. We seek to grow the market by providing first-line insulin pump therapy that is well suited to meet the needs of both diabetes patients requiring insulin and their clinicians.
| · | MODD1 is approximately 50% less expensive to manufacture than the Omnipod. This low cost allows us to spend more on patients and sampling. This will save money for payers. We can offer the pump with no upfront cost to patients. Benefits of MODD1 include: |
| · | 20% discount vs Insulet will drive preferred status; |
| · | Designed to use PBM codes as a disposable; |
| · | No new code needed to be reimbursed at launch; and |
| · | Saves provider an estimated $1,062/patient/year vs the Omnipod. |
| · | The MODD1 will be sampled and given to patients by the doctor or diabetes nurse educator at the time of the patient visit. When a patient is motivated to make change, our starter kit will make it easy for the clinician to initiate the new therapy that same day. We seek to eliminate the currently challenging “insurance journey” and product acquisition timeline and significantly reduce training time for the busy clinician, all major hurdles to pump adoption. We intend to add telehealth support to help the patient throughout adoption and use and to facilitate greater collaboration between patients and their physicians. |
Europe represents another large potential market for MODD1. Approximately 60 million people in Europe live with diabetes, and approximately $161 billion is spent annually in diabetes healthcare costs in Europe. At present, cost containment is restricting pump uptake across Europe. Current pump usage hovers between 10% and 20% in many markets. Single payor healthcare systems across the Europe traditionally attempt to contain costs in the short term and seek low price technologies with moderate medical benefits. MODD1 will offer a rebalance of this risk/reward strategy in that payors will incur only minor incremental short-term costs with the benefit of longer -term cost savings associated with reliable pump use. We intend to employ a partnership strategy across Europe following in-house managed regulatory and pricing activities in the major markets (e.g., the United Kingdom) and more cost receptive markets (e.g., the Nordic countries). We are targeting European and United Kingdom approval towards early 2023. Our initial target market for our insulin pump is the Almost Pumper population located in the United States followed quickly by an effort to obtain Conformitè Europëenne, or CE, mark approval for distribution throughout Europe.
| 11 |
Marketing
MODD1 tackles the most significant barriers to pump use-access and affordability-and makes it easier for clinicians, caregivers and individuals to manage diabetes care. Our commercialization plan will drive adoption and is designed to expand the market and is intended to do the following:
| · | Maximize adoption with a comprehensive frictionless launch program. We will seek to decrease the level of reimbursement effort and cost to encourage HCPs to offer our pumps and encourage patient trials. Our product candidate reduces the technical hurdles to widen appeal, new starts and increase adherence. We will encourage MDI patients who want or need more control to make the switch to the pump earlier in their treatment-ideally right at diagnosis. |
| · | Leverage technology to support sales and new patient acquisition. We intend to set up tech enabled sales teams backed with a full omnichannel program to drive awareness and trial with HCPs and patients. We will focus on educating providers that our product candidate is simple to teach and easy to support making it an ideal front line offering. |
| · | Facilitate patient trials. To facilitate patient trials, we intend to: |
| · | Provide a free pump and a 30-day supply of cartridges, insurance verification, co-pay coupons and telehealth support to patients thereby reducing outlay of time and money |
| · | Partner with connected care companies to provide superb support of patients from trial through the first year |
We believe that MODD1 will be the only insulin pump that patients can take home immediately from the doctor’s office.
| · | Leverage MODD1 300-unit chamber to increase adoption with Type 2 patients. MODD1 has a major advantage over existing patch pumps in that the chamber carries enough insulin to meet the high doses many type 2 patients need. We intend to promote this advantage and capture a significant share of the existing type 2 pump users as well as new starts. |
| · | Work with key organizations and policy makers to pave the way for greater access to pumps. We will promote MODD1 technology among the underserved, who are typically low users of health technology. We will identify individuals, patient organizations, professional societies, and policy and DEI organizations that are critically important to the adoption of new technologies in the diabetes space and build relationships with these influential stakeholders. |
| · | Initiate a clinical study program (with key diabetes centers) to provide additional clinical support for MODD1 in special patient types and clinical setting. After obtaining 510(k) clearance, we intend to conduct a soft launch and clinical research program in major markets to pave the way for the full launch in late 2022. We will work with our advisors and key diabetes associations to educate the community about the MODD1. In addition, we will conduct clinical studies to develop competitive claims and market expansion. |
| · | Work with major health plans to establish MODD1 as the first line pump for Type 2 patients. We believe MODD1 will be payor preferred for both type 1 and type 2 patients. It was designed to attain preferential reimbursement and avoid the coverage pitfalls many other pumps have experienced. |
| · | Payors want a simple product that is less expensive. We will launch with a discount program for payers of 20% less than Omnipod to drive uptake. |
| · | Designed to use existing PBM codes as a disposable |
| · | No new code: Reimbursed at launch |
Tie-in with the massive movement to telehealth.
2020 saw personal telehealth go from beta test to mainstream. Customers and providers have become comfortable with it. There are only 4,000 patient-facing endocrinologists in the United States. The treatment of diabetes will be significantly enhanced with telehealth to drive more volume and clinical enhancements through their practices. Telemedicine is a force multiplier for a small group of doctors to better serve a large market. MODD1 was designed to be affordable enough for free sampling and trial, and simple enough for self-guided user training. We believe that by combining telehealth support with MODD1, we will decrease the burden of diabetes care and improve the lives of people with diabetes.
| 12 |
Pre-Launch/Trial
We intend to initiate a “soft launch” following FDA clearance of the MODD1 device. Our plan is to select a group of clinicians who are well trained, experienced and have the support infrastructure to take on initial patients and monitor them carefully to provide clinical feedback on our performance to further refine our product candidate and support infrastructure prior to full commercial launch. Many of these clinicians will have been those who assisted in the development of the MODD1 offering.
We intend to continue to modify, refine and finalize our system to best meet:
| · | The general needs and preferences of our almost pumper target market based upon our knowledge of the diabetes industry and information available and/or obtained by us from Almost Pumpers and their caregivers; and |
| · | The general guidelines of third-party payors, private and public insurance companies, preferred provider organizations and other managed care providers with particular focus on the guidelines established by the Center for Medicare and Medicaid Services, or CMS, which administrates the United States Medicare program, or Medicare. To assist us in making such modifications and refinements, we have retained independent consultants to focus on ensuring that our product candidate satisfies the existing coverage and reimbursement criteria of such third-party payors. |
Manufacturing
Manufacturing requires the production of pumps, cartridges, and baseplates as well as assembly with sets. In connection therewith:
| · | We plan to build an automation machine for implementation in Southern California, close to the design engineers, that will be capable of assembling the cassettes at a rate sufficient to supply 50,000 patients in a single shift (500,000 per month). This equipment will require nine months to design and build and three months to verify and validate into our manufacturing process. |
| · | The packaging equipment and boxing will start as manual operations while the automation is refined. This equipment will be purchased and implemented as the second phase of automation of the cassette. |
| · | The sets will be purchased through third party suppliers with expertise in the product to time and cost-effectively introduce the product and focus on our core expertise. |
| · | The standard cost of the cartridge is estimated to be $7.68 at the point we are manufacturing for 3,000 users or more. The pump is estimated to cost $34.00 at similar volumes. |
| · | Our internal estimates project potential gross margins as high as 78% and a 20% operating margin, approximately 30 months after launch. |
The pumps will be built and tested in our San Diego facility while we build volume and expertise. When the production methodology has matured and the volumes have risen, we will consider a transition to outside and offshore manufacturing, as appropriate.
FDA Clearance
The FDA requires us to meet all applicable regulations for insulin pumps, a subcategory of infusion pumps, which are generally considered Class 2 devices. The design of the MODD1 pump has been completed, units have been built and testing is underway to verify that the design meets all FDA requirements. There are 17 specific tests required to submit for 510(k) clearance. We break these required tests into four testing categories: wetted surface, electrical safety, usability and internal. Appropriate design control and standard operating procedures have been implemented to allow us, when testing is completed, to submit for clearance under the premarket notification (or 510(k)) process. To achieve this, we will continue to work closely with our regulatory consultants to complete, finalize and file our submission to the FDA for 510(k) clearance and all other documentation necessary to obtain marketing authorization of our insulin pump.
| 13 |
| · | We have engaged the FDA in two pre-submission conferences to ensure that we understand and meet the FDA’s requirements, expectations and standards with regard to clearance of our product candidate. At these meetings, our team, including our FDA regulatory consultant, received FDA comments and guidance regarding our proposed submission during the pre-market notification period for 510(k) clearance (including any suggested modifications to the device description, indications for use or summary of supporting data contained in the notification); |
| · | We are currently preparing and ensuring that our premarket notification, which will be part of our FDA submission in order to demonstrate that our insulin pump is substantially equivalent to an insulin pump previously cleared by the FDA and legally marketed to the public and generally safe and effective for its intended use. We are also preparing our submission to the FDA, which will include the relevant results of our performance and human factor tests (relating to, among other things, user effectiveness, sterility, pump efficiency and shipping compatibility) demonstrating the accuracy and usability of our insulin pump, which we believe will satisfy the mandates of the FDCA and any applicable performance standards. |
Commercialization Steps
While we have substantially completed the general engineering and mechanical aspects of our insulin pump prototype, prior to commercializing, we still must successfully complete a number of material steps including:
| · | Continue to modify, refine and finalize our prototype so that it meets: |
| · | the general needs and preferences of our almost-pumper target market based upon our knowledge of the diabetes industry and information available and/or obtained by us from Almost Pumpers and their caregivers; and |
| · | the general guidelines of third-party payors, private and public insurance companies, preferred provider organizations and other managed care providers with particular focus on the guidelines established by CMS, which administers Medicare. To assist us in making such modifications and refinements, we have retained independent consultants to focus on ensuring that our product candidate satisfies the existing coverage and reimbursement criteria of such third-party payors. |
| · | Refine our manufacturing process during the submission process to identify and select a manufacturer of our insulin pump through a competitive bidding process, as we prepare for our product introduction; |
| · | Take such actions, if any, as may be required by the FDA as a condition to granting approval and providing 510(k) clearance for our insulin pump; and |
| · | Hire and retain appropriate sales and marketing personnel to develop, implement and launch a promotional campaign for our insulin pump substantially focused on our target market. |
As with any medical device attempting to enter and successfully compete with existing products in an established and competitive marketplace, we will face significant hurdles to accomplish the above steps to commercialization including:
| · | Obtaining FDA 510(k) clearance to market and sell our insulin pump to the public; |
| · | Obtaining any other FDA-required authorizations with regard to our product candidate, as required by the FDCA; |
| · | Educating endocrinologists, physician’s assistants, nurse practitioners and nurse educators, who typically prescribe pump usage, and certified diabetes educators and dieticians, who provide education and guidance to diabetes patients, as to what we believe to be the superior qualities of our product candidate; |
| · | Demonstrating to select general practitioners, who have historically been skeptical of the heightened support inherent in insulin pumps, our product candidate’s ease of use and convenience; |
| · | Ensuring that our final product does, in fact, meet the needs of Almost-Pumpers; |
| · | Overcoming the historic obstacles and reluctance of Almost-Pumpers to using insulin pumps to treat their diabetes; and |
| · | Ensuring that third party payors agree to cover all or a substantial portion of the purchase price and recurring costs of the use of our insulin pump. |
| 14 |
Looking Forward
Going forward, we expect to continue to evolve the MODD1 pumps and their capabilities and functionality, both in response to patient needs and as part of our current platform roadmap.
| · | In our next generation product, or MODD2, we will seek to add phone-based control and alternate controller enabled, or ACE, and automated insulin delivery, or AID, capability to allow integration with popular continuous glucose monitors. This will expand our available market to include many existing pumpers. The new model has the same modular design and low-cost components as MODD1 and provides a much desired breakthrough for patients - two-factor command authentication that allows the wearer to use his/her own cell phone as the controller. |
| · | Additionally, adds AID control functionality via an ACE designation |
| · | Any approved algorithm controller can drive insulin delivery in “auto” mode |
| · | CGM integration allows the controller to potentially adjust basal insulin rate for meals and exercise with an approved algorithm. |
| · | With MODD2, we will seek to move to a full featured multi chamber pump optimized for high time in range fully autonomous close loop insulin delivery utilizing the form factor and cost advantages of its pumping designs to create an affordable, easy to use drug delivery system to realize the aspiration of true “artificial pancreas” systems. We envision moving to a drug prefill model such that cartridges can be filled with therapeutics and shipped cold chain to patients, further simplifying the use process. |
Government Regulation
Our operations are subject to comprehensive federal, state, and local laws and regulations in the jurisdictions in which we or our research and development partners do business. The laws and regulations governing our business and interpretations of those laws and regulations and are subject to frequent change. Our ability to operate profitably will depend in part upon our ability, and that of our research and development partners and affiliates, to operate in compliance with applicable laws and regulations. The laws and regulations relating to medical products and healthcare services that apply to our business and that of our partners and affiliates continue to evolve, and we must, therefore, devote significant resources to monitoring developments in legislation, enforcement, and regulation in such areas. As the applicable laws and regulations change, we are likely to make conforming modifications in our business processes from time to time. We cannot provide assurance that a review of our business by courts or regulatory authorities will not result in determinations that could adversely affect our operations or that the regulatory environment will not change in a way that restricts our operations.
FDA Regulation
In the United States, medical devices are strictly regulated by the FDA. Under the FDCA, a medical device is defined as “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component, part or accessory which is, among other things: intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals; or intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.” This definition provides a clear distinction between a medical device and other FDA regulated products such as drugs. If the primary intended use of a medical product is achieved through chemical action or by being metabolized by the body, the product is usually a drug or biologic. If not, it is generally a medical device.
We are currently developing an insulin pump delivery system, which is regulated by the FDA as a medical device under the FDCA, as implemented and enforced by the FDA. The FDA regulates the development, testing, manufacturing, labeling, packaging, storage, installation, servicing, advertising, promotion, marketing, distribution, import, export, and market surveillance of our medical devices.
| 15 |
Device Premarket Regulatory Requirements
Before being introduced into the U.S. market, each medical device must obtain marketing clearance or approval from the FDA through the premarket notification (or 510(k)) process, the de novo classification process, or the premarket approval, or PMA, process, unless they are determined to be Class I devices or to otherwise qualify for an exemption from one of these available forms of premarket review and authorization by the FDA. Under the FDCA, medical devices are classified into one of three classes - Class I, Class II or Class III - depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurance of safety and effectiveness. Classification of a device is important because the class to which a device is assigned determines, among other things, the necessity and type of FDA review required prior to marketing the device. Class I devices are those for which reasonable assurance of safety and effectiveness can be maintained through adherence to general controls which include compliance with the applicable portions of the FDA’s Quality System Regulation, or the QSR, as well as regulations requiring facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials. The Class I designation also applies to devices for which there is insufficient information to determine that general controls are sufficient to provide reasonable assurance of the safety and effectiveness of the device or to establish special controls to provide such assurance, but that are not life-supporting or life-sustaining or for a use which is of substantial importance in preventing impairment of human health, and that do not present a potential, unreasonable risk of illness or injury.
Class II devices are those for which general controls alone are insufficient to provide reasonable assurance of safety and effectiveness and there is sufficient information to establish “special controls.” These special controls can include performance standards, post-market surveillance requirements, patient registries and FDA guidance documents describing device-specific special controls. While most Class I devices are exempt from the premarket notification requirement, most Class II devices require a premarket notification prior to commercialization in the United States; however, the FDA has the authority to exempt Class II devices from the premarket notification requirement under certain circumstances. As a result, manufacturers of most Class II devices must submit premarket notifications to the FDA under Section 510(k) of the FDCA (21 U.S.C. § 360(k)) in order to obtain the necessary clearance to market or commercially distribute such devices. To obtain 510(k) clearance, manufacturers must submit to the FDA adequate information demonstrating that the proposed device is “substantially equivalent” to a “predicate device” that is already on the market. A predicate device is a legally marketed device that is not subject to PMA, meaning, (i) a device that was legally marketed prior to May 28, 1976 (“pre-amendments device”) and for which a PMA is not required, (ii) a device that has been reclassified from Class III to Class II or I or (iii) a device that was found substantially equivalent through the 510(k) process. If the FDA agrees that the device is substantially equivalent to the predicate device identified by the applicant in a premarket notification submission, the agency will grant 510(k) clearance for the new device, permitting the applicant to commercialize the device. Premarket notifications are subject to user fees, unless a specific exemption applies.
If there is no adequate predicate to which a manufacturer can compare its proposed device, the proposed device is automatically classified as a Class III device. In such cases, a device manufacturer must then fulfill the more rigorous PMA requirements or can request a risk-based classification determination for its device in accordance with the de novo classification process.
Devices that are intended to be life sustaining or life supporting, devices that are implantable, devices that present a potential unreasonable risk of harm or are of substantial importance in preventing impairment of health, and devices that are not substantially equivalent to a predicate device and for which safety and effectiveness cannot be assured solely by the general controls and special controls are placed in Class III. Such devices generally require FDA approval through the PMA process, unless the device is a pre-amendments device not yet subject to a regulation requiring premarket approval. The PMA process is more demanding than the 510(k) process. For a PMA, the manufacturer must demonstrate through extensive data, including data from preclinical studies and one or more clinical trials, that the device is safe and effective for its proposed indication. The PMA must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed labeling. Following receipt of a PMA submission, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review and determine whether the proposed device can be approved for commercialization, although in practice, PMA reviews often take significantly longer, and it can take up to several years for the FDA to issue a final decision. Before approving a PMA, the FDA generally also performs an on-site inspection of manufacturing facilities for the product to ensure compliance with the QSR.
| 16 |
The de novo classification process allows a manufacturer whose novel device is automatically classified into Class III to request down-classification of its device to Class I or Class II, on the basis that the device presents low or moderate risk, as an alternative to following the typical Class III device pathway requiring the submission and approval of a PMA application. Under the Food and Drug Administration Safety and Innovation Act of 2012, the FDA is required to classify a device within 120 days following receipt of the de novo classification request from an applicant; however, the most recent FDA premarket review goals state that in fiscal year 2021, FDA will attempt to issue a decision within 150 days of receipt on 65% of all de novo classification requests received during the year and on 70% of de novo requests received during fiscal year 2022. If the manufacturer seeks reclassification into Class II, the classification request must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. The FDA may reject the classification request if it identifies a legally marketed predicate device that would be appropriate for a 510(k) notification or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed.
Clinical trials are almost always required to support PMAs and are sometimes required to support 510(k) and de novo classification submissions. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption, or IDE, regulations that govern investigational device labeling, prohibit promotion of investigational devices, and specify recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk,” as defined by the FDA, the agency requires the study sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical trials. The IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA denies the application or notifies the sponsor that the investigation is on hold and may not begin until the sponsor provides supplemental information about the investigation that satisfies the agency’s concerns. If the FDA determines that there are deficiencies or other concerns with an IDE that require modification of the study, the FDA may permit a clinical trial to proceed under a conditional approval. The FDA may also notify the sponsor that the study is approved as proposed or approved with specific requested modification. Furthermore, the agency may withdraw approval of an IDE under certain circumstances. In addition, the study must be approved by, and conducted under the oversight of, an institutional review board, or IRB, for each clinical site. If the device presents a non-significant risk to the patient according to criteria established by the FDA as part of the IDE regulations, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate authorization from the FDA, but must still comply with abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements.
Post-Marketing Restrictions and Enforcement
After a device is placed on the market, numerous regulatory requirements apply. These include, but are not limited to:
| · | submitting and updating establishment registration and device listings with the FDA; |
| · | compliance with the QSR, which requires manufacturers to follow stringent design, testing, control, documentation, record maintenance, including maintenance of complaint and related investigation files, and other quality assurance controls during the manufacturing process; |
| · | unannounced routine or for-cause device facility inspections by the FDA, which may include our suppliers’ facilities; |
| · | labeling regulations, which prohibit the promotion of products for uncleared or unapproved (or “off-label”) uses and impose other restrictions relating to promotional activities; |
| · | corrections and removal reporting regulations, which require that manufacturers report to the FDA field corrections or removals if undertaken to reduce a risk to health posed by a device or to remedy a violation of the FDCA that may present a risk to health; and |
| · | post-market surveillance regulations, which apply to certain Class II or III devices when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
In addition, under the FDA medical device reporting, or MDR, regulations, medical device manufacturers are required to report to the FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or a similar device of such manufacturer were to recur. The decision to file an MDR involves a judgment by the manufacturer. If the FDA disagrees with the manufacturer’s determination, the FDA can take enforcement action.
| 17 |
The MDR requirements also extend to health care facilities that use medical devices in providing care to patients, or “device user facilities,” which include hospitals, ambulatory surgical facilities, nursing homes, outpatient diagnostic facilities, or outpatient treatment facilities, but not physician offices. A device user facility must report any device-related death to both the FDA and the device manufacturer, or any device-related serious injury to the manufacturer (or, if the manufacturer is unknown, to the FDA) within 10 days of the event. Device user facilities are not required to report device malfunctions that would likely cause or contribute to death or serious injury if the malfunction were to recur but may voluntarily report such malfunctions through MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program.
The FDA also has the authority to require the recall of commercialized medical device products in the event of material deficiencies or defects in design or manufacture. The authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious adverse health consequences or death. Manufacturers may, under their own initiative, recall a product if any distributed devices fail to meet established specifications, are otherwise misbranded or adulterated under the Federal Food, Drug, and Cosmetic Act, or the FDCA, or if any other material deficiency is found. The FDA requires that certain classifications of recalls be reported to the FDA within ten working days after the recall is initiated.
The failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
| · | warning letters, fines, injunctions or civil penalties; |
| · | recalls, detentions or seizures of products; |
| · | operating restrictions; |
| · | delays in the introduction of products into the market; |
| · | total or partial suspension of production; |
| · | delay or refusal of the FDA or other regulators to grant 510(k) clearance, PMA approvals, or other marketing authorization to new products; |
| · | withdrawals of marketing authorizations; or |
| · | in the most serious cases, criminal prosecution. |
To ensure compliance with regulatory requirements, medical device manufacturers are subject to market surveillance and periodic, pre-scheduled and unannounced inspections by the FDA, and these inspections may include the manufacturing facilities of subcontractors.
Federal Trade Commission Regulatory Oversight
Our advertising for our products and services is subject to federal truth-in-advertising laws enforced by the Federal Trade Commission, or the FTC, as well as comparable state consumer protection laws. Under the Federal Trade Commission Act, or FTC Act, the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution.
Healthcare Law and Regulation
United States
If our MODD1 product candidate or our other future product candidates are approved in the United States, we will have to comply with various U.S. federal and state laws, rules and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws and physician self-referral laws, rules and regulations. Violations of the fraud and abuse laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state healthcare programs, including Medicare and Medicaid. These laws include the following:
| · | the federal Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; |
| 18 |
| · | the federal False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| · | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| · | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| · | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| · | the federal transparency requirements under the Physician Payments Sunshine Act require manufacturers of FDA-approved drugs, devices, biologics and medical supplies covered by Medicare or Medicaid to report, on an annual basis, to the Department of Health and Human Services information related to payments and other transfers of value to physicians, teaching hospitals, and certain advanced non-physician health care practitioners and physician ownership and investment interests; and |
| · | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by nongovernmental third-party payors, including private insurers. |
Some state laws require pharmaceutical or medical device companies to comply with the relevant industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug and device manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures.
State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. We also may be subject to, or may in the future become subject to, U.S. federal and state, and foreign laws and regulations imposing obligations on how we collect, use, disclose, store and process personal information. Our actual or perceived failure to comply with such obligations could result in liability or reputational harm and could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our customer base and thereby decrease our future revenues.
The European Union, or EU, approves the use of medical devices in a very different way. They have similar regulations and requirements to adhere to, however a Notified Body, in the form of a private company, will represent their interests and is required to have sufficient expertise to review all applications and the company’s internal processes to ensure the safety of the product for which approval is being requested. We are in the process of identifying a Notified Body to represent us, and we will follow our FDA submission process with regard to preparing the materials and processes required to meet the regulations and gain clearance.
| 19 |
European Union
European Economic Area
In the European Economic Area (which is comprised of the 27 member states of the European Union plus Norway, Iceland and Liechtenstein), or the EEA, manufacturers of medical devices need to comply with the Essential Requirements laid out in Annex I to the EU Medical Devices Directive (Council Directive 93/42/EEC) or with the General Safety and Performance Requirements (GSPR) of the new EU Medical Devices Regulation (EU 2017/745). Compliance with these requirements is a prerequisite to be able to affix the CE mark to medical devices, without which they cannot be marketed or sold in the EEA. To demonstrate compliance with the Essential Requirements and the GSPR and obtain the right to affix the CE Mark, manufacturers of medical devices must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low-risk medical devices (Class I with no measuring function and which are not sterile), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the Essential Requirements and the GSPR, a conformity assessment procedure requires the intervention of a Notified Body, which is an organization designated by a competent authority of an EEA country to conduct conformity assessments. Depending on the relevant conformity assessment procedure, the Notified Body would audit and examine the Technical File and the quality system for the manufacture, design and final inspection of the devices. The Notified Body issues a CE Certificate of Conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the Essential Requirements and GSPR. This Certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity. As a general rule, demonstration of conformity of medical devices and their manufacturers with the Essential Requirements and GSPR must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence.
All manufacturers placing medical devices into the market in the EEA must comply with the EU Medical Device Vigilance System. Under this system, incidents must be reported to the relevant authorities of the member states of the EEA, and manufacturers are required to take Field Safety Corrective Actions, or FSCAs, to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. An incident is defined as any malfunction or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labeling or the instructions for use which, directly or indirectly, might lead to or might have led to the death of a patient or user or of other persons or to a serious deterioration in their state of health. An FSCA may include the recall, modification, exchange, destruction or retrofitting of the device. FSCAs must be communicated by the manufacturer or its legal representative to its customers and/or to the end users of the device through Field Safety Notices. Where appropriate, our products commercialized in Europe are CE marked and classified as either Class I or Class II.
In 2017, the European Parliament passed the Medical Devices Regulation, which repeals and replaces the EU Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA member states, the regulations would be directly applicable (i.e., without the need for adoption of EEA member State laws implementing them) in all EEA member states and are intended to eliminate current differences in the regulation of medical devices among EEA member States. The Medical Devices Regulation, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and in vitro diagnostic devices and ensure a high level of safety and health while supporting innovation.
The Medical Device Regulation was meant to become applicable three years after publication (in May 2020). However, in April 2020, to allow EEA national authorities, notified bodies, manufacturers and other actors to focus fully on urgent priorities related to the COVID-19 pandemic, the European Council and Parliament adopted Regulation 2020/561, postponing the date of application of the Medical Device Regulation by one year. The Medical Device Regulation became applicable on May 26, 2021. Devices lawfully placed on the market pursuant to the EU Medical Devices Directive prior to May 26, 2021 may generally continue to be made available on the market or put into service until May 26, 2025. The Medical Devices Regulation, among other things:
| · | strengthens the rules on placing devices on the market and reinforces surveillance once they are available; |
| · | establishes explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| 20 |
| · | improves the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; |
| · | sets up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and |
| · | strengthens rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market. |
Competition
Today, in the United States, only three companies are commercializing insulin pumps to type 1 diabetes patients and insulin treated type 2 diabetes patients:
| · | Medtronic - commercializes the durable Minimed 770G also offering older durable pumps still in use (e.g., the 670G, 630G etc.). In 2020, they held approximately 51% of the US insulin pump market. |
| · | Tandem - commercializes the durable t:slim X2 pump (with or without algorithms - Basal-IQ and Control-IQ). In 2020, they held approximately 28% of the US insulin pump market. |
| · | Insulet - commercializes the disposable Omnipod patch pump with about 19% of the US market in 2020. |
Older insulin pumps are also still being used by a minority of patients previously provided by Roche or Animas, though these pumps are not commercialized any longer. To a lesser extent, the pumps described below are also used in small numbers.
These three insulin pump offerings are vying for the attention of the most motivated and well insured in hope of converting them away from their reliance on multi-day insulin injections. The t:slim X2 and Minimed 770G each have a ~$5,000 list price that is covered through Durable Medical Equipment (DME) reimbursement; daily consumables and insulin are also required to complete these offerings. These products have controllers integrated into the pump, making them cumbersome and bulky, along with long (>20 inch) tubing between the pump and the cannular site. The Omnipod is the third offering, a patch pump that attaches to the body for 72 hours and uses a separate controller to manage the insulin delivery process. Insurance coverage can be provided via DME but also via Pharmacy Benefit (PB). The Omnipod patch pump is more expensive per day and less accurate than other insulin pumps. Around 32% of people living with T1D are currently using insulin pumps; of these, the vast majority are using one of these three offerings, a statistic that has not changed significantly over the last 5+ years.
All of these pump products require extensive training to initiate and two to four hours per day to use and manage on an ongoing basis. This level of sophistication and effort along with the cost and awkwardness of these products contribute to the limited uptake.
Although there are purely mechanical pumps available to patients with a small percentage of T2D patients are using the Zealand V-Go patch pump, a fixed basal rate and a button to deliver small boluses. This pump is simple to use though gives little performance decision to the user (no possibility to change the basal rate, no possibility to stop bolus doses, small reservoir, pump that needs to be changed every day, etc.). The last available patch pump is provided by Cequr, called Simplicity, a bolus-only delivery option without basal delivery that is yet to be available.
In the future, Medtronic intends to launch a new version of their insulin pump, the Minimed 780G, already available in some European countries with an advanced algorithm, but no obvious change in hardware. Tandem is currently developing a patch pump called t:sport, coupled with an algorithm with potential launch expected in summer 2022. The t:sport should have a small 2mL reservoir and would be controlled by a separate unit as is the current Omnipod. Insulet should launch in the coming quarters the Omnipod 5, a similar patch pump to their offering today, that includes an algorithm.
Approximately 71% of the people who rely upon MDI choose to not administer a shot outside of their house, which creates a poorly controlled group. Our MODD1 product is designed to focus upon a segment of these people and mobilize them via a simple, easy to use, affordable product.
| 21 |
Intellectual Property
Our success depends in part on our ability to obtain patents and trademarks, maintain trade secret and know-how protection, enforce our proprietary rights against infringers, and operate without infringing on the proprietary rights of third parties. Because of the length of time and expense associated with developing new products and bringing them through the regulatory approval process, the health care industry places considerable emphasis on obtaining patent protection and maintaining trade secret protection for new technologies, products, processes, know-how, and methods.
As of March 31, 2022, we had one issued U.S. utility patent, five published U.S. utility patents, two pending foreign patent applications, and two pending international PCT patent applications covering various aspects of our technology, including our proprietary fluid movement technology. There can be no assurance that the pending patent applications will result in the issuance of patents, that patents issued to or licensed by us will not be challenged or circumvented by competitors, or that these patents will be found to be valid or sufficiently broad to protect our technology or provide us with a competitive advantage.
Available Information
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to such reports filed or furnished pursuant to section 13(a) or 15(d) of the Securities Exchange Act of 1934, as well as section 16 reports on Form 3, 4, or 5, are available free of charge on our website at www.modular-medical.com. as soon as it is reasonably practicable after they are filed or furnished with the SEC. Our Code of Business Conduct and Ethics and the charters for the Audit Committee, Compensation Committee and Nominating and Governance Committee are also available on our website. The Code of Business Conduct and charters are also available in print to any shareholder upon request without charge. Requests for such documents should be directed to Corporate Secretary, at Modular Medical, Inc., 16772 W. Bernardo Drive, San Diego CA 92127. Our Internet website and the information contained on it or connected to it are not part of, or incorporated by, reference into this prospectus. Our filings with the SEC are also available on the SEC’s website at http://www.sec.gov.
Corporate History and Background
We were formed as a corporation under the laws of the State of Nevada in October 1998 under the name Bear Lake Recreation Inc. We had no material business operations from 2002 until July 2017, when we acquired Quasuras, Inc., a Delaware corporation, in the Control Block Acquisition (as defined below). Prior to the Control Block Acquisition, we were a shell company, as defined in Rule 12b-2 promulgated under the Securities Exchange Act of 1934 (the “Exchange Act”).
The Control Block Acquisition. On April 26, 2017, pursuant to a Common Stock Purchase Agreement, dated as of April 5, 2017, by and among Manchester Explorer, LP, a Delaware limited partnership, we and certain persons named therein, Manchester Explorer, LP purchased from us 966,667 shares of our Common Stock representing in excess of a majority of our then issued and outstanding Common Stock, for a purchase price of $375,000 (the “Control Block Acquisition”), resulting in a change in control of the Company. In connection with the Control Block Acquisition, James E. Besser was appointed president and a director and Morgan C. Frank was appointed the chief executive officer, chief financial officer, secretary, treasurer and a director of ours and immediately following such appointments, our then officers and directors resigned. Mr. Besser is the managing member of and Mr. Frank is the portfolio manager and a consultant to Manchester Management Company, LLC, a Delaware limited liability company also referred to herein as MMC. MMC is the general partner of Manchester Explorer, LP and Jeb Partners, L.P. (Jeb Partners, and together with Manchester Explorer, LP, collectively, the Purchasing Funds).
The Acquisition. On July 24, 2017, pursuant to a Reorganization and Share Exchange Agreement, by and among us, Paul M. DiPerna, the sole officer, director and a controlling stockholder of Quasuras, Messrs. Besser and Frank (Messrs. Besser, Frank and DiPerna, collectively, the “3 Quasuras Shareholders”), and Quasuras, Inc. (the “Share Exchange Agreement”), we acquired all of the issued and outstanding shares of Quasuras, Inc. owned by the 3 Quasuras Shareholders, resulting in Quasuras, Inc. becoming our wholly-owned subsidiary (the “Acquisition”). Simultaneously with the closing of the Acquisition, Manchester Explorer, LP cancelled the 2,900,000 shares of our Common Stock purchased in the Control Block Acquisition, Mr. Besser resigned as our president and a director and Mr. Frank resigned as our chief executive officer, chief financial officer, secretary, and treasurer, but remained a director, and Mr. DiPerna was appointed our chairman of the board of directors, chief executive officer, chief financial officer, president, secretary and treasurer. Mr. DiPerna served as our chief executive officer until August 2021 and as our secretary until October 2021.
| 22 |
Subsidiaries
Quasuras, Inc., a Delaware corporation, is our only subsidiary.
Employees
As of March 31, 2022, we had 25 employees all of whom are located in the United States, consisting of 23 in research and development and manufacturing operations and 2 in general and administrative functions.
Properties
Our corporate facility is leased and located at 16772 West Bernardo Drive, San Diego, CA 92127. The 39-month lease term commenced April 1, 2020, and provides for an initial monthly rent of approximately $12,400 with annual rent increases of approximately 3%. In addition to the minimum lease payments, we are responsible for property taxes, insurance and certain other operating costs. We believe that our existing facility is adequate to meet our current needs.
Corporate Information
We are a Nevada corporation. Our corporate headquarters and operating facilities are located at 16772 West Bernardo Drive, San Diego, CA 92127 Our telephone number is (858) 800-3500. We maintain a website at www.modular-medical.com.
Competition
Medtronic, Inc., Tandem Diabetes Care, Inc. and Insulet Corporation are all much larger companies with substantially greater resources than us that make similar products for the more sophisticated, technically capable person with diabetes. We do not intend to directly compete for those individuals with diabetes, instead we intend to offer a simple to use more cost-effective solution to attract the more mainstream patients.
Smaller Reporting Company
We are subject to the reporting requirements of Section 13 of the Exchange Act and to the disclosure requirements of Regulation S-K of the SEC, as a “smaller reporting company.” Such designation relieves us of some of the disclosure requirements of Regulation S-K.
ITEM 1A. RISK FACTORS
We are a developmental stage medical device company and have a history of significant operating losses; we expect to continue to incur operating losses, and we may never achieve or maintain profitability.
As a development-stage enterprise, we do not currently have revenues to generate cash flows to cover operating expenses. Since our inception, we have incurred operating losses in each year due to costs incurred in connection with research and development activities and general and administrative expenses associated with our operations. For the years ended March 31, 2022 and 2021, we incurred net losses of approximately $18.6 million and $7.4 million, respectively. At March 31, 2022, we had an accumulated deficit of approximately $34.6 million. As a result, we will need to raise additional capital in the future, which may or may not be available to us at all or only on unfavorable terms.
We expect to incur losses for the foreseeable future, as we continue the development of, and seek regulatory clearance and approvals for, our insulin pump. As our prototype insulin pump is currently our only product, if it fails to gain regulatory approval and market acceptance, we will not be able to generate any revenue, or explore other opportunities to enhance shareholder value, such as through a sale. If we fail to generate revenue and eventually become profitable, or if we are unable to fund our continuing losses, our shareholders could lose all or a substantial part of their investment.
| 23 |
The full effects of COVID-19 and other potential future public health crises, epidemics, pandemics or similar events are uncertain and could have a material and adverse effect on our business, financial condition, operating results and cash flows.
The global outbreak of the coronavirus disease 2019, or COVID-19, was declared a pandemic by the World Health Organization and a national emergency by the U.S. government in March 2020. This has negatively affected the world economy, disrupted global supply chains, significantly restricted travel and transportation, resulted in mandated closures and orders to “shelter-in-place” and created significant disruption of the financial markets. The extent of the impact on our operational and financial performance will depend on future developments, including the duration and spread of the pandemic and related actions taken by U.S. and foreign government agencies to prevent disease spread, all of which are uncertain, out of our control and cannot be predicted.
We have been complying with county and state orders and, until May 2021, had implemented a teleworking policy for our employees and contractors and significantly minimized the number of employees who visit our office. However, a facility closure, work slowdowns or temporary stoppage at one of our suppliers could occur, which could have a longer-term impact and could delay our prototype production and ability to conduct business.
If our workforce is unable to work effectively, including because of illness, quarantines, absenteeism, government actions, facility closures, travel restrictions or other restrictions in connection with the COVID-19 pandemic, our operations will be negatively impacted. We may be unable to develop our product candidate, and our costs may increase as a result of the COVID-19 outbreak. The impacts could worsen if there is an extended duration of any COVID-19 outbreak or a resurgence of COVID-19 infection in affected regions after they have begun to experience improvement.
We rely on other companies to provide components and to perform services for us. An extended period of supply chain disruption caused by the response to COVID-19 could impact our ability to produce our initial product quantities, and, if we are not able to implement alternatives or other mitigations, product deliveries would be adversely impacted and negatively impact our business, financial condition, operating results and cash flows. Limitations on government operations can also impact regulatory approvals that are necessary for us to operate our business.
The continued spread of COVID-19 has also led to disruption and volatility in the global capital markets. We were recently able to raise additional capital through equity offerings in February 2022 and May 2022, however, we will need to raise additional capital to support our operations in the future. We may be unable to access the capital markets, and additional capital may only be available to us on terms that could be significantly detrimental to our existing stockholders and to our business.
| 24 |
We will need substantial additional funding to complete subsequent phases of our insulin pump product and to operate our business and such funding may not be available or, if it is available, such financing is likely to substantially dilute our existing shareholders.
The discovery, development, and commercialization of new medical devices, such as our insulin pump, entails significant costs. While we believe that we have generally completed the engineering and mechanical aspects of our insulin pump prototype, we still must modify, refine and finalize our insulin pump to, among other things, meet the general needs and preferences of the almost pumper marketplace and the guidelines of third-party payors. To enable us to accomplish these and other related items and continue to operate our business, we will need to raise substantial additional capital and/or enter into strategic partnerships or joint ventures to enable us to:
| · | fund clinical studies and seek regulatory approvals; |
| · | build or access manufacturing and commercialization capabilities; |
| · | develop, test, and, if approved, market our product candidate; |
| · | acquire or license additional internal systems and other infrastructure; and |
| · | hire and support additional management, engineering and scientific personnel. |
Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never achieve, we expect to finance our cash needs primarily through public or private equity offerings, debt financings or through the establishment of possible strategic alliances. We may in the future seek additional capital from public or private offerings of our capital stock or borrow additional amounts under new credit lines or from other sources. If we issue equity or debt securities to raise additional funds, our existing stockholders may experience dilution, we may incur significant financing costs, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional funds through collaborations, licensing, joint ventures, strategic alliances, partnership arrangements or other similar arrangements, it may be necessary to relinquish valuable rights to our potential future products or proprietary technologies or grant licenses on terms that are not favorable to us.
We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are not able to secure additional equity funding when needed, we may have to delay, reduce the scope of, or eliminate one or more of our clinical studies, development programs or future commercialization initiatives. In addition, any additional equity funding that we do obtain will dilute the ownership held by our existing equity holders. The amount of this dilution may be substantially increased if the trading price of our common stock is lower at the time of any financing. Regardless, the economic dilution to shareholders will be significant if our stock price does not increase significantly, or if the effective price of any sale is below the price paid by a particular shareholder. Any debt financing that we obtain in the future could involve substantial restrictions on activities and creditors could seek a pledge of some or all of our assets. We have not identified potential sources for such financing that we will require, and we do not have commitments from any third parties to provide any future debt financing. If we fail to obtain funding as needed, we may be forced to cease or scale back operations, and our results, financial condition and stock price would be adversely affected.
We have a limited operating history and historical financial information upon which you may evaluate our performance.
You should consider, among other factors, our prospects for success in light of the risks and uncertainties encountered by companies that, like us, are in their early stages of development. We may not successfully address these risks and uncertainties or successfully complete our studies and/or implement our existing and new products. If we fail to do so, it could materially harm our business and impair the value of our common stock. Unanticipated problems, expenses and delays are frequently encountered in establishing a new business, conducting research, and developing new products. These include, but are not limited to, inadequate funding, failure to obtain regulatory approval, unforeseen research issues, lack of consumer acceptance, competition, sluggish product development, and inadequate sales and marketing. The failure by us to meet any of these conditions would have a materially adverse effect upon us and may force us to reduce or curtail operations. No assurance can be given that we can or will ever operate profitably.
| 25 |
The amount of financing we require will depend on a number of factors, many of which are beyond our control. Our results of operations, financial condition and stock price are likely to be adversely affected if our funding requirements increase or are otherwise greater than we expect.
Our future funding requirements will depend on many factors, including, but not limited to:
| · | the testing costs for our insulin pump product and other development activities conducted by us directly, and our ability to successfully conclude the studies and activities and achieve favorable results; |
| · | our ability to attract future strategic partners to pay for or share costs related to our product development efforts; |
| · | the costs and timing of seeking and obtaining regulatory clearance and approvals for our product candidate; |
| · | the costs of filing, prosecuting, maintaining and enforcing any patents and other intellectual property rights that we may have and defending against potential claims of infringement; |
| · | decisions to hire additional scientific, engineering or administrative personnel or consultants; |
| · | our ability to manage administrative and other costs of our operations; and |
| · | the presence or absence of adverse developments in our research program. |
If any of these factors cause our funding needs to be greater than expected, our operations, financial condition, ability to continue operations and stock price may be adversely affected.
Our future cash requirements may differ significantly from our current estimates.
Our cash requirements may differ significantly from our estimates from time to time, depending on a number of factors, including:
| · | the costs and results of our clinical studies regarding our insulin pump product candidate; |
| · | the time and costs involved in obtaining regulatory clearance and approvals; |
| · | whether we are able to obtain funding under future licensing agreements, strategic partnerships, or other collaborative relationships, if any; |
| · | the costs of compliance with laws, regulations, or judicial decisions applicable to us; and |
| · | the costs of general and administrative infrastructure required to manage our business and protect corporate assets and shareholder interests. |
If we fail to raise additional funds on a timely basis, we will need to scale back our business plans, which would adversely affect our business, financial condition, and stock price, and we may even be forced to discontinue our operations and liquidate our assets.
Technological breakthroughs in diabetes monitoring, treatment or prevention could render our insulin pump obsolete.
The diabetes treatment market is subject to rapid technological change and product innovation. Our insulin pump is based on our proprietary technology, but a number of companies, medical researchers and existing pharmaceutical companies are pursuing new delivery devices, delivery technologies, sensing technologies, procedures, drugs and other therapeutics for the monitoring, treatment and/or prevention of insulin-dependent diabetes. Any technological breakthroughs in diabetes monitoring, treatment or prevention could render our insulin pump obsolete, which, since our insulin pump is our only product, would have a material adverse effect on our business, financial condition and results of operations and could result in shareholders losing their entire investment.
Any failure to attract and retain skilled directors, executives, employees and consultants could impair our product development and commercialization activities.
Our business depends on the skills, performance, and dedication of our directors, executive officers and key engineering, scientific and technical advisors. Many of our current engineering or scientific advisors are independent contractors and are either self-employed or employed by other organizations. As a result, they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, which may affect their ability to provide services to us in a timely manner. We will need to recruit additional directors, executive management employees, and advisers, particularly engineering, scientific and technical personnel, which will require additional financial resources. In addition, there is currently intense competition for skilled directors, executives and employees with relevant engineering, scientific and technical expertise, and this competition is likely to continue. If we are unable to attract and retain persons with sufficient engineering, scientific, technical and managerial experience, we may be forced to limit or delay our product development activities or may experience difficulties in successfully conducting our business, which would adversely affect our operations and financial condition.
| 26 |
We have limited internal research and development personnel, making us dependent on consulting relationships.
We consider research and development to be an important part of the process of designing, developing, obtaining regulatory required approvals and the eventual commercialization of our insulin pump. We continue to incur increased research and development expenditures, which are attributable to effort and expenses incurred in designing and developing our innovative insulin pump. We expect to continue to incur substantial costs related to research and development.
We will need to outsource and rely on third parties for various aspects relating to the development, manufacture, sales and marketing of our insulin pump as well as in connection with assisting us in the preparation and filing of our FDA submission, and our future success will be dependent on the timeliness and effectiveness of the efforts of these third parties.
We are dependent on consultants for important aspects of our product development strategy. We do not have the required financial resources and personnel to carry out independently the development of our product candidate, and do not have the capability or resources to manufacture, market or sell our current product candidate. As a result, we contract with and rely on third parties for important functions, including in connection with the development and finalization of our insulin pump, the preparation and filing of our FDA submission and eventual manufacturing and commercialization of our product candidate. We have recently entered into several agreements with third parties for such services. If problems develop in our relationships with third parties, or if such parties fail to perform as expected, it could lead to delays or lack of progress in obtaining FDA clearance, significant cost increases, changes in our strategies, and even failure of our product initiatives.
We may not be able to identify, negotiate and maintain the strategic alliances necessary to develop and commercialize our products and technologies, and we will be dependent on our corporate partners if we do.
We may seek to enter into a strategic alliance with a diabetes related service providing company for the further development and approval of our insulin pump product candidate. At this time, we have not entered into any such strategic alliance. Strategic alliances, if entered into, could potentially provide us with additional funds, expertise, access, and other resources in exchange for exclusive or non-exclusive licenses or other rights to the product that we are currently developing or a product we may explore in the future. We cannot give any assurance that we will be able to enter into strategic relationships with a diabetes related service providing company or others in the near future or at all. In addition, we cannot assure you that any agreements that we do reach will achieve our goals or be on terms that prove to be economically beneficial to us. When we do enter into strategic or contractual relationships, we become dependent on the successful performance of our partners or counter-parties. If they fail to perform as expected, such failure could adversely affect our financial condition, lead to increases in our capital needs, or hinder or delay our development efforts.
We may not receive the necessary regulatory clearance or approvals for our insulin pump, and failure to timely obtain necessary clearances and/or approvals could harm our then operations, including our ability to commercialize our product candidate.
Before we can market a new medical device, such as our insulin pump, we must first receive clearance under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or the FDCA. In the 510(k) clearance process, before a device may be marketed, the FDA must determine that such proposed device is “substantially equivalent” to a legally-marketed “predicate” device, which includes a device that has been previously cleared through the 510(k) process, a device that was legally marketed prior to May 28, 1976 (pre-amendments device), a device that was originally on the U.S. market pursuant to an approved pre-market approval (PMA) and later down-classified, or a 510(k)-exempt device. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device.
Certain future modifications made to our product, which we currently expect to be cleared through 510(k), may require a new 510(k) clearance. The 510(k) clearance process can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process usually takes from three to 12 months, but can last longer. Despite the time, effort and cost, a device may not be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory authorizations could harm our business, including our ability to commercialize our product candidate and our shareholders could lose their entire investment. Furthermore, even if we are granted the required regulatory authorizations, such authorizations may be subject to significant limitations on the indicated uses for the device, which may limit the market for our product candidate.
| 27 |
If the FDA requires us to go through a lengthier, more rigorous examination for our product candidate than we had expected, product introductions or modifications could be delayed or canceled, which could adversely affect our ability to grow our business.
The FDA can delay, limit or deny clearance or approval for our insulin pump medical device for many reasons, including:
| · | our inability to demonstrate to the satisfaction of the FDA that our product candidate is substantially equivalent to the proposed predicate device; |
| · | the disagreement of the FDA with the design or implementation of our performance testing protocols or the interpretation of data from our performance testing; |
| · | the data from performance testing may be insufficient to support a determination of substantial equivalence or that our device meets required special controls or applicable performance standards; |
| · | our inability to demonstrate that the benefits of our pump outweigh the risks; |
| · | the manufacturing process or facilities we intend to use may not meet applicable requirements; and |
| · | the potential for approval policies or regulations of the FDA to change significantly in a manner rendering our data or regulatory filings insufficient for clearance or approval. |
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our product candidate or impact our ability to modify our product candidate after clearance on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain clearance for our pump, increase the costs of compliance or restrict our ability to maintain our current approval.
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of data supporting the safety and performance of the product candidates during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence.
Obtaining marketing authorization in the United States will not obviate the need to obtain marketing authorization in other jurisdictions We must obtain approval from foreign regulatory authorities before we can market and sell any of our product candidates in countries outside the United States. We will incur additional costs in seeking such approvals, may experience delays in obtaining such approvals and cannot be certain that such approvals will be granted.
The development, manufacture, and marketing of our product candidates outside the United States is subject to government regulation. In most foreign countries, we must complete rigorous pre-clinical testing and extensive human clinical trials that demonstrate the safety and efficacy of a product in order to apply for regulatory approval to market the product. If foreign regulatory authorities grant regulatory approval of a product, the approval may be limited to specific indications or limited with respect to its distribution. Expanded or additional indications for approved devices may not be approved, which could limit our potential revenues. Foreign regulatory authorities may refuse to grant any approval. Consequently, even if we believe that pre-clinical and clinical data are sufficient to support regulatory approval for our products, foreign regulatory authorities may not ultimately grant approval for commercial sale in any jurisdiction. If our product candidates are not approved in such jurisdictions, our ability to generate revenues will be limited and our business will be adversely affected.
Our competitors may develop products that are more effective, safer and less expensive than ours.
Existing insulin pumps are expensive, with the more popular models having purchase prices exceeding $4,000 for individuals without health insurance and often require significant patient copays. Others have daily use costs that exceed the reimbursement rates of many health insurance plans, forcing some users to spend thousands of dollars a year in copays. We believe this makes insurers hesitant to pay for any pumps and places pumps out of reach for many patients whom cannot afford such out of pocket expenses.
| 28 |
We are engaged in the diabetes treatment sector of the healthcare marketplace, which is intensely competitive. There are current products that are quite effective at addressing the effects of diabetes, and we expect that new developments by other companies and academic institutions in the areas of diabetes treatment will continue. If approved for marketing by the FDA, depending on the approved clinical indication, our product will be competing with existing and future products related to treatments for diabetes.
Our competitors may:
| · | develop product candidates and market products that increase the levels of safety or efficacy that our product candidates will need to show in order to obtain regulatory approval; |
| · | develop product candidates and market products that are less expensive or more effective than ours; |
| · | commercialize competing products before we can launch any products we are working to develop; |
| · | hold or obtain proprietary rights that could prevent us from commercializing our products; or |
| · | introduce therapies or market medical products that render our potential product candidates obsolete. |
We expect to compete against large medical device companies, such as Medtronic, Inc., Tandem Diabetes Care, Inc. and Insulet Corporation and smaller companies that are collaborating with larger medical device companies, new companies, academic institutions, government agencies and other public and private research organizations. These competitors, in nearly all cases, produce similar products relative to the treatment of diabetes that have substantially greater financial resources than we do. Our competitors also have significantly greater experience in:
| · | developing medical device and other product candidates; |
| · | undertaking testing and clinical studies; |
| · | building relationships with key customers and opinion-leading physicians; |
| · | obtaining and maintaining FDA and other regulatory approvals; |
| · | formulating and manufacturing medical devices; |
| · | launching, marketing and selling medical devices; and |
| · | providing management oversight for all of the above-listed operational functions. |
If we fail to achieve superiority over other existing or newly developed products, we may be unable to obtain regulatory approval. If our competitors’ market medical devices that are less expensive, safer or more effective than our insulin pump, or that gain or maintain greater market acceptance, we may not be able to compete effectively. See “Our Business – Competition” below.
We expect to rely on third-party manufacturers and will be dependent on their quality and effectiveness.
Our insulin pump requires precise, high-quality manufacturing. The failure to achieve and maintain high manufacturing standards, including failure to detect or control anticipated or unanticipated manufacturing errors or the frequent occurrence of such errors, could result in patient injury or death, discontinuance or delay of ongoing or planned clinical studies, delays or failures in product testing or delivery, cost overruns, product recalls or withdrawals and other problems that could seriously hurt our business. Contract medical device manufacturers often encounter difficulties involving production yields, quality control and quality assurance and shortages of qualified personnel. These manufacturers are subject to stringent regulatory requirements, including the FDA’s current good-manufacturing-practices regulations. If our contract manufacturers fail to maintain ongoing compliance at any time, the production of our product could be interrupted, resulting in delays or discontinuance of our clinical studies, additional costs and loss of potential revenues.
We may not be able to successfully scale-up manufacturing of our product candidate in sufficient quality and quantity, which would delay or prevent us from developing our product candidate and commercializing our product candidate.
In order to conduct larger-scale or late-stage clinical studies and for commercialization of our insulin pump, if 510(k) clearance is granted, we will need to manufacture it in larger quantities. We may not be able to successfully increase the manufacturing capacity for our product in a timely or cost-effective manner, or at all. In addition, quality issues may arise during scale-up activities. If we are unable to successfully scale up the manufacture of our product in sufficient quality and quantity, the development and testing of our product candidate and regulatory approval or commercial launch may be delayed, which could significantly harm our business.
| 29 |
We may be subject to potential product liability and other claims that could materially impact our business and financial condition.
The development and sale of our insulin pump exposes us to the risk of significant damages from product liability and other claims, and the use of our product candidate in clinical studies may result in adverse effects. We cannot predict all the possible harms or adverse effects that may result. We maintain a modest amount of product liability insurance to provide some protection from claims. Nonetheless, we may not have sufficient resources to pay for any liabilities resulting from a personal injury or other claim, even if it is partially covered by insurance. In addition to the possibility of direct claims, we may be required to indemnify third parties against damages and other liabilities arising out of our development, commercialization and other business activities, which would increase our liability exposure. If third parties that have agreed to indemnify us fail to do so, we may be held responsible for those damages and other liabilities as well.
Legislative, regulatory, or medical cost reimbursement changes may adversely impact our business.
New laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions, that relate to the health care system in the U.S. and in other jurisdictions may change the nature of and regulatory requirements relating to innovations in medical devices, testing and regulatory approvals, limit or eliminate payments for medical procedures and treatments, or subject the pricing of medical devices to government control. In addition, third-party payors in the U.S. are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new products. Consequently, significant uncertainty exists as to the reimbursement status of newly approved health care products. Significant changes in the health care system in the U.S. or elsewhere, including changes resulting from adverse trends in third-party reimbursement programs, could have a material adverse effect on our projected future operating results and our ability to raise capital, commercialize products, and remain in business.
We are subject to extensive regulation by the FDA, which could restrict the sales and marketing of our insulin pump and could cause us to incur significant costs.
Our insulin pump is subject to extensive regulation by the FDA. These regulations relate to manufacturing, labeling, sale, promotion, distribution and shipping. Before a new medical device, or a new intended use of a legally marketed device, can be marketed in the United States, it must be cleared or approved by FDA through the applicable premarket review process (510(k), PMA, or de novo classification), unless an exemption applies. If we receive 510(k) clearance for our insulin pump, we may be required to obtain a new 510(k) clearance for significant post-market modifications to the pump. Each premarket submission and review process can be expensive and lengthy, and entail significant user fees, unless exempt.
Medical devices may be marketed only for the indications for which they are approved or cleared. Further, 510(k) clearances can be revoked if safety or effectiveness problems develop once the device is on the market.
The current regulatory requirements to which we are subject may change in the future in a way that adversely affects us. If we fail to comply with present or future regulatory requirements that are applicable to us, we may be subject to enforcement action by the FDA, which may include any of the following sanctions:
| · | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| · | customer notification, or orders for repair, replacement or refunds |
| · | voluntary or mandatory recall or seizure of our current or future products; |
| · | administrative detention by the FDA of medical devices believed to be adulterated or misbranded; |
| · | imposing operating restrictions, suspension or shutdown of production; |
| · | refusing our requests for 510(k) clearance, PMA, or de novo classification any new products, new intended uses or modifications to our insulin pump; |
| · | rescinding 510(k) clearance that has already been granted; and |
| · | criminal prosecution. |
The occurrence of any of these events would have a material adverse effect on our business, financial condition and results of operations and could result in shareholders losing their entire investment.
| 30 |
Although our system does not presently require clinical trials to apply to the FDA for clearance and even if a clinical trial is completed, the results of our clinical testing may not demonstrate the safety and efficacy of the device or may be equivocal or otherwise not be sufficient for us to obtain approval of our product candidate.
Clinical trials are almost always required to support a PMA application and may also be required to support 510(k) submissions although at this time ours does not require a PMA. If the device presents a “significant risk” to human health as defined by the FDA, the FDA requires the study sponsor to submit an investigational device exemption (“IDE”) application and obtain IDE approval prior to commencing human clinical trials. The IDE must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. An IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA denies the application or notifies the sponsor that the investigation is on hold and may not begin until the sponsor provides supplemental information about the investigation that satisfies the agency’s concerns. The FDA may also notify the sponsor that the study is approved as proposed. If the FDA determines that there are deficiencies or other concerns with an IDE that require modification of the study, the FDA may permit a clinical trial to proceed under a conditional approval. Furthermore, the agency may withdraw approval of an IDE under certain circumstances. Clinical trials for a significant risk device may begin once an IDE is approved by the FDA and the appropriate Institutional Review Board (“IRB”) at each clinical trial site. If the product is deemed a “non-significant risk” device, IDE approval from the FDA would not be required, but the clinical trial would need to meet other requirements including IRB approval. Our clinical trials must be conducted in accordance with FDA regulations and federal and state regulations concerning human subject protection, including informed consent and healthcare privacy. A clinical trial may be suspended by the FDA or at a specific site by the relevant IRB at any time for various reasons, including a determination that the risks to the trial participants outweigh the benefits of participation in the clinical trial. Even if a clinical trial is completed, the results of our clinical testing may not demonstrate the safety and efficacy of the device or may be equivocal or otherwise not be sufficient for us to obtain approval of our product.
Our success depends substantially upon our ability to obtain and maintain intellectual property protection relating to our product and research technologies.
We have applied to the U.S. Patent and Trademark Office for patents on our proprietary fluid movement technology and the configuration of our insulin pump. There is no assurance that these patents will be issued, and no assurance that they will prevent other companies from competing with us. We will continue to attempt to patent our innovations as appropriate to help ensure a sustainable competitive advantage.
Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering health care product inventions, our ability to enforce our existing patents and to obtain and enforce patents that may issue from any pending or future patent applications is uncertain and involves complex legal, scientific and factual questions. To date, no consistent policy has emerged regarding the breadth of claims allowed in medical device patents. Thus, we cannot be sure that any patents will issue from any pending or future patent applications owned by or licensed to us. Even if patents do issue, we cannot be sure that the claims of these patents will be held valid or enforceable by a court of law, will provide us with any significant protection against competing products, or will afford us a commercial advantage over competitive products. If, at some point in the future, one or more products resulting from our product candidates is approved for sale by the FDA and we do not have adequate intellectual property protection for those products, competitors could duplicate them for approval and sale in the United States without repeating the extensive testing required of us to obtain FDA approval.
If we are sued for infringing on third-party intellectual property rights, it will be costly and time-consuming, and an unfavorable outcome would have a significant adverse effect on our business.
Our ability to commercialize our product candidate depends on our ability to use, manufacture and sell our product candidate without infringing the patents or other proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the diabetes medical device area. There may be existing patents, unknown to us, on which our activities with our insulin pump candidate could infringe.
If a third-party claims that our actions infringe on its patents or other proprietary rights, we could face a number of issues that could seriously harm our competitive position, including, but not limited to:
| · | infringement and other intellectual property claims that, even if meritless, can be costly and time-consuming, delay the regulatory approval process and divert management’s attention from our core business operations; |
| · | substantial damages for infringement, including consequential damages for lost of profits or market share, if a court determines that our products or technologies infringe on a third party’s patent or other proprietary rights; |
| 31 |
| · | a court prohibiting us from selling or licensing our products or technologies unless the holder licenses the patent or other proprietary rights to us, which it is not required to do; and |
| · | even if a license is available from a holder, we may have to pay substantial royalties or grant cross-licenses to our patents or other proprietary rights. |
If any of these events occur, it could significantly harm our operations and financial condition and negatively affect our stock price.
If we are unable to protect the confidentiality of our proprietary information, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely on our unpatented technology, trade secrets and know-how. We generally seek to protect this information by confidentiality, non-disclosure and assignment of invention agreements with our officers, employees, contractors and other service providers and with parties with which we do business. These agreements may be breached, which breach may result in the misappropriation of such information, and we may not have adequate remedies for any such breach. We cannot be certain that the steps we have taken will prevent unauthorized use or reverse engineering of our technology.
Moreover, our trade secrets may be disclosed to or otherwise become known or be independently developed by competitors. To the extent that our officers, employees, contractors, other service providers, or other third parties with whom we do business use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. If, for any of the above reasons, our intellectual property is disclosed or misappropriated, it would harm our ability to protect our rights and have a material adverse effect on our business, financial condition, and results of operations.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to gain and maintain a competitive advantage. The following examples are illustrative:
| · | others may be able to make devices that are similar to our insulin pump but that are not covered by the claims of the patents that we own; |
| · | we or any collaborators might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own; |
| · | we might not have been the first to file patent applications covering certain of our inventions; |
| · | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
| · | it is possible that our pending patent applications will not lead to issued patents; |
| · | issued patents that we own may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges; |
| · | our competitors might conduct research and development activities in the U.S. and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights, and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; and |
| · | we may not develop additional proprietary technologies that are patentable. |
Healthcare reform laws could adversely affect our product candidate and financial condition.
In the United States, there have been, and continue to be, a number of legislative initiatives to contain healthcare costs. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (ACA), was enacted in the United States, which made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. Among other ways in which it may affect our business, the ACA implemented payment system reforms, including a national pilot program on payment bundling to encourage hospitals, physicians, and other providers to improve the coordination, quality, and efficiency of certain healthcare services through bundled payment models and expanded the eligibility criteria for Medicaid programs.
| 32 |
Since its enactment, there have been judicial, executive, and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how other healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal, or replace the ACA will impact the ACA or our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, reduced Medicare payments to providers by 2% per fiscal year, effective on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030, with the exception of a temporary suspension implemented under various COVID-19 relief legislation from May 1, 2020 through the end of 2021, unless additional Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Further, the Bipartisan Budget Act of 2018 among other things, amended the Medicare statute, effective January 1, 2019, to reduce the coverage gap in most Medicare drug plans, commonly known as the “donut hole,” by raising the manufacturer discount under the Medicare Part D coverage gap discount program to 70%. It is unclear how the ACA and its implementation, as well as efforts to repeal or replace, or invalidate, the ACA, or portions thereof, will affect our insulin pump or our business. Additional legislative changes, regulatory changes, and judicial challenges related to the ACA remain possible. It is possible that the ACA, as currently enacted or as it may be amended in the future, and other healthcare reform measures that may be adopted in the future, could have an adverse effect on our industry generally and on our ability to commercialize our insulin pump and achieve profitability.
Even if we are able to obtain all regulatory approvals and have completed all other steps needed to be taken to commercialize our insulin pump, if we or any contract manufacturers we select fails to comply with the FDA’s quality system regulations, the manufacturing and distribution of our product candidate could be interrupted, and our product sales and operating results could suffer.
A material step in the process of the commercialization of our product candidate will involve selecting a manufacturer or manufacturers for our pump. We and any future contract manufacturers of our insulin pump will be required to comply with the FDA’s quality system regulations, which impose a complex regulatory framework that covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of medical devices. The FDA enforces its quality system regulations through periodic unannounced inspections. We cannot assure you that, in the future, any manufacturing facilities owned by us or any contract manufacturer will pass any quality system inspection. In the event that our or any contract manufacturer’s facilities fails a quality system inspection, the manufacturing or distribution of our product candidate could be interrupted and our operations disrupted. Failure to take adequate and timely corrective action in response to an adverse quality system inspection could force a suspension or shutdown of any packaging and labeling operations or then manufacturing operations of any contract manufacturers, or a recall of our insulin pump. If any of these events were to occur, we at such time would not be able to provide our customers with the quantity of insulin pumps that they require on a timely basis, our reputation could be harmed and we could lose any customers we then have, any or all of which could have a material adverse effect on our business, financial condition and results of operations.
We may undertake infringement or other legal proceedings against third parties, causing us to spend substantial resources on litigation and exposing our own intellectual property portfolio to challenge.
We may come to believe that third parties are infringing on our patents or other proprietary rights. To prevent infringement or unauthorized use, we may need to file infringement and/or misappropriation suits, which are very expensive and time-consuming, could result in meritorious counterclaims against us and would distract management’s attention. Also, in an infringement or misappropriation proceeding, a court may decide that one or more of our patents is invalid, unenforceable, or both, in which case third parties may be able to use our technology without paying license fees or royalties. Even if the validity of our patents is upheld, a court may refuse to stop the other party from using the technology at issue on the grounds that the other party’s activities are not covered by our patents. See “Our Business – Patents,” below.
| 33 |
We may become involved in disputes with our present or future contract partners over intellectual property ownership or other matters, which would have a significant effect on our business.
Inventions discovered in the course of performance of contracts with third parties or contractors may become jointly owned by such third party contractors and us, in some cases, and the exclusive property of one of us, in other cases. Under some circumstances, it may be difficult to determine who owns a particular invention or whether it is jointly owned, and disputes could arise regarding ownership or use of those inventions or jointly developed improvements thereto. Other disputes may also arise relating to the performance or alleged breach of our agreements with third parties. Any disputes could be costly and time-consuming, and an unfavorable outcome could have a significant adverse effect on our business.
Assuming our insulin pump receives FDA clearance or approval, our insulin pump will still be subject to recalls, which would harm our reputation, business operations and financial results.
Even assuming we obtain FDA approval or clearance with regard to our insulin pump, the FDA has the authority to require the recall of our pump if we commence manufacturing of our insulin pump and we or any contract manufacturers we retain fail to comply with relevant regulations pertaining to manufacturing practices, labeling, advertising or promotional activities, or if new information is obtained concerning the safety or efficacy of the device. A government-mandated recall could occur if the FDA finds that there is a reasonable probability that our device would cause serious, adverse health consequences or death. A voluntary recall by us could occur as a result of manufacturing defects, labeling deficiencies, packaging defects or other failures to comply with applicable regulations. Any recall would divert management’s attention and financial resources and harm our reputation with customers. A recall involving our insulin pump would be particularly harmful to our business, financial condition and results of operations because it is currently our only product.
Any disruption and/or instability in economic conditions and capital markets could adversely affect our ability to access the capital markets, and thus adversely affect our business and liquidity.
Negative economic conditions and issues with regard to the financial markets, could have a negative impact on our ability to access the capital markets, and thus have a negative impact on our then operations and liquidity. A general shortage of liquidity and credit combined with the substantial losses in worldwide equity markets could lead to an extended worldwide recession in the future. If such occurred, we would face significant challenges if conditions in the capital markets did not improve. Our ability to access the capital markets under such circumstances could be severely restricted at a time when we need to access such markets, which could have a negative impact on our business plans. Even if we are able to raise capital under such circumstances, it may not be at a price or on terms that are favorable to us. We cannot predict the occurrence of future disruptions or how long such negative conditions might continue.
| 34 |
Because our current insulin pump prototype is still in the development stage, it does not have reimbursement and is not approved for insurance coverage. If in the future we are approved for and are otherwise able to commercialize our insulin pump, but are unable to obtain adequate reimbursement or insurance coverage for such product from third-party payors, we will be unable to generate significant revenue.
Because our current insulin pump prototype is still in the development stage, it does not have reimbursement and is not approved for insurance coverage. The future availability of insurance coverage and reimbursement for newly approved medical devices is highly uncertain. In the United States, patients using insulin pumps are generally reimbursed for all or part of the product cost by Medicare or other third-party payors. Any future commercial success of our insulin pump will be substantially dependent on whether third-party coverage and reimbursement is available for future customers. Medicare, Medicaid, health maintenance organizations and other third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement of new medical devices, and, as a result, they may not cover or provide adequate reimbursement for our insulin pump, assuming we are able to fully develop and obtain all regulatory approval to market it in the United States. In addition, in certain countries, no uniform policy of coverage and reimbursement for medical device products and services exists among third-party payors. Therefore, coverage and reimbursement for medical device products and services can differ significantly from payor to payor. In addition, payors continually review new technologies for possible coverage and can, without notice, deny coverage for these new products and procedures. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained, or maintained if obtained. Reimbursement systems in international markets vary significantly by country and by region within some countries, and reimbursement approvals must be obtained on a country-by-country basis. In many international markets, a product must be approved for reimbursement before it can be approved for sale in that country. Further, many international markets have government-managed healthcare systems that control reimbursement for new devices and procedures. Accordingly, unless government and other third-party payors provide coverage and reimbursement for our insulin pump, patients may not use it, which would cause investors to lose their entire investment.
We are subject to the oversight of the SEC and other regulatory agencies. Investigations by those agencies could divert management’s focus and could have a material adverse effect on our reputation and financial condition.
We are subject to the regulation and oversight of the SEC and state regulatory agencies, in addition to the FDA. As a result, we may face legal or administrative proceedings by these agencies. We are unable to predict the effect of any investigations on our business, financial condition or reputation. In addition, publicity surrounding any investigation, even if ultimately resolved in our favor, could have a material adverse effect on our business.
We are a “smaller reporting company” and, as a result of the reduced disclosure and governance requirements applicable to smaller reporting companies, our Common Stock may be less attractive to investors.
We are a “smaller reporting company,” and are subject to lesser disclosure obligations in our SEC filings compared to other issuers. Specifically, “smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings, are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. Decreased disclosures in our SEC filings due to our status as a “smaller reporting company” may make it harder for investors to analyze our operating results and financial prospects.
We do not expect any cash dividends to be paid on our shares of Common Stock for the foreseeable future.
We have never declared or paid a cash dividend and we do not anticipate declaring or paying dividends on our Common Stock for the foreseeable future. We expect to use future financing proceeds and earnings, if any, to fund operating expenses. Consequently, shareholders’ only opportunity to achieve a return on their investment is if the price of our stock appreciates and they sell their shares at a profit. We cannot assure shareholders of a positive return on their investment when they sell their shares or that shareholders will not lose the entire amount of their investment.
| 35 |
If the beneficial ownership of our Common Stock continues to be highly concentrated, it may prevent our shareholders from influencing significant corporate decisions.
As of March 31, 2022, our executive officers, directors and certain persons who may be deemed affiliates beneficially own in excess of 50.1% of our issued and outstanding Common Stock. As a result, such persons may exercise substantial influence over the outcome of corporate actions requiring shareholder approval including, without limitation, the election of directors, certain mergers, consolidations and sales of all or substantially all of our assets or any other significant corporate transactions. Such persons may also vote against a change of control, even if such a change of control would benefit our other shareholders.
Sale of our Common Stock by shareholders could encourage short sales by third parties, which could contribute to the further decline of our stock price.
The significant downward pressure on the price of our Common Stock that would be caused by the sale of material amounts of our Common Stock could encourage short sales by third parties. Such an event could place further downward pressure on the price of our Common Stock.
We are an emerging growth company, and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the JOBS Act). For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements and exemptions from the requirements of holding nonbinding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. We will remain an emerging growth company until the earlier of (i) the last day of the fiscal year (a) following the fifth anniversary of the completion of the first sale of shares covered by this prospectus, (b) in which we have total annual gross revenue of at least $1.07 billion or (c) in which we are deemed to be a large accelerated filer, which requires the market value of our common stock that is held by non-affiliates to exceed $700.0 million as of the prior September 30th, and (ii) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Future sales of our securities could adversely affect the market price of our Common Stock and our future capital-raising activities could involve the issuance of equity securities, which would dilute your investment and could result in a decline in the trading price of our Common Stock.
We may sell securities in the public or private equity markets at prices per share below the current market price of our Common Stock, even if we do not have an immediate need for additional capital at that time. Sales of substantial amounts of shares of our Common Stock, or the perception that such sales could occur, could adversely affect the prevailing market price of our shares and our ability to raise capital. We may issue additional shares of Common Stock in future financing transactions or as incentive compensation for our executive management and other key personnel, consultants and advisors. Issuing any equity securities would be dilutive to the equity interests represented by our then-outstanding shares of Common Stock. Moreover, sales of substantial amounts of shares in the public market, or the perception that such sales could occur, may adversely affect the prevailing market price of our Common Stock and make it more difficult for us to raise additional capital.
Our articles of incorporation allows for our board of directors to create new series of preferred stock without further approval by our shareholders, which could adversely affect the rights of the holders of our Common Stock.
Our board of directors has the authority to fix and determine the relative rights and preferences of preferred stock. Currently, our board of directors has the authority to designate and issue up to 5,000,000 shares of our preferred stock without further shareholder approval. In the future, our board of directors could authorize the issuance of one or more series of preferred stock that would grant to holders, among other rights, the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of Common Stock and the right to the redemption of our preferred shares acquired by such persons, together with a premium, prior to the redemption of our Common Stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our Common Stock or that is convertible into our Common Stock, which could decrease the relative voting power of our Common Stock or result in dilution to our existing shareholders.
| 36 |
If we fail to establish and maintain an effective system of internal controls, we may not be able to report our financial results accurately or prevent fraud. Any inability to report and file our financial results accurately and timely could harm our reputation and adversely affect the trading price of our Common Stock.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed. If we are unable to maintain effective internal controls, we may not have adequate, accurate or timely financial information, and we may be unable to meet our reporting obligations as a public company, including the requirements of the Sarbanes-Oxley Act of 2002 (the Sarbanes-Oxley Act). In addition, we may be unable to accurately report our financial results in future periods, or report them within the timeframes required by the requirements of the SEC or the Sarbanes-Oxley Act. Failure to comply with the Sarbanes-Oxley Act, when and as applicable, could also potentially subject us to sanctions or investigations by the SEC or other regulatory authorities. Any failure to maintain or implement required new or improved controls, or any difficulties we encounter in their implementation, could result in identification of additional material weaknesses or significant deficiencies, cause us to fail to meet our reporting obligations or result in material misstatements in our financial statements.
Furthermore, Section 404 of the Sarbanes-Oxley Act and related regulations require our management to evaluate the effectiveness of our internal control over financial reporting as of the end of each fiscal year. Based on its evaluation, our management concluded that our internal controls over financial reporting were effective as of March 31, 2022. We cannot provide assurance that, in the future, a material weakness or significant deficiency will not exist or otherwise be discovered. If that were to happen, it could harm our operating results and cause shareholders to lose confidence in our reported financial information. Any such loss of confidence would have a negative effect on the trading price of our securities.
Our board of directors is able to adopt recapitalizations through forward or reverse splits of our outstanding shares of Common Stock without shareholder approval.
Pursuant to our amended and restated articles of incorporation, our board of directors has the power, without obtaining shareholder approval, to effectuate recapitalizations of us through forward or reverse splits of our outstanding Common Stock. As a result of such provision, our board of directors can implement recapitalizations of us by effectuating a forward or reverse stock split of our outstanding Common Stock, which would increase or decrease each of our shareholder’s number of shares owned, and our shareholders will have no right to approve or disapprove any such action even if such actions have a material adverse effect on them.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None
ITEM 2: PROPERTIES
Our principal administrative and research and development functions are located in a leased facility in San Diego, California. We currently occupy approximately 7,300 square feet of space in the San Diego facility, and the lease extends through June 2023. We believe that our existing facility is adequate to meet our current needs.
ITEM 3: LEGAL PROCEEDINGS
We are not a party to any pending legal proceeding. To the knowledge of our management, no federal, state or local governmental agency is presently contemplating any proceeding against us. No director, executive officer or affiliate of ours or owner of record or beneficially of more than five percent of our common stock is a party adverse to us or has a material interest adverse to us in any proceeding.
ITEM 4: MINE SAFETY DISCLOSURES
Not applicable
| 37 |
PART II
ITEM 5: MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock is currently listed on the Nasdaq Capital Market under the symbol “MODD.”
Holders of Record
On May 31, 2022, we had approximately 80 stockholders of record. The actual number of stockholders is greater than this number of stockholders of record and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of stockholders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We have never declared or paid any cash dividend on our capital stock. We do not anticipate paying any cash dividends in the foreseeable future and we intend to retain all of our earnings, if any, to finance our growth and operations and to fund the expansion of our business. Payment of any dividends will be made in the discretion of our board of directors, after its taking into account various factors, including our financial condition, operating results, current and anticipated cash needs and plans for expansion. Any dividends that may be declared or paid on our common stock, must also be paid in the same consideration or manner, as the case may be, on our shares of preferred stock, if any.
Recent Sales of Unregistered Securities
Set forth below is information regarding shares of Common Stock, convertible notes and warrants issued, and options granted, by us within the past three years that were not registered under the Securities Act. Also included is the consideration, if any, received by us for such shares, convertible notes, warrants and options, and information relating to the section of the Securities Act, or rule of the Securities and Exchange Commission, under which exemption from registration was claimed.
Director Compensation
On March 31, 2022, we issued 15,250 shares of our Common Stock to non-employee members of our board of directors for service as directors in accordance with our Outside Director Compensation Plan (the “Director Plan”). On December 31, 2021, we issued 5,775 shares of our Common Stock to non-employee members of our board of directors for service as directors in accordance with the Director Plan. On September 30, 2021, we issued 3,636 shares of our Common Stock to non-employee members of our board of directors for service as directors in accordance with the Director Plan. On June 30, 2021, we issued 1,836 shares of our Common Stock to non-employee members of our board of directors for service as directors in accordance with our Outside Director Compensation Plan the Director Plan.
Service Providers
On March 23, 2022 we issued 45,000 shares of our Common Stock to a service provider. On January 5, 2022, we issued 16,666 shares of our Common Stock to service providers. In 2021, we issued a total of 52,834 shares of our Common Stock to five service providers in exchange for services rendered. In 2019, we issued 10,000 shares of our Common Stock for cash to a service provider.
Officer Purchases of Common Stock
On October 28, 2021, we sold to two of our executive officers a total of 30,865 shares of our Common Stock at a purchase price of $8.10 per share, which resulted in gross proceeds to us of approximately $250,000.
2021 Placement
Between February and May 2021, we issued to accredited investors in a private placement $6,610,550 aggregate principal amount of our 12% unsecured convertible promissory notes, due 12 months from each respective issuance date, at par and warrants to purchase in the aggregate 767,796 shares of our Common Stock at an exercise price of $24.00 per share, exercisable for a 5-year period, as provided in such warrants.
| 38 |
2020 Placement
Between March and December 2020, we sold to accredited investors in a private placement a total of 320,796 shares of our Common Stock at a purchase price of $8.61 per share. The 2020 Placement resulted in gross proceeds to us of $2,762,054.
2018 Placement
Between November 2018 and March 2019, we sold to accredited investors in a private placement a total of 618,996 shares of our Common Stock at a purchase price of $6.75 per share, resulting in gross proceeds to us of $4,142,666.
The above sales of our securities were made pursuant to exemptions from registration pursuant to Section 4(2) and/or Rule 506 of Regulation D of the Securities Act. We made such determinations based upon representations by the purchasers of such securities including, without limitation, that such purchasers were “accredited investors” as defined in the Securities Act.
ITEM 6: RESERVED
ITEM 7: MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with the financial statements and related notes included in this Annual Report on Form 10-K. Management’s Discussion and Analysis of Financial Condition and Results of Operations may contains statements that are forward-looking. These statements are based on current expectations and assumptions that are subject to risk, uncertainties and other factors. These statements are often identified by the use of words such as “may,” “will,” “expect,” “believe,” “anticipate,” “intend,” “could,” “estimate,” or “continue,” and similar expressions or variations. Actual results could differ materially because of the factors discussed in Part I, Item 1A, These risks and uncertainties may cause actual results to differ materially from those discussed in the forward-looking statements.
Our fiscal year ends on March 31 of each calendar year. Each reference to a fiscal year in this Report, refers to the fiscal year ended March 31 of the calendar year indicated (for example, fiscal 2022 refers to the fiscal year ending March 31, 2022). Unless the context requires otherwise, references to “we,” “us,” “our,” and the “Company” refer to Modular Medical, Inc. and its consolidated subsidiary.
Company Overview
We are a development-stage medical device company focused on the design, development and commercialization of an innovative insulin pump using modernized technology to increase pump adoption in the diabetes marketplace. Through the creation of a novel two-part patch pump, our MODD1 product, we seek to fundamentally alter the trade-offs between cost and complexity and access to the higher standards of care that presently-available insulin pumps provide. By simplifying and streamlining the user experience from introduction, prescription, reimbursement, training and day-to-day use, we seek to expand the wearable insulin delivery device market beyond the highly motivated “super users” and expand the category into the mass market. The product seeks to serve both the type 1 and the rapidly growing, especially in terms of device adoption, type 2 diabetes markets.
Historically, we have financed our operations principally through private placements and public offerings of our common stock and sales of convertible promissory notes. Based on our current operating plan, we believe we have adequate cash for at least the next 12 months. Our long-term ability to continue as a going concern depends on our ability to raise additional capital, through the sale of equity or debt securities, to support our future operations. If we are unable to secure additional capital, we will be required to curtail our research and development initiatives and take additional measures to reduce costs. We have provided additional disclosure in Note 1 to the consolidated financial statements in Item 1 of this Report and under Liquidity below.
| 39 |
Impacts of COVID-19
The global outbreak of the coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization and a national emergency by the U.S. government in March 2020. This has negatively affected the U.S. and global economy, disrupted global supply chains, significantly restricted travel and transportation, resulted in mandated closures and orders to “shelter-in-place” and created significant disruption of the financial markets. The full extent of the COVID-19 impact on our operational and financial performance will depend on future developments, including, without limitation, the duration and spread of the pandemic and related actions taken by U.S. and foreign government agencies to prevent disease spread, all of which are uncertain, out of our control, and cannot be predicted.
In March 2020, Santa Diego County in California, where we are based, and the state of California issued “shelter-in-place” orders (the Orders). We complied with the Orders and minimized business activities at our San Diego facility since March 2020 until May 2021. During that time, we implemented a teleworking policy for our employees and contractors to reduce on-site activity at our facility. In May 2021, our employees and certain contractors returned to work in our office. We have and continue to experience longer lead times for certain components used to manufacture initial quantities of our products for our submission to the FDA. We remain diligent in continuing to identify and manage risks to our business given the changing uncertainties related to COVID-19. While we believe that our operations personnel are currently in a position to build an adequate supply of products for our FDA submission, we recognize that unpredictable events could create difficulties in the months ahead. We may not be able to address these difficulties in a timely manner, which could delay our submission to the FDA and negatively impact our business, results of operations, financial condition and cash flows.
The continued spread of COVID-19 has also led to disruption and volatility in the global capital markets. We were recently able to raise additional capital through equity offerings in February 2022 and May 2022, however, we will need to raise additional capital to commercialize our pump product candidate and support our operations in the future. We may be unable to access the capital markets, and additional capital may only be available to us on terms that could be significantly detrimental to our existing stockholders and to our business.
For additional information on risks that could impact our future results, please refer to “Risk Factors” in Part I, Item 1A of this Report.
Results of Operations
The following discussion should be read in conjunction with our consolidated financial statements and related notes included elsewhere in this Report.
Research and Development
| Years ended March 31, | Year-over-Year Change | |||||||||||||||
| 2022 | 2021 | 2022 to 2021 | ||||||||||||||
| Research and development | $ | 7,729,240 | $ | 4,083,303 | $ | 3,645,937 | 89.3 | % | ||||||||
Our research and development expenses include personnel, materials and supplies and other costs associated with the development of our insulin pump product candidate. We expense research and development costs as they are incurred.
Research and development, or R&D, expenses increased in fiscal 2022 compared with fiscal 2021 primarily due to increased consulting costs, engineering and operations personnel, stock compensation expense and materials and supplies expenditures. Our R&D employee headcount increased to 23 at March 31, 2022, from 17 at March 31, 2021. R&D expenses included stock-based compensation expenses of $758,938 and $390,045 for fiscal 2022 and fiscal 2021, respectively. We expect R&D expenses to continue to increase in fiscal 2023, as we complete the development of our pump product candidate, engage third parties to test our product and develop a low-volume manufacturing process.
| 40 |
General and Administrative
| Years ended March 31, | Year-over-Year Change | |||||||||||||||
| 2022 | 2021 | 2022 to 2021 | ||||||||||||||
| General and administrative | $ | 7,197,162 | $ | 3,253,412 | $ | 3,943,750 | 121.2 | % | ||||||||
General and administrative expenses consist primarily of personnel and related overhead costs for marketing, finance, human resources and general management.
General and administrative expenses, or G&A, increased in fiscal 2022 compared with fiscal 2021 primarily as a result of increased personnel and consulting costs, stock-based compensation expenses and professional services fees, primarily related to our financing activities, including our public offering that was completed in February 2022. G&A expenses included stock-based compensation expenses of $3,272,964 and $837,533 for fiscal 2022 and fiscal 2021, respectively. We expect G&A expenses to continue to increase in fiscal 2023, as we will increase headcount as we expand our organization to support our anticipated growth and prepare for the expected commencement of the commercialization of our product in late fiscal 2023.
Interest Expense
| Years ended March 31, | Year-over-Year Change | |||||||||||||||
| 2022 | 2021 | 2022 to 2021 | ||||||||||||||
| Interest expense | $ | 2,752,229 | $ | 39,791 | $ | 2,712,438 | 6,816.7 | % | ||||||||
Interest expense consisted of interest expense incurred from our convertible promissory notes, including amortization of debt issuance costs, and our promissory (bridge) note. We retired our outstanding debt in February 2022. See Notes 5 and 6 to the consolidated financial statements included in Item 8 of this Report for additional disclosure.
Liquidity
As a development-stage enterprise, we do not currently have revenues to generate cash flows to cover operating expenses. Since our inception, we have incurred operating losses and negative cash flows in each year due to costs incurred in connection with R&D activities and G&A expenses associated with our operations. For the years ended March 31, 2022 and 2021, we incurred net losses of approximately $18.6 million and $7.4 million, respectively. At March 31, 2022, we had a cash balance of $9.1 million and an accumulated deficit of approximately $34.6 million. In May 2022, we completed a registered direct offering of securities for net proceeds of approximately $7.4 million. Our operating needs include the planned costs to operate our business, including amounts required to fund research and development activities, including clinical studies, working capital and capital expenditures. Our future capital requirements and the adequacy of our available funds will depend on many factors, including, without limitation, our ability to successfully commercialize our product, competing technological and market developments, and the need to enter into collaborations with other companies or acquire other companies or technologies to enhance or complement our product offerings. If we are unable to secure additional capital timely, we will be required to curtail our research and development initiatives and take additional measures to reduce costs in order to conserve our cash. We believe that our cash will be sufficient to meet our working capital and capital expenditure needs for at least the next twelve months.
In fiscal 2022, we used $10,259,528 in operating activities, which primarily resulted from our net loss of $18,632,761 less changes to operating assets and liabilities of $420,600, as adjusted for non-cash charges and gains, which included stock-based compensation expenses of $4,031,902, amortization of debt issuance costs of $1,833,618, a loss on debt extinguishment of $1,321,450, accrued interest of $666,338, $395,950 for issuances of shares of common stock in exchange for services, and depreciation and amortization expenses of $117,490, partially offset by a gain on PPP note forgiveness of $368,780 and net changes in lease assets and liabilities of $45,610 and other immaterial adjustments. The changes in operating assets and liabilities primarily related to the timing of payments to vendors. In fiscal 2021, we used $5,908,662 in operating activities, which primarily resulted from our net loss of $7,377,976 and changes to operating assets and liabilities of $61,147, as adjusted for non-cash charges and gains, which included stock-based compensation expenses of $1,227,578, $68,880 for issuance of shares of common stock in exchange for services, $109,731 related to the lease right-of-use asset and liability and depreciation and amortization expenses of $111,015. Such changes in assets and liabilities primarily related to the timing of payments to vendors. Increased cash usage during fiscal 2022 was due to increased operating activities related to the development and eventual commercialization of our product.
| 41 |
In fiscal 2022, cash used in investing activities of $54,764 was for the purchase of property and equipment. We used $109,669 of cash to purchase property and equipment in fiscal 2021.
Cash provided by financing activities for fiscal 2022 totaled $17,922,199 and was attributable to $13,535,000 of net proceeds from a public offering of our common stock in February 2022, $4,137,199 of net proceeds from the issuance of convertible notes, $2,100,000 of net proceeds from issuance of a bridge promissory note, and $250,000 of proceeds from a private placement of common stock to officers, which were partially offset by the $2,100,00 repayment of the bridge promissory note. Our financing activities for fiscal 2021 totaled $4,364,662 and were attributable to $1,785,882 of net proceeds from the sale of shares of common stock in a private placement, $368,760 of proceeds from the PPP Note and $2,210,00 of gross proceeds from the issuance of our convertible notes in the quarter ended March 31, 2021.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (GAAP). Note 1 to the consolidated financial statements in Item 8 of this Report describes the significant accounting policies and methods used in the preparation of our consolidated financial statements. We have identified the accounting policies below as some of the more critical to our business and the understanding of our results of operations. These policies may involve estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. Although we believe our judgments and estimates are appropriate, actual future results may differ from our estimates, and if different assumptions or conditions were to prevail, the results could be materially different from our reported results.
Use of estimates
The preparation of financial statements in conformity with GAAP requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Estimates may include those pertaining to accruals, stock-based compensation and income taxes. Actual results could materially differ from those estimates.
Stock-based compensation
We recognize stock-based compensation for stock options granted to employees and non-employees on a straight-line basis over the requisite service period, usually the vesting period, based on the grant-date fair value. We estimate the value of stock options on the date of grant using the Black-Scholes pricing model. The determination of fair value of share-based payment awards on the date of grant using an option-pricing model is affected by the option price, as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, the expected stock price volatility over the term of the awards, and projected stock option exercise behaviors.
Income taxes
We determine deferred tax assets and liabilities based upon the differences between the financial statement and tax bases of our assets and liabilities using tax rates in effect for the year in which we expect the differences to affect taxable income. A valuation allowance is established for any deferred tax assets for which it is more likely than not that all or a portion of the deferred tax assets will not be realized. Based on the available information and other factors, management believes it is more likely than not that our federal and state net deferred tax assets will not be fully realized, and we have recorded a full valuation allowance.
We account for uncertain tax positions in accordance with Financial Accounting Standards Board Accounting Standards Codification (ASC) Topic 740, Income Taxes. When tax returns are filed, it is likely that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the consolidated financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying consolidated balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest associated with unrecognized tax benefits is classified as interest expense and penalties are classified in general and administrative expenses in the consolidated statements of operations.
| 42 |
Leases
We account for our leases under Accounting Standards Update (ASU) No. 2016-02, Leases (ASC 842), and related ASUs, which provide supplementary guidance and clarifications. Under ASC 842, all significant lease arrangements are generally recognized at lease commencement. Operating lease right-of-use (ROU) assets and lease liabilities are recognized at the commencement date. A ROU asset and corresponding lease liability are not recorded for leases with an initial term of 12 months or less (short-term leases), and we recognize lease expense for these leases as incurred over the lease term.
ROU assets represent our right to use an underlying asset during the reasonably certain lease terms, and lease liabilities represent our obligation to make lease payments arising from the lease. Our lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. Operating lease ROU assets and liabilities are recognized at the lease commencement date based on the present value of lease payments over the lease term. We use our incremental borrowing rate, based on the information available at commencement date in determining the present value of lease payments. The operating lease ROU asset also includes any lease payments related to initial direct cost and prepayments and excludes lease incentives. Lease expense is recognized on a straight-line basis over the lease term.
Off-Balance Sheet Arrangements
We do not maintain any off-balance sheet arrangements or obligations that are reasonably likely to have a material current or future effect on our financial condition, results of operations, liquidity or capital resources.
Contractual Obligations
As a “smaller reporting company,” as defined by Item 10 of Regulation S-K, we are not required to provide the information requested by paragraph (a)(5) of this Item.
Recent Accounting Pronouncements
See Note 1 to the consolidated financial statements in Item 8 of this Report for a full description of recent accounting pronouncements.
ITEM 7A: QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not required.
| 43 |
ITEM 8: FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| 44 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee and
Stockholders of Modular Medical, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Modular Medical, Inc. (the “Company”) as of March 31, 2022 and 2021, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2022 and 2021, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
| 45 |
Going Concern
As described further in Note 1 to the financial statements, the Company has incurred losses since inception, and expects to continue to incur operating losses for the foreseeable future and incur cash outflows from operations as it continues to invest in the development and subsequent commercialization of its product. The Company expects that its research and development and general and administrative expenses will continue to increase, and, as a result, it will eventually need to generate significant product revenues to achieve profitability. As of March 31, 2022, the Company had cash balances of approximately $9,076,000, as a result of the capital raised in the public offering in February 2022. In addition, subsequent to March 31, 2022, the Company raised net proceeds from an equity offering of approximately $7,372,000. The Company has concluded that these plans alleviate the doubt related to its ability to continue as a going concern.
We identified management’s assessment of the Company’s ability to continue as a going concern as a critical audit matter due to inherent complexities and uncertainties related to the Company’s projections of operations. Auditing management’s going concern assessment involved a high degree of auditor judgment and audit effort due to the impact of these assumptions on the determination of the degree of doubt regarding the ability of the entity to continue as a going concern. The primary procedures we performed to address this critical audit matter included:
| · | We evaluated the reasonableness of key assumptions underlying management’s conclusion. |
| · | We evaluated that the disclosures included in the Form 10-K were complete and accurate and in accordance with accounting principles generally accepted in the United States of America. |
| · | We evaluated the impact of the Company’s existing financing arrangements and future capital needs over the next 12 months on its ability to continue as a going concern. |
Stock Based Compensation
As discussed in Note 8, during the year ended March 31, 2022, the Company granted 827,427 options to purchase shares of its common stock with 10-year terms and a grant-date fair value of $8,507,311 to employees, directors and consultants. Management is required to analyze the fair value of each option granted and amortize it over its vesting period.
We identified the grant of stock options as a critical audit matter. Management’s estimates regarding fair value of options result in the application of a high degree of auditor judgment.
The primary procedures we performed to address this critical audit matter included the following:
| · | We gained an understanding of Company’s processes and controls in place for determining the fair value of each granted option. |
| · | We evaluated the option price model the management selected to determine the fair value, and analyzed the underlying data used in the calculations. |
| · | We also recalculated the fair value of each option granted. |
| /s/ | |
| Firm Id |
|
| We have served as the Company’s auditor since 2018. | |
| June 28, 2022 | |
| 46 |
Modular Medical, Inc.
Consolidated Balance Sheets
| March 31, | ||||||||
| ASSETS | 2022 | 2021 | ||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Prepaid expenses | ||||||||
| Other current assets | ||||||||
| TOTAL CURRENT ASSETS | ||||||||
| Property and equipment, net | ||||||||
| Right of use asset, net | ||||||||
| Security deposit | ||||||||
| TOTAL NON-CURRENT ASSETS | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses | ||||||||
| Short-term lease liability | ||||||||
| PPP note payable | ||||||||
| Convertible notes payable | ||||||||
| TOTAL CURRENT LIABILITIES | ||||||||
| Long-term lease liability | ||||||||
| Bonus payable | ||||||||
| TOTAL LIABILITIES | ||||||||
| Commitments and Contingencies (Note 11) | ||||||||
| STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Preferred Stock, $ par value, shares authorized, issued and outstanding | ||||||||
| Common Stock, $ par value, shares authorized, shares and shares issued and outstanding as of March 31, 2022 and 2021, respectively | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | ( | ) | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | $ | $ | ||||||
The accompanying notes are an integral part of these audited consolidated financial statements
| 47 |
Modular Medical, Inc.
Consolidated Statements of Operations
| Twelve Months Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Operating expenses | ||||||||
| Research and development | $ | $ | ||||||
| General and administrative | ||||||||
| Total operating expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Other income | ||||||||
| Interest expense | ( | ) | ( | ) | ||||
| Loss on debt extinguishment | ( | ) | ||||||
| Loss before income taxes | ( | ) | ( | ) | ||||
| Provision for income taxes | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Net loss per share | ||||||||
| Basic and diluted | $ | ( | ) | $ | ( | ) | ||
| Shares used in computing net loss per share | ||||||||
| Basic and diluted | ||||||||
The accompanying notes are an integral part of these audited consolidated financial statements
| 48 |
Modular Medical, Inc.
Consolidated Statements of Stockholders’ Equity (Deficit)
| Common Stock | Additional Paid-In | Common Stock | Accumulated | Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Issuable | Deficit | Equity (Deficit) | |||||||||||||||||||
| Balance as of March 31, 2020 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||
| Placement of common stock | ( | ) | ||||||||||||||||||||||
| Shares issued for services | ||||||||||||||||||||||||
| Stock-based compensation | — | |||||||||||||||||||||||
| Net loss | — | ( | ) | ( | ) | |||||||||||||||||||
| Balance as of March 31, 2021 | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||
| Issuance of common stock upon public offering, net of issuance costs | ||||||||||||||||||||||||
| Issuance of common stock in settlement of convertible notes and accrued interest | ||||||||||||||||||||||||
| Placement of common stock | ||||||||||||||||||||||||
| Warrants issued with convertible notes | — | |||||||||||||||||||||||
| Shares issued for services | ||||||||||||||||||||||||
| Shares issued for reverse stock split | ( | ) | ||||||||||||||||||||||
| Issuance of common stock under equity incentive plan | ||||||||||||||||||||||||
| Stock-based compensation | — | |||||||||||||||||||||||
| Net loss | — | ( | ) | ( | ) | |||||||||||||||||||
| Balance as of March 31, 2022 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||
The accompanying notes are an integral part of these audited consolidated financial statements
| 49 |
Modular Medical, Inc.
Consolidated Statements of Cash Flows
| Year ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Cash Flows from operating activities | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Gain on PPP note forgiveness | ( | ) | ||||||
| Loss on debt extinguishment | ||||||||
| Stock-based compensation expense | ||||||||
| Depreciation and amortization | ||||||||
| Accrued interest | ||||||||
| Shares issued for services | ||||||||
| Amortization of lease right-of-use asset | ||||||||
| Change in lease liability | ( | ) | ||||||
| Amortization of debt issuance costs | ||||||||
| Other | ||||||||
| Changes in assets and liabilities: | ||||||||
| Other assets and prepaid expenses | ||||||||
| Accounts payable and accrued expenses | ( | ) | ||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Cash flows from investing activities | ||||||||
| Purchases of property and equipment | ( | ) | ( | ) | ||||
| Net cash used in investing activities | ( | ) | ( | ) | ||||
| Cash flows from financing activities | ||||||||
| Proceeds from private placements, net of issuance costs | ||||||||
| Proceeds from issuance of convertible notes, net of placement fees | ||||||||
| Proceeds from issuance of promissory note | ||||||||
| Repayment of promissory note | ( | ) | ||||||
| Proceeds from issuance of PPP note payable | ||||||||
| Proceeds from issuance of common stock upon public offering, net of issuance costs | ||||||||
| Net cash provided by financing activities | ||||||||
| Net increase (decrease) in cash and cash equivalents | ( | ) | ||||||
| Cash and cash equivalents, at beginning of year | ||||||||
| Cash and cash equivalents, at end of year | $ | $ | ||||||
| Supplemental disclosure: | ||||||||
| Noncash investing and financing activities: | ||||||||
| Fair value of detachable warrants issued with convertible notes | $ | |||||||
| Conversion of convertible notes and accrued interest into common stock | $ | |||||||
| Cash paid for: | ||||||||
| Income taxes | $ | $ | ||||||
| Interest paid | $ | |||||||
The accompanying notes are an integral part of these audited consolidated financial statements
| 50 |
MODULAR MEDICAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – THE COMPANY AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Modular Medical, Inc. (the Company) was incorporated in Nevada in October 1998 under the name Bear Lake Recreation, Inc. The Company had no material business operations from 2002 until approximately 2017 when it acquired all of the issued and outstanding shares of Quasuras, Inc., a Delaware corporation (Quasuras). As the major shareholder of Quasuras retained control of both the Company and Quasuras, the share exchange was accounted for as a reverse merger. As such, the Company recognized the assets and liabilities of Quasuras, acquired in the merger, at their historical carrying amounts. Prior to the acquisition of Quasuras and, since at least 2002, the Company was a shell company, as defined in Rule 12b-2 promulgated under the Securities Exchange Act of 1934 (the Exchange Act). In June 2017, the Company changed its name from Bear Lake Recreation, Inc. to Modular Medical, Inc.
The Company is a development-stage medical device company focused on the design, development and eventual commercialization of an innovative insulin pump to address shortcomings and problems represented by the relatively limited adoption of currently available pumps for insulin-dependent people with diabetes. The Company has developed a hardware technology allowing people with insulin-dependent diabetes to receive their daily insulin in two ways, through a continuous “basal” delivery allowing a small amount of insulin to be in the blood at all times and a “bolus” delivery to address meal time glucose input and to address when the blood glucose level becomes excessively high. By addressing the time and effort required to effectively treat their condition, the Company believes it can address the less technically savvy, less motivated part of the market.
As discussed in Note 7, in February 2022, the Company completed a public offering of its equity securities, and its common stock was approved to list on the Nasdaq Capital Market under the symbol “MODD” and began trading there on February 10, 2022.
Going Concern
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The realization of assets and the satisfaction of liabilities in the normal course of business are dependent on, among other things, the Company’s ability to operate profitably, to generate cash flows from operations, and to pursue financing arrangements to support its working capital requirements.
At issuance of the Company’s financial statements for the year ended March 31, 2021, management had determined that there was significant doubt as to the ability of the Company to meet its obligations and continue as a going concern. As a result of the Offering (see Note 7), which was completed in February 2022, and the Registered Offering (see Note 13), which was completed in May 2022, and resulting improved financial position, the Company believes it has sufficient liquidity to meet its obligations as they come due and conduct its business for a period of at least 12 months from the date of issuance of these financial statements.
The Company’s operating needs include the planned costs to operate its business, including amounts required to fund working capital and capital expenditures. The Company’s future capital requirements and the adequacy of its available funds will depend on many factors, including the Company’s ability to successfully commercialize its product, competing technological and market developments, and the need to enter into collaborations with other companies or acquire other companies or technologies to enhance or complement its product offering. If the Company is unable to secure additional capital, it may be required to curtail its research and development initiatives and take additional measures to reduce costs in order to conserve its cash.
Basis of Presentation
The consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America. The Company’s fiscal year ends on March 31 of each calendar year. Each reference to a fiscal year in these notes to the consolidated financial statements refers to the fiscal year ended March 31 of the calendar year indicated (for example, fiscal 2022 refers to the fiscal year ending March 31, 2022). The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, Quasuras. All significant intercompany transactions and balances have been eliminated in consolidation.
| 51 |
Reverse Stock Split
On November 24, 2021, the Company filed a certificate of amendment to its amended and restated certificate of incorporation with the Secretary of State of the State of Nevada to effect a 1-for-3 reverse stock split of the Company’s shares of common stock. Such amendment and ratio were previously approved by a majority of the Company’s stockholders and the board of directors. As a result of the reverse stock split, which was effective November 29, 2021, every three shares of the Company’s pre-reverse split outstanding common stock were combined and reclassified into one share of common stock. Proportionate voting rights and other rights of common stock holders were not affected by the reverse stock split. Any fractional shares of common stock resulting from the Reverse Split were rounded up to the nearest whole share. All stock options outstanding and common stock reserved for issuance under the Company’s equity incentive plans and warrants outstanding immediately prior to the reverse stock split were adjusted by dividing the number of affected shares of common stock by three and, as applicable, multiplying the exercise price by three, as a result of the reverse stock split. All share numbers, share prices, exercise prices and per share amounts have been adjusted, on a retroactive basis to reflect this 1-for-3 reverse stock split.
Use of Estimates
The preparation of the accompanying consolidated financial statements in conformity with U.S. generally accepted accounting principles (GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amount of revenues and expenses during the reporting period. Estimates may include those pertaining to accruals, stock-based compensation and income taxes. Actual results could differ from those estimates.
Reportable Segment
The Company operates in one business segment and uses one measurement of profitability for its business.
Research and Development
The Company expenses research and development expenditures as incurred.
General and Administrative
General and administrative expenses consist primarily of payroll and benefit costs, rent, stock-based compensation, legal and accounting fees, and office and other administrative expenses.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist primarily of cash. The Company maintains its cash at high quality financial institutions within the United States, which are insured by the Federal Deposit Insurance Corporation (FDIC) up to limits of approximately $250,000. No reserve has been made in the financial statements for any possible loss due to financial institution failure.
Risks and Uncertainties
The Company is subject to risks from, among other things, competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements, limited operating history and the volatility of public markets.
COVID-19
The global outbreak of the coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization and a national emergency by the U.S. government in March 2020. This has negatively affected the U.S. and global economy, disrupted global supply chains, significantly restricted travel and transportation, resulted in mandated closures and orders to “shelter-in-place” and created significant disruption of the financial markets. The full extent of the COVID-19 impact on the Company’s operational and financial performance will depend on future developments, including the duration and spread of the pandemic and related actions taken by U.S. and foreign government agencies to prevent disease spread, all of which are uncertain, out of the Company’s control, and cannot be predicted.
| 52 |
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and cash in demand deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Property and Equipment
Property and equipment are originally recorded at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally three to five years. Depreciation is recorded in operating expenses in the consolidated statements of operations. Leasehold improvements and assets acquired through capital leases are amortized over the shorter of their estimated useful life or the lease term, and amortization is recorded in operating expenses in the consolidated statements of operations.
Fair Value of Financial Instruments
The Company measures the fair value of financial instruments using a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels:
| · | Level 1 inputs to the valuation methodology are quoted prices for identical assets or liabilities in active markets. |
| · | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| · | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
Due to their short-term nature, the carrying values of cash equivalents, accounts payable and accrued expenses, approximate fair value.
Debt Modifications and Extinguishments
When the Company modifies or extinguishes debt, it does so in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 470-50, Debt— Modifications and Extinguishments, which requires modification to debt instruments to be evaluated to assess whether the modifications are considered “substantial modifications.” A substantial modification of terms shall be accounted for like an extinguishment. Based on the guidance relied upon and the analysis performed, if the Company believes the embedded conversion feature has no fair value on the date of issuance (measurement date) and the embedded conversion feature has no beneficial conversion feature, the embedded conversion feature does not meet the criteria in ASC 470-50-40-10 or 470-20-25 and the issuance of the convertible note payable is considered a modification, and not an extinguishment that would require the recognition of a gain or loss. If the Company determines the change in terms meet the criteria for substantial modification under ASC 470 it will treat the modification as extinguishment and recognize a loss from debt extinguishment.
Leases
Effective April 1, 2019, the Company adopted ASC No. 842, Leases (ASC 842). ASC 842 requires an entity to recognize a right-of-use asset and a lease liability for all leases with terms longer than 12 months. The Company adopted ASC 842 utilizing the modified retrospective transition method. The Company elected the practical expedient afforded in ASC 842 in which the Company did not reassess whether any contracts that existed prior to adoption have or contain leases or the classification of its existing leases.
Stock-Based Compensation
The Company recognizes stock-based compensation for stock options granted to employees and non-employees on a straight-line basis over the requisite service period, usually the vesting period, based on the grant-date fair value. The Company estimates the value of stock options on the date of grant using the Black-Scholes pricing model. The determination of fair value of share-based payment awards on the date of grant using an option-pricing model is affected by the option price, as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, the expected stock price volatility over the term of the awards, and projected stock option exercise behaviors.
| 53 |
Basic net loss per share is computed by dividing loss for the period by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share gives effect to all potentially dilutive common shares outstanding during the period. Potentially dilutive common shares consist of incremental shares of common stock issuable upon the exercise of stock options and exercise of warrants.
| March 31, | ||||||||
| 2022 | 2021 | |||||||
| Options to purchase common stock | ||||||||
| Warrants | ||||||||
| Total | ||||||||
Reclassification
Certain prior year amounts have been reclassified for consistency with the current period presentation. These reclassifications had no effect on the reported results of operations or cash flows.
Income Taxes
The Company determines deferred tax assets and liabilities based upon the differences between the financial statement and tax bases of the Company’s assets and liabilities using tax rates in effect for the year in which the Company expects the differences to affect taxable income. A valuation allowance is established for any deferred tax assets for which it is more likely than not that all or a portion of the deferred tax assets will not be realized. Based on the available information and other factors, management believes it is more likely than not that its federal and state net deferred tax assets will not be fully realized, and the Company has recorded a full valuation allowance.
The Company accounts for uncertain tax positions in accordance with FASB ASC Topic 740, Income Taxes. When tax returns are filed, it is likely that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the consolidated financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying consolidated balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest associated with unrecognized tax benefits is classified as interest expense and penalties are classified in general and administrative expenses in the consolidated statements of operations.
The Company files U.S. federal and state income tax returns in jurisdictions with varying statutes of limitations. All tax returns from 2016 to 2021 may be subject to examination by the U.S. federal and state tax authorities. As of March 31, 2022 and 2021, the Company had not recorded any liability for unrecognized tax benefits related to uncertain tax positions.
Comprehensive Loss
Comprehensive loss represents the changes in equity of an enterprise, other than those resulting from stockholder transactions. Accordingly, comprehensive loss may include certain changes in equity that are excluded from net loss. For the years ended March 31, 2022 and 2021, the Company’s comprehensive loss was the same as its net loss.
| 54 |
Recently Issued Accounting Pronouncement
In June 2016, the FASB issued Accounting Standards Update (ASU) No. 2016-13, Financial Instruments—Credit Losses. This ASU added a new impairment model (known as the current expected credit loss (CECL) model) that is based on expected losses rather than incurred losses. Under the new guidance, an entity recognizes an allowance for its estimate of expected credit losses and applies to most debt instruments, trade receivables, lease receivables, financial guarantee contracts, and other loan commitments. The CECL model does not have a minimum threshold for recognition of impairment losses and entities will need to measure expected credit losses on assets that have a low risk of loss. This update is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years for smaller reporting companies. The Company is still evaluating the impact of this accounting guidance on its results of operations and financial position.
NOTE 2 – CONSOLIDATED BALANCE SHEET DETAIL
| March 31, | ||||||||
| Property and equipment, net: | 2022 | 2021 | ||||||
| Leasehold improvements | $ | $ | ||||||
| Office equipment | ||||||||
| Computer equipment and software | ||||||||
| Machinery and equipment | ||||||||
| Less: accumulated depreciation and amortization | ( | ) | ( | ) | ||||
| $ | $ | |||||||
| March 31, | ||||||||
| Accrued expenses: | 2022 | 2021 | ||||||
| Accrued wages and bonus | $ | $ | ||||||
| Accrued placement fees | ||||||||
| Accrued interest | ||||||||
| Other | ||||||||
| $ | $ | |||||||
NOTE 3 – LEASES
The Company accounts for the lease for its corporate facility in San Diego, California in accordance with ASC 842. The 39-month lease term commenced April 1, 2020, and the lease provides for an initial monthly rent of approximately $12,400 annual rent increases of approximately 3%. In addition to the minimum lease payments, the Company is responsible for property taxes, insurance and certain other operating costs. The right-to-use asset and corresponding liability for the facility lease have been measured at the present value of the future minimum lease payments. A discount rate of 11%, which approximated the Company’s incremental borrowing rate, was used to measure the lease asset and liability. Lease expense is recognized on a straight-line basis over the lease term.
The
Company obtained a right-of-use asset of $
Future minimum payments under the facility operating lease, as of March 31, 2022, are listed in the table below.
| Annual Fiscal Years | Operating lease | |||
| 2023 | ||||
| 2024 | ||||
| Less: | ||||
| Imputed interest | ( | ) | ||
| Present value of lease liabilities | $ | |||
Cash
paid for amounts included in the measurement of lease liabilities was $
| 55 |
NOTE 4 – PPP NOTE
In April 2020, the
Company received a $
In May 2021, the Lender and the U.S. Small Business Administration notified the Company that the outstanding principal and accrued interest for the PPP Note was forgiven in full. The Company accounted for the forgiveness of the PPP Note in accordance with ASC Topic 470: Debt (ASC 470), and the amount forgiven was recorded as a gain on extinguishment and recognized in the other income line of the consolidated statement of operations.
NOTE 5 – CONVERTIBLE PROMISSORY NOTES
From
February through April 2021, the Company sold $2,310,000 of convertible promissory notes (each an Original Note and, collectively, the
Original Notes), at par in a private placement transaction effected pursuant to an exemption from the registration requirements under
the Securities Act of 1933, as amended. Effective April 30, 2021, pursuant to a revocation and replacement agreement between each holder
of an Original Note and the Company (the Revocation Agreement), the $2,310,000 of Original Notes and accrued interest thereon as of April
30, 2021 were replaced with $2,360,550 aggregate principal amount of new Notes (as defined below). The Company accounted for the replacement
of the Original Notes in accordance with ASC 470 and recorded a loss on extinguishment of $
In
April and May 2021, pursuant to a securities purchase agreement by and between the Company and each investor (the SPA), the Company sold
to investors $
Notes outstanding after the Trigger Date may be converted into shares of the Company’s common stock at an initial conversion price of $8.61 per share; provided that a Note holder may not convert any portion of its Note that would cause it to beneficially own in excess of 4.99% of the Company’s outstanding common stock. The conversion price and number of shares of Company common stock issuable upon conversion of the Notes are subject to adjustment from time to time for subdivisions and consolidations of shares and other standard dilutive and corporate events, as provided in the Notes. Subject to certain Exempt Issuances (as defined in the Notes), if while a Note is outstanding, the Company sells, issues or grants any shares of its common stock or other securities to acquire shares of common stock at a price per share less than the then conversion price, such conversion price shall be reduced to such lesser price, and the number of conversion shares issuable upon conversion of the Notes shall be increased, as provided in the Notes.
If the Company completes an offering of its common stock or other securities in excess of $12,000,000 of gross proceeds (a Qualified Capital Raise, as defined in the Notes), each Note holder will be required to convert its Adjusted Note Amount (as defined below) into the securities of such Qualified Capital Raise. Adjusted Note Amount equals the product of (i) the sum of all outstanding principal plus accrued interest on a Note, multiplied by (ii) 1.25.
| 56 |
The Notes contained a number of Company events of default (Events of Default) including, without limitation (i) failure to pay any principal or interest thereon when due, (ii) failure to timely deliver shares upon conversions, (iii) failure to comply with SEC reporting requirements under the Exchange Act, (iv) certain breaches of the SPA, the Notes, the Warrants, and the Registration Rights Agreement, (v) material restatements of the Company’s consolidated financial statements filed with the SEC, (vi) a holder’s inability to rely on Rule 144 for sales of shares underlying the Notes, (vii) the Company’s common stock is suspended or halted from trading and/or fails to be quoted or listed (as applicable) on the OTCQB, OTCQX, any tier of the NASDAQ Stock Market, the New York Stock Exchange, or the NYSE American within 10 days thereafter, (viii) failure to file with the SEC a registration statement covering the resale of shares of common stock underlying the Notes and Warrants within 60 calendar days following the Issue Date, (ix) failure to cause such registration statement to become effective within 120 calendar days following the Issue Date, or (x) certain mergers consolidations, business combinations and sales of all or substantially all of the Company’s assets in the event the Company is not the survivor of such transaction.
Upon an Event of Default, a Note holder may declare all amounts under its Note(s) due and payable, in which event the Company will be required to pay such Note holder the sum of (i) the product of (a) all then outstanding principal amount and accrued interest thereon, multiplied by (b) 125%; and (ii) all collection costs including legal fees and expenses in connection therewith. At the option of a Note holder, in the event the Company receives cash proceeds as a result of certain events, including, but not limited to, payments from customers, issuances of debt or equity securities, exercise of warrants or asset sales, the Company will be required to use such proceeds to repay all or any lesser outstanding amounts due under such holder’s Note.
The Notes include covenants, representations, warranties, other payment obligations and agreements by the Company including, without limitation, most-favored nation rights, rights of participation and first refusal and exchange rights.
In connection with the issuance of the Notes, the Company issued Warrants to purchase in the aggregate 767,796 shares of its common stock at an initial exercise price of $24.00 per share. The Warrants may be exercised for a period of five years from the Trigger Date, provided that, if prior to the Trigger Date, the Company (i) completes a Qualified Capital Raise, the outstanding Warrants shall be cancelled or (ii) prepays a holder’s Note(s) in whole or in part, such holder’s pro-rata number of Warrants shall be cancelled. The fair value of the Warrants was $3,700,632, of which $2,379,182 was recorded as a debt discount, which is being amortized to interest expense over the term of the Warrants, and $1,321,450 was recorded as a loss on debt extinguishment. The Company calculated the fair value of the Warrants utilizing the Black-Scholes valuation model with the following assumptions: volatility of , risk-free interest rate of , a term of years and a dividend yield of .
In connection with the April and May 2021 sales of the $4,250,000 aggregate principal amount of the Notes, the Company incurred debt issuance costs of $116,000, which were recorded as a debt discount and were amortized to interest expense over the term of the Notes using the effective interest rate method. The interest expense attributable to the debt discount, comprising the debt issuance costs and Warrants, during the year ended March 31, 2022 was $1,833,618.
Upon the closing of the Offering (see Note 7), which was a Qualified Capital Raise, in accordance with their terms, the Notes converted into 1,511,276 shares of common stock and the holders of the Notes received 1,511,276 Offering Warrants (as defined in Note 7). As a result of the Offering, the exercise price of the 767,796 outstanding Warrants was reduced to $6.00 per share.
NOTE 6 – PROMISSORY NOTE
In
October 2021, the Company issued a secured promissory note (the Bridge Note) to Manchester Explorer, L.P. (Manchester) that provided
the Company with a $
The principal amount of the Bridge Note and interest due thereon is payable to Manchester no later than the earlier of: (i) the Maturity Date and (ii) the date on which the Company has received proceeds in excess of $12,000,000 from a transaction or series of related transactions occurring prior to the Maturity Date, which such transactions constitute equity financings or other issuances of the Company’s equity securities. Provided that no Event of Default (as such term is defined in the Bridge Note) has occurred, on any date prior to the Maturity Date, upon no less than three days written notice by the Company specifying the draw amount, Manchester will advance the draw amount to the Company. No draw amount can be in an amount less than $100,000 or exceed an amount equal to $3,000,000 minus the aggregate principal amount outstanding under the Bridge Note at the time of such draw request. If an Event of Default occurs and is continuing, Manchester may declare all of the Bridge Note, including any interest and other amounts due, to be due and payable immediately.
| 57 |
In connection with the issuance of the Note, on October 28, 2021, the Company entered into a security agreement with Manchester (the Security Agreement) under which the Company granted Manchester a continuing and unconditional first priority security interest in and to any and all of the Company’s property of any kind or description, tangible or intangible, wheresoever located and whether now existing or hereafter arising or acquired.
During
fiscal 2022, the Company made draws on the Bridge Note of $
NOTE 7 – STOCKHOLDERS’ EQUITY (DEFICIT)
Public Offering
On
February 9, 2022, the Company entered into an underwriting agreement (the Underwriting Agreement) with Oppenheimer & Co. Inc., who
acted as the representative of the several underwriters (the Underwriters), in a firm commitment underwritten public
offering (the Offering) pursuant to which, on February 14, 2022, the Company sold to the Underwriters an aggregate of 2,500,000 shares
of the Company’s common stock and 2,500,000 warrants (the Offering Warrants and, collectively with the shares of common stock,
the Units), each to purchase one share of common stock. The price to the public in the Offering was $6.00 per Unit, before underwriting
discounts and commissions. The common stock and the Offering Warrants comprising the Units were immediately separable upon issuance and
were issued separately. The Offering Warrants were exercisable immediately, have an exercise price of $6.60 per share and expire on February
14, 2027. The gross proceeds from the Offering were $
Placements of Common Stock
Between March and December 2020, the Company completed a private placement of shares of its common stock (the 2020 Placement). The Company sold 962,387 shares of common stock, at a purchase price of $2.87 per share, for gross proceeds of $2,762,054. The Company paid placement agent fees on the 2020 Placement of $52,256 during fiscal 2021.
In
October 2021, the Company sold shares of common stock to two officers, its i) chief executive officer and ii) the chairman of
the Company’s board of directors (the Board), president, chief financial officer and treasurer, at
a purchase price of $8.10 per share, for gross proceeds of approximately $
During
the year ended March 31, 2022, the Company issued to service providers shares of common stock with a fair value of approximately $
Amended 2017 Equity Incentive Plan
In October 2017, the Company’s Board approved the 2017 Equity Incentive Plan (the Plan) with shares of common stock reserved for issuance. In January 2020 and August 2021, the Board approved increases in the number of shares reserved for issuance under the Plan by and shares, respectively. Under the Plan, eligible employees, directors and consultants may be granted a broad range of awards, including stock options, stock appreciation rights, restricted stock, performance-based awards and restricted stock units. The Plan is administered by the Board or, in the alternative, a committee designated by the Board.
Stock-Based Compensation Expense
The expense relating to stock options is recognized on a straight-line basis over the requisite service period, usually the vesting period, based on the grant date fair value. The unamortized compensation cost, as of March 31, 2022 was $ related to stock options and is expected to be recognized as expense over a weighted-average period of approximately .
During the year
ended March 31, 2022, the Company granted options to purchase shares of its common stock to employees, directors and consultants.
The options had 10-year terms and options vested immediately when granted. The grant-date fair value was determined to be $
| 58 |
The following assumptions were used in the fair-value method calculations:
| Year
Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Risk-free interest rates | - | - | ||||||
| Volatility | - | - | ||||||
| Expected life (years) | - | - | ||||||
| Dividend yield | ||||||||
The fair values of options at the grant date were estimated utilizing the Black-Scholes valuation model, which includes simplified methods to establish the fair term of options as well as average volatility of three comparable organizations. The risk-free interest rate was derived from the Daily Treasury Yield Curve Rates, as published by the U.S. Department of the Treasury as of the grant date for terms equal to the expected terms of the options. A dividend yield of zero was applied because the Company has never paid dividends and has no intention to pay dividends in the foreseeable future. In accordance with ASU No. 2016-09, the Company accounts for forfeitures as they occur.
| Shares | Options Outstanding | |||||||||||
| Available | Number of | Weighted Average | ||||||||||
| for Grant | Shares | Exercise Price | ||||||||||
| Balance at March 31, 2020 | $ | |||||||||||
| Options granted | ( | ) | ||||||||||
| Options cancelled and returned to the Plan | ( | ) | ||||||||||
| Balance at March 31, 2021 | ||||||||||||
| Additional shares authorized under the Plan | — | — | ||||||||||
| Options granted | ( | ) | ||||||||||
| Share awards | ( | ) | — | — | ||||||||
| Options cancelled and returned to the Plan | ( | ) | ||||||||||
| Balance at March 31, 2022 | $ | |||||||||||
There were no stock options exercised during the years ended March 31, 2022 and 2021. The Company issued 26,497 shares to its non-employee directors under the Company’s outside director compensation plan and approximately $172,100 was recorded as stock-based compensation expense for these share awards during the year ended March 31, 2022.
| Options Outstanding | Options Exercisable | |||||||||||||||||||||||
| Range of Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (in Years) | Weighted Average Exercise Price | Number Exercisable | Weighted Average Exercise Price | Aggregate Intrinsic value | ||||||||||||||||||
| $ - $ | 8.01 | $ | $ | $ | ||||||||||||||||||||
The intrinsic value per share is calculated as the excess of the closing price of the common stock on the Company’s principal trading market over the exercise price of the option at March 31, 2022.
The Company is required to present the tax benefits resulting from tax deductions in excess of the compensation cost recognized from the exercise of stock options as financing cash flows in the consolidated statements of cash flows. For the years ended March 31, 2022 and 2021, there were no such tax benefits associated with the exercise of stock options.
| 59 |
NOTE 9 – INCOME TAXES
The income tax provision consisted of the following:
| Year Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Current portion: | ||||||||
| Federal | $ | $ | ||||||
| State | ||||||||
| Deferred portion: | ||||||||
| Federal | ( | ) | ( | ) | ||||
| State | ( | ) | ( | ) | ||||
| ( | ) | ( | ) | |||||
| Change in valuation allowance | ||||||||
| Provision for income taxes | $ | $ | ||||||
At March 31, 2022,
the Company had net operating loss carryforwards (NOLs) of approximately $
The Company
also had federal research and development tax credit carryforwards of approximately $
A reconciliation of income taxes provided at the federal statutory rate (21% for each of fiscal 2022 and 2021) to the actual income tax provision is as follows:
| Year Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Federal statutory rate | ( | )% | ( | )% | ||||
| State tax rate, net of federal benefit | ( | )% | ( | )% | ||||
| Permanent differences | % | % | ||||||
| Research and development tax credits | ( | )% | ( | )% | ||||
| Section 179 assets | % | % | ||||||
| Change in valuation allowance | % | % | ||||||
| Effective income tax rate | % | % | ||||||
The losses before income tax provision for the years ended March 31, 2022 and 2021 were solely attributable to US operations.
Significant components of the Company’s deferred tax assets and liabilities were:
| March 31, | ||||||||
| 2022 | 2021 | |||||||
| Net operating loss carryforwards | $ | $ | ||||||
| Stock-based compensation expense | ||||||||
| Property and equipment | ( | ) | ||||||
| Reserves, accruals & other | ( | ) | ( | ) | ||||
| Research and development tax credits | ||||||||
| Total deferred tax assets | ||||||||
| Section 179 assets | ( | ) | ||||||
| Total deferred tax liabilities | ( | ) | ||||||
| Less: valuation allowance | ( | ) | ( | ) | ||||
| Deferred tax assets, net | $ | $ | ||||||
| 60 |
Based on the available information and other factors, management believes it is more likely than not that the net deferred tax assets at March 31, 2022 and 2021, will not be fully realizable. Accordingly, management has recorded a full valuation allowance against its net deferred tax assets at March 31, 2022 and 2021.
Management has evaluated and concluded that there were no material uncertain tax positions requiring recognition in the Company’s consolidated financial statements at March 31, 2022 and 2021. The Company does not expect any significant changes in its unrecognized tax benefits within twelve months of the reporting date.
NOTE 10 – ROYALTY AGREEMENT
In July 2017, the Company entered into a royalty agreement with its founder, then-chief executive officer, president and major shareholder (the Founder). Pursuant to the agreement, the Founder assigned and transferred all of his rights in the intellectual property of Quasuras in return for future royalty payments on the Company’s product. The Company is obligated to make royalty payments under the agreement to the Founder on any sales of the royalty product sold or otherwise commercialized by the Company equal to (a) $0.75 on each sale of a royalty product or (b) 5% of the gross sale price of the royalty product, whichever is less. The royalty payments will cease, and the agreement will terminate, at such time as the total sum of royalty payments actually paid to the Founder, pursuant to the agreement, reaches $10,000,000. The Company has the option to terminate the agreement at any time upon payment, to the Founder, of the difference between total royalty payments actually made to him to date and the sum of $10,000,000. All payments of the royalties, if due, for the preceding quarter, will be made by the Company to the Founder within thirty days after the end of each calendar quarter.
NOTE 11 – COMMITMENTS AND CONTINGENCIES
Litigations, Claims and Assessments
In the normal course of business, the Company may be involved in legal proceedings, claims and assessments arising in the ordinary course of business. The Company records legal costs associated with loss contingencies as incurred and accrues for all probable and estimable settlements.
Indemnification
In the ordinary course of business, the Company enters into contractual arrangements under which it may agree to indemnify the counterparties from any losses incurred relating to breach of representations and warranties, failure to perform certain covenants, or claims and losses arising from certain events as outlined within the particular contract, which may include, for example, losses arising from litigation or claims relating to past performance. Such indemnification clauses may not be subject to maximum loss clauses. The Company has also entered into indemnification agreements with its officers and directors. No amounts were reflected in the Company’s consolidated financial statements for the years ended March 31, 2022 and 2021 related to these indemnifications. The Company has not estimated the maximum potential amount of indemnification liability under these agreements due to the limited history of prior claims and the unique facts and circumstances applicable to each particular agreement. To date, the Company has not made any payments related to these indemnification agreements.
NOTE 12 – RELATED PARTY TRANSACTIONS
In
February 2021, the Company’s chairman of the Board and president and Manchester, which is represented by a member of the Company’s
board of directors, purchased $100,000 and $1,000,000, aggregate principal amount of the Original Notes, respectively. Effective April
30, 2021, the related party holders entered into revocation agreements with the Company pursuant to which their aggregate principal amount
of Original Notes and accrued interest were replaced with Notes. On February 14, 2022, Manchester and the executive officer held Notes
in an aggregate principal amount of $
In
May 2021, a member of the Board purchased $
The daughter of the
Company’s president, chief financial officer, treasurer and chairman of the Board is an employee of the Company. During fiscal
2022, the Company paid her $
| 61 |
NOTE 13 – SUBSEQUENT EVENT
On May 2, 2022, the Company entered into a securities purchase agreement (the Purchase Agreement) with an institutional investor (the Investor) pursuant to which the Company sold, in a registered direct offering (the Registered Offering), for gross proceeds of $8,000,000 an aggregate of 449,438 shares (the Shares) of the Company’s common stock, at a purchase price per Share of $4.45 and pre-funded warrants (the Pre-Funded Warrants) to purchase an aggregate of 1,348,314 shares of common stock at a purchase price per Pre-Funded Warrant of $4.44. The Pre-Funded Warrants were exercisable immediately on the date of issuance at an exercise price of $0.01 per share and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full.
In a concurrent private placement under the Purchase Agreement, the Company issued warrants (the Private Placement Warrants) to the Investor to purchase an aggregate of 1,438,202 shares of common stock at an exercise price of $6.60 per share. The Private Placement Warrants will be exercisable commencing November 5, 2022 and have a five-year term.
ITEM 9: CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A: CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are designed to ensure that information required to be disclosed in the reports filed with or furnished to the Securities and Exchange Commission, or the SEC, under the Securities Exchange Act of 1934, as amended, or the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in the reports filed under the Exchange Act is accumulated and communicated to our management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure.
Under the supervision and with the participation of our management, including our chief executive officer and our chief financial officer, we conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (the Exchange Act). Based on this evaluation, our management concluded that as of March 31, 2022, our disclosure controls and procedures were effective.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily is required to apply its judgment in evaluating the cost-benefit relationship of possible controls. Internal control over financial reporting is the process designed by, or under the supervision of, our chief executive officer and chief financial officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that: (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
| 62 |
Because of its inherent limitations, cost-effective internal controls over financial reporting may not prevent or detect misstatements. All internal control systems, no matter how well designed, have inherent limitations, including the possibility of human error and the circumvention of overriding controls. Accordingly, even effective internal control over financial reporting can provide only reasonable assurance with respect to consolidated financial statement preparation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an assessment of the effectiveness of our internal control over financial reporting as of the end of the period covered by this Annual Report on Form 10-K. In making this assessment, we used the criteria based on the framework in Internal Control—Integrated Framework (2013 Framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the assessment, our management concluded that our internal control over financial reporting was effective as of March 31, 2022.
Changes in Internal Control over Financial Reporting
There were no changes in our internal controls over financial reporting during the fourth fiscal quarter of 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B: OTHER INFORMATION
None
ITEM 9C: DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable
| 63 |
PART III
ITEM 10: DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
The names of our directors, executive officers and certain information about each of them at March 31, 2022 are set forth below.
| Name | Age | Position | ||
| James Besser | 46 | Chief Executive Officer | ||
| Paul DiPerna | 64 | President, Chief Financial Officer, Treasurer and Chairman of the Board of Directors | ||
William J. Febbo(1) |
53 | Director | ||
| Steven Felsher(2)(3) | 73 | Director | ||
| Morgan C. Frank | 50 | Director | ||
Philip Sheibley(2)(3) |
63 | Director | ||
Carmen Volkart(1)(2) |
61 | Director | ||
Ellen O’Connor Vos |
66 | Director |
| (1) | Member of Compensation Committee |
| (2) | Member of Audit Committee |
| (3) | Member of Nominating and Governance Committee |
The principal occupations and positions for at least the past five years of our directors and executive officers are described below. There are no family relationships among any of our directors or executive officers.
James “Jeb” Besser. Mr. Besser has served as our chief executive officer since February 23, 2022 and combines over 25 years of experience in alternative investments, strategic advisory, corporate strategy and corporate governance. Since 1999, he has been a Managing Member at Manchester Management Company, LLC (Manchester), an investment management firm. Mr. Besser is also currently a director of River Stone Biotech, a development stage specialty bioprocessing company. He holds a B.A. in history from Brown University. We believe that Mr. Besser is qualified to serve as member of our board of directors due to his extensive prior experience conducting financial analysis of public companies (certain of which were in the development stage), including such public companies’ management teams, products, including products in the development stage, the potential markets for such products and other factors that could affect the likelihood and timing of success and market penetration of such entities’ products as well as his capital raising activities. We believe this provides us with valuable insights into the financial markets and investment criteria of institutional and other investors as well as capital raising activities.
Paul DiPerna. Mr. DiPerna has been our chairman, chief financial officer, president and treasurer since we acquired Quasuras, Inc. (Quasuras) in July 2017. He also served as our chief executive officer from July 2017 until August 2021. In 2015, he founded Quasuras, an early-stage medical device company developing an insulin pump product, and, until its acquisition by us, he served as its chief executive officer and chairman. Prior to that, Mr. DiPerna founded Fuel Source Partners, LLC to incubate early stage medical device products and accumulate technical talent. Our current pump product was one of such proposed products and was spun-out to Quasuras in 2015. From 2012 to 2015, he served as a co-inventor at a private company with property rights in a medical device used for blood borne infection control called the Curos Cap, which was acquired by 3M Corporation. In 2003, Mr. DiPerna founded Tandem Diabetes Care, Inc. (Tandem) and held various positions, including as director, chief executive officer and chief technology officer and was primarily responsible for the design concept and development of Tandem’s initial insulin pump. Prior to that, he held executive and management positions at Baxter Healthcare Corporation (Baxter) where he was tasked with identifying synergistic opportunities in the diabetes industry. As a result, Mr. DiPerna developed substantial expertise and knowledge in the diabetes industry and led attempts by Baxter to acquire three insulin pump manufacturers. Previously, he held mechanical design engineering positions in the automated test equipment and blood separation sciences industries. Mr. DiPerna holds 70 patents in medical device and microfluidic technology and has achieved numerous product clearances with the FDA. He has also achieved multiple successful exits with previous companies. Mr. DiPerna received a Masters in Engineering Management from Northeastern University and a B.S. in Mechanical Engineering from the University of Massachusetts and has spent over 35 years in the medical-device industry. We believe that Mr. DiPerna is qualified to serve as the chairman of our board of directors due to his extensive knowledge and experience in the medical-device industry generally, and, in particular, with regard to insulin pumps and the diabetes industry, as well as his management and leadership experience from holding director and senior executive positions in other public and private companies and leading project development teams of medical device companies.
| 64 |
William J. Febbo. Mr. Febbo was appointed to our board of directors in January 2020. He is currently the Chief Executive Officer and a director of OptimizeRx Corporation, a digital health company focused on bringing life sciences support to patients and providers, having joined the company in 2016. Since April 2022, he has served as member of the board of directors of Augmedix, Inc., a Nasdaq-listed provider of automated medical documentation and data services. Mr. Febbo founded Plexuus, LLC, a payment processing business for medical professionals in September 2015 and remained its Chairman from September 2015 to December 2020. From April 2007 to September 2015, he served as Chief Operating Officer of Merriman Holdings, Inc., an investment banking firm, where he assisted with capital raises in the technology, biotechnology, clean technology, consumer and resources industries. Mr. Febbo was a co-founder of, and from September 2013 to September 2015 served as Chief Executive Officer of, Digital Capital Network, Inc. a transaction platform for institutional and accredited investors. He was a co-founder of, and from January 1999 to September 2015 was Chief Executive Officer of, MedPanel, LLC, a provider of market intelligence and communications for the pharmaceutical, biomedical, and medical device industries. Since 2017, Mr. Febbo has been a faculty member of the Massachusetts Institute of Technology’s linQ program, which is a collaborative initiative focused on increasing the potential of innovative research to benefit society and the economy. Since 2004, he has been a board member of the United Nations Association of Greater Boston, a resource for the citizens of Greater Boston on the broad agenda of critical global issues addressed by the United Nations and its agencies. He holds a B.A. in international studies and Spanish from Dickinson College. We believe that Mr. Febbo is qualified to serve on our board of directors because of his wealth of experience in building and managing health services and financial businesses. Mr. Febbo brings more than 20 years of experience in building and managing health services and financial businesses.
On January 29, 2018, the Financial Industry Regulatory Authority (FINRA) accepted a Letter of Acceptance, Waiver and Consent (the Consent) submitted by Mr. Febbo. Without admitting or denying the findings, Mr. Febbo consented to the sanctions and to the entry of findings that he permitted Merriman Capital, Inc. to conduct a securities business while below its net capital requirement. From August 2012 to October 2015, he was the Financial and Operations Principal (FinOp) for a registered broker-dealer, Merriman Capital, Inc. (Merriman). During certain months, while Mr. Febbo was FinOp, FINRA found that certain of Merriman’s net capital filings with FINRA were inaccurate because of the method by which Merriman calculated net capital and that, when corrected, it was retroactively determined that Merriman had operated below its minimum net capital requirements. Mr. Febbo, as FinOp, signed certain of these reports and was thus held responsible. Based on the Consent, in settlement, Mr. Febbo, who was then no longer registered with any broker-dealer, accepted a fine of $5,000, a 10-business day suspension from acting as FinOp for any FINRA member and required to requalify by examination for the Series 27 license before again acting in a FinOp capacity.
Steven Felsher. Mr. Felsher was appointed to our board of directors in November 2021. Mr. Felsher is an experienced executive with respect to finance, administration, governance and other aspects of public and private company management. He has served as a member of the board of directors of Signal Hill Acquisition Corp., a special purpose acquisition company, since March 2021. From August 2018 to July 2020, he served as a member of the board of directors of Sito Mobile, Inc., a publicly-traded company that provided customized, data-driven solutions for brands spanning all forms of media. From January 2011 to June 2019, Mr. Felsher was a senior advisor at Quadrangle Group LLC, a private investment firm focused on the information and communications technology sectors. He spent a substantial portion of his career with Grey Global Group Inc., a global marketing services company, where he served as a senior executive from 1979 until 2007, most recently as vice chairman and chief financial officer. He holds a BA in classical Greek from Dickinson College and a J.D. from Yale University School of Law. We believe that Mr. Felsher is qualified to serve on our board of directors because of his extensive business experience with administration, governance, capital allocation and other aspects of public and private company management.
Morgan C. Frank. Mr. Frank was appointed to our board of directors in April 2017. Mr. Frank has worked with Manchester, LP since May 2002, and, prior to such time, he was a founder and managing director at First Principles Group, a boutique consultancy and principal investor specializing in corporate restructuring, restarts, intellectual property assessment and salvage, and spin outs. Prior to such time, Mr. Frank spent approximately five years as an analyst and portfolio manager at Hollis Capital, a San Francisco based hedge fund and prior thereto, Mr. Frank worked for an independent private client group at Paine Webber specializing in primary research to develop investment ideas (particularly short sale ideas) for institutional clients. Prior to his employment at Paine Webber, Mr. Frank was a currency trader for Eastern Vanguard. Mr. Frank holds a BA in Economics and in Political Science from Brown University. We believe that Mr. Frank is qualified to serve as member of our board of directors due to his extensive prior experience conducting financial analysis of public companies (certain of which were in the development stage), including such public companies’ management teams, products, including products in the development stage, the potential markets for such products and other factors that could affect the likelihood and timing of success and market penetration of such entities’ products as well as his capital raising activities. We believe this provides us with valuable insights into the financial markets and investment criteria of institutional and other investors as well as capital raising activities.
| 65 |
Philip Sheibley. Mr. Sheibley was appointed to our board of directors in November 2021. Mr. Sheibley is an experienced executive and venture capitalist. Since 2011, he has served as a principal at Alumni Investment Partners, a private equity firm. From 1981 to 2010, Mr. Sheibley served as a management and technology consultant with Accenture, where he focused on the life sciences area, holding a variety of leadership positions, including North American industry director for life sciences and global lead for management consulting. Mr. Sheibley holds a B.S. in industrial and systems engineering with a business minor from Lehigh University. We believe that Mr. Sheibley is qualified to serve on our board of directors because of his extensive business experience in the life sciences area and experience with venture capital investment and consulting, including financing transactions for early-stage and scale-up stage companies, assisting with scale-up strategy/execution, and participating as a board member in the medical products industry.
Carmen Volkart. Ms. Volkart was appointed to our board of directors in December 2019. She has served as chief financial officer of Natureworks LLC, an advanced materials company offering a portfolio of renewably-sourced polymers, since October 2018. Ms. Volkart served as a member of the board of directors, including as a member of the audit committee of Antares Pharma, Inc., a Nasdaq-listed, specialty pharmaceutical company, from October 2021 to May 2022, when it was acquired by another Nasdaq-listed company. From October 2012 to July 2018, she served as chief financial officer and, for a portion of that time, as senior vice president of commercialization for NxThera, Inc., a medical device company pioneering the application of convective radiofrequency thermotherapy to treat endurological conditions. Ms. Volkart served as global chief financial officer of Tornier N.V. from 2010 to 2012, and was chief operating and financial officer, corporate secretary, compliance officer and treasurer of Spine Wave, Inc. from 2006 to 2010. Prior to 2006, she held various executive and financial positions at American Medical Systems, Inc., Medtronic, Inc. and Honeywell, Inc. Ms. Volkart holds a B.S. in accounting from the University of North Dakota and an MBA with a concentration in strategic management from the University of Minnesota. We believe that Ms. Volkart is qualified to serve on our board of directors because of her substantial financial and public-company experience, as she has served as chief financial officer at multiple medical device and other companies.
Ellen O’Connor Vos. Ms. Vos was appointed to our board of directors in May 2021 and served as our chief executive officer from August 2021 until February 23, 2022. Ms. Vos has served as a member of VosHealth LLC since November 2020. Prior to that, she served as the president and chief executive officer of the Muscular Dystrophy Association from October 2017 to November 2020. Previously, Ms. Vos had been chief executive officer of ghg | greyhealth group from 1996 to 2017, and she has been a champion of using digital capabilities to improve the public health. Ms. Vos also serves on the board of OptimizeRX Corporation, a publicly-traded digital health company, and the Jed Foundation, a leading nonprofit dedicated to protecting the emotional health of college students, and was a founding board member of MMRF, a pioneering cancer research foundation. Ms. Vos holds a B.S. in nursing from Alfred University. We believe that Ms. Vos is qualified to serve on our board of directors because of her executive experience and extensive executive skills in digital marketing, commercialization and communications in the healthcare industry.
Involvement in Legal Proceedings
Except with regard to Mr. Febbo, to our knowledge, none of our executive officers or our directors has, during the last ten years:
| · | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| · | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his or her involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| · | been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| · | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| 66 |
| · | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
To our knowledge, there are no material proceedings to which any director, officer or affiliate of ours, any owner of record or beneficially of more than 5% of any class of voting securities of us, or any associate of any such director, officer, affiliate of ours, or security holder is a party adverse to us or any of our subsidiaries or has a material interest adverse to us or any of our subsidiaries.
Arrangements for Appointment of Directors and Officers
Pursuant to the Reorganization and Share Exchange Agreement dated as of July 24, 2017, by and among us, Quasuras, Mr. DiPerna and the other stockholders of Quasuras (the Share Agreement), until July 24, 2022, our board of directors shall consist of no more than five and no less than two directors of which (i) Manchester has the right to appoint two directors, pursuant to which Manchester appointed Mr. Frank and Ms. Volkart and (ii) Mr. DiPerna, in addition to being our chairman of the board, has the right to appoint two additional directors, pursuant to which he appointed Messrs. Burns and Febbo. In May 2021, the parties amended the Share Agreement and removed Manchester’s and Mr. DiPerna’s rights to appoint directors. In addition, the parties agreed that Mr. DiPerna shall remain chairman of our board of directors until July 2022; provided, that in the event Mr. DiPerna resigns or is otherwise replaced as our chief executive officer, Mr. DiPerna shall remain as chairman of our board of directors for an additional period of three years. Following such amendment, our board of directors increased the size of the board to six members and, on May 18, 2021, appointed Ellen O’Connor Vos to our board.
The DiPerna Employment and Related Agreements
We entered into an employment agreement dated August 1, 2018, with Mr. DiPerna pursuant to which Mr. DiPerna was employed by us as our chief executive officer and president for an initial 2-year term with automatic one-year renewals. Pursuant to such agreement, we agreed to pay Mr. DiPerna: i) an annual salary of $200,000 in cash, ii) $100,000 per year in fully-vested stock options granted monthly at an exercise price determined by our board of directors in its sole discretion and iii) an annual bonus of $300,000, payable at the discretion of our board of directors, either in shares or in cash. If the board chooses to pay the bonus in shares, such shares will be valued at a price determined by our board of directors. Pursuant to such employment agreement (i) if (a) we terminate Mr. DiPerna’s employment without cause or he resigns with good reason, we will pay Mr. DiPerna a lump sum of $200,000, and (b) we terminate Mr. DiPerna’s employment for cause, we are not obligated to make any severance payment and Mr. DiPerna will receive only his base compensation through the last day of his employment, (ii) upon Mr. DiPerna’s death or disability, he will receive his base compensation through the last day of his employment and will remain eligible for all applicable benefits relative to death or disability pursuant to any plans that we have in place at such time, and (iii) upon a change of control (as defined in the employment agreement), Mr. DiPerna will be paid a lump sum of $100,000 within sixty days of the time at which such change of control takes place.
In May 2020, we amended our employment agreement with Mr. DiPerna to provide that in the event of a change in control:
| · | within 60 days of the date the change in control occurs, Mr. DiPerna shall be paid by us or our successor in interest a lump sum cash payment equal to 12 months of Mr. DiPerna’s then annual Base Compensation (as defined in the employment agreement); and |
| · | immediately prior to such change of control, any unvested stock options or other unvested securities of ours issued to Mr. DiPerna shall automatically accelerate and immediately become fully vested and exercisable. |
In June 2020, our board of directors approved an amendment to the employment agreement to provide that Mr. DiPerna’s base salary would be paid entirely in cash commencing July 1, 2020. The payment of the additional cash component of Mr. DiPerna’s annual base salary ($8,333.33 per month) was initially be deferred (the Deferred Salary) and accrue for Mr. DiPerna’s benefit until the Company has received $5,000,000 of cumulative gross proceeds of financing, at which time the Deferred Salary shall be paid to Mr. DiPerna and the salary deferrals will cease. The salary deferrals ceased and the Deferred Salary was paid to Mr. DiPerna in May 2021. In August 2021, Mr. DiPerna resigned as our chief executive officer, and he continues to serve as our president, chief financial officer, treasurer and chairman of our board of directors.
| 67 |
If a change of control occurred on March 31, 2022, under his employment agreement, Mr. DiPerna would be entitled to:
| · | payment of a lump sum of $300,000 within 60 days of the time at which such change of control takes place; and |
| · | accelerated vesting of 27,778 shares of common stock under an unvested stock option. The value of the shares subject to accelerated vesting is calculated as the intrinsic value per share multiplied by the number of shares that would become fully vested upon a change of control. The intrinsic value per share would be calculated as the excess of the closing price of the common stock of $4.40 on the Nasdaq Capital Market on March 31, 2022 over the exercise price of the option. As of March 31, 2022, the intrinsic value was zero. |
In connection with our acquisition of Quasuras, we entered into an Intellectual Property Transfer Agreement dated as of July 24, 2017, with Quasuras and Mr. DiPerna, pursuant to which Mr. DiPerna transferred to us all intellectual property rights owned directly and/or indirectly by him related to our business. Separately, we agreed to pay Mr. DiPerna, as part of his compensation for services to be performed for us, pursuant to a royalty agreement, certain fees based upon future sales, if any, of our potential product subject to a maximum $10,000,000 cap on the aggregate amount of fees that Mr. DiPerna could earn from such arrangement.
The Vos Employment Agreement
On August 11, 2021, we entered into a two-year employment agreement (the Agreement) with Ms. Vos for her service as our chief executive officer, and the Agreement renews for one-year terms, unless either party provides the other with 90-day prior written notice of termination. The Agreement provided that Ms. Vos was entitled to total base compensation of $300,000 annually, as follows: a cash salary of $250,000 per year (the Cash Salary), plus deferred salary of $50,000 per year (the Deferred Salary and, together with the Cash Salary, the Base Compensation).
On February 23, 2022, Ms. Vos informed our board of directors that she was resigning from her position as our chief executive officer, effective immediately (the Resignation). In connection with the Resignation, we and Ms. Vos entered into a Severance and Release Agreement dated February 23, 2022 (the Separation Agreement). Pursuant to the Separation Agreement, Ms. Vos is entitled to receive separation payments in an aggregate gross amount of $375,000. Under the terms of the Separation Agreement, the vesting of an option to purchase 362,452 shares of the Company’s common stock, which was granted to Ms. Vos on August 11, 2021, ceased on May 24, 2022 and the remaining unvested shares were forfeited.
Communications with our Board of Directors
Stockholders who desire to communicate with the board of directors, or a specific director, may do so by sending the communication addressed to either the corporate secretary, the board of directors or any individual director, c/o Modular Medical, Inc., 16772 West Bernardo Drive, San Diego, California 92127. These communications will be delivered to the board of directors, or any individual director, as specified.
Corporate Governance
Board Leadership Structure and Role in Risk Oversight
Due to the small size and early stage of the Company, we have not adopted a formal policy on whether the chairman and chief executive officer positions should be separate or combined. Since 2017, Mr. DiPerna has been serving as our chairman, and, since February 23, 2022, Mr. Besser has been serving as our chief executive officer. Our board of directors has oversight responsibility for our risk management processes. Our board of directors receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate, regarding our assessment of risks. Our board of directors will focus on the most significant risks facing us and our general risk management strategy, and also ensure that risks undertaken by us are consistent with our appetite for risk. While our board of directors oversees our risk management processes, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing us and that the leadership structure of our board of directors supports this approach.
| 68 |
We have established an audit committee, a compensation committee, and a nominating and governance committee. Each committee’s members and functions are described below.
Audit Committee
Our board of directors established the audit committee (the Audit Committee) for the purpose of overseeing the accounting and financial reporting processes and audits of our financial statements. The Audit Committee also is charged with reviewing any internal control violations under our whistleblower policy. The responsibilities of our audit committee are described in the Audit Committee Charter adopted by our board of directors, a current copy of which can be found on the investors section of our website, www.modular-medical.com.
Ms. Volkart, Mr. Felsher and Mr. Sheibley are the current members of the Audit Committee. Mr. Felsher serves as the chairperson and has been designated by the board of directors as the “audit committee financial expert,” as defined by Item 407(d)(5) of Regulation S-K under the Securities Act of 1933, as amended, and the Exchange Act. That status does not impose duties, liabilities or obligations that are greater than the duties, liabilities or obligations otherwise imposed on her as a member of the audit committee and the board of directors, however. Our board of directors has determined that each of our Audit Committee members satisfies the “independence” requirements of the Nasdaq listing rules and meets the independence standards under Rule 10A-3 under the Exchange Act.
Compensation Committee
Our board of directors established a compensation committee for the purpose of reviewing, recommending and approving our compensation policies and benefits, including the compensation of all of our executive officers and directors. Mr. Febbo and Ms. Volkart are the current members of the compensation committee, and Mr. Febbo serves as the chairperson. Each of our compensation committee members satisfies the “independence” requirements of the Nasdaq listing rules and meets the independence standards under Rule 10A-3 under the Exchange Act.
Our compensation committee is responsible for reviewing, recommending and approving our compensation policies and benefits, including the compensation of all of our executive officers and directors, and it also has the principal responsibility for the administration of our equity incentive plan. The responsibilities of our compensation committee are more fully described in the Compensation Committee Charter adopted by our board of directors, a current copy of which can be found on the investors section of our website, www.modular-medical.com.
Nominating and Corporate Governance Committee
The Nominating and Governance Committee consists of Mr. Sheibley and Mr. Felsher, and Mr. Sheibley serves as the chairperson. Each of the members of our Nominating and Governance Committee satisfies the “independence” requirements of the Nasdaq listing rules and meets the independence standards under Rule 10A-3 under the Exchange Act. The Nominating and Governance Committee will consider persons recommended by stockholders for inclusion as nominees for election to our board of directors if the information required by our bylaws is submitted in writing in a timely manner addressed and delivered to our secretary at the address of our executive offices.
The Nominating and Governance Committee will identify and evaluate nominees for our board of directors, including nominees recommended by stockholders, based on numerous factors it considers appropriate, some of which may include strength of character, mature judgment, career specialization, relevant technical skills, diversity, and the extent to which the nominee would fill a present need on our board of directors. The responsibilities of our Nominating and Governance committee are more fully described in the Nominating and Governance Committee Charter adopted by our board of directors, a current copy of which can be found on the investors section of our website, www.modular-medical.com.
Code of Business Conduct and Ethics for Employees, Executive Officers and Directors
We have adopted a Code of Business Conduct and Ethics, or the Code of Conduct, applicable to all of our employees, executive officers and members of our board of directors. The Code of Conduct is available on our website at www.modular-medical.com. Our Nominating and Governance Committee is responsible for overseeing the Code of Conduct, and our board of directors must approve any waivers of the Code of Conduct. In addition, we intend to post on our website all disclosures that are required by law concerning any amendments to, or waivers from, any provision of the Code of Conduct.
| 69 |
Board Diversity
We seek diversity in experience, viewpoint, education, skill, and other individual qualities and attributes to be represented on our board of directors. We believe directors should have various qualifications, including individual character and integrity; business experience; leadership ability; strategic planning skills, ability, and experience; requisite knowledge of our industry and finance, accounting, and legal matters; communications and interpersonal skills; and the ability and willingness to devote time to our company. We also believe the skill sets, backgrounds, and qualifications of our directors, taken as a whole, should provide a significant mix of diversity in personal and professional experience, background, viewpoints, perspectives, knowledge, and abilities. Nominees are not to be discriminated against on the basis of race, religion, national origin, sex, sexual orientation, disability, or any other basis proscribed by law. The assessment of prospective directors is made in the context of the perceived needs of our board of directors from time to time.
All of our directors have held high-level positions in business or professional service firms and have experience in dealing with complex issues. We believe that all of our directors are individuals of high character and integrity, are able to work well with others, and have committed to devote sufficient time to the business and affairs of our company. In addition to these attributes, the description of each director’s background set forth above indicates the specific qualifications, skills, perspectives, and experience necessary to conclude that each individual should continue to serve as a director of ours.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires our directors, executive officers and persons who own more than 10% of a registered class of our equity securities to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of ours. Directors, executive officers and greater than 10% holders are required by SEC regulation to furnish us with copies of all Section 16(a) reports they file. Based on our review of Forms 3 and 4 filed during fiscal 2022 (and any written representations to us by such persons), we believe that all directors, executive officers and 10% stockholders complied with all applicable Section 16(a) filing requirements during fiscal 2022, except that:
| · | Mr. DiPerna failed to timely file a Form 4; |
| · | Mr. Felsher failed to timely file a Form 3 and a Form 4; and |
| · | Mr. Febbo failed to timely file a Form 4. |
| 70 |
ITEM 11. EXECUTIVE COMPENSATION
SUMMARY COMPENSATION TABLE
The following table sets forth compensation information for fiscal 2022 and 2021 for each of our named executive officers.
| Name and Principal Position | Year | Salary ($) | Stock Awards ($) | Option Awards ($)(1) | Non-Equity Incentive Plan Compensation ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||||||
| Paul DiPerna, President, Chief Financial Officer, | 2022 | 370,833 | (3) | — | — | — | — | 370,833 | |||||||||||||||||||
| Treasurer and Chairman (2) | 2021 | 200,000 | — | 25,000 | — | 50,000 | (4) | 275,000 | |||||||||||||||||||
| James E. Besser, Chief Executive Officer (5) | 2022 | — | — | — | — | — | — | ||||||||||||||||||||
| Ellen O’Connor Vos, Chief Executive Officer (6) | 2022 | 133,654 | — | 4,414,645 | — | 409,662 | (7) | 4,957,961 | |||||||||||||||||||
| Stephen Daly, Chief Commercial Officer (8) | 2022 | 59,395 | — | — | — | 6,046 | 65,441 | ||||||||||||||||||||
| 2021 | 234,000 | — | — | — | — | 234,000 | |||||||||||||||||||||
| (1) | Award amounts reflect the aggregate grant date fair value with respect to awards granted, as determined pursuant to FASB ASC Topic 718. The assumptions used to calculate the aggregate grant date fair value of option awards are set forth in the notes to the consolidated financial statements included in item 8 of this Report. These amounts do not reflect actual compensation earned or to be earned by our named executive officers. |
| (2) | From August 2018 until June 30, 2020, Mr. DiPerna’s $300,000 annual salary was paid $200,000 in cash and $100,000 in fully-vested stock options granted monthly. |
| (3) | Includes payment of $70,833 of deferred salary. |
| (4) | Earned as a bonus of which $22,000 was paid on April 30, 2021, and the remainder was paid in quarterly installments commencing on July 15, 2021. |
| (5) | Ms. Besser was appointed our chief executive officer in February 2022 and is paid de minimis compensation of $1.00 per year. |
| (6) | Ms. Vos was appointed our chief executive officer in August 2021 at an annual cash salary of $250,000 per year plus deferred salary of $50,000 per year. She resigned as our chief executive officer in February 2022. The compensation amounts disclosed in the table above exclude amounts paid to Ms. Vos for her service as a non-employee director. |
(7)
|
Represents payment during fiscal 2022 of i) accrued holiday and vacation pay, ii) deferred salary and iii) three months of salary for the notice period and accrued severance of $300,000 that will be paid in fiscal 2023. |
| (8) | Mr. Daly became our chief commercial officer in March 2020 at an annual base salary of $250,000. In February 2021, Mr. Daly converted to part time, and his annual base salary was reduced to $125,000. Mr. Daly resigned as our Chief Commercial Officer in September 2021, and we and Mr. Daly entered into a consulting arrangement pursuant to which Mr. Daly provides services to us on a part-time basis. |
| 71 |
Outstanding Equity Awards at Fiscal Year-End
The following table shows certain information regarding outstanding equity awards held by our named executive officers as of March 31, 2022.
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price($) | Option Expiration Date(1) | ||||||||||
| Paul DiPerna | 1,155 | (2) | — | 9.48 | 6/1/2030 | |||||||||
| 1,169 | (3) | — | 9.48 | 5/1/2030 | ||||||||||
| 1,170 | (4) | — | 9.48 | 4/1/2030 | ||||||||||
| 1,660 | (5) | — | 7.44 | 3/2/2030 | ||||||||||
| 1,745 | (6) | — | 7.44 | 2/1/2030 | ||||||||||
| 1,727 | (7) | — | 7.44 | 1/1/2030 | ||||||||||
| 1,809 | (8) | — | 6.75 | 12/1/2029 | ||||||||||
| 1,811 | (9) | — | 6.75 | 11/1/2029 | ||||||||||
| 1,721 | (10) | — | 6.75 | 10/1/2029 | ||||||||||
| 1,662 | (11) | — | 6.75 | 9/15/2029 | ||||||||||
| 1,666 | (12) | — | 6.75 | 8/15/2029 | ||||||||||
| 1,660 | (13) | — | 6.75 | 7/15/2029 | ||||||||||
| 1,650 | (14) | — | 6.75 | 6/15/2029 | ||||||||||
| 1,677 | (15) | — | 6.75 | 5/15/2029 | ||||||||||
| 1,624 | (16) | — | 6.75 | 4/15/2029 | ||||||||||
| 1,694 | (17) | — | 6.75 | 3/15/2029 | ||||||||||
| 1,641 | (18) | — | 6.75 | 2/15/2029 | ||||||||||
| 1,603 | (19) | — | 6.75 | 1/15/2029 | ||||||||||
| 1,775 | (20) | — | 6.75 | 12/15/2028 | ||||||||||
| 1,775 | (21) | — | 6.75 | 11/15/2028 | ||||||||||
| 6,005 | (22) | — | 1.98 | 10/15/2028 | ||||||||||
| 6,005 | (23) | — | 1.98 | 09/15/2028 | ||||||||||
| 6,005 | (24) | — | 1.98 | 08/15/2028 | ||||||||||
| 2,222 | (25) | 27,778 | 6.75 | 11/25/2029 | ||||||||||
| (1) | The standard option term is ten years, but all of the options expire automatically unless exercised within 90 days after the cessation of service as an employee, director or consultant. |
| (2) | The option was granted on June 1, 2020, and the shares subject to this option were fully vested on the grant date. |
| (3) | The option was granted on May 1, 2020, and the shares subject to this option were fully vested on the grant date. |
| (4) | The option was granted on April 1, 2020, and the shares subject to this option were fully vested on the grant date. |
| (5) | The option was granted on March 2, 2020, and the shares subject to this option were fully vested on the grant date. |
| (6) | The option was granted on February 1,2020, and the shares subject to this option were fully vested on the grant date. |
| (7) | The option was granted on January 1, 2020, and the shares subject to this option were fully vested on the grant date. |
| (8) | The option was granted on December 1, 2019, and the shares subject to this option were fully vested on the grant date. |
| (9) | The option was granted on November 1, 2019, and the shares subject to this option were fully vested on the grant date. |
| (10) | The option was granted on October 1, 2019, and the shares subject to this option were fully vested on the grant date. |
| (11) | The option was granted on September 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (12) | The option was granted on August 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (13) | The option was granted on July 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (14) | The option was granted on June 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (15) | The option was granted on May 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (16) | The option was granted on April 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (17) | The option was granted on March 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (18) | The option was granted on February 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (19) | The option was granted on January 15, 2019, and the shares subject to this option were fully vested on the grant date. |
| (20) | The option was granted on December 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| (21) | The option was granted on November 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| 72 |
| (22) | The option was granted on October 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| (23) | The option was granted on September 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| (24) | The option was granted on August 15, 2018, and the shares subject to this option were fully vested on the grant date. |
| (25) | The option was granted on November 25, 2019, and the shares subject to this option vest monthly over three years commencing January 1, 2020, subject to continued service as an employee, director or consultant. |
Employment Agreements
We have entered into our standard form of employment, confidential information and invention assignment agreement with each of our named executive officers. We also have entered into agreements to indemnify our directors and executive officers, in addition to the indemnification provided for in our certificate of incorporation and bylaws. These agreements, among other things, provide for indemnification of our directors and certain executive officers for many expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by any such person in any action or proceeding, including any action by or in the right of the Company, arising out of such person’s services as a director or executive officer of ours, any subsidiary of ours or any other company or enterprise to which such person provided services at our request.
Director Compensation
Effective April 1, 2021, our board of directors approved our outside (non-employee) director compensation plan (the Director Plan). Pursuant to the Director Plan, outside directors are paid the following annual retainers:
| · | $ 25,000 for service as a member of the board of directors; | ||
| · | $5,000 for service as chair of the audit committee; and | ||
| · | $5,000 for service as chair of the compensation committee. |
The annual retainers will be paid in quarterly installments in either cash, options to purchase shares of our common stock or in shares of our common stock, as directed by each director based on an annual election. In addition, under the Director Plan, each director will also receive an annual service equity award of $100,000 paid in quarterly installments in either options to purchase shares of our common stock or shares of our common stock, as directed by each director based on an annual election.
In addition, upon appointment to our board of directors, we award our non-employee directors a stock option grant under our Amended 2017 Equity Incentive Plan (the 2017 Plan). During fiscal 2022, we awarded each of the new non-employee directors a stock option to purchase 16,667 shares of our common stock. These options vest annually over three years from the date of appointment to our board of directors.
The following table summarizes the compensation earned by our non-employee directors in fiscal 2022:
| Fee | Restricted Stock | Option | ||||||||||||||||||
| Compensation | Awards | Awards | All Other | Total | ||||||||||||||||
| Name | ($) | ($) | ($)(1)(2) | Compensation(3) | ($) | |||||||||||||||
| Liam Burns(4) | 18,750 | — | 222,291 | — | 241,041 | |||||||||||||||
| William Febbo | 30,000 | — | — | 100,000 | 130,000 | |||||||||||||||
| Steven Felsher(5) | — | — | 194,981 | 8,657 | 203,638 | |||||||||||||||
| Morgan Frank | — | — | 375,105 | — | 375,105 | |||||||||||||||
| Philip Sheibley(5) | 8,424 | — | 169,588 | 33,696 | 211,078 | |||||||||||||||
| Carmen Volkart | — | — | 296,423 | 29,671 | 326,094 | |||||||||||||||
| Ellen O’Connor Vos(6) | 2,953 | — | 281,339 | — | 284,292 | |||||||||||||||
| (1) | Award amounts reflect the aggregate grant date fair value with respect to awards granted, as determined pursuant to FASB ASC Topic 718. The assumptions used to calculate the aggregate grant date fair value of option awards are set forth in the notes to the consolidated financial statements included in Item 8 of this Annual Report on Form 10-K. These amounts do not reflect actual compensation earned or to be earned by our directors. |
| (2) | As of March 31, 2022, our non-employee directors each held outstanding options to purchase the following number of shares of our common stock: William Febbo, 66,667; Steven Felsher, 38,084; Morgan Frank, 100,699 ; Philip Sheibley, 16,667; Carmen Volkart; 90,558 and Ellen O’Connor Vos, 111,873 . |
| (3) | Represents stock awards; we calculated the estimated fair value of the stock awards issued to our non-employee directors using the closing price per share of our common stock on the day prior to the grant date in accordance with the Director Plan. |
| 73 |
| (4) | Mr. Burns resigned as a director on December 31, 2021. |
| (5) | Messrs. Felsher and Sheibley were appointed to our board of directors on November 29, 2021. |
| (6) | Ms. Vos was appointed to our board of directors in May 2021 and as our chief executive officer in August 2021. In February 2022, Ms. Vos resigned as our chief executive officer. |
ITEM 12: SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information as of May 31, 2022 concerning the ownership of our common stock by:
| · | each shareholder known by us to be the beneficial owner of more than 5% of the outstanding shares of our common stock (currently our only class of voting securities); |
| · | each of our directors; |
| · | each of our executive officers; and |
| · | all directors and executive officers as a group. |
Beneficial ownership is determined in accordance with Rule 13d-3 of the Exchange Act, and includes all shares over which the beneficial owner exercises voting or investment power. Shares that are issuable upon the exercise of options, warrants and other rights to acquire common stock that are presently exercisable or exercisable within 60 days of May 31, 2022 are reflected in a separate column in the table below. These shares are taken into account in the calculation of the total number of shares beneficially owned by a particular holder and the total number of shares outstanding for the purpose of calculating percentage ownership of the particular holder. We have relied on information supplied by our officers, directors and certain stockholders and on information contained in filings with the SEC. Except as otherwise indicated, and subject to community property laws where applicable, we believe, based on information provided by these persons, that the persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them. The percentage of beneficial ownership is based on 10,911,684 shares of common stock outstanding as of May 31, 2022.
Unless otherwise stated, the business address of each of our directors and executive officers listed in the table is 16772 West Bernardo Drive, San Diego, California 92127.
| Name and principal position | Number of Shares Beneficially Owned (Excluding Outstanding Options and Warrants)(1) | Number of Shares Issuable on Exercise of Outstanding Options and Warrants(2) | Percent of Class | |||||||||
| JEB Partners, L.P. | 2,720,577 | (3) | 653,511 | 29.17 | ||||||||
| Manchester Explorer, L.P. | 2,720,577 | (3) | 653,511 | 29.17 | ||||||||
| Manchester Management LLC | 2,720,577 | (3) | 653,511 | 29.17 | ||||||||
| Sio Capital Management, LLC | 449,438 | (4) | 712,000 | (5) | 9.99 | |||||||
| Directors and Officers: | ||||||||||||
| James E. Besser | 2,720,577 | 653,511 | 29.17 | |||||||||
| Paul DiPerna | 2,553,586 | (6) | 186,682 | 24.69 | ||||||||
| William Febbo | 79,105 | 113,260 | 1.74 | |||||||||
| Steven Felsher | 2,174 | 21,417 | * | |||||||||
| Morgan C. Frank | 2,720,577 | (3) | 737,554 | 29.69 | ||||||||
| Philip Sheibley | 8,139 | — | * | |||||||||
| Carmen Volkart | 3,999 | 73,892 | * | |||||||||
| Ellen O’Connor Vos(5) | 18,519 | 95,206 | 1.03 | |||||||||
| All current directors and executive officers as a group (8 persons) | 5,386,099 | 928,131 | 58.16 | |||||||||
| * | Represents less than 1% |
| (1) | Excludes shares subject to outstanding options and warrants to acquire common stock that are exercisable within 60 days of May 31, 2022. |
| (2) | Represents the number of shares subject to outstanding options and warrants to acquire common stock that are exercisable within 60 days of May 31, 2022. |
| 74 |
| (3) | Includes (i) 124,750 shares directly held by Mr. Besser, of which: (a) 60,277 shares were received in exchange for Mr. Besser’s shares as a result of our acquisition of Quasuras; (b) 29,630 shares purchased in a private placement in 2018 (the 2018 Placement) and (c) 34,843 shares were purchased in a private placement in 2020 (the 2020 placement); (ii) 2,218,077 held by Manchester Explorer, L.P. of which: (a) 1,515,152 shares were purchased in a private placement in 2017 (the 2017 Placement), (b) 157,037 shares were purchased in the 2018 Placement, (c) 11,614 were purchased in the 2020 Placement, (d) 300,000 shares were purchased in a public offering in February 2022, and (e) 234,274 shares were acquired upon the conversion of a convertible note in February 2022; (iii) 317,473 shares held by JEB Partners, L.P. of which (a) 252,526 shares were purchased in the 2017 Placement, (b) 53,333 shares were purchased in the 2018 Placement and (c) 11,614 shares were purchased in the 2020 Placement; and (iv) 60,277 shares held by Mr. Frank, which shares were received in our acquisition of Quasuras in exchange for Mr. Frank’s shares of Quasuras. Mr. Besser, as the managing member, and Mr. Frank, as the portfolio manager and consultant of Manchester Management, LLC, (MMC) the general partner of Manchester Explorer, L.P. and JEB Partners, L. P., have shared voting and dispositive power over shares held by Manchester Explorer, L.P. and JEB Partners, L.P. The address for Manchester Explorer, L.P is c/o MMC, 2 Calle Candina, No. 1701, San Juan, Puerto Rico 00907. |
| (4) | Consists of the following shares of Common Stock acquired in the Offering: (i) 144,438 shares of Common Stock held by Sio Partners LP (“Partners”), (ii) 85,000 shares of Common Stock held by Sio Partners Offshore LTD (Offshore), (iii) 81,000 shares of Common Stock held by Compass MAV LLC (Compass), (iv) 49,000 shares of Common Stock held by Compass Offshore MAV LTD (Compass Offshore), (v) 27,000 shares of Common Stock held by Walleye Manager Opportunities LLC (Walleye Manager) and (vi) 63,000 shares of Common Stock held by Walleye Opportunities Master Fund Ltd. (Walleye Master). Sio Capital Management, LLC (Sio Management) is the investment manager of Partners, Offshore, Compass, Compass Offshore, Walleye Manager and Walleye Master, and Michael Castor is the sole owner and Managing Member of Sio Management. Sio Management and Mr. Castor may be deemed to beneficially own the securities held by Partners, Offshore, Compass, Compass Offshore, Walleye Manager, and Walleye Master. Each of Sio Management and Mr. Castor disclaim beneficial ownership of any of the shares of our Common Stock they may be deemed to beneficially own except to the extent of their respective pecuniary interest therein. The address for Sio Management, Mr. Castor, Partners, Offshore, Compass, Compass Offshore, Walleye Manager and Walleye Master is 600 Third Avenue, New York, New York 10016. |
(5)
|
These shares are issuable upon exercise of outstanding pre-funded warrants to purchase shares of our Common Stock. As of May 31, 2022, Sio Management held 1,348,314 pre-funded warrants to purchase shares of Common Stock. Pursuant to the terms of the pre-funded warrants, Sio Management cannot exercise such pre-funded warrants if Sio Management would beneficially own, after such exercise, more than 9.99% of the outstanding shares of our Common Stock. Accordingly, pre-funded warrants to purchase 636,314 shares of our Common Stock have been excluded from the table above. |
| (6) | Includes (i) 2,000,000 shares directly held by the Paul DiPerna Irrevocable Trust, (ii) 333,334 shares directly held by Mr. DiPerna’s adult daughters, Kelsie DiPerna and Alaria DiPerna, which shares Mr. DiPerna has sole voting power over; (iii) 207,906 shares directly held by the Paul DiPerna Trust, of which 101,010 shares were purchased in the 2017 Placement and 23,429 shares were acquired upon the conversion off a convertible note in February 2022 and (iv) 12,346 shares held by Mr. DiPerna. The 2,000,000 shares held by the Paul DiPerna Irrevocable Trust, 333,334 shares held by Mr. DiPerna’s adult daughters and 73,480 shares held by the Paul DiPerna Trust that were issued in 2017 to Mr. DiPerna in the Control Block Acquisition and transferred to such persons in December 2020 by Mr. DiPerna. Mr. DiPerna is the chairman of our board of directors, and also serves as our president, chief financial officer and treasurer. Mr. DiPerna is the trustee of both the Paul DiPerna Irrevocable Trust and the Paul DiPerna Trust. |
| 75 |
ITEM 13: CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with Related Persons
As disclosed elsewhere in this Annual Report on Form 10-K, Mr. DiPerna, is a party to related party transactions with us, see Item 10. Mr. DiPerna’s daughter is an employee of ours, and, during fiscal 2022, we paid her $169,589, which includes the aggregate grant date fair value, as determined pursuant to FASB ASC Topic 718, of a stock option granted in November 2021.
In February 2021, Mr. DiPerna and Manchester Explorer, L.P. (the Related Party Holders), which is represented by Mr. Frank on our board of directors, purchased $100,000 and $1,000,000, aggregate principal amount of our convertible notes and received warrants to purchase 119,237 and 11,924 shares of our common stock (the Note Warrants), respectively. Effective April 30, 2021, the Related Party Holders entered into revocation agreements with the Company pursuant to which their collective $1,100,000 aggregate principal amount of convertible notes and accrued interest of $50,091 were replaced with new convertible notes. In connection with a public offering of our equity securities in February 2022, the convertible notes and accrued interest held by the Related Party Holders were converted into our equity securities and Mr. DiPerna received 23,429 shares of our common stock and a warrant to purchase 23,429 shares of our common stock at an exercise price of $6.60 per share and Manchester Explorer, L. P. received 234,274 shares of our common stock and a warrant to purchase 234, 274 shares of our common stock at an exercise price of $6.60 per share. In addition, the exercise prices of the Note Warrants were reduced to $6.00 per share.
In May 2021, Mr. Febbo purchased $200,000 aggregate principal amount of our convertible notes and received a warrant to purchase 23,229 shares of our common stock (the Director Warrant). In connection with a public offering of our equity securities in February 2022, the convertible note held by Mr. Febbo was converted into our equity securities. Upon conversion, Mr. Febbo received 45,586 shares of our common stock and a warrant to purchase 45,586 shares of our common stock at an exercise price of $6.60 per share. In addition, the exercise price of the Director Warrant was reduced to $6.00 per share.
In October 2021, we sold 12,346 shares of common stock to Mr. DiPerna and 18,519 shares to Ms. Vos at a price per share of $8.10 in a private placement.
Director Independence
Our board of directors has determined that each of the current directors, with the exception of Mr. DiPerna, Mr. Frank and Ms. Vos, is “independent,” as defined by the listing rules of the NASDAQ Stock Market, or Nasdaq, and the rules and regulations of the SEC. Our board of directors has standing Audit, Compensation and Nominating and Governance Committees, each of which is comprised solely of independent directors in accordance with the Nasdaq listing rules. No director qualifies as independent unless the board of directors affirmatively determines that he has no direct or indirect relationship with us that would impair his independence. We independently review the relationship of the Company to any entity employing a director or on whose board of directors he is serving currently.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table shows the fees billed to us by Farber Hass Hurley LLP, or Farber, our independent registered public accounting firm, for the audit of our consolidated financial statements and other services provided.
| Year ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Audit fees(1) | $ | 43,000 | $ | 34,500 | ||||
| Audit-related fees(2) | 10,200 | 3,700 | ||||||
| Total(3) | $ | 53,200 | $ | 38,200 | ||||
| (1) | Audit fees consisted of fees for professional services rendered for the audit of our annual consolidated financial statements and reviews of our quarterly consolidated financial statements |
| (2) | Audit-related fees consisted of fees for services related to our issuance of SEC registration statements and sales of common stock under registration statements. |
| (3) | Farber did not provide any non-audit or other services other than those reported under “Audit fees” and “Audit-related fees.” |
| 76 |
PART IV
ITEM 15: EXHIBITS
| 77 |
| (1) | As filed with the Registrant’s Current Report on Form 8-K filed July 28, 2017, and incorporated herein by reference. |
| (2) | As filed with the Registrant’s Current Report on Form 8-K filed May 12, 2021, and incorporated herein by reference. |
| (3) | As filed with the Registrant’s Current Report on Form 8-K filed June 29, 2017, and incorporated herein by reference. |
| (4) | As filed with the Registrant’s Current Report on Form 8-K filed December 1, 2021, and incorporated herein by reference. |
| (5) | As filed with the Registrant’s Annual Report on Form 10-K/A for the year ended June 30, 2008, and incorporated herein by reference. |
| (6) | As filed with the Registrant’s Quarterly Report on Form 10-Q filed November 12, 2018, and incorporated herein by reference. |
| (7) | As filed with the Registrant’s Current Report on Form 8-K filed April 5, 2017, and incorporated herein by reference. |
| (8) | As filed with the Registrant’s Current Report on Form 8-K filed November 20, 2018 and incorporated herein by reference. |
| (9) | As filed with the Registrant’s Registration Statement on Form S-1, as amended, originally filed June 27, 2019, declared effective October 22, 2019 (Commission File No. 333-232377), and incorporated herein by reference. |
| (10) | As filed with the Registrant’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2019, and incorporated herein by reference. |
| (11) | As filed with the Registrant’s Registration Statement on Form S-1, as amended, originally filed April 9, 2020, declared effective May 11, 2020 (Commission File No. 333-237615), and incorporated herein by reference. |
| (12) | As filed with the Registrant’s Current Report on Form 8-K filed May 27, 2020, and incorporated herein by reference. |
| (13) | As filed with the Registrant’s Current Report on Form 8-K filed May 12, 2020, and incorporated herein by reference. |
| (14) | As filed with the Registrant’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2020, and incorporated herein by reference. |
| (15) | As filed with the Registrant’s Annual Report on Form 10-K filed June 29, 2021, and incorporated herein by reference. |
| (16) | As filed with the Registrant’s Current Report on Form 8-K filed August 16, 2021, and incorporated herein by reference. |
| (17) | As filed with the Registrant’s Current Report on Form 8-K filed October 29, 2021, and incorporated herein by reference. |
| (18) | As filed with the Registrant’s Current Report on Form 8-K filed February 14, 2022, and incorporated herein by reference. |
| (19) | As filed with the Registrant’s Registration Statement on Form S-1 filed February 9, 2022, and incorporated herein by reference. |
| (20) | As filed with the Registrant’s Current Report on Form 8-K filed May 5, 2022, and incorporated herein by reference. |
| (21) | As filed with the Registrant’s Registration Statement on Form S-1 filed June 6, 2022 (Commission File No. 333-265444), and incorporated herein by reference. |
| + | Management contract, compensatory plan or arrangement. |
| * Filed herewith |
Item 16. Form 10-K Summary
Not applicable.
| 78 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on the 28th day of June, 2022.
MODULAR MEDICAL, INC.
| By: | /s/ James E. Besser | |||
| James E. Besser | ||||
| Chief Executive Officer, | ||||
| (Principal Executive Officer) |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints James E. Besser and Paul DiPerna as her/his true and lawful attorneys-in-fact and agent, with full power of substitution and resubstitution, for her and him and in her or his name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in- fact and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Title | Date | ||
| /s/ James E. Besser | Chief Executive Officer (Principal Executive Officer) | June 28, 2022 | ||
| James E. Besser | ||||
| /s/ Paul DiPerna | Chairman, President and Chief Financial Officer (Principal Financial and Accounting Officer) | June 28, 2022 | ||
| Paul DiPerna | ||||
| /s/ William Febbo | Director | June 28, 2022 | ||
| William Febbo | ||||
| /s/ Steven Felsher | Director | June 28, 2022 | ||
| Steven Felsher | ||||
| /s/ Morgan C. Frank | Director | June 28, 2022 | ||
| Morgan C. Frank | ||||
| /s/ Philip Sheibley | Director | June 28, 2022 | ||
| Philip Sheibley | ||||
| /s/ Carmen Volkart | Director | June 28, 2022 | ||
| Carmen Volkart | ||||
| /s/ Ellen O’Connor Vos | Director | June 28, 2022 | ||
| Ellen O’Connor Vos |
| 79 |