UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the fiscal year ended December 31, 2021
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from ___________ to ___________
Commission file number 000-19125
(Exact name of Registrant as specified in its charter)
|
|
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(IRS Employer Identification No.)
|
|
|
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Trading symbol
|
Name of each exchange on which registered
|
||
|
|
“
|
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of
the Securities Act. Yes ☒ No ☐
Indicate by check if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities
Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to
Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller
reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
Accelerated Filer ☐
|
|
|
Non-accelerated Filer ☐
|
Smaller Reporting Company
|
|
|
Emerging Growth Company
|
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Securities Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management assessment of the effectiveness of its internal
controls over financial reporting under Section 4049b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report ☒
Indicate by check mark whether the Registrant is a shell company
(as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The approximate aggregate market value of the voting common stock held by non-affiliates of the Registrant, based upon the last sale price of
the common stock reported on The Nasdaq Global Select Market was $4,675,204,973 as of June 30, 2021.*
The number of shares of voting common stock outstanding as of February 16, 2022 was 141,688,727 .
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement to be filed on or about April 20, 2022 with the Securities and Exchange Commission in
connection with the Registrant’s annual meeting of stockholders to be held on June 2, 2022 are incorporated by reference into Part III of this Report.
| * |
Excludes 23,819,152 shares of common stock held by directors and officers and by stockholders whose beneficial ownership is known by the Registrant to
exceed 10 percent of the common stock outstanding at June 30, 2021. Exclusion of shares held by any person should not be construed to
indicate that such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the Registrant, or that such person is controlled by or under common control with the Registrant.
|
FORWARD-LOOKING STATEMENTS
This report on Form 10-K and the information incorporated herein by reference includes forward-looking
statements regarding our business and the therapeutic and commercial potential of SPINRAZA (nusinersen), TEGSEDI (inotersen), WAYLIVRA (volanesorsen), eplontersen, olezarsen, donidalorsen, ION363, pelacarsen, tofersen and our technologies and products
in development. Any statement describing our goals, expectations, financial or other projections, intentions or beliefs, is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and
uncertainties, including those related to the impact of COVID-19 could have on our business, and particularly those inherent in the process of discovering, developing and commercializing medicines that are safe and effective for use as human
therapeutics, and in the endeavor of building a business around such medicines. Our forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause our results to differ materially from those expressed
or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in this report on Form 10-K, including those identified in Item 1A entitled “Risk Factors”.
Although our forward-looking statements reflect the good faith judgment of our management, these statements are based only on facts and factors currently known by us. As a result, you are cautioned not to rely on these forward-looking statements.
In this report, unless the context requires otherwise, “Ionis,” “Company,” “we,” “our,” and “us” refers to Ionis
Pharmaceuticals, Inc. and its subsidiaries.
Summary of Risk Factors
There are a number of risks related to our business and our securities. Below is a summary of material factors that make
an investment in our securities speculative or risky. Importantly, this summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, as well as other risks that we face, can be found
in this report on Form 10-K in Item 1A entitled “Risk Factors.”:
| ● |
the impact on our operations and financial condition from the effects of the current COVID-19 pandemic;
|
| ● |
our ability to generate substantial revenue from the sale of our medicines;
|
| ● |
our and our partners’ ability to compete effectively;
|
| ● |
the availability of adequate coverage and payment rates for our medicines;
|
| ● |
our ability to successfully manufacture our medicines;
|
| ● |
our ability to successfully develop and obtain marketing approvals for our medicines;
|
| ● |
our ability to secure and maintain effective corporate partnerships;
|
| ● |
our ability to sustain cash flows and achieve consistent profitability;
|
| ● |
our ability to protect our intellectual property; and
|
| ● |
our ability to maintain the effectiveness of our personnel.
|
TRADEMARKS
“Ionis,” the Ionis
logo, and other trademarks or service marks of Ionis Pharmaceuticals, Inc. appearing in this report are the property of Ionis Pharmaceuticals, Inc. “Akcea,” the Akcea logo, and other trademarks or service marks of Akcea Therapeutics, Inc. appearing in
this report are the property of Akcea Therapeutics, Inc., Ionis’ wholly owned subsidiary. This report contains additional trade names, trademarks and service marks of others, which are the property of their respective owners. Solely for convenience,
trademarks and trade names referred to in this report may appear without the ® or TM symbols.
CORPORATE INFORMATION
We incorporated in California in 1989 and in January 1991 we changed our state of incorporation to Delaware. In December
2015, we changed our name to Ionis Pharmaceuticals, Inc. from Isis Pharmaceuticals, Inc. Our principal offices are in Carlsbad, California. In December 2014, we formed Akcea Therapeutics, Inc., as a Delaware corporation, with its principal office in
Boston, Massachusetts. Prior to Akcea’s initial public offering, or IPO, in July 2017, we owned 100 percent of Akcea’s stock. In October 2020, we completed a merger transaction with Akcea such that following the completion of the merger, Akcea became
our wholly owned subsidiary.
We make available, free of charge, on our website, www.ionispharma.com, our reports on Forms 10-K, 10-Q, 8-K and amendments thereto, as soon as reasonably practical after we file such materials with the Securities and Exchange Commission, or SEC. Periodically, we
provide updates about the company in the Newsroom section of the Investors & Media page of our website. Any information that we include on or link to our website is not a part of this report or any registration statement that incorporates this
report by reference. The SEC maintains an internet site, www.sec.gov, that contains reports, proxy and information statements that we file
electronically with the SEC.
IONIS PHARMACEUTICALS, INC.
FORM 10-K
For the Fiscal Year Ended December 31, 2021
|
PART I
|
||
|
Page
|
||
|
Item 1.
|
Business
|
4
|
|
Item 1A.
|
Risk Factors
|
49
|
|
Item 1B.
|
Unresolved Staff Comments
|
66
|
|
Item 2.
|
Properties
|
66
|
|
Item 3.
|
Legal Proceedings
|
66
|
|
Item 4.
|
Mine Safety Disclosures
|
66
|
|
PART II
|
||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
67
|
|
Item 6.
|
Selected Financial Data
|
68
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
68
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
79
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
79
|
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
|
79
|
|
Item 9A.
|
Controls and Procedures
|
79
|
|
Item 9B.
|
Other Information
|
82
|
|
Item 9C.
|
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
|
82
|
|
PART III
|
||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
82
|
|
Item 11.
|
Executive Compensation
|
82
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
83
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
83
|
|
Item 14.
|
Principal Accountant Fees and Services
|
83
|
|
PART IV
|
||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
83
|
|
Signatures
|
92
|
PART I
Item 1. Business
Overview
We are a leader in RNA-targeted therapeutics. We believe our medicines, which are based on our novel antisense technology, have the
potential to pioneer new markets, change standards of care and transform the lives of people with devastating diseases. We currently have three marketed medicines- SPINRAZA, TEGSEDI and WAYLIVRA. We also have a rich late-stage pipeline of medicines,
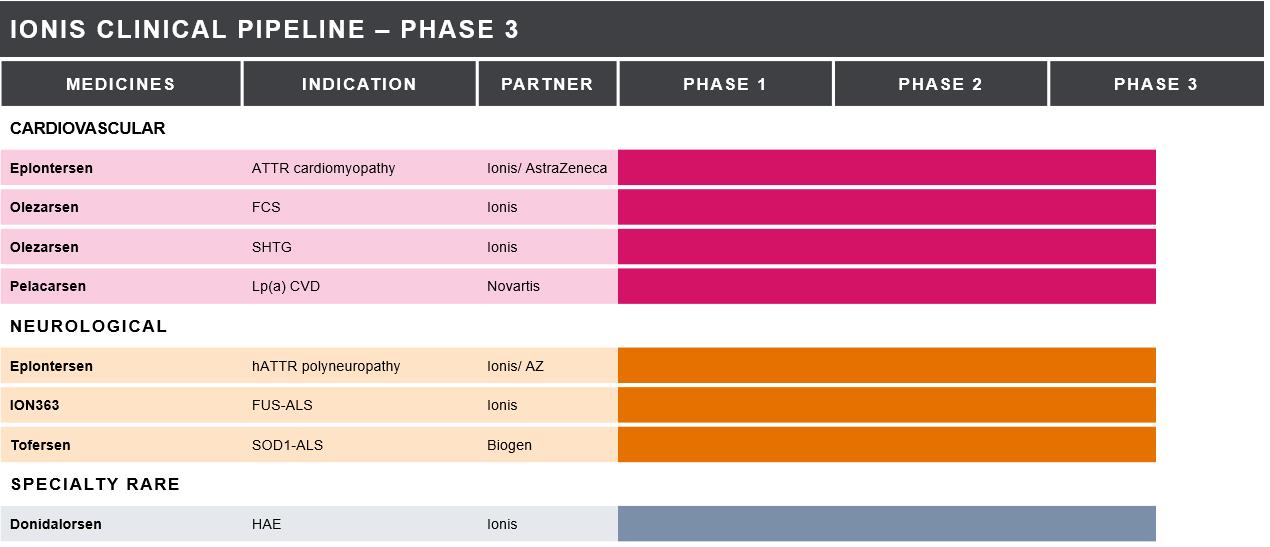
primarily focused on our cardiovascular and neurology franchises. Our late-stage pipeline consists of six medicines in Phase 3 development for eight indications.
Over the past year, we made important progress toward achieving our goal to be a leading fully integrated biotechnology company. We
advanced our commercial strategy and go-to-market plans for our near-term commercial opportunities, eplontersen, olezarsen and donidalorsen. We entered an agreement with AstraZeneca to jointly develop and commercialize eplontersen. We believe this
agreement positions eplontersen to maximize value for patients and shareholders while also enabling us to bolster our commercial organization and accelerate our preparations for our near-term product launches.
We continued to advance and expand our Phase 3 pipeline with the achievement of key enrollment milestones for eplontersen and pelacarsen,
and the addition of two new Phase 3 programs for olezarsen and donidalorsen, bringing us to 6 medicines in Phase 3 development addressing 8 indications. In 2021, we also reported data from the Phase 3 VALOR study of tofersen in patients with SOD1-ALS.
While VALOR did not achieve statistical significance in the primary endpoint, signs of reduced disease progression were observed across multiple secondary and exploratory endpoints. Biogen is actively engaged with regulators to determine the next steps
for tofersen. In addition, Roche recently announced plans to initiate a new Phase 2 study of tominersen in patients with Huntington’s disease, based on new findings from a post hoc analysis of the Phase 3 GENERATION HD1 study of tominersen.
Our mid-stage pipeline also continued to perform well, with positive data readouts from several medicines. And we invested in expanding
the reach of our technology, including obtaining exclusive rights to Bicycle Therapeutic’s peptide technology targeting transferrin receptor 1 to expand the capabilities of our Ligand Conjugated Antisense, or LICA, technology. We strengthened our
financial position and focused our resources in support of our highest priority programs through the integration of Akcea Therapeutics and our distribution agreements with Swedish Orphan Biovitrum AB, or Sobi. We accomplished all this and exceeded our
2021 financial guidance, including achieving revenues of $810 million. And we remain well capitalized with a 2021 year-end cash balance of $2.1 billion.
Our multiple sources of revenue and strong balance sheet enable us to invest in our strategic
priorities to build our commercial pipeline, expand and diversify our technology and deliver new medicines to the market. By continuing to focus on these priorities, we believe we are well positioned to drive future growth and to deliver increasing
value for patients and shareholders.
Marketed Medicines
SPINRAZA is the global foundation-of-care for the treatment of patients of all ages with spinal muscular atrophy, or SMA, a progressive,
debilitating and often fatal genetic disease. Biogen, our partner responsible for commercializing SPINRAZA worldwide, reported that as of December 31, 2021, over 11,000 patients were on SPINRAZA therapy in markets around the world. From inception
through December 31, 2021, we have earned more than $1.6 billion in revenues from our SPINRAZA collaboration, including nearly $1.2 billion in royalties on sales of SPINRAZA.
TEGSEDI is a once weekly, self-administered subcutaneous medicine approved in the U.S., Europe, Canada and Brazil for the treatment of
patients with polyneuropathy caused by hATTR, a debilitating, progressive, and fatal disease. We launched TEGSEDI in the U.S. and the European Union, or EU, in late 2018. In 2021, we began selling TEGSEDI in Europe through our distribution agreement
with Sobi. Additionally, in the second quarter of 2021, Sobi began distributing TEGSEDI in the U.S. and Canada. In Latin America, PTC Therapeutics International Limited, or PTC, is commercializing TEGSEDI in Brazil and is pursuing access in additional
Latin American countries through its exclusive license agreement with us.
WAYLIVRA is a once weekly, self-administered, subcutaneous medicine that received conditional marketing authorization in May 2019 from the
European Commission, or EC, as an adjunct to diet in adult patients with genetically confirmed familial chylomicronemia syndrome, or FCS, and at high risk for pancreatitis. We launched WAYLIVRA in the EU in the third quarter of 2019. In 2021, we began
selling WAYLIVRA in Europe through our distribution agreement with Sobi. Through our exclusive license agreement with PTC, PTC is working to provide access to WAYLIVRA across Latin America, beginning in Brazil. In the third quarter of 2021, the
National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária), or ANVISA, approved WAYLIVRA in Brazil. In December 2021, PTC submitted an application to ANVISA for approval of WAYLIVRA for the
treatment of familial partial lipodystrophy, or FPL, in Brazil. If approved, Waylivra will be the first approved treatment for patients with FPL in Brazil.
Under our distribution agreements with Sobi, we retained the marketing authorizations for TEGSEDI and WAYLIVRA. We will continue to supply
commercial product to Sobi and manage regulatory and manufacturing processes, as well as relationships with key opinion leaders. We will also continue to lead the TEGSEDI and WAYLIVRA global commercial strategy. In connection with the agreements, we
restructured our European operations in the first quarter of 2021, and we restructured our North American TEGSEDI operations in the second quarter of 2021.
We currently have six medicines in Phase 3 studies for eight indications, which include:
| ● |
Eplontersen: In July 2021, we achieved full enrollment in the NEURO-TTRansform Phase 3 study with data expected mid-2022. Enrollment is ongoing in the CARDIO-TTRansform
Phase 3 study
|
| o |
In November 2021, we entered into an agreement with AstraZeneca for eplontersen, under which we will jointly develop and commercialize eplontersen in the U.S. AstraZeneca
has exclusive rights to commercialize eplontersen in the rest of the world
|
| ● |
Olezarsen: We initiated the Phase 3 CORE study in patients with severe hypertriglyceridemia, or SHTG, in October 2021. Enrollment is ongoing in the BALANCE Phase 3 study
in patients with FCS
|
| o |
Data from the Phase 2 study of olezarsen in patients with moderate hypertriglyceridemia and at high risk for or with established cardiovascular disease were published in
the European Heart Journal
|
| ● |
Donidalorsen: Based on positive topline data from a Phase 2 study of donidalorsen in patients with hereditary angioedema which we reported in April 2021, we initiated the
Phase 3 OASIS-HAE study in November 2021
|
|
o
|
We reported additional positive results from the Phase 2 study of donidalorsen at the ACAAI annual scientific meeting in November 2021, demonstrating
rapid and sustained reductions in HAE attacks with favorable safety and tolerability
|
| ● |
ION363: In April 2021, we initiated a Phase 3 study in patients with amyotrophic
lateral sclerosis, or ALS, with mutations in the fused in sarcoma gene, or FUS, or FUS-ALS, the most common cause of juvenile-onset ALS
|
| ● |
Pelacarsen: In August 2021, Novartis achieved 50 percent enrollment in Novartis’ Lp(a) HORIZON Phase 3 cardiovascular outcome study in patients with established
cardiovascular disease and elevated lipoprotein(a), or Lp(a)
|
| ● |
Tofersen: In October 2021, Biogen reported that tofersen did not meet the primary clinical endpoint in the Phase 3 VALOR study; however, trends favoring tofersen were
seen across multiple secondary and exploratory measures of disease activity and clinical function
|
| o |
Biogen is actively engaging with regulators, the medical community, patient advocacy groups and other key stakeholders around the world to determine potential next
steps
|
| o |
Given the high unmet medical need, Biogen expanded its ongoing early access program, or EAP, to the broader SOD1-ALS population
|
| o |
The Phase 3 ATLAS study in patients with presymptomatic SOD1-ALS is ongoing
|
COVID-19
As a company focused on improving the health of people around the world, our priority during the COVID-19 pandemic
is the safety of our employees, their families, the healthcare workers who work with us and the patients who rely on our medicines. We are also focused on maintaining the quality of our studies and minimizing the impact to timelines. While the
COVID-19 pandemic has impacted some areas of our business, we believe our mitigation efforts and financial strength will enable us to continue to manage through the pandemic and execute on our strategic initiatives. Because the situation is extremely
fluid, we are continuing to evaluate the impact COVID-19 could have on our business, including the impact on our commercial products and the medicines in our pipeline.
Our Marketed Medicines – Potentially Transformational Medicines Bringing Value to Patients Today
SPINRAZA –
SPINRAZA (nusinersen) injection for intrathecal use is a survival motor neuron-2, or SMN2, directed antisense medicine indicated for the treatment of SMA in pediatric and adult patients.
SPINRAZA continues to demonstrate substantial benefit in SMA patients of all ages, supporting its position as a global foundation of care
for the treatment of SMA. Biogen, our worldwide commercial partner, reported that as of December 31, 2021, there were more than 11,000 patients on SPINRAZA therapy.
SMA is characterized by loss of motor neurons
in the spinal cord and lower brain stem, People with SMA have a deletion or defect in their SMN1 gene and rely on their SMN2 gene to produce functional SMN protein, which motor neurons need to maintain motor function and muscle strength. However, the SMN2 gene can only produce approximately 10 percent of the SMN protein critical
for motor neurons, resulting in severe and progressive loss of motor function and strength.
The rate and severity of degeneration varies depending on the amount of functional SMN protein a patient can produce. Type
1, or infantile-onset, SMA is the most severe form of the disease. Type 1 SMA patients produce very little SMN protein and often progress to death or permanent ventilation by the age of 2. Patients with Type 2 or Type 3, or later-onset, SMA produce
more SMN protein, but also suffer from a progressive loss of muscle strength and function and a reduced life expectancy.
Biogen continues to expand the body of evidence supporting SPINRAZA’s durable efficacy and well-established safety
profile to address the remaining needs of SMA patients of all ages. In the Phase 2/3 DEVOTE study, Biogen is evaluating the safety and potential to achieve increased efficacy with a higher dose of SPINRAZA compared to the currently approved dose. At
the AAN 2021 Virtual Annual meeting in April 2021, Biogen reported that initial findings from the DEVOTE study suggest no new safety concerns and support continued development of a higher dose of SPINRAZA.
In January 2021, Biogen initiated the Phase
4 RESPOND study evaluating the benefit of SPINRAZA in infants and children with a suboptimal clinical response to the gene therapy, onasemnogene abeparvovec.
And in September 2021, Biogen initiated the Phase 3b ASCEND study designed to evaluate the clinical outcomes and assess
the safety of a higher dose of SPINRAZA in children, teens and adults with later-onset SMA following treatment of risdiplam.
Additionally, Biogen continues to conduct
the Phase 2 NURTURE study, an open-label study investigating the benefit of SPINRAZA when administered before symptom onset in patients genetically diagnosed with SMA, and likely to develop Type 1 or Type 2 SMA. NURTURE was the first study to
investigate the potential to slow or stop SMA disease progression in presymptomatic SMA patients. In June 2021, Biogen reported data from an interim analysis, showing that all study patients remain alive without the need for permanent ventilation.
Additionally, at the time of the interim analysis, 92 percent of patients maintained the ability to swallow.
The approval of SPINRAZA was based on efficacy and safety data from multiple clinical studies, including two randomized,
placebo-controlled Phase 3 studies, ENDEAR, in patients with infantile-onset SMA, and CHERISH, in patients with later-onset SMA as well as from SHINE, an open-label extension, or OLE, study for patients with SMA who participated in prior SPINRAZA
studies.
TEGSEDI – TEGSEDI (inotersen)
injection is an RNA-targeted medicine indicated for the treatment of polyneuropathy due to hATTR in adults. TEGSEDI prevents the creation of TTR proteins,
reducing the amount of amyloid that builds up, which damages organs and issues.
Polyneuropathy due to hATTR is caused by the accumulation of misfolded mutated TTR protein in the peripheral nerves. Patients with
polyneuropathy due to hATTR experience ongoing debilitating nerve damage throughout their body resulting in the progressive loss of motor functions, such as walking. These patients also accumulate TTR in other major organs, which progressively
compromises their function and eventually leads to death within five to fifteen years of disease onset. There are an estimated 40,000 patients with polyneuropathy due to hATTR worldwide.
TEGSEDI is commercially available in numerous countries, including the U.S., many European countries, Canada, and Latin America. In 2021, we began selling TEGSEDI in Europe through our distribution agreement with Sobi. Additionally, in the second quarter of 2021, Sobi began distributing
TEGSEDI in the U.S. and Canada. In Latin America, PTC through its exclusive license agreement with us, is commercializing TEGSEDI in Brazil and is working to achieve access in additional Latin American countries.
The approvals of TEGSEDI were based on efficacy and safety data from the Phase 3 NEURO-TTR study in patients with hATTR amyloidosis with
stage 1 and stage 2 polyneuropathy. We also conducted an OLE study in patients with hATTR treated with TEGSEDI to evaluate the long-term efficacy and safety
profile of TEGSEDI. We reported interim data from the study that demonstrated continued efficacy in patients after two years. Results also showed that patients who started treatment earlier achieved greater long-term disease stabilization
compared to those who switched from placebo to TEGSEDI in the OLE study.
WAYLIVRA – WAYLIVRA (volanesorsen)
is an antisense medicine indicated as an adjunct to diet in adult patients with genetically confirmed FCS and at high risk for pancreatitis, in whom response to diet and triglyceride lowering therapy has been inadequate. WAYLIVRA reduces triglyceride levels by inhibiting the production of apolipoprotein C-III, or apoC-III, a protein that is a key regulator of triglyceride levels.
FCS is a rare, genetic disease estimated to affect 3,000 to 5,000 people worldwide and characterized by extremely elevated triglyceride
levels. FCS can lead to many chronic health issues including severe, recurrent abdominal pain, fatigue, high risk of life-threatening pancreatitis and abnormal enlargement of the liver or spleen. In addition, people with FCS are often unable to work,
adding to their disease burden. In severe cases, patients can have bleeding into the pancreas, serious tissue damage, infection, and cyst formation, as well as damage to other vital organs such as the heart, lungs, and kidneys.
WAYLIVRA is commercially available in multiple European countries and in Brazil. In 2021, we began selling WAYLIVRA in Europe through our distribution agreement with Sobi. In Latin America, PTC through its exclusive license agreement with us, is commercializing WAYLIVRA in Brazil and is
working to achieve access in additional Latin American countries.
WAYLIVRA’s conditional marketing authorization in the EU and approval in Brazil were based on efficacy and safety data from the Phase 3
APPROACH study, the ongoing APPROACH OLE study and supported by results from the Phase 3 COMPASS study.
Drug Discovery and Development
Introduction to Drug Discovery
Proteins are essential working molecules in a cell. Almost all human diseases result from inappropriate protein
production, improper protein activity or loss of a protein. Antisense medicines can modify the production of proteins by targeting RNAs. In this way, antisense medicines can inhibit the production of a disease-causing protein, modify the protein
produced or increase the production of a protein that, when absent, causes diseases. Antisense medicines can also treat diseases by targeting and reducing RNAs that may be causing diseases (so called “toxic RNAs”). RNAs are naturally occurring
molecules in the body that primarily act as messengers that carry the information the cell needs to produce proteins from the deoxyribonucleic acid, or DNA, to the protein making complex in the cell. When antisense medicines bind to the specific RNAs
of a particular gene, they will ultimately alter the production of the protein encoded in the target gene or, in the case of disease-causing RNAs, degrade the toxic RNAs.
Our Pipeline
We are a leader in the discovery and development of RNA-targeted therapeutics. We are focused on pioneering new markets
and changing standards of care with a focus on cardiovascular and neurological diseases. Additionally, we are developing a number of medicines that are outside these areas. We also have an emerging specialty rare disease pipeline comprised of medicines
which we believe represent a compelling opportunity for us. We are developing our medicines for systemic and local delivery (e.g., subcutaneous, intrathecal, intraocular, oral and aerosol). We plan to continue adding new investigational medicines to
our pipeline in the future.
We have built a rich pipeline of medicines designed to treat many serious diseases. To select the best candidates, we
efficiently screen many targets in parallel and apply our rational approach to selecting disease targets. With our expertise in discovering and characterizing novel antisense medicines, our scientists can optimize the properties of our antisense
medicines against each particular target. We have created LICA technology, which we designed to enhance the effective uptake and activity of our medicines in particular tissues. With our LICA technology we attach specific chemical structures or
molecules to our antisense medicines. With our first LICA conjugate, a complex sugar-like molecule called N-acetylgalactosamine, or GalNAc, we have shown an increase in medicinal potency of 20-30-fold for liver targets, compared to non-conjugated
antisense medicines. Many of the medicines in our pipeline are LICA medicines, including four LICA medicines currently in Phase 3 studies: eplontersen, olezarsen, donidalorsen and pelacarsen. We have utilized our chemistry advancements to expand the
therapeutic and commercial opportunities of our pipeline. Our antisense technology, along with our manufacturing and analytical processes that are the same across our medicines, shorten our timeline from initial concept to the first human dose, when
compared to early development timelines for other drug modalities like small molecule and monoclonal antibody medicines.
The table below lists the medicines in our clinical pipeline. We categorize patient studies to establish a medicine’s
safety profile as Phase 1/2 and those studies in healthy volunteers as Phase 1. The table includes the disease indication, a partner (if the medicine is partnered), and the development status of each medicine. We have included descriptions for each of
our medicines in Phase 2 and Phase 3 development below.

*China Only
Our Phase 3 Medicines
We currently have six medicines in Phase 3 studies for eight indications: eplontersen, olezarsen, donidalorsen, ION363, pelacarsen and
tofersen.
Eplontersen (TTR) – Eplontersen (formerly IONIS-TTR-LRx) is an
investigational LICA medicine we designed to inhibit the production of TTR protein. We are developing eplontersen as a monthly self-administered subcutaneous injection to treat all types of ATTR. ATTR amyloidosis is a systemic, progressive
and fatal disease in which patients experience multiple overlapping clinical manifestations caused by the inappropriate formation and aggregation of TTR amyloid deposits in various tissues and organs, including peripheral nerves, heart, intestinal
tract, eyes, kidneys, central nervous system, thyroid and bone marrow. The progressive accumulation of TTR amyloid deposits in these tissues and organs leads to organ failure and eventually death.
Polyneuropathy due to hATTR is caused by the accumulation of misfolded mutated TTR protein in the peripheral nerves. Patients with
polyneuropathy due to hATTR experience ongoing debilitating nerve damage throughout their body resulting in the progressive loss of motor functions, such as walking. These patients also accumulate TTR in other major organs, which progressively
compromises their function and eventually leads to death within five to fifteen years of disease onset. There are an estimated 40,000 patients with polyneuropathy due to hATTR worldwide.
ATTR cardiomyopathy is caused by the accumulation of misfolded TTR protein in the cardiac muscle. Patients experience ongoing
debilitating heart damage resulting in progressive heart failure, which results in death within 3 to 5 years from disease onset. ATTR cardiomyopathy includes both the genetic and wild-type form of the disease. There are an estimated 300,000 to
500,000 patients with ATTR cardiomyopathy worldwide.
Often patients with the polyneuropathy form of TTR amyloidosis will have TTR build up in the heart and experience cardiomyopathy
symptoms. Similarly, patients with the cardiomyopathy form of TTR amyloidosis may often have TTR build up in their peripheral nerves and experience nerve damage and progressive difficulty with motor functions.
In November 2019, we initiated the NEURO-TTRansform Phase 3 study of eplontersen in patients with polyneuropathy caused by hATTR
amyloidosis. NEURO-TTRansform is a global, multi-center, randomized, open-label study designed to evaluate the efficacy, safety and tolerability of
eplontersen. The NEURO-TTRansform study is fully enrolled with 168 patients. We expect data from the NEURO-TTRansform study in mid-2022. The current
study will be compared to the historical placebo arm from the TEGSEDI (inotersen) NEURO-TTR Phase 3 study. The NEURO-TTRansform study includes multiple primary endpoints, including the percent change from baseline in serum TTR concentration modified
Neuropathy Impairment Score +7, or mNIS+7, a measure of neuropathic disease progression and in the Norfolk Quality of Life Questionnaire-Diabetic
Neuropathy, or Norfolk QoL-DN.
In January 2020, we initiated the CARDIO-TTRansform Phase 3 cardiovascular outcome study of eplontersen in patients with ATTR
cardiomyopathy. CARDIO-TTRansform is a global, multi-center, randomized, double-blind, placebo-controlled study in up to 750 patients designed to
evaluate the efficacy, safety and tolerability of eplontersen. The CARDIO-TTRansform study includes co-primary outcome measures of cardiovascular death
and frequency of cardiovascular clinical events.
In September 2019, we reported results from the Phase 1 study with eplontersen in healthy volunteers at the Heart Failure Society of
America Annual Meeting. In this study, subjects treated with eplontersen achieved dose-dependent reductions of TTR protein of up to 94 percent and eplontersen had a favorable safety and tolerability profile supportive of continued development.
In January 2022, the FDA granted an Orphan Medicine Designation for eplontersen.
In December 2021, we entered into an agreement with AstraZeneca to jointly develop and commercialize eplontersen in the
U.S. AstraZeneca obtained exclusive rights to commercialize eplontersen outside the U.S, except for certain Latin American countries.
Olezarsen (ApoC-III) – Olezarsen (formerly IONIS-APOCIII-LRx) is an investigational LICA medicine we designed to inhibit
the production of apoC-III for patients who are at risk of disease due to elevated triglyceride levels. ApoC-III is a protein produced in the liver that regulates triglyceride metabolism in the blood. People with severely elevated triglycerides, such
as people with FCS, are at high risk for acute pancreatitis and an increased risk of CVD. It is estimated that there are between 3,000 to 5,000 patients with FCS worldwide and more than three million patients with severely high triglycerides in the
U.S.
In December 2020, we initiated our first Phase 3 study of olezarsen, BALANCE, in patients with FCS. BALANCE is a global,
multi-center, randomized, double-blind, placebo-controlled study enrolling up to 60 patients (age 18 and over) designed to assess the efficacy, safety and tolerability of olezarsen. The primary endpoint is percent change from baseline in fasting
triglyceride levels at six months compared to placebo.
In November 2021, we initiated a
second Phase 3 study of olezarsen, CORE, in patients with SHTG. CORE is a global, multi-center, randomized, double-blind, placebo-controlled study enrolling up to 450 patients designed to assess the efficacy, safety and tolerability of olezarsen. The
CORE study will compare olezarsen to placebo in patients with triglyceride levels equal to or greater than 500 mg/dL who are on currently available therapies for elevated triglycerides. The primary endpoint of the study is the percent change in fasting
triglycerides from baseline at month 6.
In January 2020, we reported positive
results from a Phase 2 clinical study in patients with hypertriglyceridemia and at high risk of or with established CVD. Olezarsen achieved statistically significant, dose-dependent reductions in fasting triglycerides compared to placebo at
all dose levels. Additionally, at the highest monthly dose, 91 percent of patients achieved serum triglycerides of ≤ 150 mg/dL, the recognized threshold for cardiovascular risk, compared to less than 5 percent of patients in the placebo group.
Olezarsen also achieved statistical significance in numerous key secondary endpoints, including significant reductions in apoC-III, very low-density lipoprotein cholesterol, or VLDL-C, and remnant cholesterol, and a statistically significant increase
in high-density lipoprotein cholesterol, or HDL-C. Olezarsen had a favorable safety and tolerability profile supportive of continued development.
Donidalorsen (PKK) – Donidalorsen (formerly IONIS-PKK-LRx) is an investigational LICA medicine we designed to inhibit the production of prekallikrein, or PKK,
to treat people with HAE. HAE is a rare genetic disease that is characterized by rapid and painful attacks of inflammation in the hands, feet, limbs, face, abdomen, larynx, and trachea and can be fatal if swelling occurs in the larynx. PKK plays an
important role in the activation of inflammatory mediators associated with acute attacks of HAE. By inhibiting the production of PKK, donidalorsen could be an effective prophylactic approach to preventing or reducing the severity of HAE attacks. It is
estimated that there are more than 20,000 patients with HAE in the U.S. and EU.
In November 2021, we initiated the Phase 3 study of donidalorsen, OASIS-HAE, in patients with HAE. OASIS-HAE is a multi-center,
randomized, double-blind placebo-controlled study in up to 84 patients designed to assess the efficacy, safety and tolerability of olezarsen. The primary endpoint is the time-normalized number of investigator-confirmed HAE attacks per month from Week 1
to Week 25.
In March 2021, we reported positive results
from a Phase 2 clinical study of donidalorsen in patients with HAE. Patients received either donidalorsen 80mg or placebo subcutaneously once monthly for 17 weeks. The Phase 2 study met its primary and secondary endpoints, achieving
significant reductions in the number of attacks suffered by patients with HAE compared to placebo. The study demonstrated a mean reduction of 90 percent in the number of monthly HAE attacks in weeks one to 17 of the study (p <0.001) and a mean
reduction of 97 percent in the number of monthly HAE attacks in weeks five to 17 (p=0.003). In weeks five to 17, 92 percent of patients treated with donidalorsen were attack-free compared to 0 percent in the placebo group (p <0.001). Additionally,
in November 2021 we reported additional data from the Phase 2 study, including that donidalorsen demonstrated an overall reduction in moderate to severe attacks starting with the second dose. For the final month of the study, all donidalorsen treated
patients were attack-free. Donidalorsen had a favorable safety and tolerability profile supportive of continued development.
In September 2020, results from the Phase 1 study of donidalorsen in healthy volunteers and a compassionate-use study of
IONIS-PKKRx and donidalorsen in patients living with severe angioedema were published in The New England Journal of Medicine. In the study, we observed that the medicines reduced plasma prekallikrein activity levels and showed evidence of clinical efficacy in reducing the number of
breakthrough attacks per month in patients over the course of the treatment, including complete resolution in a patient.
ION363 (FUS) – ION363 is an
investigational antisense medicine we designed to reduce the production of the FUS protein to treat people with ALS caused by mutations in the FUS gene. Because antisense-mediated reduction of mutant FUS protein in a FUS-ALS mouse model demonstrated
the ability to prevent motor neuron loss, it is hypothesized that reduction of FUS protein will reverse or prevent disease progression in FUS-ALS patients. It is estimated that there are approximately 350 patients with FUS-ALS in G7 countries
(comprised of Canada, France, Germany, Italy, Japan, the United Kingdom and the U.S.).
In April 2021, we initiated a Phase 3 study of ION363 in patients with FUS-ALS. The Phase 3 trial of ION363 is a global, multi-center,
randomized, double-blind, placebo-controlled study enrolling up to 64 patients designed to assess the efficacy, safety and tolerability of ION363. Part 1 of the trial will consist of patients randomized to receive a multi-dose regimen of ION363 or
placebo for 29 weeks, followed by Part 2, which will be an open-label period in which all patients in the trial will receive ION363 for 73 weeks. The primary endpoint is change from baseline as measured by the ALSFRS-R Total Score, time of rescue or
discontinuation from Part 1 and entering Part 2 due to a deterioration in function, and Ventilation Assistance-free survival, or VAFS.
Pelacarsen (Apo(a)) (TQJ230) – Pelacarsen (formerly IONIS-APO(a)-LRx) is an investigational LICA antisense
medicine we designed to inhibit the production of apolipoprotein(a), or Apo(a), in the liver to offer a direct approach for reducing Lp(a). Elevated Lp(a) is recognized as an independent, genetic cause of CVD. Lp(a) levels are determined at birth and
lifestyle modification, including diet and exercise, do not impact Lp(a) levels. Inhibiting the production of Apo(a) in the liver reduces the level of Lp(a) in blood, potentially slowing down or reversing cardiovascular disease in people with
hyperlipoproteinemia(a), a condition in which individuals have levels of Lp(a) greater than 50 mg/dL, the recognized threshold for risk of CVD. We believe antisense technology is well suited to address hyperlipoproteinemia(a) because antisense
technology specifically targets the RNA that codes for all forms of the Apo(a) molecule. Furthermore, we believe addressing elevated Lp(a) is the next important horizon in CVD risk reduction. It is estimated that there are more than eight million
people living with CVD and elevated levels of Lp(a).
In December 2019, Novartis initiated
the Phase 3 study of pelacarsen, Lp(a) HORIZON, in patients with elevated Lp(a) levels and a prior cardiovascular event. Lp(a) HORIZON is a global, multi-center, randomized, double-blind, placebo-controlled cardiovascular outcomes study in more than 8,000 patients designed to assess the efficacy,
safety and tolerability of pelacarsen. Patients will be treated with 80 mg of pelacarsen administered monthly by subcutaneous injection. The primary endpoint in Lp(a) HORIZON is the time to occurrence of first major adverse cardiovascular event, or MACE. In August 2021, we announced that the
Lp(a) HORIZON study had reached 50 percent enrollment.
In November 2018, we reported results of the Phase 2 study of pelacarsen in patients with hyperlipoproteinemia(a) at the American Heart
Association, or AHA, annual meeting. In the Phase 2 study, we observed statistically significant and dose dependent reductions from baseline in Lp(a) levels. Approximately 98 percent of patients who received the highest dose in the study demonstrated a
reduction in Lp(a) levels to below 50 mg/dL. Pelacarsen had a favorable safety and tolerability profile supportive of continued development.
In February 2019, Novartis exercised its option to license pelacarsen. As a result, Novartis is responsible for global
development, regulatory and commercialization activities, and costs for pelacarsen.
Tofersen (SOD1) (BIIB067) – Tofersen (formerly IONIS-SOD1Rx) is an investigational antisense medicine we designed to inhibit the production of superoxide dismutase 1,
or SOD1, which is a well understood genetic cause of ALS. SOD1-ALS is a rare, fatal, neurodegenerative disorder caused by a mutation in the SOD1 gene leading to a progressive loss of
motor neurons. As a result, people with SOD1-ALS experience increasing muscle weakness, loss of movement, difficulty breathing and swallowing and eventually succumb to the disease. Current treatment options for people with SOD1-ALS are extremely
limited, with no medicines that significantly slow disease progression. Tofersen is one of four medicines we have in development to treat ALS. It is estimated that there are approximately 1,400 patients with SOD1-ALS in G7 countries.
In October 2021, Biogen announced topline results of the Phase 3 VALOR study of tofersen in patients with SOD1-ALS
designed to assess the efficacy, safety and tolerability of tofersen. While tofersen did not meet the primary endpoint of change from baseline to 28 weeks in the ALSFRS-R, trends favoring tofersen were seen across multiple secondary and exploratory
measures of disease activity and clinical function. As a result, Biogen is actively engaged with regulators to determine next steps for the program. Additionally, in October 2021, Biogen announced that it would expand eligibility for its ongoing EAP to
all people with SOD1-ALS, where permitted.
In April 2021, Biogen initiated a second Phase 3 study of tofersen, ATLAS, in presymptomatic individuals with a SOD1
genetic mutation and biomarker evidence of disease activity. ATLAS is a multi-center, randomized, double-blind, placebo-controlled study enrolling up to 150 subjects designed to assess the efficacy, safety and tolerability of tofersen in presymptomatic
individuals with a SOD1 genetic mutation and biomarker evidence of disease activity.
Biogen conducted a Phase 1/2 study that demonstrated proof of biology and proof of concept. At the highest dose tested, treatment with
tofersen over a three month period resulted in a statistically significant lowering of SOD1 protein levels in the cerebrospinal fluid, or CSF, and positive numerical trends across three efficacy endpoints compared to placebo, including slowing of
clinical decline as measured by the ALSFRS-R. Tofersen had a favorable safety and tolerability profile supportive of continued development.
In December 2018, Biogen exercised its option to license tofersen, as a result, Biogen is responsible for global
development, regulatory and commercialization activities, and costs for tofersen.
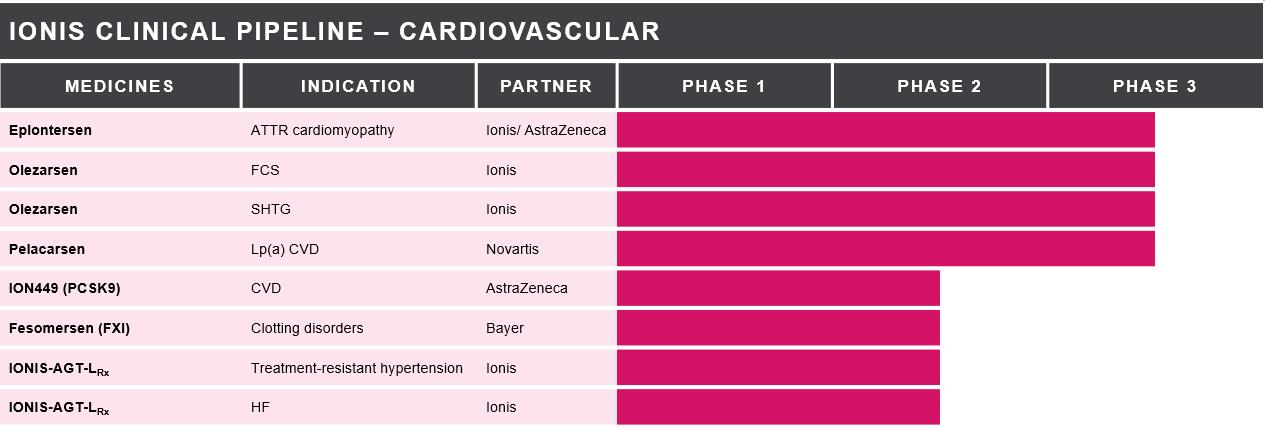
Our Cardiovascular Medicines in Development
According to the World Health Organization, or WHO, CVD remains the number one cause of death globally. An estimated 17.9 million people
died from CVD in 2019, representing approximately 30 percent of all deaths globally. Our cardiovascular medicines target the major risk factors of cardiovascular disease, including cholesterol, triglycerides, and hypertension.
Eplontersen – See the medicine
description under “Our Phase 3 Medicines” section above.
Olezarsen – See the medicine
description under “Our Phase 3 Medicines” section above.
Pelacarsen – See
the medicine description under “Our Phase 3 Medicines” section above.
ION449 (PCSK9) (AZD8233) – ION449 (formerly IONIS-AZ4-2.5-L-Rx) is an investigational LICA medicine we designed to reduce the production of proprotein convertase subtilisin/kexin type 9, or PCSK9, in the liver. PCSK9 is integrally involved in the regulation of LDL-cholesterol. Genetic studies
have shown that individuals with life-long reductions of LDL-C due to reduced function of PCSK9 have substantially reduced risk of CVD.
In November 2020, AstraZeneca initiated the Phase 2b study of ION449 in patients with LDL-C levels between 70 and 190 mg/dl and receiving statin therapy. The study is a randomized, double-blind, placebo-controlled clinical study in approximately 110 patients to assess the efficacy, safety
and tolerability of ION449. The primary objective is to assess the effect of different doses of ION449 on LDL-C compared to placebo at Week 12 in patients taking baseline statin therapy. The study will evaluate three dose levels of ION449 versus
placebo, all administered once a month by subcutaneous injection.
In November 2021, we reported positive results from the Phase 1 study of ION449 in patients with dyslipidemia. Participants were treated
with multiple ascending subcutaneous doses and ION449 demonstrated dose-dependent mean reductions in circulating plasma PCSK9 and LDL-C levels and had a favorable safety and tolerability profile supportive of continued development.
In October 2020, we reported positive results from the Phase 1 study of ION449 in healthy volunteers. Participants were treated with a
single subcutaneous dose and ION449 demonstrated dose-dependent mean reductions in circulating plasma PCSK9 and LDL-C levels and had a favorable safety and tolerability profile supportive of continued development.
We licensed ION449 to AstraZeneca under our cardiovascular, renal and metabolic diseases collaboration. As a result,
AstraZeneca is responsible for global development, regulatory and commercialization activities, and costs for ION449.
Fesomersen (FXI)
(BAY2976217) – Fesomersen (formerly IONIS-FXI-LRx) is an investigational LICA medicine we designed to inhibit the production of Factor XI. Factor XI is a clotting factor produced in the liver that is important in the growth of blood clots.
Thrombosis, characterized by the formation of a blood clot inside blood vessels, can cause heart attacks and strokes. People who are deficient in Factor XI have a lower incidence of thromboembolic events with minimal increase in bleeding risk. Although
currently available anticoagulants reduce the risk of thrombosis, physicians associate these anticoagulants with increased bleeding, which can be fatal. By inhibiting Factor XI production, we believe that fesomersen can be used broadly as an
anti-thrombotic in many different therapeutic settings for which additional safe and well tolerated anti-thrombotic medicines are needed.
In August 2020, Bayer initiated the RE-THINc Phase 2b study of fesomersen in patients with end-stage renal disease, or
ESRD, on hemodialysis. RE-THINc is a randomized, blinded, placebo-controlled study in approximately 290 patients to assess the efficacy, safety and tolerability of fesomersen. The study is designed to evaluate multiple monthly doses administered
subcutaneously. The primary endpoint is incidence of major bleeding and clinically relevant non-major bleeding.
We conducted a Phase 1, blinded, randomized, placebo-controlled, dose-escalation study of fesomersen in healthy
volunteers. In this study, fesomersen produced significant reductions in FXI activity and FXI antigen, without evidence of increased bleeding and had a favorable safety and tolerability profile supportive of continued development.
In February 2017, we licensed fesomersen to Bayer. As a result, Bayer is responsible for global development, regulatory and
commercialization activities, and costs for fesomersen.
IONIS-AGT-LRx – IONIS-AGT-LRx is an investigational LICA medicine we designed to inhibit the production of
angiotensinogen to decrease blood pressure in people with treatment resistant hypertension, or TRH. Despite the availability of antihypertensive agents, TRH is still a major contributor to cardiovascular and renal disease. Approximately 140 million
adults globally and approximately 10 million adults in the U.S. have resistant hypertension, defined as failure to achieve a blood pressure goal of 140/90 (systolic/diastolic) despite the use of three or more antihypertensive medications. People with
TRH have been found to have a three-fold higher chance of having fatal and non-fatal cardiovascular events relative to those with controlled hypertension.
We are also studying IONIS-AGT-LRx in patients with chronic heart failure with reduced ejection fraction.
Heart failure, or HF, afflicts approximately 6.5 million patients in the United States, or U.S., and 26 million worldwide. As the population ages, HF incidence is increasing, and more than 550,000 patients are diagnosed with HF each year. HF is
responsible for more hospitalizations than all forms of cancer combined and is the most common diagnosis in hospital patients 65 years and older. Every year over 1 million patients are hospitalized for HF in the U.S. and Europe, accounting for 6.5
million hospital days. High rates of hospitalizations with frequent readmission (almost 25 percent of patients with HF are readmitted within 30 days) along with other direct and indirect costs, also place an enormous economic burden on healthcare
systems. Despite new advances in medical therapy, the residual risk for patients with HF is still high.
In January 2021, we initiated a Phase 2b clinical study of IONIS-AGT-LRx in patients with hypertension
uncontrolled with three or more antihypertensive medications, including angiotensin-converting enzyme, or ACE, inhibitors or angiotensin II receptor blockers, or ARBs. The study is a randomized, double-blinded, placebo-controlled study in approximately
150 patients to assess the efficacy, safety and tolerability of IONIS-AGT-LRx. We designed the study to evaluate multiple doses administered subcutaneously. The primary endpoint is the change in systolic blood pressure, or SBP, from
baseline.
In September 2021, we initiated a Phase 2 clinical study of IONIS-AGT-LRx in patients with chronic HF with
reduced ejection fraction. The study is a randomized, double-blind, placebo-controlled study in approximately 75 patients to assess the safety, tolerability, and efficacy of IONIS-AGT-LRx. We designed the study to evaluate multiple doses
administered subcutaneously. The primary endpoint is the percent change in plasma AGT concentration from baseline.
We evaluated IONIS-AGT-LRx in two randomized, double-blinded, placebo-controlled Phase 2 studies. The first study was in
people with mild hypertension and the second was in people with TRH who were on two or three antihypertensive medications, including ACE inhibitors or ARBs.
IONIS-AGT-LRx significantly reduced AGT levels compared with placebo in both studies. Although not
powered for this endpoint, trends were noted in blood pressure reduction and IONIS-AGT-LRx had a favorable safety and tolerability profile supportive of continued development.
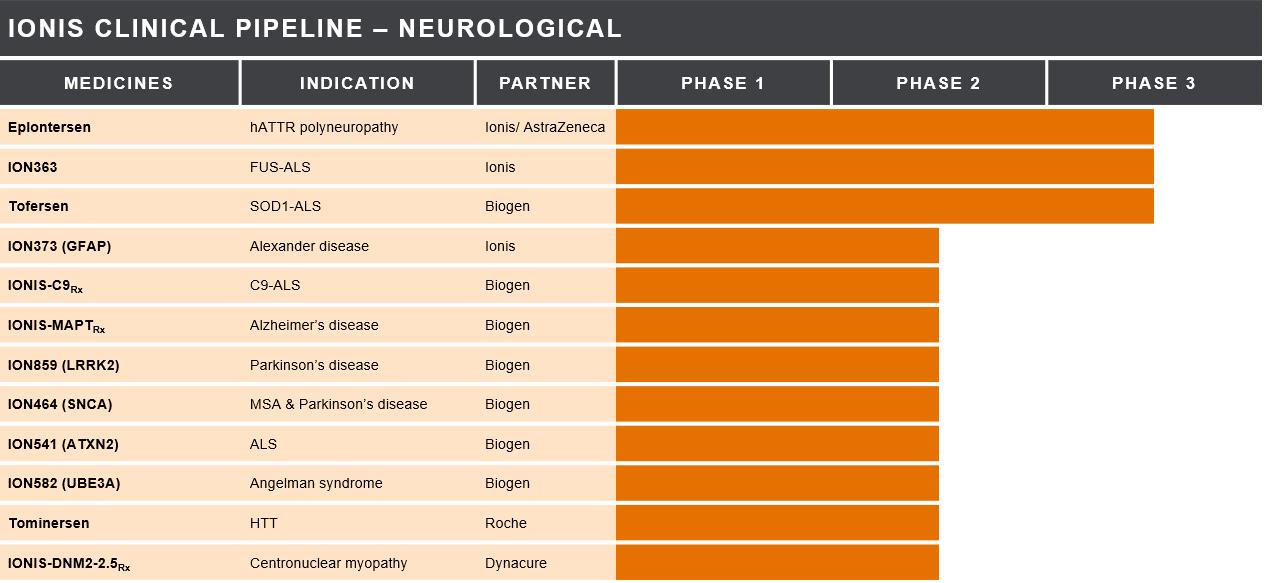
Our Neurological Medicines in Development
Our neurological medicines address a broad range of diseases in major regions of the brain and in the central nervous system, or CNS,
cell types. Our antisense medicines aim to address both large and rare patient populations. We are currently investigating potential disease-modifying treatments for common neurological diseases including Alzheimer’s disease and Parkinson’s disease. We
also have multiple investigational medicines in clinical trials for rare neurological diseases, including ALS and hATTR polyneuropathy. According to the National Institute of Neurological Disorders and Stroke, or NINDS, at the National Institutes of
Health, or NIH, a third of the 7,000 known rare diseases are neurological disorders or thought to include a neurological component.
Eplontersen – See the medicine
description under “Our Phase 3 Medicines” section above.
ION363 – See the medicine
description under “Our Phase 3 Medicines” section above.
Tofersen – See the medicine
description under “Our Phase 3 Medicines” section above.
ION373 (GFAP) – ION373 is an
investigational antisense medicine targeting glial fibrillary acidic protein, or GFAP, mRNA we designed to inhibit the production of GFAP. We are developing ION373 as a potential therapy for Alexander disease, or AxD. AxD is a rare progressive and
fatal neurological disease that affects the myelin sheath which protects nerve fibers. AxD is caused by a gain-of-function mutation in the GFAP gene and is characterized by progressive deterioration, including loss of skills and independence, generally
leading to death in childhood or early adulthood.
Two major types of AxD have been defined. Type I onset typically occurs before 4 years of age and patients can experience head
enlargement, seizures, limb stiffness, delayed or declining cognition, and lack of growth. Type II onset typically occurs after the age of 4 and symptoms can include difficulty speaking, swallowing, and making coordinated movements. AxD is most often
fatal. There are treatments that can relieve symptoms, but there is no disease modifying therapy yet available to patients.
In April 2021, we initiated a pivotal study of ION373 in patients with AxD. The Phase 2/3 study of ION373 is a multi-center,
double-blind, placebo-controlled, multiple-ascending dose study in up to 58 patients with AxD designed to assess the efficacy, safety and tolerability of ION373. Patients will receive ION373 or placebo for a 60-week period, after which all patients in
the study will receive ION373 for a 60-week open-label treatment period. The primary endpoint is the change from baseline in the 10-Meter Walk Test, or 10MWT.
IONIS-C9Rx (BIIB078) – IONIS-C9Rx is an investigational antisense medicine we designed to selectively inhibit the production of the mutated chromosome 9 open reading frame 72, or C9ORF72, gene. A mutation in this gene results in an inherited form of ALS, referred to as C9ORF72-ALS, or C9-ALS, the most prevalent genetic cause of ALS worldwide. This
mutation can lead to rapid progressive loss of motor neurons and is a fatal disease characterized by muscle weakness, loss of movement, and difficulty breathing and swallowing. IONIS-C9Rx is one of four medicines we have in development to
treat ALS.
In August 2018, Biogen initiated a Phase 1/2 clinical study of IONIS-C9Rx in adult patients with C9-ALS. The
Phase 1/2 study is a global, multi-center, randomized, double-blinded, placebo-controlled study designed to assess safety, tolerability and activity of multiple ascending doses of IONIS-C9Rx administered intrathecally.
IONIS-C9Rx is being developed under our 2013 Strategic Neurology collaboration with Biogen.
IONIS-MAPTRx (BIIB080) – IONIS-MAPTRx is an investigational antisense medicine we designed to selectively inhibit production of the microtubule-associated protein tau, or tau, protein in the brain. We are developing
IONIS-MAPTRx to treat people with Alzheimer’s disease, or AD, and potentially other neurodegenerative disorders characterized by the deposition of abnormal tau protein in the brain, such as certain forms of frontotemporal degeneration, or
FTD, and progressive supranuclear palsy, or PSP.
AD and FTD are characterized predominantly by memory impairment and behavioral changes, resulting in a person’s inability to independently
perform daily activities. PSP is characterized by problems with walking and control of movement, sleep disorder and loss of memory and ability to reason. AD generally occurs late in life and may progress to death in five to 20 years after the onset of
the disease. FTD and PSP have a more rapid disease progression. In the U.S., there are approximately five million people living with AD, approximately 55,000 people living with FTD and approximately 20,000 people living with PSP.
In July 2021, we and Biogen reported positive topline data from our Phase 1/2 study of IONIS-MAPTRx in
patients with mild Alzheimer’s disease at the Alzheimer’s Association International Conference, or AAIC. The Phase 1/2 study was a blinded, randomized, placebo-controlled, dose-escalation of IONIS-MAPTRx to evaluate the safety and activity
of once-monthly intrathecal injections of IONIS-MAPTRx in patients with mild AD. The study showed that IONIS-MAPTRx met its primary objective of safety and tolerability in patients with mild Alzheimer’s disease. The study
demonstrated robust time and dose dependent lowering of tau protein in cerebrospinal fluid over the three-month treatment period and sustained reductions during the six-month post-treatment period and IONIS-MAPTRx had a favorable safety and
tolerability profile supportive of continued development.
In December 2019, Biogen exercised its option to license IONIS-MAPTRx. We were responsible for completing the Phase 1/2 study
in patients with mild AD and a one-year long-term extension study. Biogen has responsibility for global development, regulatory and commercialization activities, and costs for IONIS-MAPTRx.
ION859 (LRRK2) (BIIB094) –
ION859 is an investigational antisense medicine we designed to inhibit the production of the Leucine Rich Repeat Kinase 2, or LRRK2, protein as a potential therapy for Parkinson’s disease, or PD. The most common genetic mutations in PD are found in the
LRRK2 protein. It is believed that increased LRRK2 protein activity could be one of the key drivers for developing PD. PD is a progressive neurodegenerative disease characterized by loss of neurons in the motor system. Patients with PD can experience
tremors, loss of balance and coordination, stiffness, slowing of movement, changes in speech and in some cases cognitive decline. PD is ultimately fatal. There are treatments that can relieve symptoms, but there is no disease modifying therapy.
In August 2019, Biogen initiated a Phase 1/2 study evaluating ION859 in adult patients with PD. The Phase 1/2 study is a global,
multi-center, randomized, double-blinded, placebo-controlled study designed to assess the safety, tolerability and activity of multiple ascending doses of ION859 administered intrathecally.
ION859 is being developed under our 2013 Strategic Neurology collaboration with Biogen.
ION464 (SNCA) (BIIB101) – ION464
is an investigational antisense medicine we designed to inhibit the production of the alpha-synuclein protein as a potential therapy for PD, Multiple System Atrophy, or MSA, and related synucleinopathies. Alpha-synuclein protein abnormally accumulates
in the brains of PD and MSA patients and is thought to be one of the key drivers of these diseases. It is believed that decreasing the production of the alpha-synuclein protein will reduce the toxic effects of gain-of-function mutations.
In July 2020, we initiated a Phase 1/2 study evaluating ION464 in patients with MSA. The current study is a multi-center, randomized,
double-blinded, placebo-controlled study designed to assess the safety and tolerability of multiple doses of ION464 administered intrathecally.
ION464 is being developed under our 2013 Strategic Neurology collaboration with Biogen.
ION541 (ATXN2) (BIIB105)
– ION541 is an investigational antisense medicine we designed to reduce the production of the ataxin-2, or ATXN2, protein for the potential treatment of ALS. The reduction of ATXN2 has been shown to decrease aggregation of TDP-43, a toxic RNA binding
protein found in most patients with ALS, including the approximately 90 percent of the ALS population with no known family history of ALS. ION541 is one of four medicines we have in development to treat ALS.
In October 2020, Biogen initiated a Phase 1/2 clinical study evaluating ION541 in this broad ALS population. The current
study is a randomized, blinded, placebo-controlled study designed to assess the safety, tolerability, and pharmacokinetics of multiple ascending doses of ION541 administered intrathecally.
ION541 is being developed under our 2013 Strategic Neurology collaboration with Biogen.
ION582 (UBE3A) (BIIB121) – ION582
is an investigational antisense medicine we designed to inhibit the expression of the UBE3A transcript, or UBE3A-ATS for the potential treatment of Angelman Syndrome, or AS. AS is a rare, genetic neurological disease caused by the loss of function of
the maternally inherited UBE3A gene. Angelman syndrome typically presents in infancy and is characterized by intellectual disability, balance
issues, motor impairment, and debilitating seizures. Some patients are unable to walk or speak. Some symptoms can be managed with existing drugs; however, there is no disease modifying therapy. It is estimated that there are more than 60,000 patients
with AS in the U.S. and EU.
In December 2021, we initiated the Phase 1/2 study, HALOS, of ION582 in patients with Angelman syndrome. The study is an open label
dose-escalation study enrolling up to 44 participants to assess the safety, tolerability and activity of multiple ascending doses of ION582.
ION582 is being developed under our 2012 Neurology collaboration with Biogen.
Tominersen (HTT) (RG6042) – Tominersen (formerly IONIS-HTTRx)
is an investigational antisense medicine we designed to target the underlying cause of Huntington’s disease, or HD, by reducing the production of all forms of the huntingtin protein, or HTT, including its mutated variant, or mHTT. HD is an inherited
genetic brain disorder that results in the progressive loss of both mental faculties and physical control. It is caused by the expansion of the CAG trinucleotide sequence in the HTT gene. The resulting mutant HTT protein is toxic and gradually destroys
neurons. Symptoms usually appear between the ages of 30 and 50 and worsen over a 10 to 25-year period. Ultimately, the weakened individual succumbs to pneumonia, heart failure or other complications. Presently, there is no effective treatment or cure
for the disease, and currently available medicines only mask the patient’s symptoms but do not slow down the underlying loss of neurons.
In January 2022, Roche announced plans to initiate a new Phase 2 trial to evaluate tominersen in patients with HD based on findings from a
post-hoc analysis of the Phase 3 GENERATION HD1 study. The findings from the post-hoc analysis suggested tominersen may benefit younger adult patients with lower disease burden. As a result, Roche is in the early stages of designing a Phase 2 clinical
trial to explore different doses of tominersen in this patient population.
Roche conducted the Phase 3 study, GENERATION HD1,of tominersen in patients with HD. The Phase 3 study was a randomized, multicenter,
double-blind, placebo-controlled study that recruited 791 participants from 18 countries around the world. In March 2021, Roche announced that dosing would be stopped in the study following a recommendation from the independent data monitoring
committee, or iDMC, based on an overall benefit/risk assessment. The study is ongoing without dosing to allow participants to be followed for safety and clinical outcomes. Roche anticipates the study will complete in March/April 2022.
Roche is also conducting the GEN-EXTEND study, an OLE study for participants coming from any prior Roche HD study. The study is ongoing
without dosing to allow participants to be followed for safety and clinical outcomes. Roche anticipates the study will complete in March/April 2022. In parallel with the OLE, Roche initiated a natural history study in a similar patient population to
the OLE aimed at further understanding the natural progression of HD.
We completed a randomized, placebo-controlled, dose escalation, Phase 1/2 clinical study of tominersen in patients with early-stage HD. In
this study, we observed dose-dependent reductions of mHTT among patients treated with tominersen and a favorable safety and tolerability profile supporting continued development. The data from this study were published in The New England Journal of Medicine in May 2019.
The European Medicines Agency, or EMA, granted PRIority MEdicines scheme, or PRIME, designation to tominersen. EMA PRIME status is granted
to medicines that may offer a major therapeutic advantage over existing treatments, or benefit patients without treatment options. The FDA and EMA granted Orphan Medicine Designation for tominersen to treat people with HD.
In December 2017, Roche exercised its option to license tominersen. As a result, Roche is responsible for global
development, regulatory and commercialization activities, and costs for tominersen.
IONIS-DNM2-2.5Rx (DYN101) – IONIS-DNM2-2.5Rx is an investigational antisense medicine we designed to inhibit the production of Dynamin 2, or DNM2, protein for the treatment of centronuclear myopathy, or CNM. CNM is a
group of rare, potentially fatal disorders of the skeletal muscle cells. It is characterized by muscle weakness, decreased muscle tone and muscle atrophy, ranging from severe to mild, and potentially life-threatening. DNM2 reduction demonstrated
improved muscle mass and muscle force, and extended lifespan in animal models of the most severe form of CNM.
In November 2019, Dynacure initiated a Phase 1/2 clinical study evaluating IONIS-DNM2-2.5Rx in patients with CNM. The current
study is an open-label study designed to assess the safety and tolerability of multiple doses of IONIS-DNM2-2.5Rx administered intravenously.
In the fourth quarter of 2017, we licensed IONIS-DNM2-2.5Rx to Dynacure. As a result, Dynacure is responsible
for global development, regulatory and commercialization activities, and costs for IONIS-DNM2-2.5Rx.
Specialty Rare Medicines in Development
Our emerging specialty rare disease pipeline is comprised of medicines that are outside of our cardiovascular and
neurological franchises, but we believe could represent a compelling opportunity for us.
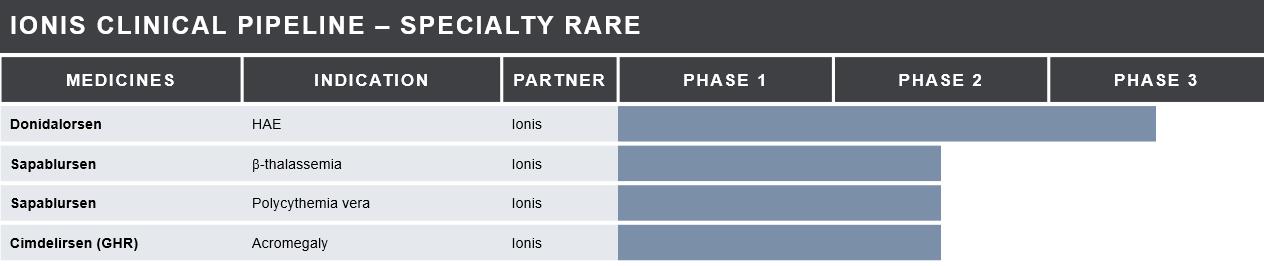
Sapablursen (TMPRSS6)
– Sapablursen (formerly IONIS-TMPRSS6-LRx) is an investigational LICA medicine we designed to target the TMPRSS6 gene to modulate the production of hepcidin, which is the key regulator of iron homeostasis. By modulating hepcidin expression,
sapablursen has the potential to positively impact diseases characterized by iron excess, such as β-thalassemia, and iron deficiency, such as polycythemia vera, or PV.
β-thalassemia is a rare, genetic and potentially fatal form of chronic anemia resulting in hepcidin deficiency, severely
reduced red blood cell production and iron toxicity. In some cases, iron accumulates in major organs, such as the heart and liver, which can be fatal. The current standard-of-care involves symptom management, including blood transfusions and iron
chelation. There are no approved disease-modifying treatments for β-thalassemia.
PV is a rare, non-genetic and potentially fatal disease caused by overproduction of red blood cells. This overproduction
leads to a thickening of the blood, which increases patients’ risk of life-threatening blood clots, including in the lungs, heart and brain. Patients with PV also experience severe iron deficiency due to hepcidin overexpression. The current
standard-of-care for PV involves symptom management. There are no approved disease-modifying treatments for PV.
In August 2020, we initiated a Phase 2 study evaluating sapablursen in patients with non-transfusion dependent, or
NTDT, β-thalassemia intermedia. The Phase 2 study is multi-center, randomized, open-label study in approximately 36 patients we designed assess the efficacy, safety, and tolerability of sapablursen administered monthly subcutaneously. The primary
endpoint is the percentage of participants with a greater than or equal to 1.0 g/dl increase from baseline in hemoglobin at week 27.
In January 2022 we initiated a Phase 2 study evaluating sapablursen in patients with Phlebotomy Dependent Polycythemia
Vera, or PD-PV. The Phase 2 study is a multi-center, randomized, open-label study in approximately 40 patients designed to assess the efficacy, safety and tolerability of sapablursen. The primary endpoint is Change in the frequency of phlebotomy
comparing baseline with the last 20 weeks of the 37-week treatment period.
In December 2018, we presented positive data from our Phase 1 study of sapablursen in healthy volunteers at the American
Society of Hematology Annual Meeting. The Phase 1 study demonstrated dose-dependent reductions of serum iron and serum transferrin saturation with sapablursen. Additionally, we observed an increase in serum hepcidin and predicted changes in hemoglobin
and sapablursen had a favorable safety and tolerability profile supportive of continued development.
Cimdelirsen (GHR) –
Cimdelirsen (formerly IONIS-GHR-LRx) is an investigational LICA medicine we designed to inhibit the production of growth hormone receptor, or GHr, to decrease the circulating level of insulin-like growth factor-1, or IGF-1. Elevated levels
of IGF-1 results in acromegaly, a chronic, slowly progressing and potentially fatal disease. Patients with acromegaly experience multiple chronic conditions, such as type 2 diabetes, hypertension, and respiratory complications and premature death.
Current treatments to block IGF-1 are often unsuccessful. Drug treatments to normalize IGF-1 levels are also available but are associated with potentially serious side effects.
In January 2021, we initiated a Phase 2
study of cimdelirsen evaluating cimdelirsen as a monotherapy in patients with acromegaly. The Phase 2 study is a multi-center, randomized, open label
study in approximately 40 patients to assess the efficacy, safety and tolerability of cimdelirsen. The primary endpoint is the percent change
from baseline in IGF-1 to week 27.
We completed a Phase 2 study evaluating
cimdelirsen as an add-on therapy in patients with uncontrolled acromegaly despite stable therapy with long-acting somatostatin receptor ligands, or SRL. Based on the results of this Phase 2 study and a preliminary analysis of the ongoing open-label
study, proof of mechanism was achieved with a strong indication of proof of concept supporting the continued development of cimdelirsen. Due to enrollment difficulties associated with the COVID-19 pandemic, the study closed early, resulting in
smaller cohort sizes than planned. While no longer powered to assess the primary endpoint (percentage of IGF- lowering at Day 141) in accordance with the protocol, the study did permit placebo-controlled evaluation of safety and efficacy. Cimdelirsen
had a favorable safety and tolerability profile supportive of continued development.
We also completed a Phase 1, blinded,
placebo-controlled, dose-escalation study of cimdelirsen in healthy volunteers. In this study, cimdelirsen demonstrated a favorable safety and tolerability profile supporting continued development.
We continue to advance other medicines in
clinical development targeting metabolic diseases, infectious diseases, renal diseases, ophthalmic diseases and cancer.
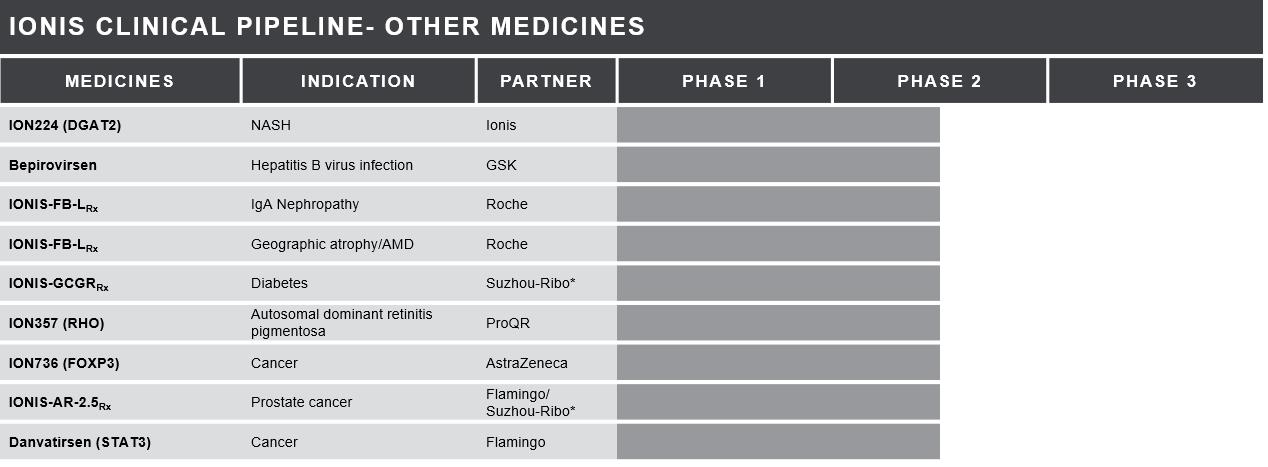
*China Only
ION224 (DGAT) – ION224 is an investigational LICA medicine designed to reduce the production of DGAT2, or diacylglycerol acyltransferase 2, to treat patients with nonalcoholic steatohepatitis,
or NASH. NASH is a common liver disease characterized by liver steatosis, inflammation and scarring and can lead to increased risk of cardiovascular disease, liver cancer, need for liver transplantation and early death. DGAT2 is an enzyme that
catalyzes the final step in triglyceride synthesis in the liver. Reducing the production of DGAT2 should therefore decrease triglyceride synthesis in the liver. In animal studies, antisense inhibition of DGAT2 significantly improved liver steatosis,
lowered blood lipid levels and reversed diet-induced insulin resistance.
NASH is sometimes considered a “silent” liver disease because people with early-stage NASH feel well, even though they are starting to
accumulate fat in their livers and may not be aware that they have the disease. However, NASH can develop into more severe diseases such as liver cirrhosis and liver failure. Currently, liver transplant is the only therapeutic option for patients with
liver cirrhosis. In addition, NASH has been shown to be a major risk factor for the development of liver cancer.
Nonalcoholic fatty liver disease, or NAFLD, describes the full spectrum of liver disease progression from fatty liver to NASH to
cirrhosis to hepatocellular carcinoma. NASH epidemiology studies have estimated 13 to 32 percent of the global population has NAFLD, 1.5 to 6.5 percent have NASH, and approximately 9 percent of NASH patients progress to advanced liver disease. There
are currently no commercially available medications to treat NASH.
In June 2021, we initiated a Phase 2 study of ION224 in patients with confirmed non-alcoholic steatohepatitis. The Phase 2 study is a multi-center, randomized, double-blind, placebo-controlled clinical study enrolling approximately 150 patients designed to assess the efficacy, safety and tolerability of multiple subcutaneous
doses of ION224 on NASH histologic improvement.
Bepirovirsen (HBV) (GSK3228836) – Bepirovirsen (formerly IONIS-HBVRx) is an investigational antisense medicine we designed to inhibit the production of viral proteins
associated with hepatitis B virus, or HBV. These include proteins associated with infection and replication, including the hepatitis B surface antigen, or HBsAg, which is present in both acute and chronic infections and is associated with a poor
prognosis in people with chronic HBV infection.
HBV infection is a serious health problem that can lead to significant and potentially fatal health conditions,
including cirrhosis, liver failure and liver cancer. Chronic HBV infection is one of the most common persistent viral infections in the world. Currently available therapies, although effective in reducing circulating HBV in the blood, do not
effectively inhibit HBV antigen production and secretion, which are associated with poor prognosis and increased risk of liver cancer.
GSK is conducting a broad Phase 2 program for bepirovirsen. The B-Clear study is a Phase 2b randomized, double-blinded,
placebo-controlled study in approximately 440 patients with chronic HBV. The primary endpoint is the percentage of patients achieving HBV surface antigen and HBV DNA less than the lower limit of quantitation. Additionally, GSK is conducting two open label Phase 2 studies and a long-term follow up study in patients with chronic HBV.
In November 2019, GSK reported results of the Phase 2a study of bepirovirsen in patients with chronic HBV infection at the American Association for the Study of Liver Diseases annual meeting. In the Phase 2a study, bepirovirsen demonstrated target engagement with dose
dependent declines in HBsAg with up to 3-log reductions in HBsAg at one month, including two patients who achieved reductions in HBsAg and HBV DNA below levels of detection. Additionally, bepirovirsen had a favorable safety and tolerability profile supportive of continued development.
In August 2019, GSK exercised its option to license our HBV program following the positive Phase 2 results described above. As a result,
GSK is responsible for global development, regulatory and commercialization activities, and costs for the HBV program.
IONIS-FB-LRx – IONIS-FB-LRx is an investigational LICA medicine we designed to inhibit the production of complement factor B, or FB. Genetic association studies have shown that overaction of this cascade has been associated with the development
of several complement-mediated diseases, including IgA nephropathy, or IgAN, and dry age-related macular degeneration, or AMD.
IgAN is one of the most common causes of inflammation that impairs the filtering ability of kidneys and is an important
cause of chronic kidney disease and renal failure. Also known as Berger’s disease, IgAN is characterized by deposits of IgA in the kidneys, resulting in inflammation and tissue damage.
AMD is the leading cause of central vision loss in developed countries. It is estimated that the disease will affect
more than three million people in the U.S. by 2026. AMD is believed to be a systemic disease with local disease manifestation at the aging retinal macula. AMD gradually destroys vision in the center of the visual field due to progressive damage of the
retina. Geographic atrophy, or GA, is an advanced form of AMD and accounts for approximately fifteen percent of all patients with cases of AMD.
In September 2019, we initiated a Phase 2
study of IONIS-FB-LRx in patients with IgA nephropathy. The Phase 2 study is a single-arm, open-label study
designed to assess the efficacy, safety and tolerability of IONIS-FB-LRx administered subcutaneously in adults with
primary IgA nephropathy. The primary endpoint is the percent reduction in 24-hour urine protein excretion from baseline to week 29.
In May 2017, we reported data from a Phase
1 study evaluating IONIS-FB-LRx in 54 healthy volunteers. The Phase 1 study was a randomized, placebo-controlled,
dose-escalation study. Subjects were treated with a single dose of IONIS-FB-LRx achieved dose-dependent reductions
in plasma FB of up to 50 percent. Treatment with multiple doses of IONIS-FB-LRx during a six-week period resulted
in greater reductions in circulating FB levels. IONIS-FB-LRx had a favorable safety and tolerability profile
supportive of continued development.
In June 2019, we initiated a Phase 2 study
evaluating IONIS-FB-LRx in patients with GA secondary to age-related macular degeneration. The study is a
randomized, masked, placebo-controlled study designed to assess the efficacy, safety and tolerability of multiple ascending doses of IONIS-FB-LRx administered subcutaneously in adults with GA. The primary endpoint is the absolute change from baseline in GA area at week 49.
IONIS-FB-LRx is being developed under our collaboration with Roche.
IONIS-GCGRRx – IONIS-GCGRRx is an investigational antisense medicine designed to inhibit the production of the glucagon receptor, or GCGR, to treat patients with type 2 diabetes. GCGR is a receptor for the hormone
glucagon. Glucagon is a hormone that opposes the action of insulin and stimulates the liver to produce glucose, particularly in type 2 diabetes. In patients with advanced diabetes, uncontrolled glucagon action can lead to significant increase in blood
glucose level. In addition, reducing GCGR produces more active glucagon-like peptide, or GLP-1, a hormone that preserves pancreatic function and enhances insulin secretion.
Diabetes is a chronic disease in which the blood glucose levels are too high. Although glucose is an important source of energy for your
body and is vital to your health, uncontrolled increases in glucose can lead to serious health problems, such as diabetes. Diabetes is separated into type 1 and type 2. In type 1 diabetes, the body does not make insulin. In type 2 diabetes, the more
common type, the body does not respond properly to insulin and, therefore, blood glucose levels are not adequately controlled.
In October 2019, Suzhou-Ribo initiated a Phase 2 clinical study evaluating IONIS-GCGRRx in patients with type 2 diabetes.
ION357 (RHO) (QR-1123)
– ION357 (formerly IONIS-RHO-2.5Rx), is an investigational antisense medicine we designed to treat patients with a genetic form of
autosomal dominant retinitis pigmentosa by inhibiting the production of the rhodopsin P23H mutant protein in the eye while allowing normal protein to be expressed.
Retinitis pigmentosa, or RP, is a group of rare inherited eye disorders causing photoreceptor degeneration that leads to
progressive vision loss. Photoreceptors are cells in the eye’s retina responsible for converting light into signals that are sent to the brain. Photoreceptors provide us our color and night vision. Affected patients first experience defective dark
adaptation during adolescence or young adulthood, followed by loss of peripheral visual field. Patients eventually have limited residual central vision, which ultimately leads to complete blindness around the age of 60.
In November 2019, ProQR initiated a Phase 1/2 clinical study evaluating ION357 in patients with RP. The Phase 1/2 study is a randomized, masked, placebo-controlled study designed to assess the safety, tolerability and activity of ION357 in adult patients with RP.
In the fourth quarter of 2018, we licensed ION357 to ProQR. As a result, ProQR is responsible for global development,
regulatory and commercialization activities, and costs for ION357.
ION736 (FOXP3)
(AZD8701) – ION736, is an investigational antisense medicine designed to reduce the production of Forkhead Box P3, or FOXP3, for the treatment of patients with cancer. FOXP3 is a protein involved in the function of immuno-suppressive T regulatory cells
(Tregs). Tregs, which are found at high levels in various types of cancers, often predict poor survival and poor response to immune checkpoint therapeutics. Preclinical studies have demonstrated that FOXP3 downregulation resulted in an increased immune
response and anti-tumor activity. Moreover, the combination of antisense inhibition of FOXP3 with other immuno-oncology drugs led to enhanced anti-tumor activity.
In August 2020, AstraZeneca initiated a first-in-human open label study of ION736 in patients with select advanced solid
tumors. The study is a multi-center, open label multi-arm study in approximately 123 patients designed to evaluate the efficacy, safety and tolerability of ION736 administered intravenously as monotherapy and in combination with durvaluamb (MEDI4736)
in patients with advanced solid tumors.
In the second quarter of 2020, we licensed ION736 to AstraZeneca. As a result, AstraZeneca is responsible for global
development, regulatory and commercialization activities, and costs for ION736.
IONIS-AR-2.5Rx – IONIS-AR-2.5Rx is an investigational antisense medicine we designed to treat people with prostate
cancer by reducing the production of all known forms of androgen receptor, or AR, including variants of the AR gene. Prostate cancer is the second
leading cause of cancer deaths in American men. Prostate cancer growth, proliferation and progression are all androgen-dependent and AR function is involved in disease progression at all stages of prostate cancer. For patients diagnosed with metastatic
prostate cancer, current treatments largely involve opposing the action of androgens by blocking the androgen receptor or removing circulating androgens. Resistance to current therapies is frequent.
An open-label, dose-escalation, Phase 1/2 clinical study of IONIS-AR-2.5Rx was completed in people with
advanced tumors for which the androgen receptor pathway is potentially a contributing factor. The study was primarily conducted in prostate cancer patients, and it showed durable responses in a number of those patients. IONIS-AR-2.5Rx had a
safety and tolerability profile supportive of continued development.
In March 2017, we licensed IONIS-AR-2.5Rx to Suzhou-Ribo to develop and commercialize the medicine in China.
In the third quarter of 2021, we entered into a license agreement with Flamingo Therapeutics for the development and commercialization of certain programs from Ionis’ oncology pipeline, including IONIS-AR-2.5Rx outside of China.
Danvatirsen (STAT3) – Danvatirsen
(formerly IONIS-STAT3-2.5Rx) is an investigational antisense medicine we designed to inhibit the production of signal transducer and activator of transcription 3, or STAT3, to treat people with cancer. STAT3 is a protein involved in the
translation of key factors critical for tumor cell growth and survival. STAT3 is over-active in a variety of cancers, including brain, lung, breast, bone, liver, and multiple myeloma. Overactivity in STAT3 prevents cancer cell death and promotes tumor
cell growth.
In October 2018, we reported data from a Phase 1/2 study of danvatirsen in combination with durvalumab in recurrent
metastatic head and neck cancer. The combination treatment resulted in seven percent of patients achieving a complete tumor response and 23 percent achieving either a partial or complete tumor response. This response rate is estimated to be double that
with durvalumab alone, based on previous studies in this difficult to treat patient population. Danvatirsen had a safety and tolerability profile supportive of continued development.
In the third quarter of 2021, we entered into a license agreement with Flamingo Therapeutics for the development and
commercialization of certain programs from Ionis’ oncology pipeline, including danvatirsen.
Phase 1 Medicines in Clinical Development
Our early-stage pipeline is comprised of medicines to treat a number of diseases, including from our cardiovascular franchise. It includes
medicines based on our latest technology advancements. As we continue to add new investigational medicines to our pipeline, we believe these medicines have the potential to expand our mid and late-stage pipelines.
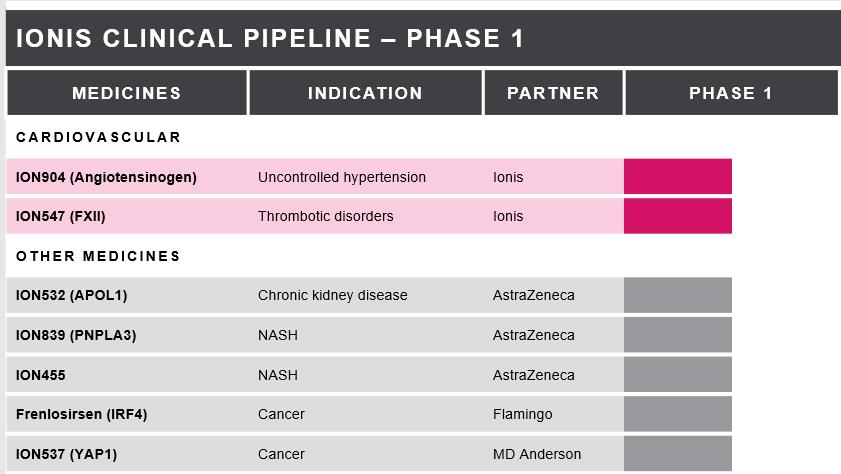
Antisense Technology
Our antisense technology is an innovative platform for discovering first-in-class and/or best-in-class medicines.
Antisense medicines target RNA, the intermediary that conveys genetic information from a gene to the protein synthesis machinery in the cell. By targeting RNA instead of proteins, we can use antisense technology to increase, decrease or alter the
production of specific proteins. The unique properties of antisense technology provide several advantages over other drug discovery technologies.
These advantages include:
| ● |
Direct intervention in the disease process at the genetic level by targeting RNA: antisense technology represents a direct route from gene to drug. The
explosion in genomic information and RNA biology has led to the discovery of many new disease-causing proteins and RNAs and has created new opportunities that are uniquely accessible by antisense technology.
|
| ● |
Precise specificity: we design antisense medicines to target a single RNA, minimizing the possibility of binding to unintended targets, which can cause
unwanted side effects.
|
| ● |
Good drug properties: antisense medicines distribute well throughout the body. They also have a long half-life, in the range of weeks to months, which
means patients and/or healthcare providers can dose our medicines weekly, monthly or even less frequently depending on the medicine and target tissue.
|
| ● |
Ability to combine with other medicines: because antisense medicines do not interact with the enzymes that metabolize or break down other medicines,
physicians can use our medicines in combination with other medicines.
|
| ● |
Broad applications to multiple disease targets, multiple tissues and multiple mechanisms: there are virtually no “undruggable” targets with antisense
technology.
|
| ● |
Efficient discovery and early development: because of the efficiency of our antisense technology, our drug discovery and early development costs and
success rates compare favorably to small molecule or antibody drug discovery and development.
|
We develop antisense medicines we believe will pioneer new markets and change standards of care. Our areas of focus
include cardiovascular and neurological diseases.
Technology Overview
We use our core technology platform to discover and develop medicines that affect targets in the body at the genetic
level. Genes contain the information necessary to produce proteins. A gene is made up of nucleotides containing the nucleoside bases: adenine, thymine, guanine, and cytosine, commonly known as A, T, G and C, which are linked together to form a
two-stranded structure that resembles a twisted ladder, known as DNA. The nucleotides on one side of the ladder bind weakly to complementary nucleotides on the other strand according to specific rules; for example, A pairs with T and G pairs with C,
creating the ladder’s rungs (Figure 1). Scientists call this highly specific nucleotide pairing hybridization. The sequence or order of these nucleotides establishes the cell’s recipes for making proteins. Each protein’s instructions reside in a
corresponding segment of DNA known as a gene.
Figure 1: Illustration of DNA.
The instructions for making a protein are transcribed from a gene, or DNA, into a different genetic molecule called
messenger RNA. This process starts with the partial uncoiling of the two complementary strands of the DNA. One strand acts as a template and information stored in the DNA template strand is copied into a complementary RNA (Figure 2) by an enzyme called
RNA polymerase, or RNAP. Messenger RNA, or mRNA, are mature, fully processed RNA that code for proteins.
Figure 2: Transcription of information contained in a gene, or DNA, to RNA.
Ribosomes, the cell’s factories for manufacturing proteins, translate mRNA into proteins. The ribosome reads the encoded
information, the mRNA’s nucleotide sequence, and in doing so, strings together amino acids to form a specific protein (Figure 3).

Figure 3: Translation of the protein-coding information contained in mRNA to protein.
We primarily use our antisense technology to interrupt the cell’s protein production process by preventing the mRNA
instructions from reaching the ribosome, thus inhibiting the production of the protein. We can also design antisense medicines to increase protein production for diseases caused by the lack of a particular protein or modify the processing (or splicing)
of the mRNA, which can alter the composition of the protein. The mRNA sequence of nucleotides that carries the information for protein production is called the ‘sense’ strand. Scientists call the complementary nucleotide chain that binds specifically
to the sense strand the “antisense” strand. We use the information contained in mRNA to design chemical structures, that we call antisense oligonucleotides, or ASOs, or antisense medicines, which resemble DNA and RNA and are the complement of RNA. Our
antisense medicines bind with high selectivity to the mRNA they were designed to target. Since each mRNA codes for a specific protein, we can design antisense medicines that selectively inhibit the disease-causing member of a protein family without
interfering with other members of the protein family that might be necessary for normal cellular or bodily functions. This unique specificity means that antisense medicines may be less toxic than traditional medicines because we can design them to
minimize the impact on unintended targets.
We have developed the majority of the medicines in our pipeline using our advanced screening methods to produce medicines with what we
believe have strong safety and tolerability profiles. We continue to advance our antisense technology to create even more potent medicines that we can use in more tissues and against more targets. These advances allow us to expand the mechanisms
through which we can use our medicines and provide us with greater opportunities to use our antisense medicines to treat a greater number of diseases and reach more patients. Today our medicines and those entering our pipeline utilize our key
technology advances, including our Generation 2.5 and our LICA technology.
Generation 2.5 chemistry, used in several medicines in our pipeline, enables up to 10-fold greater potency compared to
our medicines using our earlier chemistries. This increased potency enables broad distribution throughout the body and target engagement to multiple tissues including liver, kidney, lung, muscle, adipose, adrenal gland, peripheral nerves and tumor
tissues.
LICA is a chemical technology we developed that involves attaching a molecule called a ligand that binds with receptors
on the surfaces of cells in a highly specific manner. Because these receptors are often found only on certain cell types, LICA allows us to increase effective delivery of our antisense medicines with higher specificity to certain cell types that
express these receptors relative to non-conjugated antisense medicines. As of December 2021, we have an integrated assessment of data from multiple LICA medicines and clinical programs which demonstrates that our LICA technology for liver targets can
increase potency by 20-30-fold over our non-LICA antisense medicines.
In addition to the increase in potency, our LICA platform has consistently demonstrated favorable safety and
tolerability. Pelacarsen exemplifies these improvements. We designed this medicine to reduce the production of Apo(a) protein in the liver to offer a direct approach for reducing Lp(a). Pelacarsen was the first medicine to selectively and robustly
reduce Lp(a) levels below threshold levels associated with CVD in nearly all patients and demonstrated a favorable safety and tolerability profile in the Phase 2 study. The study included more than 280 patients, with 98 percent of patients in the high
dose group achieving levels below 50 mg/dL, the recognized risk threshold for CVD.
We can also combine our LICA technology with our Generation 2.5 chemistry, further increasing potency. In addition to
the LICA technology for liver targets, we are also developing LICA conjugation technology that we can use to target other tissues, such as pancreas and muscle, and initial results in animals are promising.
Antisense Targets and Mechanisms
There are more than a dozen different antisense mechanisms that we can utilize with our antisense technology. The
majority of the medicines in our pipeline bind to mRNAs and inhibit the production of disease-causing proteins. However, our antisense technology is broadly applicable to many different antisense mechanisms, including modulation of RNA splicing, RNA
interference, or RNAi, and enhancing protein translation to increase protein production.
When using antisense technology to inhibit the production of disease-causing proteins or reduce levels of harmful RNAs,
our antisense medicines bind to the target RNA via highly specific nucleotide pairing, or hybridization, and recruit a cellular enzyme called ribonuclease H1, or RNase H1, to degrade the target RNA. The antisense medicine itself remains intact during
this process, so it can remain active against additional target RNA molecules and repeatedly trigger their degradation (Figure 4). Examples of our antisense medicines that use the RNase H1 mechanism to reduce disease protein production include,
TEGSEDI, WAYLIVRA, eplontersen, olezarsen, donidalorsen, ION363, pelacarsen, tofersen and others.
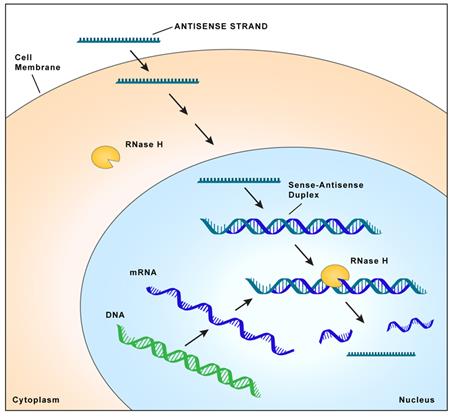
Figure 4: Antisense medicine using the RNase H mechanism of action.
SPINRAZA is an example of an antisense medicine that modulates RNA splicing to increase protein production of the SMN protein (Figure 5),
which is critical to the health and survival of nerve cells in the spinal cord that are responsible for neuro-muscular function. The SMN protein is deficient in people with SMA.
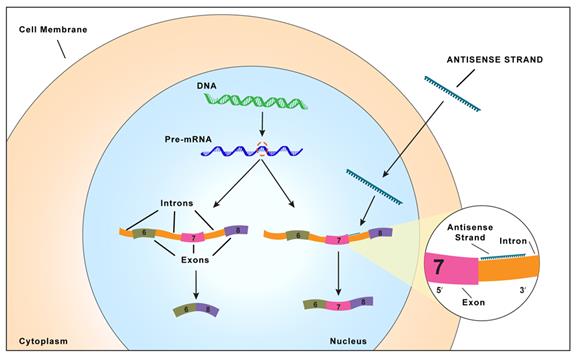
Figure 5: Antisense medicine altering splicing of the SMN2 mRNA.
We are also making progress in designing antisense medicines to target long, non-coding RNAs, or lncRNAs and RNAs that
possess a toxic function in human diseases. Many of these RNAs, such as lncRNAs, do not make proteins but often cause disease by regulating the function of other genes or proteins. In 2014, we published a paper in Nature in which we were the first to show that targeted reduction of a lncRNA with an antisense compound can ameliorate certain cognitive deficits in a mouse model of Angelman
syndrome, or AS. In 2021, we initiated the HALOS study, a Phase 1/2a study of ION582 in patients with AS.
Because the efficiency of our core technology platform can support multiple target-based antisense research programs, we
can develop antisense medicines to target a broad range of diseases, efficiently producing a large and broad proprietary portfolio of medicines. We are currently pursuing antisense drug discovery programs focused on neurological, cardiovascular, and
other diseases.
Collaborative Arrangements
We have established alliances with a cadre of leading global pharmaceutical companies. Our partners include the
following companies, among others: AstraZeneca, Bayer, Biogen, GSK, Novartis, and Roche. Through our partnerships, we have earned both commercial revenue and a broad and sustaining base of R&D revenue in the form of license fees, upfront payments
and milestone payments. In 2021, we recognized $810 million in revenue, the majority of which was from our partnered medicines and programs. We have the potential to earn more than $24 billion in future milestone payments, licensing fees and other
payments from our current partnerships. In addition, we are eligible to receive up to mid-20 percent royalties under certain partnerships. Below, we include the significant terms of our collaboration agreements. For additional details, including
other financial information, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial
Statements.
Strategic Partnership
Biogen
We have several strategic collaborations with Biogen focused on using antisense technology to advance the treatment of
neurological disorders. These collaborations combine our expertise in creating antisense medicines with Biogen’s expertise in developing therapies for neurological disorders. We developed and licensed to Biogen SPINRAZA, our approved medicine to treat
people with SMA. We and Biogen are currently developing nine investigational medicines to treat neurodegenerative diseases under these collaborations, including medicines in development to treat people with ALS, SMA, AS, Alzheimer’s disease and
Parkinson’s disease. In addition to these medicines, our collaborations with Biogen include a substantial research pipeline that addresses a broad range of neurological diseases. From inception through December 2021, we have received $3.2 billion from
our Biogen collaborations.
Spinal Muscular Atrophy Collaborations
SPINRAZA
In January 2012, we entered into a
collaboration agreement with Biogen to develop and commercialize SPINRAZA, an RNA-targeted therapy for the treatment of SMA. From inception through
December 2021, we generated more than $1.6
billion in total revenue under our SPINRAZA collaboration, including nearly $1.2 billion in revenue from SPINRAZA royalties and more than $435 million in R&D revenue. We are receiving tiered royalties ranging from 11 percent to 15 percent on
sales of SPINRAZA. We have exclusively in-licensed patents related to SPINRAZA from Cold Spring Harbor Laboratory and the University of Massachusetts. We pay Cold Spring Harbor Laboratory and the University of Massachusetts a low single digit royalty
on net sales of SPINRAZA. Biogen is responsible for global development, regulatory and commercialization activities and costs for SPINRAZA.
In December 2017, we entered into a collaboration agreement with Biogen to identify new antisense medicines for the
treatment of SMA. Biogen has the option to license therapies arising out of this collaboration following the completion of preclinical studies. Upon licensing, Biogen will be responsible for global development, regulatory and commercialization
activities and costs for such therapies. Under the collaboration agreement, we received a $25 million upfront payment in December 2017. In December 2021, we earned a $60 million license fee payment when Biogen exercised its option to license ION306. Biogen is solely responsible for the costs and expenses related to the development, manufacturing and potential future commercialization of ION306 following the option exercise.
We will receive development and regulatory milestone payments from Biogen if new medicines advance towards marketing
approval. In total over the term of our collaboration, we are eligible to receive up to $1.2 billion in license fees, milestone payments and other payments, including up to $555 million if Biogen advances ION306. In addition, we are eligible to receive
tiered royalties from the mid-teens to mid-20 percent range on net sales from any product that Biogen successfully commercializes under this collaboration.
Neurology Collaborations
2018 Strategic Neurology
In April 2018, we and Biogen entered into a strategic collaboration to develop novel antisense medicines for a broad range
of neurological diseases and entered into a Stock Purchase Agreement, or SPA. As part of the collaboration, Biogen gained exclusive rights to the use of our antisense technology to develop therapies for these diseases for 10 years. We are responsible
for the identification of antisense drug candidates based on selected targets. Biogen is responsible for conducting IND-enabling toxicology studies for the selected medicine. Biogen will have the option to license the selected medicine after it
completes the IND-enabling toxicology study. If Biogen exercises its option to license a medicine, it will assume global development, regulatory and commercialization responsibilities and costs for that medicine.
In June 2018, we received $1 billion from Biogen, comprised of $625 million to purchase our stock at an approximately 25
percent cash premium and $375 million in an upfront payment. We are eligible to receive up to $270 million in milestone payments for each medicine that achieves marketing approval. In addition, we are eligible to receive tiered royalties up to the 20
percent range on net sales from any product that Biogen successfully commercializes under this collaboration. We are currently advancing nine programs under this collaboration and through December 2021, we have generated nearly $1.1 billion in
payments, including $23 million in milestone payments generated in 2021 when Biogen advanced three programs under this collaboration.
2013 Strategic Neurology
In September 2013, we and Biogen entered into a long-term strategic relationship focused on applying antisense technology
to advance the treatment of neurodegenerative diseases. As part of the collaboration, Biogen gained exclusive rights to the use of our antisense technology to develop therapies for neurological diseases and has the option to license medicines resulting
from this collaboration. We will usually be responsible for drug discovery and early development of antisense medicines and Biogen will have the option to license antisense medicines after Phase 2 proof-of-concept. In October 2016, we expanded our
collaboration to include additional research activities we will perform. If Biogen exercises its option to license a medicine, it will assume global development, regulatory and commercialization responsibilities and costs for that medicine. We are
currently advancing six investigational medicines in development under this collaboration, including a medicine for Parkinson’s disease, three medicines for ALS, a medicine for multiple system atrophy and a medicine for an undisclosed target. In
December 2018, Biogen exercised its option to license our most advanced ALS medicine, tofersen, and as a result Biogen is now responsible for global development, regulatory and commercialization activities and costs for tofersen.
Under the terms of the agreement, we received an upfront payment of $100 million and are eligible to receive milestone
payments, license fees and royalty payments for all medicines developed under this collaboration, with the specific amounts dependent upon the modality of the molecule advanced by Biogen. For each antisense molecule that is chosen for drug discovery
and development under this collaboration, we are eligible to receive up to approximately $260 million in a license fee and milestone payments. In addition, we are eligible to receive tiered royalties up to the mid-teens on net sales from any product
that Biogen successfully commercializes under this collaboration. Through December 2021, we have generated over $280 million under this collaboration, including $10 million we received from Biogen in 2021 when Biogen advanced ION541, an investigational
medicine targeting ATXN2 to treat patients with ALS.
2012 Neurology
In December 2012, we and Biogen entered into a collaboration agreement to develop and commercialize novel antisense
medicines to treat neurodegenerative diseases. We are responsible for the development of each of the medicines through the completion of the initial Phase 2 clinical study for such medicine. Biogen has the option to license a medicine from each of the
programs through the completion of the first Phase 2 study for each program. Under this collaboration, we are currently advancing IONIS-MAPTRx for Alzheimer’s disease and ION582 for AS. If Biogen exercises its option to license a medicine,
it will assume global development, regulatory and commercialization responsibilities and costs for that medicine. In December 2019, Biogen exercised its option to license IONIS-MAPTRx and as a result Biogen is now responsible for global
development, regulatory and commercialization activities and costs for IONIS-MAPTRx.
Under the terms of the agreement, we received an upfront payment of $30 million. Over the term of the collaboration, we
are eligible to receive up to $210 million in a license fee and milestone payments per program, plus a mark-up on the cost estimate of the Phase 1 and 2 studies. In addition, we are eligible to receive tiered royalties up to the mid-teens on net sales
from any product that Biogen successfully commercializes under this collaboration. Through December 2021, we have generated over $165 million under our collaboration, including $10 million we received from Biogen for advancing ION582 during 2021.
Joint Development and Commercialization Arrangement
AstraZeneca
Eplontersen Collaboration
In December 2021, we entered into a joint development and commercialization agreement with AstraZeneca to develop and
commercialize eplontersen for the treatment of ATTR. We are jointly developing and preparing to commercialize eplontersen with AstraZeneca in the U.S. AstraZeneca obtained exclusive rights to commercialize eplontersen outside the U.S., except certain
countries in Latin America. Under the terms of the agreement, we received a $200 million upfront payment. We are eligible to receive up to $485 million in development and approval milestones, and up to $2.9 billion in sales-related milestone payments.
In addition, we are eligible to receive up to mid-20 percent royalties for sales in the U.S. and tiered royalties up to the high teens for sales outside the U.S.
The collaboration also includes
territory-specific development, commercial and medical affairs cost-sharing provisions. AstraZeneca will pay 55 percent of the costs associated with the ongoing global Phase 3 development program. As we will continue to lead the Phase 3 development
program, we will recognize as revenue the 55 percent of cost-share funding AstraZeneca is responsible for in the same period we incur the related development expenses. As AstraZeneca is responsible for the majority of the commercial and medical
affairs costs in the U.S. and all costs associated with bringing eplontersen to market outside the U.S., we will recognize cost-share funding we receive from AstraZeneca related to these activities as a reduction of our commercial and medical affairs
expenses. Through December 2021, we have generated $200 million in payments under this collaboration.
Research and Development Partners
AstraZeneca
In addition to our collaboration for eplontersen, we have two other collaborations with AstraZeneca. One is focused on the
treatment of cardiovascular, renal and metabolic diseases and the other is focused on the treatment of oncology diseases. We and AstraZeneca are currently developing six medicines under these collaborations. From inception through December 2021, we
have generated nearly $425 million from our AstraZeneca research and development collaborations.
Cardiovascular, Renal and Metabolic Diseases Collaboration
In July 2015, we and AstraZeneca formed a collaboration to discover and develop antisense therapies for treating
cardiovascular, renal and metabolic diseases. Under our collaboration, AstraZeneca has licensed five medicines from us. AstraZeneca is responsible for global development, regulatory and commercialization activities and costs for each of the medicines
it has licensed.
Under the terms of the agreement, we received a $65 million upfront payment. We are eligible to receive license fees and
milestone payments of up to more than $5.5 billion as medicines under this collaboration advance. In addition, we are eligible to receive tiered royalties up to the low teens on net sales from any product that AstraZeneca successfully commercializes
under this collaboration agreement. Through December 2021, we have generated over $280 million in payments, including $40 million we earned in 2021 for two targets that AstraZeneca is advancing for a metabolic disease.
Oncology Collaboration
In December 2012, we entered into a collaboration agreement with AstraZeneca to discover and develop antisense medicines
to treat cancer. We and AstraZeneca also established an oncology research program. In 2020, AstraZeneca licensed ION736, an investigational medicine in development targeting FOXP3 for the treatment of cancer. AstraZeneca is responsible for global
development, regulatory and commercialization activities and costs for ION736.
Under the terms of this agreement, we
received $31 million in upfront payments. We are eligible to receive license fees and milestone payments of up to more than $265 million as this collaboration advances. In addition, we are eligible to receive tiered royalties up to the low teens on
net sales from any product that AstraZeneca successfully commercializes under this collaboration agreement. Through December 2021, we have generated over $140 million in payments under this collaboration, including $13 million we earned in 2020 when
AstraZeneca licensed ION736.
Bayer
In May 2015, we entered into an exclusive license agreement with Bayer to develop and commercialize IONIS-FXIRx
for the prevention of thrombosis and we received a $100 million upfront payment. In February 2017, we amended our agreement with Bayer to advance IONIS-FXIRx and to initiate development of fesomersen (formerly IONIS-FXI-LRx),
which Bayer licensed. In conjunction with the amendment, we received a $75 million payment. In October 2019, Bayer decided to advance fesomersen following positive clinical results. Bayer is now responsible for all global development, regulatory and
commercialization activities and costs for the FXI program. We are eligible to receive additional milestone payments as the FXI program advances toward the market. Over the term of the collaboration, we are eligible to receive up to $385 million in
license fees, milestone payments and other payments. In addition, we are eligible to receive tiered royalties in the low to high 20 percent range on gross margins of both medicines combined. Through December 2021, we have generated over $190 million
under this collaboration.
GSK
In March 2010, we entered into an alliance with GSK using our antisense drug discovery platform to discover and develop
new medicines against targets for serious and rare diseases, including infectious diseases and some conditions causing blindness. Under the collaboration, we received upfront payments of $35 million. Our collaboration with GSK covers bepirovirsen,
an investigational antisense medicine we designed to reduce the production of viral proteins associated with HBV infection. In 2019, following positive Phase 2 results, GSK licensed our HBV program. GSK is responsible for all global development,
regulatory and commercialization activities and costs for the HBV program.
Under our agreement, if GSK successfully develops bepirovirsen and achieves pre-agreed sales targets, we could receive
license fees and milestone payments of more than $260 million. In addition, we are eligible to receive tiered royalties up to the mid-teens on net sales from any product that GSK successfully commercializes under this alliance. Through December 2021,
we have generated over $185 million in payments under our collaboration.
Novartis
In January 2017, we initiated a collaboration
with Novartis to develop and commercialize pelacarsen. We received a $75 million upfront payment in February 2017. In February 2019, Novartis
licensed pelacarsen and we earned a $150 million license fee. Novartis is responsible for conducting and funding future development and regulatory activities for pelacarsen, including a global Phase 3 cardiovascular outcomes study, which
Novartis initiated in December 2019. In connection with Novartis’ license of pelacarsen, we and Novartis established a more definitive framework under
which the companies would negotiate the co-commercialization of pelacarsen in selected markets. Included in this framework is an option by which Novartis could solely commercialize pelacarsen in exchange for Novartis paying us increased sales milestone
payments based on sales of pelacarsen.
Under the collaboration, we are eligible to receive up to $675 million in milestone payments related to pelacarsen. We are also eligible
to receive tiered royalties in the mid-teens to low 20 percent range on net sales of pelacarsen. Through December 2021, we have generated nearly $425 million
under our collaboration including an upfront payment, license fee, milestone payments and other payments from this collaboration, including a $25 million milestone payment we earned in 2021 when Novartis achieved 50 percent enrollment in the Lp(a)
HORIZON Phase 3 cardiovascular outcome study of pelacarsen.
In conjunction with this collaboration, we entered into a SPA with Novartis. As part of the SPA, Novartis purchased 1.6
million shares of our common stock for $100 million in the first quarter of 2017.
Roche
Huntington’s Disease
In April 2013, we formed an alliance with Hoffman-La Roche Inc. and F. Hoffmann-La Roche Ltd., collectively Roche, to
develop treatments for HD based on our antisense technology. Under the agreement, we discovered and developed tominersen, an investigational medicine targeting HTT protein. We developed tominersen through completion of our Phase 1/2 clinical study in
people with early stage HD. In December 2017, upon completion of the Phase 1/2 study, Roche exercised its option to license tominersen and is now responsible for the global development, regulatory and commercialization activities and costs
for tominersen. In March 2021, Roche discontinued dosing in the Phase 3 GENERATION HD1 study of tominersen in patients with manifest Huntington’s disease based on the results of a pre-planned review of data from the Phase 3 study conducted by an
unblinded Independent Data Monitoring Committee. In January 2022, Roche announced it is actively preparing to initiate a new Phase 2 study of tominersen in patients with HD. Post-hoc analyses from the GENERATION HD1 study suggested tominersen may
benefit younger adult patients with lower disease burden.
Under the terms of the agreement, we received an upfront payment of $30 million in April 2013. We are eligible to
receive up to $365 million in a license fee and milestone payments as tominersen advances. In addition, we are eligible to receive up to $136.5 million in milestone payments for each additional medicine successfully developed. We are also eligible to
receive tiered royalties up to the mid-teens on net sales from any product resulting from this alliance. Through December 2021, we have generated $150 million under our collaboration.
IONIS-FB-LRx for Complement-Mediated Diseases
In October 2018, we entered into a collaboration agreement with Roche to develop IONIS-FB-LRx for the treatment
of complement-mediated diseases. We are currently conducting Phase 2 studies in two disease indications for IONIS-FB-LRx, one for the treatment of patients with GA, the advanced stage of dry AMD, and a second for the treatment of patients
with IgA nephropathy. Roche has the option to license IONIS-FB-LRx at the completion of these studies. Upon licensing, Roche will be responsible for global development, regulatory and commercialization activities and costs.
Under the terms of this agreement, we received a $75 million upfront payment in October 2018. We are eligible to receive
more than $680 million including a license fee and milestone payments. In addition, we are also eligible to receive tiered royalties from the high teens to twenty percent on net sales. Through December 2021, we have generated over $75 million under our
collaboration.
Commercialization Partnerships
Swedish Orphan Biovitrum AB (Sobi)
We began commercializing TEGSEDI and WAYLIVRA in Europe in January 2021 and TEGSEDI in North America in April 2021 through
distribution agreements with Sobi. Under our agreements, we are responsible for supplying finished goods inventory to Sobi and Sobi is responsible for selling each medicine to the end customer. In exchange, we earn a distribution fee on net sales from
Sobi for each medicine.
PTC Therapeutics
In August 2018, we entered into an exclusive
license agreement with PTC Therapeutics to commercialize TEGSEDI and WAYLIVRA in Latin America and certain Caribbean countries. Under the license agreement, we are eligible to receive royalties from PTC in the mid-20 percent range on net sales for each medicine. In
December 2021, we started receiving royalties from PTC for TEGSEDI sales.
Technology Enhancement Collaboration
Bicycle License Agreement
In December 2020, we entered into a collaboration agreement with Bicycle and obtained an option to license its peptide
technology to potentially increase the delivery capabilities of our LICA medicines. In July 2021, we paid $42 million when we exercised our option to license Bicycle’s technology, which included an equity investment in Bicycle. As part of our stock
purchase, we entered into a lockup agreement with Bicycle that restricts our ability to trade our Bicycle shares for one year. In 2021, we recorded a $7 million equity investment for the shares we received in Bicycle. We recognized the remaining $35
million as R&D expense in 2021. From inception through December 2021, we have paid Bicycle $47 million under this collaboration agreement.
Other Agreements
Alnylam Pharmaceuticals, Inc.
Under the terms of our agreement with Alnylam, we co-exclusively (with ourselves) licensed to Alnylam our patent estate
relating to antisense motifs and mechanisms and oligonucleotide chemistry for double-stranded RNAi therapeutics, with Alnylam having the exclusive right to grant platform sublicenses for double-stranded RNAi. In exchange for such rights, Alnylam
gave us a technology access fee, participation in fees from Alnylam’s partnering programs, as well as future milestone and royalty payments from Alnylam. We retained exclusive rights to our patents for single-stranded antisense therapeutics and for a
limited number of double-stranded RNAi therapeutic targets and all rights to single-stranded RNAi, or ssRNAi, therapeutics. In turn, Alnylam nonexclusively licensed to us its patent estate relating to antisense motifs and mechanisms and
oligonucleotide chemistry to research, develop and commercialize single-stranded antisense therapeutics, ssRNAi therapeutics, and to research double-stranded RNAi compounds. We also received a license to develop and commercialize double-stranded RNAi
therapeutics targeting a limited number of therapeutic targets on a nonexclusive basis. Additionally, in 2015, we and Alnylam entered into an alliance in which we cross-licensed intellectual property. Under this alliance, we and Alnylam each obtained
exclusive license rights to four therapeutic programs. Alnylam granted us an exclusive, royalty-bearing license to its chemistry, RNA targeting mechanism and target-specific intellectual property for oligonucleotides against four targets, including
FXI and Apo(a) and two other targets. In exchange, we granted Alnylam an exclusive, royalty-bearing license to our chemistry, RNA targeting mechanism and target-specific intellectual property for oligonucleotides against four other targets. Alnylam
also granted us a royalty-bearing, non-exclusive license to new platform technology arising from May 2014 through April 2019 for single-stranded antisense therapeutics. In turn, we granted Alnylam a royalty-bearing, non-exclusive license to new
platform technology arising from May 2014 through April 2019 for double-stranded RNAi therapeutics.
In the fourth quarter of 2020, we completed an arbitration process with Alnylam. The arbitration panel awarded us $41
million for payments owed to us by Alnylam related to Alnylam’s agreement with Sanofi Genzyme. We recognized the $41 million payment from Alnylam as R&D revenue in the fourth quarter of 2020.
The Ludwig Institute; Center for Neurological Studies
We have a collaboration with the Ludwig Institute, the Center for Neurological Studies and researchers to discover and
develop antisense medicines for ALS and other neurodegenerative diseases. Under this agreement, we agreed to pay the Ludwig Institute and the Center for Neurological Studies modest milestone payments and royalties on any antisense medicines resulting
from the collaboration.
Manufacturing
We manufacture most of the drug product we use for our research and development activities ourselves. We have also
manufactured API and commercial supply for our approved medicines. We have dedicated significant resources to develop ways to improve manufacturing efficiency and capacity. Since we can use variants of the same nucleotide building blocks and the same
type of equipment to produce our oligonucleotide medicines, we found that the same techniques we used to efficiently manufacture one oligonucleotide medicine could help improve the manufacturing processes for our other antisense medicines. By
developing several proprietary chemical processes to scale up our manufacturing capabilities, we have greatly reduced the cost of producing oligonucleotide medicines. For example, we have significantly reduced the cost of raw materials through improved
yield efficiency, while at the same time increasing our capacity to make our medicines. Through both our internal research and development programs and collaborations with outside vendors we may achieve even greater efficiency and further cost
reductions.
Our manufacturing facility is located in a 26,800 square foot building in Carlsbad, California. We purchased this building
in 2017. In addition, we have a 25,800 square foot building that houses support functions for our manufacturing activities. We lease this facility under a lease that has a term ending in August 2026 with an option to extend the lease for an additional
five-year period. Our manufacturing facility is subject to periodic inspections by the FDA and foreign equivalents to ensure that it is operating in compliance with current Good Manufacturing Practices, or cGMP, requirements.
As part of our collaborations we may agree to manufacture clinical trial materials and/or commercial supply for our
partners. For example, in the past we have manufactured clinical supply materials for AstraZeneca, Bayer, Biogen, GSK and Novartis and commercial supply materials for Biogen.
We believe we have sufficient manufacturing capacity at our own facility or at contract manufacturing organizations, or
CMOs, to meet our current internal research, development and potential commercial needs, as well as our obligations under existing agreements with our partners for research, development and commercial material. As we continue to advance our wholly
owned medicines through Phase 3 development, we will begin to manufacture process performance qualification batches and pre-approval inspection batches of our Phase 3 medicines that may be used for regulatory submissions and, pending regulatory
approval, commercial sale. We believe our current network of CMO partners are capable of providing sufficient quantities to meet anticipated commercial demands. Additionally, we continue to evaluate relationships with additional suppliers to increase
overall capacity and diversify our supply chain. While we believe that there are alternate sources of supply that can satisfy our commercial requirements, it is possible that identifying and establishing relationships with such sources, if necessary,
could result in significant delay or material additional costs. We also could experience a disruption in supply from our current CMO partners.
CMOs are subject to the FDA’s cGMP requirements and other rules and regulations prescribed by foreign regulatory
authorities. We depend on our CMO partners for continued compliance with cGMP requirements and applicable foreign standards.
Specifically, we have the following in place for our approved medicines, SPINRAZA, TEGSEDI and WAYLIVRA and our medicines
in Phase 3 development: eplontersen, olezarsen, donidalorsen, ION363, pelacarsen and tofersen.
SPINRAZA
Biogen is responsible for SPINRAZA drug supply.
TEGSEDI and WAYLIVRA
For TEGSEDI’s commercial drug supply, we are using CMOs to produce custom raw materials, active pharmaceutical ingredient,
or API, and finished goods. For WAYLIVRA’s commercial drug supply, we have manufactured custom raw materials and API. We are using CMOs to produce the finished goods for WAYLIVRA. Our CMO partners have extensive technical expertise and cGMP experience.
We believe our we and our current network of CMO partners are capable of manufacturing sufficient quantities to meet anticipated commercial demands.
Eplontersen
Our CMO partner supplied the API and the finished drug product for eplontersen’s Phase 3 program. Pursuant to our collaboration with
AstraZeneca, we will manufacture and supply eplontersen through a CMO for the ongoing clinical trials and process qualifications. AstraZeneca is responsible for commercial supply.
Olezarsen, Donidalorsen, ION363
We have supplied the API and the finished
drug product for olezarsen, donidalorsen and ION363 that we believe will be sufficient through the completion of the Phase 3 programs for each
medicine. We plan to leverage our relationships with CMOs to procure long-term raw material and drug supplies at competitive prices in the future.
Pelacarsen
We supplied the API and the finished drug product for pelacarsen’s Phase 3 study. Pursuant to our collaboration with
Novartis, Novartis is responsible for any further pelacarsen drug supply.
Tofersen
We manufactured the first batch of API for tofersen in 2015 to support the first in human studies under our collaboration
agreement with Biogen. Pursuant to our collaboration with Biogen, Biogen is responsible for tofersen drug supply. Biogen has an oligonucleotide synthesis manufacturing facility that gives it the capability to manufacture tofersen for all subsequent
clinical studies and potential commercialization, including supplying the API for the current Phase 3 study.
Patents and Proprietary Rights
Our success depends, in part, on our ability to obtain patent protection for our products in the U.S. and other countries.
We own or have exclusively licensed a substantial patent estate with numerous issued patents worldwide protecting our products and, more generally, our platform for development and commercialization of oligonucleotide therapeutics. We focus our
resources on patents and new patent applications that drive value for our company.
We own or control patents that provide exclusivity for products in our pipeline and patents that provide exclusivity for
our core technology in the field of antisense more generally. Our core technology patents include claims to chemically modified nucleosides and oligonucleotides as well as antisense medicine designs utilizing these chemically modified nucleosides.
These core claims are independent of specific therapeutic target, nucleic acid sequence, or clinical indication. We also own a large number of patents claiming antisense compounds having nucleic acid sequences complementary to therapeutic target
nucleic acids, independent of the particular chemical modifications incorporated into the antisense compound. Most importantly, we seek and obtain issued patent claims to specifically protect each of our medicines. For example, we file and seek to
obtain claims covering each drug’s nucleic acid sequence and precise drug design. In sum, we maintain our competitive advantage in the field of antisense technology by protecting our core platform technology and by creating multiple layers of patent
protection for each of our specific medicines in development.
|
Type of Patent Claim
(Broadly Applicable to Specific)
|
||
|
● Chemically Modified Nucleosides and Oligonucleotides (target and sequence independent)
● Antisense Drug Design Motifs (target and sequence independent)
● Therapeutic Methods (sequence and chemistry independent)
● Antisense Sequence (chemistry independent)
● Drug Composition
|
 |
Chemically Modified Nucleosides and Oligonucleotides
The most broadly applicable of our patents are those that claim modified nucleosides and oligonucleotides comprising the
modified nucleosides that we incorporate into our antisense medicines to increase their therapeutic efficacy. Nucleosides and chemically modified nucleosides are the basic building blocks of our antisense medicines. Therefore claims that cover any
oligonucleotide incorporating one of our proprietary modified nucleosides can apply to a wide array of antisense mechanisms of action as well as several therapeutic targets. Of particular note are our patents covering our proprietary 2’-O-(2-methoxy)
ethyl, or “MOE,” modified nucleosides, incorporated into many of our second-generation development compounds, as well as our constrained-ethyl nucleosides, or “cEt” nucleosides incorporated into our Generation 2.5 compounds. The following are some of
our patents in this category in key jurisdictions (U.S., Europe and Japan):
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
7,101,993
|
OLIGONUCLEOTIDES CONTAINING 2’-O-MODIFIED PURINES
|
2023
|
Certain MOE nucleosides and oligonucleotides containing these nucleotides
|
||||
|
United States
|
7,399,845
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
cEt nucleosides and oligonucleotides containing these nucleoside analogs
|
||||
|
United States
|
7,741,457
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
cEt nucleosides and oligonucleotides containing these nucleoside analogs
|
||||
|
United States
|
8,022,193
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Oligonucleotides containing cEt nucleoside analogs
|
||||
|
Europe
|
1984381
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
cEt nucleosides and oligonucleotides containing these nucleoside analogs
|
||||
|
Europe
|
2314594
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Oligonucleotides containing cEt nucleoside analogs and methods of use
|
||||
|
Japan
|
5342881
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
cEt nucleosides and oligonucleotides containing these nucleoside analogs
|
||||
|
United States
|
7,569,686
|
COMPOUNDS AND METHODS FOR SYNTHESIS OF BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Methods of synthesizing cEt nucleosides
|
Antisense Drug Design Motifs
We also have patents that claim oligonucleotides comprising antisense drug design motifs, or patterns of nucleoside
modifications at specified positions in the oligonucleotide. Patent claims covering our antisense drug design motifs are independent of nucleic acid sequence, so they cover oligonucleotides having the recited motif, regardless of cellular target or
clinical indication. The claimed motifs generally confer properties that optimize oligonucleotides for a particular antisense mechanism of action, such as ribonuclease H (RNase H), RNAi, or splicing. We have designed oligonucleotides incorporating
motifs, which we refer to as chimeric compounds or gapmers, to exploit the RNase H mechanism to achieve target RNA reduction. Almost all of our medicines in clinical development, including TEGSEDI and WAYLIVRA, but excluding SPINRAZA, contain this
gapmer antisense drug design motif. We own a U.S. patent that covers all of our second-generation MOE gapmer antisense medicines until March of 2023.
In addition, we have patent claims to antisense drug design motifs incorporating bicyclic nucleosides, which include both
locked nucleic acids, or “LNA” and cEt. In Europe, we have been granted claims drawn to certain gapmer oligonucleotides with bicyclic nucleosides, which include locked nucleic acids in the wings. We have also successfully obtained issued patent claims
covering our Generation 2.5 gapmer antisense drug design motifs that incorporate our cEt modified nucleosides. The following patents are some examples of our issued patents in this category in key jurisdictions (U.S., Europe and Japan):
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
7,015,315
|
GAPPED OLIGONUCLEOTIDES
|
2023
|
Gapmer oligonucleotides having 2’-O-alkyl-O-alkyl nucleosides
|
||||
|
United States
|
7,750,131
|
5’-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Oligonucleotides having 5’-methyl BNA nucleosides
|
||||
|
Europe
|
2092065
|
ANTISENSE COMPOUNDS
|
2027
|
Gapmer oligonucleotides having 2’-modifed and LNA nucleosides
|
||||
|
Europe
|
2410053
|
ANTISENSE COMPOUNDS
|
2027
|
Gapmer oligonucleotides having wings comprised of 2’-MOE and bicyclic nucleosides
|
||||
|
Europe
|
2410054
|
ANTISENSE COMPOUNDS
|
2027
|
Gapmer oligonucleotides having a 2’-modifed nucleoside in the 5’-wing and a bicyclic nucleoside in the 3’-wing
|
||||
|
Japan
|
5665317
|
ANTISENSE COMPOUNDS
|
2027
|
Gapmer oligonucleotides having wings comprised of 2’-MOE and bicyclic nucleosides
|
||||
|
United States
|
9,550,988
|
ANTISENSE COMPOUNDS
|
2028
|
Gapmer oligonucleotides having BNA nucleosides and 2’-MOE nucleosides
|
||||
|
United States
|
10,493,092
|
ANTISENSE COMPOUNDS
|
2028
|
Gapmer oligonucleotides having BNA nucleosides and 2’-MOE nucleosides and/or 2’-OMe nucleosides
|
||||
|
Europe
|
3067421
|
OLIGOMERIC COMPOUNDS COMPRISING BICYCLIC NUCLEOTIDES AND USES THEREOF
|
2032
|
Gapmer oligonucleotides having at least one bicyclic, one 2’-modified nucleoside and one 2’-deoxynucleoside
|
LIgand-Conjugated Antisense (LICA) Technology
We also have patent claims to new chemistries created to enhance targeting of antisense medicines to specific tissues and
cells to improve a drug’s properties. We designed our GalNAc LICA medicines to provide an increase in potency for targets in the liver. We have successfully obtained issued patent claims covering our LICA technology conjugated to any modified
oligonucleotide, including gapmers, double-stranded siRNA compounds, and fully modified oligonucleotides. The following patents are some examples of our issued patents in this category:
|
Jurisdiction
|
Patent
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
9,127,276
|
CONJUGATED ANTISENSE COMPOUNDS AND THEIR USE
|
2034
|
Preferred THA LICA conjugated to any group of nucleosides, including gapmers, double-stranded siRNA compounds, and fully modified
oligonucleotides
|
||||
|
United States
|
9,181,549
|
CONJUGATED ANTISENSE COMPOUNDS AND THEIR USE
|
2034
|
Preferred THA conjugate having our preferred linker and cleavable moiety conjugated to any oligomeric compound or any nucleoside having
a 2’-MOE modification or a cEt modification
|
||||
|
Europe
|
2991661
|
CONJUGATED ANTISENSE COMPOUNDS AND THEIR USE
|
2034
|
Preferred THA LICA conjugated to any group of nucleosides, including gapmers, double-stranded siRNA compounds, and fully modified
oligonucleotides
|
Therapeutic Methods of Treatment and Antisense Drug Sequences
In addition to our broad core patents, we also own hundreds of patents, worldwide, with claims to antisense compounds
having particular sequences and compounds directed to particular therapeutically important targets or methods of achieving cellular or clinical endpoints using these antisense compounds. These “Target” patents also include claims reciting the specific
nucleic acid sequences utilized by our products, independent of chemical modifications and motifs. In addition, our product-specific patents typically include claims combining specific nucleic acid sequences with nucleoside modifications and motifs. In
this way, we seek patent claims narrowly tailored to protect our products specifically, in addition to the broader core antisense patents described above.
SPINRAZA and Survival Motor Neuron
We believe SPINRAZA is protected from generic competition in the U.S. until at least 2035 and in Europe until at least
2030 by a suite of patents. These issued patents include: (i) patents licensed from the University of Massachusetts drawn to antisense compounds having the sequence of SPINRAZA, independent of chemical modification and uses of such compounds for
treating SMA, and (ii) joint patents with Cold Spring Harbor Laboratory claiming fully modified 2’MOE compositions targeting SMN2, including the precise composition of matter of SPINRAZA and methods of using such compositions. We have filed for patent
term extension, to potentially extend the term beyond 2030. With Biogen’s license of SPINRAZA, we assigned our interest in these patents to Biogen. The table below lists some key issued patents protecting SPINRAZA in the U.S. and Europe:
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
10,266,822
|
SPINAL MUSCULAR ATROPHY (SMA) TREATMENT VIA TARGETING OF SMN2 SPLICE SITE INHIBITORY SEQUENCES
|
2025
|
Methods of increasing exon-7 containing SMN2 mRNA in a cell using an oligonucleotide having the sequence of SPINRAZA
|
||||
|
United States
|
8,110,560
|
SPINAL MUSCULAR ATROPHY (SMA) TREATMENT VIA TARGETING OF SMN2 SPLICE SITE INHIBITORY SEQUENCES
|
2025
|
Methods of using antisense oligonucleotides having sequence of SPINRAZA to alter splicing of SMN2 and/or to treat SMA
|
||||
|
Europe
|
1910395
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING
|
2026
|
Sequence and chemistry (full 2’-MOE) of SPINRAZA
|
||||
|
Europe
|
3308788
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING
|
2026
|
Pharmaceutical compositions that include SPINRAZA
|
||||
|
United States
|
7,838,657
|
SPINAL MUSCULAR ATROPHY (SMA) TREATMENT VIA TARGETING OF SMN2 SPLICE SITE INHIBITORY SEQUENCES
|
2027
|
Oligonucleotides having sequence of SPINRAZA
|
||||
|
United States
|
8,361,977
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING
|
2030
|
Sequence and chemistry (full 2’-MOE) of SPINRAZA
|
||||
|
United States
|
8,980,853
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING IN A SUBJECT
|
2030
|
Methods of administering SPINRAZA
|
||||
|
United States
|
9,717,750
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING IN A SUBJECT
|
2030
|
Methods of administering SPINRAZA to a patient
|
||||
|
Europe
|
3449926
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING IN A SUBJECT
|
2030
|
Pharmaceutical compositions that include SPINRAZA for treating SMA
|
||||
|
Europe
|
3305302
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING IN A SUBJECT
|
2030
|
Antisense compounds including SPINRAZA for treating SMA
|
||||
|
United States
|
9,926,559
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING IN A SUBJECT
|
2034
|
SPINRAZA doses for treating SMA
|
||||
|
United States
|
10,436,802
|
METHODS FOR TREATING SPINAL MUSCULAR ATROPHY
|
2035
|
SPINRAZA dosing regimen for treating SMA
|
TEGSEDI and Transthyretin
We believe TEGSEDI is protected from generic competition in the U.S. and Europe until at least 2031. Additional patent
applications designed to protect TEGSEDI in other foreign jurisdictions are being pursued. The table below lists some key issued patents protecting TEGSEDI in the U.S. and Europe:
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
8,101,743
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2025
|
Antisense sequence and chemistry of TEGSEDI
|
||||
|
United States
|
8,697,860
|
DIAGNOSIS AND TREATMENT OF DISEASE
|
2031
|
Composition of TEGSEDI
|
||||
|
United States
|
9,061,044
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Sodium salt composition of TEGSEDI
|
||||
|
United States
|
9,399,774
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Methods of treating transthyretin amyloidosis by administering TEGSEDI
|
||||
|
Europe
|
2563920
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Composition of TEGSEDI
|
WAYLIVRA and Apolipoprotein C-III
We have obtained patent claims in the U.S. and Europe drawn to the use of antisense compounds complementary to a broad
active region of human ApoC-III, including the site targeted by WAYLIVRA. We have also obtained issued patents claiming the specific sequence and chemical composition of WAYLIVRA in the U.S. and Europe. We believe the issued claims protect WAYLIVRA
from generic competition in the U.S. and Europe until at least 2023 and 2024, respectively. We are pursuing additional patent applications designed to protect WAYLIVRA worldwide. The table below lists some key issued patents protecting WAYLIVRA in the
U.S. and Europe:
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
9,624,496
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2023
|
Antisense compounds specifically hybridizable within the nucleotide region of ApoCIII targeted by WAYLIVRA
|
||||
|
United States
|
7,598,227
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2023
|
Methods of treating hyperlipidemia, lowering cholesterol levels or lowering triglyceride levels with WAYLIVRA
|
||||
|
United States
|
7,750,141
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2023
|
Antisense sequence and chemistry of WAYLIVRA
|
||||
|
Europe
|
1622597
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2024
|
Antisense sequence and chemistry of WAYLIVRA
|
||||
|
Europe
|
2441449
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2024
|
Antisense compounds specifically hybridizable within the nucleotide region of ApoCIII targeted by WAYLIVRA
|
||||
|
Europe
|
3002007
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2024
|
Compounds complementary to an ApoCIII nucleic acid for use in therapy
|
||||
|
United States
|
9,157,082
|
MODULATION OF APOLIPOPROTEIN C-III (APOCIII) EXPRESSION
|
2032
|
Methods of using ApoCIII antisense oligonucleotides for reducing pancreatitis and chylomicronemia and increasing HDL
|
||||
|
United States
|
9,593,333
|
MODULATION OF APOLIPOPROTEIN C-III (APOCIII) EXPRESSION IN LIPOPROTEIN LIPASE DEFICIENT (LPLD) POPULATIONS
|
2034
|
Methods of treating lipoprotein lipase deficiency with an ApoCIII specific inhibitor wherein triglyceride levels are reduced
|
||||
|
Europe
|
2956176
|
MODULATION OF APOLIPOPROTEIN C-III (APOCIII) EXPRESSION IN LIPOPROTEIN LIPASE DEFICIENT (LPLD) POPULATIONS
|
2034
|
ApoCIII specific inhibitors including WAYLIVRA for treating lipoprotein lipase deficiency or familial chylomicronemia syndrome
|
Eplontersen and Transthyretin
We believe eplontersen is protected from
generic competition in the U.S. and Europe until at least 2034. Additional patent applications to protect eplontersen in other foreign jurisdictions are being pursued. The table below lists some key issued patents protecting eplontersen in the U.S. and Europe:
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
10,683,499
|
COMPOSITIONS AND METHODS FOR MODULATING TTR EXPRESSION
|
2034
|
Composition of eplontersen
|
||||
|
Europe
|
3524680
|
COMPOSITIONS AND METHODS FOR MODULATING TTR EXPRESSION
|
2034
|
Composition of eplontersen
|
Olezarsen and ApoC-III
We believe olezarsen is protected from generic competition in the U.S. and Europe until at least 2034. Additional patent
applications to protect olezarsen in other foreign jurisdictions are being pursued. The table below lists some key issued patents protecting olezarsen in the U.S. and Europe.
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
9,163,239
|
COMPOSITIONS AND METHODS FOR MODULATING APOLIPOPROTEIN C-III EXPRESSION
|
2034
|
Composition of olezarsen
|
||||
|
Europe
|
2991656
|
COMPOSITIONS AND METHODS FOR MODULATING APOLIPOPROTEIN C-III EXPRESSION
|
2034
|
Composition of olezarsen
|
Donidalorsen and PKK
We believe donidalorsen is protected from generic competition in the U.S. and Europe until at least 2035. Additional
patent applications to protect donidalorsen in other foreign jurisdictions are being pursued. The table below lists some key issued patents protecting donidalorsen in the U.S. and Europe.
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
9,315,811
|
METHODS FOR MODULATING KALLIKREIN (KLKB1) EXPRESSION
|
2032
|
Methods of treating HAE
|
||||
|
Europe
|
2717923
|
METHODS FOR MODULATING KALLIKREIN (KLKB1) EXPRESSION
|
2032
|
Compounds for use in treating an inflammatory condition, including HAE
|
||||
|
United States
|
10,294,477
|
COMPOSITIONS AND METHODS FOR MODULATING PKK EXPRESSION
|
2035
|
Composition of donidalorsen
|
||||
|
Europe
|
3137091
|
COMPOSITIONS AND METHODS FOR MODULATING PKK EXPRESSION
|
2035
|
Composition of donidalorsen
|
ION363 and FUS
Patent applications designed to protect ION363 from generic competition are being pursued in the U.S. and Europe; patents issuing from
these applications would have term until at least 2040. The table below lists some key pending patent applications designed to protect ION363 in the
U.S. and Europe:
|
Jurisdiction
|
Application No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
17/613,183
|
COMPOUNDS AND METHODS FOR REDUCING FUS EXPRESSION
|
2040
|
Composition of ION363
|
||||
|
Europe
|
20815459.1
|
COMPOUNDS AND METHODS FOR REDUCING FUS EXPRESSION
|
2040
|
Composition of ION363
|
Pelacarsen and Apo(a)
We believe pelacarsen is protected from
generic competition in the U.S. and Europe until at least 2034. Additional patent protection designed to protect pelacarsen in other foreign jurisdictions is being pursued. The table below lists some key issued patents protecting pelacarsen in the U.S. and Europe:
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
9,574,193
|
METHODS AND COMPOSITIONS FOR MODULATING APOLIPOPROTEIN (A) EXPRESSION
|
2033
|
Methods of lowering Apo(a) and/or Lp(a) levels with an
oligonucleotide complementary within the nucleotide region of Apo(a) targeted by pelacarsen
|
||||
|
United States
|
10,478,448
|
METHODS AND COMPOSITIONS FOR MODULATING APOLIPOPROTEIN (A) EXPRESSION
|
2033
|
Methods of treating hyperlipidemia with an oligonucleotide
complementary within the nucleotide region of Apo(a) targeted by pelacarsen
|
||||
|
United States
|
9,884,072
|
METHODS AND COMPOSITIONS FOR MODULATING APOLIPOPROTEIN (A) EXPRESSION
|
2033
|
Oligonucleotides complementary within the nucleotide region of Apo(a) targeted by pelacarsen
|
||||
|
Europe
|
2855500
|
METHODS AND COMPOSITIONS FOR MODULATING APOLIPOPROTEIN (A) EXPRESSION
|
2033
|
Oligonucleotides complementary within the nucleotide region of Apo(a) targeted by pelacarsen for decreasing Apo(a) expression
|
||||
|
United States
|
9,181,550
|
COMPOSITIONS AND METHODS FOR MODULATING APOLIPOPROTEIN (a) EXPRESSION
|
2034
|
Composition of pelacarsen
|
||||
|
Europe
|
2992009
|
COMPOSITIONS AND METHODS FOR MODULATING APOLIPOPROTEIN (a) EXPRESSION
|
2034
|
Composition of pelacarsen
|
Tofersen and SOD-1
We believe tofersen is protected from generic competition in the U.S. and Europe until at least 2035. Additional patent applications
designed to protect tofersen in other foreign jurisdictions are being pursued. With Biogen’s license of tofersen, we assigned our interest in these patents to Biogen. The table below lists some key issued patents protecting tofersen in the U.S. and Europe:
|
Jurisdiction
|
Patent No.
|
Title
|
Expiration
|
Description of Claims
|
||||
|
United States
|
10,385,341
|
COMPOSITIONS FOR MODULATING SOD-1 EXPRESSION
|
2035
|
Composition of tofersen
|
||||
|
United States
|
10,669,546
|
COMPOSITIONS FOR MODULATING SOD-1 EXPRESSION
|
2035
|
Methods of treating a SOD-1 associated neurodegenerative disorder by administering tofersen
|
||||
|
United States
|
10,968,453
|
COMPOSITIONS FOR MODULATING SOD-1 EXPRESSION
|
2035
|
Methods of treating a SOD-1 associated neurodegenerative disorder by administering a pharmaceutical composition of tofersen
|
||||
|
Europe
|
3126499
|
COMPOSITIONS FOR MODULATING SOD-1 EXPRESSION
|
2035
|
Composition of tofersen
|
We seek patent protection in significant markets and/or countries for each medicine in development. We also seek to
maximize patent term. In some cases, the patent term can be extended to recapture a portion of the term lost during FDA regulatory review. The patent exclusivity period for a medicine will prevent generic medicines from entering the market. Patent
exclusivity depends on a number of factors including initial patent term and available patent term extensions based upon delays caused by the regulatory approval process.
Manufacturing Patents
We also own patents claiming methods of manufacturing and purifying oligonucleotides. These patents claim methods for
improving oligonucleotide drug manufacturing, including processes for large-scale oligonucleotide synthesis and purification. These methods allow us to manufacture oligonucleotides at lower cost by, for example, eliminating expensive manufacturing
steps.
We also rely on trade secrets, proprietary know-how and continuing technological innovation to develop and maintain a
competitive position in antisense therapeutics.
Government Regulation
Regulation by government authorities in the U.S. and other countries is a significant component in the development,
manufacture and commercialization of pharmaceutical products and services. In addition to regulations enforced by the FDA and relevant foreign regulatory authorities, we are also subject to regulation under the Occupational Safety and Health Act, the
Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other present and potential future federal, state and local regulations.
Extensive regulation by the U.S. and foreign governmental authorities governs the development, manufacture and sale of our
medicines. In particular, our medicines are subject to a number of approval requirements by the FDA in the U.S. under the Federal Food, Drug and Cosmetic Act, or FDCA, and other laws and by comparable agencies in those foreign countries in which we
conduct business. The FDCA and other various federal, state and foreign statutes govern or influence the research, testing, manufacture, safety, labeling, storage, recordkeeping, approval, promotion, marketing, distribution, post-approval monitoring
and reporting, sampling, quality, and import and export of our medicines. State, local, and other authorities also regulate pharmaceutical manufacturing facilities and procedures.
Our manufacturing facility and our CMOs are subject to periodic inspection by the FDA and other foreign equivalents to
ensure that they are operating in compliance with cGMP requirements. In addition, marketing authorization for each new medicine may require a rigorous manufacturing pre-approval inspection by regulatory authorities. Post approval, there are strict
regulations regarding changes to the manufacturing process, and, depending on the significance of the change, changes may require prior FDA approval. FDA regulations also require investigation and correction of any deviations from cGMP and impose
reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use.
The FDA must approve any new medicine before a manufacturer can market it in the U.S. In order to obtain approval, we and
our partners must complete clinical studies and prepare and submit an NDA to the FDA. If the FDA approves a medicine, it will issue an approval letter authorizing commercial marketing of the medicine and may require a risk evaluation and mitigation
strategy, or REMS, to help ensure the benefits of the medicine outweigh the potential risks. For example, TEGSEDI has a REMS program. The requirements for REMS can materially affect the potential market and profitability of our medicines. In foreign
jurisdictions, the drug approval process is similarly demanding.
For any approved medicine, domestic and foreign sales of the medicine depend, in part, on the availability and amount of
coverage and adequate reimbursement by third-party payers, including governments and private health plans. The process for determining whether a payer will provide coverage for a product may be separate from the process for setting the reimbursement
rate that the payer will pay for the product, or procedures which utilize such product. Private health plans may seek to manage cost and use of our medicines by implementing coverage and reimbursement limitations. For example, third-party payers may
limit coverage to specific products on an approved list, or formulary, which might not include all of U.S. FDA-approved products for a particular indication. In certain jurisdictions, governments may also regulate or influence coverage, reimbursement
and/or pricing of our medicines to control cost or affect use. Within the EU a variety of payers pay for medicines, with governments being the primary source of payment. Negotiating pricing with governmental authorities can delay commercialization.
Such pricing and reimbursement factors could impact our ability and that of our commercial partners to successfully commercialize approved medicines. Further, it is possible that additional governmental action is taken in response to the COVID-19
pandemic.
In the U.S. and foreign jurisdictions, the legislative landscape continues to evolve. There have been a number of
legislative and regulatory changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels and by foreign governments
that seek to reduce healthcare costs. There has also been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in efforts to bring more transparency to drug pricing, review
the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for medicines. Further, it is possible that additional governmental action is taken in response to the COVID-19 pandemic.
In addition, the distribution of prescription pharmaceutical products is subject to the Drug Supply Chain Security Act, or
DSCA, which regulates the distribution and tracing of prescription drugs and prescription drug samples at the federal level, and set minimum standards for the regulation of drug distributors by the states. The DSCA imposes requirements to ensure
accountability in distribution and to identify and remove counterfeit and other illegitimate products from the market.
Other healthcare laws that may affect our ability to operate include, for example, the following:
| ● |
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and
Clinical Health Act, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information;
|
| ● |
Foreign and state laws governing the privacy and security of health information, such as the General Data Protection Regulation, or GDPR, in the EU; and
the California Consumer Privacy Act, or CCPA, in California, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect; and
|
| ● |
The Physician Payments Sunshine Act, which requires manufacturers of medicines, devices, biologics, and medical supplies to report annually to the U.S.
Department of Health and Human Services information related to payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists, and chiropractors), other healthcare providers (such as
physician assistants and nurse practitioners), and teaching hospitals, and ownership and investment interests held by physicians and their immediate family members.
|
Sales and Marketing
Numerous regulatory authorities in addition to the FDA, including, in the U.S., the Centers for Medicare and Medicaid
Services, other divisions of the U.S. Department of Health and Human Services, the U.S. Department of Justice, and similar foreign, state and local government authorities, regulate sales, promotion and other activities following drug approval. As
described above, the FDA regulates all advertising and promotion activities for drugs under its jurisdiction both prior to and after approval. Only those claims relating to safety and efficacy that the FDA has approved may be used in labeling.
Physicians may prescribe legally available drugs for uses that are not described in the drug’s labeling and that differ from those we tested and the FDA approved. Such off-label uses are common across medical specialties and often reflect a physician’s
belief that the off-label use is the best treatment for the patients. The FDA does not regulate the behavior of physicians in their choice of treatments, but FDA regulations do impose stringent restrictions on manufacturers’ communications regarding
off-label uses. If we do not comply with applicable FDA requirements, we may face adverse publicity, enforcement action by the FDA, corrective advertising, consent decrees and the full range of civil and criminal penalties available to the FDA.
Promotion of off-label uses of drugs can also implicate the false claims laws described below.
In the U.S. sales, marketing and scientific/educational programs must also comply with various federal and state laws
pertaining to healthcare “fraud and abuse,” including anti-kickback laws and false claims laws. Anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, receive, or pay any remuneration in exchange for, or to induce,
the referral of business, including the purchase or prescription of a particular drug. Due to the breadth of the statutory provisions, limited statutory exceptions and regulatory safe harbors, and the absence of guidance in the form of regulations and
very few court decisions addressing industry practices, it is possible that our practices might be challenged under anti-kickback or similar laws. Moreover, recent healthcare reform legislation has strengthened these laws. For example, the Patient
Protection and Affordable Act, as amended by the Health Care and Education Reconciliation Act of 2010, or Affordable Care Act, among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes to
clarify that a person or entity does not need to have actual knowledge of this statute or specific intent to violate it. In addition, the Affordable Care Act clarifies that the government may assert that a claim that includes items or services
resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for
payment, to third-party payers (including Medicare and Medicaid) claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. Our
activities relating to the sale and marketing of our drugs may be subject to scrutiny under these laws. Violations of fraud and abuse laws may be punishable by criminal and civil sanctions, including fines and civil monetary penalties, the possibility
of exclusion from federal healthcare programs (including Medicare and Medicaid) and corporate integrity agreements, which impose, among other things, rigorous operational and monitoring requirements on companies. Similar sanctions and penalties also
can be imposed upon executive officers and employees, including criminal sanctions against executive officers under the so-called “responsible corporate officer” doctrine, even in situations where the executive officer did not intend to violate the law
and was unaware of any wrongdoing.
Given the significant penalties and fines that can be imposed on companies and individuals if convicted, allegations of
such violations often result in settlements even if the company or individual being investigated admits no wrongdoing. Settlements often include significant civil sanctions, including fines and civil monetary penalties, and corporate integrity
agreements. If the government were to allege or convict us or our executive officers of violating these laws, our business could be harmed. In addition, private individuals can bring similar actions. Our activities could be subject to challenge for the
reasons discussed above and due to the broad scope of these laws and the increasing attention being given to them by law enforcement authorities. Other healthcare laws that may affect our ability to operate include HIPAA, which prohibits, among other
things, executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, also
governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information; analogous state laws governing the privacy and security of health information, some of which are more stringent
than HIPAA and many of which differ from each other in significant ways and may not have the same effect, and the Physician Payments Sunshine Act, which requires manufacturers of drugs, devices, biologics, and medical supplies to report annually to the
U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals, and ownership and investment interests held by physicians and their immediate
family members. Further, there are an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws.
Given the lack of clarity in laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state authorities.
Similar rigid restrictions are imposed on the promotion and marketing of drugs in the E.U. and other countries. Even in
those countries where we may not be directly responsible for the promotion and marketing of our medicines, if our potential international distribution partners engage in inappropriate activity, it can have adverse implications for us.
The U.S. Foreign Corrupt Practices Act, or FCPA, prohibits certain individuals and entities, including us, from promising,
paying, offering to pay, or authorizing the payment of anything of value to any foreign government official, directly or indirectly, to obtain or retain business or an improper advantage. If we violate the FCPA, it could result in large civil and
criminal penalties as well as an adverse effect on our reputation, operations, and financial condition. We could also face collateral consequences such as debarment and the loss of export privileges.
Both the federal and state governments in the U.S. and foreign governments continue to propose and pass new legislation and regulations
designed to contain or reduce the cost of healthcare. For example, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to
Biden’s executive order, on September 9, 2021, the U.S. Department of Health and Human Services, or HHS, released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential
legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. No legislation or administrative actions have been finalized to implement these principles. Congress is also
considering additional health reform measures. Such legislation and regulations may result in decreased reimbursement, which may further exacerbate industry-wide pressure to reduce the prices charged for medical products.
Competition
Our Business in General
Some of our medicines may compete with existing therapies for market share and some of our medicines in development may
compete for patients in clinical trials. In addition, there are a number of companies pursuing the development of oligonucleotide-based technologies and the development of pharmaceuticals utilizing these technologies. These companies include
biopharmaceutical companies and large pharmaceutical companies acting either independently or together. Our medicines are differentiated from traditional small molecule medicines by their chemistry, how they move in the body, how they act in the body,
delivery technology, and formulations.
Our approved medicines and our medicines under development address numerous markets. The diseases our medicines target for which we have
or may receive marketing authorization will determine our competition. For some of our medicines, an important factor may be the timing of market introduction of competitive products. Accordingly, the relative speed with which we can develop medicines,
complete the clinical trials and marketing authorization processes and supply commercial quantities of the medicines to the market are important competitive factors. We expect to compete with products approved for sale based on a variety of factors,
including, among other things, product efficacy, safety, mechanism of action, dosing convenience, marketing and sales strategy and tactics, availability, price, and reimbursement.
Below we have included what we believe to be the competitive landscape for our marketed medicines and for the medicines we currently have
in Phase 3 trials. We have included medicines that we believe compete or may compete directly with our medicines. We included competitors, potential competitors that are past Phase 1 development or potential competitors that plan to start a pivotal
study this year. We do not believe that any medicines meet these criteria to compete with ION363.
SPINRAZA
We consider the following medicines as competitors to SPINRAZA for the indication of SMA:
|
Medicine
|
Company
|
Medicine Description (1)
|
Phase (1)
|
Route of Administration (1)
|
||||
|
Zolgensma
(Onasemnogene abeparvovec)
|
Novartis
|
Gene therapy targeting the genetic root cause of SMA by replacing the missing or nonworking SMN1 gene
|
Approved for pediatric SMA patients less than 2 years of age
|
Intravenous infusion
|
||||
|
Evrysdi
(Risdiplam)
|
Roche
|
A small molecule medicine that modulates splicing of the SMN2 gene
|
Approved for SMA patients of 2 months or older
|
Oral
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
TEGSEDI and Eplontersen
We consider the following medicines as competitors and potential future competitors to TEGSEDI and eplontersen for the
indication of hATTR amyloidosis and/or ATTR cardiomyopathy:
|
Medicine
|
Company
|
Medicine Description (1)
|
Phase (1)
|
Route of Administration (1)
|
||||
|
Onpattro
(Patisiran)
|
Alnylam
|
An RNAi medicine formulated with lipid nanoparticles to inhibit TTR mRNA
|
Approved hATTR/
Phase 3 ATTR-CM
|
Intravenous infusion
|
||||
|
Vyndaqel/Vyndamax
(Tafamidis and tafamidis meglumine)
|
Pfizer
|
A small molecule medicine to stabilize TTR protein
|
Approved in U.S., EU, Japan and select other markets for hATTR-PN and/or ATTR-CM; indications vary by region
|
Oral
|
||||
|
Vutrisiran
|
Alnylam
|
An RNAi medicine conjugated with GalNAC to inhibit TTR mRNA
|
Submitted US/EU for ATTR-PN, Phase 3 for ATTR-CM
|
Subcutaneous Injection
|
||||
|
Acoramidis
|
Bridgebio
|
Small molecule that binds and stabilizes TTR in the blood
|
Phase 3 ATTR-CM
|
Oral
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
WAYLIVRA and Olezarsen
We believe that the following medicines could compete with WAYLIVRA and olezarsen in FCS and SHTG:
|
Medicine
|
Company
|
Medicine Description (1)
|
Phase (1)
|
Route of Administration (1)
|
||||
|
ARO-APOC3
|
Arrowhead Pharmaceuticals
|
Targets APOCIII by utilizing Targeted RNAi Molecule Platform
|
3 (FCS), 2 (SHTG)
|
Subcutaneous Injection
|
||||
|
Lomitapide
|
Amryt Pharma
|
Microsomal triglyceride transfer protein (MTP) inhibitor
|
2 (FCS) (investigator led)
|
Oral
|
||||
|
Evinacumab
|
Regenerion
|
ANGPTL3 mAb
|
2 (SHTG)
|
Intravenous Infusion
|
||||
|
BIO89-100
|
Bio 89
|
FGF21 analog
|
2 (SHTG)
|
Subcutaneous Injection
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
Donidalorsen
We believe that the following medicines could compete with donidalorsen as a prophylactic treatment for patients with HAE:
|
Medicine
|
Company
|
Medicine Description (1)
|
Phase (1)
|
Route of Administration (1)
|
||||
|
Takhzyro
(lanadelumab-flyo)
|
Takeda
|
A monoclonal antibody that inhibits plasma kallikrein activity
|
Approved for HAE patients 12 years and older
|
Subcutaneous
Infusion
|
||||
|
Cinryze
(C1-esterase inhibitor)
|
Takeda
|
A human plasma protein that mediates inflammation and coagulation
|
Approved for HAE patients 6 years and older
|
Intravenous
Infusion
|
||||
|
Orladeyo
(berotralstat)
|
BioCryst
|
Oral plasma kallikrein inhibitor
|
Approved for HAE patients 12 years and older
|
Oral
|
||||
|
Haegarda
(C1 esterase inhibitor)
|
CSL Behring
|
C1 esterase inhibitor
|
Approved for HAE patients 6 years and older
|
Subcutaneous
Infusion
|
||||
|
garadacimab
|
CSL Behring
|
An anti-factor XIIa monoclonal antibody
|
3
|
Subcutaneous
Infusion
|
||||
|
KVD824
|
KalVista
|
Oral plasma kallikrein inhibitor
|
2
|
Oral
|
||||
|
NTLA-2002
|
Intellia
|
CRISPR therapeutic candidate designed to inactivate the kallikrein B1 gene
|
1/2
|
Intravenous
Infusion
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
Pelacarsen
We believe that the following medicine could compete with pelacarsen in CVD in patients with elevated LP(a):
|
Medicine
|
Company
|
Medicine Description (1)
|
Phase (1)
|
Route of Administration (1)
|
||||
|
AMG 890
|
Amgen/ Arrowhead Pharmaceuticals
|
RNAi therapeutic designed to lower Lp(a)
|
2
|
Subcutaneous Injection
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
Tofersen
We believe that the following medicines could compete with tofersen in SOD1-ALS:
|
Medicine
|
Company
|
Medicine Description (1)
|
Phase (1)
|
Route of Administration (1)
|
||||
|
Arimoclomol
|
Orphazyme
|
Provides cellular protection from abnormal proteins by activating molecular “chaperone” proteins that can repair or degrade the damaged proteins
|
3
|
Oral
|
||||
|
NI-204
|
Neurimmune
|
A human derived antibody targeting misfolded SOD1
|
2
|
Intravenous
Infusion
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
We recognize the importance of Environmental, Social and Governance, or ESG, initiatives as it relates to our business
strategy and risk assessment. During 2020 and 2021, we took steps to formalize our corporate responsibility program. In December 2021, we issued our inaugural corporate responsibility report. As part of our ongoing work, we identified the following
corporate responsibility initiatives that we believe are most important to our business:
| ● |
Safety of patients in clinical trials;
|
| ● |
Drug safety and supply chain management;
|
| ● |
Access to medicines and tackling devastating diseases;
|
| ● |
Human resources management;
|
| ● |
Diversity, equity and inclusion;
|
| ● |
Employee health and safety; and
|
| ● |
Governance and business ethics
|
We have a relatively small environmental footprint, so our stewardship programs focus on improving eco-awareness,
identifying efficiencies and integrating more sustainable practices into our daily operations. Our priority assessment considered investor and other stakeholder interests and is aligned with the requirements of ESG ratings agencies and with leading ESG
frameworks, including the Sustainability Accounting Standards Board, or SASB.
We encourage you to view our 2021 Corporate Responsibility Report published on our website for more detailed information regarding our ESG initiatives. Nothing in the report or on our website shall be deemed incorporated by reference into this Annual Report on
Form 10-K.
Employees & Human Capital
As of February 16, 2022,
we employed 660 people, the vast majority of whom reside in the U.S. A significant number of our management and professional employees have had prior experience with pharmaceutical, biotechnology or medical product companies. Our average employee
turnover rate in 2021 was 16 percent, while the turnover for life sciences/ medical device companies over this period was 19 percent according to a survey published by Radford – an Aon Hewitt Company. Given the uniqueness and complexity of our
technology, it is critical to retain the knowledge and experience of outstanding long service employees. The experience and seniority of our employees is as critical to our future success as it has been to the success we have enjoyed to date.
Collective bargaining agreements do not cover any of our employees, and management
considers relations with our employees to be good. We believe that the future will be defined by outstanding people and we are committed to recruiting, developing, motivating, and rewarding them.
We encourage you to visit our website for more detailed information regarding our Human Capital programs and initiatives.
Nothing on our website shall be deemed incorporated by reference into this Annual Report on Form 10-K.
Benefits
Employees are rewarded individually on the basis of their responsibilities and accomplishments. We offer competitive compensation and
benefits to our employees. In addition to salary and bonus programs, we also offer:
| ● |
Comprehensive medical, dental and vision insurance;
|
| ● |
401(k) matching;
|
| ● |
Stock options, RSUs and Employee Stock Purchase Plan, or ESPP;
|
| ● |
Vacation, holiday, sick time and paid time off for volunteering;
|
| ● |
Wellness programs;
|
| ● |
Flexible spending accounts for health and dependent day care needs;
|
| ● |
Life, AD&D insurance and long-term disability insurance coverage options; and
|
| ● |
Employee Assistance Program, or EAP.
|
We recognize achievements with salary increases, stock awards, promotions, and bonus opportunities.
Pay Equity
We are committed to paying our employees fairly, regardless of their gender, race, or other personal characteristics. To
ensure we are achieving our commitment, we benchmark and evaluate pay based on market data and consider factors such as an employee’s role and experience, an employee’s performance and internal equity. We also regularly review our compensation
practices, both in terms of our overall workforce and individual employees, to ensure our pay is fair and equitable.
In 2021, we engaged an independent third-party expert to perform a pay equity analysis that reviewed pay equity by gender,
race and age. We plan to continue to engage a third-party expert to review pay equity every two to three years, as we determine necessary.
Diversity, Equity and Inclusion
At Ionis, we encourage diversity in our workforce. Prejudicial barriers to human potential and productivity are foreign
to our values. We recognize that for the full potential of our workforce to be realized, we must cultivate an inclusive culture where all employees feel empowered to contribute fully in an environment that values different perspectives, leading to
better ideas and increased innovation. We have several employee-led resource groups dedicated to different aspects of diversity and a diverse management team and board of directors.
Training and Development
We designed our training and development programs to help employees gain important Ionis knowledge and develop the skills
to be successful. All of our trainings from new hire through senior leader, are focused on the Ionis culture and core principles and learning what we mean when we say: “Working the Ionis Way.”
We empower our employees to build rewarding careers at Ionis, driven by a culture of yes that encourages personal and
professional employee growth. Ionis offers robust training opportunities with course offerings and events available to every employee regardless of level or function. In addition, employees also have access to Ionis’ learning and development library
that houses important information on career growth and planning. By supporting our employees, we know that each professional development milestone enables our continued success.
Information about our Executive Officers
The following sets forth certain information regarding our executive officers as of February 16, 2022:
|
Name
|
Age
|
Position
|
||
|
Brett P. Monia, Ph.D.
|
60
|
Chief Executive Officer
|
||
|
Joseph T. Baroldi
|
44
|
Executive Vice President, Chief Business Officer
|
||
|
C. Frank Bennett, Ph.D.
|
65
|
Executive Vice President, Chief Scientific Officer
|
||
|
Onaiza Cadoret-Manier
|
57
|
Executive Vice President, Chief Product Strategy and Operations Officer
|
||
|
Richard S. Geary, Ph.D.
|
64
|
Executive Vice President, Chief Development Officer
|
||
|
Elizabeth L. Hougen
|
60
|
Executive Vice President, Finance and Chief Financial Officer
|
||
|
Patrick R. O’Neil, Esq.
|
48
|
Chief Legal Officer, General Counsel and Corporate Secretary
|
||
|
Eugene Schneider, M.D.
|
49
|
Executive Vice President, Chief Clinical Development Officer
|
||
|
Eric E. Swayze, Ph.D.
|
56
|
Executive Vice President, Research
|
BRETT P. MONIA, Ph.D.
Chief Executive Officer
Dr. Monia was promoted to Chief Executive Officer in January 2020. From January 2018 to December 2019, Dr. Monia served
as Chief Operating Officer. From January 2012 to January 2018, Dr. Monia served as Senior Vice President. From February 2009 to January 2012, Dr. Monia served as our Vice President, Drug Discovery and Corporate Development and from October 2000 to
February 2009, he served as our Vice President, Preclinical Drug Discovery. From October 1989 to October 2000 he held various positions within our Molecular Pharmacology department.
JOSEPH T. BAROLDI
Executive Vice President, Chief Business Officer
Mr. Baroldi has served as Ionis’ Executive Vice President, Chief Business Officer since January 2022. Prior to Ionis, Mr.
Baroldi was the chief operating officer at Avidity Biosciences, a biotechnology company focused on oligonucleotide-based therapies. Prior to Avidity, Mr. Baroldi was vice president of Business Development and Alliance Management at Ionis from 2009 to
2020. Mr. Baroldi has also held positions in strategic planning and scientific research for Gen-Probe Inc. and Ionis.
C. FRANK BENNETT, Ph.D.
Executive Vice President, Chief Scientific Officer
Dr. Bennett has served as Ionis’ Executive Vice President, Chief Scientific Officer since April 2020. In January 2020, Dr.
Bennett was promoted to Chief Scientific Officer. From January 2006 to December 2019, Dr. Bennett served as Senior Vice President, Antisense Research. From June 1995 to January 2006, Dr. Bennett served as our Vice President, Research. From March 1993
to June 1995, he was Director, Molecular Pharmacology, and from May 1992 to March 1993, he was an Associate Director in our Molecular and Cellular Biology department. Prior to joining Ionis in 1989, Dr. Bennett was employed by SmithKline and French
Laboratories in various research positions. He is a member of the Board of Directors for Flamingo Therapeutics and an external member of the Hereditary Disease Foundation.
ONAIZA CADORET-MANIER
Executive Vice President, Chief Product Strategy and Operations Officer
Ms. Cadoret-Manier has served as Ionis’
Executive Vice President, Chief Product Strategy and Operations Officer since February 2022. From April 2020 to February 2022, Ms. Cadoret-Manier served as our Executive Vice President, Chief Corporate Development and Commercial Officer. Ms. Cadoret-Manier joined Ionis as Chief Corporate Development and Commercial Officer in January 2020. Prior to joining Ionis, from 2018 to 2019 Ms.
Cadoret-Manier was the chief commercial officer for Grail Biosciences, an early detection genomics company. Prior to Grail, Ms. Cadoret-Manier was vice president of the Respiratory Franchise at Genentech where she worked from 2011 to 2018. Ms.
Cadoret-Manier also has held multiple senior management positions overseeing corporate strategy, alliances, and marketing and sales for numerous disease areas for Genentech, Pfizer and Amylin Pharmaceuticals.
RICHARD S. GEARY, Ph.D.
Executive Vice President, Chief Development Officer
Dr. Geary has served as Ionis’ Executive Vice President, Chief Development Officer since January 2021. From April 2020 to
December 2020, Dr. Geary served as our Executive Vice President, Development and from August 2008 to March 2020, was our Senior Vice President, Development. From August 2003 to August 2008, Dr. Geary served as our Vice President, Preclinical
Development. From November 1995 to August 2003, he held various positions within the Preclinical Development department. Prior to joining Ionis in 1995, Dr. Geary was Senior Research Scientist and Group Leader for the bioanalytical and preclinical
pharmacokinetics group in the Applied Chemistry Department at Southwest Research Institute.
ELIZABETH L. HOUGEN
Executive Vice President, Finance and Chief Financial Officer
Ms. Hougen has served as Ionis’ Executive Vice President and Chief Financial Officer since April 2020. From January 2013
to March 2020, Ms. Hougen served as our Senior Vice President, Finance and Chief Financial Officer. From January 2007 to December 2012, Ms. Hougen served as our Vice President, Finance and Chief Accounting Officer and from May 2000 to January 2007, she
served as our Vice President, Finance. Prior to joining Ionis in 2000, Ms. Hougen was Executive Director, Finance and Chief Financial Officer for Molecular Biosystems, Inc., a public biotechnology company.
PATRICK R. O’NEIL, Esq.
Chief Legal Officer, General Counsel and Corporate Secretary
Mr. O’Neil has served as Ionis’ Chief Legal Officer and General Counsel since September 2021. Mr. O’Neil also serves as
our Corporate Secretary. From March 2020 to September 2021, Mr. O’Neil served as our Executive Vice President, Legal & General Counsel and Chief Compliance Officer. From January 2013 to March 2020, Mr. O’Neil served as our Senior Vice President,
Legal and General Counsel. From September 2010 to January 2013, Mr. O’Neil served as our Vice President, Legal and General Counsel and from January 2009 to September 2010, he served as our Vice President, Legal and Senior Transactions Counsel. From
October 2001 to January 2009 he held various positions within our Legal department. Prior to joining Ionis, Mr. O’Neil was an associate at Cooley LLP.
EUGENE SCHNEIDER, M.D.
Executive Vice President, Chief Clinical Development Officer
Dr. Schneider was promoted to Executive Vice President and Chief Clinical Development Officer of Ionis in January 2021.
From August 2018 to December 2020, Dr. Schneider served as our Senior Vice President, Head of Clinical Development. From April 2015 to July 2018, Dr. Schneider was our Vice President, Clinical Development, Severe and Rare Diseases. Dr. Schneider joined
Ionis in December 2013 as Executive Director, Clinical Development. Dr. Schneider has two decades of experience in clinical development primarily in the rare diseases space. Prior to joining Ionis, Dr. Schneider was senior medical director at both
Synageva BioPharma and Biovail Technologies Ltd.
ERIC E. SWAYZE, Ph.D.
Executive Vice President, Research
Dr. Swayze has served as Ionis’ Executive Vice President, Research since April 2020 and is responsible for leading
preclinical antisense drug discovery and antisense technology research. In January 2020, Dr. Swayze was promoted to Senior Vice President of Research. Previously, Dr. Swayze was Vice President of Chemistry and Neuroscience Drug Discovery at Ionis,
overseeing the advancement of multiple programs to clinical development. He joined Ionis in 1994 and has contributed to key technology advancements, including Ionis’ Generation 2.5 chemistry and LICA technology.
Item 1A. RISK FACTORS
Investing in our securities involves a high degree of risk. You should carefully consider the
following information about the risks described below, together with the other information contained in this report and in our other public filings in evaluating our business. If any of the following risks actually occur, our business could be
materially harmed, and our financial condition and results of operations could be materially and adversely affected. As a result, the trading price of our securities could decline, and you might lose all or part of your investment.
Risks Related to the COVID-19 Pandemic
Our business could be materially adversely affected by the effects of health epidemics. To date, we believe the impacts of the recent
COVID-19 pandemic on our business are limited and manageable.
Our business could be materially adversely affected by health epidemics in regions where we or our partners are commercializing our
medicines, have concentrations of clinical trial sites or other business operations, and could cause significant disruption in the operations of third-party manufacturers and contract research organizations upon whom we rely. For example, since
December 2019, a novel strain of coronavirus, SARS-CoV-2, causing a disease referred to as COVID-19, has spread worldwide. In March 2020, the World Health Organization declared the COVID-19 outbreak a pandemic, or the COVID-19 Pandemic, and the U.S.
government imposed restrictions on travel between the U.S., Europe and certain other countries. In addition, the Governor of the State of California and the Governor of the Commonwealth of Massachusetts, the states in which our offices are located,
each declared a state of emergency related to the spread of COVID-19 and issued executive orders that directed residents to stay at home.
In response to these public health directives and orders, in March 2020, we implemented work-from-home policies for most of our
employees globally and generally suspended business-related travel. In the U.S., as vaccinations have become more widely available, states have lifted restrictions implemented as part of the pandemic response and reopened their economies. In June
2021, the Governor of California terminated the vast majority of executive actions that were put in place beginning in March 2020, leaving only a subset of provisions that facilitate the ongoing recovery. In May 2021, the Commonwealth of Massachusetts
also lifted most of its pandemic restrictions. We continue to modify our policies for our employees in California, Massachusetts, and internationally to align with current local guidance. We believe the effects of these work-from-home and travel
policies have had a limited impact on our business.
These public health directives and orders have impacted our and our partners’ sales efforts. For example, some physician and hospital
policies that have been put in place as a result of the COVID-19 Pandemic restrict in-person access by third parties, which has in some cases impacted our commercialization efforts for TEGSEDI and WAYLIVRA. Additionally, Biogen has reported that it is
monitoring the demand for SPINRAZA, including the duration and degree to which it might see delays in starting new patients on SPINRAZA due to hospitals diverting resources necessary to administer SPINRAZA to care for COVID-19 patients. These and
similar, and perhaps more severe, disruptions in our or our partner’s commercial operations could materially impact our business, operating results and financial condition in the future.
Quarantines, shelter-in-place, executive and similar government orders, or the perception that such orders, shutdowns or other
restrictions on the conduct of business operations could occur, could impact personnel at third-party manufacturing facilities in the U.S. and other countries, or the availability or cost of materials, which would disrupt our supply chain. Recently
there have been major disruptions to the global supply chain due to the COVID-19 Pandemic. To date, we have not experienced any significant consequences to our business as a result of the current supply chain disruptions, but could in the future if
such disruptions persist or worsen.
We have experienced impacts to our clinical trial operations due to the COVID-19 Pandemic; however, we believe such impacts are limited
and manageable. Some examples of these impacts include:
| ● |
delays in clinical site initiation, site monitoring and patient enrollment due to restrictions imposed as a result of the COVID-19 Pandemic;
|
| o |
For example, in March 2020, we instituted a temporary suspension of enrollment for new subjects in our Phase 3 studies of eplontersen based on advice from our trial
advisory committee; however, enrollment has resumed.
|
| ● |
some patients have not been able to meet protocol requirements, as quarantines have impeded patient movement and interrupted healthcare services;
|
| ● |
delays in site initiations due to principle investigators and site staff focusing on and prioritizing COVID-19 patient care; and
|
| ● |
delays in necessary interactions with regulators, ethics committees and other important agencies and contractors due to limitations in employee resources or forced
furlough of government or contractor personnel.
|
In addition, some of our partners have experienced impacts to their clinical trial operations as a result of the COVID-19 Pandemic. For
example, in December 2021, Novartis announced that enrollment for the Phase 3 HORIZON study had been delayed due to the COVID-19 Pandemic.
The spread of COVID-19 has caused a broad impact globally. While the potential economic impact brought by, and the duration of, the
COVID-19 Pandemic may be difficult to assess or predict, it could result in significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity. In addition, a recession
or market correction resulting from the spread of COVID-19 could materially affect our business and has and could continue to affect the value of our securities.
The global COVID-19 Pandemic continues to rapidly evolve. While we have not yet experienced material adverse effects to our business as a
result of the COVID-19 Pandemic, the ultimate impact of the COVID-19 Pandemic or a similar health epidemic is highly uncertain and subject to change. As such, we do not yet know the full extent of delays or impacts on our business, our clinical trials,
healthcare systems or the global economy as a whole. However, these effects could have a material impact on our operations, and we will continue to monitor the COVID-19 Pandemic closely.
Risks Related to the Commercialization of our Medicines
We have limited experience as a company in commercializing medicines and we will have to invest significant resources to develop these
capabilities. If we are unable to establish effective marketing, sales, market access, distribution, and related functions, or enter into agreements with third parties to commercialize our medicines, we may not be able to generate revenue from our
medicines.
We have limited experience as a company in commercializing medicines and we will have to invest significant financial
and management resources to develop the infrastructure required to successfully commercialize our medicines. There are significant risks involved in building and managing a sales organization, including our ability to hire, retain and incentivize
qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel, and effectively manage a geographically dispersed sales and marketing team. We will also need to scale-up existing internal support
functions to aid our commercialization efforts, in particular, regulatory affairs and medical affairs. Any failure to effectively build or maintain the infrastructure required to successfully commercialize our medicines, including our sales,
marketing, market access, distribution, and related capabilities, or scale-up our existing support functions, could adversely impact the revenue we generate from our medicines. In addition, if we choose to rely on third parties to assist us in
commercializing our medicines, we may not be able to enter into collaborations or hire consultants or external service providers on acceptable financial terms, or at all. If we do engage third parties to assist us in the commercialization of our
medicines, our product revenues and profitability may be lower than if we commercialized such medicines ourselves.
If the market does not accept our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA, and our medicines in development,
we are not likely to generate substantial revenues or become consistently profitable.
Even if our medicines are authorized for marketing, our success will depend upon the medical community, patients and
third-party payers accepting our medicines as medically useful, cost-effective, safe and convenient. Even when the FDA or foreign regulatory authorities authorize our or our partners’ medicines for commercialization, doctors may not prescribe our
medicines to treat patients. Furthermore, we and our partners may not successfully commercialize additional medicines.
Additionally, in many of the markets where we
or our partners may sell our medicines in the future, if we or our partners cannot agree with the government or other third-party payers regarding the price we can charge for our medicines, then we may not be able to sell our medicines in that
market. Similarly, cost control initiatives by governments or third-party payers could decrease the price received for our medicines or increase patient coinsurance to a level that makes our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA, and
our medicines in development, economically unviable. If the pricing of any of our medicines decreases for any reason, it will reduce our revenue for such medicine. For example, Biogen has disclosed that SPINRAZA revenue has decreased in part
due to lower pricing in the U.S. and certain rest of world markets.
The degree of market acceptance for our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA, and our medicines in
development, depends upon a number of factors, including the:
| ● |
receipt and scope of marketing authorizations;
|
| ● |
establishment and demonstration in the medical and patient community of the efficacy and safety of our medicines and their potential advantages over
competing products;
|
| ● |
cost and effectiveness of our medicines compared to other available therapies;
|
| ● |
patient convenience of the dosing regimen for our medicines; and
|
| ● |
reimbursement policies of government and third-party payers.
|
Based on the profile of our medicines, physicians, patients, patient advocates, payers or the medical community in general
may not accept or use any medicines that we may develop.
For example, TEGSEDI requires periodic blood and urine monitoring, is available in the U.S. only through a REMS program,
and the product label in the U.S. has a boxed warning for thrombocytopenia and glomerulonephritis. Our main competition in the U.S. market for TEGSEDI is patisiran, marketed by Alnylam Pharmaceuticals, Inc. Although patisiran requires intravenous
administration and pre-treatment with steroids, it does not have a boxed warning nor is it available only through a REMS program. Additionally, the product label for WAYLIVRA in the EU requires regular blood monitoring. In each case, these label
requirements have negatively affected our ability to attract and retain patients for these medicines. If we or our partner cannot effectively maintain patients on TEGSEDI or WAYLIVRA, including due to limitations or restrictions on the ability to
conduct periodic blood and urine monitoring of our patients as a result of the current COVID-19 Pandemic, we may not be able to generate substantial revenue from TEGSEDI or WAYLIVRA sales.
If we or our partners fail to compete effectively, our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA, and our
medicines in development, will not generate significant revenues.
Our competitors engage in drug discovery throughout the world, are numerous, and include, among others, major
pharmaceutical companies and specialized biopharmaceutical firms. Other companies are engaged in developing antisense technology. Our competitors may succeed in developing medicines that are:
| ● |
priced lower than our medicines;
|
| ● |
reimbursed more favorably by government and other third-party payers than our medicines;
|
| ● |
safer than our medicines;
|
| ● |
more effective than our medicines; or
|
| ● |
more convenient to use than our medicines.
|
These competitive developments could make our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA, and our medicines in
development, obsolete or non-competitive.
Certain of our partners are pursuing other technologies or developing other medicines either on their own or in
collaboration with others, including our competitors, to treat the same diseases our own collaborative programs target. Competition may negatively impact a partner’s focus on and commitment to our medicines and, as a result, could delay or otherwise
negatively affect the commercialization of our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA.
Many of our competitors have substantially greater financial, technical and human resources than we do. In addition, many
of these competitors have significantly greater experience than we do in conducting preclinical testing and human clinical studies of new pharmaceutical products, in obtaining FDA and other regulatory authorizations of such products and in
commercializing such products. Accordingly, our competitors may succeed in obtaining regulatory authorization for products earlier than we do.
There are several pharmaceutical and biotechnology companies engaged in the development or commercialization in certain
geographic markets of products against targets that are also targets of products in our development pipeline. For example:
| ● |
Onasemnogene abeparvovec and risdiplam compete with SPINRAZA;
|
| ● |
Patisiran, tafamidis, and tafamidis meglumine compete with TEGSEDI and could compete with eplontersen;
|
| ● |
Vutrisiran and acoramidis could compete with TEGSEDI and eplontersen;
|
| ● |
ARO-APOC3, lomitapide, evinacumab, BIO89-100, and gemcabene could compete with WAYLIVRA and olezarsen;
|
| ● |
AMG890 could compete with pelacarsen;
|
| ● |
Arimoclomol, ultomiris, mastinib and trehalose could compete with tofersen; and
|
| ● |
Lanadelumab-flyo, C1 esterase inhibitor, berotralstat, C1 esterase inhibitor
subcutaneous, garadacimab, KVD824, and NTLA-2002 could compete with donidalorsen.
|
SPINRAZA injection for intrathecal use is an antisense medicine indicated for the treatment of SMA patients of all ages approved in over
50 countries. Specifically, SPINRAZA faces competition from onasemnogene abeparvovec, a gene therapy product that was approved in the U.S. in May 2019 and in the EU in May 2020 for the treatment of SMA, as well as risdiplam, an oral product for the
treatment of SMA that was approved in the U.S. in August 2020 and in the EU in March 2021. Biogen has disclosed that SPINRAZA revenue has decreased primarily due to a reduction in demand as a result of increased competition and that future sales of
SPINRAZA may be adversely affected by competing products.
Additionally, companies that are developing medicines that target the same patient populations as our medicines in development may compete
with us to enroll participants in the clinical trials for such medicines, which could make it more difficult for us to complete enrollment for these clinical trials.
Our medicines could be subject to regulatory limitations following approval.
Following approval of a medicine, we and our partners must comply with comprehensive government regulations regarding the
manufacture, marketing and distribution of medicines. Promotional communications regarding prescription medicines must be consistent with the information in the product’s approved labeling. We or our partners may not obtain the labeling claims
necessary or desirable to successfully commercialize our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA, and our medicines in development.
The FDA and foreign regulatory bodies have the authority to impose significant restrictions on an approved medicine
through the product label and on advertising, promotional and distribution activities. For example:
| ● |
in the U.S., TEGSEDI’s label contains a boxed warning for thrombocytopenia and glomerulonephritis;
|
| ● |
TEGSEDI requires periodic blood and urine monitoring; and
|
| ● |
in the U.S., TEGSEDI is available only through a REMS program.
|
Prescription medicines may be promoted only for the approved indications in accordance with the approved label. The FDA
and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
In addition, when approved, the FDA or a foreign regulatory authority may condition approval on the performance of
post-approval clinical studies or patient monitoring, which could be time consuming and expensive. For example, in connection with the conditional marketing approval for WAYLIVRA in the EU, we are required to conduct a post-authorization safety study
to evaluate the safety of WAYLIVRA on thrombocytopenia and bleeding in FCS patients taking WAYLIVRA. If the results of such post-marketing studies are not satisfactory, the FDA, EC or other foreign regulatory authority may withdraw marketing
authorization or may condition continued marketing on commitments from us or our partners that may be expensive and time consuming to fulfill.
If we or others identify side effects after any of our medicines are on the market, or if manufacturing problems occur
subsequent to regulatory approval, or if we, our manufacturers or our partners fail to comply with regulatory requirements, we or our partners may, among other things, lose regulatory approval and be forced to withdraw products from the market, need to
conduct additional clinical studies, incur restrictions on the marketing, distribution or manufacturing of the product, and/or change the labeling of our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA.
We depend on our collaboration with Biogen for the development and commercialization of SPINRAZA.
We have entered into a collaborative arrangement with Biogen to develop and commercialize SPINRAZA. We entered into this
collaboration primarily to:
| ● |
fund our development activities for SPINRAZA;
|
| ● |
seek and obtain regulatory approvals for SPINRAZA; and
|
| ● |
successfully commercialize SPINRAZA.
|
We are relying on Biogen to obtain additional regulatory approvals for SPINRAZA, generate additional clinical data for
SPINRAZA, manufacture and successfully commercialize SPINRAZA. In general, we cannot control the amount and timing of resources that Biogen devotes to our collaboration. If Biogen fails to further develop SPINRAZA, obtain additional regulatory
approvals for SPINRAZA, manufacture or commercialize SPINRAZA, or if Biogen’s efforts are not effective, our business may be negatively affected.
Our collaboration with Biogen may not continue for various reasons. Biogen can terminate our collaboration at any time. If
Biogen stops developing or commercializing SPINRAZA, we would have to seek or spend additional funding, and SPINRAZA’s commercialization may be harmed or delayed.
Our collaboration with Biogen may not result in the continued successful commercialization of SPINRAZA. If Biogen does not
continue to successfully commercialize SPINRAZA, we will receive limited revenues for SPINRAZA.
We depend on our collaboration with AstraZeneca for the joint development and commercialization of eplontersen.
We have entered into a collaborative arrangement with AstraZeneca to develop and commercialize eplontersen. Under the
terms of the collaboration agreement, Ionis and AstraZeneca will co-develop and co-commercialize eplontersen in the U.S. and AstraZeneca will have the sole right to commercialize eplontersen in all other countries. Prior to co-commercializing
eplontersen in the U.S., we will need to negotiate a co-commercialization agreement with AstraZeneca to govern the parties’ performance of co-commercialization, which agreement will include a commercial plan and budget. As a company we do not have
experience with co-commercialization arrangements. We also do not have control over the amount and timing of resources that AstraZeneca devotes to our collaboration, particularly outside of the U.S. If the co-commercialization arrangement for
eplontersen is not successful for any reason, eplontersen may not meet our commercial objectives and our revenues for eplontersen may be limited.
In addition, a Joint Steering Committee, or JSC, having equal membership from us and AstraZeneca, and various
subcommittees oversee and coordinate the development, manufacturing, commercialization and other exploitation activities for eplontersen in the U.S. by mutual agreement. If any subcommittee cannot reach unanimous agreement on any matter within its
respective scope of authority, such matter may be referred to the JSC for resolution. If the JSC cannot come to a mutual agreement on any particular matter, this could delay our ability to develop or commercialize eplontersen.
We are relying on third parties to market, sell and distribute TEGSEDI and WAYLIVRA.
We have entered into agreements with third parties to commercialize TEGSEDI and WAYLIVRA as follows:
| ● |
In April 2021, we entered into a distribution agreement with Sobi to commercialize TEGSEDI in the U.S. and Canada;
|
| ● |
In December 2020, we entered into a distribution agreement with Sobi to commercialize TEGSEDI and WAYLIVRA in Europe; and
|
| ● |
In August 2018, we granted PTC the exclusive right to commercialize TEGSEDI and WAYLIVRA in Latin America and
certain Caribbean countries.
|
We are relying on Sobi and PTC to effectively market, sell and distribute TEGSEDI and WAYLIVRA and have less control over sales efforts
and may receive less revenue than if we commercialized TEGSEDI or WAYLIVRA by ourselves. If Sobi or PTC does not successfully commercialize TEGSEDI or WAYLIVRA, including as a result of delays or disruption caused by the current COVID-19 Pandemic, we
may receive limited revenue for TEGSEDI or WAYLIVRA in the U.S., Canada, Europe, Latin America or certain Caribbean countries, which could have a material adverse effect on our business, prospects, financial condition and results of operations.
Our operations are subject to additional healthcare laws.
Our operations are subject to additional healthcare laws, including federal and state anti-kickback laws, false claims laws, transparency
laws, such as the federal Sunshine Act, and health information privacy and security laws, which are subject to change at any time. For example, in November
2020, the U.S. Department of Health and Human Services issued a final rule modifying the anti-kickback law safe harbors for Medicare Part D plans, pharmacies, and pharmaceutical benefit managers. Efforts to ensure that our operations comply
with current applicable healthcare laws and regulations involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving
applicable fraud and abuse or other healthcare laws and regulations. Penalties for violations of applicable healthcare laws and regulations may include significant civil, criminal and administrative penalties, damages, disgorgement, fines,
imprisonment, exclusion of products from government funded healthcare programs, such as Medicare and Medicaid, and additional reporting requirements and oversight if we enter into a corporate integrity agreement or similar agreement to resolve
allegations of non-compliance with these laws. In addition, violations may also result in reputational harm, diminished profits and future earnings.
If government or other third-party payers fail to provide adequate coverage and payment rates for our medicines, including
SPINRAZA, TEGSEDI and WAYLIVRA, and our medicines in development, our revenue will be limited.
In both domestic and foreign markets, sales of our current and future products will depend in part upon the availability of coverage and
reimbursement from third-party payers. The majority of patients in the U.S. who would fit within our target patient populations for our medicines have their healthcare supported by a combination of Medicare coverage, other government health programs
such as Medicaid, managed care providers, private health insurers and other organizations. Coverage decisions may depend upon clinical and economic standards that disfavor new medicines when more established or lower cost therapeutic alternatives are
already available or subsequently become available. Assuming coverage is approved, the resulting reimbursement payment rates might not be enough to make our medicines affordable. Even if favorable coverage status and adequate reimbursement rates are
attained, less favorable coverage policies and reimbursement rates may be implemented in the future. Accordingly, SPINRAZA, TEGSEDI and WAYLIVRA, and our medicines in development, will face competition from other therapies and medicines for limited
financial resources. We or our partners may need to conduct post-marketing studies to demonstrate the cost-effectiveness of any future products to satisfy third-party payers. These studies might require us to commit a significant amount of management
time and financial and other resources. Third-party payers may never consider our future products as cost-effective. Adequate third-party coverage and reimbursement might not be available to enable us to maintain price levels sufficient to realize an
appropriate return on investment in product development.
Third-party payers, whether foreign or domestic, or governmental or commercial, are developing increasingly sophisticated
methods of controlling healthcare costs. In addition, in the U.S., no uniform policy of coverage and reimbursement for medicines exists among third-party payers. Therefore, coverage and reimbursement for medicines can differ significantly from payer to
payer. For example, the Affordable Care Act was passed in March 2010, and substantially changed the way healthcare is financed by both governmental and private insurers, and continues to significantly impact the U.S. pharmaceutical industry. There have
been judicial and Congressional challenges to certain aspects of the Affordable Care Act, as well as efforts to repeal or replace certain aspects of the Affordable Care Act. It is unclear how future litigation and healthcare reform measures will impact
the Affordable Care Act and our business.
Further, we believe that future coverage,
reimbursement and pricing will likely be subject to increased restrictions both in the U.S. and in international markets. In the U.S., recent health reform measures have resulted in reductions in Medicare and other healthcare funding, and there have
been several recent U.S. Congressional inquiries, legislation and executive orders designed to, among other things, reduce drug prices (e.g., by supporting drug price negotiation in Medicare Parts B and D, with those negotiated prices also available
to commercial plans, and progressing legislation to slow price increases over time on existing drugs), increase competition (e.g., by supporting legislation to speed the entry of biosimilar and generic drugs, including shortening the period of
exclusivity, policies in Medicare Part B to increase the prescribing of biosimilars by physicians, and a prohibition on “pay-for-delay” agreements and anti-competitive practices by drug manufacturers), lower out-of-pocket drug costs for patients
(e.g., by capping Medicare Part D beneficiary out-of-pocket pharmacy expenses), and foster scientific innovation to promote better health care and improved health (e.g., by investing in public and private research and incentivizing the market to promote discovery of valuable and accessible new treatments). At the state level, legislatures have increasingly passed legislation and implemented
regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in
some cases, designed to encourage importation from other countries and bulk purchasing. Third-party coverage and reimbursement for medicines may not be available or adequate in either the U.S. or international markets, and third-party payers, whether
foreign or domestic, or governmental or commercial, may allocate their resources to address the current COVID-19 Pandemic or experience delays or disruptions in their ability to devote resources to coverage and reimbursement matters related to our
products or medicines as a result of the COVID-19 Pandemic, which would negatively affect the potential commercial success of our products, our revenue and our profits.
If we cannot manufacture our medicines or contract with a third party to manufacture our medicines at costs that allow us
to charge competitive prices to buyers, we cannot market our products profitably.
To successfully commercialize any of our medicines, we would need to optimize and manage large-scale commercial
manufacturing capabilities either on a standalone basis or through a third-party manufacturer. We rely on third-party manufacturers to supply the drug substance and drug product for TEGSEDI and drug product for WAYLIVRA. Any delays or disruption to our
own or third-party commercial manufacturing capabilities, including any interruption to our supply chain as a result of the current COVID-19 Pandemic, could limit the commercial success of our medicines. In addition, as our drug development and
commercial pipeline increases and matures, we will have a greater need for clinical trial and commercial manufacturing capacity. For example, we have plans to expand our manufacturing infrastructure to support our wholly owned pipeline. If we are not
successful in executing this expansion, it could limit our ability to meet our manufacturing requirements and commercial objectives in the future.
Additionally, we have limited experience manufacturing pharmaceutical products of the chemical class represented by our
medicines, called oligonucleotides, on a commercial scale for the systemic administration of a medicine. There are a small number of suppliers for certain capital equipment and raw materials that we use to manufacture our medicines, and some of these
suppliers will need to increase their scale of production to meet our projected needs for commercial manufacturing. Further, we must continue to improve our manufacturing processes to allow us to reduce our drug costs. We or our partners may not be
able to manufacture our medicines at a cost or in quantities necessary to make commercially successful products.
Also, manufacturers, including us, must adhere to the FDA’s cGMP regulations and similar regulations in foreign countries,
which the applicable regulatory authorities enforce through facilities inspection programs. We, our partners and our contract manufacturers may not comply or maintain compliance with cGMP, or similar foreign regulations. Non-compliance could
significantly delay or prevent receipt of marketing authorizations for our medicines, including authorizations for SPINRAZA, TEGSEDI and WAYLIVRA, and our medicines in development, or result in enforcement action after authorization that could limit
the commercial success of our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA, and our medicines in development.
Risks Related to the Development and Regulatory Approval of our Medicines
If we or our partners fail to obtain regulatory approval for our medicines and additional approvals for SPINRAZA, TEGSEDI
and WAYLIVRA, we or our partners cannot sell them in the applicable markets.
We cannot guarantee that any of our medicines will be considered safe and effective or will be approved for
commercialization. In addition, it is possible that SPINRAZA, TEGSEDI and WAYLIVRA may not be approved in additional markets or for additional indications. We and our partners must conduct time-consuming, extensive and costly clinical studies to
demonstrate the safety and efficacy of each of our medicines before they can be approved or receive additional approvals for sale. We and our partners must conduct these studies in compliance with FDA regulations and with comparable regulations in
other countries.
We and our partners may not obtain necessary regulatory approvals on a timely basis, if at all, for our medicines. It is
possible that regulatory agencies will not approve our medicines for marketing or SPINRAZA, TEGSEDI or WAYLIVRA in additional markets or for additional indications. If the FDA or another regulatory agency believes that we or our partners have not
sufficiently demonstrated the safety or efficacy of any of our medicines, including SPINRAZA, TEGSEDI and WAYLIVRA, or our medicines in development, the agency will not approve the specific medicine or will require additional studies, which can be time
consuming and expensive and will delay or harm commercialization of the medicine. For example, in August 2018 we received a complete response letter from the FDA regarding the new drug application for WAYLIVRA in which the FDA determined that the
safety concerns identified with WAYLIVRA in our clinical development program outweighed the expected benefits of triglyceride lowering in patients with FCS. We also received a Non-W from Health Canada for WAYLIVRA in November 2018.
The FDA or other comparable foreign regulatory authorities can delay, limit or deny approval of a medicine for many
reasons, including:
| ● |
such authorities may disagree with the design or implementation of our clinical studies;
|
| ● |
we or our partners may be unable to demonstrate to the satisfaction of the FDA or other regulatory authorities that a medicine is safe and effective for
any indication;
|
| ● |
such authorities may not accept clinical data from studies conducted at clinical facilities that have deficient clinical practices or that are in
countries where the standard of care is potentially different from that in the U.S.;
|
| ● |
we or our partners may be unable to demonstrate that our medicine’s clinical and other benefits outweigh its safety risks to support approval;
|
| ● |
such authorities may disagree with the interpretation of data from preclinical or clinical studies;
|
| ● |
such authorities may find deficiencies in the manufacturing processes or facilities of third-party manufacturers who manufacture clinical and commercial
supplies for our medicines, or may delay the inspection of such facilities due to restrictions related to the COVID-19 Pandemic; and
|
| ● |
the approval policies or regulations of such authorities or their prior guidance to us or our partners during clinical development may significantly
change in a manner rendering our clinical data insufficient for approval.
|
Failure to receive marketing authorization for our medicines, or failure to receive additional marketing authorizations
for SPINRAZA, TEGSEDI or WAYLIVRA, or delays in these authorizations, could prevent or delay commercial introduction of the medicine, and, as a result, could negatively impact our ability to generate revenue from product sales.
We may not be able to benefit from orphan drug designation for our medicines.
In the U.S., under the Orphan Drug Act, the FDA may designate a medicine as an orphan drug if it is intended to treat a
rare disease or condition affecting fewer than 200,000 individuals in the U.S. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process, but it can provide financial incentives,
such as tax advantages and user-fee waivers, as well as longer regulatory exclusivity periods. The FDA has granted orphan drug designation to eplontersen for the treatment of patients with transthyretin-mediated amyloidosis. The FDA and EMA have
granted orphan drug designation to TEGSEDI for the treatment of patients with polyneuropathy due to hATTR amyloidosis, to WAYLIVRA for the treatment of patients with FCS, and to tominersen for the treatment of patients with HD. In addition, the EMA has
granted orphan drug designation to WAYLIVRA for the treatment of patients with FPL. Even if approval is obtained on a medicine that has been designated as an orphan drug, we may lose orphan drug exclusivity if the FDA or EMA determines that the request
for designation was materially defective or if we cannot assure sufficient quantity of the applicable medicine to meet the needs of patients with the rare disease or condition, or if a competitor is able to gain approval for the same medicine in a
safer or more effective form or that makes a major contribution to patient care. If we lose orphan drug exclusivity on any of our medicines, we may face increased
competition and lose market share for such medicine.
If the results of clinical testing indicate that any of our medicines are not suitable for commercial use, we may need to
abandon one or more of our drug development programs.
Drug discovery and development has inherent risks and the historical failure rate for drugs is high. Antisense medicines
are a relatively new approach to therapeutics. If we cannot demonstrate that our medicines are safe and effective for human use in the intended indication, we may need to abandon one or more of our drug development programs.
Even if our medicines are successful in preclinical and human clinical studies, the medicines may not be successful in
late-stage clinical studies.
Successful results in preclinical or initial
human clinical studies, including the Phase 2 results for some of our medicines in development, may not predict the results of subsequent clinical studies. If any of our medicines in Phase 3 clinical studies, including the studies of eplontersen, olezarsen, donidalorsen, ION363, pelacarsen and tofersen, do not show sufficient efficacy in patients with the targeted indication, or if such studies are
discontinued for any other reason, it could negatively impact our development and commercialization goals for these medicines and our stock price could decline.
In the past, we have invested in clinical
studies of medicines that have not met the primary clinical endpoints in their Phase 3 studies or have been discontinued for other reasons. For example, in October 2021, Biogen reported that tofersen did not meet the primary clinical endpoint in the Phase 3 VALOR study; however, trends favoring tofersen were seen across multiple secondary and exploratory
measures of disease activity and clinical function. In addition, in March 2021, Roche decided to discontinue dosing in the Phase 3 GENERATION HD1 study of tominersen in patients with manifest Huntington’s disease based on the results of a pre-planned
review of data from the Phase 3 study conducted by an unblinded Independent Data Monitoring Committee. Similar results could occur in clinical studies for our other medicines, including the studies of eplontersen, olezarsen, donidalorsen, ION363 and pelacarsen.
There are a number of factors that could cause a clinical study to fail or be delayed, including:
| ● |
the clinical study may produce negative or inconclusive results;
|
| ● |
regulators may require that we hold, suspend or terminate clinical research for noncompliance with regulatory requirements;
|
| ● |
we, our partners, the FDA or foreign regulatory authorities could suspend or terminate a clinical study due to adverse side effects of a medicine on
subjects or lack of efficacy in the trial;
|
| ● |
we, or our partners, may decide, or regulators may require us, to conduct additional preclinical testing or clinical studies;
|
| ● |
enrollment in our clinical studies may be slower than we anticipate;
|
| ● |
we or our partners, including our independent clinical investigators, contract research organizations and other third-party service providers on which we
rely, may not identify, recruit and train suitable clinical investigators at a sufficient number of study sites or timely enroll a sufficient number of study subjects in the clinical study;
|
| ● |
the institutional review board for a prospective site might withhold or delay its approval for the study;
|
| ● |
people who enroll in the clinical study may later drop out due to adverse events, a perception they are not benefiting from participating in the study, fatigue with the
clinical study process or personal issues;
|
| ● |
a clinical study site may deviate from the protocol for the study;
|
| ● |
the cost of our clinical studies may be greater than we anticipate;
|
| ● |
our partners may decide not to exercise any existing options to license and conduct additional clinical studies for our medicines; and
|
| ● |
the supply or quality of our medicines or other materials necessary to conduct our clinical studies may be insufficient, inadequate or delayed.
|
The current COVID-19 Pandemic could make some of these factors more likely to occur.
In addition, our current medicines, including SPINRAZA, TEGSEDI and WAYLIVRA, are chemically similar to each other. As a
result, a safety observation we encounter with one of our medicines could have, or be perceived by a regulatory authority to have, an impact on a different medicine we are developing. This could cause the FDA or other regulators to ask questions or
take actions that could harm or delay our ability to develop and commercialize our medicines or increase our costs. For example, the FDA or other regulatory agencies could request, among other things, any of the following regarding one of our
medicines: additional information or commitments before we can start or continue a clinical study, protocol amendments, increased safety monitoring, additional product labeling information, and post-approval commitments. This happened in connection
with the conditional marketing approval for WAYLIVRA in the EU, as the EC is requiring us to conduct a post-authorization safety study to evaluate the safety of WAYLIVRA on thrombocytopenia and bleeding in FCS patients taking WAYLIVRA. We have ongoing
post-marketing studies for WAYLIVRA and TEGSEDI and an EAP for WAYLIVRA. Adverse events or results from these studies or the EAPs could negatively impact our pending or future marketing approval applications for WAYLIVRA and TEGSEDI in patients with
FCS or hATTR amyloidosis or the commercial opportunity for WAYLIVRA or TEGSEDI.
Any failure or delay in our clinical studies,
including the studies of tofersen, pelacarsen, eplontersen, olezarsen, donidalorsen, and ION363, could reduce the commercial potential or viability
of our medicines.
We depend on third parties to conduct our clinical studies for our medicines and any failure of those parties to fulfill
their obligations could adversely affect our development and commercialization plans.
We depend on independent clinical
investigators, contract research organizations and other third-party service providers to conduct our clinical studies for our medicines and expect to continue to do so in the future. For example, we use clinical research organizations, such as Icon
Clinical Research Limited, Syneos Health, Inc., PPD and Medpace for the clinical studies for our medicines, including eplontersen, olezarsen,
donidalorsen, ION363, pelacarsen and tofersen. We rely heavily on these parties for successful execution of our clinical studies, but do not control many aspects of their activities. For example, the investigators are not our employees. However, we
are responsible for ensuring that these third parties conduct each of our clinical studies in accordance with the general investigational plan and approved protocols for the study. Third parties may not complete activities on schedule or may not
conduct our clinical studies in accordance with regulatory requirements or our stated protocols. The failure of these third parties to carry out their obligations, including as a result of delays or disruption caused by the current COVID-19 Pandemic
that may affect the third party’s ability to conduct the clinical studies for our medicines, or a termination of our relationship with these third parties, could delay or prevent the development, marketing authorization and commercialization of our
medicines or additional marketing authorizations for TEGSEDI and WAYLIVRA.
Since corporate partnering is a significant part of our strategy to fund the advancement and commercialization of our
development programs, if any of our collaborative partners fail to fund our collaborative programs, or if we cannot obtain additional partners, we may have to delay or stop progress on our drug development programs.
To date, corporate partnering has played a significant role in our strategy to fund our development programs and to add
key development resources. We plan to continue to rely on additional collaborative arrangements to develop and commercialize many of our unpartnered medicines. However, we may not be able to negotiate favorable collaborative arrangements for these drug
programs. If we cannot continue to secure additional collaborative partners, our revenues could decrease and the development of our medicines could suffer.
Our corporate partners are developing and/or funding many of the medicines in our development pipeline. For example, we
are relying on:
| ● |
AstraZeneca for the joint development and funding of eplontersen;
|
| ● |
Novartis for development and funding of pelacarsen;
|
| ● |
Biogen for development and funding of tofersen; and
|
| ● |
Roche for development and funding of tominersen.
|
If any of these pharmaceutical companies stops developing and/or funding these medicines, our business could suffer and we
may not have, or be willing to dedicate, the resources available to develop these medicines on our own. Our collaborators can terminate their relationships with us under certain circumstances, many of which are outside of our control. For example,
after a review of data from the global Phase 2b study of vupanorsen, Pfizer decided to discontinue the clinical development program for vupanorsen.
Even with funding from corporate partners, if our partners do not effectively perform their obligations under our
agreements with them, it would delay or stop the progress of our drug development and commercial programs.
In addition to receiving funding, we enter into collaborative arrangements with third parties to:
| ● |
conduct clinical studies;
|
| ● |
seek and obtain marketing authorizations; and
|
| ● |
manufacture, market and sell our medicines.
|
Once we have secured a collaborative arrangement to further develop and commercialize one of our drug development
programs, such as our collaborations with AstraZeneca, Bayer, Biogen, GSK, Janssen, Novartis, and Roche, these collaborations may not continue or result in commercialized medicines, or may not progress as quickly as we first anticipated.
For example, a collaborator such as AstraZeneca, Bayer, Biogen, GSK, Janssen, Novartis, or Roche, could determine that it
is in its financial interest to:
| ● |
pursue alternative technologies or develop alternative products that may be competitive with the medicine that is part of the collaboration with us;
|
| ● |
pursue higher-priority programs or change the focus of its own development programs; or
|
| ● |
choose to devote fewer resources to our medicines than it does for its own medicines.
|
If any of these occur, it could affect our partner’s commitment to the collaboration with us and could delay or otherwise
negatively affect the commercialization of our medicines, including SPINRAZA, pelacarsen, tofersen, and eplontersen.
If we do not progress in our programs as anticipated, the price of our securities could decrease.
For planning purposes, we estimate and may
disclose the timing of a variety of clinical, regulatory and other milestones, such as when we anticipate a certain medicine will enter clinical trials, when we anticipate completing a clinical study, or when we anticipate filing an application for,
or obtaining, marketing authorization, or when we or our partners plan to commercially launch a medicine. We base our estimates on present facts and a variety of assumptions, many of which are outside of our control, including the current COVID-19
Pandemic. If we do not achieve milestones in accordance with our or our investors’ or securities analysts’ expectations, including milestones related to SPINRAZA, TEGSEDI, WAYLIVRA, eplontersen, olezarsen, donidalorsen, ION363, pelacarsen and tofersen, the price of our securities could decrease.
Risks Associated with our Businesses as a Whole
Risks related to our financial condition
We have incurred losses, and our business will suffer if we fail to consistently achieve profitability in the future.
Because drug discovery and development requires substantial lead-time and money prior to commercialization, our expenses
have generally exceeded our revenue since we were founded in January 1989. As of December 31, 2021, we had an accumulated deficit of
approximately $1.2 billion and stockholders’ equity of approximately $0.8 billion. Most of our historical losses resulted from costs incurred in connection with our research and development programs and from selling, general and administrative costs associated
with our operations. Most of our income has come from collaborative arrangements, including commercial revenue from royalties and R&D revenue, with additional income from research grants and the sale or licensing of our patents, as well as interest
income. If we do not continue to earn substantial revenue, we may incur additional operating losses in the future. We may not successfully develop any additional medicines or achieve or sustain future profitability.
If we fail to obtain timely funding, we may need to curtail or abandon some of our programs.
Many of our medicines are undergoing clinical studies or are in the early stages of research and development. Most of our
drug programs will require significant additional research, development, manufacturing, preclinical and clinical testing, marketing authorizations, preclinical activities and commitment of significant additional resources prior to their successful
commercialization. These activities will require significant cash. As of December 31, 2021, we had cash, cash equivalents and short-term
investments equal to $2.1 billion. If we or our partners do not meet our goals to successfully commercialize our medicines, including SPINRAZA,
TEGSEDI and WAYLIVRA, or to license certain medicines and proprietary technologies, we will need additional funding in the future. Our future capital requirements will depend on many factors, such as the following:
| ● |
successful commercialization of SPINRAZA, TEGSEDI and WAYLIVRA;
|
| ● |
additional marketing approvals for WAYLIVRA and TEGSEDI;
|
| ● |
the profile and launch timing of our medicines, including eplontersen, olezarsen, donidalorsen, ION363, pelacarsen and tofersen;
|
| ● |
changes in existing collaborative relationships and our ability to establish and maintain additional collaborative arrangements;
|
| ● |
continued scientific progress in our research, drug discovery and development programs;
|
| ● |
the size of our programs and progress with preclinical and clinical studies;
|
| ● |
the time and costs involved in obtaining marketing authorizations;
|
| ● |
competing technological and market developments, including the introduction by others of new therapies that address our markets; and
|
| ● |
our manufacturing requirements and capacity to fulfill such requirements.
|
If we need additional funds, we may need to raise them through public or private financing. Additional financing may not
be available at all or on acceptable terms. If we raise additional funds by issuing equity securities, the shares of existing stockholders will be diluted and the price, as well as the price of our other securities, may decline. If adequate funds are
not available or not available on acceptable terms, we may have to cut back on one or more of our research, drug discovery or development programs. Alternatively, we may obtain funds through arrangements with collaborative partners or others, which
could require us to give up rights to certain of our technologies or medicines.
Risks related to our intellectual property
If we cannot protect our patent rights or our other proprietary rights, others may compete more effectively against us.
Our success depends to a significant degree upon whether we can continue to develop, secure and maintain intellectual
property rights to proprietary products and services. However, we may not receive issued patents on any of our pending patent applications in the U.S. or in other countries and we may not be able to obtain, maintain or enforce our patents and other
intellectual property rights which could impact our ability to compete effectively. In addition, the scope of any of our issued patents may not be sufficiently broad to provide us with a competitive advantage. Furthermore, other parties may
successfully challenge, invalidate or circumvent our issued patents or patents licensed to us so that our patent rights do not create an effective competitive barrier or revenue source.
We cannot be certain that the U.S. Patent and Trademark Office, or U.S. PTO, and courts in the U.S. or the patent offices and courts in
foreign countries will consider the claims in our patents and applications covering SPINRAZA, TEGSEDI, WAYLIVRA, or any of our medicines in development as patentable. Method-of-use patents protect the use of a product for the specified method. This
type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. Moreover, even if competitors do not actively promote their product
for our targeted indications, physicians may prescribe these products off-label. Although off-label prescriptions may infringe or contribute to the infringement of method-of-use patents, the practice is common and such infringement is difficult to
prevent, even through legal action.
If we or any licensor partner loses or cannot obtain patent protection for SPINRAZA, TEGSEDI, WAYLIVRA, or any of our other medicines in
development, it could have a material adverse impact on our business.
Intellectual property litigation could be expensive and prevent us from pursuing our programs.
From time to time we have to defend our intellectual property rights. If we are involved in an intellectual property
dispute, we may need to litigate to defend our rights or assert them against others. Disputes can involve arbitration, litigation or proceedings declared by the U.S. PTO or the International Trade Commission or foreign patent authorities. Even if
resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition,
there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price
of our common stock.
If a third party claims that our medicines or technology infringe its patents or other intellectual property rights, we
may have to discontinue an important product or product line, alter our products and processes, pay license fees or cease certain activities. We may not be able to obtain a license to needed intellectual property on favorable terms, if at all. There
are many patents issued or applied for in the biotechnology industry, and we may not be aware of patents or patent applications held by others that relate to our business. This is especially true since patent applications in the U.S. are filed
confidentially for the first 18 months. Moreover, the validity and breadth of biotechnology patents involve complex legal and factual questions for which important legal issues remain.
Risks related to our personnel
If our management transition is not successful our business could suffer.
In January 2020, Dr. Crooke, our founder and Chief Executive Officer, transitioned from Chief Executive Officer to Executive Chairman of
our Board of Directors, and Dr. Monia, who was our Chief Operating Officer and a member of our team since our founding over 30 years ago, began serving as our Chief Executive Officer. Following the 2021 Annual Meeting of Stockholders, Dr. Crooke
stepped down from the Board and now serves as a Strategic Advisor to the Company, providing strategic advice and continuing to participate in the Company’s scientific activities. In June 2021, Dr. Loscalzo, a member of our Board since February 2014, was appointed Chairman of the Board. If this transition is not successful, our business could suffer.
The loss of key personnel, or the inability to attract and retain highly skilled personnel, could make it more difficult
to run our business and reduce our likelihood of success.
We are dependent on the principal members of
our management and scientific staff. We do not have employment agreements with any of our executive officers that would prevent them from leaving us. The loss of our management and key scientific employees might slow the achievement of important
research and development goals. It is also critical to our success that we recruit and retain qualified scientific personnel to perform research and development work. We may not be able to attract and retain skilled and experienced scientific
personnel on acceptable terms because of intense competition for experienced scientists among many pharmaceutical and health care companies, universities and non-profit research institutions. In addition, failure to succeed in clinical studies may
make it more challenging to recruit and retain qualified scientific personnel.
Risks related to taxes
Our ability to use our net operating loss carryovers and certain other tax attributes may be limited.
Under the Internal Revenue Code of 1986, as amended, or the Code, a corporation is generally allowed a deduction for net operating losses,
or NOLs, carried over from a prior taxable year. Under the Code, we can carryforward our NOLs to offset our future taxable income, if any, until such NOLs are used or expire. The same is true of other unused tax attributes, such as tax credits.
Under the current U.S. federal income tax law, U.S. federal NOLs generated in taxable years beginning after December 31, 2017 may be
carried forward indefinitely, but the deductibility of such U.S. federal NOLs in taxable years beginning after December 31, 2020 is limited to 80 percent of taxable income. It is uncertain if and to what extent various states will conform to current
U.S. federal income tax law, and there may be periods during which states suspend or otherwise limit the use of NOLs for state income tax purposes.
In addition, under Sections 382 and 383 of the Code, and corresponding provisions of state law, if a corporation undergoes an “ownership
change,” which is generally defined as a greater than 50 percentage-point cumulative change, by value, in its equity ownership over a three-year period, the corporation’s ability to use its pre-change NOL carryforwards and other pre-change tax
attributes to offset its post-change income or taxes may be limited. We may experience ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. If an ownership change occurs
and our ability to use our NOL carryforwards or other tax attributes is materially limited, it would harm our future operating results by effectively increasing our future tax obligations. As a result of the Akcea Merger, we are subject to the separate
return limitation year, or SRLY, rules. Under the SRLY rules, our utilization of Akcea’s pre-merger NOL and tax credit carryforwards is limited to the amount of income that Akcea contributes to our consolidated taxable income. The Akcea pre-merger tax
attributes cannot be used to offset any of the income that Ionis contributes to our consolidated taxable income. In addition, at the state level, there may be periods during which the use of net operating losses is suspended or otherwise limited, which
could accelerate or permanently increase state taxes owed.
Our future taxable income could be impacted by changes in tax laws, regulations and treaties.
A change in tax laws, treaties or regulations, or their interpretation, of any country in which we operate could
materially affect us.
We could be subject to additional tax liabilities.
We are subject to U.S. federal, state, local and foreign income taxes, sales taxes in the U.S., withholding taxes and transaction taxes in
foreign jurisdictions. Significant judgment is required in evaluating our tax positions and our worldwide provision for taxes. During the ordinary course of business, there are many activities and transactions for which the ultimate tax determination
is uncertain. In addition, our tax obligations and effective tax rates could be adversely affected by changes in the relevant tax, accounting and other laws, regulations, principles and interpretations, including those relating to income tax nexus, by
recognizing tax losses or lower than anticipated earnings in jurisdictions where we have lower statutory rates and higher than anticipated earnings in jurisdictions where we have higher statutory rates, by changes in foreign currency exchange rates, or
by changes in the valuation of our deferred tax assets and liabilities. We may be audited in various jurisdictions, and such jurisdictions may assess additional taxes, sales taxes and value-added taxes against us. Although we believe our tax estimates
are reasonable, the final determination of any tax audits or litigation could be materially different from our historical tax provisions and accruals, which could have a material adverse effect on our operating results or cash flows in the period for
which a determination is made.
General risk factors
If the price of our securities continues to be highly volatile, this could make it harder to liquidate your investment and
could increase your risk of suffering a loss.
The market price of our common stock, like that of the securities of many other biopharmaceutical companies, has been and
is likely to continue to be highly volatile. These fluctuations in our common stock price may significantly affect the trading price of our securities. During the 12 months preceding December 31, 2021, the market price of our common stock ranged from $64.37 to $25.04 per share. Many factors can affect the market price of our securities, including, for example, fluctuations in our operating results, announcements
of collaborations, clinical study results, technological innovations or new products being developed by us or our competitors, the commercial success of our approved medicines, governmental regulation, marketing authorizations, changes in payers’
reimbursement policies, developments in patent or other proprietary rights and public concern regarding the safety of our medicines.
The current COVID-19 Pandemic has caused a significant
disruption of global financial markets and has resulted in increased volatility in the trading price of our common stock. Additionally, broad market and
industry factors may materially harm the market price of our common stock irrespective of our operating performance. The stock market in general, and NASDAQ and the market for biotechnology companies in particular, have experienced extreme price and
volume fluctuations that have often been unrelated or disproportionate to the operating performance of the particular companies affected. The trading prices and valuations of these stocks, and of ours, may not be predictable. A loss of investor
confidence in the market for biotechnology or pharmaceutical stocks or the stocks of other companies which investors perceive to be similar to us, the opportunities in the biotechnology and pharmaceutical market or the stock market in general, could
depress our stock price regardless of our business, prospects, financial conditions or results of operations.
Provisions in our certificate of incorporation, convertible notes documents, call spread hedge transaction documents and
Delaware law may prevent stockholders from receiving a premium for their shares.
Our certificate of incorporation provides for classified terms for the members of our board of directors. Our certificate
also includes a provision that requires at least 66 2/3 percent of our voting stockholders to approve a merger or certain other business transactions with, or proposed by, any holder of 15 percent or more of our voting stock, except in cases where
certain directors approve the transaction or certain minimum price criteria and other procedural requirements are met.
Our certificate of incorporation also requires that any action required or permitted to be taken by our stockholders must
be taken at a duly called annual or special meeting of stockholders and may not be taken by written consent. In addition, only our board of directors, chairman of the board or chief executive officer can call special meetings of our stockholders. We
have in the past, and may in the future, implement a stockholders’ rights plan, also called a poison pill, which could make it uneconomical for a third party to acquire our company on a hostile basis. In addition, our board of directors has the
authority to fix the rights and preferences of, and issue shares of preferred stock, which may have the effect of delaying or preventing a change in control of our company without action by our stockholders.
The provisions of our convertible senior notes could make it more difficult or more expensive for a third party to acquire
us. Upon the occurrence of certain transactions constituting a fundamental change, holders of the notes will have the right, at their option, to require us to repurchase all of their notes or a portion of their notes, which may discourage certain types
of transactions in which our stockholders might otherwise receive a premium for their shares over the then current market prices.
In April 2021, we completed a $632.5 million
offering of 0% Notes and used a portion of the net proceeds from the issuance of the 0% Notes to repurchase $247.9 million of our 1% Notes for $257.0 million. In
December 2019, we entered into privately negotiated exchange and/or subscription agreements with certain new investors and certain holders of our existing 1% Notes to exchange $375.6 million of our 1% Notes for $439.3 million of our 0.125% Notes, and
to issue $109.5 million of our 0.125% Notes. Additionally, in connection with the pricing of our 0% Notes and 0.125% Notes, we entered into call spread transactions in which we purchased note hedges and sold warrants. Terminating or unwinding the call spread transactions could require us to make substantial payments to the counterparties under those agreements or may increase our stock price. The
costs or any increase in stock price that may arise from terminating or unwinding such agreements could make an acquisition of our company significantly more expensive to the purchaser.
These provisions, as well as Delaware law, including Section 203 of the Delaware General Corporation Law, and other of our
agreements, may discourage certain types of transactions in which our stockholders might otherwise receive a premium for their shares over then current market prices, and may limit the ability of our stockholders to approve transactions that they think
may be in their best interests.
Future sales of our common stock in the public market could adversely affect the trading price of our securities.
Future sales of substantial amounts of our common stock in the public market, or the perception that such sales could
occur, could adversely affect trading prices of our securities. For example, we may issue approximately 17.5 million shares of our common stock upon conversion of our 0% Notes and 0.125% Notes, up to 10.9 million shares in connection with the warrant
transactions we entered into in connection with the issuance of our 0% Notes, and up to 6.6 million shares in connection with the warrant transactions we entered into in connection with the issuance of our 0.125% Notes, in each case subject to
customary anti-dilution adjustments. The addition of any of these shares into the public market may have an adverse effect on the price of our securities.
In addition, pursuant to the call spread transactions we entered into in connection with the pricing of our 0% Notes and
0.125% Notes, the counterparties are likely to modify their hedge positions from time to time at or prior to the conversion or maturity of the notes by purchasing and selling shares of our common stock, other of our securities, or other instruments,
including over-the-counter derivative instruments, that they may wish to use in connection with such hedging, which may have a negative effect on the conversion value of those notes and an adverse impact on the trading price of our common stock. The
call spread transactions are expected generally to reduce potential dilution to holders of our common stock upon any conversion of our 0% Notes or 0.125% Notes or offset any cash payments we are required to make in excess of the principal amount of the
converted 0% Notes or 0.125% Notes, as the case may be. However, the warrant transactions could separately have a dilutive effect to the extent that the market value per share of our common stock exceeds the applicable strike price of the warrants.
We are exposed to potential product liability claims, and insurance against these claims may not be available to us at a
reasonable rate in the future or at all.
Our business exposes us to potential product liability risks that are inherent in the testing, manufacturing, marketing
and sale of therapeutic products, including potential product liability claims related to SPINRAZA, TEGSEDI and WAYLIVRA, and our medicines in development. We have clinical study insurance coverage and commercial product liability insurance coverage.
However, this insurance coverage may not be adequate to cover claims against us, or be available to us at an acceptable cost, if at all. Regardless of their merit or eventual outcome, product liability claims may result in decreased demand for our
medicines, injury to our reputation, withdrawal of clinical study volunteers and loss of revenues. Thus, whether or not we are insured, a product liability claim or product recall may result in losses that could be material.
We are dependent on information technology systems, infrastructure and data, which exposes us to data security risks.
We are dependent upon our own and third-party information technology systems, infrastructure and data, including mobile technologies, to
operate our business. The multitude and complexity of our computer systems may make them vulnerable to service interruption or destruction, disruption of data integrity, malicious intrusion, or random attacks. Likewise, data privacy or security
incidents or breaches by employees or others may pose a risk that sensitive data, including our intellectual property, trade secrets or personal information of our employees, patients, customers or other business partners may be exposed to unauthorized
persons or to the public. Cyber-attacks are increasing in their frequency, sophistication and intensity, with third-party phishing and social engineering attacks in particular increasing during the COVID-19 Pandemic. Cyber-attacks could include the
deployment of harmful malware, denial-of-service, social engineering and other means to affect service reliability and threaten data confidentiality, integrity and availability. Our business partners face similar risks and any security breach of their
systems could adversely affect our security posture. A security breach or privacy violation that leads to disclosure or modification of or prevents access to patient information, including personally identifiable information or protected health
information, could harm our reputation, compel us to comply with federal and state breach notification laws and foreign law equivalents, subject us to financial penalties and mandatory and costly corrective action, require us to verify the correctness
of database contents and otherwise subject us to litigation or other liability under laws and regulations that protect personal data, any of which could disrupt our business and result in increased costs or loss of revenue. Moreover, the prevalent use
of mobile devices that access confidential information increases the risk of data security breaches, which could lead to the loss of confidential information, trade secrets or other intellectual property. While we have invested, and continue to invest,
in the protection of our data and information technology infrastructure, our efforts may not prevent service interruptions or identify breaches in our systems that could adversely affect our business and operations and result in the loss of critical or
sensitive information, which could result in financial, legal, business or reputational harm to us. In addition, our liability insurance may not be sufficient in type or amount to cover us against claims related to security breaches, cyber-attacks and
other related breaches.
Because we use biological materials, hazardous materials, chemicals and radioactive compounds, if we do not comply with
laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research, development and manufacturing activities involve the use of potentially harmful biological materials as well
as materials, chemicals and various radioactive compounds that could be hazardous to human health and safety or the environment. We store most of these materials and various wastes resulting from their use at our facilities in Carlsbad, California
pending ultimate use and disposal. We cannot completely eliminate the risk of contamination, which could cause:
| ● |
interruption of our research, development and manufacturing efforts;
|
| ● |
injury to our employees and others;
|
| ● |
environmental damage resulting in costly clean up; and
|
| ● |
liabilities under federal, state and local laws and regulations governing health and human safety, as well as the use, storage, handling and disposal of
these materials and resultant waste products.
|
In such an event, we may be held liable for any resulting damages, and any liability could exceed our resources. Although
we carry insurance in amounts and types that we consider commercially reasonable, we do not have insurance coverage for losses relating to an interruption of our research, development or manufacturing efforts caused by contamination, and the coverage
or coverage limits of our insurance policies may not be adequate. If our losses exceed our insurance coverage, our financial condition would be adversely affected.
Our business may be adversely affected by climate change, extreme weather events, earthquakes, pandemics, civil or
political unrest, terrorism or other catastrophic events.
In recent years, extreme weather events and changing weather patterns have become more common. As a result, we are
potentially exposed to varying natural disaster or extreme weather risks such as hurricanes, tornadoes, fires, droughts, floods, or other events that may result from the impact of climate change on the environment. The potential impacts of climate
change may also include increased operating costs associated with additional regulatory requirements and investments in reducing energy, water use and greenhouse gas emissions. In addition, we manufacture most of our research and clinical supplies in a
manufacturing facility located in Carlsbad, California. We manufacture the finished drug product for TEGSEDI and WAYLIVRA at third-party contract manufacturers. Biogen manufactures the finished drug product for SPINRAZA. The facilities and the
equipment we, our partners and our contract manufacturers use to research, develop and manufacture our medicines would be costly to replace and could require substantial lead time to repair or replace. Our facilities or those of our partners or
contract manufacturers may be harmed by natural disasters or other events outside our control, such as earthquakes, pandemics, war, civil or political unrest, deliberate acts of sabotage, terrorism or industrial accidents such as fire and explosion,
whether due to human or equipment error, and if such facilities are affected by a disaster or other event, our development and commercialization efforts would be delayed. Although we possess property damage and business interruption insurance coverage,
this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. In addition, our development and commercialization activities could be harmed or delayed by a shutdown
of the U.S. government, including the FDA.
Our business is subject to changing regulations for corporate governance and public disclosure that has increased both our
costs and the risk of noncompliance.
Each year we are required to evaluate our internal control systems in order to allow management to report on and our
Independent Registered Public Accounting Firm to attest to, our internal controls as required by Section 404 of the Sarbanes-Oxley Act. As a result, we continue to incur additional expenses and divert our management’s time to comply with these
regulations. In addition, if we cannot continue to comply with the requirements of Section 404 in a timely manner, we might be subject to sanctions or investigation by regulatory authorities, such as the SEC, the Public Company Accounting Oversight
Board, or PCAOB, or The Nasdaq Global Select Market. Any such action could adversely affect our financial results and the market price of our common stock.
The SEC and other regulators have continued to adopt new rules and regulations and make additional changes to existing
regulations that require our compliance. On July 21, 2010, the Dodd-Frank Wall Street Reform and Protection Act, or the Dodd-Frank Act, was enacted. There are significant corporate governance and executive compensation-related provisions in the
Dodd-Frank Act that require the SEC to adopt, or where the SEC has adopted, additional rules and regulations in these areas such as “say on pay” and proxy access. Stockholder activism, the current political environment and the current high level of
government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business.
Negative conditions in the global credit markets and financial services and other industries may adversely affect our
business.
The global credit markets, the financial services industry, the U.S. capital markets, and the U.S. economy as a whole are
currently experiencing substantial turmoil and uncertainty characterized by unprecedented intervention by the U.S. federal government in response to the COVID-19 Pandemic. In the past, the failure, bankruptcy, or sale of various financial and other
institutions created similar turmoil and uncertainty in such markets and industries. It is possible that a crisis in the global credit markets, the U.S. capital markets, the financial services industry or the U.S. economy may adversely affect our
business, vendors and prospects, as well as our liquidity and financial condition. More specifically, our insurance carriers and insurance policies covering all aspects of our business may become financially unstable or may not be sufficient to cover
any or all of our losses and may not continue to be available to us on acceptable terms, or at all. In addition, due to the rapidly rising inflation rate, we may experience increased costs of goods and services for our business.
A variety of risks associated with operating our business and marketing our medicines internationally could adversely affect our business.
In addition to our U.S. operations, we are commercializing TEGSEDI in the EU, Canada, Latin America and certain Caribbean countries, and WAYLIVRA in the EU, Latin America and certain Caribbean countries. We face risks associated with our international
operations, including possible unfavorable regulatory, pricing and reimbursement, political, tax and labor conditions, which could harm our business. Because we have international operations, we are subject to numerous risks associated with
international business activities, including:
| ● |
compliance with differing or unexpected regulatory requirements for our medicines and foreign employees;
|
| ● |
complexities associated with managing multiple payer reimbursement regimes, government payers or patient self-pay systems;
|
| ● |
difficulties in staffing and managing foreign operations;
|
| ● |
in certain circumstances, increased dependence on the commercialization efforts and regulatory compliance of third-party distributors or strategic
partners;
|
| ● |
foreign government taxes, regulations and permit requirements;
|
| ● |
U.S. and foreign government tariffs, trade restrictions, price and exchange controls and other regulatory requirements;
|
| ● |
anti-corruption laws, including the Foreign Corrupt Practices Act, or the FCPA, and its equivalent in foreign jurisdictions;
|
| ● |
economic weakness, including inflation, natural disasters, war, events of terrorism, political instability or public health issues or pandemics, such as
the current COVID-19 Pandemic, in particular foreign countries or globally;
|
| ● |
fluctuations in currency exchange rates, which could result in increased operating expenses and reduced revenue, and other obligations related to doing
business in another country;
|
| ● |
compliance with tax, employment, privacy, immigration and labor laws, regulations and restrictions for employees living or traveling abroad;
|
| ● |
workforce uncertainty in countries where labor unrest is more common than in the U.S.; and
|
| ● |
changes in diplomatic and trade relationships.
|
The United Kingdom’s exit from the E.U. could increase these risks.
Our business activities outside of the U.S. are subject to the FCPA and similar anti-bribery or anti-corruption laws, regulations or rules
of other countries in which we operate, including the United Kingdom’s Bribery Act 2010. In many other countries, the healthcare providers who prescribe pharmaceuticals are employed by their government, and the purchasers of pharmaceuticals are
government entities; therefore, any dealings with these prescribers and purchasers may be subject to regulation under the FCPA. There is no certainty that all employees and third-party business partners (including our distributors, wholesalers, agents,
contractors and other partners) will comply with anti-bribery laws. In particular, we do not control the actions of manufacturers and other third-party agents, although we may be liable for their actions. Violation of these laws may result in civil or
criminal sanctions, which could include monetary fines, criminal penalties, and disgorgement of past profits, which could have an adverse impact on our business and financial condition.
The impact on us of the vote by the United Kingdom to leave the European Union cannot be predicted.
The withdrawal of the UK from the EU, commonly referred to as “Brexit,” may adversely impact our ability to obtain regulatory approvals of
our medicines in the EU, result in restrictions or imposition of taxes and duties for importing our medicines into the EU, and may require us to incur additional expenses in order to develop, manufacture and commercialize our medicines in the EU.
Following the result of a referendum in 2016, the UK left the EU on January 31, 2020. Pursuant to the formal withdrawal arrangements
agreed between the UK and the EU, the UK was subject to a transition period that ended December 31, 2020, or the Transition Period, during which EU rules continued to apply. A trade and cooperation agreement, or the Trade and Cooperation Agreement,
that outlines the future trading relationship between the UK and the EU was signed in December 2020.
Since a significant proportion of the regulatory framework in the UK applicable to our business and our medicines is derived from EU
directives and regulations, Brexit has had, and may continue to have, a material impact upon the regulatory regime with respect to the development, manufacture, importation, approval and commercialization of our medicines in the UK or the EU. For
example, Great Britain is no longer covered by the centralized procedures for obtaining EU-wide marketing authorization from the EMA, and a separate marketing authorization will be required to market our medicines in Great Britain. It is currently
unclear whether the Medicines & Healthcare products Regulatory Agency in the UK is sufficiently prepared to handle the increased volume of marketing authorization applications that it is likely to receive. Any delay in obtaining, or an inability to
obtain, any marketing approvals, as a result of Brexit or otherwise, would delay or prevent us from commercializing our medicines in the UK or the EU.
While the Trade and Cooperation Agreement provides for the tariff-free trade of medicinal products between the UK and the EU, there may be
additional non-tariff costs to such trade which did not exist prior to the end of the Transition Period. Further, should the UK diverge from the EU from a regulatory perspective in relation to medicinal products, tariffs could be put into place in the
future. We could therefore, both now and in the future, face significant additional expenses (when compared to the position prior to the end of the Transition Period) to operate our business.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
As of February 16, 2022,
the following are the primary facilities in which we operate:
|
Property Description
|
Location
|
Square
Footage
|
Owned
or Leased
|
Initial Lease
Term End Date
|
Lease
Extension Options
|
|||||
|
Laboratory and office space facility
|
Carlsbad, CA
|
176,000
|
Owned
|
|||||||
|
Office and meeting space facility
|
Carlsbad, CA
|
74,000
|
Owned
|
|||||||
|
Manufacturing facility
|
Carlsbad, CA
|
26,800
|
Owned
|
|||||||
|
Manufacturing support facility
|
Carlsbad, CA
|
25,800
|
Leased
|
2026
|
One, five-year option to extend
|
|||||
|
Office and storage space facility
|
Carlsbad, CA
|
18,700
|
Leased
|
2023
|
One, five-year option to extend
|
|||||
|
Office space facility
|
Boston, MA
|
14,300
|
Leased
|
2029
|
One, five-year option to extend
|
|||||
|
Office space facility
|
Carlsbad, CA
|
5,800
|
Leased
|
2023
|
One, five-year option to extend
|
|||||
|
341,400
|
We believe that our current and future facilities will be adequate for the foreseeable future.
Item 3. Legal Proceedings
For details of legal proceedings, see Note 10, Legal Proceedings, in the Notes to the Consolidated Financial Statements.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information and Dividends
Our common stock is traded publicly through The Nasdaq Global Select Market under the symbol “IONS.” As of February 16, 2022, there were approximately 495 stockholders of record of our common stock. Because many of our shares are held by brokers and other
institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
We have never paid dividends and do not anticipate paying any dividends in the foreseeable future.
Performance Graph (1)
Set forth below is a table and chart comparing the total return on an indexed basis of $100 invested on December 31, 2016
in our common stock, the Nasdaq Composite Index (total return) and the Nasdaq Biotechnology Index. The total return assumes reinvestment of dividends.
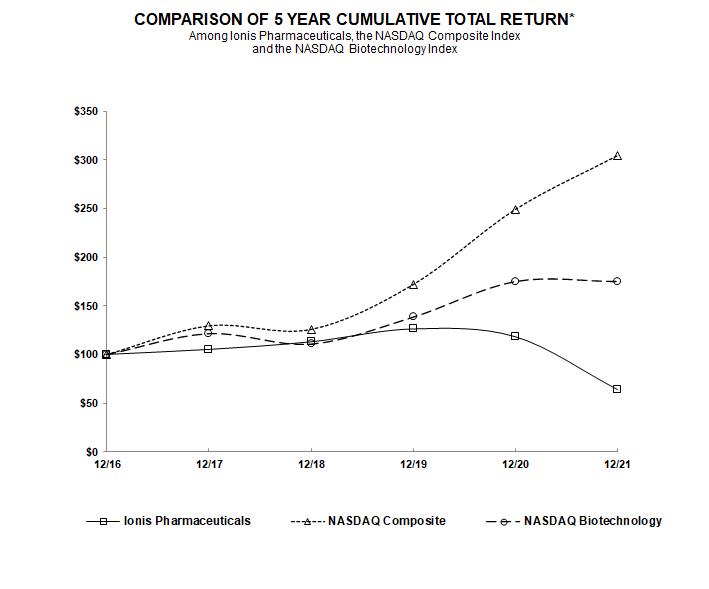
* $100 invested on December 31, 2016 in stock or index, including reinvestment of dividends. Fiscal year ending December 31.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN
Among Ionis Pharmaceuticals, Inc., the Nasdaq Composite Index,
and the Nasdaq Biotechnology Index
|
Dec-16
|
Dec-17
|
Dec-18
|
Dec-19
|
Dec-20
|
Dec-21
|
|||||||||||||||||||
|
Ionis Pharmaceuticals, Inc.
|
$
|
100.00
|
$
|
105.16
|
$
|
113.03
|
$
|
126.30
|
$
|
118.21
|
$
|
63.62
|
||||||||||||
|
Nasdaq Composite Index
|
$
|
100.00
|
$
|
129.64
|
$
|
125.96
|
$
|
172.17
|
$
|
249.51
|
$
|
304.85
|
||||||||||||
|
Nasdaq Biotechnology Index
|
$
|
100.00
|
$
|
121.63
|
$
|
110.85
|
$
|
138.69
|
$
|
175.33
|
$
|
175.37
|
||||||||||||
________________
| (1) |
This section is not “soliciting material,” is not deemed “filed” with the SEC, is not subject to the liabilities of Section 18 of the Exchange Act and is
not to be incorporated by reference in any of our filings under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
|
Item 6. Selected Financial Data
Refer to our financial data contained within Item 7, Management’s Discussion and Analysis, our financial statements and within other parts of this document.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This financial review presents our operating results for each of the two years in the period ended December 31, 2021, and our financial condition at December 31, 2021.
Refer to our 2020 Form 10-K for our results of operations for 2020 compared to 2019. Except for the historical information contained herein, the following discussion contains forward-looking statements that are subject to known and unknown risks,
uncertainties and other factors that may cause our actual results to differ materially from those expressed or implied by such forward-looking statements. We discuss such risks, uncertainties and other factors throughout this report and specifically
under Item 1A of Part I of this report, “Risk Factors.” In addition, the following review should be read in conjunction with the information presented in our consolidated financial statements and the related notes to our consolidated financial
statements as indexed on page F-1.
Overview
As noted in our Business Overview in Part I of this report, we are a leader in RNA-targeted
therapeutics. We believe our medicines, which are based on our novel antisense technology, have the potential to pioneer new markets, change standards of care and transform the lives of people with devastating diseases. We currently have three marketed
medicines- SPINRAZA, TEGSEDI and WAYLIVRA. We also have a rich late-stage pipeline of medicines, primarily focused on our cardiovascular and neurology franchises. Within our late-stage pipeline, we have six medicines in Phase 3 development for eight
indications. For further details on our business refer to the Business section of Part I of this report.
Financial Highlights
The following is a summary of our financial results (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
(as revised*)
|
||||||||
|
Total revenue
|
$
|
810.5
|
$
|
729.3
|
||||
|
Total operating expenses
|
$
|
840.6
|
$
|
901.3
|
||||
|
Loss from operations
|
$
|
(30.2
|
)
|
$
|
(172.1
|
)
|
||
|
Net loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(28.6
|
)
|
$
|
(479.7
|
)
|
||
|
Cash, cash equivalents and short-term investments
|
$
|
2,115.0
|
$
|
1,892.4
|
||||
| * |
We revised our 2020 amounts to reflect the simplified convertible instruments accounting guidance, which we adopted retrospectively. Refer to Note 1, Organization and Significant Accounting Policies, for further information.
|
Our revenue for 2021 increased compared to 2020 due to significant partner payments across our cardiology and neurology
franchises. Our commercial revenue for 2021 included SPINRAZA royalties, TEGSEDI and WAYLIVRA revenue and licensing and other royalty revenue. As a result of our distribution agreements with Sobi for TEGSEDI and WAYLIVRA, our commercial revenue from
product sales shifted to revenue from distribution fees based on net sales generated by Sobi. We completed the transition of our TEGSEDI and WAYLIVRA commercial operations in Europe and our TEGSEDI commercial operations in North America to Sobi in the
first and second quarters of 2021, respectively.
We earn our R&D revenue from multiple sources that can fluctuate depending on the timing of events. Our R&D
revenue increased in 2021 compared to 2020 primarily due to the joint development and commercialization collaboration we entered into with AstraZeneca in 2021.
Our operating expenses, excluding $90 million of expenses related to the Akcea Merger and restructured European operations we incurred in
2020, increased in 2021 compared to 2020 due to an increase in R&D expenses, partially offset by a decrease in SG&A expenses. Higher R&D expenses were primarily driven by our ongoing investments in advancing our Phase 3 programs, expanding
the number of Phase 3 studies and advancing and expanding our mid-stage pipeline. Additionally, we invested in our technology resulting in higher R&D expenses, which was primarily driven by the $35 million we paid in 2021 to license Bicycle’s
technology. As anticipated, our SG&A expenses were lower in 2021 compared to 2020 due to operating efficiencies we achieved from integrating Akcea and restructuring our commercial operations.
At December 31, 2021, we had $2.1 billion in cash and short-term investments, compared with $1.9 billion as of December 31, 2020, enabling us to accelerate investments in our strategic priorities, while maintaining a strong
financial foundation.
Business Segment
In 2021, we began operating as a single segment, Ionis operations, because our chief decision maker reviews operating
results on an aggregate basis and manages our operations as a single operating segment. Previously, we had operated as two operating segments, Ionis Core and Akcea Therapeutics. We completed the Akcea Merger in October 2020 and fully integrated Akcea’s
operations into ours as of January 1, 2021.
Critical Accounting Estimates
We prepare our consolidated financial statements in conformity with accounting principles generally accepted in the U.S.
As such, we make certain estimates, judgments and assumptions that we believe are reasonable, based upon the information available to us. These judgments involve making estimates about the effect of matters that are inherently uncertain and may
significantly impact our quarterly or annual results of operations and financial condition. Each quarter, our senior management reviews the development, selection and disclosure of such estimates with the audit committee of our board of directors. In
the following paragraphs, we describe the specific risks associated with these critical accounting estimates and we caution that future events rarely develop exactly as one may expect, and that best estimates may require adjustment. Our significant
accounting policies are outlined in Note 1, Organization and Significant Accounting Policies, in the Notes to the Consolidated Financial
Statements.
The following are our significant accounting estimates, which we believe are the most critical to aid in fully
understanding and evaluating our reported financial results:
| ● |
Assessing the propriety of revenue recognition and associated deferred revenue; and
|
| ● |
Determining the appropriate cost estimates for unbilled preclinical studies and clinical development activities
|
In 2021, we determined the estimation of our income taxes was no longer a critical accounting estimate because we
recorded a valuation allowance against the entirety of our net deferred tax assets in the fourth quarter of 2020.
The following are descriptions of our critical accounting estimates.
Revenue Recognition
We earn revenue from several sources. The judgements and estimates we make vary between each source of our revenue. At
contract inception, we analyze our collaboration arrangements to assess whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards
dependent on the commercial success of such activities and therefore within the scope of ASC Topic 808, Collaborative Arrangements (ASC 808). For collaboration arrangements within the scope of ASC 808 that contain multiple elements, we first determine
which elements of the collaboration reflect a vendor-customer relationship and therefore within the scope of ASC 606. When we determine elements of a collaboration do not reflect a vendor-customer relationship, we consistently apply the reasonable and
rational policy election we made by analogizing to authoritative accounting literature.
We evaluate the income statement classification for presentation of amounts due from or owed to other participants
associated with multiple activities in a collaboration arrangement based on the nature of each separate activity. For example, in our eplontersen collaboration with AstraZeneca, we recognize funding received from AstraZeneca for co-development
activities as revenue. While, we recognize cost sharing payments to and from AstraZeneca associated with co-commercialization activities and co-medical affairs activities as SG&A expense and research and development expense, respectively
The following is a summary of the critical accounting estimates we make with respect to each of our significant revenue
sources.
Commercial Revenue: SPINRAZA royalties and Licensing and other royalty revenue
We estimate our commercial revenue from SPINRAZA royalties based on reporting we receive from Biogen each quarter. We use
this reporting to calculate our royalty revenue based on our tiered contractual royalty rate for the given period based on annual cumulative net sales. We record our royalty revenue in the same period in which Biogen sells SPINRAZA. We also estimate
commercial revenue from licensing and other royalty revenue.
Commercial Revenue: TEGSEDI and WAYLIVRA revenue, net
We recognize product sales in the period when our customer obtains control of our products. Prior to our distribution
agreements with Sobi, we recorded TEGSEDI and WAYLIVRA commercial revenue at our net sales price, or transaction price, which included estimated reserves for discounts, returns, chargebacks, rebates and other allowances that we offered within contracts
between us and our customers, wholesalers, distributors, health care providers and other indirect customers. Our reserves reflected our best estimates under the terms of our respective contracts. Our historical reserve estimates have not been
materially different from our actual amounts. Under our agreements with Sobi, we transferred all reserves to Sobi and Sobi is responsible for any applicable reserves.
Research and development revenue under collaborative agreements
We recognize R&D revenue from numerous collaboration agreements. Our collaboration agreements typically contain
multiple elements, or performance obligations, including technology licenses or options to obtain technology licenses, R&D services, and manufacturing services. Upon entering into a collaboration agreement, we are required to make the following
judgements:
| ● |
Identifying the performance obligations contained in the agreement
|
Our assessment of what constitutes a separate
performance obligation requires us to apply judgement. Specifically, we have to identify which goods and services we are required to provide under the contract are distinct.
| ● |
Determining the transaction price, including any variable consideration
|
To determine the transaction price, we review the amount of consideration we are eligible to earn under the agreement. We
do not typically include any payments we may receive in the future in our initial transaction price since the payments are typically not probable because they are contingent upon certain future events.
We are required to reassess the total transaction price at each reporting period to determine if we should include
additional payments in the transaction price that have become probable. For example, in the fourth quarter of 2021, we achieved a milestone payment for $7.5 million under our 2018 strategic neurology collaboration with Biogen. Prior to achieving this
milestone payment, we did not consider this payment probable. Upon achieving the milestone payment, we reassessed the total transaction price of our 2018 strategic neurology collaboration. We added this milestone payment to our total transaction price
under our collaboration.
| ● |
Allocating the transaction price to each of our performance obligations
|
When we allocate the transaction price to more than one performance obligation, we make estimates of the relative stand-alone selling
price of each performance obligation because we do not typically sell our goods or services on a stand-alone basis. The estimate of the relative stand-alone selling price requires us in some cases to make significant judgements. For example, when we
deliver a license at the start of an agreement, we use valuation methodologies, such as the relief from royalty method, to value the license. Under this method we are required to make estimates including: future sales, royalties on future product
sales, contractual milestones, expenses, income taxes and discount rates. Additionally, when we estimate the selling price for R&D services, we make estimates, including: the number of internal hours we will spend on the services, the cost of work
we and third parties will perform and the cost of clinical trial material we will use.
The R&D revenue we recognize each period is comprised of several types of revenue, including amortization from upfront
payments, milestone payments, license fees and other services. Each of these types of revenue require us to make various judgements and estimates.
Amortization from Upfront Payments
We recognize revenue from the amortization of upfront payments as we perform R&D services. We
use an input method to estimate the amount of revenue to recognize each period. This method requires us to make estimates of the total costs we expect to incur to complete our R&D services performance obligation or the total length of time it will
take us to complete our R&D services performance obligation. If we change our estimates, we may have to adjust our revenue. Refer to Note 6, Collaborative Arrangements and Licensing Agreements, for further discussion of the cumulative catch up adjustment we made.
Milestone Payments
When recognizing revenue related to milestone payments we typically make the following judgements and estimates:
| ● |
Whether the milestone payment is probable (discussed in detail above under “Determining the transaction price, including any variable consideration”); and
|
| ● |
Whether the milestone payment relates to services we are performing or if our partner is performing the services:
|
| ● |
If we are performing services, we recognize revenue over our estimated period of performance in a similar manner to the amortization of upfront payments (discussed above
under “Amortization of Upfront payments”).
|
| ● |
Conversely, we recognize in full those milestone payments that we earn based on our partners’ activities when our partner achieves the milestone event and we do not have
a performance obligation.
|
License Fees
When we grant a license for a medicine in clinical development, we generally recognize as R&D revenue the total amount
we determine to be the relative stand-alone selling price of a license when we deliver the license to our partner. For example, in 2021, we received a $200 million upfront payment when we entered into an agreement with AstraZeneca to jointly develop
and commercialize eplontersen. Refer to Note 1, Organization and Significant Accounting Policies, for our revenue recognition policy. We discuss
the estimates we make related to the relative stand-alone selling price of a license in detail above under “Allocating the transaction price to each of our performance obligations.”
Estimated Liability for Clinical Development Costs
We have numerous medicines in preclinical studies and/or clinical trials at clinical sites throughout the world. On at
least a quarterly basis, we estimate our liability for preclinical and clinical development costs we have incurred and services that we have received but for which we have not yet been billed and maintain an accrual to cover these costs. These costs
primarily relate to third-party clinical management costs, laboratory and analysis costs, toxicology studies and investigator grants. We estimate our liability using assumptions about study and patient activities and the related expected expenses for
those activities determined based on the contracted fees with our service providers. The assumptions we use represent our best estimates of the activity and expenses at the time of our accrual and involve inherent uncertainties and the application of
our judgment. Upon settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements. Our historical accrual estimates have not been materially different from our actual amounts.
As of December 31, 2021, a hypothetical 10.0 percent increase in our liability for preclinical and clinical development
costs would have resulted in an increase in our loss before income tax benefit and accrued liabilities by approximately $6.6 million.
Results of Operations
Below we have included our results of operations for 2021 compared to 2020. Refer to our 2020 Form 10-K for our results of
operations for 2020 compared to 2019.
Years Ended December 31, 2021 and December 31, 2020
Revenue
Total revenue for 2021
was $810.5 million compared to $729.3
million in 2020 and was comprised of the following (amounts in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Revenue:
|
||||||||
|
Commercial revenue:
|
||||||||
|
SPINRAZA royalties
|
$
|
267.8
|
$
|
286.6
|
||||
|
TEGSEDI and WAYLIVRA revenue, net
|
55.5
|
70.0
|
||||||
|
Licensing and other royalty revenue
|
19.1
|
8.1
|
||||||
|
Total commercial revenue
|
342.4
|
364.7
|
||||||
|
R&D revenue:
|
||||||||
|
Amortization from upfront payments
|
77.5
|
79.6
|
||||||
|
Milestone payments
|
88.3
|
182.6
|
||||||
|
License fees
|
291.3
|
86.0
|
||||||
|
Other services
|
11.0
|
16.4
|
||||||
|
Total R&D revenue
|
468.1
|
364.6
|
||||||
|
Total revenue
|
$
|
810.5
|
$
|
729.3
|
||||
Our revenue for 2021 increased compared to 2020 due to significant partner payments across our cardiology and neurology franchises. Our
commercial revenue for 2021 included SPINRAZA royalties, TEGSEDI and WAYLIVRA revenue and licensing and other royalty revenue. As a result of our distribution agreements with Sobi for TEGSEDI and WAYLIVRA, our commercial revenue from product sales
shifted to revenue from distribution fees based on net sales generated by Sobi. We completed the transition of our TEGSEDI and WAYLIVRA commercial operations in Europe and our TEGSEDI commercial operations in North America to Sobi in the first and
second quarters of 2021, respectively.
We earn our R&D revenue from multiple
sources that can fluctuate depending on the timing of events. Our R&D revenue increased in 2021 compared to 2020 primarily because we earned more revenue from license fees in 2021 than in 2020. Our R&D revenue in 2021 was comprised of $252
million from our cardiovascular franchise, including $200 million from AstraZeneca for its license of eplontersen and a $25 million milestone payment from Novartis when Novartis achieved 50 percent enrollment in the Phase 3 Lp(a) HORIZON study of
pelacarsen. Additionally, our R&D revenue in 2021 included $168 million from our neurology franchise, with $60 million
from Biogen for advancing ION306, our medicine in development for SMA based on new Ionis chemistry, and from advancing several other neurology targets.
Operating Expenses
Operating expenses for 2021
were $840.6 million, and decreased compared to $901.3
million for 2020. The decrease was principally due to $89.6
million of operating expenses related to the Akcea Merger and restructured European operations we incurred in 2020. Excluding expenses related to the Akcea Merger and restructured European operations, our operating expenses for 2021 increased
compared to 2020 due to an increase in R&D expenses, partially offset by a decrease in SG&A expenses. Higher R&D expenses were primarily driven by our investments in advancing our Phase 3 programs. Additionally, we recognized $35 million in
R&D expense in 2021 for licensing Bicycle’s technology. Lower SG&A expenses primarily reflected operating efficiencies achieved from integrating Akcea and restructuring our commercial operations.
Our operating expenses were as follows (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Operating expenses, excluding non-cash compensation expense related to equity awards
|
$
|
696.0
|
$
|
640.9
|
||||
|
Restructuring expenses
|
23.9
|
30.3
|
||||||
|
Total operating expenses, excluding non-cash compensation expense related to equity awards
|
719.9
|
671.2
|
||||||
|
Non-cash compensation expense related to equity awards
|
120.7
|
170.8
|
||||||
|
Restructuring expenses related to acceleration of Akcea’s stock-based compensation expense due to
Akcea Merger
|
—
|
59.3
|
||||||
|
Total operating expenses
|
$
|
840.6
|
$
|
901.3
|
||||
In order to analyze and compare our results of operations to other similar companies, we believe it is important to
exclude non-cash compensation expense related to equity awards from our operating expenses. We believe non-cash compensation expense related to equity awards is not indicative of our operating results or cash flows from our operations. Further, we
internally evaluate the performance of our operations excluding it.
Cost of Sales
Our cost of sales consisted of manufacturing costs, including certain fixed costs, transportation and freight,
indirect overhead costs associated with the manufacturing and distribution of TEGSEDI and WAYLIVRA and certain associated period costs.
Our cost of sales were as follows (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Cost of sales, excluding non-cash compensation expense related to equity awards
|
$
|
10.4
|
$
|
10.0
|
||||
|
Non-cash compensation expense related to equity awards
|
0.4
|
1.9
|
||||||
|
Total cost of sales
|
$
|
10.8
|
$
|
11.9
|
||||
Our cost of sales, excluding non-cash compensation expense related to equity awards, for 2021 were consistent with
2020.
Research, Development and Patent Expenses
Our research, development and patent expenses consist of expenses for antisense drug discovery, antisense drug
development, manufacturing and development chemistry and R&D support expenses.
The following table sets forth information on research, development and patent expenses (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Research, development and patent expenses, excluding non-cash compensation expense related to
equity awards
|
$
|
547.4
|
$
|
411.3
|
||||
|
Restructuring expenses
|
8.5
|
8.2
|
||||||
|
Total research, development and patent expenses, excluding non-cash compensation expense related to
equity awards
|
555.9
|
419.5
|
||||||
|
Non-cash compensation expense related to equity awards
|
87.6
|
115.6
|
||||||
|
Total research, development and patent expenses
|
$
|
643.5
|
$
|
535.1
|
||||
Antisense Drug Discovery
We use our proprietary antisense technology to generate information about the function of genes and to determine the value
of genes as drug discovery targets. We use this information to direct our own antisense drug discovery research, and that of our partners. Antisense drug discovery is also the function that is responsible for advancing our antisense core technology.
This function is also responsible for making investments in complementary technologies to expand the reach of antisense technology.
Our antisense drug discovery expenses were as follows (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Antisense drug discovery expenses, excluding non-cash compensation expense related to equity awards
|
$
|
136.6
|
$
|
89.2
|
||||
|
Non-cash compensation expense related to equity awards
|
21.4
|
24.2
|
||||||
|
Total antisense drug discovery expenses
|
$
|
158.0
|
$
|
113.4
|
||||
Antisense drug discovery expenses, excluding non-cash compensation expense related to equity awards, increased in 2021
compared to 2020 primarily due to $35 million in R&D expense that we recognized in 2021 for licensing Bicycle’s technology as discussed above.
Antisense Drug Development
The following table sets forth drug development expenses, including expenses for our marketed medicines and those in Phase
3 development for which we have incurred significant costs (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
TEGSEDI and WAYLIVRA
|
$
|
11.4
|
$
|
20.3
|
||||
|
Eplontersen
|
79.1
|
34.0
|
||||||
|
Olezarsen
|
22.0
|
5.6
|
||||||
|
Donidalorsen
|
6.7
|
6.4
|
||||||
|
ION363
|
7.7
|
2.6
|
||||||
|
Other antisense development projects
|
104.5
|
69.9
|
||||||
|
Development overhead expenses
|
83.7
|
85.9
|
||||||
|
Restructuring expenses
|
7.7
|
8.0
|
||||||
|
Total antisense drug development, excluding non-cash compensation expense related to equity awards
|
322.8
|
232.7
|
||||||
|
Non-cash compensation expense related to equity awards
|
39.2
|
63.7
|
||||||
|
Total antisense drug development expenses
|
$
|
362.0
|
$
|
296.4
|
||||
Our development expenses, excluding non-cash compensation expense related to equity awards, increased in 2021 compared to
2020 primarily due to our numerous ongoing Phase 3 programs in addition to our advancing and expanding mid-stage pipeline.
We may conduct multiple clinical trials on a drug candidate, including multiple clinical trials for the various
indications we may be studying. Furthermore, as we obtain results from trials, we may elect to discontinue clinical trials for certain drug candidates in certain indications in order to focus our resources on more promising drug candidates or
indications. Our Phase 1 and Phase 2 programs are clinical research programs that fuel our Phase 3 pipeline. When our medicines are in Phase 1 or Phase 2 clinical trials, they are in a dynamic state in which we may adjust the development strategy for
each medicine. Although we may characterize a medicine as “in Phase 1” or “in Phase 2,” it does not mean that we are conducting a single, well-defined study with dedicated resources. Instead, we allocate our internal resources on a shared basis across
numerous medicines based on each medicine’s particular needs at that time. This means we are constantly shifting resources among medicines. Therefore, what we spend on each medicine during a particular period is usually a function of what is required
to keep the medicines progressing in clinical development, not what medicines we think are most important. For example, the number of people required to start a new study is large, the number of people required to keep a study going is modest and the
number of people required to finish a study is large. However, such fluctuations are not indicative of a shift in our emphasis from one medicine to another and cannot be used to accurately predict future costs for each medicine. And, because we always
have numerous medicines in preclinical and early stage clinical research, the fluctuations in expenses from medicine to medicine, in large part, offset one another. If we partner a medicine, it may affect the size of a trial, its timing, its total cost
and the timing of the related costs.
Manufacturing and Development Chemistry
Expenditures in our manufacturing and development chemistry function consist primarily of personnel costs, specialized
chemicals for oligonucleotide manufacturing, laboratory supplies and outside services. Our manufacturing and development chemistry function is responsible for providing drug supplies to antisense drug development and our collaboration partners. Our
manufacturing procedures include testing to satisfy good laboratory and good manufacturing practice requirements.
Our manufacturing and development chemistry expenses were as follows (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Manufacturing and development chemistry expenses, excluding non-cash compensation expense related
to equity awards
|
$
|
47.2
|
$
|
55.7
|
||||
|
Restructuring expenses
|
0.8
|
0.2
|
||||||
|
Total manufacturing and development chemistry expenses, excluding non-cash compensation expense
related to equity awards
|
48.0
|
55.9
|
||||||
|
Non-cash compensation expense related to equity awards
|
11.5
|
10.9
|
||||||
|
Total manufacturing and development chemistry expenses
|
$
|
59.5
|
$
|
66.8
|
||||
Manufacturing and development chemistry expenses, excluding non-cash compensation expense related to equity awards,
decreased in 2021 compared to 2020 due to costs we incurred to manufacture API for olezarsen and eplontersen in 2020.
R&D Support
In our research, development and patent expenses, we include support costs such as rent, repair and maintenance for
buildings and equipment, utilities, depreciation of laboratory equipment and facilities, amortization of our intellectual property, informatics costs, procurement costs and waste disposal costs. We call these costs R&D support expenses.
The following table sets forth information on R&D support expenses (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Personnel costs
|
$
|
17.7
|
$
|
14.7
|
||||
|
Occupancy
|
13.1
|
10.2
|
||||||
|
Patent expenses
|
5.3
|
4.1
|
||||||
|
Insurance
|
3.2
|
2.4
|
||||||
|
Computer software and licenses
|
1.8
|
2.9
|
||||||
|
Other
|
7.3
|
7.4
|
||||||
|
Restructuring expenses
|
0.1
|
—
|
||||||
|
Total R&D support expenses, excluding non-cash compensation expense related to equity awards
|
48.5
|
41.7
|
||||||
|
Non-cash compensation expense related to equity awards
|
15.5
|
16.8
|
||||||
|
Total R&D support expenses
|
$
|
64.0
|
$
|
58.5
|
||||
R&D support expenses, excluding non-cash compensation expense related to equity awards, increased in 2021 compared to
2020. The increase was primarily related to increased personnel and occupancy costs to support advancing our pipeline and our technology.
Selling, General and Administrative Expenses
Selling, general and administrative, or SG&A, expenses include personnel and outside costs associated with the
pre-commercialization and commercialization activities for our medicines and costs to support our company, our employees and our stockholders including, legal, human resources, investor relations, and finance. Additionally, we include in selling,
general and administrative expenses such costs as rent, repair and maintenance of buildings and equipment, depreciation and utilities costs that we need to support the corporate functions listed above. We also include fees we owe under our in-licensing
agreements related to SPINRAZA.
The following table sets forth information on SG&A expenses (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Selling, general and administrative expenses, excluding non-cash compensation expense related to
equity awards
|
$
|
138.1
|
$
|
219.7
|
||||
|
Restructuring expenses
|
15.4
|
22.1
|
||||||
|
Total selling, general and administrative expenses, excluding non-cash compensation related to
equity awards
|
153.5
|
241.8
|
||||||
|
Non-cash compensation expense related to equity awards
|
32.8
|
112.5
|
||||||
|
Total selling, general and administrative expenses
|
$
|
186.3
|
$
|
354.3
|
||||
SG&A expenses, excluding non-cash compensation expense related to equity awards, decreased in 2021 compared to 2020 due to operating
efficiencies achieved from the Akcea Merger and restructuring our commercial operations. Non-cash compensation expense related to equity awards decreased in 2021 compared to 2020 due to reduced headcount as a result of the Akcea Merger and
restructuring our commercial operations. In addition, our SG&A expenses in 2020 included non-cash stock-based compensation expense of $42.0 million related
to the Akcea Merger and restructured European operations.
Investment Income
Investment income for 2021
was $10.0 million compared to $30.6
million for 2020. The decrease in investment income was primarily due to a decrease in interest rates during 2021 compared to 2020.
Interest Expense
The following table sets forth information on interest expense (in millions):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
(as revised*)
|
||||||||
|
Convertible senior notes:
|
||||||||
|
Non-cash amortization of the debt discounts and debt issuance costs
|
$
|
4.9
|
$
|
3.2
|
||||
|
Interest expense payable in cash
|
1.9
|
3.8
|
||||||
|
Interest on mortgage for primary R&D and manufacturing facilities
|
2.4
|
2.4
|
||||||
|
Other
|
0.1
|
0.1
|
||||||
|
Total interest expense
|
$
|
9.3
|
$
|
9.5
|
||||
| * |
We revised our 2020 amounts to reflect the simplified convertible instruments accounting guidance, which we adopted retrospectively. Refer to Note 1, Organization and Significant Accounting Policies, for further information.
|
Gain on Investments
Gain on investments for 2021 was $10.1 million compared to $16.5 million for 2020. During 2021, we revalued our investments in Bicycle and ProQR because we recognize publicly traded equity securities at fair value and recognized gains of $7.1 million and
$1.8 million on our investments, respectively. During 2020, we revalued our investments in three privately held companies, Dynacure, Suzhou-Ribo and Aro Biotherapeutics
because the companies sold additional equity securities that were similar to the equity we own. As a result of these observable price changes in 2020, we recognized a total gain of $14.8 million on our investments in these companies during 2020
because the sales were at higher prices compared to our recorded value.
Early Retirement of Debt
As a result of the debt offering and debt repurchase completed in April 2021, we recorded an $8.6 million loss on early
retirement of debt, reflecting the early retirement of a portion of our 1% Notes. The loss on the early retirement of our debt is the difference between the amount we paid to retire our 1% Notes and the net carrying balance of the liability at the time
that we retired the debt.
Income Tax Expense (Benefit)
We recorded an income tax benefit of $0.6 million for 2021 compared to an income tax expense of $345.2 million for 2020.
Our 2020 income tax expense included a non-cash tax expense of $341 million related to an increase in the valuation allowance recorded against Ionis’ U.S. federal net deferred tax assets in 2020. We now maintain a valuation allowance against all our
consolidated U.S. federal and state net deferred tax assets. Refer to Note 5, Income Taxes, in the Notes to our consolidated financial statements
for further details on our valuation allowance.
Net Loss
We generated a net loss of $28.6
million for 2021 compared to $479.7
million for 2020. Our net loss decreased for 2021 compared to 2020 primarily due to the valuation allowance we recorded in 2020 as a result of
the Akcea Merger, as discussed above in the income tax expense (benefit) section. In addition, our revenue increased and expenses decreased year-over-year, as discussed above in the revenue and expenses sections, respectively.
Net Loss Attributable to Noncontrolling Interest in Akcea Therapeutics, Inc.
Our noncontrolling interest in Akcea on our statement of operations for 2020 was a net loss of $35.5 million. This amount represents the portion of Akcea’s net loss that third parties owned for the period from January 1, 2020 until we acquired 100 percent of Akcea in October 2020.
After we completed the Akcea Merger in October 2020, we no longer recorded any adjustment related to noncontrolling interest for Akcea’s net loss.
Net Loss Attributable to Ionis Pharmaceuticals, Inc. Common Stockholders and Net Loss per Share
We had a net loss attributable to our common stockholders of $28.6 million for 2021 compared to $444.3 million in 2020. Basic and diluted net loss per share for 2021 were each $0.20. Basic and diluted net loss per share for 2020 were each $3.18.
Liquidity and Capital Resources
We have financed our operations primarily
from research and development collaborative agreements. We also finance our operations from commercial revenue from SPINRAZA royalties and TEGSEDI and WAYLIVRA commercial revenue. From our inception through December 31, 2021, we have earned approximately $5.8 billion in revenue. We have also financed our operations
through the sale of our equity securities and the issuance of long-term debt. From the time we were founded through December 31, 2021, we have raised net proceeds of approximately $2.0 billion from the sale of our equity securities. Additionally, we borrowed approximately $2.1 billion under long-term debt arrangements to finance a portion of our operations over the same
time period.
Our cash, cash equivalents and short-term
investments, debt obligations and working capital increased from 2020 to 2021, primarily as a result of receiving more than $760 million in payments from partners in 2021 and issuing $632.5 million of 0% Notes (due in April 2026). This increase was
partially offset by our repurchase of $247.9 million of our 1% Notes in April 2021 and payment of the remaining principal balance of our 1% Notes with $62.0 million of cash at maturity in November 2021. At December 31, 2021, we had $2.1 billion of
cash and short-term investments on hand. We believe our cash and short-term investment balance is sufficient to fund our operations in the short-term and in the longer-term. In 2021 our working capital increased because our cash and
investments increased as discussed above.
The following table summarizes our contractual obligations as of December 31, 2021. The table provides a breakdown of when obligations become due. We provide a more detailed description of the major components of our debt in Note 3, Long-Term Obligations and Commitments.
|
Contractual Obligations
|
Payments Due by Period
(in millions)
|
|||||||||||
|
(selected balances described below)
|
Total
|
Less than 1 year
|
More than 1 year
|
|||||||||
|
0% Notes (principal payable)
|
$
|
632.5
|
$
|
—
|
$
|
632.5
|
||||||
|
0.125% Notes (principal and interest payable)
|
550.9
|
0.7
|
550.2
|
|||||||||
|
Building mortgage payments (principal and interest payable)
|
73.4
|
2.7
|
70.7
|
|||||||||
|
Operating leases
|
27.5
|
4.1
|
23.4
|
|||||||||
|
Other obligations (principal and interest payable)
|
0.8
|
0.1
|
0.7
|
|||||||||
|
Total
|
$
|
1,285.1
|
$
|
7.6
|
$
|
1,277.5
|
||||||
Our contractual obligations consist primarily of our convertible debt. In addition, we also have facility mortgages, facility leases,
equipment financing arrangements and other obligations. Due to the uncertainty with respect to the timing of future cash flows associated with our unrecognized tax benefits, we are unable to make reasonably reliable estimates of the period of cash
settlement with the respective taxing authorities. Therefore, we have excluded our gross unrecognized tax benefits from our contractual obligations table above. We have not entered into, nor do we currently have, any off-balance sheet arrangements (as
defined under SEC rules).
Refer to our Convertible Debt and Call Spread accounting
policies in Note 1, Organization and Significant Accounting Policies, and Note 3, Long-Term Obligations and Commitments,
in the Notes to our consolidated financial statements for the significant terms of each convertible debt instrument.
Research and Development and Manufacturing Facilities
Refer to Note 3, Long-Term Obligations
and Commitments, in the Notes to our consolidated financial statements for further details on our research and development and manufacturing facilities.
Operating Leases
Refer to Note 3, Long-Term Obligations and
Commitments, in the Notes to our consolidated financial statements for further details on our operating leases.
Other Obligations
In addition to contractual obligations, we had outstanding purchase orders as of December 31, 2021 for the purchase of
services, capital equipment and materials as part of our normal course of business.
We may enter into additional collaborations with partners which could provide for additional revenue to us and we may
incur additional cash expenditures related to our obligations under any of the new agreements we may enter into. We currently intend to use our cash, cash equivalents and short-term investments to finance our activities. However, we may also pursue
other financing alternatives, like issuing additional shares of our common stock, issuing debt instruments, refinancing our existing debt, or securing lines of credit. Whether we use our existing capital resources or choose to obtain financing will
depend on various factors, including the future success of our business, the prevailing interest rate environment and the condition of financial markets generally.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to changes in interest rates
primarily from our investments in certain short-term investments. We primarily invest our excess cash in highly liquid short-term investments of the U.S. Treasury and reputable financial institutions, corporations, and U.S. government agencies with
strong credit ratings. We typically hold our investments for the duration of the term of the respective instrument. We do not utilize derivative financial instruments, derivative commodity instruments or other market risk sensitive instruments,
positions or transactions to manage exposure to interest rate changes. Accordingly, we believe that, while the securities we hold are subject to changes in the financial standing of the issuer of such securities, we were not subject to any material
risks arising from changes in interest rates, foreign currency exchange rates, commodity prices, equity prices or other market changes that affect market risk sensitive instruments as of December 31, 2021 and will not be subject to any material risks
arising from these changes in the foreseeable future.
Item 8. Financial Statements and Supplementary Data
We filed our consolidated financial statements and supplementary data required by this item as exhibits hereto, and listed
them under Item 15(a)(1) and (2), and incorporate them herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange
Act of 1934, as amended, or Exchange Act) that are designed to ensure that information we are required to disclose in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and
forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. We designed and evaluate our
disclosure controls and procedures recognizing that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance and not absolute assurance of achieving the desired control objectives.
As of the end of the period covered by this report on Form 10-K, we carried out an evaluation of our disclosure controls
and procedures under the supervision of, and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer. Based on our evaluation, our Chief Executive Officer and Chief Financial Officer concluded that
our disclosure controls and procedures were effective as of December 31, 2021.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as
defined in Exchange Act Rules 13a-15(f). Our internal control over financial reporting is a process designed under the supervision of our Chief Executive Officer and Chief Financial Officer to provide reasonable assurance regarding the reliability of
financial reporting and the preparation of our financial statements for external purposes in accordance with U.S. generally accepted accounting principles.
As of December 31, 2021,
we assessed the effectiveness of our internal control over financial reporting based on the criteria for effective internal control over financial reporting under the 2013 “Internal Control—Integrated Framework,” issued by the Committee of Sponsoring
Organizations, or COSO, of the Treadway Commission, under the supervision of, and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer. Based on that assessment, our management concluded that we
maintained effective internal control over financial reporting as of December 31, 2021.
Ernst & Young LLP, an independent registered public accounting firm, audited the effectiveness of our internal control
over financial reporting as of December 31, 2021, as stated in their attestation report, which is included elsewhere herein.
Changes in Internal Control over Financial Reporting
The above assessment did not identify any change in our internal control over financial reporting that occurred during our
latest fiscal quarter and that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of Ionis Pharmaceuticals, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Ionis Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2021, based on criteria established in Internal
Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Ionis Pharmaceuticals, Inc. (the Company) maintained, in all material respects, effective
internal control over financial reporting as of December 31, 2021, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance
sheets of the Company as of December 31, 2021 and 2020, the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2021, and the
related notes and our report dated February 24, 2022 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of
internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our
audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange
Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable
assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and
evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our
opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the
maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of
financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide
reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation
of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
San Diego, California
February 24, 2022
Item 9B. Other Information
Not applicable.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
We incorporate by reference the information
required by this Item with respect to directors and the Audit Committee from the information under the caption “ELECTION OF DIRECTORS,” including in particular the information under “Nominating, Governance and Review Committee” and “Audit Committee,”
contained in our definitive Proxy Statement, which we will file with the Securities and Exchange Commission within 120 days after the end of the fiscal year ended December 31, 2021, or the Proxy Statement.
We include information concerning our executive officers in the section titled, Information about our Executive Officers, in this report on the Form 10-K in Item 1 titled “Business.”
We incorporate by reference the required information concerning our Code of Ethics from the information under the caption
“Code of Ethics and Business Conduct” contained in the Proxy Statement. Our Code of Ethics and Business Conduct is posted on our website at www.ionispharma.com(1). We intend to disclose future amendments to, or waivers from, our Code of
Ethics and Business Conduct on our website.
________________
|
(1)
|
Any information that is included on or linked to our website is not part of this Form 10-K.
|
Delinquent Section 16(a) Reports
Item 1, Part I of this Report contains information concerning our executive officers. We incorporate by reference the
information required by this Item concerning compliance with Section 16(a) of the Exchange Act from the information under the caption “Delinquent Section 16(a) Reports” contained in the Proxy Statement.
Item 11. Executive Compensation
We incorporate by reference the information required by this item to the information under the caption “EXECUTIVE
COMPENSATION,” “Compensation Committee Interlocks and Insider Participation” and “COMPENSATION COMMITTEE REPORT” contained in the Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
We incorporate by reference the information required by this item to the information under the captions “SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT” contained in the Proxy Statement.
Securities Authorized for Issuance under Equity Compensation Plans
The following table sets forth information regarding outstanding options and shares reserved for future issuance under our
equity compensation plans as of December 31, 2021.
|
Plan Category
|
Number of Shares to
be Issued Upon Exercise
of Outstanding Options
|
Weighted Average
Exercise Price of
Outstanding Options
|
Number of Shares
Remaining Available
for Future Issuance
|
|||||
|
Equity compensation plans approved by stockholders (a)
|
14,088,816
|
$
|
54.04
|
11,102,267
|
(b)
|
|||
|
Total
|
14,088,816
|
$
|
54.04
|
11,102,267
|
||||
________________
| (a) |
Consists of five Ionis plans: 1989 Stock Option Plan, Amended and Restated 2002 Non-Employee Directors’ Stock Option Plan, 2011 Equity Incentive Plan,
2020 Equity Incentive Plan and Employee Stock Purchase Plan, or ESPP.
|
| (b) |
Of these shares, 588,529 were available for purchase under the ESPP as of December 31, 2021.
|
For additional details about our equity compensation plans, including a description of each plan, see Note 4, Stockholders’ Equity, in the Notes to the Consolidated Financial Statements.
Item 13. Certain Relationships and Related Transactions, and Director Independence
We incorporate by reference the information required by this item to the information under the captions “Independence of
the Board of Directors” and “Certain Relationships and Related Transactions” contained in the Proxy Statement.
Item 14. Principal Accountant Fees and Services
We incorporate by reference the information required by this item to the information under the caption “Ratification of
Selection of Independent Auditors” contained in the Proxy Statement.
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a)(1) Index to Financial Statements
We submitted the consolidated financial statements required by this item in a separate section beginning on page F-1 of
this Report.
(a)(2) Index to Financial Statement Schedules
We omitted these schedules because they are not required, or are not applicable, or the required information is shown in
the consolidated financial statements or notes thereto.
(a)(3) Index to Exhibits
INDEX TO EXHIBITS
|
Exhibit Number
|
Description of Document
|
|
|
2.1
|
Agreement and Plan of Merger, dated as of August 30, 2020, among Akcea Therapeutics, Inc., Ionis Pharmaceuticals, Inc. and Avalanche Merger Sub, Inc., filed as an exhibit to the Registrant’s Current Report on Form 8-K filed August 31, 2020 and incorporated herein by reference.
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation filed June 19, 1991, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2017 and incorporated herein by reference.
|
|
|
3.2
|
Certificate of Amendment to Restated Certificate of Incorporation, filed as an exhibit to the Registrant’s Notice of Annual Meeting and Proxy Statement, for the 2014 Annual Meeting of
Stockholders, filed on April 25, 2014 and incorporated herein by reference.
|
|
|
3.3
|
Certificate of Amendment to Restated Certificate of Incorporation, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 18, 2015 and incorporated herein by reference.
|
|
|
3.4
|
Amended and Restated Bylaws, filed as an exhibit
to the Registrant’s Current Report on Form 8-K filed March 29, 2021 and incorporated herein by reference.
|
|
|
4.1
|
Certificate of Designation of the Series C Junior Participating Preferred Stock, filed as an exhibit to Registrant’s Current Report on Form 8-K filed December 13, 2000 and incorporated herein by reference.
|
|
|
4.2
|
Specimen Common Stock Certificate, filed as an exhibit to
the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2017 and incorporated herein by reference.
|
|
|
4.3
|
Indenture, dated as of November 17, 2014, between the Registrant and Wells Fargo Bank, National Association, as trustee, including Form of 1.00 percent
Convertible Senior Note due 2021, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed November 21, 2014 and incorporated
herein by reference.
|
|
|
4.4
|
Indenture, dated as of December 19, 2019, by and between the Registrant and U.S. Bank National Association, as trustee, including Form of 0.125 percent
Convertible Senior Note due 2024, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 23, 2019 and incorporated
herein by reference.
|
|
|
4.5
|
Indenture, dated as of April 12, 2021, by and between the Registrant and U.S. Bank National Association, as trustee, including Form of 0 percent
Convertible Senior Note due 2026, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed April 13, 2021 and incorporated herein
by reference.
|
|
|
4.6
|
Form of Exchange and/or Subscription Agreement for Convertible Senior Notes due 2024, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 12, 2019 and incorporated herein by reference.
|
|
|
4.7
|
Form of Convertible Note Hedge Transactions Confirmation
for Convertible Senior Notes due 2024, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 12, 2019 and
incorporated herein by reference.
|
|
|
4.8
|
Form of Convertible Note Hedge Confirmation for Convertible Senior Notes due 2026, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed April 13, 2021 and incorporated herein by reference.
|
|
|
4.9
|
Form of Warrant Transactions Confirmation for Convertible
Senior Notes due 2024, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 12, 2019 and incorporated
herein by reference.
|
|
|
4.10
|
Form of Warrant Confirmation for Convertible Senior Notes due 2026, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed April 13, 2021 and incorporated herein by reference.
|
|
|
Description of the Registrant’s Securities.
|
||
|
10.1
|
Amended Board Compensation Policy, filed as an exhibit to the
Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2021 and incorporated herein by reference.
|
|
|
10.2
|
Form of Indemnity Agreement entered into between the Registrant and its Directors and Officers with related schedule, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|||
|
10.3*
|
Registrant’s 1989 Stock Option Plan, as amended,
filed as an exhibit to Registrant’s Notice of Annual Meeting and Proxy Statement for the 2012 Annual Meeting of Stockholders, filed on April 16, 2012 and incorporated herein by reference.
|
|||
|
10.4*
|
Registrant’s Amended and Restated 2000 Employee Stock Purchase Plan, filed as an exhibit to Registrant’s Current Report on Form 8-K filed on March 26, 2019 and incorporated herein by reference.
|
|||
|
10.5
|
Form of Employee Confidential Information and Inventions Agreement, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2017 and incorporated herein by reference.
|
|||
|
10.6
|
Amendment #1 to the Research, Development and License Agreement dated May 11, 2011 by and between the Registrant and Glaxo Group Limited, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2011 and incorporated herein by reference. Portions
of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|||
|
10.7
|
Amended and Restated Collaboration and License Agreement between the Registrant and Antisense Therapeutics Ltd dated February 8, 2008, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2008 and incorporated herein by reference. Portions
of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|||
|
10.8
|
Strategic Collaboration, Option and License Agreement by and among Akcea Therapeutics, Inc. and Novartis Pharma AG, dated January 5, 2017,
filed as an exhibit to Akcea Therapeutics, Inc.’s Form S-1 filed March 27, 2017 and incorporated herein by reference.
|
|||
|
10.9
|
Amendment No. 1 to the Strategic Collaboration, Option and License Agreement between Akcea Therapeutics, Inc. and Novartis Pharma AG dated February
22, 2019, filed as an exhibit to Akcea Therapeutics, Inc.’s Quarterly Report on Form 10-Q for the quarter ended March 30, 2019 and incorporated herein by reference.
|
|||
|
10.10
|
Stock Purchase Agreement among the Registrant, Akcea Therapeutics, Inc. and Novartis Pharma AG dated January 5, 2017, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2017 and incorporated herein by reference.
|
|||
|
10.11
|
Amendment #1 between the Registrant and Bayer AG dated February 10, 2017, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2017 and incorporated herein by reference. Portions of this exhibit have been omitted and separately filed with the SEC with a
request for confidential treatment.
|
|||
|
10.12
|
Registrant’s Amended and Restated 10b5-1 Trading Plan dated September 12, 2013, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference.
|
|||
|
10.13*
|
Registrant’s Amended and Restated 2002 Non-Employee Directors’ Stock Option Plan, as amended, filed as an exhibit to the Registrant’s Notice of Annual Meeting and Proxy Statement for the 2020 Annual Meeting of Stockholders, filed on April 24, 2020 and incorporated herein by reference.
|
|||
|
10.14*
|
Form of Restricted Stock Unit Agreement for Restricted Stock Units granted under the Ionis Pharmaceuticals, Inc. Amended and Restated 2002
Non-Employee Directors’ Stock Option Plan, filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed on August 7, 2020 and
incorporated herein by reference.
|
|||
|
10.15
|
Research Collaboration, Option and License Agreement between the Registrant and Biogen MA Inc. dated December 19, 2017, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2017 and incorporated herein by reference. Portions of this exhibit have been
omitted and separately filed with the SEC with a request for confidential treatment.
|
|||
|
10.16*
|
Amended and Restated Ionis Pharmaceuticals, Inc. 2011 Equity Incentive Plan, filed as an exhibit to the Registrant’s Notice of 2021 Annual Meeting of Stockholders and Proxy Statement filed on April 23, 2021 and incorporated herein by reference.
|
|||
|
10.17*
|
Form of Option Agreement under the 2011 Equity Incentive Plan,
filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.18*
|
Form of Time-Vested Restricted Stock Unit Agreement for Restricted Stock Units granted under the 2011 Equity Incentive Plan, filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed on August 8, 2011 and incorporated herein by reference.
|
|
|
Forms of Performance Based Restricted Stock Unit Grant
Notice and Performance Based Restricted Stock Unit Agreement for Performance Based Restricted Stock Units granted under the 2011 Equity Incentive Plan.
|
||
|
10.20*
|
Ionis Pharmaceuticals, Inc. 2020 Equity Incentive Plan, filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed on December 31, 2020 and incorporated herein by reference.
|
|
|
10.21*
|
Form of Global Option Agreement for options granted under the Ionis Pharmaceuticals, Inc. 2020 Equity Incentive Plan, filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed on December 31, 2020 and incorporated herein by reference.
|
|
|
10.22*
|
Form of Global Restricted Stock Unit Agreement for restricted stock units granted under the Ionis Pharmaceuticals, Inc. 2020 Equity
Incentive Plan, filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed on December 31, 2020 and incorporated
herein by reference.
|
|
|
10.23*
|
Forms of Restricted Stock Unit Grant Notice, Stock Option Grant Notice and Stock Option Exercise Notice for options granted under the Ionis
Pharmaceuticals, Inc. 2020 Equity Incentive Plan, filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed on December
31, 2020 and incorporated herein by reference.
|
|
|
10.24
|
Loan Agreement between Ionis Gazelle, LLC and UBS AG dated July 18, 2017, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.25*
|
Form of Option Agreement under the 1989 Stock Option Plan,
filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.26*
|
Form of Option Agreement for Options granted under the 2002 Non-Employee Director’s Stock Option Plan, filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed on August 7, 2020 and incorporated herein by reference.
|
|
|
10.27
|
Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated March 30, 2010, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010 and incorporated herein by reference. Portions of this exhibit have
been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.28
|
Loan Agreement between Ionis Faraday, LLC and UBS AG dated
July 18, 2017, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.29
|
Research Agreement dated August 10, 2011 between the Registrant and CHDI Foundation, Inc, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2011 and incorporated herein by reference. Portions of this exhibit have been omitted and
separately filed with the SEC with a request for confidential treatment.
|
|
|
10.30
|
Guaranty between the Registrant and UBS AG dated July 18,
2017, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.31
|
Development, Option and License Agreement between the Registrant and Biogen Idec International Holding Ltd. dated January 3, 2012, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2012 and incorporated herein by reference. Portions
of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.32
|
DMPK Research, Development, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated June 27, 2012, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2012 and incorporated herein by reference. Portions
of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
10.33
|
Amendment #2 to Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated October 30, 2012, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference. Portions of
this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.34
|
Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated December 7, 2012, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference. Portions of this exhibit have been
omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.35
|
Amended and Restated Neurology Drug Discovery and Development Collaboration, Option and License Agreement by and between the Registrant and Biogen MA Inc.
dated July 12, 2021, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2021 and
incorporated herein by reference. Portions of this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
||
|
10.36
|
HTT Research, Development, Option and License Agreement among the Registrant, F. Hoffmann-La Roche Ltd and Hoffman-La Roche Inc. dated April 8,
2013, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and incorporated herein by
reference. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.37
|
Letter Agreement between the Registrant and CHDI Foundation, Inc. dated April 8, 2013, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and incorporated herein by reference. Portions of this exhibit have been omitted and separately
filed with the SEC with a request for confidential treatment.
|
||
|
10.38
|
Amendment #1 to Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated August 13, 2013, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference.
Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.39
|
Amendment No. 3 to the Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated July 10, 2013, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference. Portions
of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.40
|
Amendment #4 to the Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated April 10, 2014, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference. Portions of this exhibit
have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.41
|
Amendment #5 to the Research, Development and License Agreement among the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property
Development Limited dated June 27, 2014, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30,
2014 and incorporated herein by reference. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.42
|
Exclusive License Agreement between the Registrant and the University of Massachusetts dated January 14, 2010, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference. Portions of this exhibit have been
omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.43
|
Amended and Restated Collaboration and License Agreement between the Registrant and Cold Spring Harbor Laboratory dated October 26, 2011, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference.
Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.44
|
Amendment to Amended and Restated Collaboration and License Agreement between the Registrant and Cold Spring Harbor Laboratory dated March 14, 2014, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference.
Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.45
|
Research Collaboration, Option and License Agreement between the Registrant and Janssen Biotech Inc. dated December 22, 2014, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2020 and incorporated herein by reference. Portions of this exhibit have been omitted because they are both
(i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.46
|
Amendment No.2 to the Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated October 15, 2014, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2014 and incorporated herein by reference. Portions of
this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.47
|
Strategic Collaboration Agreement between the Registrant and AstraZeneca AB dated July 31, 2015, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference. Portions of this exhibit have been omitted and
separately filed with the SEC with a request for confidential treatment.
|
|
|
10.48
|
Amendment #6 to Research, Development and License Agreement between the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property Development
Limited dated September 2, 2015, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015
and incorporated herein by reference. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.49
|
Amendment Number One to the Second Amended and Restated Strategic Collaboration and License Agreement between the Registrant and Alnylam Pharmaceuticals, Inc.
dated July 13, 2015, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and
incorporated herein by reference. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.50
|
License Agreement between the Registrant and Bayer Pharma AG dated May 1, 2015, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2015 and incorporated herein by reference. Portions of this exhibit have been omitted and separately filed with the SEC
with a request for confidential treatment.
|
|
|
10.51
|
Second Amended and Restated Strategic Collaboration and License Agreement between the Registrant and Alnylam Pharmaceuticals, Inc. dated January 8, 2015, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015 and incorporated herein by reference. Portions
of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.52
|
Amendment #1 to HTT Research, Development, Option and License Agreement between the Registrant, F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc. dated
January 9, 2015, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015 and incorporated
herein by reference. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.53
|
Amendment No.3 to the Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated January 18, 2016, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 and incorporated herein by reference. Portions
of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.54
|
Amendment #7 to the Research, Development and License Agreement among the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property
Development Limited dated March 4, 2016, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31,
2016 and incorporated herein by reference. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.55
|
First Amendment to Research Collaboration, Option and License Agreement between the Registrant and Janssen Biotech Inc. dated December 21, 2016, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2020 and incorporated herein by reference. Portions of this exhibit have been
omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.56
|
Letter Agreement between the Registrant and Biogen MA Inc. dated October 28, 2016, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2016 and incorporated herein by reference. Portions of this exhibit have been omitted and separately filed
with the SEC with a request for confidential treatment.
|
|
|
10.57
|
Guaranty between the Registrant and UBS AG dated July 18, 2017,
filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
||
|
10.58
|
Environmental Indemnity Agreement among the Registrant, Ionis Gazelle, LLC and UBS AG dated July 18, 2017, filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
||
|
10.59*
|
Registrant’s Severance Benefit Plan and Summary Plan Description dated October 18, 2018, filed as an exhibit to the Registrant’s Current Report on form 8-K filed October 18, 2018 and incorporated herein by reference.
|
||
|
Fourth Amended and Restated Strategic Advisory Services Agreement by and between the Registrant and B. Lynne Parshall, dated February
22, 2022.
|
|||
|
10.61
|
Development, Commercialization, Collaboration, and License Agreement by and between the Registrant and Akcea Therapeutics, Inc., dated March 14, 2018, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2018 and incorporated herein by reference.
|
||
|
10.62
|
Amended and Restated Services Agreement by and between the Registrant and Akcea Therapeutics, Inc., dated March 14, 2018, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2018 and incorporated herein by reference.
|
||
|
10.63
|
New Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement by and between the Registrant and Biogen MA Inc., dated April 19, 2018, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 and incorporated herein
by reference. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.64
|
Stock Purchase Agreement by and between the Registrant and Biogen MA Inc., dated April 19, 2018, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 and incorporated herein by reference.
|
||
|
10.65
|
Second Amendment to Research, Collaboration, Option and License Agreement by and between the Registrant and Janssen Biotech Inc., dated August 7, 2018, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2018 and incorporated herein by reference.
Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.66
|
Factor B Development Collaboration, Option and License Agreement by and between the Registrant, F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc.,
dated October 9, 2018, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2018 and incorporated
herein by reference. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.67
|
Second Amended and Restated Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement by and between the Registrant
and Biogen MA Inc., dated October 17, 2018, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31,
2018 and incorporated herein by reference. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.68
|
Amendment #1 to the Strategic Collaboration Agreement by and between the Registrant and AstraZeneca AB, dated October 18, 2018, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2018 and incorporated herein by reference. Portions of
this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.69
|
Amendment #4 to the Collaboration, License and Development Agreement by and between the Registrant and AstraZeneca AB, dated October 18, 2018, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2018 and incorporated herein by reference. Portions of
this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.70
|
Amendment #1 to Second Amended and Restated Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement by and between the
Registrant and Biogen MA Inc., dated May 2, 2019, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended
June 30, 2019 and incorporated herein by reference.
|
||
|
10.71
|
Amendment #1 to the New Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement between the Registrant and Biogen MA
Inc., dated August 16, 2019, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2019 and
incorporated herein by reference. Portions of this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.72
|
Amendment #8 to the Research, Development and License Agreement between the Registrant, Glaxo Group Limited and Glaxosmithkline Intellectual Property
Development Limited, dated July 29, 2019, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September
30, 2019 and incorporated herein by reference. Portions of this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.73
|
Consent to Collateral Addition and Amendment to Loan Documents between the Registrant, Ionis Gazelle, LLC, Wells Fargo Bank, National Association, as Trustee
for the Benefit of the Registered Holders of UBS Commercial Mortgage Trust 2017-C3, Commercial Mortgage Pass-Through Certificates, Series 2017-C3, dated August 1, 2019, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2019 and incorporated herein by reference.
|
|
|
10.74
|
License Agreement by and among Akcea Therapeutics, Inc. and Pfizer Inc. dated October 4, 2019, filed as an exhibit to Akcea Therapeutics, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2019 and incorporated herein by reference.
|
|
|
10.75
|
Letter Agreement between the Registrant, Akcea Therapeutics, Inc., and Pfizer Inc., dated October 4, 2019, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2019 and incorporated herein by reference. Portions of this exhibit have been omitted
because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.76
|
Side Letter dated June 11, 2020 to the Second Amended and Restated Strategic Neurology Drug Discovery and Development Collaboration, Option and License
Agreement by and between the Registrant and Biogen MA Inc. dated October 17, 2018, filed as an exhibit to the Registrant’s Quarterly Report on
Form 10-Q for the quarter ended June 30, 2020 and incorporated herein by reference. Portions of this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.77
|
Amendment No. 2 dated April 30, 2020 to the Strategic Collaboration Agreement by and between the Registrant and AstraZeneca AB dated July 31, 2015, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2020 and incorporated herein by reference. Portions
of this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.78
|
Letter agreement dated October 21, 2020 to the License Agreement by and among Akcea Therapeutics, Inc. and Pfizer Inc. dated October 4, 2019, filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2021 and incorporated herein by reference. Portions
of this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.79
|
Amendment No. 3 dated December 17, 2020 to the Strategic Collaboration Agreement by and between the Registrant and AstraZeneca AB dated July 31, 2015, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2020 and incorporated herein by reference. Portions of
this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.80
|
Strategic Advisory Services Agreement by and between the Registrant and Stanley T. Crooke, dated December 17, 2020, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2020 and incorporated herein by reference.
|
|
|
10.81
|
Side Letter dated December 31, 2020 to the New Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement by and
between the Registrant and Biogen MA Inc. dated April 19, 2018, filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year
ended December 31, 2020 and incorporated herein by reference. Portions of this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
|
|
10.82
|
Collaboration and License Agreement by and between the Registrant and BicycleTX Limited dated July 9, 2021, filed as an exhibit to the Registrant’s
Quarterly Report on Form 10-Q for the quarter ended September 30, 2021 and incorporated herein by reference. Portions of this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly
disclosed.
|
|
|
Amendment No. 1 dated December 17, 2021 to the Collaboration and License Agreement by and between the Registrant and BicycleTX
Limited dated July 9, 2021. Portions of this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
||
|
Collaboration and License Agreement by and between Akcea Therapeutics, Inc. and AstraZeneca AB dated December 6, 2021. Portions of
this exhibit have been omitted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
||
|
List of Subsidiaries for the Registrant.
|
||
|
Consent of Independent Registered Public Accounting Firm.
|
||
|
24.1
|
Power of Attorney – Included on the signature page of this Annual Report on Form 10-K.
|
|
|
Certification by Chief Executive Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley
Act of 2002.
|
||
|
Certification by Chief Financial Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley
Act of 2002.
|
||
|
32.1+
|
Certification Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101
|
The following financial statements from the Ionis Pharmaceuticals, Inc. Annual Report on Form 10-K for the year ended December 31, 2021, formatted in Extensive Business Reporting Language (XBRL): (i) consolidated balance sheets, (ii) consolidated statements of operations,
(iii) consolidated statements of comprehensive income (loss), (iv) consolidated statements of stockholders’ equity (v) consolidated statements of cash flows, and (vi) notes to consolidated financial statements (detail tagged).
|
|
|
104
|
Cover Page Interactive Data File (formatted in iXBRL and included in exhibit 101).
|
| * |
Indicates management compensatory plans and arrangements as required to be filed as exhibits to this Report pursuant to Item 14(c).
|
| + |
This certification is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that
section, nor shall it be deemed incorporated by reference into any filing under the Securities Act of 133, as amended, or the Securities Exchange Act of 1934, as amended.
|
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused
this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on the 24th day of February, 2022.
|
IONIS PHARMACEUTICALS, INC.
|
||
|
By:
|
/s/ BRETT P. MONIA
|
|
|
Brett P. Monia, Ph.D.
|
||
|
Chief Executive Officer (Principal executive officer)
|
||
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Brett P. Monia and
Elizabeth L. Hougen, or any of them, his or her attorney-in-fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendments to this Report, and to file the same, with exhibits thereto and other documents in
connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his or her substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following
persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Signatures
|
Title
|
Date
|
||
|
/s/ BRETT P. MONIA
|
Director and Chief Executive Officer
|
February 24, 2022
|
||
|
Brett P. Monia, Ph.D.
|
(Principal executive officer)
|
|||
|
/s/ ELIZABETH L. HOUGEN
|
Executive Vice President, Finance and Chief Financial Officer
|
February 24, 2022
|
||
|
Elizabeth L. Hougen
|
(Principal financial and accounting officer)
|
|||
|
/s/ JOSEPH LOSCALZO
|
Chairman of the Board
|
February 24, 2022
|
||
|
Joseph Loscalzo, M.D., Ph.D.
|
||||
|
/s/ SPENCER R. BERTHELSEN
|
Director
|
February 24, 2022
|
||
|
Spencer R. Berthelsen, M.D.
|
||||
|
/s/ ALLENE M. DIAZ
|
Director
|
February 24, 2022
|
||
|
Allene M. Diaz
|
||||
|
/s/ MICHAEL HAYDEN
|
Director
|
February 24, 2022
|
||
|
Michael Hayden, CM OBC MB ChB PhD FRCP(C) FRSC
|
||||
|
/s/ JOAN E. HERMAN
|
Director
|
February 24, 2022
|
||
|
Joan E. Herman
|
||||
|
/s/ JOSEPH KLEIN
|
Director
|
February 24, 2022
|
||
|
Joseph Klein, III
|
||||
|
/s/ FREDERICK T. MUTO
|
Director
|
February 24, 2022
|
||
|
Frederick T. Muto, Esq.
|
||||
|
/s/ B. LYNNE PARSHALL
|
Director and Senior Strategic Advisor
|
February 24, 2022
|
||
|
B. Lynne Parshall, J.D.
|
|
/s/ JOSEPH H. WENDER
|
Lead Independent Director
|
February 24, 2022
|
||
|
Joseph H. Wender
|
IONIS PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Page
|
|
|
Report of Independent Registered Public Accounting Firm
(PCAOB ID )
|
F-2
|
|
Consolidated Balance Sheets at December 31, 2021 and 2020
|
F-4
|
|
Consolidated Statements of Operations for the years ended
December 31, 2021, 2020
and 2019
|
F-5
|
|
Consolidated Statements of Comprehensive Income (Loss) for
the years ended December 31, 2021, 2020
and 2019
|
F-6
|
|
Consolidated Statements of Stockholders’ Equity for the
years ended December 31, 2021, 2020
and 2019
|
F-7
|
|
Consolidated Statements of Cash Flows for the years ended
December 31, 2021, 2020
and 2019
|
F-8
|
|
Notes to Consolidated Financial Statements
|
F-10
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of Ionis Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Ionis Pharmaceuticals, Inc. (the Company) as of December 31, 2021 and 2020,
the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2021, and the related notes (collectively referred to as the “consolidated
financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2021 and 2020, and the results of its operations and its cash flows for each
of the three years in the period ended December 31, 2021, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the
Company’s internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and
our report dated February 24, 2022 expressed an unqualified opinion thereon.
Adoption of ASU No. 2020-06
As discussed in Note 1 to the consolidated financial statements, the Company changed its method of accounting for convertible instruments for
all years presented, 2019 through 2021, due to the adoption of ASU No. 2020-06, Debt–Debt with Conversion and Other Options (Subtopic 470-20) and
Derivatives and Hedging–Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and
regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether
due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the
accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were
communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The
communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing a separate opinion on the critical
audit matters or on the accounts or disclosures to which they relate.
|
AstraZeneca – Eplontersen Collaboration
|
|
Description of the Matter
|
As discussed in Note 6 to the consolidated financial statements, the Company entered into a joint development and commercialization
agreement with AstraZeneca AB (“AstraZeneca”), referred to as the “AstraZeneca agreement”, which resulted in the recognition of $200 million in revenue for the year ended December 31, 2021. The Company determined that there were four material
components of the AstraZeneca agreement: (i) license granted to AstraZeneca to develop and commercialize eplontersen; (ii) the parties’ co-development activities for eplontersen; (iii) the parties’ co-commercialization activities for
eplontersen; and (iv) the parties’ co-medical affairs activities for eplontersen.
|
|
Auditing management’s initial application of the relevant US GAAP guidance under Accounting Standards Codification (ASC) 606, Revenue
from Contracts With Customers, and ASC 808, Collaborative Arrangements, related to the AstraZeneca Agreement was especially challenging due to the complex nature of its terms and conditions. In particular, determining the distinct performance
obligations with a customer was highly judgmental.
|
||
|
How We Addressed the Matter in Our Audit
|
We obtained an understanding, evaluated the design and tested the operating effectiveness of internal controls over management’s
review of the terms and conditions of the AstraZeneca Agreement, identification of performance obligations, and consideration of the appropriate accounting guidance in determining the appropriate conclusions.
To test management’s initial application of the accounting guidance to the AstraZeneca Agreement, we performed audit procedures that
included, among others, reading the contractual agreement and assessing management’s application of the appropriate accounting guidance in their evaluation. Our procedures included evaluating management’s identification of distinct performance
obligations with a customer. We also evaluated alternative views and any contrary or corroborative evidence associated with management’s evaluation, and discussed with management the underlying business objectives of the AstraZeneca Agreement.
|
|
|
Estimated Liability for Clinical Development Costs
|
||
|
Description of the Matter
|
As of December 31, 2021, the Company accrued $65.7 million for accrued clinical development costs. As discussed in Note 2 to the
consolidated financial statements, the Company records costs for clinical trial activities based upon estimates of costs incurred through the balance sheet date that have yet to be invoiced related to clinical management costs, laboratory and
analysis costs, toxicology studies and investigator grants.
Auditing the Company’s accruals for clinical development costs is especially complex as the information necessary to estimate the
accruals is accumulated from multiple sources. In addition, in certain circumstances, the determination of the nature and level of services that have been received during the reporting period requires judgment because the timing and pattern of
vendor invoicing does not correspond to the level of services provided and there may be delays in invoicing from vendors.
|
|
|
How We Addressed the Matter in Our Audit
|
We obtained an understanding and evaluated the design and tested the operating effectiveness of controls over the accounting for
accrued clinical development costs. This included controls over management’s assessment of the assumptions and accuracy of data underlying the accrued clinical development expenses estimate.
To test the accuracy of the Company’s accrued clinical development costs, we performed audit procedures that included, among other
procedures, obtaining supporting evidence of the research and development activities performed for significant clinical trials. We corroborated the status of significant clinical development costs through meetings with accounting and clinical
project managers. We compared the costs for a sample of transactions against the related invoices and contracts, and examined a sample of subsequent payments to evaluate the accuracy of the accrued clinical development costs and compared the
results to the current year accrual.
|
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 1989.
February 24, 2022
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share data)
|
December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
(as revised*)
|
||||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
|
$
|
|
||||
|
Short-term investments
|
|
|
||||||
|
Contracts receivable
|
|
|
||||||
|
Inventories
|
|
|
||||||
|
Other current assets
|
|
|
||||||
|
Total current assets
|
|
|
||||||
|
Property, plant and equipment, net
|
|
|
||||||
|
Patents, net
|
|
|
||||||
|
Deposits and other assets
|
|
|
||||||
|
Total assets
|
$
|
|
$
|
|
||||
|
LIABILITIES
AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
|
$
|
|
||||
|
Accrued compensation
|
|
|
||||||
|
Accrued liabilities
|
|
|
||||||
|
Income taxes payable
|
|
|
||||||
|
|
|
|
||||||
|
Current portion of long-term obligations
|
|
|
||||||
|
Current portion of deferred contract revenue
|
|
|
||||||
|
Total current liabilities
|
|
|
||||||
|
Long-term deferred contract revenue
|
|
|
||||||
|
|
|
|
||||||
|
|
|
|
||||||
|
Long-term obligations, less current portion
|
|
|
||||||
|
Long-term mortgage debt
|
|
|
||||||
|
Total liabilities
|
|
|
||||||
|
Stockholders’ equity:
|
||||||||
|
Common stock, $
|
|
|
||||||
|
Additional paid-in capital
|
|
|
||||||
|
Accumulated other comprehensive loss
|
(
|
)
|
(
|
)
|
||||
|
Accumulated deficit
|
(
|
)
|
(
|
)
|
||||
|
Total stockholders’ equity
|
|
|
||||||
|
Total liabilities and stockholders’ equity
|
$
|
|
$
|
|
||||
| * |
We revised our 2020 amounts to reflect the simplified convertible instruments accounting guidance, which we adopted retrospectively. Refer to Note 1, Organization and Significant Accounting Policies, for further information.
|
See accompanying notes.
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except for per share amounts)
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
(as revised*)
|
(as revised*)
|
|||||||||||
|
Revenue:
|
||||||||||||
|
Commercial revenue:
|
||||||||||||
|
SPINRAZA royalties
|
$
|
|
$
|
|
$
|
|
||||||
|
TEGSEDI and WAYLIVRA revenue, net
|
|
|
|
|||||||||
|
Licensing and other royalty revenue
|
|
|
|
|||||||||
|
Total commercial revenue
|
|
|
|
|||||||||
|
Research and development revenue under collaborative agreements
|
|
|
|
|||||||||
|
Total revenue
|
|
|
|
|||||||||
|
Expenses:
|
||||||||||||
|
Cost of sales
|
|
|
|
|||||||||
|
Research, development and patent
|
|
|
|
|||||||||
|
Selling, general and administrative
|
|
|
|
|||||||||
|
Total operating expenses
|
|
|
|
|||||||||
|
Income (loss) from operations
|
(
|
)
|
(
|
)
|
|
|||||||
|
Other income (expense):
|
||||||||||||
|
Investment income
|
|
|
|
|||||||||
|
Interest expense
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Gain on investments
|
|
|
|
|||||||||
|
Loss on early retirement of debt
|
(
|
)
|
|
(
|
)
|
|||||||
|
Other expenses
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Income (loss) before income tax benefit (expense)
|
(
|
)
|
(
|
)
|
|
|||||||
|
Income tax benefit (expense)
|
|
(
|
)
|
(
|
)
|
|||||||
|
Net income (loss)
|
(
|
)
|
(
|
)
|
|
|||||||
|
Net (income) loss attributable to noncontrolling interest in Akcea Therapeutics, Inc.
|
|
|
(
|
)
|
||||||||
|
Net income (loss) attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
|
Basic net income (loss) per share
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
|
Shares used in computing basic net income (loss) per share
|
|
|
|
|||||||||
|
Diluted net income (loss) per share
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
|
Shares used in computing diluted net income (loss) per share
|
|
|
|
|||||||||
| * |
We revised our 2020 and 2019 amounts to reflect the simplified convertible instruments accounting guidance, which we adopted retrospectively. Refer to
Note 1, Organization and Significant Accounting Policies, for further information.
|
See accompanying notes.
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(In thousands)
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
(as revised*)
|
(as revised*)
|
|||||||||||
|
Net income (loss)
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
|
Unrealized gains (losses) on investments, net of tax
|
(
|
)
|
|
|
||||||||
|
Currency translation adjustment
|
(
|
)
|
|
|
||||||||
|
Adjustments to other comprehensive loss from purchase of noncontrolling interest of Akcea
Therapeutics, Inc.
|
|
(
|
)
|
|
||||||||
|
Comprehensive income (loss)
|
(
|
)
|
(
|
)
|
|
|||||||
|
Comprehensive income (loss) attributable to noncontrolling interest in Akcea Therapeutics, Inc.
|
|
(
|
)
|
|
||||||||
|
Comprehensive income (loss) attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
| * |
We revised our 2020 and 2019 amounts to reflect the simplified convertible instruments accounting guidance, which we adopted retrospectively. Refer to
Note 1, Organization and Significant Accounting Policies, for further information.
|
See accompanying notes.
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
Years Ended December 31, 2021,
2020 and 2019
(In thousands)
|
Common Stock
|
Additional
|
Accumulated Other
|
Accumulated
|
Total Ionis
Stockholders’
|
Noncontrolling
Interest in Akcea
|
Total
Stockholders’
|
||||||||||||||||||||||||||
|
Description
|
Shares
|
Amount
|
Paid in Capital
|
Comprehensive Loss
|
Deficit
|
Equity
|
Therapeutics, Inc.
|
Equity
|
||||||||||||||||||||||||
|
Balance at December 31, 2018 (as revised*)
|
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
|
|||||||||||||||
|
Net income
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Change in unrealized losses, net of tax
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Foreign currency translation
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Issuance of warrants
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Purchase of note hedges, net of tax
|
—
|
|
(
|
)
|
|
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||||
|
Repurchases and retirements of common stock
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||
|
Stock-based compensation expense
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Payments of tax withholdings related to vesting of employee stock awards and exercise of employee
stock options
|
(
|
)
|
|
(
|
)
|
|
|
(
|
)
|
|
(
|
)
|
||||||||||||||||||||
|
Noncontrolling interest in Akcea Therapeutics, Inc.
|
—
|
|
(
|
)
|
|
|
(
|
)
|
|
|
||||||||||||||||||||||
|
Balance at December 31, 2019 (as revised*)
|
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
|
|||||||||||||||
|
Net loss
|
—
|
|
|
|
(
|
)
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||||
|
Change in unrealized gain, net of tax
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Foreign currency translation
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Purchase of noncontrolling interest of Akcea Therapeutics, Inc., including cash payments for
cancellation of Akcea Therapeutics, Inc. equity awards
|
—
|
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||||||||||||
|
Repurchases and retirements of common stock
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||
|
Stock-based compensation expense
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Payments of tax withholdings related to vesting of employee stock awards and exercise of employee
stock options
|
(
|
)
|
|
(
|
)
|
|
|
(
|
)
|
|
(
|
)
|
||||||||||||||||||||
|
Deferred tax liability adjustment due to purchase of noncontrolling interest of Akcea Therapeutics,
Inc.
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Noncontrolling interest in Akcea Therapeutics, Inc.
|
—
|
|
(
|
)
|
(
|
)
|
|
(
|
)
|
|
(
|
)
|
||||||||||||||||||||
|
Balance at December 31, 2020 (as revised*)
|
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
|
|||||||||||||||
|
Net loss
|
—
|
|
|
|
(
|
)
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||||
|
Change in unrealized gains, net of tax
|
—
|
|
|
(
|
)
|
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||||
|
Foreign currency translation
|
—
|
|
|
(
|
)
|
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Issuance of warrants
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Purchases of note hedges
|
—
|
|
(
|
)
|
|
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Payments of tax withholdings related to vesting of employee stock awards and exercise of employee
stock options
|
(
|
)
|
|
(
|
)
|
|
|
(
|
)
|
|
(
|
)
|
||||||||||||||||||||
|
Balance at December 31, 2021
|
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
|
|||||||||||||||
| * |
We revised our 2018, 2019 and 2020 amounts to reflect the simplified convertible instruments accounting guidance, which we adopted retrospectively. Refer
to Note 1, Organization and Significant Accounting Policies, for further information.
|
See accompanying notes.
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
(as revised*)
|
(as revised*)
|
|||||||||||
|
Operating activities:
|
||||||||||||
|
Net income (loss)
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
|
Adjustments to reconcile net income (loss) to net cash provided by operating activities:
|
||||||||||||
|
Depreciation
|
|
|
|
|||||||||
|
Amortization of right-of-use operating lease assets
|
|
|
|
|||||||||
|
Amortization of patents
|
|
|
|
|||||||||
|
Amortization of premium (discount) on investments, net
|
|
|
(
|
)
|
||||||||
|
Amortization of debt issuance costs
|
|
|
|
|||||||||
|
Stock-based compensation expense
|
|
|
|
|||||||||
|
Loss on early retirement of debt
|
|
|
|
|||||||||
|
Gain on investments
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Deferred income taxes, including changes in valuation allowance
|
|
|
|
|||||||||
|
Non-cash losses related to patents
|
|
|
|
|||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Contracts receivable
|
|
(
|
)
|
(
|
)
|
|||||||
|
Inventories
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Other current and long-term assets
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Long-term income taxes receivable (payable)
|
|
(
|
)
|
|
||||||||
|
Accounts payable
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Income taxes
|
(
|
)
|
(
|
)
|
|
|||||||
|
Accrued compensation
|
(
|
)
|
|
|
||||||||
|
Accrued liabilities and other current liabilities
|
(
|
)
|
|
|
||||||||
|
Deferred contract revenue
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Net cash provided by operating activities
|
|
|
|
|||||||||
|
Investing activities:
|
||||||||||||
|
Purchases of short-term investments
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Proceeds from the sale of short-term investments
|
|
|
|
|||||||||
|
Purchases of property, plant and equipment
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Acquisition of licenses and other assets, net
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Purchases of strategic investments
|
(
|
)
|
|
(
|
)
|
|||||||
|
Net cash provided by (used in) investing activities
|
|
|
(
|
)
|
||||||||
|
Financing activities:
|
||||||||||||
|
Proceeds from equity, net
|
|
|
|
|||||||||
|
Payments of tax withholdings related to vesting of employee stock awards and exercise of employee
stock options
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Proceeds from the issuance of
|
|
|
|
|||||||||
|
Proceeds from the issuance of
|
|
|
|
|||||||||
|
|
(
|
)
|
|
|
||||||||
|
|
|
|
(
|
)
|
||||||||
|
Repurchase of $
|
(
|
)
|
|
|
||||||||
|
Repayment of remaining principal amount of
|
(
|
)
|
|
|
||||||||
|
Proceeds from issuance of warrants
|
|
|
|
|||||||||
|
Purchase of note hedges
|
(
|
)
|
|
(
|
)
|
|||||||
|
Repurchases and retirements of common stock
|
|
(
|
)
|
(
|
)
|
|||||||
|
Purchase of noncontrolling interest of Akcea Therapeutics, Inc., including cash payments for cancellation of Akcea Therapeutics, Inc. equity awards
|
|
(
|
)
|
|
||||||||
|
Principal payments on line of credit
|
|
|
(
|
)
|
||||||||
|
Net cash provided by (used in) financing activities
|
|
(
|
)
|
|
||||||||
|
Effects of exchange rates on cash
|
(
|
)
|
|
|
||||||||
|
Net increase (decrease) in cash and cash equivalents
|
|
(
|
)
|
|
||||||||
|
Cash and cash equivalents at beginning of year
|
|
|
|
|||||||||
|
Cash and cash equivalents at end of year
|
$
|
|
$
|
|
$
|
|
||||||
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
Supplemental disclosures of cash flow information:
|
||||||||||||
|
Interest paid
|
$
|
|
$
|
|
$
|
|
||||||
|
Income taxes paid
|
$
|
|
$
|
|
$
|
|
||||||
|
Supplemental disclosures of non-cash investing and financing activities:
|
||||||||||||
|
Right-of-use assets obtained in exchange for lease liabilities
|
$
|
|
$
|
|
$
|
|
||||||
|
Amounts accrued for capital and patent expenditures
|
$
|
|
$
|
|
$
|
|
||||||
|
|
$
|
|
$
|
|
$
|
|
||||||
|
|
$
|
|
$
|
|
$
|
|
||||||
| * |
We revised our 2020 and 2019 amounts to reflect the simplified convertible instruments accounting guidance, which we adopted retrospectively. Refer to
Note 1, Organization and Significant Accounting Policies, for further information.
|
See accompanying notes.
IONIS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Significant Accounting Policies
Basis of Presentation
In our consolidated financial statements we included the accounts of Ionis Pharmaceuticals, Inc. and the consolidated
results of our subsidiary, Akcea Therapeutics, Inc. and its wholly owned subsidiaries (“we”, “us” or “our”). We formed Akcea in December 2014. In July 2017, Akcea completed an initial public offering, or IPO, which reduced our ownership of Akcea’s
common stock below 100 percent. In October 2020, we completed a merger transaction with Akcea such that following the completion of the merger,
Akcea became our wholly owned subsidiary. We will refer to this transaction as the Akcea Merger throughout the remainder of this document. We reflected changes in our ownership percentage in our financial statements as an adjustment to noncontrolling
interest in the period the changes occurred.
Organization and Business Activity
We incorporated in California on January 10, 1989. In conjunction with our IPO, we reorganized as a Delaware corporation
in April 1991. We were organized principally to develop human therapeutic medicines using antisense technology. In December 2015, we changed our name from Isis Pharmaceuticals, Inc. to Ionis Pharmaceuticals, Inc.
Basic and Diluted Net Income (Loss) per Share
Basic net income (loss) per share
We compute basic net income (loss) per share by dividing the total net income (loss) attributable to our common
stockholders by our weighted-average number of common shares outstanding during the period. For the year ended December 31, 2021, we did not have to consider Akcea results separately in our calculation because we owned 100 percent of Akcea for the entire period. Our basic net loss per share for the year ended December 31, 2021 was $0.20 .
For the years ended December 31, 2020 and 2019, we calculated total net income (loss) attributable to our common
stockholders for each year using our net income (loss) for Ionis on a stand-alone basis plus our share of Akcea’s net income (loss) for the period. To calculate the portion of Akcea’s net income (loss) attributable to our ownership for each year, we
multiplied Akcea’s income (loss) per share by the weighted average shares we owned in Akcea during the period. As a result of this calculation, our total net income (loss) available to Ionis common stockholders for the calculation of net income (loss)
per share is different than net income (loss) attributable to Ionis Pharmaceuticals, Inc. common stockholders in our consolidated statements of operations for each year.
We calculated our basic net loss per share for the year ended December 31, 2020 as follows (in thousands, except
per share amounts):
|
Year Ended December 31, 2020
|
Weighted
Average Shares
Owned in Akcea
|
Akcea’s
Net Loss
Per Share
|
Basic Net Loss
Per Share
Calculation
|
|||||||||
|
Akcea’s net loss in the pre-merger period attributable to our ownership
|
|
$
|
(
|
)
|
$
|
(
|
)
|
|||||
|
Akcea’s net loss in the post-merger period attributable to our ownership
|
(
|
)
|
||||||||||
|
Akcea’s total net loss attributable to our ownership
|
$
|
(
|
)
|
|||||||||
|
Ionis’ stand-alone net loss
|
(
|
)
|
||||||||||
|
Net loss available to Ionis common stockholders
|
$
|
(
|
)
|
|||||||||
|
Weighted average shares outstanding
|
|
|||||||||||
|
Basic net loss per share
|
$
|
(
|
)
|
|||||||||
We calculated our basic net income per share for the year ended December 31, 2019 as follows (in thousands, except per share amounts):
|
Year Ended December 31, 2019
|
Weighted
Average Shares
Owned in Akcea
|
Akcea’s
Net Income
Per Share
|
Basic Net Income
Per Share
Calculation
|
|||||||||
|
Common shares
|
|
$
|
|
$
|
|
|||||||
|
Akcea’s net income attributable to our ownership
|
$
|
|
||||||||||
|
Ionis’ stand-alone net income
|
|
|||||||||||
|
Net income available to Ionis common stockholders
|
$
|
|
||||||||||
|
Weighted average shares outstanding
|
|
|||||||||||
|
Basic net income per share
|
$
|
|
||||||||||
Diluted net income (loss) per share
For the years ended December 31, 2021 and 2020, we incurred a net loss; therefore, we did not include dilutive common
equivalent shares in the computation of diluted net loss per share because the effect would have been anti-dilutive. Common stock underlying the following would have had an anti-dilutive effect on net loss per share:
| ● |
|
| ● |
Note hedges related to the
|
| ● |
|
| ● |
Dilutive stock options;
|
| ● |
Unvested restricted stock units, or RSUs;
|
| ● |
Unvested performance restricted stock units, or PRSUs; and
|
| ● |
Employee Stock Purchase Plan, or ESPP.
|
For the year ended December 31, 2021, common stock underlying the following would also have had an anti-dilutive effect on
net loss per share:
| ● |
|
| ● |
Note hedges related to the
|
Additionally as of December 31, 2021, we had warrants related to our 0 percent and 0.125 percent Notes outstanding. We will include the shares issuable
under these warrants in our calculation of diluted earnings per share when the average market price per share of our common stock for the reporting period exceeds the strike price of the warrants.
For the
year ended December 31, 2019, we reported net income available to Ionis common stockholders. As a result, we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding
during each period. We calculated our diluted net income per share as follows (in thousands except per share amounts):
|
Year Ended December 31, 2019
|
Net Income Available
to Ionis Common
Stockholders
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
Net income available to Ionis common stockholders
|
$
|
|
|
$
|
|
|||||||
|
Effect of dilutive securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
|
||||||||||
|
Shares issuable related to our 0.125 percent convertible notes
|
|
|
||||||||||
|
Shares issuable related to our 1 percent convertible notes
|
|
|
||||||||||
|
$
|
|
|
$
|
|
||||||||
Revenue Recognition
Our Revenue Sources
We generally recognize revenue when we have satisfied all contractual obligations and are reasonably assured of collecting
the resulting receivable. We are often entitled to bill our customers and receive payment from our customers in advance of recognizing the revenue. In the instances in which we have received payment from our customers in advance of recognizing revenue,
we include the amounts in deferred revenue on our consolidated balance sheet.
At contract inception, we analyze our collaboration arrangements to assess whether such arrangements involve joint
operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards dependent on the commercial success of such activities and therefore within the scope of ASC Topic 808,
Collaborative Arrangements (ASC 808). For collaboration arrangements within the scope of ASC 808 that contain multiple elements, we first determine which elements of the collaboration reflect a vendor-customer relationship and therefore within the
scope of ASC 606. When we determine elements of a collaboration do not reflect a vendor-customer relationship, we consistently apply the reasonable and rational policy election we made by analogizing to authoritative accounting literature.
We evaluate the income statement classification for presentation of amounts due from or owed to other participants
associated with multiple activities in a collaboration arrangement based on the nature of each separate activity. For example, in our eplontersen collaboration with AstraZeneca, we recognize funding received from AstraZeneca for co-development
activities as revenue. While, we recognize cost sharing payments to and from AstraZeneca associated with co-commercialization activities and co-medical affairs activities as SG&A expense and research and development expense, respectively.
Commercial Revenue: SPINRAZA royalties and Licensing and other royalty revenue
We earn commercial revenue primarily in the form of royalty payments on net sales of SPINRAZA. We will also recognize as
commercial revenue sales milestone payments and royalties we earn under our other partnerships.
Commercial Revenue: TEGSEDI and WAYLIVRA revenue, net
We began commercializing TEGSEDI and WAYLIVRA in Europe in January 2021 and TEGSEDI in North America in April 2021 through distribution
agreements with Swedish Orphan Biovitrum AB, or Sobi. Under our agreements, we are responsible for supplying finished goods inventory to Sobi and Sobi is responsible for selling each medicine to the end customer. As a result of these agreements, we
earn a distribution fee on net sales from Sobi for each medicine.
Prior to the second quarter of 2021 in North America, we sold TEGSEDI through exclusive distribution agreements with third-party logistics
companies, or 3PLs, that took title to TEGSEDI. The 3PLs then distributed TEGSEDI to a specialty pharmacy and a specialty distributor, which we collectively refer to as wholesalers, who then distributed TEGSEDI to health care providers and patients. In
the United States, or U.S., we had a single 3PL as our sole customer and in Canada we also had a single 3PL as our sole customer. Prior to 2021 in Europe, we sold TEGSEDI and WAYLIVRA to hospitals and pharmacies, which were our customers, using 3PLs as
distributors.
Under our collaboration agreement with PTC
Therapeutics International Limited, or PTC, PTC is responsible for commercializing TEGSEDI and WAYLIVRA in Latin America and Caribbean countries. In the third quarter of 2021, we earned a $4 million milestone payment from PTC when WAYLIVRA was approved in Brazil, which we included in TEGSEDI and WAYLIVRA revenue in our consolidated statement of operations. Under our agreement, we
started receiving royalties from PTC for TEGSEDI sales beginning in December 2021.
Research and development revenue under collaborative agreements
We often enter into collaboration agreements to license and sell our technology on an exclusive or non-exclusive basis.
Our collaboration agreements typically contain multiple elements, or performance obligations, including technology licenses or options to obtain technology licenses, research and development, or R&D, services, and manufacturing services.
We provide details about our collaboration agreements in Note 6, Collaborative Arrangements and Licensing Agreements. For each collaboration, we discuss our specific revenue
recognition conclusions, including our significant performance obligations under each collaboration.
Steps to Recognize Revenue
We use a five-step process to determine the amount of revenue we should recognize and when we should recognize it. The
five step process is as follows:
| 1. |
Identify the contract
|
Accounting rules require us to first determine if we have a contract with our partner, including confirming that we have met each of the
following criteria:
| ● |
We and our partner approved the contract and we are both committed to perform our obligations;
|
| ● |
We have identified our rights, our partner’s rights and the payment terms;
|
| ● |
We have concluded that the contract has commercial substance, meaning that the risk, timing, or amount of our future cash flows is expected to change as a result of the
contract; and
|
| ● |
We believe collectability of the consideration is probable.
|
| 2. |
Identify the performance obligations
|
We next identify our performance obligations, which represent the distinct goods and services we are required to provide under the
contract.
Often we enter into a collaboration agreement
in which we provide our partner with an option to license a medicine in the future. We may also provide our partner with an option to request that we provide additional goods or services in the future, such as active pharmaceutical ingredient, or
API. We evaluate whether these options are material rights at the inception of the agreement. If we determine an option is a material right, we will consider the option a separate performance obligation. Historically, we have concluded that the
options we grant to license a medicine in the future or to provide additional goods and services as requested by our partner are not material rights because these items are contingent upon future events that may not occur and are not priced at a
significant discount. When a partner exercises its option to license a medicine or requests additional goods or services, then we identify a new performance obligation for that item.
In some cases, we deliver a license at the start of an agreement. If we determine that our partner has full use of the license and we do
not have any additional material performance obligations related to the license after delivery, then we consider the license to be a separate performance obligation. For example, in the fourth quarter of 2021, we received a $200 million upfront payment when we entered into an agreement with AstraZeneca to jointly develop and commercialize eplontersen. We recognized the upfront
payment in full in the fourth quarter of 2021 because we did not have any remaining performance obligations after we delivered the license to AstraZeneca.
| 3. |
Determine the transaction price
|
We then determine the transaction price by reviewing the amount of consideration we are eligible to earn under the
collaboration agreement, including any variable consideration. Under our collaboration agreements, consideration typically includes fixed consideration in the form of an upfront payment and variable consideration in the form of potential milestone
payments, license fees and royalties. At the start of an agreement, our transaction price usually consists of only the upfront payment. We do not typically include any payments we may receive in the future in our initial transaction price because
the payments are not probable and are contingent on certain future events. We reassess the total transaction price at each reporting period to determine if we should include additional payments in the transaction price.
Milestone payments are our most common type
of variable consideration. We recognize milestone payments using the most likely amount method because we will either receive the milestone payment or we will not, which makes the potential milestone payment a binary event. The most likely amount
method requires us to determine the likelihood of earning the milestone payment. We include a milestone payment in the transaction price once it is probable we will achieve the milestone event. Most often, we do not consider our milestone payments
probable until we or our partner achieve the milestone event because the majority of our milestone payments are contingent upon events that are not within our control and/ or are usually based on scientific progress which is inherently uncertain. For
example, in the fourth quarter of 2021, we earned a $10 million milestone payment from AstraZeneca when AstraZeneca advanced a target for a metabolic disease. We did not consider the milestone payment probable until AstraZeneca achieved the milestone event because advancing the target was
contingent on AstraZeneca initiating a Phase 1 study and was not within our control. We recognized the milestone payment in full in the period the milestone event was achieved because we did not have any remaining performance obligations related to
the milestone payment.
| 4. |
Allocate the transaction price
|
Next, we allocate the transaction price to
each of our performance obligations. When we have to allocate the transaction price to more than one performance obligation, we make estimates of the relative
stand-alone selling price of each performance obligation because we do not typically sell our goods or services on a stand-alone basis. We then allocate the transaction price to each performance obligation based on the relative stand-alone selling
price. We do not reallocate the transaction price after the start of an agreement to reflect subsequent changes in stand-alone selling prices.
We may engage a third party, independent valuation specialist to assist us with determining a stand-alone selling price for collaborations in which we deliver a license at the start of an agreement.
We estimate the stand-alone selling price of these licenses using valuation methodologies, such as the relief from royalty method. Under this method, we estimate the amount of income, net of taxes, for the license. We then discount the projected
income to present value. The significant inputs we use to determine the projected income of a license could include:
| ● |
Estimated future product sales;
|
| ● |
Estimated royalties we may receive from future product sales;
|
| ● |
Estimated contractual milestone payments we may receive;
|
| ● |
Expenses we expect to incur;
|
| ● |
Estimated income taxes; and
|
| ● |
A discount rate.
|
We typically estimate the selling price of R&D services by using our internal estimates of the cost to perform the
specific services. The significant inputs we use to determine the selling price of our R&D services include:
| ● |
The number of internal hours we estimate we will spend performing these services;
|
| ● |
The estimated cost of work we will perform;
|
| ● |
The estimated cost of work that we will contract with third parties to perform; and
|
| ● |
The estimated cost of API we will use.
|
For purposes of determining the stand-alone selling price of the R&D services we perform and the API we will deliver,
accounting guidance requires us to include a markup for a reasonable profit margin.
| 5. |
Recognize revenue
|
We recognize revenue in one of two ways, over time or at a point in time. We recognize revenue over time when we are
executing on our performance obligation over time and our partner receives benefit over time. For example, we recognize revenue over time when we provide R&D services. We recognize revenue at a point in time when our partner receives full use
of an item at a specific point in time. For example, we recognize revenue at a point in time when we deliver a license or API to a partner.
For R&D services that we recognize over
time, we measure our progress using an input method. The input methods we use are based on the effort we expend or costs we incur toward the satisfaction of our performance obligation. We estimate the amount of effort we expend, including the time we
estimate it will take us to complete the activities, or costs we incur in a given period, relative to the estimated total effort or costs to satisfy the performance obligation. This results in a percentage that we multiply by the transaction price to
determine the amount of revenue we recognize each period. This approach requires us to make numerous estimates and use significant judgement. If our estimates or judgements change over the course of the collaboration, they may affect the timing and
amount of revenue that we recognize in the current and future periods. Refer to Note 6, Collaborative Arrangements and Licensing Agreements,
for further discussion of the cumulative catch up adjustment we made.
The following are examples of when we typically recognize revenue based on the types of payments we receive.
Commercial Revenue: SPINRAZA royalties and Licensing and other royalty revenue
We recognize royalty revenue, including
royalties from SPINRAZA sales, in the period in which the counterparty sells the related product and recognizes the related revenue, which in certain cases may require us to estimate our royalty revenue.
Commercial Revenue: TEGSEDI and WAYLIVRA revenue, net
Under our distribution agreements with Sobi we concluded that our performance obligation is to provide services to
Sobi over the term of the agreement, which includes supplying finished goods inventory to Sobi and because we retained the marketing authorization for TEGSEDI and WAYLIVRA we are responsible for leading the global commercial strategy for each
medicine. We view this performance obligation as a series of distinct activities that are substantially the same. Therefore, we recognize as revenue the price Sobi pays us for the inventory when we deliver the finished goods inventory to Sobi. We
also recognize distribution fee revenue based on Sobi’s net sales of TEGSEDI and WAYLIVRA in the period in which the sales occurred. Under our agreements with Sobi, Sobi does not generally have a right of return.
Prior to our distribution agreements with
Sobi, we recognized TEGSEDI and WAYLIVRA commercial revenue in the period when our customer obtained control of our products, which occurred at a point in time upon transfer of title to the customer. We classified payments to customers or other
parties in the distribution channel for services that were distinct and priced at fair value as selling, general and administrative, or SG&A, expenses in our consolidated statements of operations. We classified payments to customers or other
parties in the distribution channel that did not meet those criteria as a reduction of revenue, as discussed further below. We excluded from revenues taxes collected from customers relating to TEGSEDI and WAYLIVRA commercial revenue and remitted
these amounts to governmental authorities.
Reserves for TEGSEDI and WAYLIVRA commercial revenue
Prior to our distribution agreements with Sobi, we recorded TEGSEDI and WAYLIVRA commercial revenue at our net sales
price, or transaction price. We included in our transaction price estimated reserves for discounts, returns, chargebacks, rebates and other allowances that we offered within contracts between us and our customers, wholesalers, distributors, health
care providers and other indirect customers. We estimated our reserves using the amounts we have earned or we could claim on the associated sales. We classified our reserves as a reduction of accounts receivable when we were not required to make a
payment or as a current liability when we were required to make a payment. In certain cases, our estimates included a range of possible outcomes that were probability weighted for relevant factors such as our historical experience, current
contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. Overall, our reserves reflected our best estimates under the terms of our respective contracts. When
calculating our reserves and related TEGSEDI and WAYLIVRA commercial revenue, we only recognized amounts to the extent that we considered it probable that we would not have to reverse a significant amount of the cumulative sales we previously
recognized in a future period. Under our agreements with Sobi, we transferred all reserves to Sobi.
The following were the components of variable consideration related to TEGSEDI and WAYLIVRA product sales prior to our agreements with Sobi:
Chargebacks: In the U.S., we estimated obligations resulting from contractual commitments with the government and other entities to sell products to qualified healthcare
providers at prices lower than the list prices charged to our U.S. customer. Our U.S. customer charged us for the difference between what it paid for the product and the selling price to the qualified healthcare providers. We also estimated the
amount of chargebacks related to our estimated product remaining in the distribution channel at the end of the reporting period that we expected our customer to sell to healthcare providers in future periods. We recorded these reserves as a reduction
to contracts receivable on our consolidated balance sheet.
Government rebates: We were subject to discount obligations under government programs, including Medicaid and Medicare programs in the U.S. and we recorded reserves for government
rebates based on statutory discount rates and estimated utilization. We estimated Medicaid and Medicare rebates based on a range of possible outcomes that were probability weighted for the estimated payer mix. We recorded these reserves as an accrued
liability on our consolidated balance sheet with a corresponding offset reducing our product sales in the same period we recognized the related sale. For Medicare, we also estimated the number of patients in the prescription drug coverage gap for
whom we would owe an additional liability under the Medicare Part D program. On a quarterly basis, we updated our estimates and recorded any adjustments in the period that we identified the adjustments.
Managed care rebates: We were subject to rebates in connection with agreements with certain contracted commercial payers. We recorded these rebates as a liability on our consolidated
balance sheet in the same period we recognized the related revenue. We estimated our managed care rebates based on our estimated payer mix and the applicable contractual rebate rate.
Trade discounts: We provided customary invoice discounts on product sales to our U.S. customer for prompt payment. We recorded this discount as a reduction of product sales in the
period in which we recognized the related product revenue.
Distribution services: We received and paid for various distribution services from our U.S. and European customers (prior to our agreement with Sobi) and wholesalers in the U.S. We
classified the costs for services we received that are either not distinct from the sale of the product or for which we could not reasonably estimate the fair value as a reduction of product sales. To the extent that the services we received are
distinct from the sale of the product, we classified the costs for such services as SG&A expenses.
Product returns: Our U.S. customer had return rights and the wholesalers had limited return rights primarily related to the product’s expiration date. We estimated the amount of
product sales that our customer may return. We recorded our return estimate as an accrued refund liability on our consolidated balance sheet with a corresponding offset reducing our product sales in the same period we recognized the related sale.
Based on our distribution model for product sales, contractual inventory limits with our customer and wholesalers and the price of the product, we had minimal returns. Our European customers generally only took title to the product after they
received an order and therefore they did not maintain excess inventory levels of our products. Accordingly, we had limited return risk in Europe and we did not estimate returns in Europe.
Research and development revenue under collaboration agreements:
Upfront payments
When we enter into a collaboration agreement
with an upfront payment, we typically record the entire upfront payment as deferred revenue if our only performance obligation is for R&D services we will provide in the future. We amortize the upfront payment into revenue as we perform the
R&D services. For example, under our collaboration agreement with Roche to develop IONIS-FB-LRx for the treatment of complement-mediated diseases, we received a $75 million upfront payment in the fourth quarter of 2018. We allocated the upfront payment to our
single performance obligation, R&D services. We are amortizing the $75 million upfront payment using an input method over the estimated period of time we are providing R&D services.
Milestone payments
We are required to include additional consideration in the transaction price when it is probable. We typically
include milestone payments for R&D services in the transaction price when they are achieved. We include these milestone payments when they are achieved because typically there is considerable uncertainty in the research and development
processes that trigger these payments. Similarly, we include approval milestone payments in the transaction price once the medicine is approved by the applicable regulatory agency. We will recognize sales-based milestone payments in the period in
which we achieve the milestone under the sales-based royalty exception allowed under accounting rules.
We recognize milestone payments that relate
to an ongoing performance obligation over our period of performance. For example, in the fourth quarter of 2021, we achieved a $7.5 million milestone payment from Biogen when we advanced a target
under our 2018 strategic collaboration. We added this payment to the transaction price and allocated it to our R&D services performance obligation. We are
recognizing revenue related to this milestone payment over our estimated period of performance.
Conversely, we recognize in full those milestone payments that we earn based on our partners’ activities when our partner achieves the
milestone event and we do not have a performance obligation. For example, in the fourth quarter of 2021, we recognized $15 million in milestone
payments when Biogen advanced two targets under our 2018 strategic collaboration. We concluded that the milestone payments were not related to our R&D services performance obligation. Therefore, we recognized the milestone payments in full in the fourth quarter of 2021.
License fees
We generally recognize as revenue the total
amount we determine to be the relative stand-alone selling price of a license when we deliver the license to our partner. This is because our partner has full use of the license and we do not have any additional performance obligations related to the
license after delivery. For example, in the fourth quarter of 2021, we earned a $60 million license fee from Biogen when Biogen licensed ION306, an investigational medicine in development to treat SMA.
Sublicense fees
We recognize sublicense fee revenue in the
period in which a party, who has already licensed our technology, further licenses the technology to another party because we do not have any performance obligations related to the sublicense. For example, in the fourth quarter of 2020, we
earned a $41.2 million sublicense fee from Alnylam Pharmaceuticals for its sublicense of our technology to Sanofi Genzyme.
Amendments to Agreements
From time to time we amend our collaboration agreements. When this occurs, we are required to assess the following items
to determine the accounting for the amendment:
| 1) |
If the additional goods and/or services are distinct from the other performance obligations in the original agreement; and
|
| 2) |
If the goods and/or services are at a stand-alone selling price.
|
If we conclude the goods and/or services in the amendment are distinct from the performance obligations in the
original agreement and at a stand-alone selling price, we account for the amendment as a separate agreement. If we conclude the goods and/or services are not distinct and are sold at a stand-alone selling price, we then assess whether the remaining
goods or services are distinct from those already provided. If the goods and/or services are distinct from what we have already provided, then we allocate the remaining transaction price from the original agreement and the additional transaction
price from the amendment to the remaining goods and/or services. If the goods and/or services are not distinct from what we have already provided, we update the transaction price for our single performance obligation and recognize any change in our
estimated revenue as a cumulative adjustment.
For example, in May 2015, we entered into an exclusive license agreement with Bayer to develop and commercialize IONIS-FXIRx
for the prevention of thrombosis. As part of the agreement, Bayer paid us a $100 million upfront payment. At the onset of the agreement, we were
responsible for completing a Phase 2 study of IONIS-FXIRx in people with end-stage renal disease on hemodialysis and for providing an initial supply of API. In February 2017, we amended our agreement with Bayer to advance IONIS-FXIRx
and to initiate development of fesomersen, which Bayer licensed. As part of the 2017 amendment, Bayer paid us $75 million. We are also eligible
to receive milestone payments and tiered royalties on gross margins of IONIS-FXIRx and fesomersen. Under the 2017 amendment, we concluded we had a new agreement with three performance obligations. These performance obligations were to deliver the license of fesomersen, to provide R&D services and to deliver API. We allocated the $75 million transaction price to these performance obligations. Refer to Note 6, Collaborative Arrangements and Licensing Agreements, for further discussion of the Bayer collaboration.
Multiple agreements
From time to time, we may enter into separate agreements at or near the same time with the same partner. We evaluate such
agreements to determine whether we should account for them individually as distinct arrangements or whether the separate agreements should be combined and accounted for together. We evaluate the following to determine the accounting for the agreements:
| ● |
Whether the agreements were negotiated together with a single objective;
|
| ● |
Whether the amount of consideration in one contract depends on the price or performance of the other agreement; or
|
| ● |
Whether the goods and/or services promised under the agreements are a single performance obligation.
|
Our evaluation involves significant judgment to determine whether a group of agreements might be so closely related that accounting
guidance requires us to account for them as a combined arrangement.
For example, in the second quarter of 2018, we entered into two separate agreements with Biogen at the same time: a new strategic neurology collaboration
agreement and a stock purchase agreement, or SPA. We evaluated the Biogen agreements to determine whether we should treat the agreements separately or combine them. We considered that the agreements were negotiated concurrently and in contemplation
of one another. Based on these facts and circumstances, we concluded that we should evaluate the provisions of the agreements on a combined basis.
Contracts Receivable
Our contracts receivable balance represents
the amounts we have billed our partners or customers and that are due to us unconditionally for goods we have delivered or services we have performed. When we bill our partners or customers with payment terms based on the passage of time, we consider
the contracts receivable to be unconditional. We typically receive payment within one of billing our partner or customer.
As of December 31, 2021, approximately 93.8 percent of our contracts receivables were from two significant customers. As of December 31, 2020, approximately 99.5 percent of our contracts receivables were from two
significant customers.
Unbilled SPINRAZA Royalties
Our unbilled SPINRAZA royalties represent our right to receive consideration from Biogen in advance of when we are
eligible to bill Biogen for SPINRAZA royalties. We include these unbilled amounts in other current assets on our consolidated balance sheet.
Deferred Revenue
We are often entitled to bill our customers
and receive payment from our customers in advance of our obligation to provide services or transfer goods to our partners. In these instances, we include the amounts in deferred revenue on our consolidated balance sheet. During the years ended
December 31, 2021 and 2020, we recognized $98.1 million and $100.4 million of revenue from amounts that were in our beginning deferred revenue balance for each respective period. For further discussion, refer to our revenue recognition policy above.
Cost of Sales
Our cost of sales includes manufacturing costs, transportation and freight costs and indirect overhead costs associated with the
manufacturing and distribution of our products. We also may include certain period costs related to manufacturing services and inventory adjustments in cost of sales. We also may include certain period costs related to manufacturing services and
inventory adjustments in cost of sales.
Research, Development and Patent Expenses
Our research and development expenses include wages, benefits, facilities, supplies, external
services, clinical trial and manufacturing costs and other expenses that are directly related to our research and development operations. We expense research and development costs as we incur them. When we make payments for research and development
services prior to the services being rendered, we record those amounts as prepaid assets on our consolidated balance sheet and we expense them as the services are provided. For the years ended December 31, 2021, 2020 and 2019, research and development expenses were $638.2 million, $531.0 million and $461.5 million, respectively. A
portion of the costs included in research and development expenses are costs associated with our partner agreements.
We capitalize costs consisting principally of outside legal costs and filing fees related to obtaining
patents. We amortize patent costs over the useful life of the patent, beginning with the date the U.S. Patent and Trademark Office, or foreign equivalent, issues the patent. The weighted average remaining amortizable life of our issued patents was 10.2 years at December 31, 2021.
The cost of our patents capitalized on our consolidated balance sheet at December 31, 2021 and 2020 was $38.4 million and $37.0 million, respectively.
Accumulated amortization related to patents was $9.4 million and $9.1 million at December 31, 2021 and 2020, respectively.
Based on our existing patents, we estimate amortization expense related to patents in each of
the next five years to be the following:
|
Year Ending December 31,
|
Amortization
(in millions)
|
|||
|
2022
|
$
|
|
||
|
2023
|
$
|
|
||
|
2024
|
$
|
|
||
|
2025
|
$
|
|
||
|
2026
|
$
|
|
||
We review our capitalized patent costs regularly to ensure that they include costs for patents and patent applications
that have future value. When we identify patents and patent applications that we are not actively pursuing, we write off any associated costs. In 2021,
2020 and 2019, patent expenses
were $5.3 million, $4.1 million and $4.2 million, respectively, and included non-cash charges related to the write-down of our patent costs to their estimated net realizable values of $2.7 million, $1.9 million and $2.2 million, respectively.
Accrued Liabilities
Our accrued liabilities consisted of the following (in thousands):
|
December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Clinical expenses
|
$
|
|
$
|
|
||||
|
In-licensing expenses
|
|
|
||||||
|
Commercial expenses
|
|
|
||||||
|
Other miscellaneous expenses
|
|
|
||||||
|
Total accrued liabilities
|
$
|
|
$
|
|
||||
Estimated Liability for Clinical Development Costs
We have numerous medicines in preclinical studies and/or clinical trials at clinical sites throughout the world. On at least a quarterly
basis, we estimate our liability for preclinical and clinical development costs we have incurred and services that we have received but for which we have not yet been billed and maintain an accrual to cover these costs. These costs primarily relate to
third-party clinical management costs, laboratory and analysis costs, toxicology studies and investigator grants. We estimate our liability using assumptions about study and patient activities and the related expected expenses for those activities
determined based on the contracted fees with our service providers. The assumptions we use represent our best estimates of the activity and expenses at the time of our accrual and involve inherent uncertainties and the application of our judgment. Upon
settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements. Our historical accrual estimates have not been materially different from our actual amounts.
Noncontrolling Interest in Akcea Therapeutics, Inc.
Since Akcea’s IPO in July 2017 and prior to
the Akcea Merger in October 2020, the shares of Akcea’s common stock third parties owned represented an interest in Akcea’s equity that we did not control. During this period our ownership ranged from 68 percent to 77 percent. However, as we maintained overall control of Akcea through our voting interest, we
reflected the assets, liabilities and results of operations of Akcea in our consolidated financial statements. Since Akcea’s IPO in July 2017 and through the closing of the Akcea Merger, we reflected the noncontrolling interest attributable to other
owners of Akcea’s common stock on a separate line on our statement of operations and a separate line within stockholders’ equity in our consolidated balance sheet. In addition, through the closing of the Akcea Merger, we recorded a noncontrolling
interest adjustment to account for the stock options Akcea granted, which if exercised, would have diluted our ownership in Akcea. This adjustment was a reclassification within stockholders’ equity from additional paid-in capital to noncontrolling
interest in Akcea equal to the amount of stock-based compensation expense Akcea had recognized. Additionally, we reflected changes in our ownership percentage in our financial statements as an adjustment to noncontrolling interest in the
period the change occurred.
Concentration of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash equivalents,
short-term investments and receivables. We place our cash equivalents and short-term investments with reputable financial institutions. We primarily invest our excess cash in commercial paper and debt instruments of the U.S. Treasury, financial
institutions, corporations, and U.S. government agencies with strong credit ratings and an investment grade rating at or above A-1, P-1 or F-1 by Moody’s, Standard & Poor’s, or S&P, or Fitch, respectively. We have established guidelines
relative to diversification and maturities that maintain safety and liquidity. We periodically review and modify these guidelines to maximize trends in yields and interest rates without compromising safety and liquidity.
Cash, Cash Equivalents and Investments
We consider all liquid investments with maturities of three months or less when we purchase them to be cash equivalents.
Our short-term investments have initial maturities of greater than three months from date of purchase. We classify our short-term debt investments as “available-for-sale” and carry them at fair market value based upon prices on the last day of the
fiscal period for identical or similar items. We record unrealized gains and losses on debt securities as a separate component of comprehensive income (loss) and include net realized gains and losses in gain (loss) on investments in our consolidated
statement of operations. We use the specific identification method to determine the cost of securities sold.
We also have equity investments of less than 20 percent ownership in publicly and privately held biotechnology companies
that we received as part of a technology license or partner agreement. At December 31, 2021, we held equity investments in three publicly held companies, Antisense Therapeutics Limited, or ATL, Bicycle Therapeutics plc, or Bicycle, and ProQR Therapeutics N.V., or ProQR. We
also held equity investments in seven privately-held companies, Aro Biotherapeutics, Atlantic Pharmaceuticals Limited, Dynacure SAS, Empirico,
Inc., Flamingo Therapeutics BV, YourBio Health, Inc. (formerly Seventh Sense Biosystems) and Suzhou-Ribo Life Science Co, Ltd.
We are required to measure and record our equity investments at fair value and to recognize the changes in fair value in our consolidated
statement of operations. We account for our equity investments in privately held companies at their cost minus impairments, plus or minus changes resulting from observable price changes in orderly transactions for the identical or similar investment of
the same issuer. For example, during 2020, we revalued our investments in three privately held companies, Dynacure, Suzhou-Ribo and Aro Biotherapeutics because the companies
sold additional equity securities that were similar to the equity we own. As a result of these observable price changes, we recognized a $6.3 million gain on our investment in Dynacure, a $3.0 million gain on our investment in Suzhou-Ribo and a $5.5 million gain on our investment in Aro Biotherapeutics in our consolidated statement of operations during 2020 because the sales were at higher prices compared to
our recorded value.
Inventory Valuation
We reflect our inventory on our consolidated balance sheet at the lower of cost or net realizable value under the first-in, first-out
method, or FIFO. We capitalize the costs of raw materials that we purchase for use in producing our medicines because until we use these raw materials, they have alternative future uses, which we refer to as clinical raw materials. We include in
inventory raw material costs for medicines that we manufacture for our partners under contractual terms and that we use primarily in our clinical development activities and drug products. We can use each of our raw materials in multiple products and,
as a result, each raw material has future economic value independent of the development status of any single medicine. For example, if one of our medicines failed, we could use the raw materials for that medicine to manufacture our other medicines. We
expense these costs as R&D expenses when we begin to manufacture API for a particular medicine if the medicine has not been approved for marketing by a regulatory agency. Our raw materials- commercial inventory includes API for our commercial
medicines. We capitalize material, labor and overhead costs as part of our raw materials- commercial inventory.
We review our inventory periodically and reduce the carrying value of items we consider to be slow moving or obsolete to
their estimated net realizable value based on forecasted demand compared to quantities on hand. We consider several factors in estimating the net realizable value, including shelf life of our inventory, alternative uses for our medicines in development
and historical write-offs. We recorded an amount of inventory write-offs during the years ended December 31, 2021 and 2020.
Our inventory consisted of the following (in thousands):
|
December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
Raw materials:
|
||||||||
|
Raw materials- clinical
|
$
|
|
$
|
|
||||
|
Raw materials- commercial
|
|
|
||||||
|
Total raw materials
|
|
|
||||||
|
Work in process
|
|
|
||||||
|
Finished goods
|
|
|
||||||
|
Total inventory
|
$
|
|
$
|
|
||||
Property, Plant and Equipment
We carry our property, plant and equipment at cost and depreciate it on the straight-line method
over its estimated useful life, which consists of the following (in thousands):
|
Estimated Useful
|
December 31,
|
|||||||||||
|
Lives (in years)
|
2021
|
2020
|
||||||||||
|
Computer software, laboratory, manufacturing and other equipment
|
|
$
|
|
$
|
|
|||||||
|
Building, building improvements and building systems
|
|
|
|
|||||||||
|
Land improvements
|
|
|
|
|||||||||
|
Leasehold improvements
|
|
|
|
|||||||||
|
Furniture and fixtures
|
|
|
|
|||||||||
|
|
|
|||||||||||
|
Less accumulated depreciation
|
(
|
)
|
(
|
)
|
||||||||
|
|
|
|||||||||||
|
Land
|
|
|
||||||||||
|
Total
|
$
|
|
$
|
|
||||||||
We depreciate our leasehold improvements using the shorter of the estimated useful life or remaining lease term.
Fair Value of Financial Instruments
We have estimated the fair value of our financial instruments. The amounts reported for cash, accounts receivable,
accounts payable and accrued expenses approximate the fair value because of their short maturities. We report our investment securities at their estimated fair value based on quoted market prices for identical or similar instruments.
Leases
We determine if an arrangement contains a lease at inception. We currently only have operating leases. We recognize a
right-of-use operating lease asset and associated short- and long-term operating lease liability on our consolidated balance sheet for operating leases greater than one year. Our right-of-use assets represent our right to use an underlying asset for
the lease term and our lease liabilities represent our obligation to make lease payments arising from the lease arrangement. We recognize our right-of-use operating lease assets and lease liabilities based on the present value of the future minimum
lease payments we will pay over the lease term. We determine the lease term at the inception of each lease, and in certain cases our lease term could
include renewal options if we concluded we were reasonably certain that we will exercise the renewal option. When we exercise a lease option that was not previously included in the initial lease term, we reassess our right-of-use asset and lease
liabilities for the new lease term.
As our leases do not provide an interest rate implicit in the lease, we used our incremental borrowing rate, based on the
information available on the date we adopted Topic 842 (January 2019), as of the lease inception date or at the lease option extension date in determining the present value of future payments. We recognize rent expense for our minimum lease payments on
a straight-line basis over the expected term of our lease. We recognize period expenses, such as common area maintenance expenses, in the period we incur the expense.
Long-Lived Assets
We evaluate long-lived assets, which include property, plant and equipment and patent costs, for impairment on at least a
quarterly basis and whenever events or changes in circumstances indicate that we may not be able to recover the carrying amount of such assets. We recorded charges of $2.7 million, $1.9 million and $2.2 million for the years ended December 31, 2021, 2020 and 2019, respectively, related to the
write-down of patents.
Use of Estimates
We prepare our consolidated financial statements in conformity with accounting principles generally accepted in the U.S.
that require us to make estimates and assumptions that affect the amounts reported in our consolidated financial statements and accompanying notes. Actual results could differ from our estimates.
Stock-Based Compensation Expense
We measure stock-based compensation expense for equity-classified awards, principally related to stock options, RSUs,
PRSUs and stock purchase rights under our ESPP based on the estimated fair value of the award on the date of grant. We recognize the value of the portion of the award that we ultimately expect to vest as stock-based compensation expense over the
requisite service period in our consolidated statements of operations. We reduce stock-based compensation expense for estimated forfeitures at the time of grant and revise in subsequent periods if actual forfeitures differ from those estimates. We use
the Black-Scholes model to estimate the fair value of stock options granted and stock purchase rights under our ESPP.
On the grant date, we use our stock price and assumptions regarding a number of variables to determine the estimated fair
value of stock-based payment awards. These variables include, but are not limited to, our expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors. The expected term of stock options
granted represents the period of time that we expect them to be outstanding. We estimate the expected term of options granted based on historical exercise patterns.
We recognize compensation expense for stock options granted, RSUs, PRSUs and stock purchase rights under the ESPP using
the accelerated multiple-option approach. Under the accelerated multiple-option approach (also known as the graded-vesting method), we recognize compensation expense over the requisite service period for each separately vesting tranche of the award as
though the award were in substance multiple awards, which results in the expense being front-loaded over the vesting period.
In December 2020, we amended and restated the
Akcea 2015 equity plan, including renaming the plan as the Ionis Pharmaceuticals, Inc. 2020 Equity Incentive Plan, or 2020 Plan. As a result, all employees are now under an Ionis stock plan and subject to the same Black-Scholes assumptions.
RSU’s:
The fair value of RSUs is based on the market price of our common stock on the date of grant. The RSUs we have granted to
employees vest annually over a four-year period. The RSUs we granted to our board of directors prior to June 2020 vest annually over a four-year period. RSUs granted to our board of directors after June 2020 fully vest after one year .
PRSU’s:
Beginning in 2020, we added PRSU awards to the compensation for our Chief Executive Officer, Dr. Brett Monia. Under the
terms of the grants, of the PRSUs may vest at the end of three separate performance periods spread over the three years following the date of
grant (i.e., the one-year period commencing on the date of grant and ending on the first anniversary of the date of grant; the two-year period commencing on the date of grant and ending on the second anniversary of the date of grant; and the three-year period commencing on the date of grant and ending on the third anniversary of the date of grant) based on our relative total shareholder return, or TSR, as compared to a
peer group of companies, and as measured, in each case, at the end of the applicable performance period. Under the terms of the grants no number
of PRSUs is guaranteed to vest and the actual number of PRSUs that will vest at the end of each performance period may be anywhere from zero to
150 percent of the target number depending on our relative TSR.
We determined the fair value of Dr. Monia’s PRSUs using a Monte Carlo model because the performance target is based on our
relative TSR, which represents a market condition. We are recognizing the grant date fair value of these awards as stock-based compensation expense using the accelerated multiple-option approach over the vesting period. The weighted-average grant date
fair value of PRSUs granted to Dr. Monia for the years ended December 31, 2021 and 2020 were $77.17 and $93.09 per share, respectively.
See Note 4, Stockholders’
Equity, for additional information regarding our stock-based compensation plans.
Accumulated Other Comprehensive Loss
Accumulated
other comprehensive loss is comprised of unrealized gains and losses on investments, net of taxes and currency translation adjustments. The following table summarizes changes in accumulated other comprehensive loss for the years ended December 31, 2021, 2020 and 2019 (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
Beginning balance accumulated other comprehensive loss
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
|||
|
Unrealized gains (losses) on securities, net of tax (1)
|
(
|
)
|
|
|
||||||||
|
Currency translation adjustment
|
(
|
)
|
|
|
||||||||
|
Adjustments to other comprehensive loss from purchase of noncontrolling interest of Akcea
Therapeutics, Inc.
|
|
(
|
)
|
|
||||||||
|
Net other comprehensive loss for the period
|
(
|
)
|
|
|
||||||||
|
Ending balance accumulated other comprehensive loss
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
|||
________________
|
(1)
|
|
Convertible Debt
Adoption of ASU 2020-06
In August 2020, the FASB issued ASU 2020-06, which simplifies the accounting for convertible debt instruments, amends the
guidance on derivative scope exceptions for contracts in an entity’s own equity, and modifies the guidance on diluted earnings per share calculations. We adopted ASU 2020-06 on January 1, 2021 under the full retrospective approach, which required us to
revise our prior period financial statements. This guidance impacted our accounting for outstanding convertible debt. At January 1, 2021, we had two
outstanding convertible notes, our 0.125 % Notes, which mature in December 2024, and our 1 % Notes, which matured in November 2021. In April 2021, we completed a $632.5 million
offering of 0 % Notes primarily to repurchase a majority of our 1 % Notes. We accounted for our 0 % Notes under ASU 2020-06 at issuance. Refer to Note
3, Long-Term Obligations and Commitments, for further information.
The updated guidance eliminates the cash conversion accounting model we previously followed in Accounting Standard
Codification, or ASC, 470-20, which required us to separate each of our convertible debt instruments at issuance into two units of accounting, a liability component, based on our nonconvertible debt borrowing rate at issuance, and an equity component.
Under ASU 2020-06, we now account for each of our convertible debt instruments as a single unit of accounting, a liability, because we concluded that the conversion features do not require bifurcation as a derivative under ASC 815-15 and we did not
issue our convertible debt instruments at a substantial premium. Since we adopted ASU 2020-06 using the full retrospective approach, we were required to apply the guidance to all convertible debt instruments we had outstanding as of January 1, 2019. We
recomputed the basis of each convertible debt instrument as if we accounted for each as a single unit of accounting at issuance. This update included recalculating the amortization of debt issuance costs using an updated effective interest rate. As a
result of adopting ASU 2020-06, we recorded a cumulative adjustment to decrease our additional paid in capital and our accumulated deficit at January 1, 2019. We have updated these financial statements to reflect the cumulative adjustment for the
periods presented. We have labeled our prior period financial statements “as revised” to indicate the change required under the new accounting guidance. Below is a summary of the change in our balance sheet at December 31, 2020 and statement of
operations from the years ended December 31, 2020 and 2019 under the ASC 470-20 legacy guidance compared to the new ASU 2020-06 guidance we adopted:
The following table summarizes the adjustments we made to the consolidated balance sheet we
originally reported at December 31, 2020 to adopt ASU 2020-06 (in thousands):
|
December 31, 2020
|
||||||||||||
|
As Previously
Reported
|
ASU 2020-06
Adjustment
|
As Revised
|
||||||||||
|
|
$
|
|
$
|
|
$
|
|
||||||
|
|
$
|
|
$
|
|
$
|
|
||||||
|
Additional paid-in-capital
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||
|
Accumulated deficit
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
||||
Under ASU 2020-06, our revised
ending balances for our 1 % Notes and 0.125 % Notes as of December 31, 2020 represent the principal
balance of each convertible debt instrument less debt issuance costs. Additionally, because we have deferred tax assets related to our convertible debt instruments, we also adjusted these amounts as part of our adoption of ASU 2020-06. However,
because we have a full valuation allowance on our deferred tax assets, there was no impact to our consolidated balance sheet related to our deferred tax assets.
|
Year Ended December 31, 2020
|
||||||||||||
|
As Previously
Reported
|
ASU 2020-06
Adjustment
|
As Revised
|
||||||||||
|
Interest expense
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
||||
|
Loss before income tax expense
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
||||
|
Income tax expense
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
|||
|
Net loss
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
||||
|
Net loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
||||
|
Basic and diluted net loss per share
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
||||
|
Year Ended December 31, 2019
|
||||||||||||
|
As Previously
Reported
|
ASU 2020-06
Adjustment
|
As Revised
|
||||||||||
|
Interest expense
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
||||
|
Loss on early retirement of debt
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Income before income tax benefit (expense)
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||
|
Income tax expense
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
|||
|
Net income
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||
|
Net income attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||
|
Basic net income per share
|
|
(
|
)
|
|
||||||||
|
Diluted net income per share
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||
Under ASU 2020-06, our revised interest expense is lower because we are no longer recording non-cash
interest expense related to a debt discount. This decrease was partially offset by the increase in interest expense related to the amortization of debt issuance costs because we no longer allocate a portion of our debt issuance costs to stockholders’
equity at issuance. Instead, the entire debt issuance costs were recorded as a contra-liability on our consolidated balance sheet at issuance and we are amortizing them over the contractual term using an updated effective interest rate. Our updated
effective interest rates for our 1 % Notes and 0.125 %
Notes were 1.4 percent and 0.5 percent,
respectively.
The following tables summarize the adjustments we made to our consolidated statements of
stockholders’ equity we originally reported at December 31, 2020 and 2019 to adopt ASU 2020-06 (in thousands):
|
December 31, 2020
|
||||||||||||
|
As Previously
Reported
|
ASU 2020-06
Adjustment
|
As Revised
|
||||||||||
|
Additional paid-in-capital
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||
|
Accumulated deficit
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
||||
|
Total stockholders’ equity
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||
|
December 31, 2019
|
||||||||||||
|
As Previously
Reported
|
ASU 2020-06
Adjustment
|
As Revised
|
||||||||||
|
Additional paid-in-capital
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||
|
Accumulated deficit
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
||||
|
Total stockholders’ equity
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||
Call Spread
In conjunction with the issuance of our 0 % Notes and 0.125 % Notes in April 2021 and December 2019, respectively, we entered
into call spread transactions, which were comprised of purchasing note hedges and selling warrants. We account for the note hedges and warrants as separate freestanding financial instruments and treat each instrument as a separate unit of accounting.
We determined that the note hedges and warrants do not meet the definition of a liability using the guidance contained in ASC Topic 480; therefore, we account for the note hedges and warrants using the Derivatives and Hedging – Contracts in Entity’s
Own Equity accounting guidance contained in ASC Topic 815. We determined that the note hedges and warrants meet the definition of a derivative, are indexed to our stock and meet the criteria to be classified in shareholders’ equity. We recorded the
aggregate amount paid for the note hedges and the aggregate amount received for the warrants as additional paid-in capital in our consolidated balance sheet. We reassess our ability to continue to classify the note hedges and warrants in shareholders’
equity at each reporting period.
Segment Information
In 2021, we began operating as a single segment, Ionis operations, because our chief decision maker reviews operating
results on an aggregate basis and manages our operations as a operating segment. Previously, we had operated as two operating segments, Ionis Core and Akcea Therapeutics. We completed the Akcea Merger in October 2020 and fully integrated Akcea’s operations into ours
as of January 1, 2021.
Fair Value Measurements
We use a three-tier fair value hierarchy to prioritize the inputs used in our fair value measurements. These tiers
include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets, which includes our money market funds and treasury securities classified as available-for-sale securities and our investment in equity
securities in publicly held biotechnology companies; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable, which includes our fixed income securities and commercial paper classified as
available-for-sale securities; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring us to develop our own assumptions. We classify most of our securities as Level 2. We obtain the fair value of our
Level 2 investments from our custodian bank or from a professional pricing service. We validate the fair value of our Level 2 investments by understanding the pricing model used by the custodian banks or professional pricing service provider and
comparing that fair value to the fair value based on observable market prices.
The following tables present the major security types we held at December 31, 2021 and 2020 that we regularly measure and carry at fair value. As of December 31, 2021, our Bicycle investment was subject to trading restrictions that extend to the third quarter of 2022; as
a result, we included a lack of marketability discount in valuing this investment, which is a Level 3 input. As of December 31, 2020, we did no t
have any investments that we valued using Level 3 inputs. The following tables segregate each security type by the level within the fair value hierarchy of the valuation techniques we utilized to determine the respective securities’ fair value (in
thousands):
|
At
December 31, 2021
|
Quoted Prices in
Active Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
Significant
Unobservable Inputs
(Level 3)
|
|||||||||||||
|
Cash equivalents (1)
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Corporate debt securities (2)
|
|
|
|
|
||||||||||||
|
Debt securities issued by U.S. government agencies (2)
|
|
|
|
|
||||||||||||
|
Debt securities issued by the U.S. Treasury (2)
|
|
|
|
|
||||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (3)
|
|
|
|
|
||||||||||||
|
Other municipal debt securities (2)
|
|
|
|
|
||||||||||||
|
Investment in Bicycle Therapeutics plc (4)
|
|
|
|
|
||||||||||||
|
Investment in ProQR Therapeutics N.V. (4)
|
|
|
|
|
||||||||||||
|
Total
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
At
December 31, 2020
|
Quoted Prices in
Active Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
||||||||||
|
Cash equivalents (1)
|
$
|
|
$
|
|
$
|
|
||||||
|
Corporate debt securities (5)
|
|
|
|
|||||||||
|
Debt securities issued by U.S. government agencies (2)
|
|
|
|
|||||||||
|
Debt securities issued by the U.S. Treasury (6)
|
|
|
|
|||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (2)
|
|
|
|
|||||||||
|
Other municipal debt securities (2)
|
|
|
|
|||||||||
|
Investment in ProQR Therapeutics N.V. (4)
|
|
|
|
|||||||||
|
Total
|
$
|
|
$
|
|
$
|
|
||||||
________________
| (1) |
|
| (2) |
|
| (3) |
|
| (4) |
|
| (5) |
|
| (6) |
|
Convertible Notes
Our 0.125 % Notes and 0 % Notes had a fair value of $495.4 million and $559.2 million at December 31, 2021, respectively. We determine the fair value of our notes based on quoted market prices for these notes, which are Level 2
measurements because the notes do not trade regularly.
Income Taxes
We account for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities
for the expected future tax consequences of events that have been recognized in our financial statements or tax returns. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development
credit carryforwards. We record a valuation allowance when necessary to reduce our net deferred tax assets to the amount expected to be realized.
We apply the authoritative accounting guidance prescribing a threshold and measurement attribute for the financial recognition and
measurement of a tax position taken or expected to be taken in a tax return. We recognize liabilities for uncertain tax positions based on a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight
of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step requires us to estimate and measure the tax benefit
as the largest amount that is more than 50 percent likely to be realized upon ultimate settlement.
We are required to use significant judgment in evaluating our uncertain tax positions and determining our provision for income taxes.
Although we believe our reserves are reasonable, no assurance can be given that the final tax outcome of these matters will not be different from that which is reflected in our historical income tax provisions and accruals. We adjust these reserves for
changing facts and circumstances, such as the closing of a tax audit or the refinement of an estimate. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences may impact the provision for
income taxes in the period in which such determination is made.
We are also required to use significant judgment in determining any valuation allowance recorded against our deferred tax assets. In
assessing the need for a valuation allowance, we consider all available evidence, including scheduled reversal of deferred tax liabilities, past operating results, the feasibility of tax planning strategies and estimates of future taxable income. We
base our estimates of future taxable income on assumptions that are consistent with our plans. The assumptions we use represent our best estimates and involve inherent uncertainties and the application of our judgment. Should actual amounts differ from
our estimates, the amount of our tax expense and liabilities we recognize could be materially impacted. We record a valuation allowance to reduce the balance of our net deferred tax assets to the amount we believe is more-likely-than-not to be
realized.
We do not provide for a U.S. income tax liability and foreign withholding taxes on undistributed foreign earnings of our foreign
subsidiaries.
Impact of Recently Issued Accounting Standards
As disclosed in the “Convertible Debt” policy above within this footnote, we adopted the simplified accounting for
convertible debt instrument guidance (ASU 2020-06) on January 1, 2021. Refer to the section above for the impact of adoption. We do not expect any other recently issued accounting standards to have a material impact to our financial results.
2. Investments
The following table summarizes the contract maturity of the available-for-sale securities we held as of
December 31, 2021:
|
|
|
%
|
||
|
After
|
|
%
|
||
|
After
|
|
%
|
||
|
Total
|
|
%
|
As illustrated above, at December 31, 2021, 85 percent of our available-for-sale securities had a maturity of less than two years .
All of our available-for-sale securities are available to us for use in our current operations. As a result, we categorize
all of these securities as current assets even though the stated maturity of some individual securities may be one year or more beyond the balance sheet date.
At December 31, 2021,
we had an ownership interest of less than 20 percent in seven
private companies and three public companies with which we conduct business. The privately-held companies are Aro Biotherapeutics,
Atlantic Pharmaceuticals Limited, Dynacure SAS, Empirico, Inc., Flamingo Therapeutics BV, YourBio Health, Inc. and Suzhou-Ribo Life Science Co, Ltd. The publicly traded companies are Antisense Therapeutics Ltd., Bicycle and ProQR.
The following is a summary of our investments (in thousands):
|
Amortized
|
Gross Unrealized
|
Estimated
|
||||||||||||||
|
December 31, 2021
|
Cost
|
Gains
|
Losses
|
Fair Value
|
||||||||||||
|
Available-for-sale securities:
|
||||||||||||||||
|
Corporate debt securities (1)
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
|
Debt securities issued by U.S. government agencies
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by the U.S. Treasury (1)
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
|
|
(
|
)
|
|
|||||||||||
|
Total securities with a maturity of one year or less
|
|
|
(
|
)
|
|
|||||||||||
|
Corporate debt securities
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by U.S. government agencies
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by the U.S. Treasury
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
|
|
(
|
)
|
|
|||||||||||
|
Other municipal debt securities
|
|
|
(
|
)
|
|
|||||||||||
|
Total securities with a maturity of more than one year
|
|
|
(
|
)
|
|
|||||||||||
|
Total available-for-sale securities
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
|
Equity securities:
|
||||||||||||||||
|
Total equity securities included in other current assets (2)
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
|
Total equity securities included in deposits and other assets (3)
|
|
|
|
|
||||||||||||
|
Total equity securities
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
|
Total available-for-sale and equity securities
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
|
Amortized
|
Gross Unrealized
|
Estimated
|
||||||||||||||
|
December 31, 2020
|
Cost
|
Gains
|
Losses
|
Fair Value
|
||||||||||||
|
Available-for-sale securities:
|
||||||||||||||||
|
Corporate debt securities (1)
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
|
Debt securities issued by U.S. government agencies
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by the U.S. Treasury (1)
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
|
|
(
|
)
|
|
|||||||||||
|
Other municipal debt securities
|
|
|
(
|
)
|
|
|||||||||||
|
Total securities with a maturity of one year or less
|
|
|
(
|
)
|
|
|||||||||||
|
Corporate debt securities
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by U.S. government agencies
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by the U.S. Treasury
|
|
|
(
|
)
|
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
|
|
(
|
)
|
|
|||||||||||
|
Other municipal debt securities
|
|
|
|
|
||||||||||||
|
Total securities with a maturity of more than one year
|
|
|
(
|
)
|
|
|||||||||||
|
Total available-for-sale securities
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
|
Equity securities:
|
||||||||||||||||
|
Total equity securities included in other current assets (2)
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
|
Total equity securities included in deposits and other assets (3)
|
|
|
|
|
||||||||||||
|
Total equity securities
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
|
Total available-for-sale and equity securities
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
|||||||
________________
| (1) |
|
| (2) |
|
| (3) |
|
The following is a summary of our investments we
considered to be temporarily impaired at December 31, 2021 (in thousands). All of these investments have less than 12 months of temporary impairment. We believe that the decline in value of these securities is temporary and is primarily related to the change in market interest rates since
purchase. We believe it is more likely than not that we will be able to hold our debt securities to maturity. Therefore, we anticipate full recovery of our debt securities’ amortized cost basis at maturity.
|
Number of
Investments
|
Estimated
Fair Value
|
Unrealized
Losses
|
||||||||||
|
Corporate debt securities
|
|
$
|
|
$
|
(
|
)
|
||||||
|
Debt securities issued by U.S. government agencies
|
|
|
(
|
)
|
||||||||
|
Debt securities issued by the U.S. Treasury
|
|
|
(
|
)
|
||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
|
|
(
|
)
|
||||||||
|
Other municipal debt securities
|
|
|
(
|
)
|
||||||||
|
Total temporarily impaired securities
|
|
$
|
|
$
|
(
|
)
|
||||||
3. Long-Term Obligations and Commitments
The carrying value of our long-term obligations was as follows (in thousands):
|
December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
(as revised*)
|
||||||||
|
|
$
|
|
$
|
|
||||
|
|
|
|
||||||
|
|
|
|
||||||
|
Long-term mortgage debt
|
|
|
||||||
|
Leases and other obligations
|
|
|
||||||
|
Total
|
$
|
|
$
|
|
||||
|
Less: current portion (1)
|
(
|
)
|
(
|
)
|
||||
|
Total Long-Term Obligations
|
$
|
|
$
|
|
||||
________________
| (1) |
|
| * |
We revised our 2020 amounts to reflect the simplified convertible instruments accounting guidance, which we adopted retrospectively. Refer to Note 1, Organization and Significant Accounting Policies, for further information.
|
Convertible Debt and Call Spread
In April 2021, we completed a $632.5
million offering of convertible senior notes. We used a portion of the net proceeds from the issuance of the 0 % Notes to repurchase $247.9 million in principal of our 1 % Notes for $257.0 million.
At December 31, 2021,
we had the following 0 % Notes outstanding (amounts in
millions except interest rate and price per share data):
|
|
||||
|
Outstanding principal balance
|
$
|
|
||
|
Unamortized debt issuance costs
|
$
|
|
||
|
Maturity date
|
|
|||
|
Interest rate
|
|
|||
|
Effective interest rate
|
|
|||
|
Conversion price per share
|
$
|
|
||
|
Effective conversion price per share with call spread
|
$
|
|
||
|
Total shares of common stock subject to conversion
|
|
|||
In conjunction with the April 2021 offering, we entered into a call spread transaction, which was comprised of purchasing
note hedges and selling warrants, to minimize the impact of potential economic dilution upon conversion of our 0 % Notes by increasing the effective conversion price on our 0 % Notes. We increased our effective conversion price to $76.39
with the same number of underlying shares as our 0 % Notes.
The call spread cost us $46.9 million, of which $136.7
million was for the note hedge purchase, offset by $89.8 million we received for selling the warrants. Similar to our 0 % Notes, our note hedges are subject to adjustment. Additionally, our note hedges
are exercisable upon conversion of the 0 % Notes. The note
hedges will expire upon maturity of the 0 % Notes, or April
2026. The note hedges and warrants are separate transactions and are not part of the terms of our 0 % Notes. The holders of the 0 % Notes do not have any rights with respect to the note hedges and warrants.
We recorded the amount we paid for the note hedges and the amount we received for the warrants in additional paid-in
capital in our consolidated balance sheet. See our Call Spread accounting policy in Note 1, Organization and Significant Accounting Policies, in the Notes to the Consolidated Financial Statements. We reassess our ability to continue to classify the note hedges and warrants in
shareholders’ equity at each reporting period.
In December 2019, we entered into privately negotiated exchange and/or subscription agreements with certain new investors
and certain holders of our existing 1 % Notes to exchange $375.6 million of our 1 % Notes for $439.3 million of our 0.125 % Notes, and to issue $109.5 million of our 0.125 % Notes.
At December 31, 2021,
we had the following 0.125 % Notes outstanding with interest payable semi-annually (amounts in millions except interest rate and price per share
data):
|
|
||||
|
Outstanding principal balance
|
$
|
|
||
|
Unamortized debt issuance costs
|
$
|
|
||
|
Maturity date
|
|
|||
|
Interest rate
|
|
|||
|
Effective interest rate
|
|
|||
|
Conversion price per share
|
$
|
|
||
|
Effective conversion price per share with call spread
|
$
|
|
||
|
Total shares of common stock subject to conversion
|
|
|||
In conjunction with the issuance of our 0.125 % Notes in December 2019, we entered into a call spread
transaction, which was comprised of purchasing note hedges and selling warrants, to minimize the impact of potential economic dilution upon conversion of our 0.125 % Notes by increasing the effective conversion price on our 0.125 % Notes. We increased our effective conversion price to $123.38 with the same number of underlying shares as our 0.125 % Notes. The call spread cost us $52.6 million, of which $108.7 million was for the note hedge purchase, offset by $56.1 million we received for selling the warrants. Similar to our 0.125 %
Notes, our note hedges are subject to adjustment. Additionally, our note hedges are exercisable upon conversion of the 0.125 % Notes. The note hedges will expire upon maturity of the
0.125 % Notes, or December 2024. The note
hedges and warrants are separate transactions and are not part of the terms of our 0.125 % Notes. The holders of the 0.125 % Notes do not have any rights with respect to the note hedges and warrants.
We recorded the amount we paid for the note hedges and the amount we received for the warrants in additional paid-in
capital in our consolidated balance sheet. See our Call Spread accounting policy in Note 1, Organization and Significant Accounting Policies, in the Notes to the Consolidated Financial Statements. We reassess our ability to continue to classify the note hedges and warrants in
shareholders’ equity at each reporting period.
In November 2014, we completed a $500 million offering of convertible senior notes, which matured in 2021 and beared interest at 1 percent with interest payable semi-annually. In December 2016, we issued an additional $185.5 million of 1 % Notes in exchange for the redemption of a portion of our previously outstanding 2.75 % convertible senior notes, or 2.75 % Notes. In December 2019, we exchanged a
portion of our 1 % Notes for new 0.125 %
Notes. As a result, the principal balance of 1 % Notes was $309.9 million. Additionally, we recorded a $66.2 million non-cash loss on the early
retirement of debt, reflecting the early retirement of a significant portion of our 1 % Notes in December 2019. The non-cash loss on the early
retirement of our debt is the difference between the amount paid to exchange our 1 % Notes and the net carrying balance of the liability at the
time that we completed the debt exchange.
In April 2021, we repurchased $247.9 million in aggregate principal amount of our 1 % Notes in privately negotiated
transactions. As a result of the repurchase, we recognized an $8.6 million loss on early retirement of debt, reflecting the early retirement of a
significant portion of our 1 % Notes. The loss on the early retirement of our debt is the difference between the amount paid to retire our 1 % Notes and the net carrying balance of the liability at the time that we retired the debt. We paid the remaining principal balance of our 1 % Notes with $62.0 million of cash at maturity
in November 2021.
Other Terms of Convertible Senior Notes
The 0 % and 0.125 % Notes are convertible under certain conditions, at the option of the note holders. We can settle conversions of the notes, at our election, in cash,
shares of our common stock or a combination of both. We may not redeem the notes prior to maturity, and we do not have to provide a sinking fund for them. Holders of the notes may require us to purchase some or all of their notes upon the occurrence of
certain fundamental changes, as set forth in the indentures governing the notes, at a purchase price equal to 100 1 % Notes were subject to similar terms.
Our total interest expense for our outstanding senior convertible notes for the years ended December 31, 2021, 2020 and 2019 included $4.9 million, $3.2 million and $2.9 million, respectively, of
non-cash interest expense related to the amortization of debt issuance costs for our convertible notes.
Financing Arrangements
Research and Development and Manufacturing Facilities
In July 2017, we purchased the building that houses our primary R&D facility for $79.4 million and our manufacturing facility for $14.0 million. We financed the purchase
of these two facilities with mortgage debt of $60.4
million in total. Our primary R&D facility mortgage has an interest rate of 3.88 percent. Our manufacturing facility mortgage has an
interest rate of 4.20 percent. During the first five years
Maturity Schedules
Annual debt and other obligation
maturities, including fixed and determinable interest, at December 31, 2021 are as follows (in thousands):
|
2022
|
$
|
|
||
|
2023
|
|
|||
|
2024
|
|
|||
|
2025
|
|
|||
|
2026
|
|
|||
|
Thereafter
|
|
|||
|
Subtotal
|
$
|
|
||
|
Less: current portion
|
(
|
)
|
||
|
Less: fixed and determinable interest
|
(
|
)
|
||
|
Less: debt issuance costs
|
(
|
)
|
||
|
Plus: lease liabilities
|
|
|||
|
Plus: other liabilities
|
|
|||
|
Total long-term debt
|
$
|
|
Operating Leases
Carlsbad Leases
We lease a facility adjacent to our manufacturing facility that has laboratory and office space that we use to support our
manufacturing facility. We lease this space under a non-cancelable operating lease. In May 2020, we exercised our option to extend our lease, extending our lease term from June 2021 to August 2026. We have one remaining option to extend the lease for an additional five-year
period.
We also lease additional office spaces in Carlsbad. We lease these spaces under non-cancelable operating leases with
initial terms ending in 2023 with options to extend each of the leases for one five-year period.
Boston Leases
We entered into an operating lease agreement for office space located in Boston, Massachusetts in the second quarter of
2018. The lease commencement date was in August 2018 and we took occupancy in September 2018. We are leasing this space under a non-cancelable operating lease with an initial term ending after 123 months and an option to extend the lease for an additional five-year term. Under
the lease agreement, we received a three-month free rent period, which commenced on August 15, 2018, and a tenant improvement allowance up to $3.8 million.
In January 2022, we entered into a sublease agreement for our office space located in Boston, Massachusetts. The sublease commencement
date was in January 2022 when the office space was ready for our tenant’s occupancy. We are subleasing this space under a non-cancelable operating sublease with a sublease term ending 83 months following the sublease commencement date with no option to extend the sublease. Under the sublease agreement we provided a seven-month free rent period, which commenced on January 6, 2022. We will receive lease payments over the sublease term totaling $9.6 million.
In September 2021, we entered into an operating lease agreement for another office space located in Boston, Massachusetts. The lease
commencement date was in November 2021 when the office space was ready for our occupancy. We are leasing this space under a non-cancelable operating lease with an initial term ending 91 months following the lease commencement date and an option to extend the lease for
additional five-year term. Under the lease agreement, we will receive a seven-month free rent period, which commenced on November 1, 2021. Our lease payments over the initial term total $6.8 million. We recognized a right-of-use lease asset and lease liability in the fourth quarter of 2021 upon the lease commencement date.
When we determined our lease term for our operating lease right-of-use assets and lease liabilities for these leases, we
did not include the extension options for these leases in the original lease term.
Amounts related to our operating leases were as follows (dollar amounts in millions):
|
At December 31, 2021
|
||||
|
Right-of-use operating lease assets (1)
|
$
|
|
||
|
Operating lease liabilities (2)
|
$
|
|
||
|
Weighted average remaining lease term
|
|
|||
|
Weighted average discount rate
|
|
%
|
||
________________
| (1) |
|
| (2) |
|
During the years ended December 31, 2021, 2020, and 2019 we paid $3.3 million, $3.8 million and $3.9 million of lease payments, which were included in
operating activities in our consolidated statements of cash flows.
As of December 31, 2021, the future payments for our operating lease liabilities are as follows (in thousands):
|
Operating Leases
|
||||
|
Year ending December 31,
|
$ | |||
|
2022
|
|
|||
|
2023
|
|
|||
|
2024
|
|
|||
|
2025
|
|
|||
|
2026
|
|
|||
|
Thereafter
|
|
|||
|
Total minimum lease payments
|
|
|||
|
Less:
|
||||
|
Imputed interest
|
(
|
)
|
||
|
Total operating lease liabilities
|
$
|
|
||
Rent expense was $3.4
million, $3.7 million and $3.6 million
for the years ended December 31, 2021, 2020
and 2019, respectively.
4. Stockholders’ Equity
Preferred Stock
We are authorized to issue up to 15 million shares of “blank check” Preferred Stock. As of December 31, 2021, there
were no shares of Preferred Stock outstanding. We have designated Series C Junior Participating Preferred Stock but have no
Common Stock
At December 31, 2021
and 2020, we had 300 141.2 140.4 46.2 million.
During the years ended December 31, 2021, 2020 and 2019, we issued 1.1 million, 1.7 million and 3.1 million shares of common stock, respectively, for stock option
exercises, vesting of restricted stock units, and ESPP purchases. We received net proceeds from these transactions of $11.6 million, $52.0 million and $119.7 million in 2021, 2020 and 2019, respectively.
Share Repurchase Program
In September 2019, our board of directors approved a share repurchase program of up to $125 million of our common stock. In 2019, we repurchased 535,000
shares for $34.4 million. In the first quarter of 2020, we repurchased an additional 1.5 million shares for $90.5 million.
Stock Plans
1989 Stock Option Plan
In June 1989, our Board of Directors adopted, and the stockholders subsequently approved, a stock option plan that, as
amended, provides for the issuance of non-qualified and incentive stock options for the purchase of up to 20.0 million shares of common stock to
our employees, directors, and consultants. The plan expires in January 2024. The 1989 Plan does not allow us to grant stock bonuses or restricted stock awards and prohibits us from repricing any options outstanding under the plan unless our
stockholders approve the repricing. Options vest over a four-year period, with 25 percent exercisable at the end of one year from the date of the grant and the
balance vesting ratably, on a monthly basis, thereafter and have a term of seven years . At December 31, 2021, a total of 28 thousand options were outstanding, of which
options to purchase 28 thousand shares were exercisable, and 49 thousand shares were available for future grant under the 1989 Plan.
2011 Equity Incentive Plan
In March 2011, our Board of Directors adopted, and the stockholders subsequently approved, a stock option plan that
provides for the issuance of stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, and performance cash awards to our employees, directors, and consultants. In June 2015, May 2017 and June 2019, after
receiving approval from our stockholders, we amended our 2011 Equity Incentive Plan, or 2011 Plan, to increase the total number of shares reserved for issuance. We
increased the shares available under our 2011 Equity Incentive Plan from 5.5 million to 11.0 million in June 2015, from 11.0 million to 16.0 million in May 2017 and from 16.0 million to 23.0 million in June 2019. In the second quarter of 2021, after receiving approval from our stockholders, we amended our 2011 Plan. The amendment increased the total number of shares of common stock authorized for issuance under the 2011 Plan from 23.0 million to 29.7 million and added a fungible share counting ratio whereby the share reserve will be reduced by 1.7 shares for each share of common stock issued pursuant to a full value award (i.e., RSU or PRSU) and increased by
1.7 shares for each share of common stock returning from a
full value award. The plan expires in June 2031. The 2011 Plan does not allow us to reduce the exercise price of any outstanding stock options or stock appreciation rights or cancel any outstanding stock options or stock appreciation rights
that have an exercise price or strike price greater than the current fair market value of the common stock in exchange for cash or other stock awards unless our stockholders approve such action. Currently we anticipate awarding only stock options, RSU
and PRSU awards to our employees, directors and consultants. Options vest over a four-year period, with 25 percent exercisable at the end of one year from the date of the grant
and the balance vesting ratably, on a monthly basis, thereafter and have a term of seven years . Options granted after December 31, 2021 have a
term of ten years . We have granted restricted stock unit awards to our employees under the 2011 Plan which vest annually over a four-year period. At December 31, 2021, a
total of 12.8 million options were outstanding, of which 8.3 million were exercisable, 2.5 million restricted stock unit awards were
outstanding, and 8.5 million shares were available for future grant under the 2011 Plan.
Under the 2011 Plan, we may issue a stock award with additional acceleration of vesting and exercisability upon or after a
change in control. In the absence of such provisions, no such acceleration will occur. The stock options and restricted stock unit awards we issued to Dr. Stanley T. Crooke in his former role as chief executive officer and certain stock options and
restricted stock unit awards we issued to B. Lynne Parshall in her former role as chief operating officer have accelerated vesting upon a change of control, as defined in the 2011 Plan. In addition, we implemented a change of control and severance
benefit plan that provides for change of control and severance benefits to our executive officers, including our chief executive officer and chief financial officer. If we terminate one of our executive officers or if an executive officer resigns for good reason during the period that begins three months before and ends twelve months following a change in control of the
company, the impacted executive officers’ stock options and RSUs vesting will accelerate for options and RSUs outstanding as of the termination date.
2020 Equity Incentive Plan
In connection with the Akcea Merger in October 2020, we
assumed the unallocated portion of the available share reserve under the Akcea 2015 Equity Incentive Plan. In December 2020, we amended and restated the Akcea 2015 equity plan, including renaming the plan as the Ionis Pharmaceuticals, Inc. 2020
Equity Incentive Plan, or 2020 Plan. The 2020 Plan provided for the issuance of up to 2.6 million shares of our Common Stock to our employees, directors and consultants who were employees of Akcea prior to the Akcea Merger. In the second quarter of 2021, our Compensation Committee approved an amendment to the 2020 Plan. The amendment decreased the total number of shares of common stock authorized for issuance under the 2020 Plan from
approximately 2.6 million to 1.6 million. We assumed the 2020 Plan in connection with Ionis’
reacquisition of all of the outstanding shares of Akcea Therapeutics, Inc. as part of the Akcea Merger.
The plan expires in December 2025. The 2020 Plan does not allow us to reduce the exercise price of any outstanding stock
options or stock appreciation rights or cancel any outstanding stock options or stock appreciation rights that have an exercise price or strike price greater than the current fair market value of the common stock in exchange for cash or other stock
awards unless our stockholders approve such action. Currently we anticipate awarding only stock options and RSU awards to our eligible employees, directors and consultants. Options vest over a four-year period, with 25 percent exercisable at the end of one year from the date of the grant and the balance vesting ratably, on a monthly basis, thereafter and have a term of seven years . Options granted after December 31, 2021 have a term of ten years .
We have granted restricted stock unit awards to our employees under the 2020 Plan which vest annually over a four-year period. At December 31, 2021, a total of 0.2 million
options were outstanding, of which none were exercisable, 0.1 million restricted stock unit awards were outstanding, and 1.3 million shares were
available for future grant under the 2020 Plan.
Under the 2020 Plan, we may issue a stock award with additional acceleration of vesting and exercisability upon or after a
change in control. In the absence of such provisions, no such acceleration will occur.
Corporate Transactions and Change in Control under 2011 and 2020 Plans
In the event of certain significant corporate transactions, our Board of Directors has the discretion to take one or more
of the following actions with respect to outstanding stock awards under the 2011 and 2020 Plans:
| ● |
arrange for assumption, continuation, or substitution of a stock award by a surviving or acquiring entity (or its parent company);
|
| ● |
arrange for the assignment of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award to the
surviving or acquiring corporation (or its parent company);
|
| ● |
accelerate the vesting and exercisability of a stock award followed by the termination of the stock award;
|
| ● |
arrange for the lapse of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award;
|
| ● |
cancel or arrange for the cancellation of a stock award, to the extent not vested or not exercised prior to the effective date of the corporate
transaction, in exchange for cash consideration, if any, as the Board, in its sole discretion, may consider appropriate; and
|
| ● |
arrange for the surrender of a stock award in exchange for a payment equal to the excess of (a) the value of the property the holder of the stock award
would have received upon the exercise of the stock award, over (b) any exercise price payable by such holder in connection with such exercise.
|
2002 Non-Employee Directors’ Stock Option Plan
In September 2001, our Board of Directors adopted, and the stockholders subsequently approved, an amendment and
restatement of the 1992 Non-Employee Directors’ Stock Option Plan, which provides for the issuance of non-qualified stock options and restricted stock units to our non-employee directors. The name of the resulting plan is the 2002 Non-Employee
Directors’ Stock Option Plan, or the 2002 Plan. In June 2015, after receiving approval from our stockholders, we amended our 2002 Plan to increase the total number of shares reserved for issuance from 1.2 million to 2.0 million. In June 2020, after receiving approval from our
stockholders, we further amended our 2002 Plan. The amendments included:
| ● |
An increase to the total number of shares reserved for issuance under the
plan from
|
| ● |
A reduction to the amount of the automatic awards under the plan;
|
| ● |
A revision to the vesting schedule of new awards granted; and
|
| ● |
An extension of the term of the plan.
|
Options under this plan expire 10 years from the date of grant. At December 31, 2021, a total of 1.0 million options were outstanding, of which 0.8
million were exercisable, 0.1 million restricted stock unit awards were outstanding, and 0.7 million shares were available for future grant under the 2002 Plan.
Employee Stock Purchase Plan
In June 2009, our Board of Directors adopted, and the stockholders subsequently approved, the amendment and restatement of
the ESPP and we reserved an additional 150,000 shares of common stock for issuance thereunder. In each of the subsequent years until 2019, we
reserved an additional 150,000 shares of common stock for the ESPP resulting in a total of 3.2 million shares authorized under the plan as of December 31, 2021. The ESPP
permits full-time employees to purchase common stock through payroll deductions (which cannot exceed 10 percent of each employee’s compensation)
at the lower of 85 percent of fair market value at the beginning of the purchase period or the end of each purchase period. Under the amended
and restated ESPP, employees must hold the stock they purchase for a minimum of six months from the date of purchase. During 2021, employees purchased and we issued to employees 0.07
million shares under the ESPP at a weighted average price of $39.26 per share. At December 31, 2021, there were 0.6 million shares available for purchase under the
ESPP.
Stock Option Activity
The following table summarizes the stock option activity under our stock plans for the year ended December 31, 2021 (in thousands, except per share and contractual life data):
|
Number
of Shares
|
Weighted
Average Exercise
Price Per Share
|
Average
Remaining
Contractual Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding at December 31, 2020
|
|
$
|
|
|||||||||||||
|
Granted
|
|
$
|
|
|||||||||||||
|
Exercised
|
(
|
)
|
$
|
|
||||||||||||
|
Cancelled/forfeited/expired
|
(
|
)
|
$
|
|
||||||||||||
|
Outstanding at December 31, 2021
|
|
$
|
|
|
$
|
|
||||||||||
|
Exercisable at December 31, 2021
|
|
$
|
|
|
$
|
|
||||||||||
The weighted-average estimated fair values of options granted were $24.35 , $29.43 and $28.76 for the years ended December 31, 2021, 2020 and 2019, respectively. The total intrinsic value of options
exercised during the years ended December 31, 2021, 2020 and 2019 were $2.5 million, $15.5 million and $83.8 million, respectively, which we determined as of the date of exercise. The amount of cash received from the exercise of stock options was $8.5 million, $43.7 million and $105.9 million for the years ended December 31, 2021, 2020 and 2019, respectively. For the year
ended December 31, 2021, the weighted-average fair value of options exercised was $50.13 . As of December 31, 2021, total unrecognized compensation cost related to
non-vested stock options was $49.6 million. We expect to recognize this cost over a weighted average period of 1.1 years. We will adjust the total unrecognized compensation cost for future changes in estimated forfeitures.
Restricted Stock Unit Activity
The following table summarizes the RSU activity for the year ended December 31, 2021 (in thousands, except per share data):
|
Number
of Shares
|
Weighted Average
Grant Date Fair
Value Per Share
|
|||||||
|
Non-vested at December 31, 2020
|
|
$
|
|
|||||
|
Granted
|
|
$
|
|
|||||
|
Vested
|
(
|
)
|
$
|
|
||||
|
Cancelled/forfeited
|
(
|
)
|
$
|
|
||||
|
Non-vested at December 31, 2021
|
|
$
|
|
|||||
For the years ended December 31, 2021, 2020 and 2019, the weighted-average grant date fair value of RSUs granted was $57.69 , $60.86 and $60.23 per RSU, respectively. As of
December 31, 2021, total unrecognized compensation cost related to RSUs was $57.0 million. We expect to recognize this cost over a weighted average period of 1.2
years. We will adjust the total unrecognized compensation cost for future changes in estimated forfeitures.
Stock-based Compensation Expense and Valuation Information
The following table summarizes stock-based compensation expense for the years ended December 31, 2021, 2020 and 2019 (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
Cost of sales
|
$
|
|
$
|
|
$
|
|
||||||
|
Research, development and patent
|
|
|
|
|||||||||
|
Selling, general and administrative
|
|
|
|
|||||||||
|
Total
|
$
|
|
$
|
|
$
|
|
||||||
In October 2020, as part of the Akcea Merger, Akcea’s outstanding equity awards vested under Akcea’s Plan. As a result, in
the fourth quarter of 2020, we recognized all unrecognized stock-based compensation ($59.3 million) under Akcea’s Plan. See Note 7, Akcea Merger, in the Notes to the Consolidated Financial Statements for further details.
In the third quarter of 2019, three Akcea executive officers terminated their employment and entered into
separation agreements with Akcea. As a result, in the third quarter of 2019, Akcea reversed $19.1 million of stock-based compensation expense it had previously recognized related to the executive officers’ stock options and RSUs that were no longer going to vest.
Determining Fair Value
Valuation. We
measure stock-based compensation expense for equity-classified awards, principally related to stock options, RSUs, PRSUs and stock purchase rights under the ESPP at the grant date, based on the estimated fair value of the award and we recognize the
expense over the employee’s requisite service period. We value RSUs based on the market price of our common stock on the date of grant. See Note 1, Organization
and Significant Accounting Policies, in the Notes to the Consolidated Financial Statements for further details on how we determine the fair value of PRSUs.
We use the Black-Scholes model to estimate the fair value of stock options granted and stock purchase rights under our
ESPP. The expected term of stock options granted represents the period of time that we expect them to be outstanding. We estimate the expected term of stock options granted based on actual and projected exercise patterns. We recognize compensation
expense for stock options granted, RSUs, PRSUs and stock purchase rights under the ESPP using the accelerated multiple-option approach. Under the accelerated multiple-option approach (also known as the graded-vesting method), we recognize compensation
expense over the requisite service period for each separately vesting tranche of the award as though the award were in substance multiple awards, which results in the expense being front-loaded over the vesting period.
For the years ended December 31, 2021, 2020 and 2019, we used the following weighted-average assumptions in our Black-Scholes calculations:
Ionis Employee Stock Options:
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
Risk-free interest rate
|
|
%
|
|
%
|
|
%
|
||||||
|
Dividend yield
|
|
%
|
|
%
|
|
%
|
||||||
|
Volatility
|
|
%
|
|
%
|
|
%
|
||||||
|
Expected life
|
|
|
|
|||||||||
Ionis Board of Director Stock Options:
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
Risk-free interest rate
|
|
%
|
|
%
|
|
%
|
||||||
|
Dividend yield
|
|
%
|
|
%
|
|
%
|
||||||
|
Volatility
|
|
%
|
|
%
|
|
%
|
||||||
|
Expected life
|
|
|
|
|||||||||
Ionis ESPP:
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
Risk-free interest rate
|
|
%
|
|
%
|
|
%
|
||||||
|
Dividend yield
|
|
%
|
|
%
|
|
%
|
||||||
|
Volatility
|
|
%
|
|
%
|
|
%
|
||||||
|
Expected life
|
|
|
|
|||||||||
Risk-Free Interest Rate.
We base the risk-free interest rate assumption on observed interest rates appropriate for the term of our stock option plans or ESPP.
Dividend Yield. We
base the dividend yield assumption on our history and expectation of dividend payouts. We have not paid dividends in the past and do not expect to in the future.
Volatility. We use
an average of the historical stock price volatility of our stock for the Black-Scholes model. We computed the historical stock volatility based on the expected term of the awards.
Expected Life. The
expected term of stock options we have granted represents the period of time that we expect them to be outstanding. We estimated the expected term of options we have granted based on actual and projected exercise patterns.
Forfeitures. We
reduce stock-based compensation expense for estimated forfeitures. We estimate forfeitures at the time of grant and revise, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We estimate forfeitures based on
historical experience.
5. Income Taxes
Income (loss) before income taxes is comprised of (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
(as revised*)
|
(as revised*)
|
|||||||||||
|
United States
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
|
Foreign
|
|
|
|
|||||||||
|
Income (loss) before income taxes
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
Our income tax expense (benefit) was as follows (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
(as revised*)
|
(as revised*)
|
|||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
|
State
|
(
|
)
|
|
|
||||||||
|
Foreign
|
|
|
|
|||||||||
|
Total current income tax expense (benefit)
|
(
|
)
|
|
|
||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
|
|
|
|||||||||
|
State
|
|
|
|
|||||||||
|
Total deferred income tax benefit
|
|
|
|
|||||||||
|
Total income tax expense (benefit)
|
$
|
(
|
)
|
$
|
|
$
|
|
|||||
Our expense (benefit) for income taxes differs from the amount computed by applying the U.S. federal
statutory rate to income (loss) before taxes. The sources and tax effects of the differences are as follows (in thousands):
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
2021
|
2020
|
2019
|
||||||||||||||||||||||
|
(as revised*)
|
(as revised*)
|
|||||||||||||||||||||||
|
Pre-tax income (loss)
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||||||||||||||
|
Statutory rate
|
(
|
)
|
|
%
|
(
|
)
|
|
%
|
|
|
%
|
|||||||||||||
|
State income tax net of federal benefit
|
|
(
|
)%
|
(
|
)
|
|
%
|
|
|
%
|
||||||||||||||
|
Foreign
|
|
(
|
)%
|
|
|
%
|
|
|
%
|
|||||||||||||||
|
Net change in valuation allowance
|
|
(
|
)%
|
|
(
|
)%
|
(
|
)
|
(
|
)%
|
||||||||||||||
|
Loss on debt transactions
|
|
(
|
)%
|
|
|
|
|
%
|
||||||||||||||||
|
Impact from outside basis differences
|
|
|
|
|
(
|
)
|
(
|
)%
|
||||||||||||||||
|
Tax credits
|
(
|
)
|
|
%
|
(
|
)
|
|
%
|
(
|
)
|
(
|
)%
|
||||||||||||
|
Deferred tax true-up
|
(
|
)
|
|
%
|
(
|
)
|
|
%
|
|
|
%
|
|||||||||||||
|
Tax rate change
|
|
(
|
)%
|
(
|
)
|
|
%
|
|
|
%
|
||||||||||||||
|
Non-deductible compensation
|
|
(
|
)%
|
|
(
|
)%
|
|
|
%
|
|||||||||||||||
|
Other non-deductible items
|
|
(
|
)%
|
|
(
|
)%
|
|
|
%
|
|||||||||||||||
|
Stock-based compensation
|
|
(
|
)%
|
|
(
|
)%
|
(
|
)
|
(
|
)%
|
||||||||||||||
|
Foreign-derived intangible income benefit
|
|
|
|
|
(
|
)
|
(
|
)%
|
||||||||||||||||
|
Impacts from Akcea Merger
|
|
|
(
|
)
|
|
%
|
|
|
||||||||||||||||
|
Other
|
(
|
)
|
|
%
|
(
|
)
|
|
%
|
(
|
)
|
(
|
)%
|
||||||||||||
|
Effective rate
|
$
|
(
|
)
|
|
%
|
$
|
|
(
|
)%
|
$
|
|
|
%
|
|||||||||||
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and
liabilities for financial reporting purposes and the amounts used for income tax purposes.
Significant components of our deferred tax assets and liabilities as of December 31, 2021 and 2020 are as follows (in thousands):
|
Year Ended December 31,
|
||||||||
|
2021
|
2020
|
|||||||
|
(as revised*)
|
||||||||
|
Deferred Tax Assets:
|
||||||||
|
Net operating loss carryovers
|
$
|
|
$
|
|
||||
|
Tax credits
|
|
|
||||||
|
Deferred revenue
|
|
|
||||||
|
Stock-based compensation
|
|
|
||||||
|
Intangible and capital assets
|
|
|
||||||
|
Convertible debt
|
|
|
||||||
|
Interest expense limitation
|
|
|
||||||
|
Other
|
|
|
||||||
|
Total deferred tax assets
|
$
|
|
$
|
|
||||
|
Deferred Tax Liabilities:
|
||||||||
|
Fixed assets
|
(
|
)
|
(
|
)
|
||||
|
Other
|
(
|
)
|
(
|
)
|
||||
|
Net deferred tax asset
|
$
|
|
$
|
|
||||
|
Valuation allowance
|
(
|
)
|
(
|
)
|
||||
|
Total net deferred tax assets and liabilities
|
$
|
|
$
|
|
||||
| * |
We revised our 2020 and 2019 amounts to reflect the simplified convertible instruments accounting guidance, which we adopted retrospectively. Refer to
Note 1, Organization and Significant Accounting Policies, for further information.
|
We evaluate our deferred tax assets regularly to determine whether adjustments to the valuation allowance are appropriate
due to changes in facts or circumstances, such as changes in expected future pre-tax earnings, tax law, interactions with taxing authorities and developments in case law. In making this evaluation, we rely on our recent history of pre-tax earnings. Our
material assumptions are our forecasts of future pre-tax earnings and the nature and timing of future deductions and income represented by the deferred tax assets and liabilities, all of which involve the exercise of significant judgment. Although we
believe our estimates are reasonable, we are required to use significant judgment in determining the appropriate amount of valuation allowance recorded against our deferred tax assets.
Ionis and Akcea filed separate U.S. federal income tax returns from the date of Akcea’s IPO in 2017 through October 12,
2020, the date on which we completed the Akcea Merger. As a result of the Akcea Merger, Ionis and Akcea now file a consolidated U.S. federal income tax return, and we now assess our U.S. federal and state valuation allowance requirements on a
consolidated basis.
We assessed our valuation allowance requirements and recorded a valuation allowance of $341 million against all of Ionis’ U.S. federal net deferred tax assets in the fourth quarter of 2020, due to uncertainties related to our ability to realize
the tax benefits associated with these assets. We based this determination largely on Akcea rejoining the Ionis consolidated U.S. federal tax group in the fourth quarter of 2020. Due to Akcea’s historical and projected financial statement losses, and
the expected negative impact this will have on Ionis’ consolidated taxable income, we are uncertain if we will generate sufficient consolidated pre-tax income in future periods to realize the Ionis deferred tax benefits. We also expect that Ionis’
pre-tax income in future periods will be lower due to significant investments in research and development associated with our pipeline of wholly owned medicines. We now maintain a valuation allowance against all our consolidated U.S. federal and state
net deferred tax assets.
Our valuation allowance increased by $39 million from December 31, 2020 to December 31, 2021. The increase was primarily related to increases in our deferred tax assets for tax credits and convertible debt offset against a decrease in our
deferred tax asset for deferred revenue.
At December 31, 2021,
we had federal and state, primarily California, tax net operating loss carryforwards of $271.5 million and $333.8 million, respectively. Our federal tax loss carryforwards are available indefinitely. Our California tax loss carryforwards will begin to expire in 2031. At December 31, 2021, we also had federal and California research and development tax credit carryforwards of $225.5 million and $99.7 million, respectively. Our federal research and development tax
credit carryforwards will begin to expire in 2034. Our California research and development tax credit carryforwards are available indefinitely.
Utilization of the net operating loss and tax credit carryforwards may be subject to an annual limitation due to the ownership change
limitations provided by the Internal Revenue Code of 1986, as amended, and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization.
We analyze filing positions in all U.S. federal, state and foreign jurisdictions where we file income tax returns, and all open tax years
in these jurisdictions to determine if we have any uncertain tax positions on any of our income tax returns. We recognize the impact of an uncertain tax position on an income tax return at the largest amount that the relevant taxing authority is
more-likely-than not to sustain upon audit. We do not recognize uncertain income tax positions if they have less than 50 percent likelihood of the applicable tax authority sustaining our position.
The following table summarizes our gross unrecognized tax benefits (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
Beginning balance of unrecognized tax benefits
|
$
|
|
$
|
|
$
|
|
||||||
|
Decrease for prior period tax positions
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Increase for prior period tax positions
|
|
|
|
|||||||||
|
Increase for current period tax positions
|
|
|
|
|||||||||
|
Ending balance of unrecognized tax benefits
|
$
|
|
$
|
|
$
|
|
||||||
Included in the balance of unrecognized tax benefits at December 31, 2021, 2020 and 2019 was $6.2 million, $6.4 million and $0.4 million respectively, that if we recognized, could impact our effective tax rate, subject to our remaining valuation allowance.
We do not foresee any material changes to our gross unrecognized tax benefits within the next twelve months.
We recognize interest and/or penalties related to income tax matters in income tax expense. During the year ended December
31, 2021 and 2020, we recognized $0.5
million and $0.3 million, respectively, of accrued interest and penalties related to gross unrecognized tax benefits. We did no t record any accrued interest and penalties for the years ended December 31, 2019.
We are subject to taxation in the U.S. and various state and foreign jurisdictions. Our tax years for 1999 through 2020
are subject to examination by the U.S. federal, state and foreign tax authorities.
We do not provide for a U.S. income tax
liability and foreign withholding taxes on undistributed foreign earnings of our foreign subsidiaries as we consider those earnings to be permanently reinvested. It is not practicable for us to calculate the amount of unrecognized deferred tax
liabilities associated with these earnings.
6. Collaborative Arrangements and Licensing Agreements
Strategic Partnership
Biogen
We have several strategic collaborations with Biogen focused on using antisense technology to advance the treatment of
neurological disorders. These collaborations combine our expertise in creating antisense medicines with Biogen’s expertise in developing therapies for neurological disorders. We developed and licensed to Biogen SPINRAZA, our approved medicine to treat
people with spinal muscular atrophy, or SMA. We and Biogen are currently developing nine investigational medicines to treat neurodegenerative
diseases under these collaborations, including medicines in development to treat people with ALS, SMA, AS, Alzheimer’s disease and Parkinson’s disease. In addition to these medicines, our collaborations with Biogen include a substantial
research pipeline that addresses a broad range of neurological diseases. From inception through December 2021, we have received more than $3.1 billion from our Biogen collaborations.
Spinal Muscular Atrophy Collaborations
SPINRAZA
In January 2012, we entered into a
collaboration agreement with Biogen to develop and commercialize SPINRAZA, an RNA-targeted therapy for the treatment of SMA. From inception through
December 2021, we earned more than $1.6
billion in total revenue under our SPINRAZA collaboration, including nearly $1.2 billion in revenue from SPINRAZA royalties and more than $435 million in R&D revenue. We are receiving tiered royalties ranging from 11 percent to 15 percent on net sales of SPINRAZA. We have exclusively in-licensed patents related to SPINRAZA from Cold Spring Harbor Laboratory and the University of Massachusetts. We pay Cold Spring Harbor
Laboratory and the University of Massachusetts a low single digit royalty on net sales of SPINRAZA. Biogen is responsible for all global development, regulatory and commercialization activities and costs for SPINRAZA. We completed our performance
obligations under our collaboration in 2016.
New antisense medicines for the treatment of SMA
In December 2017, we entered into a
collaboration agreement with Biogen to identify new antisense medicines for the treatment of SMA. Biogen has the option to license therapies arising out of
this collaboration following the completion of preclinical studies. Upon licensing, Biogen will be responsible for global development, regulatory and
commercialization activities and costs for such therapies. Under the collaboration agreement, we received a $25 million upfront payment in the fourth quarter of 2017. In December 2021, we earned a $60 million license fee payment when Biogen exercised its option to license ION306. We will receive development and regulatory milestone payments from Biogen if new medicines advance towards marketing approval. In total over the term of our collaboration, we are eligible to receive up to $1.2 billion in license fees, milestone payments and other payments, including up to $555 million in payments if Biogen advances ION306, which includes up to $45 million for the achievement of development milestones, up to $110 million for the achievement of regulatory milestones and up
to $400 million for the achievement of
sales milestones. In addition, we are eligible to receive tiered royalties from the mid-teens to mid-20 percent range on net sales. We will achieve the next payment of up to $45 millionfor the initiation of a Phase 3 trial under this collaboration.
At the commencement of this collaboration, we identified one performance obligation, which was to perform R&D services for Biogen. We determined the transaction price to be the $25 million upfront payment we received when we entered into the collaboration. We allocated the transaction price to our single performance obligation. In the fourth quarter of 2019, we
completed our R&D services performance obligation under this collaboration. We recognized revenue as we performed services based on our effort to satisfy our performance obligation relative to the total effort expected to satisfy our performance
obligation. We completed our performance obligation earlier than we previously estimated, as a result, we recognized $8.3 million of additional
revenue in the fourth quarter of 2019.
In the fourth quarter of 2021, we identified another performance obligation upon Biogen’s license of ION306 because the license we granted
to Biogen is distinct from our other performance obligations. We recognized the $60 million license fee for ION306 as revenue at that time
because Biogen had full use of the license without any continuing involvement from us. Additionally, we did not have any further performance obligations related to the license after we delivered it to Biogen. Biogen is solely responsible for the costs
and expenses related to the development, manufacturing and potential future commercialization of ION306 following the option exercise. We do not have any remaining performance obligations under this collaboration.
Neurology Collaborations
2018 Strategic Neurology
In April 2018, we and Biogen entered into a
strategic collaboration to develop novel antisense medicines for a broad range of neurological diseases and entered into a SPA. As part of the collaboration, Biogen gained exclusive rights to the use of our antisense technology to develop therapies
for these diseases for 10 years. We are
responsible for the identification of antisense drug candidates based on selected medicines. Biogen is responsible for conducting IND-enabling toxicology studies for the selected medicine. Biogen will have the option to license the selected medicine after it completes the IND-enabling toxicology study. If Biogen exercises its option to license a medicine, it will assume global development, regulatory and commercialization responsibilities and costs for that medicine.
In the second quarter of 2018, we received $1 billion from Biogen, comprised of $625 million
to purchase our stock at an approximately 25 percent cash premium and $375 million in an upfront payment. We are eligible to receive up to $270 million in milestone payments for each medicine
that achieves marketing approval. In addition, we are eligible to receive tiered royalties up to the 20 percent range on net sales. We are currently advancing nine
programs under this collaboration and from inception through December 2021, we have received nearly $1.1 billion in payments under this
collaboration. We will achieve the next payment of $7.5 million if Biogen designates or advances another programunder this collaboration.
At the commencement of this collaboration, we
identified one performance obligation, which was to perform R&D services for Biogen. We determined our transaction price to be $552 million, comprised of $375 million from the upfront payment and $177 million for the premium paid by Biogen for its purchase of our common stock. We determined the fair value of the premium we received by using the stated premium in the SPA and applying a lack of marketability
discount. We included a lack of marketability discount in our valuation of the premium because Biogen received restricted shares of our common stock. We allocated the transaction price to our single performance obligation.
From inception through December 2021, we have included $616 million in payments in the transaction price for our R&D services performance obligation
under this collaboration, including $23
million of milestone payments we achieved in 2021 and $11 million of milestone payments we achieved in 2020. These milestone payments did not create new performance obligations because they are part of our original R&D services performance obligation. Therefore, we included these
amounts in our transaction price for our R&D services performance obligation in the period we achieved the milestone payment. We are recognizing revenue for our R&D services performance obligation as we perform services based on our effort to
satisfy our performance obligation relative to our total effort expected to satisfy our performance obligation. We currently estimate we will satisfy our performance obligation at the end of the contractual term in June 2028.
2013 Strategic Neurology
In September 2013, we and Biogen entered into a long-term strategic relationship focused on applying antisense technology
to advance the treatment of neurodegenerative diseases. As part of the collaboration, Biogen gained exclusive rights to the use of our antisense technology to develop therapies for neurological diseases and has the option to license medicines resulting
from this collaboration. We will usually be responsible for drug discovery and early development of antisense medicines and Biogen has the option to license antisense medicines after Phase 2 proof-of-concept. In October 2016, we expanded our
collaboration to include additional research activities we will perform. If Biogen exercises its option to license a medicine, it will assume global development, regulatory and commercialization responsibilities and costs for that medicine. We are
currently advancing six investigational medicines in development under this collaboration, including medicine for Parkinson’s disease (ION859), three medicines for ALS
(tofersen, IONIS-C9Rx and ION541), medicine for multiple system atrophy (ION464) and medicine for an undisclosed target. In the fourth quarter of 2018, Biogen exercised its option to license our most advanced ALS medicine, tofersen, our medicine in Phase 3
development for SOD1 ALS. As a result, Biogen is now responsible for global development, regulatory and commercialization activities and costs for tofersen.
Under the terms of the agreement, we received an upfront payment of $100 million and are eligible to receive milestone payments, license fees and royalty payments for all medicines developed under this collaboration, with the specific amounts dependent upon the
modality of the molecule advanced by Biogen. For each antisense molecule that is chosen for drug discovery and development under this collaboration, we are eligible to receive up to approximately $260 million in a license fee and milestone payments per program. The $260 million per
program consists of approximately $60 million in development milestones, including amounts related to the cost of clinical trials, and up to $130 million in milestone payments if Biogen achieves pre-specified regulatory milestones. In addition, we are eligible to receive tiered royalties up to the
mid-teens on net sales from any antisense medicines developed under this collaboration. From inception through December 2021, we have received over $280
million in upfront fees, milestone payments and other payments under this collaboration. We will achieve the next payment of up to $70 million if
Biogen licenses a medicine under this collaboration.
At the commencement of our 2013 strategic neurology collaboration, we identified one performance obligation, which was to perform R&D services for Biogen. At inception, we determined the transaction price to be the $100 million upfront payment we received and allocated it to our single performance obligation. As we achieve milestone payments for our R&D services, we
include these amounts in our transaction price for our R&D services performance obligation. We recognized revenue for our R&D services performance obligation based on our effort to satisfy our performance obligation relative to our total effort
expected to satisfy our performance obligation. In the third quarter of 2019, we updated our estimate of the total effort we expect to expend to
satisfy our performance obligation. As a result, we recorded a cumulative catch up adjustment of $16.5 million to decrease revenue in the third
quarter of 2019. During 2020, we completed our remaining research and development services and recognized the remaining revenue related to this performance
obligation. From inception through the completion of our R&D services performance obligation in 2020, we included $145 million in
total payments in the transaction price for our R&D services performance obligation.
Under this collaboration, we have also
generated additional payments that we concluded were not part of our R&D services performance obligation. We recognized each of these payments in full in the respective quarter we generated the payment because we did not have any performance
obligations for the respective payment. For example, in the second quarter of 2021, we earned a $10 million milestone payment when Biogen advanced ION541, which we recognized in full because we did not have any performance obligations related to this milestone payment.
2012 Neurology
In December 2012, we and Biogen entered into a collaboration agreement to develop and commercialize novel antisense
medicines to treat neurodegenerative diseases. We are responsible for the development of each of the medicines through the completion of the initial Phase 2 clinical study for such medicine. Biogen has the option to license a medicine from each of the
programs through the completion of the first Phase 2 study for each program. Under this collaboration, we are currently advancing IONIS-MAPTRx for Alzheimer’s disease and ION582 for AS. If Biogen exercises its option to license a medicine,
it will assume global development, regulatory and commercialization responsibilities and costs for that medicine. In the fourth quarter of 2019, Biogen exercised its option to license IONIS-MAPTRx. We are responsible for completing the Phase
1/2 in study patients with mild AD and a one-year long-term extension study. Biogen will have responsibility for global development, regulatory
and commercialization activities and costs for IONIS-MAPTRx.
Under the terms of the agreement, we received
an upfront payment of $30 million. Over the
term of the collaboration, we are eligible to receive up to $210 million in a license fee and milestone payments per program, plus a mark-up on the cost estimate of the Phase 1 and 2 studies. The $210 million per program consists of up to $10 million in development milestone payments, plus a mark-up on the cost estimate of the Phase 1 and
2 studies and up to $130 million in
milestone payments if Biogen achieves pre-specified regulatory milestones. In addition, we are eligible to receive tiered royalties up to the mid-teens on net sales of any medicines resulting from each of the three programs. From inception through December 2021, we have
received $155 million in payments under
this collaboration, including $19.5 million
we received from Biogen for achieving milestones for advancing IONIS-MAPTRx during 2020. We will
achieve the next payment of $25 million
if Biogen advances a medicine under this collaboration.
Under our collaboration, we determined we had a performance obligation to perform R&D services. We allocated $40 million in total payments to the transaction price for our R&D services performance obligation. In the third quarter of 2019, we completed our
R&D services performance obligation when we designated a development candidate and Biogen accepted the development candidate. Biogen’s decision to accept the development candidate was not within our control. We were recognizing revenue as we
performed services based on our effort to satisfy our performance obligation relative to the total effort expected to satisfy our performance obligation. Because Biogen accepted the development candidate earlier than when we were previously estimating,
we recognized $6.3 million of accelerated revenue in the third quarter of 2019.
When we commenced development for IONIS-MAPTRx we identified our development work as a separate performance obligation. We are
recognizing our IONIS-MAPTRx development performance obligation based on the percentage of completion. From inception through December 2021, we have included $57 million in the transaction price for our IONIS-MAPTRx development performance obligation, including $19.5 million milestone payments we earned from Biogen in 2020 when we advanced IONIS-MAPTRx. We currently estimate we will satisfy our performance obligation in 2022.
In the fourth quarter of 2019, we identified another performance obligation upon Biogen’s license of IONIS-MAPTRx because the
license we granted to Biogen is distinct from our other performance obligations. We recognized the $45 million license fee for IONIS-MAPTRx
as revenue at that time because Biogen had full use of the license without any continuing involvement from us. Additionally, we did not have any further performance obligations related to the license after we delivered it to Biogen.
In the fourth quarter of 2021, we earned a $10 million milestone payment when Biogen advanced ION582, which
we recognized in full because we did not have any performance obligations related to this milestone payment.
During the years ended December 31, 2021, 2020 and 2019, we earned the following revenue from our relationship with Biogen (in millions, except percentage amounts):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
SPINRAZA royalties (commercial revenue)
|
$
|
|
$
|
|
$
|
|
||||||
|
R&D revenue
|
|
|
|
|||||||||
|
Total revenue from our relationship with Biogen
|
$
|
|
$
|
|
$
|
|
||||||
|
Percentage of total revenue
|
|
%
|
|
%
|
|
%
|
||||||
Our consolidated balance sheet at
December 31, 2021 and 2020 included deferred revenue of $407.5 million and $465.8 million, respectively, related to our relationship with Biogen.
Joint Development and Commercialization Arrangement
AstraZeneca
Eplontersen Collaboration
In December 2021, we entered into a joint development and commercialization agreement with AstraZeneca to develop and
commercialize eplontersen for the treatment of ATTR. We are jointly developing and preparing to commercialize eplontersen with AstraZeneca in the U.S. We granted AstraZeneca exclusive rights to commercialize eplontersen outside the U.S., except certain
countries in Latin America. Under the terms of the agreement, we received a $200 million upfront payment. We are eligible to receive up to $485 million in development and approval milestones, and up to $2.9
billion in sales-related milestone payments. The agreement also includes territory-specific development, commercial and medical affairs cost-sharing provisions. In addition, we are eligible to receive up to mid-20 percent royalties for sales in the U.S. and tiered royalties up to the high teens for sales outside the U.S.
We evaluated our eplontersen collaboration under ASC 808 and identified four material components: (i) the license we granted to AstraZeneca in 2021, (ii) the co-development activities that we and AstraZeneca will perform, (iii) the co-commercialization
activities that we and AstraZeneca will perform and (iv) the co-medical affairs activities that we and AstraZeneca will perform.
We determined that we had a vendor-customer relationship within the scope of ASC 606 for the license we granted to
AstraZeneca and as a result we had one performance obligation. For our sole performance obligation, we determined the transaction price was the
$200 million upfront payment we received. We recognized the upfront payment in full in 2021 because we did not have any remaining performance
obligations after we delivered the license to AstraZeneca.
We also concluded that the co-development activities, the co-commercialization activities and the co-medical affairs
activities are within the scope of ASC 808 because we and AstraZeneca are active participants exposed to the risks and benefits of the activities under the collaboration. AstraZeneca will pay 55 percent of the costs associated with the ongoing global Phase 3 development program. As we will continue to lead the Phase 3 development program, we will recognize as revenue the 55 percent of cost-share funding AstraZeneca is responsible for in the same period we incur the related development expenses. As AstraZeneca is responsible
for the majority of the commercial and medical affairs costs in the U.S. and all costs associated with bringing eplontersen to market outside the U.S., we will recognize cost-share funding we receive from AstraZeneca related to these activities as a
reduction of our commercial and medical affairs expenses.
We will achieve the next payment of up to $50 million upon the first regulatory approval under this collaboration. Through December 2021, we have generated $200 million in payments under this collaboration.
Research and Development Partners
AstraZeneca
In addition to our collaboration for eplontersen, we have two other collaborations with AstraZeneca. One is focused on the treatment of
cardiovascular, renal and metabolic diseases and the is focused on the treatment of oncology diseases. We and AstraZeneca are currently
developing six medicines under these collaborations, including medicines in development to treat people with ATTR amyloidosis, cardiovascular
disease, a genetically associated form of kidney disease, NASH and cancer. From inception through December 2021, we have received more than $386 million from our AstraZeneca research and
development collaborations.
Cardiovascular, Renal and Metabolic Diseases Collaboration
In July 2015, we and AstraZeneca formed a collaboration to discover and develop antisense therapies for treating
cardiovascular, renal and metabolic diseases. Under our collaboration, AstraZeneca has licensed five medicines from us. AstraZeneca is
responsible for global development, regulatory and commercialization activities and costs for each of the medicines it has licensed from us.
Under the terms of the agreement, we received a $65 million upfront payment. We are eligible to receive license fees and milestone payments of up to more than $5.5 billion as medicines under this collaboration advance, including up to $1.1 billion
for the achievement of development milestones, up to $2.9 billion for regulatory milestones and up to $1.5 billion for commercial milestones. In addition, we are eligible to receive tiered royalties up to the low teens on net sales from any product that AstraZeneca successfully
commercializes under this collaboration agreement. We will achieve the next payment of $10 million under this collaboration if AstraZeneca
advances a medicine under this collaboration. From inception through December 2021, we have received over $282 million in an upfront fee, license fees, milestone payments, and other payments under this collaboration, including $30 million we earned in 2021 when AstraZeneca licensed a target for a metabolic disease and $10
million we earned in 2021 when AstraZeneca advanced a target for a metabolic disease.
At the commencement of this collaboration, we identified one performance obligation, which was to perform R&D services for AstraZeneca. We determined the transaction price to be the $65 million upfront payment we received and we allocated it to our single performance obligation. We recognized revenue for our R&D services performance obligation as we performed services
based on our effort to satisfy this performance obligation relative to our total effort expected to satisfy our performance obligation. We completed our
performance obligation in the fourth quarter of 2021. As we achieve milestone payments for our R&D services, we include these amounts in our transaction price for our R&D services performance obligation. From inception through December 2021, we have included $90 million in payments
in the transaction price for our R&D services performance obligation.
Under this collaboration, we have also generated additional payments that we concluded were not part of our R&D
services performance obligation. We recognized each of these payments in full in the respective quarter we generated the payment because the payments were distinct and we did not have any performance obligations for the respective payment. For example,
in the fourth quarter of 2021, we earned a $30 million license fee when AstraZeneca licensed a target for a metabolic disease. We recognized the
license fee as revenue at that time because AstraZeneca had full use of the license without any continuing involvement from us. Additionally, we did not have any further performance obligations related to the license after we delivered it to
AstraZeneca.
Oncology Collaboration
In December 2012, we entered into a collaboration agreement with AstraZeneca to discover and develop antisense medicines
to treat cancer. We and AstraZeneca also established an oncology research program. In 2020, AstraZeneca licensed ION736, an investigational medicine in development targeting FOXP3 for the treatment of cancer. AstraZeneca is responsible for global
development, regulatory and commercialization activities and costs for ION736.
Under the terms of this agreement, we
received $31 million in upfront payments.
We are eligible to receive milestone payments and license fees up to $160 million under this collaboration, including up to $42 million for the achievement of development milestones and up to $105 million for the achievement of regulatory milestones. In addition, we are eligible to receive tiered royalties up to the low teens on net sales from any product that AstraZeneca
successfully commercializes under this collaboration agreement. From inception through December 2021, we have received over $141 million in upfront fees, milestone payments, and other payments under this oncology collaboration, including $13 million we earned when AstraZeneca licensed ION736 in 2020. We will achieve the next payment of $12 million if AstraZeneca advances ION736 in development.
We completed all of the performance obligations we identified under this collaboration in the first quarter of 2018.
Under this collaboration, we have also
generated additional payments that we concluded were not part of other performance obligations discussed above. We recognized each of these payments in full in the respective quarter we generated the payment because the payments were distinct and we
did not have any performance obligations for the respective payment. In 2020, we earned a $13 million license fee when AstraZeneca licensed ION736 because AstraZeneca had full use of the license without any continuing involvement from us.
During the years ended December 31, 2021, 2020 and 2019, we earned the following revenue from our relationship with AstraZeneca (in millions, except percentage amounts):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
R&D revenue
|
$
|
|
$
|
|
$
|
|
||||||
|
Percentage of total revenue
|
|
%
|
|
%
|
|
%
|
||||||
We did no t have any deferred revenue from our relationship with
AstraZeneca at December 31, 2021. Our consolidated balance sheet at December 31, 2020 included deferred revenue of $10.0 million from our relationship with AstraZeneca.
Bayer
In May 2015, we entered into an exclusive license agreement with Bayer to develop and commercialize IONIS-FXIRx
for the prevention of thrombosis. We were responsible for completing a Phase 2 study of IONIS-FXIRx in people with end-stage renal disease on hemodialysis. Under the terms of the agreement, we received a $100 million upfront payment in the second quarter of 2015. In February 2017, we amended our agreement with Bayer to advance IONIS-FXIRx and to
initiate development of fesomersen, which Bayer licensed. In conjunction with the decision to advance these programs, we received a $75 million
payment from Bayer. In October 2019, Bayer decided it would advance fesomersen following positive clinical results. Bayer is now responsible for all global development, regulatory and commercialization activities and costs for the FXI program.
We are eligible to receive up to $385 million in license fees, milestone payments and other payments, including up to $125
million for the achievement of development milestones and up to $110 million for the achievement of sales milestones. In addition, we are
eligible to receive tiered royalties in the low to high 20 percent range on gross margins of both medicines combined. From inception through
December 2021, we have received over $191 million from this collaboration. We will achieve the next payment of $20 million if Bayer initiates a Phase 3 study for the FXI program.
At the commencement of this collaboration, we identified three performance obligations, the license of IONIS-FXIRx, R&D services and delivery of API, all of which we completed in 2016.
In February 2017, when we amended our collaboration with Bayer, we identified two new performance obligations, one for the license of fesomersen and one for R&D services. We determined the transaction price to be the $75 million payment. We allocated $64.9 million to
the license of fesomersen based on its estimated relative stand-alone selling price and recognized the associated revenue upon our delivery of the license in
the first quarter of 2017. We allocated $10.1 million to our R&D services performance obligation based on an estimated relative stand-alone selling price. We recognized revenue for our R&D services performance obligation as we performed services based on our effort to
satisfy our performance obligation relative to our total effort expected to satisfy our performance obligation. We completed our obligation in the third quarter of 2019.
In the fourth quarter of 2019, we earned a $10 million milestone payment when Bayer decided it would advance fesomersen. We recognized this milestone payment in full in the fourth quarter of 2019 because we did not have any performance
obligations related to this milestone payment.
During the years ended December 31, 2021, 2020 and 2019, we earned the following revenue from our relationship with Bayer (in millions, except percentage amounts):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
R&D revenue
|
$
|
|
$
|
|
$
|
|
||||||
|
Percentage of total revenue
|
|
%
|
|
%
|
|
%
|
||||||
Our consolidated balance sheet at December 31, 2021 included an amount of deferred revenue related to our relationship with Bayer. We did no t
have any deferred revenue from our relationship with Bayer at December 31, 2020.
GSK
In March 2010, we entered into an alliance with GSK using our antisense drug discovery platform to discover and develop
new medicines against targets for serious and rare diseases, including infectious diseases and some conditions causing blindness. Under the terms of the agreement, we received upfront payments of $35 million. Our collaboration with GSK currently includes two medicines targeting
hepatitis B virus, or HBV: bepirovirsen and IONIS-HBV-LRx. We designed these medicines to reduce the production of viral proteins associated with HBV infection. In the third quarter of 2019, following positive Phase 2 results, GSK licensed
our HBV program. GSK is responsible for all global development, regulatory and commercialization activities and costs for the HBV program.
Under our agreement, if GSK successfully develops these medicines and achieves pre-agreed sales targets, we could receive
license fees and milestone payments of more than $260 million, including up to $47.5 million for the achievement of development milestones, up to $120 million for the
achievement of regulatory milestones and up to $70 million for the achievement of sales milestones. In addition, we are eligible to receive
tiered royalties up to the mid-teens on net sales from any product that GSK successfully commercializes under this alliance. From inception through December 2021, we have received more than $190 million in payments under this alliance with GSK. We will achieve the next payment of $15
million if GSK initiates a Phase 3 study of a medicine under this program.
We completed our R&D services performance
obligations under our collaboration in the first quarter of 2015. We identified a new performance obligation when we granted GSK the license of the HBV program and assigned related intellectual property rights in the third quarter of 2019
because the license was distinct from our other performance obligations. We recognized the $25 million license fee for the HBV program as revenue
at that time because GSK had full use of the license without any continuing involvement from us. Additionally, we did not have any further performance obligations related to the license after we delivered it to GSK.
We do not have any remaining performance obligations under our collaboration with GSK; however, we can still earn additional payments and
royalties as GSK advances the HBV program.
During the years ended December 31, 2021, 2020 and 2019, we earned the following revenue from our relationship with GSK (in millions, except percentage amounts):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
R&D revenue
|
$
|
|
$
|
|
$
|
|
||||||
|
Percentage of total revenue
|
|
|
%
|
|
%
|
|||||||
We did no
Novartis
In January 2017, we initiated a collaboration
with Novartis to develop and commercialize pelacarsen and olezarsen. We received a $75 million
upfront payment in the first quarter of 2017. In the first quarter of 2019, Novartis licensed pelacarsen and we earned a $150 million license fee. Novartis is responsible for conducting and funding future development and regulatory activities for pelacarsen, including a global
Phase 3 cardiovascular outcomes study that Novartis initiated in the fourth quarter 2019. In connection with Novartis’ license of pelacarsen, we and
Novartis established a more definitive framework under which the companies would negotiate the co-commercialization of pelacarsen in selected markets. Included in this framework is an option by which Novartis could solely commercialize pelacarsen in
exchange for Novartis paying us increased sales milestone payments based on sales of pelacarsen. When Novartis decided to not exercise its option for olezarsen,
we retained rights to develop and commercialize olezarsen.
Under the collaboration, we are eligible to receive up to $675 million in milestone payments, including $25 million for the achievement of a
development milestone, up to $290 million for the achievement of regulatory milestones and up to $360 million for the achievement of sales milestones. From inception through December
2021, we have received nearly $425 million
in upfront payments, milestone payments, license fees and other payments from this collaboration. We are also eligible to receive tiered royalties in the mid-teens to low 20 percent range on net sales of pelacarsen. In August 2021, we earned a $25 million
milestone payment from Novartis when Novartis achieved 50 percent enrollment in the Lp(a) HORIZON Phase 3 cardiovascular outcome study of
pelacarsen. We recognized the milestone payment in full in the third quarter of 2021 because we did not have any remaining performance obligations related to the milestone payment. We will achieve the next payment of up to $75 million if Novartis advances regulatory activities for
pelacarsen.
In conjunction with this collaboration, we entered into a SPA with Novartis. As part of the SPA, Novartis purchased 1.6 million shares of our common stock for $100
million in the first quarter of 2017.
At the commencement of this collaboration, we identified four separate performance obligations:
| ● |
R&D services for pelacarsen;
|
| ● |
R&D services for olezarsen;
|
| ● |
API for pelacarsen; and
|
| ● |
API for olezarsen.
|
We determined that the R&D services for each medicine and the API for each medicine were distinct performance obligations.
We determined our transaction price to be $108.4
million, comprised of the following:
| ● |
$
|
| ● |
$
|
| ● |
$
|
We allocated the transaction price based on the estimated stand-alone selling price of each performance obligation as follows:
| ● |
$
|
| ● |
$
|
| ● |
$
|
| ● |
$
|
We completed our R&D services performance obligations for olezarsen and pelacarsen in 2019. As such, we recognized all
revenue we allocated to the olezarsen and pelacarsen R&D services as of the end of 2019.
We recognized revenue related to the R&D
services for pelacarsen and olezarsen performance obligations as we performed services based on our effort to satisfy our performance obligations relative to our total effort expected to satisfy our performance obligations.
During the years ended December 31, 2021, 2020 and 2019, we earned the following revenue from our
relationship with Novartis (in millions, except percentage amounts):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
R&D revenue
|
$
|
|
$
|
|
$
|
|
||||||
|
Percentage of total revenue
|
|
%
|
|
%
|
|
%
|
||||||
We did no
Roche
Huntington’s Disease
In April 2013, we formed an alliance with Hoffman-La Roche Inc. and F. Hoffmann-La Roche Ltd., collectively Roche, to
develop treatments for HD based on our antisense technology. Under the agreement, we discovered and developed tominersen, an investigational medicine targeting HTT protein. We developed tominersen through completion of our Phase 1/2 clinical study in
people with early stage HD. In the fourth quarter of 2017, upon completion of the Phase 1/2 study, Roche exercised its option to license tominersen. Roche is responsible for all global development, regulatory and commercialization activities and
costs for tominersen.
Under the terms of the agreement, we received an upfront payment of $30 million in April 2013 and an additional $3 million payment in 2017. We are eligible
to receive up to $365 million in a license fee and milestone payments including up to $70 million for the achievement of development milestones, up to $170 million for the
achievement of regulatory milestones and up to $80 million for the achievement of sales milestones. In addition, we are eligible to receive up to
$136.5 million in milestone payments for each additional medicine successfully developed. We are also eligible to receive tiered royalties up to
the mid-teens on net sales of any product resulting from this alliance. From inception through December 2021, we have received $150 million in upfront fees, milestone payments and license fees under this collaboration. We will achieve the next payment of $15 million if Roche advances tominersen into registration.
At the commencement of this collaboration, we identified one performance obligation, which was to perform R&D services for Roche. We determined the transaction price to be the $30 million upfront payment we received and allocated it to our single performance obligation. As we achieved milestone payments for our R&D services, we included these amounts in our
transaction price for our R&D services performance obligation. We recognized revenue for our R&D services performance obligation over our period of performance, which ended in the third quarter of 2017.
Under this collaboration, we have also generated additional payments that we concluded were not part of our R&D
services performance obligation. We recognized each of these payments in full in the respective quarter in which we generated the payment because the payments were distinct and we did not have any performance obligations for the respective payment. In
2019, we earned $35 million in milestone payments when Roche dosed the first patient in the Phase 3 study of tominersen.
In January 2022, Roche announced it is
actively preparing to initiate a new Phase 2 study of tominersen in patients with HD. Post-hoc analyses from the GENERATION HD1 study suggested tominersen may benefit younger adult patients with lower disease burden. In March 2021, Roche
decided to discontinue dosing in the Phase 3 GENERATION HD1 study of tominersen in patients with manifest HD based on the results of a pre-planned review of data from the Phase 3 study conducted by an unblinded iDMC. We do not have any remaining
performance obligations related to tominersen under this collaboration with Roche; however, we can still earn additional payments and royalties as Roche advances tominersen.
IONIS-FB-LRx for Complement-Mediated Diseases
In October 2018, we entered into a
collaboration agreement with Roche to develop IONIS-FB-LRx for the treatment of complement-mediated diseases. We are currently conducting Phase 2 studies in two disease indications for IONIS-FB-LRx, one for the treatment of patients with GA, the advanced stage
of dry AMD, and a for the treatment
of patients with IgA nephropathy. Roche has the option to license IONIS-FB-LRx at the completion of these studies. Upon licensing, Roche will be responsible for global development, regulatory and commercialization activities and costs.
Under the terms of
this agreement, we received a $75 million upfront payment in the fourth quarter of 2018. We are eligible to receive more than $680 million in development, regulatory and sales milestone payments and license fees. In addition, we are also eligible to receive tiered royalties
from the high teens to 20 percent on net sales. From inception through December 2021, we have received $75 million in upfront fees, milestone payments and license fees under this collaboration. We will achieve the next payment of $20 million if we further advance the Phase 2 study in patients with dry AMD.
At the commencement of this collaboration, we identified one performance obligation, which was to perform R&D services for Roche. We determined the transaction price to be the $75 million upfront payment we received and allocated it to our single performance obligation. We are recognizing revenue for our R&D services performance obligation as we perform services
based on our effort to satisfy our performance obligation relative to our total effort expected to satisfy our performance obligation. During the fourth quarter of 2020, we updated our estimate of the total effort we expected to expend to satisfy our performance obligation under this collaboration. In the
fourth quarter of 2020, we recorded a cumulative catch up adjustment of $9.2 million to decrease revenue because we updated our total cost estimate to complete the Phase 2 study of IONIS-FB-LRx for
the treatment of patients with GA. We currently
estimate we will satisfy our performance obligation in the fourth quarter of 2023.
During the years ended December 31, 2021, 2020 and 2019, we earned the following revenue from our relationship with Roche (in millions, except percentage amounts):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
R&D revenue
|
$
|
|
$
|
|
$
|
|
||||||
|
Percentage of total revenue
|
|
%
|
|
%
|
|
%
|
||||||
Our consolidated balance sheet at December 31, 2021 and 2020 included deferred revenue of $31.6 million and $47.2
million related to our relationship with Roche, respectively.
Commercialization Partnerships
Swedish Orphan Biovitrum AB (Sobi)
We began commercializing TEGSEDI and WAYLIVRA in Europe in January 2021 and TEGSEDI in North America in April 2021 through
distribution agreements with Sobi. Under our agreements, we are responsible for supplying finished goods inventory to Sobi and Sobi is responsible for selling each medicine to the end customer. In exchange, we earn a distribution fee on net sales from
Sobi for each medicine.
PTC Therapeutics
In August 2018, we entered into an exclusive
license agreement with PTC Therapeutics to commercialize TEGSEDI and WAYLIVRA in Latin America and certain Caribbean countries. Under the license agreement, we are eligible to receive royalties from PTC in the mid-20 percent range on net sales for each medicine. In
December 2021, we started receiving royalties from PTC for TEGSEDI sales.
Technology Enhancement Collaboration
Bicycle License Agreement
In December 2020, we entered into a collaboration agreement with Bicycle and obtained an option to license its peptide technology to
potentially increase the delivery capabilities of our LICA medicines. In July 2021, we paid $42 million when we exercised our option to license
Bicycle’s technology, which included an equity investment in Bicycle. As part of our stock purchase, we entered into a lockup agreement with Bicycle that restricts our ability to trade our Bicycle shares for one year . In 2021, we recorded a $7.2 million equity investment for the
shares we received in Bicycle. We recognized the remaining $34.8 million as R&D expense in 2021. From inception through December 31, 2021, we
have paid Bicycle $46.6 million under this collaboration agreement.
Other Agreements
Alnylam Pharmaceuticals, Inc.
Under the terms of our agreement with Alnylam, we co-exclusively (with ourselves) licensed to Alnylam our patent estate
relating to antisense motifs and mechanisms and oligonucleotide chemistry for double-stranded RNAi therapeutics, with Alynylam having the exclusive right to grant platform sublicenses for double-stranded RNAi. In exchange for such rights, Alynylam
gave us a technology access fee, participation in fees from Alnylam’s partnering programs, as well as future milestone and royalty payments from Alnylam. We retained exclusive rights to our patents for single-stranded antisense therapeutics and for a
limited number of double-stranded RNAi therapeutic targets and all rights to single-stranded RNAi, or ssRNAi, therapeutics. In turn, Alnylam nonexclusively licensed to us its patent estate relating to antisense motifs and mechanisms and
oligonucleotide chemistry to research, develop and commercialize single-stranded antisense therapeutics, ssRNAi therapeutics, and to research double-stranded RNAi compounds. We also received a license to develop and commercialize double-stranded RNAi
therapeutics targeting a limited number of therapeutic targets on a nonexclusive basis. Additionally, in 2015, we and Alnylam entered into an alliance in which we cross-licensed intellectual property. Under this alliance, we and Alnylam each obtained
exclusive license rights to four therapeutic programs. Alnylam granted us an exclusive, royalty-bearing license to its chemistry, RNA targeting mechanism and target-specific intellectual property for oligonucleotides against four targets, including
FXI and Apo(a) and two other targets. In exchange, we granted Alnylam an exclusive, royalty-bearing license to our chemistry, RNA targeting mechanism and target-specific intellectual property for oligonucleotides against four other targets. Alnylam
also granted us a royalty-bearing, non-exclusive license to new platform technology arising from May 2014 through April 2019 for single-stranded antisense therapeutics. In turn, we granted Alnylam a royalty-bearing, non-exclusive license to new
platform technology arising from May 2014 through April 2019 for double-stranded RNAi therapeutics.
In the fourth quarter 2020, we completed an arbitration process with Alnylam. The arbitration panel awarded us $41.2 million for payments owed to us by Alnylam related to Alnylam’s agreement with Sanofi Genzyme. We recognized the $41.2 million payment from Alnylam as revenue in the fourth quarter of 2020 because we did not have any performance obligations for the respective payment.
During the years ended December 31, 2021, 2020 and 2019, we
earned the following revenue from our relationship with Alnylam (in millions, except percentage amounts):
|
Year Ended December 31,
|
||||||||||||
|
2021
|
2020
|
2019
|
||||||||||
|
R&D revenue
|
$
|
|
$
|
|
$
|
|
||||||
|
Percentage of total revenue
|
|
|
%
|
|
%
|
|||||||
We did no
7. Akcea Merger
Purchase Price and Direct Transaction Costs Accounting for the Akcea Merger
In October 2020, we reacquired the shares of Akcea’s common stock we did not own, increasing our ownership from 76 percent to 100 percent. Under the purchase
agreement, we purchased 24.8 million shares at $18.15
per share, resulting in a total purchase price of $450.6 million.
To reflect our 100 percent
ownership, we accounted for the increase in our ownership by eliminating the noncontrolling interest adjustment in stockholders’ equity in accordance with the Consolidation accounting guidance (ASC Topic 810). We recognized the difference between the
purchase price and the adjustment to noncontrolling interest in stockholders’ equity as additional-paid-in capital. Refer to our Statement of
Stockholders’ Equity for detailed amounts.
We accounted for the transaction costs related to the Akcea Merger as a direct charge to stockholders’ equity. We incurred
$40.6 million of direct transaction costs from the Akcea Merger, primarily comprised of banking and legal fees.
Equity Award Payouts related to the Akcea Merger
In October 2020, as part of the Akcea Merger,
Ionis cancelled all of Akcea’s equity awards. In exchange for the cancelled awards, if eligible under the terms of the Akcea Merger, we paid holder’s a cash payment. We paid $18.15 for each outstanding RSU. For each outstanding option with an exercise price less than $18.15 , we paid $18.15 less the exercise price. As a result, we paid out $53.4 million in the fourth quarter of 2020 related to Akcea’s
cancelled equity awards. We accounted for these payments as part of the transaction costs recorded to stockholders’ equity in the fourth quarter of 2020. Because we did not replace the Akcea awards, we recognized all unrecognized non-cash
stock-based compensation ($59.3 million) under Akcea’s Plan in our statement of operations in the post-merger period in the fourth quarter of
2020.
Severance and Retention Costs related to the Akcea Merger
As a result of the Akcea Merger, we incurred severance and retention expenses of $27.0 million. During 2021 and 2020, we recorded $11.7 million and $15.3 million of severance and retention related costs in operating expenses, respectively. As of December 31, 2021, we have recognized all severance and
retention costs related to the Akcea Merger.
The following table summarizes the severance and retention expenses related to the Akcea Merger that we recognized for the periods indicated (in
millions):
|
Year Ended
December 31, 2021
|
Year Ended
December 31, 2020
|
|||||||
|
R&D expenses
|
$
|
|
$
|
|
||||
|
SG&A expenses
|
|
|
||||||
|
Total
|
$
|
|
$
|
|
||||
The following table summarizes the severance and retention reserve amounts related to the Akcea Merger that we included in
accrued compensation for the period indicated (in millions):
|
Year Ended
December 31, 2021
|
||||
|
Beginning balance as of January 1, 2021
|
$
|
|
||
|
Amount expensed during the year
|
|
|||
|
Reserve adjustments during the year
|
(
|
)
|
||
|
Net amount expensed during the year
|
|
|||
|
Amounts paid during the year
|
(
|
)
|
||
|
Ending balance as of December 31, 2021
|
$
|
|
||
The reserve adjustments during the period primarily related to forfeitures of severance and retention payments as a result of employee
terminations before they earned the amounts.
8. Severance and Retention Costs related to our
Restructured Operations
Restructured European Operations
In the fourth quarter of 2020, we
entered into a distribution agreement with Sobi to commercialize TEGSEDI and WAYLIVRA in Europe. Under the distribution agreement, Sobi took over all material distribution operations at the end of January 2021. We remain the marketing authorization holder for TEGSEDI and WAYLIVRA in Europe. We will continue to maintain limited European operations including regulatory, manufacturing, and the
management of relationships with key opinion leaders. We will also continue to lead the TEGSEDI and WAYLIVRA global commercial strategy.
As a result of this change, we incurred severance and retention expenses of $14.2 million. During 2021 and 2020, we recorded $1.7 million and $12.5 million of severance and retention related costs in operating expenses, respectively. As of December 31, 2021, we have recognized all severance and
retention costs related to this agreement.
The following table summarizes the
severance and retention expenses related to our restructured European operations that we recognized for the periods indicated (in millions):
|
Year Ended
December 31, 2021
|
Year Ended
December 31, 2020
|
|||||||
|
R&D expenses
|
$
|
|
$
|
|
||||
|
SG&A expenses
|
|
|
||||||
|
Total
|
$
|
|
$
|
|
||||
The following table summarizes the severance and retention reserve amounts related to our restructured European operations
that we included in accrued compensation for the period indicated (in millions):
|
Year Ended
December 31, 2021
|
||||
|
Beginning balance as of January 1, 2021
|
$
|
|
||
|
Amount expensed during the year
|
|
|||
|
Reserve adjustments during the year
|
(
|
)
|
||
|
Net amount expensed during the year
|
|
|||
|
Amounts paid during the year
|
(
|
)
|
||
|
Ending balance as of December 31, 2021
|
$
|
|
||
The reserve adjustments during the period primarily related to tax expense adjustments.
Restructured North American TEGSEDI Operations
In April 2021, we entered into a distribution agreement with Sobi for TEGSEDI in North America. Under the terms of the
distribution agreement, we will retain the marketing authorizations for TEGSEDI in the U.S. and Canada. We will continue to supply commercial product to Sobi and manage regulatory and manufacturing processes, as well as relationships with key opinion
leaders. We will also continue to lead the TEGSEDI global commercial strategy. Sobi will otherwise have responsibility for commercializing TEGSEDI in the U.S. and Canada.
In connection with restructuring our North American TEGSEDI operations, or Restructured North American TEGSEDI Operations,
we enacted a plan to reorganize our Akcea workforce in North America to better align with the needs of our business and to focus on our wholly owned pipeline.
The following table summarizes the severance expenses related to our
Restructured North American TEGSEDI Operations that we recognized for the period indicated (in millions):
|
Year Ended
December 31, 2021
|
||||
|
R&D expenses
|
$
|
|
||
|
SG&A expenses
|
|
|||
|
Total
|
$
|
|
||
We recognized all severance expenses related to our Restructured North American
TEGSEDI Operations during the three months ended June 30, 2021.
The following table summarizes the severance reserve amounts related to our
Restructured North American TEGSEDI Operations that we included in accrued compensation for the period indicated (in millions):
|
Year Ended
December 31, 2021
|
||||
|
Beginning balance as of January 1, 2021
|
$
|
|
||
|
Net amount expensed during the year
|
|
|||
|
Amounts paid during the year
|
(
|
)
|
||
|
Ending balance as of December 31, 2021
|
$
|
|
||
9. Employment Benefits
We have employee 401(k) salary deferral plans covering all employees. Employees could make contributions by withholding a
percentage of their salary up to the IRS annual limits of $20,500 and $27,000 in 2021 for employees under 50 years old and employees 50 years old or over,
respectively. We made approximately $5.5 million, $5.7
million and $6.4 million in matching contributions for the years ended December 31, 2021, 2020 and 2019, respectively.
10. Legal Proceedings
From time to time, we are involved in legal proceedings arising in the ordinary course of our business. Periodically, we
evaluate the status of each legal matter and assess our potential financial exposure. If the potential loss from any legal proceeding is considered probable and the amount can be reasonably estimated, we accrue a liability for the estimated loss.
Significant judgment is required to determine the probability of a loss and whether the amount of the loss is reasonably estimable. The outcome of any proceeding is not determinable in advance. As a result, the assessment of a potential liability and
the amount of accruals recorded are based only on the information available to us at the time. As additional information becomes available, we reassess the potential liability related to the legal proceeding, and may revise our estimates.
On August 5, 2021, four purported former stockholders of Akcea filed an action in the Delaware
Court of Chancery captioned John Makris, et al. v. Ionis Pharmaceuticals, Inc., et al., C.A. No. 2021-0681, or the “Delaware Action.” The plaintiffs in the Delaware Action assert claims against (i) former members of Akcea’s board of directors; and
(ii) Ionis, or collectively, the “Defendants.” The plaintiffs assert putatively direct claims on behalf of a purported class of former Akcea stockholders. The plaintiffs in the Delaware Action assert that the Defendants breached their fiduciary
duties in connection with the October 2020 take-private transaction that we and Akcea entered into, in which Akcea became a wholly-owned subsidiary of Ionis. We believe that the claims asserted in the Delaware Action are without merit and filed a
motion to dismiss the claims in November 2021. Briefing on the motion to dismiss is ongoing, and pursuant to an agreed-upon scheduling order that has been entered by the Court, argument on the motion to dismiss is expected later in the first quarter
of 2022.
On January 19, 2022, a purported stockholder of Ionis filed a stockholder derivative complaint in the Delaware Court of Chancery captioned
Leo Shumacher, et al. v. Joseph Loscalzo, et al., C.A. No. 2022-0059, or the “Action.” The complaint names as defendants the current members of Ionis’ board of directors, collectively the Directors. The company is a nominal defendant. Plaintiff asserts
a breach of fiduciary duty claim against the Directors for awarding and receiving allegedly excessive compensation. Plaintiff also asserts an unjust enrichment claim against the non-employee Directors as a result of the compensation they received. The
complaint seeks, among other things, damages, restitution, attorneys’ fees and costs, and such other relief as deemed just and proper by the court. Defendants have not yet responded to the complaint in this Action.
11. Fourth Quarter Financial Data (Unaudited)
The following financial information reflects all normal recurring adjustments, which are,
in the opinion of management, necessary for a fair statement of the results of the interim periods. Summarized fourth quarter data for 2021
and 2020 are as follows (in thousands, except per share data).
|
Three Months Ended December 31,
|
2021
|
2020
|
||||||
|
Revenue
|
$
|
|
$
|
|
||||
|
Operating expenses
|
$
|
|
$
|
|
||||
|
Income (loss) from operations
|
$
|
|
$
|
(
|
)
|
|||
|
Net income (loss)
|
$
|
|
$
|
(
|
)
|
|||
|
Net income (loss) attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
|
$
|
(
|
)
|
|||
|
Basic net income (loss) per share (1) (2)
|
$
|
|
$
|
(
|
)
|
|||
|
Diluted net income (loss) per share (1) (3)
|
$
|
|
$
|
(
|
)
|
|||
________________
| (1) |
|
| (2) |
|
Our basic net loss per share for the fourth quarter of 2020 was calculated as follows (in thousands, except per share amounts):
|
Three Months Ended December 31, 2020
|
Weighted
Average Shares
Owned in Akcea
|
Akcea’s
Net Loss
Per Share
|
Basic Net Loss
Per Share
Calculation
|
|||||||||
|
Akcea’s net loss in the pre-merger period attributable to our ownership
|
|
$
|
(
|
)
|
$
|
(
|
)
|
|||||
|
Akcea’s net loss in the post-merger period attributable to our ownership
|
(
|
)
|
||||||||||
|
Akcea’s total net loss attributable to our ownership
|
$
|
(
|
)
|
|||||||||
|
Ionis’ stand-alone net loss
|
(
|
)
|
||||||||||
|
Net loss available to Ionis common stockholders
|
$
|
(
|
)
|
|||||||||
|
Weighted average shares outstanding
|
|
|||||||||||
|
Basic net loss per share
|
$
|
(
|
)
|
|||||||||
| (3) |
|
|
Three Months Ended December 31, 2021
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
Net income available to Ionis common stockholders
|
$
|
|
|
$
|
|
|||||||
|
Effect of dilutive securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
|
||||||||||
|
Shares issuable related to our
|
|
|
||||||||||
|
Shares issuable related to our
|
|
|
||||||||||
|
Shares issuable related to our
|
|
|
||||||||||
|
Income available to Ionis common stockholders, plus assumed conversions
|
$
|
|
|
$
|
|
|||||||