UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
☒
For the fiscal year ended
☐
For the transition period from ____________ to ____________
Commission file number
HENRY SCHEIN, INC
.
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of
(I.R.S. Employer Identification No.)
incorporation or organization)
,
(Address of principal executive offices)
(Zip Code)
)
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class
Trading Symbol(s)
Name of each exchange on which registered
The Nasdaq Global Select Market
Securities registered pursuant to Section 12(g) of the Act: None
YES
:
☒
☐
YES:
☐
NO
:
☒
during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing
requirements for the past 90 days.
YES
:
☒
☐
Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
YES
:
☒
☐
emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth
company” in Rule 12b-2 of the Exchange Act.
:
☒
Accelerated filer:
☐
☐
Smaller reporting company:
☐
growth company:
☐
new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
☐
control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared
or issued its audit report. YES:
☐
YES:
☐
☒
quoted on the Nasdaq Global Select Market on June 27, 2020, was approximately $
.
Documents Incorporated by Reference:
Portions of the Registrant’s definitive proxy statement to be filed pursuant to Regulation 14A not later than 120 days after the end of the fiscal year
(December 26, 2020) are incorporated by reference in Part III hereof.
2
TABLE OF CONTENTS
Page
Number
3
24
37
38
38
38
39
41
43
67
69
124
124
127
127
127
128
128
128
128
136
137
3
PART I
ITEM 1. Business
General
Henry Schein, Inc. is a solutions company for health care professionals powered by a network of people and
technology. We believe we are the world’s largest provider of health care products and services primarily to office-
based dental and medical practitioners, as well as alternate sites of care. Our philosophy is grounded in our
commitment to help customers operate a more efficient and successful business so the practitioner can provide
better clinical care.
With more than 88 years of experience distributing health care products, we have built a vast set of small, mid-sized
and large customers in the dental and medical markets, serving more than one million customers worldwide across
dental practices and laboratories and physician practices, as well as government, institutional health care clinics and
other alternate care clinics.
We are headquartered in Melville, New York, employ more than 19,000 people (of which approximately 9,800 are
based outside the United States) and have operations or affiliates in 31 countries and territories, including the
United States, Australia, Austria, Belgium, Brazil, Canada, Chile, China, the Czech Republic, France, Germany,
Hong Kong SAR, Ireland, Israel, Italy, Japan, Liechtenstein, Luxembourg, Malaysia, the Netherlands, New
Zealand, Poland, Portugal, Singapore, South Africa, Spain, Sweden, Switzerland, Thailand, United Arab Emirates
and the United Kingdom. This broad global footprint has evolved over time through our organic success as well as
through contribution from strategic acquisitions.
Our business extends far beyond our supply chain capabilities across the globe. We provide a wide breadth
of products, value-added solutions and support to customers, including consumables and equipment. Through
Henry Schein One, we offer dental practice management, patient engagement and demand creation software
solutions. We also offer a broad range of financial services for our customers to help them operate and expand their
business operations. We believe our hands-on consultative approach to support practice decision-making is a key
differentiator for our business.
We offer a comprehensive selection of more than 120,000 branded products and Henry Schein private brand
products in stock, as well as more than 180,000 additional products available as special-order items.
As the market continues to evolve toward solutions that offer ease and convenience for ordering products and
communicating with our solutions teams, we are investing in digital enhancements to our e-commerce platforms
and our web capabilities.
We have established over 3.5 million square feet of space in 28 strategically located distribution centers around the
world to enable us to better serve our customers and increase our operating efficiency. Our infrastructure allows us
to provide rapid and accurate order fulfillment. Historically, approximately 99% of items have been shipped
without back ordering and were shipped on the same business day the order is received. Due to the significant
increase in demand for personal protective equipment (“PPE”), as a result of the COVID-19 pandemic, during the
year ended December 26, 2020, approximately 93% of items ordered were shipped without back ordering and 90%
were shipped on the same business day the order was received. As the demand for PPE stabilizes, we expect our
percentage of items shipped without back ordering and shipped on the same day to return to historic levels. This
infrastructure, together with broad product and service offerings at competitive prices, and a strong commitment to
customer service, enables us to be a single source of supply for our customers’ needs.
We conduct our business through two reportable segments: (i) health care distribution and (ii) technology and
value-added services. These segments offer different products and services to the same customer base.
The health care distribution reportable segment aggregates our global dental and medical operating segments. This
combined dental and medical segment distributes consumable products, small equipment, laboratory products, large
equipment, equipment repair services, branded and generic pharmaceuticals, vaccines, surgical products, diagnostic
4
tests, infection-control products and vitamins. Our global dental group serves office-based dental practitioners,
dental laboratories, schools, government and other institutions. Our global medical group serves physician offices,
urgent care centers, ambulatory care sites, emergency medical technicians, dialysis centers, home health, federal
and state governments and large enterprises, such as group practices and integrated delivery networks, among other
providers across a wide range of specialties. While our primary go-to-market strategy is in our capacity as a
distributor, we also manufacture certain dental specialty products in the areas of implants, orthodontics and
endodontics. We have achieved scale in these global businesses primarily through acquisitions as manufacturers of
these products typically do not utilize a distribution channel to serve customers.
As an alternative to branded product options, we also market under our own private label portfolio of cost-effective,
high-quality consumable merchandise products for our dental and medical customers. Sales of our private label
products generally achieve gross profit margins that are higher than the average margin on the other products we
sell.
Our global technology and value-added services group provides software, technology and other value-added
services to health care practitioners. Henry Schein One, the largest contributor of sales to this category, offers
software systems for dental practitioners. This segment also includes a small medical software business known as
MicroMD. In addition, we offer physicians a broad suite of electronic health records, integrated revenue cycle
management, and patient communication services. Finally, our value-added practice solutions include financial
service offerings, which include practice finance solutions such as credit card billing and facilitation of customer
loans (on a non-recourse basis) to acquire equipment and technology, as well as solutions to broker dental practice
transitions. We do not take on the liability of such loans but instead receive an origination fee for coordinating
loans between practice customers and third-party banking groups.
Recent Developments
See “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Recent
Developments” herein for a discussion related to the COVID-19 pandemic and recent corporate transactions.
Industry
The global health care distribution industry, as it relates to office-based health care practitioners, is fragmented and
diverse. The industry ranges from sole practitioners working out of relatively small offices to mid-sized and large
group practices ranging in size from a few practitioners to several hundred practices owned or operated by dental
support organizations (DSOs), hospital systems, or integrated delivery networks (IDNs).
Due in part to the inability of office-based health care practitioners to store and manage large quantities of supplies
in their offices, the distribution of health care supplies and small equipment to office-based health care practitioners
has been characterized by frequent, small quantity orders, and a need for rapid, reliable and substantially complete
order fulfillment. The purchasing decisions within an office-based health care practice are typically made by the
practitioner, hygienist or office manager. Supplies and small equipment are generally purchased from more than
one distributor, with one generally serving as the primary supplier.
The health care distribution industry continues to experience growth due to demand driven by the aging population,
increased health care awareness and the importance of preventative care, an increasing understanding of the
connection between good oral health and overall health, improved access to care globally, the proliferation of
medical technology and testing, new pharmacology treatments and expanded third-party insurance coverage,
partially offset by the effects of unemployment on insurance coverage and technological improvements, including
the advancement of software and services, prosthetic solutions and telemedicine. In addition, the non-acute market
continues to benefit from the shift of procedures and diagnostic testing from acute care settings to alternate-care
sites, particularly physicians’ offices and ambulatory surgery centers.
We believe that consolidation within the industry will continue to result in a number of distributors, particularly
those with limited financial, operating and marketing resources, seeking to combine with larger companies that can
provide growth opportunities. This consolidation also may continue to result in distributors seeking to acquire
5
companies that can enhance their current product and service offerings or provide opportunities to serve a broader
customer base.
In addition, customer consolidation will likely lead to multiple locations under common management and the
movement of more procedures from the hospital setting to the physician or alternate care setting as the health care
industry is increasingly focused on efficiency and cost containment. This trend has benefited distributors capable
of providing a broad array of products and services at low prices. It also has accelerated the growth of HMOs,
group practices, other managed care accounts and collective buying groups, which, in addition to their emphasis on
obtaining products at competitive prices, tend to favor distributors capable of providing specialized management
information support. We believe that the trend towards cost containment has the potential to favorably affect
demand for technology solutions, including software, which can enhance the efficiency and facilitation of practice
management.
Competition
The distribution and manufacture of health care supplies and equipment is highly competitive. Many of the health
care products we sell are available to our customers from a number of suppliers. In addition, our competitors could
obtain exclusive rights from manufacturers to market particular products. Manufacturers also could seek to sell
directly to end-users, and thereby eliminate or reduce our role and that of other distributors. In certain parts of the
dental end market, such as those related to dental specialty products, manufacturers already sell directly to end
customers.
In North America, we compete with other distributors, as well as several manufacturers, of dental and medical
products, primarily on the basis of price, breadth of product line, e-commerce capabilities, customer service and
value-added products and services. In the dental market, our primary competitors in the U.S. are the Patterson
Dental division of Patterson Companies, Inc. and Benco Dental Supply Company. In addition, we compete against
a number of other distributors that operate on a national, regional and local level. Our primary competitors in the
U.S. medical market, which accounts for the large majority of our global medical sales, are McKesson Corporation
and Medline Industries, Inc., which are national distributors. We also compete with a number of regional and local
medical distributors, as well as a number of manufacturers that sell directly to physicians. With regard to our dental
software, we compete against numerous companies, including the Patterson Dental division of Patterson
Companies, Inc., Carestream Health, Inc., Open Dental Software, Inc., PlanetDDS LLC, Good Methods Global Inc.
(d.b.a. CareStack) and Curve Dental, LLC. In other software end markets, including revenue cycle management,
patient relationship management and patient demand generation, we compete with companies such as Vyne
Therapeutics Inc., EDI-Health Group, Inc. (d.b.a. Dental X Change, Inc.), Weave Communications,
Inc., Solutionreach, Inc., ZocDoc, Inc., LocalMed Inc. and Prosites Inc. The medical practice management and
electronic medical records market is very fragmented and we compete with numerous companies such as the
NextGen division of Quality Systems, Inc., eClinicalWorks, Allscripts Healthcare Solutions, Inc., and Epic Systems
Corporation.
Outside of the U.S., we believe we are the only global distributor of supplies and equipment to dental practices, and
our competitors are primarily local and regional companies. We also face significant competition internationally,
where we compete on the basis of price and customer service against several large competitors, including the
GACD Group, Proclinic SA, Lifco AB, Planmeca Oy and Billericay Dental Supply Co. Ltd., as well as a large
number of other dental and medical product distributors and manufacturers in international countries and territories
we serve.
Competitive Strengths
We have more than 88 years of experience in distributing products to health care practitioners resulting in strong
awareness of the Henry Schein
®
A focus on meeting our customers’ unique needs
. We are committed to providing customized solutions to our
customers that are driven by our understanding of the end markets we serve and reflect the technology-driven
products and services best suited for their practice needs. We are committed to continuing to enhance these
6
offerings through organic investment in our products and our teams, as well through as the acquisition of new
products and services that may help us better serve our customers.
Direct sales and marketing expertise.
customer relationships through personal or virtual visits by field sales representatives, frequent direct marketing and
telesales contact, emphasizing our broad product lines, including exclusive distribution agreements, competitive
prices and ease of order placement, particularly through our e-commerce platforms. The key elements of our direct
sales and marketing efforts are:
•
Field sales consultants.
covering major North American, European and other international markets. These consultants complement
our direct marketing and telesales efforts and enable us to better market, service and support the sale of
more sophisticated products and equipment.
•
Marketing.
through a combination of owned, earned and paid digital channels, as well as through catalogs, flyers,
direct mail, and other promotional materials. Our strategies included an emphasis on educational content
through webinars and content marketing initiatives. We continue to enhance our marketing technology to
improve our targeting capability and the relevance of messaging and offers.
•
Telesales.
telesales representatives, who facilitate order processing, generate new sales through direct and frequent
contact with customers and stay abreast of market developments and the hundreds of new products,
services and technologies introduced each year to educate practice personnel.
•
Electronic commerce solutions.
competitive e-commerce solutions. We continue to invest in our e-commerce platform to offer enhanced
content management so customers can more easily find the products they need and to enable an engaging
purchase experience, supported by excellent customer service.
•
Social media.
various social media platforms, which are an important element of our communications and marketing
efforts. We continue to expand our social media presence to raise awareness about issues, engage
customers beyond a sale and deliver services and solutions to specialized audiences.
Broad product and service offerings at competitive prices.
customers, at competitive prices, in the following categories:
•
Consumable supplies and equipment.
customers. We offer over 180,000 additional SKUs to our customers in the form of special order items.
•
Technology and other value-added products and services.
engagement, and patient demand creation software solutions to our dental customers. Our practice
management solutions provide practitioners with electronic medical records, patient treatment history,
billing, accounts receivable analyses and management, appointment calendars, electronic claims processing
and word processing programs, network and hardware services, e-commerce and electronic marketing
services, sourcing third party patient payment plans, transition services and training and education
programs for practitioners. We also sell medical software for practice management, certified electronic
health records (“EHR”) and e-Prescribe medications and prescription solutions through MicroMD®. We
have approximately 800 technical representatives supporting customers using our practice management
solutions and services. As of December 26, 2020, we had an active user base of approximately 94,500
practices and 374,000 consumers, including users of AxiUm, Dentally®, Dentrix Ascend®, Dental
Vision®, Dentrix® Dental Systems, Dentrix® Enterprise, Easy Dental®, EndoVision®, Evolution® and
EXACT®, Gesden®, Julie® Software, Oasis, OMSVision®, Orisline®, PerioVision®, Power Practice®
Px, PowerDent, and Viive® and subscriptions for Demandforce®, Sesame, and Lighthouse360® for dental
practices and DentalPlans.com® for dental patients; and MicroMD® for physician practices.
7
•
Repair services.
repair, installation and technical services for our health care customers. Our over 2,000 technicians provide
installation and repair services for: dental handpieces; dental and medical small equipment; table top
sterilizers; and large dental equipment.
•
Financial services.
providing access to a number of financial services and products provided by third party vendors (including
non-recourse financing for equipment, technology and software products; non-recourse patient financing;
collection services and credit card processing) at rates that we believe are generally lower than what our
customers would be able to secure independently. We also provide consulting services, dental practice
valuation and brokerage services.
Commitment to superior customer service
. We maintain a strong commitment to providing superior customer
service. We frequently monitor our customer service through customer surveys, focus groups and statistical
reports. Our customer service policy primarily focuses on:
•
Exceptional order fulfillment
. We ship an average of approximately 128,000 cartons daily. Historically,
approximately 99% of items have been shipped without back ordering and were shipped on the same
business day the order is received. Due to the significant increase in demand for PPE, as a result of
COVID-19, during the year ended December 26, 2020, approximately 93% of items ordered were shipped
without back ordering and 90% were shipped on the same business day the order was received. As the
demand for PPE stabilizes, we expect our percentage of items shipped without back ordering and shipped
on the same day to return to historical levels.
•
Comprehensive ordering process
. Customers may place orders 24 hours a day, 7 days a week via e-
commerce solutions, telephone, fax, e-mail, and mail.
Integrated management information systems
. Our information systems generally allow for centralized management
of key functions, including accounts receivable, inventory, accounts payable, payroll, purchasing, sales, order
fulfillment and financial and operational reporting. These systems allow us to manage our growth, deliver superior
customer service, properly target customers, manage financial performance and monitor daily operational statistics.
Cost-effective purchasing
. We believe that cost-effective purchasing is a key element to maintaining and enhancing
our position as a competitively priced provider of health care products. We continuously evaluate our purchase
requirements and suppliers’ offerings and prices in order to obtain products at the lowest possible cost. In 2020,
our top 10 health care distribution suppliers and our single largest supplier accounted for approximately 30% and
4%, respectively, of our aggregate purchases.
Efficient distribution
. We distribute our products from our strategically located distribution centers. We strive to
maintain optimal inventory levels in order to satisfy customer demand for prompt delivery and complete order
fulfillment. These inventory levels are managed on a daily basis with the aid of our management information
systems. Once an order is entered, it is electronically transmitted to the distribution center nearest the customer’s
location for order fulfillment.
8
Products
The following table sets forth the percentage of consolidated net sales by principal categories of products offered
through our health care distribution and technology reportable segments:
December 26,
December 28,
December 29,
2020
2019
2018
Health care distribution:
Dental products
(1)
58.4
%
64.2
%
67.4
%
Medical products
(2)
35.8
29.8
28.3
Total health care distribution
94.2
94.0
95.7
Technology and value-added services:
Software and related products and
(3)
5.1
5.2
4.3
Total excluding Corporate TSA revenues
99.3
99.2
100.0
Corporate TSA revenues
(4)
0.7
0.8
-
Total
100.0
100.0
100.0
(1)
Includes infection-control products, handpieces, preventatives, impression materials, composites, anesthetics, teeth, dental implants,
gypsum, acrylics, articulators, abrasives, dental chairs, delivery units and lights, X-ray supplies and equipment, personal protective
equipment, equipment repair and high-tech and digital restoration equipment.
(2)
Includes branded and generic pharmaceuticals, vaccines, surgical products, diagnostic tests, infection-control products, X-ray products,
equipment, personal protective equipment, and vitamins.
(3)
Consists of practice management software and other value-added products, which are distributed primarily to health care providers, and
financial services on a non-recourse basis, e-services, continuing education services for practitioners, consulting and other services.
(4)
Corporate TSA revenues represents sales of certain products to Covetrus under the transition services agreement entered into in
connection with the Animal Health spin-off, which ended in December 2020.
Business Strategy
Our objective is to continue to expand as a global value-added provider of health care products and services to
office-based dental and medical practitioners by increasing their efficiency and success. To accomplish this, we
will apply our competitive strengths in executing the following strategies:
•
Increase penetration of our existing customer base.
intend to increase sales to our existing customer base and enhance our position as their primary supplier.
We believe our offering of a broad range of products, services and support, including software solutions
that can help drive improved workflow efficiency and patient communications for practices, coupled with
our full-service value proposition, helps us to retain and grow our customer base.
•
Increase the number of customers we serve.
sales consultants and telesales team, as well as using our customer database to focus our marketing efforts
in all of our operating segments. In the dental business, we provide products and services to independent
practices, mid-market groups, and large DSOs as well as community health centers and government sites of
care. Leveraging our broad array of assets and capabilities, we offer solutions to address these new
markets. In the medical business, we have expanded to serve customers located in settings outside of the
traditional office, such as urgent care clinics, retail, occupational health and home health settings. As
settings of health care shift, we remain committed to serving these practitioners and providing them with
the products and services they need.
•
Leverage our value-added products and services.
product lines utilizing a consultative selling process. In the dental business, we have significant cross-
selling opportunities between our dental software users and our dental distribution customers. In the
medical business, we have opportunities to expand our vaccine, injectables and other pharmaceuticals sales
to health care practitioners, as well as cross-selling electronic health record and software when we sell our
core products. Our strategy extends to providing health systems, integrated delivery networks and other
large group and multi-site health care organizations, including physician clinics, these same value added
9
products and services. As physicians and health systems closely align, we have increased access to
opportunities for cross-marketing and selling our product and service portfolios.
•
Pursue strategic acquisitions and joint ventures.
companies that add new customers and sales teams, increase our geographic footprint (whether entering a
new country, such as emerging markets, or building scale where we have already invested in businesses),
and finally, those that enable us to access new products and technologies.
Markets Served
Demographic trends indicate that our markets are growing, as an aging U.S. population is increasingly using health
care services. Between 2020 and 2030, the 45 and older population is expected to grow by approximately 11%.
Between 2020 and 2040, this age group is expected to grow by approximately 22%. This compares with expected
total U.S. population growth rates of approximately 7% between 2020 and 2030 and approximately 12% between
2020 and 2040.
In the dental industry, there is predicted to be a rise in oral health care expenditures as the 45-and-older segment of
the population increases. There is increasing demand for new technologies that allow dentists to increase
productivity, and this is being driven in the U.S. by lower insurance reimbursement rates. At the same time, there is
an expected increase in dental insurance coverage.
We support our dental professionals through the many SKUs that we offer, as well as through important value-
added services, including practice management software, electronic claims processing, financial services and
continuing education, all designed to help maximize a practitioner’s efficiency.
In the medical market, there continues to be a migration of procedures from acute-care settings to physicians’
offices and home health settings, a trend that we believe provides additional opportunities for us. There also is the
continuing use of vaccines, injectables and other pharmaceuticals in alternate-care settings. We believe we have
established a leading position as a vaccine supplier to the office-based physician practitioner.
Additionally, we seek to expand our dental full-service model and our medical offerings in countries where
opportunities exist. Through our “Schein Direct” program, we also have the capability to provide door-to-door air
package delivery to practitioners in over 190 countries around the world.
For information on revenues and long-lived assets by geographic area, see
of “Notes to Consolidated Financial Statements.”
Seasonality and Other Factors Affecting Our Business and Quarterly Results
We experience fluctuations in quarterly earnings. As a result, we may fail to meet or exceed the expectations of
securities analysts and investors, which could cause our stock price to decline.
Our business is subject to seasonal and other quarterly fluctuations. Revenues and profitability generally have been
higher in the third and fourth quarters due to the timing of sales of seasonal products (including influenza vaccine,
equipment and software products), purchasing patterns of office-based health care practitioners and year-end
promotions. Revenues and profitability may also be impacted by the timing of certain annual and biennial dental
tradeshows where equipment promotions are offered. In addition, some dental practices delay equipment purchases
in the U.S. until year-end due to tax incentives. Revenues and profitability generally have been lower in the first
quarter, primarily due to increased sales in the prior two quarters. We expect our historical seasonality of sales to
continue in the foreseeable future.
10
Governmental Regulations
We strive to be substantially compliant with the applicable laws, regulations and guidance described below, and
believe we have effective compliance programs and other controls in place to ensure substantial compliance.
However, compliance is not guaranteed either now or in the future, as certain laws, regulations and guidance may
be subject to varying and evolving interpretations that could affect our ability to comply, as well as future changes,
additions, and enforcement approaches, including in light of political changes. For example, President Biden’s
administration has authorized and encouraged a freeze on certain federal regulations that have been published but
are not yet effective, as well as a review of all federal regulations issued during President Trump’s administration.
Changes with respect to the applicable laws, regulations and guidance described below may require us to update or
revise our operations, services, marketing practices, and compliance programs and controls, and may impose
additional and unforeseen costs on us, pose new or previously immaterial risks to us, or may otherwise have a
material adverse effect on our business.
Government
Certain of our businesses involve the distribution, importation, exportation, marketing and sale of, and third party
payment for, pharmaceuticals and medical devices, and in this regard, we are subject to extensive local, state,
federal and foreign governmental laws and regulations, including as applicable to our wholesale distribution of
pharmaceuticals and medical devices, and as part of our specialty home medical supply business that distributes and
sells medical equipment and supplies directly to patients. The federal government and state governments have also
increased enforcement activity in the health care sector, particularly in areas of fraud and abuse, anti-bribery and
corruption, controlled substances prescribing, medical device regulation, and data privacy and security standards.
Government and private insurance programs fund a large portion of the total cost of medical care, and there have
been efforts to limit such private and government insurance programs, including efforts, thus far unsuccessful, to
seek repeal of the entire United States Patient Protection and Affordable Care Act, as amended by the Health Care
and Education Reconciliation Act, each enacted in March 2010, as amended (the “ACA”). In addition, activities to
control medical costs, including laws and regulations lowering reimbursement rates for pharmaceuticals, medical
devices, and/or medical treatments or services, are ongoing. Many of these laws and regulations are subject to
change and their evolving implementation may impact our operations and our financial performance.
Our businesses are also generally subject to numerous other laws and regulations that could impact our financial
performance, including securities, antitrust, consumer protection, anti-bribery and anti-kickback, customer
interaction transparency, data privacy, data security, government contracting and other laws and regulations.
Failure to comply with law or regulations could have a material adverse effect on our business.
Operating, Security and Licensure Standards
Certain of our businesses involve the distribution, importation, exportation, marketing and sale of, and third party
payment for, pharmaceuticals and medical devices, and in this regard we are subject to various local, state, federal
and foreign governmental laws and regulations, including as applicable to our wholesale distribution and sale of
pharmaceuticals and medical devices, and, as part of our specialty home medical supply business that distributes
and sells medical equipment and supplies directly to patients. Among the United States federal laws applicable to
us are the Controlled Substances Act, the Federal Food, Drug, and Cosmetic Act, as amended (“FDC Act”), and
Section 361 of the Public Health Service Act, as well as laws regulating the billing of and reimbursement from
government programs, such as Medicare and Medicaid, and from commercial payers. We are also subject to
comparable foreign regulations.
The FDC Act, the Controlled Substances Act, their implementing regulations, and similar foreign laws generally
regulate the introduction, manufacture, advertising, marketing and promotion, sampling, pricing and
reimbursement, labeling, packaging, storage, handling, returning or recalling, reporting, and distribution of, and
record keeping for, pharmaceuticals and medical devices shipped in interstate commerce, and states may similarly
regulate such activities within the state. Furthermore, Section 361 of the Public Health Service Act, which provides
authority to prevent the introduction, transmission or spread of communicable diseases, serves as the legal basis for
11
the United States Food and Drug Administration’s (“FDA”) regulation of human cells, tissues and cellular and
tissue-based products, also known as “HCT/P products.”
The Federal Drug Quality and Security Act of 2013 brought about significant changes with respect to
pharmaceutical supply chain requirements. Title II of this measure, known as the Drug Supply Chain Security Act
(“DSCSA”), is being phased in over a period of ten years, and is intended to build a national electronic,
interoperable system to identify and trace certain prescription drugs as they are distributed in the United States.
The law’s track and trace requirements applicable to manufacturers, wholesalers, repackagers and dispensers (e.g.,
pharmacies) of prescription drugs took effect in January 2015, and continues to be implemented. The DSCSA
product tracing requirements replace the former FDA drug pedigree requirements and pre-empt certain state
requirements that are inconsistent with, more stringent than, or in addition to, the DSCSA requirements.
The DSCSA also establishes certain requirements for the licensing and operation of prescription drug wholesalers
and third-party logistics providers (“3PLs”), and includes the eventual creation of national wholesaler and 3PL
licenses in cases where states do not license such entities. The DSCSA requires that wholesalers and 3PLs
distribute drugs in accordance with certain standards regarding the recordkeeping, storage and handling of
prescription drugs. The DSCSA requires wholesalers and 3PLs to submit annual reports to the FDA, which include
information regarding each state where the wholesaler or 3PL is licensed, the name and address of each facility and
contact information. According to FDA guidance, states are pre-empted from imposing any licensing requirements
that are inconsistent with, less stringent than, directly related to, or covered by the standards established by federal
law in this area. Current state licensing requirements concerning wholesalers will remain in effect until the FDA
issues new regulations as directed by the DSCSA. In addition, with respect to our specialty home medical supply
business, we are subject to certain state licensure laws (including state pharmacy laws), and also certain
accreditation standards, including to qualify for reimbursement from Medicare and other third-party payers.
The Food and Drug Administration Amendments Act of 2007 and the Food and Drug Administration Safety and
Innovation Act of 2012 amended the FDC Act to require the FDA to promulgate regulations to implement a unique
device identification (“UDI”) system. The UDI rule phased in the implementation of the UDI regulations,
generally beginning with the highest-risk devices (i.e., Class III medical devices) and ending with the lowest-risk
devices. Most compliance dates were reached as of September 24, 2018, with a final set of requirements for low
risk devices being reached on September 24, 2022, which will complete the phase in. The UDI regulations require
“labelers” to include unique device identifiers (“UDIs”), with a content and format prescribed by the FDA and
issued under a system operated by an FDA-accredited issuing agency, on the labels and packages of medical
devices (including, but not limited to, certain software that qualifies as a medical device under FDA rules), and to
directly mark certain devices with UDIs. The UDI regulations also require labelers to submit certain information
concerning UDI-labeled devices to the FDA, much of which information is publicly available on an FDA database,
the Global Unique Device Identification Database. The UDI regulations and subsequent FDA guidance regarding
the UDI requirements provide for certain exceptions, alternatives and time extensions. For example, the UDI
regulations include a general exception for Class I devices exempt from the Quality System Regulation (other than
record-keeping requirements and complaint files). Regulated labelers include entities such as device
manufacturers, repackagers, reprocessors and relabelers that cause a device’s label to be applied or modified, with
the intent that the device will be commercially distributed without any subsequent replacement or modification of
the label, and include certain of our businesses.
Under the Controlled Substances Act, as a distributor of controlled substances, we are required to obtain and renew
annually registrations for our facilities from the United States Drug Enforcement Administration (“DEA”)
permitting us to handle controlled substances. We are also subject to other statutory and regulatory requirements
relating to the storage, sale, marketing, handling, reporting, record-keeping and distribution of such drugs, in
accordance with the Controlled Substances Act and its implementing regulations, and these requirements have been
subject to heightened enforcement activity in recent times. We are subject to inspection by the DEA. Certain of our
businesses are also required to register for permits and/or licenses with, and comply with operating and security
standards of, the DEA, the FDA, the United States Department of Health and Human Services (“HHS”), and
various state boards of pharmacy, state health departments and/or comparable state agencies as well as comparable
foreign agencies, and certain accrediting bodies, depending on the type of operations and location of product
distribution, manufacturing or sale. These businesses include those that distribute, manufacture and/or repackage
12
prescription pharmaceuticals and/or medical devices and/or HCT/P products, or own pharmacy operations, or
install, maintain or repair equipment.
In addition, Section 301 of the National Organ Transplant Act, and a number of comparable state laws, impose civil
and/or criminal penalties for the transfer of certain human tissue (for example, human bone products) for valuable
consideration, while generally permitting payments for the reasonable costs incurred in procuring, processing,
storing and distributing that tissue. We are also subject to foreign government regulation of such products. The
DEA, the FDA and state regulatory authorities have broad inspection and enforcement powers, including the ability
to suspend or limit the distribution of products by our distribution centers, seize or order the recall of products and
impose significant criminal, civil and administrative sanctions for violations of these laws and regulations. Foreign
regulations subject us to similar foreign enforcement powers.
EU Regulation of Medicinal and Dental Products
EU member states regulate their own healthcare systems, as does EU law. The latter regulates certain matters,
most notably medicinal products and medical devices. Medicinal products are defined, broadly, as substances or
combinations of substances having certain functionalities and may not include medical devices. EU “regulations”
apply in all Member States, whereas “directives” are implemented by the individual laws of member states.
On medicines for humans, we are regulated under Directive No. 2001/83/EC of 6 November 2001 and EU
Regulation No. 726/2004 of 31 March 2004. These rules provide for the authorization of products, and regulate
their manufacture, importation, marketing, and distribution. It implements requirements which may be
implemented without warning, as well as a national pharmacovigilance system under which marketing
authorizations may be withdrawn, and includes potential sanctions for breaches of the rules, and on other bases
such as harmfulness or inefficiency.
EU Regulation No. 1223/2009 of 30 November 2009
on cosmetic products
includes dental products) be safe for human health when used under normal or reasonably foreseeable conditions of
use and comply with certain obligations which apply to manufacturer, importer and distributor. It includes market
surveillance, and non-compliance may result in the recall or withdrawal of products, along with other sanctions.
In the European Union, the EU Medical Device Regulation No. 2017/745 (“EU MDR”) covers a wide scope of our
activities, from dental material to X-ray machines, and certain software. It was meant to become applicable three
years after publication (in May 2020). However, on April 23, 2020, to allow European Economic Area (“EEA”)
national authorities, notified bodies, manufacturers and other actors to focus fully on urgent priorities related to the
COVID-19 pandemic, the European Council and Parliament adopted Regulation 2020/561, postponing the date of
application of the EU MDR by one year (to May 2021). In the meantime, rules provided for by Directive No.
90/385/EEC of 20 June 1990
on the approximation of the laws of the member states relating to active implantable
medical devices
The EU MDR significantly modifies and intensifies the regulatory compliance requirements for the medical device
industry as a whole. Once applicable, the EU MDR will among other things:
•
Strengthen
the
rules
on
placing
devices
on
the
market
and
rein
force
surveillance
once
they
are
available;
•
Establish
explicit
provisions
on
manufacturers’
responsibilities
for
the
follow
-
up
of
the
quality,
performance and safety of devices placed on the market;
•
Improve
the
traceability
of
medical
devices
throughout
the
supply
chain
to
the
end
-
user
or
patient
through
a
unique identification number;
•
Se
t
up
a
central
database
to
provide
patients,
healthcare
professionals
and
the
public
with
comprehensive
information on products available in the EU;
•
Strengthen
rules
for
the
assessment
of
certain
high
-
risk
devices,
such
as
implants,
which
may
have
to
undergo an additional check by experts before they are placed on the market; and
•
Identify importers and distributors and medical device products through registration in a database
(EudaMed not due until 2022 and after).
13
In particular, the EU MDR imposes stricter requirements for the confirmation that a product meets the regulatory
requirements, including regarding a product’s clinical evaluation and a company’s quality systems, and for the
distribution, marketing and sale of medical devices, including post-market surveillance. Medical devices that have
been assessed and/or certified under the EU Medical Device Directive may continue to be placed on the market
until 2024 (or until the expiry of their certificates, if applicable and earlier); however, requirements regarding the
distribution, marketing and sale including quality systems and post-market surveillance have to be observed by
manufacturers, importers and distributors as of the application date.
Other EU regulations that may apply under appropriate circumstances include EU Regulation No. 1907/2006 of 18
December 2006
concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals
, which
requires importers to register substances or mixtures that they import in the EU beyond certain quantities, and the
EU Regulation No. 1272/2008 of 16 December 2008 on classification, labelling and packaging of substances and
mixtures (“CLP Regulation”), which sets various obligations with respect to the labelling and packaging of
concerned substances and mixtures.
Furthermore, compliance with legal requirements has required and may in the future require us to delay product
release, sale or distribution, or institute voluntary recalls of products we sell, each of which could result in
regulatory and enforcement actions, financial losses and potential reputational harm. Our customers are also
subject to significant federal, state, local and foreign governmental regulation, which may affect our interactions
with customers, including the design and functionality of our products.
Certain of our businesses are subject to various additional federal, state, local and foreign laws and regulations,
including with respect to the sale, transportation, storage, handling and disposal of hazardous or potentially
hazardous substances, and safe working conditions. In addition, certain of our businesses must operate in
compliance with a variety of burdensome and complex billing and record-keeping requirements in order to
substantiate claims for payment under federal, state and commercial healthcare reimbursement programs.
Certain of our businesses also maintain contracts with governmental agencies and are subject to certain regulatory
requirements specific to government contractors.
Antitrust and Consumer Protection
The federal government of the United States, most U.S. states and many foreign countries have antitrust laws that
prohibit certain types of conduct deemed to be anti-competitive, as well as consumer protection laws that seek to
protect consumers from improper business practices. At the U.S. federal level, the Federal Trade Commission
oversees enforcement of these types of laws, and states have similar government agencies. Violations of antitrust or
consumer protection laws may result in various sanctions, including criminal and civil penalties. Private plaintiffs
may also bring civil lawsuits against us in the United States for alleged antitrust law violations, including claims for
treble damages. EU law also regulates competition and provides for detailed rules protecting consumers.
Health Care Fraud
Certain of our businesses are subject to federal and state (and similar foreign) health care fraud and abuse, referral
and reimbursement laws and regulations with respect to their operations. Some of these laws, referred to as “false
claims laws,” prohibit the submission or causing the submission of false or fraudulent claims for reimbursement to
federal, state and other health care payers and programs. Other laws, referred to as “anti-kickback laws,” prohibit
soliciting, offering, receiving or paying remuneration in order to induce the referral of a patient or ordering,
purchasing, leasing or arranging for, or recommending ordering, purchasing or leasing of, items or services that are
paid for by federal, state and other health care payers and programs. Certain additional state and federal laws, such
as the federal Physician Self-Referral Law, commonly known as the “Stark Law,” prohibit physicians and other
health professionals from referring a patient to an entity with which the physician (or family member) has a
financial relationship, for the furnishing of certain designated health services (for example, durable medical
equipment and medical supplies), unless an exception applies.
The fraud and abuse laws and regulations have been subject to heightened enforcement activity over the past few
years, and significant enforcement activity has been the result of “relators” who serve as whistleblowers by filing
14
complaints in the name of the United States (and if applicable, particular states) under applicable false claims laws,
and who may receive up to 30% of total government recoveries. Penalties under fraud and abuse laws may be
severe, and could result in significant civil and criminal penalties and costs, including the loss of licenses and the
ability to participate in federal and state health care programs, and could have a material adverse effect on our
business. Also, these measures may be interpreted or applied by a prosecutorial, regulatory or judicial authority in
a manner that could require us to make changes in our operations or incur substantial defense and settlement
expenses. Even unsuccessful challenges by regulatory authorities or private relators could result in reputational
harm and the incurring of substantial costs. Most states have adopted similar state false claims laws, and these state
laws have their own penalties, which may be in addition to federal False Claims Act penalties, as well as other
fraud and abuse laws.
With respect to measures of this type, the United States government (among others) has expressed concerns about
financial relationships between suppliers on the one hand and physicians and dentists on the other. As a result, we
regularly review and revise our marketing practices as necessary to facilitate compliance.
We also are subject to certain United States and foreign laws and regulations concerning the conduct of our foreign
operations, including the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, German anti-corruption laws
and other anti-bribery laws and laws pertaining to the accuracy of our internal books and records, which have been
the focus of increasing enforcement activity globally in recent years.
While we believe that we are substantially compliant with applicable fraud and abuse laws and regulations, and
have adequate compliance programs and controls in place to ensure substantial compliance, we cannot predict
whether changes in applicable law, or interpretation of laws, or changes in our services or marketing practices in
response to changes in applicable law or interpretation of laws, or failure to comply with applicable law, could have
a material adverse effect on our business.
Affordable Care Act
The United States Patient Protection and Affordable Care Act, as amended by the Health Care and Education
Reconciliation Act, each enacted in March 2010, as amended (the “ACA”), increased federal oversight of private
health insurance plans and included a number of provisions designed to reduce Medicare expenditures and the cost
of health care generally, to reduce fraud and abuse, and to provide access to increased health coverage. The ACA
also materially expanded the number of individuals in the United States with health insurance.
The ACA has faced ongoing legal challenges, including litigation seeking to invalidate and Congressional action
seeking to repeal some of or all of the law or the manner in which it has been implemented. In 2012, the United
States Supreme Court, in upholding the constitutionality of the ACA and its individual mandate provision requiring
that people buy health insurance or else face a penalty, simultaneously limited ACA provisions requiring Medicaid
expansion, making such expansion a state-by-state decision. In addition, one of the major political parties in the
United States remains committed to seeking the ACA’s legislative repeal, but legislative efforts to do so have
previously failed to pass both chambers of Congress. Under President Trump’s administration, a number of
administrative actions were taken to materially weaken the ACA, including, without limitation, by permitting the
use of less robust plans with lower coverage and eliminating “premium support” for insurers providing policies
under the ACA. The Tax Cuts and Jobs Act enacted in 2017 (the “Tax Act”), which contains a broad range of tax
reform provisions that impact the individual and corporate tax rates, international tax provisions, income tax add-
back provisions and deductions, also effectively repealed the ACA’s individual mandate by zeroing out the penalty
for non-compliance. In the most recent ACA litigation, the federal Fifth Circuit Court of Appeals found the
individual mandate to be unconstitutional, and returned the case to the District Court for the Northern District of
Texas for consideration of whether the remainder of the ACA could survive the excision of the individual
mandate. The Fifth Circuit’s decision was appealed to the United States Supreme Court. The Supreme Court heard
argument on the appeal on November 10, 2020, and a decision is anticipated soon. Any outcome of this case that
changes the ACA, in addition to future legislation, regulation, guidance and/or Executive Orders that do the same,
could have a significant impact on the U.S. healthcare industry.
An ACA provision, generally referred to as the Physician Payment Sunshine Act or Open Payments Program (the
“Sunshine Act”), imposes annual reporting and disclosure requirements for drug and device manufacturers and
15
distributors with regard to payments or other transfers of value made to certain covered recipients (including
physicians, dentists and teaching hospitals), and for such manufacturers and distributors and for group purchasing
organizations, with regard to certain ownership interests held by physicians in the reporting entity. The Centers for
Medicare and Medicaid Services (“CMS”) publishes information from these reports on a publicly available website,
including amounts transferred and physician, dentist and teaching hospital identities. Amendments expanded the
law to also require reporting, effective January 1, 2022, of payments or other transfers of value to physician
assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse-
midwives, and this new requirement will be effective for data collected beginning in calendar year 2021. The
Sunshine Act pre-empts similar state reporting laws, although we or our subsidiaries may be required to report
under certain state transparency laws that address circumstances not covered by the Sunshine Act, and some of
these state laws, as well as the federal law, can be ambiguous. We are also subject to foreign regulations requiring
transparency of certain interactions between suppliers and their customers.
In the United States, government actions to seek to increase health-related price transparency may also affect our
business.
Another notable Medicare health care reform initiative, the Medicare Access and CHIP Reauthorization Act of
2015 (“MACRA”), enacted on April 16, 2015, established a new payment framework, which modified certain
Medicare payments to “eligible clinicians,” including physicians, dentists and other practitioners. Under MACRA,
certain eligible clinicians are required to participate in Medicare through the Merit-Based Incentive Payment
System (“MIPS”) or Advanced Alternative Payment Models (“APMs”), through which Medicare reimbursement to
eligible clinicians includes both positive and negative payment adjustments that take into account quality,
promoting interoperability, cost, and improvement activities. Data collected in the first MIPS performance year
(2017) determined payment adjustments that began January 1, 2019. MACRA standards continue to evolve, and
represent a fundamental change in physician reimbursement that is expected to provide substantial financial
incentives for physicians to participate in risk contracts, and to increase physician information technology and
reporting obligations. The implications of the implementation of MACRA are uncertain and will depend on future
regulatory activity and physician activity in the marketplace. New payment and delivery system reform programs,
including those modeled after such federal program, are also increasingly being rolled out at the state level through
Medicaid administrators, as well as through the private sector, which may further alter the marketplace and impact
our business.
Recently, in addition to other government efforts to control health care costs, there has been increased scrutiny on
drug pricing and concurrent efforts to control or reduce drug costs by Congress, the President, executive branch
agencies and various states. At the state level, several states have adopted laws that require drug manufacturers to
provide advance notice of certain price increases and to report information relating to those price increases, while
others have taken legislative or administrative action to establish prescription drug affordability boards or multi-
payer purchasing pools to reduce the cost of prescription drugs. At the federal level, several related bills have been
introduced and regulations proposed which, if enacted or finalized, respectively, would impact drug pricing and
related costs.
As a result of political, economic and regulatory influences, the health care distribution industry in the United
States is under intense scrutiny and subject to fundamental changes. We cannot predict what further reform
proposals, if any, will be adopted, when they may be adopted, or what impact they may have on us.
EU Directive on the pricing and reimbursement of medicinal products
EU law provides for the regulation of the pricing of medicinal products which are implemented by EU member
states (Directive No. 89/105/EC of 21 December 1988
relating to the transparency of measures regulating the
pricing of medicinal products for human use and their inclusion in the scope of national health insurance systems
).
Member states may, subject notably to transparency conditions and to the statement of reasons based upon
objective and verifiable criteria, regulate the price charged (or its increases) for authorized medicines and their level
of reimbursement, or they may freeze prices, place controls on the profitability of persons responsible for placing
medicinal products on the market, and include or exclude the medicine on the list of products covered by national
health insurance systems.
16
EU law does not expressly include provisions like those of the Sunshine Act in the United States, but a growing
number of EU member states (such as France since 2011) have enacted laws to increase the transparency of
relationships in the healthcare sector. The scope of these laws varies from on member state to another and may, for
example, include the relations between healthcare industry players and physicians or their associations, students
preparing for medical professions or their associations, teachers, health establishments or publishers of prescription
and dispensing assistance software.
Regulated Software; Electronic Health Records
The FDA has become increasingly active in addressing the regulation of computer software and digital health
products intended for use in health care settings. The 21st Century Cures Act (the “Cures Act”), signed into law on
December 13, 2016, among other things, amended the medical device definition to exclude certain software from
FDA regulation, including clinical decision support software that meets certain criteria. On September 27, 2019,
the FDA issued a suite of guidance documents on digital health products, which incorporated applicable Cures Act
standards, including regarding the types of clinical decision support tools and other software that are exempt from
regulation by the FDA as medical devices, and continues to issue new guidance in this area. Certain of our
businesses involve the development and sale of software and related products to support physician and dental
practice management, and it is possible that the FDA or foreign government authorities could determine that one or
more of our products is a medical device, which could subject us or one or more of our businesses to substantial
additional requirements with respect to these products.
In addition, our businesses that involve physician and dental practice management products, and our specialty home
medical supply business, include electronic information technology systems that store and process personal health,
clinical, financial and other sensitive information of individuals. These information technology systems may be
vulnerable to breakdown, wrongful intrusions, data breaches and malicious attack, which could require us to
expend significant resources to eliminate these problems and address related security concerns and could involve
claims against us by private parties and/or governmental agencies. For example, we are directly or indirectly
subject to numerous and evolving federal, state, local and foreign laws and regulations that protect the privacy and
security of personal information, such as the federal Health Insurance Portability and Accountability Act of 1996,
as amended, and implementing regulations (“HIPAA”), the Controlling the Assault of Non-Solicited Pornography
and Marketing Act, the Telephone Protection and Electronic Protection Act of 1991, Section 5 of the Federal Trade
Commission Act, the California Privacy Act (“CCPA”), and the California Privacy Rights Act (“CPRA”) that
becomes effective on January 1, 2023. Laws and regulations relating to privacy and data protection are continually
evolving and subject to potentially differing interpretations. These requirements may not be harmonized, may be
interpreted and applied in a manner that is inconsistent from one jurisdiction to another or may conflict with other
rules or our practices. Our businesses’ failure to comply with these laws and regulations could expose us to breach
of contract claims, substantial fines, penalties and other liabilities and expenses, costs for remediation and harm to
our reputation. Also, evolving laws and regulations in this area could restrict the ability of our customers to obtain,
use or disseminate patient information, or could require us to incur significant additional costs to re-design our
products to reflect these legal requirements, which could have a material adverse effect on our operations.
Also, the European Parliament and the Council of the European Union adopted the pan-European General Data
Protection Regulation (“GDPR”), effective from May 25, 2018, which increased privacy rights for individuals in
Europe (“Data Subjects”), including individuals who are our customers, suppliers and employees. The GDPR
extended the scope of responsibilities for data controllers and data processors, and generally imposes increased
requirements and potential penalties on companies, such as us, that offer goods or services to Data Subjects or
monitor their behavior (including by companies based outside of Europe). Noncompliance can result in penalties of
up to the greater of EUR 20 million, or 4% of global company revenues, and Data Subjects may seek damages. EU
member states may individually impose additional requirements and penalties regarding certain matters, such as
employee personal data. With respect to the personal data it protects, the GDPR requires, among other things,
company accountability, consents from Data Subjects or other acceptable legal basis to process the personal data,
breach notifications within 72 hours, data integrity and security, and fairness and transparency regarding the
storage, use or other processing of the personal data. The GDPR also provides rights to Data Subjects relating
notably to information, access, modification, erasure and transporting of the personal data.
17
In the United States, the CCPA, which increases the privacy protections afforded California residents, became
effective January 1, 2020. The CCPA generally requires companies, such as us, to institute additional protections
regarding the collection, use and disclosure of certain personal information of California residents. Compliance
with the new obligations imposed by the CCPA depends in part on how particular regulators interpret and apply
them, and because the CCPA is relatively new, and its implementing regulations were released in August of 2020,
there remains some uncertainty about how the CCPA will be interpreted by the courts and enforced by the
regulators. If we fail to comply with the CCPA or if regulators assert that we have failed to comply with the CCPA,
we may be subject to certain fines or other penalties and litigation, any of which may negatively impact our
reputation, require us to expend significant resources, and harm our business. Furthermore, California voters
approved the CPRA on November 3, 2020, which will amend and expand the CCPA, including by providing
consumers with additional rights with respect to their personal information, and creating a new state agency to
enforce the CCPA and the CPRA. The CPRA will come into effect on January 1, 2023, applying to information
collected by businesses on or after January 1, 2022.
Other states, as well as the federal government, have increasingly considered the adoption of similarly expansive
personal privacy laws, backed by significant civil penalties for non-compliance. While we believe we have
substantially compliant programs and controls in place to comply with the GDPR, CCPA and CPRA requirements,
our compliance with these measures is likely to impose additional costs on us, and we cannot predict whether the
interpretations of the requirements, or changes in our practices in response to new requirements or interpretations of
the requirements, could have a material adverse effect on our business.
We also sell products and services that health care providers, such as physicians and dentists, use to store and
manage patient medical or dental records. These customers, and we, are subject to laws, regulations and industry
standards, such as HIPAA and the Payment Card Industry Data Security Standards, which require the protection of
the privacy and security of those records, and our products may also be used as part of these customers’
comprehensive data security programs, including in connection with their efforts to comply with applicable privacy
and security laws. Perceived or actual security vulnerabilities in our products or services, or the perceived or actual
failure by us or our customers who use our products or services to comply with applicable legal or contractual data
privacy and security requirements, may not only cause us significant reputational harm, but may also lead to claims
against us by our customers and/or governmental agencies and involve substantial fines, penalties and other
liabilities and expenses and costs for remediation.
Various federal initiatives involve the adoption and use by health care providers of certain electronic health care
records systems and processes. The initiatives include, among others, programs that incentivize physicians and
dentists, through MIPS, to use EHR technology in accordance with certain evolving requirements, including
regarding quality, promoting interoperability, cost and improvement activities. Qualification for the MIPS
incentive payments requires the use of EHRs that are certified as having certain capabilities designated in evolving
standards adopted by CMS and by the Office of the National Coordinator for Health Information Technology of
HHS (“ONC”). Certain of our businesses involve the manufacture and sale of such certified EHR systems and
other products linked to government supported incentive programs. In order to maintain certification of our EHR
products, we must satisfy these changing governmental standards. If any of our EHR systems do not meet these
standards, yet have been relied upon by health care providers to receive federal incentive payments, we may be
exposed to risk, such as under federal health care fraud and abuse laws, including the False Claims Act. For
example, on May 31, 2017, the U.S. Department of Justice announced a $155 million settlement and 5-year
corporate integrity agreement involving a vendor of certified EHR systems, based on allegations that the vendor, by
misrepresenting capabilities to the certifying body, caused its health care provider customers to submit false
Medicare and Medicaid claims for meaningful use incentive payments in violation of the False Claims Act.
Moreover, in order to satisfy our customers, our products may need to incorporate increasingly complex
functionality, such as reporting functionality. Although we believe we are positioned to accomplish this, the effort
may involve increased costs, and our failure to implement product modifications, or otherwise satisfy applicable
standards, could have a material adverse effect on our business.
Other health information standards, such as regulations under HIPAA, establish standards regarding electronic
health data transmissions and transaction code set rules for specific electronic transactions, such as transactions
involving claims submissions to third party payers. Failure to abide by these and other electronic health data
18
transmission standards could expose us to breach of contract claims, substantial fines, penalties, and other liabilities
and expenses, costs for remediation and harm to our reputation.
Additionally, as electronic medical devices are increasingly connected to each other and to other technology, the
ability of these connected systems to safely and effectively exchange and use exchanged information becomes
increasingly important. For example, on September 6, 2017, the FDA issued final guidance to assist industry in
identifying specific considerations related to the ability of electronic medical devices to safely and effectively
exchange and use exchanged information. As a medical device manufacturer, we must manage risks including
those associated with an electronic interface that is incorporated into a medical device.
There may be additional legislative or regulatory initiatives in the future impacting health care.
E-Commerce
Electronic commerce solutions have become an integral part of traditional health care supply and distribution
relationships. Our distribution business is characterized by rapid technological developments and intense
competition. The continuing advancement of online commerce requires us to cost-effectively adapt to changing
technologies, to enhance existing services and to develop and introduce a variety of new services to address the
changing demands of consumers and our customers on a timely basis, particularly in response to competitive
offerings.
Through our proprietary, technologically-based suite of products, we offer customers a variety of competitive
alternatives. We believe that our tradition of reliable service, our name recognition and large customer base built
on solid customer relationships, position us well to participate in this significant aspect of the distribution business.
We continue to explore ways and means to improve and expand our Internet presence and capabilities, including
our online commerce offerings and our use of various social media outlets.
International Transactions
United States and foreign import and export laws and regulations require us to abide by certain standards relating to
the importation and exportation of products. We also are subject to certain laws and regulations concerning the
conduct of our foreign operations, including the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, German
anti-corruption laws and other anti-bribery laws and laws pertaining to the accuracy of our internal books and
records, as well as other types of foreign requirements similar to those imposed in the United States.
While we believe that we are substantially compliant with the foregoing laws and regulations promulgated
thereunder and possess all material permits and licenses required for the conduct of our business, there can be no
assurance that regulations that impact our business or customers’ practices will not have a material adverse effect
on our business.
See “
” for a discussion of additional burdens, risks and regulatory developments that may
affect our results of operations and financial condition.
Proprietary Rights
We hold trademarks relating to the “Henry Schein
®
” name and logo, as well as certain other trademarks. We intend
to protect our trademarks to the fullest extent practicable.
19
Employees and Human Capital
At Henry Schein, our employees are our greatest asset. We employ more than 19,000 full-time equivalent
employees, including approximately 2,250 telesales representatives, over 3,450 field sales consultants, including
equipment sales specialists, 2,000 installation and repair technicians, 3,550 warehouse employees, 800 computer
programmers and technicians, 675 management employees and 6,300 office, clerical and administrative employees.
Approximately 49% of our workforce is based in the United States and approximately 51% is based outside of the
United States.
Approximately 13% of our employees are subject to collective bargaining agreements. We believe
that our relations with our employees are excellent.
We refer to our employees as Team Schein Members, or “TSMs.” Our TSMs are the cornerstone of the Company.
Our success is built on the engagement and commitment of our team, which is dedicated to meeting the needs of
our customers, supplier partners, fellow TSMs, stockholders and society. We are committed to supporting the
personal and professional development of our TSMs, as well as providing competitive benefits and a safe, inclusive
workplace, and believe that these measures help us to retain our TSMs and attract new TSMs. As part of this
commitment, we have, among other things:
•
Developed a strong collaborative workplace culture.
communicate and cooperate across functional and departmental teams positively impacts our performance.
Each TSM’s performance is evaluated annually, based on a measure of Team Schein values, with a focus
on open communication. Our team’s performance as a whole is evaluated via a culture survey, conducted
every two years, distributed to all TSMs, which, among other things, addresses collaboration. The results
from our culture surveys are reviewed by senior leaders, reported to the Board of Directors and used to
implement programs and processes designed to further enhance our culture. We are currently in the
process of further developing our collaborative culture by, among other things, strengthening our existing
commitment to diversity and inclusion, as further described below.
•
Committed to enhance our Diversity and Inclusion (“D&I”) initiatives.
fosters innovation and cultivates an environment filled with unique perspectives. As a result, D&I helps us
meet the needs of customers around the world. We collect feedback through hosting roundtables where our
senior leaders actively listen to our TSMs on topics related to D&I, and the insights learned are used to
guide our efforts to support a diverse and inclusive environment. To guide our efforts and education
related to D&I, we have established an Executive Diversity and Inclusion Council with engagement from
our Board of Directors and Executive Management Committee. This Council drives the Company’s overall
D&I strategy. In 2020, we launched a D&I learning program to educate our TSMs on critical D&I related
topics, and management is incentivized to advance our D&I efforts. Additionally, we promote engagement
by utilizing our Employee Resource Groups as an inclusive and diverse vehicle for all TSMs to share,
connect, learn, and develop both personally and professionally. We believe that these efforts will serve as a
critical stepping stone as we continue to strengthen our D&I initiatives in an effort to meet the evolving
needs of our customers, supplier partners, TSMs, stockholders and society.
•
Committed to the professional development of our TSMs.
and provide formal and informal learning opportunities to our TSMs. All TSMs globally are offered a
broad suite of talent and professional development training programs targeted to specific learning
opportunities based on their current and potential future role within the Company. We also offer over 50
organizational and development training courses designed to aid in the overall development and
advancement of skills and competencies to enable organizational success.
•
Supported talent development and succession planning.
commitment to ensure a strong leadership pipeline across the organization. We continuously identify a
group of potential management successors as part of our succession planning process. Our senior leaders
work to develop our TSMs’ talent and focus the team to execute our long-term strategic plans. Our Board
of Directors is provided with periodic updates regarding our talent development and succession planning
efforts, participates in professional development activities with our TSMs and receives formal
documentation on these topics annually.
20
•
Supported TSM health and safety.
eligible TSMs. In addition to employee health, we are committed to providing a safe and secure work
environment for all TSMs. In response to the COVID-19 pandemic, in March 2020, we implemented
certain policy and procedure changes in an effort to protect our TSMs and customers, and to support
appropriate health and safety protocols. While TSMs at our manufacturing and distribution facilities, as
well as field sales consultants and equipment service technicians, have continued to work onsite or in the
field to provide vital services to our customers, most TSMs in administrative functions have effectively
worked remotely since mid-March. To support the health and safety of our TSMs, we, among other things,
implemented extensive cleaning and sanitation processes and face mask policies to protect TSMs at our
manufacturing and distribution facilities, instituted social distancing and face mask policies for our field
sales consultants and equipment service technicians and adopted broad work-from-home initiatives for
TSMs in administrative functions. In connection with this shift to remote working, we made investments in
equipment, technology, and security upgrades to help protect our information and enhance our team’s
ability to work remotely. Additionally, to help the team manage stress during the pandemic, we, among
other things, established a “COVID-19 Resource Center” to provide a central location for all
communications to support the health of TSMs and their families, and hold virtual Global Town Halls for
all TSMs.
Available Information
We make available free of charge through our Internet website,
www.henryschein.com
, our annual report on Form
10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, statements of beneficial ownership of
securities on Forms 3, 4 and 5 and amendments to these reports and statements filed or furnished pursuant to
Section 13(a) and Section 16 of the Securities Exchange Act of 1934 as soon as reasonably practicable after such
materials are electronically filed with, or furnished to, the United States Securities and Exchange Commission, or
SEC. Our principal executive offices are located at 135 Duryea Road, Melville, New York 11747, and our
telephone number is (631) 843-5500. Unless the context specifically requires otherwise, the terms the “Company,”
“Henry Schein,” “we,” “us” and “our” mean Henry Schein, Inc., a Delaware corporation, and its consolidated
subsidiaries.
21
Information about our Executive Officers
The following table sets forth certain information regarding our executive officers:
Name
Age
Position
Stanley M. Bergman
71
Chairman, Chief Executive Officer, Director
Gerald A. Benjamin
68
Executive Vice President, Chief Administrative Officer, Director
James P. Breslawski
67
Vice Chairman, President, Director
Michael S. Ettinger
59
Senior Vice President, Corporate & Legal Affairs and Chief of Staff, Secretary
Mark E. Mlotek
65
Executive Vice President, Chief Strategic Officer, Director
Steven Paladino
63
Executive Vice President, Chief Financial Officer, Director
Walter Siegel
61
Senior Vice President and General Counsel
Stanley M. Bergman
Mr. Bergman held the position of President from 1989 to 2005. Mr. Bergman held the position of Executive Vice
President from 1985 to 1989 and Vice President of Finance and Administration from 1980 to 1985.
Gerald A. Benjamin
director since 1994. Prior to holding his current position, Mr. Benjamin was Senior Vice President of
Administration and Customer Satisfaction since 1993. Mr. Benjamin was Vice President of Distribution
Operations from 1990 to 1992 and Director of Materials Management from 1988 to 1990. Before joining us in
1988, Mr. Benjamin was employed for 12 years at Estée Lauder, Inc., in various management positions where his
last position was Director of Materials Planning and Control.
James P. Breslawski
Breslawski was the Chief Executive Officer of our Henry Schein Global Dental Group from 2005 to 2018. Mr.
Breslawski held the position of Executive Vice President and President of U.S. Dental from 1990 to 2005, with
primary responsibility for the North American Dental Group. Between 1980 and 1990, Mr. Breslawski held
various positions with us, including Chief Financial Officer, Vice President of Finance and Administration and
Corporate Controller.
Michael S. Ettinger
since 2015. Prior to his current position, Mr. Ettinger served as Senior Vice President, Corporate & Legal Affairs
and Secretary from 2013 to 2015, Corporate Senior Vice President, General Counsel & Secretary from 2006 to
2013, Vice President, General Counsel and Secretary from 2000 to 2006, Vice President and Associate General
Counsel from 1998 to 2000 and Associate General Counsel from 1994 to 1998. Before joining us, Mr. Ettinger
served as a senior associate with Bower & Gardner and as a member of the Tax Department at Arthur Andersen.
Mark E. Mlotek
Senior Vice President and subsequently Executive Vice President of the Corporate Business Development Group
between 2000 and 2012. Prior to that, Mr. Mlotek was Vice President, General Counsel and Secretary from 1994 to
1999 and became a director in 1995. Prior to joining us, Mr. Mlotek was a partner in the law firm of Proskauer
Rose LLP, counsel to us, specializing in mergers and acquisitions, corporate reorganizations and tax law from 1989
to 1994.
Steven Paladino
his current position, Mr. Paladino was Senior Vice President and Chief Financial Officer from 1993 to 2000 and
has been a director since 1992. From 1990 to 1992, Mr. Paladino served as Vice President and Treasurer and from
1987 to 1990 served as Corporate Controller. Before joining us, Mr. Paladino was employed in public accounting
for seven years, most recently with the international accounting firm of BDO USA, LLP. Mr. Paladino is a
certified public accountant.
22
Walter Siegel
was employed with Standard Microsystems Corporation, a publicly traded global semiconductor company from
2005 to 2012, holding positions of increasing responsibility, most recently as Senior Vice President, General
Counsel and Secretary.
Other Executive Management
The following table sets forth certain information regarding other Executive Management:
Name
Age
Position
David Brous
52
President, Strategic Business Units Group and Asia Pacific & Brazil Dental
Brad Connett
62
President, U.S. Medical Group
Jonathan Koch
46
Senior Vice President and Chief Executive Officer, Global Dental Group
Lorelei McGlynn
57
Senior Vice President, Chief Human Resources Officer
James Mullins
56
Senior Vice President, Global Services
Christopher Pendergast
58
Senior Vice President and Chief Technology Officer
Michael Racioppi
66
Senior Vice President, Chief Merchandising Officer
René Willi, Ph.D.
53
President, Global Dental Surgical Group
David Brous
Mr. Brous joined us in 2002 and has held many positions within the organization, including leading and managing
the Corporate Business Development Group and the International Healthcare Group (managing our International
Animal Health business, International Medical business and Australia / New Zealand Dental business).
Brad Connett
has held a number of increasingly responsible positions at the Company. Throughout his career, he has received
numerous industry honors, including the John F. Sasen Leadership Award from the Health Industry Distributors
Association (HIDA), in recognition of his service to the industry, and induction into the Medical Distribution Hall
of Fame by Repertoire Magazine.
Jonathan Koch
2018. Prior to joining us, for the years 2006 to 2018, Mr. Koch was a senior executive at Covance, the drug
development services business of Laboratory Corporation of America. In his last role at Covance, Mr. Koch was
the Executive Vice President and Group President of Covance Clinical Development & Commercialization
Services. Prior to that, Mr. Koch was Executive Vice President and Group President of Covance Research and
Development Laboratories from 2015 to 2017. Mr. Koch was also President of Covance Central Laboratory
Services from 2010 to 2015, and Vice President at Covance, with various responsibilities, from 2006 to 2010. Prior
to Covance, Mr. Koch held senior leadership roles of increasing responsibility while employed with Charles River
Laboratories from 1998 to 2006.
Lorelei McGlynn
us in 1999, Ms. McGlynn has served as Vice President, Global Human Resources and Financial Operations from
2008 to 2013, Chief Financial Officer, International Group and Vice President of Global Financial Operations from
2002 to 2008 and Vice President, Finance, North America from 1999 to 2002. Prior to joining us, Ms. McGlynn
served as Assistant Vice President of Finance at Adecco Corporation.
James Mullins
and has held a number of key positions with increasing responsibility, including Global Chief Customer Service
Officer.
23
Christopher Pendergast
joining us, Mr. Pendergast was the employed by VSP Global from 2008 to 2018, most recently as the Chief
Technology Officer and Chief Information Officer. Prior to VSP Global, Mr. Pendergast served in roles of
increasing responsibility at Natural Organics, Inc., from 2006 to 2008, IdeaSphere Inc./Twinlab Corporation from
2000 to 2006, IBM Corporation from 1987 to 1994 and 1998 to 2000 and Rohm and Haas from 1994 to 1998.
Michael Racioppi
his current position, Mr. Racioppi was President of the Medical Division from 2000 to 2008 and Interim President
from 1999 to 2000, and Corporate Vice President from 1994 to 2008, with primary responsibility for the Medical
Group, Marketing and Merchandising departments. Mr. Racioppi served as Senior Director, Corporate
Merchandising from 1992 to 1994. Before joining us in 1992, Mr. Racioppi was employed by Ketchum
Distributors, Inc. as the Vice President of Purchasing and Marketing. He currently serves on the board of National
Distribution and Contracting and previously served on the board of Health Distribution Management Association
and Health Industry Distributors Association (HIDA).
René Willi, Ph.D.
joining Henry Schein, Dr. Willi held senior level roles with Institut Straumann AG as Executive Vice President,
Surgical Business Unit from 2005 to 2013. Prior to Straumann, he held roles of increasing responsibility in
Medtronic Plc’s cardiovascular division from 2003 to 2005 and with McKinsey & Company as a management
consultant from 2000 to 2003.
24
ITEM 1A. Risk Factors
Our business operations could be affected by factors that are not presently known to us or that we currently
consider not to be material to our operations, so you should not consider the risks disclosed in this section to
necessarily represent a complete statement of all risks and uncertainties. The Company believes that the following
risks could have a material adverse impact on our business, reputation, financial results, financial condition and/or
the trading price of our common stock. The order in which these factors appear does not necessarily reflect their
relative importance or priority.
COMPANY RISKS
Our business, results of operations, cash flows, financial condition and liquidity may be negatively impacted by
the effects of disease outbreaks, epidemics, pandemics, or similar wide-spread public health concerns and other
natural disasters
.
The COVID-19 pandemic and the responses of governments to it had, and may again have, a
material adverse effect on our business, results of operations and cash flows and may result in a material
adverse effect on our financial condition and liquidity.
Our business, results of operations, cash flows, financial condition and liquidity may be negatively impacted by the
effects of disease outbreaks, epidemics, pandemics, similar wide-spread public health concerns, and other natural
disasters. The COVID-19 pandemic has had, and continues to have, an unprecedented impact on society, worldwide
economic activity, and the health care sector (particularly, the dental market). As a global healthcare solutions
company, the COVID-19 pandemic and the governmental responses to it had, and may again have, a material
adverse effect on our business, results of operations and cash flows and may result in a material adverse effect on
our financial condition and liquidity. In March and April 2020, the dental market was severely impacted by
COVID-19, with many, if not a majority, of practices being closed or open on a limited basis only. Although dental
practice openings and patient volume recovery in the United States and many other countries have rebounded faster
than originally anticipated, patient volumes have remained below pre-COVID-19 levels. Material uncertainty
remains and the potential for additional significant resurgences of COVID-19 could cause a significant reduction in
dental practice openings and patient volume recovery, or further delay the return to normal operations. Even after
COVID-19 has subsided, we may again experience material adverse impacts to our business, results of operations
and cash flows as a result of, among other things, its global economic impact, including any recession that may
occur in the future, or a prolonged period of economic slowdown or the reluctance of patients to return for elective
dental or medical care. The impacts and potential impacts from the COVID-19 pandemic include, but are not
limited to:
•
Significant reductions in demand or significant volatility in demand for certain of our products.
March and April 2020, many dental offices in the United States performed only emergency procedures, and
rescheduled wellness exams and elective procedures. Dental offices in other countries also experienced closures or
restricted operations, as did medical offices around the world. Such closures and restrictions impacted our
customers’ spending with us and had, and if reinstated may again have, a material adverse effect on our business,
results of operations and cash flows. Although dental practice openings and patient volume recovery have
rebounded faster than originally anticipated, capacity constraints in offices and demand-side factors may again lead
to reductions in demand or significant volatility in demand for our products. Additionally, significant reduction in
demand for certain of our products or customers’ decisions to delay the purchase of large equipment may result in
us having increased inventory;
•
Shortage of Certain Personal Protective Equipment (PPE
). Supply chain disruptions for PPE and an increased
demand for these products has resulted, and may continue to result, in backorders of certain PPE and a potential
scarcity in raw materials to make certain PPE. Prices for certain PPE have been volatile. Although we believe that
most practices currently are able to access adequate supply, with some exceptions in certain markets depending on
a number of factors, including the progress of the virus and efforts to combat it, we still may be unable to supply
our customers with the quantity of certain PPE products they demand, which may lead to our customers seeking
alternative sources of supply. Furthermore, healthcare professionals’ inability to obtain a sufficient quantity of
certain PPE would adversely impact our business, results of operations and cash flows, and could materially
adversely affect our financial condition and liquidity. Conversely, we recorded significant charges throughout the
year beginning in the second quarter for PPE inventory due to volatility of pricing for PPE, and, depending upon
25
the course of the pandemic, if PPE pricing or demand decreases, our margins and the value of certain our PPE
inventory could be further negatively impacted in future periods, which could result in a material adverse impact on
our business, results of operations and cash flows and our financial condition and liquidity;
•
Reduction in Peoples’ Ability and Willingness to be in Public.
organizations, and implemented by many local governments, to slow and limit the transmission of COVID-19
(including business closures and restrictions, stay-at-home and similar measures) were implemented and then lifted
or partially lifted in some locations and reinstituted in others. Ongoing social distancing ordinances and similar
restrictions, and the actual and potential for additional resurgences of COVID-19 has in some locations and may in
other locations result in the re-imposition or tightening of governmental social distancing and other restrictions,
and/or cause people to be less willing to go to elective medical and dental appointments, which could again
materially adversely affect demand for our products. A lengthened period of materially suppressed demand could
again cause material adverse impacts on our business, results of operations and cash flows and could materially
adversely affect our financial condition and liquidity;
•
Potential delays in customer payments, or defaults on our customer credit arrangements.
products to customers with payment terms. If customers’ cash flows or operating and financial performance
deteriorate due to the impact of COVID-19, or if they are unable to make scheduled payments or obtain credit, they
may not be able to pay, or may delay payment to us. Likewise, for similar reasons, suppliers may restrict credit or
impose more stringent payment terms. The inability of current and/or potential customers to pay us for our products
and/or services or any demands by suppliers for more stringent payment terms may materially adversely affect our
business, results of operations, cash flows, financial condition and liquidity and may limit the amounts we can
borrow under our trade accounts receivable securitization;
•
Impact on third parties’ ability to meet their obligations to us; impact on our ability to meet obligations to third
parties.
contractors (including third-party shippers), banks, joint venture partners and external business partners, to meet
their obligations to us, or significant disruptions in their ability to do so, which may be caused by their own
financial or operational difficulties, or by travel restrictions and border closures, may materially adversely affect
our business, results of operations, cash flows, financial condition and liquidity. Certain of our contracts with
supply partners contain minimum purchase requirements or include rebate provisions if we satisfy certain sales or
purchasing targets that, in certain cases we have not been able to satisfy and in other cases we may not be able to
fully satisfy, due to the impact of the COVID-19 pandemic. Rebate income recognized in fiscal 2020 is less than
rebates earned over the prior fiscal year. Our failure to satisfy such contractual provisions or renegotiate more
favorable terms could materially adversely affect our business, results of operations and cash flows;
•
Negative impact on our workforce and impact of adapted business practices.
to implement temporary cost reduction measures (including a payroll cost reduction plan centered around
furloughs, reduced pay and work hours, voluntary unpaid time off, suspension of Company contributions to certain
retirement plans and job reductions), all of which have now ended (except for a small number of TSMs who remain
on furlough), modify our business practices (including employee travel, employee work locations, and cancellation
of physical participation in meetings, events and conferences), and we may take further actions as may be required
by government authorities or that we determine are in the best interests of our employees. As the COVID-19
pandemic continues to unfold, we will continue to evaluate appropriate actions for our business. Many of our
employees shifted abruptly to working remotely and our non-essential workers who are able to work from home
continue to do so. An extended period of modified business practices and remote work arrangements could have a
negative impact on employee morale, strain our business continuity plans, introduce operational risk (including but
not limited to cybersecurity risks), and impair our ability to efficiently operate our business;
•
Significant changes in political conditions.
purchase and distribute our products have occurred and are expected to continue at least during the pendency of the
pandemic, including quarantines, governmental or regulatory actions, closures or other restrictions that limit or
close our operating facilities, restrict our employees’ ability to travel or perform necessary business functions, or
otherwise constrain the operations of our business partners, suppliers, or customers, which may materially
adversely affect our business, results of operations, cash flows, financial condition and liquidity;
26
•
Potential impact on our ability to meet obligations under credit facilities.
amendments to our material credit facilities to, among other things, extend the maturity dates and temporarily
provide additional flexibility under certain covenants, an extended negative impact of COVID-19 on our business,
results of operations, cash flows, financial condition and liquidity could impact our ability to meet our obligations
under credit facilities or outstanding long term debt, which contain maximum leverage ratios, and customary
representations, warranties and affirmative covenants;
•
Volatility in the financial markets.
availability and cost of credit to us;
•
Refocusing management resources to mitigate effects of COVID-19
. Our management is focused on mitigating the
effects of COVID-19, which has required, and may continue to require for the duration of the pandemic, a large
investment of time and resources across the Company, and may delay certain strategic and other plans, which could
materially adversely affect our business;
•
Potential
increased costs associated with our self-insured medical insurance programs.
We may incur significant
employee health care costs under our self-insurance medical insurance programs if a large number of our
employees and/or their covered family members become ill from COVID-19; and
•
Reputational risk associated with response to COVID-19.
pandemic, or if customers do not perceive our response to be adequate, we could suffer damage to our reputation
and our brands, which could materially adversely affect our business.
The impact of COVID-19 may also exacerbate other risks discussed below, any of which could have a material
adverse effect on us.
We are dependent upon third parties for the manufacture and supply of substantially all of our products.
We obtain substantially all of the products we distribute from third parties, with whom we generally do not have
long-term contracts. While there is typically more than one source of supply, some key suppliers, in the aggregate,
supply a significant portion of the products we sell. In 2020, our top 10 health care distribution suppliers and our
single largest supplier accounted for approximately 30% and 4%, respectively, of our aggregate purchases.
Because of our dependence upon such suppliers, our operations are subject to the suppliers’ ability and willingness
to supply products in the quantities that we require, and the risks include delays caused by interruption in
production based on conditions outside of our control, including a supplier’s failure to comply with applicable
government requirements (which may result in product recalls and/or cessation of sales) or an interruption in the
suppliers’ manufacturing capabilities. In the event of any such interruption in supply, we would need to identify and
obtain acceptable replacement sources on a timely basis. There is no guarantee that we would be able to obtain such
alternative sources of supply on a timely basis, if at all, and an extended interruption in supply, particularly of a
high sales volume product, could result in a significant disruption in our sales and operations, as well as damage to
our relationships with customers and our reputation.
Our future growth (especially for our technology and value-added services segment) is dependent upon our
ability to develop or acquire and maintain and protect new products and technologies that achieve market
acceptance with acceptable margins.
Our future success depends on our ability to timely develop (or obtain the right to sell) competitive and innovative
(particularly for our technology and value-added services segment), products and services and to market them
quickly and cost-effectively. Our ability to anticipate customer needs and emerging trends and develop or acquire
new products, services and technologies at competitive prices requires significant resources, including employees
with the requisite skills, experience and expertise, particularly in our technology segment, including dental practice
management, patient engagement and demand creation software solutions. The failure to successfully address these
challenges could materially disrupt our sales and operations. Additionally, our software and e-services products,
like software products generally, may contain undetected errors or bugs when introduced or as new versions are
released. Any such defective software may result in increased expenses related to the software and could adversely
affect our relationships with customers as well as our reputation. While certain software and e-services that we
27
develop are protected under patent law, we rely primarily upon copyright, trademark and trade secret laws, as well
as contractual and common law protections and confidentiality obligations. We cannot provide assurance that such
legal protections will be available, adequate or enforceable in a timely manner to protect our software or e-services
products.
Our expansion through acquisitions and joint ventures involves risks and may not result in the benefits and
revenue growth we expect.
One of our business strategies has been to expand our domestic and international markets in part through
acquisitions and joint ventures, and we expect to continue to make acquisitions and enter into joint ventures in the
future. Such transactions require significant management attention, may place significant demands on our
operations, information systems and financial resources, and there is risk that one or more may not succeed. We
cannot be sure, for example, that we will achieve the benefits of revenue growth that we expect from these
acquisitions or joint ventures or that we will avoid unforeseen additional costs or expenses. Our ability to
successfully implement our acquisition and joint venture strategy depends upon, among other things, the following:
•
the availability of suitable acquisition or joint venture candidates at acceptable prices;
•
our ability to consummate such transactions, which could potentially be prohibited due to U.S. or
foreign antitrust regulations;
•
the liquidity of our investments and the availability of financing on acceptable terms;
•
our ability to retain customers or product lines of the acquired businesses or joint ventures;
•
our ability to retain, recruit and incentivize the management of the companies we acquire; and
•
our ability to successfully integrate these companies’ operations, services, products and personnel with
our culture, management policies, internal procedures, working capital management, financial and
operational controls and strategies.
Furthermore, some of our acquisitions and future acquisitions may give rise to an obligation to make contingent
payments or to satisfy certain repurchase obligations, which payments could have material adverse impacts on our
financial results individually or in the aggregate.
Certain provisions in our governing documents and other documents to which we are a party may discourage
third parties from seeking to acquire us that might otherwise result in our stockholders receiving a premium
over the market price of their shares.
The provisions of our certificate of incorporation and by-laws may make it more difficult for a third-party to
acquire us, may discourage acquisition bids and may impact the price that certain investors might be willing to pay
in the future for shares of our common stock. These provisions, among other things require:
•
approve a merger, consolidation, or a sale, lease, transfer or exchange of all or substantially all of our
assets; and
•
remove a director; and (ii) to amend or repeal our by-laws, with certain limited exceptions.
In addition, certain of our employee incentive plans provide for accelerated vesting of stock options and other
awards upon termination without cause within two years following a change in control, or grant the plan committee
discretion to accelerate awards upon a change of control. Further, certain agreements between us and our executive
officers provide for increased severance payments and certain benefits if those executive officers are terminated
without cause by us or if they terminate for good reason, in each case within two years following a change in
control or within ninety days prior to the effective date of the change in control or after the first public
announcement of the pendency of the change in control.
28
INDUSTRY RISKS
The health care products distribution industry is highly competitive (including, without limitation, competition
from third-party online commerce sites) and consolidating, and we may not be able to compete successfully.
We compete with numerous companies, including several major manufacturers and distributors. Some of our
competitors have greater financial and other resources than we do, which could allow them to compete more
successfully. Most of our products are available from several sources and our customers tend to have relationships
with several distributors. Competitors could obtain exclusive rights to market particular products, which we would
then be unable to market. Manufacturers also could increase their efforts to sell directly to end-users and thereby
eliminate or reduce our role in distribution. Industry consolidation among health care product distributors and
manufacturers, price competition, product unavailability, whether due to our inability to gain access to products or
to interruptions in manufacturing supply, or the emergence of new competitors, also could increase competition.
Consolidation has also increased among manufacturers of health care products, which could have a material
adverse effect on our margins and product availability. We could be subject to charges and financial losses in the
event we fail to satisfy minimum purchase commitments contained in some of our contracts. Additionally,
traditional health care supply and distribution relationships are being challenged by electronic online commerce
solutions. The continued advancement of online commerce by third parties will require us to cost-effectively adapt
to changing technologies, to enhance existing services and to differentiate our business (including with additional
value-added services) to address changing demands of consumers and our customers on a timely basis. The
emergence of such potential competition and our inability to anticipate and effectively respond to changes on a
timely basis could have a material adverse effect on our business.
The repeal or judicial prohibition on implementation of the Affordable Care Act could materially adversely
affect our business.
The U.S. Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation
Act, each enacted in March 2010, as amended (the “ACA”), greatly expanded health insurance coverage in the
United States and has been the target of litigation and Congressional reform efforts since its adoption. The U.S.
Supreme Court, in upholding the constitutionality of the ACA and its individual mandate provision in 2012,
simultaneously limited ACA provisions requiring Medicaid expansion, making such expansion a state-by-state
decision. In 2017, the U.S. Congress effectively repealed the ACA’s individual mandate provision by eliminating
the financial penalty for non-compliance. In the most recent ACA litigation, a federal appeals court found the
individual mandate to be unconstitutional, and returned the case to a lower federal court for consideration of
whether the remainder of the ACA could survive the excision of the individual mandate. This decision was
appealed to the U.S. Supreme Court, and a decision is expected soon. Any outcome of this case that changes the
ACA, in addition to future legislation, regulation, guidance and/or Executive Orders that do the same, could have a
significant impact on the U.S. healthcare industry and our operations.
The health care industry is experiencing changes due to political, economic and regulatory influences that could
materially adversely affect our business.
The health care industry is highly regulated and subject to changing political, economic and regulatory influences.
In recent years, the health care industry has undergone, and is in the process of undergoing, significant changes
driven by various efforts to reduce costs, including, among other factors: trends toward managed care; collective
purchasing arrangements and consolidation among office-based health care practitioners; and changes in
reimbursements to customers, including increased attention to value-based payment arrangements, as well as
growing enforcement activities (and related monetary recoveries) by governmental officials. Both our profitability
and the profitability of our customers may be materially adversely affected by laws and regulations reducing
reimbursement rates for pharmaceuticals, medical supplies and devices, and/or medical treatments or services, or
changes to the methodology by which reimbursement levels are determined. If we are unable to react effectively to
these and other changes in the health care industry, our business could be materially adversely affected.
29
Expansion of group purchasing organizations (“GPO”) or provider networks and the multi-tiered costing
structure may place us at a competitive disadvantage.
The medical products industry is subject to a multi-tiered costing structure, which can vary by manufacturer and/or
product. Under this structure, certain institutions can obtain more favorable prices for medical products than we are
able to obtain. The multi-tiered costing structure continues to expand as many large integrated health care providers
and others with significant purchasing power, such as GPOs, demand more favorable pricing terms. Additionally,
the formation of provider networks and GPOs may shift purchasing decisions to entities or persons with whom we
do not have a historical relationship and may threaten our ability to compete effectively, which could in turn
negatively impact our financial results. Although we are seeking to obtain similar terms from manufacturers to
access lower prices demanded by GPO contracts or other contracts, and to develop relationships with existing and
emerging provider networks and GPOs, we cannot guarantee that such terms will be obtained or contracts executed.
Increases in shipping costs or service issues with our third-party shippers could harm our business.
Shipping is a significant expense in the operation of our business. We ship almost all of our orders through third-
party delivery services, and typically bear the cost of shipment. Accordingly, any significant increase in shipping
rates could have a material adverse effect on our business, financial condition or operating results. Similarly, strikes
or other service interruptions by those shippers could cause our operating expenses to rise and materially adversely
affect our ability to deliver products on a timely basis.
MACRO ECONOMIC AND POLITICAL RISKS
Uncertain global macro-economic and political conditions could materially adversely affect our results of
operations and financial condition.
Uncertain global macro-economic and political conditions that affect the economy and the economic outlook of the
United States, Europe, Asia and other parts of the world could materially adversely affect our results of operations
and financial condition. These uncertainties, include, among other things:
•
election results;
•
changes to laws and policies governing foreign trade (including, without limitation, the United States-
Mexico-Canada Agreement (USMCA), the EU-UK Trade and Cooperation Agreement of December
2020, and other international trade agreements);
•
greater restrictions on imports and exports;
•
supply chain disruptions due to social issues;
•
changes in laws and policies governing health care or data privacy;
•
tariffs and sanctions;
•
changes to the relationship between the United States and China;
•
sovereign debt levels;
•
the inability of political institutions to effectively resolve actual or perceived economic, currency or
budgetary crises or issues;
•
consumer confidence;
•
unemployment levels (and a corresponding increase in the uninsured and underinsured population);
•
changes in regulatory and tax regulations;
•
increases in interest rates;
•
availability of capital;
•
increases in fuel and energy costs;
•
the effect of inflation on our ability to procure products and our ability to increase prices over time;
•
changes in tax rates and the availability of certain tax deductions;
•
increases in health care costs;
•
the threat or outbreak of war, terrorism or public unrest; and
•
changes in laws and policies governing manufacturing, development and investment in territories and
countries where we do business.
30
Additionally, changes in government, government debt and/or budget crises may lead to reductions in government
spending in certain countries, which could reduce overall health care spending, and/or higher income or corporate
taxes, which could depress spending overall. Recessionary conditions and depressed levels of consumer and
commercial spending may also cause customers to reduce, modify, delay or cancel plans to purchase our products
and may cause suppliers to reduce their output or change their terms of sale. We generally sell products to
customers with payment terms. If customers’ cash flow or operating and financial performance deteriorate, or if
they are unable to make scheduled payments or obtain credit, they may not be able to, or may delay, payment to us.
Likewise, for similar reasons suppliers may restrict credit or impose different payment terms.
REGULATORY AND LITIGATION RISKS
Failure to comply with existing and future regulatory requirements could materially adversely affect our
business.
The laws and regulations that govern our business and operations are subject to varying and evolving
interpretations, future changes, additions, and enforcement approaches (including in light of political changes, such
as with respect to the new administration of President Biden) that affect our ability to comply. For example,
President Biden’s administration has authorized and encouraged a freeze on certain federal regulations that have
been published but are not yet effective, as well as a review of all federal regulations issued during President
Trump’s administration. Changes with respect to the applicable laws and regulations may require us to update or
revise our operations, services, marketing practices, and compliance programs and controls, and may impose
additional and unforeseen costs on us, pose new or previously immaterial risks to us, or may otherwise have a
material adverse effect on our business. There can be no assurance that current and future government regulations
will not adversely affect our business, and we cannot predict new regulatory priorities, the form, content or timing
of regulatory actions, and their impact on the health care industry and on our business and operations.
Global efforts toward healthcare cost containment continue to exert pressure on product pricing. In the United
States, in addition to other government efforts to control health care costs, there has been increased scrutiny on drug
pricing and concurrent efforts to control or reduce drug costs by Congress, the President, executive branch agencies
and various states. At the state level, several states have adopted laws that require drug manufacturers to provide
advance notice of certain price increases and to report information relating to those price increases, while others
have taken legislative or administrative action to establish prescription drug affordability boards or multi-payer
purchasing pools to reduce the cost of prescription drugs. At the federal level, several related bills have been
introduced and regulations proposed which, if enacted or finalized, respectively, would impact drug pricing and
related costs.
Under the Physician Payment Sunshine Act, we are required to collect and report detailed information regarding
certain financial relationships we have with covered recipients, such as physicians, dentists and teaching hospitals.
We or our subsidiaries may be required to report information under certain state transparency laws that address
circumstances not covered by the Physician Payment Sunshine Act, and some of these state laws, as well as the
federal law, can be ambiguous. We are also subject to foreign regulations requiring transparency of certain
interactions between suppliers and their customers. While we believe we have substantially compliant programs
and controls in place satisfying the above laws and requirements, such compliance imposes additional costs on us
and the requirements are sometimes ambiguous. In the United States, government actions to seek to increase
health-related price transparency may also affect our business.
31
Our business is subject to additional requirements under various local, state, federal and international laws and
regulations applicable to the sale and distribution of, and third-party payment for, pharmaceuticals and medical
devices, human cells, tissue and cellular and tissue-based products (“HCT/P products”). Among the federal laws
with which we must comply are the Controlled Substances Act, the U.S. Food, Drug, and Cosmetic Act, as
amended (“FDC Act”), the Federal Drug Quality and Security Act, including Drug Supply Chain Security Act
(“DSCSA”), and Section 361 of the Public Health Services Act. Among other things, such laws, and the regulations
promulgated thereunder:
•
regulate the storage and distribution, labeling, packaging, handling, reporting, record keeping,
introduction, manufacturing and marketing of drugs, HCT/P products and medical devices, including
requirements with respect to unique medical device identifiers;
•
subject us to inspection by the U.S. Food and Drug Administration (“FDA”) and the U.S. Drug
Enforcement Administration (“DEA”), and similar state authorities;
•
regulate the storage, transportation and disposal of certain of our products that are considered
hazardous materials;
•
require us to advertise and promote our drugs and devices in accordance with applicable FDA
requirements;
•
require registration with the FDA and the DEA and various state agencies;
•
require record keeping and documentation of transactions involving drug products;
•
require us to design and operate a system to identify and report suspicious orders of controlled
substances to the DEA;
•
require us to manage returns of products that have been recalled and subject us to inspection of our
recall procedures and activities;
•
impose on us reporting requirements if a pharmaceutical, HCT/P product or medical device causes
serious illness, injury or death;
•
require manufacturers, wholesalers, repackagers and dispensers of prescription drugs to identify and
trace certain prescription drugs as they are distributed;
•
require the licensing of prescription drug wholesalers and third-party logistics providers; and
•
mandate compliance with standards for the recordkeeping, storage and handling of prescription drugs,
and associated reporting requirements.
The FDA has become increasingly active in addressing the regulation of computer software and digital health
products intended for use in health care settings. The 21st Century Cures Act (the “Cures Act”), signed into law on
December 13, 2016, among other things, amended the medical device definition to exclude certain software from
FDA regulation, including certain clinical decision support software. Certain of our businesses involve the
development and sale of software and related products to support physician and dental practice management, and it
is possible that the FDA or foreign government authorities could determine that one or more of our products is
subject to regulation as a medical device, which could subject us or one or more of our businesses to substantial
additional requirements, costs, and potential enforcement actions or liabilities for noncompliance with respect to
these products.
Applicable federal, state, local and foreign laws and regulations also may require us to meet various standards
relating to, among other things, licensure or registration, program eligibility, procurement, third-party
reimbursement, sales and marketing practices, product integrity and supply tracking to product manufacturers,
product labeling, personnel, privacy and security of health or other personal information, installation, maintenance
and repair of equipment and the importation and exportation of products. The FDA and DEA, as well as CMS
(including with respect to complex Medicare reimbursement requirements applicable to our specialty home medical
supplies business), have recently increased their regulatory and enforcement activities and, in particular, the DEA
has heightened enforcement activities due to the opioid crisis in the United States. Our business is also subject to
requirements of similar and other foreign governmental laws and regulations affecting our operations abroad.
The failure to comply with any of these laws and regulations, or new interpretations of existing laws and
regulations, or the imposition of any additional laws and regulations, could materially adversely affect our business.
The costs to us associated with complying with the various applicable statutes and regulations, as they now exist
and as they may be modified, could be material. Allegations by a governmental body that we have not complied
with these laws could have a material adverse effect on our businesses. While we believe that we are substantially
32
compliant with applicable laws and regulations, and believe we have adequate compliance programs and controls in
place to ensure substantial compliance, if it is determined that we have not complied with these laws, we are
potentially subject to penalties, including warning letters, substantial civil and criminal penalties, mandatory recall
of product, seizure of product and injunction, consent decrees and suspension or limitation of product sale and
distribution. If we enter into settlement agreements to resolve allegations of non-compliance, we could be required
to make settlement payments or be subject to civil and criminal penalties, including fines and the loss of licenses.
Non-compliance with government requirements could also adversely affect our ability to participate in important
federal and state government health care programs, such as Medicare and Medicaid, and damage our reputation.
The EU Medical Device Regulation may adversely affect our business.
The EU Medical Device Regulation No. 2017/745 (“EU MDR”) was meant to become applicable three years after
publication (in May 2020). However, on April 23, 2020, to allow EEA national authorities, notified bodies,
manufacturers and other actors to focus fully on urgent priorities related to the COVID-19 pandemic, the European
Council and Parliament adopted Regulation 2020/561, postponing the date of application of the EU MDR by one
year (to May 2021). The EU MDR significantly modifies and intensifies the regulatory compliance requirements
for the medical device industry as a whole. Once applicable, the EU MDR will among other things:
•
Strengthen the rules on placing devices on the market and reinforce surveillance once they are
available;
•
Establish explicit provisions on manufacturers’ responsibilities for the follow-up of the quality,
performance and safety of devices placed on the market;
•
Improve the traceability of medical devices throughout the supply chain to the end-user or patient
through a unique identification number;
•
Set up a central database to provide patients, healthcare professionals and the public with
comprehensive information on products available in the EU;
•
Strengthen rules for the assessment of certain high-risk devices, such as implants, which may have to
undergo an additional check by experts before they are placed on the market; and
•
Identify importers and distributors and medical device products through registration in a database
(EudaMed not due until 2022 and after).
In particular, the EU MDR imposes stricter requirements for the confirmation that a product meets the regulatory
requirements, including regarding a product’s clinical evaluation and a company’s quality systems, and for the
distribution, marketing and sale of medical devices, including post-market surveillance. Medical devices that have
been assessed and/or certified under the EU Medical Device Directive may continue to be placed on the market
until 2024 (or until the expiry of their certificates, if applicable and earlier); however, requirements regarding the
distribution, marketing and sale including quality systems and post-market surveillance have to be observed by
manufacturers, importers and distributors as of the application date.
The modifications created by the EU MDR may have an impact on the way we design and manufacture products
and the way we conduct our business in the European Economic Area.
If we fail to comply with laws and regulations relating to health care fraud or other laws and regulations, we
could suffer penalties or be required to make significant changes to our operations, which could materially
adversely affect our business.
Certain of our businesses are subject to federal and state (and similar foreign) health care fraud and abuse, referral
and reimbursement laws and regulations with respect to their operations. Some of these laws, referred to as “false
claims laws,” prohibit the submission or causing the submission of false or fraudulent claims for reimbursement to
federal, state and other health care payers and programs. Other laws, referred to as “anti-kickback laws,” prohibit
soliciting, offering, receiving or paying remuneration in order to induce the referral of a patient or ordering,
purchasing, leasing or arranging for, or recommending ordering, purchasing or leasing of, items or services that are
paid for by federal, state and other health care payers and programs. Certain additional state and federal laws, such
as the federal Physician Self-Referral Law, commonly known as the “Stark Law,” prohibit physicians and other
health professionals from referring a patient to an entity with which the physician (or family member) has a
33
financial relationship, for the furnishing of certain designated health services (for example, durable medical
equipment and medical supplies), unless an exception applies.
The fraud and abuse laws and regulations have been subject to heightened enforcement activity over the past few
years, and significant enforcement activity has been the result of “relators” who serve as whistleblowers by filing
complaints in the name of the United States (and if applicable, particular states) under applicable false claims laws,
and who may receive up to 30% of total government recoveries. Penalties under fraud and abuse laws may be
severe, and could result in significant civil and criminal penalties and costs, including the loss of licenses and the
ability to participate in federal and state health care programs, and could have a material adverse effect on our
business. Also, these measures may be interpreted or applied by a prosecutorial, regulatory or judicial authority in
a manner that could require us to make changes in our operations or incur substantial defense and settlement
expenses. Even unsuccessful challenges by regulatory authorities or private relators could result in reputational
harm and the incurring of substantial costs. Most states have adopted similar state false claims laws, and these state
laws have their own penalties which may be in addition to federal False Claims Act penalties, as well as other fraud
and abuse laws.
With respect to measures of this type, the United States government (among others) has expressed concerns about
financial relationships between suppliers on the one hand and physicians, dentists and other health care providers,
on the other. As a result, we regularly review and revise our marketing practices as necessary to facilitate
compliance.
In the EU, the Directive No. 2019/1937 of 23 October 2019
on the protection of persons who report breaches of
Union law
December 17, 2021. This Directive covers whistleblowers reporting breaches of certain EU laws, in particular as
regards public health, the above-mentioned Directive No. 2001/83, Regulation No. 726/2004 or, as regards data
protection, the GDPR. The Directive protects a wide range of people and includes former employees. All private
companies with 50 or more employees are required to create effective internal reporting channels.
We also are subject to certain United States and foreign laws and regulations concerning the conduct of our foreign
operations, including the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, German anti-corruption laws
and other anti-bribery laws and laws pertaining to the accuracy of our internal books and records, which have been
the focus of increasing enforcement activity globally in recent years. Our businesses are generally subject to
numerous other laws and regulations that could impact our financial results, including, without limitation,
securities, antitrust, consumer protection, and marketing laws and regulations.
In the EU, both active and passive bribery are criminalized. The EU Council Framework Decision 2003/568/JHA
of 22 July 2003
establishes more detailed rules on the liability of
legal persons and deterrent sanctions. However, the liability of legal persons is regulated at a national level.
Failure to comply with fraud and abuse laws and regulations, and other laws and regulations, could result in
significant civil and criminal penalties and costs, including the loss of licenses and the ability to participate in
federal and state health care programs, and could have a material adverse effect on our business. We may
determine to enter into settlements, make payments, agree to consent decrees or enter into other arrangements to
resolve such matters. Intentional or unintentional failure to comply with consent decrees could materially adversely
affect our business.
While we believe that we are substantially compliant with applicable fraud and abuse and other laws and
regulations, and believe we have adequate compliance programs and controls in place to ensure substantial
compliance, we cannot predict whether changes in applicable law, or interpretation of laws, or changes in our
services or marketing practices in response to changes in applicable law or interpretation of laws, could have a
material adverse effect on our business.
34
If we fail to comply with laws and regulations relating to the confidentiality of sensitive personal information or
standards in electronic health records or transmissions, we could be required to make significant changes to our
products, or incur substantial fines, penalties or other liabilities.
Our businesses that involve physician and dental practice management products, and our specialty home medical
supply business, include electronic information technology systems that store and process personal health, clinical,
financial and other sensitive information of individuals. These information technology systems may be vulnerable
to breakdown, wrongful intrusions, data breaches and malicious attack, which could require us to expend
significant resources to eliminate these problems and address related security concerns, and could involve claims
against us by private parties and/or governmental agencies.
We are directly or indirectly subject to numerous and evolving federal, state, local and foreign laws and regulations
that protect the privacy and security of personal information, such as the HIPAA, the Controlling the Assault of
Non-Solicited Pornography and Marketing Act, the Telephone Protection and Electronic Protection Act of 1991,
Section 5 of the Federal Trade Commission Act, the CCPA, and the CPRA that becomes effective on January 1,
2023. Laws and regulations relating to privacy and data protection are continually evolving and subject to
potentially differing interpretations. These requirements may not be harmonized, may be interpreted and applied in
a manner that is inconsistent from one jurisdiction to another or may conflict with other rules or our practices. Our
businesses’ failure to comply with these laws and regulations could expose us to breach of contract claims,
substantial fines, penalties and other liabilities and expenses, costs for remediation and harm to our reputation.
Also, evolving laws and regulations in this area could restrict the ability of our customers to obtain, use or
disseminate patient information, or could require us to incur significant additional costs to re-design our products to
reflect these legal requirements, which could have a material adverse effect on our operations.
In addition, the European Parliament and the Council of the European Union have adopted the GDPR, which
increases privacy rights for individuals in Europe, or “Data Subjects”, including individuals who are our customers,
suppliers and employees. The GDPR extended the scope of responsibilities for data controllers and data processors
and generally imposes increased requirements and potential penalties on companies, such as us, that offer goods or
services to Data Subjects or monitor their behavior (including by companies based outside of Europe).
Noncompliance can result in penalties of up to the greater of EUR 20 million, or 4% of global company revenues.
Data Subjects also have the right to seek compensation for damages. EU member states may individually impose
additional requirements and penalties regarding certain matters, such as employee personal data.
In the United States, the CCPA, which increases the privacy protections afforded California residents, became
effective January 1, 2020. The CCPA generally requires companies, such as us, to institute additional protections
regarding the collection, use and disclosure of certain personal information of California residents. Compliance
with the new obligations imposed by the CCPA depends in part on how particular regulators interpret and apply
them, and because the CCPA is relatively new, and its implementing regulations were released in August of 2020,
there remains some uncertainty about how the CCPA will be interpreted by the courts and enforced by the
regulators. If we fail to comply with the CCPA or if regulators assert that we have failed to comply with the CCPA,
we may be subject to certain fines or other penalties and litigation, any of which may negatively impact our
reputation, require us to expend significant resources, and harm our business. Furthermore, California voters
approved the CPRA on November 3, 2020, which will amend and expand the CCPA, including by providing
consumers with additional rights with respect to their personal information, and creating a new state agency to
enforce CCPA and CPRA. The CPRA will come into effect on January 1, 2023, applying to information collected
by businesses on or after January 1, 2022.
Other states, as well as the federal government, have increasingly considered the adoption of similarly expansive
personal privacy laws, backed by significant civil penalties for non-compliance. While we believe we have
substantially compliant programs and controls in place to comply with the GDPR, CCPA and CPRA requirements,
our compliance with these measures is likely to impose additional costs on us, and we cannot predict whether the
interpretations of the requirements, or changes in our practices in response to new requirements or interpretations of
the requirements, could have a material adverse effect on our business.
We also sell products and services that health care providers, such as physicians and dentists, use to store and
manage patient medical or dental records. These customers and we are subject to laws, regulations and industry
35
standards, such as HIPAA and the Payment Card Industry Data Security Standards, which require the protection of
the privacy and security of those records. Our products or services may be used as part of these customers’
comprehensive data security programs, including in connection with their efforts to comply with applicable data
privacy and security laws and contractual requirements. Perceived or actual security vulnerabilities in our products
or services, or the perceived or actual failure by us or our customers who use our products or services to comply
with applicable legal or contractual data privacy and security requirements, may not only cause us significant
reputational harm, but may also lead to claims against us by our customers and/or governmental agencies and
involve substantial fines, penalties and other liabilities and expenses and costs for remediation.
Under the EU GDPR, health data belong to the category of “sensitive data” and benefit from specific protections.
Processing of such data is generally prohibited, except for specific exceptions.
Certain of our businesses involve the manufacture and sale of electronic health record (“EHR”) systems and other
products linked to government supported incentive programs, where the EHR systems must be certified as having
certain capabilities designated in evolving standards, such as those adopted by CMS and by the Office of the
National Coordinator for Health Information Technology of HHS (“ONC”). In order to maintain certification of
our EHR products, we must satisfy the changing governmental standards. If any of our EHR systems do not meet
these standards, yet have been relied upon by health care providers to receive federal incentive payments, we may
be exposed to risk, such as under federal health care fraud and abuse laws, including the False Claims Act. While
we believe we are substantially in compliance with such certifications and with applicable fraud and abuse laws and
regulations and that we have adequate compliance programs and controls in place to ensure substantial compliance,
we cannot predict whether changes in applicable law, or interpretation of laws, or resulting changes in our, could
have a material adverse effect on our business.
Moreover, in order to satisfy our customers, our products may need to incorporate increasingly complex reporting
functionality. Although we believe we are positioned to accomplish this, the effort may involve increased costs,
and our failure to implement product modifications, or otherwise satisfy applicable standards, could have a material
adverse effect on our business.
Additionally, as electronic medical devices are increasingly connected to each other and to other technology, the
ability of these connected systems to safely and effectively exchange and use exchanged information becomes
increasingly important. As a medical device manufacturer, we must manage risks including those associated with
an electronic interface that is incorporated into a medical device.
Tax legislation could materially adversely affect our financial results and tax liabilities.
We are subject to the tax laws and regulations of the United States federal, state and local governments, as well as
foreign jurisdictions. From time to time, various legislative initiatives may be proposed that could materially
adversely affect our tax positions. There can be no assurance that our effective tax rate will not be materially
adversely affected by legislation resulting from these initiatives. In addition, tax laws and regulations are extremely
complex and subject to varying interpretations. Although we believe that our historical tax positions are sound and
consistent with applicable laws, regulations and existing precedent, there can be no assurance that our tax positions
will not be challenged by relevant tax authorities or that we would be successful in any such challenge.
We face inherent risk of exposure to product liability, intellectual property infringement and other claims in the
event that the use of the products we sell results in injury.
Our business involves a risk of product liability, intellectual property infringement and other claims in the ordinary
course of business, and from time to time we are named as a defendant in cases as a result of our distribution of
products. Additionally, we own interests in companies that manufacture certain dental products. As a result, we
could be subject to the potential risk of product liability, intellectual property infringement or other claims relating
to the manufacture and distribution of products by those entities. In addition, as our private-label business continues
to grow, purchasers of such products may increasingly seek recourse directly from us, rather than the ultimate
product manufacturer, for product-related claims. Another potential risk we face in the distribution of our products
is liability resulting from counterfeit or tainted products infiltrating the supply chain. In addition, some of the
products that we transport and sell are considered hazardous materials. The improper handling of such materials or
36
accidents involving the transportation of such materials could subject us to liability or at least legal action that
could harm our reputation.
GENERAL RISKS
Security risks generally associated with our information systems and our technology products and services could
materially adversely affect our business, and our results of operations could be materially adversely affected if
such products, services or systems (or third-party systems we rely on) are interrupted, damaged by unforeseen
events, are subject to cyberattacks or fail for any extended period of time.
We rely on information systems (IS) in our business to obtain, rapidly process, analyze, manage and store customer,
product, supplier and employee data to, among other things:
•
maintain and manage worldwide systems to facilitate the purchase and distribution of thousands of
inventory items from numerous distribution centers;
•
receive, process and ship orders on a timely basis;
•
manage the accurate billing and collections for thousands of customers;
•
process payments to suppliers; and
•
provide products and services that maintain certain of our customers’ electronic medical or dental
records (including protected health information of their patients).
Information security risks have generally increased in recent years, and a cyberattack that bypasses our IS security
systems (including third-party systems we rely on) causing an IS security breach may lead to a material disruption
of our IS business systems (including third-party systems we rely on) and/or the loss of business information, as
well as claims against us by affected parties and/or governmental agencies, and involve fines and penalties, costs
for remediation, and substantial defense and settlement expenses. In addition, we develop products and provide
services to our customers that are technology-based, and a cyberattack that bypasses the IS security systems of our
products or services causing a security breach and/or perceived security vulnerabilities in our products or services
could also cause significant loss of business and reputational harm, and actual or perceived vulnerabilities may lead
to claims against us by our customers and/or governmental agencies. In particular, certain of our practice
management products and services purchased by health care providers, such as physicians and dentists, are used to
store and manage patient medical or dental records. These customers are subject to laws and regulations which
require that they protect the privacy and security of those records, and our products may be used as part of these
customers’ comprehensive data security programs, including in connection with their efforts to comply with
applicable privacy and security laws. Perceived or actual security vulnerabilities in our products or services, or the
perceived or actual failure by us or our customers who use our products to comply with applicable legal
requirements, may not only cause reputational harm and loss of business, but may also lead to claims against us by
our customers and/or governmental agencies and involve damages, fines and penalties, costs for remediation, and
substantial defense and settlement expenses. In addition, a cyberattack on a third-party that we use to manage a
portion of our information systems could result in the same effects. Additionally, legislative or regulatory action
related to cybersecurity may increase our costs to develop or implement new technology products and services.
Furthermore, procedures and safeguards must continually evolve to meet new IS challenges, and enhancing
protections, and conducting investigations and remediation, may impose additional costs on us.
Finally, our business may be interrupted by shortfalls of IS systems providers engaged by our customers, such as
Internet-based services upon which our customers depend to access certain of our products.
Our global operations are subject to inherent risks that could materially adversely affect our business.
Our global operations are subject to risks that may materially adversely affect our business. The risks that our
global operations are subject to include, among other things:
•
difficulties and costs relating to staffing and managing foreign operations;
•
difficulties and delays inherent in sourcing products, establishing channels of distribution and contract
manufacturing in foreign markets;
37
•
fluctuations in the value of foreign currencies (including, without limitation, in connection with
Brexit);
•
uncertainties relating to the EU-UK Trade and Cooperation Agreement of December 2020, including
for example potential implementation problems such as border delays, as well as potential changes to
the U.K. regulatory scheme to replace EU requirements;
•
longer payment cycles of foreign customers and difficulty of collecting receivables in foreign
jurisdictions;
•
repatriation of cash from our foreign operations to the United States;
•
regulatory requirements, including without limitation, anti-bribery, anti-corruption and laws pertaining
to the accuracy of our internal books and records;
•
unexpected difficulties in importing or exporting our products and import/export tariffs, quotas,
sanctions or penalties;
•
limitations on our ability under local laws to protect our intellectual property;
•
unexpected regulatory, legal, economic and political changes in foreign markets;
•
changes in tax regulations that influence purchases of capital equipment;
•
civil disturbances, geopolitical turmoil, including terrorism, war or political or military coups; and
•
public health emergencies, including COVID-19.
Our future success is substantially dependent upon our senior management, and our revenues and profitability
depend on our relationships with capable sales personnel as well as customers, suppliers and manufacturers of
the products that we distribute.
Our future success is substantially dependent upon the efforts and abilities of members of our existing senior
management, particularly Stanley M. Bergman, Chairman and Chief Executive Officer. The loss of the services of
Mr. Bergman could have a material adverse effect on our business. We have an employment agreement with Mr.
Bergman. We do not currently have “key man” life insurance policies on any of our employees. Competition for
senior management is intense and we may not be successful in attracting and retaining key personnel. Additionally,
our future revenues and profitability depend on our ability to maintain satisfactory relationships with qualified sales
personnel as well as customers, suppliers and manufacturers. If we fail to maintain our existing relationships with
such persons or fail to acquire relationships with such key persons in the future, our business may be materially
adversely affected.
Disruptions in the financial markets may materially adversely affect the availability and cost of credit to us.
Our ability to make scheduled payments or refinance our obligations with respect to indebtedness will depend on
our operating and financial performance, which in turn is subject to prevailing economic conditions and financial,
business and other factors beyond our control. Disruptions in the financial markets may materially adversely affect
the availability and cost of credit to us.
Item 1B. Unresolved Staff Comments
We have no unresolved comments from the staff of the SEC that were issued 180 days or more preceding the end of
our 2020 fiscal year.
38
ITEM 2. Properties
We own or lease the following properties with more than 100,000 square feet:
Own or
Approximate
Lease Expiration
Property
Location
Lease
Square Footage
Date
Corporate Headquarters
Melville, NY
Lease
185,000
July 2036
Corporate Headquarters
Melville, NY
Own
105,000
N/A
Office and Distribution Center
Fiumana-Predappio, Italy
Own
183,000
N/A
Office and Distribution Center
Tours, France
Own
166,000
N/A
Office and Distribution Center
Gillingham, United Kingdom
Lease/Own
165,000
June 2033
Office and Distribution Center
Eastern Creek, New South Wales, Australia
Lease
161,000
July 2030
Office and Distribution Center
Niagara on the Lake, Canada
Lease
128,000
September 2021
Office and Distribution Center
Bastian, VA
Own
108,000
N/A
Office and Distribution Center
West Allis, WI
Lease
106,000
October 2027
Office and Distribution Center
Greer, SC
Lease
102,000
December 2028
Distribution Center
Denver, PA
Lease
624,000
December 2032
Distribution Center
Indianapolis, IN
Lease
380,000
March 2022
Distribution Center
Sparks, NV
Lease
370,000
December 2021
Distribution Center
Indianapolis, IN
Own
287,000
N/A
Distribution Center
Grapevine, TX
Lease
242,000
July 2023
Distribution Center
Gallin, Germany
Own
215,000
N/A
Distribution Center
Jacksonville, FL
Lease
212,000
February 2026
Distribution Center
Heppenheim, Germany
Lease
194,000
March 2030
The properties listed in the table above are our principal properties primarily used by our health care distribution
segment. In addition, we lease numerous other distribution, office, showroom, manufacturing and sales space in
locations including the United States, Australia, Austria, Belgium, Brazil, Canada, Chile, China, the Czech
Republic, France, Germany, Hong Kong SAR, Ireland, Israel, Italy, Japan, Liechtenstein, Luxembourg, Malaysia,
the Netherlands, New Zealand, Poland, Portugal, Singapore, South Africa, Spain, Sweden, Switzerland, Thailand,
United Arab Emirates and the United Kingdom.
We believe that our properties are in good condition, are well maintained and are suitable and adequate to carry on
our business. We have additional operating capacity at certain distribution center facilities.
ITEM 3. Legal Proceedings
For a discussion of Legal Proceedings, see
Consolidated Financial Statements included under Item 8.
ITEM 4. Mine Safety Disclosures
Not applicable.
39
PART II
ITEM 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of
Equity Securities
Our common stock is traded on the Nasdaq Global Select Market tier of the Nasdaq Stock Market, or Nasdaq,
under the symbol HSIC.
On February 8, 2021, there were approximately 235 holders of record of our common stock and the last reported
sales price was $70.78.
Purchases of Equity Securities by the Issuer
Our share repurchase program, announced on March 3, 2003, originally allowed us to repurchase up to two million
shares pre-stock splits (eight million shares post-stock splits) of our common stock, which represented
approximately 2.3% of the shares outstanding at the commencement of the program. Subsequent additional
increases totaling $3.7 billion, authorized by our Board of Directors, to the repurchase program provide for a total
of $3.8 billion of shares of our common stock to be repurchased under this program.
As of December 26, 2020, we had repurchased approximately $3.6 billion of common stock (75,563,289 shares)
under these initiatives, with $201.2 million available for future common stock share repurchases.
As a result of the COVID-19 pandemic, as previously announced, we have temporarily suspended our share
repurchase program in an effort to preserve cash and exercise caution in this uncertain period and due to certain
restrictions related to financial covenants in our credit facilities.
During the fiscal quarter ended December 26, 2020, we did not make any repurchases of our common stock. The
maximum number of shares that could be purchased under this program is determined at the end of each month
based on the closing price of our common stock at that time. The maximum number of shares that could be
repurchased as of October 31, 2020, November 28, 2020, and December 26, 2020 were 3,164,694, 3,159,724 and
3,056,528, respectively.
Dividend Policy
We have not declared any cash or stock dividends on our common stock during fiscal years 2020 or 2019. We
currently do not anticipate declaring any cash or stock dividends on our common stock in the foreseeable future.
We intend to retain earnings to finance the expansion of our business and for general corporate purposes, including
our share repurchase program. Any declaration of dividends will be at the discretion of our Board of Directors and
will depend upon the earnings, financial condition, capital requirements, level of indebtedness, contractual
restrictions with respect to payment of dividends and other factors.
Stock Performance Graph
The graph below compares the cumulative total stockholder return on $100 invested, assuming the reinvestment of
all dividends, on December 26, 2015, the last trading day before the beginning of our 2016 fiscal year, through the
end of our 2020 fiscal year with the cumulative total return on $100 invested for the same period in the Dow Jones
U.S. Health Care Index and the Nasdaq Stock Market Composite Index.
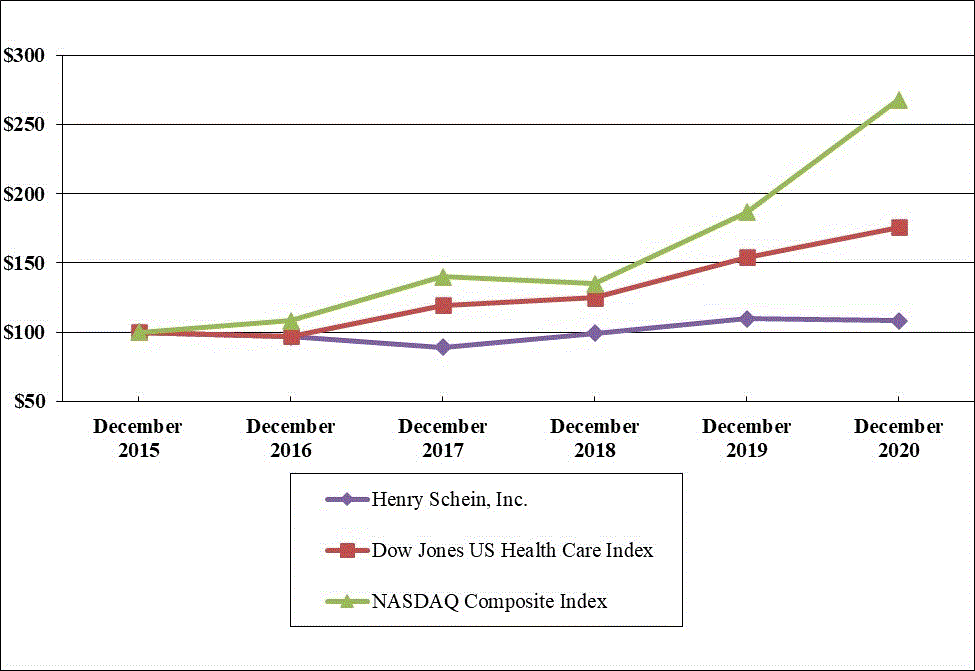
40
COMPARISON OF 5-YEAR CUMULATIVE TOTAL RETURN
ASSUMES $100 INVESTED ON DECEMBER 26, 2015
ASSUMES DIVIDENDS REINVESTED
December 26,
December 31,
December 30,
December 29,
December 28,
December 26,
2015
2016
2017
2018
2019
2020
Henry Schein, Inc.
$
100.00
$
96.58
$
88.97
$
99.20
$
109.44
$
108.21
Dow Jones U.S. Health
100.00
97.04
119.21
124.84
154.14
175.81
NASDAQ Stock Market
100.00
108.00
140.01
134.97
186.63
267.70
41
ITEM 6. Selected Financial Data
The following selected financial data, with respect to our financial position and results of operations for each of the
five fiscal years in the period ended December 26, 2020, set forth below, has been derived from, should be read in
conjunction with and is qualified in its entirety by reference to, our consolidated financial statements and notes
thereto. The selected financial data presented below should also be read in conjunction with
,
“
” and
,
“
.”
Years ended
December 26,
December 28,
December 29,
December 30,
December 31,
2020
2019
2018
2017
2016
(in thousands, except per share data)
Income Statement Data:
Net sales
$
10,119,141
$
9,985,803
$
9,417,603
$
8,883,438
$
8,218,885
Gross profit
2,814,343
3,090,886
2,910,747
2,746,662
2,605,907
Selling, general and administrative expenses
2,246,947
2,357,920
2,217,273
2,071,576
1,975,445
Litigation settlements
-
-
38,488
5,325
-
Restructuring costs (1)
32,093
14,705
54,367
-
38,621
Operating income
535,303
718,261
600,619
669,761
591,841
Other expense, net
(35,408)
(37,954)
(63,783)
(39,967)
(18,705)
Income from continuing operations before taxes, equity
499,895
680,307
536,836
629,794
573,136
Income taxes (2)
(95,374)
(159,515)
(107,432)
(308,975)
(169,311)
Equity in earnings of affiliates
12,344
17,900
21,037
15,293
17,110
Net gain (loss) on sale of equity investments (3)
1,572
186,769
-
(17,636)
-
Net income from continuing operations
418,437
725,461
450,441
318,476
420,935
Income (loss) from discontinued operations
986
(6,323)
111,685
140,817
135,460
Net income
419,423
719,138
562,126
459,293
556,395
Less: Net income attributable to noncontrolling interests
(15,629)
(24,770)
(19,724)
(25,304)
(19,651)
Less: Net (income) loss attributable to noncontrolling
-
366
(6,521)
(27,690)
(29,966)
Net income attributable to Henry Schein, Inc.
$
403,794
$
694,734
$
535,881
$
406,299
$
506,778
Amounts attributable to Henry Schein, Inc.:
Continuing operations
402,808
700,691
430,717
293,172
401,284
Discontinued operations
986
(5,957)
105,164
113,127
105,494
Net income attributable to Henry Schein, Inc.
$
403,794
$
694,734
$
535,881
$
406,299
$
506,778
Earnings (loss) per share attributable to
From continuing operations:
$
2.83
$
4.74
$
2.82
$
1.87
$
2.48
2.81
4.69
2.80
1.85
2.45
From discontinued operations:
$
0.01
$
(0.04)
$
0.69
$
0.72
$
0.65
0.01
(0.04)
0.68
0.72
0.64
Earnings per share attributable to Henry Schein, Inc.:
$
2.83
$
4.70
$
3.51
$
2.59
$
3.14
2.82
4.65
3.49
2.57
3.10
Weighted-average common shares outstanding:
142,504
147,817
152,656
156,787
161,641
143,404
149,257
153,707
158,208
163,723
42
Years ended
December 26,
December 28,
December 29,
December 30,
December 31,
2020
2019
2018
2017
2016
(in thousands)
Net Sales by Market Data:
Health care distribution (4):
$
5,912,593
$
6,415,865
$
6,347,998
$
6,047,811
$
5,554,296
3,617,017
2,973,586
2,661,166
2,497,994
2,337,661
9,529,610
9,389,451
9,009,164
8,545,805
7,891,957
Technology and value-added services (5)
514,258
515,085
408,439
337,633
326,928
Total excluding Corporate TSA revenues
10,043,868
9,904,536
9,417,603
8,883,438
8,218,885
Corporate TSA revenues (6)
75,273
81,267
-
-
-
$
10,119,141
$
9,985,803
$
9,417,603
$
8,883,438
$
8,218,885
As of
December 26,
December 28,
December 29,
December 30,
December 31,
2020
2019
2018
2017
2016
(in thousands)
Balance Sheet Data:
Total assets
$
7,772,532
$
7,151,101
$
8,500,527
$
7,863,995
$
6,811,763
Long-term debt
515,773
622,908
980,344
884,227
689,626
Redeemable noncontrolling interests
327,699
287,258
219,724
465,584
285,567
Stockholders' equity
3,984,385
3,630,137
3,541,788
2,824,410
2,800,804
1)
Restructuring costs for the year ended December 26, 2020 consist primarily of severance costs, including severance pay and benefits
of $25.8 million, facility closing costs of $5.9 million and other costs of $0.4 million. Restructuring costs for the year ended
December 28, 2019 consist primarily of severance costs, including severance pay and benefits of $13.8 million and facility closing
costs of $0.9 million. Restructuring costs for the year ended December 29, 2018 consist primarily of severance costs, including
severance pay and benefits of $50.2 million, facility closing costs of $3.2 million and other costs of $1.0 million. Restructuring costs
for the year ended December 31, 2016 consist primarily of severance costs, including severance pay and benefits of $33.8 million,
facility closing costs of $3.2 million and other costs of $1.6 million. See “Management’s Discussion and Analysis of Financial
Condition and Results of Operations – Plans of Restructuring” herein and the consolidated financial statements and related notes
contained in ITEM 8.
(2)
In 2018 we recorded (a) a $10.0 million net credit to income tax representing a change in our estimate of the transition tax on
deemed repatriated foreign earnings, (b) a one-time income tax charge of $3.9 million to income tax as a result of a reorganization of
legal entities related to Henry Schein One, (c) an income tax credit of $13.9 million ($10.6 million attributable to Henry Schein, Inc.)
resulting from a legal entity reorganization outside of the United States and (d) a one-time income tax charge of $3.1 million as a
result of the reorganization of legal entities completed in preparation for the Animal Health Spin-off. In 2017 we recorded a one-
time income tax charge of $140 million related to the transition tax on deemed repatriated foreign earnings and a one-time income
tax charge of $3.0 million for the revaluation of deferred taxes associated with U.S. tax reform legislation.
(3)
During the fourth quarter of 2019, we sold an equity investment in Hu-Friedy Mfg. Co., LLC, a manufacturer of dental instruments
and infection prevention solutions. In the fourth quarter of 2020 we received contingent proceeds of $2.1 million from the 2019 sale
of Hu-Friedy resulting in the recognition of an additional after-tax gain of $1.6 million. Our investment was non-controlling, we
were not involved in running the business and had no representation on the board of directors. During the fourth quarter of 2019, we
also sold certain other equity investments. During 2017 we sold our equity ownership in E4D Technologies resulting in a loss of
approximately $17.6 million. There was no tax benefit recognized related to this loss.
(4)
Consists of consumable products, small equipment, laboratory products, large equipment, equipment repair services, branded and
generic pharmaceuticals, vaccines, surgical products, diagnostic tests, personal protective equipment, infection-control products and
vitamins.
(5)
Consists of practice management software and other value-added products, which are distributed primarily to health care providers,
and financial services on a non-recourse basis, e-services, continuing education services for practitioners, consulting and other
services.
(6)
Corporate TSA revenues represents sales of certain products to Covetrus under the transition services agreement entered into in
connection with the Animal Health Spin-off, which ended in December 2020.
43
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Cautionary Note Regarding Forward-Looking Statements
In accordance with the “Safe Harbor” provisions of the Private Securities Litigation Reform Act of 1995, we
provide the following cautionary remarks regarding important factors that, among others, could cause future results
to differ materially from the forward-looking statements, expectations and assumptions expressed or implied
herein. All forward-looking statements made by us are subject to risks and uncertainties and are not guarantees of
future performance. These forward-looking statements involve known and unknown risks, uncertainties and other
factors that may cause our actual results, performance and achievements or industry results to be materially
different from any future results, performance or achievements expressed or implied by such forward-looking
statements. These statements are generally identified by the use of such terms as “may,” “could,” “expect,”
“intend,” “believe,” “plan,” “estimate,” “forecast,” “project,” “anticipate,” “to be,” “to make” or other comparable
terms. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in
this Annual Report on Form 10-K, and in particular the risks discussed under the caption “Risk Factors” in Item 1A
of this report and those that may be discussed in other documents we file with the Securities and Exchange
Commission (SEC). Forward looking statements include the overall impact of the Novel Coronavirus Disease 2019
(COVID-19) on the Company, its results of operations, liquidity, and financial condition (including any estimates
of the impact on these items), the rate and consistency with which dental and other practices resume or maintain
normal operations in the United States and internationally, expectations regarding personal protective equipment
(“PPE”) and COVID-19 related product sales and inventory levels and whether additional resurgences of the virus
will adversely impact the resumption of normal operations, the impact of restructuring programs as well as of any
future acquisitions, and more generally current expectations regarding performance in current and future periods.
Forward looking statements also include the (i) ability of the Company to make additional testing available, the
nature of those tests and the number of tests intended to be made available and the timing for availability, the nature
of the target market, as well as the efficacy or relative efficacy of the test results given that the test efficacy has not
been, or will not have been, independently verified under normal FDA procedures and (ii) potential for the
Company to distribute the COVID-19 vaccines and ancillary supplies.
Risk factors and uncertainties that could cause actual results to differ materially from current and historical results
include, but are not limited to: risks associated with COVID-19, as well as other disease outbreaks, epidemics,
pandemics, or similar wide spread public health concerns and other natural disasters or acts of terrorism; our
dependence on third parties for the manufacture and supply of our products; our ability to develop or acquire and
maintain and protect new products (particularly technology products) and technologies that achieve market
acceptance with acceptable margins; transitional challenges associated with acquisitions, dispositions and joint
ventures, including the failure to achieve anticipated synergies/benefits; financial and tax risks associated with
acquisitions, dispositions and joint ventures; certain provisions in our governing documents that may discourage
third-party acquisitions of us; effects of a highly competitive (including, without limitation, competition from third-
party online commerce sites) and consolidating market; the potential repeal or judicial prohibition on
implementation of the Affordable Care Act; changes in the health care industry; risks from expansion of customer
purchasing power and multi-tiered costing structures; increases in shipping costs for our products or other service
issues with our third-party shippers; general global macro-economic and political conditions, including
international trade agreements and potential trade barriers; failure to comply with existing and future regulatory
requirements; risks associated with the EU Medical Device Regulation; failure to comply with laws and regulations
relating to health care fraud or other laws and regulations; failure to comply with laws and regulations relating to
the confidentiality of sensitive personal information or standards in electronic health records or transmissions;
changes in tax legislation; litigation risks; new or unanticipated litigation developments and the status of litigation
matters; cyberattacks or other privacy or data security breaches; risks associated with our global operations; our
dependence on our senior management, as well as employee hiring and retention; and disruptions in financial
markets. The order in which these factors appear should not be construed to indicate their relative importance or
priority.
44
We caution that these factors may not be exhaustive and that many of these factors are beyond our ability to control
or predict. Accordingly, any forward-looking statements contained herein should not be relied upon as a prediction
of actual results. We undertake no duty and have no obligation to update forward-looking statements.
Where You Can Find Important Information
We may disclose important information through one or more of the following channels: SEC filings, public
conference calls and webcasts, press releases, the investor relations page of our website (www.henryschein.com)
and the social media channels identified on the Newsroom page of our website.
Recent Developments
COVID-19 Pandemic
In March 2020, the World Health Organization declared COVID-19 a pandemic. The COVID-19 pandemic has
negatively impacted the global economy, disrupted global supply chains and created significant volatility and
disruption of global financial markets. In response, many countries implemented business closures and restrictions,
stay-at-home and social distancing ordinances and similar measures to combat the pandemic, which significantly
impacted global business and dramatically reduced demand for dental products and certain medical products
beginning in the second quarter of 2020. Demand increased in the second half of 2020 resulting in slight growth
over the prior year driven by sales of PPE and COVID-19 related products.
Our consolidated financial statements reflect estimates and assumptions made by us that affect, among other things,
our goodwill, long-lived asset and definite-lived intangible asset valuation; inventory valuation; equity investment
valuation; assessment of the annual effective tax rate; valuation of deferred income taxes and income tax
contingencies; the allowance for doubtful accounts; hedging activity; vendor rebates; measurement of
compensation cost for certain share-based performance awards and cash bonus plans; and pension plan
assumptions. Due to the significant uncertainty surrounding the future impact of COVID-19, our judgments
regarding estimates and impairments could change in the future. In addition, the impact of COVID-19 had a
material adverse effect on our business, results of operations and cash flows, primarily in the second quarter of
2020. In the latter half of the second quarter, dental and medical practices began to re-open worldwide, and
continued to do so during the second half of 2020. However, patient volumes have remained below pre-COVID-19
levels and certain regions in the U.S. and internationally are experiencing an increase in COVID-19 cases. As such,
there is an ongoing risk that the COVID-19 pandemic may again materially adversely effect our business, results of
operations and cash flows and may result in a material adverse effect on our financial condition and liquidity.
However, the extent of the potential impact cannot be reasonably estimated at this time.
As part of a broad-based effort to support plans for the long-term health of our business and to strengthen our
financial flexibility, we implemented cost reduction measures that included certain reductions in payroll,
substantially decreased capital expenditures, reduced corporate spending and eliminated certain non-strategic
targeted expenditures. As our markets began to recover, we substantially ended most of those temporary expense-
reduction initiatives during the second half of 2020.
Corporate Transactions
During the fourth quarter of 2019, we sold an equity investment in Hu-Friedy Mfg. Co., LLC (“Hu-Friedy”), a
manufacturer of dental instruments and infection prevention solutions. Our investment was non-controlling, we
were not involved in running the business and had no representation on the board of directors. During the fourth
quarter of 2019, we also sold certain other equity investments. In the aggregate, the sales of these investments
resulted in a pre-tax gain in 2019 of approximately $250.2 million and an after-tax gain of approximately $186.8
million. In the fourth quarter of 2020 we received contingent proceeds of $2.1 million from the 2019 sale of Hu-
Friedy resulting in the recognition of an additional after-tax gain of $1.6 million.
On February 7, 2019 (the “Distribution Date”), we completed the separation (the “Separation”) and subsequent
merger of our animal health business (the “Henry Schein Animal Health Business”) with Direct Vet Marketing, Inc.
(d/b/a Vets First Choice, “Vets First Choice”) (the “Merger”). This was accomplished by a series of transactions
45
among us, Vets First Choice, Covetrus, Inc. (f/k/a HS Spinco, Inc. “Covetrus”), a wholly owned subsidiary of ours
prior to the Distribution Date, and HS Merger Sub, Inc., a wholly owned subsidiary of Covetrus (“Merger
Sub”). In connection with the Separation, we contributed, assigned and transferred to Covetrus certain applicable
assets, liabilities and capital stock or other ownership interests relating to the Henry Schein Animal Health
Business. On the Distribution Date, we received a tax-free distribution of $1,120 million from Covetrus pursuant to
certain debt financing incurred by Covetrus. On the Distribution Date and prior to the Animal Health Spin-off,
Covetrus issued shares of Covetrus common stock to certain institutional accredited investors (the “Share Sale
Investors”) for $361.1 million (the “Share Sale”). The proceeds of the Share Sale were paid to Covetrus and
distributed to us. Subsequent to the Share Sale, we distributed, on a pro rata basis, all of the shares of the common
stock of Covetrus held by us to our stockholders of record as of the close of business on January 17, 2019 (the
“Animal Health Spin-off”). After the Share Sale and Animal Health Spin-off, Merger Sub consummated the
Merger whereby it merged with and into Vets First Choice, with Vets First Choice surviving the Merger as a
wholly owned subsidiary of Covetrus. Immediately following the consummation of the Merger, on a fully diluted
basis, (i) approximately 63% of the shares of Covetrus common stock were (a) owned by our stockholders and the
Share Sale Investors, and (b) held by certain employees of the Henry Schein Animal Health Business (in the form
of certain equity awards), and (ii) approximately 37% of the shares of Covetrus common stock were (a) owned by
stockholders of Vets First Choice immediately prior to the Merger, and (b) held by certain employees of Vets First
Choice (in the form of certain equity awards). After the Separation and the Merger, we no longer beneficially
owned any shares of Covetrus common stock and, following the Distribution Date, will not consolidate the
financial results of Covetrus for the purpose of our financial reporting. Following the Separation and the Merger,
Covetrus was an independent, publicly traded company on the Nasdaq Global Select Market.
Executive-Level Overview
We believe we are the world’s largest provider of health care products and services primarily to office-based dental
and medical practitioners, as well as alternate sites of care. We serve more than one million customers worldwide
including dental practitioners and laboratories and physician practices, as well as government, institutional health
care clinics and other alternate care clinics. We believe that we have a strong brand identity due to our more than
88 years of experience distributing health care products.
We are headquartered in Melville, New York, employ more than 19,000 people (of which more than 9,800 are
based outside the United States) and have operations or affiliates in 31 countries and territories, including the
United States, Australia, Austria, Belgium, Brazil, Canada, Chile, China, the Czech Republic, France, Germany,
Hong Kong SAR, Ireland, Israel, Italy, Japan, Liechtenstein, Luxembourg, Malaysia, the Netherlands, New
Zealand, Poland, Portugal, Singapore, South Africa, Spain, Sweden, Switzerland, Thailand, United Arab Emirates
and the United Kingdom.
We have established strategically located distribution centers to enable us to better serve our customers and
increase our operating efficiency. This infrastructure, together with broad product and service offerings at
competitive prices, and a strong commitment to customer service, enables us to be a single source of supply for our
customers’ needs. Our infrastructure also allows us to provide convenient ordering and rapid, accurate and
complete order fulfillment.
We conduct our business through two reportable segments: (i) health care distribution and (ii) technology and
value-added services. These segments offer different products and services to the same customer base.
The health care distribution reportable segment aggregates our global dental and medical operating segments. This
segment distributes consumable products, small equipment, laboratory products, large equipment, equipment repair
services, branded and generic pharmaceuticals, vaccines, surgical products, diagnostic tests, infection-control
products and vitamins. Our global dental group serves office-based dental practitioners, dental laboratories, schools
and other institutions. Our global medical group serves office-based medical practitioners, ambulatory surgery
centers, other alternate-care settings and other institutions.
Our global technology and value-added services group provides software, technology and other value-added
services to health care practitioners. Our technology group offerings include practice management software
systems for dental and medical practitioners. Our value-added practice solutions include financial services on a
46
non-recourse basis, e-services, practice technology, network and hardware services, as well as continuing education
services for practitioners.
Industry Overview
In recent years, the health care industry has increasingly focused on cost containment. This trend has benefited
distributors capable of providing a broad array of products and services at low prices. It also has accelerated the
growth of HMOs, group practices, other managed care accounts and collective buying groups, which, in addition to
their emphasis on obtaining products at competitive prices, tend to favor distributors capable of providing
specialized management information support. We believe that the trend towards cost containment has the potential
to favorably affect demand for technology solutions, including software, which can enhance the efficiency and
facilitation of practice management.
Our operating results in recent years have been significantly affected by strategies and transactions that we
undertook to expand our business, domestically and internationally, in part to address significant changes in the
health care industry, including consolidation of health care distribution companies, health care reform, trends
toward managed care, cuts in Medicare and collective purchasing arrangements.
Our current and future results have been and could be impacted by the current economic environment and
uncertainty, particularly impacting overall demand for our products and services.
Industry Consolidation
The health care products distribution industry, as it relates to office-based health care practitioners, is fragmented
and diverse. The industry ranges from sole practitioners working out of relatively small offices to group practices
or service organizations ranging in size from a few practitioners to a large number of practitioners who have
combined or otherwise associated their practices.
Due in part to the inability of office-based health care practitioners to store and manage large quantities of supplies
in their offices, the distribution of health care supplies and small equipment to office-based health care practitioners
has been characterized by frequent, small quantity orders, and a need for rapid, reliable and substantially complete
order fulfillment. The purchasing decisions within an office-based health care practice are typically made by the
practitioner or an administrative assistant. Supplies and small equipment are generally purchased from more than
one distributor, with one generally serving as the primary supplier.
The trend of consolidation extends to our customer base. Health care practitioners are increasingly seeking to
partner, affiliate or combine with larger entities such as hospitals, health systems, group practices or physician
hospital organizations. In many cases, purchasing decisions for consolidated groups are made at a centralized or
professional staff level; however, orders are delivered to the practitioners’ offices.
We believe that consolidation within the industry will continue to result in a number of distributors, particularly
those with limited financial, operating and marketing resources, seeking to combine with larger companies that can
provide growth opportunities. This consolidation also may continue to result in distributors seeking to acquire
companies that can enhance their current product and service offerings or provide opportunities to serve a broader
customer base.
Our trend with regard to acquisitions and joint ventures has been to expand our role as a provider of products and
services to the health care industry. This trend has resulted in our expansion into service areas that complement our
existing operations and provide opportunities for us to develop synergies with, and thus strengthen, the acquired
businesses.
As industry consolidation continues, we believe that we are positioned to capitalize on this trend, as we believe we
have the ability to support increased sales through our existing infrastructure, although there can be no assurances
that we will be able to successfully accomplish this. We also have invested in expanding our sales/marketing
infrastructure to include a focus on building relationships with decision makers who do not reside in the office-
based practitioner setting.
47
As the health care industry continues to change, we continually evaluate possible candidates for merger and joint
venture or acquisition and intend to continue to seek opportunities to expand our role as a provider of products and
services to the health care industry. There can be no assurance that we will be able to successfully pursue any such
opportunity or consummate any such transaction, if pursued. If additional transactions are entered into or
consummated, we would incur merger and/or acquisition-related costs, and there can be no assurance that the
integration efforts associated with any such transaction would be successful. In response to the COVID-19
pandemic, we had taken a range of actions to preserve cash, including the temporary suspension of significant
acquisition activity. During the third and fourth quarters of 2020, as global conditions improved, we resumed our
acquisition strategy.
Aging Population and Other Market Influences
The health care products distribution industry continues to experience growth due to the aging population,
increased health care awareness, the proliferation of medical technology and testing, new pharmacology treatments
and expanded third-party insurance coverage, partially offset by the effects of unemployment on insurance
coverage. In addition, the physician market continues to benefit from the shift of procedures and diagnostic testing
from acute care settings to alternate-care sites, particularly physicians’ offices.
According to the U.S. Census Bureau’s International Data Base, in 2020 there were more than six and a half`
million Americans aged 85 years or older, the segment of the population most in need of long-term care and elder-
care services. By the year 2050, that number is projected to nearly triple to approximately 19 million. The
population aged 65 to 84 years is projected to increase by approximately 36% during the same time period.
As a result of these market dynamics, annual expenditures for health care services continue to increase in the
United States. We believe that demand for our products and services will grow, while continuing to be impacted by
current and future operating, economic and industry conditions. The Centers for Medicare and Medicaid Services,
or CMS, published “National Health Expenditure Projections 2019-2028” indicating that total national health care
spending reached approximately $3.6 trillion in 2018, or 17.7% of the nation’s gross domestic product, the
benchmark measure for annual production of goods and services in the United States. Health care spending is
projected to reach approximately $6.2 trillion in 2028, approximately 19.7% of the nation’s projected gross
domestic product.
48
Results of Operations
The following tables summarize the significant components of our operating results and cash flows from continuing
operations for each of the three years ended December 26, 2020, December 28, 2019 and December 29, 2018 (in
thousands):
Years Ended
December 26,
December 28,
December 29,
2020
2019
2018
Operating results:
Net sales
$
10,119,141
$
9,985,803
$
9,417,603
Cost of sales
7,304,798
6,894,917
6,506,856
Gross profit
2,814,343
3,090,886
2,910,747
Operating expenses:
Selling, general and administrative
2,246,947
2,357,920
2,217,273
Litigation settlements
-
-
38,488
Restructuring costs
32,093
14,705
54,367
Operating income
$
535,303
$
718,261
$
600,619
Other expense, net
$
(35,408)
$
(37,954)
$
(63,783)
Net gain on sale of equity investments
1,572
186,769
-
Net income from continuing operations
418,437
725,461
450,441
Income (loss) from discontinued operations
986
(6,323)
111,685
Net income attributable to Henry Schein, Inc.
403,794
694,734
535,881
Years Ended
December 26,
December 28,
December 29,
2020
2019
2018
Cash flows:
Net cash provided by operating activities from continuing operations
$
593,519
$
820,478
$
450,955
Net cash used in investing activities from continuing operations
(115,019)
(422,309)
(164,324)
Net cash used in financing activities from continuing operations
(181,794)
(363,351)
(402,173)
Plans of Restructuring
On July 9, 2018, we committed to an initiative to rationalize our operations and provide expense
efficiencies. These actions allowed us to execute on our plan to reduce our cost structure and fund new initiatives
to drive growth under our 2018 to 2020 strategic plan. This initiative resulted in the elimination of approximately
4% of our workforce and the closing of certain facilities.
On November 20, 2019, we committed to a contemplated initiative, intended to mitigate stranded costs associated
with the Animal Health Spin-off and to rationalize operations and to provide expense efficiencies. These activities
were originally expected to be completed by the end of 2020. As a result of the business environment brought on
by the COVID-19 pandemic, we are continuing our restructuring activities into 2021. We are currently unable in
good faith to make a determination of an estimate of the amount or range of amounts expected to be incurred in
connection with these activities in 2021, both with respect to each major type of cost associated therewith and with
respect to the total cost, or an estimate of the amount or range of amounts that will result in future cash
expenditures.
During the years ended December 26, 2020, December 28, 2019, and December 29, 2018 we recorded restructuring
charges of $32.1 million, $14.7 million and $54.4 million, respectively. The costs associated with these
restructurings are included in a separate line item, “Restructuring costs” within our consolidated statements of
income.
49
2020 Compared to 2019
Net Sales
Net sales for 2020 and 2019 were as follows (in thousands):
% of
% of
Increase / (Decrease)
2020
Total
2019
Total
$
%
Health care distribution
(1)
Dental
$
5,912,593
58.4
%
$
6,415,865
64.2
%
$
(503,272)
(7.8)
%
Medical
3,617,017
35.8
2,973,586
29.8
643,431
21.6
Total health care distribution
9,529,610
94.2
9,389,451
94.0
140,159
1.5
Technology and value-added services
(2)
514,258
5.1
515,085
5.2
(827)
(0.2)
Total excluding Corporate TSA revenues
10,043,868
99.3
9,904,536
99.2
139,332
1.4
Corporate TSA revenues
(3)
75,273
0.7
81,267
0.8
(5,994)
(7.4)
Total
$
10,119,141
100.0
$
9,985,803
100.0
$
133,338
1.3
(1)
Consists of consumable products, small equipment, laboratory products, large equipment, equipment repair services, branded and generic
pharmaceuticals, vaccines, surgical products, diagnostic tests, infection-control products, personal protective equipment and vitamins.
(2)
Consists of practice management software and other value-added products, which are distributed primarily to health care providers, and
financial services on a non-recourse basis, e-services, continuing education services for practitioners, consulting and other services.
(3)
Corporate TSA revenues represents sales of certain products to Covetrus under the transition services agreement entered into in connection
with the Animal Health Spin-off, which ended in December 2020.
The 1.3% increase in net sales for the year ended December 26, 2020 includes an increase of 1.4% local currency
growth (0.8% increase in internally generated revenue and 0.6% growth from acquisitions) partially offset by a
decrease of 0.1% related to foreign currency exchange. Excluding sales of products under the transition services
agreement with Covetrus, our net sales increased 1.4%, including local currency growth of 1.5% (0.9% increase in
internally generated revenue and 0.6% growth from acquisitions) partially offset by a decrease of 0.1% related to
foreign currency exchange. Sales for the year ended December 26, 2020 benefited from sales of PPE and COVID-
19 related products of approximately $1,298 million, an increase of approximately 208% versus the prior year.
Future PPE and COVID-19 related product sales may be lower than what we have experienced in 2020, which were
driven by rising positive COVID-19 cases and practices seeking to ensure adequate supply.
The 7.8% decrease in dental net sales for the year ended December 26, 2020 includes a decrease of 7.6% in local
currencies (8.0% decrease in internally generated revenue, partially offset by 0.4% growth from acquisitions) and a
decrease of 0.2% related to foreign currency exchange. The 7.6% decrease in local currency sales was due to
decreases in dental equipment sales and service revenues of 12.5%, all of which is attributable to a decrease in
internally generated revenue and a decrease in dental consumable merchandise sales of 6.1% (6.5% decrease in
internally generated revenue, partially offset by 0.4% growth from acquisitions). The COVID-19 pandemic
adversely impacted our dental business beginning in mid-March of 2020 as many dental offices progressively
closed or began seeing a limited number of patients, resulting in a decrease of 41.2% in second quarter dental
revenues versus the same period in the prior year. However, in the second half of the year ended December 26,
2020, our dental sales began to improve as dental practices resumed activities and patient traffic increased. Global
dental sales for the year ended December 26, 2020 benefited from sales of PPE and COVID-19 related products of
approximately $491 million, an increase of approximately 72% versus the prior year.
The 21.6% increase in medical net sales for the year ended December 26, 2020 includes an increase of 21.6% local
currency growth (20.7% increase in internally generated revenue and 0.9% growth from acquisitions). The
COVID-19 pandemic adversely impacted our medical business beginning in mid-March of 2020, but not as
significantly as our dental business as the decrease in second quarter medical revenues was only 11.2% versus the
same period in the prior year. Our medical business rebounded strongly in the second half of the year in part due to
continued strong sales of PPE, such as masks, gowns and face shields, and COVID-19 related products, such as
diagnostic test kits. Global medical sales for the year ended December 26, 2020 benefited from sales of PPE and
COVID-19 related products of approximately $807 million, an increase of approximately 490% versus the prior
year.
50
The 0.2% decrease in technology and value-added services net sales for the year ended December 26, 2020 includes
a decrease of 0.3% local currency growth (3.2% decrease in internally generated revenue, partially offset by 2.9%
growth from acquisitions) partially offset by an increase of 0.1% related to foreign currency exchange. The closure
of dental and medical offices beginning in mid-March of 2020 due to the COVID-19 pandemic resulted in a
decrease of 15.9% in second quarter technology and value-added services revenues versus the same period in the
prior year. As dental and medical practice operations, resumed in the second half of the year, the trend for
transactional software revenues improved as more patients visited practices worldwide.
Although dental and medical practices continued to re-open globally in the second half of the year, patient volumes
remain below pre-COVID-19 levels. As such, there is an ongoing risk that the COVID-19 pandemic may again
have a material adverse effect on our net sales in future periods.
Gross Profit
Gross profit and gross margins for 2020 and 2019 by segment and in total were as follows (in thousands):
Gross
Gross
Decrease
2020
Margin %
2019
Margin %
$
%
Health care distribution
$
2,448,991
25.7
%
$
2,717,574
28.9
%
$
(268,583)
(9.9)
%
Technology and value-added services
363,245
70.6
370,887
72.0
(7,642)
(2.1)
Total excluding Corporate TSA revenues
2,812,236
28.0
3,088,461
31.2
(276,225)
(8.9)
Corporate TSA revenues
2,107
2.8
2,425
3.0
(318)
(13.1)
Total
$
2,814,343
27.8
$
3,090,886
31.0
$
(276,543)
(8.9)
As a result of different practices of categorizing costs associated with distribution networks throughout our
industry, our gross margins may not necessarily be comparable to other distribution companies. Additionally, we
realize substantially higher gross margin percentages in our technology segment than in our health care distribution
segment. These higher gross margins result from being both the developer and seller of software products and
services, as well as certain financial services. The software industry typically realizes higher gross margins to
recover investments in research and development.
In connection with the completion of the Animal Health Spin-off (see
additional details), we entered into a transition services agreement with Covetrus, pursuant to which Covetrus
purchased certain products from us. The agreement, which ended in December 2020, provided that these products
would be sold to Covetrus at a mark-up that ranged from 3% to 6% of our product cost to cover handling costs.
Within our health care distribution segment, gross profit margins may vary from one period to the next. Changes in
the mix of products sold as well as changes in our customer mix have been the most significant drivers affecting
our gross profit margin. For example, sales of pharmaceutical products are generally at lower gross profit margins
than other products. Conversely, sales of our private label products achieve gross profit margins that are higher
than average. With respect to customer mix, sales to our large-group customers are typically completed at lower
gross margins due to the higher volumes sold as opposed to the gross margin on sales to office-based practitioners,
who normally purchase lower volumes at greater frequencies.
Health care distribution gross profit decreased $268.6 million, or 9.9%, for the year ended December 26, 2020
compared to the prior year period, due primarily to the COVID-19 pandemic. Health care distribution gross profit
margin decreased to 25.7% for the year ended December 26, 2020 from 28.9% for the comparable prior year
period. The overall decrease in our health care distribution gross profit is attributable to a $232.2 million decline in
gross profit due to the decrease in the gross margin rates and a $48.3 million gross profit decrease in internally
generated revenue, partially offset by $11.9 million of additional gross profit from acquisitions. Gross profit
margin was negatively affected by significant adjustments recorded for PPE inventory and COVID-19 related
products caused by volatility of pricing and demand experienced during the year, which conditions may recur and
adversely impact gross profit margins in future periods, although we do not expect material inventory adjustments
to continue into 2021. During the year, we continued to earn lower vendor rebates, due to lower purchase volumes,
in our health care distribution segment, which also contributes to the lower gross profit margin.
51
Technology and value-added services gross profit decreased $7.6 million, or 2.1%, for the year ended December
26, 2020 compared to the prior year period. Technology and value-added services gross profit margin decreased to
70.6% for the year ended December 26, 2020 from 72.0% for the comparable prior year period. The overall
decrease in our Technology and value-added services gross profit is attributable to a decrease of $11.5 million in
internally generated revenue and a decrease of $8.8 million in gross profit due to the decrease in the gross margin
rates, partially offset by $12.7 million additional gross profit from acquisitions.
Selling, General and Administrative
Selling, general and administrative expenses by segment and in total for 2020 and 2019 were as follows (in
thousands):
% of
% of
Respective
Respective
Increase / (Decrease)
2020
Net Sales
2019
Net Sales
$
%
Health care distribution
$
2,014,925
21.1
%
$
2,128,595
22.7
%
$
(113,670)
(5.3)
%
Technology and value-added services
264,115
51.4
244,030
47.4
20,085
8.2
Total
$
2,279,040
22.5
$
2,372,625
23.8
$
(93,585)
(3.9)
Selling, general and administrative expenses (including restructuring costs in the years ended December 26, 2020
and December 28, 2019) decreased $93.6 million, or 3.9%, to $2,279.0 million for the year ended December 26,
2020 from the comparable prior year period. The $113.7 million decrease in selling, general and administrative
expenses within our health care distribution segment for the year ended December 26, 2020 as compared to the
prior year period was attributable to a reduction of $151.5 million of operating costs, primarily as a result of cost-
saving measures taken in response to the COVID-19 pandemic, partially offset by $20.8 million of additional costs
from acquired companies and an increase of $17.0 million in restructuring costs. The $20.1 million increase in
selling, general and administrative expenses within our technology and value-added services segment for the year
ended December 26, 2020 as compared to the prior year period was attributable to $10.5 million of additional costs
from acquired companies and an increase of $9.6 million of operating costs. As a percentage of net sales, selling,
general and administrative expenses decreased to 22.5% from 23.8% for the comparable prior year period. The cost
savings achieved from measures taken in response to the COVID-19 pandemic are expected to diminish in future
periods as most of these measures were temporary and substantially ended during the second half of 2020.
As a component of total selling, general and administrative expenses, selling expenses decreased $86.4 million, or
5.9%, to $1,375.2 million for the year ended December 26, 2020 from the comparable prior year period, primarily
as a result of cost-saving measures taken in response to the COVID-19 pandemic. As a percentage of net sales,
selling expenses decreased to 13.6% from 14.7% for the comparable prior year period.
As a component of total selling, general and administrative expenses, general and administrative expenses
decreased $7.2 million, or 0.8%, to $903.8 million for the year ended December 26, 2020 from the comparable
prior year period. As a percentage of net sales, general and administrative expenses decreased to 8.9% from 9.1%
for the comparable prior year period.
Other Expense, Net
Other expense, net for the years ended 2020 and 2019 was as follows (in thousands):
Variance
2020
2019
$
%
Interest income
$
9,842
$
15,757
$
(5,915)
(37.5)
%
Interest expense
(41,377)
(50,792)
9,415
18.5
Other, net
(3,873)
(2,919)
(954)
(32.7)
Other expense, net
$
(35,408)
$
(37,954)
$
2,546
6.7
Interest income decreased $5.9 million primarily due to lower interest rates and reduced late fee income. Interest
expense decreased $9.4 million primarily due to lower interest rates and lower average debt balances for the year
ended December 26, 2020 as compared to the prior year.
52
Income Taxes
For the year ended December 26, 2020, our effective tax rate was 19.1% compared to 23.4% for the prior year
period. In 2020, our effective tax rate was primarily impacted by the agreement with the U.S Internal Revenue
Service on our Advanced Pricing Agreement (APA), other audit resolutions, and state and foreign income taxes and
interest expense. In 2019, our effective tax rate was primarily impacted by state and foreign income taxes and
interest expense.
Net Gain on Sale of Equity Investments
In the fourth quarter of 2020 we received contingent proceeds of $2.1 million from the 2019 sale of Hu-Friedy
resulting in the recognition of an additional after-tax gain of $1.6 million.
53
2019 Compared to 2018
Net Sales
Net sales for 2019 and 2018 were as follows (in thousands):
% of
% of
Increase
2019
Total
2018
Total
$
%
Health care distribution
(1)
Dental
$
6,415,865
64.2
%
$
6,347,998
67.4
%
$
67,867
1.1
%
Medical
2,973,586
29.8
2,661,166
28.3
312,420
11.7
Total health care distribution
9,389,451
94.0
9,009,164
95.7
380,287
4.2
Technology and value-added services
(2)
515,085
5.2
408,439
4.3
106,646
26.1
Total excluding Corporate TSA revenues
9,904,536
99.2
9,417,603
100.0
486,933
5.2
Corporate TSA revenues
(3)
81,267
0.8
-
-
81,267
-
Total
$
9,985,803
100.0
$
9,417,603
100.0
$
568,200
6.0
(1)
Consists of consumable products, small equipment, laboratory products, large equipment, equipment repair services, branded and generic
pharmaceuticals, vaccines, surgical products, diagnostic tests, infection-control products and vitamins.
(2)
Consists of practice management software and other value-added products, which are distributed primarily to health care providers, and
financial services on a non-recourse basis, e-services, continuing education services for practitioners, consulting and other services.
(3)
Corporate TSA revenues represents sales of certain products to Covetrus under the transition services agreement entered into in
connection with the Animal Health Spin-off, which ended in December 2020.
The 6.0% increase in net sales for the year ended December 28, 2019 includes an increase of 7.7% local currency
growth (4.4% increase in internally generated revenue and 3.3% growth from acquisitions) partially offset by a
decrease of 1.7% related to foreign currency exchange. Excluding sales of products under the transition services
agreement with Covetrus, our net sales increased 5.2%, including local currency growth of 6.9% (3.5% increase in
internally generated revenue and 3.4% growth from acquisitions) partially offset by a decrease of 1.7% related to
foreign currency exchange.
The 1.1% increase in dental net sales for the year ended December 28, 2019 includes an increase of 3.4% in local
currencies (2.0% increase in internally generated revenue and 1.4% growth from acquisitions) partially offset by a
decrease of 2.3% related to foreign currency exchange. The 3.4% increase in local currency sales was due to
increases in dental equipment sales and service revenues of 1.0%, all of which is attributable to an increase in
internally generated revenue and dental consumable merchandise sales growth of 4.2% (2.3% increase in internally
generated revenue and 1.9% growth from acquisitions).
The 11.7% increase in medical net sales for the year ended December 28, 2019 includes an increase of 11.9% local
currency growth (7.0% increase in internally generated revenue and 4.9% growth from acquisitions) partially offset
by a decrease of 0.2% related to foreign currency exchange.
The 26.1% increase in technology and value-added services net sales for the year ended December 28, 2019
includes an increase of 27.0% local currency growth (4.3% increase in internally generated revenue and 22.7%
growth from acquisitions) partially offset by a decrease of 0.9% related to foreign currency exchange.
54
Gross Profit
Gross profit and gross margins for 2019 and 2018 by segment and in total were as follows (in thousands):
Gross
Gross
Increase
2019
Margin %
2018
Margin %
$
%
Health care distribution
$
2,717,574
28.9
%
$
2,628,767
29.2
%
$
88,807
3.4
%
Technology and value-added services
370,887
72.0
281,980
69.0
88,907
31.5
Total excluding Corporate TSA revenues
3,088,461
31.2
2,910,747
30.9
177,714
6.1
Corporate TSA revenues
2,425
3.0
-
-
2,425
-
Total
$
3,090,886
31.0
$
2,910,747
30.9
$
180,139
6.2
As a result of different practices of categorizing costs associated with distribution networks throughout our
industry, our gross margins may not necessarily be comparable to other distribution companies. Additionally, we
realize substantially higher gross margin percentages in our technology segment than in our health care distribution
segment. These higher gross margins result from being both the developer and seller of software products and
services, as well as certain financial services. The software industry typically realizes higher gross margins to
recover investments in research and development.
In connection with the completion of the Animal Health Spin-off (see
additional details), we entered into a transition services agreement with Covetrus, pursuant to which Covetrus
purchased certain products from us. The agreement, which ended in December 2020, provided that these products
would be sold to Covetrus at a mark-up that ranged from 3% to 6% of our product cost to cover handling costs.
Within our health care distribution segment, gross profit margins may vary from one period to the next. Changes in
the mix of products sold as well as changes in our customer mix have been the most significant drivers affecting
our gross profit margin. For example, sales of pharmaceutical products are generally at lower gross profit margins
than other products. Conversely, sales of our private label products achieve gross profit margins that are higher
than average. With respect to customer mix, sales to our large-group customers are typically completed at lower
gross margins due to the higher volumes sold as opposed to the gross margin on sales to office-based practitioners,
who normally purchase lower volumes at greater frequencies.
Health care distribution gross profit increased $88.8 million, or 3.4%, for the year ended December 28, 2019
compared to the prior year period. Health care distribution gross profit margin decreased to 28.9% for the year
ended December 28, 2019 from 29.2% for the comparable prior year period. The overall increase in our health care
distribution gross profit is attributable to $73.1 million of additional gross profit from acquisitions and $30.9
million gross profit increase from growth in internally generated revenue. These increases were partially offset by
a $15.2 million decline in gross profit due to the decrease in the gross margin rates.
Technology and value-added services gross profit increased $88.9 million, or 31.5%, for the year ended December
28, 2019 compared to the prior year period. Technology and value-added services gross profit margin increased to
72.0% for the year ended December 28, 2019 from 69.0% for the comparable prior year period. Acquisitions
accounted for $80.2 million of our gross profit increase within our technology and value-added services segment
for the year ended December 28, 2019 compared to the prior year period and also accounted for the increase in the
gross profit margin. The remaining increase of $8.7 million in our technology and value-added services segment
gross profit was primarily attributable to growth in internally generated revenue.
55
Selling, General and Administrative
Selling, general and administrative expenses by segment and in total for 2019 and 2018 were as follows (in
thousands):
% of
% of
Respective
Respective
Increase / (Decrease)
2019
Net Sales
2018
Net Sales
$
%
Health care distribution
$
2,128,595
22.7
%
$
2,137,779
23.7
%
$
(9,184)
(0.4)
%
Technology and value-added services
244,030
47.4
172,349
42.2
71,681
41.6
Total
$
2,372,625
23.8
$
2,310,128
24.5
$
62,497
2.7
Selling, general and administrative expenses (including restructuring costs in the years ended December 28, 2019
and December 29, 2018, and litigation settlements in the year ended December 29, 2018) increased $62.5 million,
or 2.7%, to $2,372.6 million for the year ended December 28, 2019 from the comparable prior year period. The
$9.2 million decrease in selling, general and administrative expenses within our health care distribution segment for
the year ended December 28, 2019 as compared to the prior year period was attributable to a reduction of $73.7
million of operating costs (primarily due to $38.5 million of litigation settlement costs recorded in 2018 and a $39.7
million decrease in restructuring costs) partially offset by $64.5 million of additional costs from acquired
companies. The $71.7 million increase in selling, general and administrative expenses within our technology and
value-added services segment for the year ended December 28, 2019 as compared to the prior year period was
attributable to $70.5 million of additional costs from acquired companies and $1.2 million of additional operating
costs. As a percentage of net sales, selling, general and administrative expenses decreased to 23.8% from 24.5%
for the comparable prior year period.
As a component of total selling, general and administrative expenses, selling expenses increased $33.5 million, or
2.3%, to $1,461.6 million for the year ended December 28, 2019 from the comparable prior year period. As a
percentage of net sales, selling expenses decreased to 14.7% from 15.1% for the comparable prior year period.
As a component of total selling, general and administrative expenses, general and administrative expenses
decreased $29.0 million, or 3.3%, to $911.0 million for the year ended December 28, 2019 from the comparable
prior year period primarily due to $38.5 million of litigation settlement costs recorded in 2018 and a $39.7 million
decrease in restructuring costs partially offset by increases in general and administrative expenses. As a percentage
of net sales, general and administrative expenses decreased to 9.1% from 9.4% for the comparable prior year
period.
Other Expense, Net
Other expense, net for the years ended 2019 and 2018 was as follows (in thousands):
Variance
2019
2018
$
%
Interest income
$
15,757
$
15,491
$
266
1.7
%
Interest expense
(50,792)
(76,016)
25,224
33.2
Other, net
(2,919)
(3,258)
339
10.4
Other expense, net
$
(37,954)
$
(63,783)
$
25,829
40.5
Interest expense decreased $25.2 million primarily due to decreased borrowings under our bank credit lines.
56
Income Taxes
For the year ended December 28, 2019, our effective tax rate was 23.4% compared to 20.0% for the prior year
period. In 2019, our effective tax rate was primarily impacted by state and foreign income taxes and interest
expense. In 2018, our effective tax rate was primarily impacted by a reduction in the estimate of our transition tax
associated with the Tax Cuts and Jobs Act, tax charges and credits associated with legal entity reorganizations
outside the U.S., and state and foreign income taxes and interest expense.
Within our consolidated balance sheets, transition tax of $9.9 million was included in “Accrued taxes” for 2019 and
2018, and $94.9 million and $104.2 million were included in “Other liabilities” for 2019 and 2018 respectively.
Net Gain on Sale of Equity Investments
On October 1, 2019, we sold an equity investment in Hu-Friedy, a manufacturer of dental instruments and infection
prevention solutions. Our investment was non-controlling, we were not involved in running the business and had
no representation on the board of directors.
During the fourth quarter of 2019, we also sold certain other investments. In the aggregate, the sales of these
investments resulted in a pre-tax gain of approximately $250.2 million and an after-tax gain of approximately
$186.8 million.
Liquidity and Capital Resources
Our principal capital requirements have included funding of acquisitions, purchases of additional noncontrolling
interests, repayments of debt principal, the funding of working capital needs, purchases of fixed assets and
repurchases of common stock (which have been temporarily suspended). Working capital requirements generally
result from increased sales, special inventory forward buy-in opportunities and payment terms for receivables and
payables. Historically, sales have tended to be stronger during the third and fourth quarters and special inventory
forward buy-in opportunities have been most prevalent just before the end of the year, and have caused our working
capital requirements to be higher from the end of the third quarter to the end of the first quarter of the following
year.
The pandemic and the governmental responses to it had a material adverse effect on our cash flows in the second
quarter of 2020. In the latter half of the second quarter and continuing through year-end, dental and medical
practices began to re-open worldwide. However, patient volumes remain below pre-COVID-19 levels and certain
regions in the U.S. and internationally are experiencing an increase in COVID-19 cases. As such, there is an
ongoing risk that the COVID-19 pandemic may again have a material adverse effect on our cash flows in future
periods and may result in a material adverse effect on our financial condition and liquidity. However, the extent of
the potential impact cannot be reasonably estimated at this time.
As part of a broad-based effort to support plans for the long-term health of our business and to strengthen our
financial flexibility, we implemented cost reduction measures that included certain reductions in payroll,
substantially decreased capital expenditures, reduced corporate spending and the elimination of certain non-
strategic targeted expenditures. As our markets have begun to recover, we ended most of those temporary expense-
reduction initiatives during the second half of 2020. As the COVID-19 pandemic continues to unfold, we will
continue to evaluate appropriate actions for the business.
We finance our business primarily through cash generated from our operations, revolving credit facilities and debt
placements. Our ability to generate sufficient cash flows from operations is dependent on the continued demand of
our customers for our products and services, and access to products and services from our suppliers.
Our business requires a substantial investment in working capital, which is susceptible to fluctuations during the
year as a result of inventory purchase patterns and seasonal demands. Inventory purchase activity is a function of
sales activity, special inventory forward buy-in opportunities and our desired level of inventory. We anticipate
future increases in our working capital requirements.
57
We finance our business to provide adequate funding for at least 12 months. Funding requirements are based on
forecasted profitability and working capital needs, which, on occasion, may change. Consequently, we may change
our funding structure to reflect any new requirements.
We believe that our cash and cash equivalents, our ability to access private debt markets and public equity markets,
and our available funds under existing credit facilities provide us with sufficient liquidity to meet our currently
foreseeable short-term and long-term capital needs. We have no off-balance sheet arrangements.
On February 7, 2019, we completed the Animal Health Spin-off. On the Distribution Date we received a tax free
distribution of $1,120 million from Covetrus, which has been used to pay down our debt, thereby generating
additional debt capacity that can be used for general corporate purposes, including share repurchases and mergers
and acquisitions.
Net cash provided by operating activities was $593.5 million for the year ended December 26, 2020, compared to
$820.5 million for the prior year. The net change of $227.0 million was primarily attributable to lower net income
and lower distributions from equity affiliates, both resulting from the sale of our equity investment in Hu-Friedy in
the fourth quarter of 2019, and increased working capital requirements, specifically an increase in inventories due
to stocking of PPE and COVID-19 related products, and an increase in accounts receivable due to higher sales
volume. These working capital increases were partially offset by greater growth in accounts payable and accrued
expenses.
Net cash used in investing activities was $115.0 million for the year ended December 26, 2020, compared to $422.3
million for the prior year. The net change of $307.3 million was primarily due to decreased payments for equity
investments and business acquisitions, partially offset by decreased proceeds from sales of equity investments.
Net cash used in financing activities was $181.8 million for the year ended December 26, 2020, compared to
$363.4 million for the prior year. The net change of $181.6 million was primarily
due to increased net proceeds
from bank borrowings and lower repurchases of our common stock, partially offset by proceeds received during the
prior year related to the Animal Health Spin-off.
The following table summarizes selected measures of liquidity and capital resources (in thousands):
December 26,
December 28,
2020
2019
Cash and cash equivalents
$
421,185
$
106,097
Working capital
(1)
1,508,313
1,188,133
Debt:
Bank credit lines
$
73,366
$
23,975
Current maturities of long-term debt
109,836
109,849
Long-term debt
515,773
622,908
Total debt
$
698,975
$
756,732
Leases:
Current operating lease liabilities
$
64,716
$
65,349
Non-current operating lease liabilities
238,727
176,267
(1)
Includes $0.0 million and $127.0 million of accounts receivable which serve as security for U.S. trade accounts receivable
securitization at December 26, 2020 and December 28, 2019, respectively.
Our cash and cash equivalents consist of bank balances and investments in money market funds representing
overnight investments with a high degree of liquidity.
Accounts receivable days sales outstanding and inventory turnover
Our accounts receivable days sales outstanding from operations increased to 46.0 days as of December 26, 2020
from 44.5 days as of December 28, 2019. During the years ended December 26, 2020 and December 28, 2019, we
wrote off approximately $7.8 million and $5.9 million, respectively, of fully reserved accounts receivable against
58
our trade receivable reserve. Our inventory turnover from operations was 5.1 as of December 26, 2020 and 5.0 as
of December 28, 2019. Our working capital accounts may be impacted by current and future economic conditions.
Contractual obligations
The following table summarizes our contractual obligations related to fixed and variable rate long-term debt and
finance lease obligations, including interest (assuming a weighted average interest rate of 3.3%), as well as
inventory purchase commitments and operating lease obligations as of December 26, 2020:
Payments due by period (in thousands)
< 1 year
2 - 3 years
4 - 5 years
> 5 years
Total
Contractual obligations:
Long-term debt, including interest
$
125,797
$
43,994
$
126,464
$
435,219
$
731,474
Inventory purchase commitments
208,200
110,800
-
-
319,000
Operating lease obligations
71,801
98,719
55,046
110,228
335,794
Transition tax obligations
9,895
43,291
30,923
-
84,109
Finance lease obligations, including interest
2,503
2,138
632
920
6,193
Total
$
418,196
$
298,942
$
213,065
$
546,367
$
1,476,570
Bank Credit Lines
Bank credit lines consisted of the following:
December 26,
December 28,
2020
2019
Revolving credit agreement
$
-
$
-
Other short-term bank credit lines
73,366
23,975
Total
$
73,366
$
23,975
Revolving Credit Agreement
On April 18, 2017, we entered into a $750 million revolving credit agreement (the “Credit Agreement”), which
matures in April 2022. The interest rate is based on the USD LIBOR plus a spread based on our leverage ratio at
the end of each financial reporting quarter. We expect the LIBOR rate to be discontinued at some point
during 2021, which will require an amendment to our debt agreements to reflect a new reference rate. We do not
expect the discontinuation of LIBOR as a reference rate in our debt agreements to have a material adverse effect on
our financial position or to materially affect our interest expense. The Credit Agreement also requires, among other
things, that we maintain maximum leverage ratios. Additionally, the Credit Agreement contains customary
representations, warranties and affirmative covenants as well as customary negative covenants, subject to
negotiated exceptions on liens, indebtedness, significant corporate changes (including mergers), dispositions and
certain restrictive agreements. As of December 26, 2020 and December 28, 2019, we had no borrowings on this
revolving credit facility. As of December 26, 2020 and December 28, 2019, there were $9.5 million and $9.6
million of letters of credit, respectively, provided to third parties under the credit facility.
On April 17, 2020, we amended the Credit Agreement to, among other things, (i) modify the financial covenant
from being based on total leverage ratio to net leverage ratio, (ii) adjust the pricing grid to reflect the net leverage
ratio calculation, and (iii) increase the maximum maintenance leverage ratio through March 31, 2021.
59
364-Day Credit Agreement
On April 17, 2020, we entered into a new $700 million 364-day credit agreement, with JPMorgan Chase Bank,
N.A. and U.S. Bank National Association as joint lead arrangers and joint bookrunners. This facility matures on
April 16, 2021. As of December 26, 2020, we had no borrowings under this credit facility. We have the ability to
borrow up to an additional $200 million, from the original facility amount of $700 million, under this credit facility
on a revolving basis as needed, subject to the terms and conditions of the credit agreement. The interest rate for
borrowings under this facility will fluctuate based on our net leverage ratio. At December 26, 2020, the interest
rate on this facility was 2.50%. The proceeds from this facility can be used for working capital requirements and
general corporate purposes, including, but not limited to, permitted refinancing of existing indebtedness. Under the
terms of this agreement, we are prohibited from repurchasing our common stock until we report our financial
results for the second quarter of 2021.
Other Short-Term Credit Lines
As of December 26, 2020 and December 28, 2019, we had various other short-term bank credit lines available, of
which $73.4 million and $24.0 million, respectively, were outstanding. At December 26, 2020 and December 28,
2019, borrowings under all of these credit lines had a weighted average interest rate of 4.14% and 3.45%,
respectively.
Long-term debt
Long-term debt consisted of the following:
December 26,
December 28,
2020
2019
Private placement facilities
$
613,498
$
621,274
U.S. trade accounts receivable securitization
-
100,000
Note payable due in 2025 with an interest rate of 3.1%
at December 26, 2020
1,554
-
Various collateralized and uncollateralized loans payable with interest,
in varying installments through 2023 at interest rates
ranging from 2.62% to 4.27% at December 26, 2020 and
ranging from 2.56% to 10.5% at December 28, 2019
4,596
6,089
Finance lease obligations (see Note 7)
5,961
5,394
Total
625,609
732,757
Less current maturities
(109,836)
(109,849)
Total long-term debt
$
515,773
$
622,908
Private Placement Facilities
Our private placement facilities, with three insurance companies, have a total facility amount of $1 billion, and are
available on an uncommitted basis at fixed rate economic terms to be agreed upon at the time of issuance, from
time to time through June 23, 2023. The facilities allow us to issue senior promissory notes to the lenders at a fixed
rate based on an agreed upon spread over applicable treasury notes at the time of issuance. The term of each
possible issuance will be selected by us and can range from five to 15 years (with an average life no longer than 12
years). The proceeds of any issuances under the facilities will be used for general corporate purposes, including
working capital and capital expenditures, to refinance existing indebtedness and/or to fund potential acquisitions.
On June 29, 2018, we amended and restated the above private placement facilities to, among other things, (i) permit
the consummation of the Animal Health Spin-off and (ii) provide for the issuance of notes in Euros, British Pounds
and Australian Dollars, in addition to U.S. Dollars. The agreements provide, among other things, that we maintain
certain maximum leverage ratios, and contain restrictions relating to subsidiary indebtedness, liens, affiliate
transactions, disposal of assets and certain changes in ownership. These facilities contain make-whole provisions in
the event that we pay off the facilities prior to the applicable due dates.
60
On June 23, 2020, we amended the private placement facilities to, among other things, (i) temporarily modify the
financial covenant from being based on total leverage ratio to net leverage ratio until March 31, 2021, (ii) increase
the maximum maintenance leverage ratio through March 31, 2021, but with a 1.00% interest rate increase on the
outstanding notes if the net leverage ratio exceeds 3.0x, which will remain in effect until we deliver financials for a
four-quarter period ending on or after June 30, 2021 showing compliance with the total leverage ratio requirement,
and (iii) make certain other changes conforming to the Credit Agreement, dated as of April 18, 2017, as amended.
The components of our private placement facility borrowings as of December 26, 2020 are presented in the
following table (in thousands):
Amount of
Date of
Borrowing
Borrowing
Borrowing
Outstanding
Rate
Due Date
January 20, 2012
$
14,286
3.09
%
January 20, 2022
January 20, 2012
50,000
3.45
January 20, 2024
December 24, 2012
50,000
3.00
December 24, 2024
June 2, 2014
100,000
3.19
June 2, 2021
June 16, 2017
100,000
3.42
June 16, 2027
September 15, 2017
100,000
3.52
September 15, 2029
January 2, 2018
100,000
3.32
January 2, 2028
September 2, 2020
100,000
2.35
September 2, 2030
Less: Deferred debt issuance costs
(788)
$
613,498
(1) Annual repayments of approximately $7.1 million for this borrowing commenced on January 20, 2016.
(2) On September 2, 2020, we refinanced our $100 million private placement borrowing at 3.79%, originally due on September 2, 2020,
with a similar 10-year borrowing at 2.35% maturing on September 2, 2030.
U.S. Trade Accounts Receivable Securitization
We have a facility agreement with a bank, as agent, based on the securitization of our U.S. trade accounts
receivable that is structured as an asset-backed securitization program with pricing committed for up to three years.
Our current facility, which has a purchase limit of $350 million, was scheduled to expire on April 29, 2022. On
June 22, 2020, the expiration date for this facility was extended to June 12, 2023 and was amended to adjust certain
covenant levels for 2020. As of December 26, 2020 and December 28, 2019, the borrowings outstanding under this
securitization facility were $0.0 million and $100 million, respectively. At December 26, 2020, the interest rate on
borrowings under this facility was based on the asset-backed commercial paper rate of 0.22% plus 0.95%, for a
combined rate of 1.17%. At December 28, 2019, the interest rate on borrowings under this facility was based on
the asset-backed commercial paper rate of 1.90% plus 0.75%, for a combined rate of 2.65%.
If our accounts receivable collection pattern changes due to customers either paying late or not making payments,
our ability to borrow under this facility may be reduced.
We are required to pay a commitment fee of 25 to 45 basis points depending upon program utilization.
61
Leases
We have operating and finance leases for corporate offices, office space, distribution and other facilities, vehicles
and certain equipment. Our leases have remaining terms of less than one year to 16 years, some of which may
include options to extend the leases for up to 10 years. As of December 26, 2020, our right-of-use assets related to
operating leases were $288.8 million and our current and non-current operating lease liabilities were $64.7 million
and $238.7 million, respectively.
Stock Repurchases
From June 21, 2004 through December 26, 2020, we repurchased $3.6 billion, or 75,563,289 shares, under our
common stock repurchase programs, with $201.2 million available as of December 26, 2020 for future common
stock share repurchases.
On October 30, 2019, our Board of Directors authorized the repurchase of up to an additional $400 million in
shares of our common stock.
As a result of the COVID-19 pandemic, as previously announced, we have temporarily suspended our share
repurchase program in an effort to preserve cash and exercise caution during this uncertain period and due to
certain restrictions related to financial covenants in our credit facilities.
Redeemable Noncontrolling interests
Some minority stockholders in certain of our subsidiaries have the right, at certain times, to require us to acquire
their ownership interest in those entities at fair value. Account Standards Codification (“ASC”) 480-10 is
applicable for noncontrolling interests where we are or may be required to purchase all or a portion of the
outstanding interest in a consolidated subsidiary from the noncontrolling interest holder under the terms of a put
option contained in contractual agreements. The components of the change in the Redeemable noncontrolling
interests for the years ended December 26, 2020, December 28, 2019 and December 29, 2018 are presented in the
following table:
December 26,
December 28,
December 29,
2020
2019
2018
Balance, beginning of period
$
287,258
$
219,724
$
465,585
Decrease in redeemable noncontrolling interests due to
redemptions
(17,241)
(2,270)
(287,767)
Increase in redeemable noncontrolling interests due to
business acquisitions
28,387
74,865
4,655
Net income attributable to redeemable noncontrolling interests
13,363
14,838
15,327
Dividends declared
(12,631)
(10,264)
(8,206)
Effect of foreign currency translation loss attributable to
redeemable noncontrolling interests
(4,279)
(2,335)
(11,330)
Change in fair value of redeemable securities
32,842
(7,300)
41,460
Balance, end of period
$
327,699
$
287,258
$
219,724
Changes in the estimated redemption amounts of the noncontrolling interests subject to put options are adjusted at
each reporting period with a corresponding adjustment to Additional paid-in capital. Future reductions in the
carrying amounts are subject to a floor amount that is equal to the fair value of the redeemable noncontrolling
interests at the time they were originally recorded. The recorded value of the redeemable noncontrolling interests
cannot go below the floor level. These adjustments do not impact the calculation of earnings per share.
Additionally, some prior owners of such acquired subsidiaries are eligible to receive additional purchase price cash
consideration if certain financial targets are met. Any adjustments to these accrual amounts are recorded in our
consolidated statement of income. For the years ended December 26, 2020 and December 28, 2019, there were no
62
material adjustments recorded in our consolidated statements of income relating to changes in estimated contingent
purchase price liabilities.
On July 1, 2018, we closed on a joint venture with Internet Brands, a provider of web presence and online
marketing software, to create a newly formed entity, Henry Schein One, LLC. The joint venture includes Henry
Schein Practice Solutions products and services, as well as Henry Schein’s international dental practice
management systems and the dental businesses of Internet Brands. Internet Brands originally held a 26%
noncontrolling interest in Henry Schein One, LLC that is accounted for within stockholders’ equity, as well as a
freestanding and separately exercisable right to put its noncontrolling interest to Henry Schein, Inc. for fair value
following the fifth anniversary of the effective date of the formation of the joint venture. Beginning with the
second anniversary of the effective date of the formation of the joint venture, Henry Schein One began issuing a
fixed number of additional interests to Internet Brands, which increased Internet Brands interest to 27% effective
July 1, 2020. Henry Schein One will continue issuing additional interests to Internet Brands annually through the
fifth anniversary, ultimately increasing Internet Brands’ ownership to approximately 33.6%. Internet Brands is also
entitled to receive a fixed number of additional interests, in the aggregate up to approximately 1.6% of the joint
venture’s ownership, if certain operating targets are met by the joint venture in its fourth, fifth and sixth operating
years. These additional shares are considered contingent consideration that are accounted for within stockholders’
equity; however, these shares will not be allocated any net income of Henry Schein One until the shares vest or are
earned by Internet Brands. A Monte Carlo simulation was utilized to value the additional contingent interests that
are subject to operating targets. Key assumptions that were applied to derive the fair value of the contingent
interests include an assumed equity value of Henry Schein One, LLC at its inception date, a risk-free interest rate
based on U.S. treasury yields, an assumed future dividend yield, a risk-adjusted discount rate applied to projected
future cash flows, an assumed equity volatility based on historical stock price returns of a group of guideline
companies, and an estimated correlation of annual cash flow returns to equity returns. As a result of this transaction
with Internet Brands, we recorded $567.6 million of noncontrolling interest within stockholders’ equity.
Noncontrolling Interests
Noncontrolling interests represent our less than 50% ownership interest in an acquired subsidiary. Our net income
is reduced by the portion of the subsidiaries net income that is attributable to noncontrolling interests.
Unrecognized tax benefits
As more fully disclosed in Note 14 of “Notes to Consolidated Financial Statements,” we cannot reasonably estimate
the timing of future cash flows related to the unrecognized tax benefits, including accrued interest, of $84.0 million
as of December 26, 2020.
Critical Accounting Policies and Estimates
The preparation of consolidated financial statements requires us to make estimates and judgments that affect the
reported amounts of assets, liabilities, revenues and expenses and related disclosures of contingent assets and
liabilities. We base our estimates on historical data, when available, experience, industry and market trends, and on
various other assumptions that are believed to be reasonable under the circumstances, the combined results of
which form the basis for making judgments about the carrying values of assets and liabilities that are not readily
apparent from other sources. However, by their nature, estimates are subject to various assumptions and
uncertainties. Reported results are therefore sensitive to any changes in our assumptions, judgments and estimates,
including the possibility of obtaining materially different results if different assumptions were to be applied.
Our financial results for the year ended December 26, 2020 were affected by certain estimates we made due to the
adverse business environment brought on by the COVID-19 pandemic. During the year ended December 26, 2020,
we recorded incremental bad debt reserves of approximately $10.0 million for our global dental business. Our
stock compensation expense during the year ended December 26, 2020 was lower than in the years ended
December 28, 2019 and December 29, 2018 due to our estimate that a lower amount of performance shares granted
in 2018, 2019 or 2020 would ultimately vest as a result of the lower-than-normal earnings in 2020. Additionally, in
the year ended December 26, 2020, we recorded total impairment charges on intangible assets of approximately
$20.3 million. Although our selling, general and administrative expenses for the year ended December 26, 2020
63
represent management's best estimates and assumptions that affect the reported amounts, our judgment could
change in the future due to the significant uncertainty surrounding the macroeconomic effect of the COVID-19
pandemic.
Furthermore, during the year ended December 26, 2020, our gross profit margin was negatively affected by
significant adjustments recorded for PPE inventory and COVID-19 related products reflecting changes in our
estimates of net realizable value brought on by volatility of pricing and changes in demand experienced during the
year. Such conditions may recur and adversely impact gross profit margins in future periods, although we do not
expect material inventory adjustments to continue into 2021.
We believe that the following critical accounting policies, which have been discussed with the Audit Committee of
the Board of Directors, affect the significant estimates and judgments used in the preparation of our financial
statements:
Revenue Recognition
We generate revenue from the sale of dental and medical consumable products, equipment (health care distribution
revenues), software products and services and other sources (technology and value-added services revenues).
Provisions for discounts, rebates to customers, customer returns and other contra revenue adjustments are included
in the transaction price at contract inception by estimating the most likely amount based upon historical data and
estimates and are provided for in the period in which the related sales are recognized.
Revenue derived from the sale of consumable products is recognized at a point in time when control transfers to the
customer. Such sales typically entail high-volume, low-dollar orders shipped using third-party common carriers.
We believe that the shipment date is the most appropriate point in time indicating control has transferred to the
customer because we have no post-shipment obligations and this is when legal title and risks and rewards of
ownership transfer to the customer and the point at which we have an enforceable right to payment.
Revenue derived from the sale of equipment is recognized when control transfers to the customer. This occurs
when the equipment is delivered. Such sales typically entail scheduled deliveries of large equipment primarily by
equipment service technicians. Some equipment sales require minimal installation, which is typically completed at
the time of delivery. Our product generally carries standard warranty terms provided by the manufacturer, however,
in instances where we provide warranty labor services, the warranty costs are accrued in accordance with ASC 460
“Guarantees”.
Revenue derived from the sale of software products is recognized when products are shipped to customers or made
available electronically. Such software is generally installed by customers and does not require extensive training
due to the nature of its design. Revenue derived from post-contract customer support for software, including annual
support and/or training, is generally recognized over time using time elapsed as the input method that best depicts
the transfer of control to the customer.
Revenue derived from other sources, including freight charges, equipment repairs and financial services, is
recognized when the related product revenue is recognized or when the services are provided. We apply the
practical expedient to treat shipping and handling activities performed after the customer obtains control as
fulfillment activities, rather than a separate performance obligation in the contract.
Sales, value-add and other taxes we collect concurrent with revenue-producing activities are excluded from
revenue.
Certain of our revenue is derived from bundled arrangements that include multiple distinct performance obligations
which are accounted for separately. When we sell software products together with related services (i.e., training
and technical support), we allocate revenue to software using the residual method, using an estimate of the
standalone selling price to estimate the fair value of the undelivered elements. There are no cases where revenue is
deferred due to a lack of a standalone selling price. Bundled arrangements that include elements that are not
considered software consist primarily of equipment and the related installation service. We allocate revenue for
such arrangements based on the relative selling prices of the goods or services. If an observable selling price is not
64
available (i.e., we do not sell the goods or services separately), we use one of the following techniques to estimate
the standalone selling price: adjusted market approach; cost-plus approach; or the residual method. There is no
specific hierarchy for the use of these methods, but the estimated selling price reflects our best estimate of what the
selling prices of each deliverable would be if it were sold regularly on a standalone basis taking into consideration
the cost structure of our business, technical skill required, customer location and other market conditions.
Accounts Receivable
Accounts receivable are generally recognized when health care distribution and technology and value-added
services revenues are recognized. In accordance with the “expected credit loss” model, the carrying amount of
accounts receivable is reduced by a valuation allowance that reflects our best estimate of the amounts that will not
be collected. In addition to reviewing delinquent accounts receivable, we consider many factors in estimating our
reserve, including types of customers and their credit worthiness, experience and historical data adjusted for current
conditions and reasonable supportable forecasts.
Sales Returns
Sales returns are recognized as a reduction of revenue by the amount of expected returns and are recorded as refund
liability within current liabilities. We estimate the amount of revenue expected to be reversed to calculate the sales
return liability based on historical data for specific products, adjusted as necessary for new products. The
allowance for returns is presented gross as a refund liability and we record an inventory asset (and a corresponding
adjustment to cost of sales) for any products that we expect to be returned.
Inventories and Reserves
Inventories consist primarily of finished goods and are valued at the lower of cost or market. Cost is determined by
the first-in, first-out method for merchandise or actual cost for large equipment and high tech equipment. In
accordance with our policy for inventory valuation, we consider many factors including the condition and salability
of the inventory, historical sales, forecasted sales and market and economic trends.
From time to time, we may adjust our assumptions for anticipated changes in any of these or other factors expected
to affect the value of inventory. Although we believe our judgments, estimates and/or assumptions related to
inventory and reserves are reasonable, making material changes to such judgments, estimates and/or assumptions
could materially affect our financial results.
Acquisitions
We account for business acquisitions and combinations under the acquisition method of accounting, where the net
assets of businesses purchased are recorded at their fair value at the acquisition date and our consolidated financial
statements include their results of operations from that date. Any excess of acquisition consideration over the fair
value of identifiable net assets acquired is recorded as goodwill. The major classes of assets and liabilities that we
generally allocate purchase price to, excluding goodwill, include identifiable intangible assets (i.e., trademarks and
trade names, customer relationships and lists, non-compete agreements and product development), property, plant
and equipment, deferred taxes and other current and long-term assets and liabilities. The estimated fair value of
identifiable intangible assets is based on critical estimates, judgments and assumptions derived from: analysis of
market conditions; discount rates; discounted cash flows; customer retention rates; and estimated useful lives.
Some prior owners of such acquired subsidiaries are eligible to receive additional purchase price cash consideration
if certain financial targets are met. While we use our best estimates and assumptions to accurately value those
assets acquired and liabilities assumed at the acquisition date as well as contingent consideration, where applicable,
our estimates are inherently uncertain and subject to refinement. As a result, during the measurement period we
may record adjustments to the assets acquired and liabilities assumed with the corresponding offset to goodwill
within our consolidated balance sheets. At the end of the measurement period or final determination of the values
of such assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recognized in
our consolidated statements of operations.
65
Goodwill
Goodwill is not amortized, but is subject to impairment analysis at least once annually, or if an event occurs or
circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying
value. Such impairment analyses for goodwill require a comparison of the fair value to the carrying value of
reporting units. We regard our reporting units to be our operating segments: global dental, global medical, and
technology and value-added services. Goodwill was allocated to such reporting units, for the purposes of preparing
our impairment analyses, based on a specific identification basis.
Application of the goodwill impairment test requires judgment, including the identification of reporting units,
assignment of assets and liabilities that are considered shared services to the reporting units, and ultimately the
determination of the fair value of each reporting unit. The fair value of each reporting unit is calculated by applying
the discounted cash flow methodology and confirming with a market approach. This analysis requires judgments,
including estimation of detailed future cash flows based on budget expectations, and determination of comparable
companies to develop a weighted average cost of capital for each reporting unit. The estimates used to calculate the
fair value of a reporting unit change from year to year based on operating results, market conditions, and other
factors. Changes in these estimates and assumptions could materially affect the determination of fair value and
goodwill impairment for each reporting unit.
Supplier Rebates
Supplier rebates are included as a reduction of cost of sales and are recognized over the period they are earned. The
factors we consider in estimating supplier rebate accruals include forecasted inventory purchases and sales in
conjunction with supplier rebate contract terms which generally provide for increasing rebates based on either
increased purchase or sales volume. Although we believe our judgments, estimates and/or assumptions related to
supplier rebates are reasonable, making material changes to such judgments, estimates and/or assumptions could
materially affect our financial results.
Long-Lived Assets
Long-lived assets, other than goodwill and other definite-lived intangibles, are evaluated for impairment whenever
events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable through
the estimated undiscounted future cash flows to be derived from such assets.
Definite-lived intangible assets primarily consist of non-compete agreements, trademarks, trade names, customer
relationships and lists, and product development. For long-lived assets used in operations, impairment losses are
only recorded if the asset’s carrying amount is not recoverable through its undiscounted, probability-weighted
future cash flows. We measure the impairment loss based on the difference between the carrying amount and the
estimated fair value. When an impairment exists, the related assets are written down to fair value. Although we
believe our judgments, estimates and/or assumptions used in estimating cash flows and determining fair value are
reasonable, making material changes to such judgments, estimates and/or assumptions could materially affect such
impairment analyses and our financial results.
During the year ended December 26, 2020, we recorded total impairment charges on intangible assets of
approximately $20.3 million, nearly all of which was recorded in our technology and value-added services segment.
Stock-Based Compensation
Stock-based compensation represents the cost related to stock-based awards granted to employees and non-
employee directors. We measure stock-based compensation at the grant date, based on the estimated fair value of
the award, and recognize the cost (net of estimated forfeitures) as compensation expense on a straight-line basis
over the requisite service period. Our stock-based compensation expense is reflected in selling, general and
administrative expenses in our consolidated statements of income.
Stock-based awards are provided to certain employees and non-employee directors under the terms of our 2020
Stock Incentive Plan (formerly known as the 2013 Stock Incentive Plan), and our 2015 Non-Employee Director
66
Stock Incentive Plan (together, the “Plans”). The Plans are administered by the Compensation Committee of the
Board of Directors. Equity-based awards are granted solely in the form of restricted stock units, with the exception
of providing stock options to employees pursuant to certain pre-existing contractual obligations.
Grants of restricted stock units are stock-based awards granted to recipients with specified vesting provisions. In
the case of restricted stock units, common stock is generally delivered on or following satisfaction of vesting
conditions. We issue restricted stock units that vest solely based on the recipient’s continued service over time
(primarily four year cliff vesting, except for grants made under the 2015 Non-Employee Director Stock Incentive
Plan, which are primarily 12 month cliff vesting) and restricted stock units that vest based on our achieving
specified performance measurements and the recipient’s continued service over time (primarily three year cliff
vesting).
With respect to time-based restricted stock units, we estimate the fair value on the date of grant based on our
closing stock price. With respect to performance-based restricted stock units, the number of shares that ultimately
vest and are received by the recipient is based upon our performance as measured against specified targets over a
specified period, as determined by the Compensation Committee of the Board of Directors. Although there is no
guarantee that performance targets will be achieved, we estimate the fair value of performance-based restricted
stock units based on our closing stock price at time of grant.
The Plans provide for adjustments to the performance-based restricted stock units targets for significant events,
including, without limitation, acquisitions, divestitures, new business ventures, certain capital transactions
(including share repurchases), restructuring costs, if any, certain litigation settlements or payments, if any, changes
in tax rates in certain countries, changes in accounting principles or in applicable laws or regulations and foreign
exchange fluctuations. Over the performance period, the number of shares of common stock that will ultimately
vest and be issued and the related compensation expense is adjusted upward or downward based upon our
estimation of achieving such performance targets. The ultimate number of shares delivered to recipients and the
related compensation cost recognized as an expense will be based on our actual performance metrics as defined
under the Plans.
Although we believe our judgments, estimates and/or assumptions related to stock-based compensation are
reasonable, making material changes to such judgments, estimates and/or assumptions could materially affect our
financial results.
Unrecognized Tax Benefits
ASC Topic 740 prescribes the accounting for uncertainty in income taxes recognized in the financial statements in
accordance with other provisions contained within this guidance. This topic prescribes a recognition threshold and
a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected
to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be
sustained upon examination by the taxing authorities. The amount recognized is measured as the largest amount of
benefit that is greater than 50% likely of being realized upon ultimate audit settlement. In the normal course of
business, our tax returns are subject to examination by various taxing authorities. Such examinations may result in
future tax and interest assessments by these taxing authorities for uncertain tax positions taken in respect of certain
tax matters.
Accounting Standards Update
For a discussion of accounting standards updates that have been adopted or will be adopted in the future, please see
67
ITEM 7A. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to market risks as well as changes in foreign currency exchange rates as measured against the U.S.
dollar and each other, and changes to the credit markets. We attempt to minimize these risks by primarily using
foreign currency forward contracts and by maintaining counter-party credit limits. These hedging activities provide
only limited protection against currency exchange and credit risks. Factors that could influence the effectiveness of
our hedging programs include currency markets and availability of hedging instruments and liquidity of the credit
markets. All foreign currency forward contracts that we enter into are components of hedging programs and are
entered into for the sole purpose of hedging an existing or anticipated currency exposure. We do not enter into such
contracts for speculative purposes and we manage our credit risks by diversifying our investments, maintaining a
strong balance sheet and having multiple sources of capital.
Foreign Currency Agreements
The value of certain foreign currencies as compared to the U.S. dollar and the value of certain underlying functional
currencies of the Company, including its foreign subsidiaries, may affect our financial results. Fluctuations in
exchange rates may positively or negatively affect our revenues, gross margins, operating expenses and retained
earnings, all of which are expressed in U.S. dollars. Where we deem it prudent, we engage in hedging programs
using primarily foreign currency forward contracts aimed at limiting the impact of foreign currency exchange rate
fluctuations on earnings. We purchase short-term (i.e., generally 18 months or less) foreign currency forward
contracts to protect against currency exchange risks associated with intercompany loans due from our international
subsidiaries and the payment of merchandise purchases to foreign suppliers. We do not hedge the translation of
foreign currency profits into U.S. dollars, as we regard this as an accounting exposure, not an economic
exposure. A hypothetical 5% change in the average value of the U.S. dollar in 2020 compared to foreign currencies
would have changed our 2020 reported Net income attributable to Henry Schein, Inc. by approximately $1.3
million.
As of December 26, 2020, we had forward foreign currency exchange agreements, which expire through November
16, 2023, which include a mark-to-market loss of $9.9 million as determined by quoted market prices. Included in
the forward foreign currency exchange agreements, Henry Schein, Inc. had EUR/USD forward contracts notionally
totaling an amount of approximately €200 million, with a reported fair value of these contracts as a net liability of
$9.6 million. A 5% increase in the value of the Euro to the USD from December 26, 2020, with all other variables
held constant, would have had an unfavorable effect on the fair value of these forward contracts by decreasing the
value of these instruments by $11.9 million.
Total Return Swaps
On March 20, 2020, we entered into a total return swap for the purpose of economically hedging our unfunded non-
qualified supplemental retirement plan (“SERP”) and our deferred compensation plan (“DCP”). This swap will
offset changes in our SERP and DCP liabilities. At the inception, the notional value of the investments in these
plans was $43.4 million. At December 26, 2020, the notional value of the investments in these plans was $67.6
million. At December 26, 2020 the financing rate for this swap was based on LIBOR of 0.15% plus 0.38%, for a
combined rate of 0.53%. From March 20, 2020, the effective date of the swap, to December 26, 2020, we have
recorded a gain, within the selling, general and administrative line item in our consolidated statement of income, of
approximately $21.2 million, net of transaction costs, related to this undesignated swap for the year ended
December 26, 2020. This gain was offset by the change in fair value adjustment in deferred compensation,
resulting in a neutral impact to our results of operations. This swap is expected to be renewed on an annual basis.
Short-Term Investments
We limit our credit risk with respect to our cash equivalents, short-term investments and derivative instruments, by
monitoring the credit worthiness of the financial institutions who are the counterparties to such financial
instruments. As a risk management policy, we limit the amount of credit exposure by diversifying and utilizing
numerous investment grade counterparties.
68
Variable Interest Rate Debt
As of December 26, 2020, we had variable interest rate exposure for certain of our revolving credit facilities and
our U.S. trade accounts receivable securitization.
Our revolving credit facility which we entered into on April 18, 2017 and expires on April 18, 2022, has an interest
rate that is based on the U.S. Dollar LIBOR plus a spread based on our leverage ratio at the end of each financial
reporting quarter. As of December 26, 2020, there was $0.0 million outstanding under this revolving credit
facility. During the year ended December 26, 2020, the average outstanding balance under this revolving credit
facility was approximately $21.4 million. Based upon our average outstanding balance for this revolving credit
facility, for each hypothetical increase of 25 basis points, our interest expense thereunder would have increased by
less than $0.1 million.
Our U.S trade accounts receivable securitization, which we entered into on April 17, 2013 and was scheduled to
expire on April 29, 2022, has an interest rate that is based upon the asset-backed commercial paper rate. On June
22, 2020, the expiration date for this facility was extended to June 12, 2023. As of December 26, 2020, the
commercial paper rate was 0.22% plus 0.95%, for a combined rate of 1.17%. At December 26, 2020 the
outstanding balance was $0.0 million under this securitization facility. During the year ended December 26, 2020,
the average outstanding balance under this securitization facility was approximately $92.3 million. Based upon our
average outstanding balance for this securitization facility, for each hypothetical increase of 25 basis points, our
interest expense thereunder would have increased by $0.2 million.
69
ITEM 8. Financial Statements and Supplementary Data
INDEX TO FINANCIAL STATEMENTS
HENRY SCHEIN, INC.
Page
70
72
73
74
75
76
77
77
87
90
91
92
93
97
99
100
101
104
105
106
106
110
111
112
113
115
119
122
123
123
138
All other schedules are omitted because the required information is either inapplicable or is included in the consolidated
financial statements or the notes thereto.
70
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Stockholders and Board of Directors
Henry Schein, Inc.
Melville, NY
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Henry Schein, Inc. (the “Company”) as of
December 26, 2020 and December 28, 2019, the related consolidated statements of income, comprehensive income,
stockholders’ equity, and cash flows for each of the three years in the period ended December 26, 2020, the related
notes and schedule (collectively referred to as the “consolidated financial statements”). In our opinion, the
consolidated financial statements present fairly, in all material respects, the financial position of the Company at
December 26, 2020 and December 28, 2019, and the results of its operations and its cash flows for each of the three
years in the period ended December 26, 2020, in conformity with accounting principles generally accepted in the
United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board
(United States) (“PCAOB”), the Company's internal control over financial reporting as of December 26, 2020,
based on criteria established in
Internal Control – Integrated Framework (2013)
Sponsoring Organizations of the Treadway Commission (“COSO”) and our report dated February
1
7, 2021
expressed an unqualified opinion thereon.
Change in Accounting Principle
As discussed in Note 1 to the consolidated financial statements, effective on December 30, 2018, the Company
changed its method of accounting for leases due to the adoption of Accounting Standards Codification Topic
842,
Leases
.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is
to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public
accounting firm registered with the PCAOB and are required to be independent with respect to the Company in
accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and
Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of
material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks
of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing
procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the
amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the
accounting principles used and significant estimates made by management, as well as evaluating the overall
presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our
opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated
financial statements that was communicated or required to be communicated to the Audit Committee of the Board
of Directors and that: (1) relates to accounts or disclosures that are material to the consolidated financial
statements; and (2) involved our especially challenging, subjective or complex judgments. The communication of
critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a
whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the
critical audit matter or on the accounts or disclosures to which it relates.
71
Uncertain Tax Position
As described in Note 14 of the consolidated financial statements the Company operates in multiple jurisdictions
and is subject to transfer pricing compliance for intercompany transactions that are subject to audit by taxing
authorities. The resolution of these audits may span multiple years.
We identified the determination of uncertain tax positions related to transfer pricing from intercompany
transactions as a critical audit matter. The principal considerations for our determination included complex
judgments related to: (i) auditing assumptions applied to the interpretation of tax laws and legal rulings in
multiple tax paying jurisdictions, (ii) determining whether a transfer pricing tax position’s technical merits are
more-likely-than-not to be sustained when measuring the amount of tax benefits that qualifies for recognition,
(iii) assessing whether intercompany transactions are based on the arm’s length standard that may produce a
range of arm’s length outcomes, and (iv) assessing the adjustments to the liability for unrecognized tax benefits
associated with tax settlements or agreements. Auditing these elements involved especially subjective auditor
judgment and an increased level of audit effort, including involvement of personnel with specialized skills and
knowledge.
The primary procedures we performed to address this critical audit matter included:
●
Assessing the design and implementation and testing operating effectiveness of certain controls over
the recognition and measurement of uncertain tax positions related to transfer pricing.
●
Utilizing personnel with specialized knowledge and skill in taxation to evaluate the appropriateness of
management’s methods and assumptions used to estimate uncertain tax positions related to transfer
pricing by: (i) verifying our understanding of the relevant facts by reading the Company’s
correspondence with the relevant tax authorities and third-party advice obtained by the Company, (ii)
evaluating the reasonableness of technical merits, management’s judgments and assumptions and
assessing the overall reasonableness of conclusions reached, (iii) evaluating the ranges of arm’s length
outcomes and pricing conclusions reached within management’s transfer pricing studies, and (iv)
reviewing settlement activity or agreements with income tax authorities.
/s/ BDO USA, LLP
We have served as the Company's auditor since 1984.
New York, NY
February 17, 2021
See accompanying notes.
72
HENRY SCHEIN, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
December 26,
December 28,
2020
2019
ASSETS
Current assets:
Cash and cash equivalents
$
$
Accounts receivable, net of reserves of $
Inventories, net
Prepaid expenses and other
Total current assets
Property and equipment, net
Operating lease right-of-use assets, net
Goodwill
Other intangibles, net
Investments and other
Total assets
$
$
LIABILITIES AND STOCKHOLDERS' EQUITY
Current liabilities:
Accounts payable
$
$
Bank credit lines
Current maturities of long-term debt
Operating lease liabilities
Accrued expenses:
Payroll and related
Taxes
Other
Total current liabilities
Long-term debt
Deferred income taxes
Operating lease liabilities
Other liabilities
Total liabilities
Redeemable noncontrolling interests
Commitments and contingencies
Stockholders' equity:
Preferred stock, $
Common stock, $
Additional paid-in capital
Retained earnings
Accumulated other comprehensive loss
(108,084 )
(167,373 )
Total Henry Schein, Inc. stockholders' equity
Noncontrolling interests
Total stockholders' equity
Total liabilities, redeemable noncontrolling interests and stockholders' equity
$
$
See accompanying notes.
73
HENRY SCHEIN, INC.
CONSOLIDATED STATEMENTS OF INCOME
(in thousands, except per share data)
Years Ended
December 26,
December 28,
December 29,
2020
2019
2018
Net sales
$
$
$
Cost of sales
Gross profit
Operating expenses:
Selling, general and administrative
Litigation settlements
Restructuring costs
Operating income
Other income (expense):
Interest income
Interest expense
(41,377 )
(50,792 )
(76,016 )
Other, net
(3,873 )
(2,919 )
(3,258 )
Income from continuing operations before taxes, equity in
earnings of affiliates and noncontrolling interests
Income taxes
(95,374 )
(159,515 )
(107,432 )
Equity in earnings of affiliates
Net gain on sale of equity investments
Net income from continuing operations
Income (loss) from discontinued operations, net of tax
(6,323 )
Net Income
Less: Net income attributable to noncontrolling interests
(15,629 )
(24,770 )
(19,724 )
Less: Net (income) loss attributable to noncontrolling interests
from discontinued operations
(6,521 )
Net income attributable to Henry Schein, Inc.
$
$
$
Amounts attributable to Henry Schein Inc.:
Continuing operations
$
$
$
Discontinued operations
(5,957 )
Net income attributable to Henry Schein, Inc.
$
$
$
Earnings per share from continuing operations attributable to
Henry Schein, Inc.:
Basic
$
$
$
Diluted
$
$
$
Earnings (loss) per share from discontinued operations attributable to
Henry Schein, Inc.:
Basic
$
$
(0.04 )
$
Diluted
$
$
(0.04 )
$
Earnings per share attributable to Henry Schein, Inc.:
Basic
$
$
$
Diluted
$
$
$
Weighted -average common shares outstanding:
Basic
Diluted
See accompanying notes.
74
HENRY SCHEIN, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(in thousands)
Years Ended
December 26,
December 28,
December 29,
2020
2019
2018
Net income
$
$
$
Other comprehensive income (loss), net of tax:
Foreign currency translation gain (loss)
(4,070 )
(136,356 )
Unrealized gain (loss) from foreign currency hedging activities
(7,456 )
(3,876 )
Unrealized investment gain (loss)
(5 )
(3 )
Pension adjustment gain (loss)
(5,924 )
Other comprehensive income (loss), net of tax
(13,858 )
(132,700 )
Comprehensive income
Comprehensive income attributable to noncontrolling interests:
Net income
(15,629 )
(24,404 )
(26,245 )
Foreign currency translation loss
Comprehensive income attributable to noncontrolling interests
(12,116 )
(22,556 )
(12,249 )
Comprehensive income attributable to Henry Schein, Inc.
$
$
$
See accompanying notes.
75
HENRY SCHEIN, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY
(In thousands, except share and per share data)
Accumulated
Common Stock
Additional
Total
$.01 Par Value
Paid-in
Retained
Comprehensive
Noncontrolling
Stockholders'
Shares
Amount
Earnings
Interests
Equity
Balance, December 30, 2017
(130,067 )
Cumulative impact of adopting new accounting standards
Net income (excluding $
noncontrolling interests)
Foreign currency translation loss (excluding loss of $
attributable to Redeemable noncontrolling interests)
-
(122,360 )
(965 )
(123,325 )
Unrealized gain from foreign currency hedging activities,
net of tax benefit of $
-
Unrealized investment loss, net of tax benefit of $
-
(3 )
(3 )
Pension adjustment gain, net of tax of $
-
Dividends paid
-
(656 )
(656 )
Other adjustments
-
(19 )
Purchase of noncontrolling interests
-
(214 )
(214 )
Change in fair value of redeemable securities
-
(148,919 )
(148,919 )
Initial noncontrolling interests and adjustments related to
business acquisitions
-
Repurchase and retirement of common stock
(2,518,387 )
(25 )
(36,206 )
(163,769 )
(200,000 )
Stock issued upon exercise of stock options
Stock-based compensation expense
Shares withheld for payroll taxes
(267,772 )
(3 )
(18,140 )
(18,143 )
Settlement of stock-based compensation awards
(727 )
(727 )
Deferred tax benefit arising from acquisition of
noncontrolling interest in partnership
-
Transfer of charges in excess of capital
-
(106,146 )
Balance, December 29, 2018
(248,771 )
Cumulative impact of adopting new accounting standards
-
(274 )
(274 )
Net income (excluding $
noncontrolling interests from continuing operations
and ($
-
Foreign currency translation loss (excluding loss of $
attributable to Redeemable noncontrolling interests
and ($
-
(2,222 )
(105 )
(2,327 )
Unrealized loss from foreign currency hedging activities,
net of tax benefit of $
-
(3,876 )
(3,876 )
Unrealized investment gain, net of tax of $
-
Pension adjustment loss, net of tax benefit of $
-
(5,924 )
(5,924 )
Dividends paid
-
(535 )
(535 )
Other adjustments
-
(3 )
(3 )
Change in fair value of redeemable securities
-
Initial noncontrolling interests and adjustments related to
business acquisitions
-
Adjustment for Animal Health Spin-off
Repurchase and retirement of common stock
(8,173,912 )
(82 )
(79,785 )
(445,133 )
(525,000 )
Stock issued upon exercise of stock options
Stock-based compensation expense
Shares withheld for payroll taxes
(179,860 )
(1 )
(10,844 )
(10,845 )
Settlement of stock-based compensation awards
Share Sale related to Animal Health business
-
Separation of Animal Health business
-
(73,970 )
(543,158 )
(523,720 )
Transfer of charges in excess of capital
-
(201,457 )
Balance, December 28, 2019
(167,373 )
Cumulative impact of adopting new accounting standards
-
(412 )
(412 )
Net income (excluding $
noncontrolling interests from continuing operations)
-
Foreign currency translation gain (excluding loss of $
attributable to Redeemable noncontrolling interests )
-
Unrealized loss from foreign currency hedging activities,
net of tax benefit of $
-
(7,456 )
(7,456 )
Unrealized investment loss, net of tax benefit of $
-
(5 )
(5 )
Pension adjustment gain, including tax benefit of $
-
Dividends paid
-
(1,086 )
(1,086 )
Purchase of noncontrolling interests
-
(1,597 )
(701 )
(2,298 )
Change in fair value of redeemable securities
-
(32,842 )
(32,842 )
Initial noncontrolling interests and adjustments related to
business acquisitions
-
Repurchase and retirement of common stock
(1,200,000
(12 )
(10,949 )
(62,828 )
(73,789 )
Stock-based compensation expense
Shares withheld for payroll taxes
(236,752
(2 )
(14,475 )
(14,477 )
Settlement of stock-based compensation awards
-
(275 )
(275 )
Separation of Animal Health business
-
Transfer of charges in excess of capital
-
(1,938 )
Balance, December 26, 2020
(108,084 )
See accompanying notes.
76
HENRY SCHEIN, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands, except per share data) (unaudited)
Years Ended
December 26,
December 28,
December 29,
2020
2019
2018
Cash flows from operating activities:
Net income
$
$
$
Income (loss) from discontinued operations
(6,323 )
Income from continuing operations
Adjustments to reconcile net income to net cash provided by operating activities:
Depreciation and amortization
Impairment charge on intangible assets
Gain on sale of equity investments
(2,096 )
(250,167 )
Stock-based compensation expense
Provision for losses on trade and other accounts receivable
Benefit from deferred income taxes
(52,977 )
(4,057 )
(25,388 )
Equity in earnings of affiliates
(12,344 )
(17,900 )
(21,037 )
Distributions from equity affiliates
Changes in unrecognized tax benefits
(24,881 )
(1,169 )
Benefit from transition tax
(10,000 )
Other
Changes in operating assets and liabilities, net of acquisitions:
Accounts receivable
(189,349 )
(72,689 )
(127,201 )
Inventories
(31,817 )
(41,042 )
Other current assets
(6,479 )
(57,291 )
(165,645 )
Accounts payable and accrued expenses
Net cash provided by operating activities from continuing operations
Net cash provided by (used in) operating activities from discontinued operations
(166,391 )
Net cash provided by operating activities
Cash flows from investing activities:
Purchases of fixed assets
(48,829 )
(76,219 )
(71,283 )
Payments related to equity investments and business
acquisitions, net of cash acquired
(60,173 )
(655,879 )
(53,240 )
Proceeds from sale of equity investment
Repayments from (borrowings for) loan to affiliate
(1,243 )
(25,700 )
Other
(18,794 )
(14,175 )
(15,101 )
Net cash used in investing activities from continuing operations
(115,019 )
(422,309 )
(164,324 )
Net cash used in investing activities from discontinued operations
(2,064 )
(28,630 )
Net cash used in investing activities
(115,019 )
(424,373 )
(192,954 )
Cash flows from financing activities:
Net change in bank borrowings
(927,912 )
Proceeds from issuance of long-term debt
Principal payments for long-term debt
(611,216 )
(260,944 )
(24,735 )
Debt issuance costs
(3,879 )
(391 )
(501 )
Debt extinguishment costs
(401 )
Proceeds from issuance of stock upon exercise of stock options
Payments for repurchases of common stock
(73,789 )
(525,000 )
(200,000 )
Payments for taxes related to shares withheld for employee taxes
(14,299 )
(10,814 )
(18,023 )
Distribution received related to Animal Health Spin-off
Proceeds related to Animal Health Share Sale
Proceeds from (distributions to) noncontrolling shareholders
(7,886 )
(7,351 )
Acquisitions of noncontrolling interests in subsidiaries
(19,538 )
(2,358 )
(287,635 )
Proceeds from (payments) to Henry Schein Animal Health Business
(169,295 )
(192,745 )
Net cash used in financing activities from continuing operations
(181,794 )
(363,351 )
(402,173 )
Net cash provided by (used in) financing activities from discontinued operations
(5,391 )
(201,603 )
Net cash used in financing activities
(187,185 )
(215,980 )
(603,776 )
Effect of exchange rate changes on cash and cash equivalents from continuing operations
Effect of exchange rate changes on cash and cash equivalents from discontinued operations
(2,240 )
Net change in cash and cash equivalents from continuing operations
(101,117 )
Net change in cash and cash equivalents from discontinued operations
(23,324 )
Cash and cash equivalents, beginning of period
Cash and cash equivalents, end of period
$
$
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
77
Note 1 –Significant Accounting Policies
Nature of Operations
We distribute health care products and services primarily to office-based health care practitioners with operations or
affiliates in the United States, Australia, Austria, Belgium, Brazil, Canada, Chile, China, the Czech Republic,
France, Germany, Hong Kong SAR, Ireland, Israel, Italy, Japan, Liechtenstein, Luxembourg, Malaysia, the
Netherlands, New Zealand, Poland, Portugal, Singapore, South Africa, Spain, Sweden, Switzerland, Thailand,
United Arab Emirates and the United Kingdom.
Principles of Consolidation
Our consolidated financial statements include the accounts of Henry Schein, Inc. and all of our controlled
subsidiaries. All intercompany accounts and transactions are eliminated in consolidation. Investments in
unconsolidated affiliates, which are greater than or equal to 20% and less than or equal to 50% owned or
investments in unconsolidated affiliates of less than 20% in which we have the ability to influence the operating or
financial decisions, are accounted for under the equity method. Certain prior period amounts have been reclassified
to conform to the current period presentation.
We consolidate a Variable Interest Entity (“VIE”) where we hold a variable interest and are the primary
beneficiary. The VIE is a trade accounts receivable securitization. We are the primary beneficiary because we
have the power to direct activities that most significantly affect the economic performance and have the obligation
to absorb the majority of the losses or benefits. The results of operations and financial position of this VIE are
included in our consolidated financial statements.
For the consolidated VIE, the trade accounts receivable transferred to the VIE are pledged as collateral to the
related debt. The creditors have recourse to us for losses on these trade accounts receivable. At December 26,
2020 and December 28, 2019, trade accounts receivable that can only be used to settle obligations of this VIE were
$
were $
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United
States requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and
disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of
revenues and expenses during the reporting period. Actual results could differ from those estimates.
In March 2020, the World Health Organization declared the Novel Coronavirus Disease 2019 (“COVID-19”) a
pandemic. The COVID-19 pandemic has negatively impacted the global economy, disrupted global supply chains
and created significant volatility and disruption of global financial markets. In response, many countries
implemented business closures and restrictions, stay-at-home and social distancing ordinances and similar measures
to combat the pandemic, which significantly impacted global business and dramatically reduced demand for dental
products and certain medical products in the second quarter of 2020. Demand increased in the second half of the
year resulting in growth over the prior year driven by sales of personal protective equipment (PPE) and COVID-19
related products.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
78
Our consolidated financial statements reflect estimates and assumptions made by us that affect, among other things,
our goodwill, long-lived asset and indefinite-lived intangible asset valuation; inventory valuation; equity investment
valuation; assessment of the annual effective tax rate; valuation of deferred income taxes and income tax
contingencies; the allowance for doubtful accounts; hedging activity; vendor rebates; measurement of
compensation cost for certain share-based performance awards and cash bonus plans; and pension plan
assumptions. Due to the significant uncertainty surrounding the future impact of COVID-19, our judgments
regarding estimates and impairments could change in the future. In addition, the impact of COVID-19 had a
material adverse effect on our business, results of operations and cash flows in the second quarter of 2020. In the
latter half of the year, dental and medical practices began to re-open worldwide, and continued to do so during the
remainder of the year. However, patient volumes remain below pre-COVID-19 levels and certain regions in the
U.S. and internationally are experiencing an increase in COVID-19 cases. As such, there is an ongoing risk that the
COVID-19 pandemic may again have a material adverse effect on our business, results of operations and cash
flows and may result in a material adverse effect on our financial condition and liquidity. However, the extent of
the potential impact cannot be reasonably estimated at this time.
Fiscal Year
We report our results of operations and cash flows on a
-
The years ended December 26, 2020, December 28, 2019 and December 29, 2018 consisted of
Revenue Recognition
Revenue is recognized when a customer obtains control of promised goods or services in an amount that reflects the
consideration that we expect to receive for those goods or services. To recognize revenue, we do the following:
• identify the contract(s) with a customer;
• identify the performance obligations in the contract;
• determine the transaction price;
• allocate the transaction price to the performance obligations in the contract; and
• recognize revenue when, or as, the entity satisfies a performance obligation.
We generate revenue from the sale of dental and medical consumable products, equipment (Health care distribution
revenues), software products and services and other sources (Technology and value-added services revenues).
Provisions for discounts, rebates to customers, customer returns and other contra revenue adjustments are included
in the transaction price at contract inception by estimating the most likely amount based upon historical data and
estimates and are provided for in the period in which the related sales are recognized.
Revenue derived from the sale of consumable products is recognized at a point in time when control transfers to the
customer. Such sales typically entail high-volume, low-dollar orders shipped using third-party common carriers.
We believe that the shipment date is the most appropriate point in time indicating control has transferred to the
customer because we have no post-shipment obligations and this is when legal title and risks and rewards of
ownership transfer to the customer and the point at which we have an enforceable right to payment.
Revenue derived from the sale of equipment is recognized when control transfers to the customer. This occurs
when the equipment is delivered. Such sales typically entail scheduled deliveries of large equipment primarily by
equipment service technicians. Some equipment sales require minimal installation, which is typically completed at
the time of delivery. Our product generally carries standard warranty terms provided by the manufacturer,
however, in instances where we provide warranty labor services, the warranty costs are accrued in accordance with
Accounting Standards Codification (“ASC”) 460 “Guarantees”.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
79
Revenue derived from the sale of software products is recognized when products are shipped to customers or made
available electronically. Such software is generally installed by customers and does not require extensive training
due to the nature of its design. Revenue derived from post-contract customer support for software, including annual
support and/or training, is generally recognized over time using time elapsed as the input method that best depicts
the transfer of control to the customer.
Revenue derived from other sources, including freight charges, equipment repairs and financial services, is
recognized when the related product revenue is recognized or when the services are provided. We apply the
practical expedient to treat shipping and handling activities performed after the customer obtains control as
fulfillment activities, rather than a separate performance obligation in the contract.
Sales, value-add and other taxes we collect concurrent with revenue-producing activities are excluded from
revenue.
Certain of our revenue is derived from bundled arrangements that include multiple distinct performance obligations,
which are accounted for separately. When we sell software products together with related services (i.e., training
and technical support), we allocate revenue to software using the residual method, using an estimate of the
standalone selling price to estimate the fair value of the undelivered elements. There are no cases where revenue is
deferred due to a lack of a standalone selling price. Bundled arrangements that include elements that are not
considered software consist primarily of equipment and the related installation service. We allocate revenue for
such arrangements based on the relative selling prices of the goods or services. If an observable selling price is not
available (i.e., we do not sell the goods or services separately), we use one of the following techniques to estimate
the standalone selling price: adjusted market approach; cost-plus approach; or the residual method. There is no
specific hierarchy for the use of these methods, but the estimated selling price reflects our best estimate of what the
selling prices of each deliverable would be if it were sold regularly on a standalone basis taking into consideration
the cost structure of our business, technical skill required, customer location and other market conditions.
See
Contract Balances
Contract balances represent amounts presented in our consolidated balance sheet when either we have transferred
goods or services to the customer or the customer has paid consideration to us under the contract. These contract
balances include accounts receivable, contract assets and contract liabilities.
Accounts Receivable
Accounts receivable are generally recognized when health care distribution and technology and value-added
services revenues are recognized. In accordance with the “expected credit loss” model, the carrying amount of
accounts receivable is reduced by a valuation allowance that reflects our best estimate of the amounts that we do
not expect to collect. In addition to reviewing delinquent accounts receivable, we consider many factors in
estimating our reserve, including types of customers and their credit worthiness, experience and historical data
adjusted for current conditions and reasonable supportable forecasts.
Contract Assets
Contract assets include amounts related to any conditional right to consideration for work completed but not billed
as of the reporting date and generally represent amounts owed to us by customers, but not yet billed. Contract assets
are transferred to accounts receivable when the right becomes unconditional. The contract assets primarily relate to
our bundled arrangements for the sale of equipment and consumables and sales of term software licenses. Current
contract assets are included in Prepaid expenses and other and the non-current contract assets are included in
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
80
Investments and other within our consolidated balance sheets. Current and non-current contract asset balances as of
December 26, 2020 and December 28, 2019 were not material.
Contract Liabilities
Contract liabilities are comprised of advance payments and upfront payments for service arrangements provided
over time that are accounted for as deferred revenue amounts. Contract liabilities are transferred to revenue once
the performance obligation has been satisfied. Current contract liabilities are included in Accrued expenses: Other
and the non-current contract liabilities are included in Other liabilities within our consolidated balance sheets. At
December 28, 2019, the current portion of contract liabilities of $
Other, and $
ended December 26, 2020, we recognized substantially all of the current contract liability amounts that were
previously deferred at December 28, 2019. At December 26, 2020, the current and non-current portion of contract
liabilities were $
Deferred Commissions
Sales commissions earned by our sales force that relate to long term arrangements are capitalized as costs to obtain
a contract when the costs incurred are incremental and are expected to be recovered. Deferred sales commissions
are amortized over the estimated customer relationship period.
We apply the practical expedient related to the
capitalization of incremental costs of obtaining a contract, and recognize such costs as an expense when incurred if
the amortization period of the assets that we would have recognized is one year or less.
balances as of December 26, 2020 and December 28, 2019 were not material.
Sales Returns
Sales returns are recognized as a reduction of revenue by the amount of expected returns and are recorded as refund
liability within current liabilities. We estimate the amount of revenue expected to be reversed to calculate the sales
return liability based on historical data for specific products, adjusted as necessary for new products. The
allowance for returns is presented gross as a refund liability and we record an inventory asset (and a corresponding
adjustment to cost of sales) for any products that we expect to be returned.
Inventories and Reserves
Inventories consist primarily of finished goods and are valued at the lower of cost or net realizable value. Cost is
determined by the first-in, first-out method for merchandise or actual cost for large equipment and high tech
equipment. In accordance with our policy for inventory valuation, we consider many factors including the
condition and salability of the inventory, historical sales, forecasted sales and market and economic trends. From
time to time, we adjust our assumptions for anticipated changes in any of these or other factors expected to affect
the value of inventory.
Cash and Cash Equivalents
We consider all highly liquid short-term investments with an original maturity of three months or less to be cash
equivalents. Due to the short-term maturity of such investments, the carrying amounts are a reasonable estimate of
fair value. Outstanding checks in excess of funds on deposit of $
payments for inventory, were classified as accounts payable as of December 26, 2020 and December 28, 2019.
Direct Shipping and Handling Costs
Freight and other direct shipping costs are included in cost of sales. Direct handling costs, which represent
primarily direct compensation costs of employees who pick, pack and otherwise prepare, if necessary, merchandise
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
81
for shipment to our customers are reflected in selling, general and administrative expenses. Direct shipping and
handling costs were $
December 28, 2019 and December 29, 2018.
Advertising and Promotional Costs
We generally expense advertising and promotional costs as incurred. Total advertising and promotional expenses
were $
and December 29, 2018.
Supplier Rebates
Supplier rebates are included as a reduction of cost of sales and are recognized over the period they are earned. The
factors we consider in estimating supplier rebate accruals include forecasted inventory purchases and sales, in
conjunction with supplier rebate contract terms, which generally provide for increasing rebates based on either
increased purchase or sales volume.
Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation or amortization. Depreciation is
computed primarily under the straight-line method
for estimated useful
lives).
Amortization of leasehold improvements is computed using the straight-line method over the lesser of the
useful life of the assets or the lease term.
Capitalized software costs consist of costs to purchase and develop software. Costs incurred during the application
development stage for software bought and further customized by outside suppliers for our use and software
developed by a supplier for our proprietary use are capitalized. Costs incurred for our own personnel who are
directly associated with software development are capitalized.
Income Taxes
We account for income taxes under an asset and liability approach that requires the recognition of deferred income
tax assets and liabilities for the expected future tax consequences of events that have been recognized in our
financial statements or tax returns. In estimating future tax consequences, we generally consider all expected future
events other than enactments of changes in tax laws or rates. The effect on deferred income tax assets and
liabilities of a change in tax rates is recognized as income or expense in the period that includes the enactment date.
We file a consolidated U.S. federal income tax return with our
% or greater owned U.S. subsidiaries.
Foreign Currency Translation and Transactions
The financial position and results of operations of our foreign subsidiaries are determined using local currency as
the functional currency. Assets and liabilities of these subsidiaries are translated at the exchange rate in effect at
each year-end. Income statement accounts are translated at the average rate of exchange prevailing during the year.
Translation adjustments arising from the use of differing exchange rates from period to period are included in
Accumulated other comprehensive income in stockholders’ equity. Gains and losses resulting from foreign
currency transactions are included in earnings.
Risk Management and Derivative Financial Instruments
We use derivative instruments to minimize our exposure to fluctuations in foreign currency exchange rates. Our
objective is to manage the impact that foreign currency exchange rate fluctuations could have on recognized asset
and liability fair values, earnings and cash flows, as well as our net investments in foreign subsidiaries. Our risk
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
82
management policy requires that derivative contracts used as hedges be effective at reducing the risks associated
with the exposure being hedged and be designated as a hedge at the inception of the contract. We do not enter into
derivative instruments for speculative purposes. Our derivative instruments primarily include foreign currency
forward agreements related to certain intercompany loans, certain forecasted inventory purchase commitments with
foreign suppliers and foreign currency forward contracts to hedge a portion of our euro-denominated foreign
operations which are designated as net investment hedges.
Foreign currency forward agreements related to forecasted inventory purchase commitments with foreign suppliers
and foreign currency swaps related to foreign currency denominated debt are designated as cash flow hedges. For
derivatives that are designated and qualify as cash flow hedges, the changes in the fair value of the derivative is
recorded as a component of Accumulated other comprehensive income in stockholders’ equity and subsequently
reclassified into earnings in the period(s) during which the hedged transaction affects earnings. We classify the
cash flows related to our hedging activities in the same category on our consolidated statements of cash flows as the
cash flows related to the hedged item.
Foreign currency forward contracts related to our euro-denominated foreign operations are designated as net
investment hedges. For derivatives that are designated and qualify as net investment hedges, the changes in the fair
value of the derivative is recorded in the foreign currency translation gain (loss) component of Accumulated other
comprehensive income in stockholders’ equity until the net investment is sold or substantially liquidated.
Our foreign currency forward agreements related to foreign currency balance sheet exposure provide economic
hedges but are not designated as hedges for accounting purposes.
For agreements not designated as hedges, changes in the value of the derivative, along with the transaction gain or
loss on the hedged item, are recorded in earnings.
Total return swaps are entered into for the purpose of economically hedging our unfunded non-qualified
supplemental retirement plan (“SERP”) and our deferred compensation plan (“DCP”). This swap will offset
changes in our SERP and DCP liabilities. This swap is expected to be renewed on an annual basis.
Acquisitions
We account for business acquisitions and combinations under the acquisition method of accounting, where the net
assets of businesses purchased are recorded at their fair value at the acquisition date and our consolidated financial
statements include their results of operations from that date. Any excess of acquisition consideration over the fair
value of identifiable net assets acquired is recorded as goodwill. The major classes of assets and liabilities that we
generally allocate purchase price to, excluding goodwill, include identifiable intangible assets (i.e., trademarks and
trade names, customer relationships and lists, non-compete agreements and product development), property, plant
and equipment, deferred taxes and other current and long-term assets and liabilities. The estimated fair value of
identifiable intangible assets is based on critical estimates, judgments and assumptions derived from: analysis of
market conditions; discount rates; discounted cash flows; customer retention rates; and estimated useful lives.
Some prior owners of such acquired subsidiaries are eligible to receive additional purchase price cash consideration
if certain financial targets are met. While we use our best estimates and assumptions to accurately value those
assets acquired and liabilities assumed at the acquisition date as well as contingent consideration, where applicable,
our estimates are inherently uncertain and subject to refinement. As a result, during the measurement period we
may record adjustments to the assets acquired and liabilities assumed with the corresponding offset to goodwill
within our consolidated balance sheets. At the end of the measurement period or final determination of the values
of such assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recognized in
our consolidated statements of operations. For the years ended December 26, 2020, December 28, 2019 and
December 29, 2018, there were no material adjustments recorded in our consolidated statement of income relating
to changes in subsequent adjustments or estimated contingent purchase price liabilities.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
83
Redeemable Noncontrolling Interests
Some minority stockholders in certain of our subsidiaries have the right, at certain times, to require us to acquire
their ownership interest in those entities at fair value. Their interests in these subsidiaries are classified outside
permanent equity on our consolidated balance sheets and are carried at the estimated redemption amounts. The
redemption amounts have been estimated based on expected future earnings and cash flow and, if such earnings and
cash flow are not achieved, the value of the redeemable noncontrolling interests might be impacted. Changes in the
estimated redemption amounts of the noncontrolling interests subject to put options are reflected at each reporting
period with a corresponding adjustment to Additional paid-in capital. Future reductions in the carrying amounts are
subject to a “floor” amount that is equal to the fair value of the redeemable noncontrolling interests at the time they
were originally recorded. The recorded value of the redeemable noncontrolling interests cannot go below the floor
level. These adjustments do not impact the calculation of earnings per share.
Noncontrolling Interests
Noncontrolling interests represent our less than 50% ownership interest in an acquired subsidiary. Our net income
is reduced by the portion of the subsidiaries net income that is attributable to noncontrolling interests.
Goodwill
Goodwill is not amortized, but is subject to impairment analysis annually or more frequently if there is a triggering
event or if an event occurs or circumstances change that would more likely than not reduce the fair value of a
reporting unit below its carrying value. Such impairment analyses for goodwill requires a comparison of the fair
value to the carrying value of reporting units. We regard our reporting units to be our operating segments: global
dental; global medical; and technology and value-added services. Goodwill was allocated to such reporting units,
for the purposes of preparing our impairment analyses, based on a specific identification basis.
On December 29, 2019 we adopted Account Standards Update (“ASU”) 2017-04 Intangibles-Goodwill and Other
(Topic 350): Simplifying the Test for Goodwill Impairment, which eliminated step two from the goodwill
impairment test, thereby eliminating the requirement to calculate the implied fair value of a reporting unit. We
perform our annual goodwill impairment test by comparing the fair value of our reporting units to the carrying
value of those units. Goodwill as of December 26, 2019 and December 29, 2018 were tested under the prior
standard.
For the year ended December 26, 2020 we tested goodwill for impairment, on the first day of the fourth quarter,
using a quantitative analysis comparing the carrying value of our reporting units, including goodwill, to the
estimated fair value of our reporting units using a discounted cash flow methodology. If the fair value of a reporting
unit exceeds its carrying amount, goodwill of the reporting unit is considered not impaired. Conversely,
impairment loss would be equivalent to the excess of a reporting unit’s carrying value over its fair value limited to
the total amount of goodwill allocated to that reporting unit.
For the years ended December 26, 2019 and December 29, 2018 we tested goodwill for impairment on the first day
of the fourth quarter, using a quantitative analysis which consisted of a two-step approach. The first step of our
quantitative analysis consisted of a comparison of the carrying value of our reporting units, including goodwill, to
the estimated fair value of our reporting units using a discounted cash flow methodology. If step one resulted in the
carrying value of the reporting unit exceeding the fair value of such reporting unit, we would have then proceeded
to step two which would have required us to calculate the amount of impairment loss, if any, that we would have
recorded for such reporting unit. The calculation of the impairment loss in step two would have been equivalent to
the reporting unit’s carrying value of goodwill less the implied fair value of such goodwill.
Our use of a discounted cash flow methodology includes estimates of future revenue based upon budget projections
and growth rates which take into account estimated inflation rates. We also develop estimates for future levels of
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
84
gross and operating profits and projected capital expenditures. Our methodology also includes the use of estimated
discount rates based upon industry and competitor analysis as well as other factors. The estimates that we use in our
discounted cash flow methodology involve many assumptions by management that are based upon future growth
projections. Some factors we consider important that could trigger an interim impairment review include:
significant underperformance relative to expected historical or projected future operating results; significant
changes in the manner of our use of acquired assets or the strategy for our overall business (e.g., decision to divest a
business); or significant negative industry or economic trends.
If we determine through the impairment review process that goodwill is impaired, we record an impairment charge
in our consolidated statements of income. For the years ended December 28, 2019 and December 29, 2018, the
results of our goodwill impairment analysis did
t result in any impairments.
Long-Lived Assets
Long-lived assets, other than goodwill and other definite-lived intangibles, are evaluated for impairment whenever
events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable through
the estimated undiscounted future cash flows to be derived from such assets.
Definite-lived intangible assets primarily consist of non-compete agreements, trademarks, trade names, customer
lists, customer relationships and intellectual property. For long-lived assets used in operations, impairment losses
are only recorded if the asset’s carrying amount is not recoverable through its undiscounted, probability-weighted
future cash flows. We measure the impairment loss based on the difference between the carrying amount and the
estimated fair value. When an impairment exists, the related assets are written down to fair value.
During the year ended December 26, 2020, we recorded total impairment charges on intangible assets of
approximately $
Cost of Sales
The primary components of cost of sales include the cost of the product (net of purchase discounts, supplier
chargebacks and rebates) and inbound and outbound freight charges. Costs related to purchasing, receiving,
inspections, warehousing, internal inventory transfers and other costs of our distribution network are included in
selling, general and administrative expenses along with other operating costs.
As a result of different practices of categorizing costs associated with distribution networks throughout our
industry, our gross margins may not necessarily be comparable to other distribution companies. Total distribution
network costs were $
December 28, 2019 and December 29, 2018.
Comprehensive Income
Comprehensive income includes certain gains and losses that, under accounting principles generally accepted in the
United States, are excluded from net income as such amounts are recorded directly as an adjustment to
stockholders’ equity. Our comprehensive income is primarily comprised of net income, foreign currency
translation gain (loss), unrealized gain (loss) from foreign currency hedging activities, unrealized investment gain
(loss) and pension adjustment gain (loss).
Leases
On December 30, 2018, we adopted ASC Topic 842, Leases, using a modified retrospective approach, whereby
we continue to apply existing lease guidance during the comparative periods and apply the new lease
requirements through a cumulative-effect adjustment in the period of adoption. We elected the package of
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
85
practical expedients permitted under the transition guidance within the new standard, which, among other things,
allowed us to carry forward the historical lease classification. Information related to leases as of December 28,
2019 is presented under Topic 842, while prior period amounts are not adjusted and continue to be reported under
legacy guidance in ASC Topic 840, Leases.
The most significant impact was the recognition of ROU assets and lease liabilities for operating leases, while our
accounting for finance leases remained substantially unchanged. Adoption of the new standard resulted in the
recording of additional net operating lease assets of $
and a decrease of $
standard did not materially impact our consolidated net income and had no impact on cash flows.
We determine if an arrangement contains a lease at inception. An arrangement contains a lease if it implicitly or
explicitly identifies an asset to be used and conveys the right to control the use of the identified asset in exchange
for consideration. As a lessee, we include operating leases in Operating lease right-of-use (“ROU”) assets,
Operating lease liabilities, and Non-current operating lease liabilities in our consolidated balance sheet. Finance
leases are included in Property and equipment, Current maturities of long-term debt, and Long-term debt in our
consolidated balance sheet.
ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our
obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized
upon commencement of the lease based on the present value of the lease payments over the lease term. As most of
our leases do not provide an implicit interest rate, we generally use our incremental borrowing rate based on the
estimated rate of interest for fully collateralized and fully amortizing borrowings over a similar term of the lease
payments at commencement date to determine the present value of lease payments. When readily determinable, we
use the implicit rate.
over the lease term. Expenses associated with operating leases and finance leases are included in “Selling, general
and administrative” and “Interest expense”, respectively within our Consolidated Statement of Income.
Leases
with a lease term of 12 months or less are not capitalized.
We have lease agreements with lease and non-lease components, which are generally accounted for as a single
lease component, except non-lease components for leases of vehicles, which are accounted for separately. When a
vehicle lease contains both lease and non-lease components, we allocate the transaction price based on the relative
standalone selling price.
Accounting Pronouncements Adopted
On December 29, 2019, we adopted ASU No. 2017-04, “Intangibles-Goodwill and Other” (Topic 350) (“ASU
2017-04”). ASU 2017-04 eliminates step two from the goodwill impairment test, thereby eliminating the
requirement to calculate the implied fair value of a reporting unit. ASU 2017-04 requires us to perform our annual
goodwill impairment test by comparing the fair value of our reporting units to the carrying value of those units. If
the carrying value exceeds the fair value, we will be required to recognize an impairment charge; however, the
impairment charge should not exceed the amount of goodwill allocated to such reporting unit. Our adoption of
ASU 2017-04 did not have a material impact on our consolidated financial statements.
On December 29, 2019, we adopted ASU No. 2016-13, "Financial Instruments-Credit Losses (Topic 326):
Measurement of Credit Losses on Financial Instruments" which requires the measurement and recognition of
expected credit losses for financial assets held at amortized cost. We adopted Topic 326 using the modified-
retrospective method and recorded an immaterial cumulative-effect adjustment to the opening balance of retained
earnings. Based upon the level and makeup of our financial asset portfolio, including accounts receivable, past loan
loss activity and current known activity regarding our outstanding loans, the adoption of this ASU resulted in a
decrease of $
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
86
Recently Issued Accounting Standards
In December 2019, the FASB issued ASU No. 2019-12, “Income Taxes” (Topic 740): Simplifying the Accounting
for Income Taxes (“ASU 2019-12”). ASU 2019-12 will simplify the accounting for income taxes by removing
certain exceptions to the general principles in Topic 740. The amendments also improve consistent application of
and simplify U.S. GAAP for other areas of Topic 740 by clarifying and amending existing guidance. ASU 2019-12
is effective for fiscal years beginning after December 15, 2020. We do not expect that the requirements of this
ASU will have a material impact on our consolidated financial statements.
In August 2020, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2020-06, “Debt—Debt with
Conversion and Other Options” (Subtopic 470-20) and “Derivatives and Hedging— in Entity’s Own Equity”
(Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-
06”). ASU 2020-06 simplifies the accounting for convertible instruments. In addition to eliminating certain
accounting models, this ASU includes improvements to the disclosures for convertible instruments and earnings-
per-share (EPS) guidance and amends the guidance for the derivatives scope exception for contracts in an entity’s
own equity. ASU 2020-06 is effective for fiscal years beginning after December 15, 2021. We do not expect that
the requirements of this ASU will have a material impact on our consolidated financial statements.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
87
Note 2 – Discontinued Operations
Animal Health Spin-off
On February 7, 2019 (the “Distribution Date”), we completed the separation (the “Separation”) and subsequent
merger (“Merger”) of our animal health business (the “Henry Schein Animal Health Business”) with Direct Vet
Marketing, Inc. (d/b/a Vets First Choice, “Vets First Choice”). This was accomplished by a series of transactions
among us, Vets First Choice, Covetrus, Inc. (f/k/a HS Spinco, Inc. “Covetrus”), a wholly owned subsidiary of ours
prior to the Distribution Date, and HS Merger Sub, Inc., a wholly owned subsidiary of Covetrus (“Merger
Sub”). In connection with the Separation, we contributed, assigned and transferred to Covetrus certain applicable
assets, liabilities and capital stock or other ownership interests relating to the Henry Schein Animal Health
Business. On the Distribution Date, we received a tax-free distribution of $
certain debt financing incurred by Covetrus. On the Distribution Date and prior to the Animal Health Spin-off,
Covetrus issued shares of Covetrus common stock to certain institutional accredited investors (the “Share Sale
Investors”) for $
distributed to us. Subsequent to the Share Sale, we distributed, on a pro rata basis, all of the shares of the common
stock of Covetrus held by us to our stockholders of record as of the close of business on January 17, 2019 (the
“Animal Health Spin-off”). After the Share Sale and Animal Health Spin-off, Merger Sub consummated the
Merger whereby it merged with and into Vets First Choice, with Vets First Choice surviving the Merger as a
wholly owned subsidiary of Covetrus. Immediately following the consummation of the Merger, on a fully diluted
basis, (i) approximately
% of the shares of Covetrus common stock were (a) owned by our stockholders and the
Share Sale Investors, and (b) held by certain employees of the Henry Schein Animal Health Business (in the form
of certain equity awards), and (ii) approximately
% of the shares of Covetrus common stock were (a) owned by
stockholders of Vets First Choice immediately prior to the Merger, and (b) held by certain employees of Vets First
Choice (in the form of certain equity awards). After the Separation and the Merger, we no longer beneficially
owned any shares of Covetrus common stock and, following the Distribution Date, will not consolidate the
financial results of Covetrus for the purpose of our financial reporting. Following the Separation and the Merger,
Covetrus was an independent, publicly traded company on the Nasdaq Global Select Market.
In connection with the completion of the Animal Health Spin-off, we entered into a transition services agreement,
which ended in December 2020, with Covetrus under which we agreed to provide certain transition services for up
to twenty-four months in areas such as information technology, finance and accounting, human resources, supply
chain, and real estate and facility services.
As a result of the Separation, the financial position and results of operations of the Henry Schein Animal Health
Business are presented as discontinued operations and have been excluded from continuing operations and segment
results for all periods presented. The accompanying Notes to the Consolidated Financial Statements have been
revised to reflect the effect of the Separation and all prior year balances have been revised accordingly to reflect
continuing operations only. The historical statements of Comprehensive Income (Loss) and Shareholders' Equity
have not been revised to reflect the Separation and instead reflect the Separation as an adjustment to the balances at
December 26, 2020.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
88
Summarized financial information for our discontinued operations is as follows:
Years Ended
December 26,
December 28,
December 29,
2020
2019
2018
Net sales
$
$
$
Cost of goods sold
Gross profit
Selling, general and administrative
Operating income (loss)
(2,347 )
(9,494 )
Income tax expense (benefit)
(3,333 )
(2,181 )
Income (loss) from discontinued operations
(6,323 )
Net (income) loss attributable to noncontrolling interests
(6,521 )
Net income (loss) from discontinued operations
(5,957 )
The operating loss from discontinued operations for the year ended December 26, 2020 was primarily attributable
to costs directly related to the Animal Health Spin-off.
information.
The net income from discontinued operations for the year ended December 26, 2020 was primarily attributable to a
reduction in a liability for tax indemnification and a tax refund received during 2020 by a holding company
previously part of our Animal Health legal structure and other favorable tax resolutions.
The financial information above, for the year ended December 28, 2019, represents activity of the discontinued
operations during year-to-date through the Distribution Date. The loss from discontinued operations for the year
ended December 28, 2019 was primarily attributable to the inclusion of the transaction costs directly related to the
Animal Health Spin-off.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
89
The following are the amounts of assets and liabilities that were transferred to Covetrus as of February 7, 2019.
February 7,
2019
Cash and cash equivalents
$
Accounts receivable, net
Inventories, net
Prepaid expenses and other
Total current assets of discontinued operations
Property and equipment, net
Operating lease right-of-use asset, net
Goodwill
Other intangibles, net
Investments and other
Total long-term assets of discontinued operations
Total assets of discontinued operations
$
Accounts payable
$
Current maturities of long-term debt
Operating lease liabilities
Accrued expenses:
Payroll and related
Taxes
Other
Total current liabilities of discontinued operations
Long-term debt
Deferred income taxes
Operating lease liabilities
Other liabilities
Total long-term liabilities of discontinued operations
Total liabilities of discontinued operations
$
Redeemable noncontrolling interests
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
90
Note 3 – Property and Equipment, Net
Property and equipment, including related estimated useful lives, consisted of the following:
December 26,
December 28,
2020
2019
Land
$
$
Buildings and permanent improvements
Leasehold improvements
Machinery and warehouse equipment
Furniture, fixtures and other
Computer equipment and software
Less accumulated depreciation
(526,178 )
(468,946 )
Property and equipment, net
$
$
Estimated Useful
Lives (in years)
Buildings and permanent improvements
Machinery and warehouse equipment
-
Furniture, fixtures and other
-
Computer equipment and software
-
Property and equipment related depreciation expense for the years ended December 26, 2020, December 28, 2019
and December 29, 2018 was $
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
91
Note 4 – Goodwill and Other Intangibles, Net
The changes in the carrying amount of goodwill for the years ended December 26, 2020 and December 28, 2019
were as follows:
Health Care
Distribution
Technology and
Value-Added
Services
Total
Balance as of December 29, 2018
$
$
$
Adjustments to goodwill:
Acquisitions
Foreign currency translation
(6,969 )
(193 )
(7,162 )
Balance as of December 28, 2019
Adjustments to goodwill:
Acquisitions
Foreign currency translation
Balance as of December 26, 2020
$
$
$
Other intangible assets consisted of the following:
December 26, 2020
December 28, 2019
Accumulated
Accumulated
Cost
Amortization
Net
Cost
Amortization
Net
Non-compete agreements
$
$
(11,480 )
$
$
$
(9,327 )
$
Trademarks / trade names - definite lived
(50,893 )
(44,134 )
Customer relationships and lists
(283,469 )
(274,330 )
Product Development
(54,451 )
(42,326 )
Other
(7,662 )
(17,950 )
$
$
(407,955 )
$
$
$
(388,067 )
$
Non-compete agreements represent amounts paid primarily to key employees and prior owners of acquired
businesses, as well as certain sales persons, in exchange for placing restrictions on their ability to pose a
competitive risk to us. Such amounts are amortized, on a straight-line basis over the respective non-compete
period, which generally commences upon termination of employment or separation from us. The weighted-average
non-compete period for agreements currently being amortized was approximately
2020.
Trademarks, trade names, customer lists and customer relationships were established through business acquisitions.
Definite-lived trademarks and trade names are amortized on a straight-line basis over a weighted-average period of
approximately
intangible assets that are amortized on a straight-line basis over a weighted-average period of approximately
years as of December 26, 2020. Product development is a definite-lived intangible asset that is amortized on a
straight-line basis over a weighted-average period of approximately
Amortization expense related to definite-lived intangible assets for the years ended December 26, 2020, December
28, 2019 and December 29, 2018 was $
December 26, 2020, we recorded total impairment charges on intangible assets of approximately $
The annual amortization expense expected to be recorded for existing intangibles assets for the years 2021 through
2025 is $
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
92
Note 5 – Investments and Other
Investments and other consisted of the following:
December 26,
December 28,
2020
2019
Investment in unconsolidated affiliates
$
$
Non-current deferred foreign, state and local income taxes
Notes receivable
(1)
Capitalized costs for internally generated software for resale
Security deposits
Acquisition-related indemnification
Other long-term assets
Total
$
$
(1)
Long-term notes receivable carry interest rates ranging from
.0% to
.0% and are due in varying installments through
September 30, 2027
.
Amortization expense related to other long-term assets for the years ended December 26, 2020, December 28, 2019
and December 29, 2018 was $
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
93
Note 6 – Debt
Bank Credit Lines
Bank credit lines consisted of the following:
December 26,
December 28,
2020
2019
Revolving credit agreement
$
$
Other short-term bank credit lines
Total
$
$
Revolving Credit Agreement
On
, we entered into a $
matures in April 2022. The interest rate is based on the USD LIBOR plus a spread based on our leverage ratio at
the end of each financial reporting quarter. We expect the LIBOR rate to be discontinued at some point
during 2021, which will require an amendment to our debt agreements to reflect a new reference rate. We do not
expect the discontinuation of LIBOR as a reference rate in our debt agreements to have a material adverse effect on
our financial position or to materially affect our interest expense. The Credit Agreement also requires, among other
things, that we maintain maximum leverage ratios. Additionally, the Credit Agreement contains customary
representations, warranties and affirmative covenants as well as customary negative covenants, subject to
negotiated exceptions on liens, indebtedness, significant corporate changes (including mergers), dispositions and
certain restrictive agreements. As of December 26, 2020 and December 28, 2019, we had
revolving credit facility. As of December 26, 2020 and December 28, 2019, there were $
million of letters of credit, respectively, provided to third parties under the credit facility.
On April 17, 2020, we amended the Credit Agreement to, among other things, (i) modify the financial covenant
from being based on total leverage ratio to net leverage ratio, (ii) adjust the pricing grid to reflect the net leverage
ratio calculation, and (iii) increase the maximum maintenance leverage ratio through March 31, 2021.
364-Day Credit Agreement
On
, we entered into a new $
-day credit agreement, with JPMorgan Chase Bank,
N.A. and U.S. Bank National Association as joint lead arrangers and joint bookrunners. This facility matures on
. As of December 26, 2020, we had
borrow up to an additional $
on a revolving basis as needed, subject to the terms and conditions of the credit agreement.
. Under the
terms of this agreement, we are prohibited from repurchasing our common stock until we report our financial
results for the second quarter of 2021.
Other Short-Term Credit Lines
As of December 26, 2020 and December 28, 2019, we had various other short-term bank credit lines available, of
which $
2019, borrowings under all of these credit lines had a weighted average interest rate of
% and
%,
respectively.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
94
Long-term debt
Long-term debt consisted of the following:
December 26,
December 28,
2020
2019
Private placement facilities
$
$
U.S. trade accounts receivable securitization
Note payable due in
%
at December 26, 2020
Various collateralized and uncollateralized loans payable with interest,
in varying installments through
ranging from
% to
% at December 26, 2020 and
ranging from
% to
% at December 28, 2019
Finance lease obligations (see Note 7)
Total
Less current maturities
(109,836 )
(109,849 )
Total long-term debt
$
$
Private Placement Facilities
Our private placement facilities, with three insurance companies, have a total facility amount of $
available on an uncommitted basis at fixed rate economic terms to be agreed upon at the time of issuance, from
time to time through
. The facilities allow us to issue senior promissory notes to the lenders at a fixed
rate based on an agreed upon spread over applicable treasury notes at the time of issuance. The term of each
possible issuance will be selected by us and can range from
five
). The proceeds of any issuances under the facilities will be used for general corporate purposes, including
working capital and capital expenditures, to refinance existing indebtedness and/or to fund potential acquisitions.
On June 29, 2018, we amended and restated the above private placement facilities to, among other things, (i) permit
the consummation of the Animal Health Spin-off and (ii) provide for the issuance of notes in Euros, British Pounds
and Australian Dollars, in addition to U.S. Dollars. The agreements provide, among other things, that we maintain
certain maximum leverage ratios, and contain restrictions relating to subsidiary indebtedness, liens, affiliate
transactions, disposal of assets and certain changes in ownership. These facilities contain make-whole provisions in
the event that we pay off the facilities prior to the applicable due dates.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
95
The components of our private placement facility borrowings as of December 26, 2020 are presented in the
following table (in thousands):
Amount of
Date of
Borrowing
Borrowing
Borrowing
Outstanding
Rate
Due Date
January 20, 2012
$
%
January 20, 2022
January 20, 2012
January 20, 2024
December 24, 2012
December 24, 2024
June 2, 2014
June 2, 2021
June 16, 2017
June 16, 2027
September 15, 2017
September 15, 2029
January 2, 2018
January 2, 2028
September 2, 2020
September 2, 2030
Less: Deferred debt issuance costs
(788 )
$
(1)
.
(2) On
, we refinanced our $
%, originally due on September 2, 2020,
with a similar
% maturing on
.
U.S. Trade Accounts Receivable Securitization
We have a facility agreement with a bank, as agent, based on the securitization of our U.S. trade accounts
receivable that is structured as an asset-backed securitization program with pricing committed for up to
.
Our current facility, which has a purchase limit of $
. On
June 22, 2020, the expiration date for this facility was extended to
covenant levels for 2020. As of December 26, 2020 and December 28, 2019, the borrowings outstanding under this
securitization facility were $
borrowings under this facility was based on the asset-backed commercial paper rate of
% plus
%, for a
combined rate of
%. At December 28, 2019, the interest rate on borrowings under this facility was based on
the asset-backed commercial paper rate of
% plus
%, for a combined rate of
%.
If our accounts receivable collection pattern changes due to customers either paying late or not making payments,
our ability to borrow under this facility may be reduced.
We are required to pay a commitment fee of
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
96
As of December 26, 2020, the aggregate amounts of long-term debt, including finance lease obligations and net of
deferred debt issuance costs of $
2021
$
2022
2023
2024
2025
Thereafter
Total
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
97
Note 7 – Leases
Leases
, some of
which may include options to extend the leases for
. The components of lease expense were as
follows:
Years Ended
December 26,
December 28,
2020
2019
Operating lease cost:
$
$
Finance lease cost:
Amortization of right-of-use assets
Interest on lease liabilities
Total finance lease cost
$
$
(1)
Includes variable lease expenses.
(2)
Operating lease cost for each of the years ended December 26, 2020, and December 28, 2019, includes amortization of right-of-use
assets of $
Supplemental balance sheet information related to leases is as follows:
Years Ended
December 26,
December 28,
2020
2019
Operating Leases:
Operating lease right-of-use assets
$
$
Current operating lease liabilities
Non-current operating lease liabilities
Total operating lease liabilities
$
$
Finance Leases:
Property and equipment, at cost
$
$
Accumulated depreciation
(4,277 )
(4,581 )
Property and equipment, net of accumulated depreciation
$
$
Current maturities of long-term debt
$
$
Long-term debt
Total finance lease liabilities
$
$
Weighted Average Remaining Lease Term in Years:
Operating leases
Finance leases
Weighted Average Discount Rate:
Operating leases
%
%
Finance leases
%
%
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
98
Supplemental cash flow information related to leases is as follows:
Years Ended
December 26,
December 28,
2020
2019
Cash paid for amounts included in the measurement of lease liabilities:
Operating cash flows for operating leases
$
Operating cash flows for finance leases
Financing cash flows for finance leases
Right-of-use assets obtained in exchange for lease obligations:
Operating leases
$
Finance leases
Maturities of lease liabilities are as follows:
December 26, 2020
Operating
Finance
Leases
Leases
2021
$
$
2022
2023
2024
2025
Thereafter
Total future lease payments
Less imputed interest
(32,351 )
(232 )
Total
$
$
As of December 26, 2020, we have additional operating leases with total lease payments of $
December 26, 2020, with lease terms of
.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
99
Note 8 – Redeemable Noncontrolling Interests
Some minority stockholders in certain of our subsidiaries have the right, at certain times, to require us to acquire
their ownership interest in those entities at fair value. ASC 480-10 is applicable for noncontrolling interests where
we are or may be required to purchase all or a portion of the outstanding interest in a consolidated subsidiary from
the noncontrolling interest holder under the terms of a put option contained in contractual agreements. The
components of the change in the Redeemable noncontrolling interests for the years ended December 26, 2020,
December 28, 2019 and December 29, 2018 are presented in the following table:
December 26,
December 28,
December 29,
2020
2019
2018
Balance, beginning of period
$
$
$
Decrease in redeemable noncontrolling interests due to
redemptions
(17,241 )
(2,270 )
(287,767 )
Increase in redeemable noncontrolling interests due to
business acquisitions
Net income attributable to redeemable noncontrolling interests
Dividends declared
(12,631 )
(10,264 )
(8,206 )
Effect of foreign currency translation loss attributable to
redeemable noncontrolling interests
(4,279 )
(2,335 )
(11,330 )
Change in fair value of redeemable securities
(7,300 )
Balance, end of period
$
$
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
100
Note 9 – Comprehensive Income
Comprehensive income includes certain gains and losses that, under U.S. GAAP, are excluded from net income as
such amounts are recorded directly as an adjustment to stockholders’ equity.
The following table summarizes our Accumulated other comprehensive loss, net of applicable taxes as of:
December 26,
December 28,
December 29,
2020
2019
2018
Attributable to Redeemable noncontrolling interests:
Foreign currency translation adjustment
$
(24,617 )
$
(20,338 )
$
(18,595 )
Attributable to noncontrolling interests:
Foreign currency translation adjustment
$
$
(531 )
$
(426 )
Attributable to Henry Schein, Inc.:
Foreign currency translation adjustment
$
(76,565 )
$
(143,172 )
$
(234,799 )
Unrealized loss from foreign currency hedging activities
(11,488 )
(4,032 )
(156 )
Unrealized investment gain (loss)
(6 )
Pension adjustment loss
(20,032 )
(20,175 )
(13,810 )
Accumulated other comprehensive loss
$
(108,084 )
$
(167,373 )
$
(248,771 )
Total Accumulated other comprehensive loss
$
(132,466 )
$
(188,242 )
$
(267,792 )
The following table summarizes the components of comprehensive income, net of applicable taxes as follows:
December 26,
December 28,
December 29,
2020
2019
2018
Net income
$
$
$
Foreign currency translation gain (loss)
(4,070 )
(136,356 )
Tax effect
Foreign currency translation gain (loss)
(4,070 )
(136,356 )
Unrealized gain (loss) from foreign currency hedging activities
(10,224 )
(4,911 )
Tax effect
(396 )
Unrealized gain (loss) from foreign currency hedging activities
(7,456 )
(3,876 )
Unrealized investment gain (loss)
(6 )
(3 )
Tax effect
(2)
Unrealized investment gain (loss)
(5 )
(3 )
Pension adjustment gain (loss)
(533 )
(7,730 )
Tax effect
(1,179 )
Pension adjustment gain (loss)
(5,924 )
Comprehensive income
$
$
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
101
Our financial statements are denominated in the U.S. Dollar currency. Fluctuations in the value of foreign
currencies as compared to the U.S. Dollar may have a significant impact on our comprehensive income. The
foreign currency translation gain (loss) during the years ended December 26, 2020, December 28, 2019 and
December 29, 2018 was impacted by changes in foreign currency exchange rates of the Euro, Brazilian Real,
British Pound and Australian Dollar
.
The following table summarizes our total comprehensive income, net of applicable taxes as follows:
December 26,
December 28,
December 29,
2020
2019
2018
Comprehensive income attributable to
Henry Schein, Inc.
$
$
$
Comprehensive income attributable to
noncontrolling interests
Comprehensive income attributable to
Redeemable noncontrolling interests
Comprehensive income
$
$
$
Note 10 – Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly
transaction between market participants at the measurement date. The hierarchy for determining that distinguishes
between (1) market participant assumptions developed based on market data obtained from independent sources
(observable inputs) and (2) an entity’s own assumptions about market participant assumptions developed based on
the best information available in the circumstances (unobservable inputs).
The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices
in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3).
The three levels of the fair value hierarchy are described as follows:
• Level 1— Unadjusted quoted prices in active markets for identical assets or liabilities that are accessible at the
measurement date.
• Level 2— Inputs other than quoted prices included within Level 1 that are observable for the asset or liability,
either directly or indirectly. Level 2 inputs include: quoted prices for similar assets or liabilities in active markets;
quoted prices for identical or similar assets or liabilities in markets that are not active; inputs other than quoted
prices that are observable for the asset or liability; and inputs that are derived principally from or corroborated by
observable market data by correlation or other means.
• Level 3— Inputs that are unobservable for the asset or liability.
The following section describes the fair values of our financial instruments and the methodologies that we used to
measure their fair values.
Investments and notes receivable
There are no quoted market prices available for investments in unconsolidated affiliates and notes receivable;
however, we believe the carrying amounts are a reasonable estimate of fair value based on the interest rates in the
applicable markets.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
102
Debt
The fair value of our debt (including bank credit lines) is classified as Level 3 within the fair value hierarchy and as
of December 26, 2020 and December 28, 2019 was estimated at $
Factors that we considered when estimating the fair value of our debt include market conditions, such as interest
rates and credit spreads.
Derivative contracts
Derivative contracts are valued using quoted market prices and significant other observable and unobservable
inputs. We use derivative instruments to minimize our exposure to fluctuations in foreign currency exchange
rates. Our derivative instruments primarily include foreign currency forward agreements related to certain
intercompany loans, certain forecasted inventory purchase commitments with foreign suppliers and foreign
currency forward contracts to hedge a portion of our euro-denominated foreign operations which are designated as
net investment hedges; and a total return swap for the purpose of economically hedging our unfunded non-qualified
SERP and our DCP.
The fair values for the majority of our foreign currency derivative contracts are obtained by comparing our contract
rate to a published forward price of the underlying market rates, which is based on market rates for comparable
transactions and are classified within Level 2 of the fair value hierarchy.
Redeemable noncontrolling interests
The values for Redeemable noncontrolling interests are classified within Level 3 of the fair value hierarchy and are
based on recent transactions and/or implied multiples of earnings.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
103
The following table presents our assets and liabilities that are measured and recognized at fair value on a recurring
basis classified under the appropriate level of the fair value hierarchy as of December 26, 2020 and December 28,
2019:
December 26, 2020
Level 1
Level 2
Level 3
Total
Assets:
Derivative contracts
$
$
$
$
Total return swaps
Total assets
$
$
$
$
Liabilities:
Derivative contracts
$
$
$
$
Total liabilities
$
$
$
$
Redeemable noncontrolling interests
$
$
$
$
December 28, 2019
Level 1
Level 2
Level 3
Total
Assets:
Derivative contracts
$
$
$
$
Total assets
$
$
$
$
Liabilities:
Derivative contracts
$
$
$
$
Total liabilities
$
$
$
$
Redeemable noncontrolling interests
$
$
$
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
104
Note 11 – Business Acquisitions and Divestitures
The operating results of all acquisitions are reflected in our financial statements from their respective acquisition
dates.
We completed acquisitions during the year ended December 26, 2020, which were immaterial to our financial
statements individually. In the aggregate, these transactions resulted in consideration of $
related to business combinations, for net assets amounting to $
recorded $
interest, related to these acquisitions.
Some prior owners of acquired subsidiaries are eligible to receive additional purchase price cash consideration if
certain financial targets are met. We have accrued liabilities for the estimated fair value of additional purchase
price consideration at the time of the acquisition, none of which are material. Any adjustments to these accrual
amounts are recorded in our consolidated statements of income. For the years ended December 26, 2020,
December 28, 2019 and December 29, 2018, there were no material adjustments recorded in our consolidated
statement of income relating to changes in estimated contingent purchase price liabilities.
Divestitures of Investments
During the fourth quarter of 2019, we sold an equity investment in Hu-Friedy Mfg. Co., LLC, a manufacturer of
dental instruments and infection prevention solutions. Our investment was non-controlling, we were not involved
in running the business and had no representation on the board of directors. During the fourth quarter of 2019, we
also sold certain other equity investments. In the aggregate, the sales of these investments resulted in a pre-tax gain
of approximately $
received contingent proceeds of $
additional after-tax gain of $1.6 million. For the years ended December 28, 2019 and December 29, 2018, we
recognized approximately $
Acquisition Costs
During the years ended December 26, 2020, December 28, 2019, and December 29, 2018 we incurred $
$
In February 2019, we completed the Animal Health Spin-off. During the years ended December 26, 2020,
December 28, 2019, and December 29, 2018, we incurred $
transaction costs associated with this transaction. We do not expect to incur additional spin-off related transaction
costs after December 26, 2020. All transaction costs related to the Animal Health Spin-off have been included in
results from discontinued operations.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
105
Note 12 – Plans of Restructuring
On July 9, 2018, we committed to an initiative to rationalize our operations and provide expense
efficiencies. These actions allowed us to execute on our plan to reduce our cost structure and fund new initiatives
to drive growth under our 2018 to 2020 strategic plan. This initiative resulted in the elimination of approximately
% of our workforce and the closing of certain facilities.
On November 20, 2019, we committed to a contemplated initiative, intended to mitigate stranded costs associated
with the Animal Health Spin-off and to rationalize operations and to provide expense efficiencies. These activities
were originally expected to be completed by the end of 2020. As a result of the business environment brought on
by the COVID-19 pandemic, we are continuing our restructuring activities into 2021. We are currently unable in
good faith to make a determination of an estimate of the amount or range of amounts expected to be incurred in
connection with these activities in 2021, both with respect to each major type of cost associated therewith and with
respect to the total cost, or an estimate of the amount or range of amounts that will result in future cash
expenditures.
During the years ended December 26, 2020, December 28, 2019, and December 29, 2018 we recorded restructuring
charges of $
restructurings are included in a separate line item, “Restructuring costs” within our consolidated statements of
income.
The following table shows the amounts expensed and paid for restructuring costs that were incurred during our
2020, 2019 and 2018 fiscal years and the remaining accrued balance of restructuring costs as of December 26,
2020, which is included in Accrued expenses: Other and Other liabilities within our consolidated balance sheet:
Facility
Severance
Closing
Costs
Costs
Other
Total
Balance, December 30, 2017
$
$
$
$
Provision
Payments and other adjustments
(23,320 )
(2,865 )
(883 )
(27,068 )
Balance, December 29, 2018
$
$
$
$
Provision
Payments and other adjustments
(30,794 )
(1,714 )
(112 )
(32,620 )
Balance, December 28, 2019
$
$
$
$
Provision
Payments and other adjustments
(26,152 )
(6,309 )
(329 )
(32,790 )
Balance, December 26, 2020
$
$
$
$
The following table shows, by reportable segment, the amounts expensed and paid for restructuring costs that were
incurred during our 2020, 2019 and 2018 fiscal years and the remaining accrued balance of restructuring costs as of
December 26, 2020:
Technology and
Health Care
Value-Added
Distribution
Services
Total
Balance, December 30, 2017
$
$
$
Provision
Payments and other adjustments
(24,959 )
(2,109 )
(27,068 )
Balance, December 29, 2018
$
$
$
Provision
Payments and other adjustments
(30,853 )
(1,767 )
(32,620 )
Balance, December 28, 2019
$
$
$
Provision
Payments and other adjustments
(31,484 )
(1,306 )
(32,790 )
Balance, December 26, 2020
$
$
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
106
Note 13 – Earnings Per Share
Basic earnings per share is computed by dividing net income attributable to Henry Schein, Inc. by the weighted-
average number of common shares outstanding for the period. Our diluted earnings per share is computed similarly
to basic earnings per share, except that it reflects the effect of common shares issuable for presently unvested
restricted stock and restricted stock units and upon exercise of stock options, using the treasury stock method in
periods in which they have a dilutive effect.
A reconciliation of shares used in calculating earnings per basic and diluted share follows:
Years Ended
December 26,
December 28,
December 29,
2020
2019
2018
Basic
Effect of dilutive securities:
Stock options, restricted stock and restricted stock units
Diluted
Note 14 – Income Taxes
Income before taxes and equity in earnings of affiliates was as follows:
Years ended
December 26,
December 28,
December 29,
2020
2019
2018
Domestic
$
$
$
Foreign
Total
$
$
$
The provisions for income taxes were as follows:
Years ended
December 26,
December 28,
December 29,
2020
2019
2018
Current income tax expense:
U.S. Federal
$
$
$
State and local
Foreign
Total current
Deferred income tax expense (benefit):
U.S. Federal
(18,032 )
State and local
(4,889 )
(1,622 )
Foreign
(30,056 )
(11,287 )
(23,972 )
Total deferred
(52,977 )
(4,057 )
(25,388 )
Total provision
$
$
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
107
The tax effects of temporary differences that give rise to our deferred income tax asset (liability) were as follows:
Years Ended
December 26,
December 28,
2020
2019
Deferred income tax asset:
Investment in partnerships
$
(6,294 )
$
Net operating losses and other carryforwards
Inventory, premium coupon redemptions and accounts receivable
valuation allowances
Stock-based compensation
Uniform capitalization adjustment to inventories
Operating lease right of use asset
Other asset
Total deferred income tax asset
Valuation allowance for deferred tax assets
(1)
(40,496 )
(20,699 )
Net deferred income tax asset
Deferred income tax liability
Intangibles amortization
(118,165 )
(135,754 )
Operating lease liability
(71,343 )
(54,672 )
Property and equipment
(7,820 )
(10,555 )
Total deferred tax liability
(197,328 )
(200,981 )
Net deferred income tax asset (liability)
$
$
(41,364 )
(1)
Primarily relates to operating losses , the benefits of which are uncertain. Any future reductions of such valuation allowances will be
reflected as a reduction of income tax expense.
The assessment of the amount of value assigned to our deferred tax assets under the applicable accounting rules is
judgmental. We are required to consider all available positive and negative evidence in evaluating the likelihood
that we will be able to realize the benefit of our deferred tax assets in the future. Such evidence includes scheduled
reversals of deferred tax liabilities, projected future taxable income, tax planning strategies and the results of recent
operations. Since this evaluation requires consideration of events that may occur some years into the future, there
is an element of judgment involved. Realization of our deferred tax assets is dependent on generating sufficient
taxable income in future periods. We believe that it is more likely than not that future taxable income will be
sufficient to allow us to recover substantially all of the value assigned to our deferred tax assets. However, if future
events cause us to conclude that it is not more likely than not that we will be able to recover all of the value
assigned to our deferred tax assets, we will be required to adjust our valuation allowance accordingly.
As of December 26, 2020, we had federal, state, and foreign net operating loss carryforwards of approximately
$
carryforwards will begin to expire in various years from
. The amounts of state and foreign net
operating losses that can be carried forward indefinitely are $
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
108
The tax provisions differ from the amount computed using the federal statutory income tax rate as follows:
Years ended
December 26,
December 28,
December 29,
2020
2019
2018
Income tax provision at federal statutory rate
$
$
$
State income tax provision, net of federal income tax effect
Foreign income tax benefit
(428 )
(4,580 )
(2,558 )
Pass-through noncontrolling interest
(2,681 )
(3,931 )
(2,700 )
Valuation allowance
(79 )
Unrecognized tax benefits and audit settlements
(17,722 )
Interest expense related to loans
(11,098 )
(5,498 )
(11,700 )
Excess tax benefits related to stock compensation
(86 )
(1,008 )
Transition tax on deemed repatriation of foreign earnings
(10,000 )
Revaluation of deferred tax assets and liabilities
(1,676 )
Tax on global intangible low-taxed income ("GILTI")
Tax benefit related to legal entity reorganization outside the U.S.
(5,823 )
(13,852 )
Tax charge related to reorganization of legal entities related
to forming Henry Schein One
Tax charge (credit) related to reorganization of legal entities
completed in preparation for the Animal Health spin-off
(1,333 )
Other
Total income tax provision
$
$
$
For the year ended December 26, 2020, our effective tax rate was
% compared to
% for the prior year
period. Our effective tax rate in 2020 was primarily impacted by an Advance Pricing Agreement (“APA”) with the
U.S Internal Revenue Service (the “IRS”) in the U.S., other audit resolutions, state and foreign income taxes and
interest expense. The positive effect of the APA and the other audit resolutions are not expected to be recurring and,
as a result, we expect our effective tax rate in future periods to be higher.
In 2019, our effective tax rate of
% was primarily impacted by state and foreign income taxes and interest
expense. In 2018, our effective tax rate of
% was primarily impacted by a reduction in the estimate of our
transition tax associated with the Tax Act, tax charges and credits associated with legal entity reorganizations
outside the U.S., and state and foreign income taxes and interest expense.
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”) was enacted in
response to the COVID-19 pandemic. The CARES Act includes, but is not limited to, certain income tax
provisions that modify the Section 163(j) limitation of business interest and Net Operating Loss (“NOL”) carryover
and carryback rules. The modifications to Section 163(j) increase the allowable business interest deduction from
% of adjusted taxable income to
% of adjusted taxable income for years beginning in 2019 and 2020. The
CARES Act eliminated the NOL income limitation for years beginning before 2021 and it extended the carryback
period to five years for losses incurred in 2018, 2019 and 2020. We have analyzed the income tax provisions of the
CARES Act and have accounted for the impact in the year ended December 26, 2020, which did not have a
material impact on our consolidated financial statements. There are certain other non-income tax benefits available
to us under the CARES Act that require further clarification or interpretation that may affect our consolidated
financial statements in the future. On December 27, 2020, the Consolidated Appropriations Act was enacted into
law and extended certain non-income tax benefits under the CARES Act.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
109
On July 20, 2020, the IRS issued final regulations related to the Tax Act. The final regulations concern the global
intangible low-taxed income (“GILTI”) and subpart F income provisions of the Tax Act. To provide flexibility to
taxpayers, the IRS is permitting the application of these final regulations to prior tax years, if the taxpayer elects to
do so. We have analyzed the final regulations, which do not have a material impact to our consolidated financial
statements.
On December 22, 2017, the U.S. government passed the Tax Act. The Tax Act is comprehensive tax legislation
that implemented complex changes to the U.S. tax code including, but not limited to, the reduction of the corporate
tax rate from
% to
%, modification of accelerated depreciation, the repeal of the domestic manufacturing
deduction and changes to the limitations of the deductibility of interest. Additionally, the Tax Act moved from a
global tax regime to a modified territorial regime, which requires U.S. companies to pay a mandatory one-time
transition tax on historical offshore earnings that have not been repatriated to the U.S. The transition tax is payable
over eight years. In the fourth quarter of 2017, we recorded provisional amounts for any items that could be
reasonably estimated at the time. This included the one-time transition tax that we estimated to be $
and a net deferred tax expense of $
enacted federal income tax rate of 21%. We completed our analysis in the year ended December 29, 2018 and
recorded a net $
benefit from the revaluation of deferred taxes to reflect the new tax rate.
Within our consolidated balance sheets, transition tax of $
2019 and $
The FASB Staff Q&A, Topic 740 No. 5, Accounting for Global Intangible Low-Taxed Income, states that an entity
can make an accounting policy election to either recognize deferred taxes for temporary differences expected to
reverse as GILTI in future years or provide for the tax expense related to GILTI in the year the tax is incurred. We
elected to recognize the tax on GILTI as a period expense in the period the tax is incurred. We recorded a current
tax expense for the GILTI provision of $
respectively.
Due to the one-time transition tax and the imposition of the GILTI provisions, all previously unremitted earnings
will no longer be subject to U.S. federal income tax; however, there could be U.S. state and/or foreign withholding
taxes upon distribution of such unremitted earnings. Determination of the amount of unrecognized deferred tax
liability with respect to such earnings is not practicable.
ASC 740 prescribes the accounting for uncertainty in income taxes recognized in the financial statements in
accordance with other provisions contained within this guidance. This topic prescribes a recognition threshold and
a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected
to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be
sustained upon examination by the taxing authorities. The amount recognized is measured as the largest amount of
benefit that is greater than 50% likely of being realized upon ultimate audit settlement. In the normal course of
business, our tax returns are subject to examination by various taxing authorities. Such examinations may result in
future tax and interest assessments by these taxing authorities for uncertain tax positions taken in respect to certain
tax matters.
The total amount of unrecognized tax benefits, which are included in “Other liabilities” within our consolidated
balance sheets as of December 26, 2020 was approximately $
effective tax rate if recognized. It is possible that the amount of unrecognized tax benefits may change in the next
12 months, which may result in a material impact on our consolidated statement of income.
The tax years subject to examination by major tax jurisdictions include the years 2012 and forward by the IRS, as
well as the years 2008 and forward for certain states and certain foreign jurisdictions. All tax returns audited by the
IRS are officially closed through 2011 and 2014 through 2016. All IRS audit fieldwork has been completed for the
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
110
years 2012 and 2013. In the quarter ended December 28, 2019, we reached a settlement with the U.S. Competent
Authority to resolve certain transfer pricing issues related to 2012 and 2013. For all remaining outstanding issues
for 2012 and 2013, we have provided all necessary documentation to the Appellate Division to date and are waiting
for responses. We do not believe the final resolution will have a material impact to our consolidated financial
statements. During the quarter ended September 26, 2020 we finalized negotiations with the Advance Pricing
Division and reached an agreement on an appropriate transfer pricing methodology for the years 2014-2025. The
objective of this resolution is to mitigate future transfer pricing audit adjustments. In the fourth quarter of 2020, we
reached a favorable resolution with the IRS relating to select audit years.
The total amounts of interest and penalties are classified as a component of the provision for income taxes. The
amount of tax interest expense (credit) was approximately $(
) million, $
2019 and 2018, respectively. The total amount of accrued interest is included in “Other liabilities”, and was
approximately $
were accrued for the periods presented.
The following table provides a reconciliation of unrecognized tax benefits:
December 26,
December 28,
December 29,
2020
2019
2018
Balance, beginning of period
$
$
$
Additions based on current year tax positions
Additions based on prior year tax positions
Reductions based on prior year tax positions
(1,000 )
(1,000 )
(1,600 )
Reductions resulting from settlements with taxing authorities
(18,600 )
(4,200 )
(1,600 )
Reductions resulting from lapse in statutes of limitations
(14,300 )
(3,700 )
(16,600 )
Balance, end of period
$
$
$
Note 15 – Concentrations of Risk
Certain financial instruments potentially subject us to concentrations of credit risk. These financial instruments
consist primarily of cash equivalents, trade receivables, long-term investments, notes receivable and derivative
instruments. In all cases, our maximum exposure to loss from credit risk equals the gross fair value of the financial
instruments. We routinely maintain cash balances at financial institutions in excess of insured amounts. We have
not experienced any loss in such accounts and we manage this risk through maintaining cash deposits and other
highly liquid investments in high quality financial institutions. We continuously assess the need for reserves for
such losses, which have been within our expectations. We do not require collateral or other security to support
financial instruments subject to credit risk, except for long-term notes receivable.
We limit our credit risk with respect to our cash equivalents, short-term and long-term investments and derivative
instruments, by monitoring the credit worthiness of the financial institutions who are the counter-parties to such
financial instruments. As a risk management policy, we limit the amount of credit exposure by diversifying and
utilizing numerous investment grade counter-parties.
With respect to our trade receivables, our credit risk is somewhat limited due to a relatively large customer base and
its dispersion across different types of health care professionals and geographic areas. For the year ended
December 26, 2020, two customers accounted for slightly more than
% of our net sales from continuing
operations. For the year ended December 28, 2019, one customer accounted for slightly less than
% of our net
sales from continuing operations. With respect to our sources of supply, our top 10 health care distribution
suppliers from continuing operations and our single largest supplier from continuing operations accounted for
approximately
% and
%, respectively, of our aggregate purchases in 2020 and approximately
% and
%,
respectively, of our aggregate purchases in 2019.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
111
Our long-term notes receivable primarily represent strategic financing arrangements with certain industry affiliates
and amounts owed to us from sales of certain businesses. Generally, these notes are secured by certain assets of the
counterparty; however, in most cases our security is subordinate to other commercial financial institutions. While
we have exposure to credit loss in the event of non-performance by these counter-parties, we conduct ongoing
assessments of their financial and operational performance.
Note 16 – Derivatives and Hedging Activities
We are exposed to market risks as well as changes in foreign currency exchange rates as measured against the U.S.
dollar and each other, and changes to the credit risk of the derivative counterparties. We attempt to minimize these
risks by primarily using foreign currency forward contracts and by maintaining counter-party credit limits. These
hedging activities provide only limited protection against currency exchange and credit risks. Factors that could
influence the effectiveness of our hedging programs include currency markets and availability of hedging
instruments and liquidity of the credit markets. All foreign currency forward contracts that we enter into are
components of hedging programs and are entered into for the sole purpose of hedging an existing or anticipated
currency exposure. We do not enter into such contracts for speculative purposes and we manage our credit risks by
diversifying our counterparties, maintaining a strong balance sheet and having multiple sources of capital.
effectiveness are included in interest expense within our consolidated statements of income. The aggregate
notional value of this net investment hedge, which matures on
, is approximately €
During the years ended December 26, 2020 and December 28, 2019 we recognized approximately $
$
On
,
notional value of the investments in these plans was $
the investments in these plans was $
on LIBOR of
% plus
%, for a combined rate of
%. From March 20, 2020, the effective date of the
swap, to December 26, 2020, we have recorded a gain, within the selling, general and administrative line item in
our consolidated statement of income, of approximately $
undesignated swap for the year ended December 26, 2020. This gain was partially offset by the change in fair
value adjustment of $
March 20, 2020. This swap is expected to be renewed on an annual basis and is expected to result in a neutral
impact to our results of operations.
Fluctuations in the value of certain foreign currencies as compared to the U.S. dollar may positively or negatively
affect our revenues, gross margins, operating expenses and retained earnings, all of which are expressed in U.S.
dollars. Where we deem it prudent, we engage in hedging programs using primarily foreign currency forward
contracts aimed at limiting the impact of foreign currency exchange rate fluctuations on earnings. We purchase
short-term (i.e., generally
exchange risks associated with intercompany loans due from our international subsidiaries and the payment of
merchandise purchases to our foreign suppliers. We do not hedge the translation of foreign currency profits into
U.S. dollars, as we regard this as an accounting exposure, not an economic exposure. Our hedging activities have
historically not had a material impact on our consolidated financial statements. Accordingly, additional disclosures
related to derivatives and hedging activities required by ASC 815 have been omitted.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
112
Note 17 – Revenue from Contracts with Customers
Revenue (Net sales) is recognized in accordance with the policies discussed in
Disaggregation of Net sales
The following table disaggregates our Net sales by reportable segment and geographic area:
Year Ended
December 26, 2020
North America
International
Global
Revenues:
Health care distribution
Dental
$
Medical
Total health care distribution
Technology and value-added services
Total excluding Corporate TSA revenues
Corporate TSA revenues
Total revenues
$
$
$
Year Ended
December 28, 2019
North America
International
Global
Revenues:
Health care distribution
Dental
$
Medical
Total health care distribution
Technology and value-added services
Total excluding Corporate TSA revenues
Corporate TSA revenues
Total revenues
$
$
$
(1)
Corporate TSA revenues represents sales of certain animal health products to Covetrus under the transition services agreement
entered into in connection with the Animal Health Spin-off, which ended in December 2020.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
113
Note 18 – Segment and Geographic Data
We conduct our business through
value-added services. These segments offer different products and services to the same customer base.
The health care distribution reportable segment aggregates our global dental and medical operating segments. This
segment distributes consumable products, small equipment, laboratory products, large equipment, equipment repair
services, branded and generic pharmaceuticals, vaccines, surgical products, diagnostic tests, infection-control
products and vitamins. Our global dental group serves office-based dental practitioners, dental laboratories, schools
and other institutions. Our global medical group serves office-based medical practitioners, ambulatory surgery
centers, other alternate-care settings and other institutions. Our global dental and medical groups serve
practitioners in
Our global technology and value-added services group provides software, technology and other value-added
services to health care practitioners. Our technology group offerings include practice management software
systems for dental and medical practitioners. Our value-added practice solutions include financial services on a
non-recourse basis, e-services, practice technology, network and hardware services, as well as continuing education
services for practitioners.
The following tables present information about our reportable and operating segments:
Years Ended
December 26,
December 28,
December 29,
2020
2019
2018
Net Sales:
Health care distribution
(1)
Dental
$
$
$
Medical
Total health care distribution
Technology and value-added services
(2)
Total excluding Corporate TSA revenues
Corporate TSA revenues
(3)
Total
$
$
$
(1)
Consists of consumable products, small equipment, laboratory products, large equipment, equipment repair services, branded and
generic pharmaceuticals, vaccines, surgical products, diagnostic tests, infection-control products, personal protective equipment and
vitamins.
(2)
Consists of practice management software and other value-added products, which are distributed primarily to health care providers, and
financial services on a non-recourse basis, e-services, continuing education services for practitioners, consulting and other services.
(3)
Corporate TSA revenues represents sales of certain products to Covetrus under the transition services agreement entered into in
connection with the Animal Health Spin-off, which ended in December 2020.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
114
Years ended
December 26,
December 28,
December 29,
2020
2019
2018
Operating Income:
Health care distribution
$
$
$
Technology and value-added services
Total
$
$
$
Income from continuing operations before taxes
Health care distribution
$
$
$
Technology and value-added services
Total
$
$
$
Depreciation and Amortization:
Health care distribution
$
$
$
Technology and value-added services
Total
$
$
$
Interest Income:
Health care distribution
$
$
$
Technology and value-added services
Total
$
$
$
Interest Expense:
Health care distribution
$
$
$
Technology and value-added services
Total
$
$
$
Income Tax Expense:
Health care distribution
$
$
$
Technology and value-added services
Total
$
$
$
Purchases of Fixed Assets:
Health care distribution
$
$
$
Technology and value-added services
Total
$
$
$
As of
December 26,
December 28,
December 29,
2020
2019
2018
Total Assets:
Health care distribution
$
$
$
Technology and value-added services
Discontinued operations
Total
$
$
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
115
The following table presents information about our operations by geographic area as of and for the three years
ended December 26, 2020. Net sales by geographic area are based on the respective locations of our subsidiaries.
No country, except for the United States, generated net sales greater than
% of consolidated net sales. There
were no material amounts of sales or transfers among geographic areas and there were no material amounts of
export sales.
2020
2019
2018
Net Sales
Long-Lived
Assets
Net Sales
Long-Lived
Assets
Net Sales
Long-Lived
Assets
United States
$
$
$
$
$
$
Other
Consolidated total
$
$
$
$
$
$
Note 19 – Employee Benefit Plans
Stock-based Compensation
Our accompanying consolidated statements of income reflect pre-tax share-based compensation expense of $
million ($
million ($
and December 29, 2018.
Our accompanying consolidated statements of cash flows present our stock-based compensation expense as an
adjustment to reconcile net income to net cash provided by operating activities for all periods presented. In the
accompanying consolidated statements of cash flows, there were
excess of recognized compensation as a cash inflow from financing activities for the years ended December 26,
2020, December 28, 2019 and December 29, 2018.
Stock-based compensation represents the cost related to stock-based awards granted to employees and non-
employee directors. We measure stock-based compensation at the grant date, based on the estimated fair value of
the award, and recognize the cost (net of estimated forfeitures) as compensation expense on a straight-line basis
over the requisite service period. Our stock-based compensation expense is reflected in selling, general and
administrative expenses in our consolidated statements of income.
Stock-based awards are provided to certain employees and non-employee directors under the terms of our 2020
Stock Incentive Plan (formerly known as the 2013 Stock Incentive Plan), and our 2015 Non-Employee Director
Stock Incentive Plan (together, the “Plans”). The Plans are administered by the Compensation Committee of the
Board of Directors. Equity-based awards are granted solely in the form of restricted stock units, with the exception
of providing stock options to employees pursuant to certain pre-existing contractual obligations. As of December
26, 2020, there were
Incentive Plan and
Director Stock Incentive Plan.
Grants of restricted stock units are stock-based awards granted to recipients with specified vesting provisions. In
the case of restricted stock units, common stock is generally delivered on or following satisfaction of vesting
conditions. We issue restricted stock units that vest solely based on the recipient’s continued service over time
(primarily four year cliff vesting, except for grants made under the 2015 Non-Employee Director Stock Incentive
Plan, which are primarily
specified performance measurements and the recipient’s continued service over time (primarily three year cliff
vesting).
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
116
With respect to time-based restricted stock units, we estimate the fair value on the date of grant based on our
closing stock price. With respect to performance-based restricted stock units, the number of shares that ultimately
vest and are received by the recipient is based upon our performance as measured against specified targets over a
specified period, as determined by the Compensation Committee of the Board of Directors. Although there is no
guarantee that performance targets will be achieved, we estimate the fair value of performance-based restricted
stock units based on our closing stock price at time of grant.
The Plans provide for adjustments to the performance-based restricted stock units targets for significant events,
including, without limitation, acquisitions, divestitures, new business ventures, certain capital transactions
(including share repurchases), restructuring costs, if any, certain litigation settlements or payments, if any, changes
in tax rates in certain countries, changes in accounting principles or in applicable laws or regulations and foreign
exchange fluctuations. Over the performance period, the number of shares of common stock that will ultimately
vest and be issued and the related compensation expense is adjusted upward or downward based upon our
estimation of achieving such performance targets. The ultimate number of shares delivered to recipients and the
related compensation cost recognized as an expense will be based on our actual performance metrics as defined
under the Plans.
As a result of the Separation, the number of our unvested (as of the date of the Separation) equity-based awards
from previous grants made under our Long-term Incentive Program under the Plans was increased by a factor of
approximately
, along with a corresponding
decrease
We record deferred income tax assets for awards that will result in future deductions on our income tax returns
based on the amount of compensation cost recognized and our statutory tax rate in the jurisdiction in which we will
receive a deduction.
Stock-based compensation grants for the three years ended December 26, 2020 consisted of restricted stock/unit
grants. Certain stock-based compensation granted may require us to settle in the form of a cash payment. During
the year ended December 26, 2020, we recorded a liability of $
stock-based compensation to be settled in cash. The weighted-average grant date fair value of stock-based awards
granted before forfeitures was $
, $
December 28, 2019 and December 29, 2018.
Total unrecognized compensation cost related to non-vested awards as of December 26, 2020 was $
which is expected to be recognized over a weighted-average period of approximately
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
117
A summary of the stock option activity under the Plans is presented below:
Years Ended
December 26,
December 28,
December 29,
2020
2019
2018
Weighted
Weighted
Weighted
Average
Average
Average
Exercise
Exercise
Exercise
Shares
Price
Shares
Price
Shares
Price
Outstanding at beginning of year
$
3
$
$
Granted
Exercised
(3 )
(152 )
Forfeited
Outstanding at end of year
$
$
$
Options exercisable at end of year
$
$
$
The following table represents the intrinsic values of:
As of
December 26,
December 28,
December 29,
2020
2019
2018
Stock options outstanding
$
$
$
Stock options exercisable
The total cash received as a result of stock option exercises for the year ended December 29, 2018 was
approximately $
t realize any tax benefits for the years
ended December 26, 2020, December 28, 2019 and December 29, 2018. We settle employee stock option exercises
with newly issued common shares.
The total intrinsic value per share of restricted stock/units that vested was $
, $
years ended December 26, 2020, December 28, 2019 and December 29, 2018. The following table summarizes the
status of our non-vested restricted stock/units for the year ended December 26, 2020:
Time-Based Restricted Stock/Units
Weighted Average
Grant Date Fair
Intrinsic Value
Shares/Units
Value Per Share
Per Share
Outstanding at beginning of period
$
Granted
Vested
(298 )
Forfeited
(51 )
Outstanding at end of period
$
$
Performance-Based Restricted Stock/Units
Weighted Average
Grant Date Fair
Intrinsic Value
Shares/Units
Value Per Share
Per Share
Outstanding at beginning of period
$
Granted
(954 )
Vested
(327 )
Forfeited
(42 )
Outstanding at end of period
$
$
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
118
401(k) Plans
We offer qualified 401(k) plans to substantially all our domestic full-time employees. As determined by our Board
of Directors, matching contributions to these plans generally do not exceed
% of the participants’ contributions
up to
% of their base compensation, subject to applicable legal limits. Matching contributions consist of cash and
were allocated entirely to the participants’ investment elections on file, subject to a
% allocation limit to the
Henry Schein Stock Fund. Due to the impact of COVID-19, as part of our initiative to generate cash savings, we
suspended the matching contribution for the second half of 2020. Forfeitures attributable to participants whose
employment terminates prior to becoming fully vested are used to reduce our matching contributions and offset
administrative expenses of the 401(k) plans.
Assets of the 401(k) and other defined contribution plans are held in self-directed accounts enabling participants to
choose from various investment fund options. Matching contributions related to these plans charged to operations
during the years ended December 26, 2020, December 28, 2019 and December 29, 2018 amounted to $
$
Supplemental Executive Retirement Plan
We offer an unfunded, non-qualified SERP to eligible employees. This plan generally covers officers and certain
highly compensated employees after they have reached the maximum IRS allowed pre-tax 401(k) contribution
limit. Our contributions to this plan are equal to the 401(k) employee-elected contribution percentage applied to
base compensation for the portion of the year in which such employees are not eligible to make pre-tax
contributions to the 401(k) plan. Due to the impact of COVID-19, as part of our initiative to generate cash savings,
we suspended contributions under the SERP for the second half of 2020. The amounts charged to operations during
the years ended December 26, 2020, December 28, 2019 and December 29, 2018 amounted to $
million and $
information.
Deferred Compensation Plan
During 2011, we began to offer DCP to a select group of management or highly compensated employees of the
Company and certain subsidiaries. This plan allows for the elective deferral of base salary, bonus and/or
commission compensation by eligible employees. The amounts charged (credited) to operations during the years
ended December 26, 2020, December 28, 2019 and December 29, 2018 were approximately $
million and $
(2.3 )
information.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
119
Note 20 – Commitments and Contingencies
Purchase Commitments
In our health care distribution business, we sometimes enter into long-term purchase commitments to ensure the
availability of products for distribution. Future minimum annual payments for inventory purchase commitments as
of December 26, 2020 were:
2021
$
2022
2023
2024
2025
Thereafter
Total minimum inventory purchase commitment payments
$
Employment, Consulting and Non-Compete Agreements
We have definite-lived employment, consulting and non-compete agreements that have varying base aggregate
annual payments for the years 2021 through 2025 and thereafter of approximately $
million, $
that provide for current compensation of $
increase in 2022. In addition, some agreements have provisions for additional incentives and compensation.
Litigation
On
August 31, 2012
,
U.S. District Court for the Eastern District of Texas, Civil Action No. 2:12-CV-00572-JRG, styled as an antitrust
action under Section 1 of the Sherman Act, and the Texas Free Enterprise Antitrust Act.
. On
August 1, 2017
,
trial, to be trebled with interest and costs, including attorneys’ fees, jointly and severally, as well as injunctive
relief. On
October 30, 2017
,
On October 1, 2012, we filed a motion for an order: (i) compelling Archer to arbitrate its claims against us; (2)
staying all proceedings pending arbitration; and (3) joining the Danaher Defendants’ motion to arbitrate and stay.
On May 28, 2013, the Magistrate Judge granted the motions to arbitrate and stayed proceedings pending arbitration.
On June 10, 2013, Archer moved for reconsideration before the District Court judge. On December 7, 2016, the
District Court Judge granted Archer’s motion for reconsideration and lifted the stay. Defendants appealed the
District Court’s order. On December 21, 2017, the U.S. Court of Appeals for the Fifth Circuit affirmed the District
Court’s order denying the motions to compel arbitration. On June 25, 2018, the Supreme Court of the United States
granted defendants’ petition for writ of certiorari. On October 29, 2018, the Supreme Court heard oral arguments.
On January 8, 2019, the Supreme Court issued its published decision vacating the judgment of the Fifth Circuit and
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
120
remanding the case to the Fifth Circuit for further proceedings consistent with the Supreme Court’s opinion. On
April 2, 2019, the District Court stayed the proceeding in the trial court pending resolution by the Fifth Circuit. The
Fifth Circuit heard oral argument on May 1, 2019 on whether the case should be arbitrated. The Fifth Circuit
issued its opinion on August 14, 2019 affirming the District Court’s order denying defendants’ motions to compel
arbitration. Defendants filed a petition for rehearing en banc before the Fifth Circuit. The Fifth Circuit denied that
petition. On October 1, 2019, the District Court set the case for trial on February 3, 2020, which was subsequently
moved to January 29, 2020. On January 24, 2020 the Supreme Court granted our motion to stay the District Court
proceedings, pending the disposition of our petition for writ of certiorari, which was filed on January 31, 2020.
Archer conditionally cross petitioned for certiorari on an arbitration issue on March 2, 2020. On June 15, 2020, the
Supreme Court granted our petition for writ of certiorari, and denied Archer’s conditional petition for certiorari, and
thus the District Court proceedings remained stayed. After briefing from the parties and several amici, the case was
argued before the Supreme Court on December 8, 2020. On January 25, 2021, the Supreme Court dismissed the
writ of certiorari as improvidently granted. That action dissolved the stay the Supreme Court had previously
granted, and thus the trial of the lawsuit may proceed. The U.S. District Court for the Eastern District of Texas has
scheduled a Status Conference for February 19, 2021. Patterson and the Danaher Defendants settled with Archer
and they have been dismissed from the case with prejudice. Benco is still a defendant. We intend to defend
ourselves vigorously against this action.
On
May 29, 2018
, an amended complaint was filed in the MultiDistrict Litigation (“MDL”) proceeding In Re
National Prescription Opiate Litigation (MDL No. 2804; Case No. 17-md-2804) in an action entitled
. v. Purdue Pharma, L.P., et al., Civil Action No. 1:18-op-45090-DAP (“County of Summit
Action”), in the U.S. District Court for the Northern District of Ohio, adding Henry Schein, Inc.,
.
In addition to the County of Summit Action,
named as a defendant in multiple lawsuits (currently less than one-hundred and fifty (
)), which
within the MDL and are currently abated for discovery purposes, and others which remain pending in state courts
and are proceeding independently and outside of the MDL. On October 9, 2020, the Circuit Court of the 17th
Judicial Circuit in and for Broward County, Florida, Case No. CACE19018882, granted Henry Schein’s motion to
dismiss the claims brought against it in the action filed by North Broward Hospital District et. al. The Florida court
gave plaintiffs until November 24, 2020 to replead their claims against Henry Schein. On January 8, 2021, Henry
Schein filed a motion to dismiss the Amended Complaint. An action filed by Tucson Medical Center et al. was
previously scheduled for trial beginning on June 1, 2021 but the court has vacated that trial date. At this time, the
only case set for trial is the action filed by West Virginia University Hospitals, Inc. et al., which is currently
scheduled for a non-jury liability trial on Plaintiffs’ public nuisance claims on November 1, 2021. Of Henry
Schein’s 2020 revenue of approximately $
than one-tenth of
vigorously against these actions.
On
September 30, 2019
, the
, filed a putative class action complaint for violation of the federal securities laws
against
New York, Case No. 2:19-cv-05530-FB-RLM.
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
121
Covetrus’s capabilities as to inventory management and supply-chain services, understated the costs of integrating
the Henry Schein Animal Health Business and Vets First Choice, understated Covetrus’s separation costs from
Henry Schein, and understated the impact on earnings from online competition and alternative distribution channels
and from the loss of an allegedly large customer in North America just before the Separation and Merger. The
complaint seeks unspecified monetary damages and a jury trial. Pursuant to the provisions of the PSLRA, the court
appointed lead plaintiff and lead counsel on December 23, 2019. Lead plaintiff filed a Consolidated Class Action
Complaint on February 21, 2020. Lead plaintiff added Steve Paladino, our Chief Financial Officer, as a defendant
in the action. Lead plaintiff filed an Amended Consolidated Class Action Complaint on May 21, 2020, in which it
added a claim that Mr. Paladino is a “control person” of Covetrus. We intend to defend ourselves vigorously
against this action.
On
,
Inc. against various present and former directors and officers of Henry Schein in the U.S. District Court for the
Eastern District of New York, Case No. 1:19-cv-6485-LDH-JO.
Complaint asserted claims under the federal securities laws and state law relating to the allegations in the antitrust
actions, the In re Henry Schein, Inc. Securities Litigation, and the City of Hollywood securities class action
described in our prior filings with the SEC and/or above. The complaint sought declaratory, injunctive, and
monetary relief on behalf of Henry Schein. On January 6, 2020, one of the two law firms that filed the Finazzo
case filed another, virtually identical putative shareholder derivative action on behalf of Henry Schein against the
same defendants, asserting the same claims and seeking the same relief. That case, captioned Mark Sloan v.
Stanley M. Bergman, et al., was also filed in the U.S. District Court for the Eastern District of New York, Case No.
1:20-cv-0076. On January 24, 2020, the court consolidated the Finazzo and Sloan cases under the new caption In
re Henry Schein, Inc. Derivative Litigation, No. 1:19-cv-06485-LDH-JO, and appointed the two law firms that filed
the Finazzo case as co-lead counsel for the consolidated action. The parties agreed to a resolution of this matter
subject to various conditions, including court approval. The settlement involves the adoption of certain procedures
but does not involve the payment of any money except a fee to the plaintiffs’ attorneys that is immaterial. After the
court referred the motion to approve the settlement to a Magistrate Judge, the parties consented to having the case
assigned to the Magistrate Judge for all purposes. The Magistrate Judge to which the matter was ultimately
assigned held a fairness hearing and issued an order and judgment approving the settlement. The order and
judgment approving the settlement have become final.
On February 5, 2021, Jack Garnsey filed a putative shareholder derivative action on behalf of Covetrus, Inc. in the
U.S. District Court for the Eastern District of New York, naming as defendants Benjamin Shaw, Christine T.
Komola, Steven Paladino, Betsy Atkins, Deborah G. Ellinger, Sandra L. Helton, Philip A. Laskaway, Mark J.
Manoff, Edward M. McNamara, Ravi Sachdev, David E. Shaw, Benjamin Wolin, and Henry Schein, Inc., with
Covetrus, Inc. named as a nominal defendant. The complaint alleges that the individual defendants breached their
fiduciary duties under state law in connection with the same allegations asserted in the City of Hollywood securities
class action described above and further alleges that Henry Schein aided and abetted such breaches. The complaint
also asserts claims for contribution under the federal securities laws against Henry Schein and other defendants,
also arising out of the allegations in the City of Hollywood lawsuit. The complaint seeks declaratory, injunctive,
and monetary relief. We intend to defend ourselves vigorously against this action.
From time to time, we may become a party to other legal proceedings, including, without limitation, product
liability claims, employment matters, commercial disputes, governmental inquiries and investigations (which may
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
122
in some cases involve our entering into settlement arrangements or consent decrees), and other matters arising out
of the ordinary course of our business. While the results of any legal proceeding cannot be predicted with certainty,
in our opinion none of these other pending matters are currently anticipated to have a material adverse effect on our
consolidated financial position, liquidity or results of operations.
Note 21 – Quarterly Information (Unaudited)
The following tables present certain quarterly financial data:
Quarters ended
March 28,
June 27,
September 26,
December 26,
2020
2020
2020
2020
Net sales
$
$
$
$
Gross profit
Restructuring costs
(1)
Operating income (loss)
(7,433 )
Net gain on sale of equity investments
(2)
Net income (loss) from continuing operations
(13,852 )
Amounts attributable to Henry Schein, Inc.
from continuing operations:
Net income (loss)
(11,382 )
Earnings (loss) per share attributable to
Henry Schein, Inc. from continuing operations:
Basic
$
$
(0.08 )
$
$
Diluted
(0.08 )
Quarters ended
March 30,
June 29,
September 28,
December 28,
2019
2019
2019
2019
Net sales
$
$
$
$
Gross profit
Restructuring costs (credits)
(1)
(802 )
(1,059 )
Operating income
Net gain on sale of equity investments
(2)
Net income from continuing operations
Amounts attributable to Henry Schein, Inc.
from continuing operations:
Net income
Earnings per share attributable to Henry Schein, Inc.
from continuing operations:
Basic
$
$
$
$
Diluted
(1) See
fiscal years.
(2) See
HENRY SCHEIN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share data)
123
Note 22 – Supplemental Cash Flow Information
Cash paid for interest and income taxes was:
Years ended
December 26,
December 28,
December 29,
2020
2019
2018
Interest
$
$
$
Income taxes
For the years ended December 26, 2020, December 28, 2019 and December 29, 2018, we had $
(10.2 )
$
(4.9 )
activities, respectively.
During the third quarter of 2018, we formed Henry Schein One, LLC with Internet Brands through a non-cash
transaction resulting in approximately $
current
% minority interest and $
Henry Schein One, representing up to an additional
% ownership interests at December 26, 2020, a portion of
which is contingent upon the achievement of certain operating targets. During the third quarter of 2020, the Internet
Brands ownership interest in Henry Schein One, LLC increased to
%.
Note 23 – Related Party Transactions
In connection with the completion of the Animal Health Spin-off during our 2019 fiscal year, we entered into a
transition services agreement with Covetrus under which we have agreed to provide certain transition services for
up to twenty-four months in areas such as information technology, finance and accounting, human resources,
supply chain, and real estate and facility services
For the years ended December 26, 2020 and December 28, 2019, we recorded approximately $
$
purchased certain products from us. During the years ended December 26, 2020 and December 28, 2019, net sales
to Covetrus under the transition services agreement were approximately $
respectively. Sales to Covetrus under the transition services agreement ended in December 2020. At December 26,
2020 we had $
and 2018, we recorded $
, million, $
to this royalty agreement. As of December 26, 2020 and December 28, 2019, Henry Schein One, LLC had a net
receivable balance due from Internet Brands of $
related to the royalty agreement and other management fees.
and $
$
December 28, 2019, we had in the aggregate $
$
124
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
ITEM 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of management, including our principal executive officer and
principal financial officer, we evaluated the effectiveness of the design and operation of our disclosure controls and
procedures as of the end of the period covered by this annual report as such term is defined in Rules 13a-15(e) and
15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Based on
this evaluation, our management, including our principal executive officer and principal financial officer,
concluded that our disclosure controls and procedures were effective as of December 26, 2020 to ensure that all
material information required to be disclosed by us in reports that we file or submit under the Exchange Act is
accumulated and communicated to them as appropriate to allow timely decisions regarding required disclosure and
that all such information is recorded, processed, summarized and reported within the time periods specified in the
SEC’s rules and forms.
Changes in Internal Control over Financial Reporting
The combination of acquisitions and continued acquisition integrations undertaken during the quarter and carried
over from prior quarters as well as changes to the operating methods of some of our internal controls over financial
reporting due to the COVID-19 pandemic, when considered in the aggregate, represents a material change in our
internal control over financial reporting.
During the quarter ended December 26, 2020, we completed the acquisition of a dental business in North America
with approximate aggregate annual revenues of approximately $20 million. In addition, post-acquisition integration
related activities continued for our global dental and North American medical businesses acquired during prior
quarters, representing aggregate annual revenues of approximately $370 million. These acquisitions, the majority
of which utilize separate information and financial accounting systems, have been included in our consolidated
financial statements since their respective dates of acquisition.
All acquisitions and continued acquisition integrations involve necessary and appropriate change-management
controls that are considered in our annual assessment of the design and operating effectiveness of our internal
control over financial reporting.
In addition, as a result of a combination of continued governmental imposed and Company directed closures of
some of our facilities due to the COVID-19 pandemic, we have had to maintain a number of changes to the
operating methods of some of our internal controls. For example, moving from manual sign-offs and in-person
meetings to electronic sign-offs and electronic communications such as email and telephonic or video conference
due to out-of-office working arrangements. However, the design of our internal control framework and objectives
over financial reporting remains unchanged and we do not believe that these changes have materially affected, or
are reasonably likely to materially affect, the effectiveness of our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting,
as such term is defined in Exchange Act Rule 13a-15(f). Our internal control system is designed to provide
reasonable assurance to our management and Board of Directors regarding the preparation and fair presentation of
published financial statements. Under the supervision and with the participation of our management, including our
principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our
internal control over financial reporting based on the framework in Internal Control-Integrated Framework (2013),
updated and reissued by the Committee of Sponsoring Organizations, or the COSO Framework. Based on our
125
evaluation under the COSO Framework, our management concluded that our internal control over financial
reporting was effective at a reasonable assurance level as of December 26, 2020.
The effectiveness of our internal control over financial reporting as of December 26, 2020 has been independently
audited by BDO USA, LLP, an independent registered public accounting firm, and their attestation is included
herein.
Limitations of the Effectiveness of Internal Control
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance
that the objectives of the internal control system are met. Because of the inherent limitations of any internal control
system, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company
have been detected.
126
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Stockholders and Board of Directors
Henry Schein, Inc.
Melville, NY
Opinion on Internal Control over Financial Reporting
We have audited Henry Schein, Inc.’s (the “Company’s”) internal control over financial reporting as of December
26, 2020, based on criteria established in
Internal Control – Integrated Framework (2013)
of Sponsoring Organizations of the Treadway Commission (the “COSO criteria”). In our opinion, the Company
maintained, in all material respects, effective internal control over financial reporting as of December 26, 2020,
based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board
(United States) (“PCAOB”), the consolidated balance sheets of the Company as of December 26, 2020 and
December 28, 2019, the related consolidated statements of income, comprehensive income, stockholders’ equity,
and cash flows for each of the three years in the period ended December 26, 2020, and the related notes and
schedule and our report dated February 17, 2021 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and
for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying
“Item 9A, Management’s Report on Internal Control over Financial Reporting”. Our responsibility is to express an
opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting
firm registered with the PCAOB and are required to be independent with respect to the Company in accordance
with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange
Commission and the PCAOB.
We conducted our audit of internal control over financial reporting in accordance with the standards of the PCAOB.
Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective
internal control over financial reporting was maintained in all material respects. Our audit included obtaining an
understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and
testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our
audit also included performing such other procedures as we considered necessary in the circumstances. We believe
that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance
regarding the reliability of financial reporting and the preparation of financial statements for external purposes in
accordance with generally accepted accounting principles. A company’s internal control over financial reporting
includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail,
accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable
assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance
with generally accepted accounting principles, and that receipts and expenditures of the company are being made
only in accordance with authorizations of management and directors of the company; and (3) provide reasonable
assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the
company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect
misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that
controls may become inadequate because of changes in conditions, or that the degree of compliance with the
policies or procedures may deteriorate.
/s/ BDO USA, LLP
New York , NY
February 17, 2021
127
ITEM 9B. Other Information
Not applicable.
PART III
ITEM 10. Directors, Executive Officers and Corporate Governance
Information required by this item regarding our directors and executive officers and our corporate governance is
hereby incorporated by reference to the Section entitled “Election of Directors,” with respect to directors, and the
first paragraph of the Section entitled “Corporate Governance - Board of Directors Meetings and Committees -
Audit Committee,” with respect to corporate governance, in each case in our definitive 2021 Proxy Statement to be
filed pursuant to Regulation 14A and to the Section entitled “Information about our Executive Officers” in Part I of
this report, with respect to executive officers.
There have been no changes to the procedures by which stockholders may recommend nominees to our Board of
Directors since our last disclosure of such procedures, which appeared in our definitive 2020 Proxy Statement filed
pursuant to Regulation 14A on April 7, 2020.
Information required by this item concerning compliance with Section 16(a) of the Securities Exchange Act of
1934 is hereby incorporated by reference to the Section entitled “Delinquent Section 16(a) Reports” in our
definitive 2021 Proxy Statement to be filed pursuant to Regulation 14A, to the extent responsive disclosure is
required.
We have adopted a Code of Ethics that applies to our Chief Executive Officer, Chief Financial Officer, Chief
Accounting Officer and Controller. We make available free of charge through our Internet website,
, under the “About Henry Schein--Corporate Governance Highlights” caption, our Code of
Ethics. We intend to disclose on our Web site any amendment to, or waiver of, a provision of the Code of Ethics.
ITEM 11. Executive Compensation
The information required by this item is hereby incorporated by reference to the Sections entitled “Compensation
Discussion and Analysis,” “Compensation Committee Report” (which information shall be deemed furnished in
this Annual Report on Form 10-K), “Executive and Director Compensation” and “Compensation Committee
Interlocks and Insider Participation” in our definitive 2021 Proxy Statement to be filed pursuant to Regulation 14A.
128
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder
Matters
We maintain several stock incentive plans for the benefit of certain officers, directors and employees. All active
plans have been approved by our stockholders. Descriptions of these plans appear in the notes to our consolidated
financial statements. The following table summarizes information relating to these plans as of December 26, 2020:
Number of Common
Shares to be Issued Upon
Weighted- Average
Number of Common
Exercise of Outstanding
Exercise Price of
Shares Available for
Plan Category
Options and Rights
Outstanding Options
Future Issuances
Plans Approved by Stockholders
-
$
-
6,077,548
Plans Not Approved by Stockholders
-
-
-
Total
-
$
-
6,077,548
The other information required by this item is hereby incorporated by reference to the Section entitled “Security
Ownership of Certain Beneficial Owners and Management” in our definitive 2021 Proxy Statement to be filed
pursuant to Regulation 14A.
ITEM 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item is hereby incorporated by reference to the Section entitled “Certain
Relationships and Related Transactions” and “Corporate Governance – Board of Directors Meetings and
Committees – Independent Directors” in our definitive 2021 Proxy Statement to be filed pursuant to Regulation
14A.
ITEM 14. Principal Accounting Fees and Services
The information required by this item is hereby incorporated by reference to the Section entitled “Independent
Registered Public Accounting Firm Fees and Pre-Approval Policies and Procedures” in our definitive 2021 Proxy
Statement to be filed pursuant to Regulation 14A.
PART IV
ITEM 15. Exhibits, Financial Statement Schedules
(a)
List of Documents Filed as a Part of This Report:
1.
Financial Statements:
Our Consolidated Financial Statements filed as a part of this report are listed on the index on
Page 69.
2.
Financial Statement Schedules:
Schedule II – Valuation of Qualifying Accounts
No other schedules are required.
3.
Index to Exhibits:
See exhibits listed under Item 15(b) below.
129
(b) Exhibits
130
131
132
133
**
134
135
136
101.INS
Inline XBRL Instance Document - the instance document does not
appear in the Interactive Data File because its XBRL tags are
embedded within the Inline XBRL document.+
101.SCH
Inline XBRL Taxonomy Extension Schema Document+
101.CAL
Inline XBRL Taxonomy Extension Calculation Linkbase Document+
101.DEF
Inline XBRL Taxonomy Extension Definition Linkbase Document+
101.LAB
Inline XBRL Taxonomy Extension Label Linkbase Document+
101.PRE
Inline XBRL Taxonomy Extension Presentation Linkbase Document+
104
The cover page of Henry Schein, Inc.’s Annual Report on Form 10-K
for the year ended December 26, 2020, formatted in Inline XBRL
(included within Exhibit 101 attachments).+
_________
+ Filed or furnished herewith.
* Schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The Company
hereby agrees to furnish supplementally a copy of any of the omitted schedules and exhibits upon request
by the U.S. Securities and Exchange Commission.
** Indicates management contract or compensatory plan or agreement.
ITEM 16. Form 10-K Summary
None.
137
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly
caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Henry Schein, Inc.
By: /s/ STANLEY M. BERGMAN
Stanley M. Bergman
Chairman and Chief Executive Officer
February 17, 2021
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the
following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signature
Capacity
Date
/s/ STANLEY M. BERGMAN
Chairman, Chief Executive Officer
February 17, 2021
Stanley M. Bergman
and Director (principal executive officer)
/s/ STEVEN PALADINO
Executive Vice President, Chief Financial Officer
February 17, 2021
Steven Paladino
and Director (principal financial and accounting officer)
/s/ JAMES P. BRESLAWSKI
Vice Chairman, President and Director
February 17, 2021
James P. Breslawski
/s/ GERALD A. BENJAMIN
Director
February 17, 2021
Gerald A. Benjamin
/s/ MARK E. MLOTEK
Director
February 17, 2021
Mark E. Mlotek
/s/ MOHAMAD ALI
Director
February 17, 2021
Mohamad Ali
/s/ BARRY J. ALPERIN
Director
February 17, 2021
Barry J. Alperin
/s/ PAUL BRONS
Director
February 17, 2021
Paul Brons
/s/ DEBORAH DERBY
Director
February 17, 2021
Deborah Derby
/s/ SHIRA GOODMAN
Director
February 17, 2021
Shira Goodman
/s/ JOSEPH L. HERRING
Director
February 17, 2021
Joseph L. Herring
/s/ KURT P. KUEHN
Director
February 17, 2021
Kurt P. Kuehn
/s/ PHILIP A. LASKAWY
Director
February 17, 2021
Philip A. Laskawy
/s/ ANNE H. MARGULIES
Director
February 17, 2021
Anne H. Margulies
/s/ CAROL RAPHAEL
Director
February 17, 2021
Carol Raphael
/s/ E. DIANNE REKOW
Director
February 17, 2021
E. Dianne Rekow, DDS, Ph.D.
/s/ BRADLEY T. SHEARES, PH. D.
Director
February 17, 2021
Bradley T. Sheares, Ph. D.
138
Schedule II
Valuation and Qualifying Accounts
(in thousands)
Additions (Reductions)
Charged
Balance at
Charged to
(credited) to
Balance at
beginning of
statement of
other
end of
Description
period
income (1)
accounts (2)
Deductions (3)
period
Year ended December 26, 2020:
Allowance for doubtful accounts
and other
$
$
$
$
(7,839 )
$
Yea r ended December 28, 2019:
Allowance for doubtful accounts
and other
$
$
$
$
(5,865 )
$
Yea r ended December 29, 2018:
Allowance for doubtful accounts
and other
$
$
$
(1,158 )
$
(6,366 )
$
(1)
Represents amounts charged to bad debt expense.
(2)
Amounts charged (credited) to other accounts primarily relate to provision for late fees and the impact of foreign currency exchange rates and
the adoption of ASU No. 2016-13 effective December 29, 2019.
(3)
Deductions primarily consist of fully reserved accounts receivable that have been written off.